Superpage
Superpage Topics
Abscess
Accessory
Acute nonspecific lymphadenitis
Acute splenitis
Adipose tissue
Adult onset Still disease
ALK+ histiocytosis
Amyloid
Amyloidosis
Anatomy & histology-lymph nodes
Anatomy, histology & grossing-spleen
Angiolipomatous hamartoma
Angiomyolipoma
Angiomyomatous hamartoma
Anthracosis
B & T cells
Castleman disease
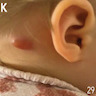
Cat scratch disease
Chronic lymphadenitis
Chronic myeloid leukemia, extramedullary
Common nonspecific abnormal features
Congestive splenomegaly
Decidual reaction
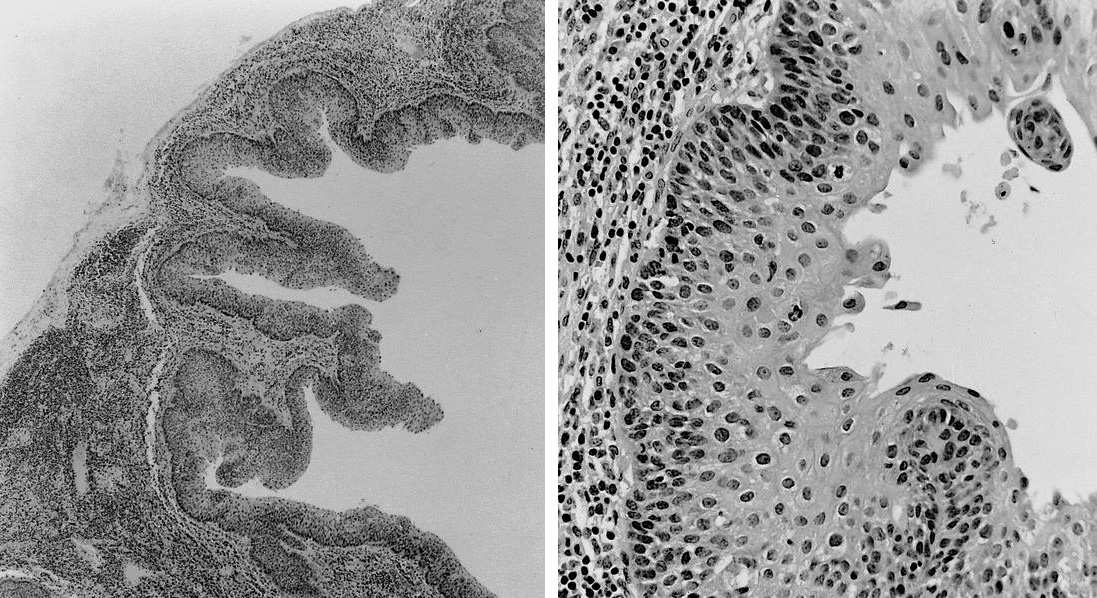
Dermatopathic lymphadenopathy
Drug hypersensitivity
EBV positive inflammatory follicular dendritic cell / fibroblastic reticular cell tumor
Epithelial cyst
Epithelial inclusions
Fibroblastic reticulum cell sarcoma
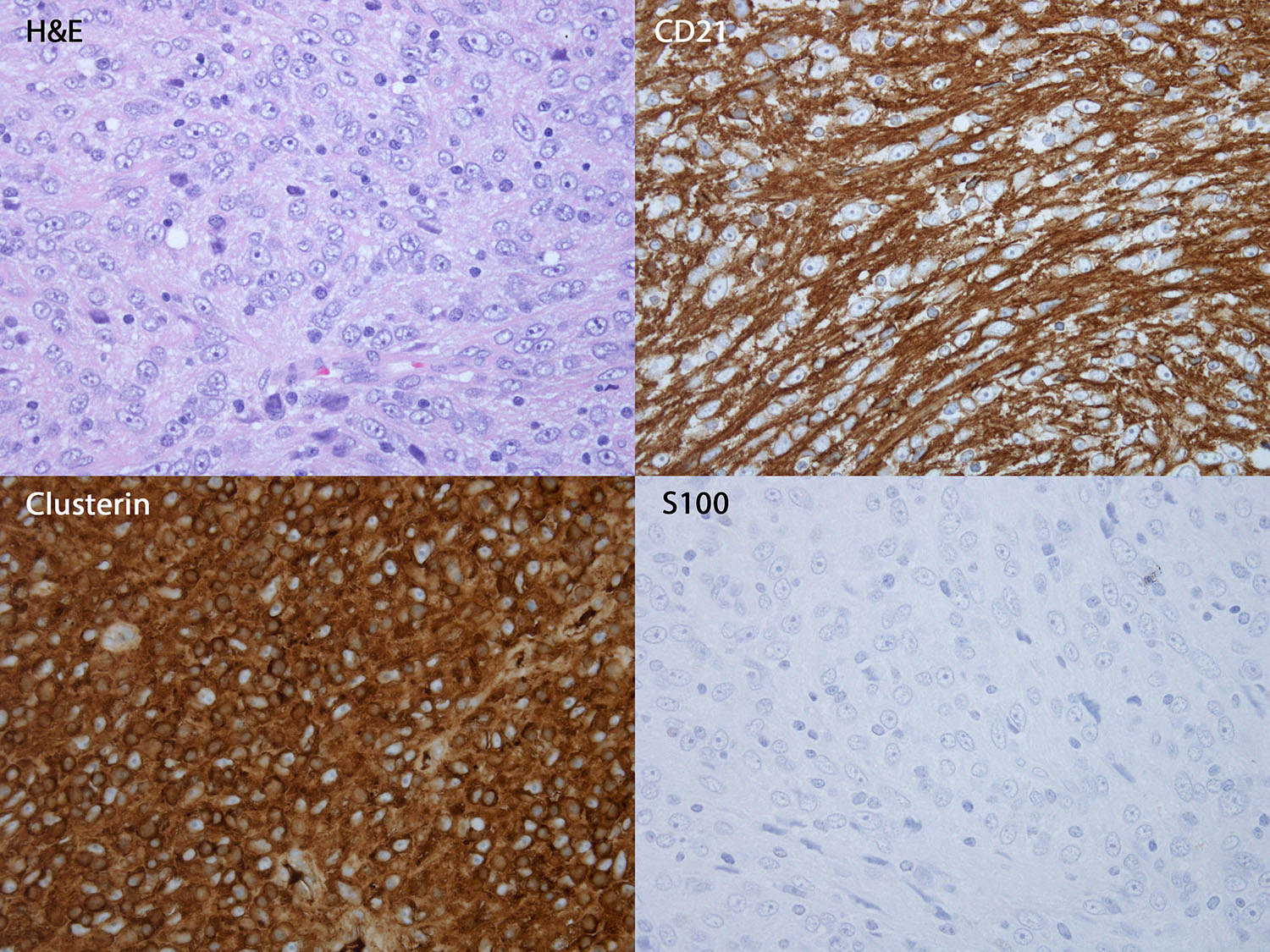
Follicular dendritic cell sarcoma (FDCS)
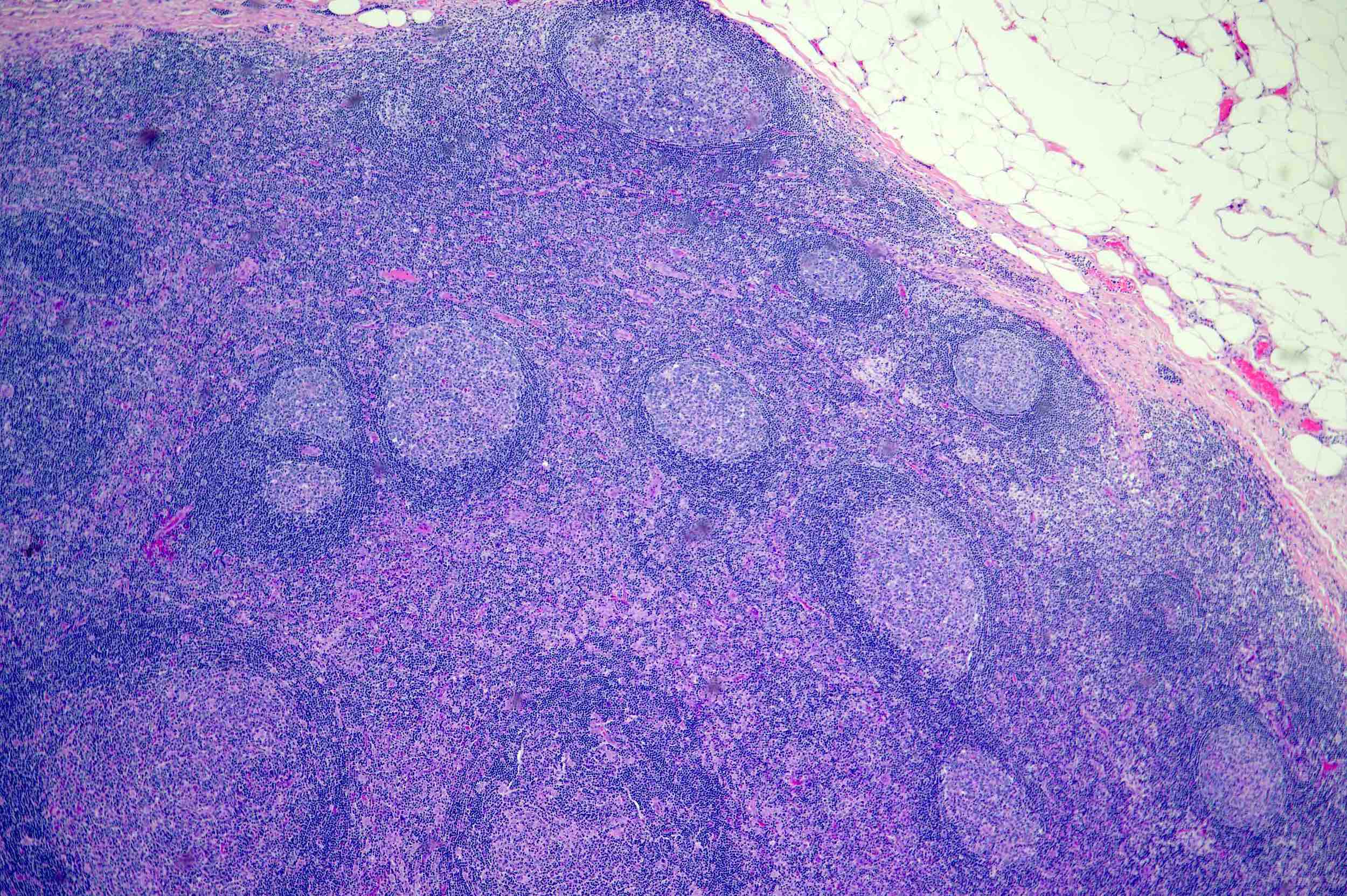
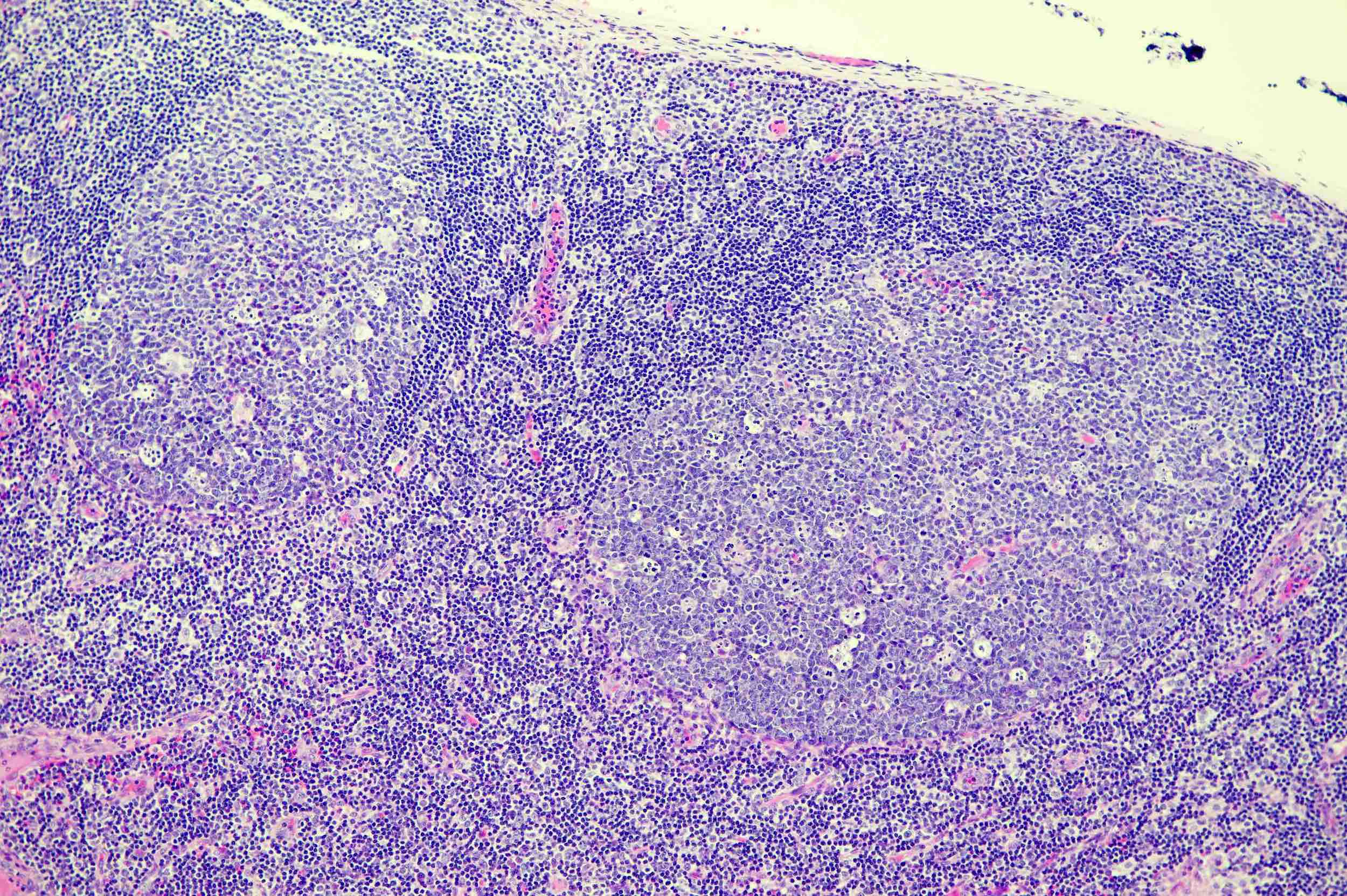
Follicular hyperplasia
Granulomatous inflammation
Granulomatous inflammation
Grossing & features to report-lymph nodes
Hamartoma
Hemangioma
Hemangioma
Hereditary spherocytosis
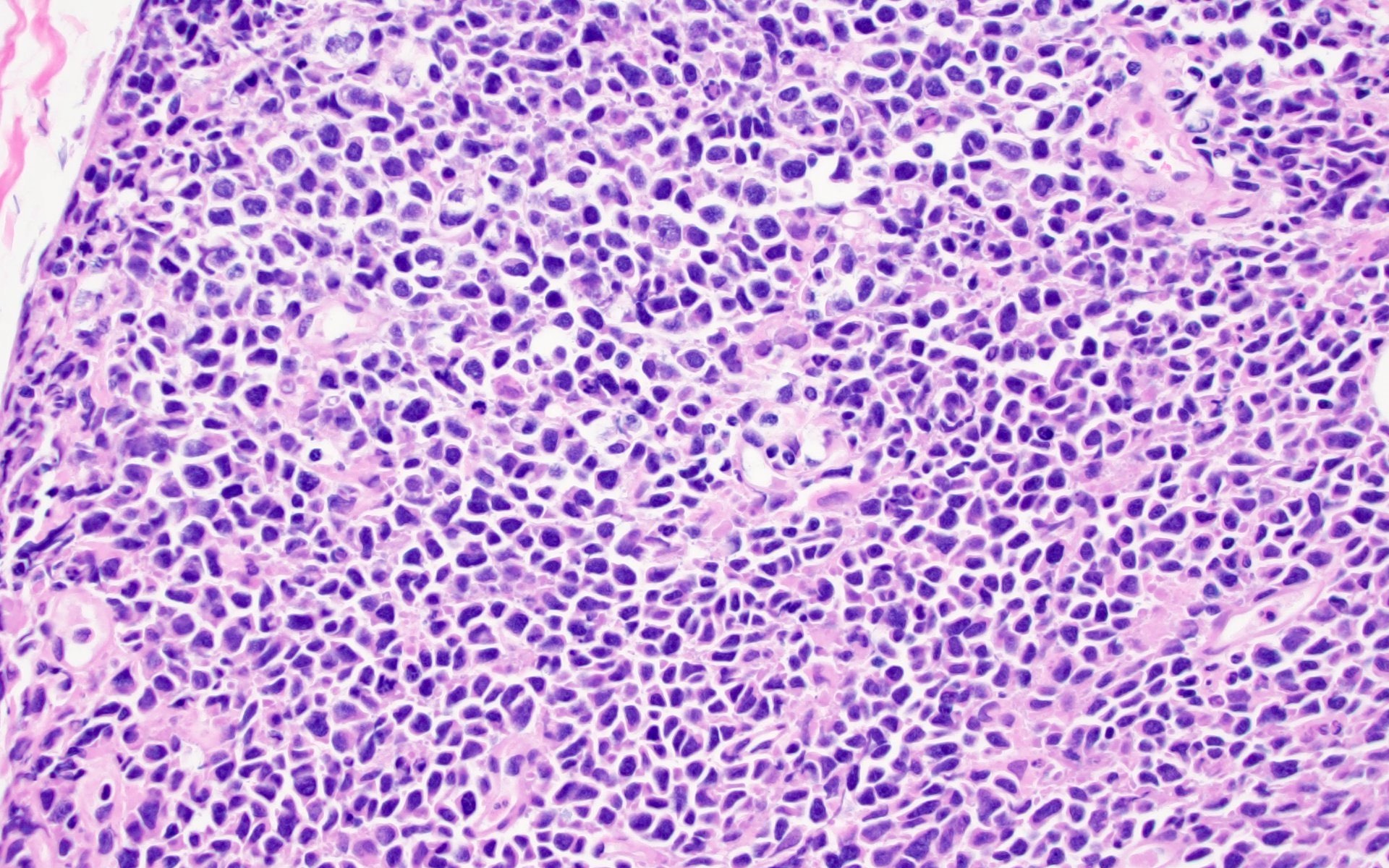
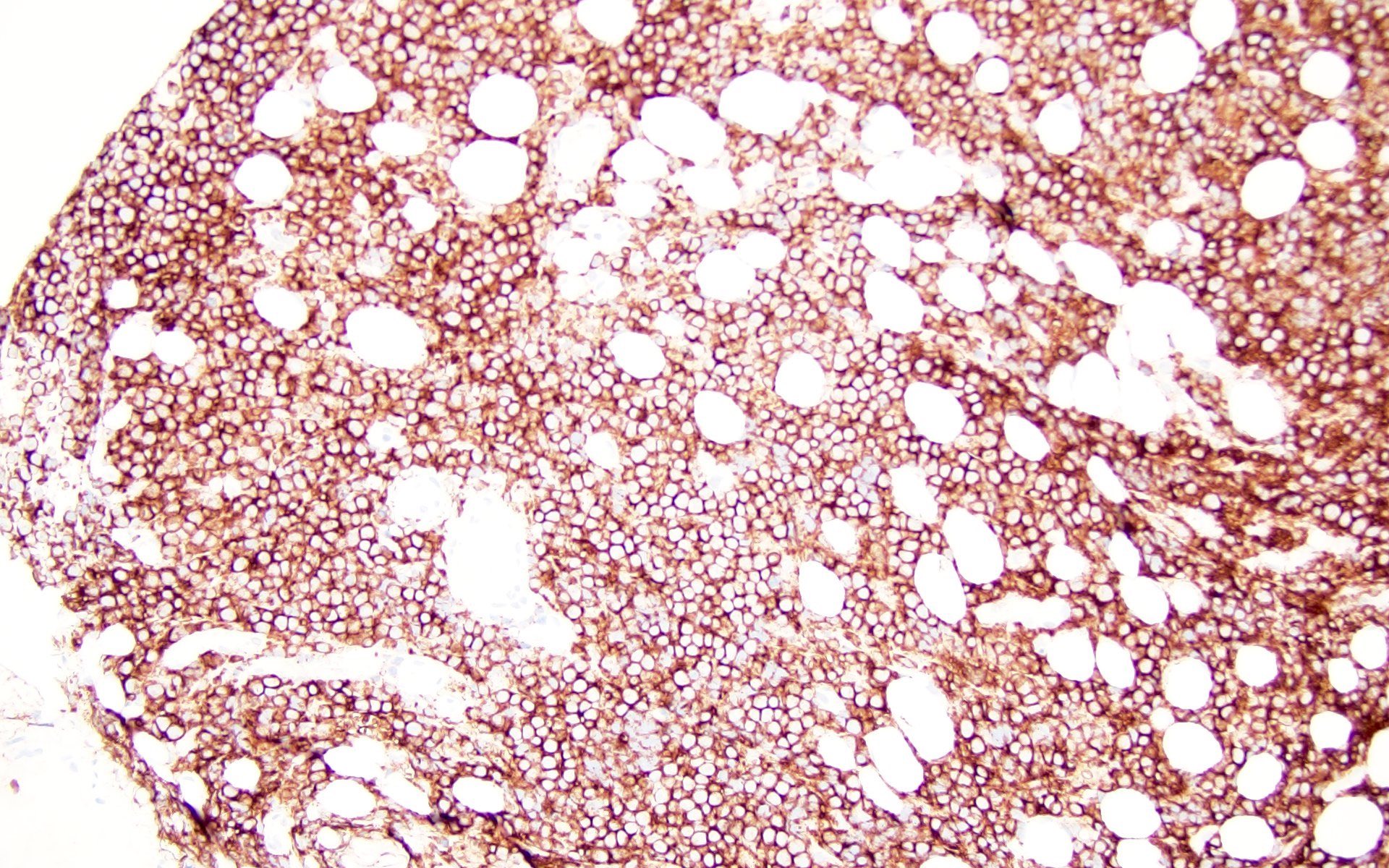
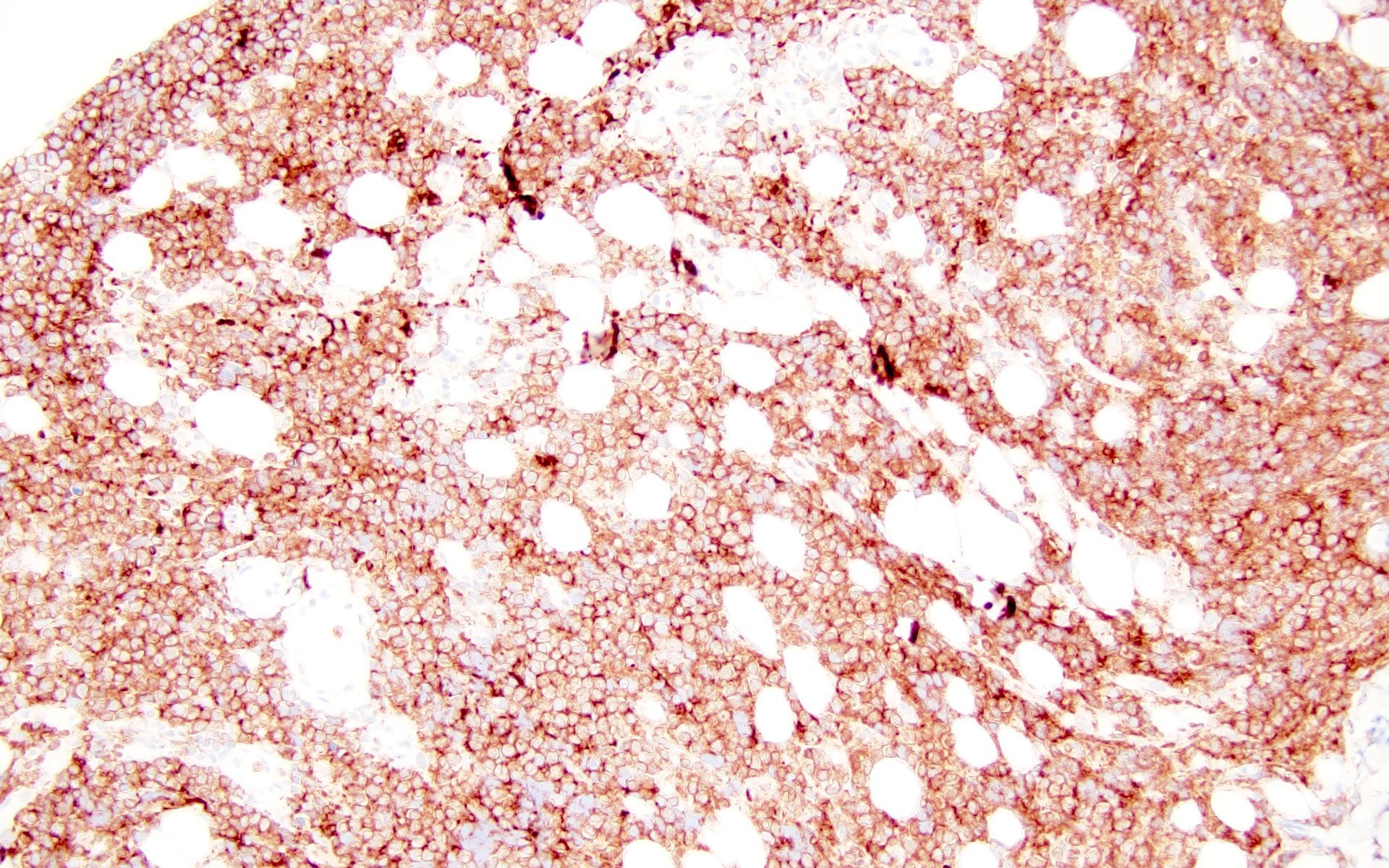
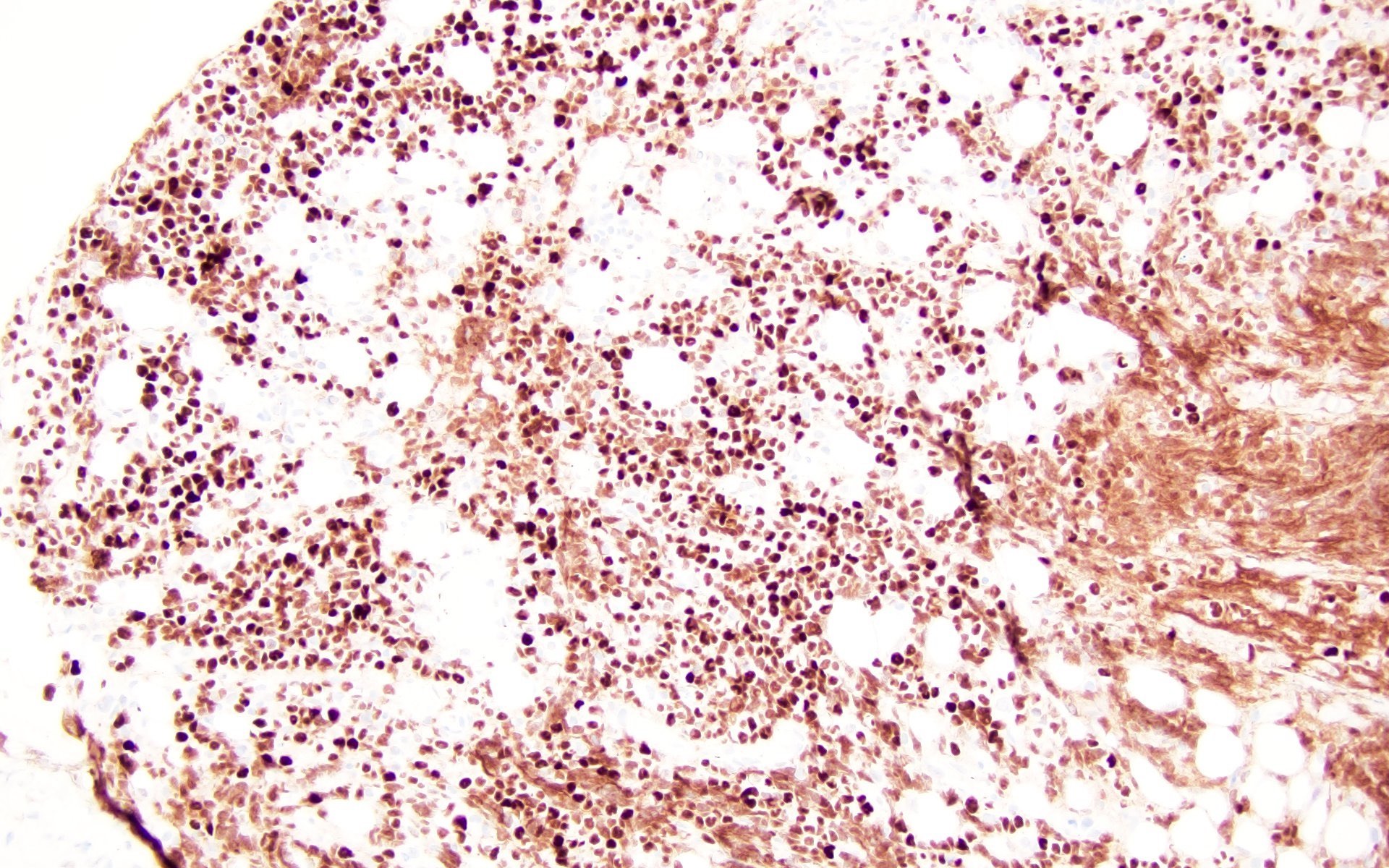
Histiocytic sarcoma
HIV
IgG4 lymphadenitis (pending)
IgG4 related lymphadenopathy
IgG4 related lymphadenopathy (pending)
Immune thrombocytopenia (ITP)
Indeterminate cell histiocytosis / indeterminate dendritic cell tumor
Indolent T lymphoblastic proliferations
Infectious mononucleosis
Interdigitating dendritic cell / reticulum cell sarcoma
Interdigitating dendritic cell tumor
Kawasaki disease
Kikuchi disease
Kimura disease
Langerhans cell histiocytosis
Lipofuscin
Littoral cell angioma
Luetic lymphadenitis
Lymphangioma
Lymphangiomyomatosis
Massive splenomegaly
Mesothelial inclusions
Metastases
Metastases
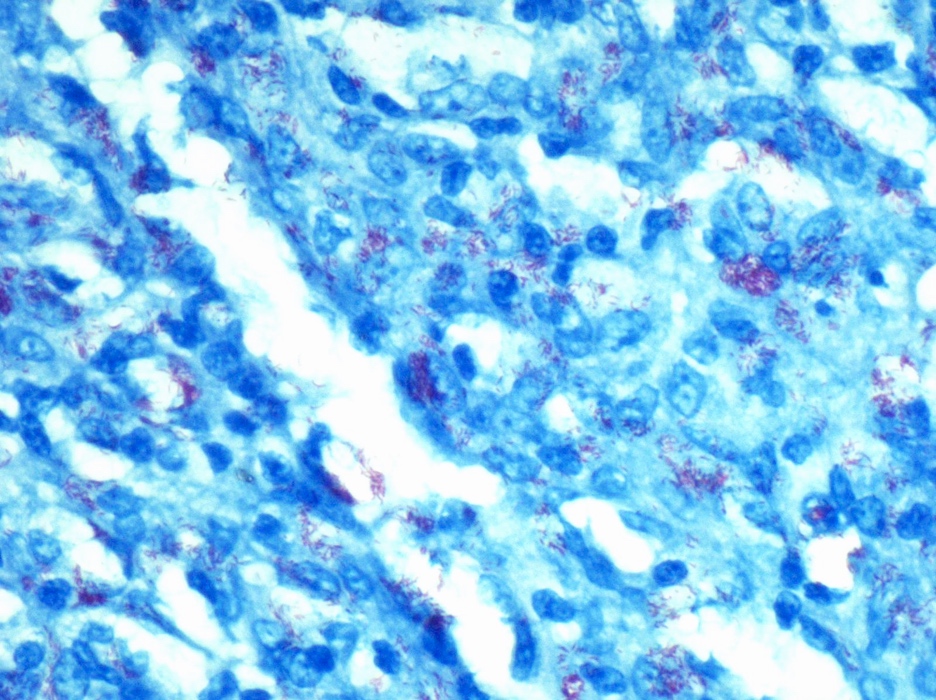
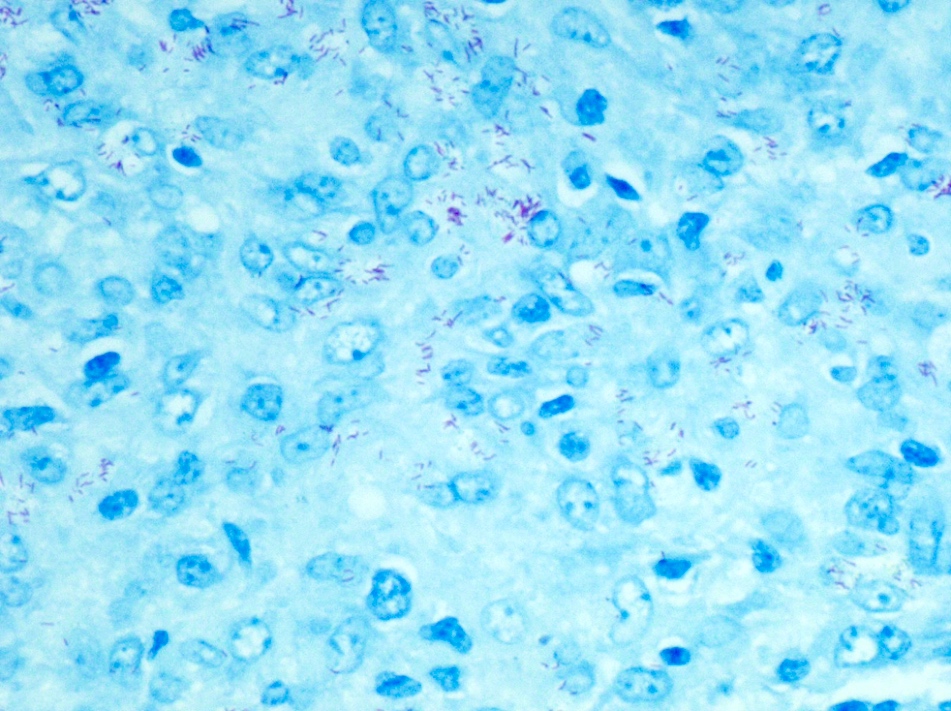
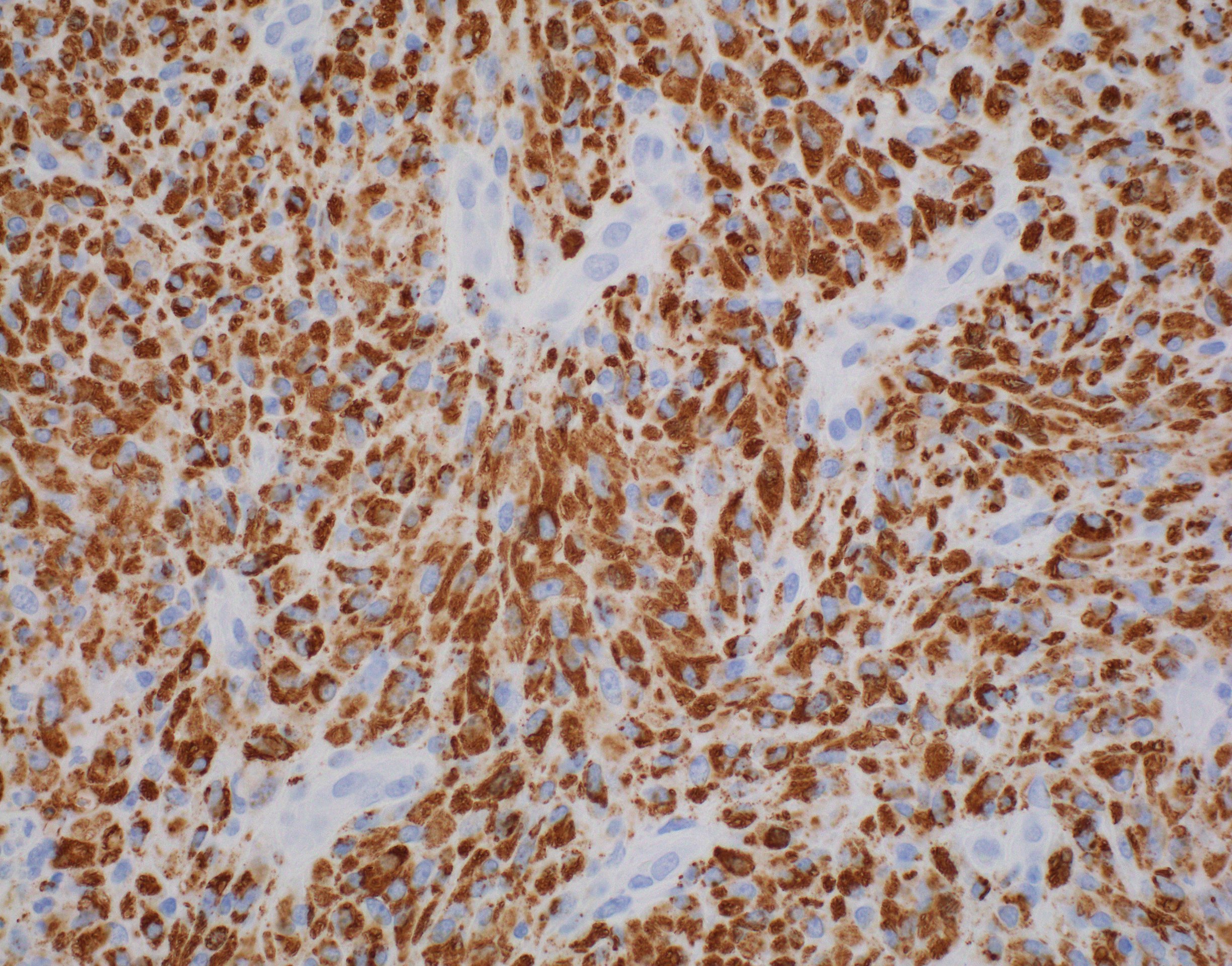
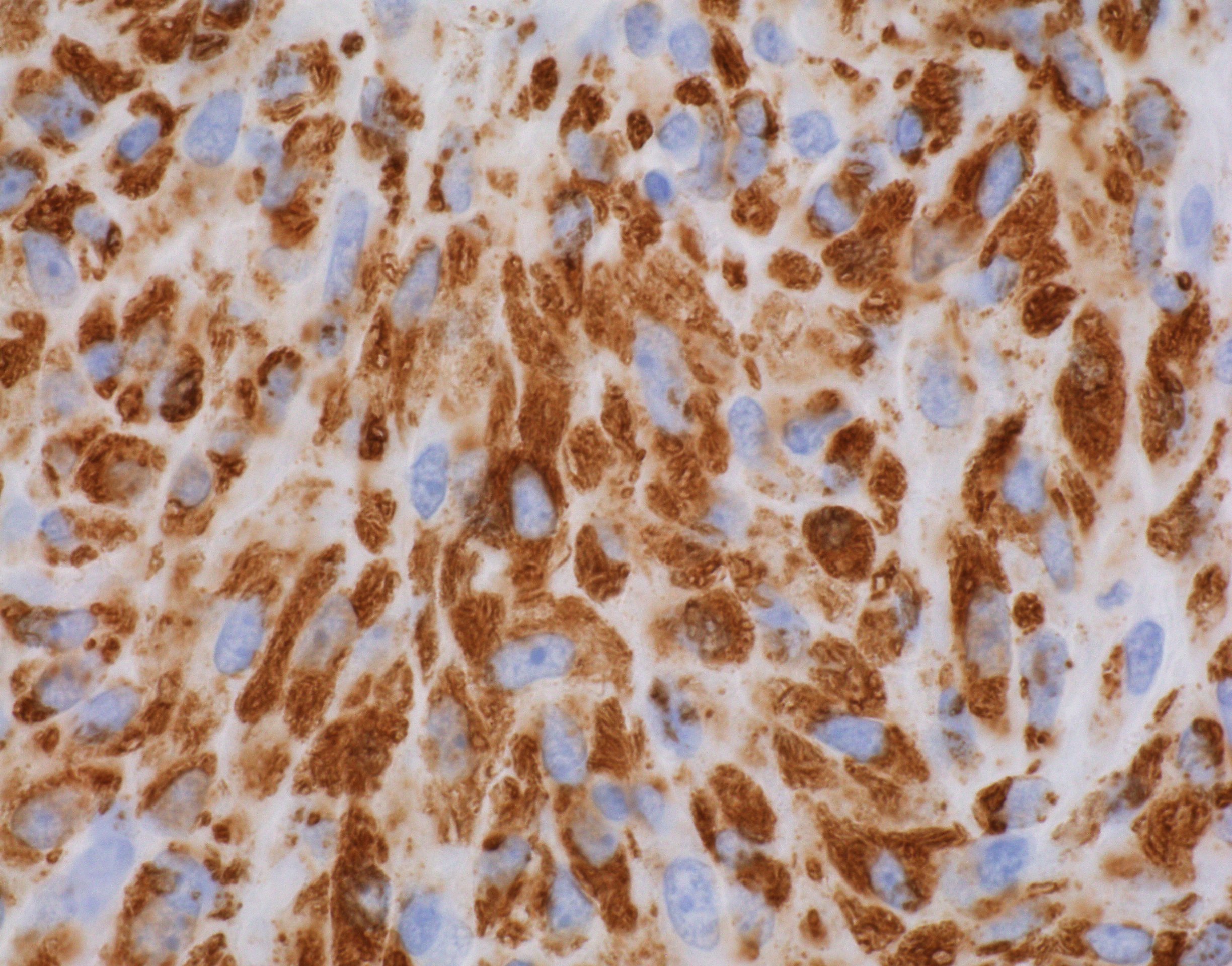
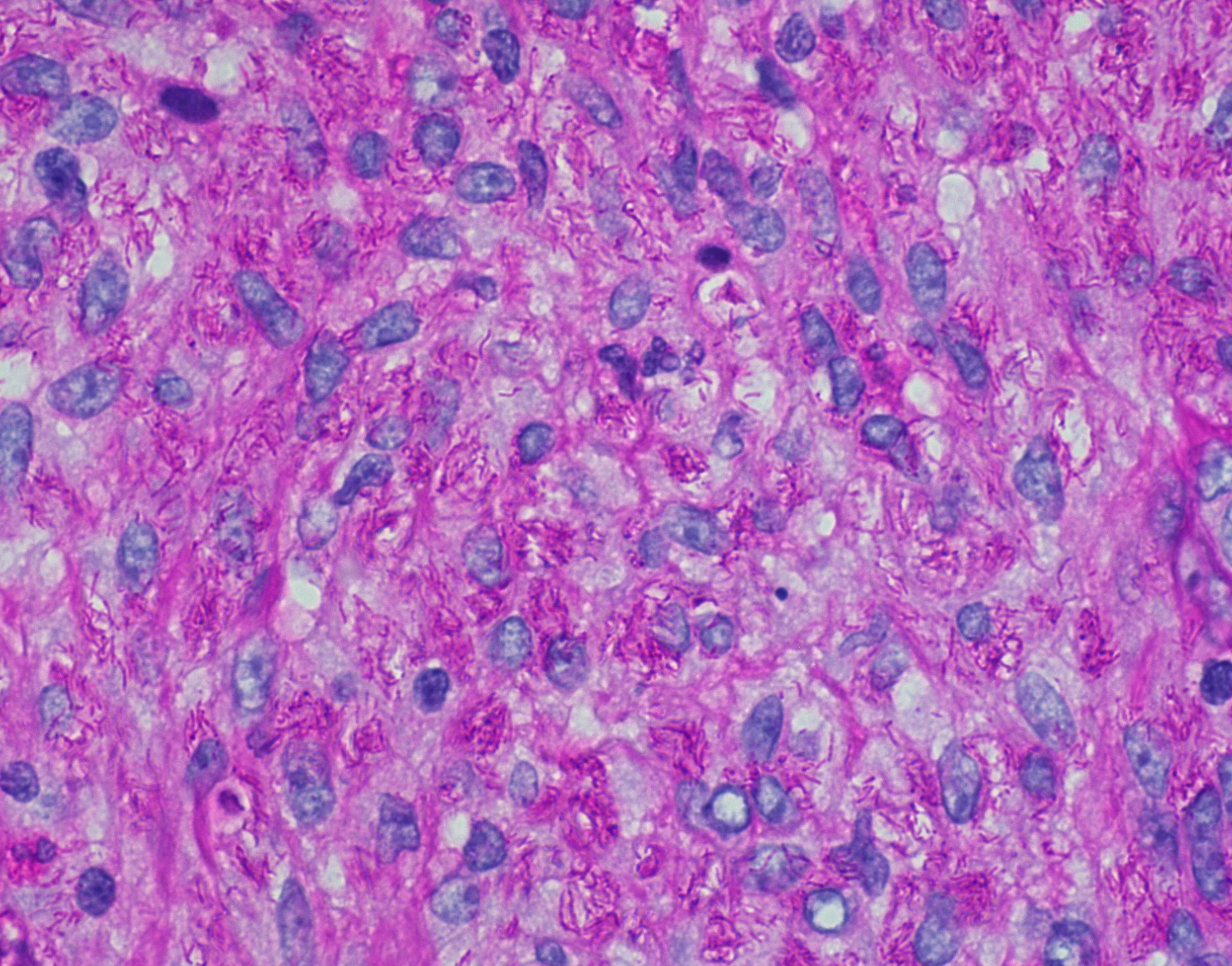
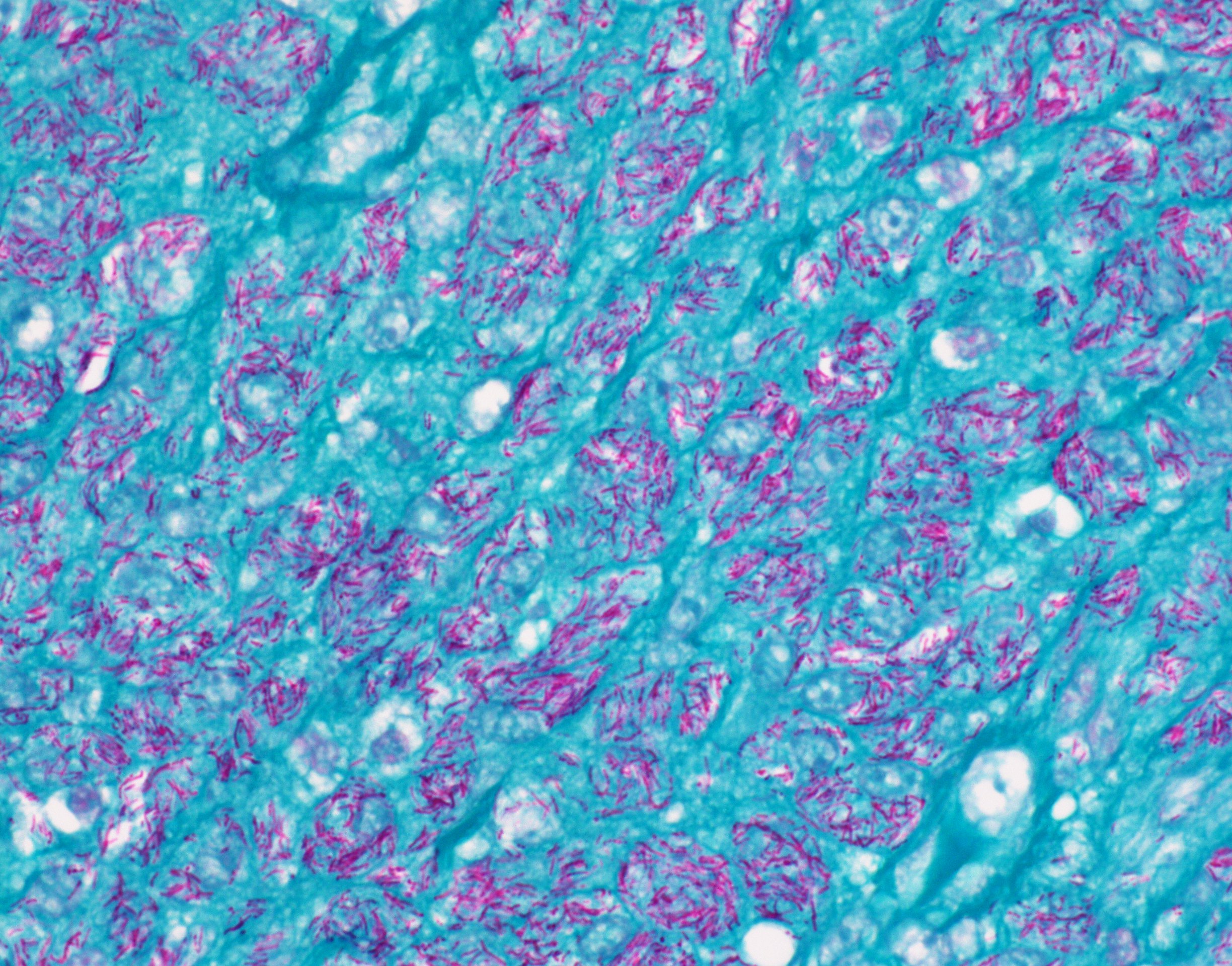
Mycobacteria-atypical / other than TB or leprosy
Mycobacteria-atypical / other than TB or leprosy
Other ectopic tissue / inclusions
Other infections
Other nonspecific findings
Other pigment / foreign material
Peliosis
Plasmacytoma
Progressive transformation of germinal centers
Progressive transformation of germinal centers
Pseudocyst
Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Reactive lymphadenopathy
Rheumatoid arthritis
Rosai-Dorfman disease (RDD)
Sclerosing angiomatoid nodular transformation
Silicone
Splendore-Hoeppli phenomenon
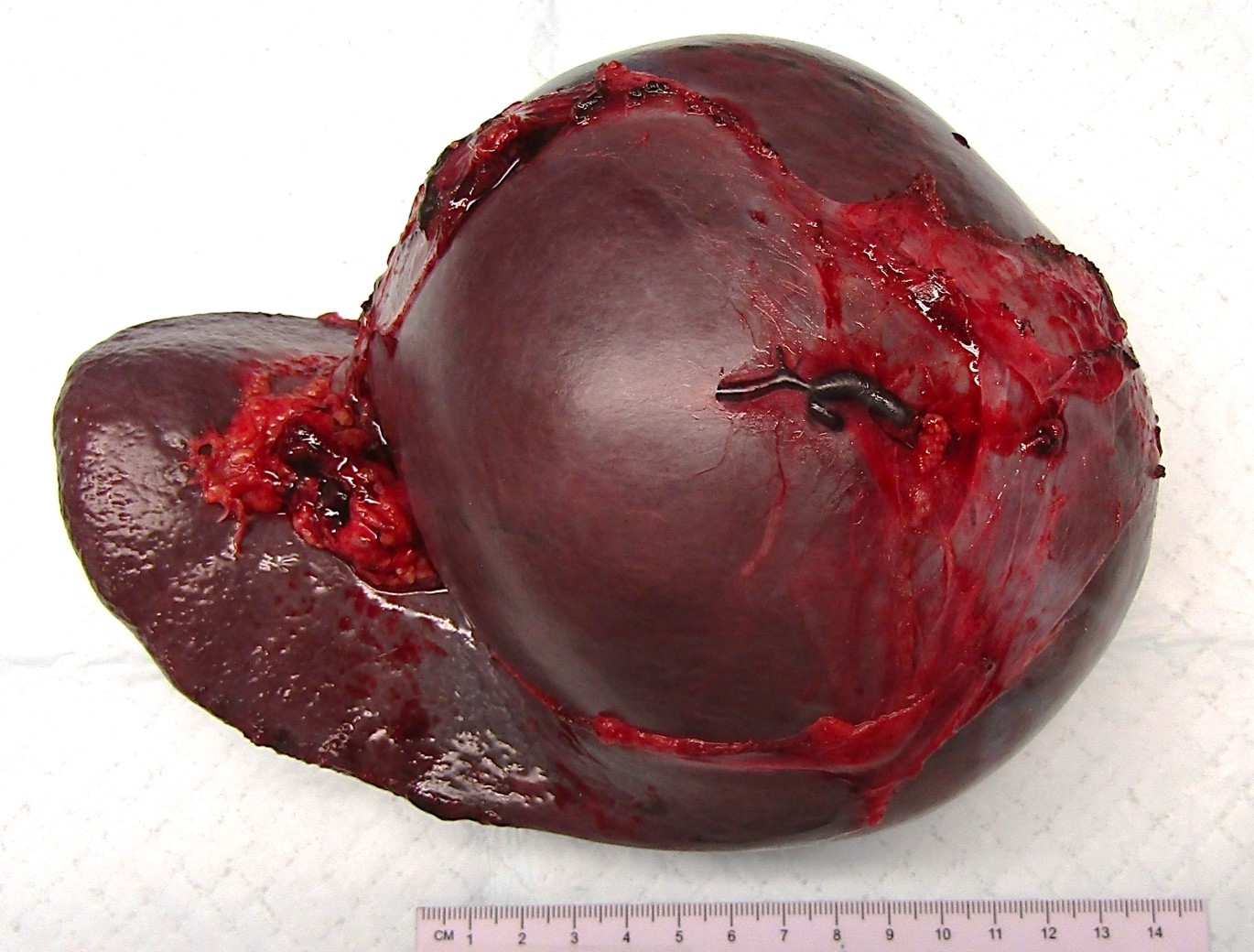
Splenectomy, rupture & splenosis
Splenic malformations
Systemic lupus erythematosus
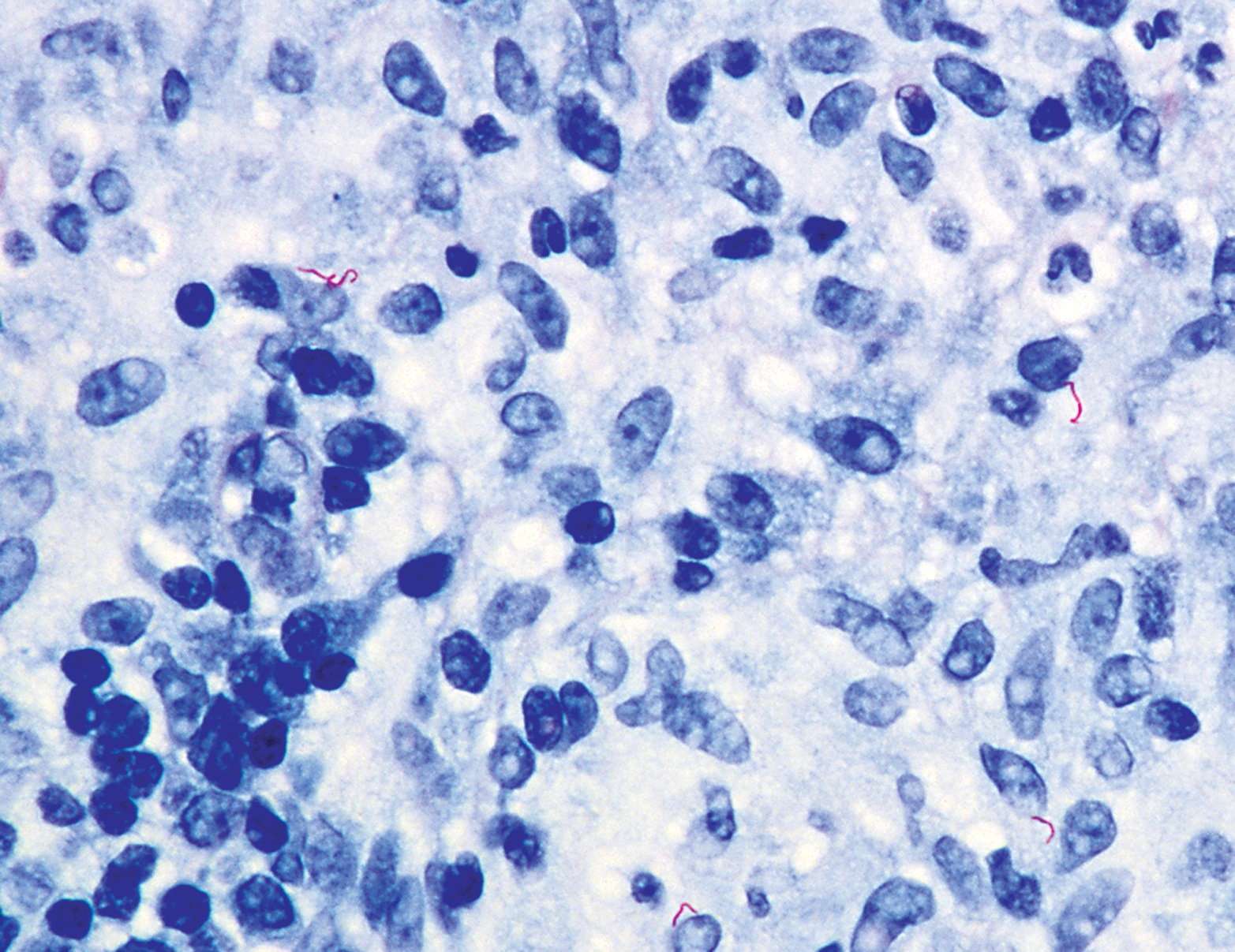
Toxoplasmosis
Uncommon infections
Vascular transformation of sinuses
WHO system for reporting lymph node cytopathology (pending)Abscess
Table of Contents
Definition / generalDefinition / general
- Very rare
- Due to trauma, subacute bacterial endocarditis or infection from another site
- Abscess often walled off
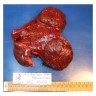
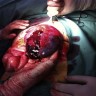
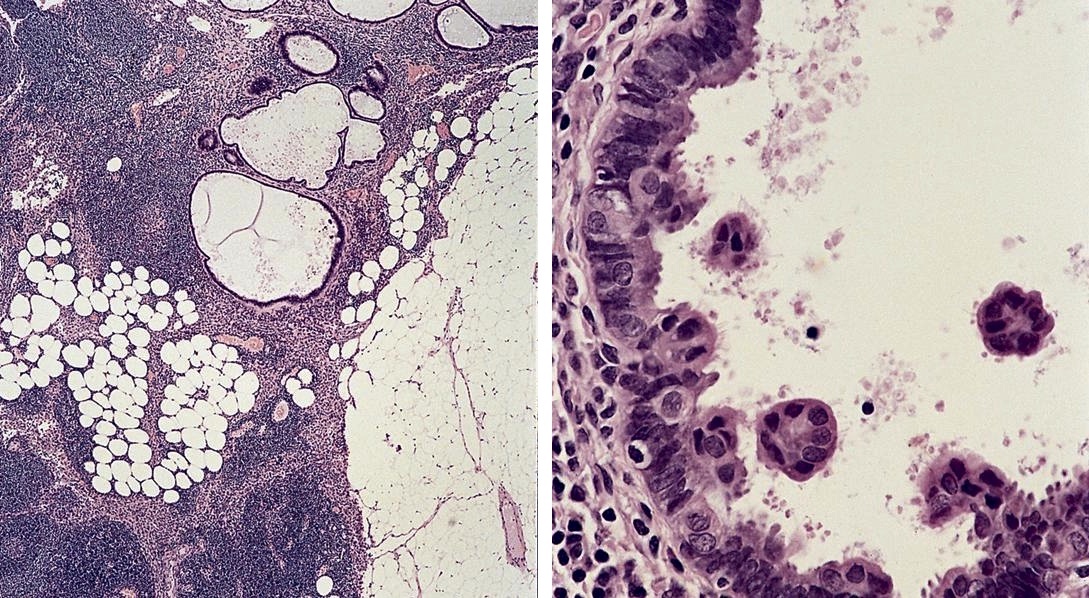
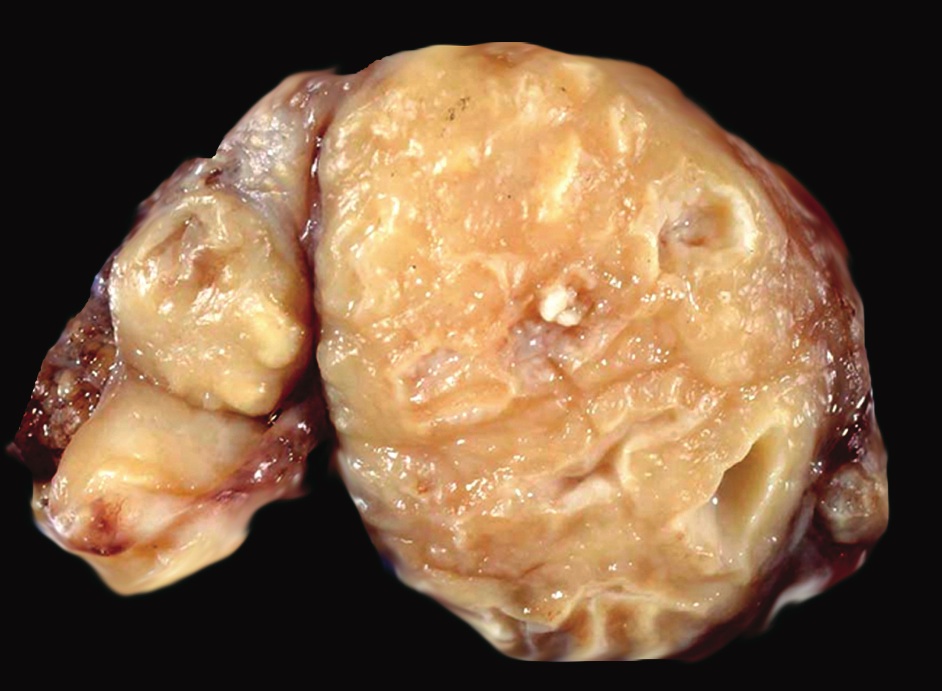
Accessory
Table of Contents
Definition / general | Case reports | Microscopic (histologic) images | Differential diagnosisDefinition / general
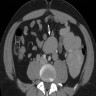
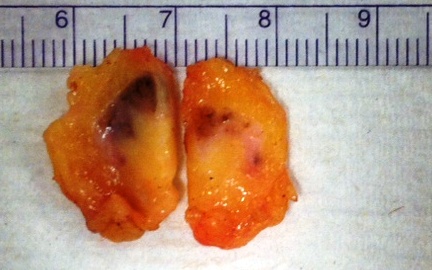
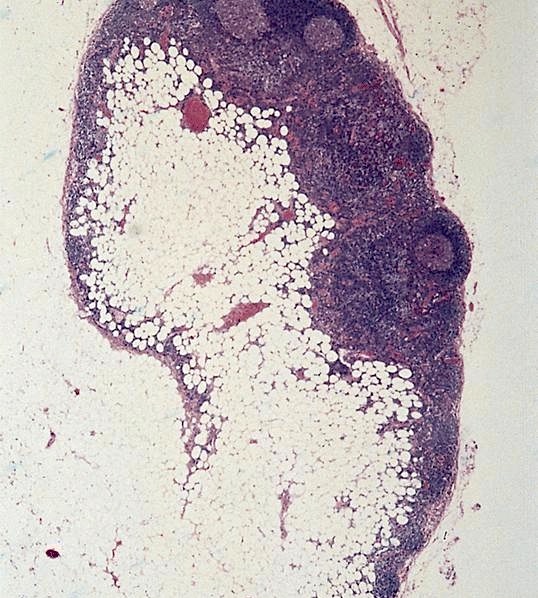
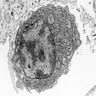
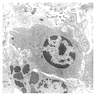
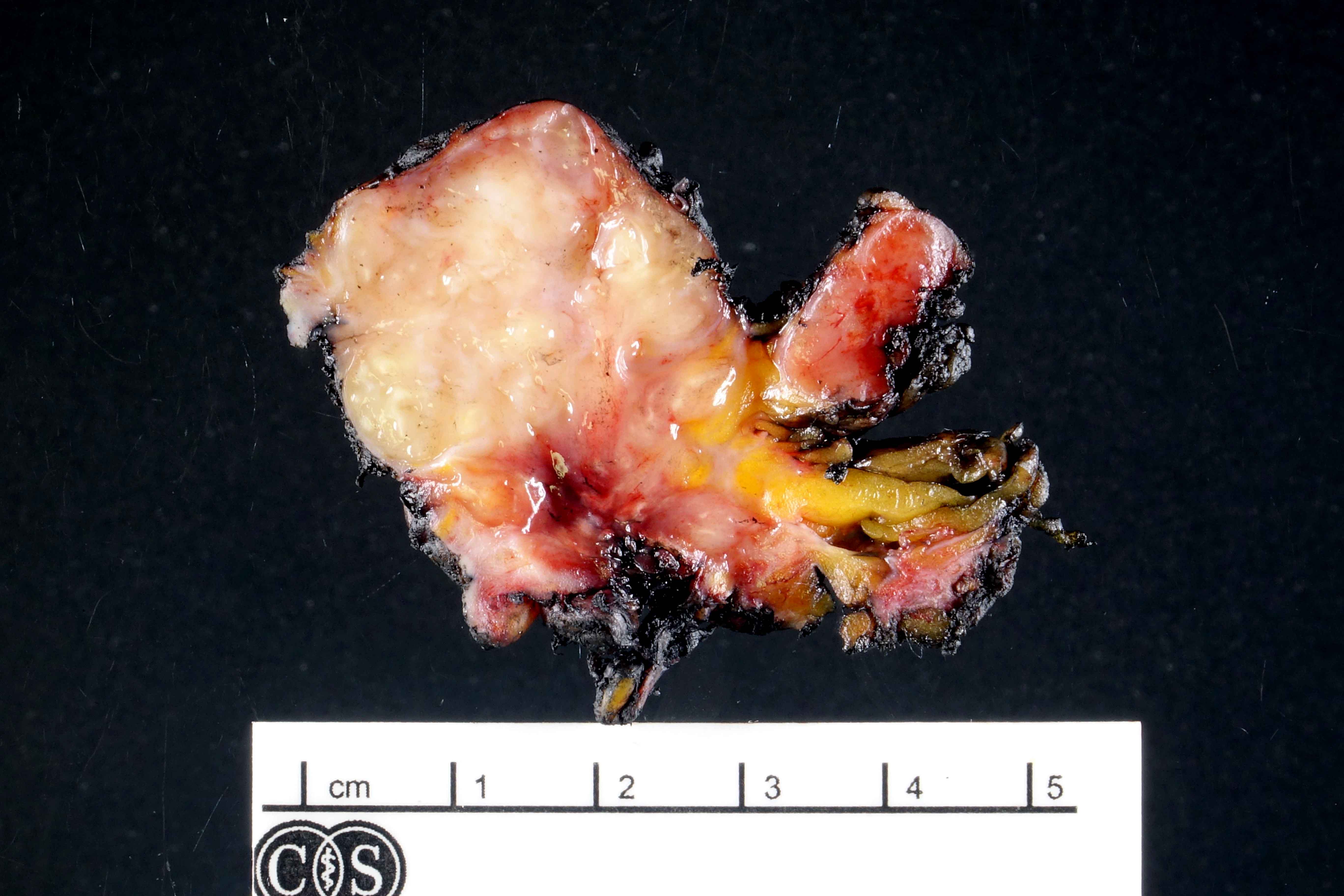
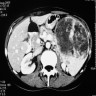
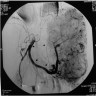
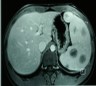
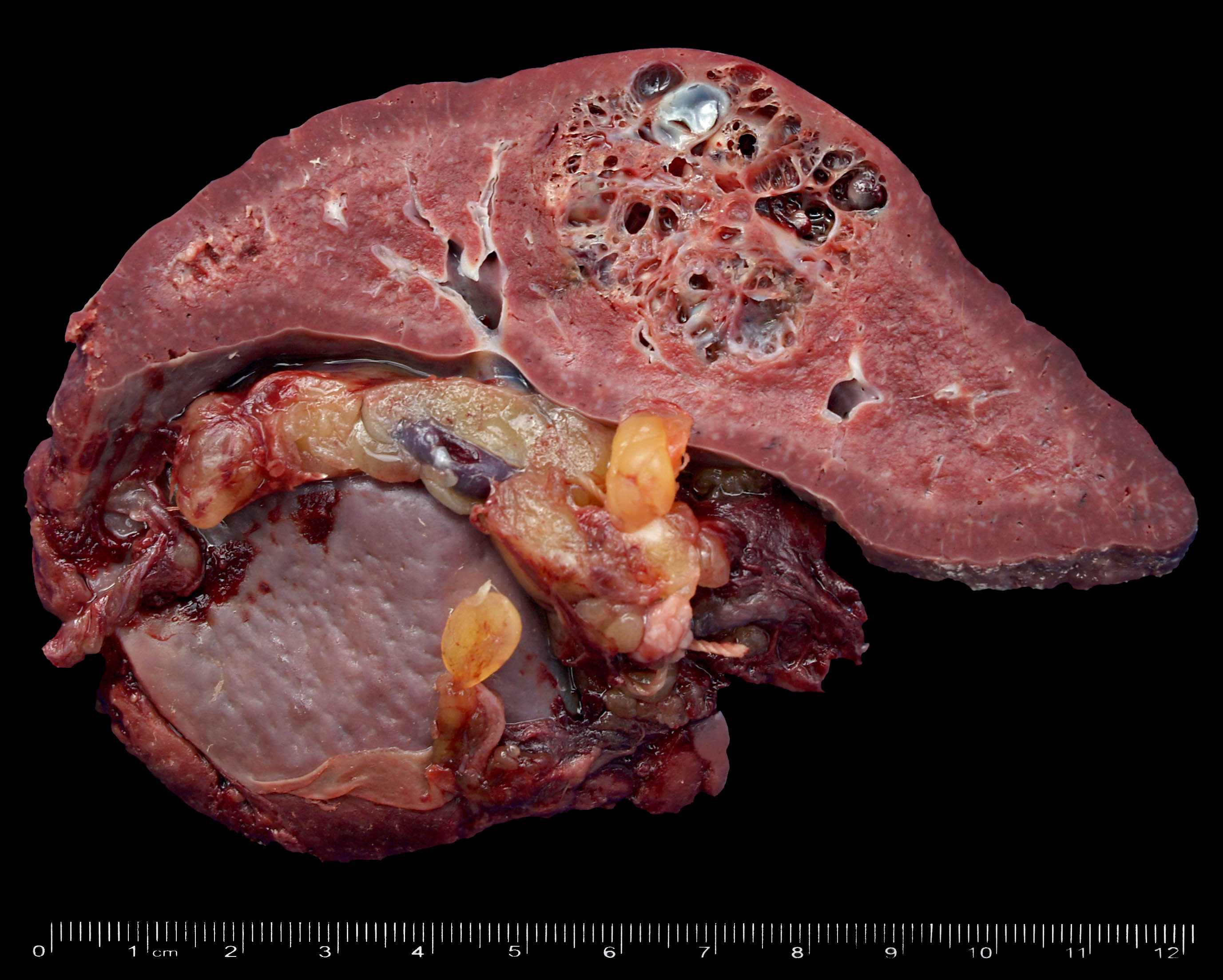
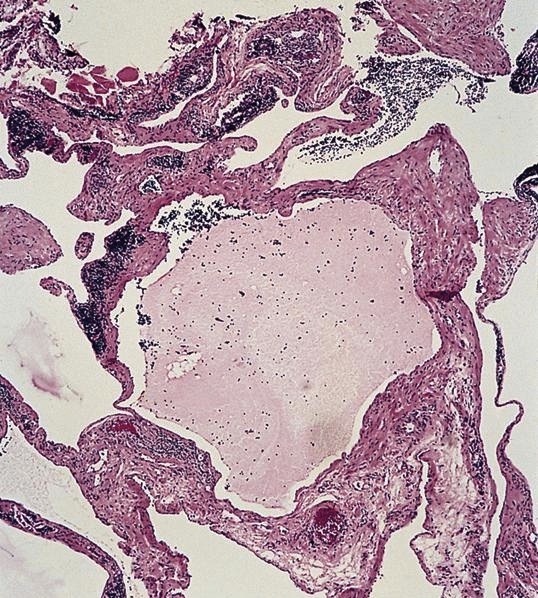
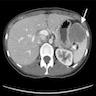
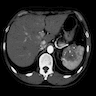
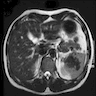
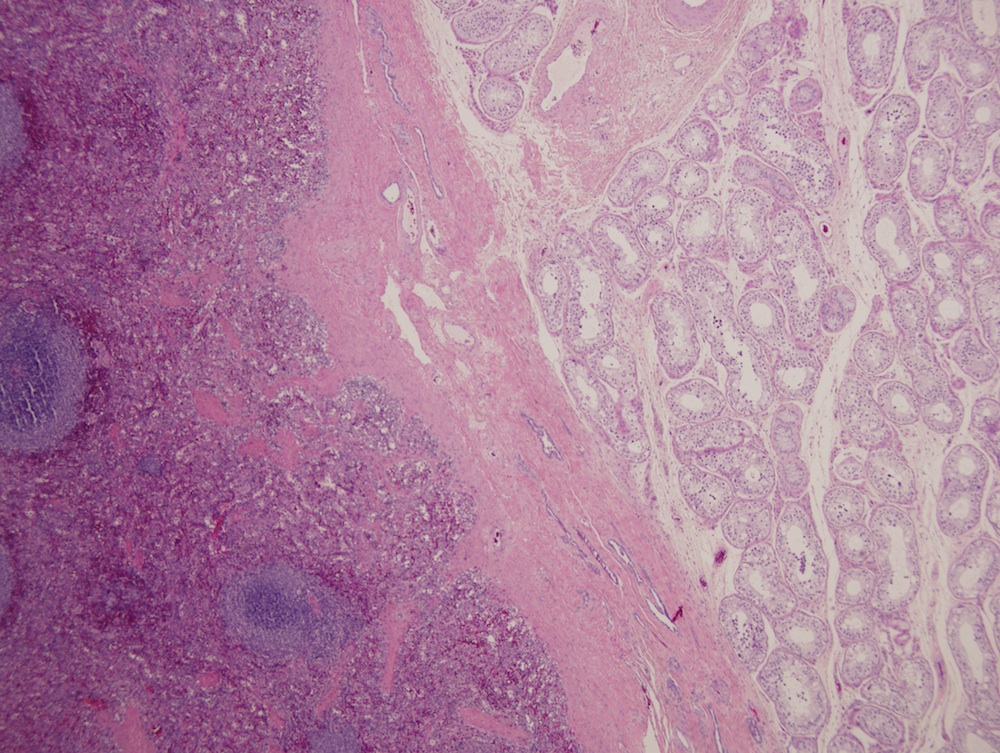
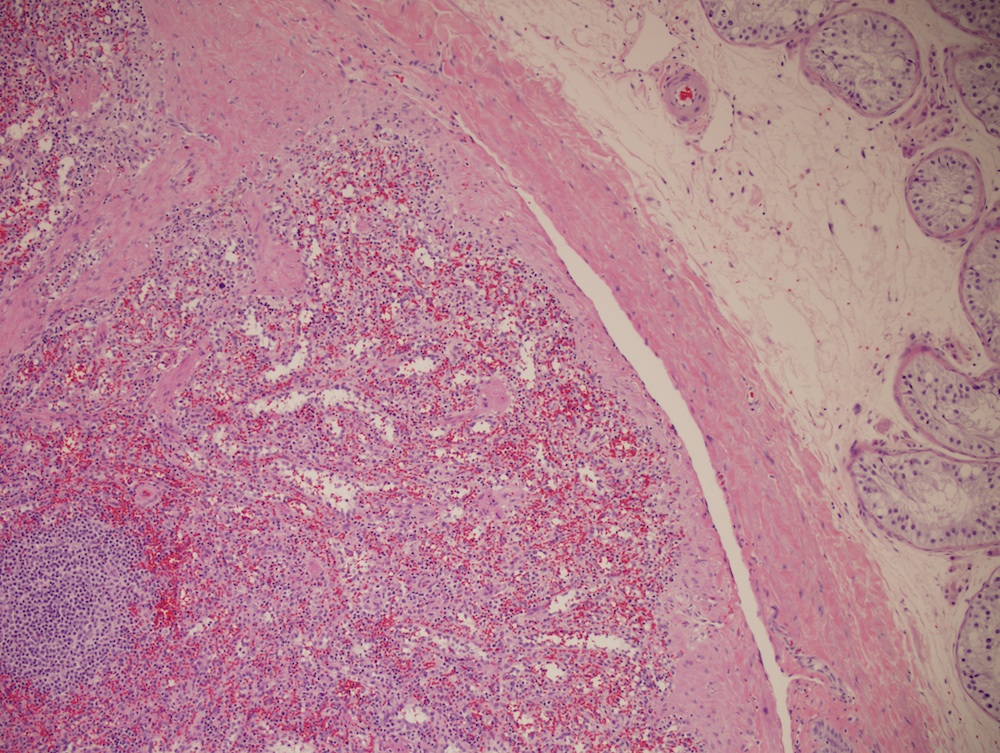
- Present in 20 - 33% of autopsies
- Usually small (up to 4 cm), resembles normal spleen macroscopically and microscopically
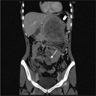
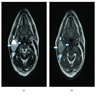
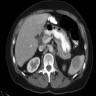
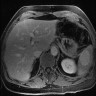
- Near splenic hilum, gastrosplenic ligament, tail of pancreas (Pancreas 2011;40:956)
- Important to document or find in patients with splenectomy for hematologic disease
- Intrapancreatic accessory spleen may contain increased fibrous stroma, pancreatic islet tissue and indistinct border with adjacent pancreatic parenchyma
- May contain epithelial cysts
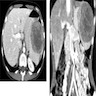
- Rare cases can result in torsion, sometimes presenting with either intermittent abdominal pain or acute abdomen
Case reports
- 3 year old girl with situs inversus and torsion of accessory spleen (J Med Invest 2012;59:220)
- 60 year old woman with 18 x 11 mm hypoechoic mass in tail of pancreas (Case of the Week #406)
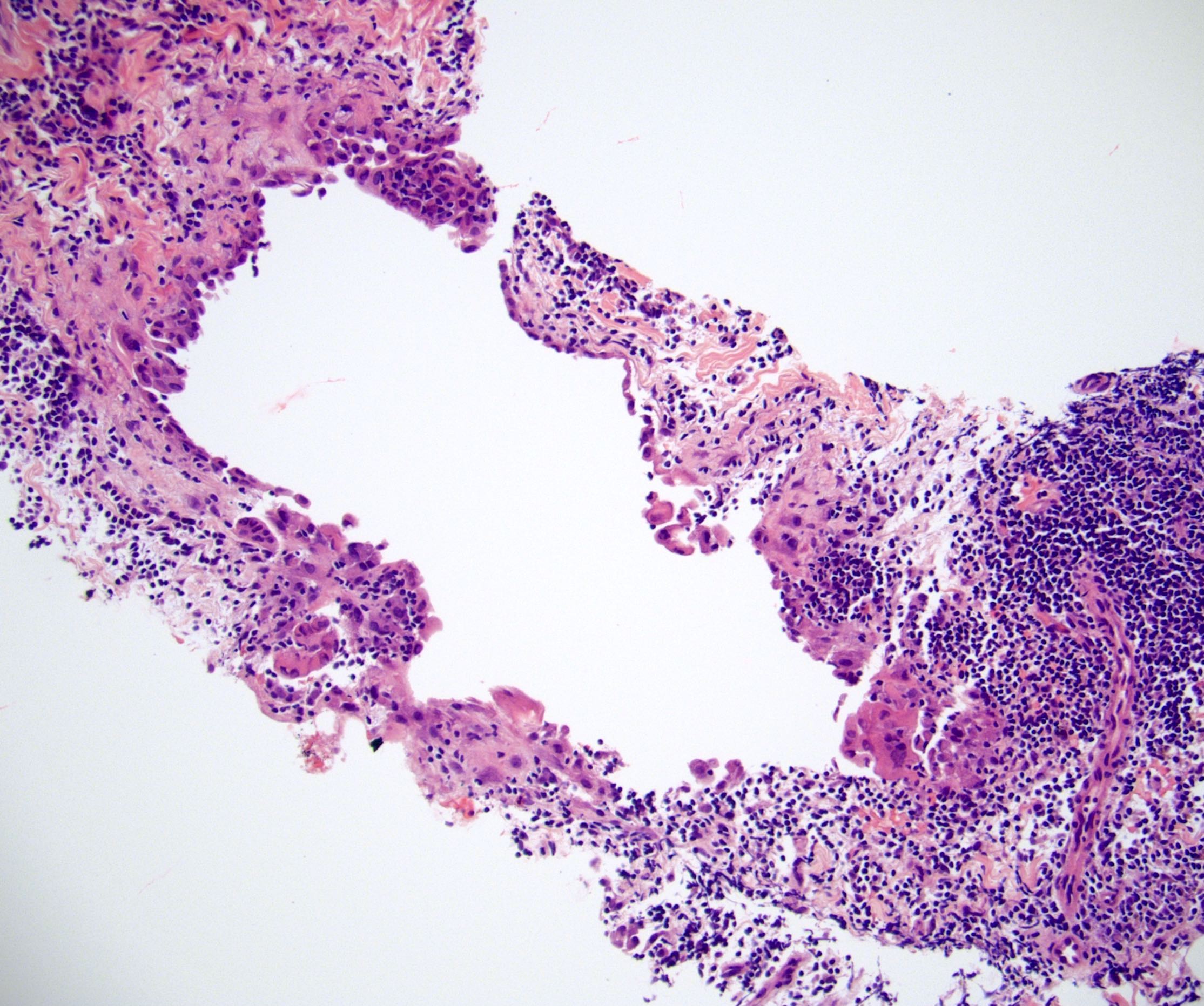
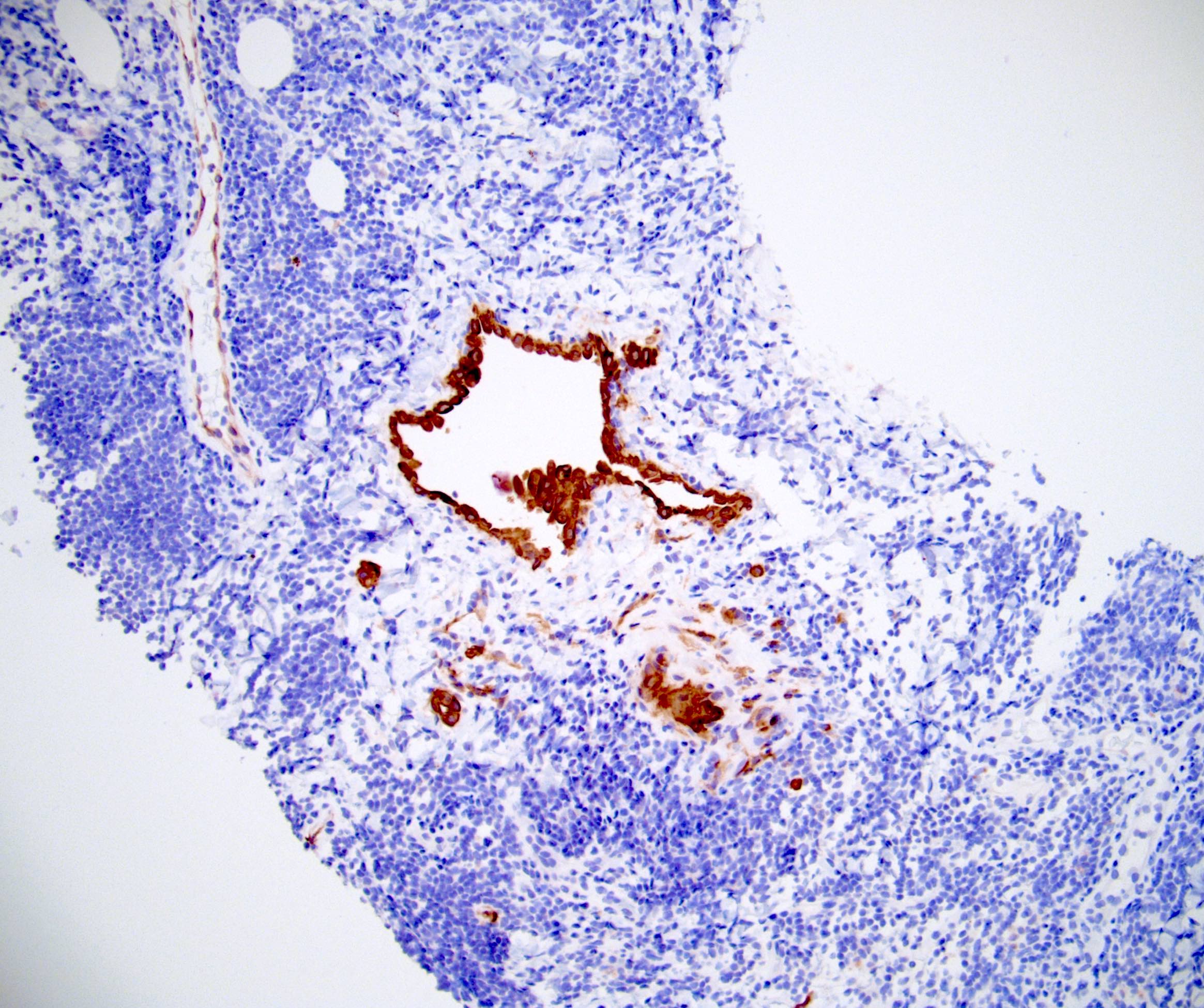
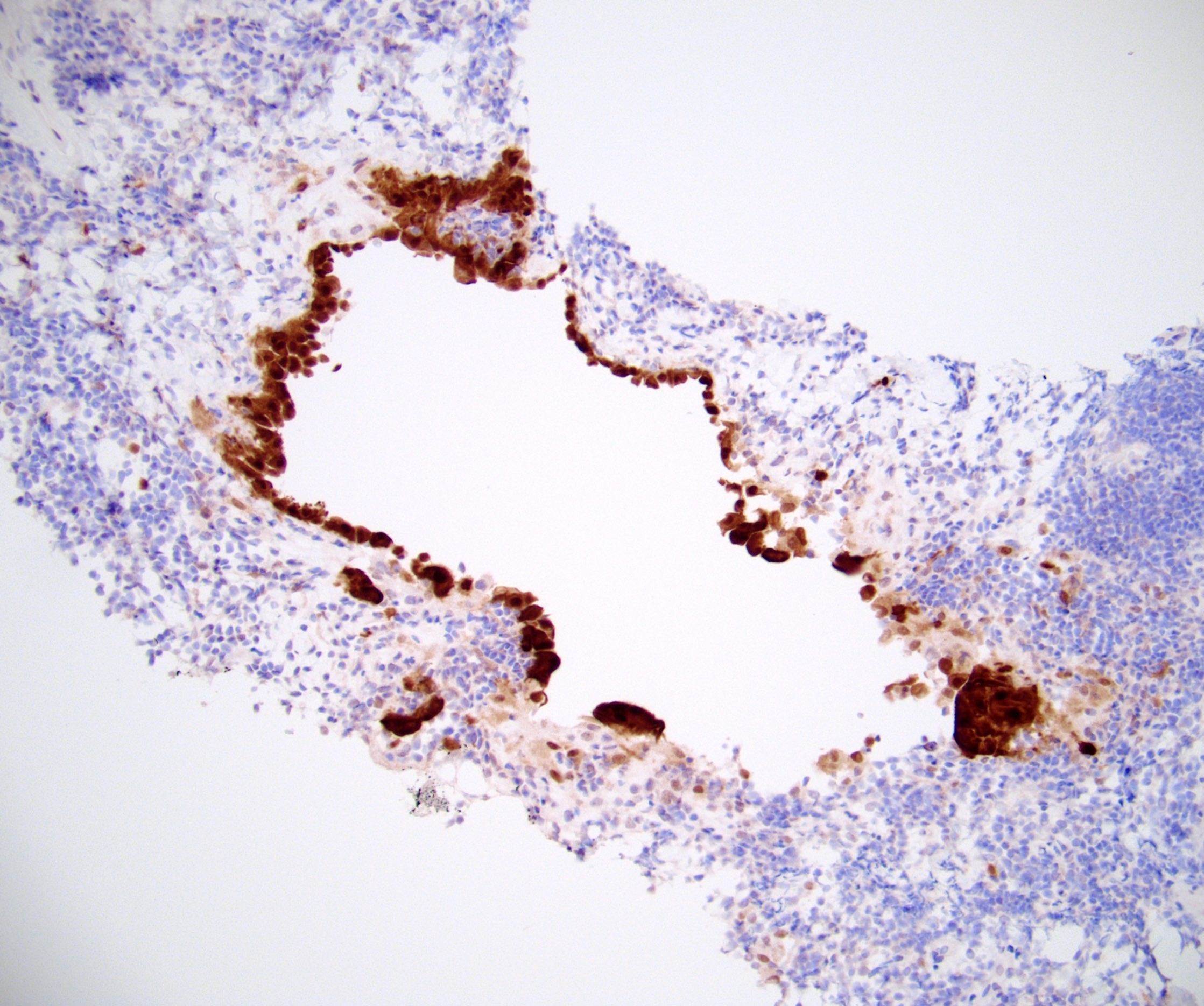
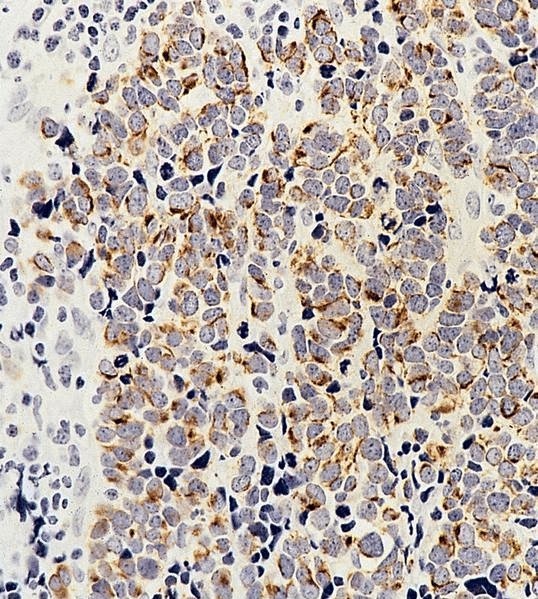
- Lymphoepithelial cyst and epidermoid cyst in accessory spleen located in pancreas (Mod Pathol 1998;11:1171)
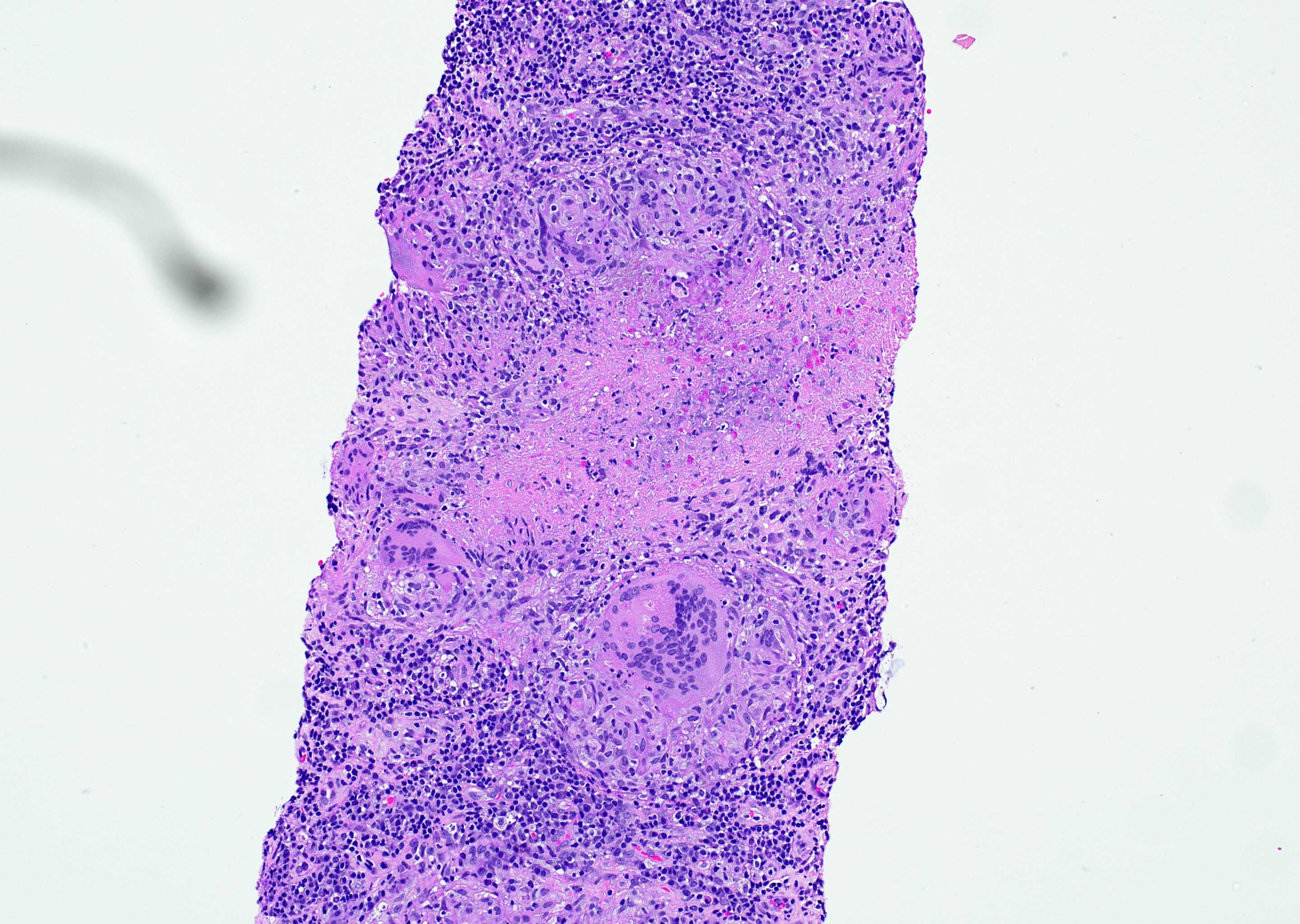
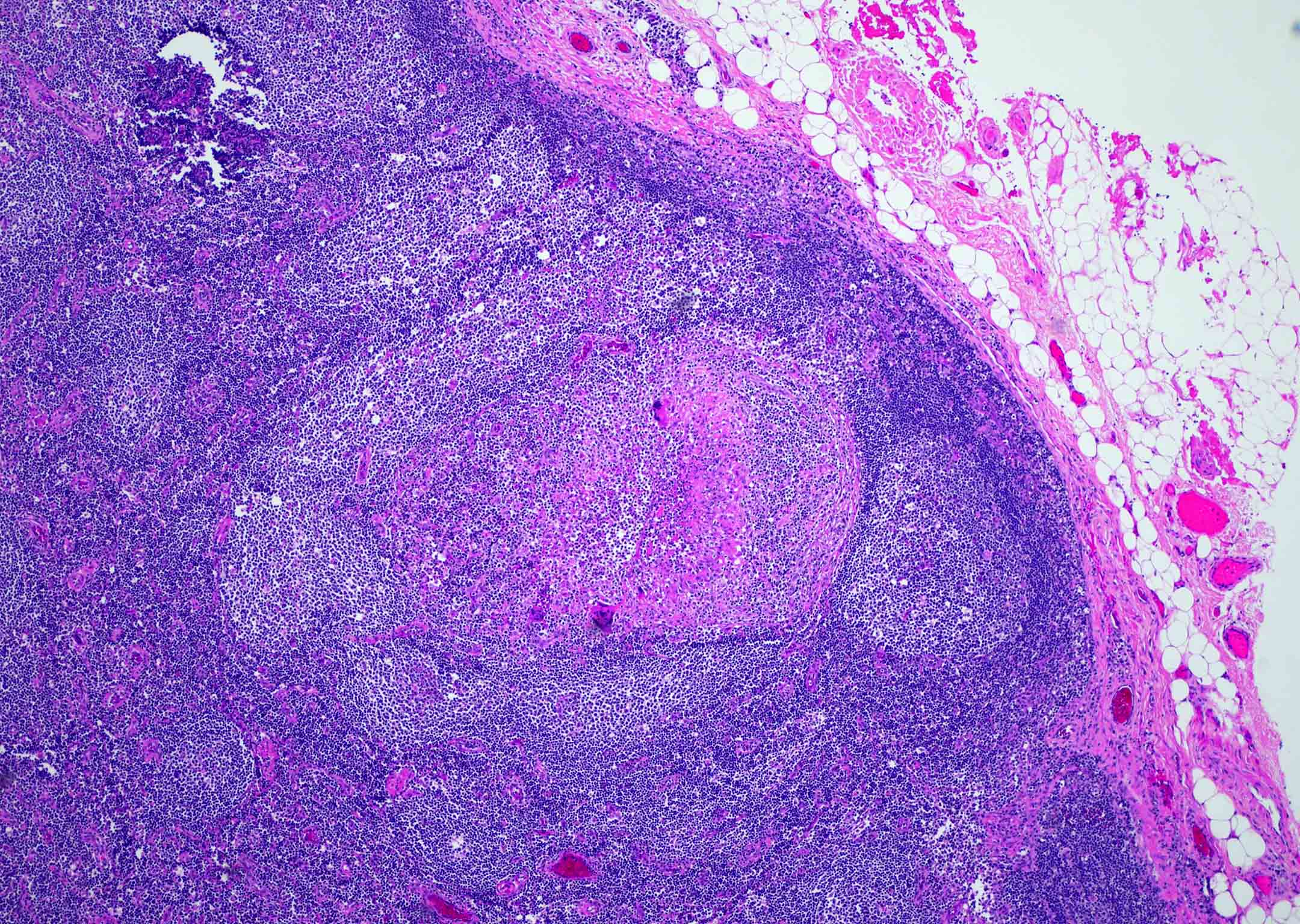
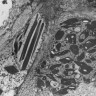
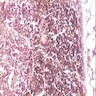
Microscopic (histologic) images
Differential diagnosis
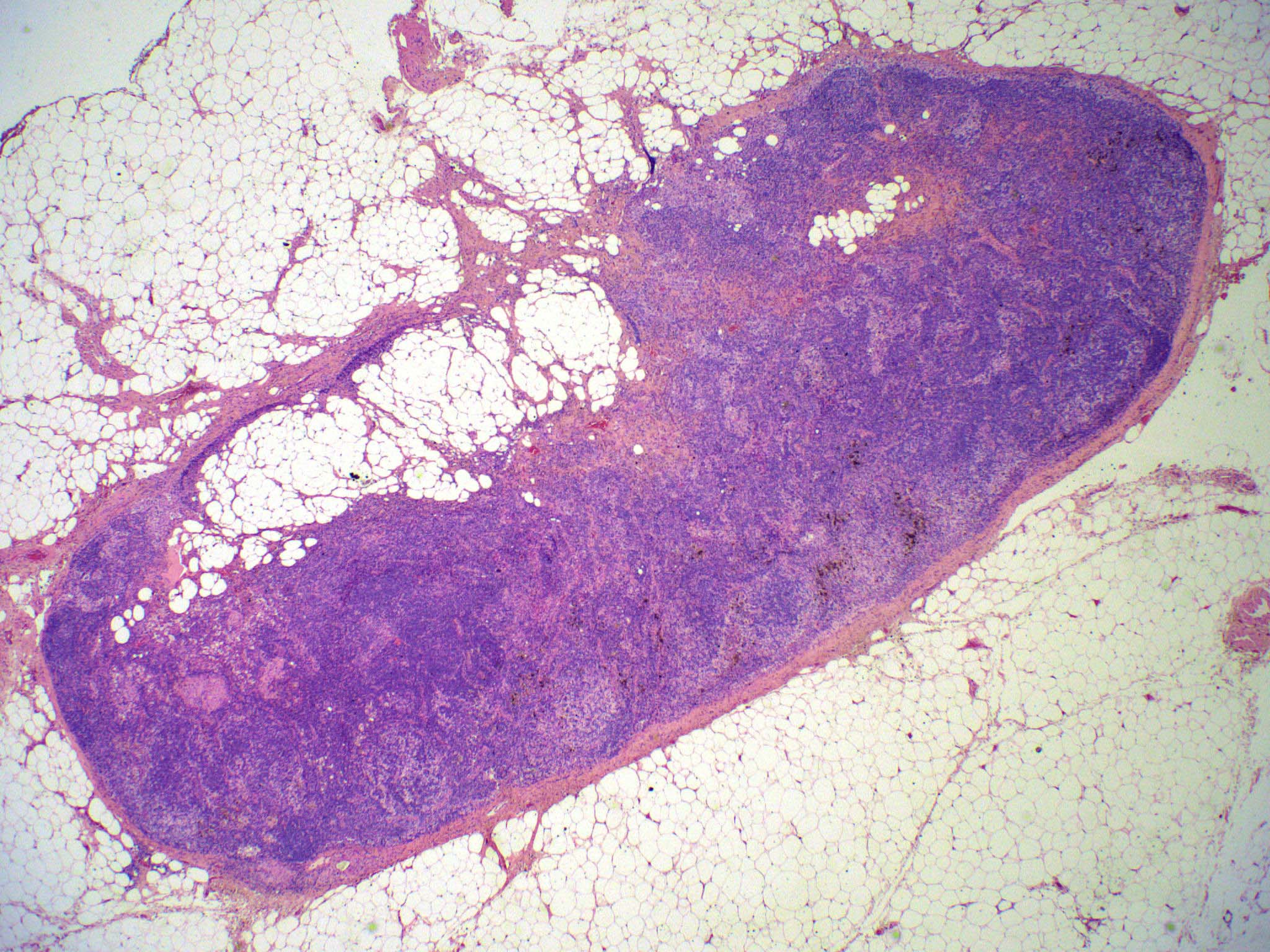
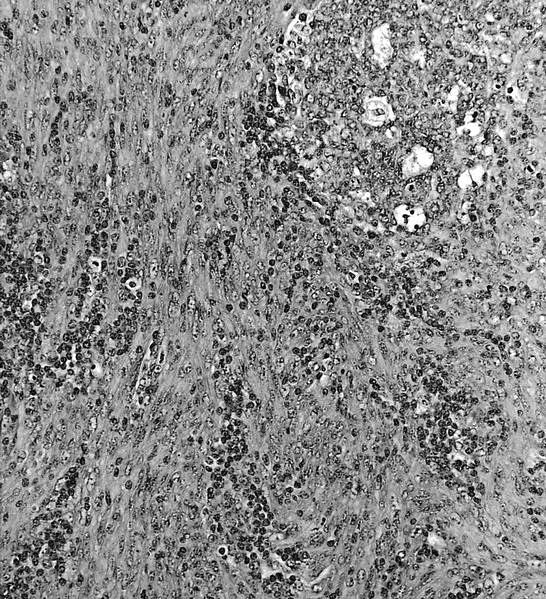
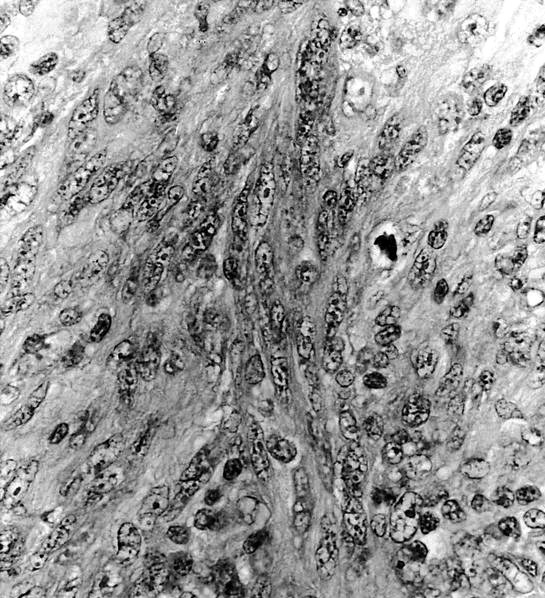
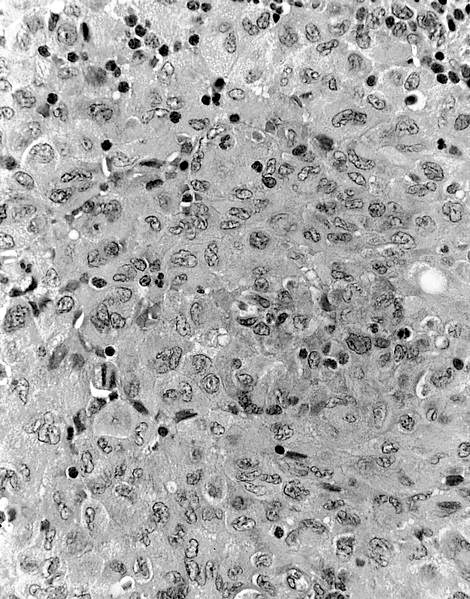
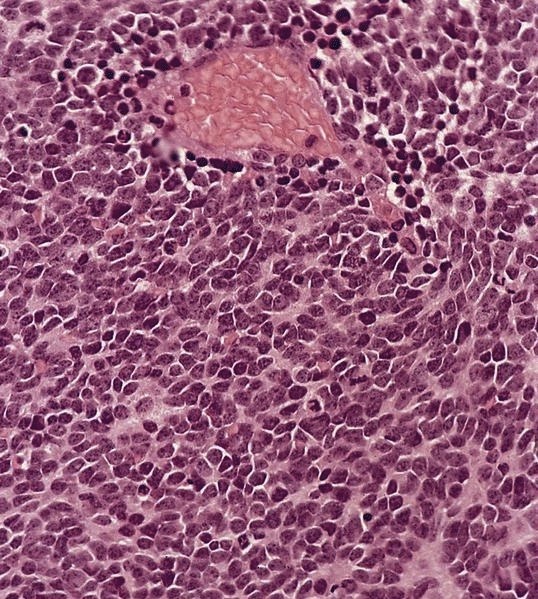
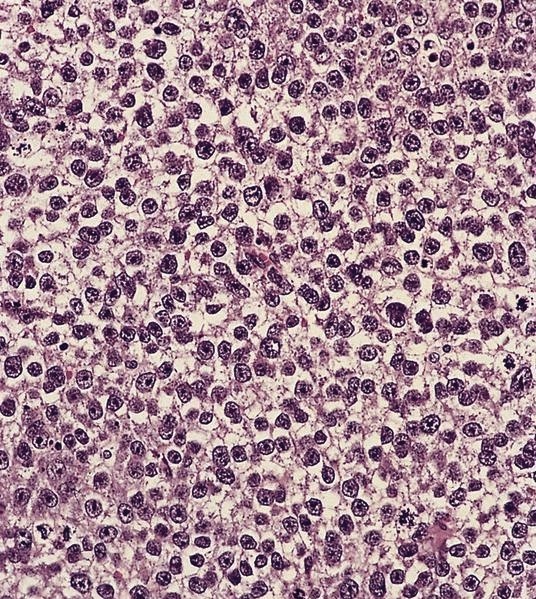
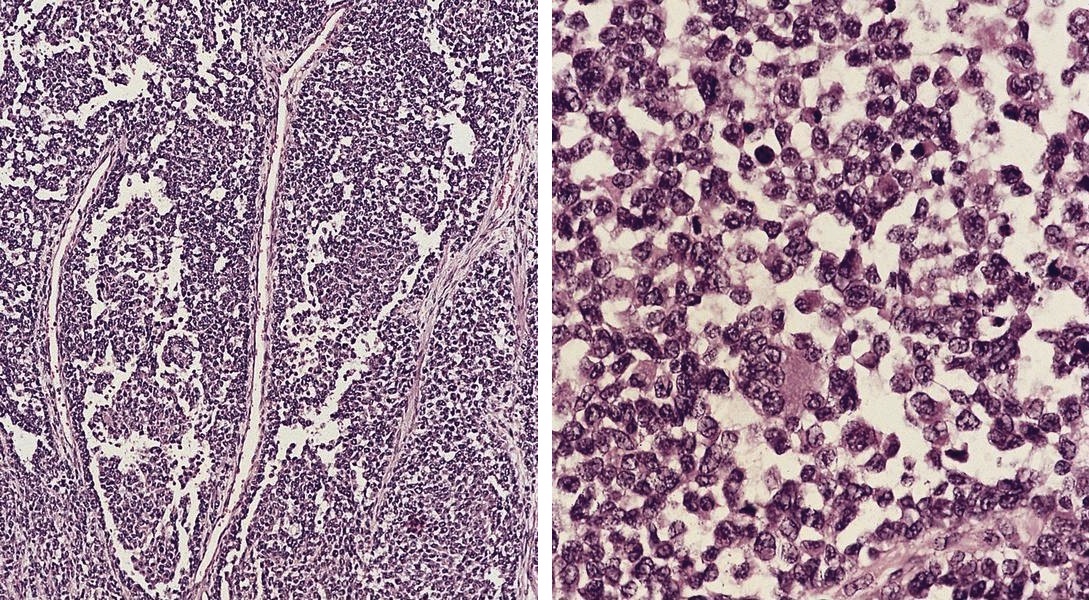
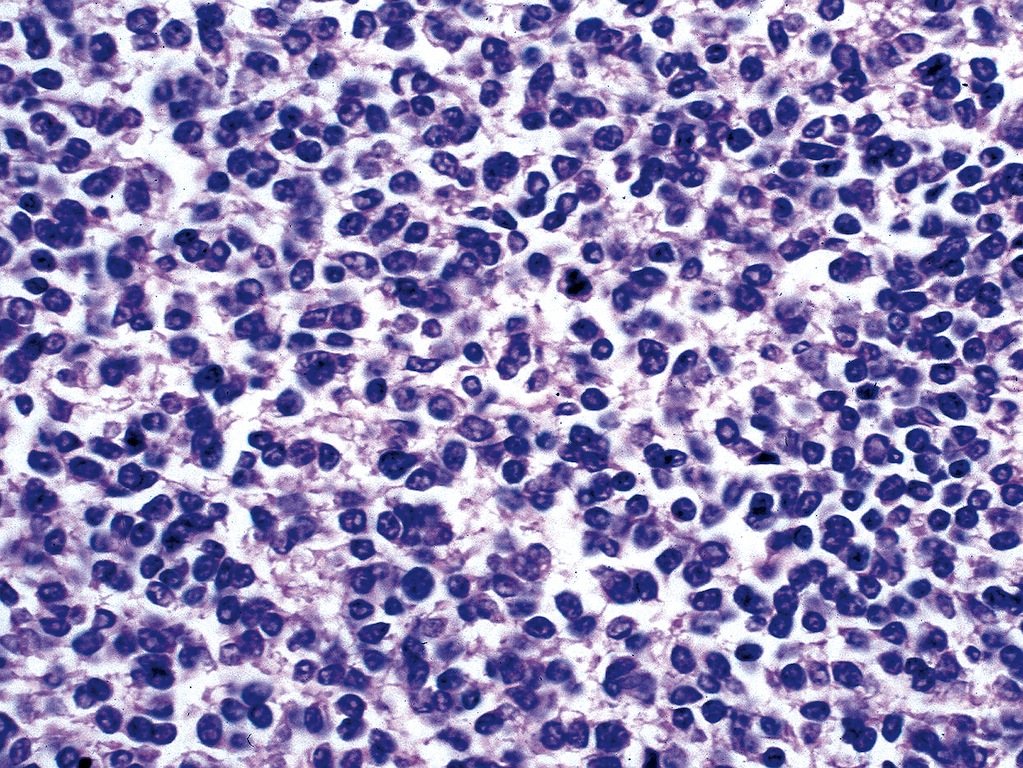
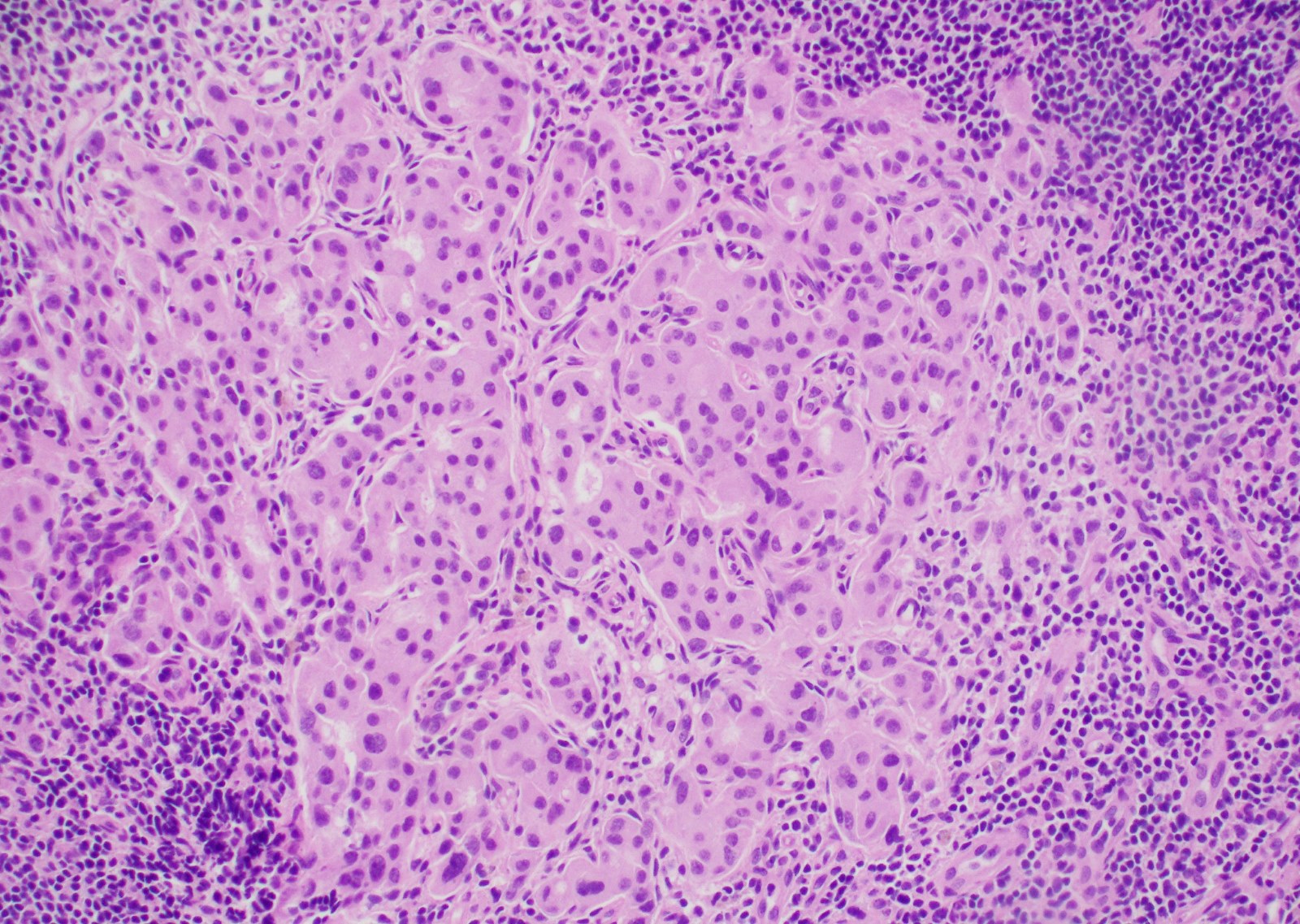
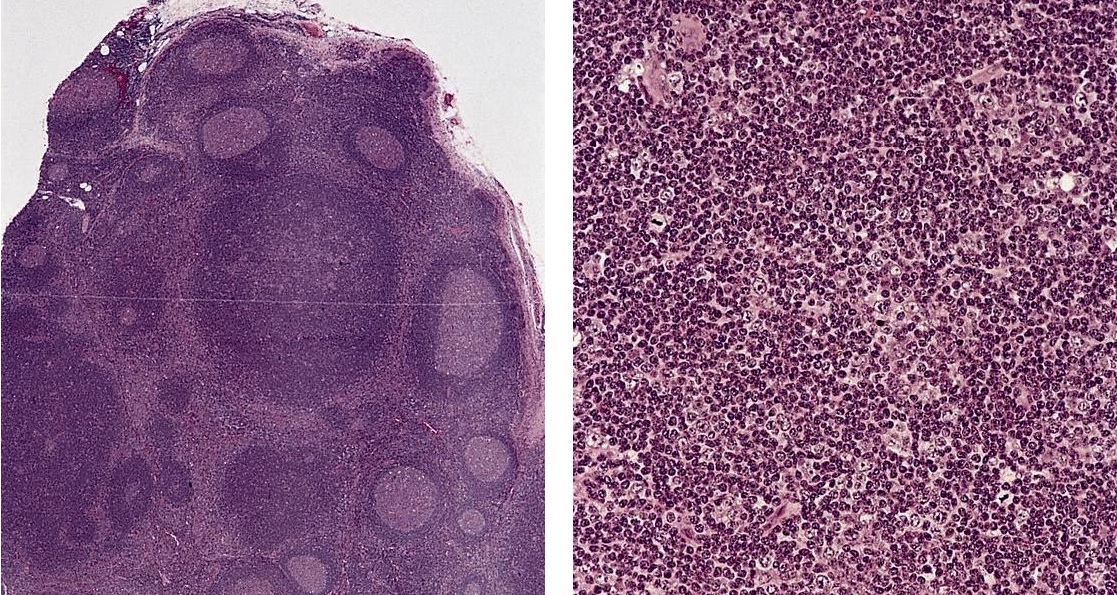
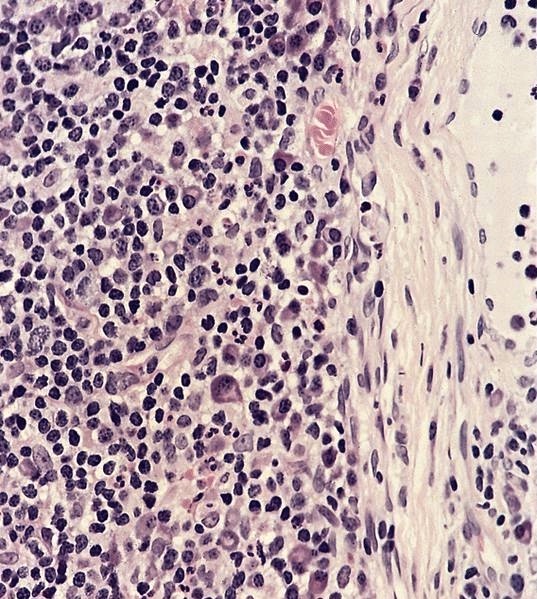
Acute nonspecific lymphadenitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
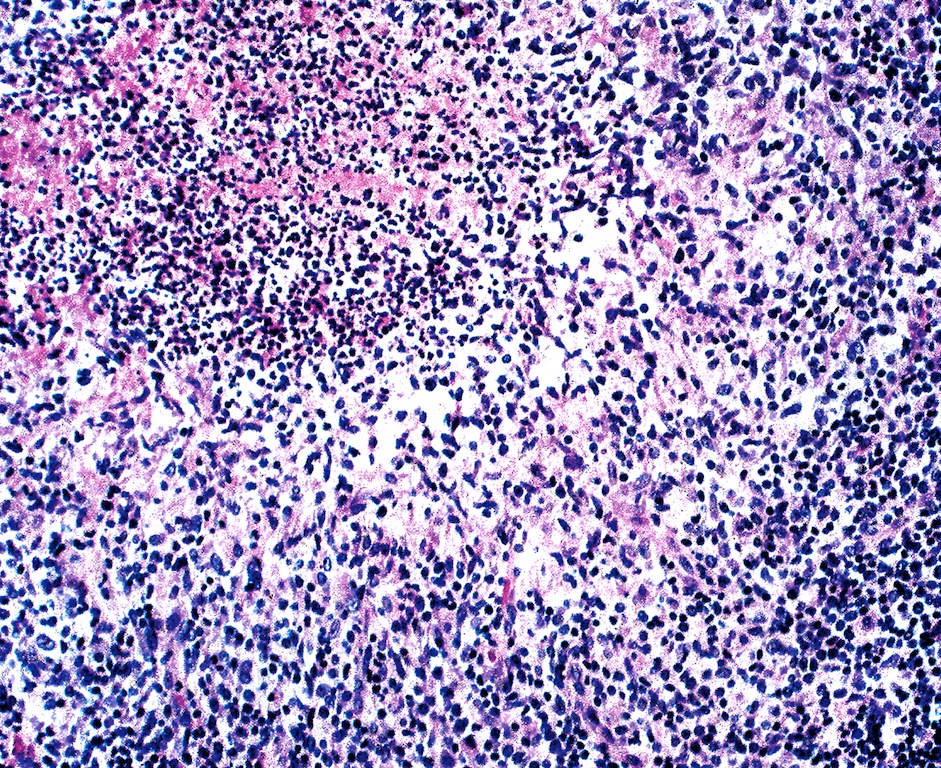
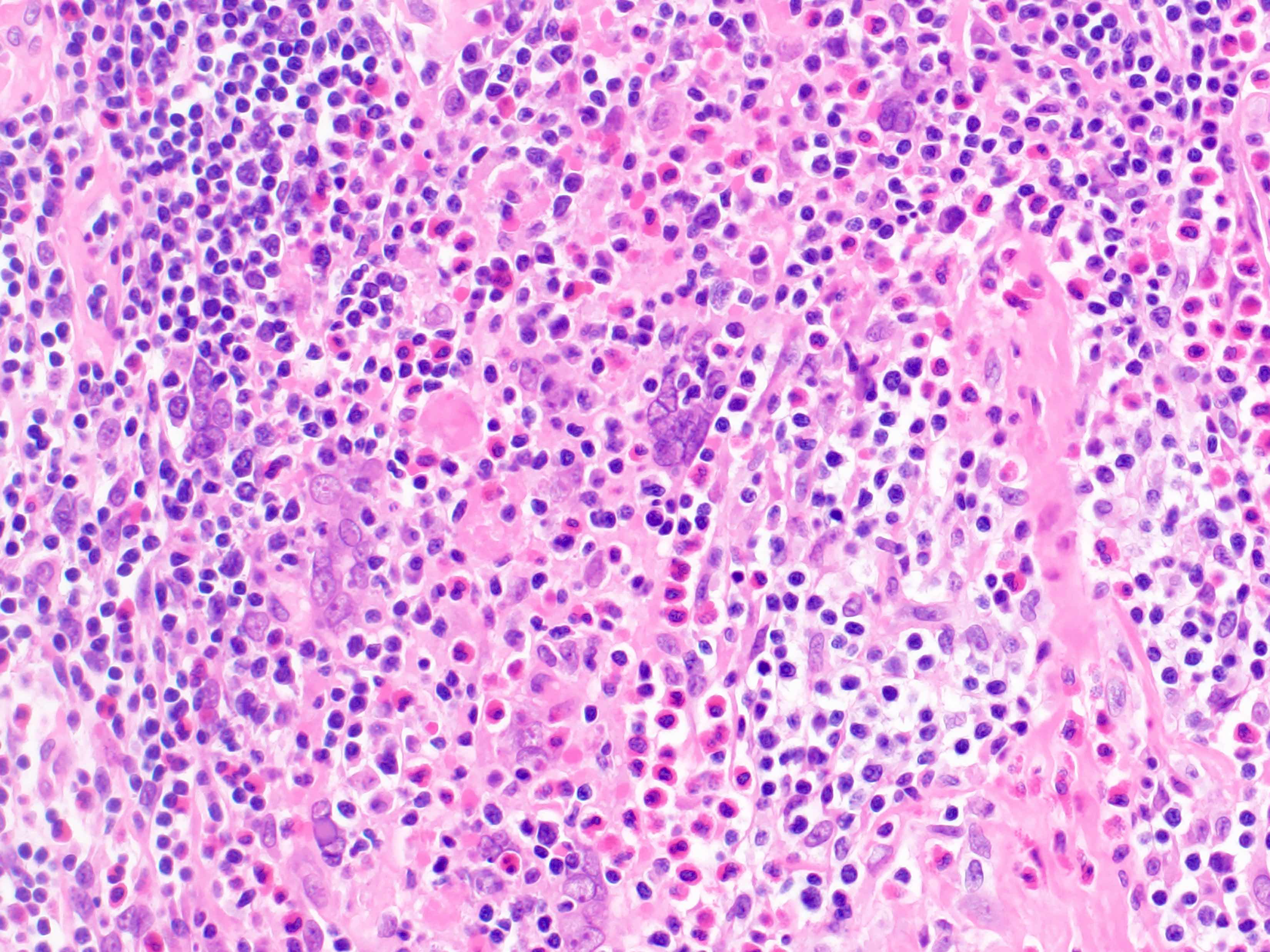
- Acute inflammation of lymph nodes
Essential features
- Enlarged and painful, tender lymph nodes
- Microscopic examination, if performed, will show sinus dilatation followed by accumulation of neutrophils, vascular dilatation and edema of the capsule
Terminology
- Acute nonspecific lymphadenitis, nontuberculous lymphadenitis
ICD coding
Epidemiology
- Common, mostly affects children
Sites
- Cervical lymph nodes are commonly affected
- Acute nonspecific mesenteric lymphadenitis is also a relatively common in children
Pathophysiology
- Acute inflammation of the involved lymph node / nodes due to an infectious or inflammatory etiology
Etiology
- Most commonly due to viral infections
- Most common bacterial causes are Staphylococcus aureus and beta hemolytic Streptococcus
- Inflammation of the draining sites or direct inflammation of the lymph nodes can be the cause
Clinical features
- Enlarged painful / tender lymph nodes, redness of overlying skin, low grade fever, malaise
Diagnosis
- Clinical examination, exclude specific causes
Laboratory
- Depending on the cause, CBC may show leukocytosis with neutrophilia or lymphocytosis, elevated ESR and bacterial / viral confirmatory tests may be positive
Prognostic factors
- Good prognosis
Treatment
- Treat the underlying cause, supportive therapy
Gross description
- Enlarged lymph node
- If bacterial infection, there can be suppuration leading to necrosis and abscess formation
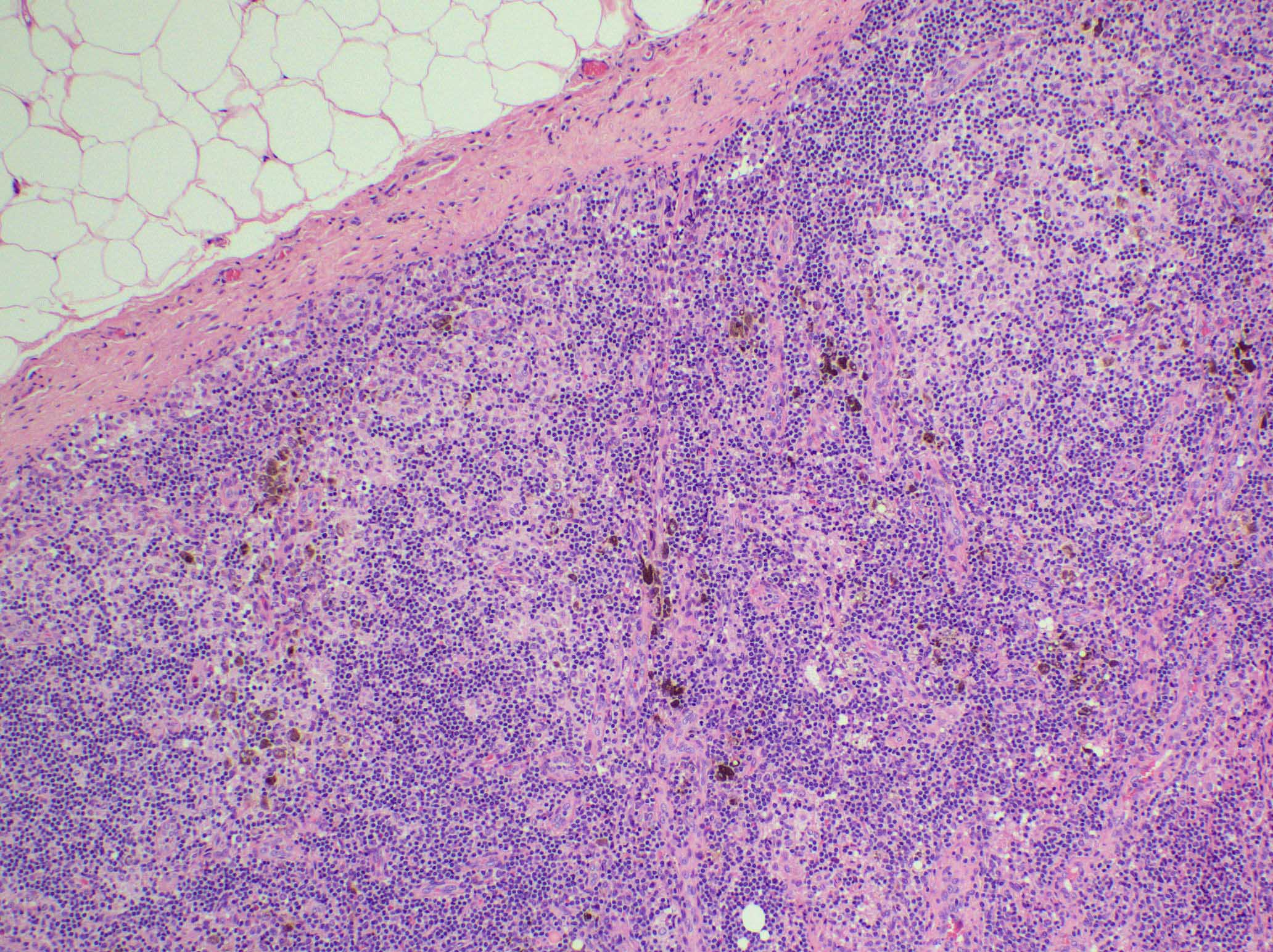
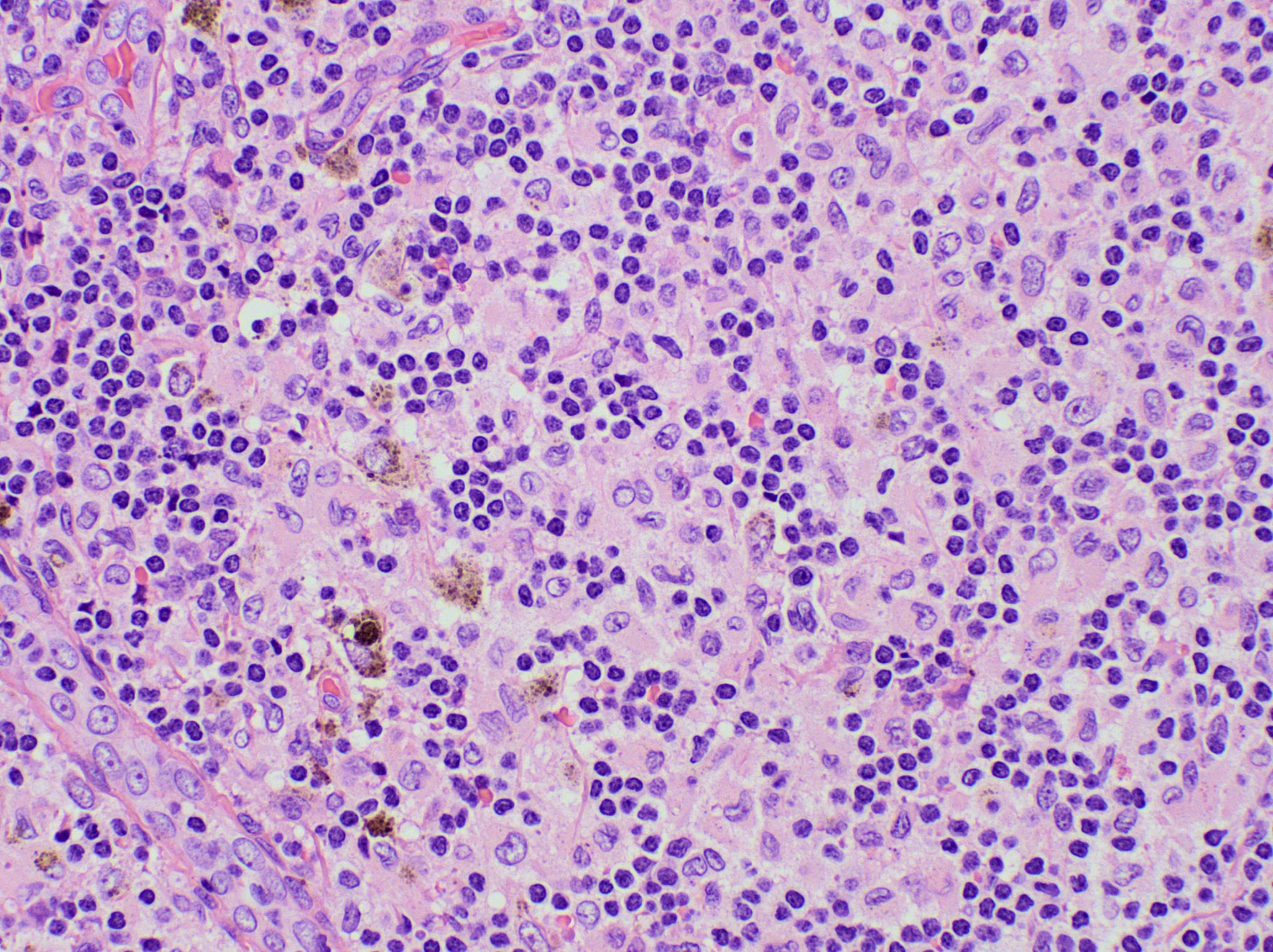
Microscopic (histologic) description
- Biopsy is rarely performed
- Microscopic examination, if performed, will show sinus dilatation followed by accumulation of neutrophils, vascular dilatation and edema of the capsule
- If bacterial origin, suppurative inflammation is present
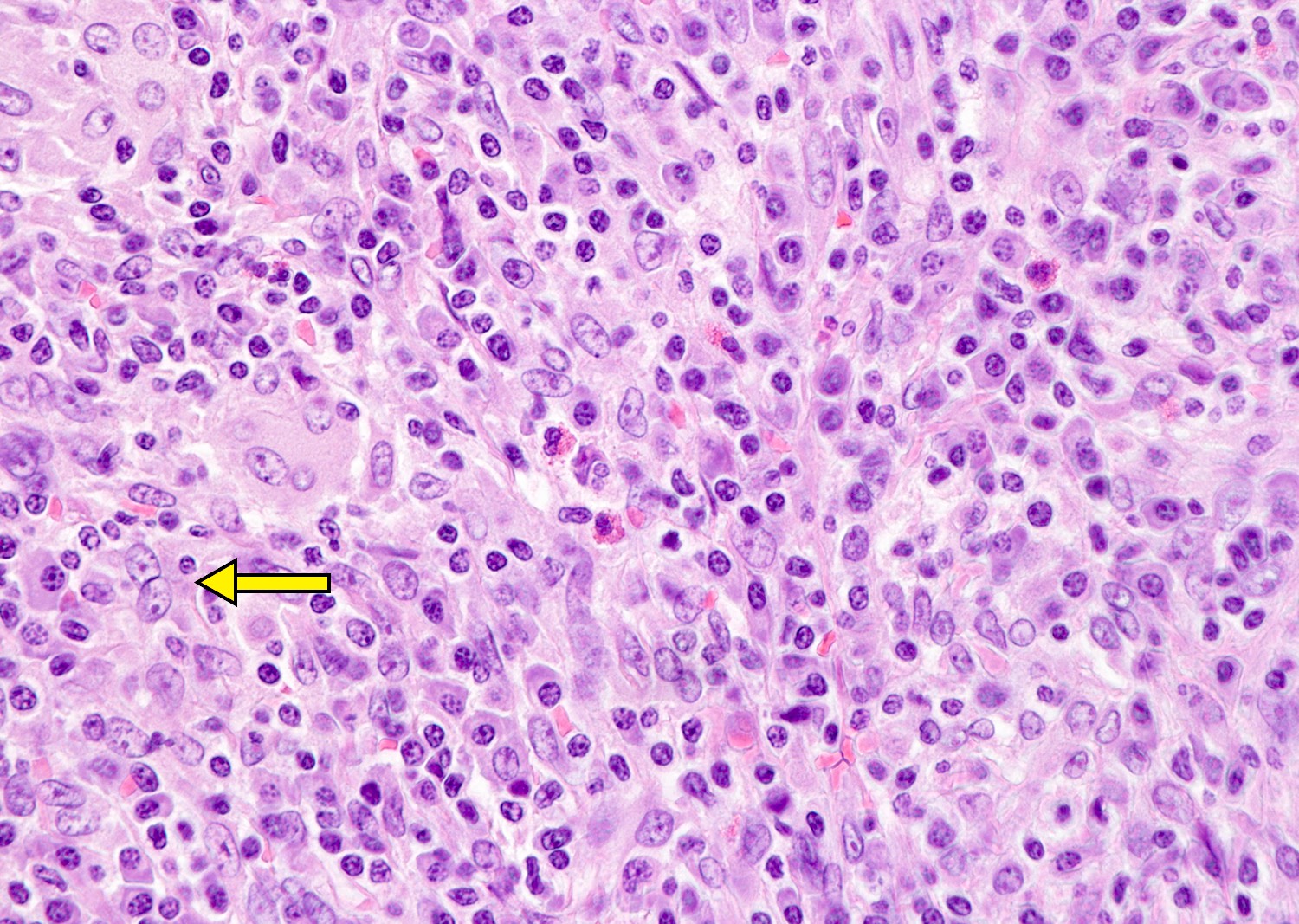
- Necrotizing inflammation can be seen in bubonic plague and tularemia, Still disease and Kikuchi necrotizing lymphadenitis
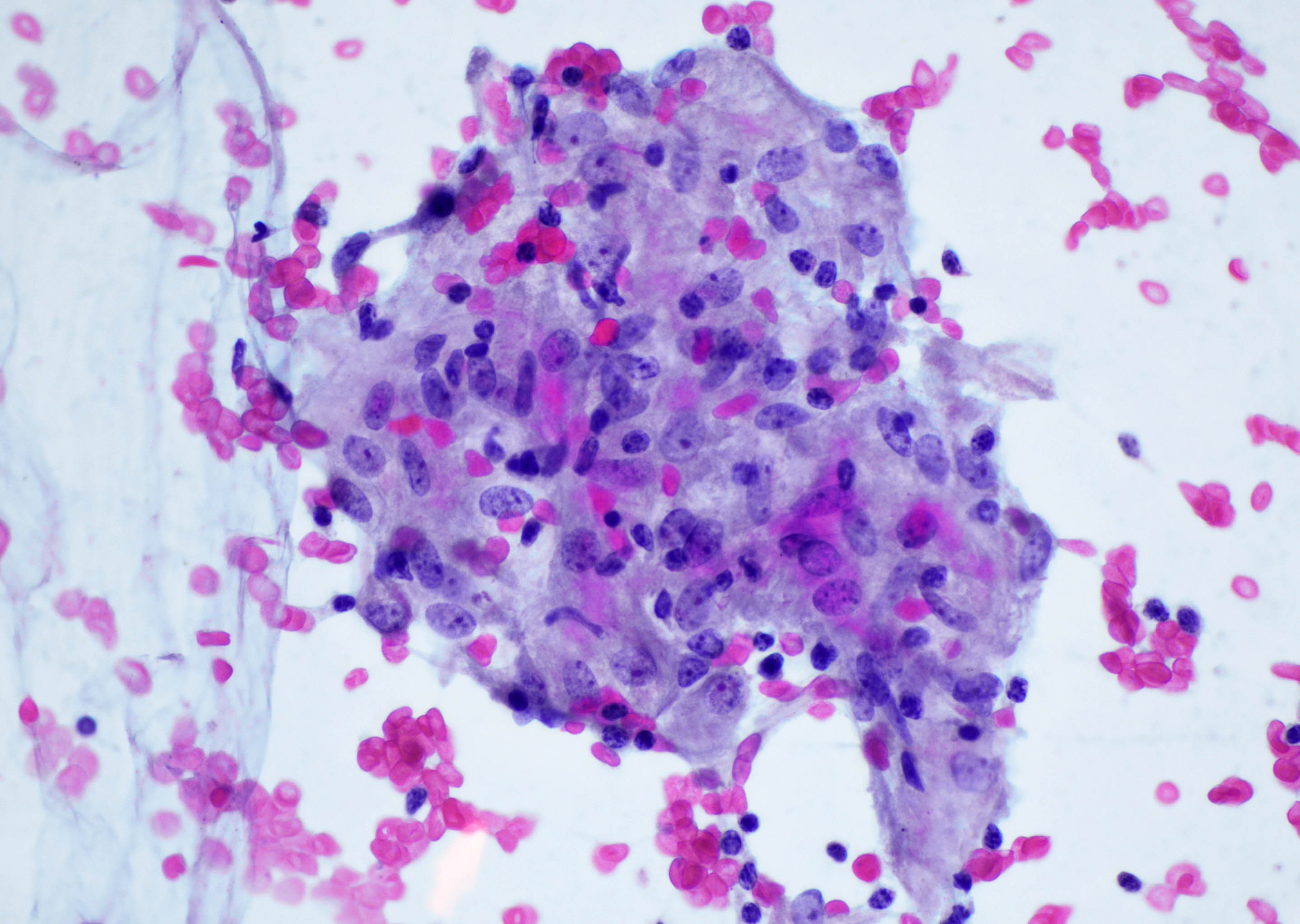
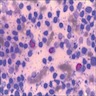
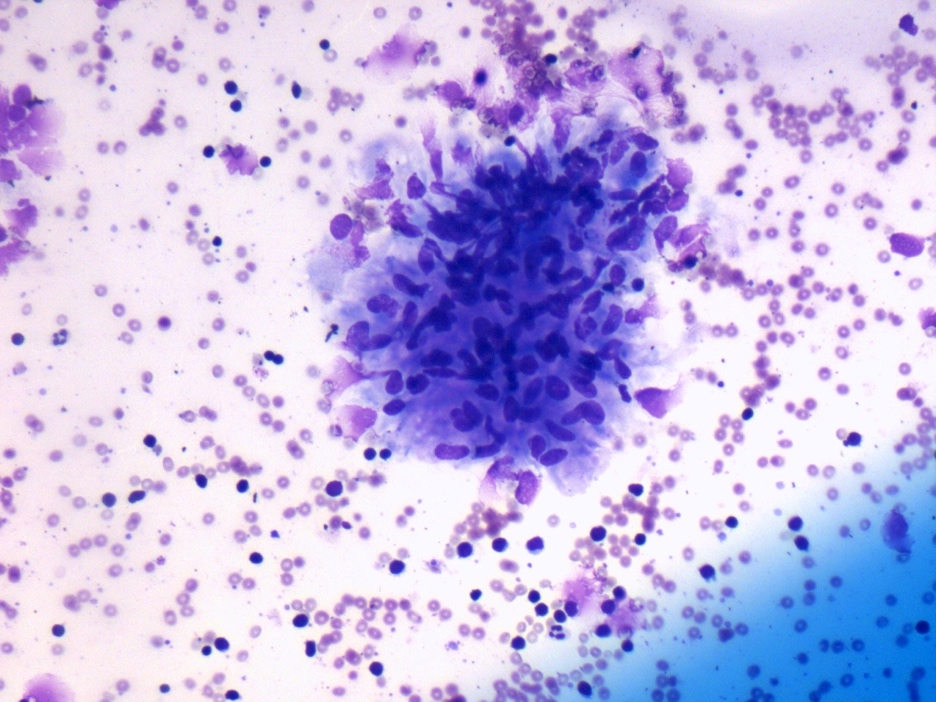
Cytology description
- Mixed small and large lymphocytes admixed with neutrophils
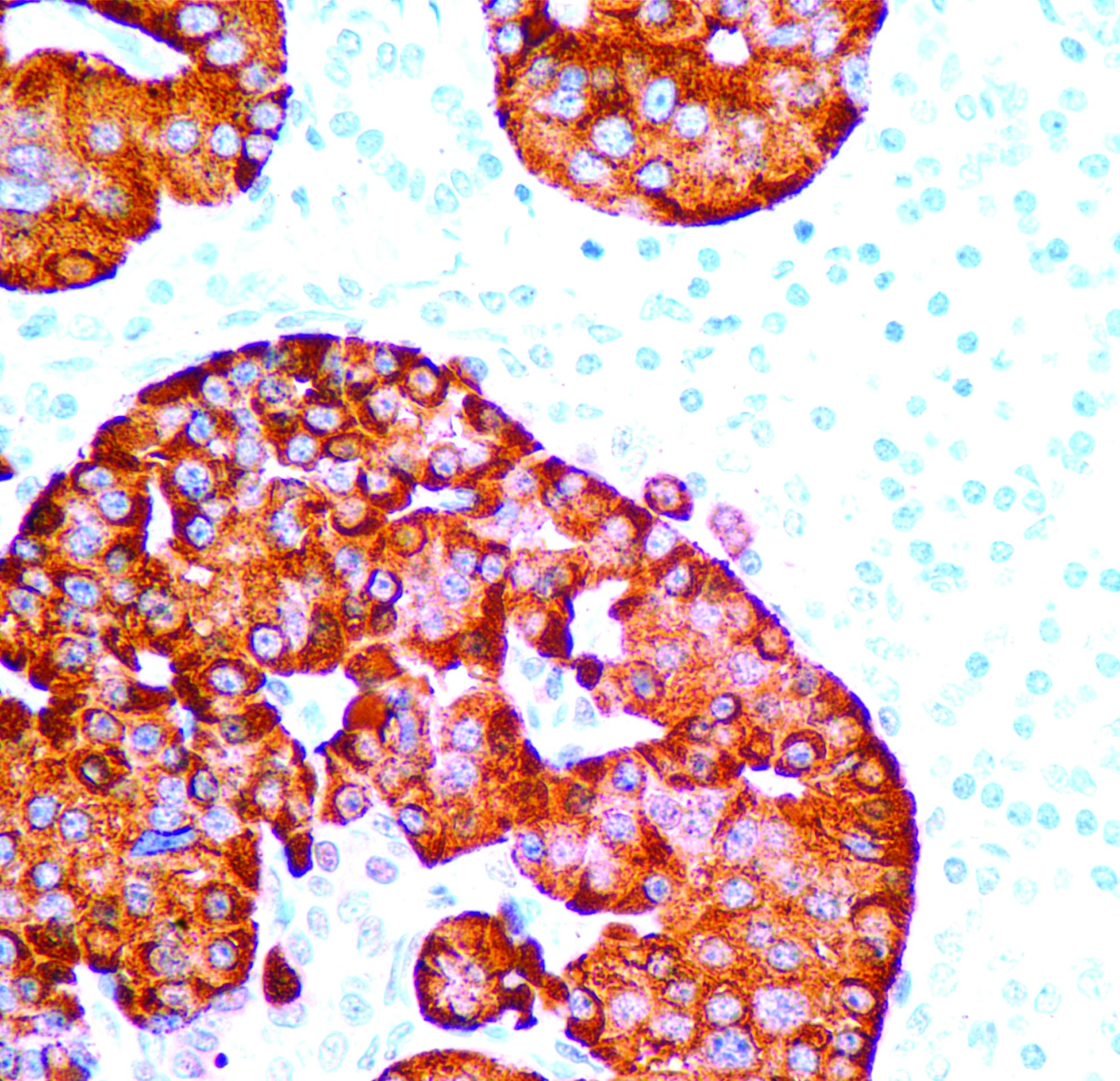
Positive stains
- Varies by etiology
- Gram stain highlights bacteria, if present
Differential diagnosis
- Specific causes should be ruled out
- Suppurative lymphadenitis caused by bacterial infections, mesenteric lymphadenitis, lymphogranuloma venereum, cat scratch disease, bubonic plague, tularemia, anthrax, typhoid fever, melioidosis
Additional references
Board review style question #1
23 year old woman presents with tender right cervical lymph node enlargement. She also complains of low grade fever and feeling tired. On examination she is found to have three 4 mm red papules on her right shoulder. On further enquiry she informs that she owns two cats. What is the best confirmatory test at this point?
A. Biopsy of the lymph node
B. Biopsy of the skin lesion
C. PCR
D. Serology
A. Biopsy of the lymph node
B. Biopsy of the skin lesion
C. PCR
D. Serology
Board review style answer #1
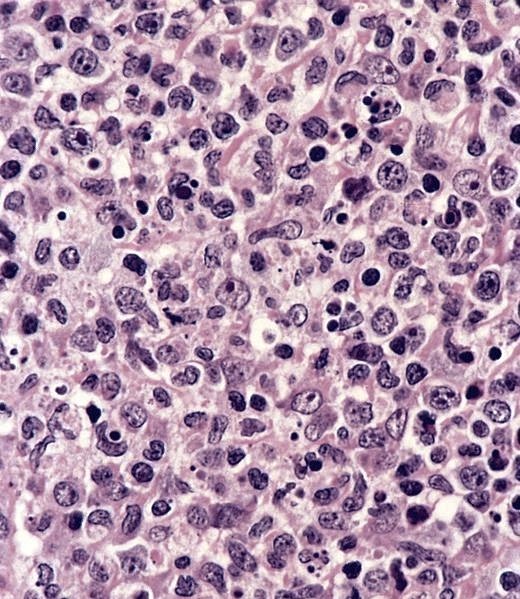
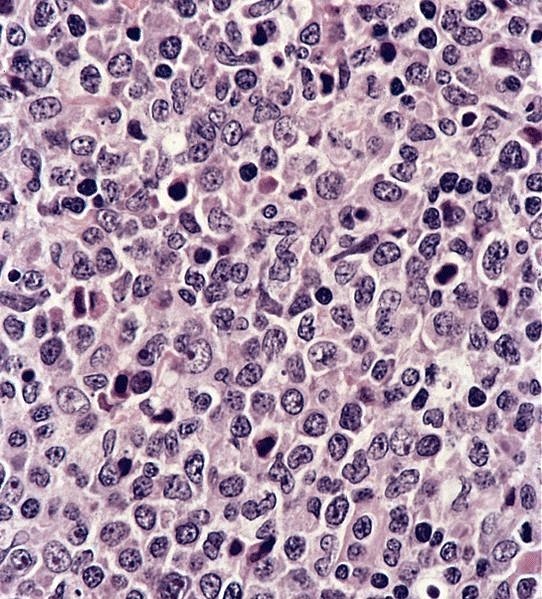
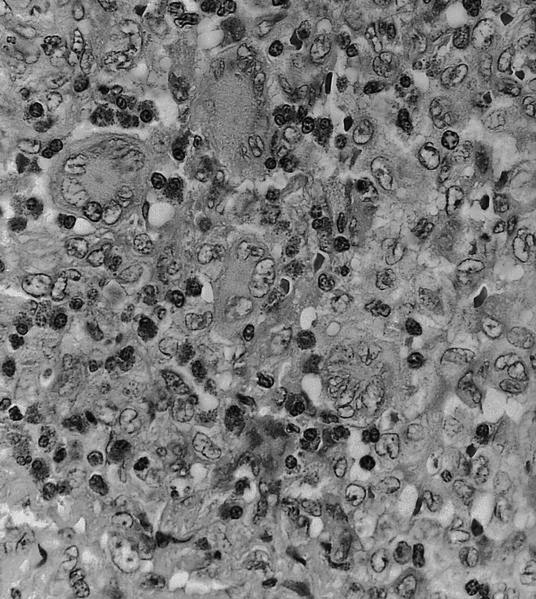
D. Serology. Serology for IgG and IgM antibodies for Bartonella henslae is confirmatory for cat scratch disease. Biopsy is rarely indicated. If performed, a biopsy will show lymphoid hyperplasia, granuloma formation, stellate abscesses and vascular proliferation depending on the stage of the disease. PCR can be done on biopsy specimen to confirm the diagnosis.
