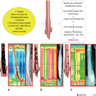
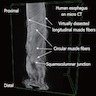
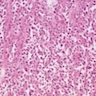
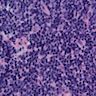
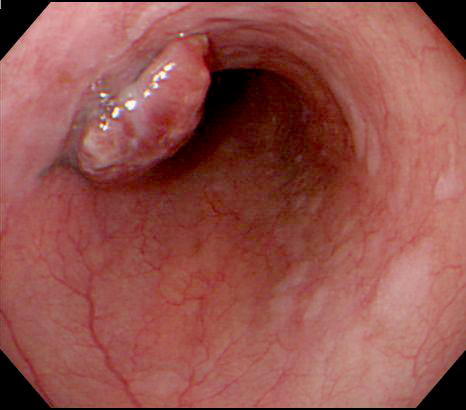
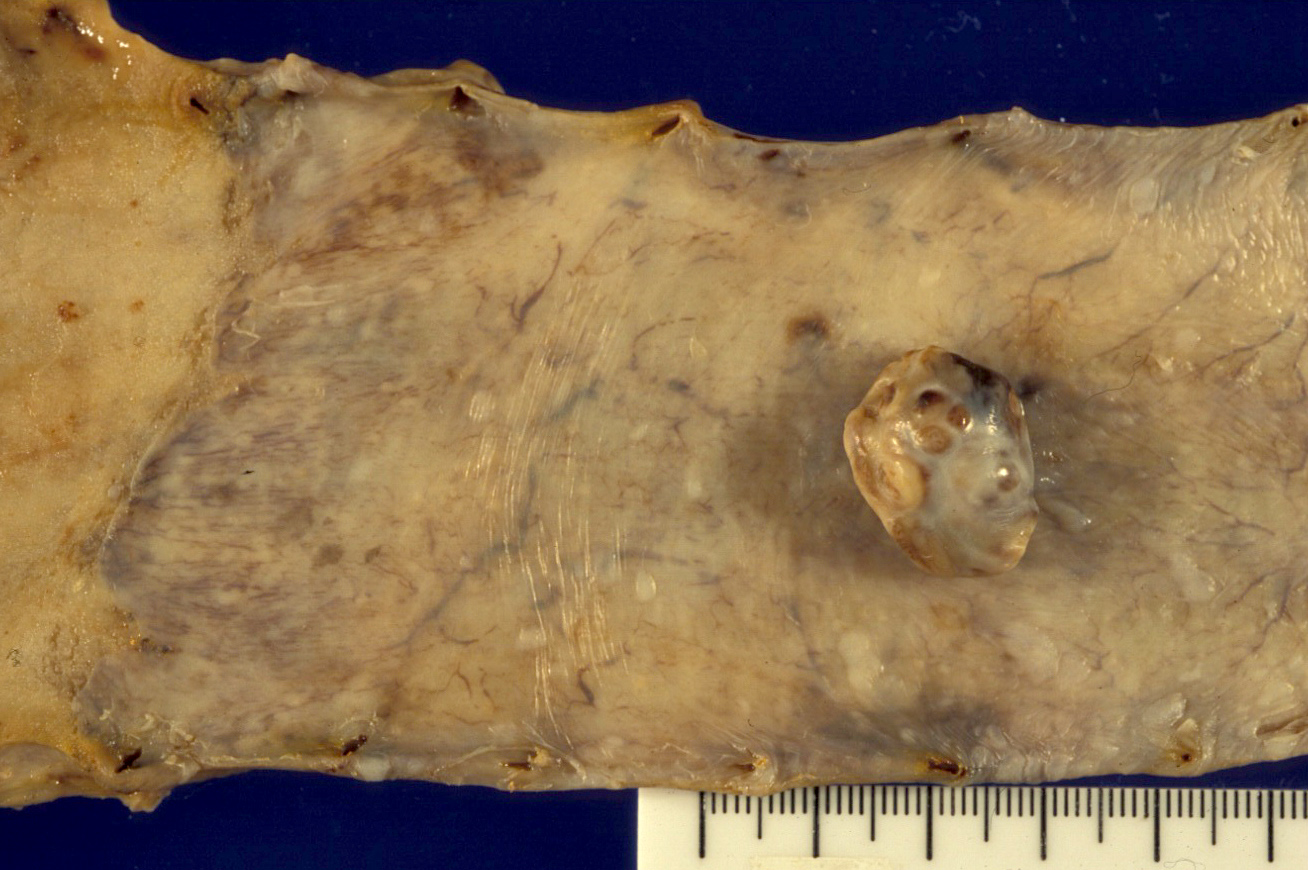
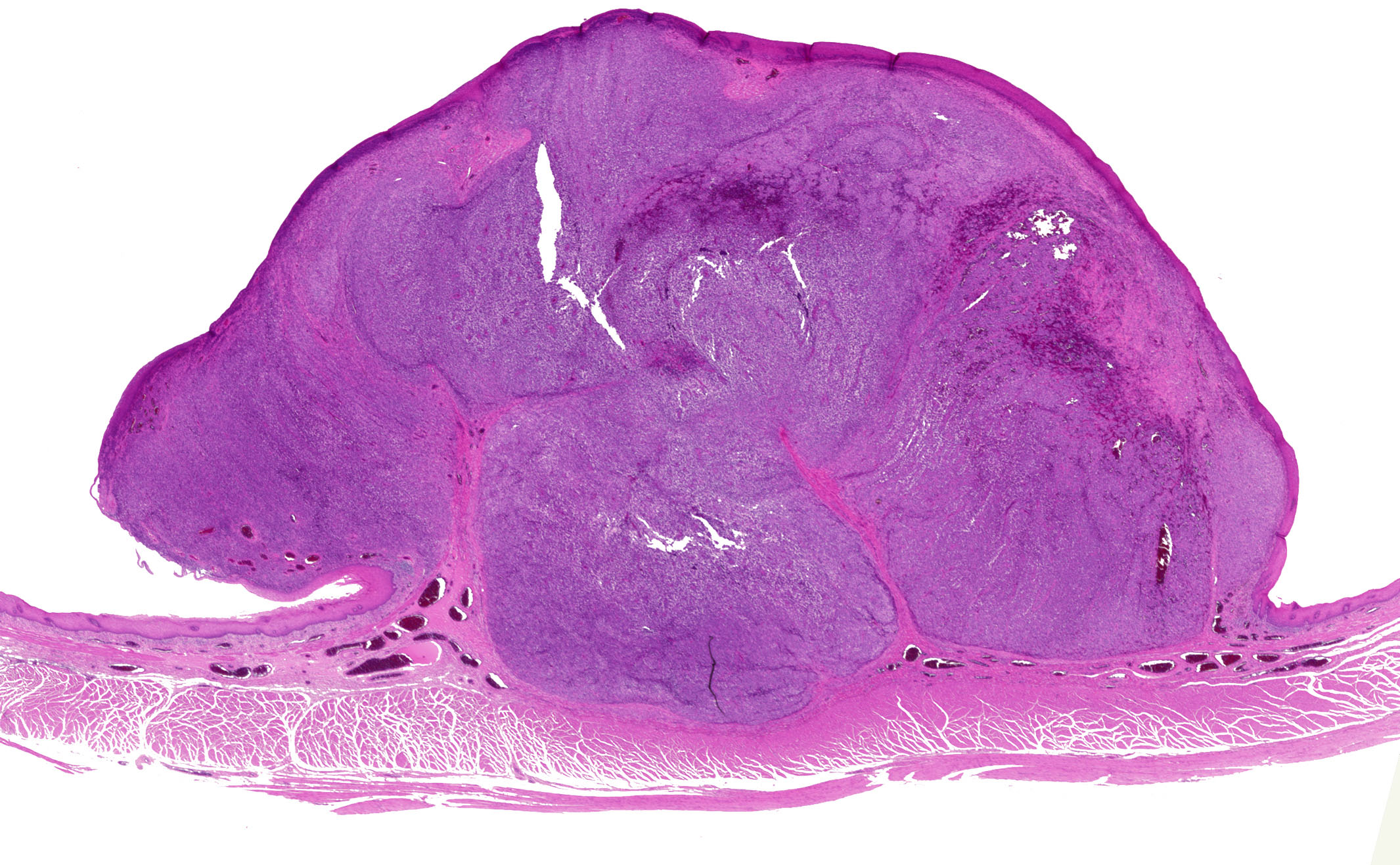
- TX: Tumor cannot be assessed
- T0: No evidence of primary tumor
- Tis: High grade dysplasia, defined as malignant cells confined to the epithelium by the basement membrane
- T1: Tumor invades the lamina propria, muscularis mucosae or submucosa
- T1a: Tumor invades the lamina propria or muscularis mucosae
- T1b: Tumor invades the submucosa
- T2: Tumor invades the muscularis propria
- T3: Tumor invades adventitia
- T4: Tumor invades adjacent structures
- T4a: Tumor invades the pleura, pericardium, azygos vein, diaphragm or peritoneum
- T4b: Tumor invades other adjacent structures, such as the aorta, vertebral body or airway
Superpage
Superpage Topics
Achalasia and motor disorders
Adenocarcinoma of the esophagus and GE junction
Adenoid cystic carcinoma
Adenosquamous carcinoma
Anatomy & embryology
Atresia and tracheoesophageal fistula (pending)
Barrett esophagus
Barrett related dysplasia
Basaloid squamous cell carcinoma
Candida
Chemical (corrosive) esophagitis
CMV
Crohn's disease
Diverticula
Eosinophilic esophagitis
Epidermoid metaplasia
Esophageal carcinoma-overview
Esophageal cysts
Esophageal manifestation of dermatologic disease (pending)
Esophageal manifestations of collagen vascular disease
Esophageal sarcoma-overview
Esophagitis dissecans superficialis
Esophagitis dissecans superficialis
Esophagitis-overview
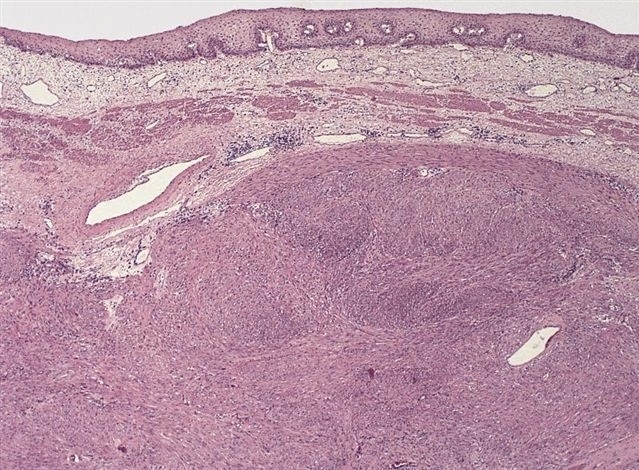
Gastrointestinal stromal tumor
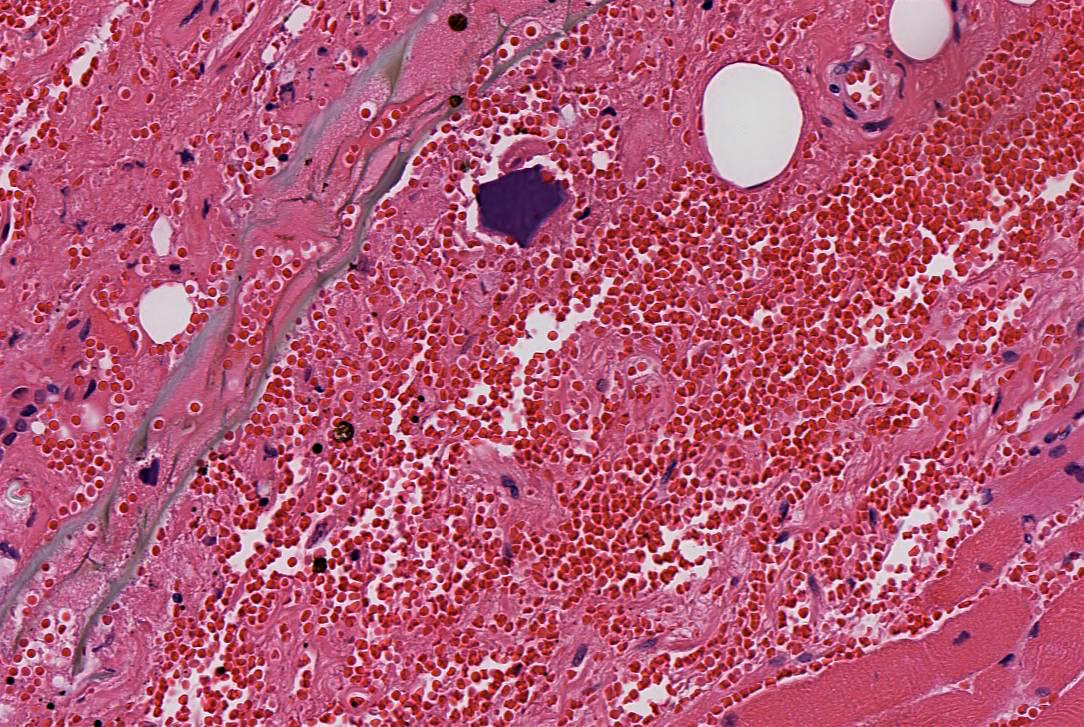
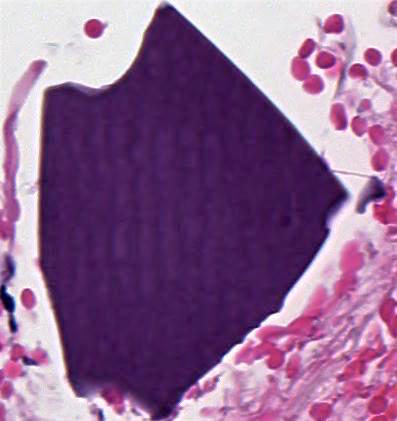
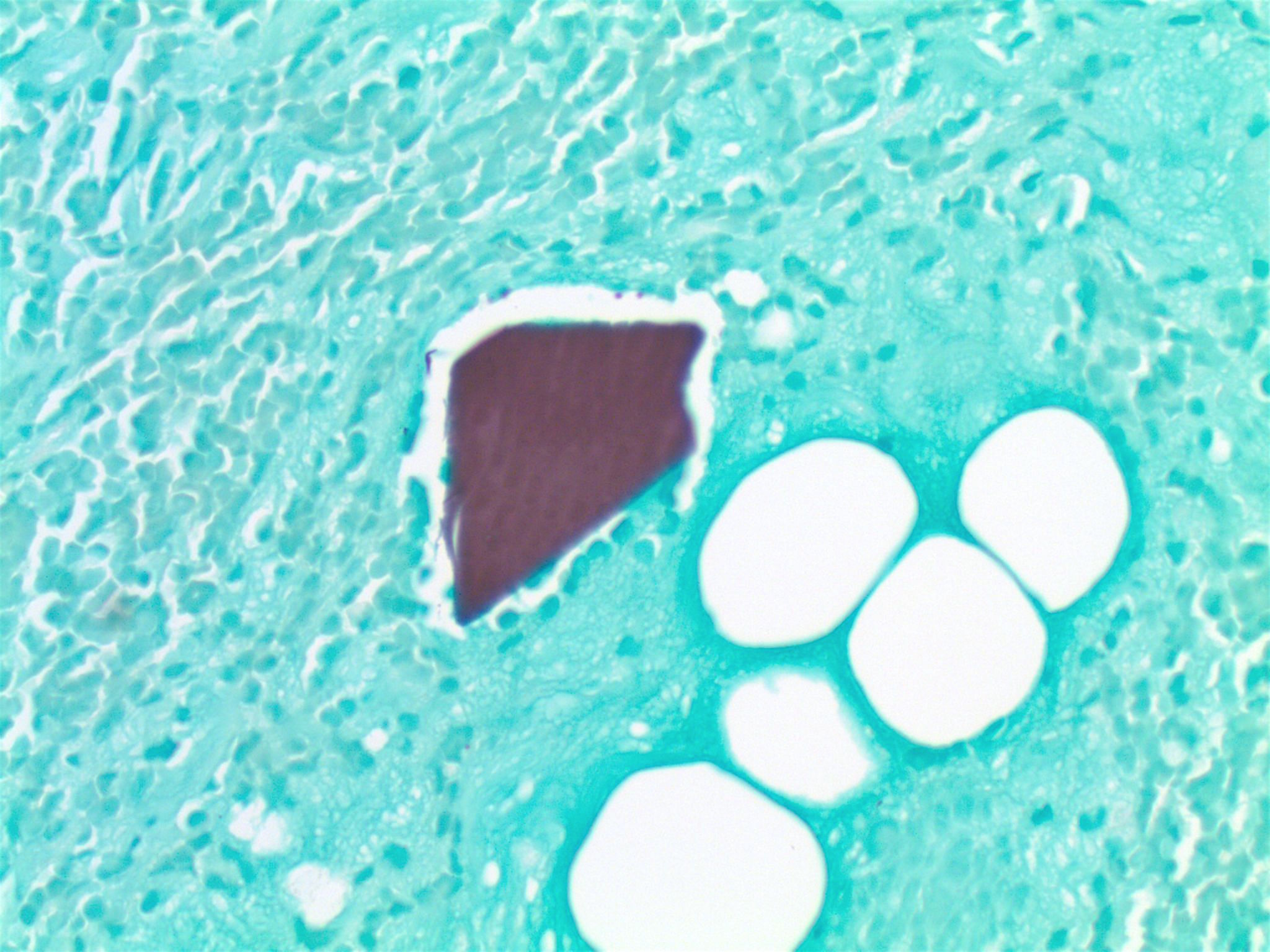
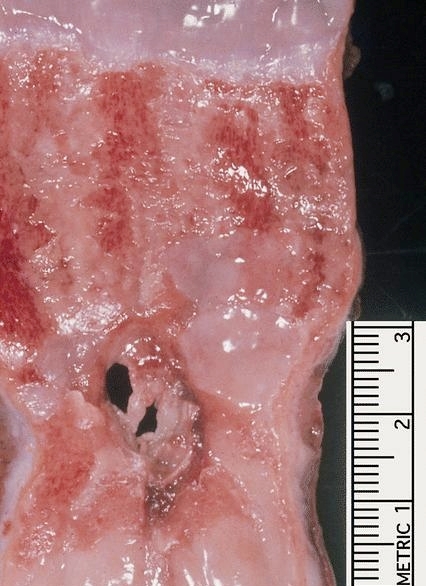
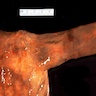
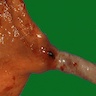
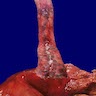
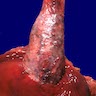
Giant fibrovascular polyp / well differentiated liposarcoma
Glycogenic acanthosis
Graft versus host disease
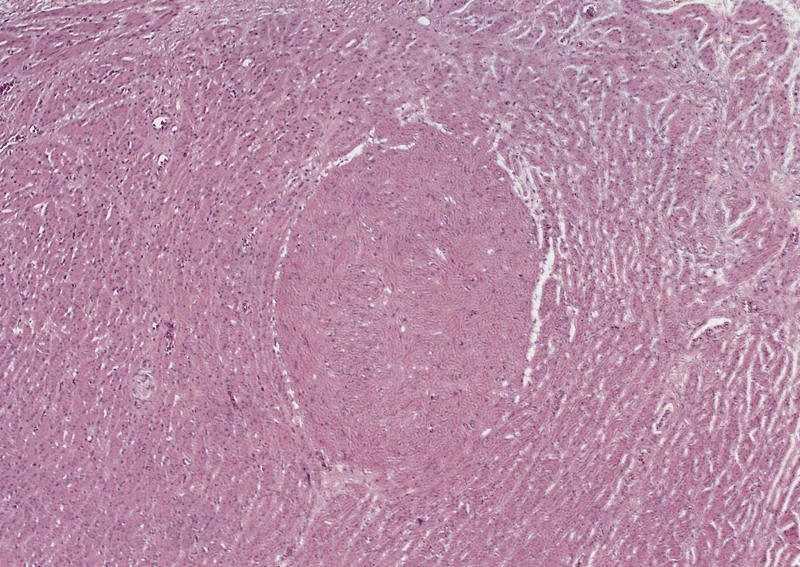
Granular cell tumor
Grossing & features to report
Heterotopic / ectopic pancreatic tissue
Heterotopic / ectopic sebaceous glands
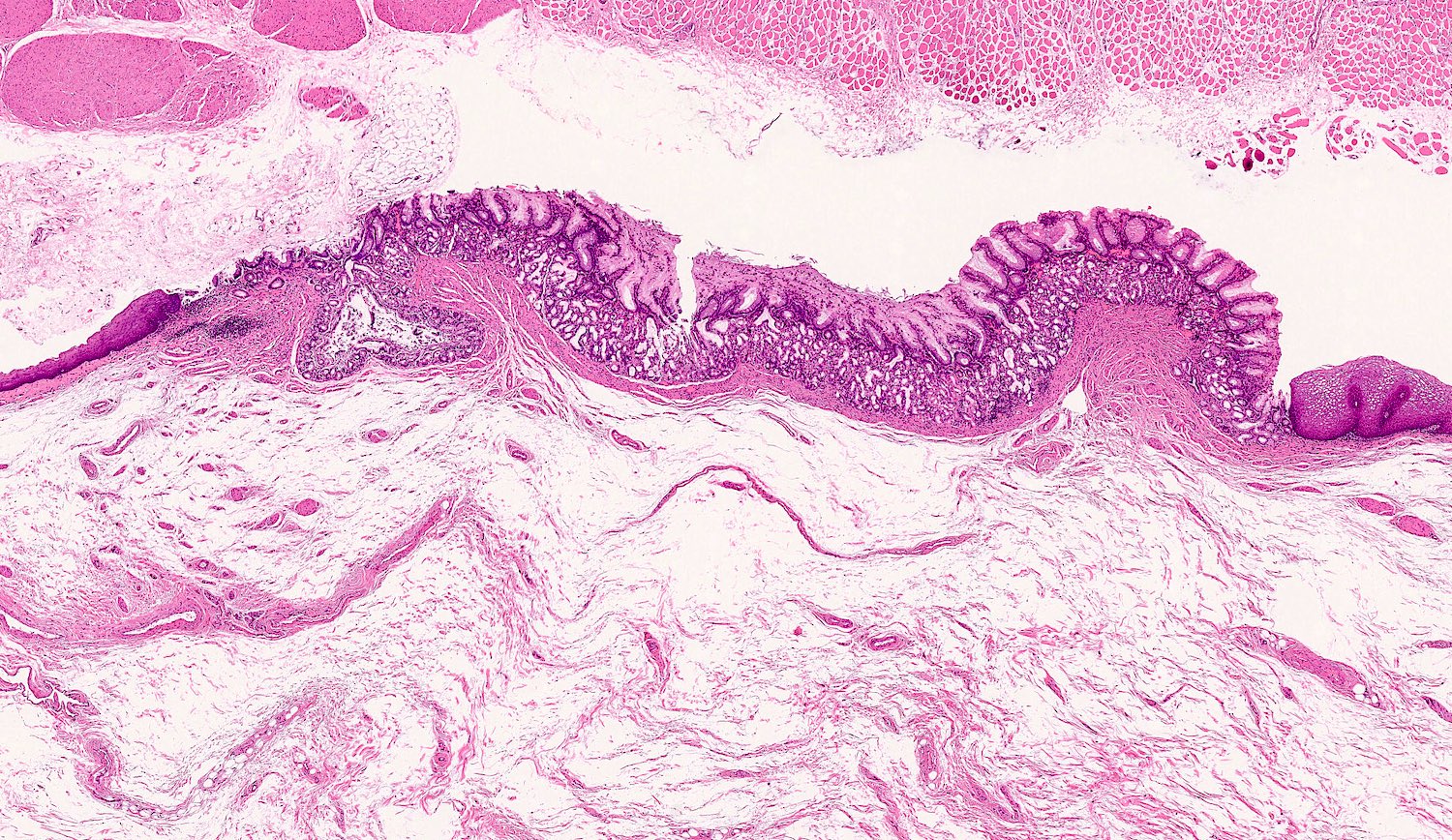
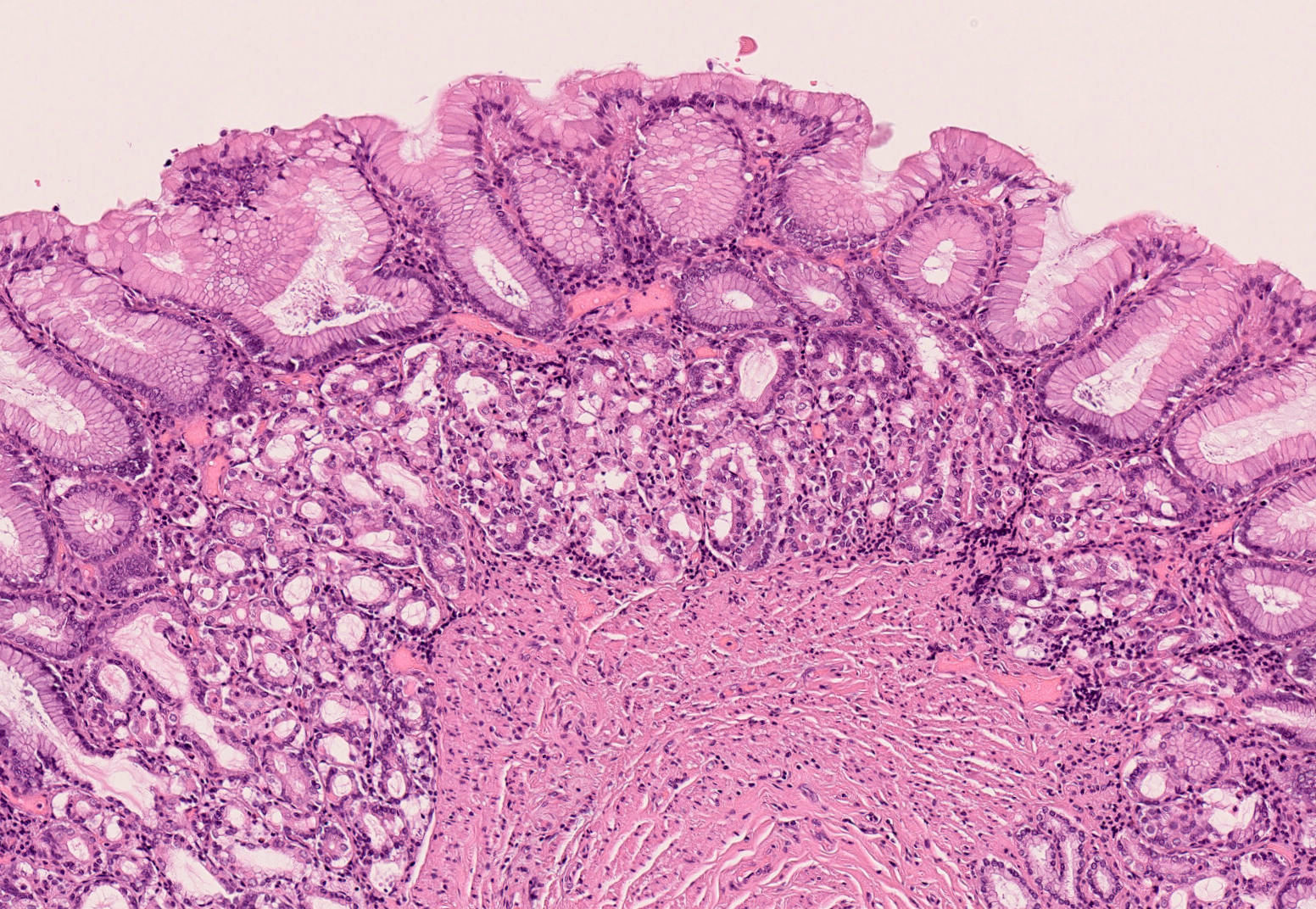
Heterotopic gastric mucosa
Histology
HSV esophagitis
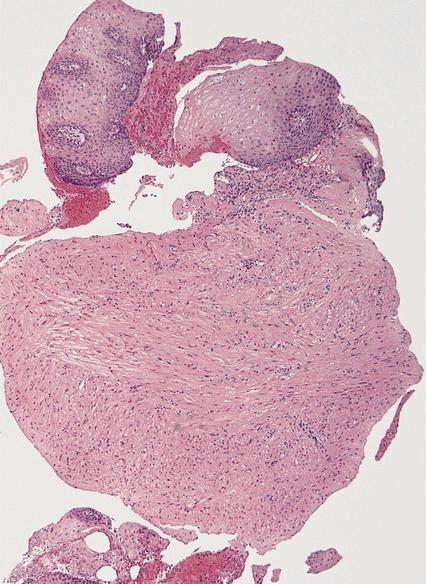
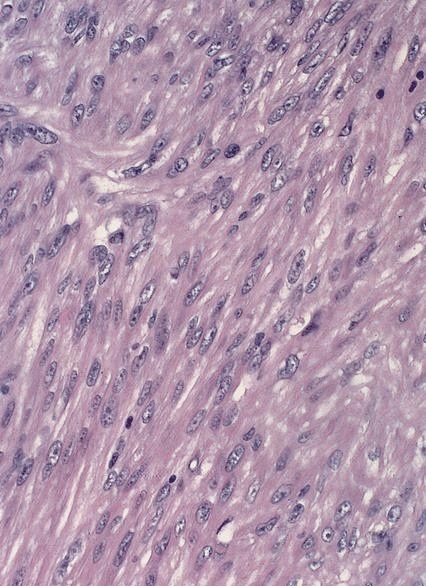
Leiomyoma
Lichenoid esophagitis
Lymphocytic esophagitis
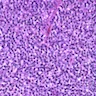
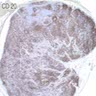
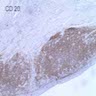
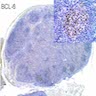
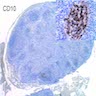
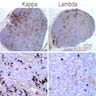
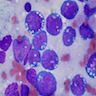
Lymphoma
Melanocytosis
Melanoma
Mucoepidermoid carcinoma (pending)
Neuroendocrine carcinoma
Neuroendocrine carcinoma
Pill induced esophagitis
Radiation esophagitis
Reflux esophagitis / gastroesophageal reflux disease
Rings and webs
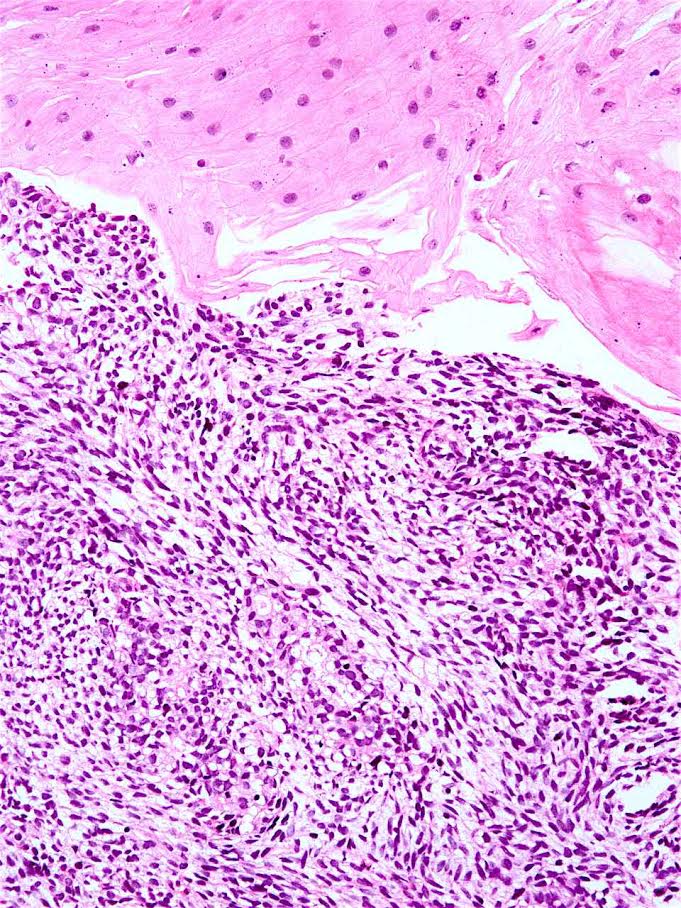
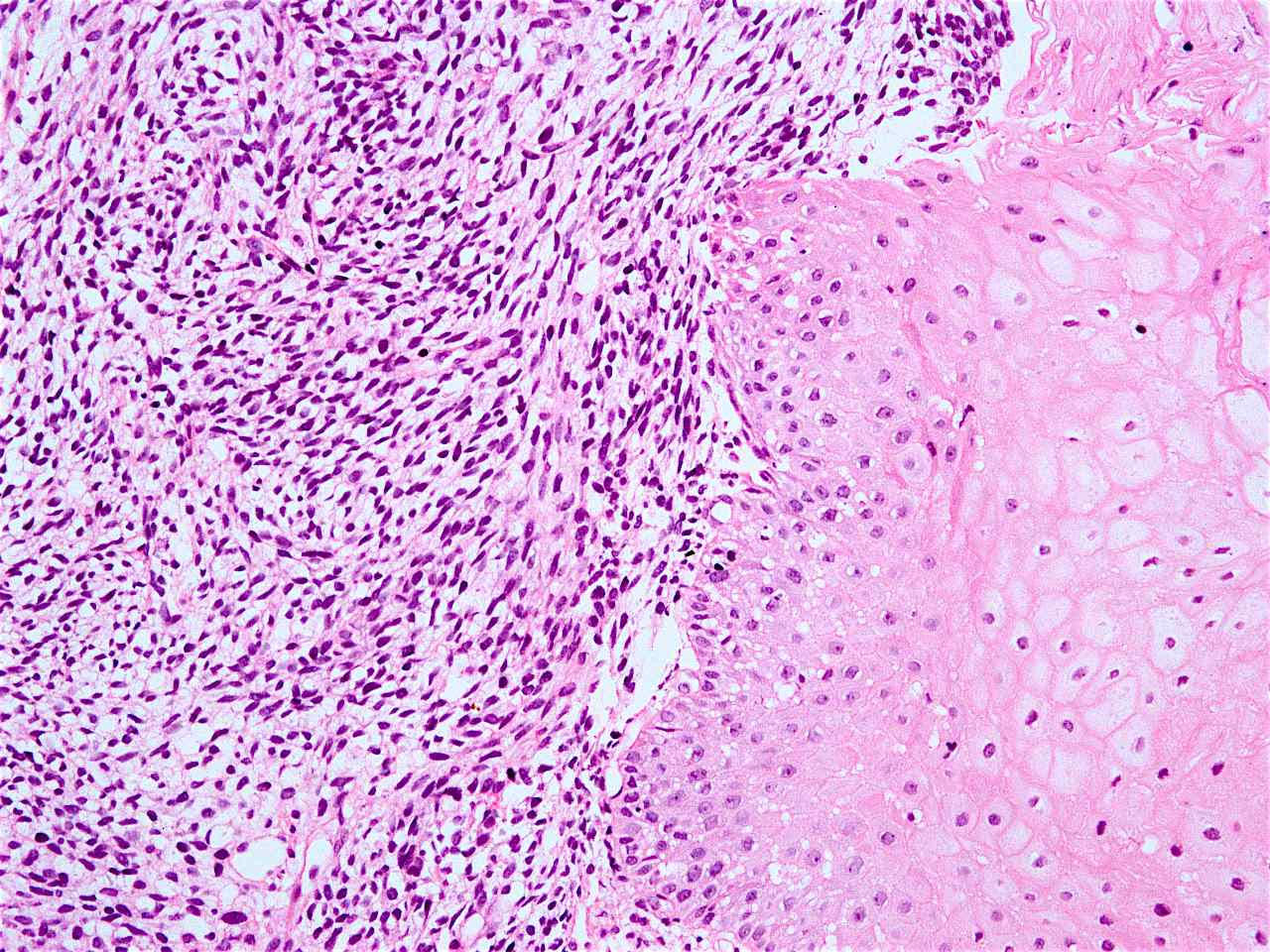
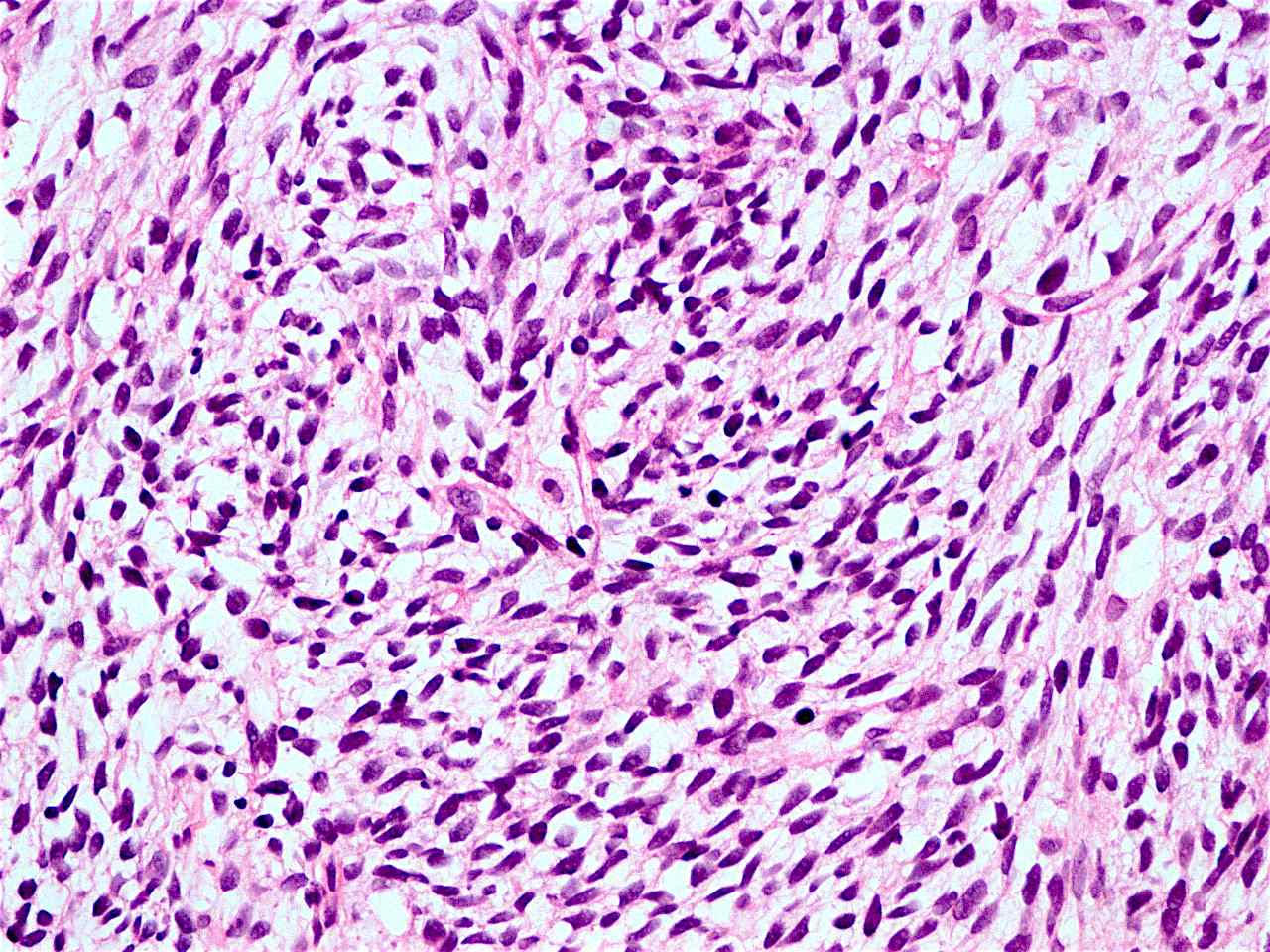
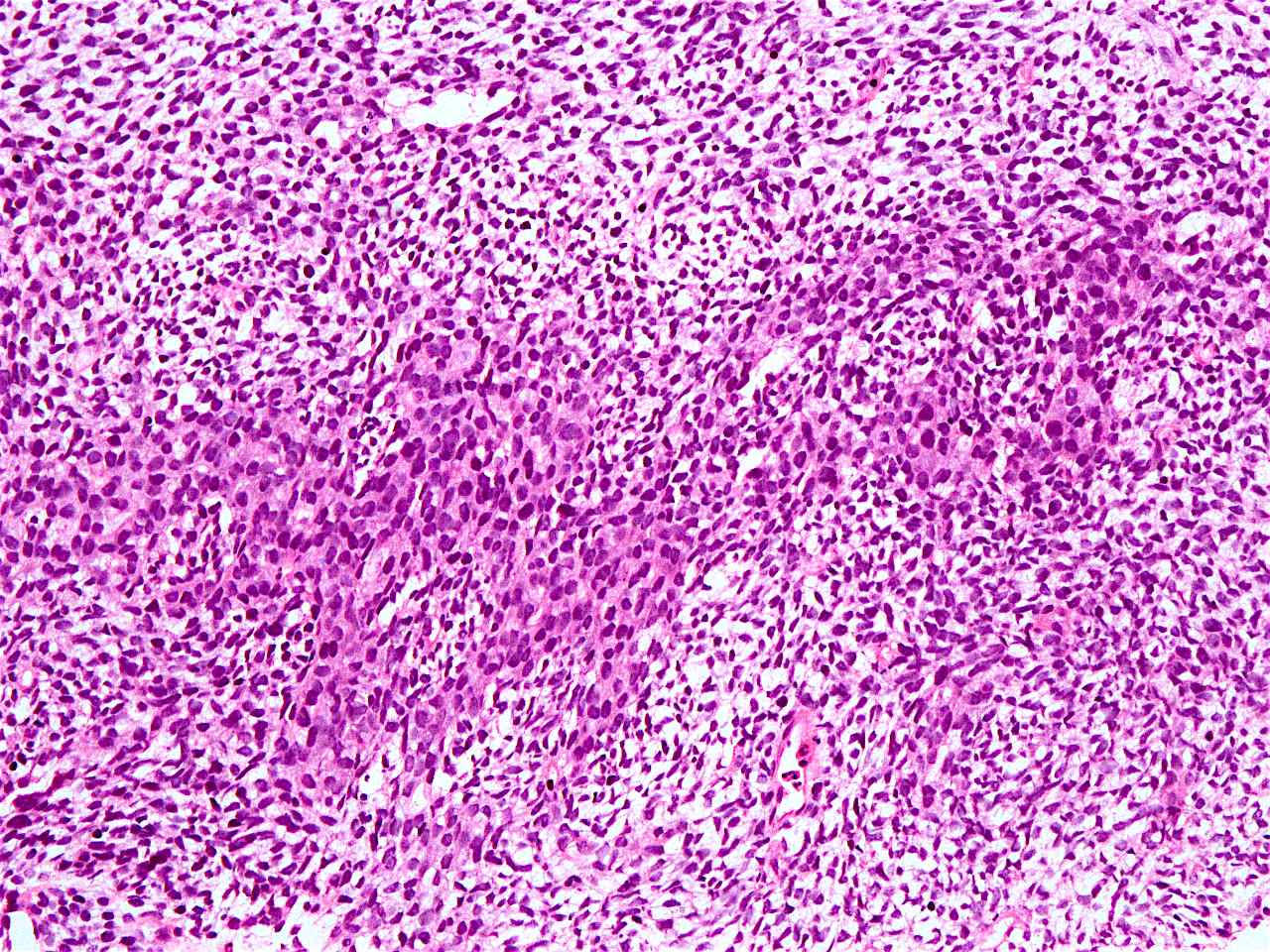
Sarcomatoid carcinoma
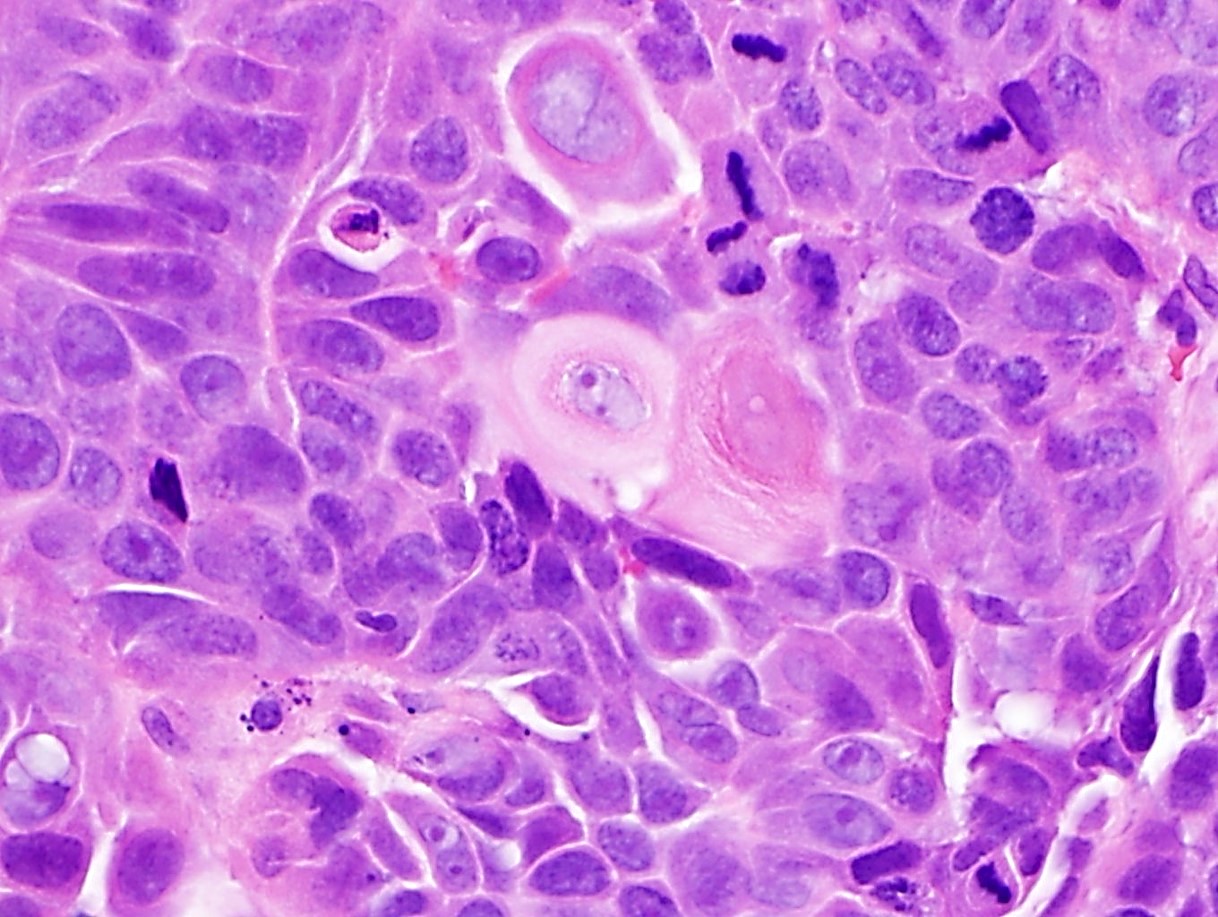
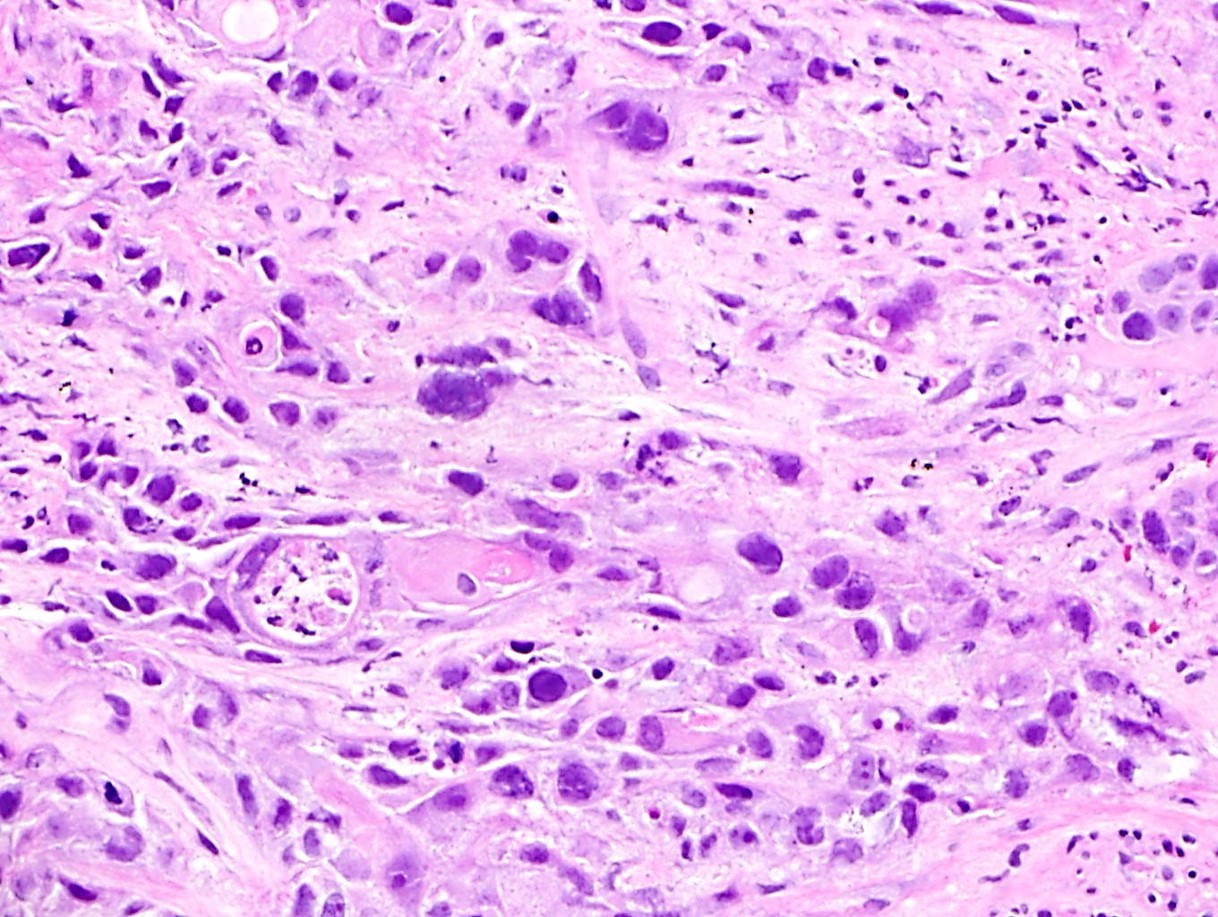
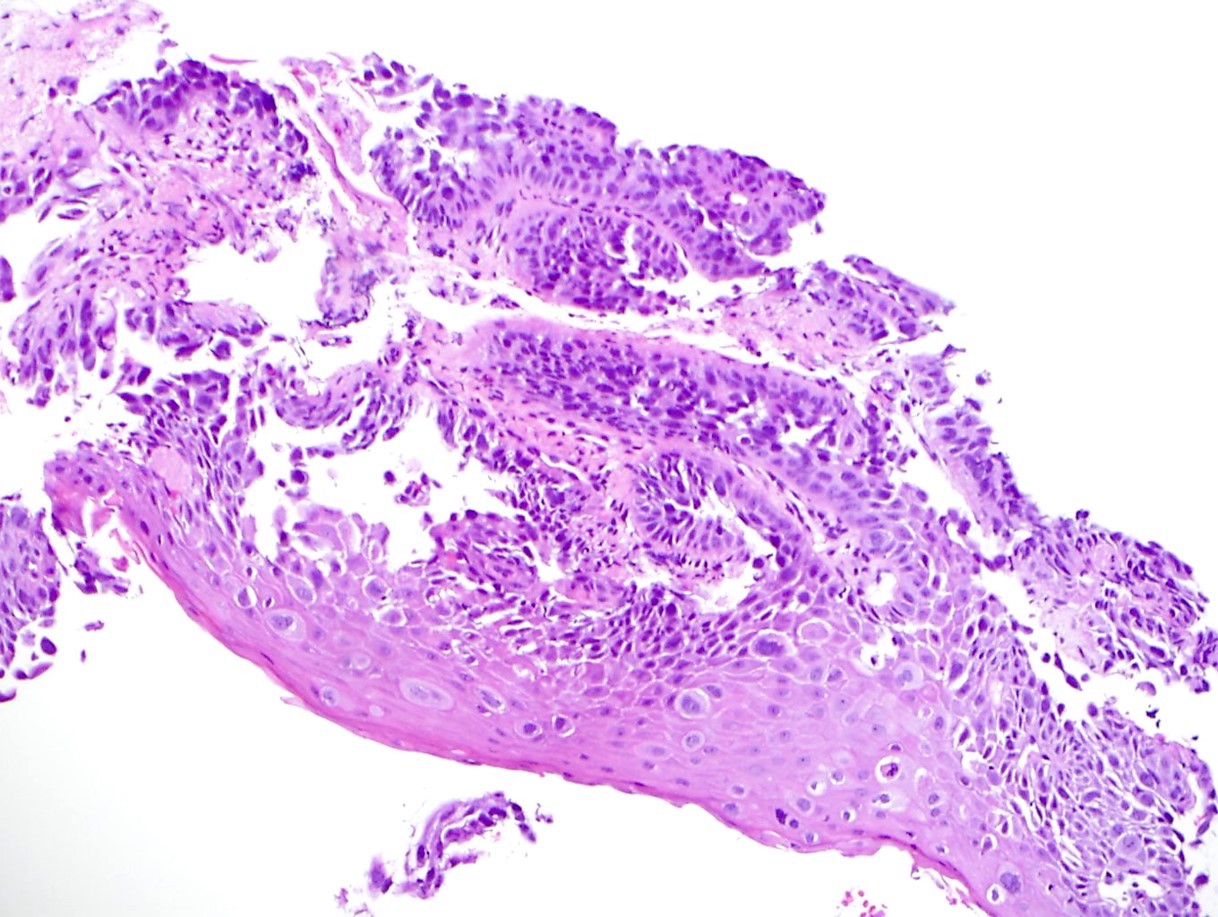
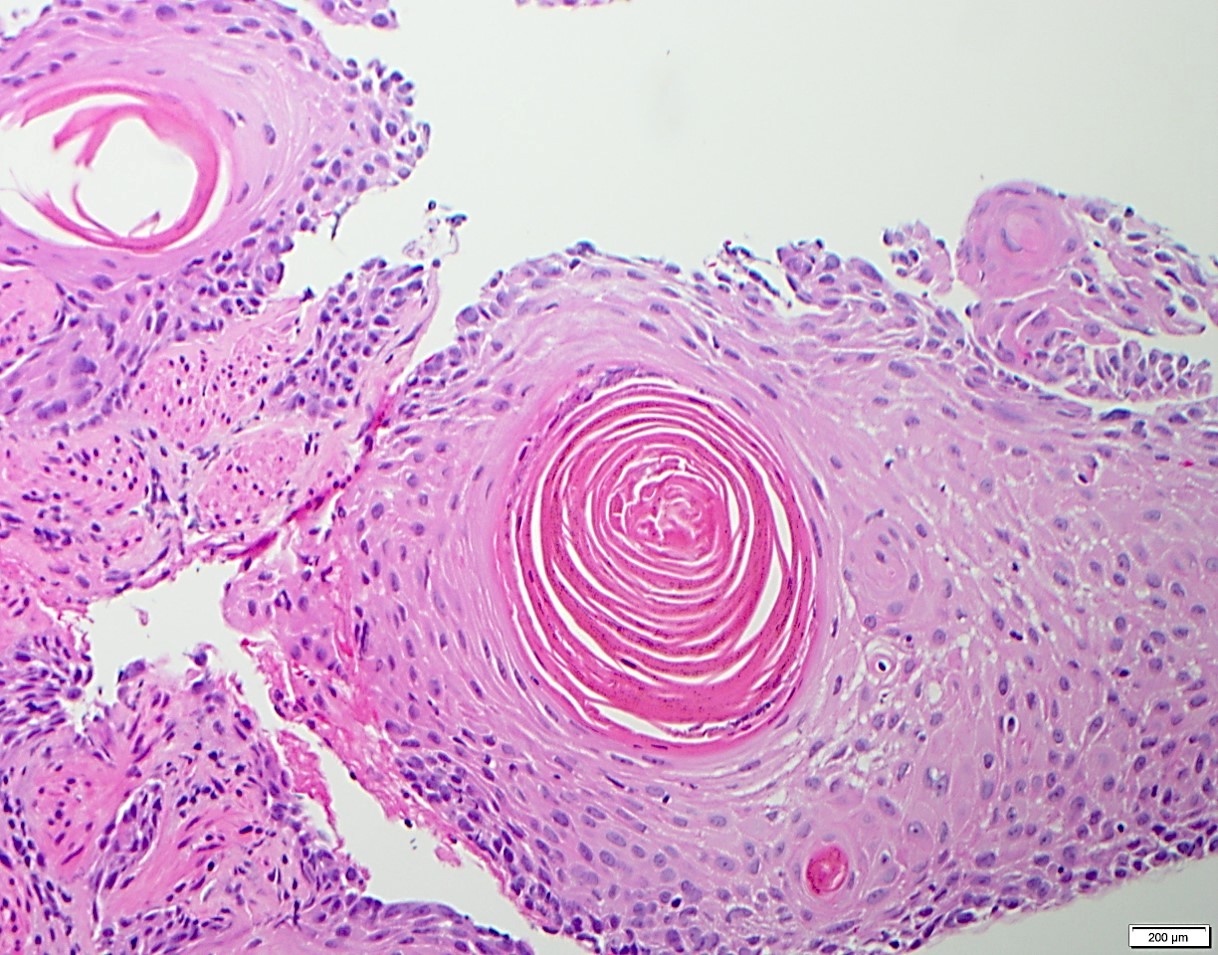
Squamous cell carcinoma
Squamous dysplasia
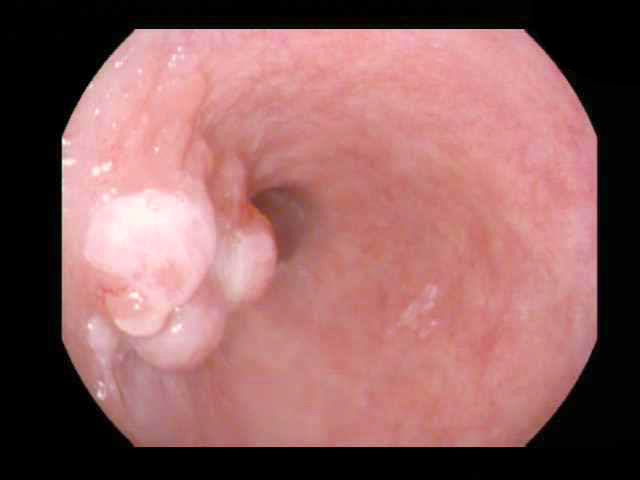
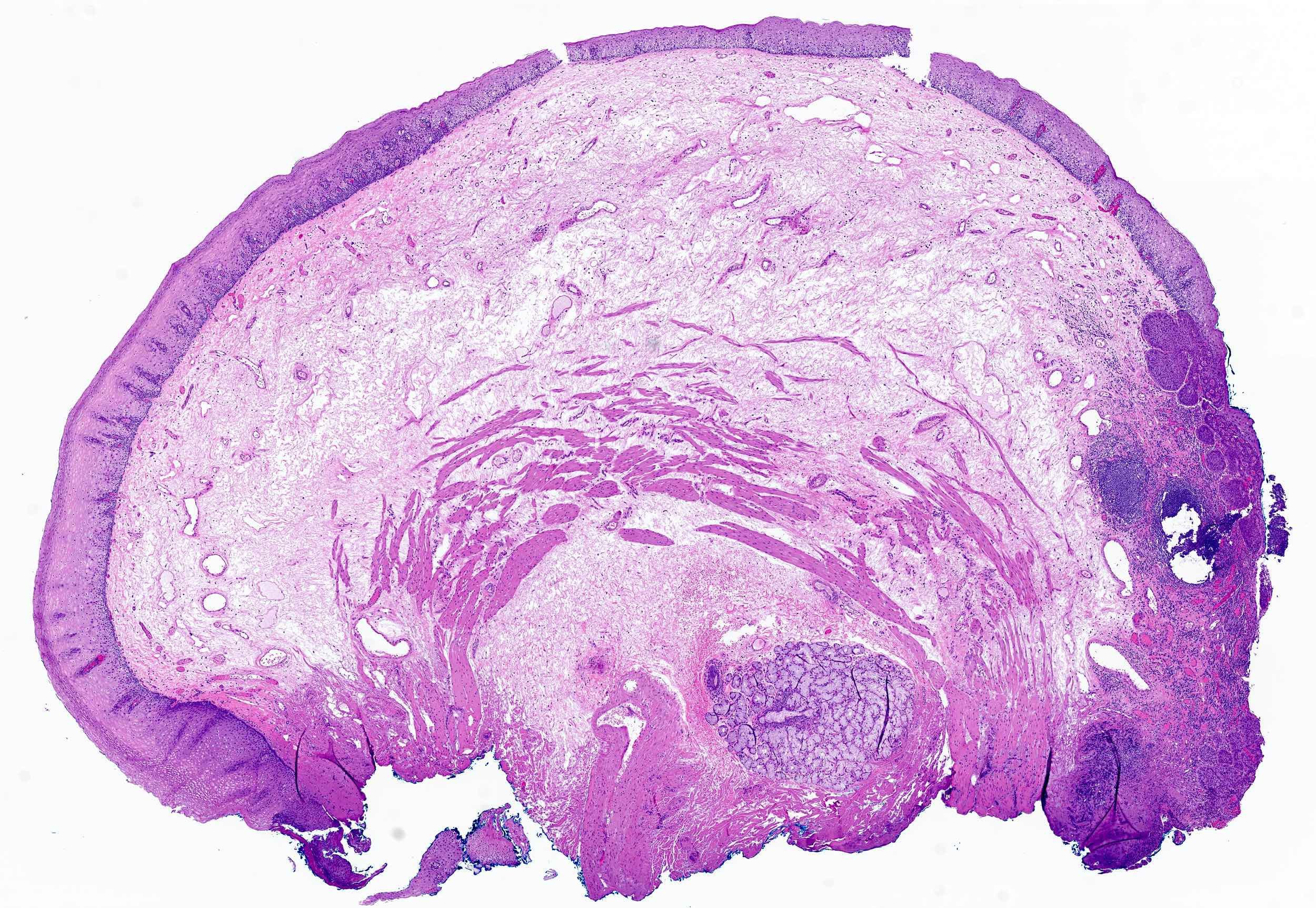
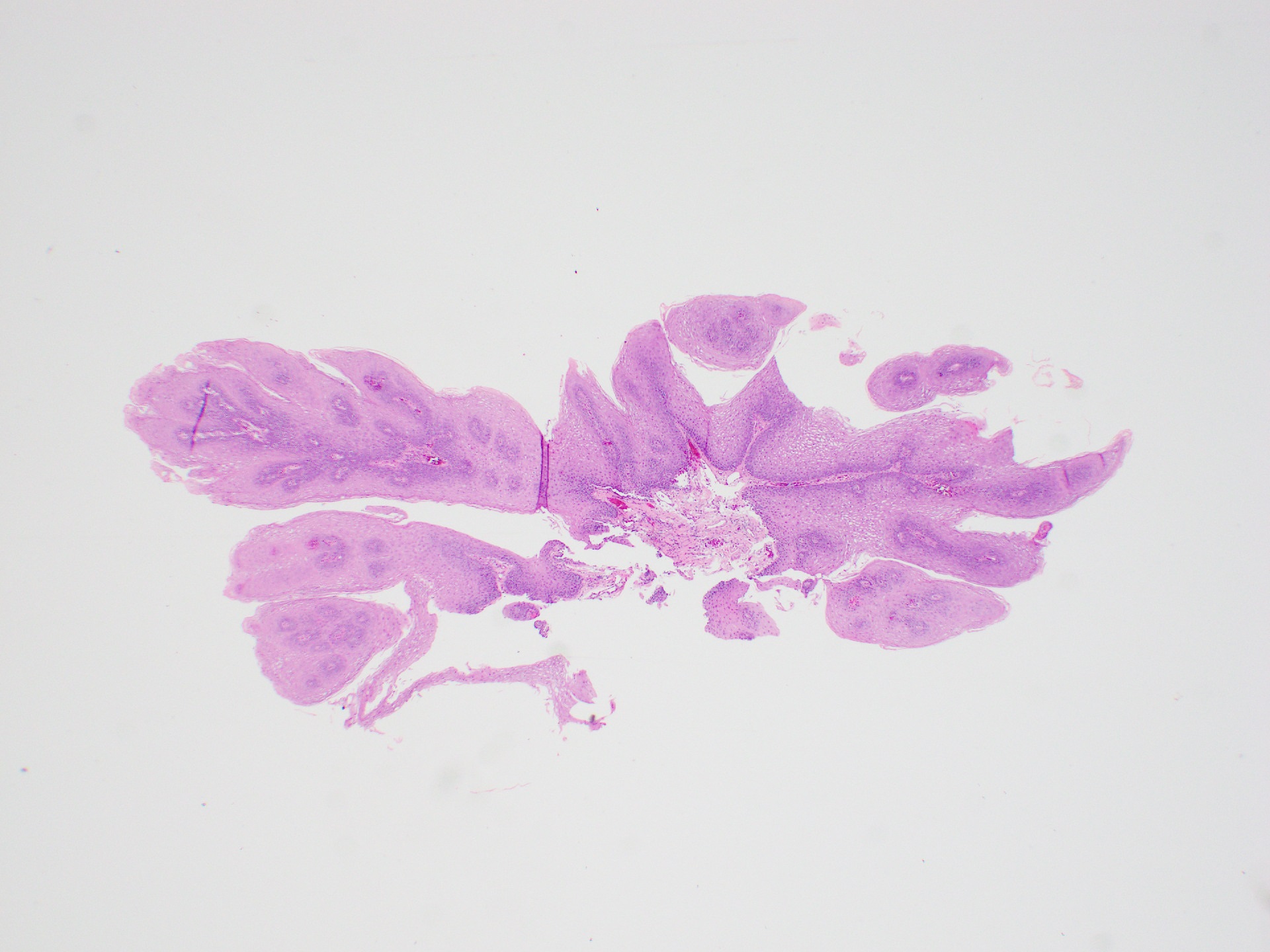
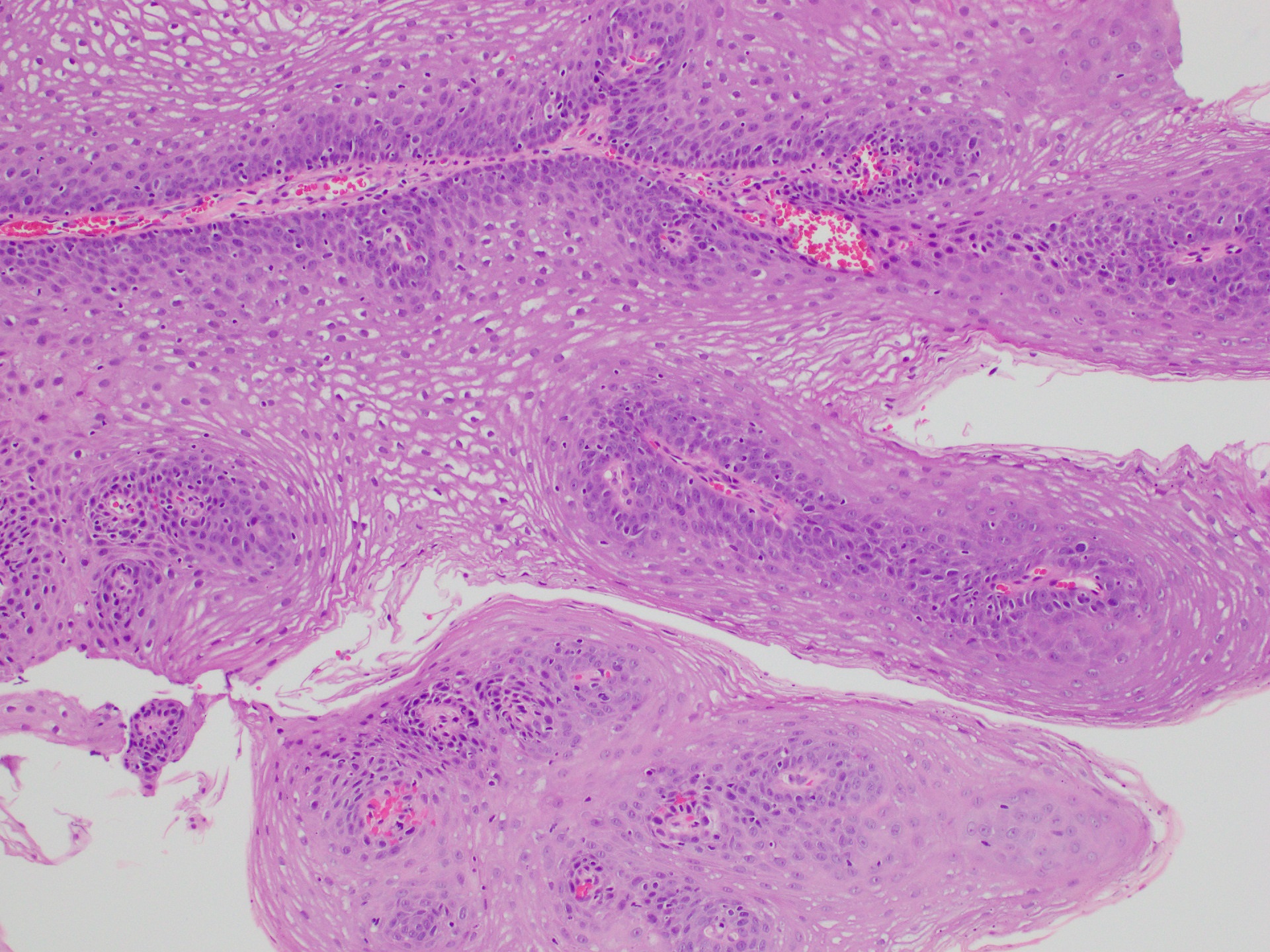
Squamous papilloma
Staging
Undifferentiated carcinoma
Varices
Verrucous squamous cell carcinoma
Well differentiated neuroendocrine tumor
WHO classificationAchalasia and motor disorders
Table of Contents
Definition / general | Terminology | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Radiology images | Treatment | Gross description | Gross images | Microscopic (histologic) description | Positive stains | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
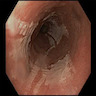
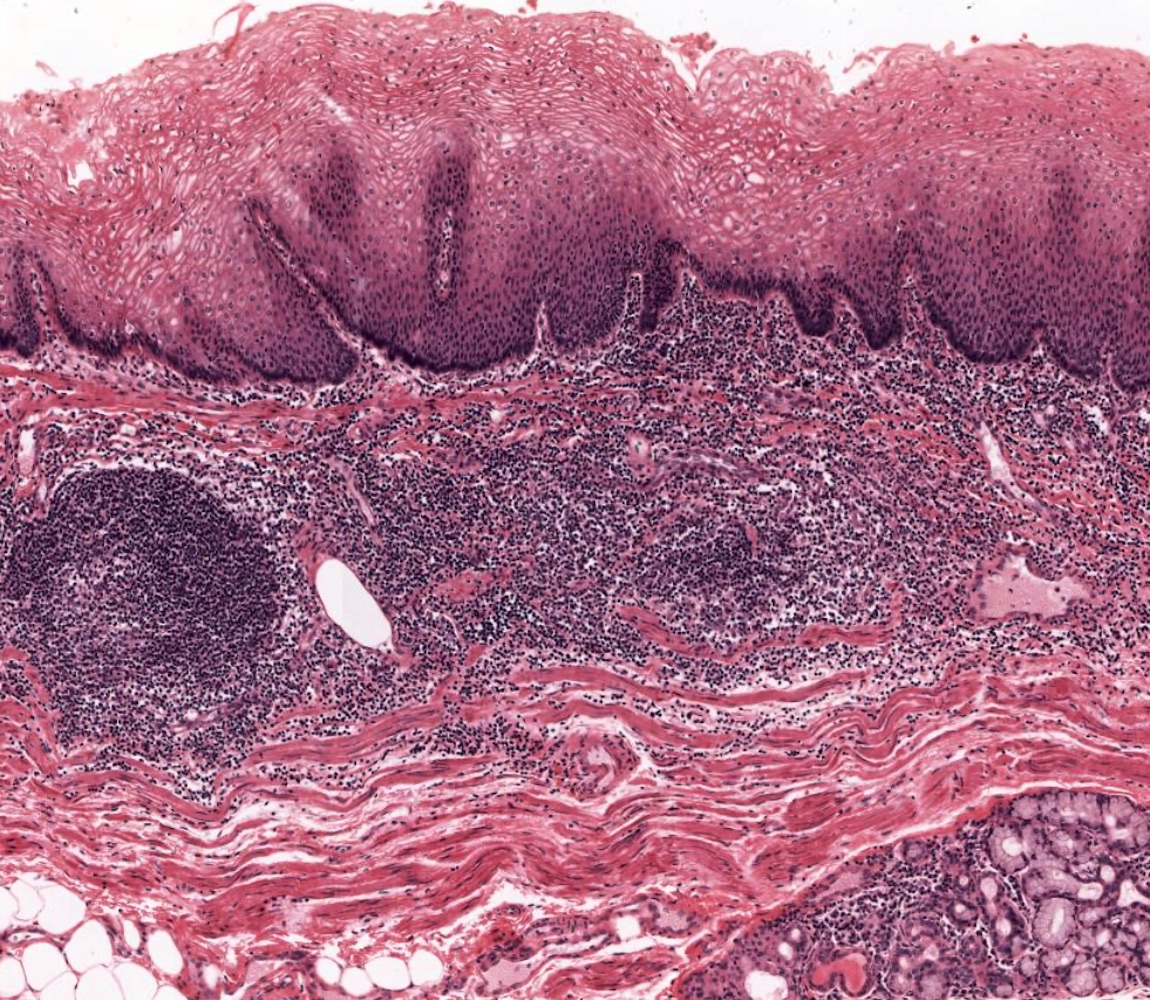
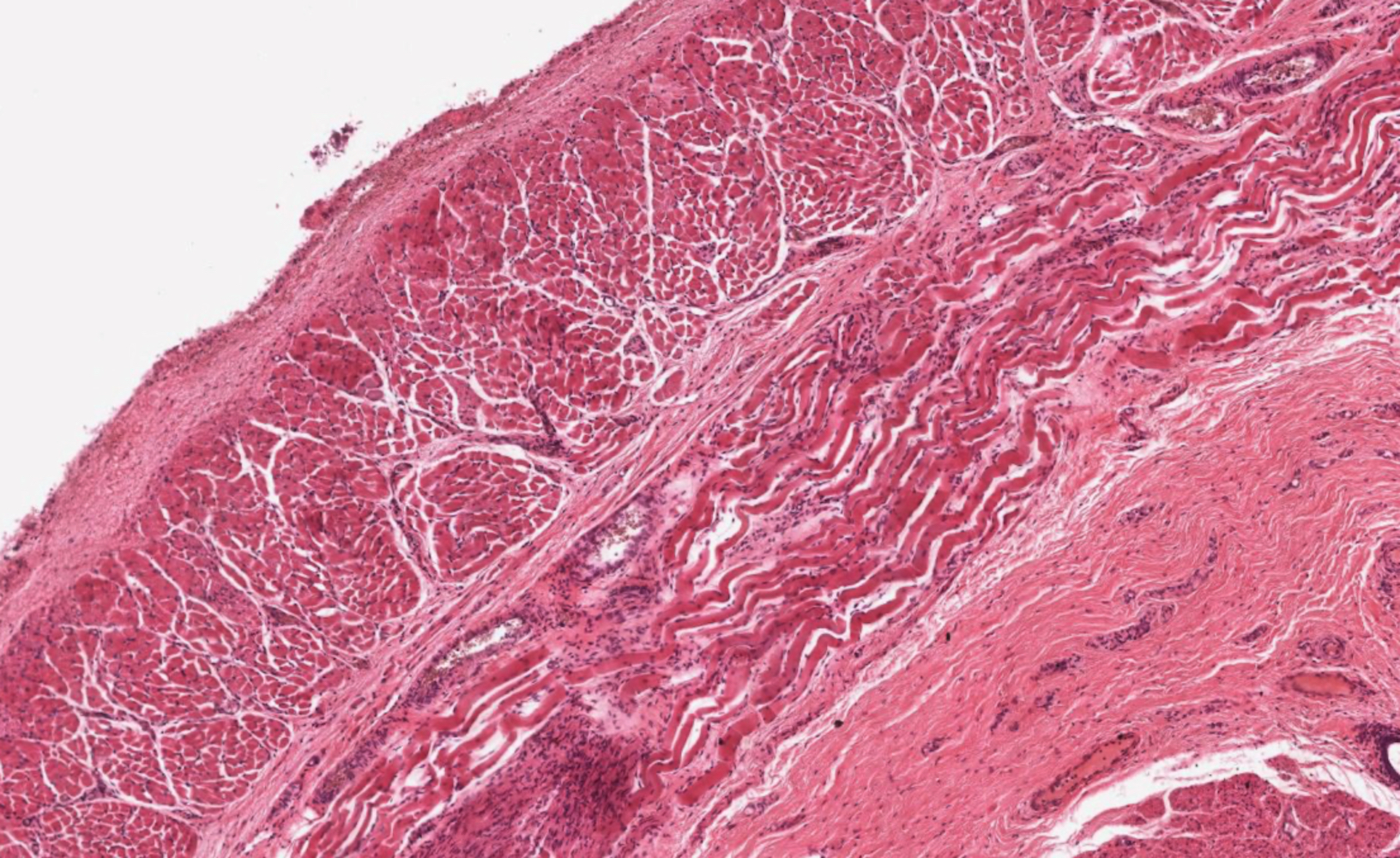
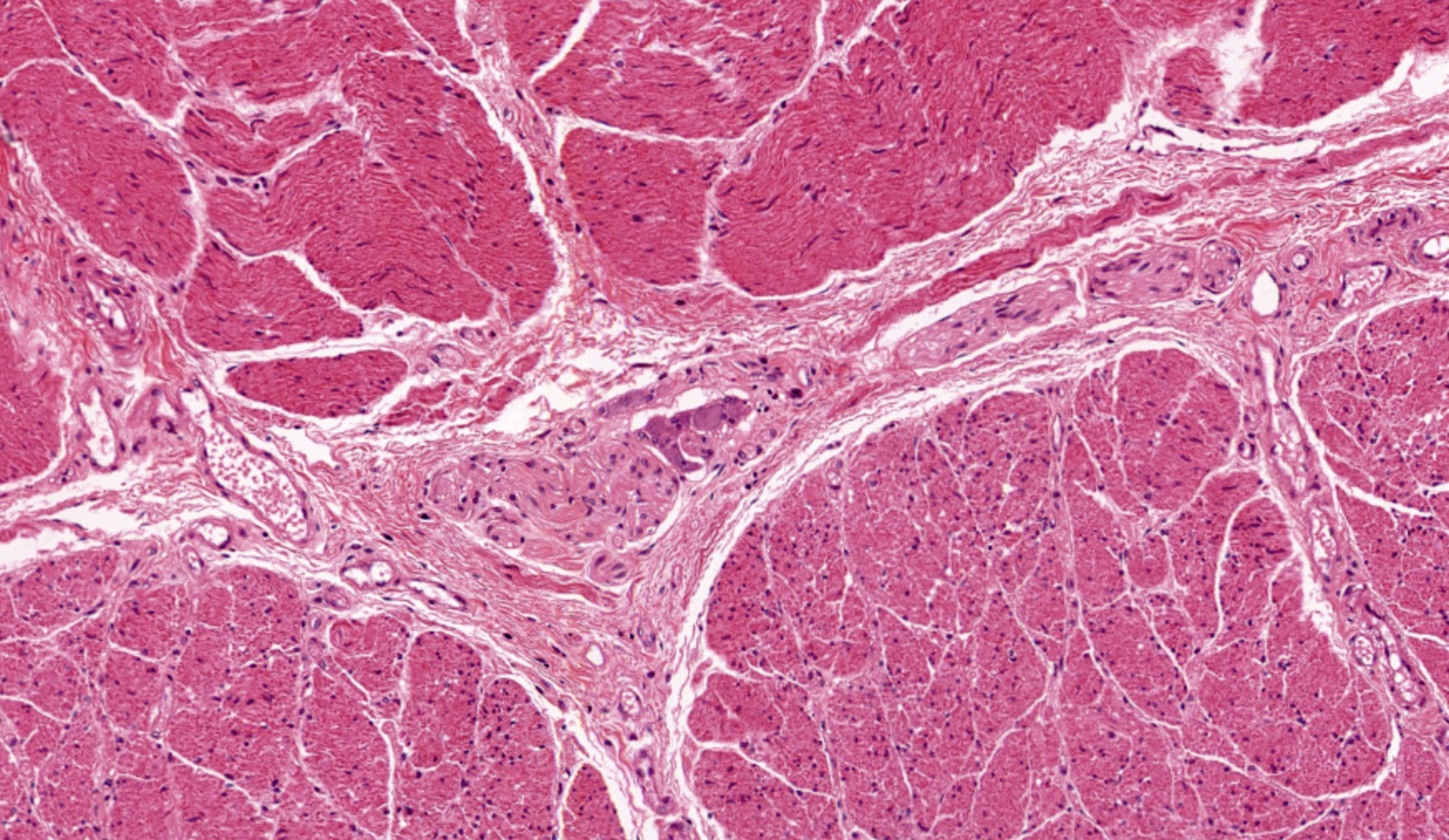
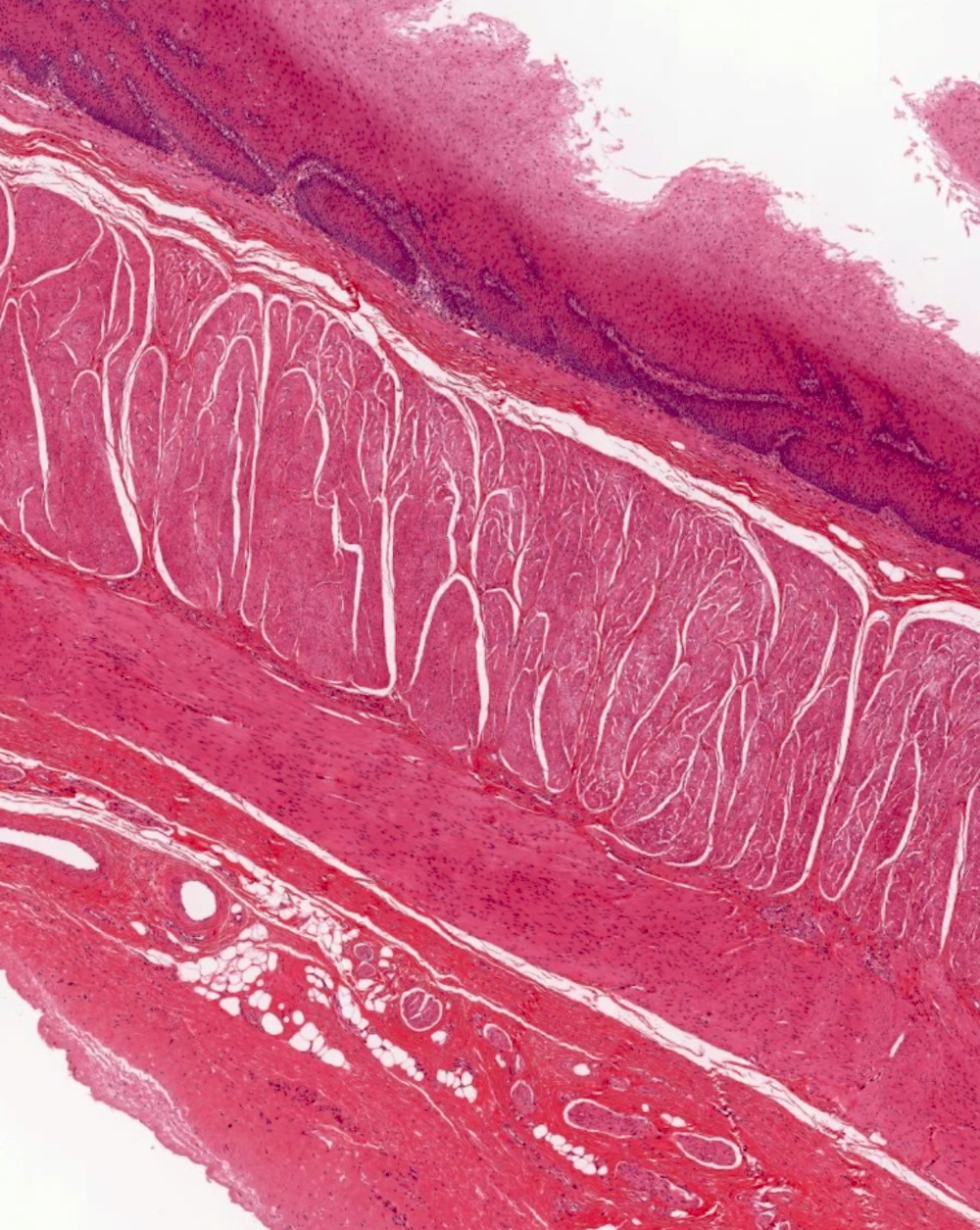
- Esophageal motor disorder characterized by lack of progressive peristalsis and partial / incomplete relaxation of lower esophageal sphincter (LES), preventing passage of food into stomach
- Preferentially involves circular layer of muscularis propria, which is hypertrophied
- Patients with achalasia may also have GERD (Eur J Gastroenterol Hepatol 2006;18:369)
- 5% risk (33x normal) of esophageal squamous cell carcinoma, at mean 21 - 28 years after diagnosis of achalasia (Anticancer Res 2000;20:3717)
- Also increased risk of aspiration, Barrett esophagus, Candida infection, gastroesophageal reflux, lower esophageal diverticula, peptic ulceration, stricture (Ann Surg 2006;243:196)
Terminology
- Also called cardiospasm, megaesophagus
Pathophysiology
- Due to T cell mediated destruction or complete absence of myenteric ganglion cells in lower third of esophagus (Am J Gastroenterol 2005;100:1404)
Etiology
- Secondary causes: Allgrove syndrome (World J Gastroenterol 2006;12:4764), amyloidosis, Chagas disease (Trypanosoma cruzi, common in South America, destroys myenteric plexus of esophagus, duodenum, colon, ureter), diabetic autonomic neuropathy, polio, sarcoidosis, surgical ablation of dorsal motor nuclei, thyroid disease (World J Gastroenterol 2007;13:594), tumor
Clinical features
- Most cases are primary, i.e. idiopathic, usually young adults with progressive dysphagia, nocturnal regurgitation and aspiration of undigested food
- Can occur in children
Treatment
- Esophagomyotomy (World J Gastroenterol 2006;12:5921, GI Motility Online: Surgical Treatment for Achalasia [Accessed 13 February 2019]), dilation (World J Gastroenterol 2006;12:5763), botulinum toxin to inhibit LES cholinergic neurons
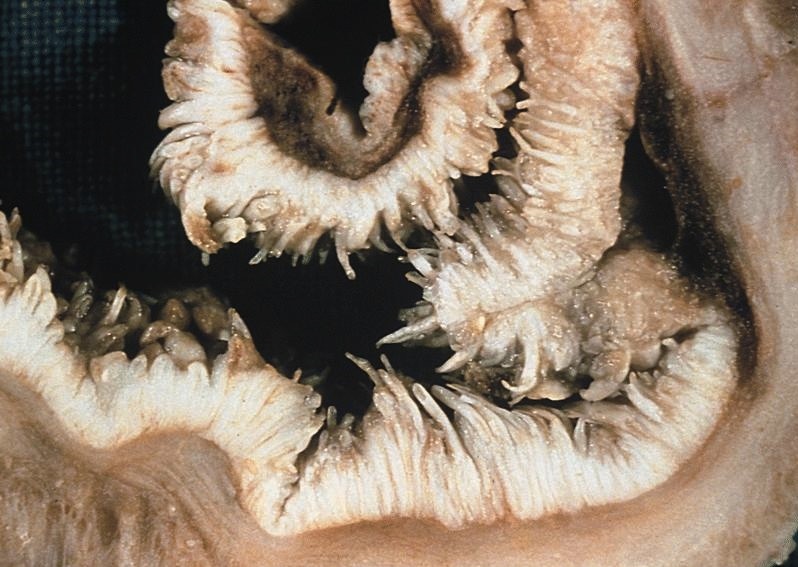
Gross description
- Progressive dilation of esophagus above LES, variable wall thickness
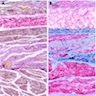
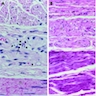
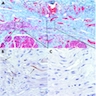
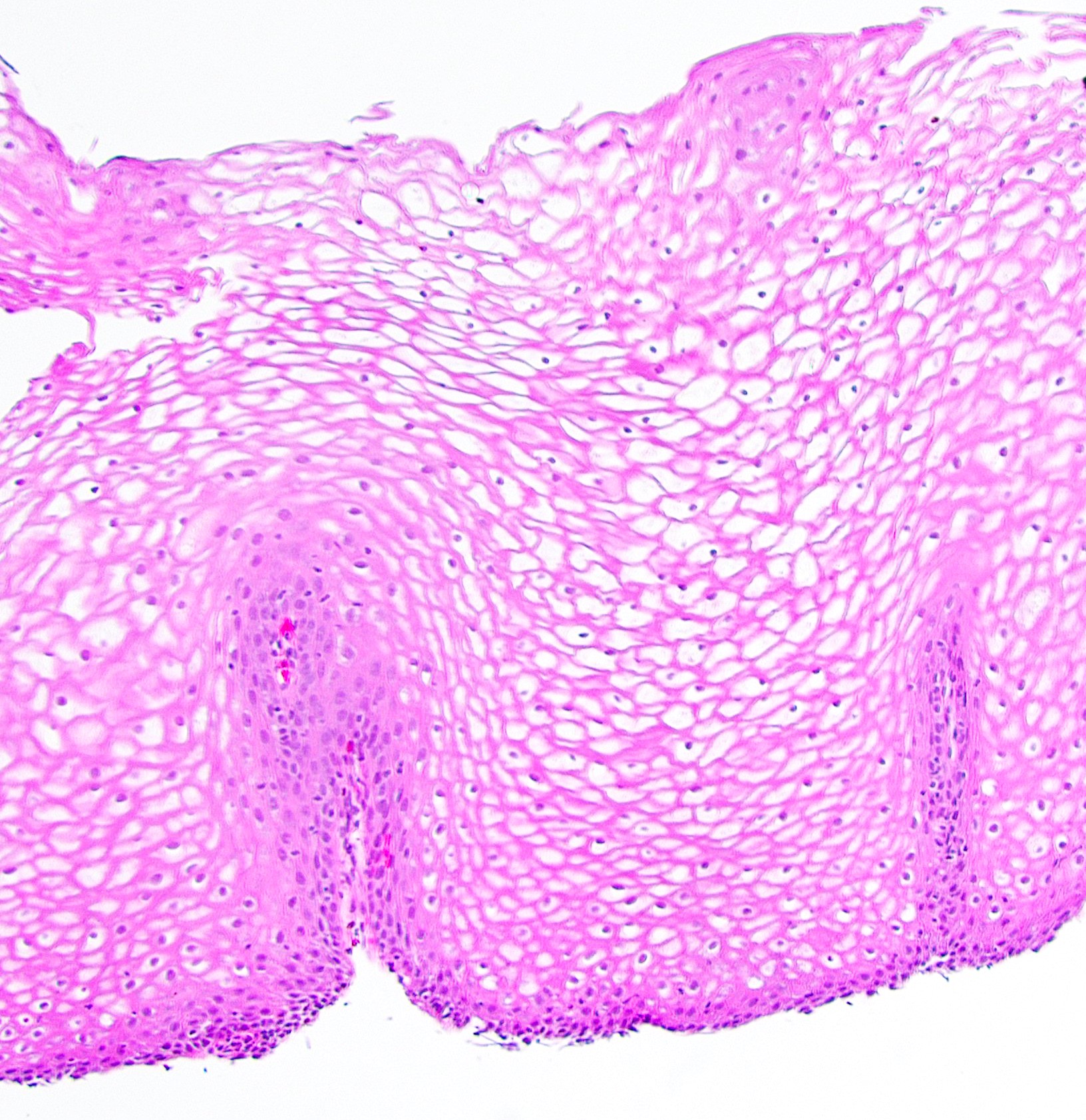
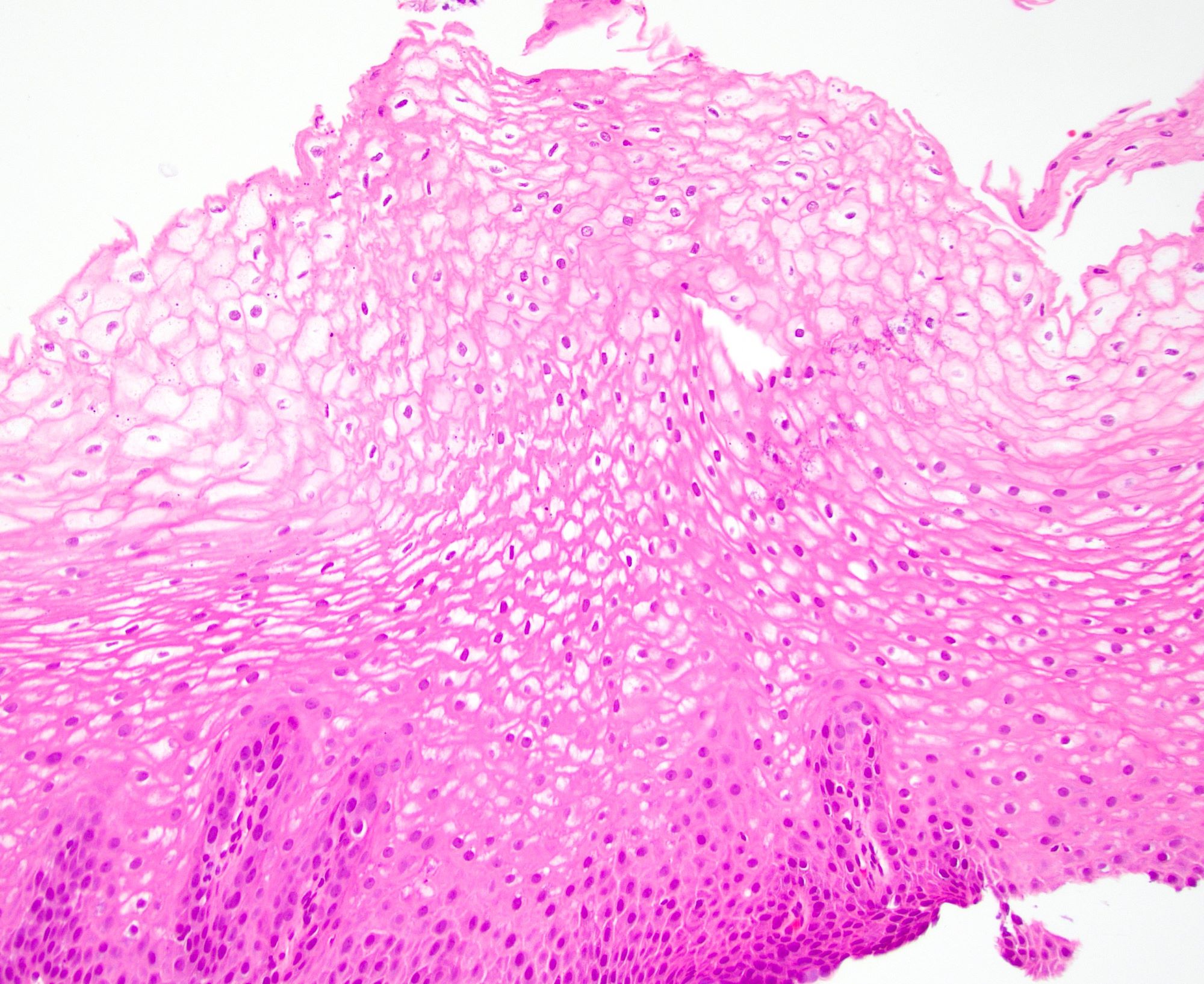
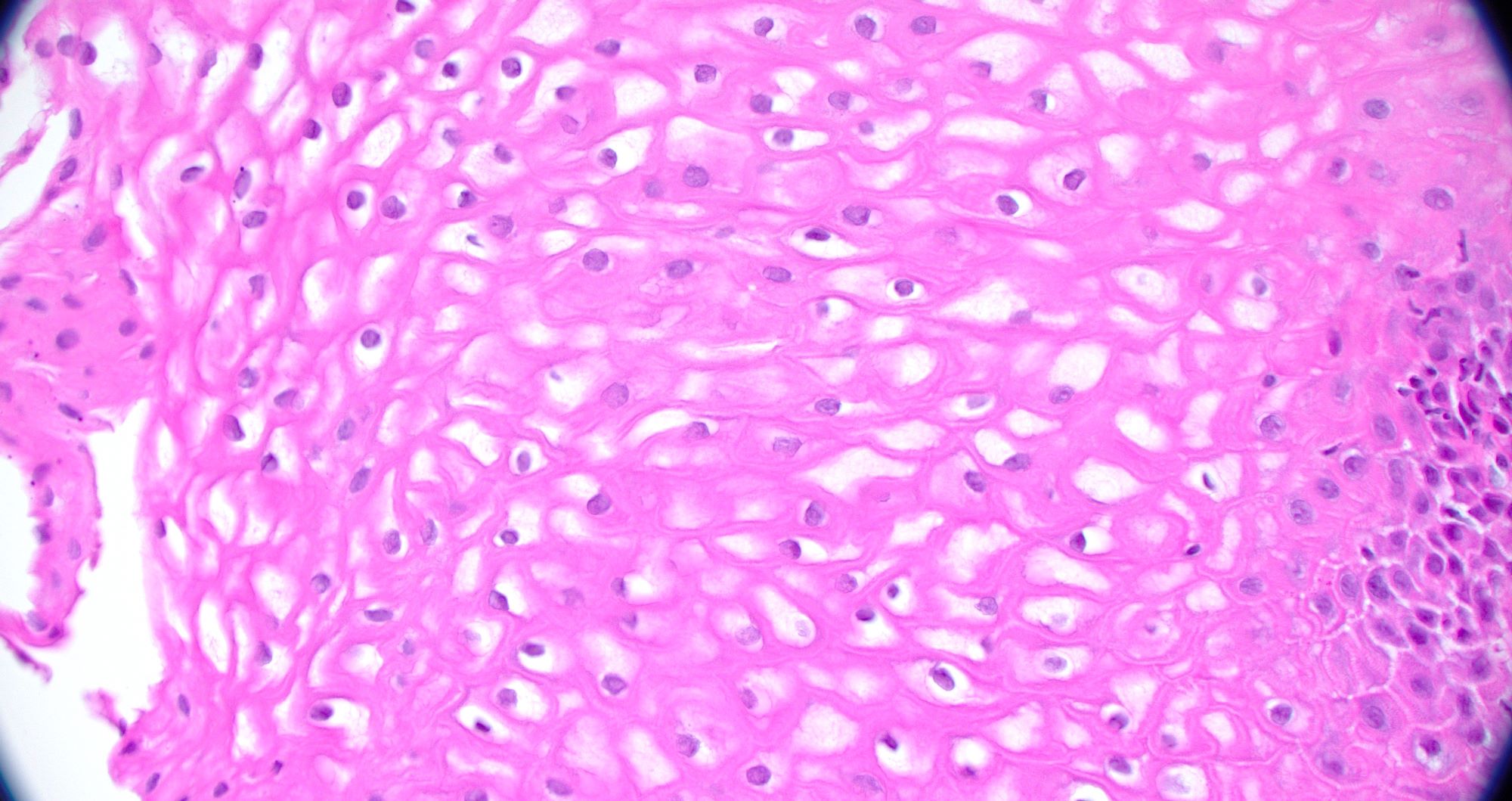
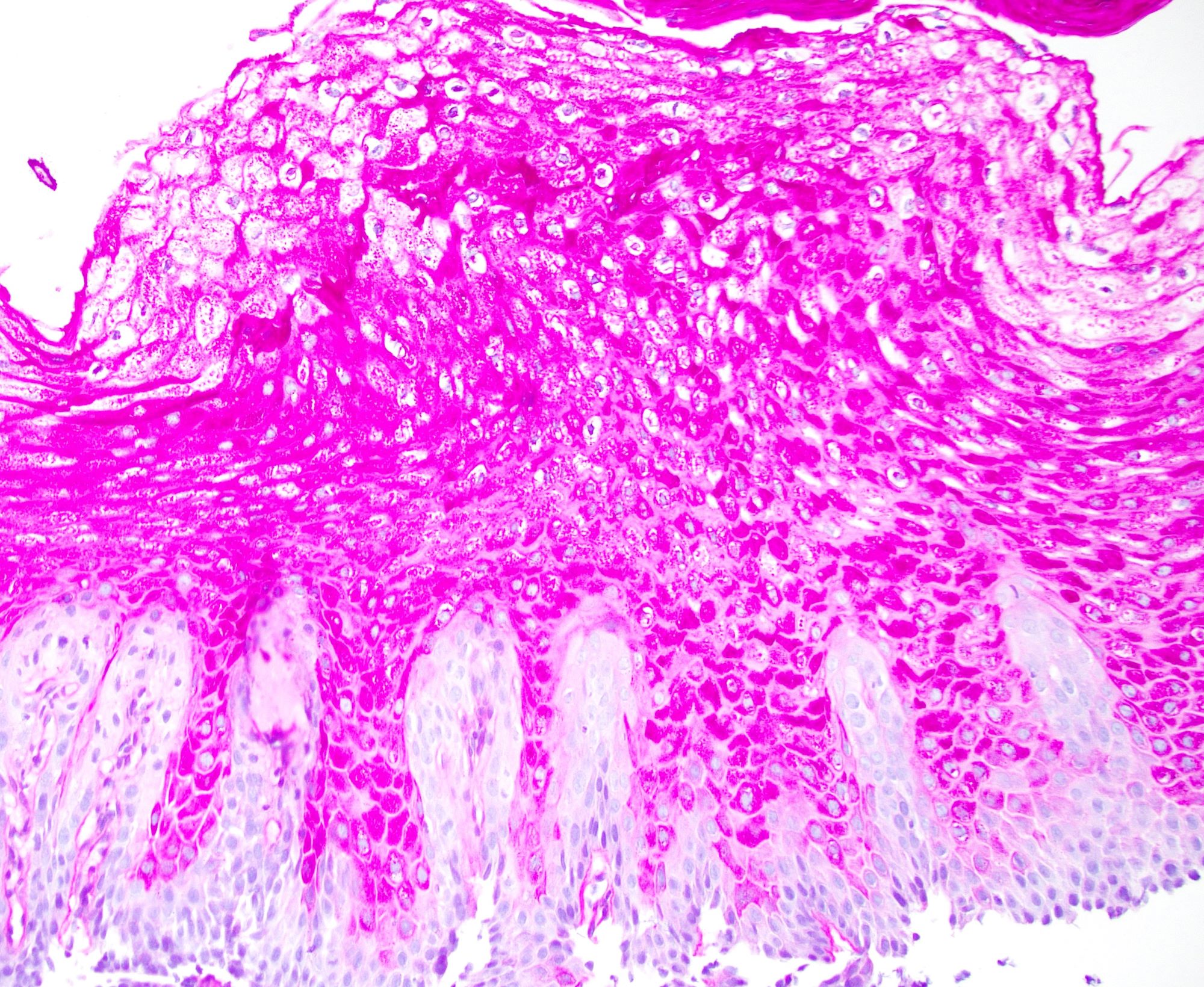
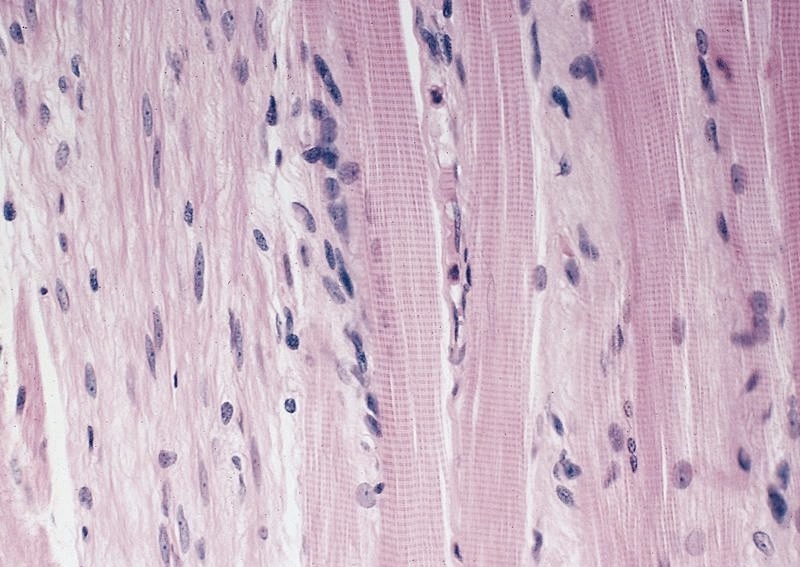
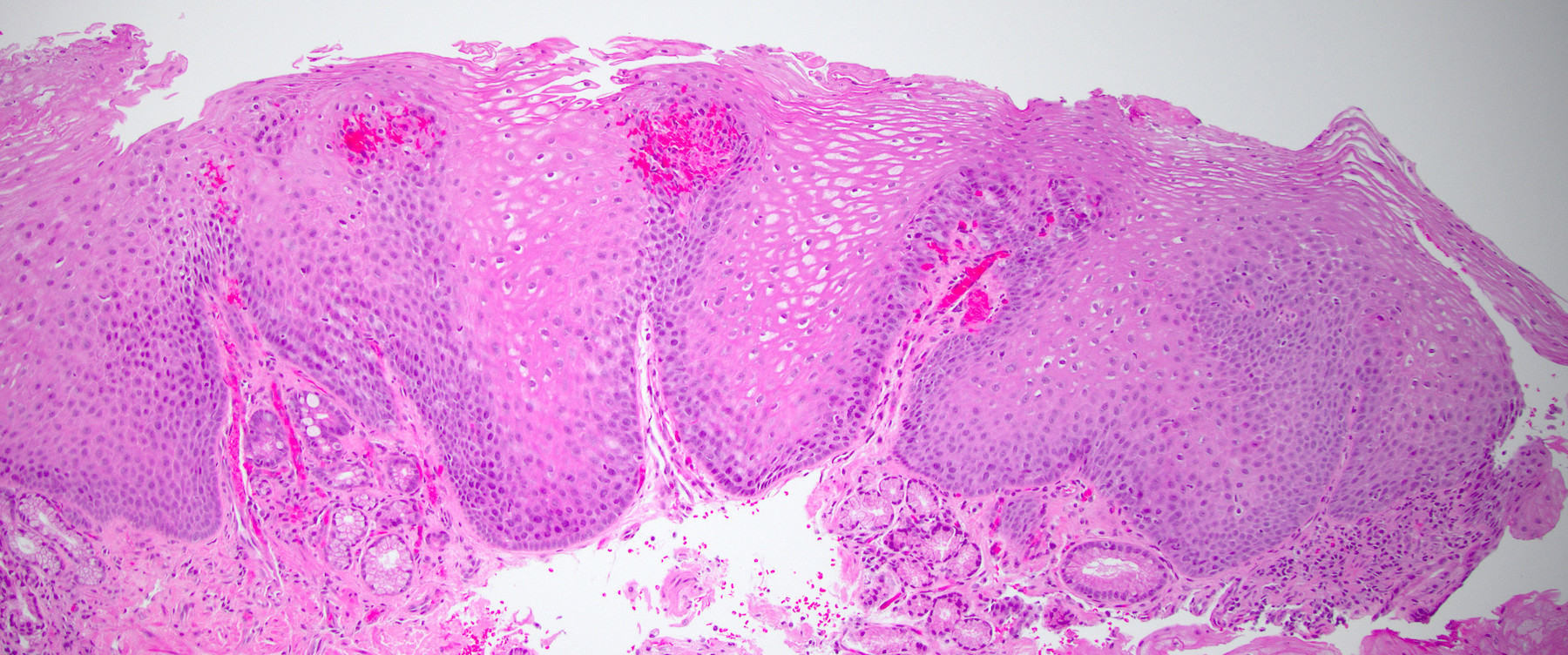
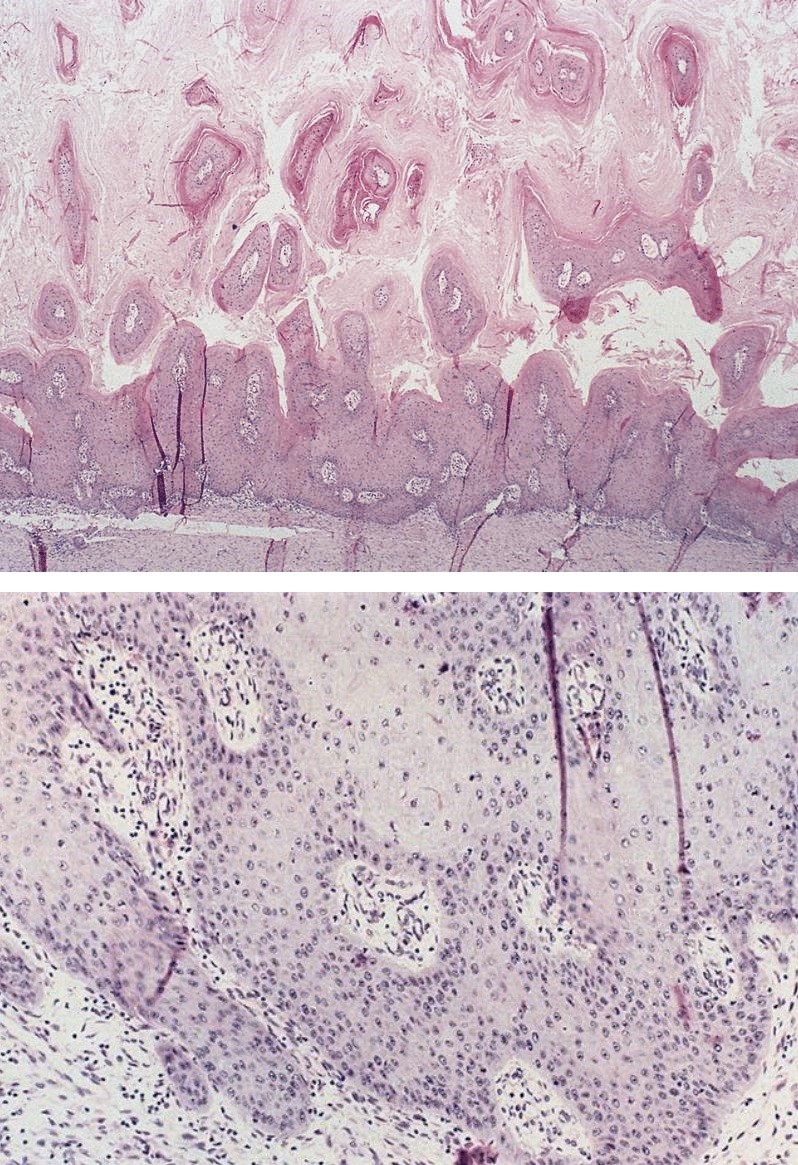
Microscopic (histologic) description
- Early: Auerbach / myenteric plexus has lymphocytic inflammation (cytotoxic T cells, eosinophils) with germinal centers and submucosal glandular atrophy
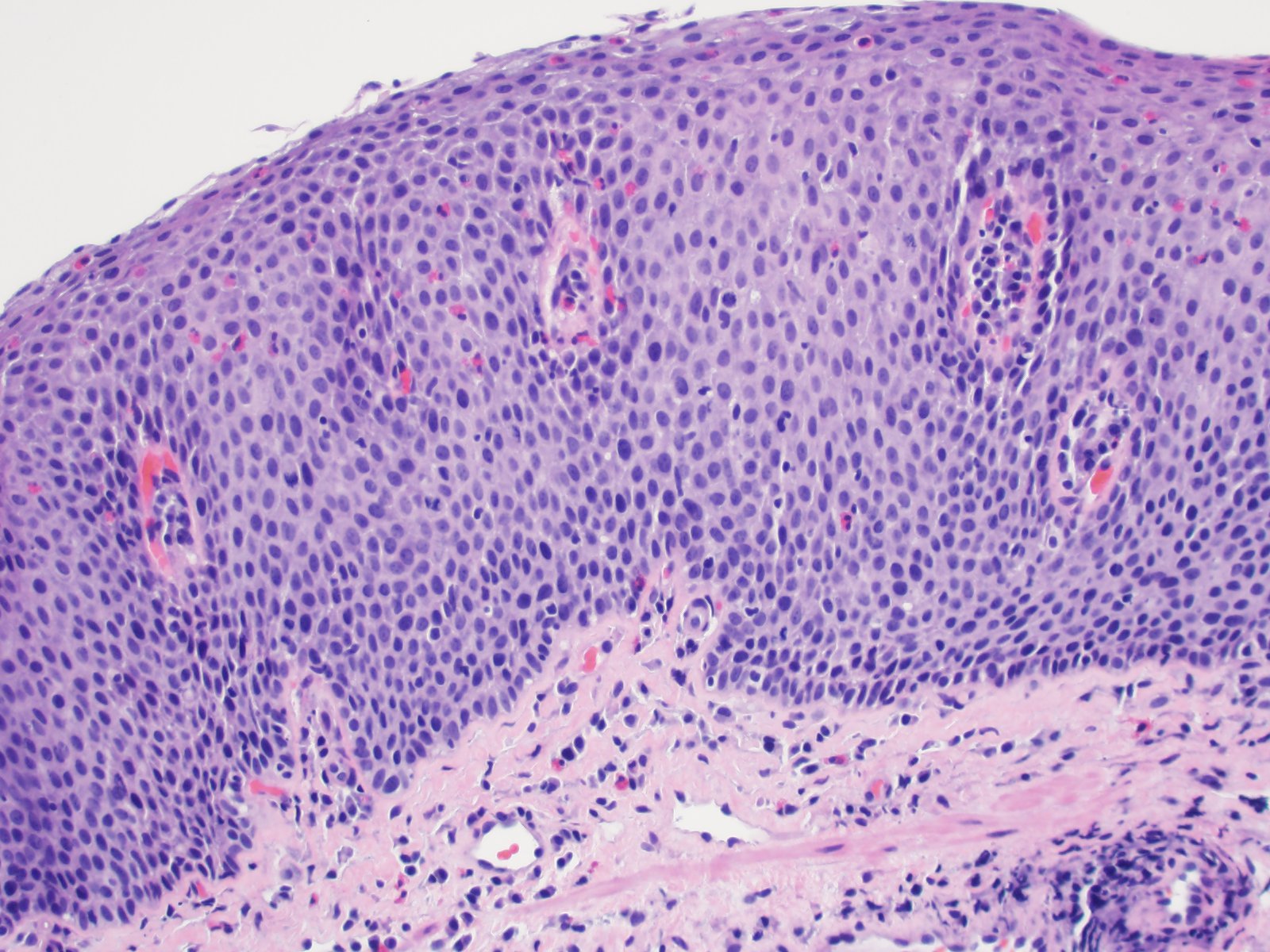
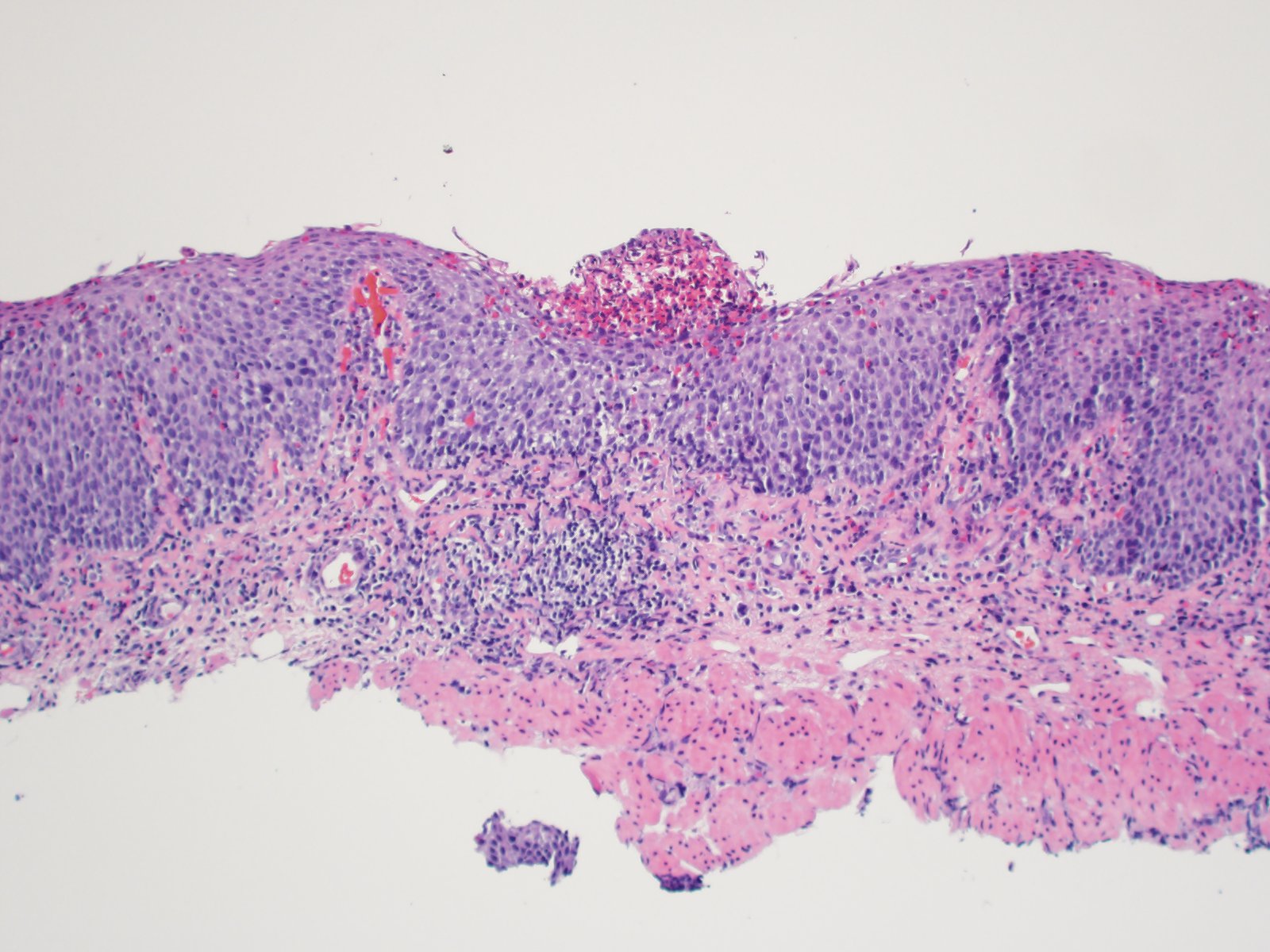
- Late: marked depletion / absence of ganglion cells in myenteric plexus (middle of esophagus, may be normal at LES) and replacement of nerves by collagen with muscular hypertrophy; squamous mucosa markedly hyperplastic with papillomatosis and basal cell hyperplasia resembling GERD (J Gastroenterol Hepatol 2006;21:727)
Positive stains
- p53, T > B cells (Am J Surg Pathol 2001;25:1413)
Electron microscopy description
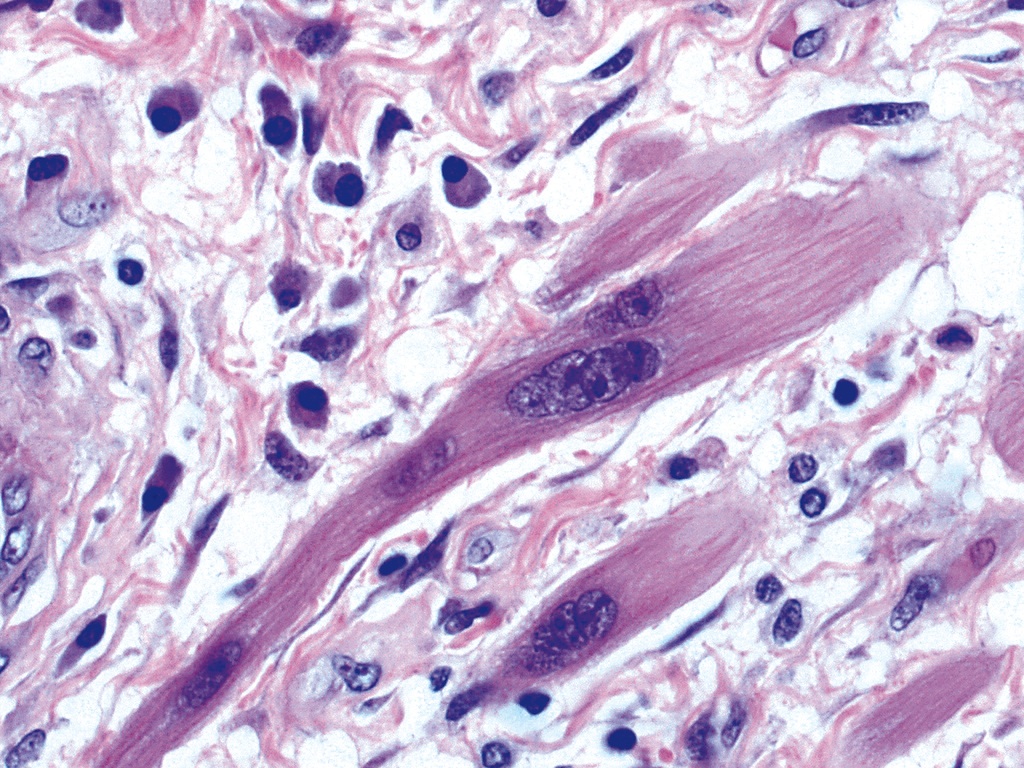
- Smooth muscle cells have nuclear and cytoplasmic inclusions, marked loss of small nerve fibers, paucity of granules in nerve fibers; also nonspecific filament disarray, mottling of myocyte fiber density, thick and long cytoplasmic dense bodies, long dense plaques (Am J Clin Pathol 1983;79:319)
Differential diagnosis
- Normal aging
- Pseudoachalasia
- Visceral neuropathies
Additional references
- Am J Surg Pathol 1994;18:327, Wikipedia: Achalasia [Accessed 13 February 2019], eMedicine: Achalasia [Accessed 13 February 2019], GI Motility Online: Pathophysiology of Achalasia and Diffuse Esophageal Spasm [Accessed 13 February 2019], Kumar: Robbins and Cotran Pathologic Basis of Disease, 8th Edition, 2009
Adenocarcinoma of the esophagus and GE junction
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Malignant epithelial neoplasm of the esophagus with glandular or mucinous differentiation
- Majority of cases occur in the lower esophagus and at the esophagogastric (GE) junction, which is defined endoscopically by the most proximal gastric fold or the distal end of the palisade vessels
Essential features
- According to WHO (5th edition), GE junction adenocarcinoma is defined as an adenocarcinoma with an epicenter within 2 cm of the GE junction and extending into the esophagus
- Rarely, adenocarcinoma can occur in the middle or upper third of the esophagus; such cases most likely develop from submucosal glands or ectopic columnar epithelium in the esophagus (also known as inlet patches or ectopic gastric mucosa)
- Evidence of glandular or mucinous differentiation within an invasive tumor originating in the esophagus and GE junction
- Barrett esophagus is an important risk factor
Terminology
- Adenocarcinoma of esophagus
- Adenocarcinoma of esophagogastric junction
ICD coding
Epidemiology
- Steady increase in incidence each year in developed countries since the 1970s through 2000, with the incidence rate eventually stabilizing in the United States through 2017 (J Natl Cancer Inst 2008;100:1184, Gastrointest Endosc Clin N Am 2021;31:1)
- Represents 15% of all esophageal cancers worldwide (the majority being esophageal squamous cell carcinoma), with men diagnosed more often than women (Gut 2020;69:1564)
- Predominance in men is greatest in the U.S., with an incidence ratio approaching 9:1 (Gut 2015;64:381, Gastroenterology 2022;163:649, Oncotarget 2016;7:38876, Clin Gastroenterol Hepatol 2016;14:338)
- Distinct geographical patterns compared with esophageal squamous cell carcinoma
- The majority of adenocarcinoma cases occur in Eastern Asia followed by North America, with China and the U.S. contributing most to the burden on a country level (Gut 2020;69:1564)
- Patients with Barrett esophagus have a 10 - 55 fold higher risk of adenocarcinoma (Dig Dis Sci 2018;63:1988)
- Rate of progression from Barrett esophagus to adenocarcinoma varies widely in the literature, within 0.07 - 3.6% and 1.5 - 2.5 fold higher when including high grade dysplasia (Gastroenterology 2015;149:577)
- Shorter segments (3 cm) of Barrett esophagus have a lower rate of progression to adenocarcinoma than longer segments (≥ 3 cm) (Endoscopy 2019;51:665)
- Unclear if proton pump inhibitors (PPI) have a protective effect on decreasing progression to adenocarcinoma (Transl Cancer Res 2021;10:1620)
- Can also occur in the setting of gastric heterotopia (Dig Liver Dis 2012;44:292)
Sites
- Esophagus
- Almost all cases occur in the lower third of the esophagus and esophagogastric junction
- Cases may occur in the upper and middle thirds of the esophagus, most likely developing from submucosal glands or ectopic columnar epithelium in the esophagus (also known as inlet patches or ectopic gastric mucosa)
Pathophysiology
- Primarily associated with Barrett esophagus, history of gastroesophageal reflux disease (GERD), obesity, tobacco smoking
- GERD is the single most important risk factor
- Intestinal metaplasia → genomic instability (often TP53 inactivation) → genome doubling and copy number alterations → malignancy (Cancer Prev Res (Phila) 2014;7:114, AORN J 1989;50:1182, Nat Genet 2015;47:1038)
- Mutations in TP53 and SMAD4 (only identified in high grade dysplasia and adenocarcinoma) occur in a stage specific manner (Nat Genet 2014;46:837)
- Next generation sequencing has identified additional genetic pathways and mutations involved, including Toll-like receptor signaling, receptor tyrosine kinase receptors and homologous recombination (PLoS Genet 2017;13:e1006808, Nat Genet 2016;48:1131)
Etiology
- ~7% of cases are familial (Cancer Epidemiol Biomarkers Prev 2006;15:1668, Dig Dis Sci 2016;61:1826, Clin Gastroenterol Hepatol 2014;12:1656)
Clinical features
- Progressive dysphagia, gastroesophageal reflux and weight loss are most common
- Iron deficiency anemia may result due to chronic gastrointestinal blood loss
- Odynophagia, nausea and vomiting are less common
- Most early (superficial) esophageal cancers are only detected serendipitously or by screening and surveillance of Barrett esophagus
- Intramucosal adenocarcinoma is not symptomatic
- ~6 - 10% are asymptomatic at the time of diagnosis (Ann Surg 2018;267:99)
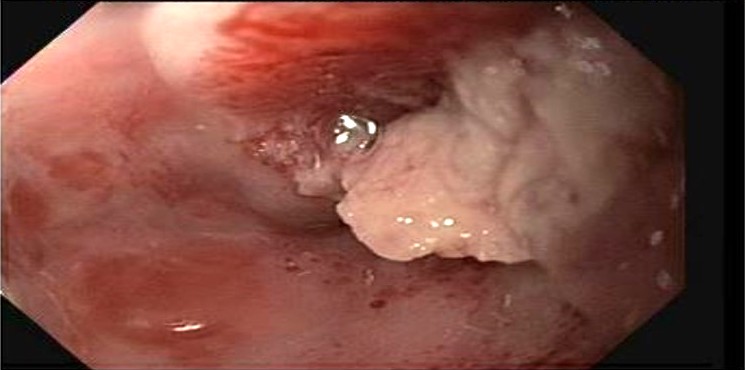
Diagnosis
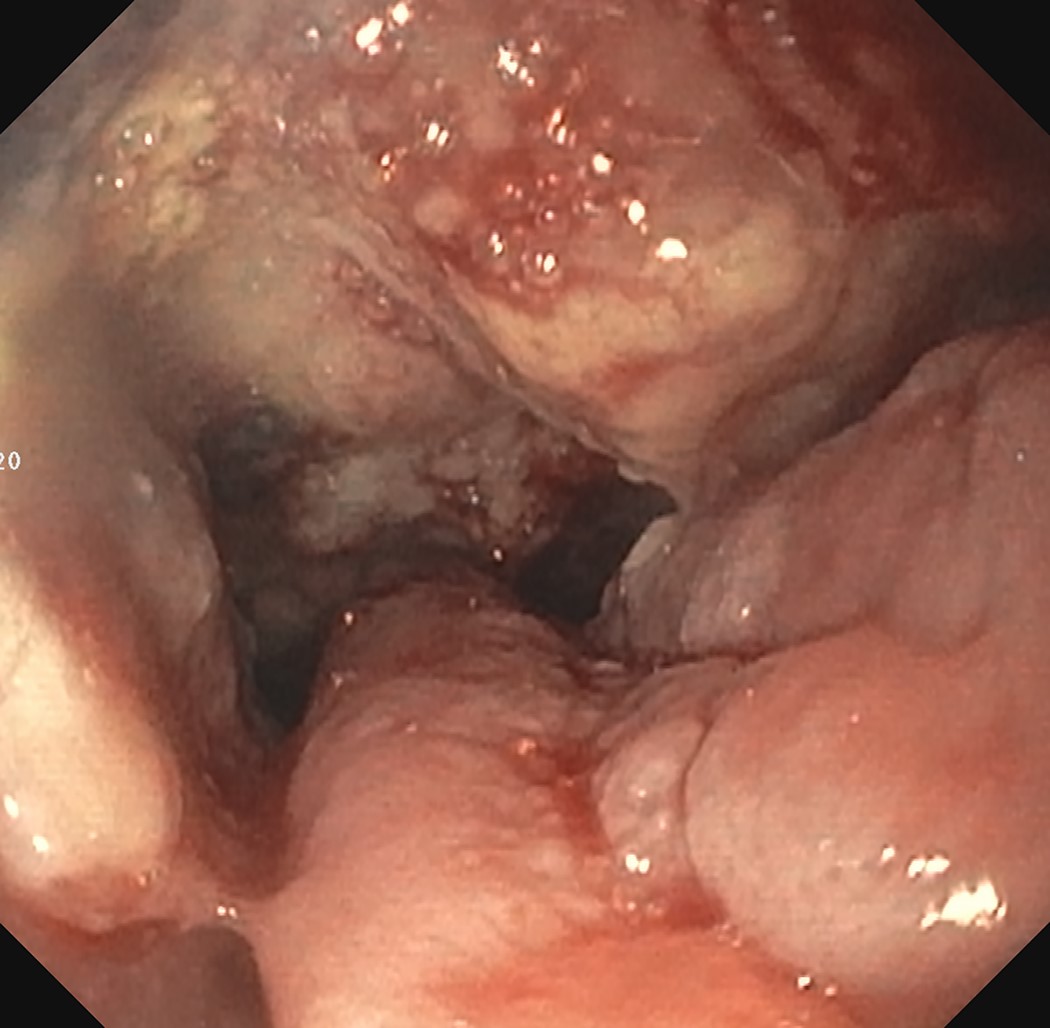
- Confirmed by endoscopic biopsy
- Radiographic imaging is necessary for staging to identify distant metastases
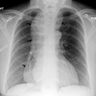
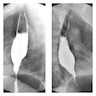
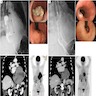
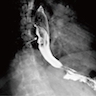
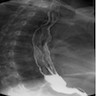
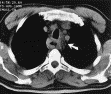
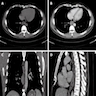
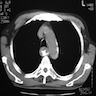
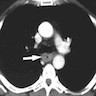
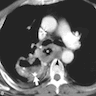
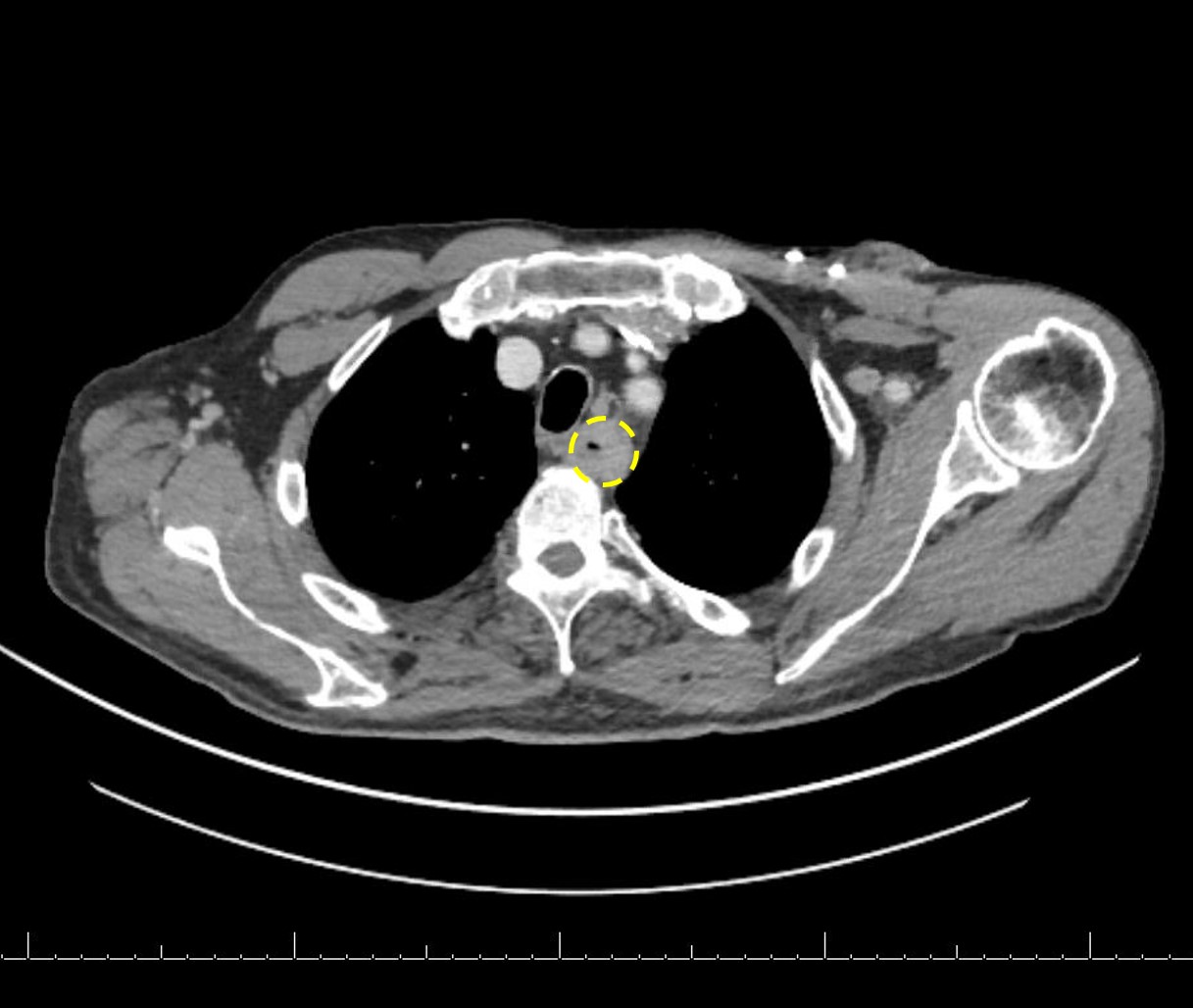
Radiology description
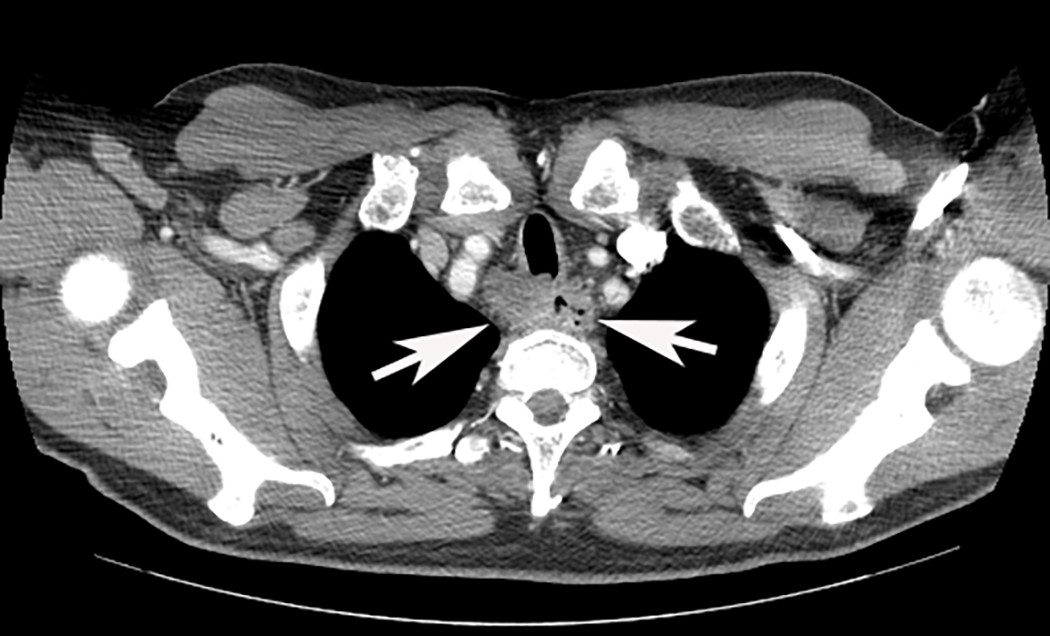
- CT may show esophageal wall thickening or a mass lesion, soft tissue with fat stranding
- Dilated proximal esophagus; oral debris may be present
- Tracheobronchial and aortic invasion may be present
