6 September 2017 - Case of the Week #435
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Week page. To subscribe or unsubscribe to Case of the Week or our other email lists, click here.
Thanks to Daniel Eligio Diaz, M.D., M.Sc., SUNY Downstate Medical Center in Brooklyn, New York for contributing this case, Dr. Belinda Lategan, St. Boniface Hospital, Winnipeg, Manitoba (Canada), for writing the discussion, and Dr. Andrey Bychkov, Chulalongkorn University, Bangkok (Thailand) for editing the discussion. To contribute a Case of the Week, first make sure that we are currently accepting cases, then follow the guidelines on our main Case of the Week page.
Advertisement
Website news:
(1) We have posted 10 new CMEs from USCAP.
(2) We continuously update the Book pages on our website with new titles and new editions. Please visit Amazon.com and purchase your books through our Amazon.com links to support PathologyOutlines.
(3) We have improved the Cite This Page feature by adding the Author(s) last name and initials. This feature is located below the Table of Contents on each Topic Page that has been converted to our new format. We will add the Author(s) names going forward as topics are updated.
Example:
Cite this page: Gellert, L. L. Paraganglioma. PathologyOutlines.com website. http://www.pathologyoutlines.com/topic/adrenalparaganglioma.html. Accessed September 2nd, 2017.
Visit and follow our Blog to see recent updates to the website.
Case of the Week #435
Clinical history:
A 26 year old African American woman with a history of hypothyroidism secondary to Hashimoto thyroiditis presented to the Emergency Department with shortness of breath and throat tightness for 1 day. She recalled a prior thyroid biopsy (report not available) a year previous that was benign. Thyroid ultrasound showed a 5.9 cm nodule in the right lobe. CT scan of the neck showed a large right thyroid nodule with tracheal deviation to the left. She underwent