Comment Here
Reference: Acute nonspecific lymphadenitis
Comment Here
Reference: Acute nonspecific lymphadenitis
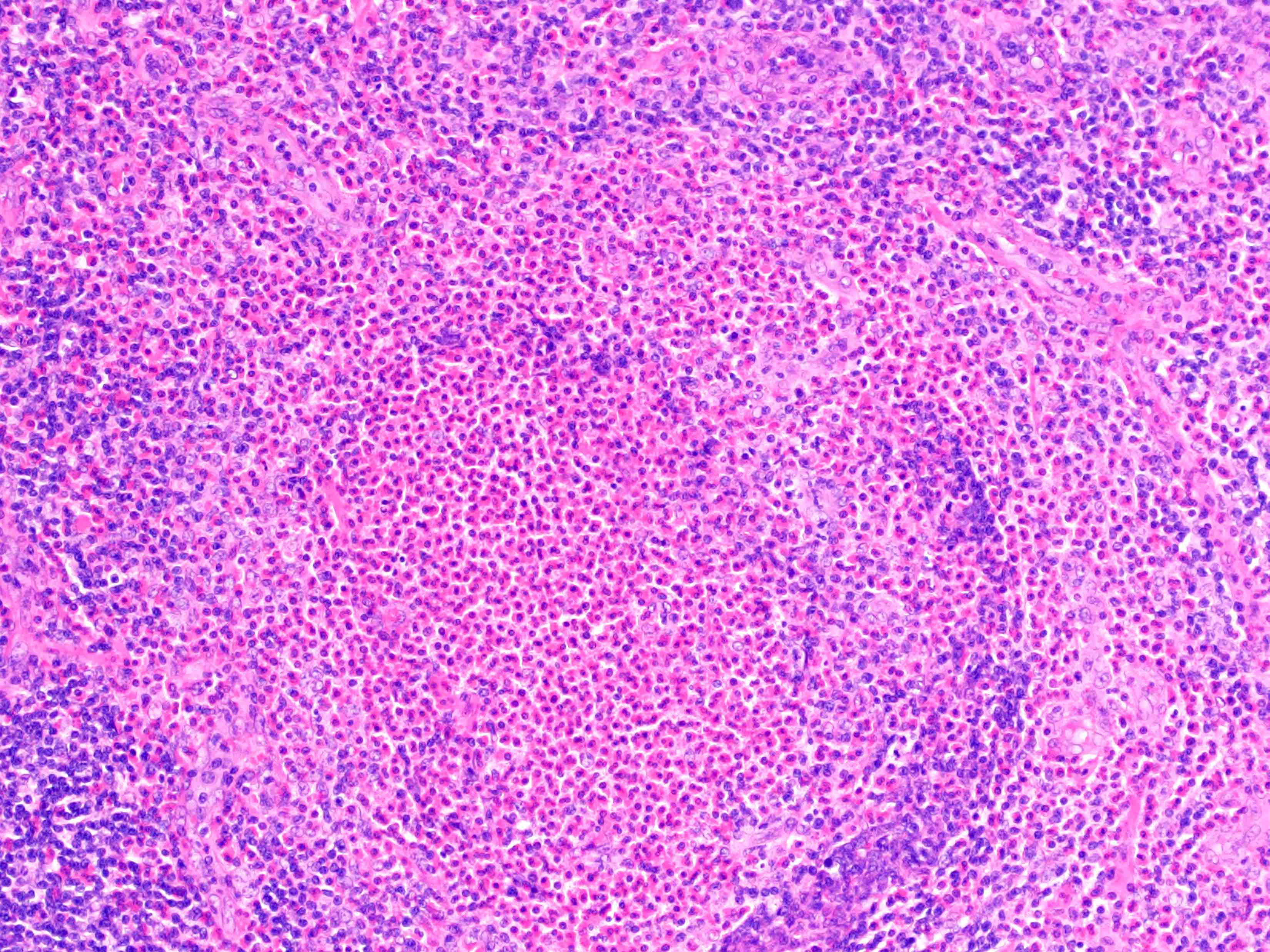
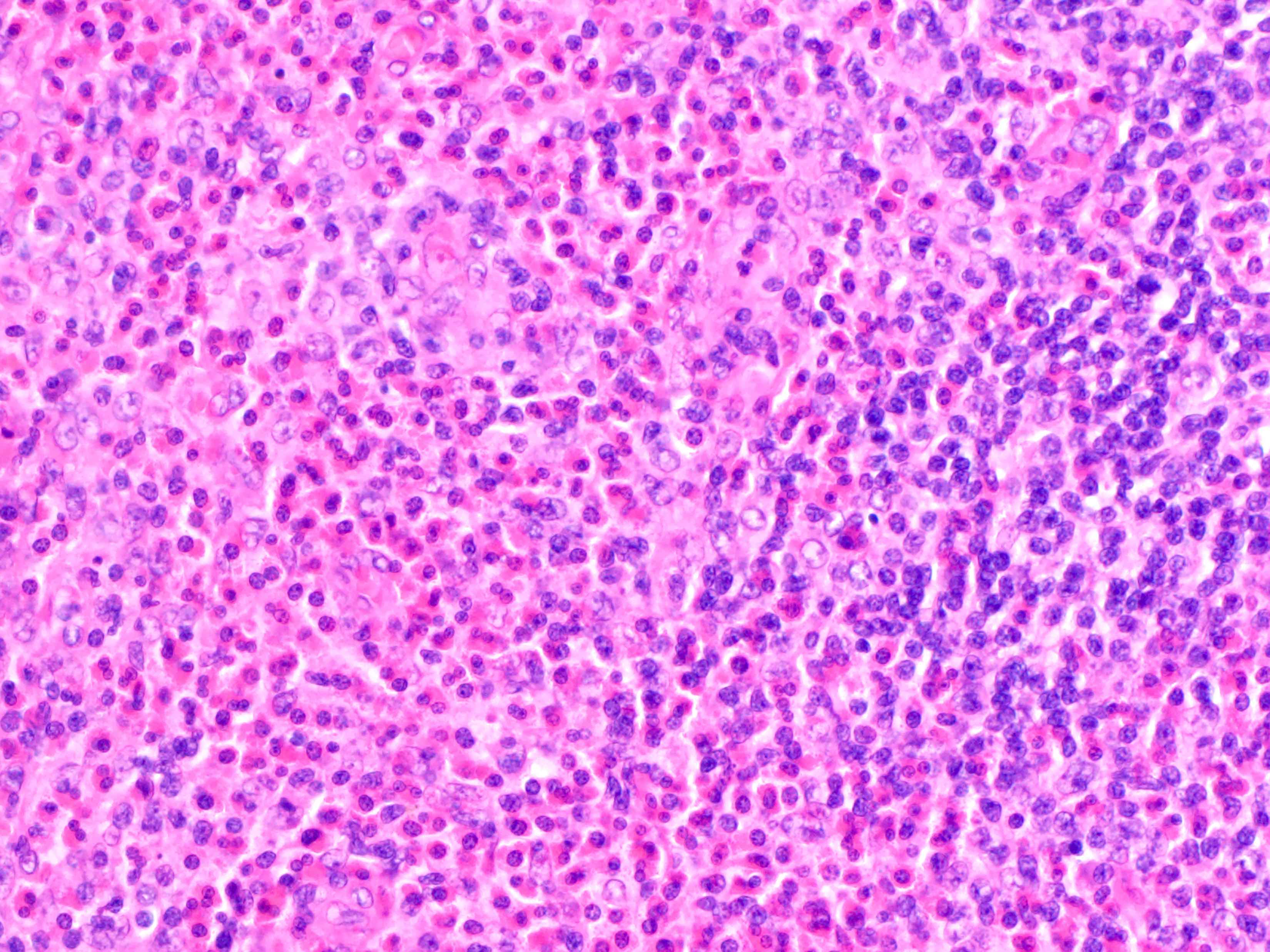
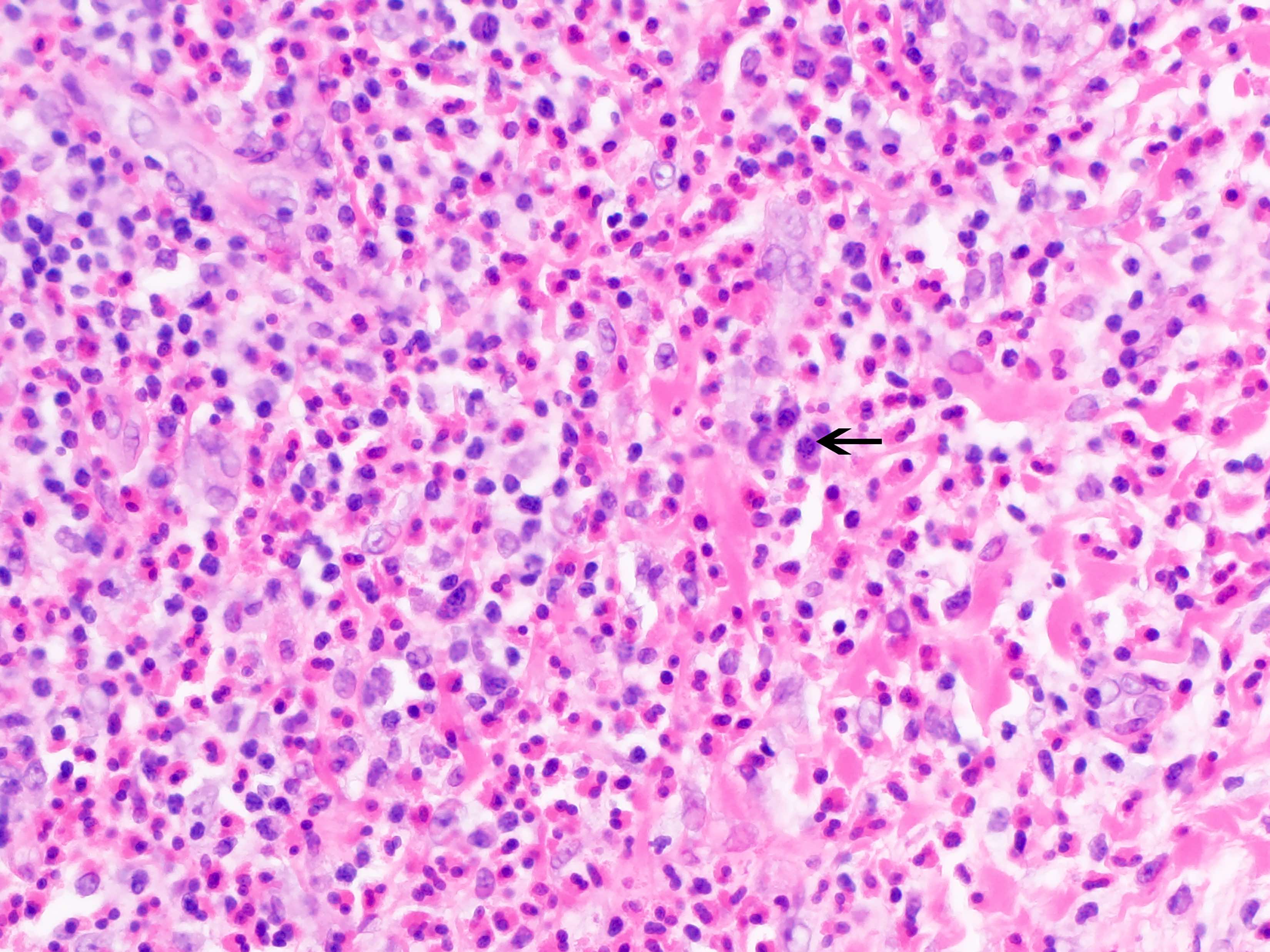
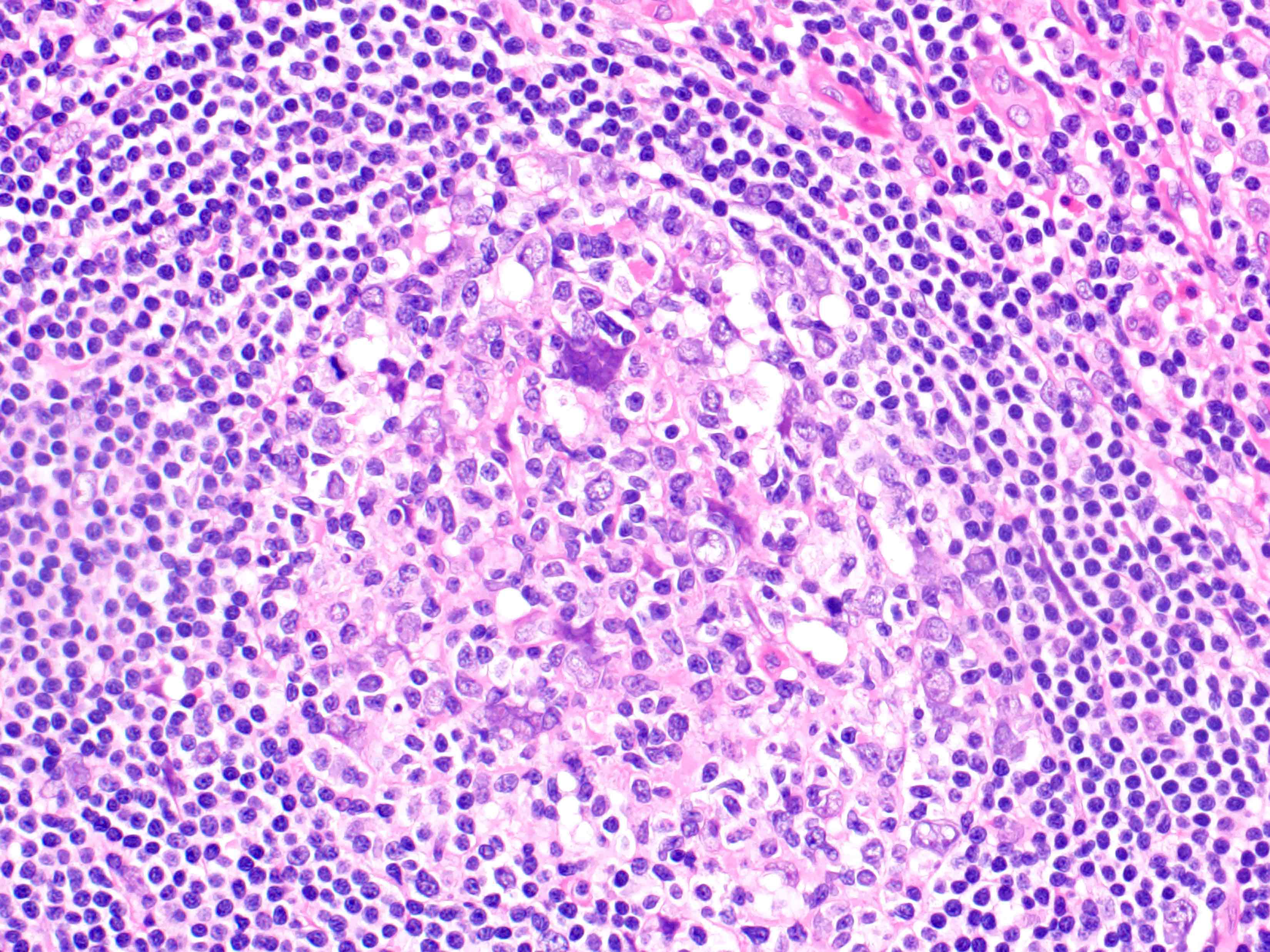
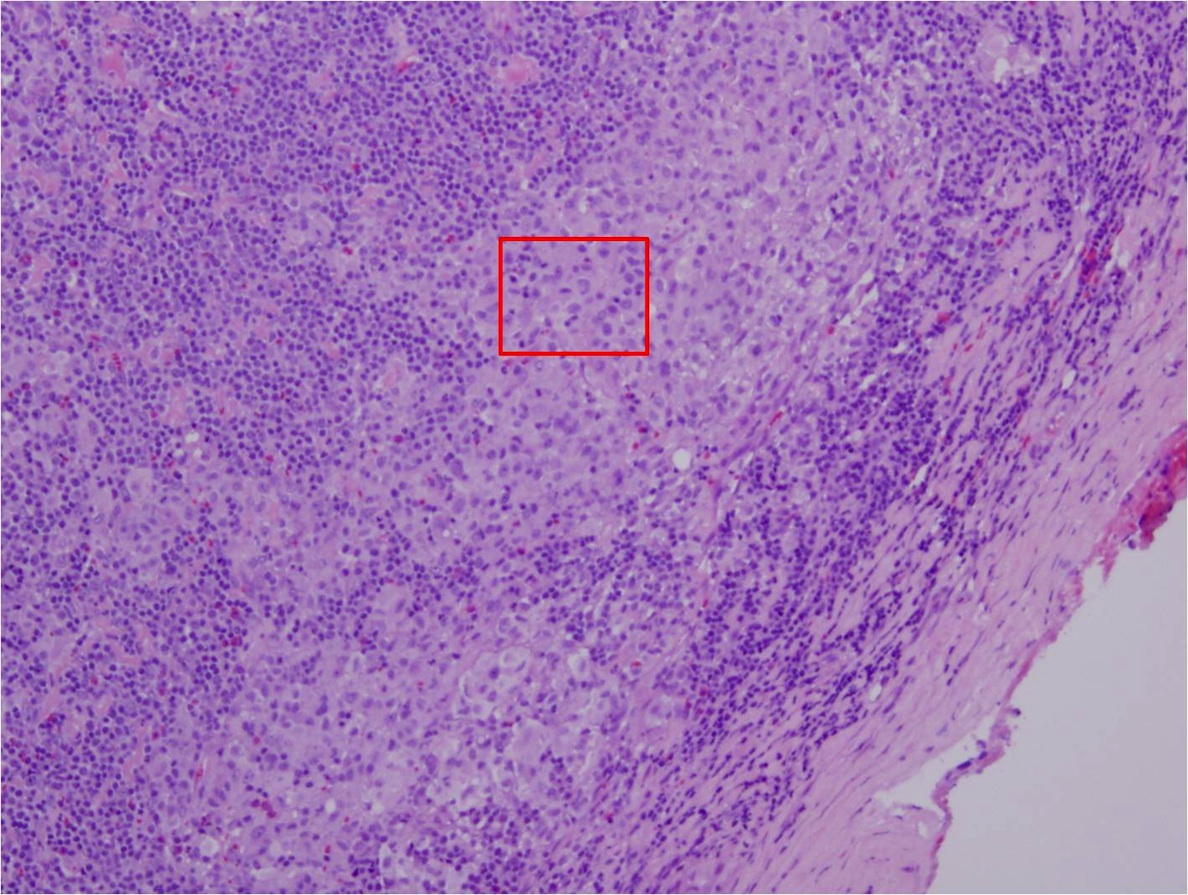
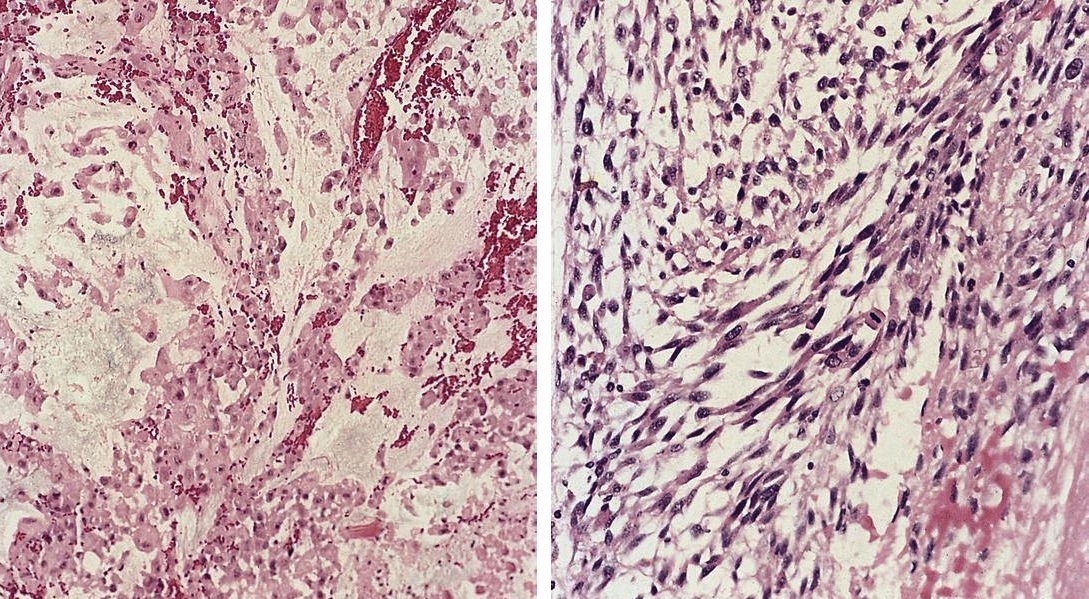
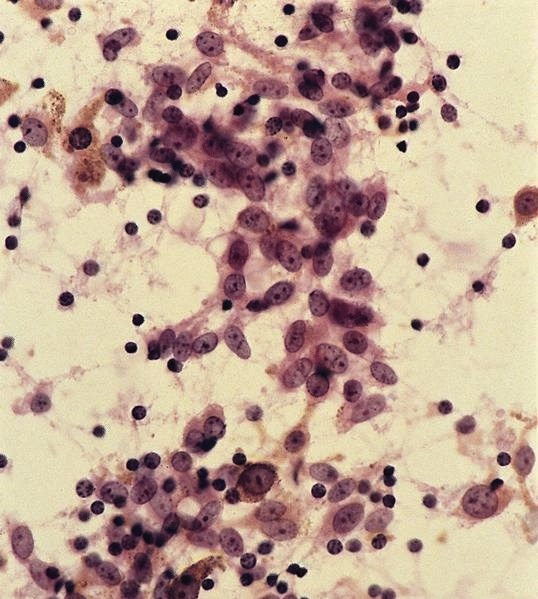
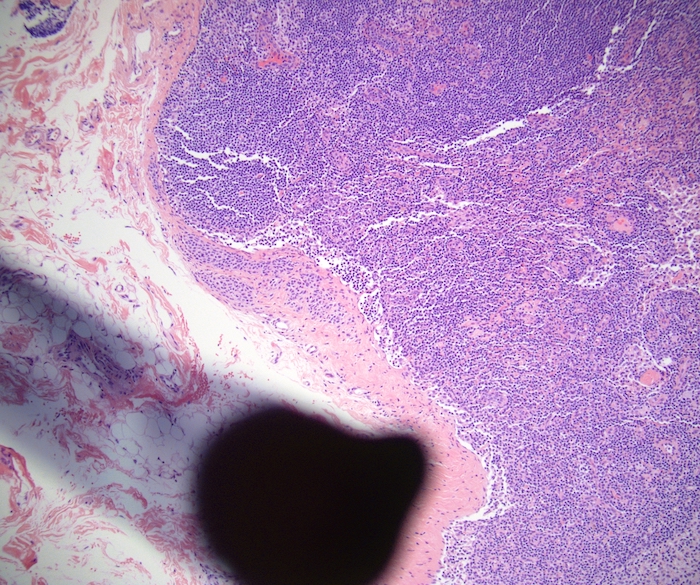
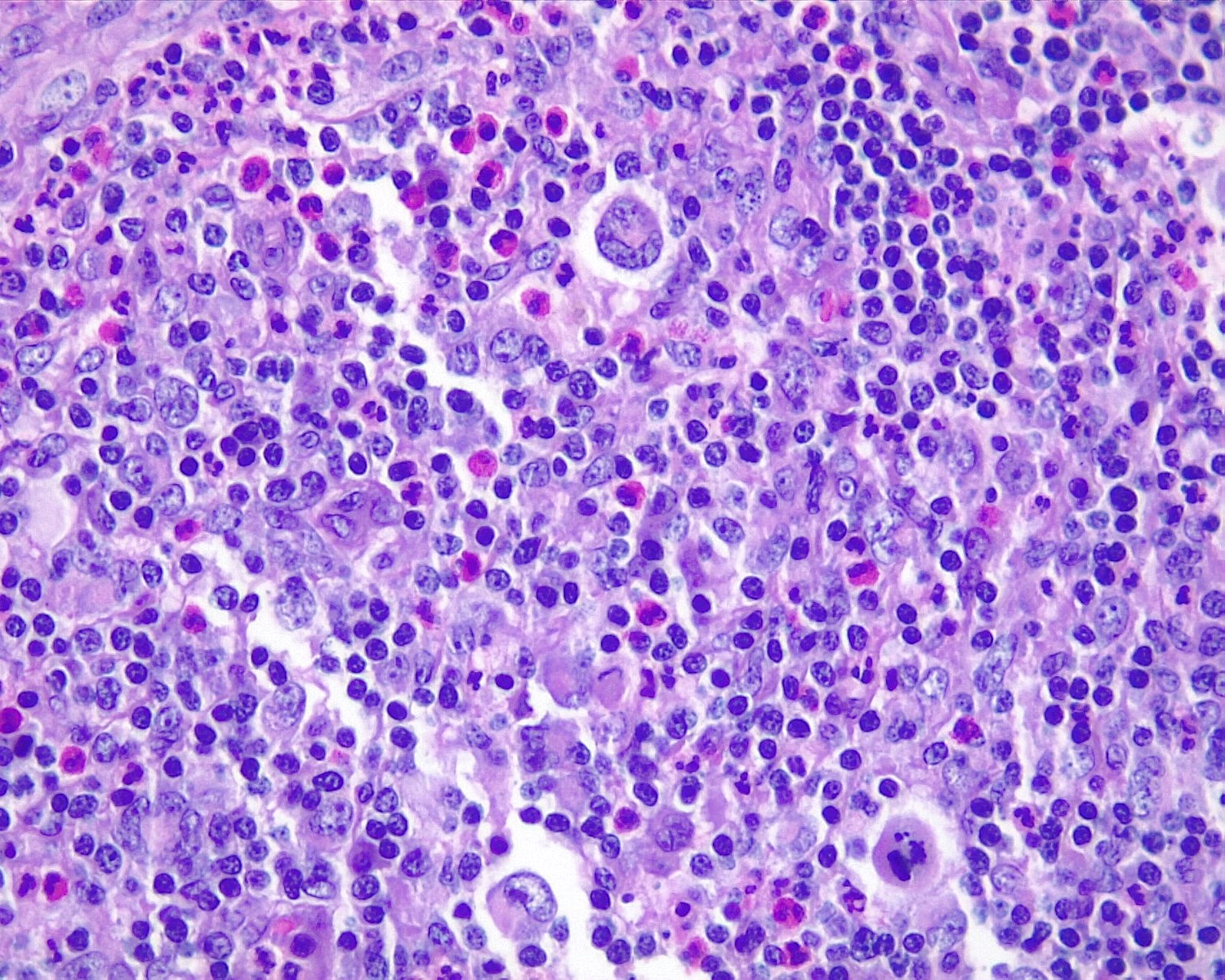
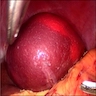
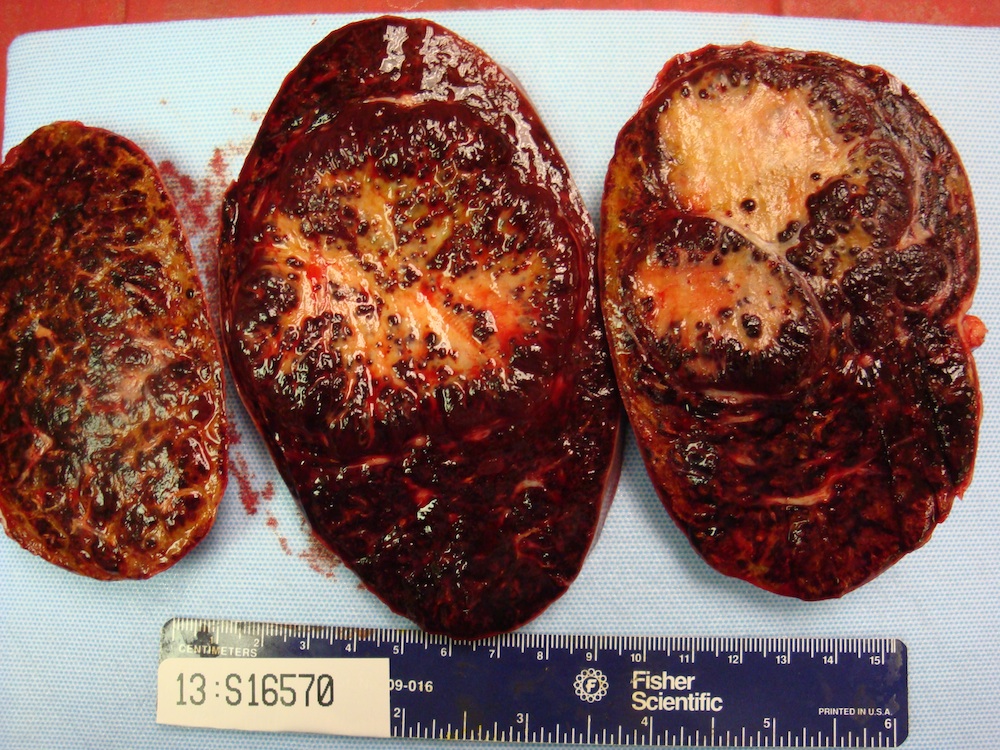
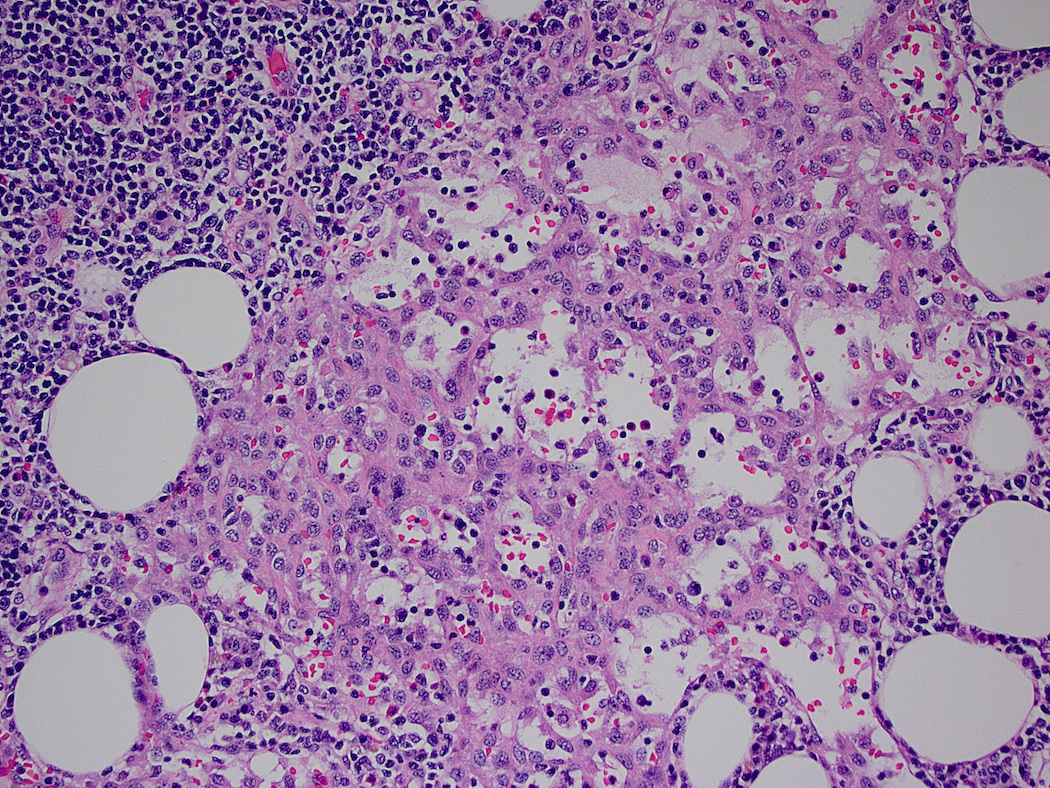
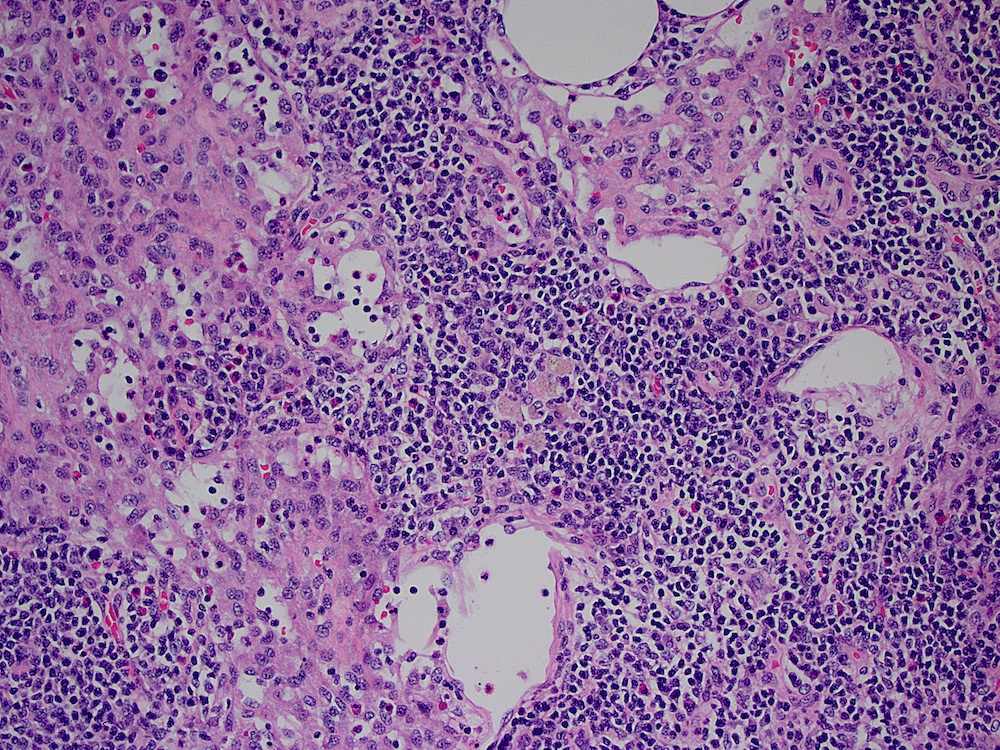
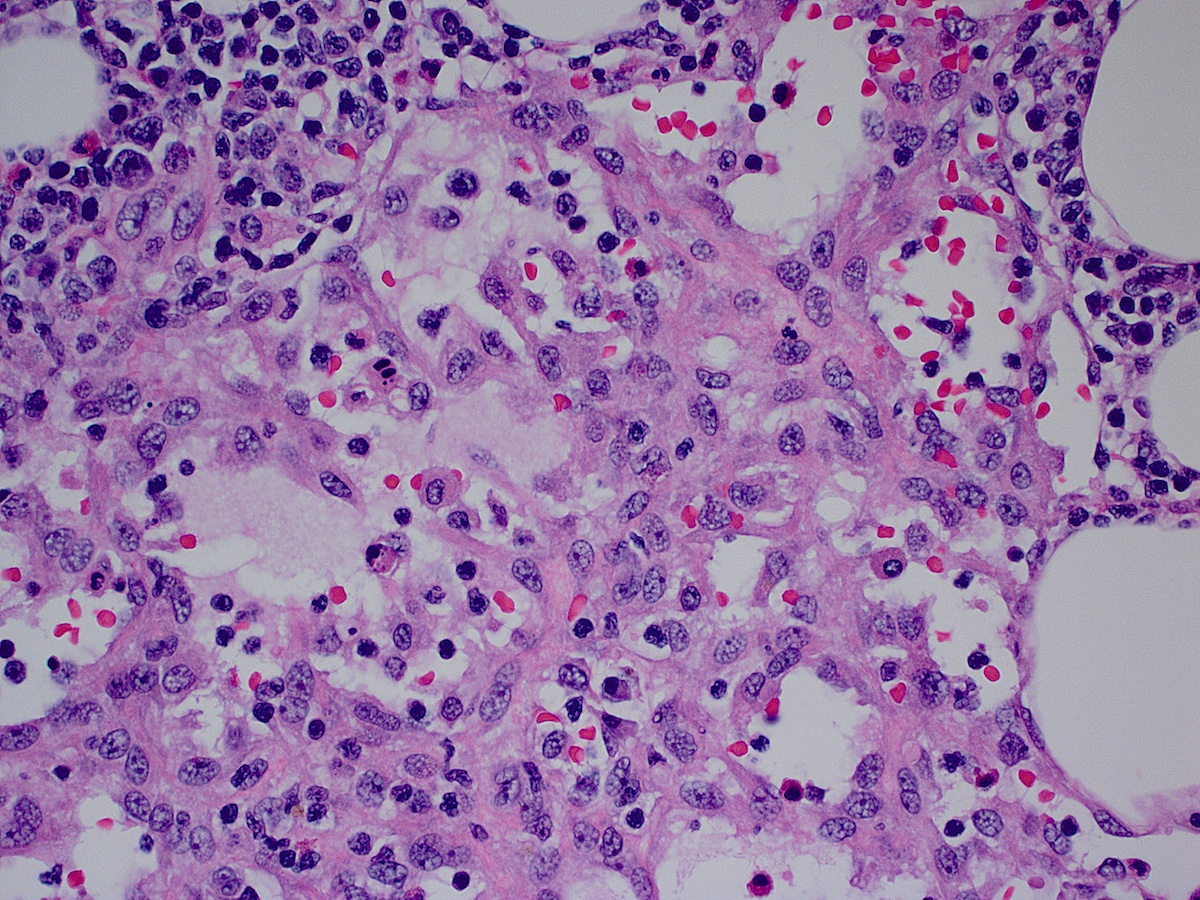
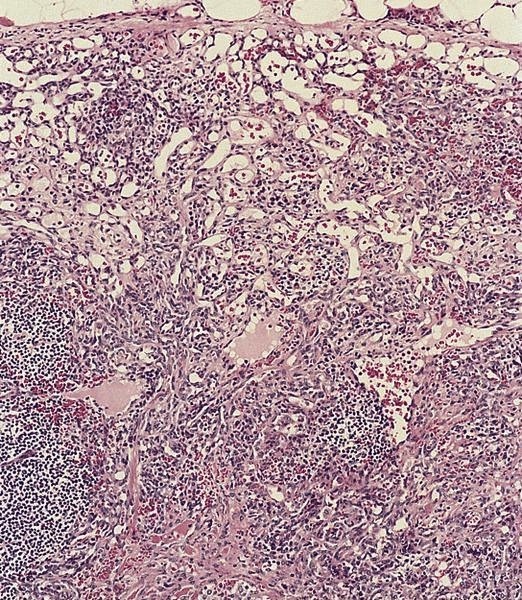
Acute splenitis
Table of Contents
Definition / general | Gross description | Microscopic (histologic) description | Differential diagnosisDefinition / general
- Also called acute splenic tumor or septic spleen
- Although traditionally associated with bacteremia, only known study shows no correlation (Arch Pathol Lab Med 2001;125:888)
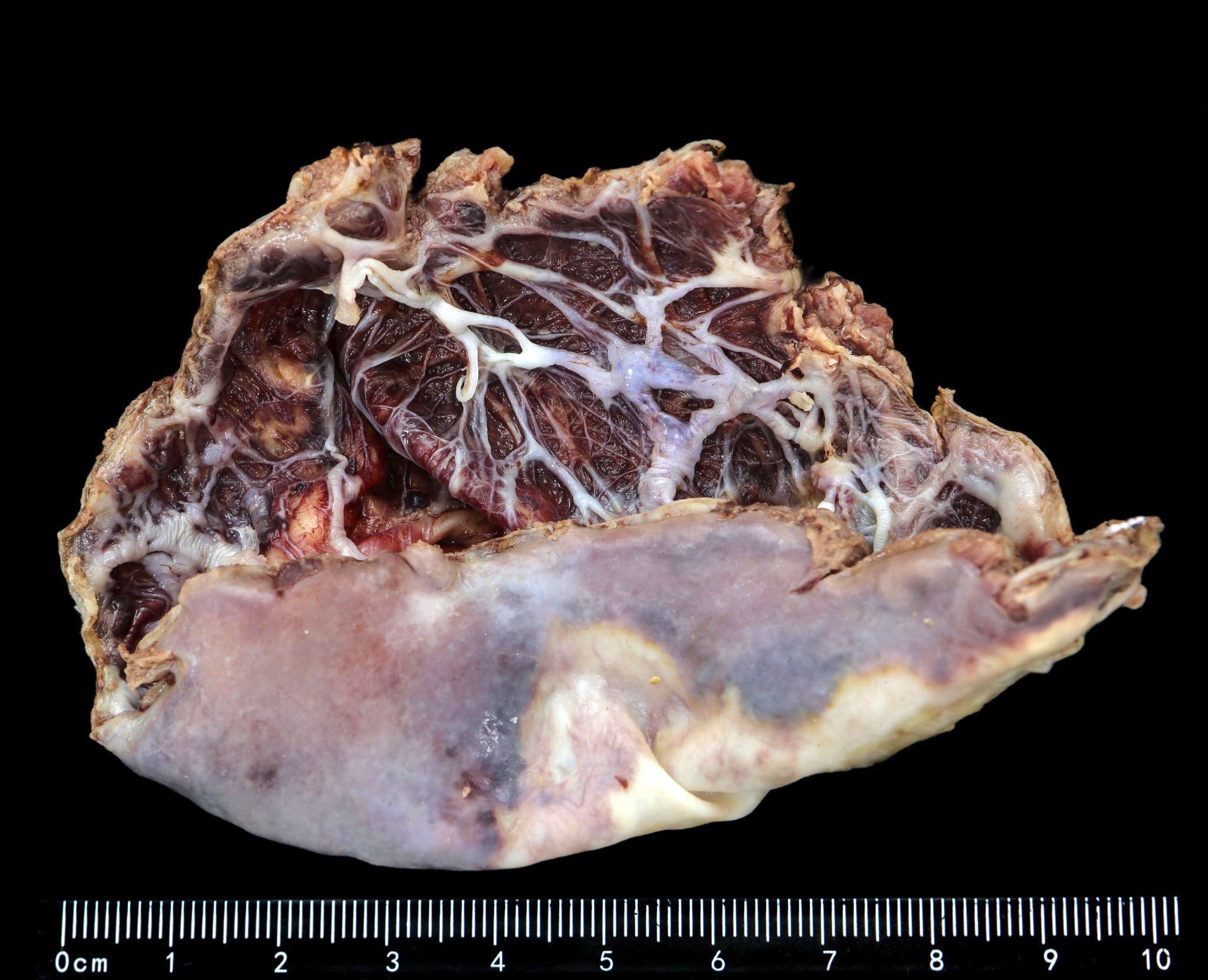
Gross description
- Splenic parenchyma may "flow" from cut surface
Microscopic (histologic) description
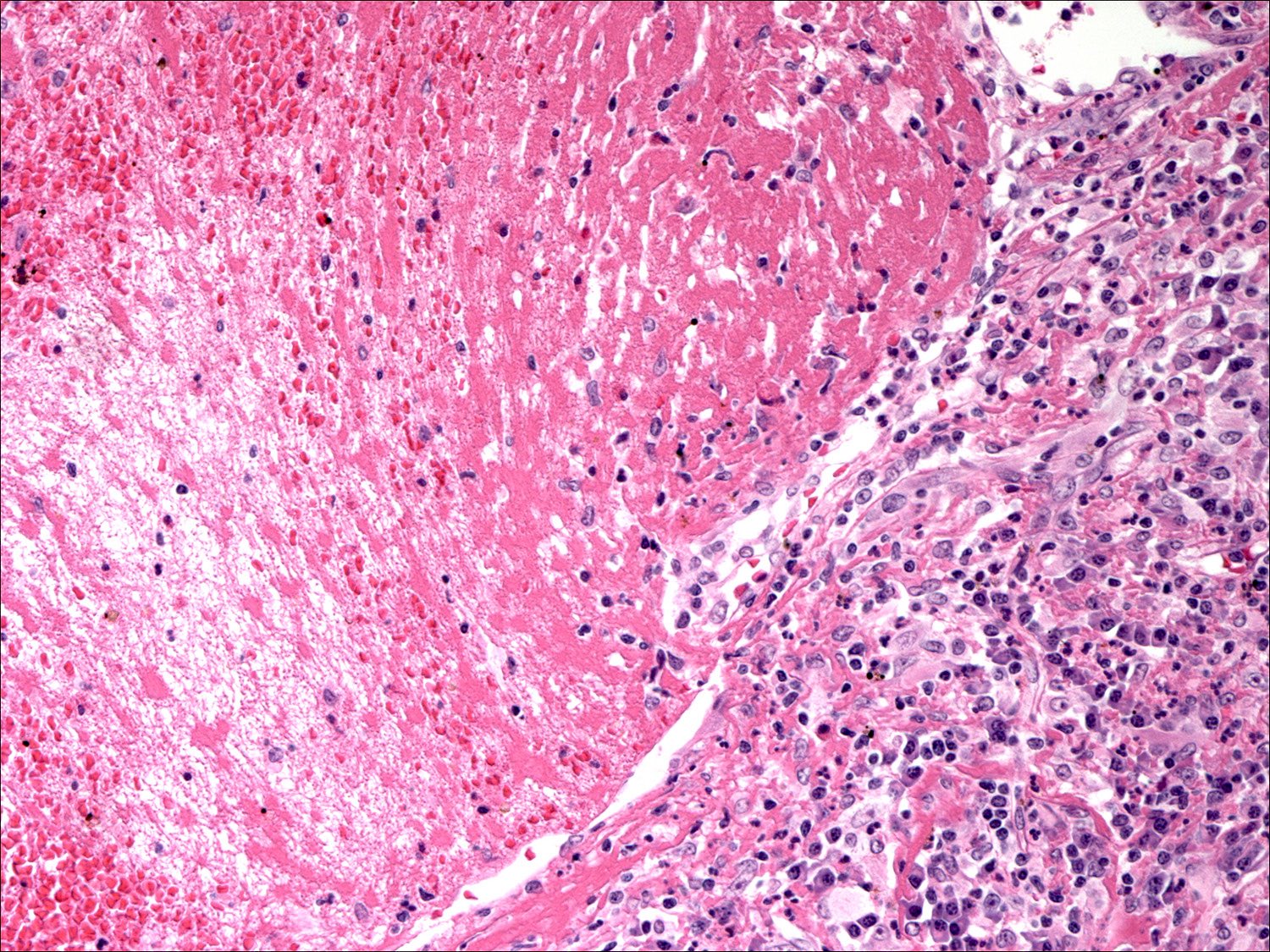
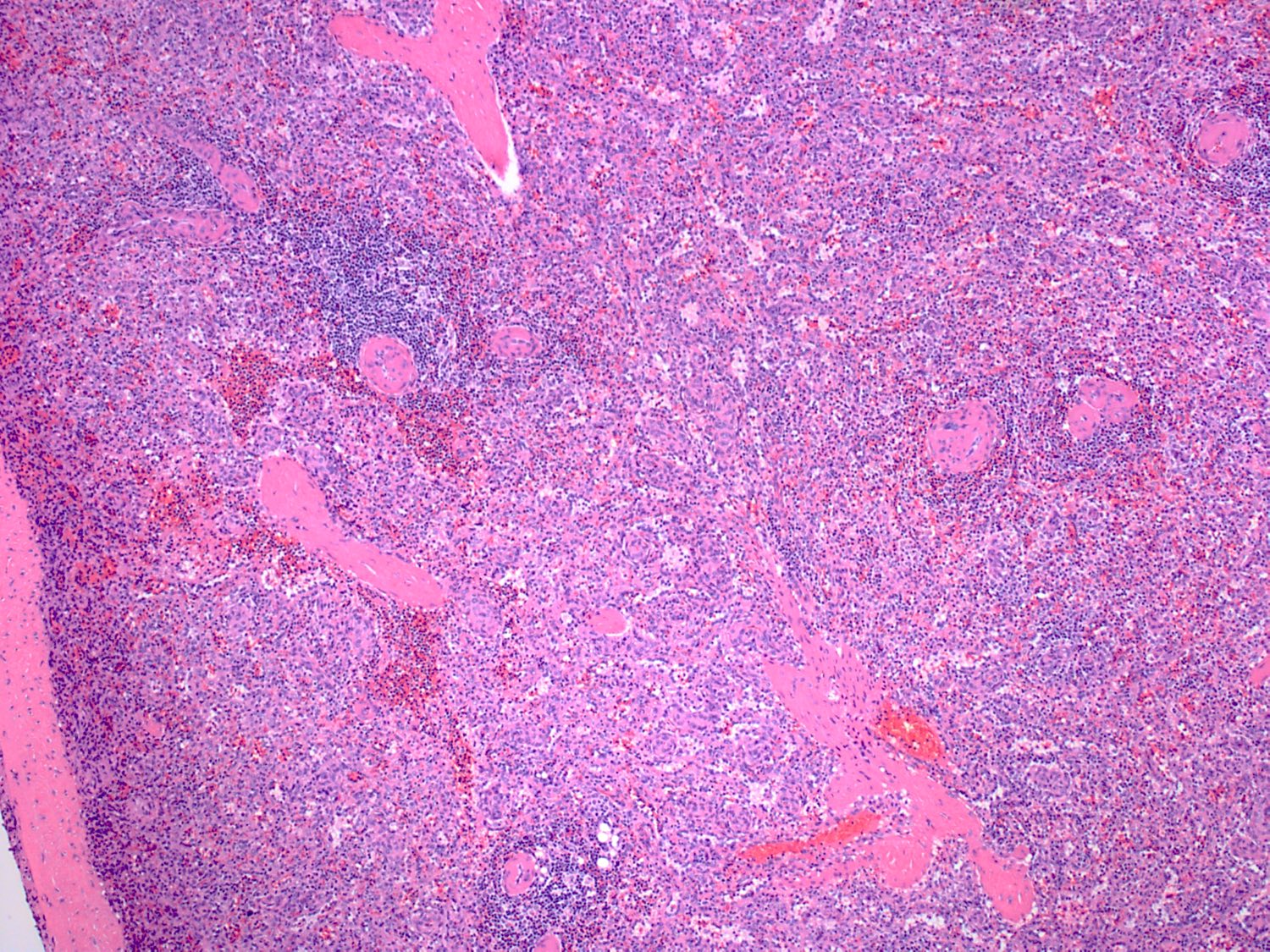
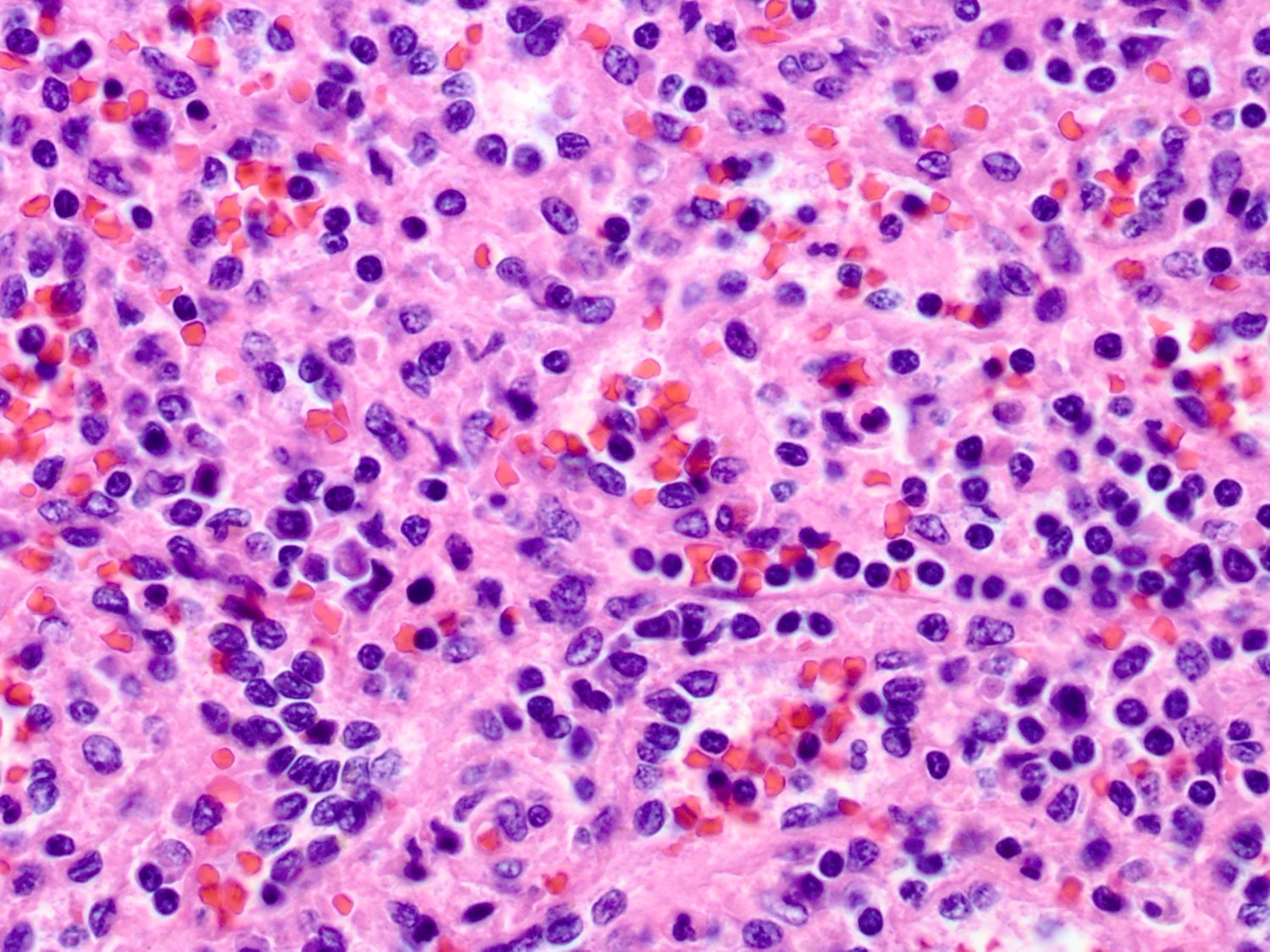
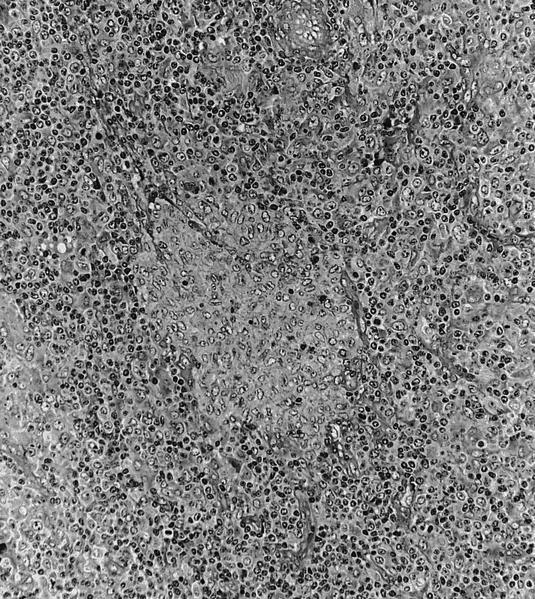
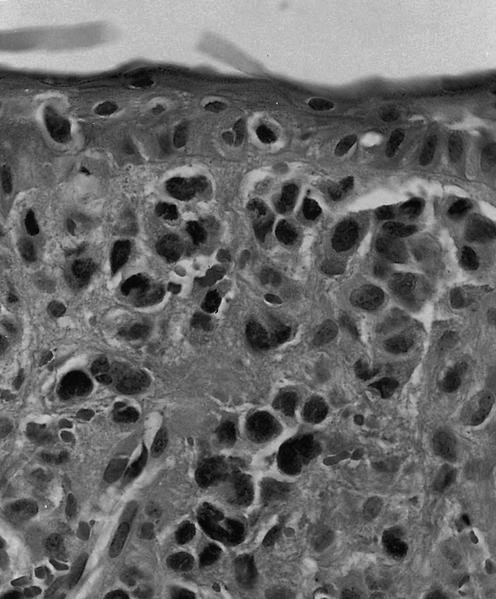
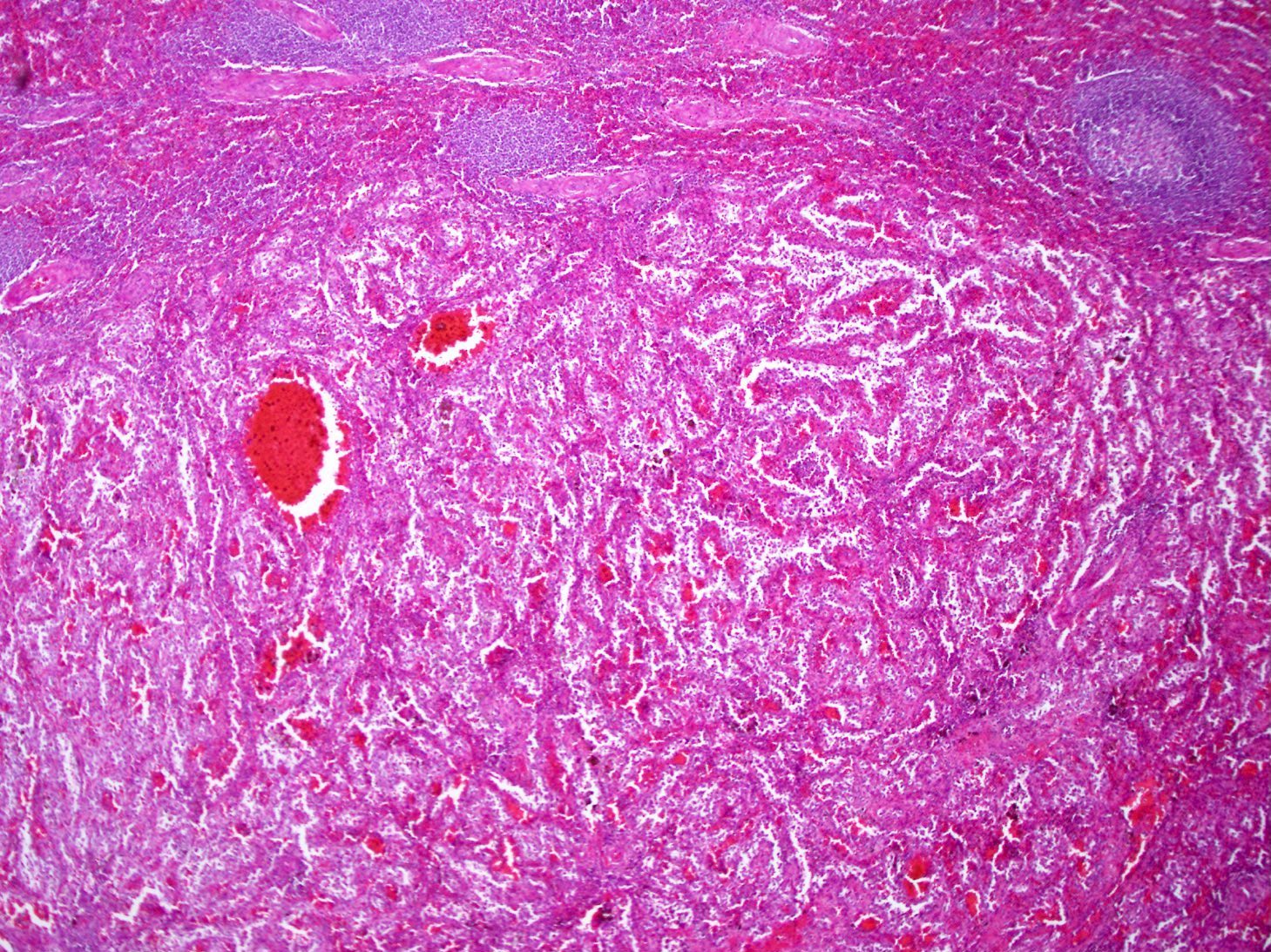
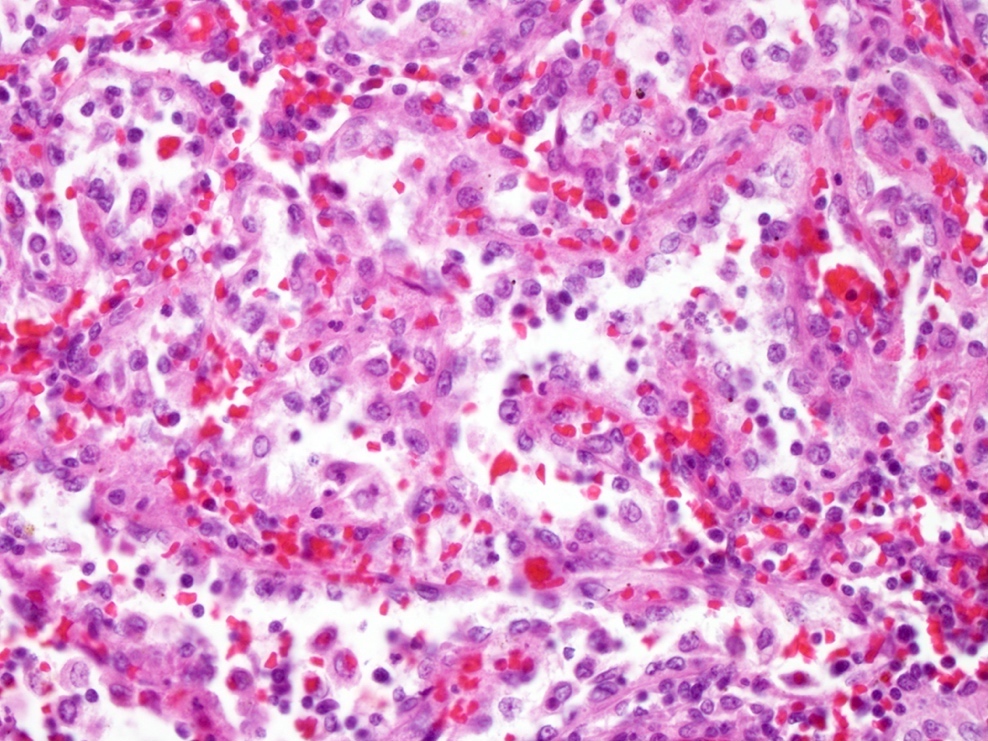
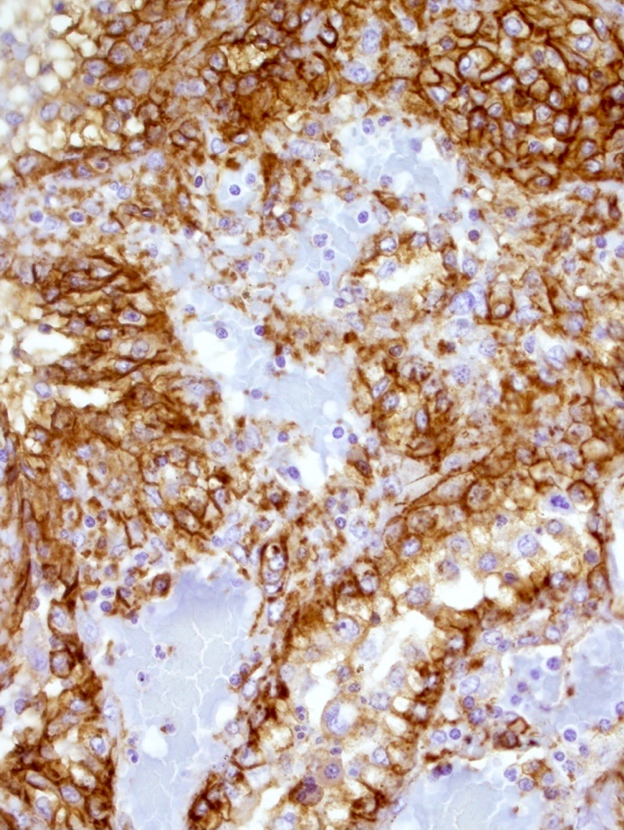
- Criteria are ill defined but traditionally are acute congestion of red pulp with numerous neutrophils in red and white pulp
- Necrosis of follicles occurs with group A streptococcal infection
- No capsular invasion by immunoblasts
Differential diagnosis
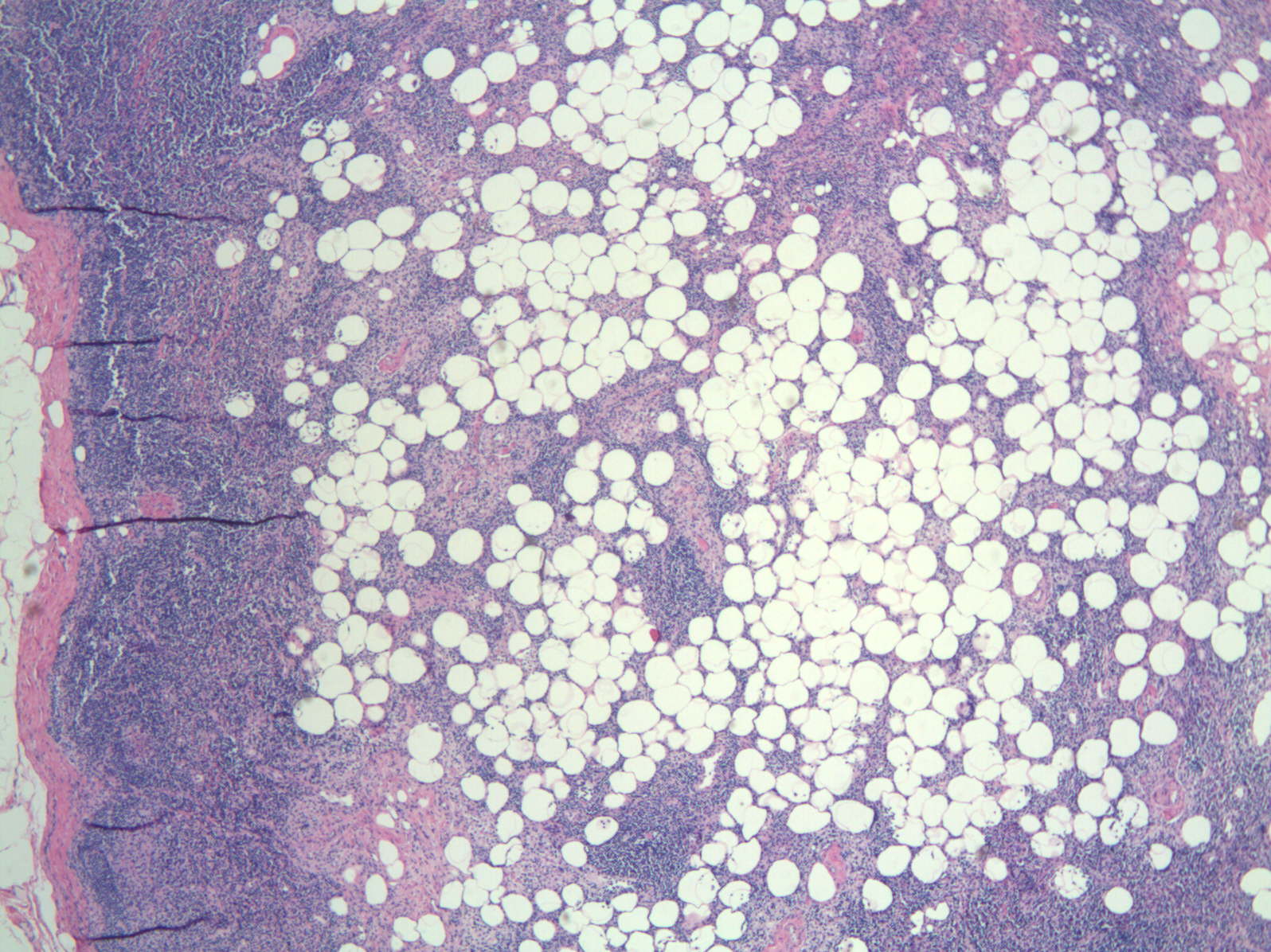
Adipose tissue
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Pathological enlargement of lymph nodes caused by abnormal accumulation of fat, due to mature, benign adipocytes within lymph node capsules
Terminology
- Lipo-lymph nodes
- Lipoplastic lymphadenopathy
Epidemiology
- Very common
Sites
- Most commonly involved sites are external iliac and obturator groups
Pathophysiology
- Accumulation of abnormal quantities of fat within lymph nodes in excess of normal aging changes
- Benign process with good prognosis
- Can cause mass effect depending on size
- May mimic lymphoma or other neoplasms
Etiology
- Unknown; postulated causes include exaggeration of normal fat deposition with aging, previous abdominal inflammatory disease, obesity
Clinical features
- Enlarged lymph nodes; may lead to formation of large masses up to 10 cm
Diagnosis
- Lymph node biopsy
Radiology description
- Progressive enlargement and increased fatty infiltration
- CAT scan and lymphangiography findings can be misleading towards neoplastic process
Case reports
- 47 year old man with abundant macroscopic fat in intra-abdominal lymph nodes (Br J Radiol 2012;85:e91)
- 49 year old woman with lipoplastic lymphadenopathy presenting as an ovarian mass (Gynecol Oncol 1987;28:345)
- 50 year old man with generalized lipomatosis of lymph nodes (Lymphology 1979;12:262)
- Middle aged women with lipolymph nodes of mesentery (Am Surg 1985;51:596)
- Lipoplastic lymphadenopathy simulating lymphoma and pelvic lipomatosis (J Urol 1975;114:788)
- Pelvic and aortic lipolymph nodes (Acta Obstet Gynecol Scand 1982;61:383)
Treatment
- Excision of involved lymph node in symptomatic cases serves both diagnostic and therapeutic purposes
Clinical images
Gross description
- Enlarged lymph node with soft greasy yellow areas within capsule, or entirely replaced by similar cut surfaces
Microscopic (histologic) description
- Benign mature adipocytes populate nodes whose capsules are thinly attenuated with fine vascular trabeculae dividing fat deposits
Microscopic (histologic) images
Differential diagnosis
- Intranodal angiolipoma (Int J Surg Pathol 2005;13:99)
- Other causes of lymphadenopathy: lymphoma, metastases, inflammatory / infectious causes
- Retroperitoneal or pelvic lipomatosis
Additional references
Adult onset Still disease
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Positive stains | Molecular / cytogenetics description | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Rare systemic inflammatory disease accompanied by a triad of spiking fever, maculopapular exanthema and arthralgia, accompanied frequently by lymphadenopathy
Essential features
- Fever, skin rash, joint pains, lymphadenopathy with paracortical hyperplasia and immunoblastic reaction
ICD coding
Epidemiology
- Rare, shows female predilection and more common in young adults
Sites
- Systemic disease
- Generalized lymphadenopathy
Pathophysiology
- Pathophysiology is yet to be clearly defined
- Interleukins, macrophage colony stimulating factor, interferon gamma and tumor necrosis factor alpha may play a role
- May also be due to genetic factors, immune dysfunction and infections
Clinical features
- Systemic inflammatory disease manifesting with spiking fever, sore throat, arthralgia, skin rash and hepatosplenomegaly
- Reactive hemophagocytic syndrome is also reported
Diagnosis
- Clinical, laboratory and imaging results
Laboratory
- Neutrophilic leukocytosis, anemia, elevated ferritin, C reactive protein (CRP), erythrocyte sedimentation rate (ESR) and abnormal liver function tests (AST and ALT)
Radiology description
- Affected joints show progressive erosions and narrowing joint space
- Commonly associated with hepatosplenomegaly
Prognostic factors
- Disease can be self limiting and the prognosis depends on the specific disease pattern
- Overall, patients with only localized disease have a better prognosis compared to those with more disseminated disabilities or severe complications
- Disease can be monocyclic - self limiting, polycyclic - symptoms recur or have chronic articular pattern
Case reports
- 28 year old woman with AOSD (Acta Med Iran 2016;54:683)
- 53 year old woman diagnosed with AOSD based on Yamaguchi criteria (Am J Case Rep 2017;18:119)
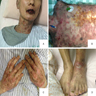
- Persistent pruritic lesions in adult onset Still disease (Am J Med Sci 2016;352:540)
- Three Japanese women ages 22, 26 and 63 with lymph node lesions from AOSD (Int J Surg Pathol 2002;10:197)
Treatment
- Mainly antiinflammatory medications, including steroids, NSAIDs and antirheumatic agents
Gross description
- Nonspecific enlargement of lymph nodes, usually small size
Microscopic (histologic) description
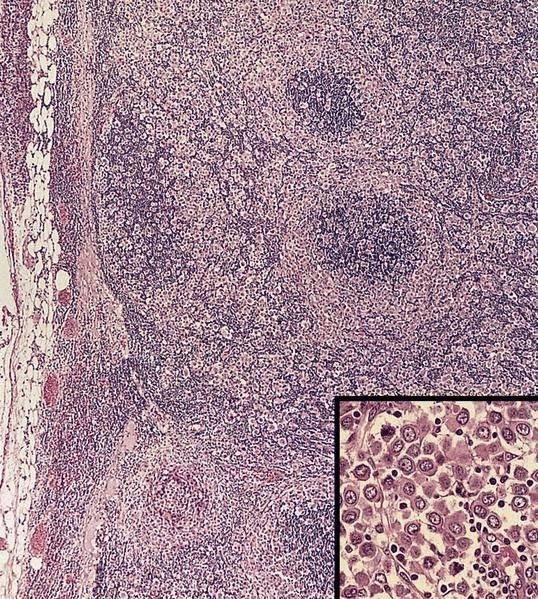
- Major histological findings limited to paracortical hyperplasia or a mixed pattern with paracortical and diffuse or paracortical, follicular and diffuse hyperplasia, with or without sinus histiocytosis
- Vascular proliferation in the interfollicular areas, immunoblastic reaction and mixed inflammatory cell infiltrations are also described commonly
- Rare findings described include pericapsular endarteritis and hemophagocytic features
Positive stains
Molecular / cytogenetics description
- Polyclonal B and T cell gene rearrangement patterns
Differential diagnosis
Additional references
Board review style question #1
- Which of the following set of markers will be helpful in differentiating a lymph node of a patient with adult onset Still disease from angioimmunoblastic T cell lymphoma?
- CD20, CD3, CD45
- CD3, CD4, CD8
- CD30, CD15, OCT2
- PD1, BCL6, CD10
Board review style answer #1
D. PD1, BCL6, CD10 - markers for follicular helper T cell phenotype. Angioimmunoblastic T cell lymphoma is of follicular helper T cell origin and is positive for PD1, BCL6 and CD10.
Comment Here
Reference: Adult onset Still disease
Comment Here
Reference: Adult onset Still disease
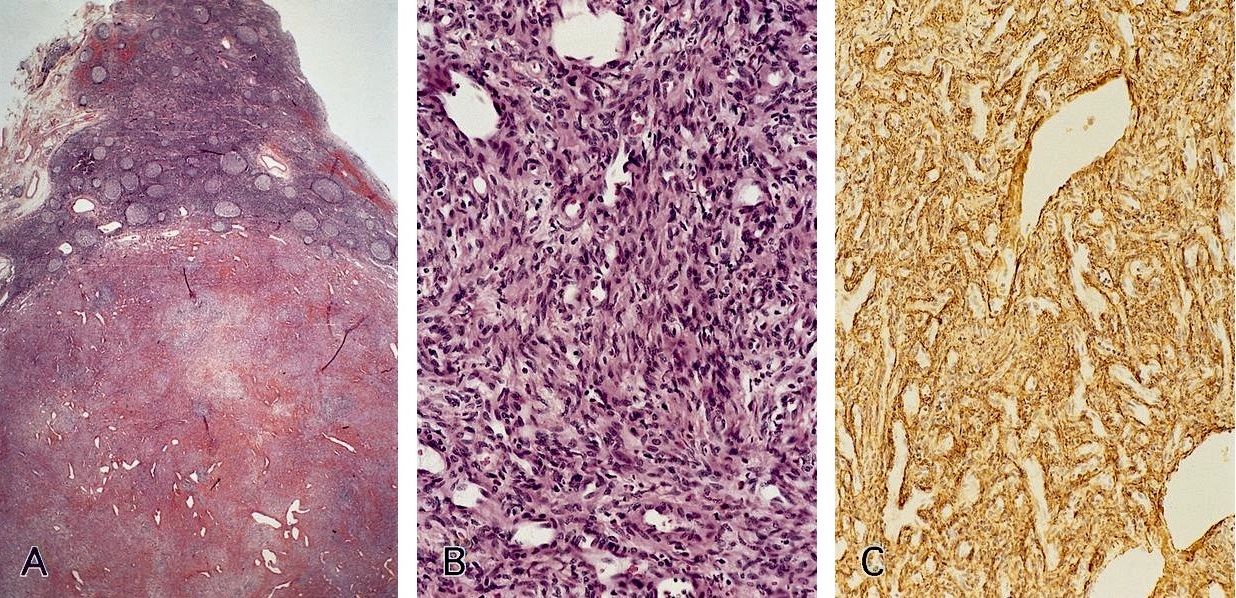
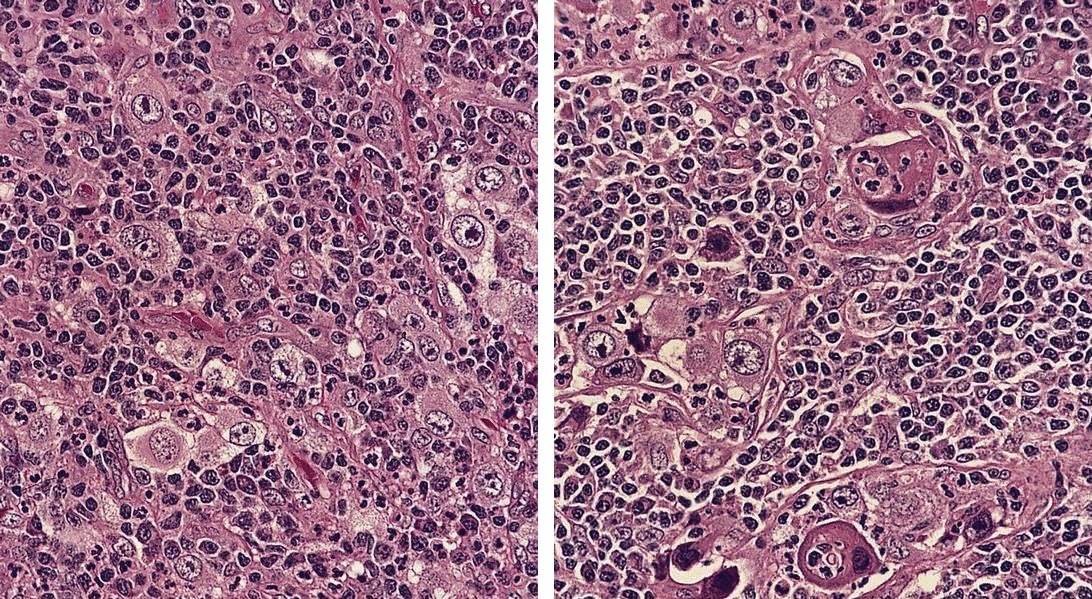
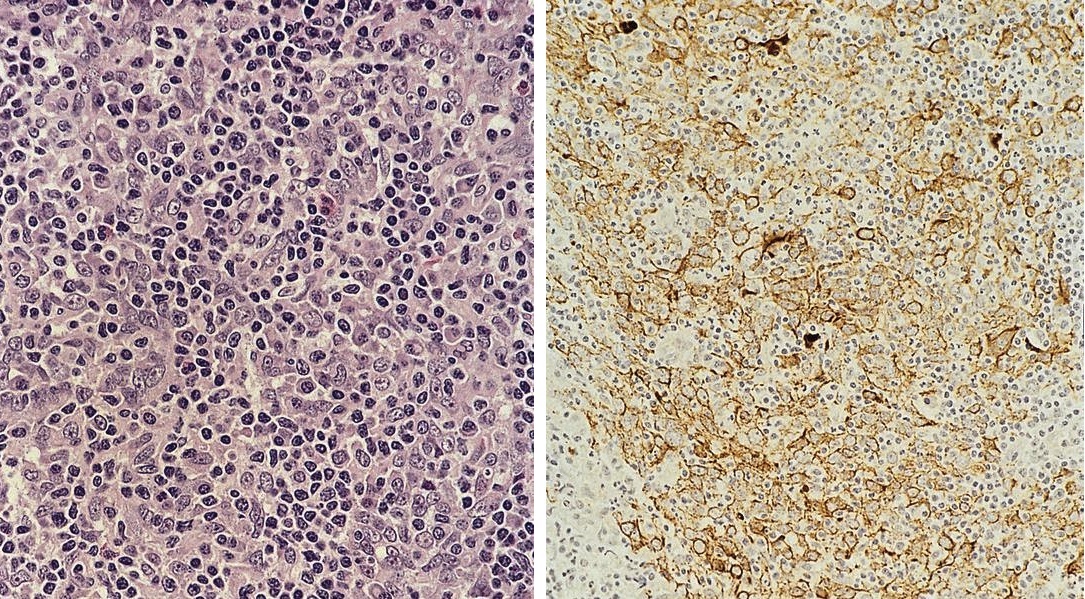
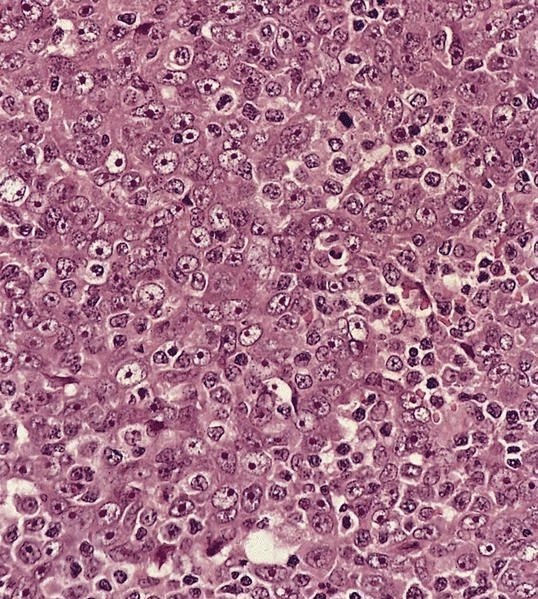
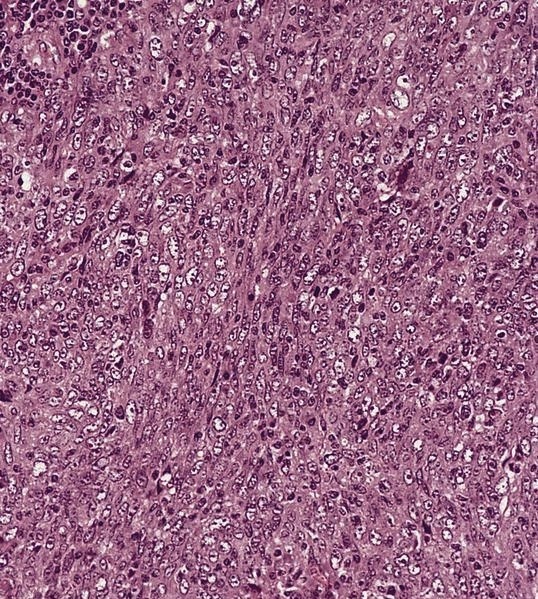
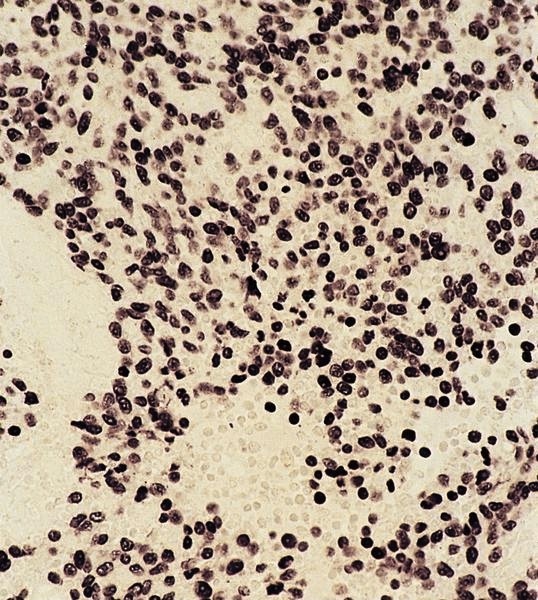
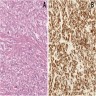
ALK+ histiocytosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
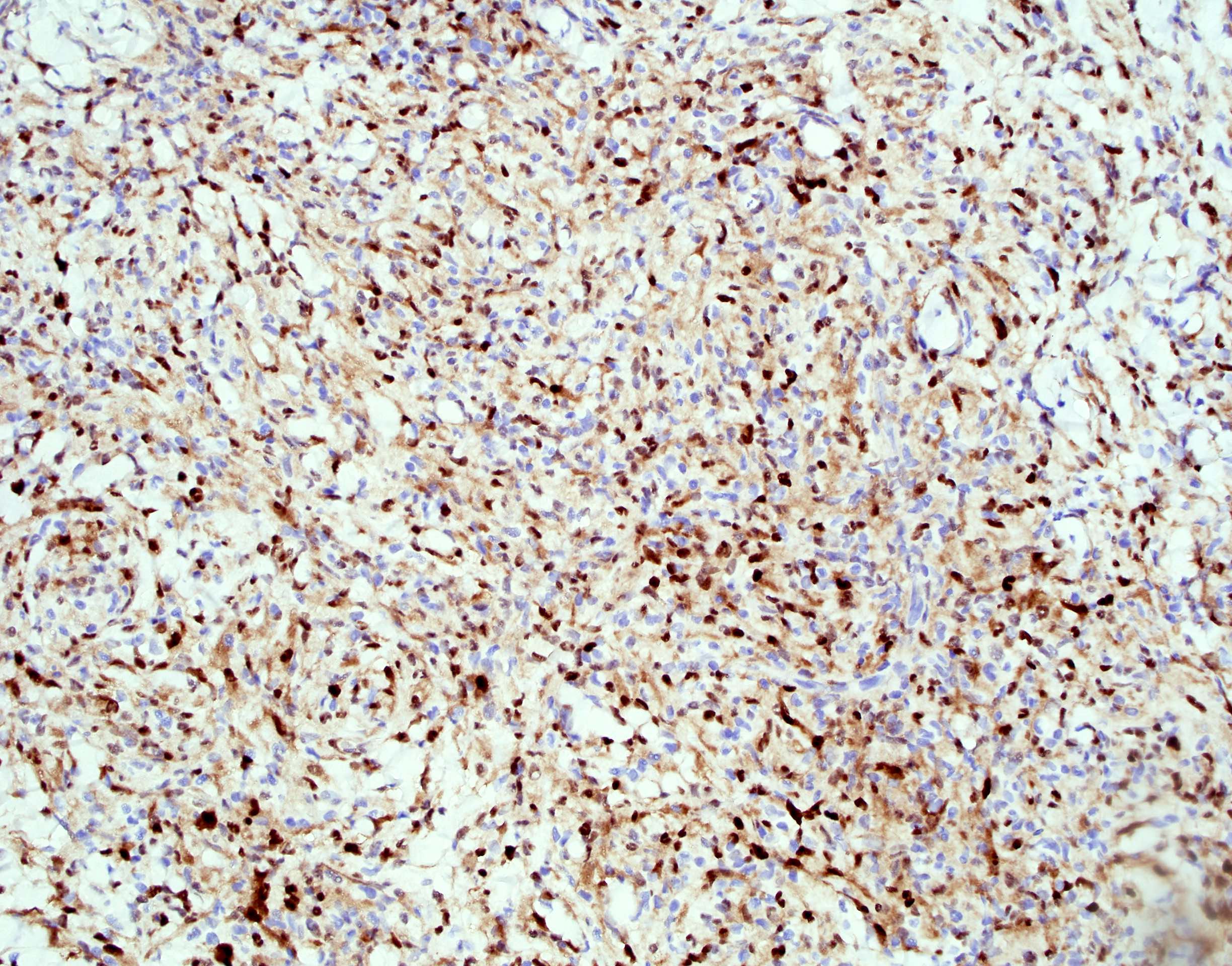
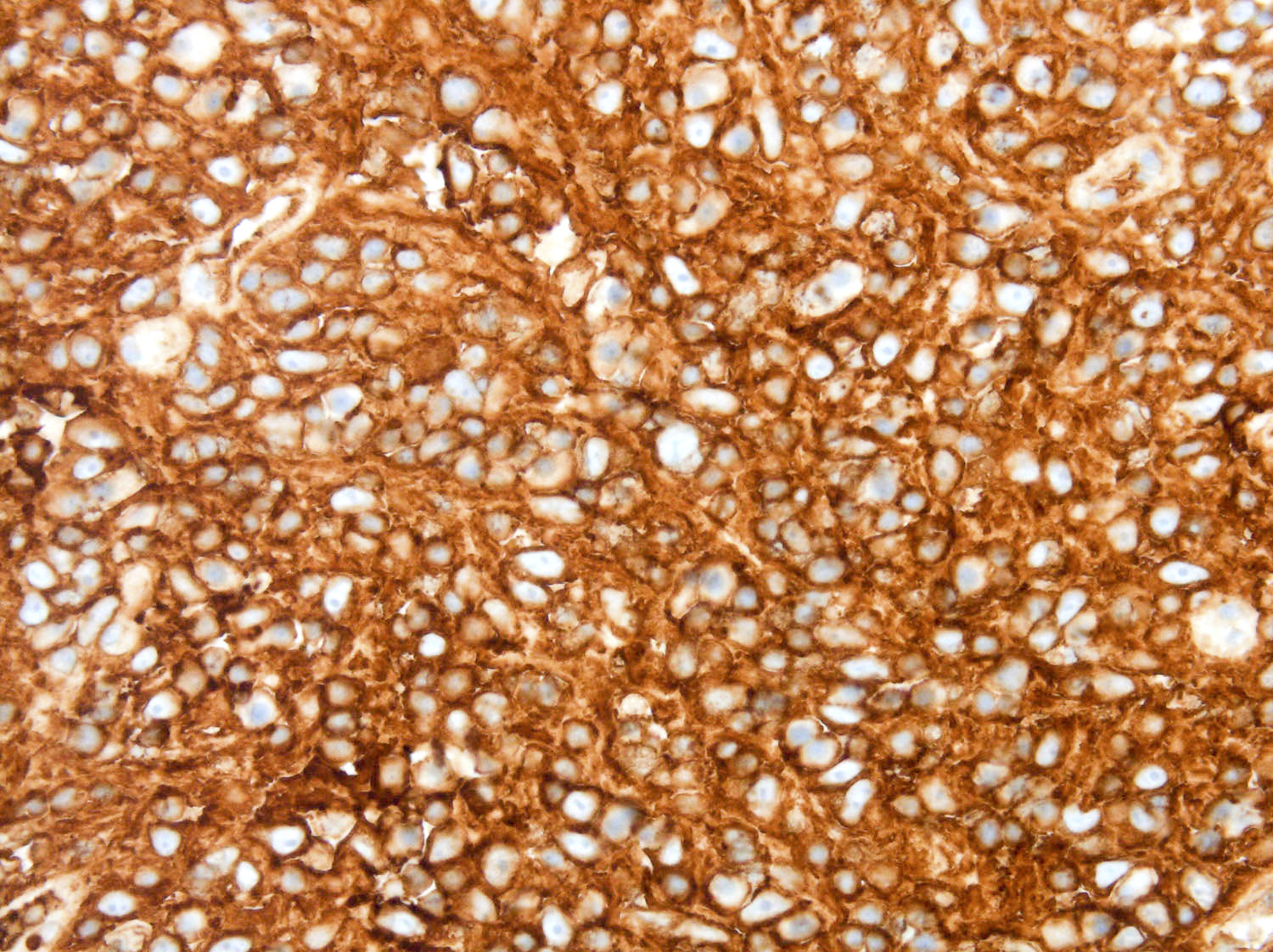
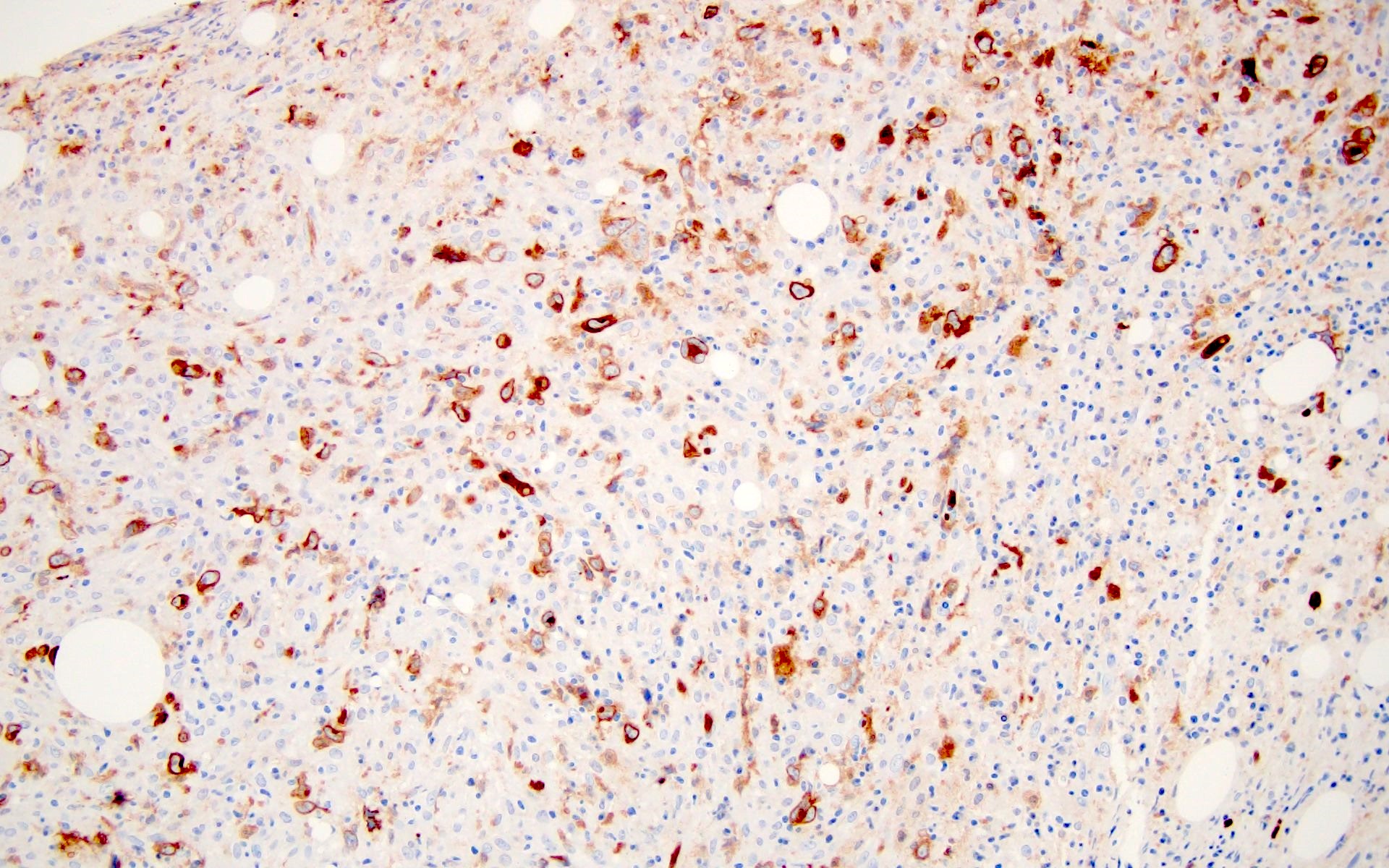
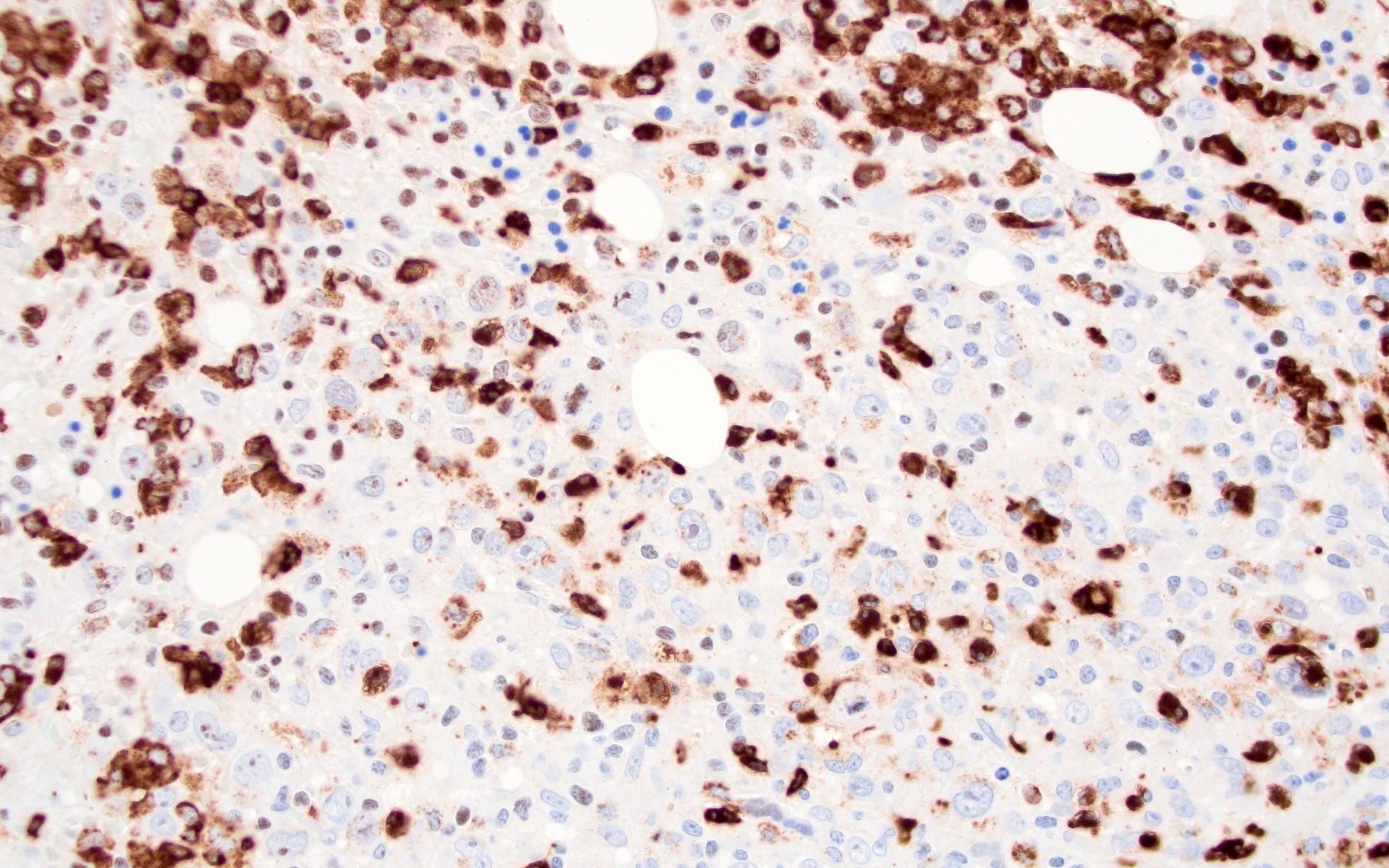
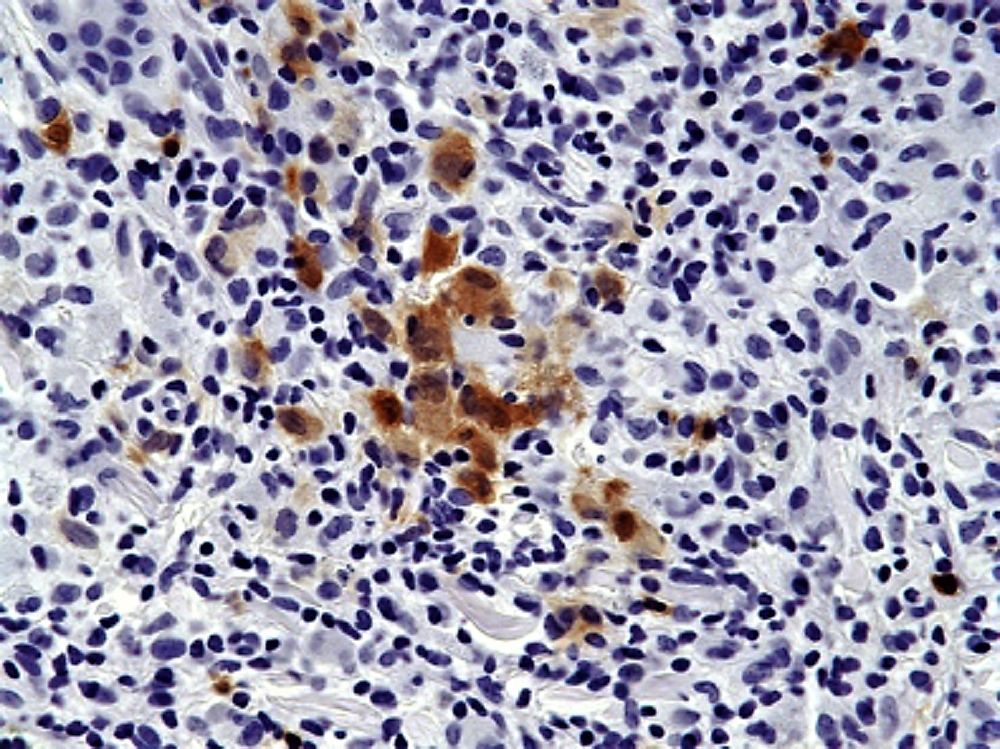
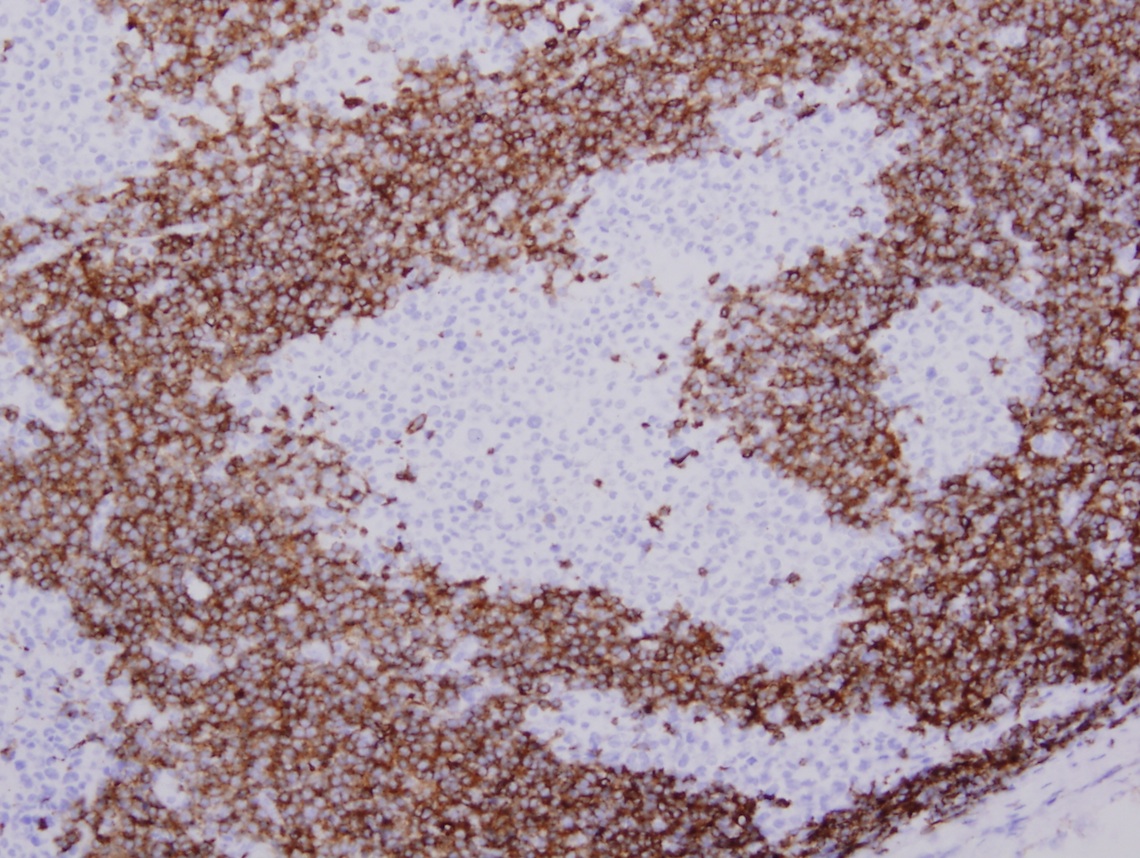
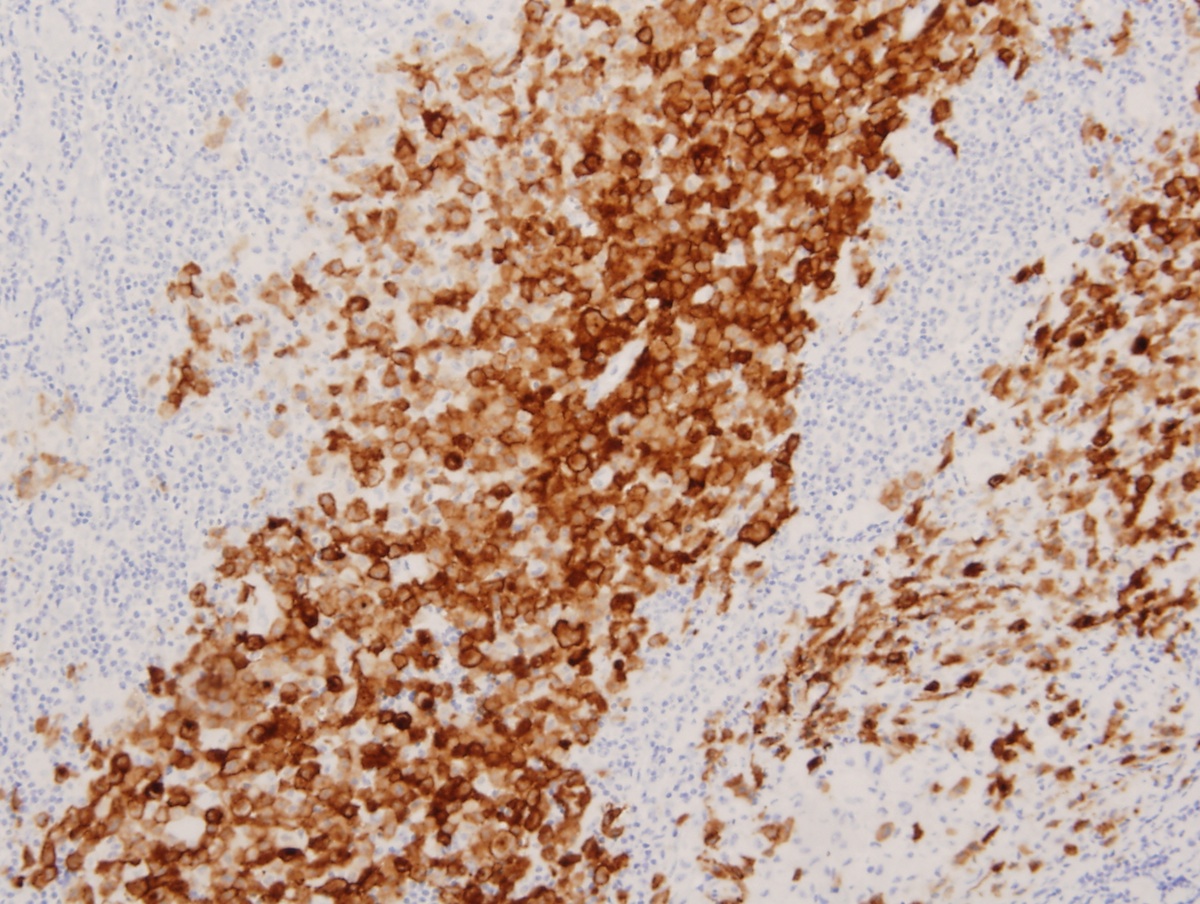
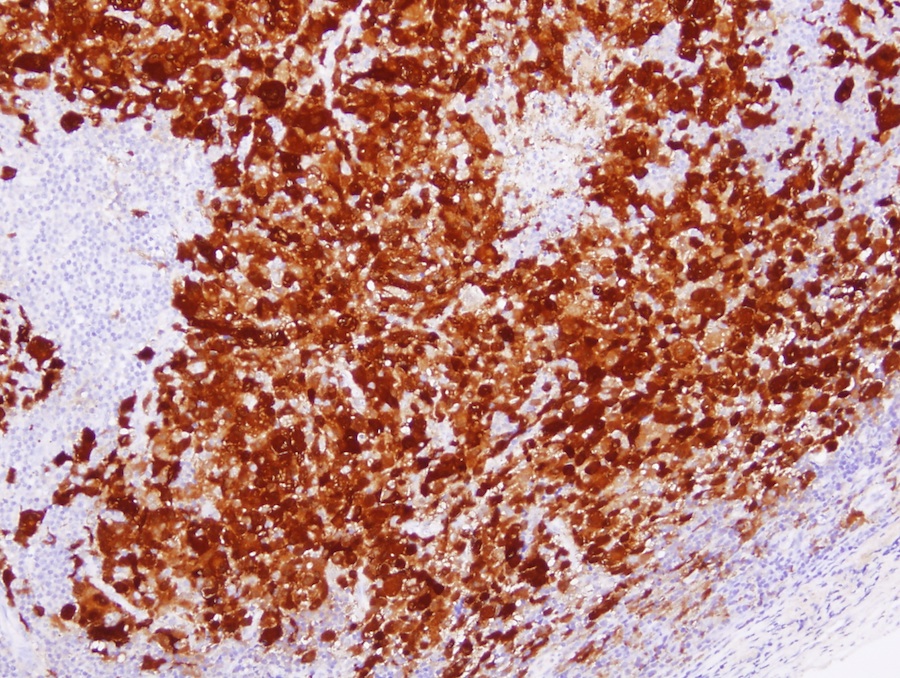
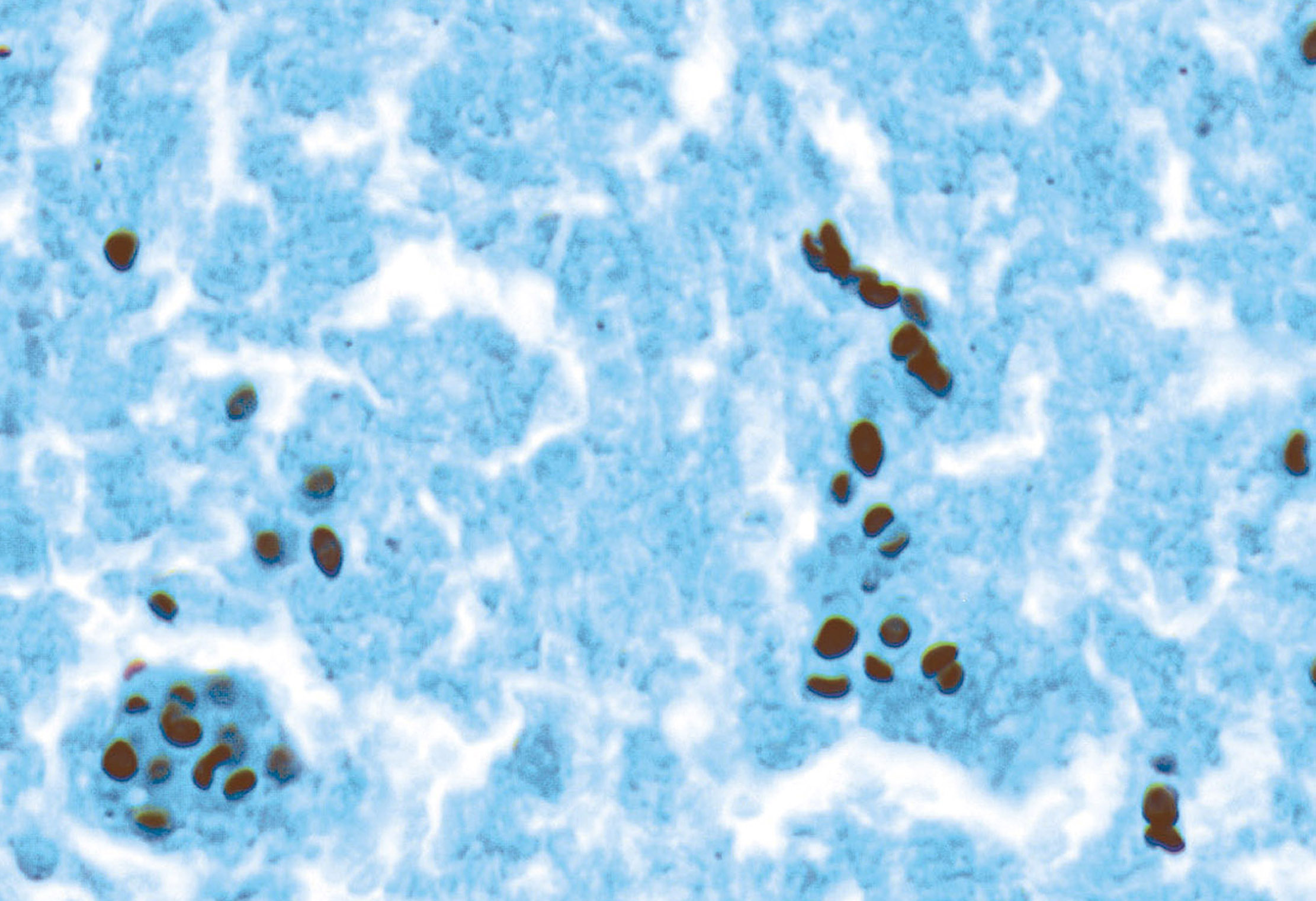
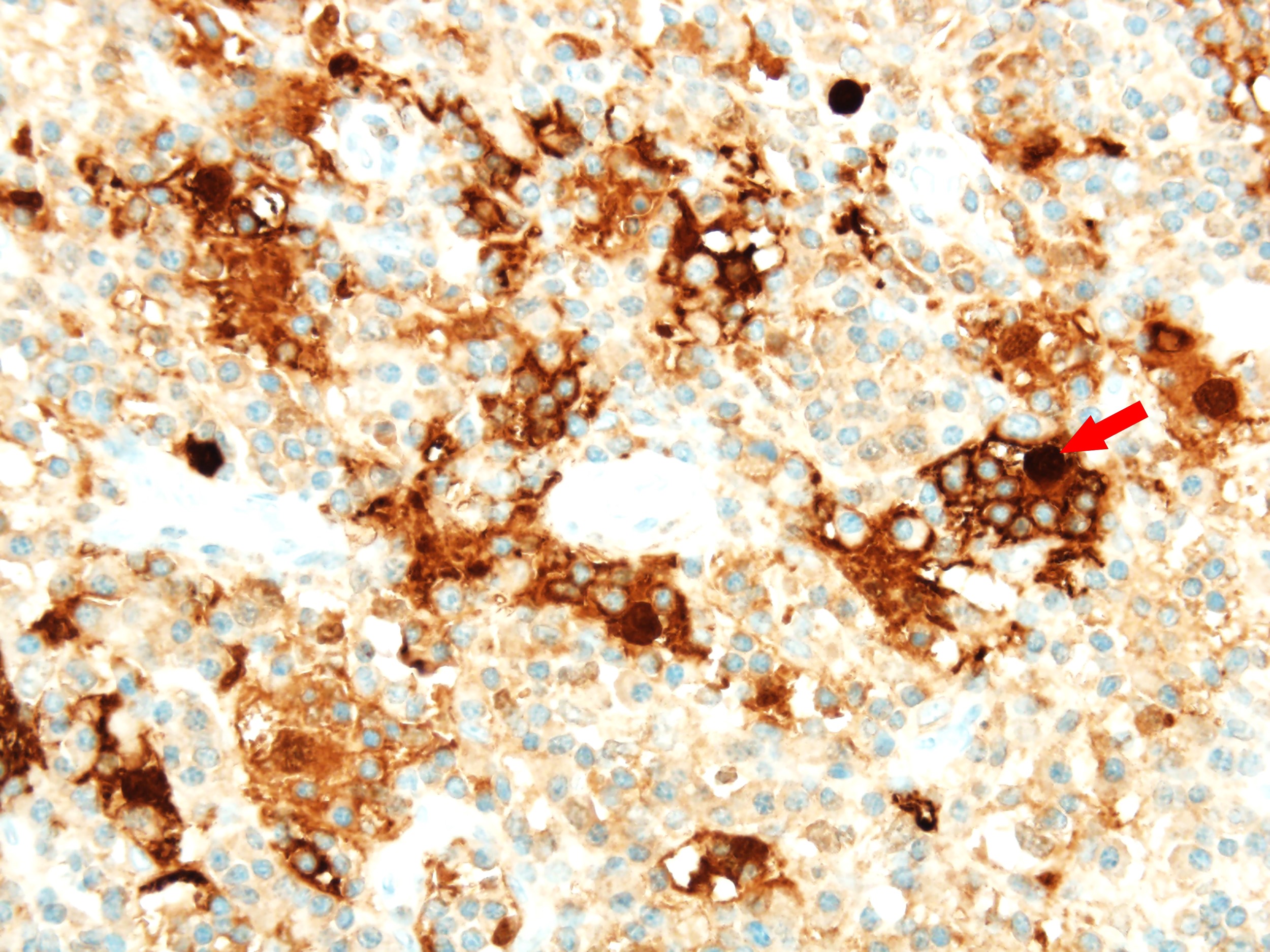
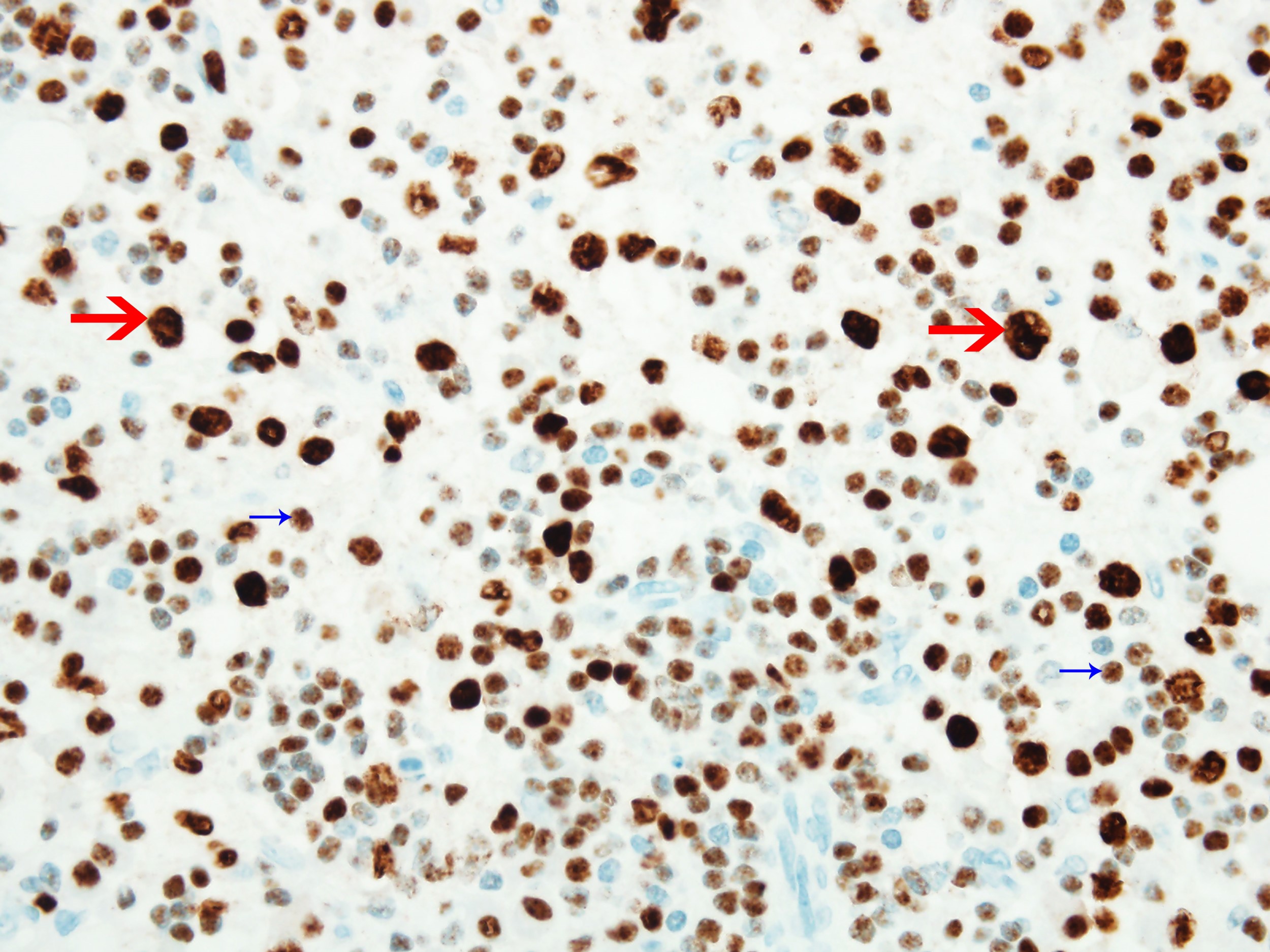
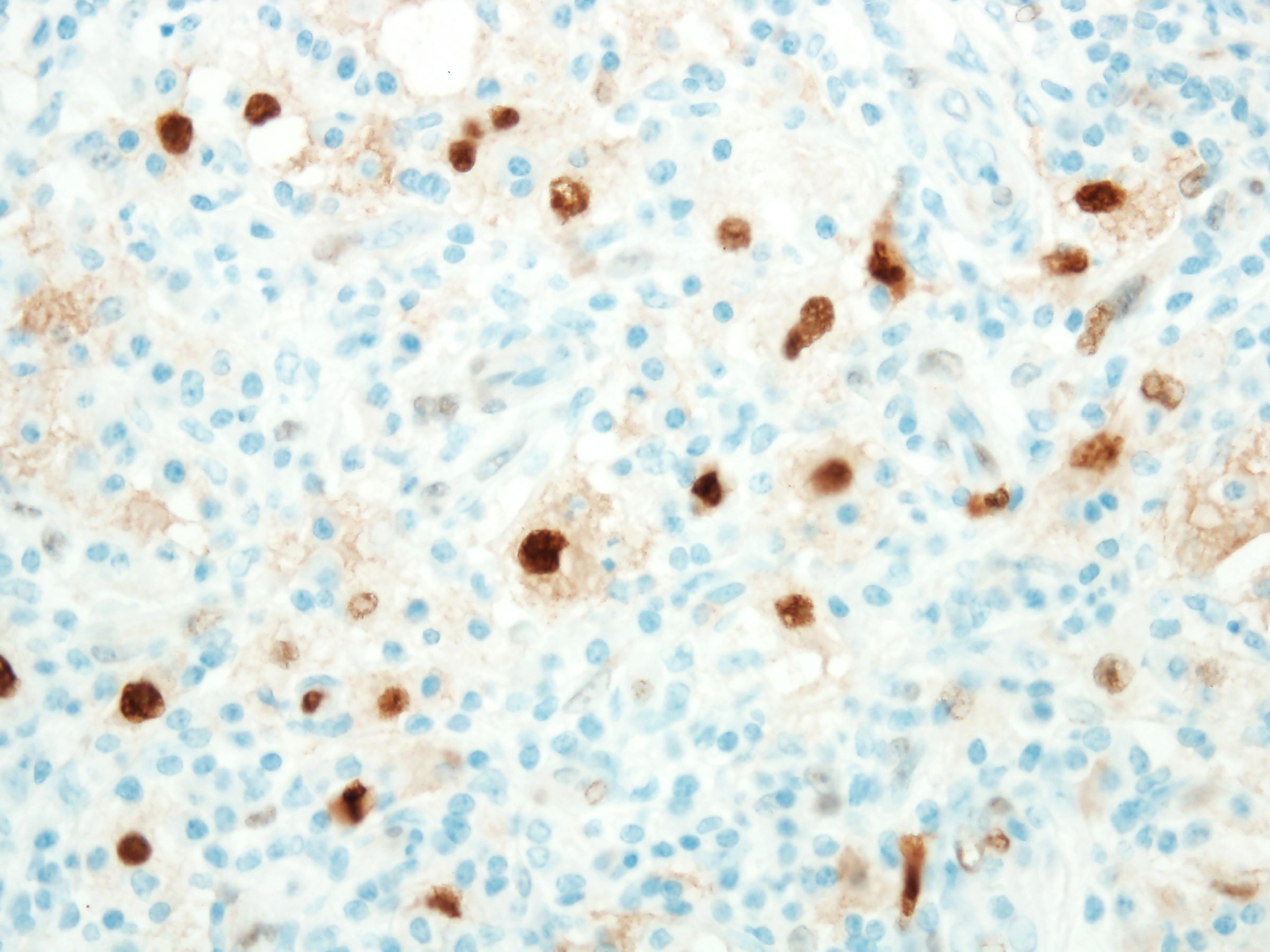
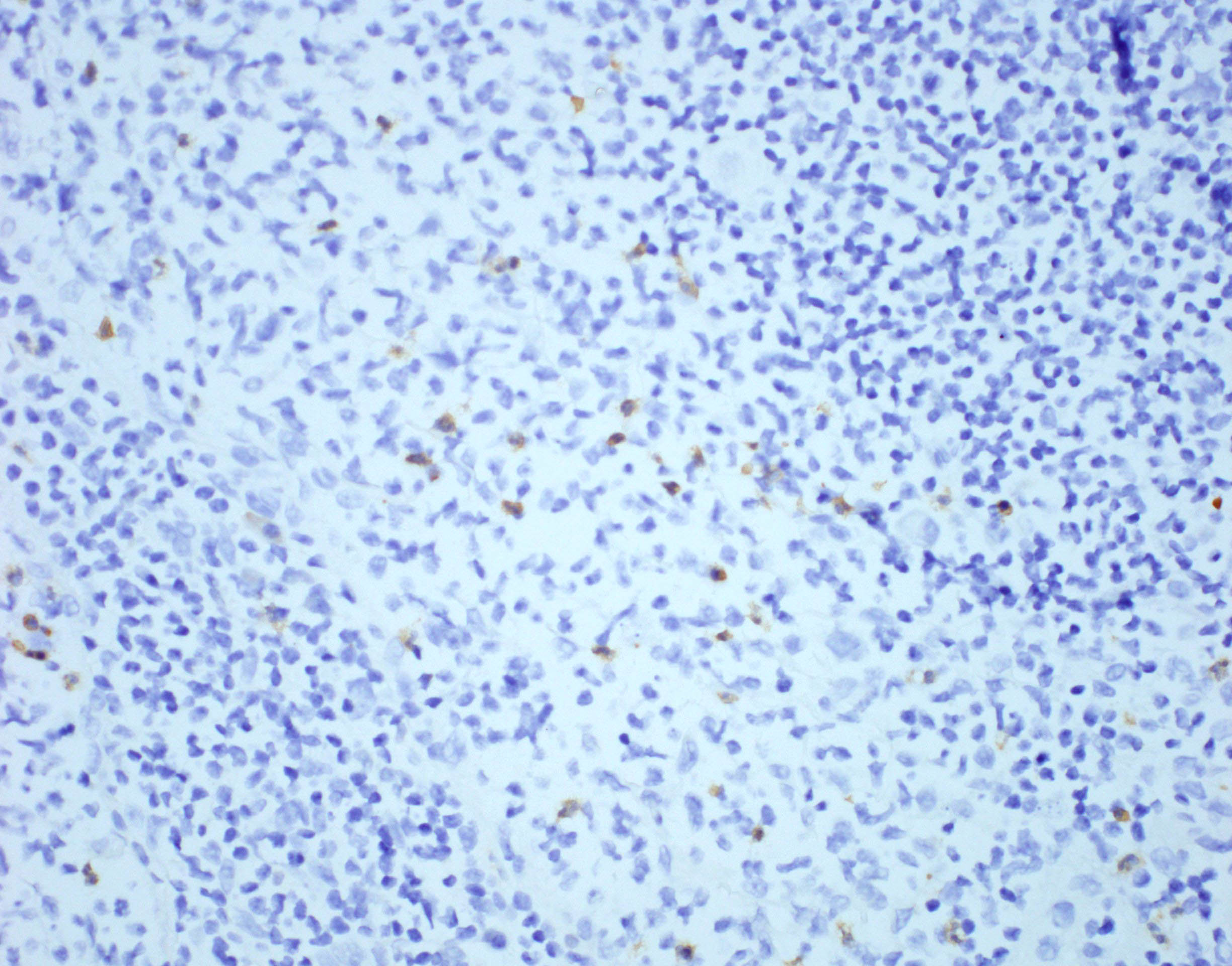
- ALK positive histiocytosis (APH) is a histiocytic neoplasm characterized by ALK (anaplastic lymphoma kinase) gene rearrangements and ALK immunoreactivity in the lesional histiocytes (Leukemia 2022;36:1703, Blood 2022;140:1229, Blood 2022;139:256)
- Initially described in 2008 presenting as systemic histiocytosis in infants (Blood 2008;112:2965)
Essential features
- Rare non-Langerhans cell histiocytic neoplasm with heterogeneous clinical features, presenting as single system disease, multisystem with systemic hematopoietic involvement (liver, spleen or marrow, skin, central nervous system [CNS]) or other multisystem disease (tumorous involvement of ≥ 2 organ systems)
- ALK expression by immunohistochemistry, predominantly in a cytoplasmic pattern, rarely in a membranous or Golgi (dot-like) pattern and practically never in a nuclear pattern
- Cytoplasmic ALK expression by immunohistochemistry with coexpression of histiocytic markers (CD68, CD163, CD4) is crucial to distinguish this entity from other histiocytic or spindle cell morphologic mimics
- Characteristic ALK gene rearrangements allow for targeted therapy with ALK inhibitors
Terminology
- ALK related histiocytosis
- ALK rearranged histiocytosis
ICD coding
- ICD-10: D76.3 - other histiocytosis syndromes
Epidemiology
- Affects any age group, including children and young adults, with a female predilection (Blood 2022;139:256)
Sites
- Multisystem with systemic hematopoietic involvement
- Systemic involvement of liver, spleen or bone marrow with or without involvement of additional sites (Blood 2022;139:256, Leukemia 2022;36:1720)
- Multisystem disease, others
- ≥ 2 organs, most commonly central and peripheral nervous system, bone, lung and skin (Leukemia 2022;36:1720, Blood 2022;139:256)
- Single system disease
- Tumorous involvement of a single organ with solitary or multiple lesions
- Most common sites include central and peripheral nervous system, skin, soft tissue and breast (Blood 2022;139:256)
Pathophysiology
- Fusion of the receptor tyrosine kinase domain of the ALK gene, most commonly to exon 24 of KIF5B, results in constitutive activation of downstream signaling, including RAS-RAF-MEK-ERK (MAPK) and PI3K / AKT / mTOR signaling pathways
- MAPK pathway activation leads to phosphorylation of downstream ERK, ultimately resulting in transcription of various effector genes, including the gene encoding for cyclin D1 (CCND1); translation of CCND1 messenger RNA to the cyclin D1 protein is mTOR dependent (Blood 2022;139:256)
Etiology
- Unknown at this time
Clinical features
- Diverse clinical presentations depending on site(s) of involvement
- Infants with multisystem disease involving the hematopoietic system present with hepatomegaly, anemia and thrombocytopenia, often accompanied by splenomegaly and coagulopathy
- Other multisystem and single system disease presents with varying mass effects depending on tumor size and location (Blood 2022;139:256)
- Neurologic involvement may present with headache, nausea / vomiting, ataxia, paresis, seizure, diplopia or trigeminal neuralgia
- Pulmonary involvement may present with dry cough or respiratory dysfunction requiring oxygen therapy
- Liver involvement may present with jaundice, coagulopathy or fever
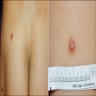
- Cutaneous lesions present as slowly growing papules or nodules
Diagnosis
- Comprehensive patient evaluation including clinical history, physical examination and imaging studies
- Coexpression of histiocytic markers and ALK should prompt molecular confirmation of ALK gene rearrangement either by RNA sequencing or by FISH (fluorescence in situ hybridization) analysis
- References: Mod Pathol 2019;32:598, Blood 2022;139:256
Laboratory
- Variable, depending on organ system(s) involved
- Liver involvement can be associated with elevated liver enzymes, elevated bilirubin and low fibrinogen (Blood 2022;139:256)
- Hematopoietic involvement can be associated with anemia, thrombocytopenia and prolonged prothrombin time and activated partial thromboplastin time (Blood 2022;139:256)
Radiology description
- Mass lesions can be identified by ultrasound, Xray, magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET) CT and other imaging modalities (Blood 2022;139:256)
- Liver, pancreas, long bone lesions appear hypermetabolic on PET CT imaging
- Liver and thyroid lesions appear hypoechoic on ultrasound
- Kidney tumors appear hypodense on CT imaging
- Tumors of the vertebral bodies and liver appear hyperintense by T2 weighted MRI
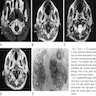
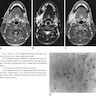
- CNS tumors rarely can mimic meningioma on MRI (Radiol Case Rep 2023;18:2259)
Radiology images
Prognostic factors
- Prognosis depends on extent of disease and location(s) of tumor(s)
- Typically favorable outcomes in cases with single system disease
- Reports of death due to multisystem disease progression include every age group (Mod Pathol 2019;32:598, Blood 2022;139:256, JCO Precis Oncol 2021;5:PO.20.00383)
Case reports
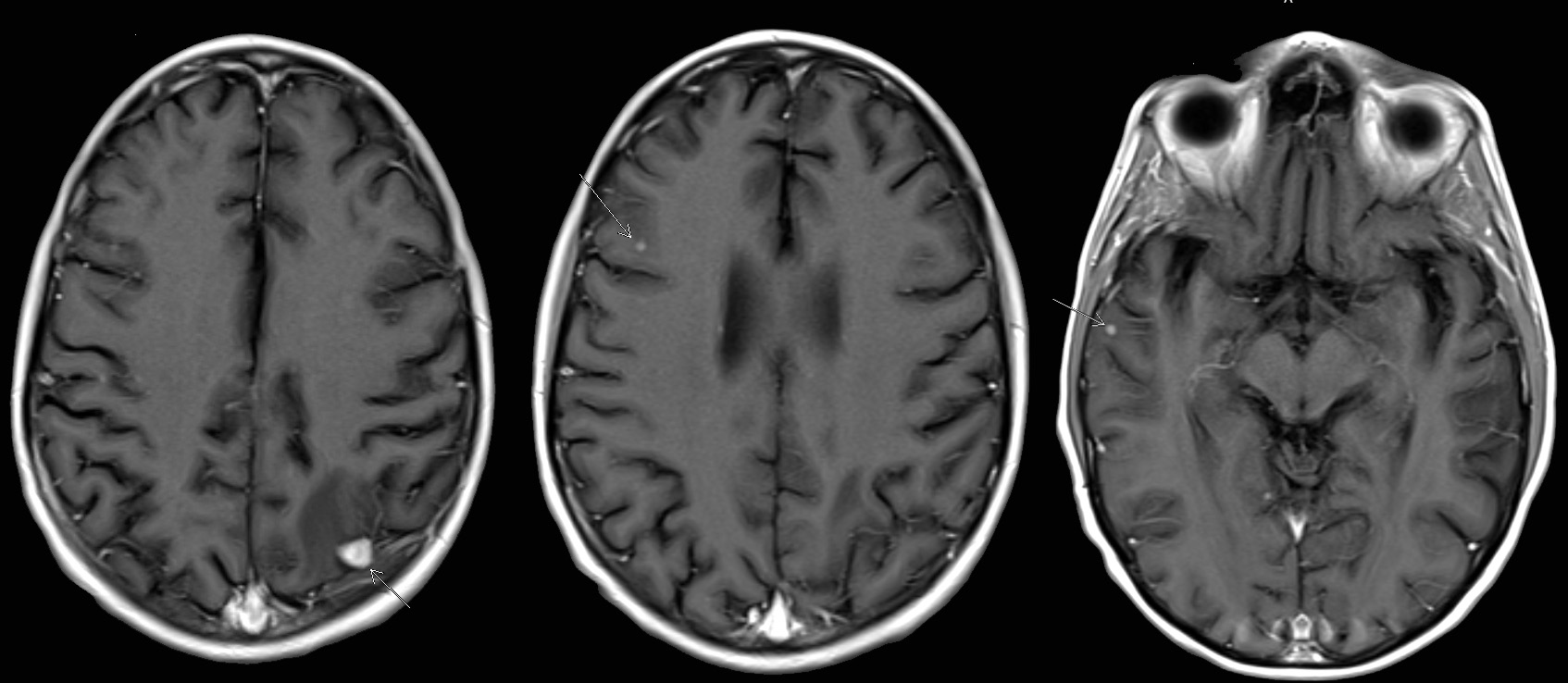
- 17 month old boy with a 5.1 cm intracranial mass, multiple nodules in the liver and bilateral lungs, as well as multiple skin papules (Orphanet J Rare Dis 2023;18:53)
- 27 year old man with a 1.5 cm intradural extramedullary enhancing lesion (Turk Patoloji Derg 2021;37:172)
- 30 year old man with cavernous sinus lesion mimicking meningioma (Radiol Case Rep 2023;18:2259)
- 38 year old woman with multiple unilateral breast lesions (Int J Surg Case Rep 2022;97:107435)
- 50 year old woman with appendiceal nodule (Haematologica 2019;104:e534)
- 51 year old woman with lesions identified in the right lung, central nervous system and iliac lymph nodes (Ann Palliat Med 2021;10:10095)
Treatment
- Management varies depending on extent of disease and site(s) of involvement
- Surgical resection is often definitive treatment for single system disease (Blood 2022;139:256)
- Systemic therapy for incompletely resected or unresectable tumors and for multisystem disease includes steroids, intravenous immune globulin (IVIG) or chemotherapy
- Targeted therapy with ALK inhibitors has been used as first or second line treatment in a majority of cases (Blood 2022;139:256, JCO Precis Oncol 2021;5:PO.20.00383, Oncologist 2017;22:1444)
- ALK inhibitors include alectinib, loratinib, brigatinib, crizotinib, ceritinib and entrectinib
- Used as monotherapy or in combination with other treatment modalities
- Rare cases have resulted in spontaneous regression (Blood 2022;139:256)
Gross description
- Tumors may be well circumscribed or ill defined with a tan, white or yellow cut surface
Microscopic (histologic) description
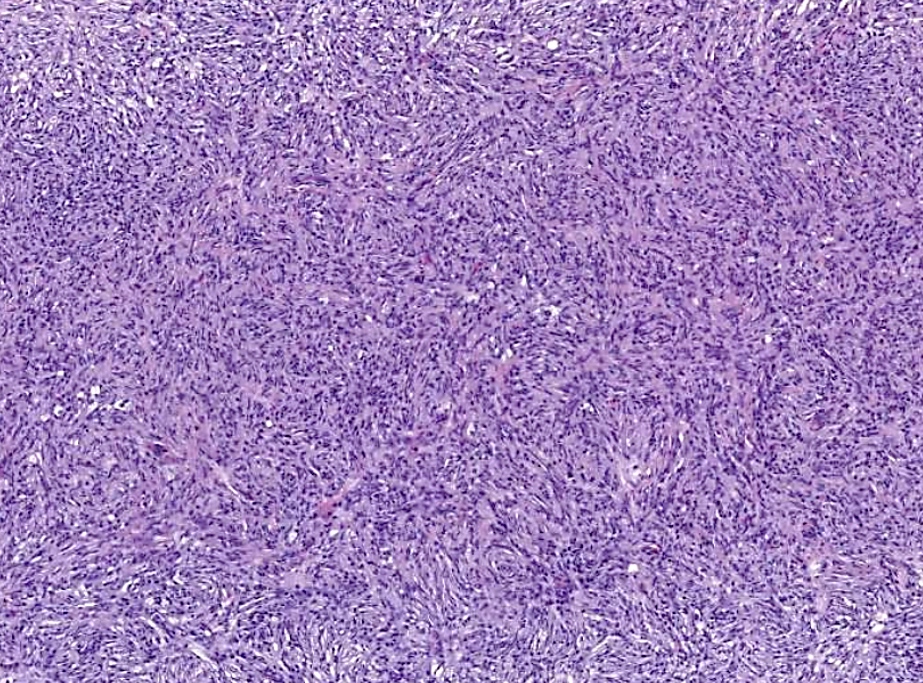
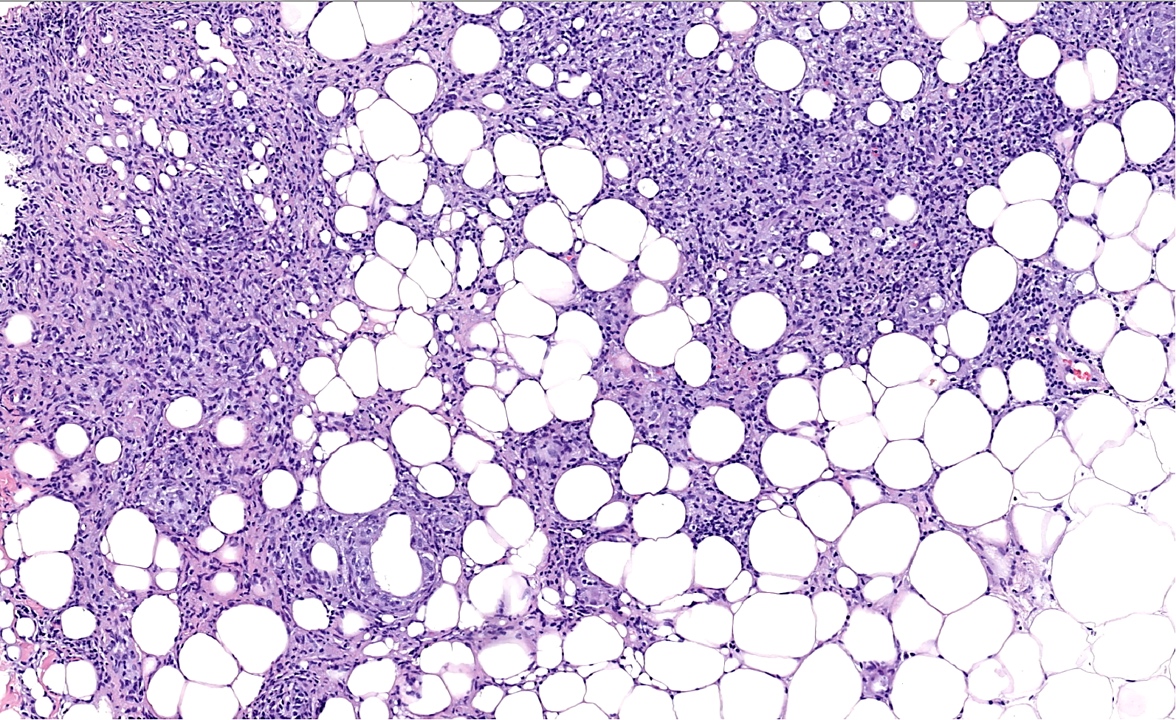
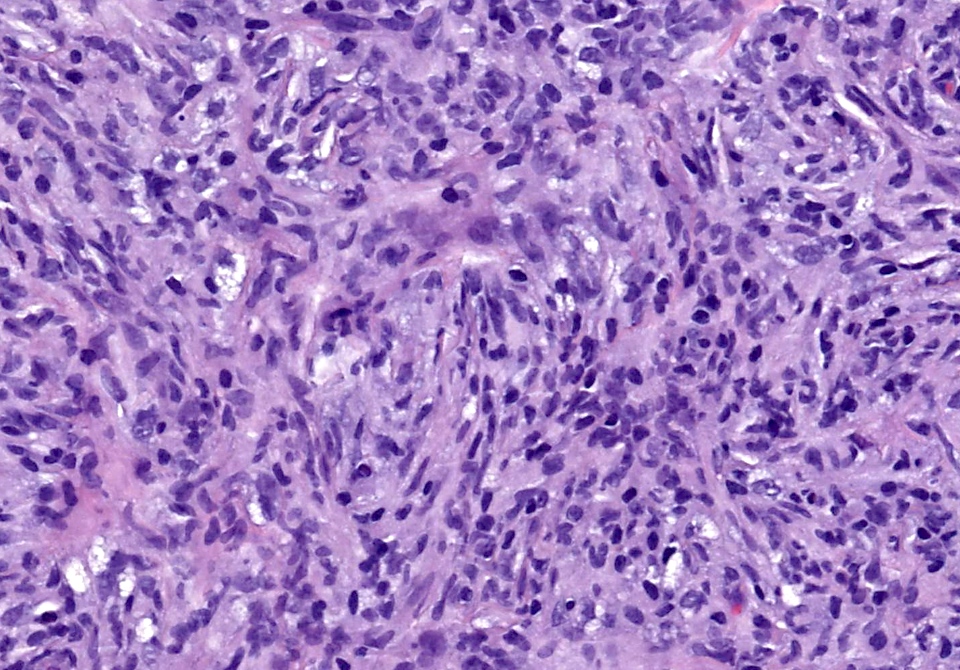
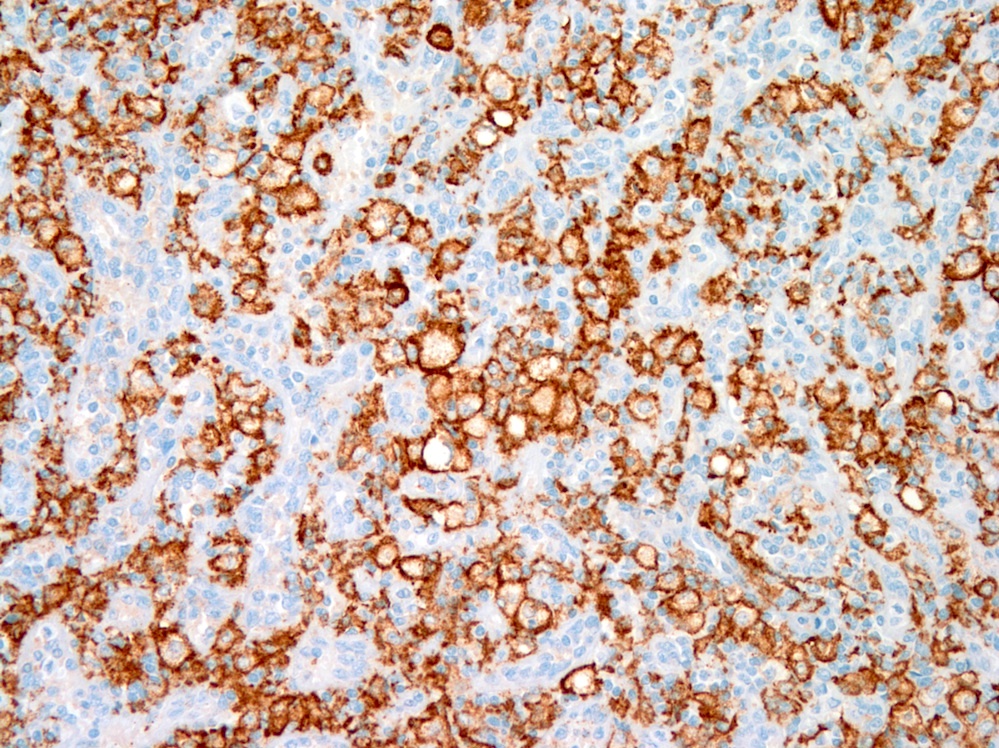
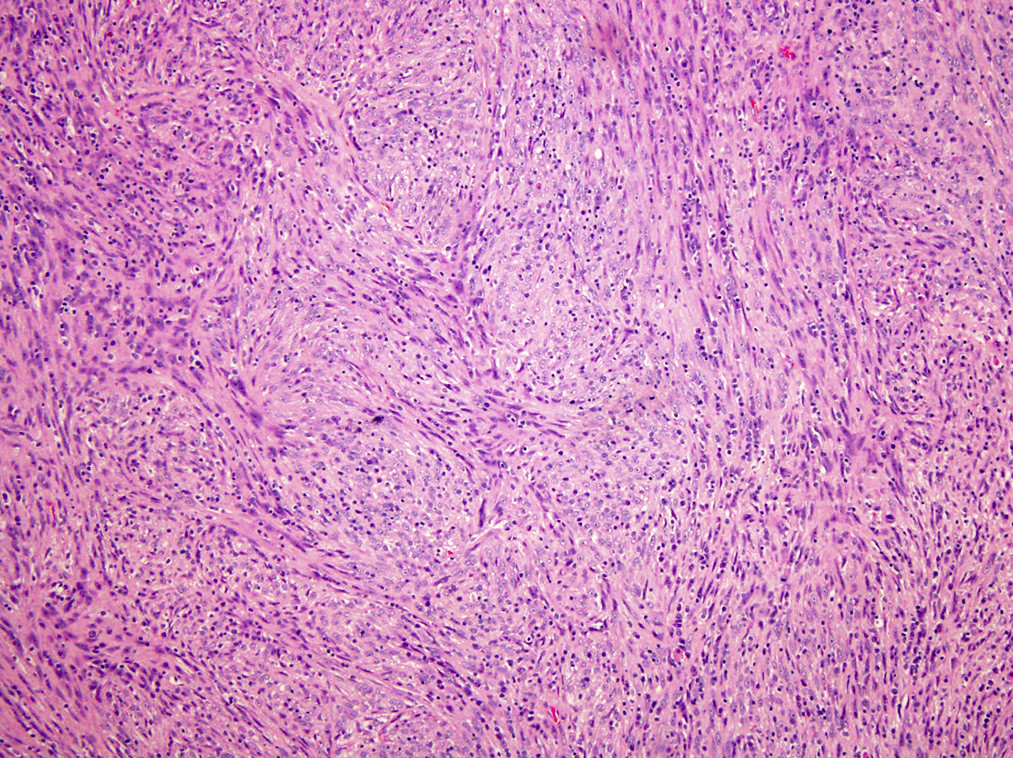
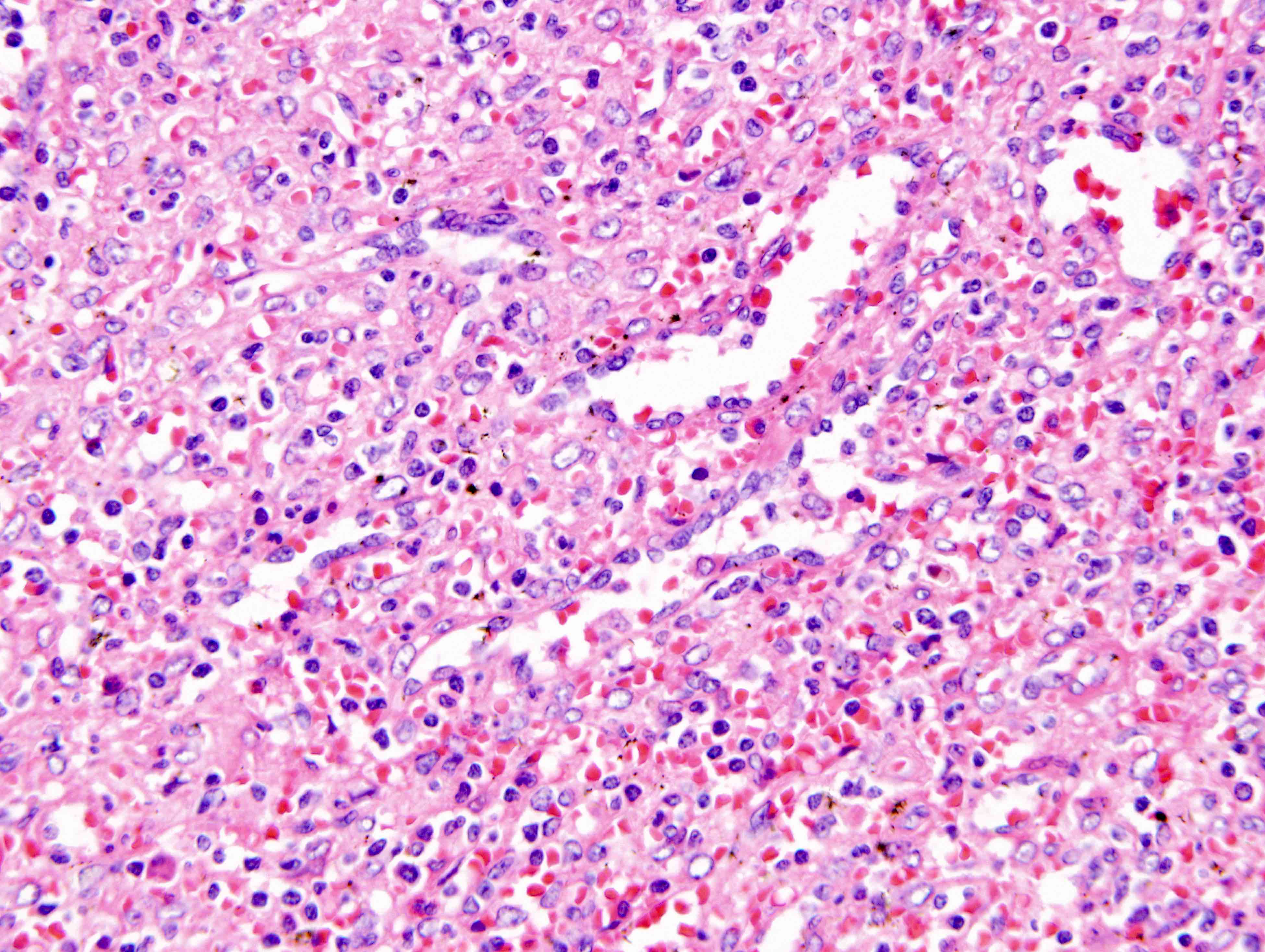
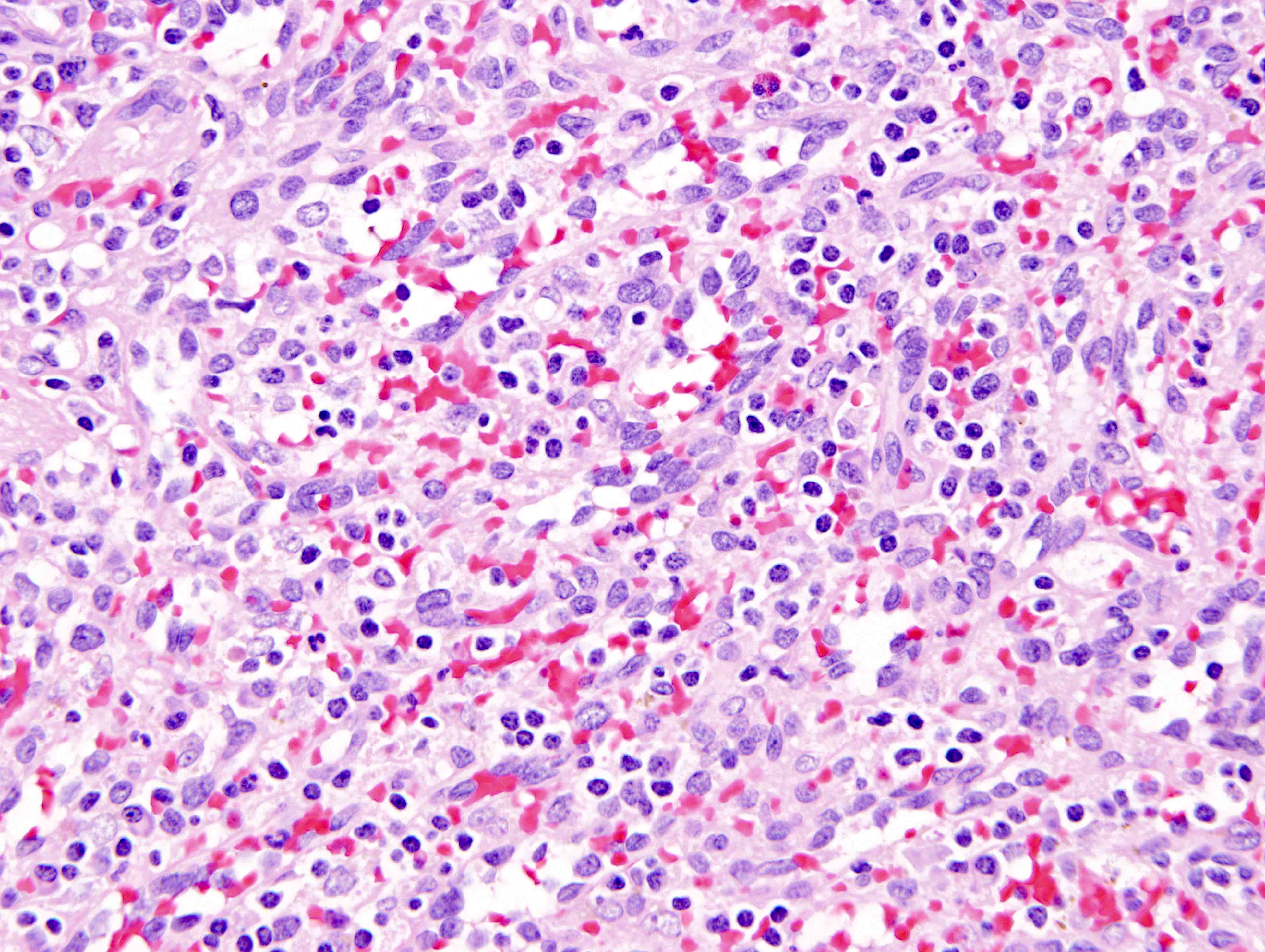
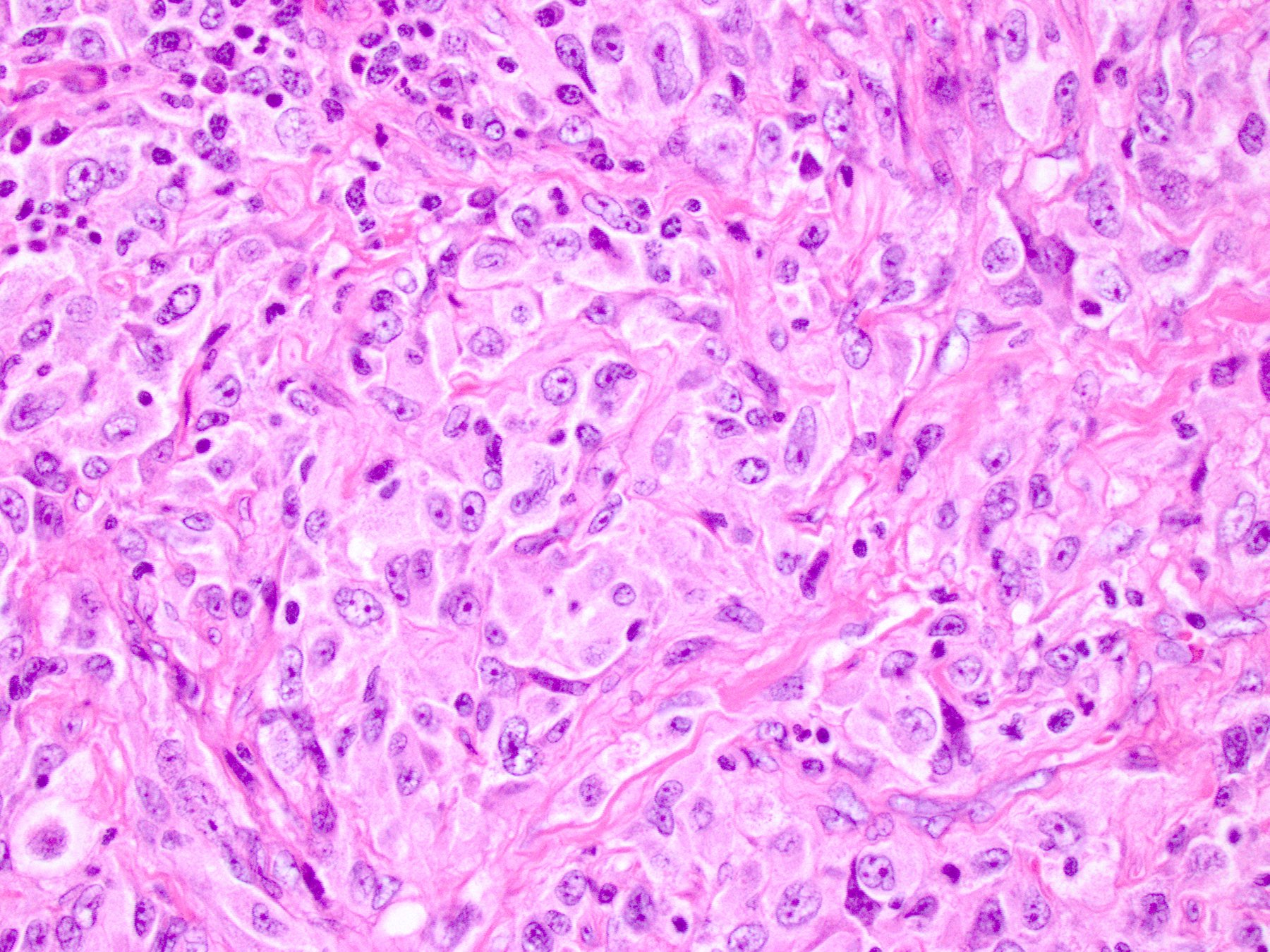
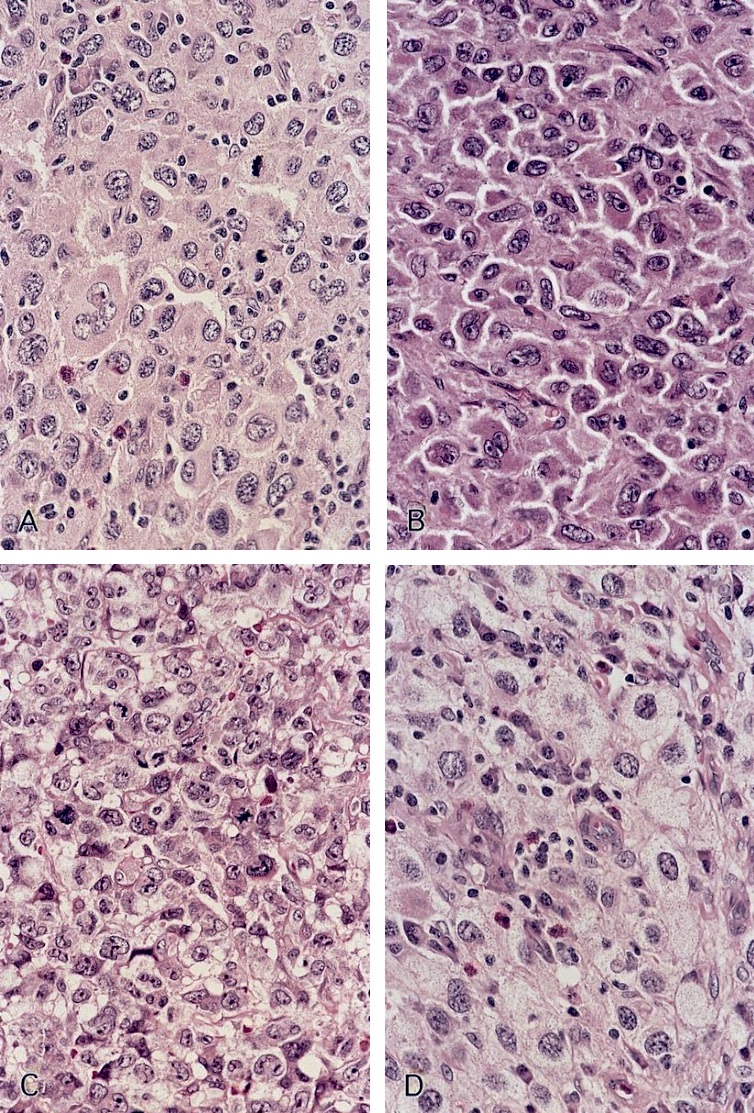
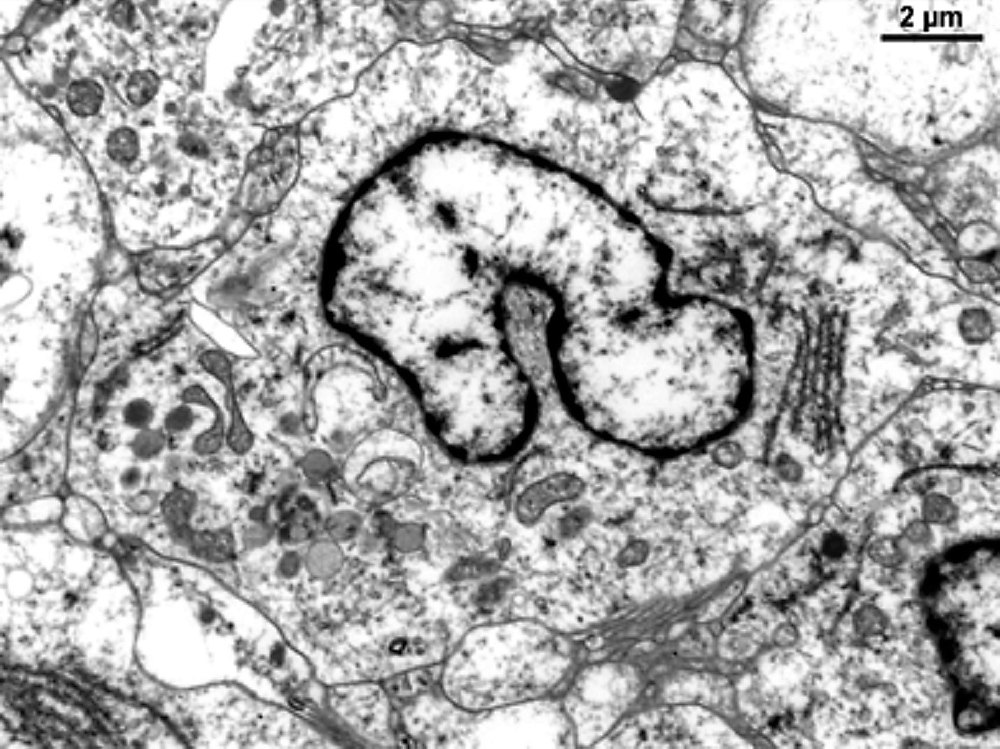
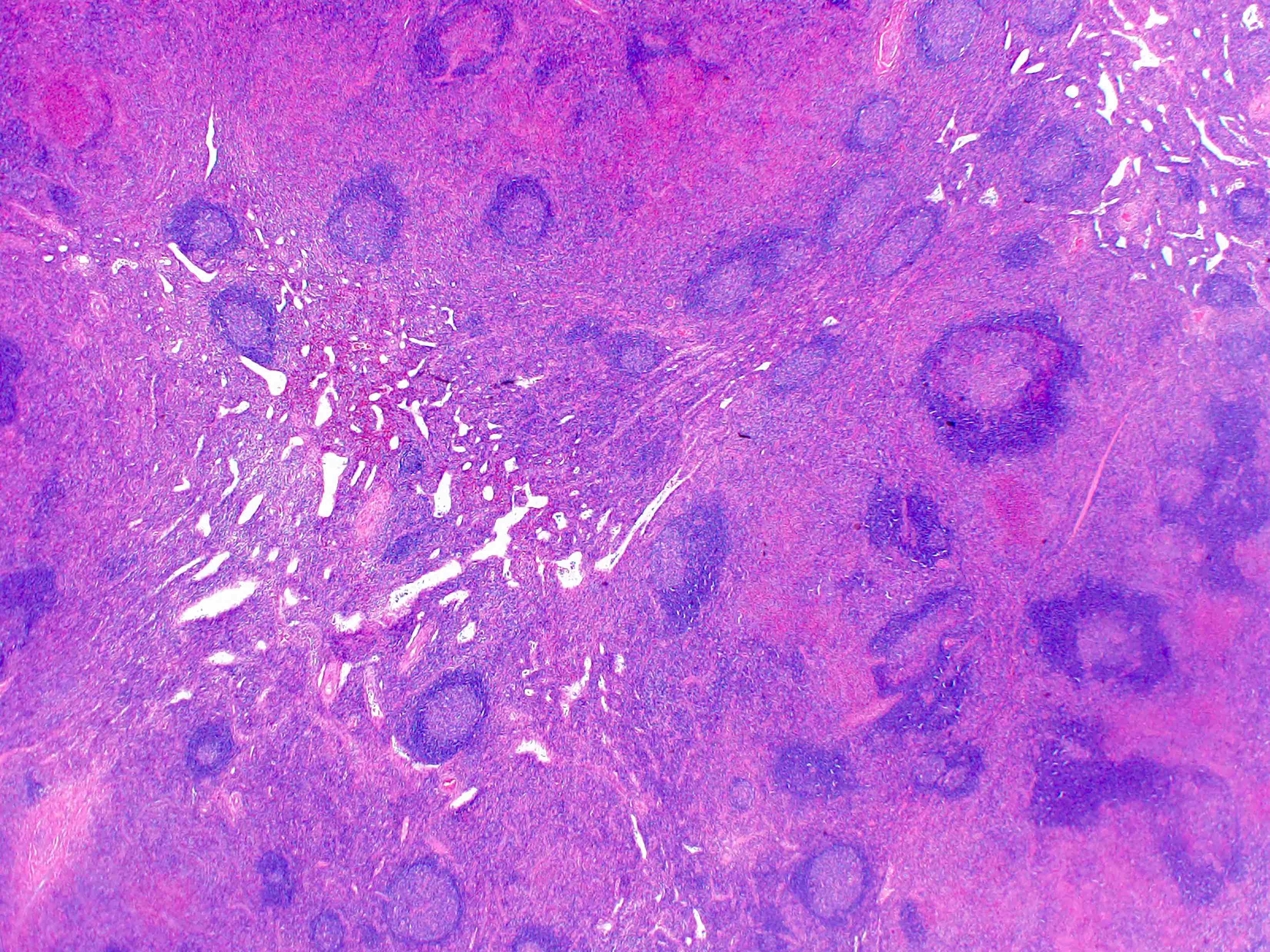
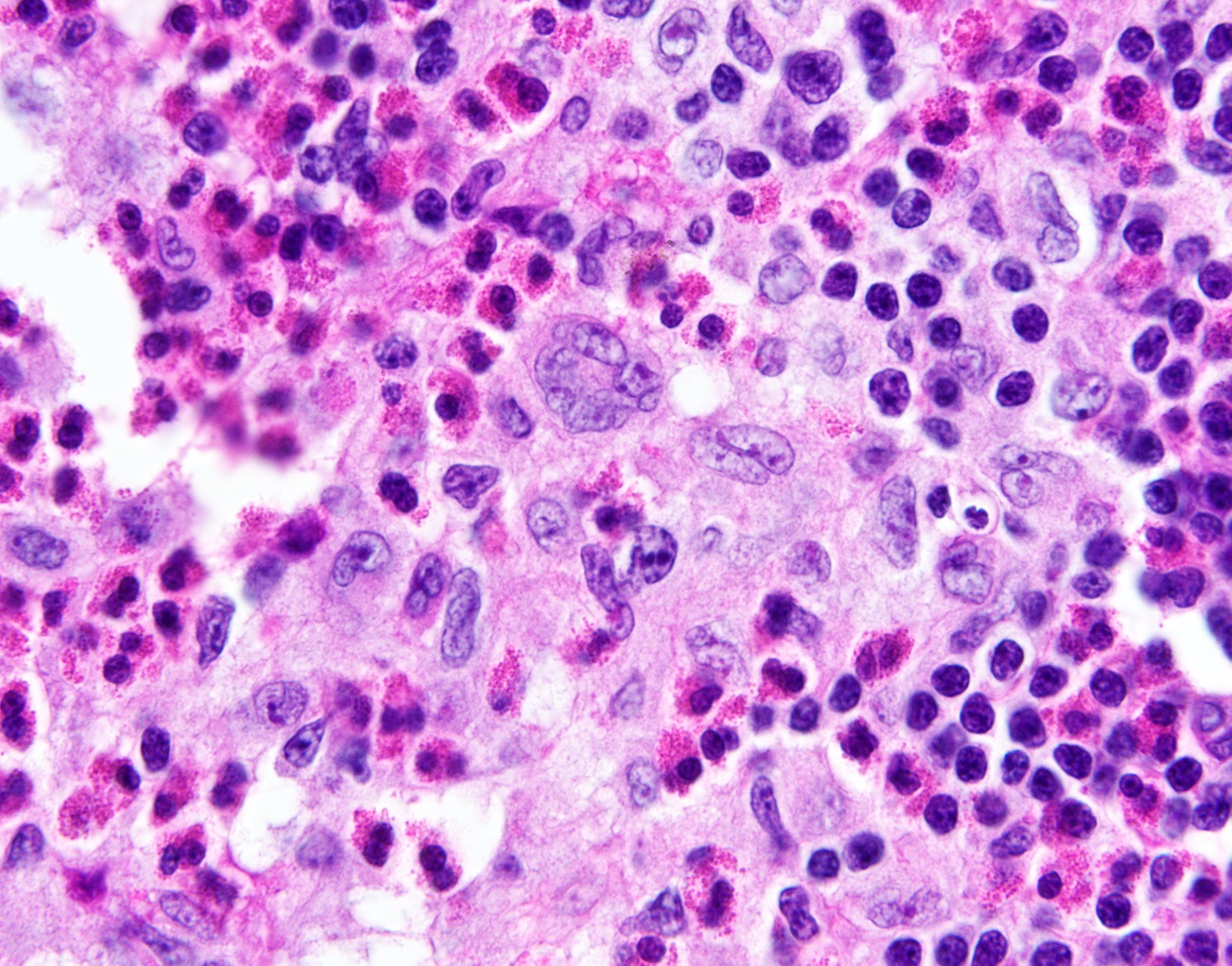
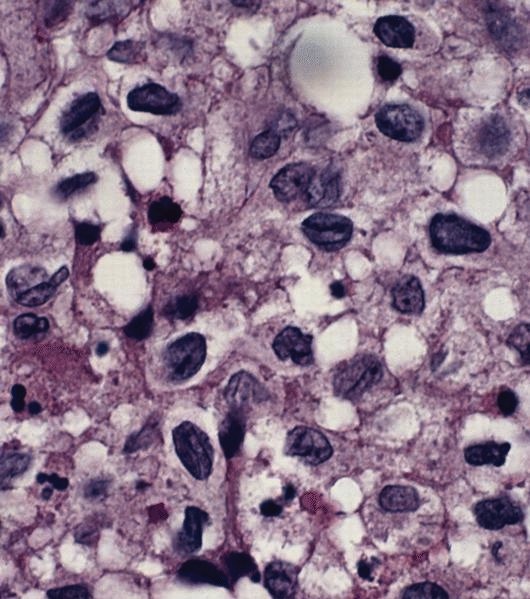
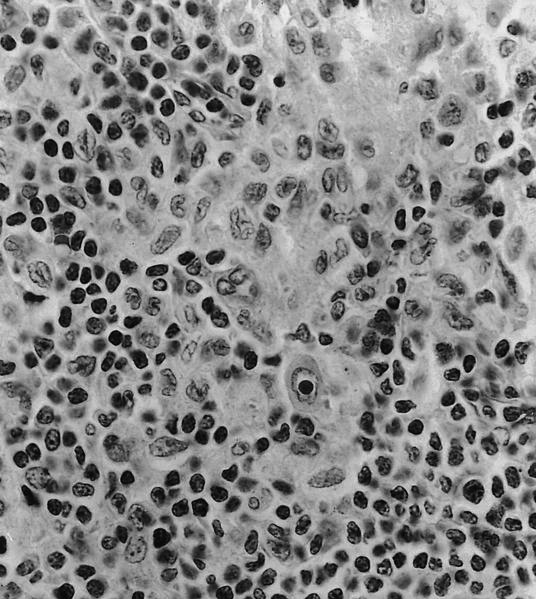
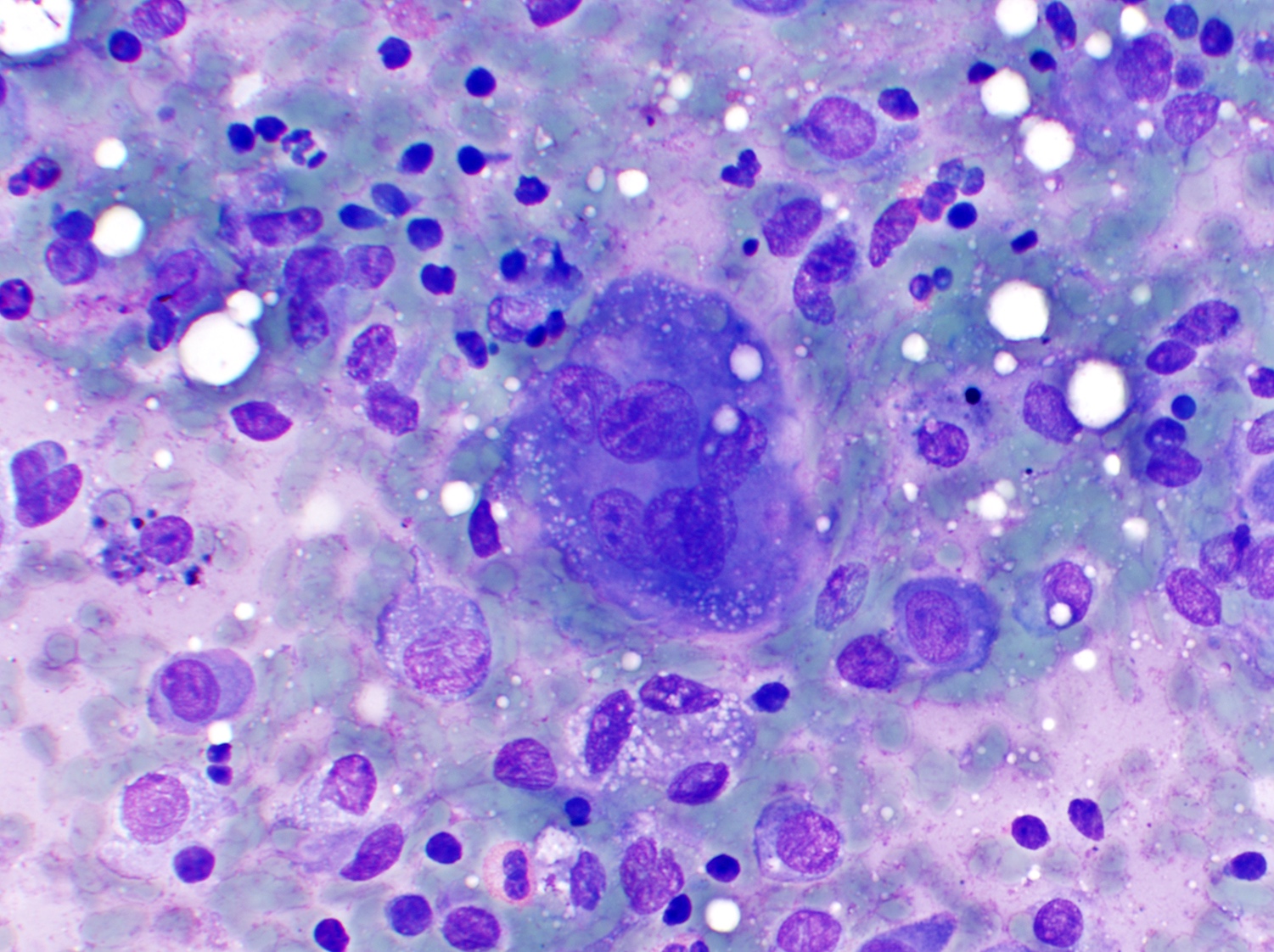
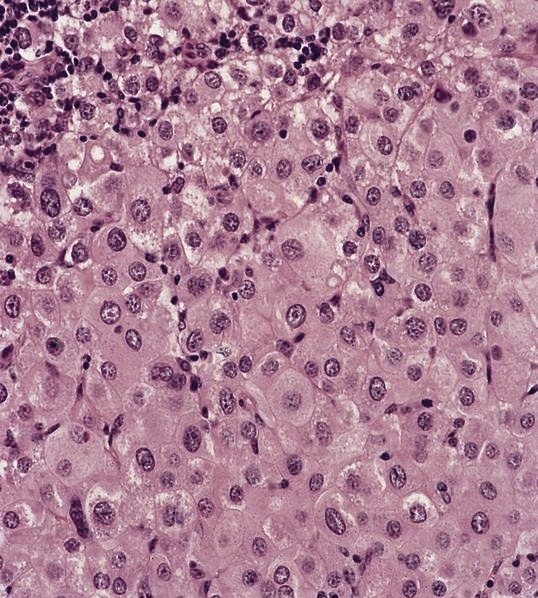
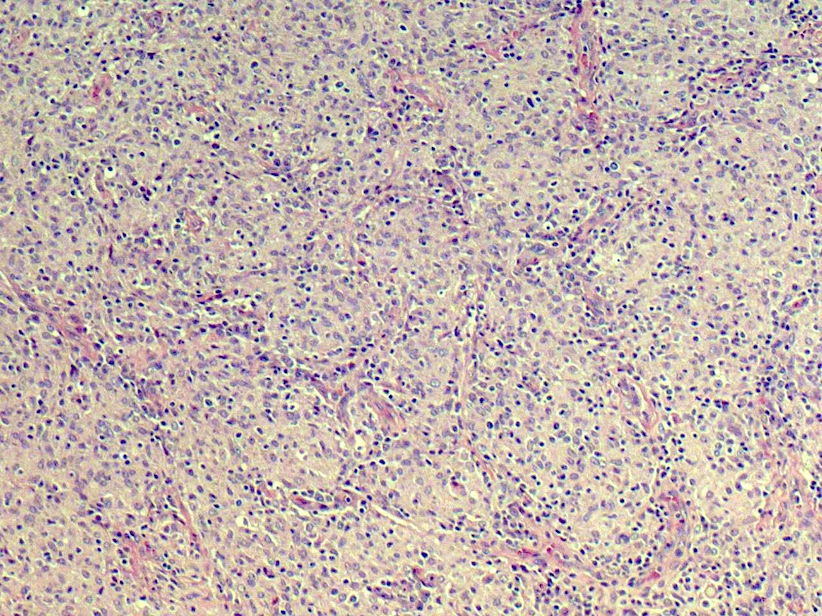
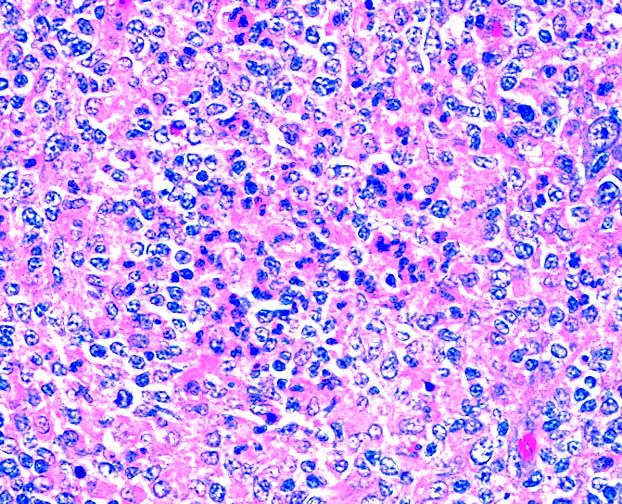
