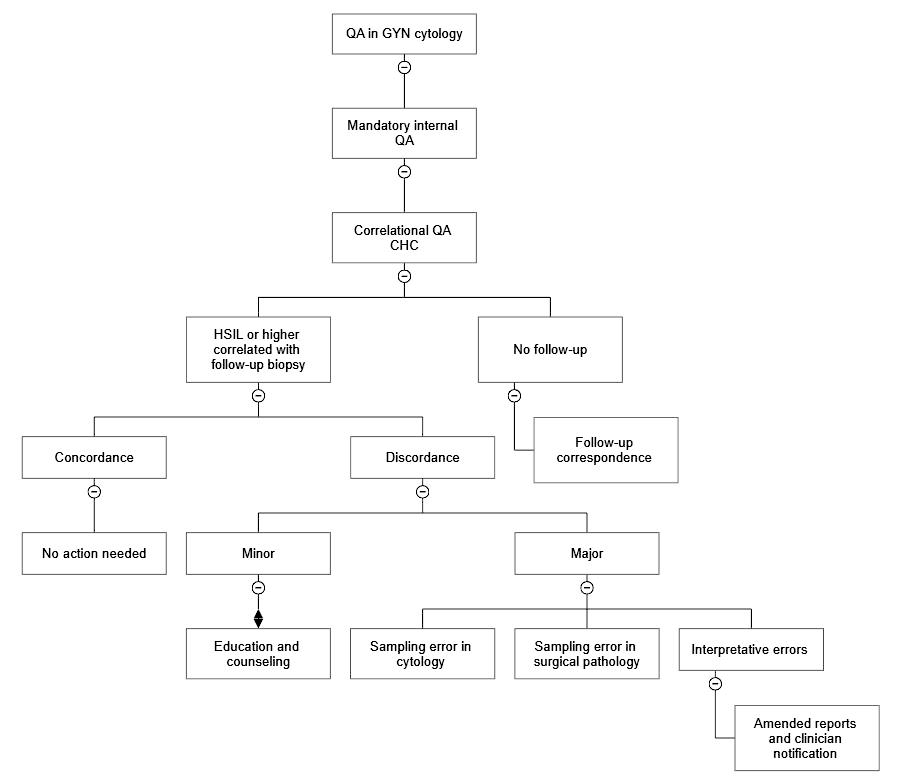
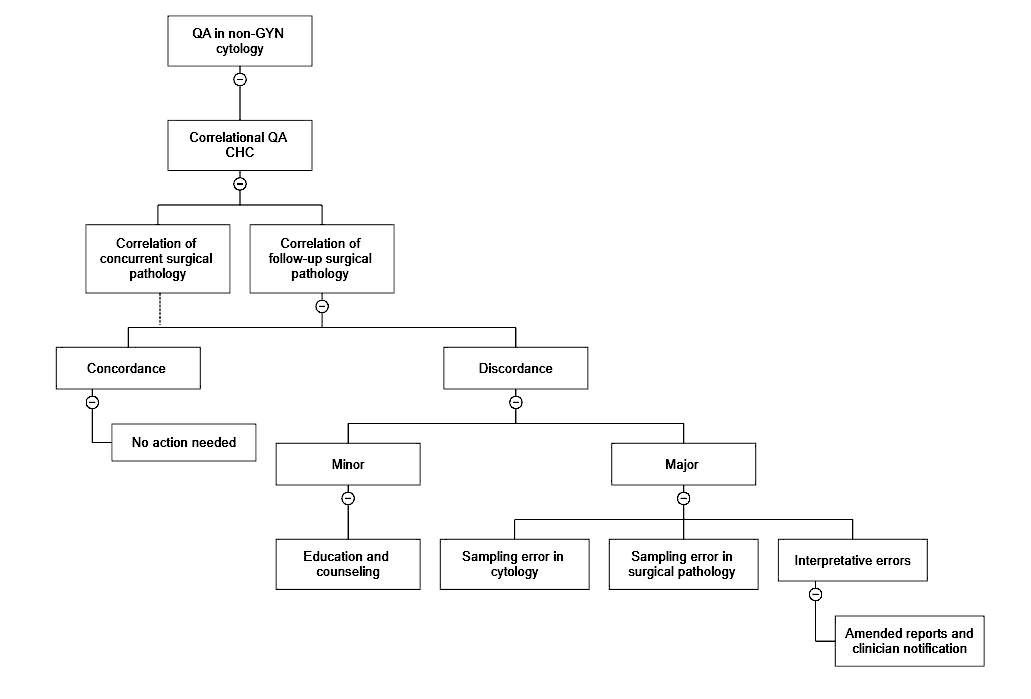
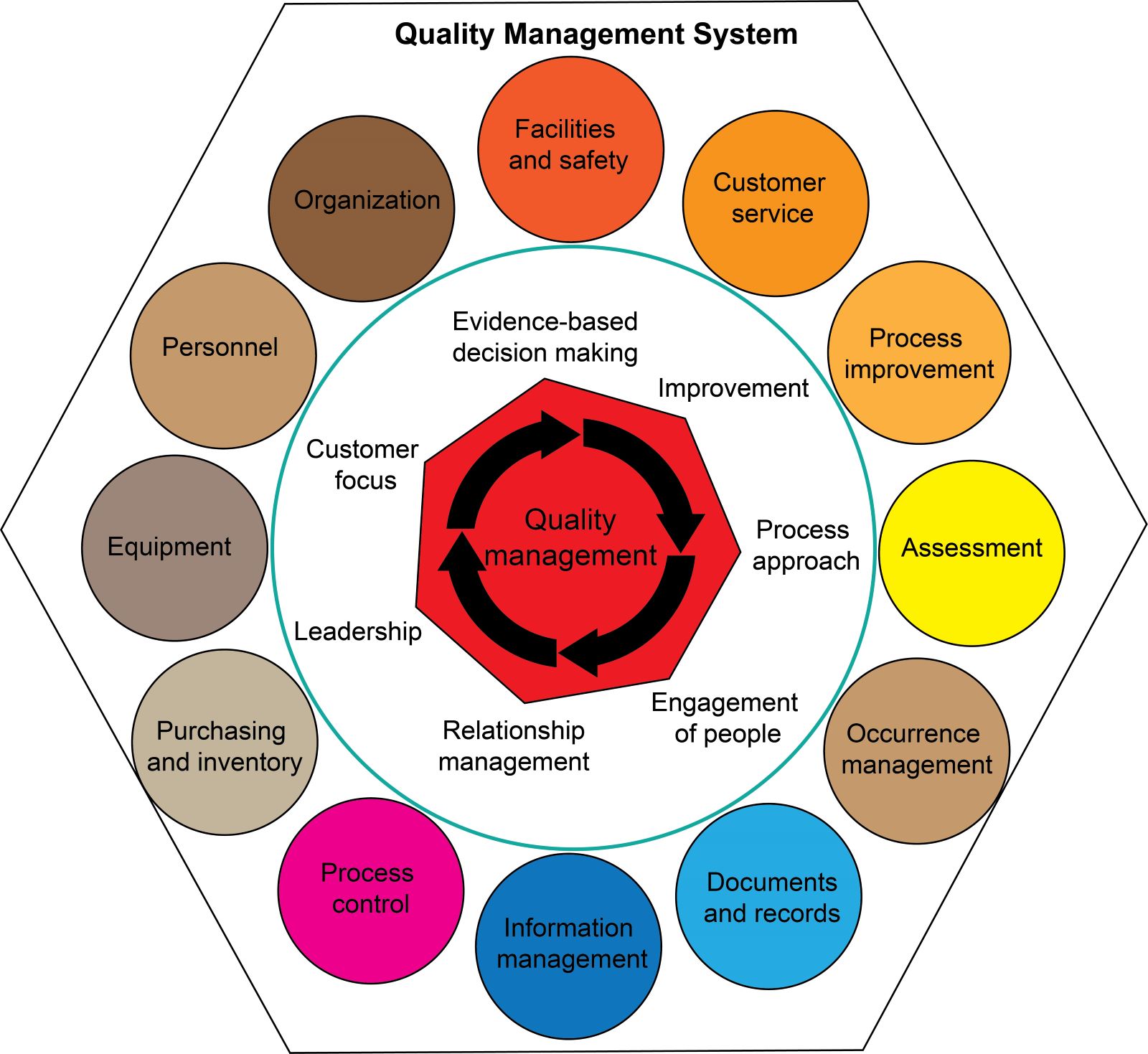
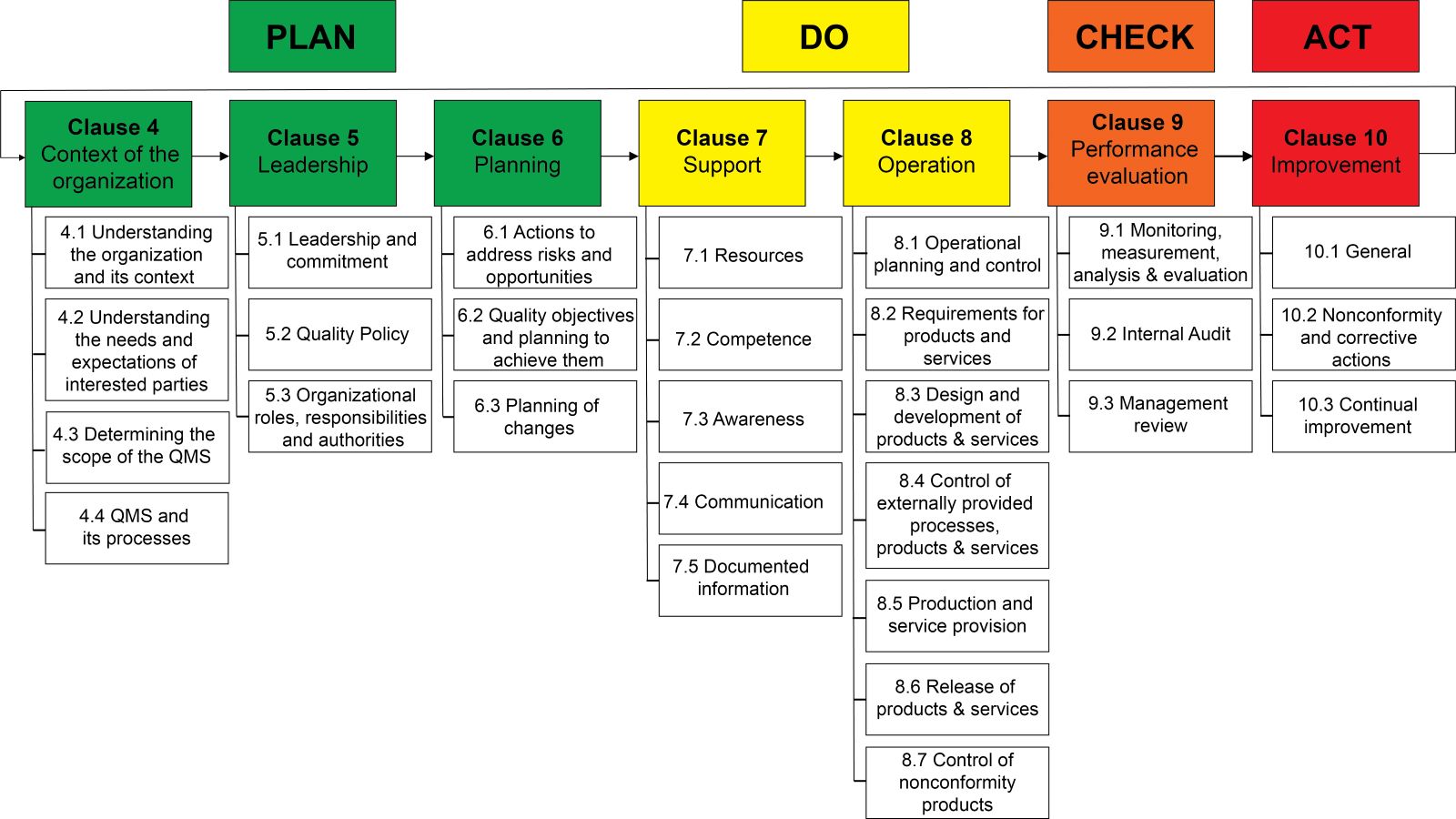
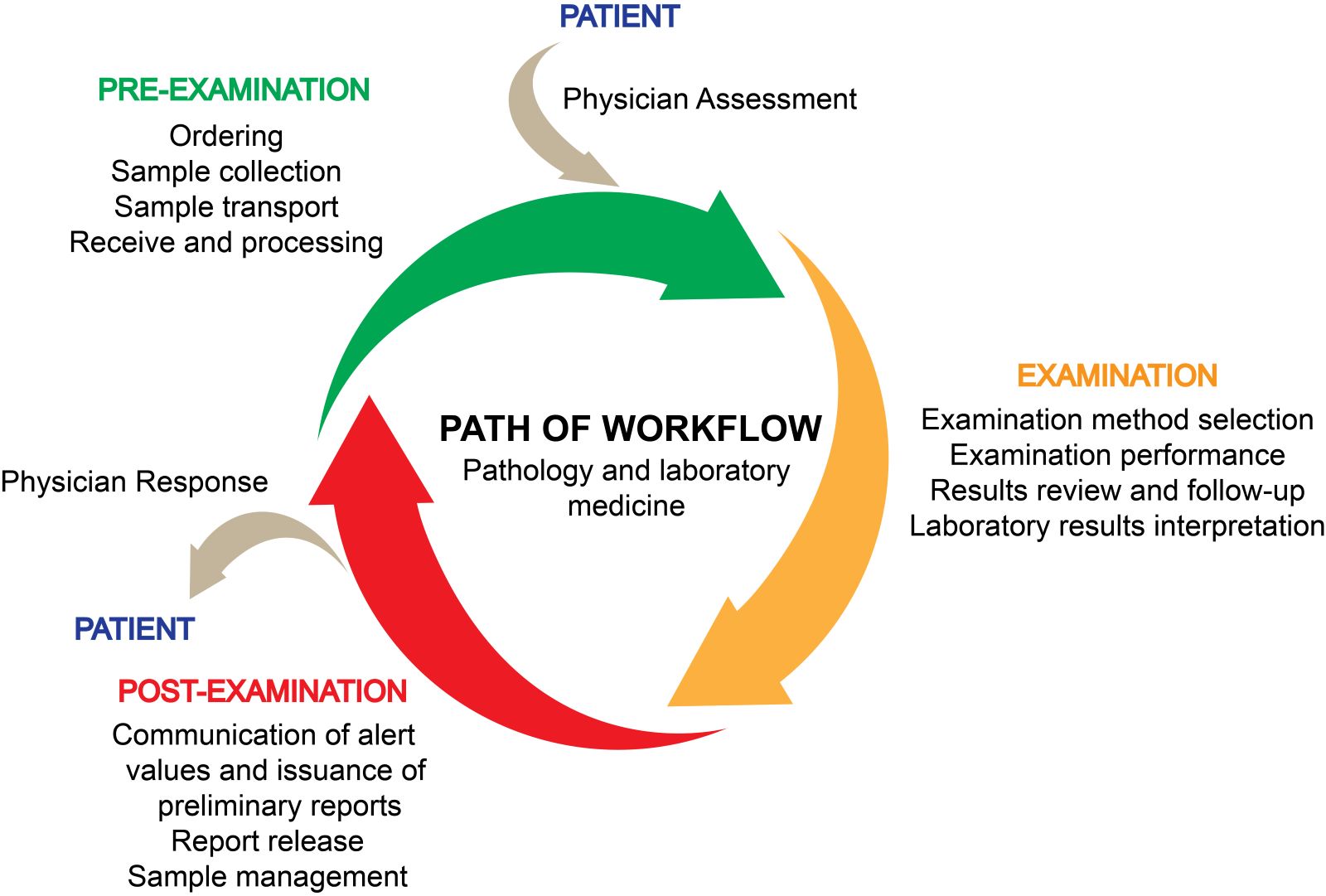
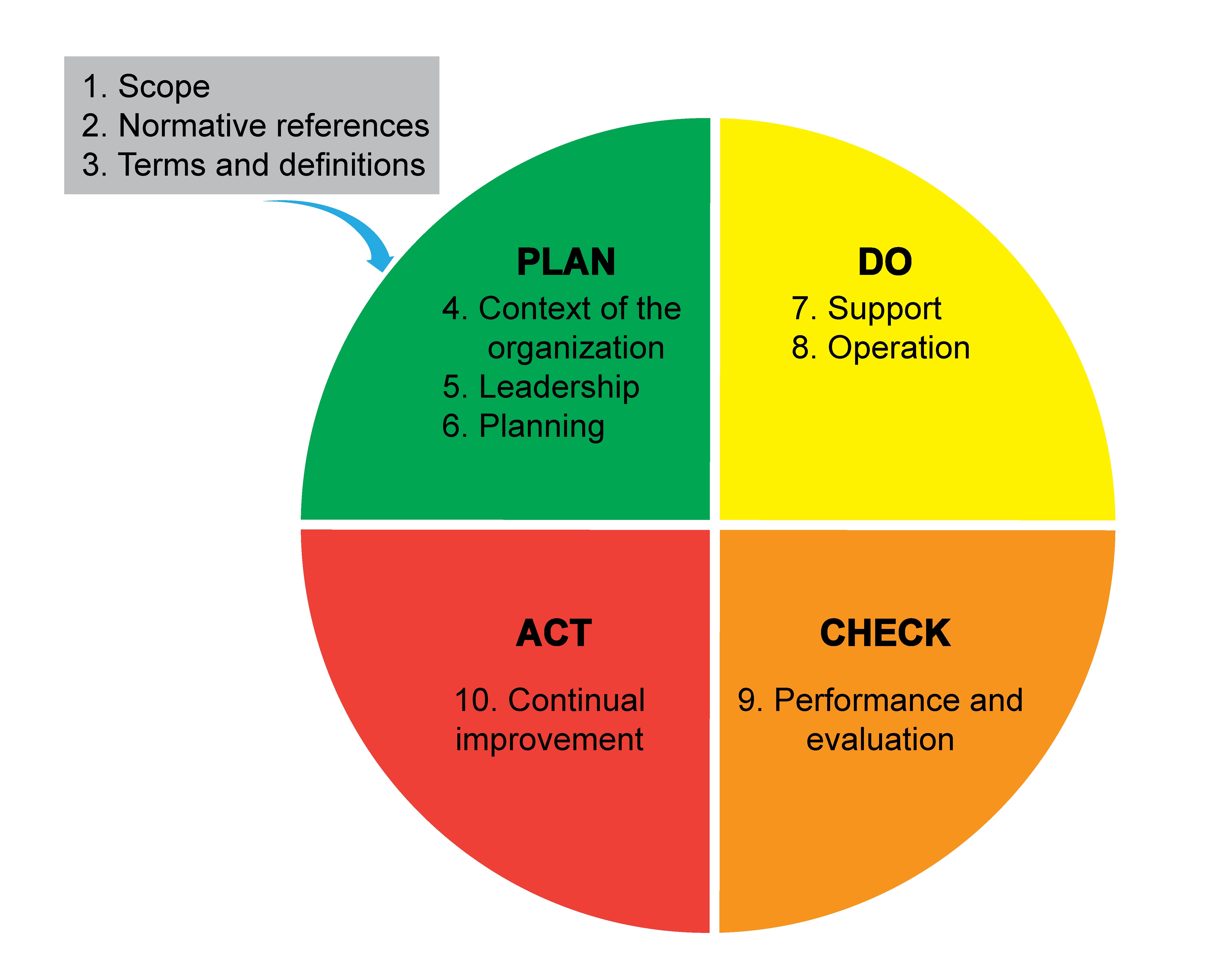
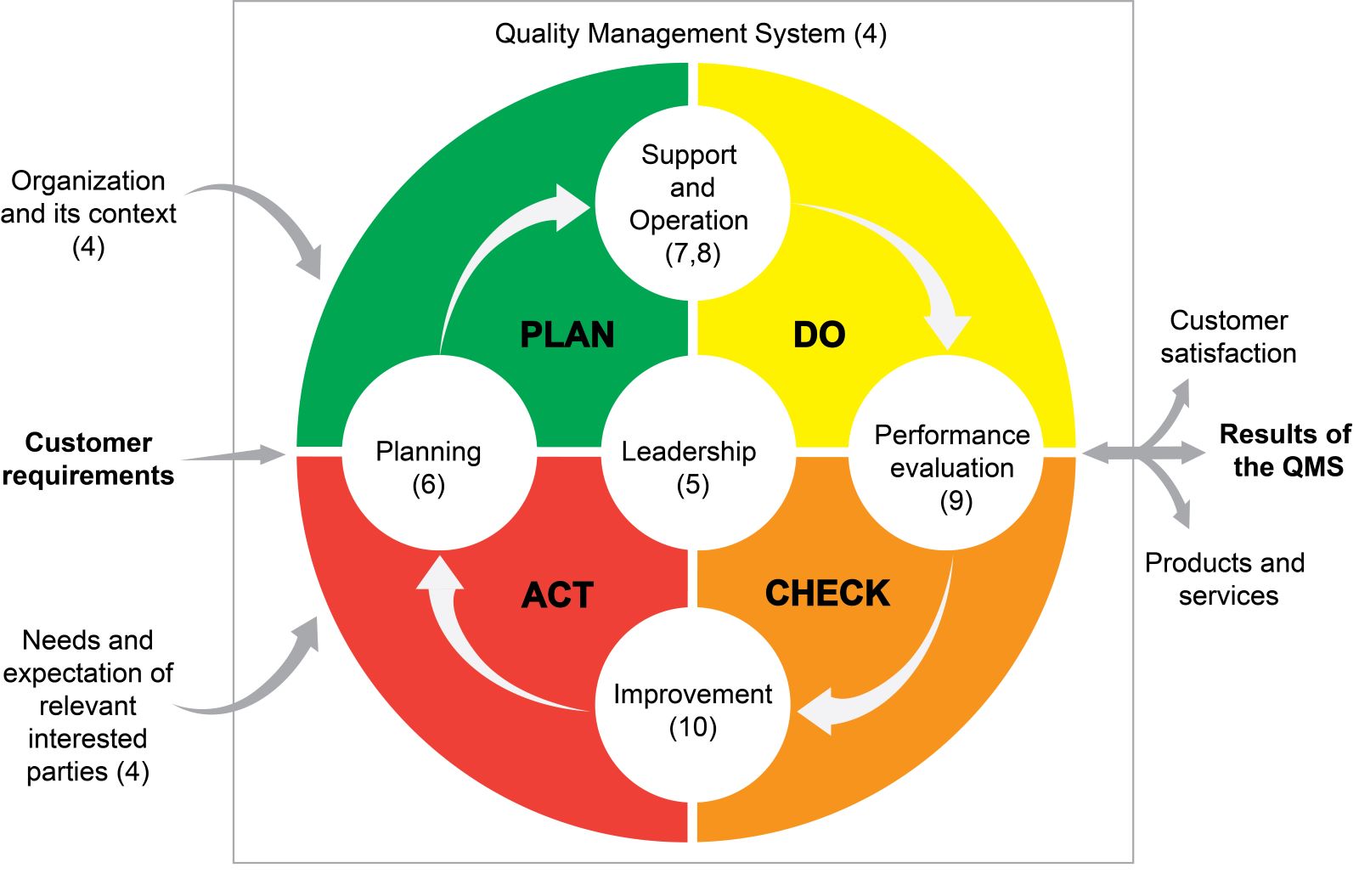
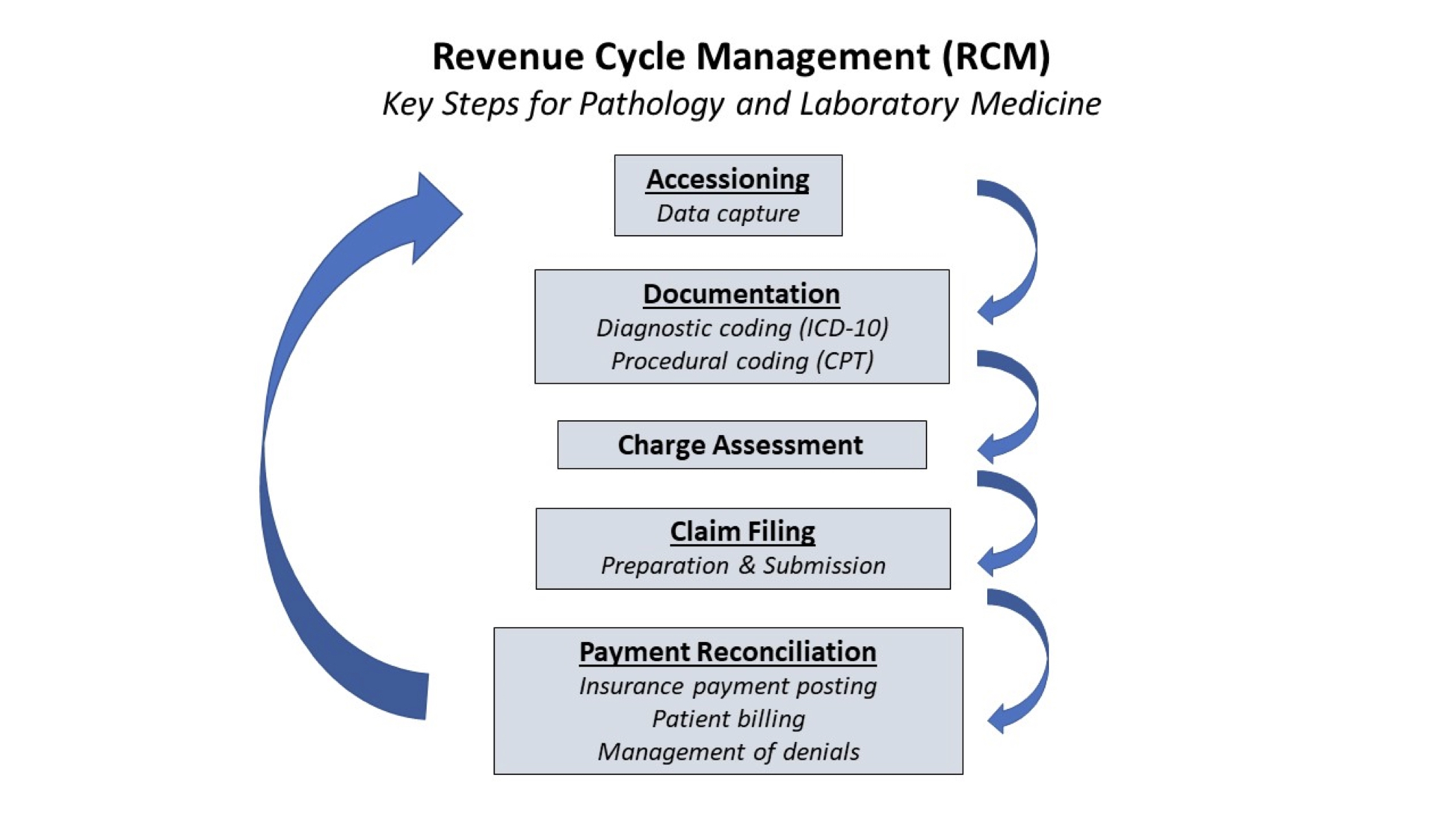
Lab safety: biosafety
Biohazard spill management
Biosafety levels
Resilience in the workplace
How to give an effective performance review
How to use SWOT analysis
Value and range of risk assessment
Employee selection to drive organizational culture
How trust drives everything in an organization
Resilience, well being and performance in the workplace by Derek Mowbray
5 why's technique
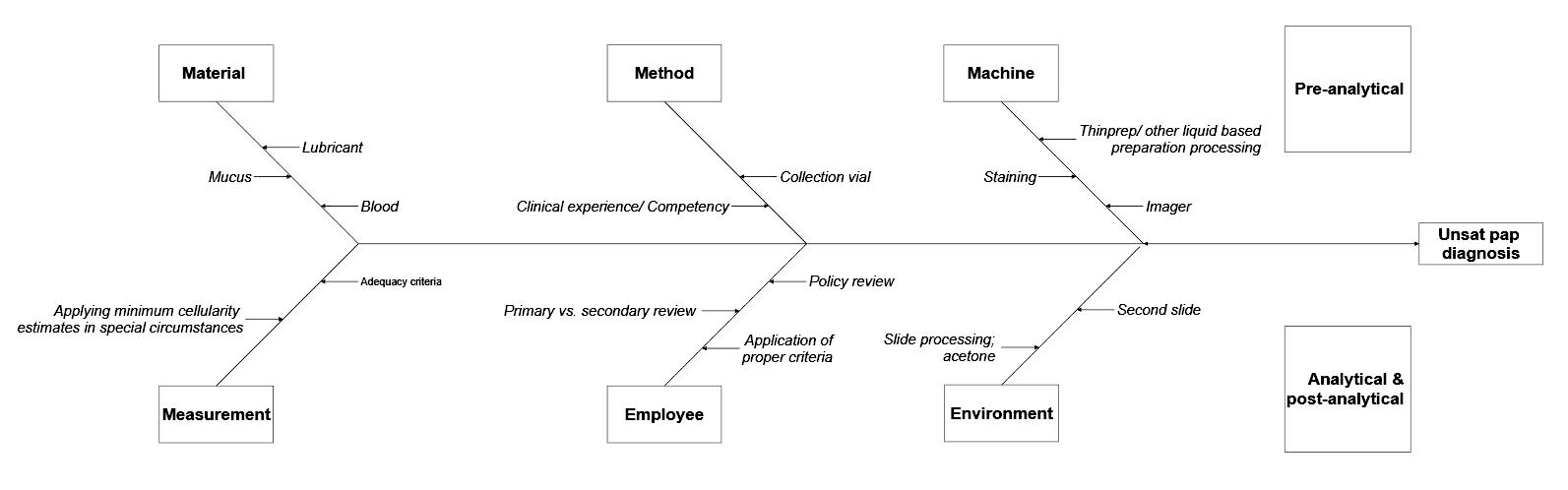
Fishbone diagram