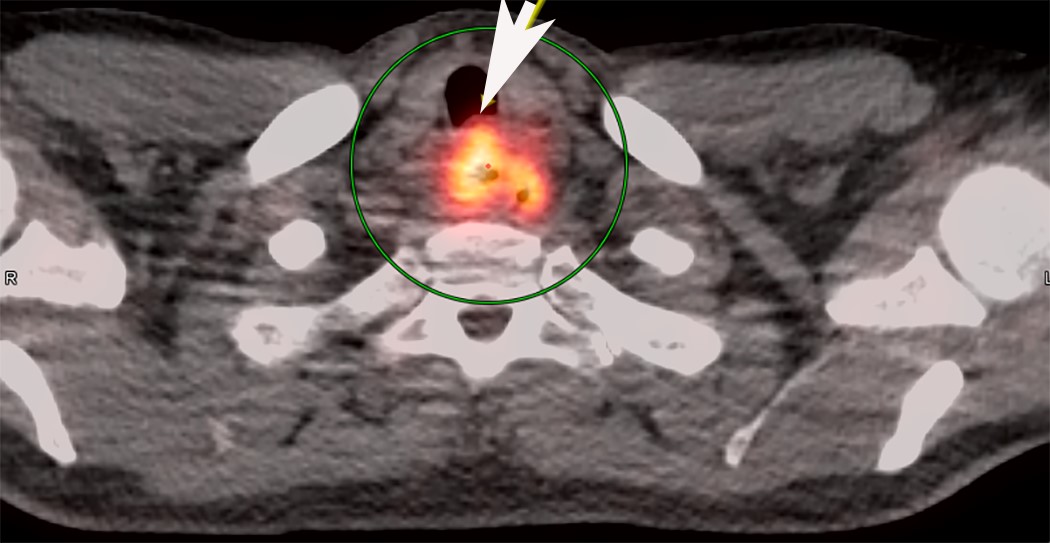
- PET CT is useful to assess lymph node and distant metastases (Mol Imaging Biol 2011;13:166)
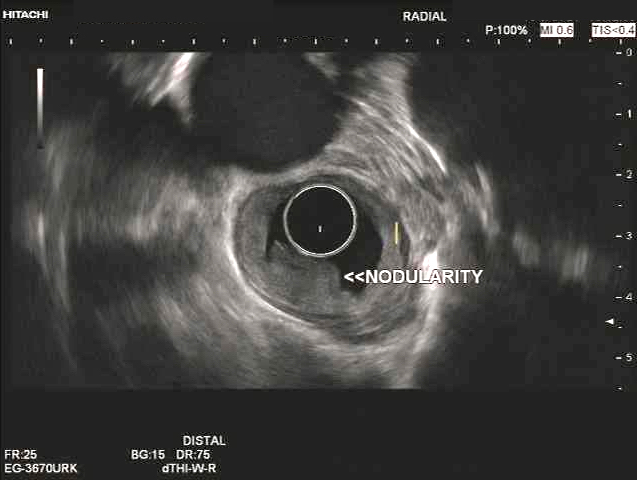
- Endoscopic ultrasound (EUS) is a modality of choice for tumor staging and locoregional lymph node involvement (Mol Imaging Biol 2011;13:166)
Prognostic factors
- Pathological stage is the most important prognostic factor
- Mortality is higher in men than women, with age standardized mortality rates of 8.2 versus 3.2 per 100,000 (Gastroenterology 2022;163:649)
- Signet ring cell morphology is associated with a poor prognosis (Ann Thorac Surg 2013;96:1927)
Case reports
- 51 year old man presented with 2 weeks of reflux, 35 pound weight loss and elevated alpha fetoprotein (AFP) (Clin J Gastroenterol 2017;10:7)
- 60 year old man presented with epigastric discomfort for one month and a mid esophageal adenocarcinoma arising from a heterotopic pancreas (Thorac Cancer 2022;13:1083)
- 71 year old man with a history of smoking and Barrett esophagus presenting with dysphagia and weight loss (Anticancer Res 2018;38:5999)
- 82 year old man presented with a 15 cm incarcerated hiatal hernia and an incidental 3 cm polypoid lesion on endoscopy at the level of the carina (BMJ Case Rep 2020;13:e235802)
- Dutch family with clustering of Barrett esophagus and esophageal adenocarcinoma (Fam Cancer 2018;17:435)
Treatment
- Dependent on tumor stage and patient's health status
- Generally less radiosensitive than esophageal squamous cell carcinoma
- Early (T1) lesions may be cured via endoscopic techniques (radiofrequency ablation, endoscopic mucosal resection, endoscopic submucosal dissection)
- Preoperative chemoradiation with or without immunotherapy targeting HER2 receptor followed by surgery is the most common treatment protocol for resectable tumors (J Natl Compr Canc Netw 2015;13:194)
- Esophagectomy and esophagogastrectomy performed in medically fit patients with localized thoracic esophageal cancer > 5 cm from the cricopharyngeus and in intra-abdominal esophageal and GE junction cancers respectively (J Natl Compr Canc Netw 2015;13:194)
- Definitive chemoradiation for cervical and cervicothoracic esophageal cancers within 5 cm of the cricopharyngeus
- Patients with esophageal adenocarcinoma who had a HER2 score of 3+ or 2+ on immunohistochemistry with positive FISH and treated with trastuzumab plus chemotherapy had a longer median survival compared with patients treated with chemotherapy alone (Lancet 2010;376:687)
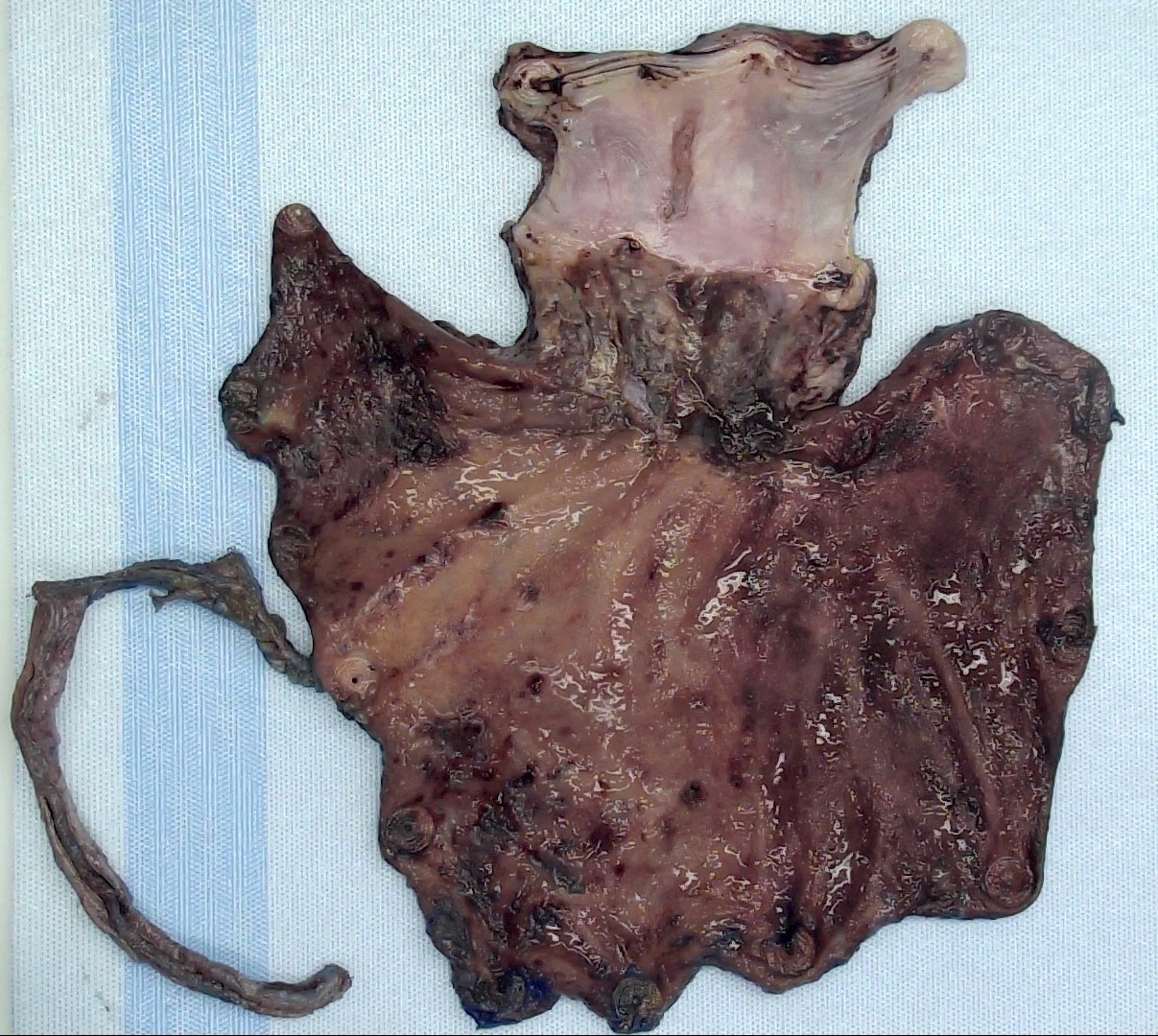
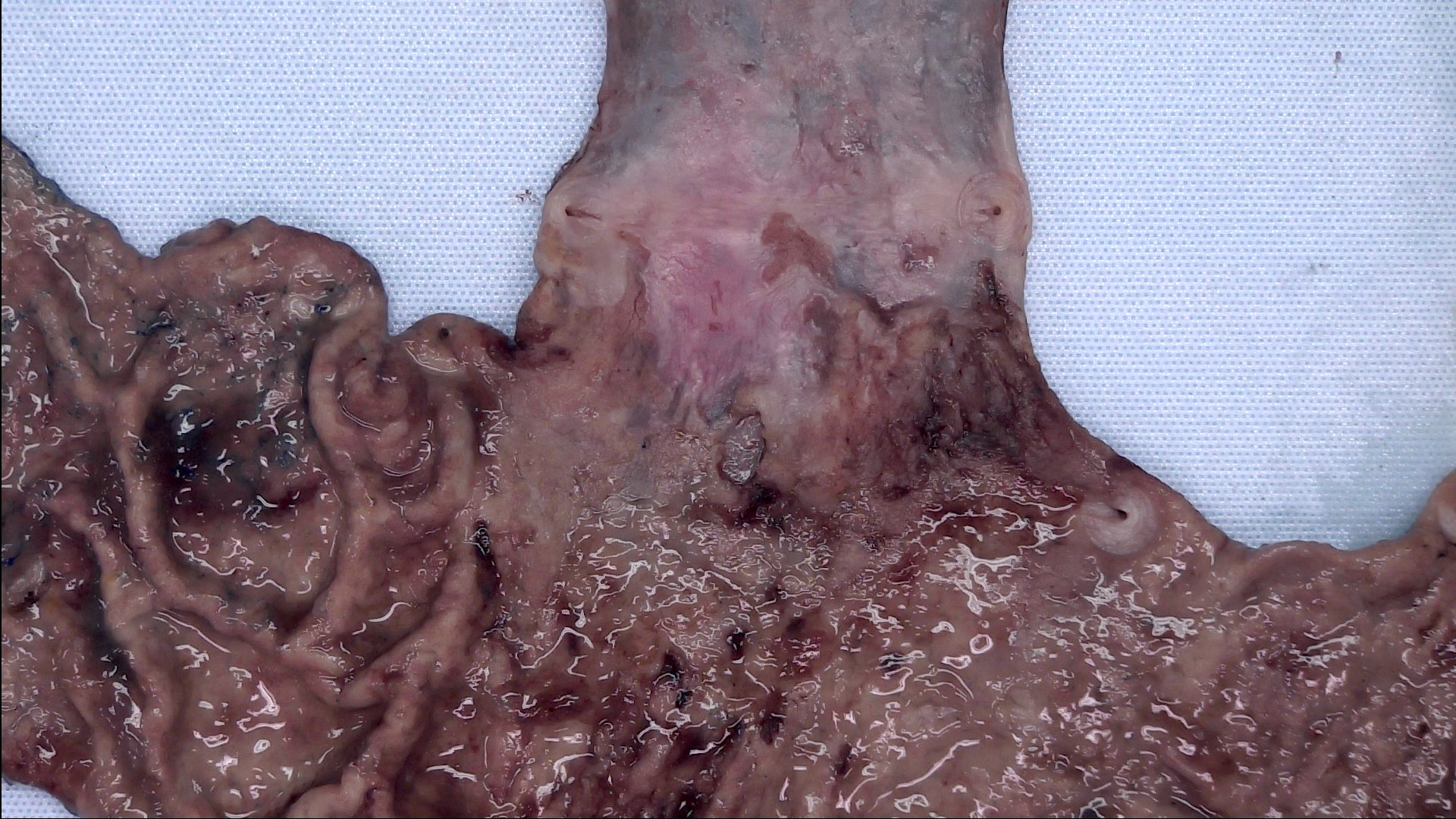
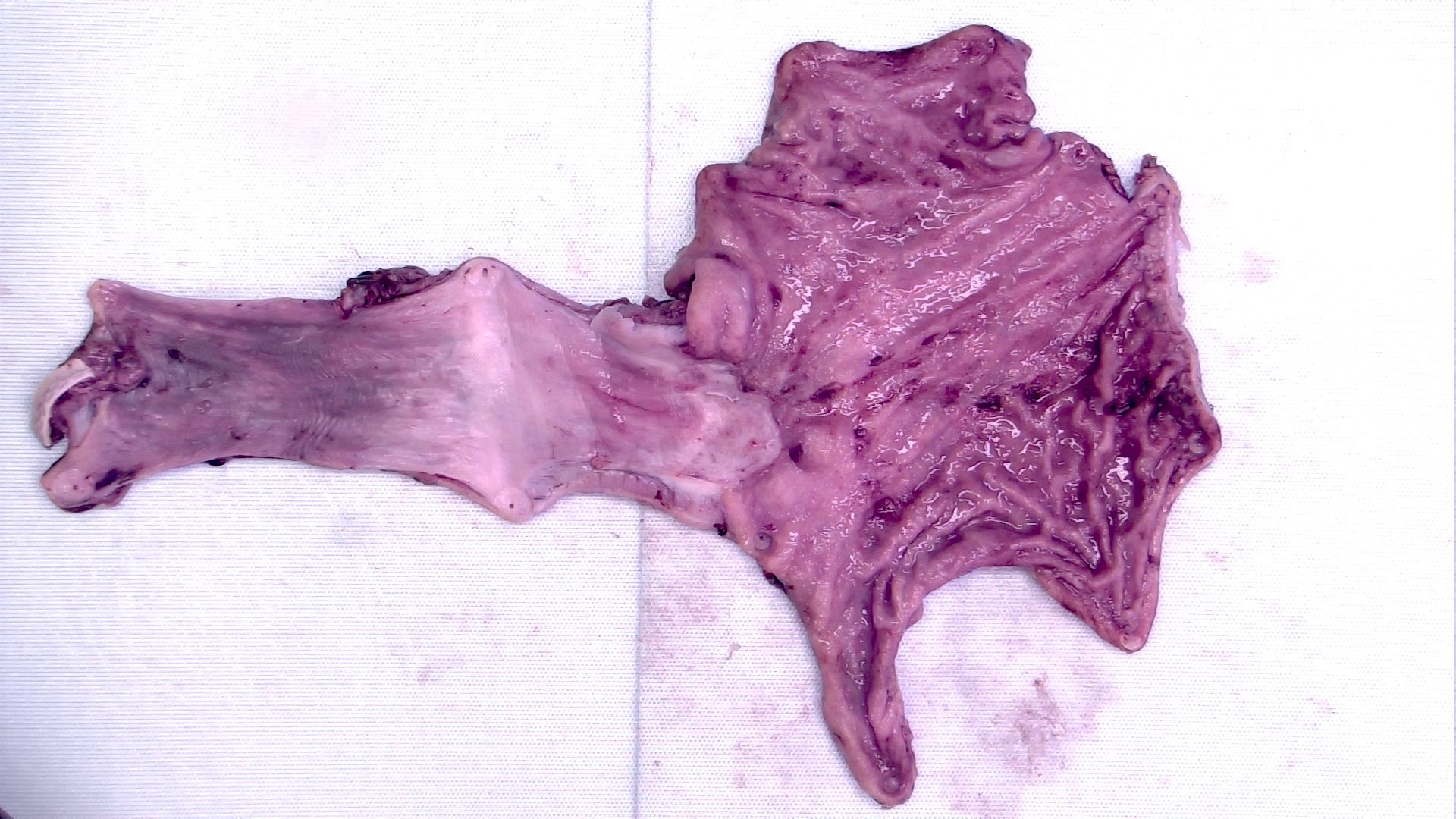
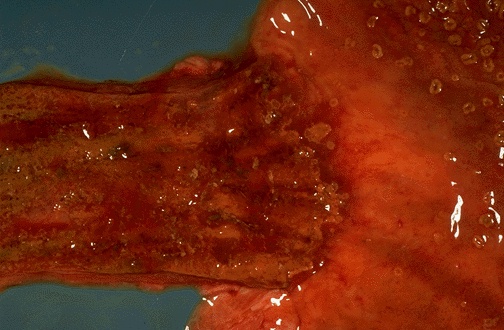
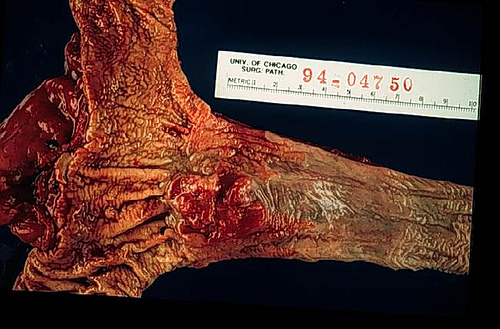
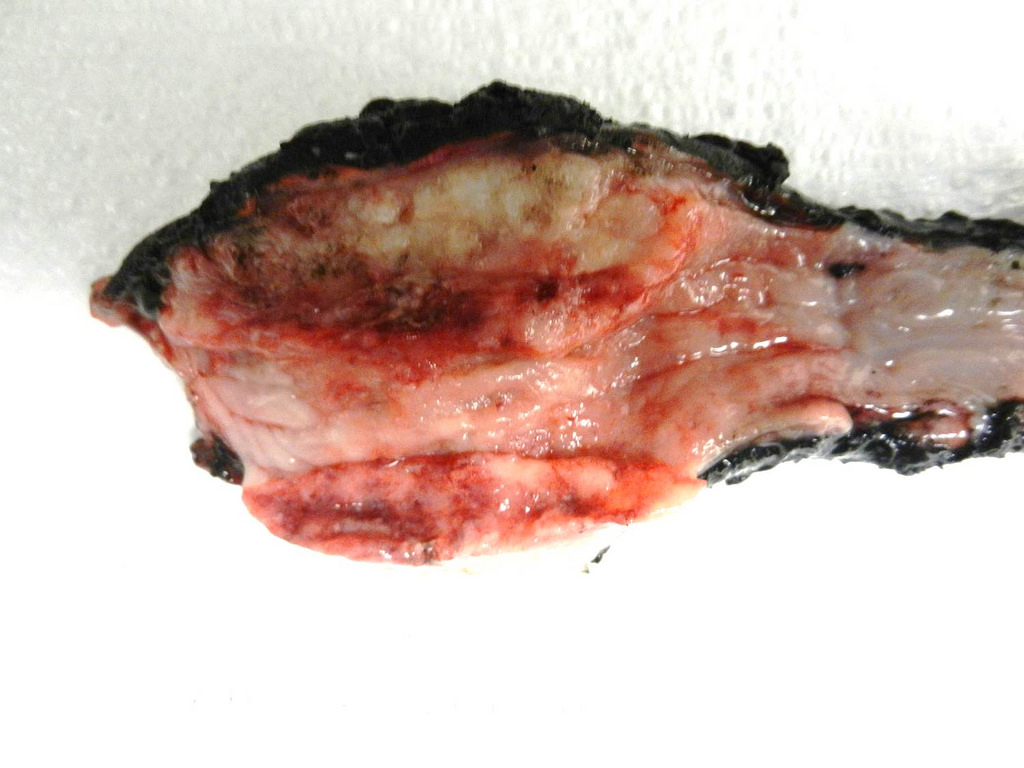
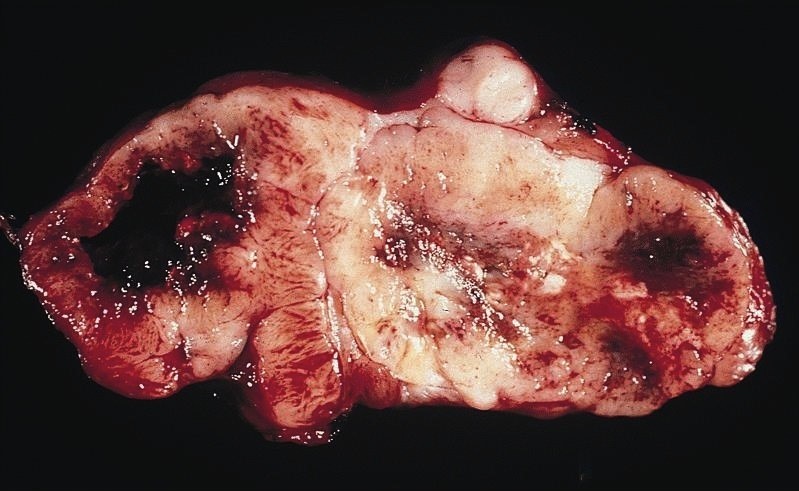
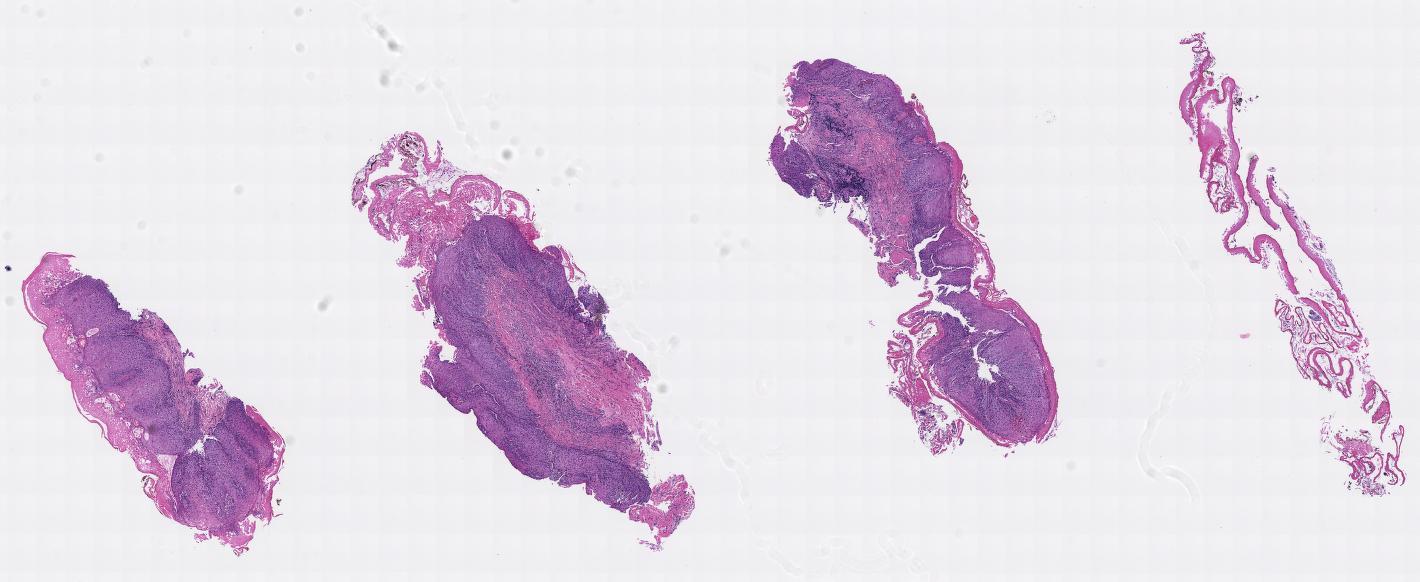
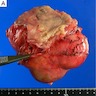
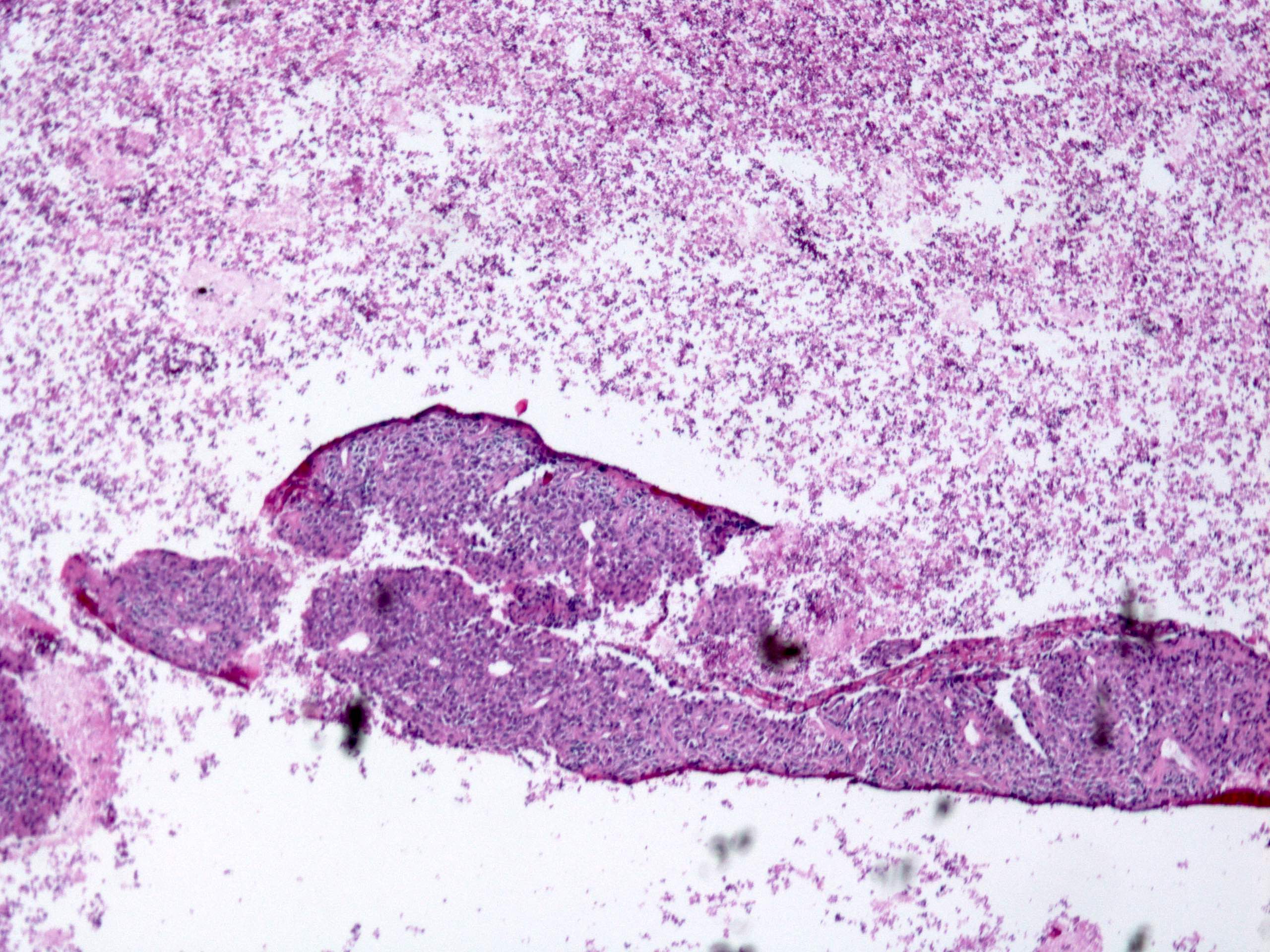
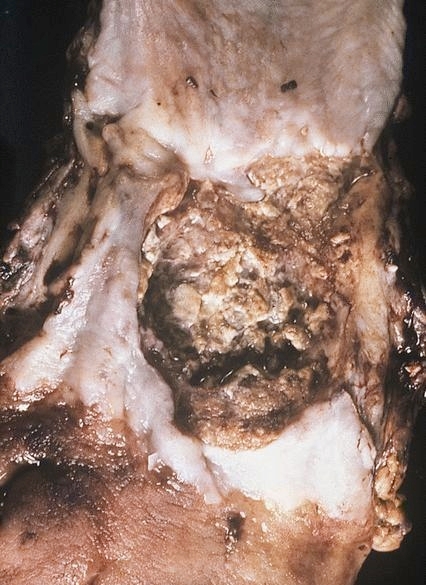
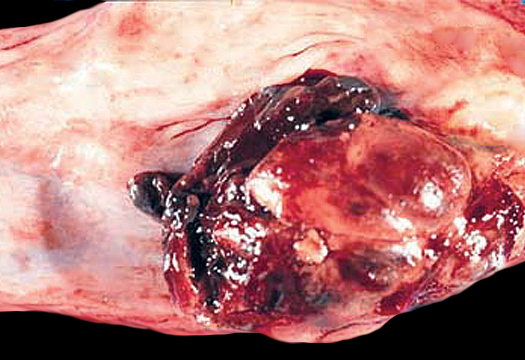
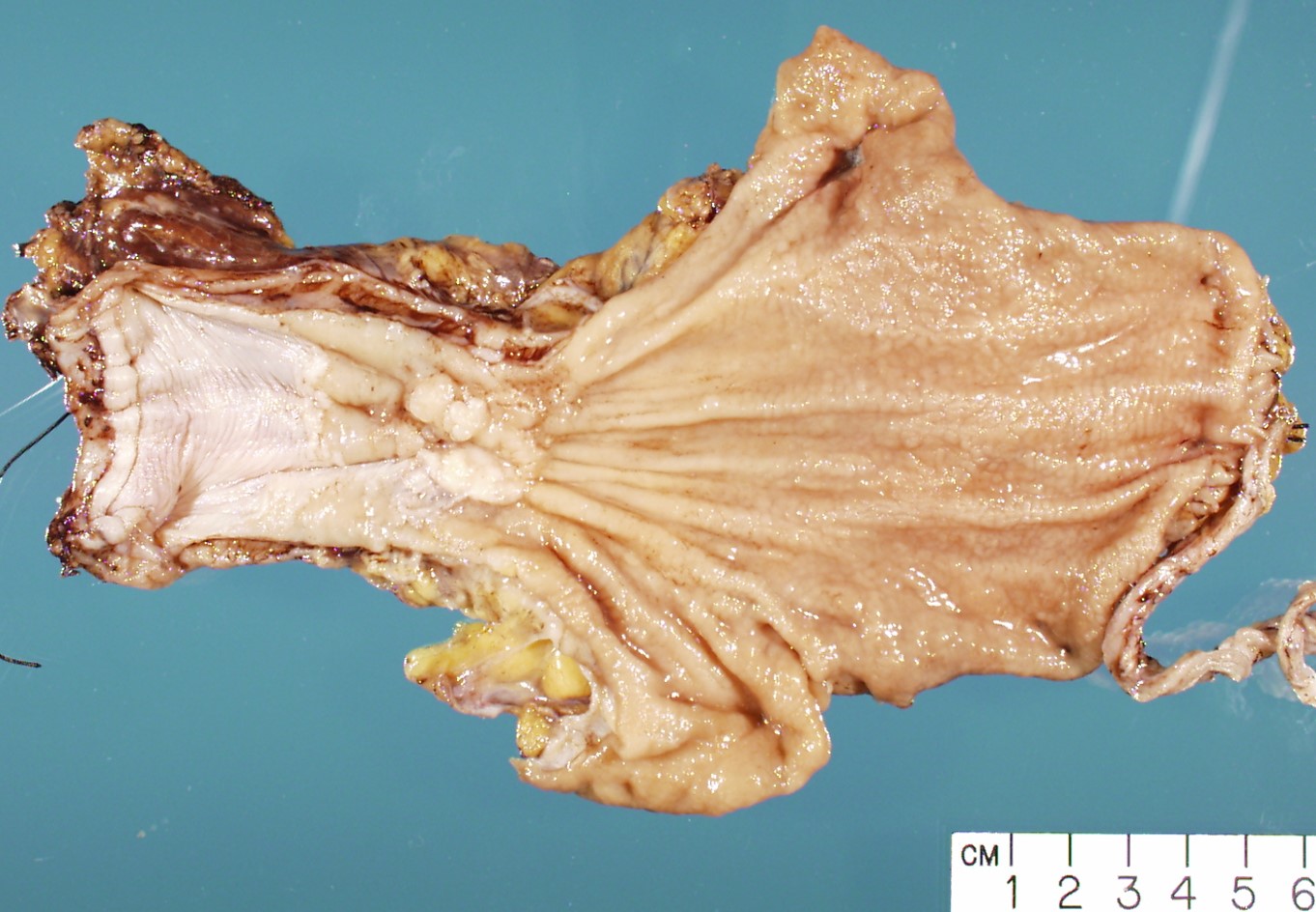
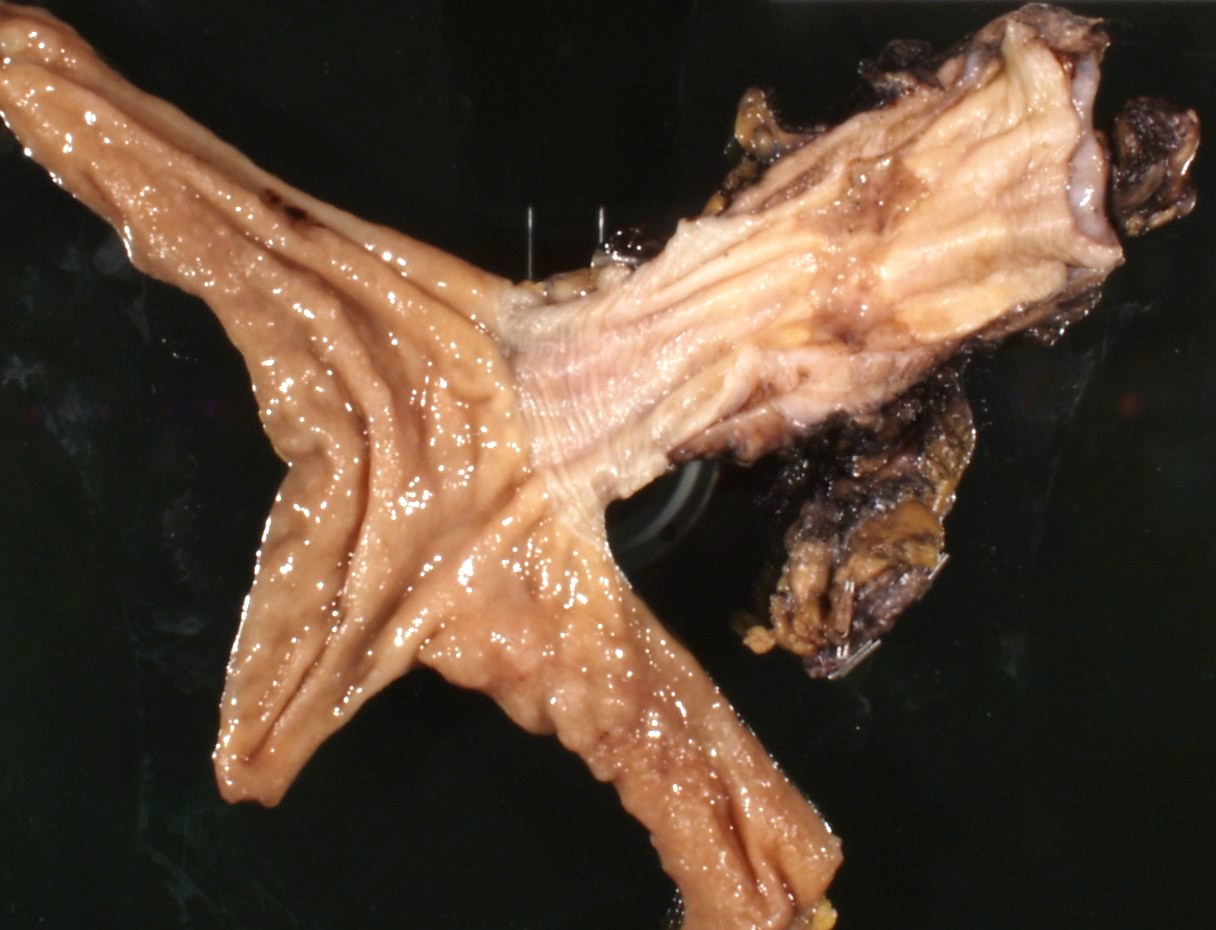
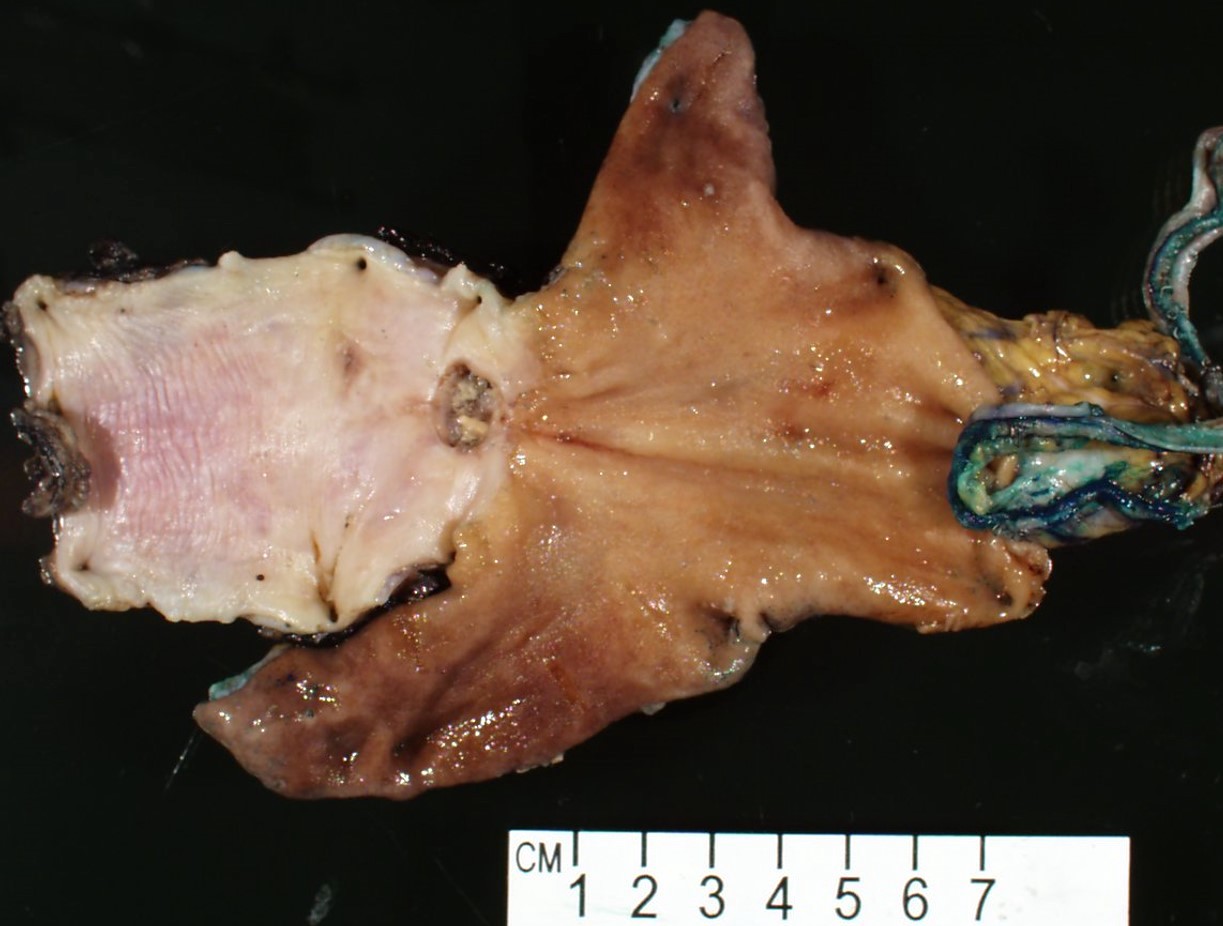
Gross description
- Often appear in advanced stages
- May be stricturing, polypoid, fungating, ulcerative or diffusely infiltrative
Gross images
Frozen section description
- Intraoperative frozen section analysis is usually performed for assessment of surgical margin status and to rule out involvement by distant metastases, as surgical management may not be effective if distant metastases are present
Frozen section images
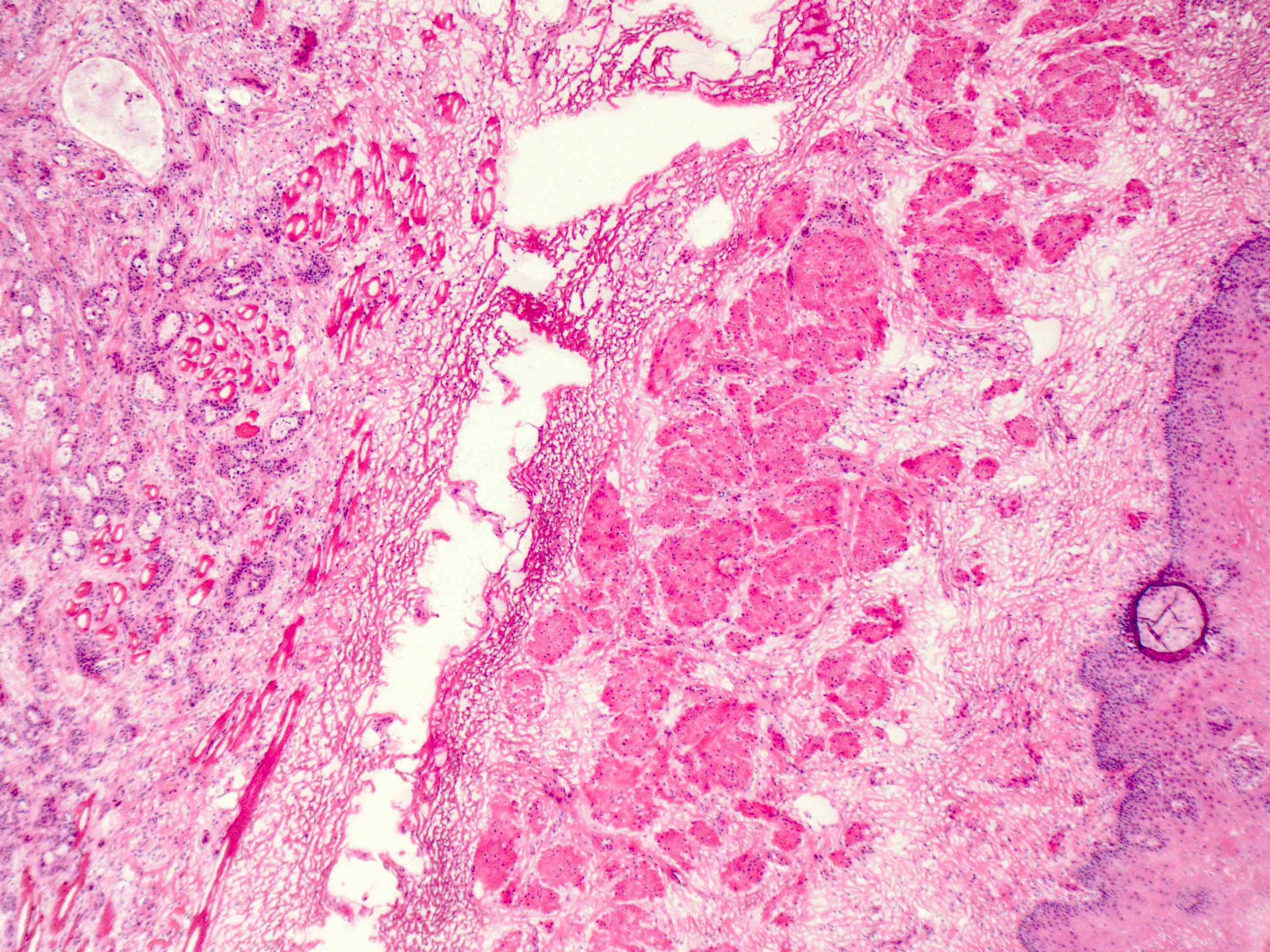
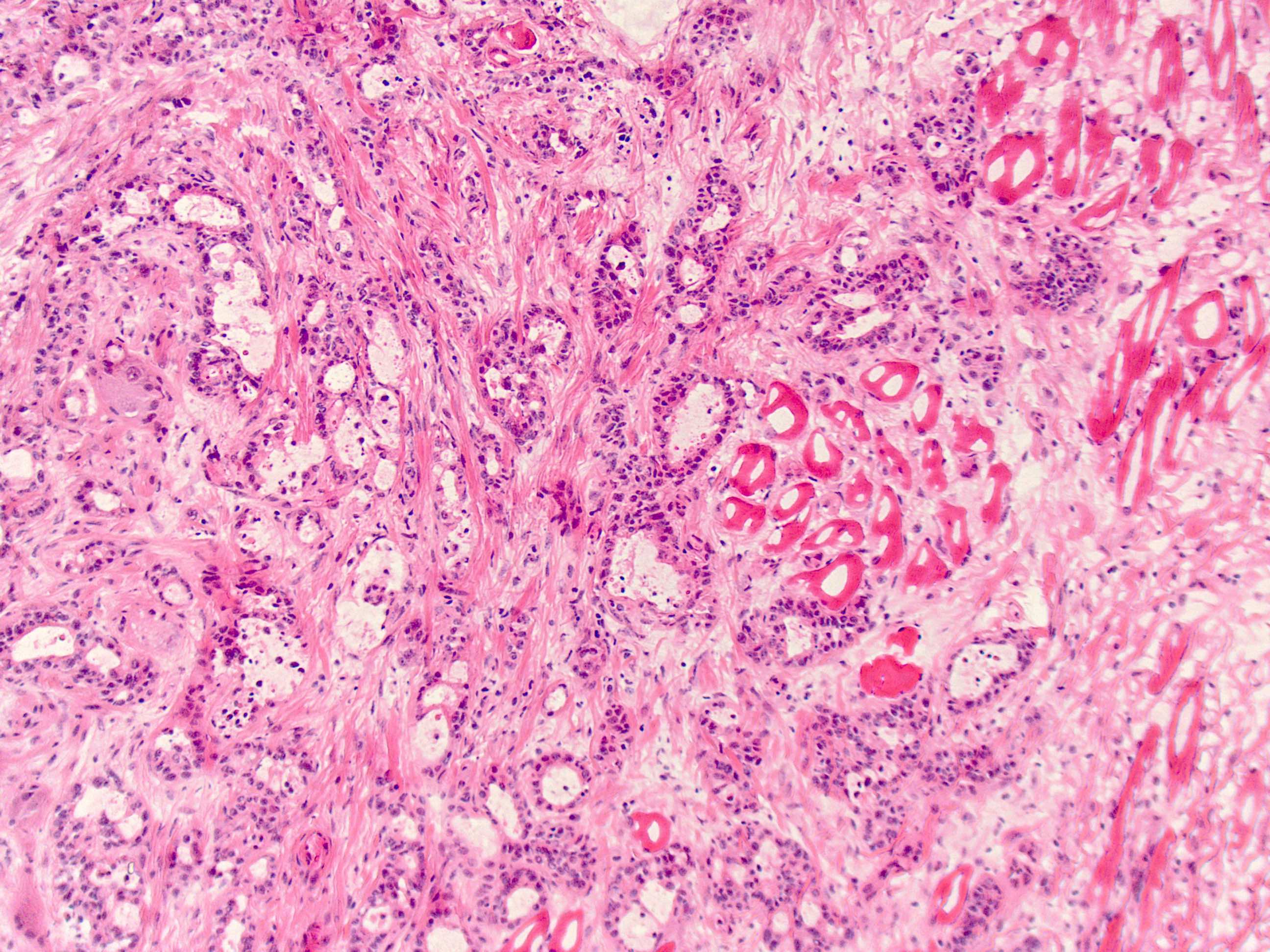
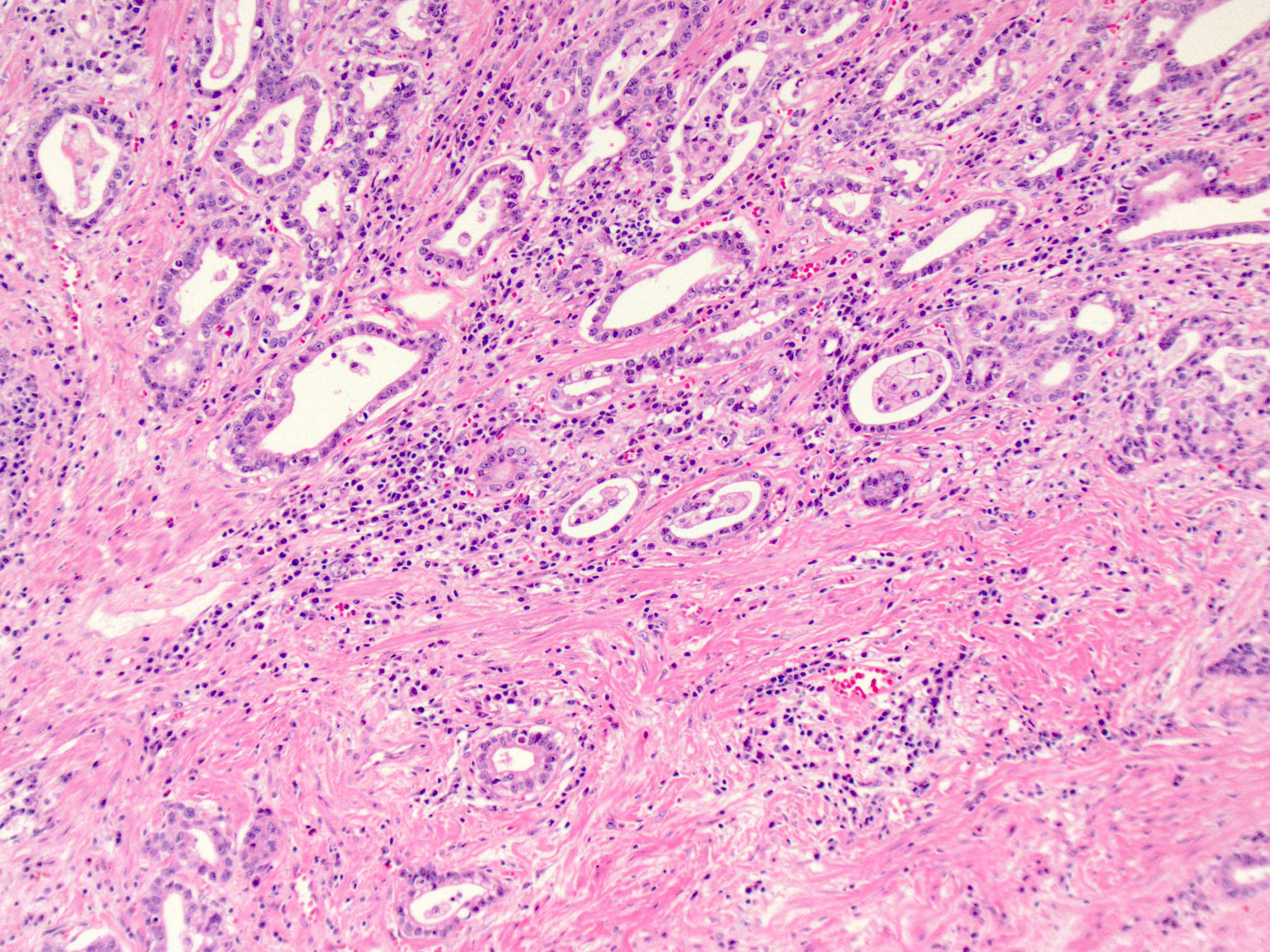
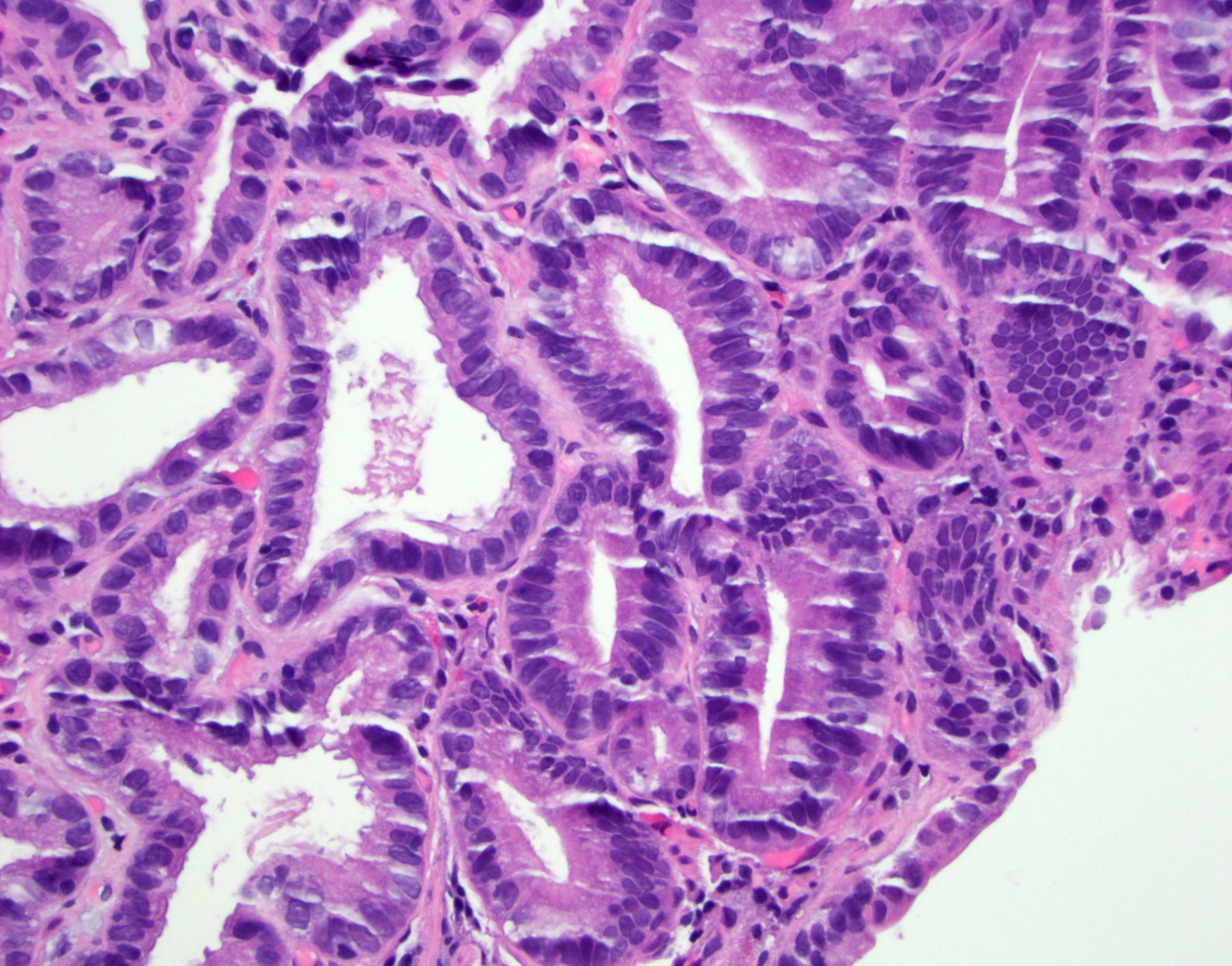
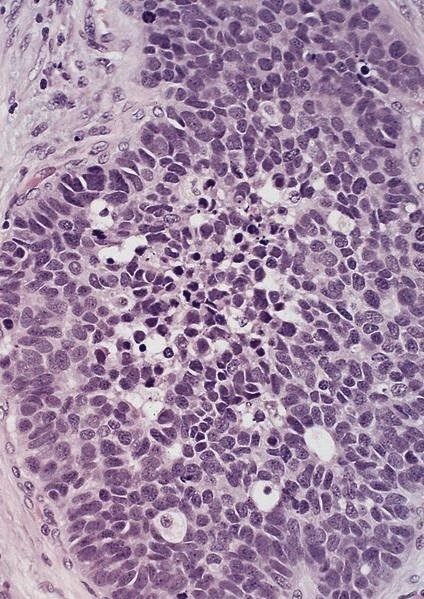
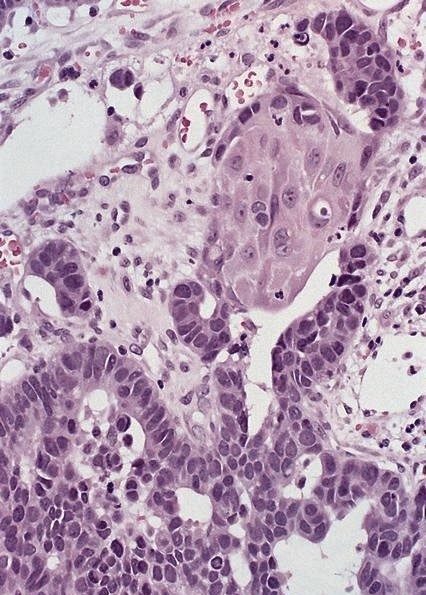
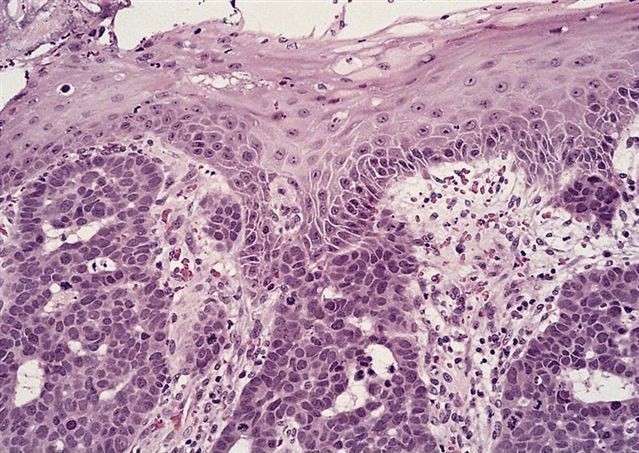
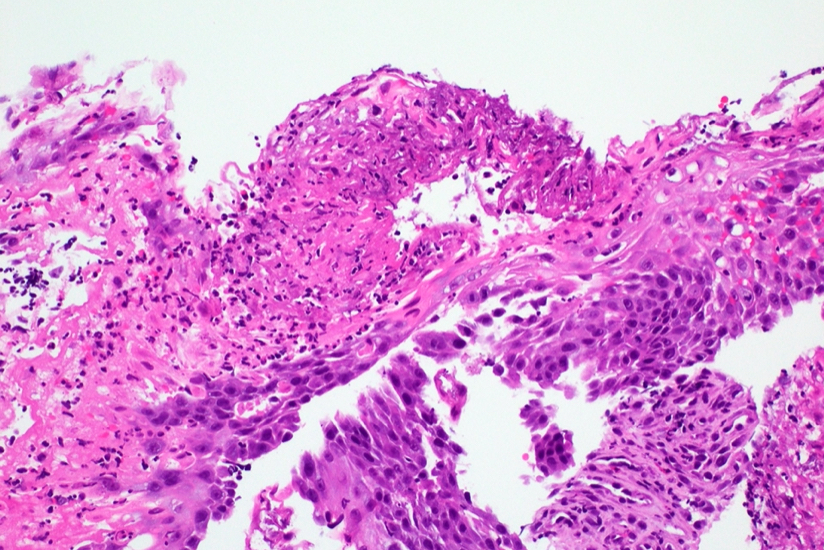
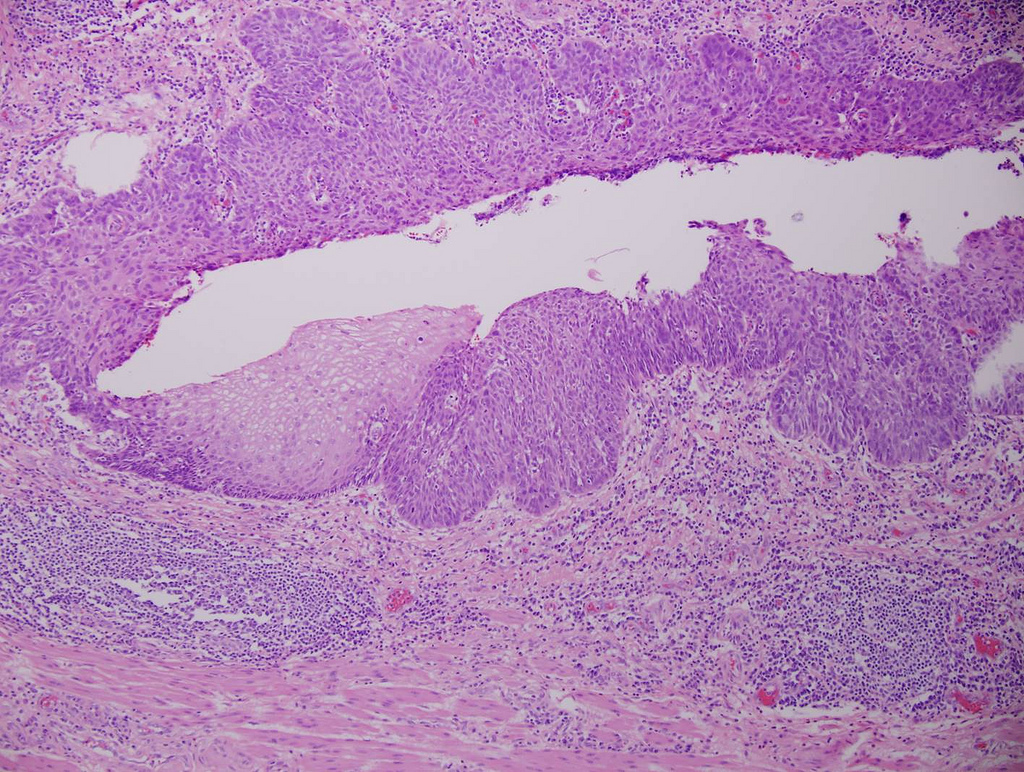
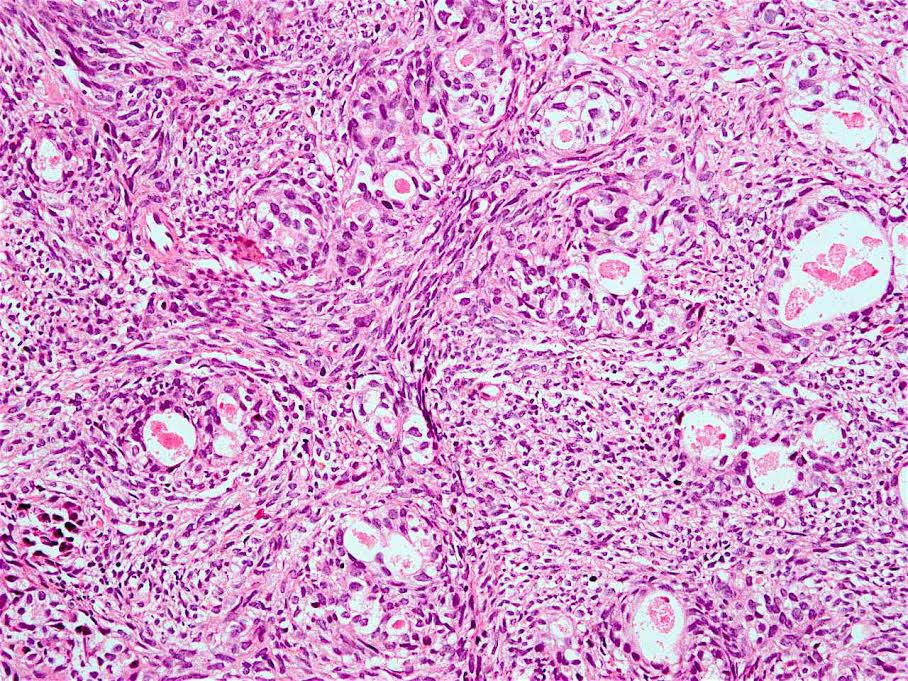
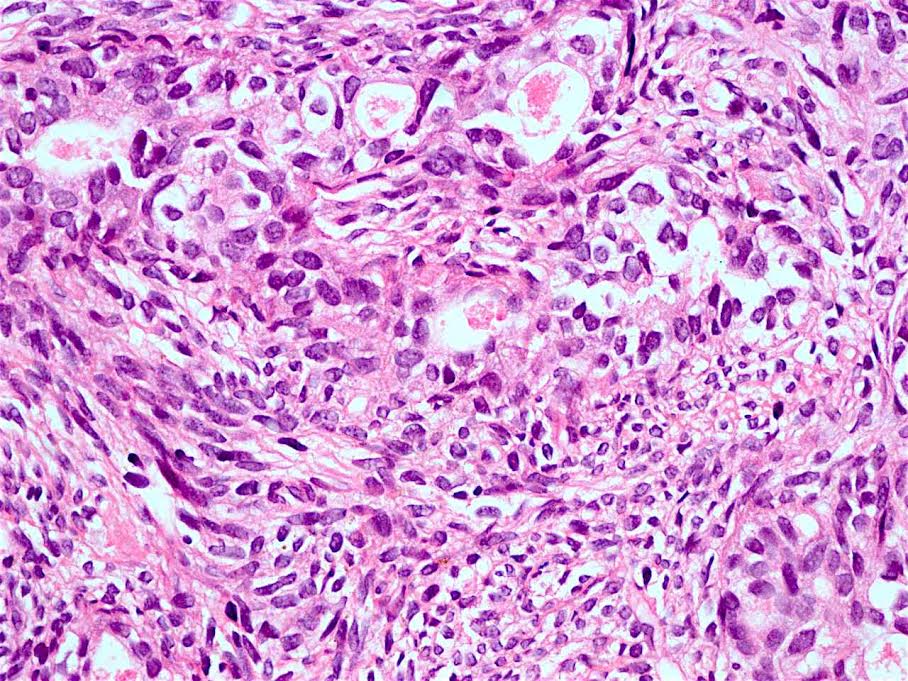
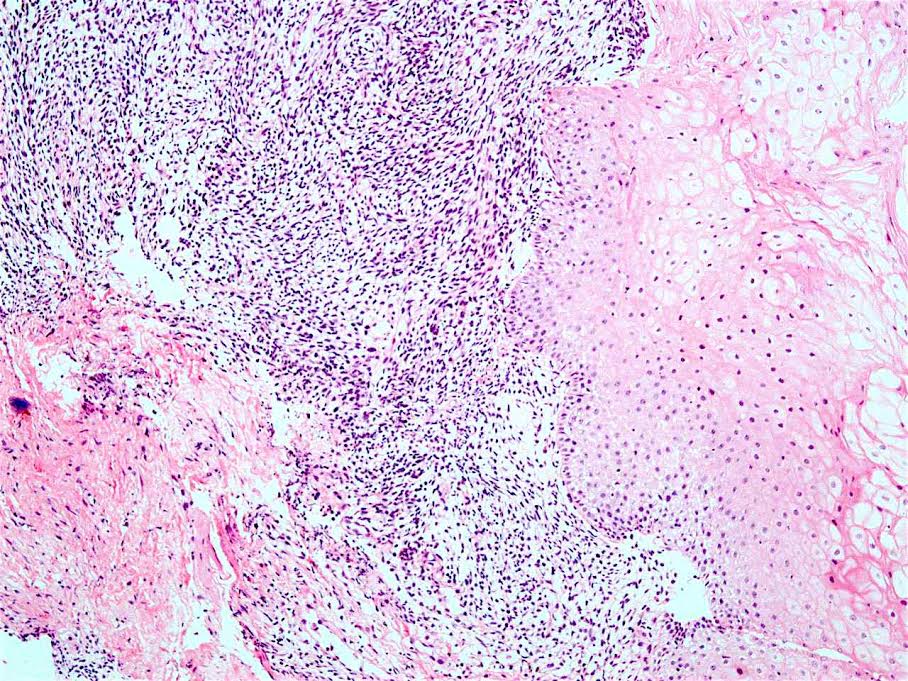
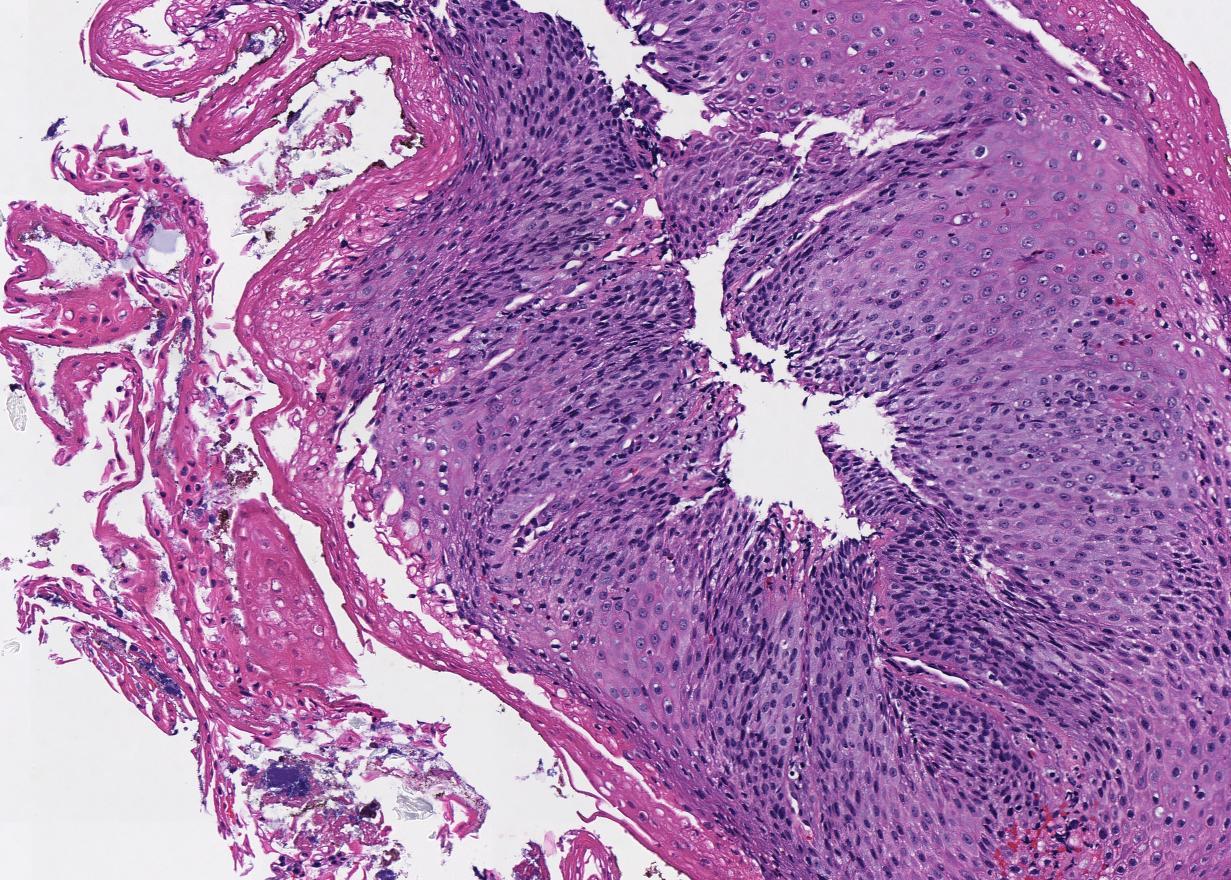
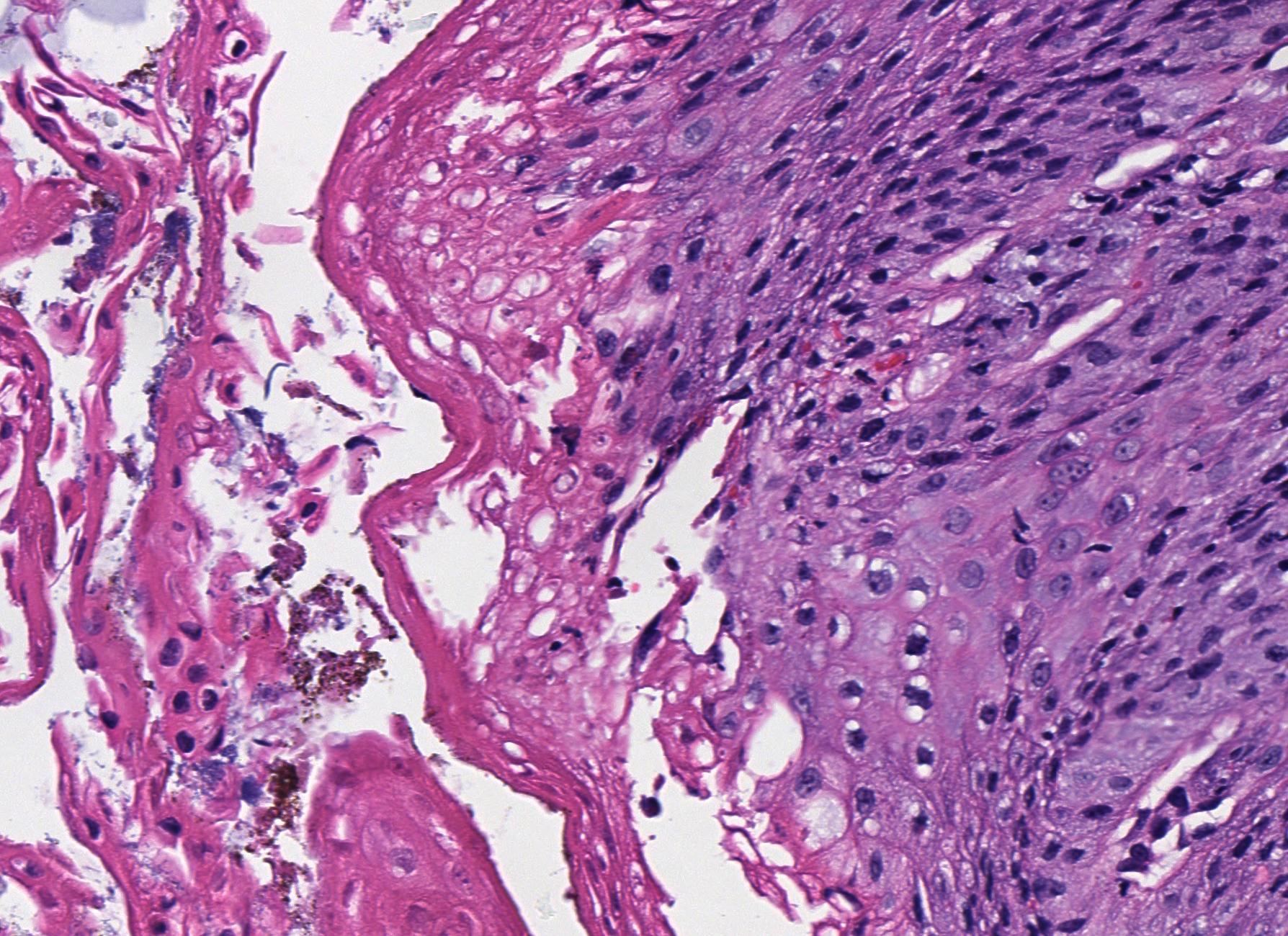
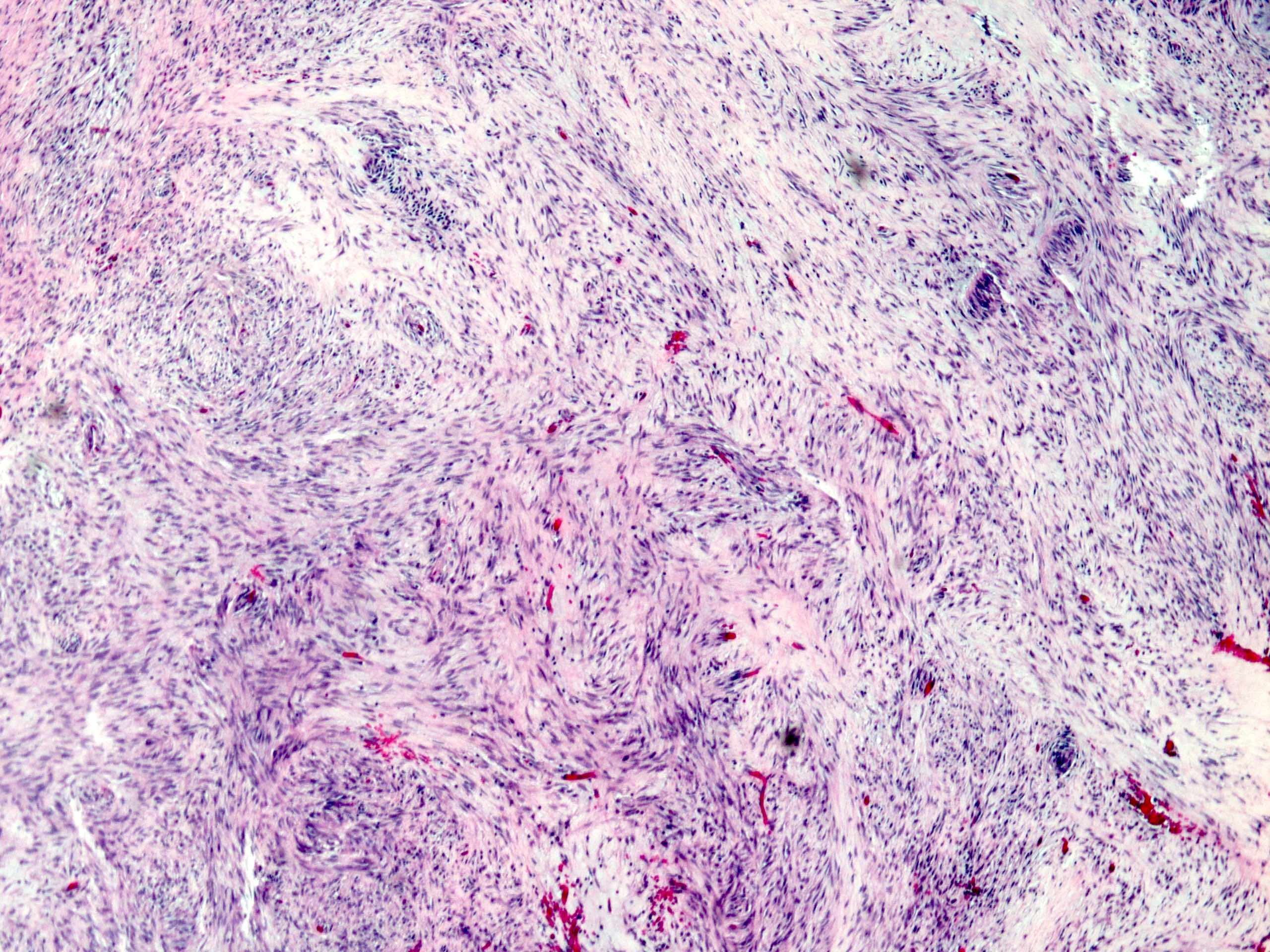
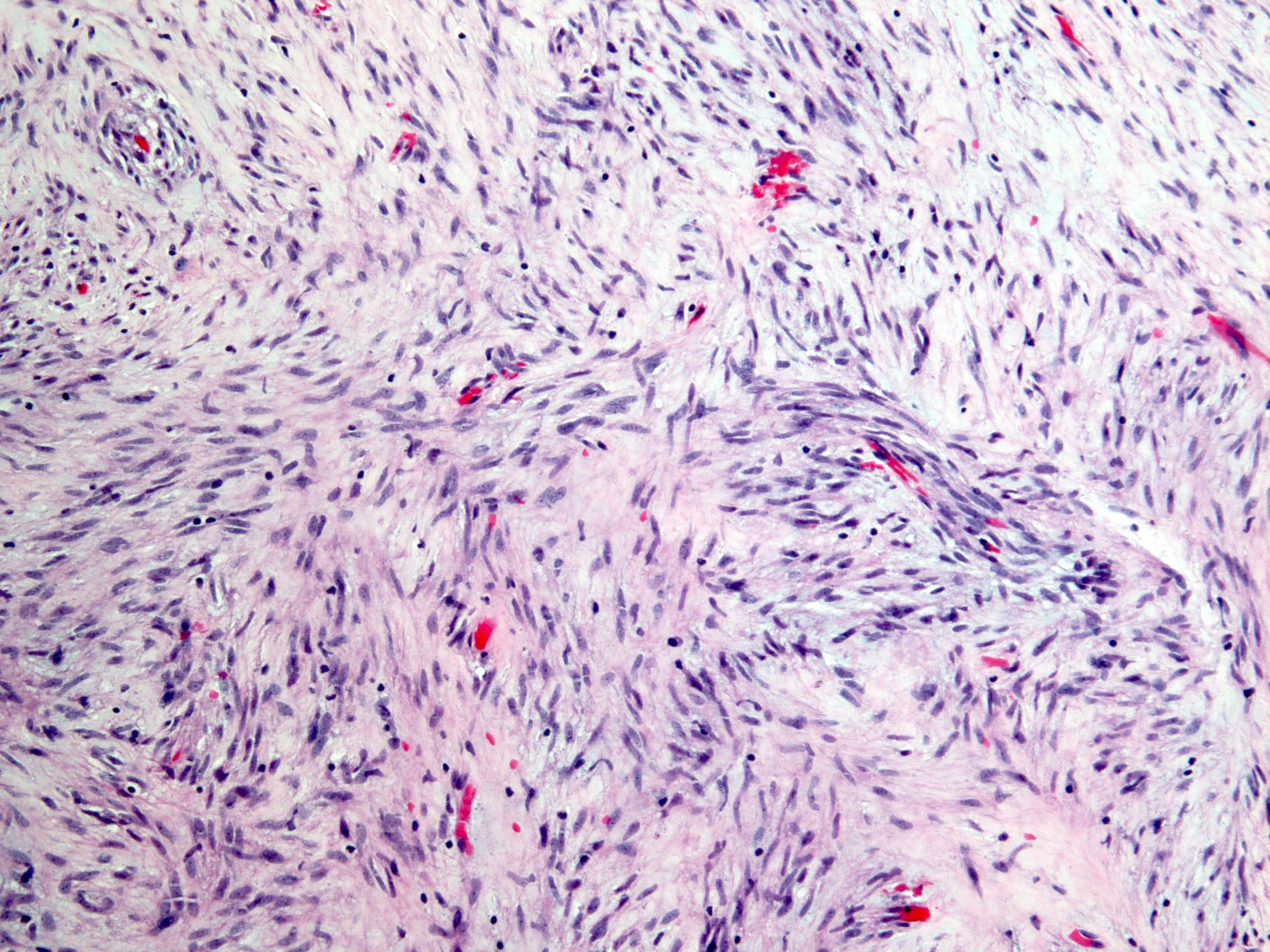
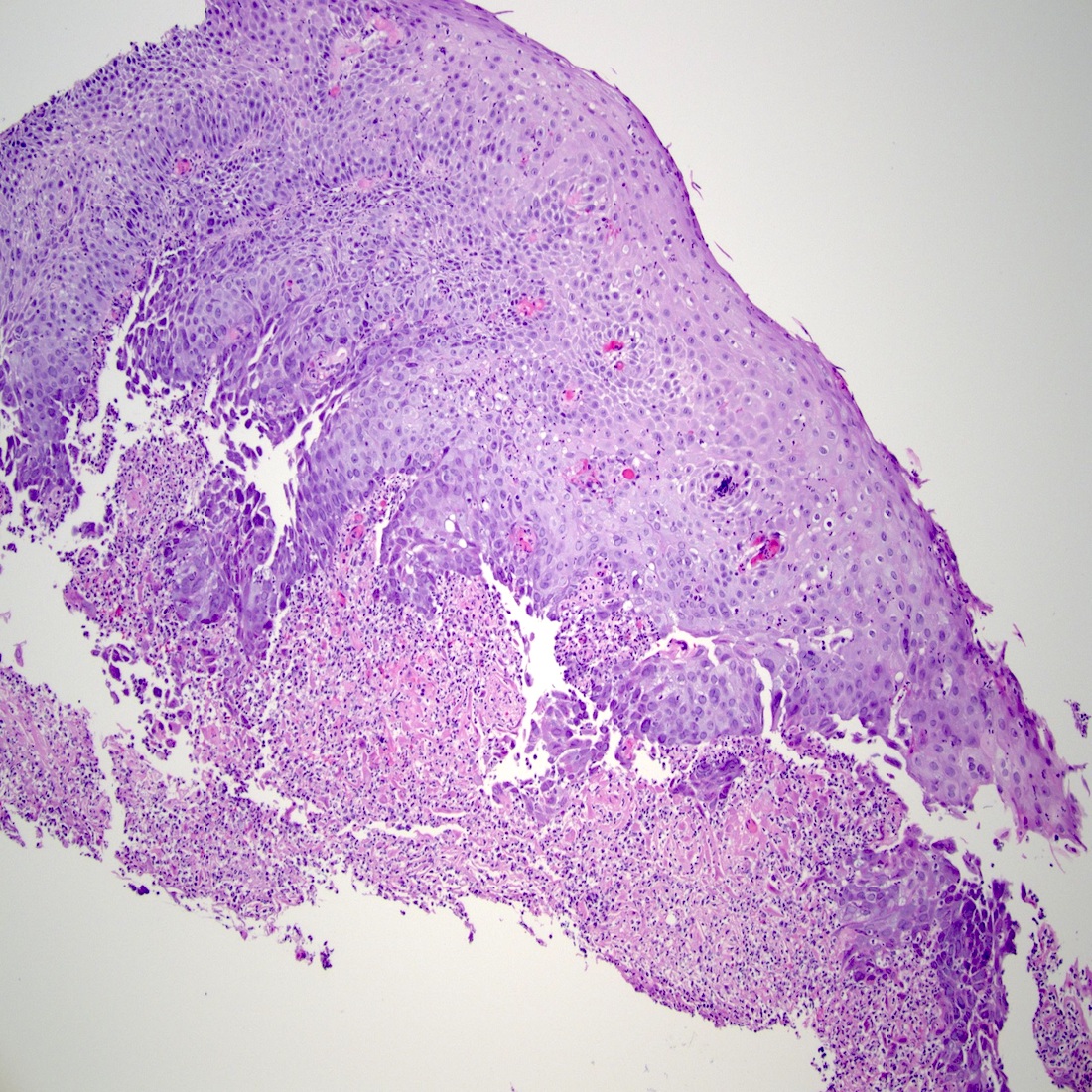
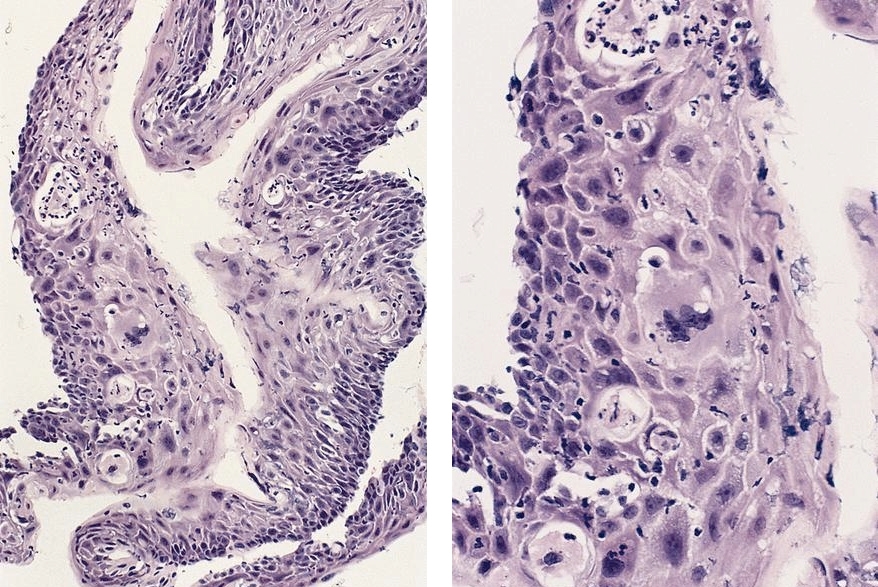
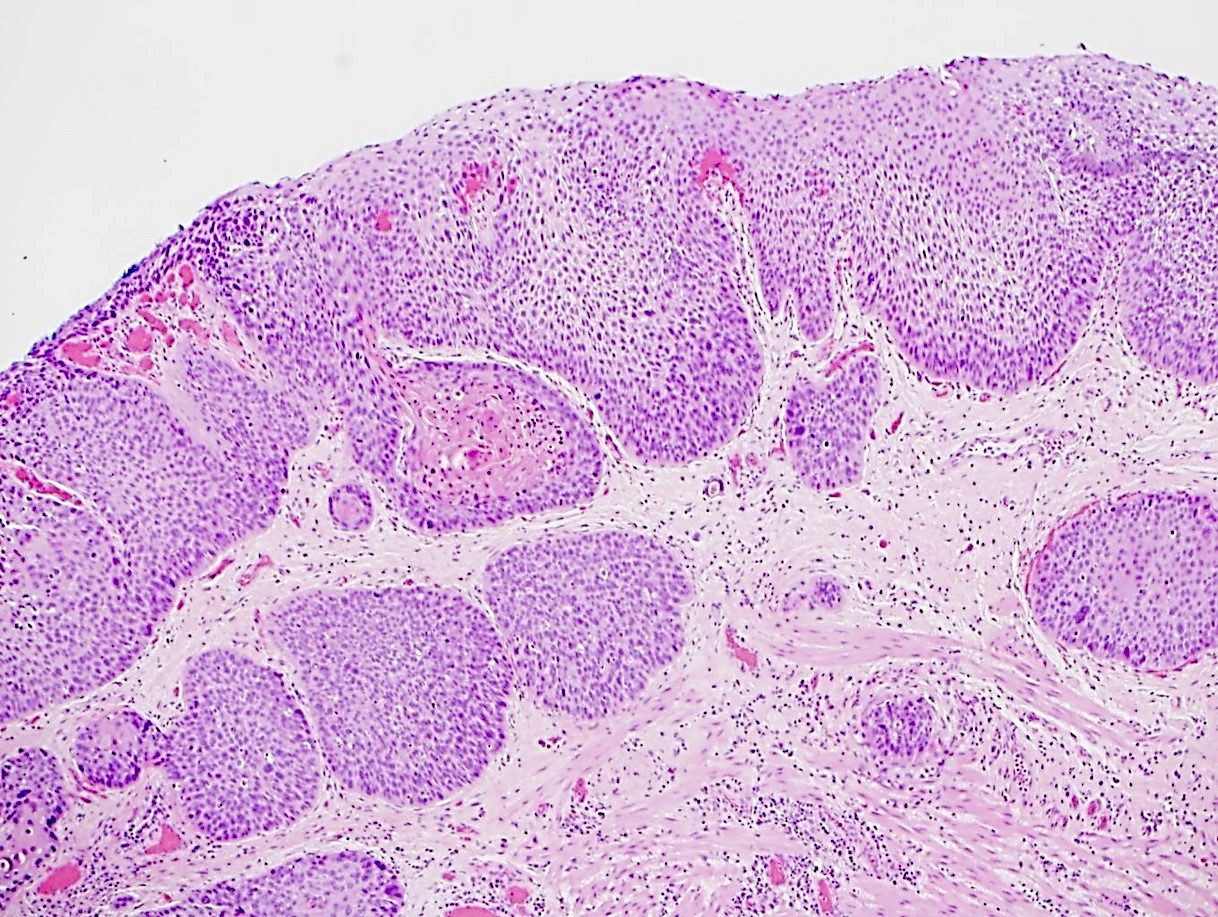
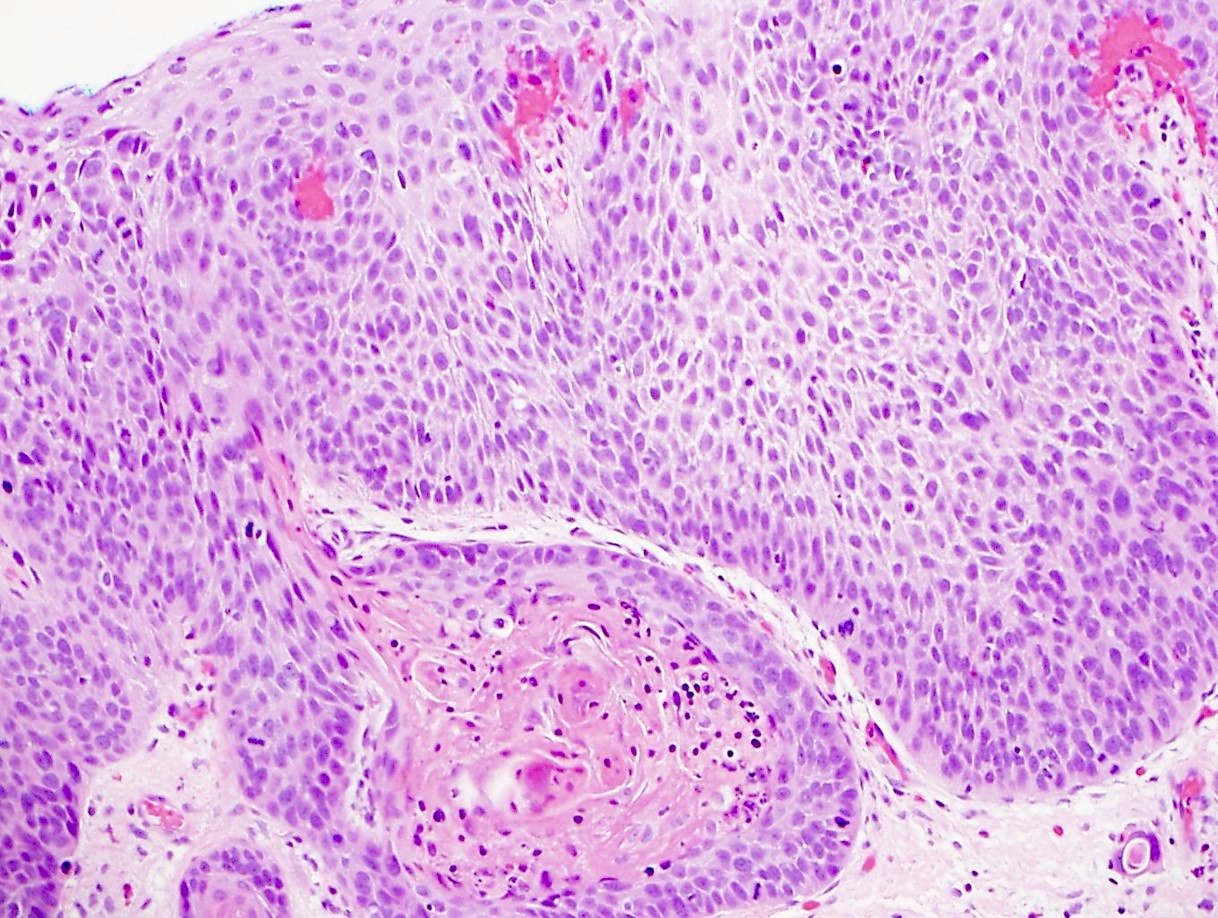
Microscopic (histologic) description
- Will show either gastric, intestinal or mixed lineage
- If in the lower esophagus, may show adjacent Barrett esophagus with intestinal metaplasia or dysplasia
- Dysplasia may be low or high grade
- Low grade will have cytological atypia but little to no architectural atypia
- High grade will have increased cytologic atypia and architectural abnormalities but the dysplastic epithelial cells are still limited by basement membrane
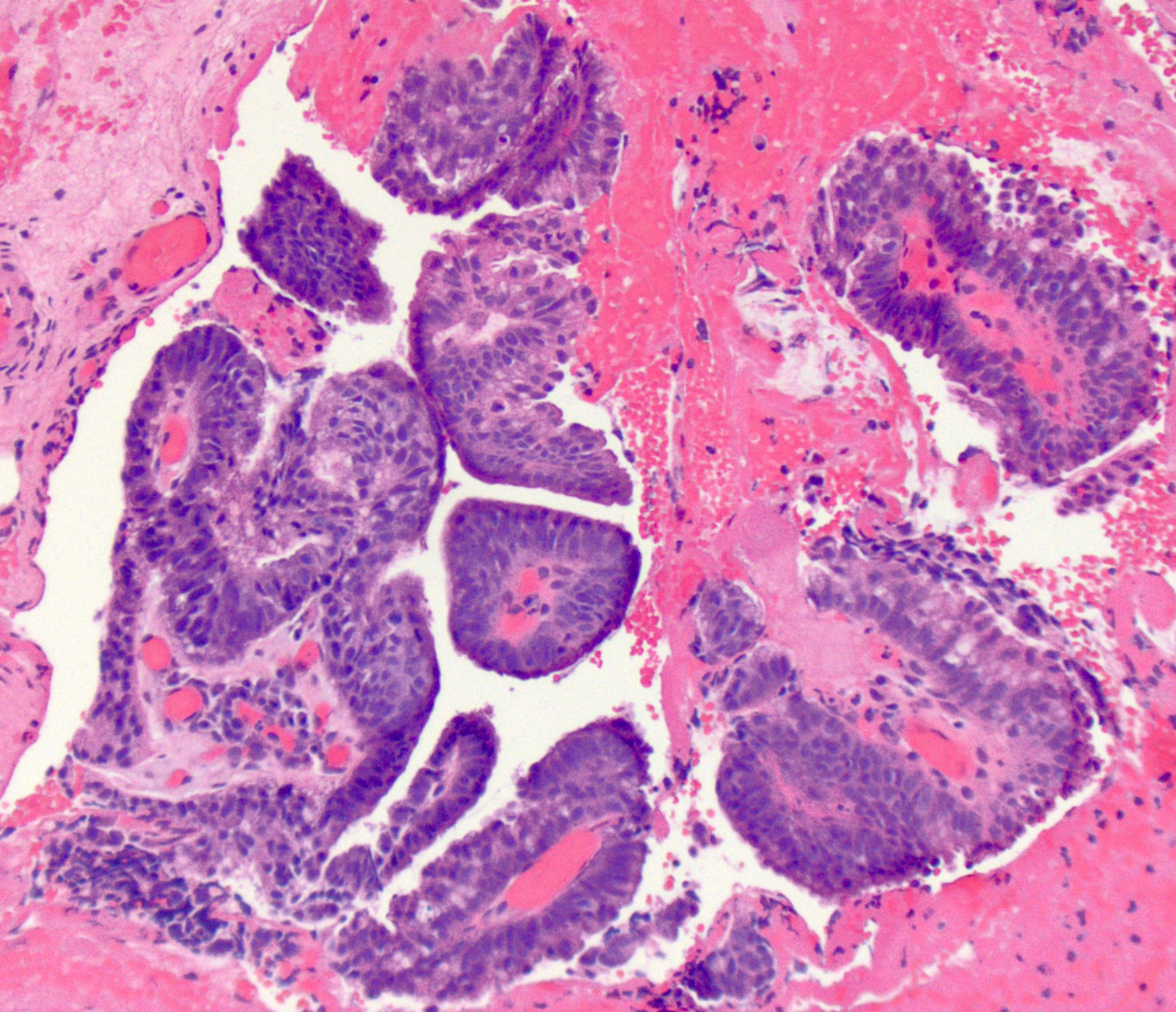
- Classified as having tubular, papillary, mucinous and signet ring cell patterns
- Papillary pattern may show micropapillary architecture (Pathol Int 2016;66:583)
- Tubular pattern is most common, with irregular, single or anastomosing tubular glandular structures and a single or stratified layer of malignant epithelium
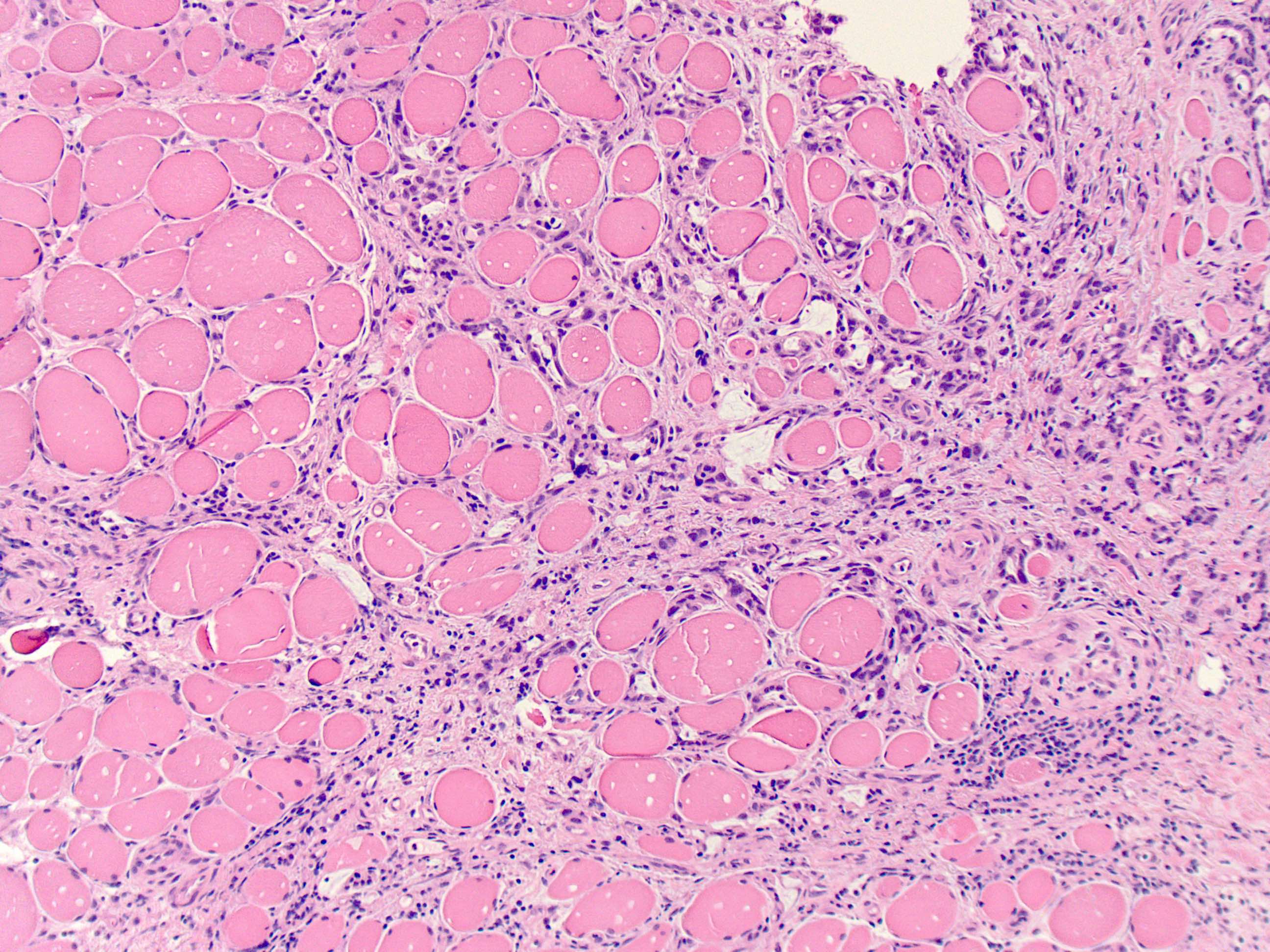
- Mucin production is variable
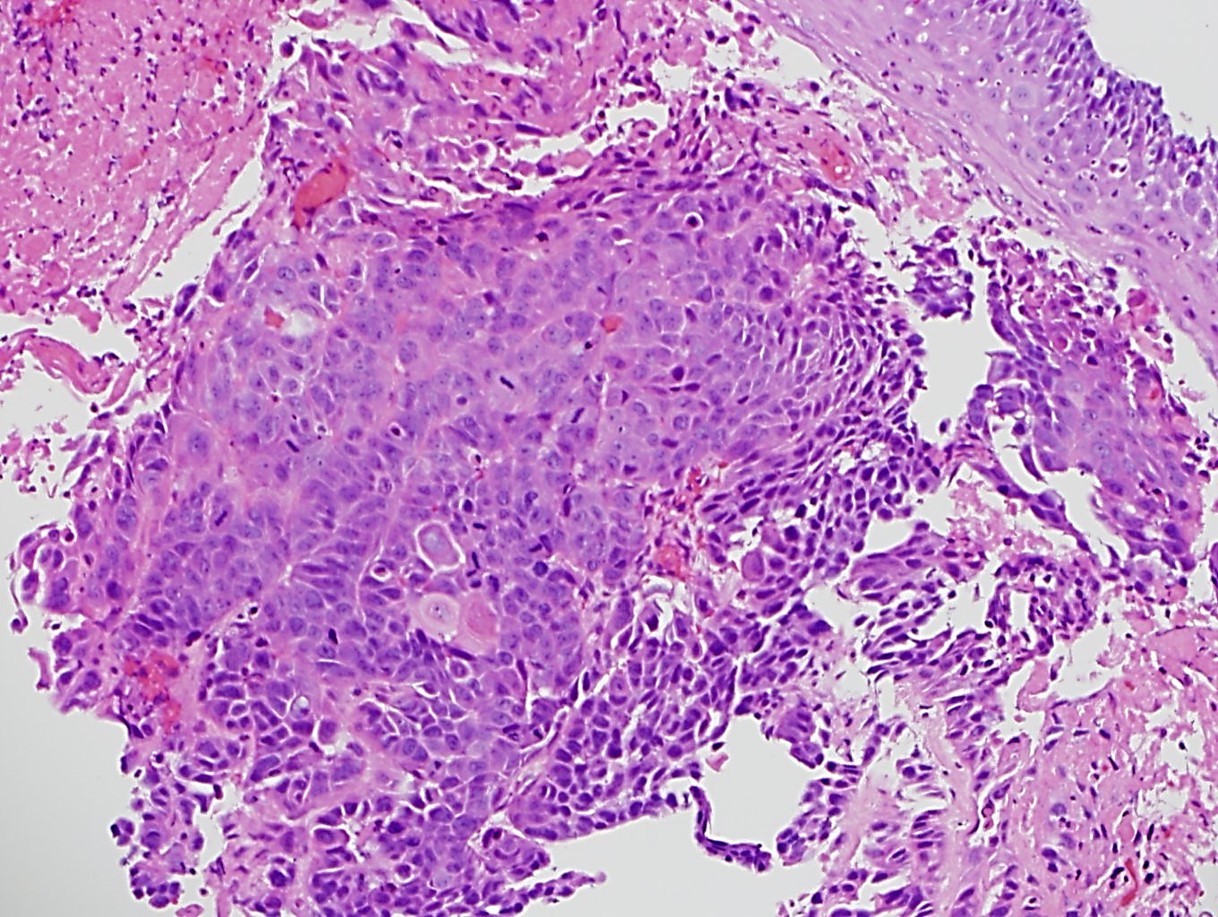
- ~50% of all esophageal adenocarcinomas are treated preoperatively with chemoradiation and therefore will show histologic changes consistent with treatment effect
- These include mucin pools that are acellular or containing small foci of residual adenocarcinoma
- Cytologic changes in the treated tumor cells include cytoplasmic eosinophilia, nuclear pyknosis and nuclear karyorrhexis
- Only residual viable tumor is considered in the determination of tumor stage and not the acellular mucin
- Similarly, lymph nodes with acellular mucin pools and therapy associated fibrosis but without viable tumor cells should be classified as no tumor within the lymph nodes (Mod Pathol 2018;31:4)
- Modified Ryan scheme for tumor regression score adapted by the College of American Pathologists (Histopathology 2005;47:141)
- 0: no viable cells (complete response)
- 1: single cells or rare groups of cancer cells (near complete response)
- 2: residual cancer with evident tumor regression but more than single cells or rare groups of cancer cells (partial response)
- 3: extensive residual cancer with no evidence of regression (poor or no response)
Microscopic (histologic) images
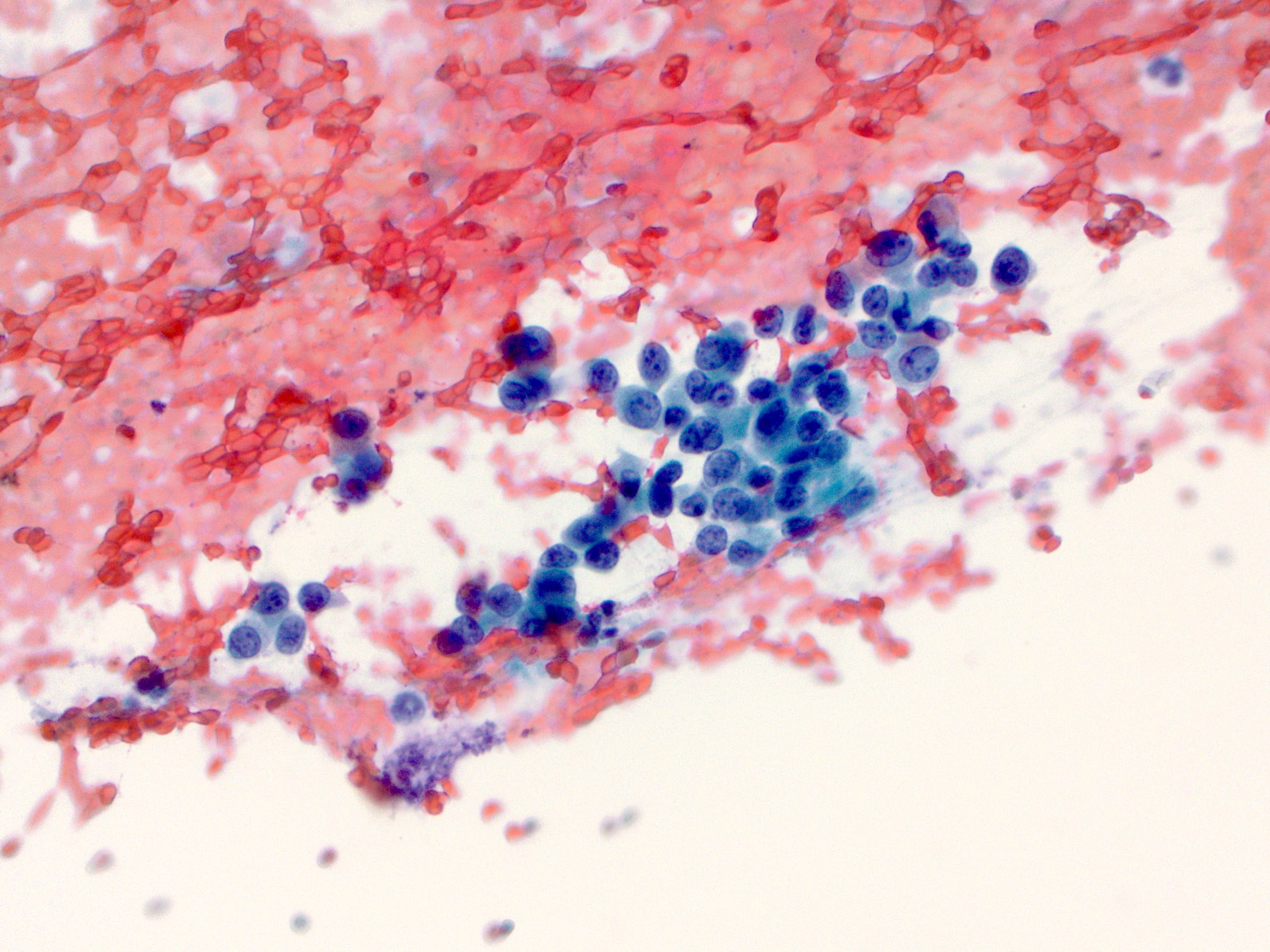
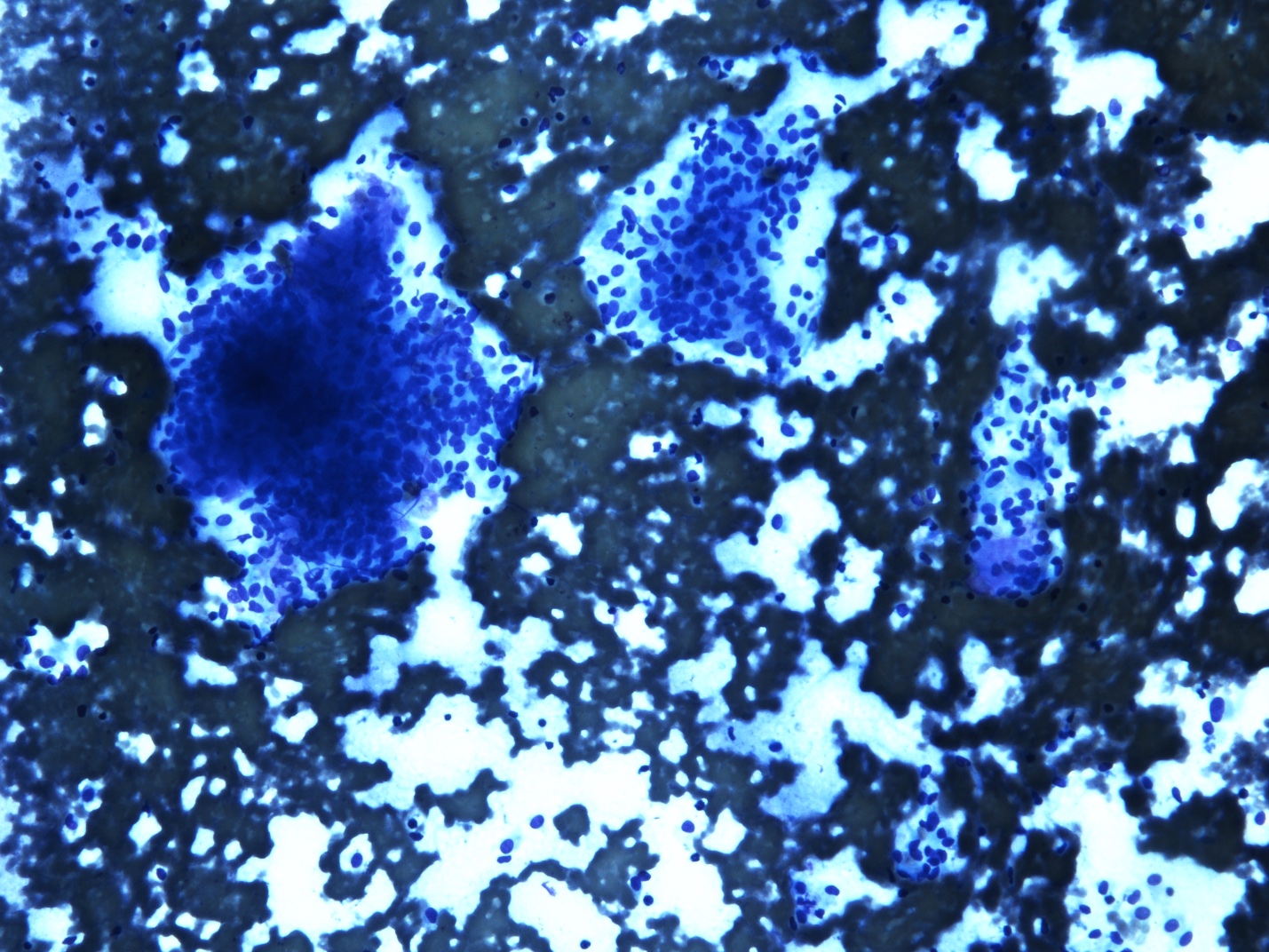
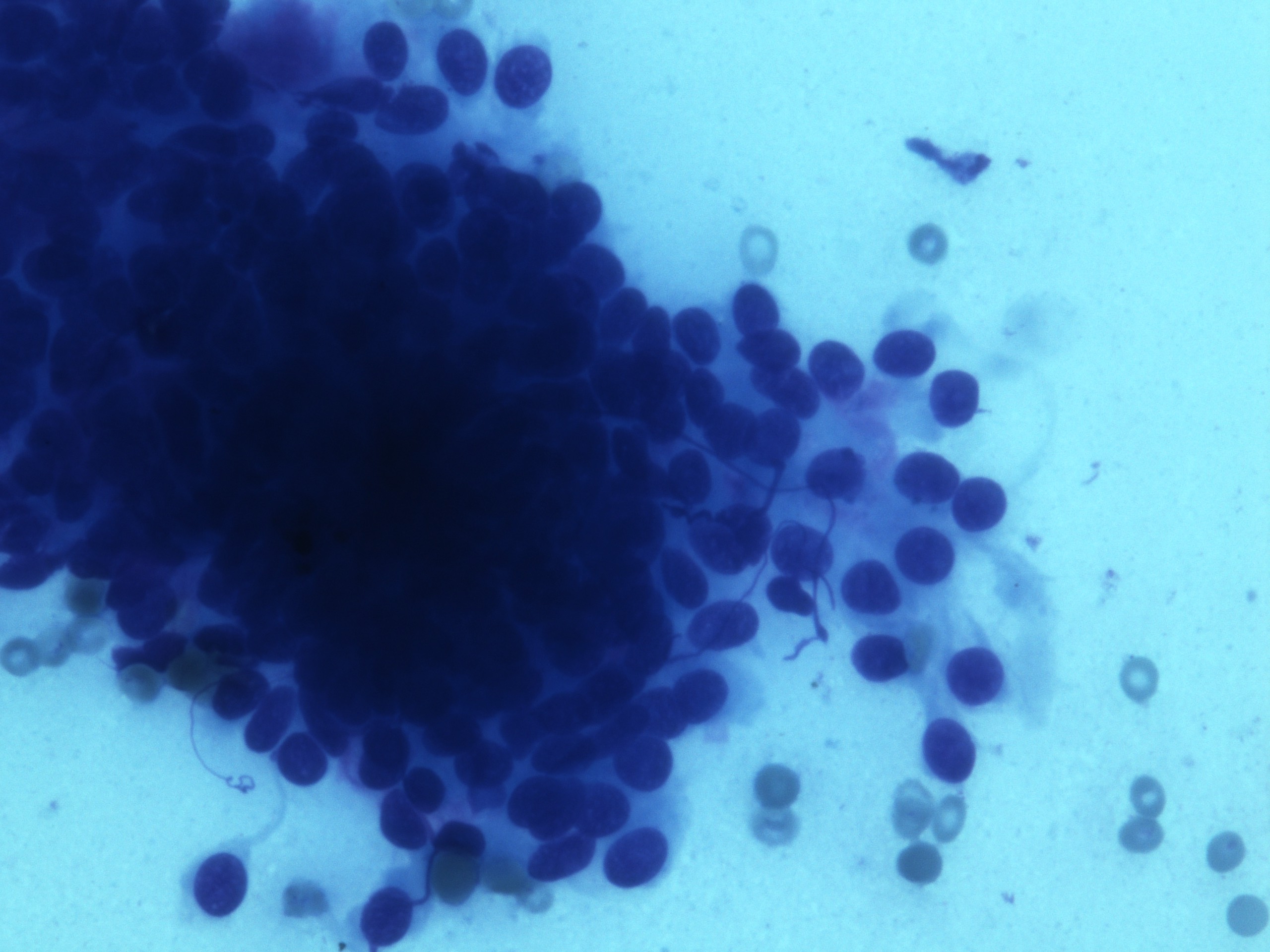
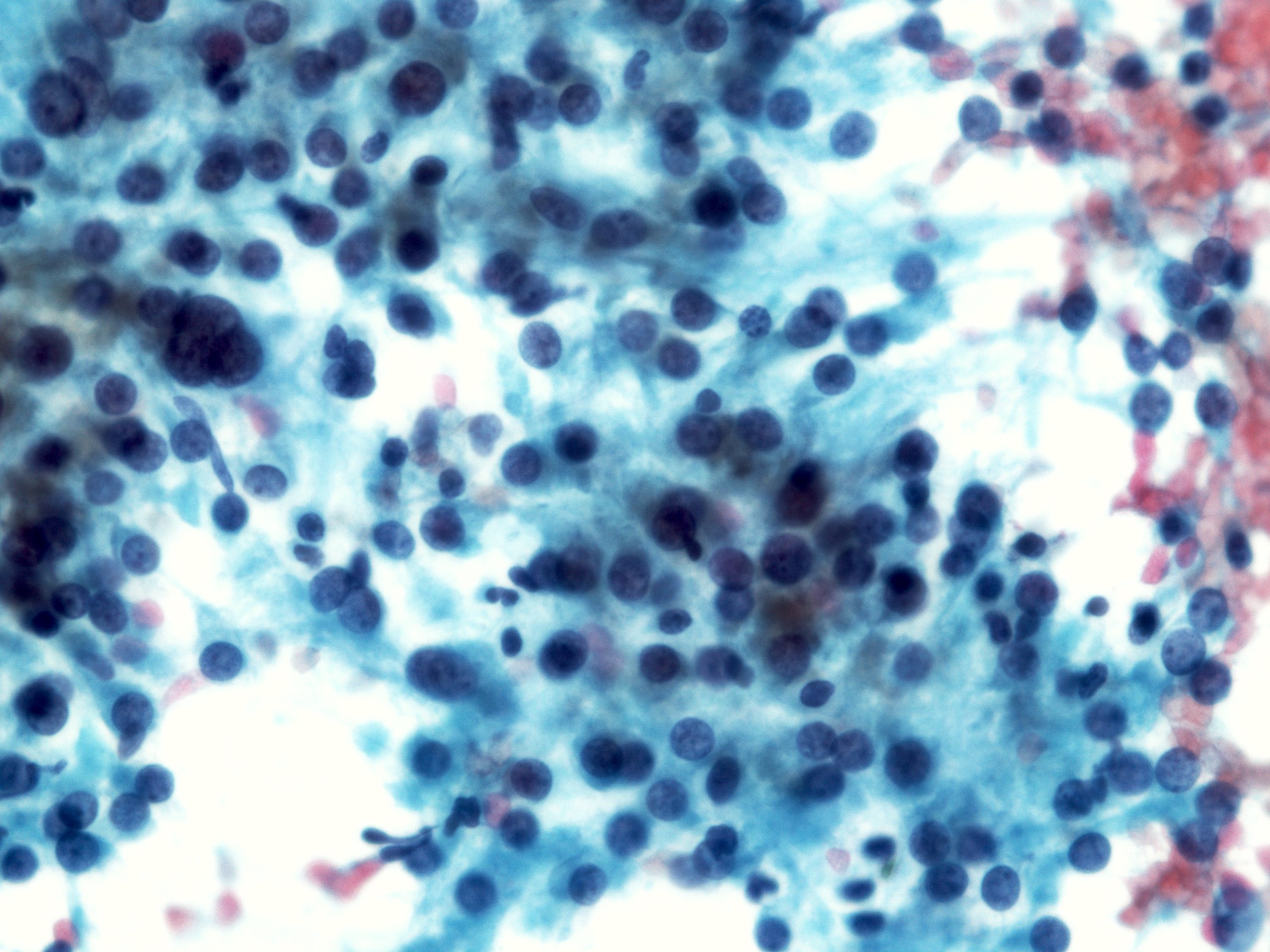
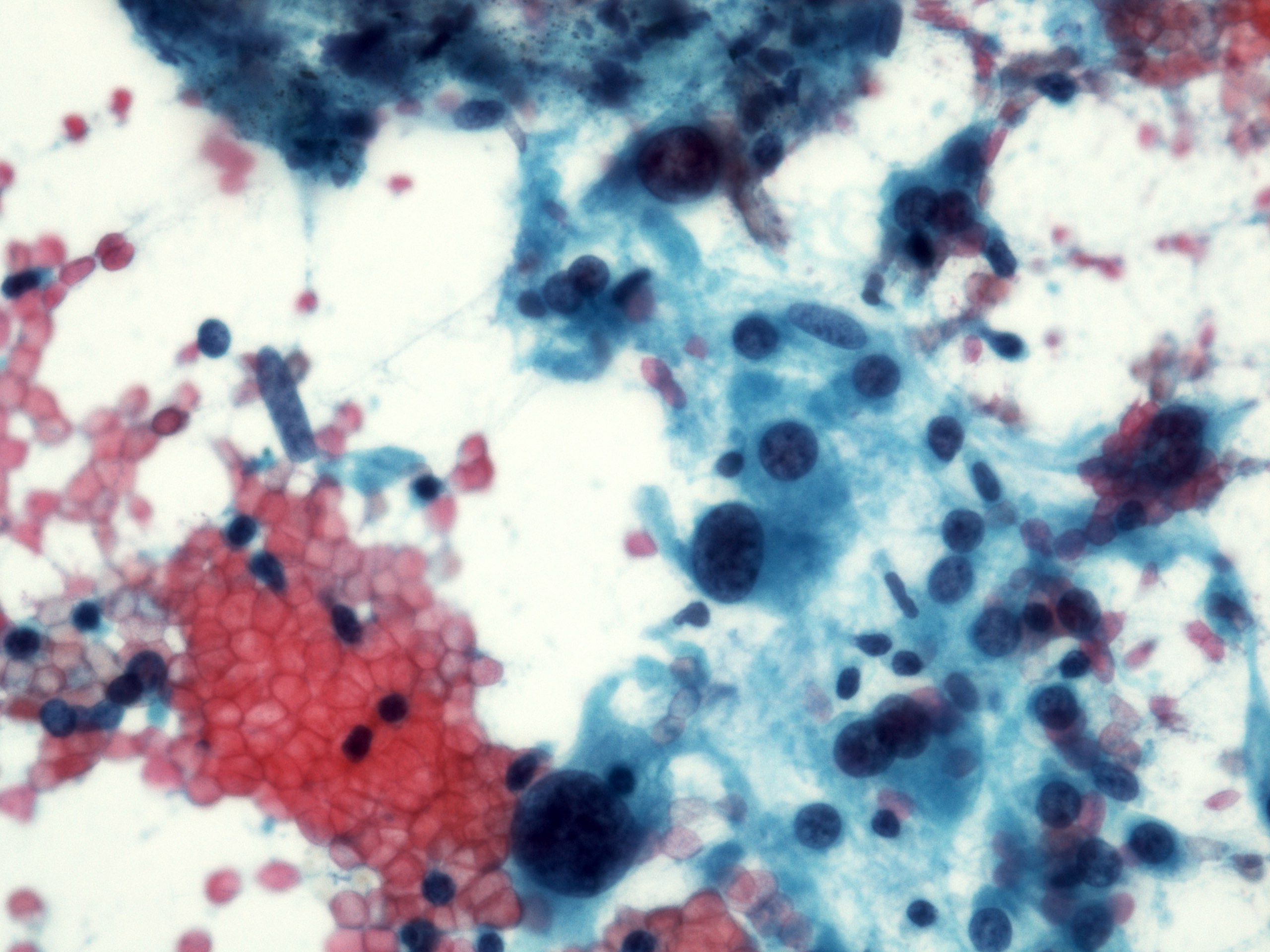
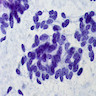
Cytology description
- Malignant glandular epithelial cells
- Smears are hypercellular with malignant cell groups showing nuclear enlargement, nuclear pleomorphism, hyperchromasia and irregular nuclear membranes
- Background may show tumor diathesis or necrosis
- Primary tumor is rarely sampled via cytology but endoscopic ultrasound guided fine needle aspiration (EUS-FNA) and transbronchial FNA sampling may be used to evaluate for paraesophageal and mediastinal / thoracic lymph node metastases (Clinics (Sao Paulo) 2011;66:1579)
Cytology images
Positive stains
- Immunohistochemistry is rarely needed for a diagnosis
- CK7+, CK19+ (this will not distinguish it from gastric or pancreaticobiliary adenocarcinoma) (Mod Pathol 2004;17:49, Am J Surg Pathol 2002;26:1213)
- TTF1 has been shown to be positive in a small subset of esophageal adenocarcinoma (Arch Pathol Lab Med 2013;137:1094)
- HER2 testing by IHC or FISH is recommended in all patients with metastatic esophagogastric junction carcinoma at diagnosis (J Natl Compr Canc Netw 2015;13:194)
- Programmed death ligand 1 (PDL1) expression is currently used as a predictive biomarker (BioDrugs 2022;36:473)
- Expression can determine candidacy for immunotherapy (i.e., pembrolizumab, humanized monoclonal PD1 blocking antibody)
- Testing is recommended if metastatic disease is documented or suspected
- PDL1 expression is considered positive if the combined positive score (CPS) ≥ 1
- Pitfalls to PDL1 IHC evaluation include spatial and temporal intratumor heterogeneity (Pathologica 2023;115:57)
- PDL1 (22C3) indicated for gastric or esophagogastric junction adenocarcinoma
Negative stains
- CK20 (may show patchy staining in a minority of cases)
Molecular / cytogenetics description
- See Positive stains
Sample pathology report
- Esophagus, distal, endoscopic biopsy:
- Invasive moderately differentiated adenocarcinoma arising in the background of high grade dysplasia and intestinal metaplasia
- Esophagus, distal, endoscopic mucosal resection:
- Invasive poorly differentiated adenocarcinoma with signet ring cell differentiation
- Tumor is invasive into the submucosa, stage pT1b
- Lateral mucosal margin is negative for carcinoma, closest approach is 3 mm
- Deep resection margin is focally positive for carcinoma
- Negative for lymphovascular and perineural invasion
- No lymph nodes are present for evaluation
- Esophagogastric junction, distal esophagus and proximal stomach, esophagogastrectomy:
- Relationship of tumor to esophagogastric junction: tumor midpoint is located at the esophagogastric junction
- Distance of tumor center from eophagogastric junction:
- Other (specify): tumor midpoint is the esophagogastric junction
- Histologic type: adenocarcinoma
- Histologic grade: G2, moderately differentiated
- Tumor size: greatest dimension in centimeters is 1.9 cm
- Tumor extent: invades muscularis propria
- Treatment effect: present, with residual cancer showing evident tumor regression but more than single cells or rare small groups of cancer cells (partial response, score 2)
- Lymphovascular invasion: present
- Perineural invasion: not identified
- Margins: all margins negative for invasive carcinoma
- Closest margin to invasive carcinoma: proximal
- Distance from invasive carcinoma to closest margin: 1.8 cm
- Margin status for dysplasia and intestinal metaplasia: all margins negative for dysplasia and intestinal metaplasia
- Regional lymph node status
- Tumor present in regional lymph node(s)
- Number of lymph node(s) with tumor: 1
- Number of lymph node(s) examined: 8
- Distant metastasis: not applicable
- Pathologic stage classification: ypT2pN1
- Additional findings:
- Intestinal metaplasia (Barrett esophagus)
- Low grade glandular dysplasia
- High grade glandular dysplasia
- Special studies: HER2 IHC: positive, 3+
Differential diagnosis
- Differentiation between high grade glandular dysplasia and invasive adenocarcinoma into the lamina propria may be difficult, especially on small biopsies
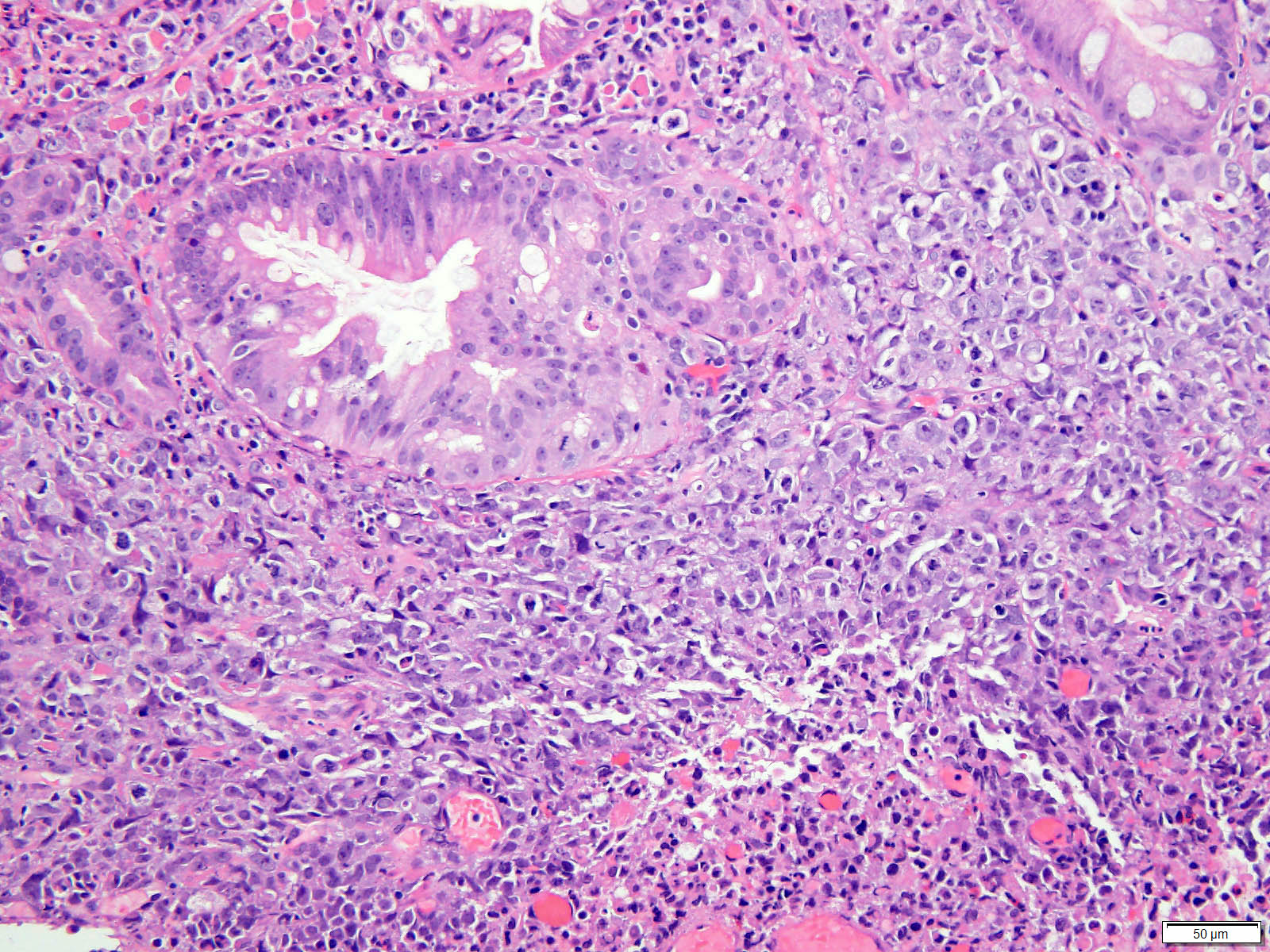
- Intramucosal adenocarcinoma:
- Shows invasion into the lamina propria or into muscularis mucosa but not through the muscularis mucosa
- Desmoplasia favors invasive carcinoma when present
- Intramucosal adenocarcinoma:
- Squamous cell carcinoma of the esophagus:
- May be difficult to distinguish from poorly differentiated adenocarcinoma with solid areas
- Squamous cell carcinoma will be positive for CK5/6, p63, p40
- Positive mucicarmine staining will favor a diagnosis of adenocarcinoma
- Adenosquamous carcinoma:
- Composed of both malignant squamous and adenocarcinoma components
- The 2 components may be intermixed or separate
- Mucoepidermoid carcinoma (J Thorac Oncol 2011;6:1426):
- Rarely arises in the esophagus
- Composed of glands and nests of malignant squamoid cells, mucin containing glandular cells and intermediate cells
- Neuroendocrine tumors, especially those with prominent pseudoglandular architecture:
- Neuroendocrine tumors will show positivity for synaptophysin, chromogranin, CD56, INSM1
Additional references
Board review style question #1
Board review style answer #1
A. Barrett esophagus / intestinal metaplasia. Patients with Barrett esophagus have a 10 - 55 times higher risk of adenocarcinoma. Candida esophagitis (B), parakeratosis (C) and proton pump inhibitor therapy (D) do not increase the risk of glandular dysplasia and adenocarcinoma. Atypical parakeratosis may overlie a focus of squamous dysplasia. Proton pump inhibitor therapy may have some protective effect but this association needs more evidence.
Comment Here
Reference: Adenocarcinoma
Comment Here
Reference: Adenocarcinoma
Board review style question #2
A biopsy of a thoracic lymph node showed a CK7+, CK20- and TTF1+ metastatic adenocarcinoma. Is this immunohistochemistry profile sufficient to distinguish between an esophageal versus a pulmonary primary?
- Likely pulmonary primary but esophageal primary needs to be excluded via clinical / endoscopic / radiographic correlation
- No, does not support either esophageal or pulmonary primary
- Yes, favors esophageal primary
- Yes, favors pulmonary primary
Board review style answer #2
A. Likely pulmonary primary but esophageal primary needs to be excluded via clinical / endoscopic / radiographic correlation. A CK7+, CK20- and TTF1+ metastatic adenocarcinoma is likely to be of pulmonary origin; however, since a minority of esophageal adenocarcinomas are TTF1+, it is prudent to rule out that possibility by correlating with clinical findings. Answers B, C and D are incorrect because a CK7+, TTF+ immunohistochemistry profile is seen in both primary lung and esophageal adenocarcinoma.