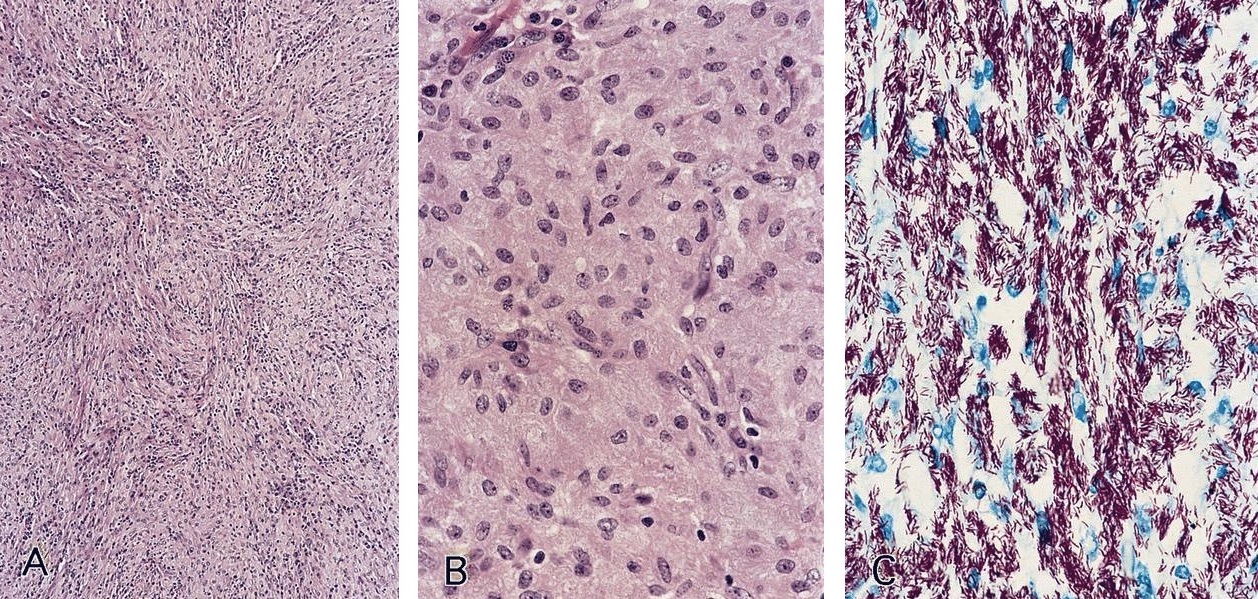
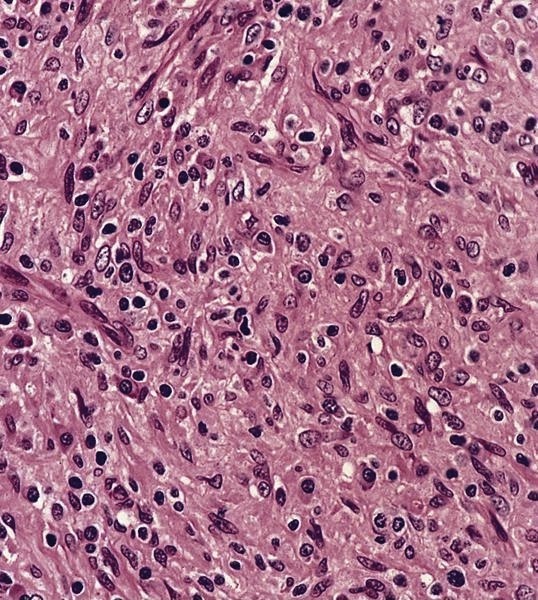
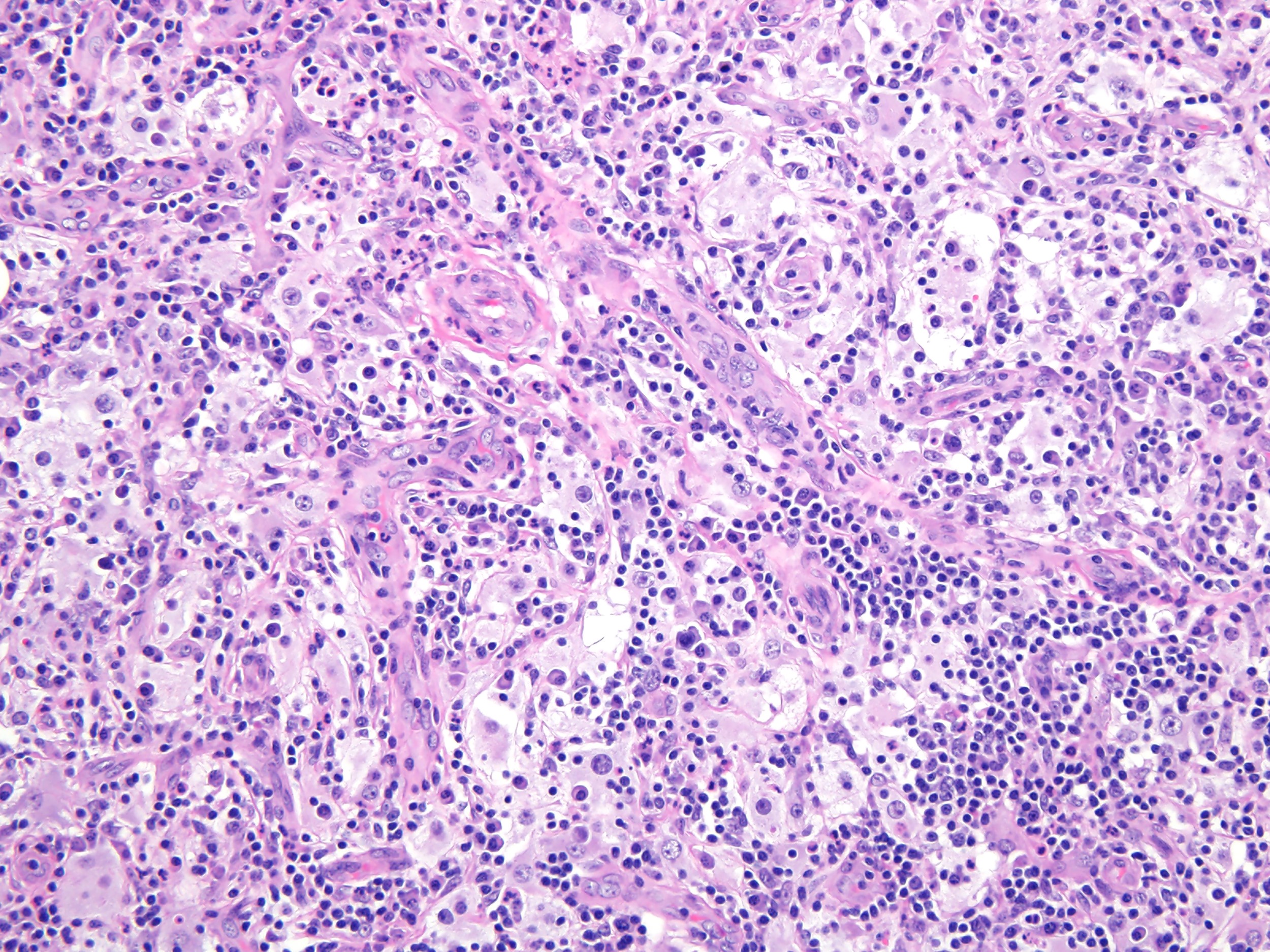
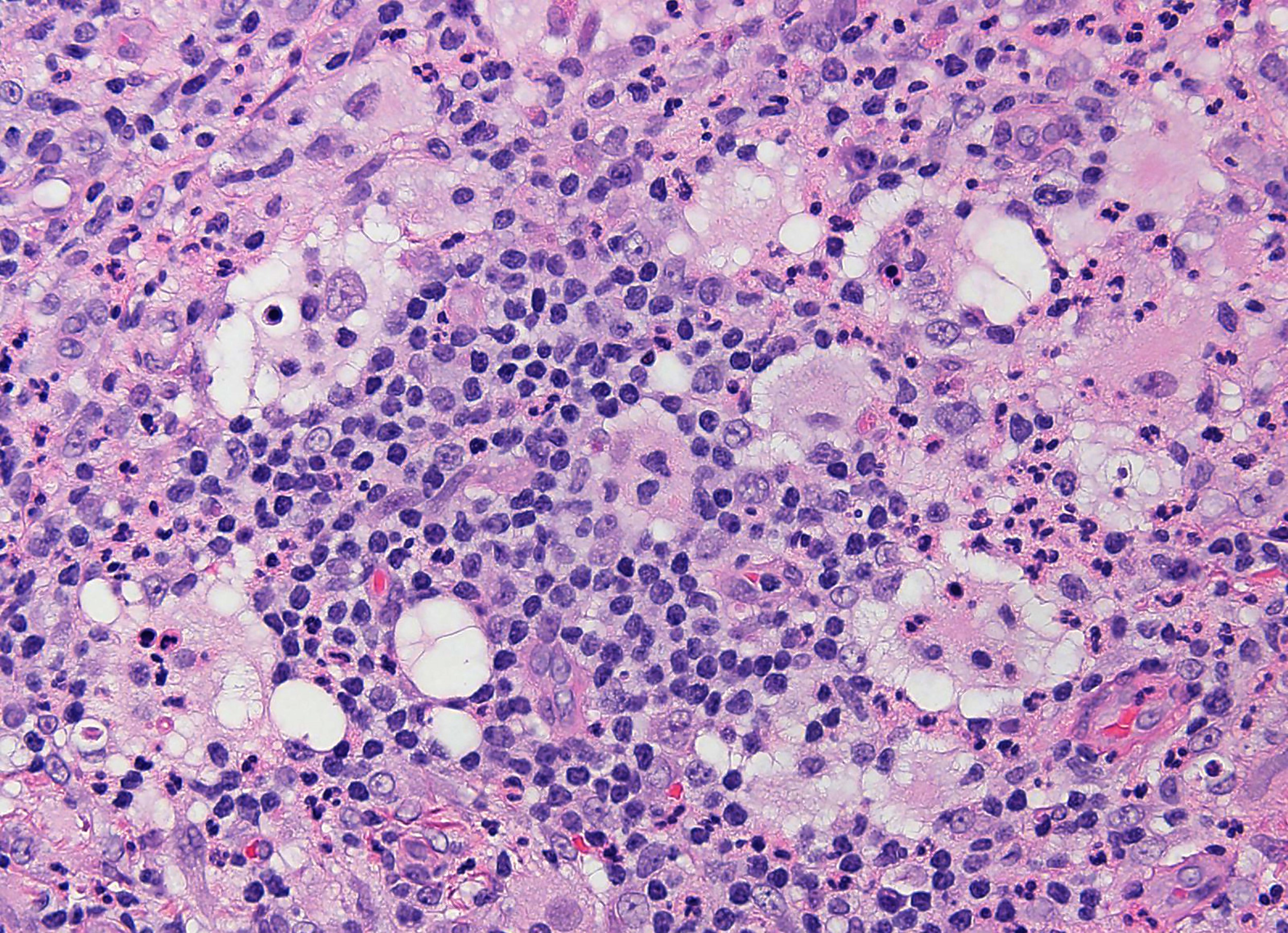
- Large oval (epithelioid), foamy or spindle cells in varying proportions without significant nuclear atypia and abundant eosinophilic cytoplasm (Mod Pathol 2019;32:598, Blood 2022;139:256)
- Large histiocytes may show irregular nuclear contours (indentation, folding, clefting, lobulation) with fine chromatin and small nucleoli (Mod Pathol 2019;32:598, Blood 2022;139:256)
- Occasional multinucleated cells, including Touton giant cells, may be present (Mod Pathol 2019;32:598, Blood 2022;139:256)
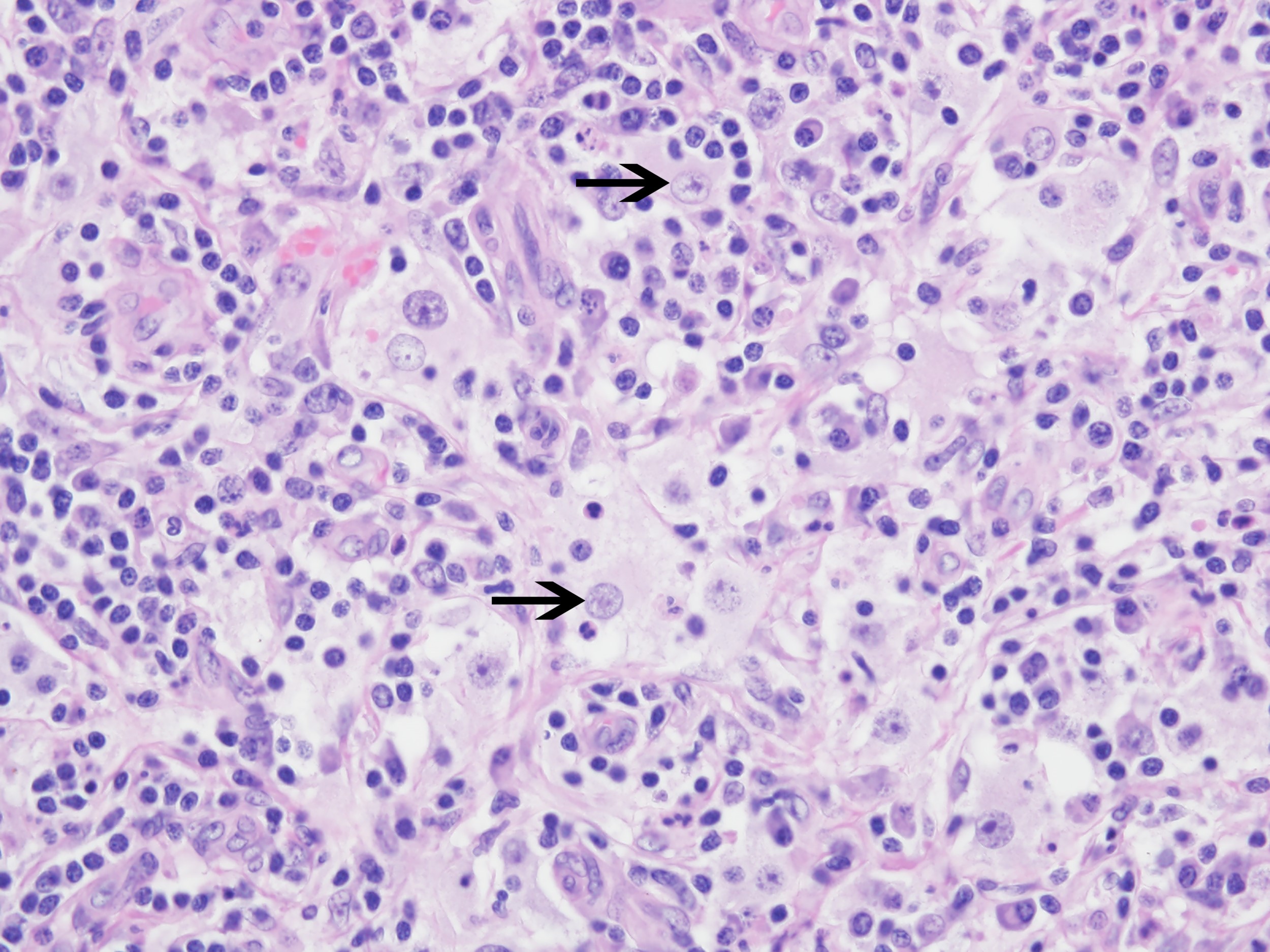
- Emperipolesis, characterized by engulfment of inflammatory cells (similar to Rosai-Dorfman disease), may be observed (Mod Pathol 2019;32:598, Blood 2022;139:256)
- Hepatic involvement may show coalescing histiocytes indistinguishable from hepatocytes (Mod Pathol 2019;32:598)
- Bone marrow infiltration may vary from focal to extensive involvement (Mod Pathol 2019;32:598)
- Skin lesions are often noncircumscribed, nonepidermotropic infiltrates composed of sheets of histiocytes with varying degree of fibrosis (Mod Pathol 2019;32:598)
- Fascicular and storiform growth patterns common in breast tumors (Am J Surg Pathol 2021;45:347)
Microscopic (histologic) images
Peripheral smear description
- In systemic cases with hematopoietic involvement, anemia, thrombocytopenia and leukocytosis are usually seen
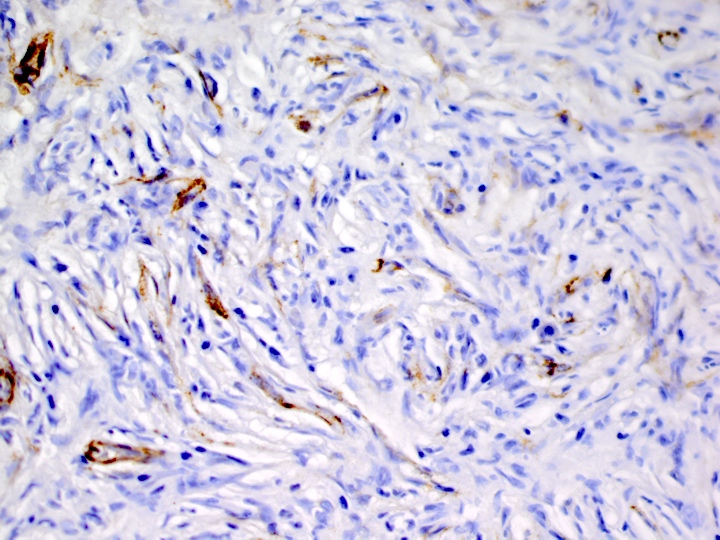
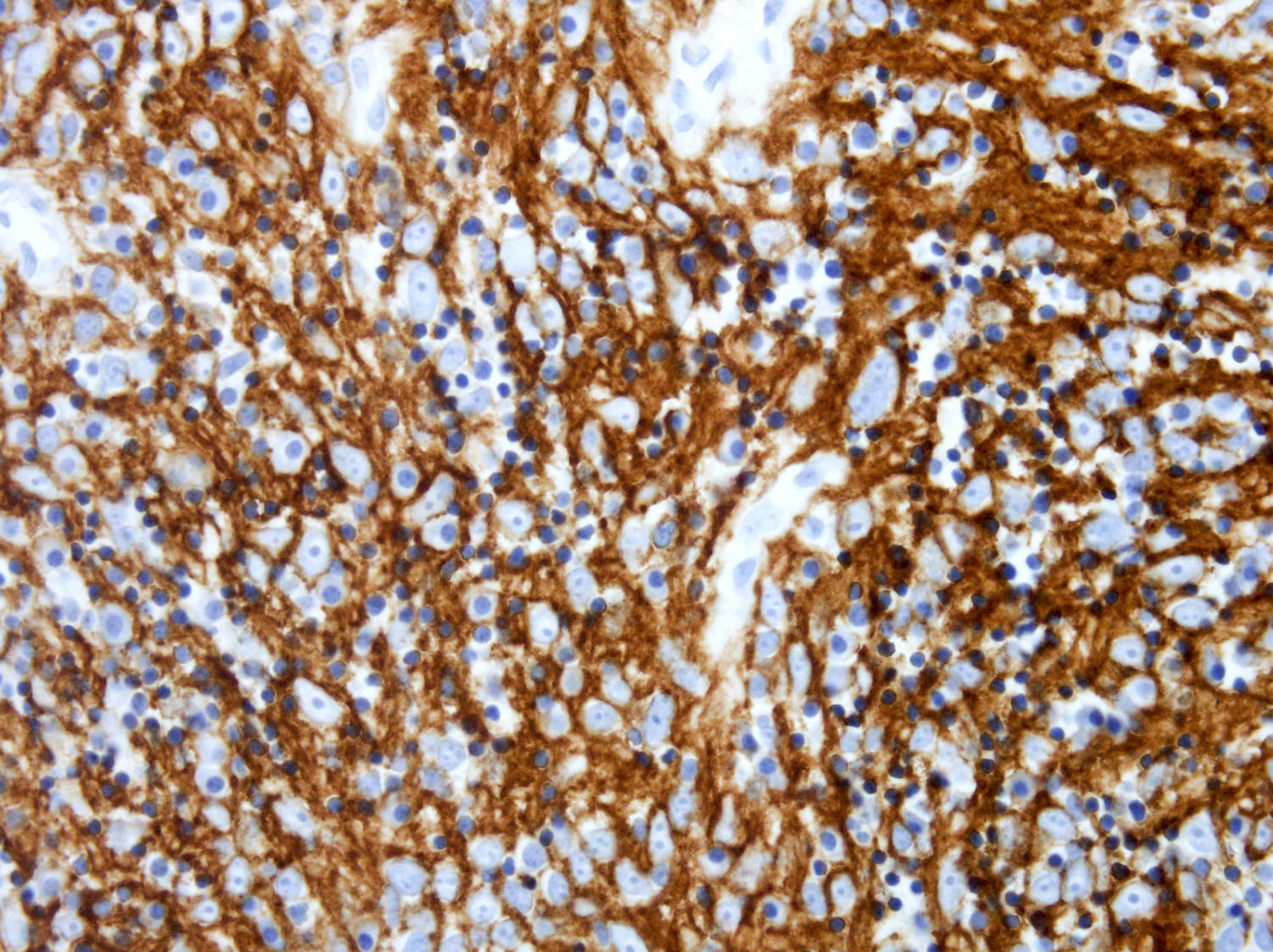
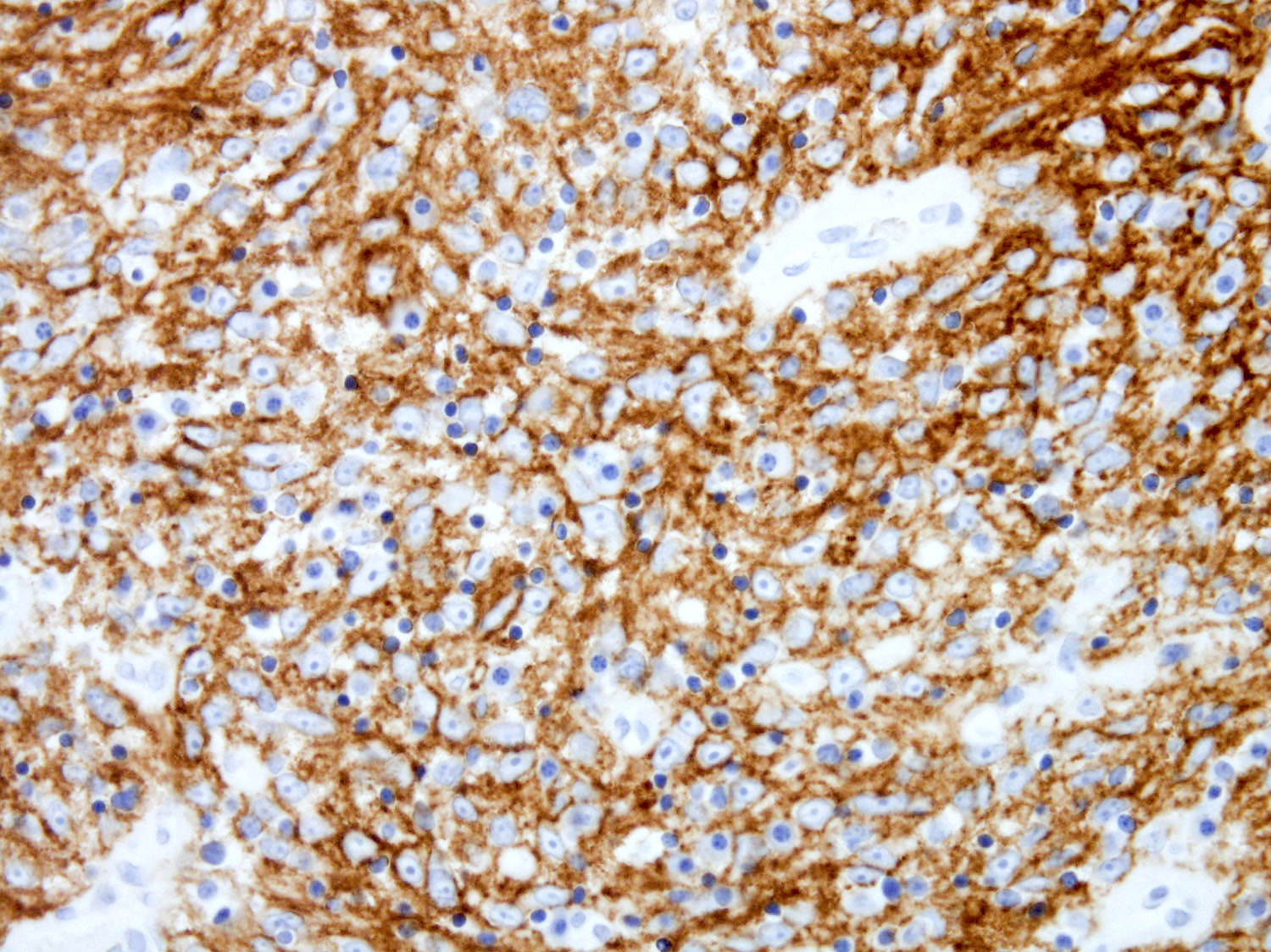
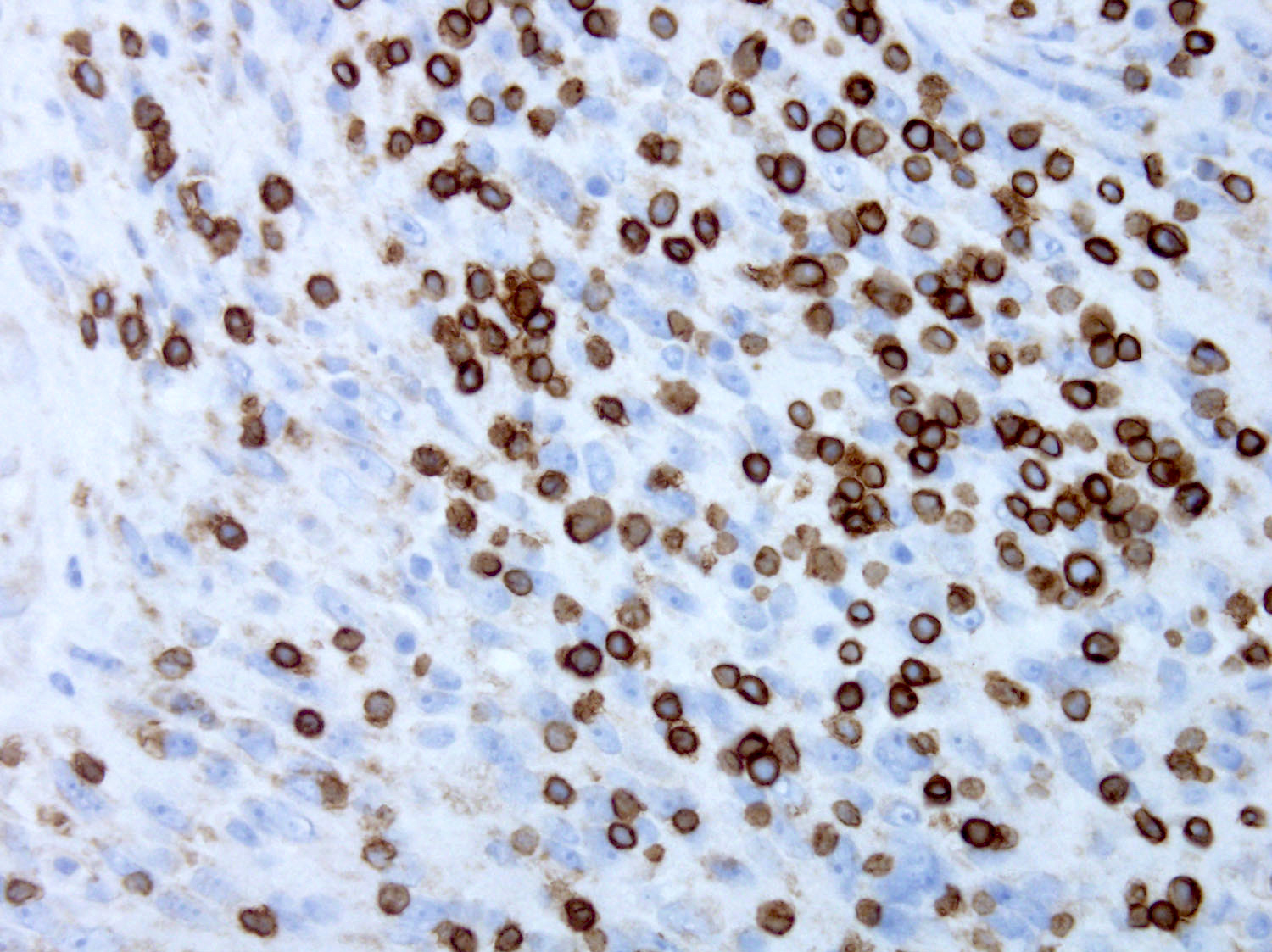
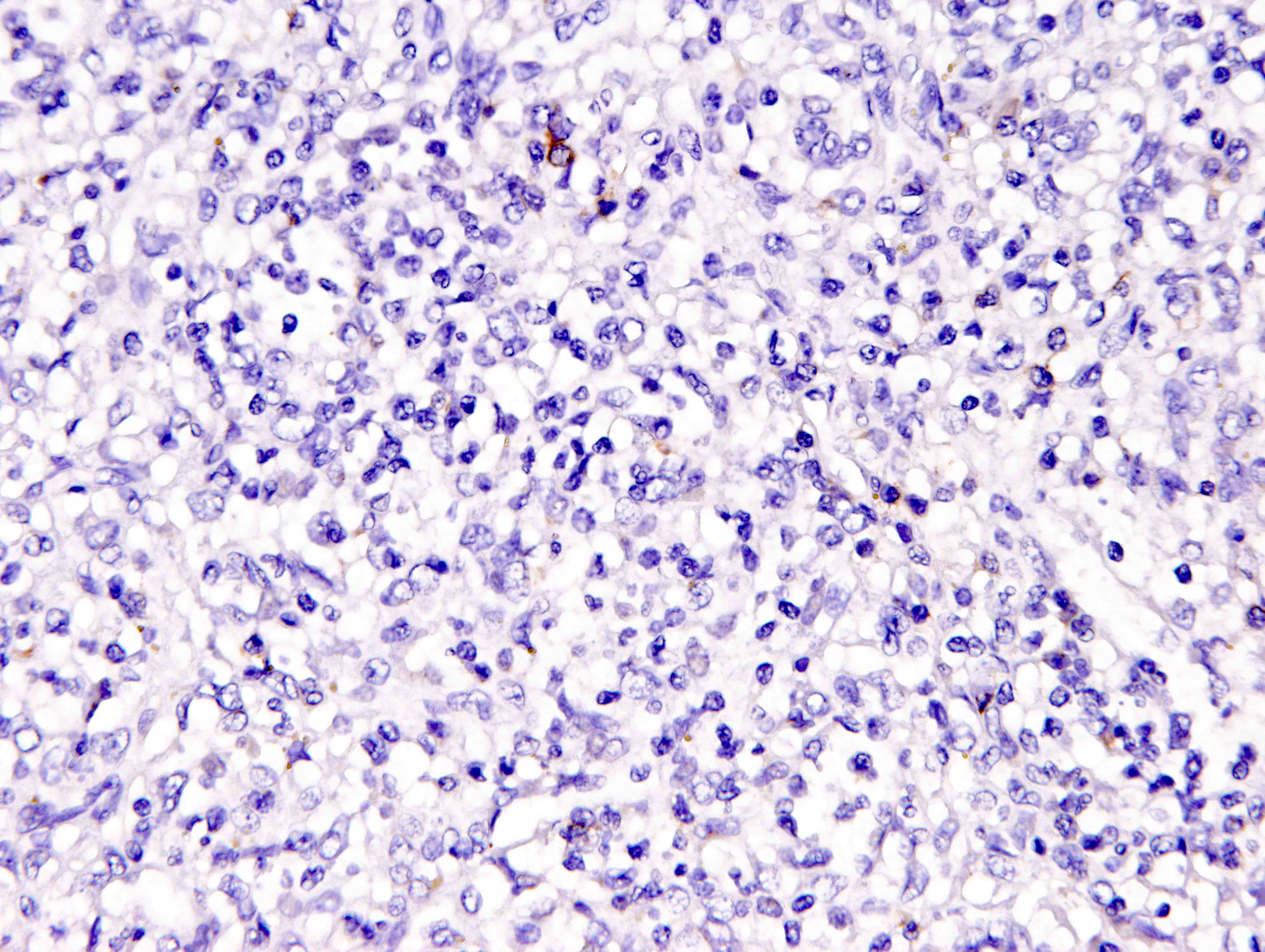
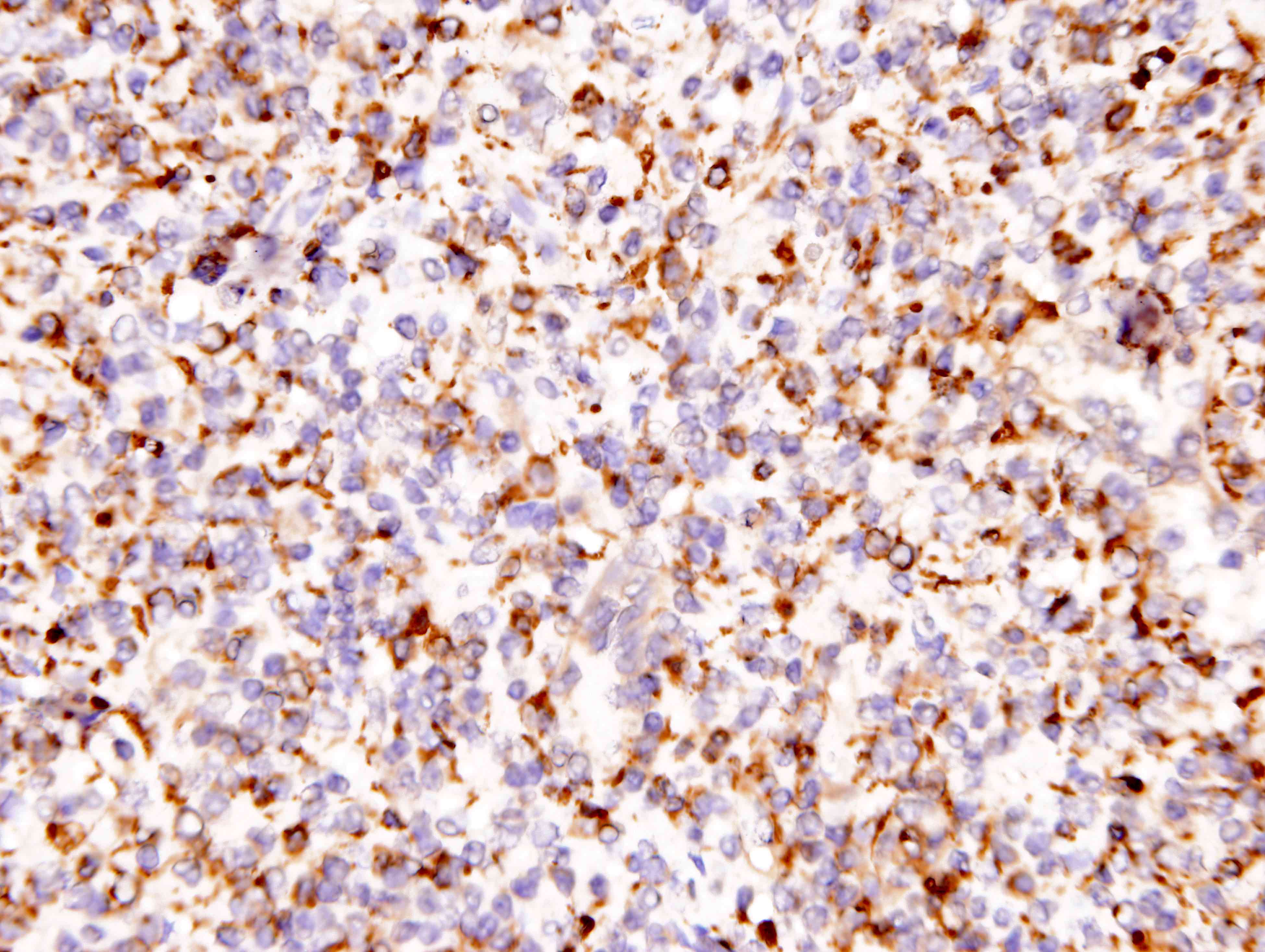
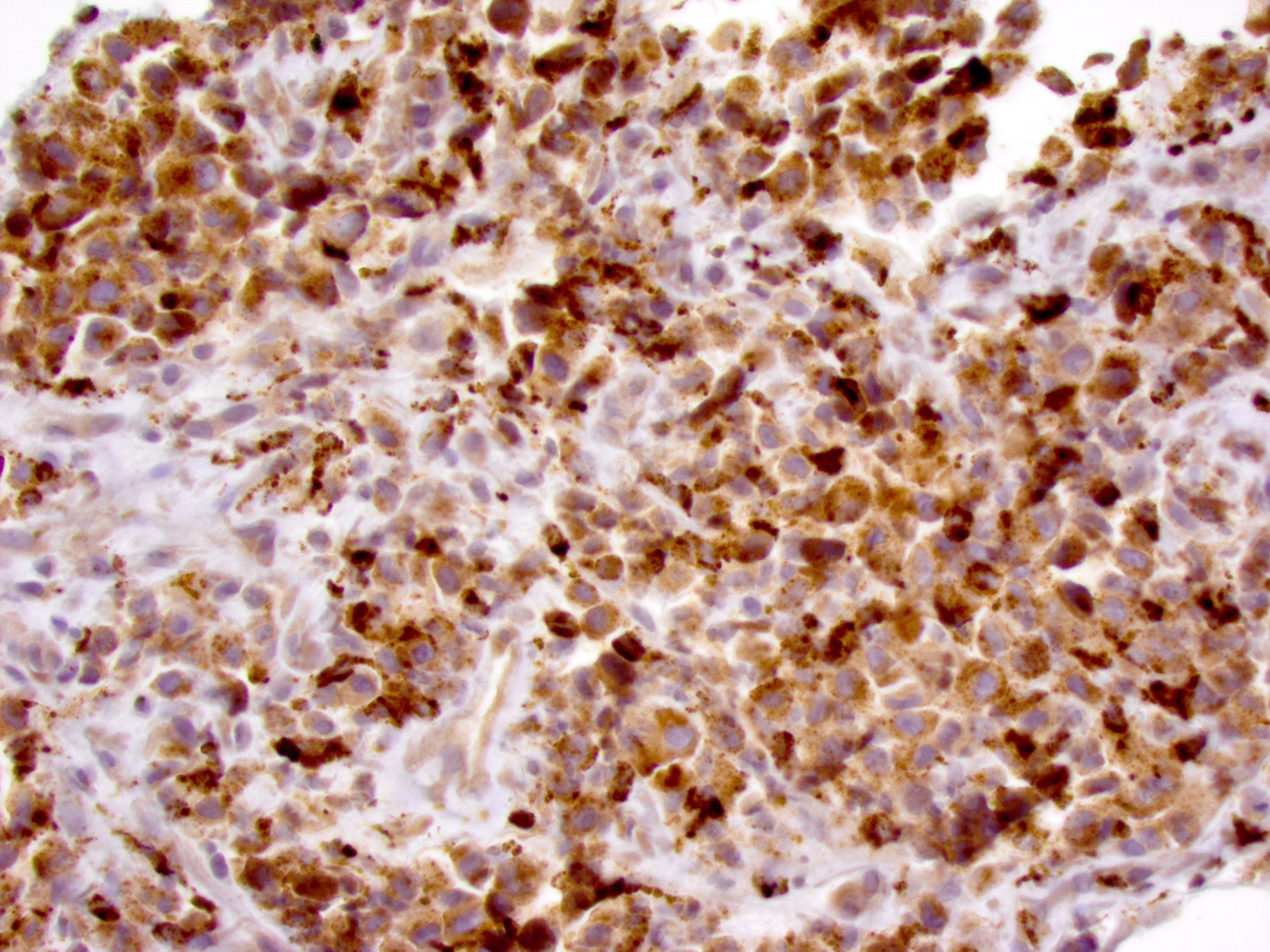
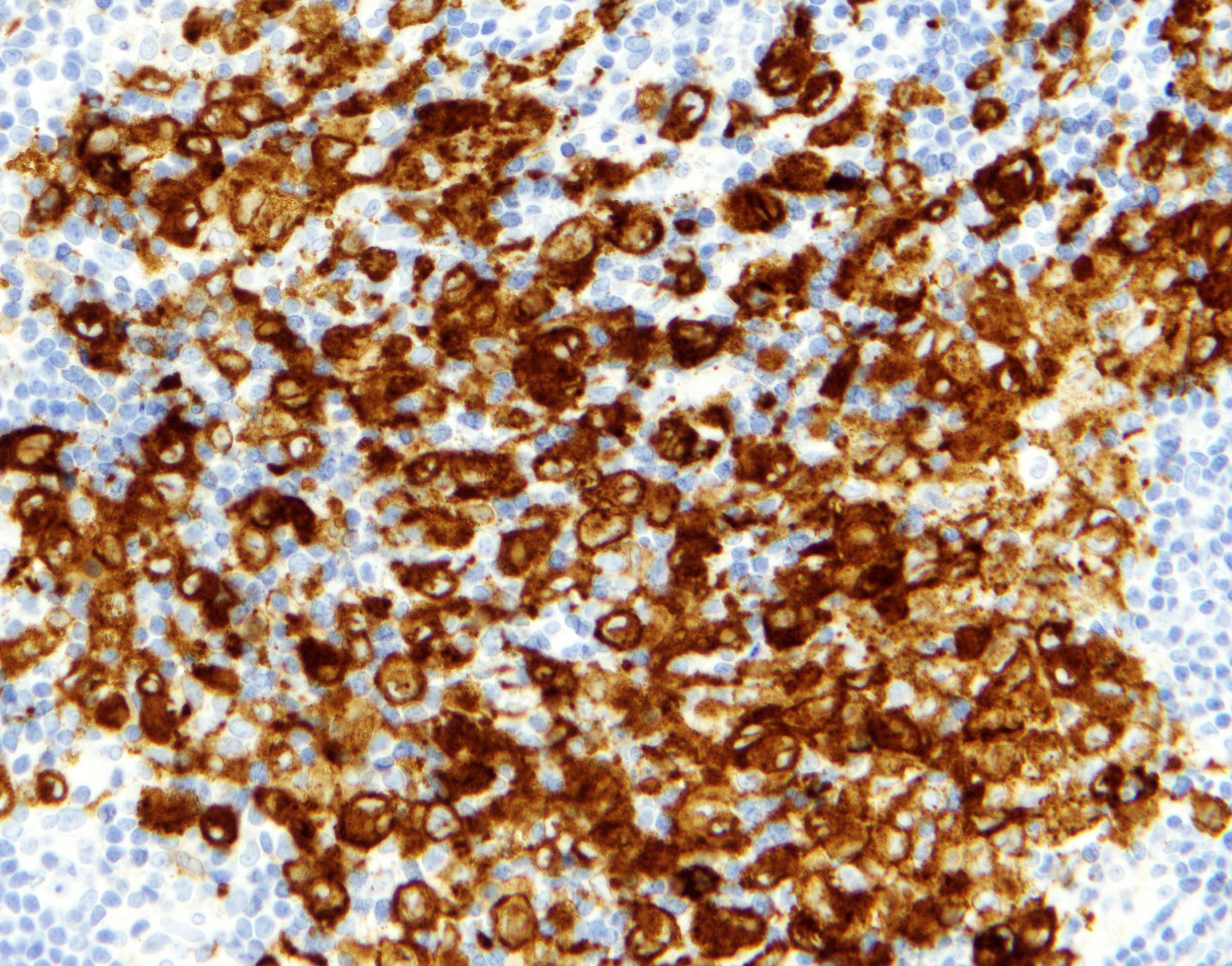
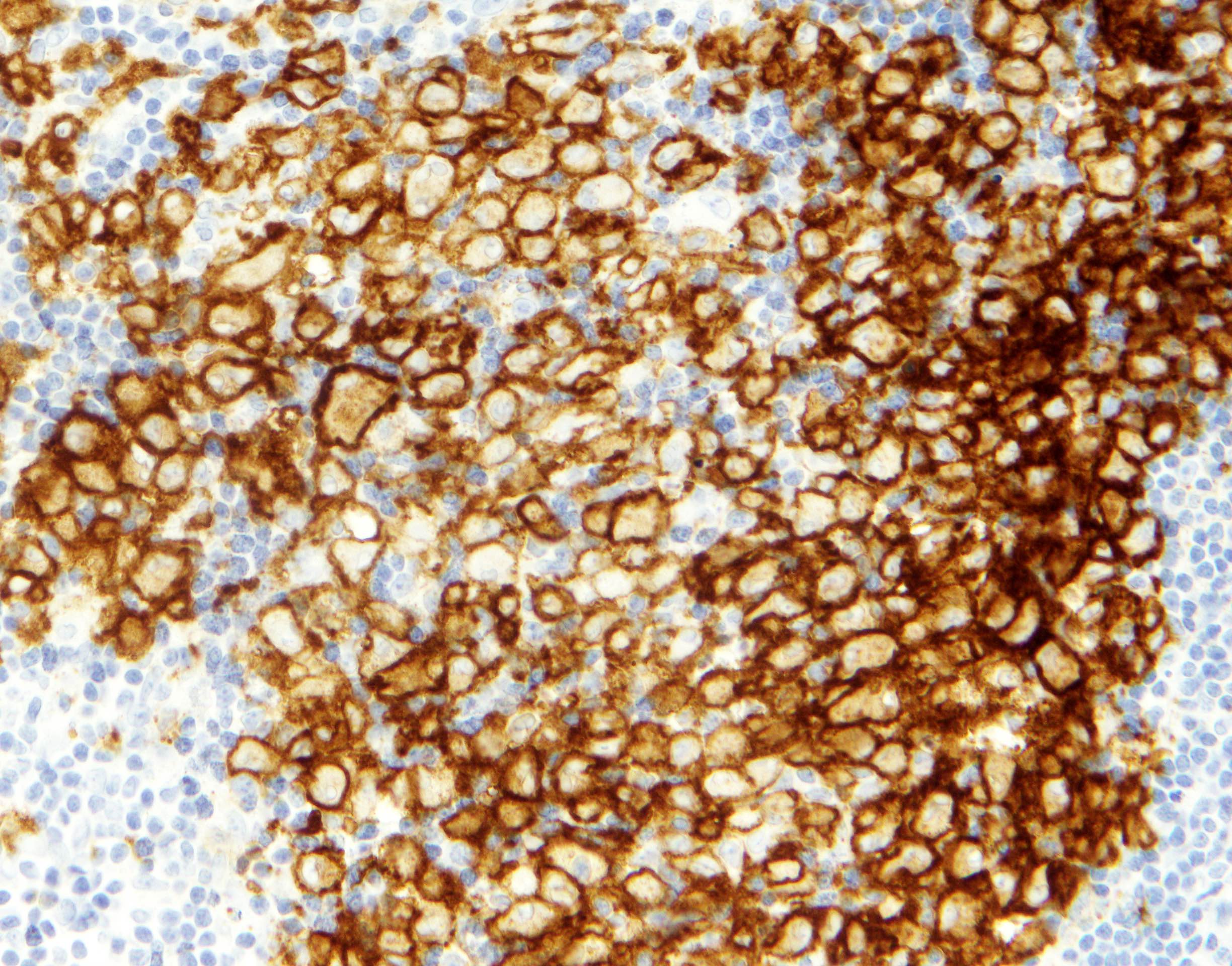
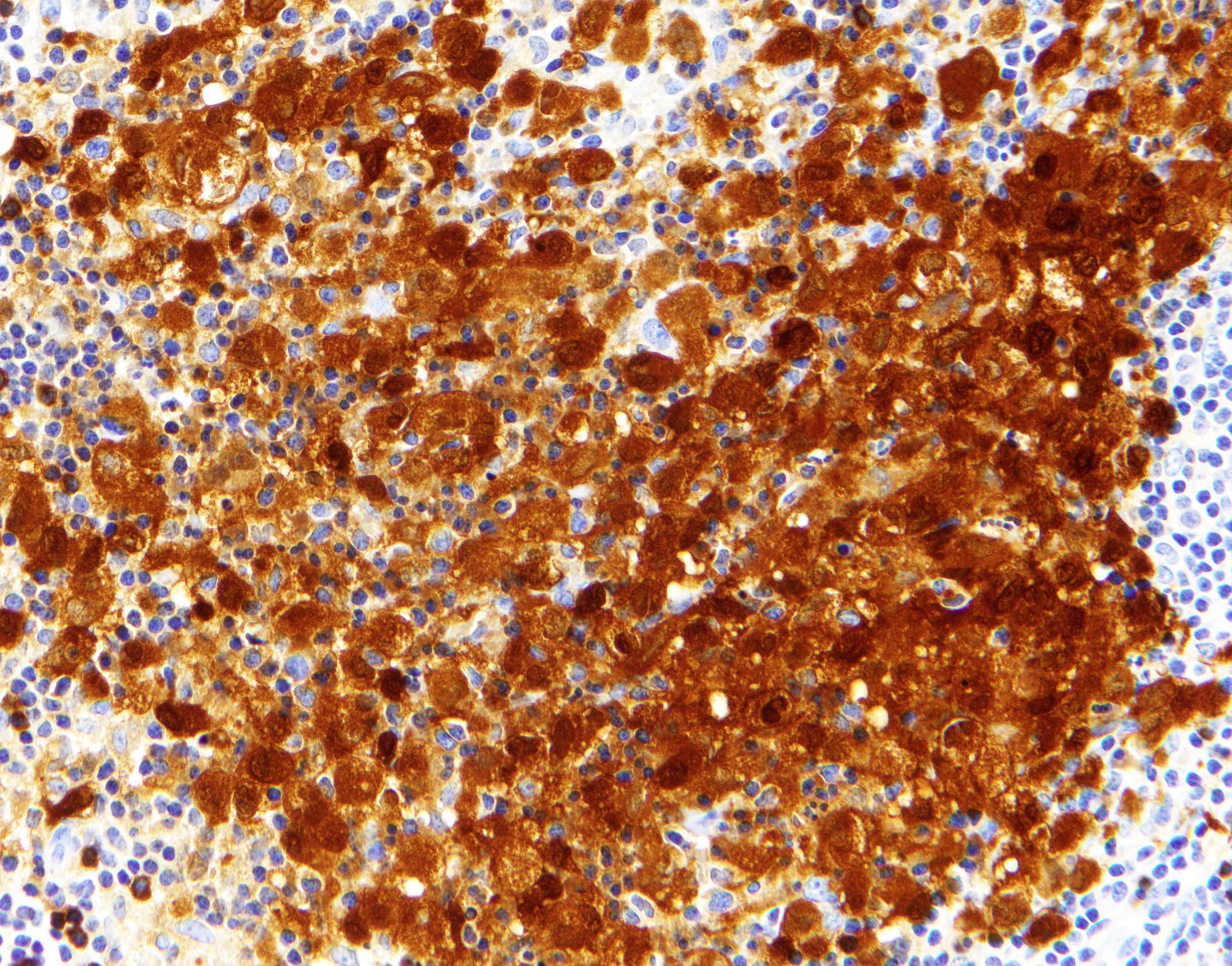
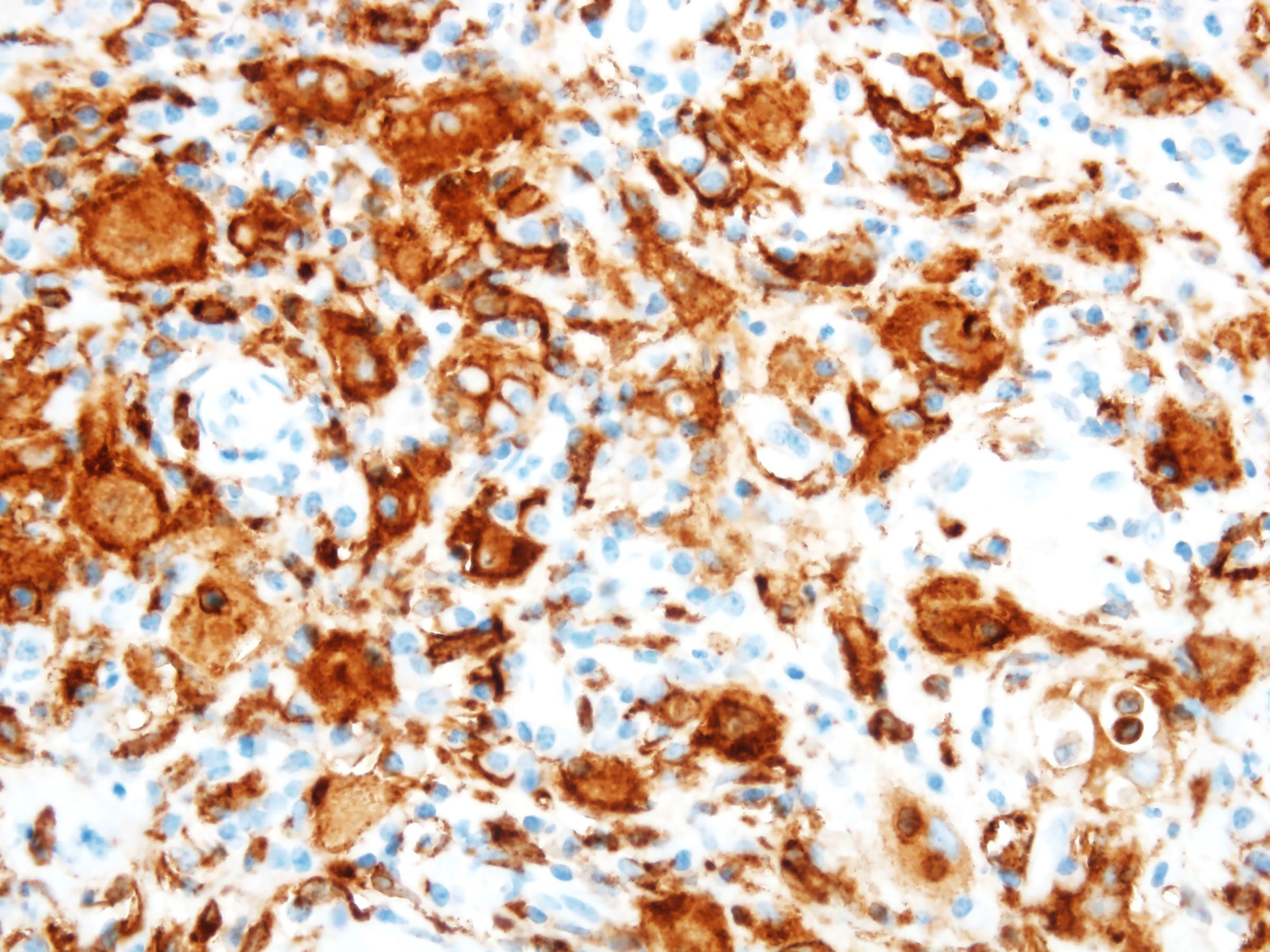
Positive stains
- ALK
- Cytoplasmic staining most common; occasionally membranous or dot-like Golgi pattern
- Lacks nuclear expression
- Can be weak or focal
- CD163, CD68, CD14, CD4, lysozyme
- Factor XIIIa, fascin, cyclin D1 (Am J Clin Pathol 2023;160:1)
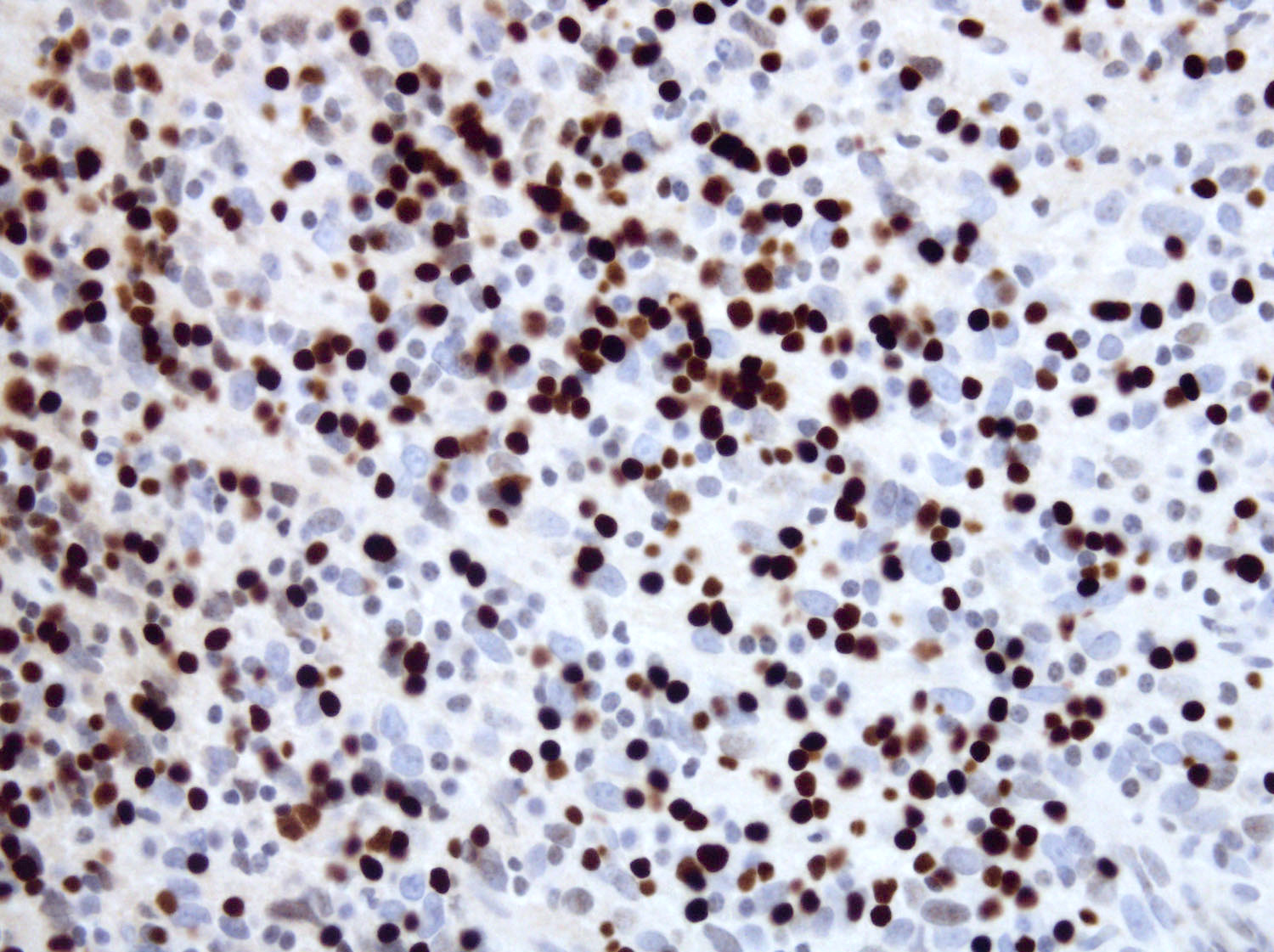
- Some cases show positivity for S100 and OCT2, mimicking Rosai-Dorfman disease (Blood 2008;112:2965, Am J Clin Pathol 2023;160:1)
- pERK expression as a consequence of MAPK pathway activation
Negative stains
Molecular / cytogenetics description
- ALK rearrangements detected by FISH
- ALK fusion partners identified via targeted RNA sequencing (Blood 2022;139:256)
- Most common: KIF5B::ALK fusion (~70 - 75% of cases)
- Other fusion partners reported: CLTC, TPM3, TFG, EML4, DCTN1, TRIM33, COL1A2
Sample pathology report
- Left breast mass, lumpectomy:
- ALK positive histiocytosis, 9 mm in greatest dimension (see comment)
- Comment: Lesional histiocytes are characterized by cytoplasmic expression of ALK with coexpression of histiocytic markers (CD68, CD163) and cyclin D1. Molecular analysis by RNA sequencing was positive for KIF5B::ALK fusion. The overall immunophenotype in conjunction with the molecular analysis supports the above diagnosis.
Differential diagnosis
- Reactive fibrohistiocytic proliferation:
- Rosai-Dorfman disease (RDD):
- Parenchymal effacement of nodal / extranodal tissue by large histiocytes with round / oval nuclei, pale chromatin, distinct nucleoli and abundant pale cytoplasm with frequent engulfment of inflammatory cells (emperipolesis)
- Abundant plasma cells and small lymphocytes; occasionally, neutrophils may also be present
- By immunohistochemistry, positive for S100, OCT2; uniformly negative for ALK
- ~10% of cases are negative for CD163 (Am J Clin Pathol 2023;160:1)
- Lacks ALK gene fusions
- Juvenile xanthogranuloma (JXG):
- Usually affects pediatric population with ~33% of cases presenting within the first year of life
- Predominantly cutaneous involvement presenting as papulonodular lesions and usually asymptomatic / self resolving over months / years
- < 5% may be systemic JXG, including CNS that may present as seizures, hydrocephalus, diabetes insipidus; bone marrow involvement presents with cytopenias
- Nonencapsulated circumscribed lesions composed of large xanthomatous (foamy) histiocytes with bland nuclear features and Touton giant cells; occasionally lesions may be composed of spindled, epithelioid or oncocytic histiocytes
- Typically has an inflammatory background with a mixed population of lymphocytes, eosinophils, plasma cells, neutrophils and mast cells
- IHC negative for ALK
- Lacks ALK gene fusions
- Erdheim-Chester disease:
- Heterogeneous clinical manifestations, most commonly presenting with bone pain, secondary to bilateral symmetric osteosclerosis of metadiaphysis of femur, tibia and fibula
- Bland appearing histiocytes characterized by abundant foamy (xanthomatous) cytoplasm with surrounding fibrosis (Mod Pathol 2018;31:581)
- Negative for OCT2 and ALK (Am J Surg Pathol 2021;45:35, Am J Clin Pathol 2023;160:1)
- Associated with MAPK pathway mutations, with BRAF V600E mutated in ~50 - 60% of cases
- Lacks ALK gene fusions
- Langerhans cell histiocytosis:
- Langerhans cells characterized by irregular, grooved, folded or indented nuclei with fine chromatin, inconspicuous nucleoli and abundant pale eosinophilic cytoplasm
- Positive for S100, CD1a and langerin; negative for factor XIIIa and ALK (Am J Clin Pathol 2023;160:1)
- Lacks ALK gene fusions
- Inflammatory myofibroblastic tumor (especially breast and soft tissue tumors):
- Proliferation of ALK positive spindle cells of myofibroblastic origin with variable prominent lymphoplasmacytic infiltrates
- Lesional cells are positive for vimentin (diffuse, strong), smooth muscle actin, muscle specific actin, calponin and desmin (variable)
- CD68 / CD163 positive histiocytes may be present in the background but are not the lesional cells
Additional references
- WHO Classification of Tumours Editorial Board: Haematolymphoid Tumours, 5th Edition, 2024, BMJ Case Rep 2018;2018:bcr2018224506, Acta Neuropathol 2019;138:335, Neuropathol Appl Neurobiol 2021;47:878, Leuk Lymphoma 2021;62:1234, J Breast Imaging 2022;4:336, J Hematopathol 2021;14:89, Hum Pathol Case Rep 2021;24:200504, Hum Pathol Case Rep 2020;21:200404
Board review style question #1
A 41 year old woman underwent a core needle biopsy for a 3 cm breast mass discovered on her first screening mammogram. The lesional spindle cells show the CD68 immunostaining pattern seen above; they are also positive for ALK and negative for SMA and desmin. Which of the following is the most likely diagnosis?
- ALK positive histiocytosis
- Erdheim-Chester disease
- Extranodal Rosai-Dorfman disease
- Inflammatory myofibroblastic tumor
- Juvenile xanthogranuloma
Board review style answer #1
A. ALK positive histiocytosis (APH) can occur as a localized mass in many anatomic locations or as multisystem disease. Morphologic features vary and may include spindled, epithelioid or foamy histiocytes with or without background lymphoplasmacytic infiltrate. Lesional histiocytes show positive immunostaining for at least 1 histiocytic marker (CD68, CD163, etc.) and for ALK. Answers B, C and E are incorrect because the lesional histiocytes in each of these lesions do not show positive immunostaining for ALK. Answer D is incorrect because the neoplastic spindle cells in inflammatory myofibroblastic tumor (IMT) are myofibroblastic in origin and are therefore positive for SMA and desmin and negative for CD68.
Comment Here
Reference: ALK+ histiocytosis
Comment Here
Reference: ALK+ histiocytosis
Amyloid
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
- Uncommon disease caused by the deposition of abnormal proteins within the soft tissues
Essential features
- Extracellular deposition of amorphous, eosinophilic, hyaline substance
- Amyloid is Congo red+
Terminology
- Amyloid, amyloidosis, amyloid lymphadenopathy
Epidemiology
- Rare
Sites
- Cervical, supraclavicular and mediastinal lymph nodes
- Any lymph node group can be affected
Pathophysiology
- Results from abnormal folding of proteins
- Proteins that form amyloid can be (a) normal proteins that have an inherent tendency to fold improperly, associate and form fibrils and do so when they are produced in increased amounts and (b) mutant proteins that are prone to misfolding and subsequent aggregation
Etiology
- As part of primary systemic amyloidosis
- As part of reactive systemic amyloidosis
- In uremic patients
- In non-Hodgkin lymphoma
- As isolated primary deposits in lymph nodes
Clinical features
- Lymph node enlargement
- In secondary cases, depending on the primary condition
Diagnosis
- Histologic demonstration of amyloid
Laboratory
- If associated with monoclonal proteins of B cell lymphoma, serum and urine protein electrophoresis will demonstrate monoclonal proteins
Radiology description
- None specific
- Enlarged lymph nodes
Prognostic factors
- Generalized amyloidosis tends to have poor prognosis
Case reports
- 46 year old man with localized lymph node light chain amyloidosis (Case Rep Hematol 2015;2015:816565)
- 77 year old woman with primary amyloidosis involving mediastinal lymph nodes diagnosed by EBUS TBNA (Respiratory Medicine CME 2009;2:51)
- 3 patients with AL amyloidosis manifesting as systemic lymphadenopathy (Amyloid 2008;15:117)
- Massive cervical and abdominal lymphadenopathy caused by localized amyloidosis (J Clin Oncol 2007;25:343)
Treatment
- Treatment of the associated condition
- Treatments inhibiting protein misfolding and fibrillogenesis are under study
Gross description
- Enlarged lymph node with firm waxy cut surfaces
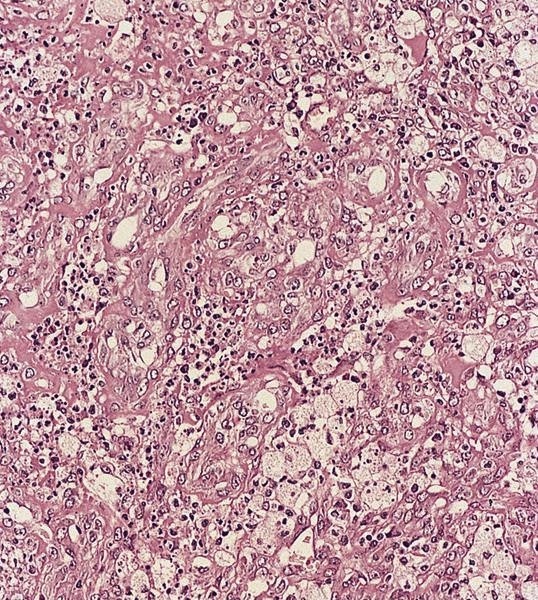
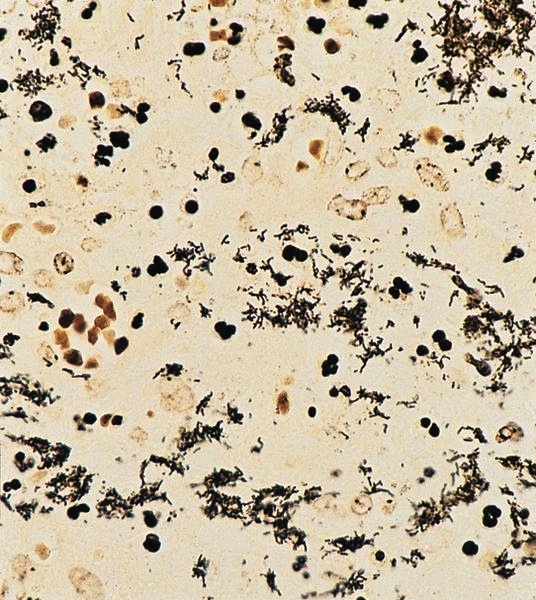
Microscopic (histologic) description
- Hyaline-like amorphous eosinophilic depositions positive for Congo red staining
- Polarizing microscope shows unique yellowish green birefringence
Microscopic (histologic) images
Cytology description
- Hyaline-like amorphous material on cytology smears
- Acellular and associated with connective tissue
- Eosinophilic to blue / green with Pap stain, deep blue with Diff-Quik
Positive stains
Electron microscopy description
- Clusters of round / oval nodules, often enclosed with cytoplasmic processes of macrophages or reticulum cells
- Fibrils are nonbranching, 7.5 nm in diameter, form parallel bundles close to cytoplasmic membranes
Differential diagnosis
Additional references
Amyloidosis
Table of Contents
Definition / general | Case reports | Gross description | Microscopic (histologic) description | Positive stains | Differential diagnosisDefinition / general
- Usually secondary; rarely localized splenic nodules
- Even with diffuse involvement, spleen may still retain "pitting" function that prevents appearance of Howell-Jolly bodies in peripheral blood smears (Arch Pathol Lab Med 1995;119:252)
- Rarely can result in splenic rupture (Am J Hematol 2003;74:131)
Case reports
- 69 year old man with coexisting malignant GIST of stomach (Arch Pathol Lab Med 2003;127:470)
- Amyloid tumor in lymphoma patient (Am J Surg Pathol 1987;11:723)
Gross description
- Firm and waxy consistency
Microscopic (histologic) description
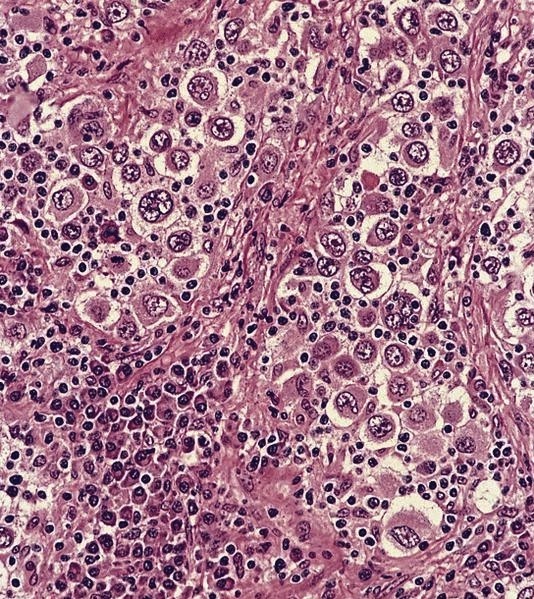
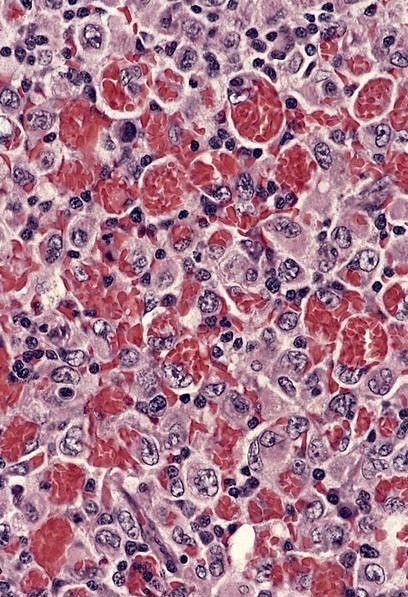
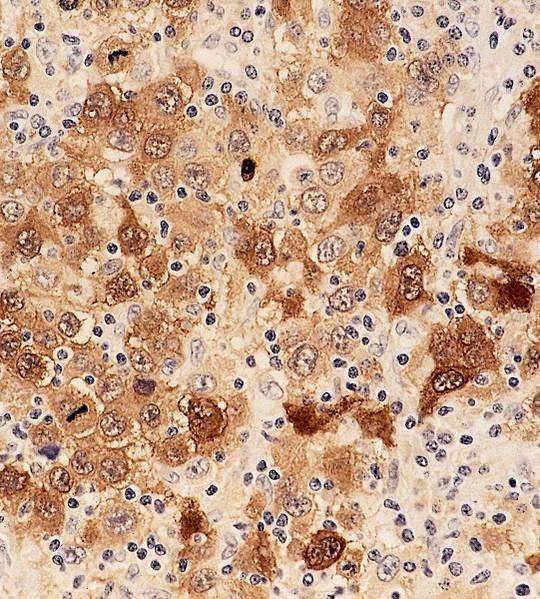
- Deposition of pink amorphous material (amyloid) limited either to germinal follicles (sago spleen) or splenic sinusoids and surrounding connective tissue (lardaceous spleen)
Positive stains
- Congo red with characteristic birefringence (apple green)
Differential diagnosis
- Hyaline adventitial thickening of splenic vessels (normal, AIDS associated, Castleman disease)
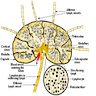
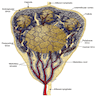
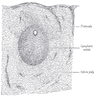
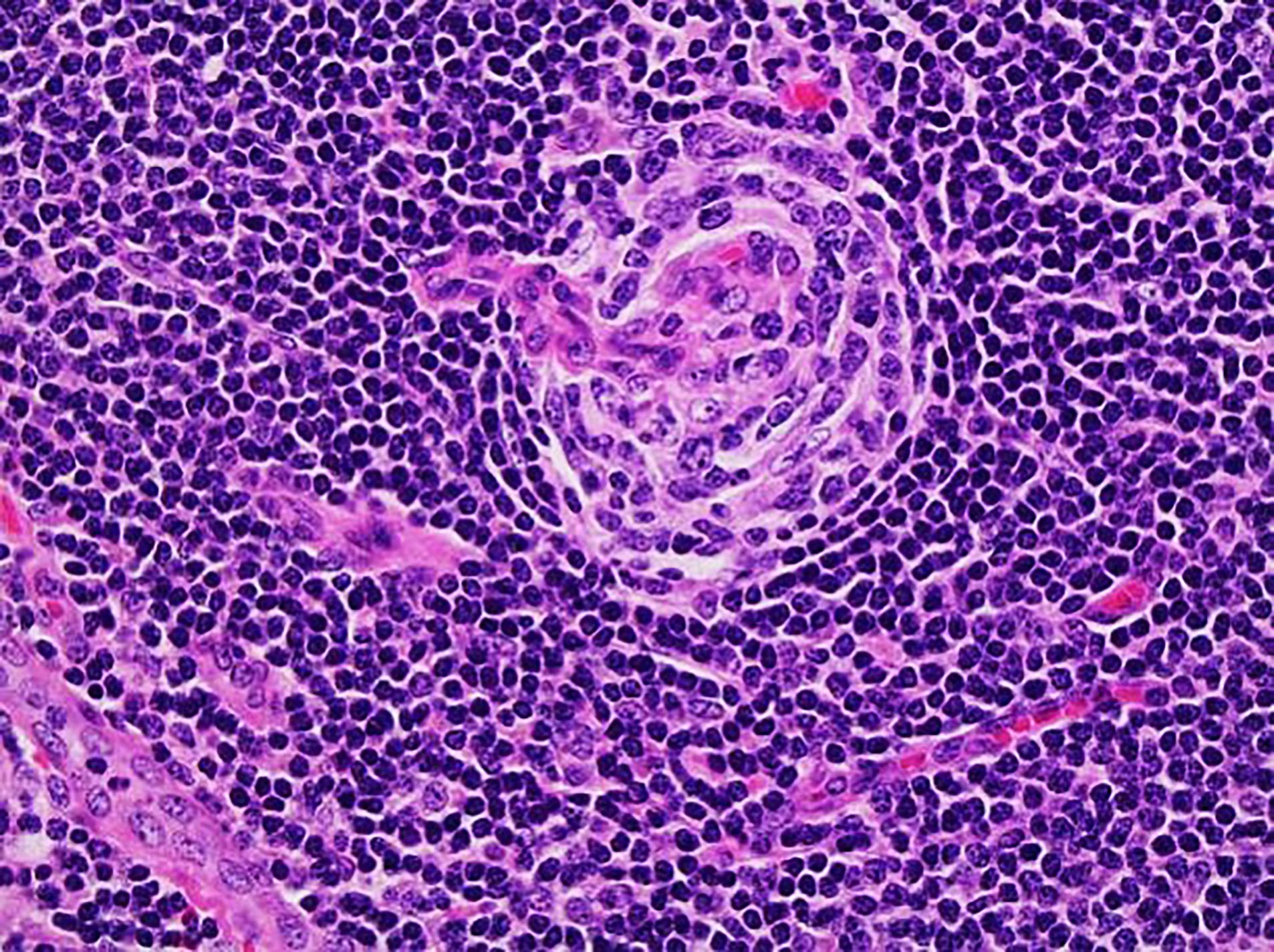
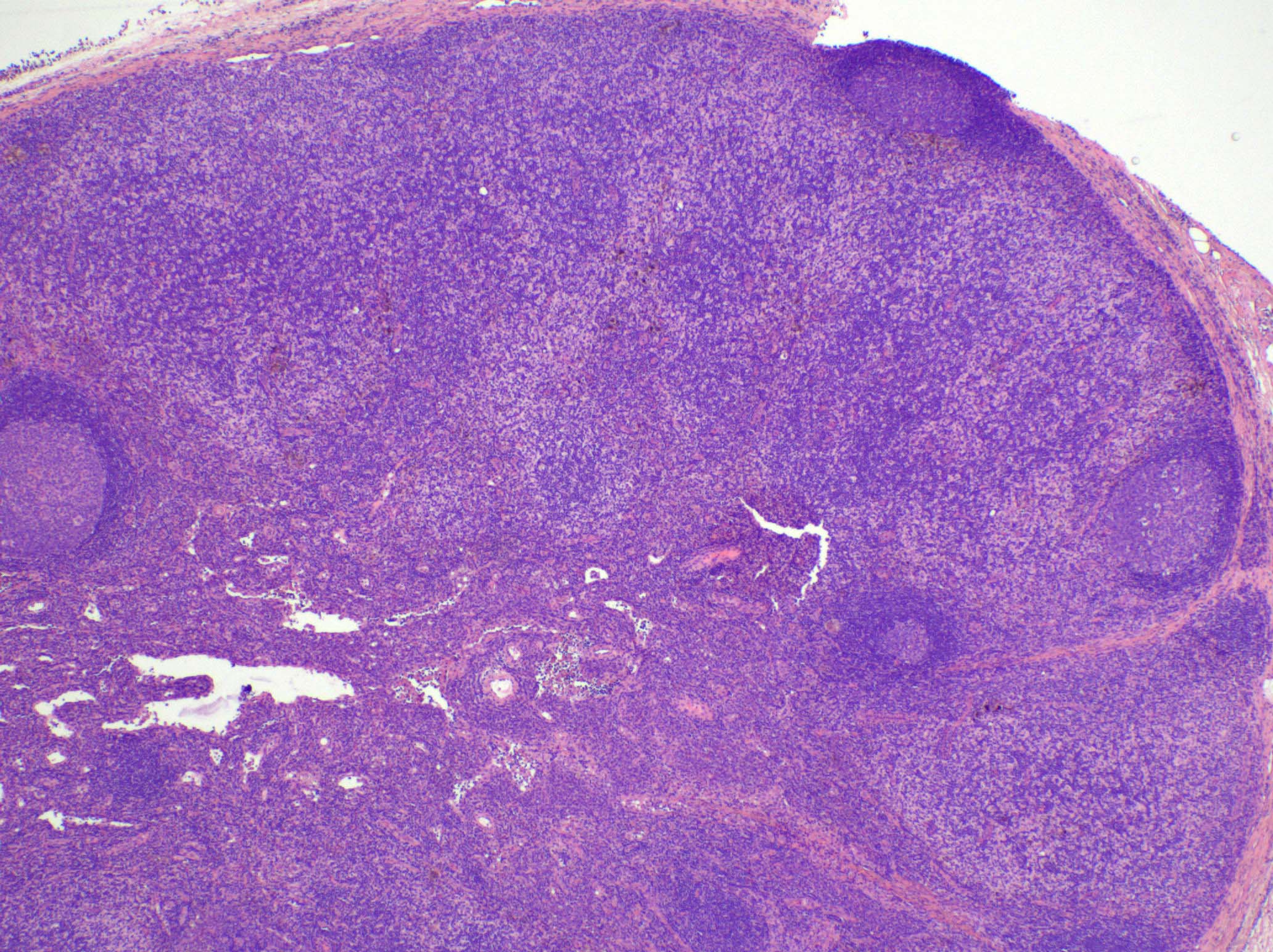
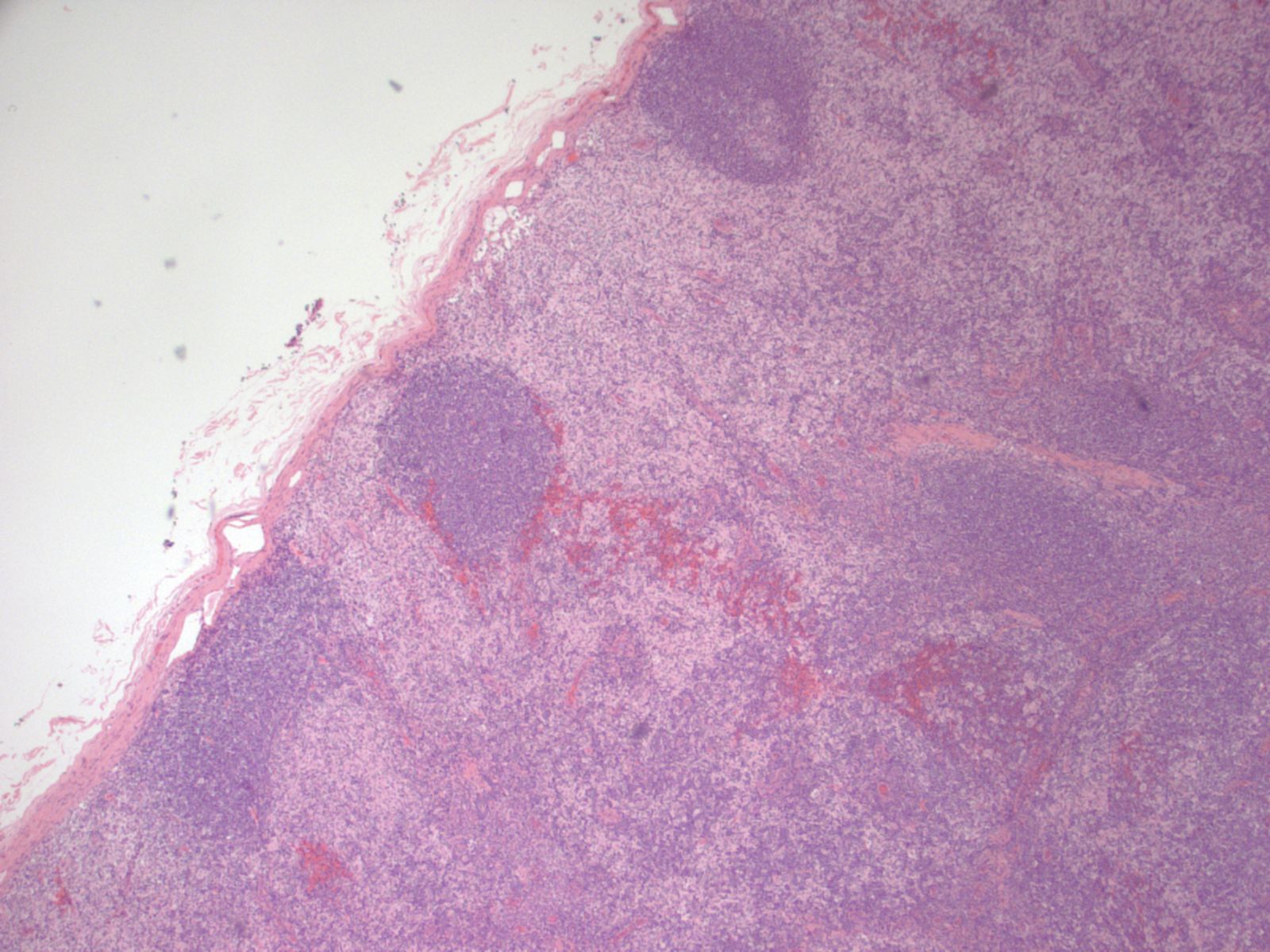
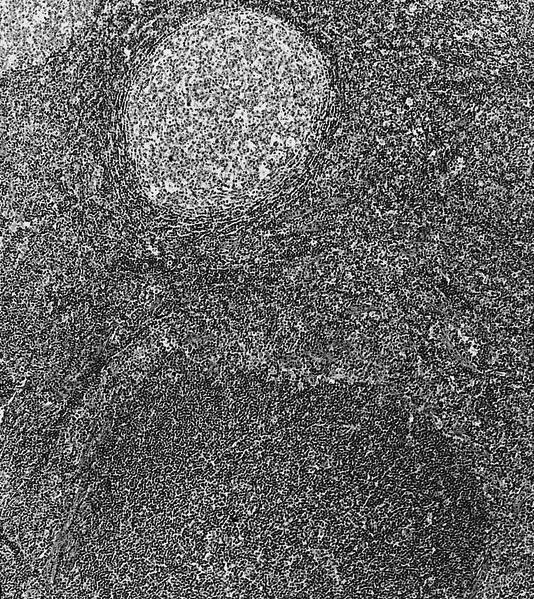
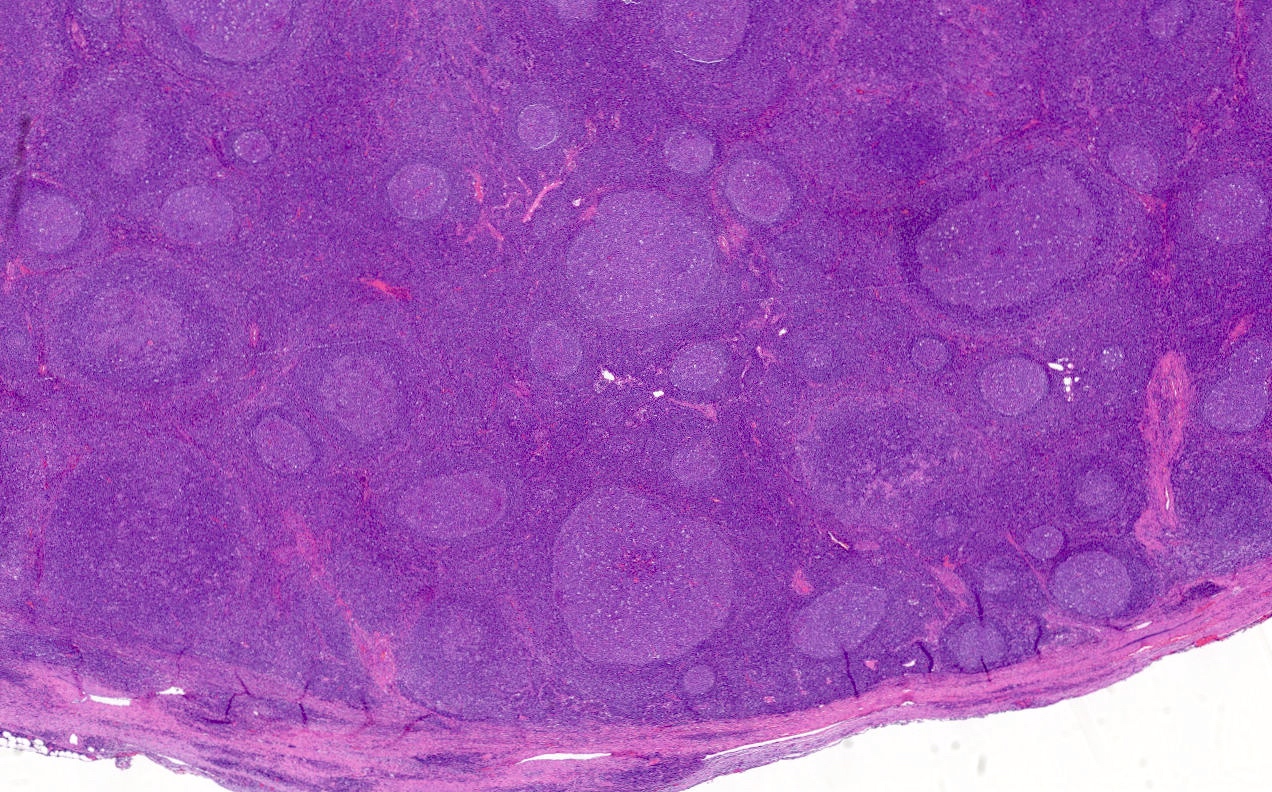
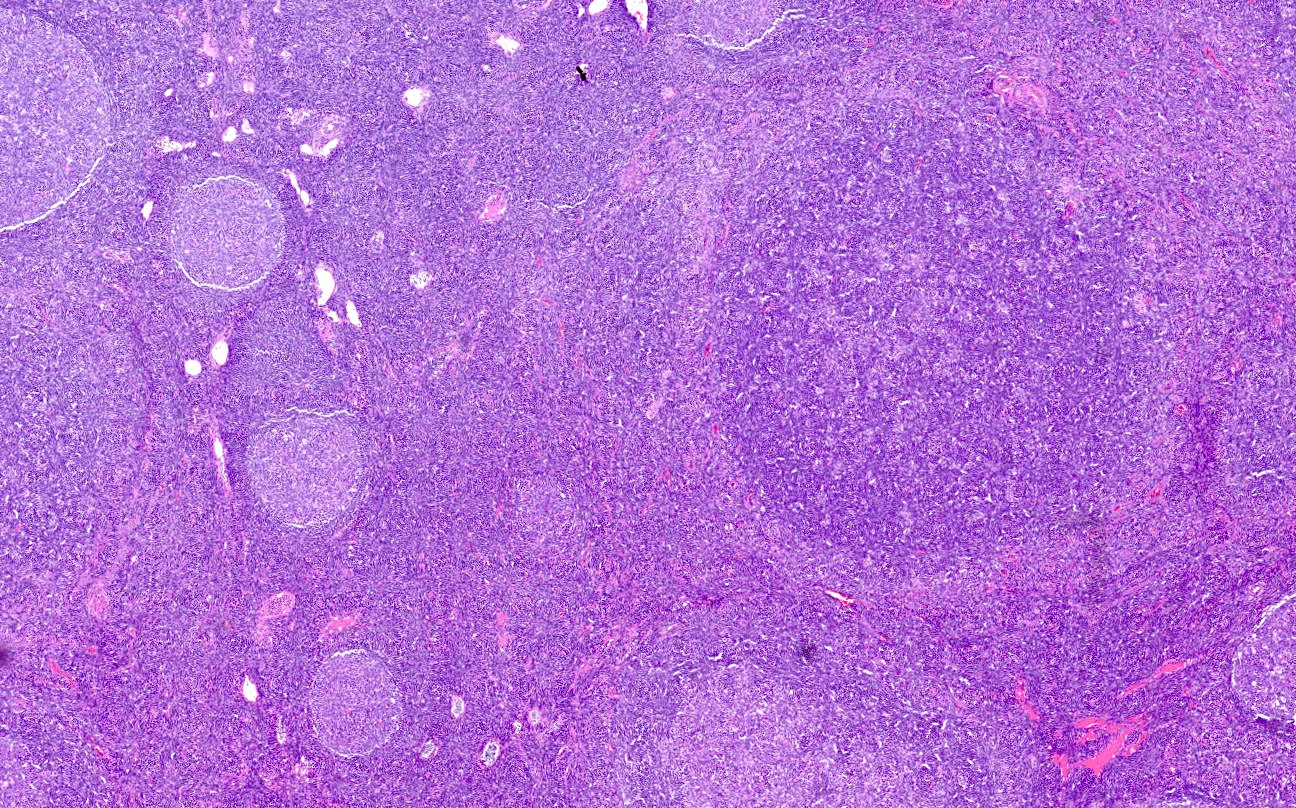
Anatomy & histology-lymph nodes
Table of Contents
Definition / general | Terminology | Embryology | Age related changes | Diagrams / tables | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy imagesDefinition / general
- A secondary lymphoid organ, where B and T cells proliferate in response to exogenous antigen; primary lymphoid organs are bone marrow and thymus
- Other secondary lymphoid organs are spleen and Peyer patches
- Tertiary lymphoid organs are tissues with few lymphocytes that recruit more when inflammation is present
- Lymph nodes are organized to detect and inactivate foreign antigens present in lymph fluid that drains skin, GI tract and respiratory tract, the major organs in contact with the environment
Terminology
- Afferent lymph vessels:
- Penetrate capsule, enter marginal sinus, communicate with intranodal sinuses, then become efferent vessels, which lack an endothelial lining
- Intranodal vessels contain littoral cells or histiocytes with phagocytic properties
- Capsule:
- Thin fibrous connective tissue covering of lymph node
- May be thicker at hilus
- Connected to fibrous trabeculae which penetrate the node
- Capsule may contain smooth muscle cells (Anat Rec 1975;183:517)
- Cortex:
- Subcapsular portion of node with largest number of follicles (primary or secondary)
- Primary follicle:
- Round aggregates of small, dark staining inactive (naïve) B lymphocytes, usually near the capsule, within a network of follicular dendritic cell processes
- No germinal center present
- Secondary follicle:
- Arises from primary follicle that develops germinal centers (see below) due to antigenic stimulation of B cells and production of antibodies
- Contains pale staining germinal center which may be polarized towards site of antigen entry
- Surrounded by mantle zone and marginal zone lymphocytes
- Germinal center:
- Contains predominantly B lymphocytes (including centroblasts and centrocytes) and scattered follicular T helper cells and T regs
- Also tingible body macrophages and follicular dendritic cells
- Mantle zone:
- Tightly packed small B lymphocytes of the primary follicles, pushed aside by the germinal centers
- Marginal zone:
- Less packed small B lymphocytes with more cytoplasm
- Light zone on outer rim of mantle zone
- Contains a mix of post-follicular memory B cells derived after stimulation of recirculating cells from T cell dependent antigen and naïve B cells
- Often not well developed in lymph nodes
- Paracortex:
- Tissue between cortical follicles and medulla (see below)
- Contains predominantly dark staining mature T cells, B immunoblasts, interdigitating dendritic cells, plasmacytoid dendritic cells, histiocytes and high endothelial venules (postcapillary venules lined by plump endothelial cells that express leukocyte adhesion molecules and contain intraluminal lymphocytes)
- Expands during cell mediated immunological reactions
- Has coarse network of reticulin fibers
- Medulla:
- Portion of node closest to hilum
- Contains the medullary cords, sinuses and vessels but minimal number of follicles
- Medullary cords:
- Found in hilar region between the sinuses, composed mostly of small B and T lymphocytes, plasmacytoid lymphocytes, plasmablasts and plasma cells
- Sinuses:
- Carry lymph from afferent to efferent lymphatics
- Subcapsular sinus is below capsule and partially lined by endothelium
- Becomes "medullary" as it approaches the hilum and is lined by macrophages
- Also contains mast cells and plasma cells
- Vessels:
- Blood enters and leaves lymph node at hilus
Embryology
- Develop from lateral plate mesoderm (on either side of intermediate mesoderm)
- First, lymphatic sacs arise from endothelial outgrowths of large central veins at week 5
- Second, lymphatic plexus develops from lymphatic sacs
- Third, plexuses are invaded by mesenchymal cells that proliferate and aggregate to form lymph nodes
- Small collections of lymphoblasts are present by first trimester
