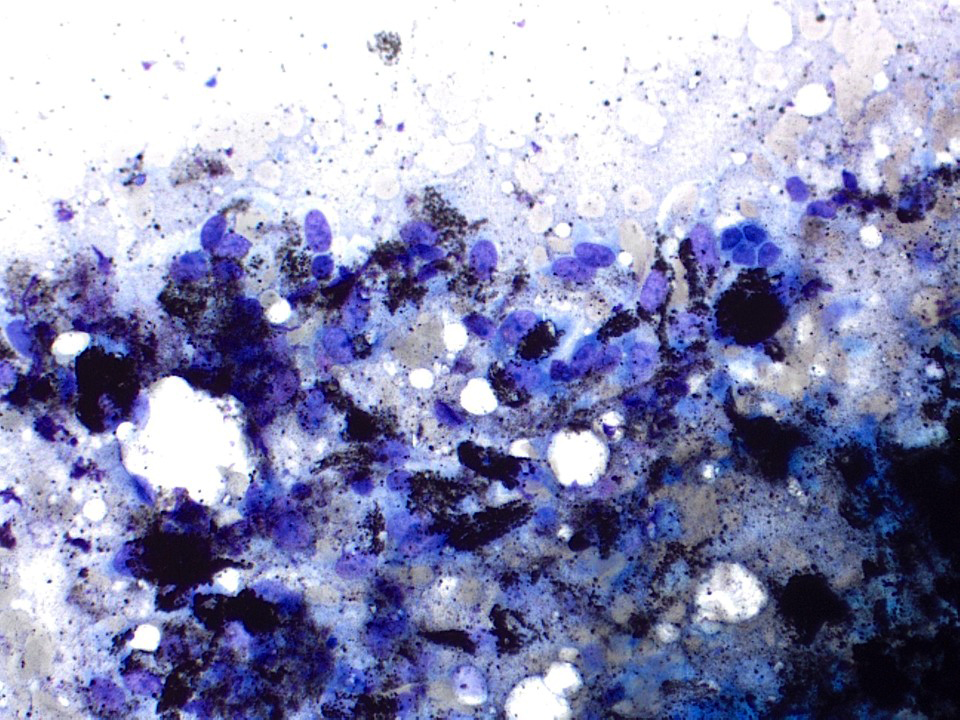
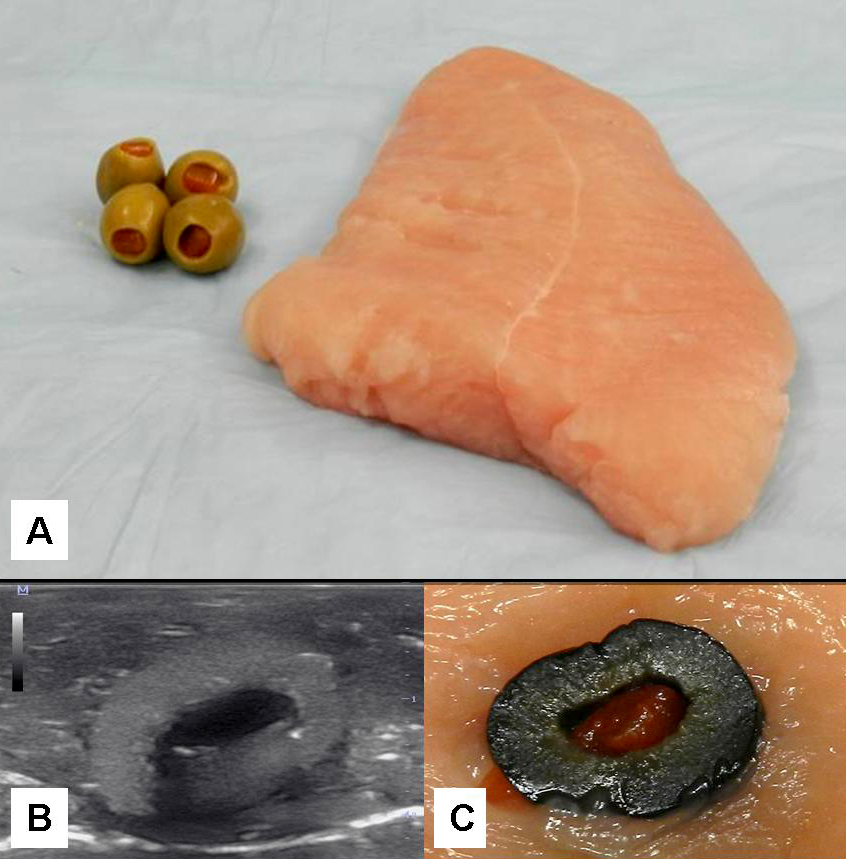
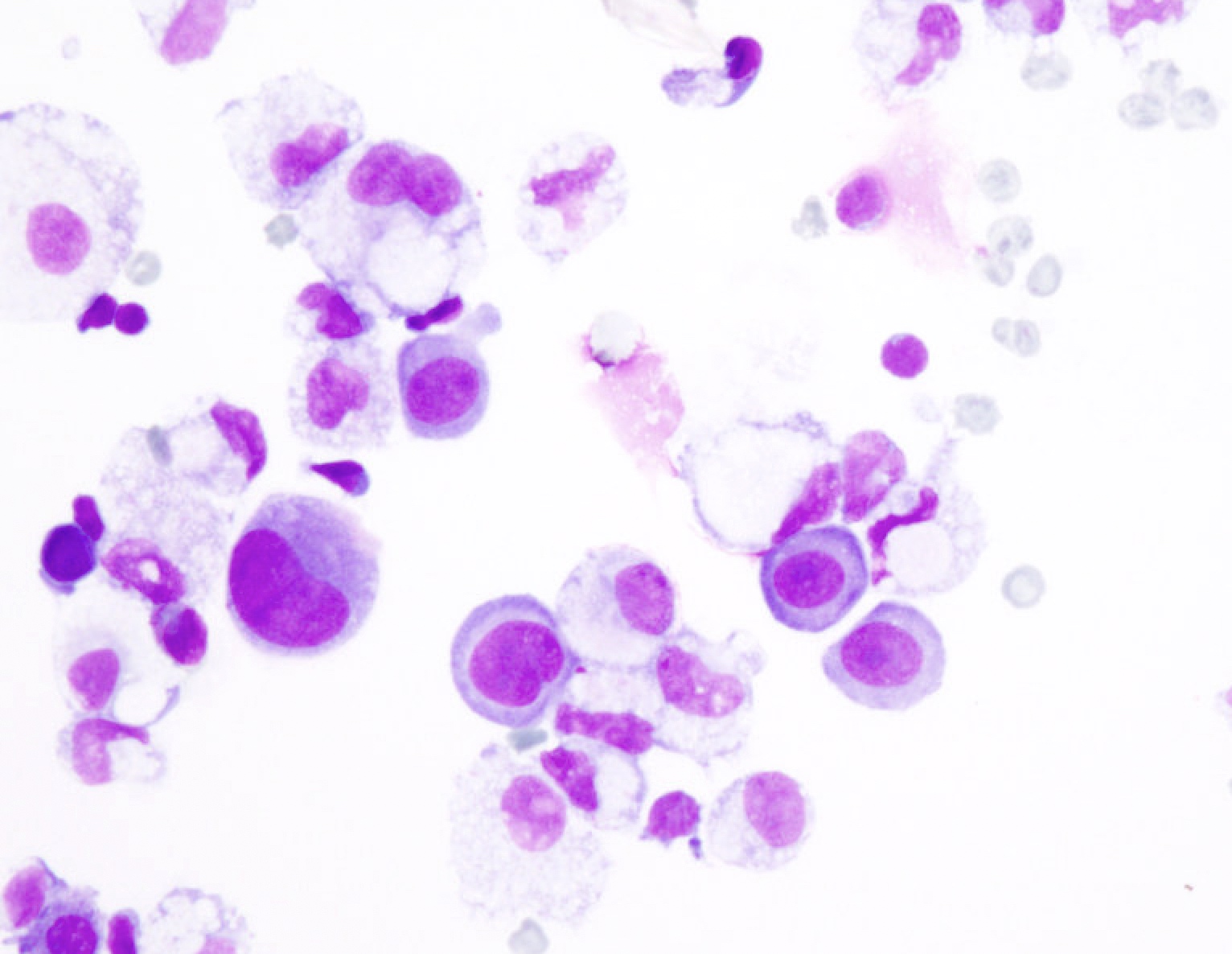
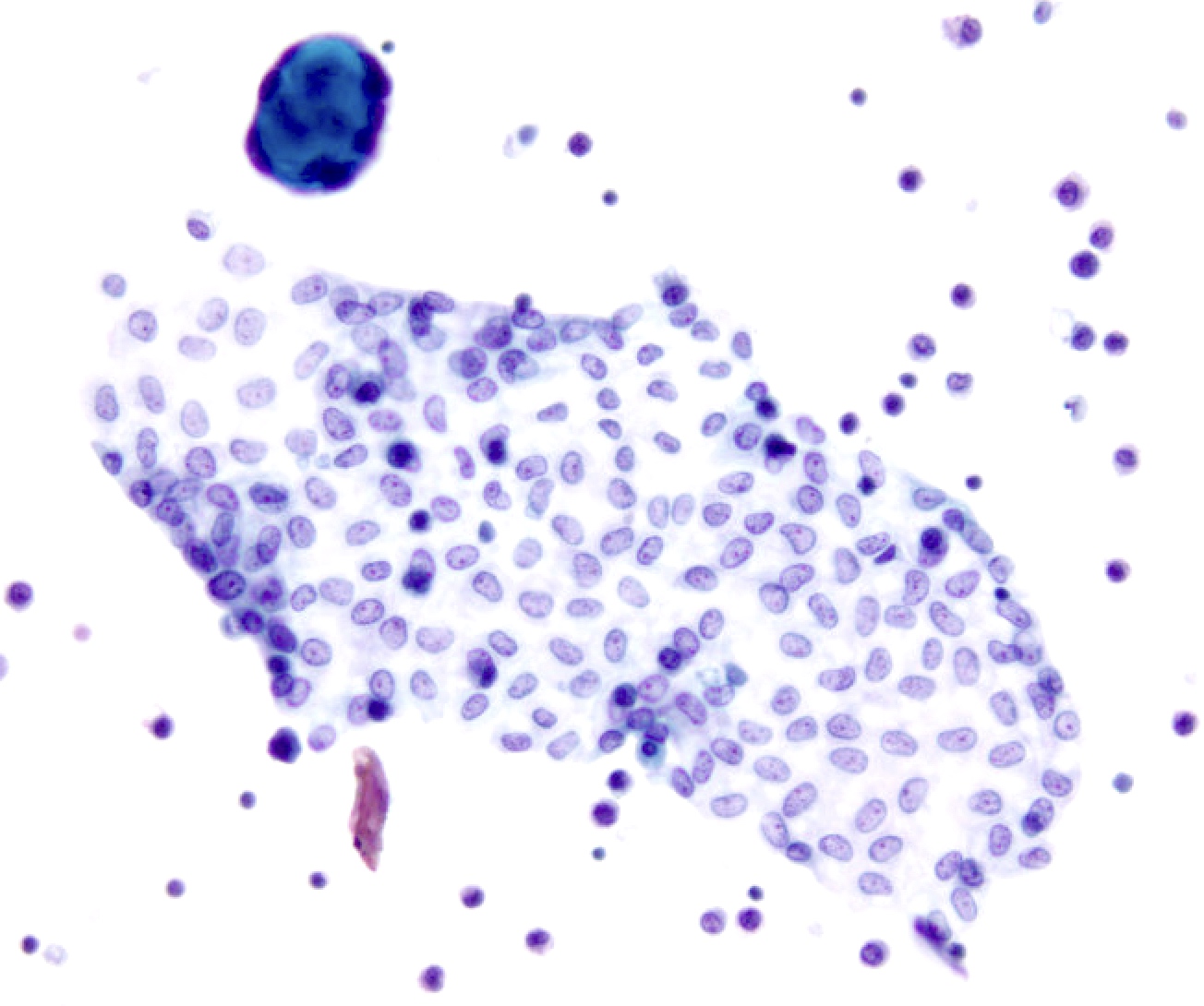
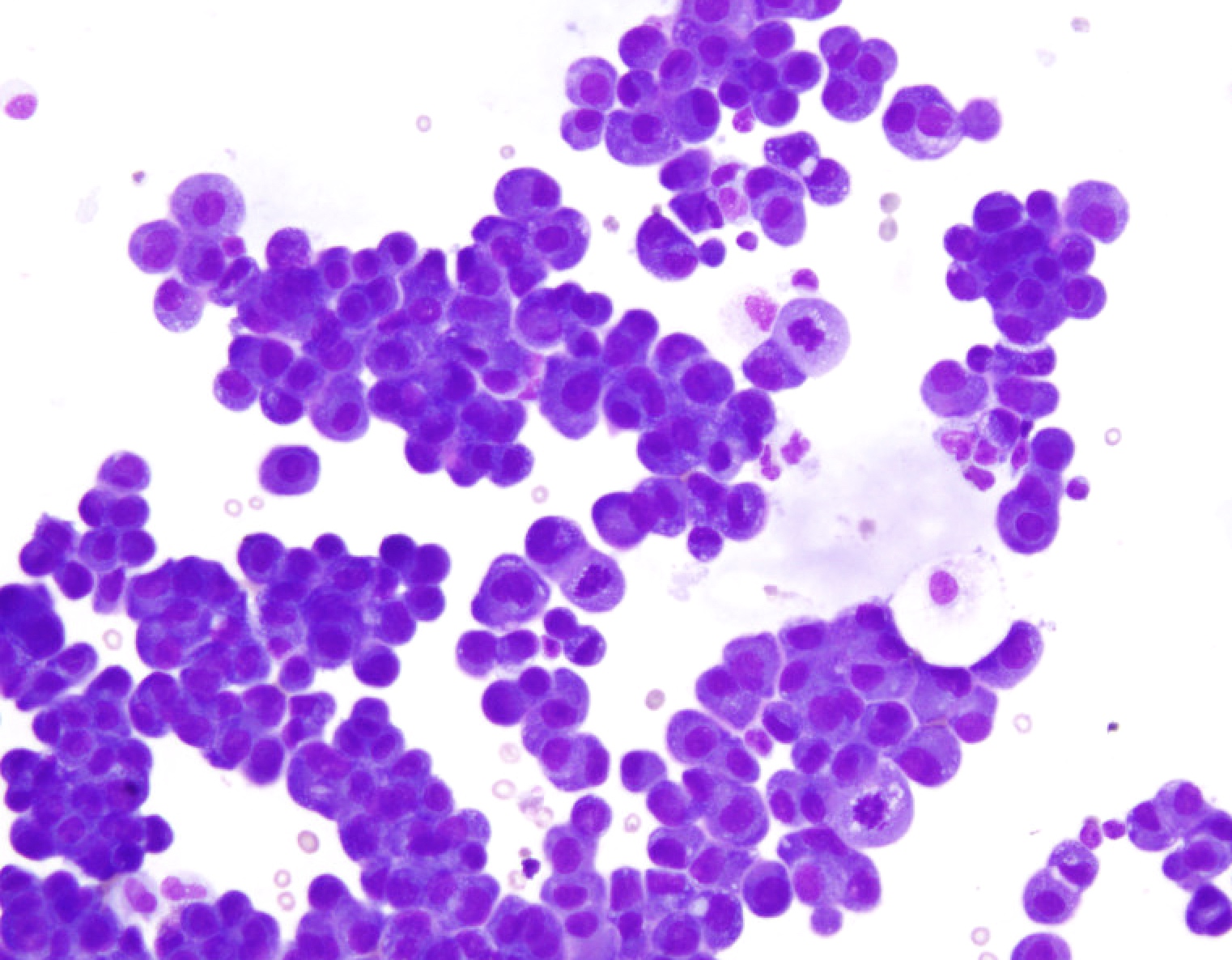
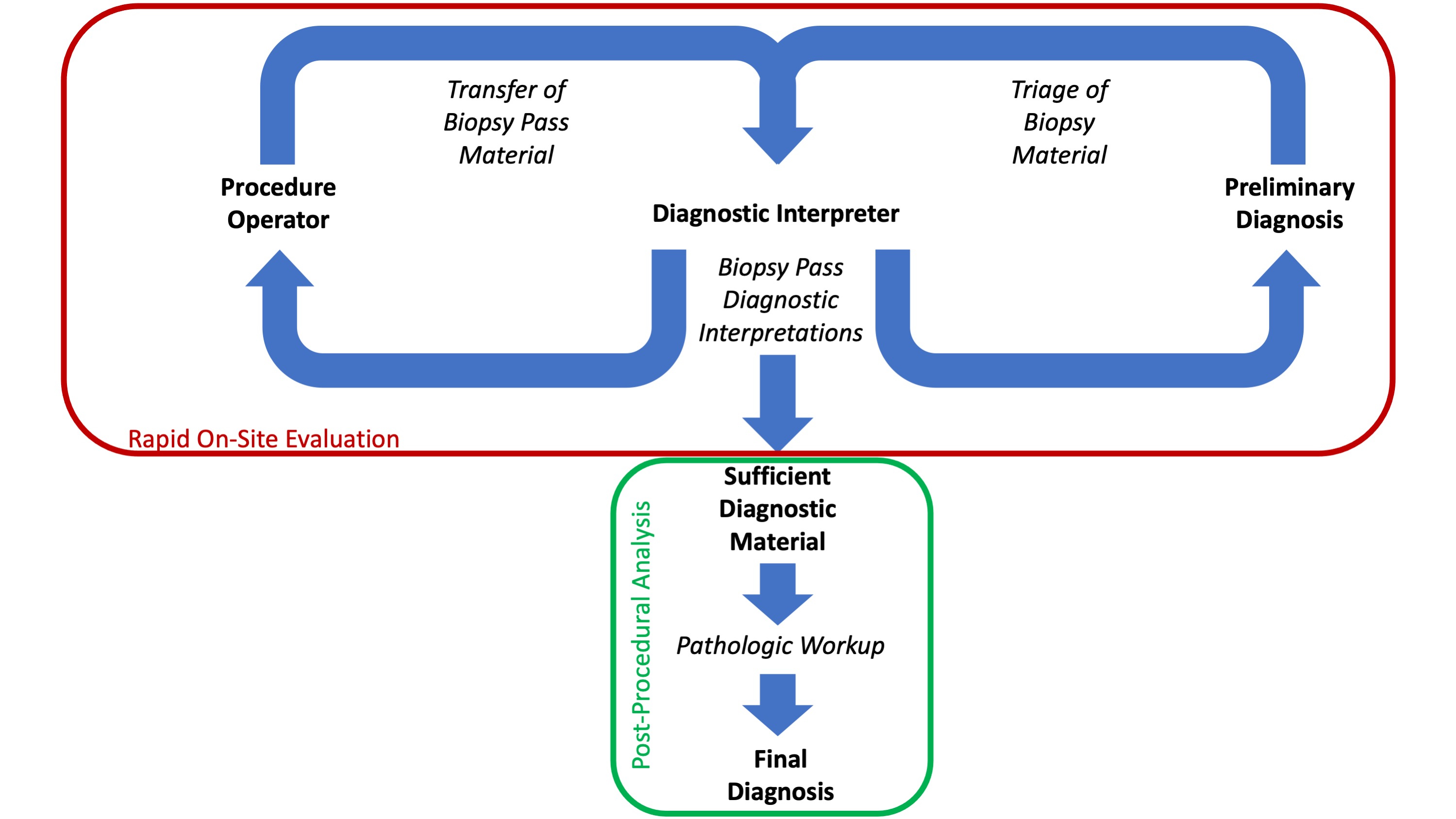
Superpage
Superpage Topics
WHO reporting system for lung cytopathology (pending)
Ancillary techniques (pending)
Ancillary techniques (pending)
Ancillary techniques (pending)
Atypical (pending)
Automation
Benign
Benign (pending)
FNA procedure
IHC panels
Immunocytochemistry (pending)
International Academy of Cytology Yokohama system (pending)
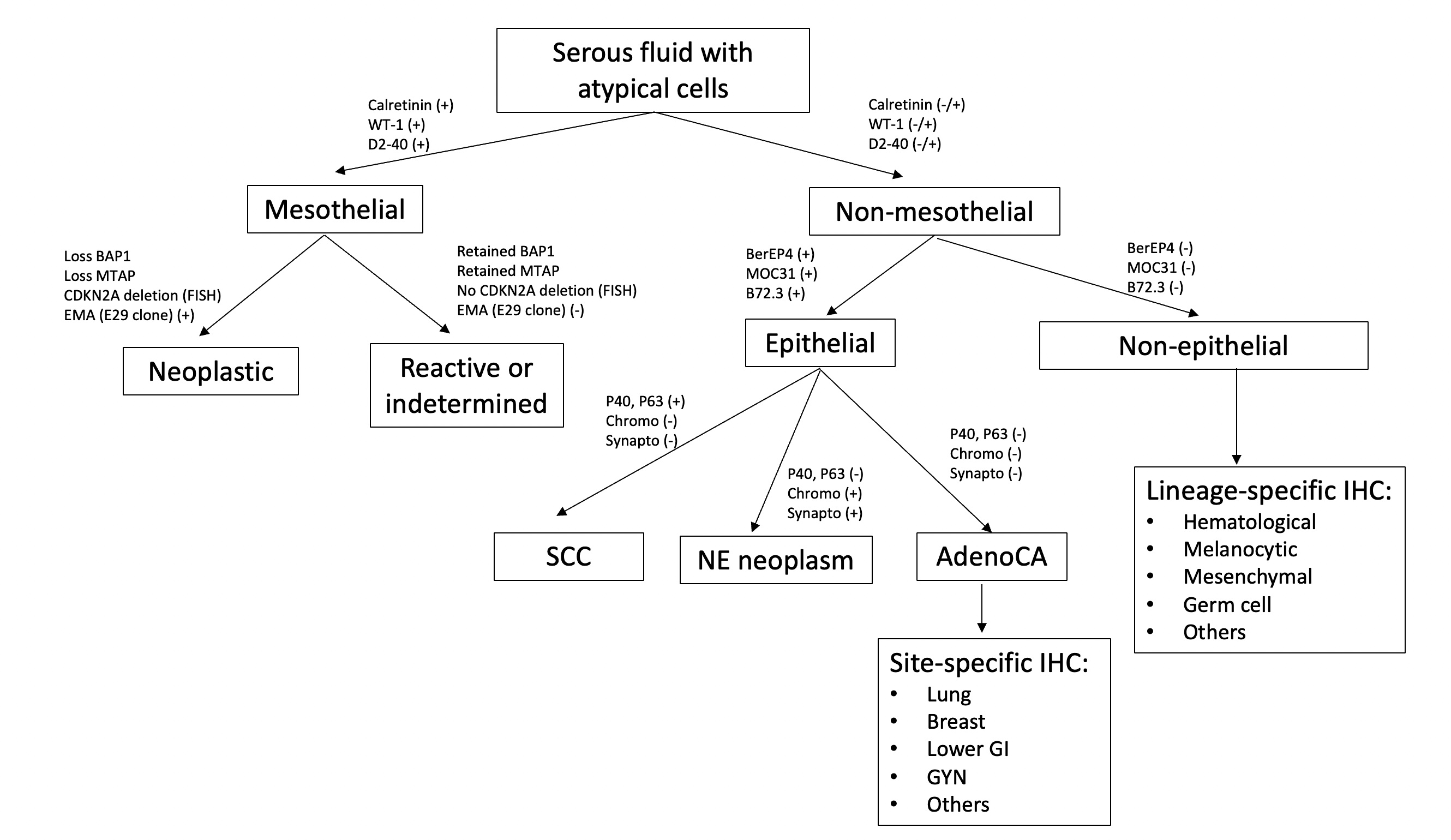
International system for reporting serous fluid cytopathology
Interventional cytopathology
Liquid based cytology
Malignant
Malignant (pending)
Molecular cytopathology (pending)
Nondiagnostic
Overview (pending)
Rapid on site evaluation (ROSE)
Smear making and techniques for recovering and dividing aspirate material
Superficial FNA procedure: contraindications and complications
Suspicious for malignancy (pending)
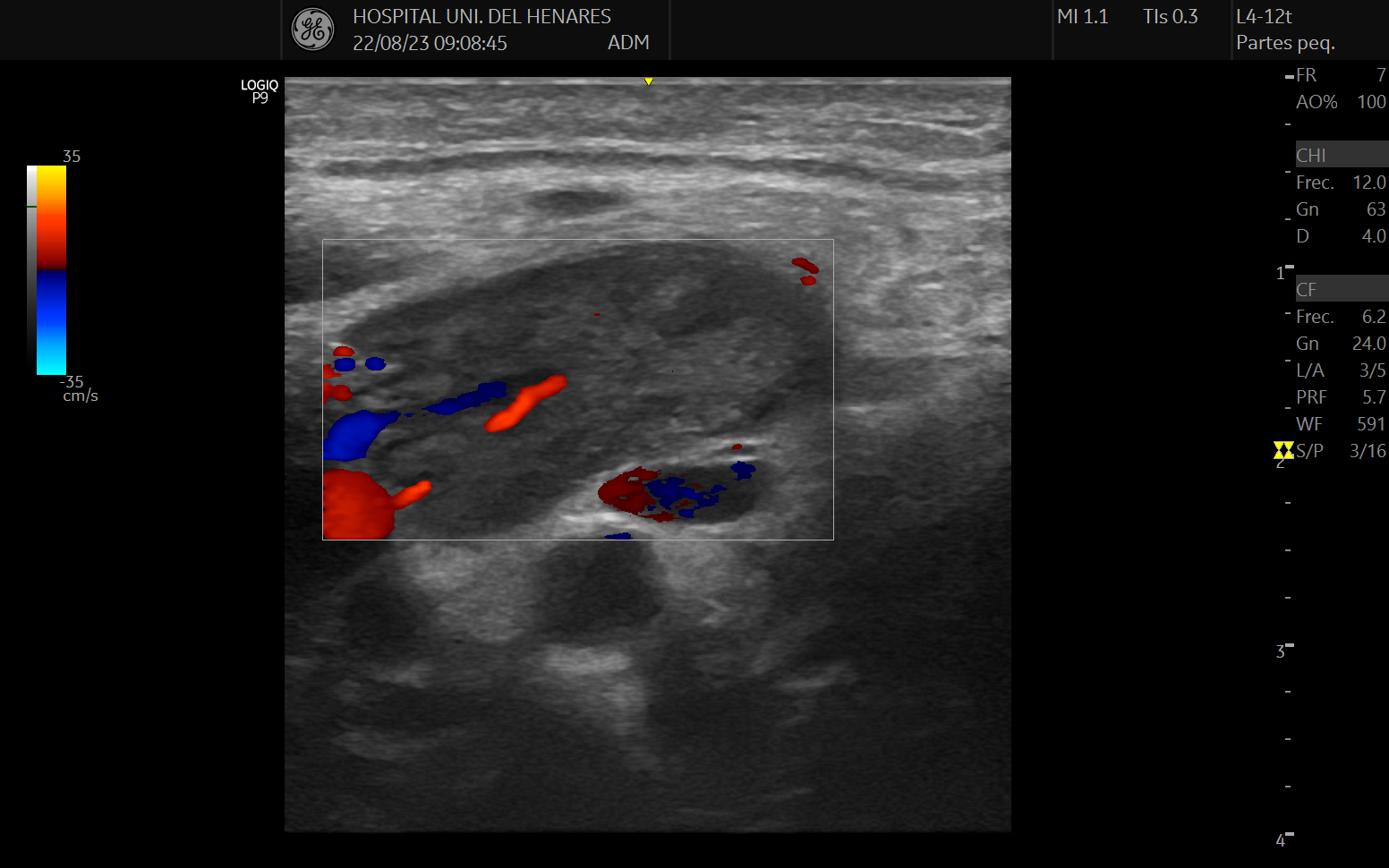
Ultrasound terminology
WHO reporting system for adrenal gland cytopathology (pending)
WHO reporting system for kidney cytopathology (pending)
WHO reporting system for liver cytopathology (pending)
WHO reporting system for soft tissue cytopathology (pending) WHO reporting system for lung cytopathology (pending)
Table of Contents
VideosVideos
Prof. Dr. Fernando Schmitt:
The WHO System for Reporting Lung Cytopathology
Ancillary techniques (pending)
[Pending]
Ancillary techniques (pending)
[Pending]
Ancillary techniques (pending)
[Pending]
Atypical (pending)
[Pending]
Automation
Table of Contents
Definition / general | Essential features | CPT coding | Terminology | Overview | Advantages of automation | Disadvantages of automation | Implementation | Effectiveness | Implication | Diagrams / tables | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Automation in cytopathology refers to the process of slide preparation (fixation and staining), image acquisition and image analysis with identification of abnormalities by automated machinery in conjunction with cytologist review
- Currently primarily utilized in gynecologic cytopathology
Essential features
- Automation has mainly been implemented in gynecologic cytopathology with the development of automation systems that utilize liquid based cytologic processing
- Automated screening has comparable sensitivity to manual screening with the added benefit of increased productivity
- Increased utilization of whole slide imaging and artificial intelligence pose additional benefits and challenges to the development of a completely autonomous digital workflow in gynecologic screening
CPT coding
Terminology
- Analytic and quantitative cytology
- Cytology image analysis techniques
- Automated screening systems in cytopathology
Overview
- Specimen collection
- Spatula / brush
- Specimen processing
- Liquid based
- ThinPrep
- Methanol based PreservCyt fixative solution
- Filtration and dispersion separate debris and mucus without adverse effect on cell appearance
- Controlled pressure deposits cell layer in 20 mm diameter circle
- SurePath
- Preservative fluid (ethanol / methanol / isopropanol)
- Centrifugation separates cells of interest
- Cell layer placed in 13 mm diameter circle via sedimentation
- ThinPrep
- Liquid based
- Image acquisition
- Prepared slides loaded into imaging station
- Cell spot image scanned and sent to image processor
- Image analysis (ACM Computing Surveys 2022;54:1)
- Segmentation approach
- Thresholding, region, contour, texture, graph, clustering, deep learning
- Segmentation free
- Support vector machine (SVM), fuzzy c-means (FCM), convolutional neural network
- Segmentation approach
- Cytotechnologist review
- Field of view (FOV) presented
- Slides without abnormality diagnosed as NILM and signed out
- Slide with abnormality marked for cytopathology review
- Slide rescreening
- Entire slide manually rescreened and signed out
Advantages of automation
- Increased productivity and cytotechnologist satisfaction
- Greater efficiency in evaluating slides
- Decreased fatigue and turnaround time
- Lower hospital cost (deriving from increased productivity)
- Reference: Diagn Cytopathol 2021;49:559
Disadvantages of automation
- Similar sensitivity to manual screening (Cytojournal 2007;4:6)
- Initial cost and maintenance
- Additional quality assurance and quality control (continued calibration and validation of machines)
- Requires additional training of cytopathologists and cytotechnologists
- Remaining fatigue, inattention and habituation with increased number of slides necessitates implementation of daily slide limitation
- Reference: Diagn Cytopathol 2021;49:559
Implementation
- Gynecologic
- PAPNET (1994, Neuromedical Systems, Inc., Suffern, NY)
- First truly digital pathology device
- Neural network technology used to identify abnormal cells in cases already screened as negative by cytotechnologist
- 128 abnormal cells / clusters identified, photographed and mailed back to laboratory as a digital tape for review by cytotechnologist
- Additional resources: Laboratory Medicine 1991;22:276
- AutoPap 300QC (1995, NeoPath Inc., precursor to BD FocalPoint GS imaging system)
- Originally developed for quality control of screened Pap smear slides to reveal false negatives
- Cell annotations used to develop algorithms to score overall slides and individual field of view (higher score, more abnormalities)
- Higher cumulative score, increased likelihood of finding abnormality on slide
- Additional resources: Acta Cytol 1996;40:45
- ThinPrep imaging system (2004, HOLOGIC, Marlborough, MA)
- FDA approved
- ThinPrep specimen processing
- ThinPrep image processer consists of imaging station, image processor controller, server and user interface
- Imaging station: scans entire slides
- Imaging processor controller: creates images and analyzes data
- Server: stores slide and imaging data
- User interface: allows operator to use the machine
- Image processor selects 22 fields of view (FOV) at 100x magnification for review by cytotechnologist
- Additional resources: HOLOGIC: ThinPrep Imaging System [Accessed 9 August 2022]
- BD FocalPoint GS imaging system (2001, Becton, Dickinson and Company, Franklin Lakes, NJ)
- FDA approved
- SurePath specimen processing
- Image processor utilizes slide profiler and review station
- Slide profiler screens slides and locations based upon likelihood of containing abnormality
- Review station arranges slides into quintiles based upon likelihood of abnormality (1 = highest risk, 5 = lowest risk)
- 10 FOVs for each slide are presented
- Lowest quintile of slides (least likely to have abnormality) can be archived without further review
- Additional resources: BD: FocalPoint GS Imaging System [Accessed 9 August 2022]
- Cytoprocessor (2017, DATEXIM, Caen, France)
- CE certified (approval in Europe)
- Utilizes any preparation protocol and liquid based cytology (LBC)
- Detects cells in whole slide images and arranges depending on abnormality
- Can be integrated into a variety of workflows without requiring additional materials
- Additional resources: DATEXIM: CYTOPROCESSOR [Accessed 9 August 2022]
- PAPNET (1994, Neuromedical Systems, Inc., Suffern, NY)
Effectiveness
- MAVARIC trial (Health Technol Assess 2011;15:1)
- Conducted in England, compared Imager / FocalPoint to manual screening for potential implementation in National Health Service (NHS)
- Significantly lower sensitivity of automated screening detection of cervical intraepithelial neoplasia grade II (CIN 2) and above (0.92) compared to manual screening
- Comparable rates of detecting CIN 2 and above, between ThinPrep and SurePath
- Increased productivity in automated screening arm (60 - 80% higher)
- Additional studies in Australia, Scotland and U.S.
- Findings:
- Significantly lower inadequate / negative reporting rates, higher low grade reporting rates
- No significant difference in detection of high grade squamous intraepithelial lesion (HSIL)
- Increased specificity with imager assisted screening compared to manual screening alone (Cytopathology 2013;24:235)
- No significant difference in positive predictive value of Imager screening versus manual screening of ThinPrep slides
- Up to 27% increase in productivity using Imager screening versus manual screening of ThinPrep slides (Diagn Cytopathol 2007;35:96)
- Findings:
Implication
- Daily screening limitations (Diagn Cytopathol 2019;47:20)
- Automated slides without abnormality count as 0.5 slides
- Abnormal automated screens requiring rescreening count as 1.5 slides
- Limits vary by country
- U.S.: 100 slides/day
- U.K.: 32 slides/day
- Italy: 25 - 50 slides/day
- Australia: 70 slides/day
- Canada: 80 slides/day
- American Society of Cytopathology guidelines (Diagn Cytopathol 2013;41:174)
- Limit Pap screening to < 7 hours/day
- Average < 70 slides/day
- Computational cytology
- Combines whole slide imaging with artificial intelligence
- Advantages:
- Potential increase in accuracy of slide interpretation
- Further increase in productivity
- Limitations (Acta Cytol 2021;65:301):
- Requires established digital workflow
- Regular calibration and maintenance of whole slide imaging and software packaging
- Cytologic processing artifact present in whole slide imaging impairs digital datasets used for whole slide analysis
- 3 dimensional clustering necessitates image capture on multiple focal planes (z stacking)
- Limited datasets for training
- Added responsibility for cytologist
- Advantages:
- Combines whole slide imaging with artificial intelligence
Diagrams / tables
Board review style question #1
Board review style answer #1
Board review style question #2
Review of slides screened as "abnormal" with automated preparation, counts as how many slides toward the daily workload limit?