Comment Here
Reference: Adenocarcinoma
Comment Here
Reference: Adenocarcinoma
Adenoid cystic carcinoma
Table of Contents
Definition / general | Epidemiology | Prognostic factors | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- Primary tumor of esophagus morphologically and immunohistochemically identical to adenoid cystic carcinoma of salivary glands
- Believed to arise from esophageal glands
Epidemiology
- Very rare, generally middle age, more common in females
Prognostic factors
- Generally favorable prognosis with excellent survival
- Superior to squamous cell carcinoma and usual adenocarcinoma of the esophagus
Case reports
- 59 year old woman (Int J Clin Pract 2005;59:1101)
- 70 year old woman (Surg Today 1997;27:238)
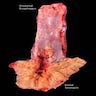
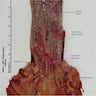
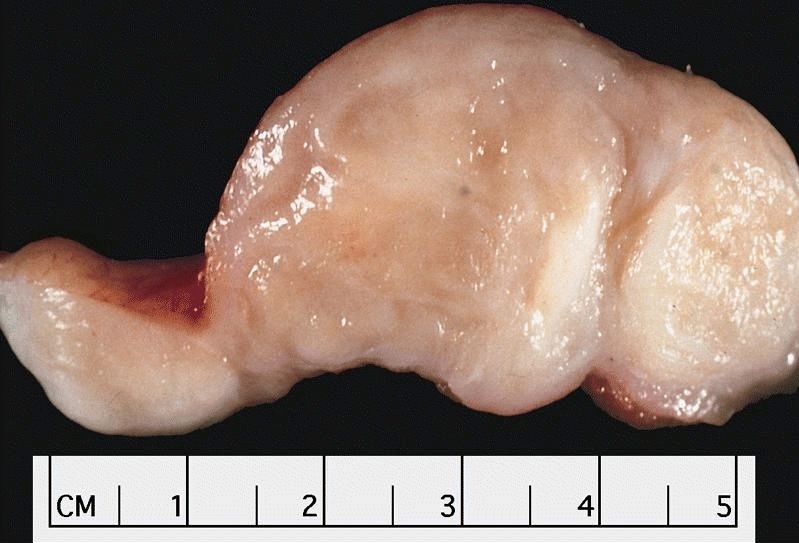
Gross description
- Generally well circumscribed nodule in submucosa
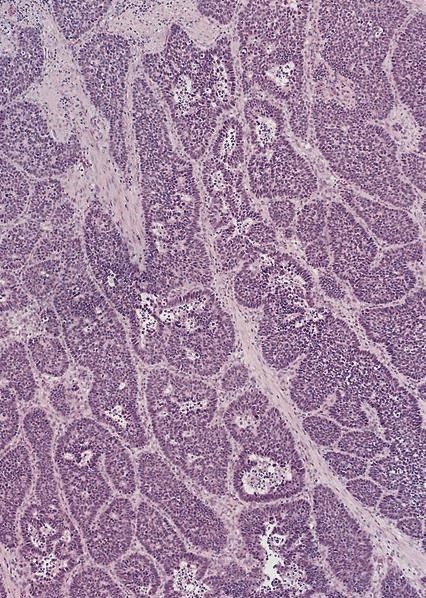
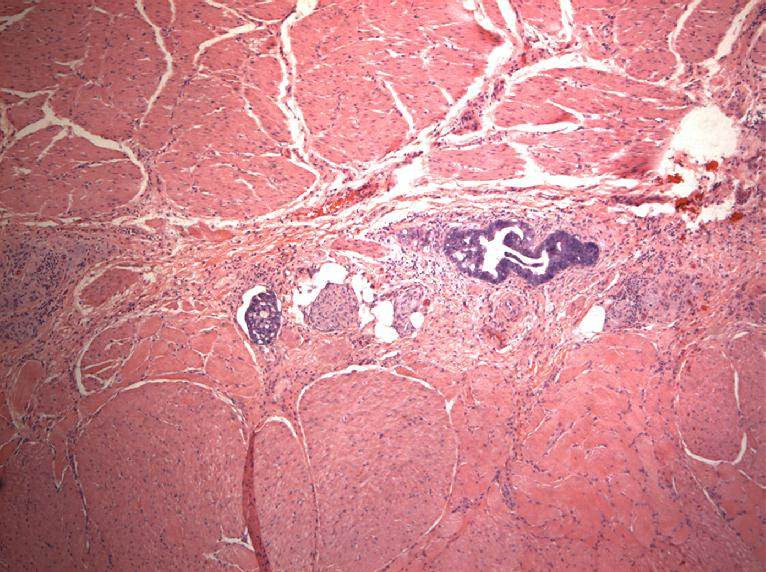
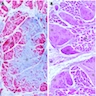
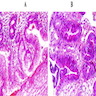
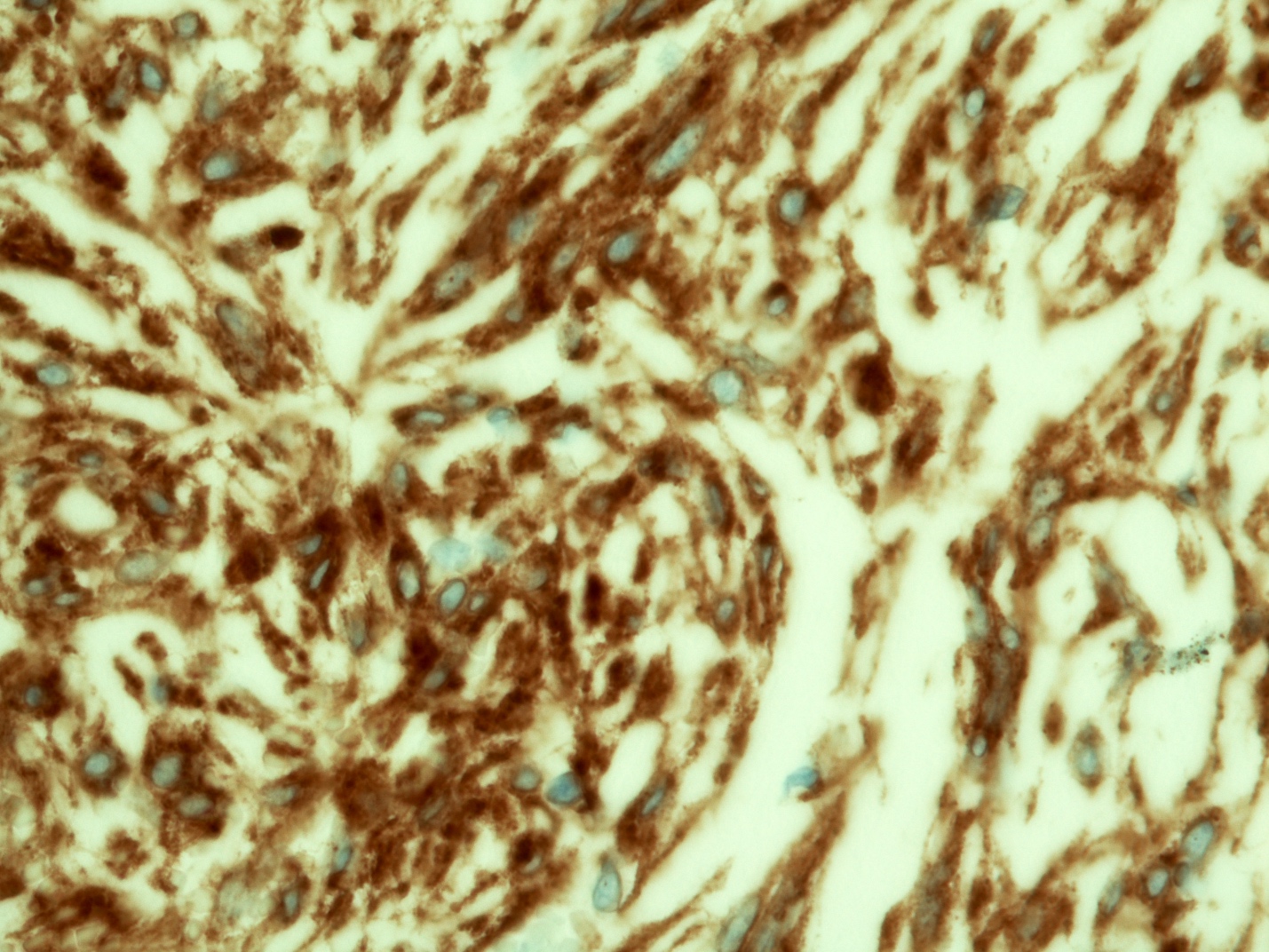
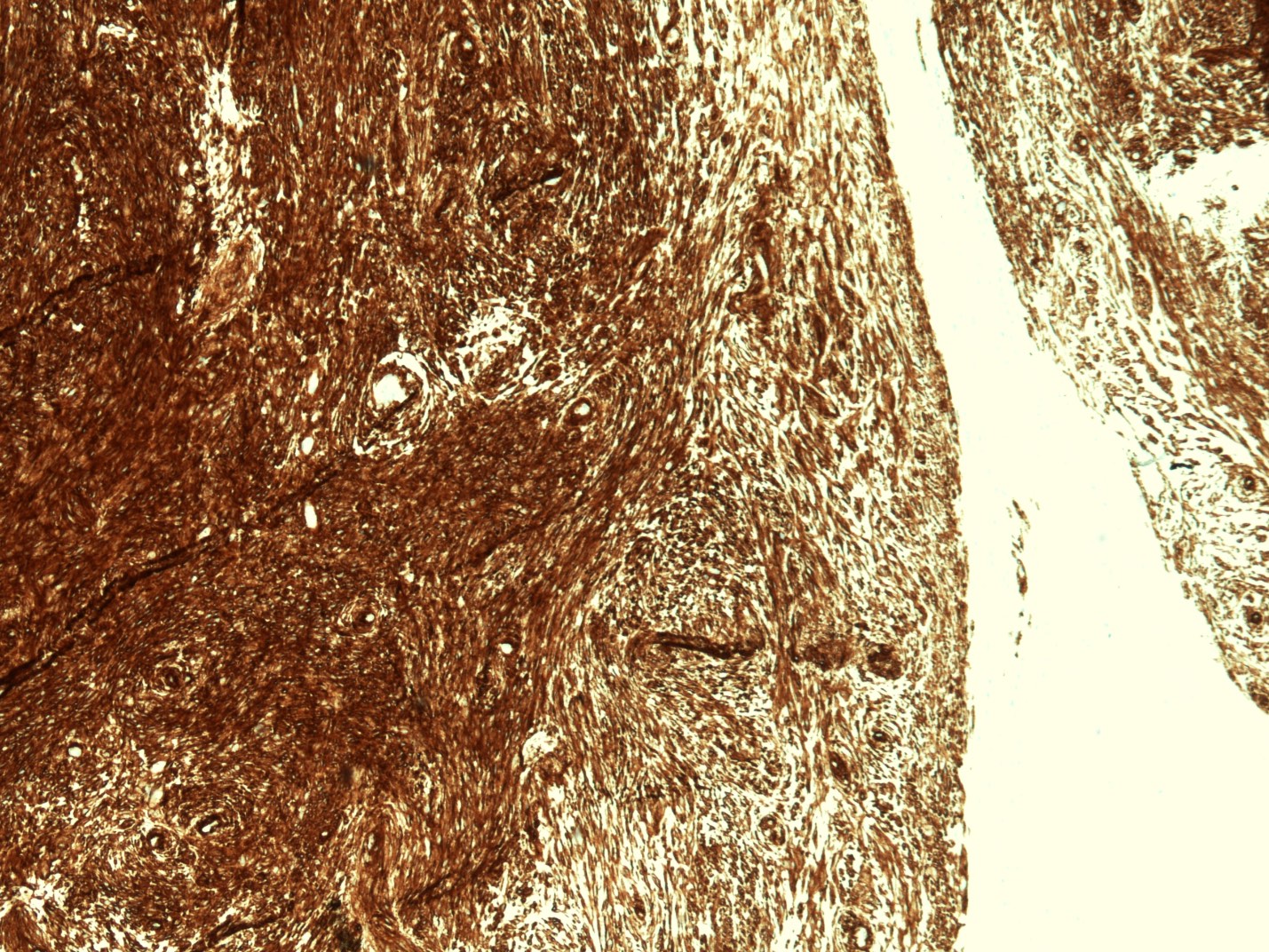
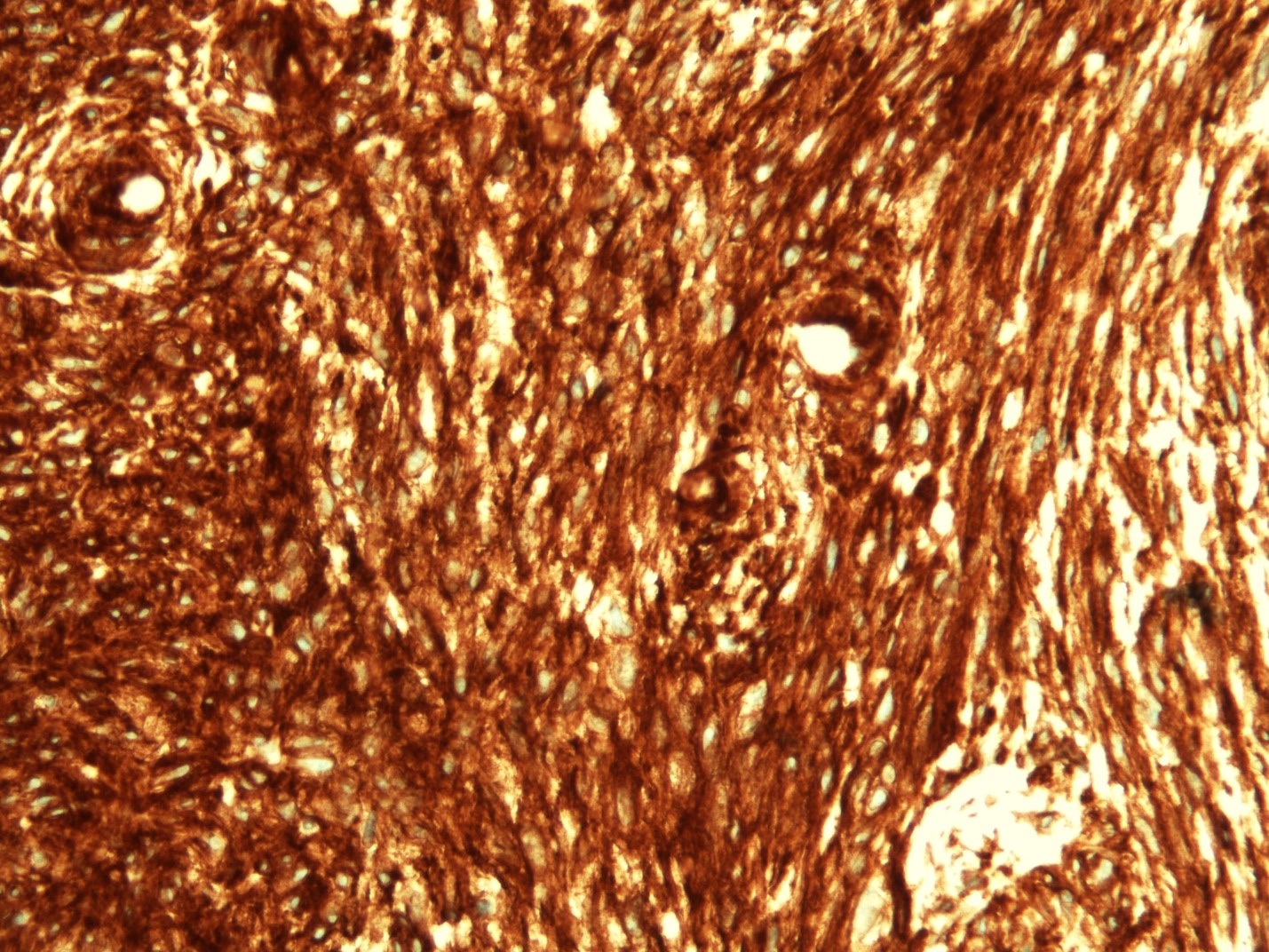
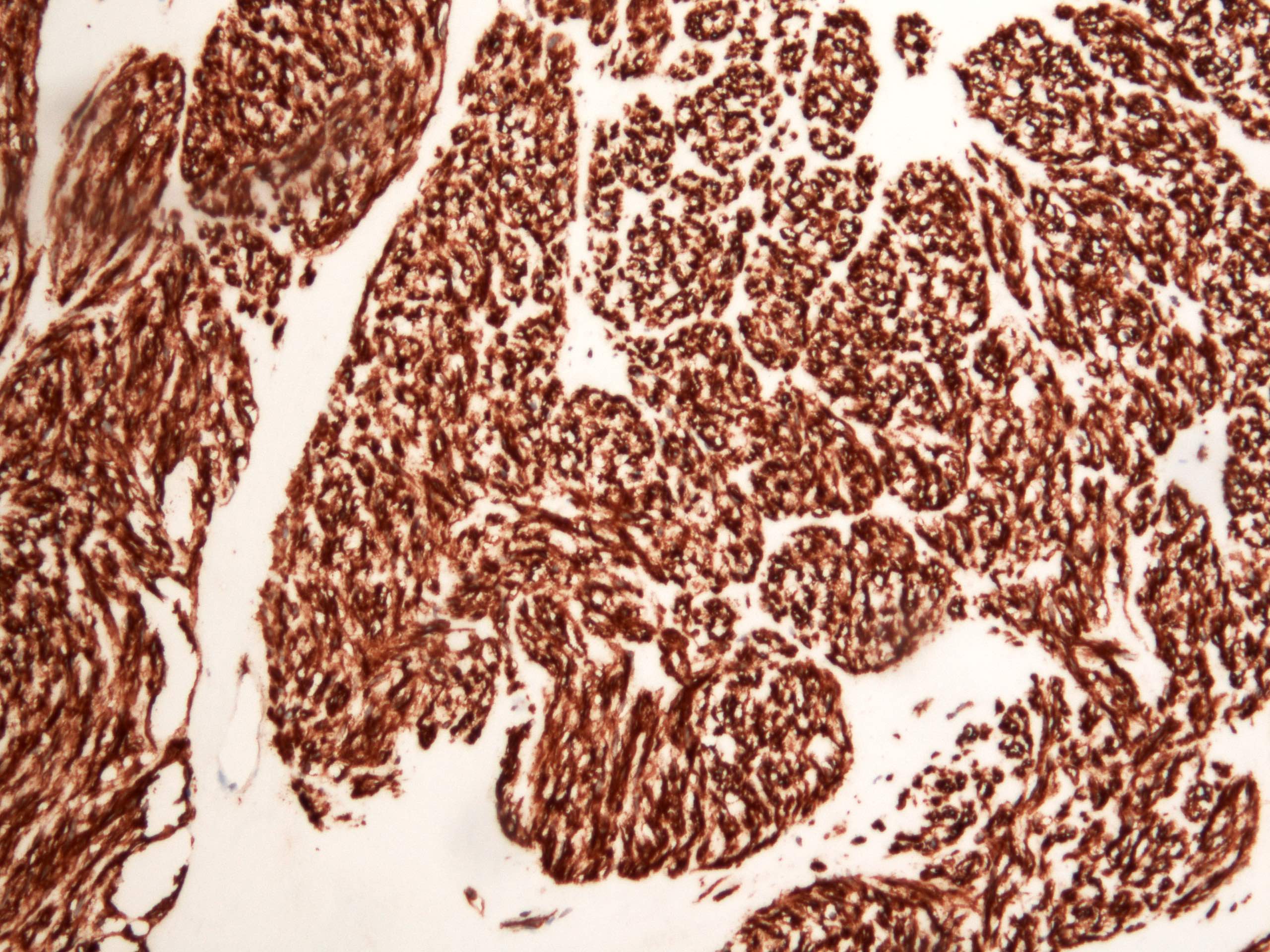
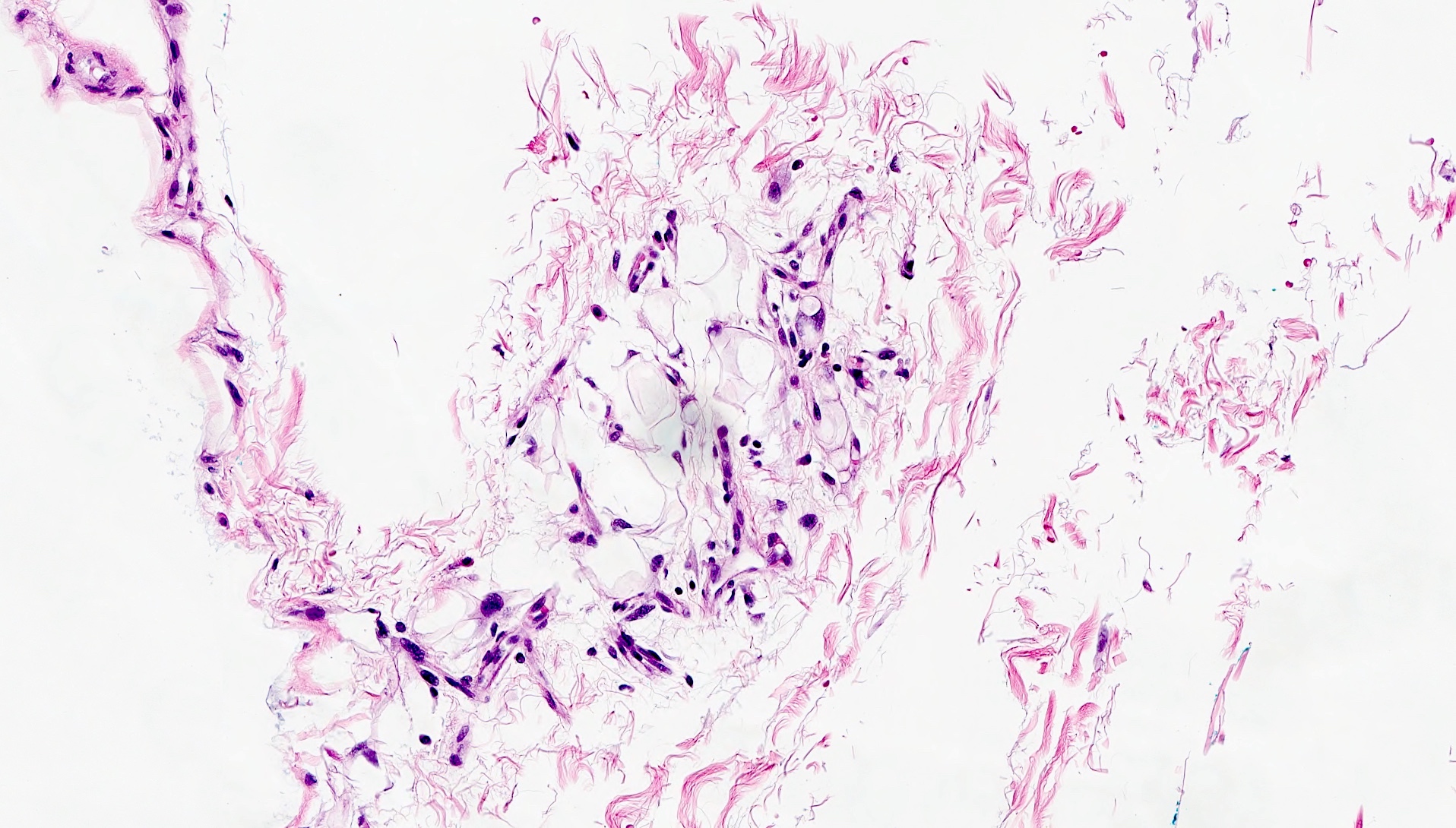
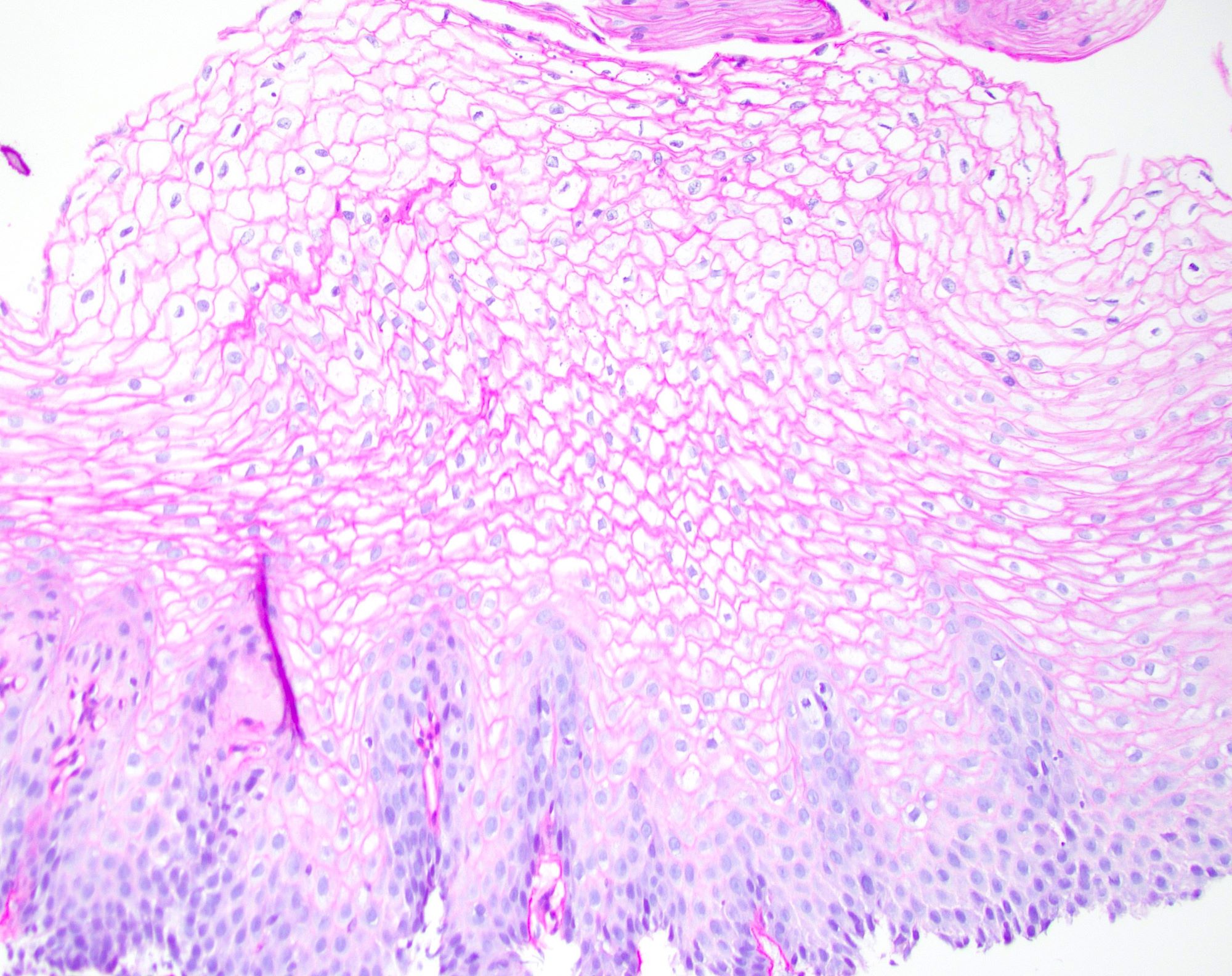
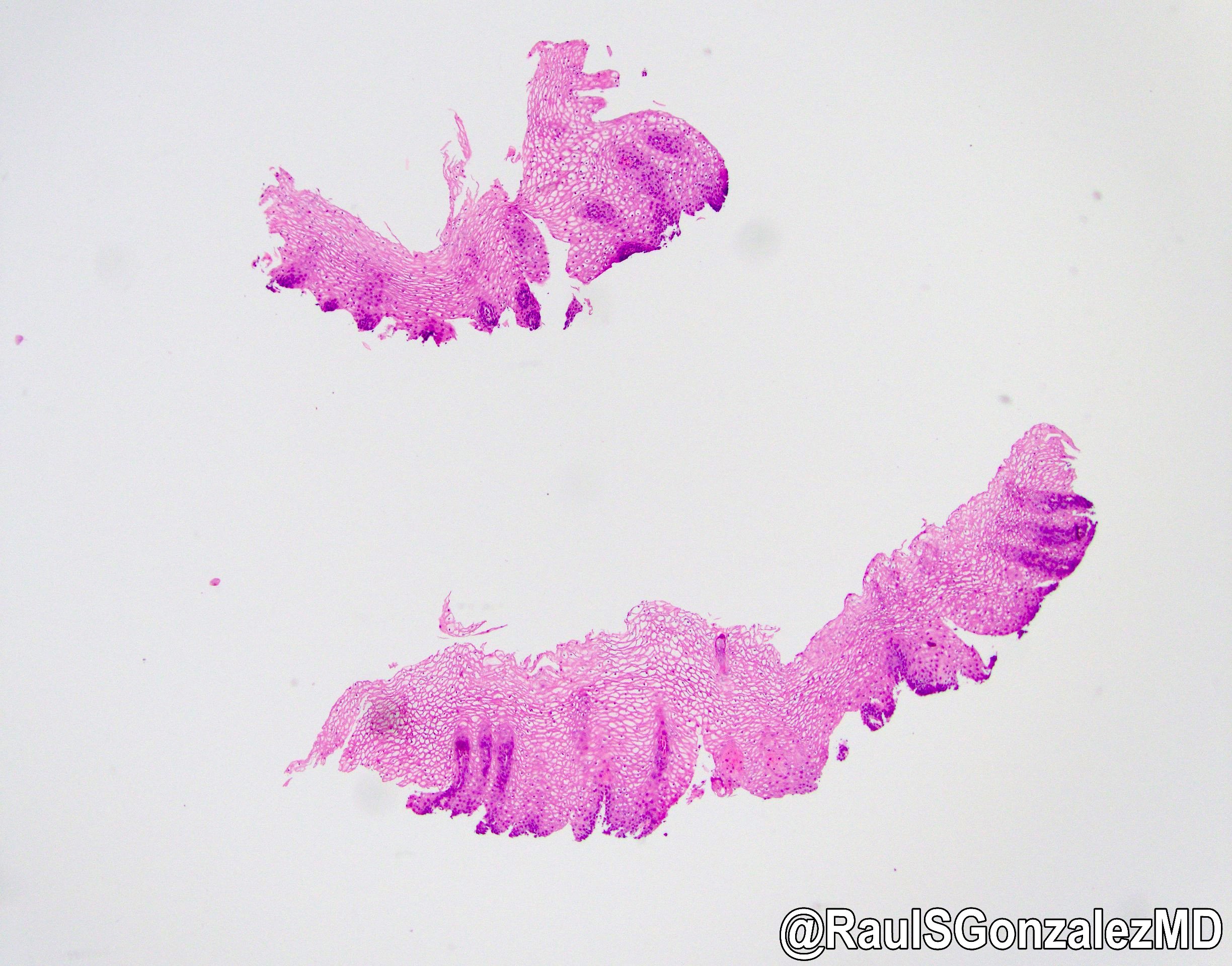
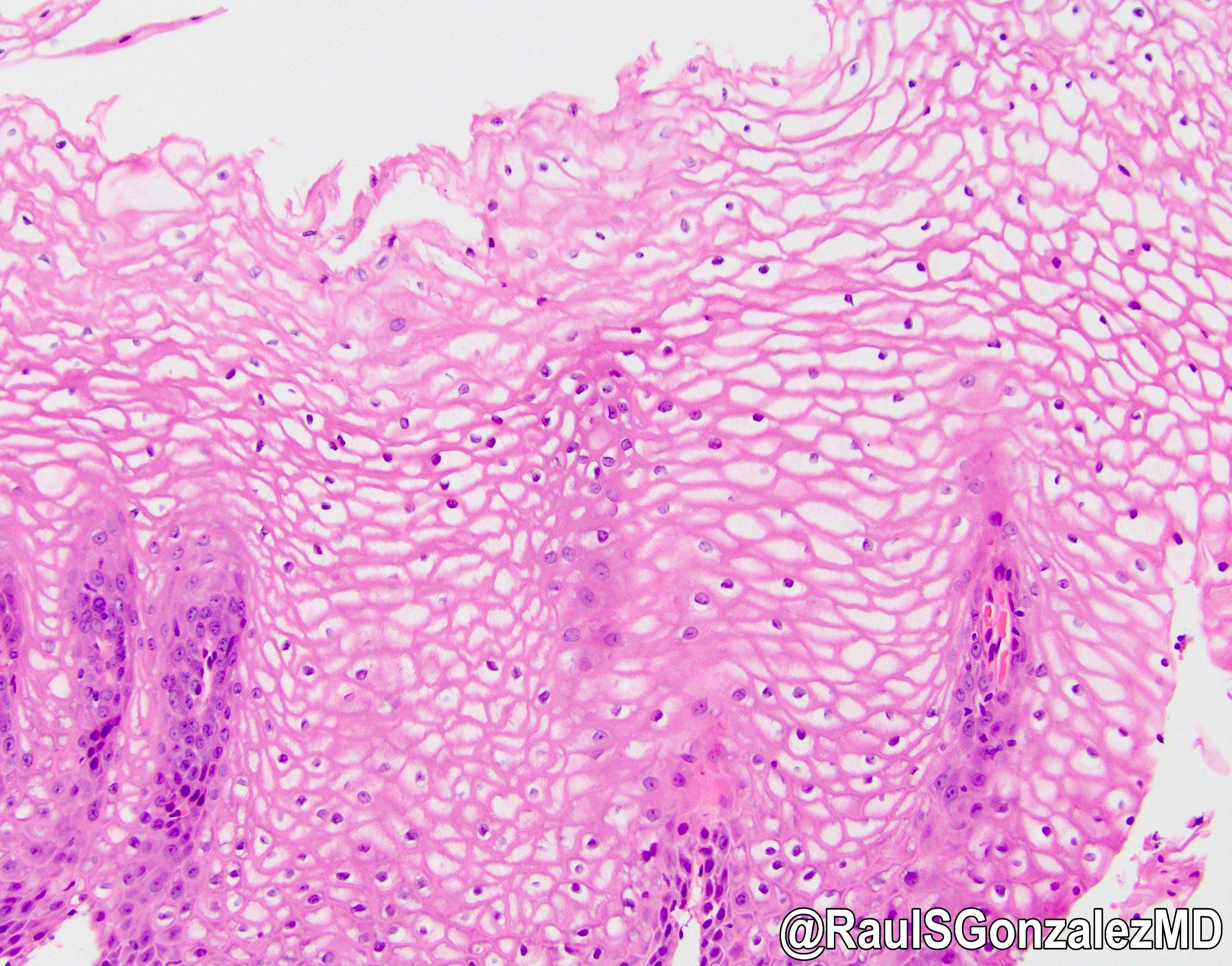
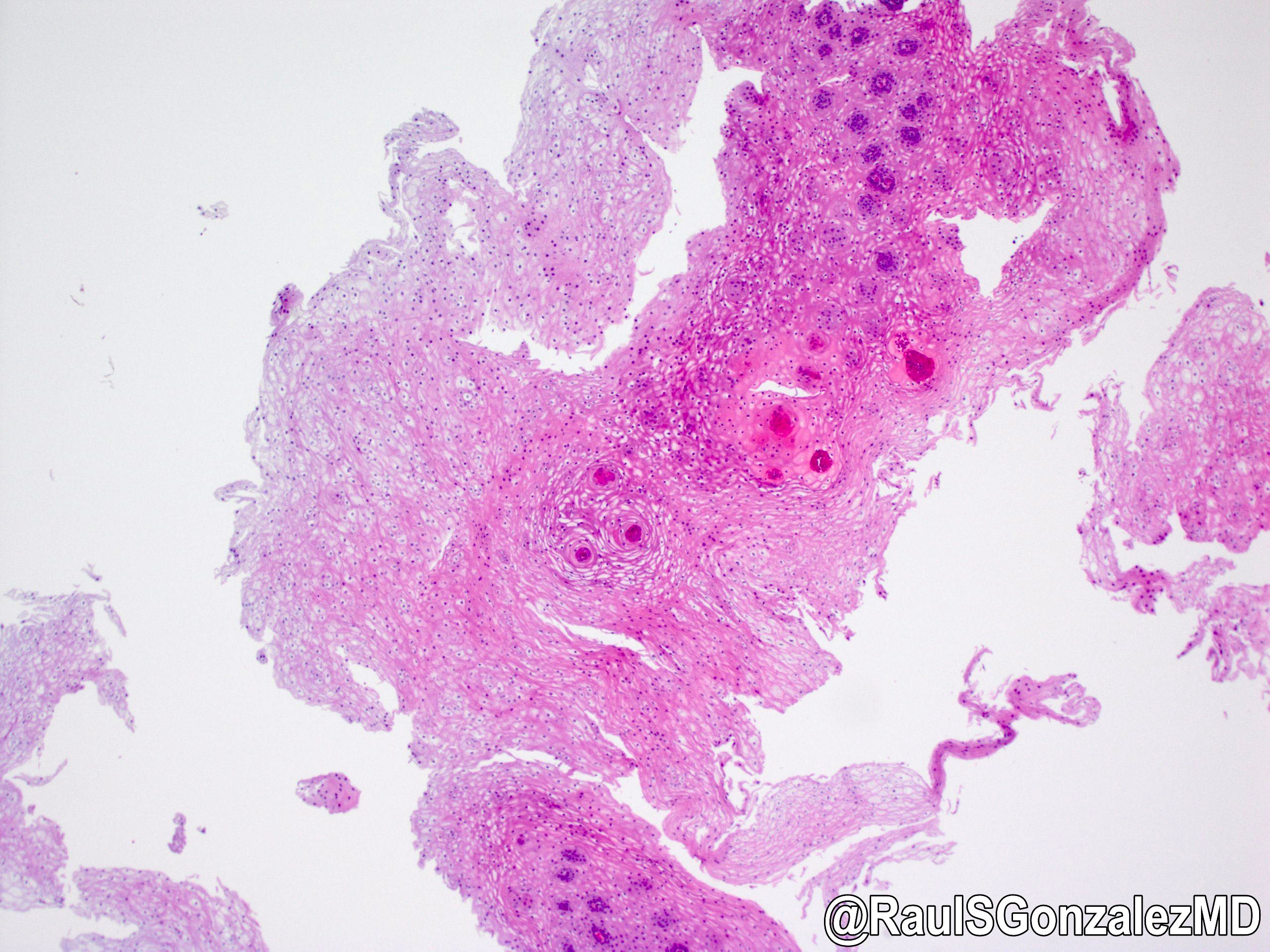
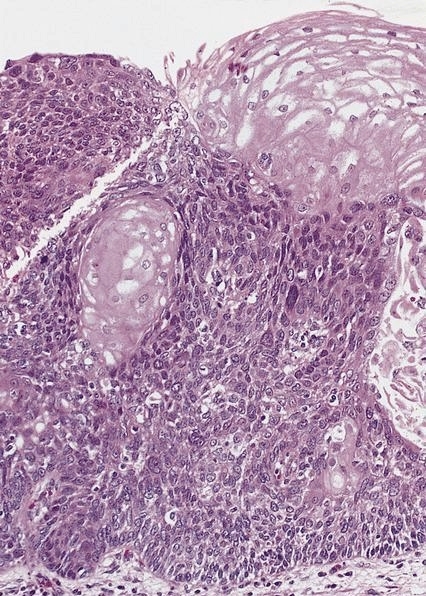
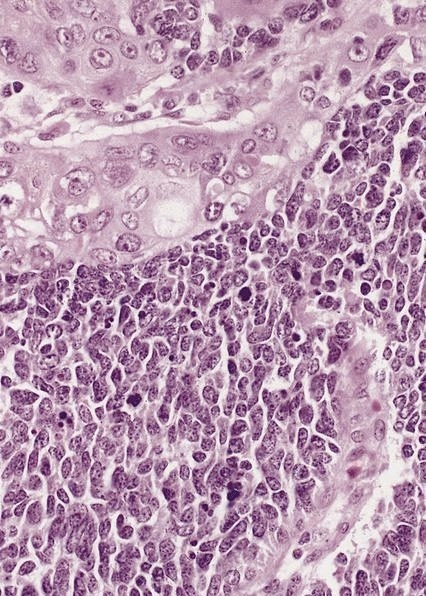
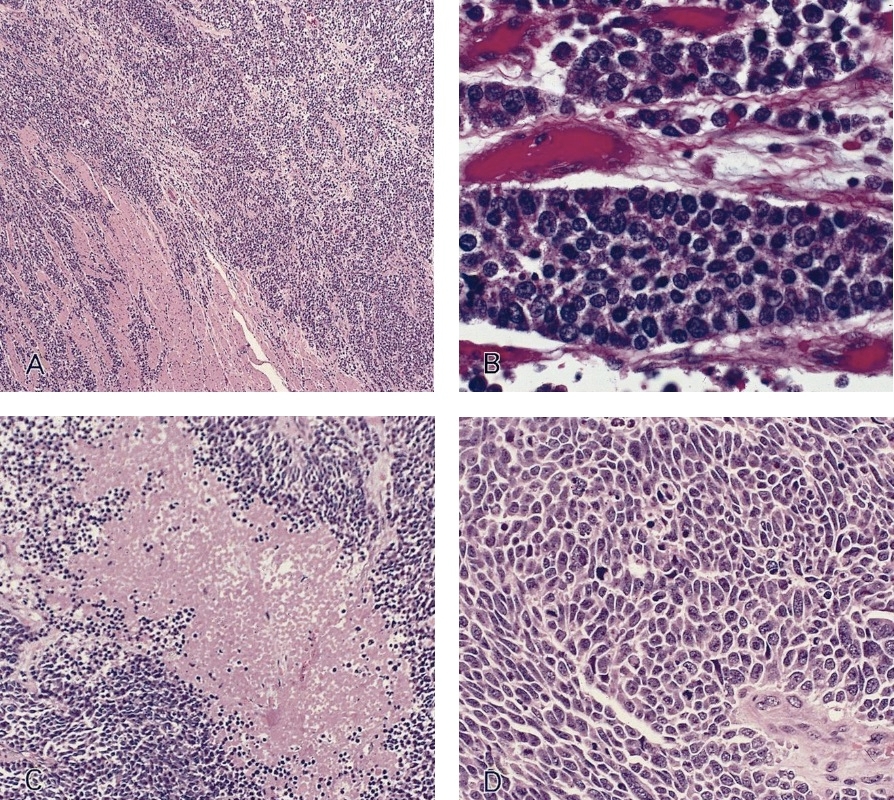
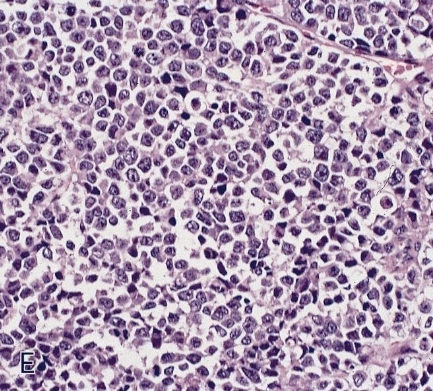
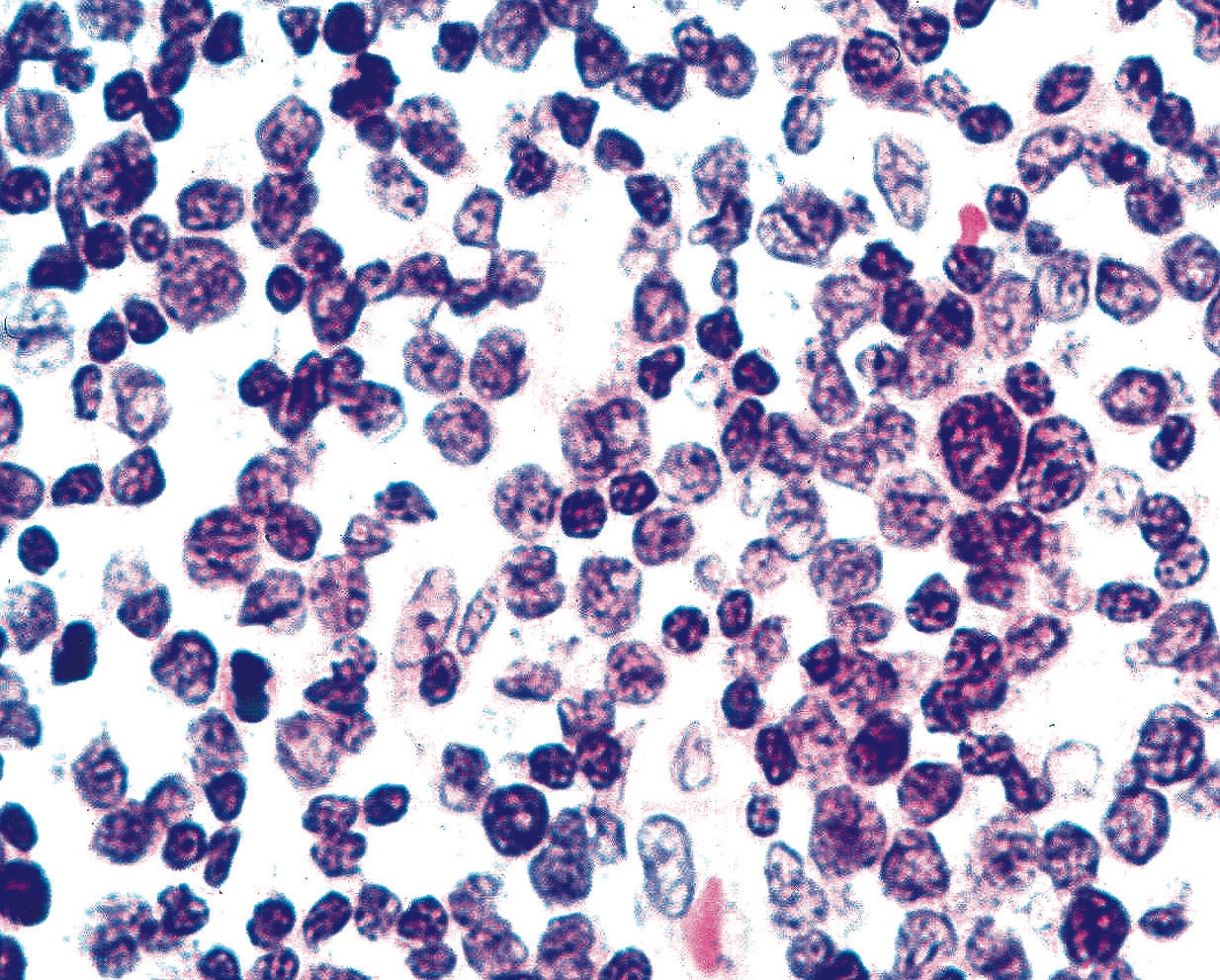
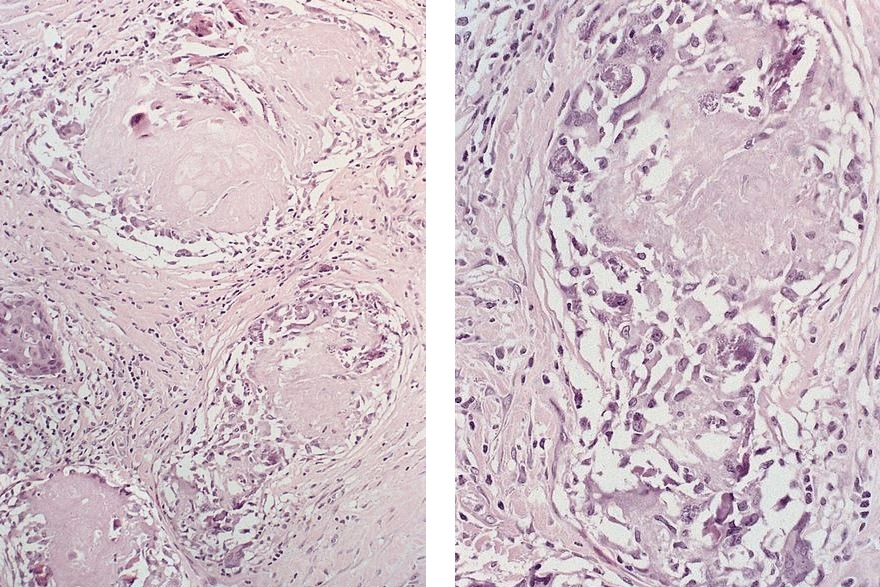
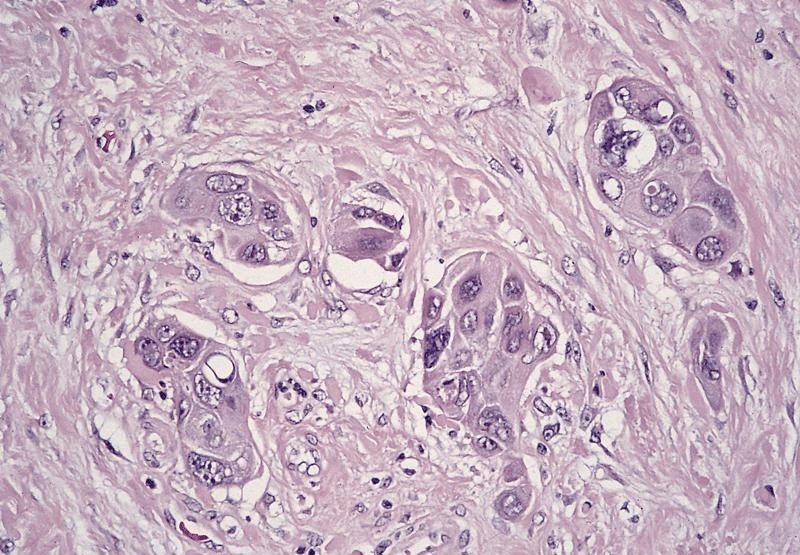
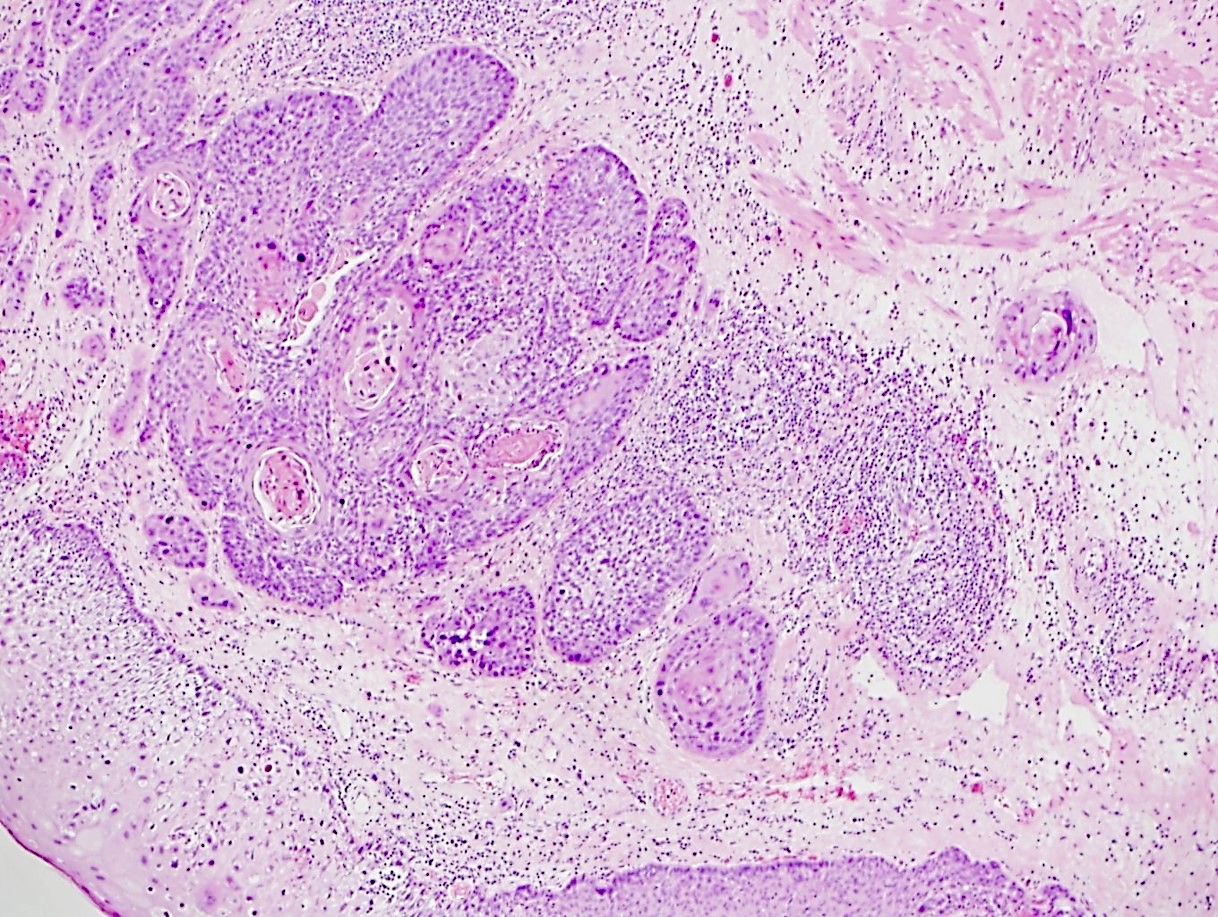
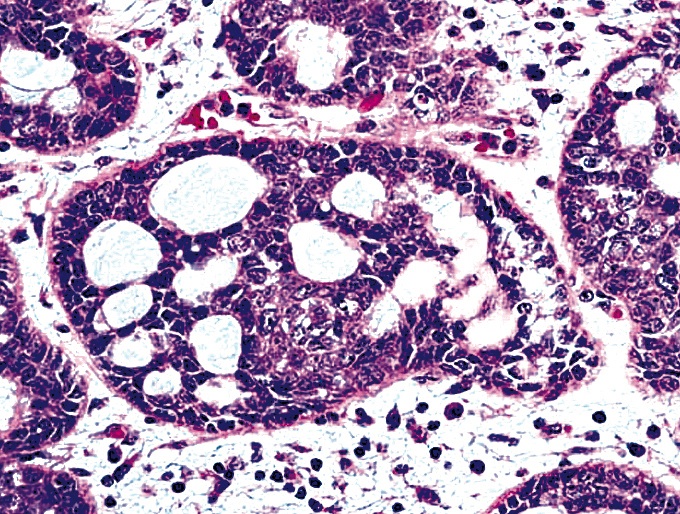
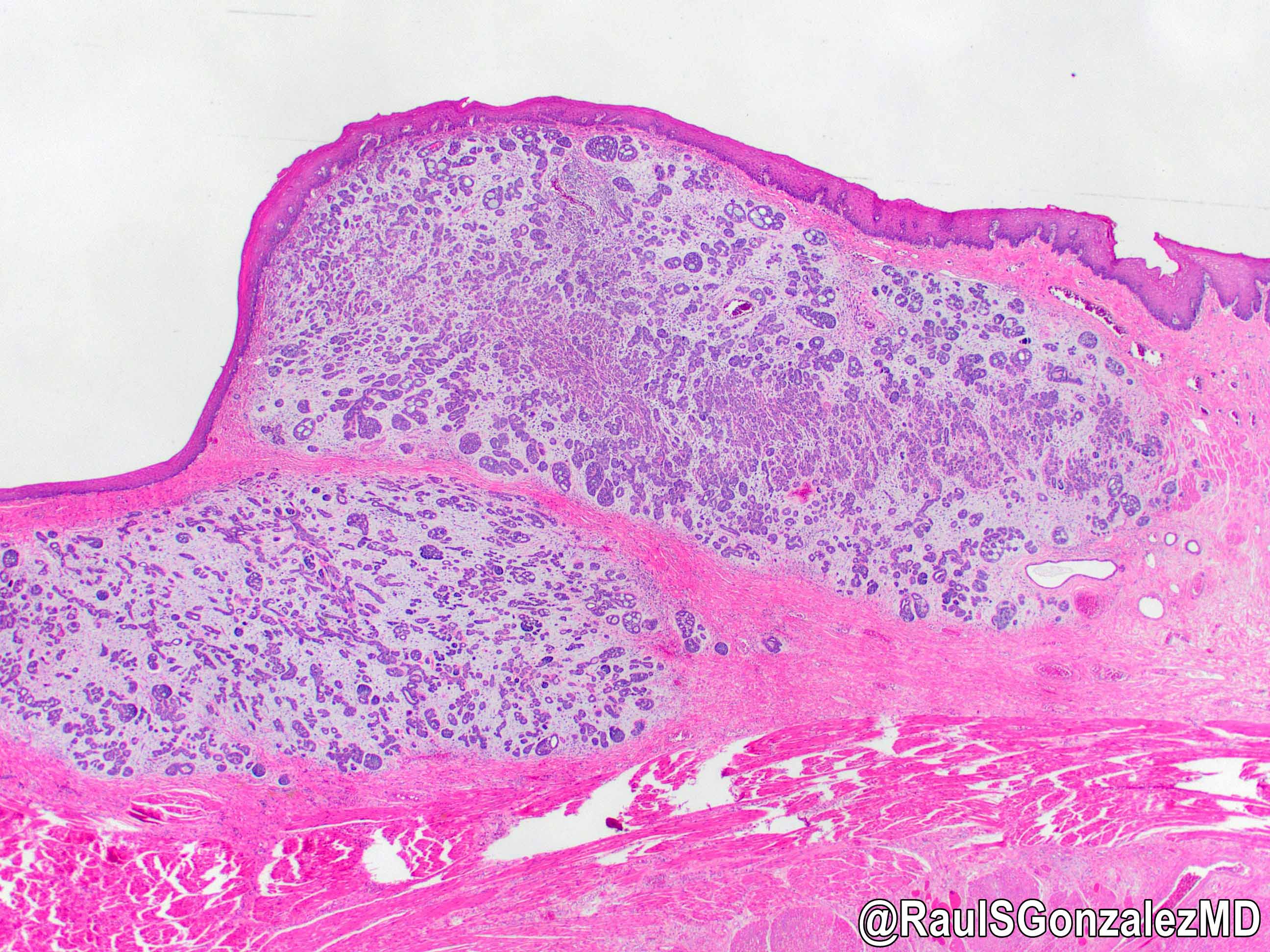
Microscopic (histologic) description
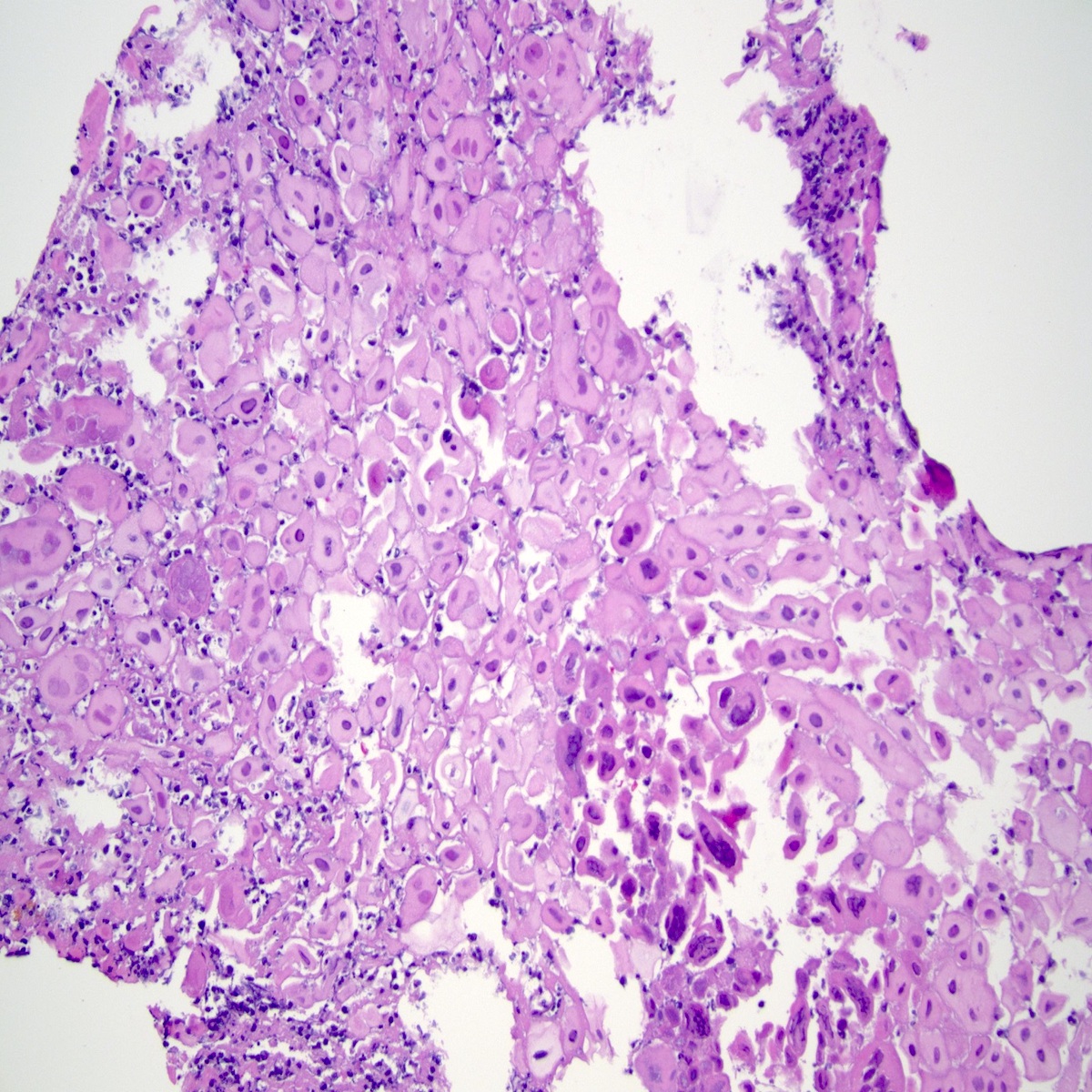
- Identical to tumor in salivary gland
- Inner ductal type epithelium and outer modified myoepithlial cells form solid nests or cribriform spaces containing balls of glyocosaminoglycans and basement membrane material
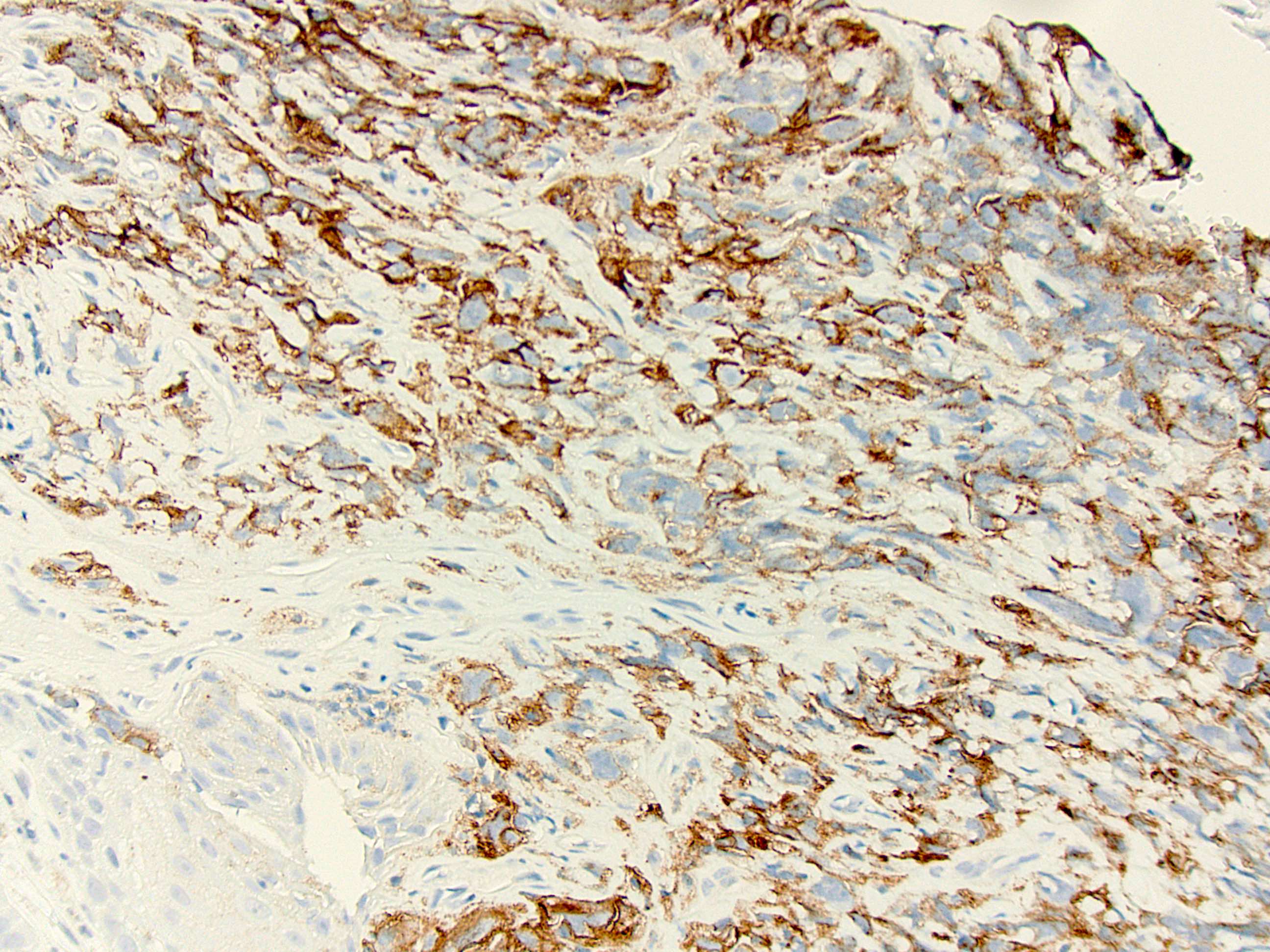
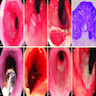
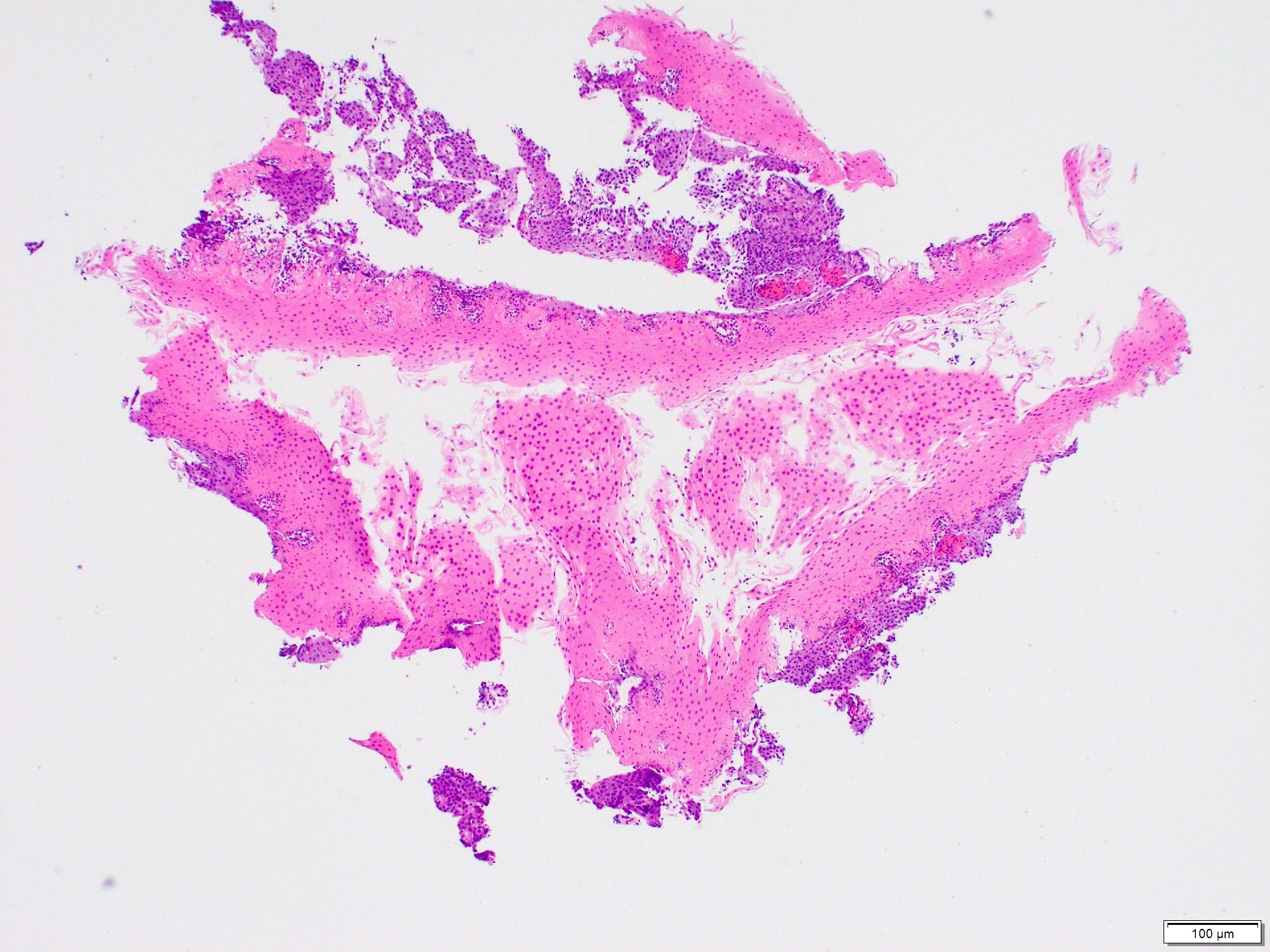
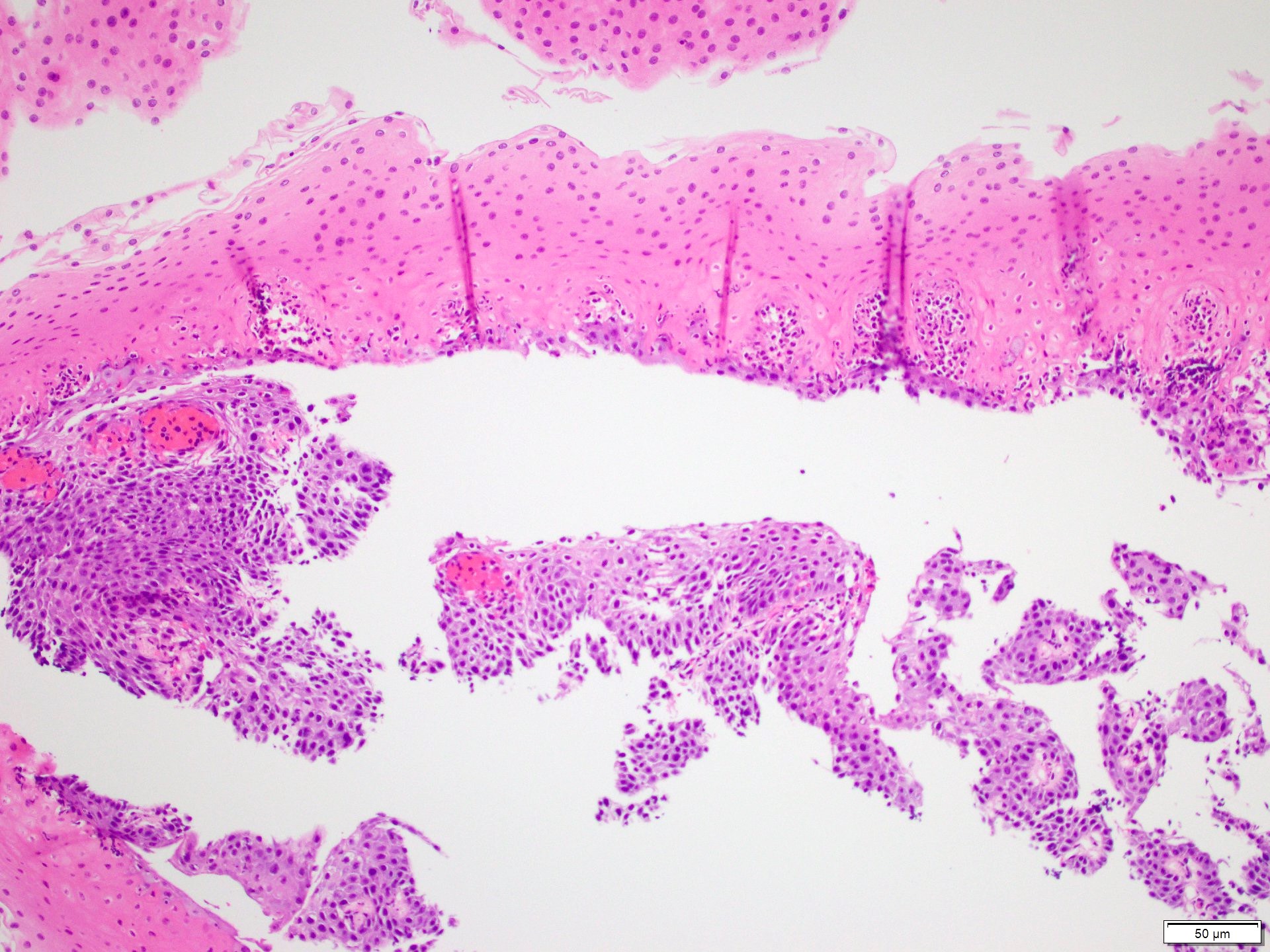
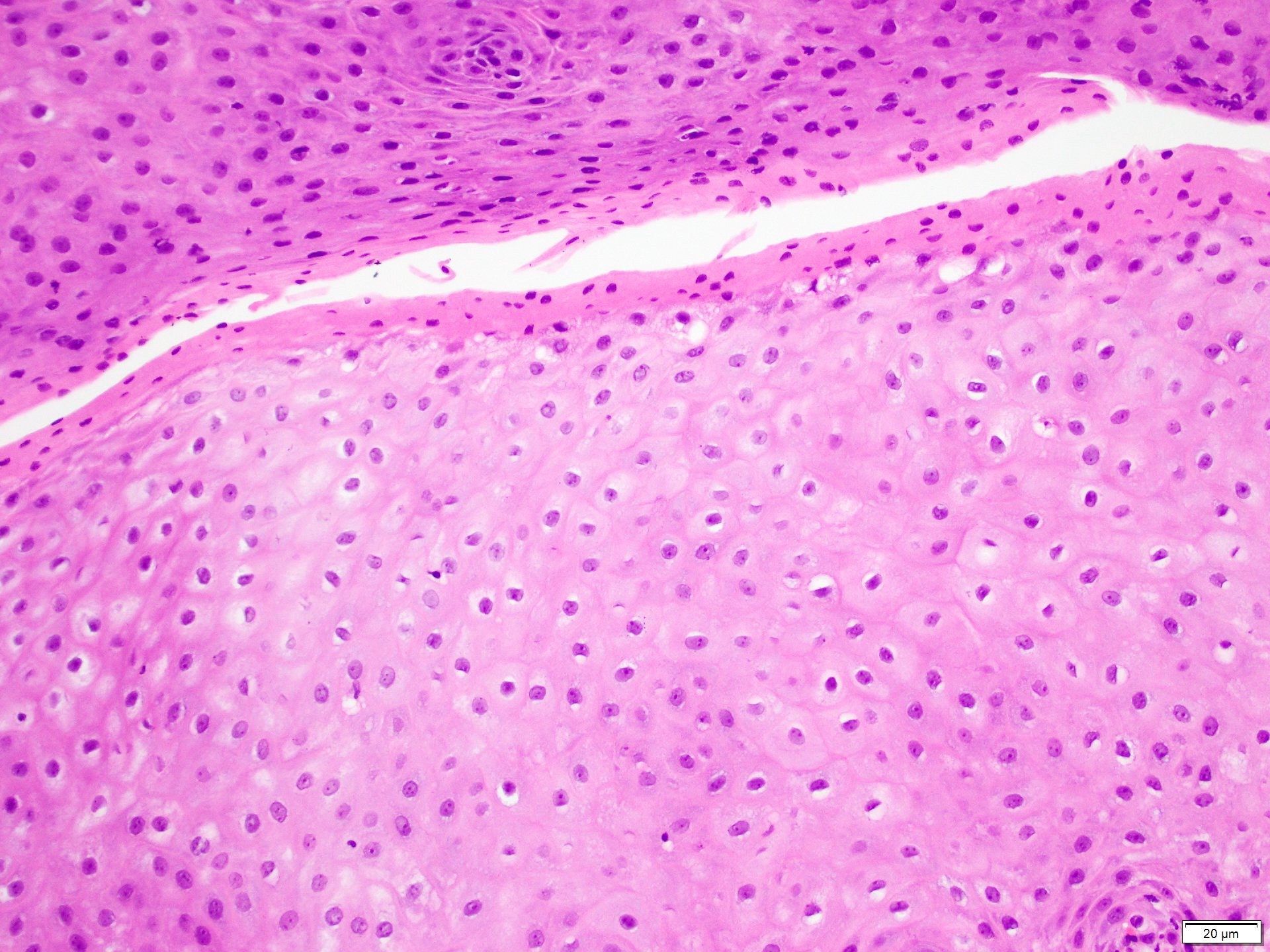
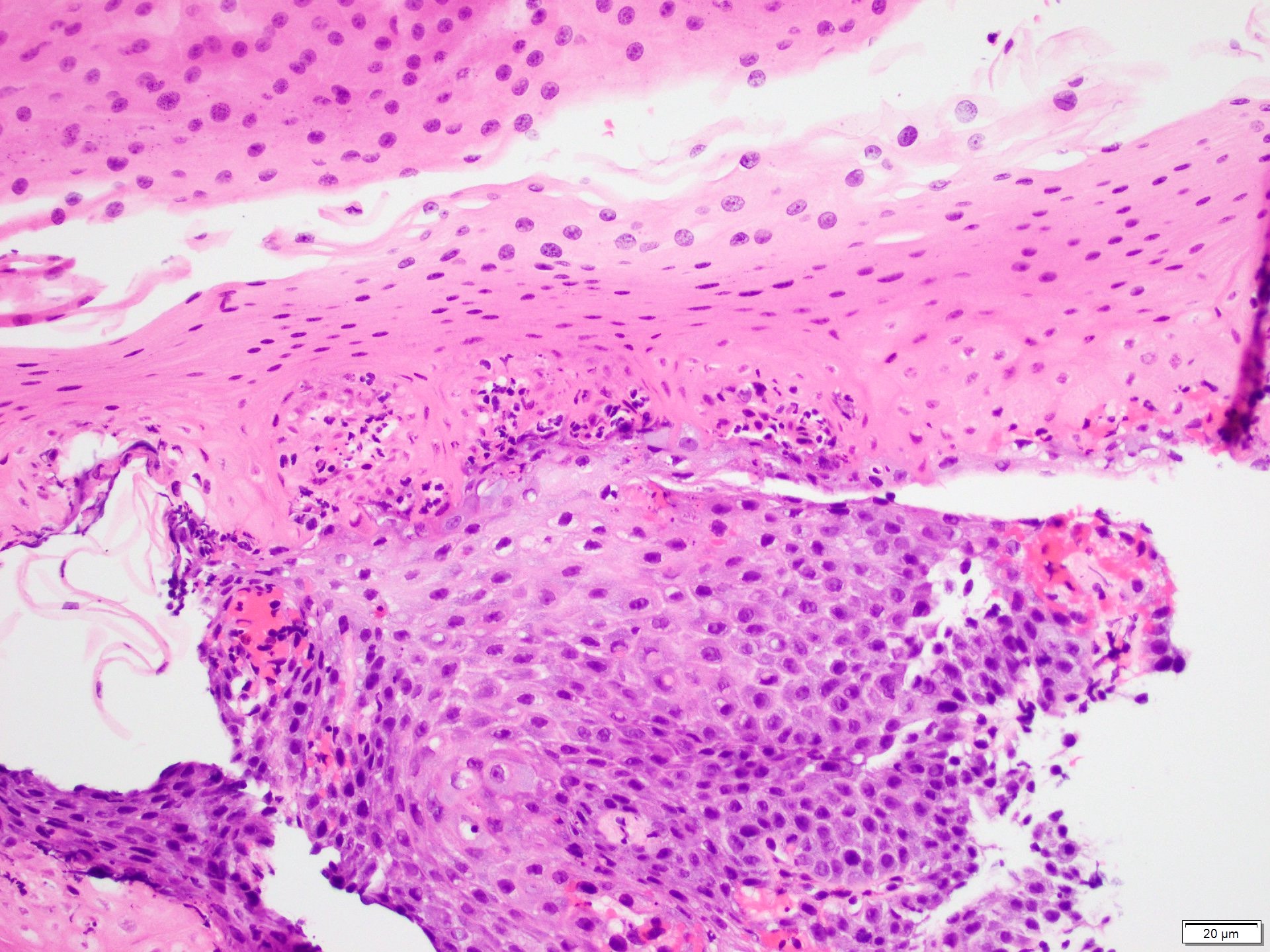
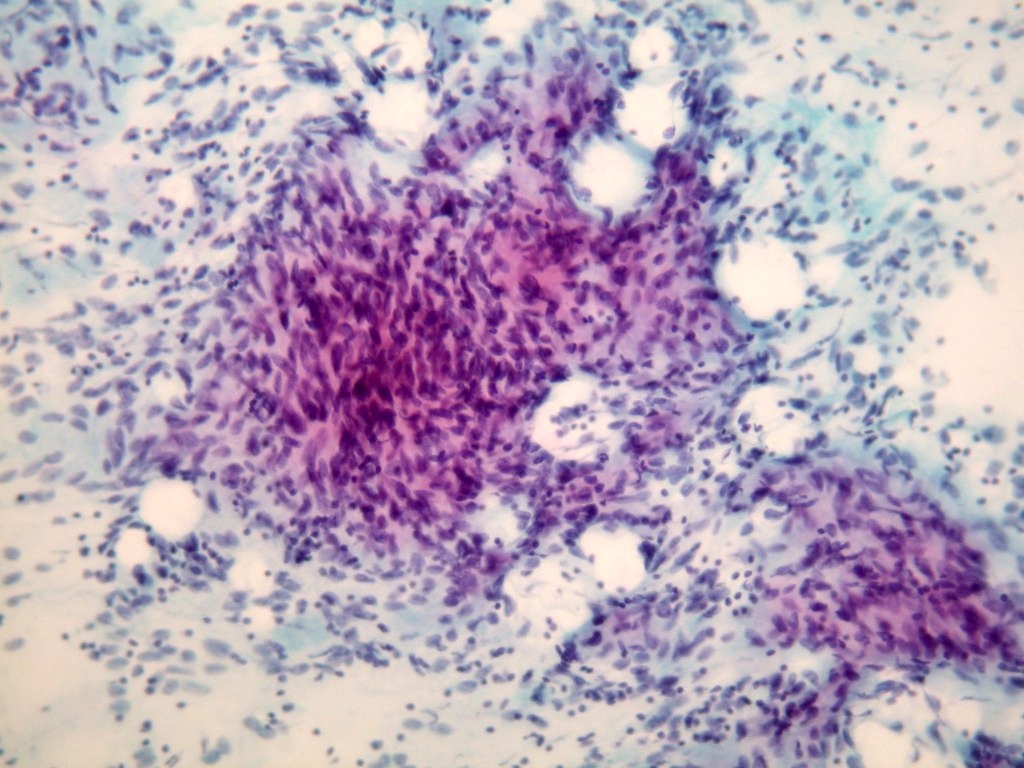
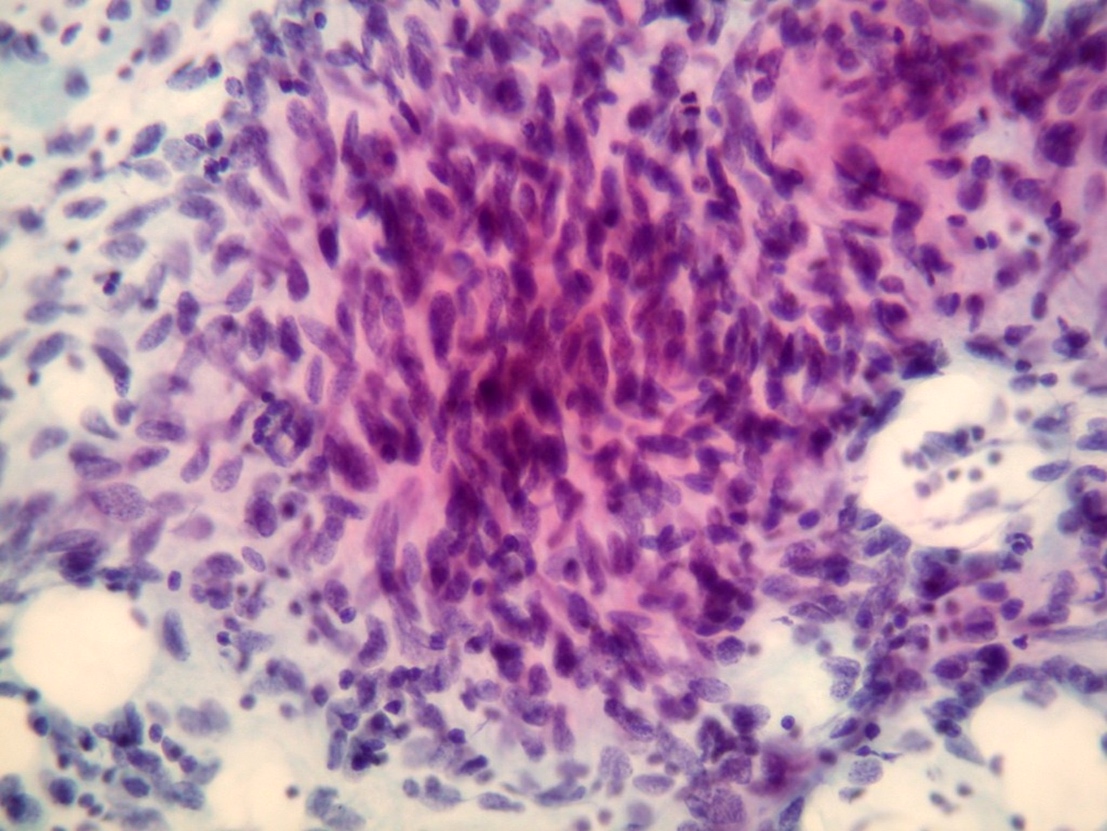
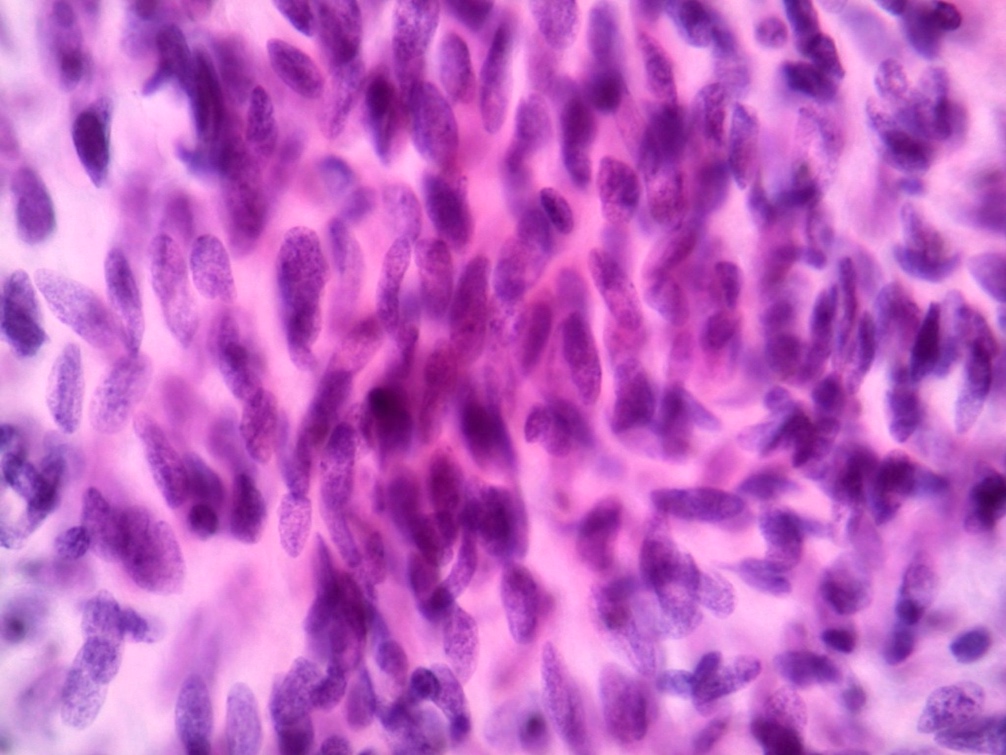
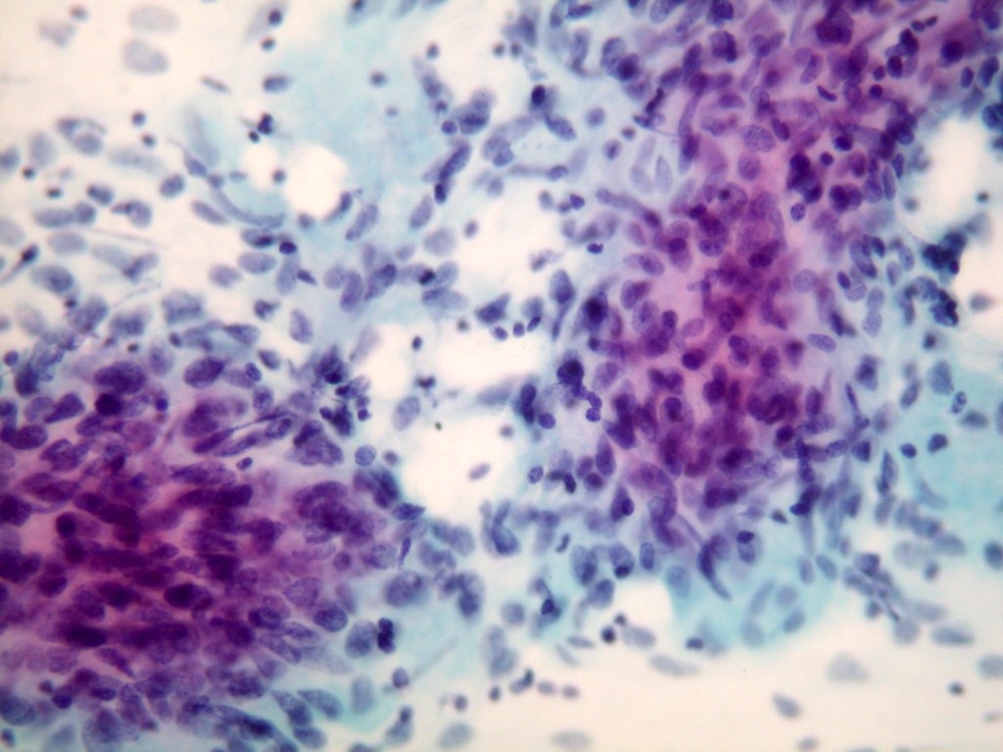
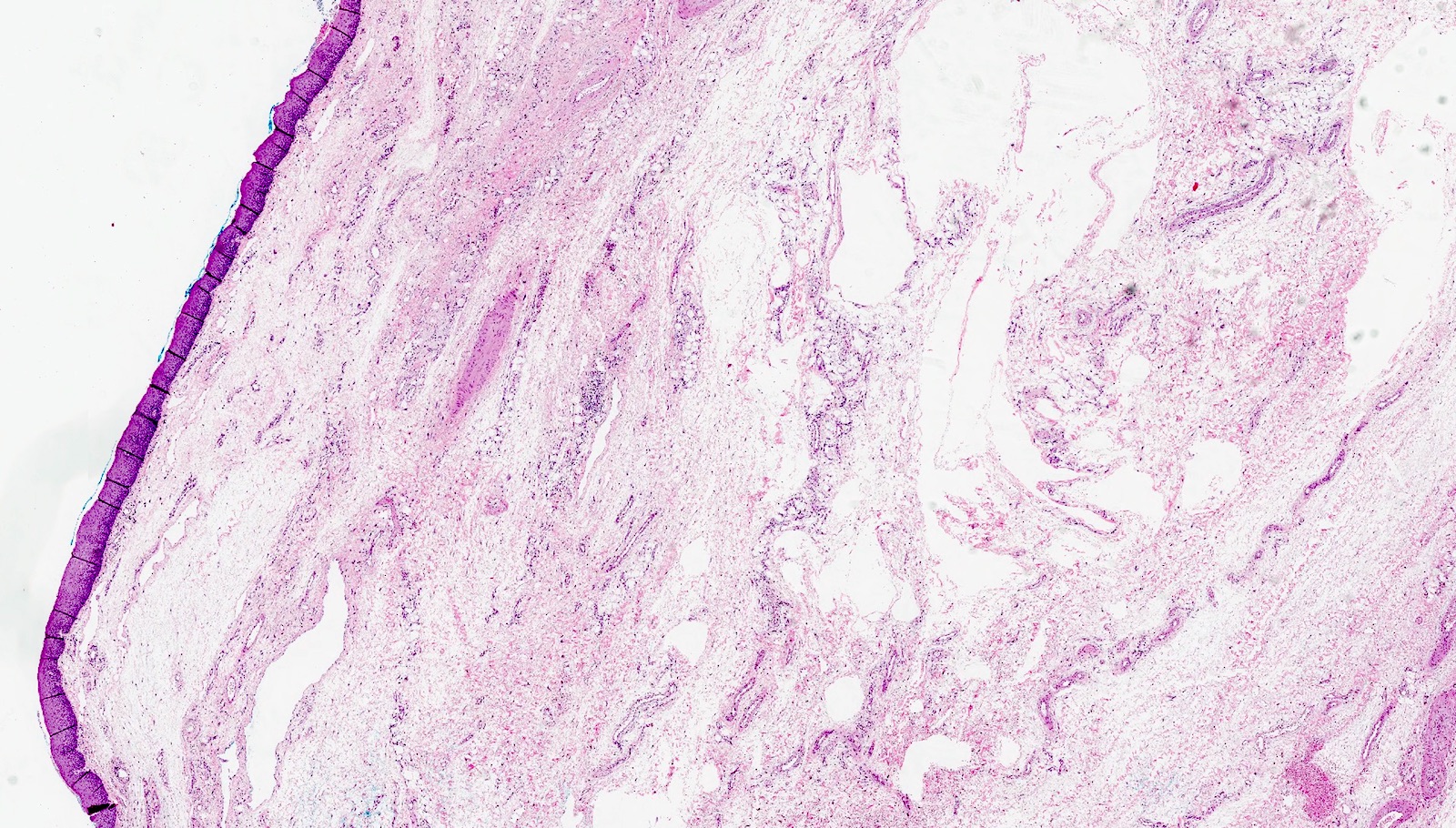
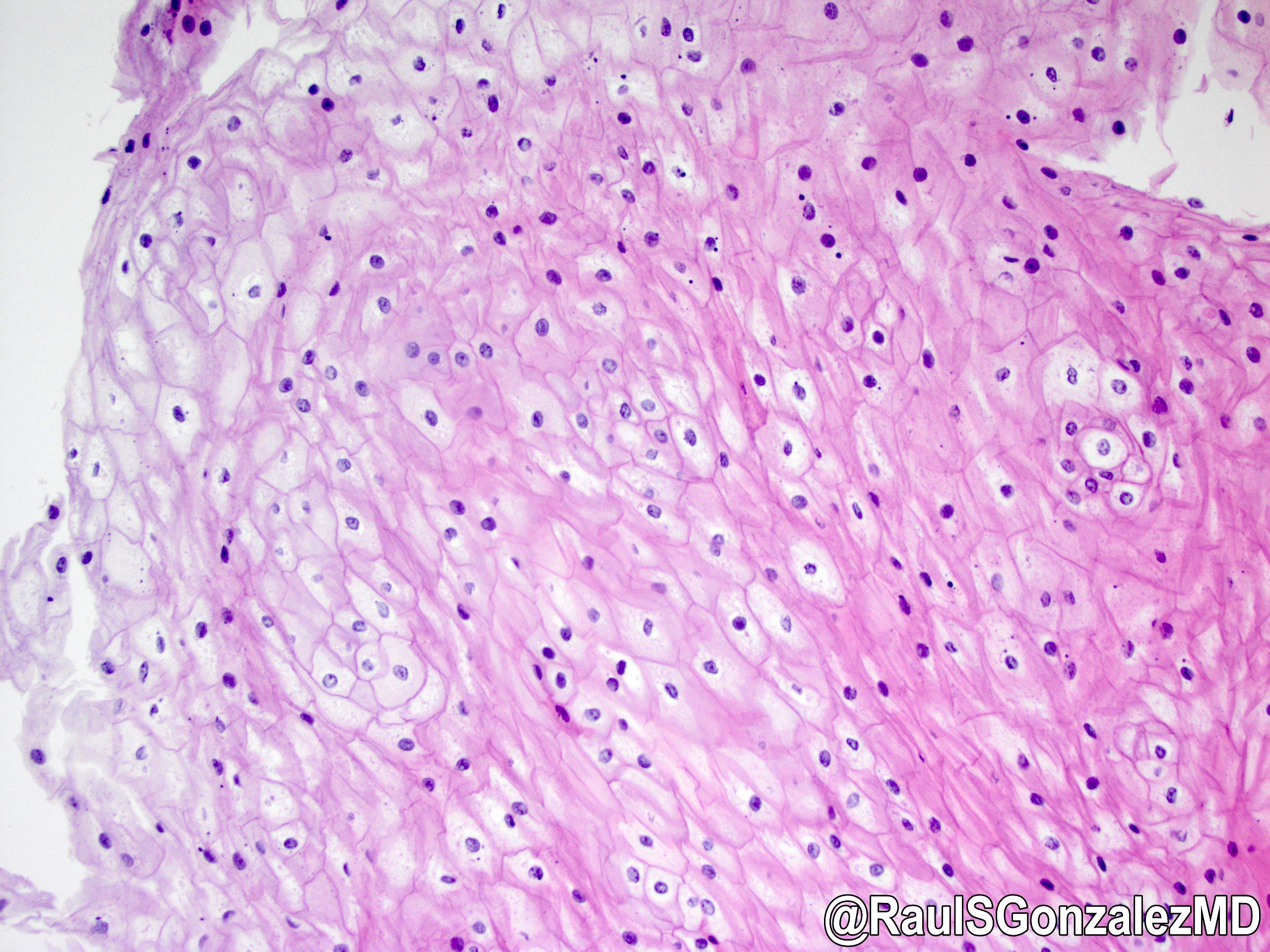
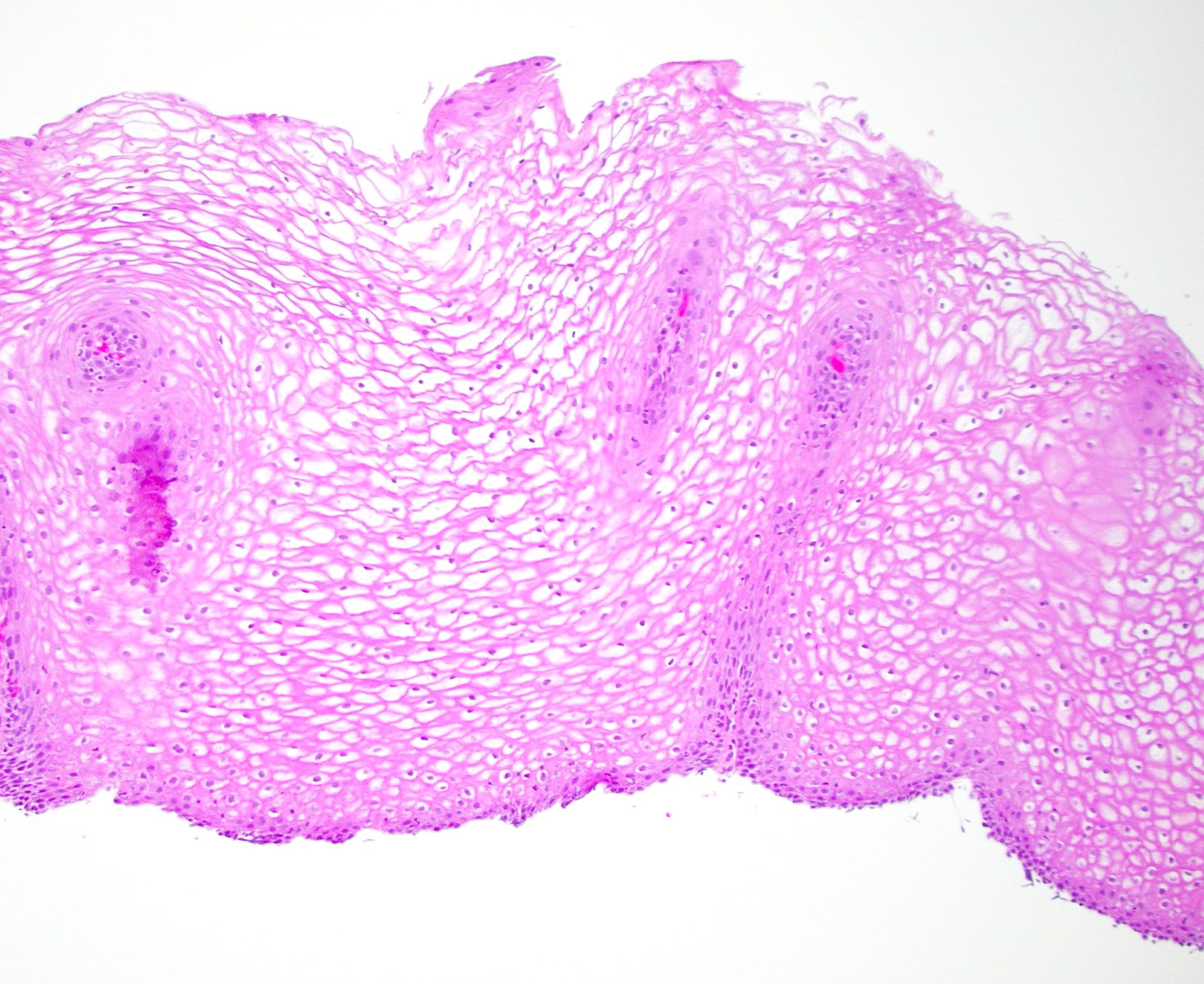
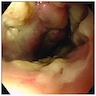
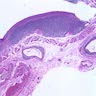
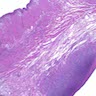
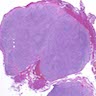
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick and @RaulSGonzalezMD on Twitter
 https://bit.ly/3zqGbca #pathology #gipath #PathTwitter #PathOutPic"
https://bit.ly/3zqGbca #pathology #gipath #PathTwitter #PathOutPic"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
Adenoid cystic carcinoma
https://bit.ly/3zqGbca #pathology #gipath #PathTwitter #PathOutPic"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">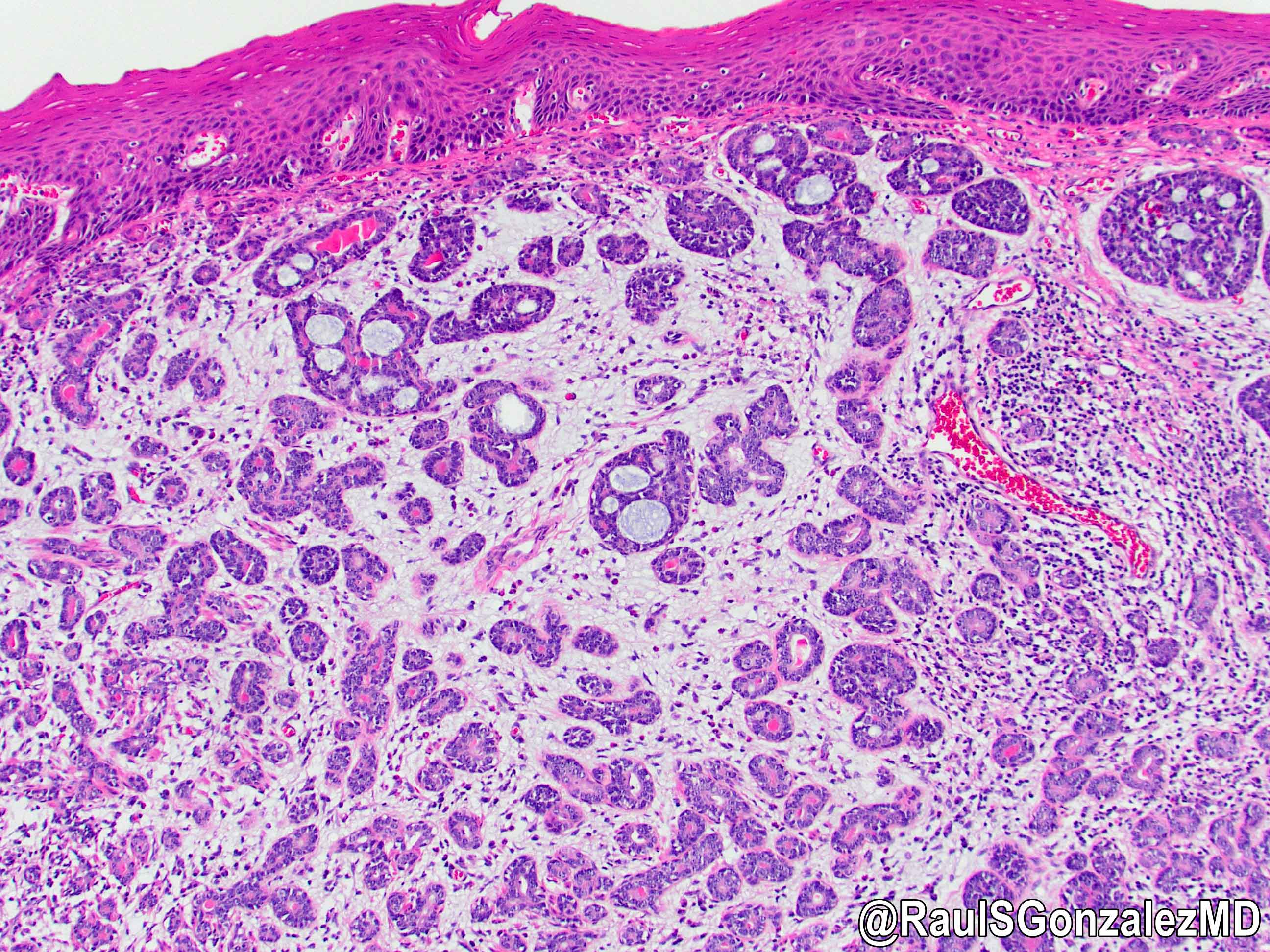 https://bit.ly/3zqGbca #pathology #gipath #PathTwitter #PathOutPic"
https://bit.ly/3zqGbca #pathology #gipath #PathTwitter #PathOutPic"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">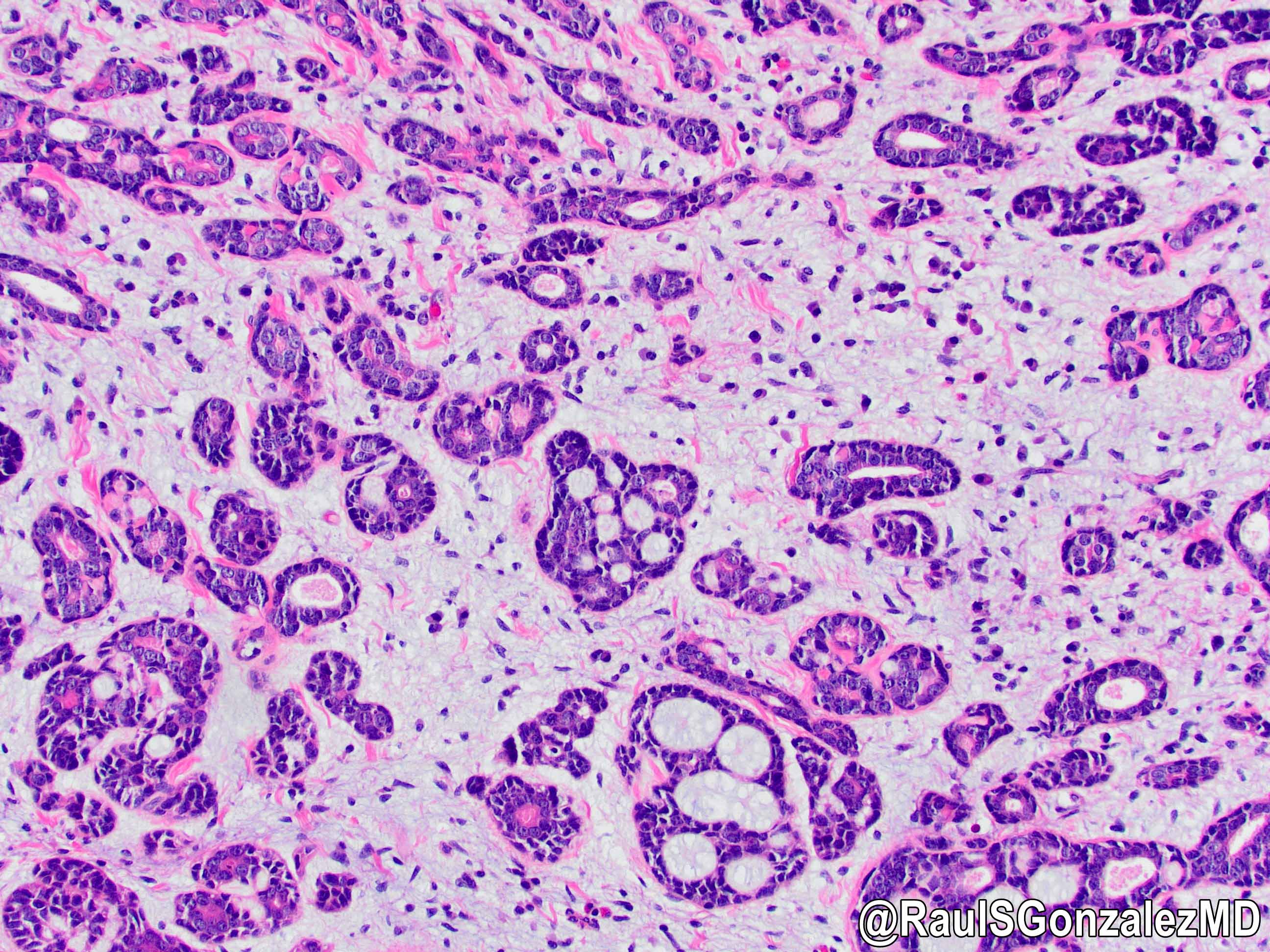
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
 https://bit.ly/3zqGbca #pathology #gipath #PathTwitter #PathOutPic"
https://bit.ly/3zqGbca #pathology #gipath #PathTwitter #PathOutPic"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">

Adenoid cystic carcinoma
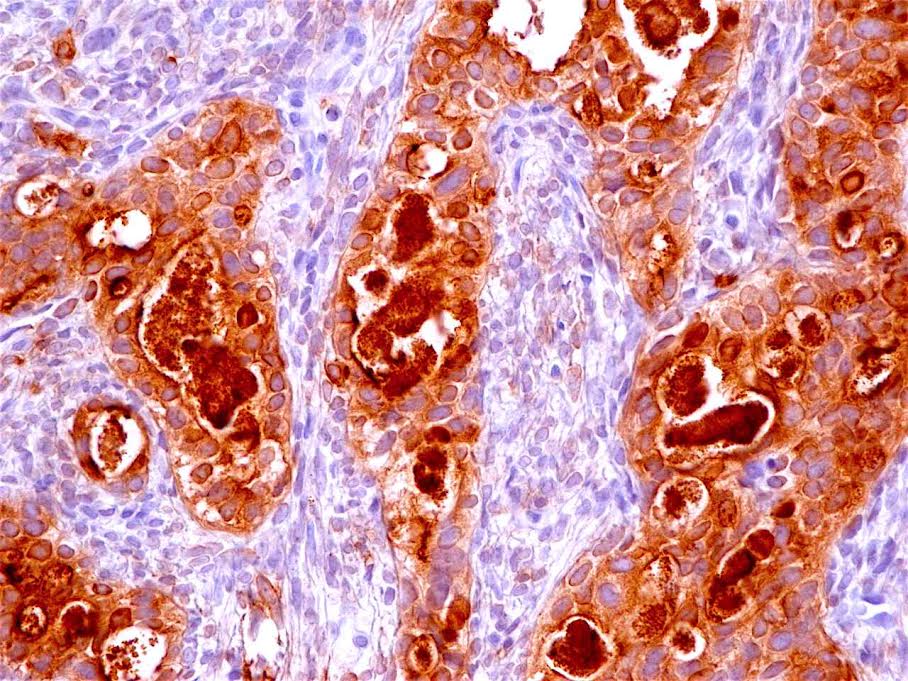
Positive stains
- Ductal epithelium is strongly cytokeratin and CEA+, while modified myoepithelial cells are weakly cytokeratin positive with strong S100, actin and vimentin positivity in modified myoepithelial cells
Differential diagnosis
- Basaloid squamous cell carcinoma
- More pleomorphic with greater mitotic activity
- Does not form true lumina and lacks CEA staining
- Generally CK19+, not seen in adenoid cystic carcinoma
Adenosquamous carcinoma
Table of Contents
Definition / general | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- Carcinoma that contains mixed elements of adenocarcinoma and squamous cell carcinoma, which remain clearly distinguishable within the tumor (WHO)
- Most authorities reserve term for tumors with substantial elements of each component as minor divergent differentiation is not uncommon
Etiology
- Believed to arise from totipotent basal stem cells that undergo heterologous differentiation (Gastroenterology 2002;122:784)
Clinical features
- Aggressive, behavior likely similar to squamous cell carcinoma (Oncology 2004;66:218)
- May present with smaller tumor size and lower stage than other carcinomas
- May be associated with Barrett esophagus (see case reports)
Diagnosis
- Endoscopic biopsy
Case reports
- 65 year old man with stage IV disease and survival of 6 years (Gan To Kagaku Ryoho 2006;33:231)
- 69 year old man with poorly differentiated tumor producing alpha fetoprotein (Anticancer Res 2003;23:3837)
- 72 year old man with Barrett esophagus (Jpn J Thorac Cardiovasc Surg 2002;50:537)
- With prominent spindle cells (Arch Pathol Lab Med 1993;117:544)
- At gastroesophageal junction (G Chir 2012;33:123)
Treatment
- Resection if possible
- Usually multimodality treatment
Microscopic (histologic) description
- Admixed squamous cell carcinoma and adenocarcinoma
Positive stains
- Rarely necessary
- CD44 (in squamous areas), Alcian blue pH 2.5 in glandular component
Differential diagnosis
- Collision tumor: rare
- Minor component of squamous differentiation in adenocarcinoma or adenocarcinoma in squamous cell carcinoma
- Mucoepidermoid carcinoma: intimate mixture of squamous carcinoma, well formed glands producing mucin and intermediate cells
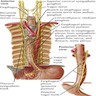
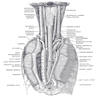
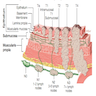
Anatomy & embryology
Table of Contents
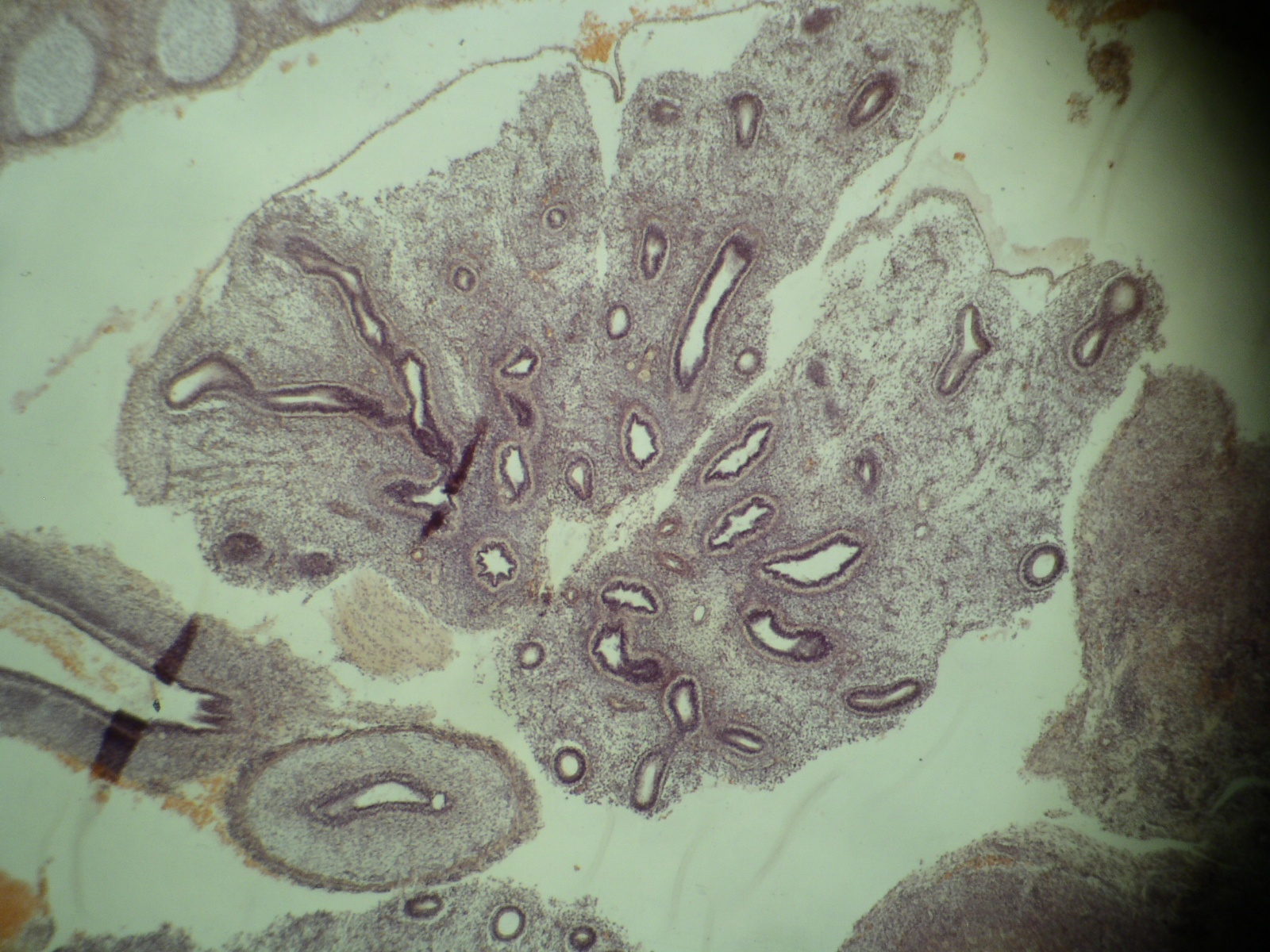
Definition / general | Embryology | Regions (AJCC) | Vessels and nerves | Diagrams / tables | Clinical images | Gross images | Microscopic (histologic) imagesDefinition / general
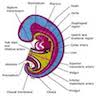
- Also called gullet
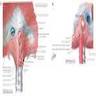
- Muscular tubular structure 25 cm long in adults, 10 - 11 cm in newborns; develops from cranial portion of the foregut; connects pharynx and stomach; has cervical, thoracic and abdominal segments
- Main purpose is to propel food from pharynx to stomach via peristalsis; secretes mucin for lubrication and to minimize reflux of gastric contents but has no other significant secretory or absorptive functions
- Extends from cricopharyngeus muscle in pharynx (level of C6) to lower esophageal sphinchter at gastroesophageal junction (T11 / T12)
Embryology
- Notocord induces formation of foregut from endoderm
- At day 21 (end of week 3), lateral walls of foregut develop septa that fuse and divide foregut into esophagus and trachea
- At week 4, myenteric plexus develops
- At weeks 5 - 6, septation of walls ends; initial lining is stratified columnar epithelium, which proliferates and almost occludes the lumen
- At weeks 6 - 7, submucosal plexus develops, circular muscular layer develops
- At weeks 6 - 7, epithelial vacuolization appears, vacuoles coalesce to form a single esophageal lumen
- At week 8, ciliated cells appear and extend to almost entire columnar epithelium
- At week 9, longitudinal muscle layer develops; interstitial cells of Cajal appear
- At week 10, a single layer of columnar cells covers entire esophagus
- At month 4, submucosal glands appear due to downward growth of columnar cells, extend distally to cardiac mucosa
- At month 5, stratified squamous epithelium initially appears in midesophagus and replaces ciliated epithelium cephalad and caudally; proximal esophagus may retain ciliated epithelium at birth
- At month 5, upper esophagus has both striated and smooth muscle
Regions (AJCC)
- Cervical (lower border of cricoid cartilage to suprasternal notch / thoracic inlet, 5 cm long, begins 15 cm from incisors); contains striated muscle
- Upper thoracic (suprasternal notch to tracheal bifurcation, 5 cm long, begins 20 cm from incisors); has striated and smooth muscle
- Midthoracic (tracheal bifurcation to diaphragmatic hiatus, 5 cm long, begins 24 cm from incisors); has striated and smooth muscle
- Lower thoracic and abdominal (10 cm long, begins 30 cm from incisors); extends past diaphragm to its junction with stomach; has smooth muscle only
- Usual points of narrowing (possible sites of food / pill lodging): cricoid cartilage (due to cricopharyngeus muscle), aortic arch, anterior crossing of left main bronchus and left atrium, where it passes through diaphragm
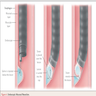
- Gastroesophageal junction: traditionally defined as macroscopic point of flaring of tubular esophagus or proximal limit of gastric rugal folds; endoscopic definition is Z ("zigzag") line at irregular boundary of squamous and columnar mucosa in distal esophagus, which is usually 2 - 3 cm proximal to macroscopic GE junction; histologic definition is proximal limit of gastric oxyntic (fundic) mucosa (Hum Pathol 2006;37:40)
- Distal 1 - 2 cm of esophagus is often composed of cardiac or cardiac - oxyntic type of mucosa; there is no consensus if this is normal or due to reflux esophagitis
- Esophageal sphincters: two areas of high pressure at rest (physiologic, not anatomic sphincters); upper esophageal sphincter is at cricopharyngeus and inferior pharyngeal constrictor muscles; lower esophageal sphincter is 2 - 4 cm proximal to esophagogastric junction at level of diaphragm (composed of intrinsic esophageal muscles, sling fibers of proximal stomach and crural diaphragm)
- Vagotomy does NOT affect tone of lower esophageal sphincter; tone is affected by gastrin, acetylcholine and serotonin
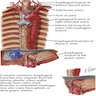
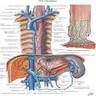
Vessels and nerves
- Arterial blood supply: cervical region - inferior thyroid artery; upper thoracic - bronchial and intercostal arteries; lower thoracic - aortic branches; abdominal - left gastric and inferior phrenic arteries; infarction is rare due to numerous anastomoses
- Venous drainage: extensive submucosal venous plexus communicates with periesophageal veins; flows into inferior thyroid (upper 1/3), azygous (middle 1/3) and gastric veins (lower 1/3); azygous vein empties into superior vena cava and gastric veins into portal system; this connection between caval and portal venous systems explains esophageal varices due to portal hypertension
- Nerves: left and right vagus nerves run lateral to esophagus, form plexi along anterior and posterior surfaces, then reunite to form anterior and posterior vagal trunks to stomach; have parasympathetic and sympathetic innervation
- Lymphatic drainage: freely anastomosing networks in submucosa, muscularis propria and occasionally lamina propria; facilitate lengthwise tumor dissemination; upper third drains into paratracheal and internal jugular nodes, middle third to mediastinal nodes, lower third to nodes around aorta and celiac axis
- Adjacent structures: cervical esophagus lies in posterior mediastinum, posterior to trachea and thyroid gland; is bounded by left and right recurrent laryngeal nerves and carotid sheaths; distal esophagus is posterior to left atrium and bounded by azygous veins; passes through opening in diaphragm called the hiatus
- Incisura / angle of His: left side of esophagus forms sharp angle where it joins the stomach
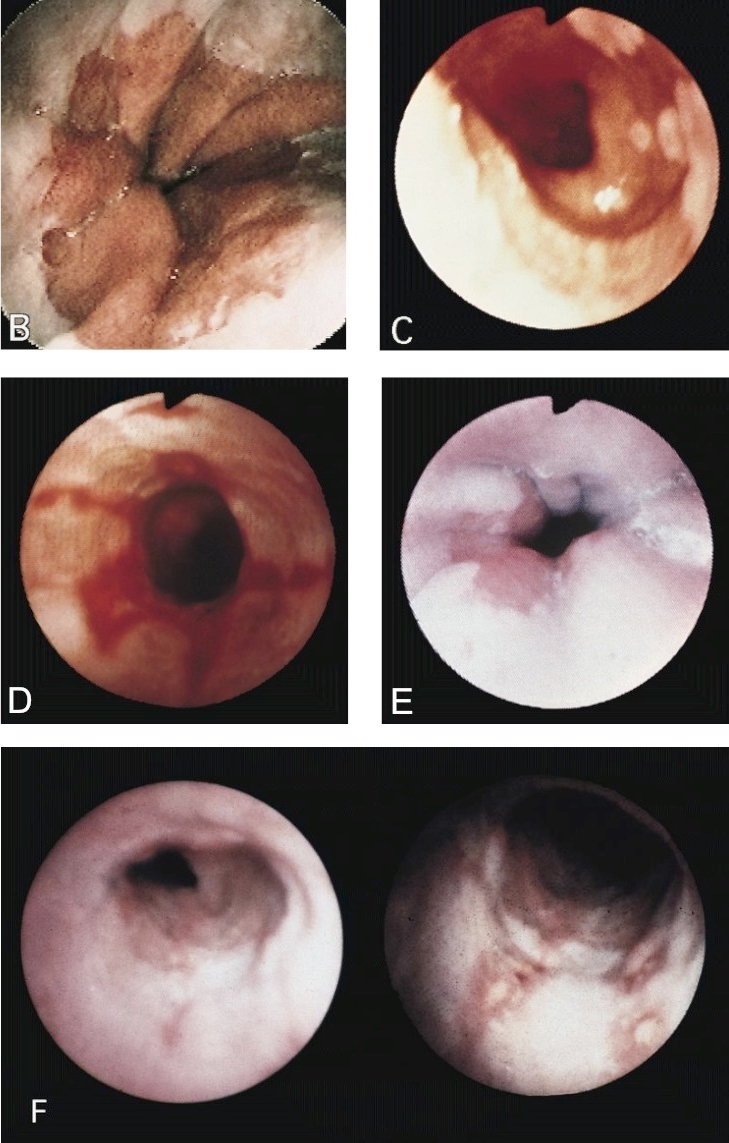
Diagrams / tables
Microscopic (histologic) images
Atresia and tracheoesophageal fistula (pending)
[Pending]
Barrett esophagus
Table of Contents
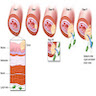
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- American College of Gastroenterology (ACG) 2016 BE definition: extension of salmon colored mucosa into the tubular esophagus extending ≥ 1 cm proximal to the gastroesophageal (GE) junction with biopsy confirmation of intestinal metaplasia (goblet cells) (Am J Gastroenterol 2016;111:30)
- British Society of Gastroenterology 2014 BE definition: columnar epithelium with or without goblet cells extending ≥ 1 cm proximal to the GE junction (Gut 2014;63:7)
Essential features
- Secondary to longstanding gastroesophageal reflux disease (GERD)
- BE requires both endoscopic (abnormal mucosa ≥ 1 cm proximal to the GE junction) and histologic (metaplastic epithelium) correlation for diagnosis (Am J Gastroenterol 2016;111:30, Am J Surg Pathol 2017;41:e8)
- Necessity of intestinal metaplasia (IM) for diagnosis of BE varies; IM required in United States and part of Europe, IM not necessary in UK and Japan (Am J Gastroenterol 2016;111:30, Gut 2014;63:7)
- BE predisposes patient to dysplasia and adenocarcinoma (Am J Surg Pathol 2017;41:e8, Am J Surg Pathol 2016;40:e45)
- Histologic assessment on H&E slide remains gold standard marker for diagnosing dysplasia and cancer prevention in BE (Am J Surg Pathol 2016;40:e83)
Terminology
- Long segment: any segment of BE measuring > 3 cm
- Short segment: any segment of BE measuring < 3 cm
- Z line: squamocolumnar junction
ICD coding
Epidemiology
- BE detected in about 10 - 15% of patients with GERD (Am J Gastroenterol 2016;111:30, Gastroenterol Hepatol (N Y) 2016;12:449)
- Symptom duration of GERD is a risk factor for BE
- Screening performed in men with chronic GERD symptoms with at least two additional risk factors (age > 50 years, white race, central obesity, smoking history or a confirmed family history of BE) (Am J Gastroenterol 2016;111:30, Gastroenterol Hepatol (N Y) 2016;12:449)
- Screening no longer indicated in women with chronic symptoms of GERD
- Screening not recommended for general population (patients without reflux symptoms)
Sites
- Distal esophagus, gastroesophageal junction
Pathophysiology
- Metaplasia in BE presumably results from cellular reprogramming
- GERD induced tissue damage reprograms immature progenitor cells to express columnar development transcription factors
- Tissue injury activates signaling pathways such as Hedgehog, BMP4 and NF-KB, and downregulates Notch signaling
- Signals lead to increased expression of SOX9 (induces columnar differentiation), FOXA2, CDX1 and CDX2 (induces intestinal differentiation) (J Clin Invest 2014;124:3767)
- Transdifferentiation (distinctive type of multilayered epithelium at the squamocolumnar junction with features of both squamous and columnar epithelium) may occur in BE (Am J Gastroenterol 2016;111:30)
Etiology
- Known risk factors (Am J Gastroenterol 2016;111:30):
- Chronic (> 5 years) GERD symptoms
- Advancing age (> 50 years)
- Male gender
- Tobacco usage
- Central obesity
- Caucasian race
- More common in first degree relatives of subjects with known BE
Diagrams / tables
Clinical features
- GERD symptoms
Diagnosis
- Characteristic endoscopic appearance (at least 1 cm segment of abnormal mucosa proximal to GE junction) plus characteristic histologic findings (metaplastic epithelium with intestinal metaplasia in USA)
- At least eight random biopsies recommended
- When eight biopsies not obtainable (as in short segment BE), at least four biopsies / cm of circumferential BE and one biopsy / cm in tongues of BE recommended
- Normal Z lines and Z lines with less than 1 cm of variability should not be biopsied (Am J Gastroenterol 2016;111:30, Gastroenterol Hepatol (N Y) 2016;12:449)
Laboratory
- Evaluate H&E step sections from biopsies to document goblet cell metaplasia
Radiology description
- Advanced imaging technologies continue to be an area of research interest
- Routine use of high definition white light endoscopy now recommended as a part of surveillance (Am J Gastroenterol 2016;111:30)
- Other advanced imaging techniques (beyond electronic chromoendoscopy) not recommended at this time
Prognostic factors
- Risk of cancer progression for nondysplastic BE is 0.2 - 0.5% per year (Am J Gastroenterol 2016;111:30)
- Annual risk of progression with low grade dysplasia (0.7% per year) and high grade dysplasia (7% per year)
Treatment
- BE patients should receive once daily proton pump inhibitor therapy
- Aspirin, other NSAIDs or antireflux surgery not routinely prescribed or performed
- Surveillance should be performed with high definition / high resolution white light endoscopy
- For BE without dysplasia, endoscopic surveillance at intervals of three to five years
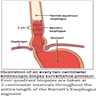
- Endoscopic surveillance should employ four quadrant biopsies at 2 cm intervals in patients without dysplasia and 1 cm intervals in patients with prior dysplasia
- For BE dysplasia, refer to BE dysplasia section for surveillance guidelines
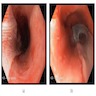
- Endoscopic mucosal resection or endoscopic ablation therapy: preferred treatment options for patients with dysplasia (refer to BE dysplasia section) (Am J Gastroenterol 2016;111:30)
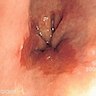
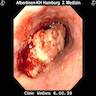
Clinical images
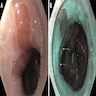
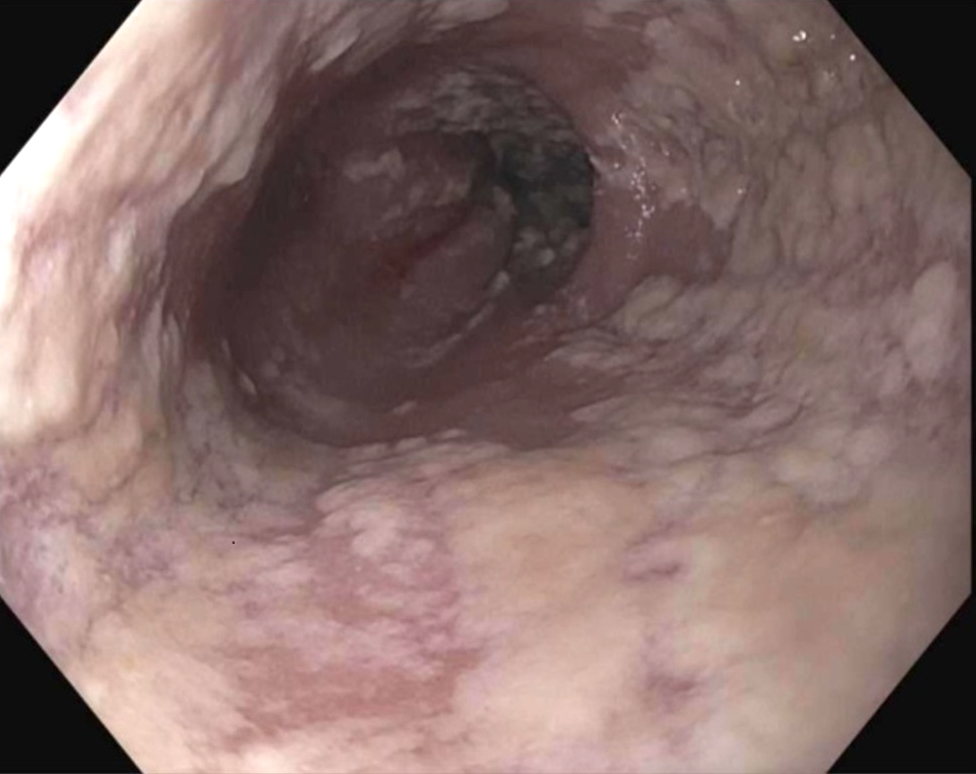
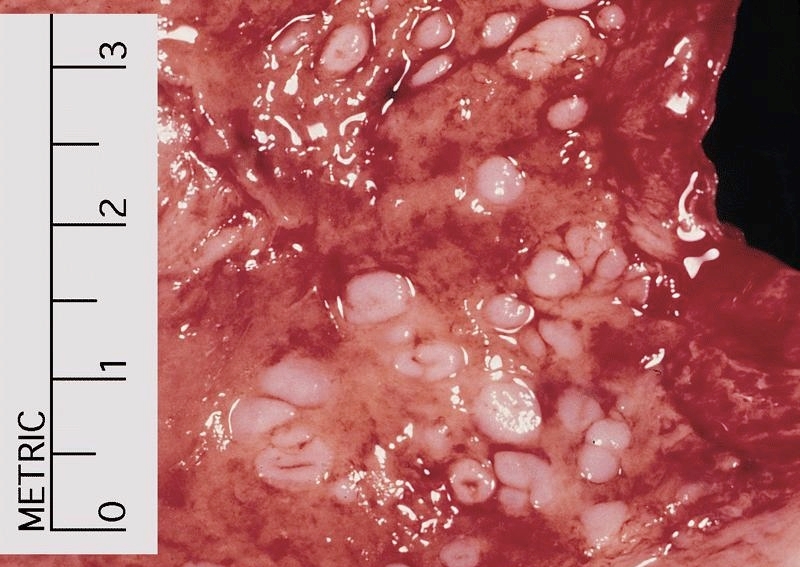
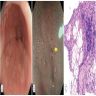
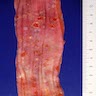
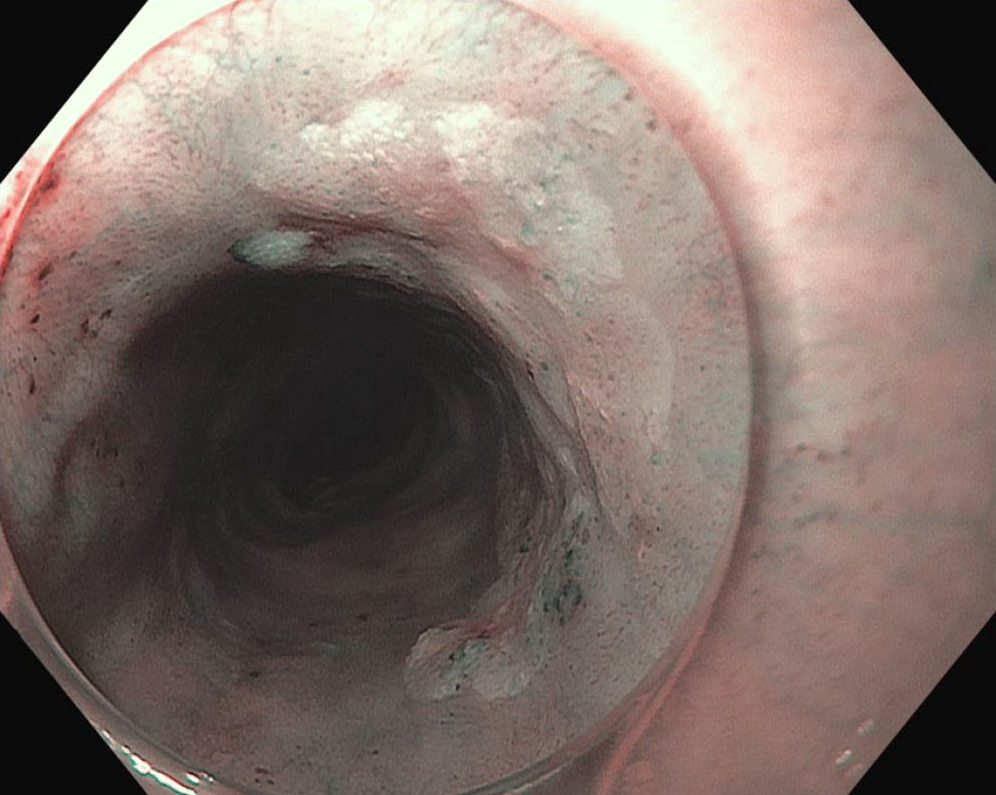
Gross description
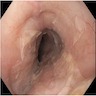
- Red / salmon colored mucosa between pale squamous mucosa of lower esophagus and lush pink gastric mucosa; may have tongues extending up from GE junction
- Endoscopists utilize the Prague classification to describe disease extent (include circumferential and maximal segment length) in Barrett mucosa (Am J Gastroenterol 2016;111:30)
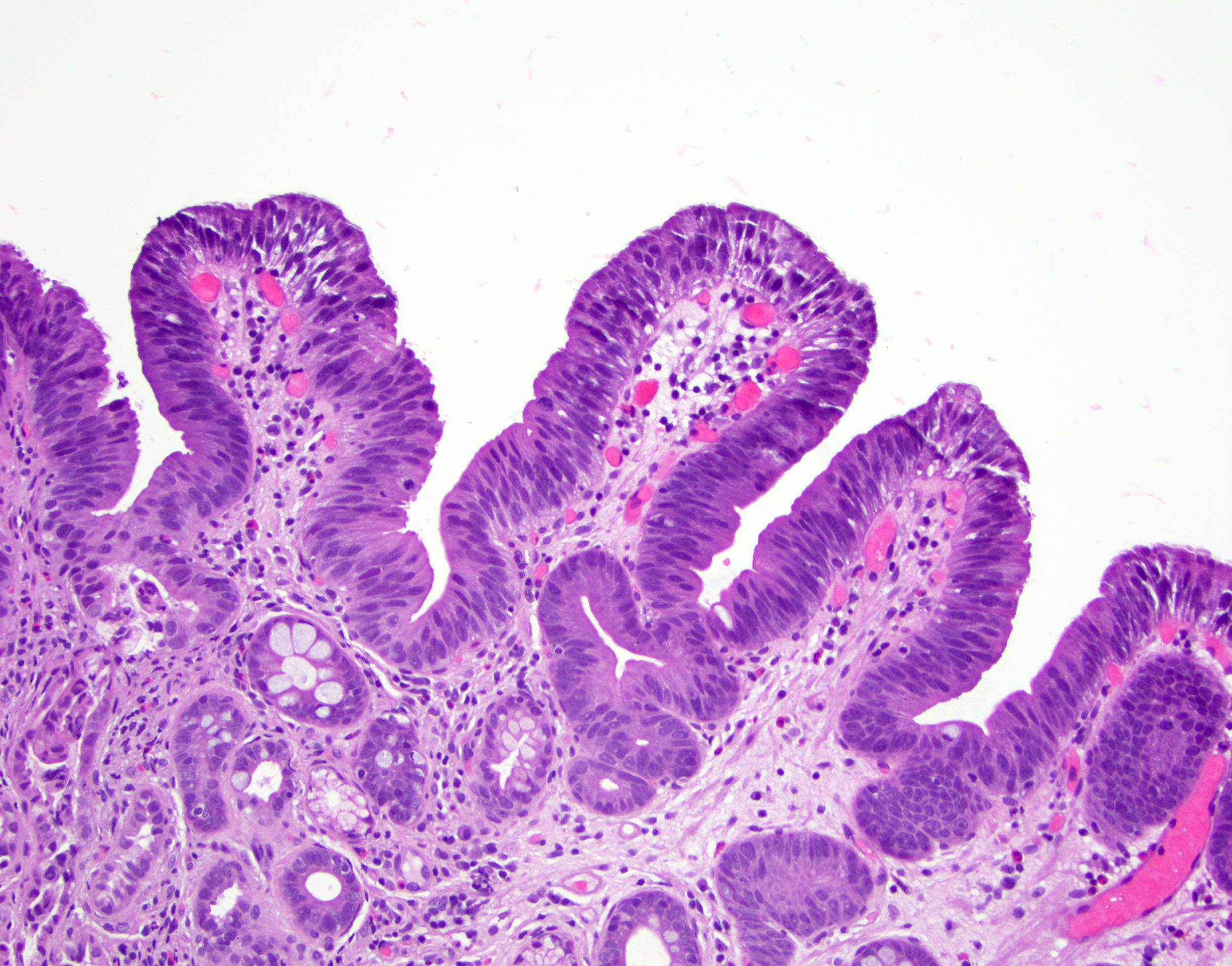
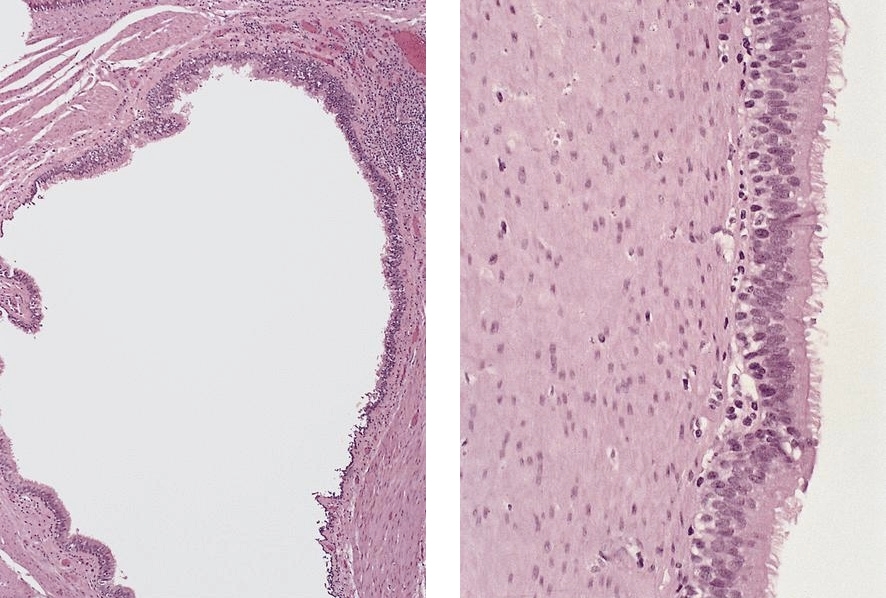
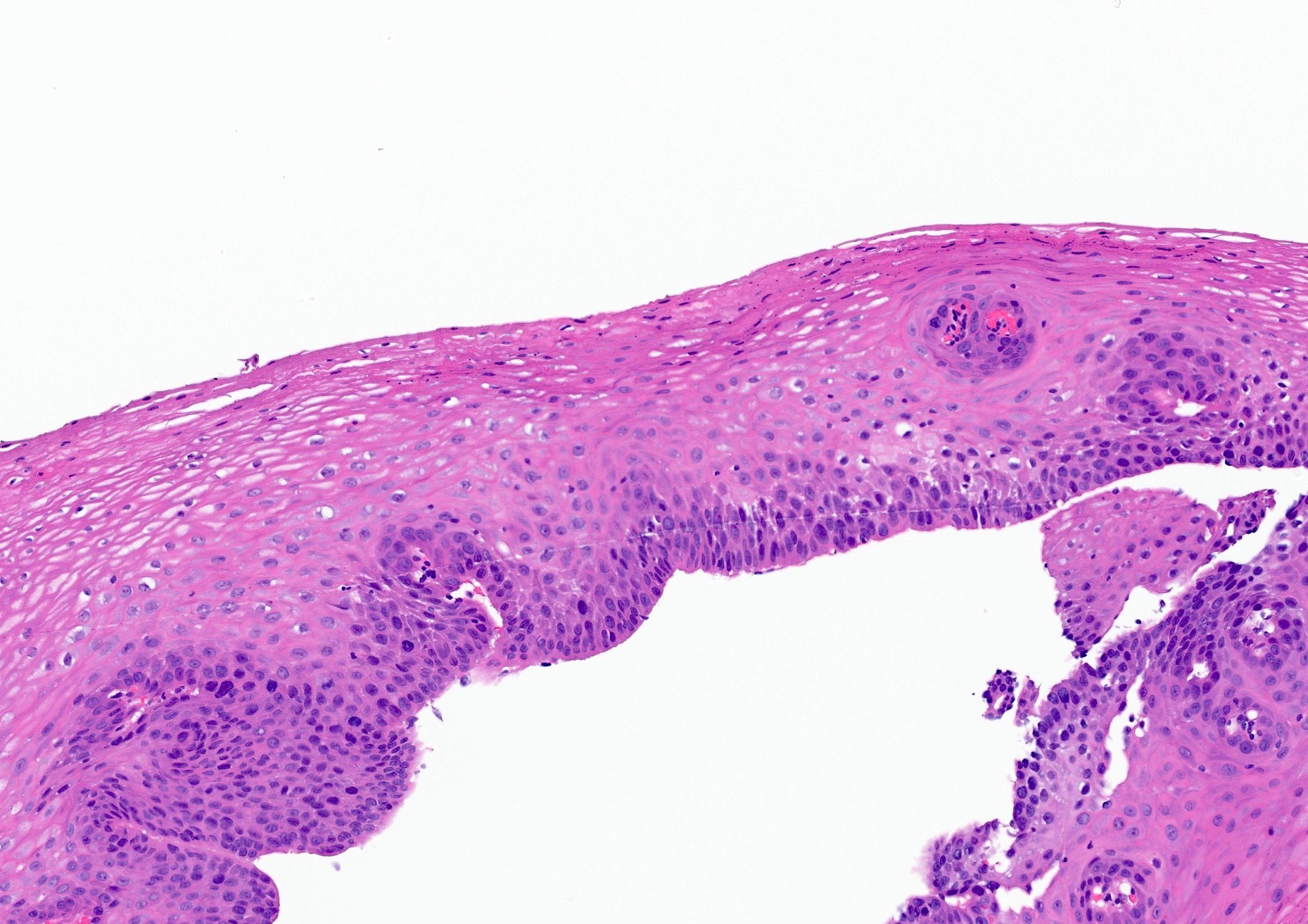
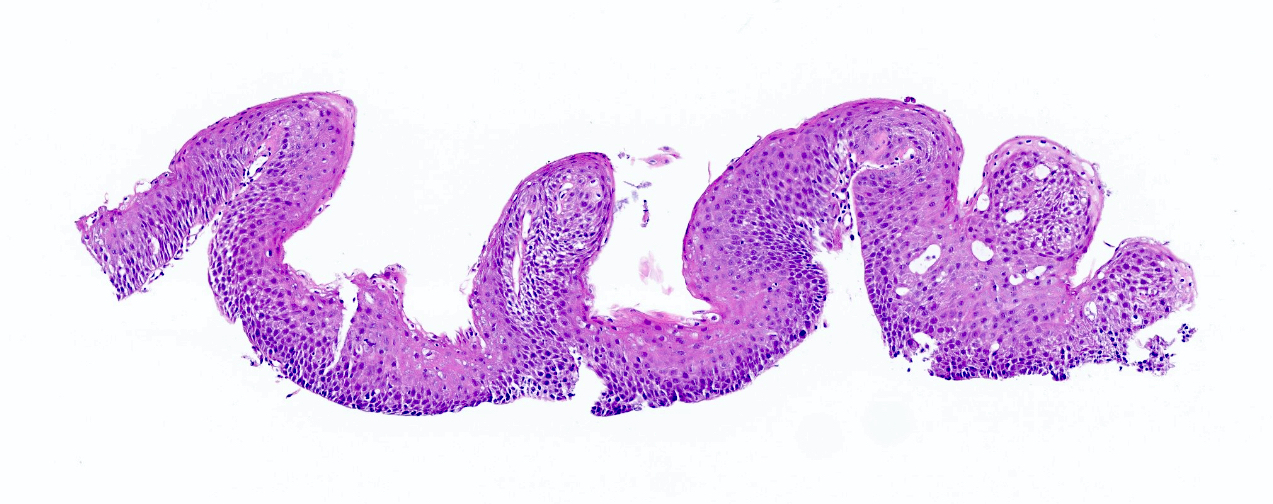
Microscopic (histologic) description
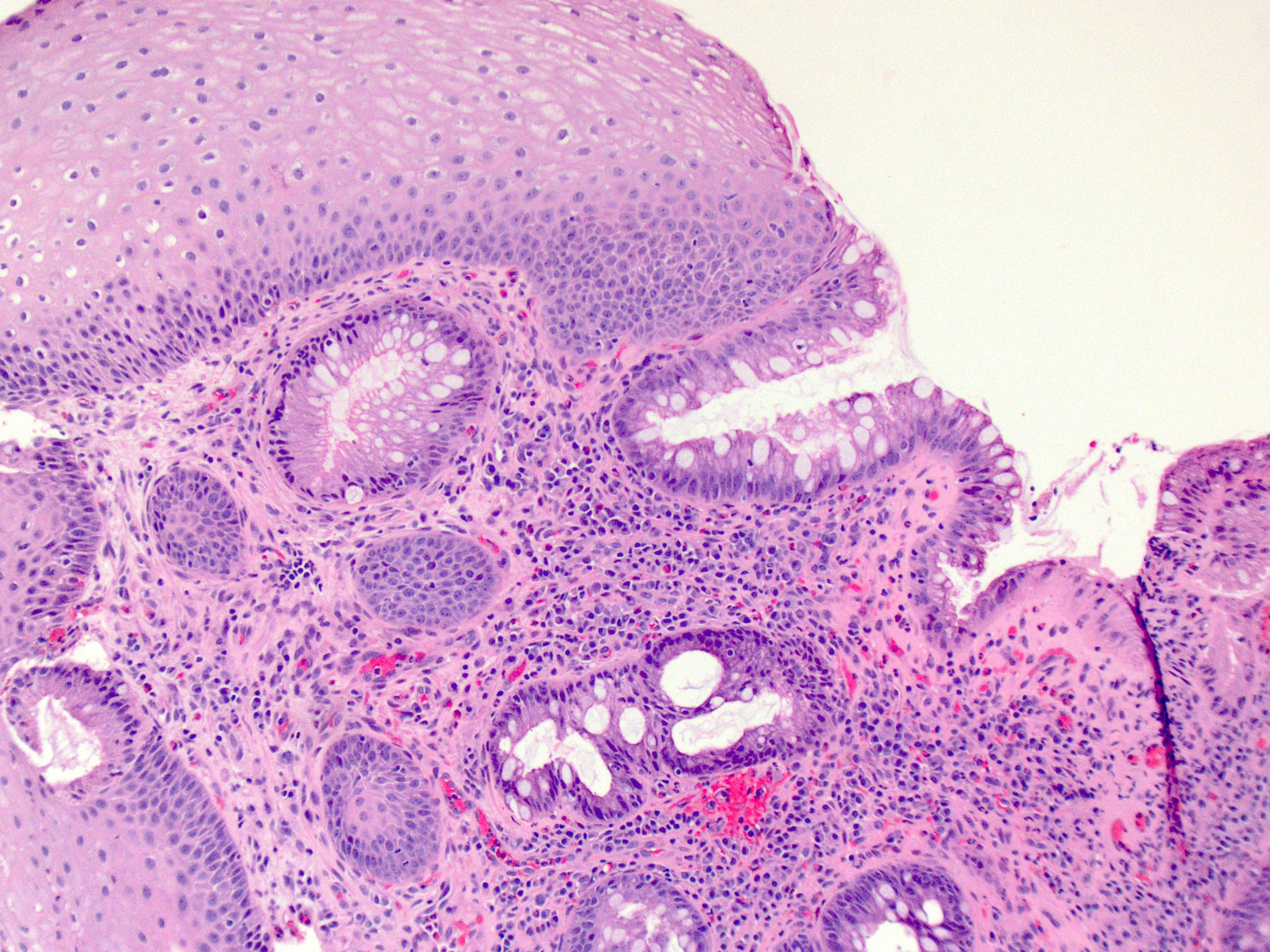
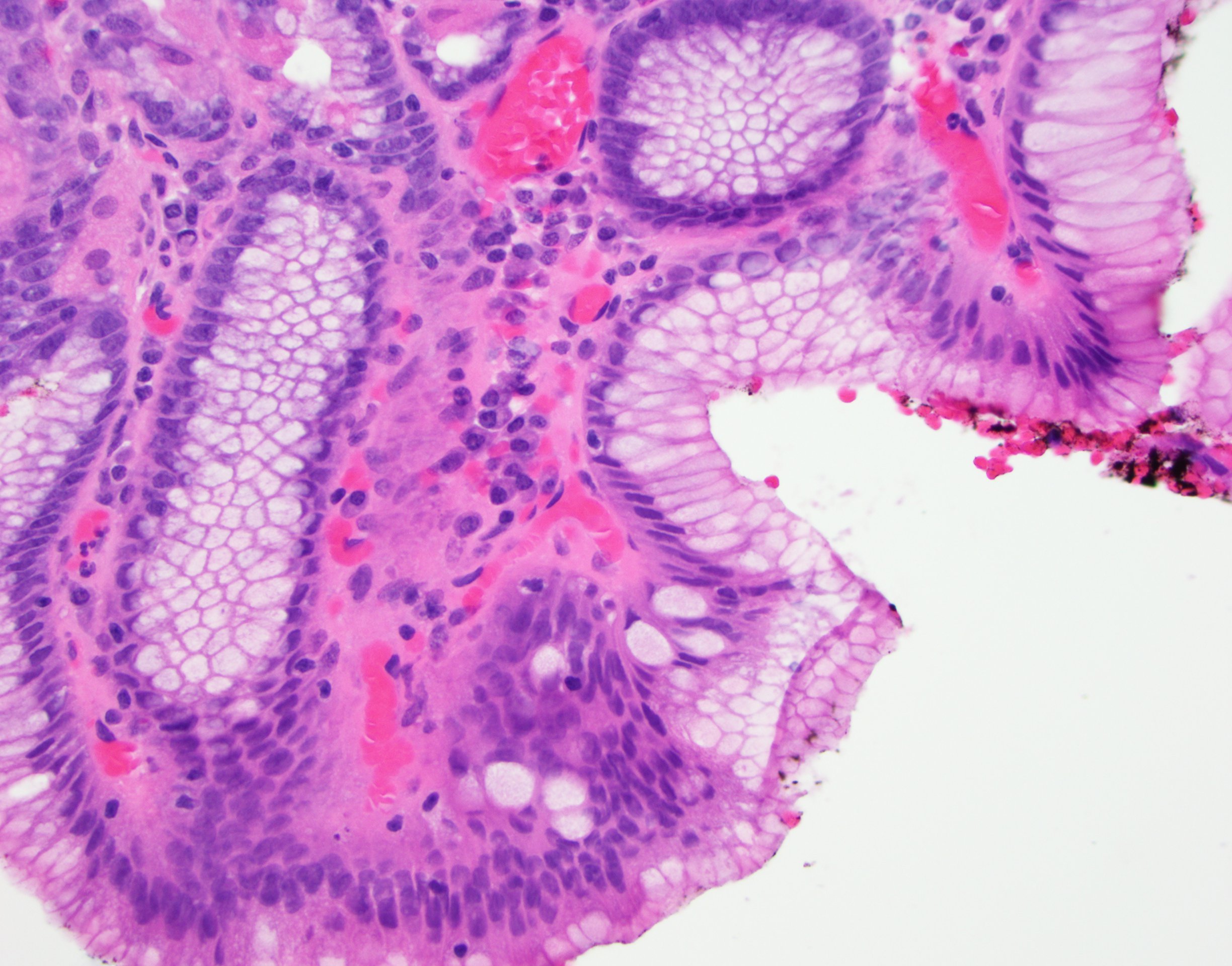
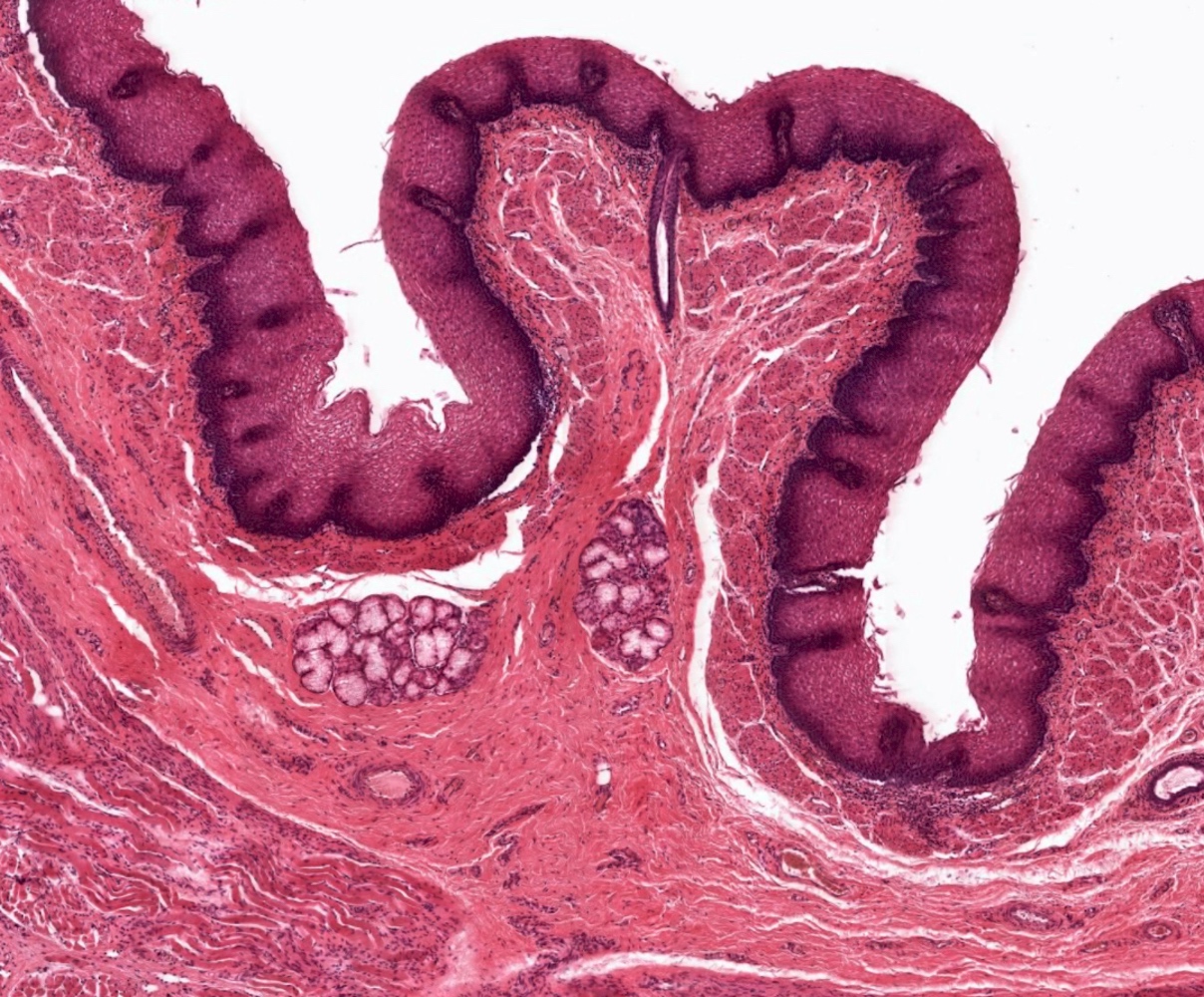
- Esophageal squamous epithelium replaced by columnar epithelium of intestinal type with goblet cells
- True goblet cells: rounded shape, clear to bluish cytoplasmic mucin, randomly scattered, mucin usually indents nucleus
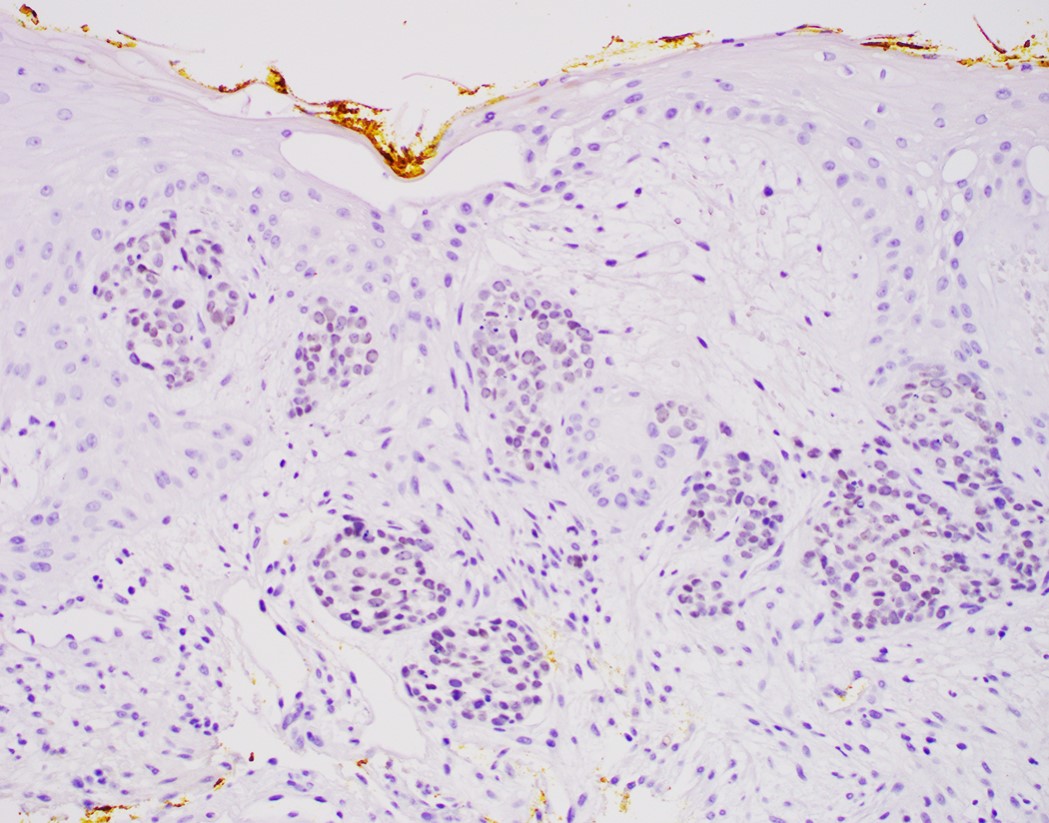
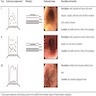
- Nondysplastic reactive BE shows the presence of four lines (Surg Pathol Clin 2017;10:781)
- First line: gastric foveolar type mucin droplet
- Second line: base of the foveolar mucin vacuole
- Third line: cytoplasm below the mucin vacuole
- Fourth line: row of nuclei
- Baseline atypia of Barrett mucosa - some basal glands may show nuclear enlargement and stratification but there is complete surface maturation; this is considered negative for dysplasia
- Duplication of muscularis mucosae characteristic finding in BE; observed in 92% of BE resections, involving 5% of the Barrett segment (Am J Surg Pathol 2007;31:1719)
- Squamous overgrowth over metaplastic epithelium, hybrid glands, presence of esophageal ducts have high specificity for BE (Am J Surg Pathol 2007;31:1733)
- Postablation histology: replacement of columnar mucosa to squamous (neosquamous) mucosa; residual metaplastic epithelium may persist beneath the squamous epithelium (known as buried Barrett’s) and progress to dysplasia or carcinoma
Microscopic (histologic) images
Cytology description
- Cytology has good sensitivity (82%) and specificity (88%) for identifying intestinal metaplasia with moderate interobserver agreement (J Am Soc Cytopathol 2015;4:113)
- Cytology may be poised to synergize with advances in other techniques for management of patients with Barrett esophagus
- Improvements in brushing devices may help to decrease the nondiagnostic rate (Diagn Cytopathol 2011;39:60)
Positive stains
- H&E remains the gold standard for diagnosis of BE and assessing Barrett dysplasia (Am J Surg Pathol 2016;40:e83)
- Alcian blue (at pH 2.5) stain acidic mucin in true goblet cells as bright purple blue; helpful in distinguishing true goblet cells from pseudogoblet cells in challenging cases, reflexive use on biopsies not justified
Negative stains
- Special stains typically not needed to diagnose BE (Am J Surg Pathol 2016;40:e83)
- Use of mucin glycoproteins (MUC2, MUC5AC) to diagnose BE currently not indicated
- Use of markers of intestinal phenotype (CDX2, Das-1, villin, HepPar1) to diagnose BE currently not indicated
- Use of CK7 and CK20 to distinguish BE from intestinal metaplasia of the gastric cardia not indicated as not adequately specific
Electron microscopy description
- Mucin granules in metaplastic cells
Sample pathology report
- No endoscopy report provided and there is intestinal metaplasia in the biopsy
- Esophagus, distal, biopsy:
- Barrett mucosa, negative for dysplasia (see comment)
- Comment: Per 2016 ACG guidelines, the diagnosis of Barrett esophagus in this case is made owing to the presence of goblet cells, with the assumption that the biopsy is taken from distal esophagus and the mucosal irregularity extends to at least 1 cm above the top of the gastric folds.