- By second trimester, cortex is distinguishable from medulla and primary follicles are present
- References: Martini: Human Anatomy, 9th Edition, 2017
Gross description
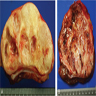
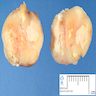
- Ovoid with gray-tan cut surface
Microscopic (histologic) description
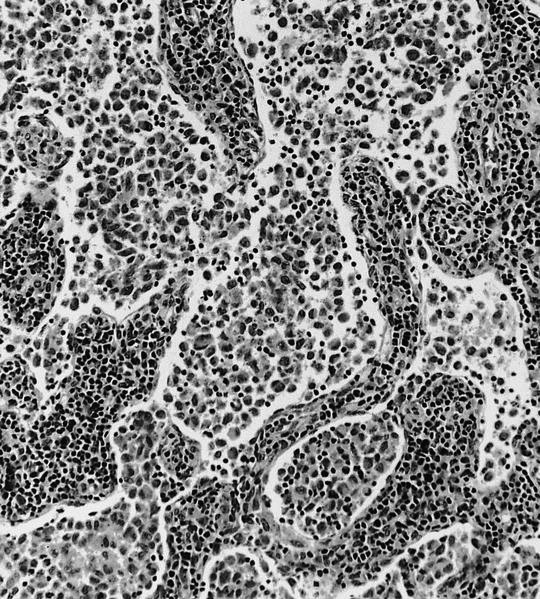
- At low power, lymph node structures are capsule, cortex and medulla, follicles, paracortex, sinuses
- Germinal center: round / oval zone containing pale staining cells, surrounded by darker cells
- Mantle zone: small unchallenged B cells surrounding pale staining germinal centers
- Marginal zone: light zone surrounding follicles; contains postfollicular memory B cells derived after stimulation of recirculating cells from T cell dependent antigen; named "marginal cells" due to location at interface of lymphoid white pulp and nonlymphoid red pulp in the spleen; however, marginal zone is rarely seen except in mesenteric nodes (APMIS 2002;110:325)
- Sinuses: direct the flow of lymph from the afferent lymphatics, to the subcapsular sinus, to the trabecular sinus, to the medullary sinus, to the efferent lymphatics (see diagrams) (Toxicol Pathol 2006;34:409)
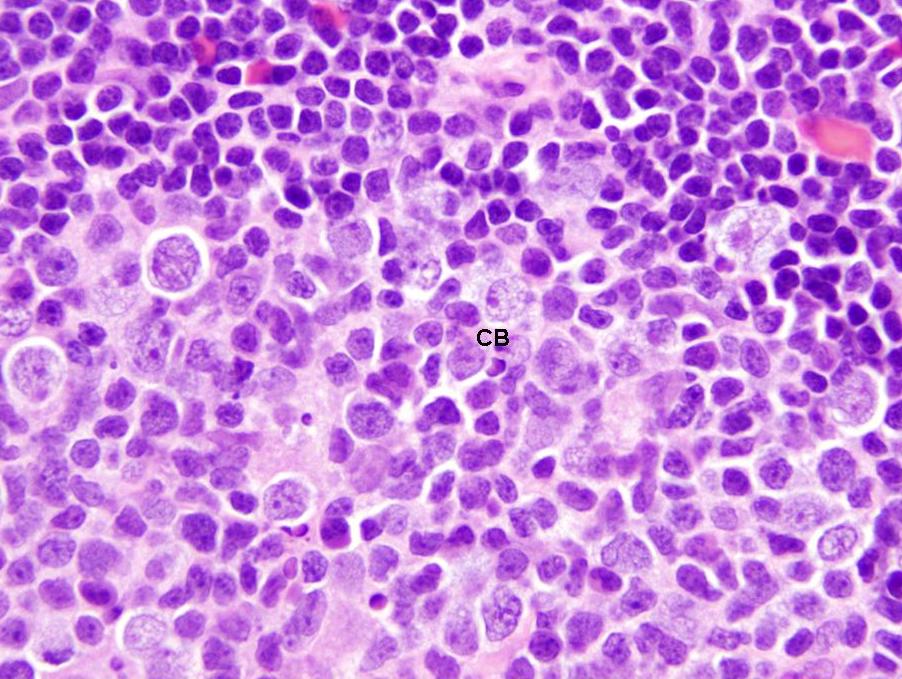
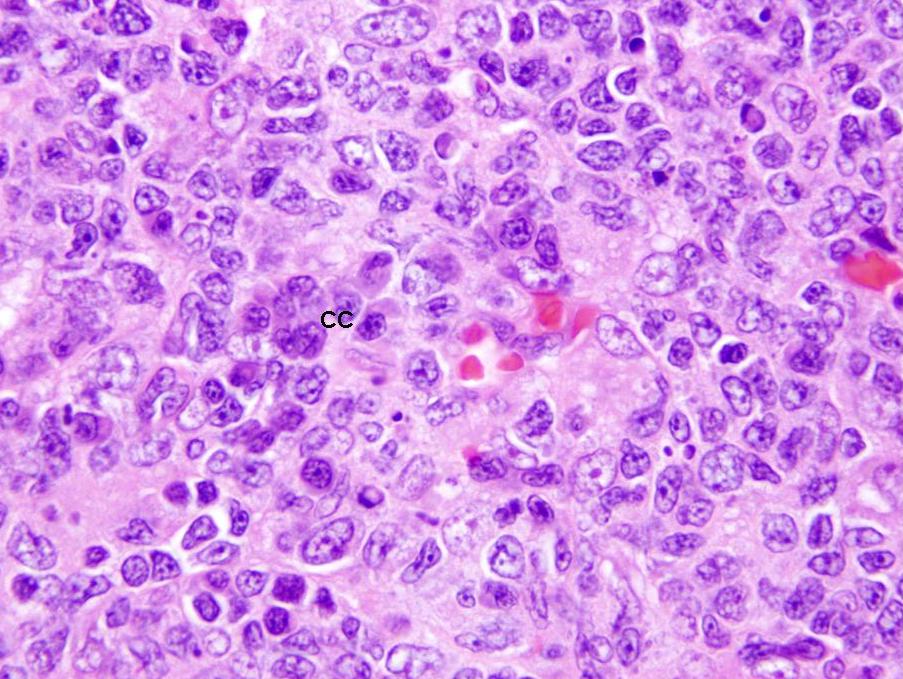
- Centroblasts:
- Large noncleaved follicular center cells (B cells) with moderate amounts of basophilic cytoplasm, large round nuclei, open chromatin, multiple peripheral nucleoli
- Frequent mitotic figures
- Centrocytes:
- Large and small cleaved follicular center cells (B cells) with scant cytoplasm and inconspicuous nucleoli
- Immunoblasts:
- Large B cells scattered throughout the paracortex
- Intermediate between small B cell and a plasma cell
- Prominent single nucleoli
- Express B cell markers (CD20, CD79a, PAX5) and CD30
- Macrophages:
- Process antigens via phagocytosis
- Related to circulatory monocytes
- Are present throughout the lymph node
- May contain thyroglobulin in lymph nodes draining thyroid tumors (J Clin Pathol 2001;54:314)
- Abundant cytoplasm with medium to large nuclei with vesicular chromatin
- Tingible body macrophages have clear cytoplasm and contain apoptotic bodies, which gives node a starry sky pattern
- Mast cells:
- Present in T cell areas (World J Surg Oncol 2003;1:25)
- Difficult to detect
- Distinct cytoplasmic boundaries, faintly granular cytoplasm, large pale nuclei
- Some cells are elongated and resemble fibroblasts
- NK cells:
- Distinct group of non T, non B lymphocytes (5 - 10% of peripheral blood lymphocytes) with large granular lymphocyte morphology on Wright-Giemsa stains
- NK cells derive from a common lymphoid progenitor with T cells
- First line of defense against various infections, by recognizing and killing target cells and producing cytokines, particularly interferon-gamma, which enhance the innate immune response
- Capable of lysing certain target cells (virally infected and tumor cells) without prior activation or major histocompatibility complex restriction (hence named "natural killers" that are part of "innate" immune system) (Wikipedia: Natural killer cell [Accessed 26 March 2021])
- Do not rearrange their receptor genes, as B / T cells do but rely on a fixed number of NK cell receptors (inhibitory and activating) that recognize MHC class I and class I-like molecules and other ligands
- Appear to have capability for memory-like responses (EMBO Rep 2009;10:1103)
- Important for immunomodulation and regulation of hematopoiesis
- Plasma cells:
- Abundant basophilic cytoplasm (due to high content of rough endoplasmic reticulum) with paranuclear hof (highlighted by Giemsa stain, due to Golgi apparatus)
- Have eccentrically placed nucleus with spoke wheel (clock face) chromatin due to small clumps of chromatin on nuclear membrane in an otherwise round and clear nucleus
- May have Russell bodies (intracytoplasmic PAS+ globules)
Microscopic (histologic) images
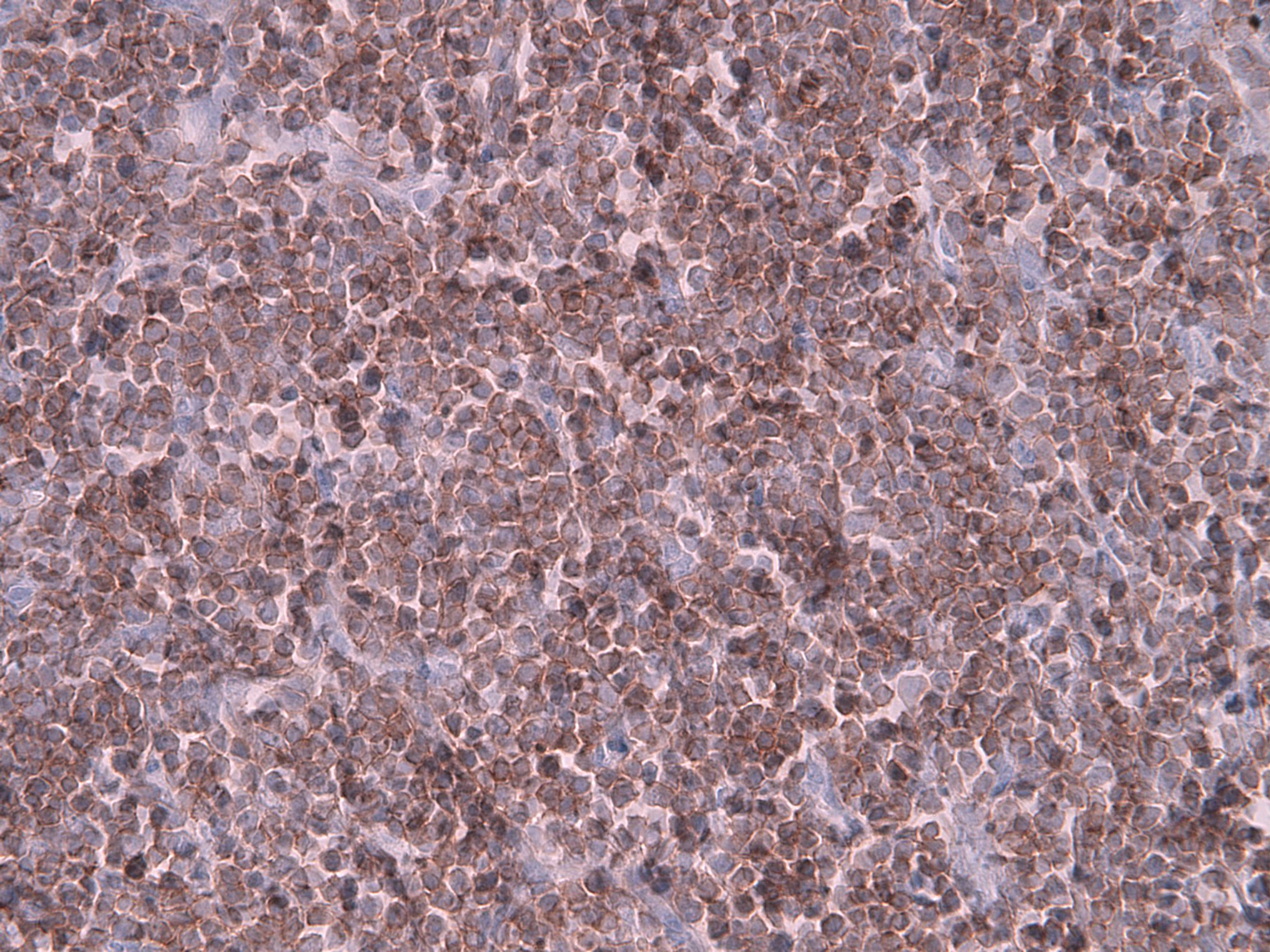
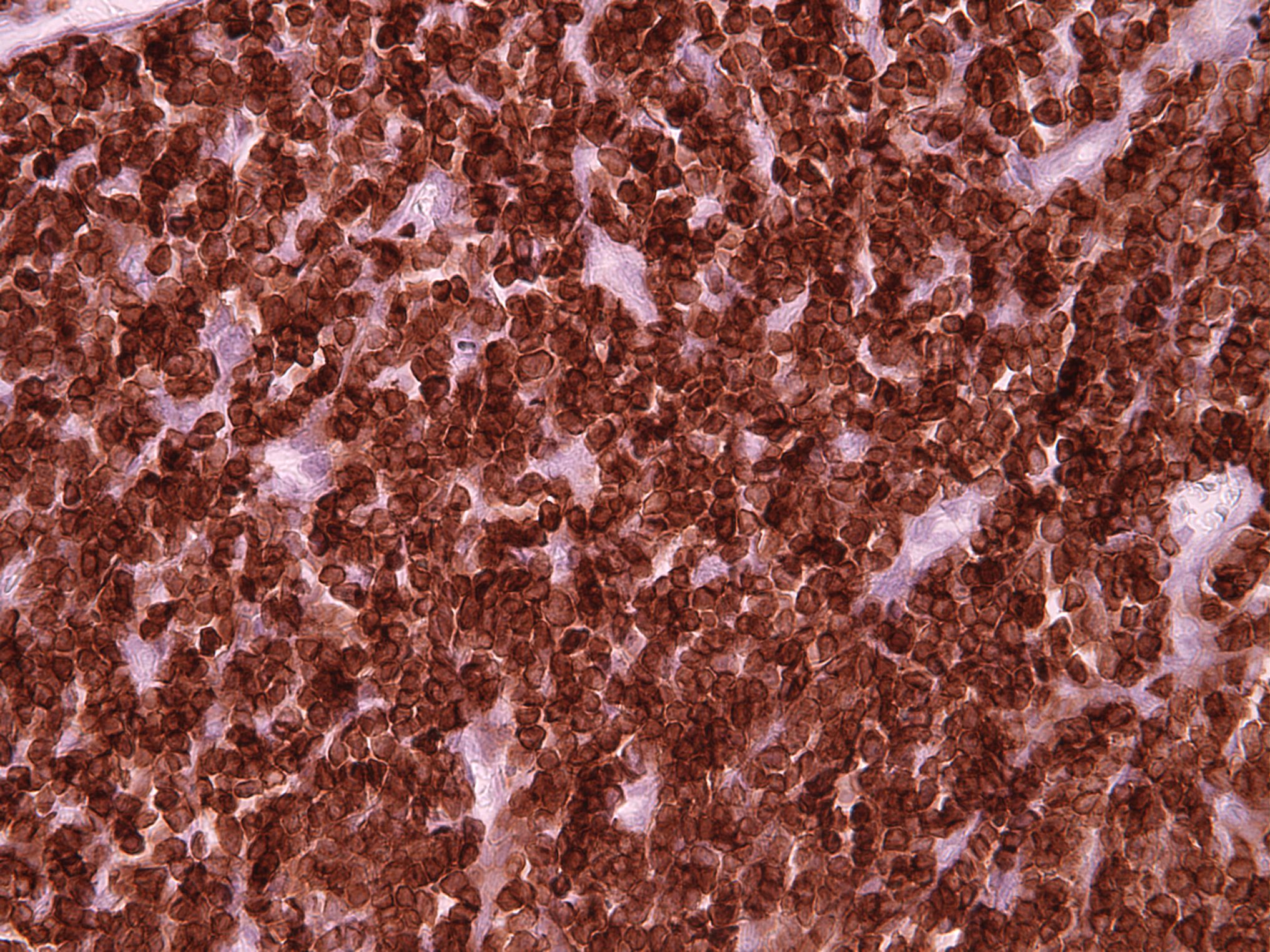
Positive stains
- B cells: CD19, CD20, CD22, CD79
- T cells: CD2, CD3, variable CD4 and CD8
- Follicular helper T cells: C3, CD4, CD57, PD1 or CD279
- T regs: CD4, CD25 and FOXP3
- Premature B and T cells: TdT (terminal deoxynucleotidyl transferase)
- Follicular dendritic cells: CD21, CD23, CD35
- Macrophages: CD68, lysozyme
- NK cells:
- Germinal centers have strong dense BCL6 and CD10 expression
Negative stains
- bcl2 (not expressed in germinal center B lymphocytes)
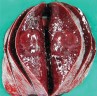
Anatomy, histology & grossing-spleen
Anatomy
- Largest lymphoid tissue of human body, accounting for 25% of total lymphocytes
- Lies between fundus of stomach and diaphragm
- Normally 150 g with thin capsule
- Pathophysiology:
- Filters foreign matter including old / damaged blood cells
- Participates in immune response to blood borne antigens
- Major repository of mononuclear phagocytic cells in red pulp, lymphoid cells in white pulp and platelets
- Produces new blood cells in infants / children or adults with severe anemia
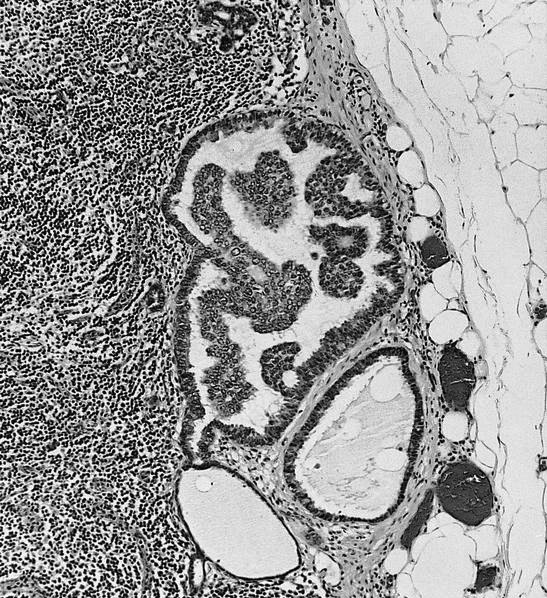
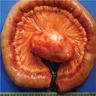
- Gross description: malpighian (splenic) follicles of white pulp are identifiable
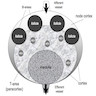
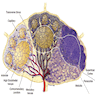
Histology
- Composed of red pulp (occupies 75% of splenic volume) and white pulp separated by marginal zone
- Red pulp:
- Filters old / damaged red blood cells
- Traversed by thin walled venous sinusoids lined by littoral cells, a type of endothelial cell which also stains with histiocytic markers and has a discontinuous wall, allowing passing of red blood cells between sinus and cords
- Sinuses are separated by splenic cords (cords of Billroth) containing a labyrinth of splenic macrophages, which filter red blood cells and ingest old (normal lifespan is 120 days), damaged (seen in hereditary spherocytosis, sickle cell anemia) or antibody coated red blood cells
- Also remove Heinz bodies or other red blood cell inclusions (peripheral blood has Howell-Jolly bodies if no functional spleen is present)
- White pulp:
- Forms sheaths of lymphoid cells around arteries (periarteriolar lymphatic sheath), composed of T cells and lymphoid follicles (B cells) with surrounding mantle zone (proliferating B cells) and outer marginal zone (memory B cells)
- Traps antigens for processing
- In young infants, immature marginal zone may contribute to increased susceptibility to bacterial infections or sudden infant death syndrome (Hum Pathol 2004;35:113)
- Blood flow:
- Arteries terminate in fine penicilliary arterioles surrounded by lymphocytes, then enter red pulp sinusoids, then to splenic veins
Grossing
- Fresh tissue preferable for special studies and flow cytometry
- Section specimen every 3 - 5 mm
- Obtain imprints after blotting with a towel to remove excess blood
- Blocks should be thin for adequate fixation, since fixative penetrates spleen slowly
- Describe apparent white pulp disorders (nodules), red pulp disorders (diffusely enlarged spleen without follicles or nodules) or other
Drawings
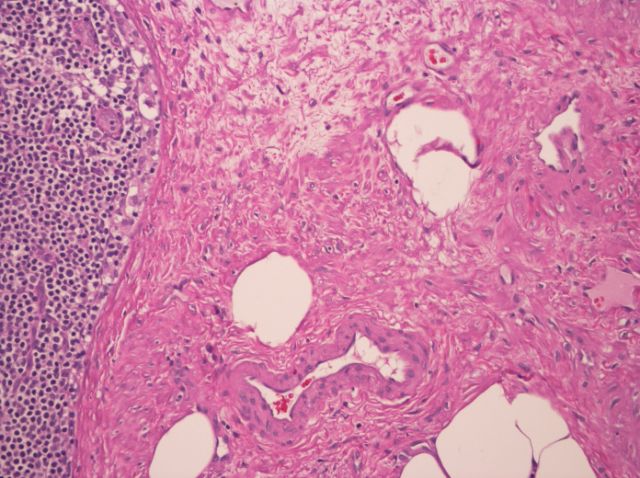
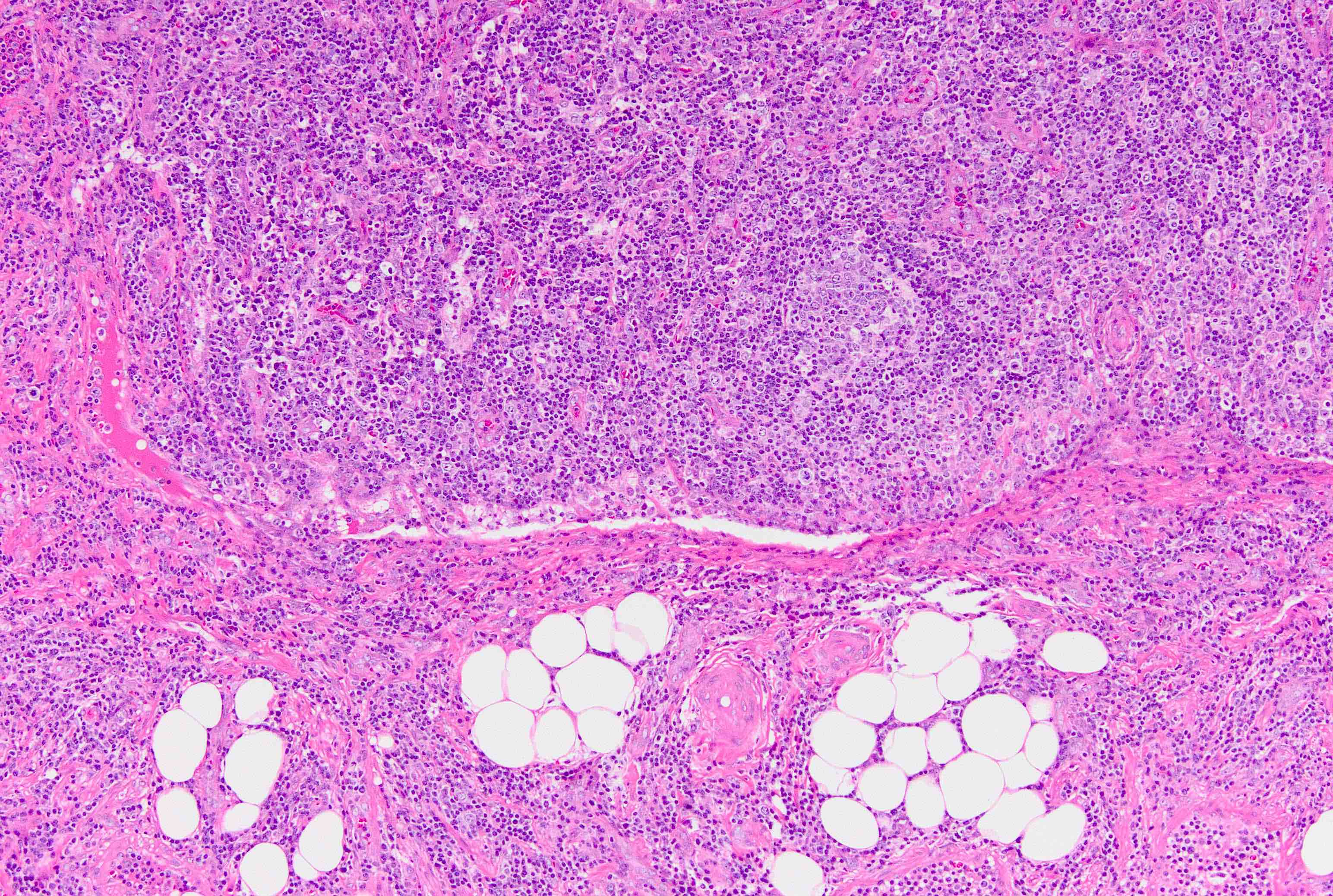
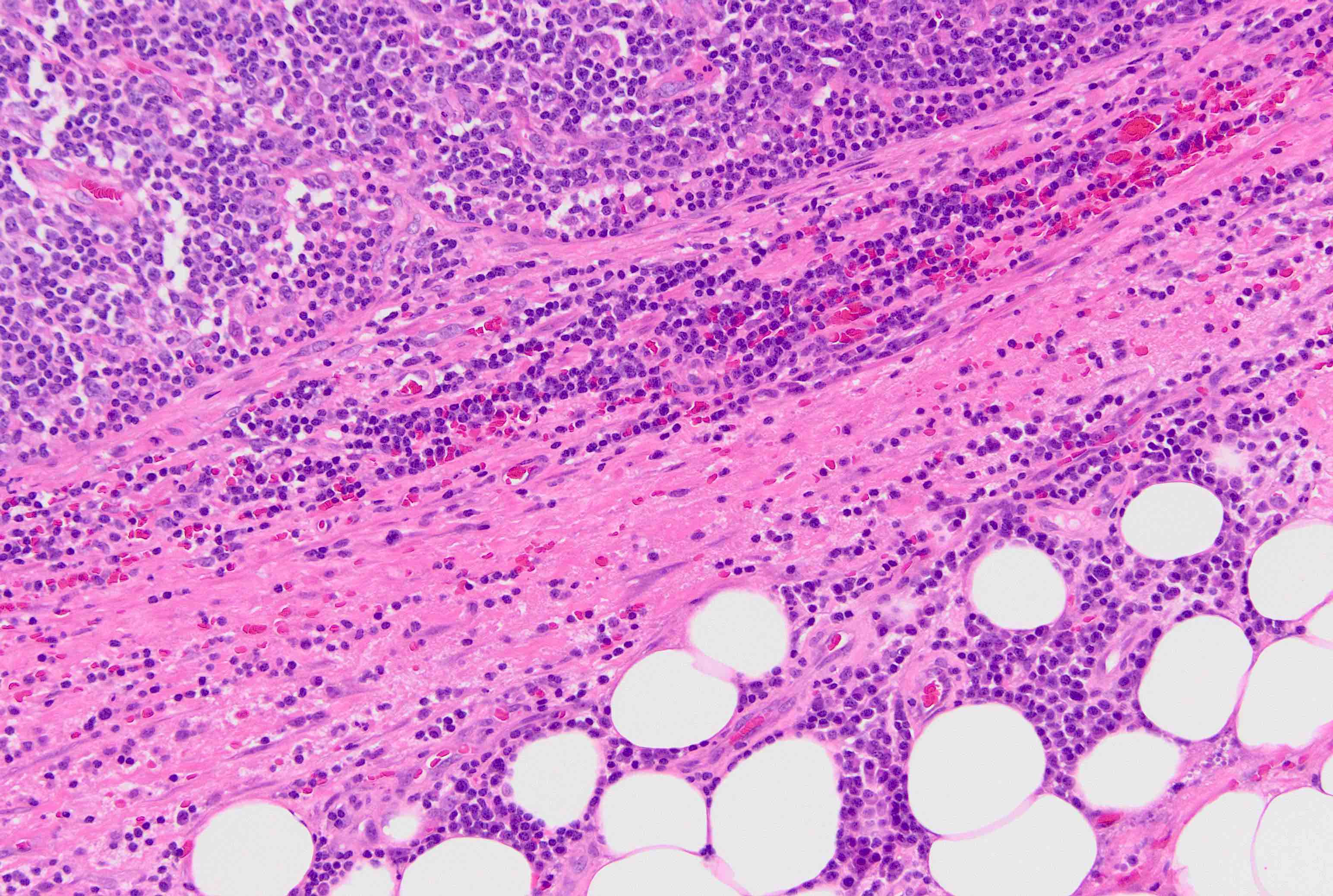
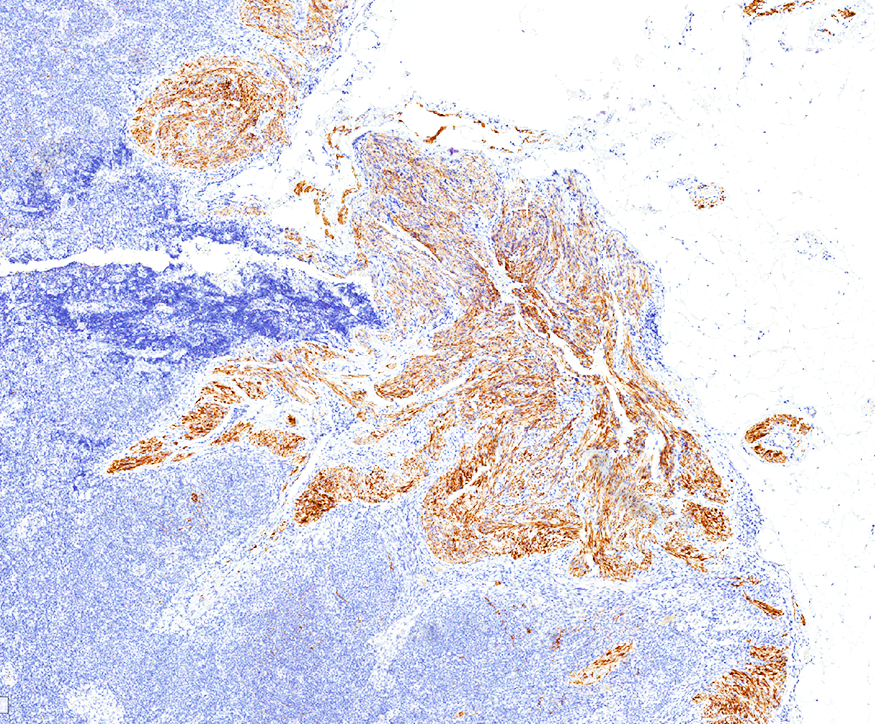
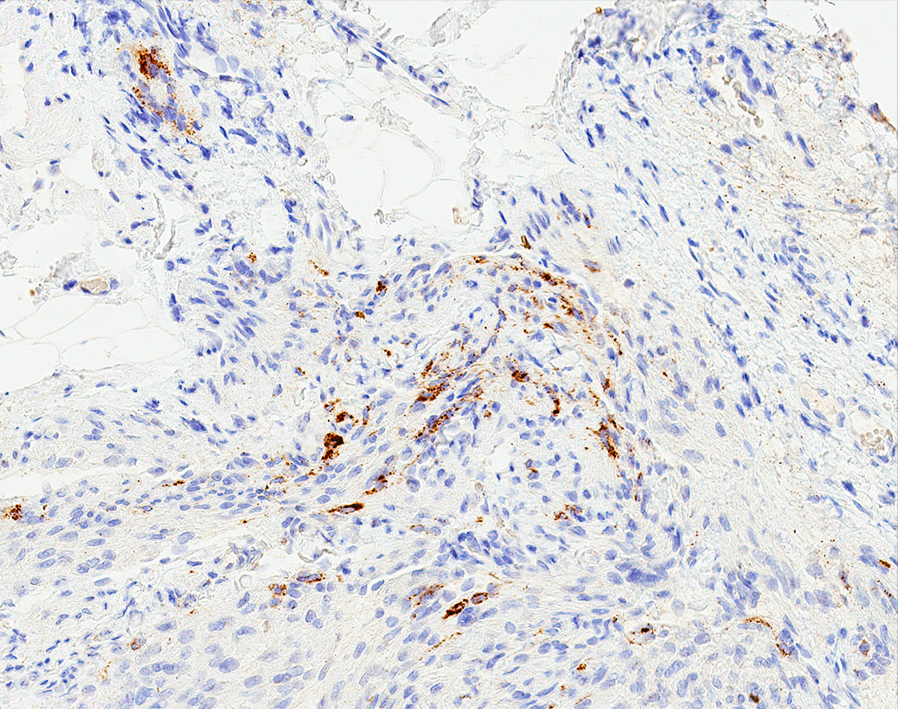
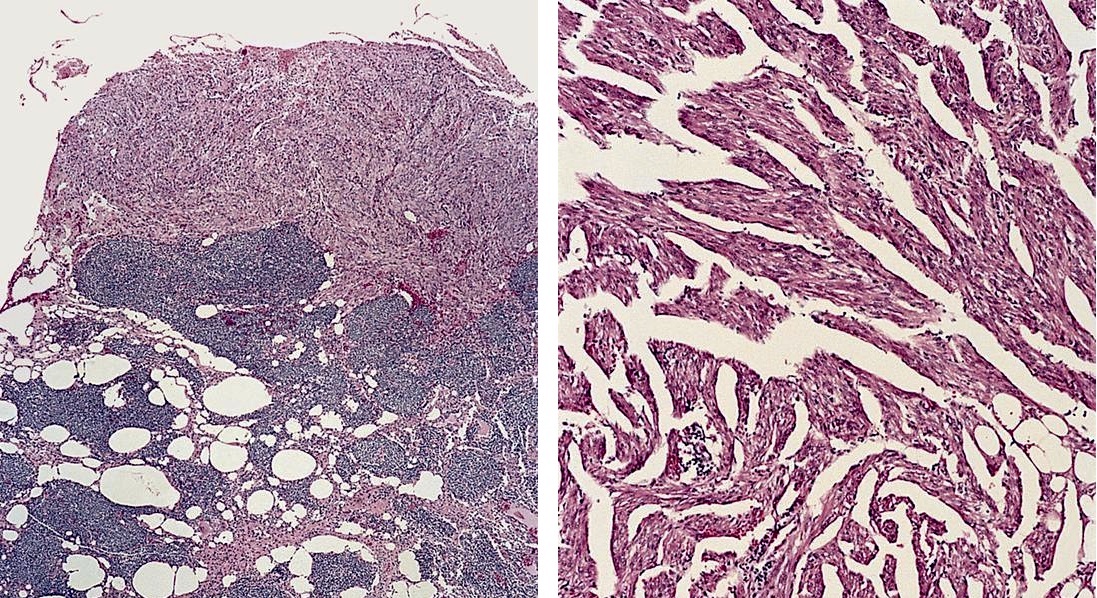
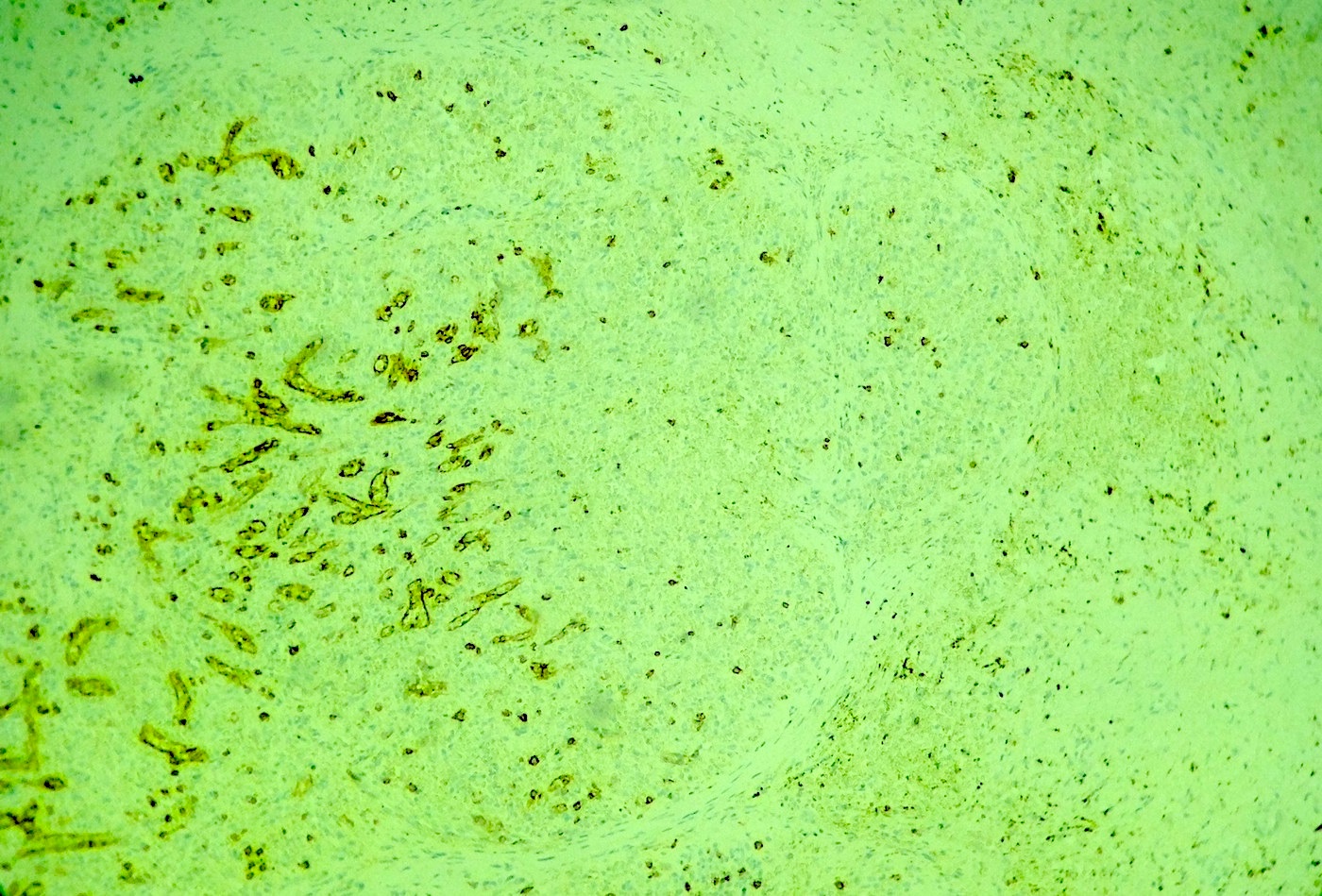
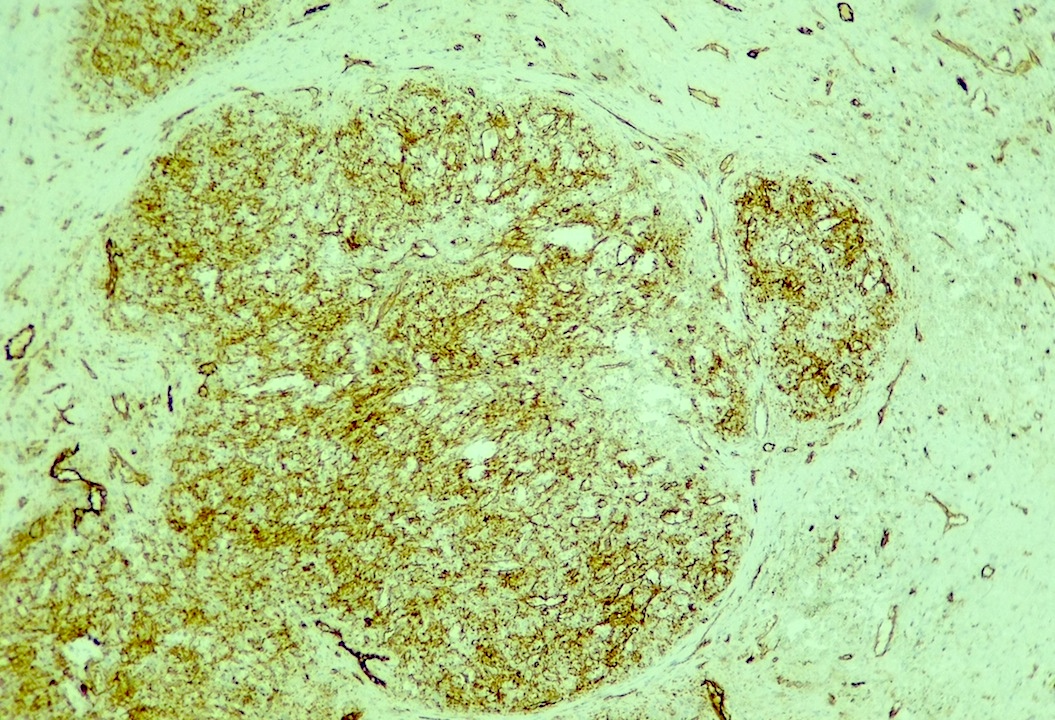
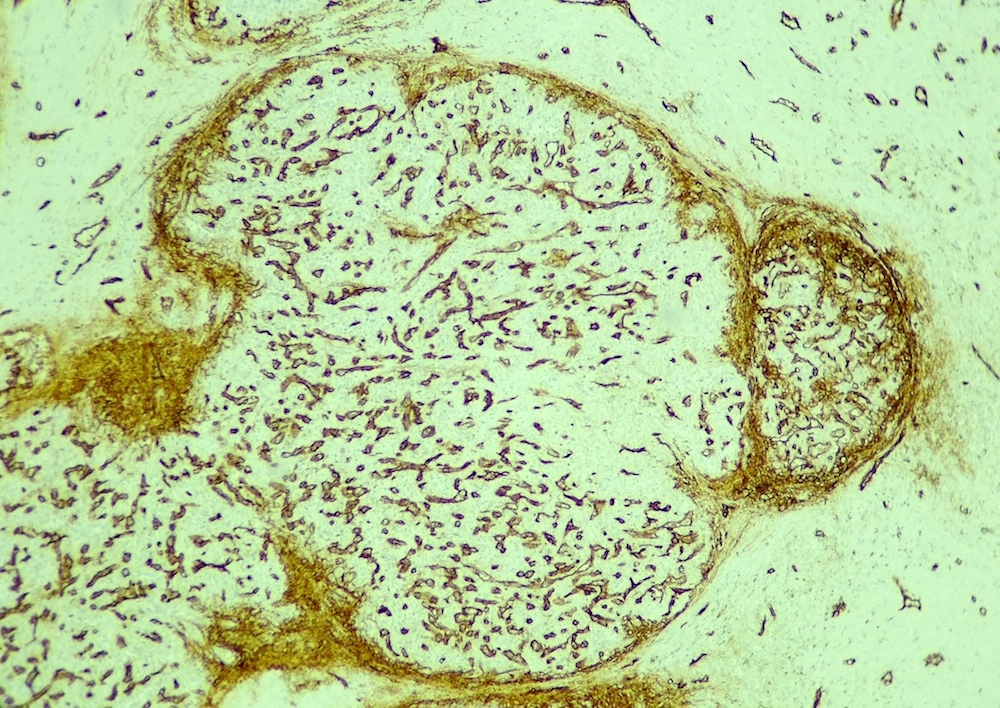
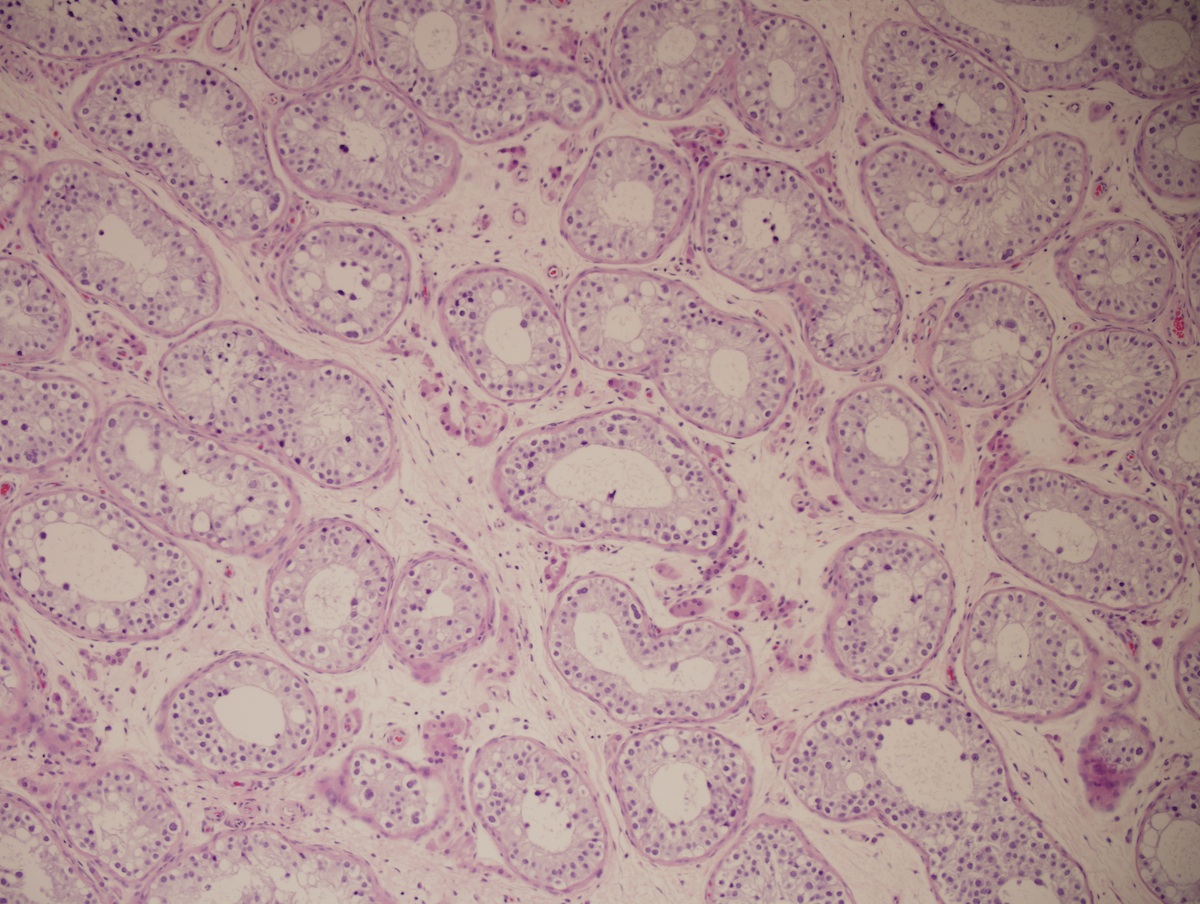
Angiolipomatous hamartoma
Table of Contents
Definition / general | Sites | Pathophysiology | Clinical features | Radiology images | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Immunohistochemistry & special stains | Differential diagnosisDefinition / general
- Benign mesenchymal proliferation with vascular and fatty components (Pathologica 2006;98:239)
- Associated with Castleman lymphadenopathy, hyaline vascular variant (Arch Pathol Lab Med 1986;110:853)
Sites
- Various nodal sites, including cervical, mediastinal and retroperitoneal lymph nodes
- Occurs in nodal parenchyma or extracapsular soft tissue
- Miscellaneous extranodal sites can be involved
Pathophysiology
- Rare disorder of unknown etiology
- Pseudotumor; not a true neoplasm
Clinical features
- Innocuous and slow growing
Case reports
- 16 year old boy with paraparesis caused by an angiolipomatous hamartoma with Proteus syndrome and scoliosis (J Neurosurg 2005;103:282)
- 60 year old man with a spindle cell sarcoma containing a focal angiolipomatous hamartoma component (J Pathol 2002;197:264)
- 70 year old man with angiolipomatous mesenchymal hamartoma (angiolipomatosis) of sigmoid mesocolon (Int J Clin Exp Pathol 2011;4:210)
- Giant lymph node hyperplasia in an angiolipomatous mediastinal mass (Arch Pathol Lab Med 1986;110:853)
- Vascular transformation of sinuses in bilateral cervical lymph nodes (Head Neck 1999;21:366)
Treatment
- Surgical excision
Gross description
- Unencapsulated single or multiple yellow and fatty nodules up to 15 cm
- Tan nodules may represent accompanying hyaline vascular Castleman lymphadenopathy
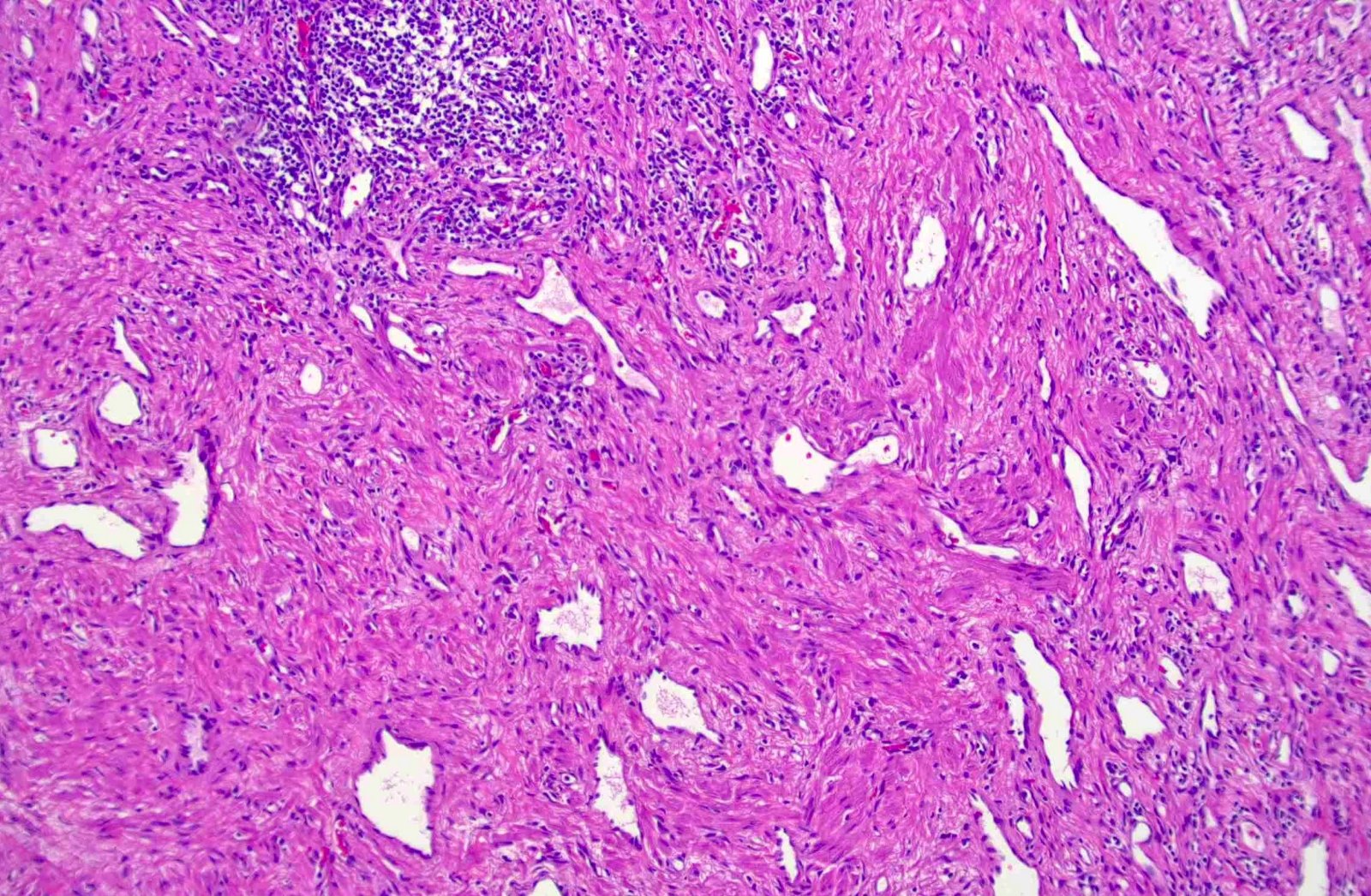
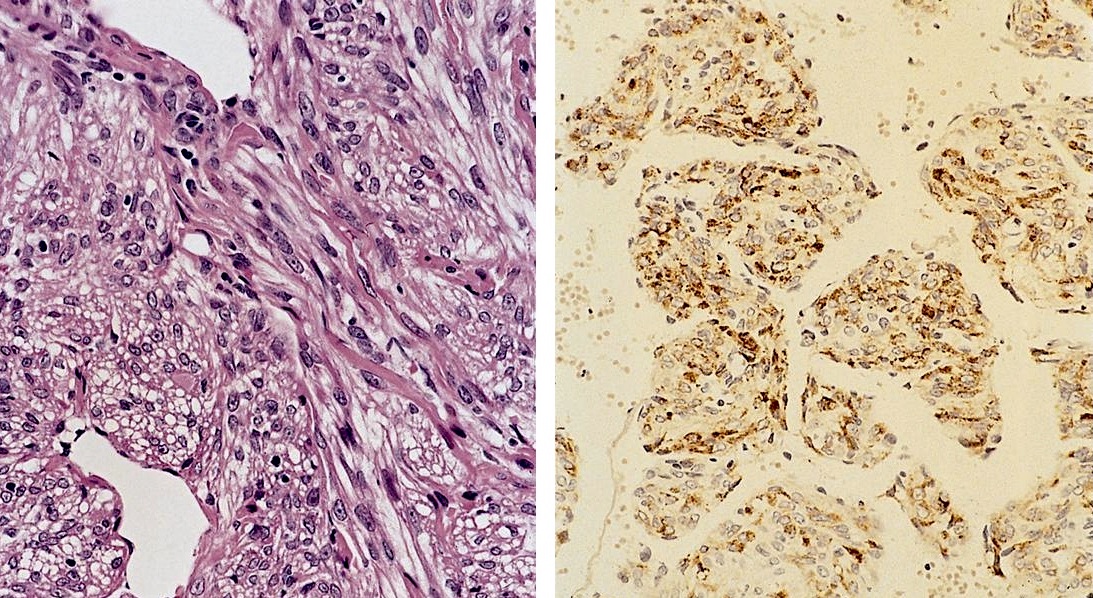
Microscopic (histologic) description
- Noncircumscribed intranodal or extranodal mass
- Mature adipose tissue and haphazard thick walled muscular vessels of varying sizes
- No thrombi within disorganized vascular channels
- Lacks other mesenchymal tissue types, such as muscle
- No necrosis, dense sclerosis, lipoblasts or marked cytologic atypia
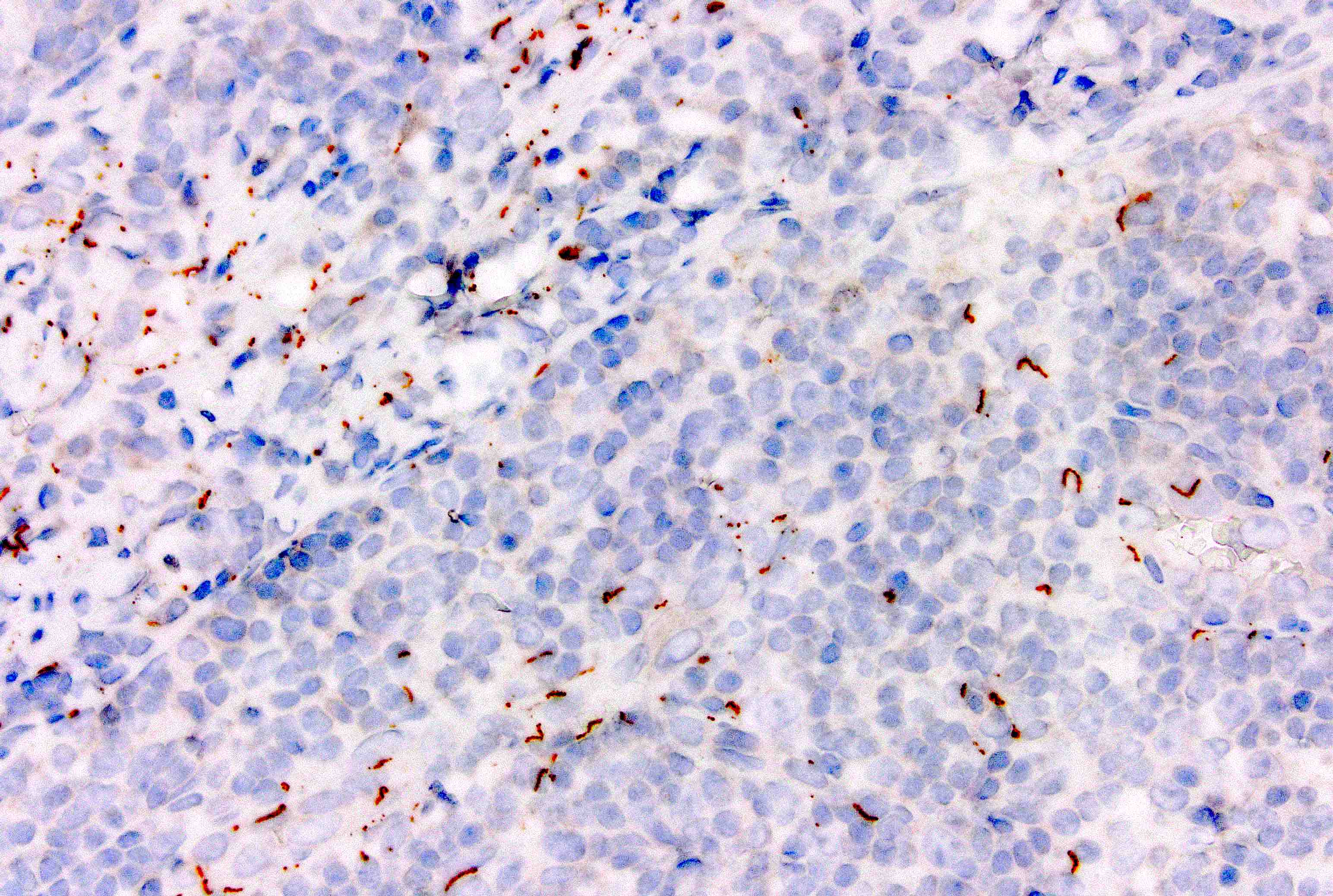
Immunohistochemistry & special stains
- Smooth muscle actin and desmin highlight muscular vessels
Differential diagnosis
- Angiomyolipoma: contains myomatous component, common in kidney
- Lipofibromatosis: pediatric soft tissue tumor with conspicuous fibrous component
- Liposarcoma: malignant tumor with lipoblasts
Angiomyolipoma
Table of Contents
Definition / general | Sites | Pathophysiology | Clinical features | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Benign tumor associated with renal angiomyolipoma
- Proliferation of adipose tissue, smooth muscle and thick walled vessels in variable proportions
- Rare nodal involvement of renal angiomyolipoma likely due to multicentric tumor rather than metastasis (Int J Urol 2000;7:386, Arch Pathol Lab Med 1990;114:65)
Sites
- Retroperitoneal and periaortic lymph nodes draining the involved kidney
Pathophysiology
- Clonal process, consistent with neoplasm rather than hamartoma
- Common progenitor cell differentiating into the 3 cell types (adipose tissue, smooth muscle and blood vessels) (Semin Diagn Pathol 1998;15:21)
Clinical features
- Presents as renal mass with regional lymphadenopathy
- May be associated with tuberous sclerosis
- Fat poor angiomyolipoma can mimic renal cell carcinoma with nodal metastasis on radiograph
- Indolent clinical course and slow growth
Case reports
- 17 year old girl with angiomyolipoma of the kidney with lymph node involvement mimicking renal cell carcinoma (Int Urol Nephrol 2001;33:617)
- 28 year old man with angiomyolipoma with regional lymph node involvement (Hinyokika Kiyo 2003;49:81)
- 34 year old woman with retroperitoneal angiomyolipoma with rapidly progressing intracystic hemorrhage and lymph node involvement (Hinyokika Kiyo 2003;49:611)
- 37 year old woman with renal angiomyolipoma with lymph node involvement (Chang Gung Med J 2003;26:607)
Treatment
- Observation
- Embolization of renal mass or renal conserving surgery
Microscopic (histologic) description
- Haphazard adipose tissue and smooth muscle cells radiating from thick walled blood vessels
- Ranges from sinusoidal involvement to massive replacement of nodal parenchyma
- Smooth muscle may be epithelioid or pleomorphic
- Infrequent mitoses
Positive stains
- Myoid cells: HMB45, MelanA / Mart1, smooth muscle actin, muscle specific actin
- Fat cells: S100
Molecular / cytogenetics description
- Diploid DNA, consistent with benign behavior
Differential diagnosis
- Angiolipomatous hamartoma: may be associated with Castleman disease
- Bacillary angiomatosis: vascular proliferation due to Bartonella infection
- Kaposi sarcoma: malignant spindle cell neoplasm with vascular slits
- Lymphangiomyomatosis: proliferation of lymphatics and smooth muscles
Additional references
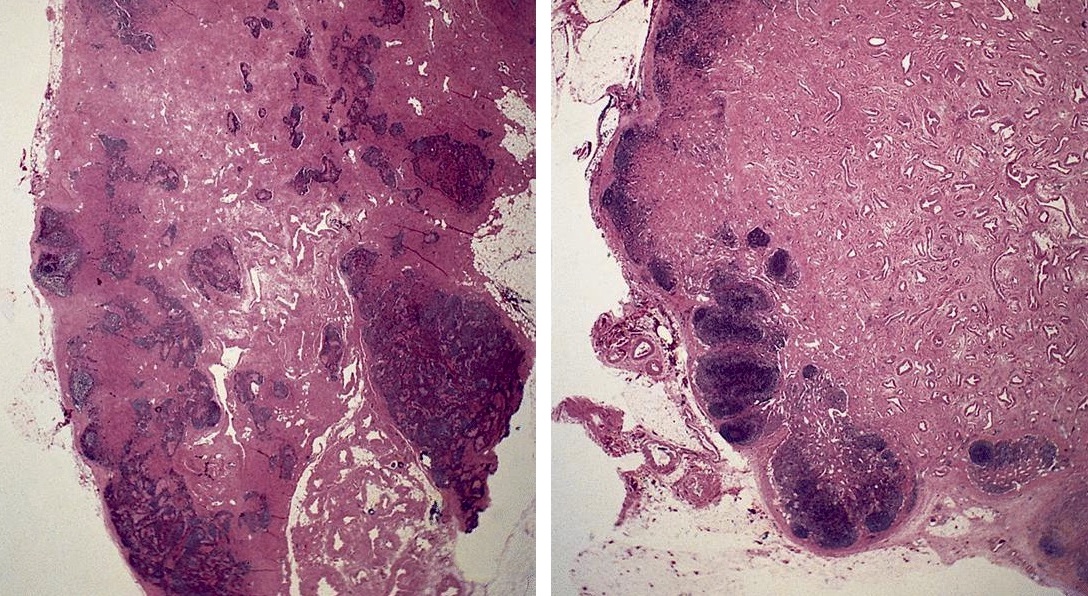
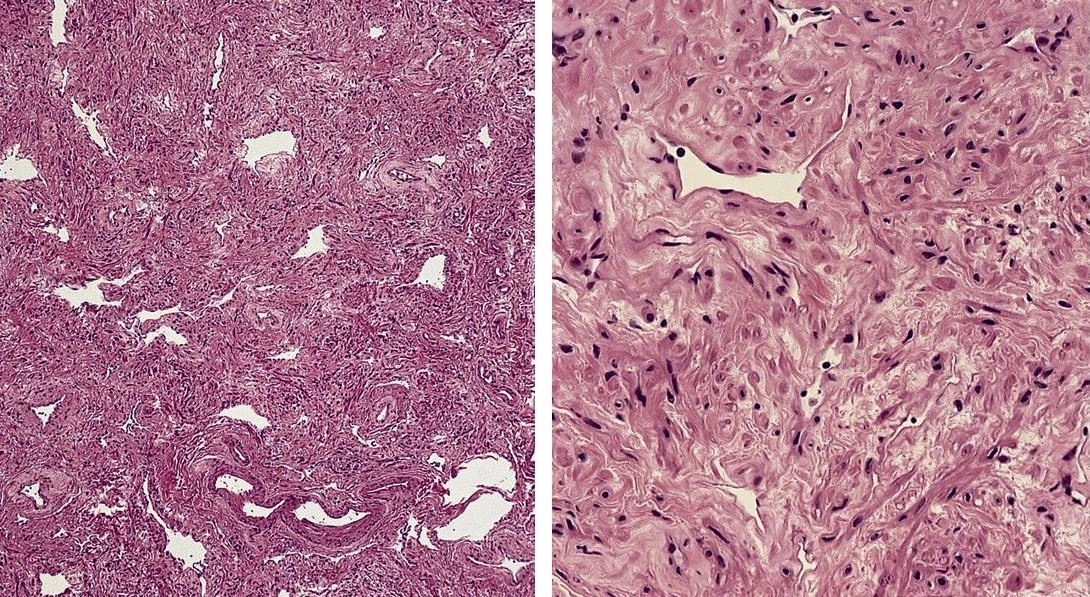
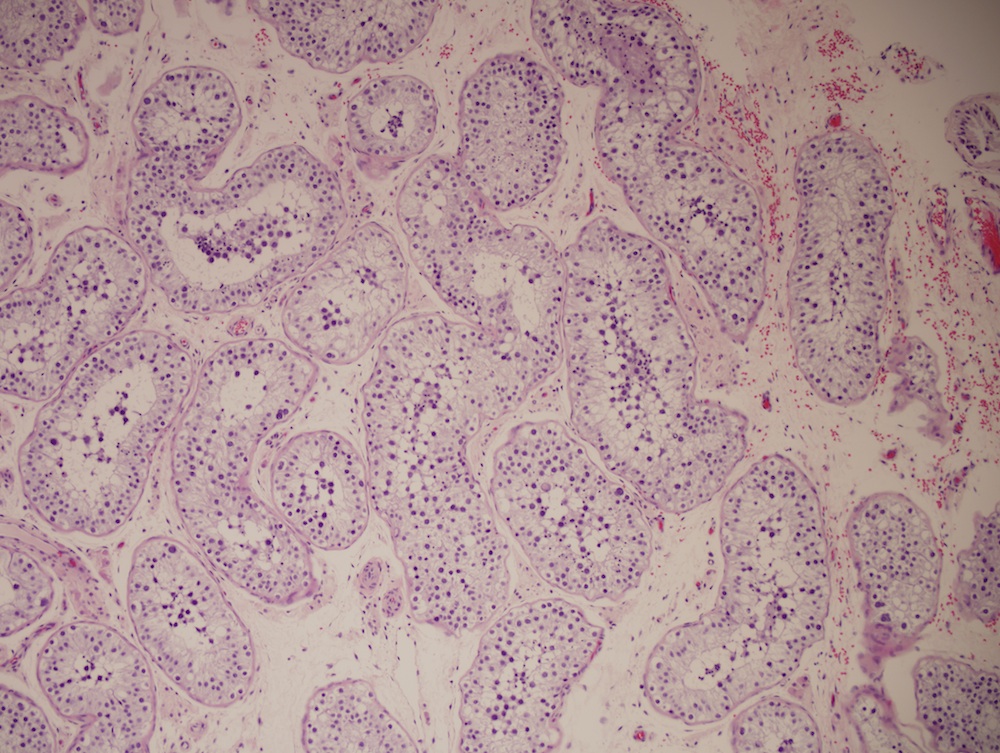
Angiomyomatous hamartoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
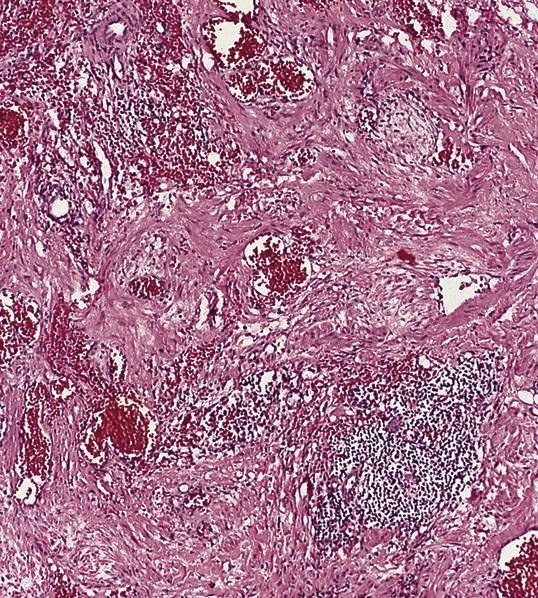
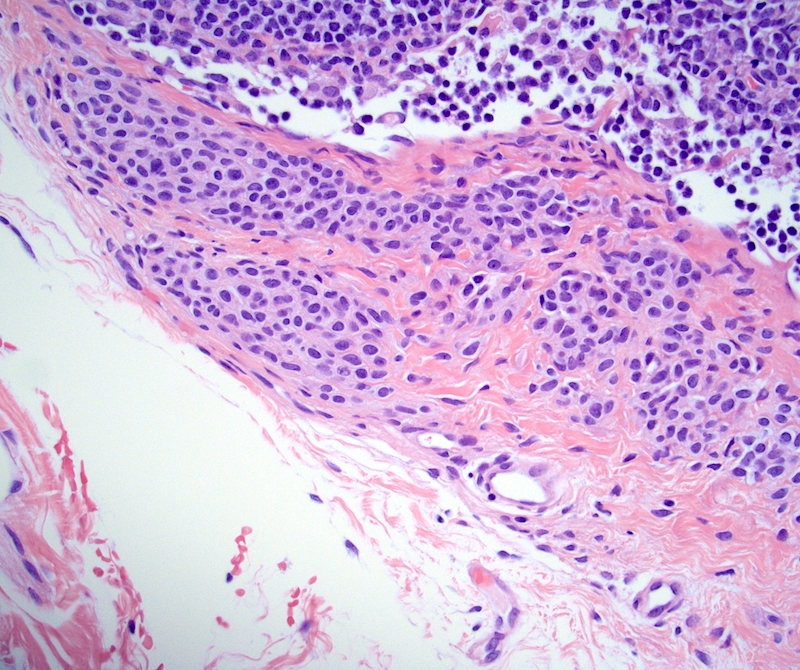
- First described by Chan et al. in 1992 (Am J Surg Pathol 1992;16:335)
- Rare and benign vascular disorder due to proliferation of blood vessels and smooth muscle in mostly the hilar region of lymph nodes, often of long duration
Essential features
- Variably sized, thick walled blood vessels and haphazardly arranged smooth muscles in sclerotic nodal parenchyma
- Benign lesion with no atypia
- Uncommon process, usually asymptomatic and often solitary
- Prominent smooth muscle infiltrate (SMA) with low proliferative fraction
- HMB45 negative (to distinguish from angiomyolipoma)
ICD coding
Epidemiology
- M > F (Blood 2020;136:1794)
- All ages, peaking at sixth decade (Hum Pathol 2017;68:175)
Sites
- Mostly involves inguinal or femoral lymph nodes, usually solitary
- May have associated limb edema; rare case report with a soft tissue mass (JAAD Case Rep 2022;27:117)
- Rare reports of cervical lymph node and popliteal lymph node involvement (Histopathology 1996;29:80, Ann Diagn Pathol 2008;12:372)
- Rarely presents as extensive lymphadenopathy (Blood 2020;136:1794)
Etiology
- Unknown etiology; may represent reparative reaction to previous nodal inflammation or chronic blockage of nodal lymphatic flow (BMC Pediatr 2012;12:172)
Clinical features
- Presents incidentally or as palpable nodule; rare report of lymphadenomegaly in different regions (Blood 2020;136:1794)
- Typically asymptomatic; rarely lymphedema of ipsilateral limb (Fed Pract 2016;33:38)
- Benign clinical course
- No known recurrence or metastasis
Diagnosis
- Excisional biopsy of lymph node usually diagnostic
Radiology description
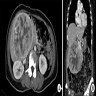
- Magnetic resonance imaging (MRI): well circumscribed solitary nodule with heterogeneous signal intensity (JAAD Case Rep 2022;27:117)
Case reports
- 33 year old man with postauricular lymph node, clinically masquerading as epidermal inclusion cyst (JAAD Case Rep 2020;7:131)
- 37 year old woman with weight loss and para-aortic lymph node (Prz Menopauzalny 2023;22:111)
- 53 year old woman with multiple neck lymph nodes showing angiomyomatous hamartoma (Int J Surg Pathol 2023 Nov 20 [Epub ahead of print])
- 60 year old man with several year history of lower leg edema (Case #118)
- 63 year old woman with right femoral subcutaneous mass and localized lymphedema (JAAD Case Rep 2022;27:117)
- 75 year old man with incidental extensive lymphadenopathy involving mediastinal, retroperitoneal, pelvic, inguinal and para-aortal lymph nodes (Blood 2020;136:1794)
Treatment
- Surgical excision is curative
Gross description
- Enlarged lymph node replaced by firm, white tissue
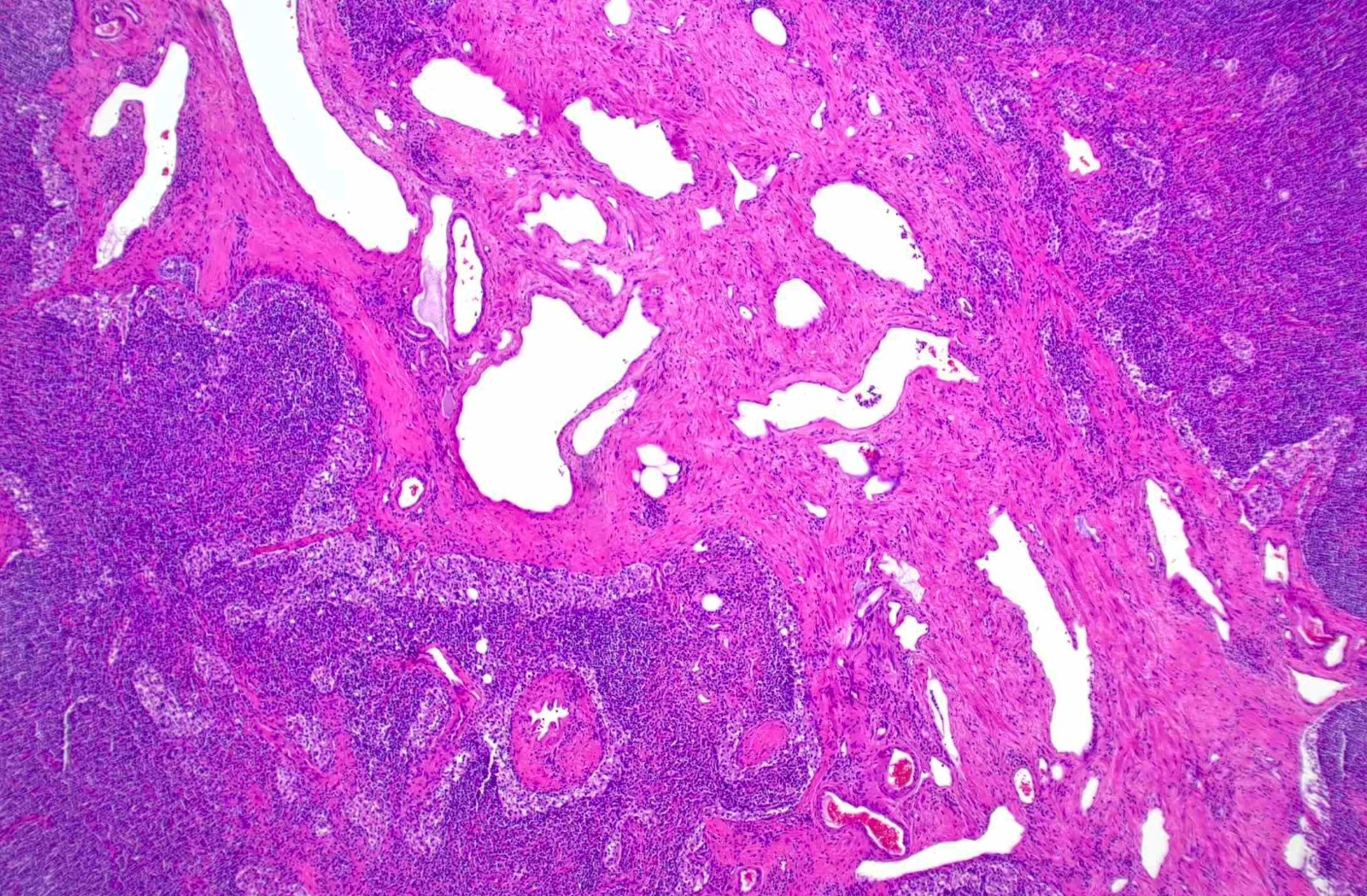
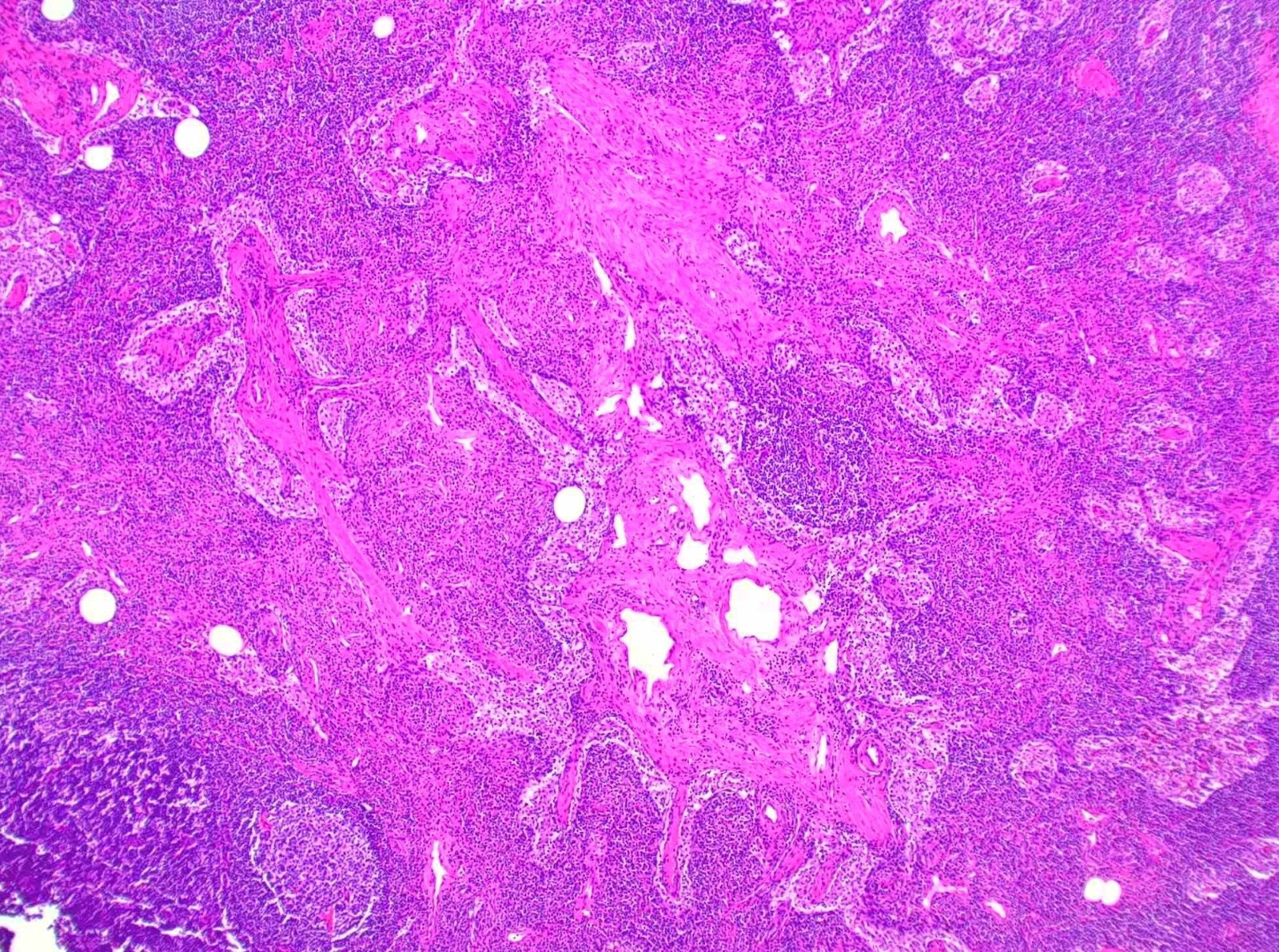
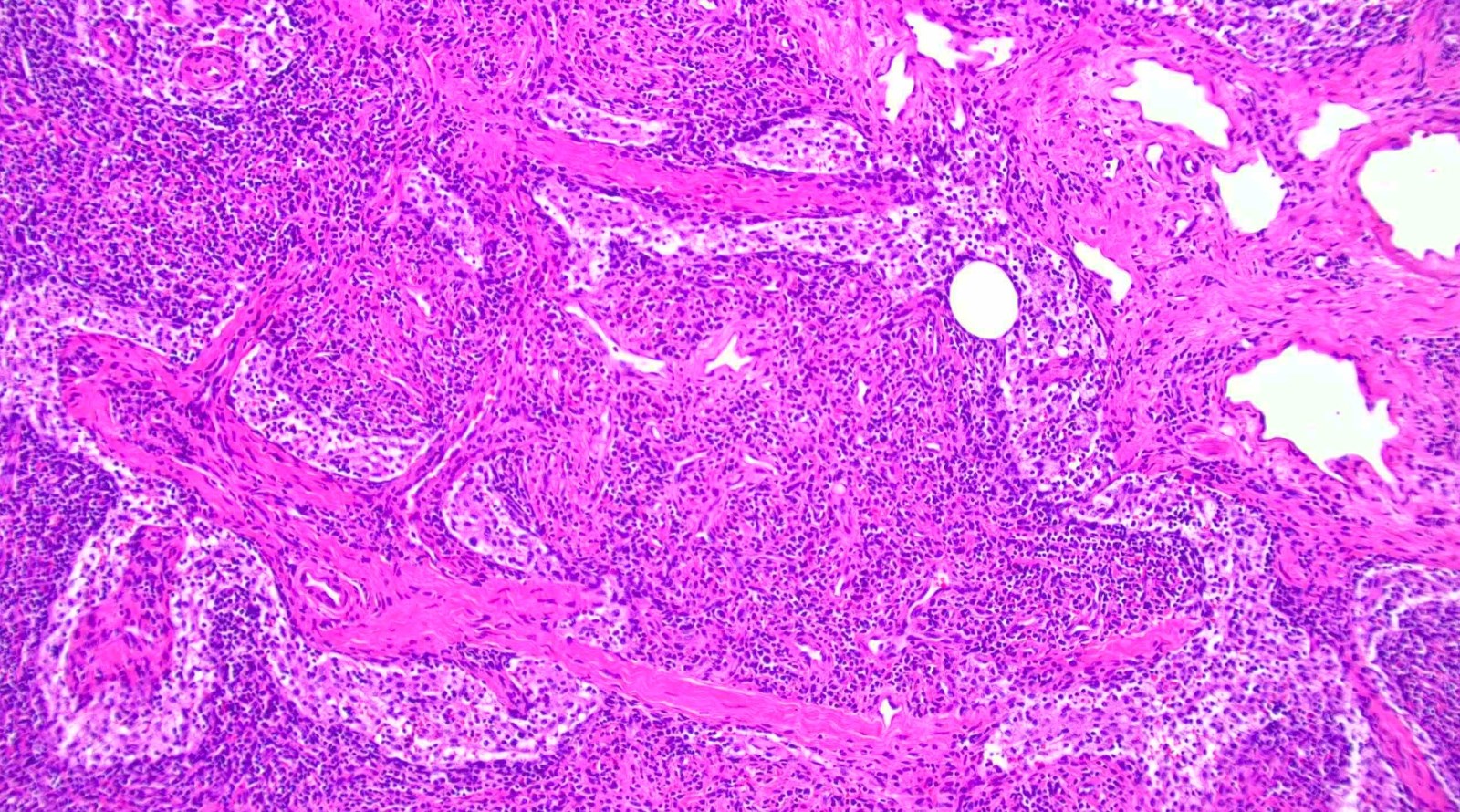
Microscopic (histologic) description
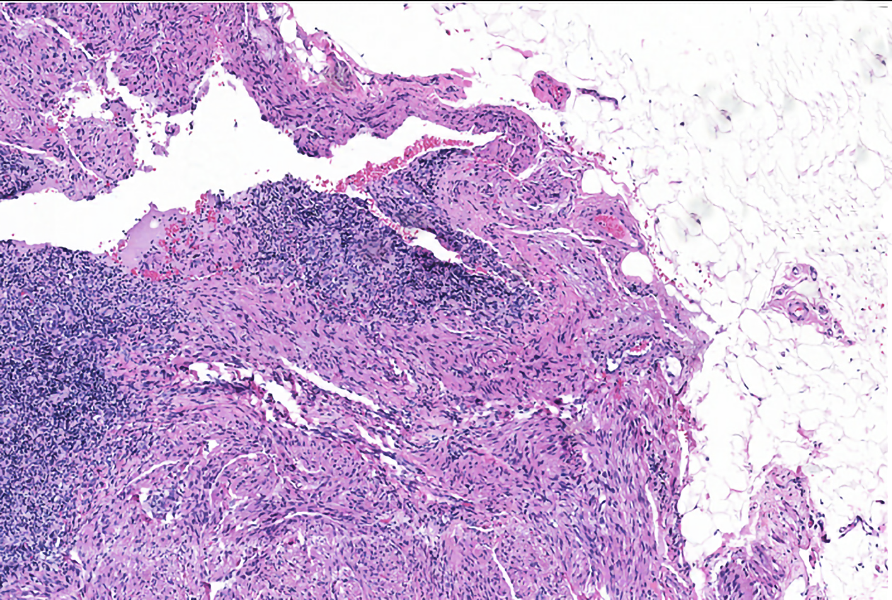
- Extensive and multifocal nodal involvement by thick walled hilar blood vessels, often with increased fibrous tissue
- Nodal parenchyma has haphazard smooth muscle fibers in sclerotic stroma
- Starts in nodal hilum and extends toward cortex
- May have admixed adipose tissue
- No cellular atypia, pleomorphism or necrosis; low mitotic rate (Hum Pathol 2017;68:175)
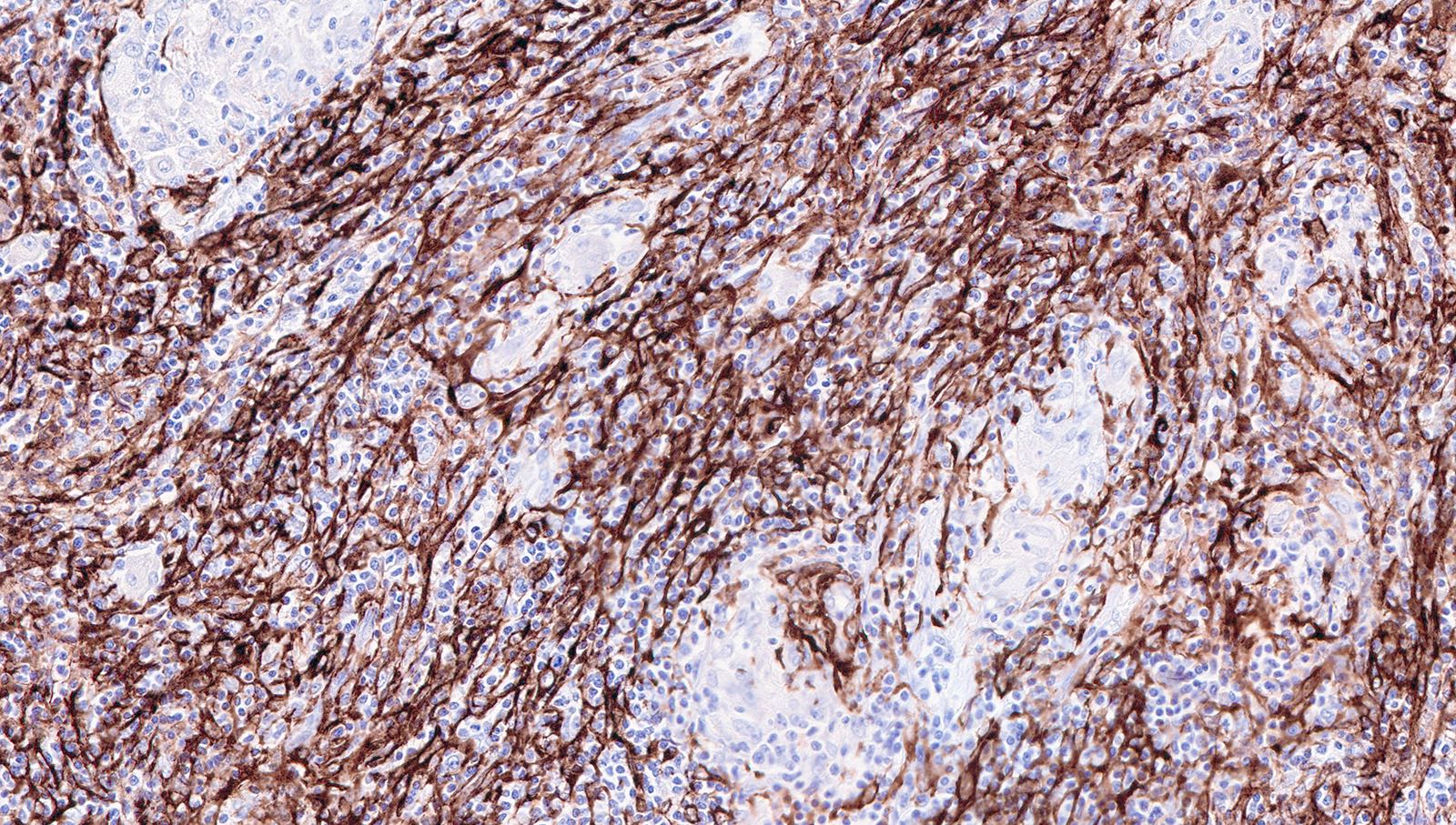
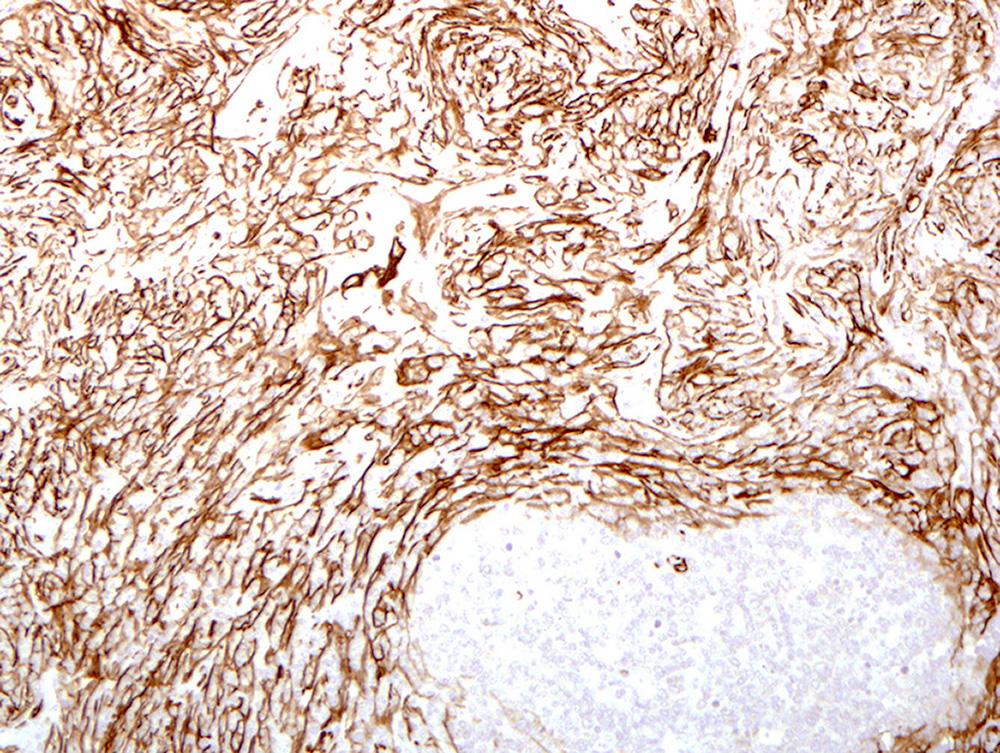
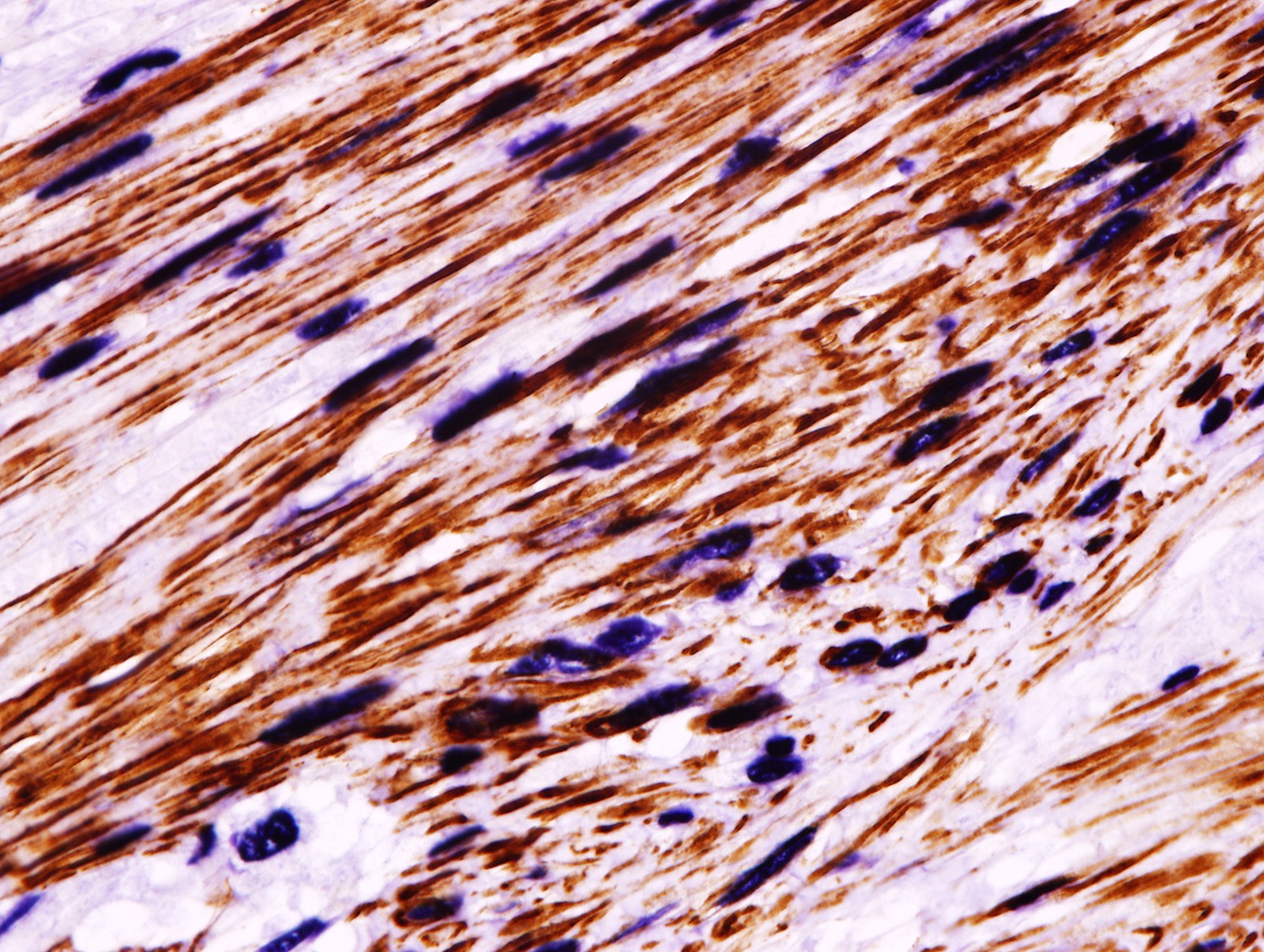
Microscopic (histologic) images
Contributed by Patricia Tsang, M.D., M.B.A., Vincent A. Graffeo, M.D. and AFIP