an FNA in the ENT clinic, with a cytological diagnosis in the Bethesda IIB (benign) category. The patient elected to have a right hemithyroidectomy due to compressive symptoms and for cosmetic defects.
Histopathology images:
What is your diagnosis?
Diagnosis:
Thyroid, right, hemithyroidectomy: encapsulated poorly differentiated thyroid carcinoma with extensive (19 foci) vascular invasion (5.3 cm)
Test question (answer at the end):
Which of the following features are not required for the diagnosis of poorly differentiated thyroid carcinoma
A. Solid, trabecular or insular growth
B. Vascular invasion, high grade nuclei and increased mitotic activity
C. Absence of conventional nuclear features of papillary thyroid carcinoma
D. Necrosis
Discussion:
Poorly differentiated thyroid carcinoma (PDTC, old term "insular carcinoma") is a rare distinct subtype of thyroid carcinoma with a prognosis and clinical behavior intermediate between the well differentiated thyroid carcinomas (follicular and papillary) and anaplastic thyroid carcinoma. PDTC arises from and often coexists with well differentiated tumors through the accumulation of key additional genetic abnormalities. These carcinomas are primary thyroid carcinomas derived from thyroid follicular cells. At least 50% of the tumor must fulfill the Turin diagnostic criteria (Am J Surg Pathol 2007;31:1256):
1. Solid, trabecular or insular growth pattern
2. No conventional nuclear features of papillary carcinoma, and
3. Demonstrate at least one of the following:
a. Convoluted nuclei
b. Mitotic activity (3 or more per 10 HPF)
c. Necrosis
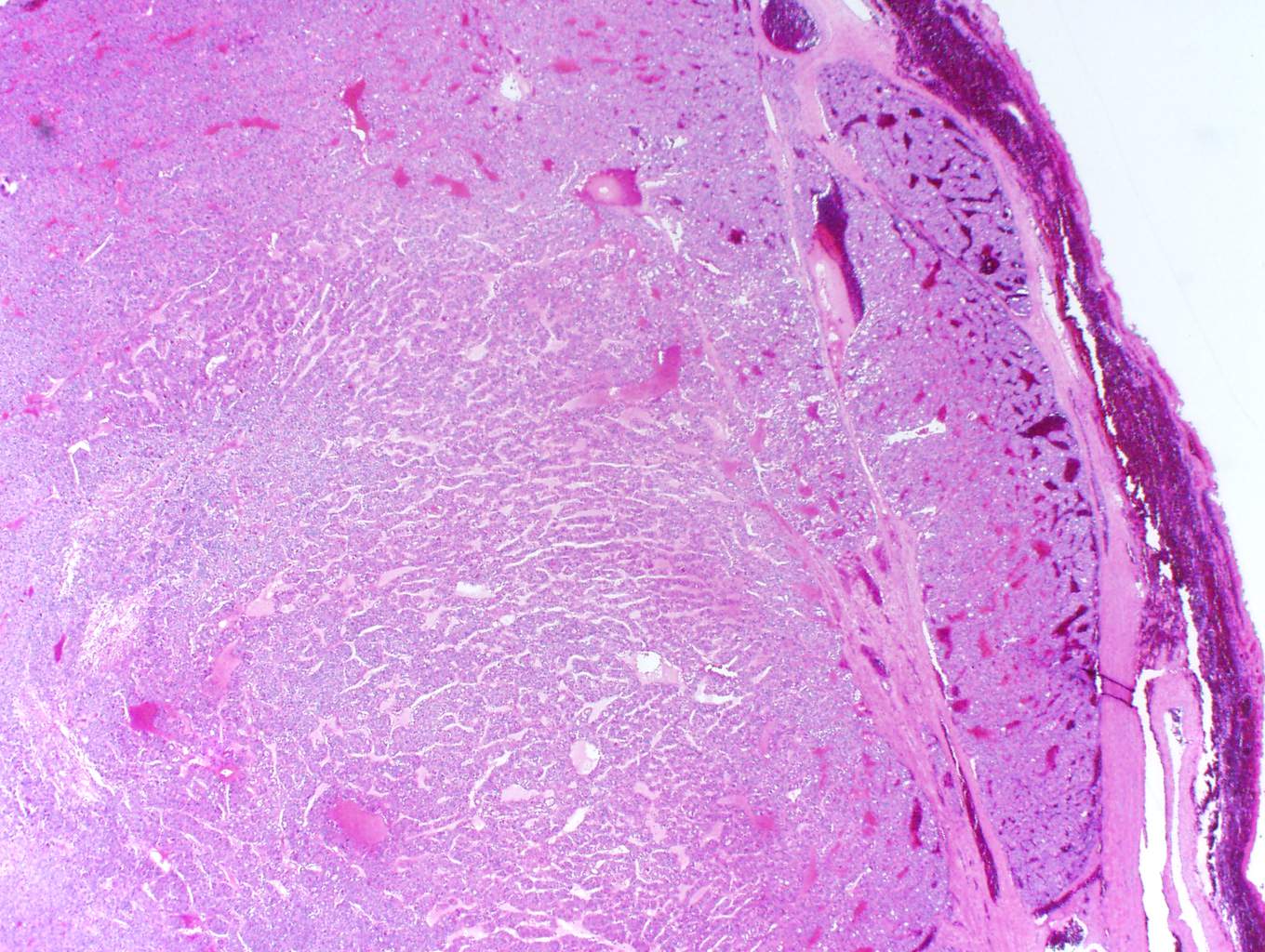
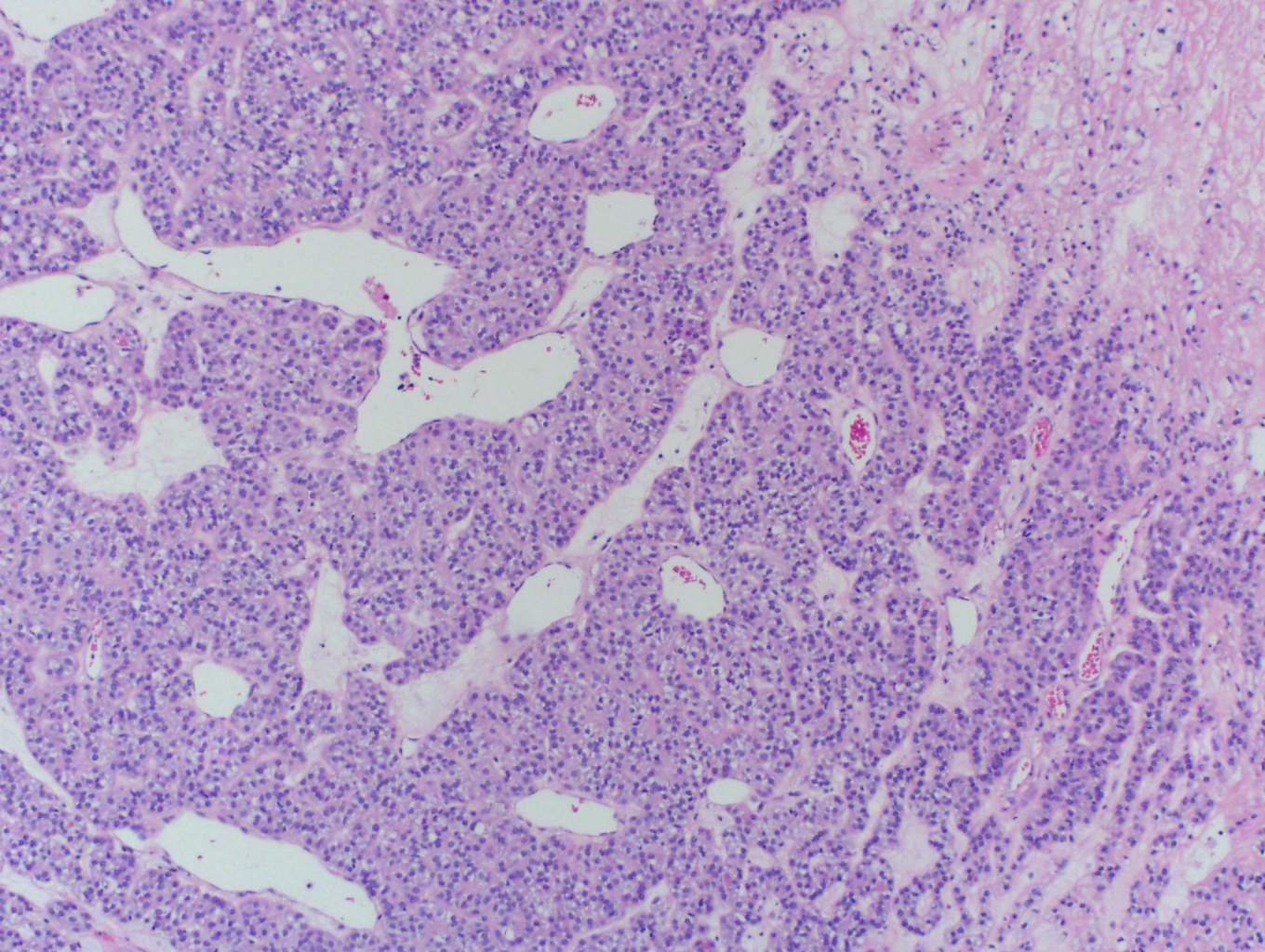
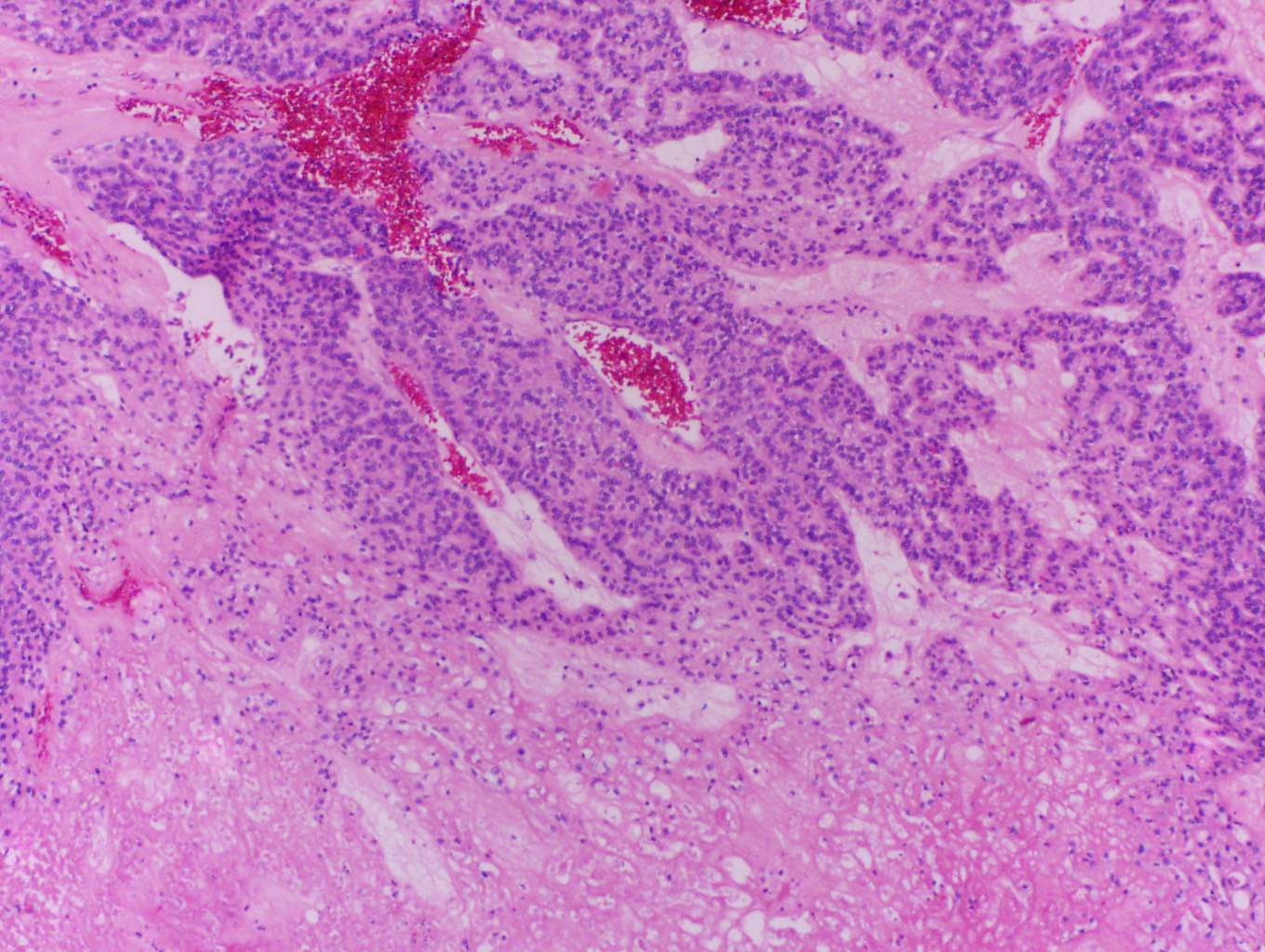
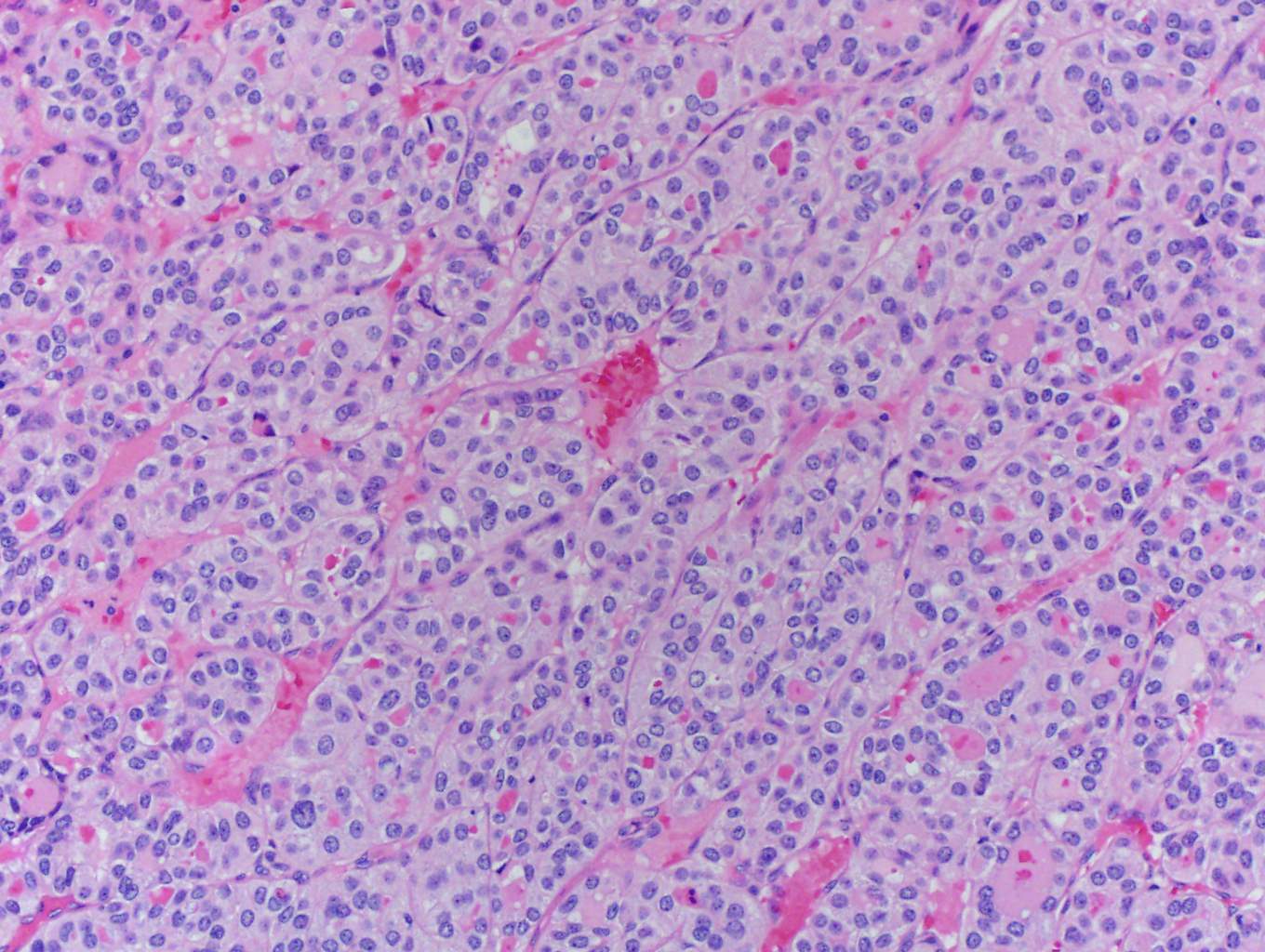
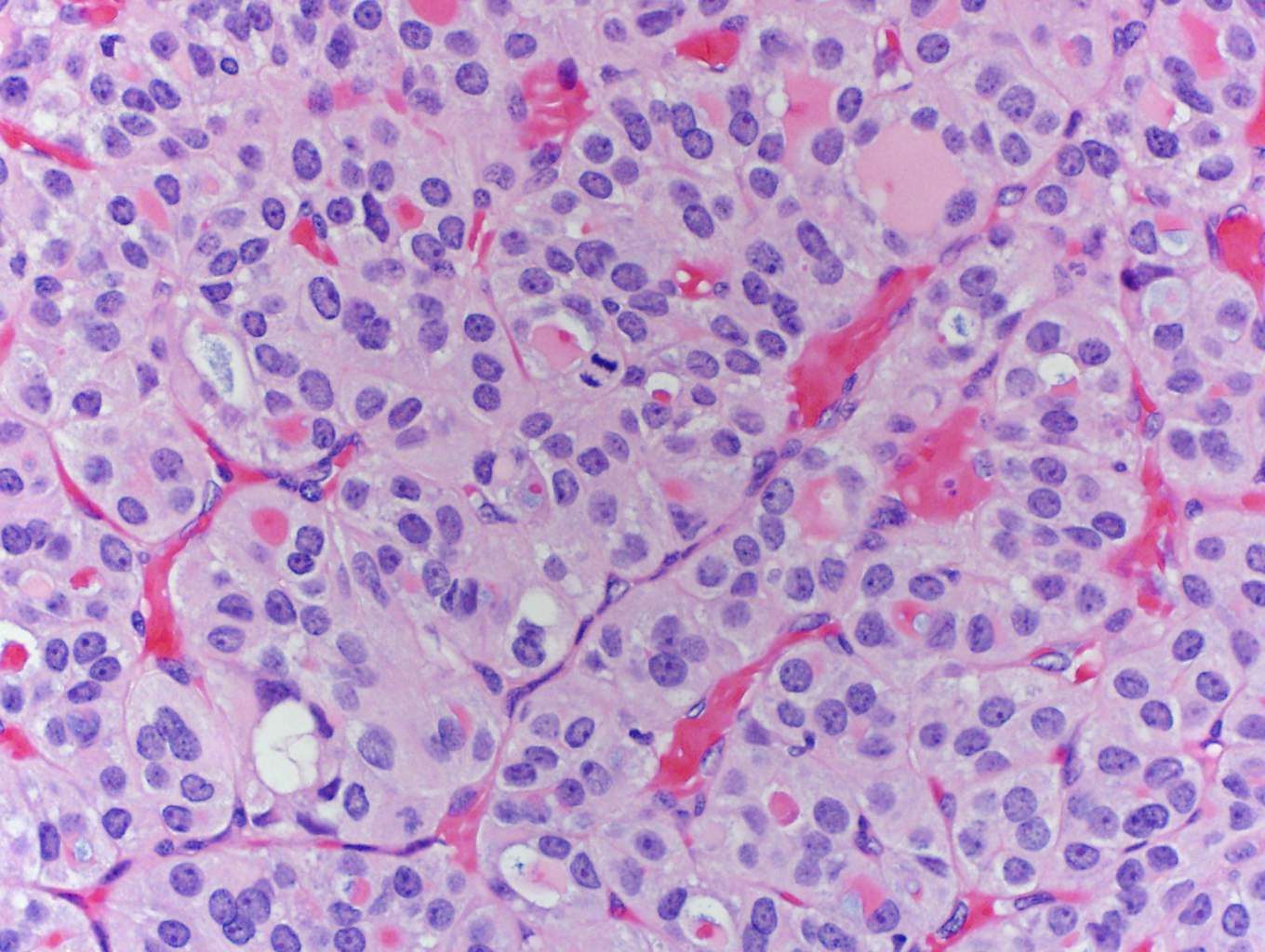
In this case, trabecular (image 1) and solid (images 4 and 5) patterns were predominant. On low power, large tumor plugs are visible within the capsular veins (image 1). High magnification disclosed microscopic areas of necrosis (images 2 and 3) and mitotic activity (image 5). This case likely progressed from a pre-existing follicular carcinoma, which implies absence of nuclear features of papillary carcinoma. The criteria also apply to tumors composed of other distinct cell morphologies like clear cell or oncocytic (Hurthle) cells (WHO Classification of Tumors of the Endocrine Organs, 2017, 4th ed, Mod Pathol 2010;23:1269). Other morphologic features of PDTC include pleomorphic tumor cells lacking a follicular (organoid) growth pattern and small globoid cells resembling fetal thyroid cells. Necrosis may also be so extensive as to result in a peritheliomatous pattern (perivascular tumor cells remain viable with extensive necrosis away from vessels). Note that any component of PDTC in an otherwise well differentiated thyroid carcinoma should be mentioned in the pathology report as it may drive prognosis.