- 0 slides
- 0.5 slide
- 1.0 slide
- 1.5 slides
- 2.0 slides
Board review style answer #2
Benign
Table of Contents
Definition / general | Essential features | CPT coding | Sites | Diagrams / tables | Laboratory | Radiology description | Radiology images | Case reports | Cytology description | Cytology images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Specimens categorized as benign demonstrate unequivocal cytopathological features, which may or may not be diagnostic of a specific process or benign neoplasm
Essential features
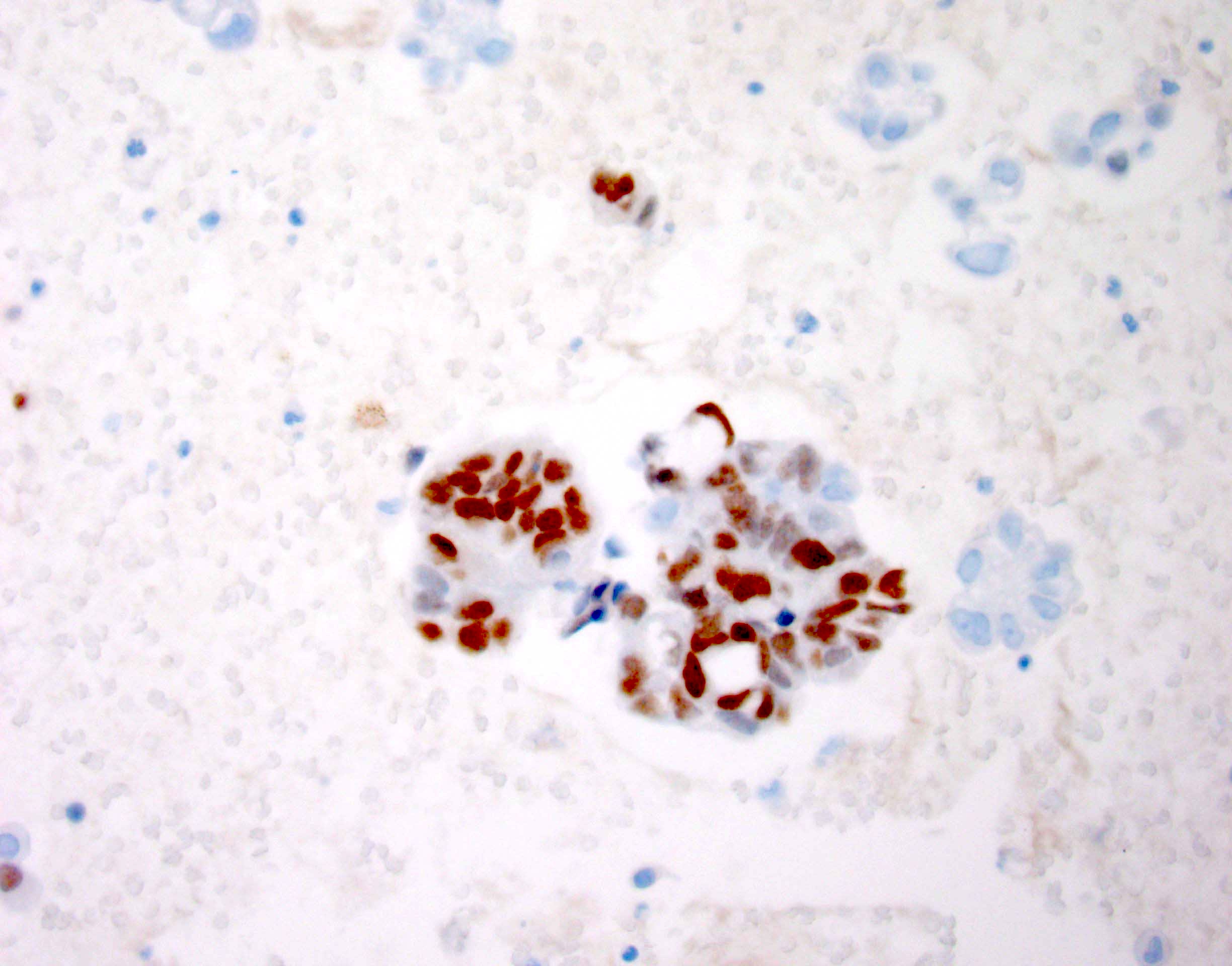
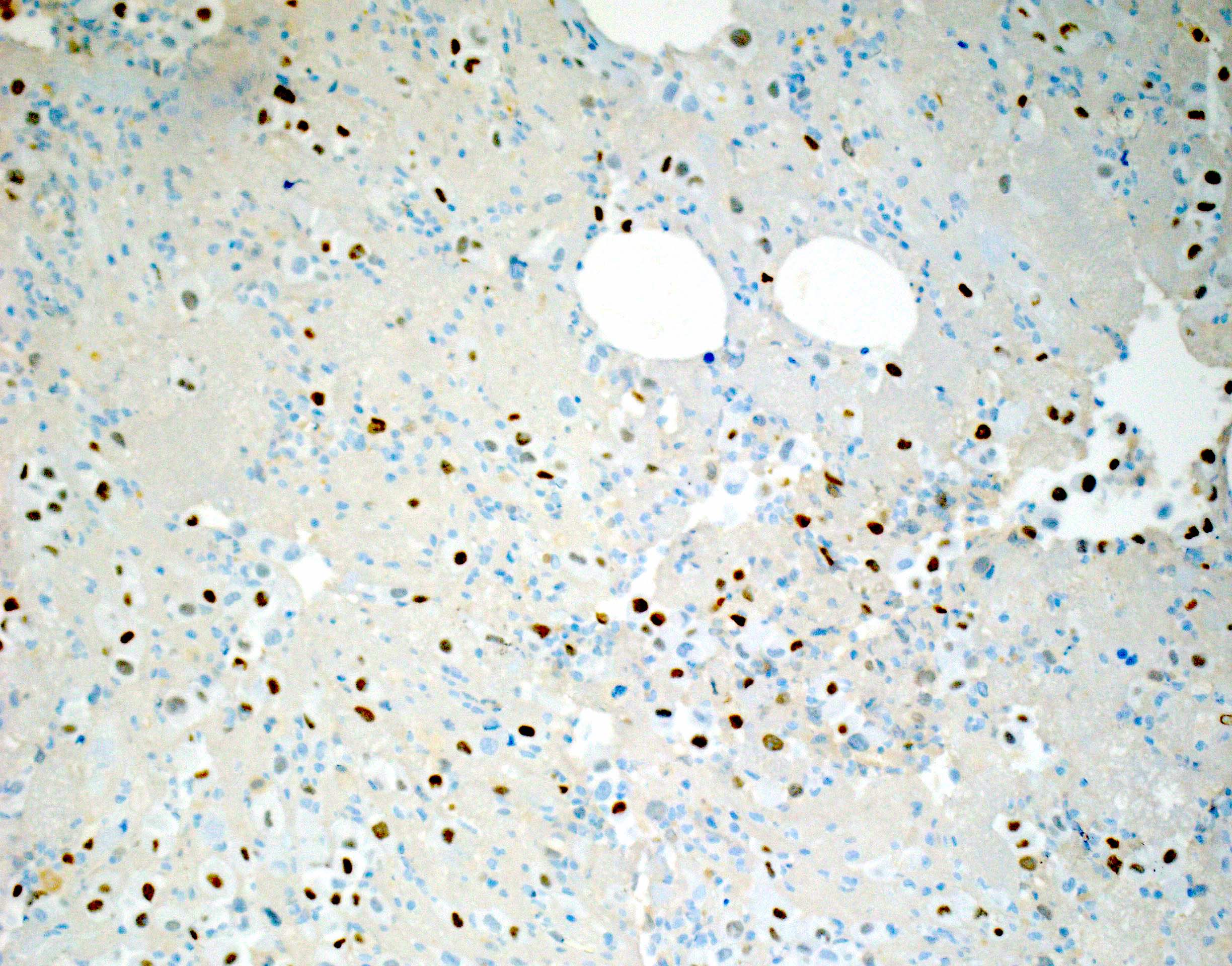
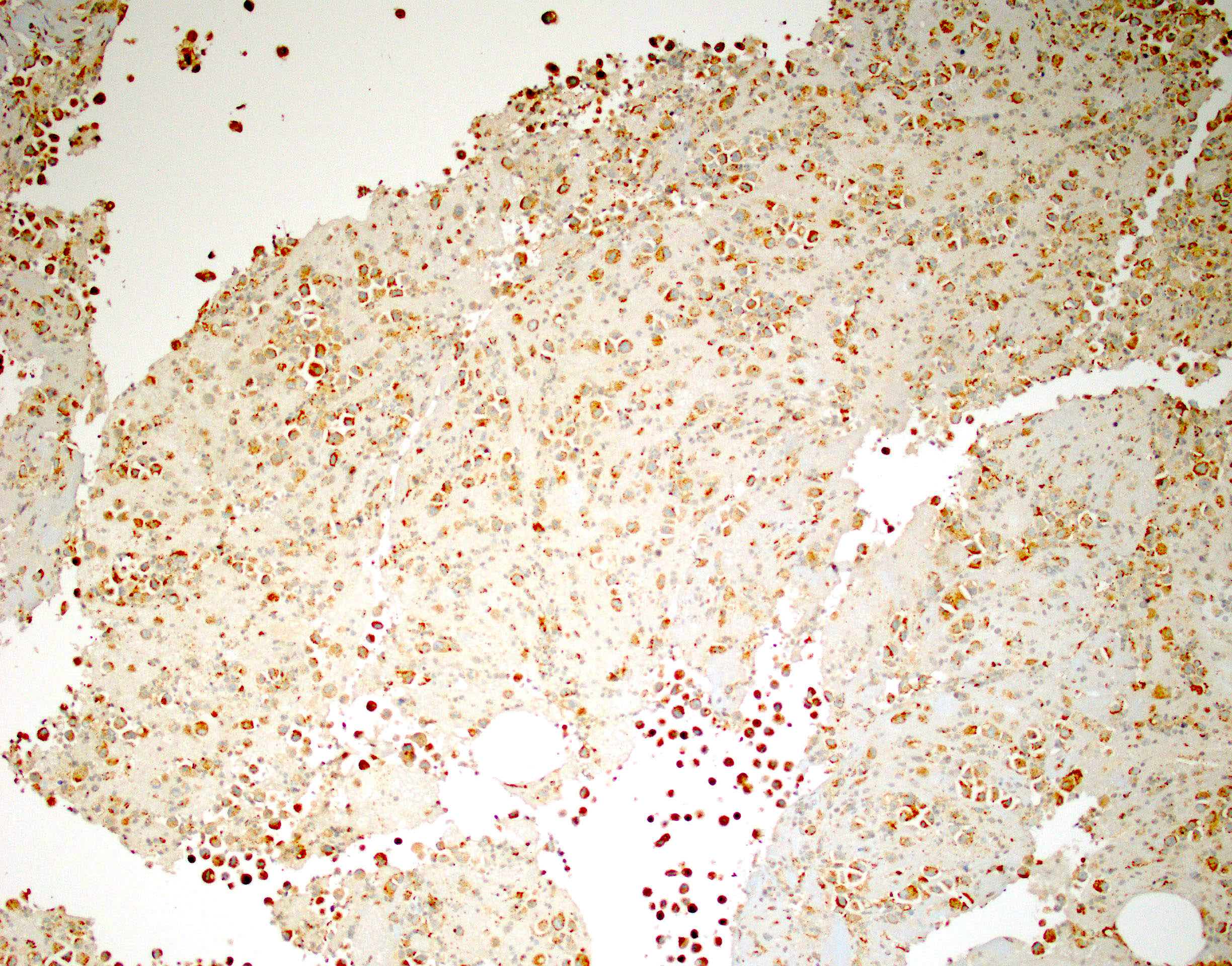
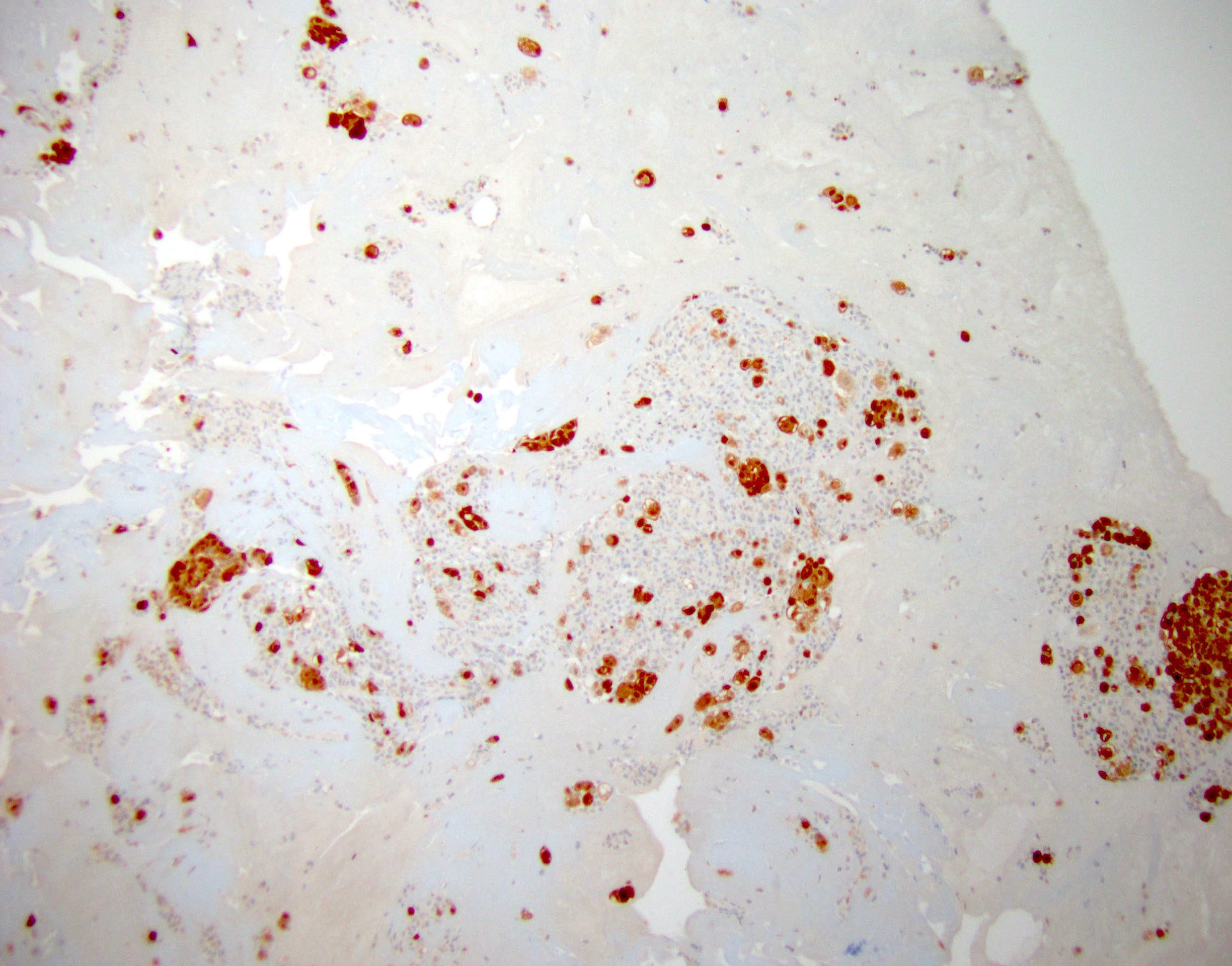
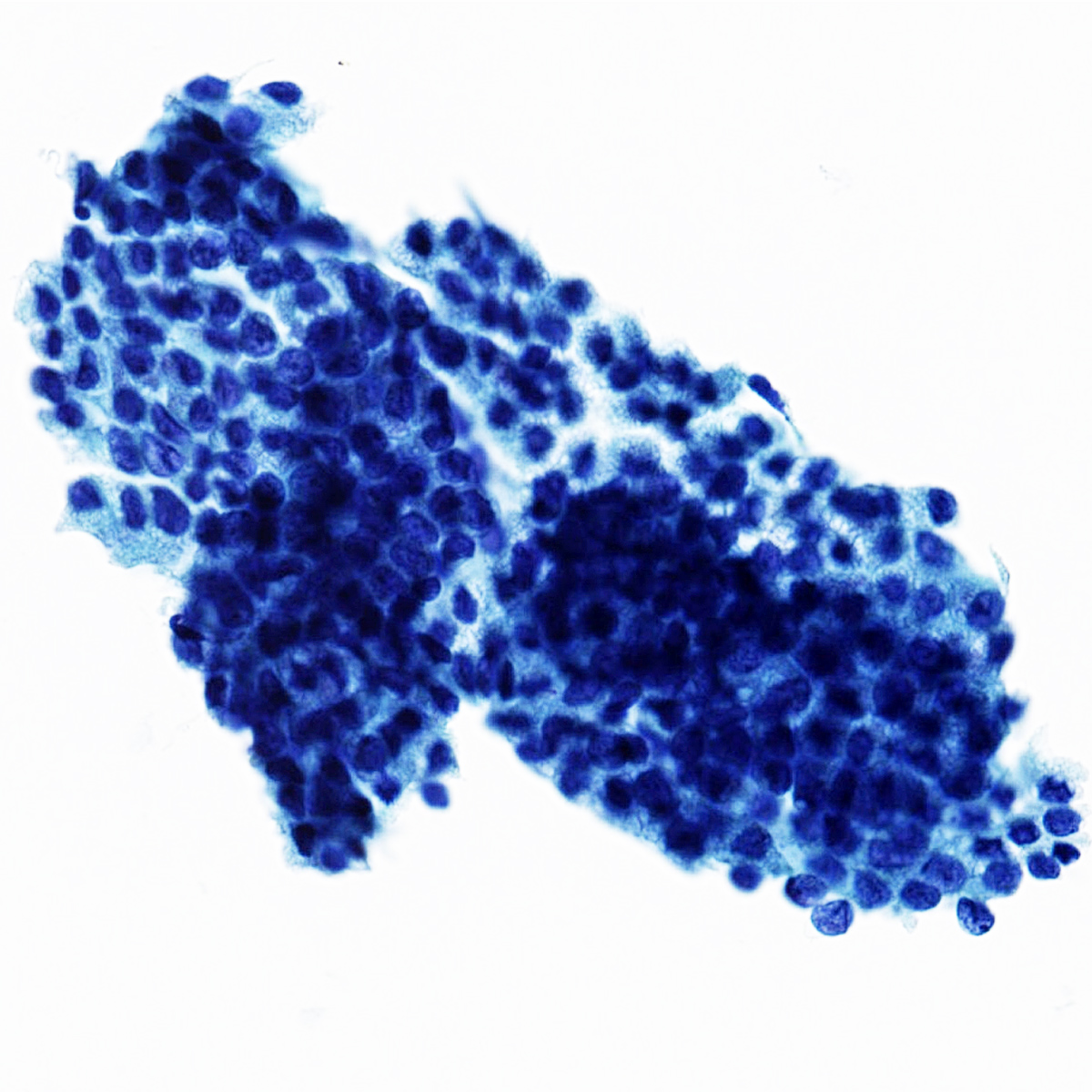
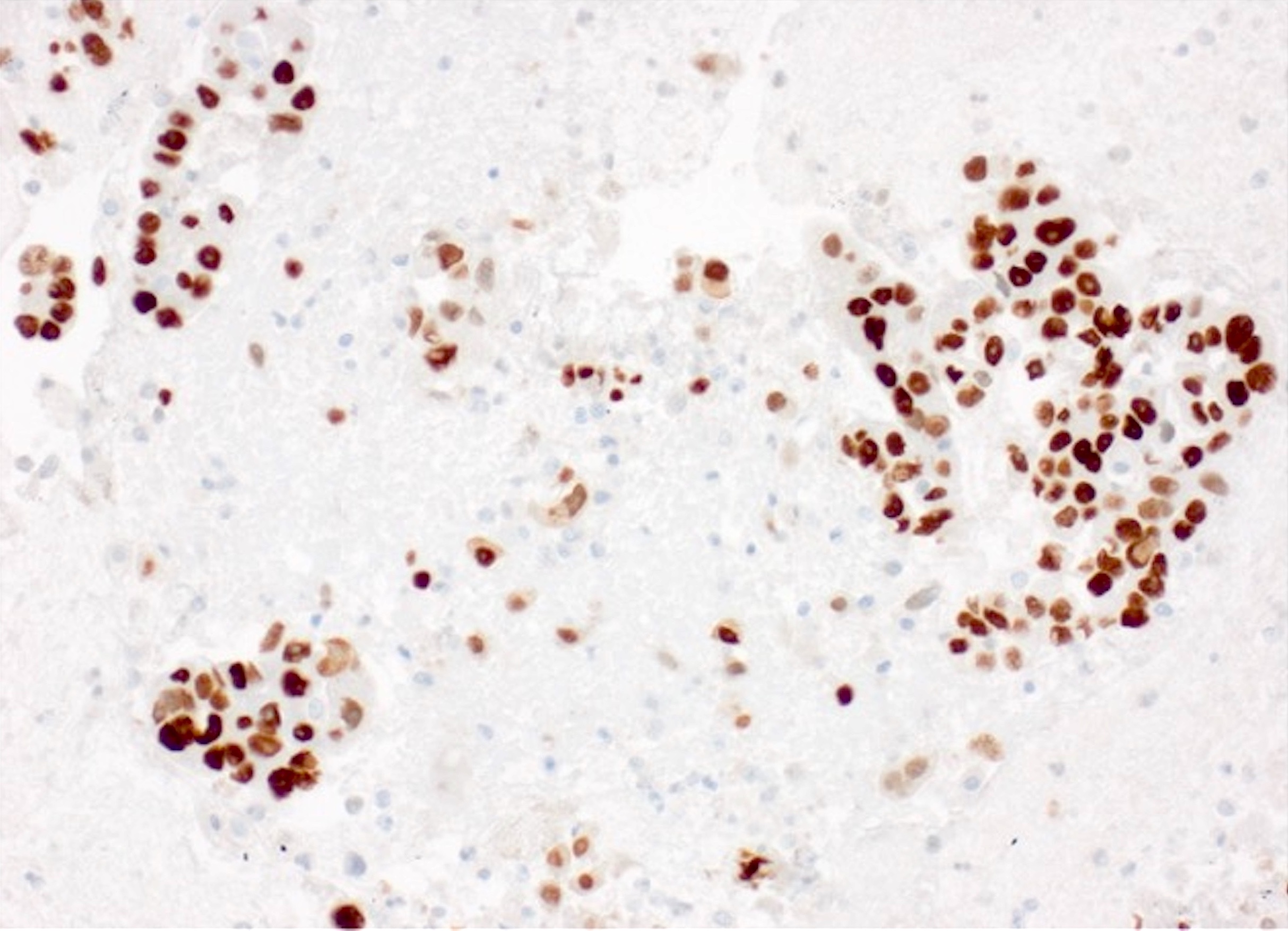
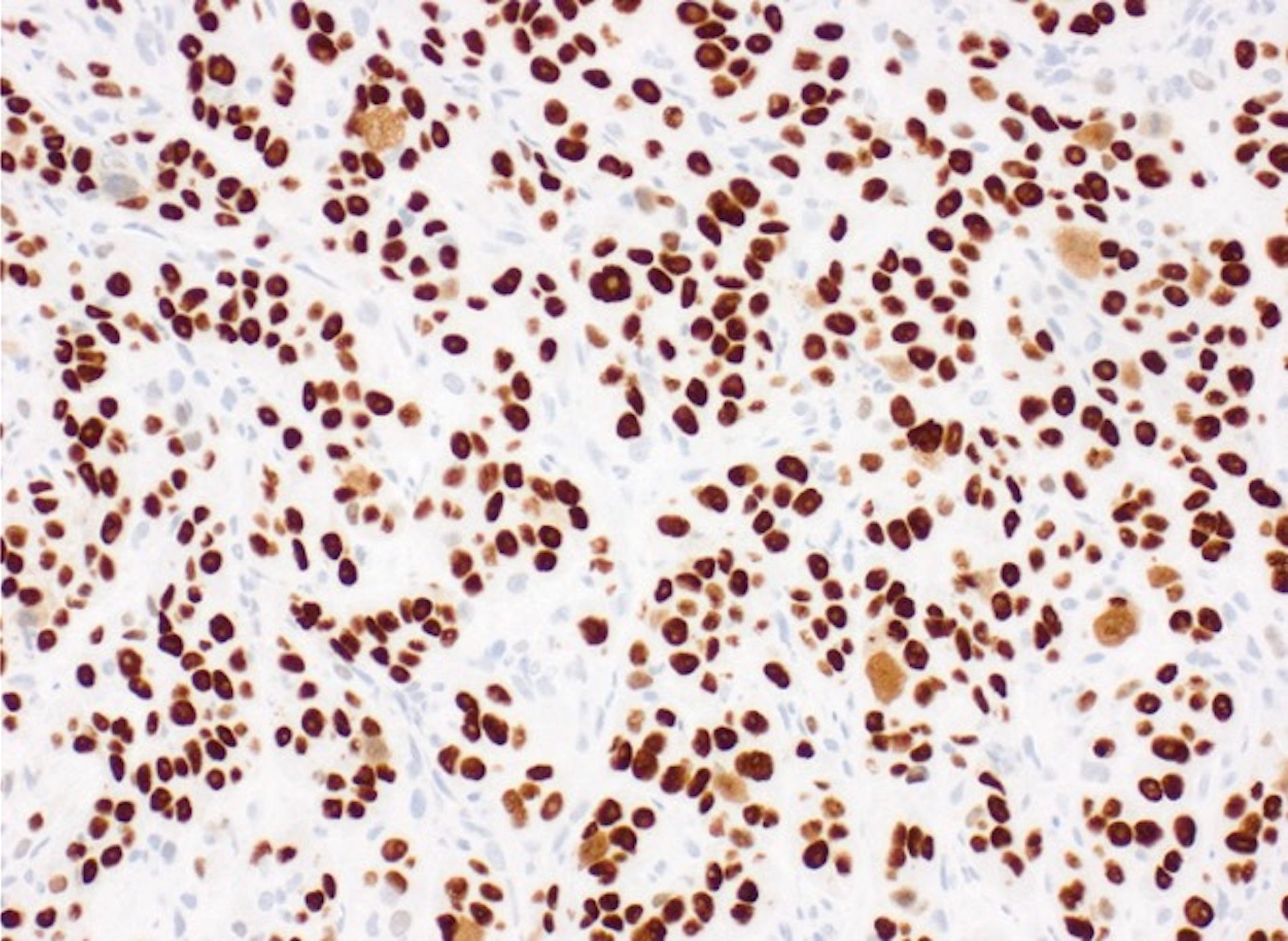
- The World Health Organization (WHO) Reporting System for Lung Cytopathology is the recommended system for reporting results (Acta Cytol 2023;67:80)
- Per the reporting system, benign includes benign bronchial elements, inflammatory or infectious processes (e.g., granulomatous inflammation) or a specific benign tumor (e.g., hamartoma)
- Rate of malignancy is reported to be in the range of 20 - 50% (Acta Cytol 2022;66:124, Diagn Cytopathol 2016;44:399, Diagn Cytopathol 2018;46:725)
CPT coding
Sites
- Lung, bronchus
- Lung, parenchyma
Diagrams / tables
N/A
Laboratory
N/A
Radiology description
N/A
Radiology images
N/A
Case reports
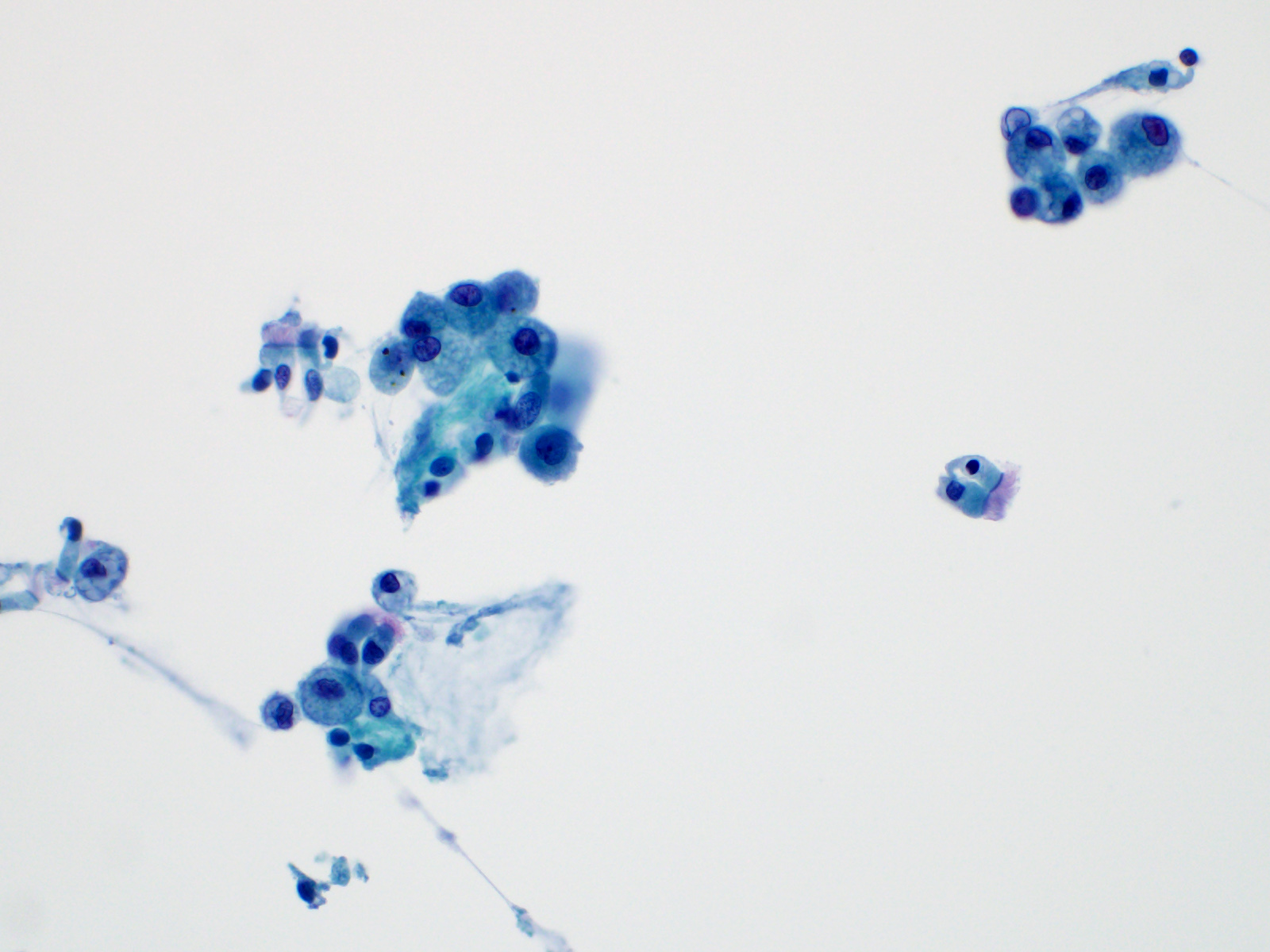
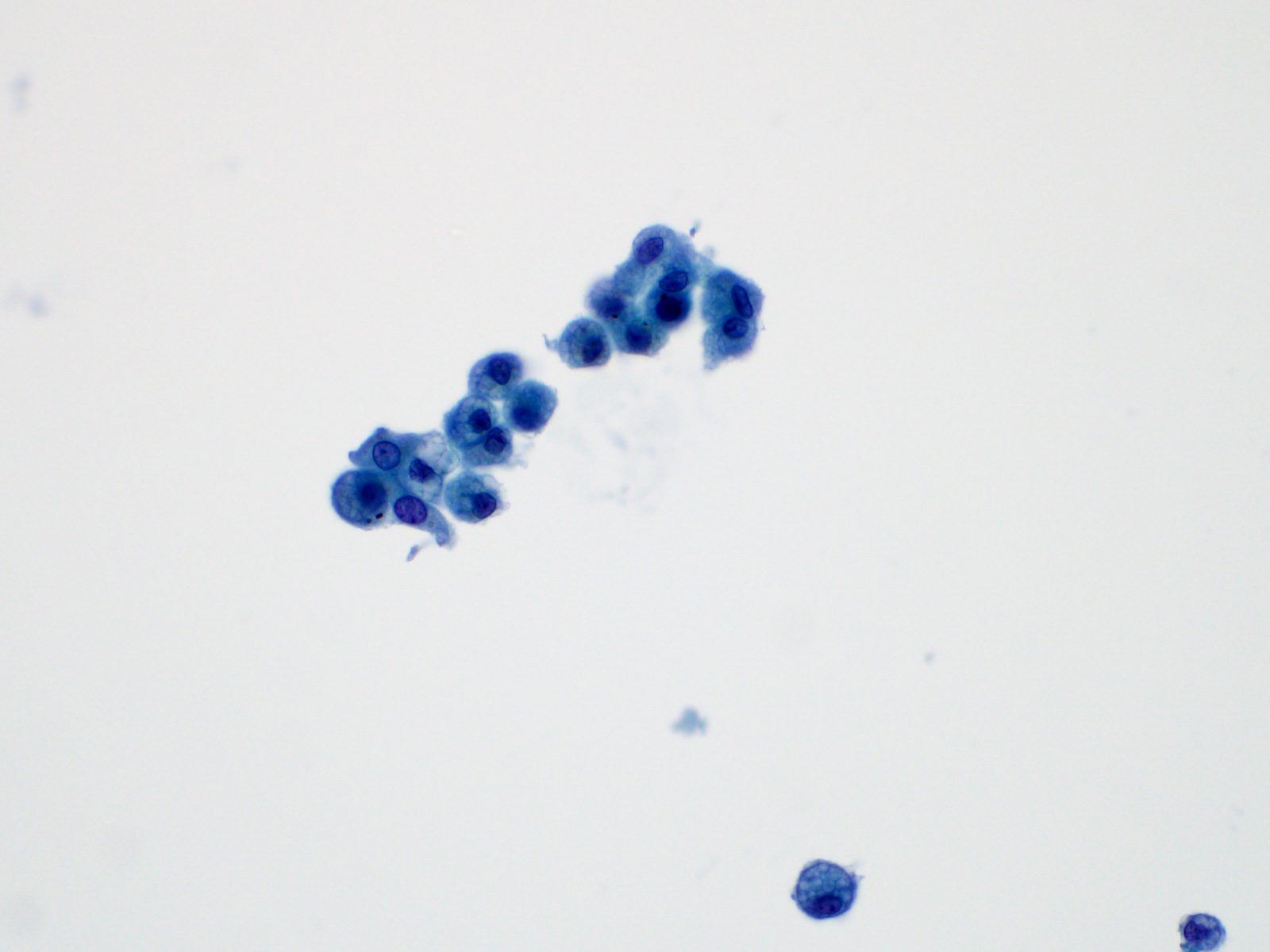
- 59 year old man with a left upper lobe lung cavitary lesion (Cytopathology 2023;34:158)
- 70 year old woman with a 3.3 cm soft tissue mass (J Cytol 2012;29:250)
- 72 year old man with ground class opacities and infiltrative shadows in bilateral lungs (Diagn Cytopathol 2021;4:E277)
Cytology description
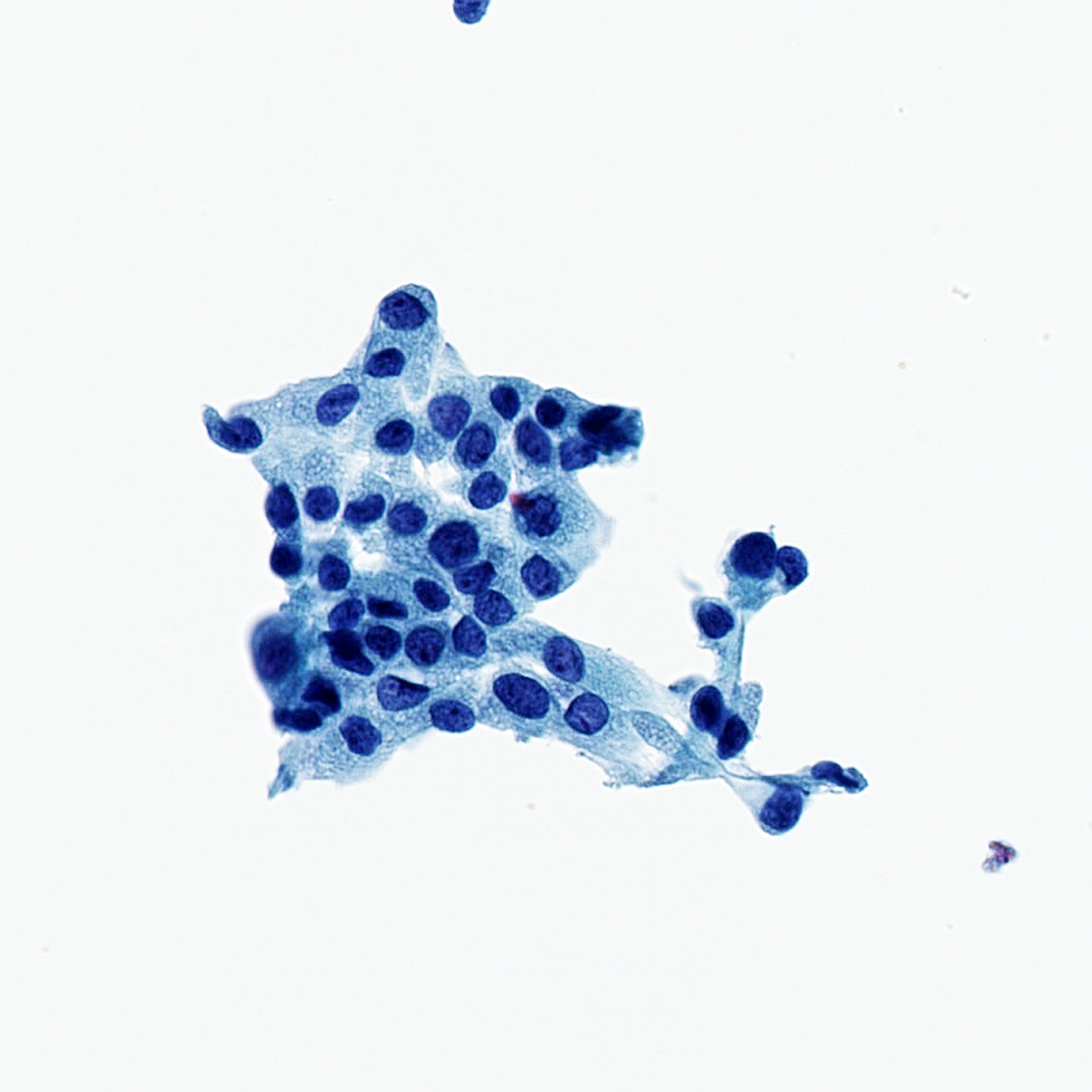
Normal benign elements
Reactive elements
Infection
Benign neoplasms
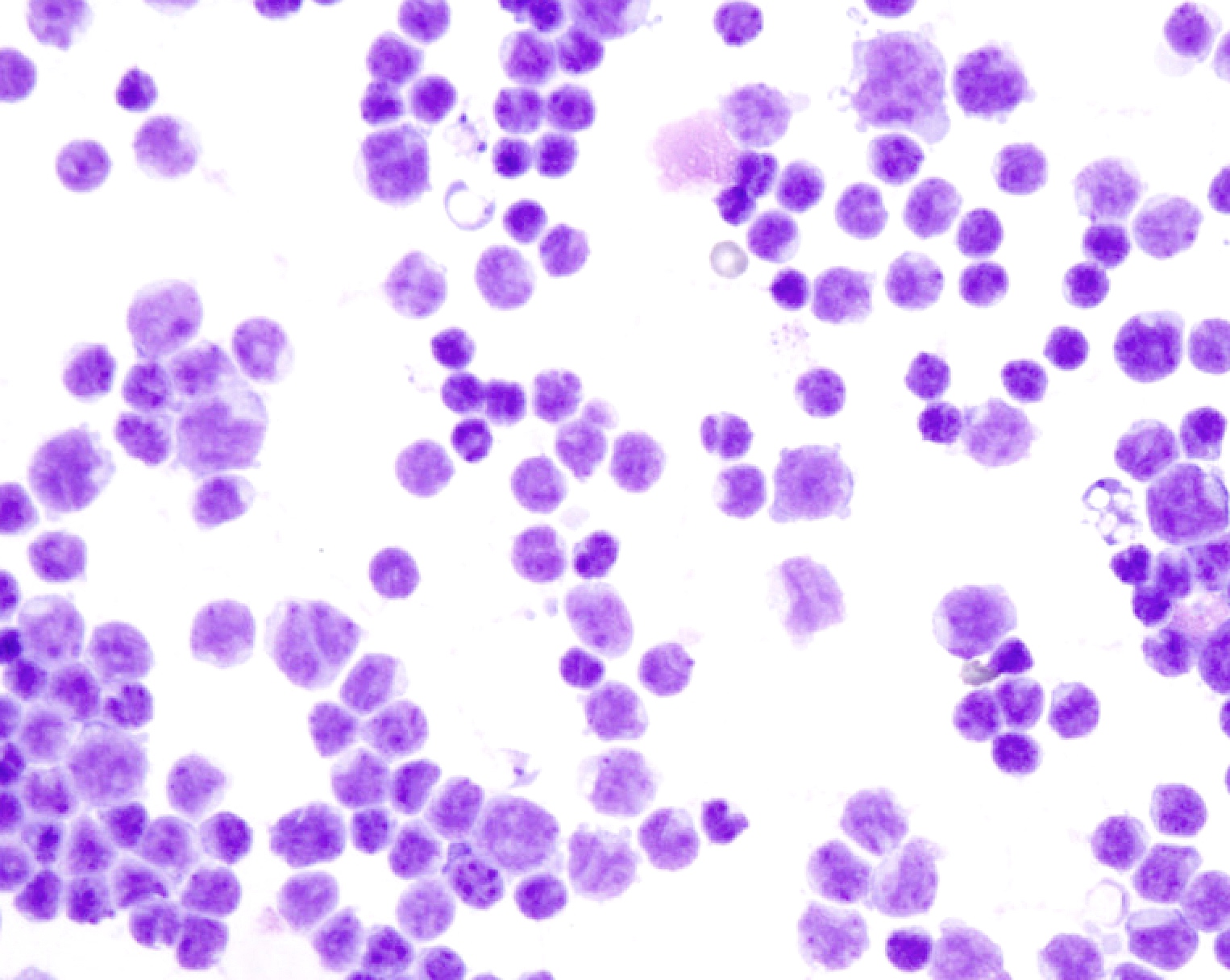
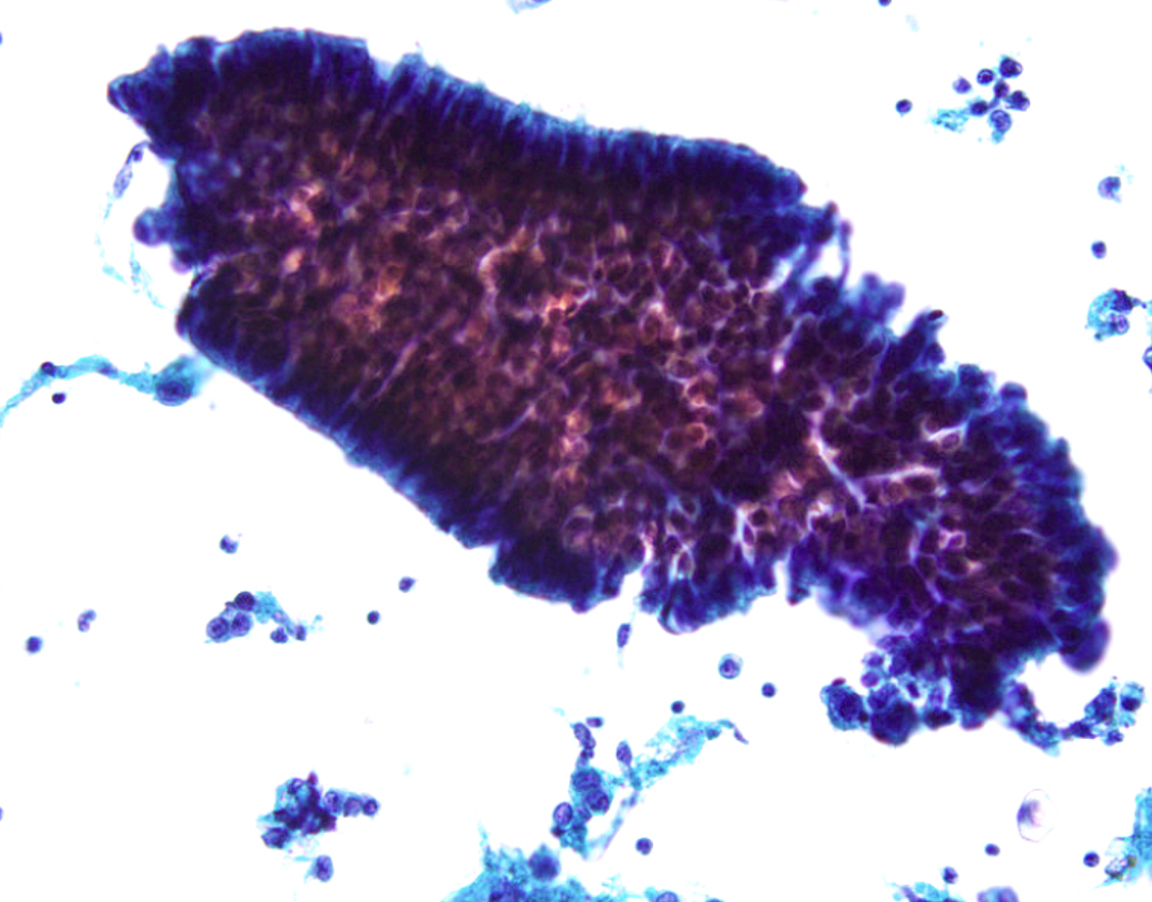
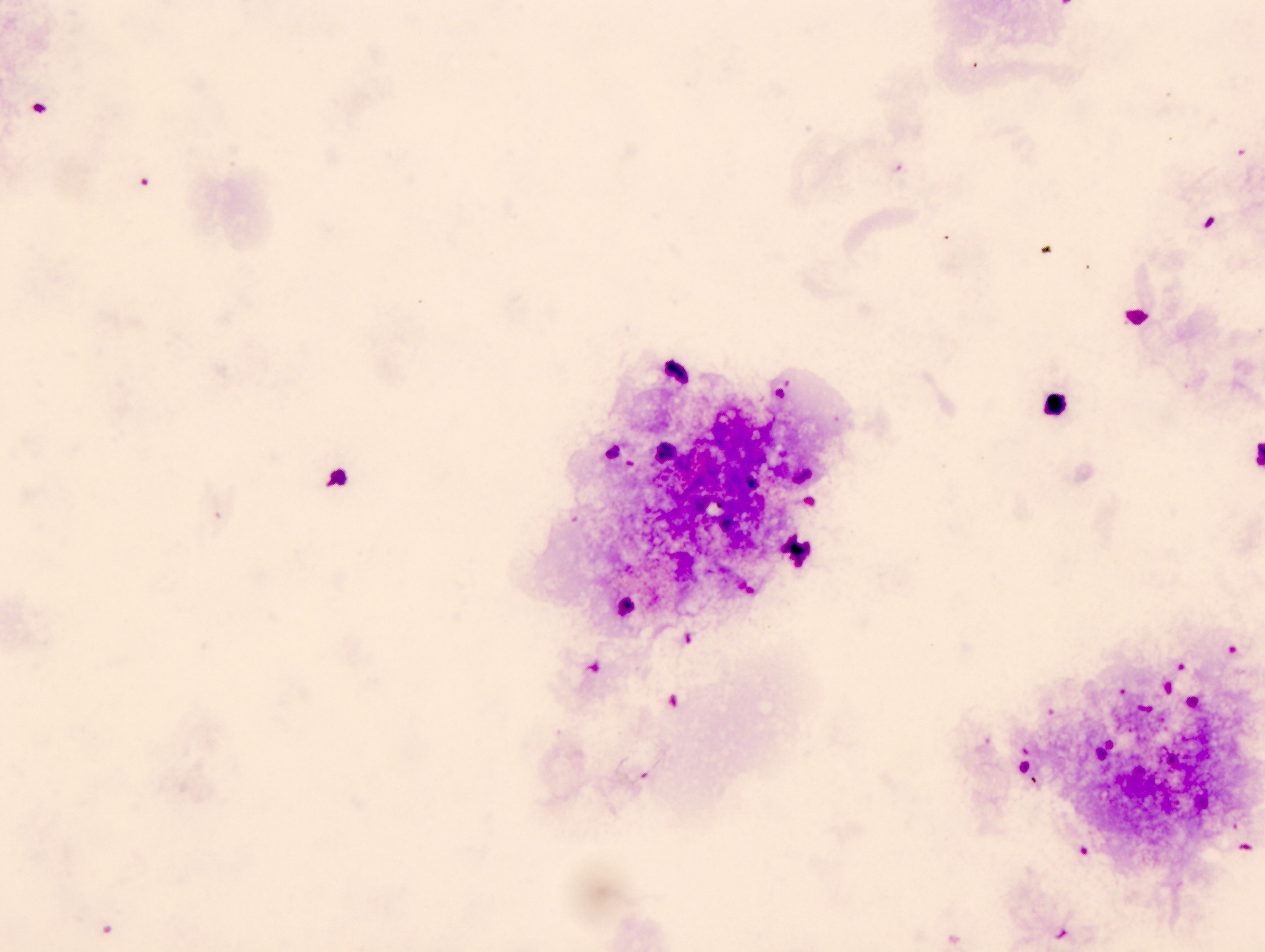
- Bronchial cells
- Columnar cells
- Small nuclei
- Abundant apical cytoplasm
- Terminal bars and cilia frequent and prominent
- Alveolar macrophages
- Round cells with pale, irregular nuclei
- Abundant, foamy cytoplasm
- Pneumocytes (Diagn Cytopathol 2010;38:297)
- Appear similar to alveolar macrophages
- Few cohesive groups with scalloped borders, intercellular windows or gaps
Reactive elements
- Basal cell hyperplasia (Diagn Cytopathol 2010;38:297)
- Small, uniform cells
- Dark round or oval nuclei, smooth nuclear borders
- Scant basophilic cytoplasm
- Occasional molding; can mimic small cell carcinoma
- Squamous metaplasia (Diagn Cytopathol 2011;39:144)
- Smudgy chromatin
- Eosinophilic cytoplasm versus organophilic
- Preserved N:C ratio
- Creola bodies (Arerugi 1989;38:542)
- Clusters of ciliated bronchial epithelial cells
- Associated with asthma and eosinophils
- Curschmann spirals (Diagn Cytopathol 1998;19:349)
- Spiral shaped mucous plugs
- Nonspecific finding: can be seen in smokers, lung cancer, chronic bronchitis
- Treatment effect: radiation therapy (Pathologica 1991;83:317)
- Clinical context is helpful in this diagnosis
- Atypical nuclei with smudgy effect
- Abundant cytoplasm, often vacuolated
- Anthracosis
- Dark pigment within macrophages
- Finer than melanin
Infection
- Bacterial
- Actinomycosis (Monaldi Arch Chest Dis 2022;92:1641)
- Dark cotton ball-like mass
- Spider leg-like projections
- Branches at acute angles
- Sulfur granules
- Nocardiosis (Diagn Cytopathol 2017;45:1105)
- Narrower than Actinomyces
- Right angle branching
- Associated with neutrophils
- Tuberculosis (Diagn Cytopathol 2014;42:993)
- Strongly associated with necrotizing granulomas
- Acid fast positive
- Actinomycosis (Monaldi Arch Chest Dis 2022;92:1641)
- Fungal
- Blastomycosis (Acta Cytol 2020;64:532)
- Thick walled, double contoured spores (8 - 15 μm)
- Broad based bud
- Histoplasmosis (Acta Cytol 2020;64:532)
- Histiocytes filled with small spores (2 - 4 μm)
- Narrow based budding with capsule
- Pneumocystis (Cancer Cytopathol 2018;126:643)
- Curved, cup shaped cysts (4 - 8 μm)
- Central dot with GMS staining
- Associated with frothy pink exudates
- Aspergillus (Cancer Cytopathol 2018;126:643)
- Septate hyphae
- 45 degree acute angle branches
- Background often has abundant neutrophils and necrosis
- Cryptococcosis (Acta Cytol 2020;64:532)