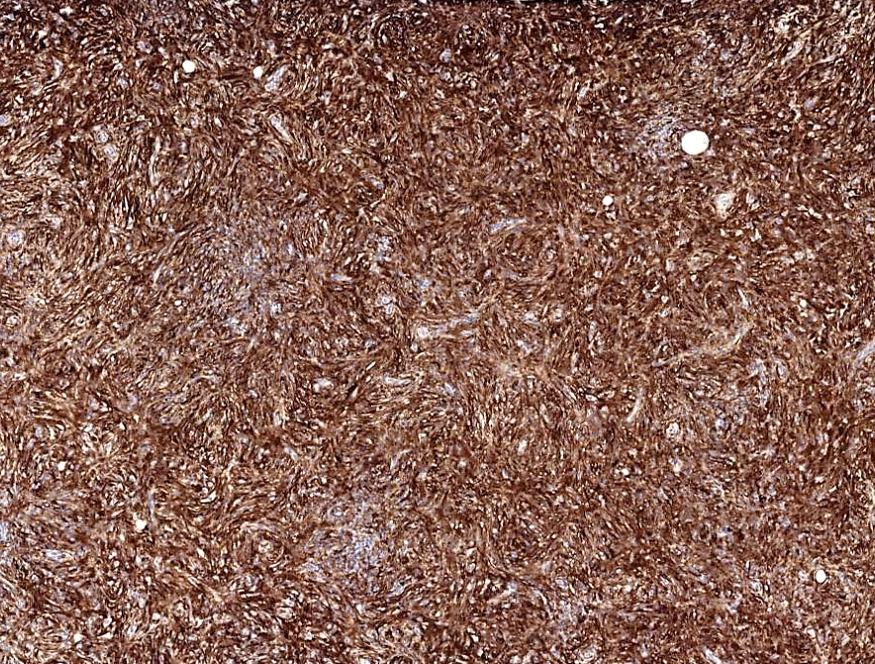
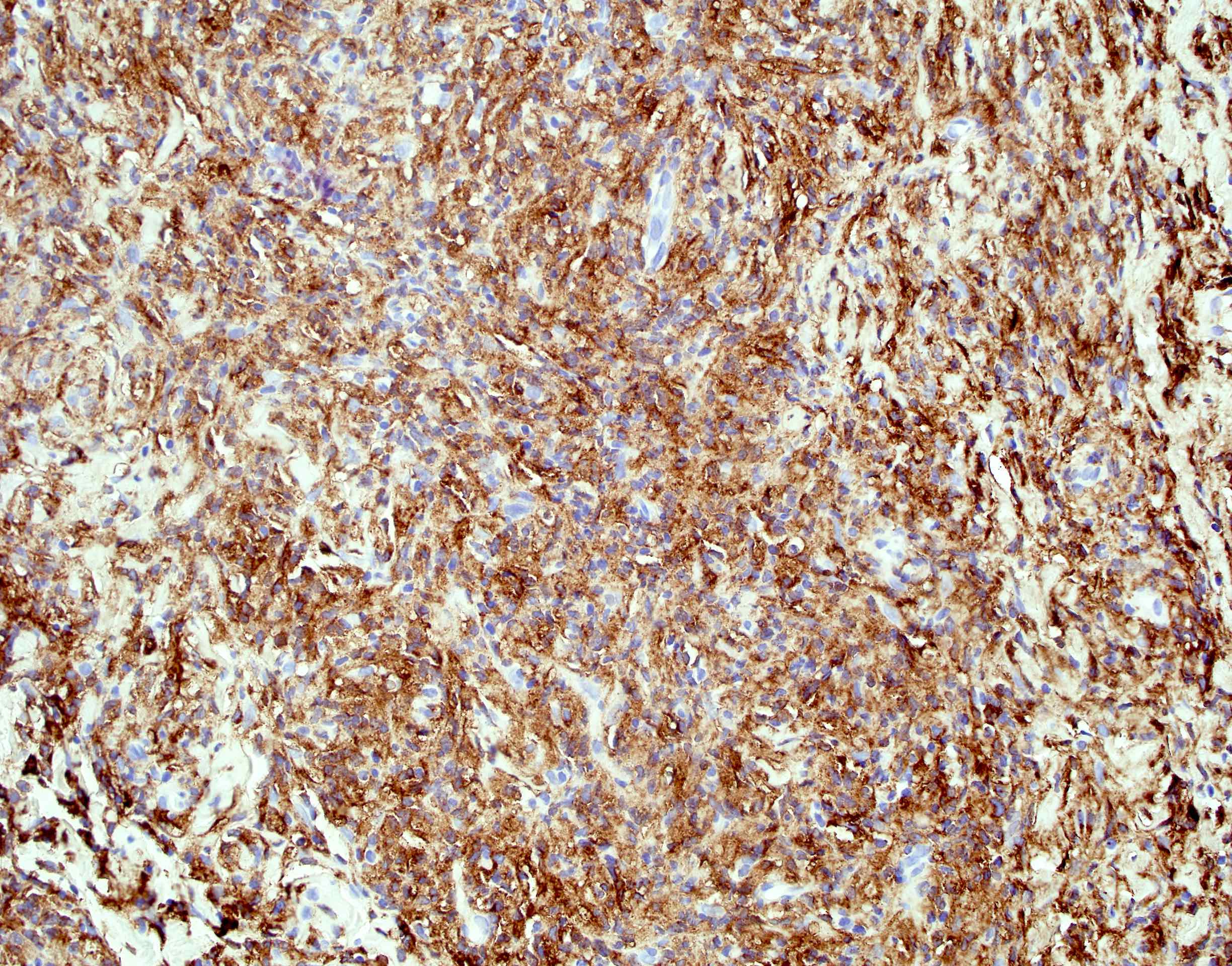
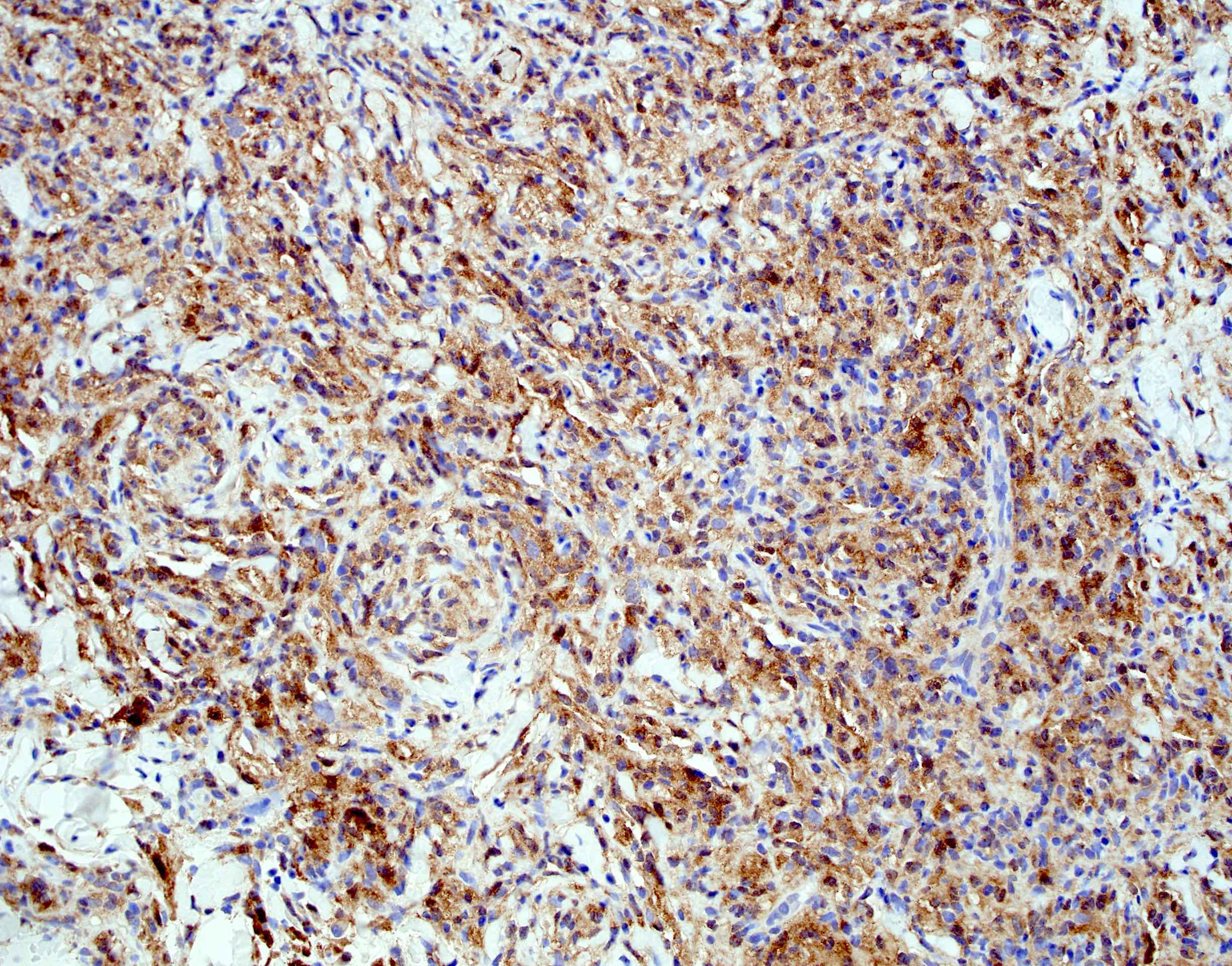
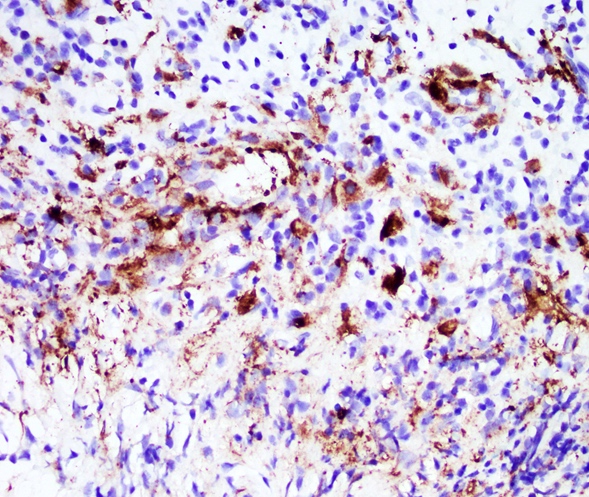
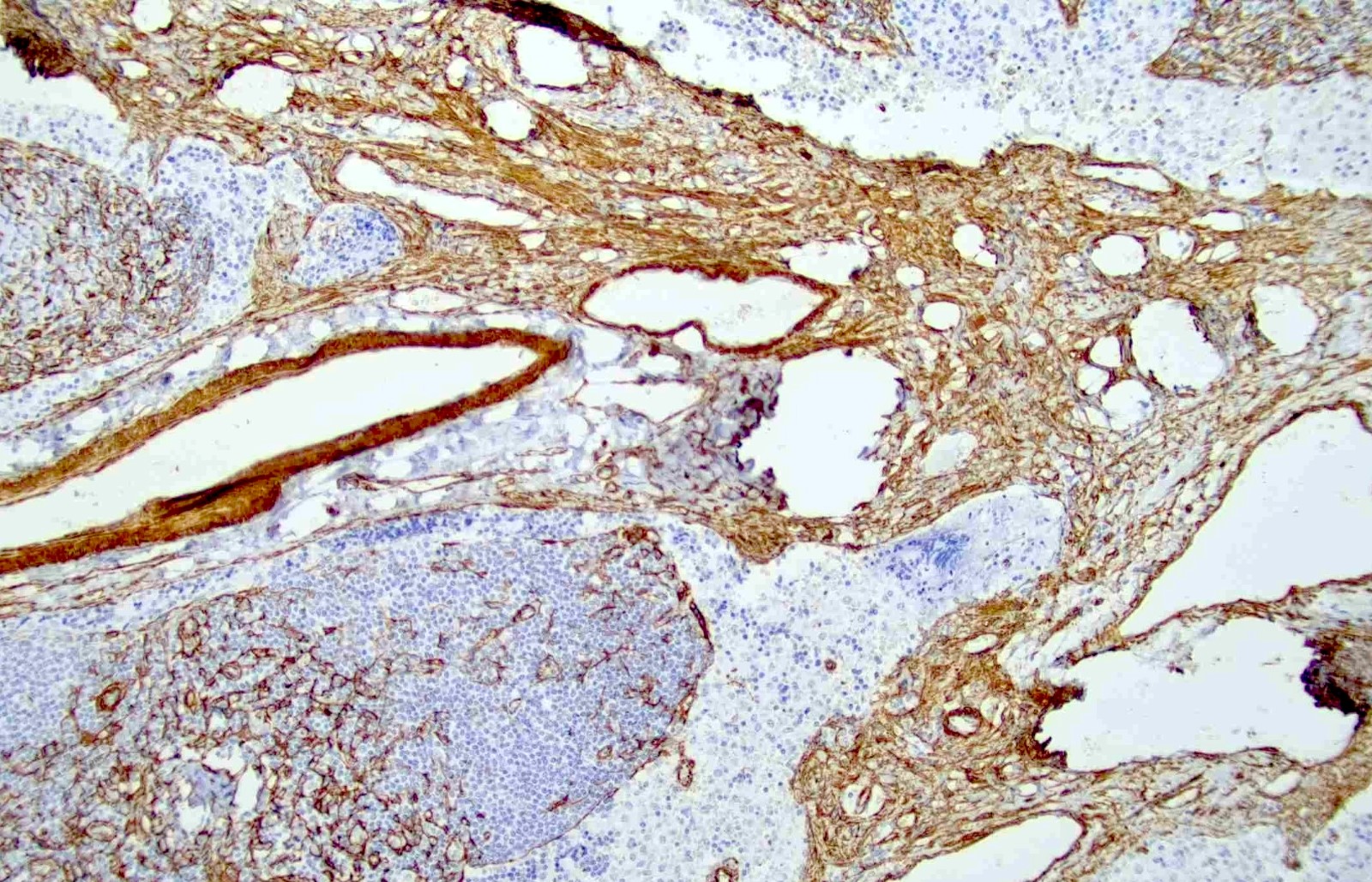
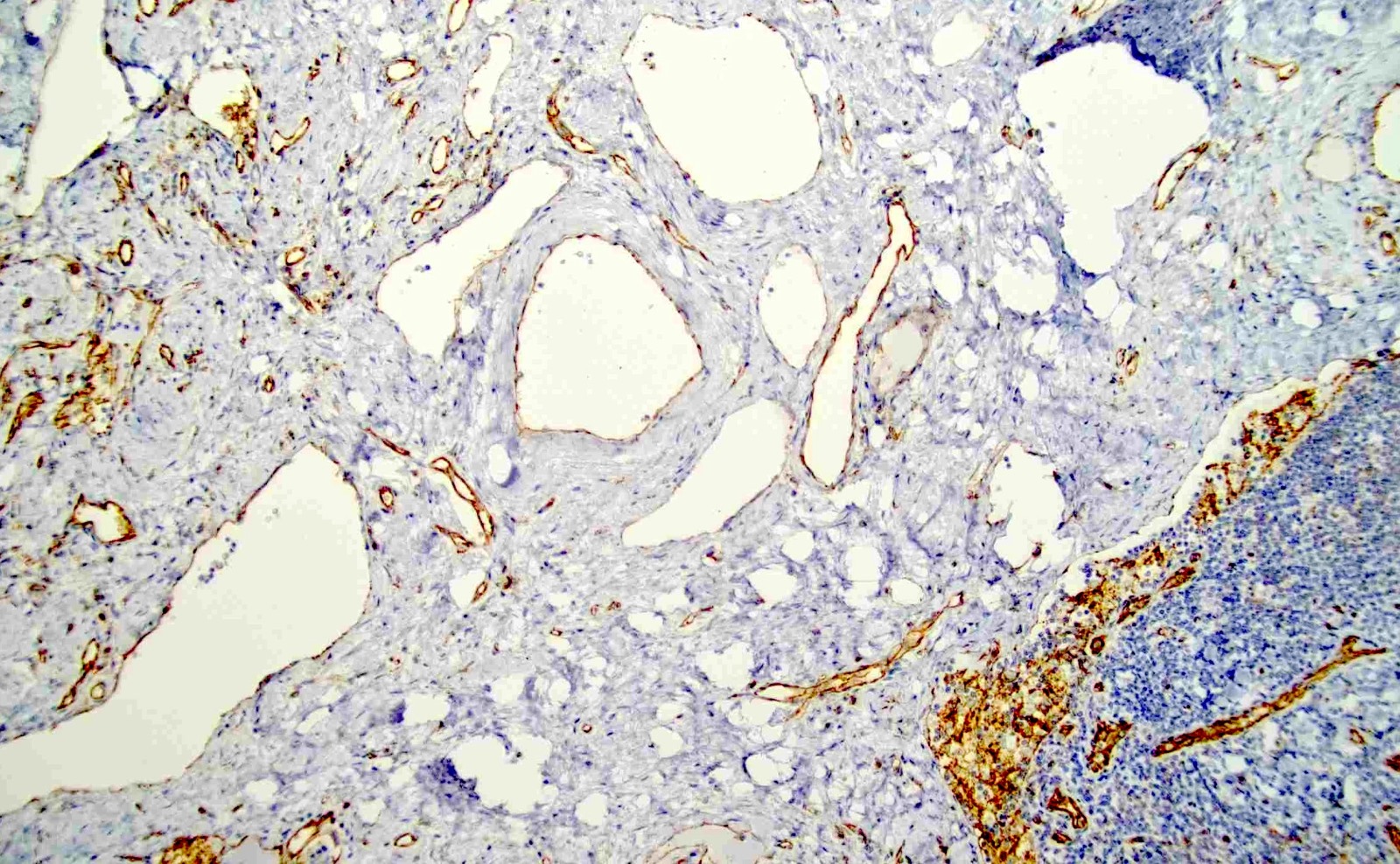
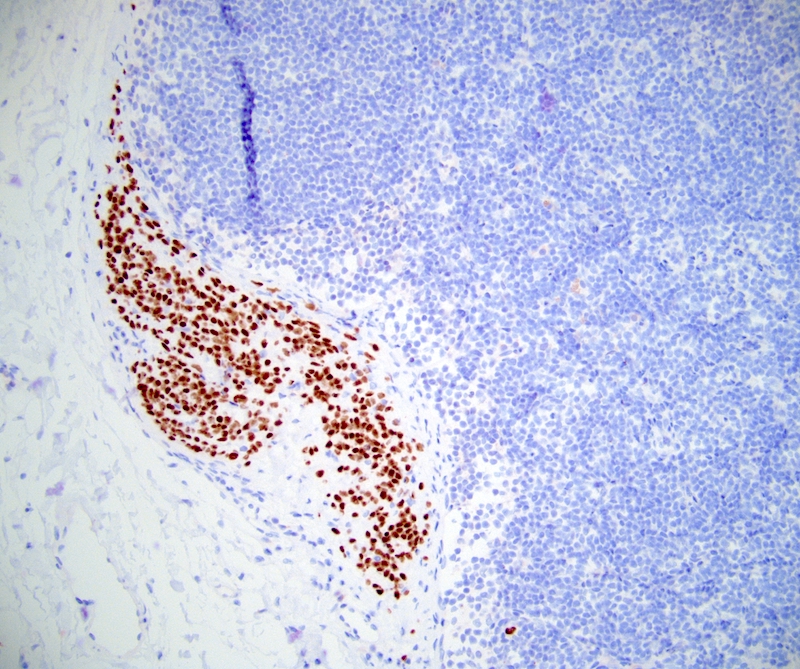
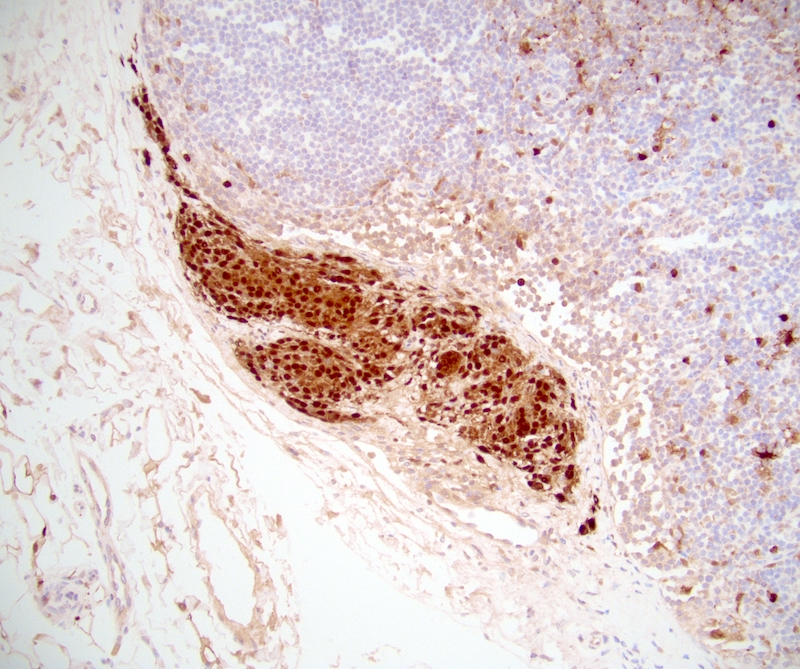
Positive stains
- Smooth muscle actin, caldesmon and desmin stain smooth muscle fibers
- CD31 and CD34 highlight endothelial cells (Int J Surg Pathol 2023 Nov 20 [Epub ahead of print])
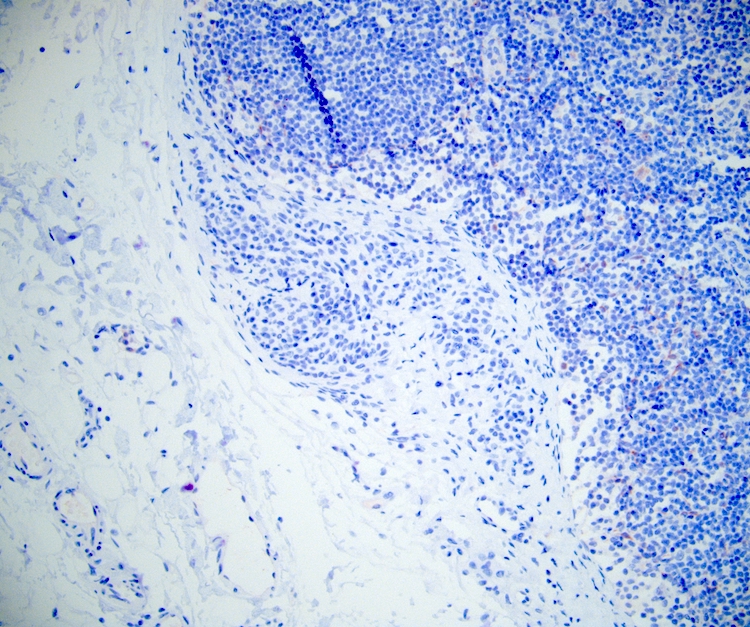
Negative stains
- HMB45 and cathepsin K, in contrast to angiomyolipoma and lymphangioleiomyomatosis
- Ki67 is mostly negative (low proliferation) (Int J Surg Pathol 2023 Nov 20 [Epub ahead of print])
Sample pathology report
- Inguinal lymph node, excision:
- Angiomyomatous hamartoma of lymph node (see comment)
- Comment: The nodal architecture is disrupted by a haphazard proliferation of thick walled vasculature and smooth muscle bundles. Occasional adipocytes are admixed. No cytologic atypia or mitoses are seen. Residual lymphoid tissue with reactive lymphoid follicles is present in the cortex. Immunohistochemistry shows CD31 and SMA highlighting the endothelial cells and smooth muscles, respectively. HMB45 is negative.
Differential diagnosis
- Angiolipomatous hamartoma:
- Various nodal sites, including cervical, mediastinal and retroperitoneal lymph nodes
- Miscellaneous extranodal sites may be involved
- Associated with Castleman disease
- Angiomyolipoma:
- HMB45+
- Typically involves retroperitoneal nodes
- Associated with renal angiomyolipoma
- Lymphangiomatosis (Lymphat Res Biol 2011;9:191):
- Commonly in children and young adults, may be congenital
- Frequently involves lung with pleural effusion and other thoracic locations; also in bone, spleen and liver; primary nodal is rare
- Smooth muscle fascicles around anastomosing dilated vascular spaces lined by flat endothelial cells
- Lymphangioleiomyomatosis:
- Female predominance
- Lungs and occasionally retroperitoneal lymph nodes
- HMB45+ and cathepsin K+
- Thin walled lymphatic channels and spindle cells in a fascicular pattern
- Vascular transformation of lymph node sinuses (Am J Surg Pathol 1991;15:732):
- Small capillary vascular channels replacing nodal subcapsular sinuses
- Reactive process often accompanied by fibrosis; no cytologic atypia
- Involves single or multiple lymph nodes in a diffuse or segmental fashion
Board review style question #1
When differentiating angiomyomatous hamartoma from angiomyolipoma, which of the following immunohistochemical stains is the most helpful?
- CD31
- CD34
- HMB45
- SMA
Board review style answer #1
C. HMB45. Angiomyomatous hamartoma is negative for melanocytic markers (HMB45 and MelanA), while angiomyolipoma is positive for these stains. Answers A and B are incorrect because both lesions contain vascular proliferation which can be highlighted by the endothelial markers, CD31 and CD34. Answer D is incorrect because both lesions show positivity for SMA, which does not help differentiate these entities.
Comment Here
Reference: Angiomyomatous hamartoma
Comment Here
Reference: Angiomyomatous hamartoma
Board review style question #2
Board review style answer #2
A. It can present as localized edema of the limb. Angiomyomatous hamartoma may be found incidentally or may present with pain or localized edema. It can be present in any location but has a predilection for the inguinal and femoral lymph nodes. Answer C is incorrect because this entity is a benign hamartoma that consists of proliferation of smooth muscles and blood vessels. Answers B and D are incorrect because there is no known association with HHV8 or melanocytic immunostain markers.
Comment Here
Reference: Angiomyomatous hamartoma
Comment Here
Reference: Angiomyomatous hamartoma
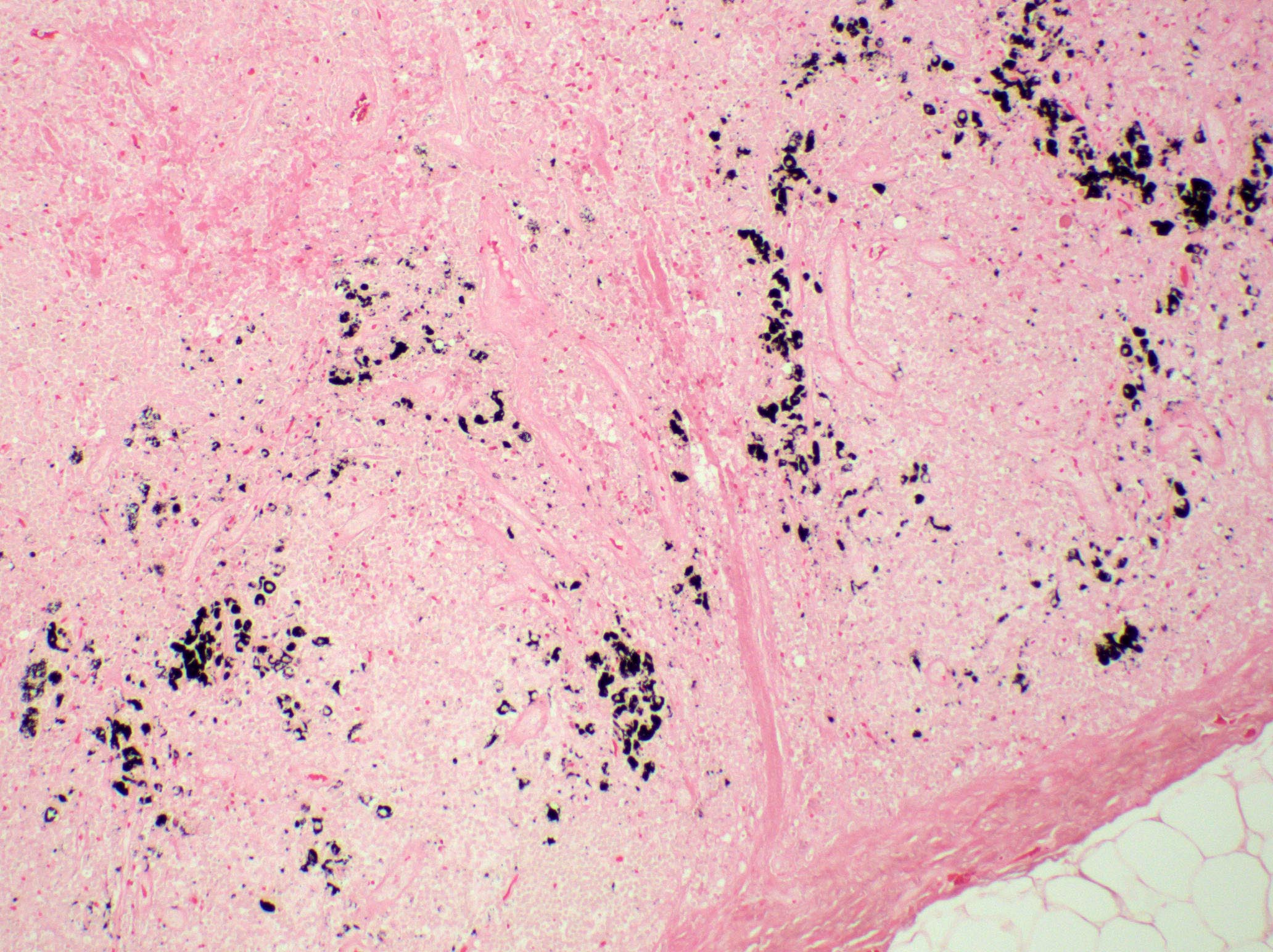
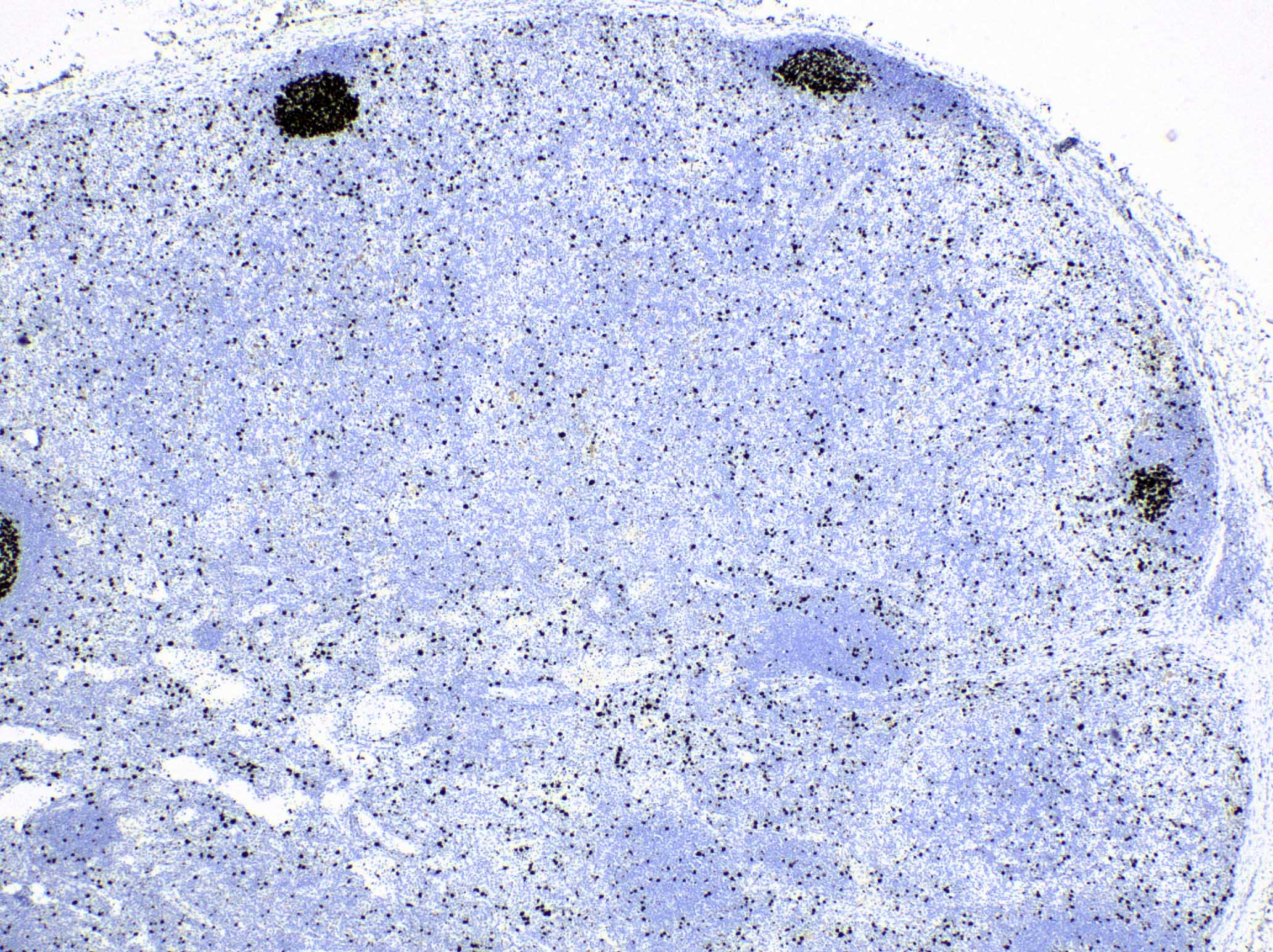
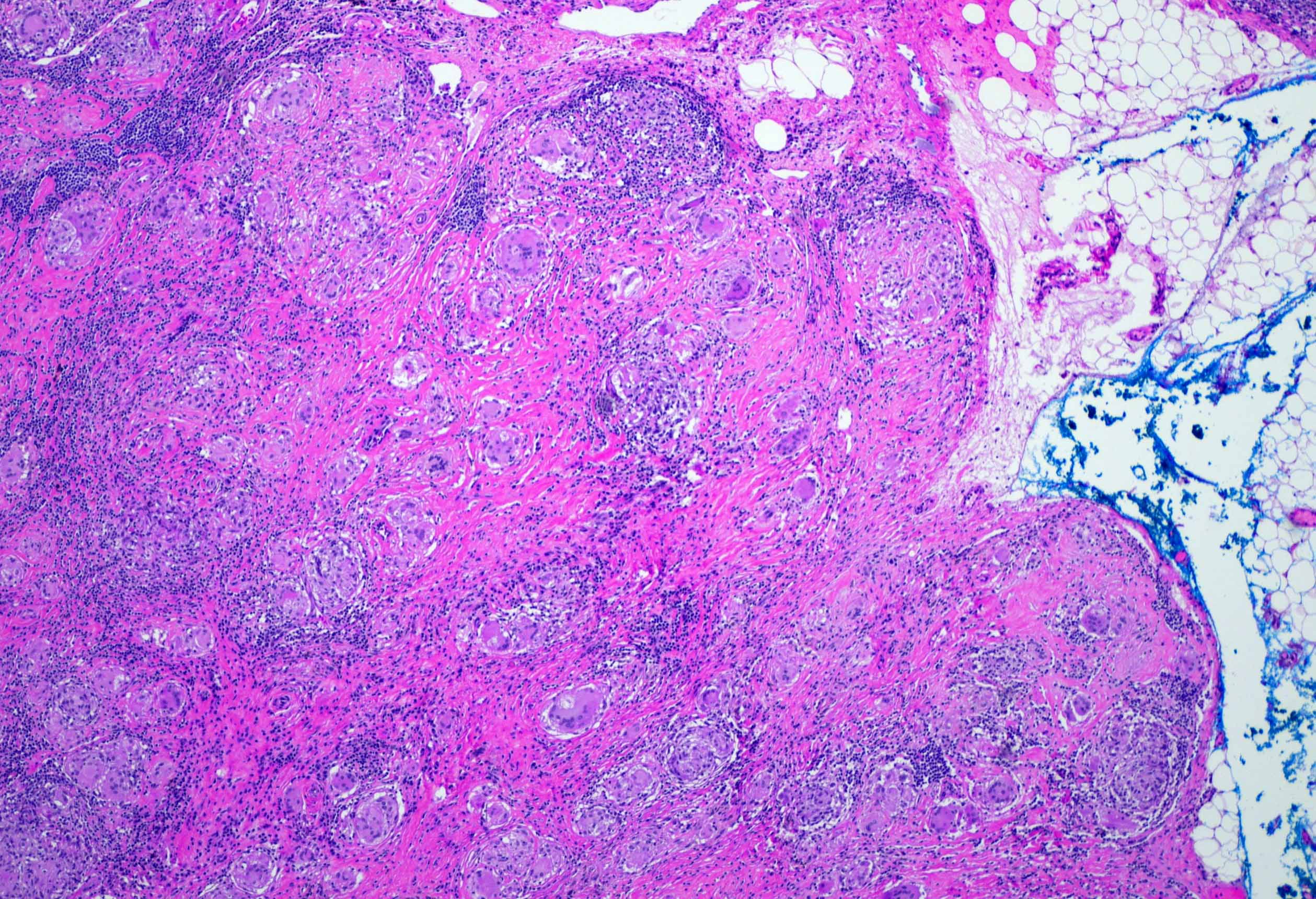
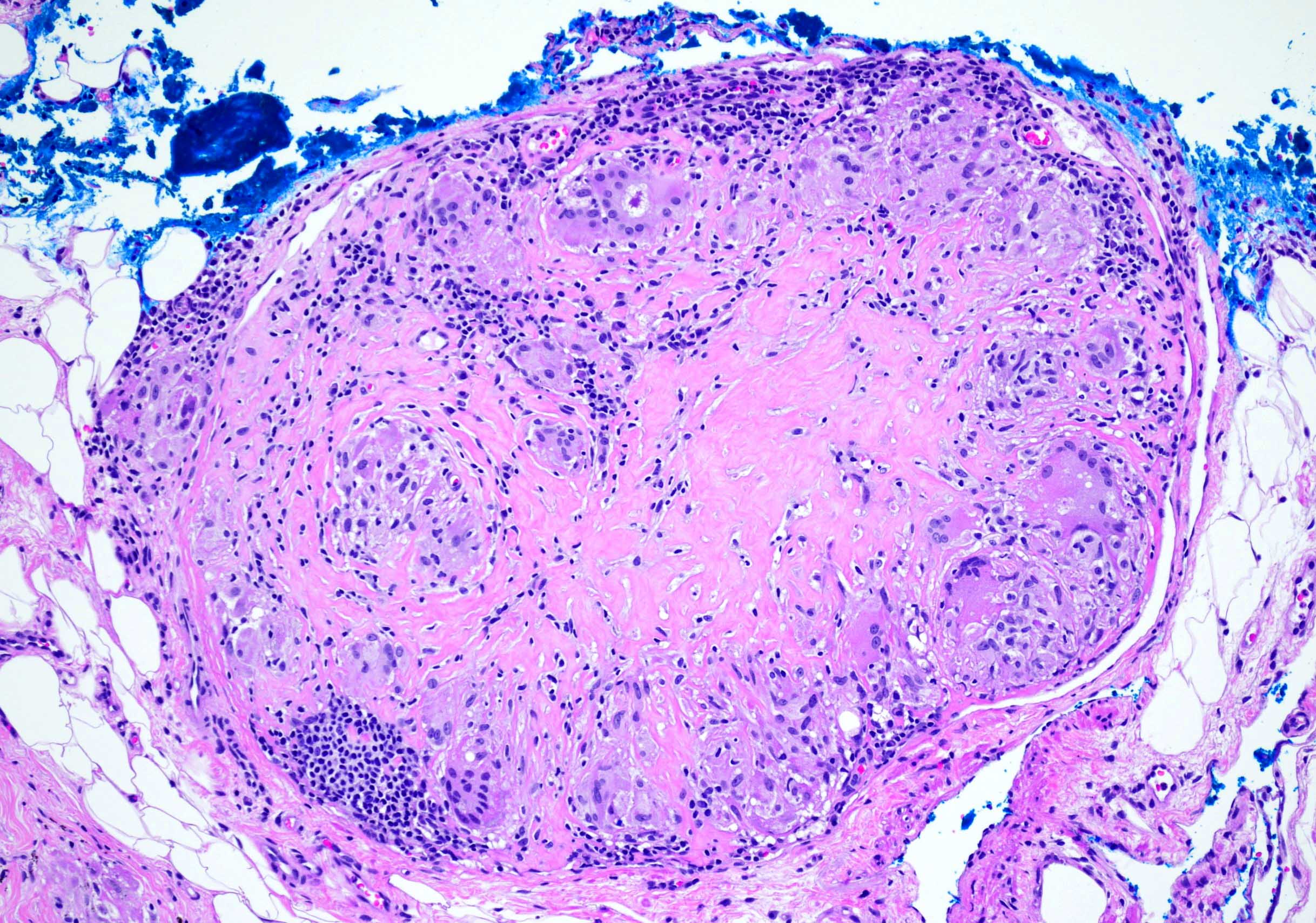
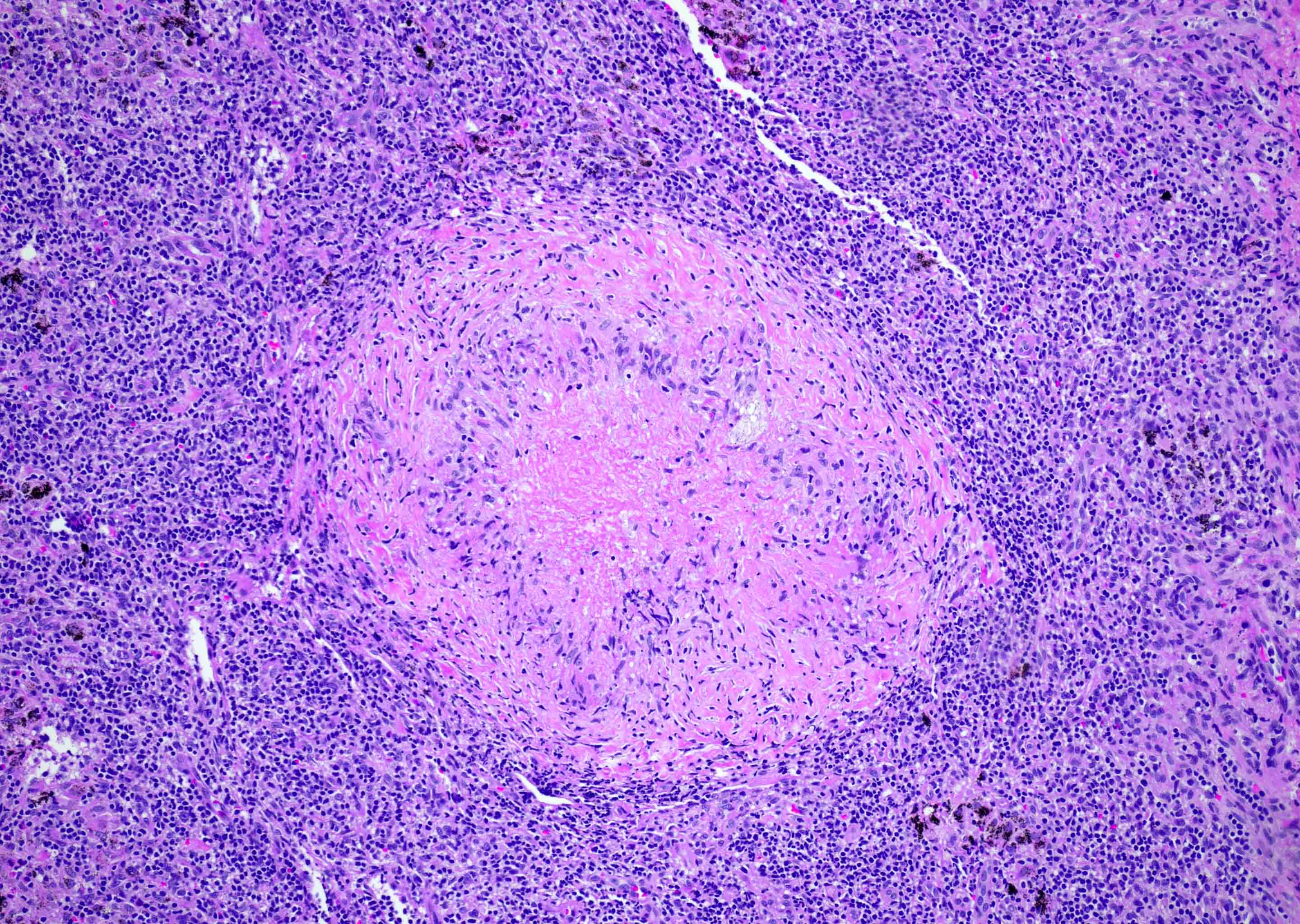
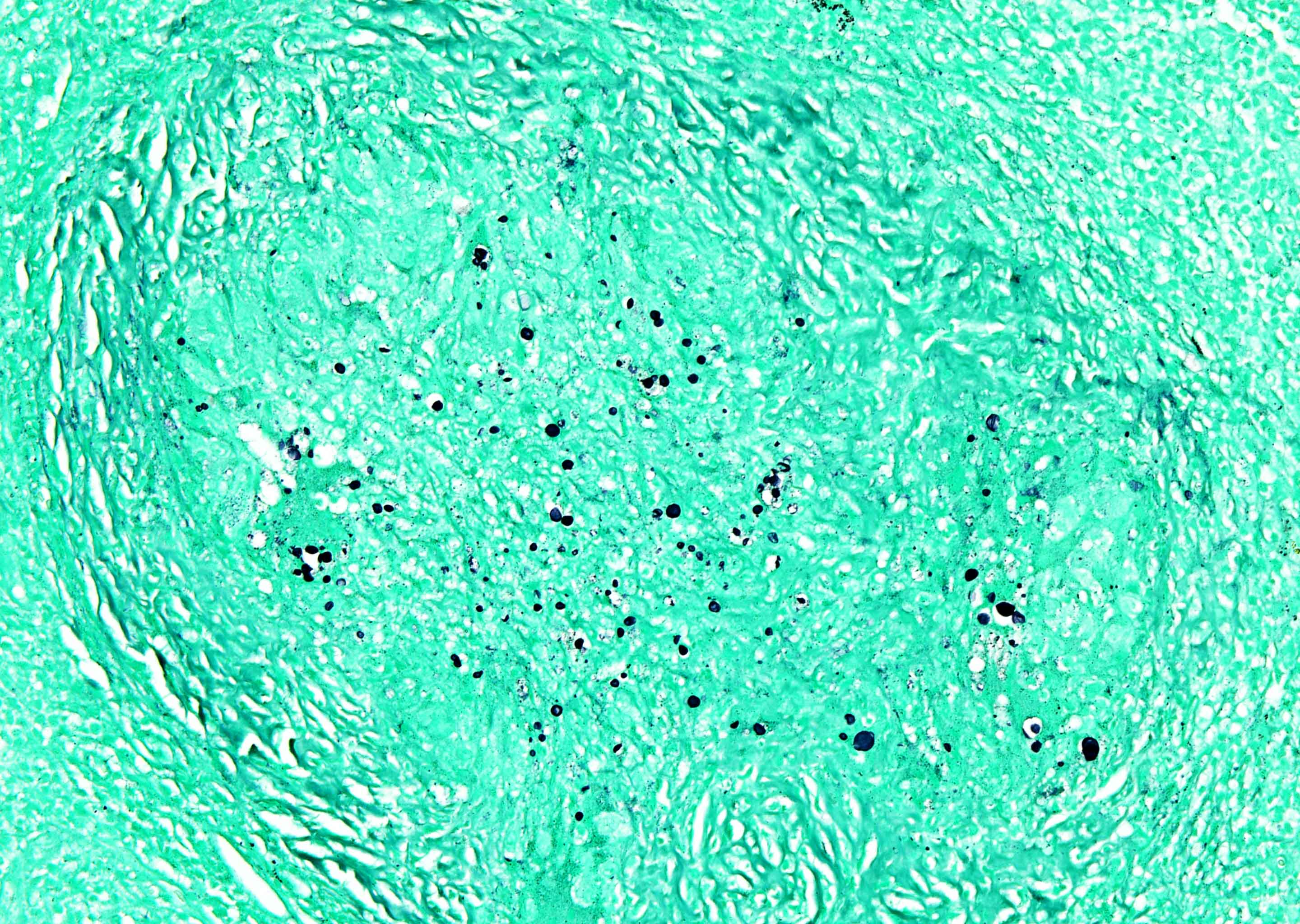
Anthracosis
Table of Contents
Definition / general | Epidemiology | Sites | Clinical features | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Cytology description | Cytology images | Positive stains | Differential diagnosis | Additional referencesDefinition / general
- Accumulation of carbon in lymph nodes, more commonly in intrapulmonary lymph nodes, due to coal dust, smoke or pollution
- May be associated with storiform pattern of histiocytes that resembles a neoplasm (Hum Pathol 1998;29:851)
- Associated with silica, although often no history of industrial exposure
- Associated with hyalinization in nodes of elderly Japanese (Histol Histopathol 2003;18:1169)
Epidemiology
- Very common
Sites
- Common in hilar and bronchial lymph nodes
Clinical features
- Enlargement of involved lymph nodes, prominent mediastinal lymphadenopathy is common
Radiology description
- Enlarged lymph nodes, especially mediastinal and bronchial lymph nodes
Radiology images
Prognostic factors
- Benign process with no significant clinical implications
Case reports
- 71 year old woman, with life-long exposure to soot from a wood cook stove, with anthracosis and large mediastinal mass with healed pulmonary tuberculosis (Clin Med Res 2010;8:99)
- Women who cooked over wood fires, with primary nodal anthracosis identified as a cause of FDG PET/CT positive mediastinal lymphadenopathy (Respiratory Medicine Case Reports 2013;10:48)
- A case of anthracosis presenting with mediastinal lymph nodes mimicking tuberculous lymphadenitis or malignancy (Eur J Intern Med 2003;14:444)
Treatment
- If significant enlargement, excision biopsy
Gross description
- Enlarged lymph nodes with firm dark brown to black cut surfaces
Microscopic (histologic) description
- Anthracotic macrophages in clusters and singly dispersed
- There may be storiform arrangement of spindle cells or granuloma like aggregates of macrophages
- Fine anthracotic pigment
- Also nodal hyaline scars and polarizable material suggestive of silica
Cytology description
- Cellular smears with a population of anthracotic macrophages that are both singly dispersed and in variously sized aggregates
- Variable foreign body type, multinucleated giant cells
- No necrosis or atypical cells
Positive stains
- CD68 (macrophages containing pigment)
Differential diagnosis
- Clinically resembles malignancy: lymphoma, metastasis
- Follicular dendritic cell tumor
- Kaposi sarcoma
- MFH
- Sarcoidosis
- Spindle cell melanoma
- Tuberculosis
Additional references
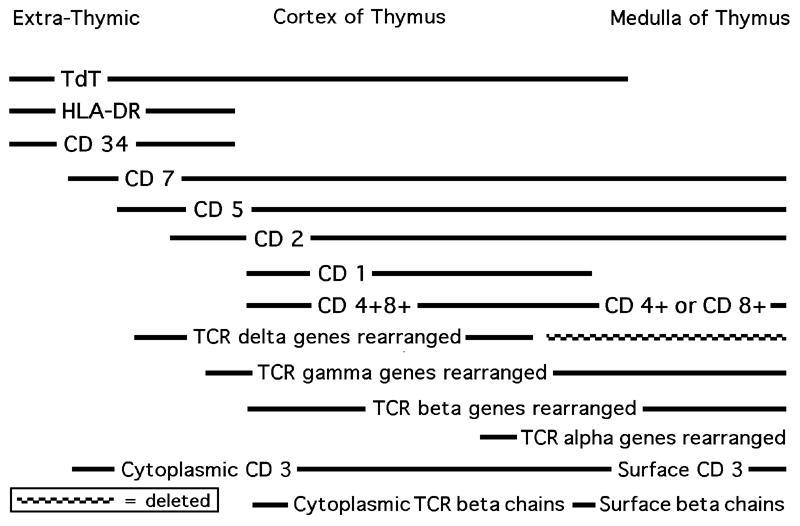
B & T cells
Table of Contents
Definition / general | Pathophysiology | Diagrams / tables | Clinical features | Positive stains | Negative stains | Additional referencesDefinition / general
-
B cells:
- Progressive maturation of hematopoietic stem cells through several stages to ultimately give rise to mature B cell pool that has been selected for reactivity against non-self antigens (Expert Rev Clin Immunol 2010;6:765)
- Develop from stem cells of yolk sac, fetal liver, spleen and bone marrow
- B cells complete most of their development within the bone marrow, in contrast to T cells that mature within the thymus
- In intersinusoidal bone marrow, hematopoietic stem cells mature to early lymphoid progenitors, the pro B, pre B stages
- Developmental stages are in close contact with slender CD10+ stromal cells or their extensions, which allows tightly regulated signaling (J Pathol 2005;205:311)
- Earliest stem cells are in subendosteum, adjacent to inner bone surface; with maturation, B lineage cells move towards central axis of marrow; final stages of development of immature B cells occur in peripheral lymphoid organs (spleen, lymph nodes)
- Develop from bone marrow, become prothymocytes, then migrate to thymus gland, where self recognizing T cells are eliminated
T cells:
Pathophysiology
-
B cells:
- B cells express surface immunoglobulin (Ig), composed of 2 heavy (H) and 2 light (L) chains (either kappa or lambda)
- B cell antigen receptor loci may have 4 types of modification: (a) recombination of variable, diversity and joining regions (VDJ); (b) somatic hypermutation of V segments; (c) immunoglobulin heavy chain gene class switching; and (d) receptor editing
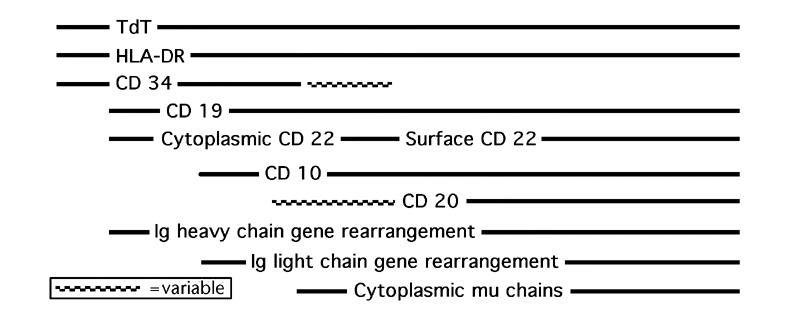
- Early B cell precursor is TdT+, CD34+, HLA-DR+, then undergoes heavy (H) chain rearrangement and adds CD19, then adds CD10, then adds IgM heavy chain; then adds light (L) chain rearrangement and adds cytoplasmic IgM with heavy and light chains, then B cells express IgM and IgD with the same binding site, then adds CD20 (now called pre B cell); then adds surface Ig, then adds CD21 and CD22 and drops TdT (now called B cell)
- If B cell encounters an antigen that interacts with its variable region, it becomes a plasma cell
- Precursor B cells contain immunoglobulin related components but not immunoglobulin; express CD179a and CD179b (precursor to light chains) as part of their pre B cell receptor, which disappears when replaced with conventional light chains
- B cells express surface immunoglobulin, consisting of 2 heavy chains and 2 light chains (kappa or lambda); immunoglobulin is associated with CD79a / CD79b complex to form a B cell antigen receptor complex
- IgH gene (heavy chain of immunoglobulin) is at 14q32; variable portion is coded by VDJ regions
- IgL gene (light chain of immunoglobulin): kappa is at 2p11, lambda is at 22q11; no diversity region is present
- Heavy chain isotype switch: determines if immunoglobulin is IgM, IgD, IgG1-4, IgA1-2 or IgE (9 constant regions); mediated by switch genes
- T cell receptors (TCR) are either alpha / beta (95%) or gamma / delta (5%) heterodimers
- T cell progenitors migrate to the thymus and undertake a highly ordered developmental program, regulated by signals derived from microenvironment
- Most immature thymocytes are CD4- / CD8- ("double negative" / DN); DN is divided into 4 stages based on CD44 / CD25 expression: DN1 is CD44+, CD25-; DN2 is CD44+, CD25+; DN3 is CD44-, CD25+; DN4 is CD44-, CD25- (Immunol Res 2010;47:45)
- Precursor cell is TdT+, CD34+, HLA-DR+, then drops HLA-DR, then adds CD2, CD5, CD7 (early thymocyte) while undergoing gamma / beta chain rearrangement, then adds CD1 and drops CD34, now a common thymocyte (CD4-, CD8-, "double negative"), then undergoes beta / alpha chain rearrangement (DN3 stage) and adds CD4 and CD8, then splits into helper (CD4) or cytotoxic (CD8) T cell ("single positive") with CD2 and CD3, and without TdT, CD1, CD5 and CD7
- T alpha and delta genes are on 14q11; T beta gene is on 7q34; T gamma gene is on 7p15
- Note: T cells and NK cells arise from common progenitor that expresses CD3 epsilon and cannot develop into B cells
T cells:
Diagrams / tables
AFIP images
Images hosted on other servers:
Clinical features
-
B cells:
- B cell lymphomas: a clonal light chain rearrangement is usually specific for the presence of a B cell neoplasm
- X linked agammaglobulinemia: no B cell development
- Defects in receiving T cell signals can cause hyper IgM syndrome (J Allergy Clin Immunol 2010;125:778)
- 90% of peripheral T cell lymphomas have rearrangements of T alpha, beta and gamma, including all cases of mycosis fungoides and Sezary syndrome
- T cell lymphomas have no distinct marker of clonality, but cells may express an abnormal immunophenotype or TCR gene rearrangement
- As there are only 10 V (variable) regions, a polyclonal population of cells can appear oligoclonal
- T cell clonality is seen in AIDS and congenital immunodeficiency syndromes, but does NOT indicate malignancy
- Rarely a clonal band may comigrate with the germline band; solution: use 2 - 3 restriction enzymes (HindIII, EcoRI, BamHI)
T cells:
Positive stains
Additional references
Castleman disease
Table of Contents
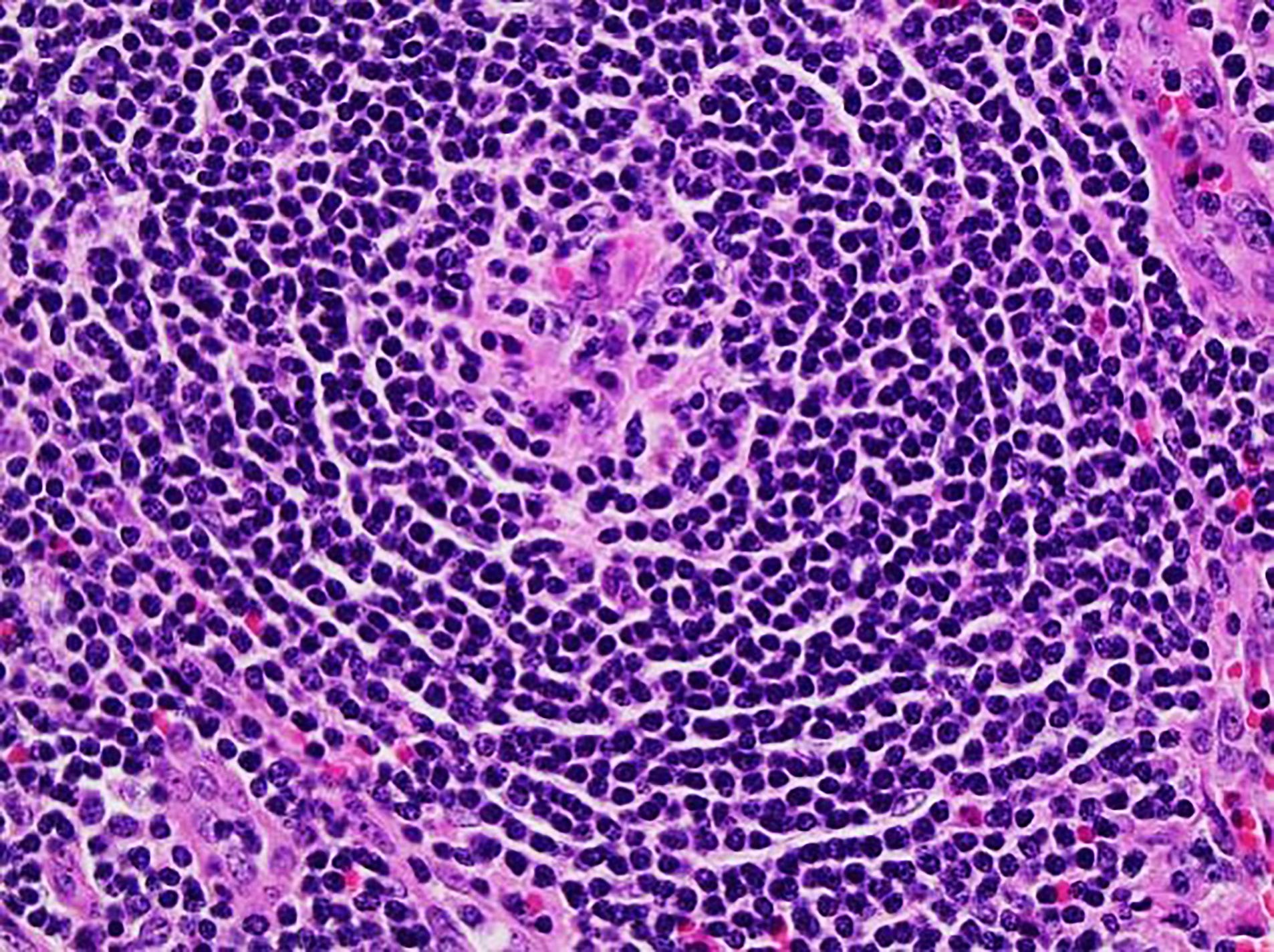
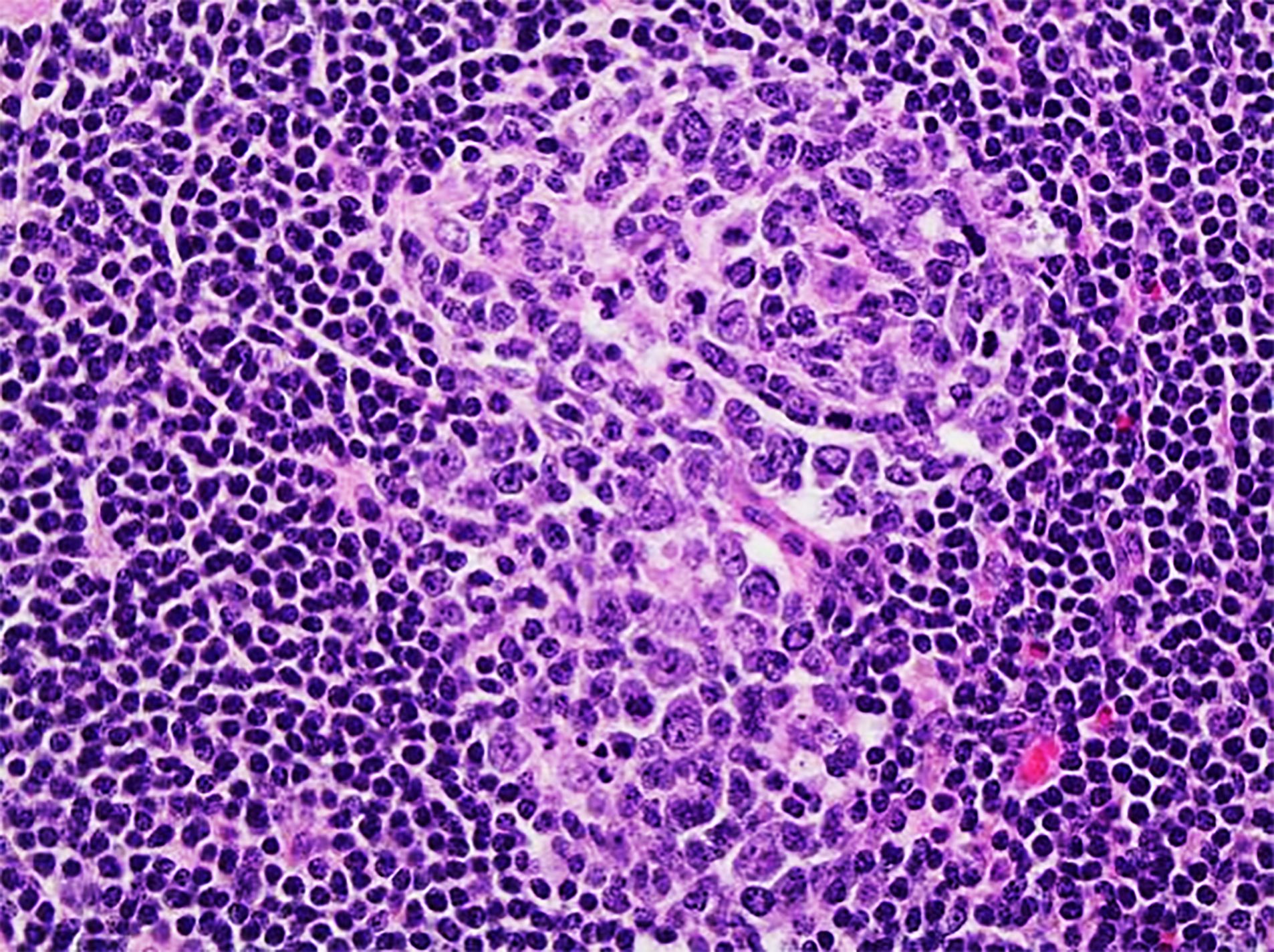
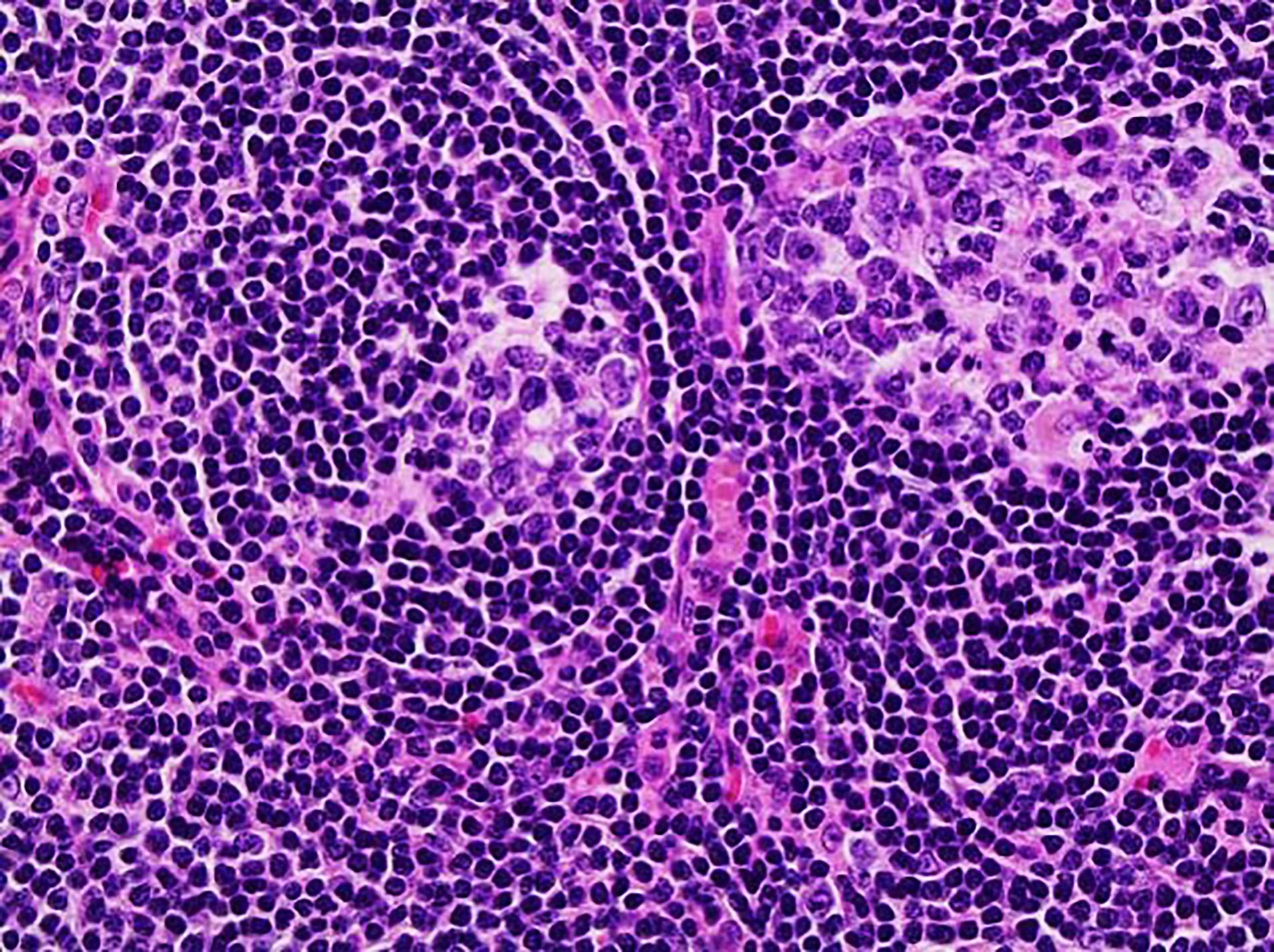
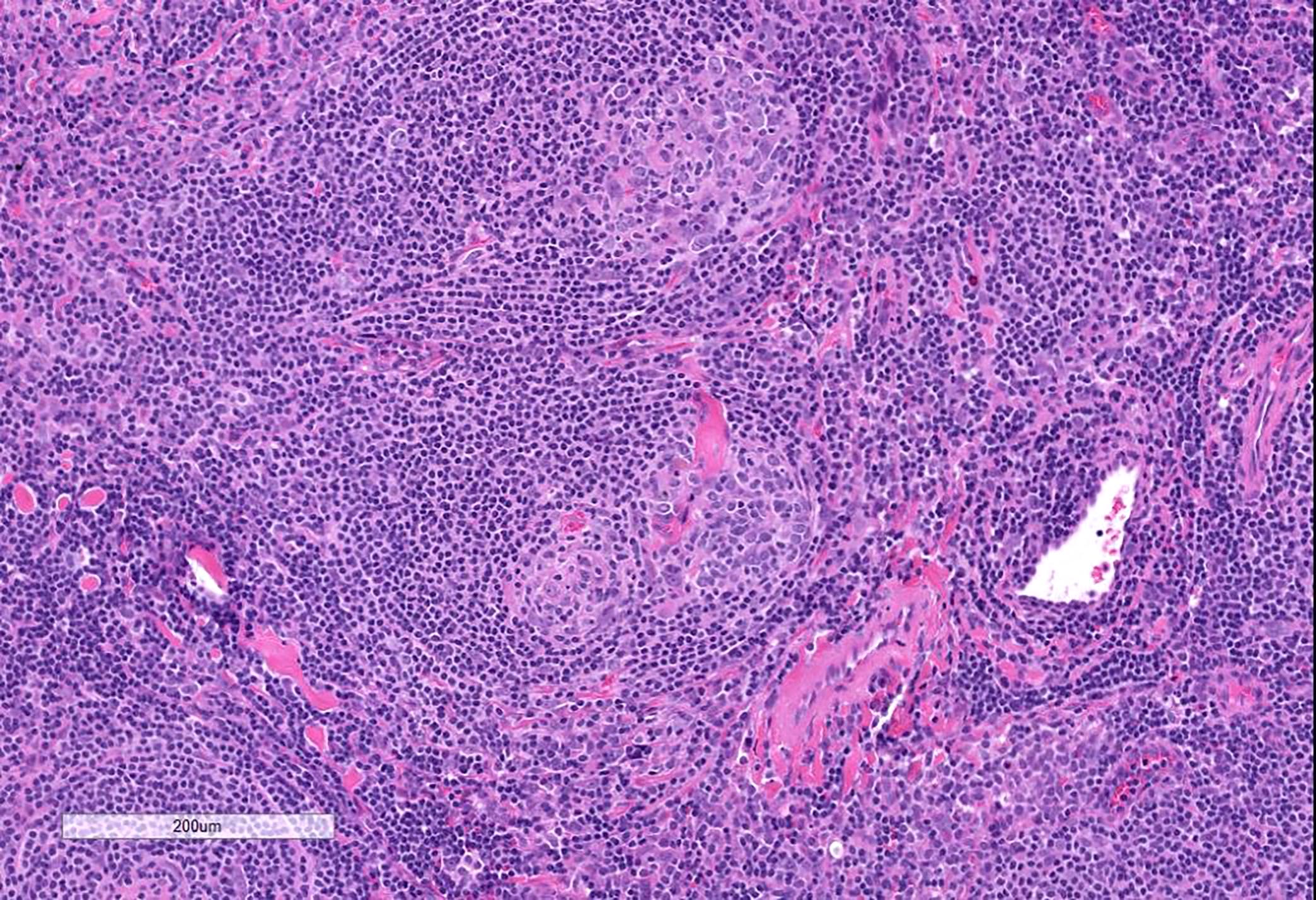
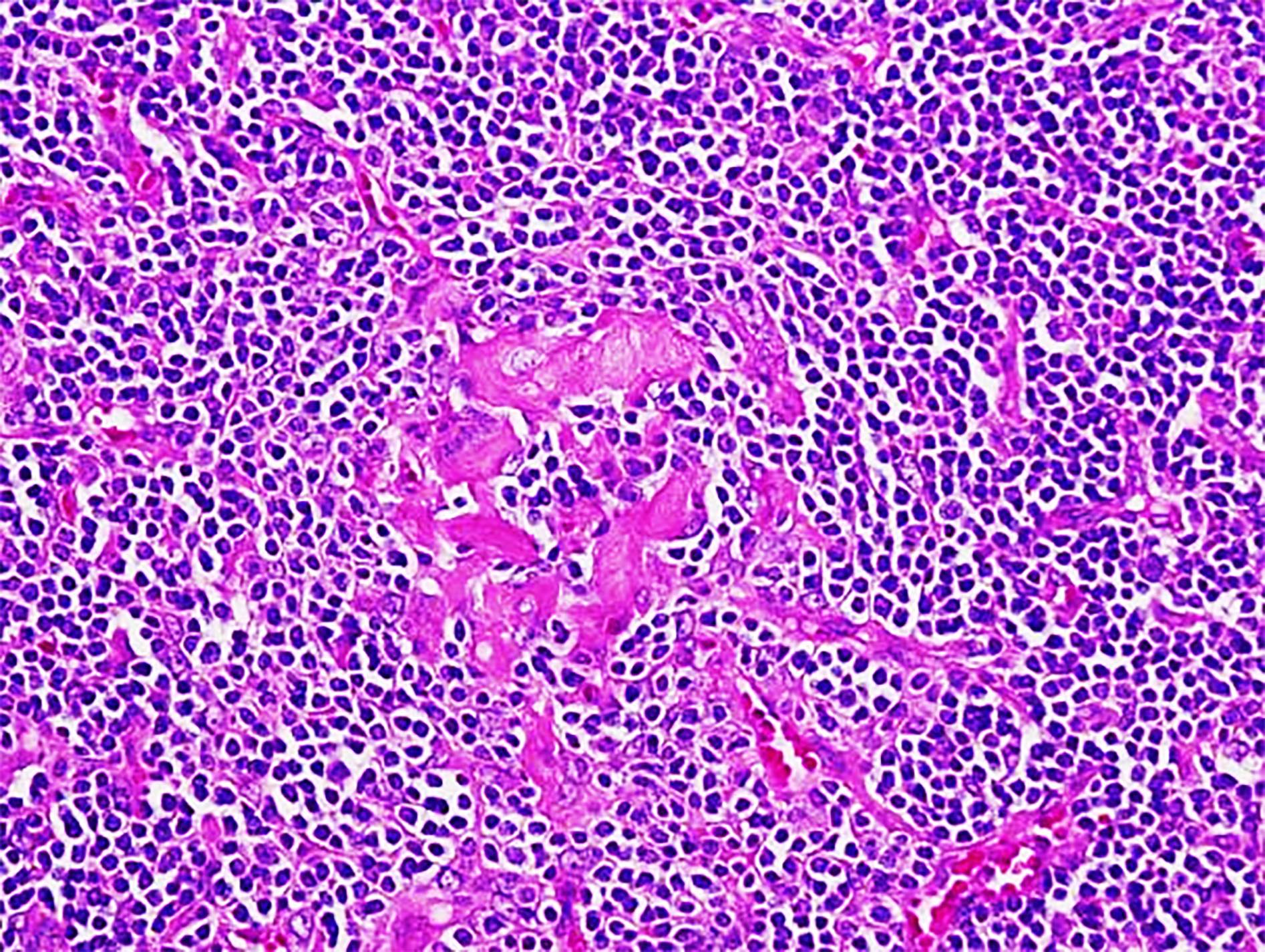
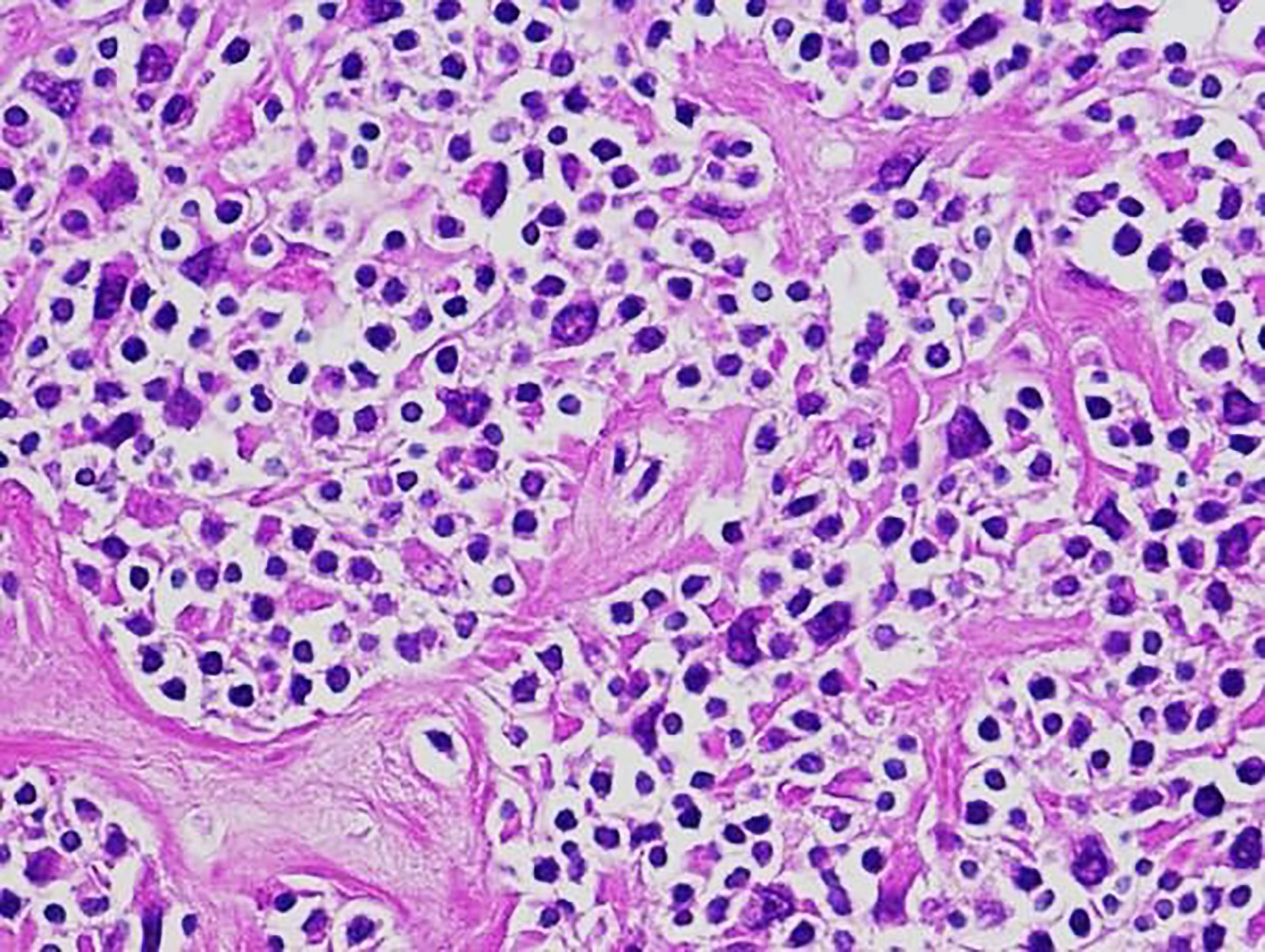
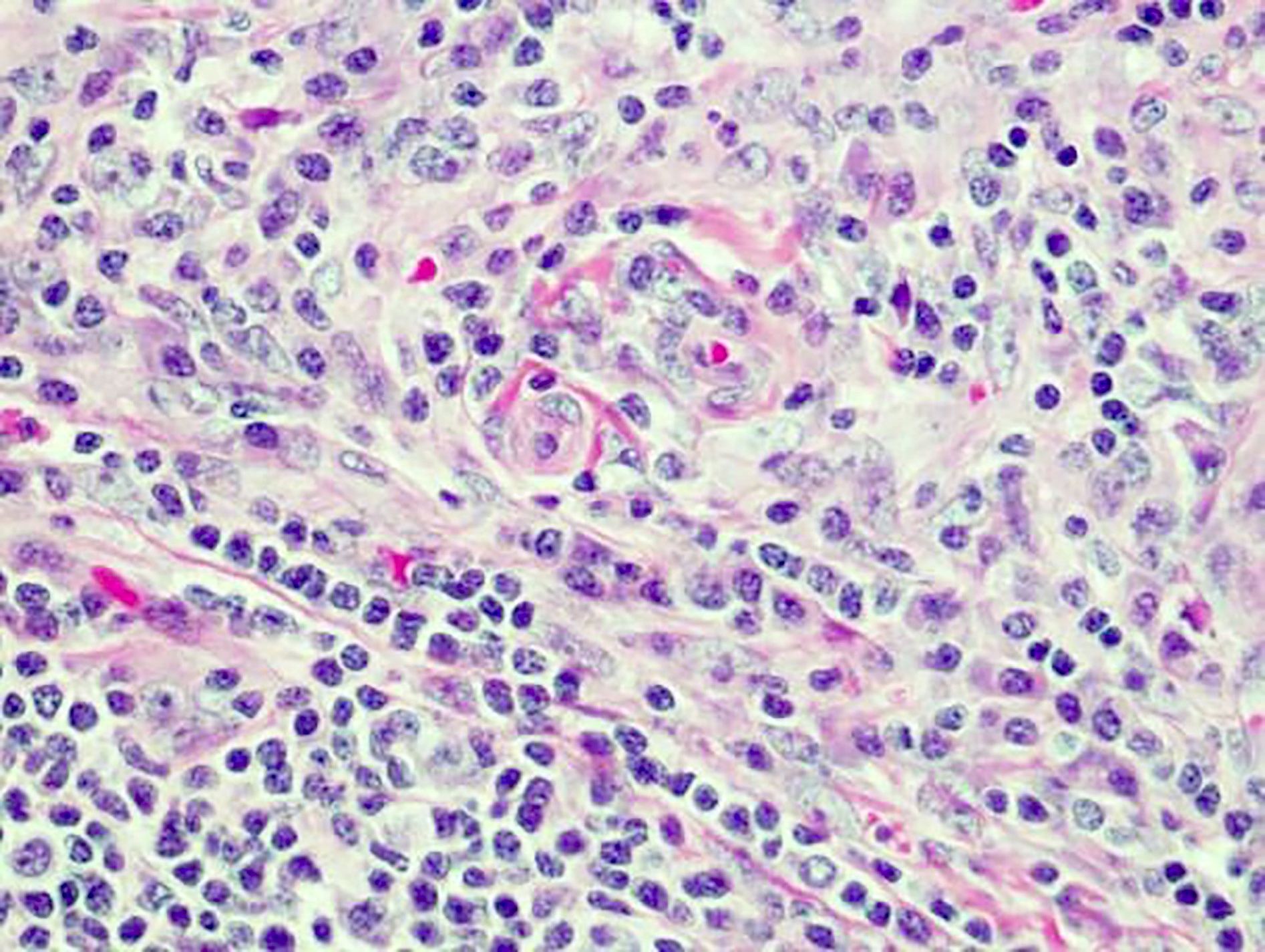
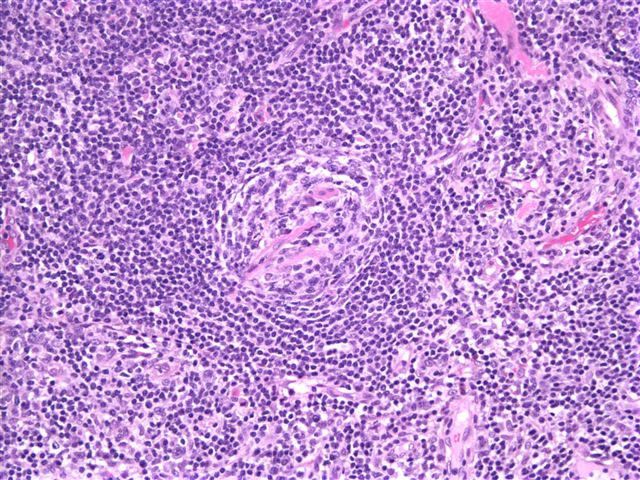
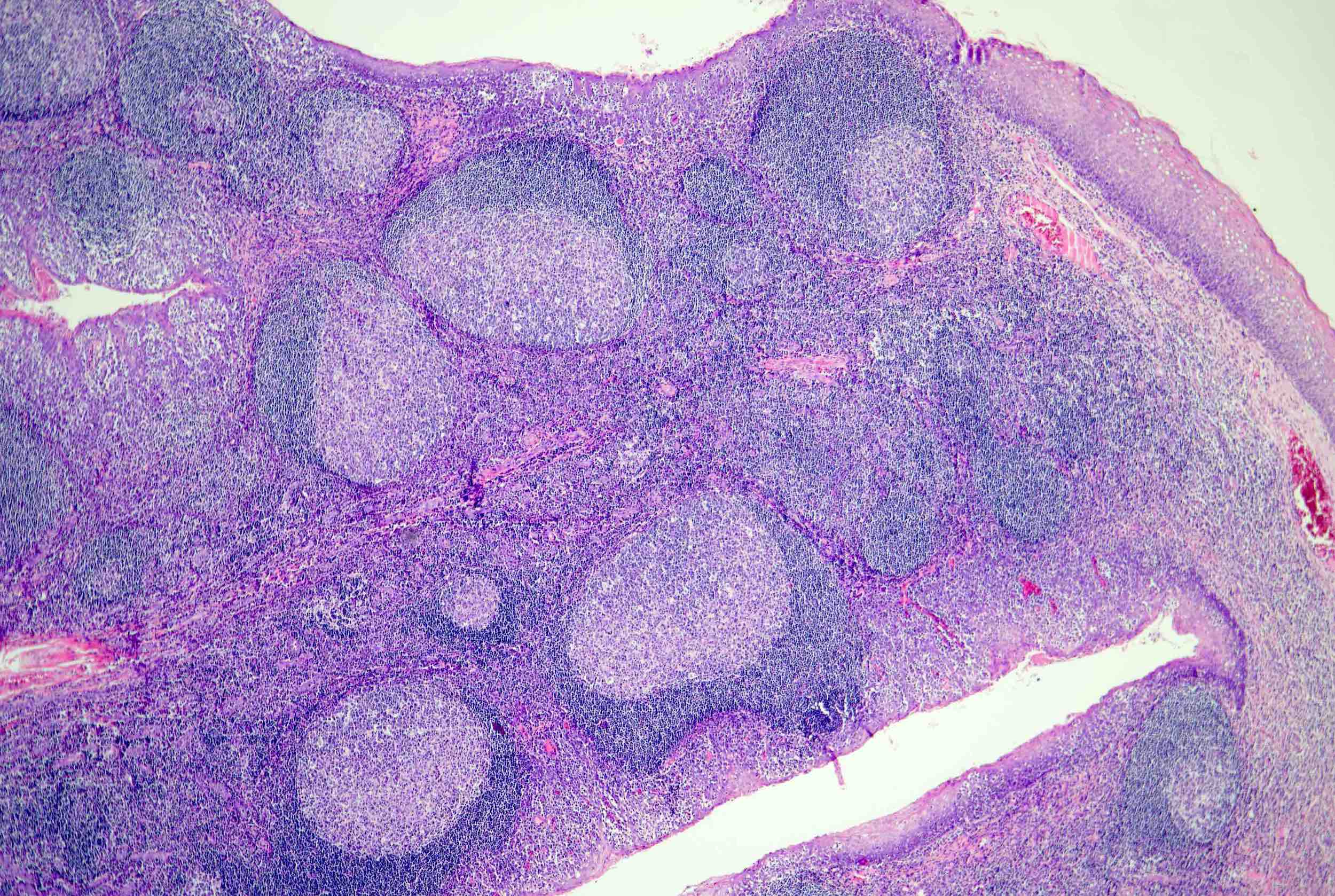
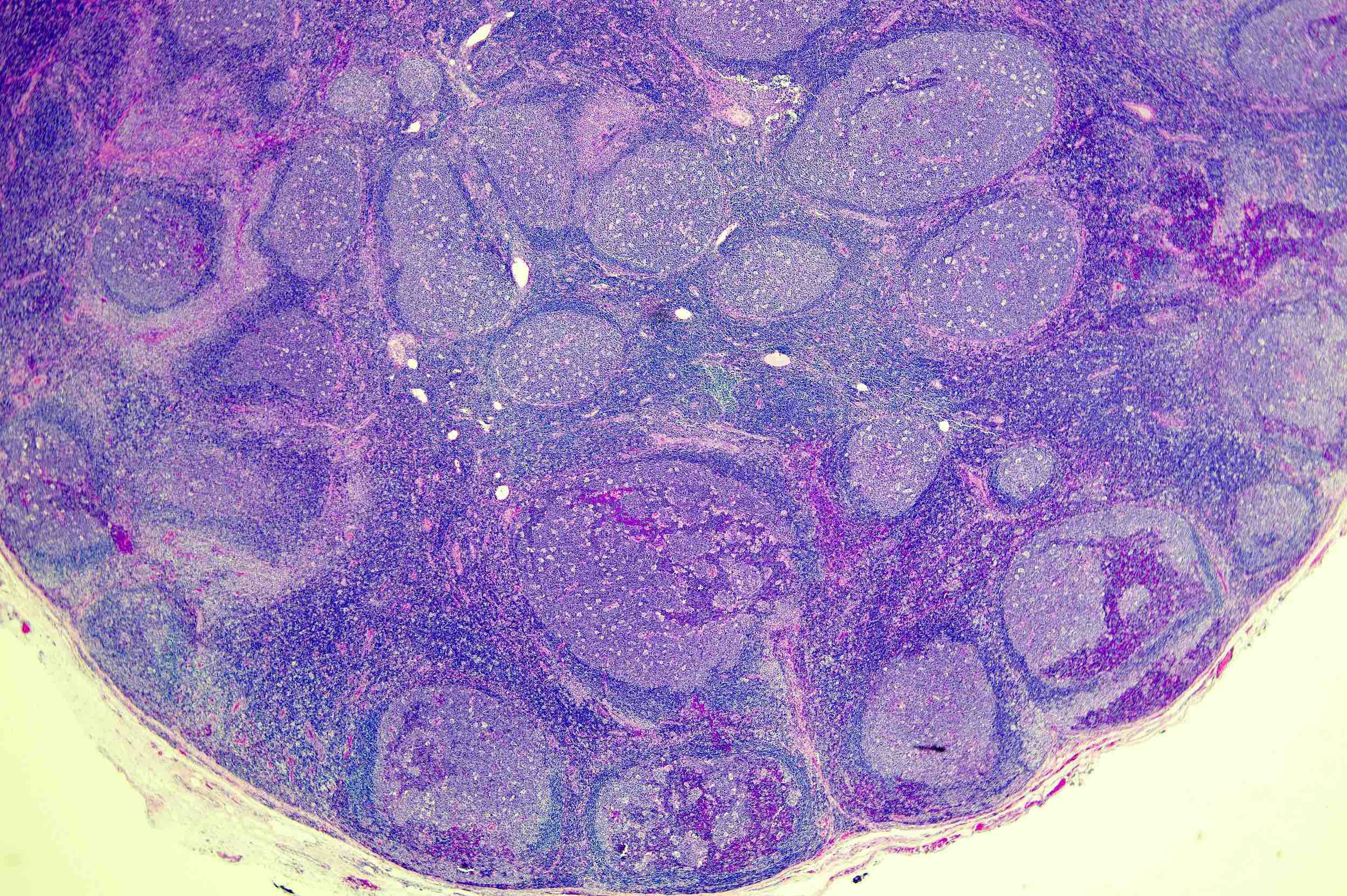
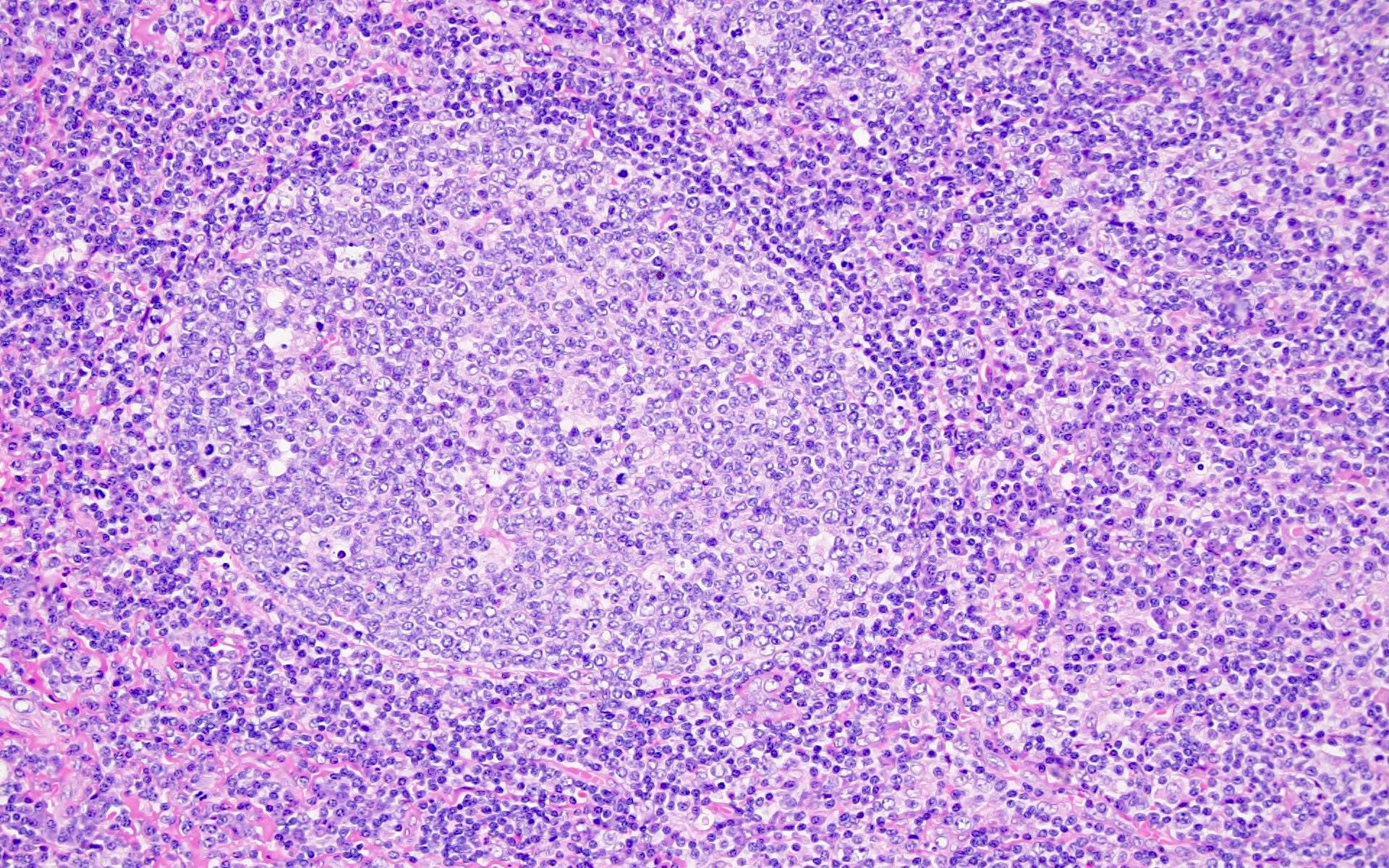
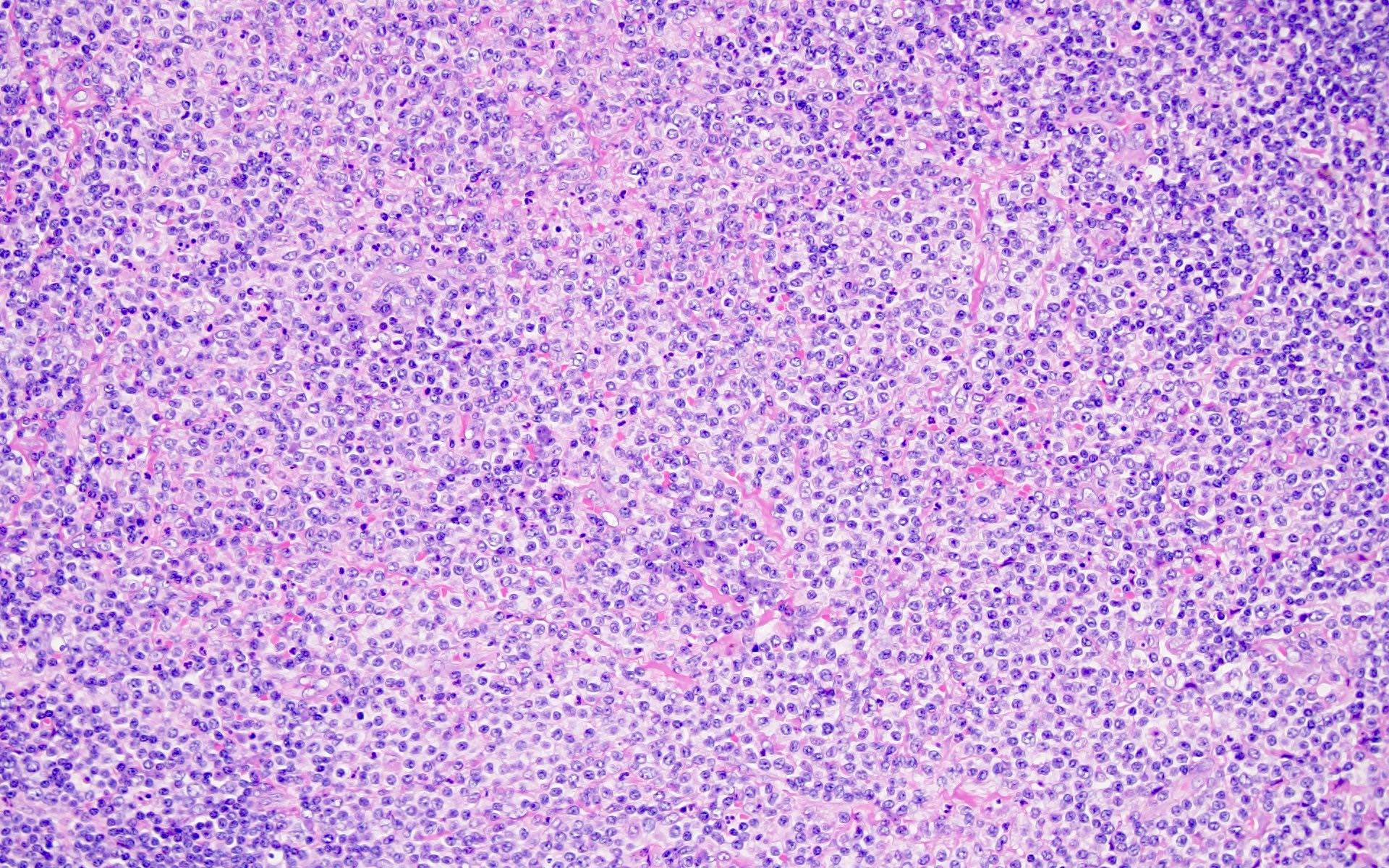
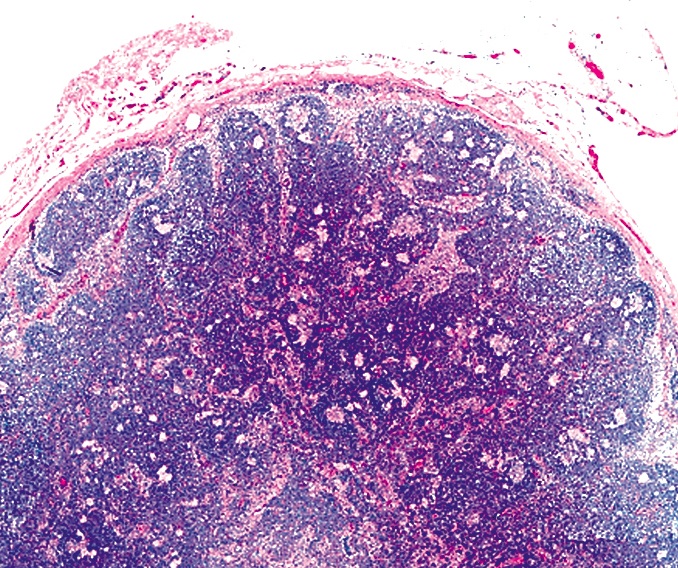
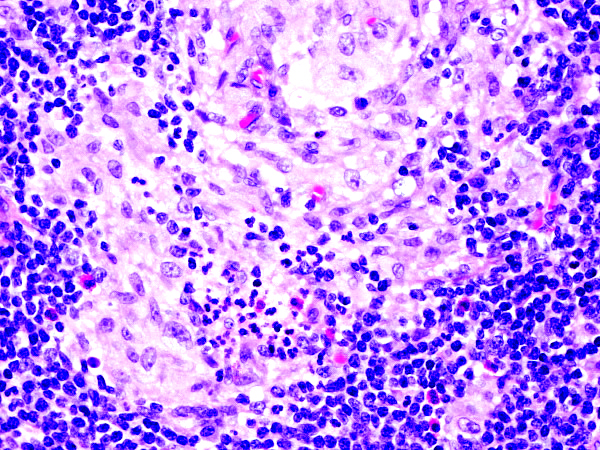
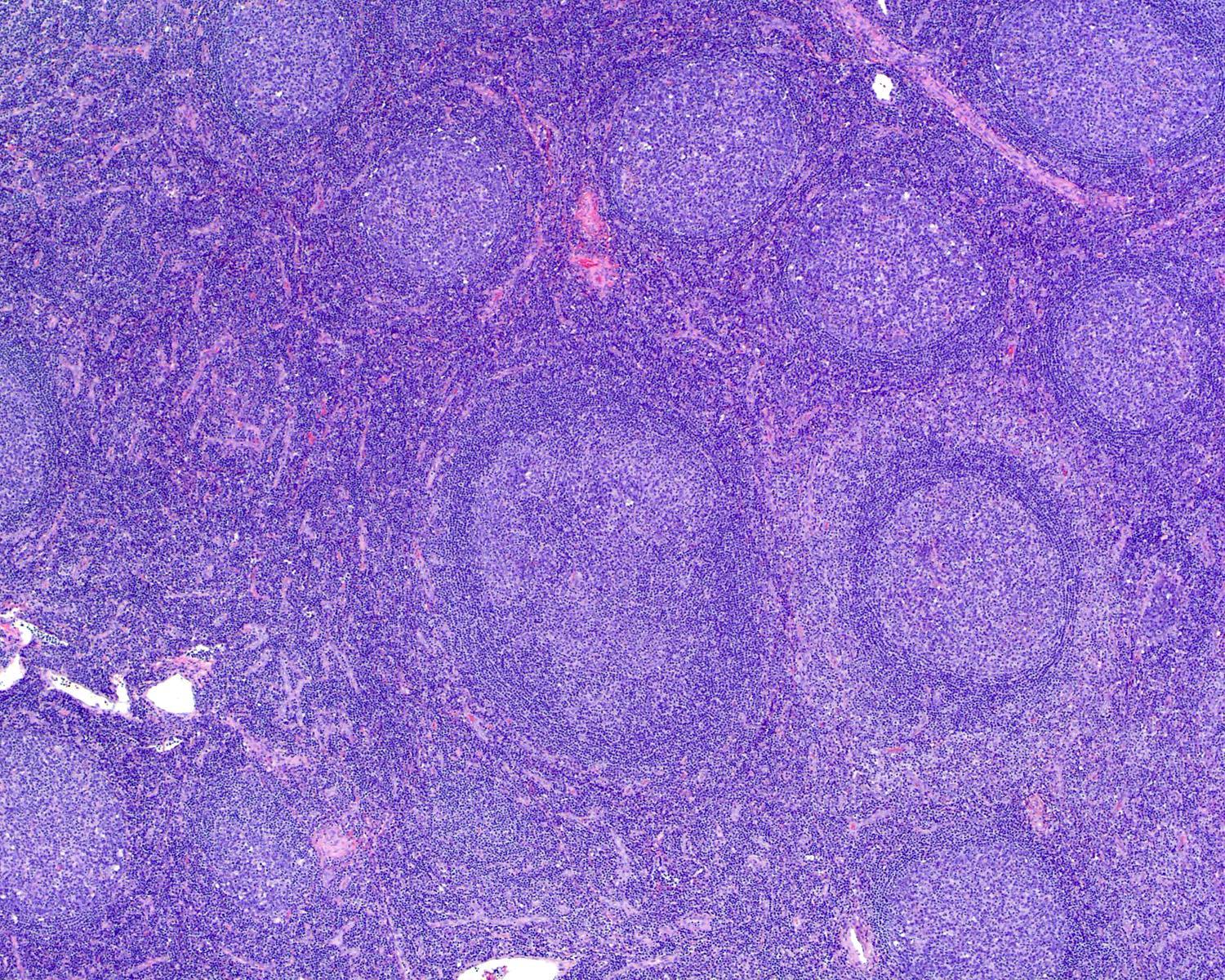
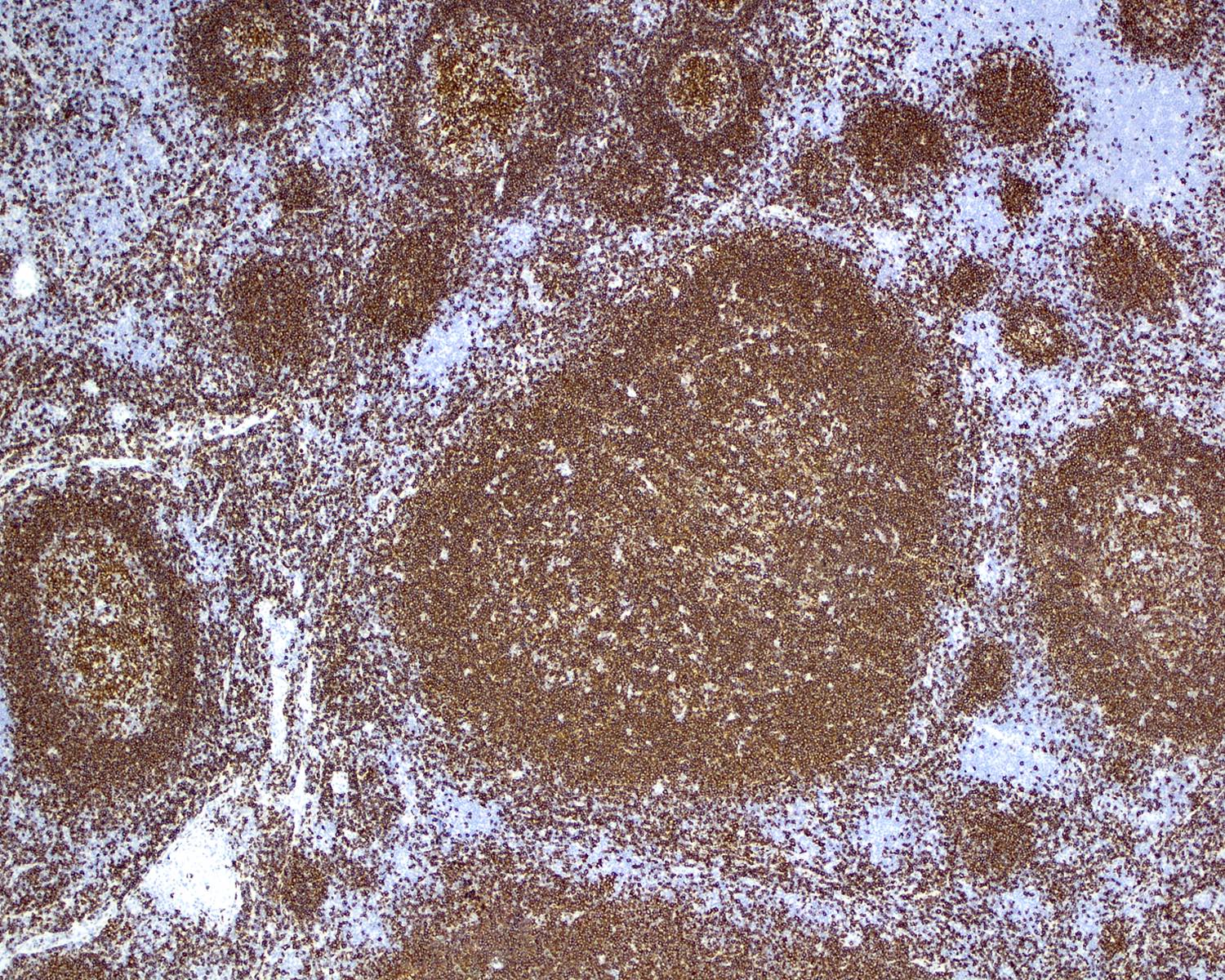
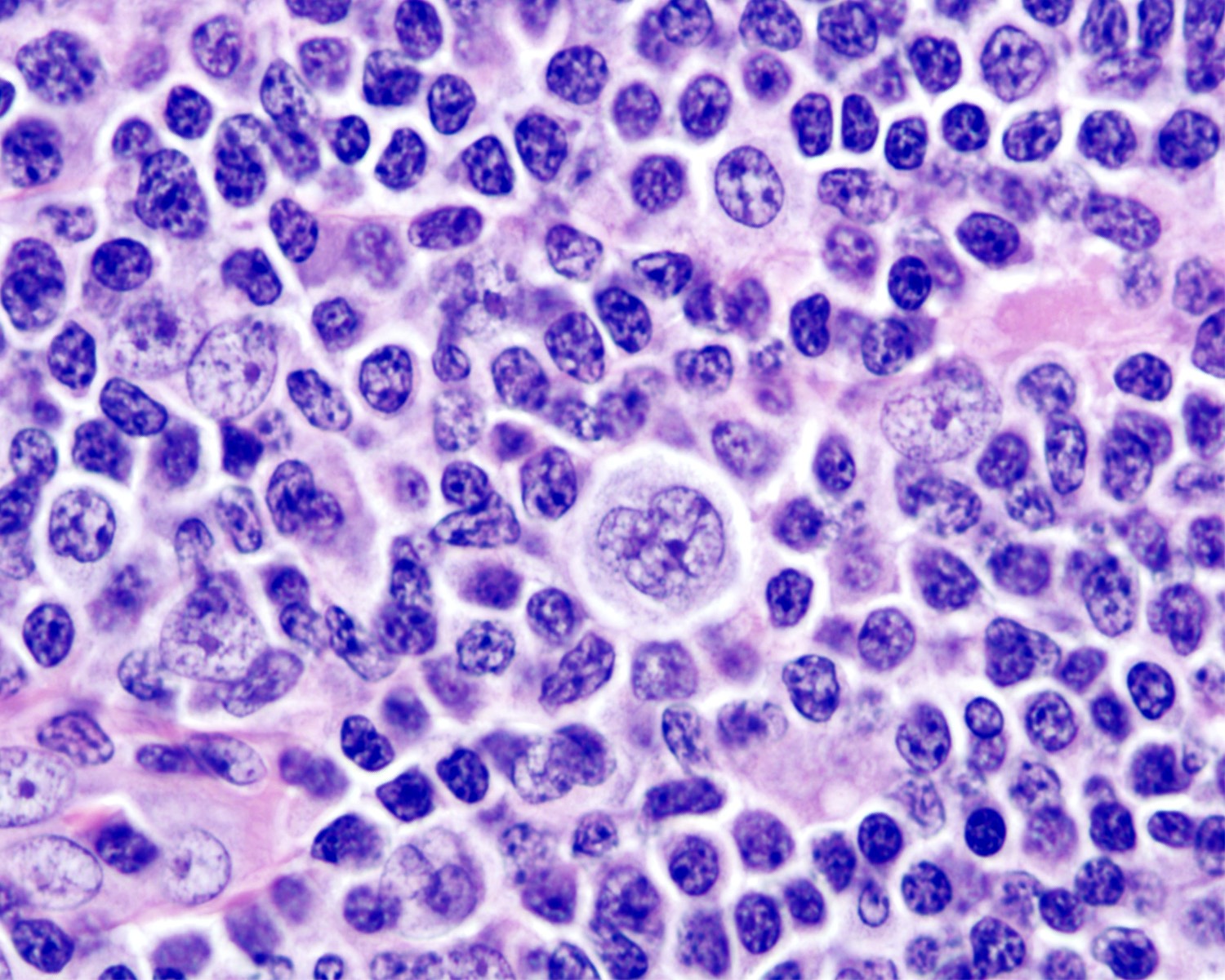
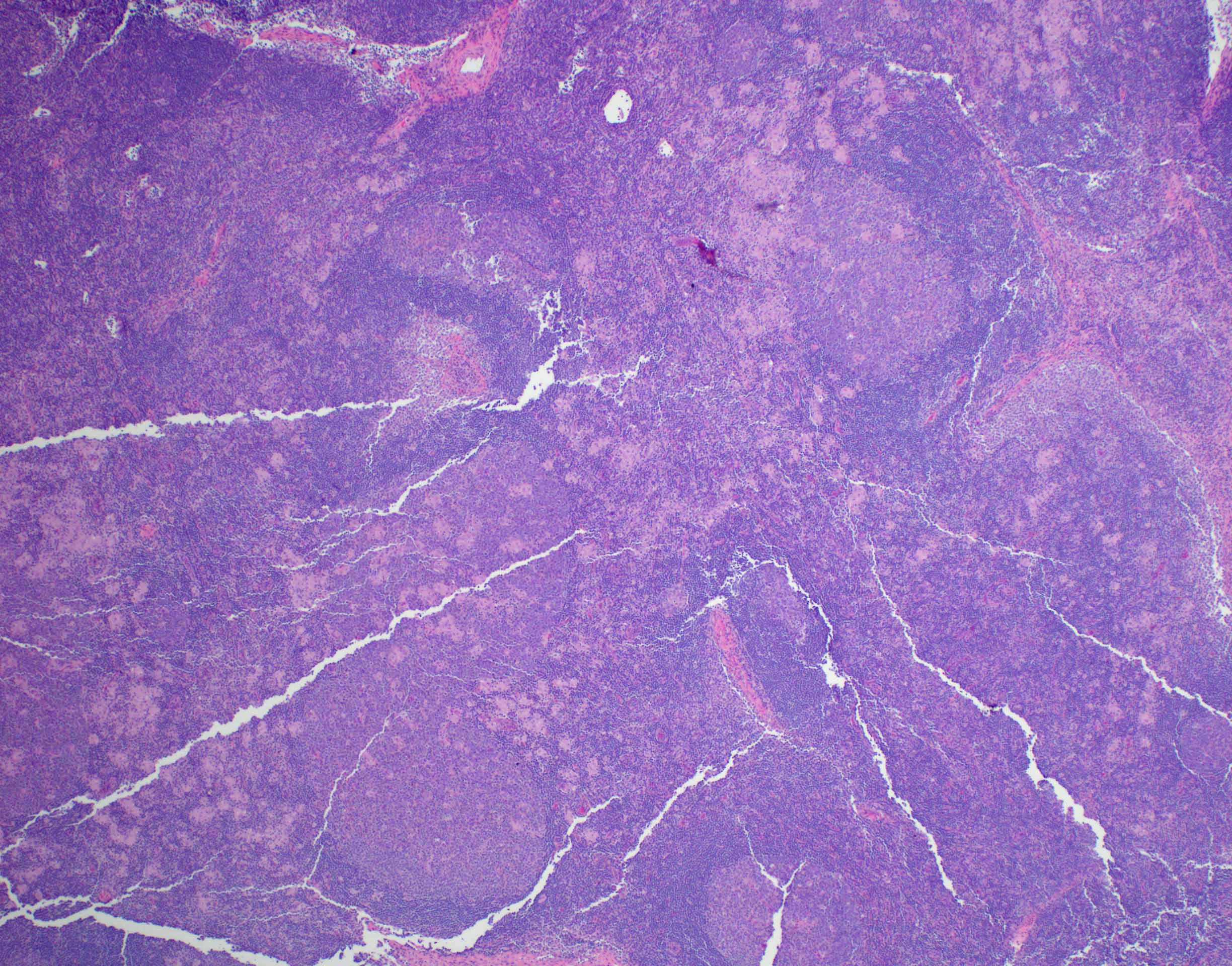
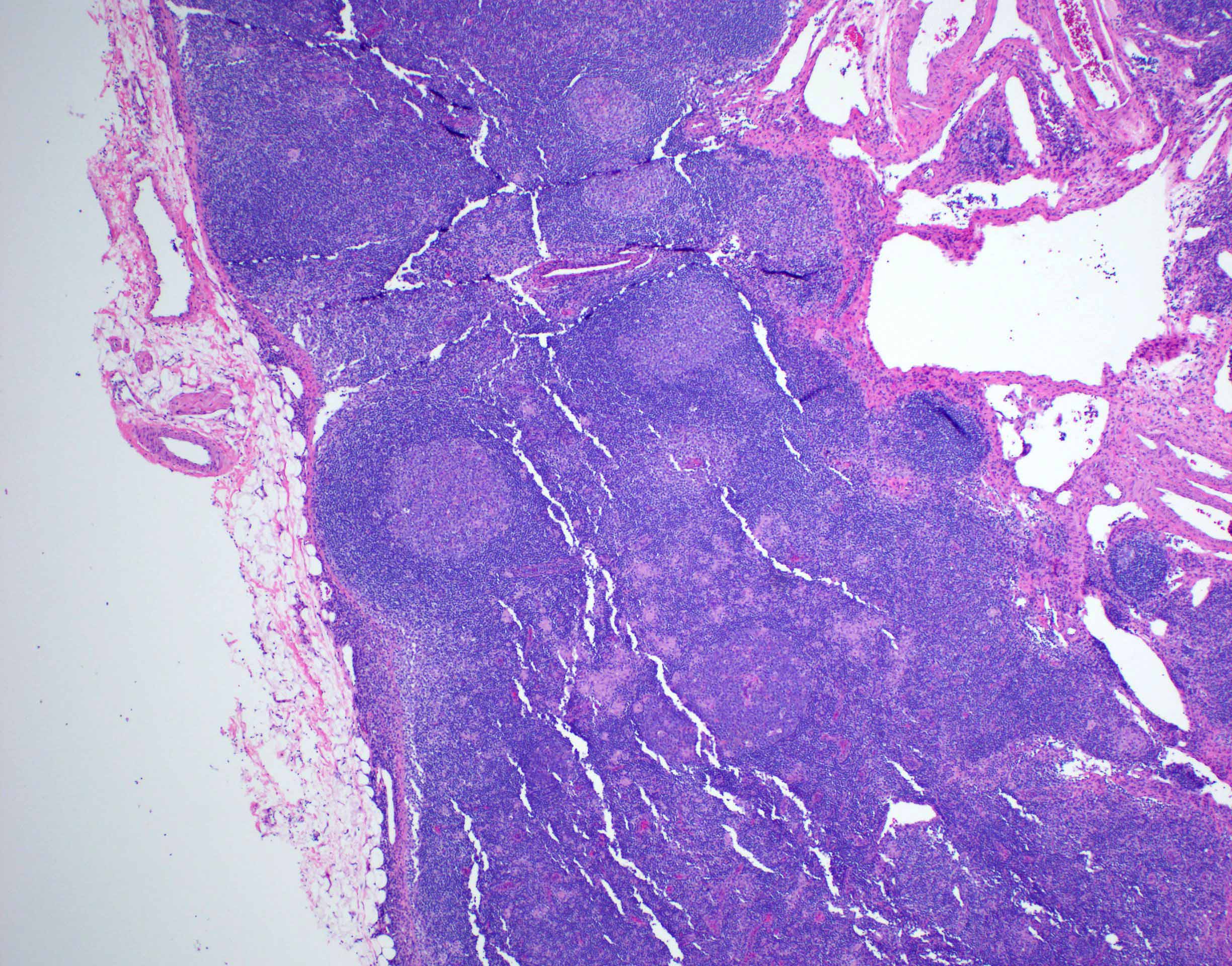
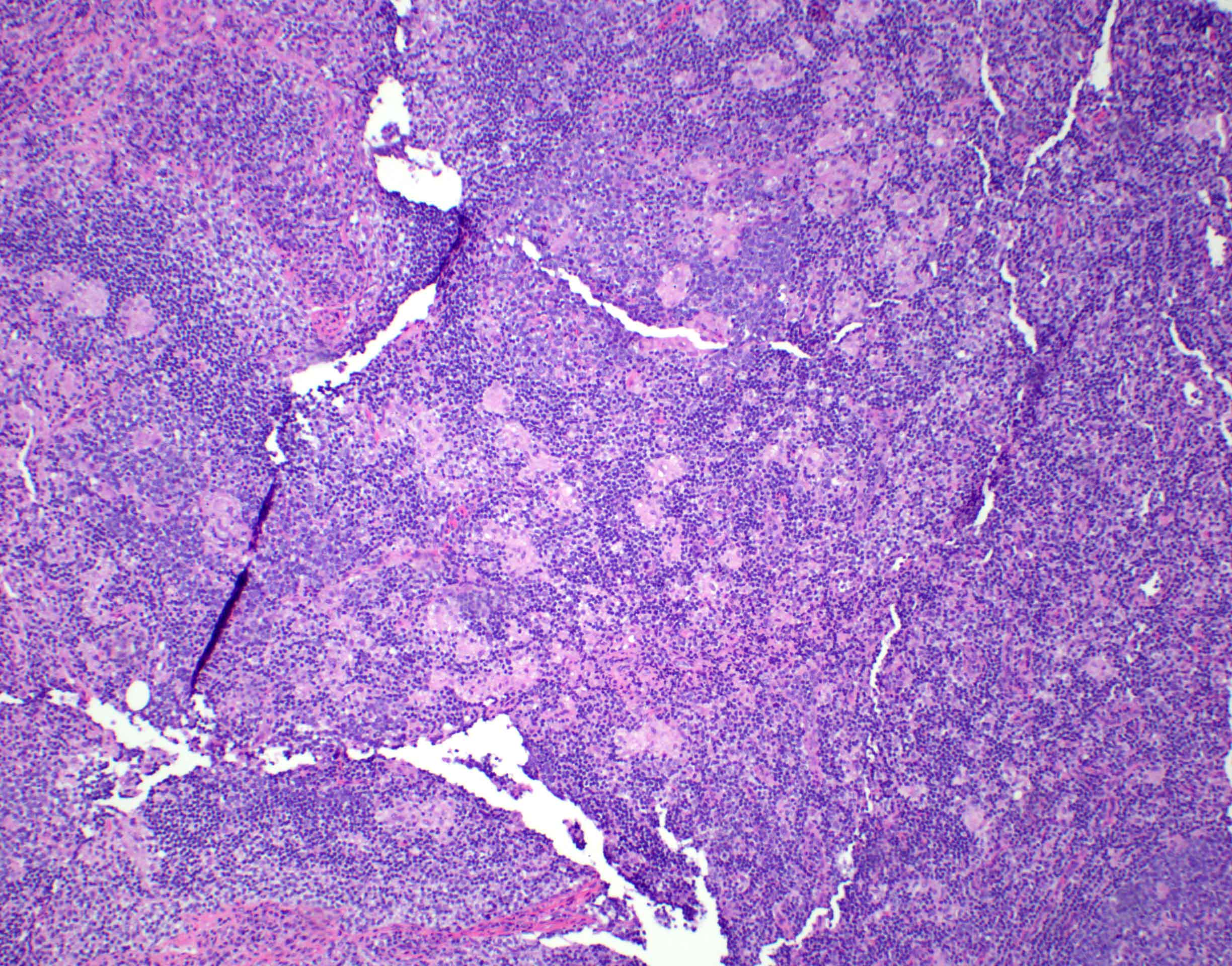
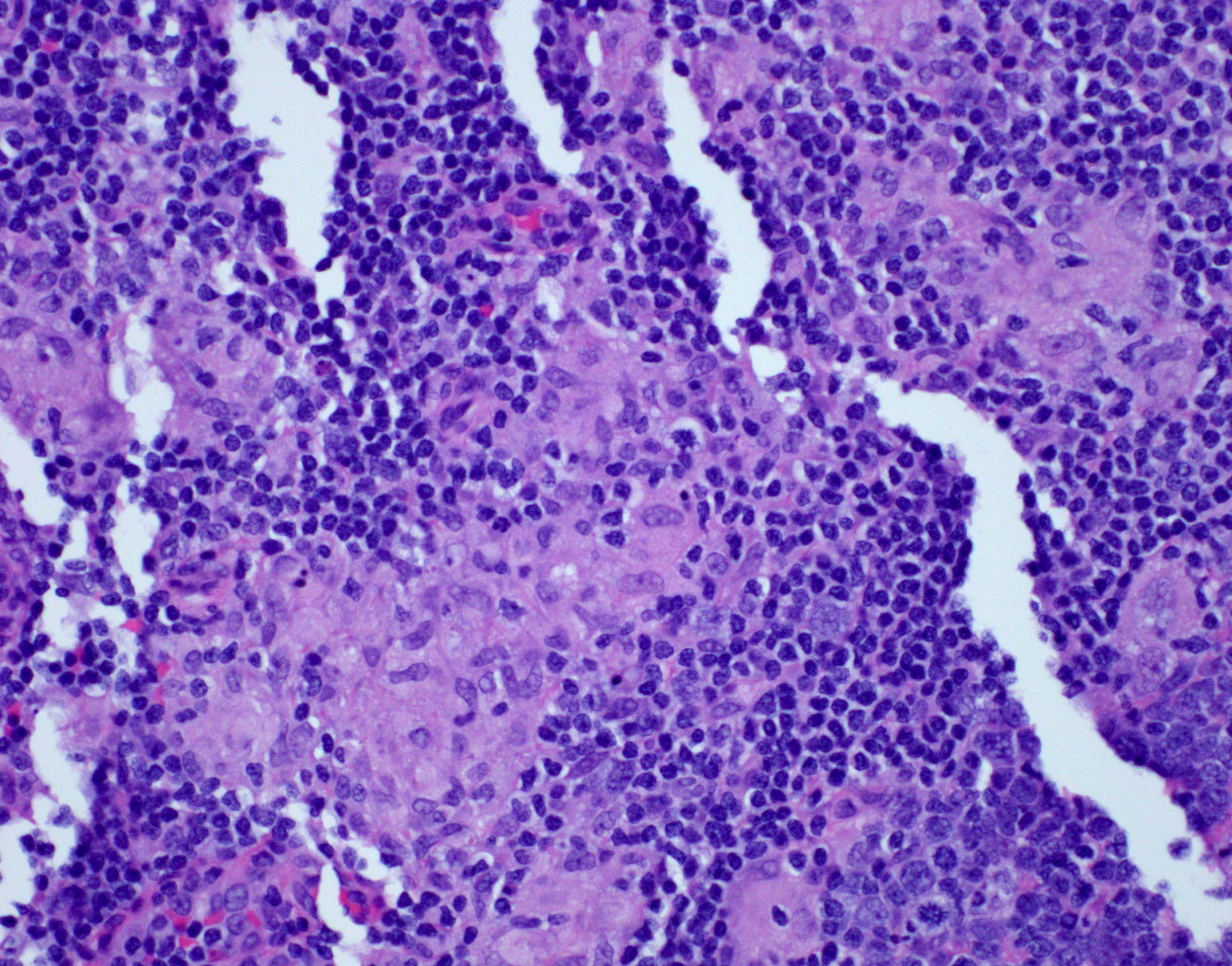
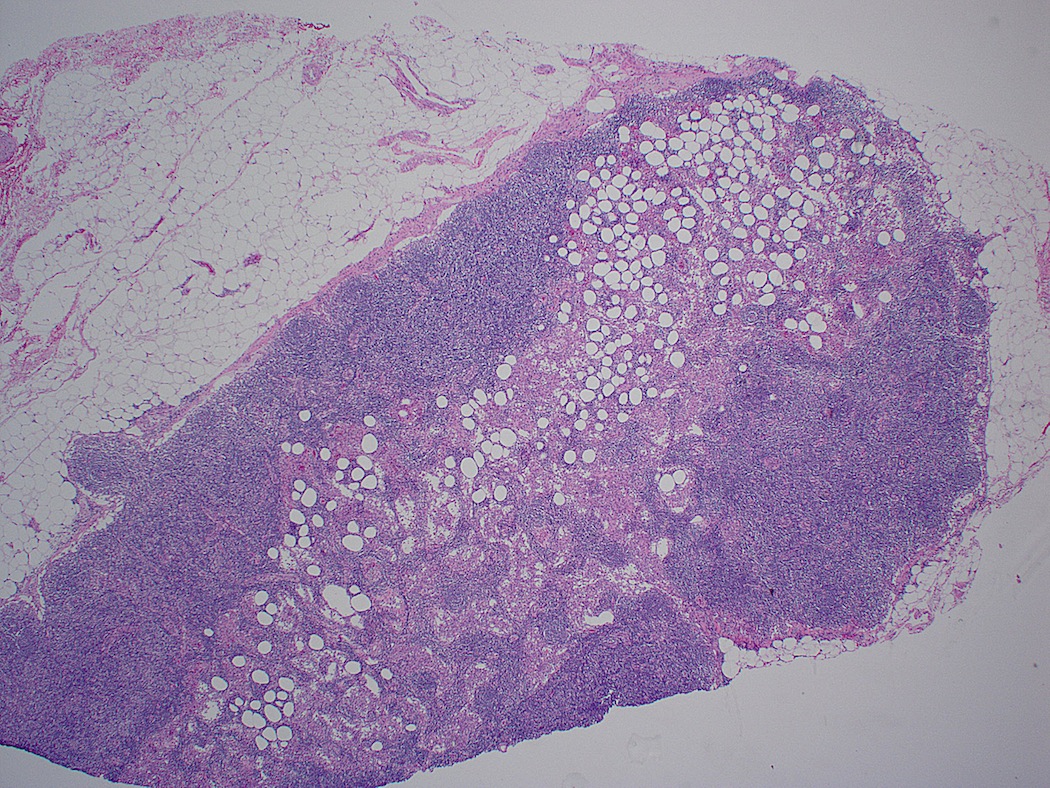
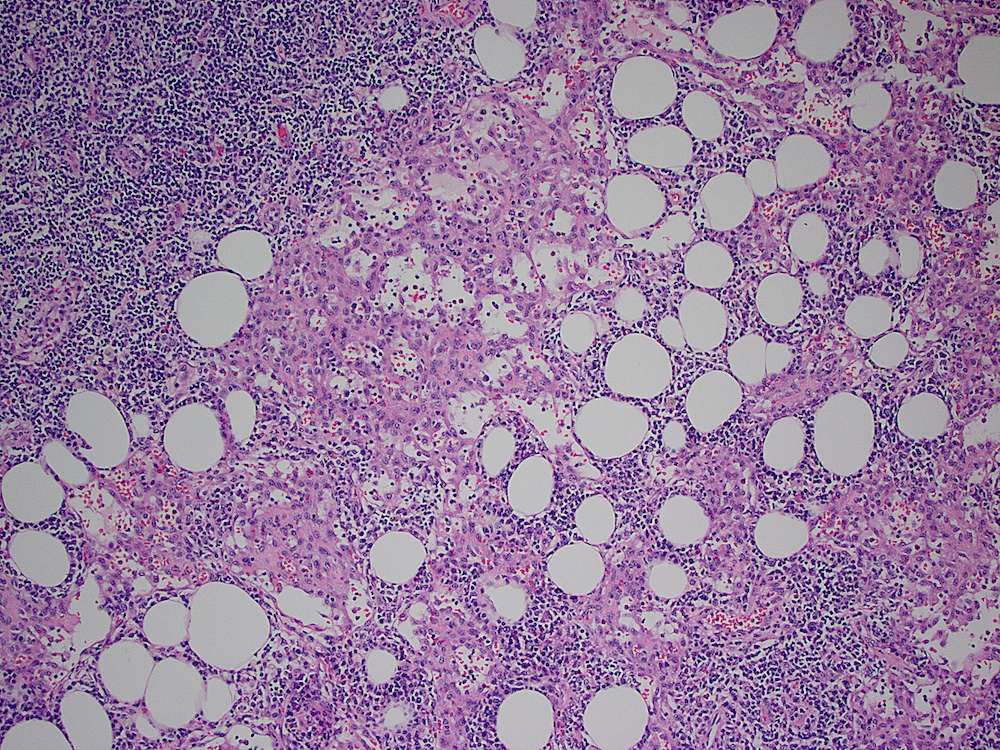
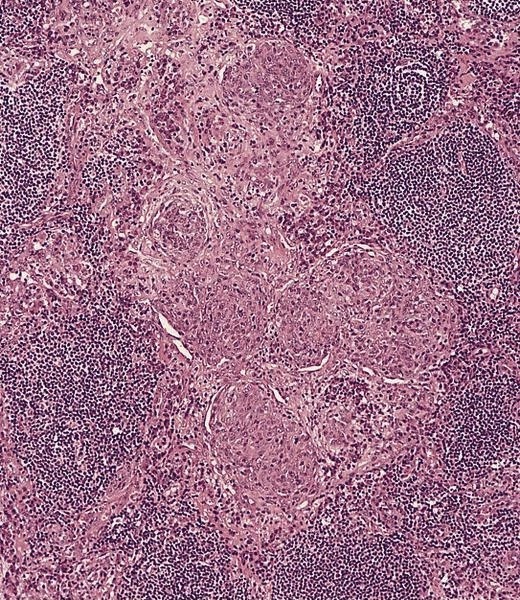
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
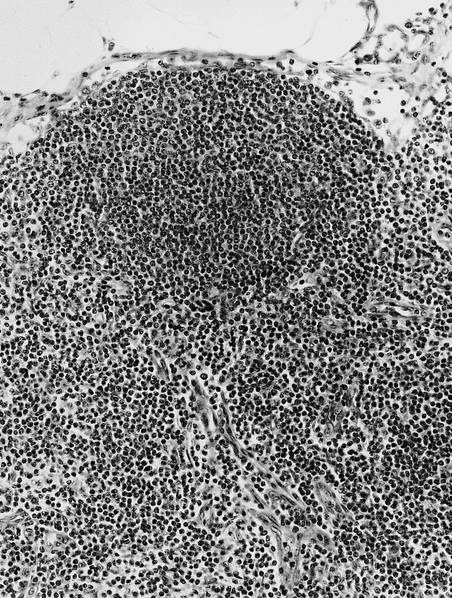
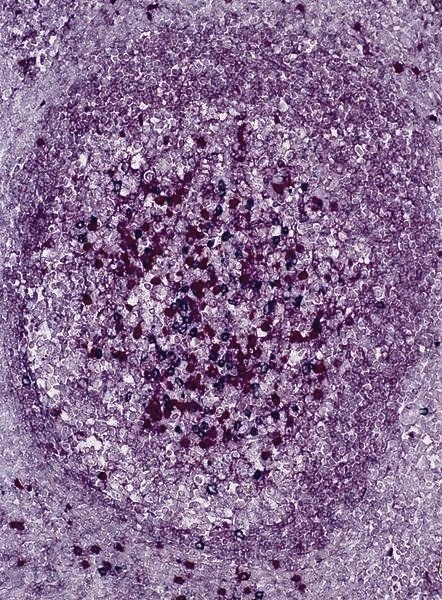
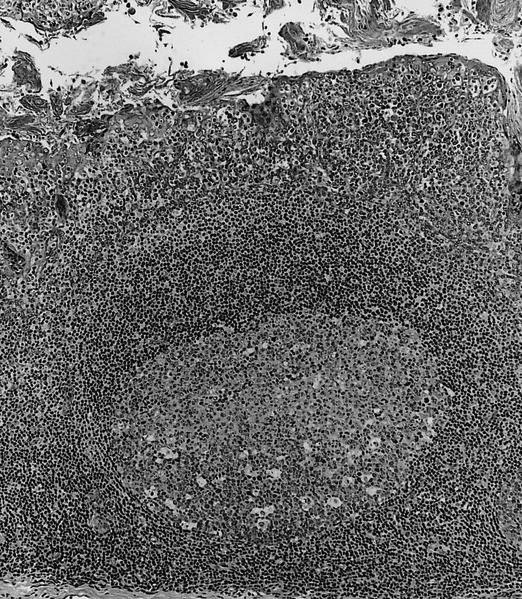
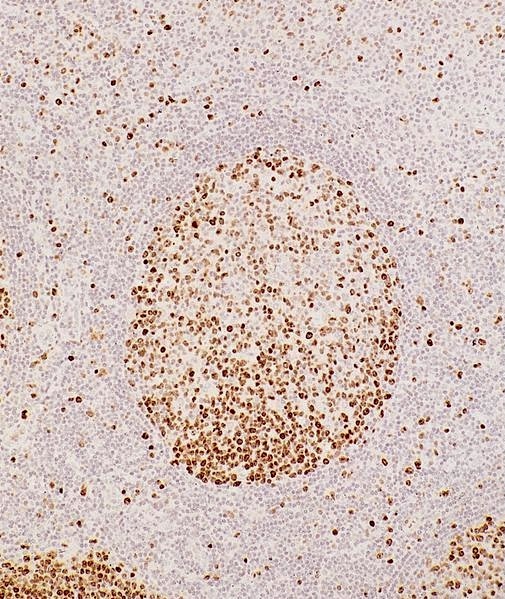
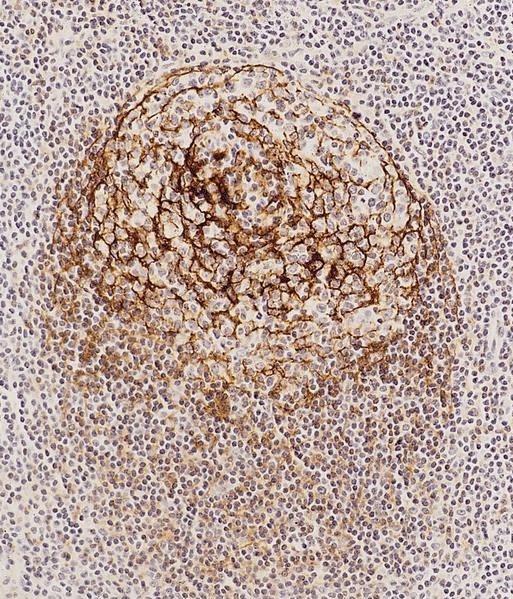
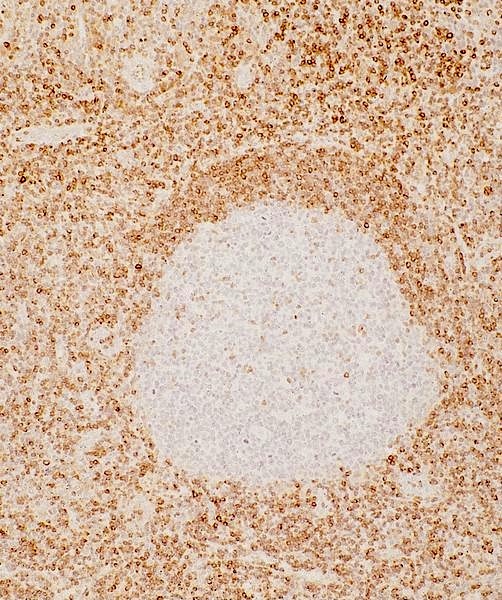
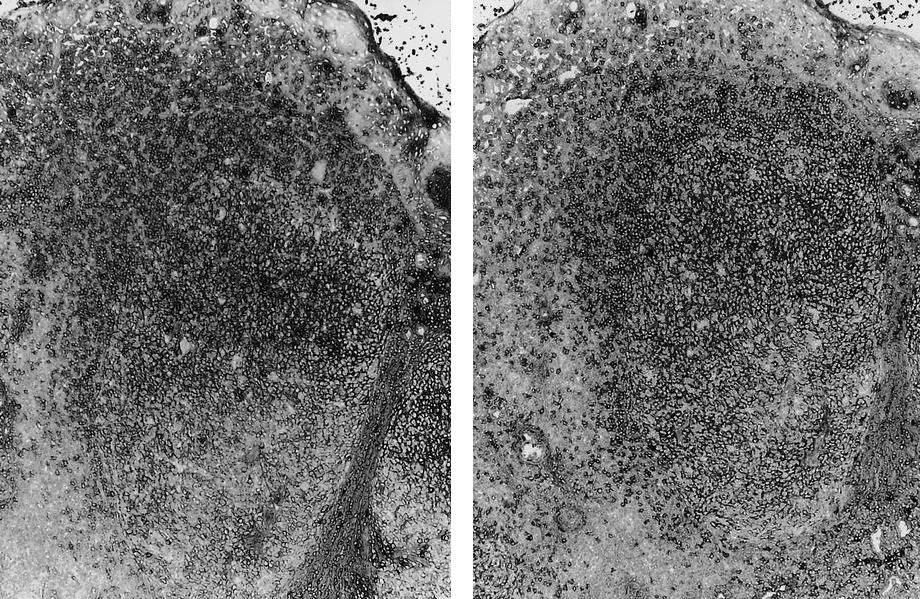
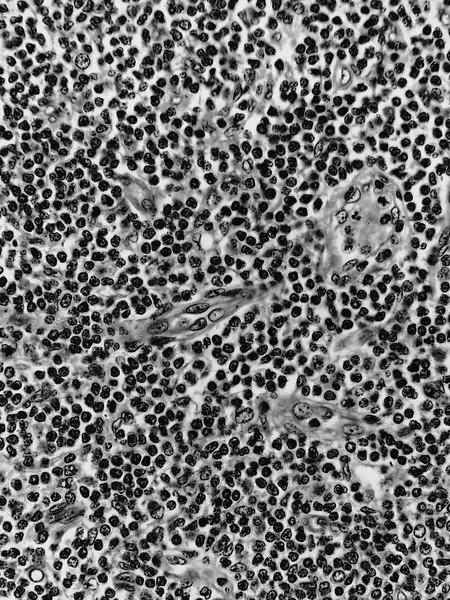
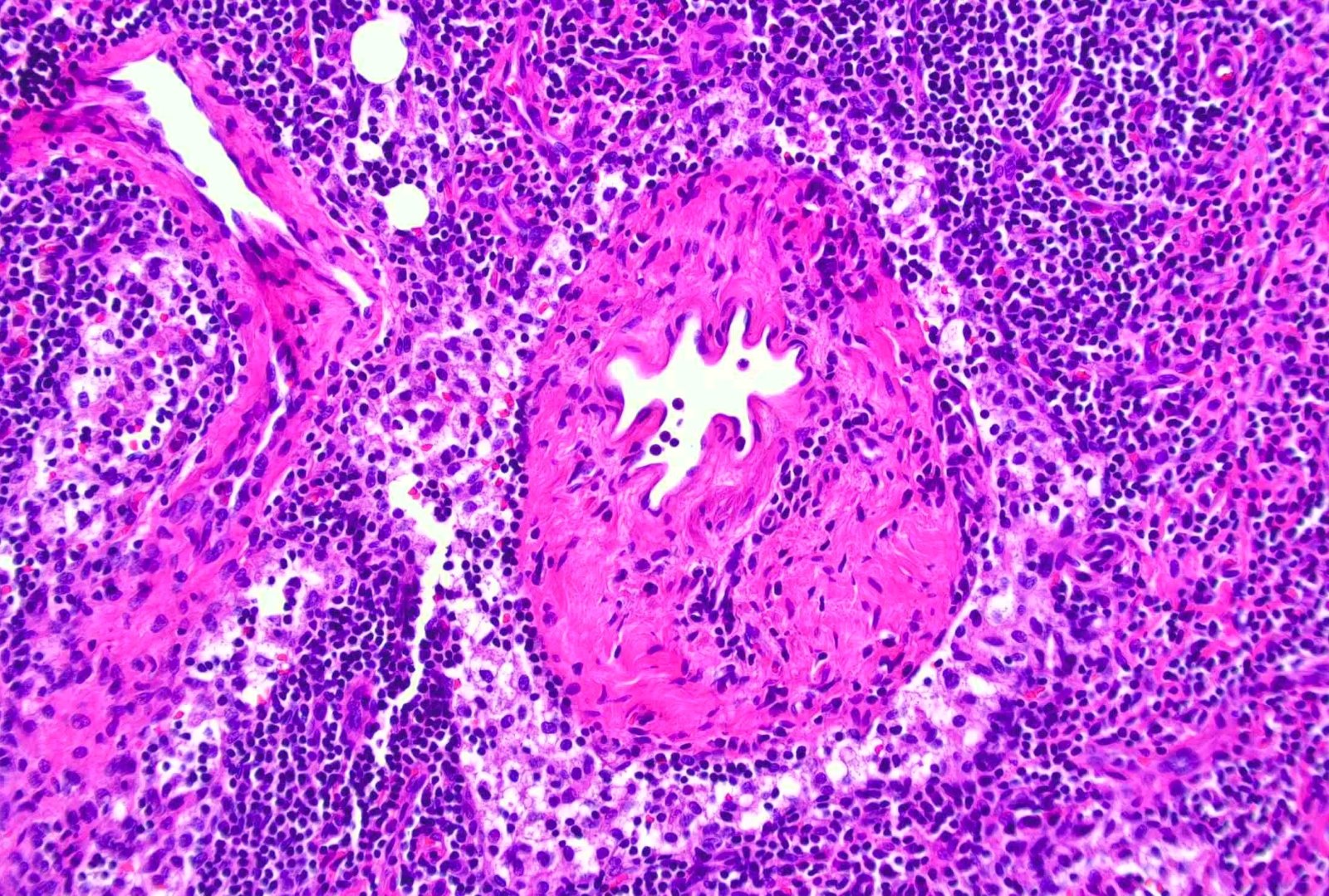
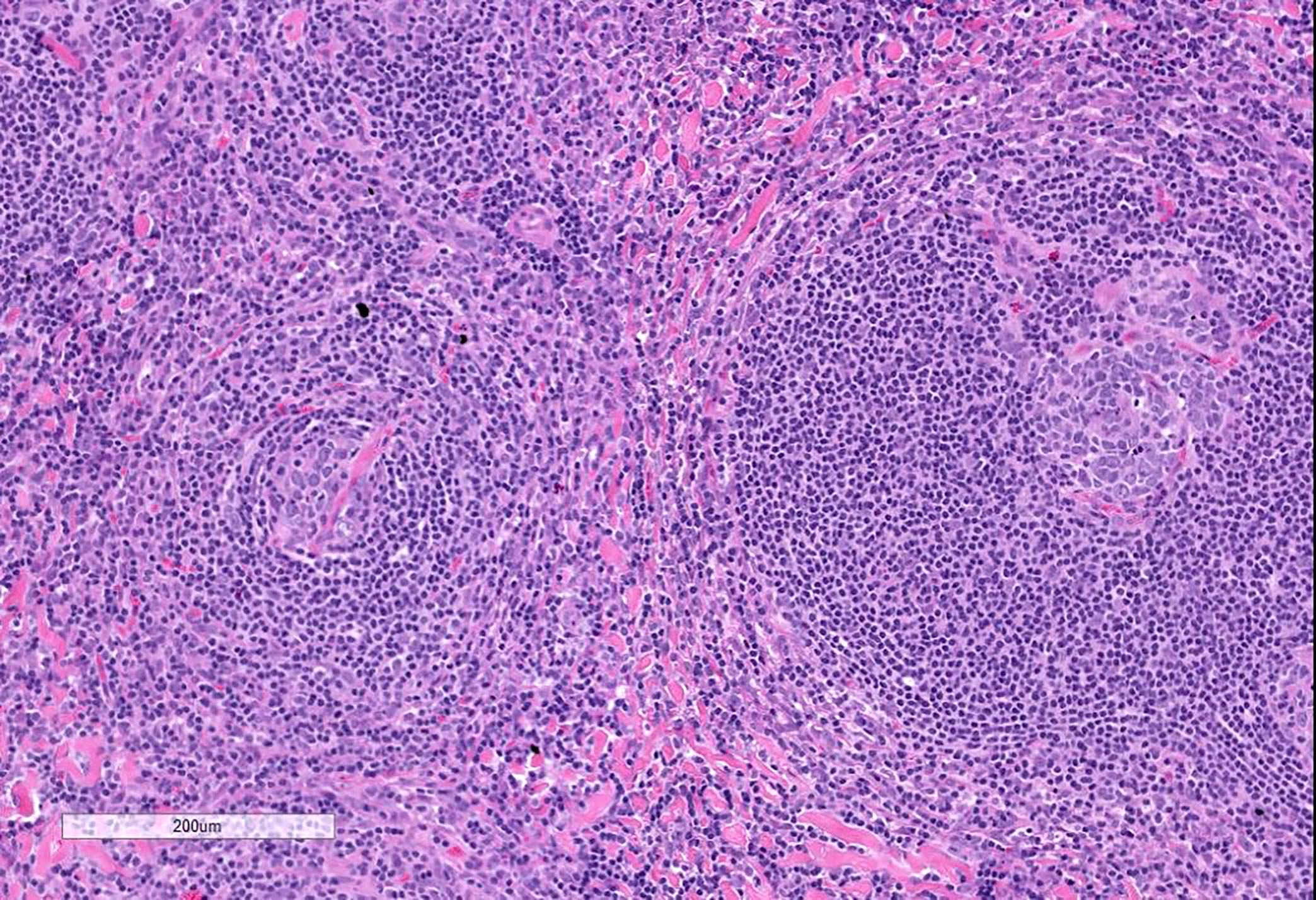
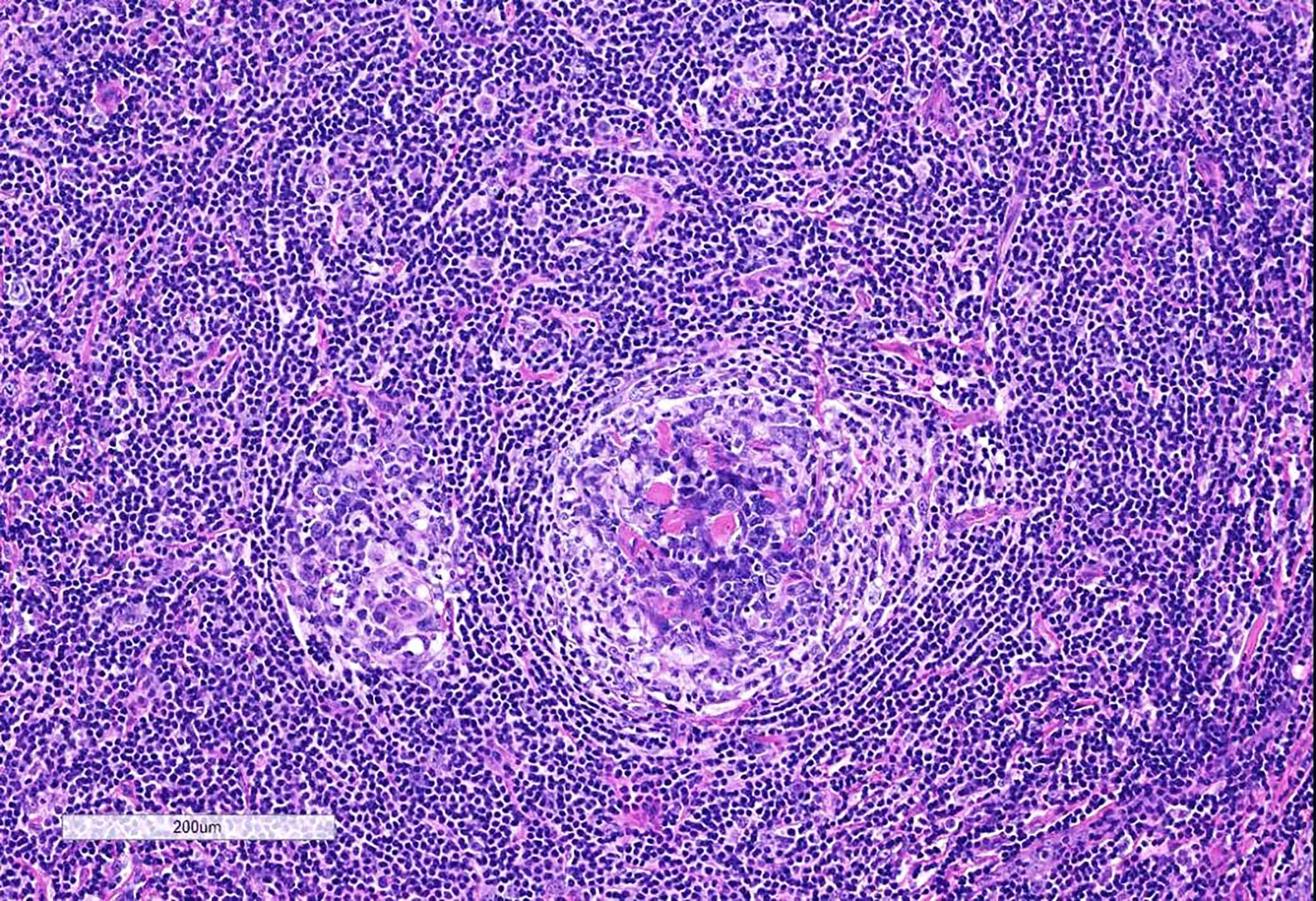
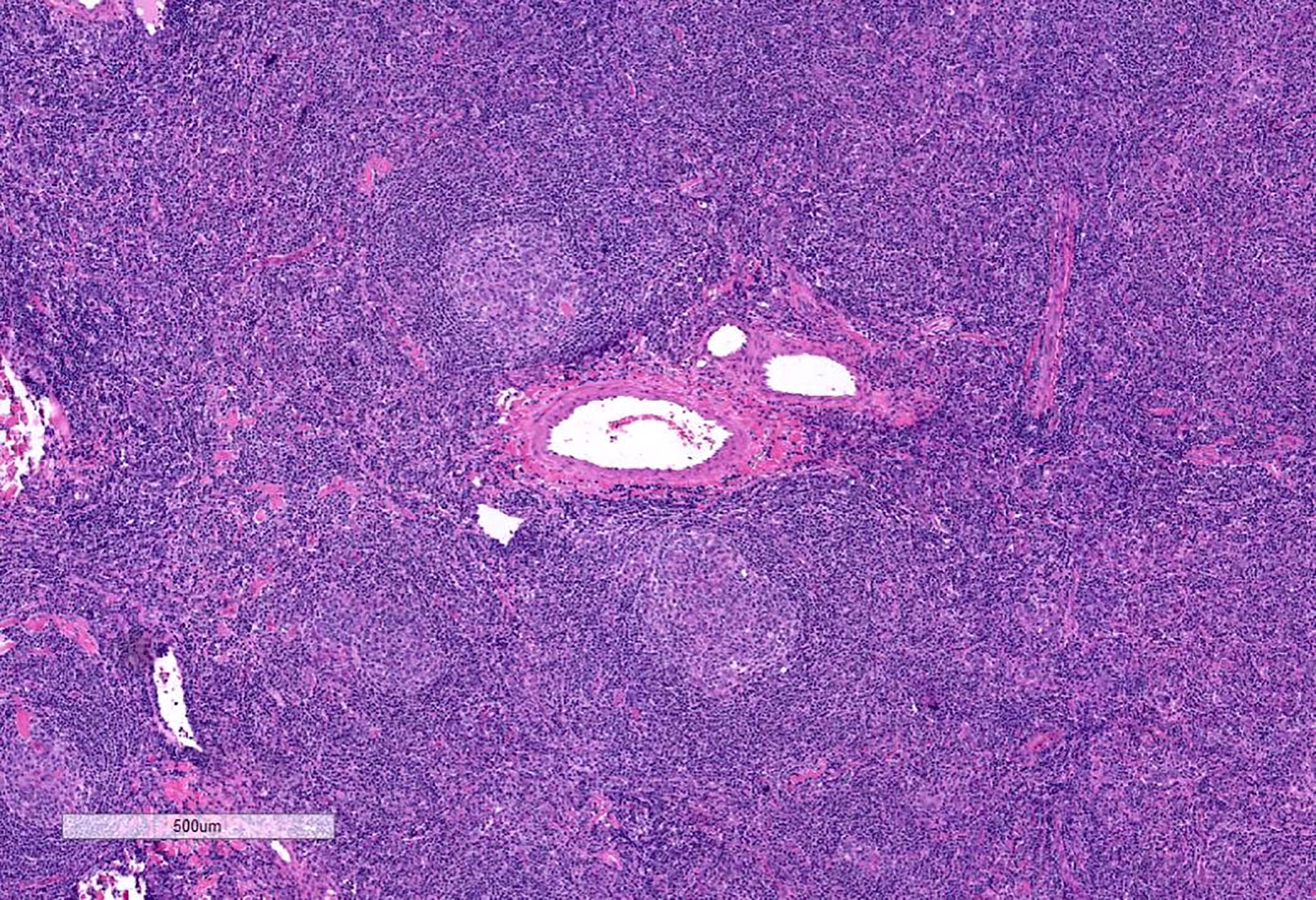
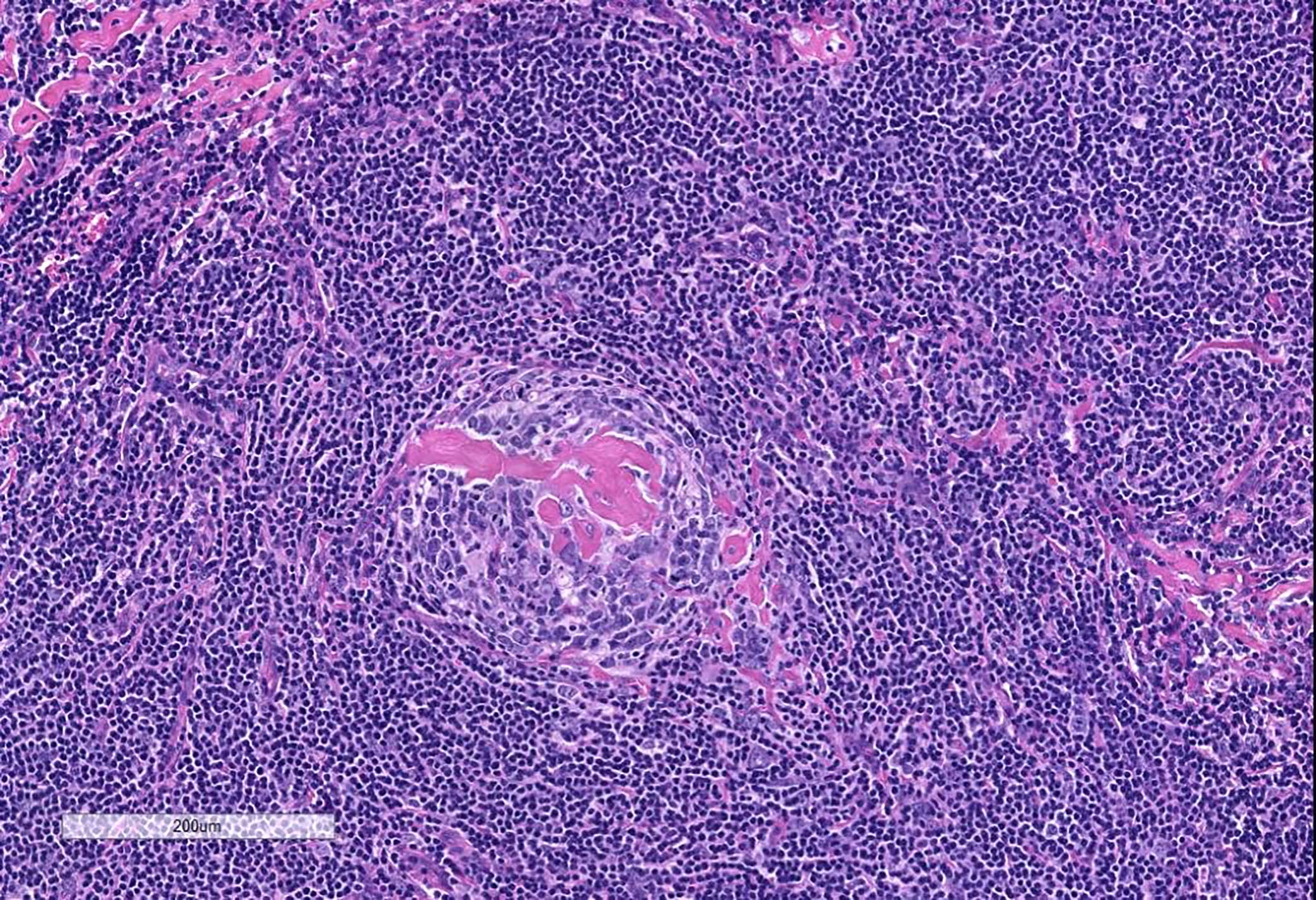
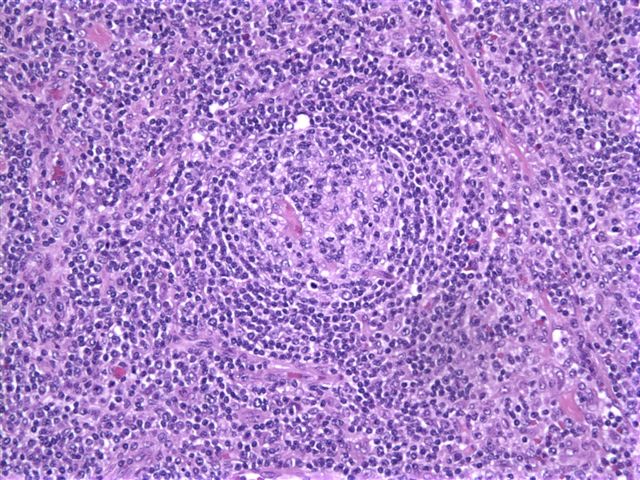
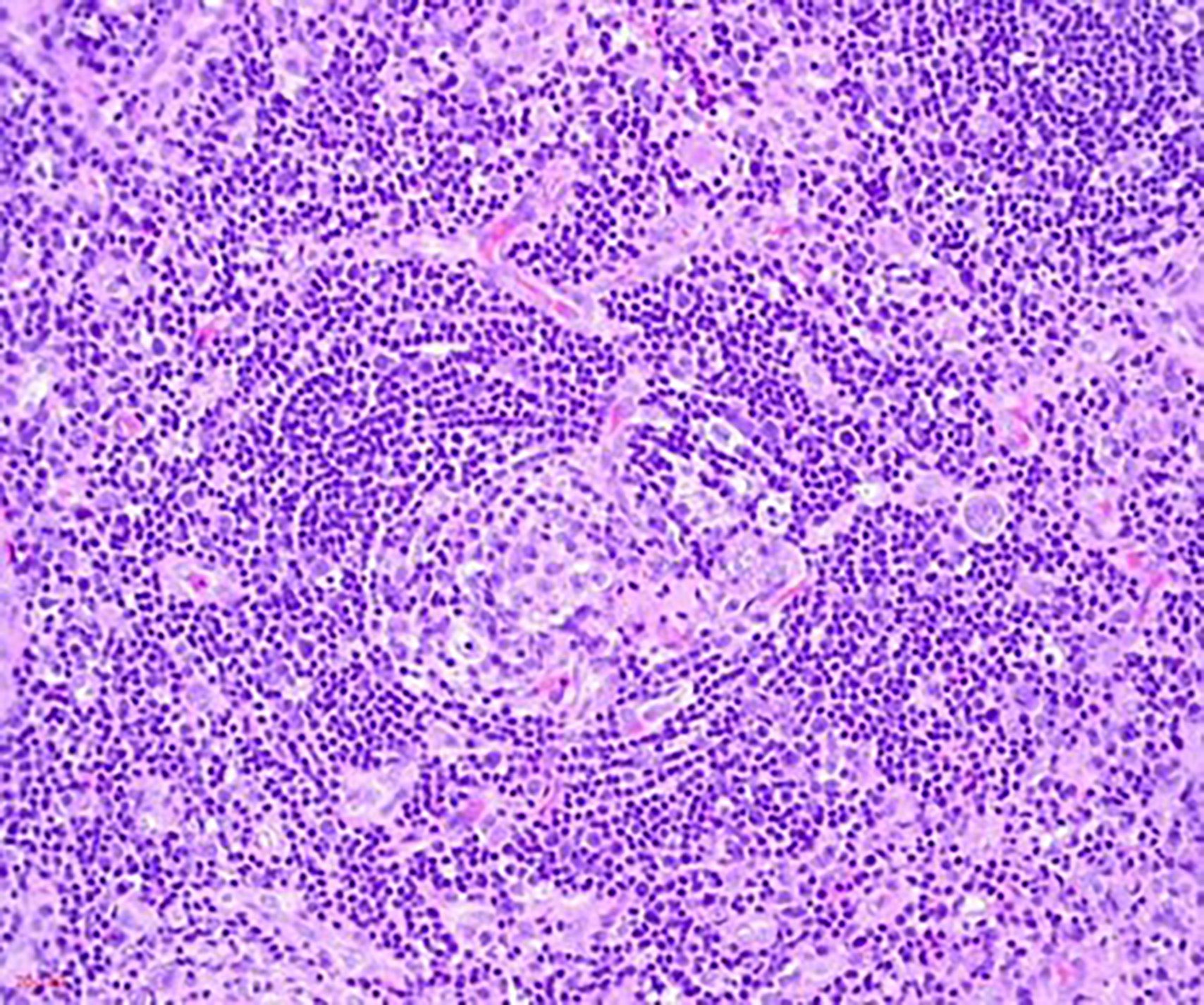
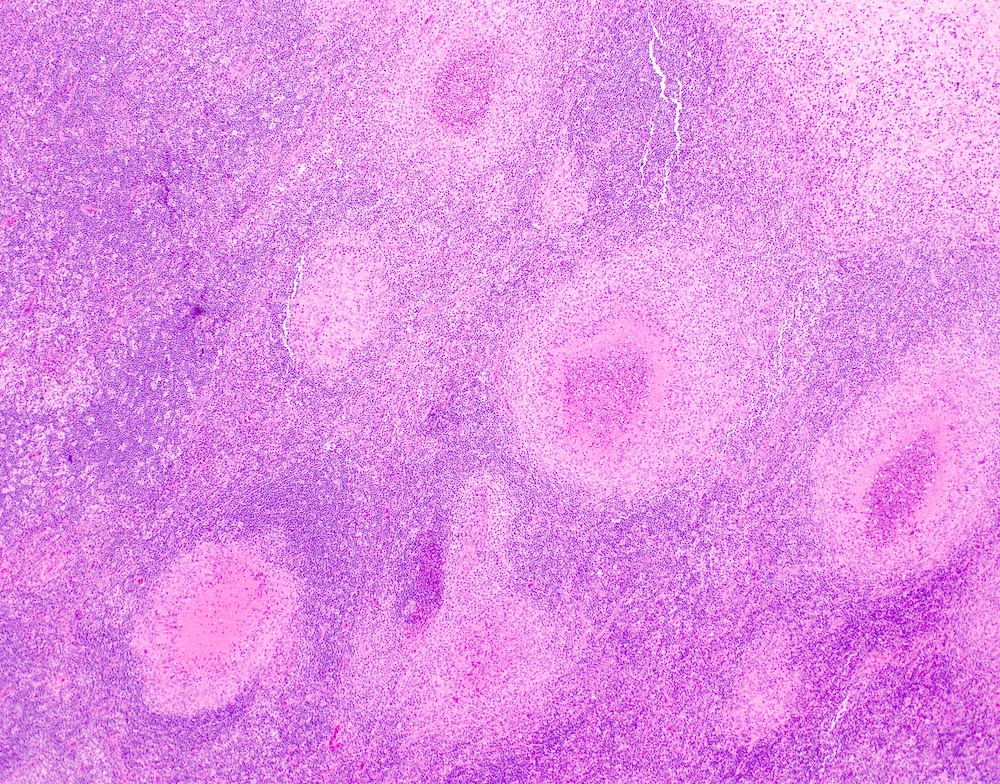
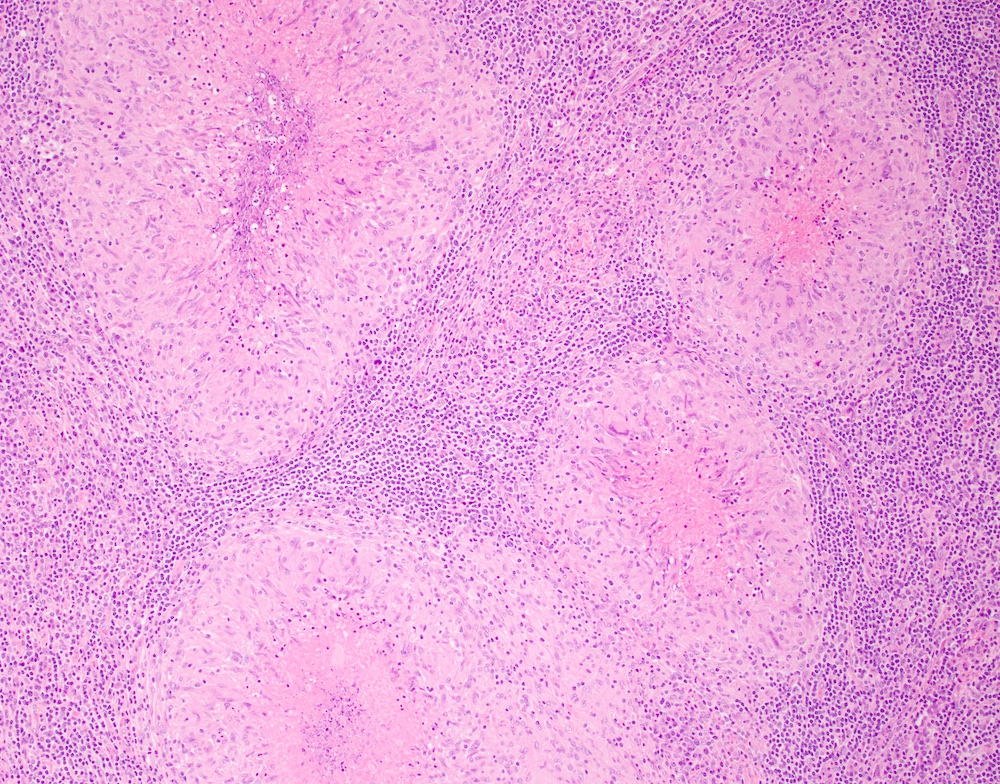
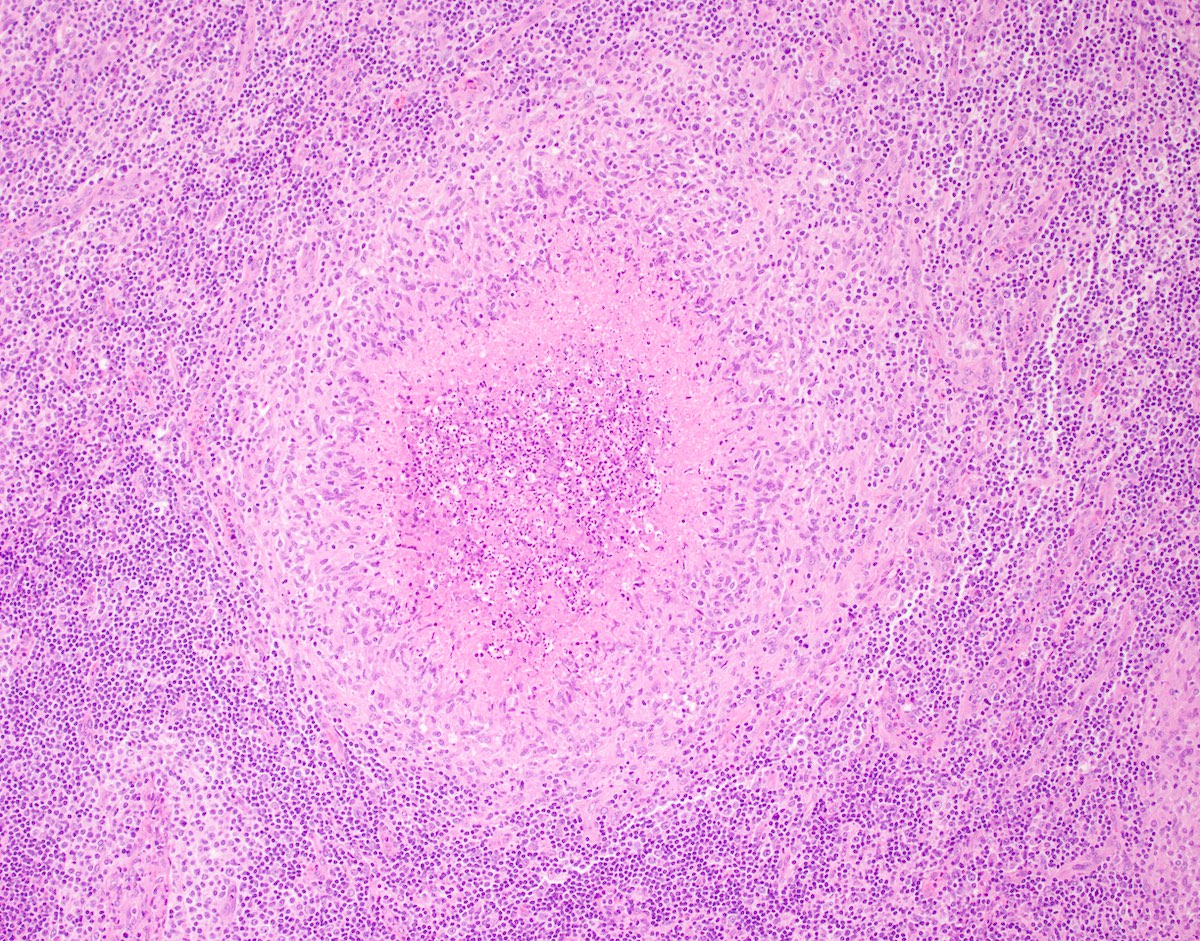
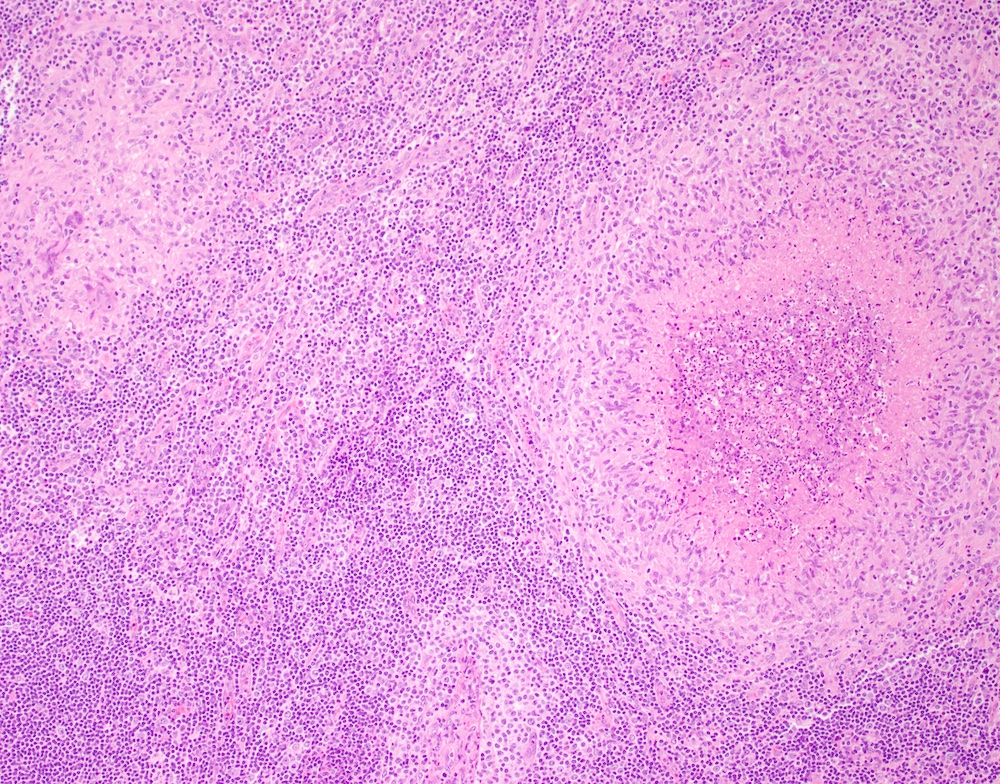
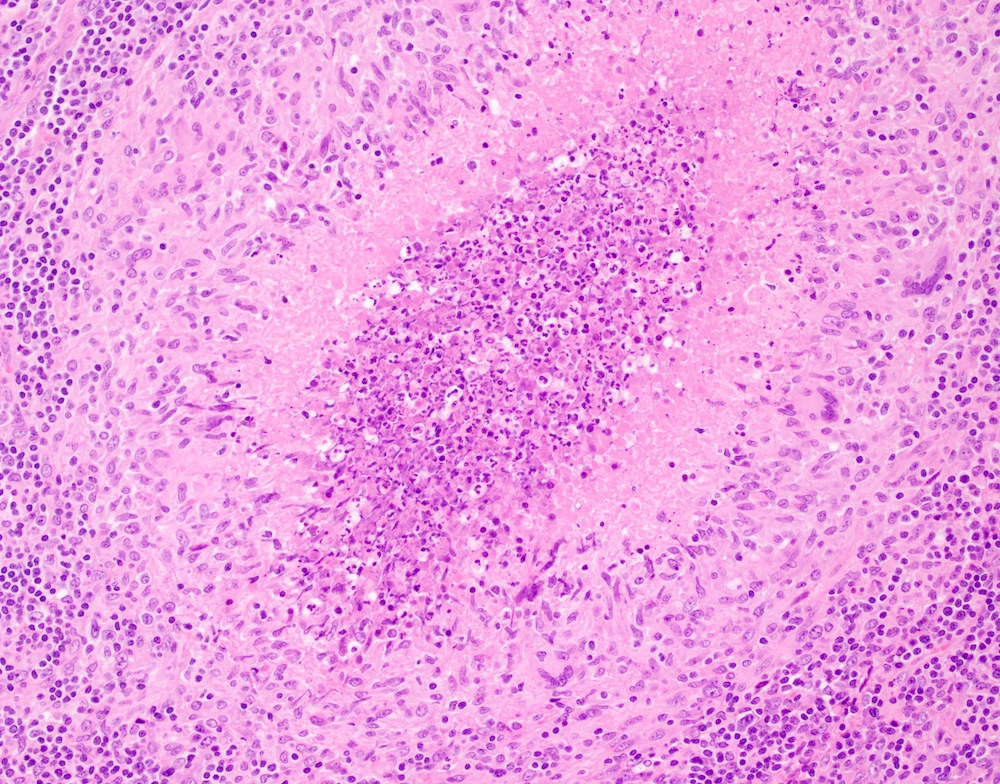
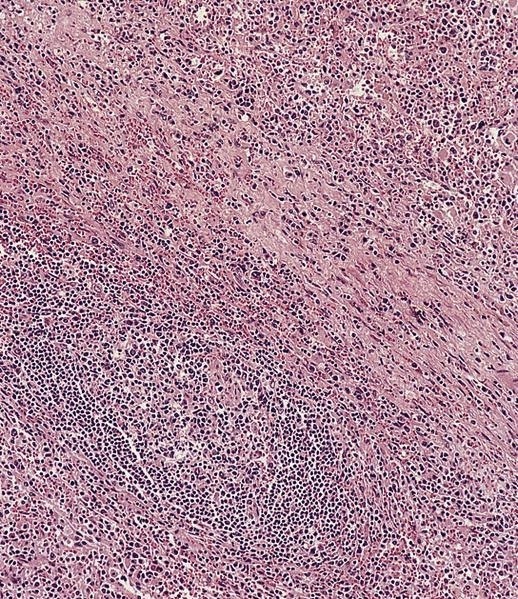
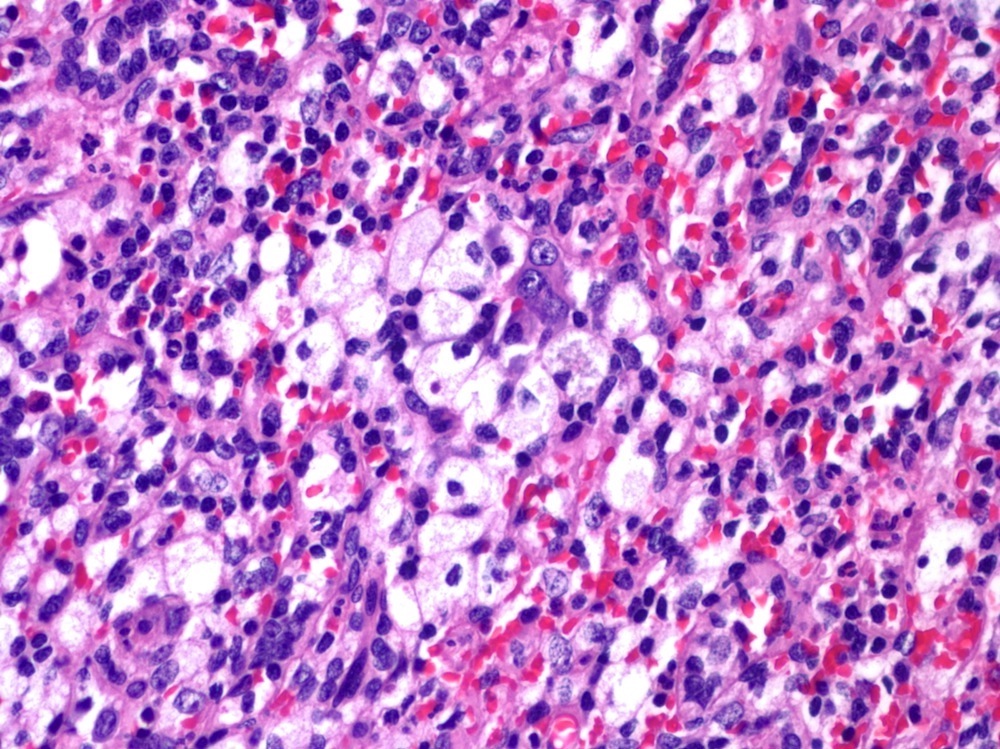
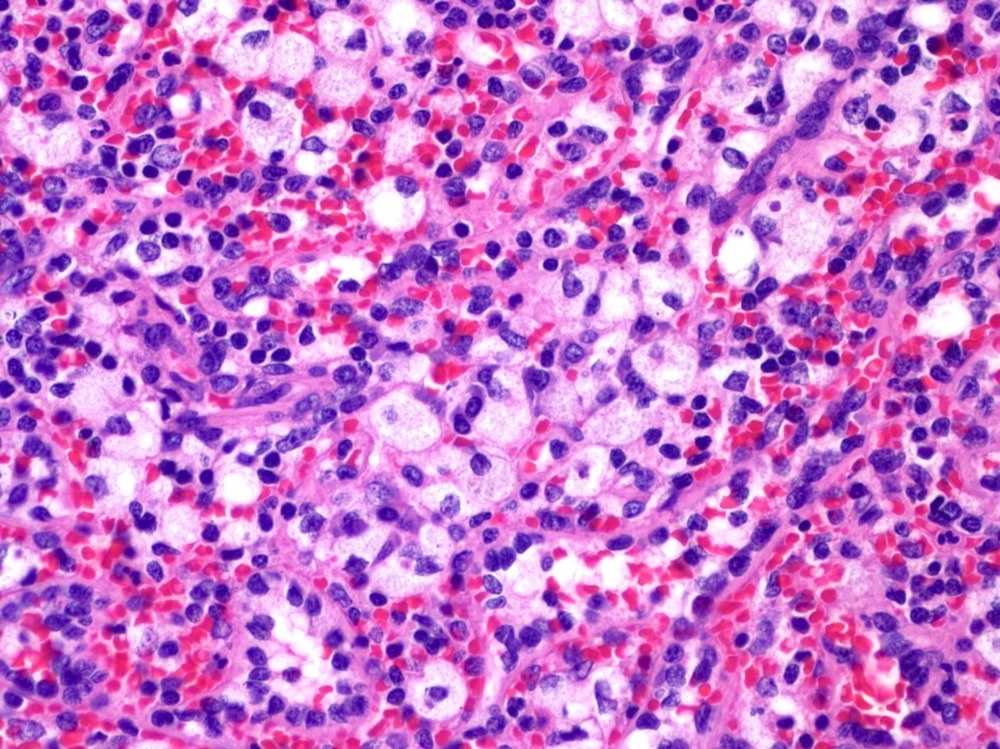
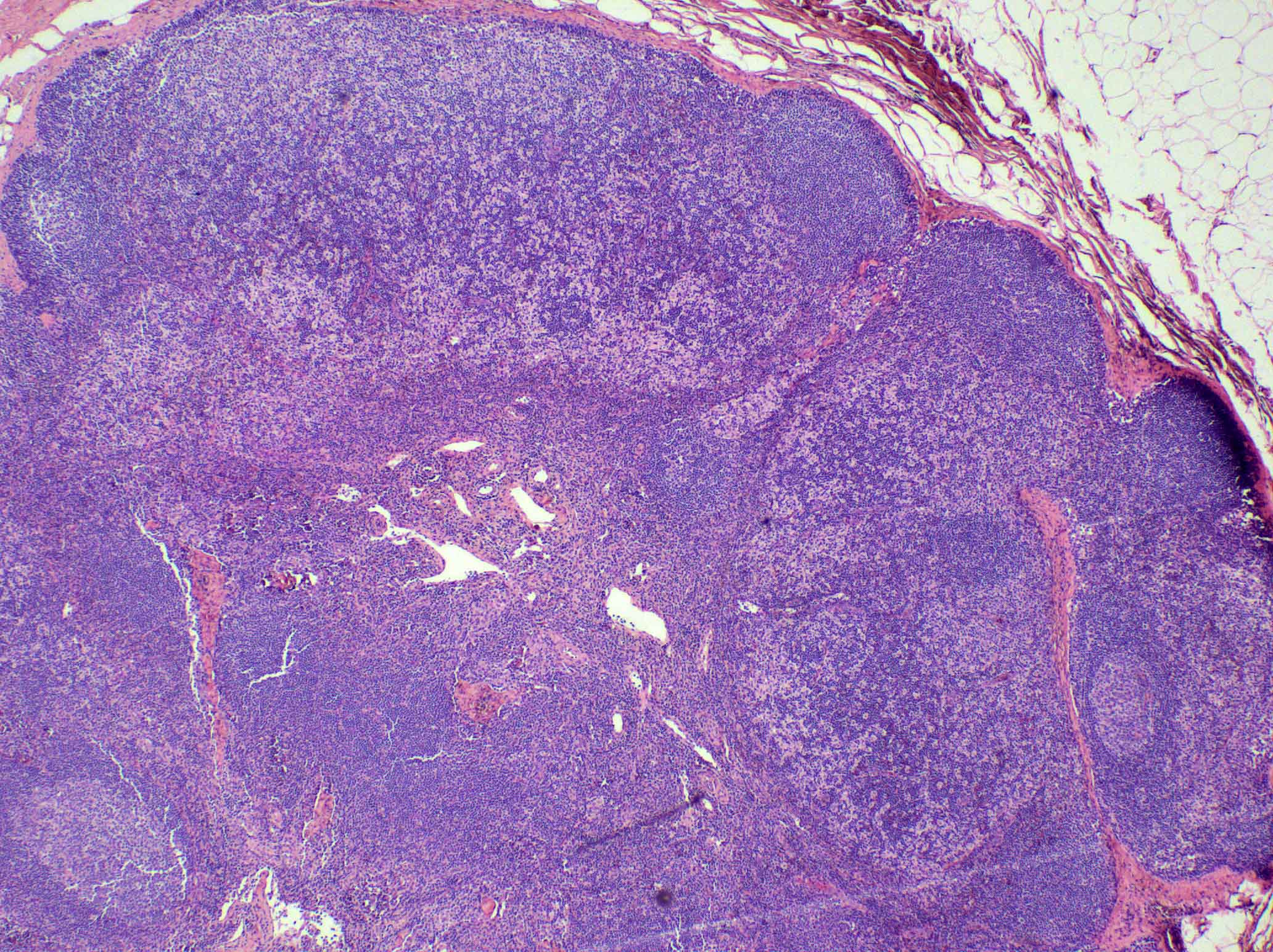
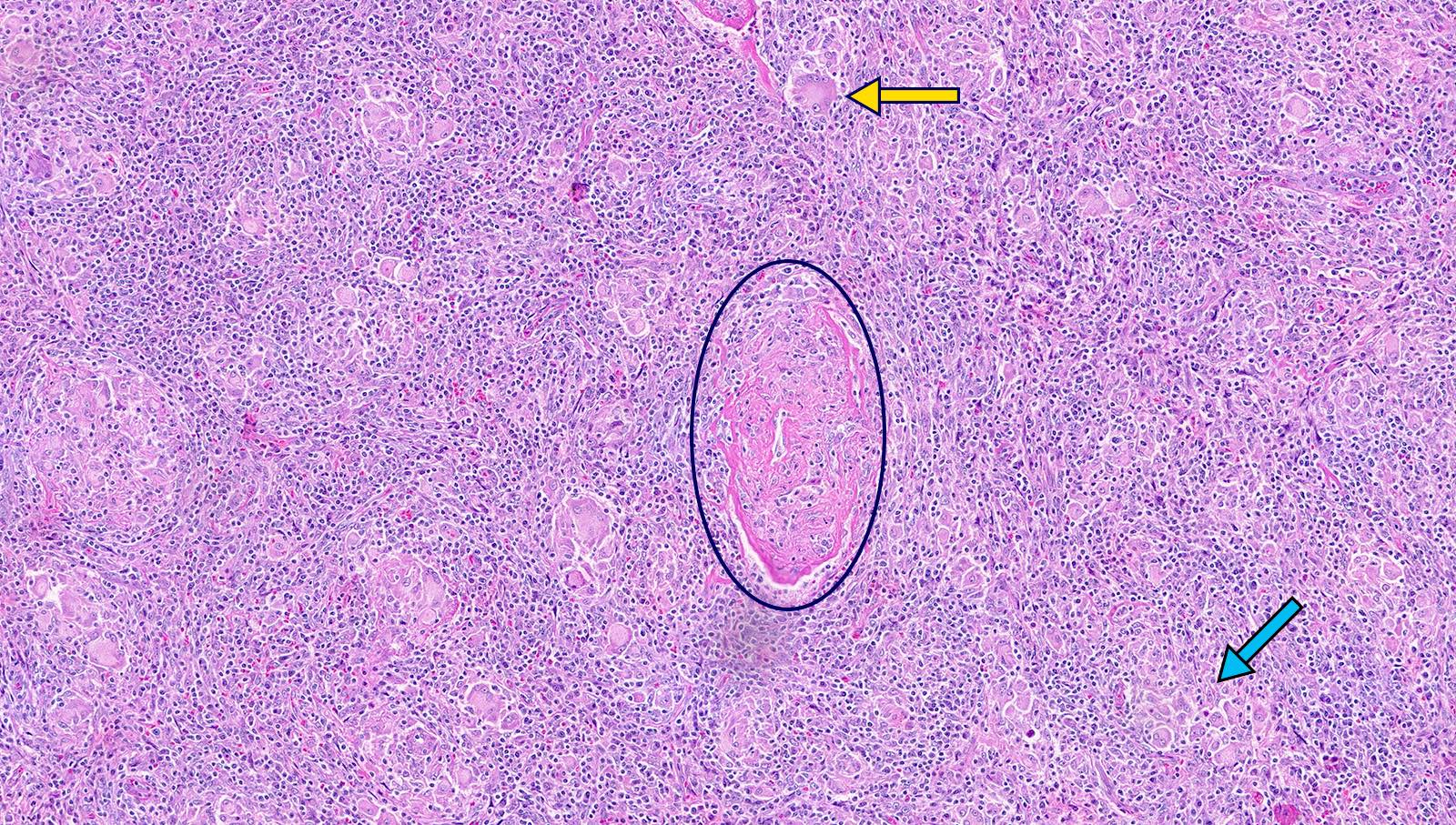
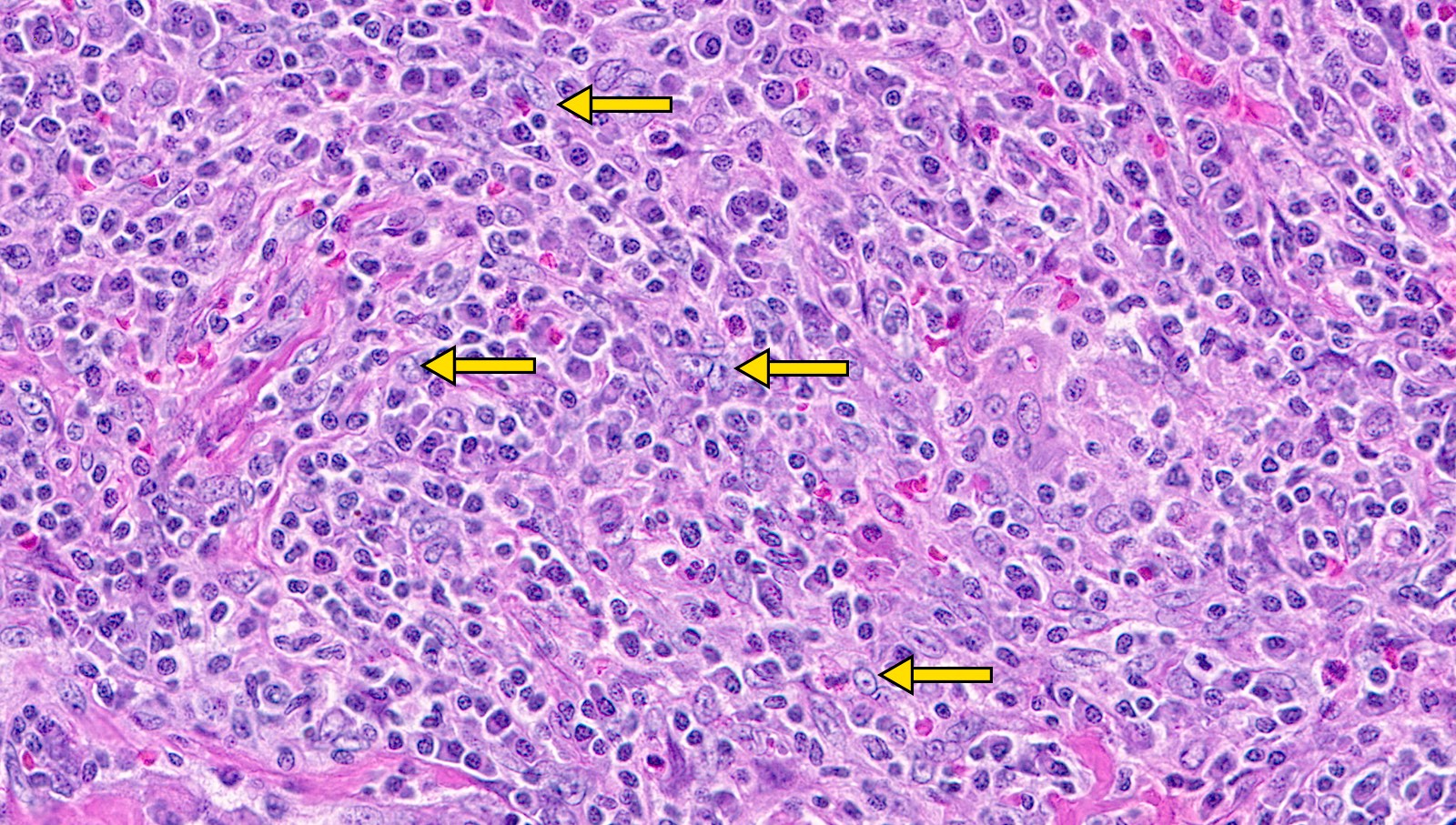
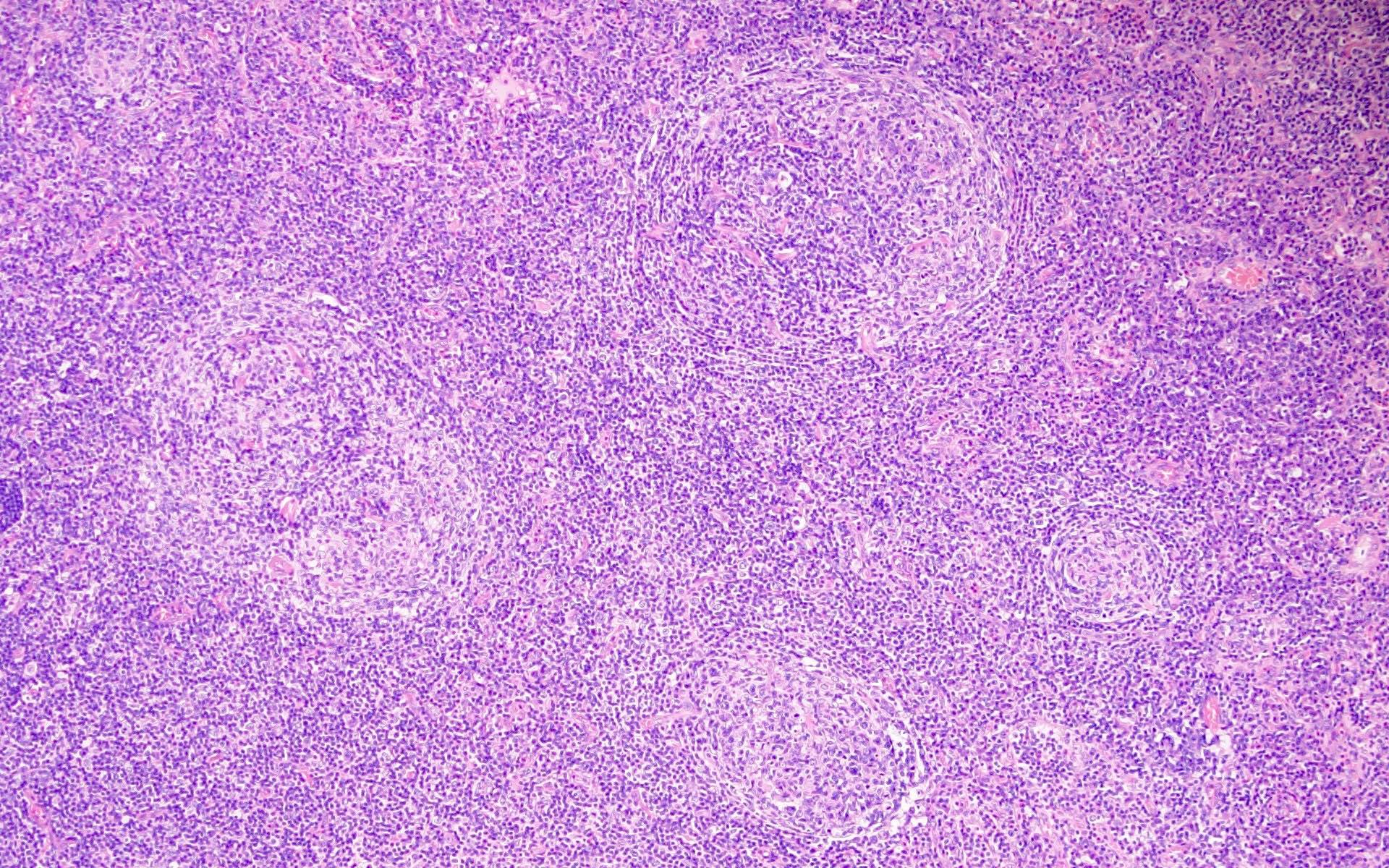
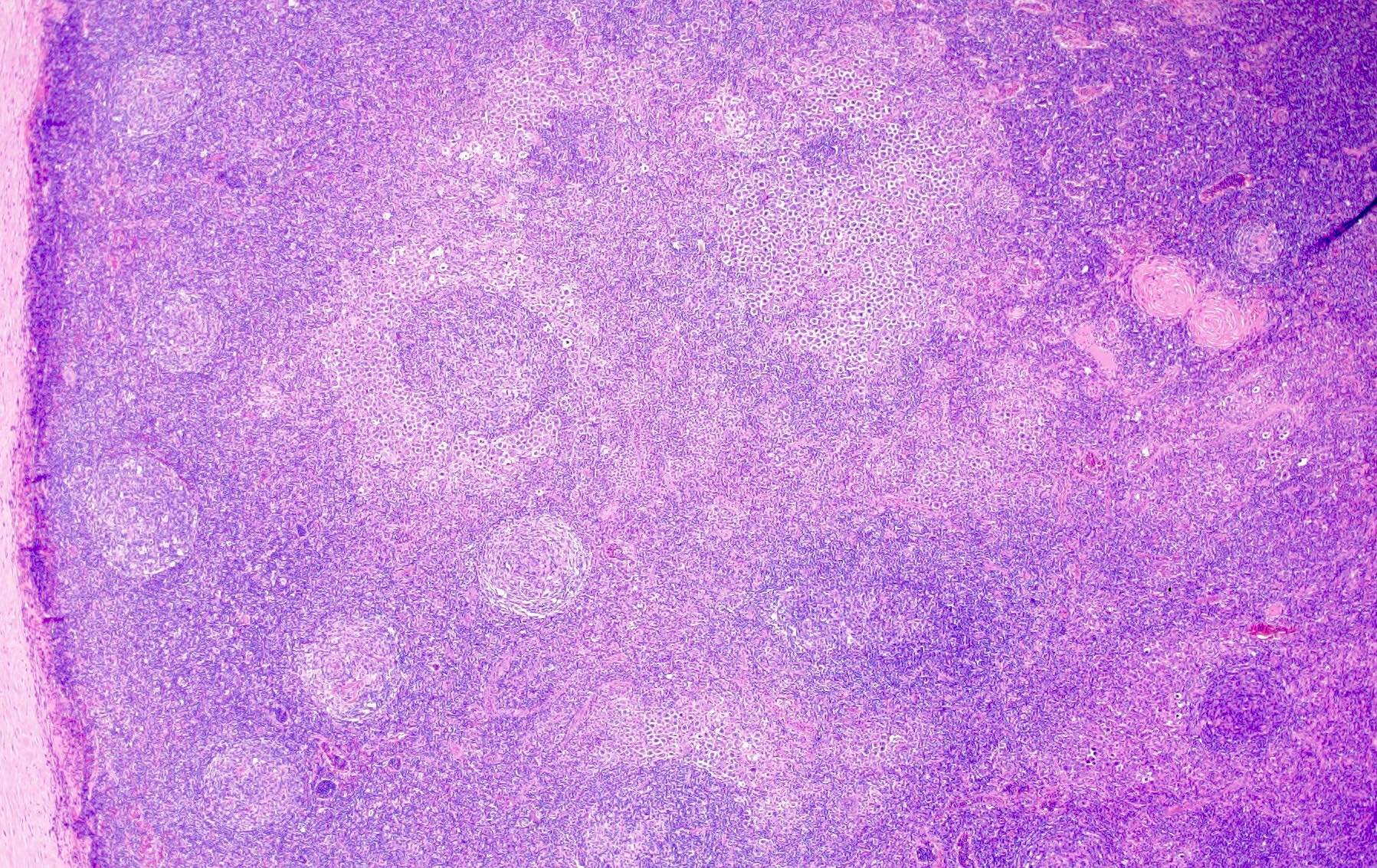
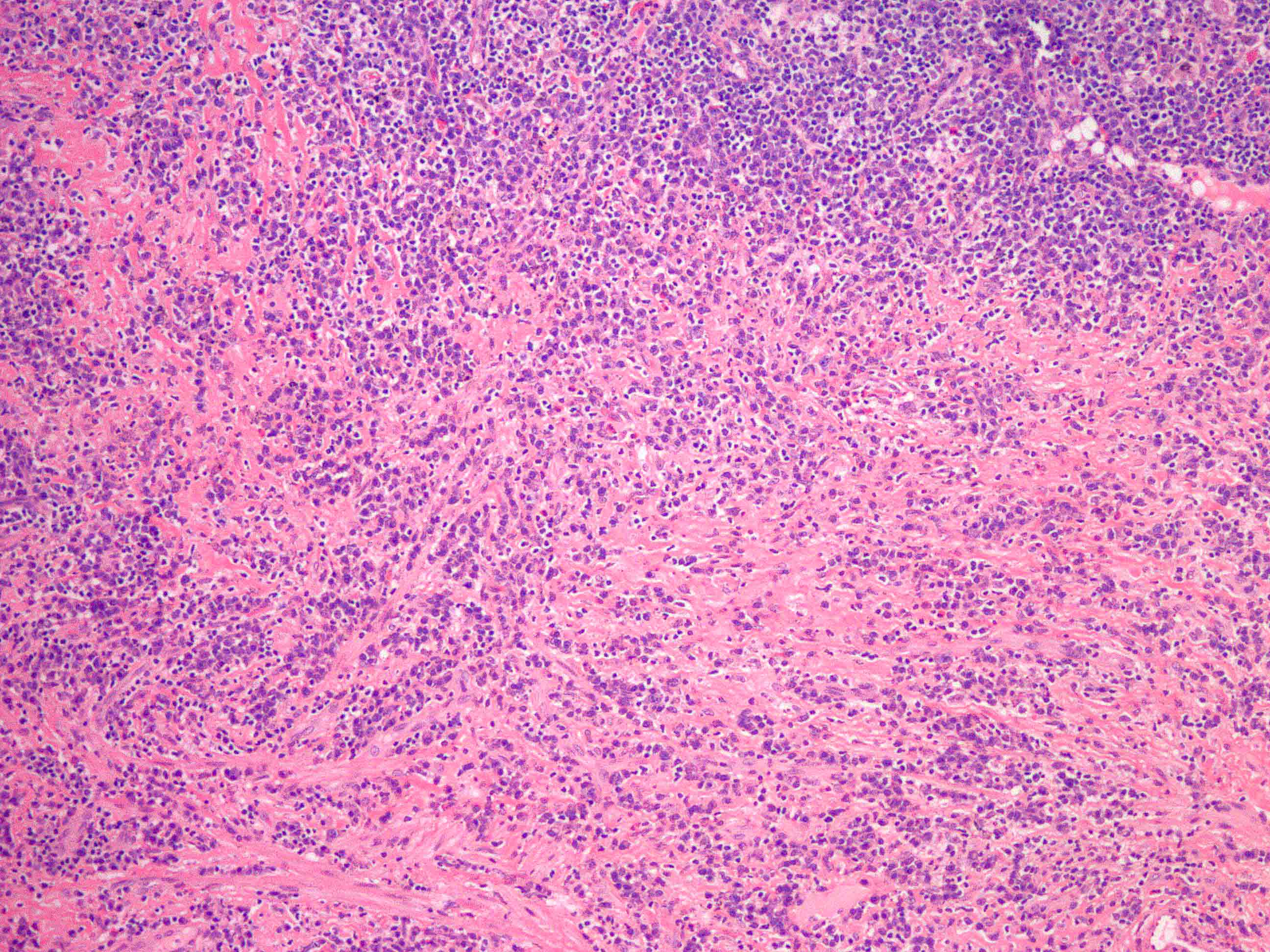
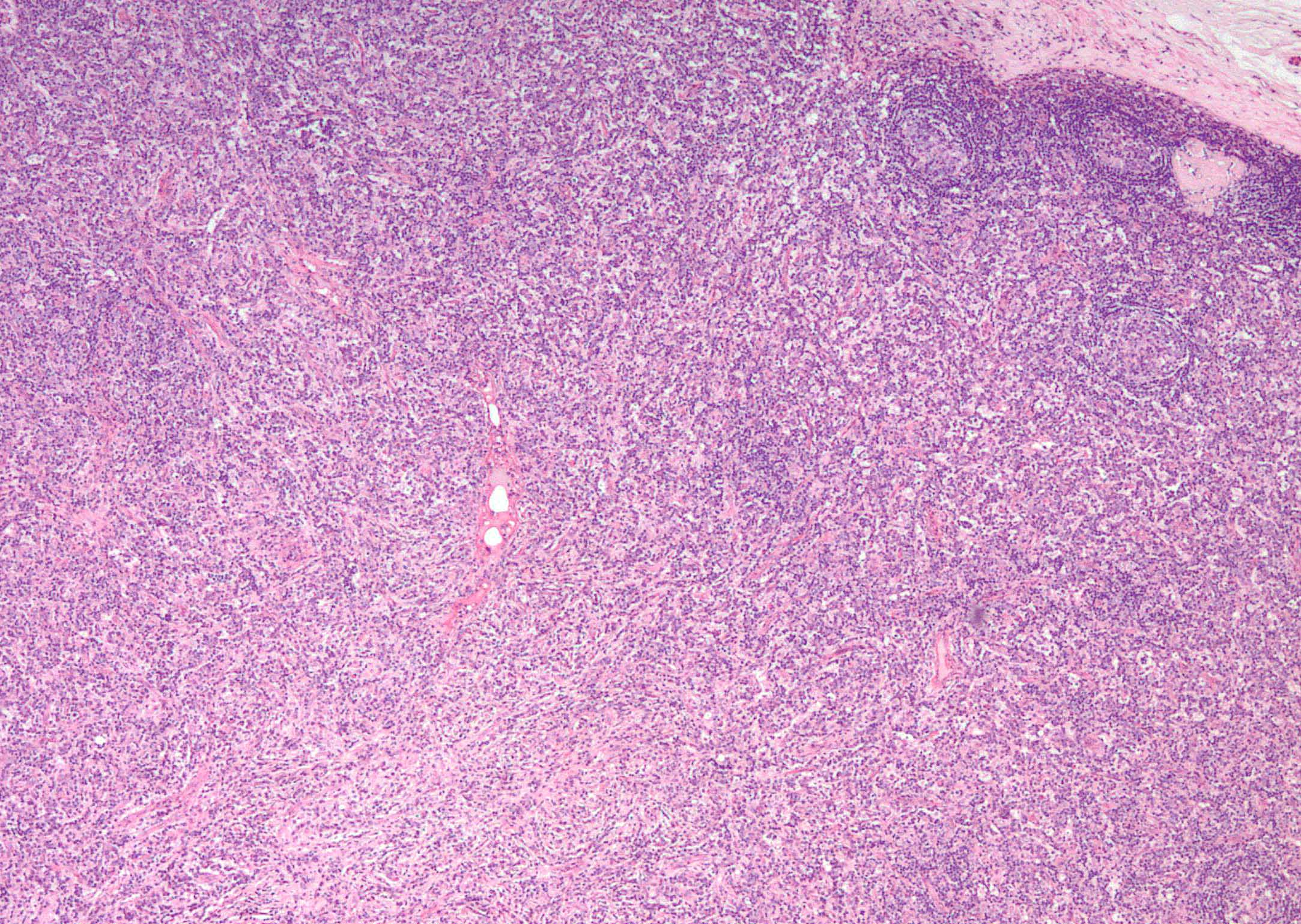
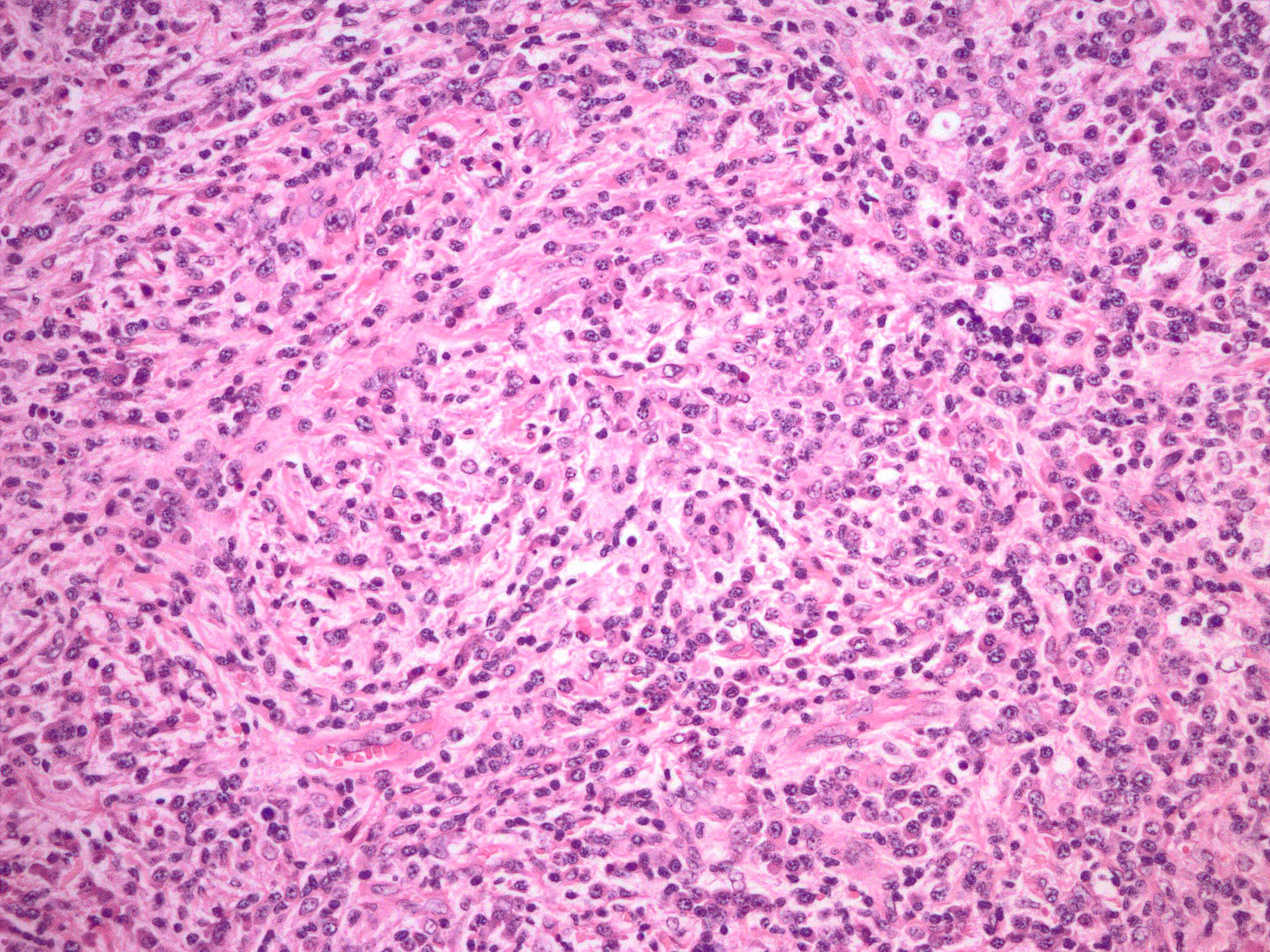
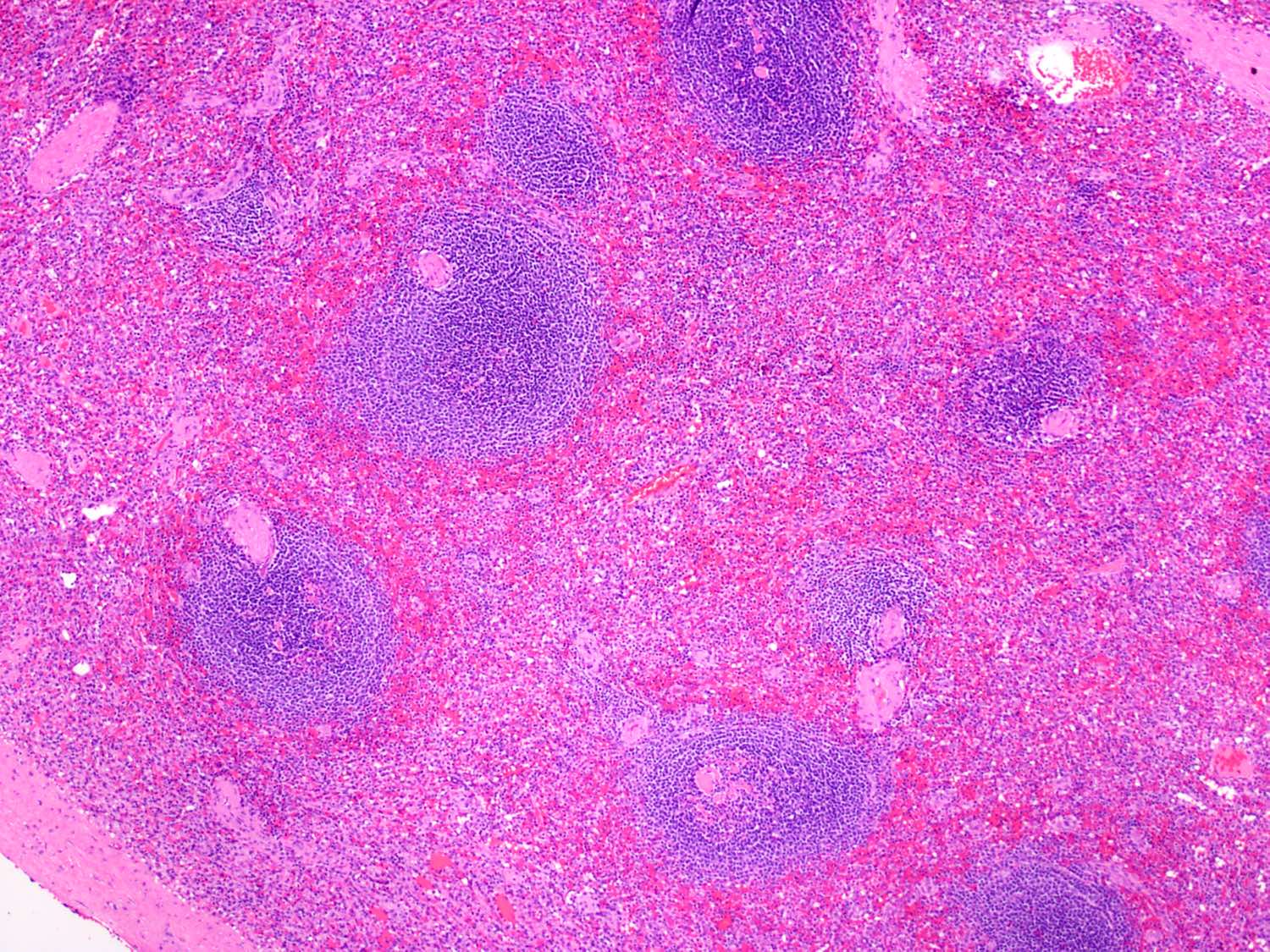
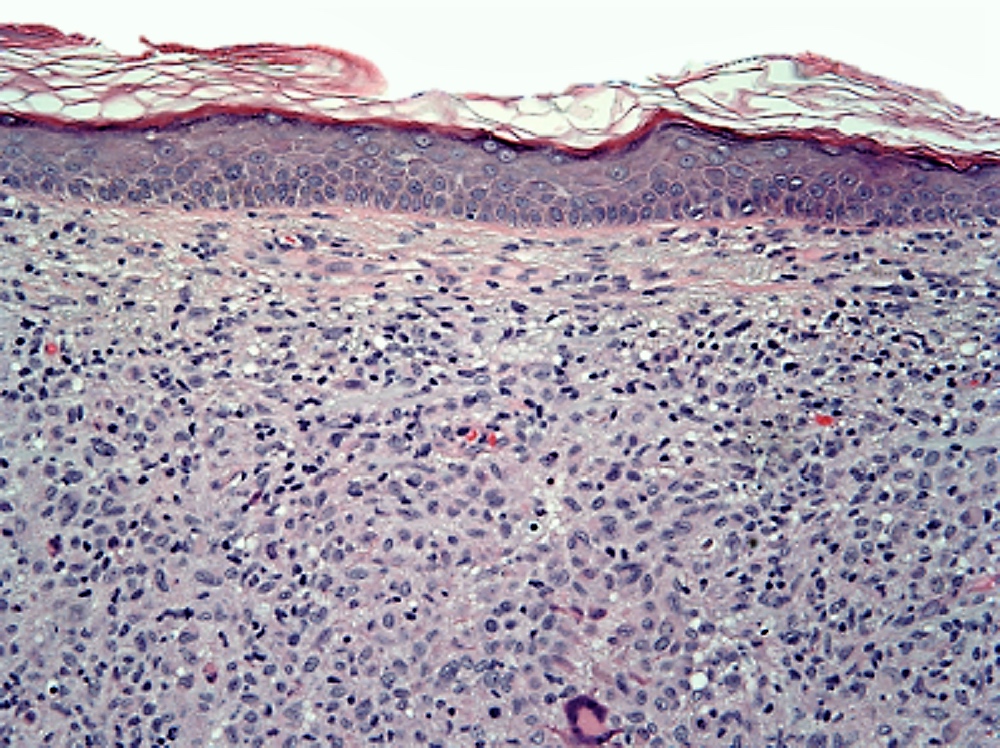
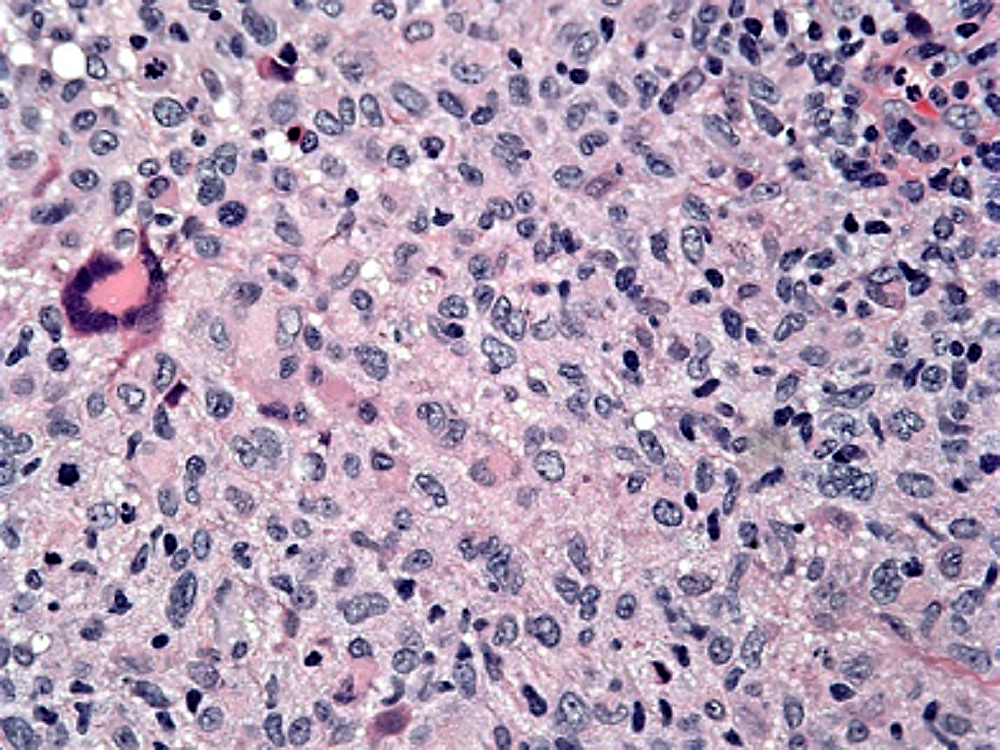
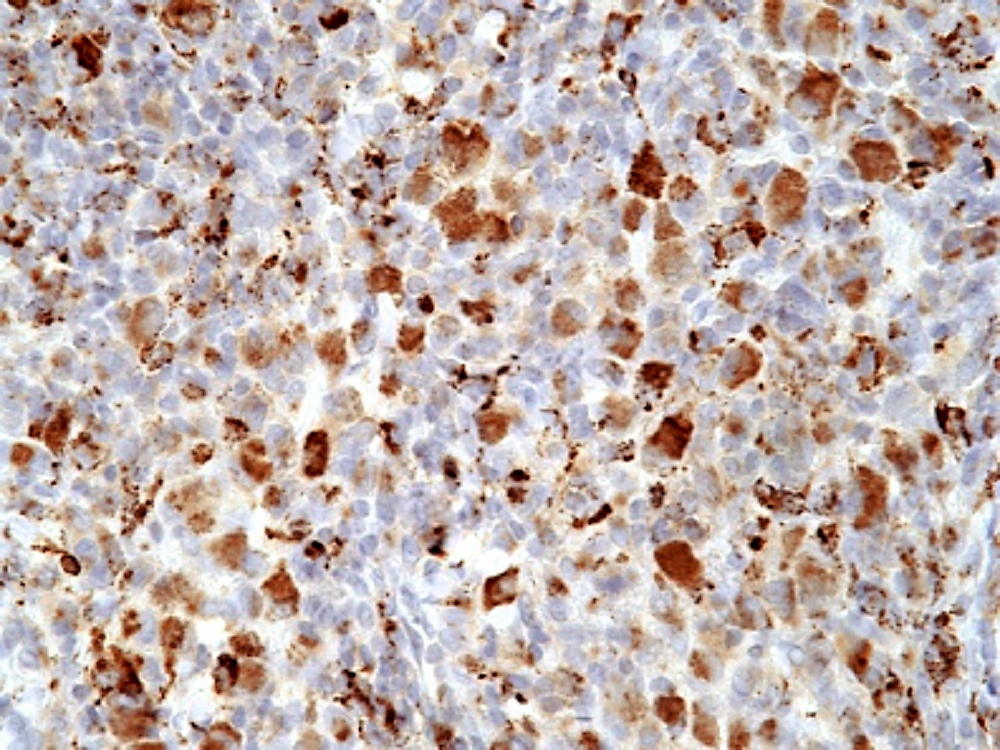
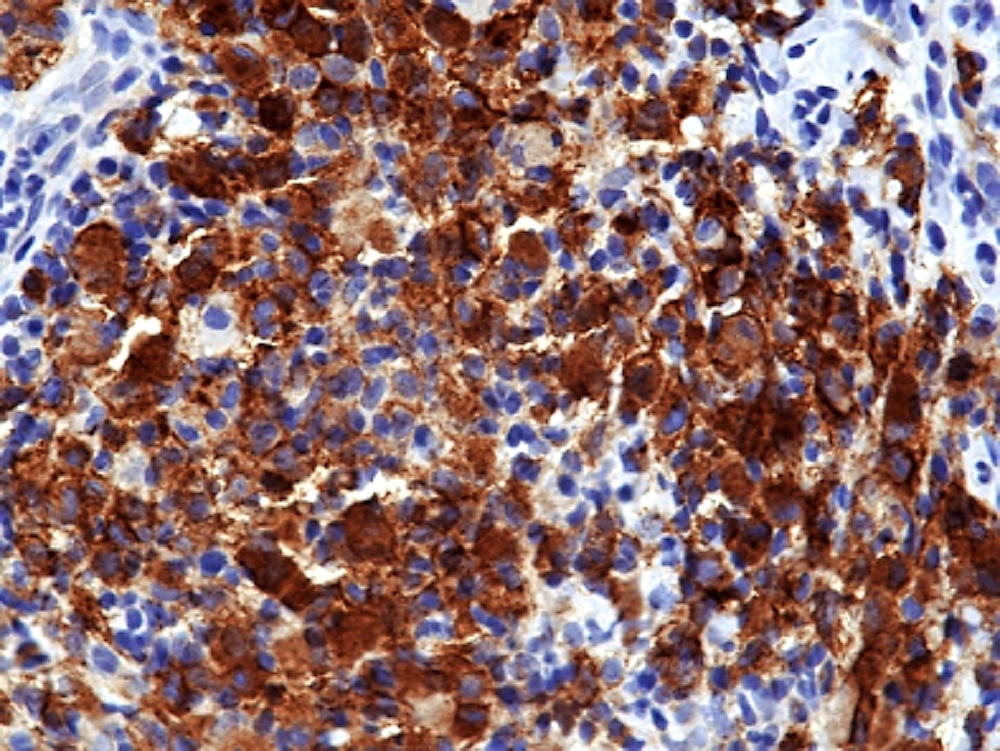
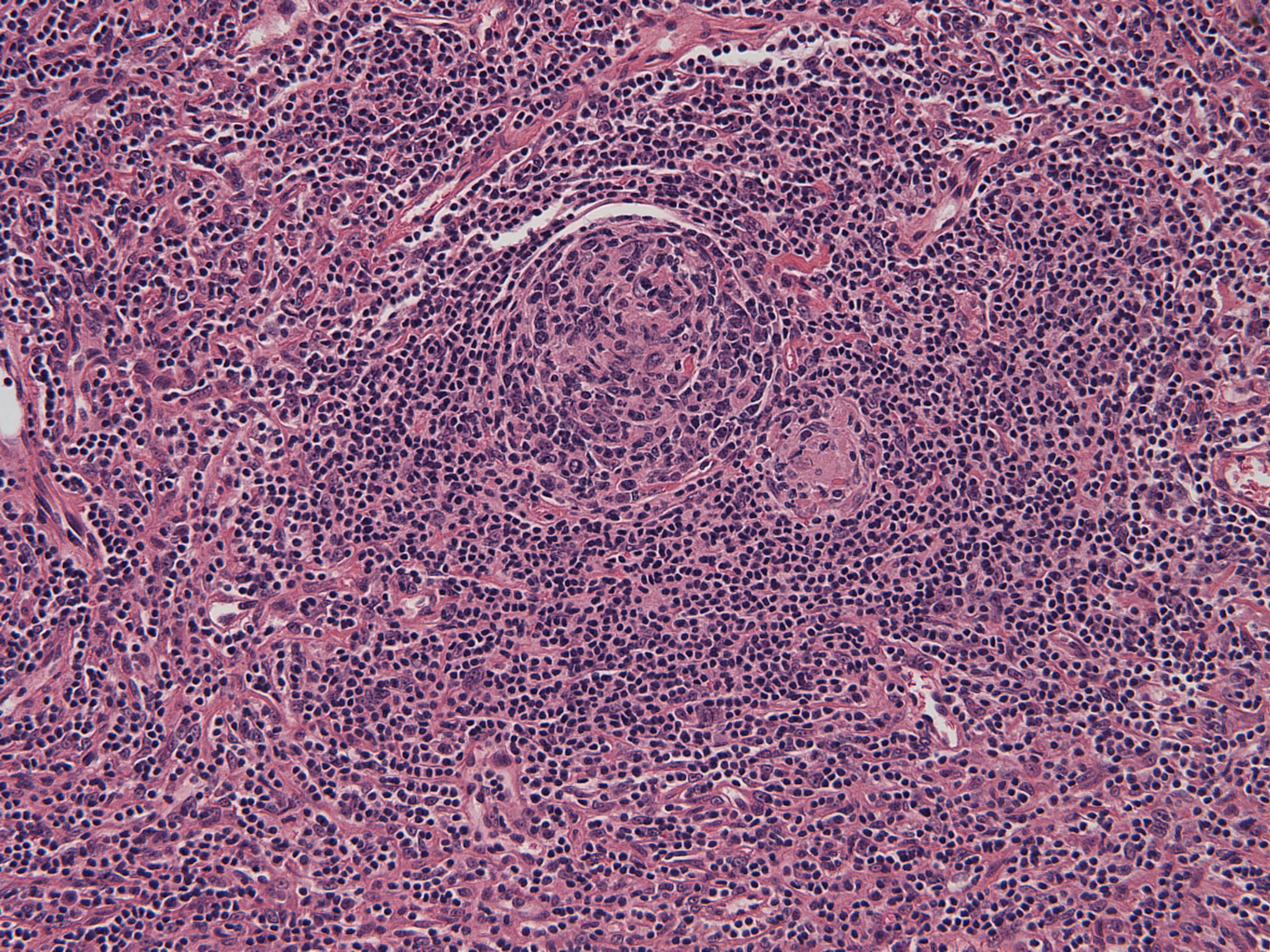
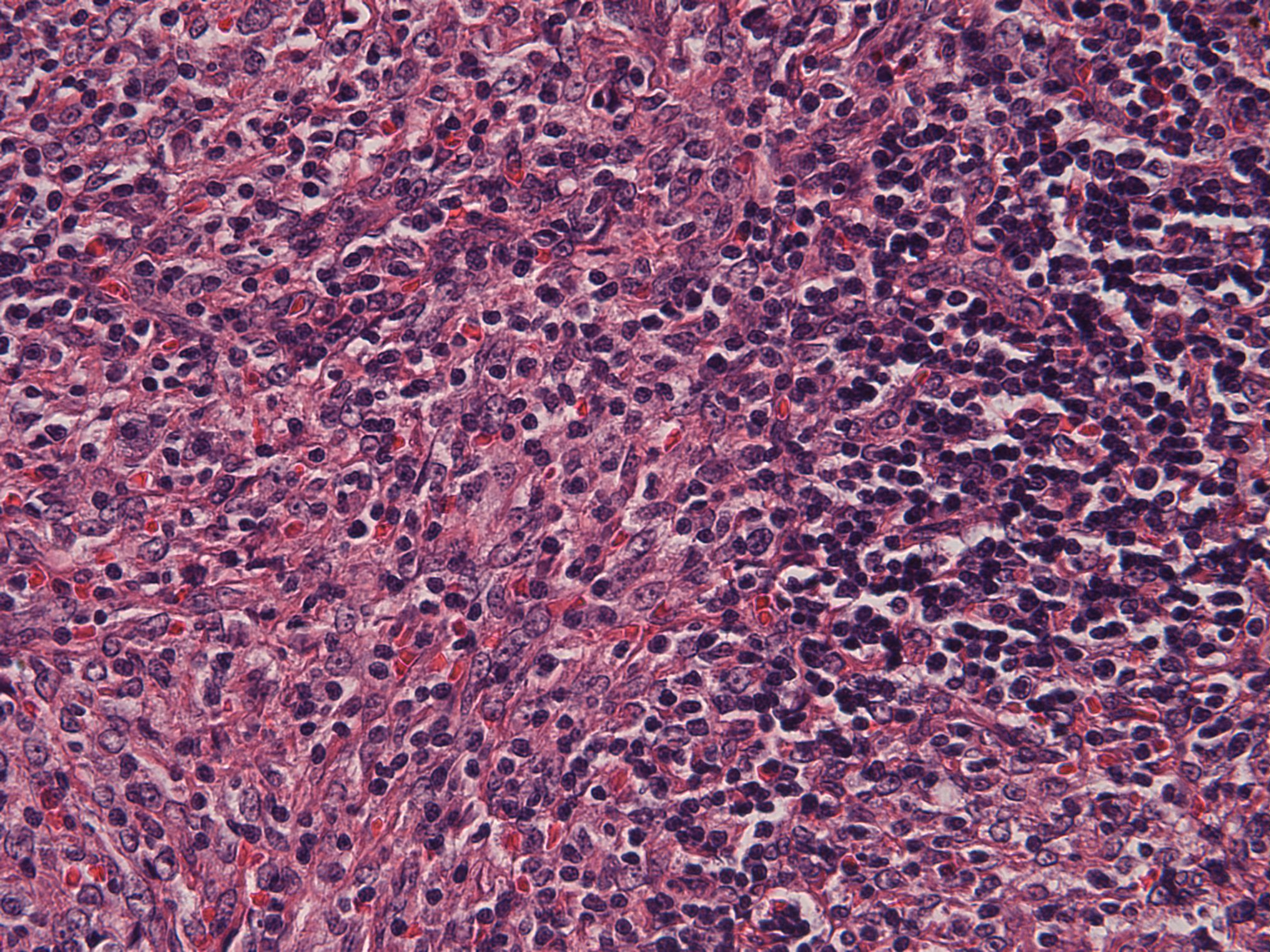
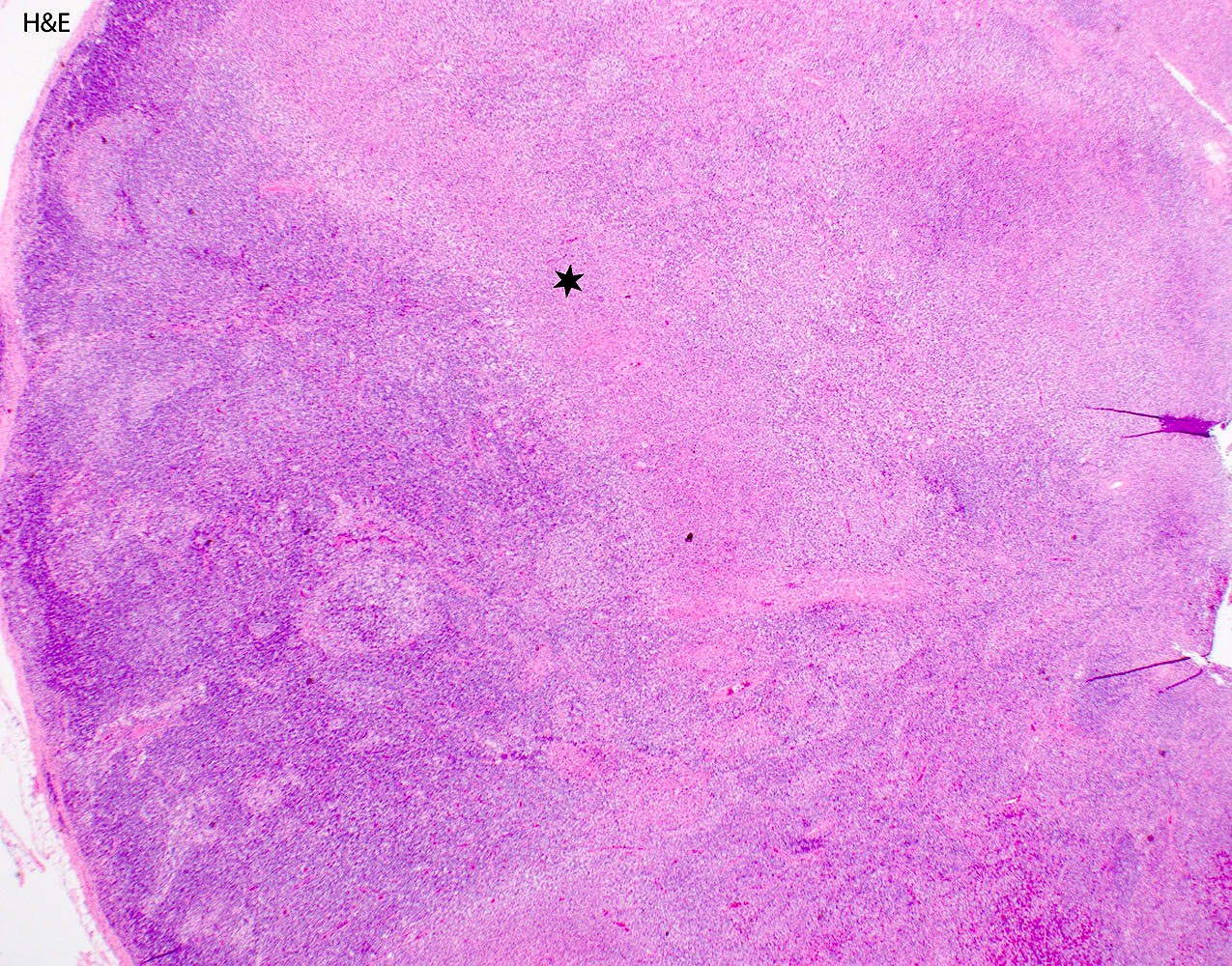
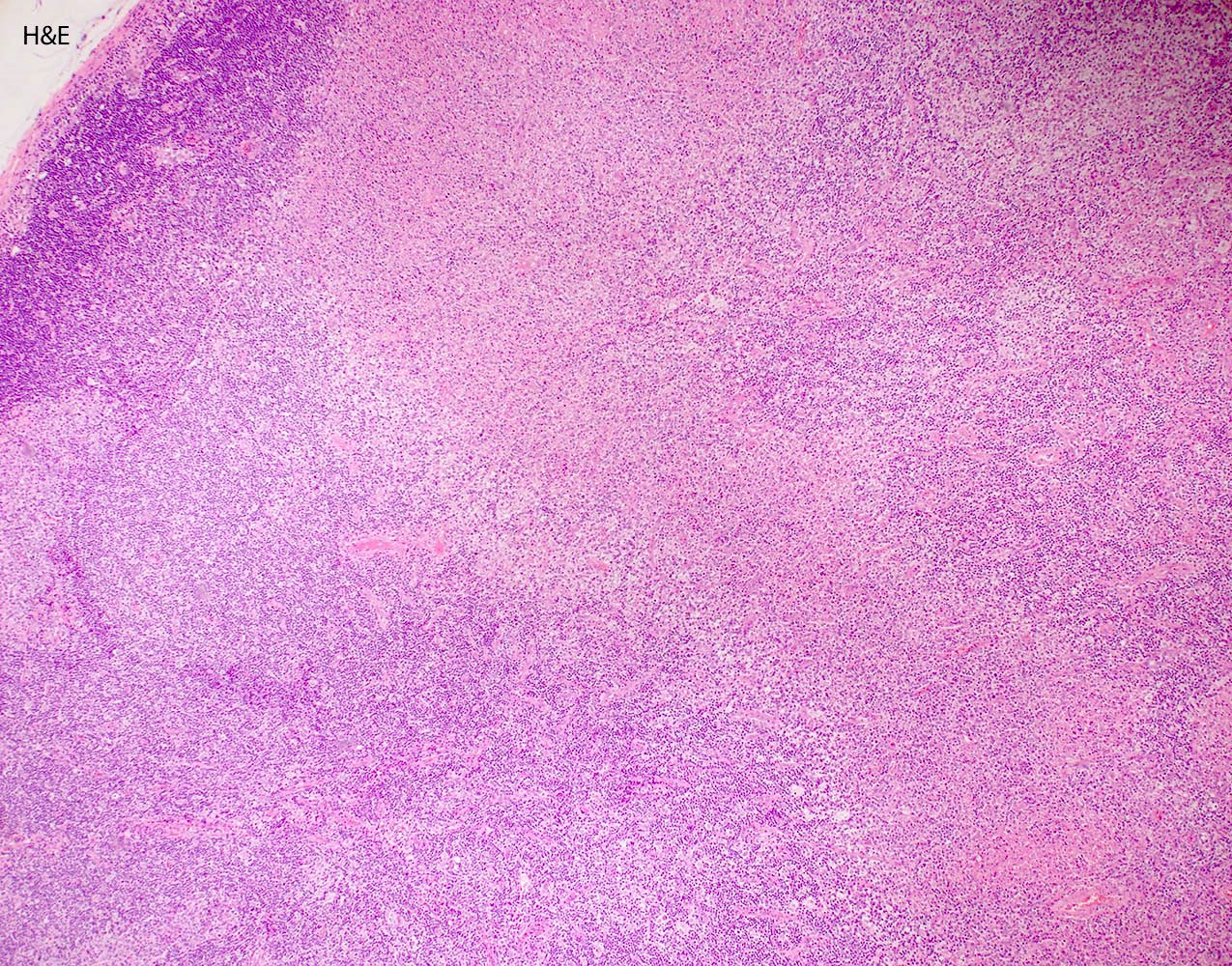
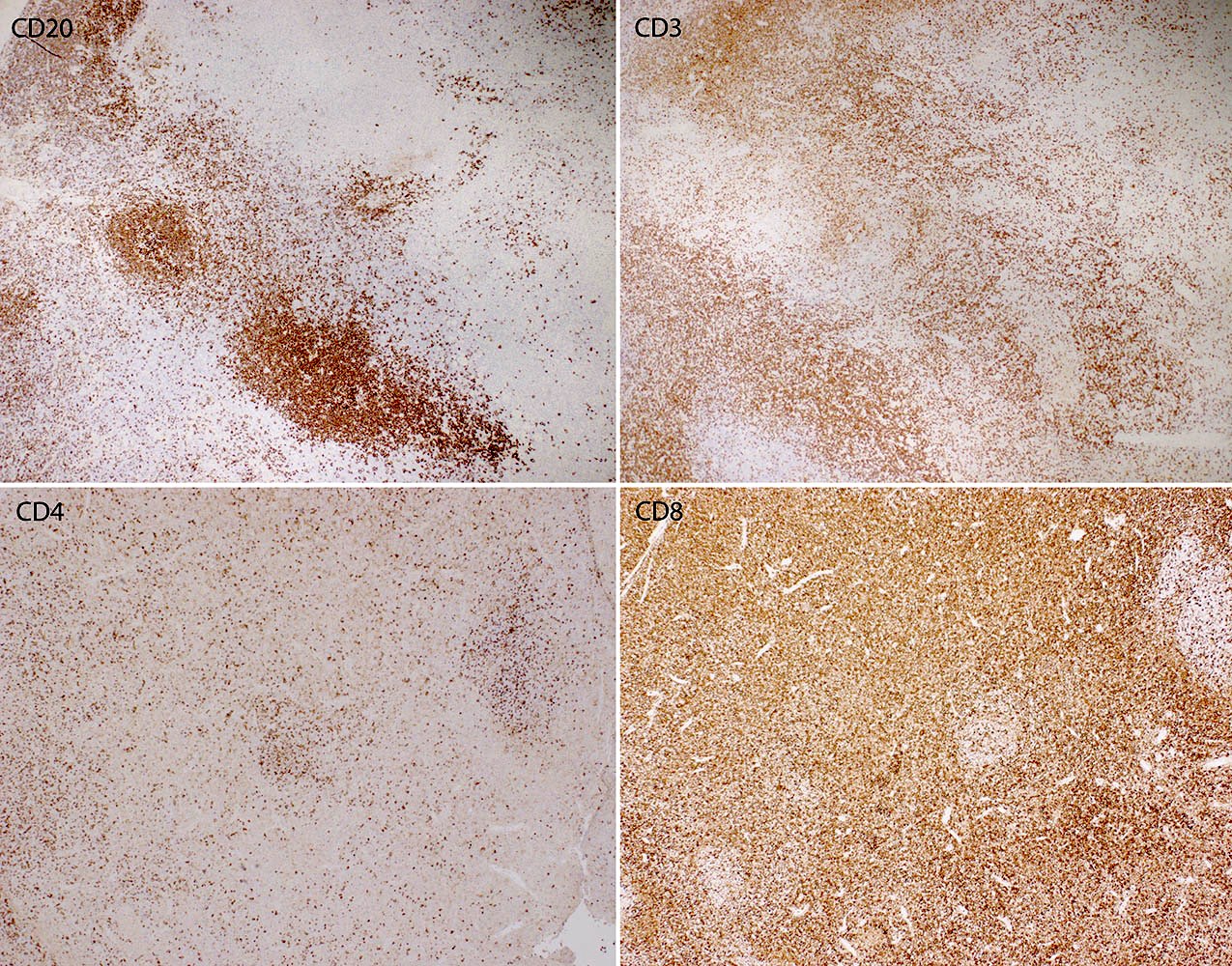
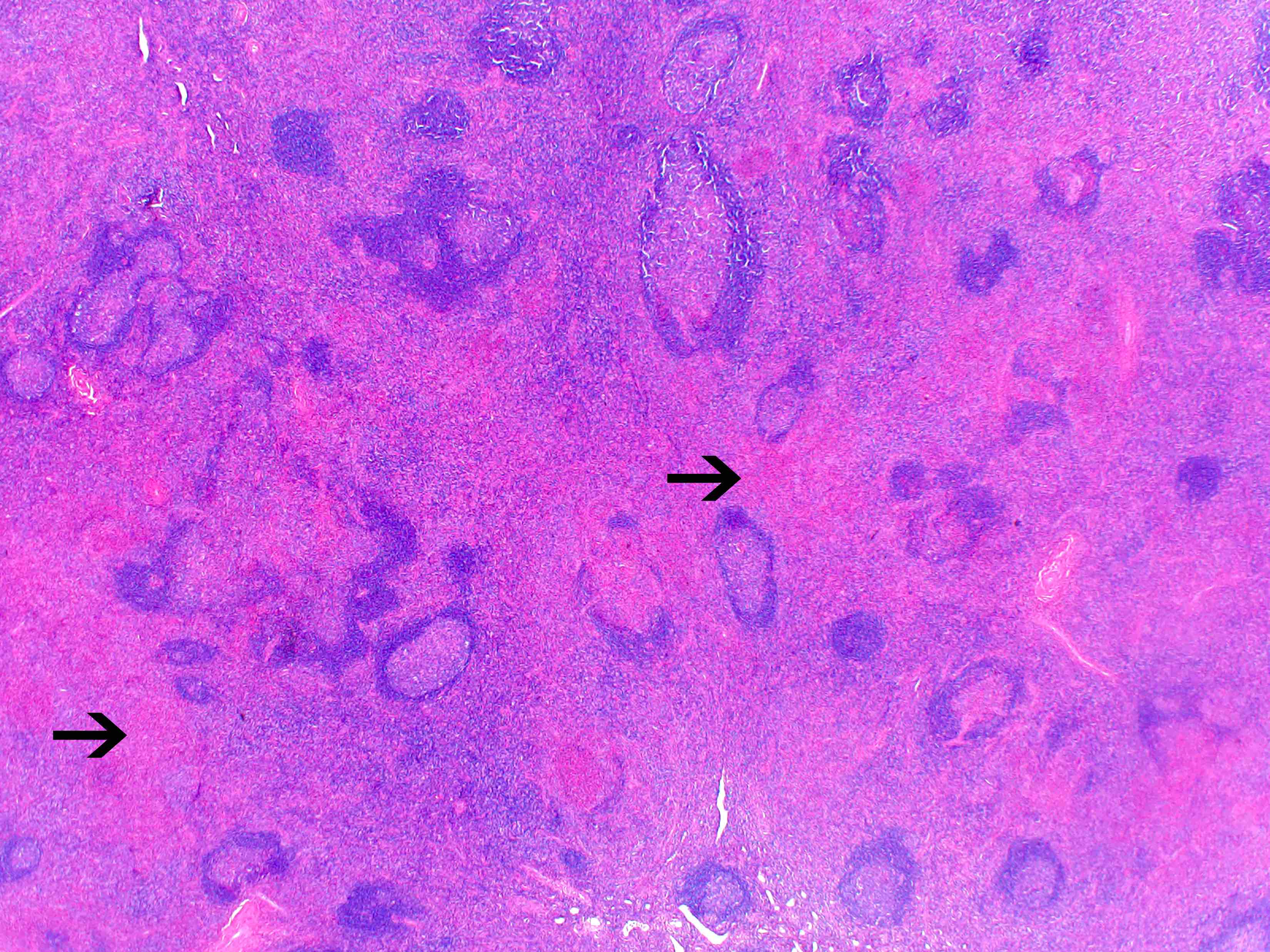
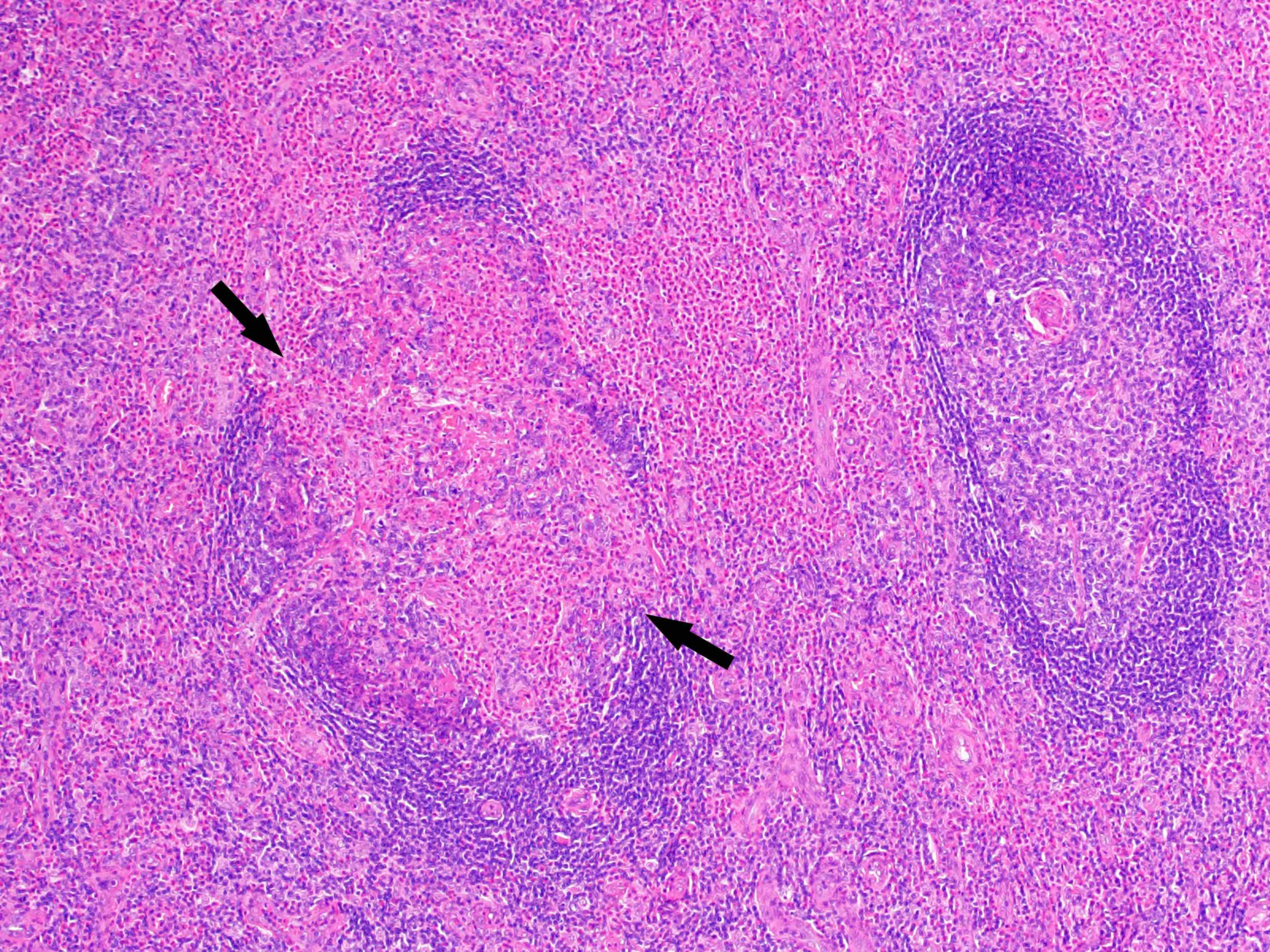
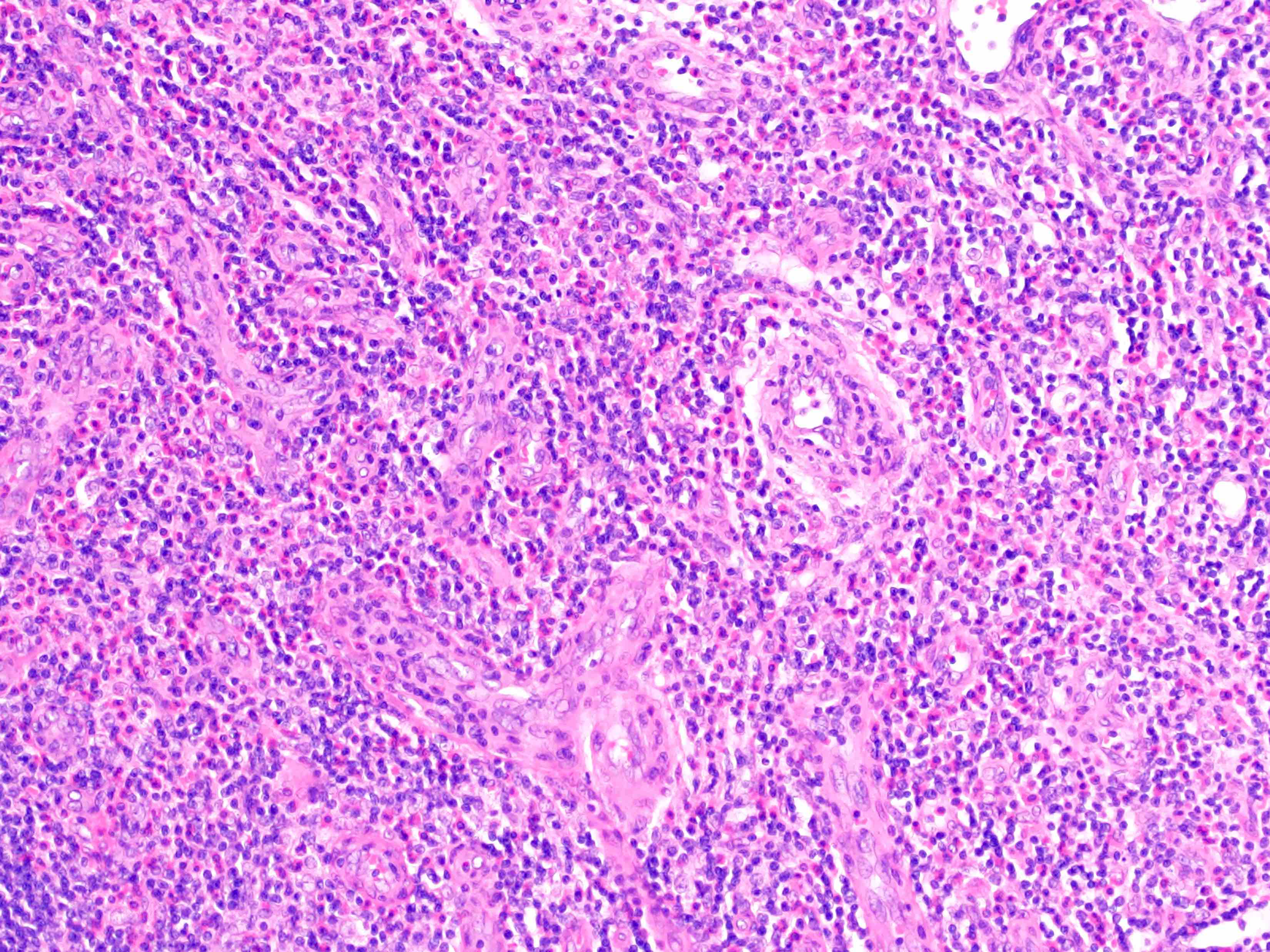
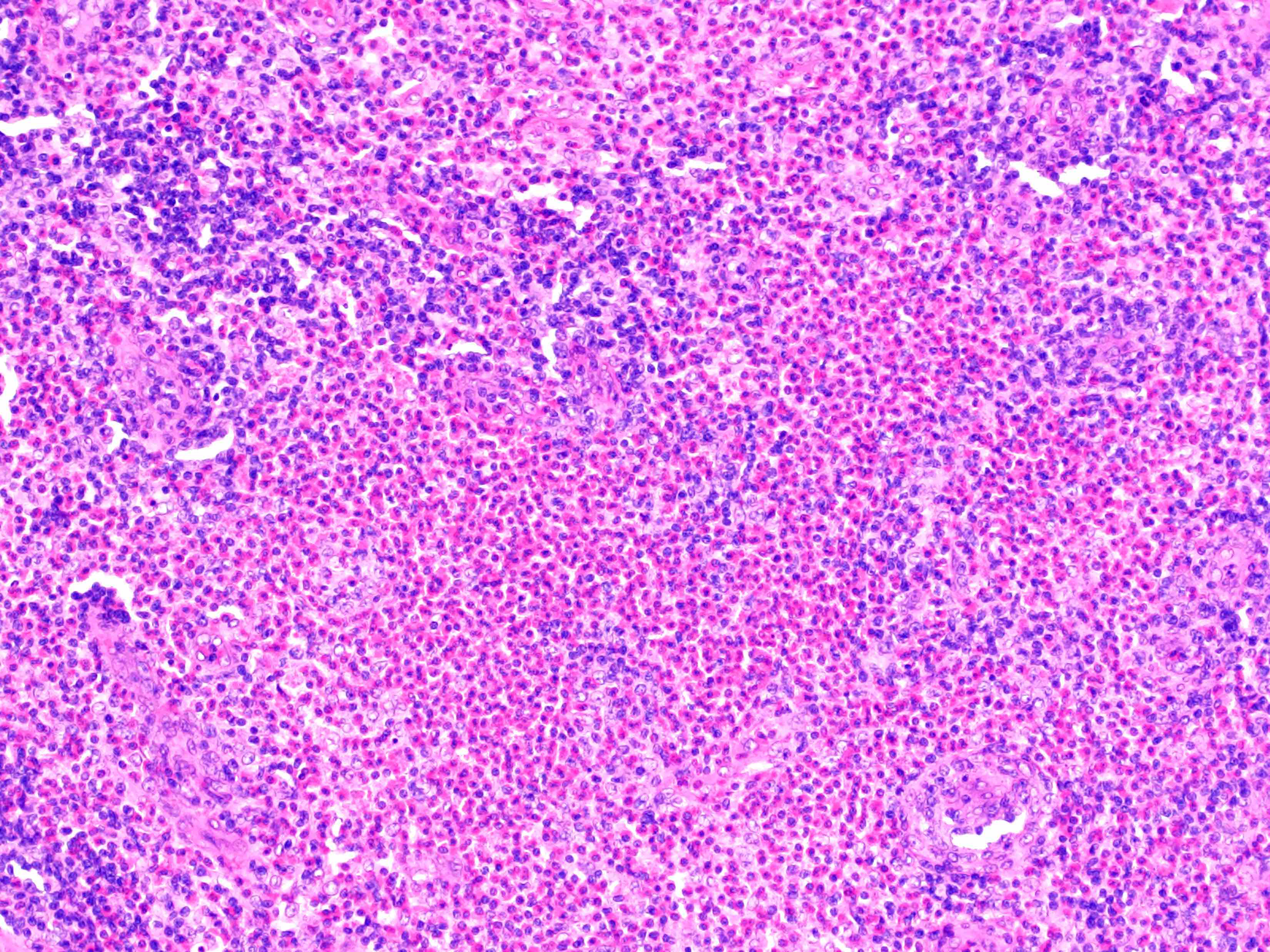
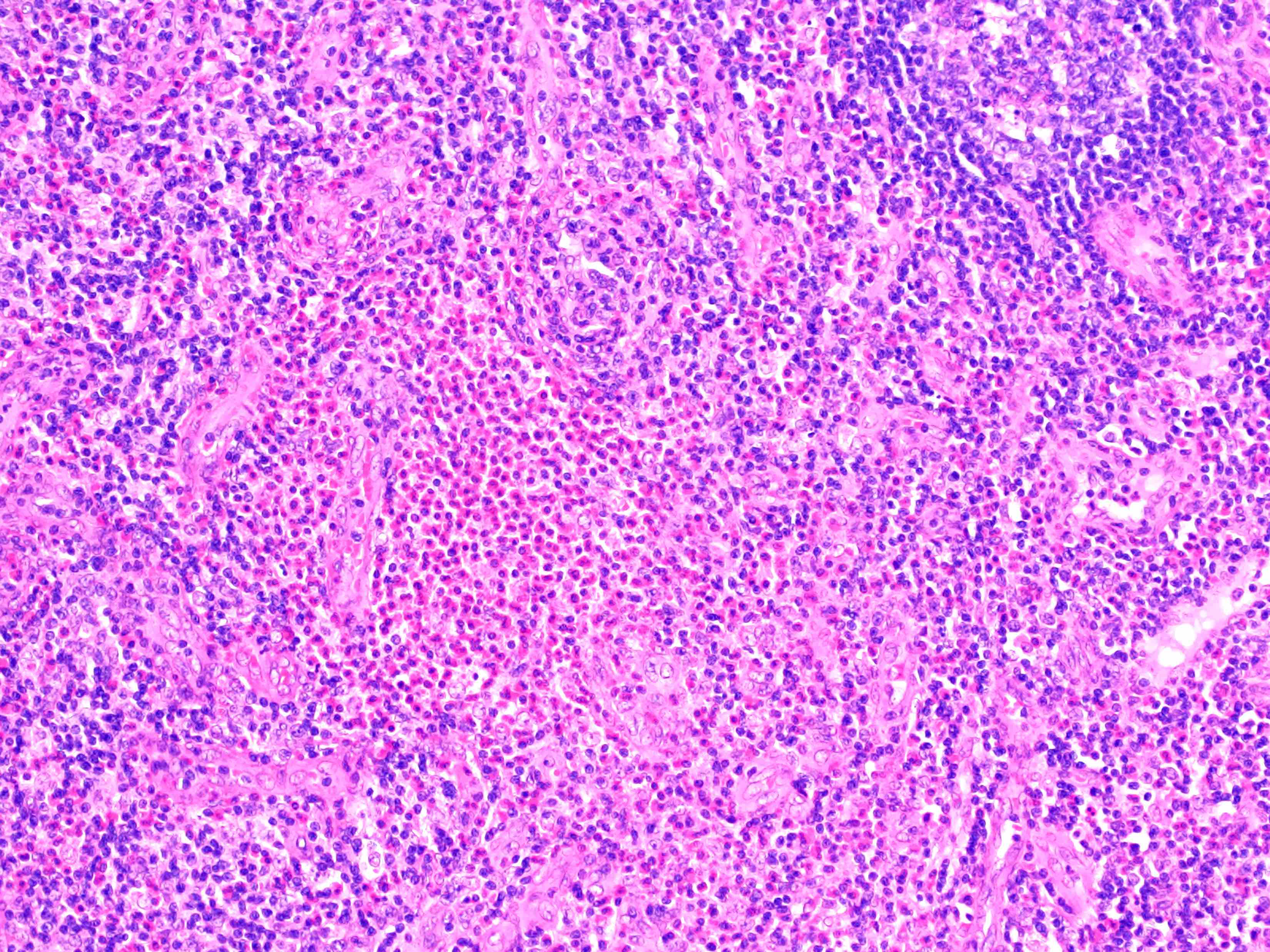
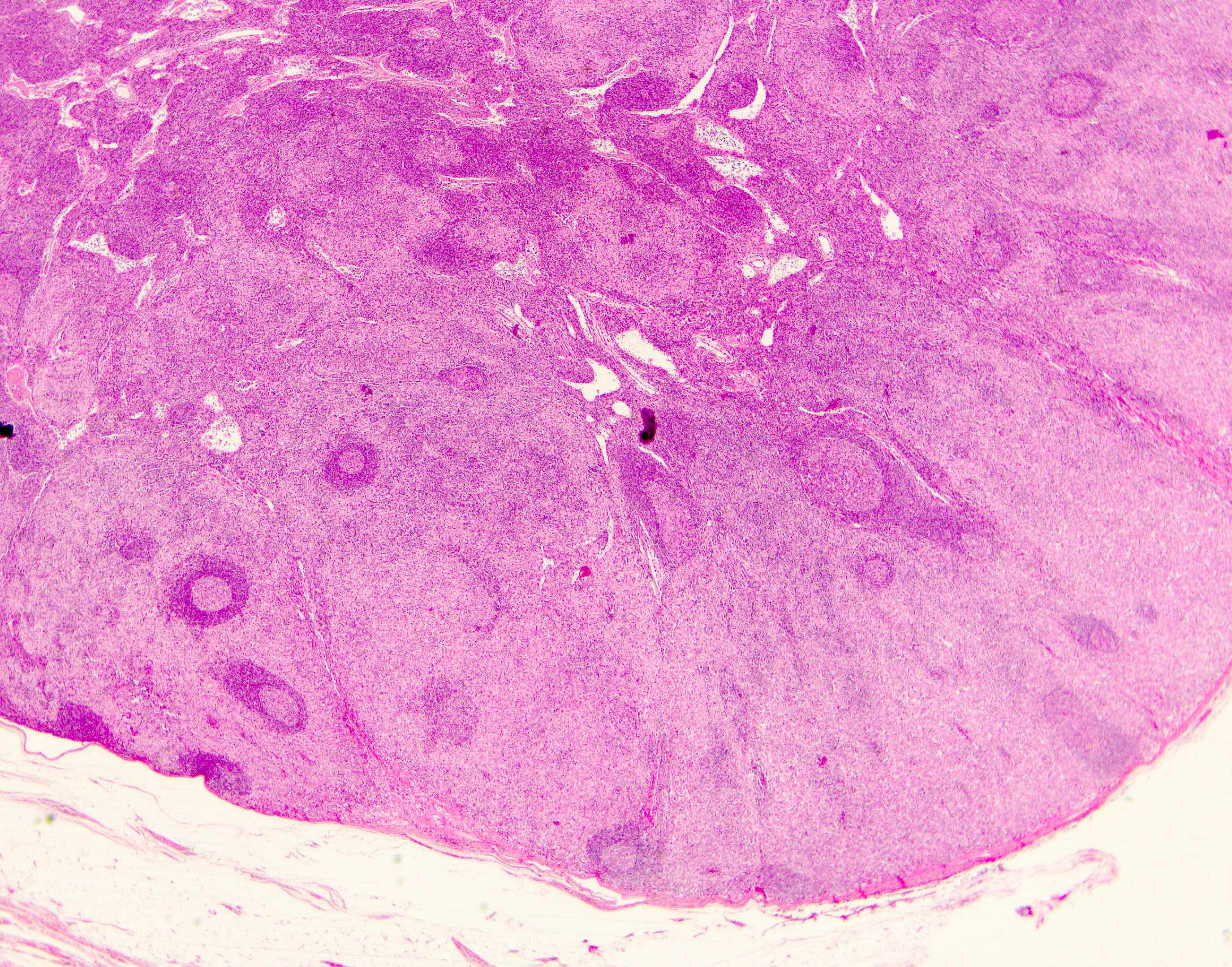
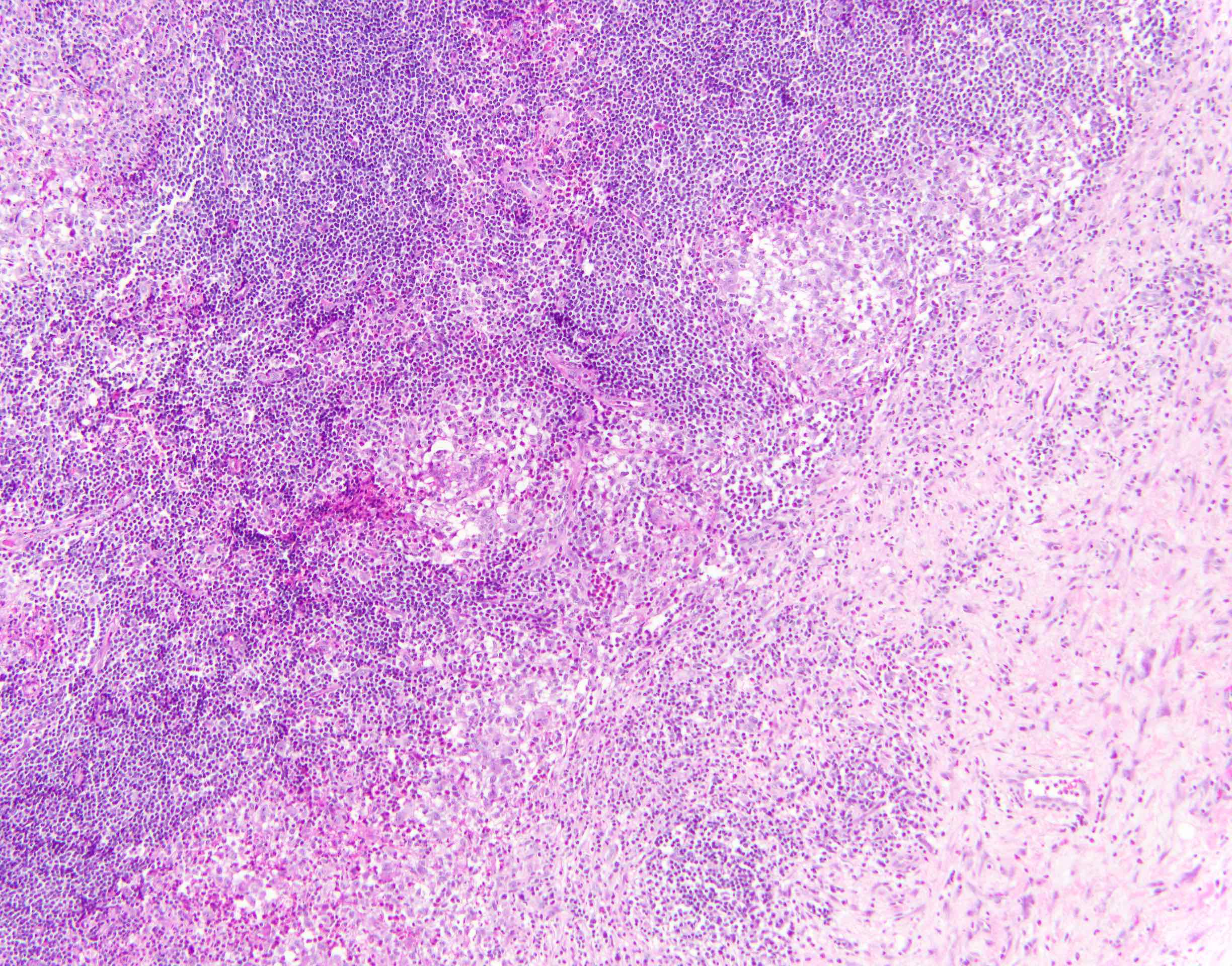
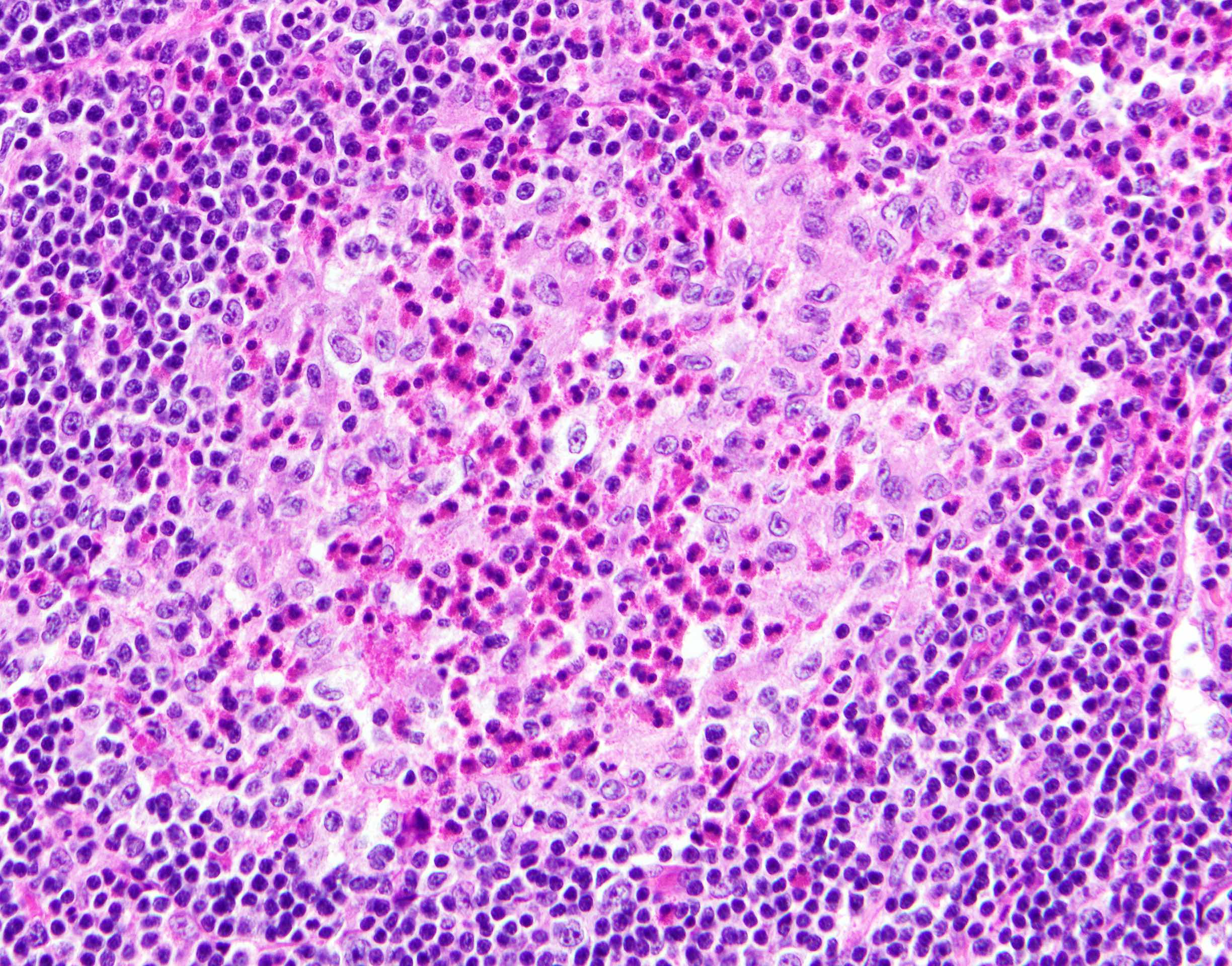
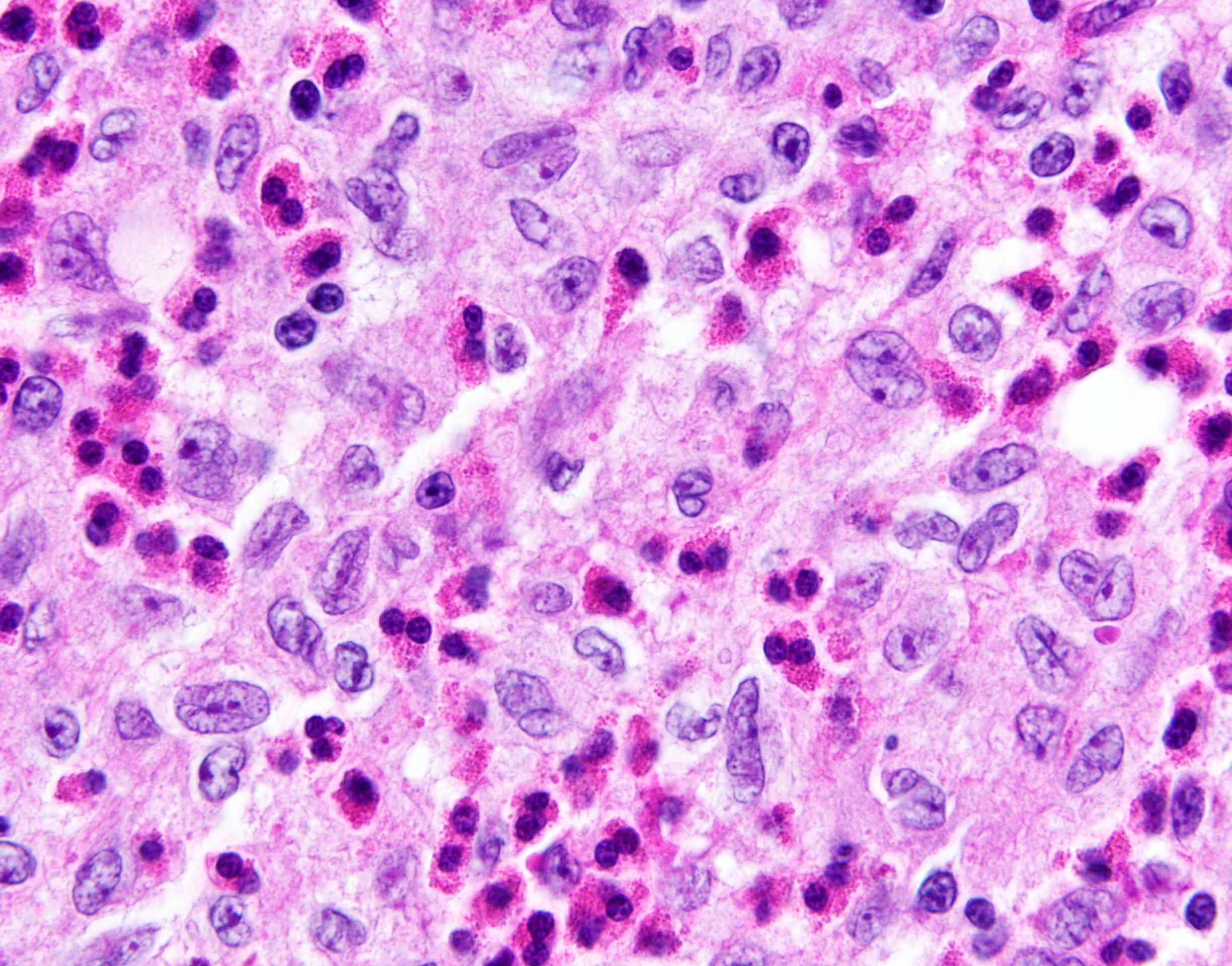
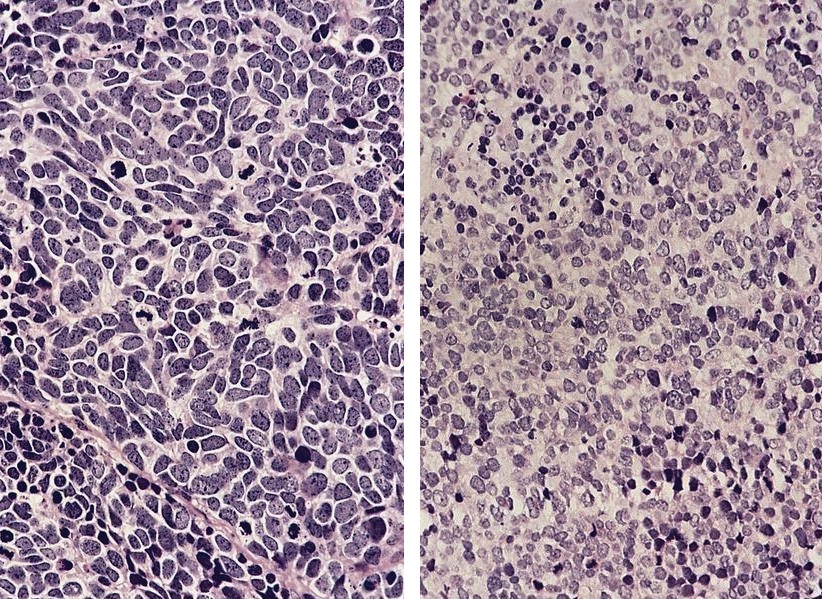
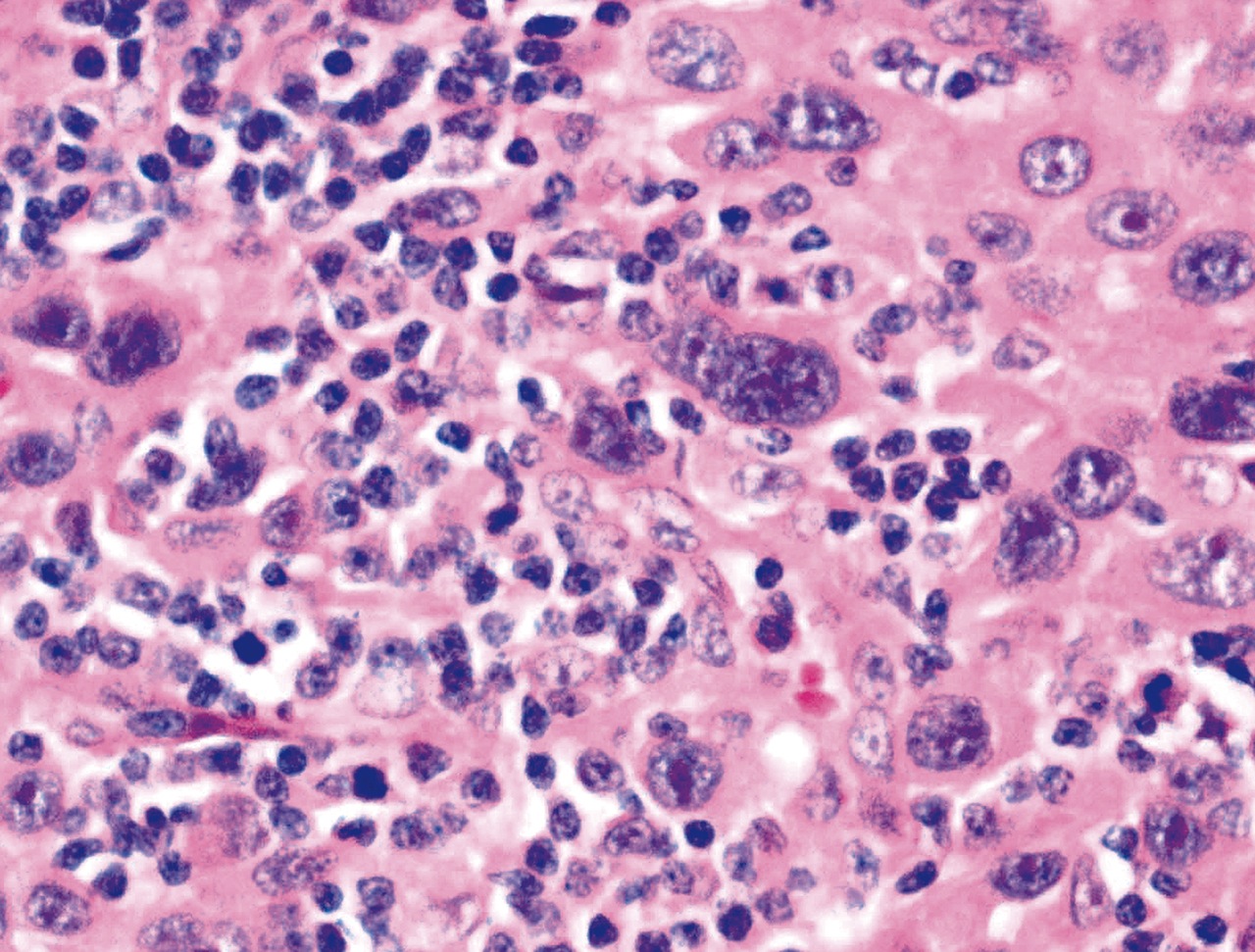
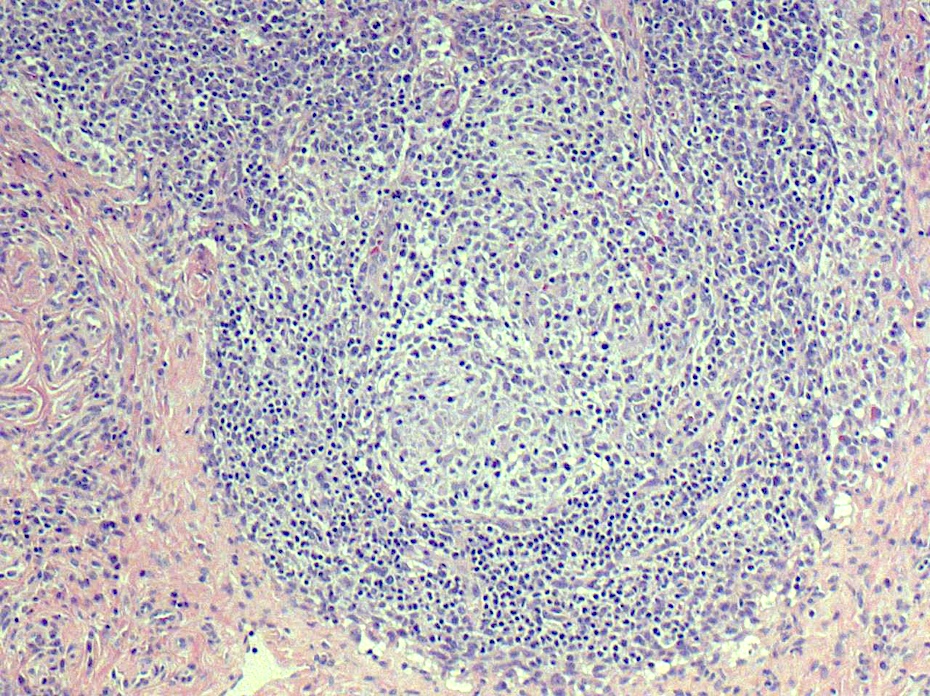
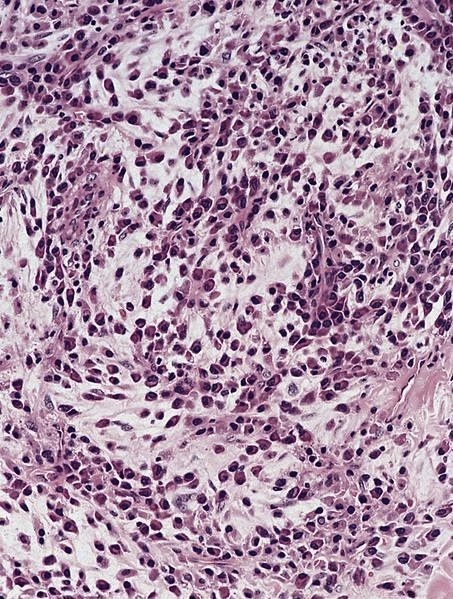
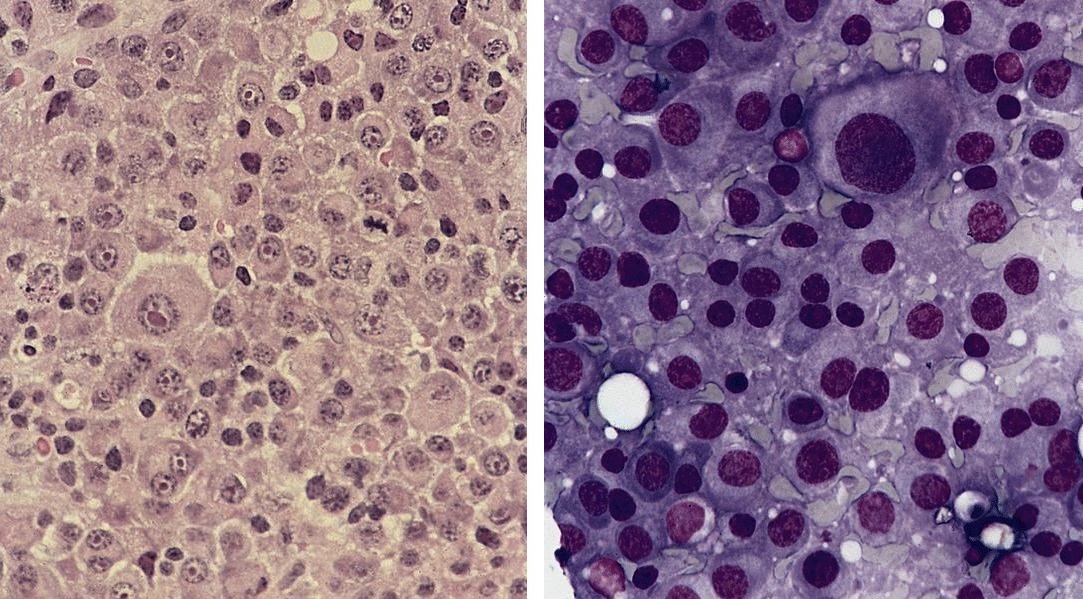
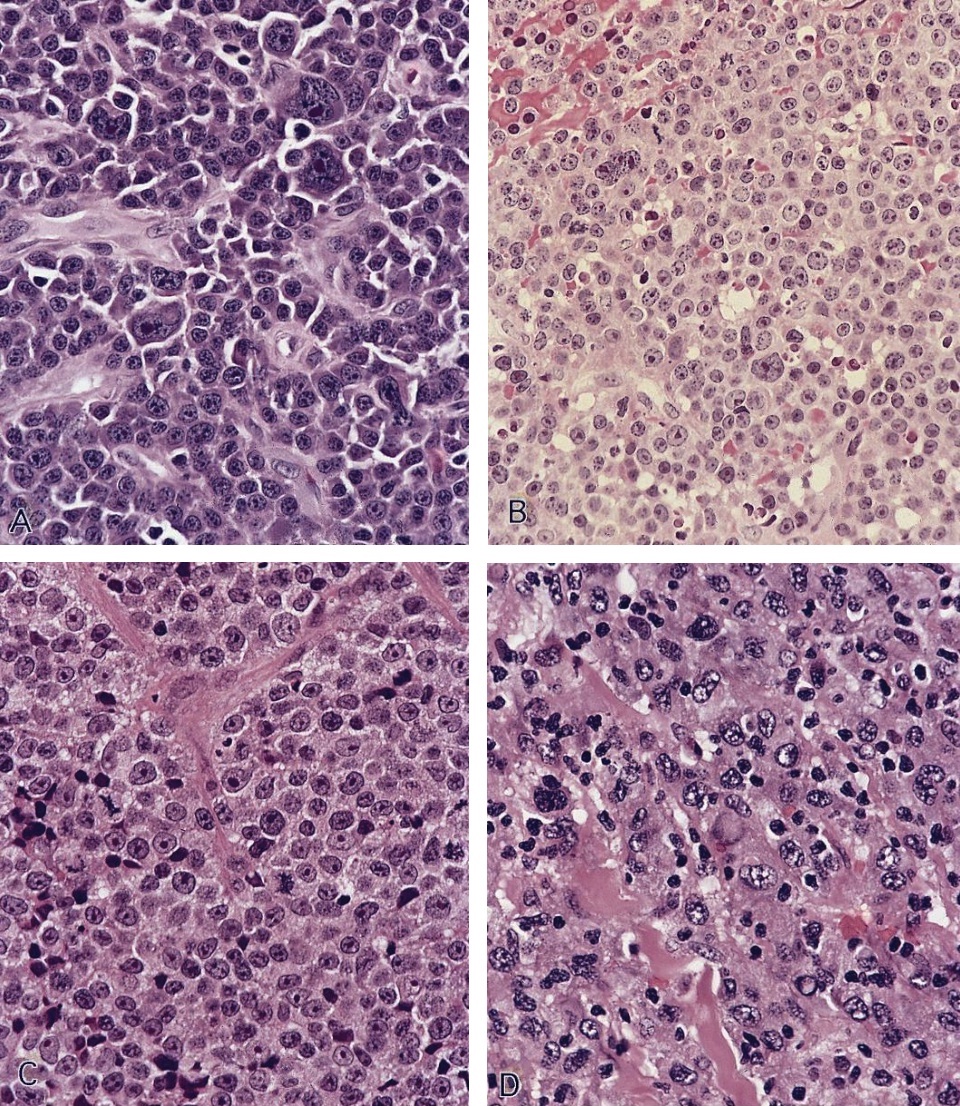
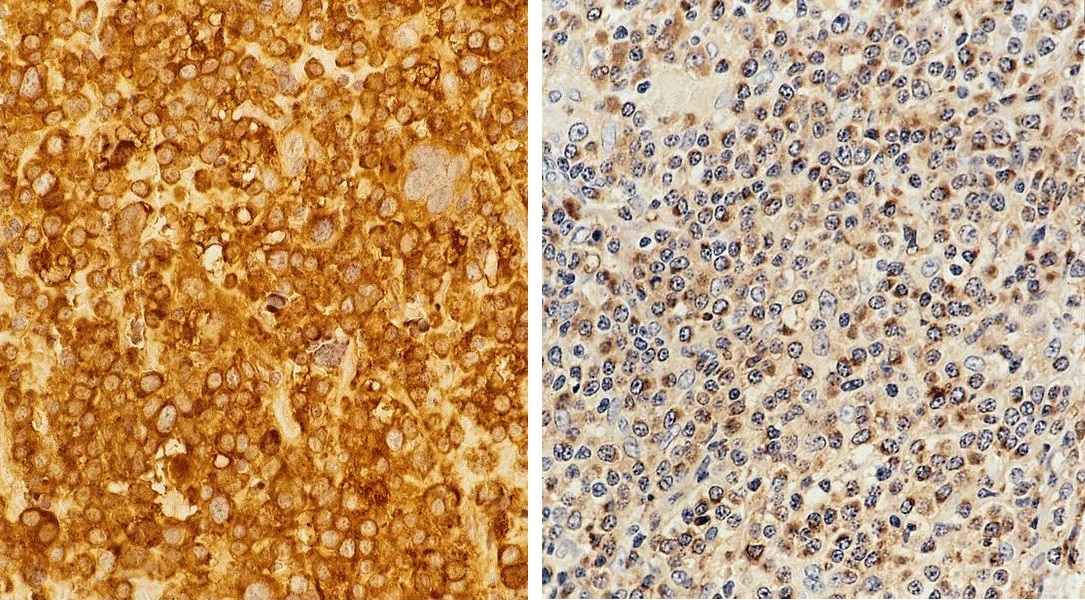
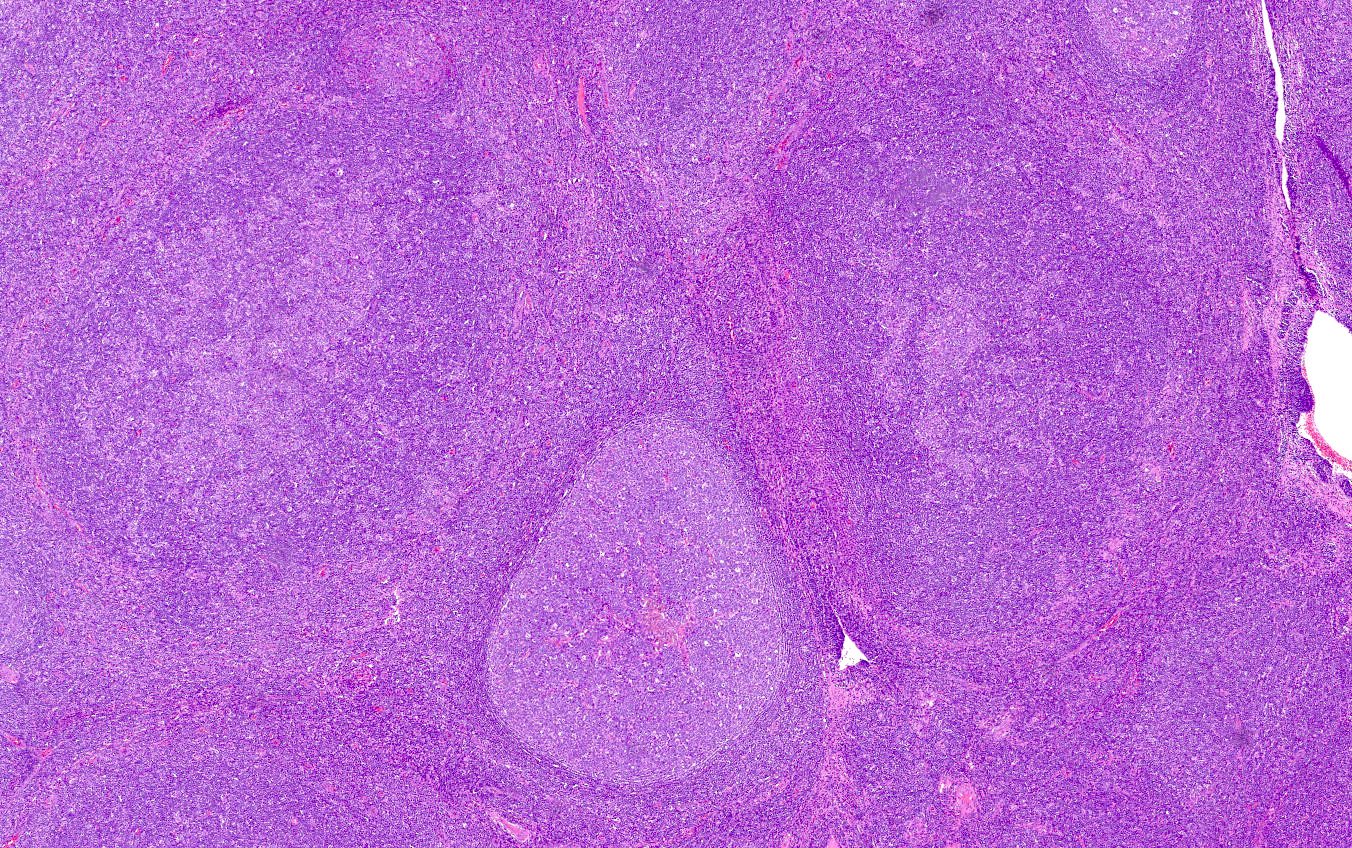
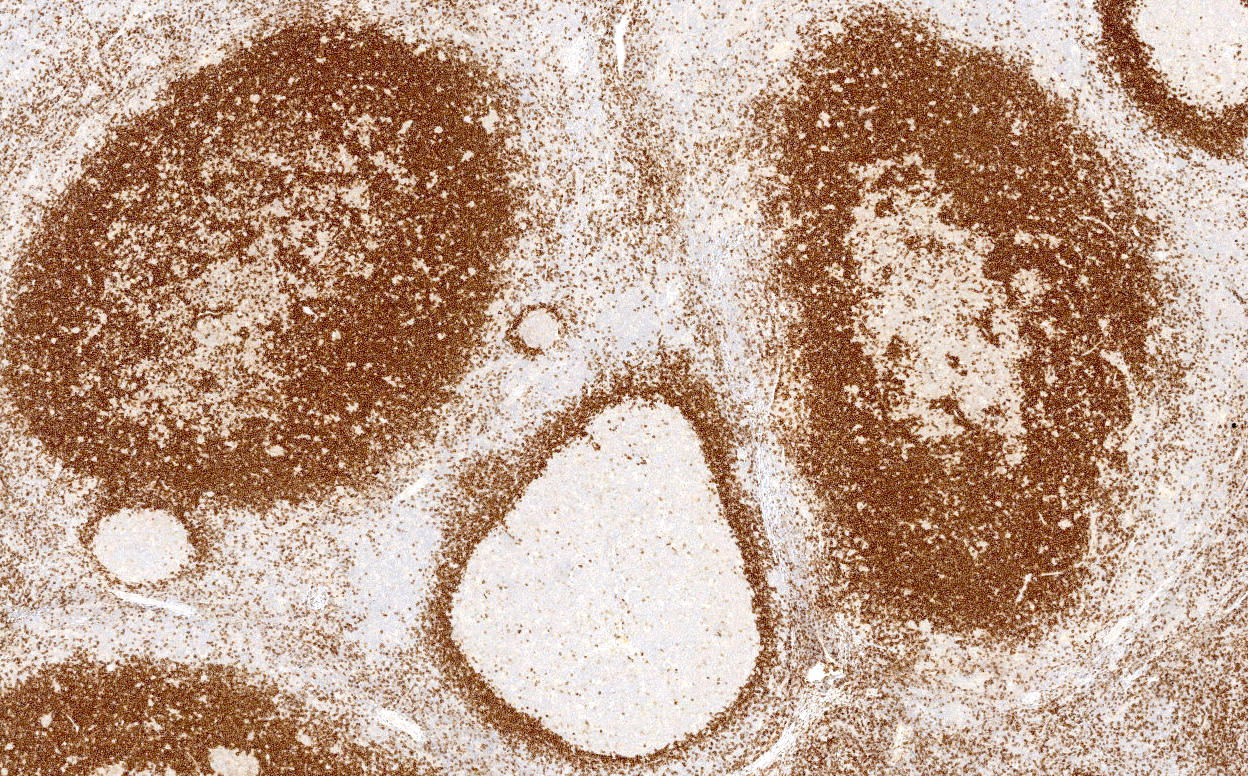
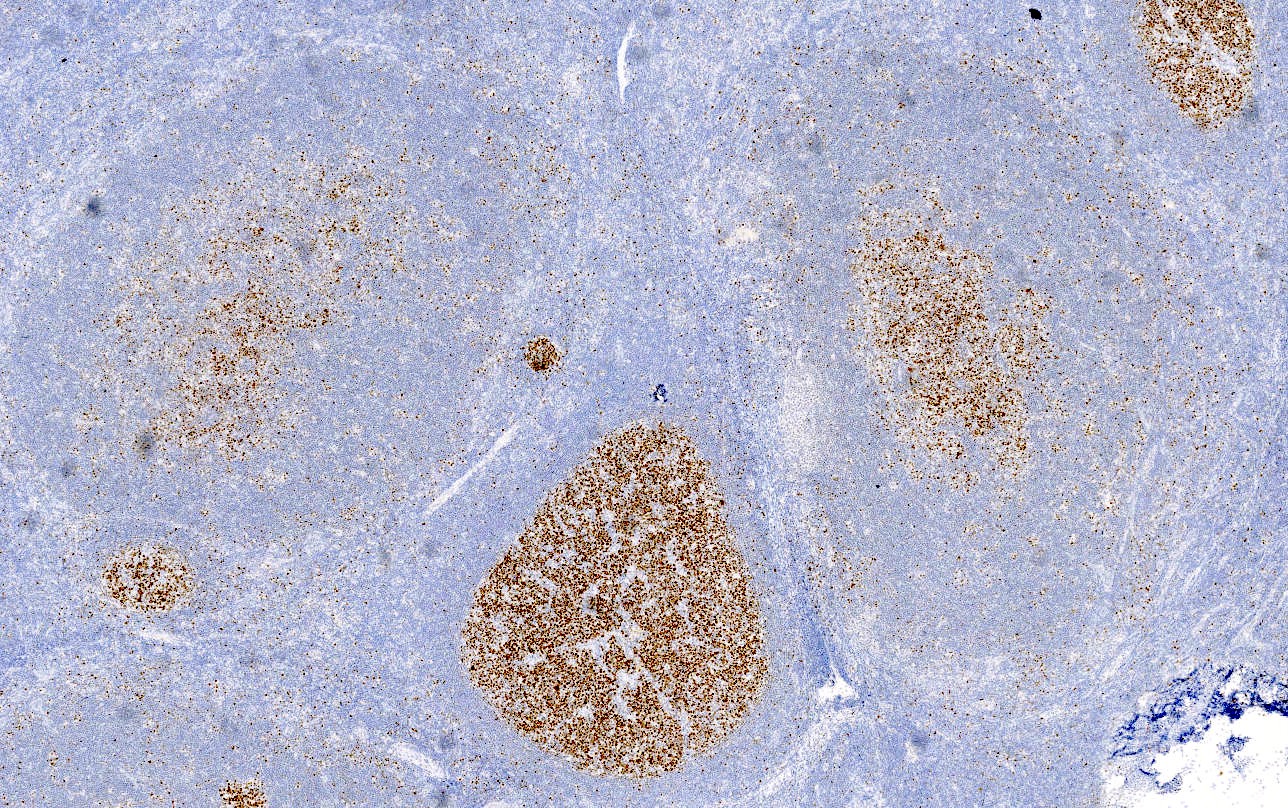
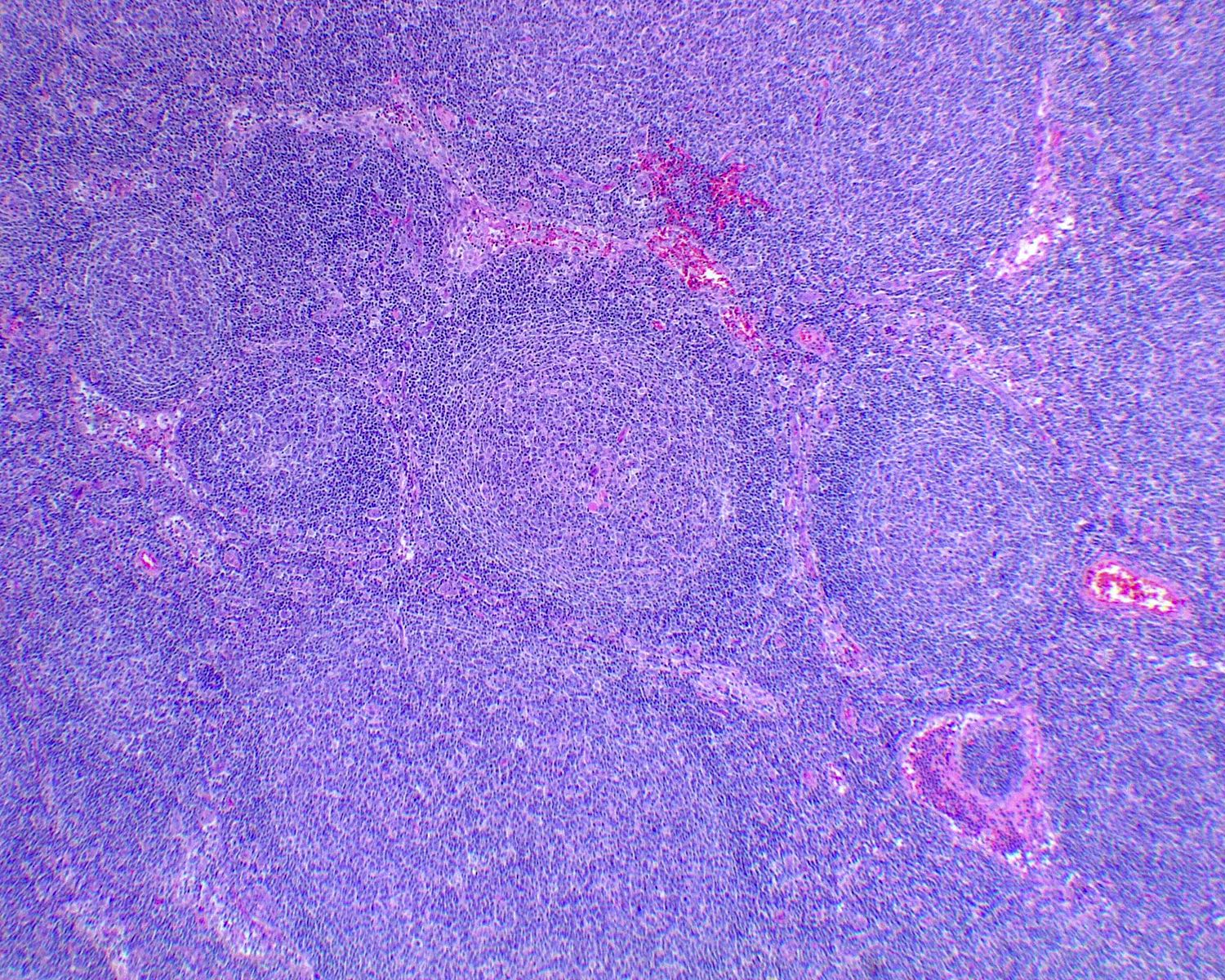
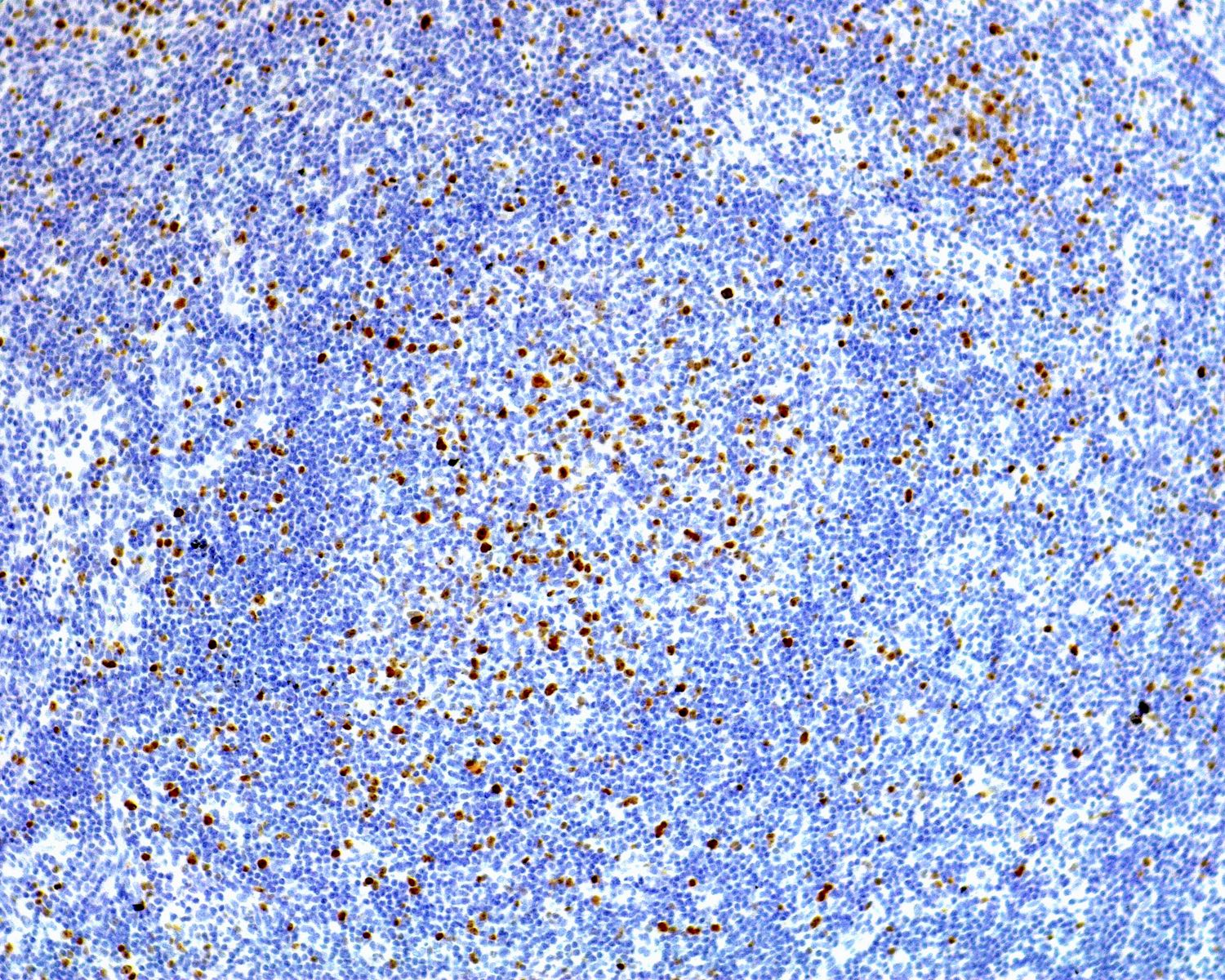
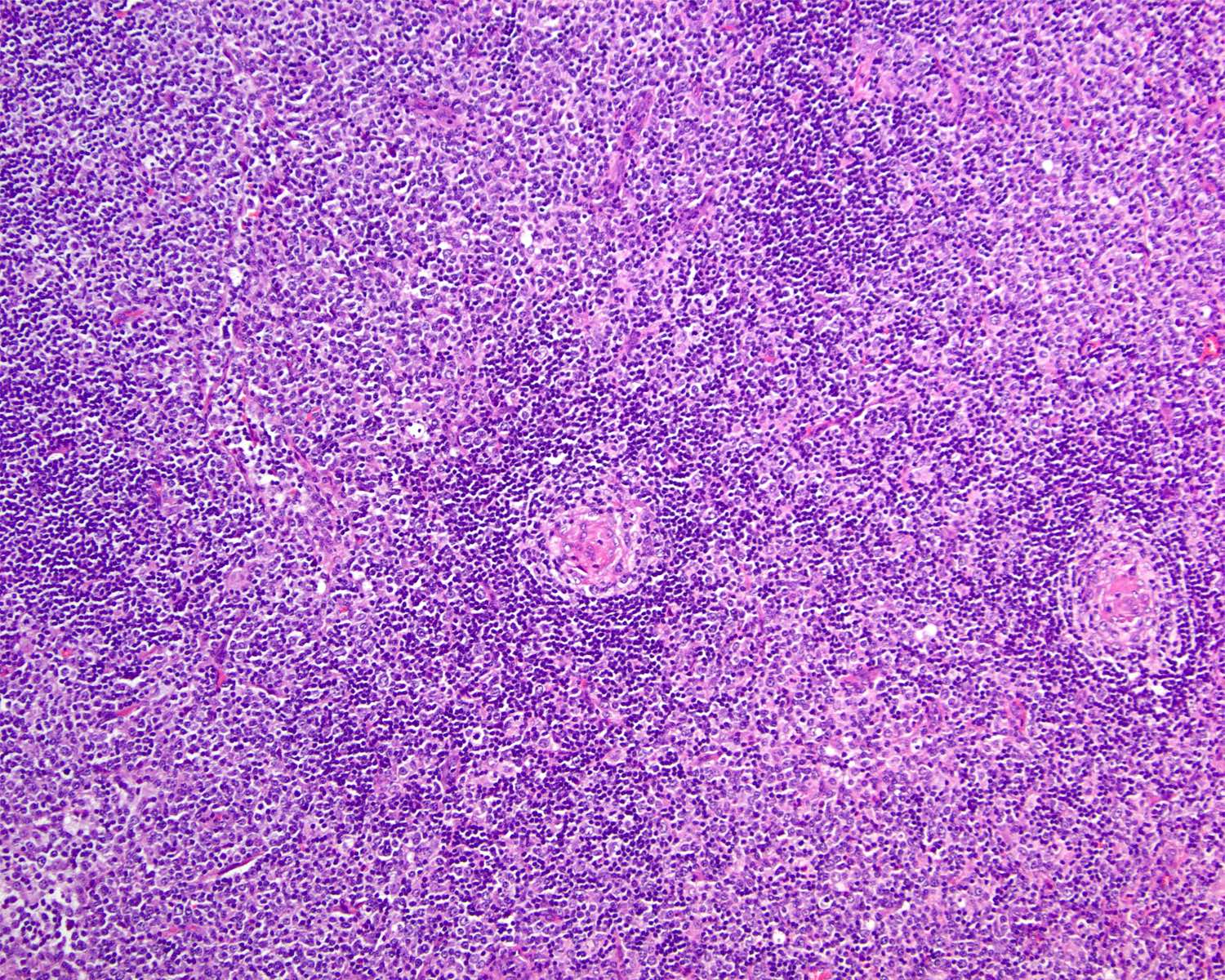
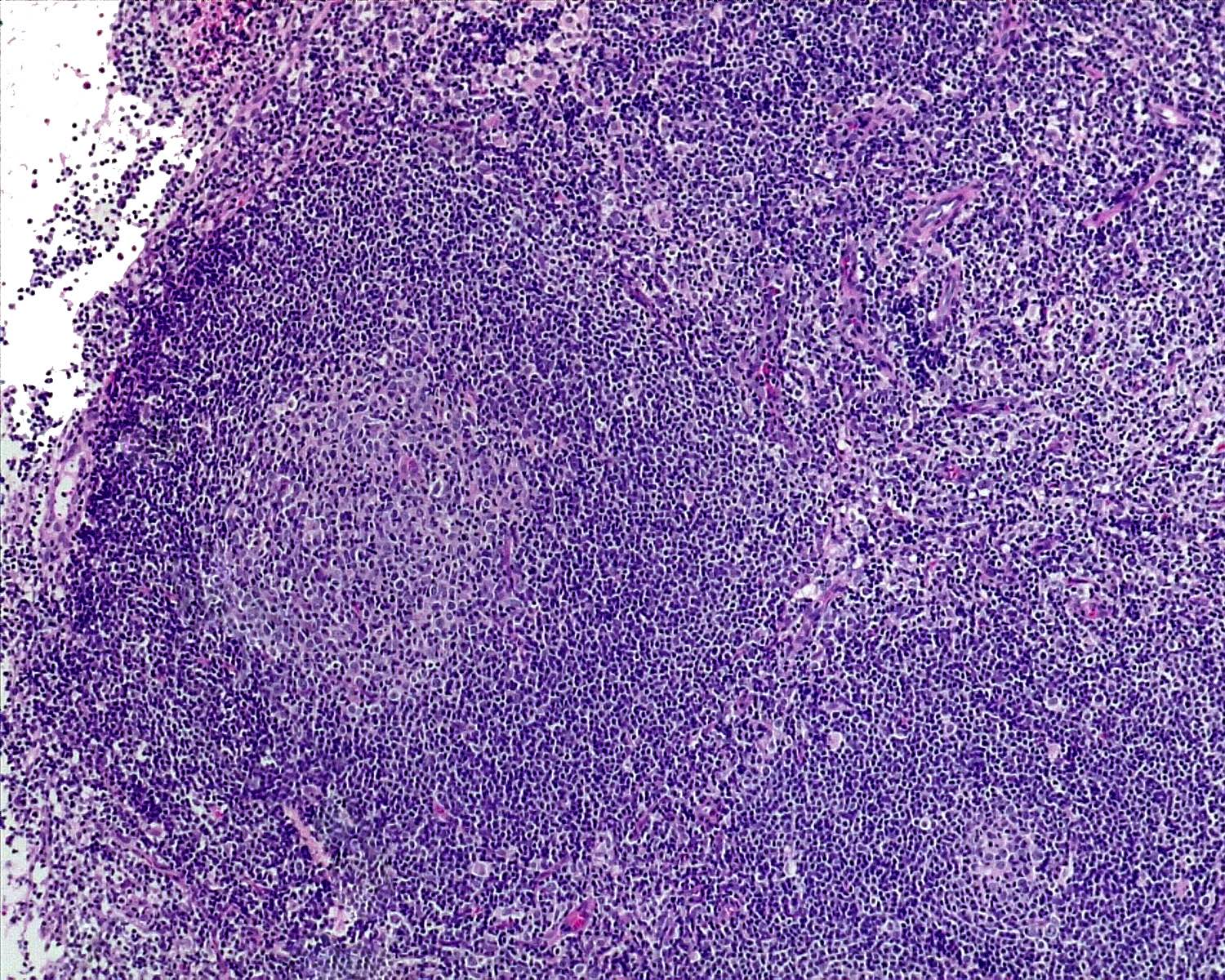
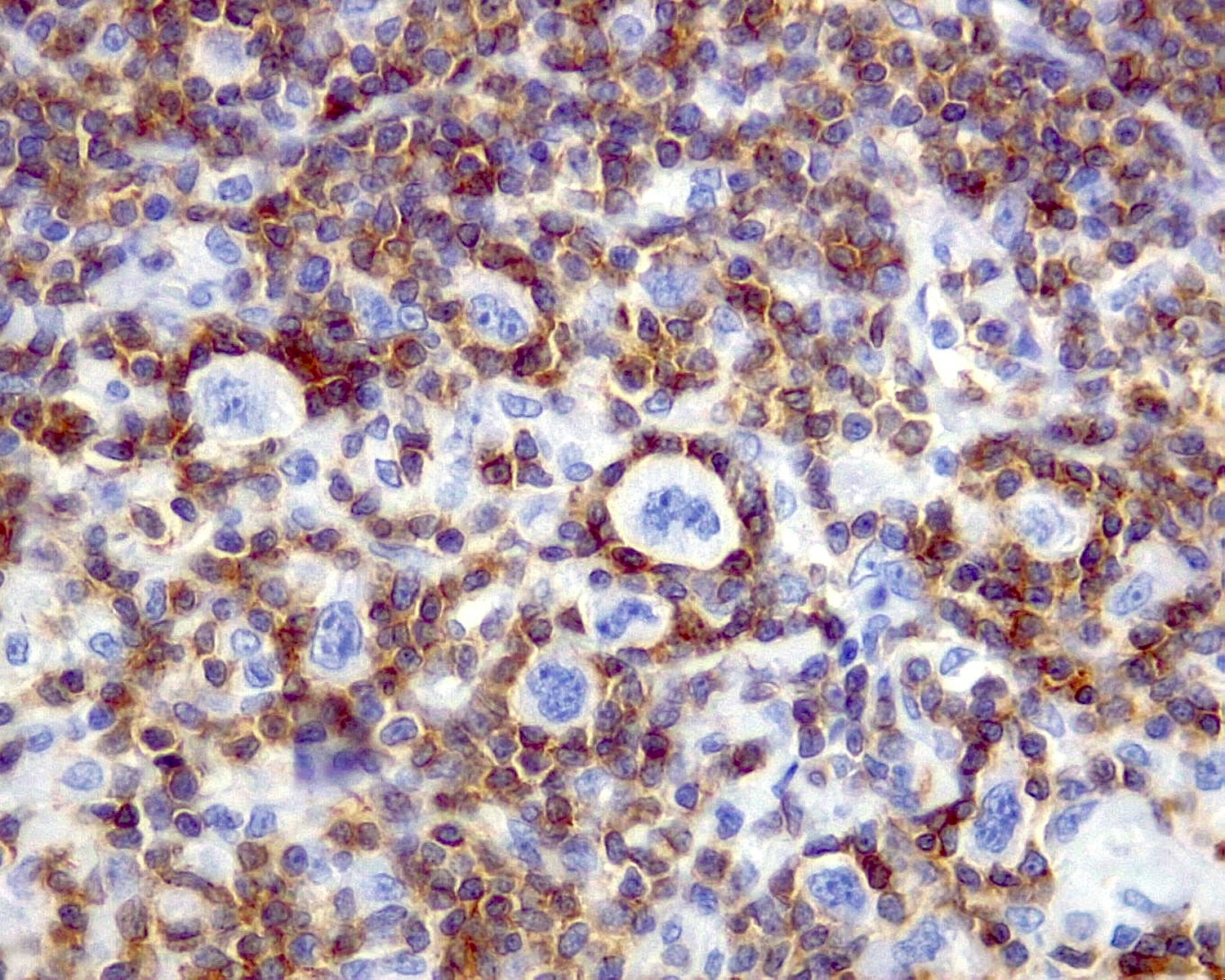
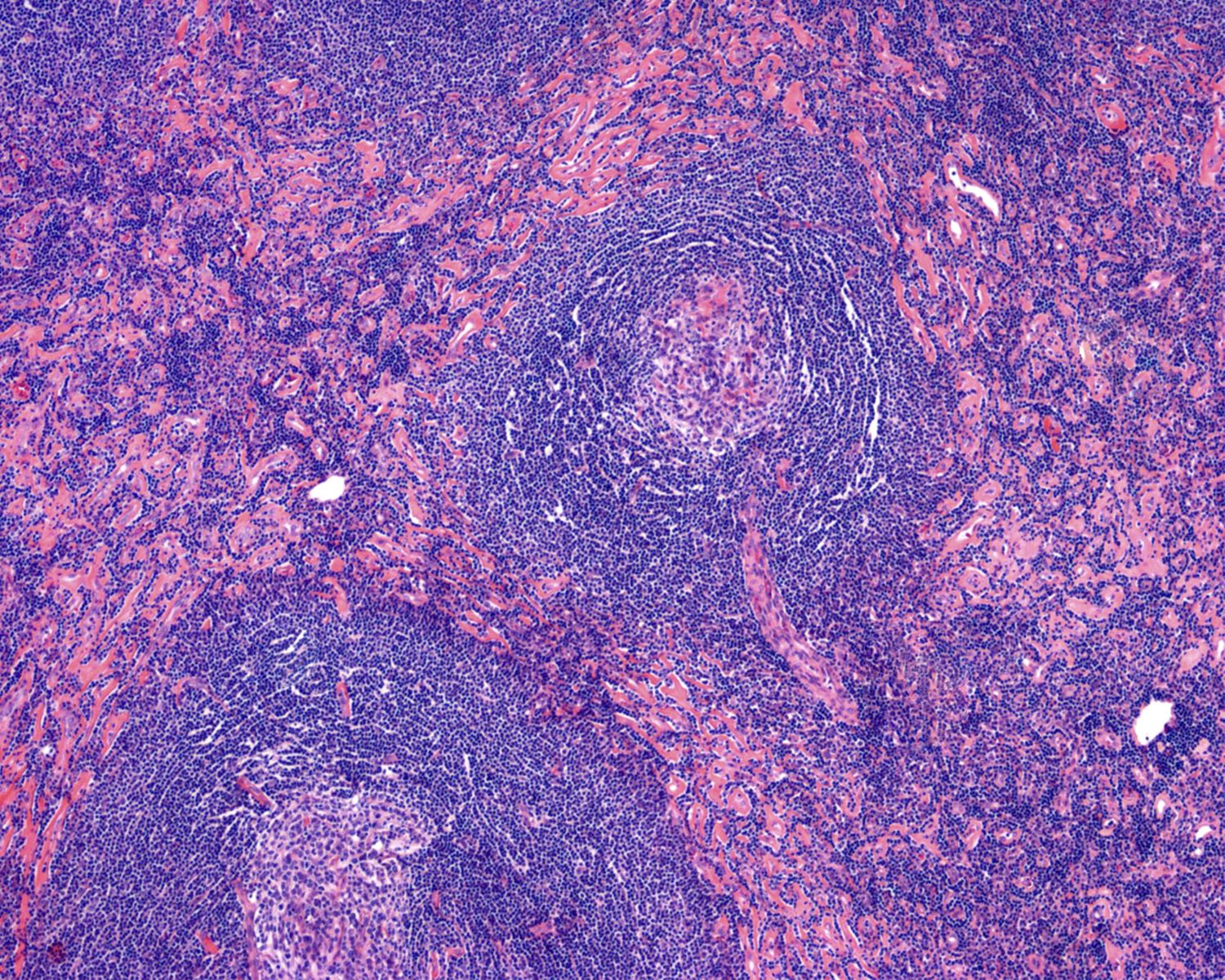
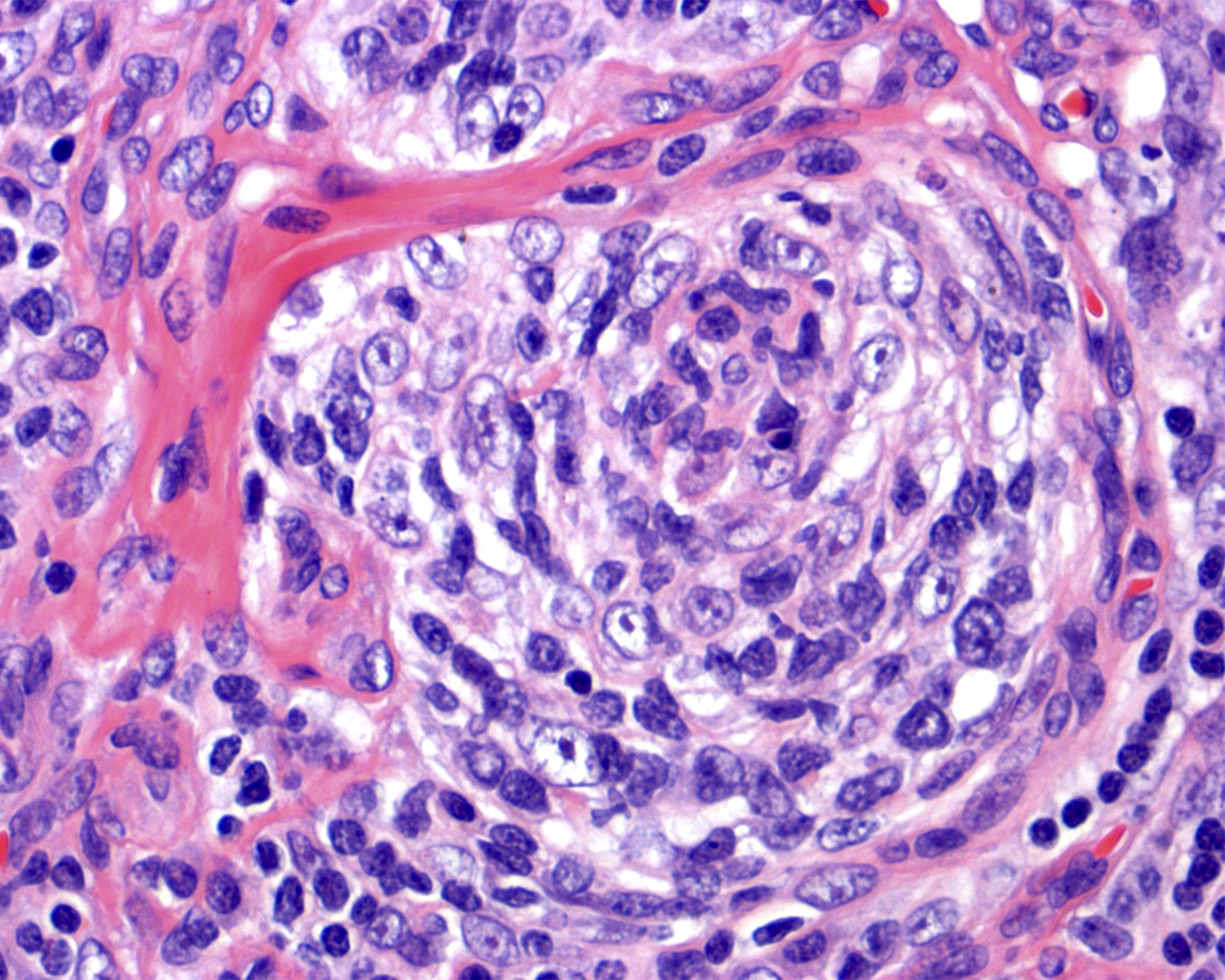
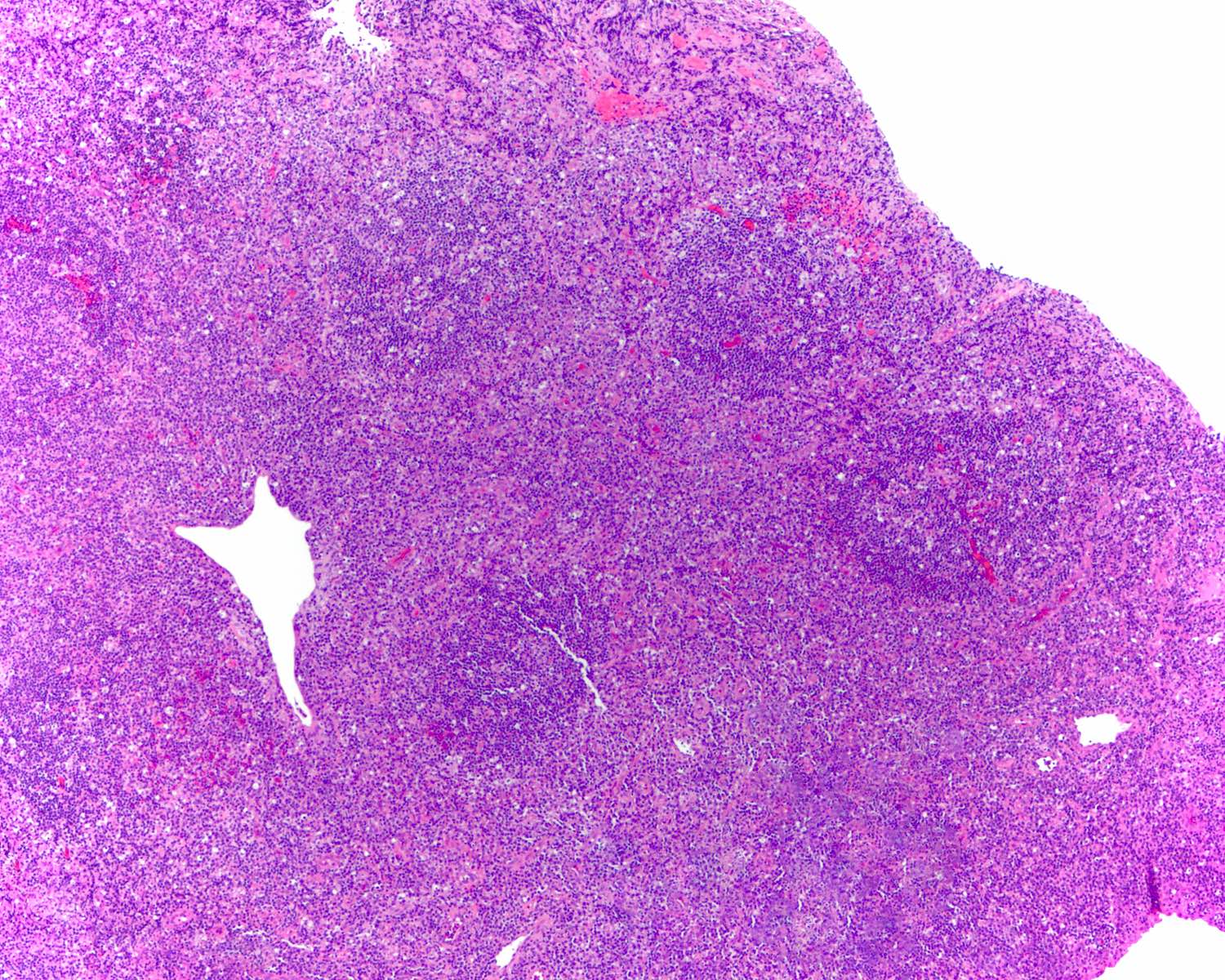
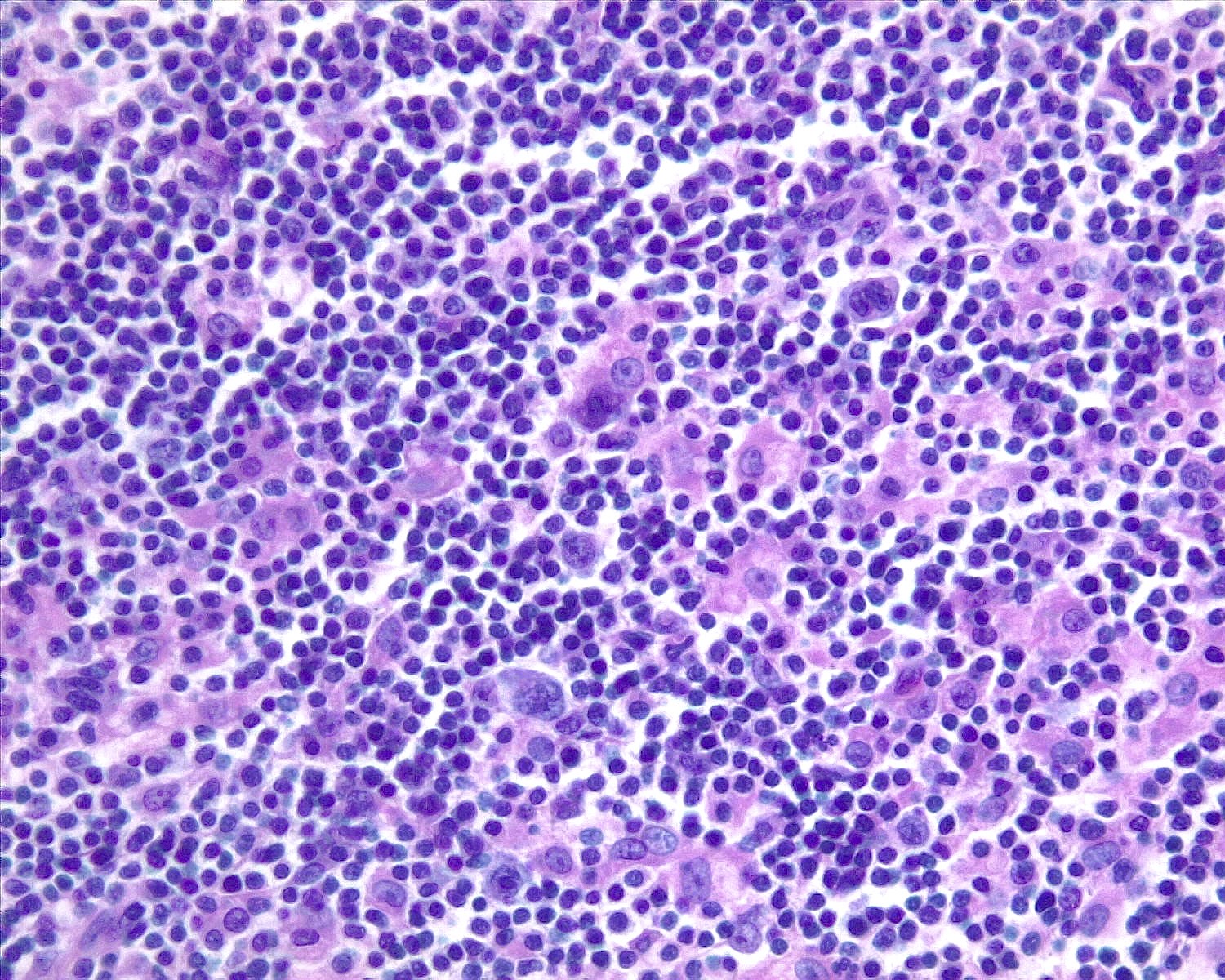
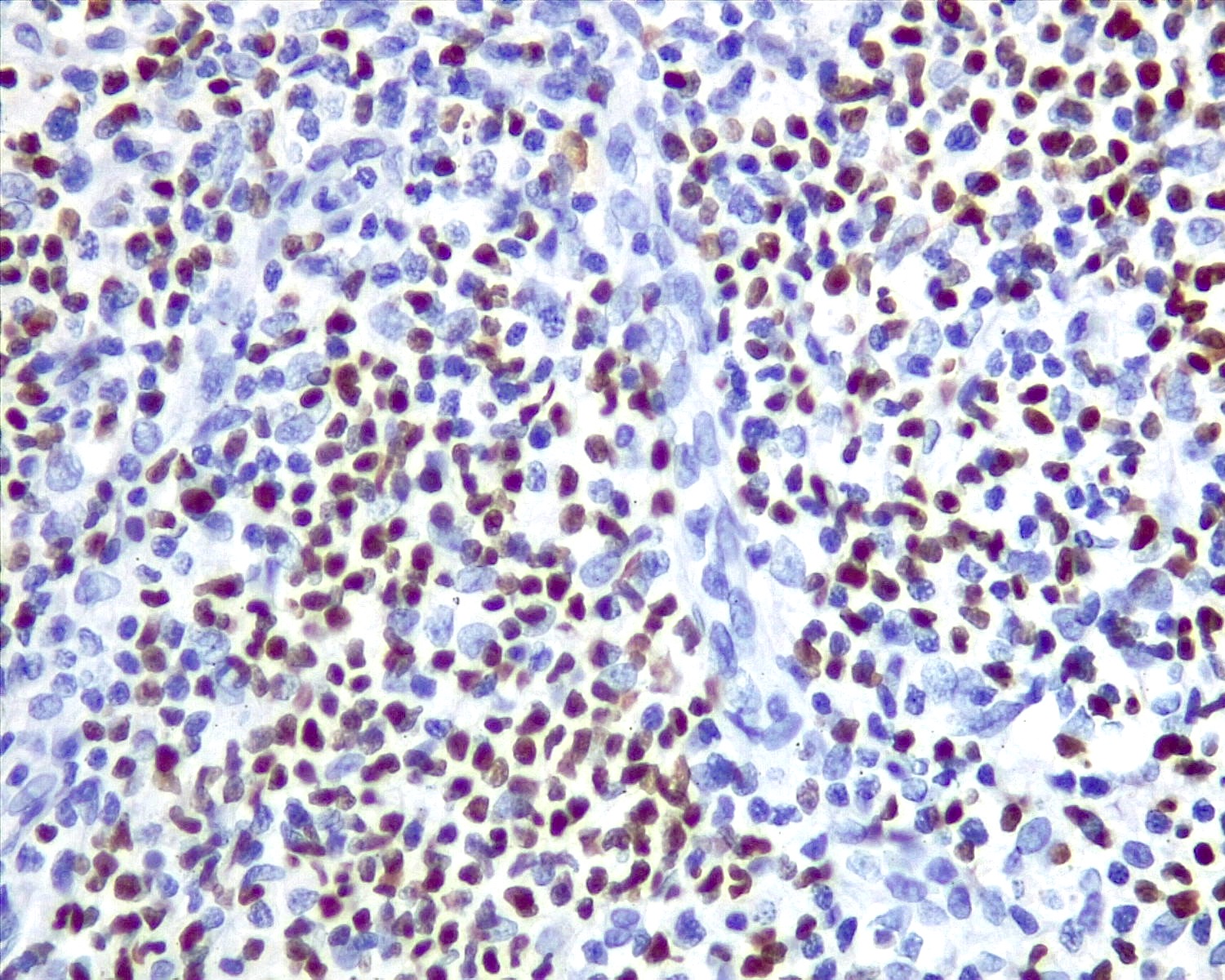
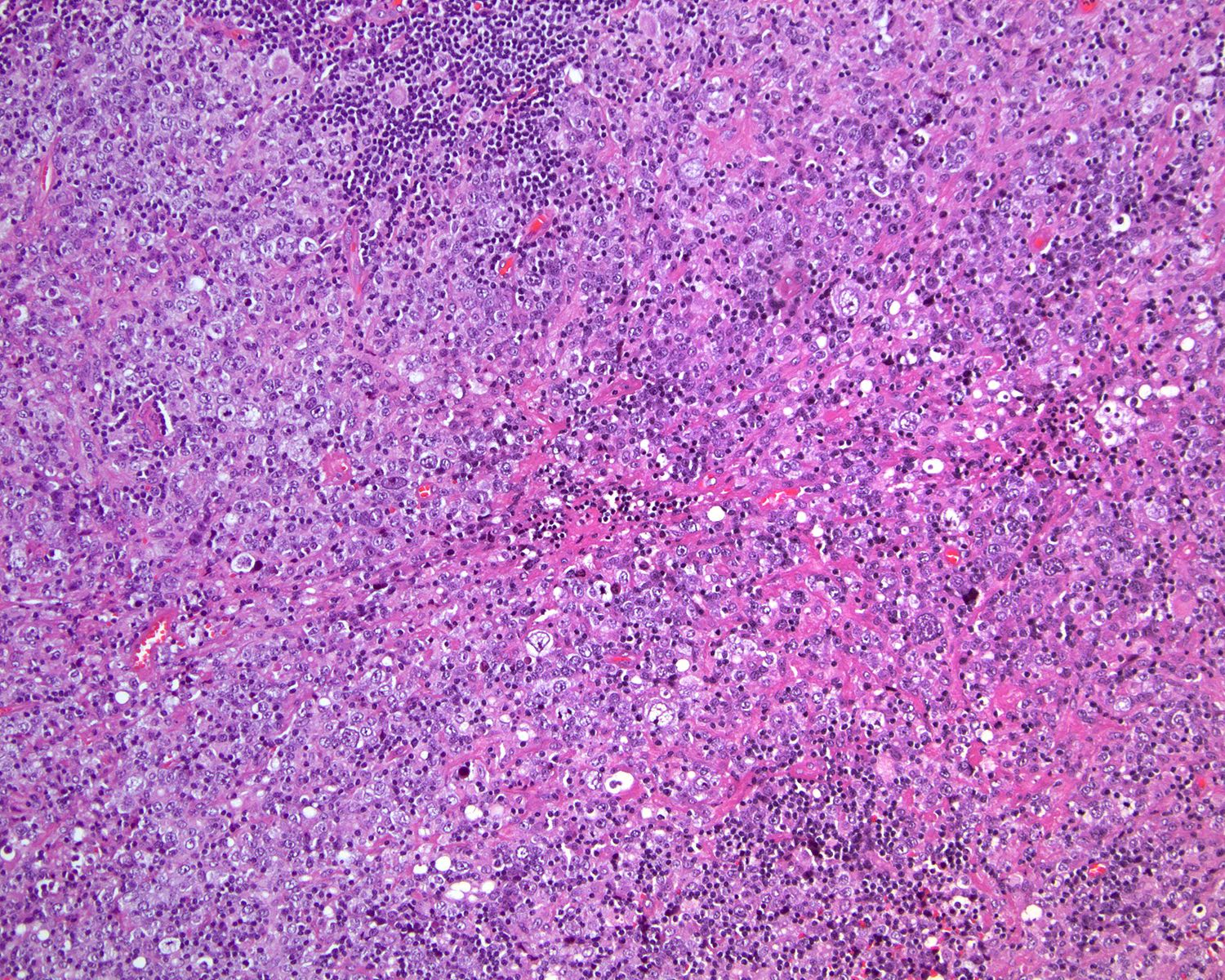
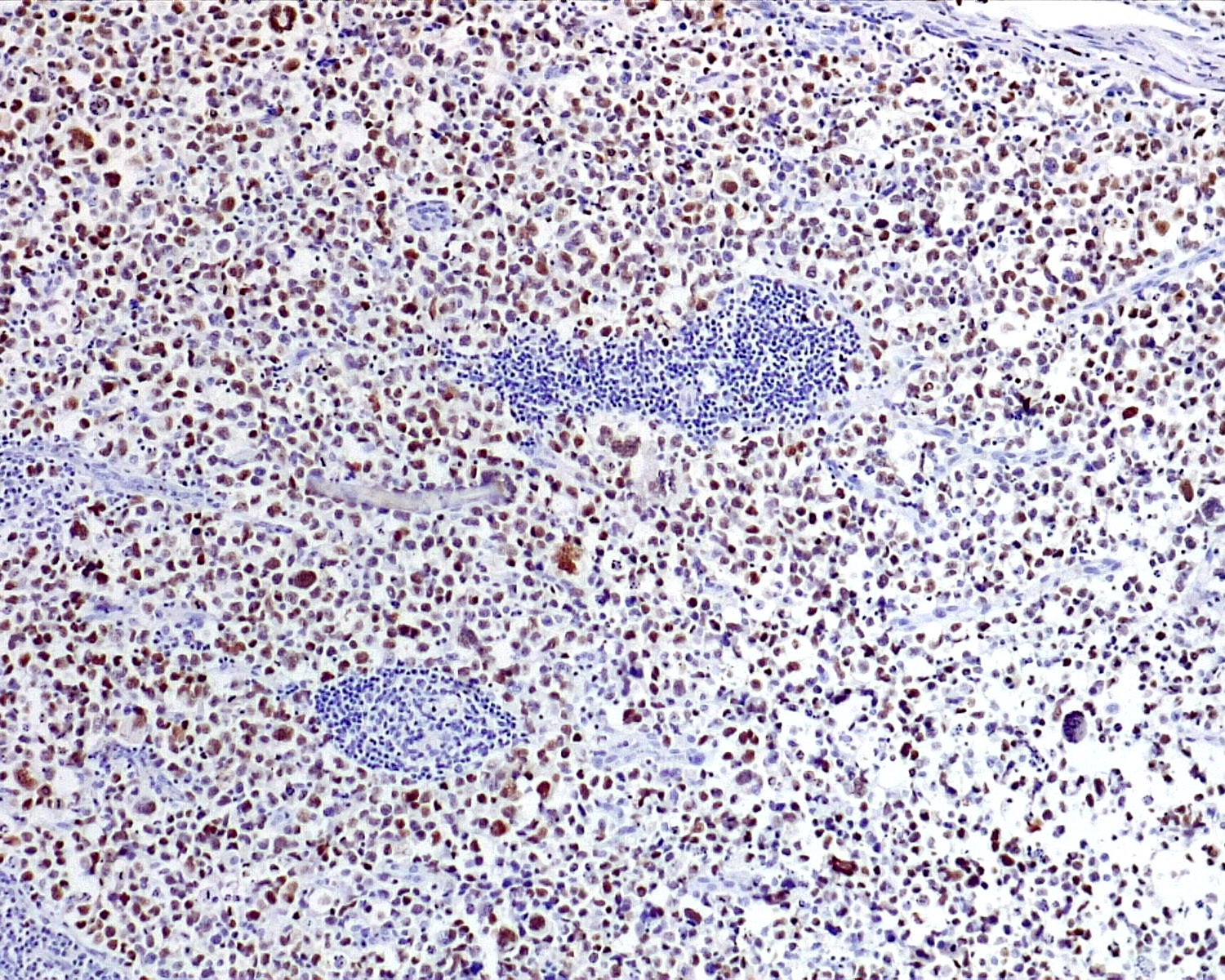
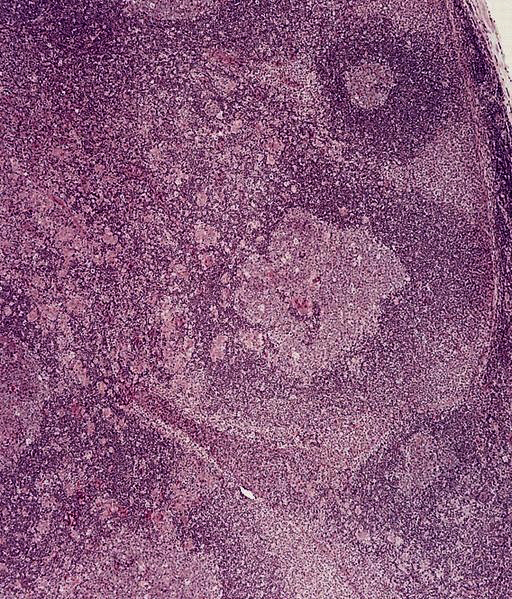
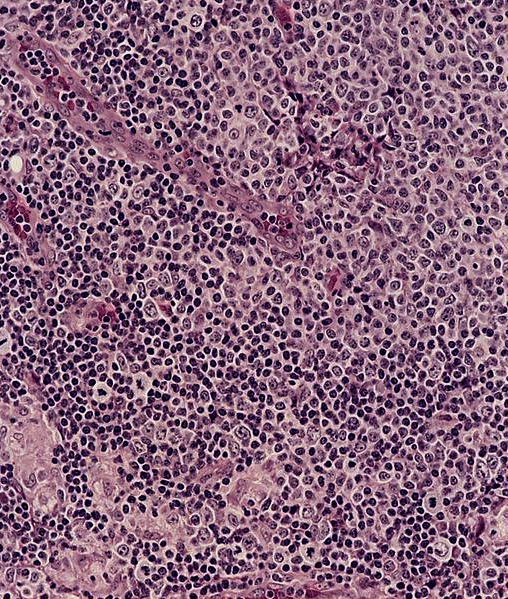
- Unusual heterogeneous group of lymphoproliferative disorders with some common morphological features involving lymph nodes or extranodal sites