There is a distinct regional preponderance of PDTC in Northern Italy and Latin America (4% - 7% of all thyroid carcinomas) compared to North America (~1.8%). Women are more commonly affected than men, as are those older than age 50. Patients may present with solitary masses, rapidly enlarging nodules in a background of previously stable nodular goiter or metastases to regional nodes, lung and bone. PDTC nodules are "cold" on scintigraphy (WHO Classification of Tumors of the Endocrine Organs, 2017, 4th ed).
PDTC is not synonymous with "high grade thyroid carcinoma" and must be differentiated from other types of thyroid carcinoma with solid growth patterns (e.g. solid variant of papillary thyroid carcinoma, medullary thyroid carcinoma and anaplastic thyroid carcinoma) as well as parathyroid neoplasms and metastatic carcinoma. Anaplastic thyroid carcinoma is "wildly" pleomorphic and displays no evidence of thyroid follicular differentiation by morphology and IHC (i.e. negative for thyroglobulin and TTF1), but PDTC is positive for thyroglobulin (often in a perinuclear dot-like pattern) and positive for TTF1. Ki-67 may help distinguish well differentiated thyroid cancer (<10%), PDTC (10-30%) and highly aggressive anaplastic carcinoma (>30%) (Pathol Int 2017;67:179). Medullary thyroid carcinoma also has a nested pattern of growth and is negative for thyroglobulin but is positive for calcitonin and also produces amyloid (birefringence under polarized light with Congo Red staining (Head Neck Pathol 2015;9:16).
Fine needle cytology aspirates (FNA) of PDTC are typically cellular with clusters of cells or trabecular arrangements (often with microfollicles), scant colloid and a necrotic background. The cells are mildly atypical with poorly defined cytoplasm and cytoplasmic vacuoles. In a representative FNA specimen, these features would result in a diagnostic Bethesda category VI (malignant), although category IV (follicular neoplasm / suspicious for follicular neoplasm) or category V (suspicious for malignancy) may also apply depending on the features present (Am J Clin Pathol 2009;132:658). In this case, the cytologic - histologic discrepancy was apparently explained by sampling error. In addition, a benign diagnosis for a 5.9 cm thyroid nodule should be treated with skepticism.
While BRAFV600E and RAS mutations remain the main drivers in aggressive thyroid carcinoma, PDTC gains additional mutations, including TERT promoter mutation (30-40%) and TP53 mutation (10-40%) (Endocr Pathol 2016;27:205). RAS mutated PDTCs (20-40%) are commonly associated with a preexisting follicular patterned thyroid carcinoma, whereas BRAF mutated PDTCs (5-30%) progress from classic or tall cell papillary carcinoma. Late genetic events are common: CTNNB1 (0 - 25%) and genes that encode effectors of the PI3K-AKT signaling pathway, including PIK3CA, AKT1 and PTEN (10 - 20% collectively).
PDTC is more clinically aggressive than differentiated thyroid cancers. It is associated with a high risk of cancer recurrence, spread to regional lymph nodes, lung, bones and increased risk of death. Thyroidectomy often followed by radioactive iodine ablation is the mainstay of treatment for PDTC. Adjuvant therapy with external beam radiation may be required in certain cases. Some patients with advanced disease may be candidates for molecular targeted therapies. PDTCs have significantly worse outcome as compared to well differentiated papillary and follicular thyroid carcinomas, with a 10 year survival of around 50% (Thyroid 2016;26:1). pT4a stage and M1 are independent predictors of worse survival (J Clin Endocrinol Metab 2014;99:1245).
