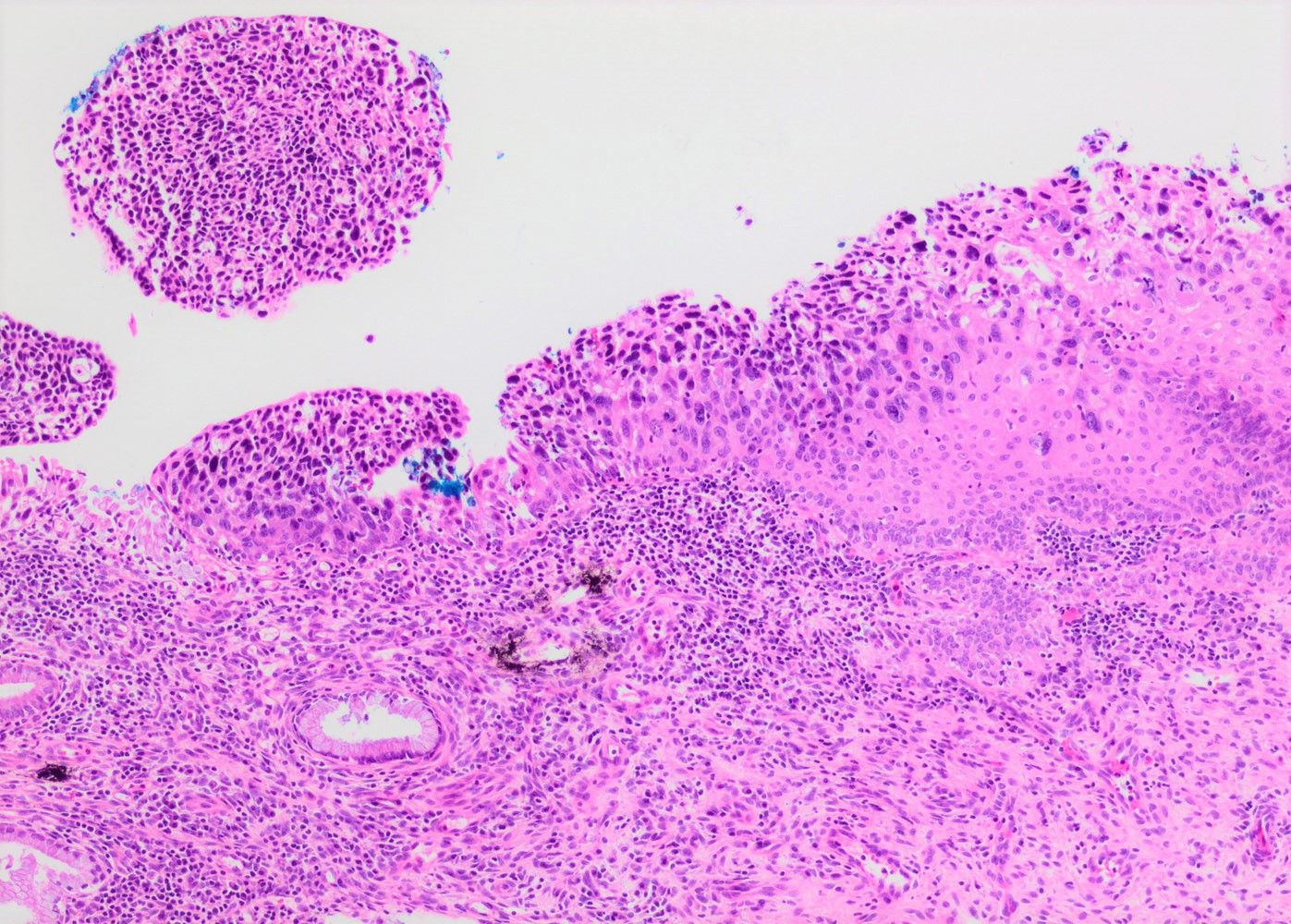
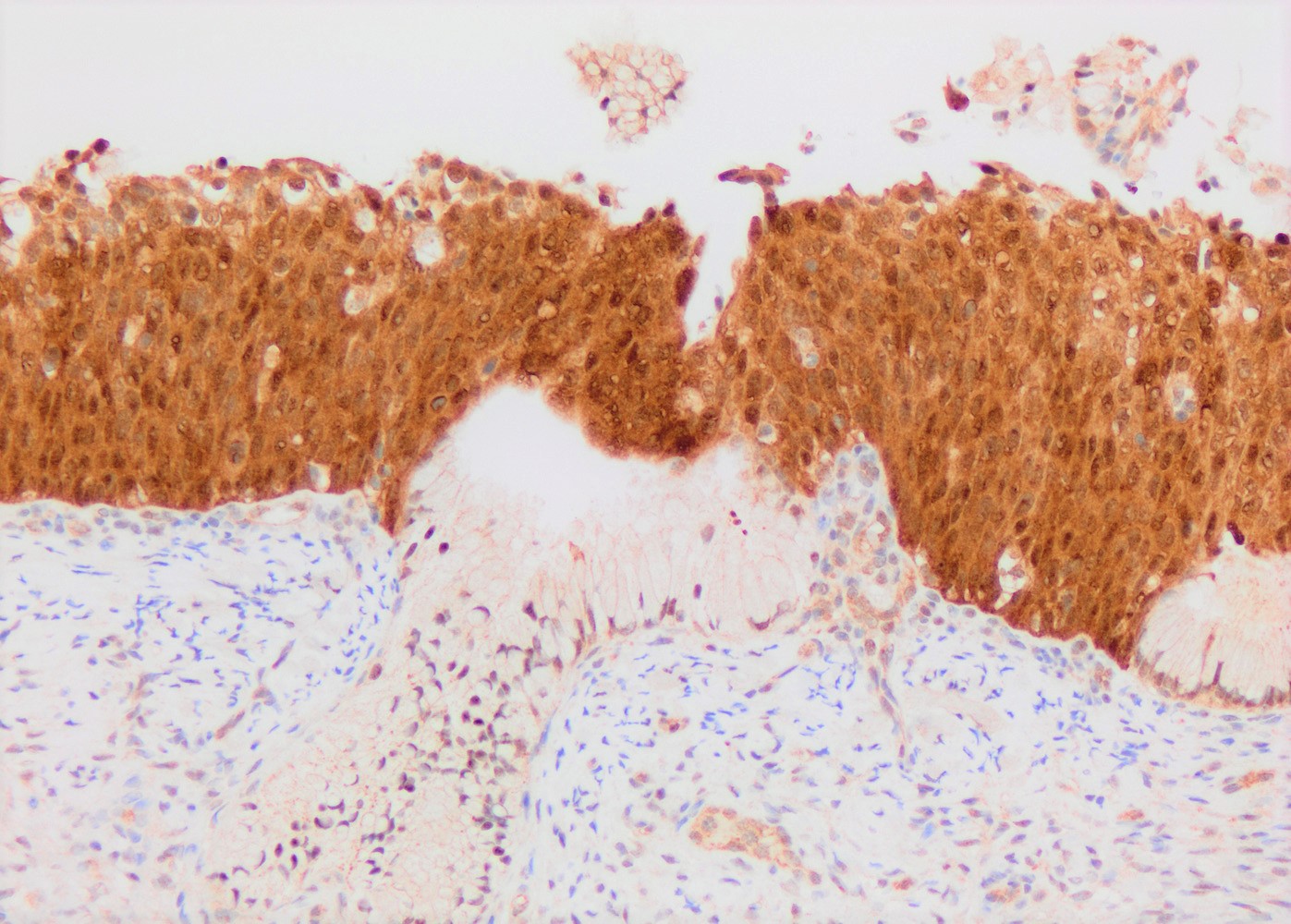
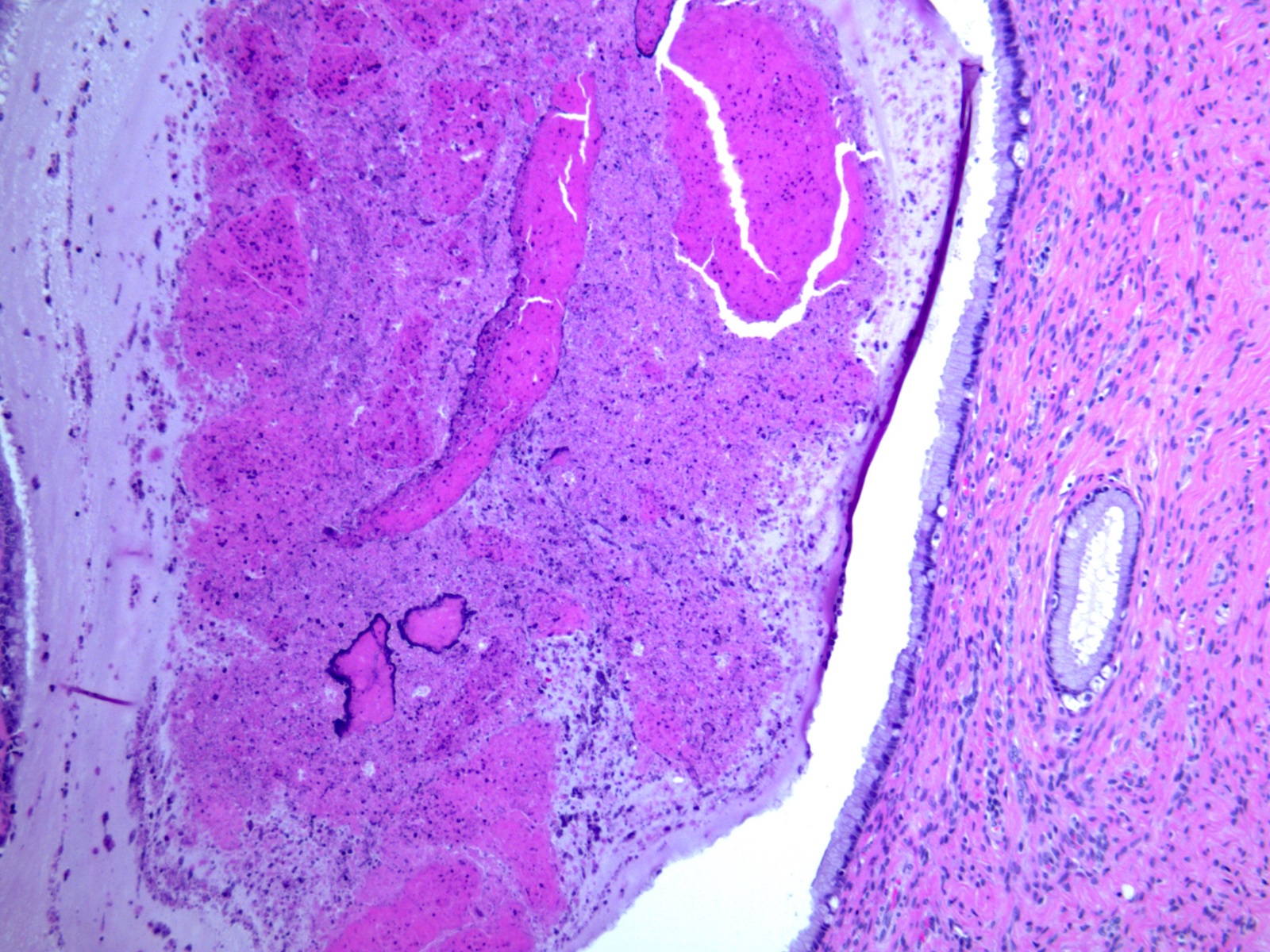
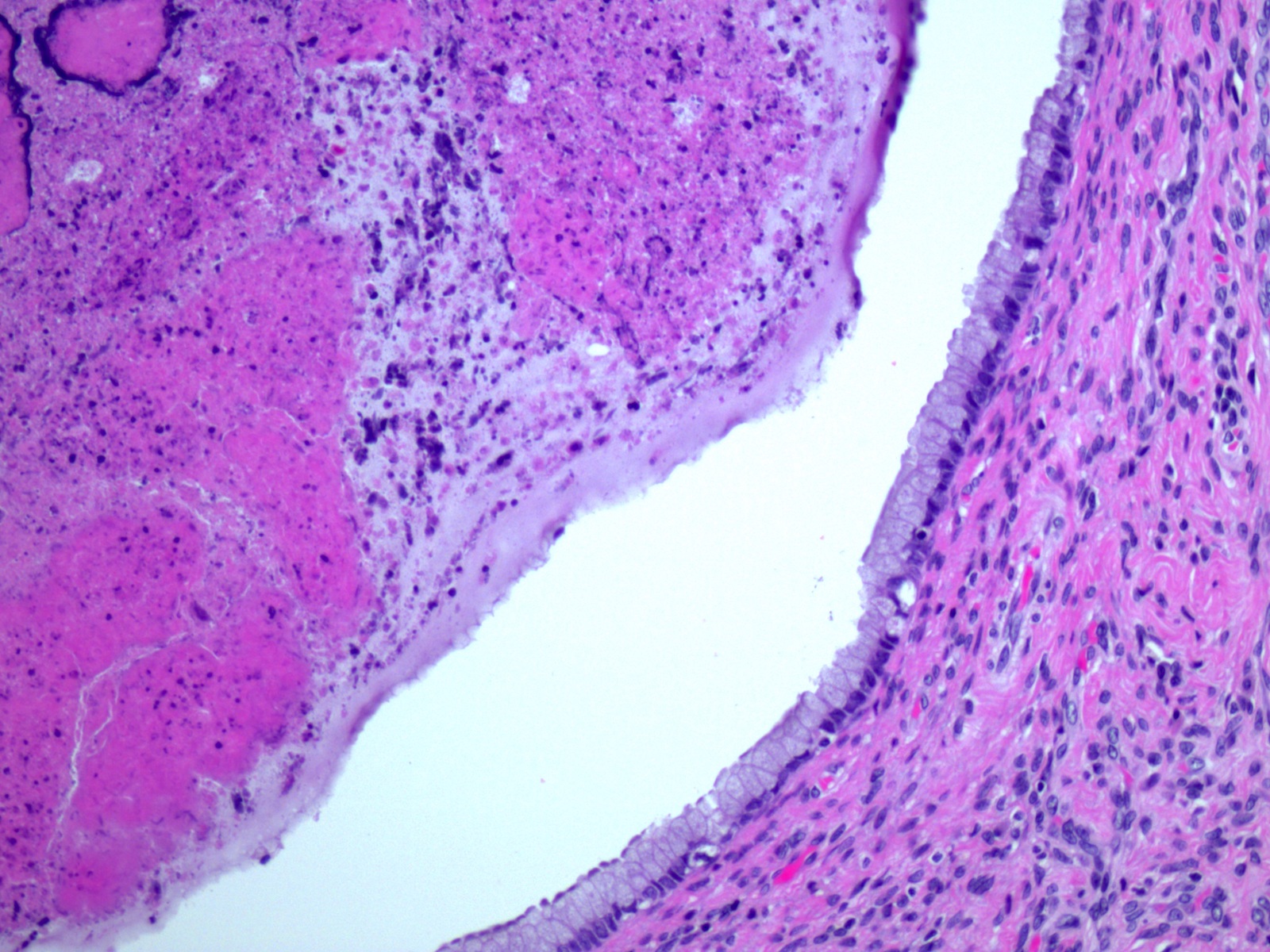
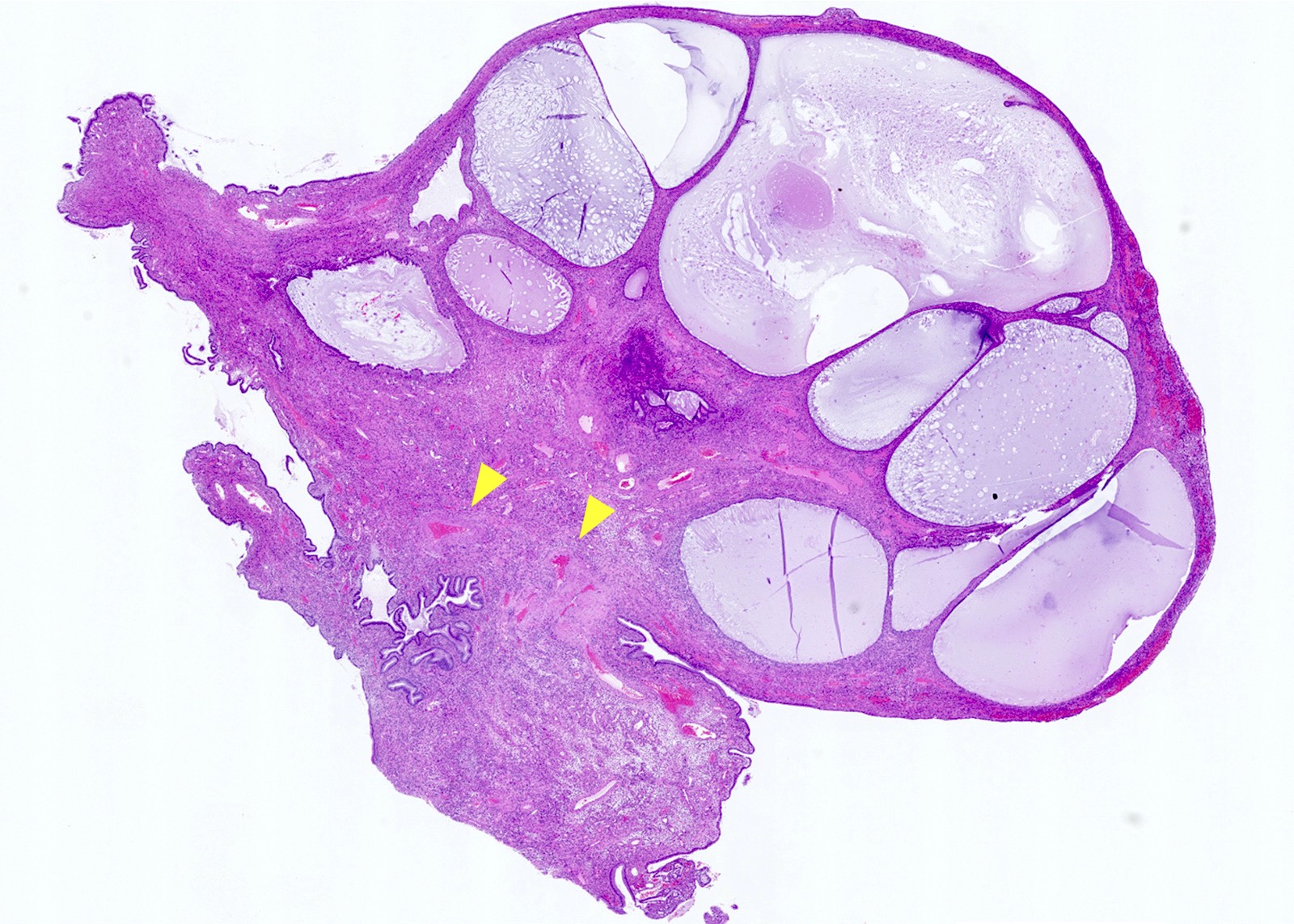
- pTX: primary tumor cannot be assessed
- pT0: no evidence of primary tumor
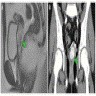
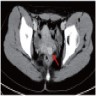
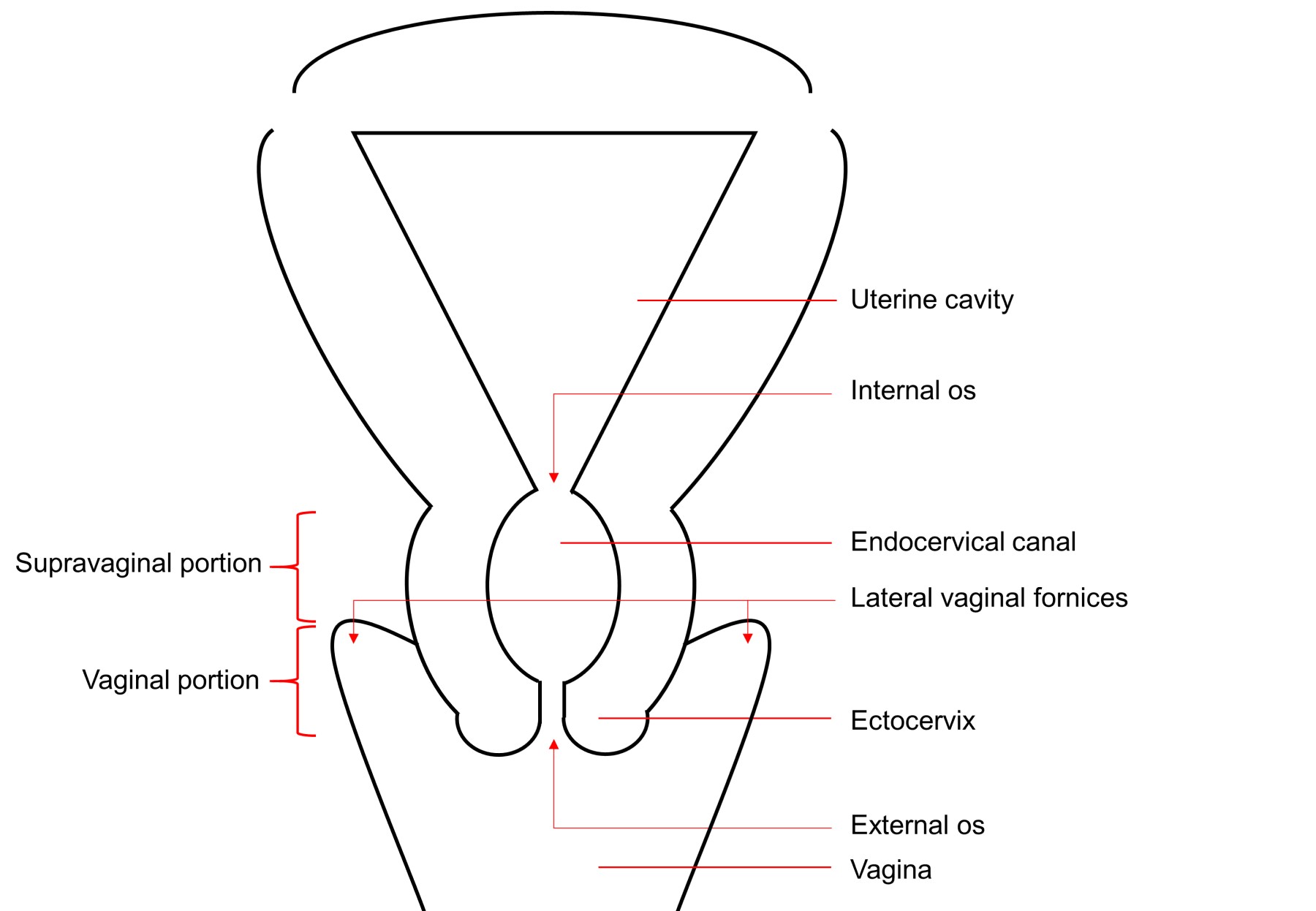
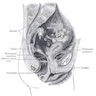
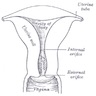
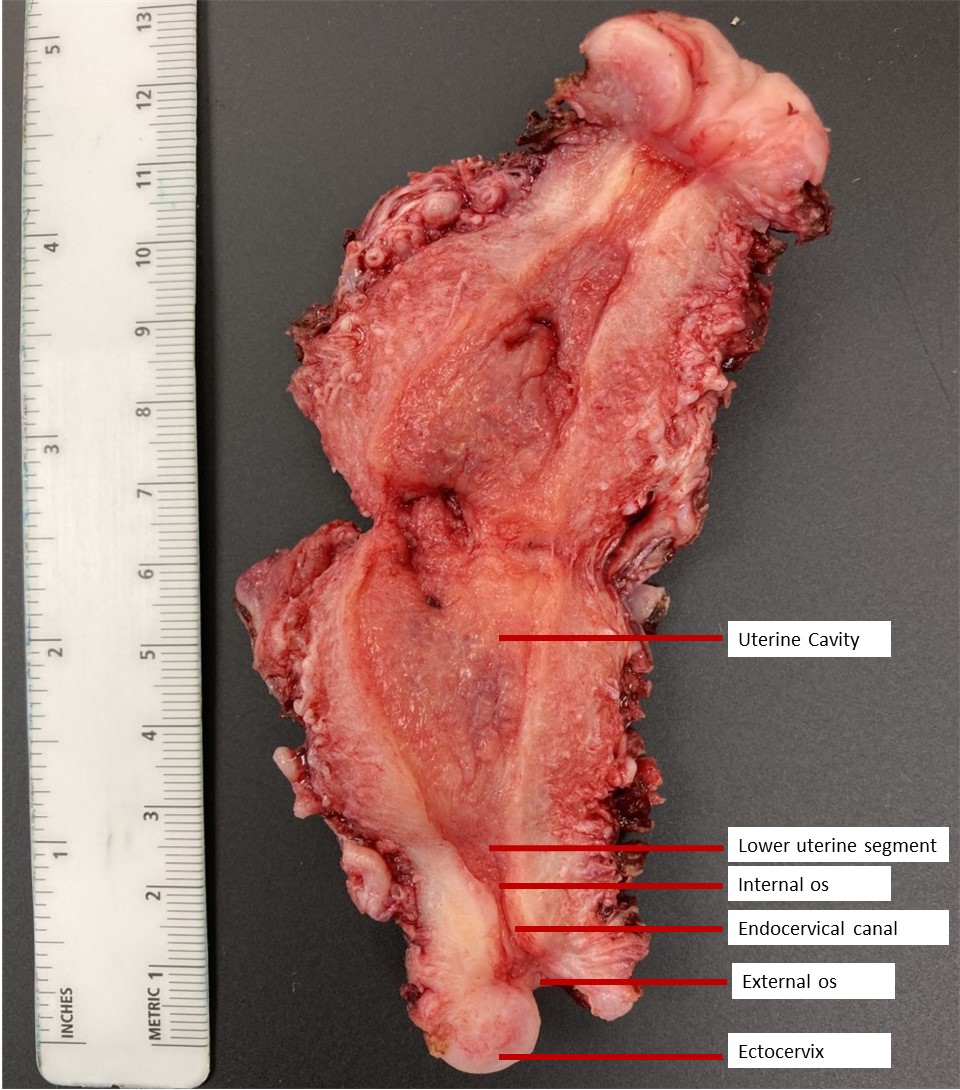
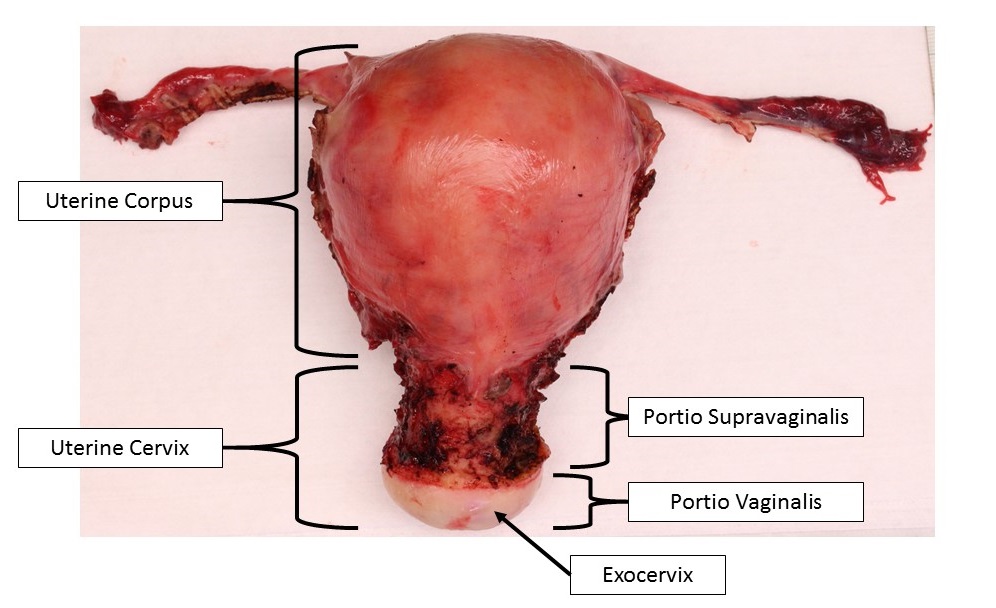
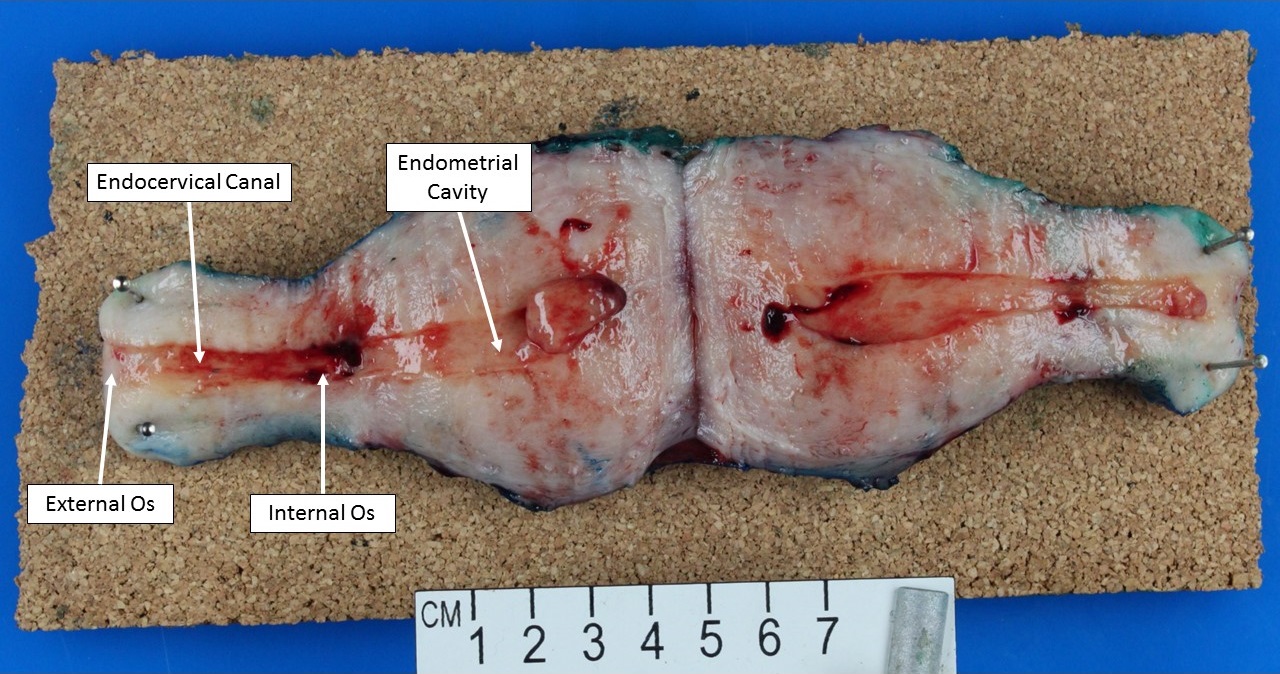
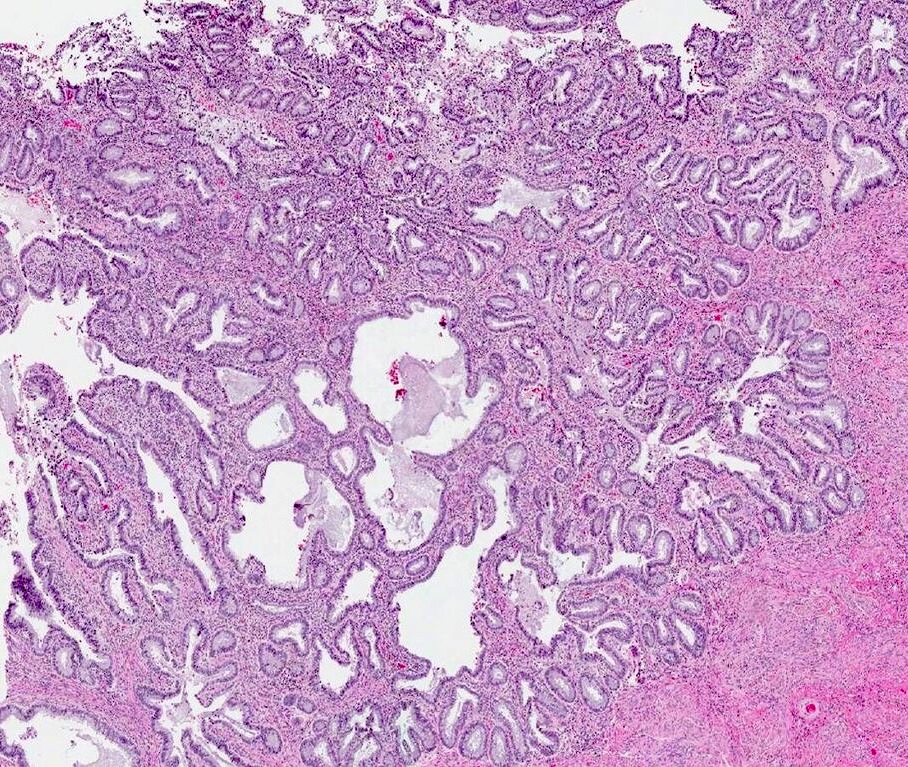
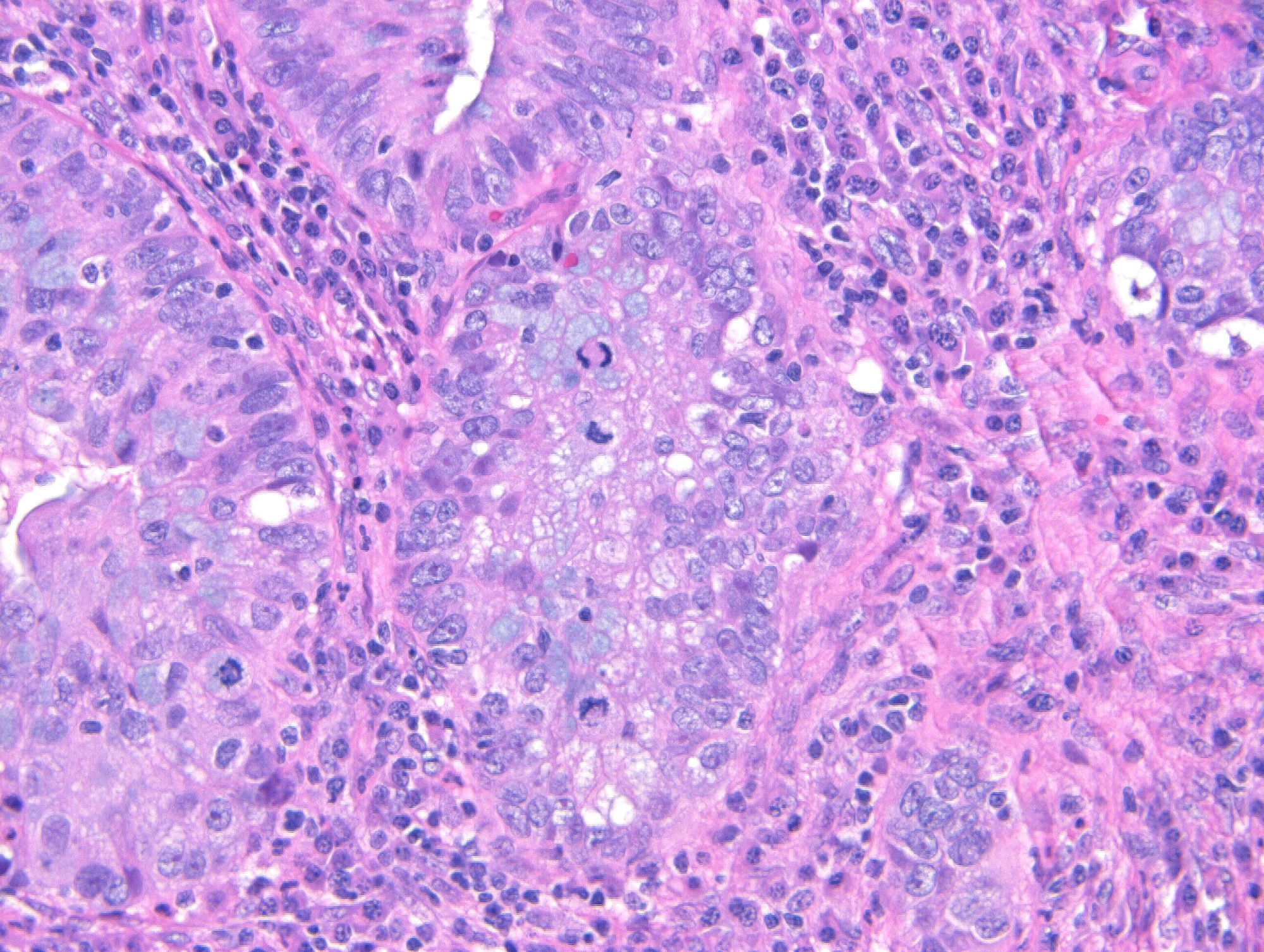
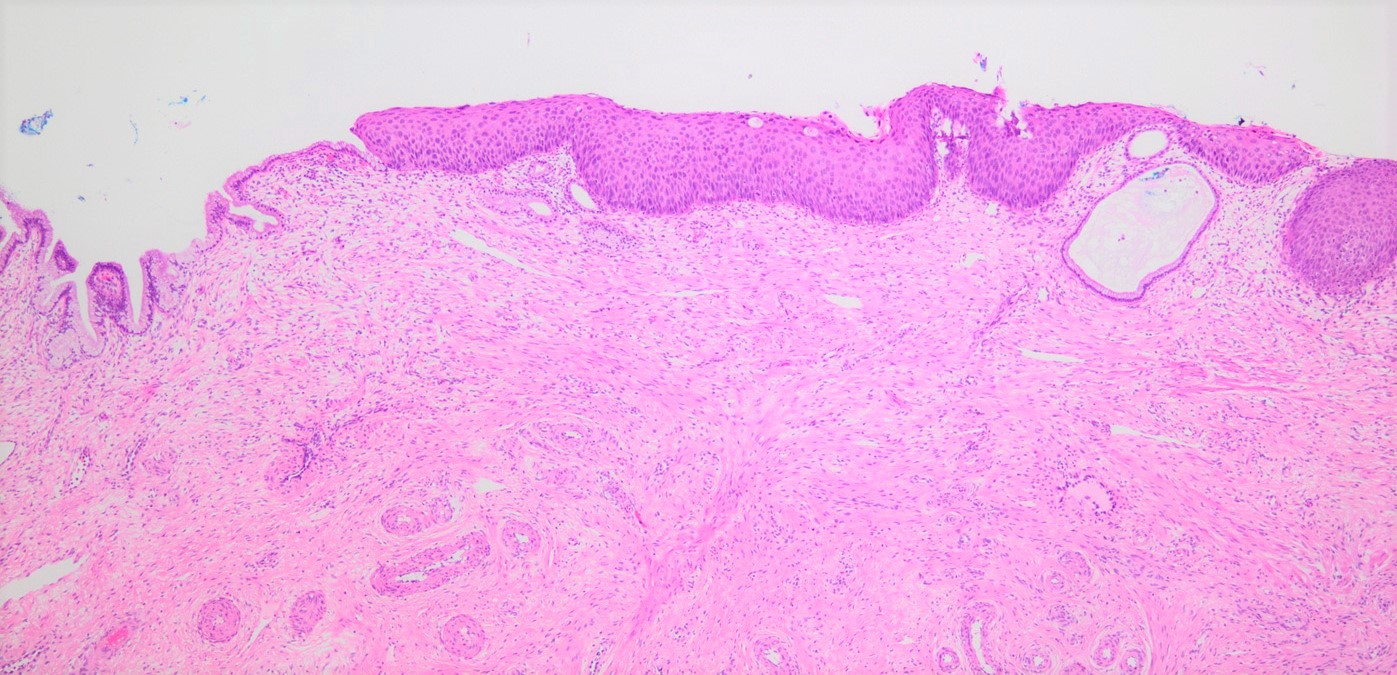
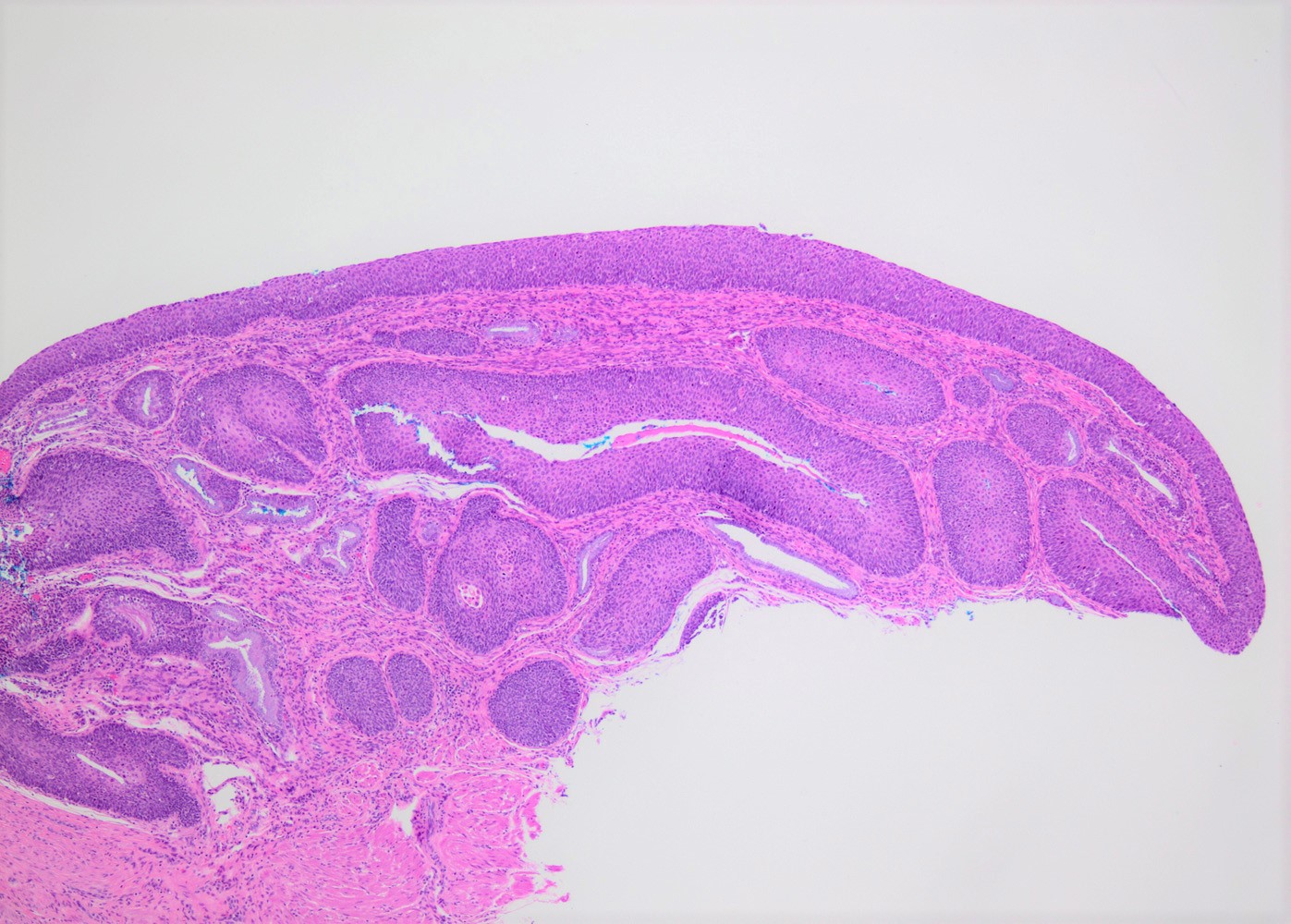