- 3 histological types: hyaline vascular variant (HVCD), plasma cell variant (PCCD) and mixed hyaline vascular and plasma cell variant
- 3 distinct clinical entities:
- Unicentric Castleman disease (UCD): localized, stable and exhibiting hyaline vascular morphology (Cancer 1972;29:670)
- Multicentric Castleman disease (MCD): a systemic progressive disease with lymphadenopathy of multiple sites (Cancer 1985;56:2446)
- MCD is divided into idiopathic (iMCD) and human herpesvirus 8 associated (HHV8 MCD) (Blood 1995;86:1276)
- Histologically, most cases show features of plasma cell variant
- Oligocentric or regional Castleman disease: with involvement of 2 or 3 adjacent lymph node regions and clinical course like UCD (Blood Adv 2020;4:6039)
Essential features
- Unusual heterogeneous group of lymphoproliferative disorders with some common morphological features involving lymph nodes or extranodal sites
- 3 distinct clinical entities: unicentric Castleman disease (UCD), multicentric Castleman disease (MCD) and oligocentric or regional Castleman disease
- MCD is divided into idiopathic (iMCD) and human herpesvirus 8 associated (HHV8 MCD)
- 3 histological types: hyaline vascular variant (HVCD), plasma cell variant (PCCD) and mixed hyaline vascular and plasma cell variant
- Clinical syndromes associated with MCD POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein spike, skin changes) and MCD TAFRO (thrombocytopenia, anasarca, fever, reticulin fibrosis, organomegaly)
- Castleman disease can show predisposition to certain neoplasms
Terminology
- Angiofollicular hyperplasia
- Giant lymph node hyperplasia
- Unicentric Castleman disease (UCD)
- Multicentric Castleman disease (MCD)
- Idiopathic (iMCD)
- Human herpesvirus 8 associated (HHV8 MCD)
- Alternative / older terminology: Kaposi sarcoma herpesvirus associated (KSHV MCD)
- Hyaline vascular type (HVCD)
- Plasma cell type (PCCD)
- MCD POEMS (polyneuropathy, organomegaly, endocrinopathy, M protein spike, skin changes)
- MCD TAFRO (thrombocytopenia, anasarca, fever, reticulin fibrosis, organomegaly)
ICD coding
- ICD-10: D47. Z2 - Castleman disease
Epidemiology
- UCD can affect all age groups and shows no gender difference with the median age of onset in the fourth decade (Hematol Oncol Clin North Am 2018;32:1)
- MCD affects patients of all ages with a median age of onset in the fifth to seventh decades
- HHV8 MCD affects mainly HIV positive individuals; however, individuals who are immunocompromised from other causes can also be affected (Leuk Lymphoma 2015;56:1252)
- HHV8 MCD in individuals who are HIV negative accounts for 2 - 50% of cases and this variation depends on the prevalence of HHV8 in the region (Blood 2020;135:1353)
- With the introduction of antiretroviral therapy (ART), the incidence of HHV8 MCD has increased
- Complex interplay between HIV and HHV8 and immune dysregulation of patients with HIV controlled by ART is thought to cause this increased incidence (Ann Oncol 2009;20:775)
- UCD is more common in childhood (75%) than iMCD and only rare cases of HHV8 MCD are reported in children (Pediatrics 2012;129:e199, Pediatr Blood Cancer 2019;66:e27613)
Sites
- UCD most commonly affects mediastinum; extrathoracic sites are also involved
- Presents as a solitary lymph node mass
- MCD affects multiple lymph node sites, predominantly in the cervical region
- Reference: Surg Pathol Clin 2019;12:849
Pathophysiology
- Etiopathogenesis remains not well known
- UCD
- Recent evidence suggests that UCD may be a neoplastic process involving follicular dendritic cells (Mod Pathol 2014;27:823)
- Among UCD patients, 17% have shown to harbor mutations in the gene PDGFRB encoding platelet receptor growth factor β (Leukemia 2019;33:1035)
- iMCD
- iMCD is proposed to involve autoinflammatory or autoimmune diseases, paraneoplastic syndromes or an unidentified viral infection leading to polyclonal lymphoproliferation and hypercytokinemia (Blood 2014;123:2924)
- Current concept is that a cytokine storm due to increased production of IL6 is associated with Castleman disease since patients have elevated levels of IL6 and symptoms improve with IL6 suppression (N Engl J Med 2020;383:2255)
- VEGF, IL1, IL2, CXCL13 and TNF have also been shown to play a role in the pathogenesis of iMCD (Blood 1994;83:2587, Br J Haematol 1999;104:482, Am J Hematol 2018;93:902)
- T cell activation, and activation of mTOR, JAK / STAT3 and type I interferon signaling pathways also thought to be pathogenetic mechanisms (J Clin Invest 2019;129:4451, Blood 2020;135:1673)
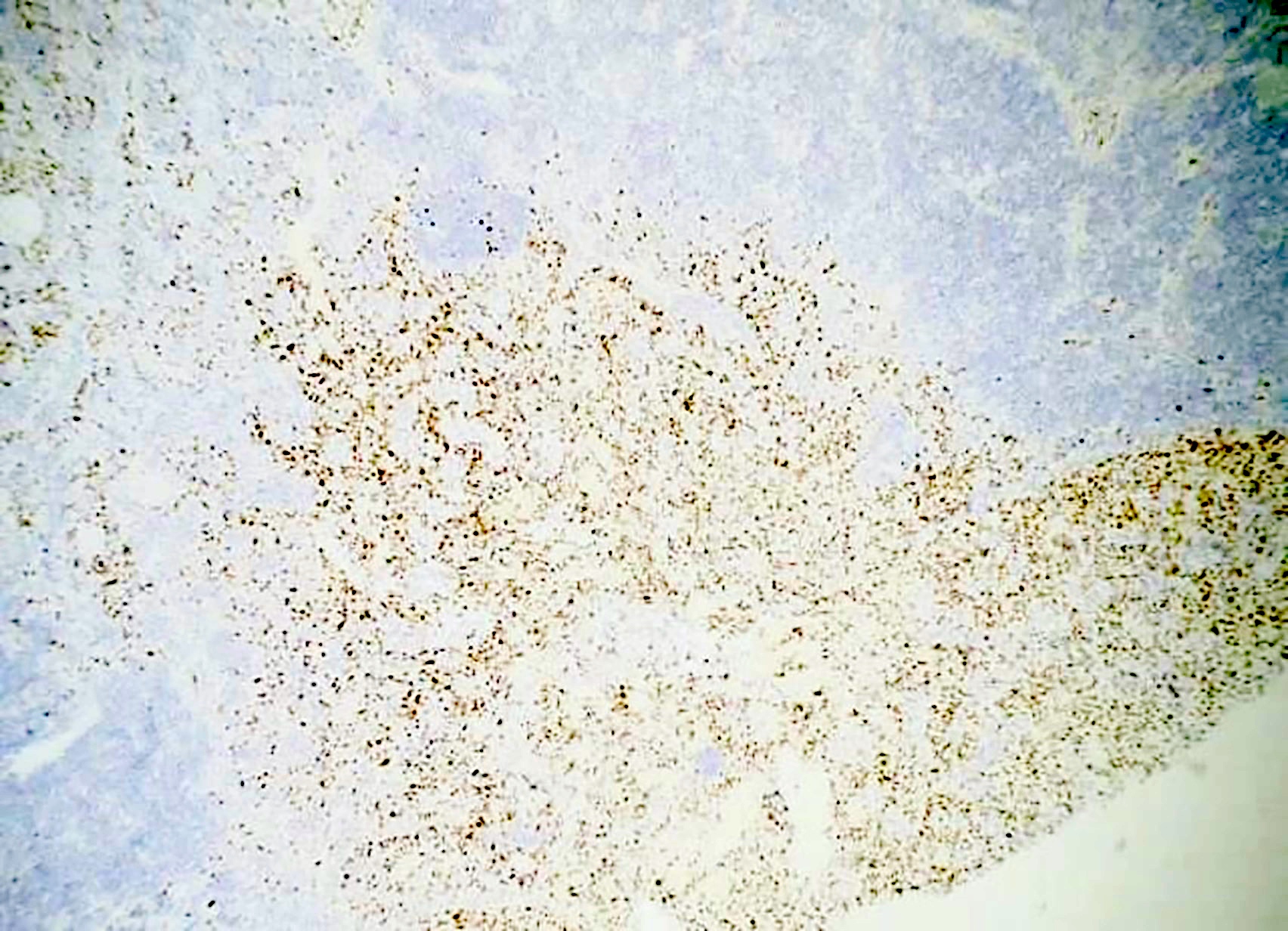
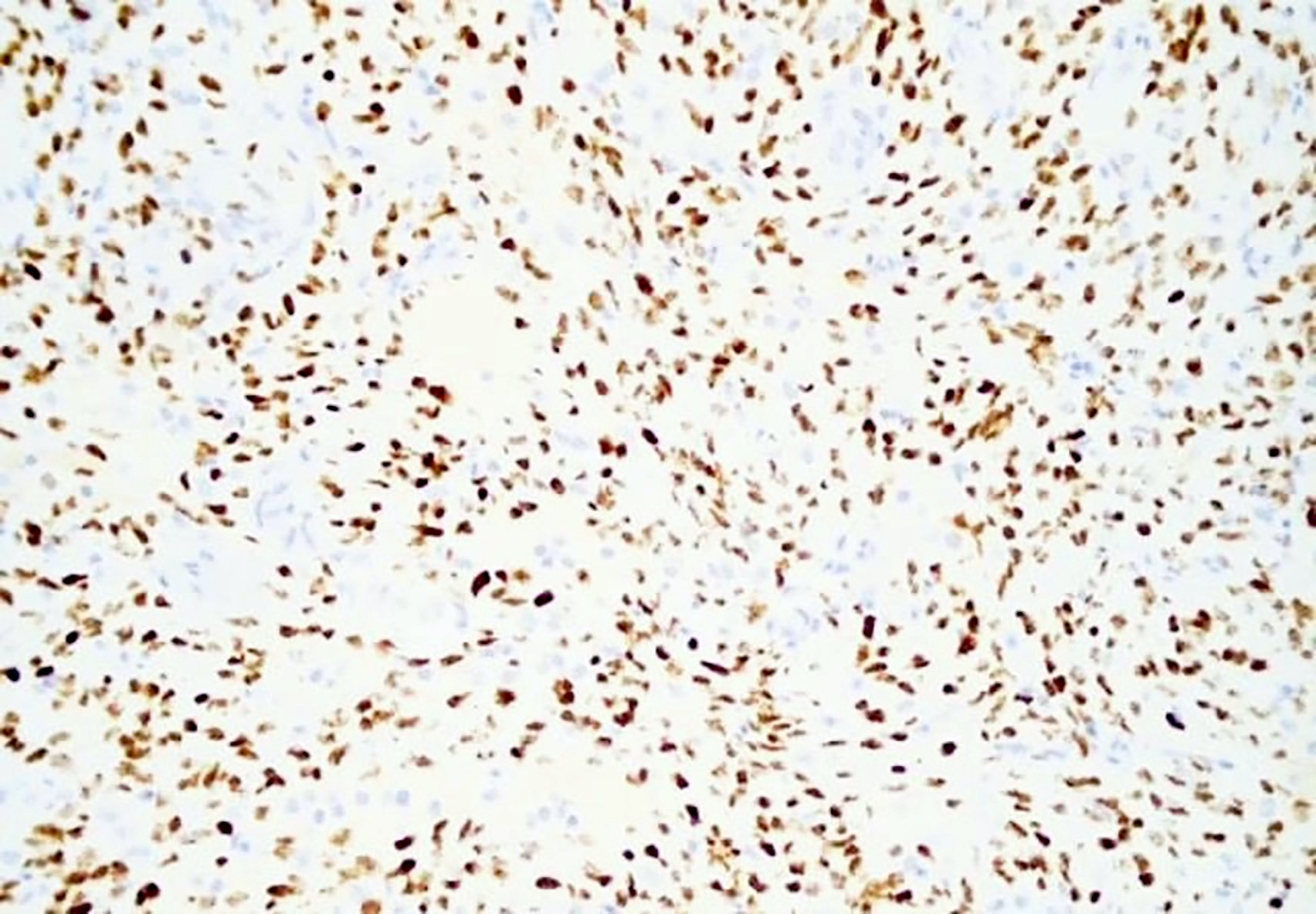
- HHV8 MCD
- HHV8 MCD occurs in HIV positive or negative individuals infected with HHV8 (Blood 2001;97:2130)
- HHV8 infected B cells in the mantle zones show expression of IgM lambda immunogobulins
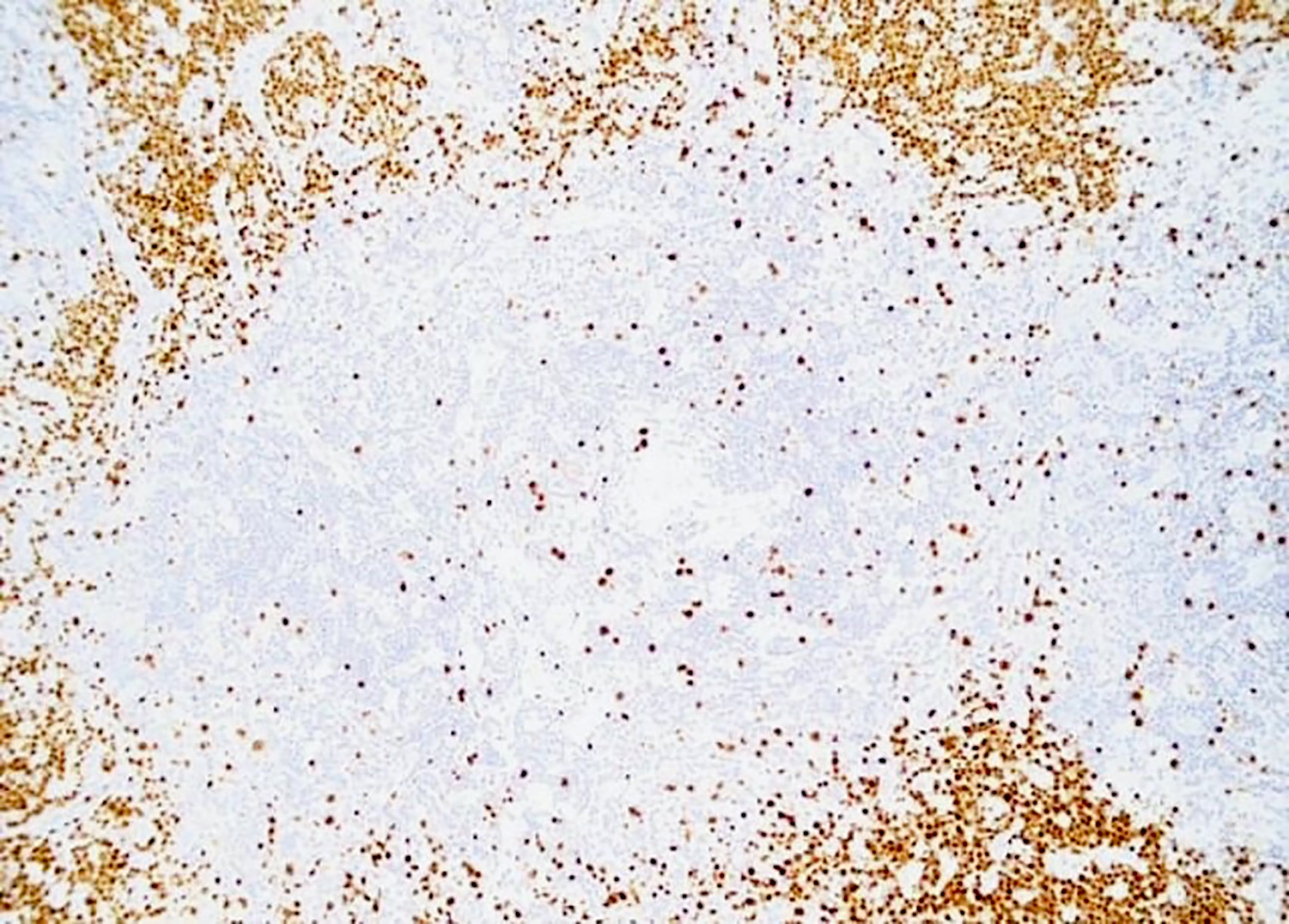
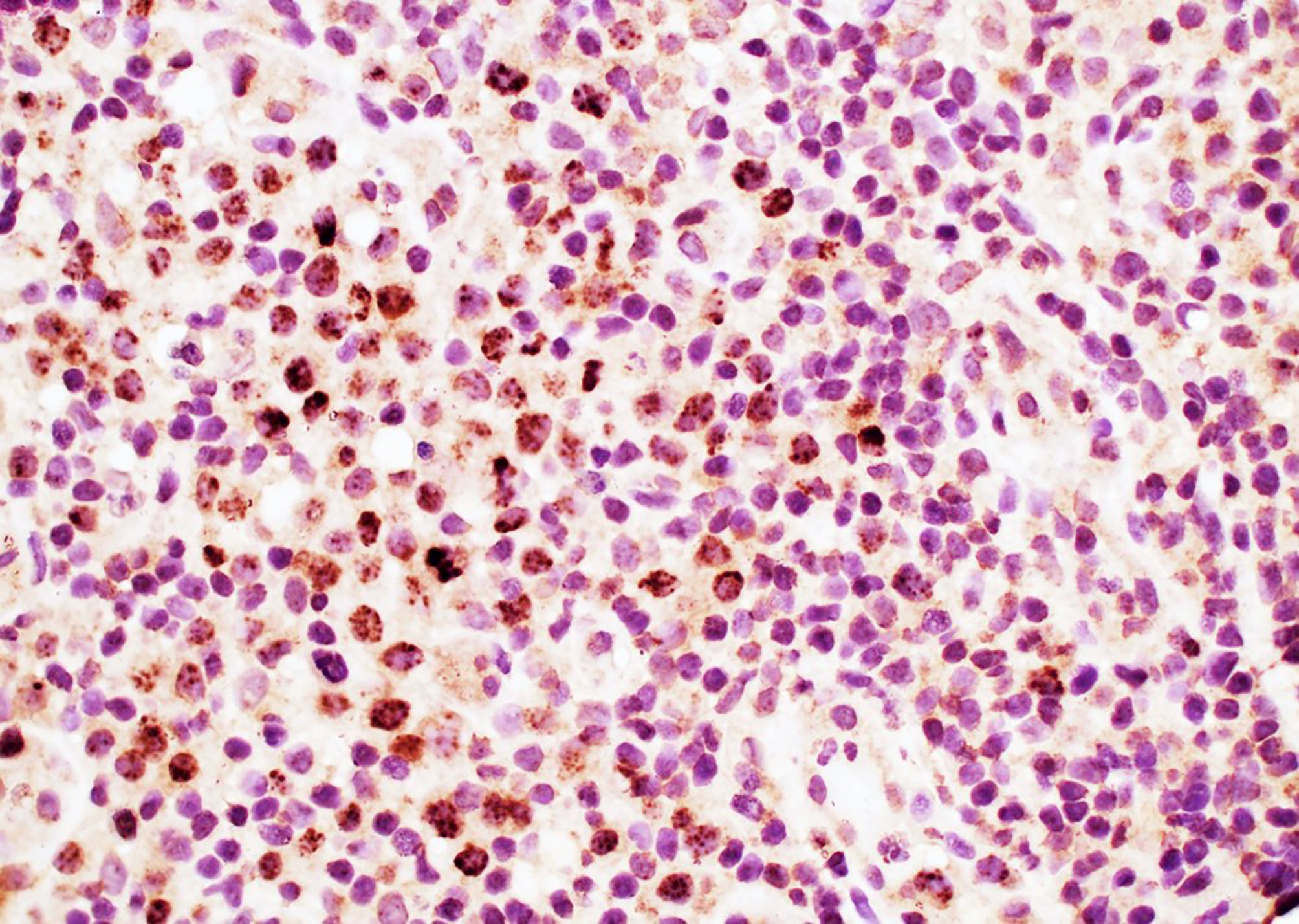
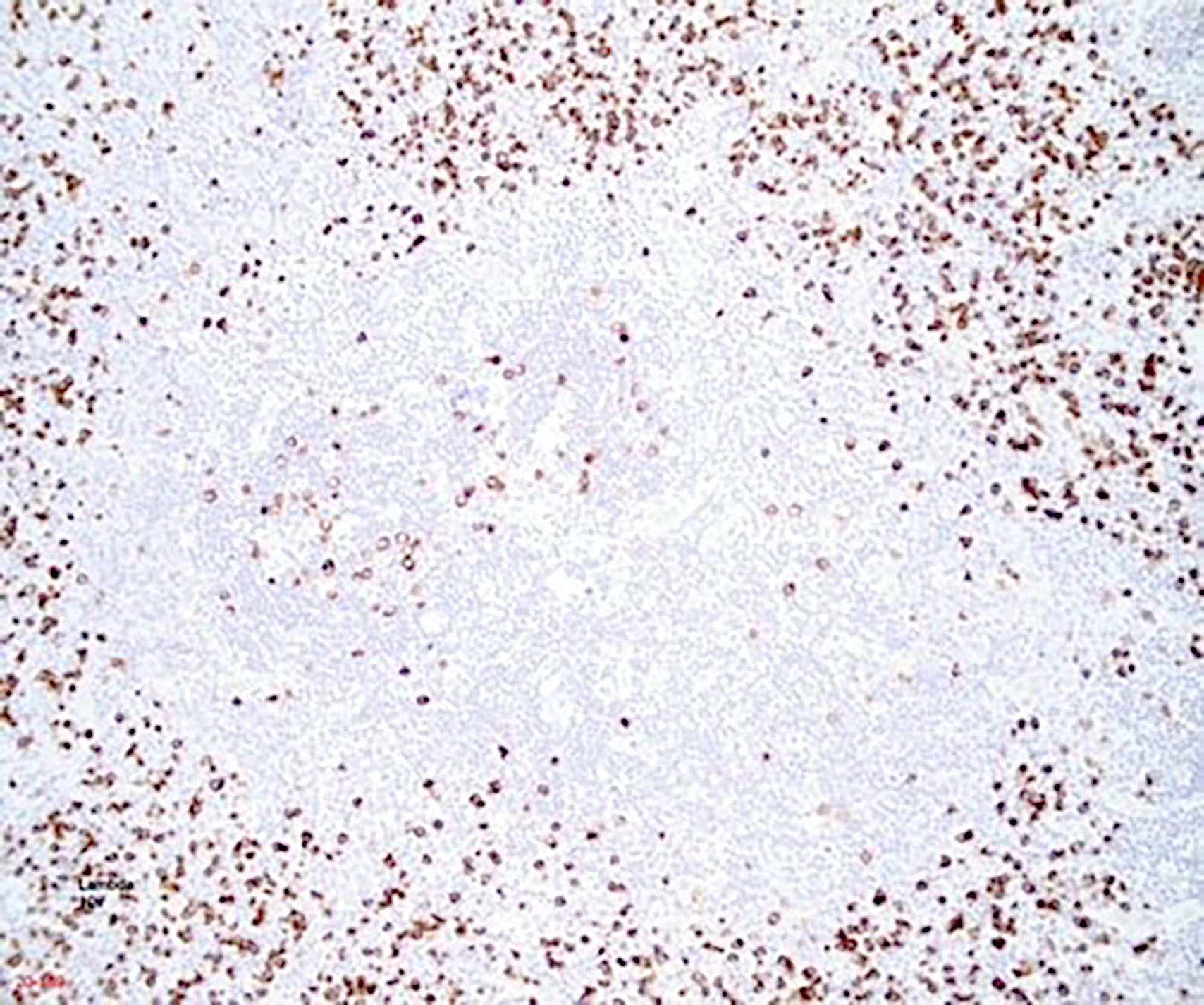
- This is by the loss of Ig kappa due to the upregulation of V(D)J recombination mediated by RAG protein and Ig lambda expression by B lymphocytes with HHV8 infection (PLoS Pathog 2018;14:e1006967)
- HHV8 encodes several viral genes; the resulting viral proteins and microRNAs stimulate cell growth, proliferation and cell survival among the infected cells, as well as among the neighboring cells by a paracrine mechanism (J Clin Invest 2016;126:3165)
- HHV8 viral lytic protein expression is proposed to be the contributing pathobiological factor of HHV8 MCD (Am J Pathol 2000;156:743)
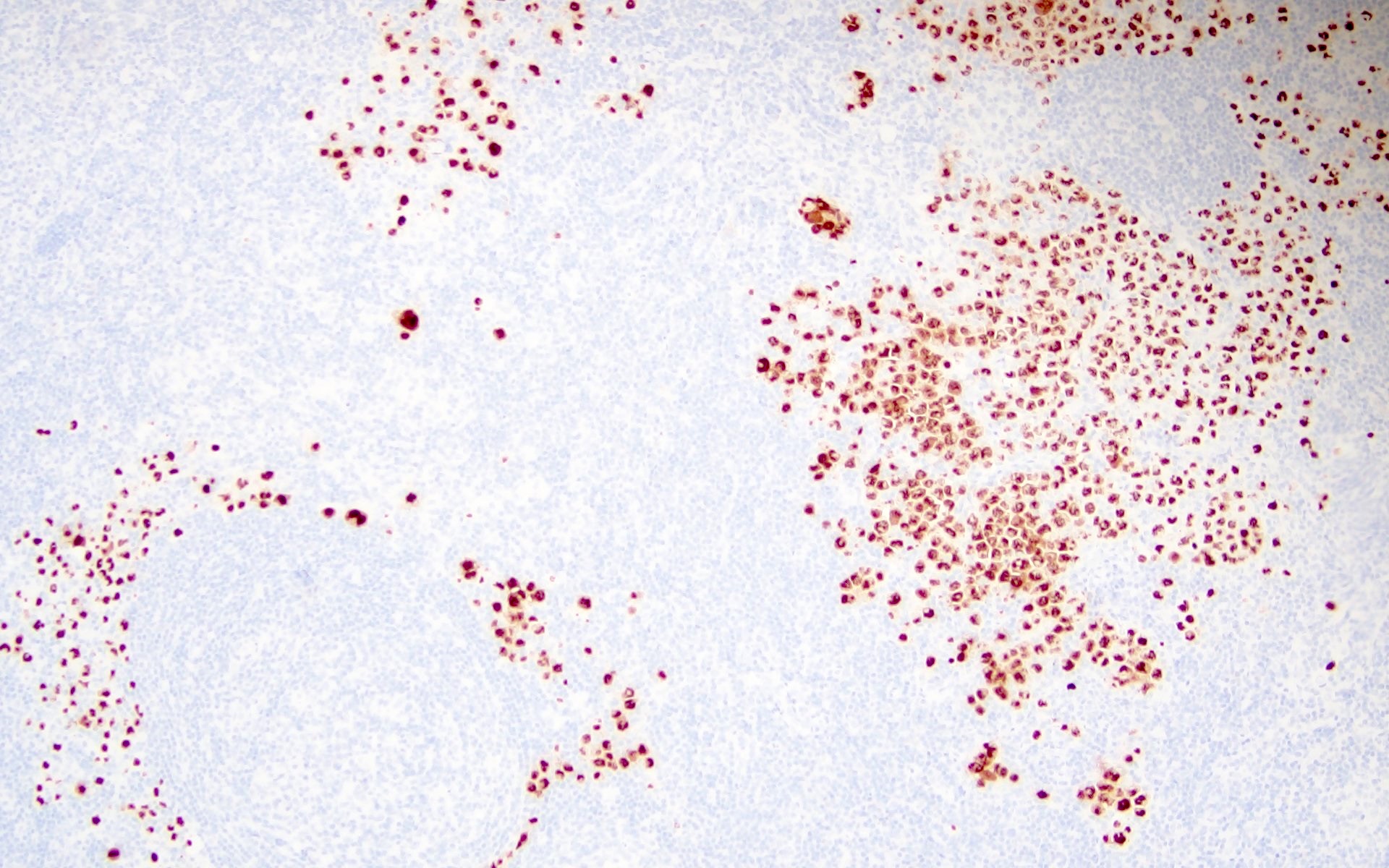
- High viral load in patients with HHV8 MCD suggests active viral replication (Blood 2000;96:2069)
- HHV8 viral protein vIL6 is expressed in high levels in plasmablasts surrounding lymphoid follicles (J Virol 1999;73:4181)
- Additionally, human cytokines are elevated in patients with HHV8 MCD, which is thought to be driven by the virus (Blood 2013;122:4189)
- Inflammatory cytokines like hIL6 and vIL6 cause anemia, fever and hypoalbuminemia (Blood 2013;122:4189)
Etiology
- UCD: etiology is unclear
- iMCD: etiology is unclear
- HHV8 MCD: HHV8 infected endothelial cell proliferation and vascularization
Clinical features
- UCD: asymptomatic or an enlarging lymph node or mass; secondary symptoms related to the mass (compression or pain) (Blood 2020;135:1353)
- MCD: all subtypes of MCD show systemic inflammatory manifestations including fever, weight loss, anasarca, generalized lymphadenopathy and hepatomegaly
- Anemia, hypoalbuminemia, cytopenias and elevated inflammatory markers are common (Blood Adv 2021;5:1660)
- MCD POEMS: a syndrome characterized by peripheral neuropathy, organomegaly, skin changes and monoclonal paraproteins occurs in some patients with MCD (Blood 1994;83:2587)
- MCD TAFRO: another syndrome characterized by thrombocytopenia, ascites, fever, reticulin fibrosis and organomegaly usually in patients with normal immunoglobulin levels and mixed or hyaline vascular histology (Sci Rep 2017;7:42316)
- iMCD, NOS: shows elevated platelet counts and immunoglobulin levels and plasmacytic histology (Blood 2020;135:1353)
Diagnosis
- Excisional biopsy of an affected lymph node with histopathological examination is the diagnostic modality of choice
- 18F fluorodeoxyglucose positron emission tomography (FDG PET) is used to exclude alternative diagnoses; also used to select the lymph node for biopsy (J Infect Dis 2015;212:1250)
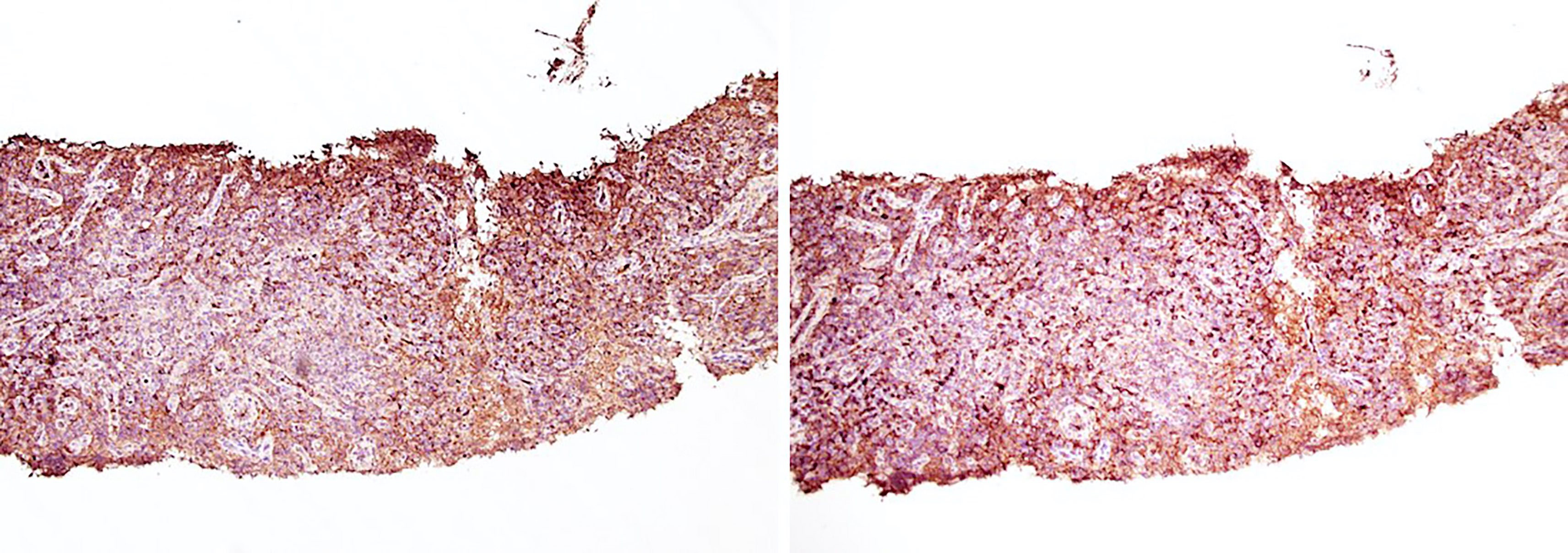
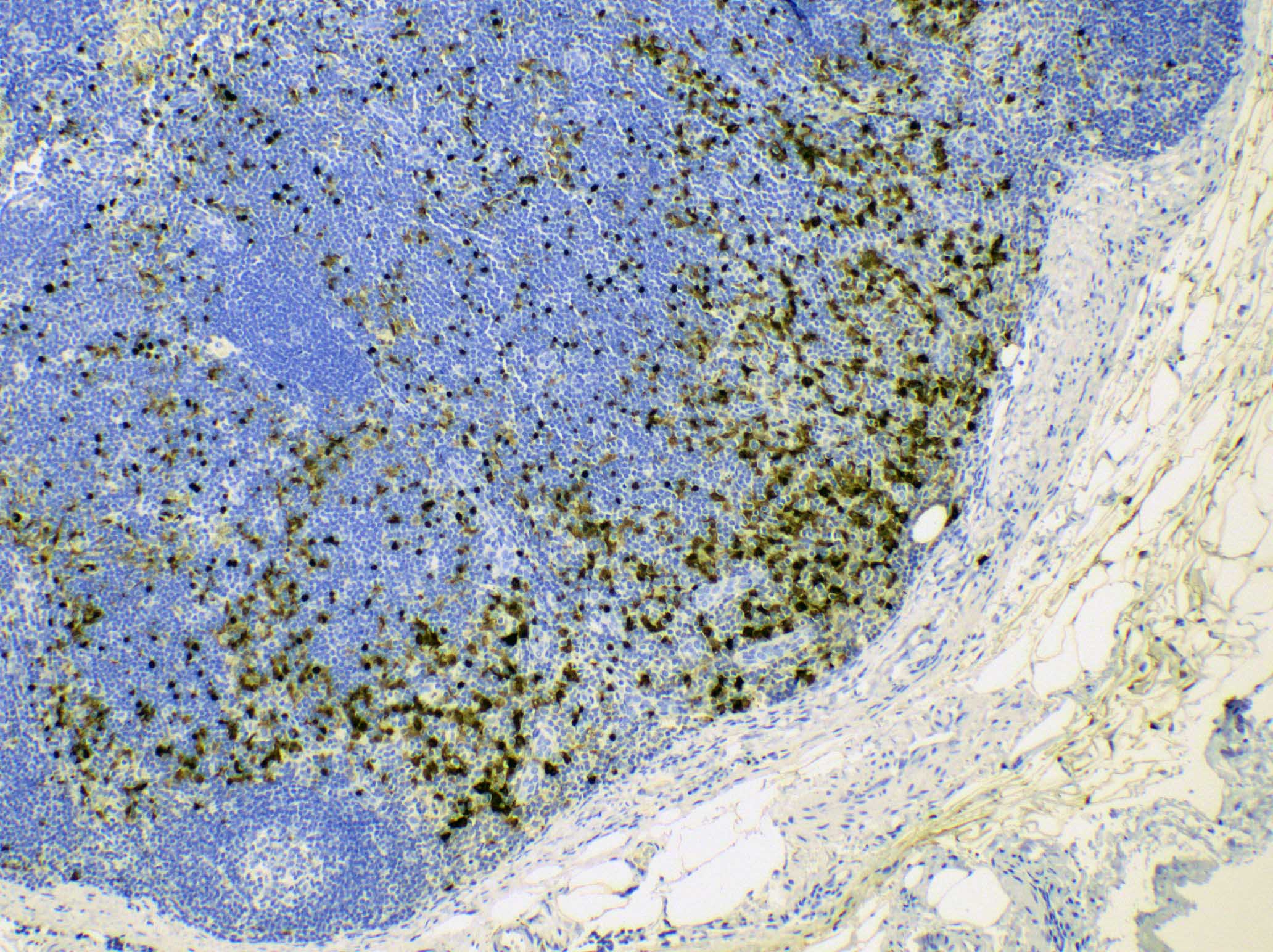
- HHV8 MCD requires the pathologic features and HHV8 positivity
- iMCD is confirmed by integration of pathological findings and clinical syndrome; a diagnosis of iMCD requires exclusion of infectious, malignant and autoimmune disorders that can mimic iMCD (Blood 2017;129:1646)
Laboratory
- UCD: usually no laboratory abnormalities
- MCD subtypes: anemia, hypoalbuminemia, renal dysfunction and liver dysfunction, dysregulation of IL6 (increased) or other cytokines like VEGF, IL1 and TNFα (Nat Rev Dis Primers 2021;7:84)
- MCD TAFRO: thrombocytopenia (Hematol Oncol Clin North Am 2018;32:37)
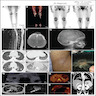
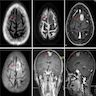
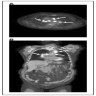
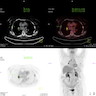
Radiology description
- MCD: 18F FDG PET / CT shows increased uptake in the affected lymph nodes
- Lymph nodes are nonconfluent with a symmetric pattern and SUV ranging from 2 - 19
- Extramedullary involvement is demonstrated as pulmonary cysts, nodules and interstitial lung disease, hypermetabolic activity in spleen and bone marrow (Nucl Med Commun 2021;42:833)
Prognostic factors
- UCD: localized and excellent response to therapy
- MCD: poorer prognosis, especially HIV positive patients and patients with MCD POEMS (Surg Pathol Clin 2019;12:849)
- Predilection for association with neoplasms (Surg Pathol Clin 2019;12:849, Blood 2020;135:1353, Indian J Pathol Microbiol 2021;64:302, Infection 2021;49:945)
- HVCD
- Follicular dendritic cell sarcoma
- Vascular tumors
- HHV8 MCD
- Kaposi sarcoma
- HHV8+ large B cell lymphoma
- Primary effusion lymphoma
- iMCD
- Classic Hodgkin lymphoma
- Diffuse large B cell lymphoma
- Mantle cell lymphoma
- Peripheral T cell lymphoma
- HVCD
- Other
- HVCD can develop indolent T lymphoblastic proliferations
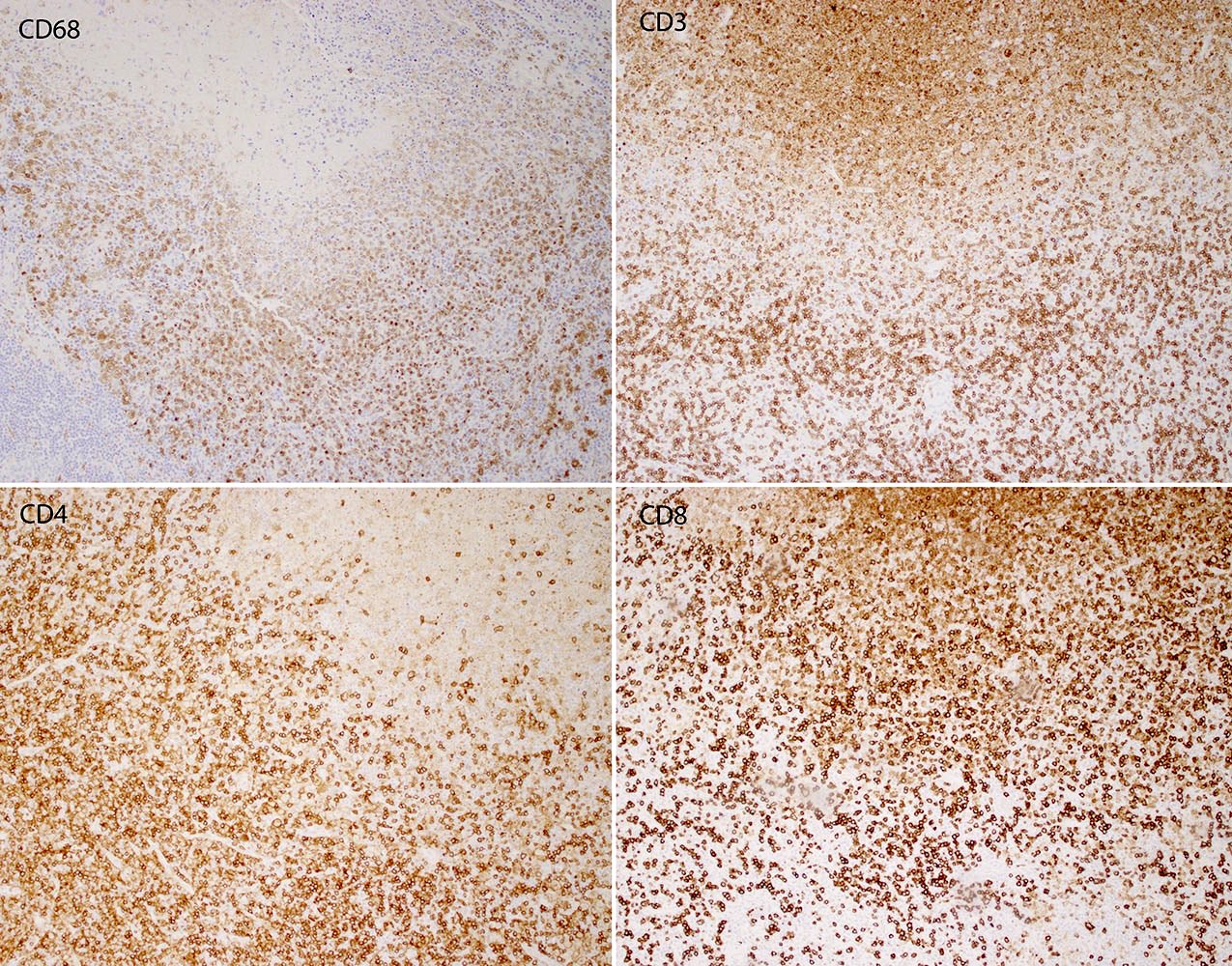
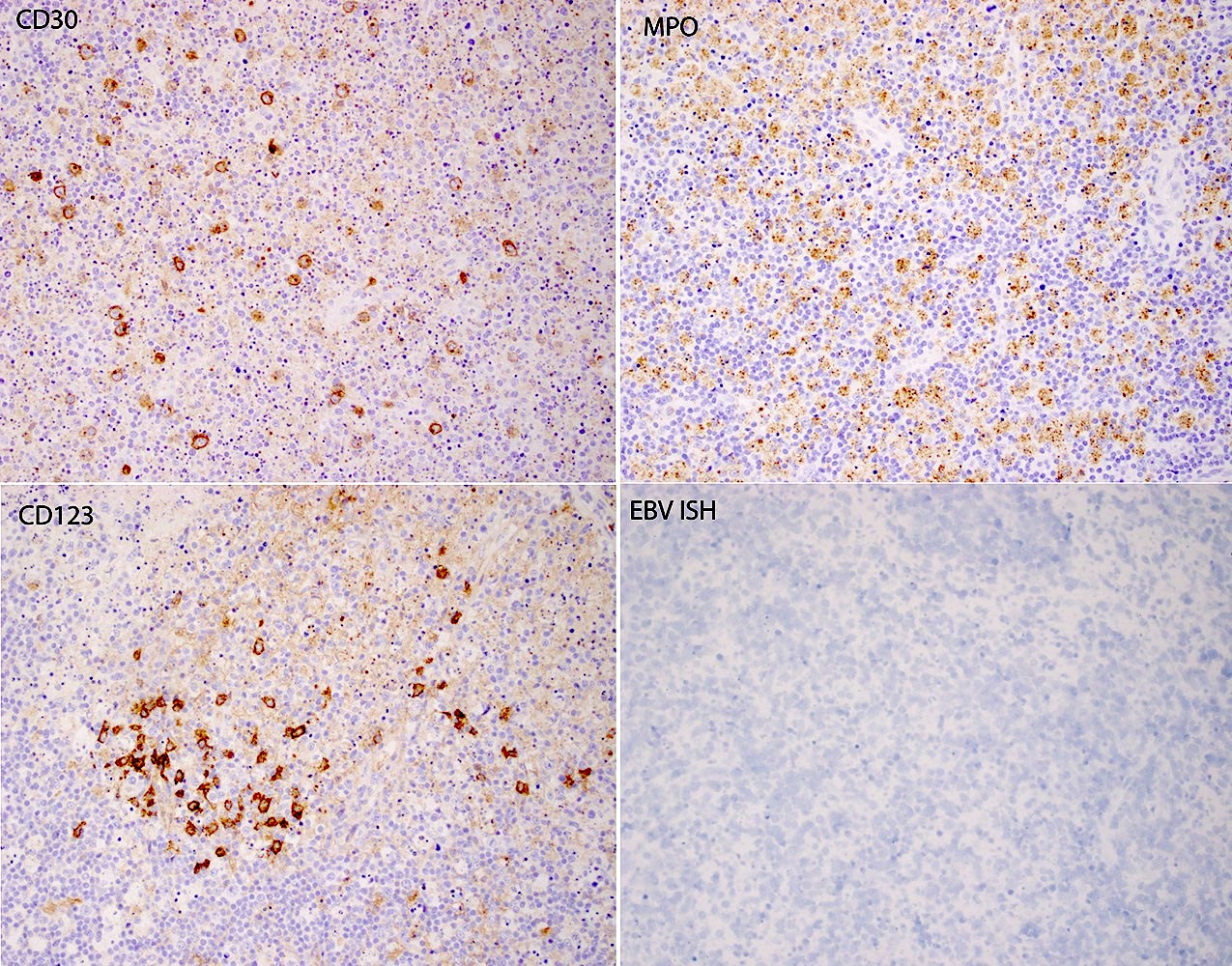
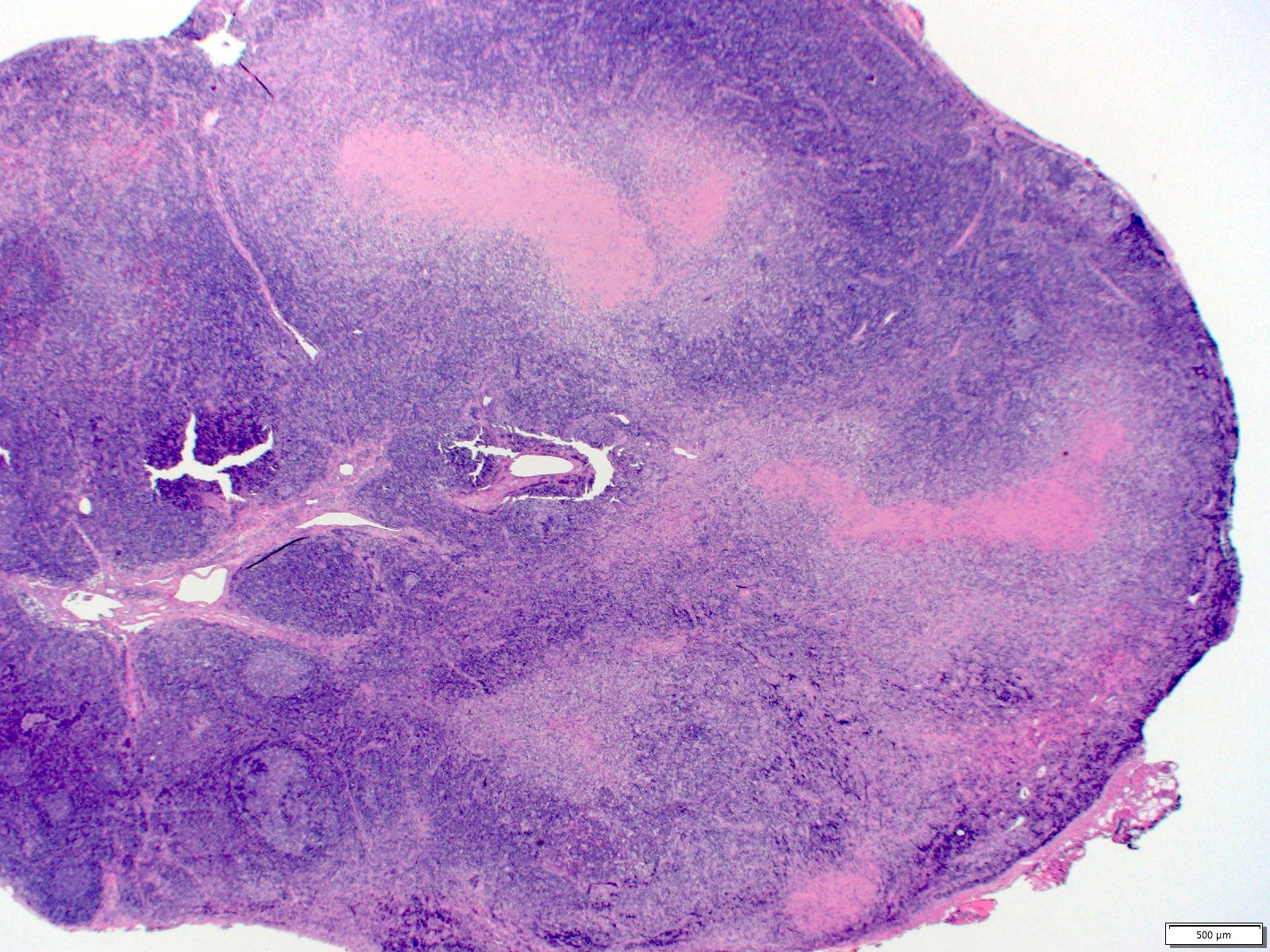
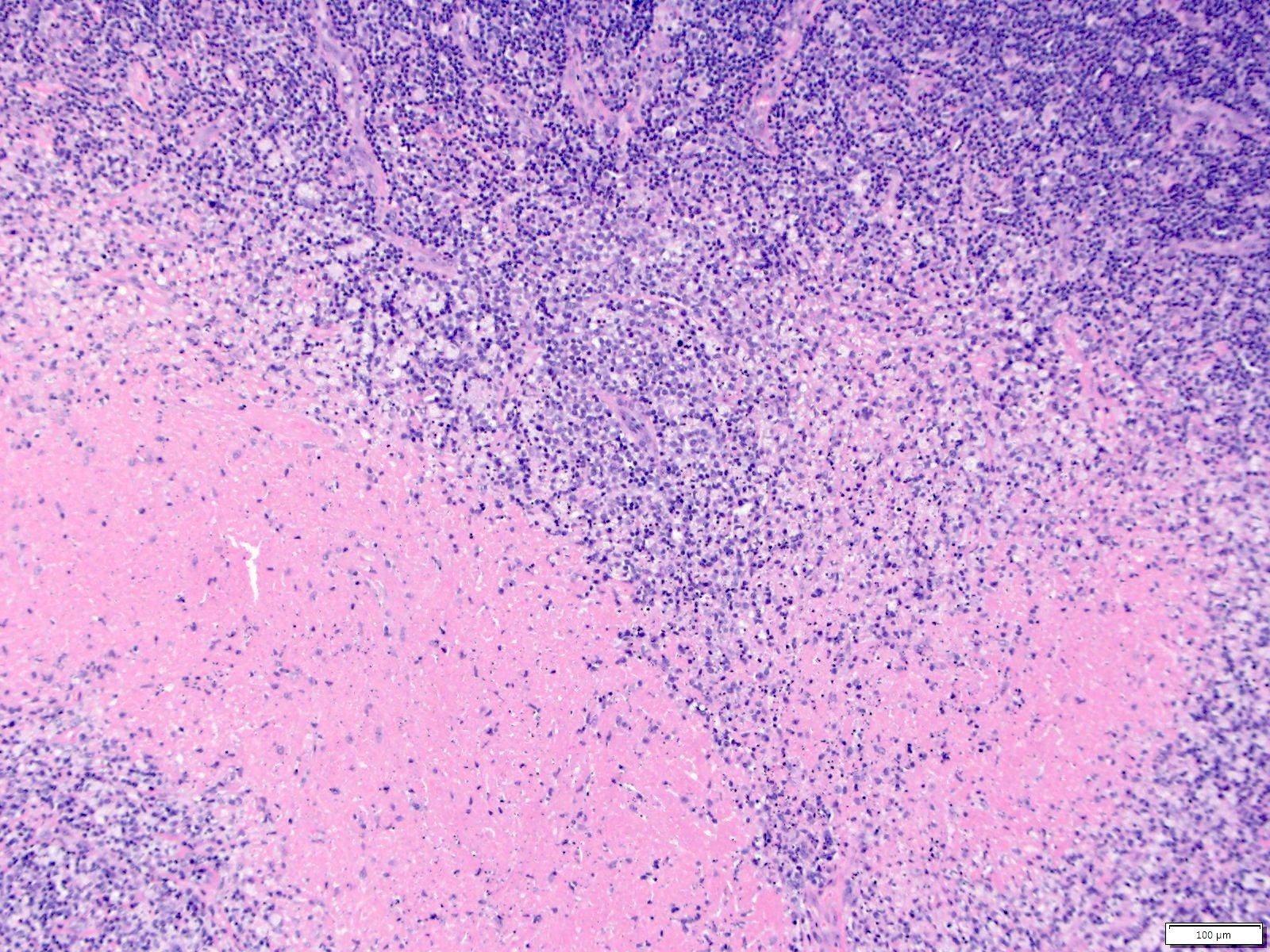
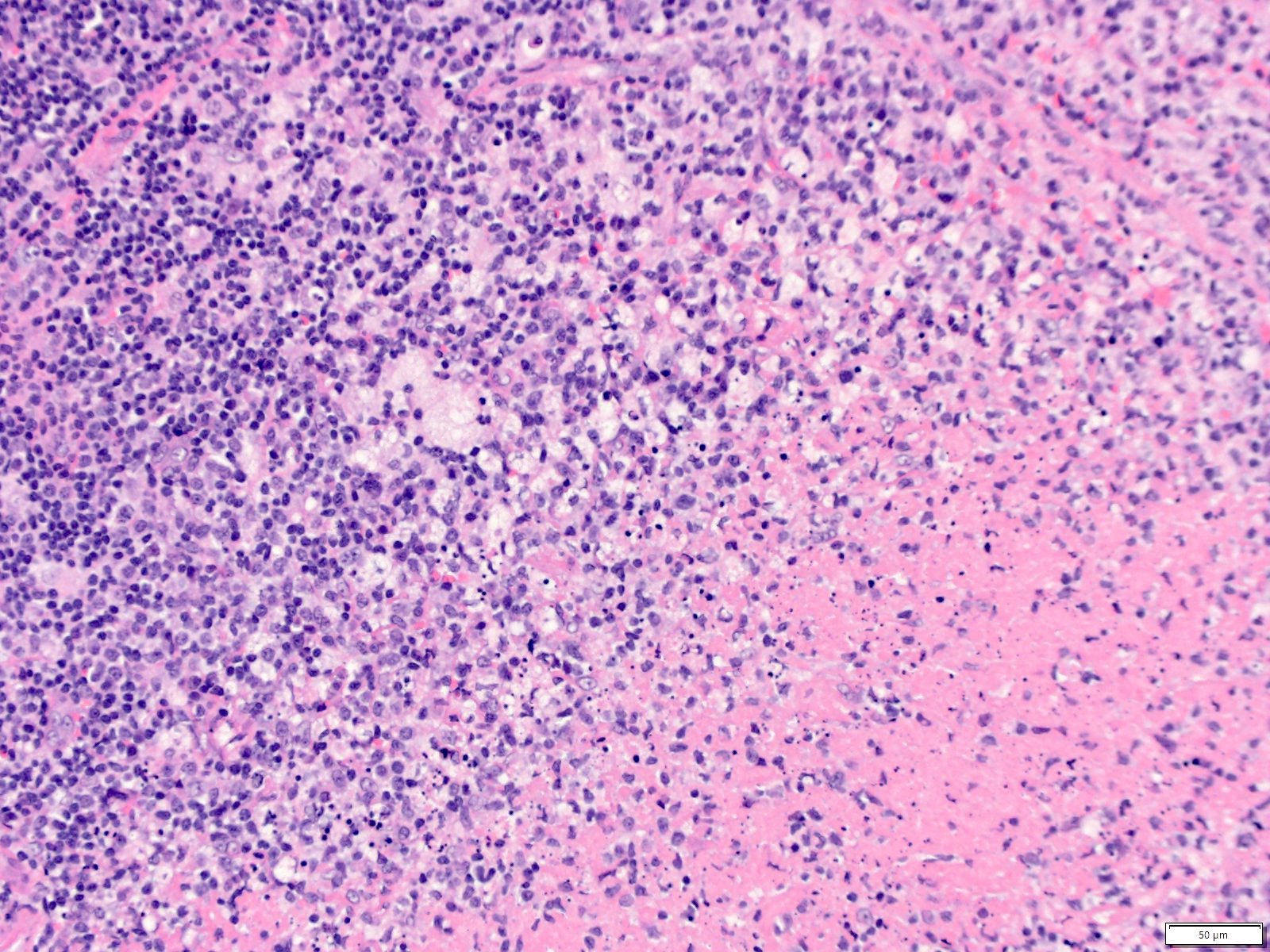
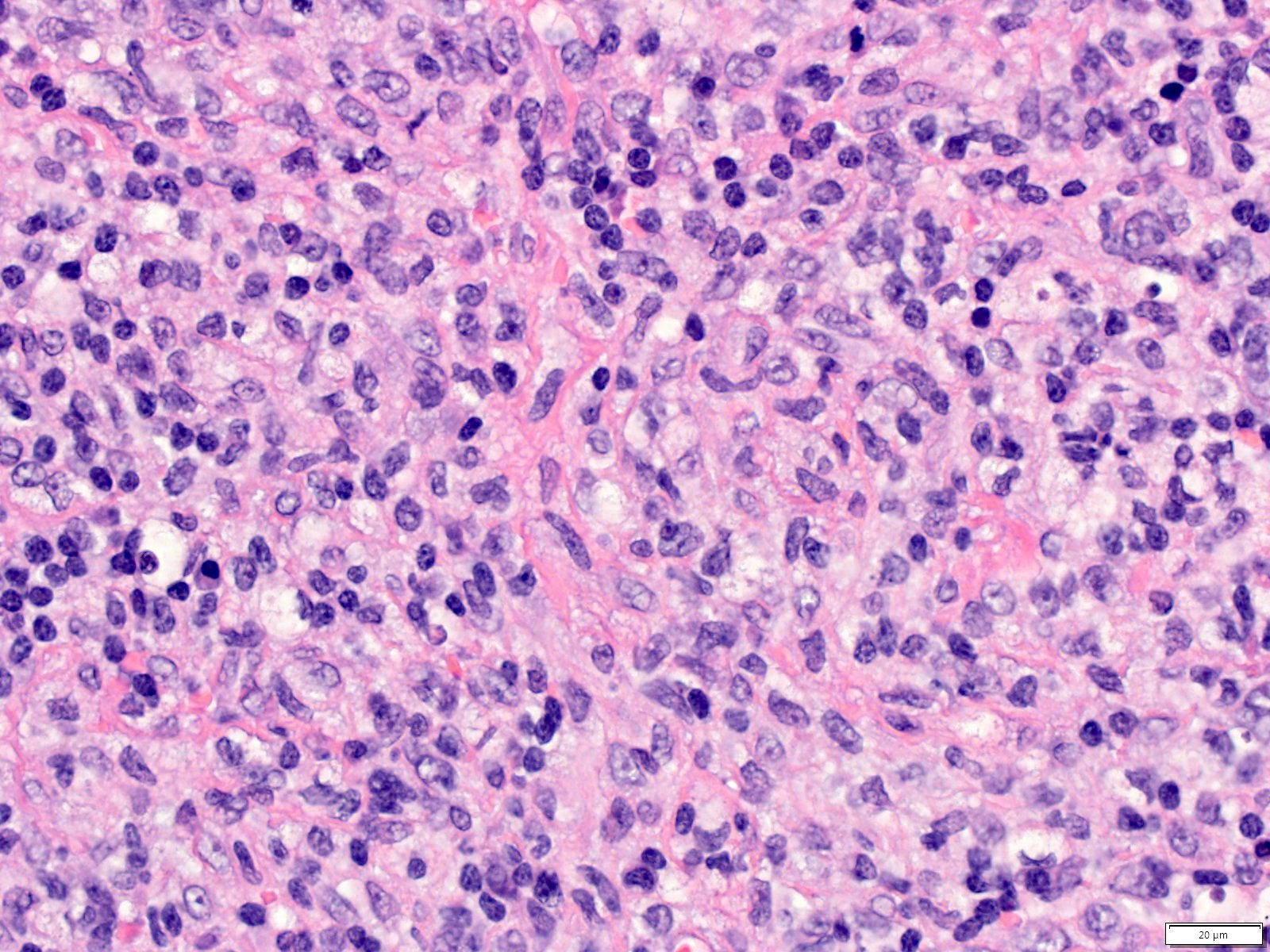
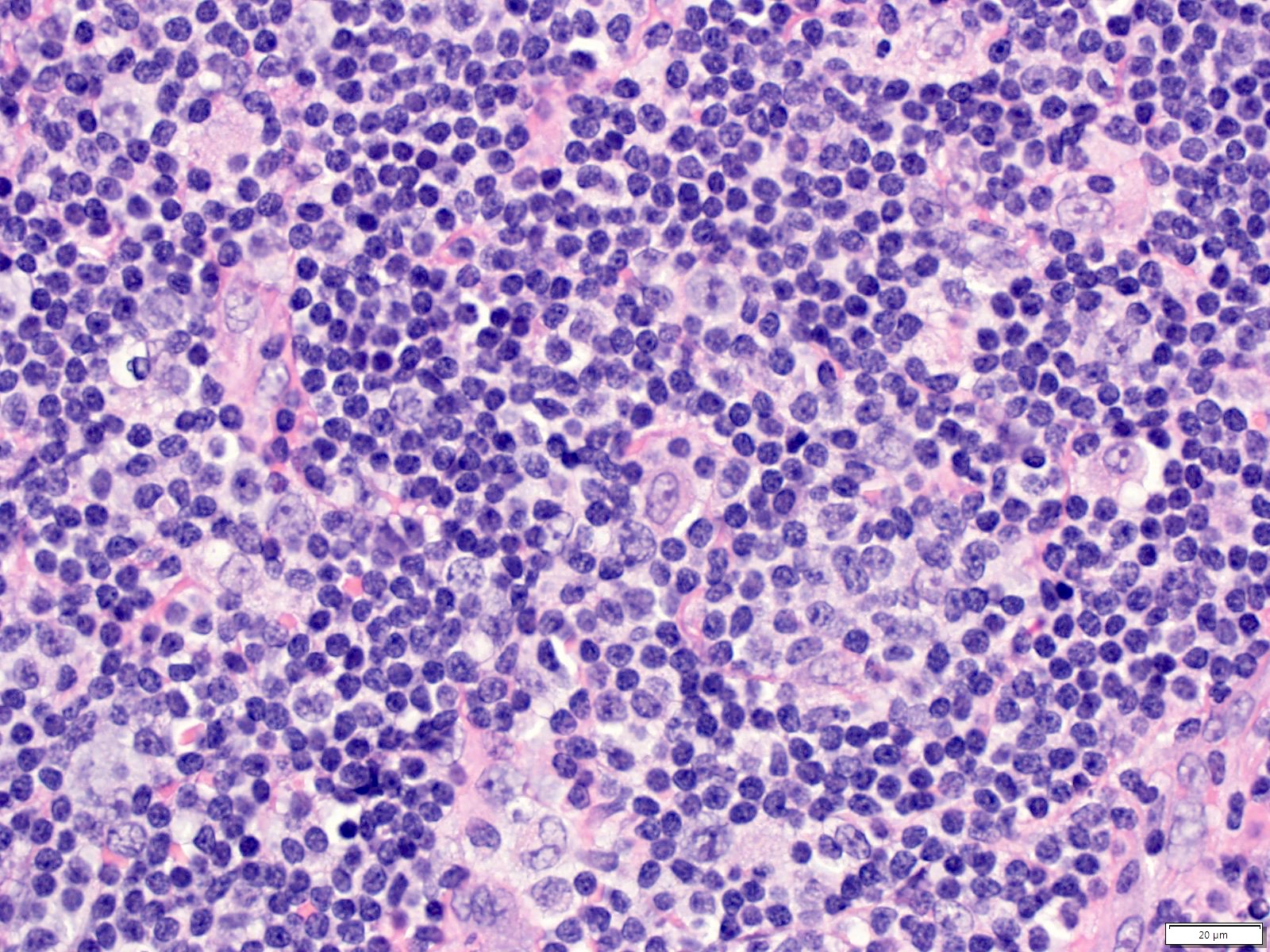
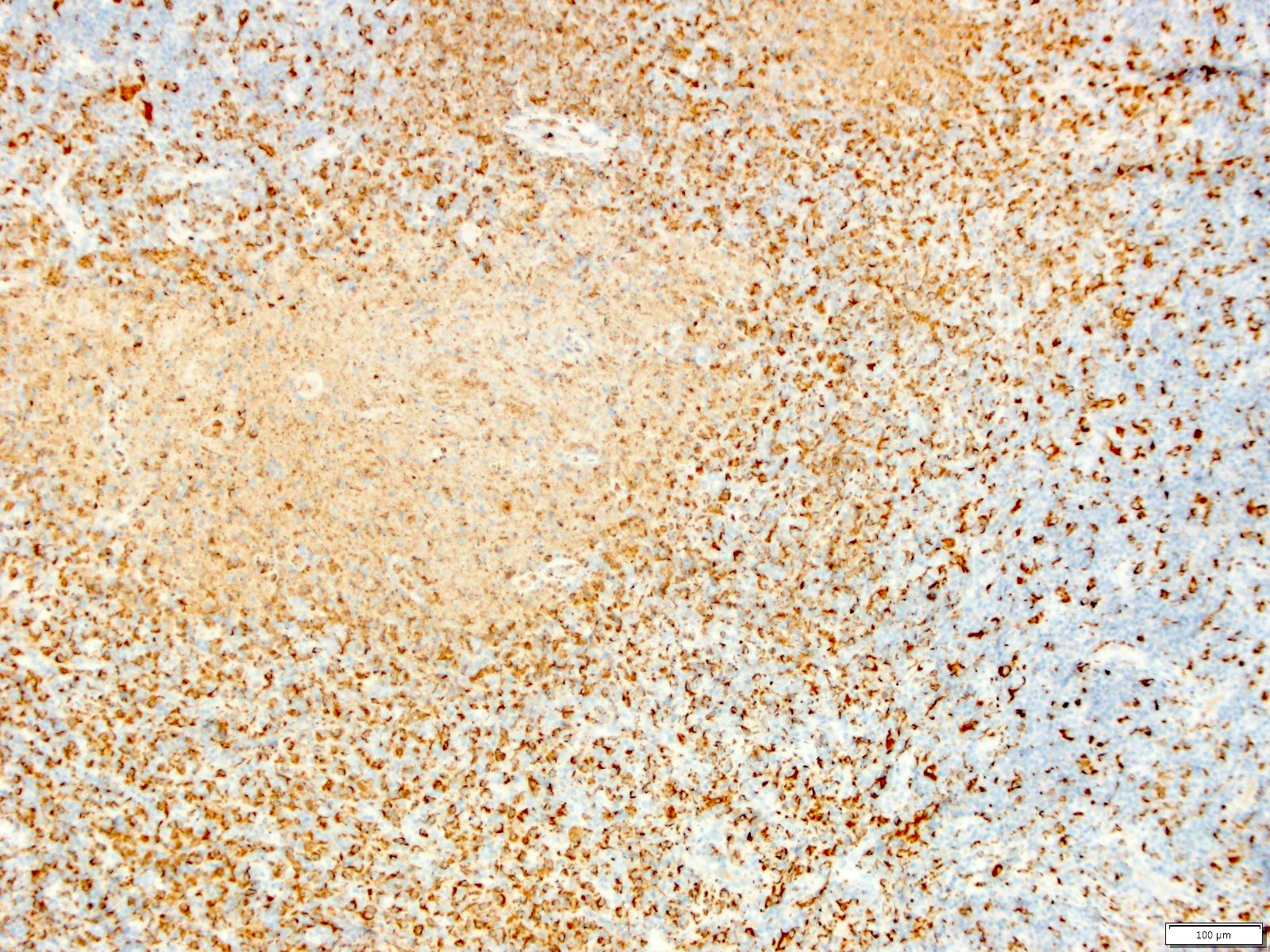
Case reports
- 26 year old woman with Castleman disease with AA amyloidosis (Medicine (Baltimore) 2020;99:e18978)
- 26 year old pregnant woman with Castleman disease and pemphigus (Medicine (Baltimore) 2021;100:e24990)
- 32 and 43 year old women with Castleman disease with spondyloarthritis treated with TNF alpha antagonist (Joint Bone Spine 2020;87:655)
- 35 year old woman with glycolytic hypermetabolism in Castleman disease by 18F FDG PET / CT (Clin Nucl Med 2020;45:868)
- 52 year old man with Castleman disease presenting as a chylous pleural effusion (Pathol Res Pract 2020;216:153209)
- 53 year old man with retroperitoneal mass (Int J Surg Case Rep 2020;70:24)
- 60 year old man with a hepatic mass and autoimmunity (Cancer Imaging 2019;19:53)
- 62 year old man with hypermetabolic mass of kidney (Clin Nucl Med 2021;46:510)
- 64 year old woman with multicentric Castleman disease and cryoglobulinemic vasculitis (J Dermatol 2021;48:e474)
- 82 year old woman with Castleman disease and eosinophilic dermatosis (Int J Dermatol 2020;59:e416)
Treatment
- UCD
- Asymptomatic patients: expectant management and follow up for disease progression; resection
- Symptomatic patients: complete surgical resection (Br J Haematol 2021;195:328)
- Unresectable symptomatic UCD: volume reduction by debulking resection, embolization or cryoablation of feeding vessels, rituximab, steroids and radiation therapy and follow up evaluation is recommended to determine the possibility of complete resection (Br J Haematol 2021;195:328, Eur J Haematol 2021;107:484)
- MCD
- Asymptomatic patients: no therapeutic intervention but follow up is needed to detect disease progression
- Symptomatic patients: depending on the presentation and disease driver (Br J Haematol 2021;195:328)
- HHV8 MCD
- Antiretroviral therapy with HHV8 MCD specific therapy
- Cytotoxic chemotherapies are used: etoposide, vincristine, vinblastine, cyclophosphamide and liposomal doxorubicin
- Chemotherapy is used in combination with rituximab
- Antiherpesvirus therapy: gancyclovir, zidovudine, valganciclovir, pomalidomide
- Anti-hIL6 therapy: siltuximab and tocilizumab are under trial (Br J Haematol 2021;195:328)
- MCD POEMS
- Therapy for the plasma cell dyscrasia: consolidation with autologous stem cell transplant
- Patients without clonal plasmacytosis in bone marrow: radiation therapy
- Patients with disseminated bone marrow involvement: systemic chemotherapy followed by adjuvant radiation; melphalan and dexamethasone, cyclophosphamide dexamethasone, lenalidomide, thalidomide and bortezomib
- Daratumumab, BCMA CAR-T, bevacizumab (anti-VEGF), interferon alpha, tamoxifen, transretinoic acid, ticlopidine, argatroban and strontium 89 are also used in rare cases
- iMCD
- Surgery, steroids, rituximab, combination chemotherapy (CHOP [cyclophosphamide, doxorubicin, vincristine (Oncovin), prednisolone] or CVAD [cyclophosphamide, vincristine, adriamycin, etoposide]-like regimens and VDTPACE [Velcade (bortezomib), dexamethasone, thalidomide, cisplatinum, adriamycin, cyclophosphamide, etoposide]), autologous stem cell transplantation, anti-IL6 monoclonal antibodies (siltuximab and tocilizumab), bortezomib, thalidomide, anakinra (IL1 antagonist), interferon alpha and all transretinoic acid are used (Hematol Oncol Clin North Am 2018;32:89, Blood 2018;132:2115)
- MCD TAFRO
- Corticosteroids, rituximab, tocilizumab, cyclosporin A
- Thrombocytopenia: romiplostim and eltrombopag
- Other options: plasma exchange, high dose cyclophosphamide, thalidomide, lenalidomide, bortezomib rapamycin and combination chemotherapy such as CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) (Ann Hematol 2022;101:485, Int J Hematol 2016;103:686)
- HHV8 MCD
Gross description
- UCD: enlarged lymph node, firm and white cut surface with or without calcification
Microscopic (histologic) description
- HVCD is characterized by prominent vascular proliferation and hyalinization of the vessel walls
- There are follicular changes and interfollicular / stromal changes described; based on the predominance of changes in each of these sites divided into follicular HVCD and stroma rich Castleman disease (Virchows Arch A Pathol Anat Histopathol 1993;423:369)
- Atretic germinal centers traversed by sclerotic penetrating vessels and hyalinization - lollipop follicles
- Mantle zones are thickened with lymphocytes arranged in layers - onion skin appearance
- Mantle zones may fuse and contain more than 1 germinal center - twinning
- Follicular dendritic cells may show proliferation and dysplastic features
- In the interfollicular areas, there is usually extensive vascular proliferation of high endothelial venules with perivascular hyalinization
- Clusters of plasmacytoid dendritic cells may be seen (Hum Pathol 2013;44:1003)
- Plasma cells, immunoblasts and eosinophils are seen in the interfollicular areas but no sheets of plasma cells
- Unapparent sinuses and obliteration of the subcapsular sinuses commonly seen
- Capsular thickening
- UCD - plasma cell type: rare (< 10% of UCDs) and MCD needs to be excluded (Surg Pathol Clin 2019;12:849)
- Interfollicular areas and medulla containing sheets of small, mature plasma cells
- Hyperplastic follicles and a subset of the follicles with hyaline vascular features
- MCD can be hyaline vascular (rare < 10%) or plasmacytic but usually shows a mix of histological features of both plasma cell and hyaline vascular Castleman disease (Blood 2017;129:1658, Blood 2017;129:1646)
- Hypervascular without dysplastic follicular dendritic cells or sclerotic vessels
- Diffuse plasma cell proliferation, germinal center hyperplasia, some follicles may show hyaline vascular changes and patent sinuses
- HHV8 MCD (Am J Surg Pathol 2003;27:91)
- Plasmacytic
- HHV8 infected cells in the mantle zones show plasmablastic or immunoblastic morphology with large nuclei, vesicular chromatin and prominent nucleoli and lambda light chain restriction
- Polyclonal plasmacytosis
- TAFRO (Hematol Oncol Clin North Am 2018;32:37)
- Mixed hyaline vascular and plasmacytic
- Lymph nodes: hypervascular
- Bone marrow: reticulin fibrosis, megakaryocytic hyperplasia and emperipolesis
Microscopic (histologic) images
Contributed by Jayalakshmi Balakrishna, M.D., Amy Duffield, M.D., Ph.D., Tapan Bhavsar, M.D., Ph.D.,
Carlos Murga-Zamalloa, M.D. and Jackie D. Sublett II, M.D.