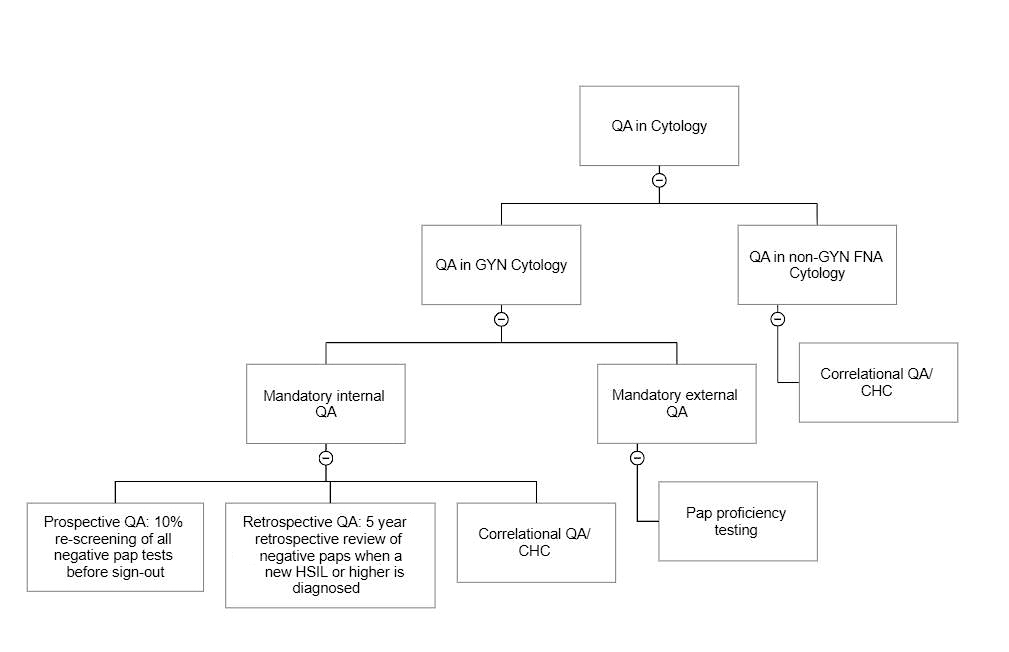
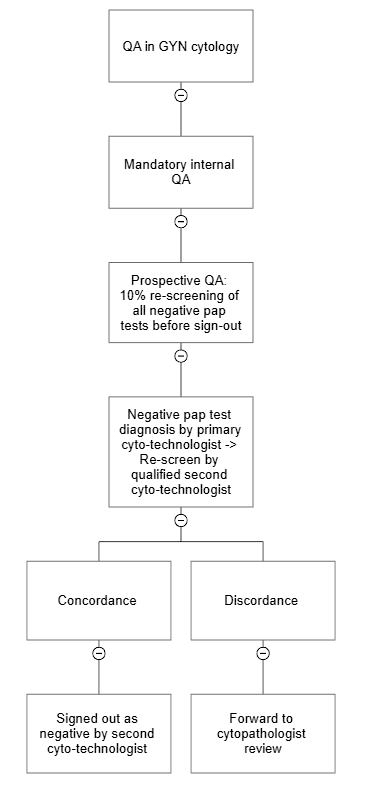
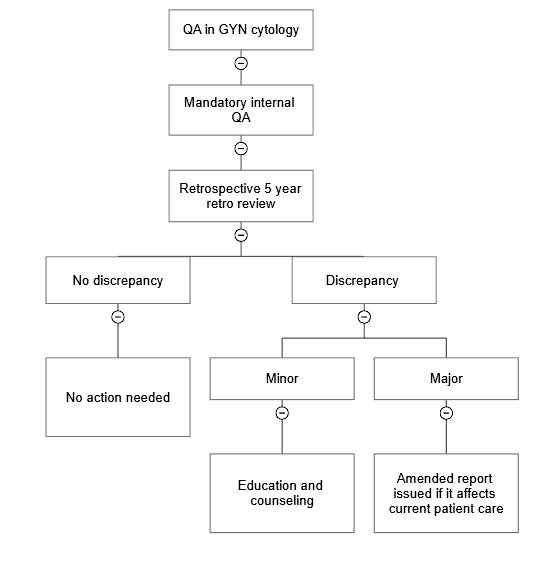
RCA2
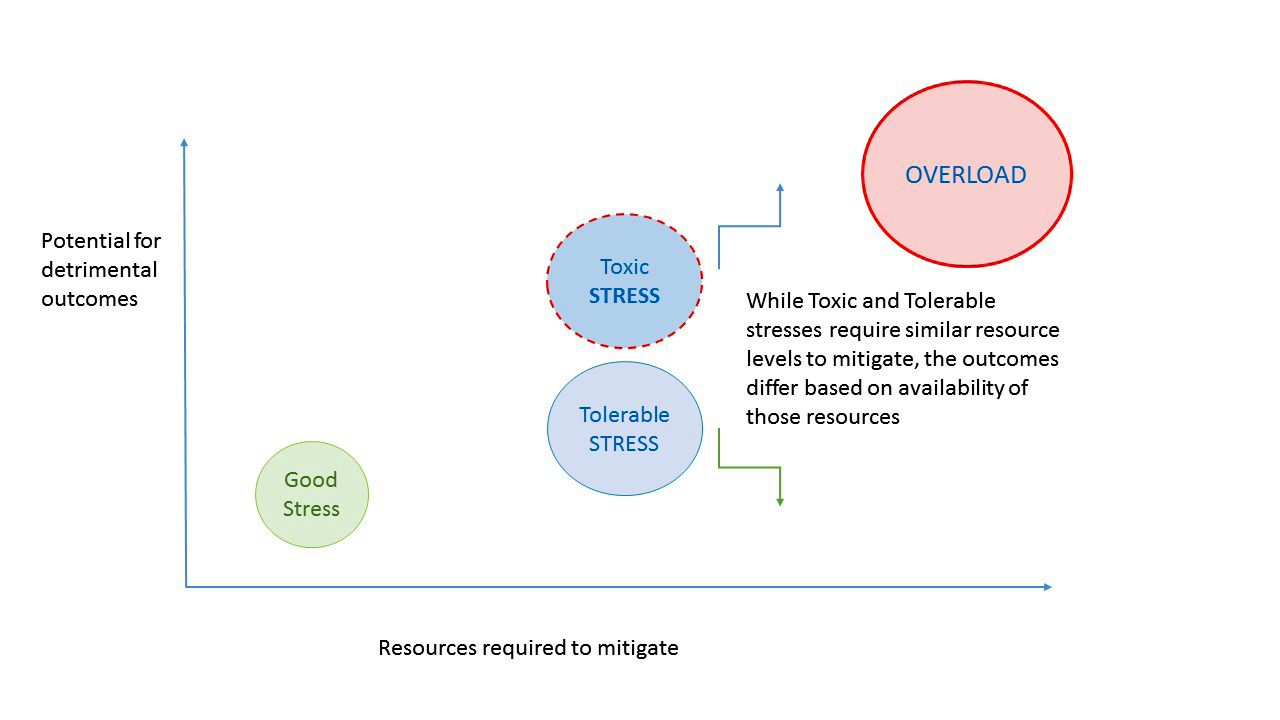
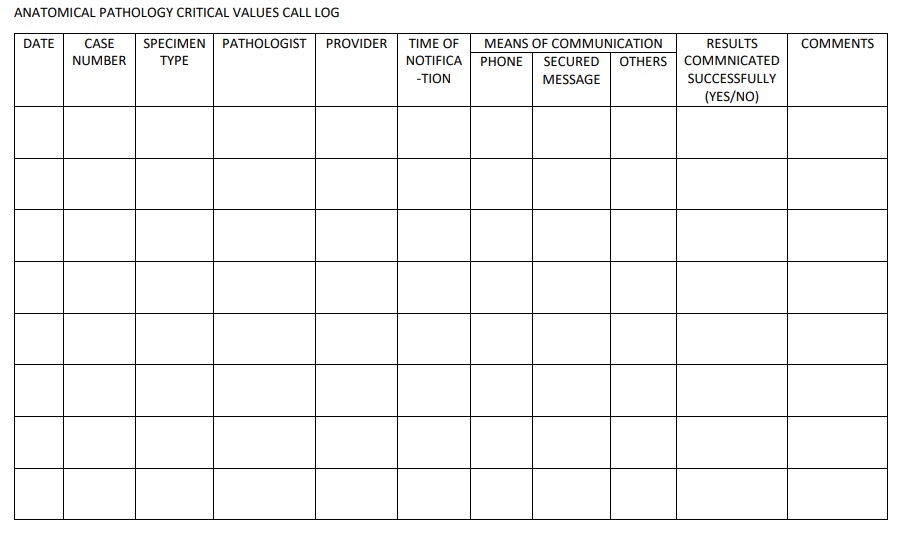
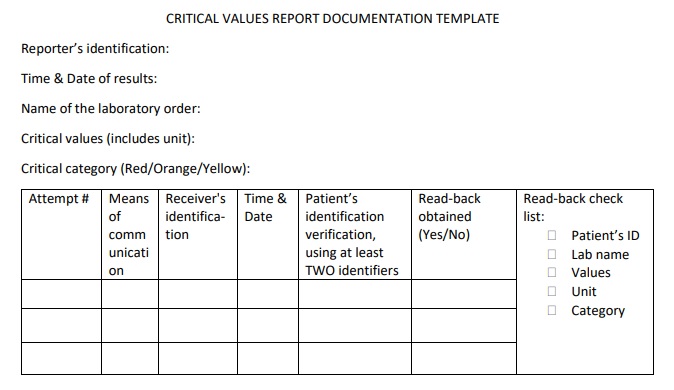
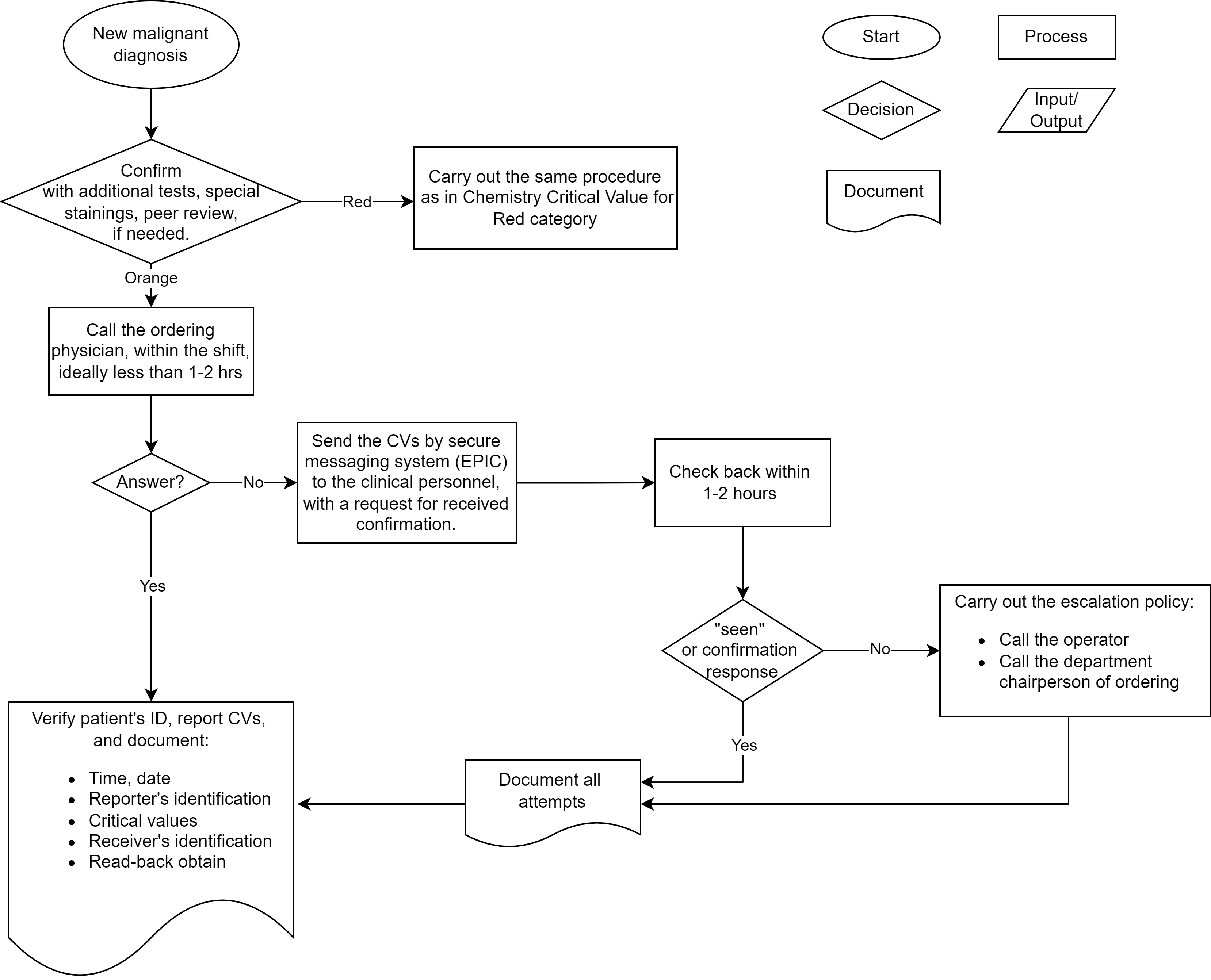
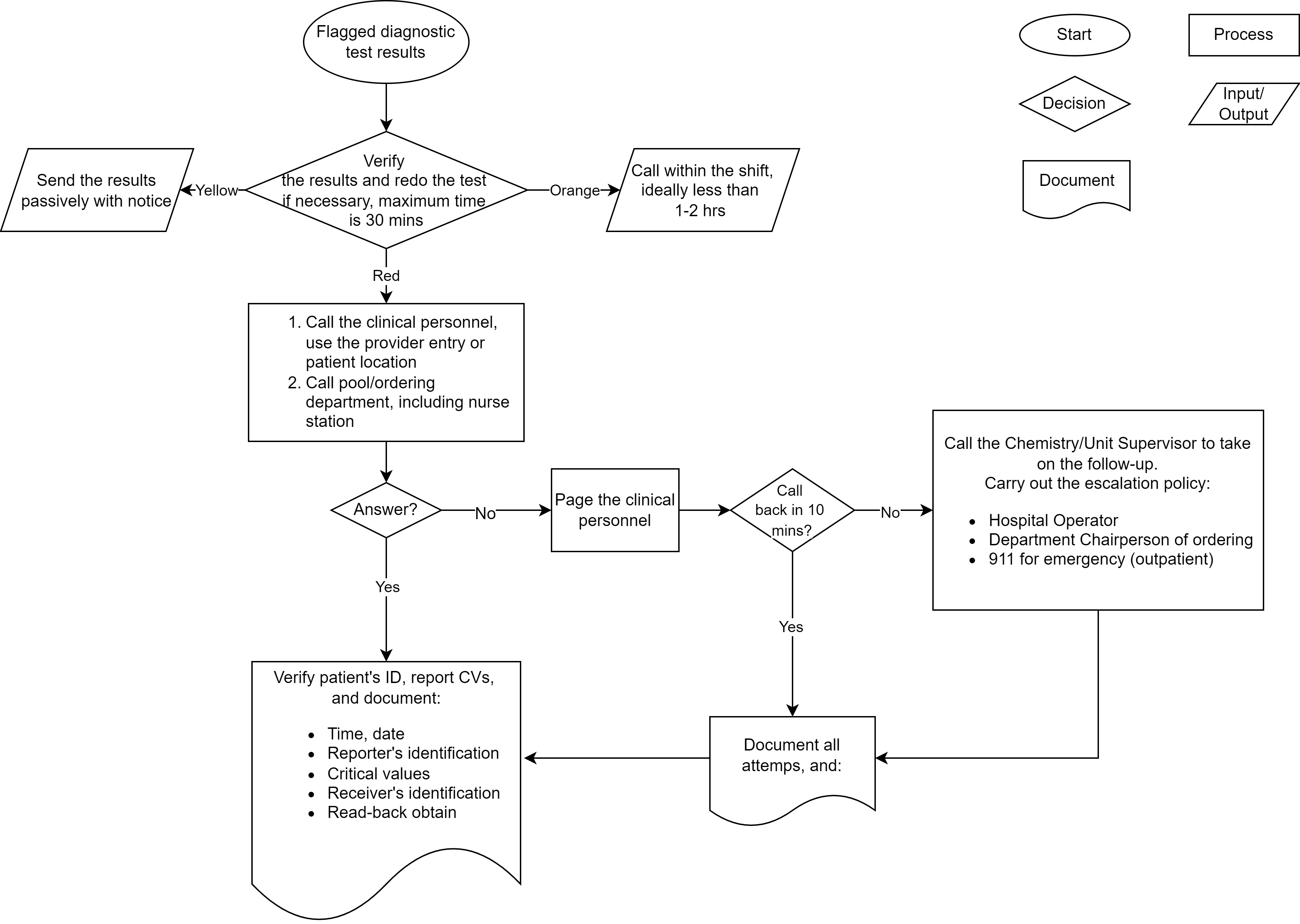
Critical values in clinical chemistry
Critical communications in healthcare
Communication in customer service
Embedding small specimens
Speed embedding
Brush technique
- Costs can be classified in different ways as shown in the following tables (McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2016):
Direct Indirect (overhead) Variable Fixed Salary Nonsalary Operating Capital Reagents ~ ~ ~ ~ Proficiency testing ~ ~ ~ ~ Analyzer service ~ ~ ~ ~ Analyzer ~ ~ ~ ~ Testing staff ~ ~ ~ ~ Management staff ~ ~ ~ ~ Rent ~ ~ ~ ~