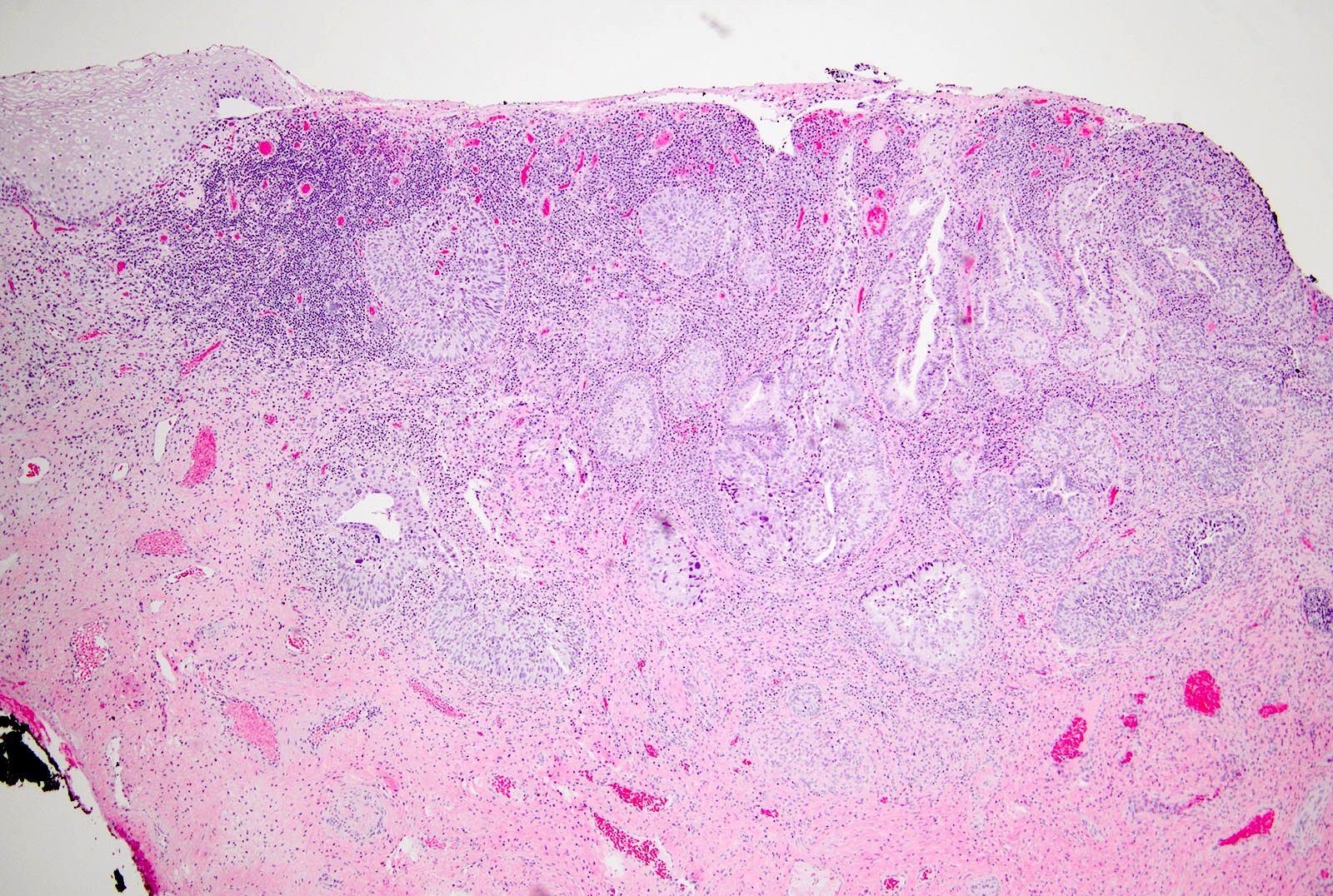
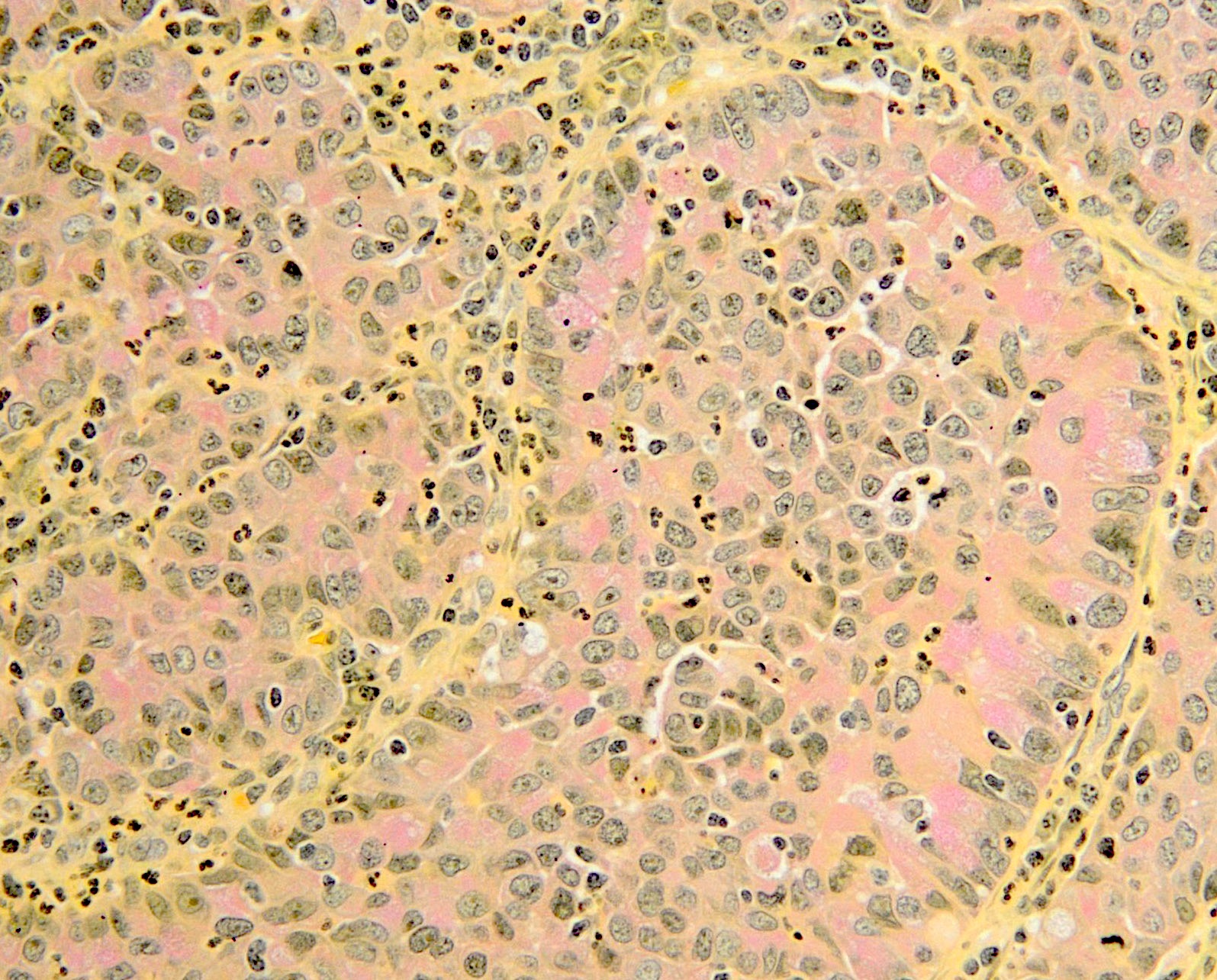
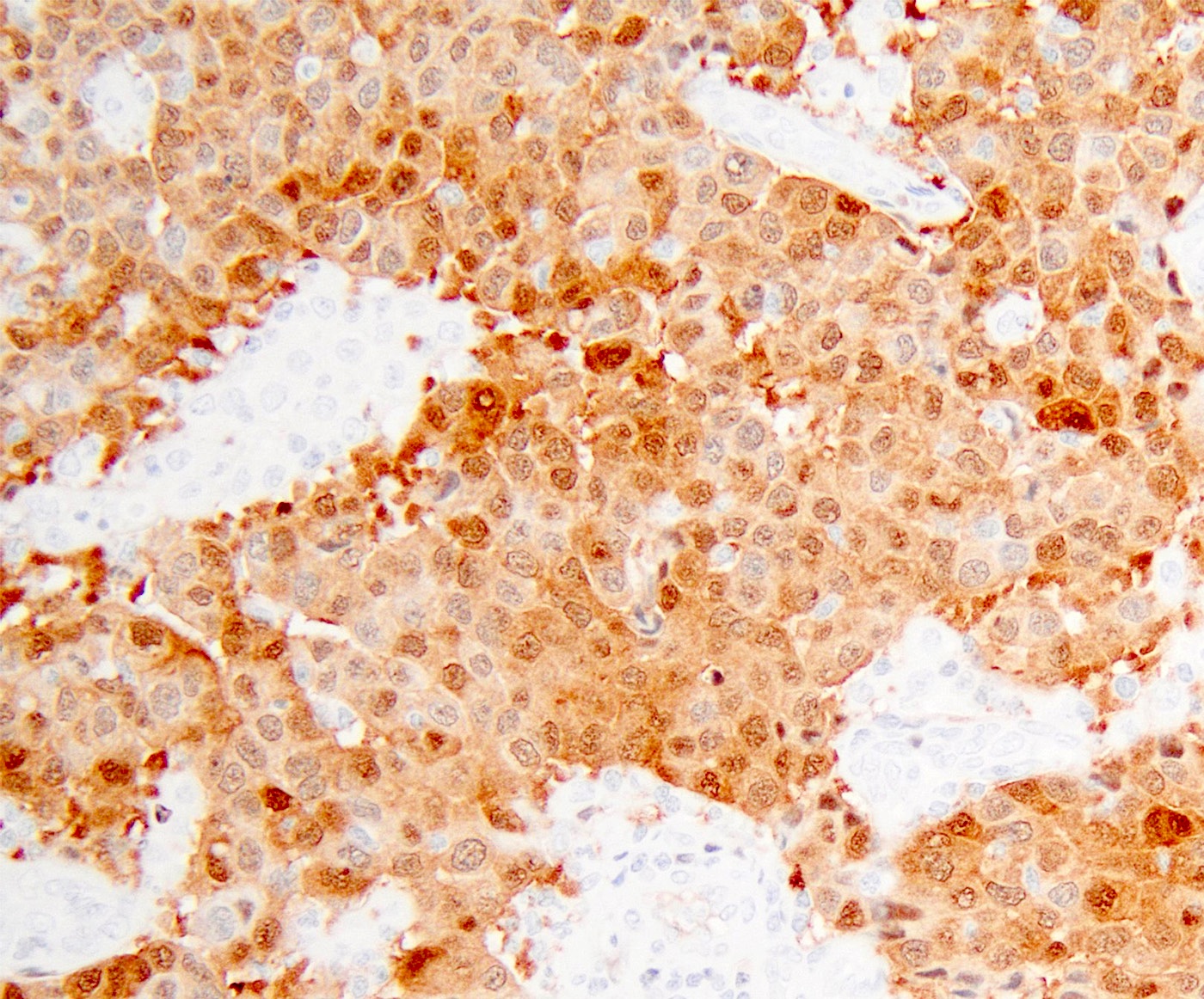
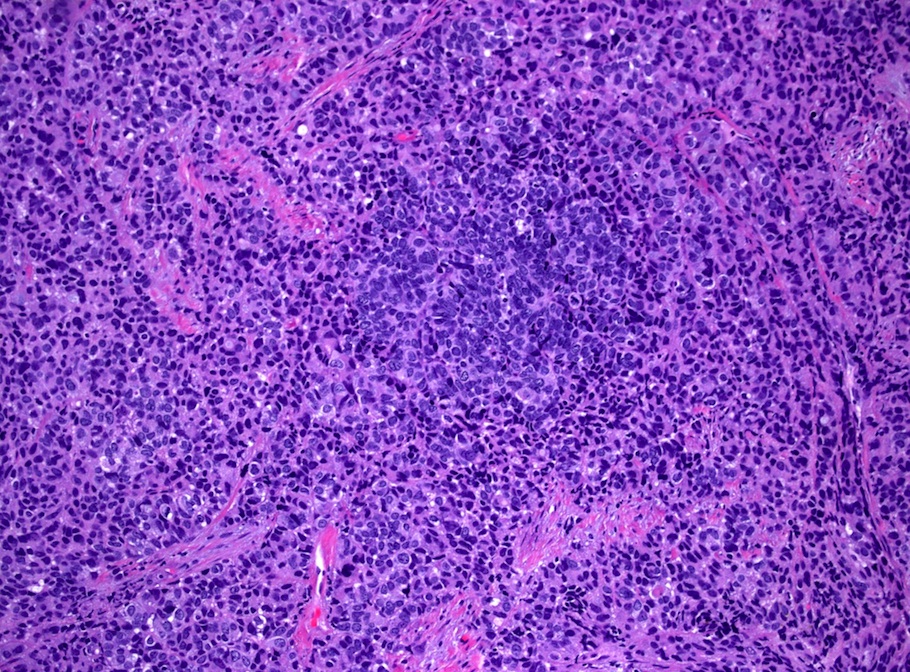
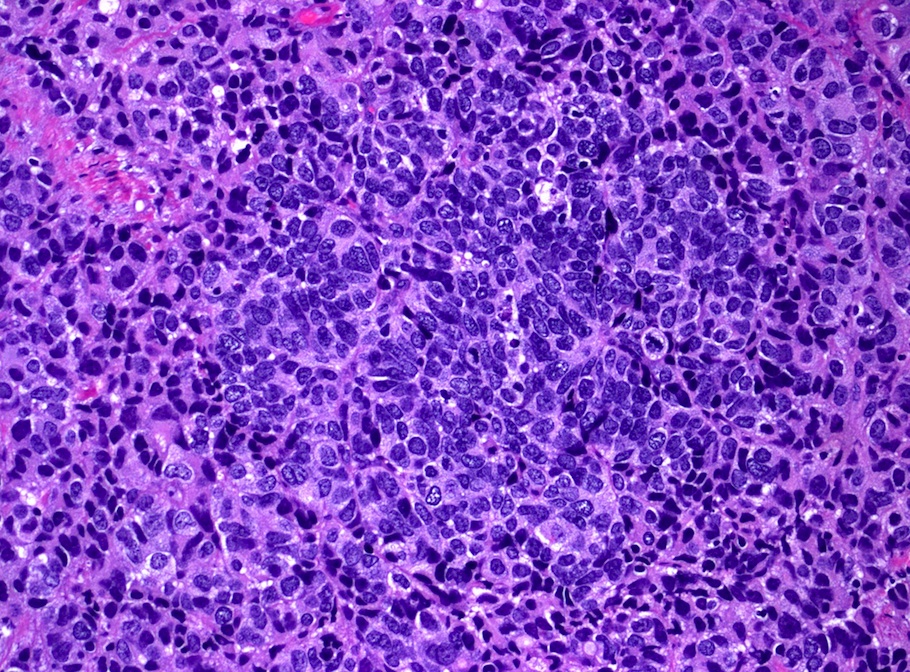
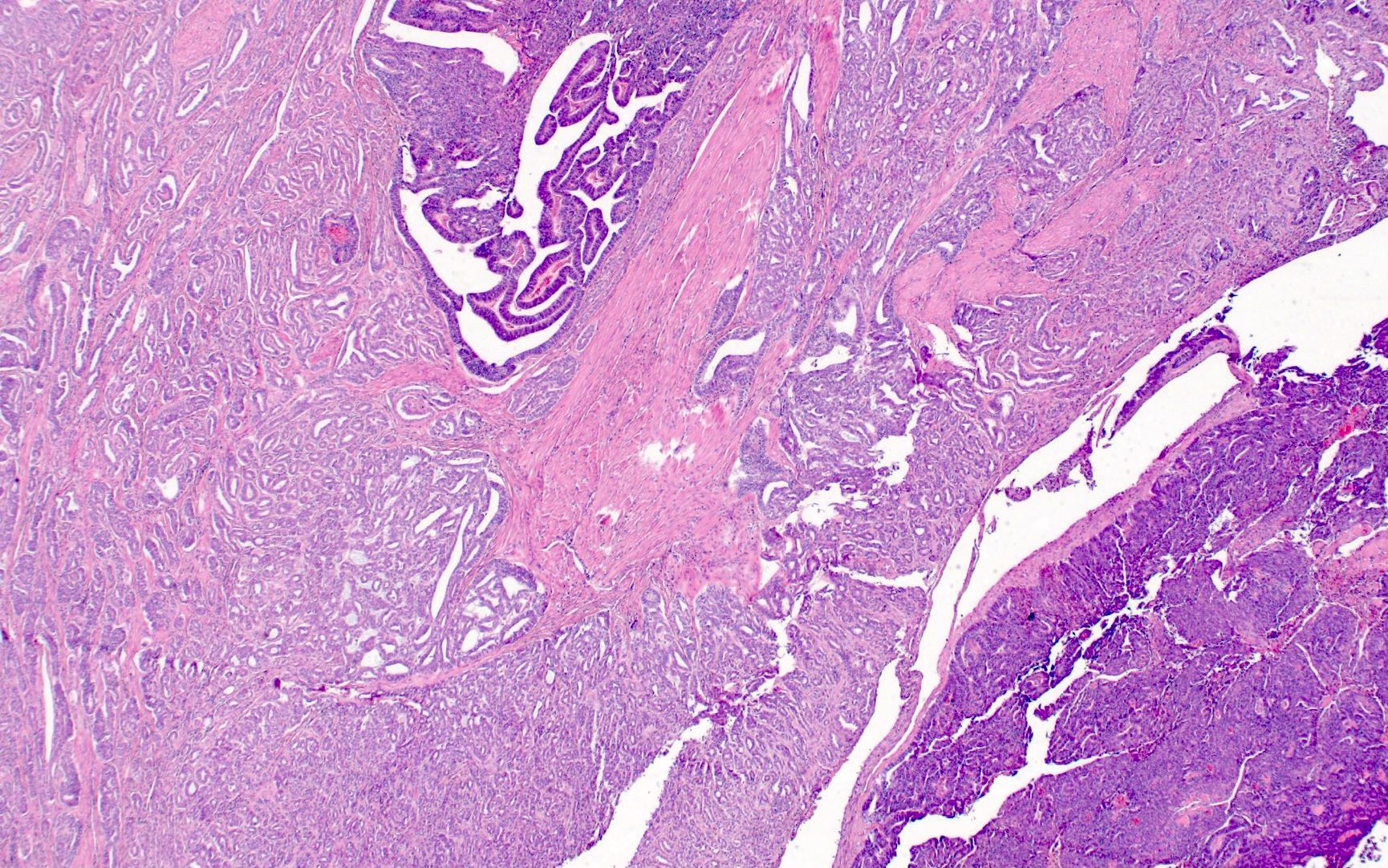
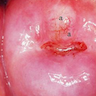
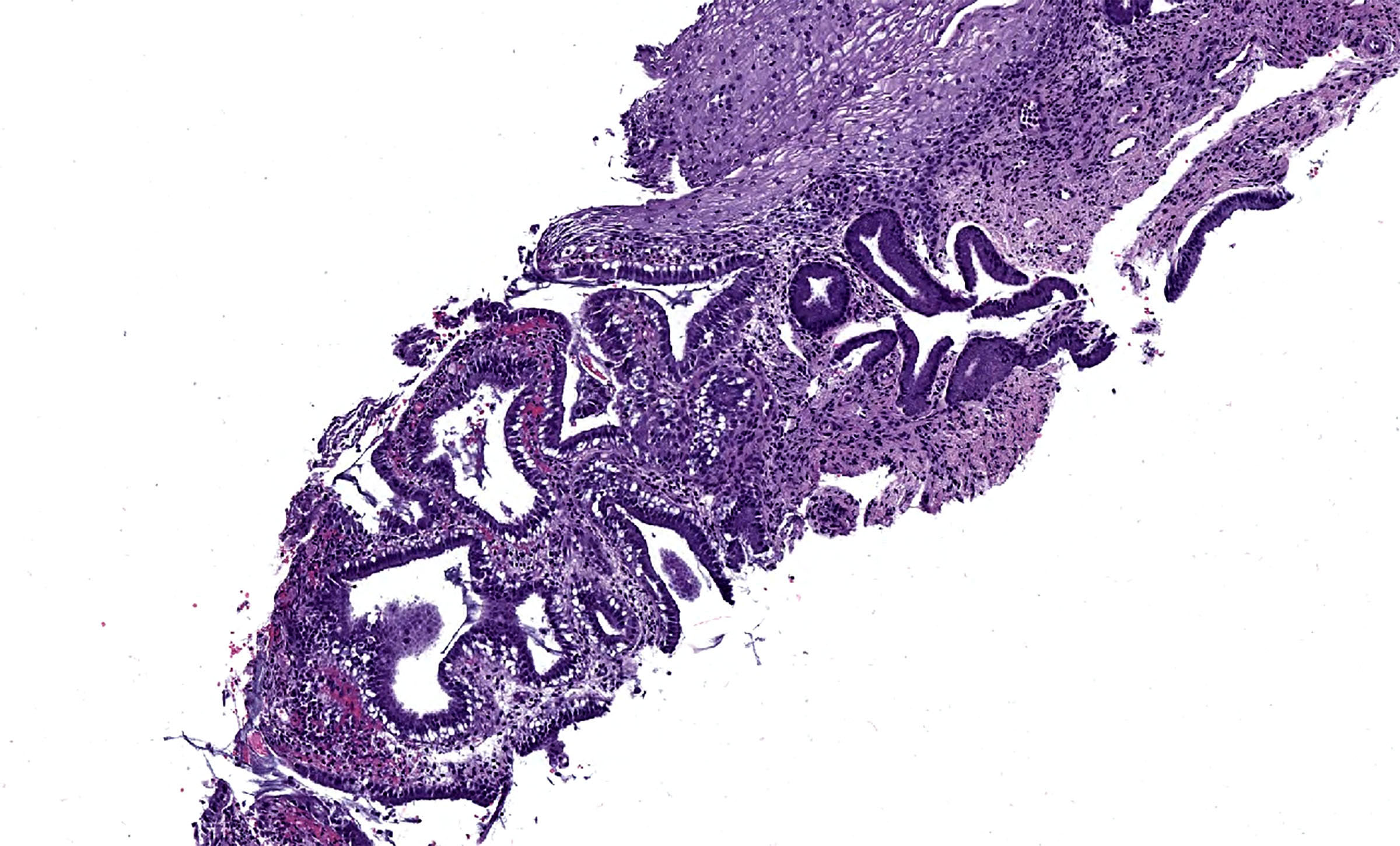
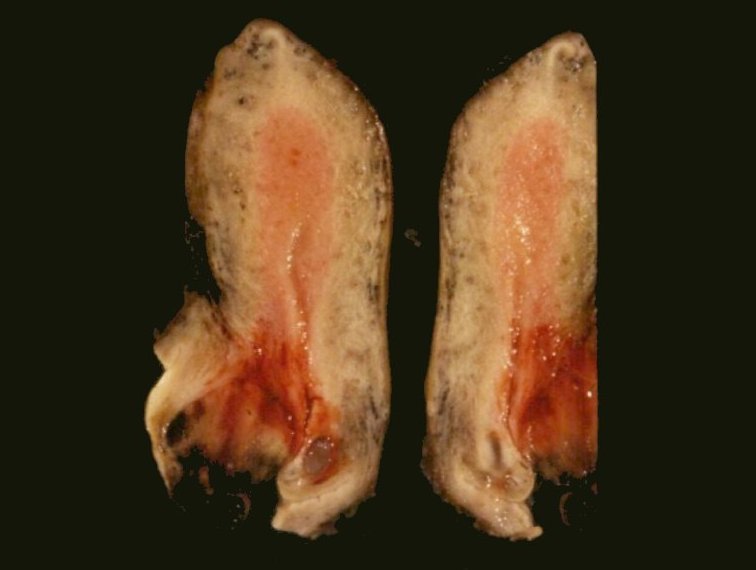
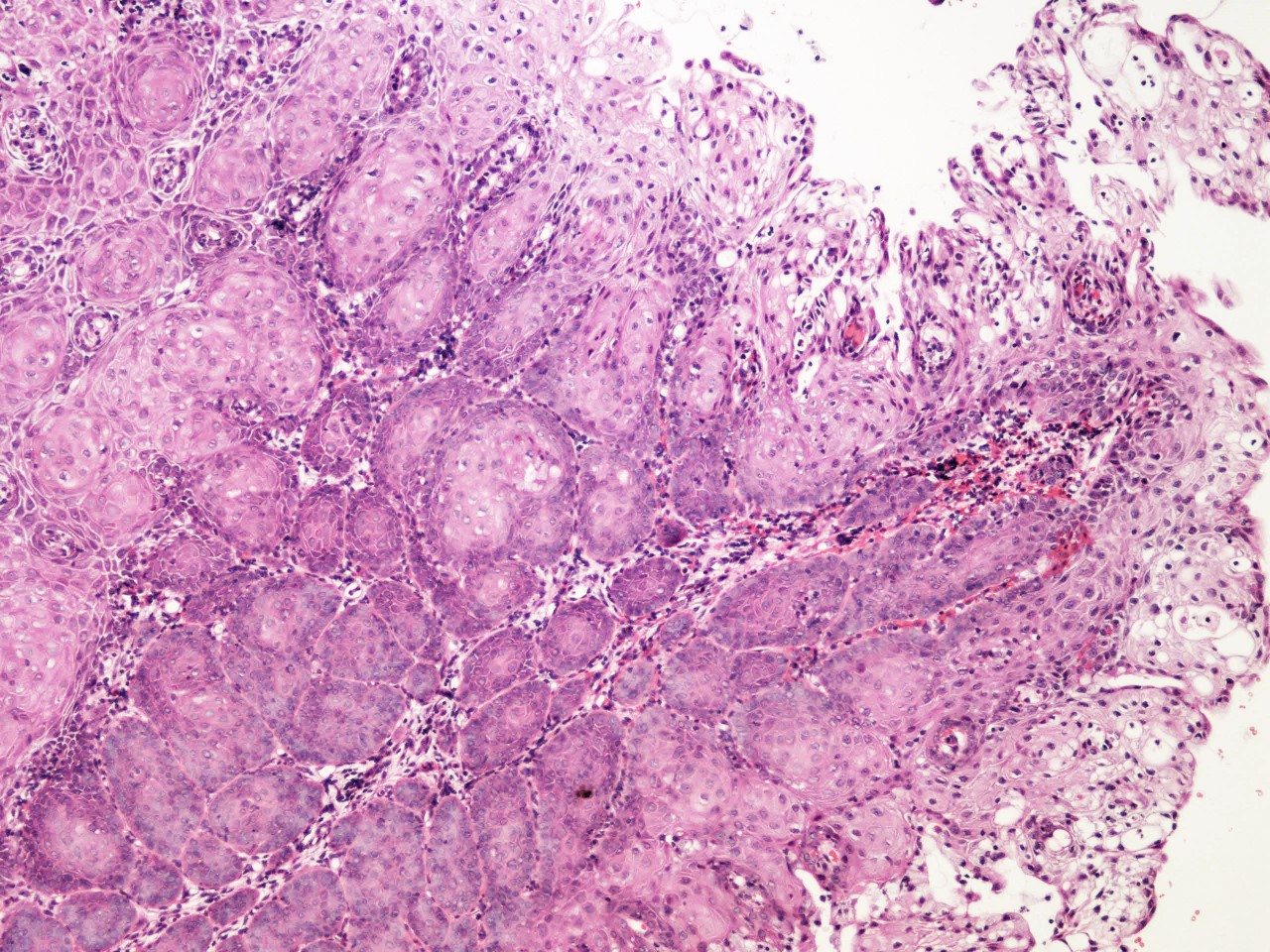
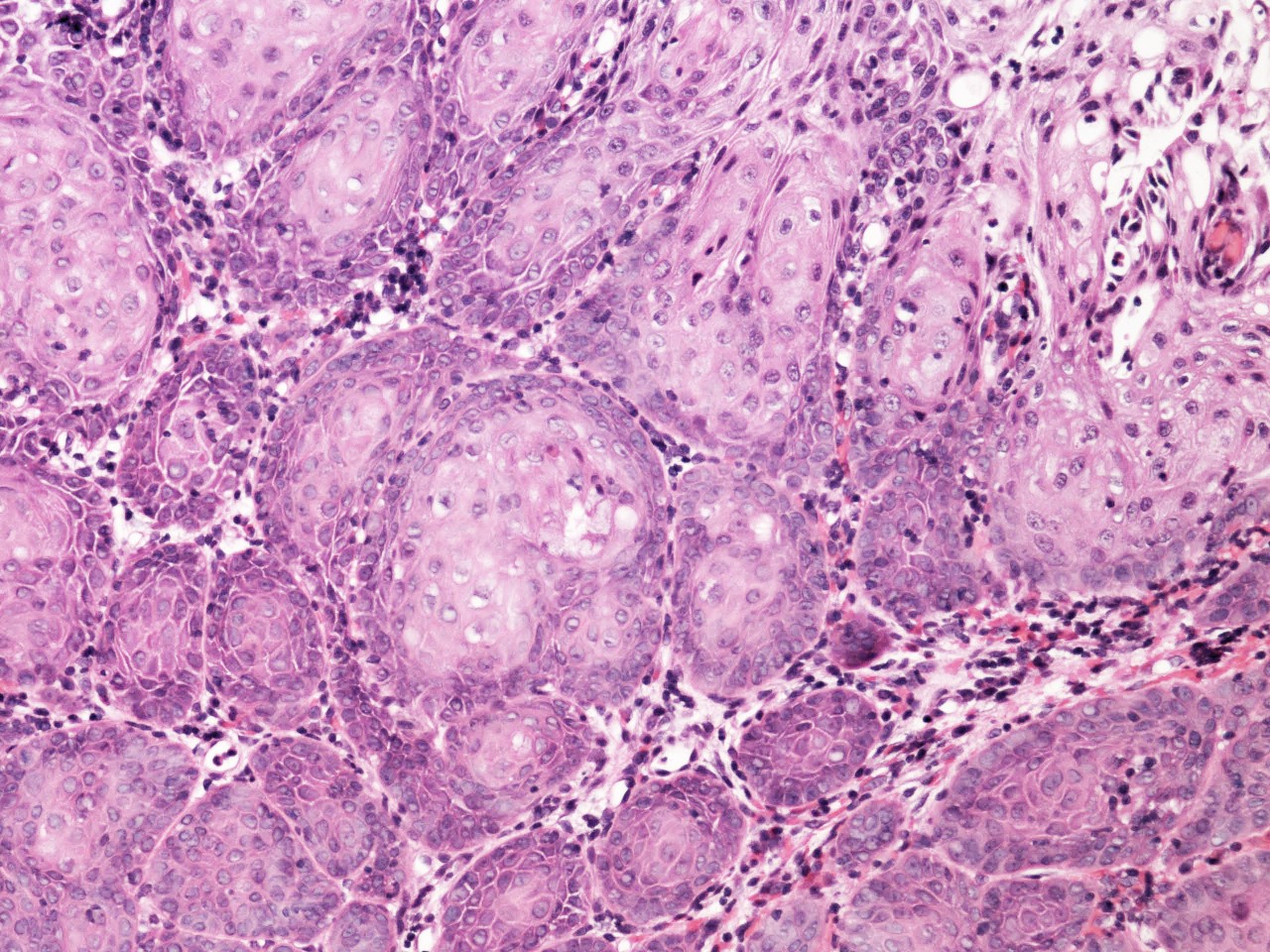
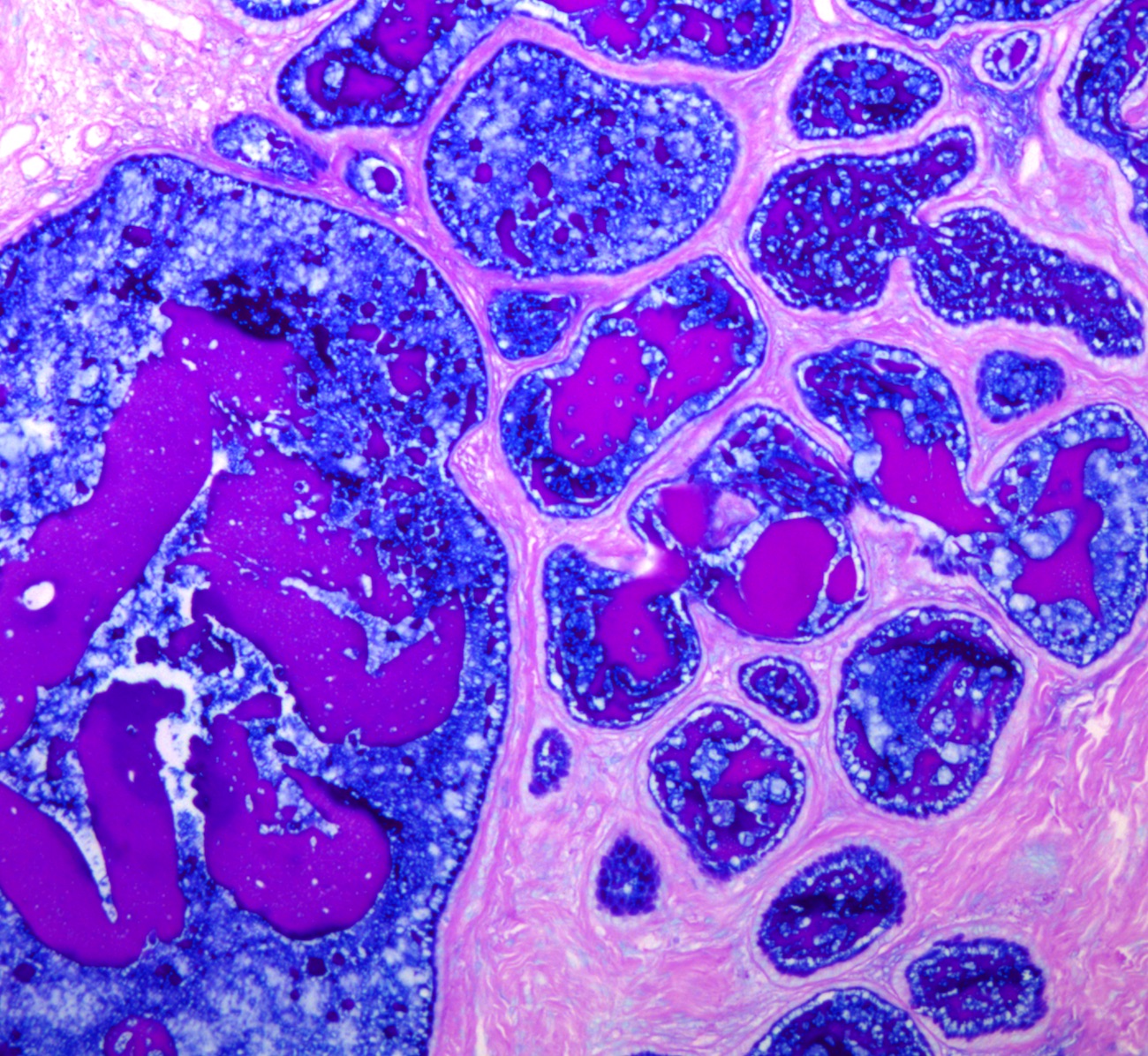
- pT1: cervical carcinoma confined to uterus (extension to corpus should be disregarded)
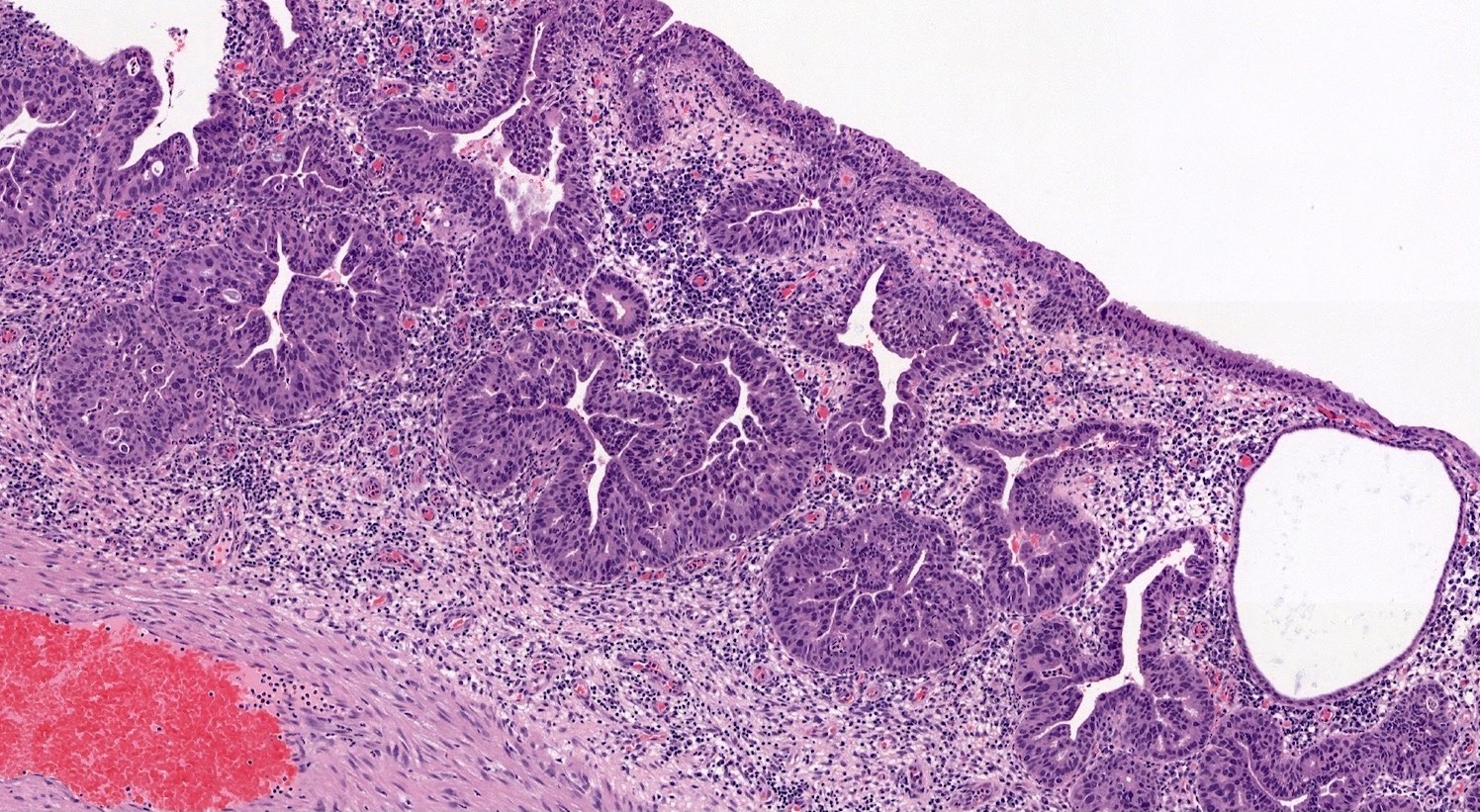
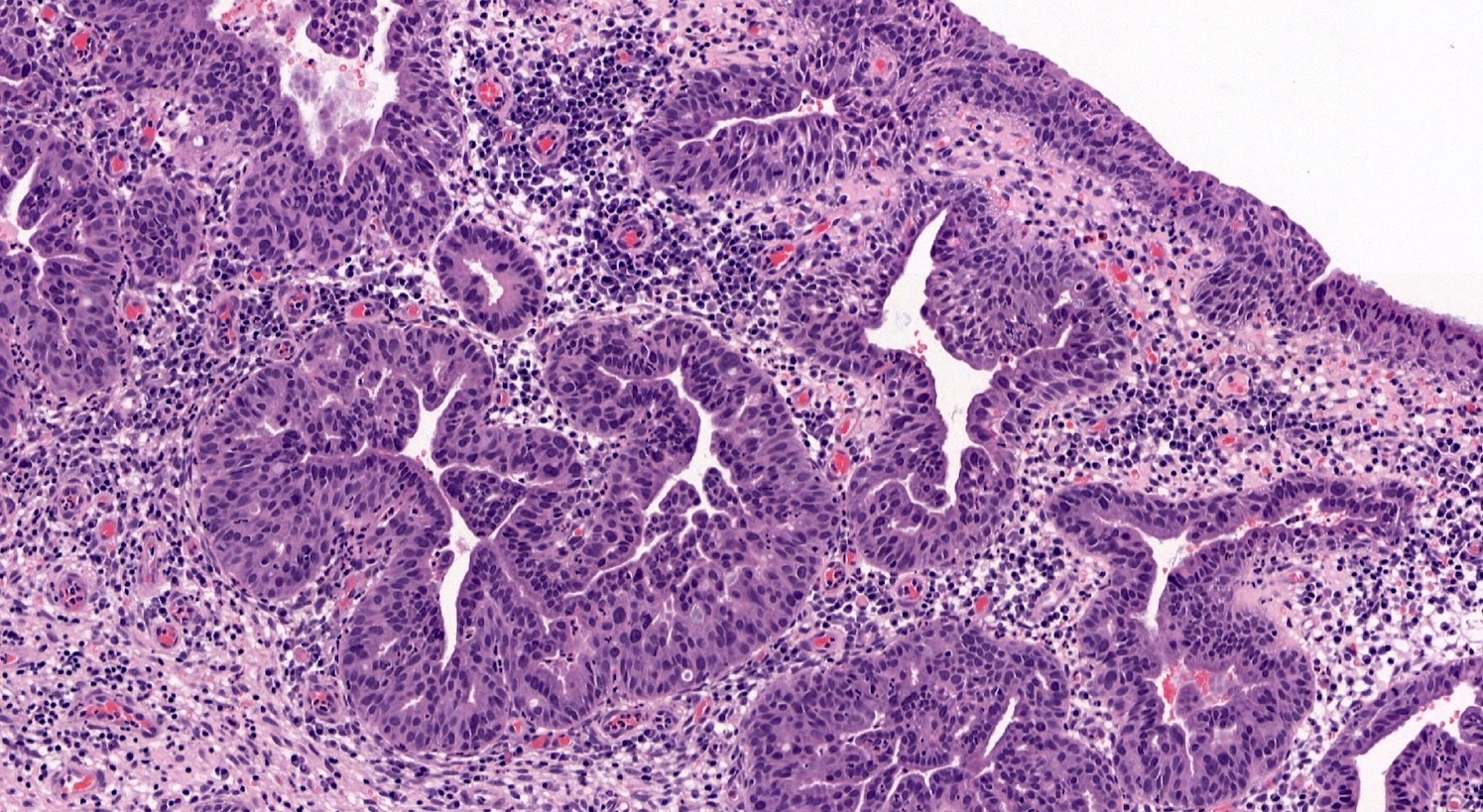
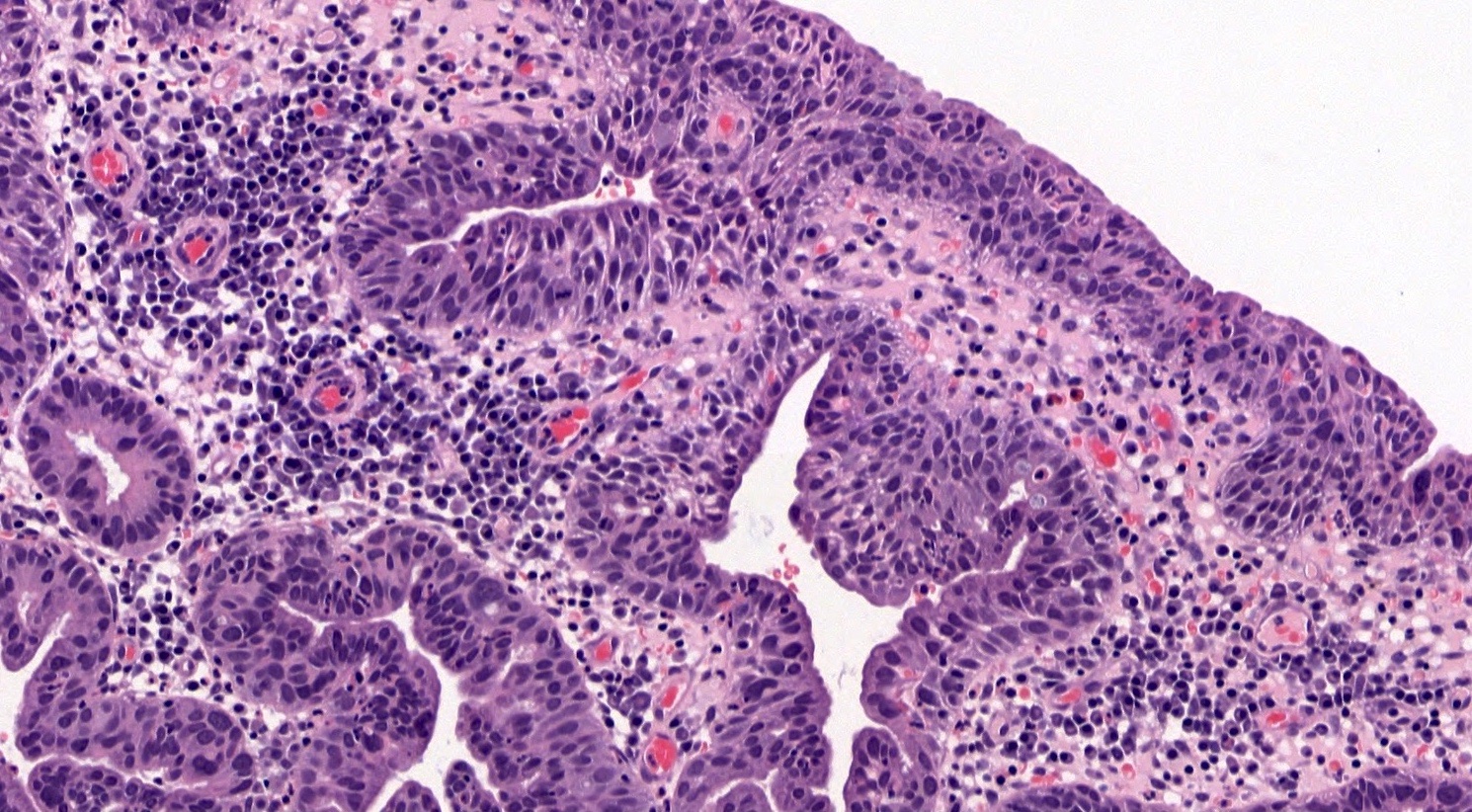
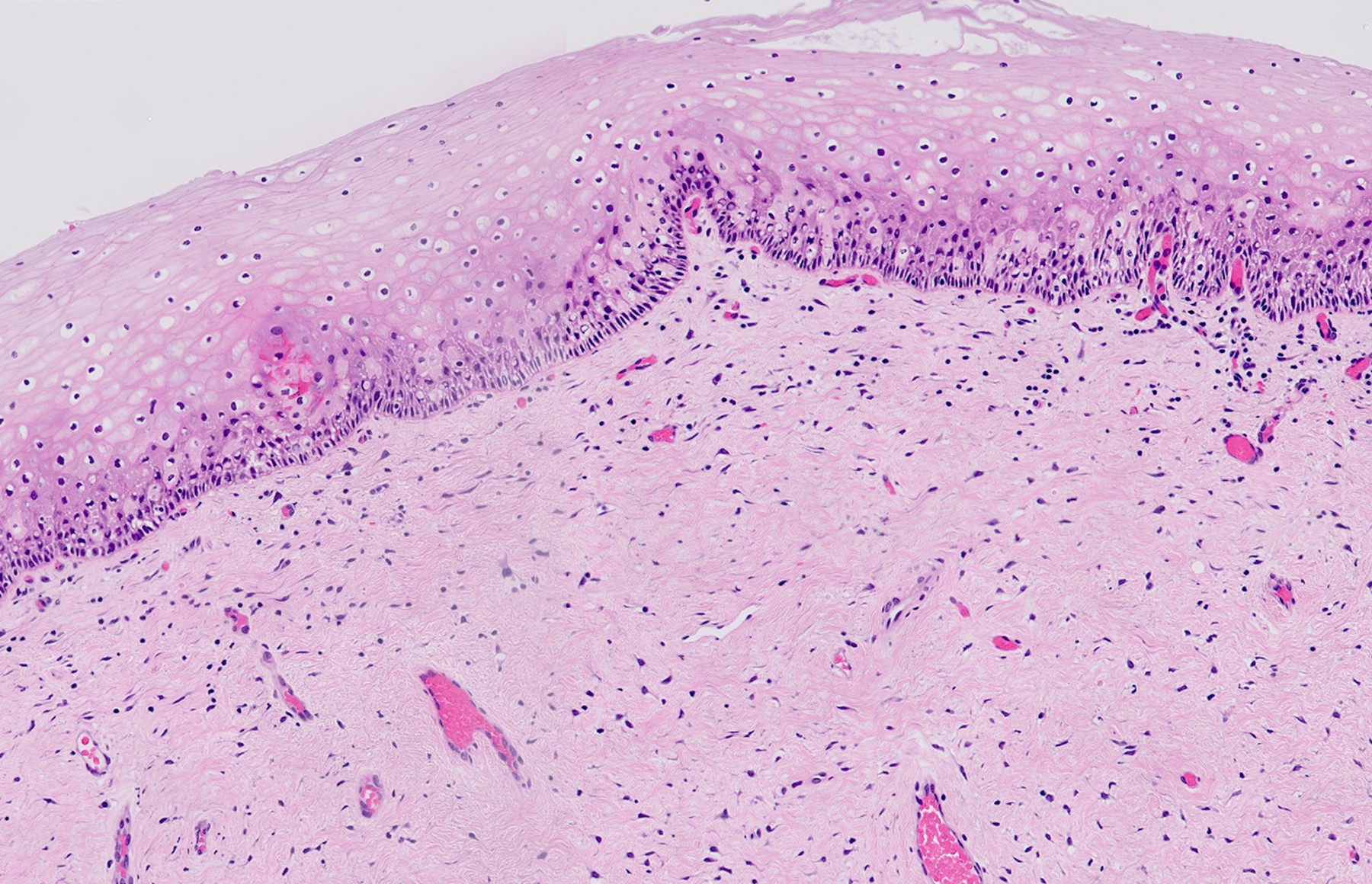