Test Question Answer:
B. Vascular invasion, high grade nuclei and increased mitotic activity.
The Turin criteria specify solid / trabecular / insular growth, lack of conventional nuclear features of papillary thyroid carcinoma, and one of the following: necrosis, convoluted nuclei and/or increased mitotic activity (3 or more mitoses/10 HPF). Vascular invasion may be seen in a variety of thyroid carcinomas and is an adverse prognostic factor regardless of histologic subtype or grade.
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Week page. To subscribe or unsubscribe to Case of the Week or our other email lists, click here.
Thanks to Daniel Eligio Diaz, M.D., M.Sc., SUNY Downstate Medical Center in Brooklyn, New York for contributing this case, Dr. Belinda Lategan, St. Boniface Hospital, Winnipeg, Manitoba (Canada), for writing the discussion, and Dr. Andrey Bychkov, Chulalongkorn University, Bangkok (Thailand) for editing the discussion. To contribute a Case of the Week, first make sure that we are currently accepting cases, then follow the guidelines on our main Case of the Week page.

February 19 - 23, 2018
Hyatt Regency Maui Resort & Spa at Kaanapali
Maui, Hawaii (USA)
A Review of GI and Liver Pathology: Evolving Concepts and Clinical Implications

Website
Hotel
Register
[#6512a]
Website news:
(1) We have posted 10 new CMEs from USCAP.
(2) We continuously update the Book pages on our website with new titles and new editions. Please visit Amazon.com and purchase your books through our Amazon.com links to support PathologyOutlines.
(3) We have improved the Cite This Page feature by adding the Author(s) last name and initials. This feature is located below the Table of Contents on each Topic Page that has been converted to our new format. We will add the Author(s) names going forward as topics are updated.
Example:
Cite this page: Gellert, L. L. Paraganglioma. PathologyOutlines.com website. http://www.pathologyoutlines.com/topic/adrenalparaganglioma.html. Accessed September 2nd, 2017.
Visit and follow our Blog to see recent updates to the website.
Case of the Week #435
Clinical history:
A 26 year old African American woman with a history of hypothyroidism secondary to Hashimoto thyroiditis presented to the Emergency Department with shortness of breath and throat tightness for 1 day. She recalled a prior thyroid biopsy (report not available) a year previous that was benign. Thyroid ultrasound showed a 5.9 cm nodule in the right lobe. CT scan of the neck showed a large right thyroid nodule with tracheal deviation to the left. She underwent an FNA in the ENT clinic, with a cytological diagnosis in the Bethesda IIB (benign) category. The patient elected to have a right hemithyroidectomy due to compressive symptoms and for cosmetic defects.
Histopathology images:
What is your diagnosis?
Diagnosis:
Thyroid, right, hemithyroidectomy: encapsulated poorly differentiated thyroid carcinoma with extensive (19 foci) vascular invasion (5.3 cm)
Test question (answer at the end):
Which of the following features are not required for the diagnosis of poorly differentiated thyroid carcinoma
A. Solid, trabecular or insular growth
B. Vascular invasion, high grade nuclei and increased mitotic activity
C. Absence of conventional nuclear features of papillary thyroid carcinoma
D. Necrosis
Discussion:
Poorly differentiated thyroid carcinoma (PDTC, old term "insular carcinoma") is a rare distinct subtype of thyroid carcinoma with a prognosis and clinical behavior intermediate between the well differentiated thyroid carcinomas (follicular and papillary) and anaplastic thyroid carcinoma. PDTC arises from and often coexists with well differentiated tumors through the accumulation of key additional genetic abnormalities. These carcinomas are primary thyroid carcinomas derived from thyroid follicular cells. At least 50% of the tumor must fulfill the Turin diagnostic criteria (Am J Surg Pathol 2007;31:1256):
1. Solid, trabecular or insular growth pattern
2. No conventional nuclear features of papillary carcinoma, and
3. Demonstrate at least one of the following:
a. Convoluted nuclei
b. Mitotic activity (3 or more per 10 HPF)
c. Necrosis
In this case, trabecular (image 1) and solid (images 4 and 5) patterns were predominant. On low power, large tumor plugs are visible within the capsular veins (image 1). High magnification disclosed microscopic areas of necrosis (images 2 and 3) and mitotic activity (image 5). This case likely progressed from a pre-existing follicular carcinoma, which implies absence of nuclear features of papillary carcinoma. The criteria also apply to tumors composed of other distinct cell morphologies like clear cell or oncocytic (Hurthle) cells (WHO Classification of Tumors of the Endocrine Organs, 2017, 4th ed, Mod Pathol 2010;23:1269). Other morphologic features of PDTC include pleomorphic tumor cells lacking a follicular (organoid) growth pattern and small globoid cells resembling fetal thyroid cells. Necrosis may also be so extensive as to result in a peritheliomatous pattern (perivascular tumor cells remain viable with extensive necrosis away from vessels). Note that any component of PDTC in an otherwise well differentiated thyroid carcinoma should be mentioned in the pathology report as it may drive prognosis.