- Narrow based budding (4 - 12 μm)
- Mucin rich capsule
- Positive for mucicarmine
- Coccidioidomycosis (Semin Diagn Pathol 2017;34:530)
- Thick walled, large spherules (10 - 80 μm)
- Can be filled with endospores
- Mucormycosis (Semin Diagn Pathol 2017;34:530)
- Exclusively in diabetic or immunocompromised patients
- Broad hyphae with thin walls
- Twisted and folded walls
- Irregular branching
- Candida (Semin Diagn Pathol 2017;34:530)
- Can occur as endobronchial growth or in abscess
- Oval yeasts
- Pseudohyphae: elongated and pinched at attachment
- Blastomycosis (Acta Cytol 2020;64:532)
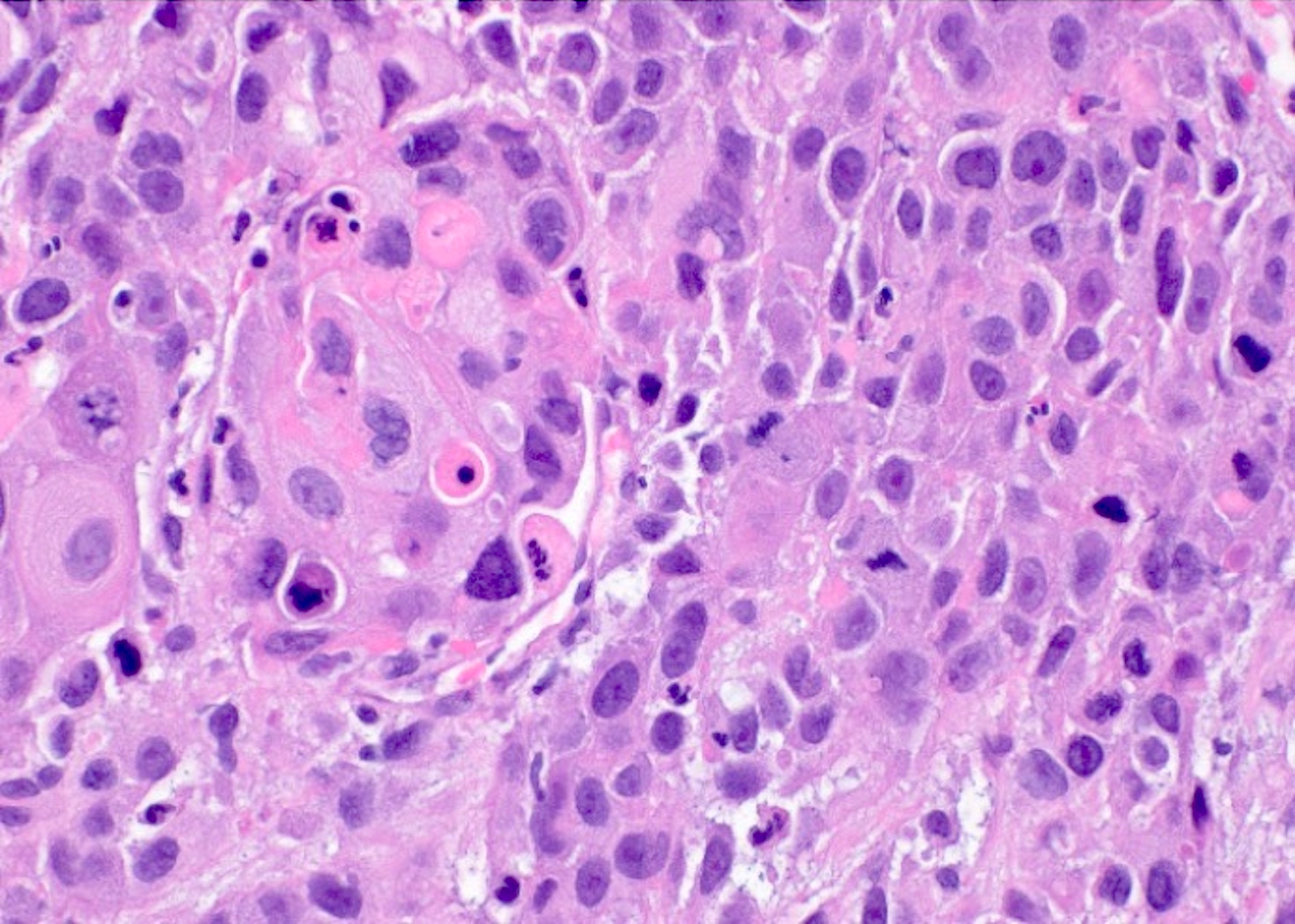
- Viral cytopathic effects
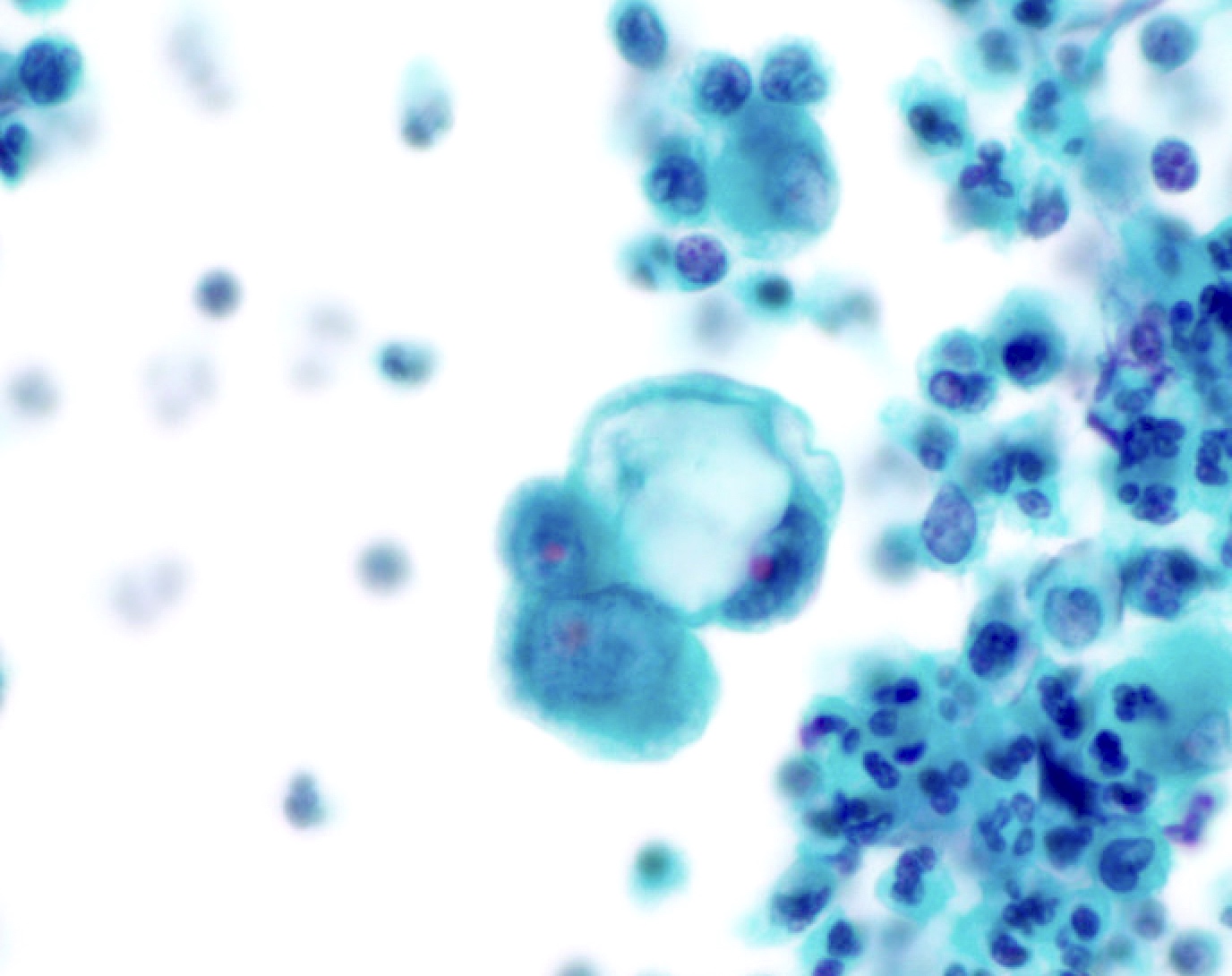
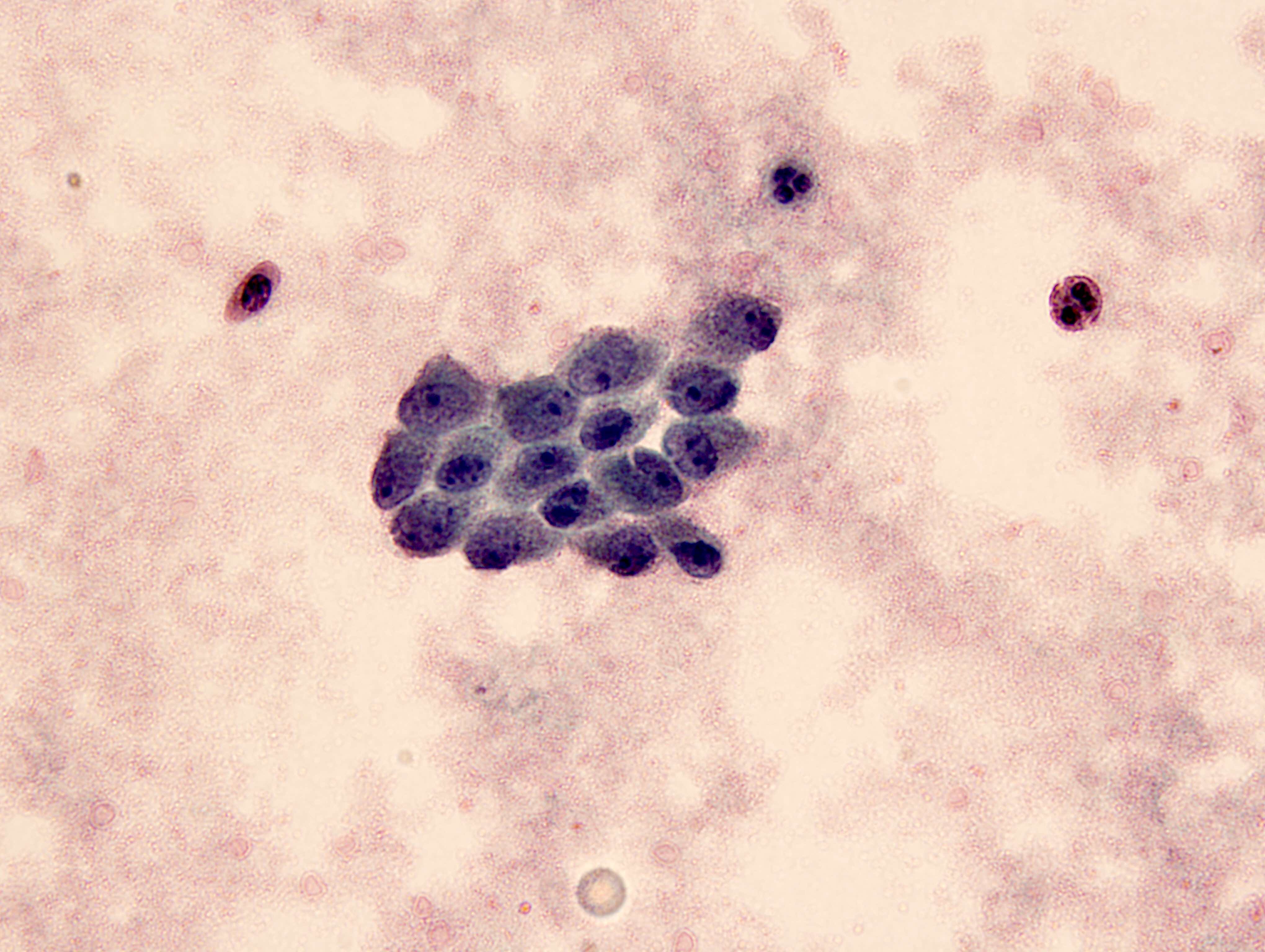
- Cytomegalovirus (Cancer Cytopathol 2018;126:643)
- Nuclear enlargement with owl eye nucleoli
- Intranuclear inclusion with surrounding halo
- Nuclear enlargement with owl eye nucleoli
- Adenovirus (Diagn Cytopathol 2017;45:614)
- Smudged nuclei
- Intranuclear basophilic inclusions surrounded by a small halo
- Herpes (Cancer Cytopathol 2018;126:643)
- Triad: multinucleation, margination, molding
- Can have significant acute inflammation and necrosis
- Cytomegalovirus (Cancer Cytopathol 2018;126:643)
- Parasitic infections
- Strongyloidiasis (Cancer Cytopathol 2018;126:643)
- Larval form in lung: filariform
- May be present in sputum
- Echinococcus (Paediatr Respir Rev 2022;43:11)
- Thick walled hydatid cyst
- 2 layers: outer PAS positive layer and endocyst layer
- Inner layer shows protoscoleces (2 circular rows of hooklets and suckers)
- Strongyloidiasis (Cancer Cytopathol 2018;126:643)
Benign neoplasms
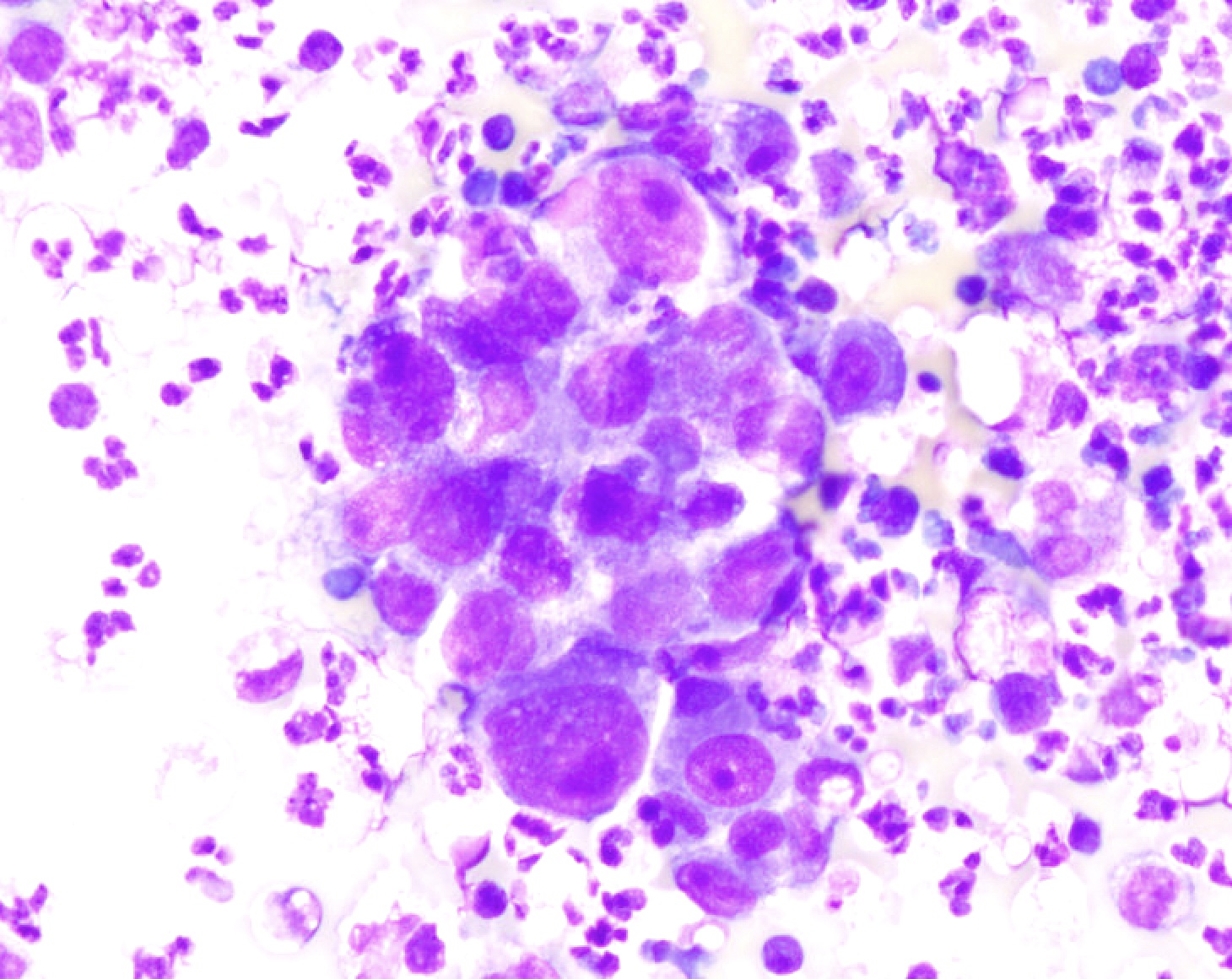
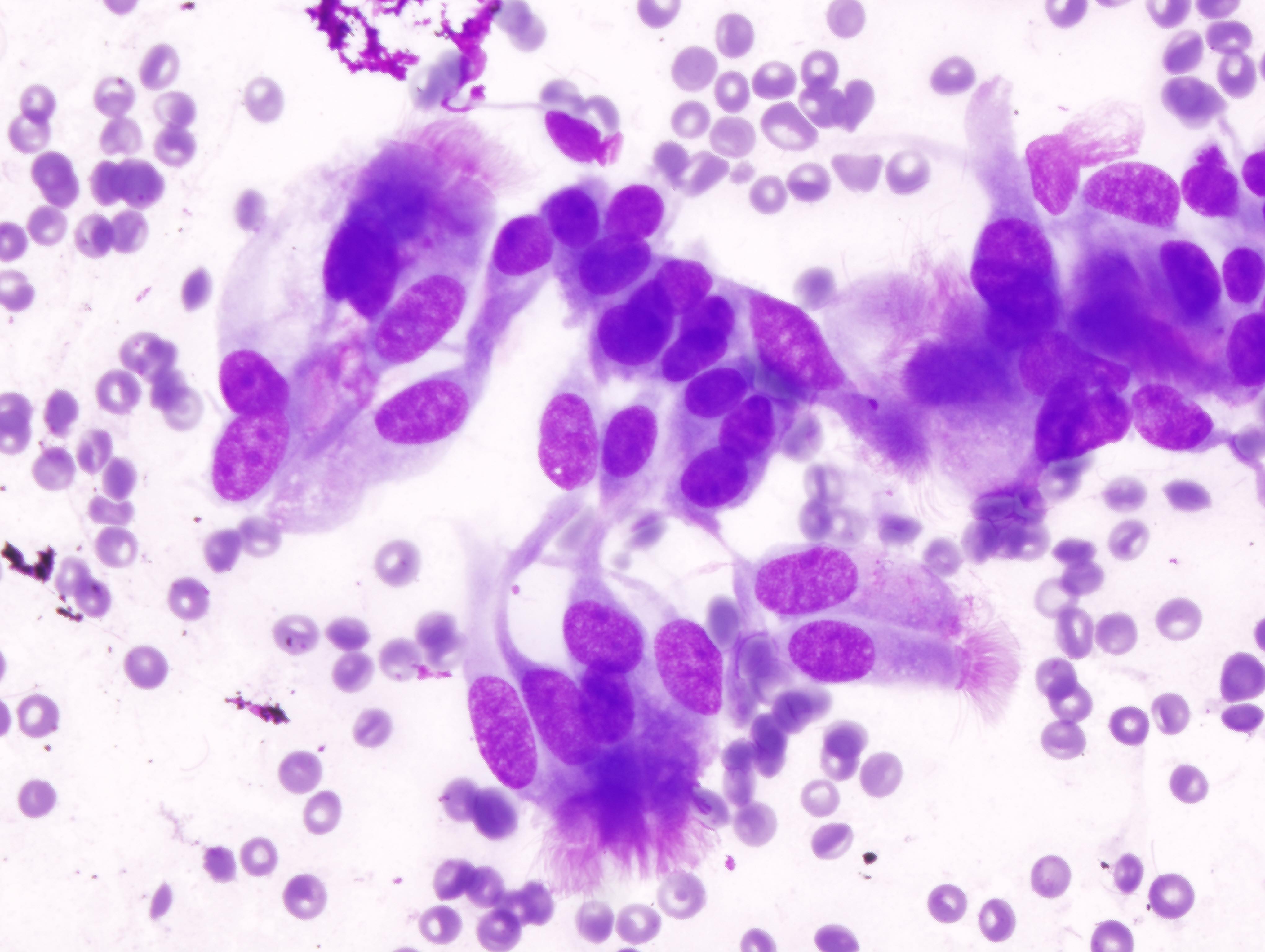
- Hamartoma (Diagn Cytopathol 2011;39:144)
- Can have varying proportions of epithelial cells and mesenchymal tissue
- Recognition of cartilage and fibromyxoid stroma is key to diagnosis
- Meningioma (Acta Cytol 1998;42:1424, Neurosurg Rev 2022;45:2671)
- Spindled shaped cells with elongated or fusiform nuclei
- Arranged in parallel or whorled formations
- Psammoma bodies
- Positive IHC: EMA, vimentin, SSTR2
- Negative IHC: S100
- Granular cell tumor (Int J Clin Exp Pathol 2014;7:5186)
- Polygonal cells with eccentric nuclei
- Eosinophilic granular cytoplasm
- Indistinct borders
- Positive IHC: S100
- Endobronchial lipoma (Ther Adv Respir Dis 2014;8:162)
- Abundant adipose tissue
- Typically polypoid and smooth on bronchoscopy
- Langerhans cell histiocytosis (N Engl J Med 2022;387:2449)
- Langerhans cells
- Foamy cytoplasm, large coffee bean shaped nuclei
- Positive IHC: S100, CD1a
- Eosinophils
- Langerhans cells
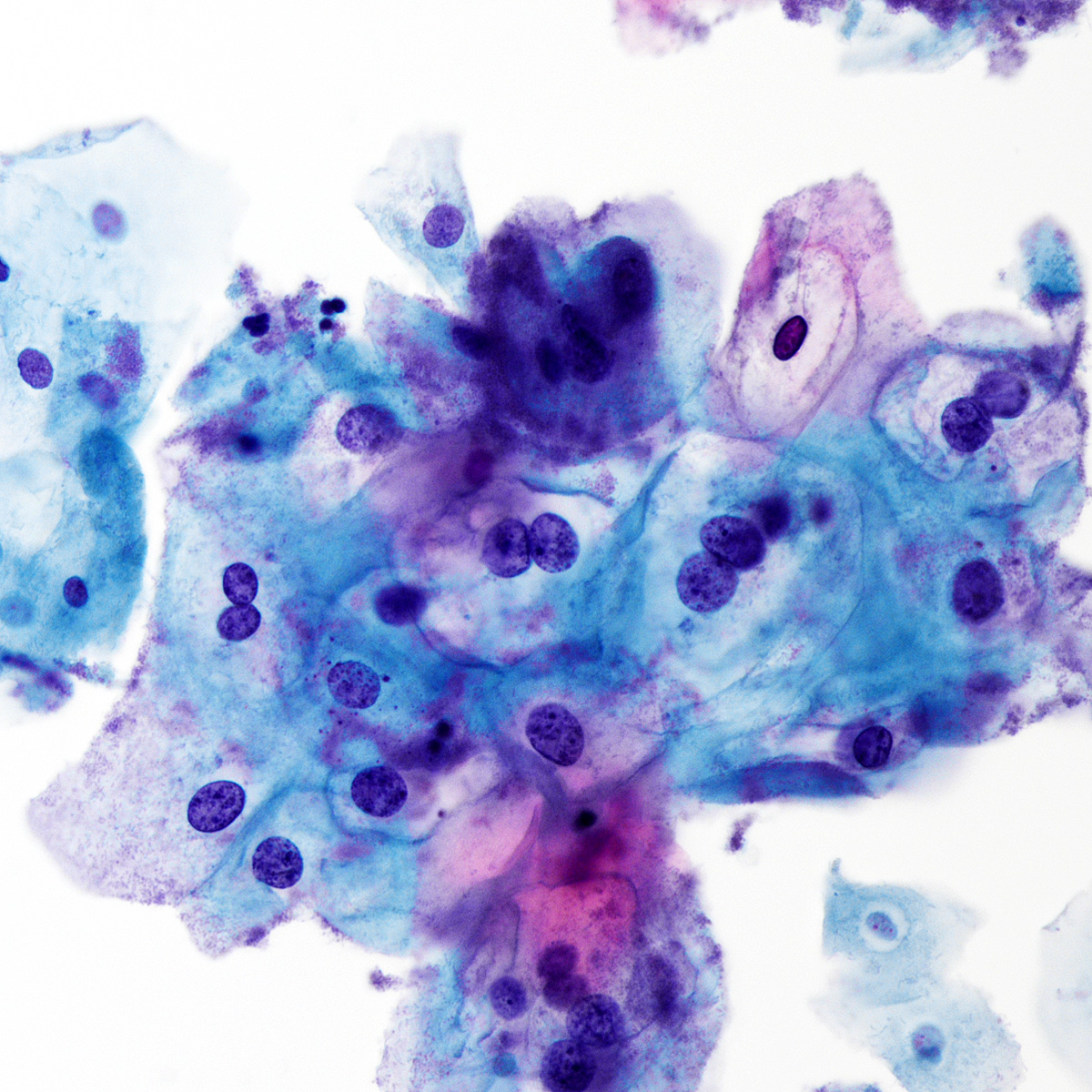
Cytology images
Molecular / cytogenetics description
N/A
Molecular / cytogenetics images
N/A
Sample pathology report
- Lung, right upper lobe, fine needle aspiration:
- Negative for carcinoma
- Granulomatous inflammation present
- Lung, left upper lobe, bronchoalveolar lavage:
- No malignant cells identified
- Predominately pulmonary macrophages and chronic inflammation
- Lung, right lower lobe, fine needle aspiration:
- Adipose, cartilage and benign reactive bronchial cells (see comment)
- No malignant cells identified
- Comment: The combination of these findings is suggestive of a hamartoma but clinicoradiographic correlation and surgical biopsy are recommended for definitive diagnosis.
Differential diagnosis
Additional references
Board review style question #1
Board review style answer #1
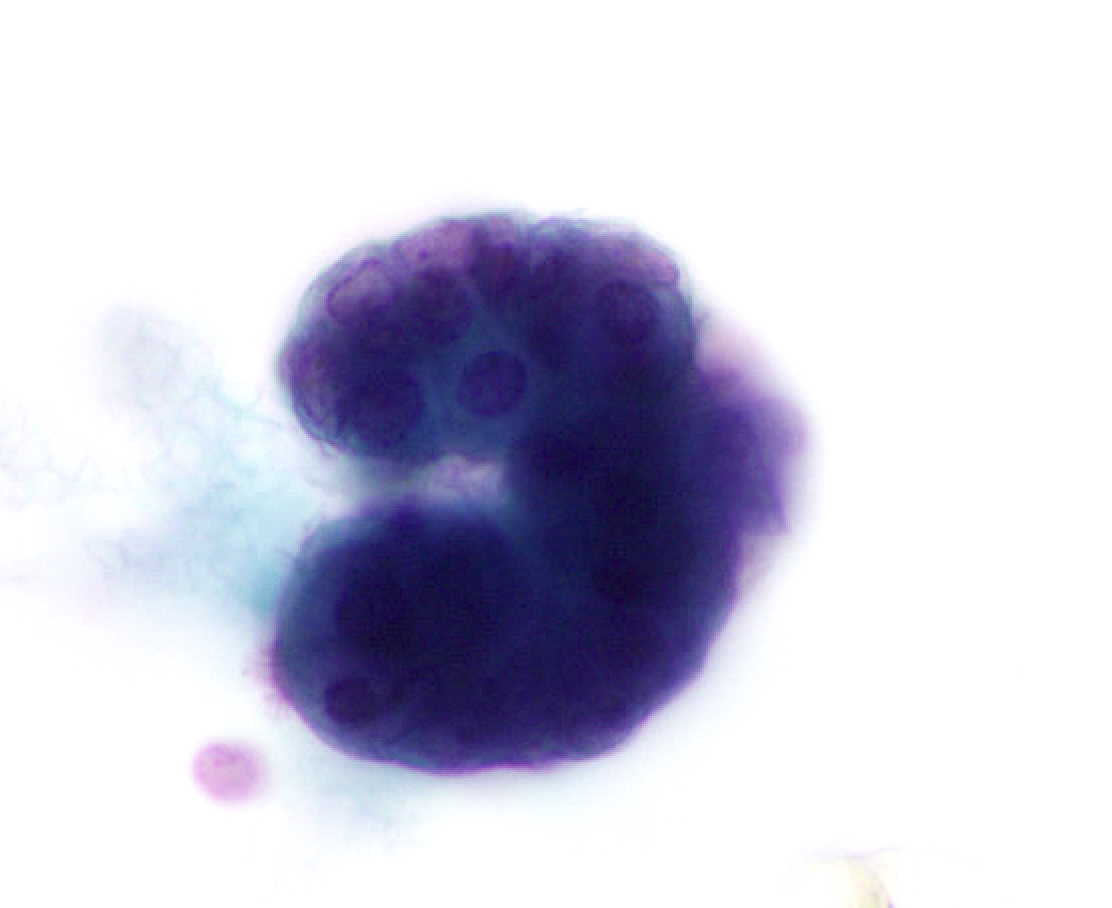
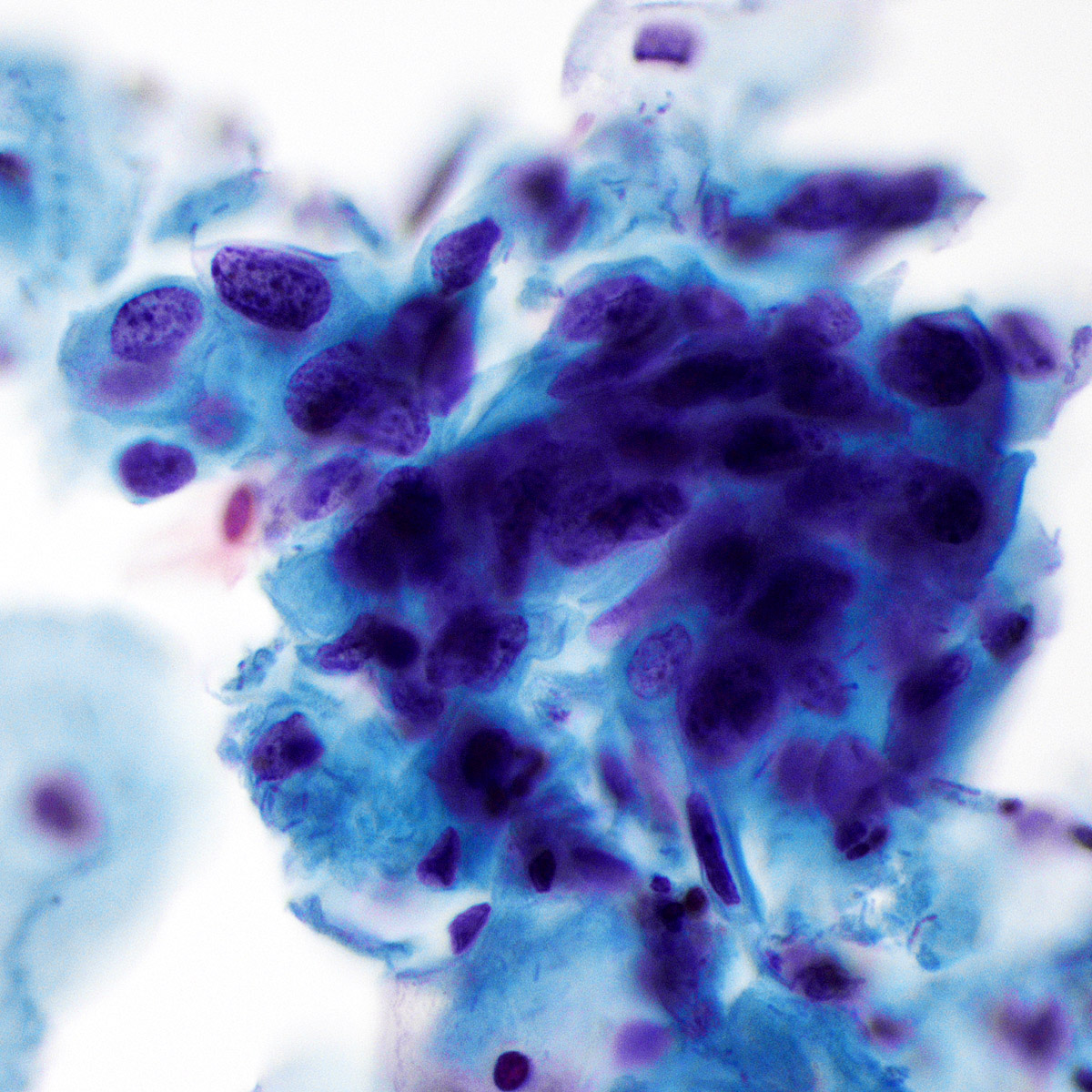
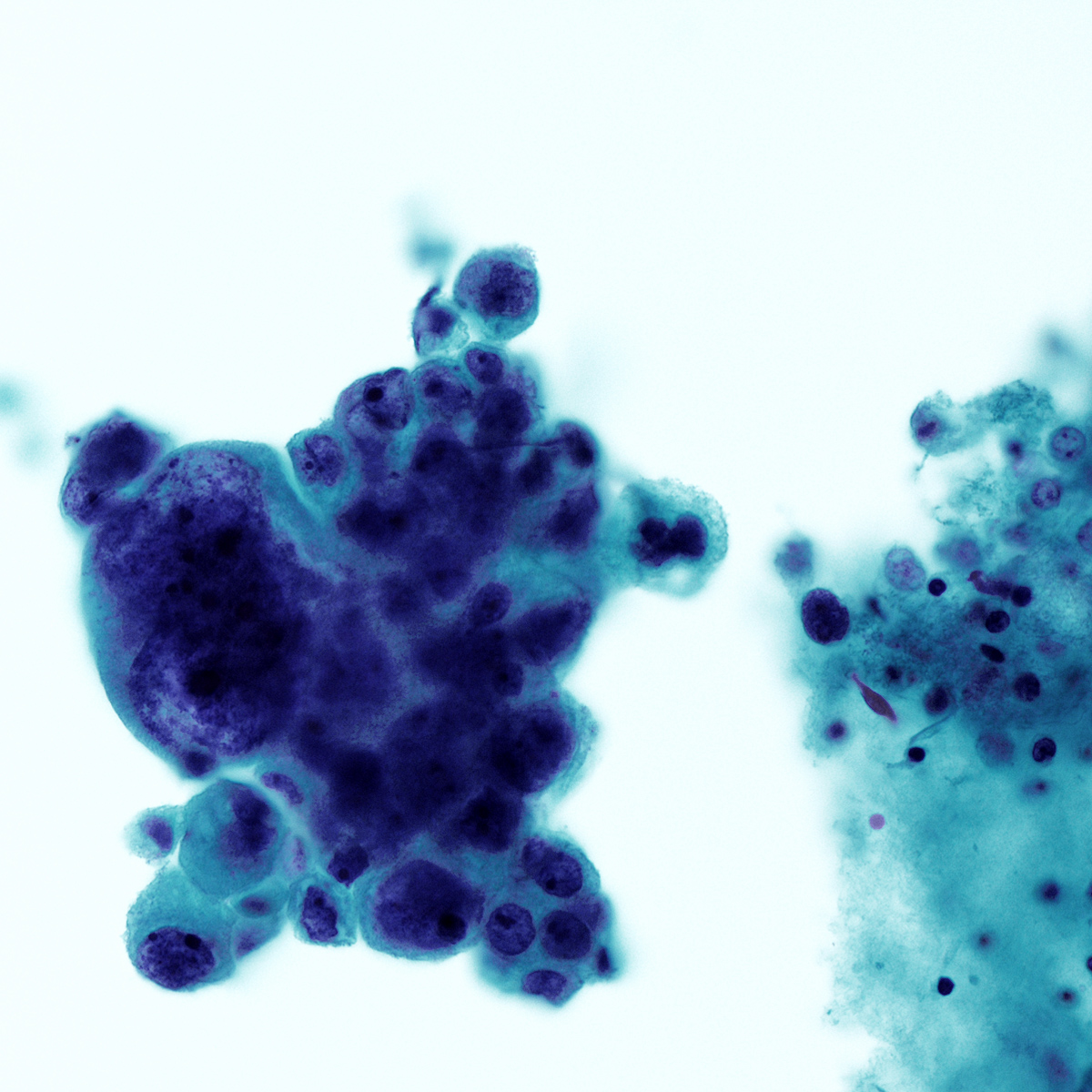
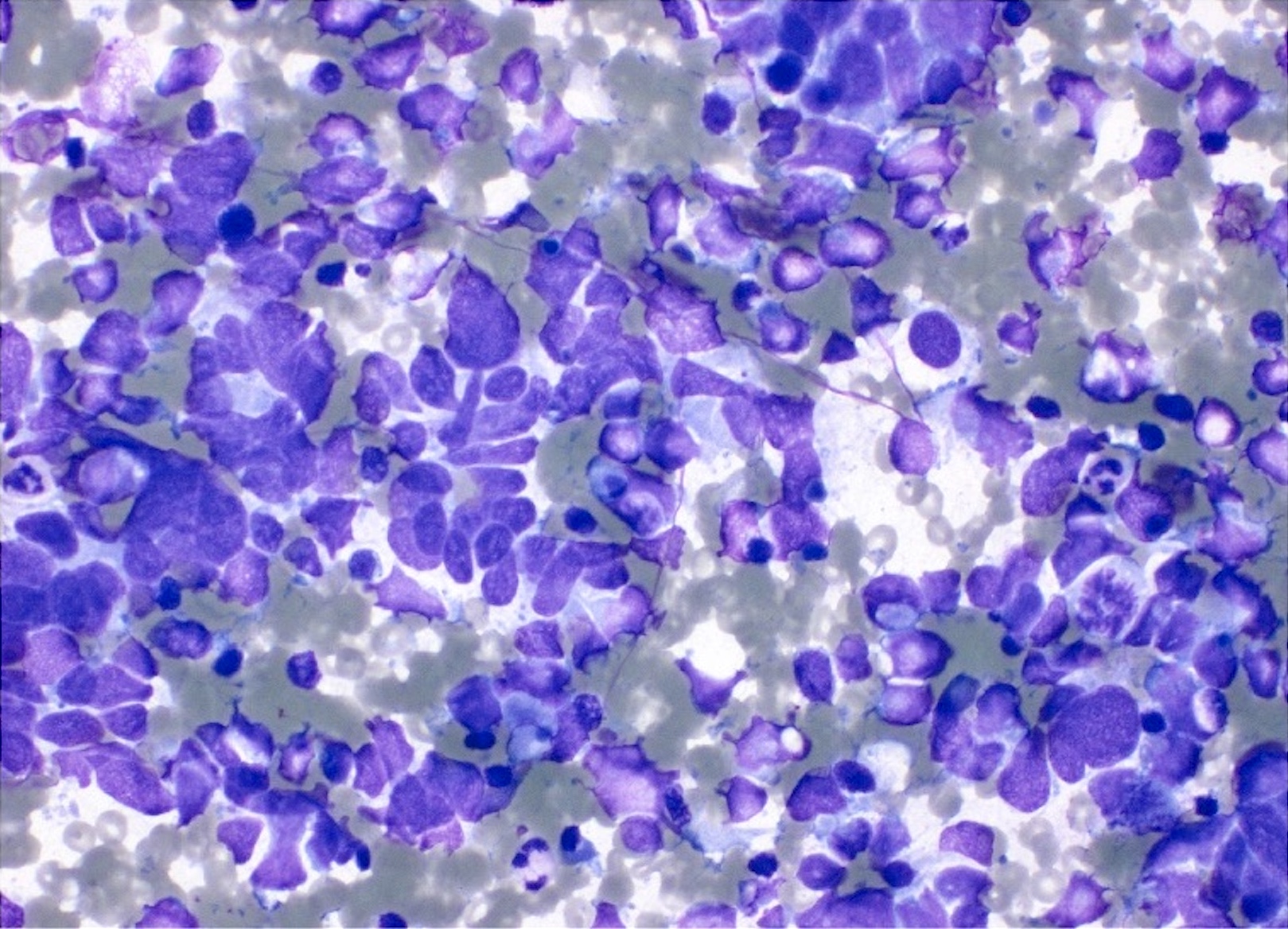
C. Benign: favor hamartoma. This FNA image comes from a 63 year old man who presented with a right upper lobe lobulated mass. A Diff-Quik smear shows benign, matrix-like material with adjacent chronic inflammation. This case was signed out as having neoplastic cells that are compatible with pulmonary hamartoma. Correlation with the imaging findings was helpful to render this diagnosis. Answers A and D are incorrect because the matrix material and adipose tissue are hallmarks for hamartoma; therefore, the categories of nondiagnostic and atypical are not appropriate in this context. Answer B is incorrect because there is abundance of matrix material and lack of bronchial cells. Answer E is incorrect because there are no small cell carcinoma cells in this sample.