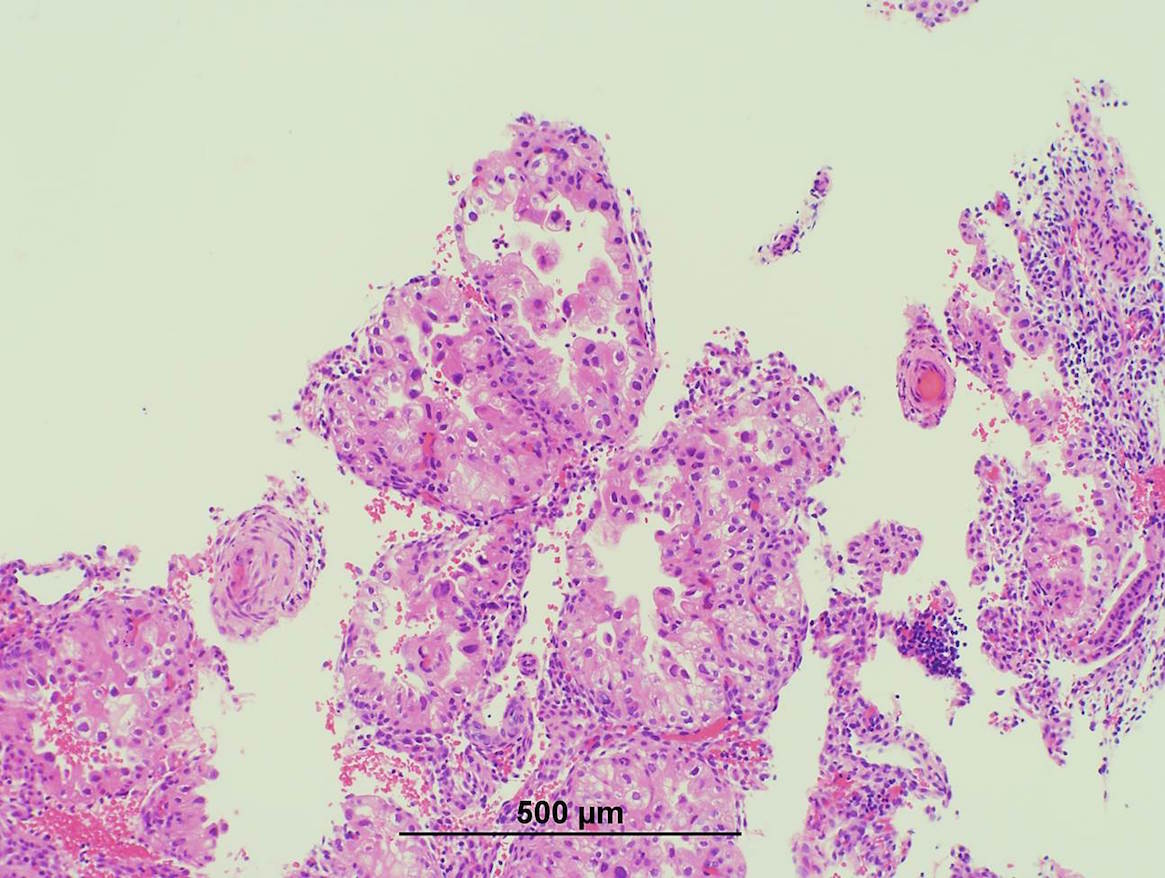
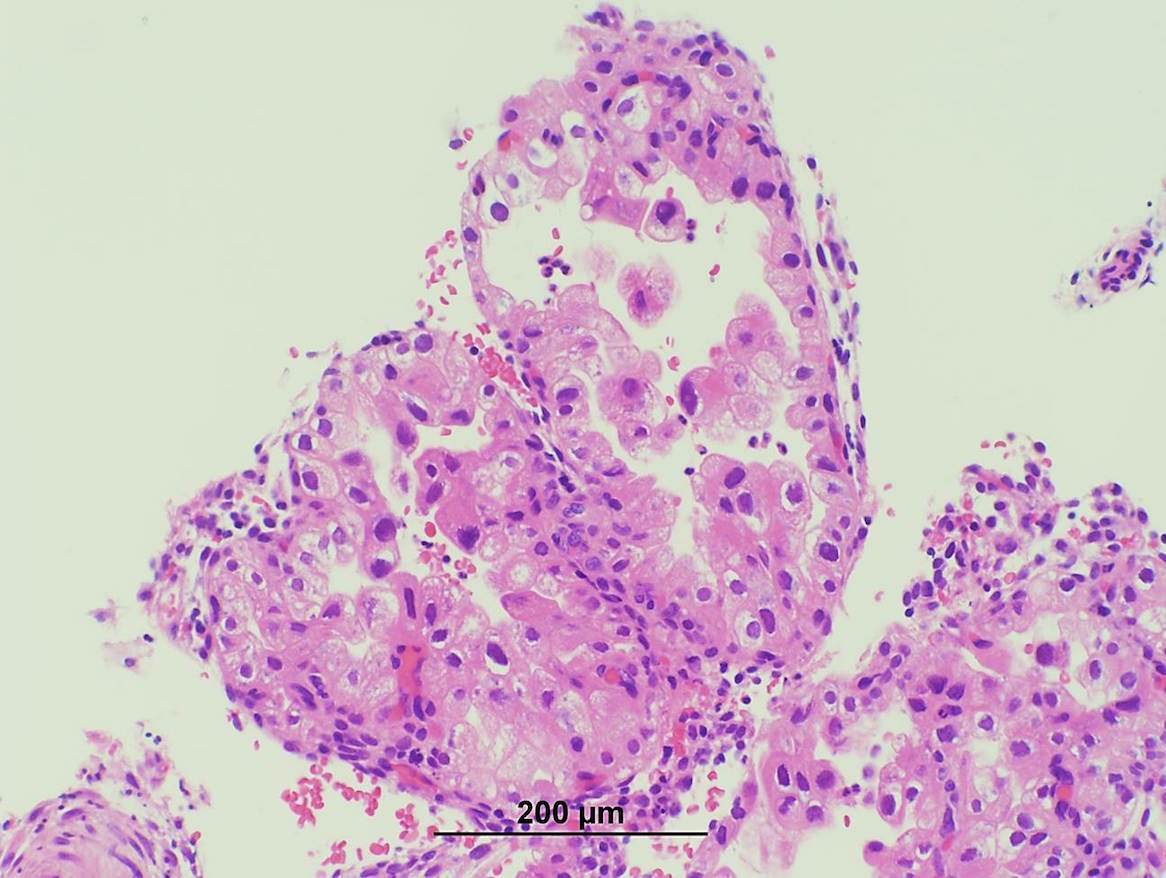
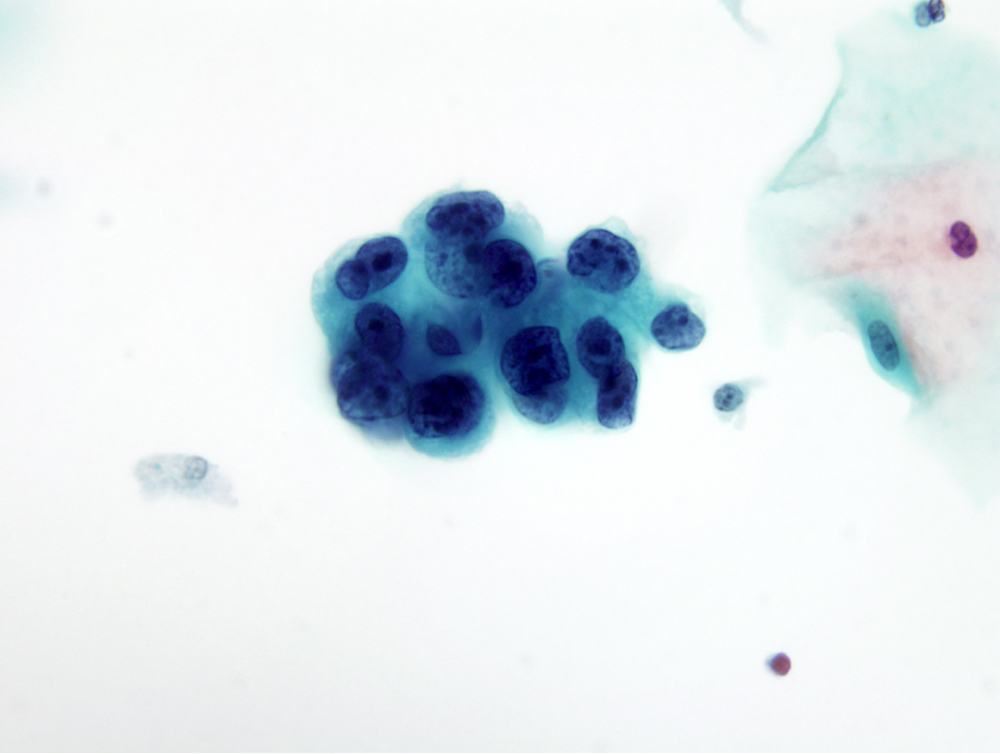
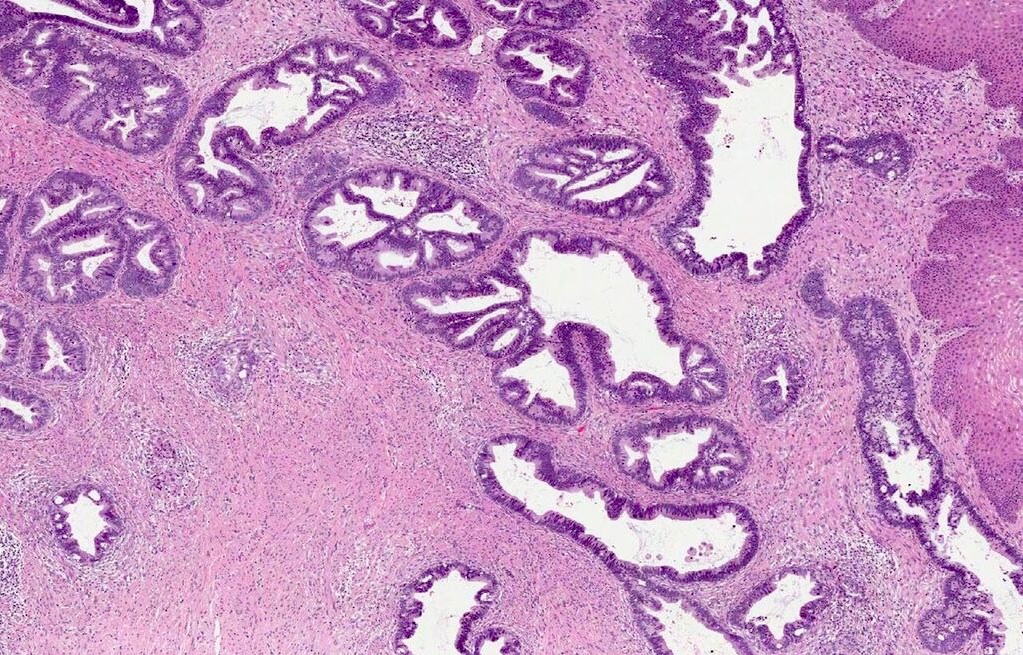
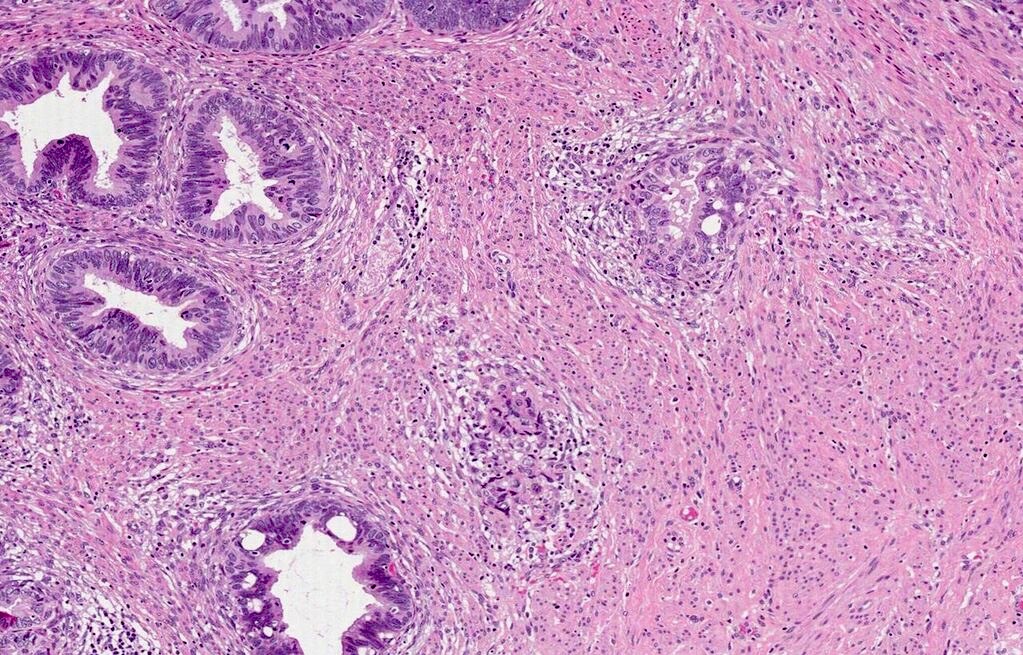
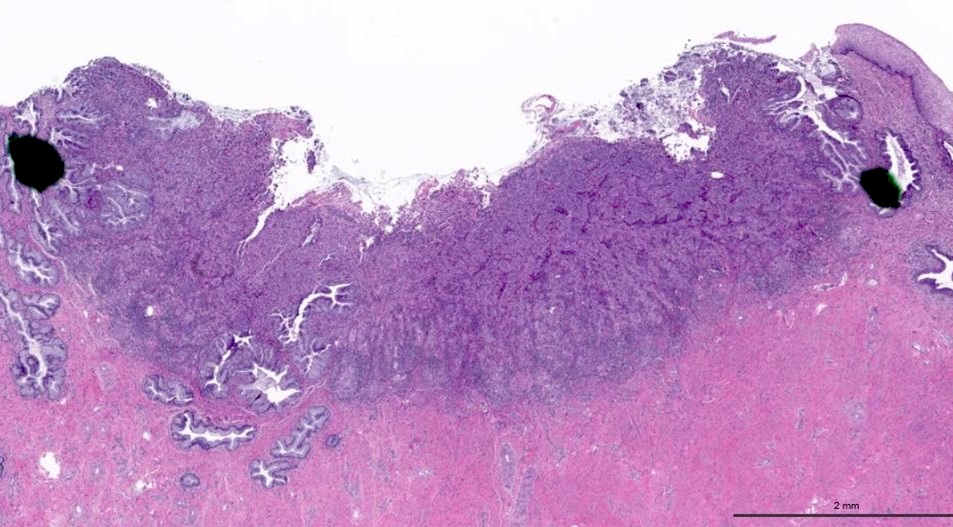
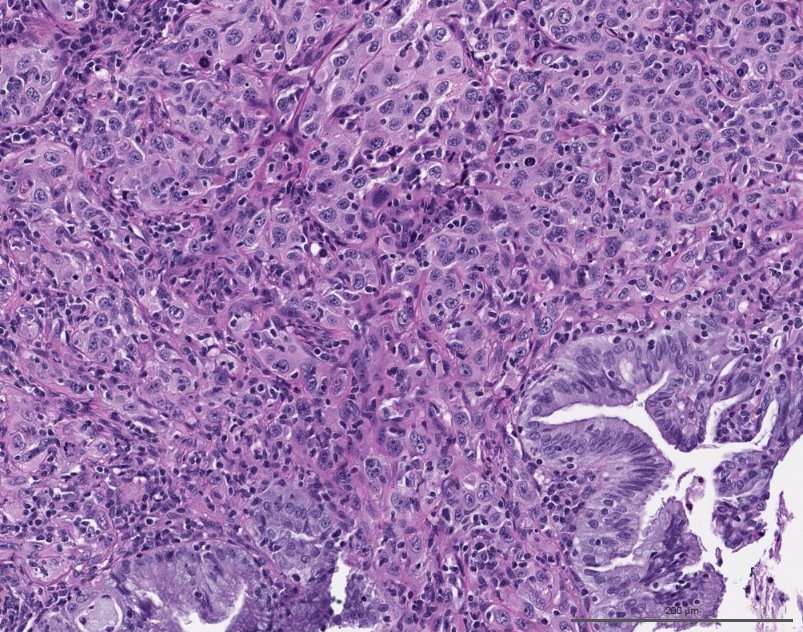
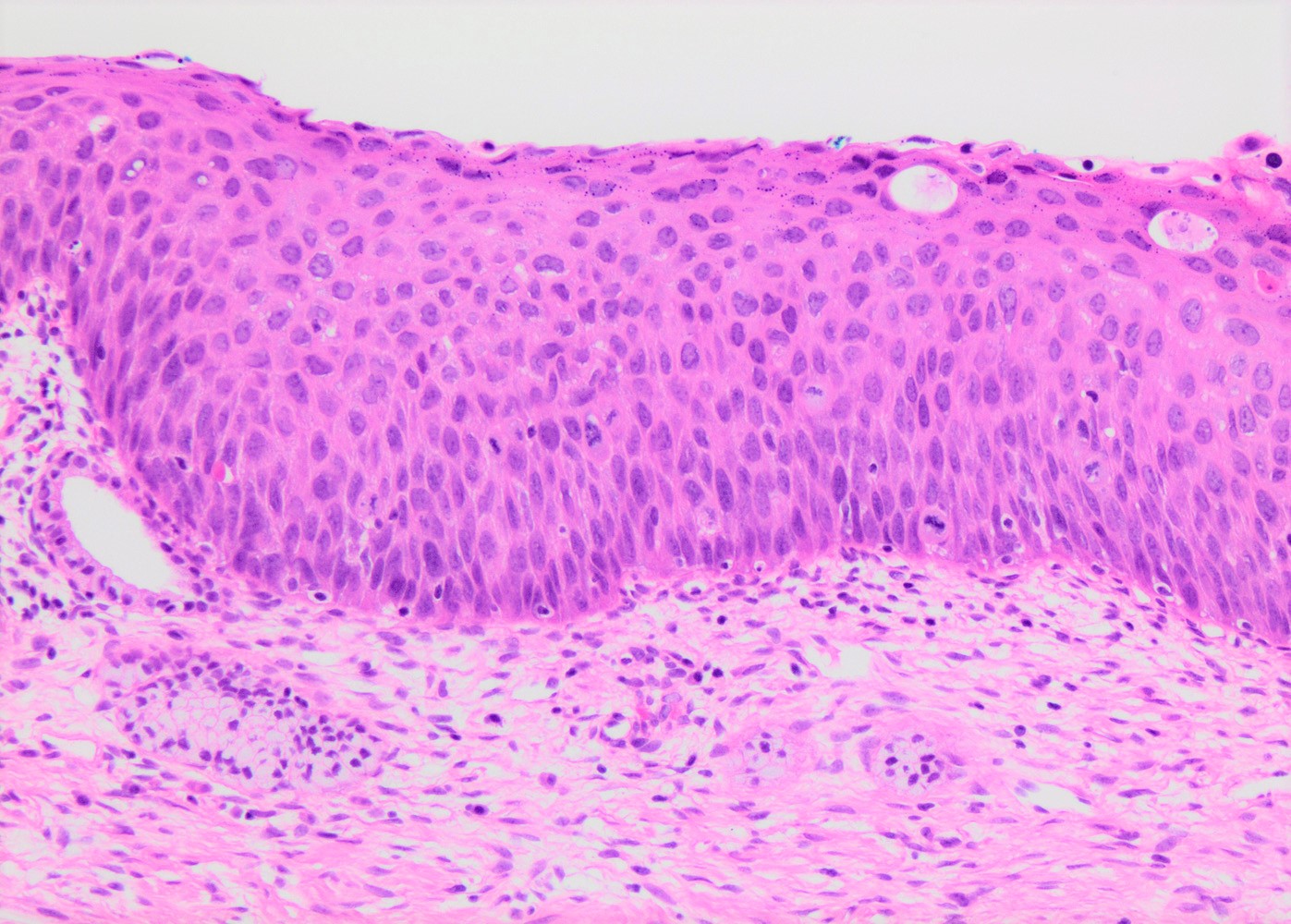
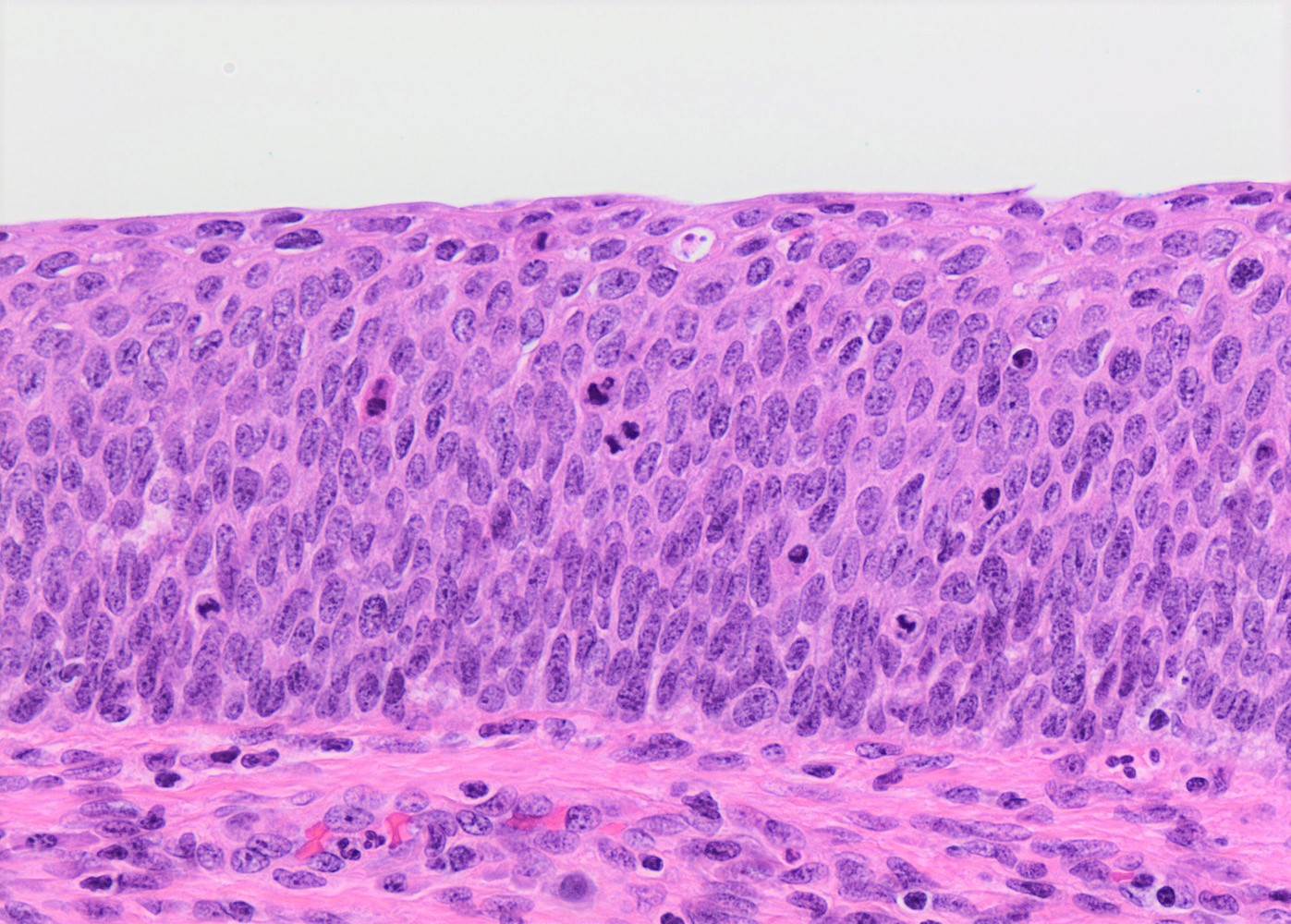
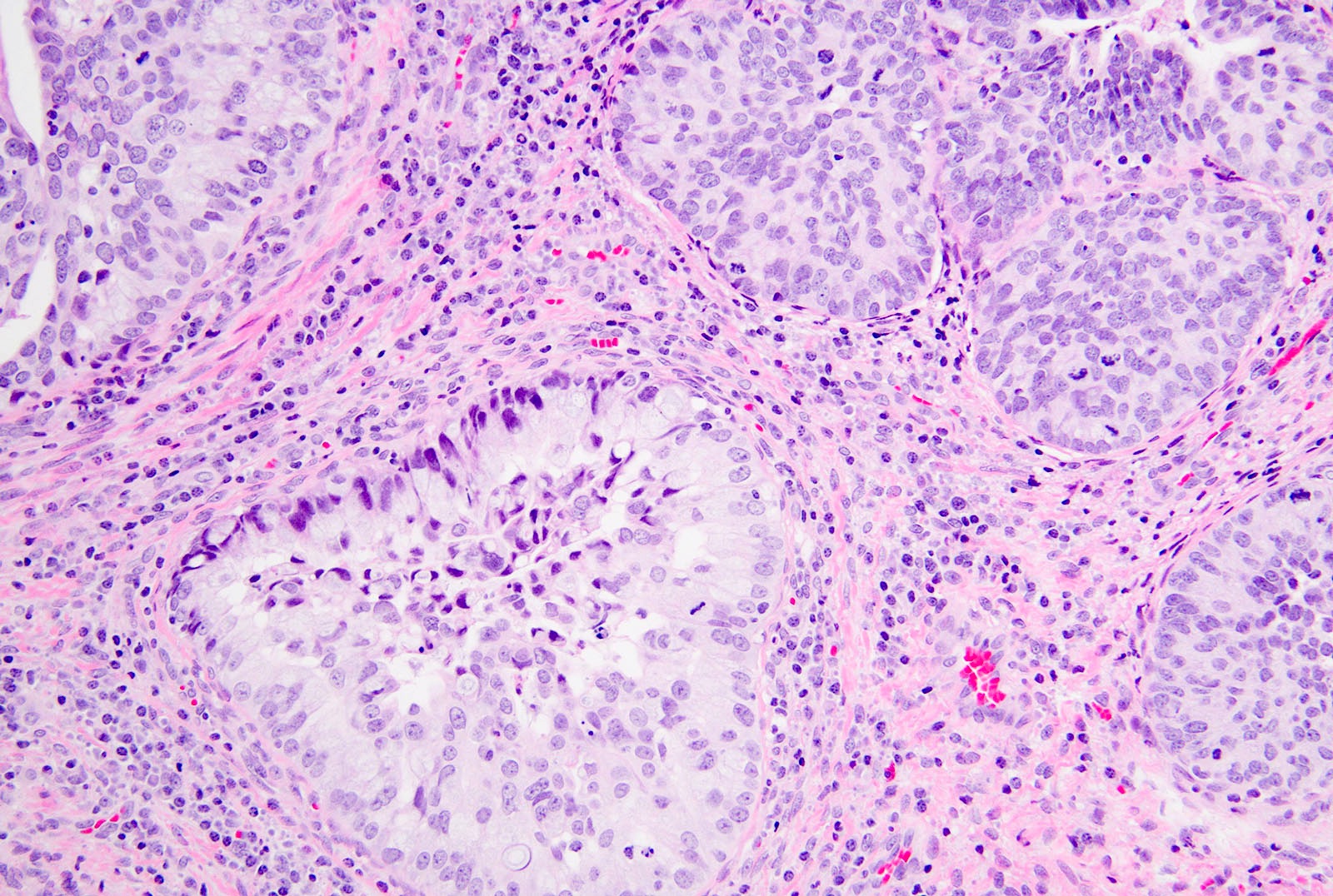
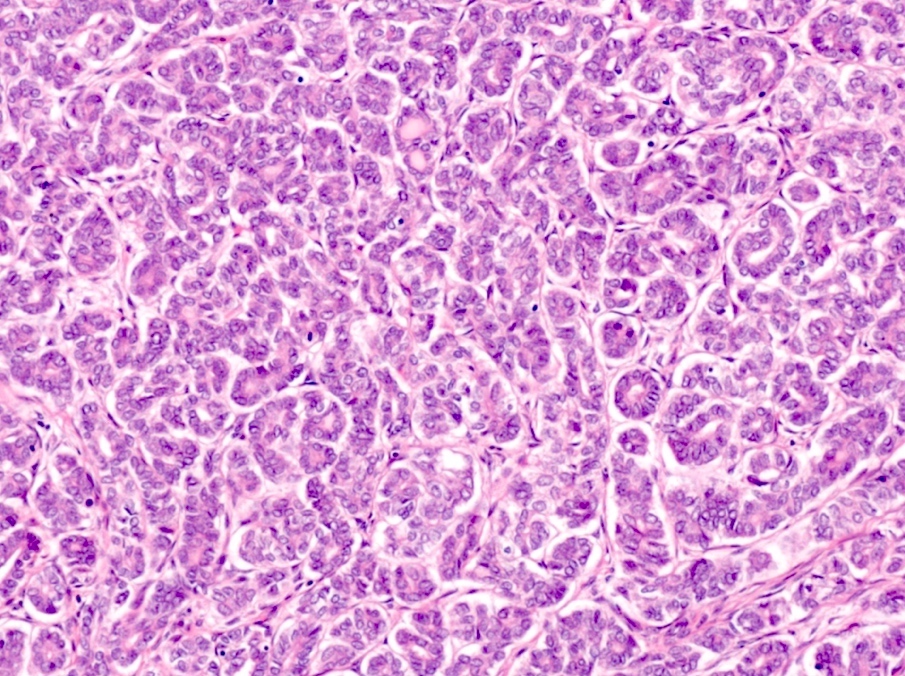
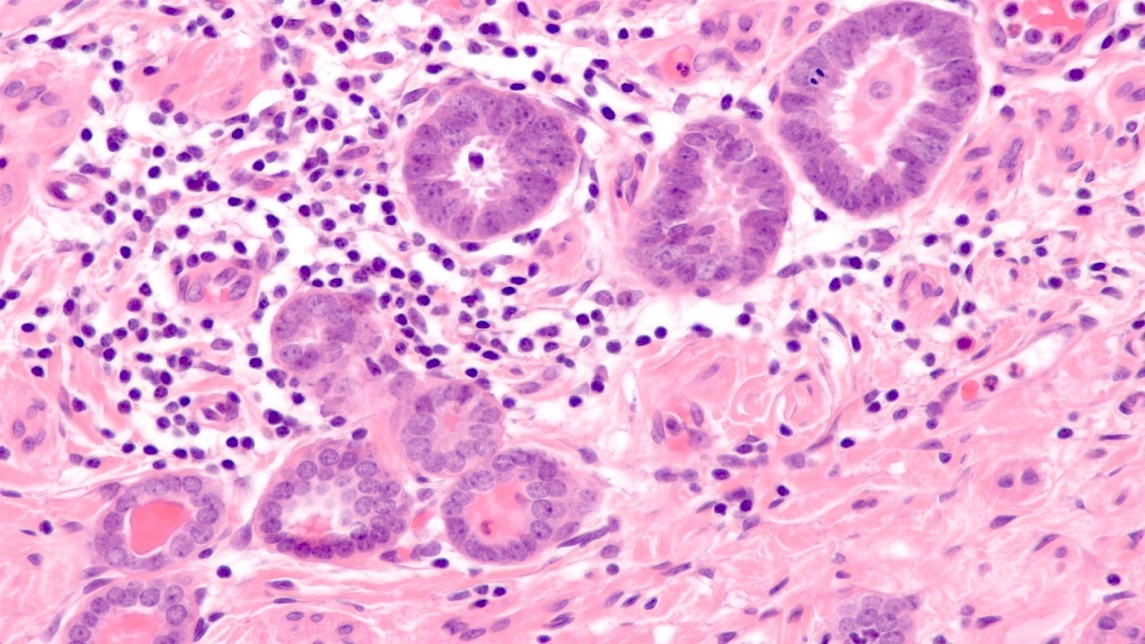
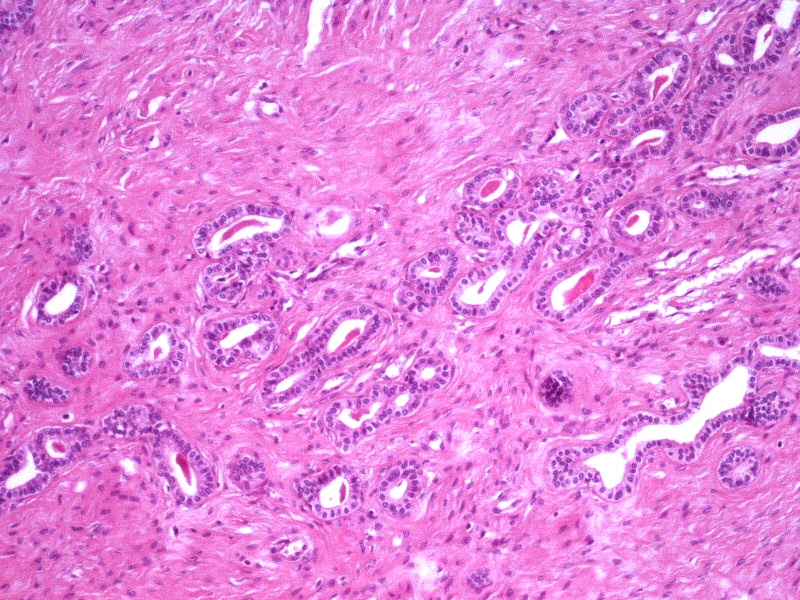
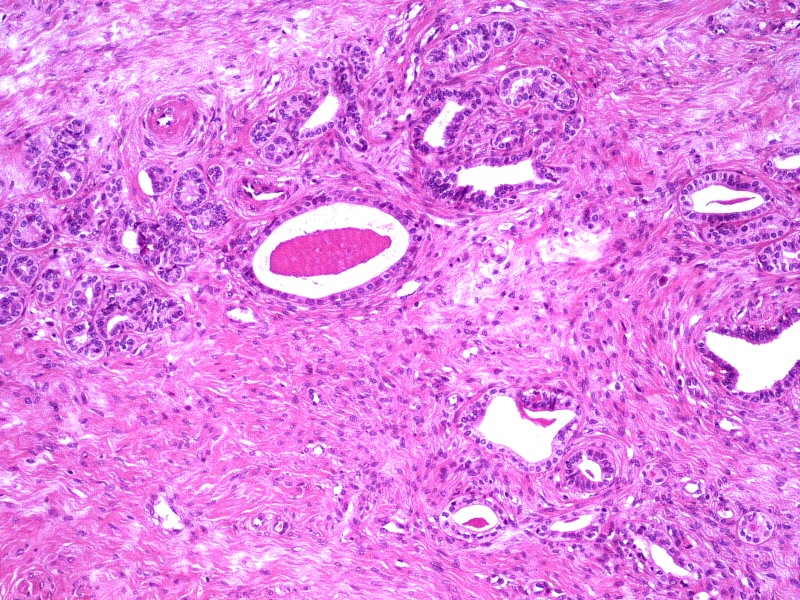
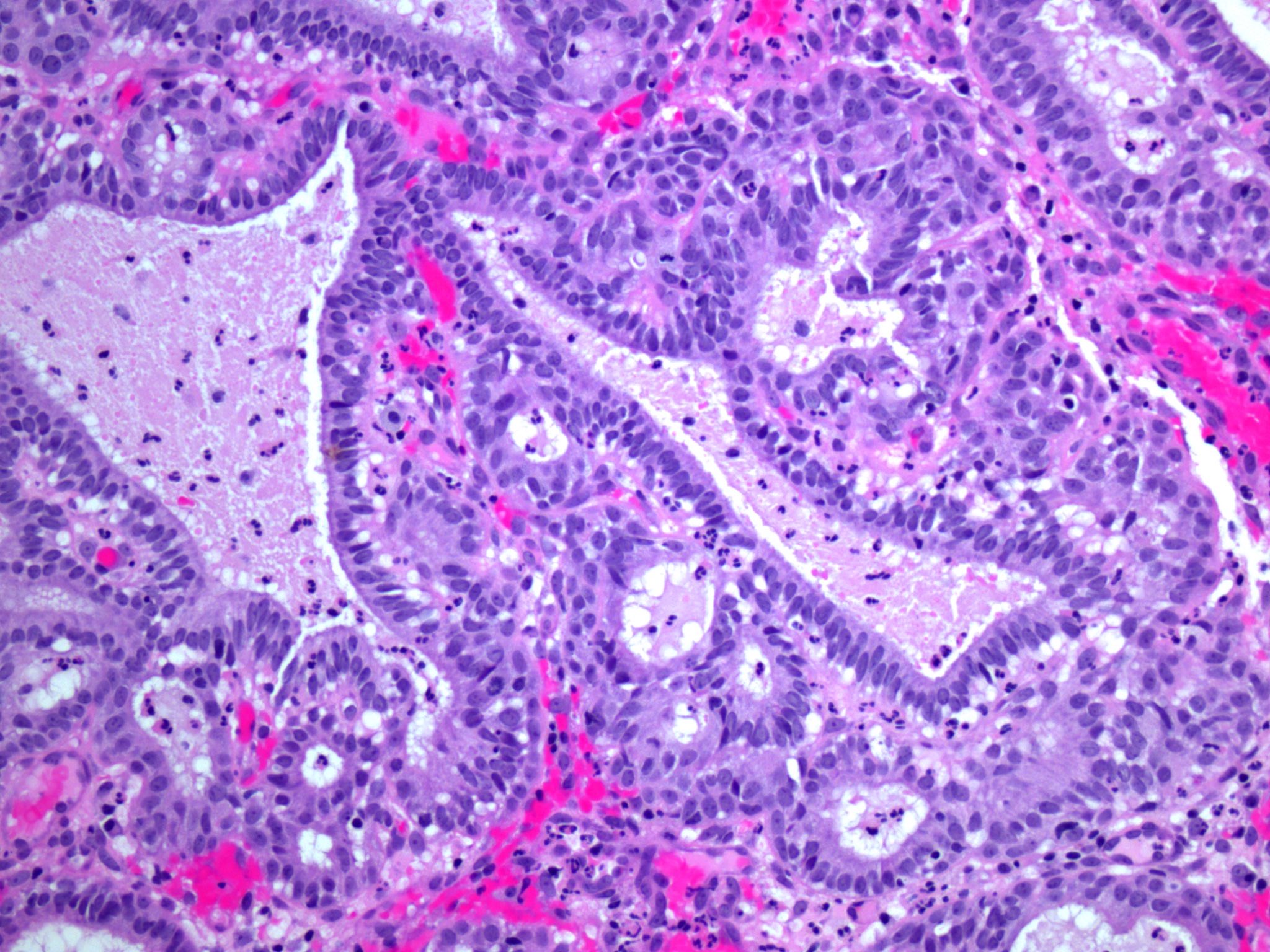
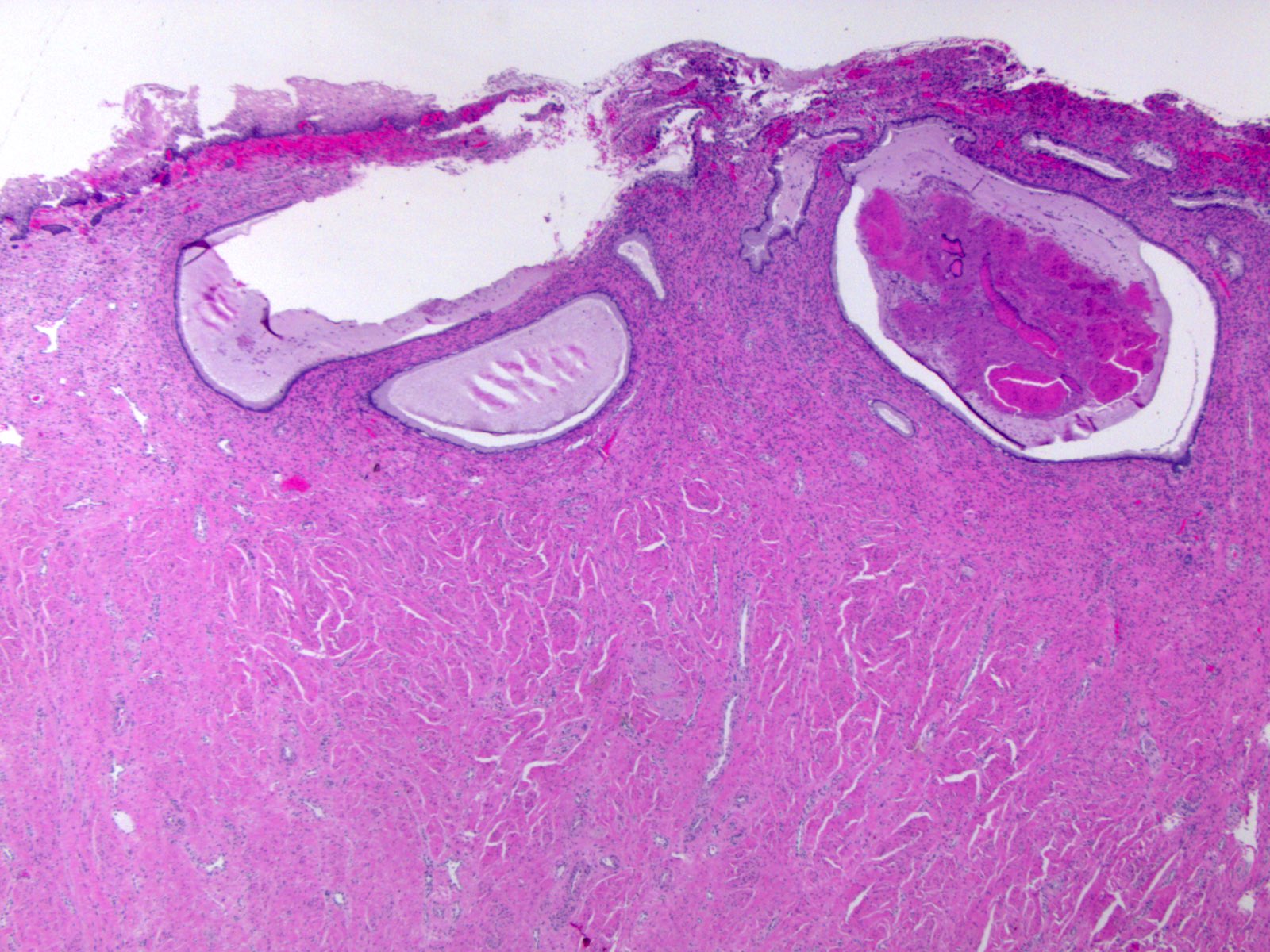
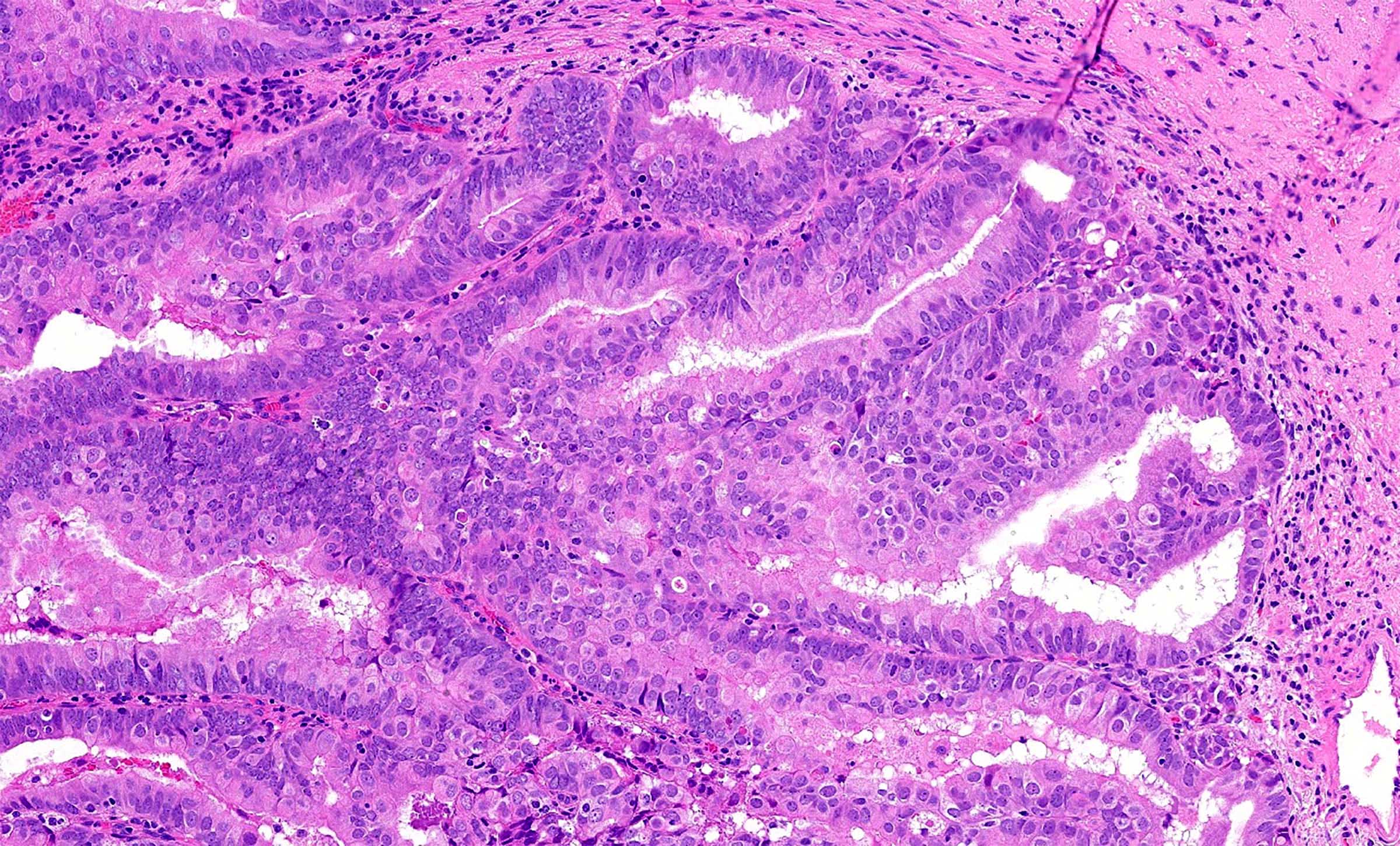
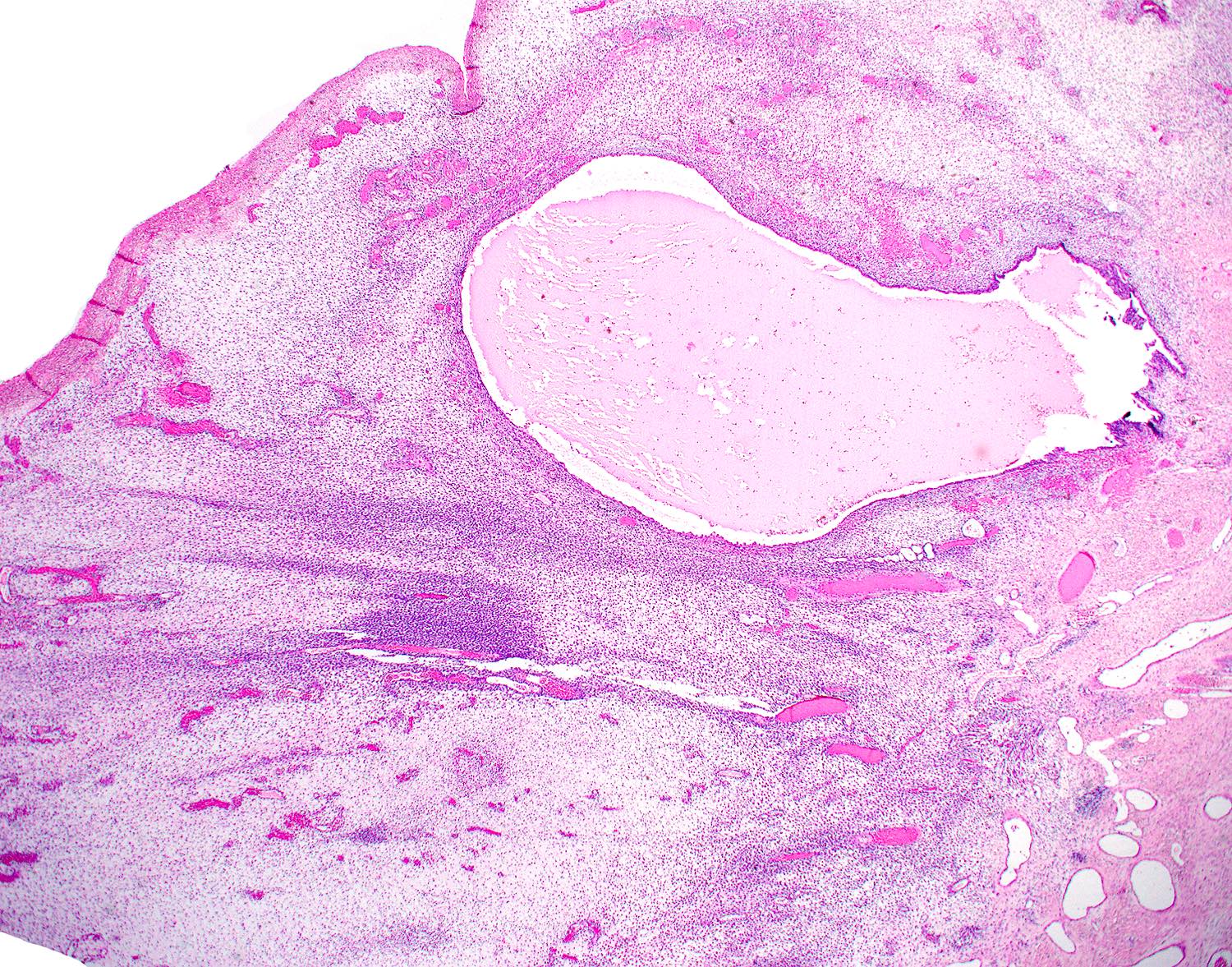