There is a distinct regional preponderance of PDTC in Northern Italy and Latin America (4% - 7% of all thyroid carcinomas) compared to North America (~1.8%). Women are more commonly affected than men, as are those older than age 50. Patients may present with solitary masses, rapidly enlarging nodules in a background of previously stable nodular goiter or metastases to regional nodes, lung and bone. PDTC nodules are "cold" on scintigraphy (WHO Classification of Tumors of the Endocrine Organs, 2017, 4th ed).
PDTC is not synonymous with "high grade thyroid carcinoma" and must be differentiated from other types of thyroid carcinoma with solid growth patterns (e.g. solid variant of papillary thyroid carcinoma, medullary thyroid carcinoma and anaplastic thyroid carcinoma) as well as parathyroid neoplasms and metastatic carcinoma. Anaplastic thyroid carcinoma is "wildly" pleomorphic and displays no evidence of thyroid follicular differentiation by morphology and IHC (i.e. negative for thyroglobulin and TTF1), but PDTC is positive for thyroglobulin (often in a perinuclear dot-like pattern) and positive for TTF1. Ki-67 may help distinguish well differentiated thyroid cancer (<10%), PDTC (10-30%) and highly aggressive anaplastic carcinoma (>30%) (Pathol Int 2017;67:179). Medullary thyroid carcinoma also has a nested pattern of growth and is negative for thyroglobulin but is positive for calcitonin and also produces amyloid (birefringence under polarized light with Congo Red staining (Head Neck Pathol 2015;9:16).
Fine needle cytology aspirates (FNA) of PDTC are typically cellular with clusters of cells or trabecular arrangements (often with microfollicles), scant colloid and a necrotic background. The cells are mildly atypical with poorly defined cytoplasm and cytoplasmic vacuoles. In a representative FNA specimen, these features would result in a diagnostic Bethesda category VI (malignant), although category IV (follicular neoplasm / suspicious for follicular neoplasm) or category V (suspicious for malignancy) may also apply depending on the features present (Am J Clin Pathol 2009;132:658). In this case, the cytologic - histologic discrepancy was apparently explained by sampling error. In addition, a benign diagnosis for a 5.9 cm thyroid nodule should be treated with skepticism.
While BRAFV600E and RAS mutations remain the main drivers in aggressive thyroid carcinoma, PDTC gains additional mutations, including TERT promoter mutation (30-40%) and TP53 mutation (10-40%) (Endocr Pathol 2016;27:205). RAS mutated PDTCs (20-40%) are commonly associated with a preexisting follicular patterned thyroid carcinoma, whereas BRAF mutated PDTCs (5-30%) progress from classic or tall cell papillary carcinoma. Late genetic events are common: CTNNB1 (0 - 25%) and genes that encode effectors of the PI3K-AKT signaling pathway, including PIK3CA, AKT1 and PTEN (10 - 20% collectively).
PDTC is more clinically aggressive than differentiated thyroid cancers. It is associated with a high risk of cancer recurrence, spread to regional lymph nodes, lung, bones and increased risk of death. Thyroidectomy often followed by radioactive iodine ablation is the mainstay of treatment for PDTC. Adjuvant therapy with external beam radiation may be required in certain cases. Some patients with advanced disease may be candidates for molecular targeted therapies. PDTCs have significantly worse outcome as compared to well differentiated papillary and follicular thyroid carcinomas, with a 10 year survival of around 50% (Thyroid 2016;26:1). pT4a stage and M1 are independent predictors of worse survival (J Clin Endocrinol Metab 2014;99:1245).
Test Question Answer:
B. Vascular invasion, high grade nuclei and increased mitotic activity.
The Turin criteria specify solid / trabecular / insular growth, lack of conventional nuclear features of papillary thyroid carcinoma, and one of the following: necrosis, convoluted nuclei and/or increased mitotic activity (3 or more mitoses/10 HPF). Vascular invasion may be seen in a variety of thyroid carcinomas and is an adverse prognostic factor regardless of histologic subtype or grade.