Sample laboratory budget
Reference: Lab Med 2003;34;515Description Current actual Current budget Variance % Variance Inpatient charges
Outpatient charges
Total revenues$246,958
$1,574,862
$1,821,820$219,370
$1,476,560
$1,695,930$27,588
$98,302
$125,89012.6%
6.7%
7.4%Salary - professional
Salary - technical regular
Salary - technical overtime
Total salary$36,484
$50,548
$9,438
$96,470$35,213
$53,030
$2,610
$90,853$(1,271)
$2,483
$(6,828)
$(5,617)-3.6%
4.7%
-261.6%
-6.2%General lab supplies
Reagents
Lease, rentals
Total supplies$24,546
$130,356
$2,070
$156,972$19,476
$75,464
$2,070
$97,010$(5,070)
$(54,892)
$0
$(59,962)-26.0%
-72.7%
0.0%
-61.8%Total expenses $253,422 $187,863 $(65,579) -34.9%
Financial management:
effective budgeting in the laboratory
- Ian McNeal, CPA
Images hosted on other servers:
Comparison: FDA versus CLIA / CMS oversight of testing (FDA: Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories - Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs) [Accessed 4 March 2024])
| U.S. FDA oversight | CLIA '88 / CMS oversight |
| Focus on devices themselves and how they perform | Focus on laboratory processes to use devices, not device quality |
| Review of analytic validity performed before test may be used on patients | Review of analytic validity performed during a 2 year inspection cycle; test may be in use for 2 years before assessment of data and test use |
| Analytic validity large in scope with thousands to tens of thousands of data points | Analytic validity may be performed on the smallest number of patients required for statistical significance |
| Requires assessment of clinical validity / utility of testing | Does not require clinical validity / utility |
| Review requires assessment of patient safety | Review does not require assessment of patient safety |
| Required demonstration of effectiveness in determining presence / absence of condition being assessed | No required demonstration of effectiveness in determining presence / absence of condition being assessed |
| Requires adverse event reporting to identify inaccurate, unsafe and ineffective devices | Does not require adverse event reporting |
| Requires removal of unsafe devices from market | Does not remove devices from the market |
Contributed by Mai Thy Tran, M.D.