Comment Here
Reference: Cytopathology - Benign (lung)
Comment Here
Reference: Cytopathology - Benign (lung)
Board review style question #2
This image is from a 35 year old woman that has a history of lung transplant and underwent a bronchial washing. Which of the following is the best diagnosis?
- Atypical cells present
- Benign: cytomegalovirus (CMV) present
- Nondiagnostic
- Positive for non-small cell carcinoma
- Suspicious for adenocarcinoma
Board review style answer #2
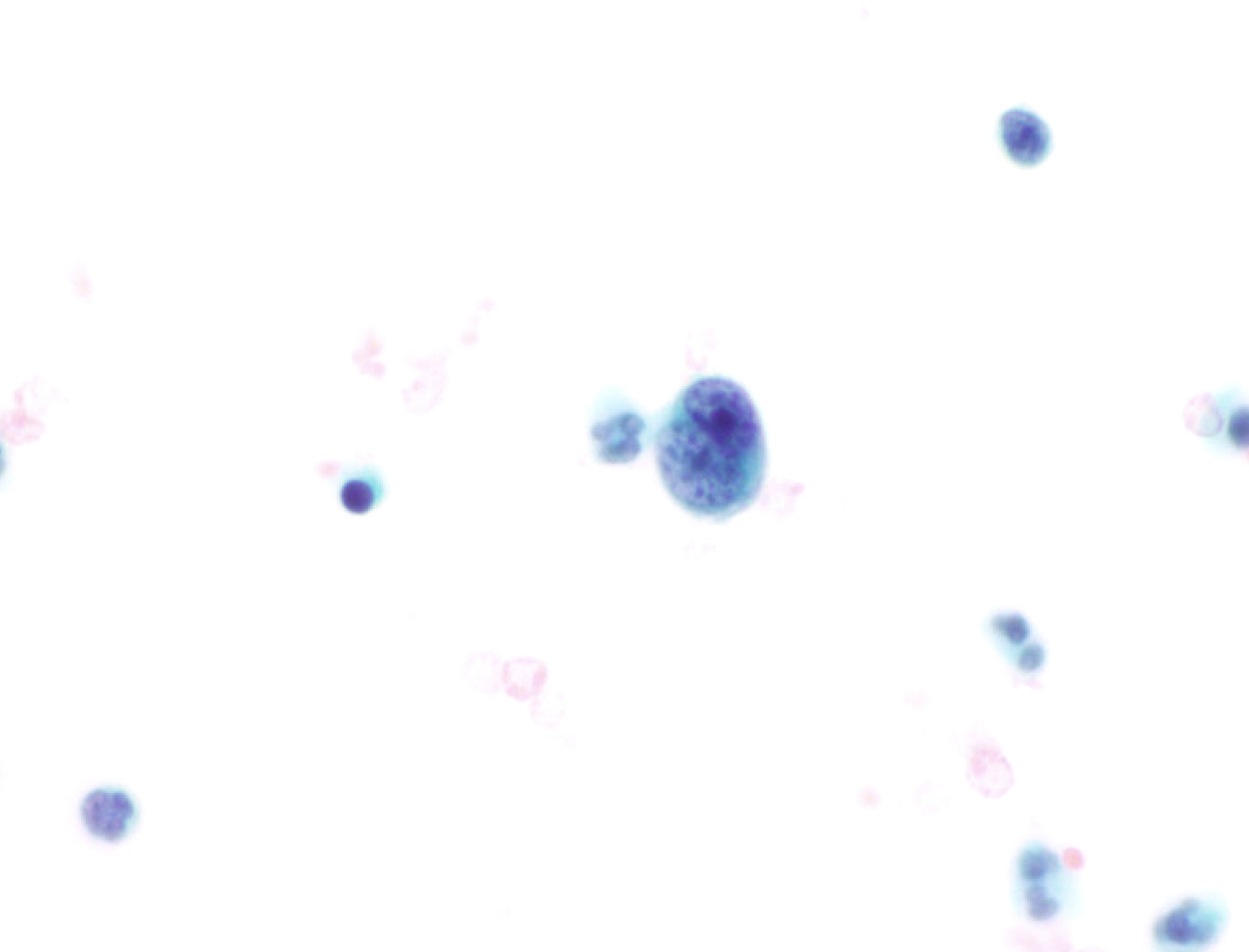
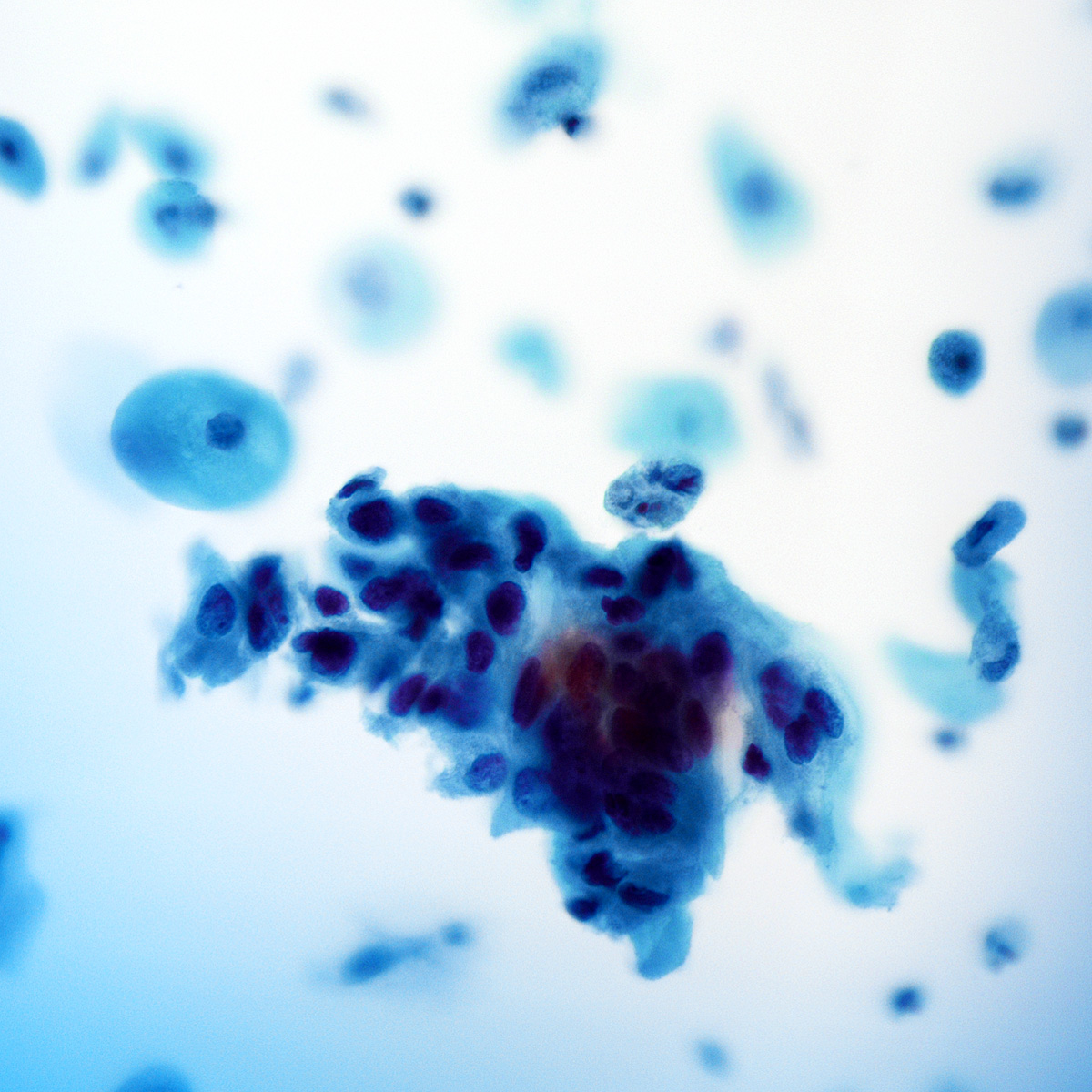
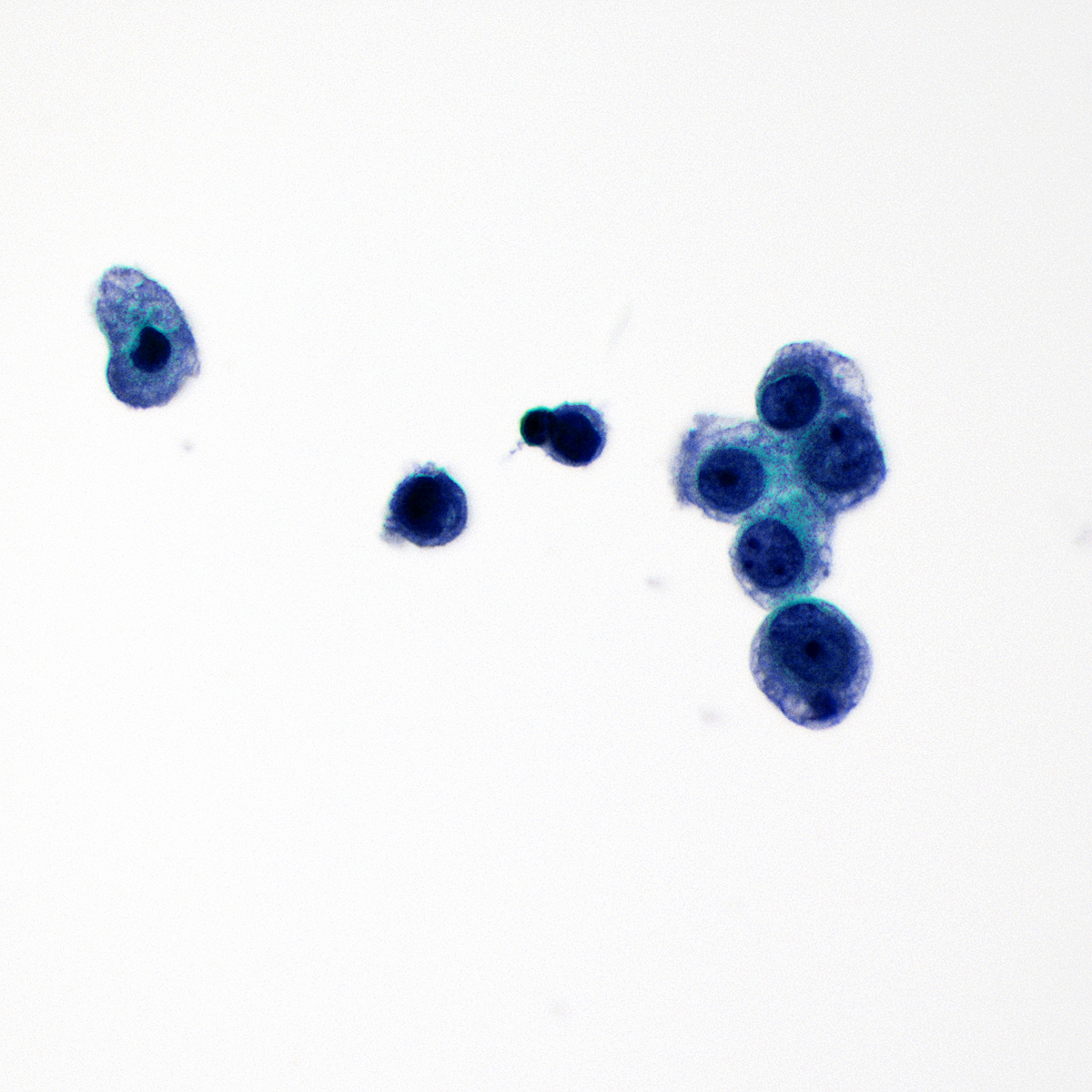
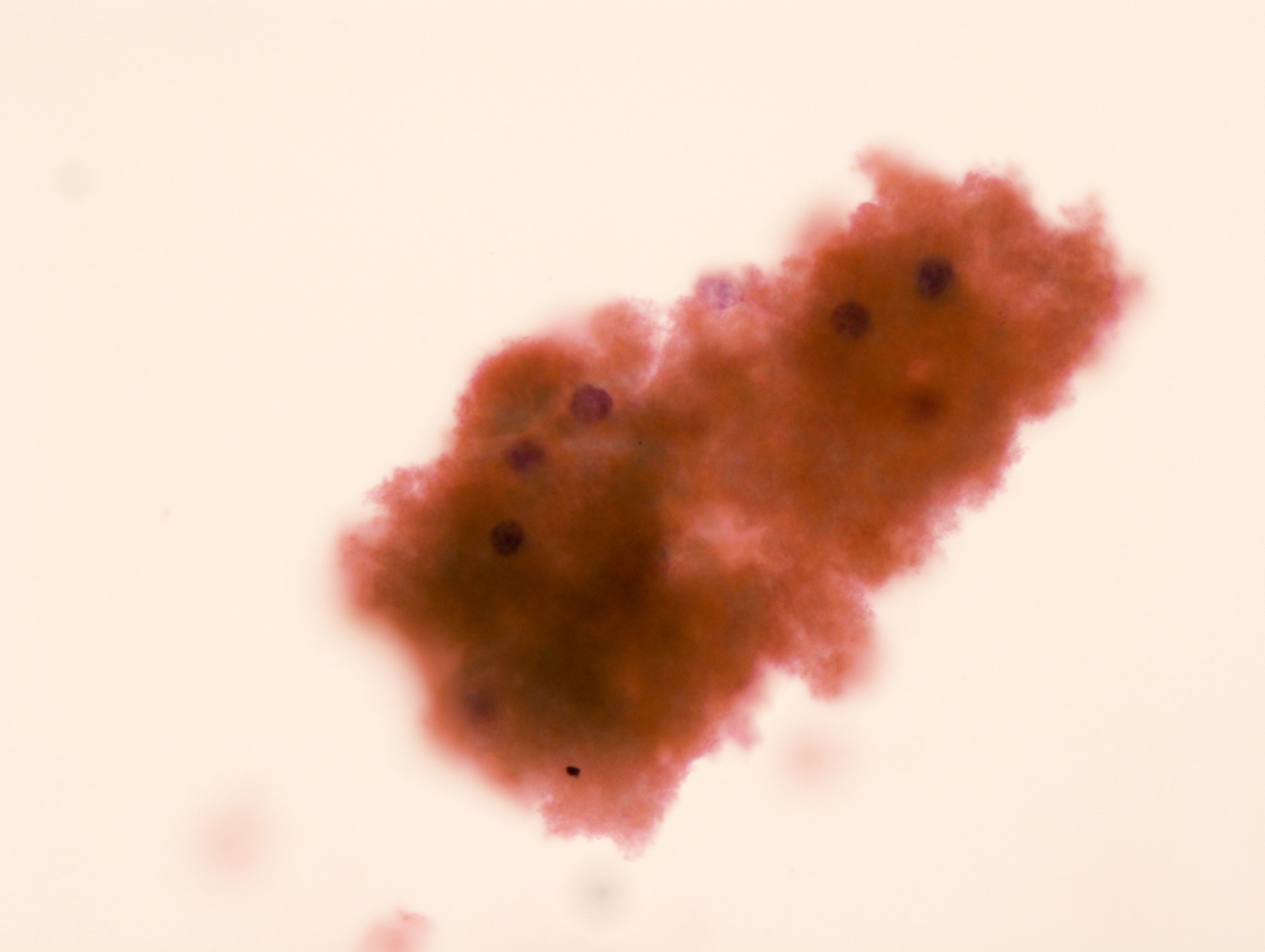
B. Benign: cytomegalovirus (CMV) present. This ThinPrep slide shows cells with owl eye inclusions characteristic of CMV. There is an enlarged nucleus with a surrounding halo, consistent with viral cytopathic changes of CMV. Answer A is incorrect because while the nuclei are atypically large, the nuclear halo helps with the diagnosis of CMV infection in benign cells. Answer C is incorrect because the cells present are diagnostic of CMV. Answers D and E are incorrect because the sample does not show neoplastic cells. The nuclei are enlarged but the changes seen, such as the nuclear halo and the preserved N:C ratio, are more supportive of CMV infection.
Comment Here
Reference: Cytopathology - Benign (lung)
Comment Here
Reference: Cytopathology - Benign (lung)
Board review style question #3
Board review style answer #3
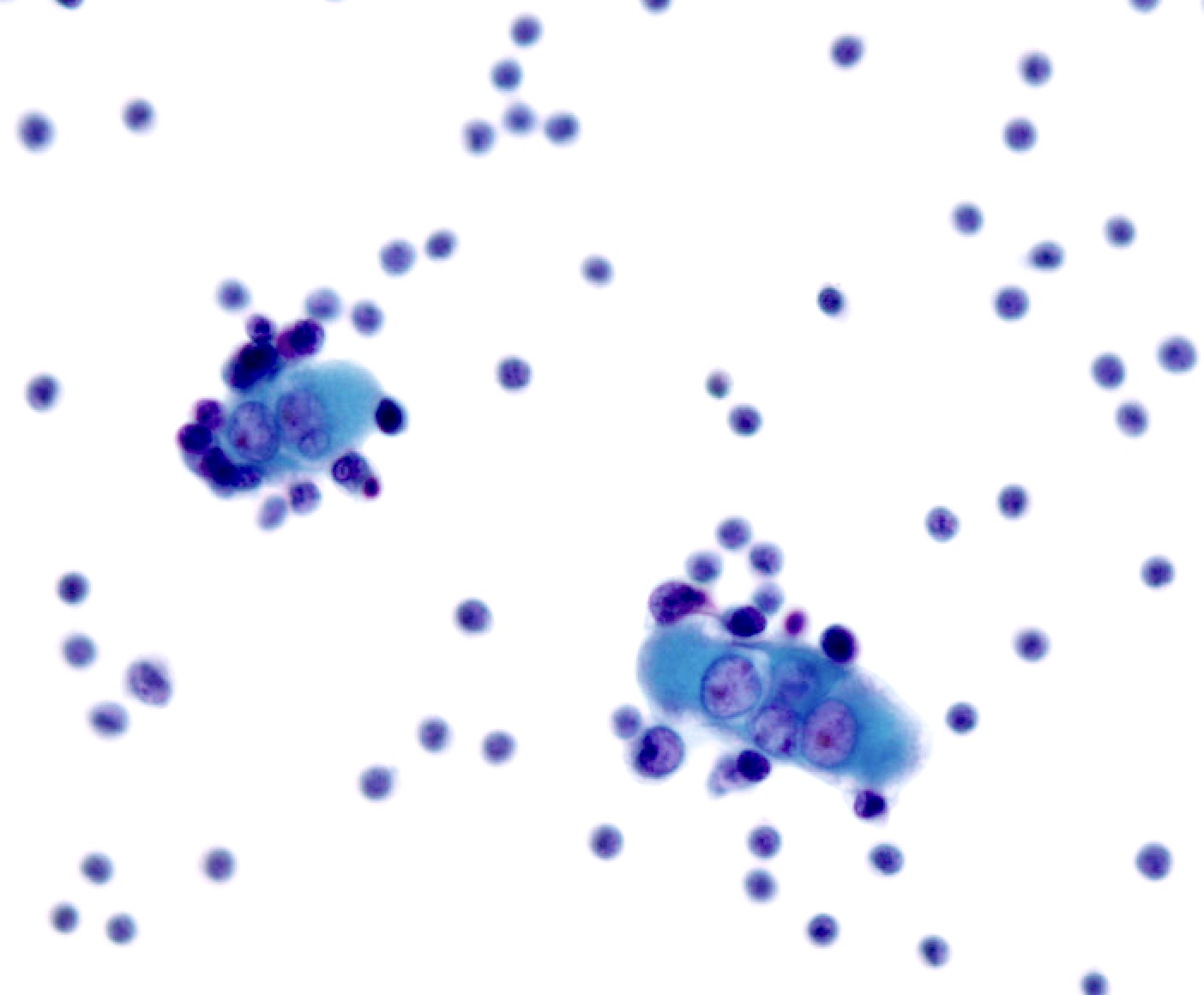
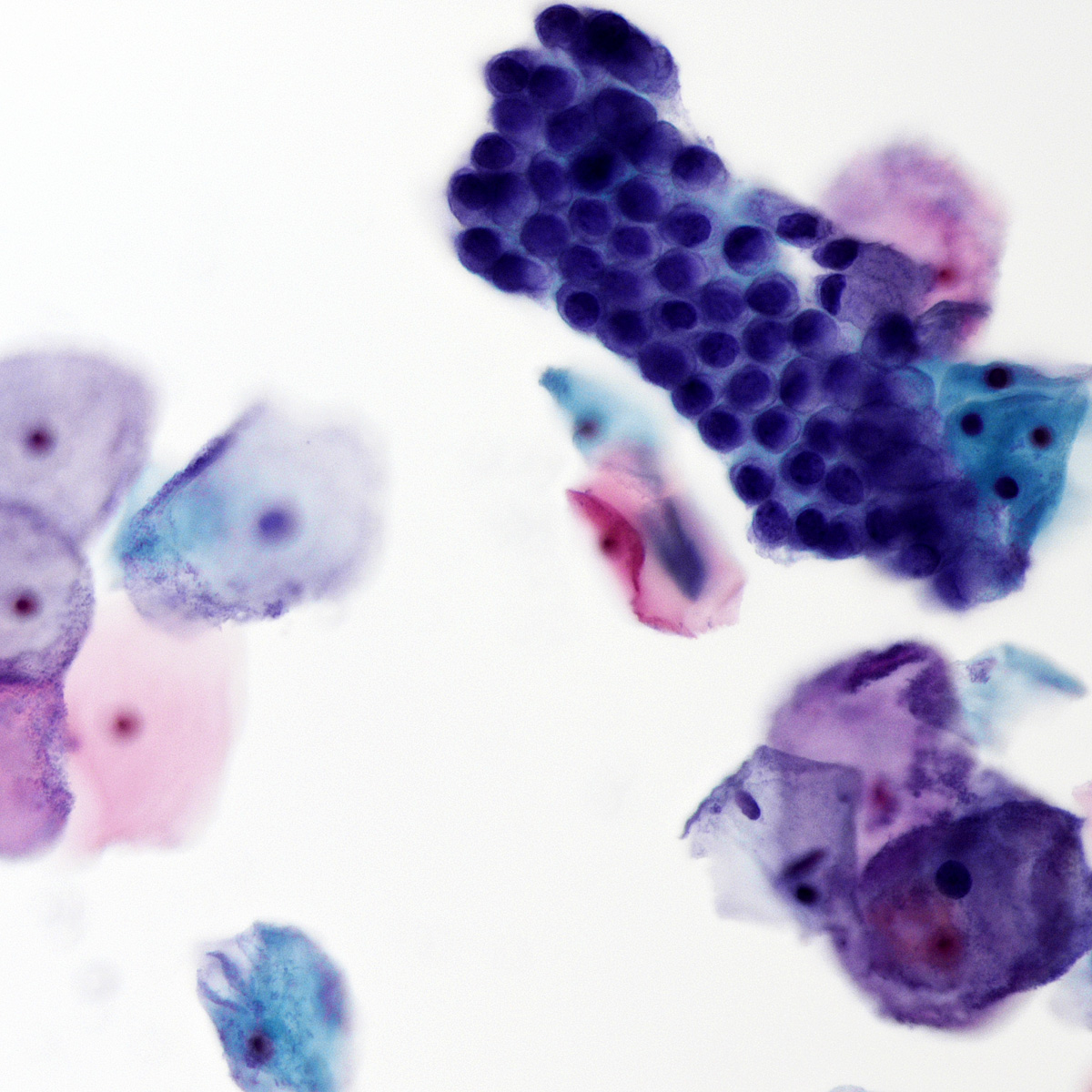
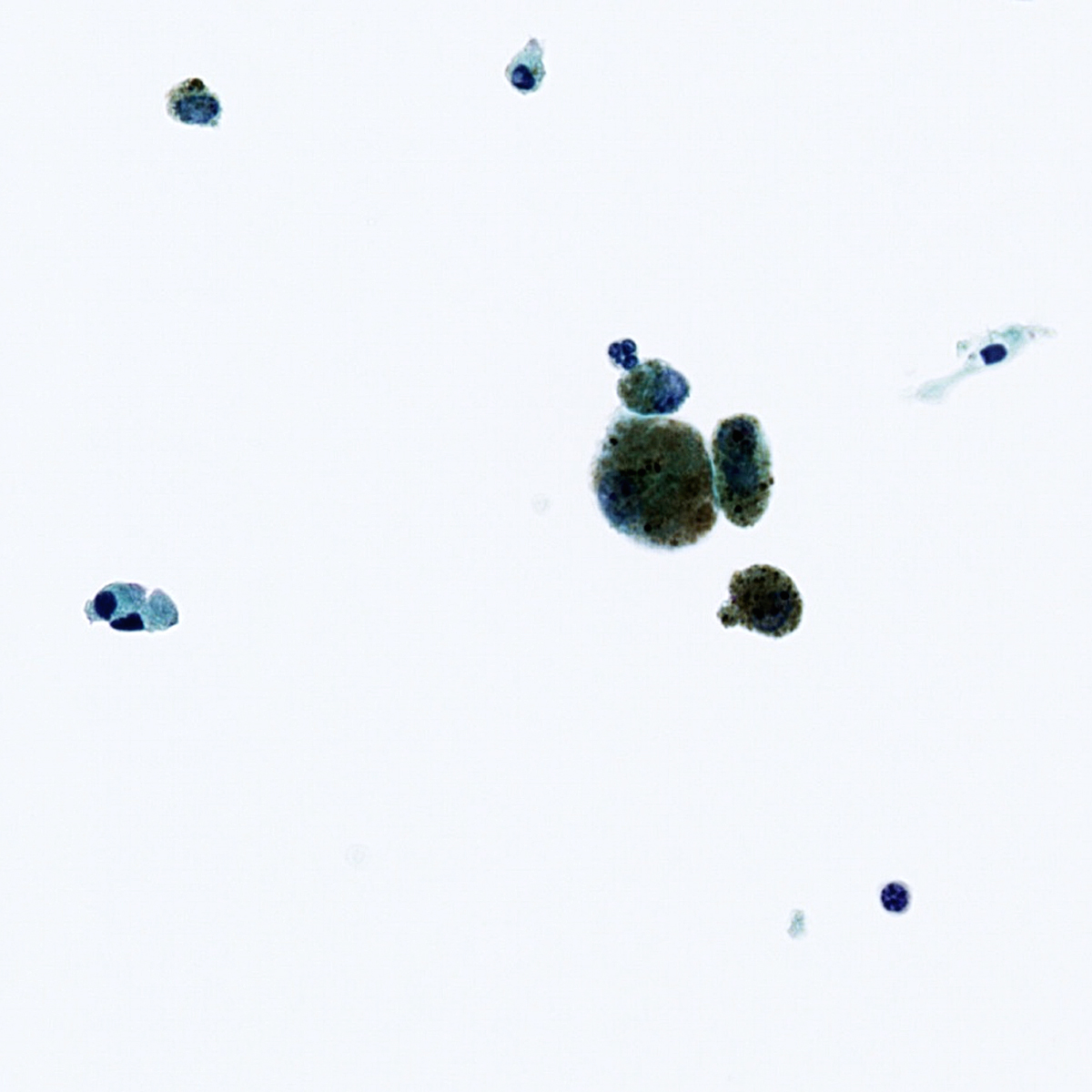
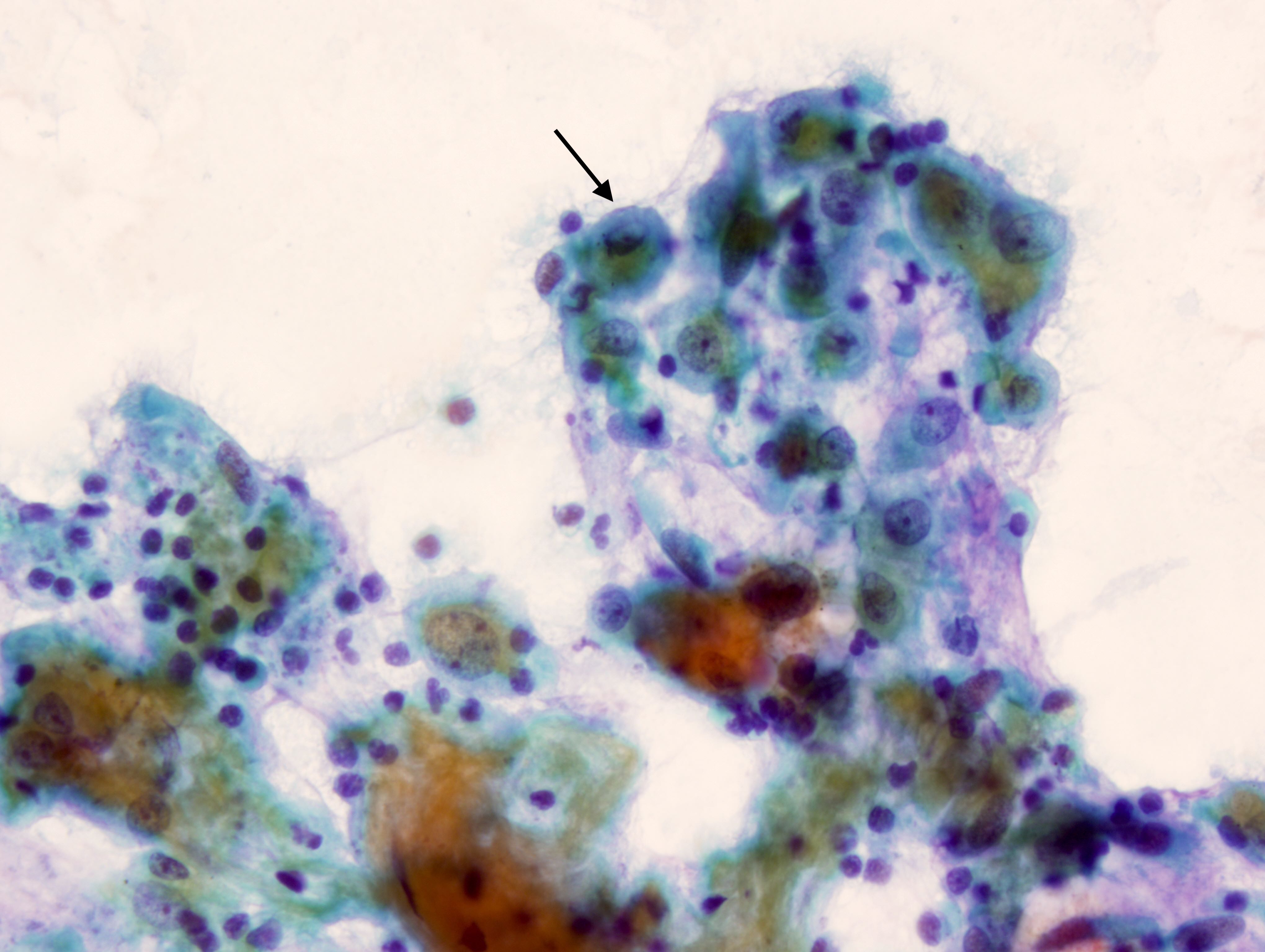
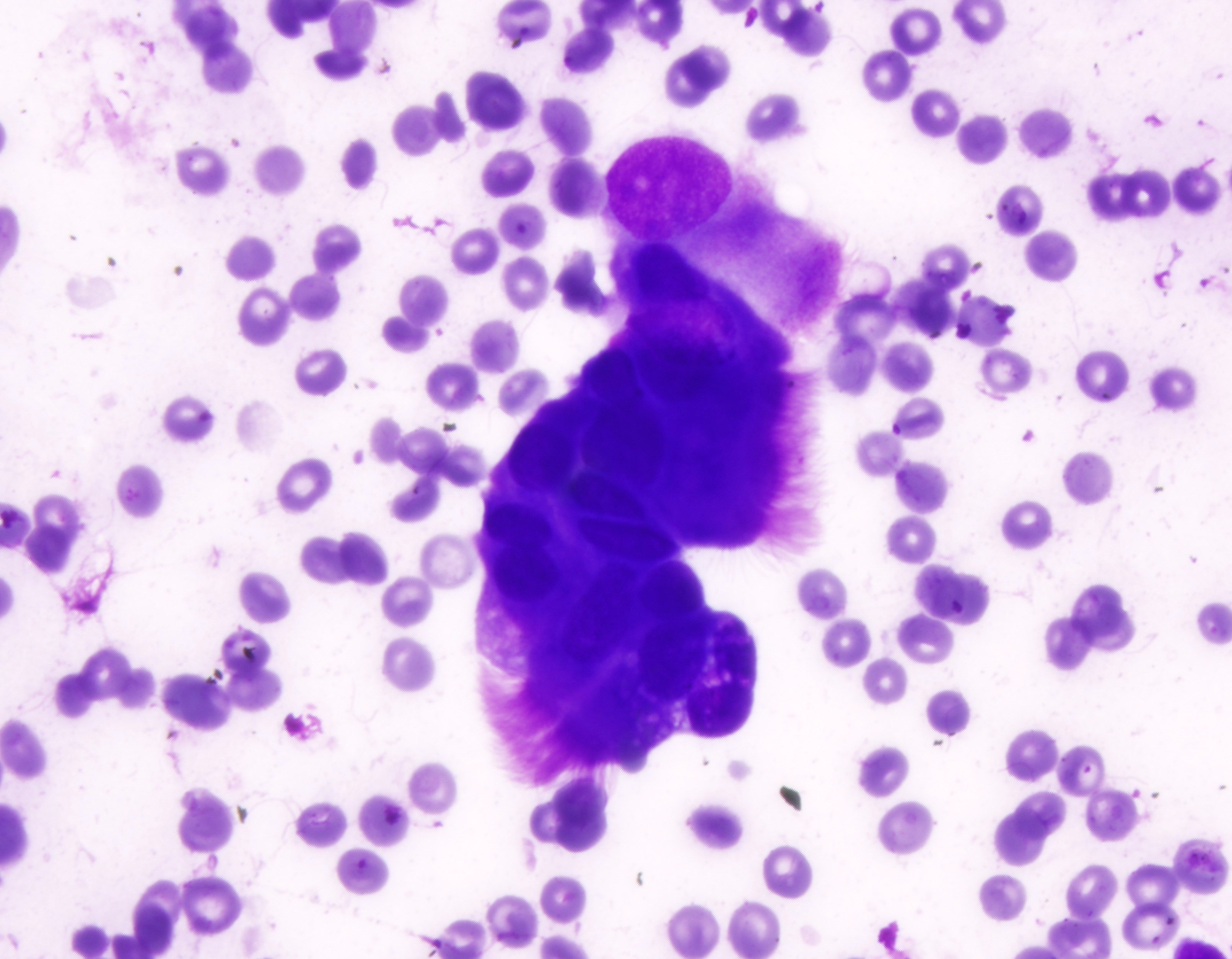
B. Benign: bronchial cells only. The cells in the image show a low N:C ratio and are columnar. Prominent cilia are present, supporting the diagnosis of benign bronchial cells. Answer A is incorrect because the cells do not show nuclear atypia. In addition, there is appropriate N:C ratio and terminal bars with cilia. Overall, these features exclude these cells from the atypical category. Answer C is incorrect because this is a bronchial washing and the presence of bronchial cells and macrophages are considered normal elements in such a specimen. Answers D and E are incorrect because the background columnar cells show preserved N:C ratio and terminal bars with cilia. The nuclei are not overlapping. Overall, these features are diagnostic of benign bronchial cells.