- Reference: Am J Gastroenterol 2016;111:30
- Esophagus, distal, biopsy:
- Endoscopy report provided and specifies that mucosal irregularity extends to at least 1 cm proximal to the gastroesophageal junction
- Esophagus, distal, biopsy:
- Barrett mucosa, negative for dysplasia
- Esophagus, distal, biopsy:
- No endoscopy report provided, there is intestinal metaplasia in the biopsy and the biopsy is labeled as gastroesophageal junction
- Gastroesophageal junction, biopsy:
- Gastric cardia type mucosa with intestinal metaplasia, negative for dysplasia (see comment)
- Comment: If the biopsy is taken from tubular esophagus and the mucosal irregularity extends to at least 1 cm above the top of gastric folds, then this represents Barrett esophagus. If the biopsy is taken from gastric cardia, then this represents intestinal metaplasia of the gastric cardia.
- Reference: Am J Gastroenterol 2016;111:30
- Gastroesophageal junction, biopsy:
- References: Ann Diagn Pathol 2019;39:111, Am J Gastroenterol 2016;111:30
Differential diagnosis
- Intestinal metaplasia of gastric cardia
- Need endoscopic correlation; lower rate of progression to dysplasia or carcinoma
- Pseudogoblet cells
- Clustered, characteristically arranged in a back to back or linear and continuous array unlike true goblet cells
- PAS / Alcian blue may be helpful, but sometimes pseudogoblet cells show nonspecific weak alcianophilia on PAS / Alcian blue
Additional references
Board review style question #1
Below is a picture of endoscopic biopsy from a 60 year old man with a clinical history of reflux symptoms and the biopsy is labeled as gastroesophageal junction. The endoscopy report is not provided. How would you sign out the case?
- Barrett esophagus, negative for dysplasia
- Gastric mucosa with intestinal metaplasia, consistent with Barrett esophagus
- Gastric cardia type mucosa with intestinal metaplasia (see comment)
- Barrett esophagus with low grade dysplasia
Board review style answer #1
C. Gastric cardia type mucosa with intestinal metaplasia, with a comment stating that endoscopic evidence of abnormal mucosa for at least 1 cm proximal to the gastroesophageal junction is required for a diagnosis of Barrett esophagus.
Comment Here
Reference: Barrett esophagus
Comment Here
Reference: Barrett esophagus
Board review style question #2
What is the best stain to diagnose Barrett esophagus on a biopsy?
- H&E stain
- PAS with Alcian blue special stain
- CDX2 immunostain
- CK7 immunostain
Board review style answer #2
Barrett related dysplasia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Unequivocally neoplastic epithelium without invasion, associated with Barrett esophagus (WHO: Digestive System Tumours, 5th Edition, 2019)
Essential features
- Presence of Barrett esophagus related dysplasia remains greatest risk factor for development of esophageal adenocarcinoma (Am J Surg Pathol 2017;41:e8)
- Pathologic diagnosis and grading of dysplasia is the gold standard marker for assessing risk of neoplastic progression (Am J Surg Pathol 2016;40:e83)
- Atypia in Barrett esophagus dysplasia can be interpreted as negative for dysplasia, indefinite for dysplasia, low grade dysplasia and high grade dysplasia (WHO: Digestive System Tumours, 5th Edition, 2019, Hum Pathol 1988;19:166)
- Diagnosis and grading of Barrett esophagus related dysplasia can be challenging, especially with coexisting inflammation (Hum Pathol 1988;19:166)
- Both low grade dysplasia and high grade dysplasia managed by endoscopic therapy, per the latest American College of Gastroenterology (ACG) guidelines (Am J Gastroenterol 2016;111:30)
Terminology
- Indefinite for dysplasia, low grade Barrett dysplasia and high grade Barrett dysplasia (WHO: Digestive System Tumours, 5th Edition, 2019, Hum Pathol 1988;19:166)
- Vienna classification uses terms noninvasive low grade neoplasia (low grade adenoma / dysplasia) for low grade dysplasia and noninvasive high grade neoplasia for high grade dysplasia (Gut 2000;47:251)
- World Health Organization recognizes both classifications (WHO: Digestive System Tumours, 5th Edition, 2019)
ICD coding
Epidemiology
- Dysplasia develops typically in the setting of Barrett esophagus (Am J Gastroenterol 2016;111:30)
- Barrett esophagus detected in ~ 10- 15% of patients with chronic gastroesophageal reflux disease (GERD) (Am J Gastroenterol 2016;111:30, Gastroenterol Hepatol (NY) 2016;12:449)
- Incidence of low grade dysplasia should be ~2 - 3% but not > 5% in prospectively evaluated patients (Am J Gastroenterol 2010;105:1523)
- Indefinite for dysplasia category should be used in only a small percentage of cases (< 3 - 5%) (Ann Diagn Pathol 2018;37:75)
- For low grade dysplasia, the annual risk of progression to cancer is ~ 0.7% per year (Am J Gastroenterol 2016;111:30)
- For high grade dysplasia, the annual risk of progression to cancer is ~ 7% per year (Am J Gastroenterol 2016;111:30)
Sites
- Distal esophagus, gastroesophageal junction (restricted to metaplastic esophageal mucosa)
Pathophysiology
- Sequential progression from inflammation to metaplasia, dysplasia and carcinoma (Am J Surg Pathol 2016;40:e45)
- Accumulation of multiple genetic and epigenetic alterations causes development and progression of dysplasia (Nat Genet 2014;46:837)
- C-myc and cyclins D1, E and B implicated as oncogenes in neoplastic progression in Barrett esophagus (Am J Surg Pathol 2016;40:e45)
- Inactivation of tumor suppressor proteins p53, p16, p15, p27 and adenomatous polyposis coli (APC) also implicated in Barrett esophagus carcinogenesis (Am J Surg Pathol 2016;40:e45)
- Other mechanisms proposed include increased telomerase expression, increased VEGFA and C, decreased membrane E-cadherin, increased MMP-7 and MMP-9, increased markers of epithelial-mesenchymal transition such as 2EB1/2EB2 and TGF-B1 (Am J Surg Pathol 2016;40:e45)
- As Barrett esophagus epithelial cells progress to cancer, they typically manifest aneuploidy, a marker of genomic instability (Am J Surg Pathol 2016;40:e45)
Etiology
- Known risk factors for development of neoplasia in Barrett esophagus (Am J Gastroenterol 2016;111:30):
- Advancing age
- Increasing length of Barrett esophagus
- Central obesity
- Tobacco usage
- Lack of nonsteroidal anti inflammatory agent use
- Lack of PPI use
- Lack of statin use
Clinical features
- Patients typically have gastroesophageal reflux disease symptoms
- No distinct clinical or radiologic manifestations of Barrett esophagus dysplasia
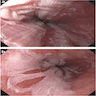
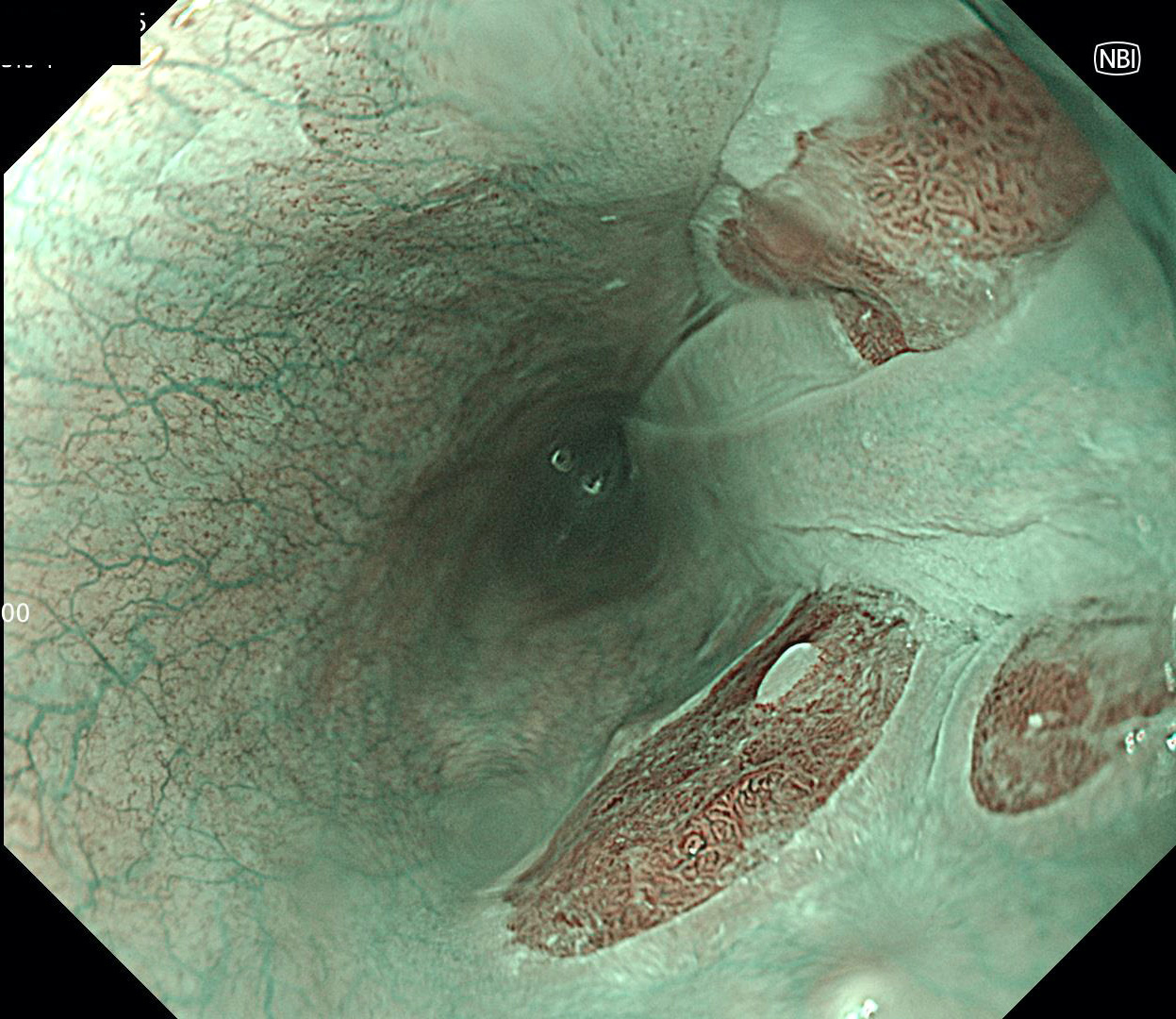
- Endoscopically, Barrett esophagus dysplasia may be visible as thickened, flat, irregular or plaque-like area, distinct from adjacent nondysplastic Barrett mucosa (Dig Dis Sci 2003;48:1537, Am J Gastroenterol 2016;111:30)
- Mucosal abnormalities (ulceration, stricture, mass, nodules, plaques) associated with increased risk of cancer (Am J Gastroenterol 2016;111:30)
Diagnosis
- Recommended for Barrett esophagus with dysplasia to be reviewed by 2 pathologists, at least 1 with specialized expertise in GI pathology (Am J Gastroenterol 2016;111:30)
- Barrett esophagus surveillance performed with high definition / high resolution white light endoscopy
- Routine use of advanced imaging techniques other than electronic chromoendoscopy not recommended for endoscopic surveillance at this time
- Endoscopic surveillance should employ 4 quadrant biopsies at 1 cm intervals in patients with prior dysplasia (Am J Gastroenterol 2016;111:30)
- Mucosal abnormalities to be sampled separately, preferably with endoscopic mucosal resection (EMR)
- Adjunct use of wide area transepithelial sampling with computer assisted 3 dimensional analysis (WATS) to forceps biopsy markedly improves detection of esophageal dysplasia (United European Gastroenterol J 2018;6:529)
- Overall detection of dysplasia by WATS reported to be increased by 242% (Dis Esophagus 2019;32:doy099)
Laboratory
- No laboratory tests assist in the detection of Barrett esophagus dysplasia
Prognostic factors
- Diagnosis of Barrett esophagus associated dysplasia remains a marker of increased risk of progression to esophageal adenocarcinoma (Am J Surg Pathol 2017;41:e8, Am J Surg Pathol 2016;40:e45)
- Progression rates of low grade dysplasia to high grade dysplasia and dysplasia to carcinoma directly proportional to number of pathologists who agree on dysplasia diagnosis (Am J Surg Pathol 2016;40:e45, Am J Gastroenterol 2007;102:483)
Treatment
- Indefinite for dysplasia:
- Repeat endoscopy after acid suppressive medication optimization for 3 - 6 months
- If indefinite for dysplasia is reconfirmed, surveillance interval of 12 months is recommended (Am J Gastroenterol 2016;111:30, Gastroenterol Hepatol (NY) 2016;12:449)
- Low grade dysplasia:
- Endoscopic eradication therapy preferred treatment modality, endoscopic surveillance every 12 months an acceptable alternative (Am J Gastroenterol 2016;111:30)
- High grade dysplasia:
- Endoscopic therapy (Am J Gastroenterol 2016;111:30, Gastroenterol Hepatol (NY) 2016;12:449)
- Nodular Barrett esophagus: recommend endoscopic mucosal resection of the nodular lesion as initial diagnostic and therapeutic maneuver (Am J Gastroenterol 2016;111:30)
- If endoscopic mucosal resection confirms high grade dysplasia, endoscopic ablative therapy of remaining Barrett esophagus (Am J Gastroenterol 2016;111:30)
- Nonnodular dysplastic Barrett esophagus: radiofrequency ablation currently preferred endoscopic ablative therapy (Am J Gastroenterol 2016;111:30)
- In patients with preablation low grade dysplasia, endoscopic surveillance recommended every 6 months in the first year following complete elimination of intestinal metaplasia (CIEM) and annually thereafter (Am J Gastroenterol 2016;111:30)
- Endoscopic surveillance following complete elimination of intestinal metaplasia for patients with preablation high grade dysplasia recommended every 3 months for the first year, every 6 months in the second year and annually thereafter (Am J Gastroenterol 2016;111:30)
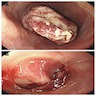
Clinical images
Gross description
- Normal appearing or nodule, erosion or polyp
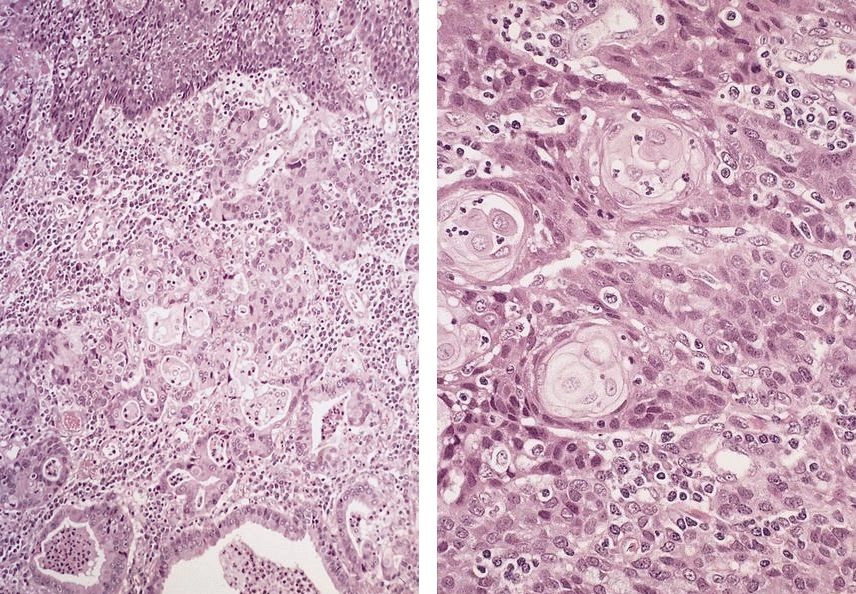
Microscopic (histologic) description
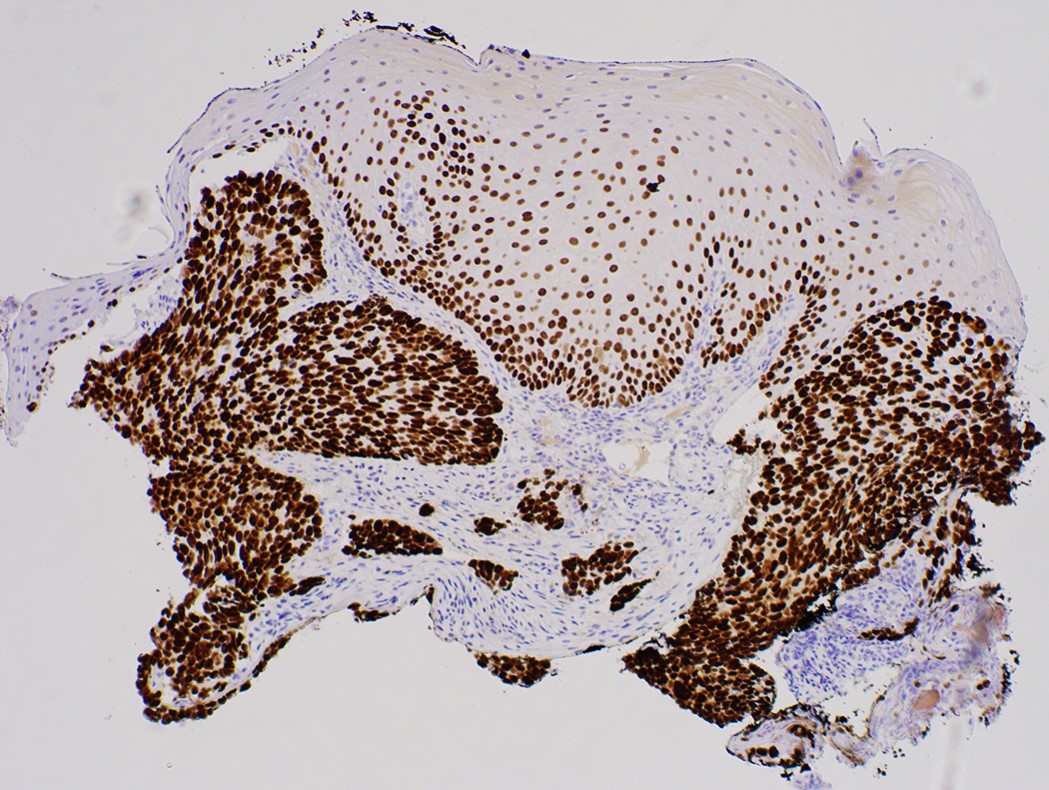
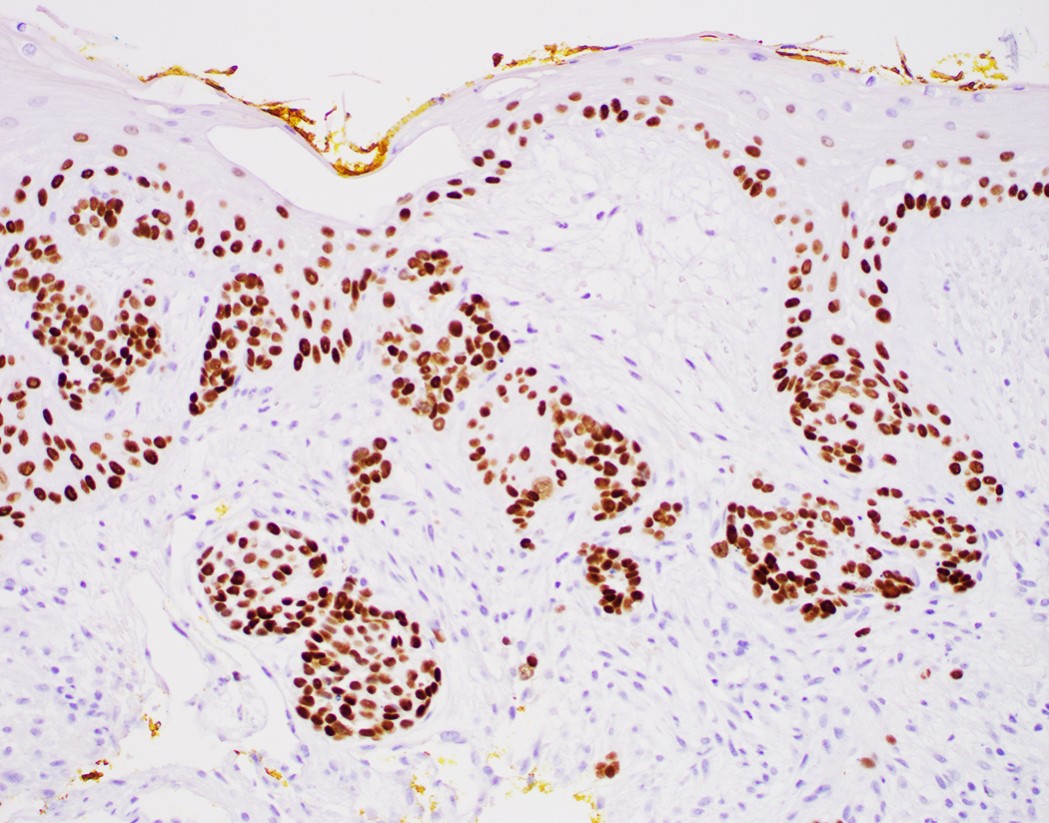
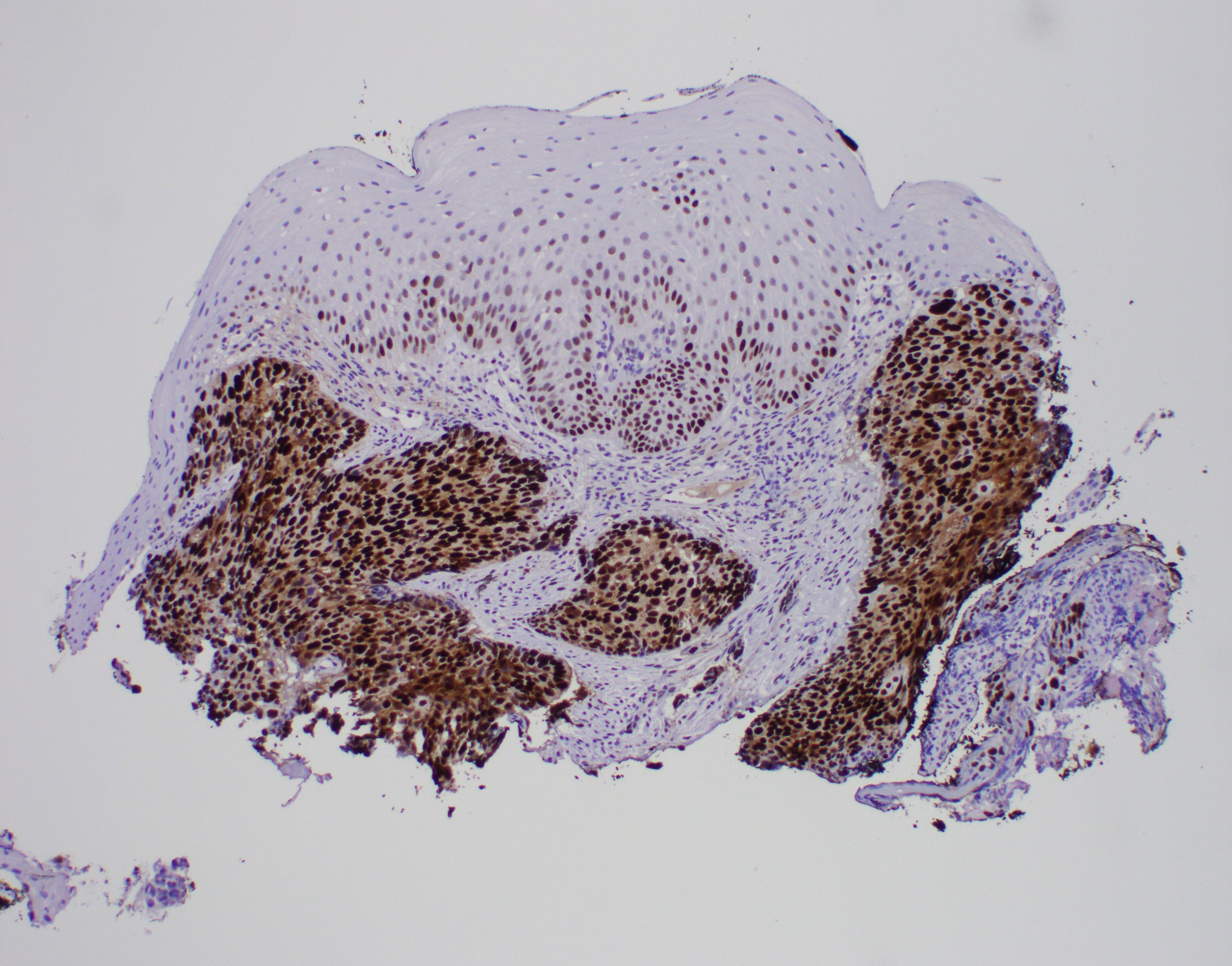
- Barrett esophagus dysplasia divided into low grade dysplasia and high grade dysplasia; indefinite for dysplasia also a valid interpretation (but not part of a histologic spectrum of progression) (Hum Pathol 1988;19:166)
- Indefinite for dysplasia (Hum Pathol 1988;19:166):
- Histologic features too marked for reactive atypia but not sufficient for a definitive diagnosis of dysplasia
- Cases with significant inflammation or ulceration in which inflammation obscures the findings
- Artifact limits interpretation (thermal effect, denuded surface epithelium, rarely technical issues)
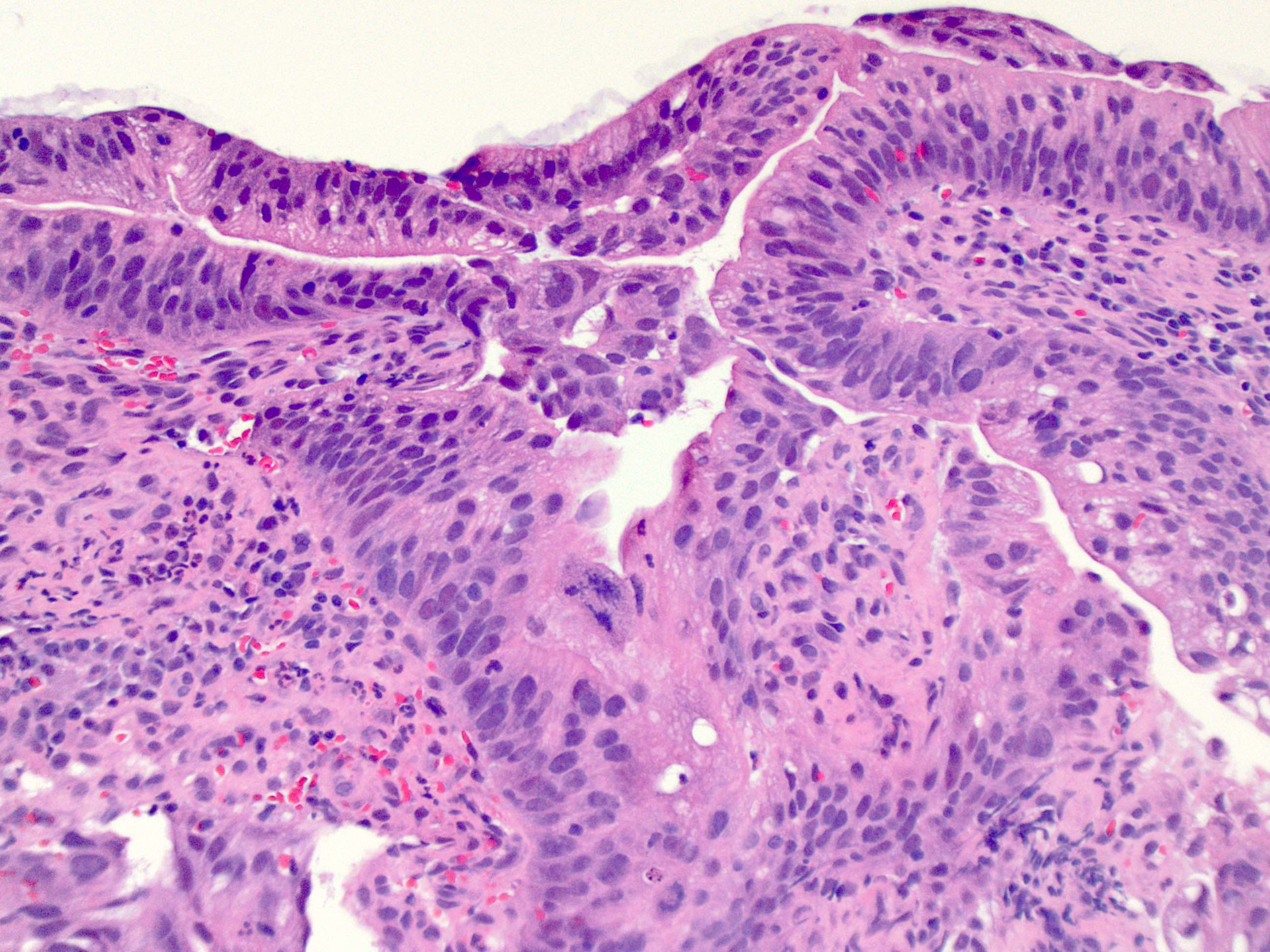
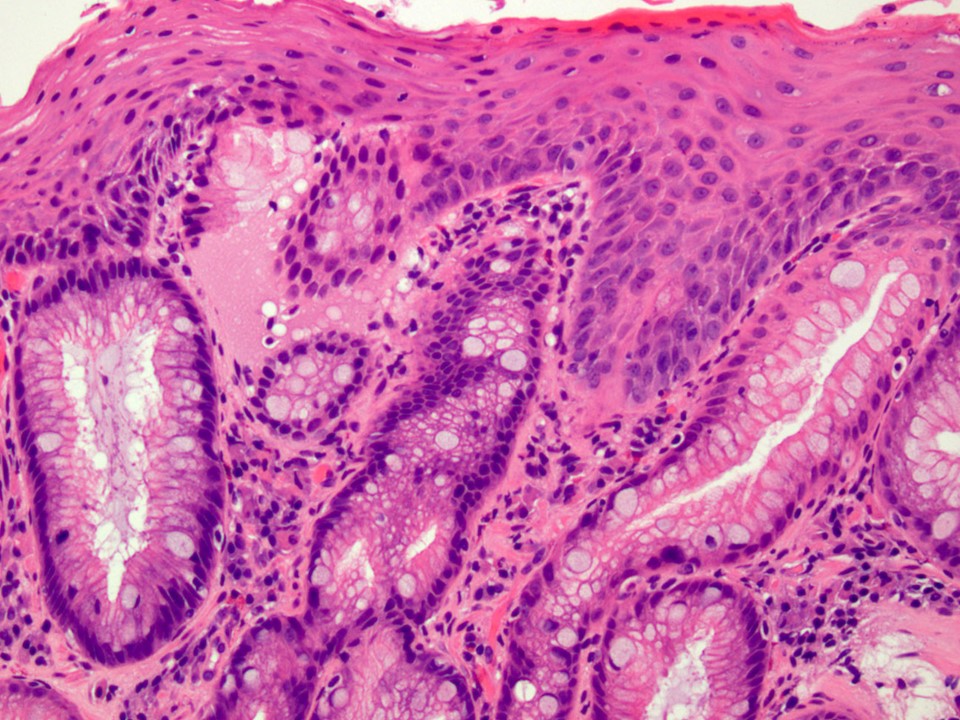
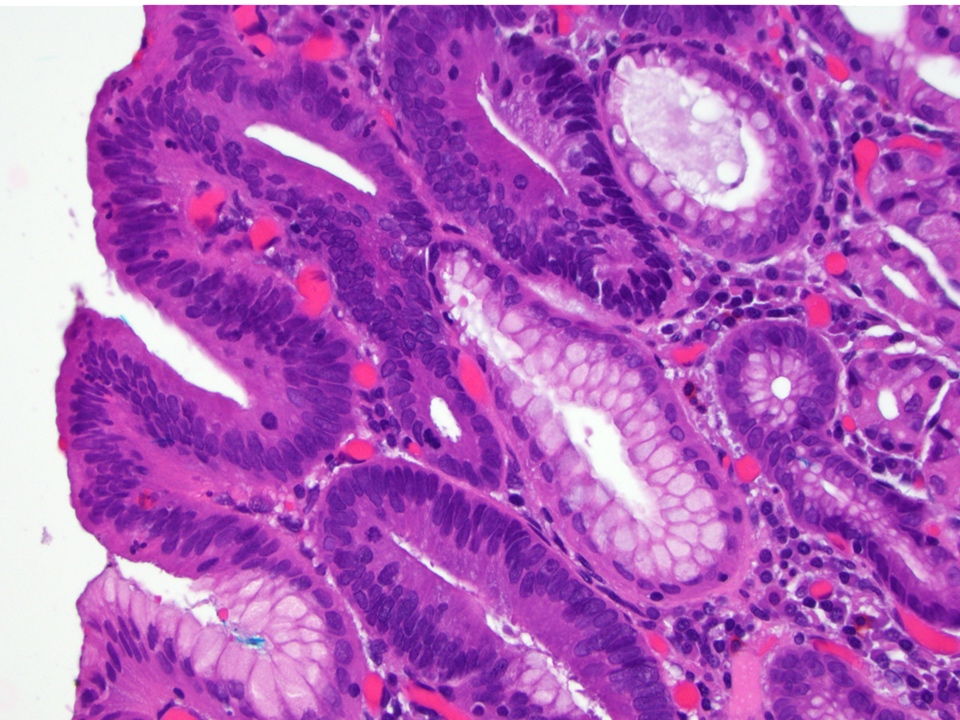
- Low grade dysplasia (Hum Pathol 1988;19:166):
- Unequivocally neoplastic
- Nuclear hyperchromasia, enlargement and stratification identified in the deeper glands and involving the surface epithelium
- Maintenance of nuclear polarity
- Abrupt transition between dysplastic and nondysplastic zones
- Effacement or loss of the 4 surface lines that characterize nondysplastic Barrett mucosa (Surg Pathol Clin 2017;10:781)
- Little (if any) architectural abnormalities
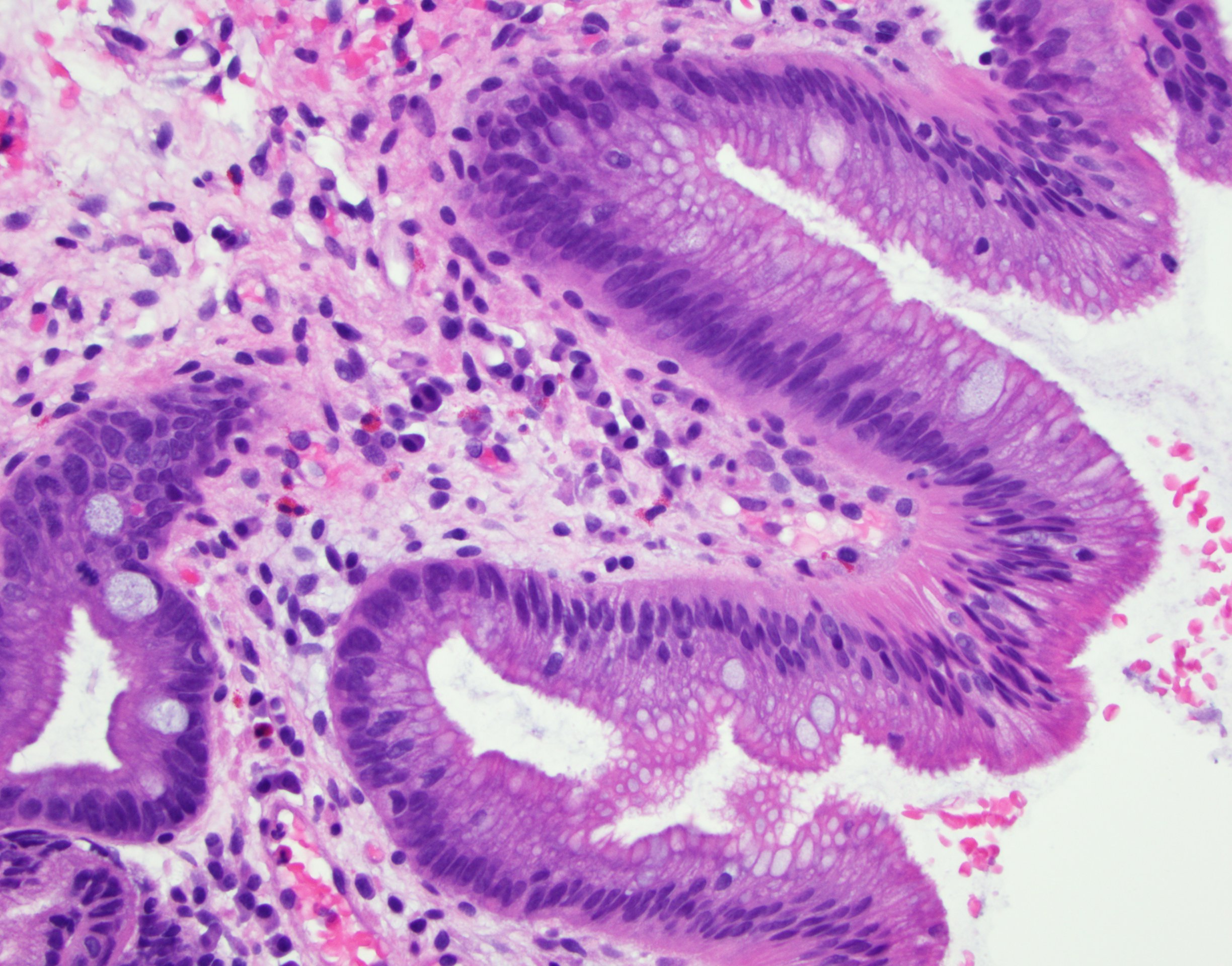
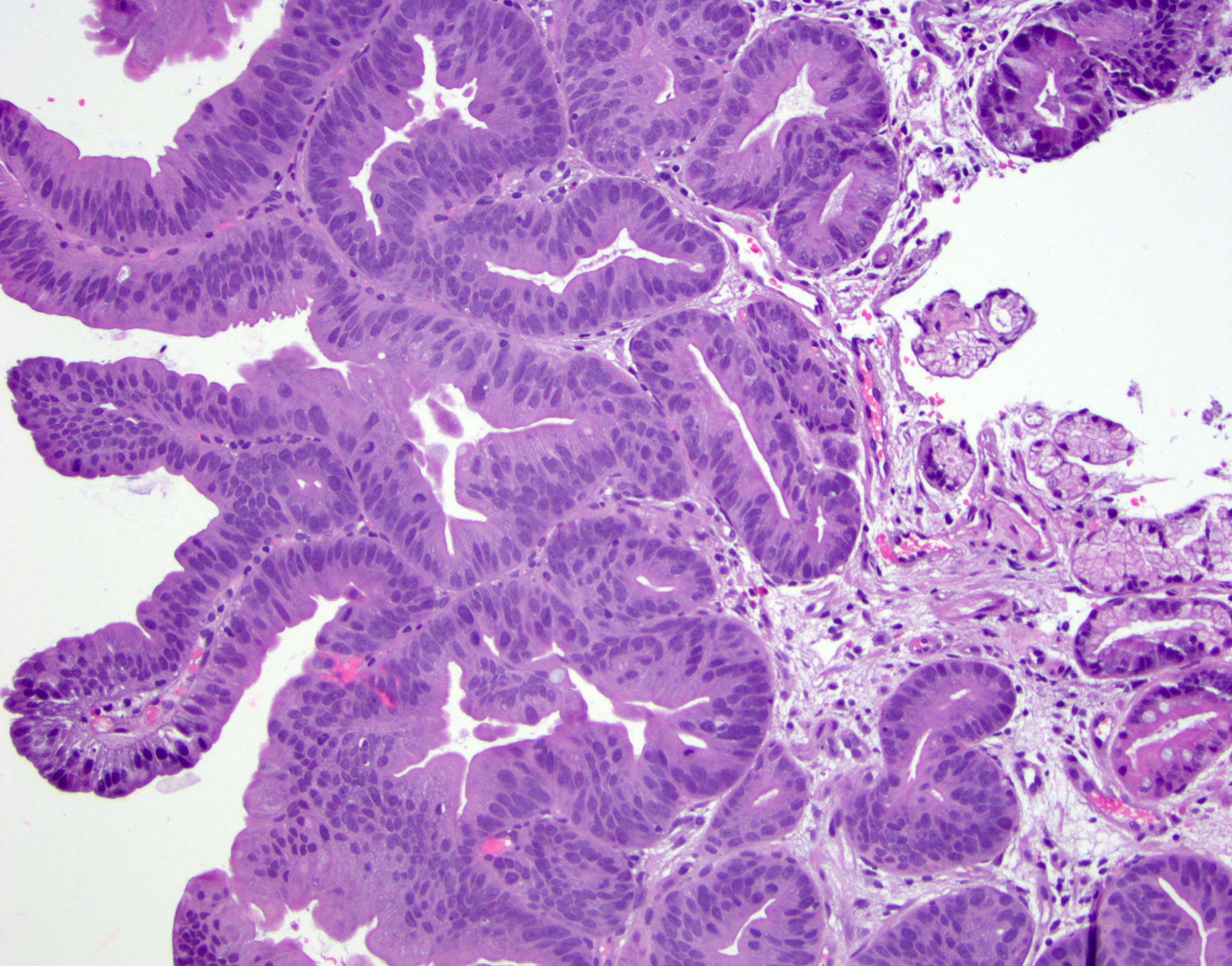
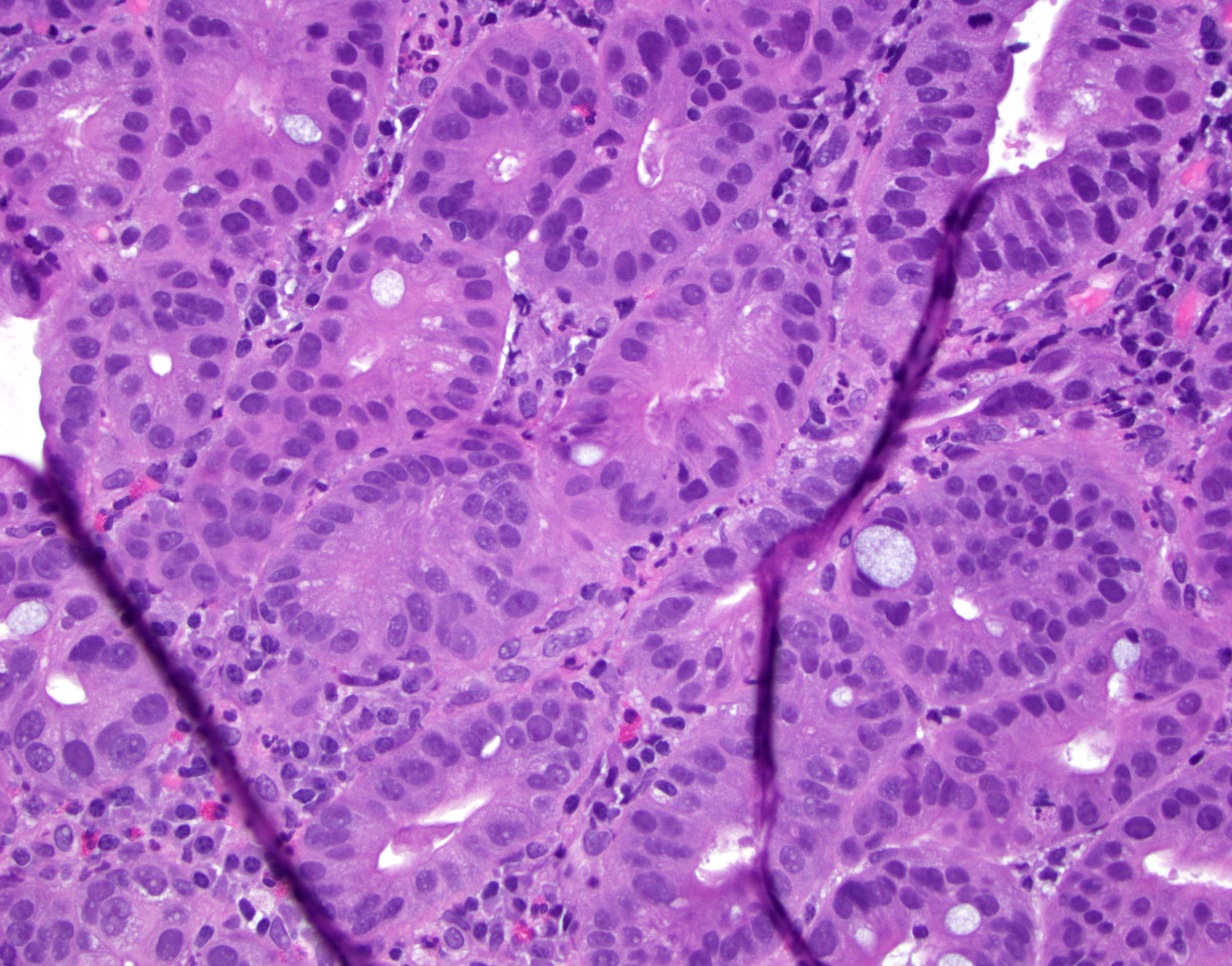
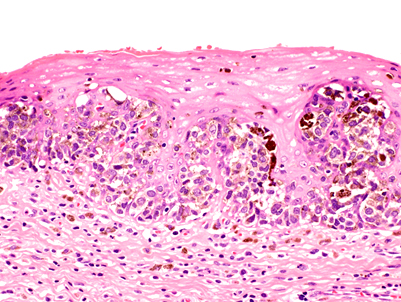
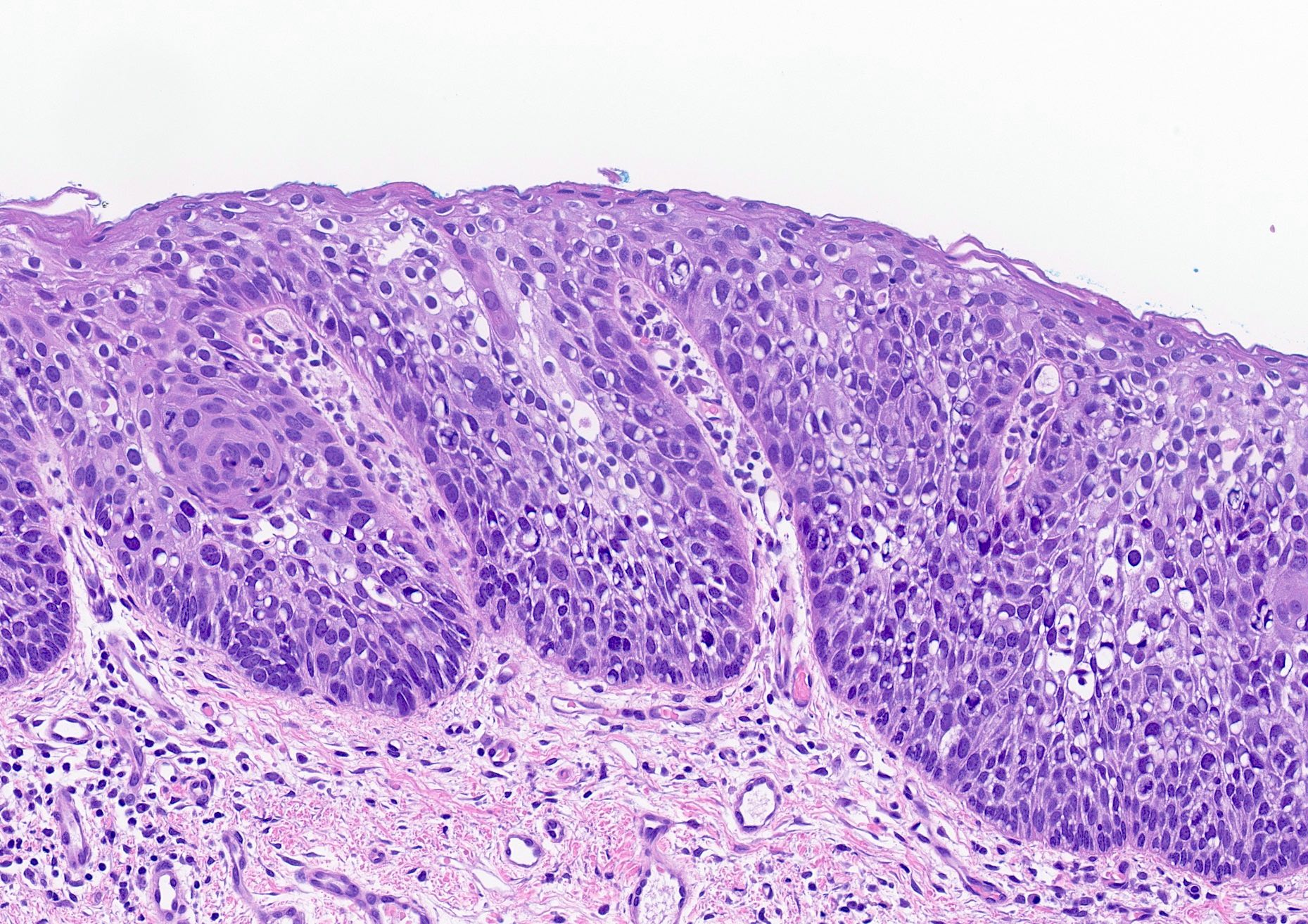
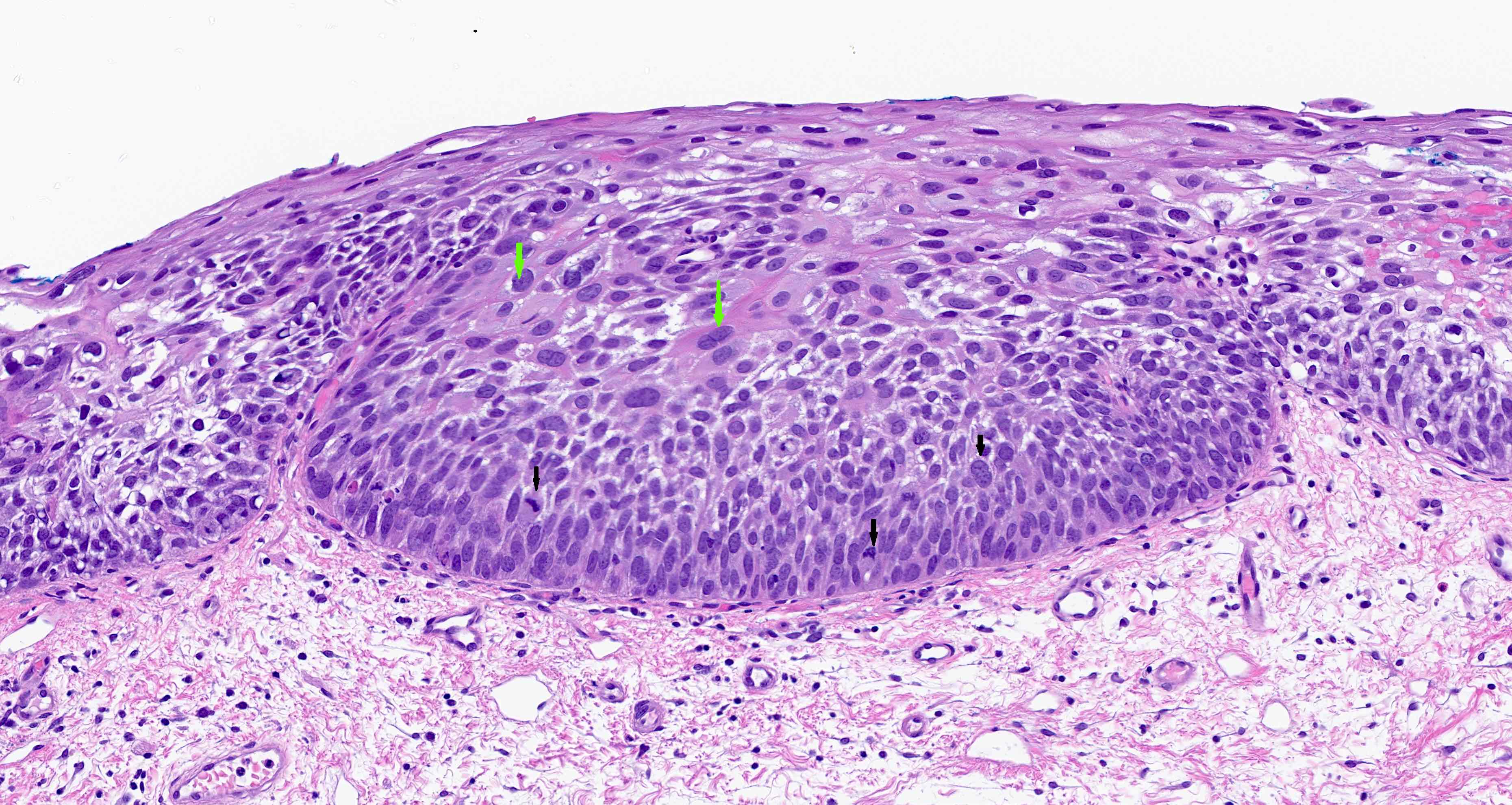
- High grade dysplasia (Hum Pathol 1988;19:166, Am J Gastroenterol 2008;103:2333):
- Greater degree of cytologic atypia in addition to architectural abnormalities
- Architectural abnormalities:
- Irregular size and shape of crypts, crowded crypts with little intervening lamina propria, intraluminal budding or cribriforming, rare dilated glands with intraluminal necrotic debris
- Cytologic features:
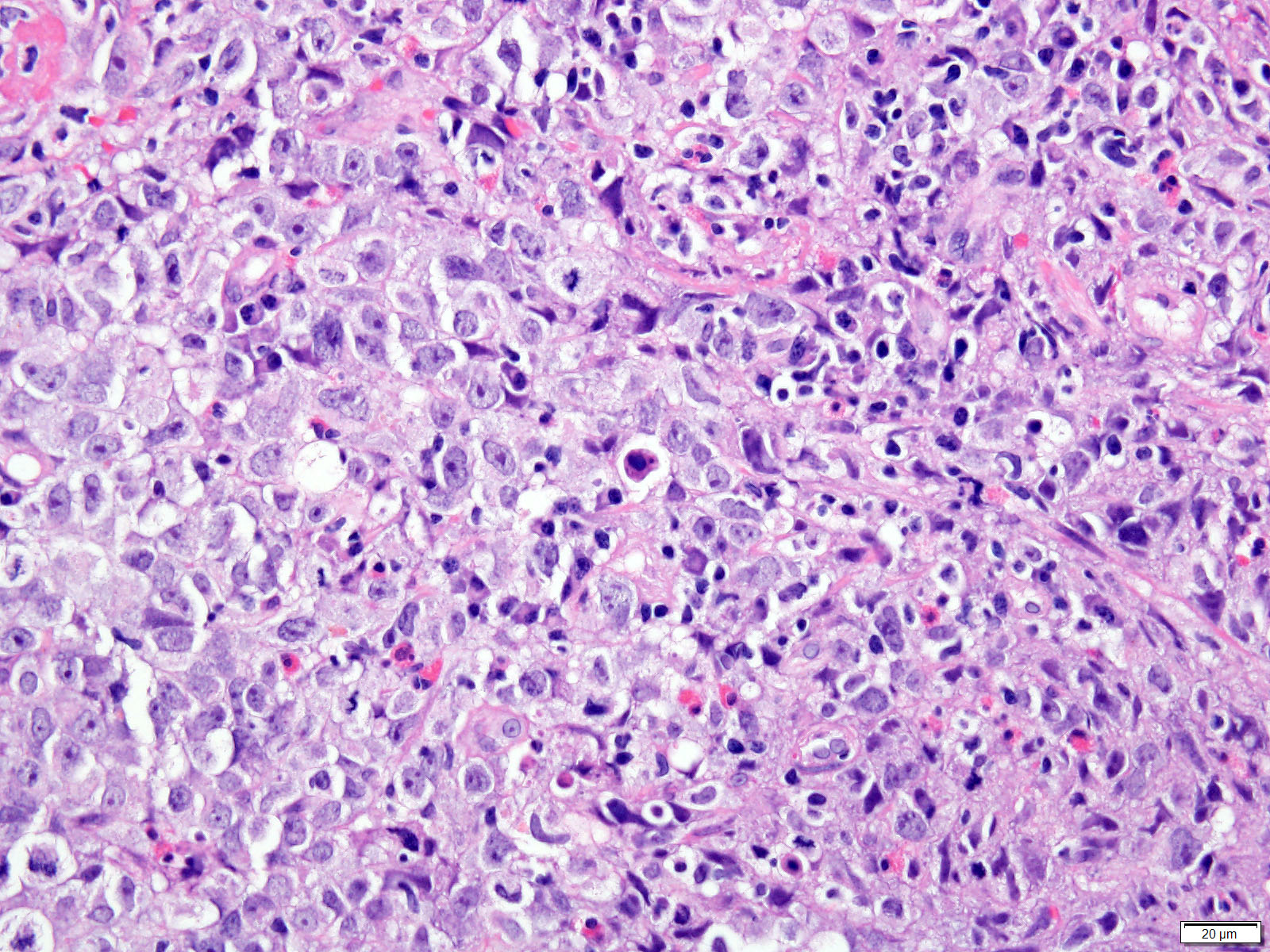
- Lack of surface maturation, loss of nuclear polarity, marked nuclear enlargement, pleomorphism and hyperchromasia, irregular nuclear contours
- Loss of nuclear polarity considered an important objective criterion to diagnose high grade dysplasia
- Basal crypt dysplasia (Am J Surg Pathol 2006;30:423):
- Controversial diagnosis
- Cytologic atypia in the basal pits with surface maturation
- Cytologic atypia may be low grade or high grade
- Some authors prefer to use the indefinite for dysplasia category for such lesions, especially with low grade atypia confined to the deep mucosa
- Finding of crypt dysplasia in one biopsy suggests high likelihood of finding conventional dysplasia in other biopsies
- Performing further deeper levels recommended
- Gastric foveolar dysplasia (non adenomatous dysplasia):
- Full thickness atypia with non stratified nuclei (Mod Pathol 2010;23:1, Hum Pathol 2013;44:1146)
- Nuclear enlargement, variably prominent nucleoli
- May appear bland and reactive at low magnification but nuclear alterations seen at high magnification
- Low grade dysplasia:
- Nuclear size 2 - 3 times the size of a small, mature lymphocyte, mostly mucinous cytoplasm and variably prominent nucleoli
- High grade dysplasia:
- Nuclear size at least 3 - 4 times the size of a small, mature lymphocyte, most cases have prominent nucleoli (Mod Pathol 2010;23:1, Hum Pathol 2013;44:1146)
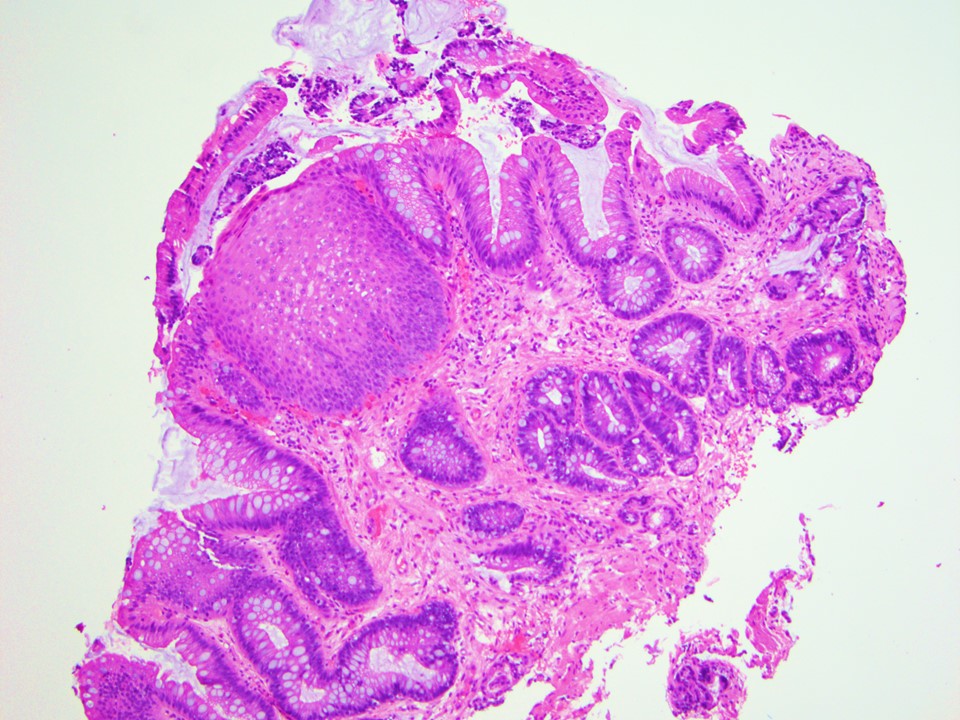
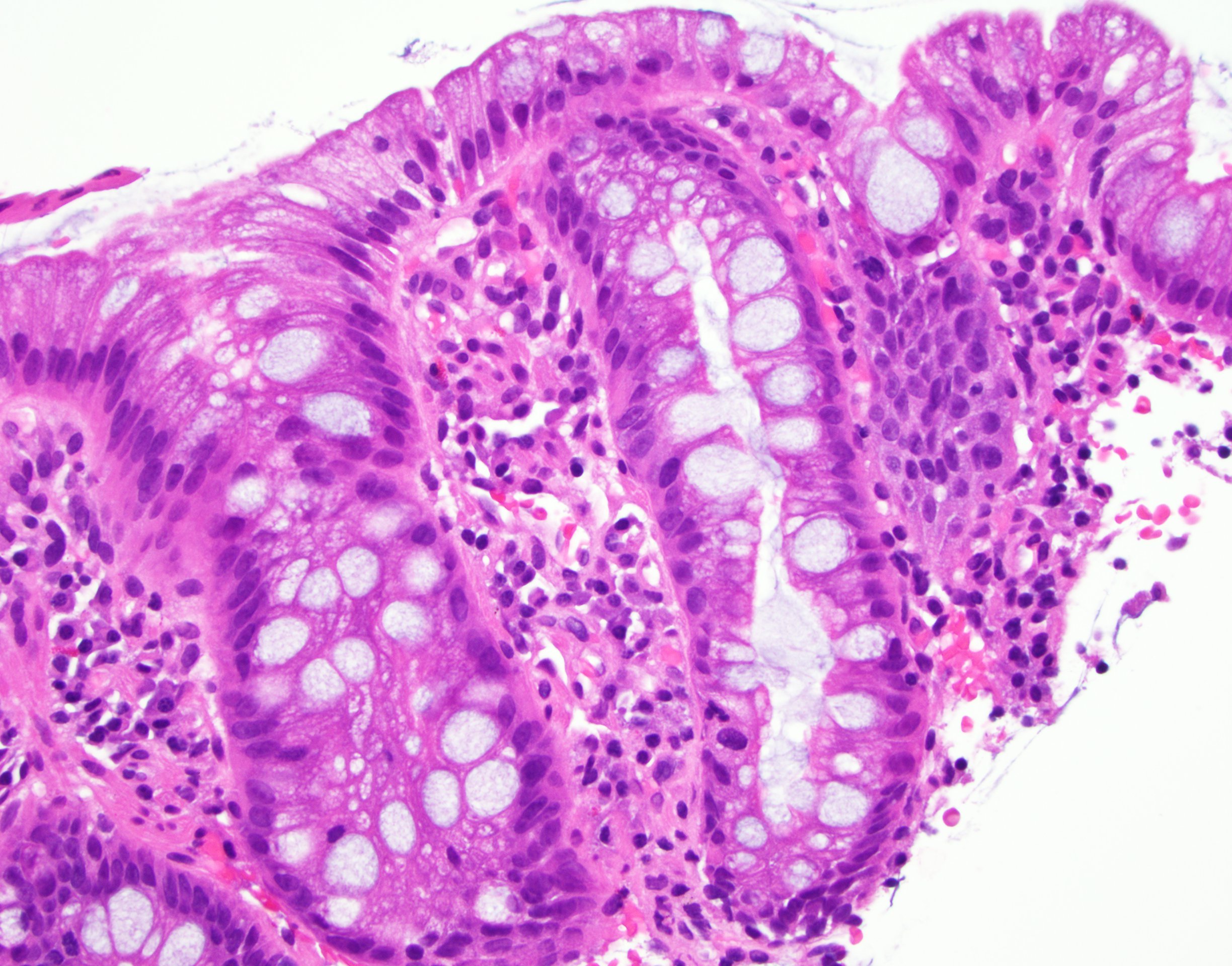
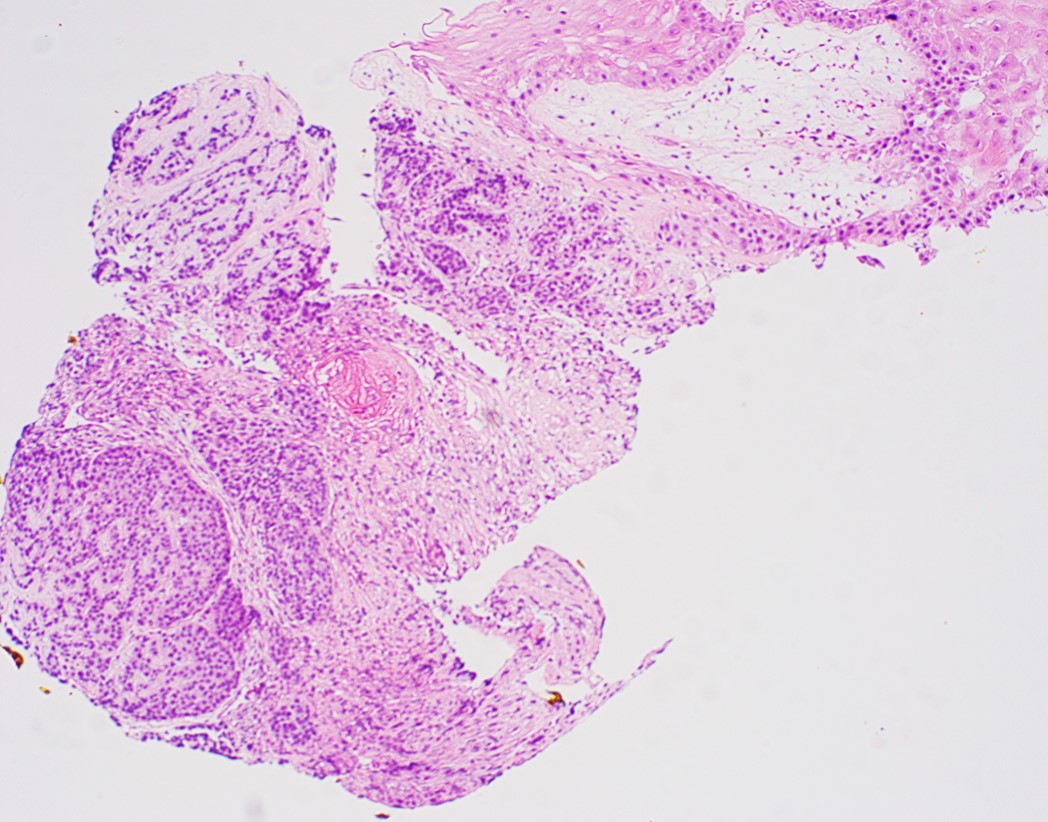
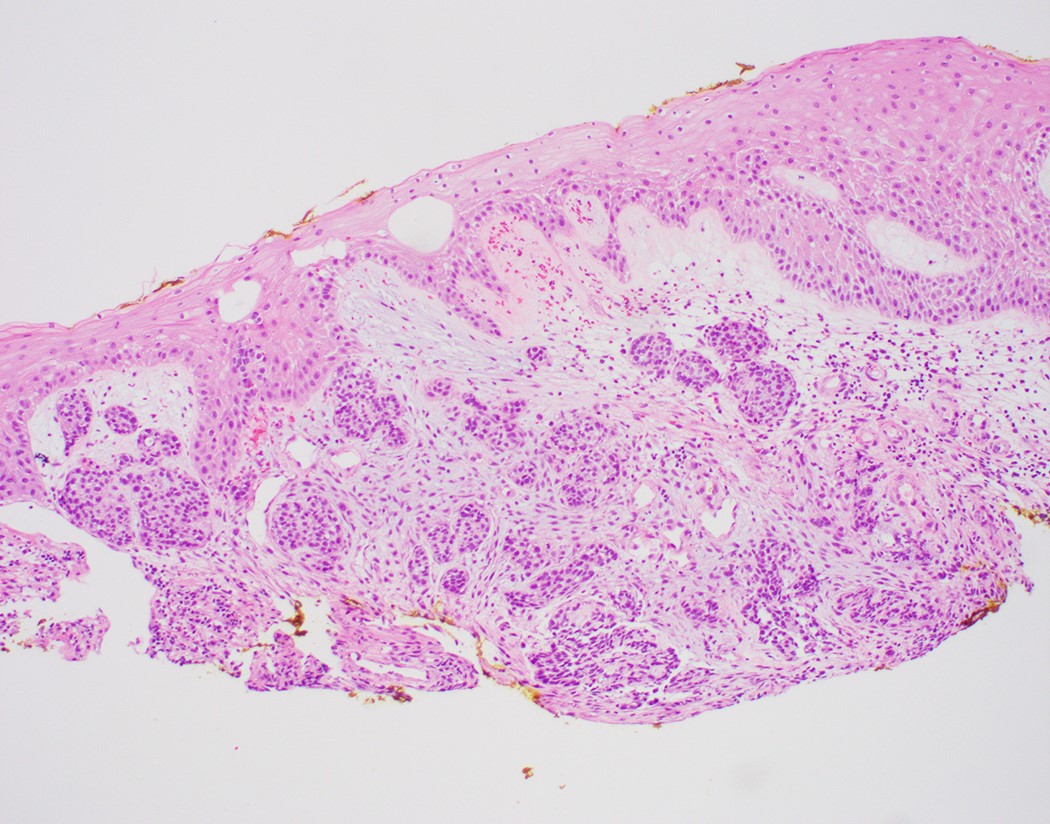
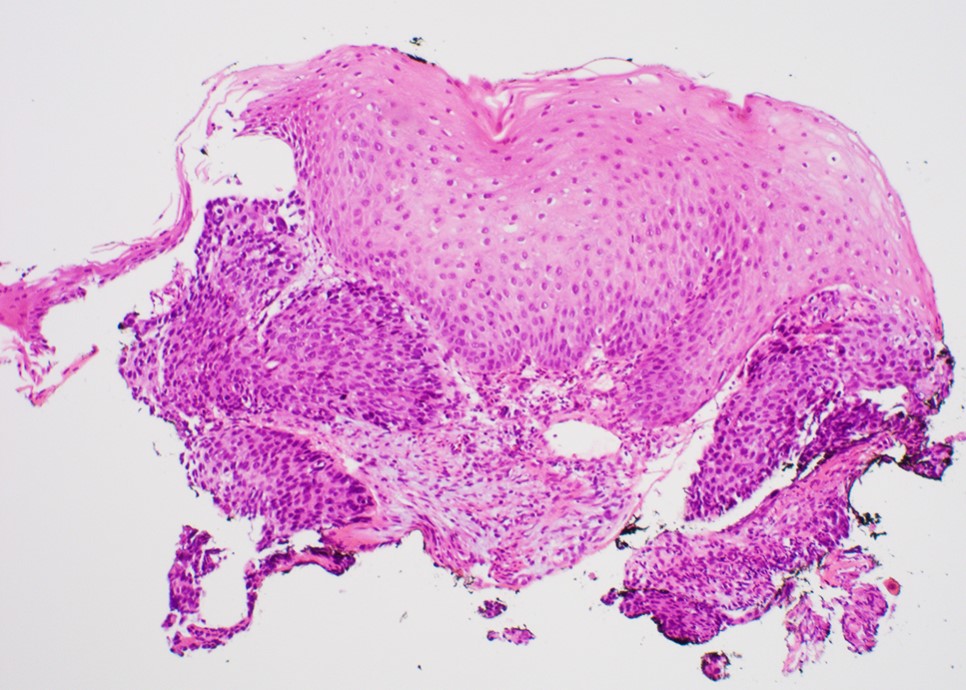
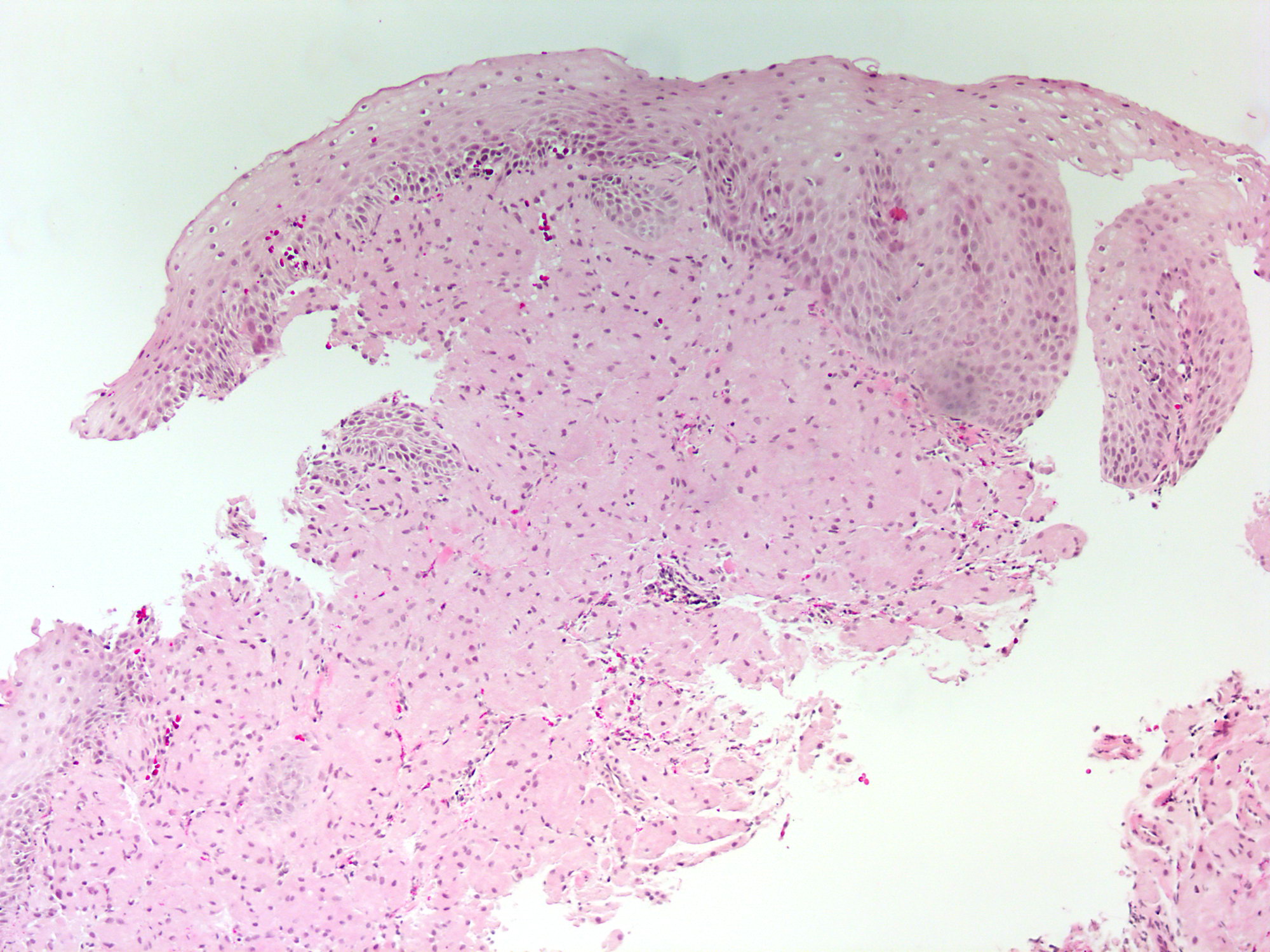
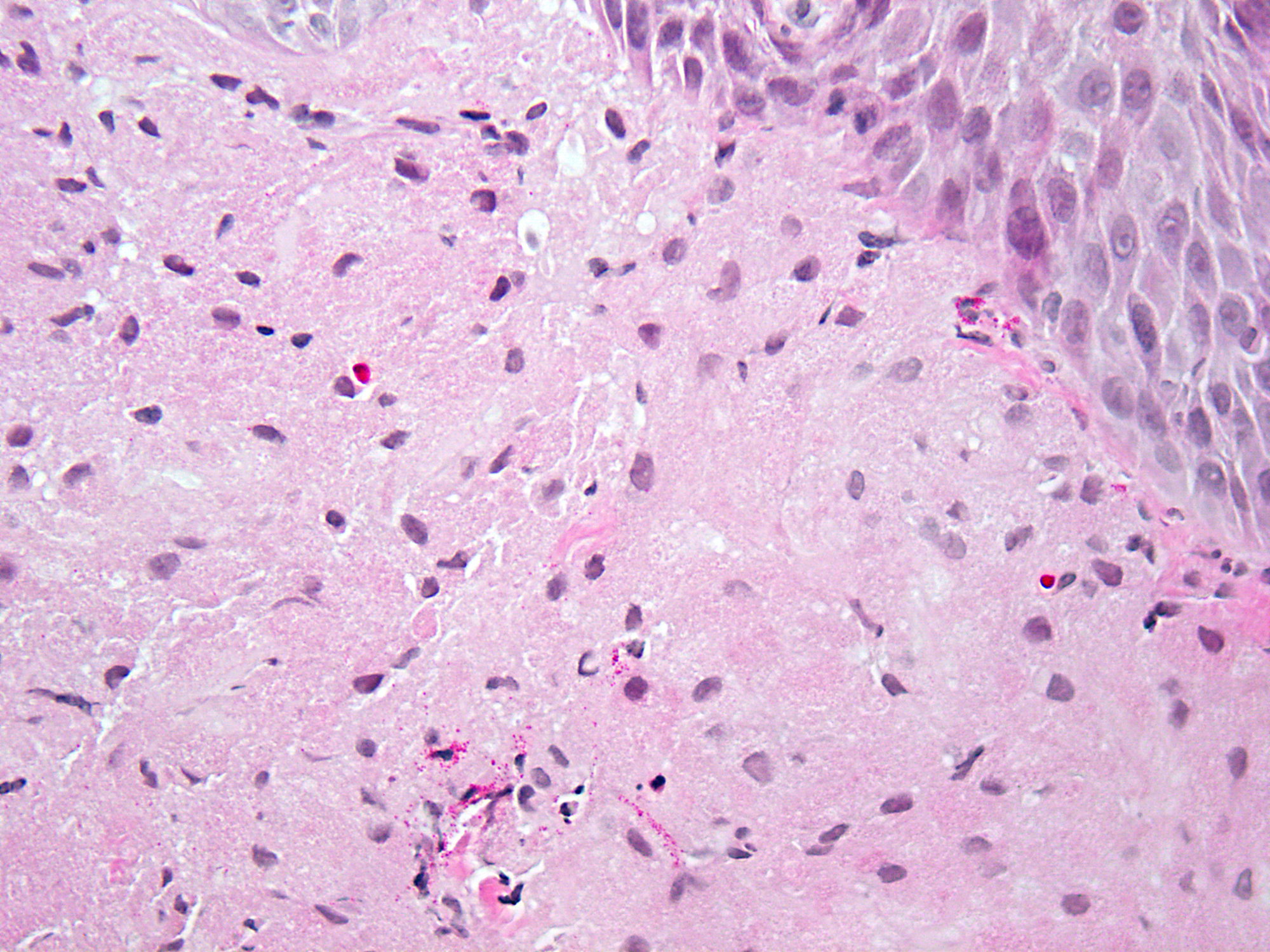
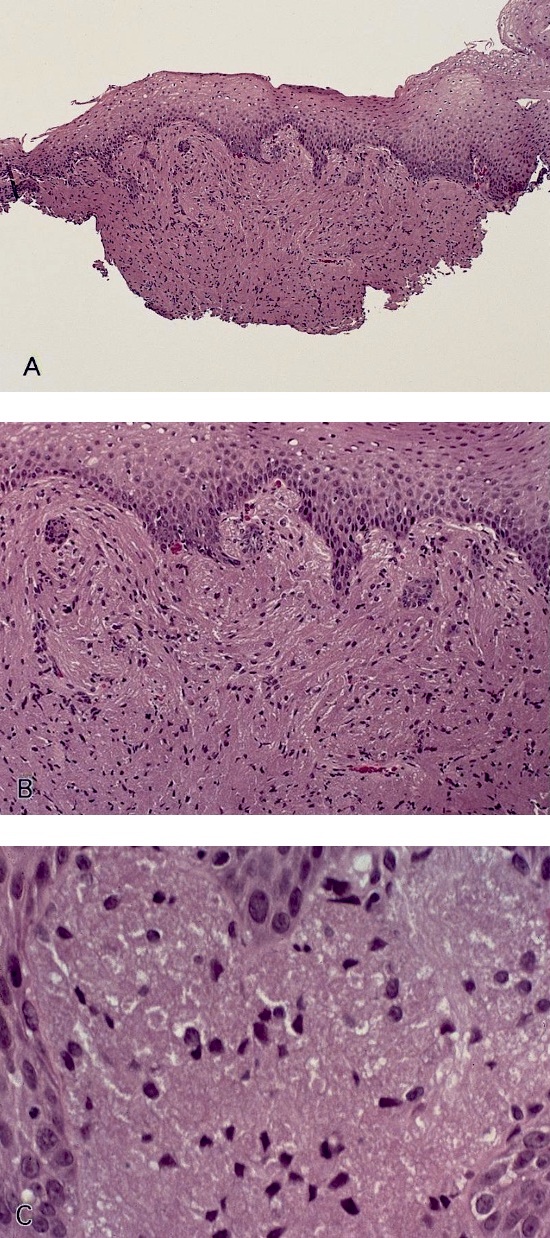
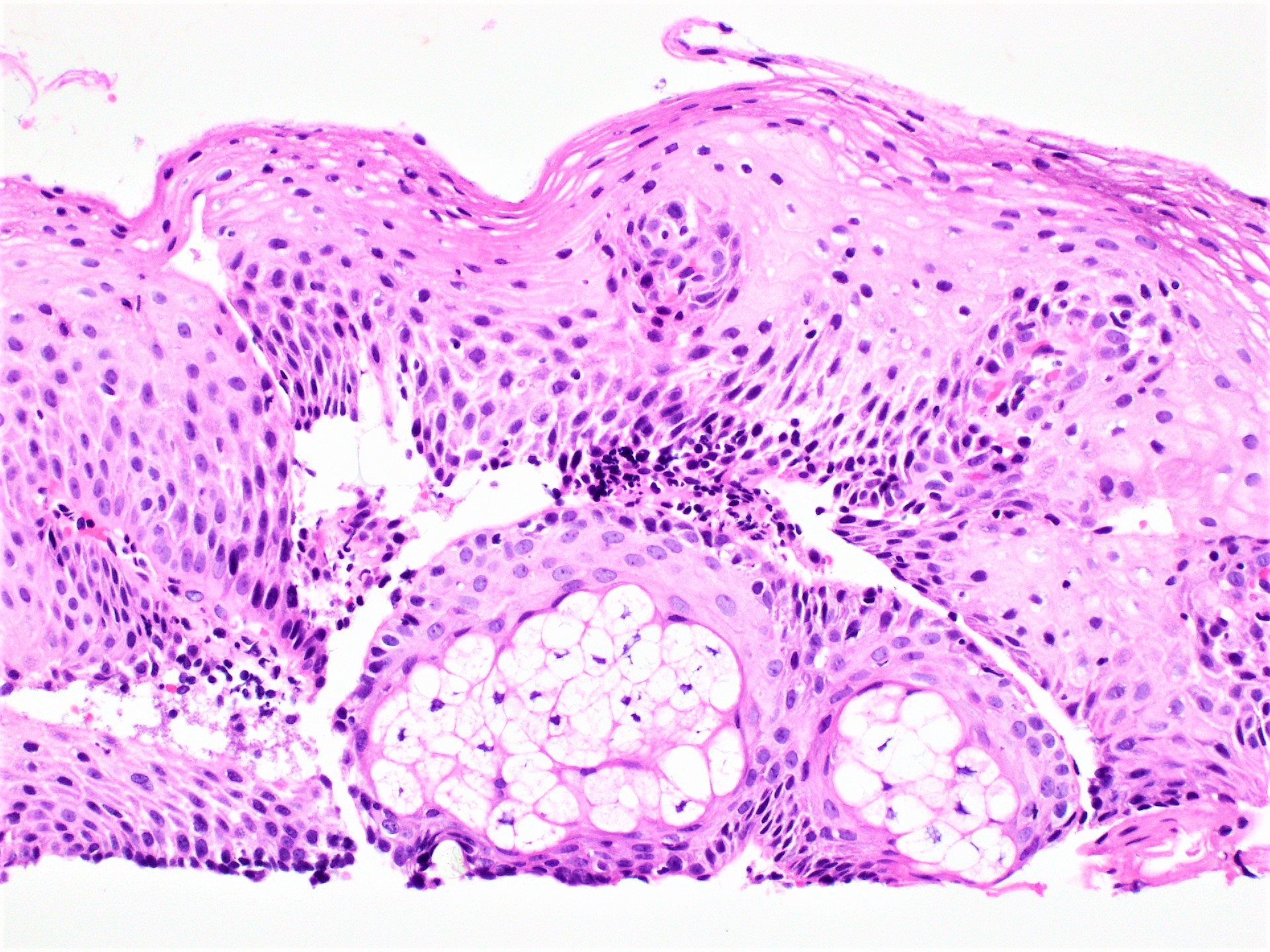
Microscopic (histologic) images
Contributed by Dipti M. Karamchandani, M.D.
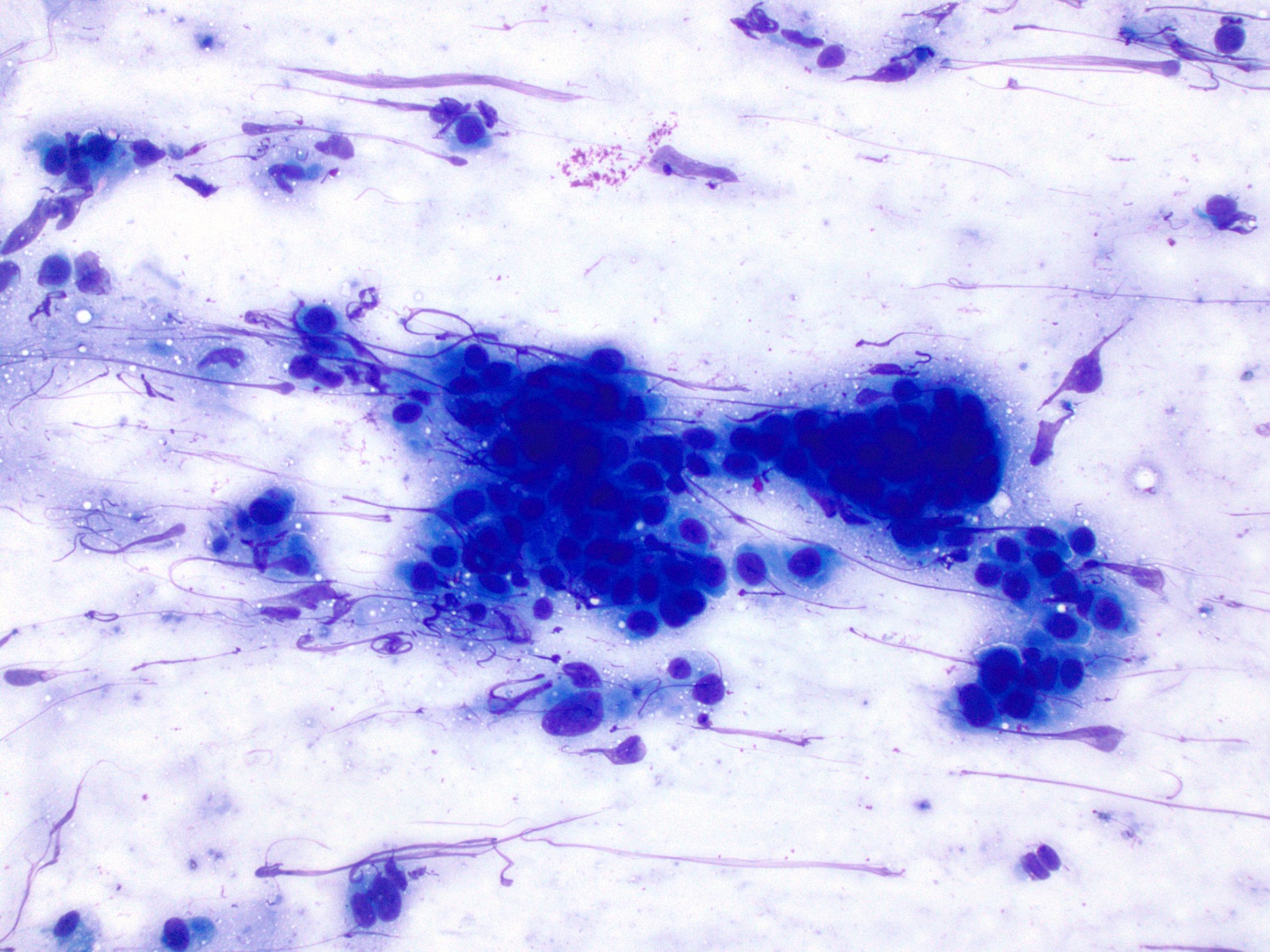
Cytology description
- Used as an adjunct to biopsy in diagnosis of Barrett esophagus and associated neoplasms (Diagn Cytopathol 2003;29:130)
- High degree of diagnostic accuracy of cytology for the diagnosis of Barrett associated high grade dysplasia, with reported sensitivity of 82% and specificity of 95% (Diagn Cytopathol 2003;29:130)
- Observed sensitivity for low grade dysplasia is low (about 31%)
- Cytologically, dysplasia shows haphazard arrangement of cells, nuclear enlargement, nuclear hyperchromasia, nuclear membrane irregularity
Positive stains
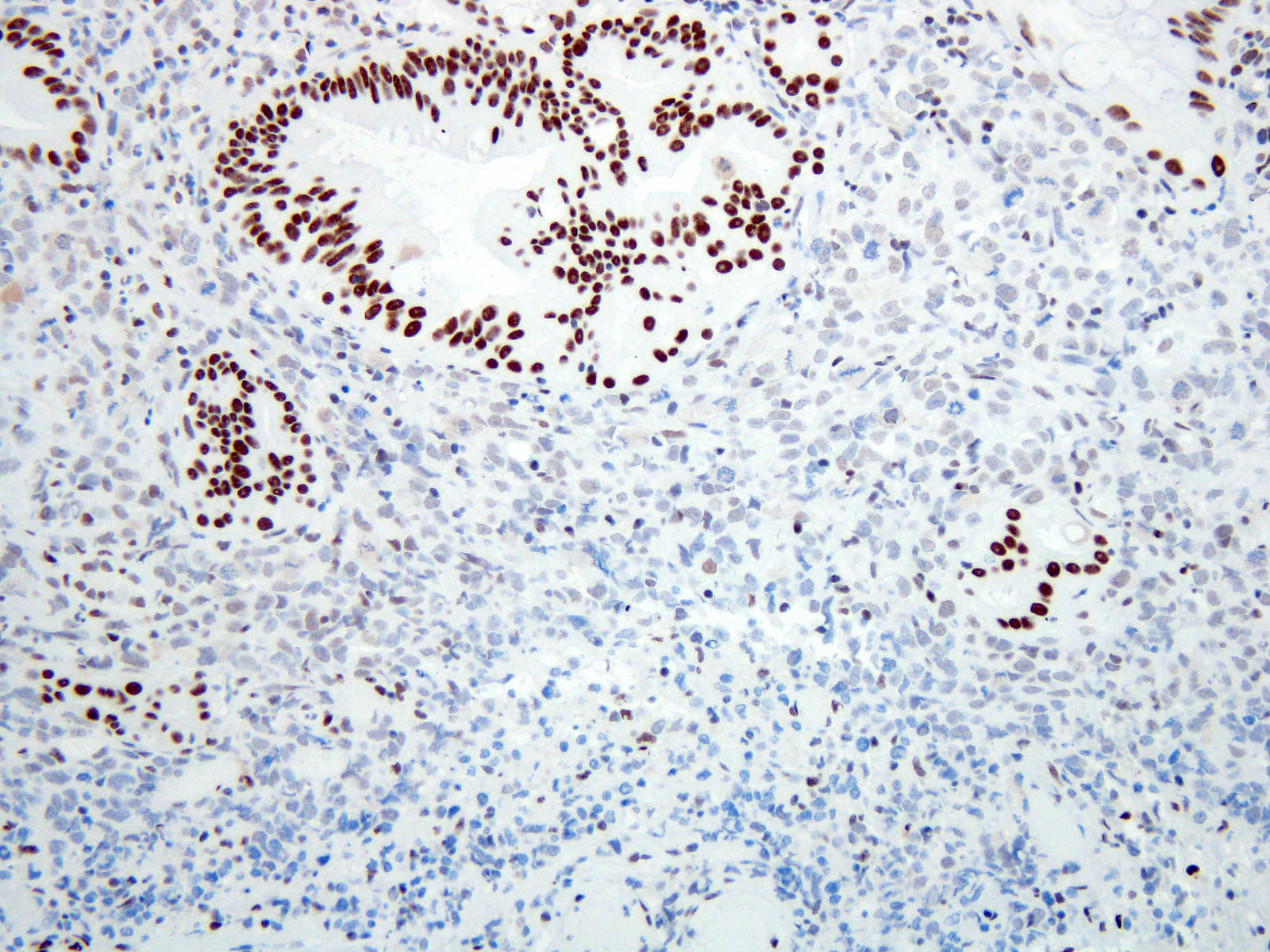
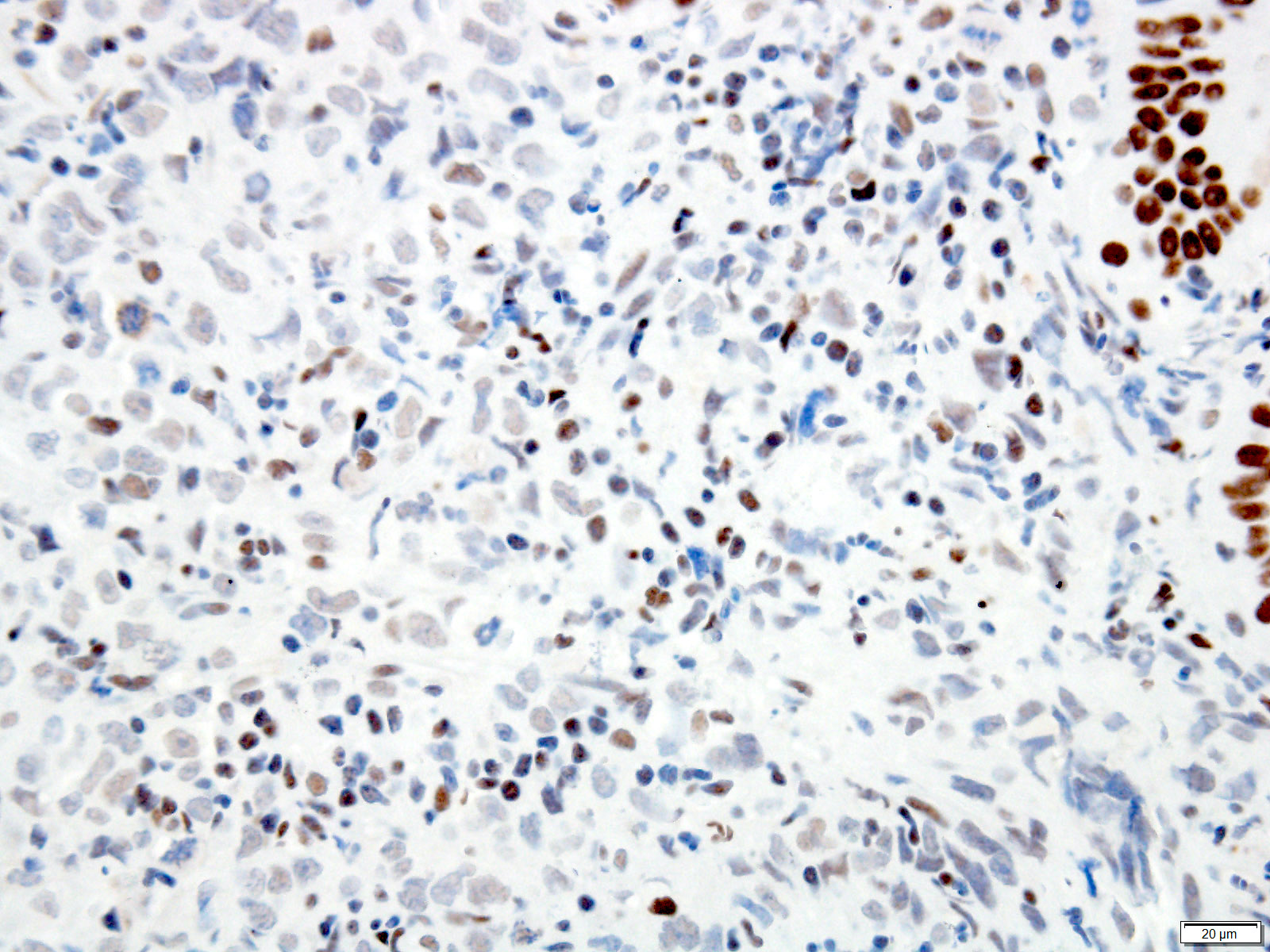
- Ancillary stains (such as AMACR and p53) have been studied for diagnosing Barrett related dysplasia; neither proved sufficiently sensitive or specific to be clinically useful in detection and classification of Barrett related dysplasia (Am J Surg Pathol 2016;40:e83, Am J Surg Pathol 2017;41:e8, Am J Surg Pathol 2016;40:e83)
- Both strong or absent (null phenotype) p53 staining considered significant (Am J Surg Pathol 2016;40:e83, Am J Surg Pathol 2017;41:e8)
- Gastrointestinal Pathology Society (GIPS) recommendation for p53 in diagnosing Barrett esophagus dysplasia:
- Additional studies are needed to develop and validate precise criteria before p53 staining can be fully endorsed and incorporated into the morphologic dysplasia diagnosis algorithm (Am J Surg Pathol 2017;41:e8)
- p53 appears promising marker in predicting disease progression but not recommended for routine use at present
Videos
Update on recently developed quality metrics
Sample pathology report
- Esophagus, 36 cm, biopsy:
- Barrett esophagus with low grade dysplasia
- Esophagus, 35 cm, biopsy:
- Barrett esophagus with high grade dysplasia
- Esophagus, 34 cm, biopsy:
- Barrett esophagus with epithelial alterations indefinite for dysplasia
Differential diagnosis
- Reactive atypia versus low grade foveolar dysplasia:
- Full thickness nuclear atypia with nonstratified nuclei suggests low grade gastric foveolar type dysplasia
- Reactive atypia is usually limited to upper mucosa
- Indefinite for dysplasia versus reactive atypia:
- Maintenance of 4 lines in a background of inflammation suggests reactive atypia (Surg Pathol Clin 2017;10:781)
- Indefinite for dysplasia versus low grade dysplasia:
- Nuclear changes extending to the surface epithelium in a background of significant inflammation suggests a diagnosis of indefinite for dysplasia
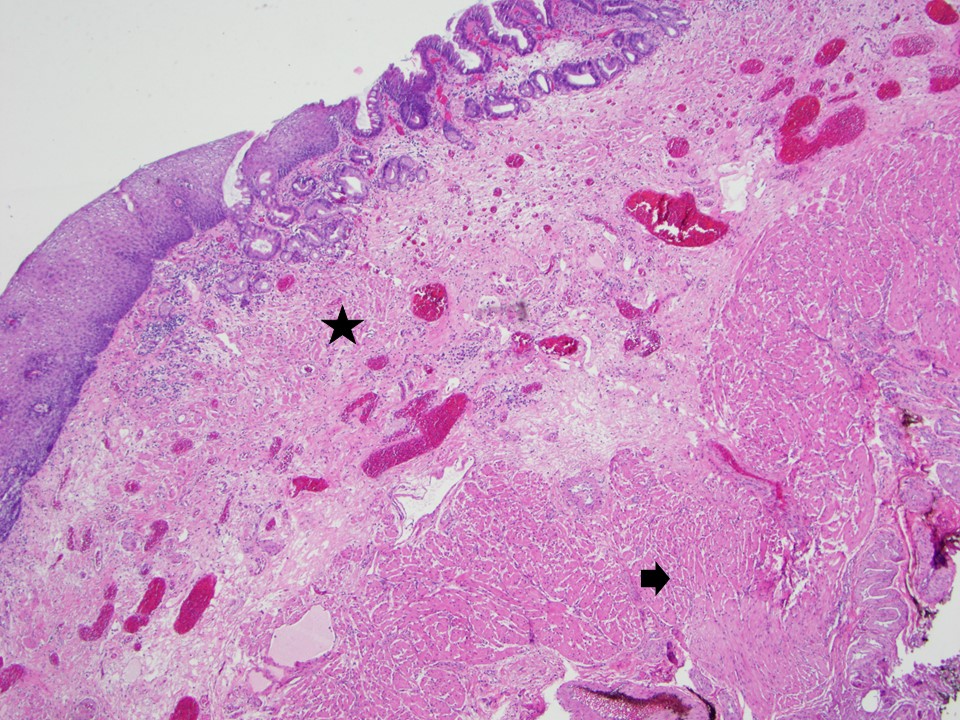
- High grade dysplasia versus intramucosal adenocarcinoma:
- Histologic features of lamina propria invasion (single cells in more than one focus, never ending glandular pattern, solid sheets of cells, significant cribriforming) is diagnostic of intramucosal adenocarcinoma (Am J Gastroenterol 2008;103:2333)
Additional references
Board review style question #1
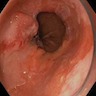
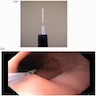
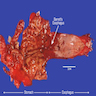
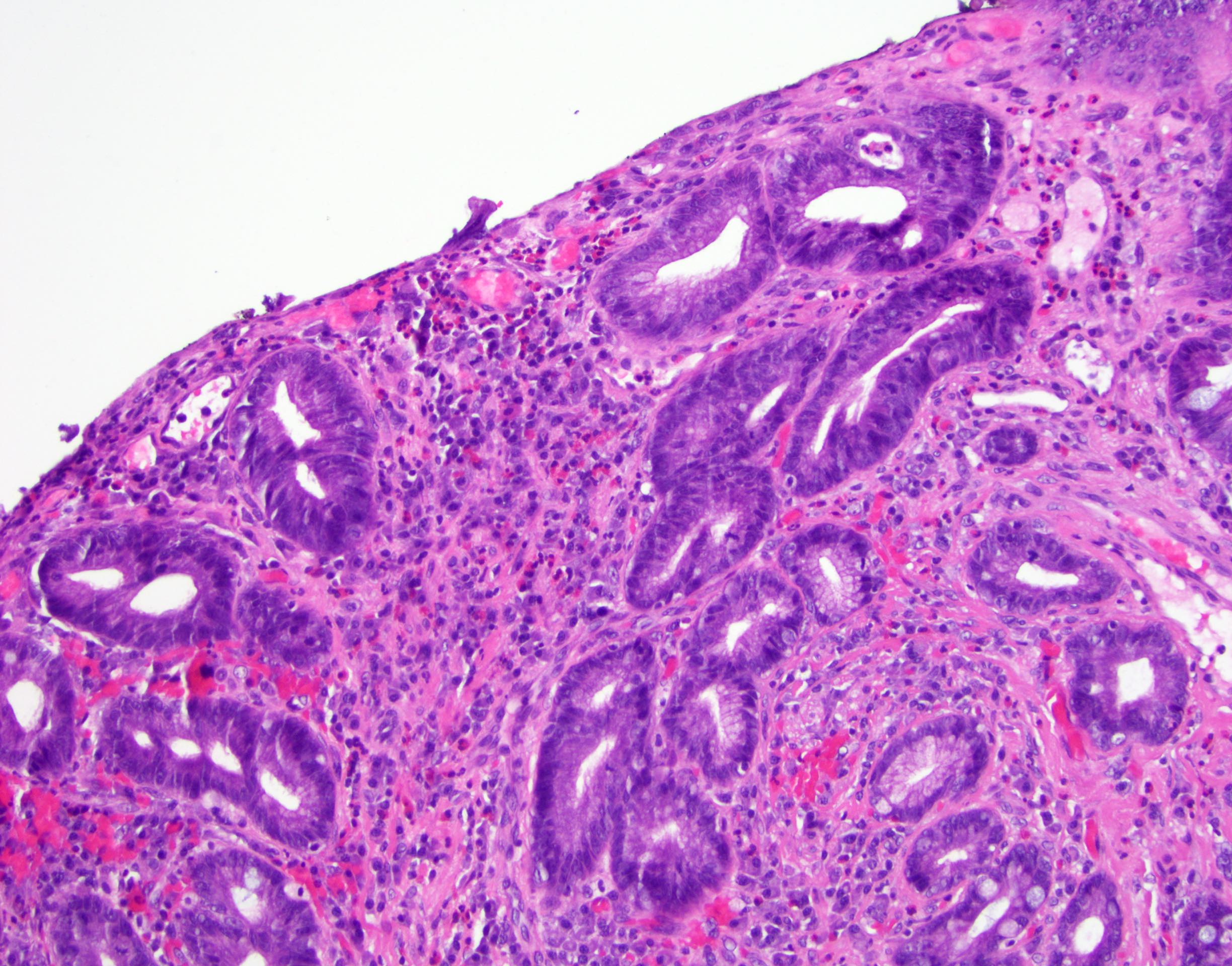
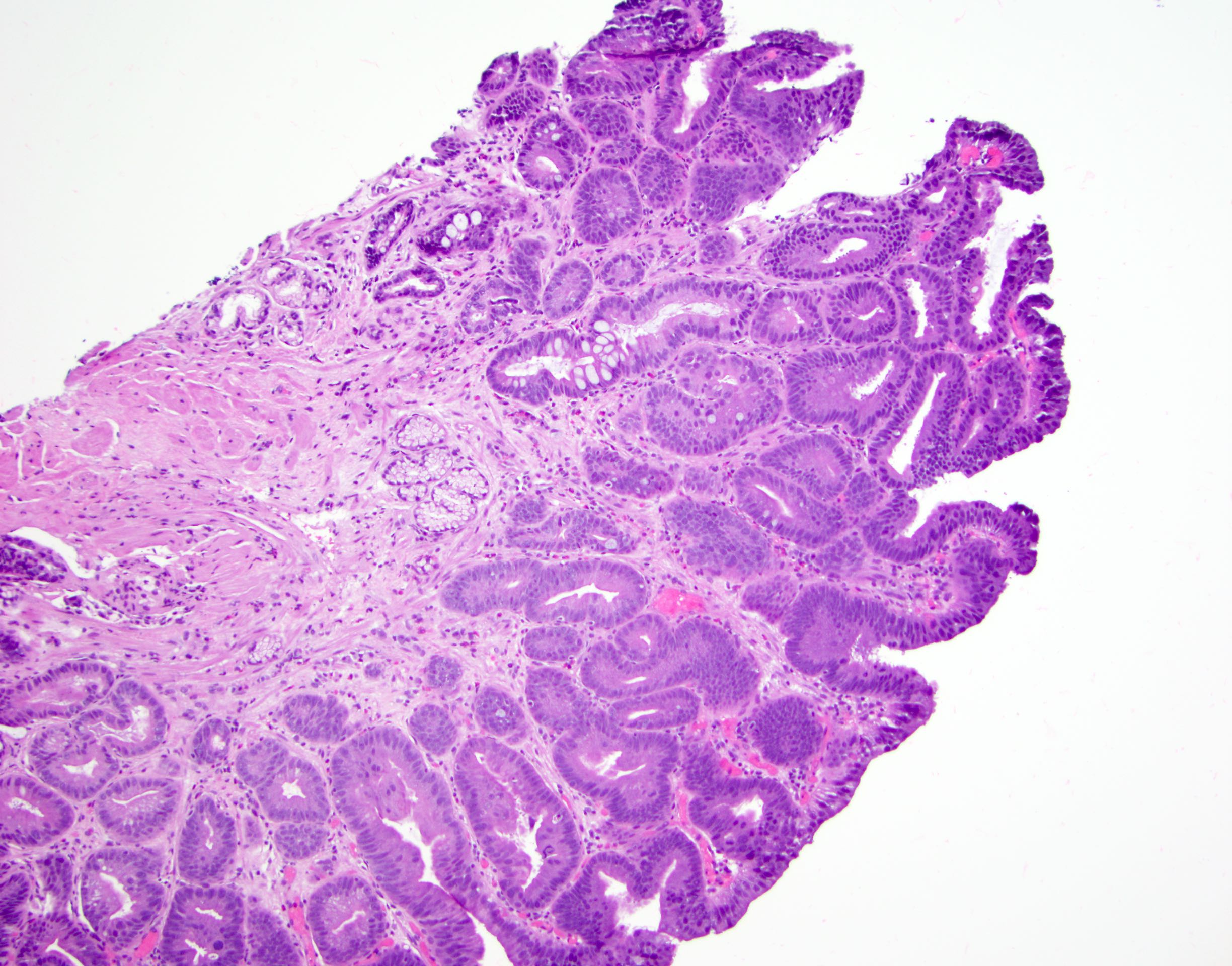
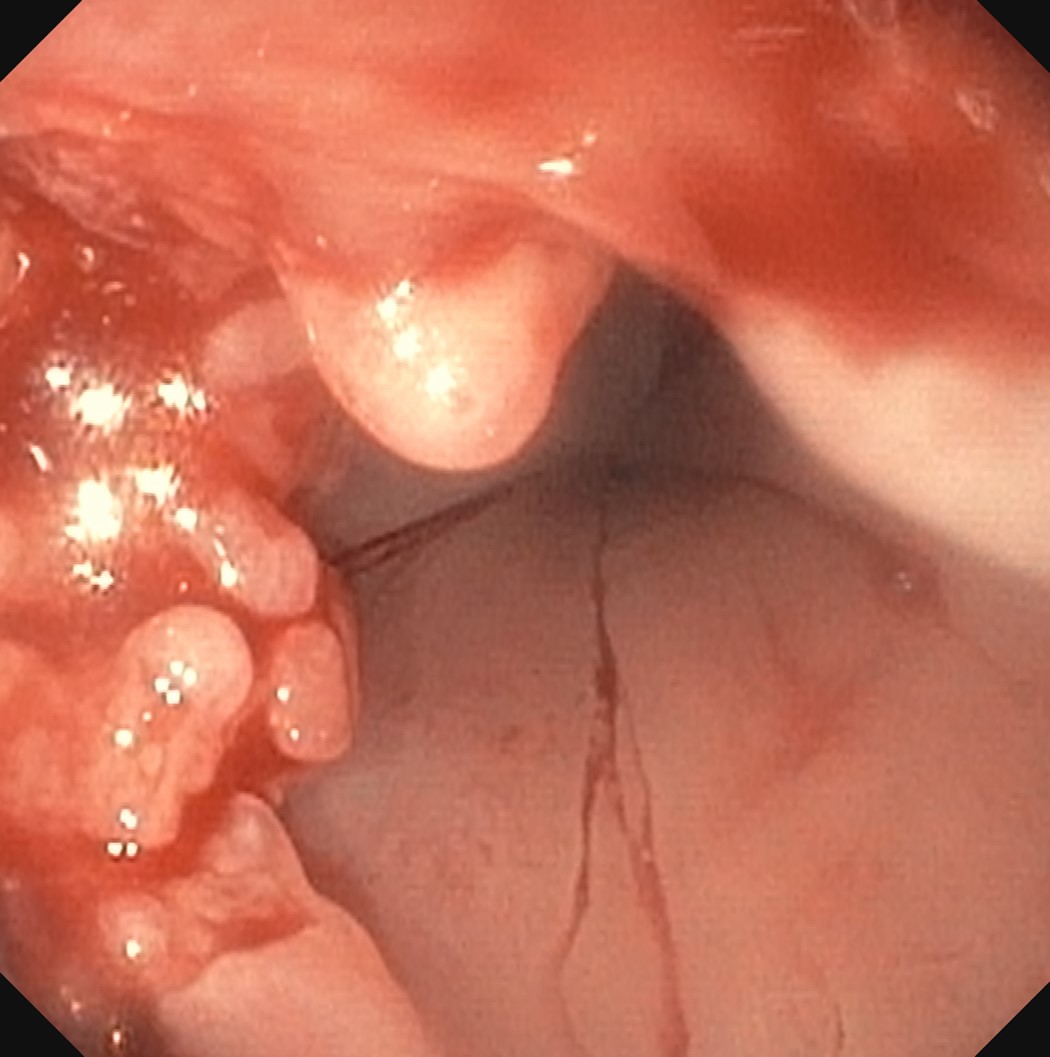
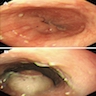
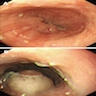
Above is a photomicrograph taken from a biopsy obtained from salmon colored mucosa in the distal esophagus extending to about 2 cm proximal to the gastroesophageal junction. What is your diagnosis?
- Barrett esophagus, negative for dysplasia
- Barrett esophagus with epithelial alterations indefinite for dysplasia
- Barrett esophagus with high grade dysplasia
- Barrett esophagus with low grade dysplasia
Board review style answer #1
Board review style question #2
A nodule is found on surveillance endoscopy of a 60 year old patient with a longstanding history of Barrett esophagus. What should be the next step in patient management?
- Endoscopic mucosal resection of the nodule
- Esophagectomy
- Radiofrequency ablation of the nodule
- Use of use of wide area transepithelial sampling with computer assisted 3 dimensional analysis (WATS)
Board review style answer #2
Board review style question #3
- A biopsy diagnosis of low grade dysplasia was made by a pathologist and confirmed by a subspecialized GI pathologist. What is the preferred appropriate management based on the ACG 2016 guidelines?