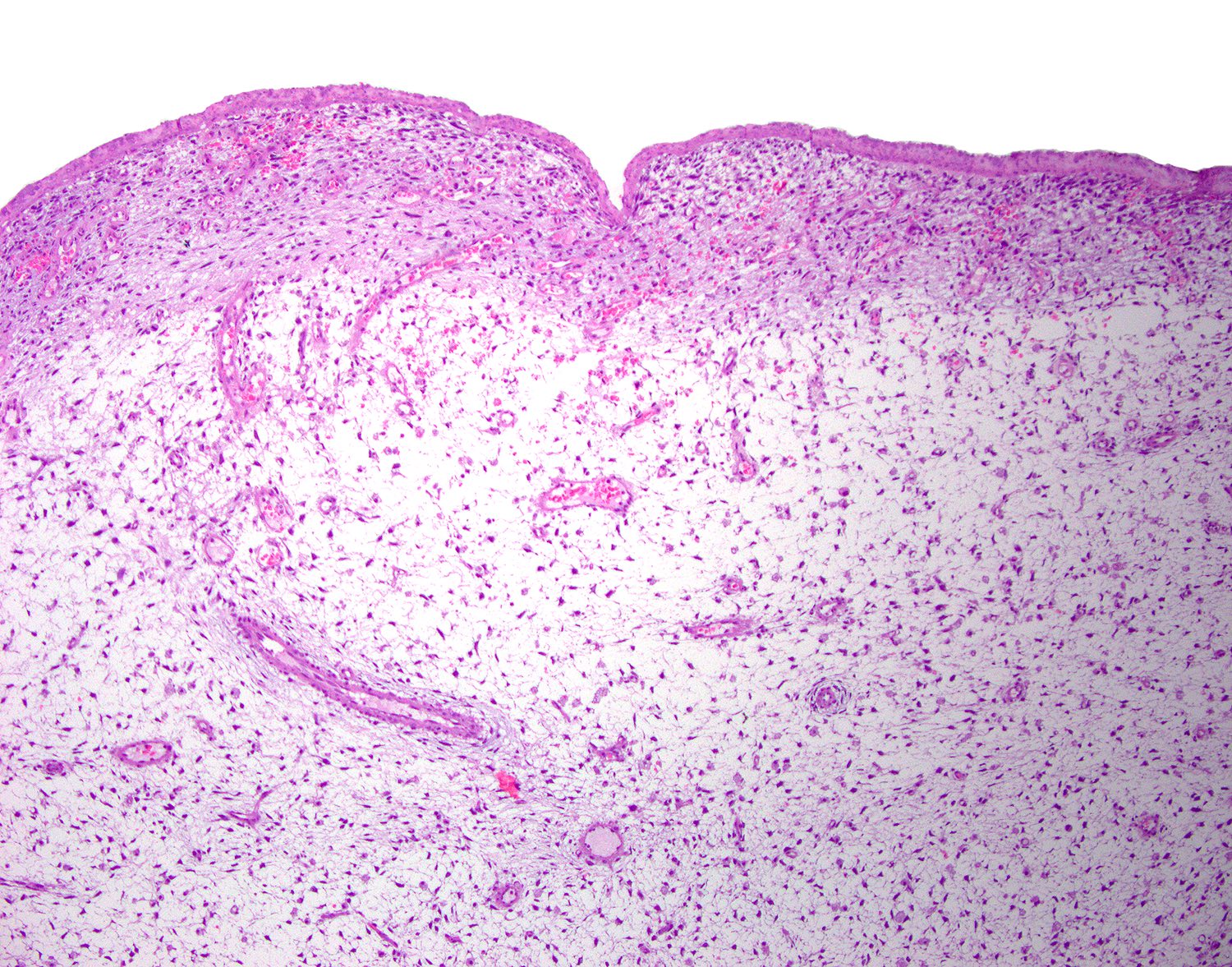
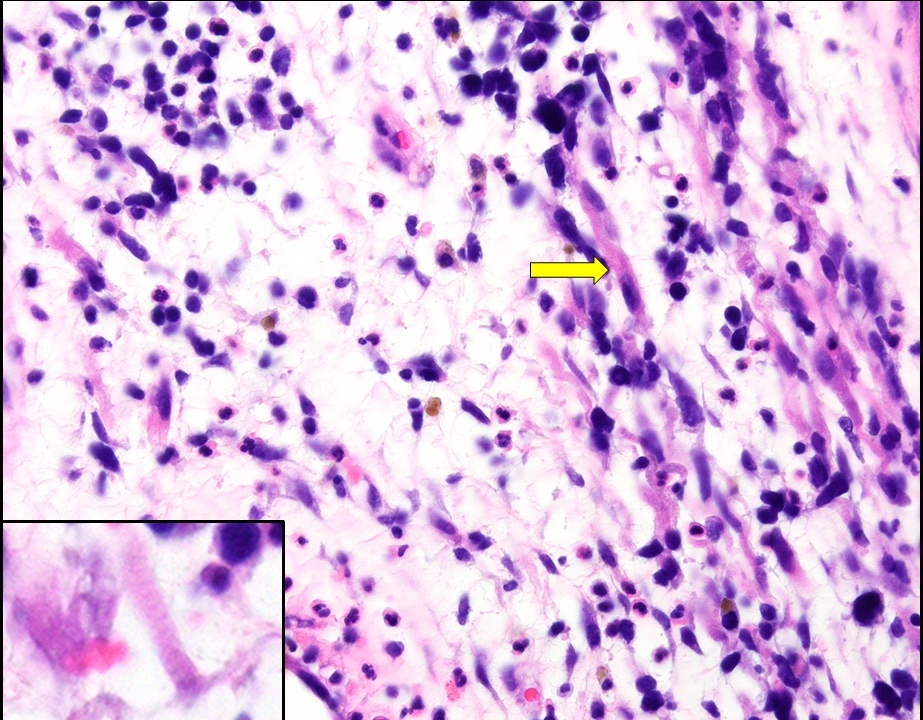
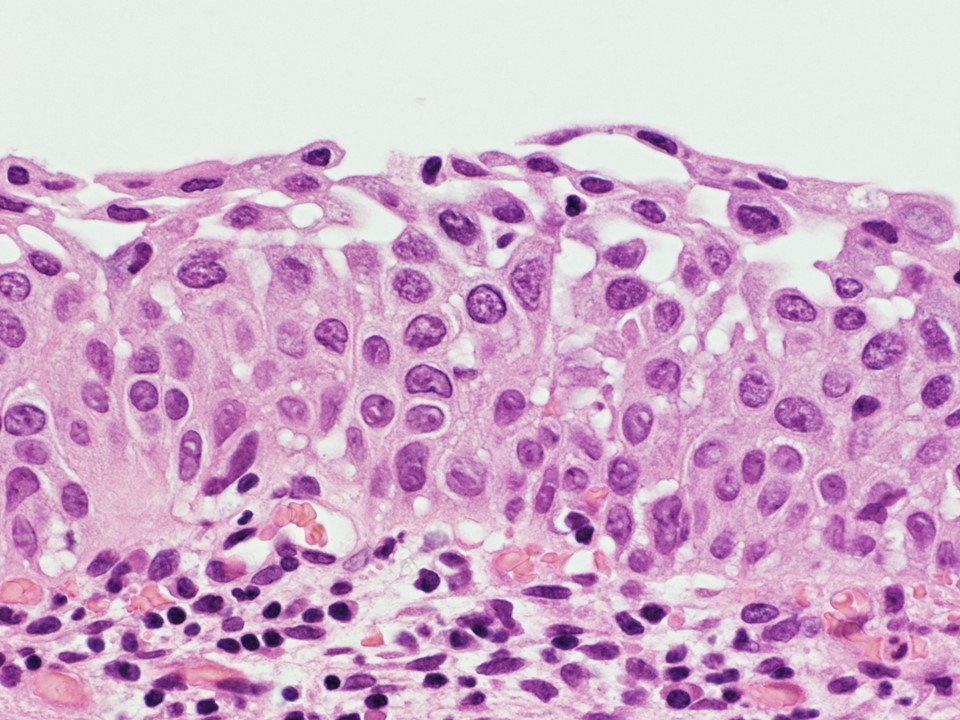
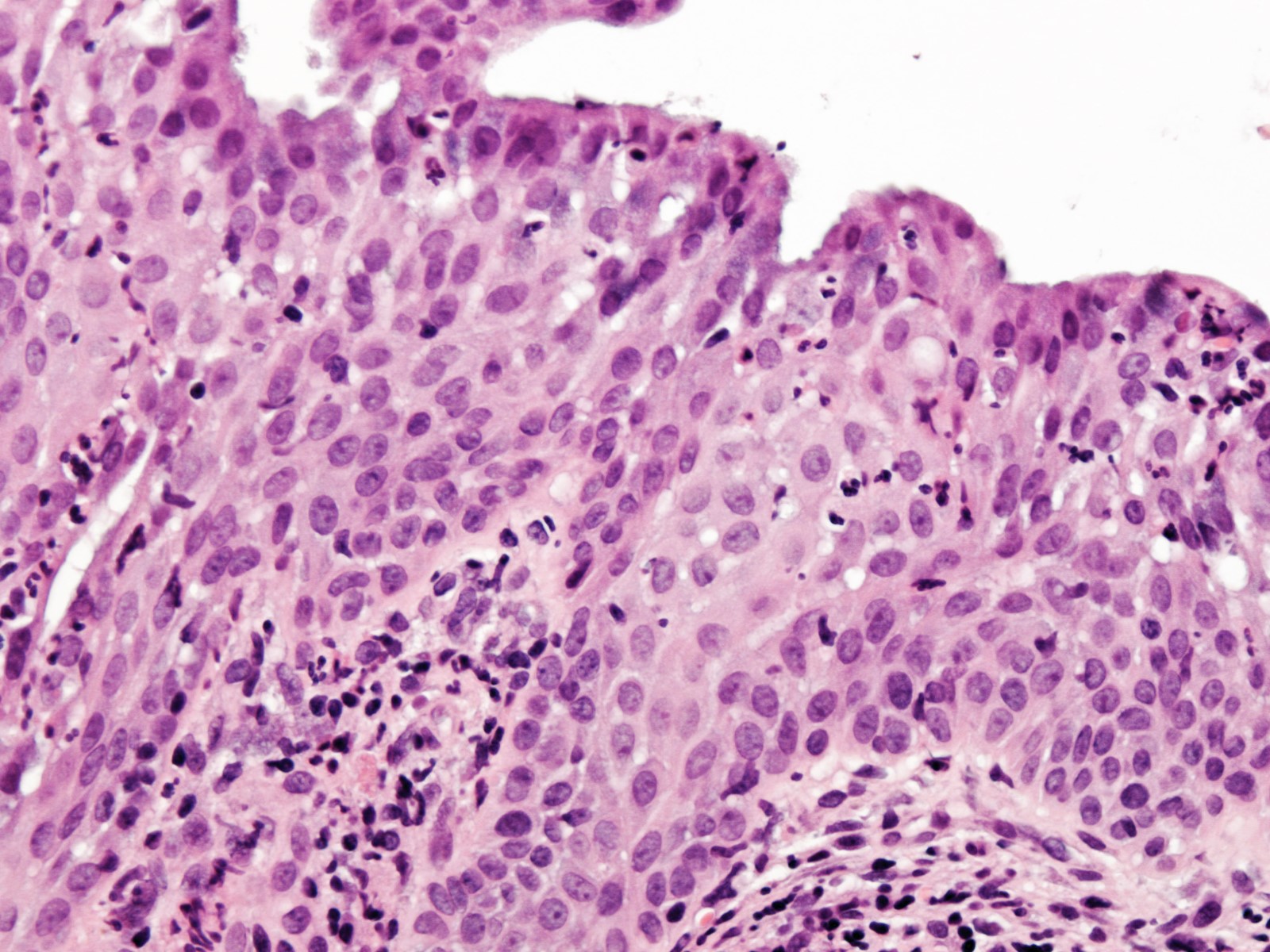
- pT1a: invasive carcinoma diagnosed by microscopy only; stromal invasion with a maximum depth of 5.0 mm measured from the base of the epithelium and a horizontal spread of 7.0 mm or less; vascular space involvement, venous or lymphatic, does not affect classification
- pT1a1: measured stromal invasion of 3.0 mm or less in depth and 7.0 mm or less in horizontal spread
- pT1a2: measured stromal invasion of more than 3.0 mm and not more than 5.0 mm, with a horizontal spread of 7.0 mm or less
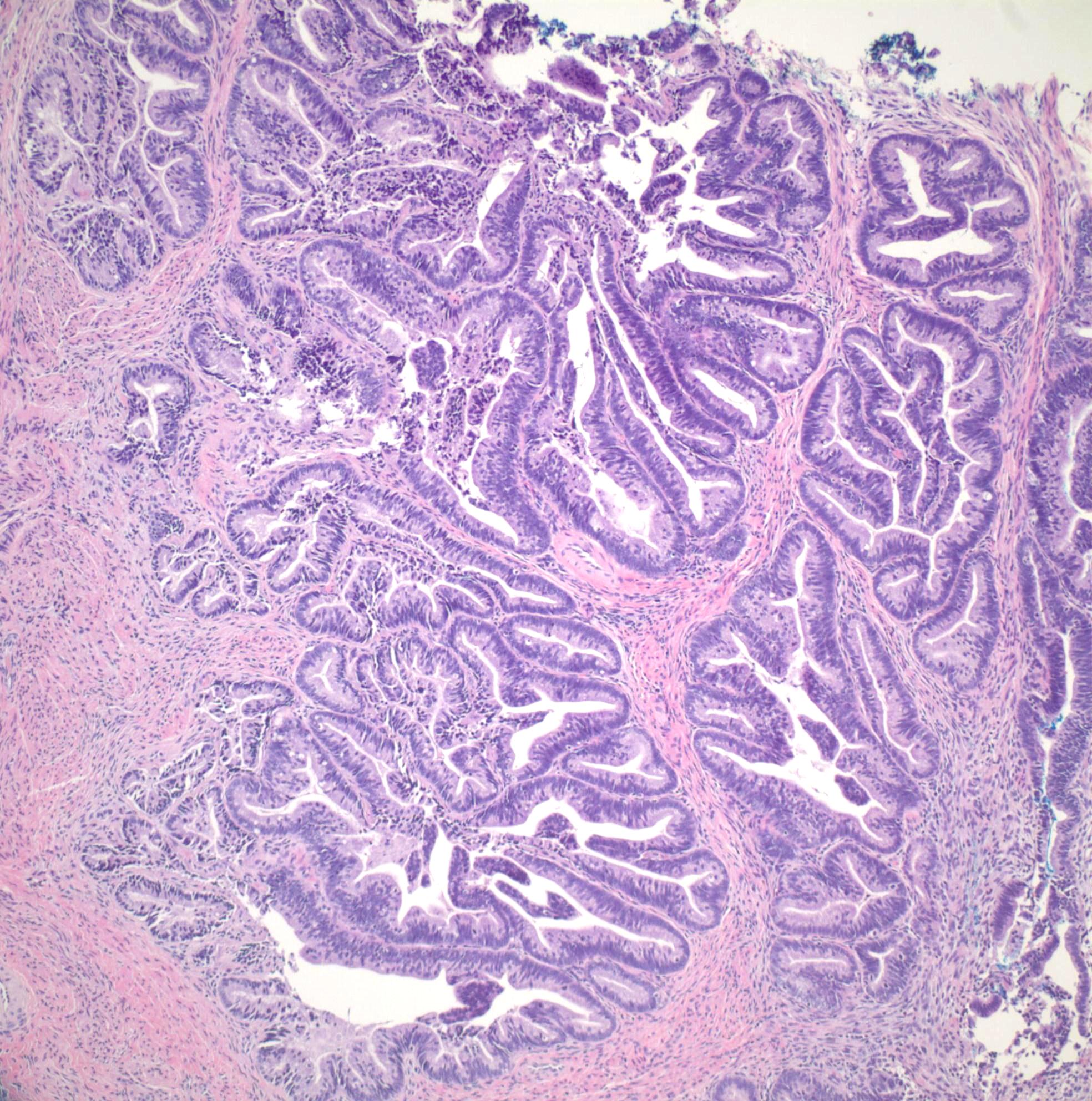
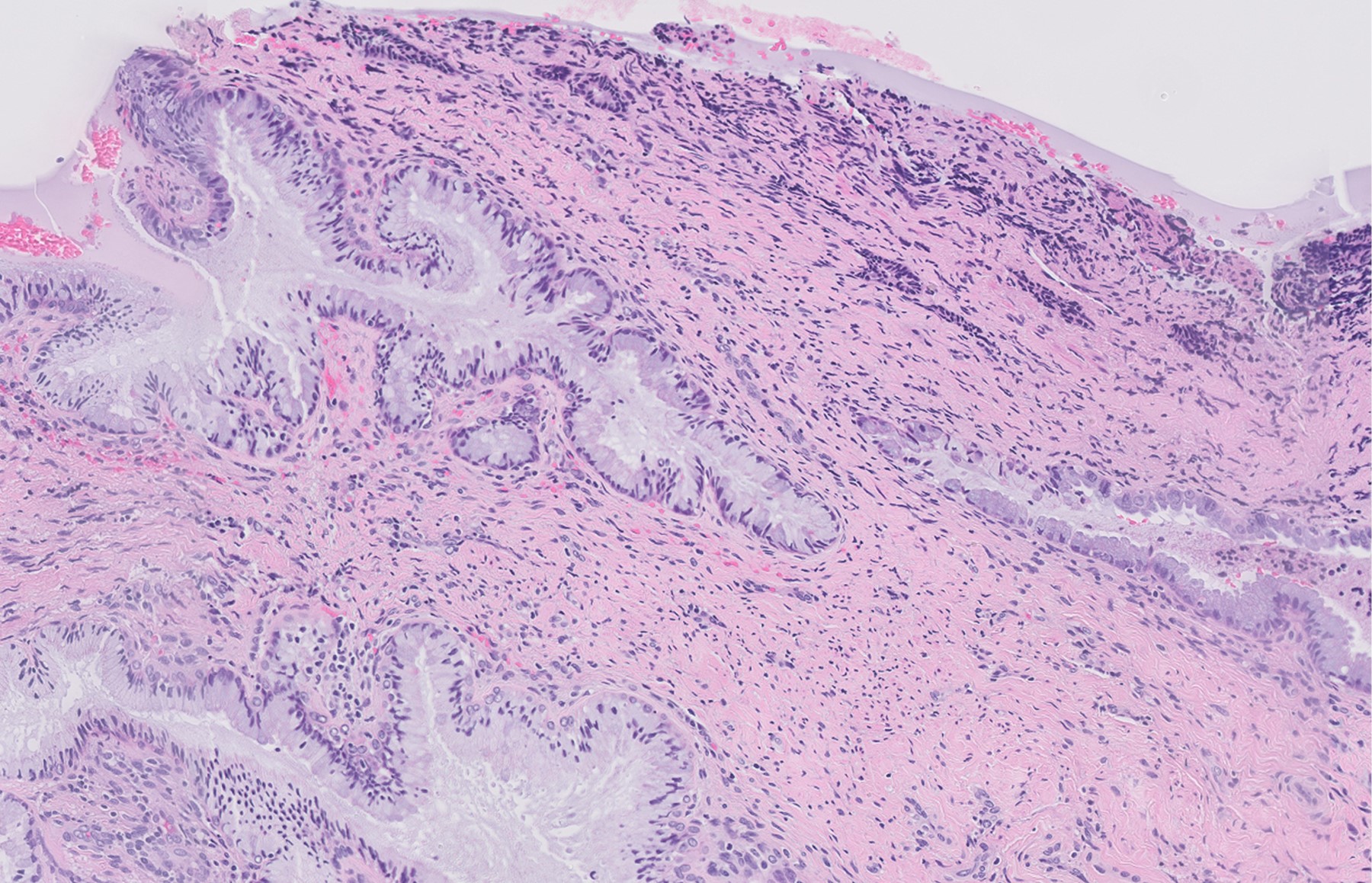
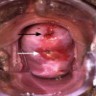