Comment Here
Reference: Cytopathology - Benign (lung)
Comment Here
Reference: Cytopathology - Benign (lung)
Benign (pending)
[Pending]
FNA procedure
Table of Contents
Definition / general | How to obtain education and hands on training in US medicine and USFNA | Background of FNA procedure | Pre FNA procedure events | Performing the FNA procedure | Clinical imagesDefinition / general
- Before making the transition to USFNA on patients, one must practice on either homemade or commercially available phantoms until the operation of US machine and needle placement skills are automatic
- To ensure the best outcome for the patient from the FNA procedure, you must:
- Clinical assess patient prior to procedure
- Adequately perform the FNA biopsy
- Make observations during the procedure
- Prepare quality smears
- Perform microscopic interpretation
- Report the results (Cancer Invest 2004;22:620, Demay: The Art & Science of Cytopathology, 2nd Edition, 2011 (vol. 2, pg. 543-47))
How to obtain education and hands on training in US medicine and USFNA
- CAP offer assessments with a certification of completion and ACE offers pathways to USFNA certification for pathologists
- College of American Pathologists (CAP): Ultrasound Guided Fine Needle Aspiration AP3 Course
- The American College of Endocrinology (ACE): Diagnostic Endocrine Neck Ultrasound and UGFNA Course
- American Association of Clinical Endocrinologists (AACE)
- United States and Canadian Academy of Pathology (USCAP)
- American Society of Cytopathology (ASC)
- American Institute of Ultrasound In Medicine (AIUM)
- Visiting a pathology practice that currently performs USFNA and offers educational training
- Attending US manufacturer sponsored workshop
- Web based US medicine education sites (e.g. sonoworld.com)
- Video based US medicine and USFNA programs (e.g. American Institute of Ultrasound In Medicine (AIUM) DVD video series)
- Becoming credentialed, for example, as a Registered Diagnostic Medical Sonographer (RDMS) through the American Registry for Diagnostic Medical Sonography (ARDMS)
Background of FNA procedure
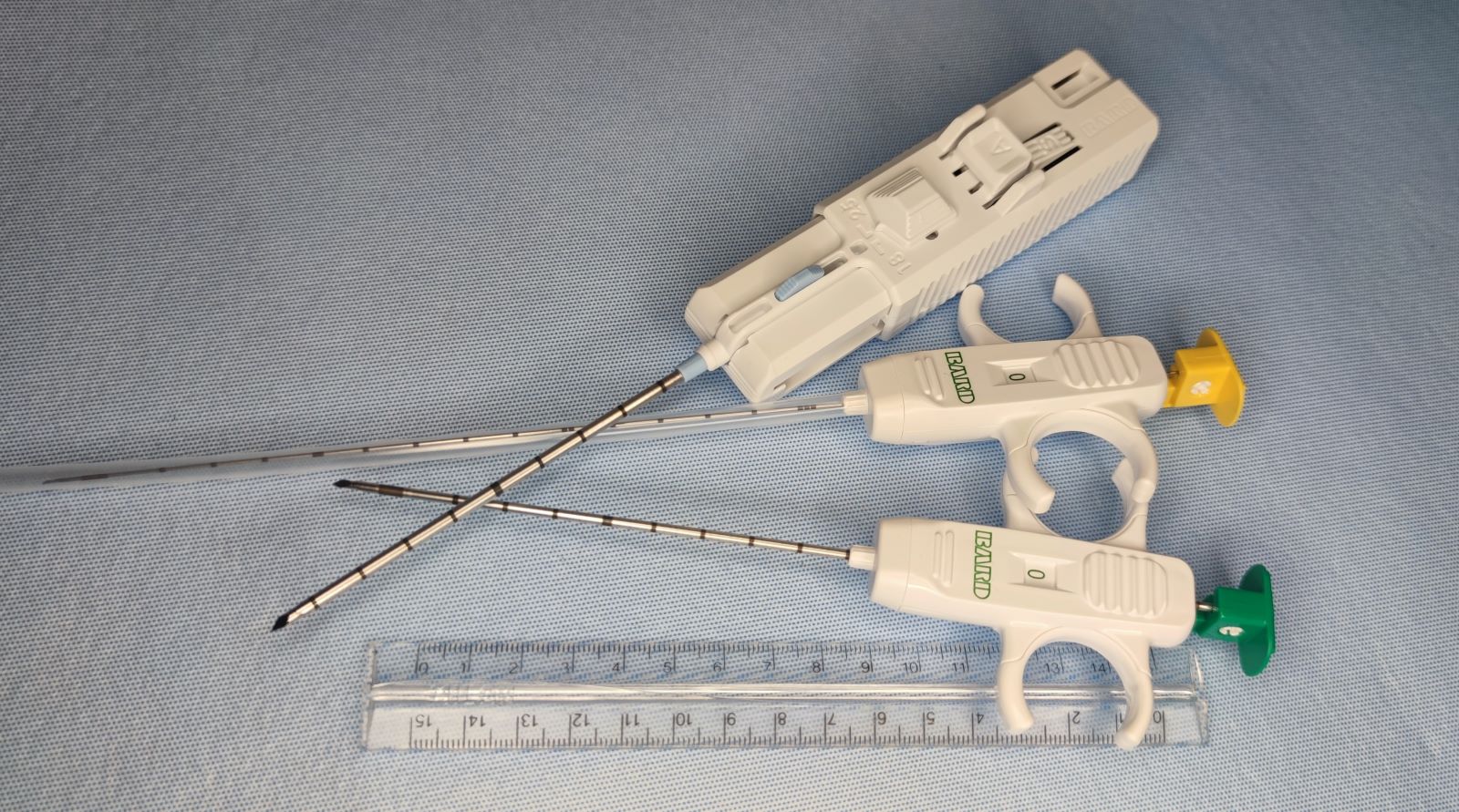
- Each single needle pass should take less than 5 - 10 seconds to complete, with 10 - 20 excursions (back and forth cutting motions of the needle) into the target being performed during this time
- When sampling vascular targets or the thyroid gland, single pass should take 2 - 5 seconds and use smaller gauge needles (e.g. 25 or 27 gauge), to decrease hemodilution of the FNA specimen
- Usually 2 - 6 passes are performed on the target for adequate sampling
- Using needle only or using an aspiration device with suction may be employed
- Number of slides produced from each pass will vary on how you are planning on triaging the material obtained and the smearing technique used:
- In general, produce 1 - 2 slides per needle pass for diagnostic purposes but usually not more than 4 per pass
- Needle rinses in a preservative solution (formalin or RPMI) or additional unstained slides may be obtained for special studies (e.g. fungal and acid fast stains)
Pre FNA procedure events
- Focused patient history and physical exam:
- Obtain patient history
- Determine site to be biopsied
- Review any pertinent radiographic imaging and laboratory studies
- Inquire about any significant medical problems including bleeding disorders, anticoagulation, previous episodes of syncope or complications and perceived problems from other previous biopsy procedures
- Inquire about special clinical requests (e.g. additional materials for hormone studies in recurrent breast cancer, material for microbial cultures, thyroglobulin wash out)
- Perform a focused physical examination of the aspiration target / site, noting the characteristics of the target:
- Location relative to other anatomical structures
- Estimated depth from skin
- Consistency (firm vs. soft or solid vs. cystic)
- Mobile vs. fixed
- Any evidence of pulsation or bruit
- Pre plan the aspiration biopsy:
- Pay special attention to:
- Proper patient positioning to access the target
- Technique to best immobilize the target
- Estimated needle length and size
- Use of suction or not during the biopsy
- Special collection methods needed (e.g. RPMI for flow cytometry, cell block, sterile container for cultures, or collection / drainage of cyst fluid)
- Explain biopsy procedure in lay terms, obtain informed consent, and reconfirm site of aspiration
- Special mention to the patient that several passes (averaging 2 - 6 passes) may be necessary to obtain adequate cells for diagnosis and any ancillary studies
- Address any patient concerns about the procedure BEFORE proceeding
- Ready aspiration setup and supplies (needles, syringes, slides, special collection tubes for any additional studies)
- Pay special attention to:
Performing the FNA procedure
- Always follow "universal precautions"
- Locate and immobilize target again with one hand
- Disinfect the skin with alcohol (70%) at site of planned needle puncture site
- Pass the needle through the skin in one quick motion
- Usually needle approach is 30 - 45 degree angle to the skin for very superficial targets and a more perpendicular approach for deep targets
- Advance the needle into the target
- In most cases, the aspirator will notice a difference in the consistency of the tissue of the target when penetrated
- If the target is small, one usually directs the needle toward the center of the target; if the target is large and there is concern for central necrosis, the needle should be aimed toward the periphery
- Once in the target, you may apply suction if using an aspiration device, and then the needle is moved in long back and forth cutting motions within the target (DO NOT let the needle come out of the skin during this motion)
- When blood or material appears in the hub of the needle the aspiration should be stopped
- PRIOR to withdrawal of the needle if using suction, negative pressure must be released to prevent suction of the material into the barrel of the syringe when the needle exits the skin
- Release the negative pressure by letting go of the plunger BEFORE removing the needle from the skin
- The plunger may or not return to the staring position
- DO NOT force the plunger down
- Remove the needle from the patient by pulling straight out so as not to lacerate the skin of the patient by angling the needle upon withdrawal
- Apply pressure to the aspiration site, preferable by an assistant
- Prepare smears (see smear making) and obtain needle rinses as needed
IHC panels
Table of Contents
Definition / general | Essential features | CPT coding | Sites | Diagrams / tables | Cytology description | Cytology images | Molecular / cytogenetics description | Molecular / cytogenetics images | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Serous cavities can be involved by metastatic neoplasms, inflammatory / infectious conditions or primary malignancies
- Immunostaining panels with correlation with cytomorphology and clinical information can aid in the differential diagnosis of serous fluid effusion
Essential features
- Clinical and radiological correlation with cytomorphology is vital to tailor an appropriate immunostain panel (Arch Pathol Lab Med 2013;137:647)
- Use of immunohistochemical panels is beneficial to avoid pitfalls as no single immunostain has 100% sensitivity or specificity
- Immunostaining can aid in the assessment of effusion cytopathology specimens by:
- Differentiating metastatic disease from mesothelioma (at least 2 markers for mesothelioma / 2 markers for metastasis) (Arch Pathol Lab Med 2013;137:647)
- Determining primary site or lineage of metastatic disease (Cancer Cytopathol 2018;126:590)
- Differentiating neoplastic from reactive mesothelial cells (Arch Pathol Lab Med 2013;137:647)
- Identifying potential therapeutic targets (Cancer Cytopathol 2018;126:590)
CPT coding
Sites
- Pleura, peritoneum, pericardium
Diagrams / tables
Cytology description
- Use of immunostaining panel with multiple stains is beneficial since no single stain has 100% sensitivity or specificity
- Positive stains are more useful than negative stains since a larger percentage of cases in effusion can lose expression as compared with primary tumors
- Cytomorphology and correlation with clinical and radiological information can tailor immunostains panel in order to make the following differential diagnoses:
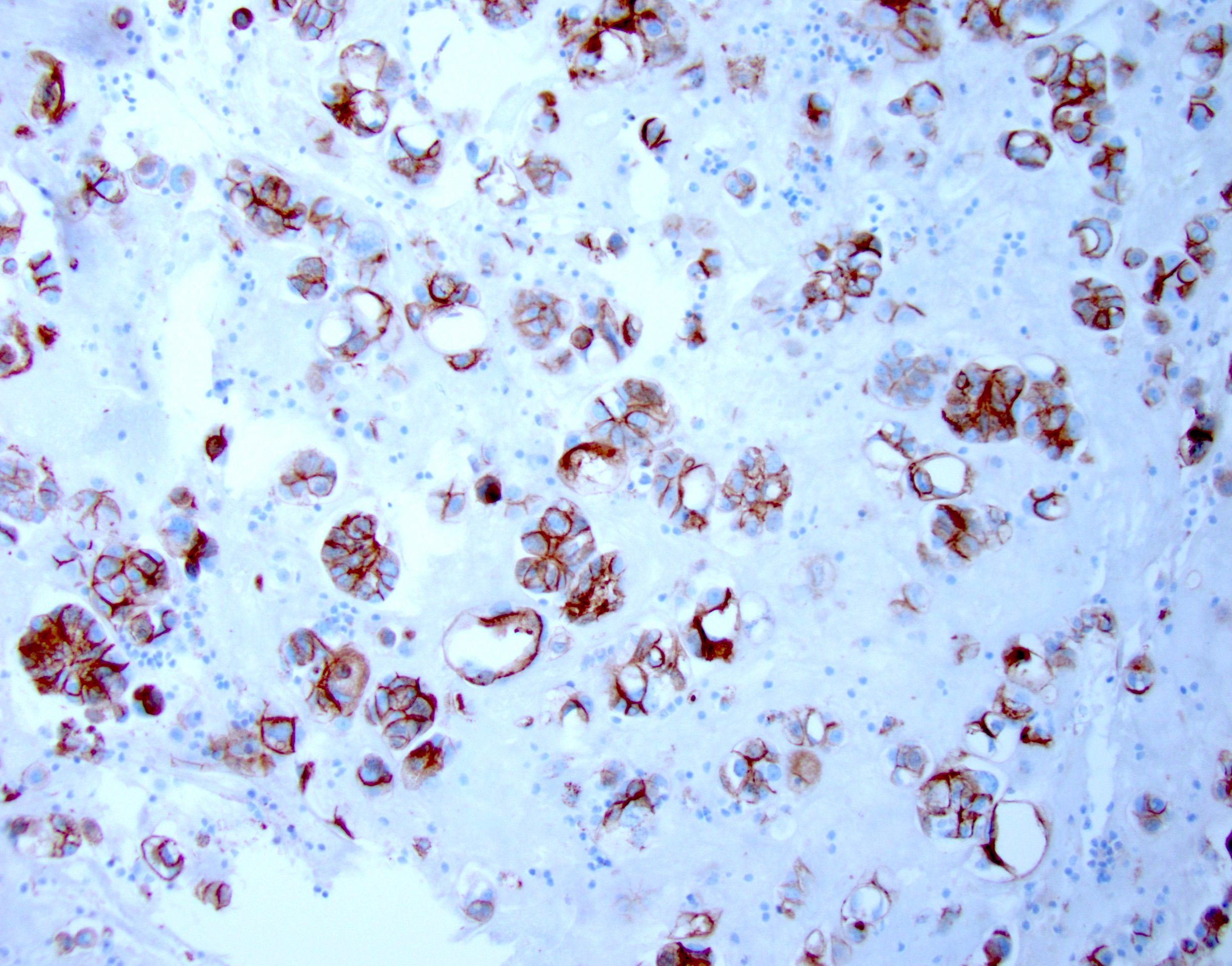
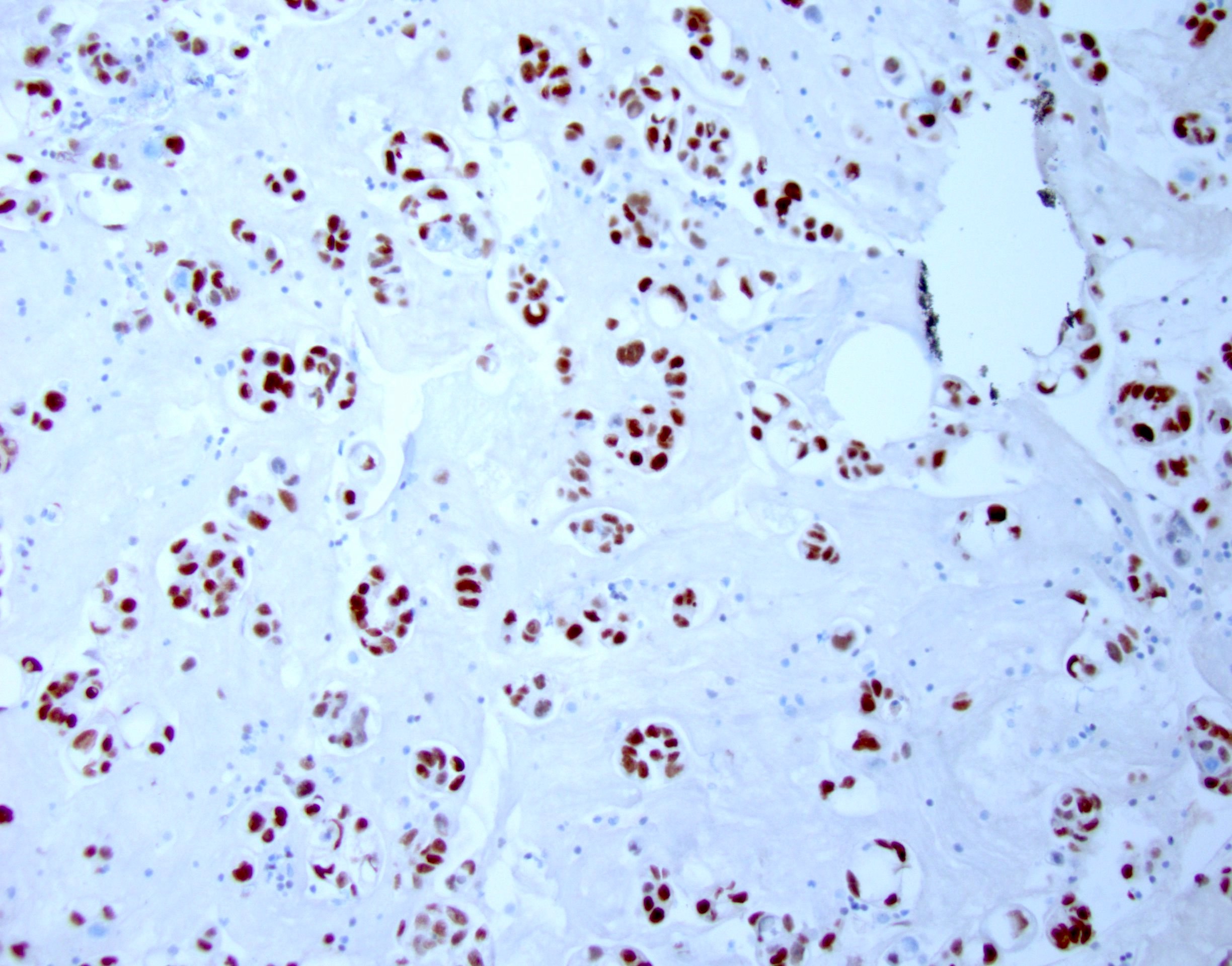
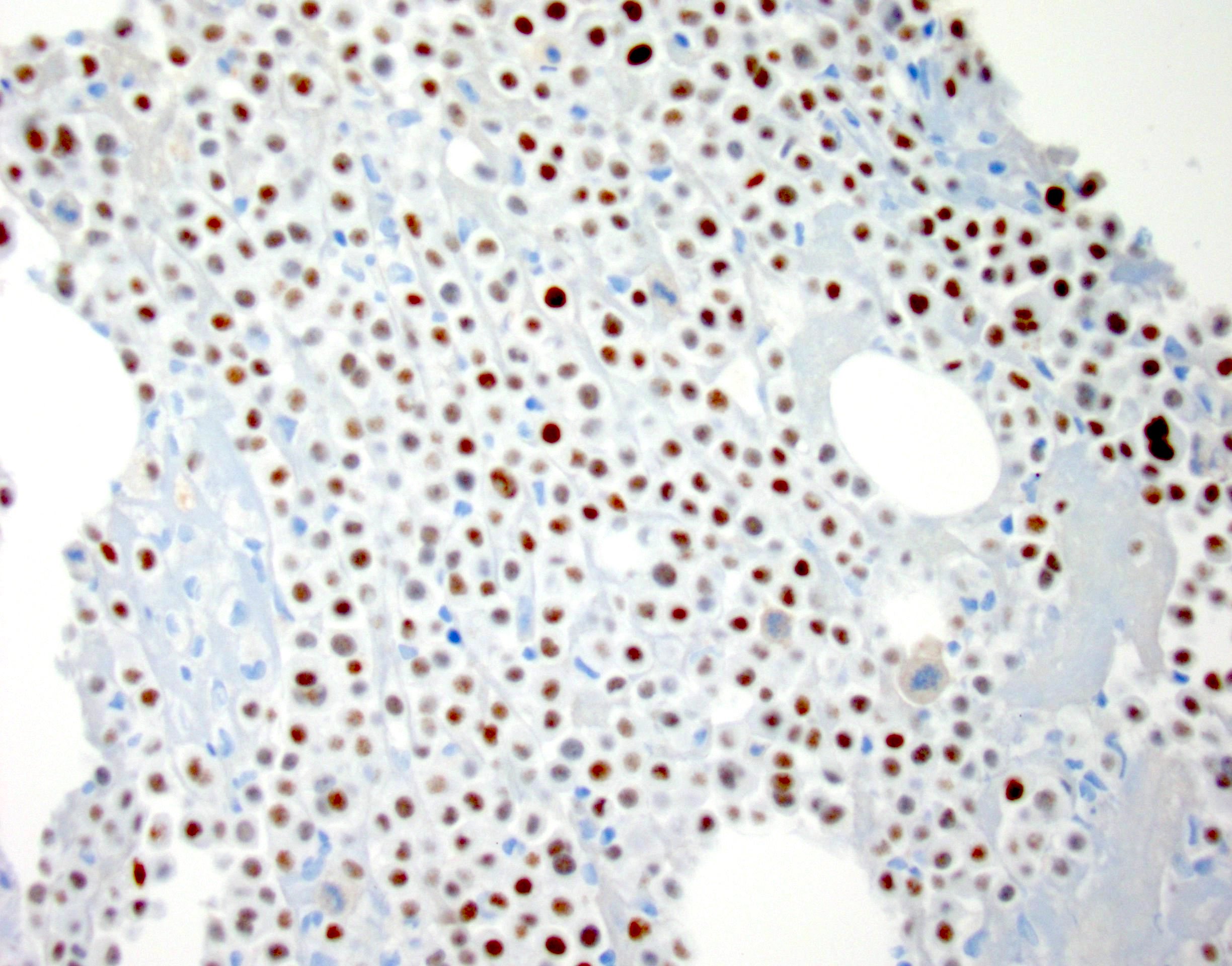
- Immunostains to differentiate mesothelioma from reactive mesothelial cells (Hum Pathol 2013;44:1, Mod Pathol 2020;33:245, Cancer Cytopathol 2018;126:54, Am J Clin Pathol 2009;131:516, Acta Cytol 2012;56:527):
Mesothelioma Reactive mesothelial cells BAP1 Lost (60% of cases) Retained MTAP Lost Retained EMA (E29 clone) Strong membranous Cytoplasmic / weak membranous Desmin Negative Positive
- Immunostains and special stains to differentiate mesothelioma from metastatic carcinoma (Hum Pathol 2013;44:1, Cancer Cytopathol 2014;122:299, Transl Lung Cancer Res 2020;9:S3):
- Ideally a panel with at least 2 mesothelial and 2 for metastatic carcinoma should be ordered (Arch Pathol Lab Med 2013;137:647)
- Pancytokeratins and CK7 should not be used in this differential diagnosis, as both metastatic carcinoma and mesothelioma can express them (Arch Pathol Lab Med 2013;137:647, Mod Pathol 2000;13:962)
Metastatic carcinoma Mesothelioma Calretinin Negative (can be positive in breast cancer, squamous cell carcinoma) Positive (nuclear and cytoplasmic) D2-40 Negative (can be positive in breast, lung and ovarian cancer) Positive (membranous) WT1 Negative (can be positive in gynecologic tumors) Positive (nuclear) CK5/6 Negative (positive in squamous cell carcinoma) Positive (cytoplasmic / membranous) Claudin4 Positive (membranous) Negative MOC31 Positive (membranous) Negative BerEP4 Positive (membranous) Negative B72.3 Positive (cytoplasmic) Negative CEA Positive (cytoplasmic) Negative PASD Positive (cytoplasmic granules) Negative Mucicarmine Positive (cytoplasmic mucin) Negative
- Immunostains to identify primary site or lineage of metastatic neoplasms (Cancer Cytopathol 2018;126:590, Adv Anat Pathol 2020;27:114, Hum Pathol 2013;44:1):
- Lung adenocarcinoma: TTF1 and Napsin A
- Thyroid: TTF1, PAX8, thyroglobulin
- Breast: mammaglobin, GCDFP-15, GATA3, ER
- Urothelial: p40 or p63 and GATA3
- Gynecological tract: PAX8, ER
- Lower gastrointestinal: CDX2, CK20 and SATB2
- Hepatocellular carcinoma: Arginase1, HepPar1, Glypican 3
- Renal cell carcinoma: PAX8, CAIX, RCC, CD10
- Prostate: NKX 3.1, PSA
- Squamous cell carcinoma: p40 and p63, CK5/6 (also positive in mesothelial cells)
- Neuroendocrine neoplasm: synaptophysin, chromogranin, INSM1, CD56
- Melanoma: SOX10, MelanA, S100, HMB45
- Hematological neoplasm: CD45, B cell marker (CD20, CD79a, PAX5), T cell marker (CD3), plasma cell marker (CD138), HHV8 in primary effusion lymphoma
- Vascular tumors: CD31, CD34, ERG
- Germ cell tumors: PLAP, OCT 3/4, SALL4, HCG
- Sex cord stromal tumors: calretinin (also positive in mesothelial cells), inhibin, SF1 and FOXL2
- Predictive and prognostic markers performed on cell block (Chandra: The International System for Serous Fluid Cytopathology, 1st Edition, 2020, J Am Soc Cytopathol 2017;6:33, Arch Pathol Lab Med 2021;145:46, J Clin Oncol 2018;36:2105, Arch Pathol Lab Med 2018;142:291, Cancer Cytopathol 2015;123:117, Cancer Cytopathol 2018;126:421, Am J Surg Pathol 2017;41:1547, Cancer Cytopathol 2017;125:896):
Immunostain Staining pattern Typically performed in Significance Additional testing ER / PR Nuclear Breast cancer Hormonal therapy for ER / PR positive tumors N/A HER2 Membranous Breast, gastric, gastroesophageal, endometrial cancer Anti HER2 therapy for HER2 positive tumors; specimens should be placed in 10% neutral buffered formalin as soon as possible and fixed for 6 - 72 hours FISH is used in equivocal results (2+) Mismatch repair proteins Nuclear Metastatic colorectal, endometrial, cholangiocarcinoma Immunotherapy for tumors with loss of mismatch repair protein PCR or NGS can be used to evaluate microsatellite instability ALK Cytoplasmic Non small cell lung cancer (NSCLC), inflammatory myofibroblastic tumor ALK inhibitors for tumors with ALK fusions or positive ALK immunostain FISH, PCR or NGS can be used as alternative to immunostaining ROS1 Cytoplasmic and membranous NSCLC ROS1 inhibitors for tumors with ROS1 fusions Immunostain is a screening method; FISH, PCR or NGS confirmation is necessary Pan TRK Nuclear, perinuclear, cytoplasmic and membranous Variety Anti NTRK therapy for tumors with NTRK fusions Immunostain is a screening method; molecular confirmation (typically NGS) is necessary PDL1 Membranous NSCLC Immunotherapy for PDL1 positive tumors; type of scoring and cut off points depend on antibody clone and type of tumor N/A
- Immunostains to differentiate mesothelioma from reactive mesothelial cells (Hum Pathol 2013;44:1, Mod Pathol 2020;33:245, Cancer Cytopathol 2018;126:54, Am J Clin Pathol 2009;131:516, Acta Cytol 2012;56:527):
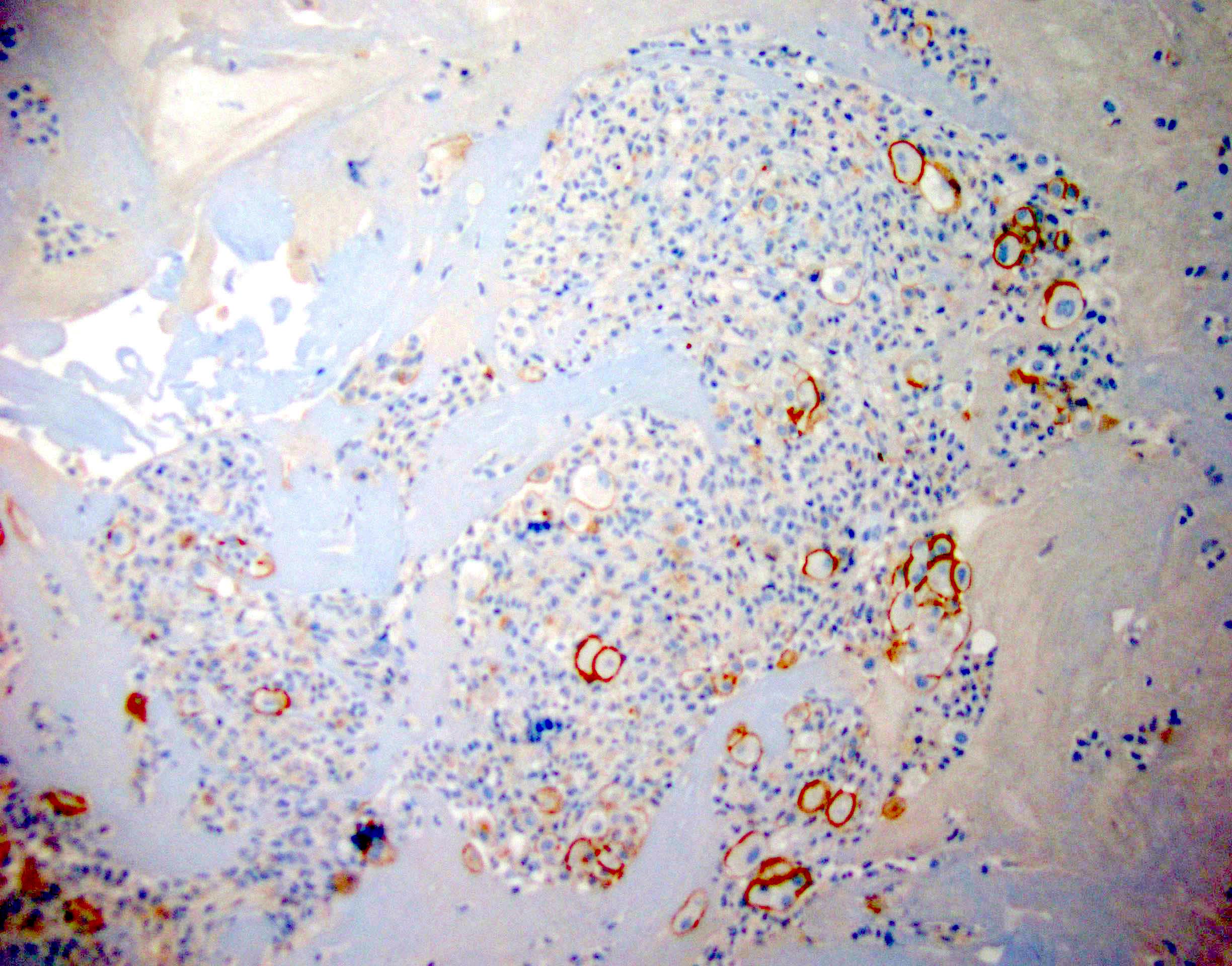
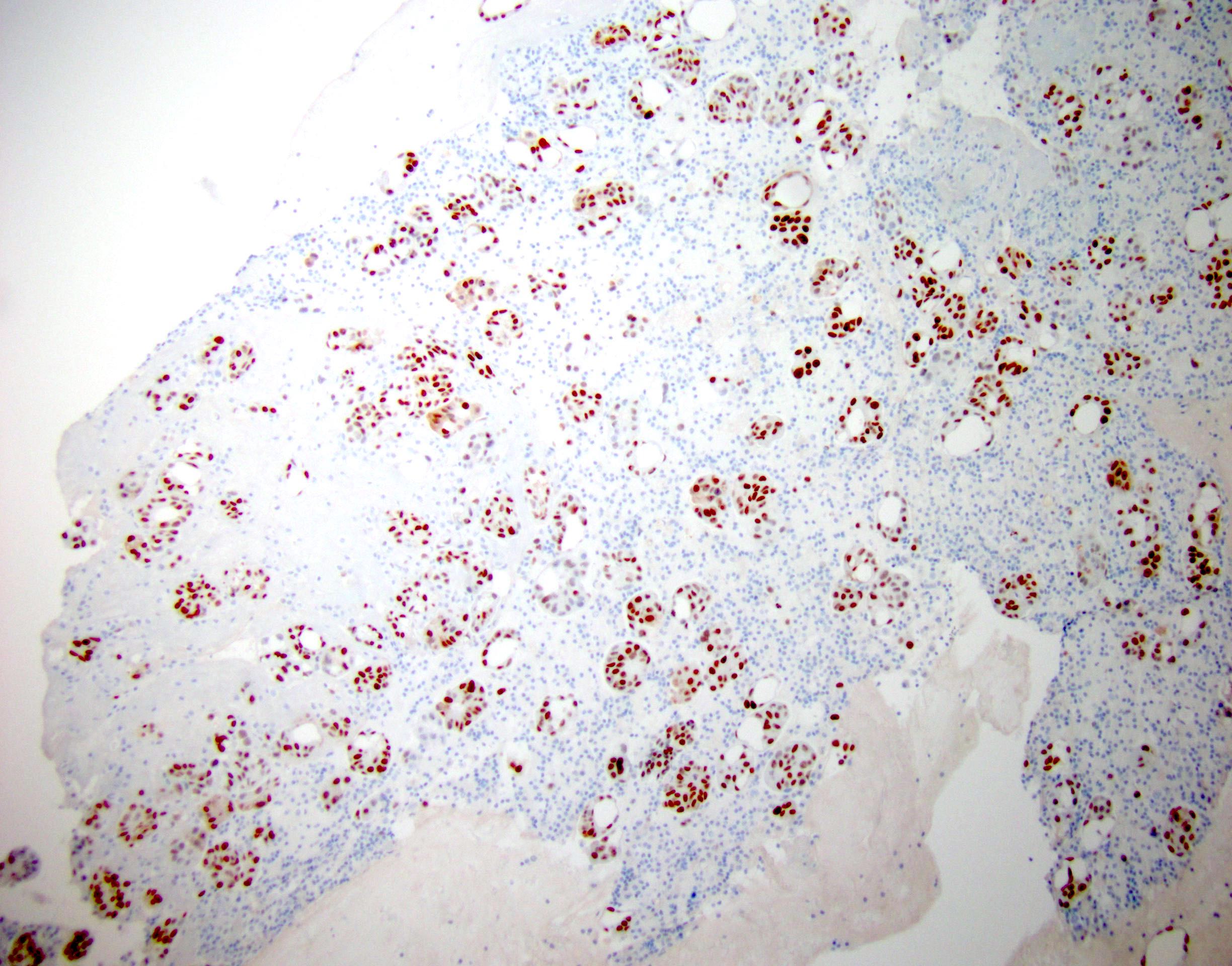
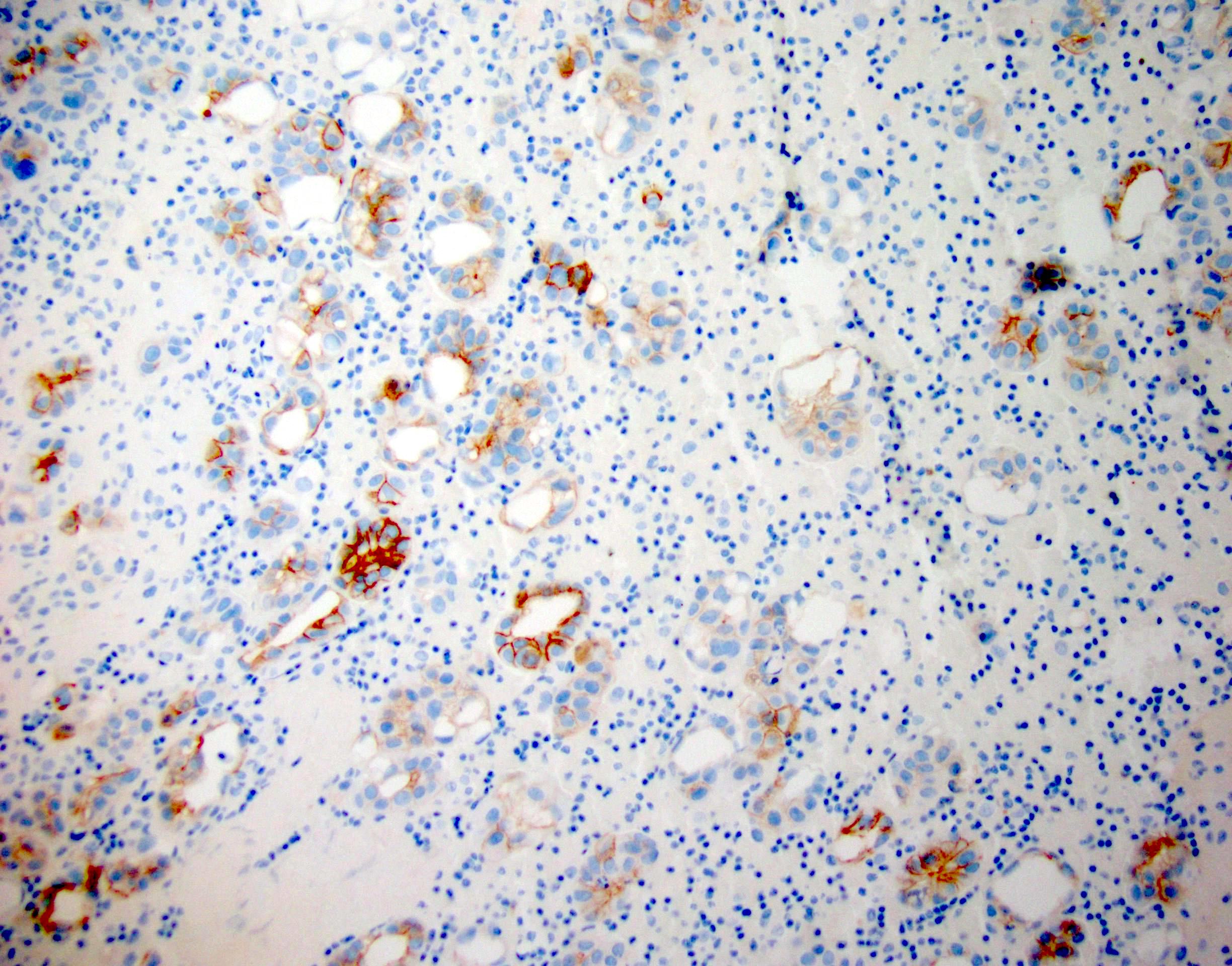
Cytology images
Contributed by Lawrence Hsu Lin, M.D., Ph.D. and Tamar C. Brandler, M.D., M.S.