- Endoscopic ablation therapy
- Endoscopic mucosal resection
- Endoscopic surveillance at 6 months interval
- Endoscopic surveillance at 12 months interval
Board review style answer #3
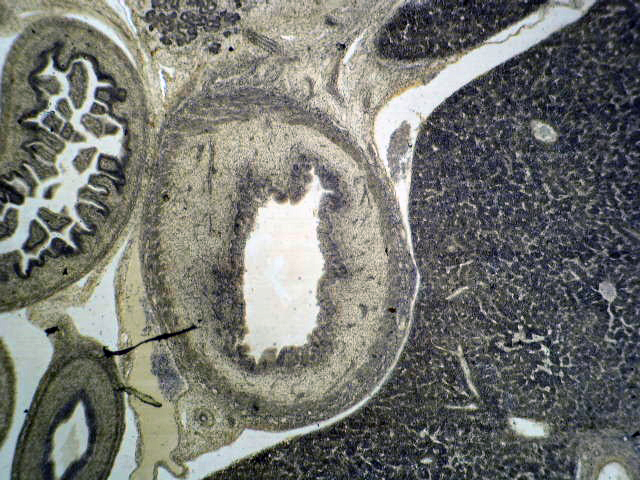
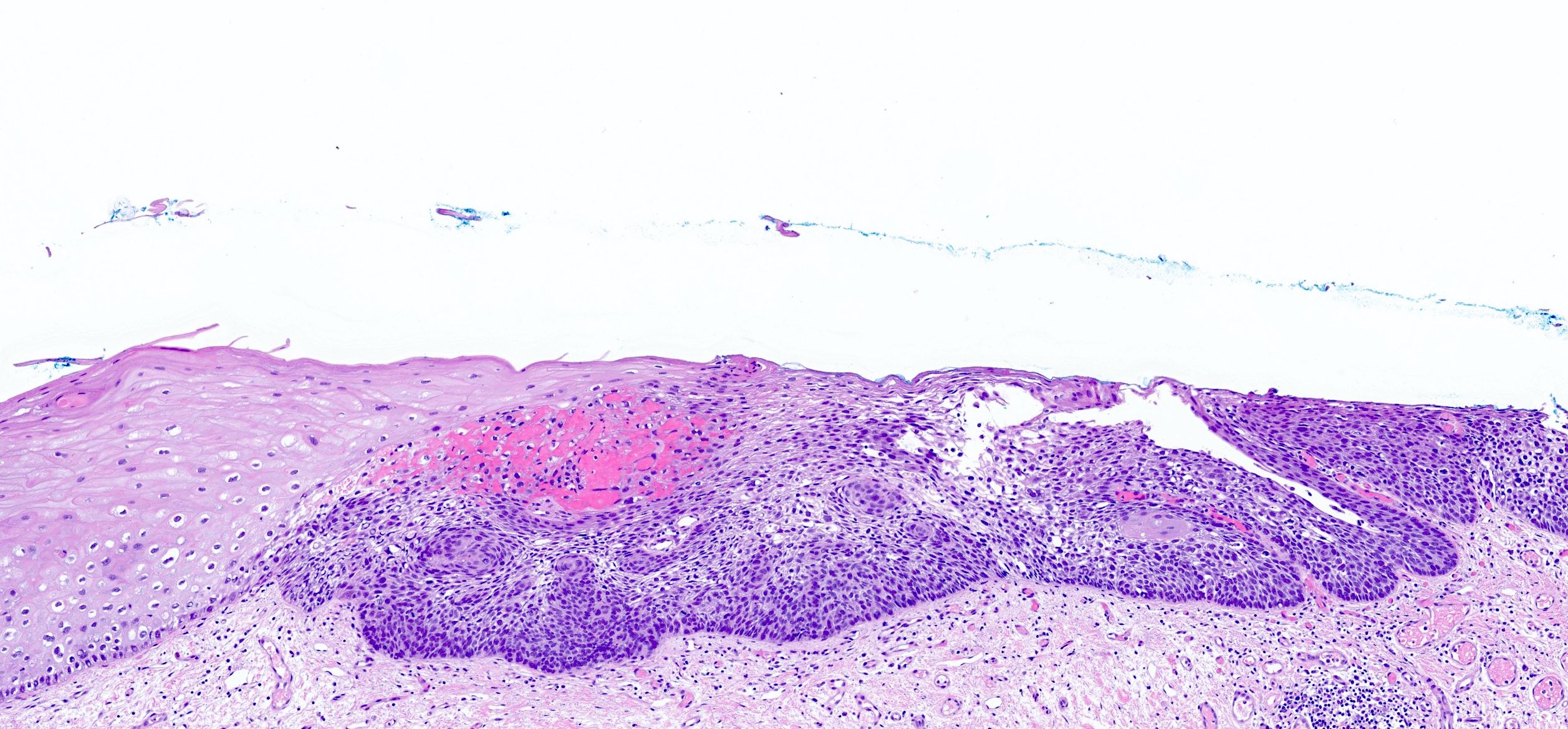
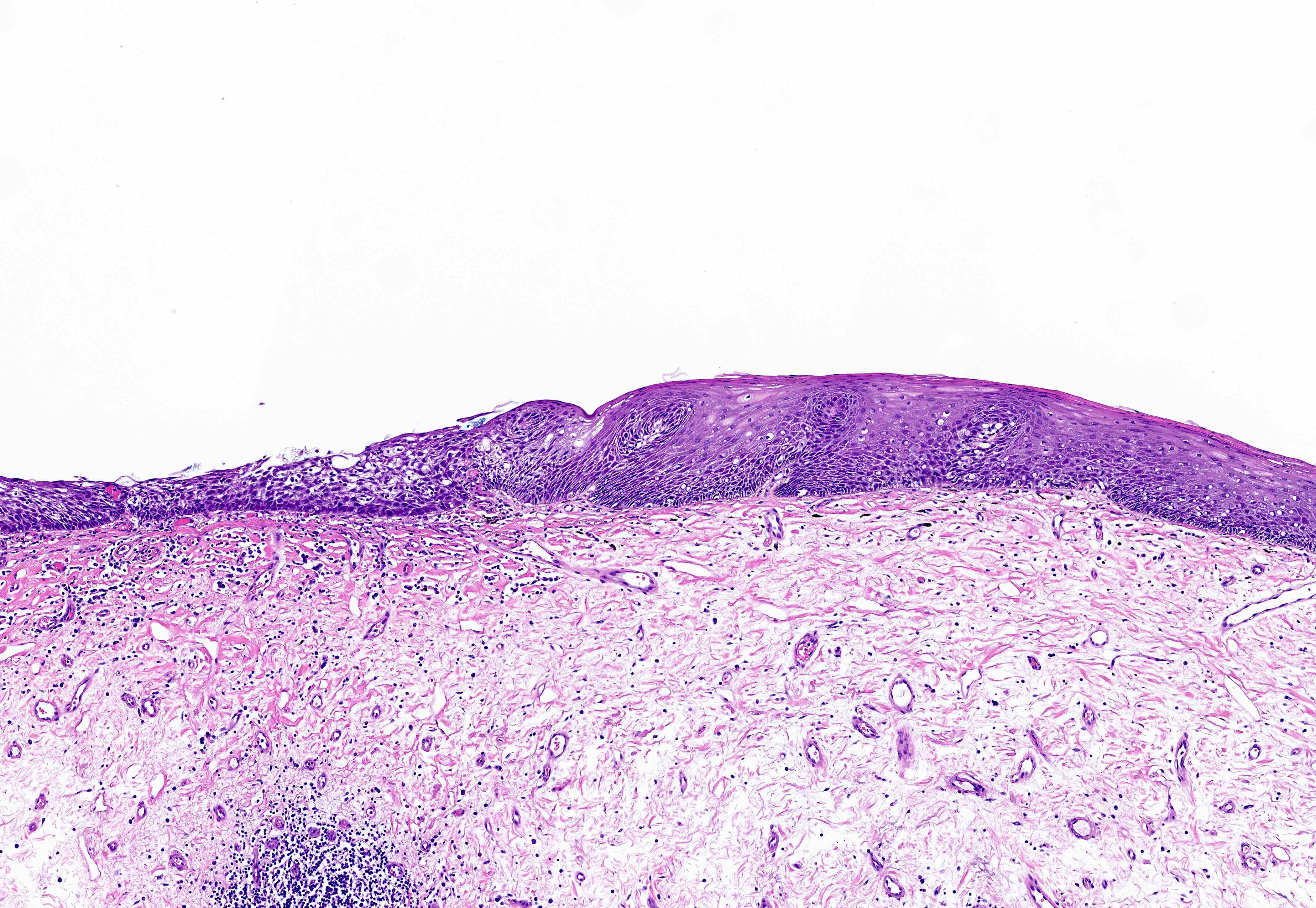
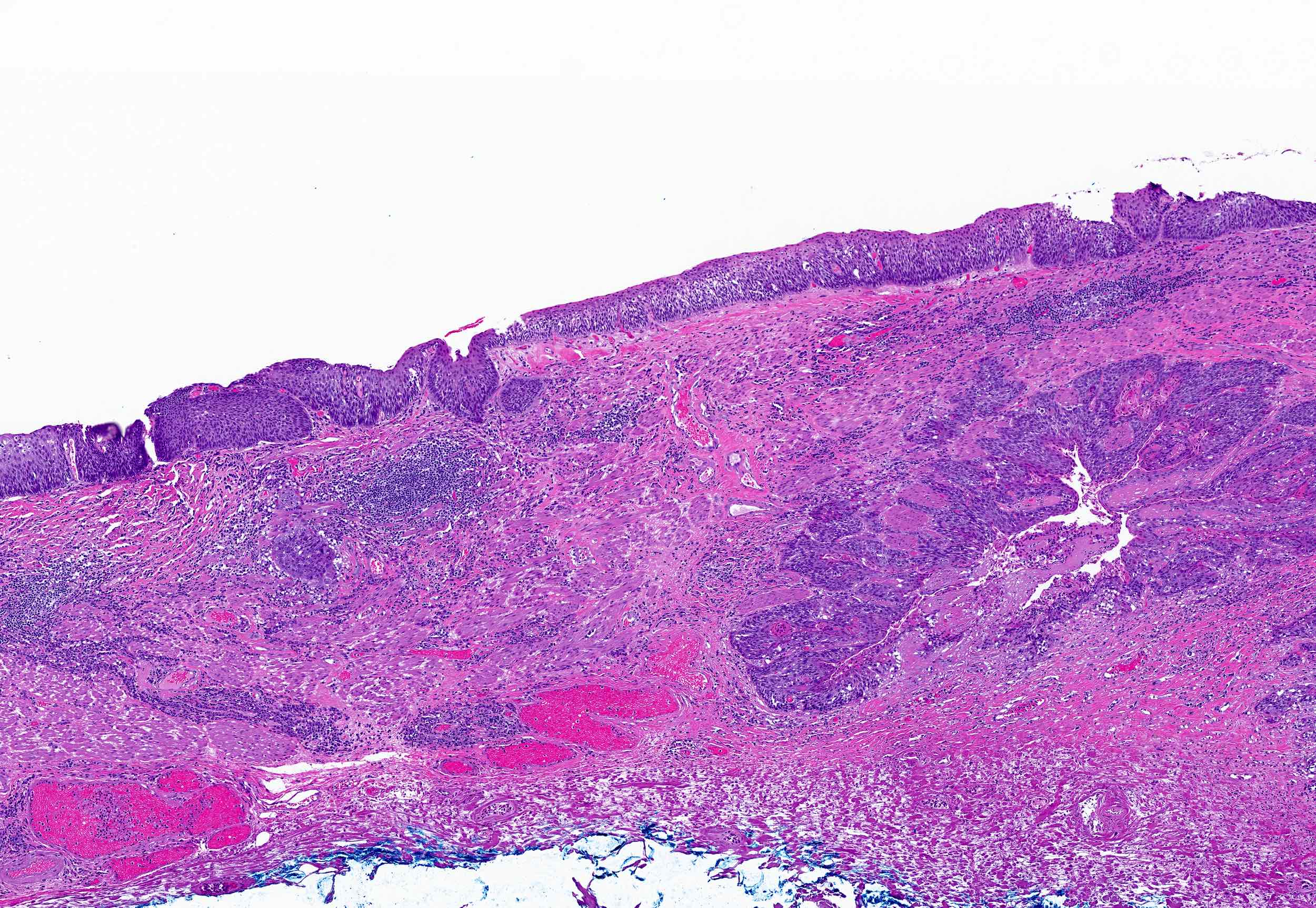
Basaloid squamous cell carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Variant of squamous cell carcinoma with distinct basaloid morphology
Essential features
- Esophageal basaloid squamous cell carcinoma (BSCC) is a rare variant of SCC
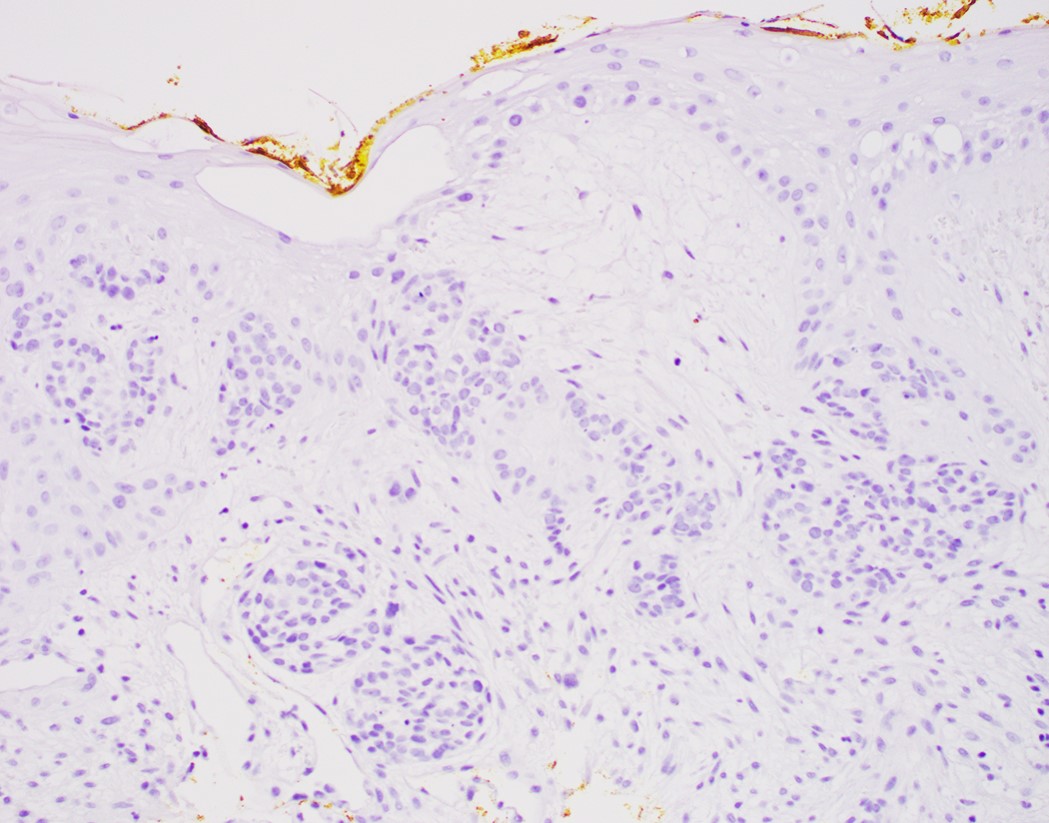
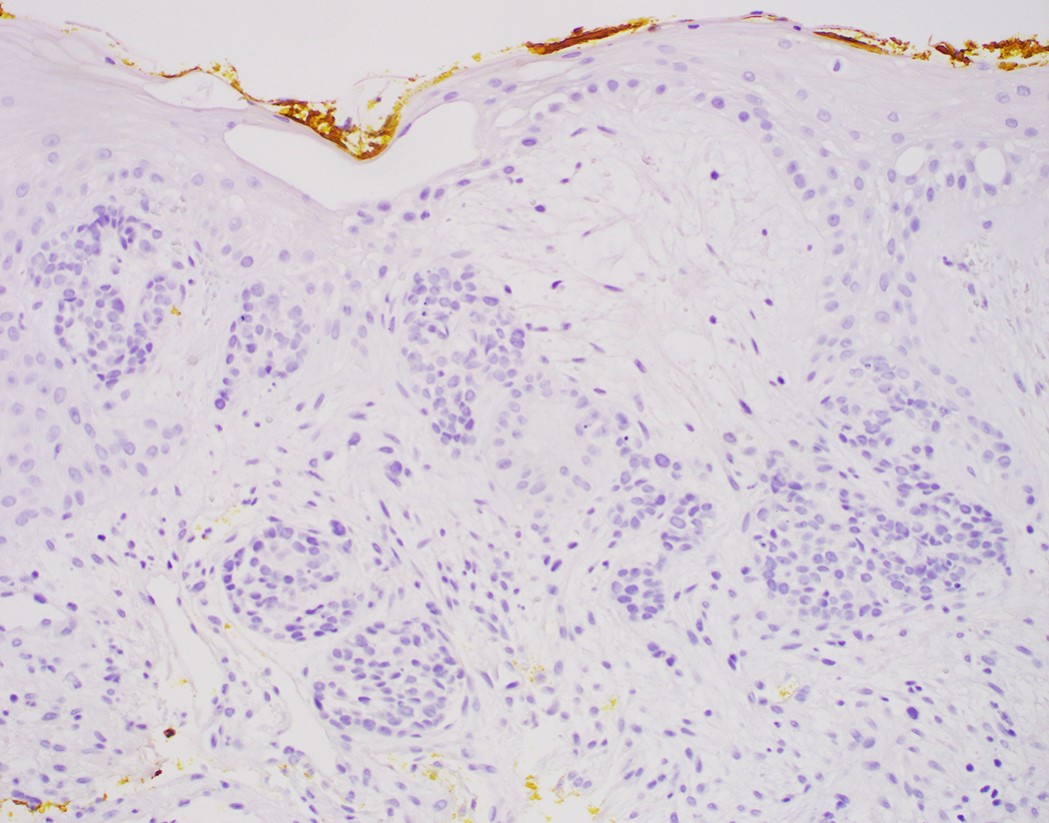
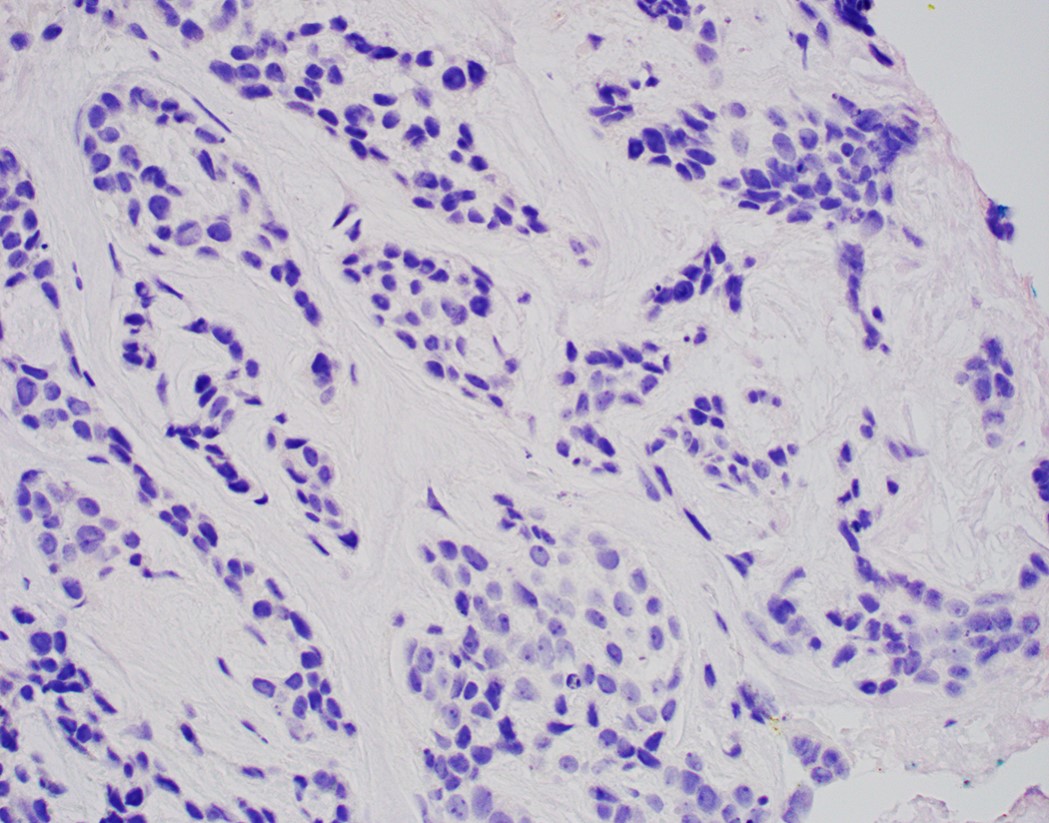
- Morphological differential diagnoses could include adenoid cystic carcinoma, neuroendocrine carcinoma (particularly small cell type), carcinosarcoma and epithelioid sarcoma
- BSCC is not human papillomavirus (HPV) related and has a relatively poor prognosis as compared to morphologically similar HPV related SCC
ICD coding
- ICD-10: C15.9 - malignant neoplasm of esophagus, unspecified
Epidemiology
- Generally older males
- Reported incidence: 1 - 11% of squamous cell carcinomas; true incidence likely ~2%
Sites
- More common in mid to distal esophagus
Pathophysiology
- Similar molecular pathology and etiology as squamous cell carcinoma (see Molecular / cytogenetics description below)
- No association with HPV (Am J Surg Pathol 2009;33:1608)
Etiology
- Genetic and environment
Clinical features
- Patients present with dysphagia and weight loss
- Usually widespread metastases at presentation
Diagnosis
- Endoscopic biopsy
Radiology description
- Positive for esophageal wall thickening or mass on CT scans
- Positive for high metabolism in PET / CT
Prognostic factors
- Very poor prognosis in some cases (J Cancer Res Clin Oncol 2012;138:1165)
- Even though more likely to be poorly differentiated at presentation, BSCC of the esophagus could have similar clinical features and survival outcomes when compared with SCC (J Am Coll Surg 2018;226:1086, Ann Surg Oncol 2015;22:3659)
- Patients with BSCC and SCC should undergo stage specific treatment to achieve optimal outcomes (J Am Coll Surg 2018;226:1086)
- Better prognosis that occurs with HPV associated BSCC of upper aerodigestive tract is different from BSCC of esophagus
Case reports
- 64 year old man with neuroendocrine and glandular differentiation (Clin J Gastroenterol 2021;14:32)
- 68 year old woman with lung metastasis of esophageal BSCC (Surg Case Rep 2020;6:199)
- 87 year old man with an esophageal subepithelial lesion (Clin J Gastroenterol 2021;14:1324)
Treatment
- Same as conventional squamous cell carcinoma
- Often unresectable due to advanced stage
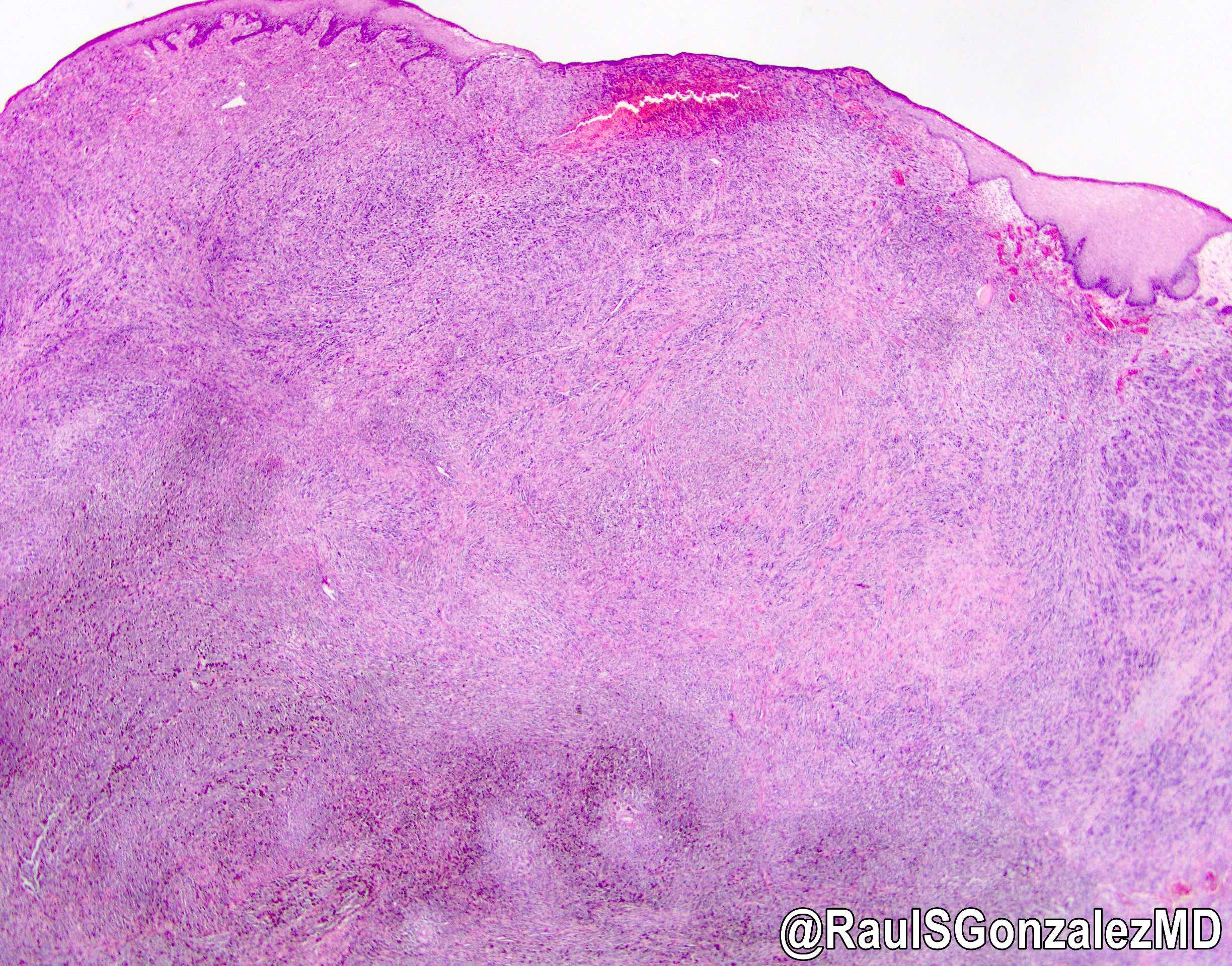
Gross description
- Generally deeply invasive, large bulky ulcerated fungating masses
Frozen section description
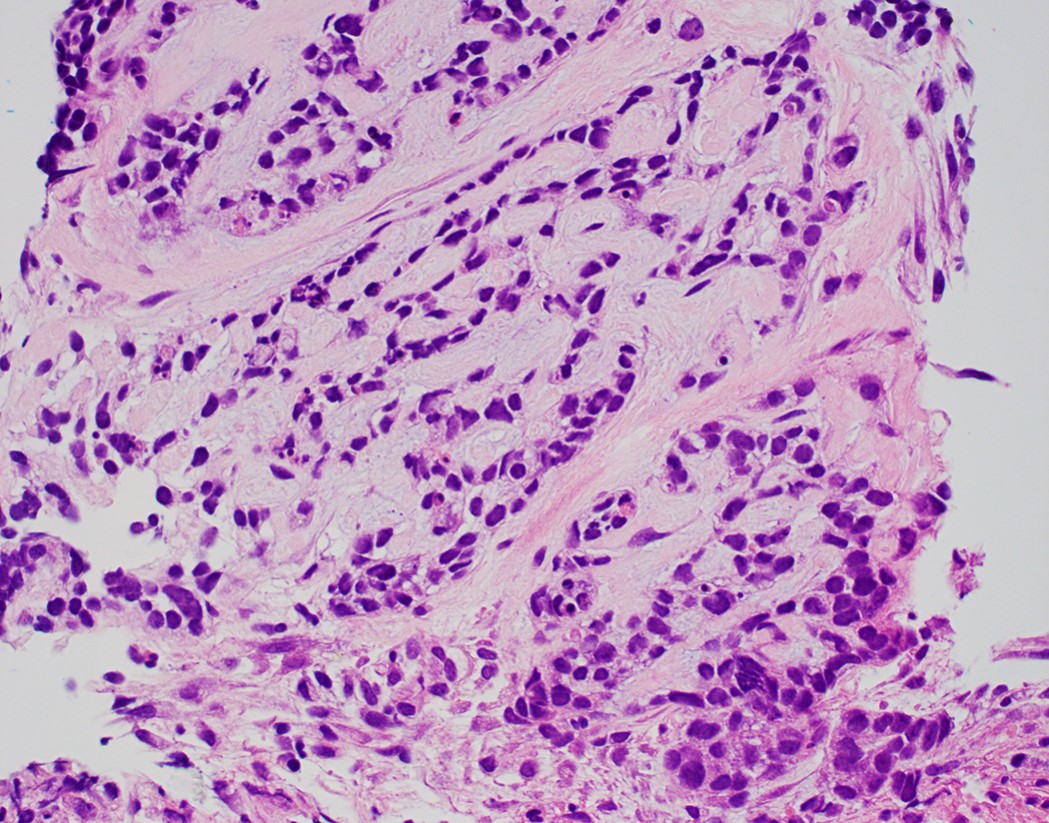
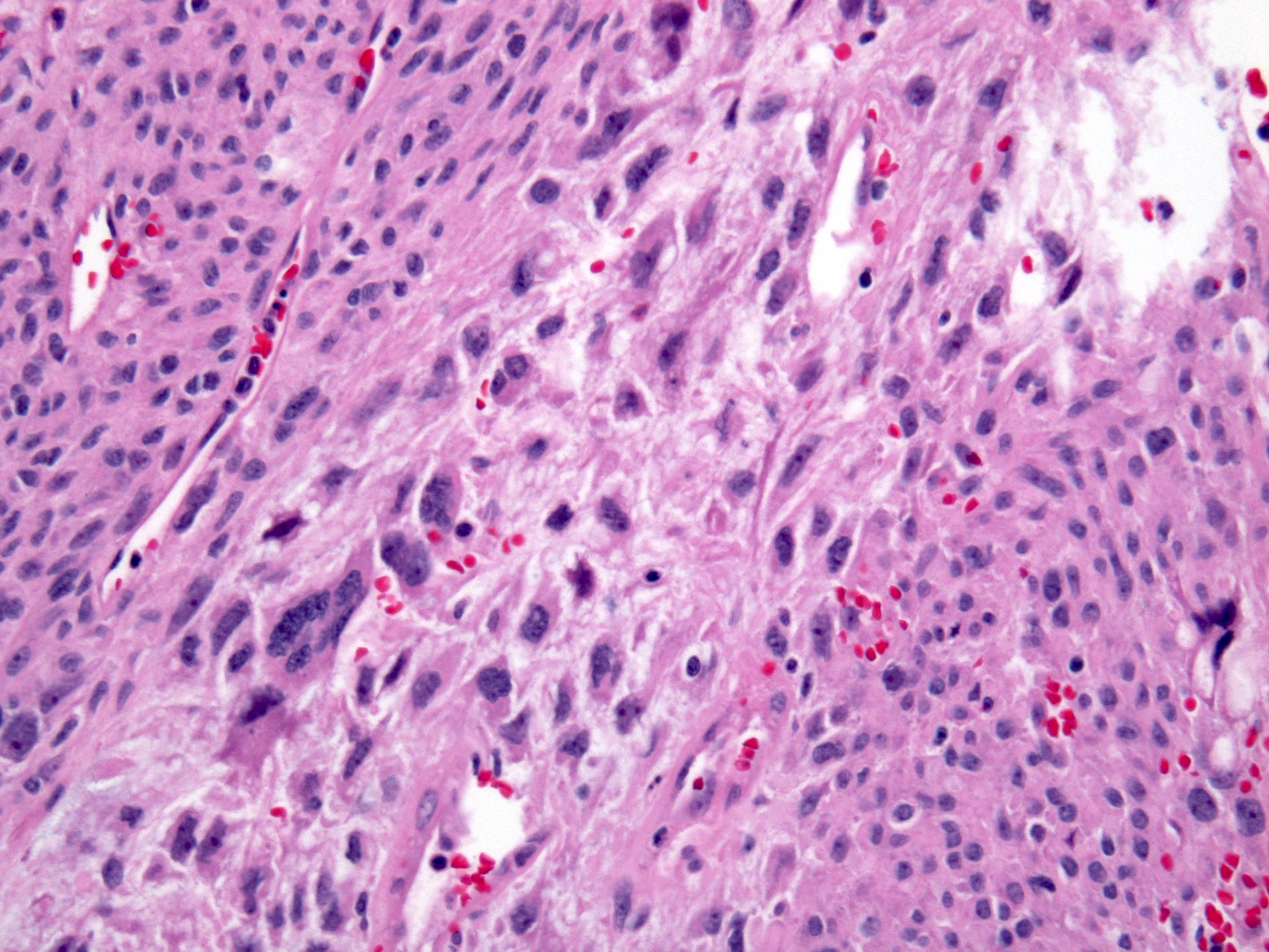
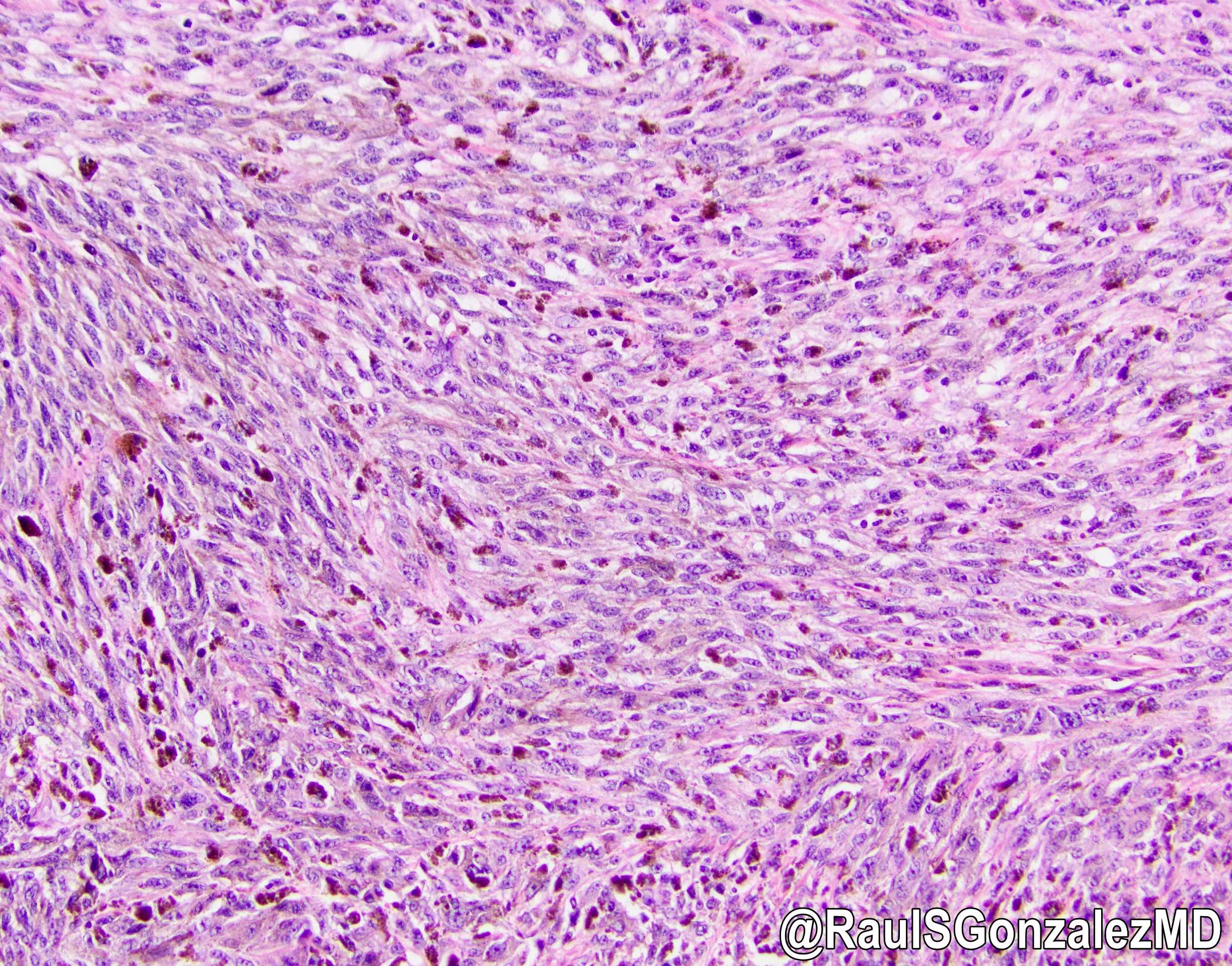
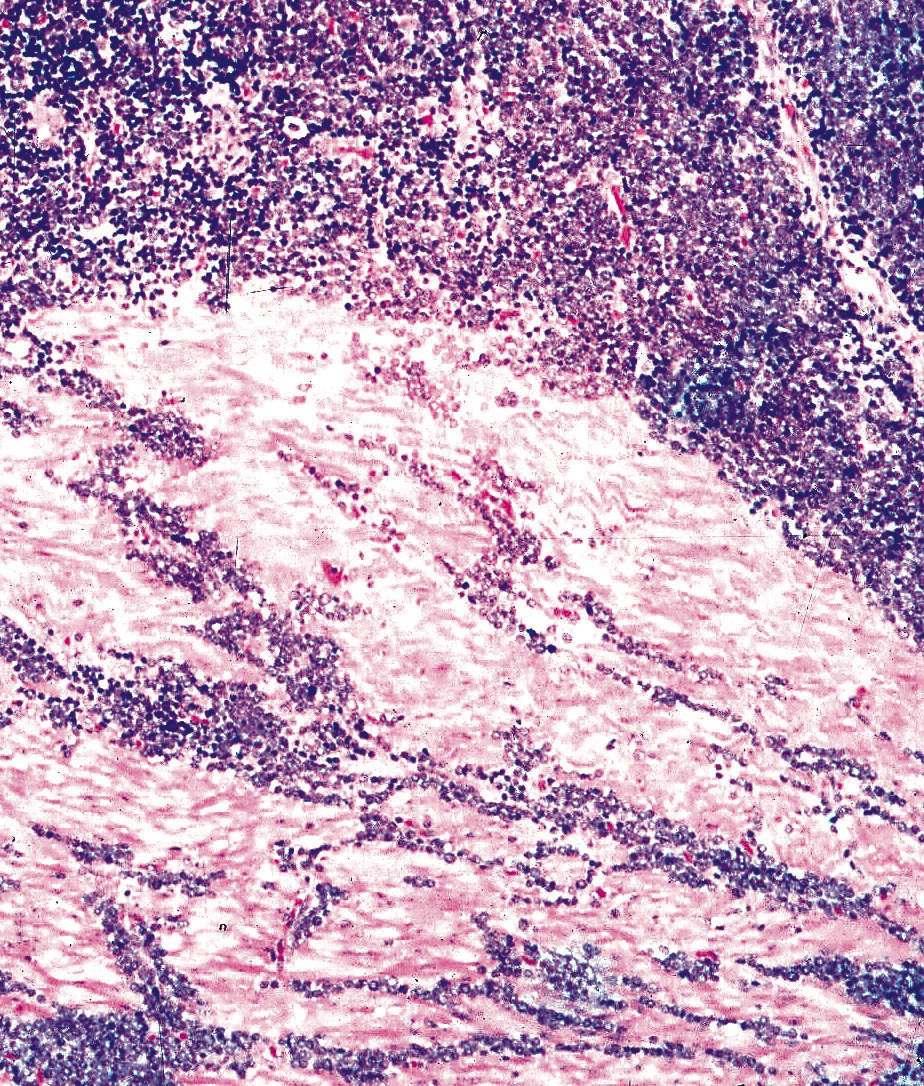
- Tumor cells show nests, trabeculae, cords and cribriform pattern infiltrating the myxoid stroma
- Morphologically it could mimic adenoid cystic carcinoma
Frozen section images
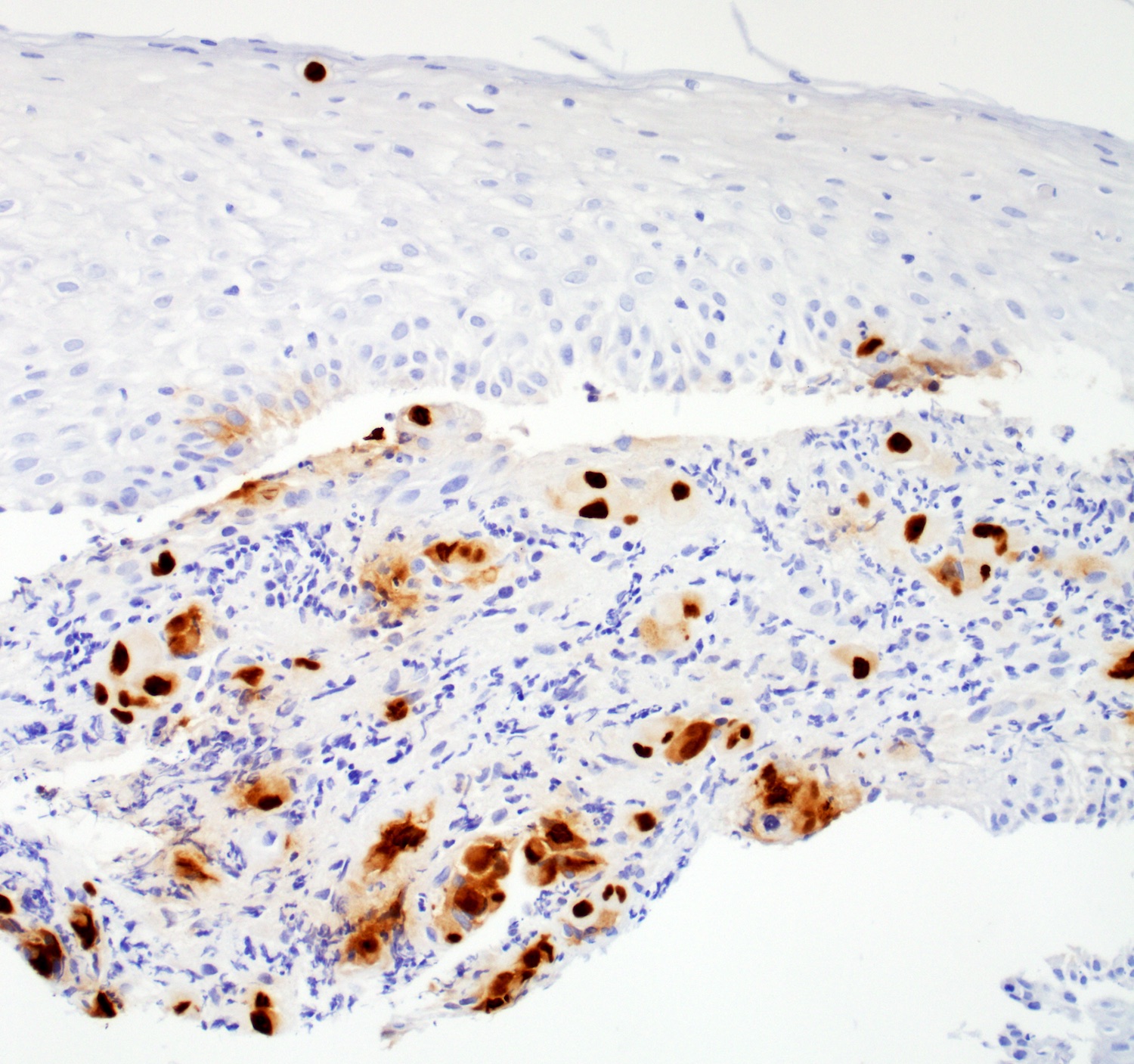
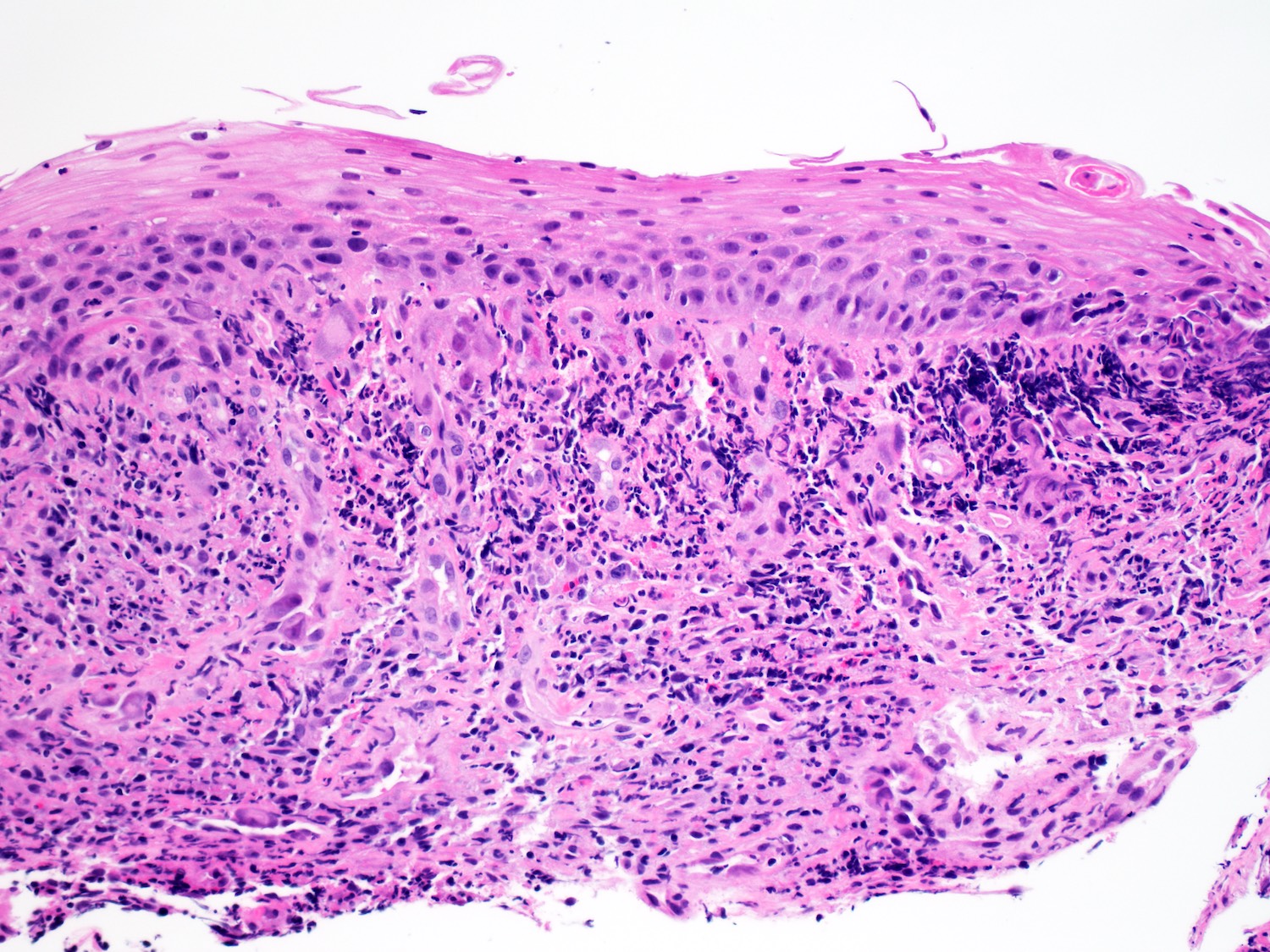
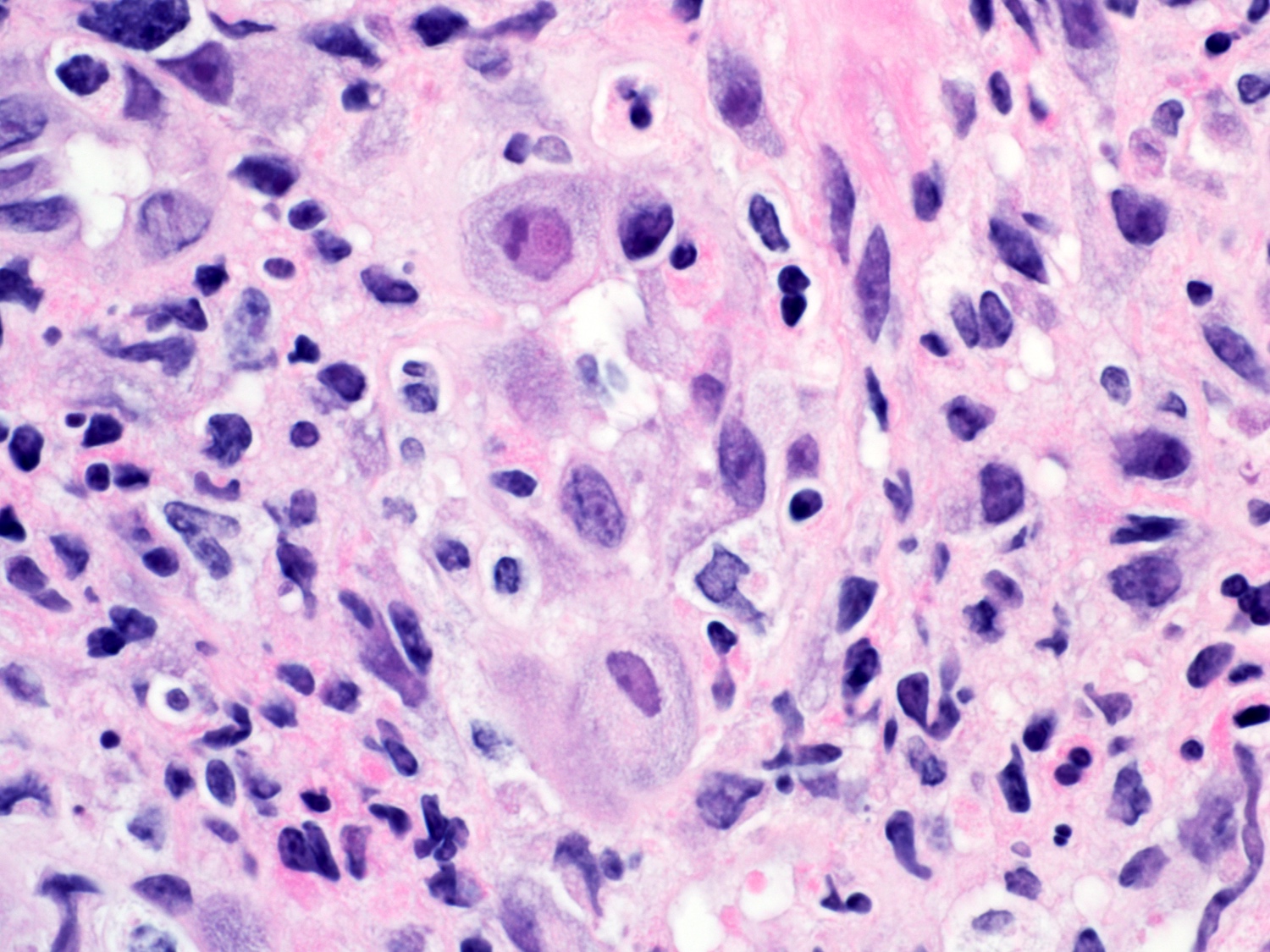
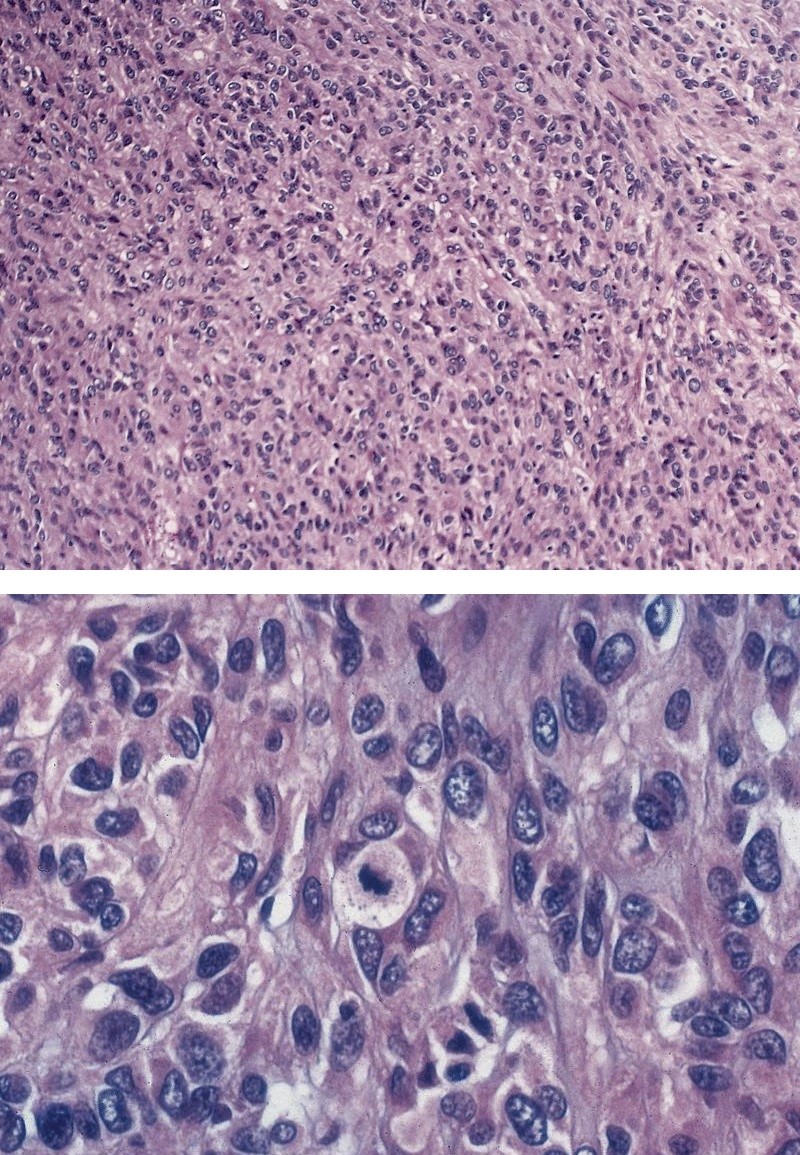
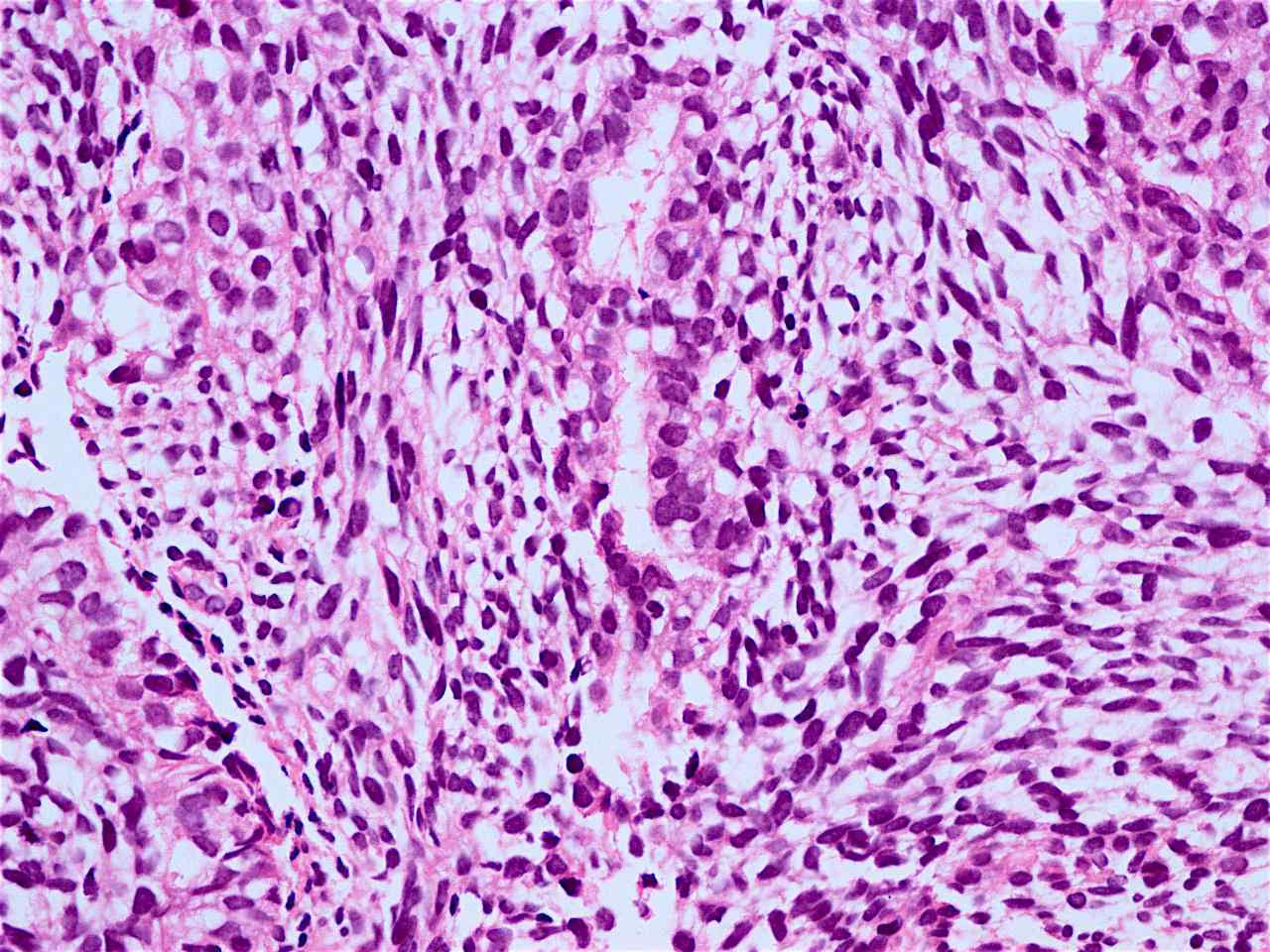
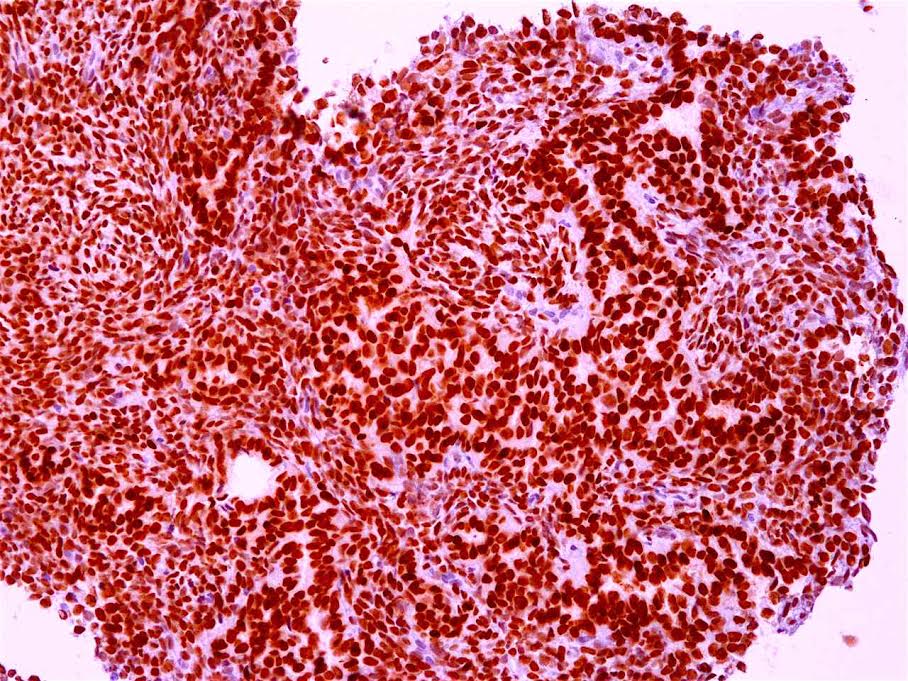
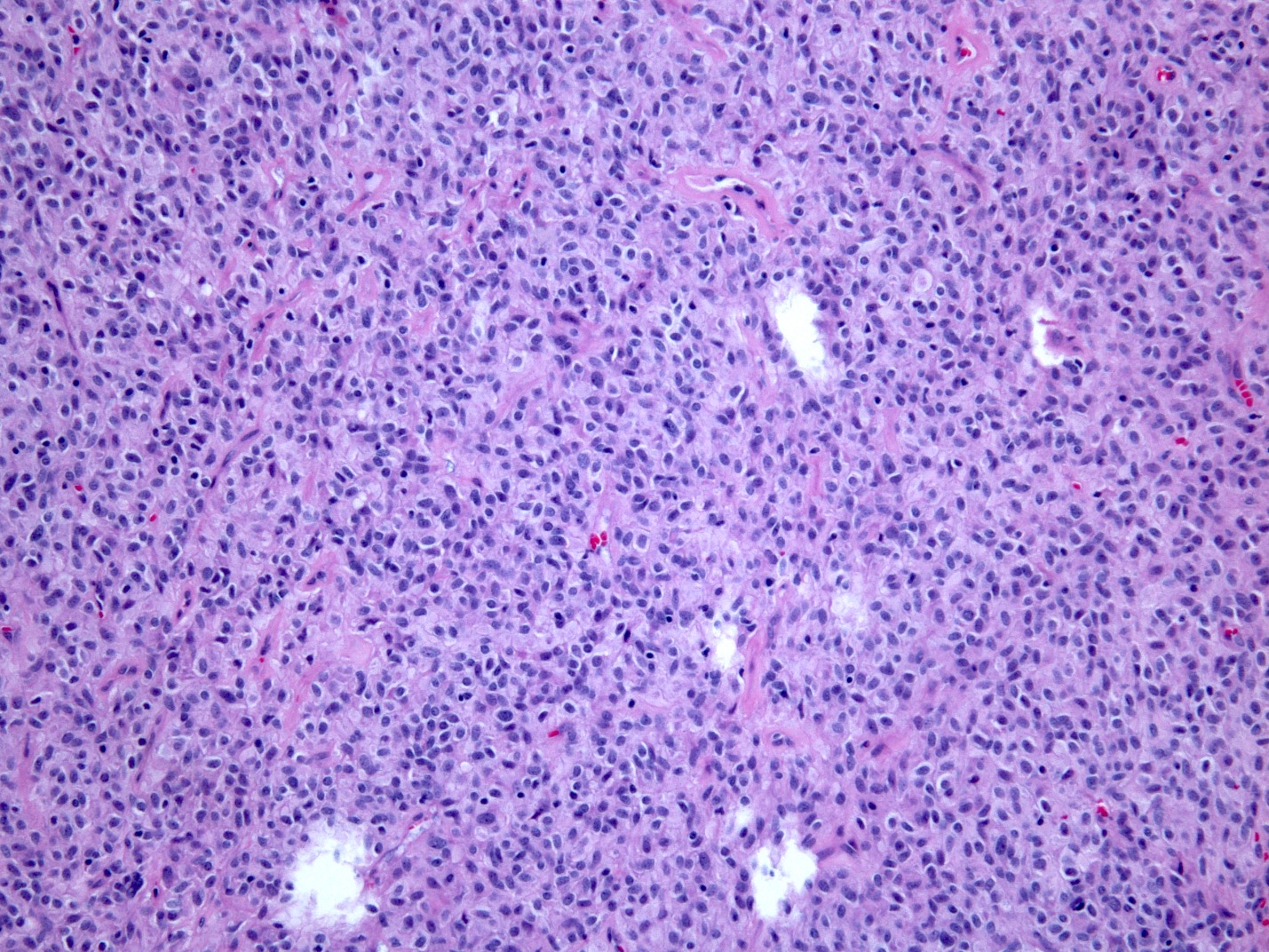
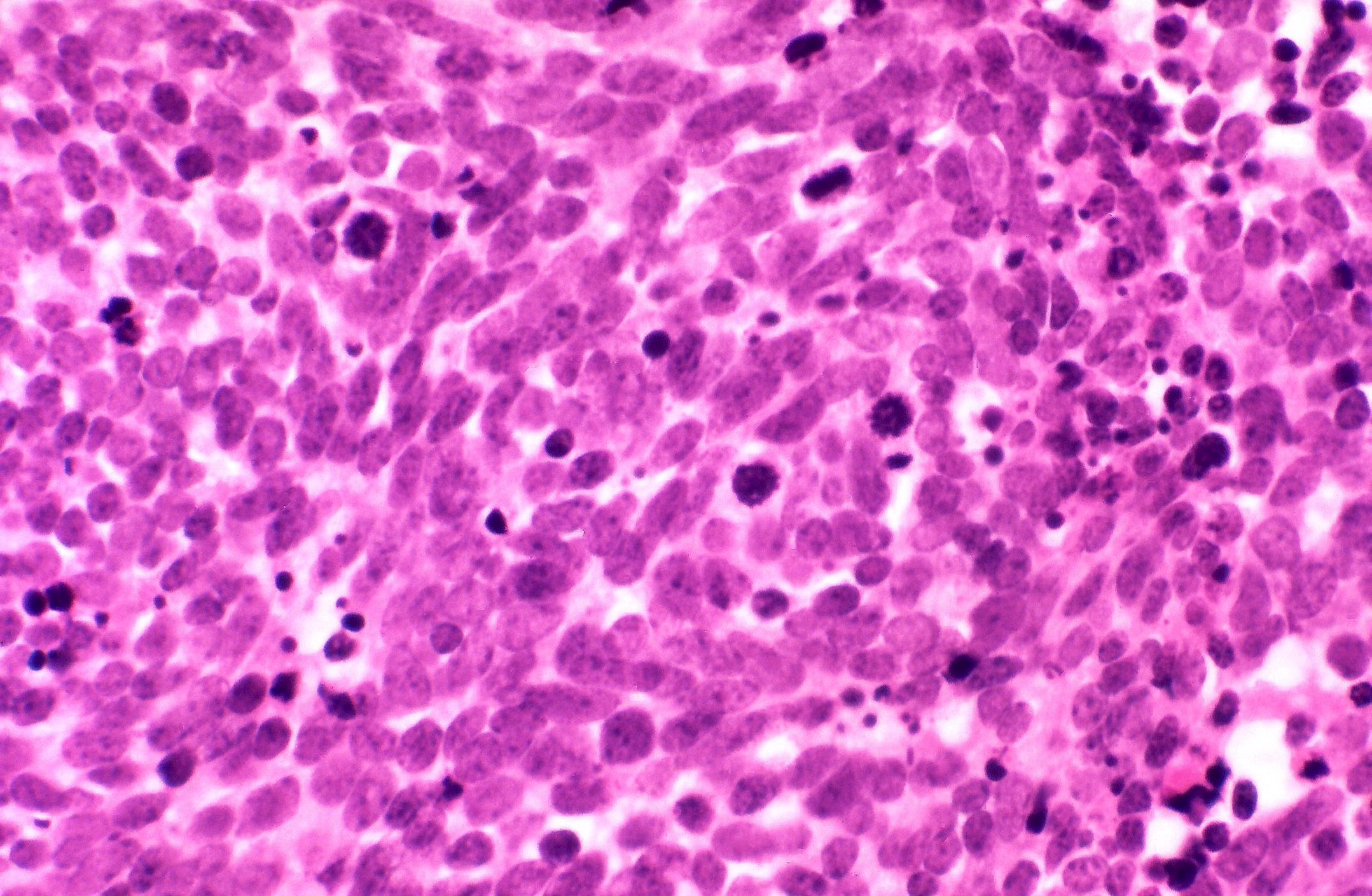
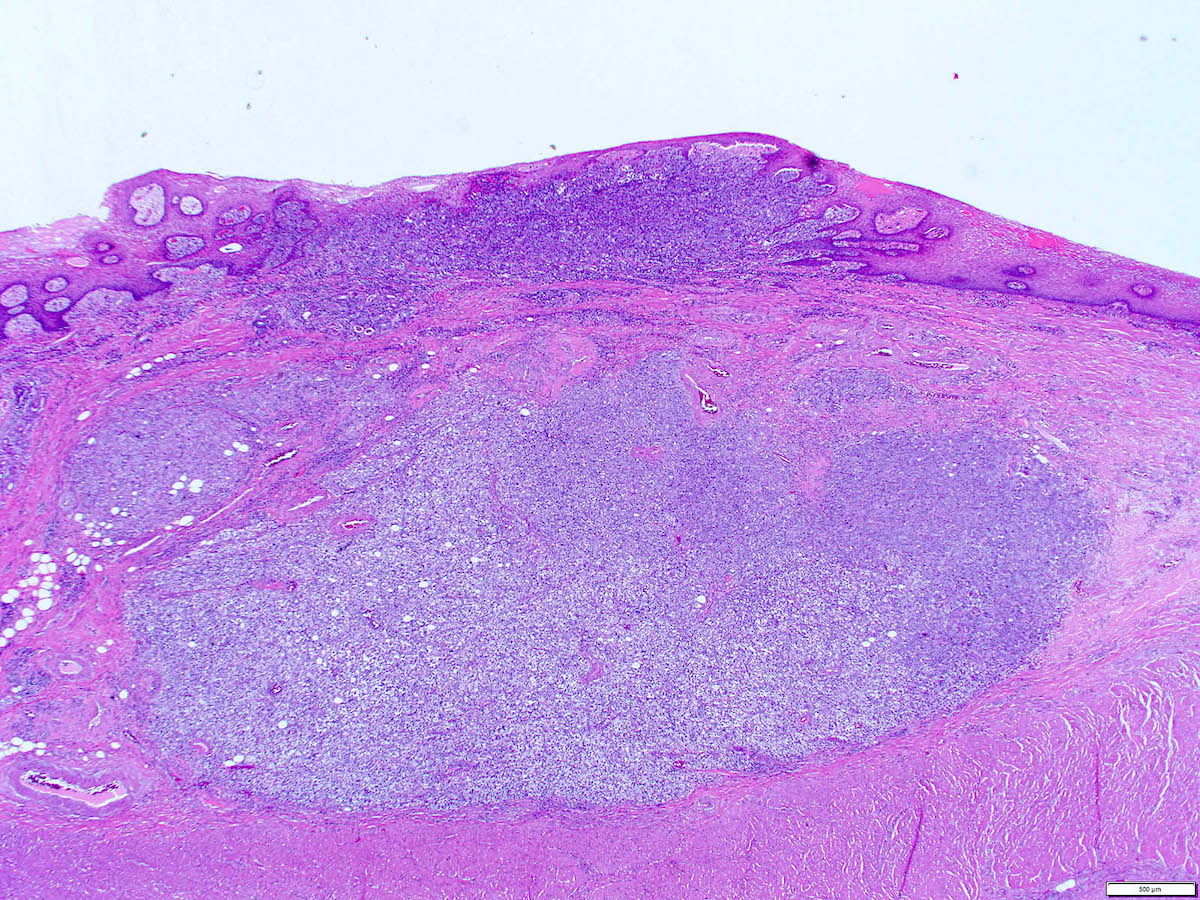
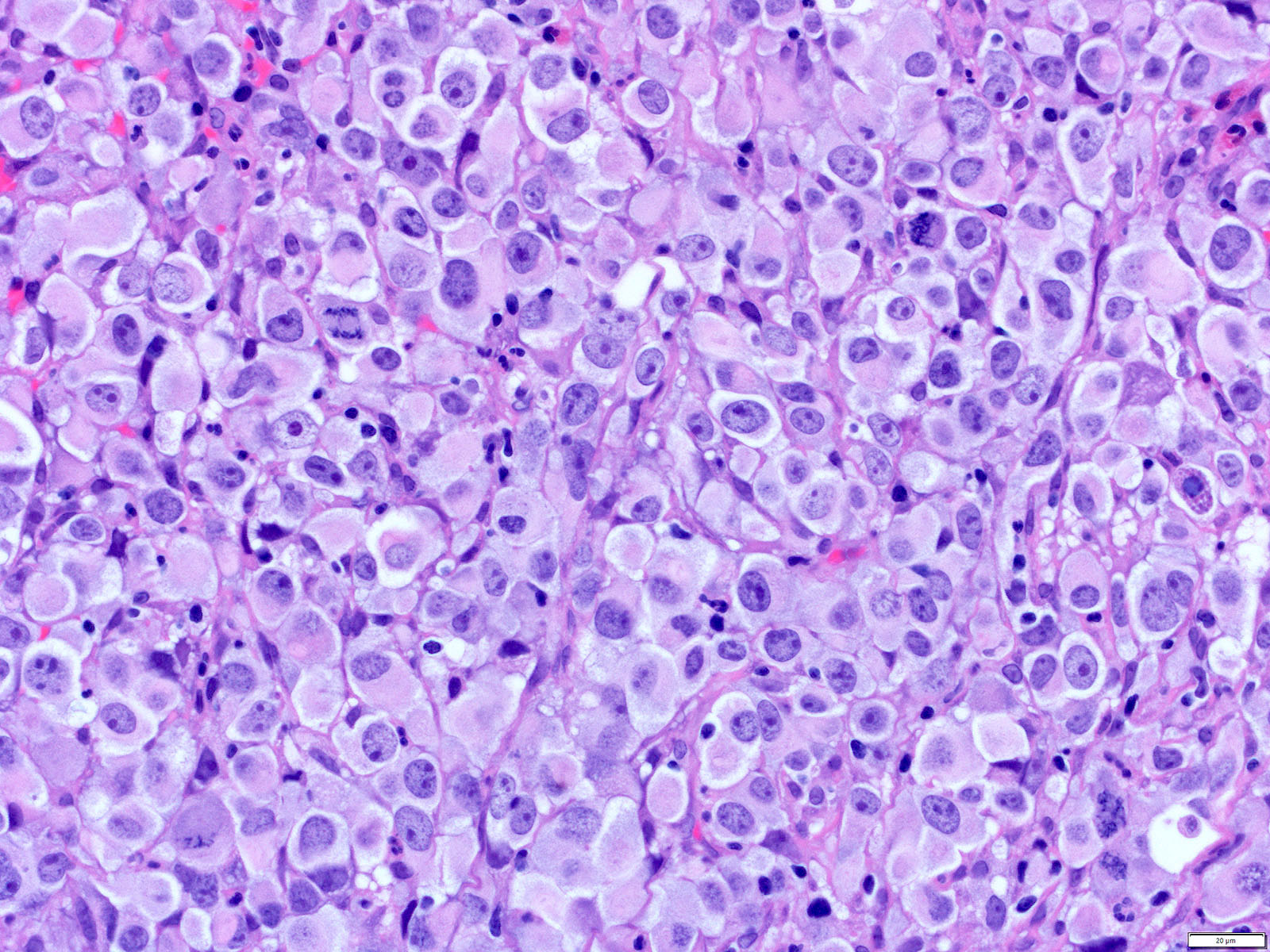
Microscopic (histologic) description
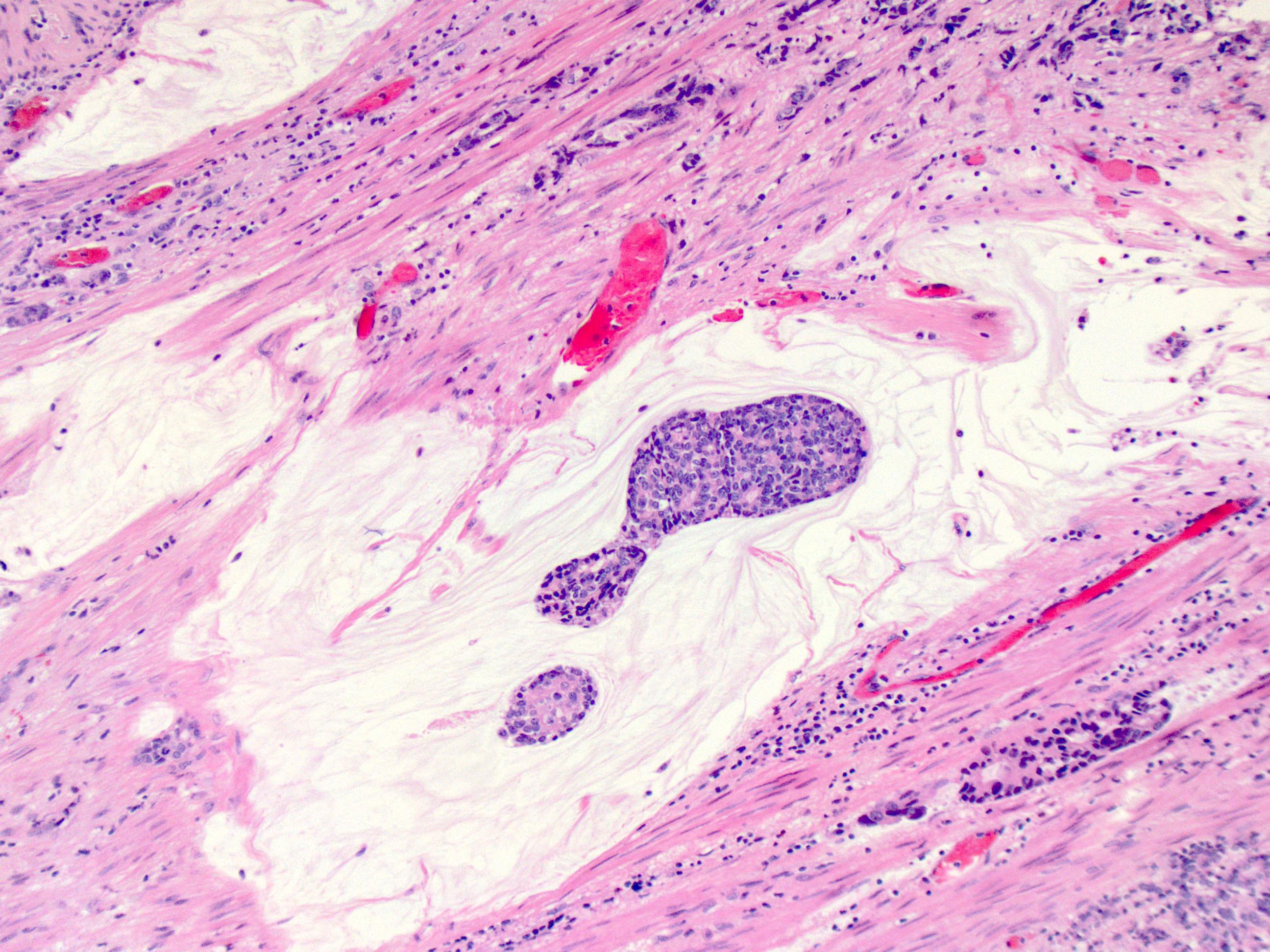
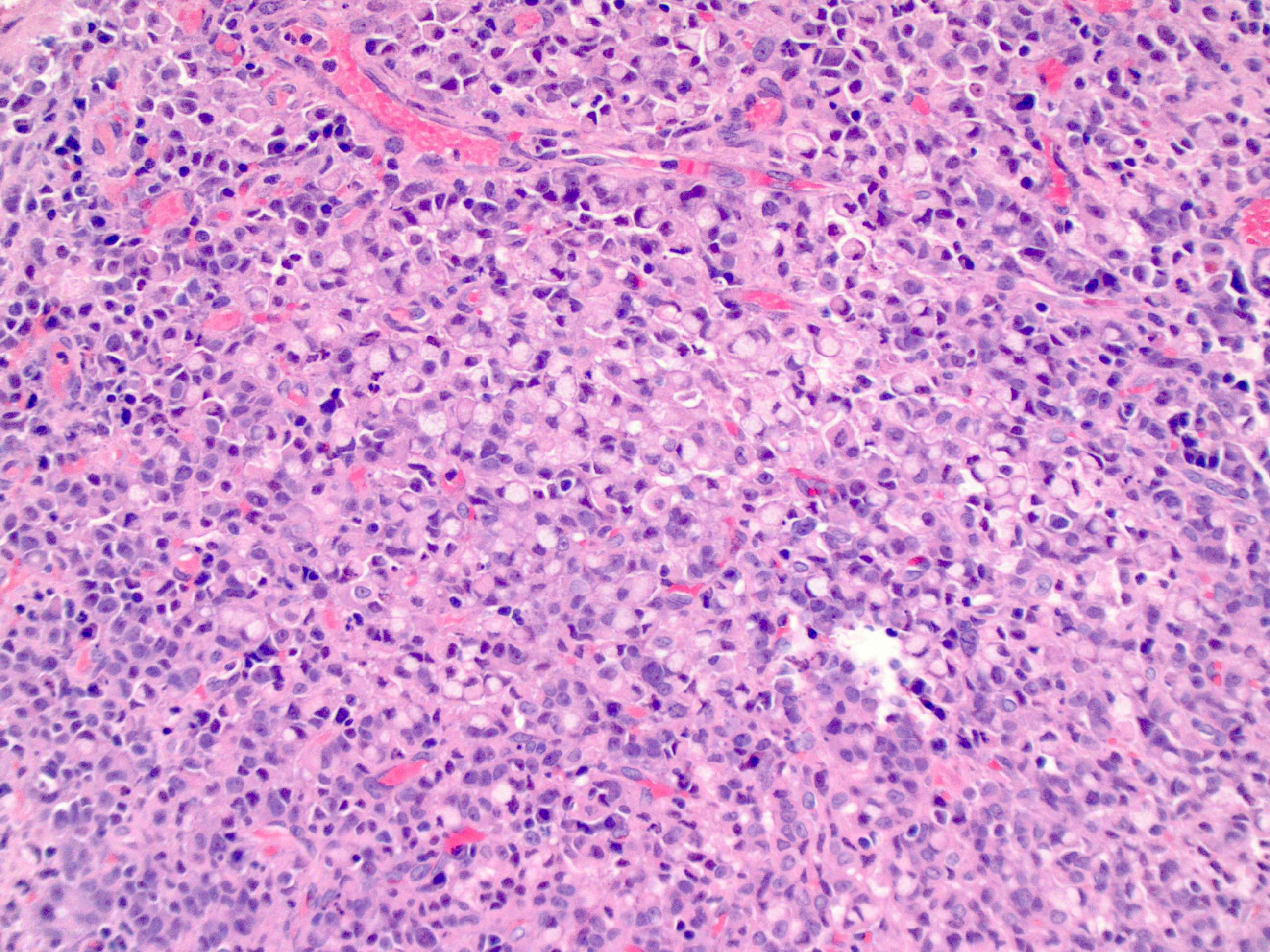
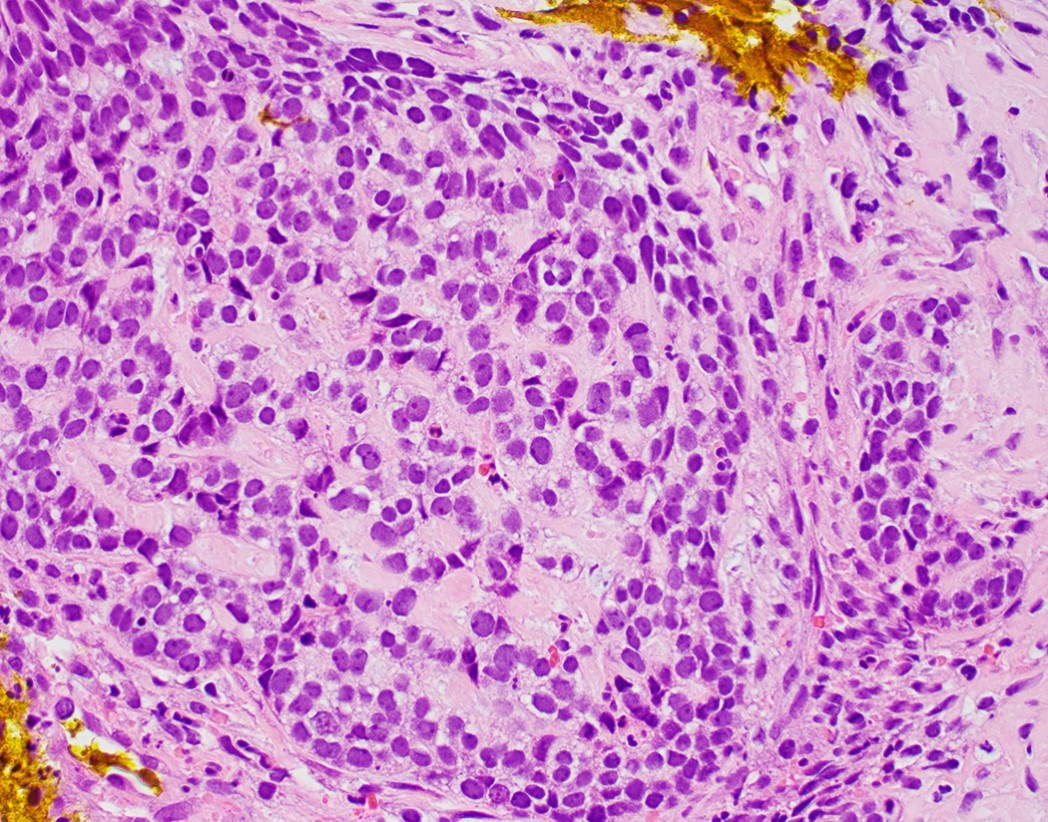
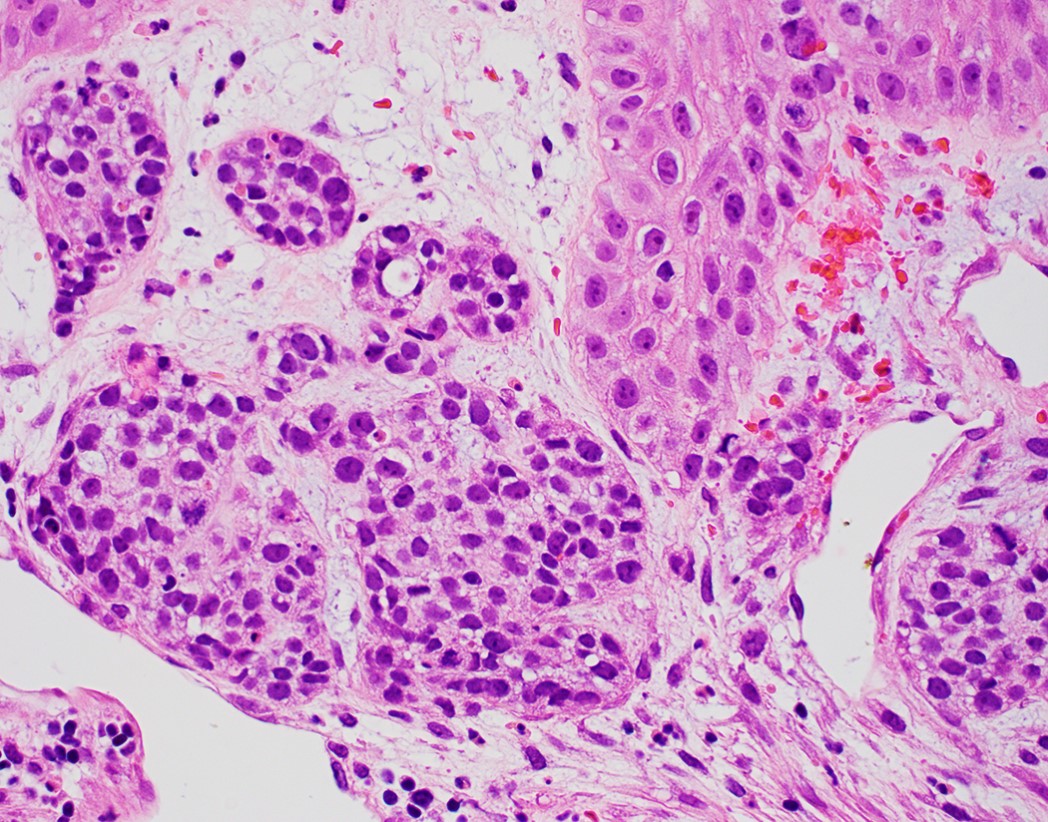
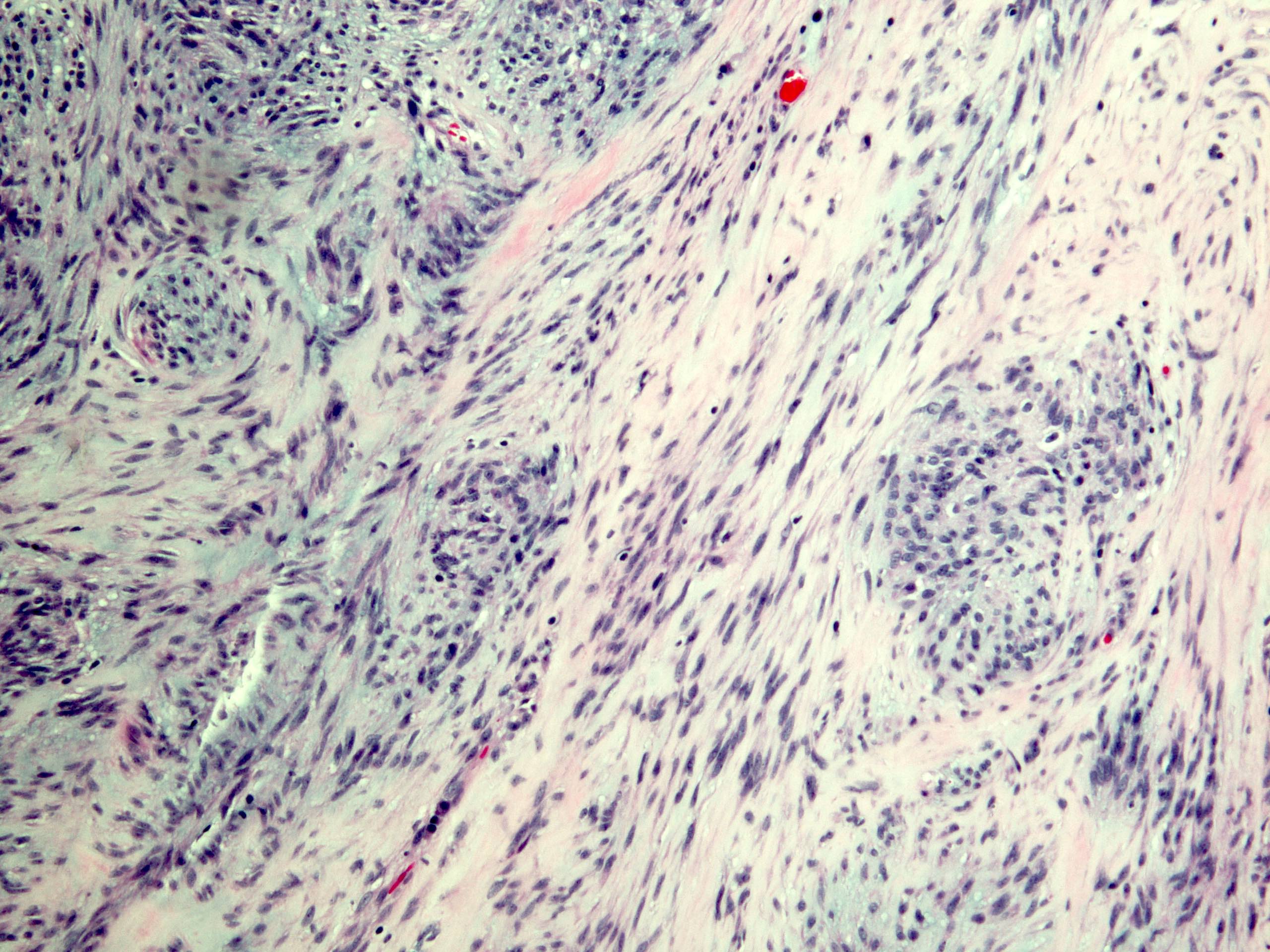
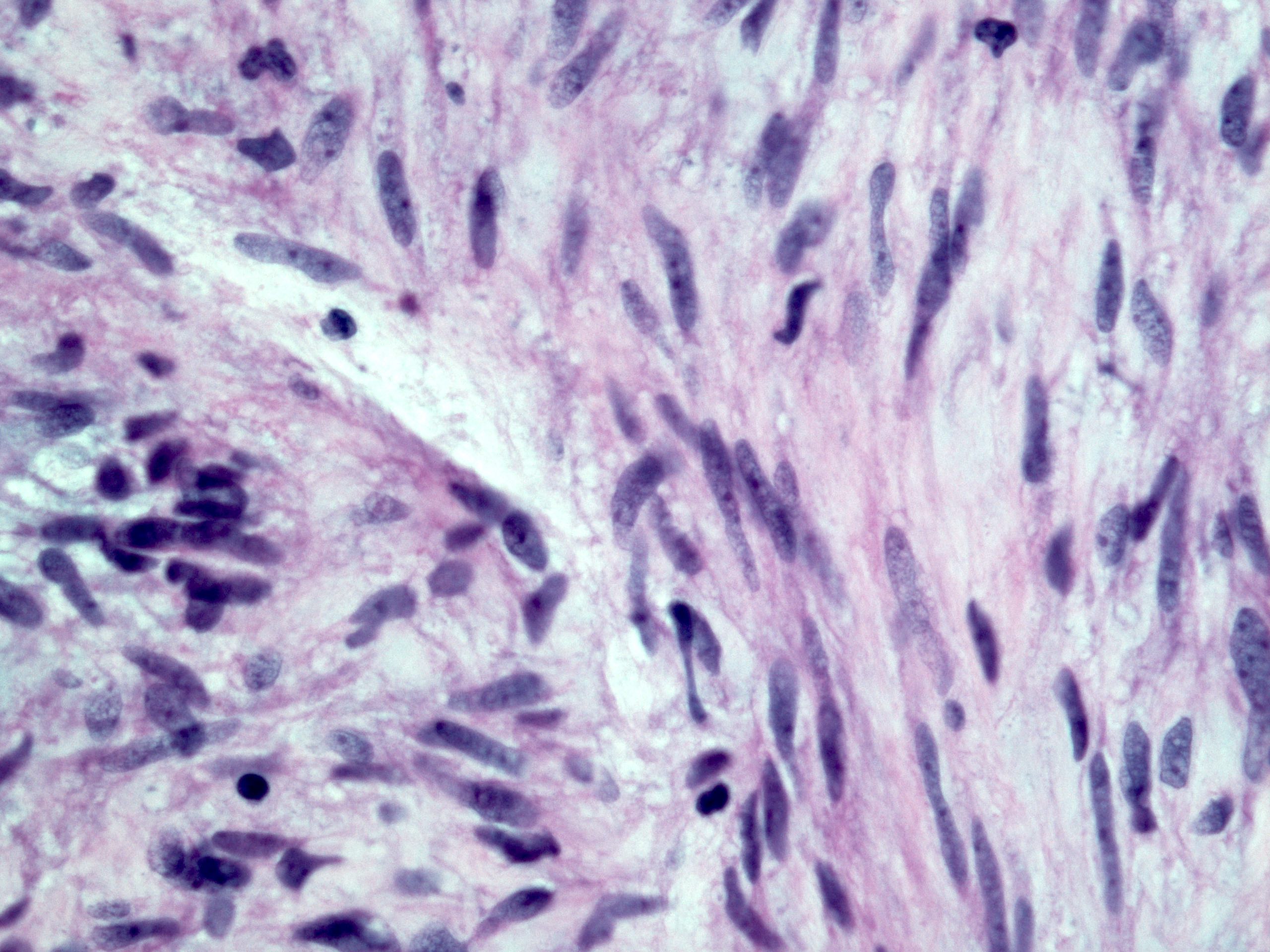
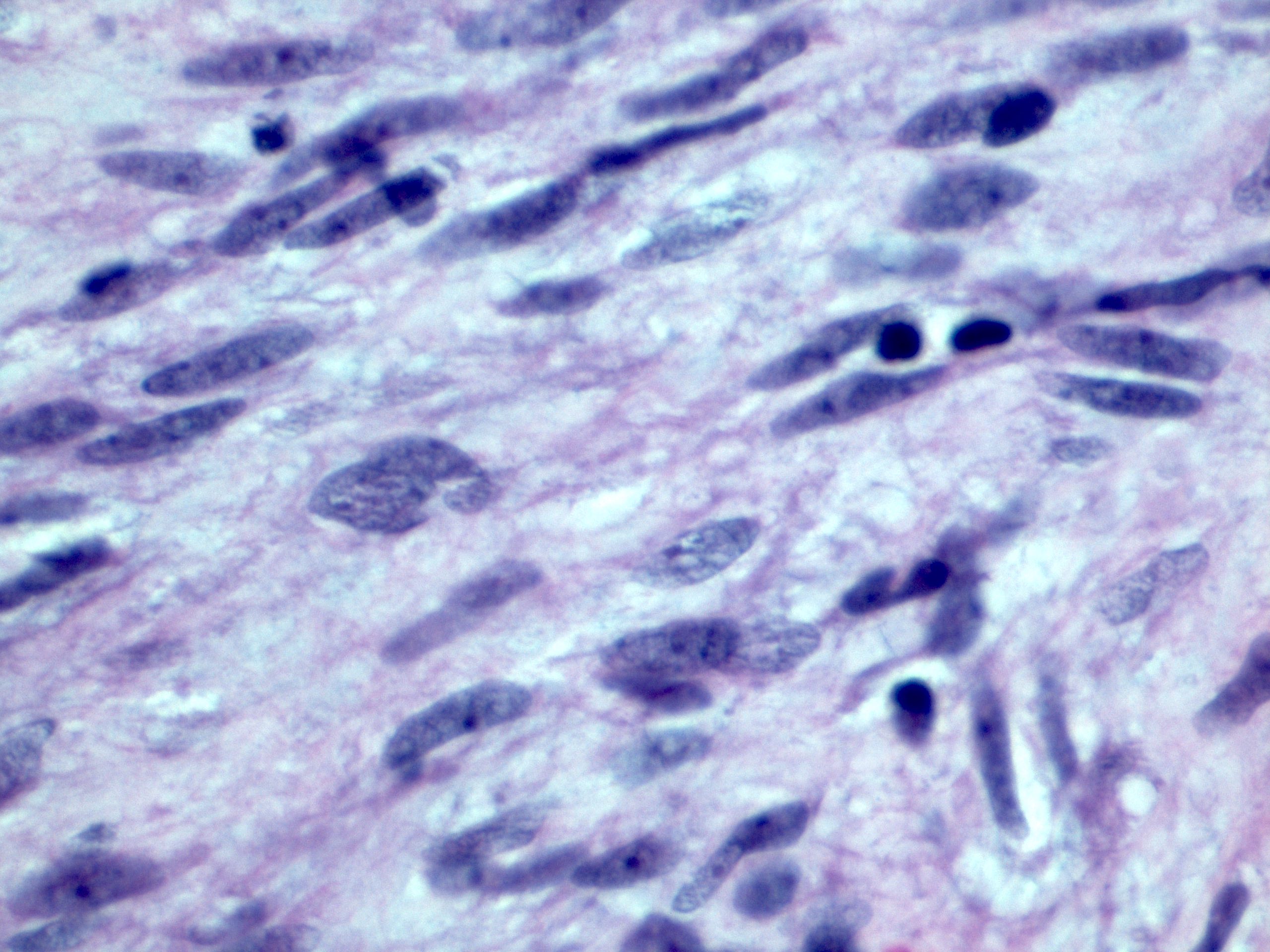
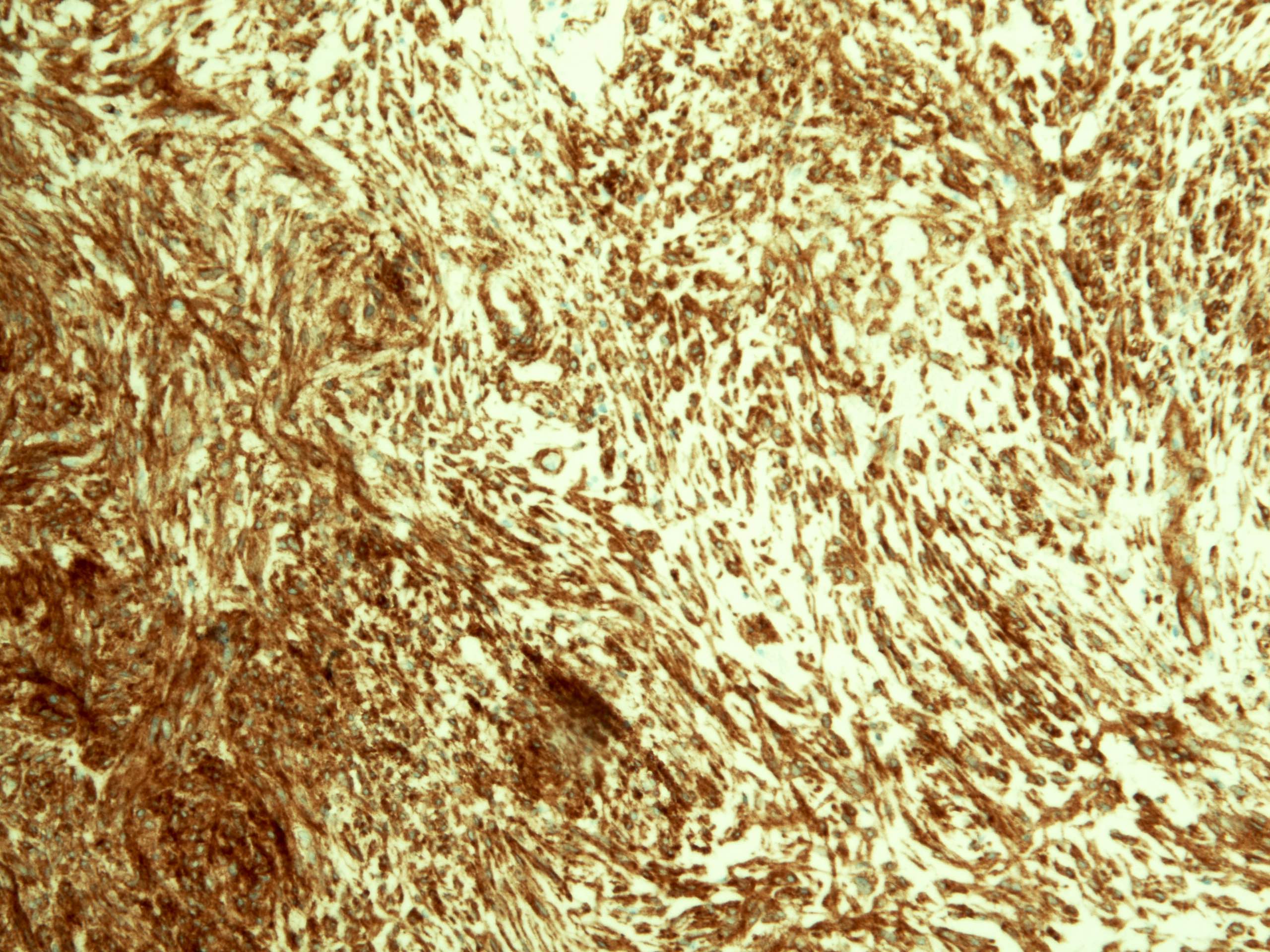
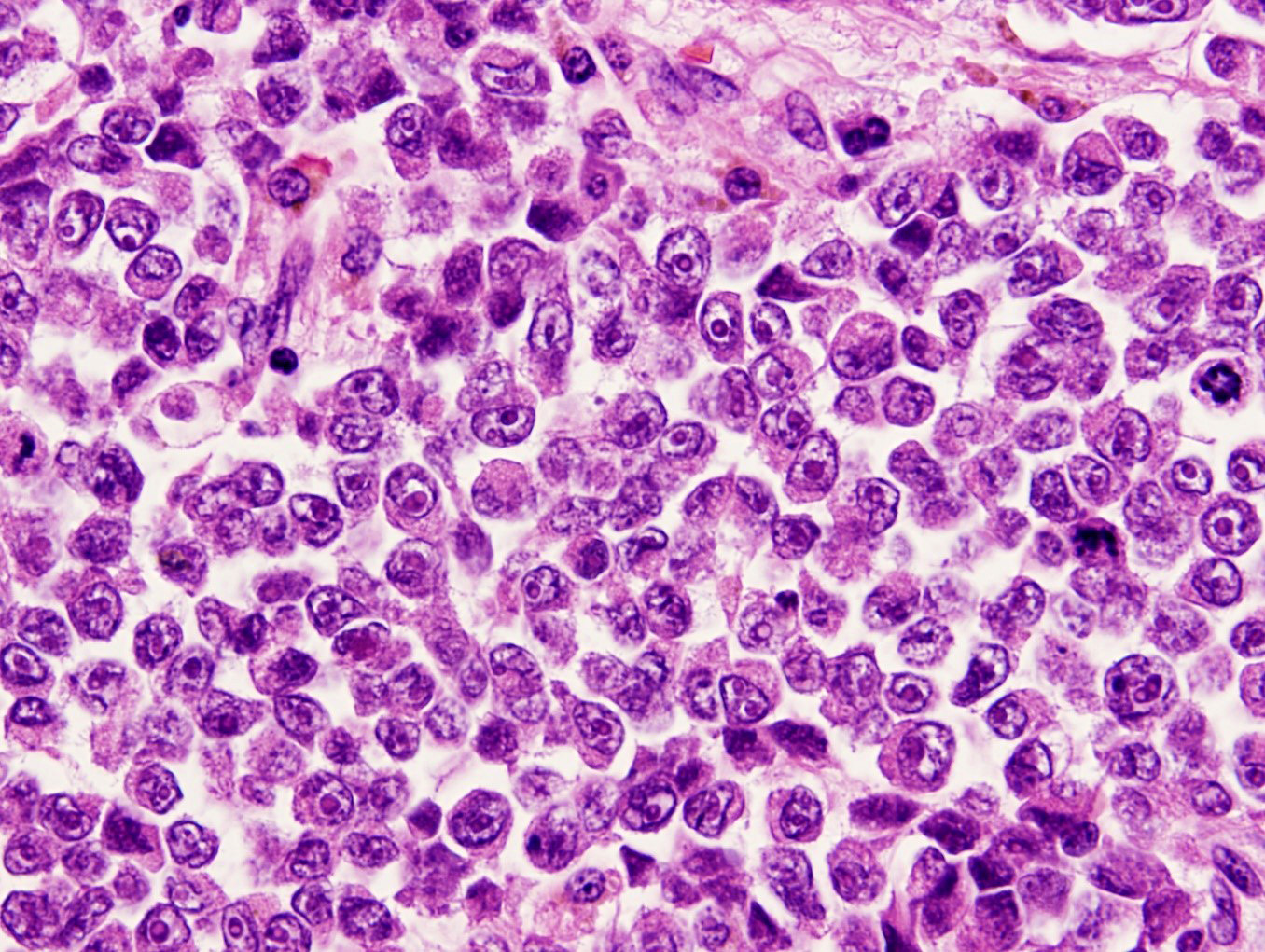
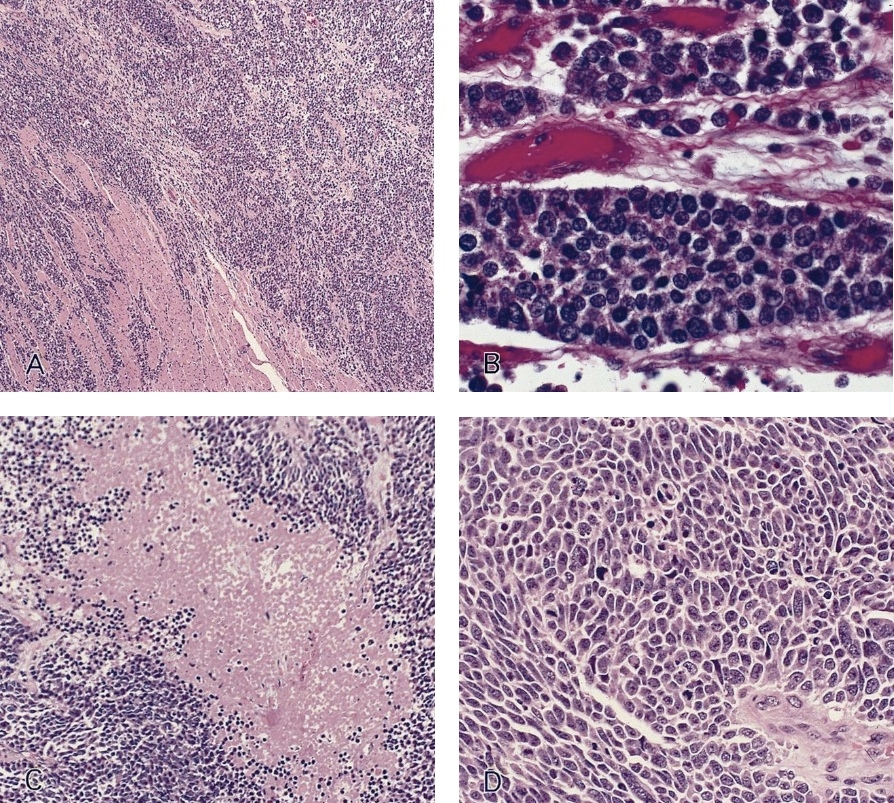
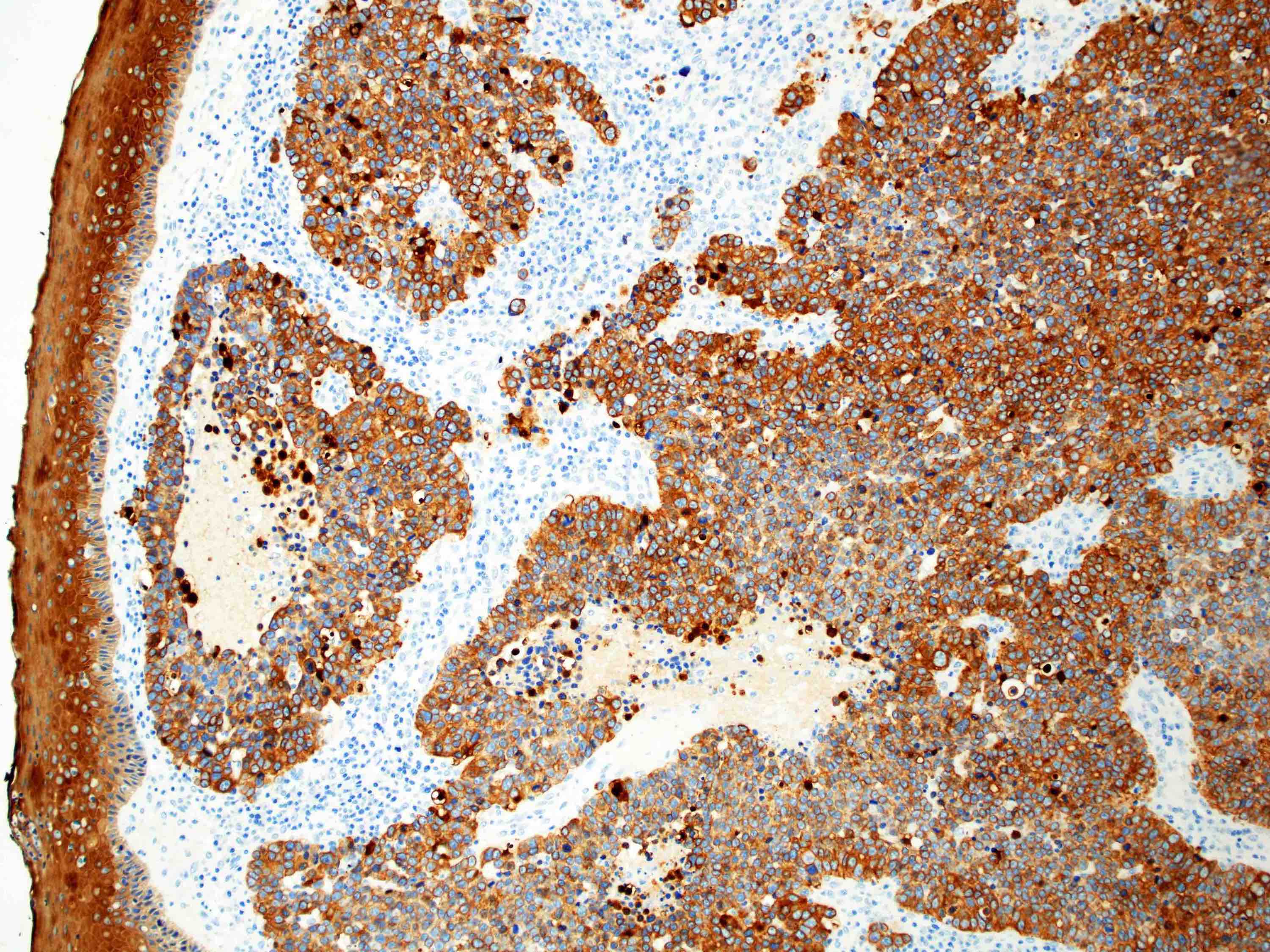
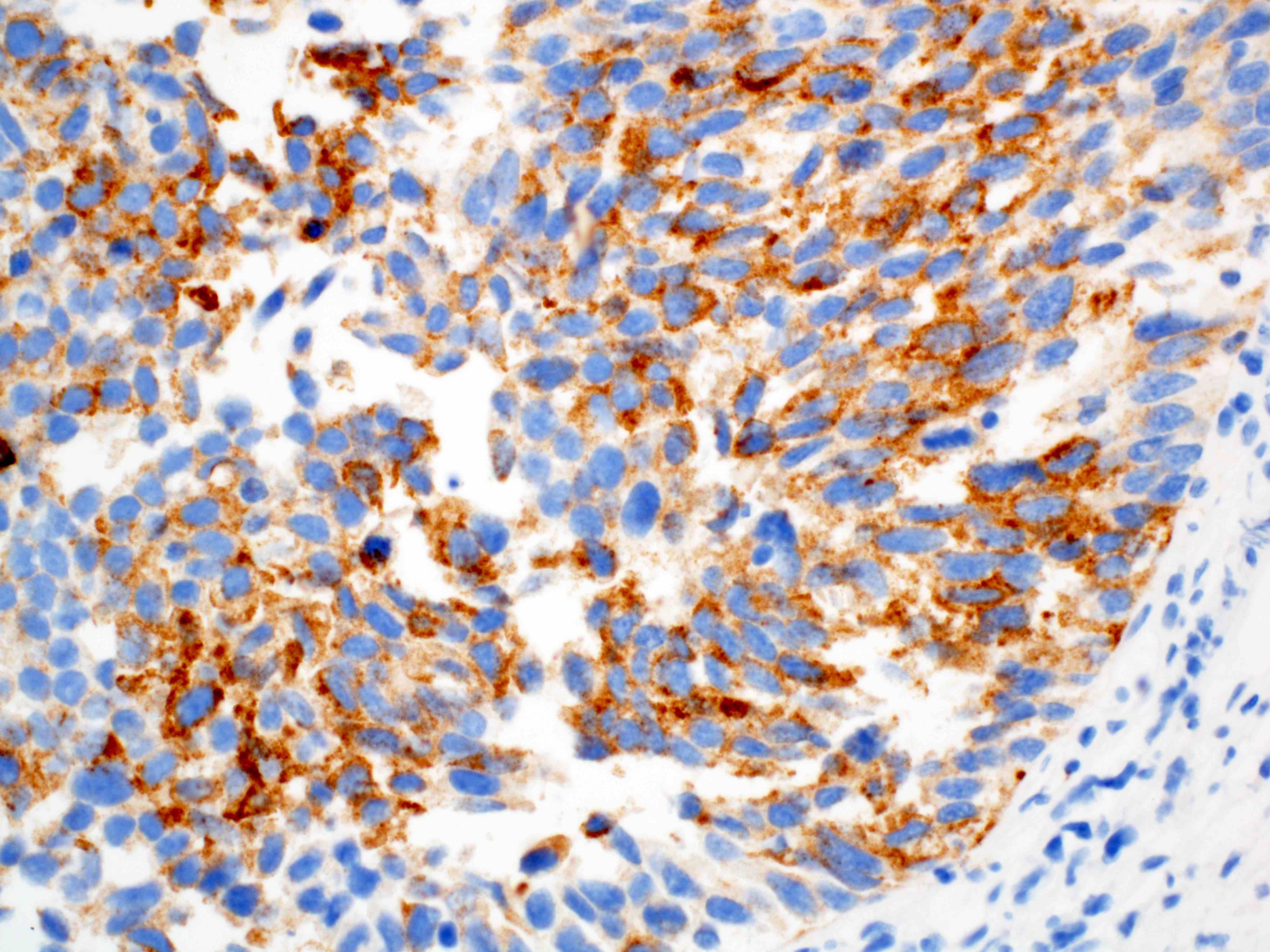
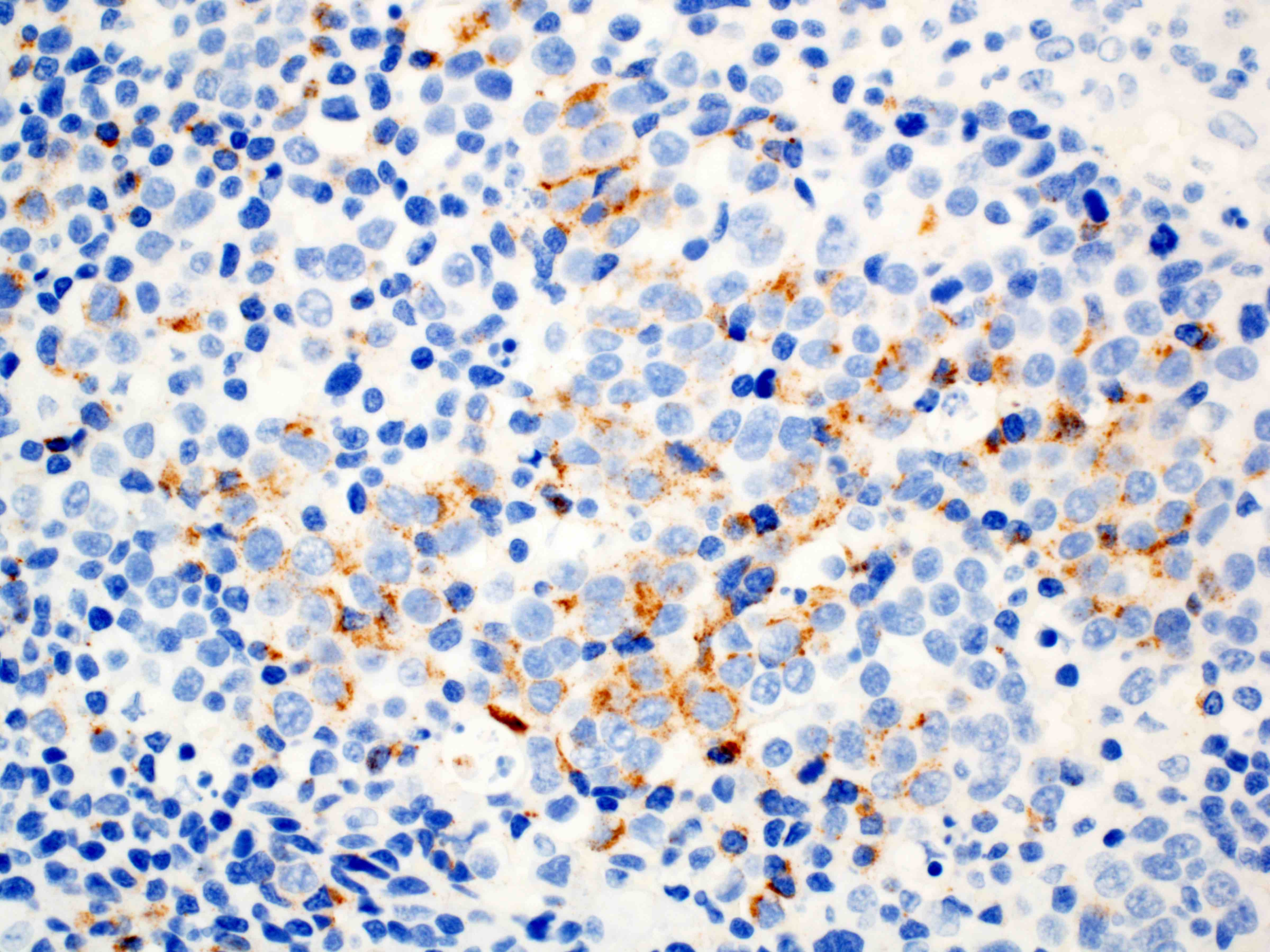
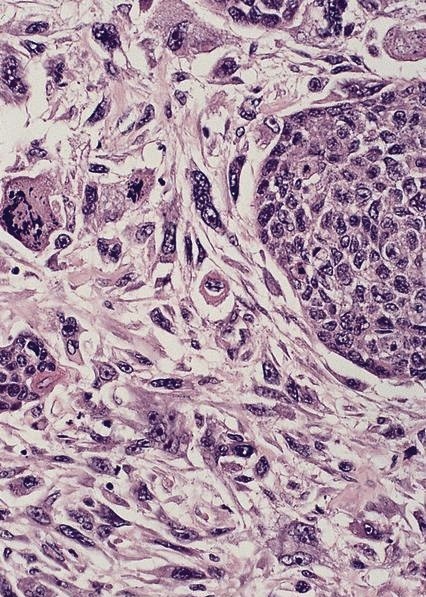
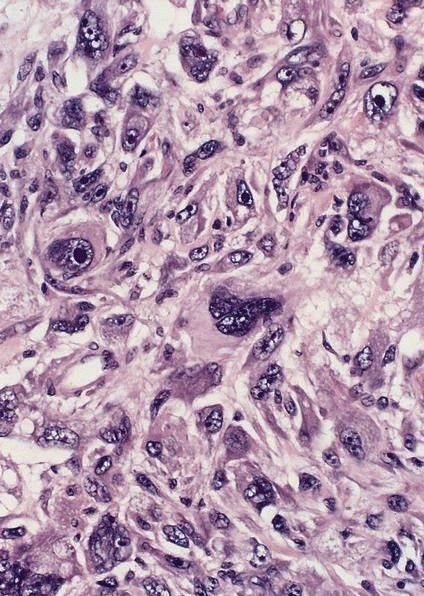
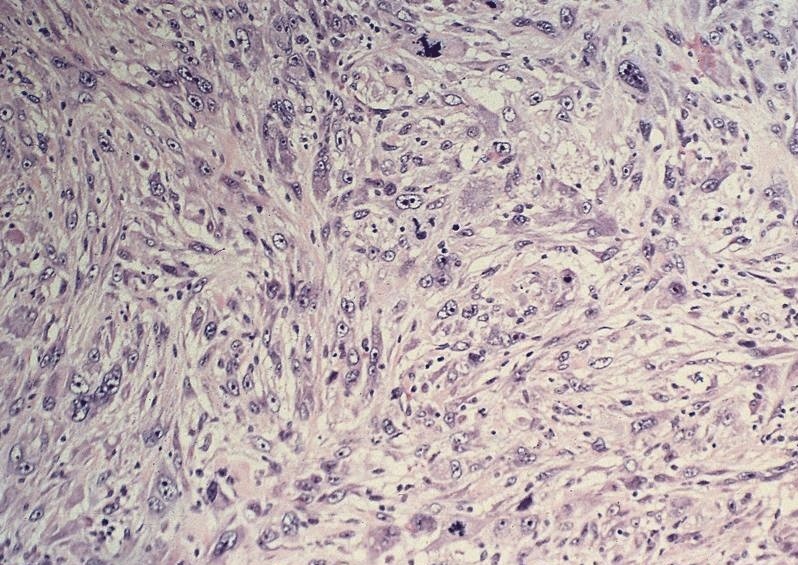
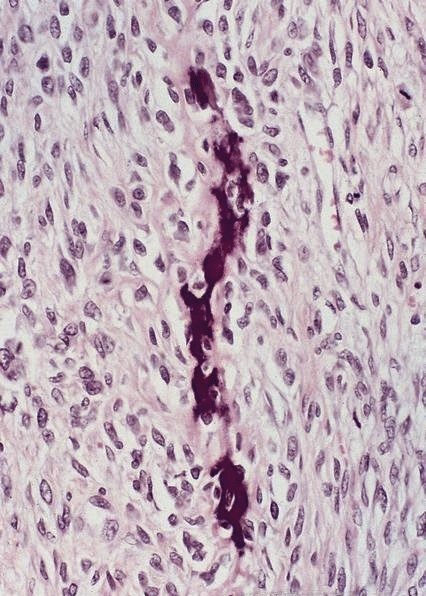
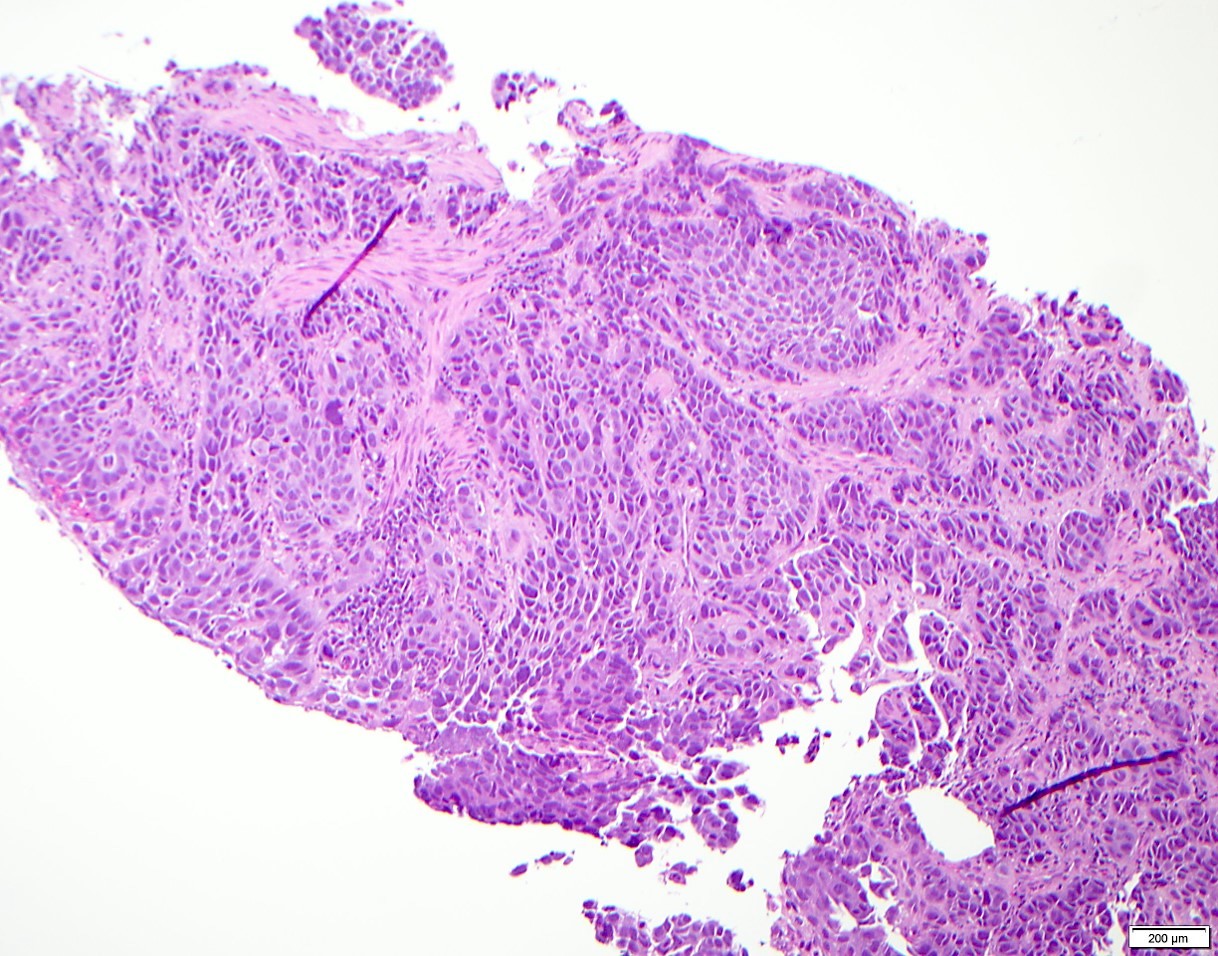
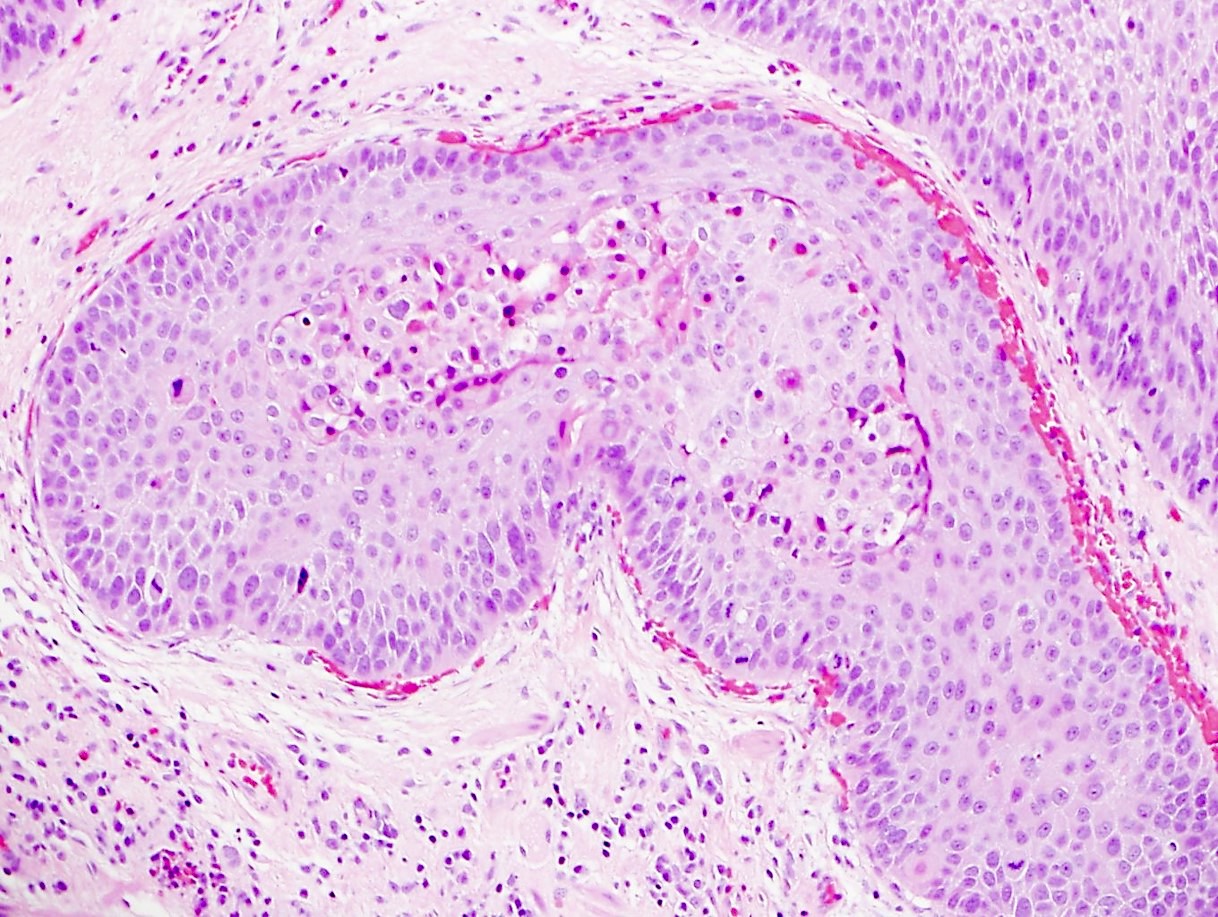
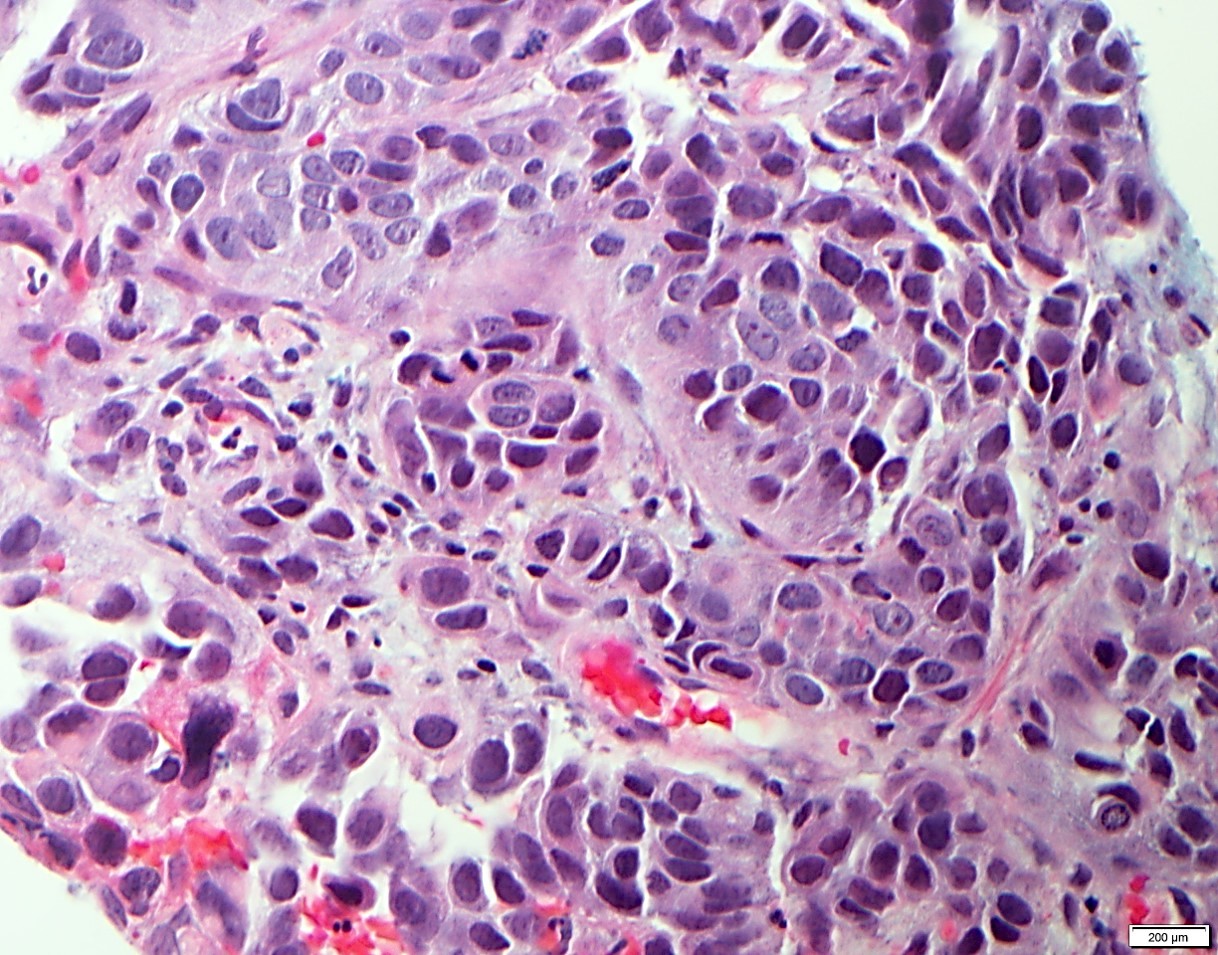
- Solid, cribriform or microcystic nests, strands, trabeculae or lobules of tumor cells (Histopathology 2000;36:331, Methods Mol Biol 2020;2129:7)
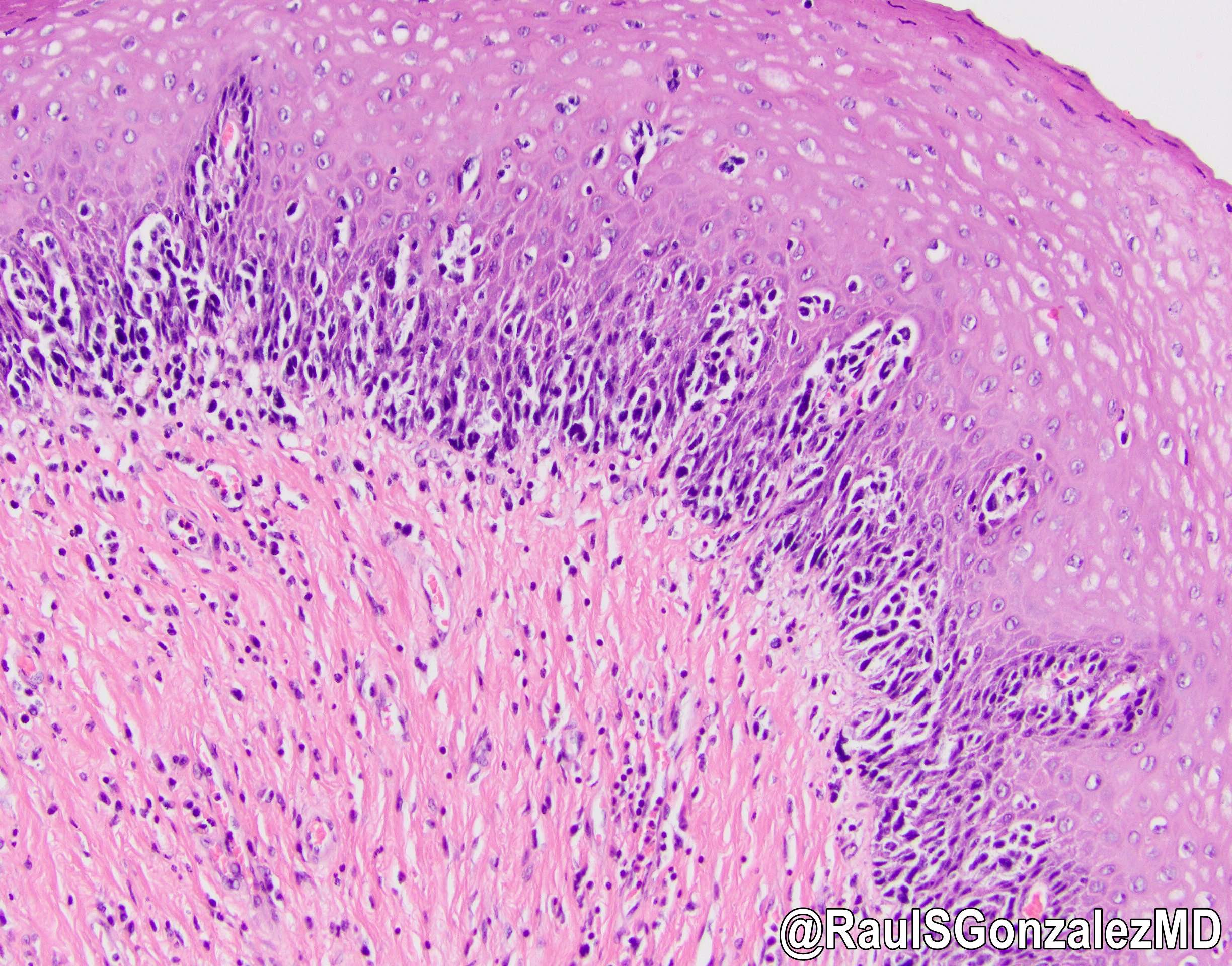
- Strands of tumor cells often connected to overlying squamous epithelium
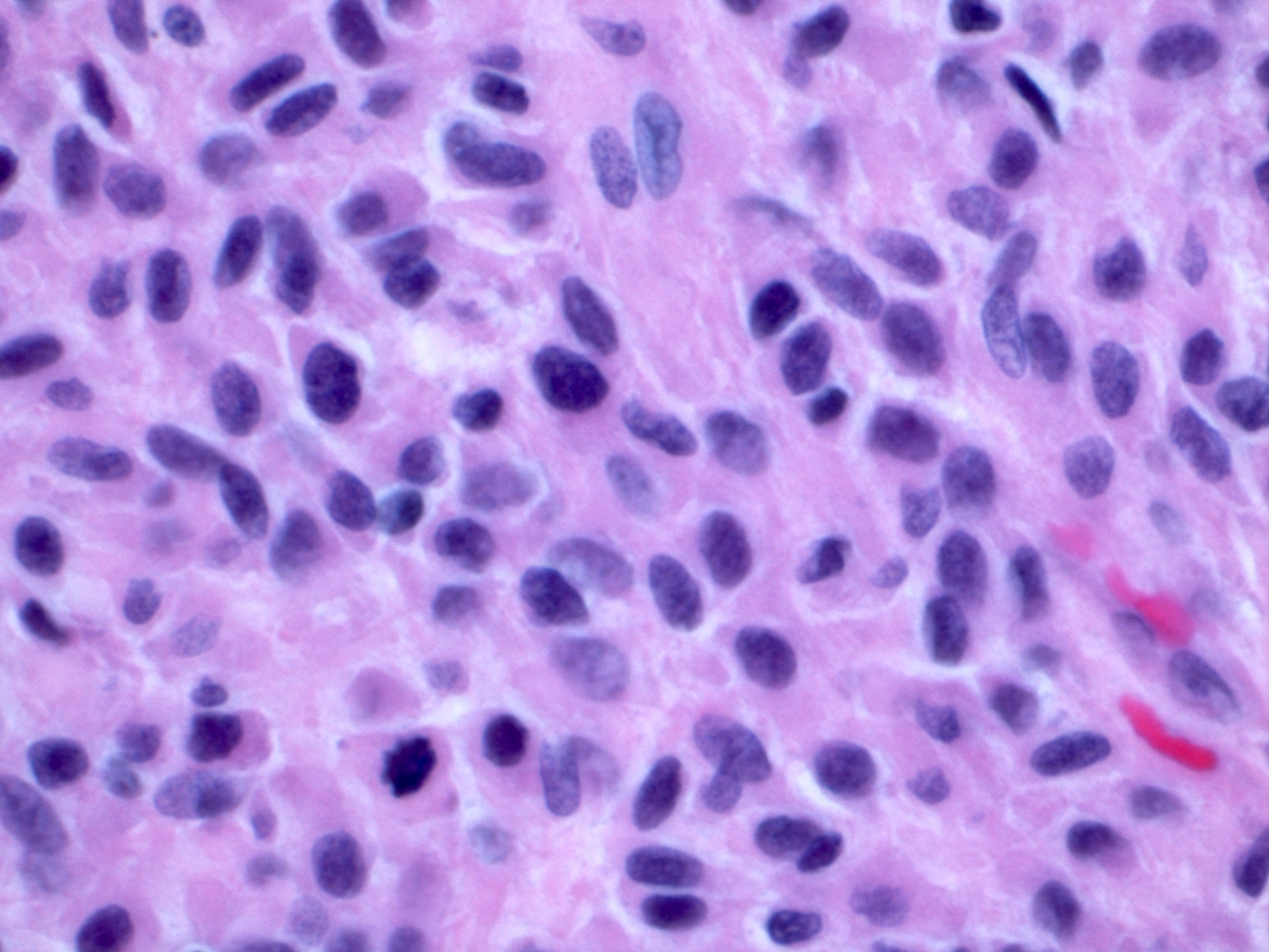
- Nuclei are round to oval, hyperchromatic and peripheral palisading, often with central comedo type necrosis
- Many mitotic figures
- Microcystic pattern contains basophilic material
- While microcysts or necrosis may cause a resemblance to lumina, true lumens are lacking
- Many have areas of stromal hyalinization
- Often admixed with conventional invasive or in situ squamous cell carcinoma and may see admixed adenocarcinoma, small cell carcinoma and spindle cell squamous cell carcinoma
Microscopic (histologic) images
Contributed by Jinping Lai, M.D., Ph.D. and AFIP images
Cytology description
- Basaloid cells
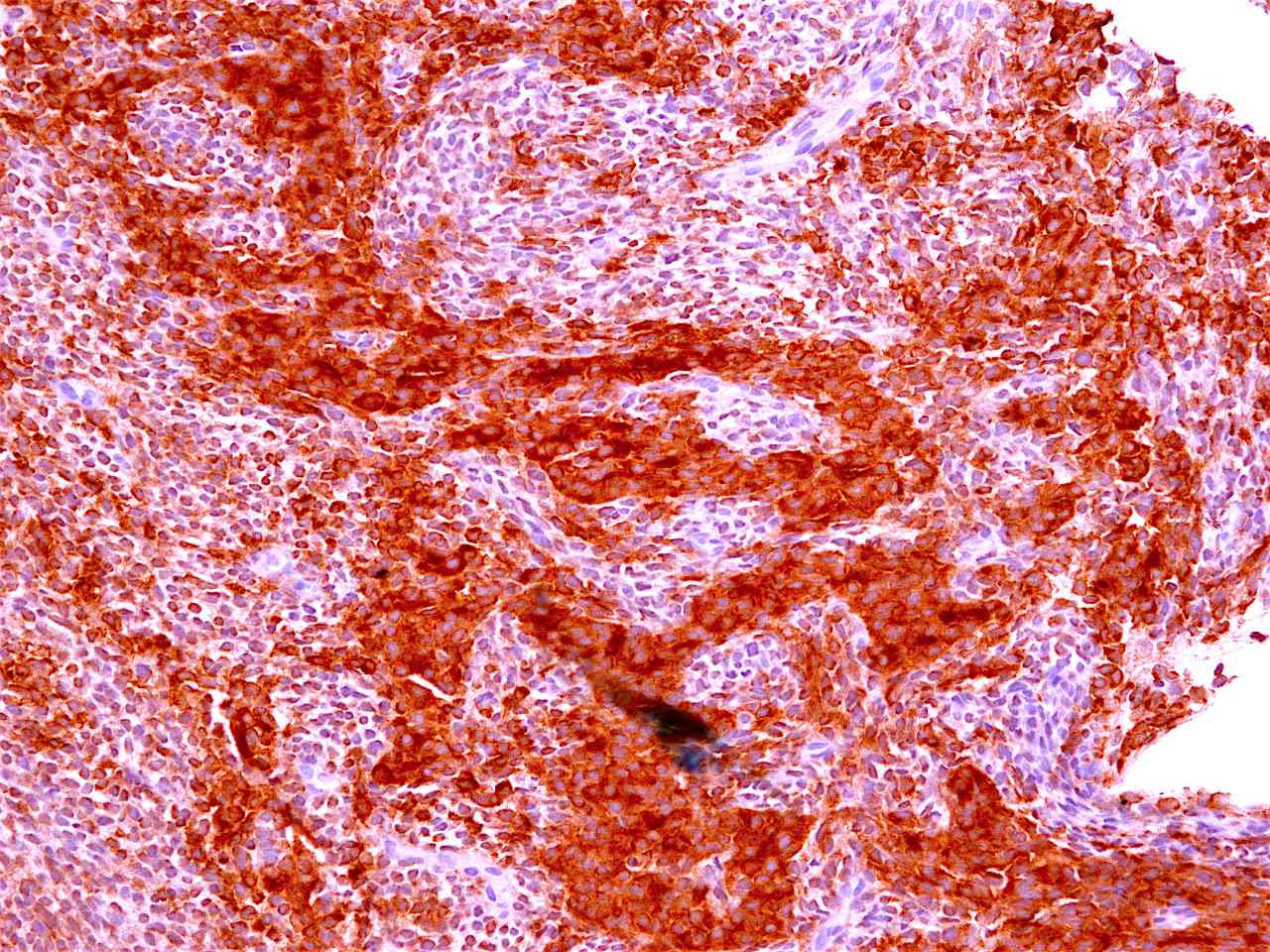
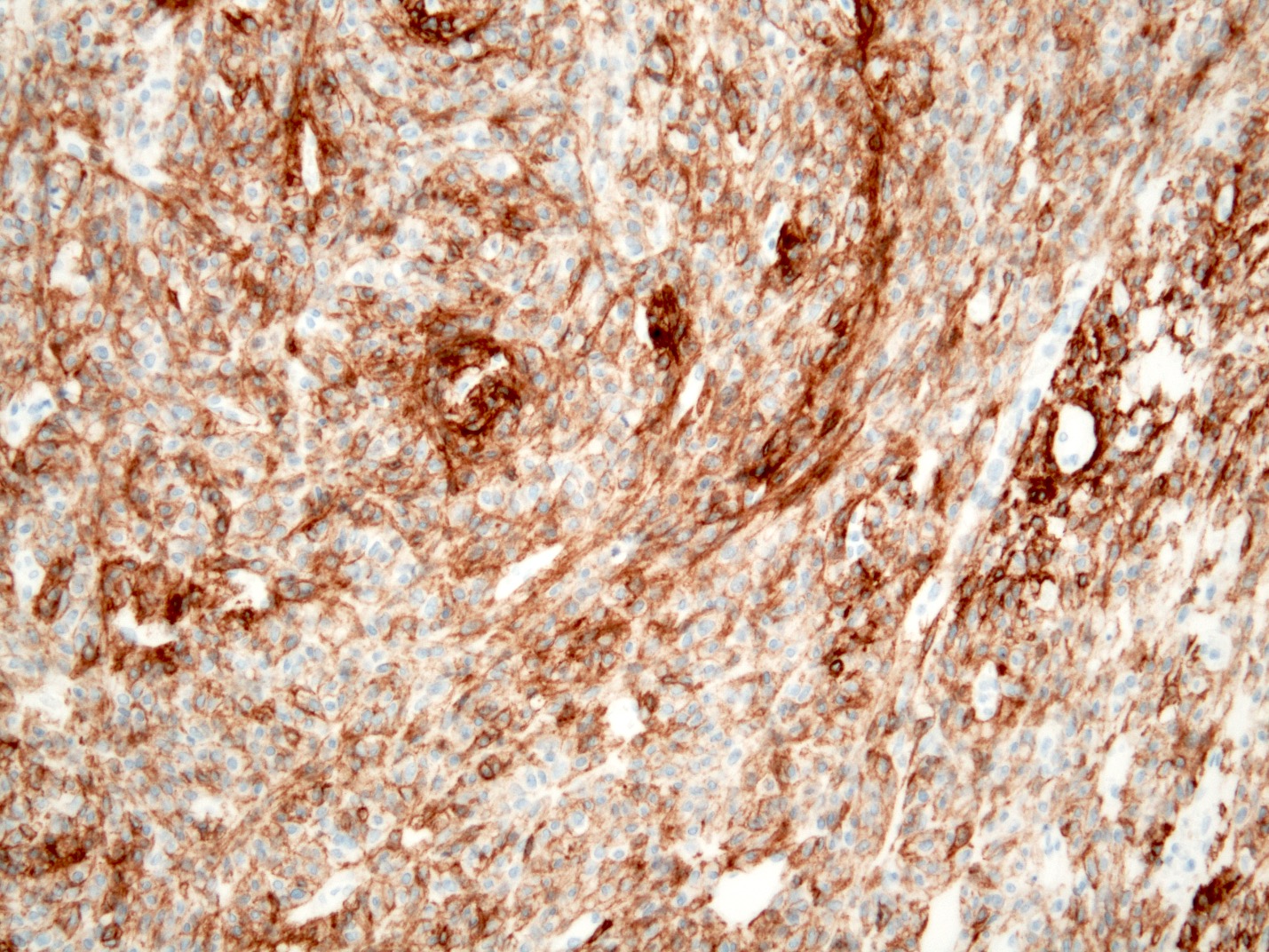
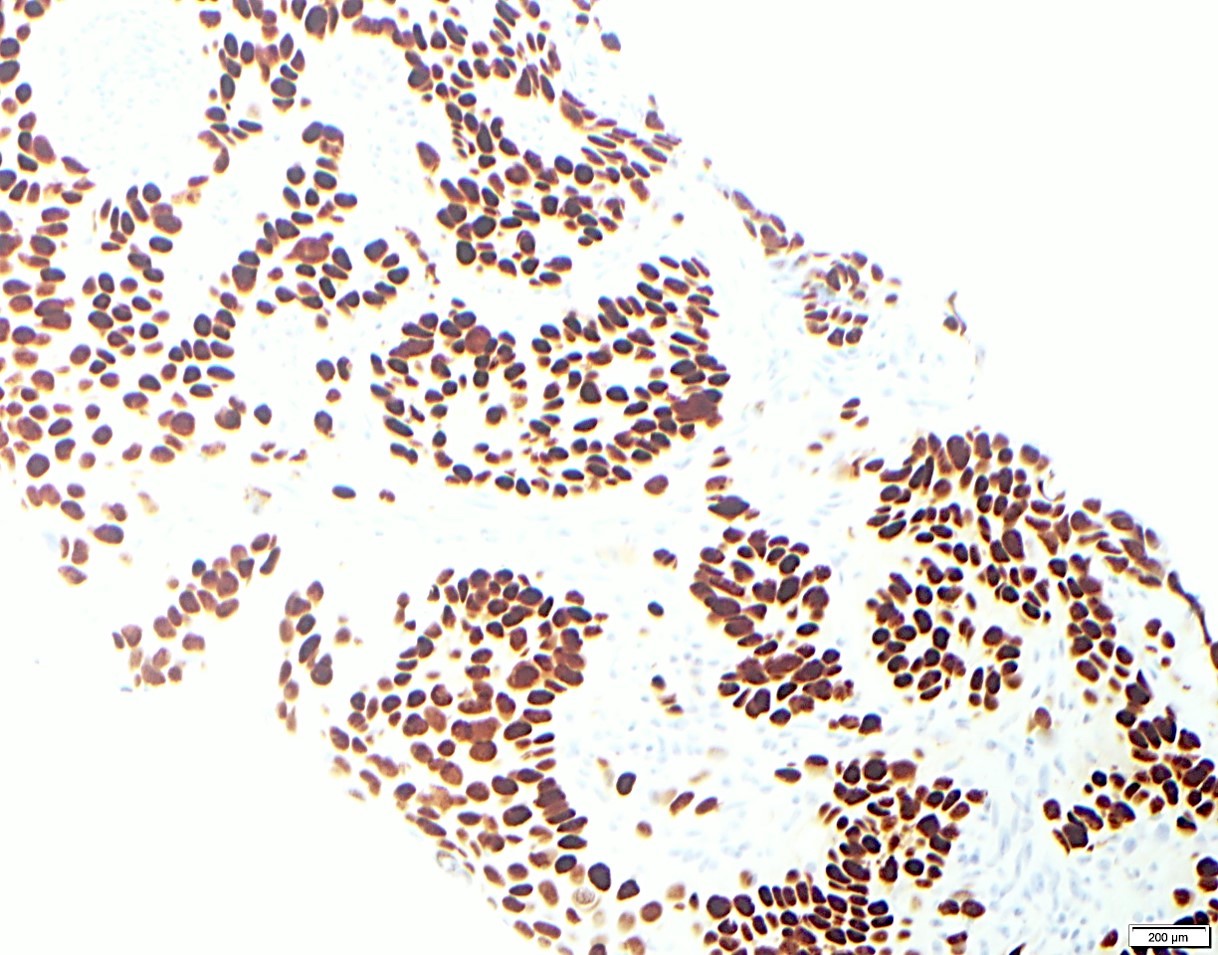
Positive stains
Negative stains
- Neuroendocrine markers
- Smooth muscle actin
- S100 (rarely weakly positive)
Electron microscopy description
- Relatively undifferentiated cellular characteristics
- Undeveloped cell organelles
- Markedly replicated basement membrane
Molecular / cytogenetics description
- p53 and Rb gene mutations similar to conventional squamous cell carcinoma (Hum Pathol 2012;43:2012)
- Up to half of esophageal BSCC cases harbor either an EGFR mutation or amplification and partial activation of the Wnt and hedgehog (HH) signaling pathways (Int J Clin Exp Pathol 2015;8:2267)
- No KRAS, BRAF or PI3K mutations observed in BSCCs, while 23% of conventional SCC harbored a PIK3CA mutation (Ann Surg Oncol 2015;22:3659)
Sample pathology report
- Esophagus, mid, mass, biopsy:
- Basaloid squamous cell carcinoma (see comment)
- Comment: Clinical photos are noted. Sections of the biopsy show subepithelial basaloid neoplasm with microcystic nests, strands, trabeculae of tumor cells infiltrating a myxoid stroma. The tumor cells are positive for p40, p63, p53 and intact INI1, while negative for p16, CK7, CK20, CDX2 and synaptophysin. The histologic features and immunoprofile support the diagnosis.
Differential diagnosis
- Adenoid cystic carcinoma:
- Much better prognosis; female predominance
- No association with in situ or invasive conventional squamous cell carcinoma
- Has myoepithelial differentiation (Medicine (Baltimore) 2019;98:e16999)
- Sarcomatoid carcinoma / carcinosarcoma:
- With spindle cell differentiation (Tohoku J Exp Med 2019;249:255, Am J Gastroenterol 2018;113:642)
- Small cell carcinoma:
- Has different morphology with finely granular chromatin, scant cytoplasm and nuclear molding, expresses neuroendocrine markers (Thorac Cancer 2020;11:1119, Am J Clin Oncol 2019;42:534)
Board review style question #1
Compared with conventional squamous cell carcinoma of the esophagus, basaloid squamous cell carcinoma of the esophagus has
- Fewer PI3KCA mutations
- More p53 mutations
- More Rb mutations
- More BRAF mutations
- More KRAS mutations
Board review style answer #1
A. No PI3KCA mutation has been observed in esophageal basaloid squamous cell carcinoma, while 23% of conventional SCCs of the esophagus harbored a PI3KCA mutation.
Comment Here
Reference: Basaloid squamous cell carcinoma
Comment Here
Reference: Basaloid squamous cell carcinoma
Board review style question #2
56 year old man with a smoking history for 20 years and dysphagia for 6 months. A biopsy is made of a lesion at his mid esophagus, shown in the photo above. The cells are diffusely nuclear positive for p40, p63, p53 and INI1, while negative for p16, CK7, CK20, CDX2 and synaptophysin. What is your diagnosis?
- Adenoid cystic carcinoma
- Basaloid squamous cell carcinoma
- Metastatic oropharyngeal HPV related squamous cell carcinoma
- Neuroendocrine carcinoma, small cell type
- SMARCB1 deficient carcinoma
Board review style answer #2
B. The most likely diagnosis is basaloid squamous cell carcinoma. The tumor cells only show 1 component and are diffusely positive for p40 and p63, making adenoid cystic carcinoma less likely. The tumor cells show eosinophilic cytoplasm with negative synaptophysin, making small cell carcinoma less likely. The tumor cells have an intact INI1, making SMARCB1 deficient carcinoma less likely. p16 is negative, making HPV related metastatic oropharyngeal squamous cell carcinoma less likely.