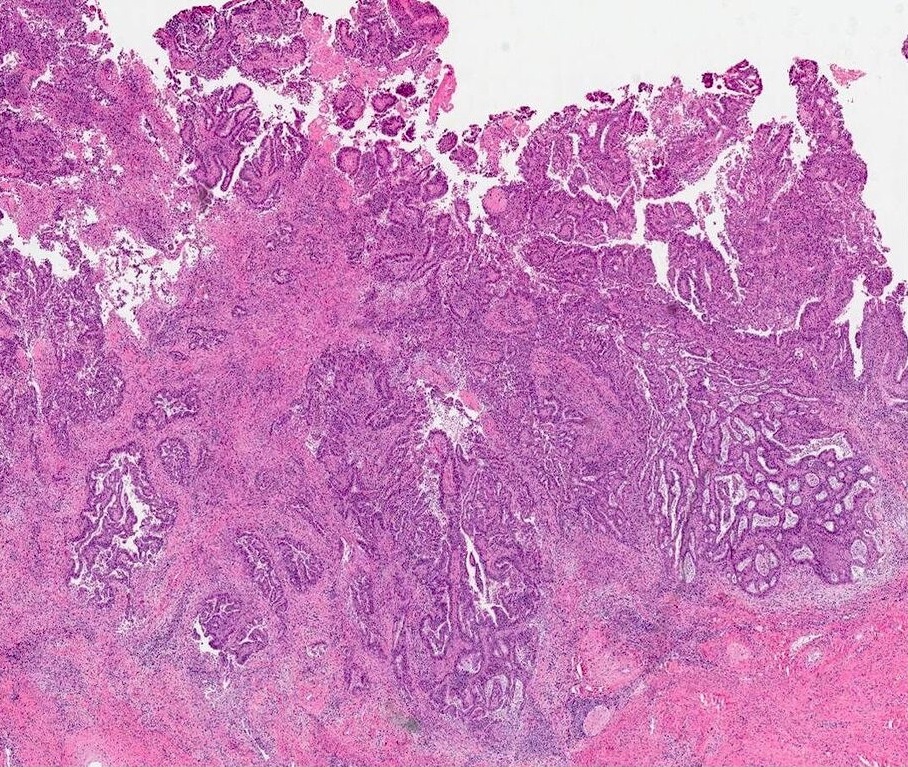
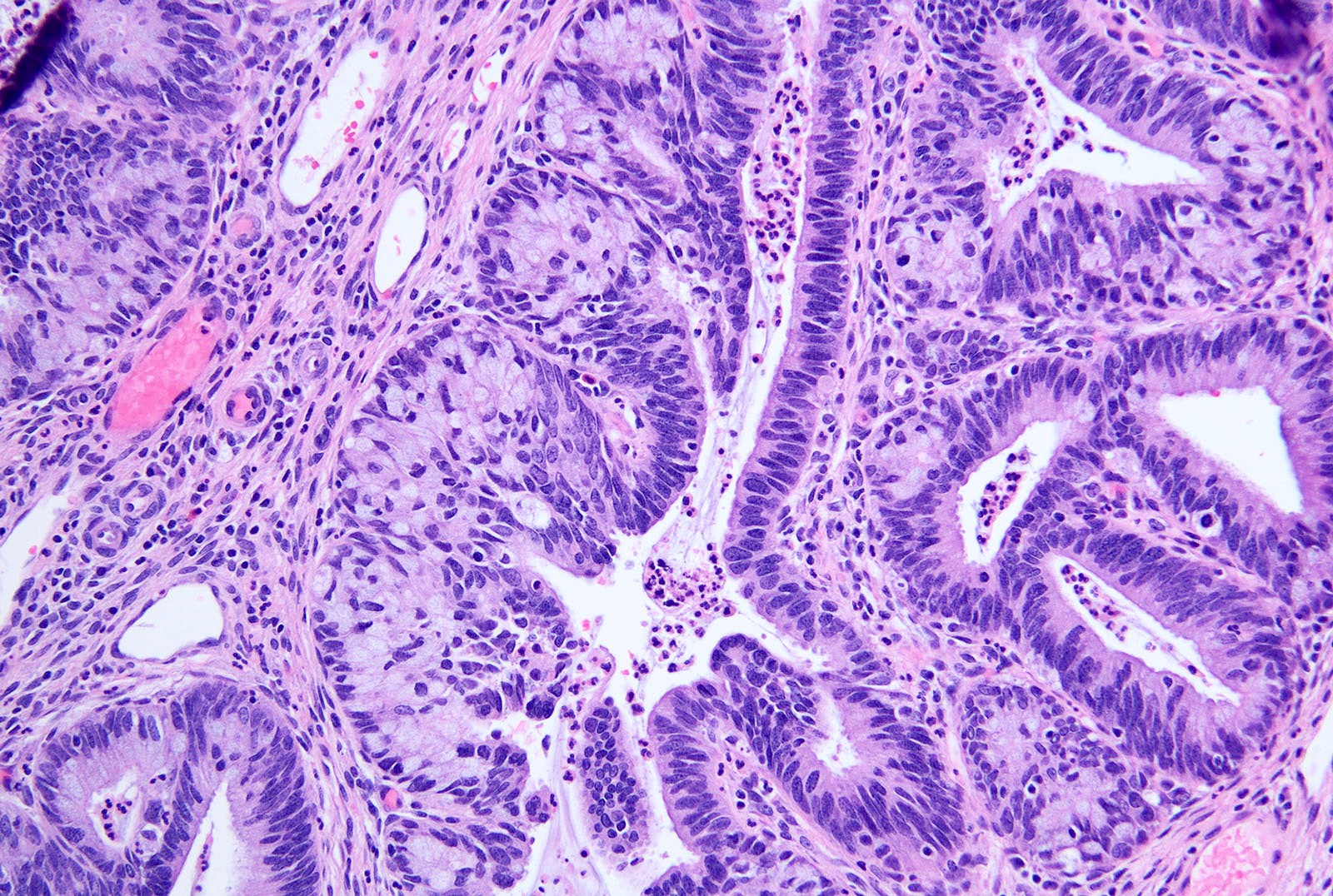
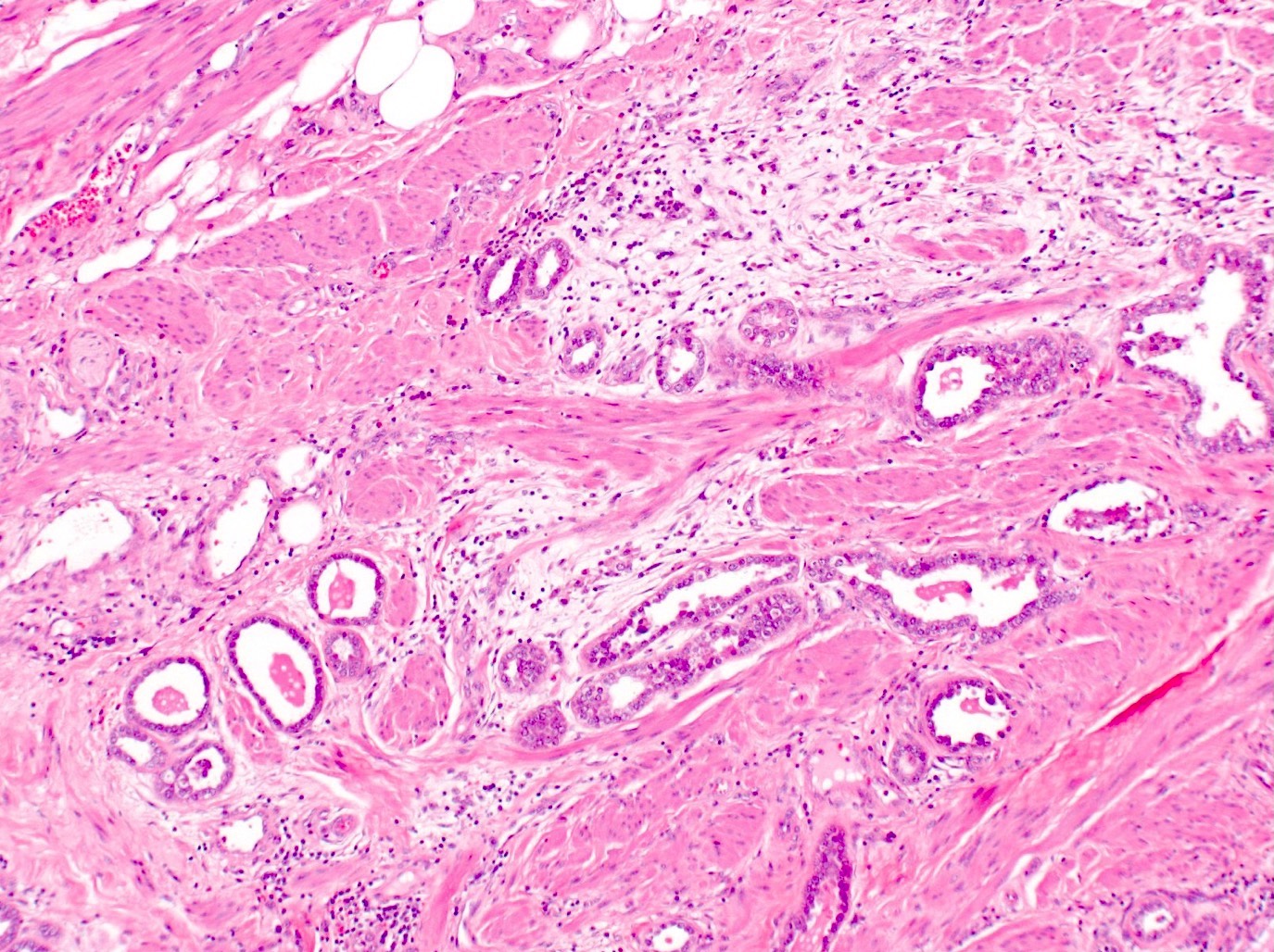
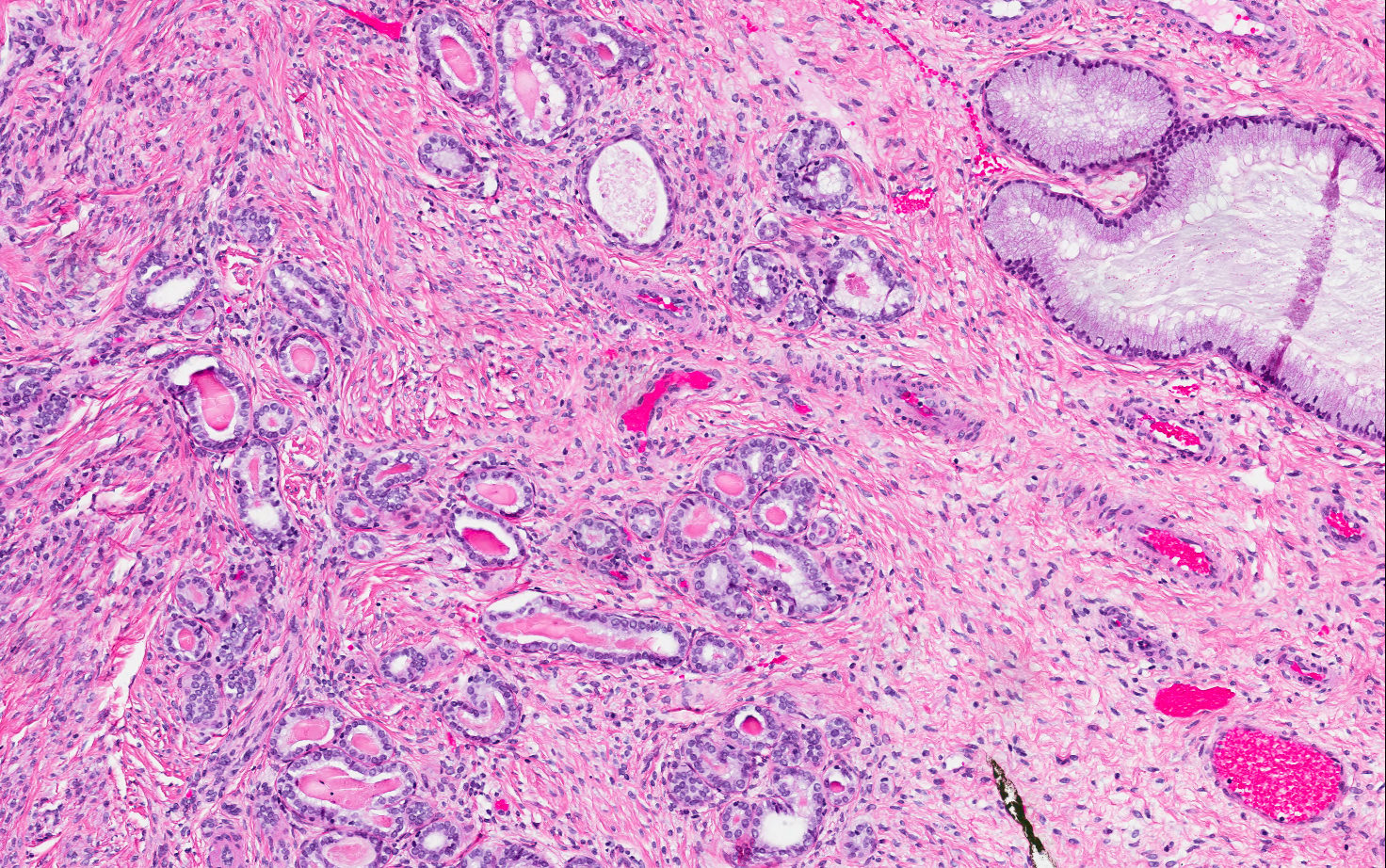
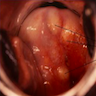
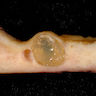
- pT1b: clinically visible lesion confined to the cervix or microscopic lesion greater than pT1a2 / IA2; includes all macroscopically visible lesions, even those with superficial invasion
- pT1b1: clinically visible lesion 4.0 cm or less in greatest dimension
- pT1b2: clinically visible lesion more than 4.0 cm in greatest dimension
- pT1a: invasive carcinoma diagnosed by microscopy only; stromal invasion with a maximum depth of 5.0 mm measured from the base of the epithelium and a horizontal spread of 7.0 mm or less; vascular space involvement, venous or lymphatic, does not affect classification