Images hosted on other servers:
Molecular / cytogenetics description
- p16 / CDKN2A deletion by fluorescent in situ hybridization (FISH) is found in up to 80% of malignant mesothelioma and can help differentiate it from reactive mesothelial cells (Cancer Sci 2015;106:1635)
Molecular / cytogenetics images
Additional references
Board review style question #1
Which immunostaining panel is most helpful in the differential diagnosis of a 60 year old male patient with unilateral pleural effusion with the above findings from the pleural fluid cytology smear stained with Papanicolaou stain?
- AE1 / AE3, CAM5.2, CK7, CK20
- BerEP4, MOC31, calretinin, D2-40, TTF1
- BerEP4, MOC31, CK7, CK20, TTF1
- Calretinin, D2-40, WT1, AE1 / AE3, CAM5.2
- TTF1, synaptophysin, chromogranin, Ki67
Board review style answer #1
Board review style question #2
Which of the following immunostains can aid in the differentiation between a reactive mesothelial cells and mesothelioma?
- BAP1
- BerEP4
- Calretinin
- D2-40
- MOC31
Board review style answer #2
Immunocytochemistry (pending)
[Pending]
International Academy of Cytology Yokohama system (pending)
Insufficient
[Pending]
Benign
[Pending]
Atypical
[Pending]
Suspicious
[Pending]
Maligant
[Pending]
International system for reporting serous fluid cytopathology
Table of Contents
Definition / general | Essential features | CPT coding | Sites | Diagrams / tables | Cytology description | Cytology images | Sample pathology report | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Effusion cytopathologic evaluation can be challenging due to multiple, different processes affecting serous cavities, ranging from benign (infectious, autoimmune) to malignant processes (primary or metastatic neoplasms)
- The international system standardized the way to report cytopathology diagnoses of serous fluids, which includes 5 different categories: nondiagnostic (ND), negative for malignancy (NFM), atypia of undetermined significance (AUS), suspicious for malignancy (SFM) and malignant (MAL)
Essential features
- The international system for reporting serous fluid cytopathology includes 5 categories with different risk of malignancy: nondiagnostic (ND), negative for malignancy (NFM), atypia of undetermined significance (AUS), suspicious for malignancy (SFM) and malignant (MAL) (Chandra: The International System for Serous Fluid Cytopathology, 1st Edition, 2020)
- For an adequate diagnosis, effusion cytopathology should be interpreted with clinical and radiologic information and correlated with ancillary techniques if needed (immunostains, molecular, flow cytometry)
- Nondiagnostic classification and adequacy evaluation depends on fluid volume, quantity of cells and quality of the preparation
- Atypia of undetermined significance and suspicious for malignancy are categories of uncertainty and can be used as preliminary diagnoses while ancillary tests are performed, in cases where there is insufficient material for ancillary testing or in cases where ancillary testing results are noncontributory or inconclusive
CPT coding
- 88108 - concentrated cytology specimen (e.g. centrifugation)
- 88112 - selective enhanced cytology specimen (e.g. liquid based slide preparation)
- 88173 - fine needle aspiration
- 88305 - cell block
- 88342 - immunohistochemistry, first stain
- 88341 - immunohistochemistry, additional stains
- 88180 - flow cytometry
Sites
- Pleura, peritoneum, pericardium
Diagrams / tables
Cytology description
- The international system standardized reporting of serous fluid cytology includes 5 categories with different malignancy risks (J Am Soc Cytopathol 2020;9:469, Chandra: The International System for Serous Fluid Cytopathology, 1st Edition, 2020)
- Cytology specimens must be interpreted in concert with clinical and radiologic information
- Ancillary studies can be utilized for further characterization (immunostains, flow cytometry, molecular) (Cancer Cytopathol 2015;123:258, Cancer Cytopathol 2018;126:590)
- Categorization is recommended whenever possible
- If the diagnosis is uncertain, a descriptive interpretation should be included
- Categories (Chandra: The International System for Serous Fluid Cytopathology, 1st Edition, 2020):
- Nondiagnostic (ND):
- Risk of malignancy: 17% (± 8.9%) (Diagn Cytopathol 2019;47:1145)
- Adequacy of specimen depends on:
- Volume of fluid: nondiagnostic category can only be used after adequate amount of fluid has been processed (there is no specific cutoff but volume < 50 mL might not be enough to exclude malignancy; higher volumes of liquid yield lower rates of nondiagnostic cases) (Cytopathology 2011;22:179)
- Cellularity: a specimen can be nondiagnostic if there are not enough cellular elements of interest to classify the sample in another diagnostic category; there is no definitive criteria of number of mesothelial cells for adequacy and the criteria might vary depending on the pathologic process (e.g. a specimen with prominent acute inflammation and scant mesothelial cells may be adequate if the clinical suspicion is empyema)
- Quality of preparation: poor cell preservation, degeneration, hemorrhage, artifacts and contaminants can impair interpretation
- Explanation for the lack of adequacy should be provided in the report
- If the specimen meets criteria for other categories (e.g. significant atypia), it should not be classified as nondiagnostic, regardless of volume / cellularity
- Negative for malignancy (NFM):
- Risk of malignancy: 21% (± 0.3%) (Diagn Cytopathol 2019;47:1145)
- Most fluids (> 80%) fall within the negative of malignancy category (Diagn Cytopathol 1987;3:8, Diagn Cytopathol 1999;20:350)
- Benign and reactive components: mesothelial and inflammatory cells
- Reactive mesothelial cells are typically single cells or small clusters and can exhibit binucleation, multinucleation and reactive cellular atypia; mesothelial cells can present as flat sheets in peritoneal lavages
- Benign cellular and noncellular findings are included in the negative for malignancy category, such as collagen balls, psammoma bodies, ciliary tufts, endosalpingiosis, endometriosis, asbestos bodies, lupus erythematosus cells, microorganisms, among others; a comment should be added explaining the finding (Acta Cytol 1992;36:466, Cancer 2004;102:87, Acta Cytol 1985;29:310)
- Unexpected second population of cells should not be present in the negative for malignancy category (expected cells include mesothelial or inflammatory cells; unexpected second population of cells often indicates malignancy)
- Majority of cases can be signed out based only on morphology but ancillary testing might be needed to rule out malignancy in particular scenarios:
- Highly atypical mesothelial cells or increased histiocytes / lipophages: immunohistochemistry to rule out adenocarcinoma
- Highly atypical mesothelial cells: clinical and radiologic correlation, immunostains and molecular to rule out mesothelioma
- Lymphocytic effusions: flow cytometry or immunostains to rule out lymphoma
- Atypia of undetermined significance (AUS):
- Risk of malignancy: 66% (± 10.6%) (Diagn Cytopathol 2019;47:1145)
- Limited nuclear or architectural atypia with overlapping features between reactive changes and malignant appearance but features are closer to benign processes
- Degree of atypia is sufficient for the specimen not to be classified as nondiagnostic
- Heterogenous group of conditions with variable criteria (Diagn Cytopathol 2019;47:1145, J Am Soc Cytopathol 2015;4:44)
- Can be used in preliminary reports before confirmatory ancillary tests; ancillary testing can lead to diagnostic upgrade (malignant or suspicious for malignancy) or downgrade (negative for malignancy)
- If ancillary tests are noncontributory or insufficient material is available for additional testing, the final diagnosis remains as atypia of undetermined significance
- Scenarios that can fall in the AUS category:
- Limited atypia that does not meet criteria for suspicious for malignancy
- Specimen with atypia, likely benign; however, due to poor preservation or low cellularity cannot confidently rule out malignancy
- Peritoneal washing with cells from benign or borderline tumors of ovary
- Peritoneal washing with epithelial cells of indeterminate origin, exhibiting benign or low grade features
- Lymphocytic effusion, favoring reactive but indefinite for lymphoproliferative disorder (when there is no confirmatory flow cytometry)
- Suspicious for malignancy (SFM):
- Risk of malignancy: 82% (± 4.8%) (Diagn Cytopathol 2019;47:1145)
- Features suspicious but not definitive for malignancy
- In comparison to the atypia of undetermined significance category, the degree of suspicion of malignancy is higher
- Can be used in preliminary reports before confirmatory ancillary tests, which can lead to upgrade (malignant) or downgrade (negative for malignancy); if the ancillary tests are noncontributory or insufficient material is available for additional testing, the final diagnosis remains as suspicious for malignancy
- Scenarios that can fall into the SFM category:
- Greater degree of atypia but cells are limited in number or by preservation of specimen
- Large number of bland appearing epithelial cells (e.g., suspicious for breast lobular carcinoma)
- Mucinous material with epithelial cells with limited atypia
- Atypical lymphoid cells, suspicious for lymphoma (when there is no confirmatory flow cytometry or flow cytometry confirms lymphoma but cytomorphology is not clear enough to term positive for malignancy)
- Malignant (MAL):
- Risk of malignancy: 99% (± 0.1%) (Diagn Cytopathol 2019;47:1145)
- Definitive evidence of malignancy
- Subclassified into:
- Primary (MAL-P): majority are mesothelioma; other neoplasms such as primary lymphoma and primary mesenchymal tumors can also occur
- Secondary (MAL-S): majority are metastatic adenocarcinoma; other metastatic neoplasms such as squamous cell carcinoma, neuroendocrine tumors, melanoma, lymphoma, mesenchymal and germ cell tumors can also occur
- In the majority of cases, immunostains can aid in the differential diagnosis (please refer to the Immunostaining panels in effusion cytology topic)
- Cytologic and architectural features of mesothelioma (Cytojournal 2015;12:26, Arch Pathol Lab Med 2018;142:893, Cancer Cytopathol 2014;122:70)
- Highly cellular smears composed of mesothelial cells (cells with dense cytoplasm with pale rim / ectoplasm [skirts], displaying a narrow space in between cells when grouped [window])
- Architecture atypia: large tissue fragments or cell clusters (papillary, morules, spheres) with numerous cells per cluster; can also present as single cells or mixture of architectures
- Cytologic atypia: nuclear enlargement and pleomorphism, irregular nuclear contours, macronucleoli, frequent binucleation or multinucleation, atypical mitosis) with intermediate to high grade features; cytologic atypia of mesothelioma can overlap with reactive mesothelial cells
- Softer signs: metachromatic / 2 tone cytoplasm, large variation in size (from normal to gigantic), pseudokeratotic cells (pyknotic, eosinophilic or orangeophilic cells), May-Grünewald-Giemsa stain showing increased perinuclear lipid vacuoles (confirmed by oil-red O stain) and pink / red granular background
- Ancillary techniques: immunostains or FISH (p16 / CDKN2A) (please refer to the Immunostaining panels in effusion cytology topic)
- Cytologic and architectural features of metastatic adenocarcinoma (Adv Anat Pathol 2006;13:174, Surg Pathol Clin 2018;11:523)
- Highly cellular smears
- Second population of cells
- 3 dimension clusters with smooth contours, papillary and glandular formations, single signet ring cells
- Highly atypical cells (nuclear enlargement, increased N/C ratio, variable pleomorphism, irregular nuclear contours, coarse chromatin); atypia can be subtle, particularly in breast lobular and gastric carcinomas
- Intracellular mucin vacuoles or extracellular mucin
- Commonly display tumor groups surrounded by retraction artifact on cell block
- Ancillary techniques: immunostains (lineage specific and site specific) (please refer to the Immunostaining panels in effusion cytology topic)
- Nondiagnostic (ND):
Cytology images
Contributed by Lawrence Hsu Lin, M.D., Ph.D. and Tamar C. Brandler, M.D., M.S.
Sample pathology report
- Lung, right, pleural effusion, thoracocentesis:
- Evaluation limited by extensive hemorrhage
- Nondiagnostic (see comment)
- Comment: The specimen is predominantly composed of blood. A repeat procedure is recommended if clinically indicated.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Negative for malignancy (see comment)
- Comment: The specimen is predominantly composed of reactive mesothelial cells and histiocytes. Immunohistochemical stains were performed on the cell block and show that the mesothelial cells are reactive for calretinin and D2-40 and cells are nonreactive for BerEp4 and MOC31.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Atypia of undetermined significance (see comment)
- Comment: The smears are composed of mixed population of mesothelial and inflammatory cells. There are a small cluster of cells with mild nuclear enlargement and mild hyperchromasia. The cell block consists predominantly of blood; therefore, immunostains could not be performed to further characterize the process. Additional sampling for further characterization should be considered if fluid reaccumulates, if clinically indicated.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Suspicious for malignancy (see comment)
- Suspicious for carcinoma
- Comment: The smears are composed of a mixed population of mesothelial and inflammatory cells. There are few small clusters of cells with marked nuclear enlargement and hyperchromasia. The cell block consists predominantly of blood; therefore, immunostains could not be performed to further characterize the process. The overall findings are concerning for malignancy.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Positive for malignancy (see comment)
- Metastatic adenocarcinoma
- Comment: The specimen is composed of highly cellular smears with a second population of markedly atypical cells arranged in large clusters. The cells exhibit nuclear enlargement, increased N/C ratio and hyperchromasia with prominent nucleoli. Immunostains show that the atypical cells are positive for MOC31, BerEP4, TTF1 and CK7 and negative for calretinin, D2-40, CK20 and p40. The overall findings and previous history of lung adenocarcinoma supports the diagnosis of metastatic lung adenocarcinoma.
- Lung, right, pleural effusion, thoracocentesis:
- Satisfactory for evaluation
- Positive for malignancy (see comment)
- Malignant mesothelioma
- Comment: The specimen is composed of highly cellular smears with predominant population of markedly atypical mesothelial cells in large clusters. The cells exhibit nuclear enlargement, increased N/C ratio and hyperchromasia with prominent nucleoli. Immunostains show that the cells are positive for calretinin and D2-40 and negative for MOC31 and BerEP4. Loss of BAP1 by immunohistochemistry and deletion of p16 / CDKN2 by FISH supports the above diagnosis.
Additional references
Board review style question #1
Board review style answer #1
C. Negative for malignancy
Comment Here
Reference: International system for reporting serous fluid cytopathology
Comment Here
Reference: International system for reporting serous fluid cytopathology
Board review style question #2
Board review style answer #2
C. Negative for malignancy
Comment Here
Reference: International system for reporting serous fluid cytopathology
Comment Here
Reference: International system for reporting serous fluid cytopathology
Board review style question #3
Which of the following is the category for the above findings in a peritoneal washing according to the international system for reporting serous fluid cytopathology in a patient with concurrent surgical pathology with ovarian serous borderline tumor?
- Atypia of undetermined significance
- Malignant
- Negative for malignancy
- Nondiagnostic
- Suspicious for malignancy
Board review style answer #3
A. Atypia of undetermined significance
Comment Here
Reference: International system for reporting serous fluid cytopathology
Comment Here
Reference: International system for reporting serous fluid cytopathology
Interventional cytopathology
Table of Contents
Definition / general | Essential features | Terminology | CPT coding | Diagrams / tables | Clinical images | Indications | Contraindications (relative) | Advantages of core biopsy over FNA | Advantages of core biopsy over open biopsy | Pitfalls of core biopsy over FNA | Pitfalls of core biopsy over open biopsy | Combined use of US guided core biopsy with US guided FNA | Set up and collection technique of US guided core biopsy | Sample handling | Complications | Treatment | Videos | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3 | Board review style question #4 | Board review style answer #4Definition / general
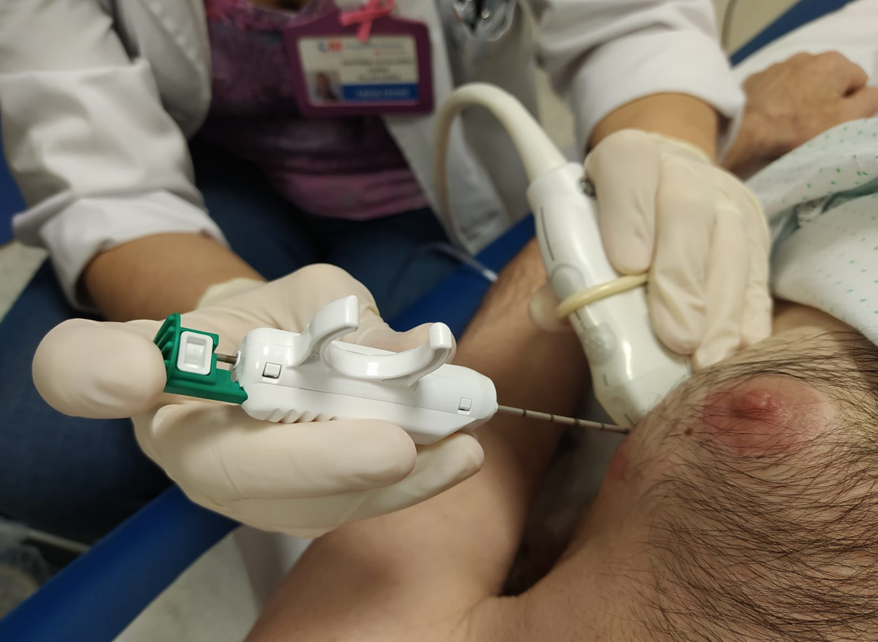
- Minimally invasive technique to obtain tissue samples from superficial organs (palpable or not)
- Recommended with ultrasound guidance to improve performance (Cytopathology 2016;27:115, Endocr Pract 2002;8:282)
- In some cases, it can minimize the need for open biopsies for diagnosis
- Can prevent complications associated with surgery for open biopsies
- Performed by many operators across the globe (oncologists, head & neck surgeons, radiologists, other surgeons and some interventional pathologists)
- Different sample gauges can be obtained with automatic and manual devices depending on the type of tissue
- Reporting with protocol templates for interventional procedures is recommended (Rev Esp Patol 2019;52:163)
Essential features
- Low risk procedure through a minimal skin incision with low morbidity and few contraindications
- Can be performed immediately after ultrasound fine needle aspiration (USFNA) following rapid on site evaluation (ROSE) assessment during the same patient's visit (Rev Esp Patol 2022;55:73)
- Can avoid open surgeries for diagnosis in some cases (Medicine (Baltimore) 2016;95:e2172)
- Same parallel approach technique as for ultrasound FNA with manual or automatic trigger devices
- Different biopsy gauges according to tissue needs and lesion size
- Biopsy of superficial nodules (palpable or not) performed by an interventional pathologist provides more efficient and timely progress through the diagnostic process for the patient (Diagn Cytopathol 2009;37:262, Acta Cytol 2021;65:453)
Terminology
- Biopsy, percutaneous biopsy, core needle biopsy, ultrasound guided biopsy, interventional, interventional pathology
CPT coding
- 76942 - ultrasonic guidance for needle placement (e.g., biopsy, aspiration, injection, localization device), imaging supervision and interpretation
- 20206 - biopsy, muscle, percutaneous needle
- 38505 - biopsy or excision of lymph node(s); by needle, superficial (e.g., cervical, inguinal, axillary)
- 60100 - biopsy thyroid, percutaneous core needle
Diagrams / tables
Clinical images
Indications
- Assessment of tumor masses for diagnosis and to perform additional studies as necessary
- Patient is not eligible for open surgery
- Indeterminate FNA
- Indeterminate features of the nodule by ultrasound (not fully benign features)
- Not possible to obtain a cell block
- Tumor relapse assessment
- Solid neoplasms, some hematolymphoid tumors, soft tissue masses and sarcomas in a multidisciplinary approach (Korean J Radiol 2017;18:361)
- Muscular biopsies for myopathies (Rev Esp Patol 2021;54:156)
Contraindications (relative)
- Deep seated organs (for inexperienced pathologists)
- Severe anxiety (anxiolytics)
- Children may require sedation
- Coagulopathy (multidisciplinary approach)
- The only absolute contraindication is the patient's refusal
- Anticoagulant therapy is not currently a contraindication (ultrasound guided core needle biopsy is considered a low risk of bleeding procedure by the current guidelines) (J Vasc Interv Radiol 2019;30:1168)
Advantages of core biopsy over FNA
- Provides larger and more intact tissue samples to evaluate tissue architecture
- Tumor subtyping and grading
- Immunohistochemistry and molecular studies in larger samples
- More comprehensive view of the tumor features
Advantages of core biopsy over open biopsy
- Less invasive
- Rapid results allowing faster preliminary diagnosis
- Real time ultrasound guidance
- Faster recovery, reduced scarring
- Local anesthesia
- Outpatient procedure
- Lower risk of infection
- Lower cost
Pitfalls of core biopsy over FNA
- More invasive
- Slightly higher risk of bleeding and bruising
- Local anesthesia challenges
- Limited use in cysts
- Generally more technically involved than FNA; might not be suitable for every healthcare setting or practitioner (Clin Orthop Relat Res 2010;468:2992)
Pitfalls of core biopsy over open biopsy
- Sampling error
- Challenging anatomical locations that might not be accessible for core biopsy
- Tumor heterogeneity (Clin Orthop Relat Res 2010;468:2992)
Combined use of US guided core biopsy with US guided FNA
- After ultrasound FNA with ROSE of the sample obtained (Acta Cytol 2016;60:1)
- To increase sensitivity, specificity and decrease nondiagnostic rate
- To overcome some pitfalls of FNA
- To obtain representative and sufficient sample for molecular diagnosis and further testing
Set up and collection technique of US guided core biopsy
- Determine if the patient is receiving antithrombotic therapy
- Follow current guidelines for antithrombotic therapy in interventional procedures (Rev Esp Cardiol (Engl Ed) 2018;71:553)
- If the patient has no specific risks for bleeding or thrombosis, ask about allergies
- Document the features of the lesion on ultrasound
- Choose the proper patient position depending on the location of the mass
- Select the appropriate device (gauge, length and trigger mechanism)
- Disinfect the skin area and apply local anesthesia (recommended with epinephrine) (Local Reg Anesth 2018;11:35)
- Make a small cut on the skin to avoid the tent effect during the insertion of the device
- Insert the needle (preloaded) in a parallel approach with the probe
- Enter the skin and take the needle tip to the edge of the nodule and deploy the reservoir of the manual device or take the shot of the automatic device
- Collect the sample and enter again repeating the procedure to take at least 2 cores
Sample handling
- Select fresh tissue for further studies if necessary
- The rest of the sample is fixed in formaldehyde
- If sufficient sample is available, sample can also be stored in freezer
- Perform touch print ROSE of the cores if needed
- Order further studies (such as IHC in advance if needed) (Diagn Cytopathol 2019;47:149, J Am Soc Cytopathol 2020;9:322, Diagn Cytopathol 2021;49:E137)
Complications
- Local pain
- Hematoma or bruise (depending on the nodule / body site)
- Puncture site infection, damage to surrounding tissues or other complications are less common (Clin Orthop Relat Res 2010;468:2992)
Treatment
- Following the core needle biopsy procedure, ultrasound can be used to scan for potential complications prior to the patient leaving the clinic / hospital
- Place suture bandages (Steri-Strips) on the skin point of entry
- Remind the patient of the aftercare
- Explain alarm symptoms
Videos
To be provided
Additional references
Board review style question #1
Board review style answer #1
B. Local pain and hematoma. Potential complications of ultrasound guided core needle biopsy of superficial nodules include bleeding, hematoma formation, infection, pain or discomfort at the biopsy site, injury to surrounding structures and rarely, pneumothorax in cases involving nodules near the lung or pleura. Answer C is incorrect because in core needle biopsy, only sterile medical devices are used, and under appropriate conditions of sterilization and disinfection, no foreign bodies that may cause adverse reactions are inserted into the body. Answer A is incorrect because bleeding in percutaneous biopsy of superficial nodules is not common. It is considered a low bleeding risk in interventional guidelines due to the possibility of tissue compression even when the lesion is not palpable. Answer D is incorrect because visual disturbances are not expected complications of diagnostic procedures such as FNA and core biopsy.
Comment Here
Reference: FNA and core biopsy procedure
Comment Here
Reference: FNA and core biopsy procedure
Board review style question #2
Which of the following is an advantage of ultrasound guided core needle biopsy over fine needle aspiration for evaluating superficial nodules?
- Ability to evaluate architectural features and tissue architecture
- Higher sensitivity in detecting malignancy
- Less risk of complications, such as hematoma or infection
- Shorter procedure time and lower cost
Board review style answer #2
A. Ability to evaluate architectural features and tissue architecture. One of the advantages of ultrasound guided core needle biopsy over fine needle aspiration is the ability to evaluate architectural features and tissue architecture. Core biopsy provides larger tissue samples, allowing for a more comprehensive evaluation of the nodule, including the assessment of tissue architecture and the presence of important features such as invasion or encapsulation. Answer B is incorrect because while it is true that in lesions that are indeterminate by FNA, the biopsy provides a more comprehensive assessment of the lesion that can identify malignancy more easily, both techniques can discriminate benign from malignant lesions with good sensitivity.
Answer C is incorrect because there is no significant difference between the two procedures in terms of risk of infection; however, it is true that percutaneous biopsy may cause hematoma more frequently than FNA, especially the larger the diameter of the device. Answer D is incorrect because the cost of doing a biopsy is slightly higher than that of doing an FNA, not only because of the use of a different device and additional supplies than an FNA
but also because the additional time spent during the procedure may lead to an increase in the cost of the required staff.
Comment Here
Reference: FNA and core biopsy procedure
Comment Here
Reference: FNA and core biopsy procedure
Board review style question #3
What is important to do when performing an ultrasound guided core needle biopsy of a superficial nodule?
- Administer prophylactic antibiotics to reduce infection risk
- Apply pressure before the procedure to prevent bleeding
- Perform a postprocedure ultrasound to evaluate for complications
- Use a larger gauge needle for improved sample size
Board review style answer #3
C. Perform a postprocedure ultrasound to evaluate for complications. After performing an ultrasound guided core needle biopsy of a superficial nodule, it is important to perform a postprocedure ultrasound to evaluate for potential complications such as hematoma formation or other abnormalities. This helps ensure patient safety and prompt management of any complications that may arise. Answer B is incorrect because pressure should be applied following the procedure and not before it is performed. Answer D is incorrect because using a larger diameter does not mean that a better sample will be obtained; in certain cases, use of a smaller diameter (by increasing the number of cores) may be considered in order to obtain a better representative sample of the lesion. Answer A is incorrect because the use of prophylactic antibiotics is not required to perform core needle procedures.
Comment Here
Reference: FNA and core biopsy procedure
Comment Here
Reference: FNA and core biopsy procedure
Board review style question #4
Board review style answer #4
C. Nodules with high suspicion for malignancy. Ultrasound guided core needle biopsy is a valuable tool for evaluating superficial nodules, particularly when there is a high suspicion for malignancy. Nodules with atypical ultrasound features or those that demonstrate suspicious characteristics such as irregular margins, microcalcifications, increased vascularity, or rapid growth should be considered for biopsy. By targeting nodules with a high likelihood of malignancy, ultrasound guided core needle biopsy can provide important diagnostic information, aid in treatment planning and potentially avoid unnecessary surgical procedures. It is crucial to carefully select nodules with suspicious features for biopsy to optimize diagnostic yield and patient management. Answer D is incorrect because nodules typically benign on ultrasound and with appropriate clinical context, are not chosen for biopsy; in any case, if there is clinical suspicion of malignancy and the ultrasound image is benign, you could start with FNA and then decide whether or not to perform the biopsy. Answer B is incorrect because sometimes there are nodules that can be seen with a pattern suggestive of malignancy on ultrasound and in the appropriate clinical context it may be appropriate to start with FNA to exclude malignancy. For example, a hematoma may have a heterogeneous ultrasound image and be suspicious for malignancy but with a history of local trauma, if FNA is performed and blood is drawn only, there is no need to do a biopsy. Answer A is incorrect because The size of the lesion is not a malignancy criterion in itself. It can be a lesion smaller than 5 cm with suspicion of malignancy that requires a biopsy or it can be a 5 cm nodule with benign FNA and it is not necessary to perform a biopsy.
Comment Here
Reference: FNA and core biopsy procedure
Comment Here
Reference: FNA and core biopsy procedure
Liquid based cytology
Table of Contents
Definition / general | Essential features | CPT coding | Sites | Laboratory | Case reports | Cytology description | Cytology images | Molecular / cytogenetics description | Sample pathology report | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Liquid based preparation (LBP): samples from various sources are collected or rinsed in a preservative solution; the cell suspension is then evenly distributed as a monolayer on a glass slide for examination
- The 2 most popular methods used today are ThinPrep (Hologic, Marlborough, Massachusetts) and SurePath (Becton Dickinson and Company, Franklin Lakes, New Jersey)
Essential features
- LBP allows for easier processing, potential for automation (for cervical pap tests), fewer slides required per case and less screening time compared with conventional / direct smears
- Improvements in identification of morphologic features with less air drying artifact, improved nuclear and cytoplasmic details, reduction in background blood and inflammation and increased cellularity
- Familiarity with known pitfalls is needed: i.e., cell clusters / papillae are broken up, smaller cell size, more prominent nucleoli in benign conditions, lack of background mucin, necrosis or change in quality of background elements
CPT coding
- Gynecological:
- Nongynecological:
- 88112 - enriched / concentrated preparation (e.g., liquid based slide preparation: ThinPrep, SurePath)
- FNA:
- No specific separate charge
Sites
- Gynecological: cervix, vagina (Pap test)
- Body cavity fluids (pleural, peritoneal, pericardial)
- Cerebrospinal fluid (CSF)
- Gastrointestinal (GI): anal (Pap test), pancreas, bile duct, liver
- Genitourinary (GU): urethra, bladder, ileal conduit, renal pelvis, ureter
- Respiratory: bronchus, lung
- Hematologic: lymph nodes
- Head and neck: thyroid, salivary gland
- Breast
Laboratory
- Specimen collection:
- Collection medium: methanol based PreservCyt (for ThinPrep Hologic, Marlborough, Massachusetts) and CytoRich Red preservative fluid (SurePath Becton Dickinson and Company, Franklin Lakes, New Jersey)
- Cervical or vaginal sample:
- ThinPrep: collection using Rovers Cervex-Brush Combi device (a green broom-like device with an integrated endocervical sampler), endocervical brush or spatula
- Following collection, the device is rinsed in the PreservCyt vial, then vigorously swirled before discarding
- SurePath: collection using broom-like devices (Rovers Cervex-Brush Device and Rovers Cervex-Brush Combi Device), endocervical brush or spatula
- Following collection, the device is placed in the vial; the device head is pulled or snapped off and remains in the vial
- ThinPrep: collection using Rovers Cervex-Brush Combi device (a green broom-like device with an integrated endocervical sampler), endocervical brush or spatula
- Anal sample:
- ThinPrep: using moistened Dacron swab or cytobrush to collect sample, rotate in collection medium, then vigorously swirl device before discarding
- SurePath: using moistened swab collect sample, place in SurePath vial; the device head remains in the vial
- Brushing (bronchial, bile duct): rinse device into collection medium
- Fine needle aspiration (FNA) sample: collect needle rinse / aspiration material directly into collection medium
- Specimen processing:
- SurePath:
- Sample is vortexed then passed through a small opening using a syringe device (causing disaggregation)
- Sample is poured into a centrifuge tube with a density gradient reagent
- Specimen is centrifuged and the pellet is resuspended; this step is repeated 5 times
- Tubes are then transferred to the PrepStain instrument
- Robotic arm transfers the fluid into a cylinder, the cells settle into a cationic polyelectrolyte coated slide, a 13 mm diameter deposit is made
- Staining is performed in the same instrument using the robotic arm
- ThinPrep:
- Vial is placed on a stage and a plastic cylinder with a 20 mm diameter; a polycarbonate filter is bonded to the surface and is inserted on top of the vial
- Rotor spins the cylinder, dispersing the cells, then a vacuum is applied to the cylinder to trap the cells onto the filter
- Instrument monitors the cell density on the filter; once a threshold is reached, the cylinder with the attached filter is inverted 180 degrees
- Filter is pressed on to a glass slide, creating a 20 mm diameter deposit
- Slide is immediately dropped into an alcohol bath
- Staining is performed in a separate device
- SurePath:
- Advantages (compared with conventional smear technique):
- General (Diagn Cytopathol 2013;41:257):
- Standardization of processing
- Minimal air drying artifact from immediate fixation
- Compatible with automated screening devices
- Allows batch processing
- Residual sample can be used for additional testing (human papillomavirus [HPV], Neisseria gonorrhoeae [GC], chlamydia, cell block, florescent in situ hybridization [FISH], molecular testing)
- Uniform distribution of cells in a monolayer with less overlap
- Lower rate of unsatisfactory samples
- Minimal cell loss
- Less screening time
- Reduction in inflammation or blood in background
- Useful when onsite evaluation is unavailable
- SurePath:
- Staining step included in processing
- Better reduction in background blood
- ThinPrep:
- T5000 processor allows for 20 samples to be processed at once
- Fully automated, hands free process
- Less operator training required
- General (Diagn Cytopathol 2013;41:257):
- Disadvantages:
- General:
- Does not allow for real time adequacy assessment
- Some ancillary tests cannot be performed: microbial culture, flow cytometry
- SurePath:
- More manual steps (centrifugation steps)
- More operator training required
- Cells have a more 3 dimensional distribution
- ThinPrep:
- Staining performed separately
- More collection training required to prevent unsatisfactory sample collection (cervical, vaginal and anal samples)
- Excess blood may be present
- Loss of background material needed for diagnosis
- General:
Case reports
- 32 year old woman with placental site nodule misinterpreted as a low grade squamous intraepithelial lesion (LSIL) (Case Rep Oncol 2020;13:1415)
- 57 year old woman with large cell neuroendocrine carcinoma of the cervix (Cytojournal 2017;14:28)
- 58 year old man with pleomorphic rhabdomyosarcoma arising in the anterior mediastinal tumor (Diagn Cytopathol 2017;45:333)
- 65 year old man with a history of Merkle cell carcinoma presents with pleural effusions post COVID-19 pneumonia (Diagn Cytopathol 2022;50:E37)
- 73 year old man with diffuse large B cell lymphoma with cryptococcal meningitis, ThinPrep of cerebrospinal fluid (CSF) (J Pathol Transl Med 2018;52:61)
Cytology description
- General features of LBP compared with conventional / direct smears (Diagn Cytopathol 2007;35:621, Diagn Cytopathol 2013;41:257):
- Fragmentation of cell groups (clusters, papillary structures)
- Smaller cells and nuclei
- More discohesive cells
- Loss of background material (necrosis, blood, inflammation, mucin, colloid)
- Retention of blood (in ThinPrep)
- Artifactual aggregation of lymphoid cells
- Prominent nucleoli in benign / reactive conditions
- Site specific features:
- Gynecological (cervix, vagina)
- Compared with conventional pap smears:
- Cells and their nuclei are smaller
- Potentially less hyperchromatic
- Nucleoli may be more visible
- Background elements may appear different or cling to cells (DeMay: The Art and Science of Cytopathology, 2nd Edition, 2011)
- Improved detection of LSIL (CIN 1) but no significant difference in detection of HSIL (CIN 2 / CIN 3) (Arch Pathol Lab Med 2003;127:200, JAMA 2009;302:1757, Obstet Gynecol 2008;111:167)
- Equivalent for detection of endocervical adenocarcinoma in situ and endometrial pathology (Cancer 2007;111:482, Diagn Cytopathol 2000;23:260)
- Decreased number of unsatisfactory specimens (Acta Cytol 1998;42:189)
- In 2020, FDA approval of primary HPV testing using Cobas 6800 / 8800 systems manufactured by Roche (Basel, Switzerland)
- Standardized reporting system and adequacy assessment: The Bethesda System for Reporting Cervical Cytology (Nayar: The Bethesda System for Reporting Cervical Cytology - Definitions, Criteria, and Explanatory Notes, 3rd Edition, 2015)
- Compared with conventional pap smears:
- Body cavity fluids (pleural, peritoneal, pericardial)
- In effusions, cells "round up"
- Blood is less likely to obscure cells
- High cellularity and discohesive pattern
- ThinPrep for pleural effusions: similar to cytospin Diff-Quik stained for distinguishing mesothelioma from adenocarcinoma (key features seen in both) (Diagn Cytopathol 2005;32:137)
- College of American Pathologist (CAP) interlaboratory comparison program: highest diagnostic concordance rate was seen with ThinPrep slides and the lowest was with conventional smears (Arch Pathol Lab Med 2018;142:53)
- Exception: pericardial fluids, which had the highest concordance rates with SurePath (Arch Pathol Lab Med 2018;142:53)
- Standardized reporting system: The International System for Serous Fluid Cytopathology (Chandra: The International System for Serous Fluid Cytopathology, 1st Edition, 2020)
- CSF
- General features of liquid based preparation compared with cytospin method (Cytopathology 2019;30:236)
- Clear background
- Enhanced cell enhancement
- Better nuclear details
- Higher cellularity per slide
- General features of liquid based preparation compared with cytospin method (Cytopathology 2019;30:236)
- GI (anal, bile duct, pancreas, liver)
- Anal pap test
- Liquid based preparation and conventional smears yield comparable results (Diagn Cytopathol 2014;42:840)
- Standardized reporting system: The Bethesda System for Reporting Cervical Cytology (Nayar: The Bethesda System for Reporting Cervical Cytology - Definitions, Criteria, and Explanatory Notes, 3rd Edition, 2015)
- Bile duct brushing
- Nuclear atypia-like conventional smear but with enhanced preservation of the nuclear features and greater 3 dimensional architecture (Arch Pathol Lab Med 2010;134:1116)
- Pancreatic FNA
- General features of liquid based preparation compared with conventional smears (Diagn Cytopathol 2004;30:71):
- Smaller cell clusters, more single cells
- Lack of background mucin - potential to under call mucinous neoplasms
- Nucleoli more conspicuous in benign cells - potential to overcall as atypical
- SurePath showed superior accuracy and sensitivity for malignant pancreatic lesions compared with conventional smears in the absence of rapid onsite evaluation (Endosc Int Open 2020;8:E1611)
- ThinPrep has a higher sensitivity in detection of pancreatic adenocarcinoma for samples obtained by endoscopic retrograde cholangiopancreatography (ERCP), with better preservation and cytological detail (Diagn Cytopathol 2005;32:70)
- Standardized reporting system: The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology (Pitman: The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology - Definitions, Criteria and Explanatory Notes, 2015 Edition, 2015)
- General features of liquid based preparation compared with conventional smears (Diagn Cytopathol 2004;30:71):
- Anal pap test
- GU (urine, bladder washing, ileal conduit)
- Morphologic features of high grade urothelial carcinoma (HGUC) cells appear to be similar in samples prepared using ThinPrep and cytospin (Thermo Fisher Scientific, Waltham, Massachusetts) methods (Cancer Cytopathol 2020;128:119)
- N/C ratio is preserved between both methods for HGUC; slightly more hyperchromasia in ThinPrep (Cancer Cytopathol 2020;128:119)
- The Paris System appears valid for ThinPrep (Cancer Cytopathol 2020;128:119)
- Standardized reporting system: The Paris System for Reporting Urinary Cytology (Rosenthal: The Paris System for Reporting Urinary Cytology, 1st Edition, 2016)
- Respiratory (bronchial wash, bronchial brush, bronchoalveolar lavage [BAL], sputum, FNA)
- Reduction of mucus, inflammation and blood
- Nuclear details are more preserved (Diagn Cytopathol 2008;36:167)
- Background may be clumped
- Morphology of microorganisms may be similar to conventional smears
- More uniform, dispersed cells
- Small cell carcinoma (Diagn Cytopathol 2003;29:8)
- Decreased, more subtle nuclear molding
- Less prominent nuclear smearing
- More noticeable amount of cytoplasm
- Diagnosis of lung tumors using ThinPrep may be improved compared with direct smears when evaluating small cell carcinoma (Surg Oncol 2006;15:173, Diagn Cytopathol 2003;29:8)
- Hematologic (lymph nodes)
- Compared with conventional smears liquid based cytology features:
- Lack of background material including necrosis, may be a disadvantage
- Comparison of SurePath and conventional smears showed no difference in cellularity or presence of monolayer sheets; better nuclear and cytoplasmic details were seen (J Cytol 2016;33:187)
- In thyroid lymphoma specimens, larger nuclei in > 10% lymphoid cells, elongated nuclei and swollen, naked nuclei helped to distinguish thyroid primary lymph from benign lymphoid cells (Acta Cytol 2018;62:93)
- Increased sensitivity for metastatic malignancy in cervical lymphadenopathy (Diagn Cytopathol 2016;44:169)
- Use of ThinPrep slides for grading of follicular lymphoma in FNA samples - counting centroblasts improved due to better fixation, improved nuclear morphology, clean background, random distribution of cells allowing of nonbiased counting (Cancer 2006;108:319)
- Compared with conventional smears liquid based cytology features:
- Head and neck (thyroid, salivary gland)
- Thyroid
- Compared with conventional smears:
- Similar accuracy for thyroid neoplasms (Am J Clin Pathol 1995;104:150, Cancer 1998;84:17)
- Superior nuclear features
- Follicles and papillary fragments may be more fragmented; follicular cells usually present in groups, single cells and sheets
- ThinPrep may have lower accuracy for detecting chronic lymphocytic thyroiditis (62% compared with 92% on direct smear) (Cancer 1998;84:17)
- Less detection of diffuse or watery colloid (Am J Clin Pathol 1995;104:150, Cancer 2001;93:179)
- Watery colloid may be present as tissue paper-like material (Diagn Cytopathol 2004;30:7)
- Standardized reporting system: The Bethesda System for Reporting Thyroid Cytopathology (Ali: The Bethesda System for Reporting Thyroid Cytopathology - Definitions, Criteria, and Explanatory Notes, 2nd Edition, 2018)
- Compared with conventional smears:
- Salivary gland
- ThinPrep compared with conventional smears (Acta Cytol 2014;58:552):
- Normal acinar groups: intact cytoplasm, no bare nuclei
- Minimal to no cellular debris (extracellular mucin)
- Difficult to evaluate nuclear detail of different lymphocytes due to smaller cell size
- Smaller, less complex groups of epithelial cells
- More background cells (epithelial and myoepithelial)
- Decrease quantity and quality of matrix / stroma - loose stroma appears as porous, moth eaten leaves
- ThinPrep compared with conventional smears (Acta Cytol 2014;58:552):
- Standardized reporting system: The Milan System for Reporting Salivary Gland Cytopathology (Faquin: The Milan System for Reporting Salivary Gland Cytopathology, 1st Edition, 2018)
- Thyroid
- Breast
- Conventional smears limited by drying artifact while ThinPrep aspirates of carcinomas show better nuclear detail and greater cellularity (Diagn Cytopathol 1999;21:137)
- ThinPrep of benign masses show greater epithelial cellularity, better nuclear detail and more specimens with myoepithelial cells (Diagn Cytopathol 1999;21:137)
- Pitfalls reported in ThinPrep compared with conventional smears (Diagn Cytopathol 2017;45:655):
- Fibroadenoma: bipolar cells can appear singly dispersed with preserved cytoplasm, resembling carcinoma
- Solid papillary carcinoma: singly dispersed cells with plasmacytoid features that mimic lobular carcinoma
- More prominent nucleoli and cytological atypia - potential for overcall
- Clumping of epithelioid histiocytes that may be interpreted as atypical
- Gynecological (cervix, vagina)
Cytology images
Molecular / cytogenetics description
- Urine cytology:
- See Cytology-general, normal findings & biomarker testing
- Residual sample from ThinPrep utilized for 69 gene, urothelial carcinoma specific next generation sequencing panel (Cancer Cytopathol 2021;129:537)
- Almost every mutation detected in the cytology specimen was present in the formalin fixed paraffin embedded (FFPE) bladder tumor sample (Cancer Cytopathol 2021;129:537)
- Biliary duct brushing:
- Combination of FISH probes 1q21, 7p12, 8q24 and 9p21 useful for the identification of biliary tract malignancy, with a significantly higher level of sensitivity (64.7%) than UroVysion probes (Gastroenterology 2015;149:1813)
- Samples collected in PreservCyt or CytoLyt
- Combination of FISH probes 1q21, 7p12, 8q24 and 9p21 useful for the identification of biliary tract malignancy, with a significantly higher level of sensitivity (64.7%) than UroVysion probes (Gastroenterology 2015;149:1813)
- Thyroid FNA:
- See Molecular testing in FNA
- Using 6 groups of 10+ follicular cells as a cutoff, nearly 97% of thyroid FNA samples collected in ThinPrep vial contained enough DNA for BRAF mutational assay (200 ng of DNA used as minimum criteria) (Cancer Cytopathol 2014;122:114)
- 18 gene next generation sequencing panel successfully performed from residual ThinPrep sample (J Cancer 2020;11:7276)
- Lower negative predictive value but a higher positive predictive value, compared to ThyroSeq v2 testing (J Cancer 2020;11:7276)
- Cellularity and storage time may affect the quantity and quality of nucleic acid extracted from thyroid FNA specimens collected in CytoLyt (Cancer Cytopathol 2020;128:656)
Sample pathology report
- Pleural fluid, thoracentesis:
- Specimen adequacy:
- Satisfactory for evaluation
- Microscopic interpretation:
- Malignant cells present
- Metastatic adenocarcinoma
- Cell block shows similar features
- Specimen adequacy:
Additional references
Board review style question #1
Board review style answer #1
E. Reduction in background blood and inflammation (Demay: The Art & Science of Cytopathology, 2nd Edition, 2011)
Comment Here
Reference: Liquid based cytology
Comment Here
Reference: Liquid based cytology
Board review style question #2
A pleural effusion sample has been entirely placed in a ThinPrep vial and processed. A single ThinPrep slide and cell block has been created. The clinical information received with the specimen is "rule out lymphoma". Which known limitation of liquid based preparation applies in this scenario?