Comment Here
Reference: Basaloid squamous cell carcinoma
Comment Here
Reference: Basaloid squamous cell carcinoma
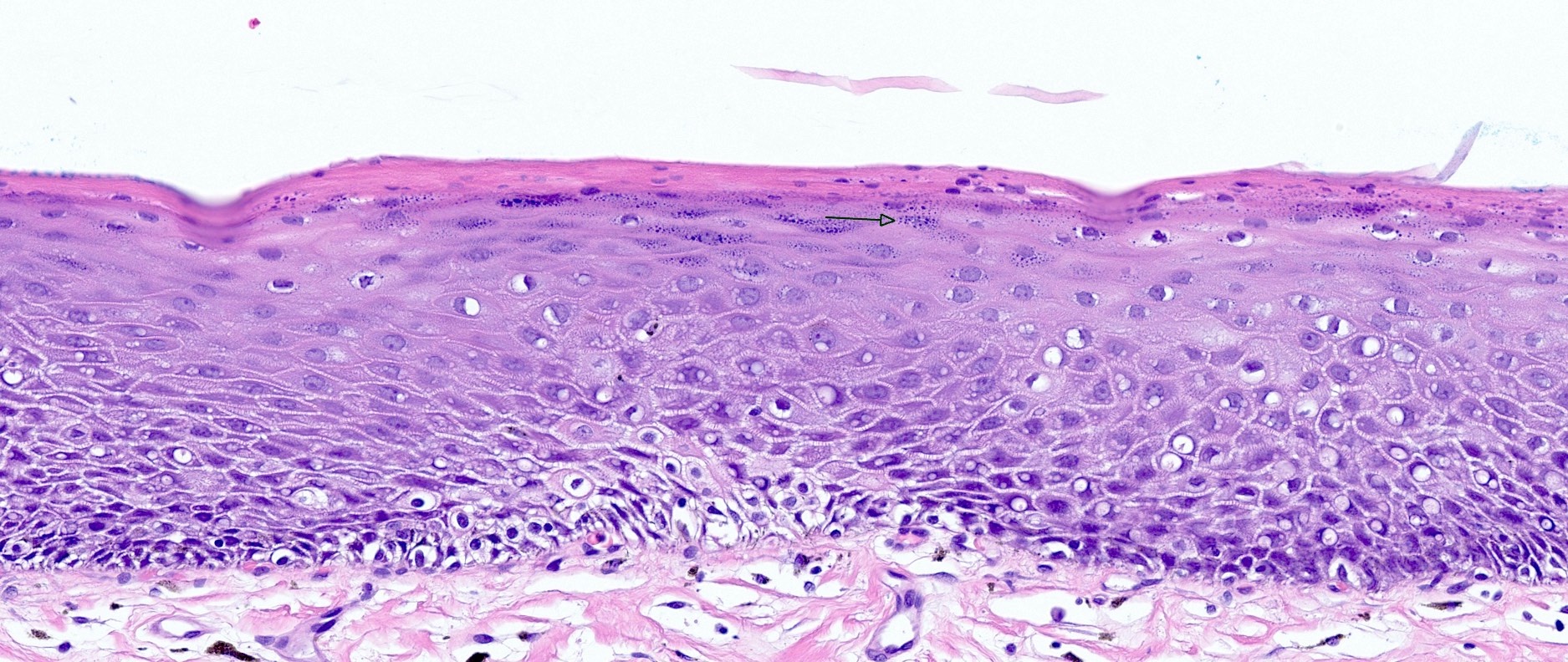
Candida
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
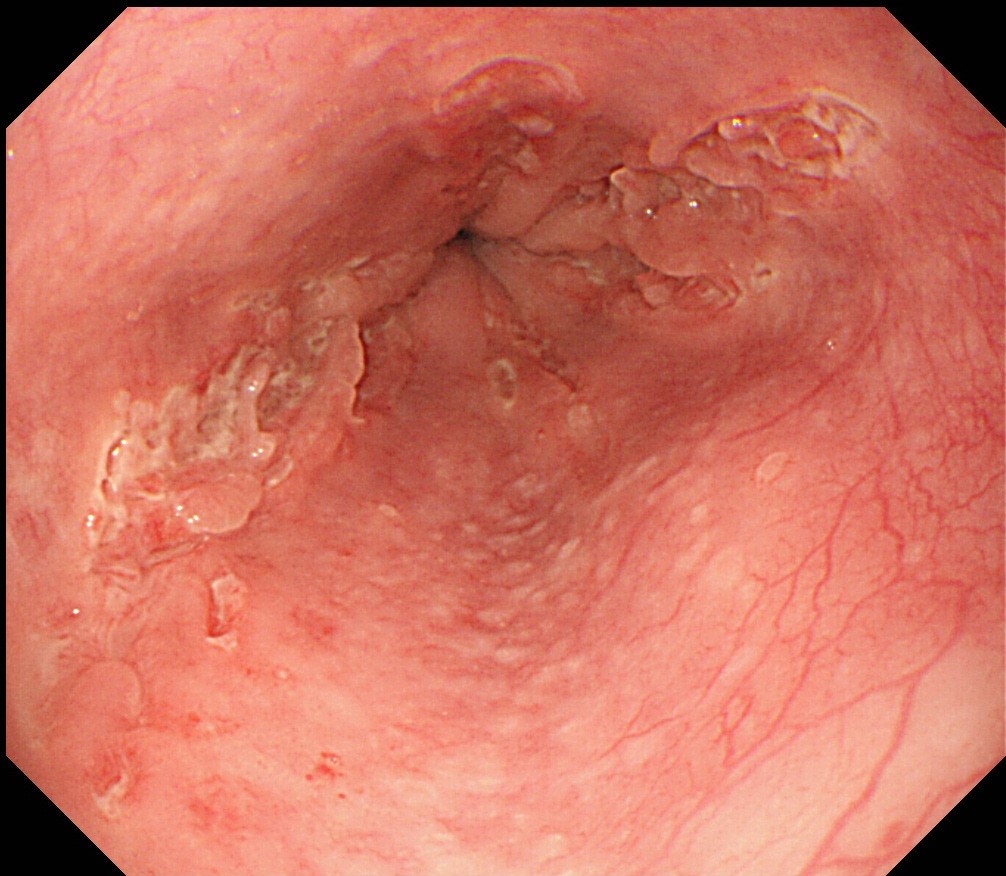
- Candida albicans, which can be locally invasive, is the most prevalent cause of infectious esophagitis
- Other common fungal species relevant to infectious esophagitis are C. tropicalis, C. glabrata, C. krusei and C. parapsilosis
Essential features
- Candida esophagitis is one of the most common types of esophagitis in immunosuppressed individuals
- Histological features include acute inflammation, intraepithelial neutrophilic abscesses and epithelial edema; parakeratosis most prominent in the superficial epithelial layers with yeast forms and pseudohyphae
- Treated with antifungals, often with good prognosis
Terminology
- Esophageal candidiasis, Candida esophagitis or esophageal moniliasis
ICD coding
Epidemiology
- Incidence rates ranging from 0.32% to 5.2% in the general population
- Increased prevalence in human immunodeficiency virus (HIV) positive patients
- M = F (Gastroenterology Res 2018;11:195)
- Risk factors
- HIV
- Diabetes mellitus
- Peptic ulcer disease
- Medications such as antibiotics and corticosteroids
- Achalasia cardia
- Pregnancy
- Proton pump inhibitors (PPI) (Clin Gastroenterol Hepatol 2019;17:200)
- Smoking
- Adrenal insufficiency
- Malignancy
Sites
- Can be diffuse or localized in esophagus (StatPearls: Esophageal Candidiasis [Accessed 27 November 2023])
Pathophysiology
- Due to impaired cell mediated immunity, the esophageal epithelial layer is susceptible to infection
- Candida colonizes, proliferates and adheres to the esophageal mucosa, forming white-yellow plaques
- Reference: Can J Gastroenterol Hepatol 2019:2019:3585136
Etiology
- Candida albicans
- Candida tropicalis
- Candida glabrata
- Candida krusei
- Candida parapsilosis
- Reference: Clin Infect Dis 2016;62:e1
Clinical features
- Typical symptoms include dysphagia, odynophagia and retrosternal pain
- Less commonly, abdominal pain, heartburn, diarrhea, nausea, vomiting and weight loss (Am J Clin Pathol 2017;147:33)
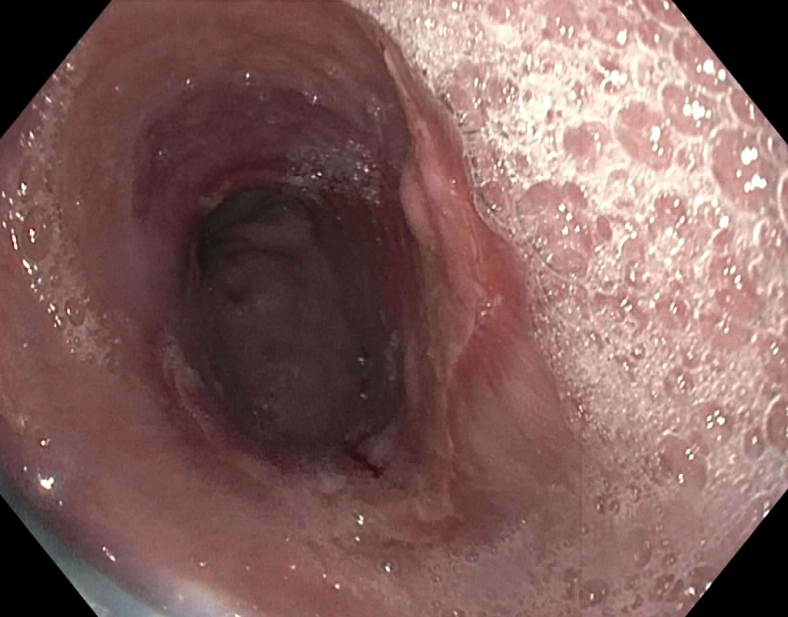
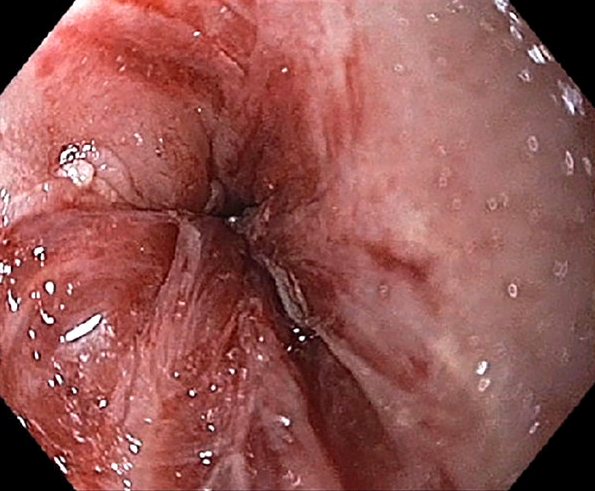
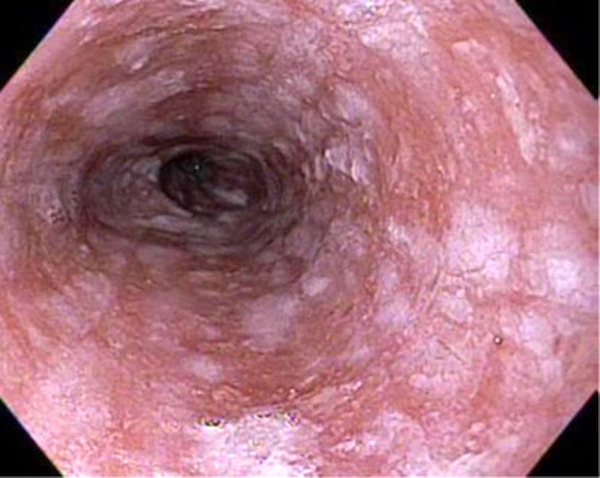
- Plaques can be seen on upper endoscopy and do not wash from the mucosa with water irrigation
- May coexist with herpes or cytomegalovirus (CMV) esophagitis, oral thrush, esophageal intramural pseudodiverticulosis
Diagnosis
- Gold standard for the diagnosis is through histological examination
- Biopsy or brushing of the esophageal mucosa is taken during endoscopy
Radiology description
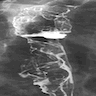
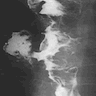
- Esophagogram
- On double contrast studies, discrete longitudinally oriented linear or irregular plaque-like lesions separated by normal mucosa with small (< 1 cm) punctuate, round or oval ulcers
- In advanced cases, the esophagus may have a grossly irregular or shaggy appearance as a result of innumerable plaques and pseudomembranes, with trapping of barium between the lesions
- Cobblestone appearance may be visible (Radiology 2005;237:414)
- Patients with scleroderma or achalasia may develop a foamy esophagus
Prognostic factors
- Poorer prognosis in the elderly (Dis Esophagus 2006;19:189)
- Esophageal obstruction, perforation and tracheoesophageal or aortoesophageal fistula formation are other rare but potentially life threatening complications
Case reports
- 29 year old Black woman, status post-deceased donor kidney transplant, with difficulty and pain in swallowing (BMJ Case Rep 2019;12:e230410)
- 36 year old man with esophageal stricture due to esophageal candidiasis (ACG Case Rep J 2021;8:e00603)
- 74 year old man with 3 day course of dysphagia (Cureus 2022;14:e24312)
- 80 year old woman with nausea, vomiting and appetite loss (JGH Open 2022;6:512)
Treatment
- Usually 2 - 3 week course of fluconazole (UpToDate: Esophageal Candidiasis in Adults [Accessed 16 November 2023])
- Treatment should continue for 1 - 2 weeks after resolution of symptoms
- Voriconazole is FDA approved for children at least 12 years of age
- Resolution of the radiographic findings sometimes lags behind the clinical recovery, so follow up barium studies may still be abnormal in patients who are asymptomatic
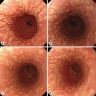
Gross description
- White or yellow patches measuring < 1 cm
- Resemble cottage cheese
- Reference: Prz Gastroenterol 2013;8:333
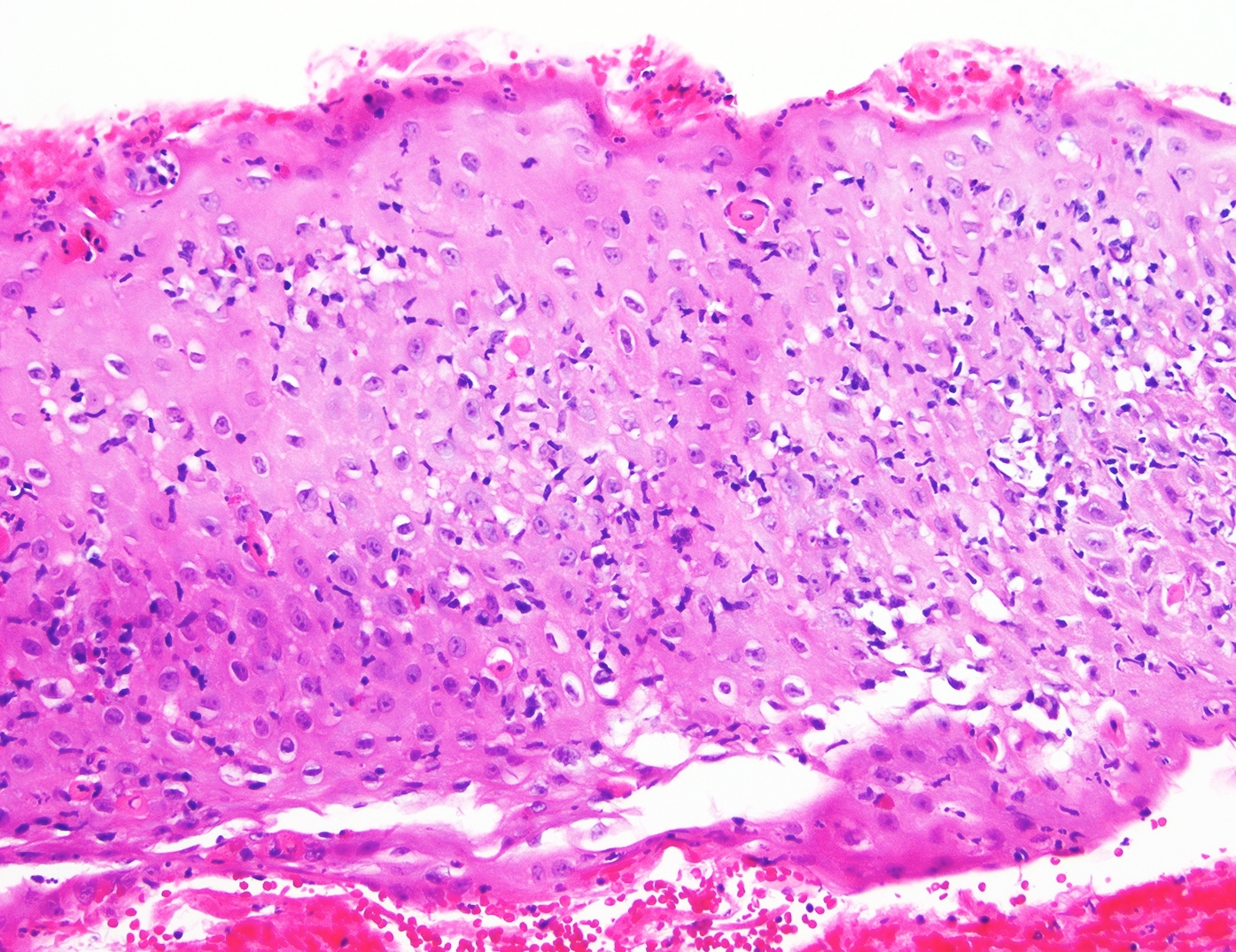
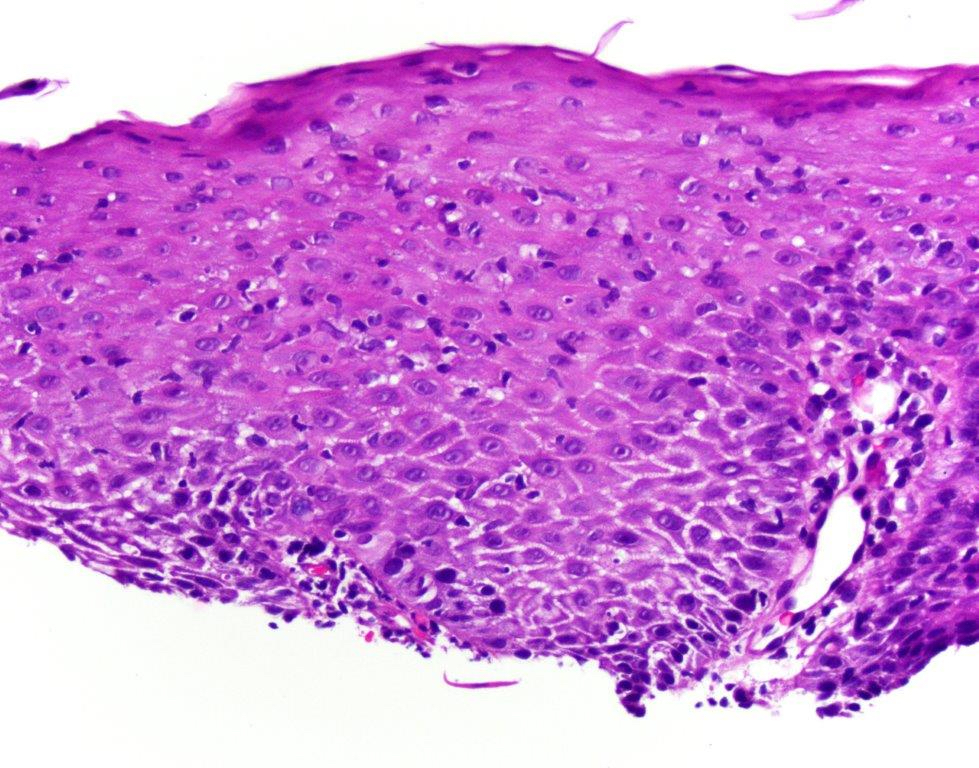
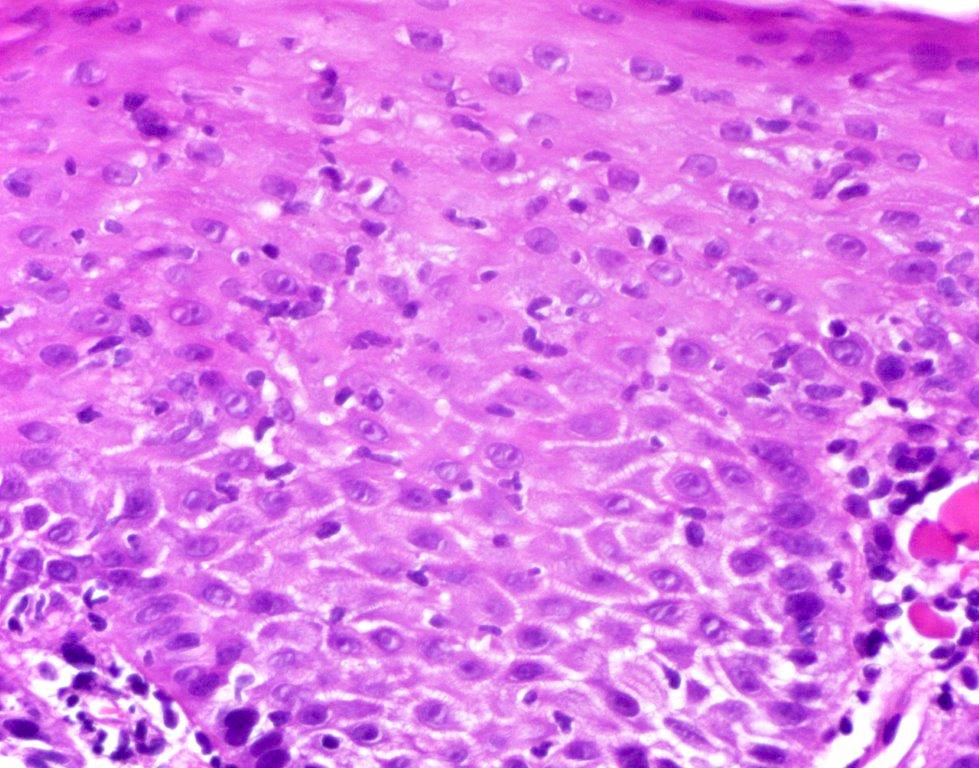
Microscopic (histologic) description
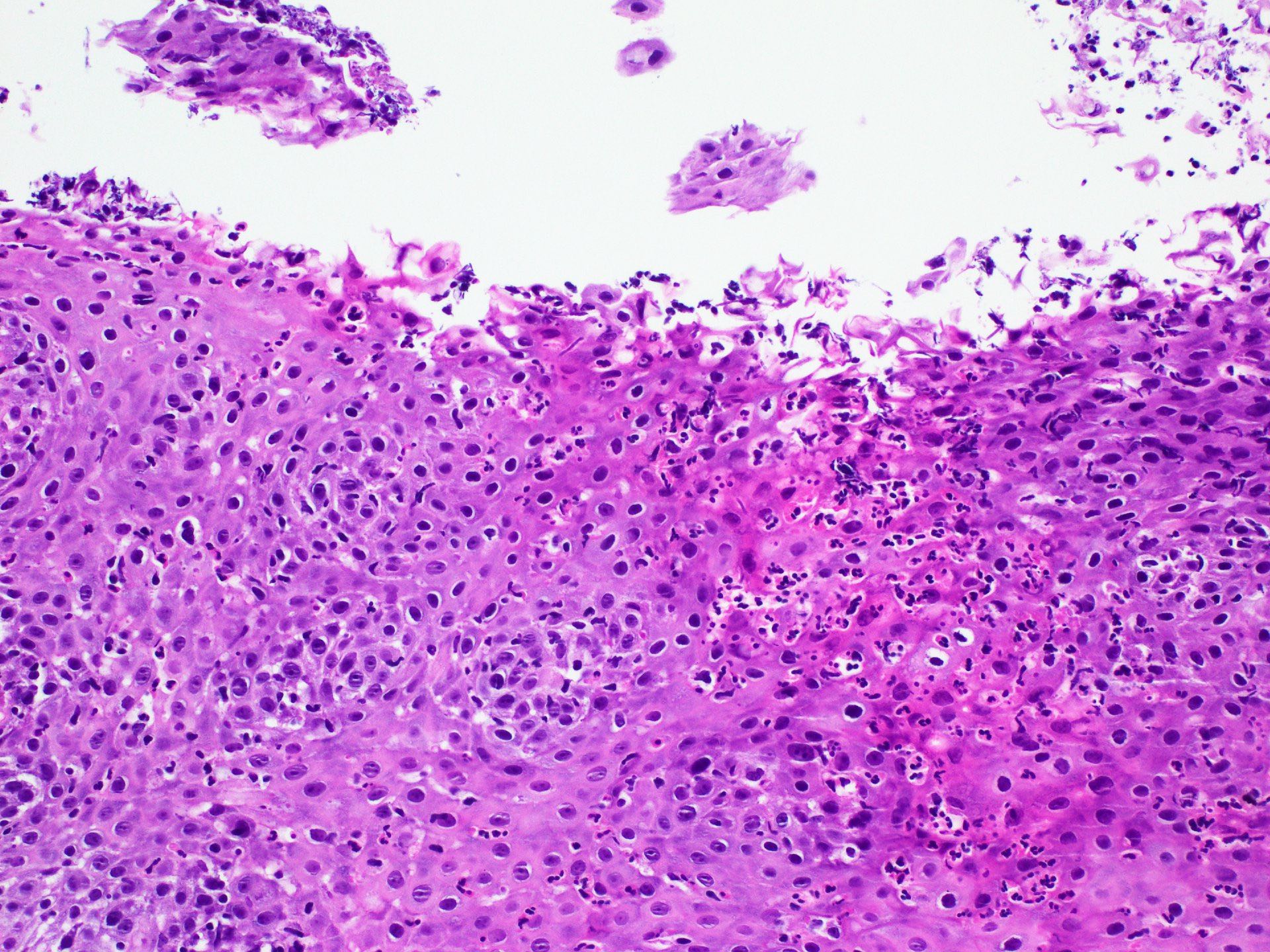
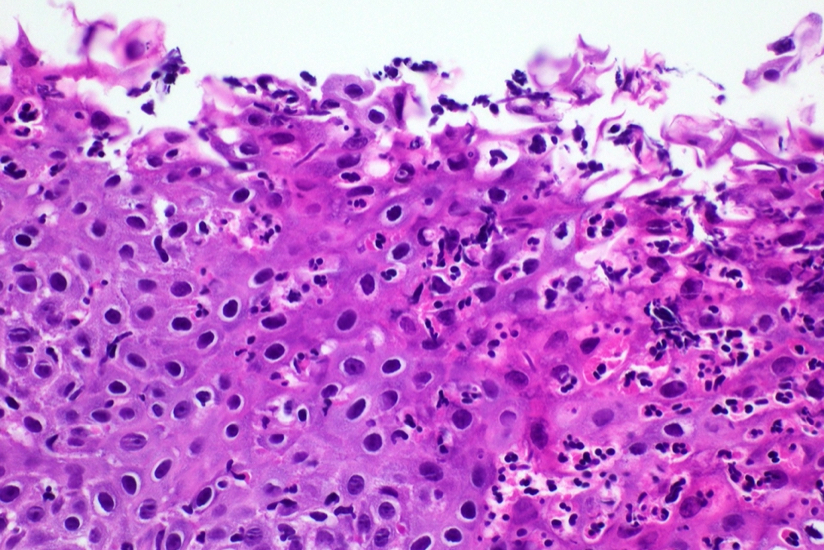
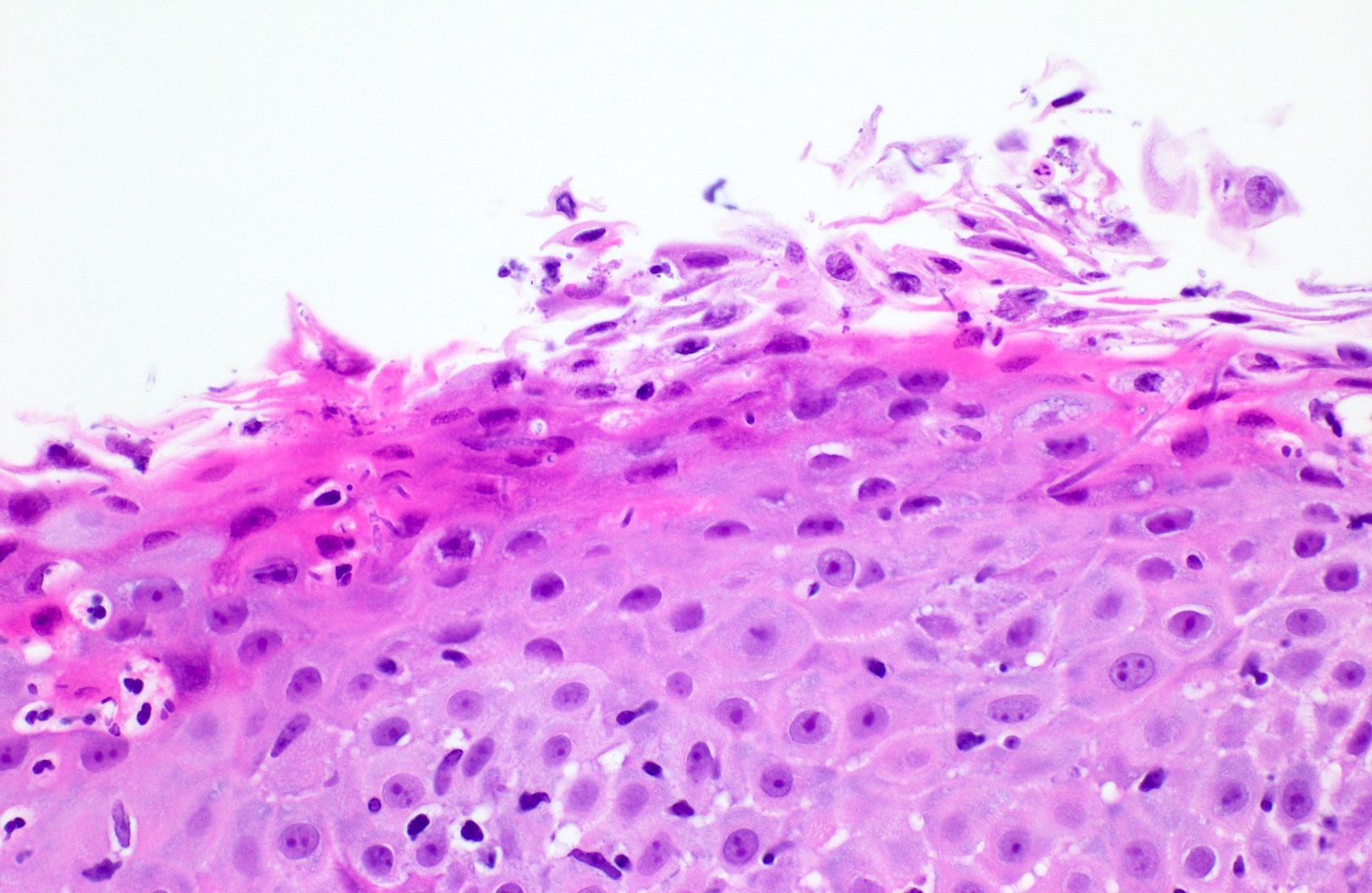
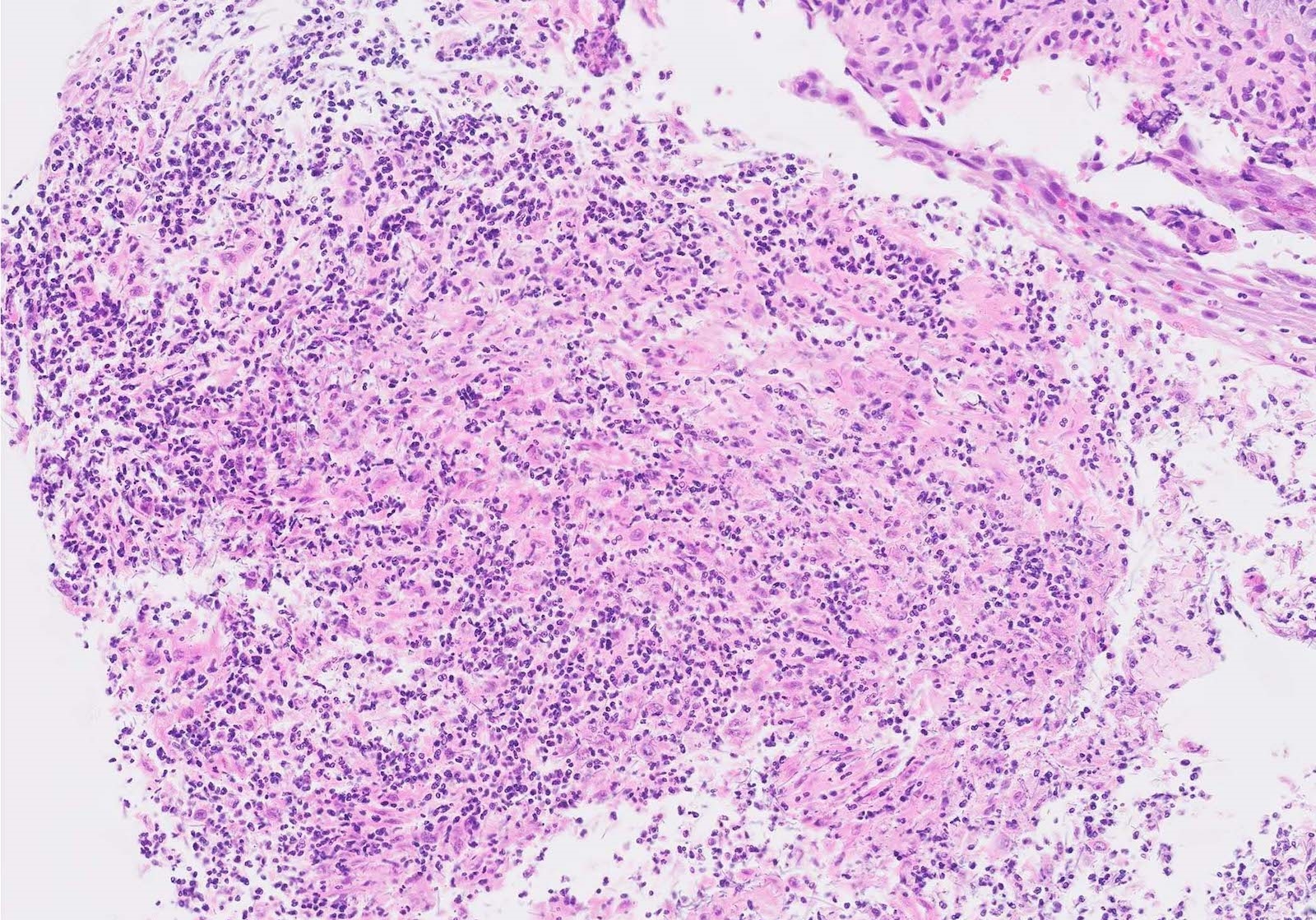
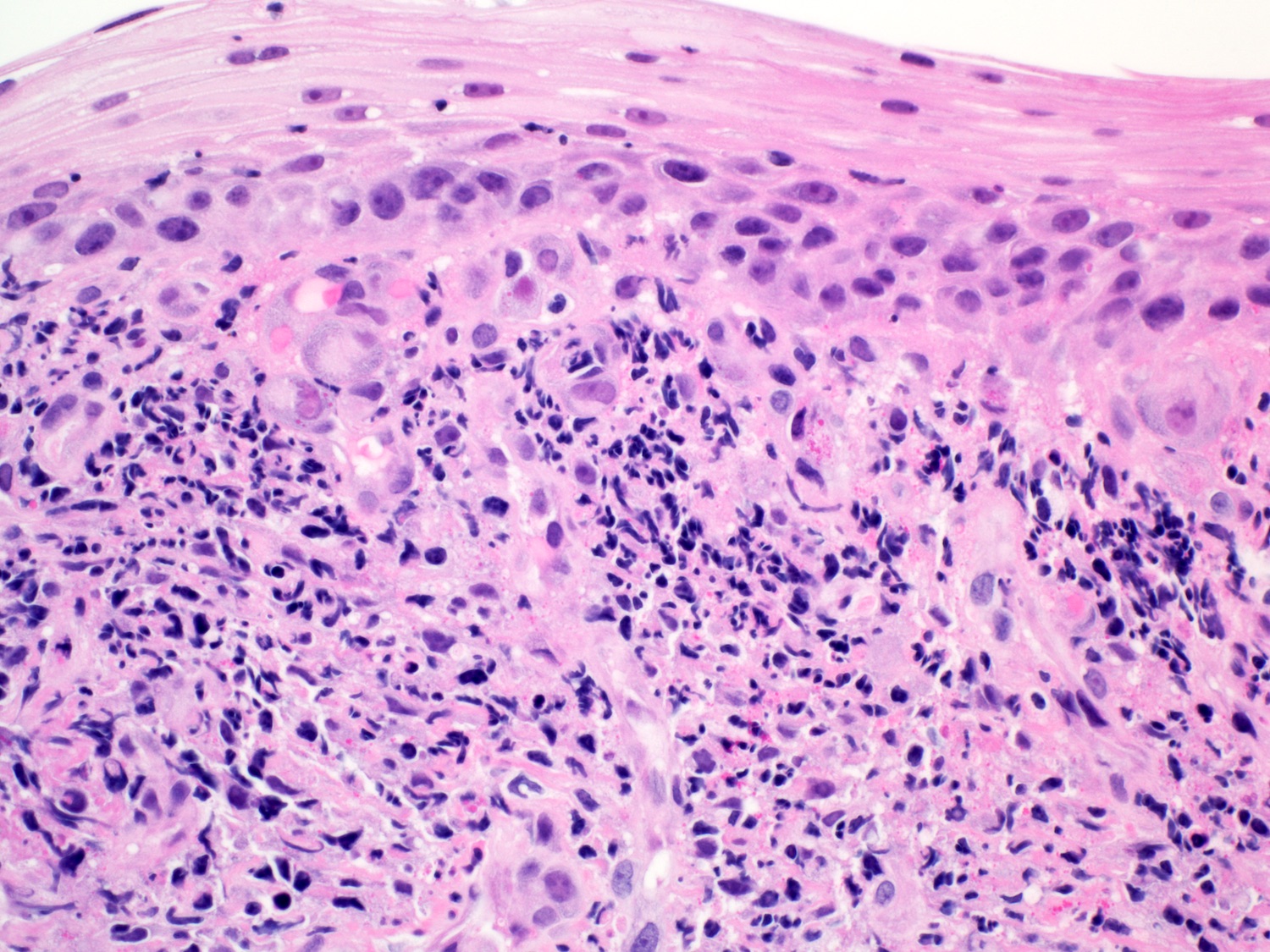
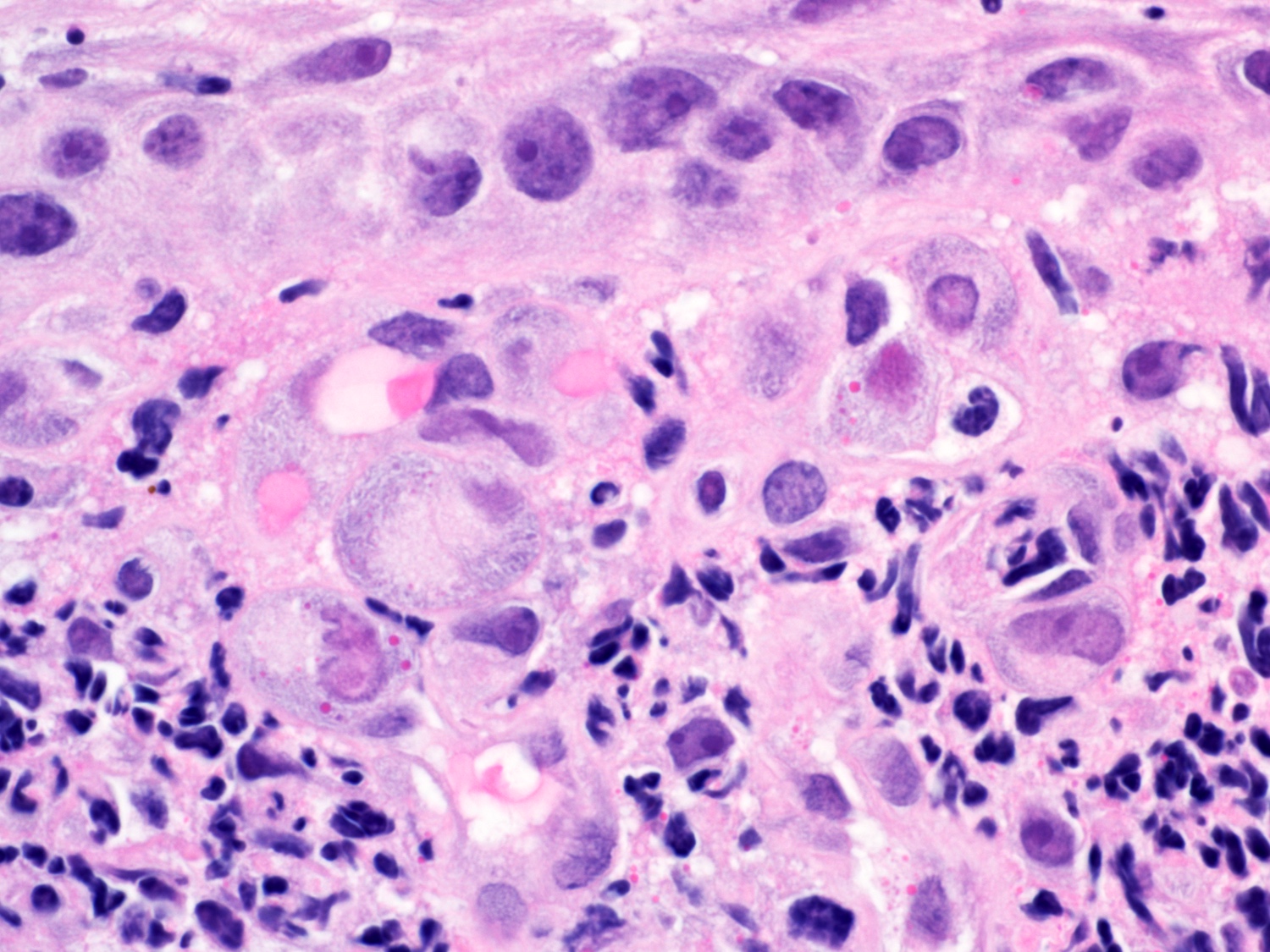
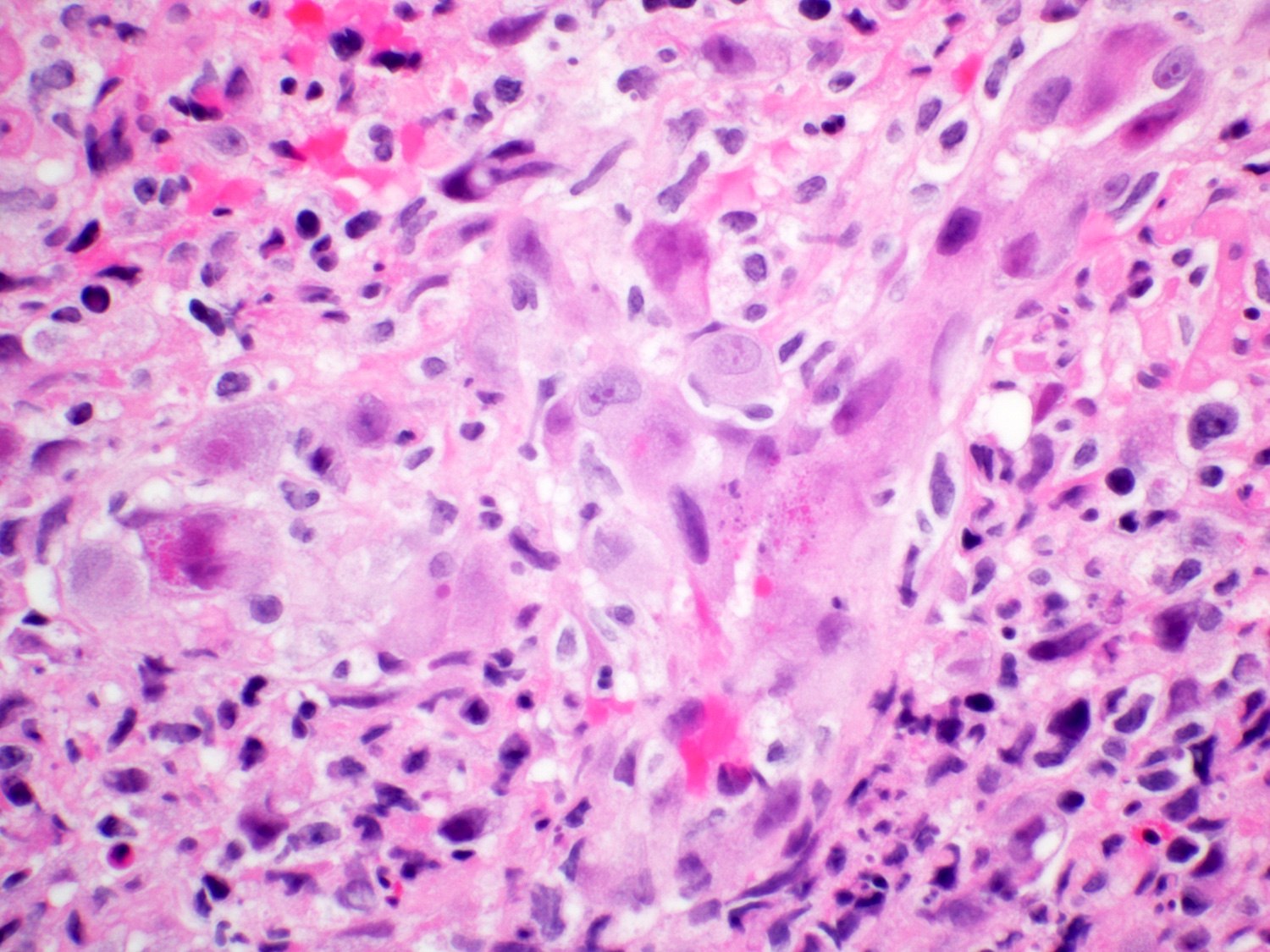
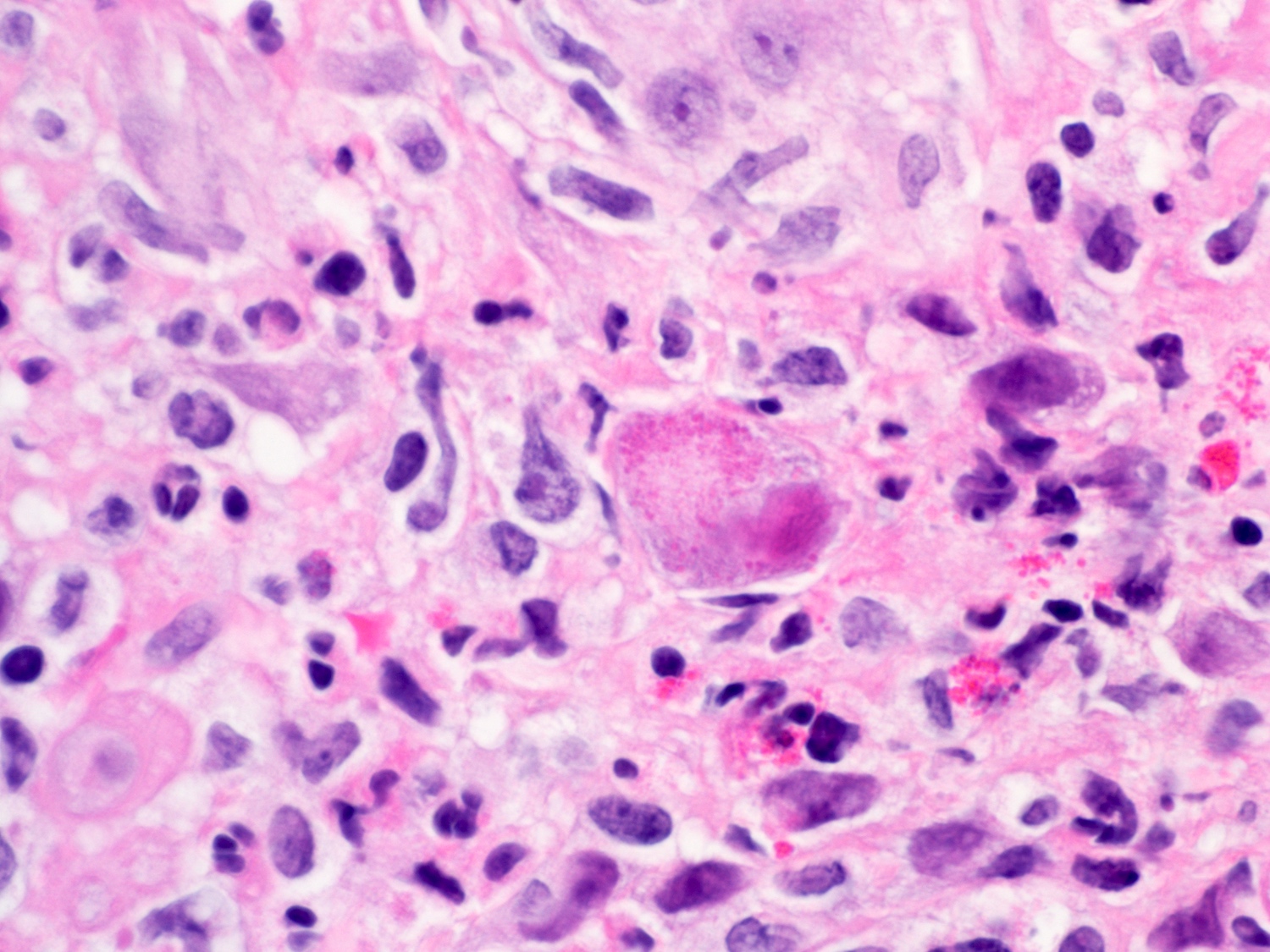
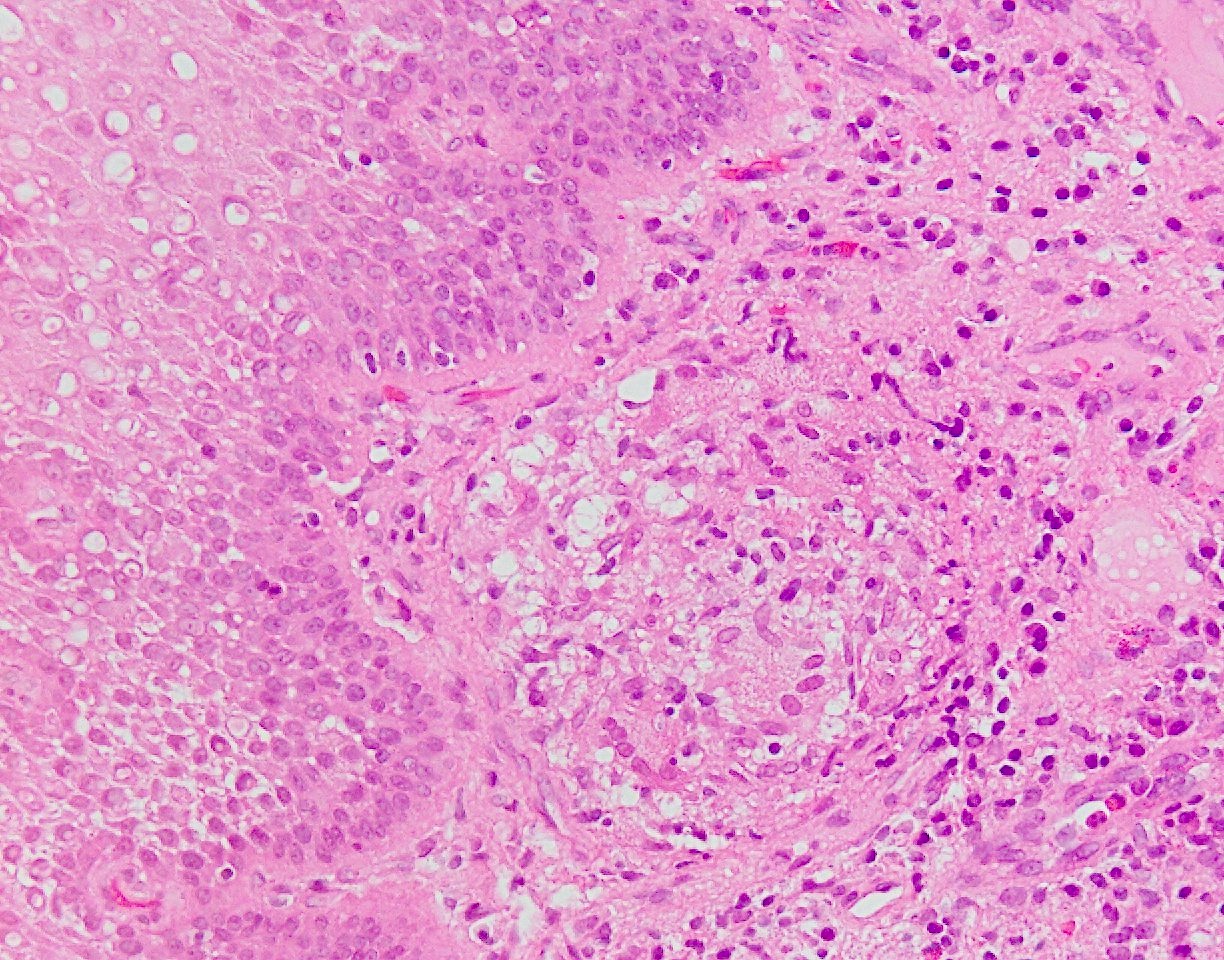
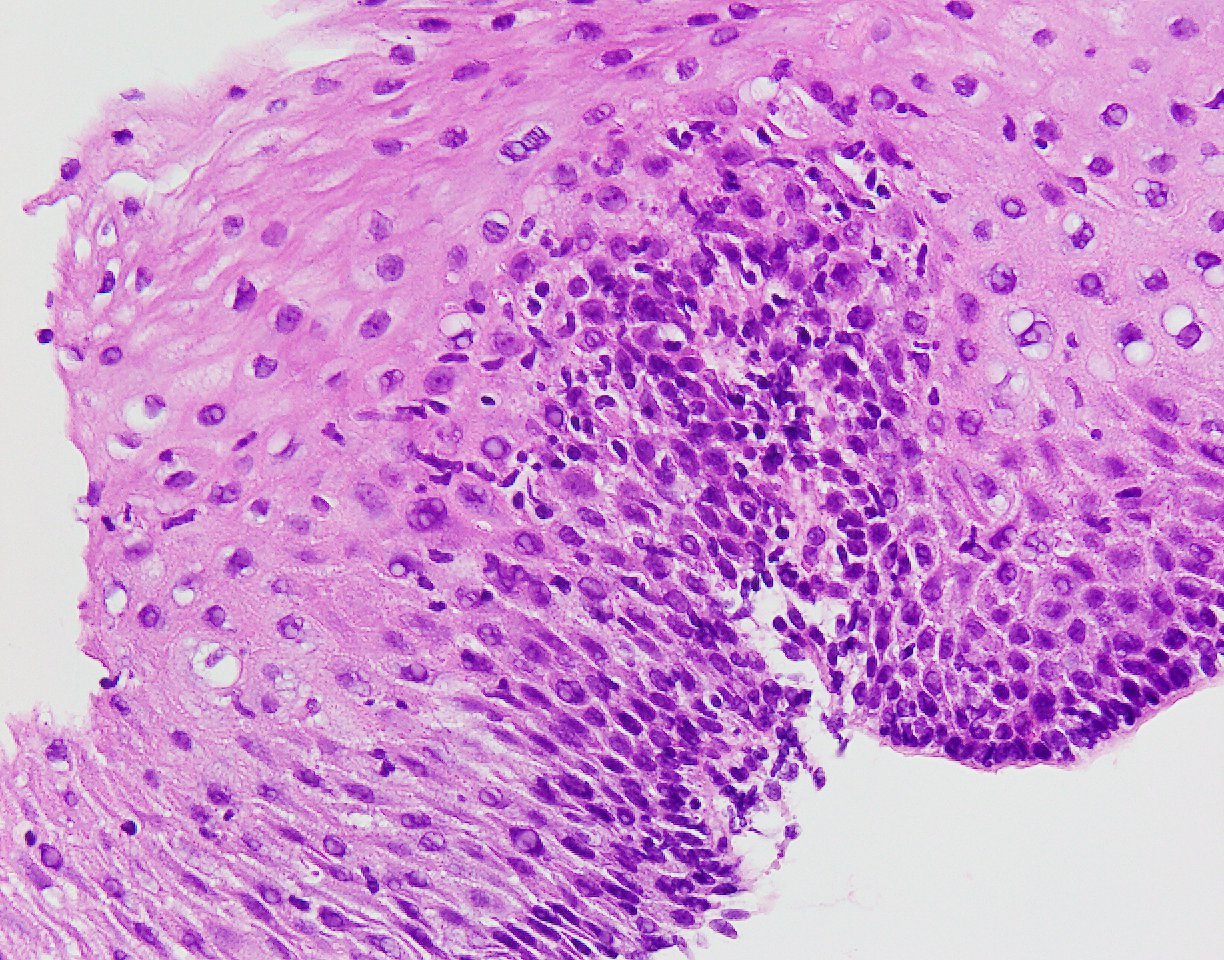
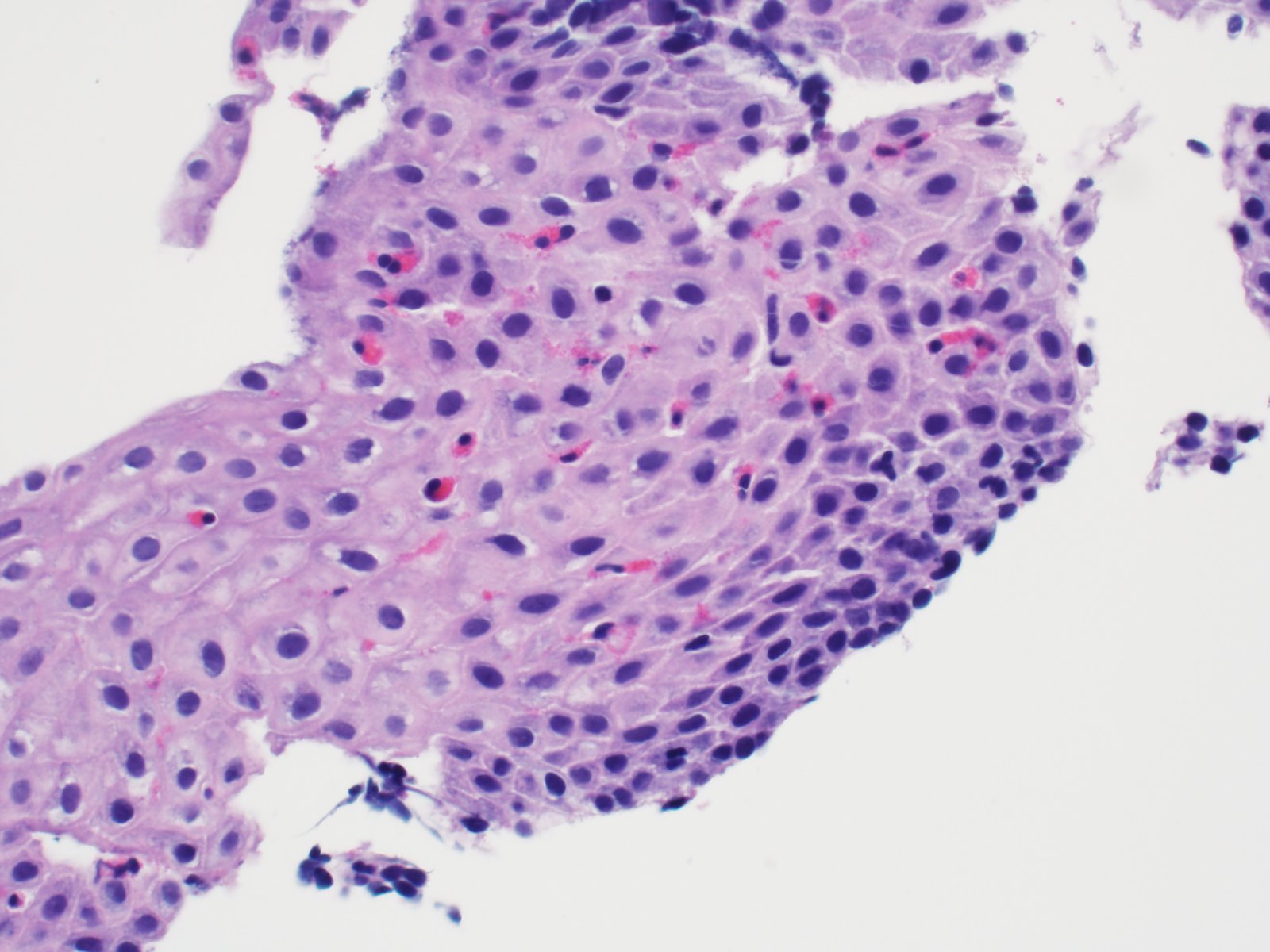
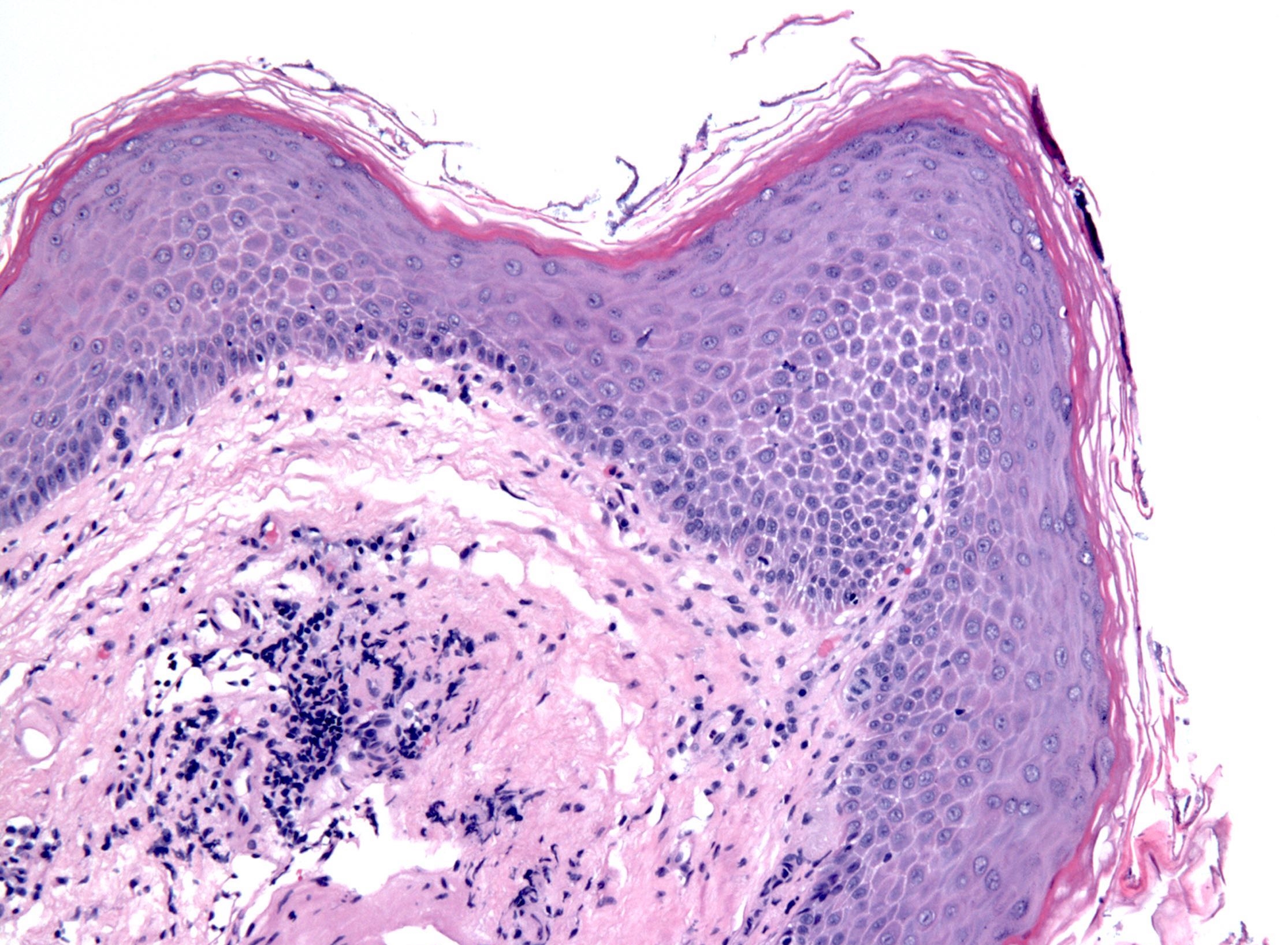
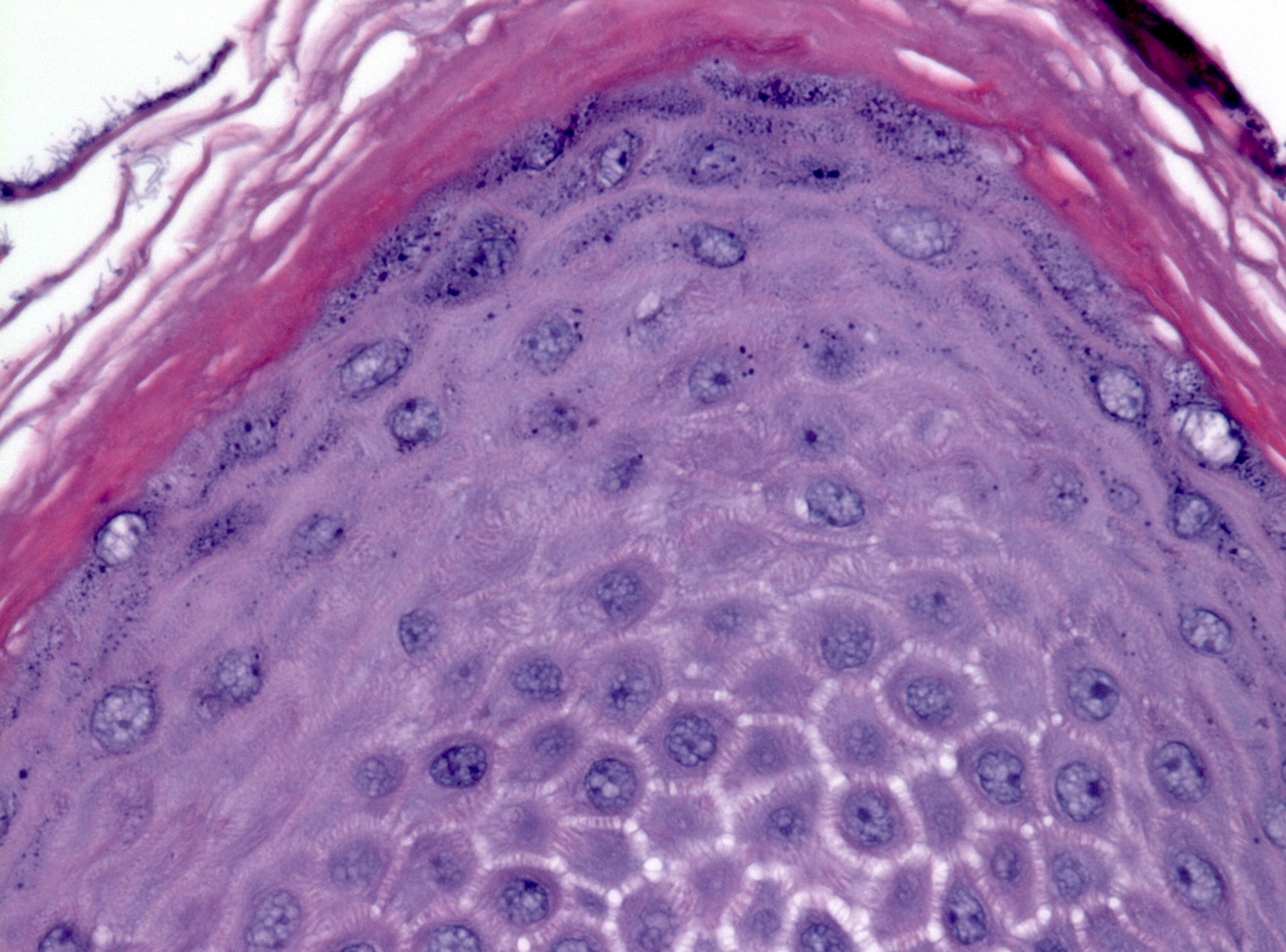
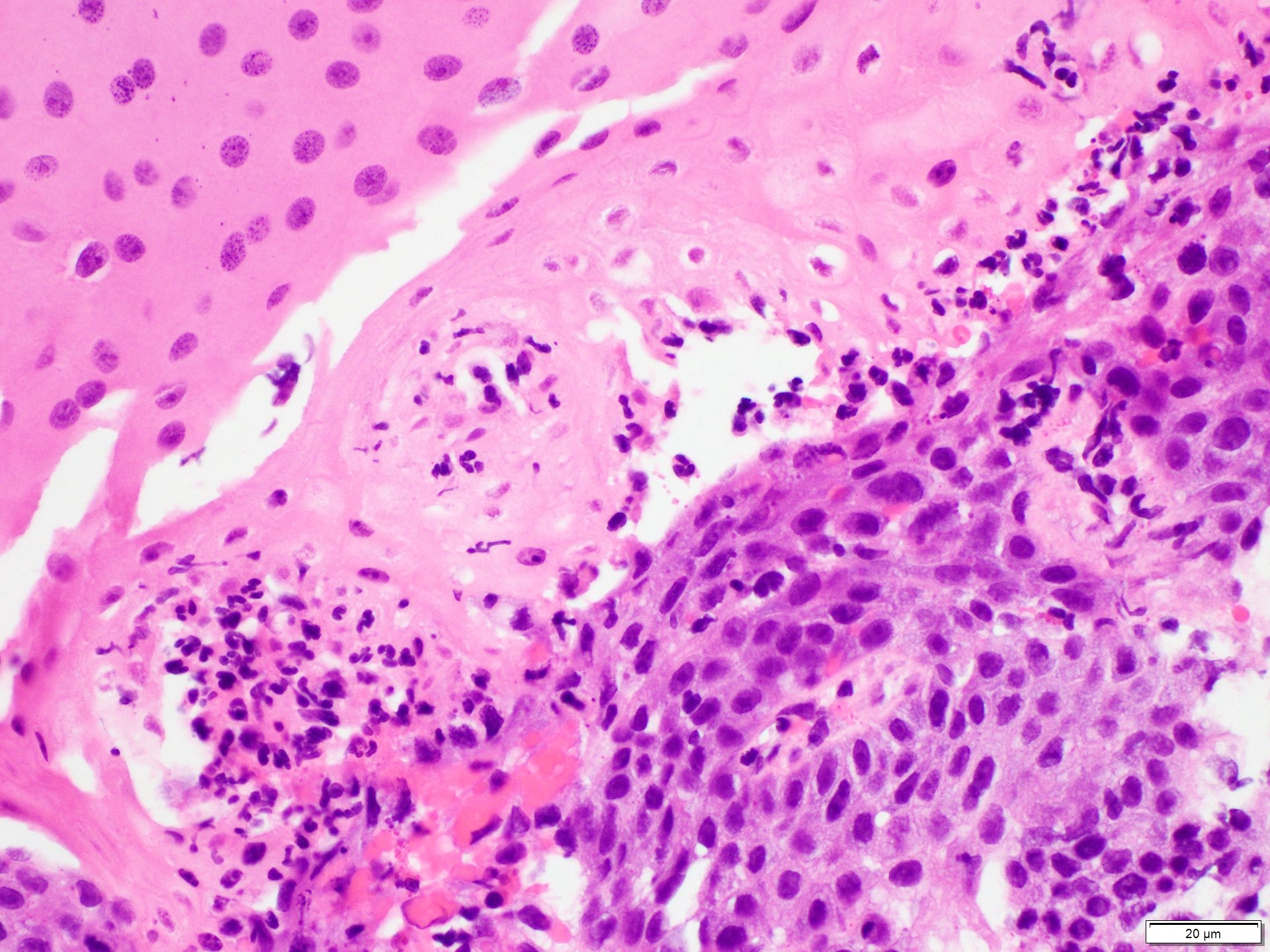
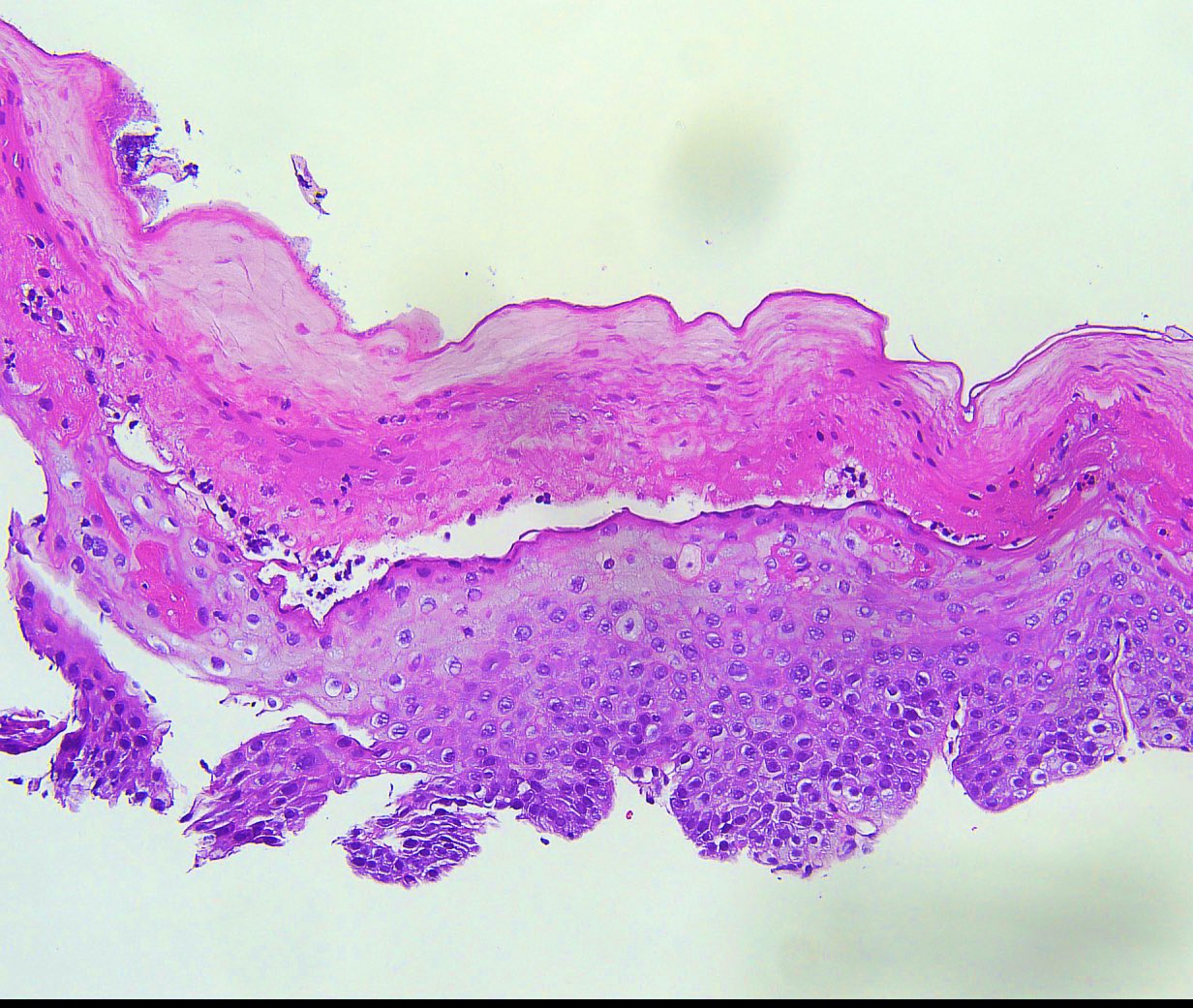
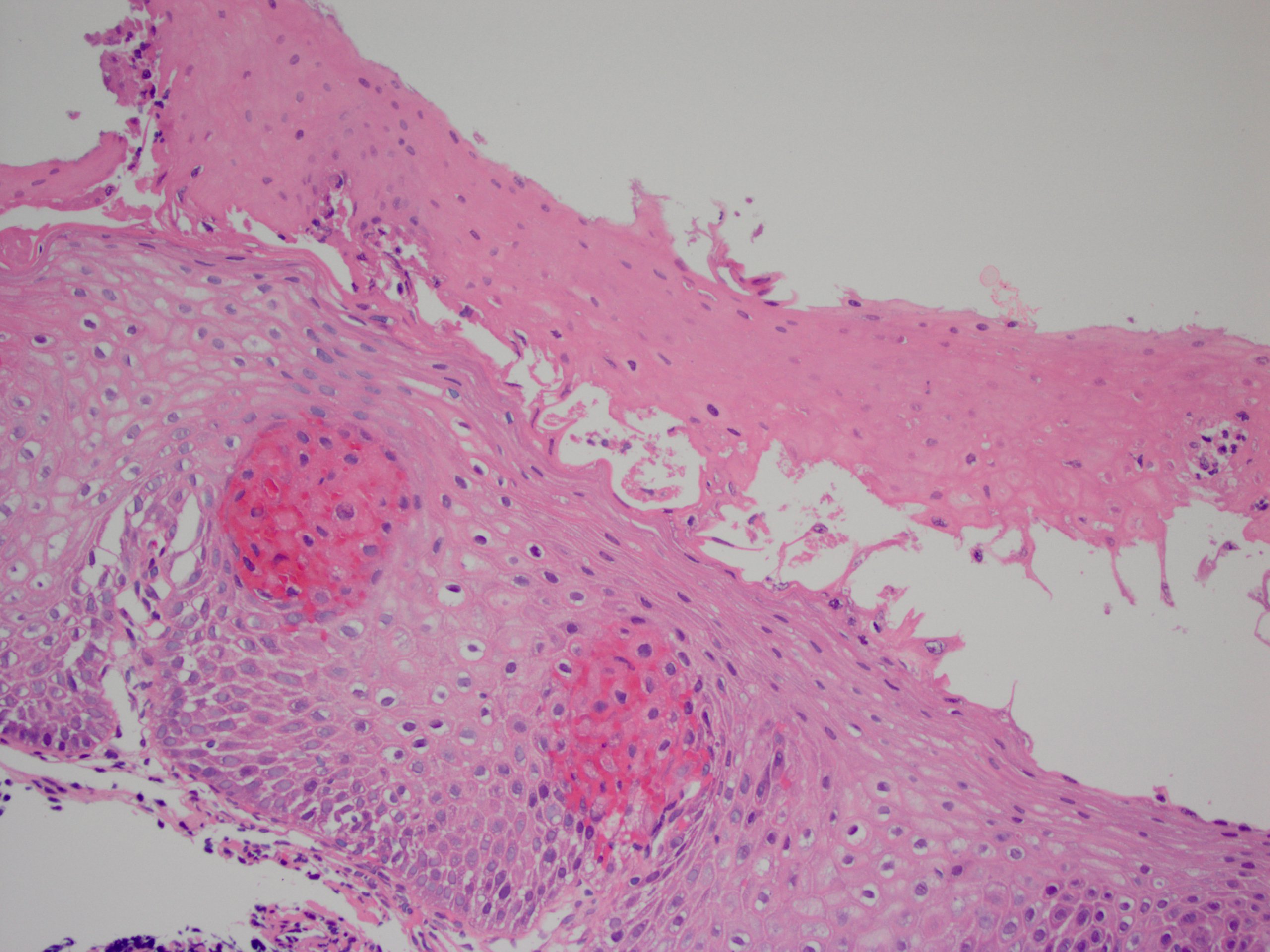
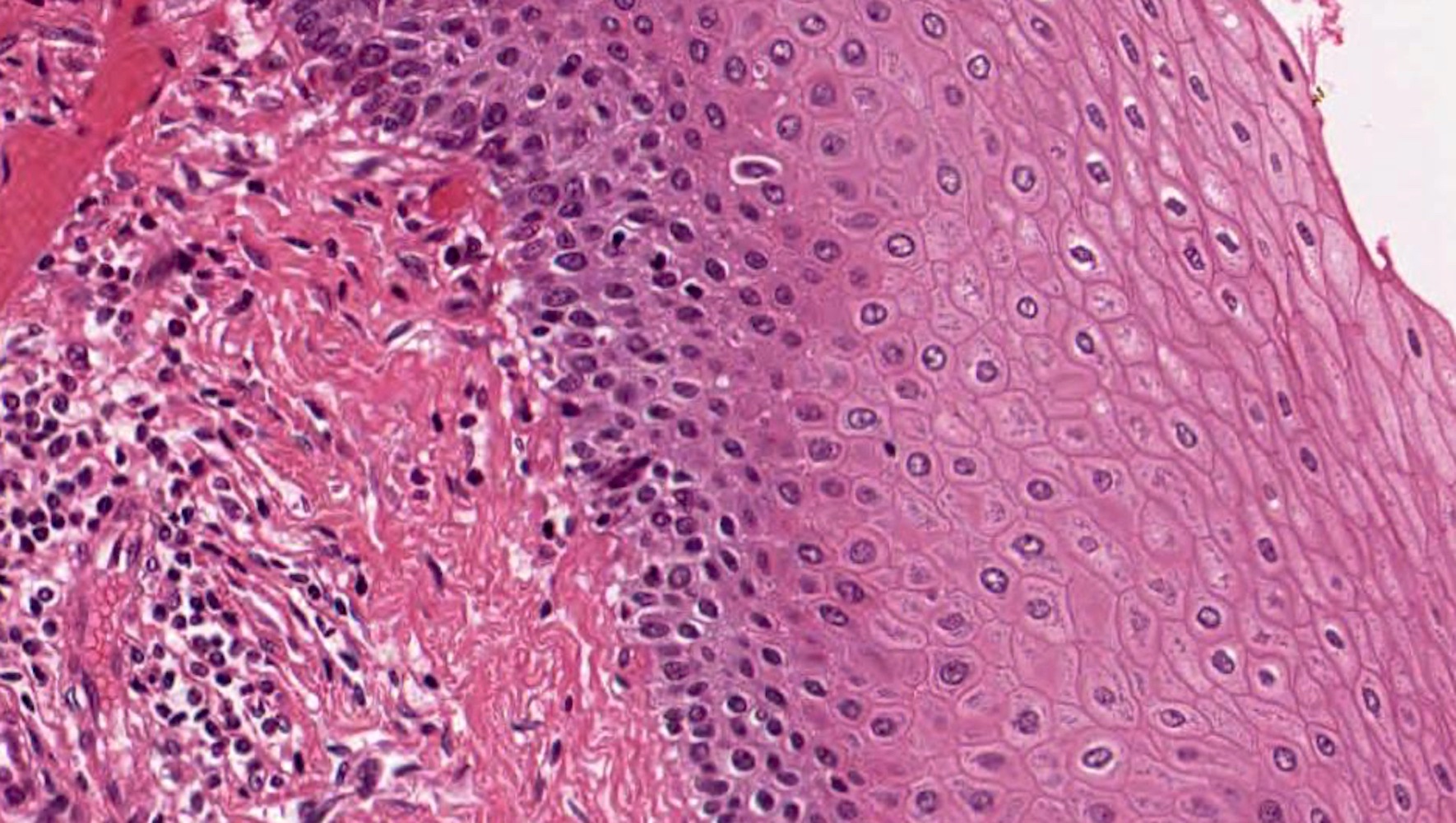
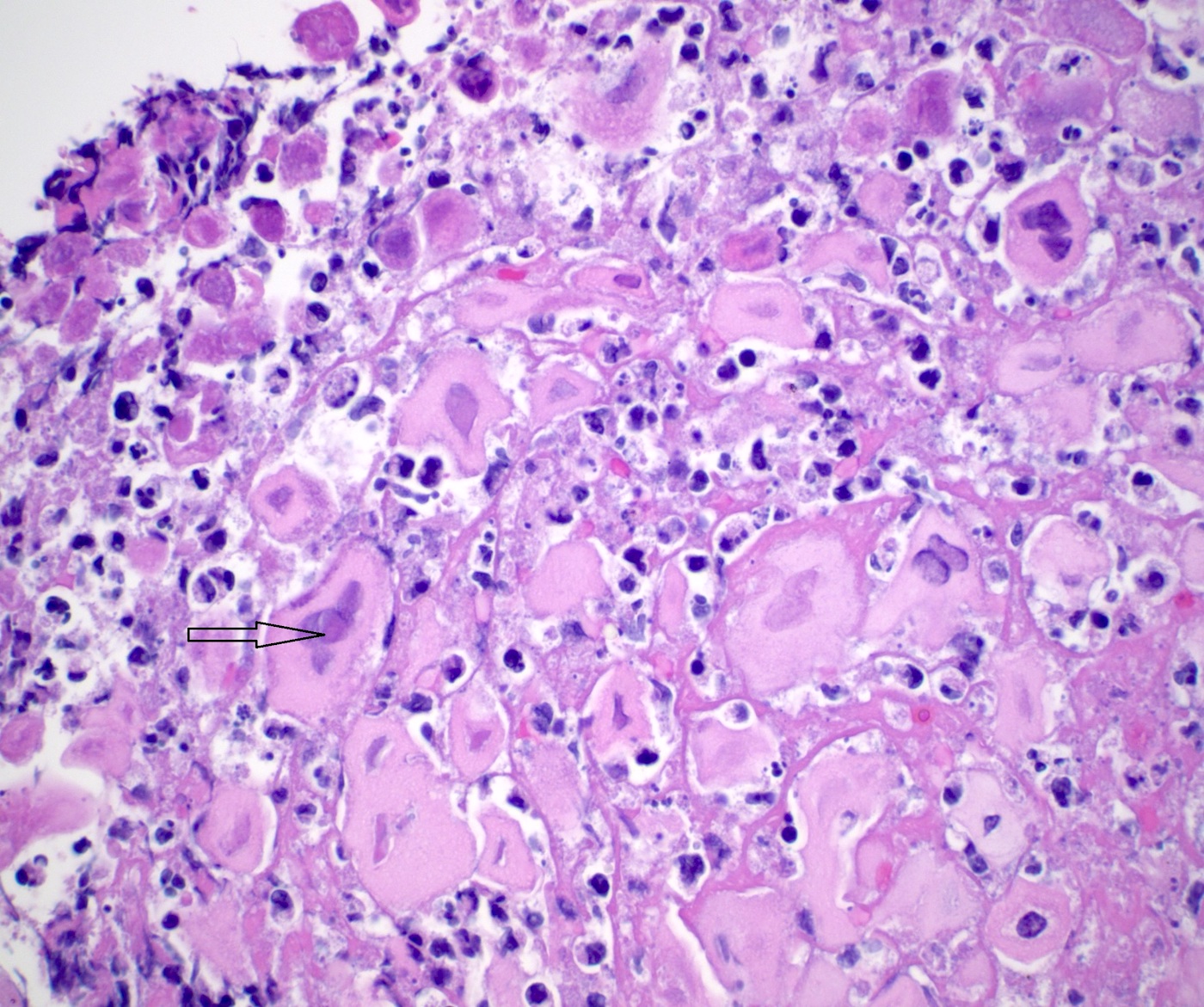
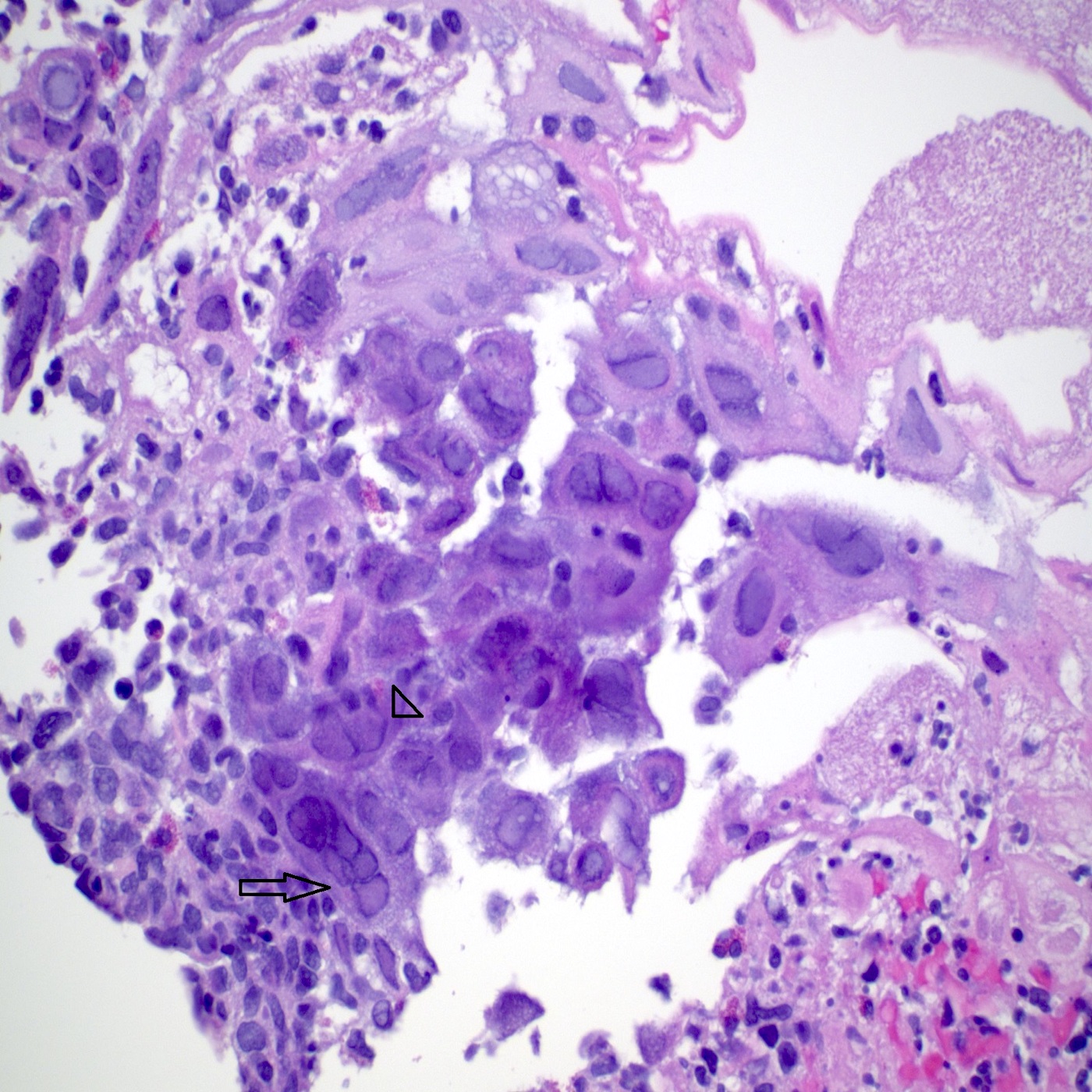
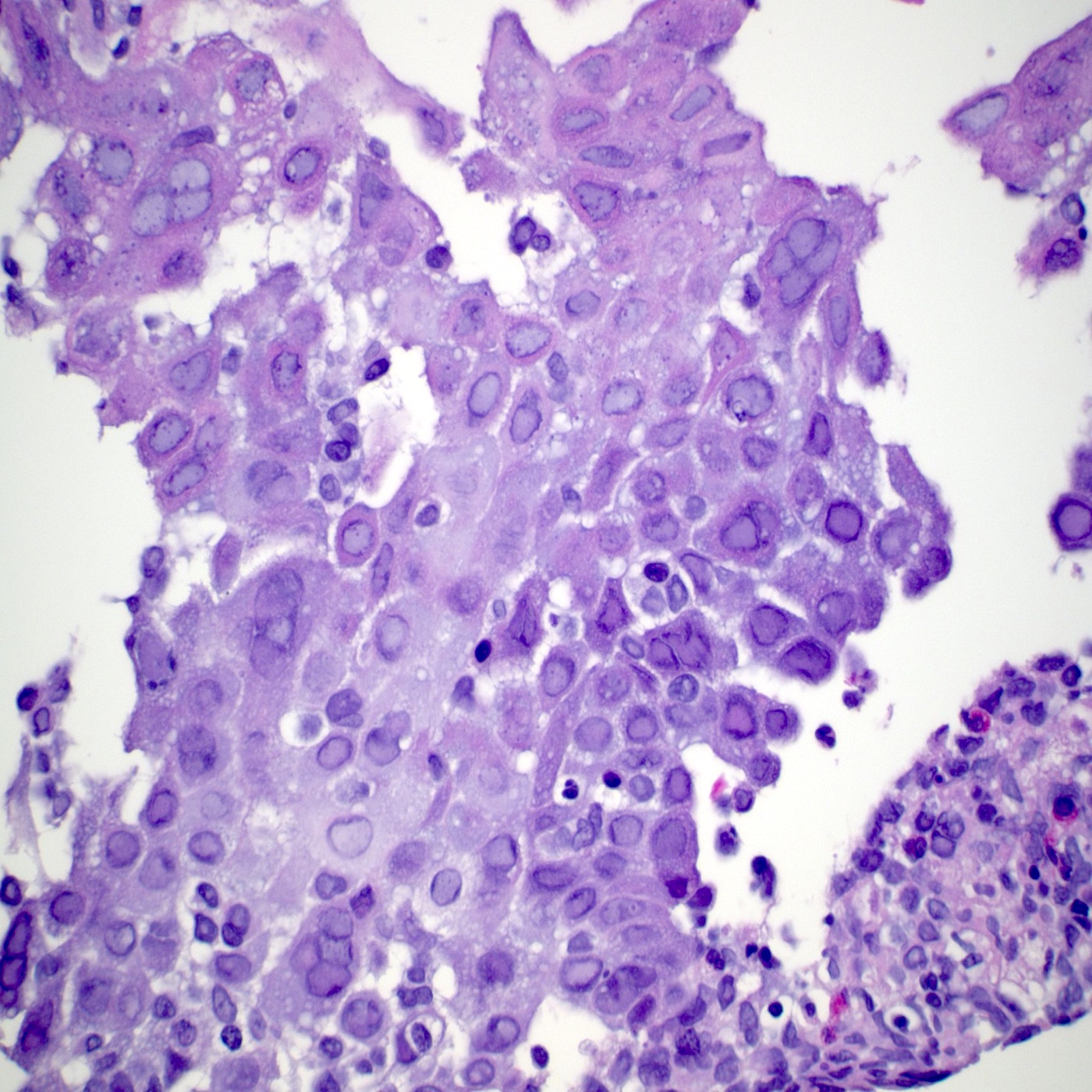
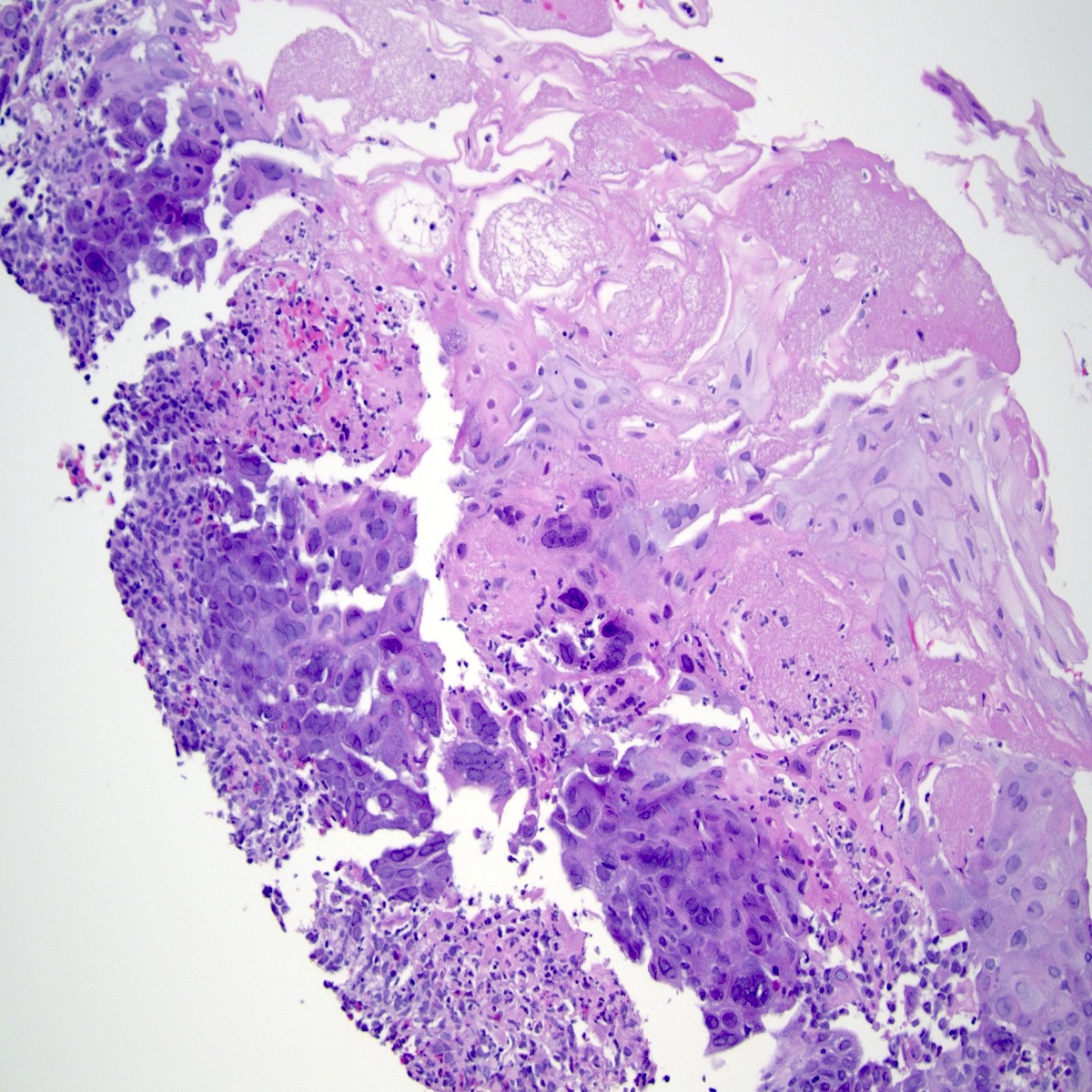
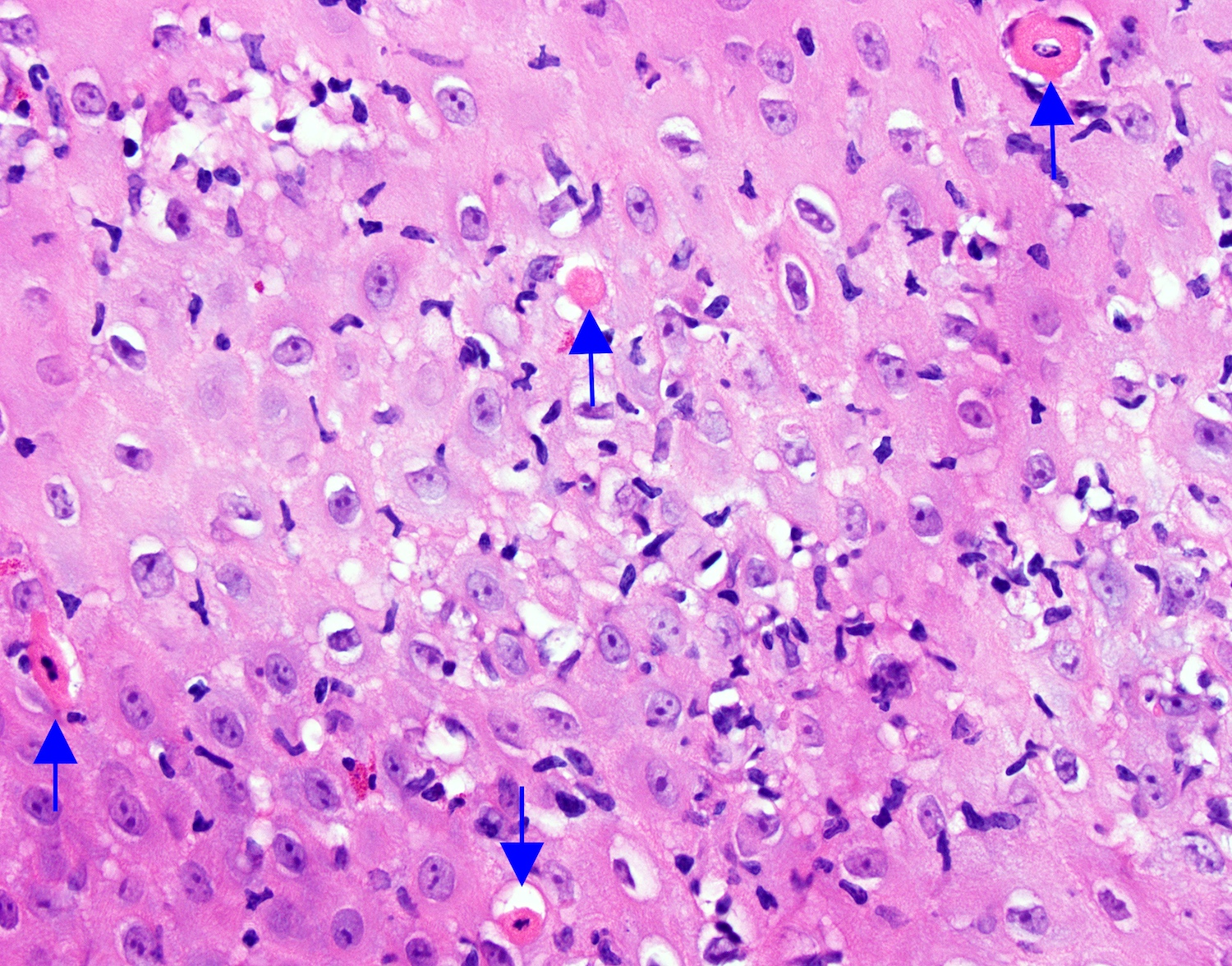
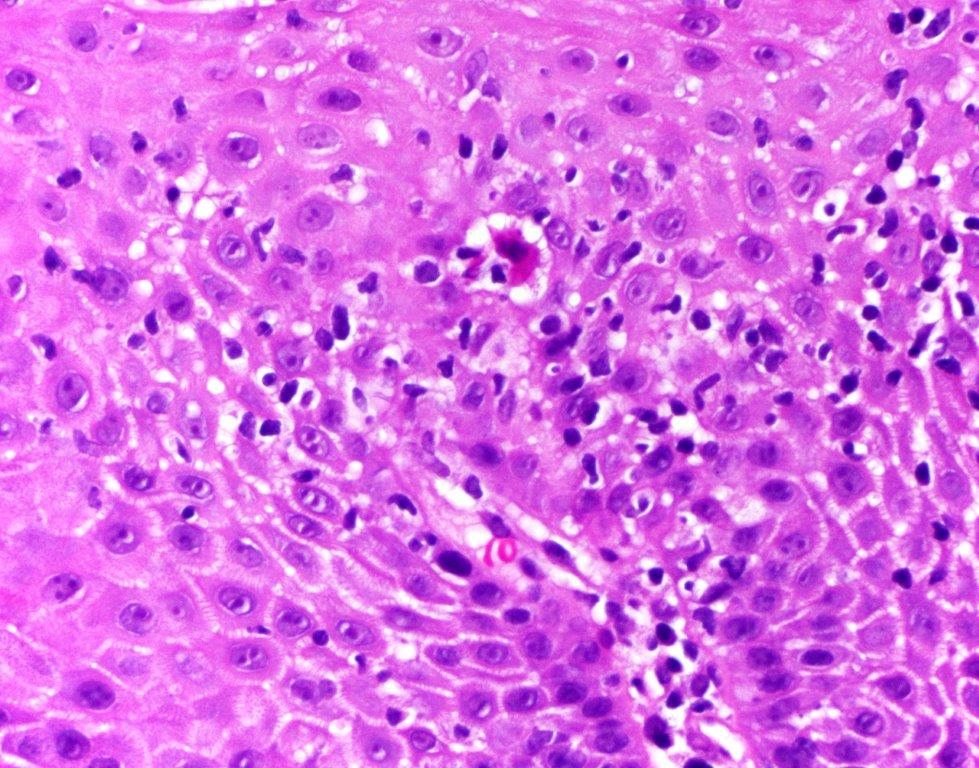
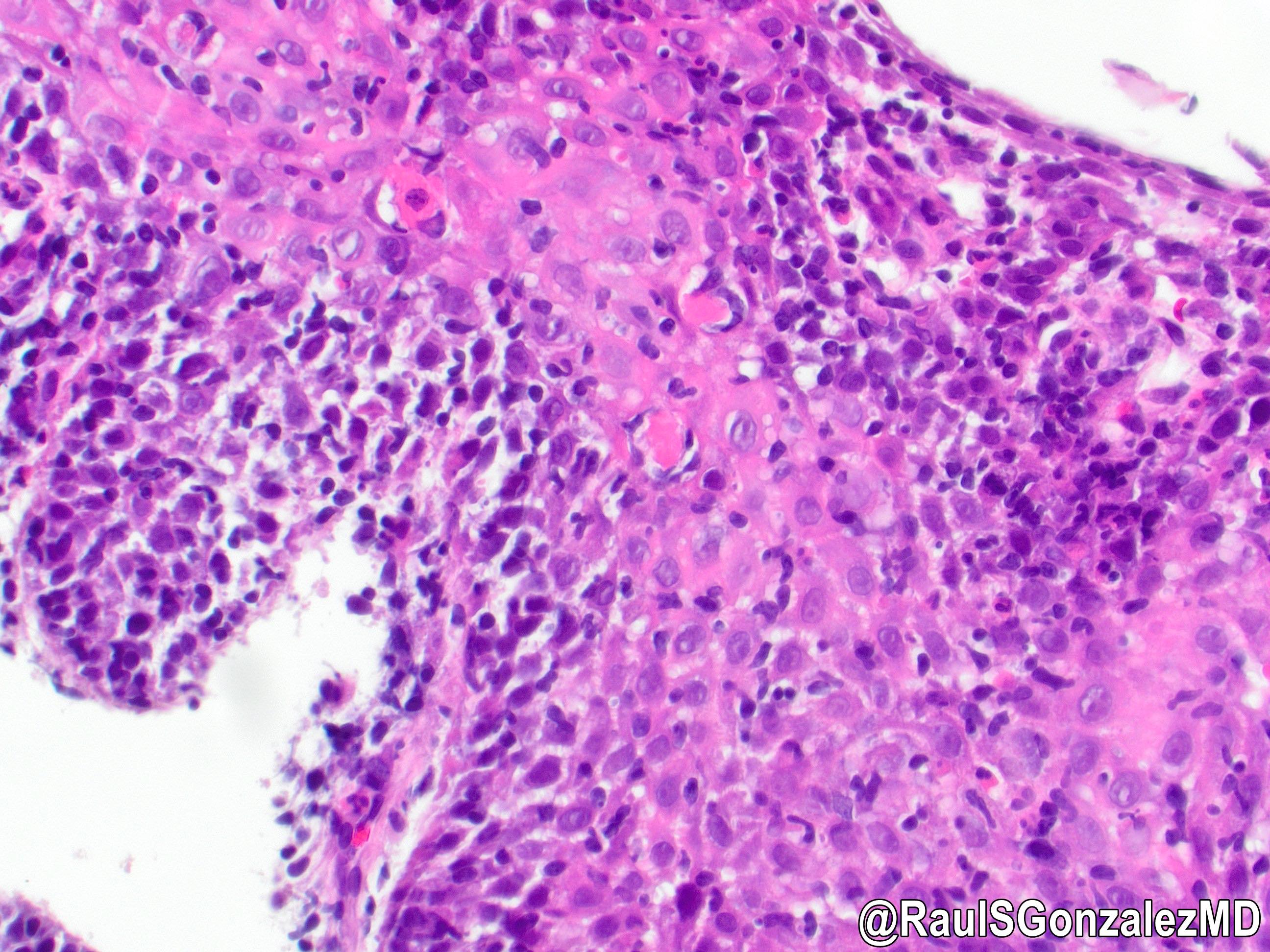
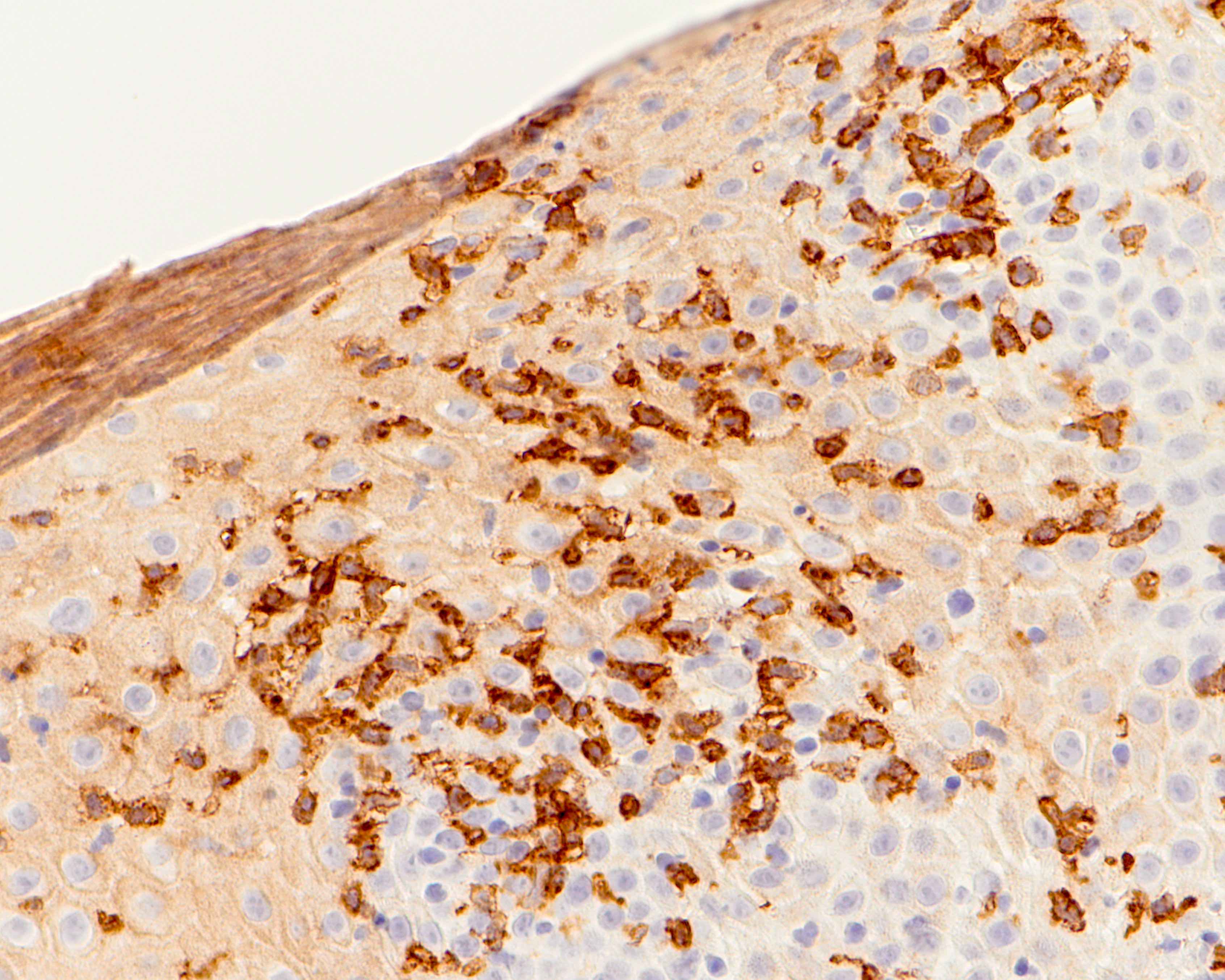
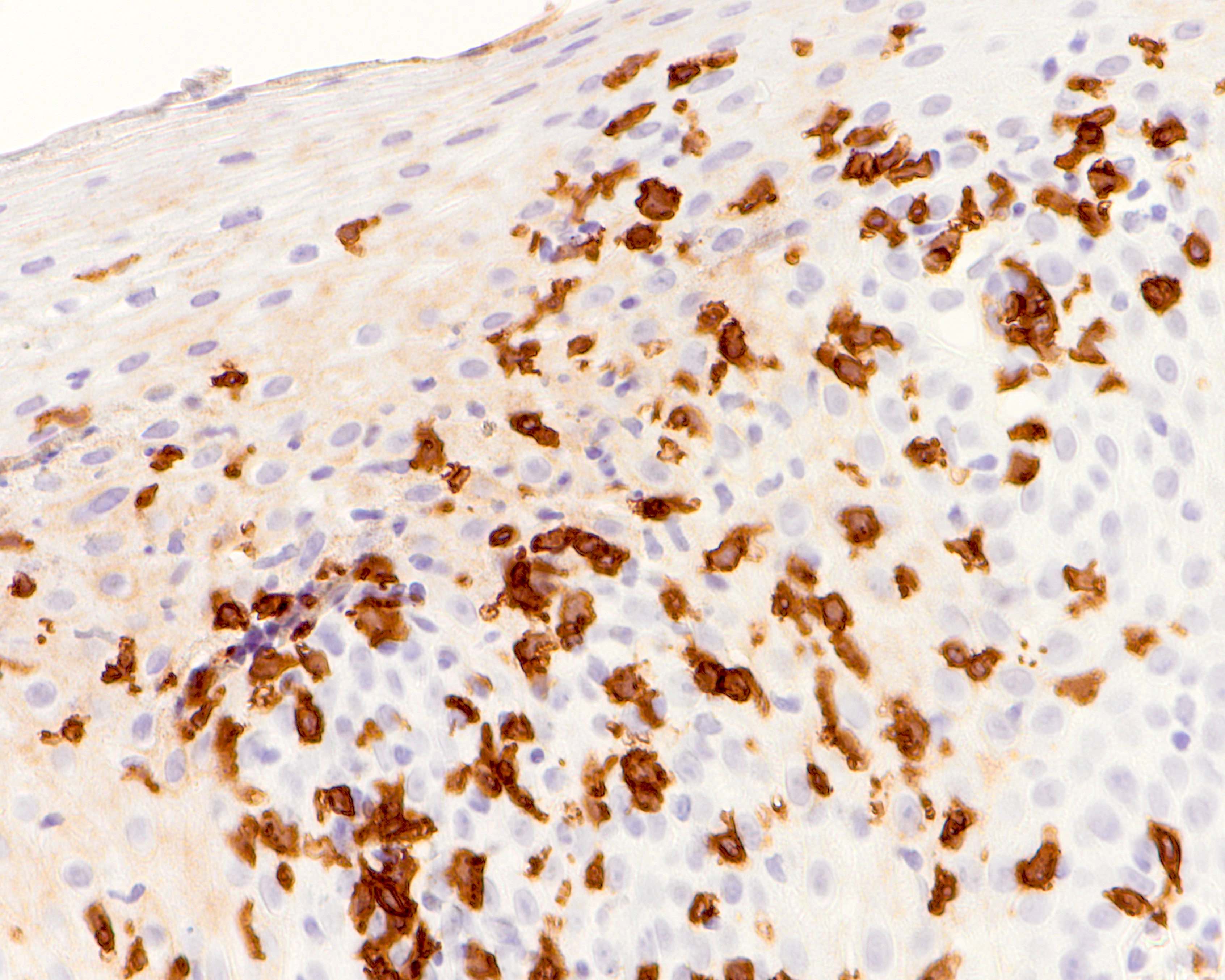
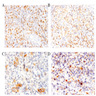
- Erosive esophagitis pattern of injury with acute inflammation, intraepithelial neutrophilic abscesses and epithelial edema most prominent in the superficial epithelial layers
- Reactive changes including basal zone hyperplasia, parakeratosis and hyperkeratosis are frequently associated
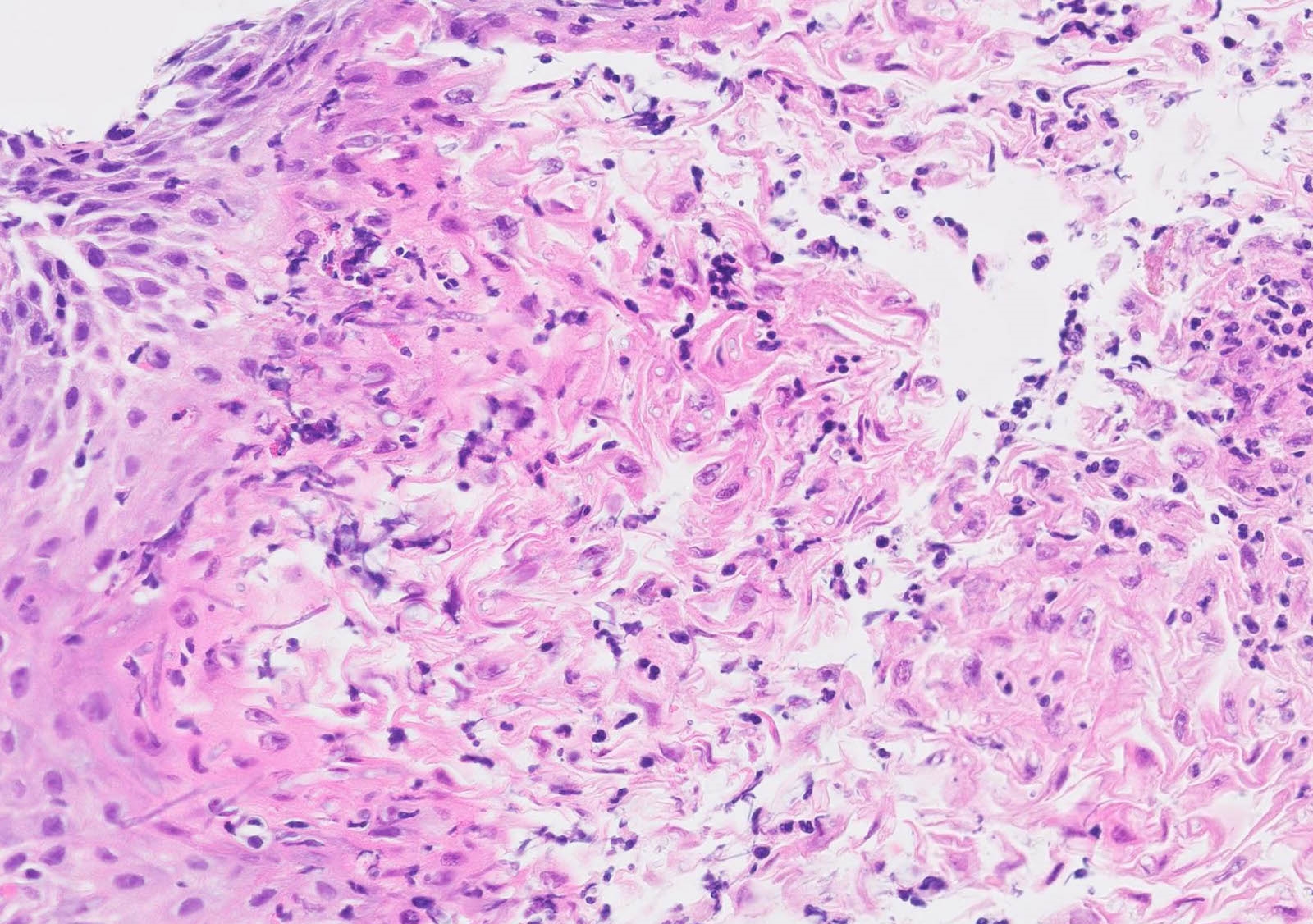
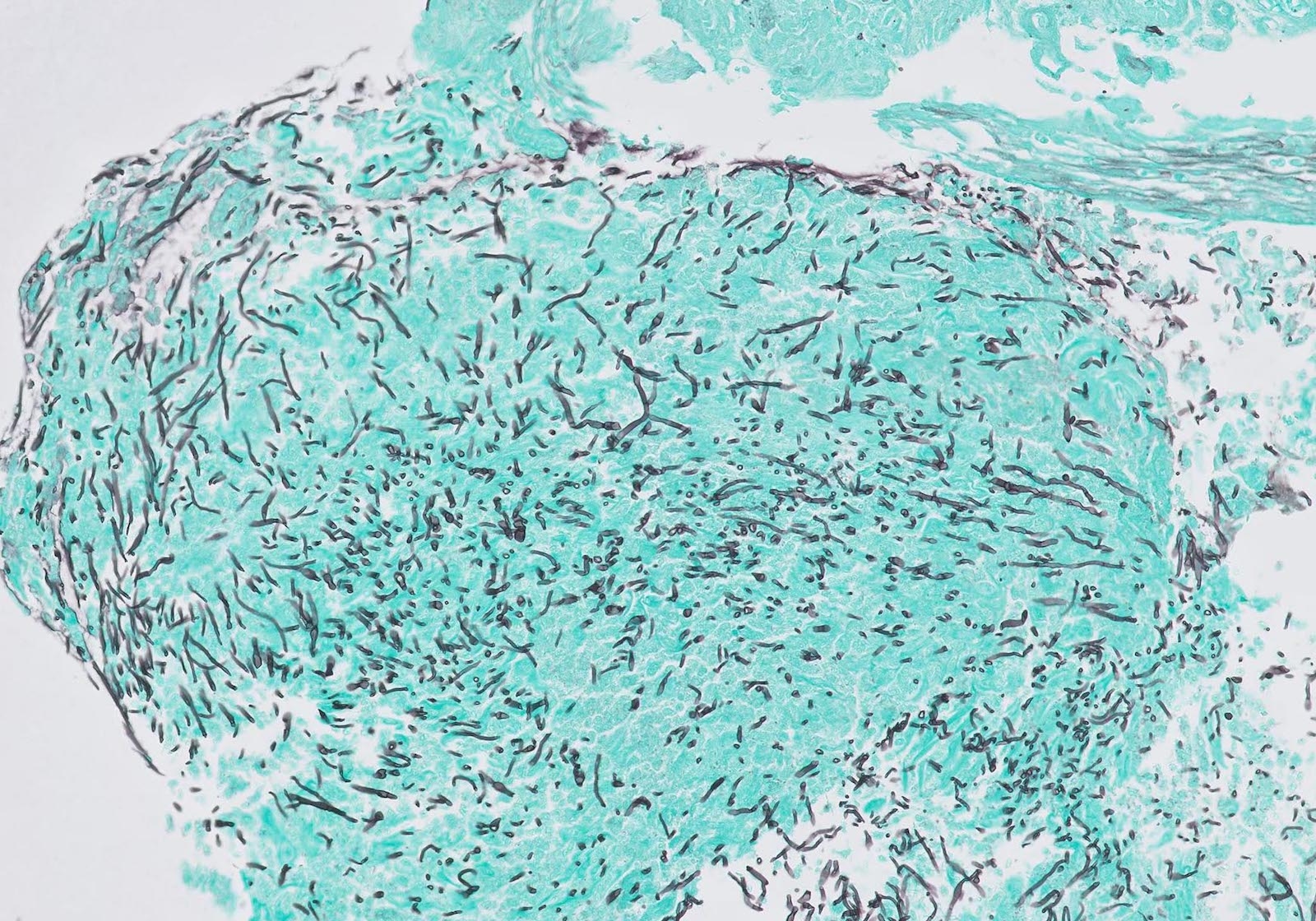
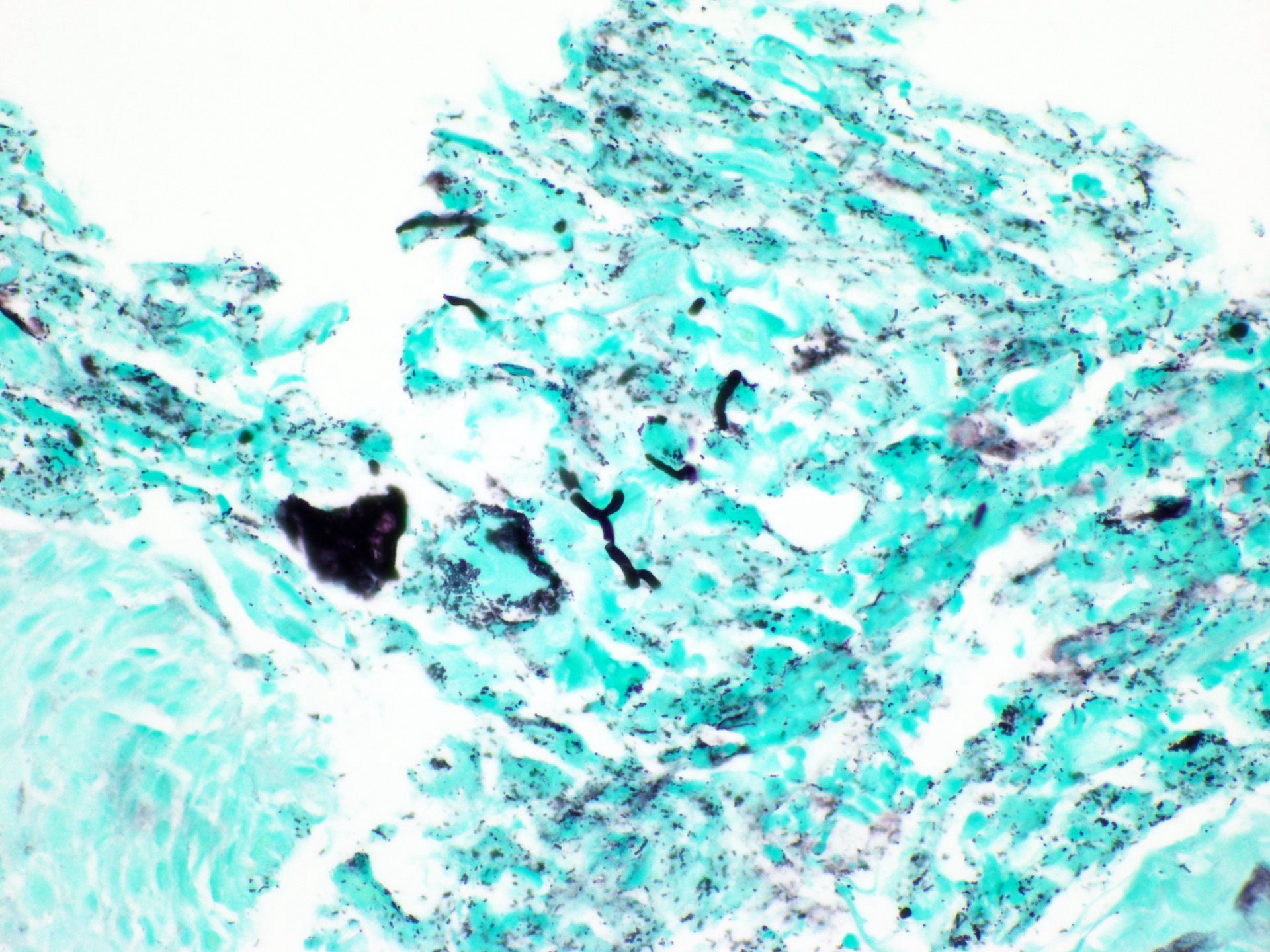
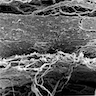
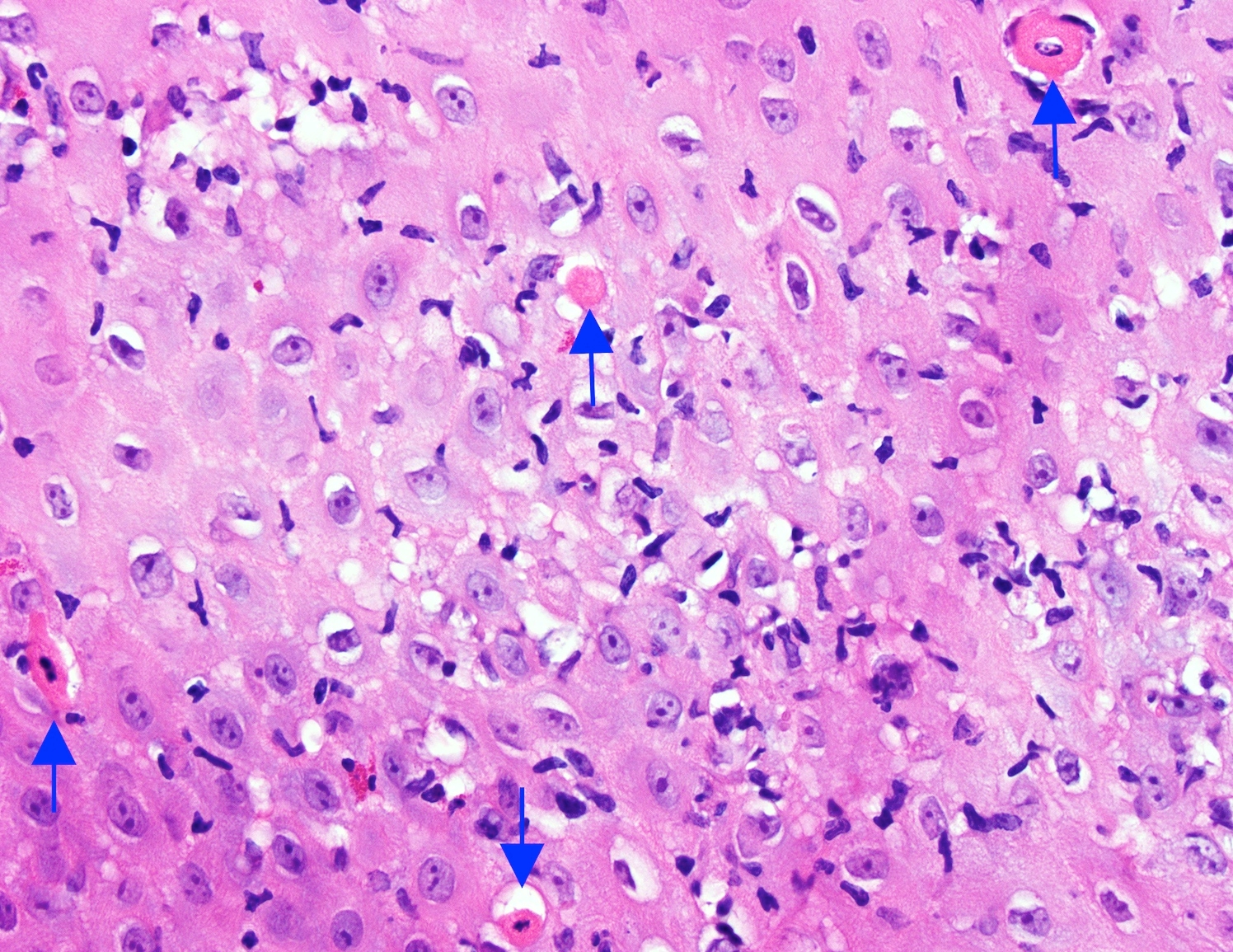
- 2 forms: yeast cells (often identified on Grocott methenamine silver stain [GMS]) and pseudohyphae
- Fungal elements are usually identified within the squamous debris, fibrinopurulent exudate or necrotic debris
- HIV patients may have invasion into muscularis propria and adventitia if untreated (Mycoses 1997;40:81)
- Histologic clues to esophageal candidiasis
- Hyper pink parakeratosis
- Shish kebab: pseudohyphae piercing through and skewer shed squames (Am J Clin Pathol 2017;147:33)
Microscopic (histologic) images
Contributed by Divya Sharma, M.D., Andrey Bychkov, M.D., Ph.D. and Jijgee Munkhdelger, M.D., Ph.D.
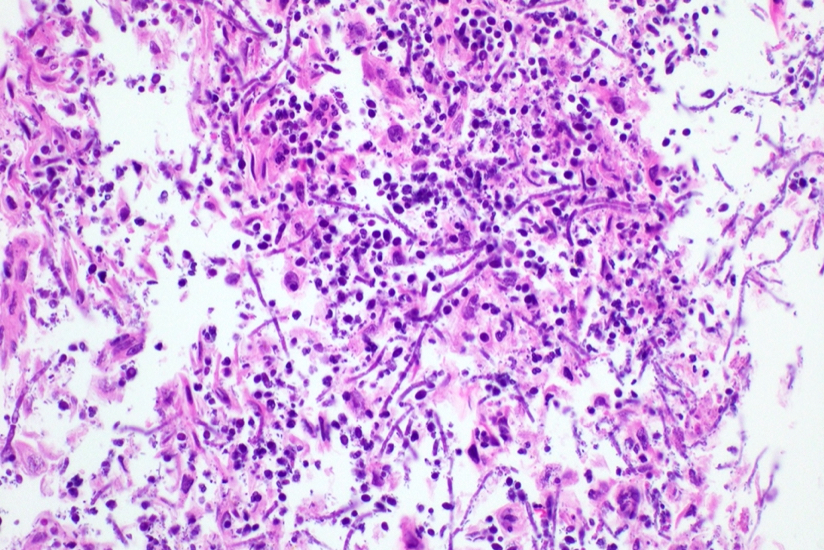
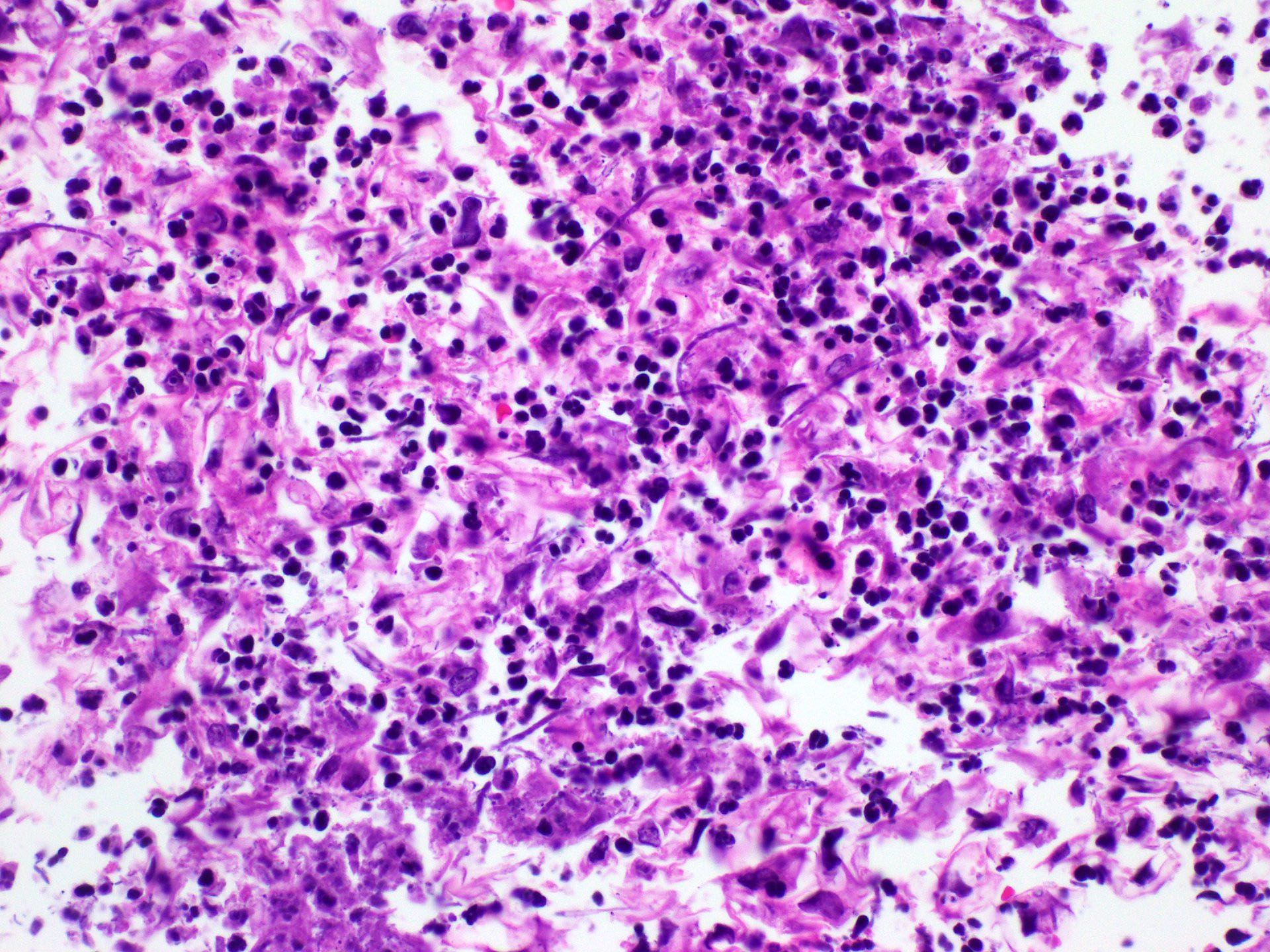
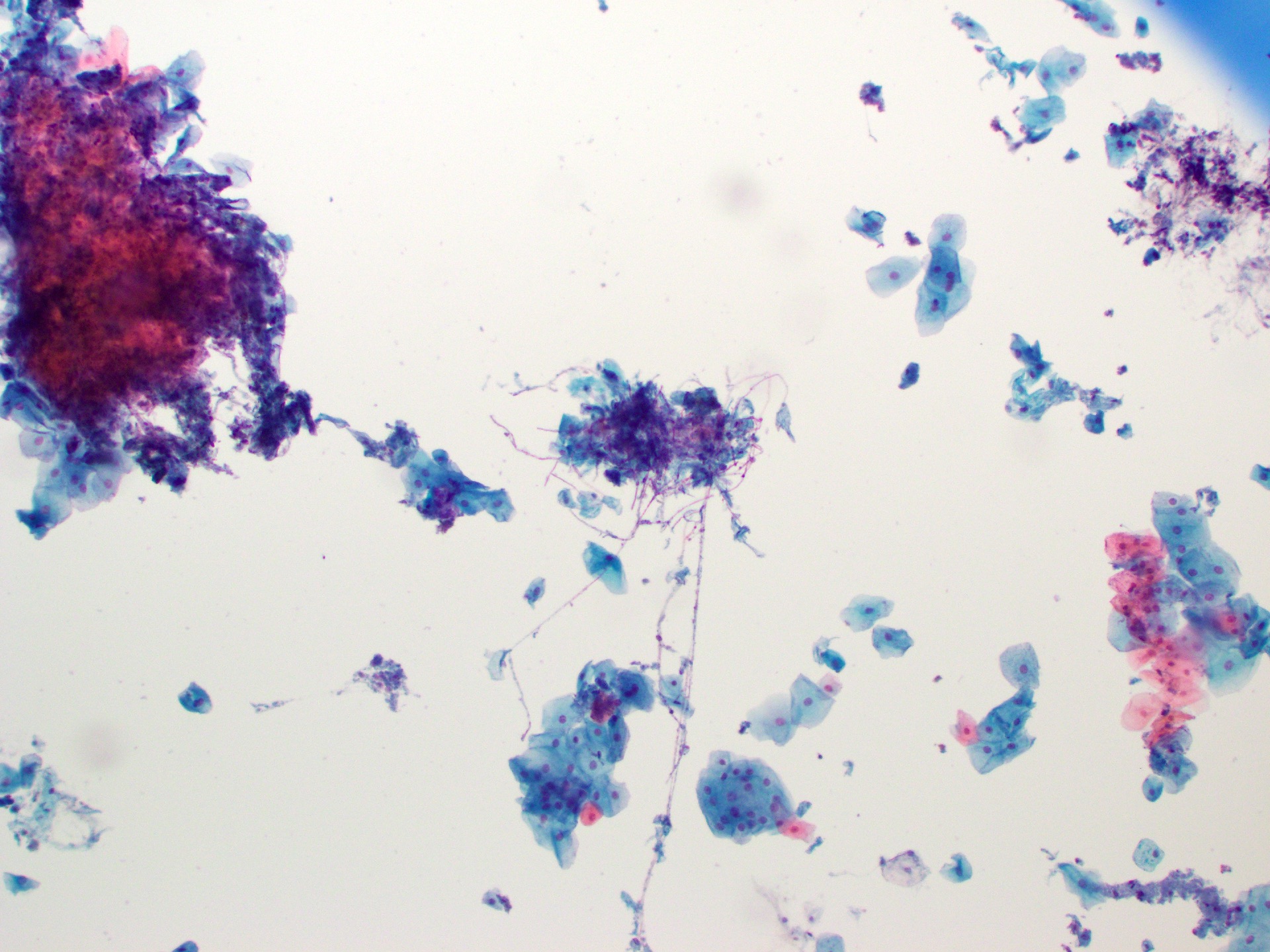
Cytology description
- Tiny budding yeast may form pseudohyphae with constrictions between adjacent cells
Videos
Candida esophagitis
Sample pathology report
- Esophagus, biopsy:
- Candida esophagitis
- Negative for intramucosal eosinophilia, dysplasia or malignancy
Differential diagnosis
- CMV esophagitis:
- More commonly presents as a single, isolated and deep, large ulcer rather than multiple small, shallow ulcerations as in herpes simplex virus (HSV) esophagitis
- Intranuclear and intracytoplasmic inclusions are more common in mesenchymal and endothelial cells than epithelial cells
- Positive CMV IHC stain
- Negative HSV IHC stain
- Herpes esophagitis:
- Herpes zoster / varicella:
Additional references
Board review style question #1
A 46 year old man with a history of human immunodeficiency virus (HIV) presented with odynophagia. Esophagogastroduodenoscopy (EGD) showed white-yellow mucosal plaques in the esophagus. The figure above is from an esophagus biopsy. Which of the following could be the causative agent?
- Candida
- Cytomegalovirus (CMV)
- Herpes simplex virus (HSV)
- Iron pills
Board review style answer #1
A. Candida. This patient's history and the EGD findings of the esophagus suggest Candida esophagitis. His history of HIV is an underlying risk factor. The biopsy of the esophagus shows scattered neutrophils with fungal elements including pseudohyphae, supporting the above entity. Answer B is incorrect because pseudohyphae and yeast cells, as shown on the biopsy, are diagnostic for Candida esophagitis. Answer C is incorrect because while HSV esophagitis can affect both immunocompetent and immunocompromised individuals, it is considerably more common in those with compromised immune function. In such cases, these viral infections typically present as ulcerative lesions in the esophagus rather than plaques. To confirm the presence of cytomegalovirus and HSV, it is advisable to perform biopsies of the ulcers. Answer D is incorrect as there was no polarizable crystalline material identified.
Comment Here
Reference: Candida
Comment Here
Reference: Candida
Board review style question #2
Which of the following gross examination findings is consistent with Candida esophagitis?
- Bleeding varices
- Sloughing of the mucosa
- Ulcer in the mid esophagus
- Yellow-white mucosal plaques
Board review style answer #2
D. Yellow-white mucosal plaques. Yellow-white plaques, which bleed upon removal, are consistent with Candida esophagitis. Answer C is incorrect as cytomegalovirus (CMV) / herpes simplex virus (HSV) are frequently associated with ulcers. Answer B is incorrect because sloughing of the mucosa can be seen in esophagitis dessecans superficialis. Answer A is incorrect because bleeding varices are associated with portal hypertension.