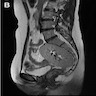
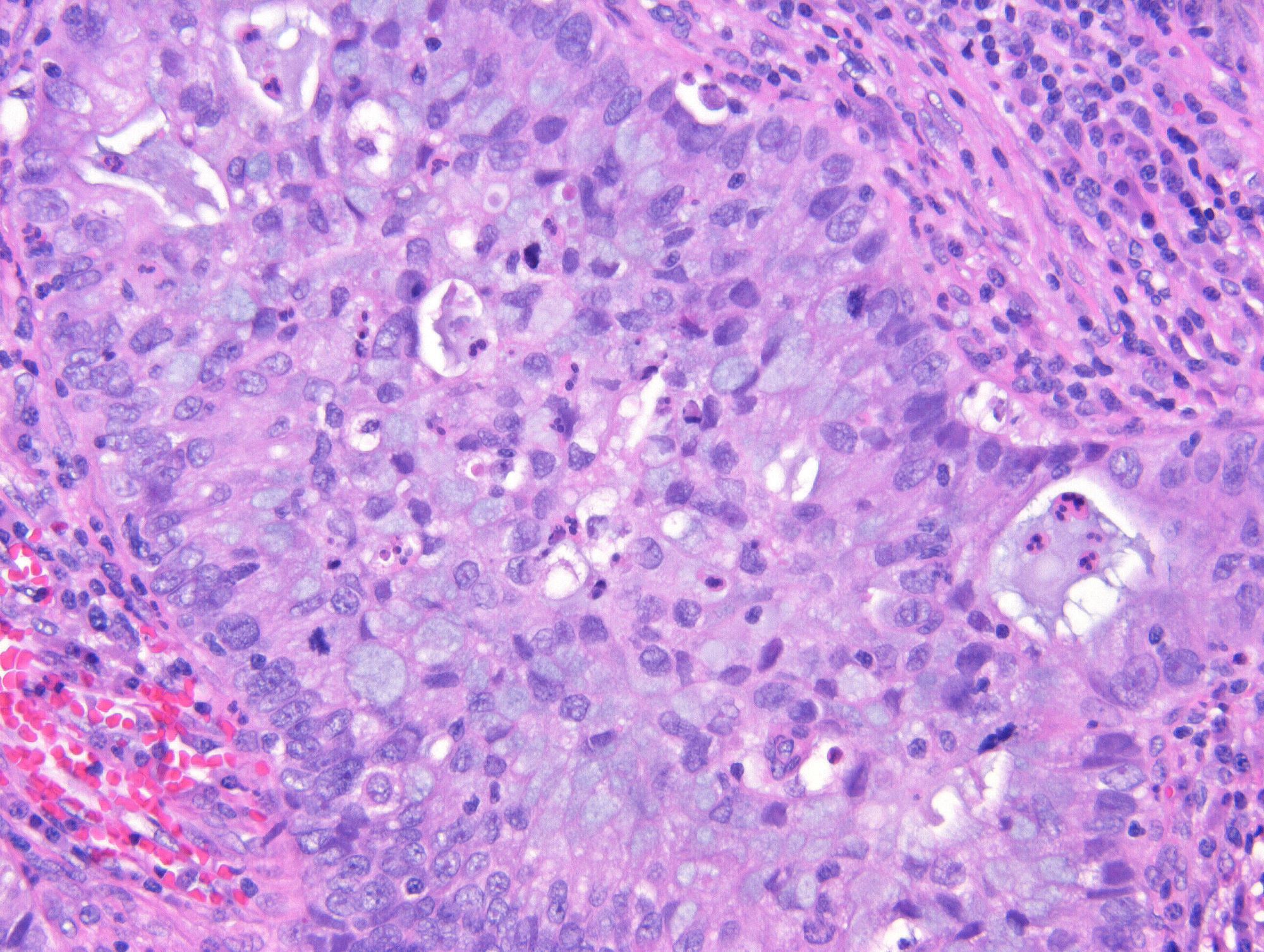
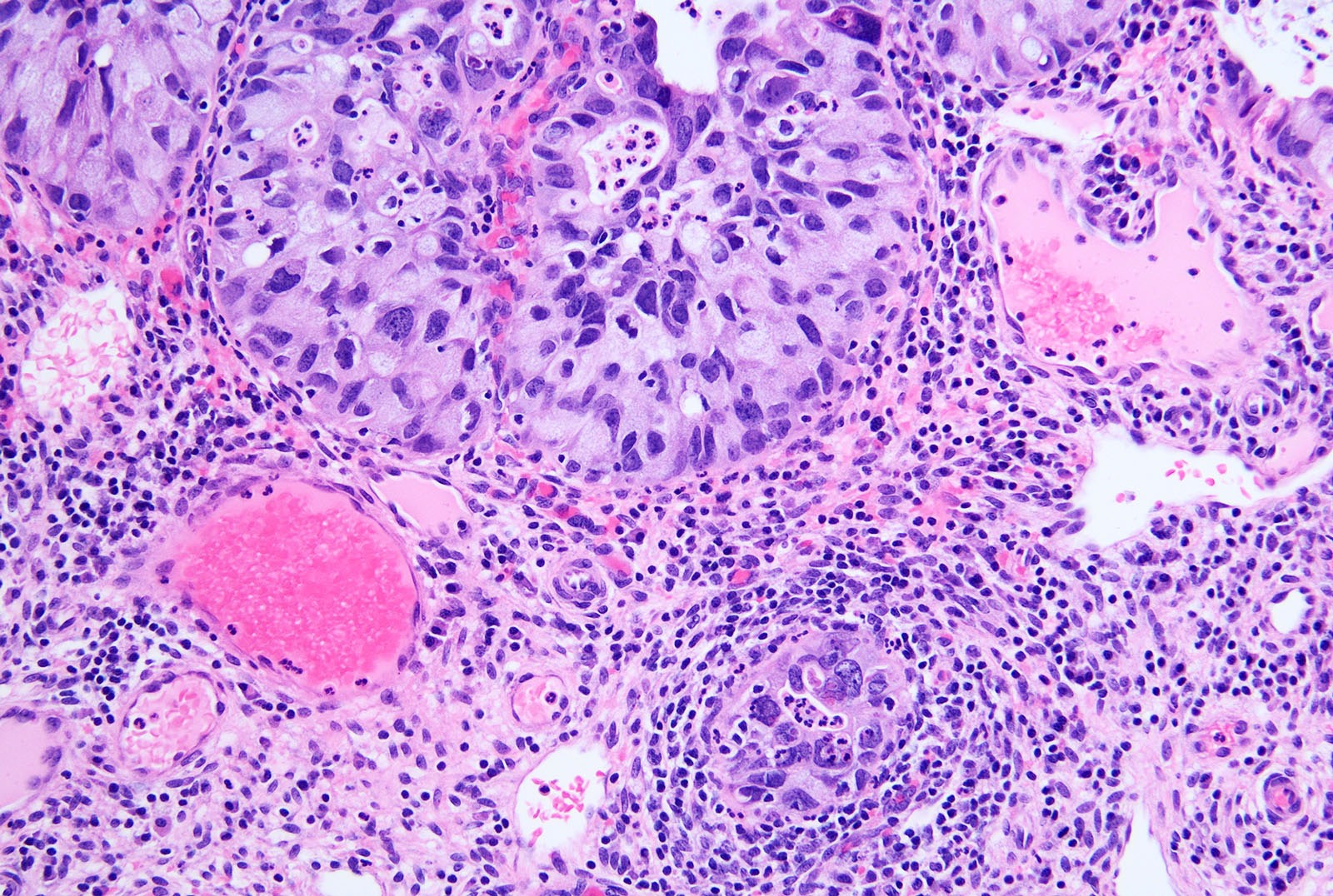
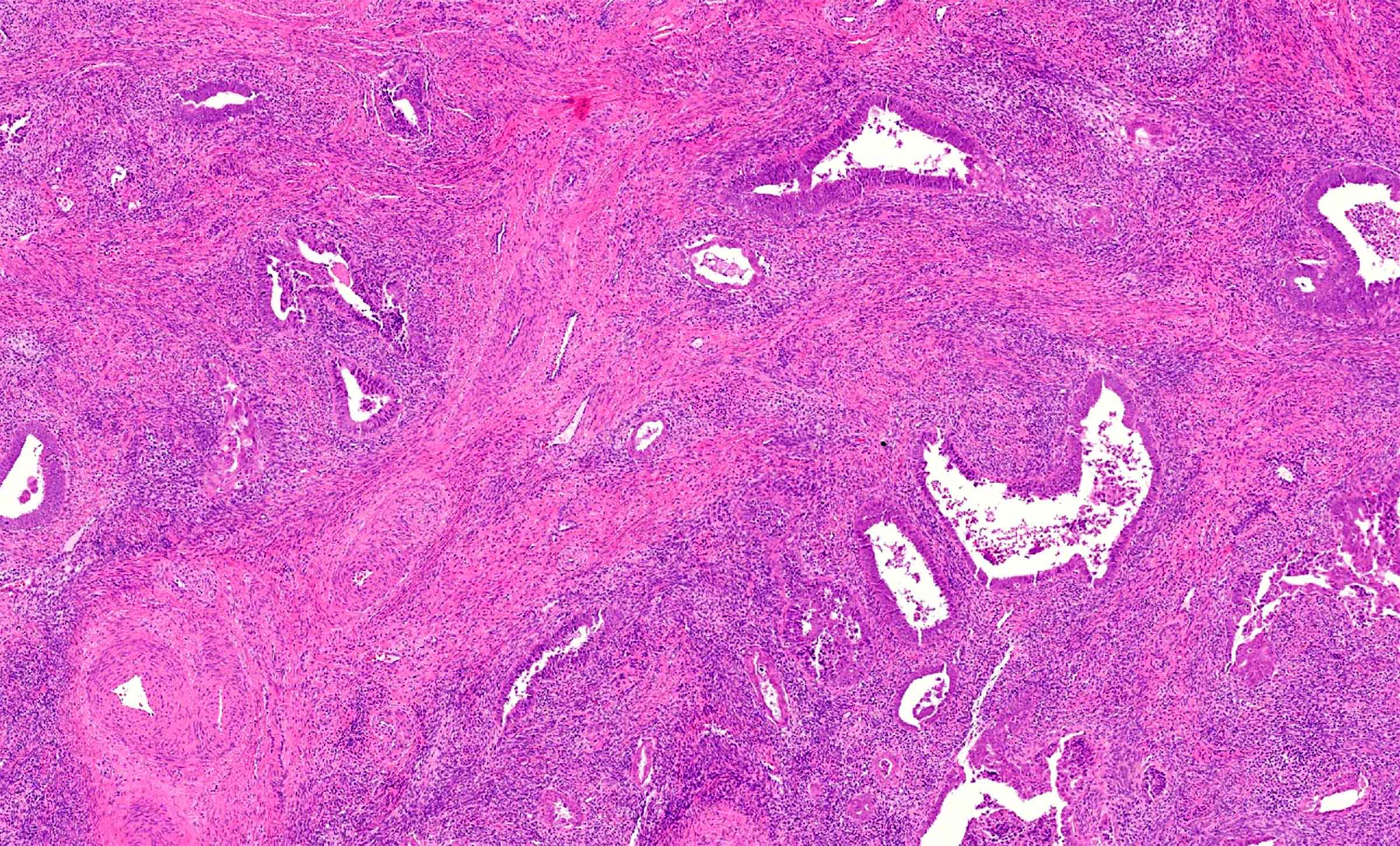
- pT2: cervical carcinoma invading beyond the uterus but not to the pelvic wall or to the lower third of the vagina
- pT2a: tumor without parametrial invasion
- pT2a1: clinically visible lesion 4.0 cm or less in greatest dimension
- pT2a2: clinically visible lesion more than 4.0 cm in greatest dimension
- pT2b: tumor with parametrial invasion
- pT2a: tumor without parametrial invasion
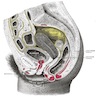