Hyaline vascular Castleman disease (HVCD)
Plasma cell Castleman disease (PCCD)
Human herpesvirus 8 associated multicentric Castleman disease (HHV8 MCD)
Virtual slides
Positive stains
- HVCD (Surg Pathol Clin 2019;12:849)
- PCCD (Histopathology 1989;14:333)
- Clusters of plasma cells in the interfollicular areas: CD138, polytypic light chains
- HHV8 MCD (Hum Pathol 2001;32:95, PLoS Pathog 2018;14:e1006967)
- HHV8-LANA1, viral IL6, lambda light chain, cytoplasmic IgM, OCT2, BLIMP1 and IRF4 / MUM1
Negative stains
Flow cytometry description
- Serum concentrations of IL2, IL4, IL6, IL10, TNFα and IFNγ cytokines can be evaluated by flow cytometry (J Med Virol 2021;93:4033)
Molecular / cytogenetics description
- HVCD
- Subset of cases shows clonal cytogenetic abnormalities: t(1;16)(p11;p11), t(1;22)(p22;q13) and t(7;8)(qter;q12) (Am J Surg Pathol 2000;24:882)
- In about 75% of cases studied HUMARA gene assays show monoclonal pattern (loss of heterozygosity) (Mod Pathol 2014;27:823)
- MCD
- Monoclonal IGH rearrangement in subset of cases (more common in HIV positive patients with HHV8 infection) (Am J Pathol 1988;131:84)
- B and T lymphocytes in Castleman disease are usually polyclonal (Histopathology 2006;48:233)
Sample pathology report
- Lymph node, left axillary, excisional biopsy:
- Kaposi sarcoma and HHV8 positive multicentric Castleman disease (see comment and report)
- Comment: The patient has a history of HIV / AIDS and presents with generalized lymphadenopathy and fever. The lymph node shows morphologic features consistent with plasma cell type Castleman disease and focal involvement by Kaposi sarcoma. Flow cytometry shows no evidence of an immunophenotypically abnormal lymphocyte or plasma cell population and molecular analysis to evaluate presence of clonal B cell population is negative ruling out B cell lymphoma and plasma cell neoplasm.
Differential diagnosis
- Systemic lupus erythematosus:
- Follicular hyperplasia, interfollicular expansion of lymphocytes and immunoblasts, and numerous plasma cells within germinal centers and medullary cords
- Coagulative necrosis with abundant karyorrhectic debris and histiocytes
- Hematoxylin bodies
- Serological diagnosis of systemic lupus erythematosus
- Rheumatoid arthritis:
- Can resemble HVCD or PCCD
- Follicular hyperplasia, interfollicular and intrafollicular plasmacytosis and neutrophils within sinuses
- Lacks thickened mantle zones, twinning of germinal centers and piercing vessels
- Clinical and serological diagnosis of autoimmune disease
- Hemophagocytic lymphohistiocytosis:
- Proliferation of benign histiocytes in the sinuses
- Lymphocyte depletion may be seen
- Phagocytosed red cells or white cells
- Adult onset Still disease (Medicine (Baltimore) 2015;94:e787):
- Paracortical hyperplasia or mixed follicular and paracortical hyperplasia
- Vascular proliferation and immunoblasts
- Pericapsular endarteritis
- Clinical and serologic diagnosis of adult onset Still disease
- IgG4 disease (Semin Diagn Pathol 2012;29:226):
- Fibroinflammatory disorder with dense lymphoplasmacytic infiltrates containing numerous IgG4+ plasma cells
- 5 different histological patterns - type resemble MCD
- Hyperplastic and regressed follicles, some with penetrating vessels
- Interfollicular areas with numerous plasma cells
- Serological diagnosis
- Follicular dendritic cell sarcoma:
- Plasma cell neoplasm:
- Monoclonal plasma cells
- HIV associated lymphadenitis:
- Clinical and serological diagnosis of HIV infection
- Angioimmunoblastic T cell lymphoma, early stages:
- Follicular lymphoma:
- Mantle cell lymphoma:
- Reactive lymph node enlargement:
- Lack characteristic histological features of CD
- Can show different histological patterns: follicular hyperplasia, sinus histiocytosis, interfollicular / mixed and diffuse patterns
Additional references
Board review style question #1
Board review style answer #1
D. Twinning of germinal centers. The features of hyaline vascular Castleman disease are atrophic follicles, penetrating vessels, onion skinning of mantle zones, twinning of germinal centers and hyalinized vessels.
Comment Here
Reference: Castleman disease
Comment Here
Reference: Castleman disease
Board review style question #2
Which of these lymphomas occurs in the background of human herpesvirus 8 associated multicentric Castleman disease (HHV8 MCD)?
- Burkitt lymphoma
- EBV positive diffuse large B cell lymphoma, NOS
- Follicular lymphoma
- HHV8 positive large B cell lymphoma
Board review style answer #2
D. HHV8 positive large B cell lymphoma is known to occur in a background of HHV8 MCD. Other neoplasms occur in this setting are Kaposi sarcoma and primary effusion lymphoma.
Comment Here
Reference: Castleman disease
Comment Here
Reference: Castleman disease
Cat scratch disease
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Majority of cases are caused by Bartonella henselae acquired via arthropod vector (cat flea) or inoculation by cat scratch (Pediatr Infect Dis J 2021;40:S11)
- Cutaneous red papule 3 - 10 days after contact that may become crusted or pustular, with subsequent development of regional lymphadenopathy 1 - 2 weeks later
- Histology is characterized by necrotizing stellate abscesses surrounded by palisading histiocytes
- Usually resolves spontaneously; rare cases may require treatment with antimicrobial therapy
Essential features
- Typically self limited lymphadenitis caused by Bartonella henselae and transmitted by cats
- Cutaneous lesion followed by usually localized lymphadenopathy
- Lymph node histopathology characterized by necrotizing stellate abscesses surrounded by palisading histiocytes
- Organism stains with Warthin-Starry or specific immunohistochemistry; confirmation by serology or PCR
ICD coding
- ICD-10: A28.1 - cat scratch disease
Epidemiology
- Adults or children can be affected but the highest incidence is in children 14 and under (Emerg Infect Dis 2016;22:1741)
- Considered the most common cause of benign lymphadenopathy in young people (Ocul Immunol Inflamm 2014;22:148)
- Estimated annual incidence of 4.7/100,000 population (Emerg Infect Dis 2016;22:1741)
- Incidence is higher in the southern United States and in the winter (thought to possibly be due to holiday adoption of kittens) (Emerg Infect Dis 2016;22:1741)
Sites
- Skin: cutaneous red papule or wheal that may become crusted or pustular
- Lymph nodes, most commonly upper extremity, followed by cervical and inguinal; patients may have involvement of 1 or multiple lymph node sites (Pediatr Infect Dis J 2021;40:S11)
- Liver, spleen or bone
- Rarely, CNS, eye, hematologic involvement / complications (Eur J Intern Med 2005;16:610)
- Parinaud oculoglandular syndrome: follicular conjunctivitis and regional lymphadenopathy, possibly caused by direct conjunctival inoculation (Ocul Immunol Inflamm 2014;22:148)
Etiology
- Majority of cases are caused by Bartonella henselae, harbored by kittens and young cats; transmitted between cats by the cat flea (Am J Clin Pathol 1994;101:607)
- Up to 100% of cat fleas harbor Bartonella and it can also often be detected in cat saliva, blood, skin, claws and feces
- Most important risk factors are owning a kitten and being bitten, scratched or licked by a kitten harboring fleas; however, up to 25% of patients with cat scratch disease do not report contact with cats
Clinical features
- Cutaneous nonpruritic red papule: 3 - 10 days after contact that may become crusted or pustular (Ocul Immunol Inflamm 2014;22:148)
- Lymphadenopathy, often of cervical or axillary nodes, develops in 1 - 2 weeks; lymphadenopathy is essentially universal but 85% have involvement of only a single node
- May have necrotizing granulomas in liver, spleen or bone
- Fever and constitutional symptoms may be present (Am J Dis Child 1985;139:1124)
- Rare complications in ~10%, including granulomatous conjunctivitis, thrombocytopenic purpura, CNS disease (Eur J Intern Med 2005;16:610, Infect Dis Clin North Am 1987;1:575, Am Fam Physician 2011;83:152)
Diagnosis
- PCR (J Clin Microbiol 2005;43:3800)
- Serology or immunofluorescence (Clin Diagn Lab Immunol 2003;10:686)
- Culture is not practical, as Bartonella are fastidious organisms that are difficult to culture (Pediatr Infect Dis J 2021;40:S11)
Radiology description
- Regional lymphadenopathy
- Liver and spleen granulomas
Prognostic factors
- Self limited disease in most; may take 2 - 6 months for lymphadenopathy to regress (Infect Dis Clin North Am 1987;1:575)
- Immunocompromised patients may present with disseminated disease or other diseases / syndromes associated with Bartonella, such as bacillary angiomatosis (BMJ Case Rep 2021;14:e239932, Am J Clin Pathol 2004;121:S71, Eur J Clin Microbiol Infect Dis 2021 Apr 12 [Epub ahead of print])
Case reports
- 16 year old boy with tender cervical lymphadenopathy (Arch Pathol Lab Med 2005;129:1065)
- 32 year old HIV infected woman with B. quintana and M. tuberculosis coinfection (J Infect 2003;46:244)
- 53 year old man with cat scratch disease encephalopathy (BMJ Case Rep 2018;2018:bcr2017223647)
- 59 year old heart transplant patient with disseminated cat scratch disease (Transpl Infect Dis 2017;19)
Treatment
- Treatment neither required nor recommended for immunocompetent patients / uncomplicated disease (Antimicrob Agents Chemother 2004;48:1921)
- Antibiotics (doxycycline, rifampin) could be considered if disseminated or complicated disease (Antimicrob Agents Chemother 2004;48:1921, Pediatr Infect Dis J 2021;40:S11)
Clinical images
Microscopic (histologic) description
- Early: histiocytes and follicular hyperplasia, microabscesses localized adjacent to the thickened capsule (Am J Clin Pathol 2004;121:S71)
- Intermediate: capsulitis and subcapsular granulomas
- Late: necrosis and abscesses, often stellate, surrounded by palisading histiocytes (Int J Dermatol 1978;17:656)
- Paracortical vascular proliferation (Am J Clin Pathol 2004;121:S71)
- Sinuses are often packed with monocytoid B cells but no epithelioid cells are present (Am J Clin Pathol 2004;121:S71)
- Small rods may be present with silver stain around small blood vessels and lymphatics; most readily identified early in disease (Science 1983;221:1403, Am J Clin Pathol 2004;121:S71)
- Skin shows dermal necrosis surrounded by histiocytes
- Also multinucleated giant cells, lymphocytes and eosinophils
- References: Calif Med 1955;82:25, Am Fam Physician 2011;83:152
Microscopic (histologic) images
Cytology description
- Similar to histologic features (granulomas, epithelioid histiocytes, neutrophils, necrosis, polymorphous lymphocytes) (Acta Cytol 1985;29:542, Diagn Cytopathol 1996;15:108)
Positive stains
- Warthin-Starry or other silver stains
- B. henslae immunohistochemistry (Am J Clin Pathol 2009;131:250)
- Combined use of Warthin-Starry and IHC may yield higher sensitivity than either alone (Appl Immunohistochem Mol Morphol 2020;28:781)
Negative stains
- Gram stain has limited utility, as Bartonella usually does not stain well (Am J Clin Pathol 2009;131:250)
Electron microscopy description
- Extracellular bacteria form small groups within bundles of collagen fibrils
- Bacteria are gram negative pleomorphic rods, with thick and homogeneous cell walls (Am J Clin Pathol 1987;87:739)
Molecular / cytogenetics description
- Causative organism may be detected by PCR (J Clin Microbiol 2005;43:3800)
Sample pathology report
- Cervical lymph node, excision:
- Necrotizing granulomatous lymphadenitis (see comment)
- Comment: The lymph node architecture is distorted by frequent stellate necrotizing granulomas with occasional multinucleated giant cells. Bacterial structures are identified by both Warthin-Starry stain and immunohistochemistry for B. henselae. The overall features are in keeping with cat scratch lymphadenitis, a condition that is generally self limited in immunocompentent patients.
Differential diagnosis
- Lymphoma, particularly classic Hodgkin lymphoma:
- May demonstrate granulomatous inflammation but will have Hodgkin / Reed-Sternberg cells and mixed inflammation, including eosinophils and plasma cells
- Sarcoidosis:
- Granulomas usually small and tight without suppuration
- Other infectious agents capable of causing granulomatous lymphadenitis:
- Kikuchi syndrome:
- No neutrophils
- Kimura disease:
- Eosinophils predominate, rather than neutrophils
Board review style question #1
A 10 year old girl presents with an enlarged, tender axillary lymph node. An excisional biopsy is performed with findings shown. Which of the following is a likely clinical history for this patient?
- Asthma, peripheral blood eosinophilia and elevated serum IgE
- Drenching night sweats, petechial rash and peripheral blood cytopenias
- Fever, pharyngitis, splenomegaly and positive monospot (heterophile antibody) test
- Low grade fever and fatigue, new kitten in the household
Board review style answer #1
D. Low grade fever and fatigue, new kitten in the household. The image shows multiple well formed suppurative granulomas. A relatively common cause of these morphologic findings is cat scratch disease, lymphadenitis transmitted by cats, almost always caused by B. henselae. Most patients with cat scratch disease are under the age of 18 and have recent exposure to a cat, with tender lymphadenopathy appearing in the draining lymph nodes from the site of a scratch. Fever, pharyngitis, splenomegaly and positive monospot (heterophile antibody) test (answer C) describes the classic presentation of infectious mononucleosis. Drenching night sweats, petechial rash and peripheral blood cytopenias (answer B) is more suggestive of a malignant process. Asthma, peripheral blood eosinophilia and elevated serum IgE (answer A) can be seen in Kimura disease.
Comment Here
Reference: Cat scratch disease
Comment Here
Reference: Cat scratch disease
Board review style question #2
Board review style answer #2
A. Cat scratch lymphadenitis. The image demonstrates lymphadenopathy with suppurative granulomas, a feature that can be seen in cat scratch lymphadenitis. Special stains (silver stain) or organism specific immunohistochemistry on tissue sections may be helpful. Clinical correlation (anatomic distribution of lymphadenopathy, presence of risk factors, such as contact with cats, etc.) and infectious disease workup (serologic studies and culture) are helpful. Kimura disease demonstrates prominent eosinophilic infiltration. Progressive transformation of germinal centers is characterized by indistinct germinal centers invaded by mantle zone cells. Treponema pallidum lymphadenopathy is characterized by plasmacytosis and capsular fibrosis.
Comment Here
Reference: Cat scratch disease
Comment Here
Reference: Cat scratch disease
Chronic lymphadenitis
Table of Contents
Definition / general | Essential features | Terminology | Sites | Clinical features | Diagnosis | Laboratory | Prognostic factors | Clinical images | Gross description | Microscopic (histologic) description | Cytology description | Positive stains | Negative stains | Flow cytometry description | Differential diagnosis | Additional referencesDefinition / general
- Common, chronic inflammatory process of lymph nodes in response to various pathogens
- May be specific or nonspecific, known or unknown
- Lymph nodes show variable amounts of necrosis, abscesses, granulomas and fibrosis
Essential features
- Nonspecific etiology
- General histological features are follicular hyperplasia, prominence of postcapillary venules, increased number of immunoblasts, plasma cells, histiocytes and fibrosis
Terminology
- Also called chronic nonspecific lymphadenitis
Sites
- Cervical or any other lymph node groups can be affected
Clinical features
- Enlarged lymph nodes (by palpation or imaging)
- Painless
Diagnosis
- Biopsy and clinical history are necessary
- Adjuvant diagnostic methods such as special stains, electron microscopy, immunohistochemistry, in situ hybridization analysis and polymerase chain reaction (PCR) techniques help delineate specific causative factors
Laboratory
- Specific findings depend on the etiology
Prognostic factors
- Good prognosis in general, although prognosis and treatment vary based on specific etiology
Gross description
- Enlarged lymph node
- Capsule may be thickened
Microscopic (histologic) description
- Follicular hyperplasia, prominence of postcapillary venules, increased number of immunoblasts, plasma cells and histiocytes and fibrosis
- Capsule may be inflamed or fibrotic
- Inflammation / fibrosis may extend into the immediate perinodal tissues
- Terms such as xanthogranulomatous lymphadenitis and eosinophilic lymphadenitis are used to describe cases with undue prominence of eosinophils, foamy macrophages or mast cells
Cytology description
- Smear derived from reactive follicle: centroblasts, centrocytes, small lymphocytes and tingible body macrophages with nuclear fragments
- Paracortical hyperplasia: spectrum of basophilic cells, ranging from mature plasma cells to immunoblasts with a background of many small lymphocytes
Positive stains
- T cell and B cell markers show reactive pattern
Negative stains
- Special stains for microorganisms
- Immunohistochemical stains / patterns for lymphoma
Flow cytometry description
- Reactive pattern: mixed / polyclonal population
Differential diagnosis
- Malignant: lymphoma, metastases
- Specific infections: atypical mycobacteria, fungal infections, leprosy, parasites, syphilis, tuberculosis
- Specific inflammatory lesions: Castleman disease, dermatopathic lymphadenitis, lupus erythematosus, rheumatoid arthritis, sarcoidosis
Additional references
Chronic myeloid leukemia, extramedullary
Table of Contents
Definition / general | Terminology | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy images | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Represents extramedullary involvement of lymph node by chronic myeloid leukemia
- CML may be in chronic phase, accelerated phase or blast crisis during nodal presentation
- Marker of poor prognosis - usually signals impending or concurrent blast crisis
Terminology
- Myeloid sarcoma or granulocytic sarcoma
Case reports
- 14 year old child with biphenotypic extramedullary blast crisis as a presenting manifestation of Philadelphia chromosome+ CML (Pediatr Hematol Oncol 2007;24:195)
- 22 year old man with concurrent chronic myelogenous leukemia and tuberculous lymphadenitis (Acta Cytol 2005;49:650)
- 59 year old man with chronic phase chronic myelogenous leukemia with an abdominal hematopoietic tumor (Am J Hematol 2004;77:167)
- 60 year old man with chronic myelogenous leukemia in the chronic phase with lymph node swelling (Rinsho Byori 2011;59:360)
Treatment
- Tyrosine kinase inhibitors, such as Gleevec® (imatinib) and Tasigna (nilotinib)
- Immunotherapy, such as interferon
- Allogeneic stem cell transplant from matched donor
- Chemotherapy for blast crisis
Microscopic (histologic) description
- Mixture of mature and immature myeloid cells effacing the nodal architecture
- May contain hematopoietic trilineages (granulocytic, erythroid and megakaryocytic)
Microscopic (histologic) images
Positive stains
Electron microscopy images
Molecular / cytogenetics description
- Philadelphia (Ph) chromosome with t(9;22) in vast majority of cases
- BCR-ABL fusion gene with uncontrolled tyrosine kinase production (molecular target for therapy)
- Methods of detection: cytogenetics, FISH, PCR
Differential diagnosis
- Anaplastic plasma cell myeloma
- Granulocytic sarcoma related to acute myeloid leukemia
- Metastatic carcinoma to lymph node
Additional references
Common nonspecific abnormal features
Table of Contents
Foamy macrophages | Follicular hyperplasia | Hypersplenism | Infarction | PerisplenitisFoamy macrophages
Definition / general
Essential features
Terminology
Case reports
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by David Lynch, M.D.
Positive stains
Negative stains
Differential diagnosis
Additional references
- Increased foamy histiocytes / macrophages, typically in the red pulp
Essential features
- Associated with a wide variety of conditions both benign and malignant
- Conditions with high cell turnover may produce foamy histiocytes
- Diagnosis typically requires clinicopathologic correlation
Terminology
- Ceroid histiocytosis - histiocytes filled with phospholipids; not specific to a single disease entity
Case reports
- 7 year old boy diagnosed with Gaucher disease by FNA of spleen (J Clin Diagn Res 2016;10:ED13)
- 52 year old man with splenomegaly (BMJ Case Rep 2015 Feb 5;2015)
Microscopic (histologic) description
- Red pulp expanded by histiocytes with abundant foamy cytoplasm
- Histiocytes scattered without forming a discrete mass
- May have basophilic cytoplasm from ingestion of platelets in idiopathic thrombocytopenic purpura (ITP)
- Finely fibrillary cytoplasm suggests underlying storage disease
- White pulp is normal in size
Microscopic (histologic) images
Contributed by David Lynch, M.D.
Positive stains
Negative stains
Differential diagnosis
- Autoimmune disease
- Chronic myelogenous leukemia: extramedullary hematopoiesis with hypolobated megakaryocytes, foamy macrophages
- Crystal storing histiocytosis: eosinophilic rhomboid crystals within histiocytes
- Hemophagocytic lymphohistiocytosis: histiocytes have ingested RBCs
- Hyperlipidemia
- Idiopathic thrombocytopenic purpura: follicular hyperplasia, increased foamy histiocytes, increased plasma cells, lipogranulomas in some cases
- Langerhans cell histiocytosis: cleaved nuclei; S100, CD1a and langerin are positive
- Mycobacterial or fungal infections: positive AFB or GMS stains
- Postchemotherapy histiocyte rich pseudotumor (Am J Clin Pathol 2009;132:342)
- Rosai-Dorfman disease: emperipolesis is present; histiocytes are S100 positive
- Storage disease (Gaucher, Niemann-Pick): histiocytes have fibrillary cytoplasm in Gaucher disease
Additional references
Follicular hyperplasia
Definition / general
Gross description
Microscopic (histologic) description
- Also called reactive follicular hyperplasia
- Focal or diffuse
- Normal in children
- In adults, due to systemic infection (malaria, measles, typhoid fever, virus, other), immune mediate disorders (hemolytic anemia, immune thrombocytopenic purpura, rheumatoid arthritis)
- Often associated with congestion and plasmacytic proliferation
- Associated with hypersplenism in Zaire, Nigeria and New Guinea, where spleens also show extramedullary hematopoiesis and marked sinusoidal dilation; may be related to malaria
- Note: graft rejection and AIDS are associated with reactive nonfollicular hyperplasia, which may resemble lymphoma but has heterogeneous lymphocytic population without atypia and without clonality
- Felty syndrome (rheumatoid arthritis): no granulocytic phagocytosis but expansion of red pulp cords and sinuses with macrophages
Gross description
- May have enlarged spleen with multiple small, pale tan nodules or solitary large nodules resembling lymphoma (Am J Surg Pathol 1983;7:373)
Microscopic (histologic) description
- Resemble nodal reactive follicles, with mixed follicular center population and tingible body macrophages
- Usually mature lymphocytes and plasma cells in red pulp
Hypersplenism
- Also called dysplenism
- Enlarged spleen leads to removal of cellular blood components (some or all), causing thrombocytopenia, neutropenia, hemolytic anemia or pancytopenia
- Due to disorders (congestive splenomegaly, Gaucher disease, hamartoma, hemangioma, Langerhans cell histiocytosis, leukemia / lymphoma, other conditions diffusely involving red pulp) causing widening of splenic cords with increase in macrophages or connective tissue, causing premature destruction of normal blood components
- Reactive follicular hyperplasia of white pulp may be present in hypersplenism associated with cytopenias
- Infectious causes include brucellosis, CMV, Echinococcus, histoplasmosis, infectious mononucleosis, leishmaniasis, malaria, schistosomiasis, syphilis, toxoplasmosis, trypanosomiasis, tuberculosis, typhoid
- May be due to abnormal cellular blood components (hereditary spherocytosis)
Infarction
Definition / general
Gross description
- Due to thrombosis of splenic vein, usually secondary to cardiac emboli
- Also associated with granulomatosis with polyangiitis (Wegener) (may cause splenic rupture), massive splenomegaly, idiopathic
Gross description
- Wedge shaped white-gray infarct involving capsule; infarcts heal as large, depressed scars
Perisplenitis
- Thick fibrous plaques coating splenic surface
- Incidental finding at autopsy or associated with portal hypertension
Congestive splenomegaly
Table of Contents
Definition / general | Treatment | Gross description | Microscopic (histologic) descriptionDefinition / general
- Caused by portal hypertension, which may be due to Budd-Chiari syndrome (thrombosis of hepatic veins), cirrhosis, congestive heart failure, portal vein stenosis, thrombosis of splenic veins, other thrombosis
- Portal vein thrombosis may be due to inflammation, inflammatory induced extrinsic pressure, trauma, tumor or idiopathic
- Portal vein stenosis may be due to extension of obliterative process at birth in umbilical vein and ductus venosus into portal vein
- Banti syndrome: idiopathic portal hypertension; controversial entity, cases in Japan and India, associated with fibroelastosis in portal tracts, dilated capillaries, phlebosclerosis; associated with hypersplenism and anemia, leukopenia and thrombocytopenia
Treatment
- Splenectomy:
- No shunt needed if coronary vein joins portal system central to point of obstruction; otherwise shunt is needed
- Possible shunts include splenic vein to renal vein, portal vein to vena cava
Gross description
- Large, firm, dark with fibrosis of capsule
Microscopic (histologic) description
- Dilated veins and sinuses, fibrosis of red pulp, hemosiderin laden macrophages
- Iron and calcium containing fibrotic nodules (Gamna-Gandy bodies) secondary to hemorrhage
- No prominent lymphoid follicles
Decidual reaction
Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Negative stains | Differential diagnosis | Additional referencesDefinition / general
- Deposits of decidual tissue without accompanying glands in lymph nodes
- Rare, incidental microscopic finding in para-aortic and pelvic lymph nodes in pregnancy
- Decidual cell reaction in the absence of pregnancy is seen only with a corpus luteum present in the ovary
- Ectopic decidual reaction in lymph nodes was first reported by Geipel in 1913
- Ectopic decidual reaction can be located in the vagina, cervix, fallopian tubes, ovary, peritoneum; also appendix, diaphragm, liver (surface), lymph nodes, mesentery, small bowel, spleen
Epidemiology
- Rare, but exact incidence unknown since it is detected as an incidental finding at autopsy or pelvic lymphadenectomy for a genital tract malignancy
- A particular problem differentiating from squamous cell carcinoma during frozen section (Arch Pathol Lab Med 2005;129:e117)
Sites
- Limited to para-aortic and pelvic lymph nodes including obturator and internal iliac lymph nodes
Etiology
- Unclear
- Theories postulated are:
- Decidual change of endometriotic sites in pregnant women
- Entrapment of or failure of migration of coelomic remanants during embryologic development and during pregnancy, these rests are sensitized by estrogen, progesterone and further stimuli inducing a decidual reaction
- Benign metastasis or lymphatic spread
Clinical features
- May be enlargement of lymph nodes
- May have associated peritoneal decidual reaction
Diagnosis
- Biopsy of the involved lymph nodes
Prognostic factors
- Typically no treatment needed
- Although extremely rare, malignant change can occur in these inclusions
Case reports
- 33 year old woman with ectopic decidua suspicious of malignancy in pregnancy (The Internet Journal of Gynecology and Obstetrics 2004;5)
- Decidual change in pelvic lymph nodes in the presence of cervical squamous cell carcinoma during pregnancy (Am J Obstet Gynecol 1977;127:674)
- Decidua in pelvic lymph nodes (Obstet Gynecol 1967;29:824)
- Ectopic decidua and metastatic squamous carcinoma (J Surg Oncol 1988;38:126)
Gross description
- Rarely is grossly visible as tiny, gray, subcapsular nodules
- Typically occupies the subcapsular sinus and superficial cortex, and less commonly, the central parts of the lymph node
Microscopic (histologic) description
- Decidual cells arranged in well circumscribed round groups, solid nests and loosely cohesive aggregates in the subcapsular sinus, as well as deep within the lymphoid tissue
- The individual large polygonal cells show abundant granular esosinophilic cytoplasm, sharply defined cell borders, small, round to oval bland nuclei with dispersed chromatin and single conspicuous nucleoli
- Mitoses or atypical features are not present
- The cells may be separated by an intervening amorphous pale staining myxoid stroma
Differential diagnosis
- Metastatic squamous cell carcinoma: small, irregular nests with well circumscribed borders of tightly cohesive, polygonal cells with small, dark staining nuclei and pleomorphism
Additional references
Dermatopathic lymphadenopathy
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Positive stains | Flow cytometry description | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Reactive lymphadenopathy characterized by paracortical expansion with increased interdigitating dendritic cells, Langerhans cells and histiocytes / macrophages, typically including melanophages
- Typically associated with chronic skin irritation, inflammation or infection
Essential features
- T cell mediated immune response to persistent cutaneous antigenic stimulation
- Paracortical expansion, often overall nodular in arrangement, with numerous interdigitating dendritic cells, Langerhans cells and histiocytes / macrophages
- Macrophages containing melanin pigment are typically present but vary widely in extent
- Classically associated with dermatologic disorders but can also present without any clinical evidence of skin disease
Terminology
- Lipomelanotic reticular hyperplasia
- Lipomelanotic reticulosis
- Dermatopathic lymphadenitis
- Pautrier-Woringer disease (Am J Dermatopathol 2004;26:499)
ICD coding
- ICD-10: I88.9 - nonspecific lymphadenitis, unspecified
Epidemiology
- Limited studies available
- Sex: initial case series suggested predilection for males, while recent case series included more women than men (Calif Med 1967;106:170, Mod Pathol 2020;33:1104)
- Age: any age, with peak in fifth to sixth decades (Calif Med 1967;106:170)
Sites
- Most commonly affects superficial axillary or inguinal lymph nodes
- Can be bilateral
- Generalized lymphadenopathy can occur uncommonly
- References: Medeiros: Ioachim's Lymph Node Pathology, 5th Edition, 2021, Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017
Pathophysiology
- Persistent cutaneous antigenic stimulation leading to local cytokine release, which mediates migration of Langerhans cells to regional lymph nodes (J Exp Med 2002;196:417)
- Partially through tumor necrosis factor alpha mediated downregulation of epithelial adhesion molecules (e.g., E-cadherin) in skin
- Antigen processing and presentation by dendritic cells and Langerhans cells, leading to a T cell mediated immune response
- Presence of melanophages in part due to pigment incontinence, melanophagocytosis and migration from skin to lymph node (Mod Pathol 2020;33:1104, Int Immunol 2001;13:695)
Etiology
- Dermatologic disorders
- Benign: atopic dermatitis, chronic contact dermatitis, erythrodermic drug reaction, psoriasis, stasis dermatitis
- Malignant: mycosis fungoides, melanoma, squamous cell carcinoma, Kaposi sarcoma
- No underlying dermatologic disorder can be identified in a subset of cases (12 - 48%) (Calif Med 1967;106:170, Mod Pathol 2020;33:1104)
Clinical features
- Moderately enlarged, firm, moveable and nontender lymph nodes
- Typically associated with chronic dermatologic disorders (Mod Pathol 2020;33:1104)
- Commonly found in patients with mycosis fungoides and Sézary syndrome
- Higher association with severe / florid dermatopathic lymphadenopathy (Mod Pathol 2020;33:1104)
- Can be found in patients with autoimmune disease / presence of autoantibodies (Int J Surg Pathol 2004;12:127)
Diagnosis
- Clinical, radiologic and histologic evaluation of lymph node biopsy
Laboratory
- Peripheral eosinophilia (up to 35%)
- Depending on the underlying condition, if any (e.g., autoimmune dermatitis could be associated with autoantibodies)
- References: Medeiros: Ioachim's Lymph Node Pathology, 5th Edition, 2021, Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017
Radiology description
- Moderately enlarged lymph node(s)
- Can be FDG avid on 18F-FDG PET / CT (World J Nucl Med 2020;20:205, Chin Med J (Engl) 2015;128:3121)
Radiology images
Prognostic factors
- Usually self resolving, depending on underlying condition
Case reports
- Newborn boy with chromosome 22q11.2 deletion syndrome and generalized skin rash (Am J Case Rep 2020;21:e924961)
- 21 year old man with fever, pruritis and nonblanching rash (Clin Case Rep 2018;6:1637)
- 50 year old woman without skin disease (J Cytol 2016;33:49)
- 56 year old woman with a history of breast carcinoma in remission (Am J Case Rep 2017;18:1330)
Treatment
- Treatment of the underlying dermatologic disorder
Gross description
- Enlarged lymph node (usually > 1.5 cm in greatest dimension)
- Yellow to tan with occasional brown pigmented foci
Microscopic (histologic) description
- There is always preserved nodal architecture with intact capsule
- Follicular hyperplasia may be present but it is usually minimal
- Spectrum of changes from mild to severe, characterized by paracortical expansion with pale, irregularly shaped areas containing numerous pale staining histiocytes, interdigitating dendritic cells, Langerhans cells and occasional immunoblasts
- Predominance of interdigitating dendritic cells over Langerhans cells, which are indistinguishable on morphology alone
- Both cell types show ill defined cell borders with fine irregular reniform nuclear contours and occasional nuclear grooves
- Paracortical or sinusoidal histiocytes and macrophages often contain cytoplasmic melanin pigment but they also can contain hemosiderin or lipid
- Medullary plasmacytosis is variable
- Distended sinuses with histiocytes, plasma cells and eosinophils can be present
- Variable, usually mild capillary hyperplasia
- Mild / early
- Increased interdigitating dendritic cells that do not form nodules or networks, admixed with Langerhans cells and histiocytes
- Hyperplastic lymphoid follicles
- Severe / florid
- Large vague nodules or sheets of interdigitating dendritic cells that can compress lymphoid follicles
- Rarely, sinuses can be partially compressed
- Severe forms are more frequently seen in patients with mycosis fungoides
- References: Medeiros: Ioachim's Lymph Node Pathology, 5th Edition, 2021, Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017
Microscopic (histologic) images
Contributed by Ingrid Tam, M.D., M.Sc., Emina Emilia Torlakovic, M.D., Ph.D. and Nikhil Sangle, M.D. (Case #396)
Peripheral smear description
- Peripheral eosinophilia in up to 35% of cases
Positive stains
- S100, CD1a, CD207 / langerin: Langerhans cells
- CD4 and CD8: typically there is great predominance of CD4+ T cells over CD8
- Fontana-Masson silver stain for melanin; melanin histochemical stain is often positive even when melanin is not readily identified on H&E sections
- S100, MUM1 / IRF4: interdigitating dendritic cells (Am J Surg Pathol 2022;46:1514)
- CD68: histiocytes and dendritic cells
- Prussian blue for hemosiderin: this is less useful as it is not as specific as melanin for this diagnosis
- Pan-T cell markers CD2, CD5 and especially CD7 may be useful if there is a suspicion of involvement by mycosis fungoides or other cutaneous T cell lymphoproliferative disease (e.g., CD7 is typically negative in mycosis fungoides)
- CD30: immunoblasts; CD30+ immunoblasts are not required for diagnosis
Flow cytometry description
- No evidence of aberrant B or T cell populations, except if involved by mycosis fungoides or other primary cutaneous lymphoproliferative disorder
Electron microscopy description
- Predominance of interdigitating cells over Langerhans cells
- Langerhans cells: cytoplasmic Birbeck granules are defining feature
- Both interdigitating cells and Langerhans cells have fine irregular nuclear contours and finger-like cytoplasmic extensions
- References: Medeiros: Ioachim's Lymph Node Pathology, 5th Edition, 2021, Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017
Molecular / cytogenetics description
- No evidence of immunoglobulin or T cell receptor gene rearrangement when associated with benign skin disease
- Monoclonal T cell receptor gene rearrangements when associated with mycosis fungoides / Sézary syndrome or other primary cutaneous lymphoproliferative disorder
Sample pathology report
- Lymph node, left axilla, excision:
- Reactive changes consistent with dermatopathic lymphadenopathy (see comment)
- Comment: The lymphoid architecture is preserved. There is evidence of paracortical hyperplasia with dendritic cells, Langerhans cells and histiocytes. Melanin pigment is noted. There is also mild focal follicular hyperplasia. The overall features are consistent with dermatopathic lymphadenopathy.
Differential diagnosis
- Mycosis fungoides:
- Neoplastic T lymphocytes with cerebriform nuclei that demonstrate partial loss of CD7
- Lymph node may be involved even if it is not possible to confirm the presence of atypical T cells by morphology or immunophenotyping; therefore, TCR clonality assay is always required in patients with primary cutaneous lymphoproliferative disorder where regional lymph nodes show dermatopathic changes (Am J Surg Pathol 1981;5:343)
- Flow cytometry: loss of CD7 is the most frequent finding; other pan-T cell markers including CD2, CD3, CD8 and CD5 may also be altered (low, high, negative, double positive CD4 / CD8, etc.); downregulated CD26 is often seen
- T cell receptor (TCR) clonality assay: monoclonal TCR gene rearrangement
- Langerhans cell histiocytosis:
- Sinusoidal involvement with partial or total effacement of lymph node architecture
- Formation of aggregates and sheets of Langerhans cells is more typical of Langerhans cell histocytosis than dermatopathic lymphadenopathy, even when the latter is florid
- Predominance of large Langerhans cells with abundant eosinophilic cytoplasm
- Often with abundant eosinophils
- Molecular: BRAF V600E in up to 50% of cases; BRAF V600E IHC assay may also be used
- Classic Hodgkin lymphoma:
- Metastatic melanoma:
- Melanoma cells are positive for HMB45 (cytoplasmic), SOX10 (nuclear) or S100 (nuclear / cytoplasmic), MelanA / MART1 (cytoplasmic)
- Ki67 proliferative index often > 10% but this is a nonspecific finding
- Reactive lymphadenopathy with paracortical hyperplasia due to viral infections:
- Positive serology, culture or in situ hybridization studies
- Usually no significant increase in interdigitating dendritic cells or Langerhans cells
- Melanophages are typically absent
- Toxoplasma lymphadenitis:
- Sinuses with monocytoid B cells
- Interfollicular and paracortical epithelioid histiocytes
- Prominent follicular hyperplasia
- No significant increase in interdigitating dendritic cells or Langerhans cells
- Melanophages typically absent
Board review style question #1
Which of the following histologic patterns is characteristically seen in dermatopathic lymphadenopathy?
- Florid reactive follicular hyperplasia
- Nonnecrotizing granulomatous inflammation
- Paracortical expansion with a predominance of interdigitating cells admixed with Langerhans cells and histiocytes
- Reactive follicular hyperplasia, interfollicular epithelioid histiocytes and monocytoid B cell hyperplasia
- Sinus expansion with histiocytes showing emperipolesis
Board review style answer #1
C. Dermatopathic lymphadenopathy is characterized by a paracortical expansion with increased interdigitating dendritic cells, Langerhans cells and histiocytes / macrophages. Florid reactive follicular hyperplasia (answer A) can be seen in a number of conditions, including HIV lymphadenitis, rheumatoid arthritis and syphilis. A variety of etiologies can lead to nonnecrotizing granulomatous inflammation (answer B), including sarcoidosis, infections and autoimmune conditions. Reactive follicular hyperplasia, interfollicular epithelioid histiocytes and monocytoid B cell hyperplasia describe the triad of Toxoplasma lymphadenitis (answer D). Sinus histiocytosis with emperipolesis (answer E), characterizes Rosai-Dorfman disease.
Comment Here
Reference: Dermatopathic lymphadenopathy
Comment Here
Reference: Dermatopathic lymphadenopathy
Board review style question #2
Board review style answer #2
E. Polyclonal population by TCR assays. In dermatopathic lymphadenopathy, T cell receptor gene rearrangement assays show a polyclonal population. There is no evidence of B or T cell clonality (answers C and D); however, dermatopathic lymphadenopathy detected in patients with mycosis fungoides may show T cell clonality. This may be the case even without morphologic evidence of involvement by mycosis fungoides (e.g., stage N1b, with histopathology Dutch grade 1). BRAF V600E mutation (answer A) is associated with several hematologic conditions, including Langerhans cell histiocytosis and hairy cell leukemia. MAP2K1 mutations (answer B) can be seen in a subset of Langerhans cell histiocytosis cases as well as in Rosai-Dorfman disease.
Comment Here
Reference: Dermatopathic lymphadenopathy
Comment Here
Reference: Dermatopathic lymphadenopathy
Drug hypersensitivity
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Flow cytometry description | Molecular / cytogenetics description | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Certain drugs may give rise to soft lymph node enlargement, predominantly in the neck (J Pathol Bacteriol 1968;95:314)
- Lymphadenopathy may occur alone or be associated with involvement of other organs (Neurology 1979;29:1480)
- May resemble lymphoma by morphology
- When the drug is stopped, the lymphadenopathy usually disappears in 1 - 2 weeks
- Can present with lymphadenopathy or with a rare systemic disorder, DIHS / DRESS (Drug Induced Hypersensitivity Syndrome / Drug Reaction with Eosinophilia and Systemic Symptoms (Rev Assoc Med Bras 2016;62:227)
Essential features
- History of drug exposure
- Polymorphous infiltrate composed of immunoblasts, small lymphocytes, plasma cells, eosinophils and histiocytes
Terminology
- Drug induced lymphadenopathy
- Hydantoin lymphadenopathy
- Pseudolymphoma syndrome
- Drug induced hypersensitivity syndrome (DIHS)
- Drug reaction with eosinophilia and systemic symptoms (DRESS)
ICD coding
Epidemiology
- Uncommon
- Pediatric population
Sites
- Predominantly cervical but all lymph node groups can be affected
- Can be generalized
Pathophysiology
- Different theories have been proposed:
- Hapten: drug molecules become antigenic when bound to a carrier protein, creating an immune response (Curr Opin Immunol 2012;24:730)
- p-i concept: direct binding of drugs and their metabolites to HLA trigger T cell responses (Curr Opin Allergy Clin Immunol 2002;2:301)
- Very strong link between HLA-B*5701 in HIV positive Caucasians and the development of hypersensitivity to abacavir
- Abacavir binds noncovalently and specifically to the peptide binding groove of HLA-B*5701 molecule (Nature 2012;486:554)
- Susceptibility to carbamazepine reactions in patients with HLA-B*1502 variant (Nature 2004;428:486)
- Drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with reactivation of inactive Herpesviridae family viruses, including human herpesvirus 6 (HHV6), Epstein-Barr virus (EBV) and cytomegalovirus (CMV) (Arch Dermatol 2001;137:301, Br J Dermatol 2006;155:344, Arch Dermatol 2009;145:1030)
Etiology
- Reaction to drug exposure, including (Am J Med 2011;124:588):
- Abacavir, allopurinol, antibiotics, carbamazepine, dapsone
- Lamotrigine, mexiletine, minocycline, nevirapine
- Oxcarbazepine, phenobarbital, phenytoin (J Pathol Bacteriol 1968;95:314, Neurology 1979;29:1480), salazosulfapyridine
- Sulfamethoxazole, sulfasalazine, strontium ranelate, vancomycin
Clinical features
- Isolated lymph node enlargement OR
- Drug Induced Hypersensitivity Syndrome (DIHS) / Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) (Allergol Int 2016;65:432):
- Fever, lymphadenopathy, facial edema, periorbital edema, conjunctival injection
- Abdominal pain, diarrhea, hepatomegaly, splenomegaly
- Skin rash (maculopapular rash, bullous, wheal and flare, plaque, patch, target lesion)
- Internal organ involvement (liver - elevated alanine aminotransferase levels, kidney, lung); also brain (Turk J Pediatr 2015;57:541), heart, muscle, pancreas, thyroid (Int J Mol Sci 2017 Jun 9;18(6) pii: E1243)
- Hematologic abnormalities (eosinophilia, atypical lymphocytes, lymphocytosis, lymphopenia, thrombocytopenia)
Diagnosis
- Clinical history of drug exposure
- Characteristic clinical and laboratory features
- Biopsy of the affected lymph node (rarely done, not required for diagnosis)
Radiology description
- Enlarged lymph nodes
Prognostic factors
- Underlying disease conditions
- Extent of drug exposure
- Drug reaction with eosinophilia and systemic symptoms (DRESS) has 4% mortality (Acta Derm Venereol 2012;92:200)
Case reports
- 17 year old girl presenting with bilateral cervical neck lymphadenopathy (Int J Pediatr Otorhinolaryngol 2017;98:82)
- 20 year old woman with fever of 38°C, prostration and drowsiness (Rev Assoc Med Bras 2016;62:227)
- 24 year old woman with lamotrigine cervical pseudolymphoma (Epilepsy Behav Case Rep 2017;7:40)
- 37 year old woman with redness and edema of inguinal area (Drug Saf Case Rep 2017;4:1)
- 60 year old man with skin rash, fever, distended abdomen and severe pruritus for 10 days (Iran Red Crescent Med J 2016;18:e36825)
Treatment
- Discontinuation of the offending drug
- For severe cases, immunosuppressive drugs / corticosteroids, IV Ig, plasma exchange
Gross description
- Enlarged lymph nodes
Microscopic (histologic) description
- Polymorphous cellular infiltrate composed of lymphocytes, plasma cells, eosinophils and histiocytes, most often with architectural distortion
- Focal hemorrhagic necrosis is usually seen without fibrosis or scarring
- Typical multinucleated Reed-Sternberg cells are absent; however, cells resembling Hodgkin / Reed-Sternberg (HRS) cells may be seen
- Blood vessels show endothelial hyperplasia
- There is variable follicular and paracortical hyperplasia (Hum Pathol 1974;5:519)
Flow cytometry description
- No aberrant immunophenotype
Molecular / cytogenetics description
- No clonal cell populations
Differential diagnosis
- For drug reaction with eosinophilia and systemic symptoms (DRESS) (Rev Assoc Med Bras 2016;62:227):
- Hypereosinophilic syndrome: manifests with urticaria and angioedema and can be a hematopoietic malignancy
- Kawasaki disease: presents with mucosal congestion and desquamation of extremities / skin and lacks eosinophilia and atypical lymphocytes
- Stevens-Johnson syndrome / toxic epidermal necrolysis: lacks eosinophilia, atypical lymphocytosis and lymphadenopathy
- On lymph node biopsy (Hum Pathol 1974;5:519):
- Angioimmunoblastic T cell lymphoma (AITL):
- Presence of vascular proliferation, immunoblasts and polymorphous infiltrate cause confusion with angioimmunoblastic T cell lymphoma
- AITL has characteristic T cells of T follicular helper (TFH) phenotype in perisinusoidal areas and T cell clones, not seen in drug hypersensitivity
- Hodgkin lymphoma:
- Presence of polymorphous inflammatory infiltrate and large Hodgkin / Reed-Sternberg-like cells may cause confusion
- However, classic Hodgkin / Reed-Sternberg cells are not identified in drug hypersensitivity
- Angioimmunoblastic T cell lymphoma (AITL):
Additional references
Board review style question #1
All of the following are proposed pathogenetic mechanisms for drug hypersensitivity, except:
- Direct toxicity
- Drug interactions with specific HLA types
- Hapten theory
- p-i concept
Board review style answer #1
A. Direct toxicity. Drug molecules causing an immune reaction are proposed mechanisms of pathogenesis of drug hypersensitivity, including hapten theory (drug molecules bind to a protein and cause antigenicity), p-i concept (drug molecules bind to HLA and cause T cell response) and drug molecules interacting with certain HLA subtypes. Direct toxicity does not manifest as a drug reaction with eosinophilia and systemic symptoms (DRESS).
Comment Here
Reference: Drug hypersensitivity
Comment Here
Reference: Drug hypersensitivity
EBV positive inflammatory follicular dendritic cell / fibroblastic reticular cell tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Epstein-Barr virus (EBV) positive inflammatory follicular dendritic cell (FDC) / fibroblastic reticular cell (FRC) tumor (ICC, 2022) / EBV positive inflammatory follicular dendritic cell (FDC) sarcoma (WHO, 5th edition) is an EBV driven indolent malignancy of FDC / FRC phenotype with a prominent inflammatory background
- Encompasses extranodal lesions previously referred to as EBV positive inflammatory pseudotumor (IPT); nodal EBV positive IPT as well as EBV negative FDC sarcoma are excluded
Essential features
- Extranodal EBV positive mesenchymal neoplasm with a phenotypic spectrum ranging from FDCs to FRCs associated with a prominent polymorphic inflammatory background
- Previously considered a variant of FDC sarcoma, it has now been recognized as a distinct entity by both the WHO, 5th edition and the ICC, 2022
- Nodal cases reported as inflammatory pseudotumor are excluded
- Usually involves middle aged women of Asian descent
- Solitary, asymptomatic splenic mass is the most common finding but it can also affect the liver and rarely, the colon and pancreas
Terminology
- Inflammatory pseudotumor of the spleen, EBV positive
- Inflammatory pseudotumor-like follicular / fibroblastic dendritic cell sarcoma
- Inflammatory pseudotumor-like follicular dendritic cell tumor
ICD coding
Epidemiology
- Lesion occurs predominantly in middle aged Asian women and is by definition associated with EBV infection
- F:M = 10:1 to 7:2 (Am J Surg Pathol 2001;25:721, Am J Surg Pathol 2021;45:765)
- Age distribution ranges between 19 and 67 years; mean age of 49 - 58 years (Am J Surg Pathol 2023;47:476, Am J Surg Pathol 2021;45:765, Am J Surg Pathol 2001;25:721)
Sites
- Spleen is the most frequent site of involvement, followed by the liver (Am J Surg Pathol 2023;47:476, Am J Surg Pathol 2021;45:765, Am J Surg Pathol 2001;25:721)
- Rare reports in the gastrointestinal (GI) tract and the lungs (Hum Pathol 2015;46:1956, Case Rep Pathol 2019:2019:2648123, Am J Surg Pathol 2023;47:476)
- Nodal IPT cases are excluded; in these cases, EBV is consistently absent in the mesenchymal component (Hum Pathol 2015;46:1956)
Pathophysiology
- Steps involved in the development of these neoplasms are poorly characterized
- CD21 may allow entry of EBV in the FDCs (Proc Natl Acad Sci U S A 1984;81:4510, Virology 2016:494:23, Nat Rev Microbiol 2019;17:691)
- Mechanism of EBV infection in cases with an FRC phenotype is less clear
- How EBV promotes neoplastic transformation in mesenchymal cells is not fully understood
- Distinct from inflammatory myofibroblastic tumors in which ALK or other tyrosine kinase receptors are involved
- MAPK pathway alterations have not been reported (Am J Surg Pathol 2001;25:721)
Etiology
- Neoplastic cells are positive for EBV encoded small RNA (EBER)
- Frequent association with EBV genotype A, f variant and with a 30 base pair deletion in exon 3 of the LMP1 gene, demonstrating the presence of a clonal EBV genome (Histopathology 2016;69:883)
- This implies that EBV plays a role in the etiology of these lesions
Clinical features
- Usually asymptomatic finding during imaging work up for other reasons
- Systemic symptoms can be seen, including fever, fatigue and weight loss
- Abdominal distension and pain with or without palpable abdominal mass
- Colonic cases can present with positive fecal occult blood test (Am J Surg Pathol 2021;45:765)
Diagnosis
- Clinical, laboratory and imaging findings are largely nonspecific
- Endoscopy shows a polypoid mass in cases involving the GI tract (Hum Pathol 2015;46:1956)
- Diagnosis requires histopathological demonstration of characteristic morphology, immunophenotype and EBER positivity by the WHO criteria; these are best evaluated on excisional specimens, including splenectomies
Laboratory
- Nonspecific inflammatory reaction, including anemia, hypergammaglobulinemia, elevated C reactive protein
Radiology description
- Solitary, well defined hypodense mass in the affected organ (J Comput Assist Tomogr 2018;42:399)
Prognostic factors
- EBV positive FDC / FRC is an indolent tumor with a propensity for local recurrences in later stages of the disease
- Location seems to be important for the prognosis, with splenic cases showing fewer recurrences than cases in the liver (Int J Clin Exp Pathol 2014;7:2421, Am J Surg Pathol 2001;25:721)
- Distant metastases are rare and have been reported in the lungs and the spine
- Overall mortality rate is 10% for liver cases
- Colonic cases do not seem to recur and can be cured by complete excision (Hum Pathol 2015;46:1956)
Case reports
- 42 year old woman with a polypoid mass in the ascending colon (Hum Pathol 2015;46:1956)
- 55 year old woman with a 20 cm liver mass (Front Med (Lausanne) 2023:10:1192998)
- 70 year old woman with a pancreatic mass (Case Rep Pathol 2019:2019:2648123)
Treatment
- Surgical excision alone is the treatment of choice (Am J Surg Pathol 2023;47:476)
Gross description
- Solitary, large and well circumscribed mass with a broad size range from 1.5 cm up to 20 cm (Am J Surg Pathol 2023;47:476, Front Med (Lausanne) 2023:10:1192998)
- Cut surface is smooth, white to tan and often shows areas of hemorrhage and necrosis
Gross images
Microscopic (histologic) description
- Neoplastic cells are spindled to oval, with vesicular chromatin, a small, central nucleolus and indistinct cytoplasmic borders
- Binucleate cells with a kissing nuclei appearance can be noted in cases with FDC differentiation
- Neoplastic cells form loose fascicles with a storiform appearance
- Rich inflammatory background composed of small lymphocytes, plasma cells and histiocytes; eosinophils can be present (Hum Pathol 1995;26:1093, Am J Surg Pathol 2001;25:721)
- In some cases, the inflammatory component can obscure the sparsely distributed neoplastic cells
- Cytologic atypia is variable; it can range from subtle, with cells showing delicate nuclear membranes, to prominent with Hodgkin / Reed-Sternberg (HRS)-like cells (Am J Surg Pathol 2001;25:721)
- Necrosis can be seen and it has not been consistently associated with more aggressive behavior (Am J Surg Pathol 2001;25:721, Histopathology 2016;69:883, Am J Surg Pathol 2021;45:765)
- Cases referred to as having a hemangioma-like appearance due to the prominence of blood vessel wall hyaline and fibrinoid degeneration have been reported (Am J Surg Pathol 2023;47:476)
Microscopic (histologic) images
Contributed by Elaine S. Jaffe, M.D., Shunyou Gong, M.D., Ph.D. and João Víctor Alves de Castro, M.D.
Cytology description
- Fine needle aspiration biopsy smears, touch imprints and scrape preparations show spindle cells with vesicular, atypical nuclei and delicate cytoplasmic processes distributed singly or in syncytial clusters (Diagn Cytopathol 2008;36:42)
- Nuclear grooves and prominent nucleoli can be seen
- Cellularity ranges from hypo to hypercellular smears
- Background is polymorphic and is rich in lymphocytes, plasma cells and histiocytes; can be hemorrhagic or necrotic
- Immunohistochemistry is mandatory for the diagnosis (see Positive stains)
Positive stains
- FDC differentiation is demonstrated by at least a subset of cells positive for one or more markers
- FRC differentiation can be suggested by positivity for smooth muscle actin (SMA)
- Cases with FRC differentiation may or may not be positive for FDC markers
- EBER positivity in the spindle cells is required for the diagnosis
- EBV latency pattern is not consistently reported but latent membrane protein 1 (LMP1) is positive in 72 - 90% of cases evaluated (Int J Clin Exp Pathol 2014;7:2421, Histopathology 2016;69:883)
- IgG4 positive plasma cells can be present (Am J Surg Pathol 2023;47:476)
Negative stains
Molecular / cytogenetics description
- ALK and other tyrosine kinase gene rearrangements are negative by FISH and sequencing methods, including NGS
Molecular / cytogenetics images
Sample pathology report
- Liver, left, partial hepatectomy:
- Epstein-Barr virus (EBV) positive inflammatory follicular dendritic cell / fibroblastic reticular cell tumor (ICC, 2022) / EBV positive inflammatory follicular dendritic cell sarcoma (see comment)
- Comment: Histologic sections reveal effacement of the liver architecture within the grossly described mass by an atypical lymphoplasmacytic infiltrate. The infiltrate is composed predominantly of small lymphocytes with mature chromatin and numerous plasma cells. There are rare scattered atypical cells with elongated nuclei. Fibrinoid deposition is present around several vessels in addition to areas of hyalinization. Within the areas of hyalinization, there are numerous large vessels. While scattered islands of hepatocytes are present, normal portal areas with bile ducts within the mass lesion are rare.
- Within the lesion, there are rare scattered atypical, spindled appearing cells that are positive for CD21 and more often SMA. Clusterin is focally positive. CD20 and CD3 highlight B and T cells in aggregates respectively, with a predominance of T cells. EBV ISH highlights numerous cells, some with a spindled morphology and others that are more rounded. CD34 and SMA highlight sinusoidal spaces while keratin 7 highlights bile ducts, which are decreased in amount.
- Given the patient's clinical presentation in conjunction with rare CD21, SMA and EBV positive spindled cells in the mass lesion, this case is best felt to represent an Epstein-Barr virus (EBV) positive inflammatory follicular dendritic cell / fibroblastic reticular cell tumor (ICC, 2022) / EBV positive inflammatory follicular dendritic cell sarcoma. The vessels and pattern of bile duct loss within the lesion are unusual. It is unclear whether the EBV positive spindle cell proliferation has distorted the underlying liver parenchyma, which then adapted to a predominantly arterial supply.
- Epstein-Barr virus (EBV) positive inflammatory follicular dendritic cell / fibroblastic reticular cell tumor is the terminology currently used for the lesion also known as inflammatory pseudotumor. These occur in extranodal locations, including spleen, liver and more rarely GI tract. While previously referred to as sarcoma, when localized, the process has a good prognosis without significant risk for dissemination. This is an EBV driven lesion, in which the EBV positive cells are thought to be either FDCs or fibroblastic reticular cells (SMA positive).
Differential diagnosis
- Inflammatory myofibroblastic tumor:
- Presence of ALK rearrangements
- EBV associated smooth muscle tumor:
- Clinical setting of immunodeficiency (HIV, posttransplant or rarely primary immunodeficiency)
- Eosinophilic cytoplasm with myoid features
- Positive for desmin
- IgG4 related disease:
- Clinical criteria, including increase in serum IgG4
- Follicular dendritic cell sarcoma:
- EBER negative
- Can present as nodal disease
- Usually much more tumor cell rich, with only a sprinkling of small lymphocytes
- Fibroblastic reticular cell tumor:
- EBER negative
- Positive for cytokeratin and desmin
- Interdigitating dendritic cell sarcoma:
- Hodgkin lymphoma:
Additional references
Board review style question #1
Epstein-Barr virus (EBV) positive inflammatory follicular dendritic cell / fibroblastic reticular cell tumor (ICC, 2022) / EBV positive inflammatory follicular dendritic cell sarcoma (WHO, 5th edition), previously referred to as EBV positive inflammatory pseudotumor (IPT), has been separated from EBV negative lesions that might also be termed IPT. Which of the following is compatible with the diagnosis of EBV positive FDC / FRC?
- Inflammatory background usually lacks plasma cells
- Lesion should be nodal in presentation, infrequently involving the spleen or liver
- Negative results for LMP1
- Neoplastic cells should have a distinct immunophenotype compatible with both follicular dendritic cell and fibroblastic reticular cell differentiation
- This lesion has a good prognosis, differing from the aggressive FDC sarcoma (EBV negative)
Board review style answer #1
E. This lesion has a good prognosis, differing from the aggressive FDC sarcoma (EBV negative). EBV positive inflammatory FDC / FRC tumor (ICC, 2022) / sarcoma (WHO, 5th edition) is an indolent neoplasm, in contrast to the aggressive FDC sarcoma. Answer B is incorrect because the lesion should be extranodal. Answer C is incorrect because EBV positivity is an essential criterion usually detected by EBER, but LMP1 can also be detected in many cases.
Answer D is incorrect because the immunophenotype is highly variable and can be of either FDC or FRC derivation. Answer A is incorrect because the inflammatory background is important for the diagnosis and usually has prominent plasma cells.
Comment Here
Reference: EBV positive inflammatory follicular dendritic cell / fibroblastic reticular cell tumor
Comment Here
Reference: EBV positive inflammatory follicular dendritic cell / fibroblastic reticular cell tumor
Epithelial cyst
Table of Contents
Definition / general | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- Usually children or young adults
- Solitary or multiple; may be associated with accessory spleen
- May mimic mucinous cystic neoplasm if in intrapancreatic accessory spleen
- Called "epithelioid" / "epidermoid" if squamous lining
- Origin unknown; may derive from metaplasia in mesothelial cysts (Am J Surg Pathol 1988;12:275)
- Often large and requires splenectomy
Case reports
- 62 year old man with epidermoid cyst in intrapancreatic accessory spleen (JOP 2011;12:279)
Gross description
- Glistening inner surface with marked trabeculation
Gross images
Microscopic (histologic) description
- Lined by squamous, columnar, cuboidal or mesothelial-like epithelium
- No skin adnexae
- Rarely mucinous and associated with pseudomyxoma peritonei
Microscopic (histologic) images
Positive stains
- CEA, CA19-9 (Am J Surg Pathol 1998;22:704)
Differential diagnosis
- Mucinous / cystic neoplasm
- Pseudocyst
Epithelial inclusions
Table of Contents
Definition / general | Terminology | Sites | Pathophysiology | Clinical features | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosis | Additional referencesDefinition / general
- Rare presence of benign, well differentiated epithelial cell clusters in lymph nodes
- May coexist with breast micrometastases (Am J Surg Pathol 2003;27:513)
- In axilla, may be due to pre-sentinel lymph node biopsy breast massage (Am J Surg Pathol 2004;28:1641, Arch Pathol Lab Med 2003;127:e25)
Terminology
- Also called benign metastasis or heterotopia
Sites
- Axillary, cervical, mediastinal, mesenteric, para-aortic, pelvic, renal lymph nodes
Pathophysiology
- Different theories regarding the pathogenesis:
- Benign metastasis
- Developmental heterotopia
- Metaplasia of multipotent cells
- Iatrogenic displacement and transport (J Clin Oncol 2006;24:2013)
Clinical features
- Asymptomatic, incidental finding
Prognostic factors
- Benign with no significant clinical implications
- Rarely may undergo disease processes and become cystic, a benign tumor or malignant
Case reports
- 44 year old woman with right breast carcinoma and benign Müllerian inclusions coexisting with breast metastatic carcinoma in an axillary lymph node (Virchows Arch 2005;446:467)
- 48 year old woman with axillary intranodal cysts associated with breast malignancy (Arch Pathol Lab Med 2004;128:361)
- 50 year old woman with unusual cystic epithelial choristoma in a celiac lymph node (Hum Pathol 1987;18:866)
- 52 year old woman with squamous inclusion cyst in a sentinel axillary lymph node associated with breast malignancy (J Coll Physicians Surg Pak 2012;22:50)
- 65, 66 and 75 year old women with endosalpingiosis in axillary sentinel lymph nodes obtained for staging of breast carcinoma (Am J Surg Pathol 2010;34:1211)
- 70 year old woman with invasive ductal carcinoma of the left breast and endosalpingiosis of axillary lymph nodes (Case Rep Pathol 2016;2016:2856358)
- 73 year old woman with ductal carcinoma in situ of the breast and epithelial inclusions in an ipsilateral axillary lymph node (Int J Surg Pathol 2018;26:564)
- Epithelial inclusion in axillary lymph node associated with a breast carcinoma (Pathol Res Pract 1999;195:263)
- Benign inclusion of axillary lymph nodes (Breast J 2009;15:664)
Treatment
- No specific treatment needed, unless associated with another disease
Gross description
- Normal unremarkable lymph nodes
Microscopic (histologic) description
- Reactive follicular hyperplasia
- Well formed epithelial formations, in or near the peripheral sinuses
- Examples include salivary gland, thyroid follicles, breast tissue, respiratory type epithelium, fallopian tube lining or endometrium
- Single or tubules of epithelial cells in subcapsular sinus of draining lymph node after surgical or needle manipulation (Am J Clin Pathol 2000;113:259)
- Also hemosiderin laden macrophages and damaged erythrocytes
Positive stains
- Keratins and specific markers for the cell lineage
Differential diagnosis
Fibroblastic reticulum cell sarcoma
Table of Contents
Definition / general | Pathophysiology | Case reports | Treatment | Microscopic (histologic) description | Cytology images | Positive stains | Negative stains | Differential diagnosis | Additional referencesDefinition / general
- Rare malignant neoplasm of nodal stromal cells
Pathophysiology
- Arises from fibroblastic reticular cells in the T cell zone of the nodal cortex
- Fibroblastic reticular cells secrete chemokines (interleukin 7 and CCL19) responsible for naive T cell survival (Nat Immunol 2007;8:1255)
Case reports
- 25 year old man with reticulum cell sarcoma of lymph node with mixed dendritic and fibroblastic features (Mod Pathol 2001;14:1059)
- 71 year old woman with tumor of fibroblastic reticular cells of lymph node coincidental with an undifferentiated endometrial stromal sarcoma (APMIS 2011;119:216)
Treatment
- Surgery
Microscopic (histologic) description
- Storiform or fascicular pattern of oval to spindle cells in a collagenized background
Positive stains
- CD31, vimentin, desmin, smooth muscle actin
- Cytokeratin variable
Differential diagnosis
Additional references
Follicular dendritic cell sarcoma (FDCS)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare mesenchymal neoplasm arising from follicular dendritic cells (FDC) of lymphoid follicles at nodal and extranodal sites
- Nodal follicular dendritic cell sarcoma (FDCS) first characterized in 1986 (Am J Pathol 1986;122:562)
- Extranodal follicular dendritic cell sarcoma first described in 1994 (Am J Surg Pathol 1994;18:148)
Essential features
- Presents as a painless solid mass in nodal and extranodal sites
- Behaves as a low grade sarcoma
- Immunohistochemistry showing expression of ≥ 2 FDC markers is essential for diagnosis
Terminology
- Previous designation: follicular dendritic cell tumor (in WHO classification prior to 2008)
- Classified under histiocytic / dendritic cell neoplasms in the WHO Classification of Haematolymphoid Tumours (4th revised edition, 2017)
- Classified under mesenchymal dendritic cell neoplasms in the category of stroma derived neoplasms of lymphoid tissues in the current WHO Classification of Haematolymphoid Tumours (5th edition, 2023)
ICD coding
- ICD-10: C96.4 - sarcoma of dendritic cells (accessory cells)
Epidemiology
- Wide age range; median age is 50 years (Crit Rev Oncol Hematol 2013;88:253)
- M = F
Sites
- Lymph nodes: cervical (most common); second most common is axillary or supraclavicular lymph nodes
- Extranodal FDCS occurs in a wide variety of sites, including head and neck, gastrointestinal tract, retroperitoneum, tonsil and lung (Adv Anat Pathol 2021;28:21)
Pathophysiology
Etiology
- May arise in the context of follicular dendritic cell proliferation in Castleman disease, hyaline vascular variant
- Cases that show association with Epstein-Barr virus (EBV) and predominantly involve the liver or spleen are now considered a distinct entity: EBV positive inflammatory follicular dendritic cell sarcoma (Am J Surg Pathol 2001;25:721)
Clinical features
- Nodal and extranodal involvement characterized by slow growing, painless solid mass
- B symptoms (fever, night sweats, weight loss) are usually absent
- Surgical resection results in complete remission in a majority of cases
- ~33% of cases recur locally
- May show late metastasis by hematologic spread to bone, lungs, liver
- Rarely, may be associated with paraneoplastic autoimmune multiorgan syndrome (PAMS) (Am J Surg Pathol 2018;42:1647)
Diagnosis
- Morphologic evaluation supplemented by immunophenotype of follicular dendritic cells is key to diagnosing FDCS
- FDCS can coexist with Castleman disease (Pathologica 2021;113:316)
Radiology description
- Nodal involvement presents as a homogeneous mass, slightly hypoattenuating on CT, hypointense or isointense on T1 weighted MRI and hyperintense on T2 weighted MRI (AJR Am J Roentgenol 2021;216:835)
- Extranodal sites of involvement appear heterogeneous with hyperattenuation on unenhanced CT or hyperintense foci on T1 weighted MRI, with or without associated necrosis and calcifications, often accompanied by regional lymphadenopathy (AJR Am J Roentgenol 2021;216:835)
Radiology images
Prognostic factors
- Poor prognostic factors include size of tumor (> 6 cm), nuclear pleomorphism, increased mitoses (> 5/10 high power fields), necrosis and intra-abdominal location (Arch Pathol Lab Med 2017;141:596)
- Association with paraneoplastic autoimmune multiorgan syndrome follows an aggressive course related to respiratory morbidity and mortality (Am J Surg Pathol 2018;42:1647)
Case reports
- Man in his early 30s presented with jaundice, abdominal pain and weight loss (J Int Med Res 2022;50:3000605221142401)
- 34 year old man with Birt-Hogg-Dubé syndrome presented with mass at the ileocecal junction (Diagn Pathol 2022;17:64)
- 66 year old woman with intermittent right upper quadrant pain (World J Clin Cases 2019;7:785)
- 66 year old man with no significant medical history presented with right cervical mass (Mol Clin Oncol 2020;13:23)
- 72 year old man presented with enlarged mediastinal lymph nodes (Cureus 2023;15:e37715)
Treatment
- Includes complete surgical resection with or without radiation therapy
- Role of adjuvant chemotherapy or radiation is unclear (Cancers (Basel) 2014;6:2275)
- Role of chemotherapy in advanced disease is not well established (Crit Rev Oncol Hematol 2013;88:253)
Gross description
- Usually round to ovoid, well circumscribed fleshy solid mass (Arch Pathol Lab Med 2016;140:186)
- Size ranges from 1 cm to 15 cm, largely dependent on the site of the tumor
- May show areas of hemorrhage and necrosis
Gross images
Microscopic (histologic) description
- Oval to spindled cells with dispersed chromatin, small nucleoli, eosinophilic and fibrillar cytoplasm with syncytial borders arranged in fascicles, whorls or storiform patterns
- Often binucleate or occasional multinucleate forms, nuclear pseudoinclusions
- Perivascular lymphocyte cuffs and admixed population of lymphocytes, eosinophils, plasma cells or neutrophils may be present (Am J Surg Pathol 2004;28:988)
Microscopic (histologic) images
Positive stains
- Most sensitive FDC markers: clusterin, CD21, CXCL13 (J Pathol 2008;216:356, Am J Surg Pathol 2004;28:988)
- Most specific FDC markers: CXCL13, CD21, CD23, CD35
- Other FDC markers: podoplanin / D2-40, SSTR2a, follicular dendritic cell secreted protein (FDCSP) and serglycin (SRGN) (Am J Clin Pathol 2007;128:776, Am J Surg Pathol 2019;43:374, Oncotarget 2017;8:16463)
- Other positive stains: fascin, PDL1, desmoplakin, EGFR and vimentin (Mod Pathol 2005;18:260)
- TdT highlights immature T cell lymphoblastic proliferation in 45% of cases; when abundant, this finding may be associated with paraneoplastic autoimmune multiorgan syndrome (Am J Surg Pathol 2018;42:1647)
Negative stains
Molecular / cytogenetics description
- Proposed molecular pathogenesis involves activation of nuclear factor κβ genes as a consequence of recurrent loss of function alterations in nuclear factor κβ regulatory genes (NFKBIA, CYLD, BIRC3, SOCS3, TRAF3, TNFAIP3), tumor suppressor genes (CDKN2A, RB1, TP53) and genes involved in immune evasion (CD274, PDCD1LG2) (Mod Pathol 2016;29:67)
- Genetic alterations, including copy number variations, somatic mutations involving oncogenes / tumor suppressors (ZBTB7A, SETD2), chromatin remodeling genes (CABIN1, NCAPH) and other genes, have been reported (Blood Adv 2018;2:481)
- BRAF V600E or other MAPK pathway mutations are uncommon (Histopathology 2014;65:261, Arch Pathol Lab Med 2023;147:896)
- Chromosome analysis typically reveals a complex karyotype (In Vivo 2013;27:211)
Sample pathology report
- Lymph node, left neck, excisional biopsy:
- Follicular dendritic cell sarcoma, completely excised (see comment)
- Comment: Lymph node architecture is partially effaced by an abnormal population of epithelioid to spindle shaped cells in a whorling pattern. The neoplastic cells have ovoid nuclei, delicate chromatin, small nucleoli and abundant eosinophilic cytoplasm. They are variably admixed with small lymphocytes. Immunohistochemical stains were performed on paraffin sections of the left neck lymph node (CD21, CD23, CD31, CD34, CD35, CD45, clusterin, CXCL13, epithelial membrane antigen, melanA, keratin [AE1 / AE3], HMB45, S100 and terminal deoxynucleotidyl transferase). The abnormal cells are positive for CD21, CD23, CD35, clusterin and CXCL13. There are small numbers of terminal deoxynucleotidyl transferase positive (TdT) cells mixed with the tumor. The neoplastic cells are negative for the other markers tested. Morphology and immunohistochemical stains support the diagnosis of follicular dendritic cell sarcoma.
Differential diagnosis
- Interdigitating dendritic cell sarcoma:
- Histologically may be indistinguishable from follicular dendritic cell sarcoma and immunohistochemical studies are required for a definite diagnosis
- Positive for S100, CD45, CD68; negative for FDC markers (Am J Surg Pathol 2004;28:988)
- Langerhans cell histiocytosis:
- Langerhans cells are oval and recognized by their grooved, folded or indented nuclei with fine chromatin, inconspicuous nucleoli and thin nuclear membrane with moderately abundant and slightly eosinophilic cytoplasm
- Positive for CD1a and langerin
- Kaposi sarcoma:
- Atypical spindled cells form slit-like vascular spaces containing red blood cells
- Positive for HHV8
- Spindle cell thymoma:
- Metastatic carcinoma:
- Metastatic melanoma:
- Inflammatory myofibroblastic tumor:
- Spindled myofibroblasts and fibroblasts with bland nuclei
- Positive for ALK1
- Pleomorphic sarcoma:
Additional references
Board review style question #1
An 88 year old man presented with significant snoring and increasing sleep apnea. Laryngoscopy revealed a pharyngeal mass. Computed tomography (CT) of the head and neck with intravenous (IV) contrast revealed a 6 cm enhancing mass in the right parapharyngeal space. An excisional biopsy was performed. Based on the immunophenotype (positive for CD21 and clusterin, negative for S100), what is the diagnosis?
- Follicular dendritic cell sarcoma
- Interdigitating dendritic cell sarcoma
- Langerhans cell sarcoma
- Pleomorphic sarcoma
Board review style answer #1
A. Follicular dendritic cell sarcoma. The immunophenotype indicated here is typical of follicular dendritic cells (FDC: CD21 positive, clusterin positive), supporting the diagnosis of follicular dendritic cell sarcoma. Answer B is incorrect because interdigitating dendritic cell sarcomas are characterized by positive expression of CD45, CD68 and S100, while they are negative for FDC markers. Answer C is incorrect because Langerhans cell sarcoma is positive for Langerhans cell markers (CD1a, Langerin) and negative for FDC markers. Answer D is incorrect because pleomorphic sarcoma is negative for FDC markers.
Comment here
Reference: Follicular dendritic cell sarcoma (FDCS)
Comment here
Reference: Follicular dendritic cell sarcoma (FDCS)
Board review style question #2
Which of the following molecular pathways is most frequently associated with follicular dendritic cell sarcoma (FDCS)?
- JAK-STAT
- MAPK-ERK
- NFκB
- PI3K-Akt
Board review style answer #2
C. NFκB. Genetic alterations involving NFκB pathway are present in over 50% of FDCS cases. Answers B and D are incorrect because MAPK-ERK and PI3K-Akt pathways are frequently associated with histiocytic / dendritic cell neoplasms, such as Erdheim-Chester disease, Rosai-Dorfman disease and Langerhans cell histiocytosis. Answer A is incorrect because JAK-STAT pathway is associated with other hematologic malignancies (such as myeloproliferative neoplasms) and not FDCS.
Comment here
Reference: Follicular dendritic cell sarcoma (FDCS)
Comment here
Reference: Follicular dendritic cell sarcoma (FDCS)
Follicular hyperplasia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Follicular hyperplasia is a benign proliferation of lymphoid follicles, which can develop wherever lymphoid tissue is present (Surg Neurol 2008;70:514)
Essential features
- Most common type of reactive lymphadenopathy (Mod Pathol 2013;26:S88)
- Associated with varying degrees of paracortical or sinus hyperplasia (Mod Pathol 2013;26:S88)
- A descriptive term that refers to an exaggerated response to antigenic stimulation, characterized by an increased number of germinal centers with variably sized mantle zones (Ann Diagn Pathol 2020;44:151421)
- Follicular hyperplasia represents stimulation of the B cell compartment of the lymph node
Terminology
- Follicular hyperplasia, reactive follicular hyperplasia (RFH), follicular lymphoid hyperplasia (FLH)
ICD coding
- ICD-10: R59.9 - enlarged lymph nodes, unspecified
Epidemiology
- Follicular hyperplasia is seen in pediatric and young adults but may also be encountered in all ages, including the elderly (Mod Pathol 1991;4:24)
Sites
- Majority of cases with follicular hyperplasia occur in lymph nodes
- However, it can affect extranodal organs
- More than 75% of lymphadenopathies are generally localized but commonly involve head and neck regions (StatPearls: Adenopathy [Accessed 7 October 2022])
Pathophysiology
- Reactive follicular hyperplasia is caused by stimulation of the B cell compartment and by abnormal cell growth of secondary follicles (Mod Pathol 2013;26:S88)
Etiology
- Causes of reactive follicular hyperplasia are often unknown; however, it can be caused by bacterial and viral infections, as well as immune mediated disorders (Miranda: Atlas of Lymph Node Pathology, 1st Edition, 2013)
Clinical features
- Lymphadenopathies such as follicular hyperplasia can present clinically as pain, swelling, warmth, tenderness, fever and chills (StatPearls: Adenopathy [Accessed 7 October 2022])
- Nonspecific reactive follicular hyperplasia involving younger patients often resolves spontaneously (Mod Pathol 2013;26:S88)
Diagnosis
- Morphologically, follicular hyperplasia can be diagnosed by observation of specific histological features such as expansion of germinal center, moderate to high mitotic rate, distinct mantle zone, polarization of the germinal center, variably shaped and sized follicles, tingible body macrophages and mixture of cell types in germinal center (Mod Pathol 2013;26:S88)
- Lymph nodes with reactive follicular hyperplasia show a lower follicular density than is seen in follicular lymphoma
Laboratory
- Leukocytosis, neutrophilia or lymphocytosis may be present in association with infections
Prognostic factors
- Prognosis of follicular hyperplasia is mostly excellent
Case reports
- 51 year old woman with asymptomatic firm mass in the left posterior maxillary site (BMC Oral Health 2019;19:243)
- 55 year old Caucasian woman with swelling in her left posterior hard palate (J Craniomaxillofac Surg 2009;37:79)
- 74 year old edentulous woman with maxillary denture presenting with palatal swelling (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;96:172)
- Reactive follicular hyperplasia in the lymph node lesions in 21 patients with systemic lupus erythematosus (Pathol Int 2000;50:304)
Treatment
- Localized lymph node enlargement without any additional symptoms will usually spontaneously regress
- Generalized lymphadenopathy usually requires additional investigation for etiology
Gross description
- Typically < 1 cm in size
- Cut surface is tan-pink homogenous
- No gross appearance of hemorrhage or necrosis
- Reference: Mod Pathol 2013;26:S88
Microscopic (histologic) description
- H&E sections of the lymph node show hyperplastic follicles with expanded germinal centers and paracortical regions
- Follicles exhibit normal polarization
- No significant cytologic atypia is identified
- Immunostains reveal a normal staining pattern and distribution
- Reference: Mod Pathol 2013;26:S88
Microscopic (histologic) images
Cytology description
- Polymorphous lymphocytes and tingible body macrophages
- Cytology to histopathology correlation is ~90%
Positive stains
- BCL6 shows densely populated germinal centers with well defined and regular borders and strong staining of follicular center cells but only rare BCL6+ cells in the interfollicular areas (Hum Pathol 1999;30:403)
- CD21, CD23 and CD35 highlight the enlargement of the dendritic meshwork (ScienceDirect: Follicular Hyperplasia [Accessed 29 September 2023])
- Ki67 highlights a predominance of large transformed germinal center cells that have a high mitotic rate (ScienceDirect: Follicular Hyperplasia [Accessed 29 September 2023])
- CD10 stains only follicular center cells and is typically much stronger in follicular lymphoma than in most follicular hyperplasias (Am J Surg Pathol 2000;24:846, ScienceDirect: Follicular Hyperplasia [Accessed 29 September 2023], Appl Immunohistochem Mol Morphol 2000;8:263)
- HGAL also stains germinal center B cells, as does GCET1 (Nat Commun 2013;4:1338)
Negative stains
- BCL2, helpful in distinguishing from follicular lymphoma (Weidner: Modern Surgical Pathology, 2nd Edition, 2009)
Flow cytometry description
- Polytypic B cell population with positive CD10 and CD23; typically, it is a subset that is CD10 positive
- Evaluation of CD38 by flow cytometry can be helpful in distinguishing follicular hyperplasia from follicular lymphoma (Cytometry B Clin Cytom 2009;76:315)
- Clonal B cell populations may be present in the histologically reactive setting of follicular hyperplasia (Am J Clin Pathol 2004;121:464)
- Combined analysis of CD20 and BCL2 by flow cytometry can distinguish follicular hyperplasia and follicular lymphoma; it may be particularly helpful if samples are limited or B cell clonality is questionable (Am J Clin Pathol 2000;114:258)
Molecular / cytogenetics description
- Unlike follicular lymphoma, there is no evidence of t(14;18)(q32;q21) or IGH::BCL2 fusion sequences found in cases with follicular hyperplasia
- IgH rearrangement was not identified in follicular lymphoma and was mainly found in lymphoplasmacytoid and follicular lymphoma (Oncotarget 2017;8:77009)
Videos
Histopathology lymph node: follicular hyperplasia
Sample pathology report
- Lymph node, excision:
- Reactive follicular hyperplasia; no carcinoma or lymphoma (see comment)
- Comment: The H&E sections of the lymph node show hyperplastic follicles with expanded germinal centers and paracortical region. The follicles exhibit normal polarization. No significant cytologic atypia is identified. The immunostains reveal a normal staining pattern and distribution. The histological and immunohistochemical features are consistent with a reactive follicular hyperplasia. Clinical and microbiological correlation is recommended for complete interpretation.
- Immunohistochemical stain:
Block Stain Result 1 CD3 Positive in T cells CD20 Positive in B cells CD10 Positive in germinal center cells BCL6 Positive in germinal center cells BCL2 Positive in nongerminal center cells CD21 Highlight follicular dendritic meshwork
- Interpretation: There is a reactive follicular hyperplasia with no evidence of a malignant lymphoma.
Differential diagnosis
- Follicular lymphoma:
- Progressive transformation of germinal centers (PTGCs):
- Morphologically, the nodules seen in PTGCs are 3 - 4 times larger than those of reactive follicular hyperplasia
- Germinal center B cells are BCL2 negative
- Hyaline vascular Castleman disease:
- Regressed germinal centers
- Sclerotic vessels
- Prominent mantle zones with onion skin appearance
- Morphological features such as twinning (2 or more germinal centers inside each follicle) and lollipop sign (a radially penetrating sclerotic blood vessel in the germinal center)
- Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL):
- Immunophenotypically, NLPHL expresses CD45, EMA and 50% of the cases with MUM1
- Morphologically distinct NLPHL can be seen with neoplastic lymphocyte predominant (LP) cells in the background of small lymphocytes and epithelioid histiocytes
- Both follicular hyperplasia and NLPHL may show a nodular proliferation at low power; however, NLPHL nodules are more irregular and have a moth eaten appearance due to the presence of clusters of epithelioid histiocytes (Pediatr Blood Cancer 2018;65:10.1002/pbc.26753)
- Follicular neoplasia in situ:
- Can be an incidental finding in a background of follicular hyperplasia (Blood 2011;118:2976)
- Pediatric variant of follicular lymphoma (PFL):
- Biologically different from common follicular lymphoma (FL)
- PFL cases have been reported exclusively in male patients in both children and young adults
- Most common site of PFL cases is head / neck region
- Morphologically, PFL cases demonstrate blastoid cytologic features with a high proliferation rate by Ki67
- By immunohistochemistry, PFL is positive for germinal center markers (CD10, BCL6, LMO2, HGAL)
- BCL2 expression by immunohistochemistry varies depending on the anatomic site: testicles (negative), lymph nodes (18% weakly positive), tonsils (63% of cases are positive)
- Majority of PFL cases demonstrate clonal immunoglobulin gene rearrangement by PCR analysis
- Absence of BCL2::IGH translocation by FISH
- MAP kinase pathway involvement has been reported in 50% cases with PFL (Blood 2016;128:1093)
- TNFRSF14 mutations have been reported in cases of PFL (Blood 2016;128:1093)
Board review style question #1
When differentiating follicular hyperplasia from follicular lymphoma, which immunohistochemical stain is most helpful?
- BCL2
- BCL6
- CD10
- Ki67
- MUM1
Board review style answer #1
A. BCL2. Follicular hyperplasia distinguishes itself from follicular lymphoma by lack of BCL2 expression whereas follicular lymphoma is classically positive for BCL2 expression.
Comment Here
Reference: Follicular hyperplasia
Comment Here
Reference: Follicular hyperplasia
Board review style question #2
Board review style answer #2
A. Polarization of germinal centers is typically seen in follicular hyperplasia along with features such as low density of follicles, variably sized follicles, mixture of cell types in the germinal centers, tingible body macrophages, moderate to high mitotic rate, intact nodal architecture. Answer B is incorrect because positive staining of BCL2 in the germinal center is seen in follicular lymphoma. Answers C and D are incorrect because multilobated popcorn cells and Reed-Sternberg (RS) cells are seen in Hodgkin lymphoma. Answer E is incorrect because twinning of the follicle is seen in Castleman disease.
Comment Here
Reference: Follicular hyperplasia
Comment Here
Reference: Follicular hyperplasia
Granulomatous inflammation
Definition / general
- Common in splenectomy specimens
- Either:
- Large active granulomas with epithelioid and Langhans giant cells, with or without central necrosis
- Widespread small, sarcoid-like epithelioid granulomas with rare giant cells and no necrosis
- Inactive granulomas with fibrosis and calcification
- Granulomas usually associated with systemic disease involving liver, bone marrow and lymph nodes; also chronic uremia, IgA deficiency, infectious mononucleosis
- Granulomas may be present in hairy cell leukemia, Hodgkin lymphoma, non-Hodgkin lymphoma, although granuloma itself does not mean spleen is involved by tumor (Arch Pathol Lab Med 1977;101:518)
- Active granulomas: in adults, associated with fever, weight loss, hepatosplenomegaly and hypersplenism
- Inactive granulomas: associated with histoplasmosis
Treatment
- Splenectomy for symptoms of hypersplenism
Granulomatous inflammation
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Specialized immune response against various inflammatory insults, involving chronic activation and organization of mononuclear phagocytic cells (macrophages) (J Clin Tuberc Other Mycobact Dis 2017;7:1)
- Histologically characterized by epithelioid histiocytes, with or without central necrosis, surrounded by acute or chronic inflammation
Essential features
- Specialized inflammatory response against various offending entities, including (StatPearls: Granuloma [Accessed 13 June 2022]):
- Infectious (usually necrotic, suppurative)
- Autoimmune / inflammatory
- Malignancy
- Others (see below)
- Histologically characterized by central epithelioid histiocytes surrounded by acute or chronic inflammation
- Microbiologic cultures of fresh sample, special stains / immunohistochemistry for microorganisms are essential when clinical history includes suspicion of infection or an immunocompromised / suppressed state
- Immunohistochemistry to evaluate for malignancies that arise as differential diagnoses (e.g., carcinoma [cytokeratin], melanoma [SOX10]) (O'Malley: Benign and Reactive Conditions of Lymph Node and Spleen, 1st Edition, 2009)
Terminology
- Lymphadenitis (LA), infectious lymphadenitis, granulomatous lymphadenitis (GLA)
ICD coding
Epidemiology
- May be associated with infectious disease or noninfectious disease (J Clin Tuberc Other Mycobact Dis 2017;7:1)
- No particular predilection for age / sex (StatPearls: Lymphadenopathy [Accessed 13 June 2022])
Sites
- Any lymph node site in the body
- Spleen (O'Malley: Benign and Reactive Conditions of Lymph Node and Spleen, 1st Edition, 2009)
- Certain etiologies have strong association with anatomical site (J Clin Tuberc Other Mycobact Dis 2017;7:1)
- Inguinal nodes in lymphogranuloma venereum
- Cervical and axillary nodes in tularemia and cat scratch disease
- Mesenteric lymph nodes in Yersinia lymphadenitis
- Pulmonary hilar lymph nodes in environmental, fungal, tuberculous lymphadenitis
Pathophysiology
- Dysregulation of immune response due to inability of macrophages to clear the offending agent (StatPearls: Granuloma [Accessed 13 June 2022])
- Results in chronic active inflammation, histologically characterized by (StatPearls: Granuloma [Accessed 13 June 2022]):
- Central collection of epithelioid histiocytes
- Rim of plasma cells and lymphocytes
- Central necrosis or suppuration, depending on etiology
- Can be conceptualized as consisting of 3 phases (J Clin Exp Hematop 2012;52:1):
- Initiation / accumulation, peak and late phase
- Initiation characterized by activation of T cells via MHC class II interactions with antigen presenting cells (APCs) (J Clin Tuberc Other Mycobact Dis 2017;7:1)
- Activated T helper 1 (Th1) cells stimulate and recruit macrophages via secretion of TNF alpha, IFN gamma, IL2, IL12 and chemokines
- Precise mechanism of defective downregulation of active inflammation is poorly understood
- With ensuing active inflammation, peak phase is characterized by rim of chronic inflammatory infiltrate consisting of:
- CD4+ T cells, CD8+ T cells, NK cells, plasma cells and occasional granulocytes
- Certain infectious GLA features prominent monocytoid B cell infiltrates (Toxoplasma lymphadenitis, Bartonella) (J Clin Exp Hematop 2012;52:1)
- Role poorly understood
- Late phase characterized by fibrosis and hyalinization
- Resolution occurs after clearance of antigenic stimulus and is characterized by a fibrous scar
Etiology
- Infectious:
- Suppurative (purulent):
- Tularemia lymphadenitis (Francisella tularensis)
- Cat scratch lymphadenitis (Bartonella henselae)
- Yersinia lymphadenitis (Yersinia enterocolitica)
- Various fungal infections
- Nonsuppurative:
- Tuberculous lymphadenitis (Mycobacterium tuberculosis spp.)
- Atypical mycobacterial infection
- Bacillus Calmette-Guérin (BCG) lymphadenitis
- Toxoplasma lymphadenitis
- Hansen disease or leprosy (M. leprae)
- Syphilis
- Brucellosis
- Fungal lymphadenitis (Coccidioides, Cryptococcus, Histoplasma, Pneumocystis)
- Suppurative (purulent):
- Noninfectious:
- Systemic sarcoidosis
- Foreign body (berylliosis, silicosis)
- Malignancies (lymphoma, carcinoma, especially breast, uterus, lung, stomach)
- Systemic inflammatory / autoimmune conditions (lupus [SLE], rheumatoid arthritis [RA], granulomatosis with polyangiitis [GPA])
- Toxic (chemotherapy, heavy metals, beryllium, zirconium, silicon)
- Histiocytic inflammatory (Rosai-Dorfman disease, Castleman disease)
- Reference: J Clin Tuberc Other Mycobact Dis 2017;7:1
Clinical features
- Painful / painless lymphadenopathy (StatPearls: Lymphadenopathy [Accessed 13 June 2022])
Diagnosis
- Clinical exam
- Imaging:
- CT
- Ultrasound
- Tissue biopsy:
- Excisional preferred over needle core biopsies, due to preservation of lymph node architecture; usually yields sufficient tissue to perform ancillary studies (microbiologic studies, special stains, immunostains)
- Fine needle aspiration (FNA):
- Can be ultrasound guided
- Reference: StatPearls: Lymphadenopathy [Accessed 13 June 2022]
Laboratory
- Dependent on suspected etiology but generally includes:
- Microbiologic studies (e.g., cultures, serology)
- Essential to obtain tissue cultures, as necrotic node usually contains high density of organisms
- Autoimmune / rheumatology workup
- Microbiologic studies (e.g., cultures, serology)
Prognostic factors
- Dependent on etiology (e.g., HIV, sarcoidosis, malignancy, infection)
Case reports
- 27 year old Greek man with suppurative necrotizing granulomatous lymphadenitis in adult onset Still disease (J Med Case Rep 2012;6:354)
- 29 year old man with necrotizing granulomatous sarcoidosis (Cureus 2020;12:e10220)
- 82 year old man with necrotizing granulomatous lymphadenitis (BMJ Case Rep 2011;2011:bcr1120103548)
Treatment
- Management of underlying disease (e.g., infection, neoplasm, inflammatory / autoimmune)
- Removal of offending agent (e.g., removal of foreign body)
Gross description
- Pale tan circumscribed nodules
- Fibrotic and firm compared to surrounding lymph node parenchyma
- Can show central necrosis, which can be dry and crumbly (caseating) or soft with a gelatinous consistency
- Can show gross suppuration
- Reference: Foucar: Tumors of the Bone Marrow, 1st Edition, 2016
Frozen section description
Microscopic (histologic) description
- Collection of epithelioid macrophages with abundant eosinophilic cytoplasm and indistinct cell borders (StatPearls: Granuloma [Accessed 13 June 2022])
- Surrounded by a rim of inflammatory cells, including lymphocytes, plasma cells and macrophages (StatPearls: Granuloma [Accessed 13 June 2022])
- Multinucleated giant cells are often present (StatPearls: Granuloma [Accessed 13 June 2022])
- Certain etiologies may demonstrate characteristic features
- May present as a characteristic triad of florid follicular hyperplasia, interfollicular monocytoid B cells and cortical / subcortical, ill defined aggregates of epithelioid cells (Toxoplasma lymphadenitis)
- Subtypes:
- Necrotizing granulomas (J Clin Exp Hematop 2012;52:1):
- Necrotic focus surrounded by a rim of chronic inflammatory cells, including epithelioid macrophages
- May show suppuration (acute inflammation) depending on etiology
- Usually associated with infectious agents (e.g., tuberculosis, lymphogranuloma venereum, tularemia, cat scratch disease, fungal)
- Important to look for organisms within granulomas
- Use special stains to highlight
- Often essential for diagnosis as serology may have low sensitivity / specificity
- Obtain tissue culture
- Nonnecrotizing (sarcoid and sarcoid-like) granulomas (J Clin Exp Hematop 2012;52:1):
- Well defined borders, cohesive solid appearance, no central necrosis
- Various types of inclusions inside macrophages (e.g., asteroid bodies)
- Etiology includes
- Sarcoidosis containing Langhans type giant cells containing nuclei arranged in a horseshoe shape along cell periphery
- Systemic / autoimmune entities
- Foreign body type lymphadenitis containing giant cells with haphazardly arranged nuclei and engulfed foreign material
- Lipogranuloma (O'Malley: Benign and Reactive Conditions of Lymph Node and Spleen, 1st Edition, 2009):
- Frequently found in spleen; can be seen in lymph node
- Focus of macrophages with ingested fat surrounded by a rim of lymphocytes and plasma cells
- Etiology is unknown
- Has been associated with excessive topical application / ingestion of mineral oils
- Necrotizing granulomas (J Clin Exp Hematop 2012;52:1):
Microscopic (histologic) images
Cytology description
- Sarcoid granulomatous lymphadenitis:
- Tight collection of epithelioid histiocytes in syncytial arrangement with curved nuclei in horseshoe, C or V shape
- Infectious type granulomatous lymphadenitis:
- Histiocyte cluster may be loose or tight
- Usually surrounded by abundant acute inflammation
- Can show suppuration or necrosis, depending on infectious etiology
- Ultimately, findings are nonspecific and microbiologic, serologic studies are needed for a definitive etiology
- Reference: Cibas: Cytology - Diagnostic Principles and Clinical Correlates, 5th Edition, 2020
Positive stains
Negative stains
- Acid fast bacilli (AFB): positive in mycobacterial etiology
- Grocott methenamine silver (GMS) and periodic acid-Schiff (PAS): positive in fungal etiology
- Cytokeratin: positive in carcinoma
- SOX10: positive in melanoma
- CD30: positive in Hodgkin lymphoma
Sample pathology report
- R4 lymph node, excision:
- Nonnecrotizing granulomatous lymphadenitis (see comment)
- No evidence of malignancy identified
- Comment: The overall findings raise the possibility of sarcoidosis; infection remains in the differential diagnosis. AFB and GMS stains are negative for acid fast organisms and fungal forms. Clinical correlation with microbiologic studies is recommended.
Differential diagnosis
- Necrotizing granulomatous inflammation:
- Suppurative:
- Nonsuppurative:
- Nonnecrotizing granulomatous inflammation:
- Mycobacterium tuberculosis
- Nontuberculous mycobacterium (including Hansen disease)
- Salmonella typhi
- Rickettsia spp.
- Coxiella
- C. albicans
- C. immitis
- Viral (cytomegalovirus, herpes, hepatitides)
- Parasitic (toxoplasmosis, schistosomiasis)
- Noninfectious:
- Most noninfectious causes of granulomatous lymphadenitis present with nonnecrotizing granulomatous inflammation, including
- Autoimmune:
- Drugs:
- Bacillus Calmette-Guérin
- NSAIDs
- Antibiotics
- Methotrexate
- Malignancy associated:
- Carcinoma
- Melanoma
- Lymphoma (e.g., classic Hodgkin lymphoma, T cell lymphoma)
- Most noninfectious causes of granulomatous lymphadenitis present with nonnecrotizing granulomatous inflammation, including
- Foreign body:
- Talc, starch, suture, hyaluronic acid (and other injectable fillers):
- Foreign material may be visualized within granuloma
- May be polarizable
- Talc, starch, suture, hyaluronic acid (and other injectable fillers):
- Reactive conditions that present with granulomatous lymphadenitis:
- Kikuchi-Fujimoto disease:
- Predominance of CD8+ T cells surrounding necrotic center
- Histiocytes with C shaped nuclei
- Plasmacytoid dendritic cells
- Kimura disease:
- Hypereosinophilia and eosinophilic abscesses
- Preservation of follicles, hyperplastic follicles
- Stromal and perivascular sclerosis
- Kikuchi-Fujimoto disease:
- Rosai-Dorfman disease (also known as sinus histiocytosis with massive lymphadenopathy):
- Histiocytic neoplasms:
- Histiocytic sarcoma:
- Rarely affects lymph nodes (usually extranodal presentation)
- Diffuse infiltration by malignant, variably pleomorphic, mature appearing histiocytes
- Nodal architecture effacement, sparing of follicles
- No well formed granulomas
- Langerhans cell histiocytosis:
- Histiocytic sarcoma:
- Reference: J Clin Tuberc Other Mycobact Dis 2017;7:1
Board review style question #1
A 30 year old pregnant woman presents to her general practitioner complaining of mild flu-like symptoms. On physical examination, there is small but palpable, tender cervical lymphadenopathy. She states that her allergies seem to be acting up lately, which may be due to her new cat. She reports a family history of lymphoma in a grandparent and would like the lymph node biopsied. Sections of the node reveal secondary follicles of varying shapes and sizes, clusters of monocytoid lymphoid cells and cortical, loose collections of epithelioid histiocytes. Based on the clinical presentation and histologic findings, what is the most likely etiology for this patient's presentation?
- Cat scratch disease
- Follicular lymphoma
- Metastatic carcinoma
- Toxoplasmosis
- Tularemia
Board review style answer #1
Board review style question #2
You receive an excisional biopsy of an enlarged superficial neck lymph node from a 55 year old homeless man who was found obtunded. He is a poor historian and is unable to provide the duration of the adenopathy but he reports feeling unwell for quite some time. On physical examination, he has somewhat tender cervical adenopathy, as well as axillary and inguinal adenopathy. In addition, imaging studies show mediastinal and intra-abdominal adenopathy. Biopsy sections of the superficial cervical node show follicular hyperplasia with scattered epithelioid granulomas that are nonnecrotizing. The expanded follicles show a monotonous proliferation of small to medium lymphocytes, with variably irregular nuclear contours, condensed chromatin and scant cytoplasm. Occasional larger lymphoid cells are seen. Interfollicular plasma cells, eosinophils and scattered epithelioid histiocytes are also seen. What additional studies could narrow your differential diagnosis?
- AFB and GMS stains
- Immunostains to evaluate for both non-Hodgkin and Hodgkin lymphomas
- PCR studies for HIV
- Serologic studies for infectious etiologies
- Tissue culture of fresh tissue sent to the microbiology laboratory
Board review style answer #2
B. Immunostains to evaluate for both non-Hodgkin and Hodgkin lymphomas
Comment Here
Reference: Granulomatous inflammation
Comment Here
Reference: Granulomatous inflammation
Grossing & features to report-lymph nodes
Table of Contents
Grossing for diagnosis | Grossing for staging | Features to report by organization | Features to reportGrossing for diagnosis
- Cut perpendicular to long axis if possible
- Avoid squeezing nodes, which may alter histology
- For culture (if clinically indicated) or for flow cytometry, use end of lymph node
- For touch imprints, fix in ethanol, stain with H&E, Wright stain or use for immunocytochemistry
- Use scrapes / cell suspension for flow cytometry, cytogenetics, molecular gene rearrangement studies, FISH
- Sections for formalin or B5 fixation should be 3 - 4 mm thick to allow for proper fixation; B5 (mercury containing fixative) provides best morphologic details
- Snap freezing is best for research, some immunohistochemistry, future molecular studies
- Formalin fixation is best for PCR
- EM is helpful only rarely to diagnose Langerhans histiocytosis or occasionally metastatic tumors
- Include extranodal fat (infiltration implies malignant process)
- Note: the most important slides to obtain are sufficient H&E for diagnosis
- Frozen sections may confirm involvement of node by a disease process, but do not use to obtain a specific diagnosis because freezing artifacts may hinder diagnosis
Grossing for staging
- Most lymph nodes are near the wall of the organ
- Be aware of minimal number of nodes required for staging some carcinomas
- May want to fix first overnight - lymph nodes stand out as white nodules
- Carnoy solution helps clear the fat
- Clearing solutions (such as ethanol, diethyl ether, glacial acetic acid and formalin) may help identify additional lymph nodes (Am J Surg Pathol 1997;21:1387, Arch Pathol Lab Med 2003;127:1552, Arch Pathol Lab Med 2001;125:642)
- For each anatomic group, describe the number of nodes, the size of the largest node and any gross features
- Submit all lymph nodes for histology; section node if 5 mm or greater in diameter
- For large nodes grossly involved by tumor, only one section needs to be submitted to demonstrate tumor and possible extranodal extension but save remainder for resampling if necessary
- For other large nodes, submission of entire node detects additional metastases in some cases (Am J Clin Pathol 1998;109:571)
- Describe number of nodes in each cassette and whether whole or sectioned
Features to report by organization
Features to report
- Clinical history: prior diagnoses of lymphoma, presence of lymphadenopathy or organomegaly, hematologic findings, constitutional symptoms, HIV status, prior immune abnormalities, autoimmune disorders, other relevant serology and other related conditions such as H. pylori infection
- Anatomic site
- Tumor type(s) (WHO classification), grade (if relevant)
- Focal or complete involvement of lymph node or other structures
- Specimen inadequacy
- Results of ancillary studies
- Diagnosis should incorporate all of the above findings or explain any inconsistencies
- Checklist (2004)
- References: Hum Pathol 2002;33:1064, Mod Pathol 2004;17:131
Hamartoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- A rare, benign parenchymal lesion composed of disorganized red pulp without well formed white pulp
Essential features
- Circumscribed nodule in spleen
- Composed of disorganized red pulp and absent white pulp
- CD8 confirms the presence of disorganized sinuses
Terminology
- Also called splenoma
ICD coding
- ICD-11: 3B81.0 - tumor-like conditions of spleen
Epidemiology
- Uncommon
- Occurs in all age groups and exhibits no sex predilection
Sites
- Spleen
Pathophysiology
- Postulated to represent a malformation, vascular neoplasm or posttraumatic reactive lesion
- Debate exists if hamartoma is a true neoplasm (Mod Pathol 2011;24:108)
- Human androgen receptor assay (HUMARA) suggests a polyclonal lesion
- However, a single case showed dic(16;21)(p13.1;p11.2)del(16)(q11.1) with gains of several chromosomes and monosomy 21 (Cancer Genet Cytogenet 2005;157:160)
Etiology
- Unknown
Clinical features
- Usually an incidental finding in an asymptomatic individual
- May infrequently present with symptoms related to mass effect, such as pain or a palpable mass
- Can present with hematologic complications associated with hypersplenism, such as thrombocytopenia and anemia (Am J Case Rep 2022;23:e937195)
- Splenic rupture is rare
- Rare cases associated with tuberous sclerosis and Alagille syndrome (Fetal Pediatr Pathol 2014;33:216)
Diagnosis
- Radiology (ultrasound, CT or MRI) demonstrates the presence of a solid mass
- Splenic biopsy (FNA, needle biopsy)
- Splenectomy
- Reference: Am J Case Rep 2022;23:e937195
Laboratory
Radiology description
- Increased blood flow on color Doppler ultrasound in the splenic lesion
- Most appear on MRI as isointense lesions on T1 weighted image and heterogeneously hyperintense on T2 weighted image
Radiology images
Prognostic factors
- Excellent prognosis
Case reports
- 19 year old man with upper abdominal pain (Am J Case Rep 2022;23:e937195)
- 21 year old man with an incidental splenic lesion on imaging (World J Clin Cases 2021;9:7231)
- 49 year old woman with a palpable painless mass on the left abdomen (Ann Med Surg (Lond) 2018;36:199)
- 65 year old man with pancytopenia (Hematol Transfus Cell Ther 2020;42:390)
Treatment
- Splenectomy or partial splenectomy is curative
Gross description
- Red circumscribed nodule, well demarcated from the uninvolved spleen (Semin Diagn Pathol 2019;36:16)
- Usually single, rarely multiple
- Median size of 50 mm (range: < 10 - 100 mm) (Semin Diagn Pathol 2021;38:159)
- Can have a bulging appearance that compresses the adjacent normal spleen
Gross images
Microscopic (histologic) description
- Benign vascular proliferation composed of disorganized red pulp elements (sinuses and cords) only (Mod Pathol 2011;24:108)
- Unencapsulated with borders that may be ill defined microscopically (Semin Diagn Pathol 2019;36:16)
- Scant fibrous trabeculae
- No well formed white pulp
- Usually lacks cytologic atypia
- CD8+ sinuses present
- Additional features may include focal sclerosis, calcifications, hemorrhage, peliosis, plasmacytosis, extramedullary hematopoiesis, eosinophils and mast cells
- Rarely, scattered bizarre cells featuring ill defined cytoplasm and irregular nuclei with vesicular chromatin that are likely dendritic or stromal myoid in nature are identified
Microscopic (histologic) images
Cytology description
- Nonspecific
- No atypia or mitosis
- Stromal cells can be bizarre
Positive stains
Molecular / cytogenetics description
- See Pathophysiology
Sample pathology report
- Spleen, splenectomy:
- Splenic hamartoma (see comment)
- Comment: Histopathologic examination of the [size] cm mass revealed a vaguely demarcated area showing red pulp without white pulp. Immunohistochemical staining was performed on block [X]. CD8 stains the endothelium of normal splenic sinuses in the normal splenic tissue as well as the disorganized sinuses in the hamartoma. CD20 and CD3 staining revealed a lack of B cells and rare T cells, respectively, within the hamartoma. Normal white pulp stains with CD20 with peripheral CD3 positive T cells. Kappa and lambda RNA staining revealed polytypic plasma cells throughout the tissue, without monotypic staining in the normal splenic white pulp. The findings support a diagnosis of splenic hamartoma. Extramedullary hematopoiesis is present and commonly found in splenic hamartomas.