- FISH studies cannot be performed
- Flow cytometry cannot be performed
- Immunohistochemistry cannot be performed
- Microbial culture cannot be performed
- Molecular testing cannot be performed
Board review style answer #2
B. Flow cytometry cannot be performed. Since the specimen was entirely placed in the methanol based ThinPrep vial, flow cytometry and microbial culture tests cannot be performed. Flow cytometry may be required for diagnosis when the clinical suspicion includes lymphoma. Immunohistochemistry, molecular testing and FISH studies can all be potentially performed on the cell block. In some instances, molecular and FISH testing can be performed on residual sample collected in ThinPrep vials.
Comment Here
Reference: Liquid based cytology
Comment Here
Reference: Liquid based cytology
Malignant
Table of Contents
Definition / general | Essential features | CPT coding | Sites | Diagrams / tables | Clinical features | Laboratory | Radiology description | Radiology images | Case reports | Cytology description | Cytology images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- This category is to be used only when a specimen has sufficient quality and quantity of cellular material that demonstrates unequivocal cytopathological features of malignancy (Acta Cytol 2023;67:80)
- Risk of malignancy with this category varies slightly based on the sampling method but generally is close to 100% (Acta Cytol 2023;67:80)
Essential features
- This category includes both primary and metastatic tumors of the lung
- Precise cytologic and molecular characterization of tumors is essential due to advancements in personalized treatment of advanced non-small cell lung cancer (NSCLC)
- All lung adenocarcinomas should be tested for mutations or rearrangements in EGFR, ALK, ROS1 and BRAF to guide treatment; additionally, IHC for PDL1 is standard of care in NSCLC to evaluate potential treatment with immune checkpoint inhibitors
- If there is insufficient material for ancillary testing or no correlation to clinical or imaging findings, then repeat fine needle aspiration biopsy (FNAB) with or without core needle biopsy should be considered (Acta Cytol 2023;67:80)
CPT coding
- 88104: cytopathology, fluids, washings or brushings, except cervical or vaginal
- 88108: sputum cytology smears
- 88112: concentrated cytology preparations (e.g., ThinPrep)
- 88173: diagnosis for a fine needle aspiration sample
- 88305: cell block, H&E
- 88342: immunohistochemical stain (qualitative), first stain
- 88341: immunohistochemical stain (qualitative), second and subsequent stains
Sites
- Lungs
- Pleura
Diagrams / tables
None
Clinical features
- This category includes both primary and metastatic tumors of the lung
- Whenever possible, the neoplasm should be subclassified based on cytopathologic features, immunohistochemistry (IHC) molecular testing and flow cytometry
- However, it is recommended to limit use of IHC for diagnosis and subtyping to preserve cells for molecular analysis (J Thorac Oncol 2011;6:244)
- Small cell carcinoma and non-small cell lung cancers (NSCLC) can be differentiated with accuracy > 70% by cytomorphology alone (Acta Cytol 2023;67:80)
- For cases of non-small cell carcinoma (NSCC) that require immunocytochemistry (ICC) to make a definitive diagnosis, they are to be reported as: NSCC, favor adenocarcinoma / squamous cell carcinoma (Acta Cytol 2023;67:80)
- In poorly differentiated carcinomas where NSCC cannot be further classified by cytomorphology or ICC, a final report of NSCC, NOS can be used (Acta Cytol 2023;67:80)
- Precise cytologic and molecular characterization of tumors is essential due to advancements in personalized treatment of advanced non-small cell lung cancer
- All lung adenocarcinomas should be tested for mutations or rearrangements in EGFR, ALK, ROS1 and BRAF to guide treatment
- Additionally, IHC for PDL1 is standard of care in NSCLC to evaluate potential treatment with immune checkpoint inhibitors (J Thorac Oncol 2018;13:323, Drugs Context 2023;12:2022, Transl Lung Cancer Res 2020;9:898, Front Med (Lausanne) 2021;8:668612)
- Overall cellularity of specimens and the proportion of tumor cells varies by specimen type as well as type and location of neoplasm
- If there is insufficient material for ancillary testing or no correlation to clinical or imaging findings, then repeat FNAB with or without core needle biopsy should be considered (Acta Cytol 2023;67:80)
Laboratory
- IHC can be used to confirm or subtype a primary lung tumor and differentiate from metastases; however, IHC use for subtyping should be minimized whenever possible to preserve samples for molecular analyses
- Use of TTF1 and p40 is considered the gold standard for subtyping NSCLC; napsin A and CK5/6 can be used as second markers for adenocarcinoma and squamous cell carcinoma (SCC), respectively (J Thorac Oncol 2019;14:377)
- p40 must stain > 50% of tumor cell nuclei to be called positive (J Thorac Oncol 2019;14:377)
- Focal TTF1 staining is enough to be called positive (J Thorac Oncol 2019;14:377)
- IHC for PDL1 is standard of care in NSCLC to evaluate potential treatment with immune checkpoint inhibitors (Transl Lung Cancer Res 2020;9:898, Front Med (Lausanne) 2021;8:668612)
- In tumors with an adenocarcinoma component, IHC may be used as an alternative to FISH for ALK testing
- IHC may be used for screening prior to molecular testing for ROS1 but is not recommended for EGFR (J Thorac Oncol 2018;13:323)
- Panel of synaptophysin, chromogranin and CD56 is the best combination to differentiate suspected neuroendocrine tumors (NETs) from other NSCLC (J Thorac Oncol 2019;14:377)
- TTF1 is also frequently positive in primary lung neuroendocrine tumors (Am J Clin Pathol 2014;142:320)
- Ki67 is used primarily to distinguish low grade NETs (Ki67: 1 - 25%) from small cell lung carcinoma (Ki67: 70 - 100%) and large cell neuroendocrine carcinomas (Ki67: 25 - 80%) (Virchows Arch 2021;478:5)
Radiology description
- Radiologic features of pulmonary nodules concerning for malignancy include (Curr Radiopharm 2020;13:238)
- Irregular, spiculated margins
- Size > 3 cm; growth over time
- Multiple nodules
- Lymphadenopathy in mediastinum or hilum
- Thickening or nodularity of the pleura adjacent to mass
- Air bronchograms suggesting an obstructing lesion
- Cavitation, seen as air - fluid level, which suggests SCC
- Small cell carcinoma and SCC tend to be located centrally, while adenocarcinoma and large cell carcinoma tend to be peripheral
Case reports
- 45 year old man with acute myeloid leukemia diagnosed by thoracentesis of a pleural effusion (Diagn Cytopathol 2021;49:E316)
- 46 year old man with primary Ewing sarcoma of the lung diagnosed on cytology (Diagn Cytopathol 2020;48:1098)
- 50 year old woman with combined small cell carcinoma with giant cell carcinoma component (Oncol Lett 2018;15:1907)
- 70 year old woman with synchronous diffuse large B cell lymphoma and lung adenocarcinoma diagnosed by effusion cytology (Respir Med Case Rep 2023;43:101852)
- 74 year old woman with primary malignant melanoma of the lung (Diagn Pathol 2020;15:11)
Cytology description
- Morphologic features based on the WHO Reporting System for Lung Cytopathology (IAC-IARC-WHO Joint Editorial Board: WHO Reporting System for Lung Cytopathology, 1st Edition, 2023)
- Adenocarcinoma
- Cells may be arranged in flat sheets, papillae with fibrovascular cores, glands, acini and micropapillae
- Tumor cells are cuboidal to columnar, with large, round to oval, eccentric nuclei with fine to coarse chromatin
- Poorly differentiated adenocarcinomas show more marked nuclear pleomorphism
- Nuclear grooves, intranuclear pseudoinclusions and one or more prominent nucleoli are commonly seen
- Cells typically have moderate to abundant amount of granular to vacuolated cytoplasm that is located toward the center of glandular structures
- Vacuoles are typically fine but may be large and contain mucin droplets
- Other common findings
- Intra and extracellular mucin
- Psammoma bodies
- Necrotic background, especially in high grade carcinomas
- Features consistent with invasive mucinous carcinoma
- Flat sheets of cells filled with mucin containing vacuoles (may be single vacuole or many small vacuoles)
- Irregular arrangement of bland nuclei with fine chromatin within sheets of cells
- Mucinous carcinoma may be negative for TTF1 but a mucin stain may aid in identifying intracellular mucin (J Thorac Oncol 2022;17:362)
- Squamous cell carcinoma (SCC)
- Keratin is eosinophilic on Pap and robin's egg blue on Giemsa; it may be present intracellularly or as keratin pearls in cohesive tissue fragments
- Keratinized cells often have pyknotic nuclei
- Cells may be arranged both singly and in multilayered sheets
- Well defined cell borders with dense cytoplasm and intercellular bridges
- Polygonal, elongated or bizarrely shaped cells; tadpole or spindle shaped cells may be present
- Central, large, hyperchromatic nuclei with irregular nuclear membranes and clumped chromatin
- Prominent nucleoli are typically absent
- Extensive background inflammation and necrosis is common
- Basaloid SCC
- Predominantly larger cell groups with variable amount of palisading at their periphery; pseudoglandular spaces may be seen
- Cells often have high N:C ratio, dense, granular chromatin and a scant rim of cytoplasm
- Focal nuclear molding may be present
- Keratin is eosinophilic on Pap and robin's egg blue on Giemsa; it may be present intracellularly or as keratin pearls in cohesive tissue fragments
- Low grade neuroendocrine tumor (NET) / carcinoid tumor
- Highly cellular specimens
- Cohesive groups of cells that may branch around thin fibrovascular strands or form rosette-like structures
- Cells are small and can be round / oval, plasmacytoid or spindled
- Nuclei are monotonous, round / oval shaped with salt and pepper chromatin and smooth nuclear outlines
- Cytoplasm: scant to moderate amount of finely granular cytoplasm
- Granules are eosinophilic on H&E, grayish blue on Pap or red to magenta on Giemsa
- Nuclear grooves, binucleation, nuclear pseudoinclusions and naked nuclei may be present
- Necrosis is typically absent
- Small cell carcinoma
- Highly cellular aspirates of loosely arranged and discohesive cells
- Small, blue cells with scant cytoplasm
- Cell size is usually < 3 times the size of a small lymphocyte
- Angulated nuclei that display nuclear molding in larger sheets of cells (best appreciated on Giemsa stain)
- Chromatin smearing is very frequent and caused by crush artifact but preserved cells have salt and pepper chromatin (best appreciated on Pap)
- Very scant cytoplasm
- Paranuclear blue bodies (Giemsa) are a distinctive feature of SCLC (Cancers (Basel) 2021;13:820)
- Mitotic rate is usually very high (10 mitoses per 10 high power fields)
- Necrosis is frequently extensive and in large zones
- Can be combined with a NSCLC component
- Large cell neuroendocrine carcinoma
- Moderate to highly cellular specimens
- Small, loosely cohesive tissue fragments in background of scattered single cells
- Pseudorosette architecture may be present
- Cells are intermediate to large in size (> 3 times the size of a small lymphocyte)
- Nuclei are enlarged with irregular nuclear membrane; molding may be present to a lesser degree than small cell carcinoma
- Moderate to abundant cytoplasm
- Chromatin is typically granular; chromatin smearing is common
- May see perinucleolar clearing in Pap
- Prominent background of necrosis
- Frequent mitoses
- Difficult to diagnose on cytology without cell blocks that demonstrate neuroendocrine morphology and architecture (i.e., nesting, palisading, rosettes)
- Other tumor types in this category
- Salivary gland type carcinomas
- Adenosquamous carcinoma
- Pleomorphic carcinoma
- Pulmonary blastoma
- Carcinosarcoma
- NUT carcinoma
- Thoracic SMARCA4 deficient undifferentiated tumor
- Lymphoproliferative malignancies
- Mesenchymal tumors
- Mesothelioma
- Angiosarcoma
- Metastatic malignancies
Cytology images
Contributed by Reima El Naili, M.D.
Molecular / cytogenetics description
- All lung adenocarcinomas should be tested for mutations or rearrangements in EGFR, ALK, ROS1 and BRAF to guide treatment (J Thorac Oncol 2018;13:323, Drugs Context 2023;12:2022)
- MET, RET, ERBB2 (HER2) and KRAS should also be included in any expanded molecular panel for lung adenocarcinomas (J Thorac Oncol 2018;13:323)
- Invasive mucinous carcinomas frequently have KRAS mutations or alterations in NRG1 or ERBB2 (J Thorac Oncol 2022;17:362)
- For primary lung cancers without an adenocarcinoma component, molecular testing may be conducted if the clinical presentation suggests a heightened likelihood of an oncogenic driver (J Thorac Oncol 2018;13:323)
- Small cell carcinoma
- 95% of tumors have loss of RB1, 91% have 3p deletions and 65% have TP53 mutations (Cancers (Basel) 2021;13:820)
- It has recently been suggested to subtype small cell lung carcinoma (SCLC) by mRNA expression of ASCL1, NEUROD1, POU2F3 and YAP1 (J Thorac Oncol 2020;15:1823)
Sample pathology report
- Lung, fine needle aspiration (FNA):
- Specimen adequacy: adequate for evaluation
- Interpretation: malignant
- Non-small cell carcinoma, favor adenocarcinoma (see comment)
- Comment: The cytology specimen demonstrates malignant cells consistent with non-small cell lung carcinoma (NSCLC). The cells exhibit features indicative of poorly differentiated adenocarcinoma, including enlarged nuclei, prominent nucleoli, high N:C ratio and intracellular mucin. Immunohistochemical staining for TTF1 is positive and staining for p40 is negative, supporting a diagnosis of adenocarcinoma. Additional studies for molecular profiling will be performed for targeted therapy selection. Clinical correlation and further evaluation with imaging studies are advised for staging and treatment planning.
- Lung, fine needle aspiration (FNA):
- Specimen adequacy: adequate for evaluation
- Interpretation: malignant
- Small cell carcinoma (see comment)
- Comment: The cellular features seen are consistent with small cell carcinoma and include small to intermediate sized cells with scant cytoplasm, nuclear molding, finely granular chromatin and frequent mitotic figures. By immunohistochemistry, the tumor cells are positive for TTF1, synaptophysin, chromogranin and CD56, while negative for CD45 and p40. Ki67 stain shows a proliferation index of > 90% within the tumor cell nuclei. The morphology and immunoprofile supports the diagnosis.
Differential diagnosis
- All from WHO Reporting System for Lung Cytopathology (IAC-IARC-WHO Joint Editorial Board: WHO Reporting System for Lung Cytopathology, 1st Edition, 2023)
- For adenocarcinoma:
- Adenocarcinoma cannot be distinguished from precursor lesions such as carcinoma in situ or atypical adenomatous hyperplasia by cytology alone
- NSCLC, NOS
- Metastatic adenocarcinoma
- Adenocarcinoma variants, including fetal, colloid and enteric adenocarcinoma
- Sclerosing pneumocytoma
- Acute or granulomatous inflammation
- Diffuse alveolar damage
- Pulmonary infarcts
- Poorly differentiated adenocarcinoma may be difficult to distinguish from poorly differentiated SCC
- For SCC:
- NSCLC, NOS
- Metastatic SCC:
- Well differentiated metastatic SCC is indistinguishable from primary lung SCC
- Correlation with clinical and imaging findings, as well as HPV testing may help differentiate
- Reactive, metaplastic and dysplastic squamous cells
- Poorly differentiated SCC may be difficult to distinguish from poorly differentiated adenocarcinoma
- For basaloid SCC:
- For low grade NET:
- Distinction between typical and atypical carcinoid is not possible in most cases and should be deferred to surgical resection (Diagn Cytopathol 2013;41:689, Acta Cytol 1995;39:1152)
- Small cell neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Adenocarcinoma
- Lymphoma
- Metastatic carcinoma
- Melanoma
- Adenoid cystic carcinoma
- Paraganglioma
- For small cell lung carcinoma:
- Other neuroendocrine neoplasms
- Basaloid SCC
- Small round cell sarcomas
- Lymphomas
- NUT carcinoma
- SMARCA4 deficient thoracic tumors
- Merkel cell carcinoma
- For large cell neuroendocrine carcinoma:
Additional references
None
Board review style question #1
A 65 year old woman with an extensive smoking history is found to have a 4.2 cm right lower lobe lung mass. Fine needle aspiration of the mass is shown above. What set of genes should always be interrogated for mutations or rearrangements in this type of tumor in order to guide treatment?
- EGFR, ALK, ROS1, BRAF
- EGFR, MET, ALK, ASCL1
- ERBB2 (HER2), KRAS, NEUROD1, BRAF
- MET, RET, ERBB2 (HER2), YAP1
Board review style answer #1
A. EGFR, ALK, ROS1, BRAF. The cytology specimen demonstrates adenocarcinoma and all adenocarcinomas should be tested for mutations or rearrangements in EGFR, ALK, ROS1 and BRAF to predict response to treatment with tyrosine kinase and BRAF inhibitors (although it may be reasonable to perform sequential testing on EGFR and ALK, followed by ROS1 testing). Additionally, IHC for PDL1 is standard of care in NSCLC to evaluate potential treatment with immune checkpoint inhibitors. Answers B - D are incorrect because it is appropriate to include MET, RET, ERBB2 (HER2) and KRAS as part of larger panels performed initially or when routine testing is negative but they are not currently required. ASCL1, NEUROD1, POU2F3 and YAP1 are genes involved in SCLC subtyping (J Thorac Oncol 2018;13:323, Drugs Context 2023;12:2022, J Thorac Oncol 2020;15:1823).
Comment Here
Reference: Cytopathology - Malignant
Comment Here
Reference: Cytopathology - Malignant
Board review style question #2
Board review style answer #2
C. TTF1-, napsin A-, p40+. The radiographic description and cytology specimen is consistent with squamous cell carcinoma, which typically stains positive for p40. Answers A, B and D are incorrect because TTF1 and napsin A are typically positive in adenocarcinomas. Of note, TTF1 can also positive in tumors of neuroendocrine lineage and may also stain background benign bronchial cells.
Comment Here
Reference: Cytopathology - Malignant
Comment Here
Reference: Cytopathology - Malignant
Board review style question #3
Fine needle aspiration of a central lung mass in a patient with an extensive smoking history is diagnosed as small cell lung carcinoma. What feature likely aided this diagnosis?
- Absence of necrosis
- Ki67 of 35%
- Light blue cytoplasmic inclusions
- Round nuclei with prominent nucleoli
Board review style answer #3
C. Light blue cytoplasmic inclusions describe paranuclear blue bodies, a distinctive finding of small cell carcinoma. Answers A and D are incorrect because small cell lung carcinomas typically have angulated nuclei that demonstrate nuclear molding and typically have a background of prominent necrosis. Answer B is incorrect because while not a diagnostic criteria, small cell carcinomas typically have a Ki67 of 70 - 100%.
Comment Here
Reference: Cytopathology - Malignant
Comment Here
Reference: Cytopathology - Malignant
Malignant (pending)
[Pending]
Molecular cytopathology (pending)
[Pending]
Nondiagnostic
Table of Contents
Definition / general | Essential features | CPT coding | Sites | Radiology description | Case reports | Cytology description | Cytology images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Nondiagnostic / inadequate / insufficient specimen lacks sufficient material in quantity or quality for a reliable diagnosis
- They should lack any atypical cell / microorganism / acellular matrix that guides to a specific malignancy, benign diagnosis and any infectious cause (Diagn Cytopathol 2016;44:399)
- Risk of malignancy is 40% for a nondiagnostic specimen (Layfield: The Papanicolaou Society of Cytopathology System for Reporting Respiratory Cytology, 1st Edition, 2019)
- The term nondiagnostic is preferred to its synonymous terms: insufficient and inadequate
Essential features
- Lung cytopathology involves the assessment of specimens that can be categorized into
- Exfoliative cytology (sputum, bronchial brushing, bronchial wash and bronchoalveolar lavage [BAL])
- Fine needle aspiration (FNA) cytology (percutaneous transthoracic and endobronchial ultrasound guided [EBUS])
- Nondiagnostic result is reported when no malignant cells are seen or a specific benign diagnosis could not be made
- Most common causes are insufficient cellularity, cellular degeneration, poor preservation of cells and hemorrhagic samples (Layfield: The Papanicolaou Society of Cytopathology System for Reporting Respiratory Cytology, 1st Edition, 2019)
- Bronchoalveolar lavage with fewer than 10 alveolar macrophages per 2 mm² using a high power field diameter of 0.5 mm, is considered inadequate (WHO Joint Editorial Board: WHO Reporting System for Lung Cytopathology, 1st Edition, 2023)
| Adequacy criteria in respiratory cytology specimens | ||
| Sample type | Normal cellular components | Adequacy criteria |
| FNA |
|
|
| Bronchoalveolar lavage |
|
|
| Bronchial wash |
|
|
| Bronchial brush |
|
|
| Sputum |
|
|
CPT coding
Sites
- Lung
Radiology description
- Usually a mass-like lesion or ground glass opacity is seen on imaging
Case reports
- 31 year old man with esophageal bronchogenic cyst (Medicine (Baltimore) 2016;95:e3111)
- Woman in her 50s presented with blood tinged sputum (Cytojournal 2019;16:23)
- 72 year old man with a subcarinal lesion (Cytojournal 2023;20:1)
- 73 year old man who received repeated chemotherapy for angioimmunoblastic T cell lymphoma (Respirol Case Rep 2022;10:e0924)
- 76 year old woman who had a demonstrable solitary pulmonary nodule and 81 year old woman with a solitary nodule in the left lower lobe of the lung (BMC Pulm Med 2003;3:2)
Cytology description
| Cell type | Location | Cytologic features |
| Alveolar macrophages | Alveoli |
|
| Type II pneumocytes | Alveoli |
|
| Basal or reserve cells | Bronchi and bronchioles |
|
| Ciliated cells | Columnar cells (bronchi) and cuboidal cells (bronchioles) |
|
- Common causes of nondiagnostic sample include a mucopurulent exudate; cellular changes due to degeneration; or presence of laboratory artifacts (J Cytol 2013;30:241)
- Fine needle aspiration cytology
- Columnar cells with terminal bar and cilia are a common contamination in an EBUS FNA
- EBUS FNA should include reactive bronchial ciliated columnar cells or > 40 lymphocytes in the area of highest cellularity (Am J Clin Pathol 2008;130:434)
- Hyperchromatic crowded groups of basal / reserve cells are a potential pitfall in interpreting a specimen as atypical or suspicious
- Crush artifact of lymphocytes is a potential pitfall for small cell carcinoma
- Presence of a cytopathologist for rapid on site evaluation and expertise of the interventional radiologist or clinician has been shown to reduce nondiagnostic FNA samples (Ann Thorac Surg 2008;86:1104, Rev Port Pneumol (2006) 2015;21:253)
- Bronchial brushings usually show fragments of epithelial cells or tumor cells since the specimen is obtained under direct vision
- Absence of the tumor cells or poor fixation and air drying of the cells leading to swelling of nuclei and loss of chromatin detail is considered nondiagnostic
- A count of < 10 alveolar macrophages per 2 mm² using a high power field diameter of 0.5 mm is generally considered inadequate for a bronchoalveolar lavage
- Adequacy criteria in sputum samples is disputable; however, a high number of alveolar macrophages (no numerical cut off point has been defined), along with ciliated columnar bronchial cells and a recommended sufficient volume to produce at least 2 smears is considered adequate
- Several studies showed a decreased sensitivity of sputum sampling in the detection of diagnostic cells for patients affected by peripherally located lung masses (Diagn Cytopathol 2008;36:167)
Cytology images
Videos
WHO reporting system for lung cytopathology
Adequacy criteria in EBUS FNA
Overview of lung cytopathology
Sample pathology report
- Lung, left, lower lobe, mass (2.0 cm, solid), endobronchial ultrasound guided fine needle aspiration (with cell block):
- Specimen adequacy:
- Inadequate
- Cytopathologic interpretation / diagnosis:
- Nondiagnostic (see comment)
- Sample comments:
- Hemodiluted and hypocellular aspirates predominantly with bronchial mucosal contamination
- Tissue fragments are entrapped in blood clot and fibrin precluding cytologic evaluation
- Hypocellular specimen showing extensive crush artifact precluding cytologic evaluation of material present
- H&E stained sections of the cell block show few poorly preserved epithelial cells
- Specimen adequacy:
- Right lower lobe, bronchoalveolar lavage:
- Nondiagnostic: inadequate for evaluation due to nonrepresentative scant bronchial epithelial cells and absence of alveolar macrophages in bronchoalveolar lavage
- Nondiagnostic: unsatisfactory for evaluation due to lack of pulmonary macrophages and poor preservation of cells
- Per IAC-IARC-WHO Cytopathology Reporting Systems, the report should always state "correlation with clinical and imaging findings is required"
Differential diagnosis
- Bronchogenic cyst:
- Shows predominance of degenerated cellular debris admixed with scattered fragments of cells showing ciliary tufts
- Carcinoid:
- Tumor cells are arranged in acini or pseudorosette structures, dispersed individual cells with monomorphic appearance
- Tumor cells reveal salt and pepper type chromatin, vesicular nuclei and inconspicuous or small nucleoli
- Pulmonary chondroma:
- Shows predominance of mature cartilage
- Clinicopathologic and radiology correlation is essential for accurate assessment of the specimen
Additional references
Board review style question #1
Which of the following, when present in an EBUS FNA from a lung mass, is considered adequate?
- Aspergillus hyphae
- Goblet cells and alveolar macrophages
- Mucin
- Tufts of columnar epithelium with terminal bar and cilia
- Type 2 pneumocytes without atypia
Board review style answer #1
A. Aspergillus hyphae. Aspergilloma is one of the causes of cavitary mass-like lesion in the lung. The presence of Aspergillus organisms explains the mass in the lung and is considered adequate for evaluation.
Answer B is incorrect because goblet cells and alveolar macrophages are part of the normal respiratory epithelial lining of the airways. Finding these in an EBUS FNA sample would likely indicate contamination with normal respiratory tract cells. These cells are not indicative of the characteristics of a lung mass and their presence would not help in evaluating the mass itself.
Answer C is incorrect because the presence of mucin in an EBUS FNA sample is generally not sufficient for evaluating a lung mass. Mucin can be present in various lung conditions but its presence alone does not provide specific information about the nature of the mass, whether it's benign or malignant or what type of mass it is.
Answer D is incorrect because in an EBUS FNA procedure, the goal is to obtain a sample that can be used to evaluate the nature and characteristics of a lung mass. The presence of respiratory epithelium, such as tufts of columnar epithelium with a terminal bar and cilia, is valuable because it indicates that the sample is from the lining of the airways. However, it does not explain the presence of a mass-like lesion.
Answer E is incorrect because type 2 pneumocytes are a type of lung cell responsible for producing surfactant and are not typically associated with the evaluation of lung masses. Finding these cells without atypia would suggest the presence of normal lung tissue but it doesn't provide information about the characteristics or pathology of the mass itself.
Comment Here
Reference: Lung - Nondiagnostic
Comment Here
Reference: Lung - Nondiagnostic
Board review style question #2
Presence of which of the following cells in a percutaneous transthoracic FNA from a well circumscribed lung mass is a reason to consider the sample adequate?
- Alveolar macrophages
- Degenerated epithelial cells
- Neutrophils
- Red blood cells
- Superficial squamous cells
Board review style answer #2
C. Neutrophils. Presence of increased number of neutrophils in a mass lesion may indicate an abscess. Neutrophils can be present in various lung conditions, and more clinical information is essential to make a definitive diagnosis.
Answer A is incorrect because alveolar macrophages are normal components of lung tissue and are not indicative of the nature of the lung mass. Their presence doesn't provide specific information about the mass.
Answer B is incorrect because in a percutaneous transthoracic FNA, the goal is to obtain a sample that can help diagnose and characterize the nature of a lung mass. While the presence of degenerated epithelial cells may indicate that the sample may be from a lung mass, a definitive diagnosis cannot be made just by the presence of degenerated epithelial cells.
Answer D is incorrect because the presence of red blood cells may suggest bleeding within the mass but doesn't, on its own, provide adequate information for characterizing the nature of the lung mass.
Answer E is incorrect because superficial squamous cells are typically part of the normal respiratory tract lining and may indicate contamination with normal cells rather than providing information about the nature of the mass.
Comment Here
Reference: Lung - Nondiagnostic
Comment Here
Reference: Lung - Nondiagnostic
Board review style question #3
Board review style answer #3
C. Nondiagnostic. It is a nondiagnostic specimen due to the lack of alveolar macrophages. Other cells and material in this specimen, such as a few chronic inflammatory and mucin, may represent contamination.
Answer A is incorrect because this option is not likely as the specimen does not show the presence of atypical cells, which would typically be associated with malignancy.
Answer B is incorrect because while the specimen may not contain clear evidence of malignancy, it cannot be confidently classified as negative for malignancy due to the lack of alveolar macrophages and the nondiagnostic nature of the sample.
Answer D is incorrect because there is insufficient evidence in the specimen to conclude that it is positive for malignancy. The absence of alveolar macrophages and a clear lack of cancerous cells makes this an unlikely interpretation.
Answer E is incorrect because similar to the other options, this interpretation is not supported due to the lack of alveolar macrophages and the nondiagnostic nature of the specimen.
In summary, the provided picture of bronchioalveolar lavage (BAL) suggests that the sample is nondiagnostic due to the absence of alveolar macrophages and any atypical cell. Further testing or sampling may be needed to establish a definitive diagnosis.
Comment Here
Reference: Lung - Nondiagnostic
Comment Here
Reference: Lung - Nondiagnostic
Overview (pending)
[Pending]
Rapid on site evaluation (ROSE)
Table of Contents
Definition / general | Essential features | CPT coding | Sites | Diagrams / tables | Laboratory | Radiology description | Case reports | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Rapid on site evaluation (ROSE) is a cytopathologic diagnostic adequacy assessment of individual biopsy passes performed during a biopsy procedure in order to optimize the procedure itself and inform subsequent patient management
Essential features
- ROSE may be performed by a cytopathologist, a pathology trainee, cytotechnologist or clinical physician with sufficient training (Cytopathology 2020;31:303)
- A central goal of ROSE is to optimize the quantity of diagnostic material procured during a biopsy procedure including triage in anticipation of any ancillary studies (e.g., flow cytometry, molecular testing, etc.)
- ROSE specimens consist of aspirate smears from fine needle aspiration (FNA) biopsies or touch preparation slides from needle core (NC) biopsies (see smear making and special techniques for recovering and dividing aspirate material)
- ROSE diagnostic statements are performed for each examined biopsy pass and can be brief and categorical (i.e., adequate or nondiagnostic) or detailed and specific (i.e., favor squamous cell carcinoma), depending on the context
- Utility of ROSE: reduce the number biopsy passes, numbers of procedures and cost to obtain a final diagnosis
CPT coding
- Note 1: ROSE CPT codes for immediate adequacy interpretations may only be billed if the diagnosis is made by an attending pathologist
- Note 2: each different anatomical site for which ROSE is performed during biopsy is billed separately
- 88172 - aspirate smear, first pass
- 88177 - aspirate smear, all subsequent passes
- 88333 - touch preparation, first pass
- 88334 - touch preparation, all subsequent passes
Sites
- ROSE may be performed during cytology biopsy procedures from any anatomic site
- Content of a ROSE statement may be dependent on the institution, anatomic site, presumptive diagnosis, biopsy operator and pathologist
Diagrams / tables
Laboratory
- ROSE can optimize the performance of biopsy procedures benefitting the patient in 3 general capacities:
- Reducing the number of passes made during a biopsy procedure and the overall number of procedures necessary to obtain a treatable diagnosis (Cancer Cytopathol 2013;121:518)
- Informing the efficient disposition of cellular material for subsequent diagnostic and ancillary testing, including obtaining sufficient material necessary for anticipated diagnostic immunohistochemical studies, flow cytometry and theragnostic molecular testing (Cancer Cytopathol 2013;121:544)
- Performance of ROSE can reduce the cost to obtain a diagnosis depending on a number of factors, including the availability of equipment, laboratory staff and procedure frequency (PLoS One 2015;10:e0135466)
- Utility of ROSE may be limited in some circumstances:
- Cysts: only small volumes of cyst fluid may be analyzed generally obviating the utility of ROSE (Cibas: Cytology - Diagnostic Principles and Clinical Correlates, 5th Edition, 2021)
- Solid pancreatic lesions sampled via needle core biopsy (Surg Endosc 2017;31:225)
- Core depletion: depletion of diagnostic material from needle cores due to improper touch preparation technique (J Am Soc Cytopathol 2020;9:322)
- Pathology ancillary studies prompted by ROSE may include:
- Recording formalin fixation time for lesions of suspected breast origin (Am J Clin Pathol 2018;149:275)
- Molecular testing (Curr Oncol 2017;24:103, Acta Cytol 2016;60:372)
- Microbiological cultures (Cancer Cytopathol 2018;126:643, Cancer Cytopathol 2015;123:612)
- Flow cytometry (Acta Cytol 2016;60:302)
- There is no standard staining protocol for performing ROSE but maintenance of the stain line should occur according to laboratory standards
- Diff-Quik Romanowsky stain is commonly used for ROSE slides (CAP Today: Cytopathology and More | FNA Cytology - Rapid On-Site Evaluation - How Practice Varies [Accessed 20 December 2021])
- Rapid or ultrafast Papanicolaou, toluidine blue, rapid hematoxylin and eosin stains or brilliant cresyl blue may be used (J Cytol 2012;29:241, J Int Med Res 2019;47:626, Diagn Cytopathol 2019;47:469)
- ROSE can be performed via telepathology following validation (Diagn Cytopathol 2019;47:206, Cytopathology 2020;31:402)
- Equipment necessary for a ROSE procedure:
- Adequate workspace
- Disposable workpad (i.e., Chux pad)
- Microscope
- Power source for microscope
- Stain line
- Coplin jar
- Pencil and pencil sharpener
- Blank slides
- Slide box
- RPMI tubes and holders
- Formalin jars
- Needles for manipulating tissue
- Steel needle holder
- Needle disposal box
- Biohazard trash receptacle
- Cleaning supplies
- Secure storage
- Note 1:
- Equipment may be brought by the pathologist or stored in the procedure room
- All equipment must be maintained according to accreditation protocols regardless of its storage site
- Note 2: see Diagrams / tables for a depiction of a portable cart containing all the above items
- Personnel:
- ROSE may be provided by a single pathologist
- An assistant may be very useful, as they can work on preparing slides while the pathologist renders interpretations
Radiology description
- ROSE can be performed in conjunction with any radiological modality incorporated into the biopsy procedure
Case reports
- 19 year old man with early cytologic diagnosis of cysticercosis using ROSE (IDCases 2018;15:e00477)
- 65 year old woman diagnosed with sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman-Destombes disease) using ROSE (Eur J Med Res 2021;26:34)
- 65 year old man with respiratory failure and metastasis of renal cell carcinoma diagnoses using endobronchial ultrasound guided transbronchial needle aspiration using ROSE (Respir Med Case Rep 2020;29:101028)
Molecular / cytogenetics description
- Molecular testing, including next generation sequencing (NGS) and microRNA classifier assessments, may be performed on uncoverslipped, stained aspirate smears with adequate cellularity of diagnostic material (Diagn Cytopathol 2019;47:469, Arch Pathol Lab Med 2018;142:291)
Sample pathology report
- See Diagrams / tables for a sample blank report form containing all necessary elements for an ROSE procedure
- ROSE pathology reports should be designated as provisional or preliminary in the patient record
- Nodule, inferior right lobe, thyroid, ultrasound guided fine needle aspiration biopsy:
- Pass 1 (aspirate smear): adequate follicular cells and colloid
- Mass, liver, CT guided fine needle aspiration and needle core biopsy:
- Pass 1 (aspirate smear): hepatocytes and blood
- Pass 2 (aspirate smear): malignant cells present
- Pass 3 (touch preparation): malignant cells present
- Passes 3, 4 and 5 were placed directly in formalin for anticipated ancillary diagnostic studies
- Lymph node, station 7, endobronchial ultrasound guided fine needle aspiration biopsy:
- Pass 1 (aspirate): blood and bronchial cells
- Pass 2 (aspirate): lymphoid elements, rare epithelioid histiocytes
- Pass 3 (aspirate): noncaseating granulomas
- Pass 4 (aspirate) was sent for culture by the clinician
- Passes 5 and 6 were placed directly in RPMI for anticipated ancillary diagnostic studies
- Mass, pancreas, endoscopic ultrasound guided fine needle aspiration biopsy:
- Pass 1 (aspirate): nondiagnostic, predominately GI contaminant
- Pass 2 (aspirate): atypical cells present
- Pass 3 (aspirate): atypical cells present
- Pass 4 (aspirate): diagnostic material present
Differential diagnosis
- ROSE preliminary diagnoses can be generally categorized:
- Nondiagnostic or inadequate:
- Material present on slides is not consistent with a definitive final diagnosis
- Diagnostic or adequate:
- Material present on the slides is diagnostic of some process
- This may or may not include malignant diagnoses
- Atypical:
- Cytologic features are present that may or not be associated with a pathologic process
- Both reactive and neoplastic entities may be suggested by this category
- Suspicious:
- Cytologic features suspicious but not definitive for malignancy, are present
- Malignant:
- Cytologic features present on the slides is consistent with a malignant process
- Nondiagnostic or inadequate:
- ROSE diagnostic classifications are generally very reliable when compared to the final diagnosis; concordance with the final diagnosis is not guaranteed, with false negatives being the more common error (J Am Soc Cytopathol 2014;3:67, Diagn Cytopathol 2019;47:367)
Board review style question #1
A radiologist performs an ultrasound guided fine needle aspiration biopsy of a liver mass obtaining 4 passes from a single anatomic site. Aspirate smears from each pass were evaluated by an attending pathologist during rapid on site evaluation (ROSE). A preliminary interpretation was given to the radiologist for each pass and was subsequently recorded in the patient record in a preliminary report. What CPT codes are appropriate for the ROSE portion of this procedure?