Comment Here
Reference: Candida
Comment Here
Reference: Candida
Chemical (corrosive) esophagitis
Table of Contents
Epidemiology | Etiology | Clinical features | Lye stricture | Treatment | Microscopic (histologic) descriptionEpidemiology
- Children or adults
Etiology
- Causes: ingestion of strong alkali;, acids or nonphosphate detergents (may be suicide attempts); cytotoxic chemotherapy (see Lye stricture) (Int J Pediatr Otorhinolaryngol 2005;69:1257)
- In Taiwan, commonly due to ingestion of alkaline oil (Pediatr Surg Int 2004;20:207)
Clinical features
- Complications: stricture, Barrett esophagus, rarely squamous cell carcinoma
Lye stricture
- Also called corrosive or caustic stricture
- Due to ingestion of lye (or other caustic substance) leads to sloughing of mucosa followed by extensive fibrosis (Sao Paulo Med J 2001;119:10, eMedicine: Caustic Ingestions [Accessed 28 June 2023])
- Usually at bifurcation of trachea
- Mean age 6 years at time of ingestion
- Over 5,000 cases of accidental caustic injury to children in the U.S. each year
- Also suicide attempts in adults
- Associated with motility disorders in children (Braz J Med Biol Res 2004;37:1623)
- Complications: carcinoma, mean 40 years later, usually at tracheal bifurcation
- Treatment: stenting, dilation or surgical resection
Treatment
- Dilation, often surgery (Int J Pediatr Otorhinolaryngol 2001;57:203)
Microscopic (histologic) description
- Mucosal or transmural injury with hemorrhage, necrosis and possible bacterial infection
CMV
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Esophageal infection of adults and children by cytomegalovirus, a double stranded DNA virus of the Herpesviridae family (human herpes virus 5 [HHV5]) spread by blood and other bodily fluids
- Rare in immunocompetent patients but causes serious disease in the setting of immunosuppression (e.g., AIDS, solid organ or bone marrow transplant, chemoradiation therapy)
- Cytomegalovirus (CMV) is the only herpes virus with both nuclear and cytoplasmic inclusions, identified on routine H&E stain
Essential features
- Severe and potentially life threatening esophageal infections occur in immunosuppressed patients (e.g., people living with HIV / AIDS, solid organ or bone marrow transplant recipients, patients undergoing chemoradiation therapy)
- Diagnosis requires endoscopy with tissue biopsy of areas of ulceration or erosion, which typically occur in the mid or distal esophagus
- Histopathologic features characterized by ulceration or erosion harboring enlarged cells with intranuclear and cytoplasmic inclusions
- Viropathic inclusions can be identified in endothelial cells, stromal fibroblasts or epithelial cells
- CMV immunohistochemistry or CMV in situ hybridization aids in the detection of viropathic inclusions
ICD coding
Epidemiology
- Ubiquitous, worldwide distribution
- Majority of adults are exposed to CMV in their lifetime; seroprevalence rates range from 45 to 100% (PLoS One 2018;13:e0200267, Rev Med Virol 2019;29:e2034)
- Can occur in children and adults secondary to viral reactivation due to immunosuppression from
- HIV / AIDS (CMV esophagitis is an AIDS defining illness)
- Underlying malignancy (particularly lung cancer) and concurrent chemoradiation therapy (Dis Esophagus 2016;29:392)
- Solid organ and hematopoietic cell transplantation
- Systemic steroid or immunosuppressive therapy
- Critical illness (e.g., shock, pneumonia, respiratory failure, intensive care unit [ICU] requirement) (J Clin Med 2022;11:1583)
- Uncommon in immunocompetent patients (Clin Gastroenterol Hepatol 2020;18:736)
Sites
- In the gastrointestinal tract, esophagus is the most common site of infection in symptomatic, immunocompromised children (J Pediatr Surg 2015;50:1874)
- Esophagus is second to the stomach as the most frequent site of upper gastrointestinal site involvement (Scand J Gastroenterol 2011;46:1228, Diagn Pathol 2022;17:9)
Pathophysiology
- CMV transmission
- Sexual contact (Sex Transm Dis 2008;35:472)
- Blood products (transfusion, transplantation) (Am J Transplant 2012;12:2457)
- Close contact with saliva and urine from infected individuals (particularly children) (J Clin Virol 2008;43:266)
- Intrauterine, after primary or nonprimary infections (Rev Med Virol 2007;17:253)
- Primary infection usually asymptomatic but can be associated with mononucleosis-like illness (J Infect Chemother 2019;25:431)
- CMV establishes latency and persists for life but can be reactivated in the setting of immunosuppression due to the dysfunction of cell mediated immunity (QJM 2012;105:401)
- Mucosal infection and replication occur in human epithelial cells, endothelial cells and tissue fibroblasts
- Infection leads to an ischemic pattern of tissue erosion or necrosis and inflammation (Ann Intern Med 1993;119:924)
Etiology
- Diseases and medications that cause immunosuppression and viral reactivation (Curr Gastroenterol Rep 2008;10:409)
Clinical features
- Third leading cause of infectious esophagitis after Candida spp. and herpes simplex virus (HSV) esophagitis (Dis Esophagus 2018;31:doy094)
- Symptoms include epigastric pain, fever, odynophagia, dysphagia, gastrointestinal bleeding (J Clin Med 2022;11:1583)
- Can occur concomitantly with reflux esophagitis (38.6%), Barrett esophagus (9.1%), esophageal candidiasis (18.2%) and benign gastric ulcers (29.5%) (J Clin Med 2022;11:1583)
- Associated with higher overall mortality and longer hospital admissions compared to those without CMV esophagitis (J Clin Med 2022;11:1583)
- Also occurs in immunosuppressed infants on prolonged antibiotic therapy with broad spectrum antibiotics (J Clin Med 2020;9:939)
- Involvement of additional visceral organs (e.g., stomach, duodenum, colon, lungs) occurs in 20% of cases (Clin Gastroenterol Hepatol 2020;18:736)
Diagnosis
- Endoscopic appearance of lesions is variable but most commonly occurs in distal esophagus (65%), followed by mid esophagus (28%) (Clin Gastroenterol Hepatol 2020;18:736)
- Requires tissue biopsy and histopathologic diagnosis
- Tissue sampling should include granulation tissue or ulcer bed, given predilection for endothelial and stromal cell infection
- H&E stain demonstrates characteristic viropathic inclusions
- CMV immunostain or CMV in situ hybridization
- Finding > 2 positive cells by CMV IHC has higher correlation with CMV viremia versus finding rare positive cells (< 2) (Gastroenterology Res 2016;9:92)
Laboratory
- CMV antigenemia is a poor predictor of gastrointestinal tract involvement but viral load by real time PCR is more reliable (Bone Marrow Transplant 2004;33:431)
Prognostic factors
- ICU requirement and acute kidney injury are risk factors for in hospital mortality
- Upper gastrointestinal tract involvement has a 17% mortality rate 1 month after diagnosis and 25% mortality rate 1 year after diagnosis according to a small study of 12 patients (GE Port J Gastroenterol 2017;24:262)
- Poor outcomes associated with ganciclovir resistant CMV infection (Transplantation 2016;100:e74)
Case reports
- 42 year old man who presented to the emergency department (ED) with a 2 week history of abdominal pain and watery diarrhea was found to have a new diagnosis of HIV / AIDS (Cureus 2022;14:e22455)
- 44 year old man with dermatomyositis on mycophenolate was admitted for acute gastrointestinal bleed (Clin Case Rep 2022;10:e6044)
- 60 year old woman on immunosuppressive therapy for pemphigus vulgaris presented with odynophagia and was found to have ulcerative CMV and HSV esophagitis (IDCases 2020:22:e00925)
- 72 year old man with kidney transplant who complained of dysphagia and unintentional weight loss was found to have distal esophageal stricture (ACG Case Rep J 2022;9:e00836)
- 77 year old immunocompetent man with erosive esophageal lesion was found to have moderately differentiated squamous cell carcinoma and CMV (Gut Pathog 2021;13:24)
Treatment
- Intravenous ganciclovir or foscarnet for 3 - 6 weeks; continued maintenance therapy is indicated for patients with concurrent retinitis or recurrent gastrointestinal disease (Am J Gastroenterol 1998;93:317, Arch Intern Med 1998;158:957)
- Antiviral prophylaxis is standard of care for high risk solid organ transplant recipients; preemptive therapy preferred for hematopoietic cell transplant recipients (Clin Transplant 2019;33:e13512)
- Ganciclovir and foscarnet resistance can occur (Transpl Infect Dis 2022;24:e13733, Case Rep Gastroenterol 2013;7:25)
Gross description
- Variably superficial to deep / punched out ulcers, serpiginous and circumferential ulcers (Medicine (Baltimore) 2019;98:e15845)
- Can be associated with esophageal strictures (IDCases 2020:21:e00795, J Pediatr Surg 2015;50:1874)
Microscopic (histologic) description
- Ulceration and inflamed granulation tissue with necroinflammatory debris
- Cytologic enlargement by both intranuclear and intracytoplasmic inclusions
- Intranuclear basophilic or amphophilic inclusions; can have owl eye appearance when associated with peripheral clearing (an artifact of fixation) and margination of nuclear chromatin (Cowdry type A body)
- Intracytoplasmic basophilic or amphophilic inclusions; can also appear as coarse, eosinophilic inclusions
- PAS and GMS stains may weakly highlight intracytoplasmic inclusions, not intranuclear inclusions (Semin Diagn Pathol 2017;34:510)
- Viropathic inclusions identified in endothelial cells, stromal cells and epithelial cells
Microscopic (histologic) images
Positive stains
- CMV immunohistochemistry or CMV in situ hybridization
- Does not supplant routine H&E evaluation (Hum Pathol 2017:60:11)
Negative stains
Electron microscopy description
- Spheroid shaped virions, diameter of 150 - 200 nm, each with dense, protein core comprised of linear, double stranded DNA genome, surrounded by a hyperlucent halo
Sample pathology report
- Esophagus, ulcer, biopsy:
- Ulcerated squamous mucosa with intranuclear and basophilic intracytoplasmic inclusions, morphologically consistent with cytomegalovirus (CMV); confirmed by CMV immunohistochemistry
Differential diagnosis
- Herpes simplex virus 1 / 2:
- More typically presents as discrete ulcers with vesicles or bullae versus deep / punched out ulcers (Medicine (Baltimore) 2019;98:e15845)
- Viropathic inclusions more common in squamous epithelial cells than in stromal and endothelial cells
- Intranuclear type A Cowdry inclusion without nuclear enlargement
- 3 Ms of nuclear changes: molding of nuclear contours, margination of chromatin and multinucleation
- No intracytoplasmic inclusions
- Varicella zoster virus:
- Features similar to HSV 1 / 2 but multinucleation is uncommon
- No intracytoplasmic inclusions
Board review style question #1
In the biopsy of the esophagus shown above, what is the most likely clinical presentation of the patient?
- 2 year old immunocompetent boy with new onset projectile vomiting
- 21 year old immunocompetent woman presenting with symptoms of gastroesophageal reflux
- 67 year old female cyclist with new onset shortness of breath and chronic cough
- 86 year old man undergoing chemoradiation for squamous cell carcinoma
Board review style answer #1
D. 86 year old man undergoing chemoradiation for squamous cell carcinoma. Underlying malignancy and chemoradiation is a risk factor for CMV infection. Answer A is incorrect because the patient is immunocompetent without reported risk factors for CMV infection. Answer B is incorrect because the patient is immunocompetent, which is an unlikely presentation for CMV esophagitis. Answer C is incorrect because the clinical symptoms suggest an acute respiratory infection.
Comment Here
Reference: CMV
Comment Here
Reference: CMV
Board review style question #2
Which herpes virus demonstrates both intranuclear and intracytoplasmic viropathic inclusions?
- Cytomegalovirus
- Epstein-Barr virus
- Herpes simplex virus 1 and 2
- Varicella zoster virus
Board review style answer #2
A. Cytomegalovirus. Only CMV virus demonstrates both intranuclear and intracytoplasmic viropathic inclusions. Answer C is incorrect because herpes simplex virus 1 and 2 causes an intranuclear inclusion (Cowdry type A) without an accompanying intracytoplasmic inclusion. Answer D is incorrect because varicella zoster virus also demonstrates an intranuclear Cowdry type A inclusion without an intracytoplasmic inclusion. Answer B is incorrect because Epstein-Barr virus does not cause inclusions in infected cells.
Comment Here
Reference: CMV
Comment Here
Reference: CMV
Crohn's disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Esophagitis associated with Crohn's disease
Essential features
- Incidence of esophageal Crohn's disease ranges from 0.3% to 10% in adults with Crohn's disease and from 4.2% to 42% in pediatric patients with Crohn's disease
- Aphthous ulcer, longitudinal ulcer, stricture and fistula
- Histologic features of esophageal Crohn's disease are generally nonspecific
- Nonnecrotizing granuloma(s) may be found
Terminology
- Esophagitis associated with Crohn's disease
ICD coding
- ICD-10: K509 - Crohn's disease, unspecified
Epidemiology
- Incidence of esophageal Crohn's disease ranges from 0.3% to 10% in adults with Crohn's disease and from 4.2% to 42% in pediatric patients with Crohn's disease (J Crohns Colitis 2020;14:624, Adv Anat Pathol 2022;29:2, J Crohns Colitis 2018;12:1073)
- Higher frequency of endoscopic and histological alterations was found when upper digestive endoscopy is performed routinely as part of the evaluation of the disease, regardless of the presence of gastrointestinal symptoms (World J Gastrointest Pharmacol Ther 2019;10:35, J Crohns Colitis 2018;12:1399, Inflamm Bowel Dis 2016;22:1896)
- Patients with proximal Crohn's disease including the esophagus were more likely to be younger at diagnosis (Am J Gastroenterol 2013;108:106, J Crohns Colitis 2018;12:1399)
- Male sex was an independent predictor for upper gastrointestinal tract involvement including the esophagus (J Crohns Colitis 2018;12:1399)
- 18 of 61 patients (30%) with lymphocyte rich inflammation in the mid or proximal esophagus had Crohn's disease (Am J Surg Pathol 2020;44:198)
- Patients in the lymphocytic esophagitis cohort had a higher than normal proportion of patients with Crohn's disease, raising the possibility that lymphocytic esophagitis could be associated with or a manifestation of inflammatory bowel disease (Am J Clin Pathol 2006;125:432, Am J Surg Pathol 2022;46:e55)
- Association between esophageal lymphocytosis and Crohn's disease has been reported in pediatric populations (Inflamm Bowel Dis 2011;17:45, Inflamm Bowel Dis 2014;20:1324, Am J Surg Pathol 2022;46:e55)
Sites
- Distal esophagus is the most common involved site, either alone or with the involvement of the entire esophagus (J Crohns Colitis 2020;14:624)
Pathophysiology
- Crohn's disease is a chronic inflammatory disease mainly affecting the intestine, especially the terminal ileum; Crohn's disease is characterized by the alternating periods of flares and remissions caused by a complex pathogenesis involving a reshaped microenvironment of the intestine (Biol Direct 2020;15:23)
Etiology
- Caused by a complex pathogenesis involving the interactions of environmental factors, the immune system, susceptibility genes and the host’s microbiome changes, leading to disruption of the intestinal mucosa; among these, inflammation plays a key role
- Etiology of esophageal Crohn’s disease remains unclear, as overall pathogenesis of Crohn’s disease remains poorly understood (Biol Direct 2020;15:23)
Clinical features
- Patients are asymptomatic or have nonspecific symptoms, such as dysphagia, odynophagia, heartburn, vomiting, chest pain and weight loss (J Crohns Colitis 2020;14:624, J Gastroenterol Hepatol 2018;33:355)
- Crohn's disease limited to the esophagus is extremely rare and almost all cases have concomitant intestinal Crohn's disease (Inflamm Bowel Dis 2015;21:2106, World J Gastrointest Pharmacol Ther 2019;10:35, J Crohns Colitis 2020;14:624, J Gastroenterol Hepatol 2018;33:355)
- Endoscopic findings are nonspecific including erosion, aphthous ulcer, granular and hyperemic mucosa and longitudinal ulcer (J Gastroenterol Hepatol 2018;33:355)
- Advanced disease may display cobblestone appearance, stricture and fistula (J Gastroenterol Hepatol 2018;33:355)
Diagnosis
- Clinicopathologic diagnosis including the patient's clinical history of Crohn's disease and endoscopic and histologic features, such as aphthous ulcer, longitudinal ulcer and granuloma(s)
Radiology description
- Aphthous ulcer, longitudinal ulcer, rose thorn fissure, a cobblestone appearance of the mucosa, stricture and fistula (Inflamm Bowel Dis 2015;21:2106, J Gastroenterol Hepatol 2018;33:355)
Prognostic factors
- Controversial
- Involvement of upper GI tract did not result in a worse outcome (J Crohns Colitis 2018;12:1399)
- Expected poor prognosis (J Crohns Colitis 2017;11:3)
Case reports
- 23 year old woman with Crohn's disease and esophageal involvement (BMJ Case Rep 2017;2017:bcr2016217960)
- 24 year old woman with Crohn's disease and esophageal involvement (ACG Case Rep J 2019;6:e00052)
- 30 year old woman with history of severe ileocolonic Crohn's disease presented with odynophagia and progressive dysphagia to liquids and solids (Dig Dis Sci 2017;62:2690)
- 33 year old woman with Crohn's disease and esophageal involvement (Clin Gastroenterol Hepatol 2022;20:A15)
- 51 year old woman with an esophageal stricture (Rev Esp Enferm Dig 2022;114:501)
Treatment
- Mild esophageal Crohn’s disease may be treated with a proton pump inhibitor only (J Crohns Colitis 2017;11:3)
- Severe or refractory disease requires additional systemic corticosteroids or anti-TNF therapy (J Crohns Colitis 2017;11:3)
- Surgery or dilatation by endoscopy are appropriate for symptomatic stricture
- Treatment is decided based on both esophageal Crohn's disease activity and extraesophageal involvement
Gross description
- Erythema, erosion, aphthous ulcer, longitudinal ulcer, stricture and fistula
Microscopic (histologic) description
- Nonnecrotizing granuloma(s) may be found in up to 50% of cases (Gastrointest Endosc 1994;40:296, Inflamm Bowel Dis 2015;21:2106)
- Frequency of nonnecrotizing granuloma(s) varies greatly depending on the type of specimen (biopsy versus resection) (Adv Anat Pathol 2022;29:2, J Gastroenterol Hepatol 2018;33:355)
- Biopsy often shows chronic inflammation only
- Transmural chronic inflammation in the esophagectomy specimen, one of the histologic hallmarks of Crohn's disease, has been reported in a patient with uncontrollable stricture due to Crohn's disease (Rev Esp Enferm Dig 2022;114:501)
- Concurrent eosinophilic and lymphocytic esophagitis has been described in patients with Crohn's disease (Adv Anat Pathol 2022;29:2, Am J Surg Pathol 2020;44:198, Am J Surg Pathol 2022;46:e55)
Microscopic (histologic) images
Negative stains
Sample pathology report
- Esophagus, ulcer, biopsy:
- Squamous mucosa with nonnecrotizing granuloma(s) (see comment)
- GMS and PASF stains, negative for fungal infection.
- AFB stain, negative for acid fast bacilli.
- Comment: Given the patient's clinical history of Crohn's disease and the endoscopic findings of longitudinal and aphthous ulcers, the biopsy findings are compatible with granulomatous inflammation associated with Crohn's disease. Clinical correlation is suggested.
Differential diagnosis
- Histologic features of esophageal Crohn's disease are typically nonspecific
- In many cases, a definitive diagnosis of esophageal Crohn's disease cannot be established based on histologic features alone and only a diagnosis of compatible with esophageal CD can be achieved
- Lymphocytic esophagitis:
- Lymphocytic esophagitis is a term suggested for the finding of intraepithelial lymphocytosis (≥ 20 lymphocytes / high power field) in an esophageal biopsy
- History of Crohn's disease
- Esophagitis secondary to gastroesophageal reflux disease:
- Endoscopic findings and pH monitoring would be helpful
- Lichenoid pattern esophagitis:
- Important cause of lymphocyte rich esophagitis, affecting the esophagus in up to 50% of patients with oral or cutaneous disease (Endoscopy 2009;41:187, Am J Surg Pathol 2020;44:198)
- Biopsy samples show dense mononuclear cell rich lamina propria inflammation accompanied by band-like chronic inflammation underneath the epithelium
- Esophageal tuberculosis:
- Caseating granuloma(s) may be found
- AFB stain, culture and polymerase chain reaction testing for mycobacterium tuberculosis
- Clinical presentation and pathologic features useful for esophageal tuberculosis diagnosis includes fever, night sweaters, transverse ulcer, ileocecal lesion and caseating granuloma(s)
- Sarcoidosis:
- Esophageal involvement is an extremely rare occurrence in sarcoidosis
- Presence of T lymphocytes, mononuclear phagocytes and noncaseating granuloma(s)
- Involvement of other organs, especially lung
- Parasite and fungal infection:
- Granuloma(s) may be found (Rev Bras Parasitol Vet 2019;28:769)
- GMS stain, PAS stain and culture
- Foreign body granuloma(s) :
- Clinical history (wound, operation, endoscopic treatment, etc.)
- Cytomegalovirus (CMV) infection:
- Cytomegaly and nucleomegaly with CMV inclusions of infected cells including endothelial cells and mesenchymal cells
- Positive CMV immunostaining
- Candida esophagitis:
- Neutrophilic infiltration with fungal pseudohyphae in sloughed epithelial debris or squamous epithelium
- Drug induced esophagitis:
- Deposition of drug fragments, such as iron pill
- Behçet disease:
- Prominent vasculitis
- Round deep ulcers with sharp borders in the ileocecal location
Board review style question #1
Which of the following statements regarding the histologic features of esophageal Crohn’s disease is true?
- Chronic inflammation with predominantly lymphocytes and plasma cells is the most common finding in biopsy samples
- Elderly patients with Crohn's disease tend to show more frequent esophageal involvement
- Proximal esophagus is the most common site of involvement
- The finding of nonnecrotizing granuloma(s) is diagnostic of Crohn’s disease
Board review style answer #1
A. Chronic inflammation with predominantly lymphocytes and plasma cells is the most common finding in biopsy samples. The biopsy findings of esophageal Crohn's disease are generally nonspecific. Biopsy often shows chronic inflammation only. Epithelioid granuloma(s) may be found up to 50% of cases. Answer C is incorrect because the distal esophagus is the most common site of involvement. Answer D is incorrect because nonnecrotizing granuloma(s) can be seen in various conditions, such as sarcoidosis, tuberculosis and foreign body reaction, among others. Answer B is incorrect because there have been no established evidence / previous reports that elderly patients with Crohn's disease tend to show more frequent esophageal involvement.
Comment Here
Reference: Crohn's disease
Comment Here
Reference: Crohn's disease
Board review style question #2
Which of the following statements about esophageal Crohn’s disease is true?
- Almost all cases have concomitant intestinal Crohn's disease
- Granuloma(s) are frequently seen in biopsy samples
- Most patients will eventually require esophagectomy
- Often shows esophageal stricture
Board review style answer #2
A. Almost all cases have been reported to have concomitant intestinal Crohn's disease. Crohn's disease limited to the esophagus is extremely rare. Answer C is incorrect because esophagectomy is rarely performed for the treatment of esophageal Crohn's disease. Answer B is incorrect because granuloma(s) can be seen in up to (at most) 50% of cases. Answer D is incorrect because esophageal stricture due to Crohn's disease is not frequently seen.
Comment Here
Reference: Crohn's disease
Comment Here
Reference: Crohn's disease
Diverticula
Table of Contents
Definition / general | Types | Case reports | Gross images | Microscopic (histologic) imagesDefinition / general
- Outpouching of alimentary tract containing all visceral layers (eMedicine: Zenker Diverticulum [Accessed 14 February 2019])
- May cause obstruction, aspiration pneumonia, abscess, infection, hemorrhage or be associated with malignancy
Types
- Zenker diverticula: also called pharyngoesophageal or pulsion diverticula; most common esophageal diverticula (~70%), more common in elderly; above upper esophageal sphincter, usually posterior wall; due to disordered cricopharyngeal motor dysfunction or weakness in esophageal wall at junction with pharynx; at junction between the pharynx and esophagus (known as the Killian triangle), may accumulate food, cause regurgitation or aspiration pneumonia or simulate a neck mass; malignancy in 0.3%
- Mid esophageal / traction diverticula: near mid esophagus at level of tracheal bifurcation; becoming uncommon; previously mostly due to tuberculosis, mediastinal lymphadenitis and scarring; may be due to motor dysfunction, congenital or alkali ingestion (Med Hypotheses 2004;62:931); better prognosis than distal disease (Dysphagia 2006;21:198)
- Epiphrenic diverticula: rare; immediately above lower esophageal sphincter (LES); due to lack of coordination of peristalsis and LES relaxation (Am J Surg 2005;190:891); often associated with hiatal hernia, may cause nocturnal regurgitation of massive amounts of fluid, obstruction, aspiration; contains mucosa, submucosa and muscularis mucosae; lined by squamous epithelium, often markedly inflamed
- False or pseudodiverticula: mucosa and submucosa only, rare, usually with diffuse esophageal spasm
Case reports
- 47 year old man with epiphrenic diverticula with fatal hemorrhage (Arch Pathol Lab Med 2006;130:867)
- Squamous cell carcinoma in Zenker diverticula (Dis Esophagus 2007;20:75)
Eosinophilic esophagitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Multifactorial, chronic eosinophilic inflammatory condition affecting the esophagus in both adult and pediatric patients that occurs in the absence of identifiable secondary causes (Med Clin North Am 2019;103:29)
- Characterized by a chronic immune reaction to environmental and food allergens leading to a deficient esophageal mucosal barrier (Arch Pediatr 2019;26:182)
Essential features
- Major diagnostic criteria of ≥ 15 eosinophils per high power field (40x magnification) in the proper clinical context (Arch Pediatr 2019;26:182)
Terminology
- Idiopathic or primary eosinophilic esophagitis
- Chronic atopic inflammatory disease of the esophagus
- Allergic or atopic esophagitis
ICD coding
- ICD-10: K20.0 - eosinophilic esophagitis
Epidemiology
- M:F = 3:1 (World J Gastroenterol 2019;25:4598)
- Peak incidence between 30 and 44 years of age (Gastroenterology 2018;154:319)
- Caucasians have threefold increased prevalence compared with other races
- Population based study looking at 7,000 patients diagnosed with eosinophilic esophagitis found 89.3% were Caucasians, 6.1% African American and 5.6% of Asian descent (Gastrointest Endosc Clin N Am 2018;28:27)
- More common in cold and arid climates and rural areas (Med Clin North Am 2019;103:29)
- Incidence:
- Incidence rates vary greatly depending on the geographic location
- Pooled incidence rate of 3.7 cases per 100,000 population per year (95% confidence interval, 1.7 - 6.5) from meta analysis (Gastroenterology 2018;154:319)
- Included population based studies in North America, Europe and Australia
- Pooled incidence rate for adults is 7.0 cases per 100,000 population per year (95% confidence interval, 1 - 18.3) (Aliment Pharmacol Ther 2016;43:3)
- Pooled incidence rate for children is 5.1 cases per 100,000 population per year (95% confidence interval, 1.5 - 10.9) (Aliment Pharmacol Ther 2016;43:3)
- Prevalence:
- Pooled prevalence of 22.7 per 100,000 population (95% confidence interval, 12.4 - 36.0) (Gastroenterology 2018;154:319)
Sites
- Can diffusely affect the entire length of the esophagus
Pathophysiology
- Intraepithelial eosinophils recruited via chemotaxis act as antigen presenting cells (APCs) recruiting T cells, activating mast cells and activating basophils (Arch Pediatr 2019;26:182)
- Eosinophils secrete a cationic protein eosinophilic peroxidase (EPO) and major binding protein (MBP) causing cell damage leading to the symptom of esophageal dysmotility (Arch Pediatr 2019;26:182)
- Concentration of eosinophilic peroxidase (EPO) correlates to increased symptomology, not the number of intraepithelial eosinophils (Arch Pediatr 2019;26:182)
- Recruited T cells differentiate into Th2 T cells and secrete IL4, IL5 and IL13 (Arch Pediatr 2019;26:182)
- IL4 promotes the differentiation of naïve T cells into Th2 T cells (Arch Pediatr 2019;26:182)
- IL5 promotes eosinophil survival (Arch Pediatr 2019;26:182)
- IL13 leads to expression of eotaxin3, which is a chemokine that induces eosinophilic activation and infiltration (Med Clin North Am 2019;103:29)
- Activated eosinophils produce TGF beta that promotes tissue remodeling and leads to fibrosis (Med Clin North Am 2019;103:29)
Etiology
- Environmental
- Food allergens
- Most common include milk, wheat, soy, eggs, peanuts / nuts, fish and shellfish (World J Gastroenterol 2019;25:4598)
- Hygiene and bacterial dysbiosis hypothesis
- Improved hygiene conditions lead to reduced bacterial exposures and alterations in the microbiota, which may alter mucosal permeability (World J Gastroenterol 2019;25:4598)
- Gastroesophageal reflux disease (GERD) leads to increased mucosa permeability to food allergens
- Proton pump inhibitor (PPI) usage
- Reduced acid secretion downregulates digestive enzyme activation
- Food antigens are not degraded, increasing the immune response to these large proteins (World J Gastroenterol 2019;25:4598)
- Food allergens
- Genetic / familial
- Genetic variants at multiple loci have been shown to increase the risk of development of atopic diseases, including eosinophilic esophagitis and asthma
- Genes found with alterations in patients with eosinophilic esophagitis:
- CCL26 - encodes eotaxin3
- Thymic stromal lymphopoietin (TSLP)
- Filaggrin (FLG)
- Desmoglein1 (DSG1)
- STAT6
- Calpain 14 (CAPN14)
- CRLF2
- Genes found with alterations in patients with eosinophilic esophagitis:
- Genetic variants at multiple loci have been shown to increase the risk of development of atopic diseases, including eosinophilic esophagitis and asthma
Clinical features
- Symptoms vary widely based on the age group:
- Pediatrics
- Infants and small children: emesis, food rejection, regurgitation, irritability, dysphagia and rarely growth retardation (World J Gastroenterol 2019;25:4598, Arch Pediatr 2019;26:182)
- School aged children and adolescents: nausea, emesis, regurgitation, chest and abdominal pain, dysphagia and food impaction (World J Gastroenterol 2019;25:4598, Arch Pediatr 2019;26:182)
- Adults
- Dysphagia, chest and abdominal pain and untreatable heartburn (World J Gastroenterol 2019;25:4598, Arch Pediatr 2019;26:182)
- Pediatrics
Diagnosis
- Eosinophilic esophagitis is a clinicopathologic diagnosis and findings should be considered in combination and not independent of each other
- Current diagnostic modalities
- Esophagogastroduodenoscopy (endoscopy)
- Endoscopy may show many characteristic but not pathognomonic changes
- Major criteria: mucosa edema, white exudate, longitudinal furrows, circular esophageal rings (also called pseudotrachea), esophageal stenosis (Gut 2013;62:489)
- Minor criteria: feline esophagus (concentric mucosal rings observed with some types of motility that disappear with air insufflation), narrow caliber esophagus, crêpe paper esophagus, mucosal laceration or fragility with passage of instruments before dilation (Gut 2013;62:489)
- Up to 25% of patients have normal endoscopic findings (World J Gastroenterol 2019;25:4598)
- Endoscopy may show many characteristic but not pathognomonic changes
- Esophageal biopsies are required for diagnosis
- Biopsies need to be taken from the proximal, mid and distal esophagus with a minimum of 2 biopsies per site
- 6 - 9 mucosal biopsies yield a near 100% diagnostic sensitivity (World J Gastroenterol 2019;25:4598)
- Single biopsy yields a sensitivity of 55% (Arch Pediatr 2019;26:182)
- Histology
- Major criteria: ≥ 15 eosinophils per high power field (40x magnification) (Arch Pediatr 2019;26:182)
- Minor criteria: extreme basal zone hyperplasia with papillary hyperplasia, eosinophils concentrated in the surface epithelium as opposed to the base, eosinophilic microabscesses, eosinophil degranulation, surface desquamation, lamina propria fibrosis (Arch Pediatr 2019;26:182)
- Esophagogastroduodenoscopy (endoscopy)
- Noninvasive diagnostic procedures being tested
- Esophageal string test (EST) (Am J Gastroenterol 2019;114:1614)
- Currently in clinical trials
- 1 hour test that can distinguish active from inactive disease in both children and adults
- Minimally invasive way to monitor disease
- Cytosponge (Curr Treat Options Gastroenterol 2019;17:48)
- Mesh sponge tethered by a string that is compressed in a dissolvable capsule
- Capsule is swallowed and then withdrawn through the mouth
- Sensitivity of 75% and specificity of 86%
- MicroRNA expression (Curr Treat Options Gastroenterol 2019;17:48)
- Esophageal brushings obtained via endoscopy or via nasogastric tube are analyzed for the presence and quantity of eosinophil derived neurotoxin, which is overexpressed in eosinophilic esophagitis
- Esophageal string test (EST) (Am J Gastroenterol 2019;114:1614)
Radiology description
- Radiology is useful for the evaluation of fibrostenotic disease (Gastrointest Endosc Clin N Am 2018;28:47)
- Barium esophagram is more sensitive than endoscopy for identification of strictures and diffuse small caliber esophagus (Gastrointest Endosc Clin N Am 2018;28:47)
- Strictures are classified by the length of fixed narrowing
- Rings are ≤ 1 cm (Gastrointest Endosc Clin N Am 2018;28:47)
- Strictures ≤ 8 cm (Gastrointest Endosc Clin N Am 2018;28:47)
- Small caliber esophagus > 8 cm (Gastrointest Endosc Clin N Am 2018;28:47)
- Strictures are classified by the length of fixed narrowing
Case reports
- 11 year old boy with allergic rhinitis and chest pain (Clin Endosc 2019;52:606)
- 34 year old man with refractory heartburn following 2 month trial of proton pump inhibitor and intermittent dysphagia (CMAJ 2016;188:893)
- 40 year old Caucasian woman with eosinophilic esophagitis and spontaneous transmural esophageal perforation (J Med Case Rep 2019;13:275)
Treatment
- Treatment revolves around the 3 Ds: drugs, diet and dilation
- Drugs
- Proton pump inhibitors:
- First line therapy
- Generally used in combination with dietary modifications as there is a high association with food allergens
- Corticosteroids:
- Second line therapy
- Topical or systemic (systemic reserved for patients with severe symptoms)
- Proton pump inhibitors:
- Diet
- Dietary modification due to the role that food allergens play in the pathogenesis of the disease
- Elemental diet
- Elimination diets
- Guided by allergy testing
- Empiric elimination diets
- Dietary modification due to the role that food allergens play in the pathogenesis of the disease
- Dilation
- Treatment for esophageal strictures and narrowing
- Improves symptoms of dysphagia only
- A meta analysis showed the risk of perforation from dilation procedures in eosinophilic esophagitis was 0.3% (Med Clin North Am 2019;103:29)
- Treatment for esophageal strictures and narrowing
Gross description
- Major criteria (Gut 2013;62:489)
- Mucosa edema
- Exudate or white plaques
- Longitudinal furrows
- Circular esophageal rings (pseudotrachea)
- Esophageal stenosis
- Minor criteria (Gut 2013;62:489)
- Feline esophagus
- Concentric mucosal rings observed with some types of motility that disappear with air insufflation
- Narrow caliber esophagus
- Crêpe paper esophagus
- Mucosal laceration or fragility with passage of instruments before dilation
- Feline esophagus
Microscopic (histologic) description
- Major criterion: ≥ 15 eosinophils per high power field (40x magnification) present in the squamous mucosa (Arch Pediatr 2019;26:182)
- Minor criteria: eosinophils concentrated in the surface epithelium as opposed to the base, extreme basal zone hyperplasia, eosinophilic microabscesses, eosinophil degranulation, surface desquamation, lamina propria fibrosis (Arch Pediatr 2019;26:182)
Microscopic (histologic) images
Virtual slides
Videos
Endoscopic features of eosinophilic esophagitis
Sample pathology report
- Esophagus, mid, biopsy:
- Eosinophil rich esophagitis (> 50 eosinophils per single high power field, maximum count) (see comment)
- Comment: The biopsy demonstrates marked reactive basal zone hyperplasia with increased eosinophils. The eosinophils have a top heavy distribution and few eosinophilic microabscesses are identified. These features are consistent with eosinophilic esophagitis in the proper clinical context.
Differential diagnosis
- Gastroesophageal reflux disease (GERD):
- Triad of findings includes:
- Hyperplasia of lamina propria papillae (greater than two - thirds of the epithelial thickness)
- Mild basal zone hyperplasia (approximately 15 - 25% of the epithelial thickness)
- Mildly increased intraepithelial eosinophils concentrated at the base of the mucosa as opposed to the surface (usually < 10 per high power field though counts are not pathognomonic for either condition) (Arch Pediatr 2019;26:182)
- Eosinophil counts in GERD are generally higher in distal esophageal biopsies compared with more proximal biopsies (this is reversed in eosinophilic esophagitis but is not pathognomonic)
- No surface eosinophilic microabscesses
- No lamina propria fibrosis
- Other intraepithelial inflammatory cells seen in some but not all cases include lymphocytes and neutrophils
- Triad of findings includes:
- Eosinophilic gastroenteritis:
- Affects any portion of the gastrointestinal tract with the stomach and small bowel being the most common locations (Lancet Gastroenterol Hepatol 2018;3:271)
- Symptoms such as diarrhea, intestinal obstruction and ascites are not common in eosinophilic esophagitis
- When seen in the esophagus, can be an exact histologic mimic of eosinophilic esophagitis so clinical presentation and correlation with other mucosal biopsies is essential
- PPI responsive esophageal eosinophilia:
- Same symptomatology and histologic findings as eosinophilic esophagitis but has complete remission with PPI therapy
- May actually be the same disease as eosinophilic esophagitis
Additional references
Board review style question #1
A 12 year old boy with a history of seasonal allergies and asthma complains of dysphagia and abdominal pain. Endoscopic biopsies reveal increased eosinophils in the esophageal squamous mucosa. What feature shown in the image favors eosinophilic esophagitis over GERD as the most likely diagnosis?
- Increased mitotic activity
- Intercellular edema
- Scattered intraepithelial lymphocytes
- Superficial concentration of eosinophils
Board review style answer #1
Board review style question #2
A 36 year old man presents to clinic with the chief complaint of intermittent dysphagia and heartburn that has not responded to over the counter medications. Endoscopy was notable for white plaques in the esophagus, longitudinal furrows and pseudotrachea. Biopsies taken during endoscopy show increased eosinophils in the distal and proximal specimens. Which chemokine producing immune cell plays an essential role in eosinophil infiltration and activation?
- B cells
- Eosinophils
- Mast cell
- Th2 T cells
Board review style answer #2
Epidermoid metaplasia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Esophageal squamous epithelium with a prominent granular layer and orthokeratosis / hyperorthokeratosis, resembling the epidermis of the skin
Essential features
- Affects middle aged to elderly individuals with a history of smoking and alcohol intake
- Well demarcated white plaque in the mid to distal esophagus
- Esophageal squamous epithelium with a prominent granular layer and orthokeratosis / hyperorthokeratosis
- Possible association with esophageal squamous cell carcinoma and dysplasia
- Follow up, unless a patient has a concurrent squamous cell carcinoma
Terminology
- Also known as epidermization, esophageal leukoplakia, orthokeratosis and hyperkeratosis
ICD coding
- ICD-10: K22.9 - Disease of esophagus, unspecified
Epidemiology
- Incidence:
- 2 cases (0.2%) of epidermoid metaplasia out of 1,048 consecutive esophageal biopsies; 50x less common than parakeratosis (11%) (Histopathology 2016;68:988)
- 37 cases (2%) of epidermoid metaplasia and hyperkeratosis without a prominent granular layer out of 1,845 consecutive esophageal biopsies (Histopathology 2013;63:463)
- Mean age: 61.5 years with a slight female predominance (Mod Pathol 2014;27:38)
- Possible association with squamous cell carcinoma (Am J Gastroenterol 2021;116:1533, Histopathology 2016;68:988, Histopathology 2013;63:463, Mod Pathol 2014;27:38)
Sites
- Mid to distal esophagus (Mod Pathol 2014;27:38)
- Most common in the mid esophagus (51%) in non-Barrett setting (Histopathology 2013;63:463)
- Most common in the distal esophagus (98%) in setting of Barrett esophagus (Histopathology 2013;63:463)
Etiology
- Remains unknown
- Associated with smoking and alcohol intake (Histopathology 2016;68:988, Histopathology 2013;63:463, Mod Pathol 2014;27:38)
Clinical features
- Dysphagia or asymptomatic
- Endoscopy findings
- Scaly or shaggy white plaque with clear borders
- Mimics crackleware china
- Lugol voiding lesion with clear demarcation, resembling superficial esophageal cancer (Hepatogastroenterology 2011;58:809)
Diagnosis
- Diagnosis is based on esophageal biopsy histologic findings
Case reports
- 53 year old man with epidermoid metaplasia and mucosal bridge formation (Endoscopy 2016;48:E325)
- 57 year old Japanese man with superficial esophageal cancer arising from epidermization (Clin J Gastroenterol 2018;11:29)
- 58 year old Japanese man with epidermization and a concurrent superficial squamous cell carcinoma (Am J Surg Pathol 1997;21:605)
- 69 year old man with an unusual appearance of epidermoid metaplasia (Endoscopy 2015;47:E100)
- 69 year old man with epidermoid metaplasia in association with esophageal intramural pseudodiverticulosis and candidiasis (Case Rep Gastroenterol 2021;15:709)
Treatment
- Follow up, unless a patient has a concurrent squamous cell carcinoma
Gross description
- Scaly or shaggy white plaque with clear borders (mean size: 2.6 cm) (Mod Pathol 2014;27:38)
Microscopic (histologic) description
- Esophageal squamous epithelium with a compact layer of orthokeratosis / hyperorthokeratosis and a prominent granular layer 1 - 4 cells thick with keratohyalin granules, resembling the epidermis of the skin
- Abrupt transition from the adjacent normal squamous epithelium
- Reference: Am J Surg Pathol 1997;21:605
Microscopic (histologic) images
Positive stains
- PAS: reduced expression of PAS+ cells compared with normal esophageal squamous epithelium (Am J Surg Pathol 1997;21:605)
- Antikeratin 1 immunostain labels the suprabasal cells of epidermization (Am J Surg Pathol 1997;21:605)
- Antikeratin 14 immunostain labels all the cell layers of epidermization (Am J Surg Pathol 1997;21:605)
Molecular / cytogenetics description
- Targeted next generation sequencing analysis of epidermoid metaplasia shows frequently mutated genes consisted of TP53, PIK3CA, EGFR, MYCN, HRAS and the TERT promoter (Mod Pathol 2017;30:1613)
Sample pathology report
- Esophagus, mid, plaque, biopsy:
- Epidermoid metaplasia / epidermization / orthokeratosis with prominent granular layer
- Negative for dysplasia or malignancy
Differential diagnosis
- Hyperkeratosis:
- No prominent granular layer
- Parakeratosis:
- Nuclei present in a keratin layer without an associated prominent granular layer
- Dysplasia:
- Variable nuclear atypia present
- Squamous cell carcinoma in situ:
- Full thickness nuclear atypia present
- Atrophic change:
- No orthokeratosis or prominent granular layer
Additional references
Board review style question #1
Which of the following statements about epidermization / epidermoid metaplasia of the esophagus is true?
- Characterized by orthokeratosis / hyperorthokeratosis with a prominent granular layer
- No association with smoking or excessive alcohol consumption
- No association with squamous cell carcinoma and dysplasia
- PAS stain highlights epidermoid metaplasia
Board review style answer #1
A. Epidermization / epidermal metaplasia is characterized by orthokeratosis / hyperorthokeratosis with a prominent granular layer, mimicking epidermis.
Comment Here
Reference: Epidermoid metaplasia
Comment Here
Reference: Epidermoid metaplasia
Board review style question #2
Which of the following statements about histologic features of epidermization / epidermoid metaplasia of the esophagus is true?
- Characterized by the presence of the stratum lucidum beneath the cornified layer
- Finding of increased thickness in the cornified layer is called parakeratosis
- Granular layer is characterized by the presence of basophilic stained granules known as keratohyalin granules
- Orthokeratosis / hyperkeratosis is more frequently seen in the esophagus than parakeratosis
Board review style answer #2
C. Epidermization / epidermoid metaplasia is histopathologically characterized by the presence of the granular layer beneath the cornified layer. The granular layer contains basophilic stained granules known as keratohyalin granules. Parakeratosis is characterized by keratosis with persistence of the cell nuclei. The stratum lucidum is seen only in soles and palms and is not seen in epidermization / epidermoid metaplasia. Parakeratosis is more frequently seen in the esophagus than orthokeratosis / hyperkeratosis.
Comment Here
Reference: Epidermoid metaplasia
Comment Here
Reference: Epidermoid metaplasia
Esophageal carcinoma-overview
Table of Contents
Definition / general | Risk factors | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Overall 5 year survival | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- < 1% of all cancers in US, ~6% of gastrointestinal cancers
- Disproportionate numbers of deaths as most patients are asymptomatic during early stages and present with advanced or metastatic disease (Wikipedia: Esophageal Cancer [Accessed 25 February 2019], eMedicine: Esophageal Cancer [Accessed 25 February 2019])
- Worldwide, 90% are squamous cell carcinoma
- In US and Western Europe, has been a shift from squamous cell carcinoma to adenocarcinoma
- Incidence of adenocarcinoma has been increasing, especially in white men and is now higher than squamous cell carcinoma in US (NIH: Esophageal Cancer [Accessed 25 February 2019]), France (Gastroenterol Clin Biol 2005;29:1258) and elsewhere
- Estimated 17,990 new cases with 15,210 deaths in US in 2013 (CA Cancer J Clin 2013;63:11)
Risk factors
- In developed world, smoking and drinking are most important risk factors
- Other risk factors are diet, especially some hot liquids, Plummer-Vinson syndrome, achalasia, stricture, webs, rings, diverticula
- Higher incidence among African Americans, city dwellers, poor
- "Esophageal carcinoma belt" extends from Northeast China to Middle East, with high incidence of squamous cell carcinoma
- Adenocarcinoma associated with Barrett esophagus
Sites
- Squamous cell carcinoma: 20% upper, 50% middle, 30% lower esophagus
- Adenocarcinoma mostly in distal esophagus
- Adenocarcinoma rarely arises in heterotopic gastric mucosa or submucosal glands
Pathophysiology
- Adenocarcinoma: dysplasia - carcinoma sequence occurs in Barrett mucosa with stepwise accumulation of genetic mutations, especially p53, also HER2 / cERB-B2, cyclin D1, cyclin E, RB, p16
- Squamous cell carcinoma: genetic alterations include p53, p16INK4a, amplification of cyclin D1, cMYC and EGFR; related to smoking, alcohol, diet, possible nutritional deficiency, genetic factors
Clinical features
- Insidious onset, dysphagia to solids, followed by dysphagia to all food
- Extreme weight loss due to loss of nutrition and tumor itself
- Squamous cell carcinoma may erode through esophagus; invades respiratory tree with fistula formation and pneumonia; aorta with exsanguination; also invades mediastinum or pericardium
- Metastasis generally occurs early even in superficial tumors due to extensive lymphatic network in esophagus that allows horizontal and longitudinal spread
- Cancers of upper esophagus metastasize to cervical lymph nodes; of mid esophagus to mediastinal, paratracheal and tracheobronchial lymph nodes; of lower esophagus to gastric and celiac lymph nodes
- Metastases to liver, lungs and pleura
- Recurrences are common
Diagnosis
- Endoscopic biopsy
- Rarely identified during resection for achalasia or for lesions not amenable to biopsy
Radiology description
- Stenosis or obstruction
- Protruding mass may be detected
Overall 5 year survival
- ~ 14% (Oncologist 2005;10:590) but 47% after resection (J Am Coll Surg 2006;202:588, Dis Esophagus 2009;22:1)
- Adenocarcinoma: < 20%
- Squamous cell carcinoma: ~9%; early detection raises to 75%
- ~0% in stage IV disease
- Presence of lymph node metastases at time of surgery greatly reduces survival
Case reports
- 47 year old man with esophageal adenocarcinoma arising from Barrett dysplasia (Clin Med Res 2006;4:184)
- 53 year old man with advanced middle esophageal cancer with multiple regional lymph node metastases (Jpn J Clin Onc 2002;32:310)
Treatment
- Generally resection if possible
- Sometimes adjuvant chemoradiation prior to resection
- Chemoradiation may achieve results similar to surgery (Ann Thorac Cardiovasc Surg 2006;12:234)
- Recommended to submit proximal margins for frozen section to rule out carcinoma underneath a normal appearing mucosa (Arch Pathol Lab Med 2005;129:1558)
- Occasionally HER2+, although this is not related to survival (Mod Pathol 2007;20:120) and anti-HER2 therapy does not appear to be effective (Int J Radiat Oncol Biol Phys 2007;67:405)
Gross description
- Adenocarcinoma is located in distal esophagus and may involve gastric cardia (note: tumors in the proximal 5 cm of the stomach are regarded as esophageal carcinomas in Edge: AJCC Cancer Staging Manual, 7th Edition, 2011)
- Flat or raised patches of intact mucosa develop into nodular masses
- Squamous cell carcinoma begins as an in situ process
- Generally starts as plaque-like lesions which grow to eventually encircle the lumen
- Mostly form protruding cauliflower-like lesions (60%), may be flat (15%) or ulcerated
Microscopic (histologic) description
- Overwhelming majority of carcinomas of esophagus are adenocarcinoma and squamous cell carcinoma
- Diagnosis usually simple
- Generally mucin secreting adenocarcinomas, less often signet ring cell carcinoma
- Usually foci of dysplastic mucosa adjacent to cancer
- Squamous cells carcinoma tends to be well or moderately differentiated; histologic variants include verrucous, spindle cell or basaloid
- Other carcinomas are adenoid cystic carcinoma, adenosquamous carcinoma, mucoepidermoid carcinoma
- Special stains only rarely needed for diagnosis
Microscopic (histologic) images
Differential diagnosis
- Chemoradiation induced atypia: enlarged hyperchromatic nuclei with prominent mitotic figures but relatively mild pleomorphism in inflammatory background
- Reparative processes: atypical cells in granulation tissue but with fine chromatin, few mitotic figures; cells mature at deeper levels, keratin negative
Esophageal cysts
Table of Contents
Definition / general | Bronchogenic cysts | Duplication cysts | Inclusion cysts | Retention cystsDefinition / general
- Rare; mostly developmental unless due to cystic degeneration of tumor
- Simple cysts are epithelial lined; duplication cysts have 2 muscle layers (eMedicine: Esophageal Cysts [Accessed 14 February 2019], Arch Pathol Lab Med 1977;101:136)
- Either intramural or attached to outer layers of esophagus
- Often asymptomatic, even if large; may cause obstructive symptoms
- See Retention cysts
Bronchogenic cysts
Definition / general
Case reports
Microscopic (histologic) images
AFIP images:
- Often young women with dysphagia or chest pain during exercise (Clin Imaging 2006;30:309)
- Developmental cysts arise from anomalous budding of foregut bronchial structures; contains cartilage and mucus glands, smooth muscle and ciliated columnar epithelium
Case reports
- 26 year old man with cystic lesion at lower esophagus (Dig Surg 2006;23:209)
Microscopic (histologic) images
AFIP images:
Duplication cysts
Definition / general
Case reports
Treatment
Clinical images
Images hosted on other servers:
Microscopic (histologic) description
Microscopic (histologic) images
Images hosted on other servers:
- Also called gastroenteric cyst, foregut cyst
- Congenital anomaly, usually lower esophagus
- Most are intramural; usually isolated anomaly; however, duplications external to esophageal wall may be associated with vertebral anomalies
- 90% do not communicate with esophagus
- Usually symptomatic causing dysphagia or respiratory difficulty
Case reports
- 22 day old boy (Indian J Pathol Microbiol 2006;49:396)
- 2 infants with respiratory distress (Pediatr Emerg Care 2005;21:854)
- 14 year old boy with fistula to lung (Eur J Cardiothorac Surg 2000;18:117)
- 52 year old woman (Yonsei Med J 2005;46:859)
- 61 year old woman with squamous cell carcinoma (Br J Radiol 2003;76:343)
Treatment
- Surgery (if symptoms) or possibly observation (Endoscopy 2005;37:870)
Clinical images
Images hosted on other servers:
Microscopic (histologic) description
- Mucosa, submucosa and muscular layers similar to GI tract; lined by either esophageal squamous, gastric, primitive, ciliated columnar or small intestinal epithelium
Microscopic (histologic) images
Images hosted on other servers:
Inclusion cysts
Definition / general
- Lined by squamocolumnar epithelium, may be ciliated
Retention cysts
Definition / general
Microscopic (histologic) description
- Also called mucocele
- Derive from obstructed submucosal gland ducts
- Small, usually in lower esophagus
- May cause intramural pseudodiverticulosis, with multiple flask-like invaginations into esophageal wall (Am J Clin Pathol 1976;65:314)
- Associated with chronic esophagitis and fibrosis; also surgically isolated segments of esophagus (Dis Esophagus 2002;15:96)
Microscopic (histologic) description
- Saccular or flask shaped dilation of submucosal gland excretory ducts; rarely reaches muscularis propria
- In large lesions, muscularis does not accompany the lesion so are not true diverticula
Esophageal manifestation of dermatologic disease (pending)
[Pending]
Esophageal manifestations of collagen vascular disease
Table of Contents
Definition / general | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Electron microscopy images | Differential diagnosisDefinition / general
- Characterized by fibrosis, inflammation, increased collagen and vasculitis
- Can be part of CREST syndrome (Calcinosis, Raynaud phenomenon, Esophageal involvement, Sclerodactyly, Telangiectasia)
- Involves esophagus in 75%+ patients, usually distal 2/3, with aperistalsis and reduced tone of lower esophageal sphincter
- Commonly causes reflux esophagitis (Semin Arthritis Rheum 2006;36:173), strictures, Candida esophagitis, Barrett esophagus (J Clin Gastroenterol 2006;40:769 but similar prevalence of Barrett as other reflux esophagitis patients, Arthritis Rheum 2005;52:2882)
Case reports
- 38 year old man with achalasia (Dtsch Med Wochenschr 2006;131:1799)
- 51 year old woman with CMV esophagitis (J Clin Rheumatol 2001;7:384)
Treatment
- No treatment for underlying disease; proton pump inhibitors for reflux esophagitis, dilation for strictures
Microscopic (histologic) description
- Resection specimens: atrophy and replacement fibrosis of inner circular layer of muscularis propria and resulting stenosis; longitudinal layer is usually involved; also submucosal fibrosis, mild inflammation, intimal proliferation of arterioles (Gut 2006;55:1697)
- Biopsies: ulcers or erosions resembling reflux esophagitis, Candida or Barrett
Microscopic (histologic) images
Electron microscopy images
Differential diagnosis
- Cricopharyngeal dysphagia
- Primary visceral myopathy
- Sjögren syndrome
- Systemic lupus erythematosus
Esophageal sarcoma-overview
Table of Contents
Definition / general | Epidemiology | Sites | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Flow cytometry description | Electron microscopy description | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Malignant mesenchymal tumors of the esophagus with variable degrees of differentiation, biologic behavior and prognosis
- Examples
- Angiosarcoma: high grade malignancy of endothelial cells
- Ewing sarcoma: highly malignant small round blue cell tumor characterized by recurrent chromosomal translocations [t(11;22) EWSR1-FLI1 or t(21;22) EWSR1-ERG] and membranous MIC2 / CD99 overexpression
- Fibrosarcoma: spindle cell neoplasm of low grade to intermediate grade malignancy
- Gastrointestinal stromal tumor (GIST): mesenchymal tumor of digestive tract, likely originating from multipotential progenitors of interstitial cells of Cajal
- Hemangiopericytoma (solitary fibrous tumor): ubiquitous mesenchymal tumor showing uncertain line of differentiation (not true microvascular pericytes)
- Kaposi sarcoma: uncommon, low grade, vascular malignancy caused by Kaposi sarcoma herpesvirus / human herpesvirus 8 (KSHV / HHV8) infection
- Leiomyosarcoma: malignant smooth muscle tumor
- Liposarcoma: malignant adipocytic / lipomatous tumor
- Malignant peripheral nerve sheath tumor (MPNST): malignant tumor arising from peripheral nerve or a neurofibroma or in extraneural soft tissue and showing nerve sheath differentiation
- Osteosarcoma: mesenchymal malignancy characterized by neoplastic cells that produce osteoid matrix
- Synovial sarcoma (SS): malignant mesenchymal tumor showing epithelial differentiation, either overtly (biphasic SS) or by IHC alone (monophasic SS)
- Undifferentiated pleomorphic sarcoma: highly malignant pleomorphic neoplasm lacking any specific line of differentiation
Epidemiology
- Extremely rare
Sites
- Likely arise from submucosal or intramural elements
Clinical features
- Asymptomatic mass
- Obstructive features: dysphagia, odynophagia, nausea, vomiting
Diagnosis
- Biopsy or resection with immunohistochemical or molecular ancillary studies
Radiology description
- Highly variable
- Destructive mass with variable hemorrhage or necrosis
- Strictures or obstruction
Radiology images
Prognostic factors
- Poor prognostic factors
- High histologic grade
- Cytologic atypia, nuclear pleomorphism
- Necrosis
- Mitotic activity
- High histologic grade
- Poor degree of differentiation
Treatment
- Treatment options depend on type of sarcoma and clinical stage
- Treatment modalities include surgery, chemotherapy, radiotherapy
Gross description
- Often large tumor with infiltrative margins and variable hemorrhage, necrosis
Microscopic (histologic) description
- Highly variable
- Round blue cell tumors: Ewing sarcoma, small cell variant of osteosarcoma
- Spindle cell tumors: angiosarcoma, fibrosarcoma, GIST, hemangiopericytoma, Kaposi sarcoma, leiomyosarcoma, MPNST, synovial sarcoma
- Adipocytic differentiation: liposarcoma
- Osteoid production: osteosarcoma
- Undifferentiated: pleomorphic sarcoma
Microscopic (histologic) images
Positive stains
- Angiosarcoma: endothelial markers (factor VIII related antigen, CD31, CD34)
- Ewing sarcoma: MIC2 / CD99 (100% sensitive but not specific), FLI1 gene product (90%), neuron specific enolase, vimentin, p53
- GIST: CD117 / c-kit, DOG1, CD34, focal SMA, focal desmin, focal S100
- Hemangiopericytoma: CD34 (95%), CD99 (70%), CD57 (50%), focal nuclear beta catenin
- Kaposi sarcoma: HHV8, factor VIII related antigen, CD34, CD31, FLI1, D2-40, VEGFR3, BCL2
- Leiomyosarcoma: smooth muscle markers (SMA, desmin, caldesmon, calponin)
- Liposarcoma: S100 (lipogenic zones); MDM2, CDK4 (well differentiated tumors)
- MPNST: S100 (weak / focal in spindled MPNST, diffuse / strong in epithelioid MPNST)
- Synovial sarcoma: focal high molecular weight cytokeratin
- Undifferentiated pleomorphic sarcoma: CD68
Flow cytometry description
- Useful to exclude hematopoietic malignancies
Electron microscopy description
- Angiosarcoma: Weibel-Palade bodies
- Ewing sarcoma: extensive cytoplasmic glycogen deposits
- Hemangiopericytoma: undifferentiated spindle cell or fibroblastic features
- Leiomyosarcoma: features of smooth muscle differentiation (thin filaments, pinocytic vesicles, attachment plaques, interrupted external lamina)
- Liposarcoma: nonmembrane bound intracytoplasmic lipid droplets of varying sizes / densities
- MPNST: tumor cells with external laminae, indicating schwannian differentiation
- Osteosarcoma: osteoid matrix comprises nonperiodic fibrils, scattered collagen fibers and hydroxyapatite calcium crystals
Molecular / cytogenetics description
- RT PCR or FISH
- Ewing sarcoma: recurrent chromosomal translocations including t(11;22)(q24;q12) EWS-FLI1, t(21;22)(q22;q12) EWS-ERG, t(7;22) EWS-ETV1, t(17;22) EWS-E1AF, t(2;22) EWS-FEV
- GIST: mutually exclusive mutations of activating KIT (95%) or platelet derived growth factor alpha (PDGFRA) receptor tyrosine kinase (5%)
- Kaposi sarcoma: HHV8 detected by PCR
- Leiomyosarcoma: no consistent genetic events reported
- Well differentiated liposarcoma: giant marker or supernumerary ring chromosomes with amplification of 12q12-15 region, including MDM2, CDK4 and other genes
- Myxoid / round cell liposarcoma: t(12;16) DDIT3-TLS or t(12;22) DDIT3-EWS balanced translocations
- Synovial sarcoma: characteristic t(X;18) SSYT-SSX1 / 2 translocations
Differential diagnosis
- Benign spindle cell proliferations, e.g.
- Inflammatory fibroid polyp: PDGFRA+, CD34+, CD117-, mixed inflammatory infiltrate (especially eosinophils)
- Leiomyoma: rare atypia, mitotic activity, or necrosis
- Ossifying fibromyxoid tumor: usually bland cytology and peripheral ossification
- Schwannoma: diffuse S100+, Verocay bodies
- Lymphoma: usually CD45 (LCA)+, flow cytometry may be diagnostic
- Melanoma: melanoma markers+ (S100, HMB45, MART, MITF, MelanA, SOX10, tyrosinase)
- Other round blue cell tumors, e.g.
- Alveolar rhabdomyosarcoma: desmin+, myogenin+, myoD1+
- Desmoplastic small round cell tumor (DSRCT): striking desmoplasia, coexpression of cytokeratin / desmin, WT1+
- Glomus tumor: round to polygonal cells with scant cytoplasm and marked cellularity uniformity; SMA+, desmin-, S100-
- Neuroendocrine (small cell) carcinoma: neuroendocrine markers+ (CD56, chromogranin, synaptophysin)
- Poorly differentiated carcinoma: focal cytokeratin+
Additional references
Esophagitis dissecans superficialis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign clinical condition characterized by sloughing of the esophageal mucosa with unclear pathogenesis
Essential features
- Rare condition diagnosed incidentally on endoscopy
- Strips of vertically sloughed mucosal fragments evident on endoscopy
- Histologically, characterized by intraepithelial splitting, prominent parakeratosis, intraepithelial cystic degeneration and basal cell hyperplasia
- 2 tone esophagus
- Resolves spontaneously without complications
Terminology
- Sloughing esophagitis, this entity was first reported by B. Rosenberg in 1982 (Abdom Radiol (NY) 2021;46:5050)
ICD coding
- ICD-10: K20.9 - esophagitis, unspecified
Epidemiology
- Usually occurs in older age group (Int J Biomed Sci 2014;10:282)
Sites
- Mid or distal part of esophagus
- However, it can affect the entire length of the esophagus
Pathophysiology
- Unknown
- Can result from ischemia or a direct insult to the esophageal mucosa caused by chemical, thermal, physical or immunological mechanisms (Gastroenterology Res 2016;9:108)
Etiology
- Mostly idiopathic
- Consumption of hot beverages and exposure to chemical irritants
- Malignancy
- Esophageal trauma
- Use of medications such as bisphosphonates, nonsteroidal anti-inflammatory drugs, psychoactive medications (e.g., SSRIs or SNRIs), methotrexate and potassium chloride
- Presence of celiac disease, collagen disorders and autoimmune bullous dermatoses (Cureus 2023;15:e43549)
- Can occur following immune checkpoint inhibitor therapy (Curr Probl Cancer Case Rep 2021;3:100044)
- Gastrointestinal side effects of COVID-19 or potentially aspirin consumption (Middle East J Dig Dis 2022;14:346)
Diagrams / tables
None
Clinical features
- Usually diagnosed incidentally on endoscopy
- Few patients can present with dysphagia, heartburn, odynophagia, regurgitation, dyspepsia, upper gastrointestinal bleeding, anemia and weight loss
- In some extreme cases, patients can vomit mucosal casts (Am J Gastroenterol 1998;93:655)
- Associated with different autoimmune conditions such as celiac disease, lupus, pemphigus vulgaris, bullous pemphigoid and Stevens-Johnson syndrome (Am J Surg Pathol 2009;33:1789)
Diagnosis
- Upper gastrointestinal endoscopy (i.e., EGD) which shows the characteristic appearance of sloughed mucosal fragments, along with a biopsy to confirm the diagnosis and rule out other possible conditions
- Meeting 3 of the following endoscopic criteria is consistent with esophagitis dissecans superficialis (EDS) (Ulster Med J 2020;89:39)
- Strip(s) of sloughed esophageal mucosa > 2 cm in length
- Normal underlying esophageal mucosa
- Lack of ulcerations or friability of immediately adjacent esophageal mucosa
Laboratory
Not relevant to this topic
Radiology description
- Sloughed out mucosal strips highlighted by barium can be seen on the barium esophagram in few cases (Abdom Radiol (NY) 2021;46:5050)
Radiology images
Image from PMID: 34137931 is not free to access
Prognostic factors
- Favorable prognosis as it commonly resolves spontaneously without complications (Curr Probl Cancer Case Rep 2021;3:100044)
Case reports
- 50 year old man with a past medical history of HIV was hospitalized due to a 3 week course of oropharyngeal dysphagia (Case Rep Infect Dis 2019;2019:4616937)
- 65 year old woman presented with nausea, vomiting, odynophagia and throat pain after ingesting a colored hair dye (Clin Pract 2021;11:185)
- 71 year old man with dysphagia and odynophagia (J Investig Med High Impact Case Rep 2019;7:2324709619892726)
- 81 year old woman with unintentional weight loss of 8 pounds (Cureus 2023;15:e44372)
Treatment
- No standard treatment is available as the condition is self limiting
- Treatment is often decided based on the severity of symptoms
- Proton pump inhibitors are commonly used, although their primary outcome seems to involve minimizing additional injuries, rather than addressing the root cause (Ann N Y Acad Sci 2016;1380:178)
- Patients with resistance to proton pump inhibitors may undergo treatment involving a trial dose of steroids (J Investig Med High Impact Case Rep 2019;7:2324709619892726)
Clinical images
Gross description
Not relevant
Gross images
Not relevant to this topic, diagnosed mostly by endoscopy followed by biopsy
Frozen section description
Not relevant to this topic
Frozen section images
Not relevant to this topic
Microscopic (histologic) description
- Characterized by intraepithelial splitting, which results in detached fragments of superficial epithelium
- Prominent parakeratosis is the most common histological finding
- Variably sized cysts and bullae (termed intraepithelial cystic degeneration by Hart et al.) (Dig Dis Sci 2015;60:2049)
- Dual tone appearance of esophageal epithelium
- Overlying superficial layer of necrotic, deeply eosinophilic squamous cells
- Deep layer of viable epithelium without degeneration or inflammation
- Basal cell hyperplasia
- Detached fragments may show an association with fungal or bacterial colonies, typically characterized by minimal or focal acute inflammatory responses
Microscopic (histologic) images
Virtual slides
I could not find a virtual slide for this topic
Cytology description
Not relevant to this topic
Cytology images
Not relevant to this topic
Immunofluorescence description
Not relevant to this topic
Immunofluorescence images
Not relevant to this topic
Positive stains
Note to author: we don't generally include H&E
Electron microscopy description
Not relevant to this topic
Electron microscopy images
Not relevant to this topic
Molecular / cytogenetics description
Not relevant to this topic
Molecular / cytogenetics images
Not relevant to this topic
Videos
Sloughing esophagitis
Sample pathology report
- Esophagus, biopsy:
- Morphologic features concerning for esophagitis dissecans superficialis / sloughing esophagitis (see comment)
- Negative for intramucosal eosinophilia, dysplasia or malignancy
- Comment: The etiology is usually unknown but condition has been associated with some medications, bullous dermatoses, motility disorders, physical / thermal injury and autoimmune diseases.
Differential diagnosis
- Candida esophagitis:
- Similar appearance of white plaques on endoscopy
- Erosive esophagitis pattern of injury with acute inflammation, intraepithelial neutrophilic abscesses and epithelial edema most prominent in the superficial epithelial layers on microscopy
- Basal zone hyperplasia, parakeratosis and hyperkeratosis are frequently associated
- Yeast forms and pseudohyphae can be identified on Grocott methenamine silver (GMS) stain
- Epidermoid metaplasia:
- Well demarcated white plaque in the mid to distal esophagus
- Esophageal squamous epithelium with a prominent granular layer and orthokeratosis / hyperorthokeratosis
- Abrupt transition from the adjacent normal squamous epithelium
- Lacks superficial necrosis, epithelial detachment
- Bullous dermatoses involving esophagus (pemphigoid, pemphigus, Stevens-Johnson syndrome):
- Characterized by immune mediated split at different anatomic levels within the basement membrane zone with inflammatory infiltrates
- Complement and Ig deposits can be identified on immunofluorescence using fresh / frozen tissues
- Iatrogenic trauma (endoscopy or specimen handling during biopsy):
- This can result in artifactual intraepithelial splitting
Additional references
Board review style question #1
Which histologic feature is commonly seen in esophagitis dissecans superficialis (EDS)?
- Dual tone appearance of esophagus
- More than 20 eosinophils per high powered field
- Prominent granular layer with orthokeratosis
- Psuedohyphae and yeast forms
Board review style answer #1
A. Dual tone appearance of esophagus is a common histological finding for EDS. Answer D is incorrect because fungal elements (pseudohyphae and yeast forms) are seen in esophageal candidiasis. Answer B is incorrect because increased eosinophils are usually seen with eosinophilic esophagitis. Answer C is incorrect because a prominent granular layer with orthokeratosis is seen in epidermoid metaplasia.
Comment Here
Reference: Esophagitis dissecans superficialis
Comment Here
Reference: Esophagitis dissecans superficialis
Board review style question #2
The image above is from an esophageal biopsy in a 46 year old man with dysphagia. Endoscopy of the esophagus shows strips of sloughed esophageal mucosa. Which of the following would be a possible etiology for these endoscopic findings?
- Allergic contact dermatitis
- Chemical exposure
- Immunosuppression
- Use of proton pump inhibitors
Board review style answer #2
B. Chemical exposure can be a causative factor for esophagitis dissecans superficialis (EDS). Answer C is incorrect because immunosuppression can predispose to cause esophageal candidiasis. Answer A is incorrect because allergic dermatitis can be associated with eosinophilic esophagitis. Answer D is incorrect because proton pump inhibitors are commonly used in the treatment of EDS and do not contribute to cause EDS.
Comment Here
Reference: Esophagitis dissecans superficialis
Comment Here
Reference: Esophagitis dissecans superficialis
Esophagitis dissecans superficialis
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Benign condition of uncertain etiology
- Superficial strips of squamous mucosa peel off into the esophageal lumen with normal underlying mucosa
- Tends to resolve without sequelae
Essential features
- Characteristic endoscopic finding of long, vertical white strips of peeling epithelium with normal underlying mucosa
- Constellation of nonspecific histologic findings
- Splitting of the squamous epithelium at different levels above the basal layer
- Variably sized cysts and bullae
- Thick layer of parakeratosis
- Basal cell hyperplasia
- Focal or minimal inflammation
Terminology
- Also called "sloughing esophagitis"
Epidemiology
- More common in older individuals (median age in the seventh decade)
Etiology
- Unknown
- Has been variably associated with certain medications (NSAIDs, bisphosphonates), polypharmacy, prior trauma, underlying motility disorders, heavy smoking, alcohol, hot beverages, immunosuppression, celiac disease, concurrent bullous skin conditions and impaired mobility
Clinical features
- Range from asymptomatic to dramatic
- Original reports described patients vomiting up casts of esophageal epithelium
- Esophageal symptoms such as dysphagia, odynophagia and reflux more common
- Can be an incidental finding
Diagnosis
- Characteristic endoscopic findings of peeling, white, vertical strips of epithelium > 2 cm long
- Normal underlying mucosa
- Lack of ulcerations or friability of adjacent mucosa
- Endoscopic appearance likened to an esophagus "filled with gift wrap paper" (Am J Surg Pathol 2009;33:1789)
- Biopsy may not be needed unless to rule out other conditions
Case reports
- 40 year old Hispanic woman with bullous systemic lupus erythematosus associated with esophagitis dissecans superficialis (Case Rep Rheumatol 2015;2015:930683)
- 67 year old man with unusual appearing esophagus on endoscopy (Case #420)
- 68 year old man with fungal esophagitis presenting with esophagitis dissecans superficialis (Gastroenterology Res 2016;9:108)
Treatment
- Typically proton pump inhibitor therapy and discontinuation of medications that may have triggered the condition
Clinical images
Microscopic (histologic) description
- Splitting of the squamous epithelium at different levels above the basal layer
- Variably sized cysts and bullae (termed "intraepithelial cystic degeneration" by Hart et al., Dig Dis Sci 2015;60:2049)
- Thick layer of parakeratosis that separates off into necrotic fragments
- "Two tone" appearance to epithelium
- Basal cell hyperplasia
- Fungal or bacterial colonies can be associated with the loose fragments
- Inflammation is typically minimal or focal (despite "esophagitis" being in the name)
Microscopic (histologic) images
Differential diagnosis
- Endoscopically:
- Candida esophagitis (white plaques)
- Iatrogenic trauma (e.g., prior endoscopy)
- Histologically:
- Artefactual splitting of epithelium due to biopsy or during grossing
- Bullous skin diseases such as pemphigus vulgaris
- Stricture (parakeratosis)
Additional references
Board review style question #1
Which histologic feature is not commonly seen in esophagitis dissecans superficialis?
- Basal cell hyperplasia
- More than 20 eosinophils per high powered field
- Splitting of the squamous epithelium at different levels above the basal layer
- Thick layer of parakeratosis
- Variably sized cysts and bullae
Board review style answer #1
B. More than 20 eosinophils per high powered field
Comment Here
Reference: Esophagitis dissecans superficialis
Comment Here
Reference: Esophagitis dissecans superficialis
Esophagitis-overview
Table of Contents
Definition / general | Epidemiology | Etiology | Microscopic (histologic) descriptionDefinition / general
- Defined as epithelial damage and inflammation (eMedicine: Esophagitis [Accessed 12 February 2019])
Epidemiology
- 5% to 11% incidence in US, although reflux symptoms in 33 - 44% of general population
- Higher incidence in northern Iran and China
Etiology
- Most common cause is gastroesophageal reflux (reflux of gastric contents into lower esophagus); infectious causes are much less common
Microscopic (histologic) description
- Histologic changes may be severe (erosion, ulcer, exudates) or subtle
Gastrointestinal stromal tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Most common mesenchymal tumor of the gastrointestinal tract (Transl Gastroenterol Hepatol 2018;3:6, World J Gastroenterol 2015;21:5630)
- Comprises 0.1 - 3.0% of all gastrointestinal tumors
Essential features
- Rare in esophagus < 1% (0.7%); most common site is stomach (Mod Pathol 2022;35:554)
- Most are due to mutations in proto-oncogene KIT (exon 11)
- 3 histologic types: spindle, epithelioid and mixed
- Diagnosis confirmed immunohistochemically using KIT and DOG1 antibodies
- Prognosis: depends on tumor size, mitotic rate and site of origin
- Treatment: surgical excision or imatinib
Terminology
- Gastrointestinal stromal tumor (GIST)
- Size range is 0.1 - 12.0 cm (median: 2.5 cm), majority are ≤ 5 cm (Mod Pathol 2022;35:554)
- Microscopic GIST: sporadic interstitial cell of Cajal hyperplasia, seedling GIST, minimal GIST
- Range in size from 4 - 10 mm in diameter and immunohistochemically positive for KIT (Am J Pathol 2002;160:1567)
- Historic terms:
- Gastrointestinal smooth muscle tumor
- Gastrointestinal autonomic nerve tumor
- Leiomyoblastoma
- Smooth muscle tumor of uncertain malignant potential
- Gastrointestinal pacemaker cell tumor
ICD coding
- ICD-10: C49.A1 - GIST, esophagus
Epidemiology
- Very rare in esophagus: < 1% of all GIST arise in esophagus (0.7%); they most frequently arise in stomach (60.3%), followed by small intestine (33.2%), rectum (3.1%) and colon (2.9%) (Mod Pathol 2022;35:554)
- Annual incidence 0.1 - 0.3 per million
- More common in men (M:F = 1.7:1)
- Average age: < 60 years (median: 67)
- 25% incidental, asymptomatic tumors at diagnosis; may be incidental finding in patients undergoing resection of upper GI neoplasms (Am J Cancer Res 2014;5:333, Can J Surg 2012;55:366, Dtsch Med Wochenschr 2005;130:2380)
- GISTs comprise < 25% of mesenchymal esophageal tumors, leiomyomas being the most common (Am J Surg Pathol 2000;24:211)
Sites
- Most common location is lower esophagus, followed by middle esophagus; rare in upper esophagus (Am J Cancer Res 2014;5:333, Medicine (Baltimore) 2016;95:e2446)
Pathophysiology
- GISTs arise from interstitial cells of Cajal (ICC)
- Driver event involves gain of function mutations in KIT and PDGFRA most frequently
- Most of these tumors respond to the tyrosine kinase inhibitor, imatinib
- Less frequently, a BRAF V600E mutant is involved as the driving force and these cases are less responsive to imatinib
- Etv1, a major transcriptional regulator of ICC - intramuscular and ICC - myenteric, induces BRAF expression in a mouse model
- Smooth muscle cells have recently been proposed as an alternative cell of origin for GIST, and these tumors are BRAF V600E driven and resistant to imatinib (J Pathol 2020;252:441)
Etiology
- Mostly sporadic
- 5% of GISTs arise in patients with:
- Neurofibromatosis type I syndrome (NF1, multiple small intestinal tumors)
- Carney triad (gastric epithelioid GISTs in young females) (Arch Pathol Lab Med 2006;130:1466)
- Familial GIST syndrome: rare, autosomal dominant genetic disorder, characterized by germline KIT or PDGFRA mutations, early onset of multiple GIST tumors, skin pigmentation abnormalities (Cancer 2015;121:2960, Arch Pathol Lab Med 2006;130:1466)
Clinical features
- Dysphagia, most common (51%), weight loss (20%), bleeding (10%) and chest pain (Diagn Interv Radiol 2010;16:217)
- Dysphagia and odynophagia (30%), gastroesophageal reflux (8%) and epigastric pain (4%) (Mod Pathol 2022;35:554)
- Esophageal mass, stricture, perforation
- Rare metastatic spread to lung, liver, bone and peritoneum (Am J Surg Pathol 2000;24:211)
Diagnosis
- May be an incidental finding during imaging or endoscopic examination for other clinical symptoms
- Endoscopy shows as a subepithelial lesion; both leiomyoma and GIST appear similar on CT and EUS (J Thorac Dis 2015;7:E648)
- Biopsy with immunohistochemical stains / molecular required for confirmation
Radiology description
- Barium studies: smooth intraluminal mass or large ulcerative mass extending intraluminally (see radiology image 1)
- Endoscopic ultrasound determines size, shape and intratumoral characteristics of the lesion, its relationship to layers of the bowel wall and enables image guided core needle biopsy or fine needle aspiration (J Gastrointestin Liver Dis 2008;17:131) (see clinical image 1)
- CT / MRI: useful for preoperative and metastatic evaluation
- Well circumscribed, hypoattenuating submucosal mass (Cancer Imaging 2012;12:100) (see radiology image 2)
- PET FDG avidity correlates with malignant potential of GIST
- Also used to monitor response to chemotherapy (tyrosine kinase inhibitor therapy) and evaluate postoperative recurrence (see radiology image 3)
Radiology images
Prognostic factors
- Nashville Risk Score is utilized in predicting progression free survival of patients with GIST (Histopathology 2022;80:874, Mod Pathol 2022;35:554)
- Risk stratification based on tumor size, mitotic activity and site of origin per 50 high power fields (HPF or 5 mm²)
- Low risk:
- < 5 cm and < 5 mitoses/50 HPF or 5 mm²
- Intermediate risk:
- < 5 cm and 6 - 10/50 HPF
- 5 - 10 cm and < 5 mitoses/50 HPF or 5 mm²
- High risk:
- > 5 cm and mitoses > 5/5 mm²
- > 10 cm and any mitotic rate
- Any size and > 10/50 HPF or 5 mm²
- Presence of rupture at the time of surgery and male sex independent risk factor
- Low risk:
| Risk stratification of esophageal GISTs using the new Nashville risk score | ||
| Risk category | Tumor size | Mitotic rate/50 HPF or 5 mm² |
| Low | < 5 cm | < 5 |
| Intermediate | < 5 cm | 6 - 10 |
| Intermediate | 5 - 10 cm | < 5 |
| High | > 5 cm | > 5 |
| High | > 10 cm | Any mitotic rate |
| High | Any size | > 10 |
Case reports
- 39 year old man with esophageal perforation (Int J Surg Case Rep 2013;4:636)
- 51 year old woman with bulky tumor in posterior mediastinum (Chirurgia (Bucur) 2015;110:300)
- 53 year old man with pulmonary and bone metastases (Diagn Interv Radiol 2010;16:217)
- 57 year old man with germline KIT mutation (Arch Pathol Lab Med 2007;131:1393)
- 86 year old man with 3 month history of dysphagia presented with a huge gastrointestinal stromal tumor (Surg Case Rep 2022;8:109)
Treatment
- Surgical resection for localized GIST (3 - 5 cm) (Surg Case Rep 2022;8:109)
- Neoadjuvant therapy, small molecule tyrosine kinase inhibitors: imatinib mesylate (STI571, Gleevec), sunitinib maleate may be given preoperatively to reduce the size of tumor
- Imatinib is considered in patients with high mitotic rates or larger tumor sizes to obtain negative microscopic margins (R0 resection) and to reduce the risk of intraoperative complications, including tumor rupture (Histopathology 2017;71:805) (see radiology image 4)
Gross description
- Lobulated, well circumscribed mass with homogenous fleshy cut surfaces (see gross image 1)
- Exophytic or intramural, centered within the muscularis propria
- Cystic degeneration, hemorrhage, necrosis (more with high grade)
- Overlying esophageal mucosa may be ulcerated (see gross image 2)
- References: Transl Gastroenterol Hepatol 2018;3:6, Int J Surg Case Rep 2013;4:636
Frozen section description
- Spindle cell neoplasm; defer to permanent for definite categorization
Microscopic (histologic) description
- Histologic heterogeneity: 3 morphologic types - spindle (70%), epithelioid (20%) and mixed (10%)
- Spindle cell type: bland spindle cells with eosinophilic cytoplasm in short fascicles or syncytia, elongated nuclei with inconspicuous nucleoli, indistinct cell borders, paranuclear cytoplasmic vacuoles (more common in gastric), stromal lymphocytes and microcystic stromal degeneration (as in schwannoma), minimal collagen, delicate thin walled vessels, stromal hemorrhage
- Subtypes: sclerosing, palisaded, vacuolated, diffuse hypercellular, can have sarcomatoid features with significant nuclear atypia and mitotic activity
- Epithelioid type: round cells with vesicular chromatin, eosinophilic or clear cytoplasm in nests or sheets, more pleomorphism than spindle type
- Subtypes: sclerosing, discohesive, hypercellular, sarcomatous with significant atypia and mitotic activity
- Mixed type: combination of cells with both spindle and epithelioid type, may have abrupt transition between the 2 or complex comingling
- Spindle cell type: bland spindle cells with eosinophilic cytoplasm in short fascicles or syncytia, elongated nuclei with inconspicuous nucleoli, indistinct cell borders, paranuclear cytoplasmic vacuoles (more common in gastric), stromal lymphocytes and microcystic stromal degeneration (as in schwannoma), minimal collagen, delicate thin walled vessels, stromal hemorrhage
- SDH deficient: epithelioid or mixed with spindle cell component, multinodular, minimal nuclear pleomorphism, occasional atypical mitosis (gastric location strictly, not seen in esophagus)
- Dedifferentiated: anaplastic morphology with unusual phenotype such as loss of KIT expression or aberrant expression of markers such as cytokeratin
- Stromal skeinoid fibers (< 20%): hyaline or fibrillary brightly eosinophilic PAS+ structures, representing nodular tangles of collagen fibers; more common in small or large bowel GIST (Case Rep Gastroenterol 2014;8:257)
- Rarely: cytologic atypia, myxoid stroma
- Variable mitotic activity
- Microscopic GIST: < 10 mm, spindle cells with hyalinized stroma and variable calcification (Pathology 2008;40:9, Histopathology 2017;70:211)
- Treated GIST can microscopically have hyalinized areas or areas of necrosis
Microscopic (histologic) images
Cytology description
- Endoscopic ultrasound guided fine needle aspiration may be primary diagnostic modality
- Cellular smears, single cells and loosely cohesive fascicles of monomorphic spindle to epithelioid cells
- Irregularly outlined clusters of cells
- Prominent vascular pattern is commonly observed
- Spindle / round / oval nuclei, vesicular chromatin, wispy cytoplasm with long extensions
- Perinuclear or paranuclear vacuoles may be present
- Nuclear palisading can occur
- Stripped spindled to epithelioid nuclei
- Cohesive fragments with admixed delicate, thin walled vessels
- Features of malignant behavior of tumor predicted by cytologic atypia, cellular dyscohesion, nuclear pleomorphism, prominent nucleoli, increased mitotic activity, prominent necrosis
- Reference: DeMay: The Art & Science of Cytopathology, 2nd Edition, 2012
Cytology images
Positive stains
- CD117 / KIT (95%), membranous and cytoplasmic or concentrated in a dot-like perinuclear pattern (globular) diffuse, strong (Am J Surg Pathol 2009;33:1401)
- DOG1 (ANO1) (98%) (Am J Pathol 2004;165:107)
- CD34 (75%), SMA (45%, focal), desmin (5%, focal), S100 (5%, focal) (Histopathology 2017;71:805, Mod Pathol 2011;24:866)
Negative stains
Electron microscopy description
- Relative lack of differentiation (Endocr Relat Cancer 2007;14:853)
- Incomplete smooth muscle or neuroaxonal differentiation
- No bundles of actin filaments (seen in true smooth muscle tumors)
Molecular / cytogenetics description
- Mutually exclusive activating mutations in the proto-oncogene KIT (75%) or platelet derived growth factor alpha (PDGFRA) receptor tyrosine kinase (10%)
- In frame deletions, point mutations, duplications, insertions
- Most common: KIT juxtamembrane domain (exon 11) mutations
- Confers a better response to tyrosine kinase inhibitor therapy
- References: Science 2003;299:708, Cancers (Basel) 2019;11:679, Semin Diagn Pathol 2006;23:91
Sample pathology report
- Esophagus, distal, esophagectomy:
- Gastrointestinal stromal tumor (GIST), mixed spindle and epithelioid type, 4.0 x 3.5 x 2.4 cm (see synoptic report)
- Margins are negative
- AJCC pathologic stage: pTxNx
- Reference: CAP: Protocol for the Examination of Specimens from Patients with Gastrointestinal Stromal Tumor (GIST) [Accessed 12 October 2022]
Differential diagnosis
- Leiomyoma / leiomyosarcoma:
- Spindle cells with cigar shaped nuclei (blunt ended), brightly eosinophilic cytoplasm, arranged in fascicles
- Desmin+, h-caldesmon+; diffusely SMA+, CD117-
- Schwannoma:
- Desmoid fibromatosis:
- Beta catenin+, CD117 variable, CD34-
- Inflammatory fibroid polyp:
- Inflammatory myofibroblastic tumor:
- ALK rearrangements
- CD117-
- Prominent inflammatory infiltrates
- Melanoma:
- MelanA+, tyrosinase+, MITF+, SOX10+
- Malignant peripheral nerve sheath tumor:
- Carcinoma:
- Solitary fibrous tumor:
Additional references
Board review style question #1
Board review style answer #1
A. 3 cm mass and mitosis < 3/5 mm². Size more than 5 cm, mitosis greater than 5 per 5 mm² and site are associated with disease progression in esophageal GIST. Tumor size > 5 cm and mitotic rate > 5/5 mm² are independent risk factors for progression. Esophageal and colonic GIST are more aggressive than gastric GIST. Of the choices above, A is low risk, D is high risk and others have moderate risk of progression (Mod Pathol 2022;35:554).
Comment Here
Reference: Gastrointestinal stromal tumor
Comment Here
Reference: Gastrointestinal stromal tumor
Board review style question #2
Succinate dehydrogenase deficient GIST is seen exclusively in which part of the gastrointestinal system?
- Colon
- Esophagus
- Ileum
- Stomach
Board review style answer #2
D. Stomach. Succinate dehydrogenase deficient GIST has a female preponderance and seen almost exclusively in stomach (predilection for distal stomach and antrum) (Am J Surg Pathol 2010;34:636).
Comment Here
Reference: Gastrointestinal stromal tumor
Comment Here
Reference: Gastrointestinal stromal tumor
Giant fibrovascular polyp / well differentiated liposarcoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Giant fibrovascular polyp used to be considered a nonneoplastic polypoid lesion of the esophagus
- The majority of giant fibrovascular polyps of the esophagus now appear to represent well differentiated and dedifferentiated liposarcoma, based on the result of MDM2 FISH (fluorescence in situ hybridization) testing (Mod Pathol 2018;31:337)
Essential features
- Pedunculated polypoid (sausage shaped) mass, up to 23 cm in length (average 13 cm) (World J Gastrointest Oncol 2016;8:835)
- Consists of fibroadipose tissue with slightly enlarged, hyperchromatic stromal cells (Mod Pathol 2018;31:337)
- MDM2 and CDK4 overexpression by immunohistochemistry and amplification by FISH, indicating diagnosis of liposarcoma (Mod Pathol 2018;31:337)
Terminology
- Giant fibrovascular polyp, fibrovascular polyp, well differentiated liposarcoma, atypical lipomatous tumor
Epidemiology
- Approximately 0.5% of all esophageal neoplasms (World J Gastrointest Oncol 2016;8:835)
- Middle aged to elderly patients with male predominance
Sites
- Proximal / cervical esophagus (Int J Surg Case Rep 2019;64:113)
Clinical features
- Middle aged to elderly patients with male predominance (Int J Surg Case Rep 2019;64:113)
- Slowly worsening dysphagia, weight loss, odynophagia and oral regurgitation of polyp
Diagnosis
- Characteristic radiological and endoscopic appearances, plus characteristic histologic findings with immunohistochemical / molecular confirmation
Prognostic factors
- Dedifferentiated type tends to recur and metastasize (Mod Pathol 2018;31:337)
Case reports
- 36 year old woman with an 11 cm giant fibrovascular polyp (Gastrointest Endosc 2020;91;442)
- 42 year old man with a 14 cm giant fibrovascular polyp (Acta Gastroenterol Belg 2019;82:437)
- 42 year old man with an 8 cm giant fibrovascular polyp (ACG Case Rep J 2019;6:e00126)
- 54 year old man with a 17.5 cm giant pedunculated liposarcoma (Int J Surg Case Rep 2019;64:113)
- 75 year old man with a 6.5 cm pedunculated, well differentiated liposarcoma (Ann R Coll Surg Engl 2017;99:e209)
Treatment
- Endoscopic or surgical resection
Gross description
- Pedunculated polypoid (sausage shaped) mass up to 23 cm in length (average 13 cm) (World J Gastrointest Oncol 2016;8:835)
Microscopic (histologic) description
- Fibroadipose tissue with slightly enlarged, hyperchromatic stromal cells, covered with intact squamous mucosa (Mod Pathol 2018;31:337)
- Mostly well differentiated type / atypical lipomatous tumor (Mod Pathol 2018;31:337)
- Occasionally dedifferentiated type (Mod Pathol 2018;31:337)
Microscopic (histologic) images
Molecular / cytogenetics description
- MDM2 and CDK4 overexpression by immunohistochemistry (World J Gastrointest Oncol 2016;8:835)
- MDM2 and CDK4 amplification by FISH (Mod Pathol 2018;31:337)
- Ring or marker chromosomes with regional amplification of chromosome 12q13-21 (Hum Pathol 2012;43:293)
Sample pathology report
- Esophagus, mass, endoscopic resection:
- Well differentiated liposarcoma / giant fibrovascular polyp (see comment)
- Resection margin negative for liposarcoma
- Comment: The slightly atypical stromal cells are positive for MDM2 and CDK4 by immunohistochemistry with appropriate controls. The results support the above diagnosis.
Differential diagnosis
- Lipoma:
- Carcinosarcoma:
- Pedunculated tumor
- Biphasic tumor with carcinomatous and sarcomatous components
Board review style question #1
Which of the following is true about this mass shown in the picture?
- Despite large size of the tumor, patients never present with dysphagia
- Gross features suggest an aggressive tumor
- Patients need an aggressive treatment, including chemotherapy and radiation
- This polypoid tumor is usually seen in the proximal / cervical esophagus
Board review style answer #1
D. Giant fibrovascular polyp / well differentiated liposarcoma is usually seen in the proximal / cervical esophagus
Comment Here
Reference: Giant fibrovascular polyp / well differentiated liposarcoma
Comment Here
Reference: Giant fibrovascular polyp / well differentiated liposarcoma
Board review style question #2
Which of the following is true about giant fibrovascular polyp of the esophagus?
- Most show MDM2 amplification
- They represent a reactive / nonneoplastic mass of the esophagus
- They usually are composed of markedly pleomorphic, hyperchromatic stromal cells
- They usually are seen in young female patients
Board review style answer #2
A. Most giant fibrovascular polyps show MDM2 amplification and CDK4 amplification supporting the diagnosis of well differentiated liposarcoma
Comment Here
Reference: Giant fibrovascular polyp / well differentiated liposarcoma
Comment Here
Reference: Giant fibrovascular polyp / well differentiated liposarcoma
Glycogenic acanthosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Glycogenic acanthosis (GA) is a benign hyperplasia of the esophageal squamous epithelium due to accumulation of intracytoplasmic glycogen of unknown etiology
- Endoscopically, affected areas display white plaques or nodular areas (usually 2 - 10 mm in diameter), which may be solitary, multiple or coalesced
- Diffuse glycogenic acanthosis may be a manifestation of PTEN hamartoma tumor syndrome (Cowden syndrome) in 20 - 80% of affected adults (Best Pract Res Clin Gastroenterol 2022;58:101792)
Essential features
- First described by Rywlin and Ortega in 1970, glycogenic acanthosis of the esophagus is a common benign lesion seen during upper gastrointestinal endoscopy
- Characterized histologically by squamous hyperplasia and intracellular glycogen accumulation
- Manifestation of PTEN hamartoma tumor syndrome (Cowden syndrome)
- References: Am J Med Genet A 2003;122A:315, Am J Gastroenterol 2003;98:1429, Am J Gastroenterol 1997;92:1038, Arch Pathol 1970;90:439, Gastrointest Radiol 1984;9:93
Terminology
- Pachyderma nodosa, esophageal leukoplakia
ICD coding
- ICD-10: K22.9 - disease of esophagus, unspecified
Epidemiology
- Most common in adults with a mean age of 52 years (range: 45 - 79 years)
- Common incidental endoscopic finding seen in up to 3.5% of upper endoscopies
- Diffuse involvement of the esophagus is associated with PTEN hamartoma tumor syndrome (Cowden syndrome) and highly predictive of PTEN mutation (80 - 90% of patients)
- PTEN hamartoma tumor syndrome (Cowden syndrome) is an autosomal dominant multiple hamartoma syndrome with increased risk of malignant tumors (breast, thyroid and endometrial) due to germline mutations in the tumor suppressor gene PTEN on 10q23
- There are rare reported cases in patients with tuberous sclerosis complex (J Clin Gastroenterol 1994;19:46)
- Reported associations with reflux esophagitis (GERD), increasing age, eosinophilic esophagitis and celiac disease
- References: World J Gastroenterol 2015;21:1091, Prz Gastroenterol 2020;15:39, Acta Paediatr 2004;93:568, J Pediatr Gastroenterol Nutr 2021;72:e161
Sites
- Esophagus, primarily in the mid esophagus (AJR Am J Roentgenol 1995;164:96)
- Rare reports of laryngeal involvement (Laryngoscope 2022;132:1641, Arch Otolaryngol Head Neck Surg 1998;124:1029, Otolaryngol Head Neck Surg 2021;164:1153)
Pathophysiology
- Unknown (see Etiology)
Etiology
- Although the etiology of glycogenic acanthosis is unknown, it is strongly associated with PTEN hamartoma tumor syndrome (Cowden) syndrome
- Glycogenic acanthosis has also been associated with tuberous sclerosis, hiatal hernia, reflux esophagitis (GERD) and increased age
- Rare reports have noted a possible association with eosinophilic esophagitis or celiac disease
- Glycogenic acanthosis has not been found to be associated with disorders of glucose metabolism (diabetes) or skin disorders (psoriasis or acanthosis nigricans)
- Given an association with GERD, a postinflammatory hypothesis was raised but has not been proven
- References: Prz Gastroenterol 2020;15:39, Am J Med Genet A 2003;122A:315, Acta Paediatr 2004;93:568, J Pediatr Gastroenterol Nutr 2021;72:e161, AJR Am J Roentgenol 1995;164:96
Clinical features
- See Etiology and Epidemiology
Diagnosis
- Ultimately, the diagnosis is based on the characteristic histologic findings in endoscopic biopsies (see Microscopic (histologic) description)
Radiology description
- May be seen in ~30% of patients undergoing double contrast radiography
- Mucosal nodules or plaques ranging from 2 to 15 mm in diameter
- Some have sharp edges that are demarcated by the barium, while others have ill defined borders that blend into the surrounding mucosa
- References: AJR Am J Roentgenol 1982;139:683, AJR Am J Roentgenol 1995;164:96
Prognostic factors
- Nonneoplastic and benign
- Solitary lesions have no clinical significance
- Prognosis of glycogenic acanthosis with PTEN hamartoma tumor syndrome (Cowden syndrome) is determined by the risk of various malignant tumors (breast, thyroid, kidney, endometrium or colorectal)
- Reference: Yantiss: Diagnostic Pathology - GI Endoscopic Correlation, 1st Edition, 2014
Case reports
- 6 year old boy and 8 year old girl with anemia and failure to thrive (Acta Paediatr 2004;93:568)
- 14 year old boy with eosinophilic esophagitis diagnosed with glycogenic acanthosis (J Pediatr Gastroenterol Nutr 2021;72:e161)
- 36 year old man with a history of gastrointestinal polyps (Eur J Gastroenterol Hepatol 2007;19:513)
- 45 year old man with a history of Cowden syndrome (Clin Gastroenterol Hepatol 2017;15:e131)
- 62 year old man with gastrointestinal polyposis (Am J Gastroenterol 2003;98:1429)
Treatment
- No treatment is required as it is a benign condition
Gross description
- Endoscopically and grossly, glycogenic acanthosis is described as raised white plaques or nodular areas (typically 2 - 10 mm in diameter), which may be single, multiple or coalesced (Gastrointest Radiol 1984;9:93)
Microscopic (histologic) description
- Squamous hyperplasia of the esophageal epithelium with intracellular glycogen accumulation
- Glycogen is positive for PAS and sensitive to diastase; a combination of PAS and PASD stains may be helpful to confirm the diagnosis (PAS positive intracellular glycogen is removed with PASD stain, which will be negative)
- References: World J Gastroenterol 2015;21:1091, Prz Gastroenterol 2020;15:39
Microscopic (histologic) images
Positive stains
Negative stains
- See Positive stains
Sample pathology report
- Esophagus, nodule, biopsy:
- Glycogenic acanthosis (see comment)
- Comment: Glycogenic acanthosis is a benign esophageal lesion that may be associated with PTEN hamartoma tumor (Cowden) syndrome but is also a common finding in upper endoscopies in nonsyndromic patients. Correlation with clinical and endoscopic findings is recommended.
Differential diagnosis
- Normal esophageal mucosa:
- Probably the most challenging histologic differential diagnosis, easily missed if lesional and nonlesional epithelium are not present
- Look for glycogenated squamous epithelial cells comprising over 50% of the mucosal thickness
- Candidal esophagitis:
Additional references
Board review style question #1
A 56 year old man underwent an esophagogastroduodenoscopy for reflux symptoms. A biopsy was taken of a small, raised white nodule in the esophagus. The histology is depicted in the H&E image shown above. What is the best diagnosis?
- Candidal esophagitis
- Epidermoid metaplasia
- Glycogenic acanthosis
- Mucosal calcinosis
- Normal esophageal squamous mucosa
Board review style answer #1
C. Glycogenic acanthosis. The H&E image demonstrates accumulation of intracytoplasmic glycogen in the squamous epithelium and the best diagnosis is glycogenic acanthosis (GA). PAS and PASD stains would be helpful to confirm the diagnosis since glycogen is PAS positive and diastase sensitive. Glycogenic acanthosis can be difficult to distinguish from normal esophageal mucosa, particularly if nonlesional tissue is not present. The endoscopic impression of a nodule is helpful and the diagnosis of glycogenic acanthosis would explain the endoscopic findings. Mucosal calcinosis is rare in the esophagus and would have calcium deposition within the epithelium or mucosa. Epidermoid metaplasia can also appear white on endoscopy but is characterized by a granular cell layer within the squamous epithelium resembling skin, hence the term epidermoid.
Comment Here
Reference: Glycogenic acanthosis
Comment Here
Reference: Glycogenic acanthosis
Board review style question #2
A 45 year old woman with a history of hamartomatous gastrointestinal polyps undergoes an esophagogastroduodenoscopy. There are diffuse white plaques and nodules in the esophagus. Biopsies are taken of some of the esophageal lesions and demonstrate hyperplastic squamous epithelium with intracytoplasmic accumulation of glycogen consistent with glycogenic acanthosis. What syndrome does this patient likely have?
- Lynch syndrome
- Maffucci syndrome
- Peutz-Jeghers syndrome
- PTEN hamartoma tumor syndrome (Cowden syndrome)
Board review style answer #2
D. PTEN hamartoma tumor syndrome (Cowden syndrome). PTEN hamartoma tumor syndrome (PTHS) is an all encompassing term for Cowden, Bannayan-Riley-Ruvalcaba, PTEN related Proteus and PTEN related Proteus-like syndromes. Cowden syndrome (CS) is an autosomal dominant syndrome caused by a germline mutation in the PTEN tumor suppressor gene on chromosome 10. Esophageal glycogenic acanthosis, particularly if diffuse, is a manifestation of Cowden syndrome and is seen in around 80 - 90% of patients. Maffucci syndrome is characterized by multiple enchondromas and hemangiomas. Peutz-Jeghers syndrome (PJS) is a hamartomatous polyposis syndrome of the gastrointestinal tract with increased risk of certain malignancies. PJS is autosomal dominantly inherited and caused by mutations in the STK11 gene. Lynch syndrome is the most common cause of hereditary colorectal cancer and is due to mutations in mismatch repair genes. Patients with Lynch syndrome are also at risk of other types of cancers. None of these, except Cowden syndrome, have glycogenic acanthosis.
Comment Here
Reference: Glycogenic acanthosis
Comment Here
Reference: Glycogenic acanthosis
Graft versus host disease
Table of Contents
Definition / general | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Common after allogeneic stem cell transplantation (Ann Hematol 2004;83:101)
Case reports
- Causing bullous esophagitis (Am J Gastroenterol 1997;92:529)
- Acute esophageal stricture after bone marrow transplantation (Bone Marrow Transplant 1988;3:513)
Gross description
- Desquamative esophagitis with web formation
Microscopic (histologic) description
- Increased intraepithelial lymphocytes, dyskeratotic squamous cells and apoptosis of individual squamous cells in noninflamed background is diagnostic
- Also have increased submucosal fibrosis associated with mucosal esophagitis and ulceration (Gastroenterology 1981;80:914)
- May be disparity between severity of clinical disease and biopsy findings
- Findings may be focal, may need serial sections
Differential diagnosis
- Scleroderma: fibrosis but no apoptotic squamous cells, different clinical history
Granular cell tumor
Table of Contents
Definition / general | Clinical features | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy descriptionDefinition / general
- Also called Abrikosoff tumor, myoblastoma
- Most common site in GI tract for these tumors
- #2 most common stromal tumor of esophagus after leiomyoma
Clinical features
- Usually incidental, in lower esophagus, 90% solitary (Ann Thorac Surg 1996;62:860)
- May cause obstruction if large
- Almost all cases of Schwannian origin
- Relatively more common in women in 40s, African Americans
- May be underdiagnosed on superficial biopsies that lack lamina propria
- Endoscopy: sessile, yellowish white, firm, intact epithelium
- 1 - 3% are malignant (locally recur)
- Associated with rapid growth, > 4 cm, tumor necrosis, increased cellularity, atypia, > 2 mitotic figures/HPF
Case reports
- 39 year old man with coexisting esophageal leiomyomas (Dig Liver Dis 2004;36:292)
- 40 year old woman with coexisting squamous cell carcinoma (Dis Esophagus 2002;15:88)
- 53 year old man with multiple tumors (J Gastroenterol 2003;38:776)
- 59 year old Japanese man with esophageal tumor 7 years after bronchial tumor (APMIS 2006;114:659)
Treatment
- Local excision
Gross description
- Intramural nodule(s), poorly circumscribed, up to 2 cm
Microscopic (histologic) description
- Identical to granular cell tumors elsewhere; sheets or packets of uniform epithelioid cells with abundant eosinophilic granular cytoplasm and small nuclei that interdigitate with overlying epithelium
- Often pseudoepitheliomatous hyperplasia, which may mimic squamous cell carcinoma on small biopsies (J Surg Oncol 1980;13:301)
- Often involves superficial lamina propria
- May extend into muscularis propria
Microscopic (histologic) images
Contributed by Dr. Oleksandr Grygoruk, AFIP and @RaulSGonzalezMD on Twitter
shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)"> shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"
shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">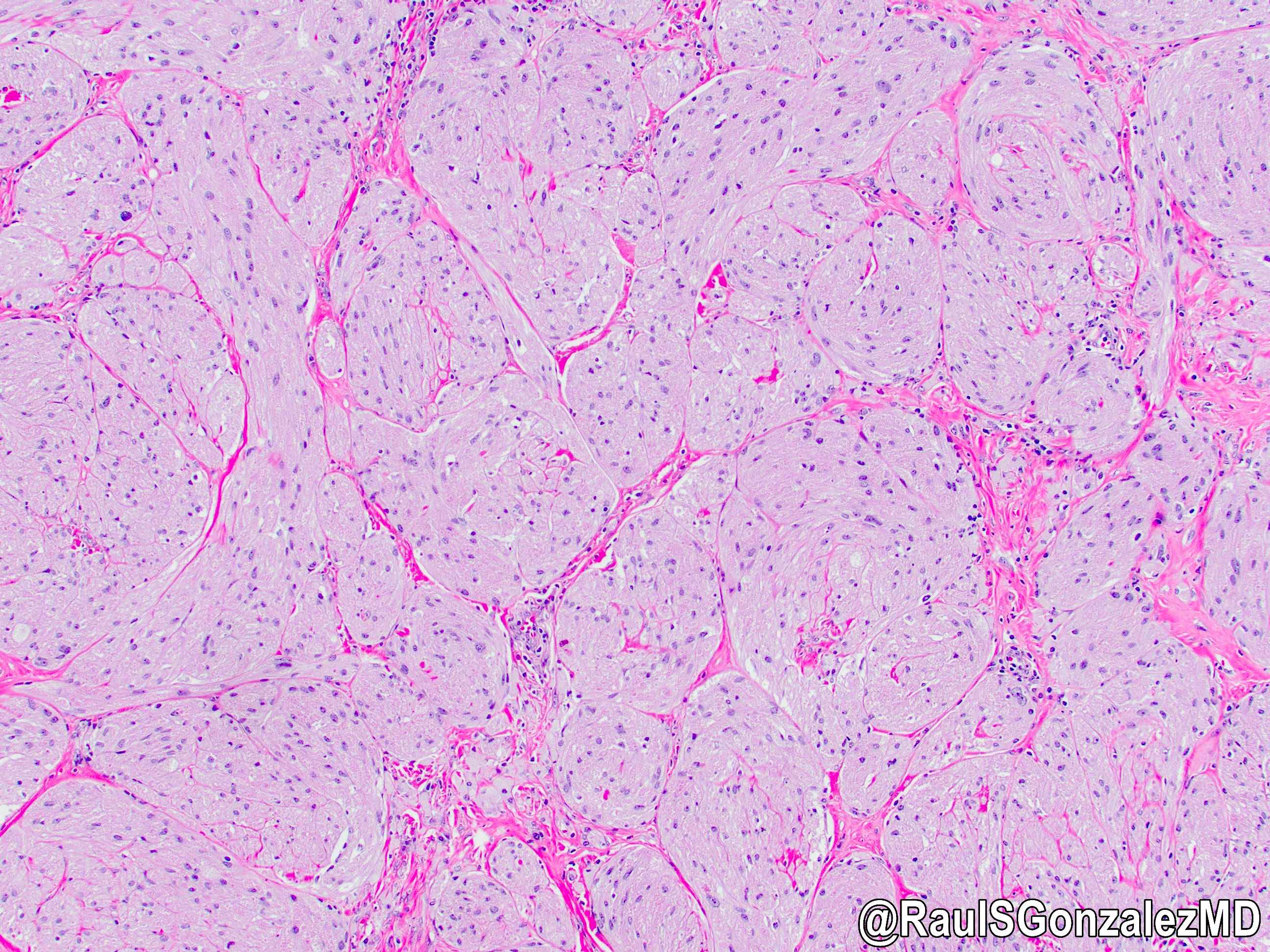 shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"
shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">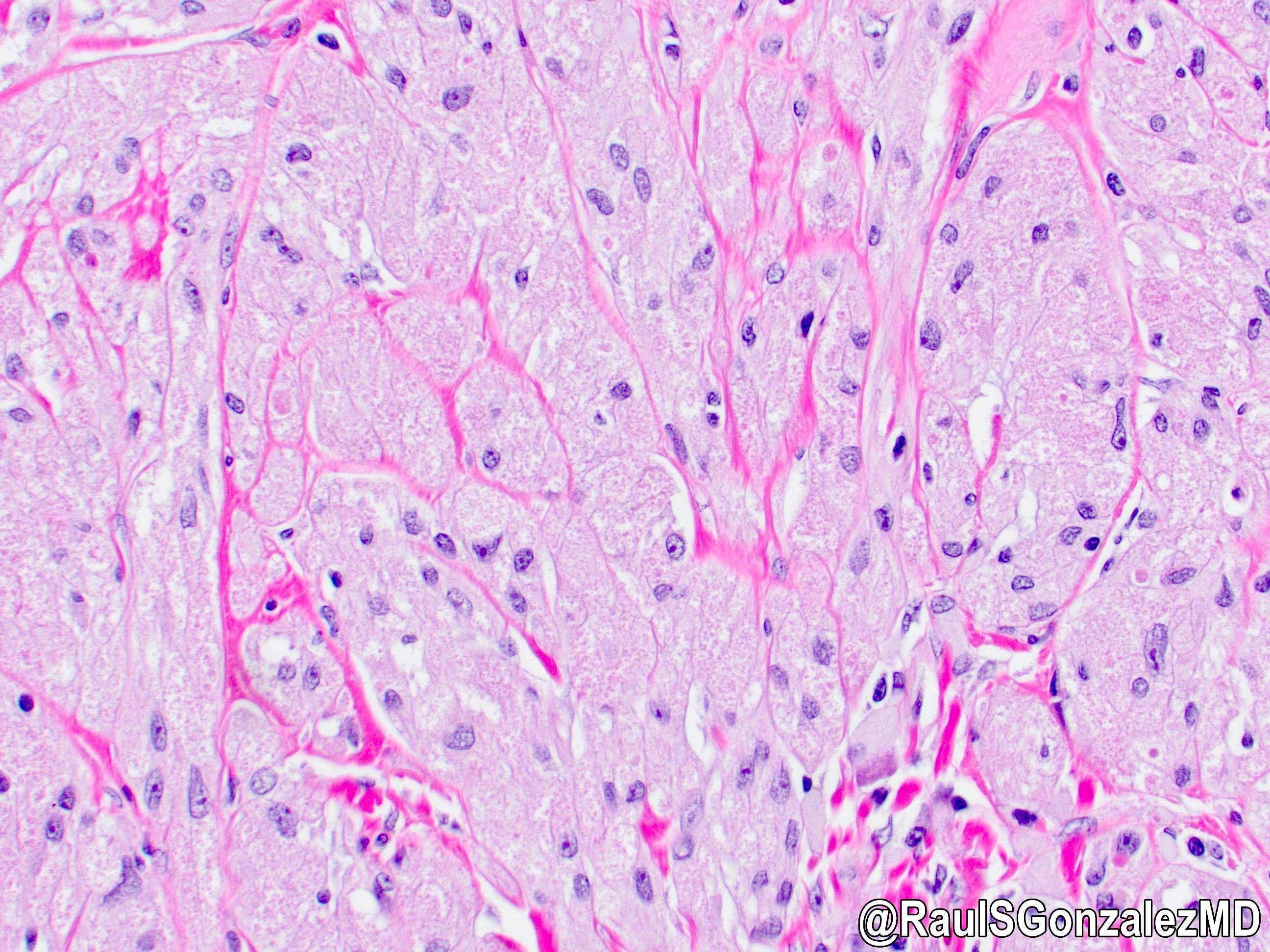
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
 shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"
shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
 shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"
shorturl.at/cdglN #pathology #gipath #PathTwitter #PathOutPic"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">

Granular cell tumor
Positive stains
Electron microscopy description
- Myelin-like tubules, cytoplasmic processes surrounded by basal lamina in layers reminiscent of Schwann cells
Grossing & features to report
Table of Contents
Definition / general | Relevant clinical history for esophageal specimens | Procedure - biopsies | Procedure - esophagectomies | Procedure - endoscopic mucosal resections | Sections to obtain | Tips | Diagrams / tables | Frozen section | Gross description | Gross images | Sample gross description report | Gross differential diagnosis | Features to report - general | Features to report - biopsies | Features to report - endoscopic resection, esophagectomy or esophagogastrectomy | Features to report - optional | Board review style question #1 | Board review style answer #1Definition / general
- This topic describes how to gross esophageal biopsies, esophagectomy specimens and endoscopic mucosal resection specimens
- Essential clinical history
- Gross differential diagnosis
- Features to report in biopsies, esophagectomies and mucosal resections (Arch Pathol Lab Med 2015;139:1446)
Relevant clinical history for esophageal specimens
- Organ / tissue resected or biopsied
- Indication for the procedure
- Endoscopic appearance of lesion / tissue sampled (e.g., mass, ulcer, stricture)
- Gross appearance of the organ / lesion / tissue sampled
- Location of biopsies:
- Upper (cervical), middle (midthoracic) or lower esophagus (lower thoracic)
- How many centimeters from the incisors on esophagogastroduodenoscopy (EGD)
- Relevant clinical and imaging findings (e.g., history of caustic ingestion)
- Prior surgery / biopsies and results (e.g., history of Barrett esophagus)
- Prior malignancy
- Prior treatment (e.g., neoadjuvant therapy, chemo or radiotherapy)
- Immune status (e.g., history of HIV, transplant)
- References: Pathology Resident Wiki: Gastrointestinal Grossing [Accessed 21 June 2022], Lester: Manual of Surgical Pathology, 3rd Edition, 2010, Gupta: Ace My Path - Gross Dictation Handbook, 1st Edition, 2022, Arch Pathol Lab Med 2015;139:1446
Procedure - biopsies
- Biopsies performed for evaluation of heartburn, dysphagia or surveillance of dysplasia in a patient with history of Barrett esophagus
- Patient identification: patient name, (last, first) label - designated specimen
- Received in formalin / unfixed
- Count the number of tissue fragments and describe their color
- Filter small fragments through tissue paper / biopsy bags
- Record the aggregate measurement or give the range, if multiple pieces
- Submit all tissue for processing in 1 cassette, if < 6 pieces
- Reference: Arch Pathol Lab Med 2015;139:1446
Procedure - esophagectomies
- Esophagectomies are performed with or without proximal stomach for severe dysplasia or for neoplasms; less frequently for strictures
- Patient identification: patient name, (last, first) label - designated specimen
- Received in formalin / unfixed / fresh for intraoperative consultation
- Confirm procedure as esophagectomy or other
- Orientation: identify anatomic structures, only esophagus or esophagus and portion of stomach or other; look for any sutures to identify the margins
- Remove the staple line with scissors, as close to the staples as possible
- Locate the lesion by gently palpating the luminal surface
- Open the specimen longitudinally
- Avoid cutting across any palpable lesions
- Measure the length and diameter or circumference and wall thickness of the esophagus and attached proximal stomach, if present
- Describe the mucosa, look for any areas of ulceration, glandular mucosa (pink or tan, appears different from pale squamous mucosa) strictures and tumors and the narrowing of the lumen caused by these lesions
- Note the dimensions of any lesions and their location, with reference to the proximal / distal / deep margins, the squamocolumnar junction (Z line) and the gastroesophageal (GE) junction
- This should be measured on the fresh specimen due to retraction following fixation
- Z line is the intersection of squamous and glandular mucosa
- GE junction is the junction of the tubular esophagus and saccular stomach, irrespective of the types of mucosa present
- Proximal displacement of the Z line above the GE junction suggests Barrett esophagus
- Describe the external surface and note areas of retraction, induration, perforation by the tumor or any lymph nodes
- Photograph lesions (as appropriate)
- Ink the proximal, distal and deep (adventitial) margins underneath the tumor, pin the specimen on a board and fix in 10% formalin; if the tumor is large, make longitudinal cuts to aid in fixation
- Fix the specimen in formalin overnight
- Describe any lesions including:
- Size
- Color
- Configuration (e.g., exophytic, ulcerated, infiltrative)
- Depth of invasion: after fixation cut through the tumor or ulcer to assess the depth of invasion into the esophageal wall
- Location (including relationship to squamocolumnar junction) (see note)
- Note: tumors that have their epicenter at the gastroesophageal junction and extend no more than 2 cm into the proximal stomach are staged as esophageal, while tumors with an epicenter more than 2 cm into the proximal stomach are staged as primary gastric carcinoma even if the tumor involves the gastroesophageal junction
- Percent of circumference involved
- Luminal diameter at the lesional site
- Degree of proximal dilatation
- Describe uninvolved mucosa:
- Normal squamous esophagus: glistening smooth white mucosa
- Normal glandular stomach: velvety pink / red mucosa with rugal folds
- Barrett (glandular) mucosa: pale pink / salmon, finely granular, may be discontinuous
- Adventitial soft tissue / fat should be thoroughly searched and sectioned for lymph nodes
- Nodes may be very small and close to the esophageal or gastric outer surfaces
- Reference: Arch Pathol Lab Med 2015;139:1446
Procedure - endoscopic mucosal resections
- Indications:
- Barrett esophagus, with or without dysplasia or intramucosal carcinoma
- Lesions that are ≤ 2 cm, well to moderately differentiated and limited to the mucosa or superficial submucosa (Middle East J Dig Dis 2017;9:5, Gastroenterology 2015;149:1599, Endoscopy 2007;39:24)
- Procedure:
- Should arrive in the laboratory in a fresh state, with margins oriented by the endoscopist
- Specimen is usually round or oval
- Ink base and lateral mucosal margins with different colors
- Stretch the specimen gently and fix overnight in 10% formalin on a rigid board, with mucosal surface up
- After fixation the specimens should be serially sectioned at 2 - 3 mm intervals parallel to the long axis (not < 2 mm)
Sections to obtain
- Submit tumor: 1 section per centimeter, including maximal depth of invasion and relation to proximal / distal margins
- Longitudinal sections preferred
- If no gross tumor present (which is often the case after neoadjuvant therapy of the GE junction tumors), then the entire ulcerated / fibrotic area should be blocked off and submitted
- Margins: en face sections of proximal / distal margins unless tumor is < 2 cm from the margin then margin can be best demonstrated in perpendicular sections
- Esophagus and stomach: representative sections of uninvolved areas
- Other gross lesions (including Barrett esophagus): representative sections
- Lymph nodes: submit entirely
- For endoscopic mucosal resection (EMR): entire specimen should be submitted in a sequential manner (World J Gastrointest Endosc 2012;4:489)
Tips
- If no gross tumor present (which is often the case after neoadjuvant therapy of the GE junction tumors), then the entire ulcerated / fibrotic area should be blocked off and submitted
- Examine the specimen for Barrett mucosa, presenting as salmon / pink, granular mucosa (similar to gastric mucosa) above the GE junction, replacing the glistening white squamous mucosa
- Tumors that have their epicenter at the gastroesophageal junction and extend no more than 2 cm into the proximal stomach are staged as esophageal, while tumors with an epicenter more than 2 cm into the proximal stomach are staged as primary gastric carcinoma even if the tumor involves the gastroesophageal junction (Amin: AJCC Cancer Staging Manual, 8th Edition, 2017)
- 15 lymph nodes are recommended for esophageal or GE junction cancer; however, if not found submit entire adventitial tissue for lymph nodes (Ann Surg Oncol 2020;27:1227)
- For EMR, immediate pinning and fixing of the specimen helps preserve the size, shape and orientation of the lesion
Diagrams / tables
Frozen section
- Assessment of margins
- Assessment of depth of invasion and margin in an EMR (Clin Gastroenterol Hepatol 2006;4:173)
- Confirmation of pathology
- Volume of cancer cells for tumor banking (Methods Mol Biol 2020;2129:83)
Gross description
- Portions of esophagus are usually resected to remove neoplasms and less frequently strictures
- After checking the history, indication for the procedure and most recent endoscopic findings, proceed as below
Gross images
Sample gross description report
- The specimen designated esophagus is received fresh in a container labeled with the patient's name and medical record number and consists of an esophagectomy and partial gastrectomy, including esophagus (20.0 cm length x 3.5 cm circumference) with attached proximal stomach (4.0 cm length x 10.0 cm circumference). There is a tan-pink, firm, fungating mass (2.5 x 2.0 cm), which invades through the muscularis propria into the adjacent soft tissue. The tumor is 15.0 cm from the proximal resection margin, 2.5 cm from the gastroesophageal junction, 6.5 cm from the distal resection margin and 0.2 cm from the deep resection margin (inked in black). The adjacent esophageal mucosa is tan-pink and finely granular from the gastroesophageal junction, up to within 10.0 cm of the proximal margin. The remainder of the mucosal surfaces are unremarkable. The attached soft tissue contains four palpable lymph nodes (range 0.3 - 0.5 cm). Gross photographs are taken. Section code:
- Cassette 1: proximal resection margin, en face
- Cassette 2: distal resection margin, en face
- Cassettes 3 - 4: tumor with deepest extent of invasion and deep margin, longitudinal
- Cassette 5: tumor with adjacent esophageal mucosa, longitudinal, representative
- Cassette 6: tumor with adjacent gastric mucosa, longitudinal
- Cassette 7: abnormal esophageal mucosa, representative
- Cassette 8: normal gastric mucosa, representative
- Cassettes 9 - 10: submit all lymph nodes, entirely
Gross differential diagnosis
- Normal esophageal mucosa white, smooth and glistening
- Adenocarcinoma:
- Close to the GE junction
- Grossly resemble colonic adenocarcinomas
- Tan / pink, polypoid, central ulceration
- May invade the submucosa and undermine proximal / distal normal appearing mucosa
- Barrett mucosa:
- Pale pink / salmon colored
- Finely granular
- Extends proximally from the GE junction
- May be discontinuous
- May be associated with adenocarcinoma
- Squamous cell carcinoma:
- Occurs in upper, middle and lower esophagus
- Exophytic (intraluminal), infiltrative / ulcerating or circumferential thickening
- Preoperative radiation therapy given in most cases
- Residual tumor difficult to detect and may be present as shallow ulceration, erosion, fibrosis or granular mucosa
- Leiomyoma:
- Typically arises from muscularis propria
- Well circumscribed tumor with white-pink, whorled cut surfaces
- Intramural or intraluminal / polypoid
Features to report - general
- Editorial note
- Lymphoma (consider the Hodgkin or non-Hodgkin protocol)
- Gastrointestinal stromal tumor (consider the GIST protocol)
- Non-GIST sarcomas (consider the soft tissue protocol)
- Others (report using separate TNM staging systems or CAP protocols)
Features to report - biopsies
- Specimen type (procedure)
- Site of biopsy (if known)
- Histologic type
- Histologic grade
- Microscopic tumor extension
- Additional findings
- Reference: Arch Pathol Lab Med 2015;139:1446
Features to report - endoscopic resection, esophagectomy or esophagogastrectomy
- Specimen:
- Esophagus
- Proximal stomach
- Other (specify)
- Not specified
- Procedure:
- Endoscopic resection
- Esophagectomy
- Esophagogastrectomy
- Other (specify)
- Not specified
- Tumor site:
- Cervical (proximal) esophagus
- Mid esophagus:
- Upper thoracic esophagus
- Mid thoracic esophagus
- Distal (lower thoracic) esophagus
- Esophagogastric junction (EGJ)
- Proximal stomach and EGJ
- Other (specify)
- Not specified
- Esophagus not otherwise specified
- Relationship of tumor to EGJ:
- Tumor is confined to the tubular esophagus and does not involve the EGJ
- Tumor midpoint is in the distal esophagus and tumor involves the EGJ
- Tumor midpoint is at the EGJ
- Tumor midpoint is in the proximal stomach or cardia and tumor involves the EGJ
- Not specified
- Cannot be assessed
- Distance of tumor center from EGJ (if applicable): __ cm
- Tumor size:
- Greatest dimension: __ cm
- Cannot be determined
- Histologic type:
- Squamous cell carcinoma
- Adenocarcinoma
- Adenosquamous carcinoma
- High grade neuroendocrine carcinoma:
- Large cell neuroendocrine carcinoma
- Small cell neuroendocrine carcinoma
- Adenoid cystic carcinoma
- Mucoepidermoid carcinoma
- Basaloid squamous cell carcinoma
- Spindle cell squamous cell carcinoma
- Verrucous squamous cell carcinoma
- Lymphoepithelioma-like carcinoma
- Mixed squamous cell carcinoma - neuroendocrine carcinoma
- Mixed adenocarcinoma - neuroendocrine carcinoma
- Mixed adenocarcinoma - neuroendocrine tumor
- Undifferentiated carcinoma
- Other (specify)
- Carcinoma, type cannot be determined
- Histologic grade:
- Not applicable
- GX: cannot be assessed
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
- G4: undifferentiated
- Microscopic tumor extension:
- Cannot be assessed
- No evidence of primary tumor
- High grade dysplasia (carcinoma in situ)
- Tumor invades lamina propria
- Tumor invades muscularis mucosae
- Tumor invades submucosal
- Tumor invades muscularis propria
- Tumor invades through muscularis propria into periesophageal soft tissue (adventitia)
- Tumor directly invades adjacent structures (specify)
- Margins (Middle East J Dig Dis 2017;9:5):
- If all margins uninvolved by invasive carcinoma, dysplasia and intestinal metaplasia
- Margins examined (proximal, distal, radial, mucosal, deep, others):
- Distance of invasive carcinoma from closest margin: __ cm
- Specify closest margin
- Proximal / distal margin involved by:
- Invasive carcinoma
- Dysplasia:
- Squamous dysplasia: low / high grade
- Intestinal metaplasia (Barrett esophagus) with dysplasia: low grade / high grade
- Intestinal metaplasia (Barrett esophagus) without dysplasia
- Circumferential (adventitial / deep) margin:
- Cannot be assessed
- Uninvolved by invasive carcinoma
- Involved by invasive carcinoma
- Other margins (specify):
- Cannot be assessed
- Uninvolved by invasive carcinoma
- Involved by invasive carcinoma
- For endoscopic resection specimens only:
- Mucosal margin:
- Cannot be assessed
- Involved by invasive carcinoma
- Uninvolved by invasive carcinoma
- Uninvolved by dysplasia
- Involved by:
- Squamous dysplasia: low / high grade
- Intestinal metaplasia (Barrett esophagus) with dysplasia: low grade / high grade
- Mucosal margin:
- Treatment effect (carcinomas treated with neoadjuvant therapy) (Dis Esophagus 2006;19:329):
- No prior treatment
- Present:
- No viable cancer cells (complete response, score 0)
- Single cells or rare small groups of cancer cells (near complete response, score 1)
- Residual cancer with evident tumor regression (partial response, score 2)
- No definite response / absent with extensive residual cancer with no evident tumor regression (poor / no response, score 3)
- Cannot be determined
- Treatment history not known
- Lymphovascular invasion:
- Not identified
- Present
- Indeterminate
- Perineural invasion:
- Not identified
- Present
- Indeterminant
- Regional lymph nodes:
- Lymph node examination required only if lymph nodes present in the specimen
- No nodes submitted or found
- Number of lymph nodes involved
- Number of lymph nodes examined
- Lymph node examination required only if lymph nodes present in the specimen
- Pathologic staging (pTNM):
- TNM descriptors:
- m (multiple primary tumors)
- r (recurrent)
- y (posttreatment)
- Primary tumor (pT)
- Regional lymph nodes (pN)
- Distant metastasis (pM)
- TNM descriptors:
- Additional pathologic findings:
- None
- Intestinal metaplasia (Barrett esophagus)
- Squamous or glandular dysplasia: low grade / high grade
- Esophagitis
- Gastritis
- Other (specify)
- Ancillary studies (specify):
- HER2 neu status: for HER2 reporting, CAP gastric HER2 template should be used
- Clinical history:
- Barrett esophagus
- Other (specify)
- Not known
- References: Edge: AJCC Cancer Staging Manual, 7th Edition, 2009, CAP: Cancer Protocol Templates [Accessed 21 June 2022], CAP: Protocol for the Examination of Specimens from Patients with Carcinoma of the Esophagus [Accessed 21 June 2022], Surg Oncol Clin N Am 2006;15:751
Features to report - optional
- Size of largest tumor involvement in metastatic lymph node (Ann Surg Oncol 2002;9:1010, Ann Thorac Surg 2007;83:1265)
- Extracapsular lymph node involvement (Am J Surg Pathol 2006;30:171)
- Other features (esophagitis, Barrett esophagus, dysplasia, specific types of infection)
Board review style question #1
For esophageal or gastroesophageal junction cancers, what is the number of lymph nodes recommended for pathological evaluation?
- At least 5
- At least 5 - 10
- Not less than 15
- No number is required but entire adventitia should be submitted
Board review style answer #1
C. The current evidence supports the goal of obtaining at least 15 lymph nodes for pathological evaluation in both primary esophagectomy and postinduction therapy cases. Earlier, no number was required for esophageal or gastroesophageal cancer. If no lymph node is found, the entire adventitia should submitted (Ann Surg Oncol 2020;27:1227).
Comment Here
Reference: Esophagus - Grossing & features to report
Comment Here
Reference: Esophagus - Grossing & features to report
Heterotopic / ectopic pancreatic tissue
Table of Contents
Definition / general | Microscopic (histologic) description | Cytology description | Positive stainsDefinition / general
- Also called pancreatic heterotopia
- At gastroesophageal junction in 16% of pediatric / young adult patients (Arch Pathol Lab Med 2000;124:1165)
- May be congenital and independent of Barrett esophagus (Am J Surg Pathol 1996;20:1507)
- Usually no clinical significance but theoretically may exacerbate reflux esophagitis; rarely exhibits pathologic changes of pancreatic tissue, including ductal carcinoma (Arch Pathol Lab Med 1994;118:568), other malignancy (Ann Thorac Surg 2000;69:259) or pancreatitis (Surg Laparosc Endosc Percutan Tech 2005;15:345, Dig Dis Sci 1995;40:2373)
Microscopic (histologic) description
- Benign appearing pancreatic ducts and acini
Cytology description
- Clusters of benign appearing ducts and small acini mixed with inflammatory cells (Diagn Cytopathol 2004;31:175)
Positive stains
- Trypsin, chymotrypsin, amylase and lipase (Am J Surg Pathol 1995;19:1172)
Heterotopic / ectopic sebaceous glands
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Heterotopic / ectopic sebaceous glands in the esophagus
Essential features
- Incidentally found, small (3 - 4 mm) yellowish plaques with granular or lobulated appearance; the number of lesions varies from single to more than 100
- Mature sebaceous gland with excretory duct within the lamina propria of the esophagus
- Asymptomatic and benign; no need for treatment
Terminology
- Ectopic sebaceous gland
- Heterotopic sebaceous gland
ICD coding
- ICD-10: K22.8 - other specified diseases of esophagus
Epidemiology
- Reported incidence is low:
- 0.005% (World J Clin Cases 2014;2:311)
- 0.05% (Dis Esophagus 2017;30:1)
- Reported age range:
- 45 - 79 years (World J Gastroenterol 2015;21:1091, Clin Endosc 2018;51:495, Case Rep Gastroenterol 2012;6:217, Pathol Int 1999;49:364)
- Has not been reported in pediatric patients
- Reported median age:
- 52 years (range 45 - 69 years) (World J Gastroenterol 2015;21:1091)
- 57.6 years (46 - 71 years) (World J Clin Cases 2014;2:311)
- Reported male to female ratios:
Sites
- Middle and lower esophagus (World J Clin Cases 2014;2:311, Dig Dis Sci 2019;64:2049, Case Rep Gastroenterol 2012;6:217, Clin Endosc 2018;51:495, Pathol Int 1999;49:364)
Etiology
- Whether heterotopic / ectopic sebaceous glands in the esophagus are the result of congenital anomaly or metaplastic change of squamous epithelium remains unknown (Pathol Int 1999;49:364)
- Since the esophagus is of endodermal origin, while sebaceous glands are of ectodermal origin, metaplastic change of squamous epithelium is suspected
Clinical features
- Incidentally found, small, yellowish plaques
- Usually asymptomatic
Diagnosis
- Confirmed via biopsy (Clin Endosc 2018;51:495, Case Rep Gastroenterol 2012;6:217)
Case reports
- 47 year old man with several yellow plaques and 63 year old man with multiple yellowish patches (Clin Endosc 2018;51:495)
- 56 year old man with more than 100 yellowish plaques measuring 1 - 20 mm in diameter (Case Rep Gastroenterol 2012;6:217)
- 78 year old woman with numerous 3 - 4 mm plaques in the middle and distal esophagus (Dig Dis Sci 2019;64:2049)
- 79 year old man with numerous esophageal heterotopic sebaceous glands accompanied by superficial esophageal cancer (Pathol Int 1999;49:364)
Treatment
- No treatment required
Gross description
- Randomly distributed, small (3 - 4 mm) yellowish plaques with granular or lobulated appearance
- Number of lesions varies from single to more than 100
- References: Pathol Int 1999;49:364, Clin Endosc 2018;51:495, Case Rep Gastroenterol 2012;6:217
Microscopic (histologic) description
- Mature sebaceous glands characterized by large polygonal cells with clear, vacuolated cytoplasm within the lamina propria
- A squamous lined excretory duct may also be present
- Associated hair follicles or other skin appendages are absent
Microscopic (histologic) images
Sample pathology report
- Esophagus, mid, biopsy:
- Heterotopic / ectopic sebaceous gland
- Negative for dysplasia or carcinoma
Differential diagnosis
- Xanthoma (Clin Endosc 2014;47:358):
- Grossly yellowish plaques, commonly solitary
- Aggregates of foamy macrophages beneath the squamous epithelium
- Glycogenic acanthosis:
- Acanthotic squamous epithelium with clear (glycogen containing) cytoplasm
- Squamous papilloma:
- Wart-like exophytic lesion
- Papillary proliferation of benign squamous epithelium
- Heterotopic / ectopic gastric mucosa:
- Salmon colored mucosa located just below the upper esophageal sphincter composed of heterotopic / ectopic gastric fundic glands
- Granular cell tumor:
- Small submucosal nodule composed of polygonal cells with small nuclei and abundant eosinophilic granular cytoplasm
- Positive for S100, calretinin and CD68
- Candidiasis:
- Whitish plaques with histologic evidence of pseudohyphae and yeast forms
- Well differentiated neuroendocrine tumor:
- Extremely rare in the esophagus
- Positive for neuroendocrine markers
Board review style question #1
Board review style answer #1
B. Excretory ducts can be seen in heterotopic / ectopic sebaceous glands of the esophagus.
Comment Here
Reference: Heterotopic / ectopic sebaceous glands
Comment Here
Reference: Heterotopic / ectopic sebaceous glands
Board review style question #2
Which of the following is true about heterotopic / ectopic sebaceous glands of the esophagus?
- Characterized by small (3 - 4 mm) yellowish plaques with granular or lobulated appearance
- Gastroesophageal reflux disease is one of the etiologic factors
- Most patients are elderly males with abnormal lipid metabolism disorders
- Often seen in pediatric patients
Board review style answer #2
A. Small (3 - 4 mm) yellowish plaques with granular or lobulated appearance are characteristic endoscopic features.
Comment Here
Reference: Heterotopic / ectopic sebaceous glands
Comment Here
Reference: Heterotopic / ectopic sebaceous glands
Heterotopic gastric mucosa
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Heterotopic gastric mucosa, most commonly seen in the postcricoid / cervical esophagus
- Usually composed of fundic / oxyntic type gastric mucosa admixed with mucous glands
Essential features
- Can be seen in both genders at all ages
- Usually asymptomatic
- Most common in the postcricoid region / cervical esophagus
- Single or multiple sharply demarcated circular pink to red area(s) (usually ~1 cm in size) in the cervical esophagus
- Usually composed of fundic / oxyntic type gastric mucosa admixed with mucous glands
- No treatment unless symptomatic
Terminology
- Inlet patch
- Cervical inlet patch
- Ectopic gastric mucosa
ICD coding
- ICD-10: K22 - other diseases of esophagus
Epidemiology
- Can be seen in all ages and both genders with an incidence of 1 - 15% (Adv Med Sci 2021;66:170, BMC Gastroenterol 2017;17:87, J Pediatr 2016;176:99)
Sites
- Most common in the postcricoid region / cervical esophagus (BMC Gastroenterol 2017;17:87, J Pediatr 2016;176:99)
Pathophysiology
- Congenital abnormality resulting from an incomplete embryonic esophageal epithelization process (the embryonic esophagus lined by columnar epithelium undergoes gradual replacement of columnar epithelium with squamous epithelium starting from the middle of the esophagus with vertical extension in both directions)
Etiology
- No known etiologic factors to cause above mentioned embryonic abnormality
Clinical features
- Can be seen in both genders at all ages (no significant difference)
- Usually asymptomatic but rare patients present with dysphasia, globus sensation, cough, sore throat, hoarseness, excessive throat clearing, among others (Adv Med Sci 2021;66:170, BMC Gastroenterol 2017;17:87, J Pediatr 2016;176:99)
Diagnosis
- Esophagogastroduodenoscopy with biopsy
- Narrow band imaging (NBI) increases the detection rate (Adv Med Sci 2021;66:170)
Case reports
- 44 year old woman with chronic dysphagia and globus sensation due to multiple inlet patches underwent esophageal dilation and proton pump inhibitor therapy, resulting in a resolution of her symptoms (Cureus 2023;15:e33459)
- 52 year old man with dysphagia caused by Helicobacter pylori associated inlet patch ulcer (ACG Case Rep J 2020;7:e00405)
- 59 year old man with esophageal adenocarcinoma arising from circumferential ectopic gastric mucosa (J Gastroenterol Hepatol 2022;37:47)
- 65 year old man with alpha fetoprotein (AFP) producing esophageal carcinoma arising from ectopic gastric mucosa in the cervical esophagus (J Gastrointestin Liver Dis 2017;26:193)
Treatment
- No treatment for asymptomatic patients
- Eradication of H. pylori if positive for H. pylori in heterotopic gastric mucosa
- Proton pump inhibitor (PPI) for symptomatic patient (Frontline Gastroenterol 2018;9:214)
- Argon plasma coagulation (APC) or radiofrequency ablation (RFA) for symptomatic patient (Gastrointest Endosc 2016;84:1022, Dig Endosc 2018;30:212, J Pediatr 2016;176:99)
- Endoscopic resection for dysplasia and adenocarcinoma arising from heterotopic gastric mucosa (Expert Rev Gastroenterol Hepatol 2016;10:405, Gastrointest Endosc 2015;81:1297)
Gross description
- Single or multiple sharply demarcated circular pink to red area(s) in the postcricoid region / cervical esophagus
Microscopic (histologic) description
- Heterotopic gastric mucosa with fundic / oxyntic type glands admixed with mucous glands surrounded by squamous mucosa
- Helicobacter pylori and intestinal metaplasia can be rarely seen
Microscopic (histologic) images
Sample pathology report
- Esophagus, proximal, biopsy:
- Heterotopic gastric mucosa
- Negative for dysplasia or malignancy
Differential diagnosis
- Barrett esophagus:
- Specialized columnar mucosa at the esophagogastric junction / lower esophagus
- Heterotopic pancreatic tissue:
- Presence of pancreatic acinar cells
- Heterotopic sebaceous glands:
- Presence of sebaceous glands
- Adenocarcinoma:
- Cytological and structural atypia with invasive growth
Board review style question #1
Which of the following statements regarding the histologic features of heterotopic gastric mucosa of the esophagus is true?
- Characterized by the presence of gastric type mucosa with intestinal metaplasia
- Double muscularis mucosae is one of the characteristic findings
- Most often contains oxyntic type gastric glands
- Usually associated with significant inflammation
Board review style answer #1
C. Most often contains oxyntic type gastric glands. Heterotopic gastric mucosa is most often composed of an admixture of oxyntic type gastric glands and mucous glands. Intestinal metaplasia, significant inflammation and double muscularis mucosae are not common findings of heterotopic gastric mucosa.
Comment Here
Reference: Heterotopic gastric mucosa
Comment Here
Reference: Heterotopic gastric mucosa
Board review style question #2
Which of the following statements about heterotopic gastric mucosa of the esophagus is true?
- Often associated with Barrett esophagus
- Patients often present with dysphagia
- Rare cases of adenocarcinoma arising from heterotopic gastric mucosa have been reported
- Surrounding background mucosa should be examined carefully due to possible association with squamous cell carcinoma or squamous dysplasia
Board review style answer #2
C. Rare cases of adenocarcinoma arising from heterotopic gastric mucosa have been reported. Adenocarcinoma arising from heterotopic gastric mucosa is extremely rare but rare cases of adenocarcinoma arising from heterotopic gastric mucosa have been reported. Association with Barrett esophagus is controversial. No association with squamous cell carcinoma or squamous dysplasia. Most patients are asymptomatic.