- pT3: tumor extending to the pelvic sidewall or involving the lower third of the vagina or causing hydronephrosis or nonfunctioning kidney
- pT3a: tumor involving the lower third of the vagina but not extending to the pelvic wall
- pT3b: tumor extending to the pelvic wall or causing hydronephrosis or nonfunctioning kidney
- pT4: tumor invading the mucosa of the bladder or rectum or extending beyond the true pelvis (bullous edema is not sufficient to classify a tumor as pT4)
- Reference: Amin: AJCC Cancer Staging Manual, 8th Edition, 2017
Notes:
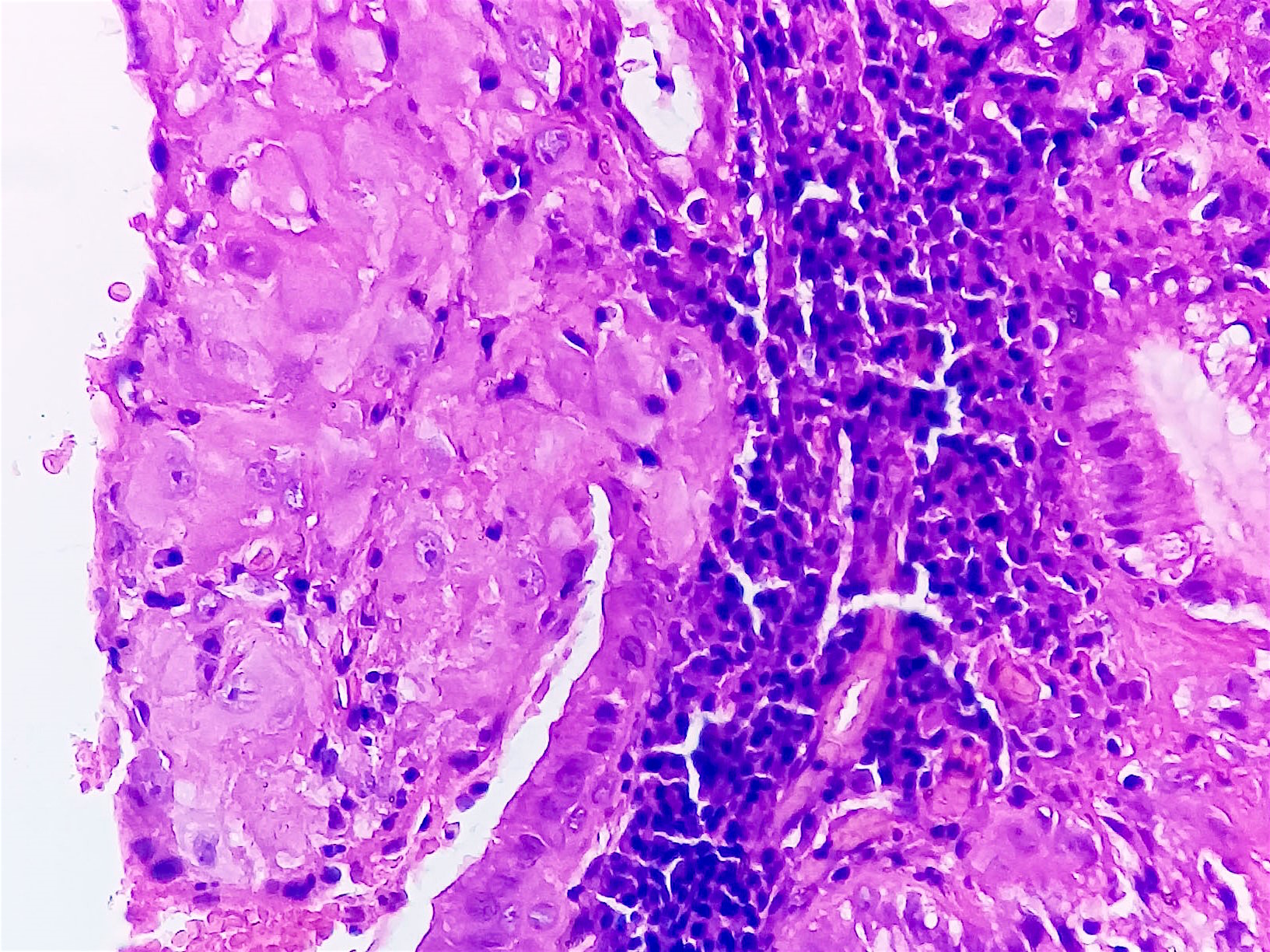
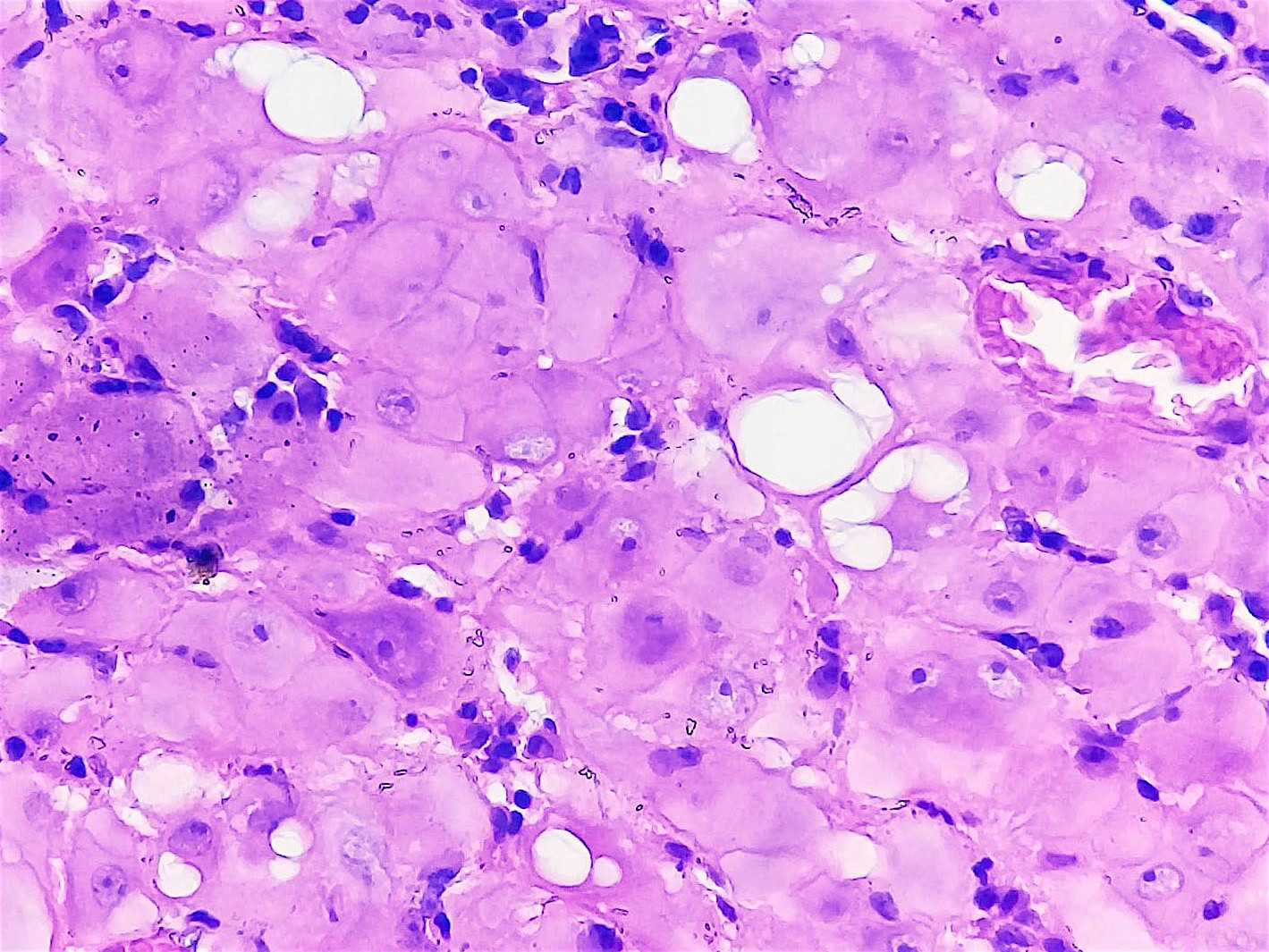
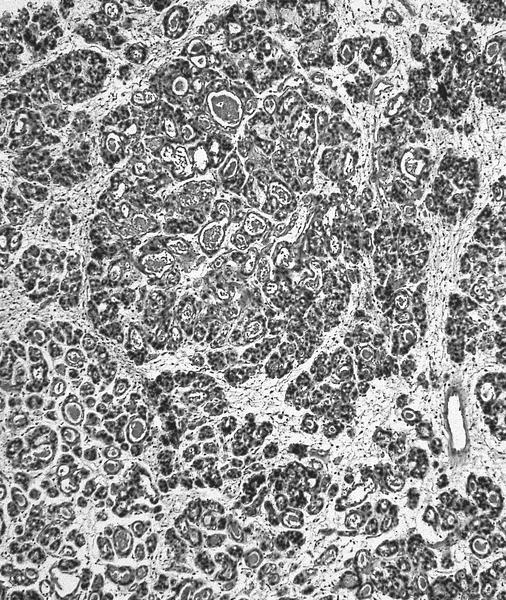
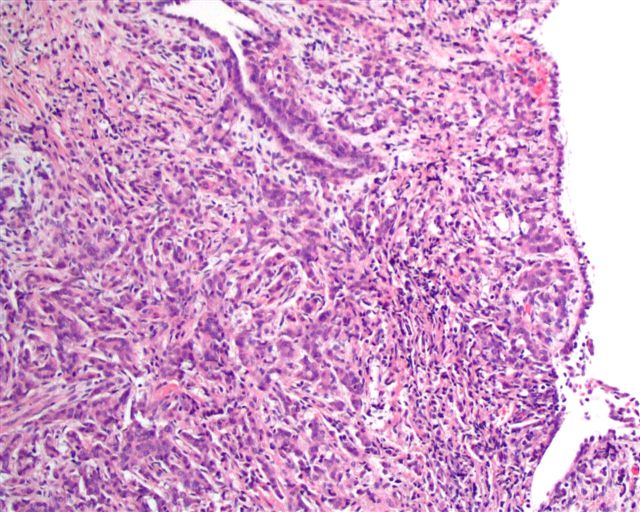
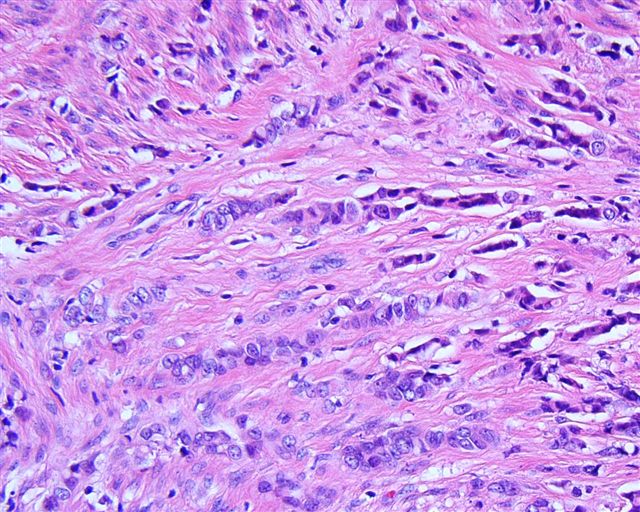
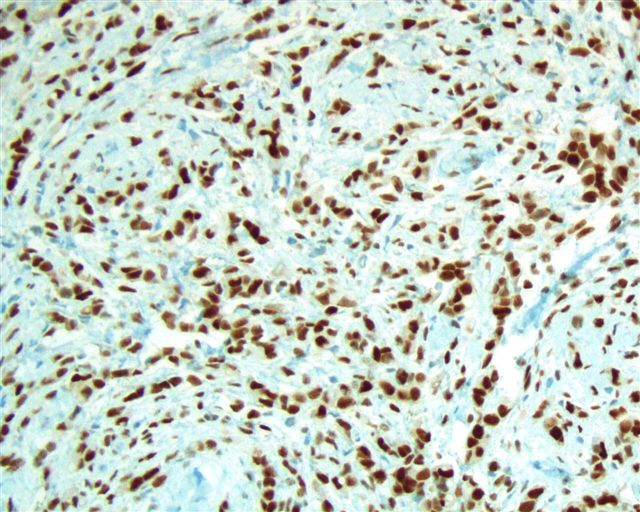