Images hosted on other servers:
Options for lab accreditation
| CLIA | CAP | ISO 15189 | COLA | JC | |
| Purpose |
|
|
|
|
|
| Inspector | CLIA inspectors (usually state department of health personnel) | Peer inspections by CAP inspectors and self inspections by staff | Certified inspectors | COLA surveyor (most often medical technologists) | JC surveyors |
| Accreditation cycle | 2 years | 2 years | 3 years | 2 years | 2 years |
| Frequency of inspection | Every 2 years | Peer inspections every 2 years and annual self inspection | Annual internal audit | Every 2 years | Every 2 years |
| Cost | One time registration fee of $100; certification fee based on the annual testing volume and number of laboratory specialties performed ($180 - $9,500 / year) | Nonrefundable one time application fee of $1,200 (domestic) or $1,500 (international); CAP's annual accreditation fee is determined based on the laboratory's size and complexity | The fee schedule will include an annual base fee and fees for assessments; cost will vary depending on the size and scope of the lab | Enrollment fee and certification fee | Onsite survey fee; annual fee based on the number of specialties the lab provides and the number of locations |
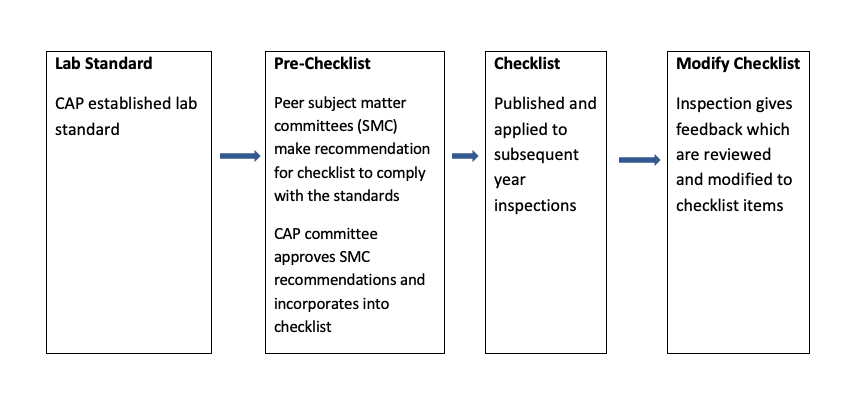
Example for lab accreditation check list
| Lab accreditation - general check list | Yes | No | N/A |
| Specimen collection, handling and reporting | |||
| Proficiency testing | |||
| Quality management | |||
| Result reporting and referral of testing | |||
| Quality of water and glassware washing | |||
| Reagents (storage, handling, labeling) | |||
| Instrument and equipment maintenance / function check | |||
| Personnel | |||
| Laboratory computer services | |||
| Physical facility | |||
| Laboratory safety |
CAP15189
UKAS accreditation
CAP accreditation
What is CLIA?
What is the Joint Commission?
Who is FDA?
CDC
CMS
What is Lean?
What is Six Sigma?
5 whys
Lean / Six Sigma - waste, DMAIIC, etc.
Managers, steps and duties
Descriptive statistics
Five S
Value stream mapping basics
What is Kanban?
What is DMAIIC
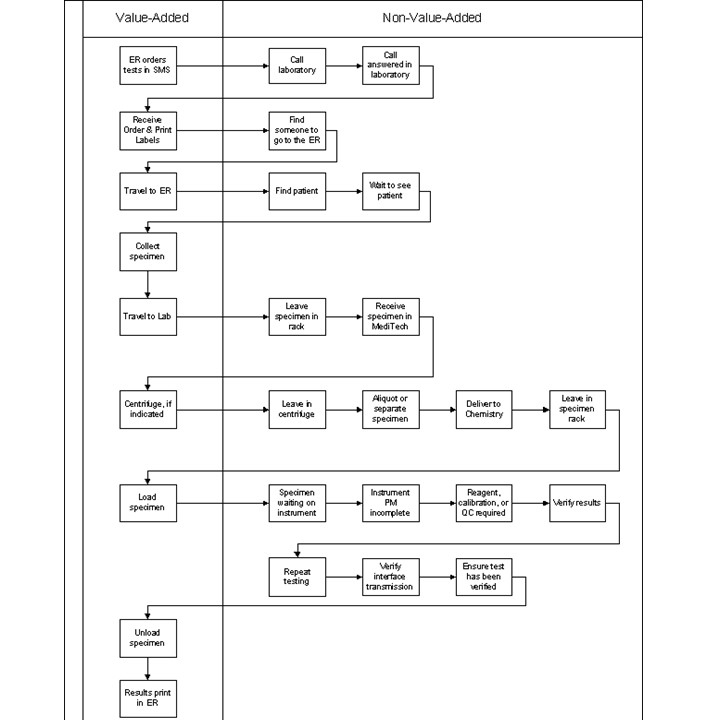
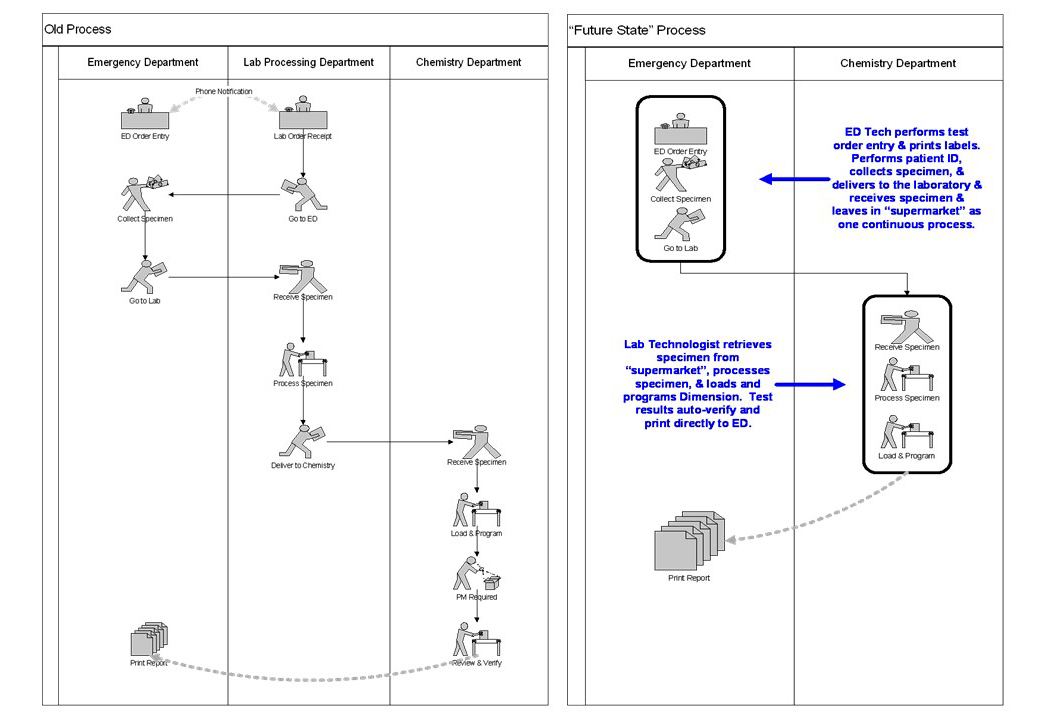
- Spaghetti diagrams or workflow charts can be created in order to maximize the utility and efficiency of work space (see Lean workflow)
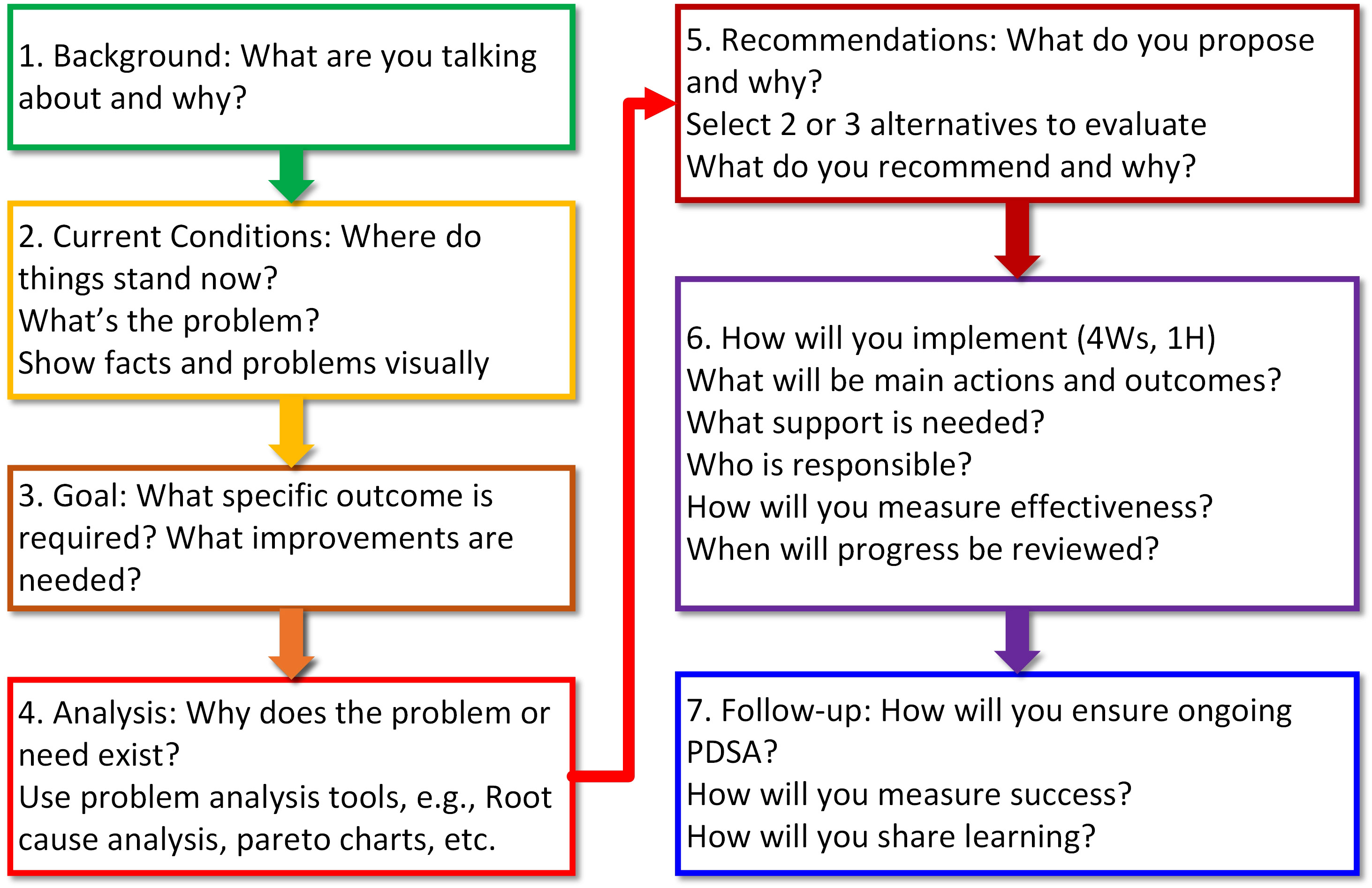
- Root cause analysis (RCA): problem → ask why (and then why again and again and again) → root cause → corrective action
Images hosted on other servers:
Workflow examples
Root cause analysis (RCA)
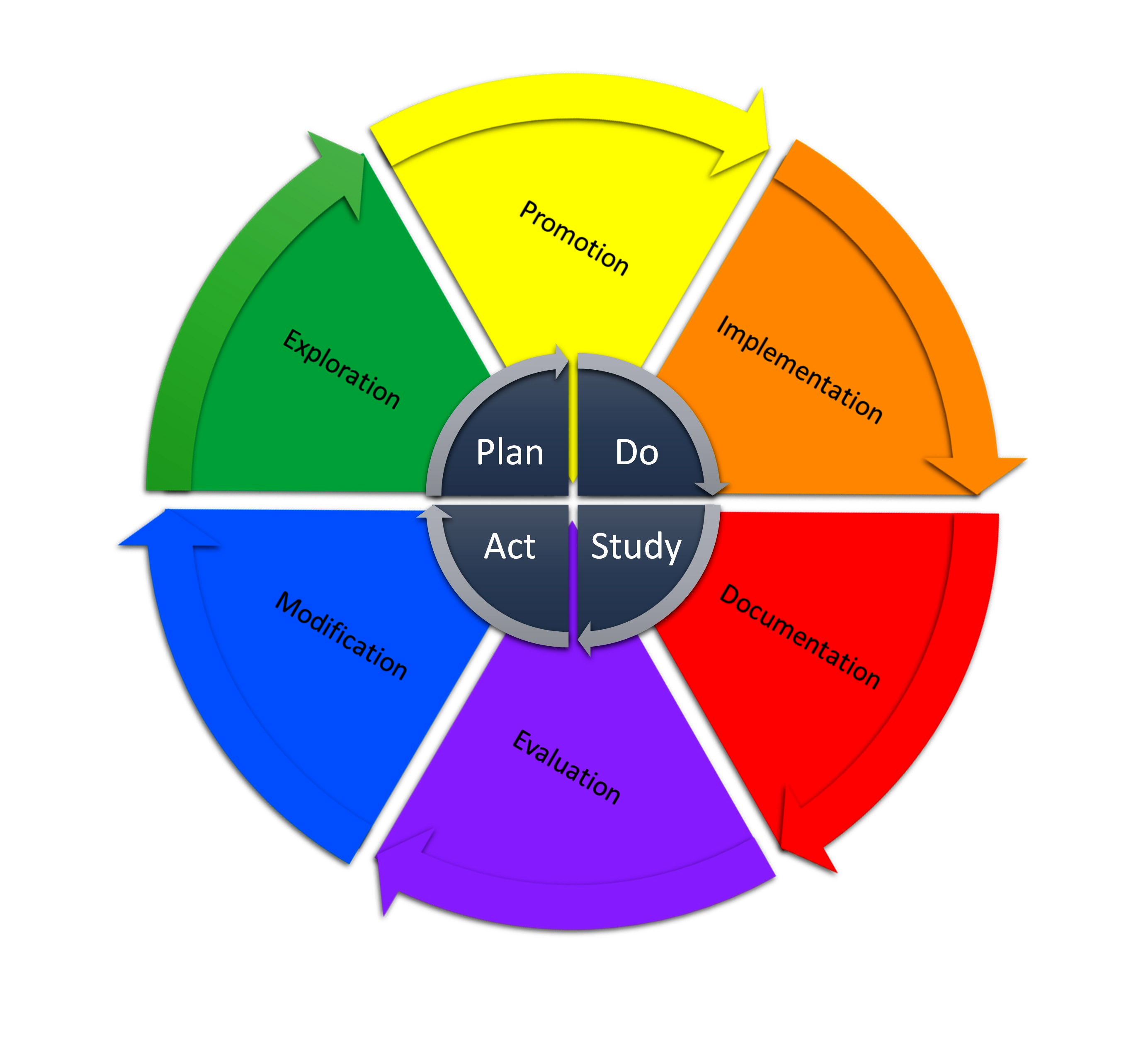
Plan, Do, Study, Act (PDSA) cycle
Failure mode and effects analysis (FMEA)
Root cause analysis techniques
| Formal quality assurance programs | Informal quality assurance programs |
|---|---|
| Retrospective case review | Autopsy |
| Proficiency testing | Diagnostic consult |
| Prospective case review | Patient referral |
| Characteristic | Proficiency testing | Internal case review (retrospective) | Internal case review (prospective) | External peer case review by subspecialist (retrospective) |