Comment Here
Reference: Heterotopic gastric mucosa
Comment Here
Reference: Heterotopic gastric mucosa
Histology
Table of Contents
Definition / general | Essential features | Physiology | Diagrams / tables | Clinical features | Laboratory | Radiology images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Videos | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Esophagus is a muscular tube that extends from the pharynx to the gastroesophageal junction; it has typical gastrointestinal (GI) tract layering (mucosa, submucosa, muscularis propria / externa, adventitia) around a central lumen as well as 2 muscular sphincters (upper and lower)
Essential features
- Esophageal tissue is arranged in 4 concentric layers following typical GI layering: mucosa, submucosa, muscularis propria / externa, adventitia
- Mucosa lines the lumen of the esophagus and is comprised of epithelium (nonkeratinized stratified squamous), lamina propria, muscularis mucosae
- Submucosa lies just deep to the mucosa and is comprised of connective tissue, nerves, Meissner plexus, lymph and blood vessels, and submucosal glands
- Muscularis propria / externa is composed 2 concentric layers of muscle cells; these muscle cells are a variable mixture of skeletal and smooth muscle with a greater proportion of skeletal muscle at the proximal end of the esophagus transitioning to solely smooth muscle at the distal end
- Esophagus is primarily retroperitoneal and is covered in a connective tissue adventitia and fascia
Physiology
- Esophagus is a muscular tube that extends from the oral cavity / pharynx to the stomach; in an adult it is ~25 cm in length (Richter: The Esophagus, 5th Edition, 2012)
- Esophagus has cervical, thoracic and abdominal portions; it transitions from the cervical to thoracic region at the suprasternal notch and then passes from the thorax to the abdomen through the diaphragmatic hiatus
- In axial cross section, esophagus is located posterior to the trachea and anterior to the vertebral column
- Embryologically, esophagus develops from foregut at ~10 weeks; upper esophagus is derived from branchial arches 4 - 6
- Arterial blood supply of the esophagus is generally arranged in segments with the inferior thyroid artery supplying the upper esophageal sphincter and cervical esophagus, the aortic esophageal arteries supplying the thoracic esophagus and the left gastric artery and a branch of the left phrenic artery supplying the distal esophagus and lower esophageal sphincter
- Esophageal infarction is rare due to the abundant blood supply
- Parasympathetic and sympathetic innervation of the esophagus is via the vagus and spinal nerves (sympathetic chain) (J Clin Gastroenterol 2008;42:610)
- Auerbach plexus (that lies between the longitudinal and circular layers of the muscularis propria) regulates contraction of the outer muscular layers of the esophagus
- Meissner plexus (submucosa) regulates contraction of the muscularis mucosae as well as glandular secretion
Diagrams / tables
Clinical features
- Esophageal tumors spread to involve local organs more easily than in other areas of the GI tract because the esophagus is not covered in a serosa; this also makes these tumors harder to treat surgically (Silverman: Oncologic Imaging - A Multidisciplinary Approach, 1st Edition, 2012)
- Gastroesophageal reflux disease (GERD) can cause chronic esophageal irritation that induces epithelial metaplasia (i.e., Barrett esophagus); this induces an epithelial replacement of cells from the normal stratified squamous to intestinal columnar epithelium with goblet cells (Gastrointest Endosc 2017;85:889)
Laboratory
- Biopsy is performed via endoscopy for evaluation of esophageal tissue; diagnostic accuracy is high for identification of malignant versus infectious diseases, as well as in evaluation for dysplasia (Gastrointest Endosc Clin N Am 2000;10:713)
Gross description
- Esophagus is a muscular tube that extends from the oral cavity / pharynx to the stomach; in an adult, it is ~25 cm in length (Richter: The Esophagus, 5th Edition, 2012)
- Esophagus has typical GI layering: mucosa, submucosa, muscularis propria / externa, adventitia
- 2 muscular sphincters are present: upper and lower esophageal sphincters
- Primarily retroperitoneal and is therefore covered in a connective tissue adventitia and fascia
- In axial cross section, esophagus is located posterior to trachea and anterior to the vertebral column
Microscopic (histologic) description
- Esophagus is arranged in 4 concentric layers following a typical GI layering scheme; listed from the lumen outward, these layers are mucosa, submucosa, muscularis propria / externa, adventitia
- Esophageal mucosa
- Epithelium: nonkeratinized stratified squamous epithelium
- Innermost layer of the esophageal mucosa, lining the lumen
- Other cells present: melanocytes, endocrine cells, Langerhans cells, Merkel cells, T cells
- Basement membrane separates the epithelium from the underlying lamina propria
- Basal layer: < 15% of the mucosal thickness, usually 2 - 3 cells thick, comprised of basophilic proliferative cells with minimal cytoplasm, mitotic figures are found only here (under normal conditions) (Richter: The Esophagus, 5th Edition, 2012)
- Intermediate layer: these glycogen rich squamous cells have more cytoplasm than those of the basal layer (i.e., decreased N:C ratio)
- Superficial layer: cells appear more flattened as they mature and approach the lumen, many cells are anucleate
- Lamina propria: composed of loose fibrovascular connective tissue
- Situated between the epithelium and muscularis mucosae
- Folds form papillae; these usually extend ~one - third to half of epithelial thickness (Richter: The Esophagus, 5th Edition, 2012)
- Scattered inflammatory cells may be present
- Muscularis mucosae: composed of smooth muscle
- Begins proximally at the cricoid cartilage and becomes thickened distally
- In the lower esophagus, it may play a protective role against stomach acid erosion (Ann N Y Acad Sci 2013;1300:261)
- May resemble muscularis propria / externa which is important for accurate American Joint Committee on Cancer (AJCC) staging of adenocarcinoma in this region (J Thorac Dis 2014;6:S289)
- Epithelium: nonkeratinized stratified squamous epithelium
- Submucosa: connective tissue
- Contains fibroblasts / collagen, nerves, sparse ganglia (Meissner plexus), lymphatic channels, blood vessels and submucosal (seromucous) glands
- Submucosal glands are comprised of mucinous cells surrounding a central lumen that produce acid mucin and empty into the esophagus via squamous lined ducts
- Submucosal glands are a significant identifying landmark of esophagus on biopsy or resection specimen (Richter: The Esophagus, 5th Edition, 2012)
- Muscularis propria (i.e., muscularis externa)
- Composed of striated skeletal muscle in the proximal third of the esophagus, smooth muscle in the distal third and a mixture of both skeletal and smooth muscle in the middle third
- Inner layer is oriented circumferentially while the outer layer is oriented longitudinally; this allows for both segmental contraction and peristalsis
- Myenteric plexuses (nerves and ganglia) are found between the inner and outer layer
- Adventitia: composed of loose irregular connective tissue
- Esophagus is primarily retroperitoneal and the majority is surrounded by fascia
Microscopic (histologic) images
Contributed by Nicole Stringham, Ph.D. (sources: University of Michigan virtual slide box and Duke University virtual slide collection) and AFIP
Virtual slides
Cytology description
- Esophageal brushing specimens are usually composed of mature superficial type and intermediate type squamous cells with abundant cytoplasm and small round, regular nuclei
- Superficial type nuclei are dense and pyknotic; intermediate type contain finely granular chromatin and inconspicuous nucleoli
- Cells present as loosely cohesive aggregates, individual cells, large sheets
- Brush cytology may be helpful in the diagnosis of Barrett esophagus (Diagn Cytopathol 2011;39:60)
Positive stains
- PASD and Alcian blue (at pH 2.5) are positive in submucosal glands
- CK7, CK8/18, CK14 and CK19 are positive in ducts
- CK10 and CK13 are positive in normal stratified squamous epithelium (Prz Gastroenterol 2015;10:61)
Negative stains
- Methylene blue is helpful for distinguishing areas of dysplastic columnar epithelium associated with Barrett esophagus (95% accuracy) (Gastrointest Endosc 1996;44:1)
- CDX2 negative in normal stratified epithelium (positive in 87.5% of Barrett esophagus) (Prz Gastroenterol 2015;10:61)
Electron microscopy description
- Cytoarchitecture of the muscularis mucosae and distribution of lymphatic vessels varies between regions of the esophagus (i.e., cervical, thoracic, abdominal); this may affect esophageal cancer infiltration and lymph node metastasis (Esophagus 2019;16:44)
Electron microscopy images
Videos
Normal esophagus histology
Additional references
Board review style question #1
Which of the following cell types comprise the innermost lumen lining layer of a normal esophagus?
- Columnar epithelium with goblet cells
- Keratinized stratified squamous epithelium
- Low columnar epithelium
- Nonkeratinized stratified squamous epithelium
- Simple columnar epithelium
Board review style answer #1
D. Nonkeratinized stratified squamous epithelium. The innermost layer of the esophageal mucosa, lining the lumen, is made up of nonkeratinized stratified squamous epithelium. Answer A is incorrect because columnar epithelium is normally found in the small intestine but can be found in the esophagus in cases of dysplasia (Barrett esophagus). Answer B is incorrect because keratinized epithelium is generally associated with skin (epithelium on outward facing surfaces of the body). Answer C is incorrect because low columnar epithelium is a type of simple columnar epithelium and is typical of the stomach and intestines, not the esophagus. Answer E is incorrect because simple columnar epithelium is usually found in other areas of the gastrointestinal (GI) tract.
Comment Here
Reference: Esophagus - Histology
Comment Here
Reference: Esophagus - Histology
Board review style question #2
The image shown above is in need of evaluation. Features that are present, both in the image and in subjacent areas include gastrointestinal (GI) layering, Auerbach plexus, submucosal glands and 2 distinct layers of muscularis propria made of smooth and skeletal muscle. What is the anatomical origin of this sample?
- Anus
- Duodenum
- Large intestine
- Pylorus
- Upper esophagus
Board review style answer #2
E. Upper esophagus. The upper esophagus is the only listed option that has nonkeratinized stratified squamous epithelium (shown in the image) in addition to all of the other listed features. Answer A is incorrect because the anus does not have submucosal glands. Answer B is incorrect because although the duodenum has many of these features, including submucosal glands, it does not have skeletal muscle and is lined by simple columnar (enterocytes) and goblet epithelial cells. Answer C is incorrect because the large intestine is lined by enterocytes with goblet cells and has mucosal rather than submucosal glands. Answer D is incorrect because the pylorus does not have 2 layered muscularized propria with skeletal muscle and the epithelium is simple columnar, not squamous.
Comment Here
Reference: Esophagus - Histology
Comment Here
Reference: Esophagus - Histology
HSV esophagitis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Infectious esophagitis is the second most common cause of esophagitis after gastroesophageal reflux disease (GERD) (Gastroenterol Hepatol (N Y) 2013;9:517)
- The most common causes of infectious esophagitis are Candida (88%), herpes simplex virus (10%) and cytomegalovirus (2%)
- Herpes simplex virus (HSV) is the most common cause of viral esophagitis (StatPearls: Esophagitis [Accessed 10 January 2023])
Essential features
- Majority of the cases are due to reactivation of HSV1 (Case Rep Gastrointest Med 2016;2016:7603484)
- Biopsy should be performed from the margin / edge of the ulcer rather than from the base (Clin Microbiol Infect 1997;3:397)
- Confirmation of the diagnosis is obtained by histology or viral culture of esophageal specimens (Clin Microbiol Infect 1997;3:397)
- IHC stain is only required when histologic features are equivocal
- PAS or GMS stains can be performed to rule out concomitant fungal infection
Epidemiology
- HSV esophagitis occurs most frequently in immunocompromised hosts, such as:
- Solid organ and bone marrow transplant recipients
- After the prolonged use of corticosteroid or immunosuppressive drugs
- HIV / AIDS
- Self limited in immunocompetent patients
- More frequent in men than women (World J Gastroenterol 2017;23:3011)
- Incidence of 1.8% in an autopsy series (Medicine (Baltimore) 2010;89:204)
Sites
- Herpes simplex virus infection in the gastrointestinal tract primarily affects the esophagus (Medicine (Baltimore) 2016;95:e3187)
- Lower third of the esophagus is more commonly involved (68.3%); diffuse esophagitis can occur (Case Rep Gastrointest Med 2016;2016:7603484)
Pathophysiology
- More commonly occurs after reactivation of latent HSV with spread of virus to the esophageal mucosa by way of the vagus nerve or by direct extension of oral - pharyngeal infection into the esophagus (Prz Gastroenterol 2013;8:333)
- Primary HSV infection is less common
Etiology
- Most infections are related to HSV1, although HSV2 has occasionally been reported (Case Rep Gastrointest Med 2016;2016:7603484)
Clinical features
- Most common presentations are:
- Odynophagia
- Dysphagia
- Esophageal pain
- Can have coexistent herpes labialis, glossitis or oropharyngeal ulcers
- May be associated with various opportunistic diseases, such as candidiasis, CMV and Kaposi sarcoma
- Endoscopic findings include multiple discrete or coalescent small ulcers, which may be superficial, punched out or volcano-like in appearance (Case Rep Gastrointest Med 2016;2016:7603484)
Diagnosis
- Histopathological examination from the margins of the ulcers (Clin Microbiol Infect 1997;3:397)
- Endoscopic examination
- Tissue culture
- Polymerase chain reaction (PCR) to detect HSV DNA
- In situ hybridization (ISH) (Hum Pathol 1990;21:443)
- Immunohistochemistry (Appl Immunohistochem Mol Morphol 2021;29:713)
Prognostic factors
- Overall good
- Disseminated disease is rare but may be fatal
Case reports
- 17 year old immunocompetent boy with HSV esophagitis (Glob Pediatr Health 2021;8:2333794X211052914)
- 21 year old woman with HSV esophagitis presenting as dysphagia (World J Gastroenterol 2007;13:2756)
- 28 year old man with HSV2 esophagitis (Case Rep Gastrointest Med 2016;2016:7603484)
- 62 year old female ICU patient with HSV esophagitis presenting as hematochezia (ACG Case Rep J 2020;7:e00372)
Treatment
- Treatment is always indicated in immunosuppressed patients
- Acyclovir (Clin Infect Dis 1996;22:926)
- Foscarnet in cases with acyclovir resistance (Bone Marrow Transplant 1993;11:177)
Clinical images
Gross description
- According to macroscopic appearance, HSV esophagitis is divided into 3 types (Medicine (Baltimore) 2016;95:e3187):
- Type I: small, punched out lesions with raised margins usually coated with yellowish exudate
- Type II: small, punched out lesions but no raised margins or exudate
- Type III: multiple ulcers, with a map-like, confluent appearance over the entire esophagus
- Typical ulcers are superficial, with size varying from a few millimeters up to 2 cm (Clin Microbiol Infect 1997;3:397)
- Most lesions are located in the middle to distal esophagus (Medicine (Baltimore) 2016;95:e3187)
- Nonulcerated erythematous mucosa and pseudomembranes have been noted occasionally
- See Diagrams / tables for gross appearance versus endoscopic images
Microscopic (histologic) description
- Typical histologic findings are only present at the edge of the ulcer (Clin Microbiol Infect 1997;3:397)
- Early lesions show nuclear swelling of keratinocytes and individual cell necrosis
- Ulcer bed demonstrates prominent necrosis and acute inflammatory cell infiltrate
- Marked mononuclear cell infiltrate adjacent to the infected squamous epithelium
- Infected squamous epithelial cells show 3 Ms:
- Molding of nuclear contours
- Margination of chromatin to the periphery of nuclei
- Multinucleation
- Intranuclear inclusions:
- Cowdry type A: acidophilic inclusion with surrounding clear halo
- Equivocal cases require an HSV immunohistochemical (IHC) stain to confirm the diagnosis
Microscopic (histologic) images
Positive stains
- HSV complex (HSV1 and HSV2) IHC stain: stains infected squamous epithelial cells
Negative stains
- CMV IHC stain
- VZV IHC stain
- PAS special stain (Clin Microbiol Infect 1997;3:397)
- GMS special stain
Videos
Overview of HSV esophagitis
Gross and histopathologic findings of HSV esophagitis
Sample pathology report
- Esophagus, biopsy:
- HSV esophagitis
Differential diagnosis
- CMV esophagitis:
- More commonly presents as a single, isolated and deep, large ulcer rather than multiple small, shallow ulcerations as in HSV esophagitis
- Intranuclear and intracytoplasmic inclusions are more common in mesenchymal and endothelial cells than epithelial cells
- Positive CMV IHC stain
- Negative HSV IHC stain
- Herpes zoster / varicella:
- Candida esophagitis:
- Varicella zoster virus (VZV) esophagitis (Am J Surg Pathol 2021;45:209):
- Viral cytopathic changes are similar to HSV, but it is often pronounced in peripapillary epithelium as compared to superficial epithelium in HSV esophagitis
- Esophageal ulcers are more hemorrhagic and less inflammatory as compared to HSV
- Clinically, patients often have the cutaneous manifestations of chicken pox or shingles
- Positive VZV IHC stain
- Negative HSV IHC stain
Additional references
Board review style question #1
A patient who underwent liver transplantation had a recent episode of Candida esophagitis treated with fluconazole. Symptoms of pain and dysphagia persisted and repeat endoscopy showed multiple sharply demarcated and superficial ulcers ranging from 2 mm to 2 cm. What is the biopsy diagnosis?
- Cytomegalovirus (CMV) esophagitis
- Herpes simplex virus (HSV) esophagitis
- Nonspecific ulcer
- Recurrent or persistent Candida esophagitis
Board review style answer #1
B. HSV esophagitis. The latter image shows the classic histologic findings of herpes esophagitis, including molding of nuclei, margination of nuclear chromatin and multinucleation. CMV infected cells are large and show distinctive intranuclear inclusions in mesenchymal cells. Candida esophagitis shows pseudohyphae or budding spores.
Comment Here
Reference: Herpes simplex esophagitis
Comment Here
Reference: Herpes simplex esophagitis
Board review style question #2
A 63 year old woman presented with dysphagia and esophageal ulcers. The image above shows a section stained with a polyclonal antibody to herpes simplex virus type 1 (HSV1). Which of the following statements about HSV immunohistochemistry is true?
- Immunohistochemistry is more sensitive and specific than in situ hybridization for HSV1 and HSV2
- The section is likely to react with anti-HSV2 antibody because of cross reactivity
- The section is likely to stain positively with an anti-HSV2 antibody because a combined HSV1 and HSV2 infection is common
- The section is not likely to stain with an anti-HSV2 antibody because a combined infection with HSV1 and HSV2 is uncommon
Board review style answer #2
B. The section is likely to react with anti-HSV2 antibody because of cross reactivity. Immunohistochemistry of HSV is useful when histology is equivocal. Most antibodies stain both HSV1 and HSV2 precluding reliable identification of the specific HSV. In situ hybridization allows the distinction of HSV1 and HSV2 but is more labor intensive. HSV antibodies may weakly cross react with varicella zoster virus (VZV) but this is usually not a problem.
Comment Here
Reference: Herpes simplex esophagitis
Comment Here
Reference: Herpes simplex esophagitis
Leiomyoma
Table of Contents
Definition / general | Clinical features | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Most common benign tumor of esophagus (eMedicine: Esophageal Leiomyoma [Accessed 15 February 2019], Surg Clin North Am 1983;63:625), 8% incidence in autopsy studies (Hum Pathol 1981;12:1006)
Clinical features
- Median age 35 years, 2/3 men, usually single, 24% multiple (seedling tumors)
- Usually arises from inner circular muscle; most common in distal esophagus, rarely polypoid
- Benign behavior (Ann Thorac Surg 2005;79:1122)
- Minute (1 - 2 mm "seedling") tumors are often near the gastroesophageal junction and are asymptomatic
- Multiple tumors are associated with MEN1 syndrome (Am J Pathol 2001;159:1121)
- Large tumors may cause obstructive symptoms
Case reports
- 26 year old woman with multinodular growth pattern simulating carcinoma (Dis Esophagus 2007;20:187)
- 64 year old man with coexisting early squamous cell carcinoma (Jpn J Clin Oncol 2004;34:751)
- 75 year old man with coexisting leiomyosarcoma (J Exp Clin Cancer Res 2005;24:487)
Treatment
- Excise if significant symptoms (Ann Thorac Cardiovasc Surg 2007;13:78), endoscopic enucleation for small tumors (Singapore Med J 2006;47:901), esophagectomy for large tumors (World J Gastroenterol 2005;11:4258)
Gross description
- Circumscribed, mural, solitary mass, 2 - 5 cm (surgical specimens), bulges into lumen, may be polypoid
- Pinkish gray white with whorled cut surface; mucosal surface is only rarely ulcerated
Gross images
Microscopic (histologic) description
- Similar to classic endometrial leiomyoma; circumscribed lesion of circular muscularis propria or muscularis mucosae composed of intersecting fascicles of bland spindle cells with abundant cytoplasm
- Variable fibrosis in center of large leiomyomas
- Occasional calcification; no / rare mitotic figures; no atypia, no cellular foci
Microscopic (histologic) images
AFIP images
Images hosted on other servers:
Positive stains
Differential diagnosis
- GIST: very rare; solid, myxoid and perivascular patterns; more cellular by H&E and cytology, CD117+, CD34+, variable desmin and actin immunoreactivity (Am J Surg Pathol 2000;24:211)
Lichenoid esophagitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Immunofluorescence description | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Lichenoid esophagitis is an umbrella term to cover a histologic pattern of injury; it is not a specific diagnosis
- Lichen planus can cause this pattern of injury
- In cases without evidence of skin disease or positive immunofluorescence (IF), the etiology can be quite variable, including:
- Infections (especially HIV and viral hepatitis)
- Crohn's disease
- Medications (NSAIDs, antihypertensives, checkpoint inhibitors, antimalarial drugs)
- Pill esophagitis
- Rheumatologic disorders
Essential features
- Umbrella term with many different etiologies, describing a pattern of injury
- Proximal and mid esophagus of middle aged or elderly women
- Endoscopy often shows white plaque or streaks
- Histology shows Civatte bodies and characteristic distribution of lymphocytes
Terminology
- Findings can be described as “lichenoid esophagitis pattern” (LEP) in patients without lichen planus (who have “lichen planus esophagitis”)
ICD coding
- ICD-10: K20.9 - esophagitis, unspecified
Epidemiology
- More frequent in females (71%)
- Median age 58 years (Am J Surg Pathol 2013;37:1889)
Sites
- Mid esophagus more frequently involved in lichenoid esophagitis pattern
- Upper and lower esophagus more frequently involved in true lichen planus esophagitis
Pathophysiology
- Lichen planus appears to be mediated by cytotoxic CD8 T cells that attack an antigen in the basal epithelium in a manner resembling graft versus host disease (GVHD) (Dis Esophagus 2003;16:47)
- Predisposing factors for oral lichen planus include medications such as antimalarial drugs, NSAIDs, antihypertensive agents and dental materials such as amalgam
Etiology
- Lichen planus
- Infections (especially HIV and viral hepatitis)
- Upper tract Crohn's disease
- Medications: nivolumab and other checkpoint inhibitors, antimalarial drugs, gold, NSAIDs and thiazides (ACG Case Rep J 2017;4:e57)
- Mucosa physically damaged by nearby pill esophagitis
- Rheumatologic disorders
Clinical features
- Rheumatologic diseases more common in patients with lichen planus esophagitis (24%) versus lichenoid esophagitis pattern (11%) (Am J Surg Pathol 2013;37:1889)
- Viral hepatitides and human immunodeficiency virus (HIV) infections are associated with lichenoid esophagitis pattern (Am J Surg Pathol 2013;37:1889)
Diagnosis
- Histologic assessment of biopsy
Prognostic factors
- Squamous dysplasia and carcinoma have been described; periodic surveillance may be useful (Eur J Gastroenterol Hepatol 2006;18:1111, Am J Surg Pathol 2013;37:1889)
- Stricture: risk of stricture formation is higher in patients with lichen planus esophagitis (38%) versus lichenoid esophagitis pattern (9%) (Am J Surg Pathol 2013;37:1889)
Case reports
- 63 year old woman with dysphagia and areas of friable mucosa and stricture at the upper / mid esophagus (World J Gastroenterol 2013;19:1652)
- 68 year old patient with isolated esophageal involvement (Z Gastroenterol 2004;42:379)
- 69 year old woman with dysphagia and odynophagia; endoscopy revealed severe strictures and web-like areas in esophagus (World J Gastroenterol 2013;19:2278)
- Two siblings with lichen planus and squamous cell carcinoma of the esophagus (Eur J Gastroenterol Hepatol 2006;18:1111)
Treatment
- Corticosteroids for esophageal lichen planus
- Esophageal dilation for patients with stricture
Clinical images
Gross description
- At endoscopy (Am J Surg Pathol 2013;37:1889)
- White plaques or streaks are present, mimicking candidiasis or glycogenic acanthosis
- In addition, it may cause ulceration in mid or proximal esophagus
- Strictures can also be present
Microscopic (histologic) description
- Prominent band-like lymphocytic infiltrate involving the interface of basal epithelium and lamina propria (this feature cannot be evaluated in biopsies lacking lamina propria) (Am J Surg Pathol 2013;37:1889)
- Intraepithelial lymphocytosis
- Dyskeratotic keratinocytes (Civatte bodies)
- Acanthosis
- Junctional split seen at the junction of the epithelium and lamina propria (unlike sloughing esophagitis)
Microscopic (histologic) images
Immunofluorescence description
- Negative in lichenoid esophagitis pattern
- In lichen planus esophagitis, globular IgM deposits at the junction of the squamous epithelium and lamina propria as well as complement staining in apoptotic keratinocytes
Positive stains
Negative stains
- GMS (no fungal microorganisms)
Sample pathology report
- Proximal esophagus, biopsy:
- Esophageal squamous mucosa with lichenoid esophagitis pattern of injury (see comment)
- Comment: The biopsy demonstrates a prominent band-like lymphocytic infiltrate involving the interface of lamina propria and epithelium, intraepithelial lymphocytes and dyskeratotic keratinocytes. Unfortunately, this pattern of injury is not entirely specific and can be seen as an esophageal manifestation of lichen planus, viral infections (particularly HIV and viral hepatitis), Crohn's disease, medications (NSAIDs, antihypertensive agents, antimalarial drugs) or pill esophagitis. Correlation with the clinical findings (in particular with cutaneous, oral or genital lesions) is suggested. If lichen planus is a consideration, additional biopsies with fresh tissue for immunofluorescence studies are worth performing. No fungal organisms were identified on the GMS stain. There is no dysplasia.
Differential diagnosis
- Candida esophagitis:
- Endoscopic findings (white plaques) can be similar to lichenoid esophagitis pattern
- Microscopic similarities to lichenoid esophagitis pattern: numerous intraepithelial lymphocytes, superficial neutrophils, parakeratosis and Civatte bodies
- Desquamated and hyperpink parakeratosis
- Pseudohyphae and budding yeast: GMS positive (should always be performed when entertaining a diagnosis of lichenoid esophagitis pattern)
- Gastroesophageal reflux disease (GERD):
- Predilection for distal esophagus
- Basal cell hyperplasia, spongiosis and elongated papillae
- Intraepithelial lymphocytes can be seen but are accompanied by eosinophils and rare neutrophils
- Apoptotic keratinocytes not a diagnostic feature
- Lymphocytic esophagitis:
- Lymphocytes tend to have a peripapillary distribution while in lichenoid esophagitis pattern prominent band-like lymphocytic infiltrate involves the interface of basal epithelium and lamina propria
- In biopsies lacking lamina propria it is a challenge to assess the distribution of lymphocytes
- Eosinophilic esophagitis:
- Endoscopically similar findings to lichenoid esophagitis pattern
- Prominent eosinophils rather than lymphocytes
- Sloughing esophagitis:
- Intraepithelial split while in lichenoid esophagitis pattern split is at the junction of the epithelium and lamina propria
- Inflammation not prominent
- Isolated esophageal mucous membrane pemphigoid:
- Positive immunofluorescence with linear deposits of IgG
- Serum anti BP230 or BP180 antibodies
- Treatment requires systemic immunosuppression and may be worsened by repeated dilations alone; should be considered in patients with unexplained dysphagia accompanied by mucosal sloughing, strictures and ulcerations (Am J Gastroenterol 2019;114:1695)
- Esophageal GVHD:
- History of hematopoietic stem cell transplant
- Intraepithelial lymphocytosis, basilar vacuolization, epithelial apoptosis and necrosis in severe disease
- Esophageal webs or strictures in upper or mid third of the esophagus can be seen in chronic GVHD (Best Pract Res Clin Haematol 2008;21:251)
Additional references
Board review style question #1
- A 45 year old woman with dysphagia undergoes an upper endoscopy which demonstrates mucosal breaks involving at least 75% of esophageal circumference in mid esophagus. Tissue submitted for immunofluorescence study is negative and the patient has no skin, oral or genital lesions. An esophageal biopsy demonstrates the findings shown above. Which one of the following has been associated with this pattern of injury?
- Cytomegalovirus
- Herpes virus I/II
- HIV and viral hepatitis
- Sarcina ventriculi
Board review style answer #1
C. HIV and viral hepatitis
HIV and viral hepatitis have been associated with lichenoid esophagitis pattern, shown here. Sarcina ventriculi is a Gram positive coccus, recognized in gastric biopsies, particularly of patients with delayed gastric emptying characterized by basophilic staining and tetrad arrangement. Cytomegalovirus causes viral cytopathic effect seen at the base of esophageal ulcers involving endothelial cells. Herpes virus I/II would show multinucleation, margination and molding (3 M’s) within superficial epithelial cells.
Comment Here
Reference: Lichenoid esophagitis
HIV and viral hepatitis have been associated with lichenoid esophagitis pattern, shown here. Sarcina ventriculi is a Gram positive coccus, recognized in gastric biopsies, particularly of patients with delayed gastric emptying characterized by basophilic staining and tetrad arrangement. Cytomegalovirus causes viral cytopathic effect seen at the base of esophageal ulcers involving endothelial cells. Herpes virus I/II would show multinucleation, margination and molding (3 M’s) within superficial epithelial cells.
Comment Here
Reference: Lichenoid esophagitis
Board review style question #2
- Which of the followings is true regarding lichenoid esophagitis pattern of injury?
- Dysphagia is not a common symptom in lichenoid esophagitis pattern patients
- Stricture formation in mid esophagus can be seen in upper endoscopy
- There are no reports of an association with squamous cell neoplasia
- White plaques in distal esophagus of young men is the typical clinical scenario
Board review style answer #2
B. Stricture formation in mid esophagus can be seen in upper endoscopy
Lichenoid esophagitis pattern has been rarely reported in association with dysplasia and carcinoma and periodic surveillance should be suggested. White plaques in proximal esophagus of middle aged women is a common endoscopic finding. Endoscopically it may mimic Candida esophagitis or eosinophilic esophagitis. Stricture formation can be seen but is not a common finding in lichenoid esophagitis pattern unlike lichen planus esophagitis. Mid esophagus is more frequently involved in lichenoid esophagitis pattern. Dysphagia is a common symptom at the time of endoscopy.
Comment Here
Reference: Lichenoid esophagitis
Lichenoid esophagitis pattern has been rarely reported in association with dysplasia and carcinoma and periodic surveillance should be suggested. White plaques in proximal esophagus of middle aged women is a common endoscopic finding. Endoscopically it may mimic Candida esophagitis or eosinophilic esophagitis. Stricture formation can be seen but is not a common finding in lichenoid esophagitis pattern unlike lichen planus esophagitis. Mid esophagus is more frequently involved in lichenoid esophagitis pattern. Dysphagia is a common symptom at the time of endoscopy.
Comment Here
Reference: Lichenoid esophagitis
Lymphocytic esophagitis
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Uncommon esophageal histologic pattern of injury related to different etiologies (Am J Surg Pathol 2022;46:e55, Ann N Y Acad Sci 2018;1434:185)
- Controversial diagnosis as a new distinct disease rather than a pattern of injury associated with more prevalent conditions (Am J Surg Pathol 2022;46:e55)
- More common in older women and patients with underlying immunologic abnormalities (Dig Dis Sci 2021;66:3976, Am J Surg Pathol 2022;46:e55)
- Gastroesophageal reflux disease (GERD), Crohn's disease, dysmotility, achalasia and medication induced esophagitis are some of the underlying conditions associated with lymphocytic esophagitis pattern of injury (Am J Surg Pathol 2022;46:e55)
Essential features
- Dysphagia is the most common clinical presentation (Am J Surg Pathol 2020;44:198)
- Histologically characterized by a triad of intraepithelial lymphocytosis, with epithelial damage and no significant granulocytic inflammation
- Unequivocal mucosal lymphocytosis
- Rare to absent granulocytes
- Epithelial damage consists of dyskeratotic keratinocytes (Civatte bodies) and intracellular edema (spongiosis) (Am J Surg Pathol 2022;46:e55)
ICD coding
- ICD-10: K20.90 - esophagitis, unspecified without bleeding
Epidemiology
- Prevalence is estimated to be 0.1% in adult population and 5.7% in pediatric population (Cureus 2022;14:e30300)
- F > M, with median age in the sixth decade (J Clin Gastroenterol 2012;46:828, Gut 2012;61:1108)
- In pediatric population, there is a strong association between lymphocytic esophagitis and Crohn's disease (Inflamm Bowel Dis 2014;20:1324, Inflamm Bowel Dis 2011;17:45)
Sites
- Any level within the tubular esophagus can be affected (Am J Surg Pathol 2022;46:e55)
Pathophysiology
- Pathophysiology is currently not well understood
Etiology
- Several conditions and diseases are associated with this pattern of injury, from autoimmune diseases such as Crohn's, rheumatoid arthritis, dysmotility disorder (achalasia) to medication effect (Dig Dis Sci 2021;66:3976)
Clinical features
- Dysphagia is the most common presentation (Dig Dis Sci 2021;66:3976)
- On endoscopy, features can vary based on the underlying cause from normal appearing esophagus to strictures and edematous esophagus (Am J Surg Pathol 2022;46:e55, Am J Surg Pathol 2015;39:1558, Am J Surg Pathol 2020;44:198)
Diagnosis
- Diagnosis is based on histological findings
Case reports
- 28 year old woman with polyvalent sensitization to food and inhalant allergens presented with intermittent dysphagia (Allergy Asthma Clin Immunol 2021;17:56)
- 66 year old woman complaining of dysphagia for several weeks with the loss of ability to swallow solid foods (Cureus 2022;14:e30300)
- 71 year old man with a medical history of squamous cell carcinoma of the tongue treated with external beam radiation 17 years ago presented with dysphagia (Cureus 2018;10:e2153)
Treatment
- No definitive targeted treatment for lymphocytic esophagitis
- Management is pointed towards the underlying condition
- 1 case report listed empiric therapy with oral steroids, proton pump inhibitor and balloon dilation (Cureus 2022;14:e30300)
Microscopic (histologic) description
- Characterized by a triad of intraepithelial lymphocytosis, with epithelial damage and no significant granulocytic inflammation
- Unequivocal mucosal lymphocytosis; studies have reported various cutoff values for lymphocytes, ranging from 20 - 50/HPF (Am J Surg Pathol 2022;46:e55)
- Rare to absent granulocytes (if present abundantly it argues against the diagnosis of lymphocytic esophagitis) (Am J Surg Pathol 2022;46:e55)
- Epithelial damage consists of dyskeratotic keratinocytes (Civatte bodies) and nonspecific changes such as intracellular edema (spongiosis) (Am J Surg Pathol 2022;46:e55, Am J Surg Pathol 2020;44:198)
- Depending on the underlying disease, the T cell population may differ
- Crohn's and most GERD patients can have a high CD8:CD4 ratio while dysmotility patients tend to show predominance of CD4 helper T cells with a CD4:CD8 ratio > 1 (neither sensitive nor specific) (Arch Pathol Lab Med 2021;145:1138, Am J Surg Pathol 2015;39:1558)
Microscopic (histologic) images
Contributed by Monica T. Garcia-Buitrago, M.D., Omar Aljuboori, M.B.B.S. and @RaulSGonzalezMD on Twitter
Negative stains
Sample pathology report
- Esophagus, biopsy:
- Esophageal squamous mucosa with lymphocytic esophagitis (see comment)
- Comment: Lymphocytic esophagitis pattern of injury is associated with rheumatological diseases, Crohn's disease, hypothyroidism, immune mediated conditions, medications (i.e., checkpoint inhibitors) and dysmotility disorders.
Differential diagnosis
- Gastroesophageal reflux disease (GERD):
- Histologic features that are associated with GERD include inflammatory cell infiltrates and classically eosinophils
- Basal cell hyperplasia with elongated vascular papillae and esophageal spongiosis
- Candida esophagitis:
- These patients are usually immunocompromised
- On endoscopy, there are visible white plaques that can be scraped off easily from the underlying mucosa
- Neutrophilic infiltration is seen in biopsy along with fungal pseudohyphae in sloughed epithelial debris or invading intact squamous epithelium perpendicularly
- Radiation esophagitis:
- Acute postradiation esophagitis usually shows mucosal necrosis and neutrophilic infiltration
- In chronic esophagitis, there is submucosal fibrosis, capillary telangiectasia and vascular hyalinization
- Esophageal lichen planus:
- On endoscopy, mucosal denudation, trachealization and hyperkeratosis are seen; esophageal stenosis may occur
- Prominent band-like lymphocytic infiltrate involving the interface of lamina propria and epithelium, intraepithelial lymphocytes and dyskeratotic keratinocytes (Am J Surg Pathol 2013;37:1889)
- Direct immunofluorescence shows diagnostic globular IgM deposits and fibrinogen deposits along the basement membrane zone
- Esophageal graft versus host disease (GVHD):
- History of hematopoietic stem cell or lymphocyte rich transplant
- On endoscopy: esophageal webs or strictures in upper or mid third of the esophagus in chronic GVHD
- Intraepithelial lymphocytosis, basilar vacuolization, epithelial apoptosis and necrosis in severe disease (Best Pract Res Clin Haematol 2008;21:251)
Board review style question #1
A 60 year old woman underwent an upper endoscopy for dysphagia. Endoscopy revealed stricture in the mid esophagus. In addition to the findings pictured, no neutrophilic / eosinophilic infiltrate was present. What is the most probable diagnosis?
- Candida esophagitis
- Gastroesophageal reflux disease (GERD)
- Lymphocytic esophagitis
- Radiation esophagitis
Board review style answer #1
C. Lymphocytic esophagitis. The presence of increased lymphocytic infiltrates, esophageal swelling and absence of neutrophilic and eosinophilic infiltrate point towards the diagnosis of lymphocytic esophagitis. Answer B is incorrect because eosinophilic and neutrophilic infiltrates associated with basal epithelial changes are characteristic of gastroesophageal reflux disease (GERD). Answer A is incorrect because neutrophilic infiltration along with fungal pseudohyphae in sloughed epithelial debris is a hallmark of Candida esophagitis. Answer D is incorrect because radiation esophagitis is usually associated with mucosal necrosis and marked neutrophilic infiltration.
Comment Here
Reference: Lymphocytic esophagitis
Comment Here
Reference: Lymphocytic esophagitis
Board review style question #2
A 71 year old man with a known history of squamous cell carcinoma of the tongue treated with external beam radiation 17 years prior, presented to the clinic for esophagogastroduodenoscopy (EGD) with dilation for esophageal strictures. His only complaint at this time was dysphagia. A biopsy was taken and showed the findings above. Which of the following are characteristic features of lymphocytic esophagitis?
- Basal cell hyperplasia, spongiosis and elongated papillae with intraepithelial lymphocytes can be seen but are accompanied by eosinophils and rare neutrophils
- Intraepithelial lymphocytosis, basilar vacuolization, epithelial apoptosis and necrosis with a history of hematopoietic stem cell transplant
- Intraepithelial lymphocytosis, epithelial damage and paucity of granulocytes
- Prominent band-like lymphocytic infiltrate involving the interface of lamina propria and epithelium, intraepithelial lymphocytes and dyskeratotic keratinocytes
Board review style answer #2
C. Intraepithelial lymphocytosis, epithelial damage and paucity of granulocytes. The presence of lymphocytic infiltrate, esophageal swelling and absence of neutrophilic and eosinophilic infiltrate are the clinical features of lymphocytic esophagitis. Answer D is incorrect because these are the features of esophageal lichenoid planus. Answer B is incorrect because these are the features of graft versus host disease (GVHD). Answer A is incorrect because these are the features of gastroesophageal reflux disease (GERD).
Comment Here
Reference: Lymphocytic esophagitis
Comment Here
Reference: Lymphocytic esophagitis
Board review style question #3
Which of the following stains highlights most of the cells in lymphocytic esophagitis?
- CD3
- CD20
- CD68
- CD138
Board review style answer #3
A. CD3. CD3 stain identifies T cells, increased in lymphocytic esophagitis cases. Answer B is incorrect because CD20 stain shows rare B cells in lymphocytic esophagitis. Answer C is incorrect because CD68 stain is expressed in histiocytic and monocytic cells. Answer D is incorrect because CD138 stain is expressed in plasma cells.
Comment Here
Reference: Lymphocytic esophagitis
Comment Here
Reference: Lymphocytic esophagitis
Lymphoma
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Risk factors | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Peripheral smear description | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Lymphomas comprise a diverse group of clonal (malignant) lymphoproliferative disorders, classified by WHO based on lymphocytic origin (lymphoma)
- Hodgkin lymphomas arise from precursor B cells (Reed-Sternberg cells)
- Classic Hodgkin lymphoma - 95%
- Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) - 5%
- Non-Hodgkin lymphomas arise from monoclonal expansion of malignant B or T cells
- B cell lymphomas
- B cell acute lymphoblastic lymphoma (ALL)
- Chronic lymphocytic lymphoma / small lymphocytic leukemia (CLL / SLL)
- Mantle cell lymphoma
- Follicular lymphoma
- Marginal zone B cell lymphoma
- Extranodal MALT type
- Hairy cell leukemia
- Plasmacytoma / plasma cell myeloma
- Diffuse large B cell lymphoma (DLBCL)
- Burkitt lymphoma
- T / NK cell lymphomas
- T cell acute lymphoblastic lymphoma (ALL)
- T cell CLL
- Mycosis fungoides / Sézary syndrome
- Peripheral T cell lymphoma
- Angioimmunoblastic T cell lymphoma
- Enteropathy associated intestinal T cell lymphoma
- Hepatosplenic T cell lymphoma
- Anaplastic large cell lymphoma (ALCL)
- Extranodal NK / T cell lymphoma, nasal type
- B cell lymphomas
- Hodgkin lymphomas arise from precursor B cells (Reed-Sternberg cells)
Terminology
- Primary esophageal lymphoma
- Arises de novo in esophageal mucosa associated lymphoid tissue (MALT)
- Secondary esophageal lymphoma
- Spreads to esophagus by either direct extension or metastasis
Epidemiology
- GI tract is most common site of extranodal non-Hodgkin lymphoma (4 - 20%)
- Esophagus is least common site of GI involvement
- < 0.2% of all extranodal non-Hodgkin lymphoma
- Esophagus is least common site of GI involvement
- Lymphomas comprise < 1% of all esophageal cancers
- Secondary lymphoma (contiguous spread from mediastinal or cervical lymph nodes) >> primary esophageal disease
- Most originate from mature B cells
- Non-Hodgkin lymphoma >> Hodgkin lymphoma
- Mostly DLBCL and extranodal marginal zone B cell lymphoma, MALT type
- Males > females
- Wide age range: 17 - 86 years but usually middle age to older adults
- Younger age of onset in HIV+ patients (40 vs. 60 years)
Sites
- Primary lymphoma:
- Arises in submucosal lymphoid patches
- Secondary lymphoma
- Proximal esophageal involvement may result from adjacent cervical lymphadenopathy
- Middle esophageal involvement may be due to mediastinal lymphadenopathy
- Distal esophageal involvement often secondary to gastric disease
Risk factors
- Chronic immune suppression or dysregulation
- HIV infection
- Chronic hepatitis C infection
Clinical features
- Features of primary lymphoma (Dawson criteria):
- No palpable superficial lymphadenopathy
- Normal chest radiograph with no evidence of lymphadenopathy
- Normal peripheral white blood cell count
- Primary esophageal lesion with only regional adenopathy
- No liver or spleen involvement
- Insidious onset
- Dysphagia (most common)
- Odynophagia
- Chest / abdominal pain
- Weight loss
- Complications (Ann Otol Rhinol Laryngol 1994;103:843)
- Hemorrhage
- Vocal cord paralysis / hoarseness
- Stricture / obstruction
- Perforation with esophagomediastinal or esophagotracheobronchial fistula or mediastinitis
- Vocal cord paralysis
- Relatively poor prognosis
Diagnosis
- Biopsy with ancillary studies (immunohistochemistry, flow cytometry) to confirm histologic type
Laboratory
- Complete blood count may show:
- Leukocytosis ± blasts (leukemic spread)
- Pancytopenia (bone marrow infiltration)
Radiology description
- Nonspecific radiographic features
- Local extension of gastric lymphoma may cause distal esophageal stricture
- Barium swallow / CT: stricture, polypoid or intramural mass
- PET useful for staging
Radiology images
Images hosted on other servers:
Strictures:
Esophageal thickening ± mass lesions:
Endoscopic ultrasound:
Primary esophageal Hodgkin lymphoma:
Prognostic factors
- Stage at diagnosis
- Feasibility of surgery or chemotherapy
- Successful management depends on accurate diagnosis and appropriate subsequent treatment
- Histologic type: MALT lymphoma and Hodgkin lymphoma have better prognoses than DLBCL or T cell lymphomas
- HIV / AIDS associated with worse outcomes
- Median survival: 4 - 6 months
Case reports
- 3 year old boy with anaplastic large cell lymphoma of esophagus (Pediatr Radiol 2012;42:627)
- 53 year old man with multiple lymphomatous polyposis with widespread involvement of GI tract (Arch Pathol Lab Med 2003;127:1028)
- 53 year old man with primary MALT lymphyoma of esophagus, manifesting as a submucosal tumor (Korean J Gastroenterol 2013;62:117)
- 56 year old man with primary esophageal lymphoma (Ann Thorac Surg 1998;66:1418)
- 58 year old man with primary extramedullary plasmacytoma of esophagus (Ann Diagn Pathol 2003;7:174)
- 59 year old man with MALT lymphoma of esophagus (Int J Clin Oncol 2012;17:174)
- 60 year old woman with dysphagia (Endoscopy 2012;44:E167)
- 61 year old men with Hodgkin lymphoma of esophagus (Am J Clin Pathol 1997;108:593, AJR Am J Roentgenol 2003;180:1335)
- 62 year old woman with MALT lymphoma arising in esophagus, stomach and lung (Gen Thorac Cardiovasc Surg 2011;59:826)
- 63 year old man with T cell lymphoma presenting as esophageal obstruction and bronchoesophageal fistula (Med Sci Monit 2011;17:CS66)
- 65 year old man with MALT lymphoma of esophagus coexistent with bronchus associated lymphoid tissue lymphoma of lung (Yonsei Med J 2005;46:562)
- 67 year old man with multiple polyposis of esophagus (Clin Gastroenterol Hepatol 2012;10:e65)
- 70 year old man with esophageal MALT lymphoma (Dis Esophagus 2013;26:349)
- 70 year old man with CD8+ mycosis fungoides with esophageal involvement (Oncol Lett 2013;5:73)
- 77 year old man with primary isolated non-Hodgkin lymphoma of esophagus (World J Gastroenterol 2009;15:1901)
- 80 year old woman with esophageal perforation and MALT lymphoma (University of Pittsburgh: Esophageal Perforation with an Unusual Etiology [Accessed 31 January 2018])
- 6 cases of primary esophageal lymphoma (Dig Dis Sci 2006;51:77)
- Indolent T cell lymphoproliferative disease of GI tract (Blood 2013;122:3599)
- Esophageal leukemic infiltration in children (J Pediatr Gastroenterol Nutr 2011;52:781)
- Two immunocompetent patients with primary esophageal lymphoma (World J Radiol 2010;2:334)
Treatment
- Primary esophageal lymphoma: local and systemic therapy
- Radiotherapy, chemotherapy
- Non-Hodgkin lymphoma: CHOP (cyclophosphamide, vincristine, prednisone, doxorubicin), rituximab (anti-CD20 monoclonal antibody)
- Hodgkin lymphoma: ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or Stanford V (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, prednisone)
- Surgical resection: diagnostic, curative or palliative
- Endoscopic resection for early lesions (Dig Endosc 2014;26:478)
- Radiotherapy, chemotherapy
- Secondary esophageal lymphoma: chemotherapy
Clinical images
Gross description
- Submucosal mass with variable ulceration or exophytic growth
Gross images
Microscopic (histologic) description
- DLBCL: large centrocytes, centroblasts, immunoblasts and anaplastic large B cells
- Surface Ig+, CD20+, BCL6±, CD10±, CD43±, PAX5+
- Marginal zone B cell lymphoma, MALT type: small lymphocytes, marginal zone B cells, plasma cells, reactive follicles, lymphoepithelial lesions
- Surface and cytoplasmic Ig+ (IgM > IgG or IgA), CD20+, CD5-, CD10-, BCL6-, BCL2+, CD43±, cyclin D1-
- Mantle cell lymphoma: small to medium sized, slightly irregular cells with scant cytoplasm
- Surface IgMD+, CD20+, CD5+, CD10-, CD43+, cyclin D1+
- Burkitt lymphoma: medium sized atypical lymphoid cells with round nuclei, basophilic cytoplasm and tingible body macrophages (starry sky appearance)
- Cytoplasmic lipid droplets are Oil red O+
- Surface IgM+, CD20+, CD10+, BCL6+, BCL2-, Ki67 ~100%
- Follicular lymphoma: mixtures of centrocytes and centroblasts, follicular dendritic cells
- Surface Ig+, CD20+, CD10+, BCL6+, BCL2+, CD5-, CD43-, cyclin D1-
- Classic Hodgkin lymphoma: Reed-Sternberg cells and variants in a reactive background
- R-S cells: CD30+, CD15±, PAX5+, ALK-, CD45-, CD3-
- Nodular lymphocyte predominant Hodgkin lymphoma: vague nodules of small B cells and interspersed large tumor cells (LP cells) with thin nuclear membranes, fine chromatin and variable nucleoli
- LP cells: CD45+, CD20+, CD15-, CD30-
Microscopic (histologic) images
Images hosted on other servers:
MALT lymphoma:
Cytology description
- Discohesive, single abnormal lymphoid cells
Peripheral smear description
- Peripheral blasts if leukemic spread
Positive stains
- CD45 (leucocyte common antigen): most hematopoietic neoplasms
- Notable exceptions: ALCL, classic Hodgkin lymphoma, plasma cell myeloma
- CD2, CD3, CD4: most T cell neoplasms
- CD5: CLL / SLL, mantle cell lymphoma
- CD10: follicular lymphoma, Burkitt lymphoma, some DLBCL
- CD19, CD20, CD79a: most B cell neoplasms
- CD23: SLL / CLL
- CD30: ALCL, classic Hodgkin lymphoma
- Cyclin D1: mantle cell lymphoma
- EBV: classic Hodgkin lymphoma
- EMA: ALCL, plasmacytoma, LP cells in NLPHL, some T cell lymphomas
- MUM1: classic Hodgkin lymphoma, plasmablastic lymphoma
- PAX5: B cell marker, classic Hodgkin lymphoma
Negative stains
- Most cytokeratins
- Melanocytic markers (S100, HMB45, MelanA, MITF)
- Smooth muscle markers (desmin, caldesmon, smooth muscle actin)
- Neuroendocrine markers (chromogranin, synaptophysin, NSE)
Flow cytometry description
- Useful to determine surface and cytoplasmic CD markers
Molecular / cytogenetics description
- Common genetic features of mature B cell lymphomas
- Marginal zone B cell lymphoma, MALT type
- IGH clonally rearranged
- t(11;18)
- DLBCL
- IGH clonally rearranged
- t(8;14) c-myc-IGH
- t(14;18) IGH-BCL2
- Burkitt lymphoma
- IGH clonally rearranged
- t(8;14)
- t(2;8)
- t(8;22)
- Mantle cell lymphoma
- IGH clonally rearranged
- t(11;14) BCL1-IGH
- Marginal zone B cell lymphoma, MALT type
Differential diagnosis
- Reactive lymphoid hyperplasia: polyclonal by IHC / flow cytometry
Additional references
Melanocytosis
Table of Contents
Definition / general | Gross description | Microscopic (histologic) description | Positive stains | Negative stains | Electron microscopy description | Differential diagnosisDefinition / general
- Melanocytes are present in 8% of normal esophageal specimens (Virchows Arch A Pathol Anat Histopathol 1990;417:137) but grossly visible lesion in less than 0.2% of upper endoscopy patients (Endoscopy 1990;22:94, J Gastroenterol Hepatol 2011;26:1463)
- Benign, involves mid / lower esophagus
- More common in men
- May be due to gastroesophageal reflux disease
- May be a precursor of esophageal melanoma
- Recommended to not use term "melanosis" (Arch Pathol Lab Med 2006;130:552)
Gross description
- No abnormalities
Microscopic (histologic) description
- Melanocytic proliferation in basal layer of esophageal squamous epithelium with increased quantity of melanin; overlying epithelium may demonstrate hyperplasia, acanthosis or hyperkeratosis; variable inflammation, melanophages, fibrosis and telangiectasia; no atypia
Positive stains
Electron microscopy description
- Long dendritic cytoplasmic processes that extend between keratinocytes, melanosomes; mildly hyperchromatic nuclei with uniform chromatin and indented nuclei; no desmosomes or tonofilaments (Virchows Arch A Pathol Anat Histopathol 1991;418:515)
Differential diagnosis
Melanoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Primary malignant melanoma developing from the esophageal mucosa / melanocyte
Essential features
- Middle aged to elderly patients, with male predominance
- Protruding tumor with pigmentation / black discoloration in the middle or lower esophagus
- Epithelioid or spindle melanoma cells with junctional melanocytic activity / junctional melanocytic component
- Aggressive tumor
Terminology
- Primary malignant melanoma of the esophagus
ICD coding
- ICD-10: C15.9 - malignant neoplasm of the esophagus, unspecified
Epidemiology
- ~0.3 - 0.8% of all esophageal tumors (Esophagus 2021;18:1, Histopathology 2021;78:240)
- Middle aged to elderly patients, with male predominance (Mod Pathol 2019;32:957, Thorac Cancer 2019;10:950, Histopathology 2021;78:240)
Sites
- Most common in the distal third of the esophagus (Mod Pathol 2019;32:957, Dis Esophagus 2019 Oct 30 [Epub ahead of print])
- Lower esophagus (58% [44/76 cases] to 60% [12/20 cases]); middle esophagus (34% [26/76 cases] to 35% [7/20 cases]) (Thorac Cancer 2019;10:950, Medicine (Baltimore) 2020;99:e20957)
Pathophysiology
Etiology
Clinical features
- Middle aged to elderly patients, with male predominance (Mod Pathol 2019;32:957, Thorac Cancer 2019;10:950, Histopathology 2021;78:240)
- Progressive dysphagia, weight loss and chest pain (Mod Pathol 2019;32:957, Dis Esophagus 2019 Oct 30 [Epub ahead of print])
Diagnosis
- Biopsy with immunohistochemistry
Radiology description
- Bulky, polypoid intraluminal masses (Radiology 1998;209:455)
Prognostic factors
- TNM staging (Medicine (Baltimore) 2020;99:e20957)
Case reports
- 68 year old man with a 20 mm amelanotic malignant melanoma of the esophagus (Oncol Lett 2018;15:9087)
- 74 year old man with an amelanotic malignant melanoma of the esophagus treated with immunotherapy (J Surg Case Rep 2021;2021:rjab393)
- 81 year old woman with a 30 mm primary malignant melanoma treated with esophagectomy and nivolumab (J Med Case Rep 2021;15:237)
- 86 year old woman with a 58 mm amelanotic malignant melanoma of the esophagus (Surg Case Rep 2019;5:4)
Treatment
- Surgical resection
- Endoscopic resection
- Immunotherapy (PD-1 inhibitor systemic treatment) (Thorac Cancer 2019;10:950)
- Chemotherapy
- Targeted therapy
Gross description
- Protruding / polypoid lesion with pigmentation / black discoloration (Oncol Lett 2019;18:1872, Histopathology 2021;78:240, Dis Esophagus 2019 Oct 30 [Epub ahead of print])
Microscopic (histologic) description
- Mostly epithelioid tumor cells with at least focal melanin pigment (Mod Pathol 2019;32:957)
- Associated melanoma in situ component (junctional melanocytic activity / junctional melanocytic component / tumor nests at the epithelium - lamina propria junction / horizontal tumor spread in the basal layer of the epithelium) and melanocytosis / melanosis (Mod Pathol 2019;32:957, Ann Thorac Surg 2013;96:1002)
Microscopic (histologic) images
Positive stains
- Melanoma markers (S100, HMB45, MelanA / MART1, MITF, SOX10, tyrosinase)
Negative stains
- Cytokeratin (mostly negative; however, anomalous cytokeratin expression has been reported) (Mod Pathol 1990;3:494, J Cutan Pathol 2021;48:1246, Am J Dermatopathol 2019;41:502)
- Squamous cell markers (p40 and p63)
- Neuroendocrine markers (chromogranin and synaptophysin); neuroendocrine markers can be expressed in malignant melanomas / malignant melanomas with neuroendocrine differentiation have been reported (Histopathology 2005;47:402)
- CD45
- CD68
Molecular / cytogenetics description
- Contrary to cutaneous melanoma, no (0% [0/22]) or few (up to 6.6%) BRAF mutations identified (Mod Pathol 2019;32:957, Thorac Cancer 2019;10:950, Virchows Arch 2009;454:513)
- NRAS mutations: 33 - 35% (Mod Pathol 2019;32:957, Virchows Arch 2009;454:513)
- Relatively common KIT mutations (10 - 50%) by next generation sequencing (NGS) analysis (Histopathology 2021;78:240 Mod Pathol 2019;32:957)
- NGS analysis revealed NF1 (30%), SF3B1 (20%), KRAS (10%), BRCA2 (10%), KIT (10%) and TP53 (10%) mutations; no BRAF mutations were detected (Histopathology 2021;78:240)
Sample pathology report
- Esophagus, mass, biopsy:
- Malignant melanoma (see comment)
- Comment: The infiltrating epithelioid tumor cells are positive for S100, HMB45, MelanA and negative for AE1 / AE3, CAM5.2, synaptophysin and chromogranin. The morphology and immunoprofile are consistent with malignant melanoma.
Differential diagnosis
- Poorly differentiated squamous cell carcinoma or adenocarcinoma:
- Keratin positive and melanoma marker negative
- Neuroendocrine carcinoma (small cell neuroendocrine carcinoma and large cell neuroendocrine carcinoma):
- Keratin positive; neuroendocrine marker positive and melanoma marker negative
- Basaloid squamous carcinoma:
- Keratin positive and melanoma marker negative
Board review style question #1
Which of the following is true about primary malignant melanoma of the esophagus?
- Junctional melanocytic activity suggests a primary malignant melanoma of the esophagus
- Melanin pigments should be always found in the tumor cells by definition
- No melanocytes are found in the normal esophagus
- SOX10 is a useful marker to differentiate benign nevi from malignant melanomas
Board review style answer #1
A. Junctional melanocytic activity (shown in the picture) suggests a primary malignant melanoma of the esophagus
Comment Here
Reference: Esophageal melanoma
Comment Here
Reference: Esophageal melanoma
Board review style question #2
Which of the following is true about primary malignant melanoma of the esophagus?
- BRAF mutation is the most common mutation, as in cutaneous melanoma
- Cervical esophagus is the most common site
- Most cases show protruding lesions
- Smoking and alcohol consumption are important risk factors
- Usually seen in young female patients
Board review style answer #2
Mucoepidermoid carcinoma (pending)
[Pending]
Neuroendocrine carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare high grade epithelial tumor with neuroendocrine differentiation divided into small cell or large cell types by morphology; follows an aggressive clinical course and has a poor prognosis
Essential features
- Rare malignant tumor of the esophagus with poor prognosis and frequent locoregional or distant metastasis at the time of diagnosis
- Usually involves mid or distal third of the esophagus
- Positive for either synaptophysin or chromogranin
- Divided into either small cell or large cell type by morphology, using the same criteria as for other sites in the gastrointestinal tract
- Small cell: minimal cytoplasm, nuclei with fine chromatin and nuclear molding, inconspicuous nucleoli, frequent mitoses, necrosis
- Large cell: moderately abundant cytoplasm, nuclei with clumped chromatin and prominent nucleoli, frequent mitoses, necrosis
Terminology
- Poorly differentiated endocrine carcinoma, small cell carcinoma, large cell carcinoma
ICD coding
Epidemiology
- Small carcinoma in the esophagus was first described in 1952 (J Pathol Bacteriol 1952;64:889)
- Most esophageal neuroendocrine neoplasms are high grade tumors (Clinics (Sao Paulo) 2011;66:1671, J Oncol 2016:2016:2402417)
- Extremely rare, accounts for < 1% of all malignant neoplasms and between 0.4 - 2% of esophageal malignancies (BMC Cancer 2014:14:569, Crit Rev Oncol Hematol 2019:137:92, PLoS One 2017;12:e0173501, J Oncol 2016:2016:2402417)
- M:F = 2.5 - 3:1 (Am J Surg Pathol 2013;37:467, J Thorac Dis 2016;8:1250)
- Mean age: 60 - 70 years (Crit Rev Oncol Hematol 2019:137:92)
Sites
- Middle or lower third of the esophagus, corresponding to distribution of neuroendocrine cells (Am J Surg Pathol 2013;37:467, Int J Clin Exp Pathol 2013;6:485, J Thorac Dis 2016;8:1250, Crit Rev Oncol Hematol 2019:137:92)
Pathophysiology
- Unknown but small studies suggest origin from totipotent progenitor cell (Hum Pathol 1984;15:460, J Thorac Cardiovasc Surg 1985;90:351)
Etiology
- Associated with high alcohol intake and smoking (Arq Gastroenterol 2017;54:4)
Diagrams / tables
Not available
Clinical features
- Dysphagia (most common), weight loss, appetite loss, pain (retrosternal, epigastric, abdominal) (Int J Clin Exp Pathol 2013;6:485, Crit Rev Oncol Hematol 2019:137:92)
- Regional lymph node metastasis or widespread metastasis is common at the time of diagnosis (Am J Surg Pathol 2008;32:1404, BMC Cancer 2014:14:569)
- Metastatic sites include lungs, liver, adrenal, peritoneal, bones (Arq Gastroenterol 2017;54:4)
- Rare cases demonstrate an association with paraneoplastic neurologic syndrome (Jpn J Clin Oncol 2006;36:109)
Diagnosis
- Typically diagnosed via endoscopy with biopsy or less commonly after surgical resection (Crit Rev Oncol Hematol 2019:137:92)
Laboratory
- Testing for calcium, phosphate, alkaline phosphatase can aid in evaluating for bone metastasis
Radiology description
- Imaging studies can help define the extent of esophageal involvement
- Barium esophagography can be used in the initial identification of tumors (Crit Rev Oncol Hematol 2019:137:92)
- Computed tomography (CT) imaging for defining regional or distant lymph node metastasis and systemic disease
- Whole body positron emission tomography (PET) scans to evaluate for distant metastases (Front Med (Lausanne) 2020:6:336)
Radiology images
Not available
Prognostic factors
- Neuroendocrine carcinomas (NECs) are highly aggressive with poor prognosis as in other body sites
- Patients with locoregional disease have better overall survival than those with distant metastases (Am J Surg Pathol 2008;32:1404, Dis Esophagus 2014;27:152)
- Prognosis is better in patients with mixed NEC (having both NEC and squamous cell carcinoma or adenocarcinoma) (Am J Surg Pathol 2008;32:1404)
- Median survival is 3 to 20 months (BMC Cancer 2007;7:38, Arch Pathol Lab Med 2000;124:228, Dis Esophagus 2014;27:152)
- 5 year survival is ≤ 8% (Chin Med J (Engl) 2007;120:355, Ann Surg Oncol 2013;20:4239)
Case reports
- 51 year old man with collision tumor between papillary adenocarcinoma and large cell neuroendocrine carcinoma (Arch Pathol Lab Med 2000;124:411)
- 55 year old man with large cell NEC of the esophagus (Cureus 2022;14:e28842)
- 63 year old woman with esophageal small cell carcinoma coexisting with paraneoplastic neurological syndrome (Jpn J Clin Oncol 2006;36:109)
- 66 year old man with rare collision tumor of squamous carcinoma and small cell carcinoma in esophagus with separate lymph node involvement (J Thorac Dis 2013;5:E203)
- 67 year old man found to have NEC in lower esophagus with metastasis (J Med Case Rep 2023;17:144)
- 79 year old man with dysphagia (BMJ Case Rep 2013;2013:bcr2013200468)
Treatment
- No standardized treatment
- Multimodal therapy (surgery, platinum based chemotherapy or radiation) improves survival in patients with locoregional and metastatic disease (Dis Esophagus 2014;27:152)
- Folinic acid, fluorouracil and irinotecan (FOLFIRI) can be considered for second line therapy after platinum etoposide first line chemotherapy (Lancet Oncol 2023;24:297)
Gross description
- Ulcerative, fungating mass with a gray-white cut surface (J Investig Med High Impact Case Rep 2020:8:2324709620918245)
Gross images
Frozen section description
Not available
Frozen section images
Not available
Microscopic (histologic) description
- Poorly differentiated (high grade) carcinomas
- Divided into small cell or large cell types according to WHO 2019 classification
- Small cell carcinoma
- Small round to oval cells with scant cytoplasm, prominent nuclear molding, fine granular chromatin, absent or inconspicuous nucleoli
- Solid, rosette or palisading pattern of infiltration
- Brisk mitotic activity and necrosis
- Large cell carcinoma
- Medium to large tumor cells with enlarged nuclei, prominent nucleoli and moderately abundant basophilic cytoplasm
- Brisk mitotic activity and necrosis
- Small cell carcinoma
- Neuroendocrine carcinoma may have small component(s) of adenocarcinoma or squamous cell carcinoma differentiation (Arq Gastroenterol 2017;54:4)
Microscopic (histologic) images
Contributed by Gillian L. Hale, M.D., M.P.H., Mark R. Wick, M.D. and AFIP
Virtual slides
Not available
Cytology description
- Small cell type: obvious pleomorphism, marked nuclear molding, hyperchromatic nuclei, inconspicuous nucleoli
- Large cell type: pleomorphic, medium to large cells, moderate cytoplasm, coarse chromatin, prominent nucleoli
- Numerous mitoses, crush artifact, necrosis
- Apoptotic figures, blue bodies
- References: Cancer 2000;90:148, Mod Pathol 1992;5:555
Immunofluorescence description
Not available
Immunofluorescence images
Not available
Positive stains
- Keratins (e.g., CAM 5.2, AE1 / AE3)
- Neuroendocrine markers: synaptophysin, chromogranin, CD56, NSE
- Synaptophysin is more sensitive than chromogranin (Am J Surg Pathol 2008;32:1404)
- Proliferative index assessed by Ki67 / MIB1 immunohistochemistry is typically > 60% (Pathol Res Pract 2021:226:153614)
- TTF1 positive in 71% of esophageal neuroendocrine carcinoma (Am J Surg Pathol 2013;37:467)
- Other stains reportedly positive include p63 (55%), CD117 (86%), HER2 (16%) and p16 (84%) (Am J Surg Pathol 2013;37:467)
Negative stains
Electron microscopy description
- Neurosecretory granules
Molecular / cytogenetics description
- Associated with a high rate of inactivating mutations in 5 well known genes: TP53, RB1, NOTCH1, FAT1, FBXW7 and 3 genes not previously associated with esophageal carcinogenesis: PDE3A, PTPRM and CBLN2 (Cell Res 2018;28:771)
Molecular / cytogenetics images
Not available
Videos
Not available
Sample pathology report
- Esophagus, mass, biopsy:
- Poorly differentiated neuroendocrine carcinoma, small cell type (see comment)
- Comment: Sections show sheets of crowded tumor cells with round to oval nuclei, inconspicuous nucleoli, prominent nuclear molding and numerous mitoses. There are broad zones of necrosis and necroinflammatory debris within the tumor. The tumor cells are highlighted by stains for pankeratin (strong, diffuse), synaptophysin (strong, diffuse) and chromogranin A (weak, patchy). Ki67 highlights a proliferative index of > 80%. The morphologic features and immunophenotype are those of a poorly differentiated neuroendocrine carcinoma, small cell type.
Differential diagnosis
- High grade carcinomas:
- Metastatic pulmonary neuroendocrine carcinoma:
- There are no reliable means of differentiating a primary esophageal neuroendocrine carcinoma from a metastatic pulmonary neuroendocrine carcinoma (Hum Pathol 2020:96:8)
- Lymphoma:
- CD45 positive
- Melanoma:
- Positive for S100, HMB45, MelanA
- Typically negative for keratin markers, rare keratin expression has been reported in dedifferentiated melanoma (Am J Dermatopathol 2019;41:502)
- Basaloid squamous cell carcinoma:
- Immunopositivity for p40 and CK5/6; however, small cell carcinoma can focally express p40 in ~34% of cases (Thorac Cancer 2019;10:1188)
- Metastatic pulmonary neuroendocrine carcinoma:
Additional references
Board review style question #1
For the lower esophageal tumor depicted in the image above, which of the following statements is true?
- Synaptophysin immunohistochemistry has greater sensitivity than chromogranin in detection
- There are established consensus guidelines on the treatment of this large cell carcinoma
- This tumor has a favorable prognosis with a median survival of 10 years
- Tumors with a concomitant adenocarcinoma or squamous cell carcinoma component have a worse prognosis
Board review style answer #1
A. Synaptophysin immunohistochemistry has greater sensitivity than chromogranin in detection. This is an esophageal small cell carcinoma, which expresses synaptophysin or chromogranin, although synaptophysin has greater sensitivity than chromogranin. Answer C is incorrect because this tumor has a poor prognosis. Answer D is incorrect because tumors with an adenocarcinoma or squamous cell carcinoma component tend to have a better prognosis than pure small cell carcinoma. Answer B is incorrect because the image depicts a small cell carcinoma (not large cell carcinoma) and there is no consensus approach to the treatment of neuroendocrine carcinomas given the rarity of the tumor and lack of randomized controlled trials; however, treatment often includes a combination of approaches including surgery, platinum based chemotherapy and radiation.
Comment Here
Reference: Neuroendocrine carcinoma
Comment Here
Reference: Neuroendocrine carcinoma
Board review style question #2
N/A
Board review style answer #2
N/A
Neuroendocrine carcinoma
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Clinical features | Grading | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy images | Differential diagnosisDefinition / general
- Epithelial neoplasm with prominent neuroendocrine differentiation
- Pathologic spectrum from low grade neuroendocrine tumors (carcinoid, atypical carcinoid) to high grade neuroendocrine carcinoma (small cell or large cell neuroendocrine carcinoma)
Small cell neuroendocrine carcinoma:
- Small cell neuroendocrine carcinoma is analogous to pulmonary small cell carcinoma
- WHO definition
- Malignant epithelial tumor consisting or small cells with scant cytoplasm, ill defined cell borders, finely granular nuclear chromatin and absent or inconspicuous nucleoi
- Cells are round, oval and spindle shaped
- Nuclear molding is prominent
- Necrosis is typically extensive and the mitotic count is high
Terminology
- Synonyms
- Neuroendocrine tumor (low grade) = carcinoid tumor, atypical carcinoid tumor
- Neuroendocrine carcinoma (high grade) = small cell carcinoma, large cell carcinoma
Epidemiology
- Extremely rare in esophagus: about 100 reported cases (mostly high grade / small cell carcinomas)
- M:F ratio = 3:1 (Am J Surg Pathol 2013;37:467)
- Mean age: 62 years
Sites
- Middle or lower esophagus (Am J Surg Pathol 2013;37:467, Int J Clin Exp Pathol 2013;6:485)
Clinical features
- Usually incidental / unexpected finding on radiologic studies or upper GI endoscopy
- Dysphagia, weight loss, chest pain with high grade carcinoma (Int J Clin Exp Pathol 2013;6:485)
- Typically diagnosed via biopsy or (less commonly) surgical resection
Small cell neuroendocrine carcinoma:
- Very aggressive (Dis Esophagus 2014;27:152, Hepatogastroenterology 2005;52:1738)
- Median survival 18 months (BMC Cancer 2007;7:38)
- 5 year survival 8% or less (Chin Med J (Engl) 2007;120:355, Ann Surg Oncol 2013;20:4239, Hum Pathol 1999;30:216)
Grading
- Grade 1: < 2 mitoses/10 HPF or < 2% Ki67 index
- Grade 2: 2 - 20 mitoses/10 HPF or 3 - 20% Ki67 index
- Grade 3: > 20 mitoses/10 HPF or > 20% Ki67 index (UpToDate: Classification of Neuroendocrine Neoplasms GI Tract [Accessed 27 February 2019], Pancreas 2010;39:707)
Prognostic factors
- Mitotic rate and Ki67 index determine grade
- Low grade lesions have favorable prognosis
- High grade carcinomas are very aggressive, as in other body sites
Case reports
- 51 year old man with collision tumor between papillary adenocarcinoma and large cell neuroendocrine carcinoma (Arch Pathol Lab Med 2000;124:411)
- 54 year old man with atypical carcinoid of the esophagus (Ann Thorac Cardiovasc Surg 2002;8:302)
Small cell neuroendocrine carcinoma:
- 63 year old woman with coexisting paraneoplastic neurological syndrome (Jpn J Clin Oncol 2006;36:109)
- 66 year old man with rare collision tumor of squamous carcinoma and small cell carcinoma in esophagus in separate lymph nodes (J Thorac Dis 2013;5:E203)
- 79 year old man (BMJ Case Rep 2013 Sep 3;2013)
Treatment
- Surgical resection
- Chemoradiation for high grade carcinoma (Rare Tumors 2013;5:e6)
Gross description
- Polypoid or ulcerated mass on upper endoscopy
Gross images
Microscopic (histologic) description
- Well differentiated (low grade) tumors
- Uniform, small, bland tumor cells in solid, trabecular, gyriform or glandular patterns
- May have Paneth cell differentiation
- Poorly differentiated (high grade) carcinomas
- Large cell type: nests of pleomorphic, large cells with prominent nucleoli and a moderate amount of cytoplasm
- Small cell type: sheets and nests of small cells with hyperchromatic nuclei and a minimal to moderate amount of cytoplasm; prominent crush artifact and Azzopardi phenomenon, as in small cell carcinomas at other sites
- Necrosis
- Increased mitotic activity
- Angiolymphatic invasion common
- Solid to cribriform growth
- Usually in lamina propria
- May be associated with heterotopic oxyntic mucosa or Barrett esophagus (large cell carcinoma)
- Neuroendocrine carcinoma may have small component(s) of adenocarcinoma or squamous cell carcinoma differentiation
Microscopic (histologic) images
Cytology description
- Low grade tumors
- Flat sheets or loosely cohesive groups / cords of monotonously uniform plasmacytoid cells
- Eccentric nuclei, coarsely stippled (salt and pepper) chromatin, finely granular cytoplasm
- High grade carcinomas
- Obvious pleomorphism, marked nuclear molding, hyperchromatic nuclei, inconspicuous nucleoli
- Numerous mitoses, crush artifact, necrosis
- Apoptotic figures, blue bodies
Cytology images
Positive stains
- Keratins (e.g., CAM 5.2, AE1 / AE3)
- Neuroendocrine markers: synaptophysin, chromogranin, CD56, NSE
- Ki67 (essential for tumor grading see above)
- TTF1 positive in 71% of esophageal neuroendocrine carcinoma (Am J Surg Pathol 2013;37:467)
Negative stains
Differential diagnosis
- Low grade tumors: glomus tumor, paraganglioma
- High grade carcinomas:
- Metastatic pulmonary neuroendocrine carcinoma: exclude clinically, lymphoma, melanoma, basaloid squamous cell carcinoma
Pill induced esophagitis
Table of Contents
Definition / general | Kayexalate damage | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Pills sticking in esophagus cause local injury, often in mid esophagus (J Clin Gastroenterol 1999;28:298)
- Often doxycycline and other antibiotics, aspirin and NSAIDs, slow release potassium, ferrous salts, alendronate (Fosamax) (Mod Pathol 1999;12:1152)
- Associated with diabetes and ischemic heart disease, advanced age; more common in women (Endoscopy 2005;37:740)
- May rarely cause strictures
- Prevention: take pills when upright and with adequate fluids (Curr Treat Options Gastroenterol 2004;7:71, Drug Saf 2000;22:237)
- Endoscopy: erosions, kissing ulcers, multiple small ulcerations with bleeding, mainly in mid esophagus
Kayexalate damage
- Kayexalate (sodium polystyrene sulfonate) in sorbitol is used to treat hyperkalemia
- Symptoms are from sorbitol, given orally though NG tube or as an enema, may crystallize in esophagus, stomach or duodenum and produce endoscopic ulcers and erosions resembling carcinoma or Candida (Am J Surg Pathol 2001;25:637)
- Colonic infarction and erosions reported
- Crystals are lightly basophilic with a faint crystalline mosaic pattern, better seen with PAS / Alcian blue
- Crystals are refractile but not polarizable, luminal and adherent to intact surface epithelium or mixed with inflammatory exudate in patients with ulcer or erosion
Case reports
- 43 year old man with case resulting from telithromycin (Turk J Gastroenterol 2006;17:113)
- 65 year old man with history of esophageal cancer and end stage renal disease (Case #460)
- 70 year old man with case resulting from rifampin (Ann Pharmacother 1999;33:27)
- 2 cases resulting from alendronate and doxycycline (Rom J Gastroenterol 2005;14:159)
- Woman with case resulting from oral contraceptive Diane 35 (Int J Clin Pract Suppl 2005;147:79)
- Procardia extended release (J Assoc Acad Minor Phys 1997;8:38)
- Tamsulosin for prostatic hypertrophy (Dig Liver Dis 2004;36:632)
Microscopic (histologic) description
- Inflammatory exudate, ulceration, inflamed granulation tissue, prominent eosinophils, necrotic squamous epithelium, polarizable crystalline foreign material, multinucleated giant cells
- Fungi or viral inclusions present in 20% of cases due to alendronate
Microscopic (histologic) images
Additional references
Board review style question #1
Which of the following microscopically identifiable medication materials typically shows a 2 tone appearance with a fish scale pattern on H&E?
- Cholestyramine
- Crospovidone
- Sevelamer
- Sodium polystyrene sulfonate
Board review style answer #1
Radiation esophagitis
Table of Contents
Definition / general | Gross images | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
- Complication of treatment for cancer of lung, mediastinum or esophagus with doses of 30 gray or higher
- Radiation esophagitis is primary dose limiting acute toxicity for radiation therapy of thoracic neoplasms (Semin Radiat Oncol 2004;14:280)
- Use of cytotoxic chemotherapy has an additive effect (World J Gastroenterol 2005;11:2626)
- Acute damage: mucosal necrosis, submucosal edema
- Chronic injury: due to fractionated doses of 60 gray or more; submucosal fibrosis, capillary telangiectasia, thick walled arterial vessels, mucus glandular atrophy, atypical fibroblasts; grossly are ulcers, strictures or fistulas
Gross images
Microscopic (histologic) description
- Variably sized cells in all layers, often increased cytoplasm but usually normal nuclear to cytoplasmic ratio
- Nuclei enlarged with bizarre shapes, may be hyperchromatic or smudged
- May have large eosinophilic nucleoli; usually no mitotic figures
Microscopic (histologic) images
Reflux esophagitis / gastroesophageal reflux disease
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Reflux esophagitis (RE) / gastroesophageal reflux disease (GERD) is the reflux of gastric contents into the esophagus that results in symptoms or complications; reflux esophagitis / gastroesophageal reflux disease is objectively defined as mucosal injury seen at endoscopy or abnormal esophageal acid exposure on a reflux monitoring study
- Remains one of the most common diseases seen by gastroenterologists, surgeons and primary care physicians (Am J Gastroenterol 2022;117:27)
Essential features
- One of the most common diseases in everyday pathology practice
- May affect all ages and is more common in men
- Caused by reflux of gastric contents into the distal esophagus
- Minimum criteria for histologic diagnosis have not been established
- 3 main histologic features are basal cell hyperplasia, elongation of the lamina propria papillae and inflammatory infiltrates
- Mainstay of therapy is proton pump inhibitors
ICD coding
Epidemiology
- Affects an estimated 20% of the United States population
- Prevalence is increasing worldwide
- Males affected more commonly than females
- All ages, including infants and children
- One of the most costly gastrointestinal diseases in the United States, exceeding $14 billion dollars annually
- References: Adv Anat Pathol 2009;16:161, World J Surg 2017;41:1698, Surg Clin North Am 2015;95:515
Sites
- Esophageal mucosa
Pathophysiology
- Lower esophageal sphincter (LES) is a circular muscle layer at the distal esophagus that creates a higher pressure than the intra-abdominal pressure
- Physiologic reflux does occur and is termed transient lower esophageal sphincter relaxations (TLESRs)
- TLESRs are a normal mechanism for the stomach to vent and may be activated by things such as gastric distension
- TLESRs are increased in those with reflux esophagitis / gastroesophageal reflux disease
- Inappropriate relaxation of the LES allows gastric acid to enter the distal esophagus
- Chemoreceptors are stimulated and irritation occurs, causing symptoms
- Symptoms result from mucosal injury and are directly related to frequency of the reflux, duration of reflux and the injurious potential of the refluxate
- Certain drugs may relax the tone of the LES and worsen reflux
- Hiatal hernias are associated with reflux esophagitis / gastroesophageal reflux disease
- Reference: Surg Clin North Am 2015;95:515
Etiology
- Caused by reflux of gastric contents into the lower esophagus
Clinical features
- May be asymptomatic
- Typical symptoms are heartburn and regurgitation
- May have so called extraesophageal symptoms, including hoarseness, cough, odynophagia, reflux induced asthma, laryngitis and aspiration pneumonia
- Atypical or severe symptoms (hematemesis, weight loss, dysphagia) may indicate a stricture or malignancy, endoscopic evaluation is recommended as the first test
- References: Histopathology 2012;60:864, Surg Clin North Am 2015;95:515, Am J Gastroenterol 2022;117:27
Diagnosis
- No gold standard for diagnosis
- Combination of symptoms, endoscopy, pH monitoring and response to therapy
- Classic endoscopic findings include erosions / ulcers, erythema and mucosal edema
- Some have normal or near normal endoscopy with typical reflux symptoms, so called nonerosive reflux disease (NERD)
- References: Adv Anat Pathol 2009;16:161, Am J Gastroenterol 2022;117:27
Case reports
- 34 year old man, a Swiss bagpipe player with chronic cough (Gut 2018;67:1792)
- 35 year old man with drenching night sweats (Can Fam Physician 2020;66:901)
- 54 year old woman with idiopathic pulmonary fibrosis and gastroesophageal reflux disease (Respir Med Case Rep 2016;17:40)
Treatment
- Diet and lifestyle modifications: weight loss for obese patients, elevating the head of the bed, tobacco and alcohol cessation, avoiding late night meals and bedtime snacks, staying upright after eating and not ingesting foods known to make reflux worse (coffee, chocolate, carbonated beverages, spicy foods, acidic foods and fatty foods)
- Proton pump inhibitors (PPIs) are the mainstay of therapy
- Histamine 2 receptor antagonists may be added to PPIs for persistent nocturnal symptoms
- Surgical treatment (e.g., fundoplication) is an option for those with chronic gastroesophageal reflux disease whose symptoms do not respond to medical management or have a defective lower esophageal sphincter (LES) (Goldblum: Rosai and Ackerman’s Surgical Pathology, 11th Edition, 2017)
- Reference: Am J Gastroenterol 2022;117:27
Microscopic (histologic) description
- The 3 major features of reflux esophagitis / gastroesophageal reflux disease are:
- Inflammatory cells in the squamous epithelium
- Elongation of the lamina propria papillae
- Basal cell hyperplasia
- Inflammatory infiltrate may be composed of neutrophils, eosinophils and lymphocytes
- Numerous neutrophils suggest severe injury; eosinophils are seen in 50% of cases
- Lymphocytes are present as squiggle cells, in between the squamous epithelial cells
- Normal basal cell layer is only 1 - 4 cells in thickness and makes up only the bottom 10 - 15% of the epithelial thickness; basal cell hyperplasia is defined as more than 15% of the thickness of the epithelium
- Lamina propria papillae elongation, to a height > two - thirds of the epithelial thickness, is commonly seen in reflux esophagitis / gastroesophageal reflux disease
- Capillaries within the lamina propria papillae may show congestion and red blood cell extravasation
- Distended squamous epithelial cells are nonspecific and termed balloon cells
- Spongiosis is common and may be a marker of early injury without endoscopic findings; this is seen in up to 80% of NERD cases
- Notably, none of these features are specific for reflux esophagitis / gastroesophageal reflux disease
- Minimum criteria for a diagnosis of reflux esophagitis / gastroesophageal reflux disease remain undefined
- References: Adv Anat Pathol 2009;16:161, Histopathology 2012;60:864
Microscopic (histologic) images
Sample pathology report
- Esophagus, biopsy:
- Changes consistent with reflux esophagitis
Differential diagnosis
- Eosinophilic esophagitis:
- More eosinophils, proximal and mid esophagus should be involved
- Must exclude reflux esophagitis / gastroesophageal reflux disease, after trial of high dose PPIs
- Lymphocytic esophagitis:
- Increased intraepithelial lymphocytes
- Uncertain clinical significance
- May be associated with Crohn's disease or achalasia
- Infectious esophagitis if prominent neutrophilic infiltrate or ulcer / erosion:
- Exclude with special stains for fungal or viral causes
- Pill esophagitis:
- May have crystalline polarizable or nonpolarizable material
- Systemic diseases such as collagen vascular disease, Crohn's disease, Stevens-Johnson syndrome, various bullous diseases, lichen planus and graft versus host disease
- Trauma and other injuries:
- Chemotherapy, radiation:
- Telangiectasia, bizarre cells
- Chemotherapy, radiation:
Board review style question #1
A 43 year old man complains of several months of reflux and regurgitation that is worse after eating and while lying down. Upper endoscopy shows mucosal erythema and erosions of the distal esophagus. After esophageal pH monitoring, the patient is diagnosed with gastroesophageal reflux disease. A histologic image of the esophageal biopsy is shown above. What are the 3 main histologic features of reflux esophagitis / gastroesophageal reflux disease?
- Basal cell hyperplasia, elongation of the lamina propria papillae, inflammatory infiltrates
- Basal cell hyperplasia, loss of polarity, > 15 - 20 eosinophils per high power field
- Elongation of the lamina propria papillae, necrosis, increased apoptotic bodies
- Full thickness atypia, inflammatory infiltrates, > 15 - 20 eosinophils per high power field
Board review style answer #1
A. Basal cell hyperplasia, elongation of the lamina propria papillae, inflammatory infiltrates
Comment Here
Reference: Reflux esophagitis / gastroesophageal reflux disease
Comment Here
Reference: Reflux esophagitis / gastroesophageal reflux disease
Board review style question #2
What is the mainstay of therapy for reflux esophagitis / gastroesophageal reflux disease?
- Behavioral modification
- Fundoplication
- Histamine 2 receptor antagonists
- NSAIDs
- Proton pump inhibitors
Board review style answer #2
E. Proton pump inhibitors
Comment Here
Reference: Reflux esophagitis / gastroesophageal reflux disease
Comment Here
Reference: Reflux esophagitis / gastroesophageal reflux disease
Rings and webs
Table of Contents
Definition / general | Diagrams / tables | Case reports | Treatment | Clinical imagesDefinition / general
- Initially described as a constant, symmetrical and diaphragm-like narrowing at the GE junction, associated with a small sliding hiatus hernia (Am J Roentgenol Radium Ther Nucl Med 1953;70:911)
- Now defined as concentric, smooth, thin (3 - 5 mm) protrusions of normal esophageal tissue (with mucosa, submucosa and muscularis propria) into the lumen (eMedicine: Esophageal Webs and Rings [Accessed 8 February 2019], eMedicine: Schatzki Ring [Accessed 8 February 2019], GI Motility Online: Pharyngeal and Esophageal Diverticula, Rings and Webs [Accessed 8 February 2019], Wikipedia: Schatzki Ring [Accessed 8 February 2019])
- Usually in distal esophagus
- May be congenital or a scar from drinking caustic liquids
- Type A: lower muscular ring; rare; thickened circular smooth muscle with overlying squamous mucosa; usually 1.5 cm proximal to squamocolumnar junction, usually asymptomatic (Am J Gastroenterol 2000;95:43)
- Type B: lower mucosal ring / Schatzki ring; 5% of normal esophagi; upper surface is lined by stratified squamous epithelium, undersurface is lined by columnar type epithelium; ring contains connective tissue and fibers of muscularis mucosae; located at squamocolumnar junction at proximal margin of hiatal hernia; associated with meat impaction ("steakhouse syndrome," Surg Endosc 1989;3:195), "swallow syncope" (Dysphagia 2005;20:273) and a reduced incidence of Barrett esophagus (Dig Dis Sci 2004;49:770)
- Type C: rare; refers to indentation on radiographic studies caused by diaphragmatic crura; usually asymptomatic
Case reports
- 16 year old boy with Schatzki ring causing dysphagia (J Postgrad Med 1987;33:99)
- 54 year old man with dysphagia (Dis Chest 1968;54:465)
Treatment
- Dilation, possibly antireflux medical therapy or surgery (Ann Thorac Surg 1984;37:103)
Clinical images
Sarcomatoid carcinoma
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Differential diagnosisDefinition / general
- Squamous cell carcinoma with a variable spindle cell component (WHO definition)
Terminology
- WHO uses the term "spindle cell (squamous) carcinoma"
- Spindle cell carcinoma (Odze) and sarcomatoid carcinoma (Rosai) are also commonly used
- Synonyms include carcinosarcoma, pseudosarcomatous squamous cell carcinoma, polypoid carcinoma, metaplastic carcinoma, squamous cell carcinoma with a spindle cell component, Lane tumor, carcinoma with mesenchymal stroma and pseudosarcoma
Epidemiology
- Uncommon, usually middle aged to elderly men
Sites
- Typically in mid esophagus
Pathophysiology
- In the vast majority of cases the epithelial component is squamous and the lesion is generally regarded as a variant of squamous cell carcinoma
- Very rare examples of spindle cell carcinoma associated with adenocarcinoma are known
- Spindle cell component dominates, has growth advantage over epithelial component, although both evolve from same clone
Clinical features
- Patients generally present with short history of dysphagia, weight loss and pain
Diagnosis
- Endoscopic biopsy or resection
Radiology description
- Large polypoid lesion, typically with narrow pedicle leads to characteristic "cupola" sign
Prognostic factors
- Better survival than typical squamous cell carcinoma, overall survival about 50%
- Tends to present at low stage with intraluminal instead of intramural growth
- 30% have nodal metastases at presentation (usually epithelial or both components)
- Sarcomatous component thought to represent sarcomatous metaplasia of malignant epithelial cells; has malignant potential
Case reports
- 46 year old man whose tumor had extensive osseous differentiation (Indian J Pathol Microbiol 2003;46:49)
- 57 year old man with polypoid tumor (Am J Surg Pathol 1978;2:201)
- 64 year old man with esophageal carcinosarcoma with basaloid squamous carcinoma and rhabdomyosarcoma components with p53 mutation (Pathol Int 2004;54:803)
- Polypoid squamous carcinoma of the esophagus (Am J Surg Pathol 1983;7:495)
- So called pseudosarcoma of the esophagus (Arch Pathol Lab Med 1977;101:604)
- True carcinosarcoma of the esophagus (Dis Esophagus 2006;19:48)
Gross description
- Bulky, exophytic and polypoid mass that grows into lumina, often with short pedicle, mean 6 cm
- Surrounding mucosa is grossly normal
Microscopic (histologic) description
- Biphasic carcinomatous and spindle cell components
- Spindle cells predominate, are elongated with blunt nuclei and atypia resembling undifferentiated pleomorphic sarcoma (MFH)
- May have bizarre giant cells, bone, cartilage, strap cells
- Stroma is edematous with scattered or abundant collagen and mucopolysaccharides
- Epithelial component usually is squamous or basaloid, rarely adenocarcinoma and often is limited with only superficial invasion or in situ disease
- Prominent mitotic activity
- May have neuroendocrine differentiation
- Metastasis may have one or both components
Microscopic (histologic) images
Positive stains
Negative stains
Electron microscopy description
- Dilated cisternae of rough endoplasmic reticulum, peripheral cytoplasmic intermediate filaments
- Also some intermediate type junctions and subplasmalemmal linear densities
- Sarcomatous cells may retain epithelial features including tonofibril bundles or desmosomes or lack epithelial elements and have fibrohistiocytic differentiation (Hum Pathol 1987;18:692)
- Spindle cells may resemble myofibroblasts
Molecular / cytogenetics description
- Derived from single clone (Hum Pathol 2004;35:322), sarcoma component is more often aneuploid than carcinoma component (Hum Pathol 1998;29:863)
Differential diagnosis
- Gastrointestinal stromal tumor
- Leiomyosarcoma
- Melanoma
- Sarcoma: must section extensively to rule out presence of epithelial component
Squamous cell carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- WHO definition: a malignant epithelial tumor displaying squamous cell differentiation characterized by keratinocyte type cells with intercellular bridges or keratinization
Essential features
- Esophageal squamous cell carcinoma (ESCC) is the most common esophageal cancer worldwide
- Distinct from esophageal adenocarcinoma in terms of cell origin, epidemiology, etiology, pathogenesis and location in the esophagus
- Rich lymphovascular network facilitates early tumor spread
- Early diagnosis is critical to improve survival and endoscopic surveillance with biopsy evaluation is the standard of care in high risk groups
ICD coding
Epidemiology
- Incidence worldwide, the most common esophageal cancer
- Great regional and ethnic variation
- Highest incidence regions (> 50 cases/100,000 person years) stretching from Eastern to Central Asia
- Intermediate rates (10 - 50 cases/100,000 person years) observed along the Indian coast of Africa, in southern Brazil and Uruguay and in parts of the Caribbean
- Trending down and less common in the U.S., Europe, Australia and many other Western countries than esophageal adenocarcinoma
- Predominates in Eastern Europe, Japan and South America (Gastroenterology 2018;154:360)
- Age
- Rare before 30 years
- Median age: 65 years
- Sex
- Overall, male predominant
- Ratio varies with the M:F ratio reaching 4:1 in low risk areas (such as U.S.) and the ratio being lower or even exceeding 1:1 in high incidence areas of China and Iran (Gastroenterology 2018;154:360)
- Ethnicity
- In the U.S., 2 - 3 times more common in African Americans than others (Cancer Detect Prev 2008;32:87)
Sites
- Most commonly in middle third of esophagus, followed by lower third and upper third, respectively
Pathophysiology
- Develops by stepwise progression, with accumulating genetic abnormalities driving progression from histologically normal squamous mucosa to low grade intraepithelial neoplasia (dysplasia) to high grade intraepithelial neoplasia and finally to invasive squamous cell carcinoma
- TP53 mutation is a key early driver mutation developing at the stage of intraepithelial neoplasia
- Other key genetic abnormalities, including cell cycle regulators (e.g., CDKN2A [p16], RB1, NFE2L2, CHECK1, CHECK2, CCND1, CKD4, CDK6 and MDM2), EGFR signaling pathway, PI3Kinase signaling pathway, histone modification, NOTCH and WNT pathway, Hedgehog signaling and other genetic changes, including aneuploidy, copy number alterations (Ann N Y Acad Sci 2018;1434:342)
- Specific mutations required for the invasion beyond the basement membrane are still unknown
- Acquisition of invasive and migratory capability via epithelial mesenchymal transition is important; EIF5A2 amplification has been shown to be a factor in inducing this phenotype
Etiology
- Multifactorial and strongly population dependent
- Low socioeconomic status is one of the most consistent factors, even after adjusting other confounding factors (e.g., tobacco use and alcohol consumption)
- Tobacco use
- Remains a key risk factor in developed countries but a weaker effect in developing countries
- Dependent on both exposure duration and intensity, with duration outweighing intensity
- Tobacco specific nitrosamines (TSNA) and polycyclic aromatic hydrocarbons (PAH) are thought to be the major carcinogenic substances in tobacco
- Alcohol beverage
- Alcoholic beverage consumption has been causally linked to ESCC by International Agency for Research on Cancer (IARC)
- Consumption of > 3 drinks per day is associated with a risk almost 5 times that in individuals who consume 1 drink per day
- Acetaldehyde, a metabolite of ethanol, is linked to the increase in risk of ESCC, particularly in Eastern Asian population, which was found to exclusively have ALDH2 and ALDH1A1 mutations leading to accumulation of acetaldehyde following alcohol consumption
- Other risk factors: betel quid, diets low in fruit and vegetables, pickled vegetables, deficiency of selenium or riboflavin, hot foods and beverages, PAH, increased BMI, poor oral health
- Associated medical conditions: Plummer-Vinson syndrome, achalasia, caustic ingestion injury, radiotherapy to the chest area and Fanconi anemia
- Esophageal leukoplakia or epidermoid metaplasia has been associated with esophageal squamous cell carcinoma and dysplasia (Mod Pathol 2014;27:38)
- Genetic factors: tylosis, a keratosis disorder caused by an autosomal dominant mutation in RHBDF2al, 17q25.3, is highly associated with ESCC (> 90% of patients develop ESCC by the age of 65 years)
- HPV infection is unlikely to be a substantial risk factor in the current consensus
- Reference: Gastroenterology 2018;154:360
Clinical features
- Most common symptom is progressive dysphagia and weight loss
- Insidious onset with dysphagia to solids, followed by dysphagia to all food
- Chest pain, painful swallowing and weight loss can also present
- Usually advanced at presentation
- Chronic gastrointestinal blood loss from ESCC is common and may result in iron deficiency anemia
- Hoarseness or cough may occur if the recurrent laryngeal nerve is invaded
- May erode the esophageal wall, causing fistulas; the adjacent respiratory tree, causing pneumonia; the aorta, causing exsanguination or the mediastinum and pericardium
- Horizontal and longitudinal spread are facilitated by rich lymphovascular network
- Lymph node metastases vary by region:
- Upper third: cervical nodes
- Middle third: mediastinal, paratracheal and tracheobronchial node
- Lower third: gastric and celiac nodes
- Most common sites for distant metastasis are the lungs, liver, bones, adrenal glands, kidneys
- References: Goldblum: Rosai and Ackerman's Surgical Pathology, 11th Edition, 2017, UpToDate: Clinical Manifestations, Diagnosis, and Staging of Esophageal Cancer [Accessed 28 October 2021]
Diagnosis
- Upper gastrointestinal tract endoscopy and biopsy for histological analysis, with or without brushings for cytological examination (UpToDate: Clinical Manifestations, Diagnosis, and Staging of Esophageal Cancer [Accessed 28 October 2021])
- If metastases are present, by image guided biopsy of a metastatic site (UpToDate: Clinical Manifestations, Diagnosis, and Staging of Esophageal Cancer [Accessed 28 October 2021])
- CT and fluorodeoxyglucose PET are useful for staging more advanced disease and response after chemoradiation (AJR Am J Roentgenol 2020;215:1072)
Radiology description
- Fluoroscopy / barium swallow
- Irregular stricture
- Prestricture dilatation with hold up
- Shouldering of the stricture
- CT is the best initial modality for detection of distant metastasis, gross direct invasion and enlarged lymph nodes
- Eccentric or circumferential wall thickening > 5 mm
- Periesophageal soft tissue and fat stranding
- Dilated fluid and debris filled esophageal lumen is proximal to an obstructing lesion
- Tracheobronchial invasion appears as a displacement of the airway as a result of mass effect by the esophageal tumor
- Aortic invasion
- Endoscopic ultrasound is the most sensitive modality for assessment of the depth of invasion and regional enlarged lymph nodes
- Approximately 20% of squamous cell carcinomas are superficial and frequently multicentric
- Early lesions: nodule, polyp, plaque
- Advanced tumors: exophytic, ulcerative, infiltrative
- PET has a primary role in the depiction of distant sites of metastatic disease; also can be useful for restaging after the initial neoadjuvant therapy
- References: Radiographics 2009;29:403, AJR Am J Roentgenol 2020;215:1072
Radiology images
Prognostic factors
- Stage is most important; American Joint Committee on Cancer / Union for International Cancer Control (AJCC / UICC) TNM the 8th edition (2017) is the current staging system to predict prognosis (Ann Cardiothorac Surg 2017;6:119)
- Females have better outcomes than males in several studies (Thorac Cancer 2014;5:204)
- Tumor length is an independent predictor of mortality when controlled for depth of invasion in patients with localized disease (Thorac Cancer 2014;5:204, Cancer 2002;95:1434)
- TP53 hotspot mutation p.R213* and TENM3 mutation are reported as independent prognostic factors (Ann Oncol 2018;29:938)
- Pathologic response to therapy is an independent prognostic factor (Eur J Surg Oncol 2017;43:1607)
- Tumor grade and location do not significantly affect patient survival (Thorac Cancer 2014;5:204)
- Overall 5 year survival of all stages combined is 20% according to the Surveillance, Epidemiology and End Results (SEER) database (American Cancer Society: Survival Rates for Esophageal Cancer [Accessed 28 October 2021])
- Early detection when the cancer is localized improves survival to 47%, compared to 5% for patients with distant metastasis (American Cancer Society: Survival Rates for Esophageal Cancer [Accessed 28 October 2021])
Case reports
- 43 year old man with synchronous triple squamous cell carcinoma of the esophagus (Int J Surg Case Rep 2018;49:34)
- 56 year old woman with verrucous carcinoma of the esophagus (Surg Case Rep 2020;6:35)
- 58 year old woman with simultaneous esophageal squamous cell carcinoma and adenocarcinoma (Middle East J Dig Dis 2015;7:257)
- 64 year old man with esophageal squamous cell carcinoma with neuroendocrine, basaloid and ciliated glandular differentiation (Clin J Gastroenterol 2021;14:32)
- 67 year old man with esophageal squamous cell carcinoma arising from esophageal squamous papillomatosis (Int J Surg Case Rep 2020;71:335)
Treatment
- Early lesion (T1) can be managed by endoscopic submucosal dissection or esophagectomy
- Neoadjuvant chemoradiation followed by esophagectomy is widely accepted for advanced lesion (Surg Clin North Am 2019;99:479)
- Definitive chemoradiation is an acceptable alternative in patients who are not surgical candidates or patients who wish to avoid surgery (Surg Clin North Am 2019;99:479)
- Although salvage therapy is a viable option, longterm outcomes are unclear but it can be considered in select patients (Surg Clin North Am 2019;99:479)
- By integrating molecular profiling as part of the management of patients with esophageal cancer, future treatment guidelines will provide a more personalized and effective approach to this disease
Clinical images
Gross description
- Fungating / exophytic / polypoid lesions
- Ulcerative or infiltrative (intramural causing thick, rigid esophageal wall with luminal narrowing, linitis plastica pattern and only minor mucosal defect, associated with stricture)
- References: Cancer 1983;51:2139, Goldblum: Rosai and Ackerman's Surgical Pathology, 11th Edition, 2017
Gross images
Microscopic (histologic) description
- Essential diagnostic criteria: histological evidence of vertical and horizontal growth of neoplastic squamous epithelium, with definite evidence of invasion
- Staging (TNM) (J Thorac Oncol 2017;12:36)
- Should be staged using the 8th editions (2017) of the UICC / AJCC cancer staging manual
- Depth of invasion provides a clinically relevant division of esophageal squamous cell carcinoma into superficial (or early) disease versus advanced (or late) disease
- Superficial disease is invasion restricted to the mucosa and submucosa; it has a low risk of regional lymph node metastasis
- Advanced disease is invasion beyond the muscularis propria, with a high risk of regional or systemic metastasis
- Grading is based on the degree of cytological atypia, mitotic activity and presence of keratinization
- Grade 1 (well differentiated):
- Contains enlarged cells with abundant eosinophilic cytoplasm and keratinization
- Cytological atypia is minimal and the mitotic rate is low
- Invasive margin is pushing and the cells remain well ordered
- Grade 2 (moderately differentiated):
- Has evident cytological atypia and the cells are less ordered
- Mitotic figures are easily identified
- Grade 3 (poorly differentiated):
- Consists predominantly of basal-like cells forming nests with or without central necrosis
- Grade 1 (well differentiated):
- Treatment effect often involved in both tumor cells and the peritumor stroma
- Cellular changes include nuclear enlargement or shrinkage, nuclear vacuolation, apoptosis and necrosis
- Neutrophilic or chronic inflammatory cell response may be seen
- There is fibrosis and sometimes stromal elastosis
- Subtypes:
- Verrucous squamous cell carcinoma: usually at the gastroesophageal junction, associated with reflux; shows a very well differentiated histology with a pushing border and surface papillary projection and metastases are uncommon
- Spindle cell squamous cell carcinoma: biphasic morphology with conventional squamous cell carcinoma and a high grade spindle cell component with heterogeneous differentiation
- Basaloid squamous cell carcinoma: shows a solid or nested growth pattern of basaloid cells, sometimes with central comedonecrosis and occasionally with pseudoglandular cribriform formations
Microscopic (histologic) images
Contributed by Xiaoqin Liu, M.D., Ph.D. and Aaron R. Huber, D.O.
Cytology description
- Nuclear membrane alterations, nuclear hyperchromasia and enlargement, loss of nuclear polarity, prominent nucleoli
Positive stains
- Immunohistochemistry is useful in confirming poorly differentiated and spindle cell squamous cell carcinoma
- Pancytokeratin, CK5/6, p63, p40
Negative stains
- Negative Kreyberg or mucicarmine stain can help to rule out poorly differentiated adenocarcinoma
- Negative synaptophysin and chromogranin stains can help to rule out neuroendocrine carcinoma
Molecular / cytogenetics description
- At present, no molecular tests are required
- TP53 mutation found in 59 - 93% of all cases
- NOTCH1 and NOTCH3 mutations detected in up to 28% of cases
- EGFR overexpression reported in 59.6 - 76% of cases
- Mutations or amplification in RAS and AKT family seen in at least 75% of cases
- Genetic alterations involved in cell cycle regulation, epigenetic factors, copy number alterations, chromosome aneuploidy (in particular, gains in chromosome 3, 10, 12 and 20) were reported
- Associated with tylosis and Fanconi anemia
Sample pathology report
- Esophagus, at 35 to 28 cm, biopsy:
- Invasive moderately differentiated squamous cell carcinoma (see comment)
- Comment: Immunohistochemical / special stains were performed and show the tumor is positive for p40 and negative for Kreyberg for intracellular mucin. The morphology and immunoprofile are consistent with squamous cell carcinoma.
Differential diagnosis
- Pseudoepitheliomatous hyperplasia:
- Lacks paradoxical maturation
- Intact basal layer
- High grade neuroendocrine carcinoma:
- Basaloid variant can resemble this entity
- Synaptophysin, chromogranin, CD56, INSM1 can be helpful
- Sarcomas and spindle cell melanoma:
- Immunohistochemistry for S100 for melanoma
- CD117 for gastrointestinal stromal tumor
- Squamous cell carcinoma from other sites:
- From lung with direct extension into esophagus
- Cannot be differentiated based on morphology or ancillary test
- Clinical history or imaging can be helpful
- Atypical regenerative hyperplasia in biopsy:
- No stromal infiltration
- Postradiation chemotherapy changes:
- Usually monomorphic and background has inflammation and granulation tissue
- Reactive changes in ulcer beds:
- Atypical mesenchymal cells in biopsy specimens are pleomorphic and hyperchromatic but usually not mitotically active, are cytokeratin negative
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
What is the most important prognostic factor for esophageal squamous cell carcinoma?
- Tumor grade
- Tumor length
- Tumor location
- Tumor stage
Board review style answer #2
Squamous dysplasia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Neoplastic alteration of squamous esophageal mucosa without invasion
- Precursor lesion to squamous cell carcinoma (SCC)
- Low grade dysplasia is characterized by atypia limited to the lower half of the epithelium while high grade dysplasia is characterized by atypia involving greater than half of the epithelium
Essential features
- Precursor lesion to squamous cell carcinoma
- Occurs predominantly in the middle third of the esophagus
- Often encountered at the periphery of invasive squamous cell carcinoma
- Comprises both architectural and cytologic abnormalities
Terminology
- Squamous intraepithelial neoplasia, squamous carcinoma in situ
ICD coding
- ICD-O
- ICD-11
- 2E92.0 & XH3Y37 - benign neoplasm of esophagus & esophageal squamous intraepithelial neoplasia (dysplasia), low grade
- 2E60.1 & XH9ND8 - carcinoma in situ of esophagus & esophageal squamous intraepithelial neoplasia (dysplasia), high grade
Epidemiology
- More prevalent in certain geographic regions, with a higher incidence in parts of Asia (including China and Iran) and parts of Africa
- Risk is highest in North Central China where 25% of adults ≥ 35 years of age are found to have the lesion (Cancer Epidemiol Biomarkers Prev 2013;22:540)
- Diets rich in nitrates and nitrosamines may be predisposing factor
- U.S. is a low risk area for both squamous dysplasia and SCC
- In the U.S., squamous dysplasia is more common in Black patients than in White patients
- Mostly seen in heavy alcohol users and smokers (Int J Clin Oncol 2010;15:135)
Sites
- Can occur anywhere in the esophagus but more common in mid esophagus (Gastroenterol Rep 2017;5:247)
Etiology
- Environmental exposures, predisposing conditions and genetic factors interplay in the pathogenesis of squamous dysplasia; risk factors are similar to those of SCC (Gastroenterology 2018;154:360)
- Substantial alcohol use and smoking
- Chronic esophageal irritation associated with conditions such as achalasia, esophageal diverticula, gastroesophageal reflux disease (GERD), Plummer-Vinson syndrome and chronic strictures
- Low socioeconomic status
- Dietary factors and drinking very hot beverages
- Increased body mass index
- Male sex
- Genetic conditions
- Nonepidermolytic palmoplantar keratoderma (tylosis), a rare autosomal dominant disorder with hyperkeratosis of palms and soles, confers a > 90% risk of development of SCC (Orphanet J Rare Dis 2015;10:126)
- Epidermoid metaplasia, also termed esophageal leukoplakia, is thought to represent a precursor lesion to squamous dysplasia
- Magnitude of risk is not known, although targeted next generation sequencing supports that epidermoid metaplasia shares molecular signatures with squamous dysplasia and squamous cell carcinoma (Mod Pathol 2017;30:1613)
- Current consensus is that human papillomavirus (HPV) is unlikely to be a substantial risk factor (Gastroenterology 2018;154:360)
Clinical features
- Patients may present with dysphagia or be asymptomatic
- Endoscopically, dysplasia is usually flat and requires enhancing techniques such as methylene blue or Lugol iodine with chromoendoscopy or narrow band imaging (Ann Gastroenterol 2015;28:41)
Diagnosis
- Endoscopic biopsy with histologic examination
Prognostic factors
- Patients with high grade dysplasia have a greater risk of progressing to SCC
- In a study of Chinese patients over a period of 3.5 years, 5% of patients with mild dysplasia progressed to SCC versus 65% with severe dysplasia (Cancer 1994;74:1686)
Case reports
- 62 year old man with liver cirrhosis (Transl Cancer Res 2022;11:2433)
- 62 year old man with upper gastrointestinal (GI) bleed (Rev Esp Enferm Dig 2023;115:194)
- 76 year old woman with a past medical history of GERD with esophagitis (Am J Gastroenterol 2020;115:S962)
- 77 year old woman with history of achalasia (Cureus 2022;14:e23735)
Treatment
- Endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) is the definitive treatment with endoscopic surveillance for recurrence or progression
- Alternatively, for superficial dysplasia, radiofrequency ablation can be performed (Gastrointest Endosc 2017;85:322, Gastrointest Endosc 2017;85:330)
Clinical images
Gross description
- Erythema, nodular or irregular mucosa, erosive appearance or plaque-like white patches
- Discolored, granular mucosa
Microscopic (histologic) description
- Nuclear enlargement and irregularity, anisonucleosis and nuclear pleomorphism
- Hyperchromasia with irregular nuclear contours
- Increased N:C ratio
- Increased mitotic activity
- Abnormal epithelial maturation with increased cellularity of superficial mucosa
- 2 tiered microscopic grading system
- Low grade: involvement of the lower half of the epithelium only, with mild atypia
- High grade: neoplastic cells occupy > 50% of epithelial thickness or severe cytologic atypia
- Reference: Mod Pathol 2017;30:S112
Microscopic (histologic) images
Positive stains
- p53 mutant pattern, especially in cases of high grade dysplasia (77 - 93% of high grade dysplasia cases) (Dig Dis 2023;41:685, Hum Pathol 2011;42:1430)
- Increased Ki67 proliferation (Hum Pathol 2011;42:1430)
Molecular / cytogenetics description
- Overexpression of p53 and hypermethylation of CDKN2A (Cancer Epidemiol Biomarkers Prev 2013;22:540)
Videos
Squamous dysplasia screening and treatment
Sample pathology report
- Esophagus, endoscopic biopsy:
- High grade squamous dysplasia
- Esophagus, endoscopic mucosal resection:
- High grade squamous dysplasia
- Margins are negative for dysplasia
Differential diagnosis
- Reparative epithelial changes or pseudoepitheliomatous hyperplasia:
- Prominent nucleoli and accompanying inflammatory process suggests a reactive process
- Benign hyperplasia of the epithelial rete ridges that mimics dysplasia but lacks cytological atypia
- Squamous papilloma:
- Endoscopically present as a papillary wart-like growth
- Histologically characterized by papillary hyperplasia of the squamous layer without dysplasia
- Microinvasive squamous cell carcinoma:
- More atypia and pleomorphism
- Invasion beyond basement membrane
Additional references
Board review style question #1
Board review style answer #1
A. Epidermoid metaplasia. Epidermoid metaplasia (leukoplakia) is thought to represent a precursor lesion to esophageal squamous dysplasia, although the magnitude of risk is unknown. Answer B is incorrect because intestinal metaplasia (i.e., Barrett esophagus) is a precursor for glandular dysplasia in the esophagus. Answer C is incorrect because pancreatic acinar metaplasia is usually an incidental finding seen at the gastroesophageal (GE) junction with no known neoplastic risk. Answer D is incorrect pyloric gland metaplasia is usually seen in the intestine and not in the esophagus.
Comment Here
Reference: Squamous dysplasia
Comment Here
Reference: Squamous dysplasia
Board review style question #2
The image above shows an endoscopic biopsy of a lesion noted as a white plaque that did not stain with Lugol iodine in the middle third of the esophagus. What is your diagnosis?
- Barrett esophagus with dysplasia
- High grade squamous dysplasia
- Low grade squamous dysplasia
- Reactive atypia, negative for dysplasia
Board review style answer #2
B. High grade squamous dysplasia. A full thickness high grade squamous esophageal dysplasia with cytologic atypia is seen on the right side of the image with sharp demarcation from the normal nondysplastic squamous epithelium on the left. Answer C is incorrect because low grade dysplasia encompasses less than half of the mucosa. Answer A is incorrect because Barrett esophagus or intestinal metaplasia, which by definition is columnar epithelium with or without goblet cells that extends ≥ 1 cm proximal to the gastroesophageal junction, is commonly seen in distal esophagus and gastroesophageal (GE) junction. Answer D is incorrect because the full thickness atypia present exceeds what would be expected for reactive atypia.
Comment Here
Reference: Squamous dysplasia
Comment Here
Reference: Squamous dysplasia
Squamous papilloma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign esophageal epithelial polyp composed of squamous epithelium with a papillary growth pattern
Essential features
- Papillary proliferation of nondysplastic squamous epithelium with fibrovascular cores of lamina propria
- Etiologic factors include irritation (alcohol consumption, smoking and reflux esophagitis, metal stent and chronic food impaction), infection (HPV infection) and genetic syndromes (Goltz-Gorlin syndrome and angioma serpiginosum)
- Human papilloma virus infection rate: 10.5 - 57%
- Often seen in the distal esophagus in the U.S. (58.3 - 70%) and the middle esophagus in Asian countries (52.6 [Japan] - 57.5% [Taiwan])
Terminology
- Squamous cell papilloma
ICD coding
- ICD-O: 8052/0 - squamous cell papilloma, NOS
Epidemiology
- Incidence: 0.20 - 0.42% (World J Clin Cases 2017;5:134, World J Gastroenterol 2016;22:2349, Acta Histochem Cytochem 2006;39:23 )
- Average age: 48.9 - 59.2 years (World J Clin Cases 2017;5:134, Dis Esophagus 2017;30:1, World J Gastroenterol 2016;22:2349, Acta Histochem Cytochem 2006;39:23, Am J Surg Pathol 1993;17:803)
- Reported male to female ratio varies from roughly 5:2 to 1:5 (World J Gastroenterol 2016;22:2349, Am J Surg Pathol 1993;17:803)
- Average size: 3.8 - 5.0 mm (World J Clin Cases 2017;5:134, World J Gastroenterol 2016;22:2349, Acta Histochem Cytochem 2006;39:23, Am J Surg Pathol 1993;17:803)
- Most cases solitary (80 - 92%) (Dis Esophagus 2017;30:1, World J Gastroenterol 2016;22:2349, Acta Histochem Cytochem 2006;39:23, Am J Surg Pathol 1993;17:803)
Sites
- Distal esophagus in the U.S. (58.3 - 70%) (Am J Surg Pathol 1993;17:803, Dis Esophagus 2017;30:1)
- Middle esophagus in Asian countries (52.6 [Japan] - 57.5% [Taiwan]) (Acta Histochem Cytochem 2006;39:23, World J Gastroenterol 2016;22:2349)
Pathophysiology
- Chronic mucosal irritation or HPV infection followed by hyperregenerative response / proliferation of squamous epithelium
Etiology
- Hyperplasia of the squamous epithelium due to chemical irritation (alcohol consumption, smoking and reflux esophagitis), mechanical irritation (metal stent and chronic food impaction), infection (HPV infection), genetic syndromes (Goltz-Gorlin syndrome and angioma serpiginosum) (World J Gastroenterol 2017;23:2246, Eur J Hum Genet 2007;15:543, Am J Surg Pathol 1993;17:803, Acta Histochem Cytochem 2006;39:23)
- Most papillomas in the lower esophagus are associated with gastroesophageal reflux disease (GERD) (Am J Surg Pathol 1993;17:803)
- Human papilloma virus infection rate varies (10.5 - 57%) (Am J Surg Pathol 1993;17:803, Acta Histochem Cytochem 2006;39:23, Dis Esophagus 2017;30:1)
Clinical features
- Usually asymptomatic
- Rarely, dysphasia and heartburn (Clin Gastroenterol Hepatol 2016;14:A21)
- Incidentally found small warty polypoid lesion in the esophagus
Diagnosis
- Incidentally found on endoscopy
- Confirmed on biopsy
Prognostic factors
- No known prognostic factors (progression to dysplasia or carcinoma is extremely rare)
Case reports
- 3 year old girl with history of Goltz syndrome presented with esophageal squamous papillomatosis (J Pediatr Gastroenterol Nutr 2018;66:e132)
- 42 year old man with multiple esophageal squamous papillomas (ACG Case Rep J 2019;6:e00180)
- 44 year old woman with squamous cell carcinoma in situ arising from esophageal squamous papilloma (Clin Endosc 2019;52:72)
- 64 year old man with history of Cronkhite-Canada syndrome presented with papilloma-like lesions of the esophagus (Dig Liver Dis 2020;52:352)
- 65 year old woman with carcinoma cuniculatum initially misdiagnosed as benign squamous papilloma (Endoscopy 2014;46:E531)
Treatment
- Endoscopic resection / polypectomy
- Ablation by radiofrequency (World J Gastrointest Endosc 2015;7:290)
- Cryotherapy (ACG Case Rep J 2019;6:1)
Clinical images
Gross description
- Small (usually < 5 mm), sessile, whitish, warty polypoid lesion
Microscopic (histologic) description
- Papillary proliferation of nondysplastic squamous epithelium with fibrovascular cores of lamina propria
- Koilocytes can be seen
- Most commonly, exophytic but rarely, endophytic and tentacular type proliferation (Mayo Clin Proc 2019;94:1551)
- No dysplasia is seen (progression to dysplasia or carcinoma is extremely rare)
Microscopic (histologic) images
Sample pathology report
- Esophagus, mid, polyp, biopsy:
- Squamous papilloma (see comment)
- Comment: It is negative for dysplasia or carcinoma.
Differential diagnosis
- Verrucous carcinoma / carcinoma cuniculatum (Endoscopy 2014;46:E531):
- Usually large mass
- Hyperkeratosis, dyskeratosis and nuclear atypia in addition to papillary growth and acanthosis
- Squamous cell carcinoma:
- Cytological and architectural atypia
- Glycogenic acanthosis:
- Acanthosis with clear cytoplasm
- No papillary proliferation
- Epidermoid metaplasia / epidermization:
- Occasionally, shows papillary proliferation but usually with prominent granular layer and orthokeratosis
Additional references
Board review style question #1
Board review style answer #1
A. Koilocytes can be seen in squamous papilloma of the esophagus.
Comment Here
Reference: Squamous papilloma
Comment Here
Reference: Squamous papilloma
Board review style question #2
Which of the following is true about squamous papilloma of the esophagus?
- Almost all squamous papillomas of the esophagus are associated with human papilloma virus infection
- Gastroesophageal reflux disease is one of the etiologic factors
- Most patients are young women with human papilloma virus infection
- Usually it is seen in the cervical esophagus
Board review style answer #2
B. Gastroesophageal reflux disease is one of the etiologic factors.
Comment Here
Reference: Squamous papilloma
Comment Here
Reference: Squamous papilloma
Staging
Table of Contents
Definition / general | Essential features | ICD coding | Primary tumor (pT) | Regional lymph nodes (pN) | Distant metastasis (pM) | Location (squamous cell carcinoma only) | Prefixes | Stage grouping | Registry data collection variables | Histologic grade | Histopathologic type | Residual tumor | Diagrams / tables | Additional references | Board review style question #1 | Board review style answer #1Pathologic TNM staging of carcinomas of the esophagus, AJCC 8th edition
Definition / general
- All carcinomas of the esophagus, including poorly differentiated neuroendocrine carcinomas, are covered by this staging system
- Esophagus cancers are defined as having an epicenter within the esophagus, within the gastroesophageal junction or no more than 2 cm into the proximal stomach
- Well differentiated neuroendocrine tumors of the true esophagus are extremely rare and do not have a separate staging system, unlike other gastrointestinal organs
- Well differentiated neuroendocrine tumors of the gastroesophageal junction and cardia are staged using the stomach neuroendocrine system
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory (Amin: AJCC Cancer Staging Manual, 8th Edition, 2018)
ICD coding
- C15.9: Malignant neoplasm of esophagus, unspecified
Primary tumor (pT)
Regional lymph nodes (pN)
- NX: Regional lymph nodes cannot be assessed
- N0: No regional lymph node metastasis
- N1: Metastasis in 1 or 2 regional lymph nodes
- N2: Metastasis in 3 - 6 regional lymph nodes
- N3: Metastasis in 7+ regional lymph nodes
- Notes: regional lymph nodes include lower cervical paratracheal, upper / lower paratracheal, subcarinal, upper / middle / lower thoracic paraesophageal, pulmonary ligament, diaphragmatic, paradarcial, left gastric, common hepatic, splenic, celiac and cervical periesophageal level VI / VII nodes
Distant metastasis (pM)
- M0: No distant metastasis
- M1: Distant metastasis
Location (squamous cell carcinoma only)
- X: Location unknown
- Upper: Cervical esophagus to lower border of azygous vein
- Middle: Lower border of azygous vein to lower border of inferior pulmonary vein
- Lower: Lower border of inferior pulmonary vein to stomach, including gastroesophageal junction
Prefixes
- y: Preoperative radiotherapy or chemotherapy
- r: Recurrent tumor stage
- m: Multiple / skip lesions
Stage grouping
| Squamous cell carcinoma, clinical (cTNM) | |||
| Stage 0: | cTis | cN0 | cM0 |
| Stage I: | cT1 | cN0 - 1 | cM0 |
| Stage II: | cT2 | cN0 - 1 | cM0 |
| cT3 | cN0 | cM0 | |
| Stage III: | cT3 | cN1 | cM0 |
| cT1 - 3 | cN2 | cM0 | |
| Stage IVA: | cT4 | cN0 - 2 | cM0 |
| any cT | cN3 | cM0 | |
| Stage IVB: | any cT | any cN | cM1 |
| Squamous cell carcinoma, pathological (pTNM) | |||||
| Stage 0: | pTis | pN0 | pM0 | G N/A | any location |
| Stage IA: | pT1a | pN0 | pM0 | G1, GX | any location |
| Stage IB: | pT1a | pN0 | pM0 | G2 - 3 | any location |
| pT1b | pN0 | pM0 | G1 - 3, GX | any location | |
| pT2 | pN0 | pM0 | G1 | any location | |
| Stage IIA: | pT2 | pN0 | pM0 | G2 - 3, GX | any location |
| pT3 | pN0 | pM0 | any G | lower | |
| pT3 | pN0 | pM0 | G1 | upper / middle | |
| Stage IIB: | pT3 | pN0 | pM0 | G2 - 3 | upper / middle |
| pT3 | pN0 | pM0 | GX | any location | |
| pT3 | pN0 | pM0 | any G | location X | |
| pT1 | pN1 | pM0 | any G | any location | |
| Stage IIIA: | pT1 | pN2 | pM0 | any G | any location |
| pT2 | pN1 | pM0 | any G | any location | |
| Stage IIIB: | pT2 | pN2 | pM0 | any G | any location |
| pT3 | pN1 - 2 | pM0 | any G | any location | |
| pT4a | pN0 - 1 | pM0 | any G | any location | |
| Stage IVA: | pT4a | pN2 | pM0 | any G | any location |
| pT4b | pN0 - 2 | pM0 | any G | any location | |
| any pT | pN3 | pM0 | any G | any location | |
| Stage IVB: | any pT | any pN | pM1 | any G | any location |
Squamous cell carcinoma, postneoadjuvant therapy (ypTNM)
| Stage I: | ypT0 - 2 | ypN0 | ypM0 |
| Stage II: | ypT3 | ypN0 | ypM0 |
| Stage IIIA: | ypT0 - 2 | ypN1 | ypM0 |
| Stage IIIB: | ypT3 | ypN1 | ypM0 |
| ypT0 - 3 | ypN2 | ypM0 | |
| ypT4a | ypN0 | ypM0 | |
| Stage IVA: | ypT4a | ypN1 - 2, X | ypM0 |
| ypT4b | ypN0 - 2 | ypM0 | |
| any ypT | ypN3 | ypM0 | |
| Stage IVB: | any ypT | any ypN | ypM1 |
| Adenocarcinoma, clinical (cTNM) | |||
| Stage 0: | cTis | cN0 | cM0 |
| Stage I: | cT1 | cN0 | cM0 |
| Stage IIA: | cT1 | cN1 | cM0 |
| Stage IIB: | cT2 | cN0 | cM0 |
| Stage III: | cT2 | cN1 | cM0 |
| cT3 | cN0 - 1 | cM0 | |
| cT4a | cN0 - 1 | cM0 | |
| Stage IVA: | cT1 - 4a | cN2 | cM0 |
| cT4b | cN0 - 2 | cM0 | |
| any cT | cN3 | cM0 | |
| Stage IVB: | any cT | any cN | cM1 |
| Adenocarcinoma, pathological (pTNM) | ||||
| Stage 0: | pTis | pN0 | pM0 | G N/A |
| Stage IA: | pT1a | pN0 | pM0 | G1, GX |
| Stage IB: | pT1a | pN0 | pM0 | G2 |
| pT1b | pN0 | pM0 | G1 - 2, GX | |
| Stage IC: | pT1 | pN0 | pM0 | G3 |
| pT2 | pN0 | pM0 | G1 - 2 | |
| Stage IIA: | pT2 | pN0 | pM0 | G3, GX |
| Stage IIB: | pT1 | pN1 | pM0 | any G |
| pT3 | pN0 | pM0 | any G | |
| Stage IIIA: | pT1 | pN2 | pM0 | any G |
| pT2 | pN1 | pM0 | any G | |
| Stage IIIB: | pT2 | pN2 | pM0 | any G |
| pT3 | pN1 - 2 | pM0 | any G | |
| pT4a | pN0 - 1 | pM0 | any G | |
| Stage IVA: | pT4a | pN2 | pM0 | any G |
| pT4b | pN0 - 2 | pM0 | any G | |
| any pT | pN3 | pM0 | any G | |
| Stage IVB: | any pT | any pN | pM1 | any G |
| Adenocarcinoma, postneoadjuvant therapy (ypTNM) (same as for squamous cell carcinoma) | |||
| Stage I: | ypT0 - 2 | ypN0 | ypM0 |
| Stage II: | ypT3 | ypN0 | ypM0 |
| Stage IIIA: | ypT0 - 2 | ypN1 | ypM0 |
| Stage IIIB: | ypT3 | ypN1 | ypM0 |
| ypT0 - 3 | ypN2 | ypM0 | |
| ypT4a | ypN0 | ypM0 | |
| Stage IVA: | ypT4a | ypN1 - 2, X | ypM0 |
| ypT4b | ypN0 - 2 | ypM0 | |
| any ypT | ypN3 | ypM0 | |
| Stage IVB: | any ypT | any ypN | ypM1 |
Registry data collection variables
- Squamous cell carcinoma:
- Clinical staging modalities (endoscopy and biopsy, EUS, EUS-FNA, CT, PET / CT)
- Tumor length
- Depth of invasion
- Number of nodes involved, clinical
- Number of nodes involved, pathological
- Location of nodal disease, clinical
- Location of nodal disease, pathological
- Sites of metastases, if applicable
- Presence of skip lesions: T(m)
- Perineural invasion
- LVI (lymphatic, vascular, both)
- Extranodal extension
- Type of surgery
- Chemotherapy
- Chemoradiation therapy (for ypTNM)
- Surgical margin (negative, microscopic, macroscopic)
- Adenocarcinoma:
- Clinical staging modalities (endoscopy and biopsy, EUS, EUS-FNA, CT, PET / CT)
- Tumor length
- Depth of invasion
- Number of nodes involved, clinical
- Number of nodes involved, pathological
- Location of nodal disease, clinical
- Location of nodal disease, pathological
- Sites of metastases, if applicable
- Presence of skip lesions: T(m)
- Perineural invasion
- LVI (lymphatic, vascular, both)
- Extranodal extension
- HER2 status (positive or negative)
- Type of surgery
- Chemotherapy
- Chemoradiation therapy (for ypTNM)
- Surgical margin (negative, microscopic, macroscopic)
Histologic grade
- GX: Grade not available
- G1: Well differentiated
- G2: Moderately differentiated
- G3: Poorly differentiated
Histopathologic type
- Squamous:
- Squamous cell carcinoma
- Basaloid squamous cell carcinomas
- Adenosquamous carcinoma
- Spindle cell (squamous) carcinoma
- Verrucous (squamous) carcinoma
- Undifferentiated carcinoma with squamous component
- Adenocarcinoma:
- Adenocarcinoma
- Adenoid cystic carcinoma
- Mucoepidermoid carcinoma
- Mixed adenoneuroendocrine carcinoma
- Undifferentiated carcinoma with glandular component
- Other:
- Neuroendocrine tumor, G1
- Neuroendocrine tumor, G2
- Neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Small cell neuroendocrine carcinoma
Residual tumor
- R0: No evidence of residual tumor
- R1: Microscopic tumor at margins (including at inked radial margin)
- R2: Macroscopically visible tumor at margins
Diagrams / tables
Additional references
Board review style question #1
Which of the following variables has no impact on staging esophageal squamous cell carcinoma?
- Histologic grade
- Margin status
- Neoadjuvant therapy use
- Tumor location within esophagus
Board review style answer #1
Undifferentiated carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Rare and aggressive esophageal carcinoma
- Histologically characterized by sheets of cells with high grade features, frequent rhabdoid appearance and monotony
Essential features
- Neoplastic cells appear high grade with frequent rhabdoid appearance
- Background Barrett esophagus with or without dysplasia may be identified
- May have limited or absent expression of pancytokeratins
- Characterized by frequent loss of SMARCA4 or SMARCA2
- Dismal prognosis with frequent recurrence or metastasis
ICD coding
Epidemiology
- M > F
- Median age in seventh decade
- Likely related to Barrett esophagus associated adenocarcinomas
- Very rare: as low as 0.15% of esophageal carcinomas (Dis Esophagus 2009;22:1)
Sites
- Distal esophagus
- Gastroesophageal junction
Pathophysiology
- Likely dedifferentiation / high grade progression of adenocarcinoma
- Subset of cases has associated Barrett esophagus and Barrett associated dysplasia
- Mutant p53 pattern by immunohistochemistry
- Frequent loss of SMARCA4 or SMARCA2 by immunohistochemistry
Etiology
- Same as Barrett esophagus associated adenocarcinoma
Clinical features
- Presentation similar to other esophageal cancers
- Progressive dysphagia
- Symptoms of gastroesophageal reflux disease
- Unexplained weight loss (Hum Pathol 2015;46:366)
- Typically locally advanced at diagnosis
Diagnosis
- Seen as exophytic mass on esophagogastroduodenoscopy
- Confirmed by histology and immunohistochemistry
Prognostic factors
- Poor prognosis overall: currently 20% 1 year survival rate
- As many as 67% will have local recurrence or metastasis (Hum Pathol 2015;46:366)
Case reports
- 67 year old man with ulcerative mass of gastroesophageal junction (Surg Case Rep 2019;5:8)
- 71 year old woman with mass of middle esophagus (Int J Clin Exp Pathol 2020;13:1902)
- 79 year old man with EBV infection (Int J Clin Exp Med 2013;6:219)
Treatment
- Neoadjuvant chemotherapy followed by esophagogastrectomy
- Palliative care
Gross description
- Exophytic mass
- Raised edges
- Central ulceration
- Pushing border
Microscopic (histologic) description
- Nests and sheets of cells
- Cytology is high grade with large nuclei and high nucleus to cytoplasm ratio
- Can have rhabdoid features
- Sheets of necrosis
- Lymphoepithelioma-like carcinoma a distinct subtype
- Sheets of epithelioid cells surrounded by dense lymphoplasmacytic inflammatory infiltrate
- Absence of identifiable signs of differentiation (adenocarcinoma, squamous cell carcinoma, neuroendocrine carcinoma)
Microscopic (histologic) images
Positive stains
- Pancytokeratin: variable expression, ranging from diffuse to patchy or negative (Hum Pathol 2015;46:366, Am J Surg Pathol 2021;45:414)
- p53 can display mutant pattern of expression (either diffusely strong positive or total loss) (Am J Surg Pathol 2021;45:414)
- EBV positivity can be seen in lymphoepithelioma-like variant
Negative stains
- SMARCA4 (BRG1), SMARCA2 (BRM) or SMARCB1 (INI1) aberrant loss (Dis Esophagus 2009;22:1, Am J Surg Pathol 2021;45:414)
Sample pathology report
- Esophagus, biopsy:
- Undifferentiated carcinoma (see comment)
- Comment: SMARCA4 loss is seen by immunohistochemistry.
Differential diagnosis
- Poorly differentiated neuroendocrine carcinoma:
- Potentially also with nested / sheet-like architecture but would express synaptophysin and chromogranin
- Poorly differentiated squamous cell carcinoma:
- Stains for squamous markers (e.g. p40)
- More consistent pattern of cytokeratin positivity
- Malignant melanoma:
- Can also show nested architecture but would express melanocytic markers such as MelanA
- Hematolymphoid malignancy:
- Also would be negative for pancytokeratin but would express hematolymphoid markers (e.g. CD34 / LCA)
Additional references
Board review style question #1
A patient undergoes esophagogastrectomy for a tumor of the distal esophagus. A representative hematoxylin and eosin photomicrograph of the lesion is shown. The tumor lacks any immunohistochemical signs of differentiation, including negativity for pancytokeratin, MelanA, synaptophysin, chromogranin and CD34. An esophageal carcinoma of this subtype is characterized by what immunophenotype?
- Keratin 7 expression
- Wild type p53
- Loss of SMARCA4 or SMARCA2
- Expression of Rb
Board review style answer #1
C. Loss of SMARCA4 or SMARCA2. This is an undifferentiated carcinoma of the esophagus.
Comment Here
Reference: Undifferentiated carcinoma
Comment Here
Reference: Undifferentiated carcinoma
Varices
Table of Contents
Definition / general | Clinical features | Treatment | Gross description | Gross images | Microscopic (histologic) images | Differential diagnosis hematemesisDefinition / general
- Dilated tortuous vessels, usually submucosal, that develop due to portal hypertension (prolonged or severe), which induces formation of collaterals between portal and caval systems (Wikipedia: Esophageal Varices [Accessed 15 February 2019], eMedicine: Portal Hypertension [Accessed 15 February 2019], eMedicine: Esophageal Varices Imaging [Accessed 15 February 2019], Mayo Clinic: Esophageal Varices [Accessed 15 February 2019])
- Collaterals in lower esophagus divert flow from portal vein, through coronary veins of stomach, into esophageal veins, then azygous veins, then into vena cava
Clinical features
- Present in 90% of cirrhotic patients, usually due to alcoholism; acute alcohol intake may cause death, often outside the hospital (Arch Pathol Lab Med 2002;126:1197)
- May rupture and cause massive hemorrhage (cause of death in 50% with advanced cirrhosis)
- 40% die in first episode of bleeding varices; rebleeding occurs in 50% of survivors within 1 year with 40% mortality
- Blood stains at death scene and unusual body positions of deceased are clues to fatal esophageal variceal hemorrhage
- #2 cause of bleeding varices worldwide is hepatic schistosomiasis
- "Downhill" varices occur in upper esophagus from other causes (Rev Esp Enferm Dig 2006;98:359)
Treatment
- Repeated sclerotherapy (injection of thrombotic agents, Ann Surg 2006;244:764), banding / ligation (Arq Gastroenterol 2005;42:72), balloon tamponade, beta blockers or octreotide to reduce portal blood flow, TIPS (Transjugular Intrahepatic Portosystemic Shunt) procedure, liver transplant
Gross description
- Varices may protrude into lumen, mucosa may be normal or inflamed
Gross images
Differential diagnosis hematemesis
- Esophageal laceration
- Gastritis
- Peptic ulcer
Verrucous squamous cell carcinoma
Table of Contents
Definition / general | Epidemiology | Pathophysiology / etiology | Clinical features | Diagnosis | Radiology images | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Highly differentiated variant of squamous cell carcinoma that is locally invasive with a pushing, as opposed to an infiltrative border, that is identical to lesions of the same name in the upper aerodigestive tract
- Metastases are uncommon
Epidemiology
- Extremely rare
- Male predominance
- Ages 36 - 76 years
Pathophysiology / etiology
- Cases related to chronic mucosal irritation, lye ingestion, diverticular disease, reflux esophagitis (Gastroenterology 1996;110:904)
- Reported case with positive HPV stain (Gastroenterol Hepatol 2013;36:311)
Clinical features
- Slow growing, nodal metastases are uncommon and distant metastases are not reported but still has high mortality because of advanced local disease with the development of fistulas (J Gastroenterol Hepatol 1993;8:107, J Clin Gastroenterol 1991;13:102, Minerva Chir 2005;60:61)
Diagnosis
- Tissue biopsy, knowledge of clinical and endoscopic findings may be essential to prevent misdiagnosis
Case reports
- 51 year old man with dysphagia
- 58 year old woman (Case Rep Gastroenterol 2013;7:498)
Treatment
- Resection, possible endoscopic resection (Dis Esophagus 2014;27:452)
Gross description
- Large, exophytic, polypoid, generally circumferential mass
Microscopic (histologic) description
- Similar to oral cavity counterpart
- Well differentiated with papillary fronds covered by squamous epithelium
- Bland cytologic features with mild atypia
- Variable parakeratosis and hyperkeratosis
- Pushing type of invasion with blunt epithelial projections
Microscopic (histologic) images
Differential diagnosis
- Carcinoma cuniculatum: deeply penetrating and burrowing growth pattern (Ann Diagn Pathol 2005;9:134)
- Superficial biopsies may be diagnosed as squamous papillomas or other benign lesions
- Papillomas are generally under 3 cm in dimension, generally do not demonstrate circumferential growth, lack ulceration, do not cause stricture and do not invade submucosa or muscularis, which are common findings in verrucous carcinoma (Can J Gastroenterol 2004;18:459, Am J Gastroenterol 1996;91:1031)
Well differentiated neuroendocrine tumor
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Clinical features | Grading | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy images | Differential diagnosisDefinition / general
- Well differentiated epithelial neoplasm with prominent neuroendocrine differentiation
- Pathologic spectrum from low grade neuroendocrine tumor (carcinoid, atypical carcinoid) to high grade neuroendocrine carcinoma (small or large cell carcinoma)
Terminology
- Synonym: neuroendocrine tumor (low grade) = carcinoid tumor, atypical carcinoid tumor
Epidemiology
- Extremely rare in esophagus: < 50 reported cases
- 0.002% of all carcinoid tumors
- Wide age range: 30 - 82 years, average age 60 years
- M:F = 6:1
Sites
- Distal esophagus
Clinical features
- Usually incidental / unexpected finding on radiologic studies or upper GI endoscopy
- Dysphagia is most common symptom
- Carcinoid syndrome is rare
Grading
- Grade 1 (carcinoid): < 2 mitoses/10 HPF or < 2% Ki67 index
- Grade 2 (atypical carcinoid): 2 - 20 mitoses/10 HPF or 3 - 20% Ki67 index (UpToDate: Classification of Neuroendocrine Neoplasms GI Tract [Accessed 26 February 2019])
Diagnosis
- Endoscopic biopsy
Prognostic factors
- Mitotic rate and Ki67 index determine grade
- Low grade lesions have favorable prognosis
Case reports
- 54 year old man with atypical carcinoid of esophagus (Ann Thorac Cardiovasc Surg 2002;8:302)
Treatment
- Surgical resection
Gross description
- Polypoid or ulcerated mass on upper endoscopy
Microscopic (histologic) description
- Well differentiated (low grade) tumor
- Uniform, small, bland tumor cells in solid, trabecular, gyriform or glandular pattern
- May have Paneth cell differentiation
- Solid to cribriform growth
- Usually in lamina propria
Cytology description
- Low grade tumors:
- Flat sheets or loosely cohesive groups / cords of monotonously uniform plasmacytoid cells
- Eccentric nuclei, coarsely stippled (salt and pepper) chromatin, finely granular cytoplasm
Positive stains
- Keratins (e.g. CAM 5.2, AE1 / AE3)
- Neuroendocrine markers: synaptophysin, chromogranin, CD56, NSE
- Ki67 (essential for tumor grading - see above)
Negative stains
Differential diagnosis
- Atypical carcinoid
- Glomus tumor
- Paraganglioma
- Small cell (neuroendocrine) carcinoma
WHO classification
Definition / general
- WHO classification of tumors of the esophagus
- Currently on 5th edition, published in 2019
- Based on histologic appearance, not molecular characteristics
WHO (2019)
-
Benign epithelial tumors and precursors ICD-O codes
- Squamous cell papilloma, NOS 8052/0
- Squamous papillomatosis 8060/0
- Esophageal glandular dysplasia (intraepithelial neoplasia), low grade 8148/0
- Esophageal glandular dysplasia (intraepithelial neoplasia), high grade 8148/2
- Esophageal squamous intraepithelial neoplasia (dysplasia), low grade 8077/0
- Esophageal squamous intraepithelial neoplasia (dysplasia), high grade 8077/2
-
Malignant epithelial tumors ICD-O codes
- Adenocarcinoma, NOS 8140/3
- Adenoid cystic carcinoma 8200/3
- Mucoepidermoid carcinoma 8430/3
- Adenosquamous carcinoma 8560/3
- Squamous cell carcinoma, NOS 8070/3
- Carcinoma, undifferentiated, NOS 8020/3
- Lymphoepithelioma-like carcinoma 8082/3
- Neuroendocrine tumor, NOS 8240/3
- Neuroendocrine tumor, grade 1 8240/3
- Neuroendocrine tumor, grade 2 8249/3
- Neuroendocrine tumor, grade 3 8249/3
- Neuroendocrine carcinoma, NOS 8246/3
- Large cell neuroendocrine carcinoma 8013/3
- Small cell neuroendocrine carcinoma 8041/3
- Mixed neuroendocrine - nonneuroendocrine neoplasm (MiNEN) 8154/3
- Combined small cell - adenocarcinoma 8045/3
- Combined small cell - squamous cell carcinoma 8045/3
Recent Esophagus Pathology books
Find related Pathology books: GI, liver