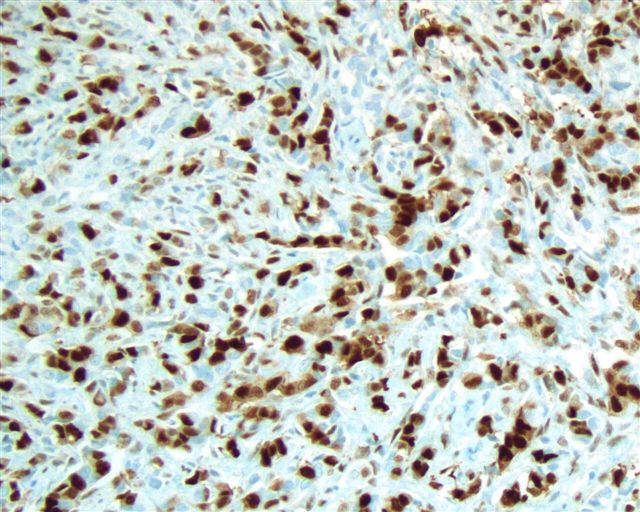
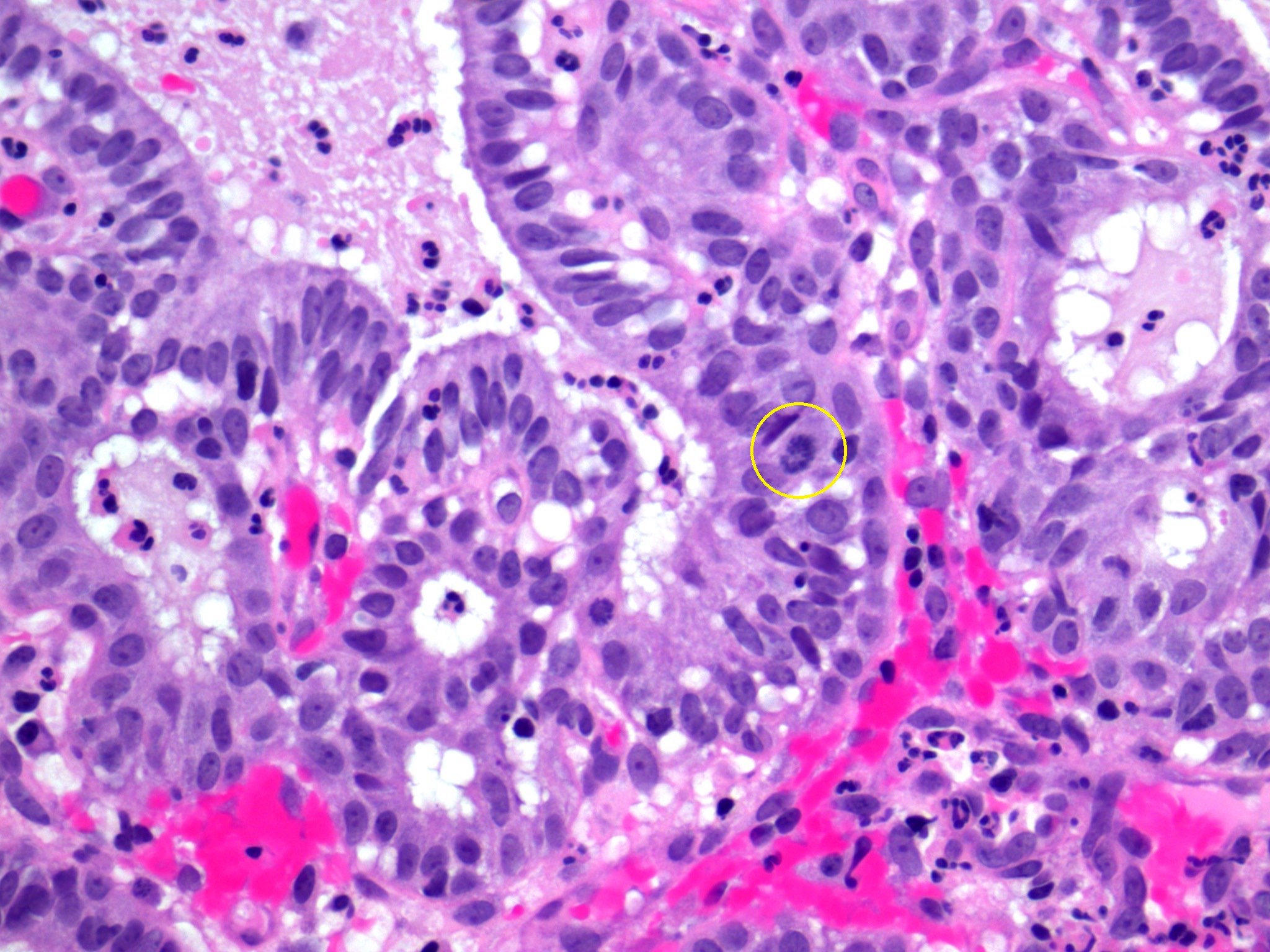
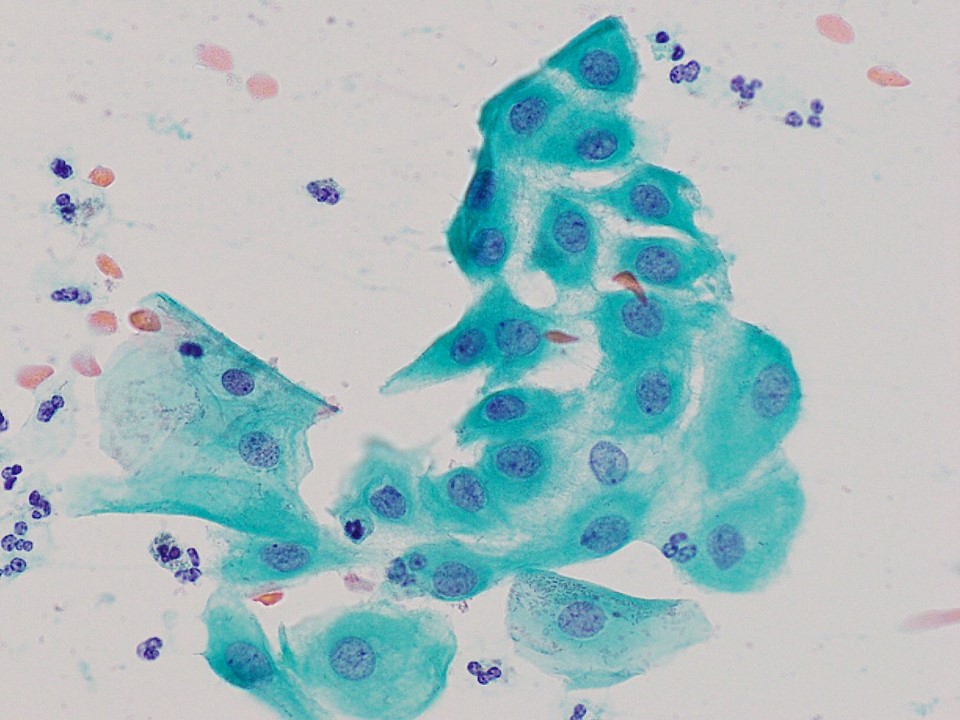
- Stromal invasion needs to be documented in millimeters for tumors that cannot be measured grossly
- This step is not necessary in larger tumors that can be measured grossly
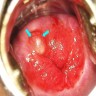
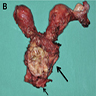
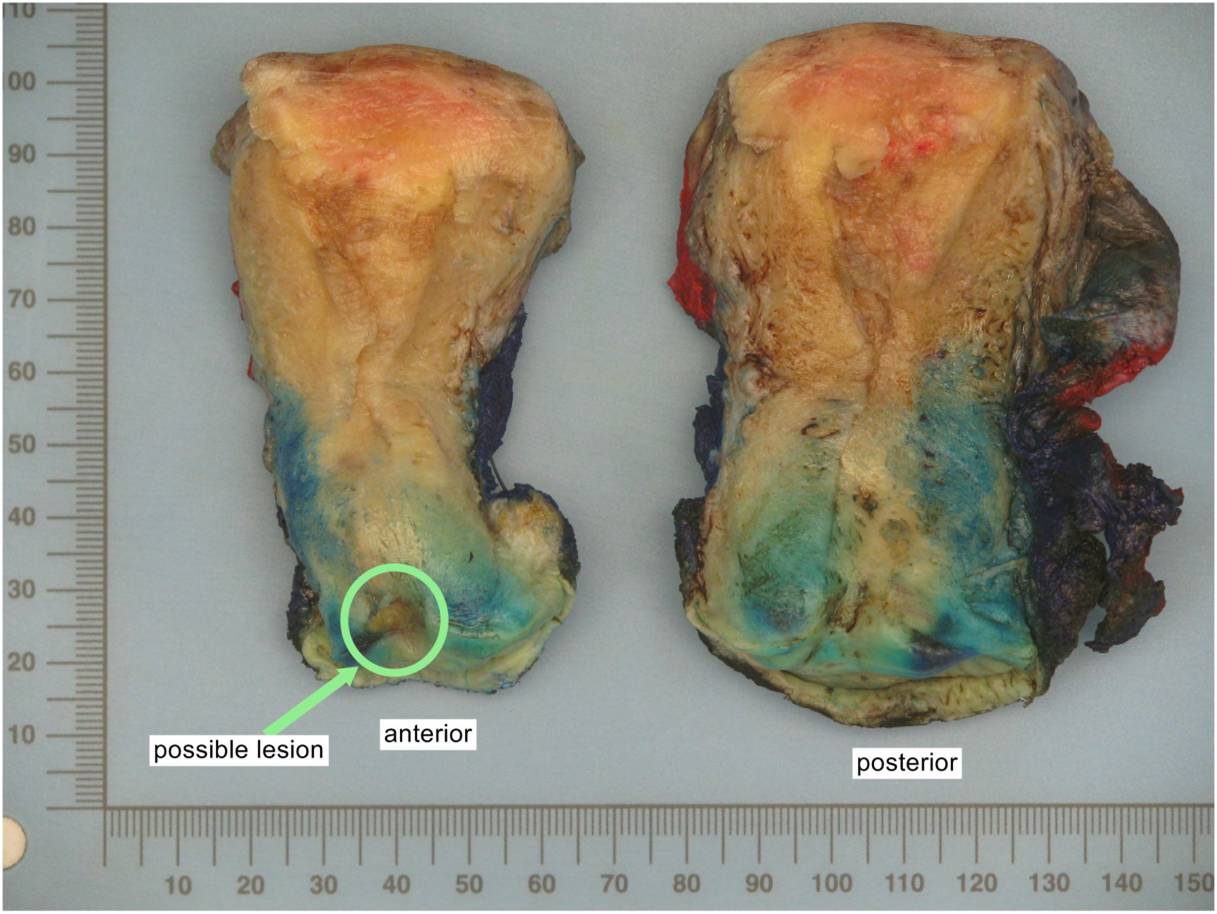
- All macroscopically visible lesions - even with only superficial invasion - are at least FIGO IB
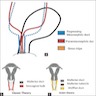
- Pelvic sidewall is defined as the muscle, fascia, neurovascular structures and skeletal portions of the bony pelvis; on rectal examination, there is no cancer free space between the tumor and pelvic sidewall