|---|---|---|---|---|
| Adds to the pathologist workload | ✔ | ✔ | ✔ | |
| Peer reviewed | ✔ | ? | ? | ✔ |
| Standardized | ✔ | ✔ | ||
| False negative & positive cases | ✔ | ✔ | ||
| QA total process | ✔ | ✔ | ✔ | |
| Benchmarking | ✔ | ✔ | ||
| Ability to influence the diagnosis in real / near real time | ✔ | |||
| Key positive feature(s) | Established minimum quality tool | Most common QA practice | Real time | External subspecialist review, does not use pathologist time |
| Negative consideration | Does not QA the full case detail from gross to report | Demanding on pathologist and technologist time | Most demanding on pathologist and technologist time | Program needs to be double blinded for confidentiality |
| Best demonstrated practice | CAP and ASCP proficiency programs | ADASP guidelines on QC & QA in AP quality assurance | UPMC | QualityStar™ external QA case review by subspecialist |
WHO: LQSI series - implementing a laboratory quality management system
Laboratory quality management and standards
CAP: quality management
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Can include images on our server or other servers
- Add a
between the table and section header - If adding a table that does not have a thumbnail available, use the PathOut table thumbnail: https://www.pathologyoutlines.com/thumb/table.jpg
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
- If adding in a table from an author's word doc, use the following table format
- Start the table using
- Add rows using
- Add cells using
- Double borders will be added automatically
- Width automatically adjusts as more text is added
- To include a specific width in a cell, add width="#px" or width="#%", e.g.,
or - To center text horizontally, use
- To center text vertically, use
- To center text both horizontally and vertically, use
tags are obsolete, use style="text-align:center" instead - End the table using
- To center text horizontally, use
Table title in bold (no italics)Heading Heading Heading Text text text text text Text text text text text Text text text text text Text Text Text - Add rows using
- Start the table using
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Common abbreviations are ok to use, such as CT, MRI and PET
- Xray should be formatted with capital X and no space before ray
- Can include images on our server or other servers
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Includes intraoperative images or images of the patient
- Can include images on our server or other servers
- Does not include radiology images, which belong in the Radiology images section
- Does not include gross images, which belong in the Gross images section
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Images of excised specimen
- Can include images on our server or other servers
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
Notes:
- Images on our server
- Format heading
- Use the "Contributed by" heading (class="f11bi"), no colon at end
- Heading should have one
before and one
after - Use periods for degrees such as M.D., Ph.D.