- 88172x1 and 88177x3
- 88172x4
- 88307x1
- 88333x1 and 88334x3
- 88333x4
Board review style answer #1
Board review style question #2
A radiologist performs a CT guided needle core biopsy of a soft tissue mass present in the quadriceps muscle of a patient obtaining 3 passes. Each needle core is transferred to an attending pathologist during rapid on site evaluation (ROSE). The pathologist gently manipulates each needle core on a glass slide to produce touch preparations and then transfers them to a formalin container for posterior processing in the cytology laboratory. Following staining an examination of the touch preparation slides, a preliminary interpretation of "scant spindle cells and blood" is provided to the radiologist following each pass. These results were recorded in the patient record in a preliminary report. Examination of the formalin fixed, paraffin embedded needle core tissue with H&E demonstrates a schwannoma which correlates well with the clinical and radiological impression. No immunohistochemical studies were necessary to produce the final diagnosis. What CPT codes should be applied to this pathology case?
- 88172x1, 88177x2, 88305x1
- 88172x3, 88305x1
- 88307x1
- 88333x1, 88334x2, 88307x1
- 88333x3, 88307x1
Board review style answer #2
D. 88333x1, 88334x2, 88307x1 (see CPT coding)
Comment Here
Reference: Rapid on site evaluation (ROSE)
Comment Here
Reference: Rapid on site evaluation (ROSE)
Board review style question #3
The practices and terminologies used to perform rapid on site evaluation (ROSE) are
- Delineated by CLIA '88 and the College of American Pathologists
- Regulated and informed by standards established by the American Society of Cytopathology
- Universal and standardized
- Vary widely between institutions
Board review style answer #3
D. Vary widely between institutions. The practice, terminologies and equipment to perform ROSE vary widely between institutions and may even vary within institutions depending on the specific needs of clinical practices. Variation is influenced by organ site, clinical capabilities, local convention and individual judgement of the ROSE provider.
Comment Here
Reference: Rapid on site evaluation (ROSE)
Comment Here
Reference: Rapid on site evaluation (ROSE)
Smear making and techniques for recovering and dividing aspirate material
Table of Contents
Overview | Type of FNA material obtained for smear making | Transferring FNA material from needle onto glass slide | Types of smear making methods | One step smear method | Two step pull method | Pop off method | Simple wick concentration method | Simple incline concentration method | Two step method for concentration | Modified two step method for concentration | Method(s) for dividing a single smear into several smears | Additional special techniques for recovering aspirate materialOverview
- Special studies may be applied to the aspirate material obtained from FNA but most aspirators just spread a portion of FNA material on a glass slide for examination by routine light microscopy
- Preparation of aspirate smears is by far the least expensive and most rapid type of preparation of FNA material for diagnosis
- IMPORTANT:
- The preparation of high quality smears is one of the most important parts of the entire FNA procedure
- Although the smearing techniques may seem tedious, they can be mastered with practice and executed within a few seconds
- High quality smears:
- Are prepared by methods tailored to physical properties of FNA material, for example cyst fluid versus semisolid material
- Cells are spread out in a monolayer and stains so they easily transmit light
- Specimen is concentrated enough in a small portion of slide for ease of screening and microscopic review
- Cells are well preserved without crush artifact
- Any semisolid tissue fragments are spread out with gentle pressure with some remaining intact
- Cellular material is not excessively trapped in blood clot
- Slides are properly labeled with at least two patient identifiers
- Several stains and special methods may be applied to the material on aspirate smear slides:
- Air dry and stain with rapid Romanowsky type stains (e.g. Diff-Quik)
- Immediate fixation (e.g. 95% ethanol) or rehydration of air dried smears for H&E or Papanicolaou staining
- Histochemical staining (e.g. AFB, Congo Red, GMS)
- Immunocytochemistry
- Molecular testing (e.g. FISH, mutational analysis such as KRAS, EGFR)
Type of FNA material obtained for smear making
- Three general categories of physical properties of FNA material which suggest how to triage the material and what smear making methods to use:
Solids:
- Distinct firm fragments of visible material that appear in background of other fluid or semisolid material or are expressed out as a single "core" or as fragments in a clean background
- Often difficult or impossible to smear as a monolayer
- May roll about the slide, leave "streak marks" or be extensively crushed if smearing is attempted
- Often create air bubbles under cover slips as they are very thick
- Examples: tissue fragments from very firm sarcomas, fibrous / scar tissue or even normal skeletal muscle, bone, cartilage, foreign body or calcified material
IMPORTANT: This firm solid material may be best triaged by picking it off the slide and submitting it for cell block or perhaps performing a touch preparation or a squash preparation instead of attempting to make a smear
Semisolid and special types of semisolids:
- Most common aspirate material obtained, consisting of visible minute soft particulate material often in a slightly bloody background
- Tends to be moderate to highly cellular material and often yields good material for diagnosis unless necrotic
- Particulate material tends to spread easily about the slide when smeared
- May cause "thick smear" with difficult interpretation if too much material placed on slide for smearing or if concentrated too much in a limited area
- Creates low to moderate hydrostatic forces between smearing slides
- Examples: tumor and tumor like conditions including solid thyroid nodule, lymph node, breast tumor, abscess, epidermal inclusion cyst
- Adipose tissue and fatty / lipid containing material:
- Often accompanied by oily droplets on smear slide
- Does not air dry well and stays "greasy"
- Is hydrophobic, and does not stay on slide very well after staining
- Examples: aspirates from fat containing tumors such as lipoma or well differentiated liposarcoma
Fluid:
- Watery like material that does not "stick" to the slide very well
- Tends to run all over the slide when slide is tilted even slightly
- Usually has very low concentration of cells
- Creates high hydrostatic forces between smearing slides
- Examples: thin serous fluid from thyroid cysts, fibrocystic changes in breast, seroma
Transferring FNA material from needle onto glass slide
General:
IMPORTANT: No smearing technique can correct clotting artifact
Techniques to ensure best results in transferring FNA material from needle to glass slide include:
- FNA material must be rapidly transferred from needle onto glass slide to make good quality smears for diagnosis
- Clotting of FNA material within needle, if transfer is delayed, may cause material to be trapped within needle or hub or smear to be excessively thick or clotted, causing a limited or obscurred microscopic evaluation
IMPORTANT: No smearing technique can correct clotting artifact
Techniques to ensure best results in transferring FNA material from needle to glass slide include:
- Keep most of FNA material within needle shaft during aspiration and stop the aspiration when material is seen entering the hub
- Perform aspiration in rapid manner (typically less than 5 - 10 seconds)
- Set up FNA procedure so that material is expressed onto glass slide within seconds of needle removal from patient
- Make sure needle is easily removed from syringe tip (especially with Leur lock tips) BEFORE aspiration occurs
- Place glass slides for smear making as close as safely possible to aspiration site
- Clear everything / everyone from path between aspiration site and slide making site as you will be moving rapidly between those areas with a "dirty needle"
- Make smears immediately after you place material onto glass slide (seconds count!)
- When expressing the material out of the needle using positive pressure from the syringe, keep the needle tip's beveled edge in contact with the glass slide surface using a 45 to 90 degree angle to the surface of the slide
- Do not "spray" the FNA material over the glass slide so that material is broken up into tiny droplets about the slide, which will cause them to dry much faster than if a single small droplet is placed
Types of smear making methods
- Several smear making methods and variations have been documented
- Personal preference and teaching exposure influence the method of choice but the physical properties of the FNA material (including the concentration of hypocellular material or cyst fluid) can also dictate the most useful method of triaging and smear making
General:
- Express the FNA material for smear making on the slide so that it is about 2/3 the way up from the non frosted end of the slide
- Generally, one to two drops forming a single large droplet of semisolid FNA material should suffice per slide (1 drop = ~.05 mL)
- For cyst fluid or liquid material, several drops up to 0.5 mL may be placed on the slide and concentrated using the special methods below
One step smear method
General:
Technique:
Smear problems:
- Excellent for semisolid and small volume FNA material
- Typically only one slide is produced that has smeared material for evaluation
- Smear usually occupies less surface area than Two step pull method but this technique is more difficult than Two step pull method
Technique:
- Hold up a slide with the droplet of FNA material (the "non spreader" slide); the drop should face you and the non frosted end should point at a steep angle towards the floor; use your non dominant hand by holding the frosted end pinched between your thumb and index finger and use the remaining fingertips of this hand to support the back of the slide
- A second slide, called the "spreader slide", is then brought up with the dominant hand by grasping it by the frosted end pinched between the thumb, index and third finger to a position that is perpendicular above the "non spreader" slide
- Keeping this perpendicular orientation, the middle of the "spreader slide" is then poised over the specimen droplet on the "non spreader" slide with its lower edge of the long axis touching the "non spreader" slide and forming a hinge like contact between them
- "Spreader slide" is then rotated forward (while still maintaining the hinge like contact) onto the droplet with gentle pressure to flatten but not crush it
- "Spreader slide" is then pulled down the surface of the "non spreader" slide (which is held steady) to smear the droplet using constant and gentle pressure on the "spreader slide" while continuing to maintain the perpendicular orientation of the two slides
- The "non spreader" slide should have a good quality ovoid shaped smear
- The "spreader slide" should have little to no material on it and may be discarded
Smear problems:
- "Edge artifact": caused by uneven pressure on either edge of the "spreader slide" in which a single or multiple thick smear lines will be created; this results in an area that is difficult to evaluate due to excessive cell thickness; correct by holding the "smear slide" with even pressure throughout the smearing process so that the flat surfaces of the "smear slide" and "non smear slide" remain parallel
- "Crush artifact": caused by too much pressure being placed on flat surfaces between the "spreader slide" and "non spreader" slide OR by too much hydrostatic forces between the slides (e.g. watery fluid); correct by decreasing the thumb pressure on "smear slide" and increase the speed of the smearing action; for cyst fluid, try using another smearing or concentration technique before smearing and also lessen the contact time between the initial lowering of the "smear slide" with the "non smear slide" and increasing the speed of the smearing action
Two step pull method
General:
Technique:
Smear problems:
- Excellent for semisolid and small volume FNA material
- Usually two slides are produced that have smeared material for evaluation
- Usually produces a smear that occupies more surface area than the One step method
- Technically less challenging than the One step method
Technique:
- The slide with the droplet of FNA material is held up so it is essentially parallel with the floor by using the non dominant hand to hold the frosted end pinched between your thumb, index and third finger
- A second slide is then brought up with the dominant hand in an inverted fashion by grasping it by the frosted end pinched between thumb, index and third finger
- This inverted slide is then poised over the specimen droplet of the slide below in a parallel fashion with the edge of the non frosted end of the inverted slide touching just below the frosted end of the droplet slide forming a hinge like contact
- The inverted slide is then lowered (while still maintaining the hinge like contact of the lower edge of the long axis) onto the droplet with gentle pressure to flatten but not to crush the droplet
- Both slides are then pulled apart to smear the droplet while continuing to maintain the parallel arrangement of the slides using constant and gentle pressure
- Both slide should have a good quality ovoid shape of smear material
Smear problems:
- "Edge artifact": caused by uneven pressure on either non frosted edge of the slides when they are pulled apart in which a single or multiple thick smear lines will be created, results in an area that is difficult to evaluate due to excessive thickness; correct by holding both slides with even pressure throughout the smearing process so their flat surfaces remain parallel throughout the pulling process
- "Crush artifact": caused by too much pressure being placed on the flat surfaces between the slides OR by too much hydrostatic forces between the slides (e.g. watery cyst fluid); correct by decreasing the pressure on one or both of the slides and increasing the speed of the smearing action; for cyst fluid try using another smearing or concentration technique before smearing and also lessen the contact time between the initial lowering of the inverted slide and increase the speed of the pulling action
Pop off method
General:
Technique:
- Rarely used technique (mostly of historical value only)
- Does not involve smearing the FNA material
- Produces a small thick and rounded button of FNA material for evaluation
- Good at keeping "tissue like" and cellular fragments together and thus giving some semblance of a histologic tissue section
- Two slides are produced that have material for evaluation
- Technically easy to produce
- Main disadvantage is that the button produced is composed of thick cells / tissue fragments that can make cytologic evaluation difficult
Technique:
- The slide with a small droplet of FNA material is held up essentially parallel to the floor by using the non dominant hand by holding the frosted end pinched between your thumb and index finger and the remaining finger tips of this hand supporting the back of the slide
- A second slide is then brought up with the dominant hand, grasping it by the frosted end pinched between the thumb, index and third finger and holding it in a perpendicular orientation over the bottom slide containing the droplet
- Keeping this perpendicular orientation, the middle of the second slide is then poised over the specimen droplet with its lower edge of the long axis touching the droplet slide and forming a hinge like contact between them
- This second top slide is then quickly rotated forward (while still maintaining the hinge like contact) and onto the droplet on the bottom slide with gentle pressure to flatten but not to crush the droplet
- The second top slide is then quickly rotated back off the flattened out droplet (while still maintaining the hinge like contact)
- Both slides should then have an irregular rounded button of material for staining and microscopic examination
Simple wick concentration method
General:
Technique:
- Good for concentrating non bloody fluids and large volume FNA material
- Technically very easy
Technique:
- The slide with the expressed fluid droplet of FNA material is allowed to sit on the table for a few seconds in order to allow the cells to sediment
- Leaving the slide stationary on the table, a corner of a piece of gauze is then placed very minimally into one edge of the fluid droplet and the fluid is allowed to wick into the gauze
- After a few seconds the gauze is removed and discarded
- The concentrated material may then be smeared using the One step or the Two step pull method
Simple incline concentration method
General:
Technique:
- Good for concentrating non bloody fluids and large volume FNA material
- Technically very easy
Technique:
- The slide with the expressed fluid droplet of FNA material is allowed to sit on the table for a few seconds in order to allow the cells to sediment
- The slide is then picked up slowly by holding it parallel to the floor by using the non dominant hand with the frosted end pinched between your thumb, index and middle finger
- Using your dominant hand, a piece of gauze is placed along the long axis of the slide with the fluid droplet
- The slide with the fluid droplet is then gently rotated in the direction of the gauze and the fluid on the slide should flow and wick into the gauze
- After a few seconds the gauze is removed and discarded
- The slide with the concentrated material may then be smeared using the One step or the Two step pull method
Two step method for concentration
General:
Technique:
- Good for concentrating fluids and large volume FNA material essentially using an a combination of surface tension and an incline type method of specimen concentration
- Technically more challenging than simple incline concentration methods, but may concentrate the FNA material within a smaller area on the slide for ease of screening and examination
Technique:
- A slide with the droplet of FNA material (the "non spreader" slide) is held up (drop facing you and with the non frosted end pointed at small angle down towards the floor) by using your non dominant hand by holding the frosted end pinched between your thumb and index finger and with the remaining finger tips of this hand supporting the back of the slide
- A second slide, called the "spreader slide", is then brought up with the dominant hand by grasping it by the upper edge of the long axis near the frosted end by pinching between the thumb, index and third finger to a position that is parallel to the "non spreader" slide with the frosted ends facing each other
- Keeping this parallel orientation, the non frosted end of the "spreader slide" is used to form a 45 degree, hinge like contact between the "non spreader" slide below the specimen droplet
- "Spreader slide" is slid up into the FNA droplet pool (while still maintaining the hinge like contact) and paused until the material spreads along its edge
- "Spreader slide" is then pushed down the surface of the "non spreader" slide (which is held steady) to about the middle lower end while keeping the parallel orientation of the two slides
- The spreader slide is removed and what is left is a line of fluid material on the "non spreader slide"
- The "non spreader slide" is then quickly inverted so that the fluid runs from the line of material towards the frosted end
- The now concentrated line of material on the "non spreader slide" is then smeared up towards the non frosted end of the "non spreader slide" using the One step method
- There will be some material left on the distal edge of the "spreader slide" that may be recovered by spreading on another clean slide using the Two step pull method
Modified two step method for concentration
General:
Technique:
- Good for concentrating fluids of large volume FNA material (up to 0.5 mL) using a wick and spreader slide method of concentration
- Technically more challenging than simple wick concentration methods but may have the advantage of concentrating the FNA material within a smaller area on the slide for ease of screening
Technique:
- The slide with the expressed fluid droplet of FNA material is allowed to sit on the table for a few seconds in order to allow the cells to sediment
- The slide is then picked up slowly by holding it parallel to the floor by using the non dominant hand with the frosted end pinched between your thumb, index and middle finger
- Using your dominant hand a piece of gauze is placed along the long axis of the slide with the fluid droplet
- The slide with the fluid droplet is then gently rotated in the direction of the gauze and the fluid on the slide should flow and wick into the gauze
- After a few seconds the gauze is removed and discarded
- A second slide, called the "spreader slide", is then brought up with the dominant hand by grasping it at the frosted end by pinching between the thumb, index and third finger to a position that is parallel to the "non spreader" slide with the frosted ends facing each other
- Keeping this parallel orientation, the non frosted end of the "spreader slide" is used to form a 45 degree, hinge like contact between the "non spreader" slide below the concentrated specimen
- "Spreader slide" is then slid up through the concentrated specimen pool (while still maintaining the hinge like contact) towards the frosted end which will carry the material along its edge
- The spreader slide is removed and what is left is a line of concentrated material on the "non spreader slide"
- The concentrated line of material may then be smeared using the One step or the Two step pull method
- Any material left on the distal edge of the "spreader slide" may be recovered by spreading on another clean slide using the Two step pull method
Method(s) for dividing a single smear into several smears
General:
Technique #1:
Technique #2:
- Excellent for semisolid and large volume FNA material
- Employed when too much FNA material is expressed onto a single slide and helps avoid making overly thick smears
- Multiple slides may be produced that have smear material for evaluation
Technique #1:
- Essentially a combination of the Pop off method followed by smear making using the One step or the Two step pull method
- The slide with the droplet of FNA material is held up essentially parallel to the floor using the non dominant hand by holding the frosted end pinched between your thumb and index finger and with the remaining finger tips of this hand supporting the back of the slide
- A second slide is then brought up with the dominant hand, grasping it by the frosted end pinched between the thumb and index finger and third finger, and holding it in a perpendicular position over the bottom slide containing the droplet
- The middle of this second top slide is then poised over the specimen droplet with the lower edge of the long axis touching the bottom slide forming a hinge like contact
- This second top slide is then quickly rotated forward (while still maintaining the hinge like contact) and onto the droplet on the bottom slide with gentle pressure to flatten but not to crush the droplet
- The second top slide is then quickly rotated back off the flattened out droplet (while still maintaining the hinge like contact)
- This second slide is then lifted entirely off the bottom slide
- The bottom slide is quickly put down on the table and the free non dominant hand can pick up a clean slide and the second slide with the divided material is then smeared onto this clean slide using the One step or the Two step pull method
- The bottom slide that was initially put down on the table can then be picked back up and smeared with another clean slide by using the One step or the Two step pull method; OR, if there is still enough material on this slide, can be divided again as above and additional smears created
Technique #2:
- Simple and crude method of using the bottom edge of a clean spreader slide to split the FNA material
- The slide with the droplet of FNA material ("non spreader slide") is held up and slightly tilted up along its long axis from parallel to the floor using the non dominant hand by pinching the frosted end between your thumb and index finger and with the remaining finger tips of this hand supporting the back of the slide
- A second spreader slide is then brought up with the dominant hand, grasping it by the frosted end pinched between the thumb and index and third finger, and holding its non frosted edge in a perpendicular position over the droplet on the bottom slide
- The spreader slide's flat edge is then lowered into a portion of the droplet on the bottom slide until it makes a 45 degree contact angle with the bottom slide
- The spreader slide is then pulled upwards and off the long axis edge of the bottom slide
- The spreader slide should now have a portion of the original droplet material on its bottom edge and this may be smeared onto another clean slide using the One step or the Two step pull method
- The non spreader slide may then be smeared by using the One step or the Two step pull method; OR, partitioned again using this technique
Additional special techniques for recovering aspirate material
- Ideally, the aspirate material obtained during FNA should be collected in the needle shaft and the aspiration stopped when material is seen entering the hub
- At times, however, diagnostic material may be trapped in the hub that cannot be forced out by using positive pressure from a syringe or material may have inadvertently entered the tip or barrel of the syringe
- Use these special collection methods to recover trapped material to make additional slide smears:
Needle snap technique: to recover material trapped in the hub of the needle
- First, recover as much material as possible from needle shaft by forcefully blowing air through it with the syringe and onto the glass slide
- Then secure the needle tip in a needle protection device (e.g. Point-Lok Device from SIMS Portex Inc)
- Then pick up the needle protection device and position it just beyond the end of a new glass slide so the hub of the needle points at a small angle down and touches the glass slide surface about 1/3 of the way up from the non frosted end of the slide
- Your free hand can grab the hub of the needle and pull it upward while the hand holding the needle tip protector remains steady which will result in bending of the shaft of the needle in a slight arch and the resulting tension will "load" the hub
- The needle hub is then quickly released and it is allowed to "snap" rapidly and forcefully against the glass slide and this will expel any FNA material that was trapped in the hub onto the slide
- The hub may then be rapidly grabbed again and "snapped" in a similar matter as above to remove any more material that may be trapped in the hub
- The additional material obtained may now be quickly smeared using the one or two step smearing technique
- Alternatively, you can secure the needle tip as above, then grab the hub and forcefully hit the open end of it against the surface of the slide to dislodge the material onto the slide
Syringe pop technique: to recover material trapped in the tip or barrel of the syringe by rapidly and forcefully blowing it onto the surface of the glass slide
- The syringe without the needle is held in your hand as if making a fist with the tip pointing down and just above the surface of the glass slide
- The plunger is pulled out gently with the free hand to fill the barrel with air
- Using the palm of the free hand the plunger is then rapidly, forcefully and audibly pushed into the barrel which is being held steady so the tip remains just above the glass slide surface
- CAUTION: if the tip of the syringe slams against the glass slide during this technique, the glass slide may shatter
- The additional material that has been expelled from the syringe and onto the glass slide may now be quickly smeared using the one or two step smearing technique
Superficial FNA procedure: contraindications and complications
Table of Contents
Definition / general | Essential features | Terminology | Sites | Contraindications | Limitations | Complications of FNA on superficial masses | Organ specific complications | Case reports | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Fine needle aspiration (FNA) is a minimally invasive method for acquiring a cellular specimen involving the insertion of a fine needle into a mass, extracting cellular material and then providing a cytological diagnosis (StatPearls: Fine Needle Aspiration [Accessed 1 September 2023])
- Superficial FNA procedure is a minimally invasive, safe, fast, and cost effective technique that enhances clinical decision making and appropriate patient triage by distinguishing an inflammatory process from a neoplastic lesion, broadly
- Although not many major contraindications or complications are associated with superficial FNA procedures, it is important to address the few complications that are relevant to the clinical context
Essential features
- FNA is a minimally invasive diagnostic method and complications are relatively rare when dealing with superficial lumps compared to deep seated lesions (StatPearls: Fine Needle Aspiration [Accessed 1 September 2023])
- Patient consent and cooperation are necessary before starting the procedure
- FNA cytology can be performed safely on most of the palpable superficial lumps and cysts
- Knowledge of site specific FNA procedural complications helps with effective communication and establishing clinical rapport
- Awareness of potential diagnostic limitations aids in minimizing false positive and false negative FNA cytology diagnosis and improving cytology histology correlation
- Rapid on site sample adequacy evaluation (ROSE) or immediate cytologic assessment can increase adequacy rates and reduce needle passes (PLoS One 2015;10:e0135466)
- FNA cytology diagnosis when performed in the outpatient clinics and inpatient units is time and cost effective and enhances patient triage (Diagn Cytopathol 2002;27:1)
Terminology
- Fine needle aspiration cytology (FNAC) or fine needle aspiration biopsy (FNAB)
- Rapid on site sample adequacy evaluation (ROSE)
Sites
- FNA can be performed on any palpable superficial lesions percutaneously by a pathologist or clinician; deep body site FNAs are performed under image guidance
- Common superficial body sites
- Head and neck including salivary gland masses or cysts
- Cervical lymph nodes
- Subcutaneous mass or cyst
- Breast lesions of low clinical suspicion for breast cancer (e.g., cyst, abscess or other benign processes)
- Suspected recurrent malignancy in the chest wall (Arch Pathol Lab Med 2021;145:825)
- Discrete thyroid nodules rarely
Contraindications
- Uncooperative patient
- No valid consent
- Pulsatile neck masses due to vascular etiology (e.g., cervical aortic arch [CAA], high riding brachiocephalic artery, vascular malformations, etc.) (BMJ Case Rep 2018;2018:bcr2018224515)
- Infections, such as hydatid cysts, which can trigger severe anaphylactic reactions due to inadvertent rupture of cyst wall
- Infected skin overlying the mass (StatPearls: Fine Needle Aspiration [Accessed 1 September 2023])
Limitations
- Superficial FNA procedure is safe with relatively fewer major complications but there are a few limitations to this time and cost efficient procedure, which include the following
- When there is a high degree of suspicion that cytology will not be sufficient to make the diagnosis and histology is needed (e.g., suspected lymphoma) (StatPearls: Fine Needle Aspiration [Accessed 1 September 2023])
- Using breast FNA for primary diagnosis of breast lesions and prognostic / predictive biomarkers in breast carcinoma as it can be very challenging to interpret immunohistochemistry in breast pathology using FNA cell block material (Arch Pathol Lab Med 2021;145:825)
- Distinguishing between in situ and invasive breast carcinoma using breast FNA (Diagn Cytopathol 2001;25:73)
- Patients with severe coagulopathic disorders due to relative bleeding risks, which should be addressed carefully during the preprocedural decision making process (Endosc Int Open 2019;7:E15)
- Risk of false negative result due to sampling error especially in thyroid and breast lesions (Thyroid 2004;14:207)
- Risk of false positive results due to diagnostic misinterpretation especially in breast lesions; breast fine needle aspiration cytology (Cancer Cytopathol 2013;121:561)
- Ethical dilemma regarding when to disclose the preliminary result to the patient as opposed to deferring the disclosure (AMA J Ethics 2016;18:779)
Complications of FNA on superficial masses
- Overall complications related to FNA are infrequent, especially using smaller gauge needles and include the following
- Pain
- Bleeding (especially in hypervascular tumors such as paraganglioma)
- Bruising
- Vasovagal reaction (StatPearls: Fine Needle Aspiration [Accessed 1 September 2023])
- Damage to surrounding structures
- Formation of fistula
- Seeding of malignant cells (Br J Oral Maxillofac Surg 2016;54:260)
Organ specific complications
Parotid gland
Breast
Thyroid
- Parotitis
- Facial nerve paresis (Br J Oral Maxillofac Surg 2016;54:260)
- Seeding of malignant cells
- Very rare complication due to displacement of tumor cells immediately after FNAC or core needle biopsy
- It is not clear if disseminated malignant cells mature into tumor masses (along the needle track or in subcutaneous fat away from the tumor)
- Histological features (associated with an inflammatory and multinucleated foreign body type giant cell response) have been suggested as diagnostic of seeding along the needle track (Br J Oral Maxillofac Surg 2016;54:260)
- Lack of specific criteria for this entity and a defined interval after which the tumor developed make this complication clinically less relevant
Breast
- Bleeding
- Bruising
- Swelling at the aspirated site
- Pain
- Pneumothorax, in rare instances if the needle is advanced deep in the chest area, especially in patients with thin chest wall and chest wall deformities (Int Semin Surg Oncol 2005;2:14)
- Pneumothorax is a relatively rare complication of breast FNA since the ribs form a much larger area of the chest wall surface than the intercostal muscles; furthermore, the pectoral muscles provide additional protective structures
- Pneumothorax after breast FNA may be seen more frequently in patients who have thin body habitus and small sized breasts
- Patients with deep central lesions or lesions in the tail of the breast are also at higher risk for developing this complication
Thyroid
- Bleeding
- Local pain at the aspirated site
- Cervical neuritis preceded by post-FNA hematoma
- Tumor seeding (Br J Oral Maxillofac Surg 2016;54:260)
- Acute or delayed transient swelling of the thyroid gland
- Recurrent laryngeal nerve injury
- One of the infrequent complications
- Incidence rate is still unknown
- Multiple mechanisms have been proposed (nerve stretching by hematoma, inflammation, thrombosis in small arterioles and rarely direct injury to nerve) (Radiol Diagn Imaging 2017;1:1)
- Thyrotoxicosis (Clin Endocrinol (Oxf) 2009;71:157)
- Cutaneous sinus formation (Case Rep Endocrinol 2014;2014:923438)
- Very rarely, pseudoaneurysm in superior thyroid artery (Clin Endocrinol (Oxf) 2009;71:157)
- Very rarely, focal carotid intramural hematoma
- Acute bacterial thyroiditis
- Rare complication representing only 0.7% of all thyroid FNA related complications
- Can result in serious consequences (systemic sepsis, endangering the airways, cardiac arrhythmias)
- Common bacteria involved are Staphylococcus aureus and Streptococcus sp. (Case Rep Endocrinol 2020:2020:7104806)
Case reports
- 35 year old woman presented with hoarseness, laryngeal stridor and dyspnea without fever that emerged ~3 days after a first diagnostic FNA (Int J Endocrinol Metab 2016;14:e39174)
- 49 year old woman with background history of hypertension, hyperlipidemia and presumed transient ischemic attack (TIA) presented with an anterior neck lump of 1 year duration (Case Rep Endocrinol 2020;2020:7104806)
- 49 year old man presented with acute swelling and severe tenderness of the parotid gland accompanied by skin hyperemia, which had started 1 day after FNA (Eur J Case Rep Intern Med 2019;6:001147)
Board review style question #1
Which of the following is a contraindication for FNA?
- Palpable cervical lymph node
- Pulsatile neck lesions
- Salivary gland mass or cyst
- Superficial palpable skin and subcutaneous nodule
Board review style answer #1
B. Pulsatile neck lesions. Pulsatile neck lesions are associated with higher risk of bleeding due to their vascular nature. The other options are incorrect as they are not contraindications for FNA. Answer C is incorrect because FNA is a helpful tool for differentiating neoplastic and nonneoplastic conditions and diagnosing most benign salivary gland neoplasms. Answer A is incorrect because FNA of palpable cervical lymph node is useful in the diagnosis of metastatic carcinomas and granulomatous processes. Answer D is incorrect because FNA is a reliable and useful procedure for the diagnosis of superficial palpable skin and subcutaneous nodules. Complications are quite rare, especially when using the small needle.
Comment Here
Reference: Superficial FNA procedure: contraindications and complications
Comment Here
Reference: Superficial FNA procedure: contraindications and complications
Board review style question #2
Which of the following statements regarding FNA is correct?
- Complications are lower with superficial masses when compared to the deep seated masses
- It cannot distinguish between in situ and invasive breast carcinoma
- It cannot distinguish between neoplastic and nonneoplastic lesions
- Patient understanding and consent are not necessary before the procedure
Board review style answer #2
A. Complications are lower with superficial masses when compared to deep seated masses. Answer A is correct because complications, such as spread of infection or seeding of neoplastic cells through other tissue layers, are lower when FNA is performed on superficial masses as compared to the deep seated masses. Answer B is incorrect because FNA cannot reliably distinguish in situ from invasive carcinoma, as invasive features require documentation of stromal invasion in tissue biopsy. Answer C is incorrect because the FNA technique is very helpful in distinguishing between neoplastic and nonneoplastic lesions. Answer D is incorrect becausepatient understanding and consent are absolute requirements before performing the FNA.
Comment Here
Reference: Superficial FNA procedure: contraindications and complications
Comment Here
Reference: Superficial FNA procedure: contraindications and complications
Suspicious for malignancy (pending)
[Pending]
Ultrasound terminology
Table of Contents
General imaging terminology | Basic ultrasound machine function terminology | Key ultrasound terms used to describe the sonographic image and US wave interactions with tissuesGeneral imaging terminology
- DICOM (Digital Imaging and COmmunication in Medicine) standard: an imaging standard with a specific file format and a network communications protocol that allows collected images including epidemiological information to be interconnected and transferred to other systems for viewing, printing, and electronic storage
- PACS (Picture Archiving and Communication System): a medical imaging technology which provides electronic storage, retrieval, and access to images in which the universal format of the input data is in DICOM standard
Basic ultrasound machine function terminology
- Calipers: a cursor that is electronically superimposed over the cross sectional image that is used to calculate size of the scanned structure; measurement is typically given in centimeters
- Depth control: increases or decreases the depth of the field of view and is typically a running scale at one margin of the sonogram with the depth measurement in centimeters; as the depth is increased with a linear array transducer, the width of the field of view will remain constant but the image on the screen will become narrower and the structures smaller
- Doppler ultrasound: allows assessment of tissue vascularity
- Several different types of Doppler US exist but the pathologist will find that color flow Doppler or power Doppler will suit their most basic needs:
- Color flow doppler: based on the Doppler principle to detect moving objects (red blood cells within vascular spaces) and assigns a color to the movement relative to the transducer
- Gives the direction and velocity of blood flow
- Blood flow moving away from the transducer will be blue and blood flow towards the transducer will be red (note: the corresponding colors, however, can be set by the operator on the US machine)
- Absence of blood flow is indicated by the absence of color
- Angle of transducer with respect to the blood flow will affect detection of flow (at a 90 degree perpendicular scanning angle, flow may appears absent!)
- Important to keep the on screen color Doppler box as small as possible when evaluating the area of interest to maximize sensitivity
- In general, the pathologist will not be interested in the direction or velocity of flow in a tissue or target, but in the presence or absence of flow, the distribution of flow within a target (peripheral versus central), and the overall quantity of flow (minimal, moderate, or high flow)
- Power doppler: useful adjuvant to color flow Doppler; essentially measures the "magnitude" of blood flow (essentially related to the number of RBC creating the Doppler shift) without regard to direction or velocity of blood flow
- Considered more sensitive than color flow Doppler for detection of low blood flow
- Less angle dependence than color flow Doppler in detecting blood flow
- Disadvantage is that it is more susceptible to tissue and patient movement artifact (so called flash artifact) due to higher sensitivity
- Focal zone: a point where the US beam emitted from the transducer is at its narrowest and gives you the best lateral resolution
- Can be adjusted manually to a selected depth on most US machines and is usually indicated by a single marker at the edge of the depth measurement
- When manually adjusting, the focal zone should be placed at or slightly below the target
- An inappropriately positioned focal zone may cause subtle masses to be less visible, sharp edges of a target to appear ill defined, and cysts to appear as solid tissue
- Some US machines have the capability of applying more than one focal zone on an image, however, this may slow down real time scanning and may decrease image resolution
- Frequency adjustment: a keyboard control used to either increase or decrease the US frequency of the transducer within a narrow range of a few Megahertz
- High US frequencies have less tissue penetration
- Lower frequencies have more tissue penetration
- As a rule of thumb, deeper targets are imaged using lower US frequencies and superficial targets, higher frequencies
- There is an inverse relationship between sonographic resolution and scanning US frequency
- Higher frequencies have a better sonographic resolution
- Lower frequencies have a poorer sonographic resolution
- During US scanning, you will need to select a scanning frequency that optimizes both tissue penetration and sonographic resolution
- Linear array US transducer: a high frequency transducer with a linear set of piezoelectric crystals that gives good resolution in superficial tissues but does sacrifice tissue penetration as these two properties are inversely proportional; the sonogram produced is rectangular and the extent of visualization is usually limited to the footprint area
- On screen indicator: a colored mark or symbol in the upper left hand corner of the US display screen used for orientation purposes with the transducer indicator
- On screen orientation: by convention this is the proper orientation during US scanning in which the transducer indicator corresponds to the on screen indicator
- Overall gain: allows you to increase or decrease the brightness of the entire sonogram similar to the "brightness" control on a computer monitor
- Piezoelectric crystals: found under the footprint area of the transducer, has two functions:
- Vibrates when a voltage pulse is applied and creates the mechanical US waves (1% of the time during US scanning)
- Receives the pressure changes from the reflected US waves from the tissue (99% of the time during US scanning) that induce a mechanical vibration in the "listening" crystals which is converted into an electrical signal and processed by the US machine into the sonographic image
- Sonogram: 2 dimensional, grey scale, digital image representing both the strength and depth of the reflected US waves back to the transducer
- Time gain compensation (TGC): allows stepwise control of the brightness of the reflected US waves from different depths of the tissue to produce a desired uniform brightness of the sonogram
- Transducer footprint: portion of the transducer area that makes contact with the patient's skin and area in which the piezoelectric crystals are found
- Transducer frequency: usually a narrow range of a few Megahertz (e.g. 8 - 12 MHz) of scanning US frequencies that may be increased or decreased with a keyboard control on the US machine
- Transducer indicator: a colored mark or palpable protuberance at one end of the long axis of the transducer casing used for orientation purposes with the display screen
- Transducer scanning positions:
- Transverse: axial (right to left) orientation of the ultrasound probe with the transducer indicator notch towards the patient's right such that the left side of the sonogram should display structures on the patient's right side
- Longitudinal (also called Sagittal): cranial to caudal orientation of the ultrasound probe with the transducer indicator notch towards the patient's head such that the left side of the sonogram should display structures nearer to the patient's head
- Radial: used in breast US and follows a wagon wheel pattern of scanning with the nipple in the center to visualize the breast plane parallel to the ductal system
- Antiradial: used in breast US and is a scanning plane 90 degrees to the radial scanning pattern and is used to visualize the breast plane perpendicular to the ductal system
- Ultrasound transducer: produces the mechanical US waves and also receives the returning US echoes reflected from the patient's tissue boundaries and interfaces
Key ultrasound terms used to describe the sonographic image and US wave interactions with tissues
- Acoustic impedance: the resistance of tissue boundaries and interfaces to the passage of the US waves
- Anechoic: without returning echoes; appears completely black on US
- Echogenicity: the ability of a structure or tissue to return reflected US waves
- Edge shadow artifact: narrow hypoechoic lines that run parallel to the US beam and appear at the lateral edges of tissue, cysts, or masses that are smooth and rounded
- Heterogeneous: nonuniform in echogenicity and is a mixture of hyperechoic and hypoechoic areas
- Homogenous: uniform or equally distributed echogenicity
- Hyperechoic: highly reflective tissue with stronger returning echoes then the surrounding tissues; appears as a lighter shade of grey or white on US
- Hypoechoic: less reflective tissue with lower returning echoes then the surrounding tissues; appears a darker shade of grey on US
- Isoechoic: has the same shade of grey as the surrounding tissue of reference
- Posterior acoustic enhancement: a hyperechoic area in the sonogram at the posterior interface of the tissue or structures
- Posterior acoustic shadowing: a hypoechoic or anechoic area in the sonogram at the posterior interface of the tissue or structures
- Reverberation artifact: multiple hyperechoic lines that are perpendicular to the US beam that occur deep to interfaces
WHO reporting system for adrenal gland cytopathology (pending)
[Pending]
WHO reporting system for kidney cytopathology (pending)
[Pending]
WHO reporting system for liver cytopathology (pending)
[Pending]
WHO reporting system for soft tissue cytopathology (pending)
[Pending]
Recent Cytopathology Pathology books
Find related Pathology books: breast, cytopathology, GI, GU/adrenal, gynecologic, head & neck/endocrine, lung