- Include only the contributor's name and degrees, not their university or country
- For multiple contributors, list their names separated by a comma in the same heading (the lightbox will indicate whose image it is)
- If images are from an old PathOut Case, use the heading "Case #__" and link to the Case, e.g., Case #300
- For new Case images, use the heading "Contributed by __ (Case #__)"
- If images are from AFIP, use the heading "AFIP images" (and also use this in the legend)
- Exception: if there is also an author in the heading, use this format: Contributed by Author Name and AFIP
- If images are taken from virtual slides, use the heading "Contributed by Author name (source: virtual slide source)"
- Subheadings should use format class="f10bi" (both bolded and italicized)
- Load images to our server
- Edit the files using "auto levels" to even out the colors
- Be sure to resize images so that the files are 400 - 600 KB
- Load to the imgau directory or the imgau/chapter directory
- We are no longer adding legends to the image files
- Save the image file as chaptertopicauthor#.jpg, e.g., lymphomanonBanaplasticbalakrishna01.jpg
- Add lightbox
- Each section should have a unique lightbox code in the format data-lightbox="chaptertopicsection"
- Add in depth description as a legend in the data-title section
- The legend should be formatted in complete sentences with periods
- Ok to include magnification in image legend (2x, 4x, 10x, 20x, 40x, etc.)
- Stain and magnification should be at the end of the image legend and formatted as (stain, magnification).
- To include a reference link in the legend, use this code (Journal Year;Volume:Page)
- After the legend, include a
and the Contributed by text (only include the contributor of that specific image if there are multiple contributors)
- Add captions
- Include a few words as a caption in the html p tag
- Should not include magnification, H&E or figure #
- To make images share a caption, add the appropriate div class with between each image (each image still needs its own legend)
- (for special formatting, can add style="text-align:center" to the div and p tags to center the caption, be sure to add in
s)(for 1 image, 1 caption)(for 2 images, 1 caption)(for 3 images, 1 caption)(for 4 images, 1 caption)(for 5 images, 1 caption)(for 6 images, 1 caption)- Captions should include no more than 4 lines of text; if the caption cannot be trimmed down, use
and add
s to divide the words onto 4 lines- Add style="text-align:center" into the div and p tags to center the caption: and
- Add alt text
- Copy and paste the text from the caption into the alt text (after width and height, add alt="caption")
- Images on other servers (for author topics, delete all micro images from other servers)
- Use the other servers heading (class="f11bi"), colon at end, one
before heading (two
if after other row of images), one
after heading - Fill in the href
- Link to the image on the page by finding the id in the source code
- To link to an image in a PDF, add #page=NUMBER to the end of the URL and fill in "NUMBER" with the page number, e.g., https://www.gastrojournal.org/action/showPdf?pii=S0016-5085%2897%2900366-1#page=5
- For the src, add the thumbnail to our server (see thumbnail procedure on page 60 of Procedure manual)
- If it is a composite image, first crop the image so that it only shows the relevant image
- Resize to 96 x 96
- Load to thumb directory
- For composite images in multiple sections, crop the image for each section and link to same image
- For composite images in the same section, only include one link / thumbnail (showing all relevant composite images)
- Include a few words as a caption in the html p tag
- Copy and paste the text from the caption into the alt text (after width and height, add alt="caption")
- Link to images that include a description; do not link to images on other servers if they don't have a description (unless there are no other images)
- Remove images from sites that are not stable, such as Archives of Pathology and WebPathology
- Remove images from sites that bypass paywalls, such as Sci-Hub
- General notes
- We now require at least 6 micro images per topic
- Include up to 6 images per row, then
onto next line - Include up to 6 rows (we no longer use the scroll bar)
- If images are on the PathOut Flickr account, add them to our server
- Also add Flickr images from the following pathologists who have given us permission: Yale Rosen, Nicole Andeen, Maria Tretiakova, Debra Zynger, Jian-Hua Qiao or the author of the topic
- We prefer images on our server but we allow images on other servers in every section except for micro images
- Click here to view the different types of images
- Add alt text
Cytology images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:- Can include images on our server or other servers
- Format Diff-Quik with hyphen
- Always capitalize Pap
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
- For CNS tumor topics only, cytology and frozen section images should be grouped together in frozen section rather than separated (use heading "Intraoperative frozen / smear cytology images" for these)
Flow cytometry images- Composite images are allowed for this section only
Electron microscopy images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:- Can include images on our server or other servers
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
Molecular / cytogenetics images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:- Can include images on our server or other servers
- Common abbreviations are ok to use, e.g., DNA, RNA, PCR, FISH, CISH, ISH
- Italicize all genes, e.g., TFE3
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
Videos
Short caption
Shared caption
Notes:- For the src, be sure to use the link with "embed" in it (e.g., https://www.youtube.com/embed/v80kA_dt6EE)
- To link to a specific portion of a video, add the start time in seconds (?start=XXXs), e.g., https://www.youtube.com/embed/AO9LKJdCpFQ?start=960
- Add a
between the video and section header
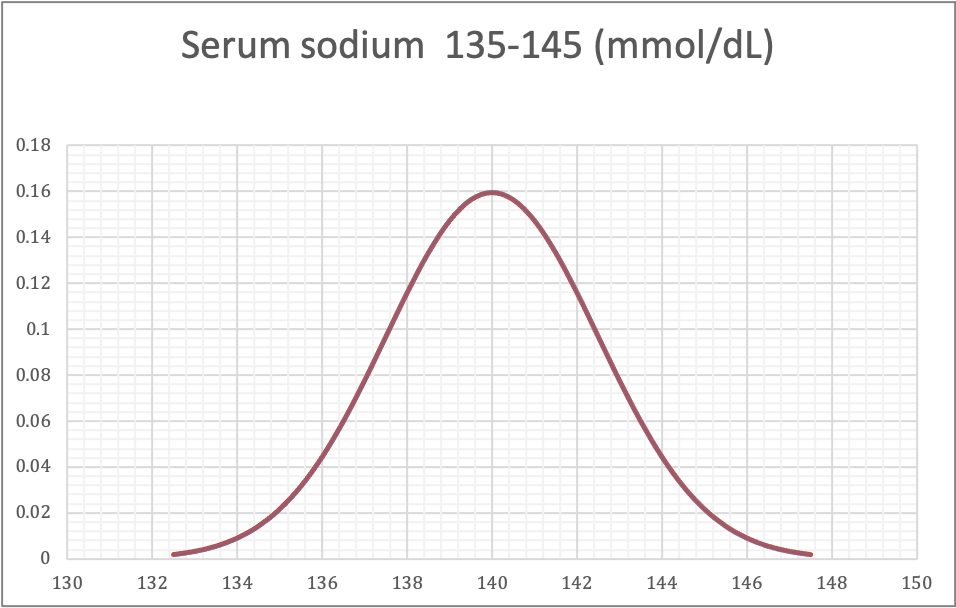
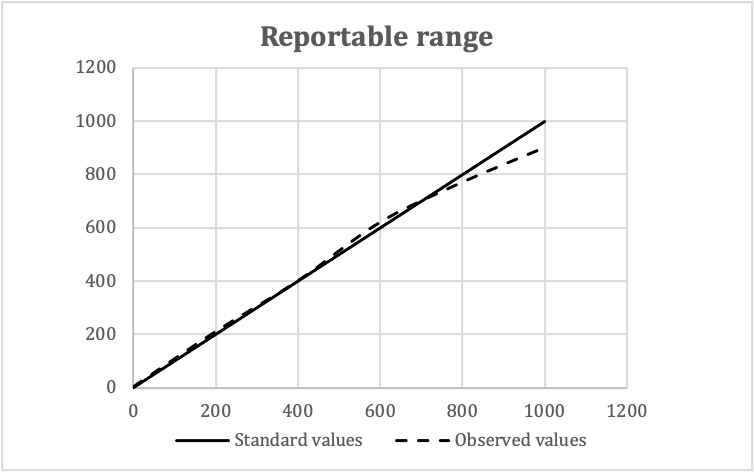
Validation of reference intervals and reportable rangeDiagrams / tables
Contributed by Duy K. Doan, M.D. and Lewis A. Hassell, M.D.
Validation of reportable rangesStandard values Observed values Means Replicate 1 Replicate 2 Replicate 3 0 2 0 4 3 200 220 210 200 210 400 400 400 400 400 600 620 630 610 620 800 760 760 790 770 1,000 920 900 880 900
- In this example:
- Expected reportable ranges: 0 - 1,000 mg/dL
- Number of concentration levels: 5
- Number of replicates: 3
Back to topVideos
Reference intervals, the basics
Recent Laboratory Administration & Management of Pathology Practices Pathology books
Find related Pathology books: general surgical pathology, lab medicine, management, frozen section
- Format heading