Differential diagnosis
- Splenic hemangioma:
- Littoral cell angioma:
- Sclerosing angiomatoid nodular transformation of the spleen (SANT):
- Benign vascular lesion of uncertain etiology, likely reactive
- Predominantly affects adults
- Typically presents as a single mass with a central stellate scar
- Multiple angiomatoid nodules containing a mixture of capillaries, sinusoids and veins
- Interspersed fibrosclerotic stroma
- Endothelial cells can be prominent
- Peliosis:
- Can present as an isolated finding but most commonly presents in association with peliosis hepatis
- Multiple blood filled spaces that are haphazardly scattered in the red pulp
- Spaces are lined by inconspicuous endothelial cells; however, endothelial cells may be completely absent
- Key differentiating factor is the presence of white pulp
- If an endothelial lining is present: CD34+, factor VIII+ and CD31+
Additional references
Board review style question #1
Board review style answer #1
C. Red pulp without white pulp. This is an essential feature of splenic hamartoma. Answer A is incorrect because anastomosing vessels are seen with splenic angiosarcoma. Answer B is incorrect because dilated sinuses are seen with congestive splenomegaly. Answer D is incorrect because sclerosing nodules and entrapped vessels are seen in sclerosing angiomatoid nodular transformation of the spleen (SANT).
Comment Here
Reference: Hamartoma
Comment Here
Reference: Hamartoma
Hemangioma
Table of Contents
Definition / general | Epidemiology | Clinical features | Diagnosis | Radiology description | Radiology images | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Nonencapsulated benign proliferation of vascular channels that range from capillary to cavernous in size
- Most common primary tumor of spleen
Epidemiology
- Average age is 63 years (range 23 - 94 years) (J Gastrointest Surg 2000;4:611)
Clinical features
- Usually < 2 cm, incidental mass, asymptomatic
- May present with a palpable mass or abdominal pain / discomfort
- May be associated with hemangiomas at other sites
- May be associated with anemia, thrombocytopenia, Kasabach-Merritt syndrome (thrombocytopenia caused by platelet sequestration and destruction in large cavernous hemangiomas, usually infants, rarely adults) (Srp Arh Celok Lek 2012;140:777)
- Rarely is large, multiple or involves entire spleen (angiomatosis)
- Rarely presents with splenic rupture and massive hemorrhage
Diagnosis
- By ultrasound examination and CT, confirmation by histopathology
Radiology description
- At CT, hemangiomas appear as hypodense well circumscribed masses with marked homogeneous enhancement of solid components
Radiology images
Case reports
- 18 year old man with elective laparoscopic splenectomy for giant hemangioma (Cases J 2009;2:10)
- 42 year old woman with coexisting giant splenic hemangioma and multiple hepatic hemangiomas (J Med Case Rep 2008;2:147)
- 68 year old man with noncalcified splenic hemangioma identified by radionuclide bone scan (J Nucl Med 1989;30:1111)
Treatment
- Splenectomy
Gross description
- Well defined lesions, 0.3 - 7 cm, rarely diffuse
- Usually solid, larger lesions can be partly cystic
- Large lesions may have calcifications
Gross images
Microscopic (histologic) description
- Capillary or cavernous
- Vascular spaces lined by single layer of bland endothelial cells, without mitoses
- Thrombosis and infraction can be seen
- When organized, infarcted hemangioma may resemble leiomyoma
Microscopic (histologic) images
Positive stains
- Factor VIII, CD31, CD34
- CD68 (diffuse hemangiomas)
Differential diagnosis
- Angiosarcoma: marked atypia, anastomosing vascular spaces
- Splenic hamartoma: well circumscribed lesion with disorganized blood vessels of varying sizes intermingled with splenic red pulp element; also entrapped adipocytes, focal extramedullary hematopoiesis; CD8+
Hemangioma
Table of Contents
Definition / general | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Rare as primary lymph node tumor, usually an extension of soft tissue tumor
- May represent a metastasis from epithelioid hemangioma of bone (Int J Surg Pathol 2006;14:9) or pulmonary sclerosing hemangioma (Arch Pathol Lab Med 2003;127:321, Ann Thorac Surg 2005;80:2351)
Case reports
- 4 year old boy with inguinal node hemangioma (Arch Pathol Lab Med 1989;113:804)
- 21 year old woman with hemangiomas of small bowel and local lymph nodes (J Clin Pathol 2000;53:552)
Microscopic (histologic) description
- Resembles hemangiomas at other sites
- Discrete mass lesion, either capillary or cavernous
- May be highly cellular or epithelioid
Microscopic (histologic) images
Differential diagnosis
- Kimura disease: inflammatory process with prominent vessels
- Other vascular tumors
- Vascular transformation of sinuses: limited to sinuses
Additional references
Hereditary spherocytosis
Table of Contents
Definition / general | Diagrams / tables | Treatment | Gross description | Microscopic (histologic) description | Peripheral smear imagesDefinition / general
- Congenital hemolytic anemia due to genetically determined abnormal spectrin and ankyrin molecules, leading to defects in red blood cell membrane, causing spherical shape and lack of plasticity
- Red blood cells become trapped within spleen and have less than usual 120 day lifespan
- Splenic function is normal
- Osmotic fragility: increased; basis for diagnostic testing
Treatment
- Splenectomy (prolongs survival of red blood cells, although they still have membrane defects)
Gross description
- Firm, deep red tissue, thin capsule, no grossly identifiable Malpighian follicles, 100 - 1000 g
Microscopic (histologic) description
- Marked congestion in cords
- Sinuses appear empty but actually contain ghost red blood cells
- May have prominent endothelial lined sinuses, hemosiderin deposition, erythrophagocytosis
Histiocytic sarcoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Malignant proliferation of cells with morphologic and immunophenotypic features of mature histiocytes, excluding dendritic cell neoplasms, Langerhans cell neoplasms and myeloid sarcomas with monocytic differentiation
- Mostly in extranodal locations, including skin, soft tissue, lung and central nervous system
- A small subset (~17%) in lymph nodes
- Diagnosis requires histopathologic evidence of neoplasm and confirmation of cell origin by special studies
Essential features
- Malignant neoplasm of mature histiocytes
- May show highly pleomorphic or spindle cytology, mimicking pleomorphic or spindle cell sarcoma
- Can be dedifferentiated from B cell lymphomas, myeloid neoplasms and rarely T cell lymphomas
- Positive BRAF V600E mutation and clonal IGH or TRG rearrangements in a subset of cases
Terminology
- Histiocytic medullary reticulosis, malignant histiocytosis, true histiocytic lymphoma, true histiocytic sarcoma
Epidemiology
- < 1% of all hematolymphoid neoplasms (Leuk Lymphoma 2019;60:553)
- Incidence: 0.17 cases per 1,000,000 individuals
- Usually adults, median age ~61 years
- Slight male predilection, M:F = 5:3
Sites
- Mainly extranodal locations, including skin, soft tissue, lung and central nervous system (Leuk Lymphoma 2019;60:553)
- ~17% of cases in lymph nodes
Etiology
- No specific etiologic agents discovered
- ~25% of cases transdifferentiated from B cell lymphomas, including small lymphocytic lymphoma / chronic lymphocytic leukemia, follicular lymphoma, mantle cell lymphoma, extranodal marginal zone lymphoma and diffuse large B cell lymphoma (Hum Pathol 2016;55:39, Eur J Haematol 2016;97:9)
- Rare cases associated with B cell or T cell lymphoblastic lymphoma / acute lymphoblastic leukemia, myeloid neoplasms and mediastinal nonseminomatous germ cell tumors (Lancet Oncol 2004;5:248, Medicine (Baltimore) 2016;95:e2515)
Clinical features
- Mostly advanced diseases with B symptoms (fever, fatigue and weight loss)
- Physical examinations: lymphadenopathy, hepatosplenomegaly and skin lesions
- Lytic bone lesions and rare central nervous system involvement
- Median overall survival, 6 months (Leuk Lymphoma 2019;60:553)
Diagnosis
- Presence of mature histiocytic cytology with variable degree of pleomorphism
- Positive for histiocytic markers, particularly CD68, CD163 and lysozyme
- Exclusion of myeloid sarcoma with monocytic differentiation, Langerhans cell neoplasm and other mimicries
Prognostic factors
- Surgery alone or in combination with radiotherapy was associated with a prolonged overall survival in patients with skin and connective tissue disease (Leuk Lymphoma 2019;60:553)
- Surgical treatment showed some statistically insignificant improvement in overall survival in patients with gastrointestinal and lung disease
- Patients with hematopoietic and reticuloendothelial system involvement had a poor prognosis even with systemic chemotherapy
- Compared with de novo cases, secondary histiocytic sarcomas have a more aggressive course and shorter overall survival (Clin Lymphoma Myeloma Leuk 2018;18:e427)
Case reports
- 47 year old woman with primary central nervous system histiocytic sarcoma with PDGFR mutation and PDL1 / PDL2 expression (Radiat Oncol 2018;13:167)
- 50 year old African American woman with clonally related histiocytic sarcoma and B lymphoblastic leukemia / lymphoma (Am J Clin Pathol 2019;152:486)
- 63 year old white woman with histiocytic sarcoma associated with splenic marginal zone lymphoma (J Med Case Rep 2017;11:92)
- 72 year old man with histiocytic sarcoma secondary to chronic myeloid leukemia (Eur J Haematol 2016;97:9)
- 74 year old man with histiocytic sarcoma arising from hairy cell leukemia (J Clin Oncol 2014;32:e117)
- 75 year old man with histiocytic sarcoma associated with follicular lymphoma (Clin Lymphoma Myeloma Leuk 2019;19:e1)
Treatment
- Localized unifocal disease: surgical resection if possible, followed by adjuvant radiotherapy and chemotherapy
- Disseminated disease: more intense chemotherapy regimens, including ifosfamide, carboplatin and etoposide; CHOP-like (cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy; autologous hematopoietic stem cell transplantation (Clin Lymphoma Myeloma Leuk 2019;19:522)
- Central nervous system cases: surgery, chemotherapy and focal radiation (Radiat Oncol 2018;13:167, Leuk Lymphoma 2016;57:1961)
- Cases with activated RAS / MEK / ERK pathway: MEK inhibitor, trametinib (Int J Hematol 2019;109:228)
Microscopic (histologic) description
- Diffuse infiltration in the soft tissue or characteristic sinusoidal infiltration in the lymph nodes, liver and spleen
- Effacement of nodal architecture or commonly sinus expansion with sparing of follicles
- Large tumor cells with variable pleomorphism but often resembling mature histiocytes
- Oval or irregular and eccentrically placed nuclei with vesicular chromatin, large nucleoli and occasional grooves
- Abundant eosinophilic cytoplasm, often foamy or vacuolated
- Rarely with multinucleated giant tumor cells, erythrophagocytosis or focal spindling cytology
- Increased mitotic figures and apoptotic cells and presence of tumor necrosis
- Mixed inflammatory background with variable numbers of reactive lymphocytes, plasma cells, benign histiocytes and eosinophils
- Reference: Surg Pathol Clin 2019;12:805
Microscopic (histologic) images
Positive stains
Negative stains
- B cell and T cell markers
- Follicular dendritic markers: CD21, CD23 and CD35
- Langerhans cell markers: CD1a and CD207
- S100 typically negative albeit focal reactivity in some cases
- CD34, CD30, HMB45, myeloperoxidase and cytokeratins
- Reference: Surg Pathol Clin 2019;12:805
Electron microscopy description
- Abundant surface activity, microfilaments, short segments of rough endoplasmic reticulum, scattered haloed granules
- No Birbeck granules
- Reference: Surg Pathol Clin 2019;12:805
Molecular / cytogenetics description
- Clonal IGH and TRG rearrangements in a subset of bona fide cases and cases dedifferentiated from lymphomas (Oncotarget 2016;7:78355)
- Cases transdifferentiated from B cell lymphomas retain the same genetic alterations in the prior B cell lymphoma, including t(14;18) in follicular lymphoma and del(13q) in small lymphocytic lymphoma / chronic lymphocytic leukemia (Diagn Cytopathol 2015;43:659)
- BRAF V600E mutation detected in up to 63% of cases (Histopathology 2014;65:261)
- Rare cases associated with mediastinal nonseminomatous germ cell tumors harbor isochromosome 12p (Medicine (Baltimore) 2016;95:e2515)
Sample pathology report
- Lung, right upper lobe, wedge biopsy:
- Histiocytic sarcoma, dedifferentiated from follicular lymphoma (see comment)
- Comment: The patient had a history of follicular lymphoma (grade 1 - 2 of 3) 3 years ago, and the current pulmonary histiocytic sarcoma demonstrates IGH-BCL2 rearrangement by FISH studies and clonal IGH rearrangements by PCR assays. Therefore, this histiocytic sarcoma represents dedifferentiation from previous follicular lymphoma, which has been reported in the literature (Leukemia 2014;28:1937).
- Microscopic description: The wedge biopsy reveals a diffuse infiltrate of large, pleomorphic cells. The tumor cells have round or irregular nuclei, vesicular chromatin and prominent nucleoli. The cytoplasm is abundant and pale staining. Frequent mitotic figures and apoptotic cells are noted. No small lymphoma cells are present within the specimen.
- Immunohistochemical stains: The large tumor cells are diffusely positive for CD45, CD68, CD163 and lysozyme. S100 only stains a small subset of cells. All other markers studied are negative, including CD1a, CD3, CD20, cytokeratins (AE1 / AE3, CK7 and CK20) and melanocytic markers (SOX10, HMB45 and MelanA).
- Flow cytometric assays: No evidence of monotypic B cell or abnormal T cell population detected.
- FISH studies: Positive for IGH-BCL2 gene rearrangements in 47% of 200 cells analyzed.
- Molecular studies: Positive for clonal IGH gene rearrangement. Negative for clonal TRG gene rearrangement.
Differential diagnosis
- Diffuse large cell lymphoma:
- Anaplastic large cell lymphoma:
- Rosai-Dorfman disease:
- Langerhans cell histiocytosis:
- Metastatic carcinoma with anaplastic features:
- Clinical history of carcinoma
- Positive for cytokeratin
- Metastatic melanoma:
- Myeloid sarcomas with monocytic differentiation:
- Reactive histiocytosis and storage diseases:
- May present with a marked histiocytic infiltration in the sinuses
- Lack of significant cytologic atypia
Additional references
Board review style question #1
Which of the following immunohistochemical stains are mostly positive in histiocytic sarcoma?
- CD4, CD1a, S100
- CD21, CD23, CD35
- CD34, myeloperoxidase, CD117
- CD45, CD68, CD163
Board review style answer #1
Board review style question #2
Which of the following statements is true regarding histiocytic sarcoma?
- Cases dedifferentiated from B cell lymphomas usually retain the expression of B cell markers
- Clonal IGH or TRG rearrangements detected in a subset of cases
- Most cases initially occur in the lymph nodes
- Typically expresses CD68, S100 and CD1a
Board review style answer #2
B. Clonal IGH or TRG rearrangements detected in a subset of cases
Comment Here
Reference: Histiocytic sarcoma
Comment Here
Reference: Histiocytic sarcoma
HIV
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Human immunodeficiency viruses (HIV) include HIV1 and HIV2, which belong to Lentivirus (a subgroup of retrovirus)
- HIV1 causes acquired immunodeficiency syndrome (AIDS), a condition characterized by progressive failure of the immune system leading to opportunistic infections and cancers (PLoS One 2016;11:e0162704)
- World Health Organization (WHO) defines advanced HIV disease as CD4 cell count < 0.2 x 109/L or patient has disease manifestations of WHO stage 3 or 4 in adults and adolescents (WHO: HIV and AIDS [Accessed 29 February 2024])
Essential features
- HIV infection is classified into different stages: 3 stages per The U.S. Centers for Disease Control and Prevention (CDC) and 4 stages per WHO
- Viral load and CD4+ T cell count is useful for disease follow up
- Per WHO, an estimated 39.0 million (33.1 - 45.7 million) people were living with HIV (LWH) at the end of 2022
- In 2022, estimated that 630,000 (480,000 - 880,000) people died from HIV related causes and 1.3 million (1.0 - 1.7 million) people acquired HIV (WHO)
ICD coding
Epidemiology
- HIV1 is the most common subtype and the most common subtype associated with AIDS throughout most of the world
- HIV2 is less common and is found mostly in Western Africa (J Gen Virol 2002;83:1253)
- Clinical AIDS defining conditions include some of the following (refer to the complete list in MMWR Recomm Rep 2014;63:1)
- Kaposi sarcoma
- Lymphomas: Burkitt, immunoblastic, CNS
- Cervical carcinoma, invasive
- Encephalopathy, HIV related
- Wasting syndrome, HIV related
- Recurrent pneumonia
- Opportunistic infections caused by the following: Candida, Cryptococcus, Cryptosporidium, Histoplasma, herpes simplex virus, Mycobacterium, Pneumocystis, Toxoplasma, etc.
- HIV infection has been classified into different stages per the CDC and WHO (MMWR Recomm Rep 2014;63:1, Virtual Mentor 2010;12:202)
CDC Stage 0 Negative HIV test within 6 months of the first HIV diagnosis and remains 0 until 6 months after diagnosis Stages Age specific CD4+ T cell testing < 1 year 1 - 5 years 6 years - adult Cells/µL % Cells/µL % Cells/µL % 1 ≥ 1,500 ≥ 34 ≥ 1,000 ≥ 30 ≥ 500 ≥ 26 2 750 - 1,499 26 - 33 500 - 999 22 - 29 200 - 499 14 - 25 3 < 750 > 26 < 500 < 22 < 200 < 14 Unknown stage CD4 test results are missing
WHO Stage 1 Asymptomatic or generalized lymphadenopathy of at least 2 sites for longer than 6 months Stage 2 Unexplained weight loss of < 10% of body weight and recurrent respiratory / skin infections Stage 3 Unexplained weight loss of > 10% of body weight, diarrhea (> 1 month), pulmonary tuberculosis (TB), mucocutaneous and systemic bacterial infections Stage 4 AIDS defining illnesses, HIV wasting syndrome, pneumocystis pneumonia, recurrent severe bacterial pneumonia, extrapulmonary TB, Kaposi sarcoma, CNS toxoplasmosis, HIV encephalopathy, others
Pathophysiology
- HIV is a spherical virus that attaches to the host cells with glycoproteins; the virus then integrates its chromosomal material into the host's cell, leading to generation of viral proteins and genetic material (StatPearls: Acquired Immune Deficiency Syndrome [Accessed 13 December 2023])
- Cells in the lymph nodes that are infected include monocytes, macrophages and follicular dendritic cells
- Progressive loss of CD4+ T cells occurs during the course of disease
- B cell activation during early disease leads to hypergammaglobulinemia, autoimmune phenomenons and later decreased antibody production
- Natural killer cell activity is also progressively decreased
Etiology
- There are 4 modes of acquiring HIV infection
- Sexual contact with an infected partner
- Parenteral drug use
- Exposure to infected blood or blood products
- Perinatal exposure
Clinical features
- Acute retroviral syndrome
- Headache, fever, rash, fatigue noted with initial HIV infection
- Occurs 1 - 3 weeks after infection and may last for 1 - 2 weeks
- Generalized lymphadenopathy
- Early / asymptomatic HIV disease
- Latency period that may last for years
- Advanced symptomatic HIV disease
- AIDS defining conditions
- References: World Health Organization: WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV Related Disease in Adults and Children [Accessed 5 January 2024], MMWR Recomm Rep 2014;63:1
Diagnosis
- Screening test: performed on peripheral blood or body fluids
- Detection of antibodies to HIV glycoproteins by enzyme linked immunoassay (EIA) (J Infect Dis 2012;205:521, Curr Opin HIV AIDS 2012;7:125, J Infect Dis 2010;202:S270)
- Positive in about 2 - 4 weeks after infection
- Confirmatory tests: antibodies to HIV through enzyme linked immunoassay fourth generation (15 - 20 days from infection) or HIV viral load test cutoff 50 copies/mL (10 - 15 days) or ultrasensitive cutoff 1 - 5 copies/mL (5 days from infection)
- Latest recommendations: testing with immunoassay (both HIV1 and HIV2 antibodies) and p24 antigen
- Negative result: rules out infection within specific ranges of exposure and test sensitivity
- Positive result may have 4 scenarios
- HIV1+ & HIV2-: HIV1 antibodies present
- HIV1- & HIV2+: HIV2 antibodies present
- HIV1+ & HIV2+: both HIV1 and HIV2 antibodies present
- HIV1- or indeterminate & HIV2-: in this case nucleic acid test is done to identify the presence or absence of HIV1 acute infection
- Once tested positive, viral load and CD4+ T cell counts are performed (Internist (Berl) 2016;57:773)
Laboratory
- Anemia, neutropenia or thrombocytopenia
- If the white blood cell and lymphocyte counts are within the reference ranges, then the CD4 count is most likely normal
- If the absolute lymphocyte count is < 0.95 x 109/L, then a CD4 count < 0.2 x 109/L is likely (Acad Emerg Med 2011;18:385)
- Based on the symptoms and organ involvement, different tests are performed
- Complete metabolic profile: baseline renal and hepatic function
- EKG, cardiac biomarkers and echocardiography
- Chest radiography, computed tomography of chest
- Arterial blood gas analysis
- Testing for tuberculosis (TB)
- Esophagogastroduodenoscopy, colonoscopy
- Testing for ova, parasites, bacteria and C. difficile toxins
- Computed tomography of the head and lumbar puncture with cerebrospinal fluid (CSF) analysis
- Positron emission tomography (PET)
Radiology description
- Chest imaging: consolidation, ground glass opacity, cystic lesions, nodules and mass (AJR Am J Roentgenol 2012;198:1295)
- Brain imaging: ring enhancing lesions, encephalitis and mass (Jpn J Radiol 2021;39:1023)
- PET / CT: generalized lymphadenopathy, PET avid lesions in other organs
Prognostic factors
- Prognosis of adults with HIV or AIDS is now approaching that of the general population; however, it depends on the effectiveness of combination antiretroviral therapy (cART) (J Hosp Med 2015;10:608, JAMA 2008;300:51)
- Factors associated with poor prognosis from AIDS related conditions (Public Health Rep 2015;130:468, J Infect Dis 2009;199:991, J Int Assoc Physicians AIDS Care (Chic) 2011;10:5, HIV Clin Trials 2014;15:133)
- African American or mixed races
- Opportunistic infections
- Poor functional and nutritional status
- Anemia
- Substance abuse
- Low CD4+ count
- High HIV viral load
- Type of malignancy also plays an important role in overall survival and progression free survival
Case reports
- 25 year old man presented with sudden onset of psychotic symptoms associated with fever, cough and general asthenia (Front Immunol 2021:12:669723)
- 40 year old man was diagnosed HIV positive in 2008 but his viral load estimation every year since then had always been below lower detection limit and he remained healthy without treatment (Case Rep Infect Dis 2018:2018:5279595)
- 40 year old man with a history of HIV on cART presented with dyspnea and hypertension (SAGE Open Med Case Rep 2020:8:2050313X20965423)
- 46 year old man with HIV infection on cART presented with fever off and on, splenomegaly, lymphadenopathy and severe thrombocytopenia (Int J STD AIDS 2021;32:286)
- 78 year old woman of African descent from Mtwara Region, south of Tanzania presented for evaluation of leukopenia and anemia (J Med Case Rep 2021;15:341)
Treatment
- All individuals should be offered antiretroviral therapy (ART), regardless of the CD4 count or viral load
- Treatment results in sustained suppression of HIV RNA, improved cellular immunity (i.e., CD4 count), reduced HIV immune activation (e.g., proinflammatory cytokines, chronic inflammation and T cell activation) and decreased HIV transmission to others; however, access to care and treatment outcomes can differ depending upon demographic and psychosocial factors
- cART / ART: includes reverse transcriptase inhibitors (RTIs), integrase inhibitors (IIs) and protease inhibitors (PIs) (Br J Haematol 2001;112:900, AIDS 2014;28:881, Cold Spring Harb Perspect Med 2012;2:a007161)
- Latency reversing agents (LRAs): target the process of cell death via virus mediated cytolysis or immune associated clearance (Cell Host Microbe 2018;23:14, Nature 2012;487:482, PLoS Pathog 2014;10:e1004473)
- Gene therapy: works by inserting a healthy gene into the cells, which acts by repressing the mutant RNA or correcting the mutant gene (Einstein (Sao Paulo) 2017;15:369)
- Treatment of opportunistic infections and malignancies
Microscopic (histologic) description
Reactive and opportunistic infection
KSHV / HHV8 associated multicentric Castleman disease
Polymorphic lymphoproliferative disorder arising in HIV
HIV associated lymphomas
- HIV patients with CD4+ cell counts < 0.2 x 109/L, are prone to a wide variety of bacterial, viral, fungal and protozoal infections, which include
- Toxoplasma gondii, Pneumocystis jirovecii (previously Pneumocystis carinii), Cryptococcus neoformans, Mycobacterium avium, Mycobacterium tuberculosis, cytomegalovirus, herpes simplex viruses and Histoplasma capsulatum infections (StatPearls: HIV-1-Associated Opportunistic Infections [Accessed 13 December 2023])
- Pathological findings depend on the organ involved and the type of infection
- Most have lymphadenopathy, which includes florid follicular hyperplasia, paracortical hyperplasia, monocytoid B cell proliferation, granulomatous inflammation and necrosis
- HIV lymphadenitis has 3 histologic patterns, which correspond to clinical stages: acute, chronic and burnout (Medeiros: Ioachim's Lymph Node Pathology, 5th Edition, 2021)
- Pattern A
- Enlarged reactive follicles
- Hyperplastic germinal centers
- Diminished / absent mantle zones
- Folliculolysis - disruption of germinal centers by small lymphocytes
- Aggregates of monocytoid cells
- Pattern B
- Disruption of follicular dendritic cell meshwork
- Effacement of follicles
- Involution of germinal centers
- Increased plasma cells
- Depletion of lymphocytes
- Vascular proliferation
- Pattern C
- Atrophic follicles
- Hyalinized germinal centers
- Excessive plasma cells
- Marked loss of lymphocytes
- Fibrosis
- Pattern A
KSHV / HHV8 associated multicentric Castleman disease
- Associated with 80% of HIV+ patients (Neth J Med 2015;73:202)
- Seen in cases with low HIV viral load and median CD4 count (between 0.15 and 0.3 x 109 cells) (J Clin Oncol 2011;29:2481, Blood 2011;118:3499, Blood 2013;122:4189)
- Predominantly lymphadenopathy is noted with classic features
- Regressed follicles along with plasmacytosis
- Presence of plasmablasts in the mantle zones, which are medium to large cells with 1 - 2 nucleoli and a moderate amount of amphophilic cytoplasm
- Plasmablasts display negative to dim levels of CD20, positive expression for MUM1 and latency associated nuclear antigen (LANA) (a gene product of KSHV / HHV8 ORF73) and express cytoplasmic IgM lambda (Histopathology 2008;53:513, Blood 2001;97:2130)
- Foci of Kaposi sarcoma may be seen (Blood 2000;95:1406, Am J Pathol 1997;151:1517, AIDS Rev 2008;10:25)
- Increased risk of developing lymphoma
Polymorphic lymphoproliferative disorder arising in HIV
- Incidence in HIV is < 5% (Am J Surg Pathol 2003;27:293, Virchows Arch 2012;461:93)
- Architectural distortion by polymorphous infiltrate consisting of spectrum of B cells, including small lymphocytes, plasmacytoid cells, plasmablasts and immunoblasts along with T cells and histiocytes
- May have Reed-Sternberg-like cells
- Epstein-Barr virus (EBV) expression seen in both small and large cells
- Responds well to cART
HIV associated lymphomas
- Lymphomas arising in HIV+ patients now are classified under lymphomas arising in immune deficiency / dysregulation per 2022, WHO classification
- HIV+ patients present with advanced clinical stage, bulky disease with a high tumor burden
- Lymphomas arising in HIV+ patients include the following
- B cell lymphomas
- Diffuse large B cell lymphoma (DLBCL)
- Burkitt lymphoma
- Extranodal marginal zone lymphoma
- Primary effusion lymphoma
- Plasmablastic lymphoma
- KSHV / HHV8 positive diffuse large B cell lymphoma
- T cell lymphomas
- Peripheral T cell lymphoma, not otherwise specified
- Anaplastic large cell lymphoma
- Nodal T follicular helper cell lymphoma, angioimmunoblastic type (nTFHL AI)
- Hepatosplenic T cell lymphoma (HSTCL)
- Mycosis fungoides
- Extranodal NK / T cell lymphoma (ENKTCL)
- Classic Hodgkin lymphoma
- B cell lymphomas
- Large B cell lymphoma in CNS (PCNS lymphoma) and diffuse large B cell lymphoma (DLBCL) are associated with low CD4+ T cell count
- Burkitt lymphoma (BL) and classic Hodgkin lymphoma (CHL) noted in more immunocompetent patients (Adv Clin Path 1997;1:13, Blood 2001;98:2339, Lancet HIV 2020;7:e641)
- Plasmablastic lymphoma, KSHV / HHV8 associated lymphomas such as primary effusion lymphomas (PEL) and KSHV / HHV8+ DLBCL occur predominantly in HIV+ patients
- With the advent of cART, the incidence of PCNS lymphoma and DLBCL have declined, while CHL cases have increased and BL incidence has been stable (Blood 2001;98:2339, AIDS 2006;20:1645, J Natl Cancer Inst 2007;99:962, J Acquir Immune Defic Syndr 2016;72:177, AIDS Patient Care STDS 2013;27:259)
- Most of the lymphomas are usually indistinguishable from their counterparts that arise in immunocompetent patients with some of the following differences
Lymphoma Histologic features of lymphomas affecting HIV+ patients BL - More variation in size and shape (pleomorphism)
- Plasmacytoid differentiation: eccentric nucleus, single central nucleolus and abundant cytoplasm (Ann Oncol 1991;2:289)
- Stronger EBV association (Semin Diagn Pathol 2017;34:352)
DLBCL - Extranodal or disseminated presentation
- Immunoblastic / plasmablastic morphology more common
CHL - Nodular sclerosis type comprises 50% of CHL cases in people living with HIV
- Lymphocyte depleted type specifically seen
- Effective cART has negated the impact of HIV related prognostic factors and has led to better therapy for most of the aggressive lymphomas, contributing to better survival outcomes comparable to lymphoma occurring in the general population (Br J Haematol 2015;168:806, Blood 2015;125:1226, Ann Oncol 2015;26:958, Blood 2015;126:160, Blood Adv 2021;5:2852)
Microscopic (histologic) images
Contributed by Barina Aqil, M.D.
Positive stains
- p24 (Histopathology 2010;56:530)
- Antibody to HIV core protein
- Localized in reactive germinal centers along follicular dendritic cell processes
- p15 and p17 (Am J Pathol 1988;133:516)
- Staining of follicular dendritic cells
- Radiolabeled HIV RNA in situ hybridization (J Infect Dis 1991;164:1051)
- Found in high concentrations in germinal centers
Sample pathology report
- Lymph node, left supraclavicular, needle core biopsy:
- Classic Hodgkin lymphoma, EBV+ arising in an HIV+ patient
- Flow cytometric analysis performed on the left supraclavicular lymph node biopsy reveals a polytypic B cell population and a T cell population without evidence of immunophenotypic abnormality based on the markers assayed
- Microscopic description: Histologic sections show cylindrical cores of lymphoid tissue with scattered large mononuclear cells with prominent macronucleoli in the background of histiocytes, small lymphocytes, plasma cells and eosinophils. Rare mummified cells are noted.
- Immunohistochemistry results: CD3- in large atypical cells (shows numerous small T cells), CD20- in large atypical cells (shows scattered small B cells), PAX5+ with strong staining in background B cells (dim in large atypical cells), CD30+ in large atypical cells, CD15+ in large atypical cells, MUM1+ in large atypical cells, EBER+ in scattered large atypical cells
Differential diagnosis
- Infectious mononucleosis:
- Similarly reactive follicles as in HIV acute pattern
- Serologic testing positive for EBV
- Negative HIV antibodies
- Cytomegalovirus, measles and varicella lymphadenitis:
- CMV inclusions seen in CMV infection
- Giant multinucleated cells (Warthin-Finkeldey) seen in both measles and HIV; so serologic testing is required
- Toxoplasma lymphadenitis:
- Monocytoid hyperplasia noted in Toxoplasma as well as in HIV infection
- Perifollicular and intrafollicular clusters of epithelioid cells only noted in Toxoplasma
- Castleman lymphadenopathy:
- Mimics pattern B and C of HIV lymphadenopathy
- Lacks depletion of lymphocytes
- Negative for HIV antibodies
- Nodal TFH cell lymphoma, angioimmunoblastic type:
- Paracortical expansion with vascular proliferation and effaced follicles reminiscent to HIV chronic and burnout patterns
- No depletion of lymphocytes
- Proliferation of immunoblasts
Additional references
- CDC: About HIV [Accessed 14 December 2023], CDC: PrEP (Pre-exposure Prophylaxis) [Accessed 14 December 2023], Bennett: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 9th Edition, 2019, Goldman: Goldman-Cecil Medicine, 27th Edition, 2023, Clinicalinfo: Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV [Accessed 14 December 2023]
Board review style question #1
The patient is a 42 year old man who presented with fever, cough and weight loss. Imaging showed diffuse lymphadenopathy. An excisional lymph node biopsy was performed that showed the above morphology (H&E stains and LANA immunohistochemistry). What is the diagnosis?
- Burnout pattern of HIV lymphadenitis
- Diffuse large B cell lymphoma (DLBCL)
- KSHV / HHV8 associated multicentric Castleman disease
- Toxoplasma lymphadenitis
Board review style answer #1
C. KSHV / HHV8 associated multicentric Castleman disease. The lymph node showed regressed germinal centers with hyalinized arterioles, vascular proliferation and plasmablasts in the mantle zones. The plasmablasts are positive for CD138, MUM1, LANA and negative for B cell markers (CD20, PAX5, etc.).
Answer D is incorrect because toxoplasmosis has reactive follicles, monocytoid hyperplasia and perifollicular / intrafollicular clusters of epithelioid histiocytes. Answer A is incorrect because later stages of HIV lymphadenitis (patterns B and C) show markedly attenuated follicles with vascular proliferation and loss of lymphocytes. Answer B is incorrect because DLBCL has diffuse infiltrate of large neoplastic B cells, which can have variable cellular morphology, including centroblastic, immunoblastic or anaplastic. The neoplastic cells retain B cell markers and are LANA negative.
Comment Here
Reference: HIV
Answer D is incorrect because toxoplasmosis has reactive follicles, monocytoid hyperplasia and perifollicular / intrafollicular clusters of epithelioid histiocytes. Answer A is incorrect because later stages of HIV lymphadenitis (patterns B and C) show markedly attenuated follicles with vascular proliferation and loss of lymphocytes. Answer B is incorrect because DLBCL has diffuse infiltrate of large neoplastic B cells, which can have variable cellular morphology, including centroblastic, immunoblastic or anaplastic. The neoplastic cells retain B cell markers and are LANA negative.
Comment Here
Reference: HIV
Board review style question #2
The patient is an adult who was found unresponsive at the bus stop and brought over to the emergency department by the paramedics. HIV serologies were positive. Imaging showed generalized lymphadenopathy, pulmonary infiltrates and bilateral pleural effusion. Lung sampling showed the above morphology (H&E stains and Gomori methenamine silver). What is the diagnosis?
- Alveolar proteinosis
- Blastomycosis
- Mycobacterium avium intracellulare (MAI)
- Pneumocystis jirovecii infection
Board review style answer #2
D. Pneumocystis jirovecii infection is a fungal infection seen in immunocompromised patients. Lung specimen shows pink foamy amorphous material within the alveolar spaces and the GMS (Grocott-Gomori methenamine silver) reveals thin walled, oval, round and crescentic yeast forms about the size of red blood cells (7 μm) with a small central dot.
Answer A is incorrect because intra-alveolar PAS (periodic acid Schiff) positive and GMS negative material is noted in alveolar proteinosis. Answer B is incorrect because blastomycosis is also a fungal infection characterized by granulomatous inflammation and GMS shows 12 μm spherical yeast forms with broad based buds. Answer C is incorrect because MAI is a bacterial infection that may have necrotizing granulomas or just foamy histiocytes with red stained acid fast bacilli seen on special stain (AFB).
Comment Here
Reference: HIV
Answer A is incorrect because intra-alveolar PAS (periodic acid Schiff) positive and GMS negative material is noted in alveolar proteinosis. Answer B is incorrect because blastomycosis is also a fungal infection characterized by granulomatous inflammation and GMS shows 12 μm spherical yeast forms with broad based buds. Answer C is incorrect because MAI is a bacterial infection that may have necrotizing granulomas or just foamy histiocytes with red stained acid fast bacilli seen on special stain (AFB).
Comment Here
Reference: HIV
IgG4 lymphadenitis (pending)
[Pending]
IgG4 related lymphadenopathy
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) description | Virtual slides | Cytology description | Cytology images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Lymphadenopathy attributable to IgG4 related disease, with distinct clinicopathologic features
Essential features
- Refers to lymphadenopathy that occurs in patients with established IgG4 related disease and is attributable to that disorder
- Because increased IgG4 positive plasma cells are relatively nonspecific in lymph nodes, the term IgG4 related lymphadenopathy should not be used as a general descriptor for a lymph node with increased IgG4 positive plasma cells in a patient without established extranodal IgG4 related disease
- Involved lymph nodes demonstrate both an absolute (> 100 IgG4 positive plasma cells per high power field [HPF]) and relative (IgG4:IgG plasma cell ratio > 40%) increase in IgG4 positive plasma cells
- Various morphologic patterns are described, many of which are nonspecific: 1) Castleman disease-like, 2) follicular hyperplasia, 3) progressive transformation of germinal centers, 4) interfollicular expansion, 5) inflammatory pseudotumor-like as well as infrequent patterns, including infectious mononucleosis-like features
- Features thought to be relatively specific for IgG4 related lymphadenopathy include increased IgG4 positive plasma cells (> 100 per HPF) and IgG4:IgG ratio (> 40%) within expanded interfollicular or fibrotic regions (i.e., interfollicular expansion and inflammatory pseudotumor-like patterns)
ICD coding
Epidemiology
- Most common in middle aged to older adults with a male predominance
- Extremely rare in children
- Lymphadenopathy occurs in at least 40 - 60% of patients with IgG4 related disease (Am J Surg Pathol 2010;34:1812, Arthritis Rheumatol 2015;67:2466)
Sites
- Localized lymphadenopathy in anatomic regions near the site of extranodal IgG4 related disease is most common but distant lymphadenopathy and multifocal / generalized lymphadenopathy occurs in some cases
Pathophysiology
- Pathophysiology of IgG4 related disease is poorly understood
- Both aberrant B cell and T cell activity has been implicated
- B cell depletion as a treatment for IgG4 related disease implicated a role of activated B cells in IgG4 related disease, though recent studies have favored abnormal T cells to be the underlying immunologic defect
- T follicular helper cells have been implicated in contributing to IgG4 related disease through cytokines IL21 and IL4, which are thought to contribute to lymphoid follicle formation, class switch to IgG4 and plasmablast induction (Arthritis Rheumatol 2015;67:2476, Arthritis Res Ther 2016;18:167)
- Circulating Tfh2 cells have been shown to be increased in IgG4 related disease and to correlate with serum IgG4 levels (Arthritis Rheumatol 2015;67:2476)
- Treg cells also may contribute to IgG4 related disease through secretion of IL10 and TGF beta resulting in IgG4 class switching and fibrosis (Clin Nephrol 2010;73:385)
- Oligoclonal expansions of CD4+ cytotoxic T cells have been found in blood and tissues of IgG4 related disease patients and have been implicated in contributing to fibrosis (Autoimmunity 2017;50:19)
- Role of IgG4 positive plasma cells in the disease is uncertain; given that IgG4 is a relatively inactive immunoglobulin subclass that cannot fix complement or crosslink, some have suggested that IgG4 positive plasma cells are a secondary phenomenon and may be produced in an attempt to dampen the inflammatory process (Clin Exp Allergy 2009;39:469, Gut 2018;67:728)
Etiology
- Lymph node involvement by IgG4 related disease
Diagrams / tables
not applicable
Clinical features
- IgG4 related disease usually presents due to enlargement of an exocrine organ, most commonly submandibular glands, orbit / lacrimal glands or pancreaticobiliary tract, with systemic involvement in ~33% of cases
- Orbit is the most common site in children (Pediatr Rheumatol Online J 2016;14:18)
- Organ enlargement is detected by a visible mass in superficial sites (i.e., orbit or submandibular gland) or due to organ dysfunction from mass effect at deeper sites (i.e., pancreas)
Diagnosis
- Physical exam and imaging play a central role in the diagnosis of IgG4 related disease through demonstration of mass-like lesions and for investigation of lymphadenopathy
- In cases with characteristic organ involvement, physical exam or imaging may prompt consideration of IgG4 related disease and subsequent laboratory testing or biopsy
- However, in many cases the etiology of the tumefactive lesion of IgG4 related disease is not recognized and there may be clinical suspicion for a neoplastic process, prompting biopsy
Laboratory
- Elevated serum IgG4 levels are commonly touted (especially among pathologists) as defining IgG4 related disease; while useful, elevated serum IgG4 is neither sensitive nor specific for the diagnosis of IgG4 related disease
- Sensitivity of elevated serum IgG4 for the diagnosis of IgG4 related disease has been reported from about 50 to 90% and specificity ~60 - 80%, with higher IgG4 levels being more specific (Pediatr Rheumatol Online J 2016;14:18, Ann Rheum Dis 2015;74:14)
- Serum IgG4 levels may also be elevated in other inflammatory and immune disorders and neoplasms, including IgG4 positive plasma cell neoplasms and IgG4 positive B cell neoplasms with plasmacytic differentiation; in the latter, the elevated IgG4 may be associated with a clonal M component on serum protein electrophoresis (SPEP) / immunofixation electrophoresis (IFE) (Mod Pathol 2014;27:375, Am J Clin Pathol 2017;148:215)
- In contrast, the elevated serum IgG4 in IgG4 related disease is polyclonal
- Other useful laboratory values often seen in IgG4 related disease include elevated serum IgE or CRP, decreased complement (C3, C4) levels and peripheral eosinophilia (Pediatr Rheumatol Online J 2016;14:18)
Radiology description
- Imaging findings of mass-like enlargement or fibrosis plays a key role in diagnosis of IgG4 related disease
Radiology images
None
Prognostic factors
- IgG4 related lymphadenopathy is generally treated in the context of IgG4 related disease in an attempt to prevent irreversible organ damage rather than as an attempt to correct the lymphadenopathy
- Natural history of IgG4 related disease is variable, ranging from spontaneous improvement in rare cases, a good and durable response to initial therapy in many cases, to progressive organ damage in some cases
- Involvement of vital organs including the heart, aorta and kidneys may be associated with more severe outcomes
- Flares of activity may result in complications such as exocrine dysfunction of various organs and diabetes mellitus in IgG4 related pancreatitis
- Morbidities associated with treatment, such as long term steroid therapy, are also a consideration
Case reports
- 48 year old woman with longstanding lymphadenopathy and development of pancreatitis and kidney disease (Mod Rheumatol Case Rep 2023;7:192)
- 58 year old man with jaundice, elevated transaminases and inguinal and axillary lymphadenopathy (Surgical and Experimental Pathology 2020;3:30)
- 66 year old woman with a thyroid mass and lymphadenopathy (Diagn Pathol 2018;13:3)
Treatment
- Treatment varies from watchful waiting in cases of IgG4 related disease with isolated lymphadenopathy or minimal salivary gland enlargement to prompt treatment of cases in which vital organs are involved
- Most patients have good responses to glucocorticoids but may relapse after treatment
- Rituximab is used as a second line agent in cases of recurrent or refractory disease (Arthritis Rheumatol 2015;67:1688)
- Other agents have been used in combination with glucocorticoids including cyclophosphamide, azathioprine or methotrexate
Gross description
- Lymph nodes are enlarged with variable cut surface ranging from fleshy to nodular and fibrotic
Gross images
none available
Microscopic (histologic) description
- Enlarged lymph node with various reactive morphologic patterns (described below), increased plasma cells typically in extrafollicular locations (i.e., between follicles) and variable fibrosis that may be capsular, parenchymal or inflammatory pseudotumor-like (Am J Surg Pathol 2021;45:178)
- Increased number (> 100 per HPF) of IgG4 positive plasma cells and increased ratio of IgG4:IgG positive plasma cells (> 40%) is required for the diagnosis of IgG4 related lymphadenopathy
- These parameters are increased in the lymph nodes of most patients with enlarged lymph nodes and established extranodal IgG4 related disease; however, in a subset of patients with known IgG4 related disease and enlarged lymph nodes, the lymph nodes will not show an increase of IgG4 positive plasma cells
- Eosinophils are almost always present admixed with the plasma cells in IgG4 related disease and IgG4 related lymphadenopathy, a useful morphologic clue
- Presence of increased IgG4 positive plasma cells and IgG4:IgG ratio in regions of nodal fibrosis or in extrafollicular regions appears more specific for true IgG4 related disease than an isolated increase in intrafollicular IgG4 positive plasma cells; increased IgG4 positive cells and IgG4:IgG ratio found only within follicles / germinal centers can be seen in many nonspecific causes of lymphadenopathy (Am J Surg Pathol 2021;45:178)
- 5 main morphologic patterns of IgG4 related lymphadenopathy are classically described
- These patterns are nonspecific in the absence of increased IgG4 immunostaining parameters as described above
- On H&E alone, none of these patterns are specific for IgG4 related disease but the presence of these patterns along with nodal plasmacytosis should prompt work up for IgG4 related lymphadenopathy
- Multicentric Castleman disease-like pattern
- Increased IgG4 positive cells outside of follicles
- Lollipop follicles with a penetrating vessel and onion skin arrangement of mantle zone B cells
- Variable fibrosis
- In true Castleman disease follicles are atrophic, whereas in this pattern of IgG4 related disease follicles are more often normal sized or hyperplastic
- Follicular hyperplasia pattern
- Enlarged reactive follicles
- Classically described with increased extrafollicular IgG4 positive plasma cells
- Interfollicular expansion pattern
- Markedly expanded interfollicular zones with few widely separated residual follicles
- Interfollicular zones contain small lymphocytes, plasma cells, eosinophils, histiocytes and immunoblasts
- Plasma cells are predominantly IgG4 positive
- Morphologic features may be reminiscient of angioimmunoblastic T cell lymphoma
- This pattern has been shown to be highly specific for IgG4 related disease (Am J Surg Pathol 2021;45:178)
- Progressive transformation of germinal centers (PTGC) pattern
- Follicular hyperplasia with frequent PTGC
- This pattern is classically associated with increased intrafollicular IgG4 positive plasma cells
- Some studies have suggested that when this pattern is present in submandibular / cervical lymph nodes it is associated with concurrent of subsequent development of submandibular IgG4 related sclerosing sialadenitis (Mod Pathol 2012;25:956)
- Inflammatory pseudotumor-like pattern
- Broad areas of fibrosis replacing lymph node parenchyma
- Storiform fibrosis is common
- Increased IgG4 positive plasma cells and IgG4:IgG ratio within the fibrosis
- This pattern has been shown to be highly specific for IgG4 related disease (Am J Surg Pathol 2021;45:178)
- Multicentric Castleman disease-like pattern
- Other uncommon patterns have been described, including infectious mononucleolsis-like and rarely Rosai-Dorfman-like changes (Am J Surg Pathol 2018;42:977)
- Other morphologic features that may be present include ring-like perifollicular granulomas or phlebitis
- Significant neutrophilic inflammation is typically not present in IgG4 related disease or IgG4 related lymphadenopathy
Microscopic (histologic) description
Virtual slides
none found
Cytology description
not applicable
Cytology images
not applicable
Peripheral smear description
not applicable
Peripheral smear images
not applicable
Positive stains
- IgG4: the presence of > 100 IgG4 positive plasma per HPF in the regions with the highest number of IgG4 positive cells*
- IgG4:IgG: IgG4 positive plasma cells are > 40% of total IgG positive plasma cells in the regions with the highest number of IgG4 positive cells*
- CD138: may be useful to aid in quantification if IgG stain shows high background staining
- Kappa / lambda IHC or ISH: plasma cells are polytypic in IgG4 related disease; if monotypic, then consider an IgG4 positive plasma cell or B cell neoplasm with plasmacytic differentiation
- Elastic stain may be useful to demonstrate obliterative phlebitis, a useful feature for extranodal IgG4 related disease but uncommonly present in lymph nodes
Negative stains
- Spirochete / treponemal IHC and silver stains (syphilitic lymphadenitis may mimic IgG4 related lymphadenopathy)
- HHV8 (excludes HHV8 positive Castleman disease)
- ALK1
- ROS1
Flow cytometry description
- Reactive pattern with polytypic B cells and plasma cells and immunophenotypically normal T cells
Flow cytometry images
not applicable
Electron microscopy description
not applicable
Electron microscopy images
not applicable
Molecular / cytogenetics description
- IgG4 related disease and IgG4 related lymphadenopathy are nonneoplastic disorders and should not show molecular or cytogenetic abnormalities
- When the differential diagnosis includes neoplastic entities, including IgG4 positive plasma cell neoplasms or IgG4 positive B cell lymphomas with plasmacytic differentiation, inflammatory myofibroblastic tumor or others, molecular / cytogenetic testing may be useful
Molecular / cytogenetics images
not applicable
Videos
not applicable
Sample pathology report
- Lymph node, excision:
- Enlarged lymph node with fibrosis and increased IgG4 positive plasma cells (see comment)
- Comment: The lymph node is enlarged with follicular hyperplasia, capsular fibrosis and foci of parenchymal fibrosis. There are frequent plasma cells with fewer admixed eosinophils within the regions of fibrosis and present between follicles. Immunohistochemistry for IgG4 and IgG show increased IgG4 positive plasma cells (up to 165 per HPF) and IgG4:IgG ratio of approximately 70% within interfollicular and fibrotic regions. Plasma cells demonstrate polytypic expression of kappa and lambda light chains by in situ hybridization. A spirochete (Treponema) immunostain is negative.
- Increased IgG4 positive plasma cells may be seen in lymph nodes in various conditions including IgG4 related disease, Castleman disease and other inflammatory and infectious processes. The presence of increased IgG4 staining parameters within fibrosis and interfollicular regions of enlarged lymph nodes, as seen in this case, has been described as a relatively specific finding for IgG4 related disease. Therefore, in this patient without a history of established extranodal IgG4 related disease, the findings are suspicious for IgG4 related lymphadenopathy; however, further investigation for an extranodal site of IgG4 related disease and laboratory testing including serum IgG4, IgE and complement (C3 / C4) levels may be useful for definitive diagnosis of IgG4 related disease.
Differential diagnosis
- Increased IgG4 positive plasma cells can be seen in various reactive and neoplastic disorders involving lymph nodes
- Rosai-Dorfman disease (RDD):
- May have increased IgG4 positive plasma cells and IgG4:IgG ratio
- Hallmark is enlarged histiocytes with atypical nuclei and emperipolesis
- RDD histiocytes are positive for S100, OCT2 and cyclin D1 (Am J Surg Pathol 2021;45:35, Br J Haematol 2019;186:837)
- Histiocyte emperipolesis may be seen extremely rarely in IgG4 related lymphadenitis as a focal finding and without cytologic atypia
- Castleman disease:
- Lymph nodes in hyper-IL6 syndromes, including Castleman disease and rheumatoid arthritis, may contain increased IgG4 positive plasma cells, typically in the setting of interfollicular plasmacytosis
- Plasma cell type of Castleman disease typically contains regions with sheets of plasma cells without admixed eosinophils and with associated hemosiderin, a finding that would be uncommon in IgG4 related disease
- Neutrophils may be seen in lymph nodes of hyper-IL6 syndromes and are not seen in IgG4 related lymphadenopathy
- Castleman disease follicles are typically atrophic while follicles in the Castleman disease-like pattern of IgG4 are more often normal sized to hyperplastic
- HHV8 positive plasmablasts within Castleman disease type follicles supports the diagnosis of HHV8 associated Castleman disease
- Plasma cell neoplasm expressing IgG4:
- IgG4 positive plasma cells are clonal by IHC / ISH for kappa and lambda light chains
- Serum IgG4 may be elevated
- SPEP / IFE may show M component
- B cell lymphoma with plasmacytic differentiation expressing IgG4:
- Usually nodal or extranodal marginal zone lymphoma
- IgG4 positive plasma cells are clonal by IHC / ISH for kappa and lambda light chains
- Serum IgG4 may be elevated
- SPEP / IFE may show M component
- Inflammatory myofibroblastic tumor (Am J Surg Pathol 2019;43:314):
- May demonstrate marked morphologic overlap with IgG4 related disease, including storiform fibrosis, plasmacytosis with increased IgG4 positive plasma cells and IgG4:IgG ratio and even obliterative phlebitis
- Stromal cells demonstrate cytologic atypia, a finding not seen in IgG4 related disease
- ALK1 and ROS1 immunohistochemistry or fusion analysis may be useful when inflammatory myofibroblastic tumor is suspected
- Angioimmunoblastic T cell lymphoma (AITL):
- Morphologic overlap with interfollicular expansion pattern of IgG4 related lymphadenopathy
- IgG4 positive plasma cells are not typically increased in AITL
- T cells demonstrate a Tfh immunophenotype and clonal TCR gene rearrangement, may demonstrate aberrant loss of pan-T cell markers and are associated with expanded follicular dendritic cell meshworks
- Syphilitic lymphadenitis:
- May closely mimic the morphology of IgG4 related disease and IgG4 related lymphadenopathy, including plasmacytosis, fibrosis and phlebitis
- Treponemal immunohistochemistry demonstrates spirochetes
- Laboratory for syphilis may be useful
- IgG4 positive plasma cells are usually not increased in syphilis but can be in some cases (Am J Surg Pathol 2018;42:472)
- Chronic infections including mastoiditis, aortitis
- Kimura disease:
- More extensive eosinophil infiltrates including eosinophilic microabscesses
- IgE deposition in follicles
- IgG4 positive plasma cells not typically increased in Kimura disease
- Angiolymphoid hyperplasia with eosinophilia / epithelioid hemangioma:
- Nonspecific inflammation adjacent to other tumors:
- Increased IgG4 positive plasma cells can be seen in soft tissue adjacent to other tumors; correlation with imaging and clinical suspicion is needed in such cases to avoid misdiagnosis as IgG4 related disease
Board review style question #1
A 65 year old man presents with bilateral enlargement of the submandibular glands and submandibular lymph nodes. An enlarged submandibular lymph node is excised. What stain is most likely to aid in rendering the correct diagnosis?
- Cyclin D1
- EBER in situ hybridization
- HHV8
- IgG and IgG4 immunostains
- Toxoplasma immunostain
Board review style answer #1
D. IgG and IgG4 immunostains. The photomicrographs show marked fibrosis with a mixed inflammatory cell infiltrate including many plasma cells and eosinophils. Areas of storiform fibrosis are present. Along with the clinical history of bilateral submandibular gland enlargement, the morphologic findings should prompt consideration of the inflammatory pseudotumor-like pattern of IgG4 related lymphadenopathy. IgG4 and IgG immunostains are necessary for further evaluation.
Comment Here
Reference: IgG4 related lymphadenopathy
Comment Here
Reference: IgG4 related lymphadenopathy
Board review style question #2
A 60 year old woman presents with jaundice, a pancreatic mass and abdominal lymphoadenopathy. Biopsy of the pancreas and peripancreatic lymph node demonstrates marked fibrosis with a storiform pattern, frequent plasma cells and obliterative phlebitis in the pancreas. What is the most likely diagnosis?
- Desmoplastic small round cell tumor
- IgG4 related disease and IgG4 related lymphadenopathy
- Lymphoma
- Pancreatic ductal carcinoma
- Pancreatic neuroendocrine tumor
Board review style answer #2
B. IgG4 related disease and IgG4 related lymphadenopathy. The morphologic description, including storiform fibrosis, obliterative phlebitis and plasmacytosis, is classic for IgG4 related disease. Immunohistochemistry for IgG4 and IgG will show increased IgG4 positive plasma cells and IgG4:IgG ratio.
Comment Here
Reference: IgG4 related lymphadenopathy
Comment Here
Reference: IgG4 related lymphadenopathy
IgG4 related lymphadenopathy (pending)
[Pending]
Immune thrombocytopenia (ITP)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Acquired autoimmune bleeding disorder characterized by isolated thrombocytopenia (peripheral blood platelet count < 100 × 109/L) (Blood 2009;113:2386)
- Mixed cell mediated and antibody mediated: abnormal T cell responses resulting in platelet destruction primarily in the spleen, mainly due to antiplatelet autoantibodies (J Clin Med 2017;6:16)
Essential features
- Immune thrombocytopenia (ITP) is a diagnosis of exclusion with an isolated thrombocytopenia of < 100 × 109/L (Blood 2009;113:2386)
- Affected sites predominantly include skin and mucous membranes (petechiae, wet / dry purpura) and spleen (primary site of platelet clearance)
- Impairment in CD4+ T regulatory cells and dendritic cells leads to the initiation and perpetuation of ITP (Curr Opin Hematol 2020;27:423)
- Thrombocytopenia is due to IgG antiplatelet autoantibodies (principle mechanism of antibody mediated platelet phagocytosis), cytotoxic CD8+ T cells and impaired megakaryocyte function (J Clin Med 2017;6:16)
- Primary diagnostic tests include complete blood counts, peripheral blood smear and testing to exclude HIV and hepatitis C virus; antiplatelet antibody testing is among the supportive tests that may be performed (UpToDate: Immune Thrombocytopenia (ITP) in Adults - Clinical Manifestations and Diagnosis [Accessed 16 February 2021])
Terminology
- Formerly known as idiopathic thrombocytopenic purpura
ICD coding
- ICD-10: D69.3 - Immune thrombocytopenic purpura
Epidemiology
- Annual incidence: ~1 - 6 per 100,000 adults (Am J Hematol 2012;87:848)
- Prevalence: ~12 per 100,000 in adults and 8 per 100,000 in children (Am J Hematol 2012;87:848)
- Increased incidence with increasing age (Am J Hematol 2012;87:848, Br J Haematol 2009;145:235)
- Incidence: M = F when > 60 years of age
- Childhood ITP is a clinically distinct condition from adult ITP with a higher likelihood of spontaneous remission
Sites
- Skin:
- Petechiae (flat, red, discrete lesions that do not blanch under pressure), often lower legs in ambulatory patients or sacral area in recumbent patients
- Dry purpura (coalescence of petechiae, nonpalpable)
- Mucous membranes (such as oral mucosa):
- Wet purpura (hemorrhagic blisters)
- Severe bleeding:
- Affects a minority of patients (J Thromb Haemost 2015;13:457)
- Associated with marked thrombocytopenia and new diagnosis of ITP
- Extensive mucosal bleeding often present
- Intracranial hemorrhage uncommon
- Spleen: primary site of platelet clearance in most patients
- Liver, bone marrow or lymph nodes: may also be sites of platelet clearance
Pathophysiology
- Impairment in CD4+ T regulatory cells and dendritic cells leads to the initiation and perpetuation of ITP
- Decreased CD4+ CD25+ FOXP3+ T regulatory cell levels and function in patients with active disease (Curr Opin Hematol 2020;27:423)
- Also dendritic cells and myeloid derived suppressor cells have been described to be impaired in ITP (Curr Opin Hematol 2020;27:423, Blood 2016;127:1587)
- Thrombocytopenia due to:
- IgG antiplatelet autoantibodies (principle mechanism) bind to platelet surface antigens (such as glycoprotein [GP] αIIbβ3 [GPIIbIIIA] and GPIb-IX-V)
- Platelets with bound autoantibodies are subsequently recognized by macrophages bearing Fcγ receptors (FcγRs), resulting in antibody mediated platelet phagocytosis and destruction primarily in the spleen (Haematologica 2021;106:250)
- Cytotoxic CD8+ T cells may directly lyse platelets (Nat Med 2003;9:1123)
- Impaired megakaryocyte function resulting in impaired platelet production due to targeting by antiplatelet autoantibodies or CD8+ T cells in the bone marrow (Haematologica 2015;100:623, Blood 2008;112:1078)
Diagnosis
- ITP is a diagnosis of exclusion; an isolated thrombocytopenia is present (peripheral blood platelet count < 100 × 109/L) without anemia or leukopenia and without another apparent cause of thrombocytopenia
Laboratory
- Complete blood counts, peripheral blood smear: to confirm thrombocytopenia and to exclude platelet morphology abnormalities (e.g. lack of platelet granules or uniformly large or small platelets) suggestive of hereditary platelet disorders or myelodysplasia
- HIV and hepatitis C virus testing: may explain the thrombocytopenia and treating the underlying infection might improve the platelet count
- Additional testing for patients with suspected ITP may include:
- Helicobacter pylori testing: in case of gastrointestinal symptoms suggestive of infection due to association between ITP and H. pylori infection
- Bone marrow investigation: not required for typical presentation
- Normal or increased number of megakaryocytes
- In some patients, a shift towards younger megakaryocytes with hypolobation or lesser degrees of nuclear polyploidy and less evidence of platelet production
- Vitamin B12 and folate levels: required for hematopoiesis and their deficiency may present with mild thrombocytopenia
- Coagulation studies: to investigate and treat other potential causes of thrombocytopenia (e.g. liver disease) and bleeding (e.g. vitamin K deficiency)
- Immunologic studies (e.g. testing for antinuclear antibodies or quantitative immunoglobulin levels to identify underlying common variable immune deficiency)
- Antiplatelet antibody testing (not always detectable)
- Reference: Curr Opin Hematol 2020;27:423, UpToDate: Immune Thrombocytopenia (ITP) in Adults - Clinical Manifestations and Diagnosis [Accessed 16 February 2021]
Prognostic factors
- Adult ITP: majority stable, safe platelet count due to spontaneous remission (up to 10%) or therapy, often within first 6 months (Br J Haematol 2003;122:966)
- Childhood ITP: spontaneous remission (up to 50%) after months or even years of chronic ITP
Case reports
- 3 year old boy and girl with intravenous immunoglobulin refractory ITP receiving chemotherapy for high risk neuroblastoma (J Pediatr Hematol Oncol 2019;41:e257)
- 41 year old woman with anti-GPVI mediated ITP (Res Pract Thromb Haemost 2017;1:291)
- 48 year old man critically ill with COVID-19 (Int J Infect Dis 2020;99:269)
- 65 and 71 year old men presenting with lethal diffuse alveolar hemorrhage (Case Rep Hematol 2019;2019:5170282)
Treatment
- First line includes corticosteroids, intravenous immunoglobulin and anti-D immunoglobulin
- Subsequent treatments include rituximab, splenectomy or thrombopoietin receptor agonists
- Treatment goals: maintaining platelet counts > 20 - 30 × 109/L for at least symptomatic patients (Blood Adv 2019;3:3780)
Microscopic (histologic) description
- Splenic white pulp is prominent, with focal germinal centers and hyperplastic marginal zones
- Red pulp is cellular with histiocytes, activated lymphocytes (immunoblasts) and neutrophils
- Increased number of cordal macrophages, with phagocytosis of (apparent) antibody coated platelets
- Cords of Billroth may have a dirty appearance due to granular platelet debris both within macrophages and extracellularly
- Reference: HemaSphere: The spleen in immune thrombocytopenia [Accessed 22 February 2021]
Microscopic (histologic) images
Sample pathology report
- ITP is a clinical diagnosis of exclusion, typically not based primarily on a pathology specimen.
Differential diagnosis
- ITP is a diagnosis of exclusion; an isolated thrombocytopenia is present (peripheral blood platelet count < 100 × 109/L) without anemia or leukopenia and without another apparent cause of thrombocytopenia such as:
- Drug induced thrombocytopenia, including heparin induced thrombocytopenia
- Infections
- Liver disease
and hypersplenism - Microangiopathic processes
- Hereditary thrombocytopenia
- Myelodysplastic syndromes and other acquired bone marrow disorders
Board review style question #1
A previously healthy 38 year old woman comes to the physician because of petechiae and bruising on her arms and legs for 3 weeks. CBC shows a platelet count of 15 × 109/L as the sole abnormality. What should be the next step for the physician?
- A bone marrow aspirate should be conducted to investigate megakaryocytes for confirmation of diagnosis of ITP
- Other causes of thrombocytopenia should be excluded first to confirm the diagnosis of ITP
- Patient should be started on corticosteroids for treatment of ITP
- Patient should be tested for the presence of antiplatelet autoantibodies to confirm the diagnosis of ITP
Board review style answer #1
B. Other causes of thrombocytopenia should be ruled out first, which includes hereditary platelet disorders, HIV and hepatitis C virus infection.
Bone marrow investigation is not required in cases of typical presentation. It could be supportive as in some ITP patients a shift towards younger megakaryocytes with lesser degrees of nuclear polyploidy and less evidence of platelet production may be seen (answer A). Before considering any treatments for ITP, the diagnosis of ITP should be confirmed first. ITP is a diagnosis of exclusion. An isolated thrombocytopenia should be present (peripheral blood platelet count < 100 × 109/L) without anemia or leukopenia and without another apparent cause of thrombocytopenia (answer C). The presence of antiplatelet autoantibodies could be supportive but is not required for the diagnosis of ITP. These autoantibodies are also not always detectable (answer D).
Comment Here
Reference: Immune thrombocytopenia
Bone marrow investigation is not required in cases of typical presentation. It could be supportive as in some ITP patients a shift towards younger megakaryocytes with lesser degrees of nuclear polyploidy and less evidence of platelet production may be seen (answer A). Before considering any treatments for ITP, the diagnosis of ITP should be confirmed first. ITP is a diagnosis of exclusion. An isolated thrombocytopenia should be present (peripheral blood platelet count < 100 × 109/L) without anemia or leukopenia and without another apparent cause of thrombocytopenia (answer C). The presence of antiplatelet autoantibodies could be supportive but is not required for the diagnosis of ITP. These autoantibodies are also not always detectable (answer D).
Comment Here
Reference: Immune thrombocytopenia
Board review style question #2
Which of the following is true regarding the bleeding disorder ITP?
- Certain drugs can trigger the onset of ITP
- Cytotoxic CD8+ T cells may play a pathogenetic role in platelet breakdown
- Platelet breakdown occurs predominantly in the bone marrow
- CD4+ CD25+ FOXP3+ T regulatory cells levels are increased in number and function in active disease
Board review style answer #2
B. Not only antiplatelet autoantibodies but also cytotoxic CD8+ T cells can be responsible for the platelet destruction.
When drugs trigger the onset of ITP, the diagnosis is drug induced thrombocytopenia, which should always be considered in the differential diagnosis of ITP (answer A). While the spleen is the primary site for platelet destruction, platelet clearance can also occur in liver, bone marrow or lymph nodes. ITP may still (re)occur after splenectomy (answer C). There is an impairment in CD4+ T regulatory cells which leads to the initiation and perpetuation of ITP, so CD4+ CD25+ FOXP3+ T regulatory cells are decreased in number and function in patients with active disease (answer D) (Curr Opin Hematol 2020;27:423).
Comment Here
Reference: Immune thrombocytopenia
When drugs trigger the onset of ITP, the diagnosis is drug induced thrombocytopenia, which should always be considered in the differential diagnosis of ITP (answer A). While the spleen is the primary site for platelet destruction, platelet clearance can also occur in liver, bone marrow or lymph nodes. ITP may still (re)occur after splenectomy (answer C). There is an impairment in CD4+ T regulatory cells which leads to the initiation and perpetuation of ITP, so CD4+ CD25+ FOXP3+ T regulatory cells are decreased in number and function in patients with active disease (answer D) (Curr Opin Hematol 2020;27:423).
Comment Here
Reference: Immune thrombocytopenia
Indeterminate cell histiocytosis / indeterminate dendritic cell tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Flow cytometry description | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Indeterminate cell histiocytosis is an extremely rare neoplastic proliferation of cells of dendritic / histiocytic lineage that share immunophenotypic features of Langerhans cells but lack Birbeck granules and langerin expression
Essential features
- Skin involvement can be solitary or multifocal
- Usually restricted to skin, with rare organ or lymph node involvement
- Indeterminate cells are histopathologically and antigenically similar to Langerhans cells but lack Birbeck granules and langerin (CD207) expression
- Clinical course varies: spontaneous regression, stable disease, rapid progression or recurrence
Terminology
- Indeterminate cell histiocytosis
- Indeterminate cell tumor
- Indeterminate dendritic cell tumor
ICD coding
- ICD-10: D76.3 - other histiocytosis syndromes
Epidemiology
- Rare neoplasm, with 85 cases reported between 1985 - 2016 (Am J Dermatopathol 2018;40:736)
- Reported in all age groups, with a median age of 45
- Near equal M:F
Sites
- Cutaneous involvement (88%) is most common (Am J Dermatopathol 2018;40:736)
- Lymph node (9%) and spleen (2.3%) involvement less common (Am J Dermatopathol 2018;40:736)
Etiology
- Etiology remains uncertain
- Multiple hypotheses on the relationship between indeterminate cells and Langerhans cells, including:
- Indeterminate cells are Langerhans cells that have lost their Birbeck granules
- Indeterminate cells are immature Langerhans cells
- A subset of cases express dendritic cell marker ZBTB46, raising the possibility that a subset of cases arise directly from bone marrow progenitors as opposed to embryonic precursors that locally renew in the skin (Mod Pathol 2018;31:1479)
- Association between other hematologic neoplasms has been reported (including B cell lymphoma, T cell lymphoma, acute myeloid leukemia and chronic myelomonocytic leukemia) (Am J Surg Pathol 2008;32:1868, J Cutan Pathol 2017;44:958, Am J Dermatopathol 2019;41:461, Dermatol Res Pract 2010;2010:569345)
- Indeterminate cell histiocytosis can share clonal abnormality of associated lymphoma or leukemia (Am J Surg Pathol 2008;32:1868)
Clinical features
- Reported in all age groups, with a median age of 45
- Near equal M:F patients
- Single cutaneous papulonodule or multiple lesions (few to innumerable)
- Can have plaques and tumors with leonine facies (J Cutan Pathol 2016;43:158)
- Usually restricted to skin with rare organ or lymph node involvement
- Clinical course varies: spontaneous regression, stable disease, rapid progression or recurrence
- Reference: Am J Dermatopathol 2018;40:736
Diagnosis
- Skin biopsy for localized lesions
- Systemic workup to exclude deep involvement
- CT scans and bone marrow biopsy
Laboratory
- Can have elevated lactate dehydrogenase, erythrocyte sedimentation rate and C reactive protein (Serbian J Dermatology Venereol 2018;10:18)
Radiology description
- True cutaneous indeterminate cell histiocytosis typically lacks evidence of deep organ involvement on imaging
Prognostic factors
- Cases restricted to the skin typically behave indolently
- Involvement of deep or visceral tissues or bone marrow may portend worse prognosis
Case reports
- 6 year old girl with polypoid and verrucoid lesions of the vulva (Am J Dermatopathol 2018;40:736)
- 13 year old girl with pityriasis rosea type rash (Acta Derm Venereol 2002;82:288)
- 28 year old man presented after a mosquito bite (Medicine (Baltimore) 2015;94:e1443)
- 48 year old woman with a later diagnosis of mycosis fungoides (Am J Dermatopathol 2019;41:461)
- 55 year old woman with a history of burning and confluent erythematous papules on the face and arms (Cureus 2021;13:e12850)
- 55 year old woman presented with leonine facies (J Cutan Pathol 2016;43:158)
- 55 year old man with complete response to ultraviolet phototherapy (J Cutan Pathol 2017;44:958)
- 61 year old man with recurrence after excision (J Pathol Transl Med 2018;52:243)
- 72 year old man with chronic myelomonocytic leukemia (J Cutan Pathol 2017;44:958)
- 74 year old woman with a prior history of follicular lymphoma demonstrating t(14;18) translocation (Am J Surg Pathol 2008;32:1868)
- 90 year old man with aggressive case mimicking angiosarcoma (Ann Dermatol 2017;29:614)
Treatment
- Surgical excision for isolated lesions
- Psoralen and ultraviolet A therapy (PUVA) (J Cutan Pathol 2017;44:958, Autops Case Rep 2016;6:33, Kaohsiung J Med Sci 2004;20:24)
- Chemotherapy can be considered for more aggressive disease or those cases associated with concurrent myeloid or lymphoid neoplasm
Clinical images
Microscopic (histologic) description
- Dermal infiltration by pale eosinophilic histiocytic cells with reniform nuclei
- Background reactive lymphocytes
- Scant to absent eosinophils
- Can have multinucleated cells
- Spindled morphology is less common
- References: Am J Dermatopathol 2018;40:736, Blood 2015;126:2344, Arch Pathol Lab Med 2003;127:748, Am J Surg Pathol 2008;32:1868, Cureus 2021;13:e12850
Microscopic (histologic) images
Positive stains
- Positive for S100, CD1a, CD68
- Variably express CD4, CD45, CD56, lysozyme
- References: Am J Dermatopathol 2018;40:736, Blood 2015;126:2344, Arch Pathol Lab Med 2003;127:748, Am J Surg Pathol 2008;32:1868
Negative stains
- Negative for langerin (CD207)
- Negative for histiocytic marker CD163
- B cell and T cell markers, follicular dendritic cell markers (CD21, CD23 and CD35), CD30 and MPO
- References: Am J Dermatopathol 2018;40:736, Blood 2015;126:2344, Arch Pathol Lab Med 2003;127:748, Am J Surg Pathol 2008;32:1868
Flow cytometry description
- No known abnormalities in peripheral blood flow cytometry in true cutaneous indeterminate cell histiocytosis
- For flow cytometry information for cases of chronic myelomonocytic leukemia with an indeterminate cell-like immunophenotype, please refer to chronic myelomonocytic leukemia
Electron microscopy description
- Histiocytic cells show nuclear infoldings with peripheral villi (Rare Tumors 2013;5:e13)
- Lamellated dense bodies but no Birbeck granules (Acta Derm Venereol 2002;82:288)
Molecular / cytogenetics description
- ETV3::NCOA2 in a subset (Blood 2015;126:2344, BMJ Case Rep 2017;2017:bcr2017221538)
- BRAF V600E in rare cases (Ann Diagn Pathol 2015;19:113, Virchows Arch 2017;471:467)
- Can share clonal abnormality of associated lymphoma or leukemia (Am J Surg Pathol 2008;32:1868)
- TP53 in 1 case (Leukemia 2022;36:573)
Sample pathology report
- Skin, left arm, biopsy:
- Compatible with indeterminate cell histiocytosis (see comment)
- Comment: The presence of histiocytes that express CD1a without langerin is compatible with a diagnosis of indeterminate cell histiocytosis (ICH). The definitive etiology of ICH is not known. ICH may represent a distinct entity, as suggested by the detection of ETV3::NCOA2 in a subset of cases or a variant of Langerhans cell histiocytosis (LCH) that has lost its Birbeck granules (Blood 2015;126:2344). Furthermore, chronic myelomonocytic leukemia (CMML) presenting with skin lesions rarely may mimic the immunohistochemical phenotype of ICH (J Cutan Pathol 2017;44:1075). If clinically indicated, correlation with genomic sequencing to assess for molecular aberrancies characteristic of ICH, LCH or CMML is recommended.
- Microscopic description: The histologic sections show skin with a nodular infiltrate of epithelioid cells with abundant eosinophilic cytoplasm, vesicular and variably reniform nuclei and prominent nucleoli present in sheets in the superficial and mid-dermis. On immunohistochemical staining, the lesional cells are positive for CD1a, S100 and CD68 and are negative for langerin, MPO and BRAF V600E.
Differential diagnosis
- Langerhans cell histiocytosis:
- CD1a+, langerin (CD207)+ (100%), S100+, CD68+, BRAF VE1 (~50%)
- Juvenile xanthogranuloma:
- CD1a-, langerin (CD207)-, S100 variable, CD68+
- Multicentric reticulohistiocytosis:
- CD1a-, langerin (CD207)-, S100 variable, CD68+
- Interdigitating dendritic cell sarcomas:
- CD1a-, langerin (CD207)-, S100+, SOX10+
- Histiocytic sarcoma:
- Significant cytologic atypia, CD1a-
- Chronic myelomonocytic leukemia secondarily involving the skin:
- Can have identical morphologic and immunohistochemical findings, correlate with molecular studies (J Cutan Pathol 2017;44:1075)
- Reactive indeterminate cell infiltrates:
Board review style question #1
Which of the following electron microscopy findings is most consistent with a diagnosis of indeterminate cell histiocytosis (ICH)?
- Lack of Birbeck granules
- Presence of Birbeck granules
- Presence of characteristic cytoplasmic inclusions
- Proliferation of Langerhans cells
Board review style answer #1
A. Lack of Birbeck granules. Electron microscopy shows a lack of Birbeck granules in indeterminate cell histiocytosis.
Comment Here
Reference: Indeterminate cell histiocytosis / indeterminate dendritic cell tumor
Comment Here
Reference: Indeterminate cell histiocytosis / indeterminate dendritic cell tumor
Board review style question #2
Board review style answer #2
D. CD207 (langerin) positivity in the Langerhans cells is specific for this disorder. Langerhans cells also typically stain for S100, CD1a and CD68 but CD1a is also expressed in indeterminate cell histiocytosis and CD68 and S100 can be seen in other histiocytic neoplasms. CD45 is a nonspecific leukocyte marker. CD20 is a B cell marker.
Comment Here
Reference: Indeterminate cell histiocytosis / indeterminate dendritic cell tumor
Comment Here
Reference: Indeterminate cell histiocytosis / indeterminate dendritic cell tumor
Indolent T lymphoblastic proliferations
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign expansion of immature terminal deoxynucleotidyl transferase (TdT) positive T cells in extramedullary and extrathymic tissues
- Can be seen concurrently with other hematologic disorders or epithelial malignancies, such as Castleman disease, follicular dendritic cell sarcomas, angioimmunoblastic T cell lymphoma, hepatocellular carcinoma, acinic cell carcinoma, etc.
Essential features
- Benign proliferation of extrathymic immature T cells that lack significant morphologic atypia but mimic malignant T lymphoblastic lymphoma / leukemia
- Nonclonal process without evidence of TCR γ or β gene rearrangements
- Lack of aberrant immunophenotypic or karyotypic abnormalities
Terminology
- Immature T lymphoblastic proliferation
- iT-LBP
Epidemiology
- Age, gender, ethnicity, incidence / prevalence are undefined at this point
Sites
- Typically presents with localized lymphadenopathy or a discrete mass
- Common anatomical locations: mandible, cervical, supraclavicular, abdominal, retroperitoneal and oropharyngeal regions (Adv Anat Pathol 2013;20:137)
- Rare cases have shown disseminated multinodal involvement (Am J Surg Pathol 2014;38:1298)
- Does not involve mediastinum
- Peripheral blood and bone marrow are not involved
Clinical features
- Clinical presentation varies but patients are generally asymptomatic and healthy
- Can be associated with other underlying neoplastic pathologic conditions, such as carcinomas, Castleman disease, follicular dendritic cell tumors and angioimmunoblastic T cell lymphoma (Am J Surg Pathol 2012;36:1619)
Diagnosis
- Major criteria (Adv Anat Pathol 2013;20:137):
- No aberrant antigen expression (immunophenotype is of normal immature cortical thymocytes)
- Nonclonal TdT+ T cells
- No evidence of T cell gene rearrangements (TCR α and TCR β)
- Clinical evidence of indolence, > 6 months of follow up without progression in the absence of treatment
Prognostic factors
- Clinically indolent
- May persist in the involved tissue
Case reports
- 35 year old man with indolent T lymphoblastic proliferation associated with low grade follicular dendritic cell sarcoma and Castleman disease (Pathology 2018;50:351)
- 37 year old woman with an indolent T lymphoblastic proliferation involving the upper aerodigestive tract (Int J Clin Exp Pathol 2014;7:6350)
- 41 year old woman with pelvic pain found to have localized Castleman disease with concomitant iT-LBP (Ann Pathol 2019;39:29)
Treatment
- Typical lesions show no signs of progression and require no treatment
- Treatment for secondary disease (if present) may be needed
Gross description
- Typically forms a tumor mass, varies in size from ≤ 1 cm to ≥ 9 cm in greatest dimension
- External surface may be firm and fibrous or nonencapsulated
- Displays pink-tan firm fleshy cut surfaces
- Necrotic (yellow) areas may be seen in cases associated with hepatocellular carcinoma (HCC)
- Reference: Adv Anat Pathol 2013;20:137
Microscopic (histologic) description
- Preservation of overall tissue architecture (Adv Anat Pathol 2013;20:137)
- Lymph nodes: benign lymphoblasts localize predominantly to interfollicular / paracortical regions
- Carcinomas: lymphoblasts are interspersed or clustered between malignant epithelial cells without definitive evidence of lymphoepithelial lesions
- Small to intermediate sized lymphoid cells with immature fine / blastoid chromatin lacking significant atypia
- Inconspicuous nucleoli
- Numerous mitotic figures may be present
- Histiocytes are interspersed evenly throughout the proliferation
Microscopic (histologic) images
Cytology description
- Small to intermediate sized monotonous population with fine dispersed chromatin pattern and scant cytoplasm; occasional variably sized nucleoli may be present
Positive stains
Flow cytometry description
- Incidental benign TdT+ T cell populations may be detected by flow cytometry
- Immunophenotype can show the entire range of T cell differentiation from the most immature dual CD4- / CD8- / CD7 bright+ / CD34+ / cytoplasmic CD3+ / surface CD3- forms to surface CD3+ forms with coexpression of CD4 and CD8 (Cytometry B Clin Cytom 2020;98:282)
- Proportion of T lymphoblasts (identified by cytoplasmic CD3 and TdT expression) can vary from < 1% of events to > 50% of events
Molecular / cytogenetics description
- iT-LBP have nonclonal T cell receptor (TCR) gene rearrangement studies (TCR γ or β)
- No definitive chromosomal abnormalities
- Initial reported case reported chromosome 9 inversion of uncertain significance (Am J Surg Pathol 1999;23:977)
Sample pathology report
- Lymph node, inguinal, excision:
- Castleman disease, hyaline vascular variant
- Indolent T lymphoblastic proliferation (see comment)
- Comment: Sections from the inguinal node show predominantly preserved nodal architecture with atretic follicles and increased vascularity with a subset of hyalinized vessels penetrating regressed germinal centers. Interspersed within the paracortical areas are increased CD3+ / TdT+ positive T cells without aberrant antigen expression. The presence of immature T cells in association with hyaline vascular Castleman disease has been reported and typically shows indolent behavior. Clinical correlation is recommended. (Am J Surg Pathol 2012;36:1619)
Differential diagnosis
- T lymphoblastic leukemia / lymphoma:
- Typically effaces nodal architecture or will infiltrate relatively nondiscriminately throughout entire node obliterating residual follicles
- Usually expresses LMO2 on malignant lymphoblasts (Histopathology 2020;77:984)
- Ectopic thymic tissue:
- Thymoma:
- Lobulated architecture
- Presence of thymic epithelial meshworks
- No expression of LMO2 on lymphoblasts
- Presence in the mediastinum
Additional references
Board review style question #1
Which of the following antigens do indolent T lymphoblastic proliferations lack?
- CD3
- CD20
- CD33
- CD45
- TdT
Board review style answer #1
B. CD20. CD20 is a B cell marker which is not expressed by T lymphoblasts. CD3 is incorrect as it is a T cell marker expressed by indolent T lymphoblastic proliferations. CD33 is a myeloid associated marker which has been described as expressed by a subset of indolent T lymphoblastic proliferations. CD45 is a pan-hematopoietic cell marker which is expressed by indolent T lymphoblastic proliferations. TdT is incorrect as it is an immature marker expressed by indolent T lymphoblastic proliferations.
Comment Here
Reference: Indolent T lymphoblastic proliferations
Comment Here
Reference: Indolent T lymphoblastic proliferations
Board review style question #2
Board review style answer #2
B. Indolent T lymphoblastic proliferation. This image shows a lymph node typical of Castleman disease. Indolent T lymphoblastic proliferations have been described in association with Castleman disease. Chronic lymphadenitis is incorrect as it is not a disease well known to occur in association with Castleman disease. Kikuchi-Fujimoto disease is incorrect as this is a necrotizing lymphadenitis of unknown etiology and association. Kimura disease is incorrect as it would show numerous eosinophils and eosinophilic granulomas and is not associated with Castleman disease. Rosai-Dorfman disease is incorrect as it shows large histiocytic cells with round to ovoid nuclei and distinct central nucleoli that exhibit emperipolesis.
Comment Here
Reference: Indolent T lymphoblastic proliferations
Comment Here
Reference: Indolent T lymphoblastic proliferations
Infectious mononucleosis
Table of Contents
Definition / general | Case reports | Clinical images | Microscopic (histologic) description | Positive stains | Differential diagnosisDefinition / general
- May cause spontaneous splenic rupture and death, often 10 - 21 days after disease onset
- Rupture may be due to increased intrasplenic pressure due to congestion and weakening of splenic capsule from infiltration by immunoblasts
Case reports
- 16 year old girl with spontaneous splenic rupture (Int J Surg Case Rep 2012;3:97)
Microscopic (histologic) description
- Expansion of red pulp with immunoblastic proliferation
- Immunoblasts may have strikingly atypical morphology, resemble Reed-Sternberg cells, infiltrate subintima of intratrabecular veins, occasionally exhibit hemophagocytosis
Positive stains
Differential diagnosis
- Hodgkin lymphoma
- Leukemia
- Non-Hodgkin lymphoma
Interdigitating dendritic cell / reticulum cell sarcoma
Table of Contents
Definition / general | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
- Also called interdigitating dendritic cell tumor
- Very rare
- Usually arises in lymph nodes with widespread lymphadenopathy but also in bladder, bowel, intestine, nasopharynx, salivary glands, skin, spleen, testis and tonsils
- Aggressive behavior, with half dying of disease after an average of 7 months
- Have same morphologic and immunohistochemical features as nonneoplastic interdigitating dendritic cells
- Presence of another hematologic neoplasm is common
Case reports
- 20 year old woman with multifocal lymphadenopathy (Arch Pathol Lab Med 1994;118:183)
- 30 year old woman with cervical nodal involvement (Saudi Med J 2002;23:1281)
- 38 year old woman with cervical lymph node and breast involvement (Virchows Arch 2005;446:546)
- 41 year old man with cervical lymph node involvement (Zhonghua Xue Ye Xue Za Zhi 2005;26:232)
- 79 year old man with coexisting SLL in axillary node (Arch Pathol Lab Med 2006;130:544)
Treatment
- Chemotherapy (Leuk Lymphoma 2002;43:817)
Microscopic (histologic) description
- Fascicles or whorls of paracortical tumor cells that spare the follicles and sinuses
- Tumor cells are large and pleomorphic with abundant pale, eosinophilic cytoplasm and bizarre, grooved nuclei
- May have tumor giant cells, mitotic activity, inflammatory infiltrate
Positive stains
Electron microscopy description
- Interdigitating cytoplasmic processes but no desmosomes or other junctional complexes, no Birbeck granules
Differential diagnosis
Additional references
Interdigitating dendritic cell tumor
Table of Contents
Definition / general | Epidemiology | Sites | Pathophysiology | Clinical features | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Differential diagnosisDefinition / general
- Rare tumor of hematopoietic origin arising from interdigitating dendritic cells (IDCs), a type of immune accessory cell found in lymphoid and nonlymphoid organs
- IDCs present antigens to T cells and regulate cellular immune responses (Crit Rev Oncol Hematol 2013;88:253)
- Usually arises in lymph nodes (Am J Surg Pathol 1999;23:1141)
Epidemiology
- IDC tumors have been reported in all age groups (median age 56 years)
Sites
- Can be nodal or extranodal; have been described throughout the body
- Only two reported cases of testicular involvement
Pathophysiology
- Dysregulation of immune system may facilitate malignant transformation of IDCs (Crit Rev Oncol Hematol 2013;88:253)
- Recent studies show a clonal relationship between IDC tumors and low grade B cell lymphomas in patients harboring both diseases synchronously or metachronously
- A group of IDC tumors likely develops secondarily after the malignant transformation and trans differentiation of B cells, rather than transformation of IDCs themselves
- IDC tumors have developed following the use of calcineurin inhibitors, tacrolimus and pimecrolimus; these drugs dampen the responses of T cells to which IDCs present antigens
Clinical features
- Painless testicular mass
Laboratory
- Serum tumor markers for testicular germ cell tumors (hCG and alpha fetoprotein) are normal
Radiology description
- Testicular ultrasound is imaging modality of choice for a scrotal mass (Gordetsky: Gordo's Guide to GUPathology: A Resource for Urology and Pathology Residents, 2013)
Prognostic factors
- Worse outcomes seen in younger patients and patients with intraabdominal involvement
- Stage at presentation is most important prognostic factor (Crit Rev Oncol Hematol 2013;88:253)
Case reports
- 37 year old man with painless testicular mass (Int J Surg Pathol 2011;19:104)
- 74 year old man with a painless unilateral testicular mass (Am J Surg Pathol 1999;23:1141)
Treatment
- Surgery with adjuvant chemotherapy
Gross description
- Light tan, solid, may replace entire testis
Microscopic (histologic) description
- Whorls and fascicles of spindle cells mixed with small lymphocytes
Microscopic (histologic) images
Negative stains
Electron microscopy description
- Complex interdigitating cytoplasmic dendritic processes, abundant rough endoplasmic reticulum, abundant mitochondria
Differential diagnosis
- Germ cell tumors with a sarcomatous component
- Sex cord stromal tumors
- Infiltration by paratesticular spindle cell neoplasms
- Metastasis
Kawasaki disease
Table of Contents
Definition / general | Treatment | Microscopic (histologic) description | Additional referencesDefinition / general
- Also called mucocutaneous lymph node syndrome
- Febrile disorder of unknown etiology usually affecting children
- High incidence (39 per 100K children below age 5) in Hong Kong (Hong Kong Med J 2005;11:331)
- Fever, pharyngitis and conjunctivitis, erythematous skin rashes, cervical adenopathy (25%); also arthritis (40%), coronary arteritis with persistent damage (15 - 25%) which may cause death
Treatment
- IV gamma globulin, high dose aspirin
Microscopic (histologic) description
- Fibrin thrombin in smaller vessels with patchy infarcts (Am J Surg Pathol 1982;6:493)
Additional references
Kikuchi disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign, self limited lymphadenitis of unknown etiology with distinct histopathologic features
Essential features
- Benign, self limited lymphadenitis of unknown etiology
- Predominantly affects young adults, with cervical lymphadenopathy, fever and often leukopenia
- Involved lymph nodes show histiocytes, plasmacytoid dendritic cells, immunoblasts and necrosis with karyorrhectic debris
- Histologic features overlap with autoimmune disease associated lymphadenitis, especially systemic lupus erythematosus
- Clinically and histologically may mimic lymphoma
Terminology
- Kikuchi-Fujimoto disease
- Kikuchi lymphadenitis
- Histiocytic necrotizing lymphadenitis
- Subacute necrotizing lymphadenitis
ICD coding
- ICD-10: I88 - nonspecific lymphadenitis
Epidemiology
- M:F = 1:1 - 4
- Primarily young adults (average 20 - 30 years) but any age may be affected
- Initially described in Asia; now known to occur worldwide
- References: Semin Diagn Pathol 1988;5:329, Int J Infect Dis 2009;13:322, Acta Pathol Jpn 1993;43:635, Am J Surg Pathol 1995;19:798, Otolaryngol Head Neck Surg 2003;128:650, Clin Rheumatol 2007;26:50, Semin Arthritis Rheum 2012;41:900, Cureus 2020;12:e7383
Sites
- Cervical lymph nodes, especially posterior
- Other lymph nodes less commonly involved (axillary, mediastinal, abdominal, inguinal)
- Rarely, extranodal sites, including skin
Pathophysiology
- Necrosis is due to apoptotic cell death mediated by cytotoxic T cells (Acta Otolaryngol Suppl 1998;538:250, Mod Pathol 1997;10:231)
Etiology
- Etiology unknown
- May represent a T cell and histiocyte mediated immune reaction to an infectious agent; however, no definitive causal relationship with a specific infection established (Pathologica 2016;108:120, Cancer Control 2014;21:313, Clin Rheumatol 2007;26:50)
- May represent a self limited autoimmune process (Semin Diagn Pathol 1988;5:329, Semin Arthritis Rheum 2012;41:900)
- May be more likely in genetically predisposed individuals: HLA class II alleles DPA1*01 and DPB1*0202 more frequent among Japanese patients with Kikuchi disease than controls (Tissue Antigens 1999;54:246)
Clinical features
- Acute or subacute onset
- Lymphadenopathy (typically painful), usually cervical; may affect lymph nodes at other sites or be generalized
- Fever is common
- Additional symptoms may include weight loss, night sweats, fatigue, malaise, erythematous rash, headache, upper respiratory tract symptoms, joint pain, diarrhea, nausea, vomiting (J Microbiol Immunol Infect 2010;43:366, Pathologica 2016;108:120, Clin Rheumatol 2007;26:50, Semin Diagn Pathol 1988;5:329, Int J Infect Dis 2009;13:322)
- May co-occur with or precede a diagnosis of systemic lupus erythematosus (2 - 23% of patients) or another autoimmune condition (Sjögren syndrome, polymyositis, rheumatoid arthritis, vasculitis, antiphospholipid syndrome, Still disease)
- If associated with systemic lupus erythematosus, may be best diagnosed as lymphadenitis associated with systemic lupus erythematosus (Semin Arthritis Rheum 2012;41:900, Case Rep Hematol 2018;2018:1791627, Int J Infect Dis 2009;13:322, Otolaryngol Head Neck Surg 2003;128:650)
Diagnosis
- Lymph node excision with pathologic examination
Laboratory
- No definitive diagnostic laboratory test
- Leukopenia
- Leukocytosis, especially in young children (Cureus 2020;12:e7383)
- Anemia
- Elevated lactate dehydrogenase
- Elevated erythrocyte sedimentation rate; elevated C reactive protein
- Antinuclear antibodies (ANA), anti-DNA antibodies and rheumatoid factor (RF) typically negative
- Viral serologies typically negative
- References: Otolaryngol Head Neck Surg 2003;128:650, Semin Diagn Pathol 1988;5:329, Semin Arthritis Rheum 2012;41:900, Clin Rheumatol 2007;26:50, Pathologica 2016;108:120, J Microbiol Immunol Infect 2010;43:366
Prognostic factors
- Usually self limited; resolves within 1 - 6 months
- May recur (3 - 21% of patients) (Am J Surg Pathol 1995;19:798, Am J Surg Pathol 1994;18:219, Int J Infect Dis 2009;13:322, Semin Diagn Pathol 1988;5:329, J Microbiol Immunol Infect 2010;43:366)
- Rare fatalities reported (Pathologica 2016;108:120, Cancer 1989;63:1856, Clin Rheumatol 2007;26:50)
Case reports
- 20 month old boy with enlarged axillary lymph node and rash (Cureus 2020;12:e7383)
- 4 year old boy with Kikuchi disease and hemophagocytic lymphohistiocytosis (Medicine (Baltimore) 2020;99:e23500)
- 20 year old man with fevers and cervical adenopathy (Blood 2017;129:917)
- 38 year old woman with cervical lymphadenopathy (Head Neck Pathol 2020;14:272)
Treatment
- Observation or supportive care (for example, nonsteroidal anti-inflammatory drugs) (Arch Pathol Lab Med 2010;134:289, Arch Pathol Lab Med 2018;142:1341)
- May include corticosteroid therapy (J Laryngol Otol 2000;114:709, Medicine (Baltimore) 2014;93:372)
- Followup is recommended, along with relevant laboratory studies, if indicated, to monitor for development of systemic lupus erythematosus
Microscopic (histologic) description
- Partial or complete lymph node involvement by irregularly shaped, pale areas composed of histiocytes, plasmacytoid dendritic cells, eosinophilic granular material and abundant karyorrhectic debris (nuclear dust), often surrounding a central zone of overt necrosis
- Karyorrhectic areas may extend beyond nodal capsule or involve residual germinal centers
- Regions between pale areas include small lymphocytes admixed with immunoblasts and clusters of plasmacytoid dendritic cells, causing a mottled or starry sky appearance
- Histiocytes include phagocytic cells with eosinophilic cytoplasm and crescent shaped nuclei (crescentic histiocytes) and nonphagocytic cells with twisted or reniform nuclei (Am J Surg Pathol 1994;18:219)
- Plasmacytoid dendritic cells are intermediate in size with amphophilic cytoplasm, round nuclei, small nucleoli; difficult to recognize on H&E (Diagn Cytopathol 2010;38:521)
- No neutrophils; rare to absent plasma cells (Clin Rheumatol 2007;26:50)
- Overt necrosis not required for diagnosis (Am J Surg Pathol 1994;18:219)
- 3 stages described based on histology (Am J Surg Pathol 1995;19:798, Pathol Int 2004;54:237):
- Proliferative: proliferation of plasmacytoid dendritic cells, immunoblasts and histiocytes
- Necrotizing: areas of necrosis with karyorrhectic debris
- Xanthomatous: foamy histiocytes predominate; may represent histologic variant
Microscopic (histologic) images
Contributed by Elizabeth Courville, M.D. and Amy Beckman, M.D.
Lymph node from a 38 year old woman:
Cervical lymph node from a 40 year old woman:
AFIP images
Virtual slides
Cytology description
- Phagocytic histiocytes with peripheral nuclei; plasmacytoid dendritic cells
Positive stains
- CD3, CD4, CD8 highlight abundant T cells; CD8+ T cells > CD4+ T cells
- Lysozyme, CD4 (weak) are positive in histiocytes
- CD4 (weak), CD123 (bright) and TCL1 highlight plasmacytoid dendritic cells (Diagn Cytopathol 2010;38:521)
- CD20 stains B cells present in uninvolved areas but absent from karyorrhectic areas
- Myeloperoxidase: may stain histiocytes; emphasizes absence of granulocytes / neutrophils
Negative stains
- Fite acid fast stain, Gomori methenamine silver (GMS)
- HSV, CMV, in situ hybridization for EBV encoded RNA (EBER ISH)
- Reference: Arch Pathol Lab Med 2018;142:1341
Electron microscopy description
- Tubuloreticular structures present in cytoplasm of immunoblasts, endothelial cells and histiocytoid cells (Am J Pathol 1982;107:292)
- Cells at various stages of apoptosis; histiocytes containing phagocytosed karyorrhectic debris / apoptotic bodies (Acta Otolaryngol Suppl 1998;538:250, Acta Pathol Jpn 1993;43:635)
Electron microscopy images
Sample pathology report
- Lymph node, cervical, excision:
- Necrotizing lymphadenitis (see comment)
- Comment: The findings favor a diagnosis of Kikuchi disease, a benign and typically self limited lymphadenitis. There is no evidence of lymphoma. Histologically, changes associated with autoimmune disease may mimic those seen in Kikuchi disease. Clinical correlation, along with evaluation for a possible autoimmune disease, such as systemic lupus erythematosus, is recommended.
- Microscopic description: H&E stained sections show a lymph node with architecture distorted by pale areas composed of eosinophilic, granular material, karyorrhectic debris and interspersed lymphocytes and histiocytes. These areas are surrounded by histiocytic cells (some with twisted nuclei and others with so called crescentic nuclei) and cells with morphology suggestive of plasmacytoid dendritic cells. Outside the pale areas, there is an expansion of small lymphocytes admixed with immunoblasts. Residual primary and secondary follicles are present but neutrophils are not seen and plasma cells are rare to absent. Neither hematoxylin bodies nor aggregates of large atypical lymphocytes are identified. Immunohistochemical stains are performed with appropriate controls. CD3 highlights numerous T cells; CD8 positive cells outnumber CD4 positive cells. CD20 stains residual nodules of B cells. CD68 stains histiocytes within and adjacent to karyorrhectic areas. CD123 highlights plasmacytoid dendritic cells, which surround and form clusters outside of karyorrhectic areas. CD4 shows weak staining in both histiocytes and plasmacytoid dendritic cells.
Differential diagnosis
- Lupus lymphadenitis:
- Infectious lymphadenitis:
- Neutrophils, granulomatous inflammation, viral cytopathic changes
- Lymphoma (e.g., diffuse large B cell lymphoma, peripheral T cell lymphoma, NOS, Hodgkin lymphoma):
- Nodal effacement by monotonous or atypical lymphocytes or presence of Reed-Sternberg / Hodgkin cells
- Pattern of staining seen with a panel of immunohistochemical stains (for example, CD3, CD20, CD30, PAX5) and interpreted in the context of morphology on routine hematoxylin and eosin stain is important in making the distinction from Kikuchi disease
Additional references
Board review style question #1
Which disease shows similar morphologic and immunohistochemical features to Kikuchi lymphadenitis?
- Classic Hodgkin lymphoma
- Rheumatoid arthritis
- Systemic lupus erythematosus
- Toxoplasmosis
Board review style answer #1
Board review style question #2
Board review style answer #2
Kimura disease
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign chronic inflammatory disorder of unknown etiology (Am J Surg Pathol 2004;28:505)
- Eosinophilic infiltrates forming microabscesses in a background of polymorphous inflammatory cells
Essential features
- Benign disorder of unknown etiology with an indolent clinical course
- Predominantly affects young Asian males
- Involves nodal and extranodal sites, most commonly skin, head and neck
- Commonly recurs postsurgical excision
Terminology
- First described in 1937 by Kimm and Szeto as eosinophilic hyperplastic lymphogranuloma
- In 1948, Kimura described it as "unusual granulation combined with hyperplastic changes in lymphoid tissue"; subsequently, this process was widely known as Kimura disease (Am J Otolaryngol 2017;38:626)
ICD coding
- ICD-10: D21.9 - Kimura disease
Epidemiology
- Predominantly affects males
- Peak incidence between ages 20 - 40 years (Int J Oral Maxillofac Surg 2017;46:350)
- Most common in Asians
Sites
- Nodal and extranodal
- Extranodal commonly includes skin and subcutaneous tissue, head and neck sites, including major salivary glands
Pathophysiology
- Th1 / Th2 cytokine imbalance has been postulated as an underlying pathogenesis
- Th2 cells may result in increased IgE production through interleukins (IL4 and IL5) and promote activation of eosinophils
- Reference: Auris Nasus Larynx 2011;38:77
Etiology
- Cause unknown
- Presumed to be a consequence of an infection triggering an abnormal immune response
Clinical features
- Insidious onset with an indolent clinical course
- Slow growing nodules / masses, often painless and nontender
- Systemic symptoms uncommon
- Nephrotic syndrome may occur in a subset (Ren Fail 2019;41:126)
- Recurrence is common after surgical excision
Diagnosis
- Morphologic diagnosis characterized by hyperplastic reactive follicles surrounded by eosinophilic infiltrates in a background of polymorphous inflammatory cells
Laboratory
- Elevated serum immunoglobulin IgE levels (Arch Pathol Lab Med 2007;131:650)
- Peripheral blood eosinophilia (Am J Otolaryngol 2017;38:626)
Radiology description
- Ultrasonography (US) and magnetic resonance imaging (MRI) studies are useful to detect extent of disease
- CT findings may demonstrate the following findings (AJNR Am J Neuroradiol 1996;17:382)
- Enlarged lymph nodes with homogeneous enhancement
- Major salivary glands and soft tissue may show heterogeneous enhancement
- US findings include the following findings (AJNR Am J Neuroradiol 2001;22:513)
- Enlarged lymph nodes appear as solid, hypoechoic and maintain hilar architecture
- Nodal involvement often exhibits hilar vascularity and occasionally a mixed hilar and capsular vascular pattern
- Soft tissue masses appear hypoechoic and predominantly solid but may occasionally have a cystic component with homogenous or heterogeneous internal architecture
- MRI findings may demonstrate varying signal intensity proportionate to amount of fibrosis and vascular proliferation (AJNR Am J Neuroradiol 1996;17:382)
Prognostic factors
- Benign entity with an indolent course
- Recurrence is common after surgical excision (Int J Oral Maxillofac Surg 2017;46:350)
Case reports
- 4 year old boy with bilateral upper eyelid swelling (Allergy Asthma Clin Immunol 2021;17:48)
- 7 year old boy with paralysis of bilateral lower extremities (BMC Surg 2020;20:209)
- 14 year old boy with submandibular swelling (Med Pharm Rep 2019;92:195)
- 18 year old man with nontender, mobile subcutaneous nodule below right ear (J Clin Diagn Res 2017;11:ME01)
- 28 year old man with multiple subcutaneous swellings of head and neck (Indian J Otolaryngol Head Neck Surg 2019;71:589)
Treatment
- Conservative management in asymptomatic cases
- Surgery followed by radiotherapy has been shown to result in longer period of remission (Int J Oral Maxillofac Surg 2017;46:350)
- Other options include corticosteroids with or without immunosuppressants (e.g. cyclosporine)
Gross description
- Enlarged lymph nodes
- Soft tissue involvement presents as large, tumor-like masses
Frozen section description
- Reactive lymphoid hyperplasia admixed with abundant eosinophils
- Other entities need to be evaluated on permanent sections prior to classification as Kimura disease (see Differential diagnosis)
Microscopic (histologic) description
- Hyperplastic follicles with germinal centers associated with expansion of interfollicular areas by abundant eosinophils, often forming eosinophilic microabscesses (Arch Pathol Lab Med 2007;131:650)
- Disruption of follicles by increased eosinophils causing folliculolysis
- Proteinaceous material may be present in germinal center due to increased IgE deposition
- Admixed with polymorphous populations of lymphocytes, plasma cells, mast cells and histiocytes
- Polykaryocytes of Warthin-Finkeldey type may be present (Am J Surg Pathol 1989;13:177)
- Vascular proliferation in interfollicular areas
- Necrosis may be present
- Varying degree of sclerosis may be seen
Microscopic (histologic) images
Cytology description
- Polymorphous population comprised of markedly increased eosinophils admixed with inflammatory cells (lymphocytes, plasma cells and occasionally histiocytes)
- Cytologic findings are not sufficient to diagnose; biopsy is required for definitive characterization (Acta Cytol 2002;46:357)
Positive stains
- Diagnosed on morphology; immunostains often not required
- Involved tissue shows a reactive background with a mixture of CD3 positive T cells and CD20 positive B cells
- IgE deposits in germinal centers (Am J Surg Pathol 1989;13:177)
Flow cytometry description
- Absence of abnormal B cells, aberrant T cell population or increased blasts
Molecular / cytogenetics description
- No known clonal gene rearrangements
- Negative for gene translocations
Sample pathology report
- Lymph node, right groin, excisional biopsy:
- Kimura disease (see comment)
- Comment: The right groin lymph node is involved by a proliferation of enlarged but well spaced secondary lymphoid follicles that maintain the normal zoning pattern of follicle center cells. The paracortex (interfollicular areas) is expanded by numerous eosinophils accompanied by small lymphocytes and occasional immunoblasts. Eosinophilic microabscesses are present and eosinophils also infiltrate some of the follicle centers. Additionally, the paracortex shows vascular proliferation. No malignant cells of any type are seen.
Differential diagnosis
- Reactive follicular hyperplasia:
- Clinical history is important
- May occur in a variety of conditions, such as infections, medications, autoimmune diseases
- Progressive transformation of germinal center:
- Hyperplastic germinal center follicles (4 - 5 times the normal) in a background of reactive follicular hyperplasia
- Eosinophilic microabscesses absent
- Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia):
- Benign vascular neoplasm, common in superficial dermis
- Morphology shows lobular proliferation of capillaries that are lined by plump, epithelioid endothelial cells, usually associated with eosinophilic and lymphocytic inflammatory infiltrate
- Subset shows FOSB gene rearrangements
- Castleman disease, hyaline vascular variant:
- Large follicles with regressed germinal centers surrounded by concentric rings of mantle zone
- Interfollicular areas show increased vascular proliferation and plasmacytoid dendritic cells
- Eosinophilic microabscesses absent
- Dermatopathic lymphadenopathy (Clin Case Rep 2018;6:1637):
- Commonly involves axillary and inguinal lymph nodes
- Paracortical expansion of lymph node parenchyma by mixed population of Langerhans cells, pigment laden histiocytes and small lymphocytes
- Eosinophilic microabscesses absent
- Langerhans cell histiocytosis:
- Hodgkin lymphoma, mixed cellularity type:
Board review style question #1
A 30 year old previously healthy Asian man presented with enlarged cervical lymph node (see images above). Infectious and autoimmune workup were unrevealing. He was not taking any medications. Immunohistochemistry showed a mixture of CD3 positive T cells and CD20 positive B cells. Which of the following is the best diagnosis?
- HIV lymphadenitis
- Kikuchi-Fujimoto disease
- Kimura disease
- Langerhans cell histiocytosis
- Rosai-Dorfman disease
Board review style answer #1
Board review style question #2
Microabscesses in Kimura disease are composed of which of the following inflammatory cells?
- Eosinophils
- Langerhans cells
- Lymphocytes
- Neutrophils
- Plasma cells
Board review style answer #2
Langerhans cell histiocytosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Langerhans cell histiocytosis (LCH) is a clonal proliferation of cells that morphologically and immunophenotypically resemble Langerhans cells
Essential features
- Langerhans cell histiocytosis is a clonal proliferation of cells that morphologically and immunophenotypically resemble Langerhans cells
- More common in childhood (1 - 3 years old) and involves nodal and extranodal sites (most common site is bone)
- Lesional cells show prominent nuclear grooves with admixed eosinophils and are positive for CD1a, langerin (CD207) and S100
- Most cases harbor BRAF V600E mutations and other RAS / MAPK pathway activating mutations (Blood 2010;116:1919)
- Unifocal disease is generally associated with a good prognosis, whereas multisystem and multifocal disease is associated with poor prognosis
Terminology
- Histiocytosis X
- Eosinophilic granuloma (solitary bone lesion)
- Hand-Schüller-Christian disease (multiple lesions)
- Hashimoto-Pritzker disease
- Letterer-Siwe disease (disseminated or visceral involvement)
ICD coding
Epidemiology
- More common in childhood (1 - 3 years old)
- Incidence: 3 - 5 per million children, 1 - 2 per million adults (Blood 2015;126:26)
- M > F
- Pulmonary Langerhans cell histiocytosis strongly associated with smoking
Sites
- Can be unifocal, multifocal but involving a single organ system or involve multiple organs (Blood 2015;126:26)
- The most common sites involved are bone and adjacent soft tissue
- The following sites can also be involved, particularly in multisystem disease (in order of decreasing frequency): bone, skin, bone marrow, lymph nodes, liver, spleen, oral mucosa, lung, central nervous system / pituitary and gastrointestinal tract
- Brain involvement can result in a neurodegenerative syndrome; pituitary involvement can present with diabetes insipidus (Brain 2005;128:829)
Pathophysiology
- The majority of cases are clonal and harbor driver mutations involving the RAS / MAPK pathway (Blood 2010;116:1919)
- Misguided myeloid / dendritic cell model
- Clinical severity and distribution of Langerhans cell histiocytosis lesion(s) may be defined by the cellular stage of myeloid differentiation during which the somatic BRAF V600E or other activating kinase mutation arises and results in pathological extracellular signal regulated kinases (ERK) activation (Br J Haematol 2015;169:3)
Etiology
- Pulmonary Langerhans cell histiocytosis is strongly associated with smoking (N Engl J Med 2002;346:484)
- Causes for other forms are unknown
Diagnosis
- Imaging studies (Blood 2015;126:26):
- Skeletal survey
- PET-CT scan
- MRI: most effective for brain lesions
- Bone marrow biopsy / aspiration in patients with cytopenias
- Endoscopy to rule out gastrointestinal involvement in patients with evidence of malabsorption
- Biopsy of lesion(s): complete excision is not required, particularly for bone lesions
Laboratory
- Complete blood count: cytopenias suggest bone marrow involvement (Blood 2015;126:26)
- Liver function studies: abnormal if liver involved
Radiology description
- Plain radiographs / CT: solitary or multiple punched out lytic lesions without sclerotic rim
- MRI: T1 hypointense to isointense, T2 hyperintense, T1 contrast enhancing (Dähnert: Radiology Review Manual, 6th Edition, 2007)
Radiology images
Prognostic factors
- Localized (single system) disease: good outcome
- Multiorgan (2 or more organs) or multisystem disease including liver, spleen or bone marrow: poor outcome (Blood 2015;126:26)
Case reports
- 2 year old boy with generalized lymphadenopathy, hepatosplenomegaly and lytic lesions (J Cancer Res Ther 2015;11:1028)
- 7 year old girl with cervical lymphadenopathy (AABS 2016:3:C79)
- 41 year old woman with skin lesions and generalized lymphadenopathy (Postepy Dermatol Alergol 2015;32:225)
- 52 year old woman with inguinal lymphadenopathy (J Clin Pathol 2005;58:104)
- 68 year old woman with small lymphocytic lymphoma / chronic lymphocytic leukemia and rapidly expanding inguinal node (Case #314)
Treatment
- Surgical resection may be sufficient for single system disease (Blood 2015;126:26)
- Curettage may be sufficient for isolated bone lesions
- Systemic chemotherapy (vinblastine, prednisone, mercaptopurine)
- Smoking cessation for pulmonary Langerhans cell histiocytosis
Microscopic (histologic) description
- Partial effacement of lymph node with preservation of follicular centers
- Infiltration of sinuses by Langerhans cells: 12 - 15 microns in diameter with abundant, pale eosinophilic cytoplasm, irregular and elongated nuclei with prominent nuclear grooves and folds, fine chromatin and indistinct nucleoli (Arch Pathol Lab Med 2015;139:1211)
- Occasionally multinucleated
- Sinuses commonly have foci of necrosis, often surrounded by rim of eosinophils
- Variable mitotic activity
Microscopic (histologic) images
Contributed by Vignesh Shanmugam, M.D.
Contributed by Catherine Hagen, M.D.
AFIP images
Case #314
Cytology images
Positive stains
- CD1a (Histopathology 2002;41:1)
- Langerin (Histopathology 2002;41:1)
- S100 (Histopathology 2002;41:1)
- CD68 (Histopathology 2002;41:1, Blood 2001;97:1241, Mod Pathol 2010;23:1616)
- BRAF VE1 (~50% of cases) (Hematol Oncol 2018;36:307)
- CD45 (Histopathology 2002;41:1)
- CD4 (Histopathology 2002;41:1)
- Fascin (Am J Clin Pathol 2002;118:335)
- Cyclin D1 (Am J Surg Pathol 2017;41:1390)
Negative stains
- CD21 (Histopathology 2002;41:1)
- CD30 (Histopathology 2002;41:1)
- B and T lineage markers (except CD4) (Histopathology 2002;41:1)
Electron microscopy description
- Birbeck granules: tennis racquet shaped, 200 - 400 nm long and 33 nm wide, with a zipper-like appearance (Mol Biol Cell 2002;13:317)
Electron microscopy images
Molecular / cytogenetics description
- ~50% of cases harbor BRAF V600E mutations (Blood 2010;116:1919)
- ~35% of BRAF wild type cases show other activating mutations involving the MAPK pathway in a mutually exclusive manner (MAP2K1 mutations, BRAF fusions, ARAF mutations, ERBB3 mutations) (Blood 2016;128:2533, Blood 2014;124:3007, Blood 2014;123:3152, Blood 2014;124:1655)
- Most cases do not show any cytogenetic abnormalities (Genes Chromosom Cancer 2009;48:239)
Sample pathology report
- Lymph node, right inguinal, excisional biopsy:
- Langerhans cell histiocytosis (see comment)
- Comment: The sections show lymph node tissue with an infiltrate of epithelioid cells involving the interfollicular areas and sinuses. The infiltrate is composed of cells with irregular to folded nuclei with prominent nuclear grooves, vesicular chromatin, distinct nucleoli and moderate amounts of pale eosinophilic cytoplasm. Frequent admixed eosinophils are seen. Reactive appearing germinal centers are relatively preserved.
- Immunohistochemical studies demonstrate that the lesional cells are strongly positive for CD1a, langerin, S100 and cyclin D1.
Differential diagnosis
- Dermatopathic change:
- Pigment laden macrophages, lacks eosinophils, CD1a+ dendritic cells that are cyclin D1- (Am J Surg Pathol 2017;41:1390)
- Cat scratch disease:
- Granulomas with necrosis
- Kimura disease:
- Lacks a significant population of Langerhans cells
- Juvenile xanthogranuloma:
- Erdheim-Chester disease:
- Rosai-Dorfman disease:
- Langerhans cell sarcoma:
- Pleomorphic and highly atypical Langerhans cells
Additional references
Board review style question #1
Board review style answer #1
B. BRAF. The image shows histiocytoid cells in a background of eosinophils and multinucleated cells characteristic of Langerhans cell histiocytosis. The most frequent driver mutation in Langerhans cell histiocytosis is the BRAF V600E mutation (50% of cases).
Comment Here
Reference: Langerhans cell histiocytosis
Comment Here
Reference: Langerhans cell histiocytosis
Board review style question #2
A 2 year old boy presents with generalized lymphadenopathy, hepatosplenomegaly and lytic bone lesions. A diagnostic lymph node biopsy is performed which shows a sinusoidal infiltrate. Electron microscopy reveals club shaped structures in the lesional cells. Which of the following clinical findings can be seen in this entity?
- Bilateral sclerotic long bone lesions
- Elevated white blood cell count
- Hairy kidney
- Neurodegeneration
- Serum monoclonal paraprotein
Board review style answer #2
D. Neurodegeneration. The clinical scenario describes an aggressive presentation of multisystem Langerhans cell histiocytosis in a young patient. The histologic findings are characterized by sinusoidal involvement and electron microscopy shows Birbeck granules, i.e. club shaped or tennis racquet shaped structures in the neoplastic Langerhans cells. Brain involvement by Langerhans cell histiocytosis can result in neurodegenerative syndrome or diabetes insipidus secondary to involvement of the hypothalamic pituitary axis. Hairy kidney and sclerotic bone lesions are characteristic of Erdheim-Chester disease. Serum monoclonal paraproteins are typically seen in association with B cell lymphomas and plasma cell neoplasms.
Comment Here
Reference: Langerhans cell histiocytosis
Comment Here
Reference: Langerhans cell histiocytosis
Lipofuscin
Table of Contents
Definition / general | Sites | Pathophysiology | Etiology | Clinical features | Prognostic factors | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
- Common incidental finding
- Lipofuscin is a golden brown, finely granular pigment derived chiefly from the breakdown products of lipids, usually those derived from cell membranes
- Ceroid is a variant of lipofuscin that is acid fast and autofluorescent
Sites
- Portal lymph nodes and other nodes draining the liver
- Also in other lymph node groups
Pathophysiology
- Accumulation of lipofuscin in post mitotic cells by accretion of oxidized, cross linked proteins
- Conspicuous in conditions associated with atrophy of an organ
Etiology
- Various inflammatory, necrotic, neoplastic lesions, including chronic cholestatic lesions like primary biliary cirrhosis and primary sclerosing cholangitis
Clinical features
- Enlarged lymph nodes
Prognostic factors
- Although previously thought to have no significance, there is increasing evidence that lipofuscin may both cause and be due to increased oxidant stress in cells and may impair proteasomal and lysosomal functions
Gross description
- Enlarged lymph nodes with brown pigment
Microscopic (histologic) description
- Sinus histiocytosis with granular deposits of brown pigment in sinus macrophages
- Varying degrees of follicular hyperplasia
Microscopic (histologic) images
Positive stains
- Sudan Black B, Schmorl reaction, oil red O, carbol lipofuscin stain, periodic acid-Schiff, Ziehl-Neelsen acid fast stain, autofluorescence or the lysosomal acid phosphatase and esterase stains
Negative stains
Electron microscopy description
- Membrane limited, electron dense, spherical inclusions
Differential diagnosis
- Iron / hemosiderin
- Melanin
- Tattoo pigment
Littoral cell angioma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnostic criteria | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, typically benign splenic vascular tumor
- Originates from littoral cells lining splenic red pulp sinuses
Essential features
- Strong association with diverse immunologic and neoplastic disorders
- Presentation varies from asymptomatic and incidental to fever, pain and cytopenias related to hypersplenism
- Dual endothelial and histiocytic differentiation of tumor cells, similar to normal splenic littoral cells
- CD34 is characteristically absent while other endothelial cell antigens are expressed
Terminology
- Littoral cell angioma of the spleen
ICD coding
- ICD-10: D73.89 - other diseases of spleen
Epidemiology
- Mostly in middle aged adults
- Median age: 50 years (range: 1 - 86 years)
- M = F (Int J Clin Exp Pathol 2015;8:8516)
Sites
- Unique to the spleen
Pathophysiology
- Immunodysregulation is postulated to explain the clinical association with various malignancies or immune / autoimmune disorders (Histopathology 2016;69:762)
Etiology
- Unknown etiology
Clinical features
- Almost always benign with exceedingly rare malignant transformation
- 50% asymptomatic (World J Gastroenterol 2015;21:6660)
- 50% present with splenomegaly, hypersplenism associated thrombocytopenia and anemia, abdominal pain, fever or weight loss (Histopathology 2016;69:762, World J Gastroenterol 2015;21:6660)
- 36% associated with immune / autoimmune / metabolic disorders, such as Crohn's disease or immune thrombocytopenic purpura (Blood 2017;129:1564)
- 40 - 60% associated with malignancies, such as colonic adenocarcinoma, pancreatic tumor, lung carcinoma, renal cell carcinoma, lymphoma, myelodysplasia, aplastic anemia or testicular seminoma (Histopathology 2016;69:762)
Diagnostic criteria
- Splenomegaly with nodules composed of benign tortuous vascular channels
- Dual endothelial and histiocytic immunophenotype lacking CD34
Laboratory
- Thrombocytopenia, anemia in subset of patients
Radiology description
- Enlarged spleen
- Ultrasound varies from heterogeneous echotexture without specific nodules to hyperechogenic, hypoechogenic or isoechogenic lesions
- CT shows solitary or multiple hypoattenuating nodules (BMJ Case Rep 2015;2015:bcr2015212882)
- MRI shows hypodense lesions on T1 and T2 weighted scans due to hemosiderin (Int J Clin Exp Pathol 2015;8:8516)
Radiology images
Prognostic factors
- Typically benign with excellent prognosis postsplenectomy
- Increased risk of malignant transformation with massive splenomegaly (> 1,500 g or > 20 cm long) (Minerva Chir 2014;69:229)
- Rare reports of recurrence in accessory spleen or metastasis (Medicine (Baltimore) 2018;97:e0378)
Case reports
- 30 year old man presented with abdominal pain, fever and multiple splenic masses (Acta Chir Belg 2021;121:357)
- 38 year old man with splenic, renal and hepatic cysts (Arch Pathol Lab Med 2001;125:1505)
- 43 year old man with prior pulmonary sarcoidosis (World J Surg Oncol 2011;9:106)
- 45 year old man with obstructive jaundice (Indian J Pathol Microbiol 2012;55:109)
- 46 year old women and 52 year old man with littoral cell angioma detected during physical examination (Chin Med J (Engl) 2011;124:3423)
- 58 year old woman with large solitary littoral cell angioma (J Clin Imaging Sci 2012;2:69)
- 60 year old man with rapid increase in size of spleen (Rare Tumors 2010;2:e17)
- 61 year old woman with metastasis to the liver treated with chemotherapy (Medicine (Baltimore) 2018;97:e0378)
Treatment
- Splenectomy
- Management of any potential complications of asplenia (e.g., severe infection and thrombosis)
- Clinical evaluation for any coexisting neoplastic or immune disorders (World J Gastroenterol 2015;21:6660)
Gross description
- Solitary or multiple distinct splenic nodules (solitary to multiple ratio is ~0.3:1) (Front Oncol 2022;12:790332)
- Minute to large nodules of variable consistency
- Various colors (yellow / brown / red / black) depending on degree of necrosis, cyst formation, thrombi and fibrosis (Int J Clin Exp Pathol 2015;8:8516)
- Spongy / cystic (J Cytol 2017;34:121)
- Well demarcated but lacking a fibrous capsule
Gross images
Microscopic (histologic) description
- Proliferation of anastomosing, tortuous, blood filled vascular channels (Int J Clin Exp Pathol 2015;8:8516)
- Irregular channel lumina, often with papillary projections and cystic spaces
- Lined by tall endothelial cells with variable hemophagocytosis (BMJ Case Rep 2015;2015:bcr2015212882)
- Sloughing of endothelial cells into vascular spaces
- No sclerosis or cytologic atypia
Microscopic (histologic) images
Cytology description
- 3 dimensional, bland appearing, epithelioid foamy cells with low N:C ratio (J Cytol 2017;34:121)
- May contain intracytoplasmic hemosiderin pigment
Positive stains
- Dual endothelial (factor VIII, CD31, von Willebrand factor) and histiocytic (CD4, CD68, CD163, FLI1, MAC387, HAM56, lysozyme) differentiation (Histopathology 2016;69:762)
- Langerin (CD207) (Hum Pathol 2019;83:43)
- CD21
- Vimentin
- Cyclin D1
- Low Ki67 proliferative index
- S100 (variable; 20%) (Hum Pathol 2019;83:43)
Negative stains
- CD34 (characteristically absent despite expression of other endothelial cell antigens)
- ERG and WT1 vascular endothelial cell markers (Ann Diagn Pathol 2015;19:143)
- CD45, CD8 (usually negative), CD117, HHV8, CD1a, cytokeratins
Molecular / cytogenetics description
- No specific molecular findings
Sample pathology report
- Spleen, splenectomy:
- Littoral cell angioma (see comment)
- Comment: The nodule in the splenic red pulp is composed of tortuous blood vessels lined by plump endothelial cells that form papillary projections into the lumina. No overt cytologic atypia or mitoses are seen. The white pulp is histologically unremarkable.
- Immunohistochemistry demonstrates dual endothelial and histiocytic differentiation of the vascular lining cells. The phenotypic profile is as follows: CD31+, factor VIII+, CD68+, lysozyme+, langerin+. Not expressed are CD34, CD117 and HHV8. The proliferative fraction based on Ki67 is low (about 15%).
- The overall findings are characteristic of splenic littoral cell angioma, which is benign in most cases. However, this lesion may be seen in association with a variety of neoplastic and immune / autoimmune conditions. Clinical correlation and follow up are suggested.
Differential diagnosis
- Angiosarcoma:
- Atypical sarcomatous cells with hyperchromatic nuclei
- Frequent tumor necrosis, high mitotic rate and poor demarcation
- CD34+
- Hamartoma:
- Disorganized blood vessels with entrapped adipocytes
- CD8+
- Splenic littoral cell hemangioendothelioma:
- Typically mild to moderate cytologic atypia
- May have solid areas
- Potentially malignant or metastasizing
- Kaposi sarcoma:
- Hemangioma:
- Hemangiopericytoma:
- Lymphangioma:
- Cystic, malformed lymphatic channels, often subcapsular
- Attenuated endothelial lining
- Lacks histiocytic markers and langerin
Additional references
Board review style question #1
Board review style answer #1
B. CD34. The photomicrograph shows anastomosing vascular channels in the spleen with sloughing of plump endothelial cells into the lumina, morphologically consistent with littoral cell angioma. The proliferative fraction is low. CD34 is characteristically negative in littoral cell angioma, while other vascular endothelial cell associated markers (CD31 and factor VIII) are positive. The histiocytic marker CD68 is also characteristically expressed.
Comment Here
Reference: Littoral cell angioma
Comment Here
Reference: Littoral cell angioma
Board review style question #2
Which of the following statements is true about littoral cell angioma of the spleen?
- Hypersplenism related anemia or thrombocytopenia is a well recognized manifestation
- It is pathogenetically linked to human herpesvirus 8 (HHV8)
- It originates from the white pulp vascular lining cells of the spleen
- Malignant transformation is common among middle aged and elderly patients
Board review style answer #2
A. Hypersplenism related anemia or thrombocytopenia is a well recognized manifestation. Hypersplenism in patients with littoral cell angioma can cause entrapment of RBCs and platelets in the spleen, leading to anemia and thrombocytopenia.
Comment Here
Reference: Littoral cell angioma
Comment Here
Reference: Littoral cell angioma
Luetic lymphadenitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Peripheral smear description | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Lymph node pathology caused by Treponema pallidum (syphilis) infection
Essential features
- Lymphadenitis caused by Treponema pallidum
- Characteristic histologic features include extensive capsular fibrosis with pericapsular chronic inflammation, follicular hyperplasia with numerous plasma cells, phlebitis with endarteritis and occasional poorly formed granulomas
- Organisms stain with Warthin-Starry or specific immunohistochemical stains; confirmation by serology or PCR
Terminology
- Syphilitic lymphadenitis
ICD coding
- ICD-10: A52.79 - other symptomatic late syphilis
Epidemiology
- In 2021 there were > 171,000 cases of all stages of syphilis, representing a 68% increase from 2017 (Infect Dis Clin North Am 2023;37:195)
- Sexually transmitted infection or congenital syphilis acquired from infected mothers
- Screening recommended for men who have sex with men, in the setting of HIV and in pregnant patients
Sites
- Lymphadenitis associated with secondary syphilis (see Clinical features)
- Inguinal (most common), axillae, cervical and occipital (Br Med J 1970;4:67)
Pathophysiology
- Spread of T. pallidum organisms from the primary site of inoculation (chancre, painless ulcer with indurated margins) to regional or distant lymph nodes resulting in lymphadenopathy
- Modes of transmission include sexual contact and infection passed from mother to child
- T. pallidum infection normally triggers a robust humoral and cellular immune response but may take weeks or months to gain control of infection which is often incomplete with common infectious relapses (PLoS Negl Trop Dis 2012;6:e1717)
- Macrophages appear to be the primary means of bacterial killing in the skin (PLoS Negl Trop Dis 2012;6:e1717)
- Spirochetal membrane, including lipoproteins, is key in interacting with the host immune system, first triggering an innate immune response
- Spirochetal lipoproteins, such as outer surface protein B (OspB), may inhibit neutrophil function and prevent oxidative burst; others may activate neutrophils, induce neutrophil extracellular traps and activate monocytes / macrophages and dentritic cells through CD14 and Toll-like receptor signaling pathways resulting in a proinflammatory response and cytokine production (Front Immunol 2017:8:364, PLoS Negl Trop Dis 2012;6:e1717)
- However, the outer membrane of T. pallidum is unique with limited exposed lipoproteins and antigenic variation within the population in lipoprotein expression, resulting in overall poor reaction with antibodies present in syphilitic sera and enabling T. pallidum to evade the immune system (Infect Immun 2010;78:5178)
- Secondary syphilis occurs as a result of T. pallidum’s ability to escape immune recognition while causing inflammation (PLoS Negl Trop Dis 2012;6:e1717)
- T. pallidum may also trigger systemic immunologic abnormalities (PLoS Negl Trop Dis 2012;6:e1717)
- Syphilis infection increases the likelihood of HIV infection; may increase HIV viral load and decrease CD4 count in HIV infected patients (Neurol Clin Pract 2014;4:114)
Etiology
- Treponema pallidum (gram negative spirochete) infection
Clinical features
- Primary syphilis
- Painless chancre with or without regional lymphadenopathy typically within 3 - 6 weeks of inoculation of spirochete in the site of infection (Sex Transm Dis 1997;24:185, CMAJ 2011;183:2015)
- Secondary syphilis
- Disseminated skin rash (maculopapular or with pustular scales) or localized to the palms and soles of the feet, typically within 4 - 10 weeks after the appearance of the chancre (Genitourin Med 1989;65:1)
- Cutaneous well demarcated hypertrophic papules or plaques (condyloma lata) (JAAD Case Rep 2021;10:18)
- May also be associated with diffuse lymphadenopathy, hepatosplenomegaly, hepatitis or nephrotic syndrome (Am J Case Rep 2018;19:238)
- Tertiary syphilis
- Occurs 10 - 20 years post infections in untreated patients
- Cardiovascular syphilis (aneurysm or aortic insufficiency), granulomatous lesions invading and causing tissue destruction (gummas) and neurosyphilis (Infect Dis Clin North Am 2023;37:195)
- Latent syphilis
- Lack of clinical symptoms
Diagnosis
- Darkfield examinations and molecular tests for detecting T. pallidum directly from lesion exudate or tissue are the definitive methods for diagnosing early syphilis and congenital syphilis (CDC: Syphilis [Accessed 11 October 2023])
- 2 types of serologic test for syphilis are classified based on the type of antigen to which they are directed and are required for presumptive diagnosis (Neurol Clin Pract 2014;4:114, CDC: Syphilis [Accessed 11 October 2023])
- Treponemal tests detect antibody to T. pallidum proteins (reactive versus nonreactive); may show false positive in inflammatory diseases
- Treponemal tests include microhemagglutination assay, particle agglutination, hemagglutination assay, fluorescent treponemal antibody absorbed (FTA ABS) test, chemoluminescence immunoassays and enzyme immunoassays
- Nontreponemal tests detect antibodies (IgM or IgG) against lipoidal antigens, damaged host cells and possibly from treponemes and are semiquantitative, reflecting disease activity (Neurol Clin Pract 2014;4:114)
- Nontreponemal tests include rapid plasma reagin (RPR), the venereal disease research laboratory (VDRL) test and the toluidine red unheated serum test
- No single test is sufficient with both tests being used to confirm infection or determine disease activity
- Treponemal tests detect antibody to T. pallidum proteins (reactive versus nonreactive); may show false positive in inflammatory diseases
Laboratory
- Histology and immunohistochemical staining (J Biol Chem 1988;263:6363, J Invest Dermatol 2007;127:2345)
- Silver stain (Warthin-Starry) or antibodies to identify spirochetes in formalin fixed paraffin
- Embedded tissues from primary or secondary syphilis infections
- PCR (J Biol Chem 1988;263:6363)
- Complimentary test to serology in the diagnosis of early syphilis or congenital syphilis
- Dark field microscopy (Toxicol Sci 2004;81:3)
- Immunofluorescence is required for diagnosis but has the ability to distinguish between Treponema pallidum from nonpathogenic treponemes
- Culture (Can J Infect Dis Med Microbiol 2005;16:45)
- Technically impractical for routine diagnosis
Radiology description
- Lymphadenopathy, regional or distant sites
Prognostic factors
- Favorable prognosis with appropriate antibiotic treatment
Case reports
- 23 year old man with painless enlarged inguinal lymph node (Hum Pathol 2018;13:9)
- 27 year old man with right sided neck mass (Am J Case Rep 2018;19:238)
- 29 year old woman with painful unilateral neck swelling (Cureus 2023;15:e36065)
- 35 year old man with cervical syphilis with Pringer-Kuchinka-like lymphadenitis (Br J Oral Maxillofac Surg 2014;52:e141)
- 40 year old man with syphilitic cervical lymphadenitis with abscess formation (Blood 2018;131:707)
- 48 year old woman with acute onset of bilateral inguinal enlargement (Histopathology 2010;56:656)
- 48 and 72 year old men with nontender, progressively enlarging neck masses (J Int Med Res 2012;40:1988)
- 56 year old man with a firm enlarged inguinal lymph node and histologic features mimicking lymphogranuloma venereum (S Afr Med J 2016;106:49)
- 71 year old with primary syphilis and painful inguinal lymphadenopathy without cutaneous manifestations (Radiol Case Rep 2022;18:280)
- 2 brothers presented with isolated unilateral cervical lymphadenitis (J Infect 2009;58:76)
Frozen section description
- Similar findings to microscopic with reactive follicles and increased plasma cells
- Organisms can be seen on Giemsa stains (Intern Med 2017;56:2083)
Frozen section images
Microscopic (histologic) description
- Preserved or partial architectural distortion of lymphoid architecture (Pathol Int 2016;66:142)
- Thickened capsule with pericapsular chronic inflammation, plasma cells and extensive fibrosis (Histopathology 2010;56:656)
- Marked follicular hyperplasia with interfollicular, sinusoidal, perinodal and perivascular plasmacytosis (Arch Otolaryngol Head Neck Surg 1992;118:757)
- Phlebitis with endarteritis in the capsule and nodular tissue
- Variable histiocytes, noncaseating granulomas may be present (Pathol Int 2016;66:142, Am J Clin Pathol 1970;53:304)
- Unusual formation of abscess containing spirochetes may occur (Blood 2018;131:707, Am J Clin Pathol 1970;53:304)
Microscopic (histologic) images
Virtual slides
Cytology description
- Branching / arborizing vessels with perivascular plasma cell cuffing, presence of neutrophils and granulomas (Diagn Cytopathol 2023;51:E199)
Peripheral smear description
- Leukocytosis, anemia and thrombocytopenia especially in neonates in the setting of congenital acquired syphilis (AJP Rep 2017;7:e167)
Positive stains
- Warthin-Starry silver stain or modified Steiner stain highlights spirochetes (Hum Pathol 2018;13:9)
- T. pallidum IHC (Hum Pathol 2018;13:9)
- Giemsa stain (Intern Med 2017;56:2083)
Negative stains
- Difficult to visualize with Gram stain (Intern Med 2017;56:2083)
- Acid fast stain (Medeiros: Ioachim's Lymph Node Pathology, 5th Edition, 2021)
Electron microscopy description
- Complex structure with 2 layered outer wall (Bull World Health Organ 1966;35:223)
Sample pathology report
- Inguinal lymph node, excisional biopsy:
- Reactive follicular hyperplasia with increased plasma cells, capsular thickening and fibrosis (see comment)
- Positive for T. pallidum consistent with syphilitic lymphadenitis
- Comment: The patient is a 60 year old man presenting with an indurated inguinal mass. Concurrent flow cytometry demonstrates no evidence of a monoclonal B cell or aberrant T cell population. The morphologic findings together with the organisms visualized by a special stain for T. pallidum are consistent with syphilitic (luetic) lymphadenitis. Correlation with clinical presentation and history are recommended. Confirmation with serologic or molecular studies can be considered if clinically indicated.
Differential diagnosis
- Lymphogranuloma venereum:
- Sexually transmitted disease caused by Chlamydia trachomatis
- Diagnosis may be complicated by coinfection with T. pallidum
- Hyperplastic lymphoid follicles with increased plasma cell infiltrates and suppurative lymphadenitis which often involves the lymph node capsule and surrounding tissues
- Stellate abscesses are characteristic but not specific (S Afr Med J 2016;106:49)
- PCR for chlamydial DNA or complement fixation with titers > 1:256
- No commonly available stain for the tissue identification of C. trachomatis (Histopathology 2021;78:392)
- Necrotizing granulomatous lymphadenitis:
- Lymphadenitis characterized by acute inflammatory cells and necrosis with giant cells and granulomas
- Clinical conditions include tuberculosis, cat scratch disease, histoplasmosis and leprosy
- Etiologic agents can be determined through serology, specific antibodies or identification of specific antigens using PCR
- Inflammatory pseudotumor:
- Etiology unknown
- Prominent benign lymphadenopathy affecting 1 or several nodes
- Reactive node with mixed inflammation, vascular proliferation with phlebitis and varying degrees of storiform sclerosis
- Negative stains: Warthin-Starry or anti-Treponema pallidum antibodies, ALK and HHV8
- Cat scratch lymphadenitis:
- Caused by Bartonella henselae acquired from arthropod vector or inoculation by scratch from infected cat
- Lymph nodes show follicular hyperplasia with deposition of amorphous intercellular proteinaceous material in follicles and characteristic stellate necrotizing granulomas (Am J Clin Pathol 1962;38:513)
- Positive stain with Warthin-Starry (rod shape rather than spiral)
- Monoclonal anti-B. henselae on paraffin fixed tissue or PCR analysis of bacterial DNA
- Toxoplasma lymphadenitis:
- Lymphadenitis caused by Toxoplasma gondii
- Preserved lymph node architecture, with no capsular involvement
- Characteristic triad of florid follicular hyperplasia, monocytoid B cell hyperplasia and epithelioid histiocytes and microgranulomas
- Difficult to identify organisms in nodes but infection can be confirmed by serology for IgM and IgG antibodies against T. gondii cell wall antigens
- Highly sensitive and specific Sabin-Feldman dye test (mainly used by reference laboratories)
- HIV lymphadenitis:
- Similar patient population and may co-occur
- Prominent follicular hyperplasia
- Capsular fibrosis is not a characteristic feature
- Distinguished by a lack of detectable organisms, serology
- Reference: Medeiros: Ioachim's Lymph Node Pathology, 5th Edition, 2021
Board review style question #1
A 40 year old man presents with a right sided inguinal lymphadenopathy several weeks after a new sexual encounter. No monoclonal B cell or aberrant T cell populations are detected by flow cytometry. Histologic sections of a lymph node and an IHC stain are shown. Which of the following is most consistent with the timing, region and morphologic features of this process?
- Lymphogranuloma venereum
- New HIV infection
- Primary syphilis
- Secondary syphilis
- Tertiary syphilis
Board review style answer #1
D. Secondary syphilis. The findings are most suggestive of syphilitic lymphadenitis, which is associated with secondary syphilis. Answer B is incorrect because while this may overlap with an HIV infection, acute HIV will typically show robust follicular hyperplasia without increased capsular fibrosis and without identifiable organisms. Answer A is incorrect because lymphogranuloma venereum may show some overlapping features but typically shows more suppurative features. Overlapping conditions may occur and so clinical correlation and serologic studies are recommended. Answers C and E are incorrect because timing and clinical features described are not consistent with primary or tertiary syphilis.
Comment Here
Reference: Luetic lymphadenitis
Comment Here
Reference: Luetic lymphadenitis
Board review style question #2
Which morphologic feature is least characteristic of syphilitic (luetic) lymphadenitis?
- Ability to visualize organisms by special stains
- Acute inflammation with necrotizing granulomas
- Increased plasma cells
- Reactive follicular hyperplasia
- Thickened, fibrotic capsule
Board review style answer #2
B. Acute inflammation with necrotizing granulomas. Answers A and C - E are incorrect because syphilitic lymphadenitis is characterized by follicular hyperplasia, increased plasma cells, thickened fibrotic capsule and the ability to visualize organisms by silver stain or IHC for T. pallidum. Granulomatous inflammation can also be seen but necrotizing granulomas with acute inflammation are more characteristic of cat scratch lymphadenitis. Toxoplasma lymphadenitis may show follicular hyperplasia, epithelioid granulomas and increased monocytoid cells along vessels. Toxoplasma organisms are not usually well visualized by special stains in affected lymph nodes.
Comment Here
Reference: Luetic lymphadenitis
Comment Here
Reference: Luetic lymphadenitis
Lymphangioma
Table of Contents
Definition / general | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stainsDefinition / general
- Usually an extension of a soft tissue tumor
- Rare as primary of lymph node
Case reports
- 12 year old girl with growing neck mass (Kulak Burun Bogaz Ihtis Derg 2013;23:187)
- 60 year old man with slow growing, upper laterocervical, painless enlargement (Acta Cytol 1999;43:442)
Microscopic (histologic) description
- Dilated, thin walled lymphatic vessels, lined by attenuated endothelial cells
- Lumina are empty or filled with proteinaceous material, lymphocytes or erythrocytes
- Surrounding stroma shows lymphocytic infiltrate
Cytology description
- Uniform population of small and round nonatypical lymphocytes with occasional histiocytes, centrocytes, centroblasts and plasma cells
- No mitotic figures, no atypia (Acta Cytol 1999;43:442)
Positive stains
Lymphangiomyomatosis
Table of Contents
Definition / general | Terminology | Epidemiology | Etiology | Diagrams / tables | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Differential diagnosis | Additional referencesDefinition / general
- Multisystem disorder affecting mainly middle age females, causing pulmonary and extrapulmonary disease
- Its pathologic features result from the proliferation of neoplastic cells (LAM cells), which have characteristics of both smooth muscle cells and melanocytes
- It was originally classified as benign but now is considered a "low grade, destructive metastasizing neoplasm" (Am J Respir Crit Care Med 2012;186:1210)
Terminology
- Also called lymphangioleiomyomatosis
Epidemiology
- Estimated median transplant free survival time for LAM patients in the U.S. is 29 years from onset of symptoms and 23 years from diagnosis
- Estimated 10 year survival transplant free is 86% (Lung 2013;191:35)
- About 80% of women with TSC develop cystic disease in lungs (Chest 2013;144:578), compared to 13% of male patients (Clin Radiol 2011;66:625)
- Relationship between pulmonary and extrapulmonary LAM needs further investigation
Etiology
- Associated with tuberous sclerosis complex (TSC), an autosomal dominant disorder caused by mutations in the TSC1 or TSC2 genes, characterized by intellectual disability, autism, seizures, facial angiofibroma, cortical tubers, cardiac rhabdomyoma, asrocytoma
Case reports
- 47, 59 and 71 year old women with extrapulmonary lymphangioleiomyomatosis in pelvic and paraaortic lymph nodes (Int J Gynecol Pathol 2011;30:470)
- 55 year old woman with extrapulmonary lymphangioleiomyomatosis (Case of the Month #522)
- 59 year old woman with "malignant" uterine perivascular epithelioid cell tumor, pelvic lymph node lymphangioleiomyomatosis and gynecological pecomatosis (Int J Gynecol Pathol 2008;27:86)
- Extrapulmonary lymphangioleiomyomatosis in pelvic lymph nodes associated with uterine cancer (Zhonghua Bing Li Xue Za Zhi 2015;44:527)
- Incidental pelvic and paraaortic lymph node lymphangioleiomyomatosis (Am J Surg Pathol 2015;39:1015)
Microscopic (histologic) description
- Single or multiple lymph nodes, median size is 3.5 cm
- Mostly affecting nodal parenchyma but can be seen in hilum, subcapsular sinuses or extranodal extension
- LAM cells are either spindle or epitheliod
- Epithelioid cells are arranged in nests or swirls, separated by clefts which resemble lymphatic spaces
- Spindled cells show fascicular growth with some nesting and less prominent lymphatic channels
- No atypia, no necrosis, no mitosis
Microscopic (histologic) images
Contributed by Anna Sarah Erem, M.D. and Gulisa Turashvili, M.D., Ph.D. (Case #522), @katcollmd on Twitter and AFIP
Cytology description
- Relatively large monomorphic spindle cells (Acta Cytol 1997;41:877)
Positive stains
Differential diagnosis
- Metastatic well differentiated leiomyosarcoma: no prominent vascular channels, more atypia and mitotic figures, HMB45-
Additional references
Massive splenomegaly
Table of Contents
Definition / generalDefinition / general
- Spleen > 1000 g
- Due to chronic myeloid leukemia, Gaucher disease, hairy cell leukemia, marginal zone B cell lymphoma, myelofibrosis, plasmacytoma, prolymphocytic leukemia
Mesothelial inclusions
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Differential diagnosis | Additional referencesDefinition / general
- Inclusions of benign mesothelial cells in lymph nodes
- Often missed on routine H&E sections (Am J Surg Pathol 1999;23:1264)
- Hyperplastic mesothelial cells in nodal tissue may derive from reactive serosal mesothelium that is dislodged into draining lymphatics (Arch Pathol Lab Med 2000;124:609)
- Often associated with serosal fluid collection (pericardial, pleural, abdominal) at time of nodal biopsy (Hum Pathol 1998;29:339), including episodes of intraperitoneal hemorrhage and ascites (Pathology 2001;33:239), perhaps because effusion allows for mesothelial cell migration into lymphatics (Diagn Cytopathol 2003;29:163)
Terminology
- Benign mesothelial inclusions
- Benign metastasizing mesothelial cells
Epidemiology
- Very rare occurrence
Sites
- Mediastinal and abdominal lymph nodes
- Rare - cervical lymph nodes
Etiology
- Transportation of these cells through the lymphatics to the lymph node during injury or manipulation at the primary site of the origin
- They undergo a degeneration, and thus it becomes difficult to find them
Clinical features
- Incidental finding
- Enlarged lymph nodes
- Site specific symptoms
Diagnosis
- Biopsy plus immunohistochemistry
Radiology images
Prognostic factors
- Benign process with no significant clinical implications
Case reports
- 12 year old boy with diffuse hyperplastic mesothelial cells in multiple lymph nodes (Int J Clin Exp Pathol 2013;6:926)
- 16 year old boy with mesothelial cell inclusions mimicking adenocarcinoma (Indian J Med Paediatr Oncol 2010;31:62)
- 18 year old woman with benign metastasizing mesothelial cells (J Clin Oncol 2011;29:e546)
- 52 year old woman with mesothelial pelvic lymph node inclusions mimicking metastatic thyroid carcinoma (Gynecol Oncol 1998;68:210)
- Mesothelial cell inclusions in mediastinal lymph nodes mimicking metastatic carcinoma (Am J Clin Pathol 1990;93:741)
- Mesothelial cell inclusions within mediastinal lymph nodes (Histopathology 1994;25:483)
- Mesothelial pelvic lymph node inclusion in a patient with ovarian microinvasive borderline mucinous tumor (Int J Gynecol Cancer 2007;17:917)
- Benign hyperplastic mesothelial cells in lymph node (Int J Surg Pathol 2007;15:297)
Treatment
- Depends upon the clinical presentation / underlying cause
- In incidental findings, none necessary
Gross description
- Enlarged lymph nodes with yellowish tan to gray white, smooth to mottled surface
Microscopic (histologic) description
- Small clusters and singly scattered, round to polygonal cells, seen in the subcapsular and interfollicular sinuses of the nodes
- These cells show a round, vesicular nucleus with small nucleolus
- The nuclear - cytoplasmic ratio is low
- No mitotic activity is detected
- There is no extranodal or parenchymal infiltration of the cells
- Tiny spaces are seen in between the cells of the clusters (mesothelial windows)
Microscopic (histologic) images
Positive stains
- Cytokeratin AE1 / AE3, cytokeratin 7 (CK7)
- Calretinin (nuclear and cytoplasmic), WT1, CK5 / 6, CAIX (membranous)
- EMA (weak and cytoplasmic)
Negative stains
Differential diagnosis
- Lymphangioma: multicystic, has smooth muscle and lymphocytes in cyst wall and lumen, keratin+
- Metastatic adenocarcinoma
- Metastatic mesothelioma
- Mucinous cystadenoma: ovarian-like stroma, mucin+, CEA+, negative for hyaluronic acid
- Müllerian inclusions: ER+; negative for calretinin, CK5 / 6; PAX8 can be positive in both
- Simple hemorrhagic cyst: keratin-
- Sinus histiocytosis
Metastases
Table of Contents
Definition / general | Microscopic (histologic) images | Adenocarcinoma | Breast adenocarcinoma | Hepatocellular carcinoma | Melanoma | Mesothelioma | Nasopharyngeal carcinoma | Pancreatic adenocarcinoma | Papillary thyroid carcinoma | Paraganglia | Plexiform fibrohistiocytic tumor | Rhabdomyosarcoma | Serous borderline tumor | Small cell neuroendocrine carcinoma | Squamous cell carcinomaDefinition / general
- Lymph nodes are common site of metastatic disease
- Presence of metastases is important for TNM staging
- Must determine when examining lymph nodes: tumor present or not; tumor primary or metastatic; possible sites of primary; number of metastatic nodes; size of largest metastatic deposit often helpful; presence of extranodal or vascular involvement
- Nodal metastases are common with carcinoma, melanoma or germ cell tumors; rare with CNS tumors and sarcoma (except for angiosarcoma, clear cell sarcoma, epithelioid sarcoma, MFH, rhabdomyosarcoma or synovial sarcoma)
- Must also rule out lymphoma; compared to metastatic disease, lymphomas are more likely multifocal with diffuse penetration of vessel wall, minimal necrosis, no nesting, not limited to sinuses, not limited to intravascular invasion; differentiate based on special stains / immunostains (see below), touch preparations (clumping favors carcinoma), EM for epithelial features
- Rarely, there is passive transport of benign neoplastic cells to lymph nodes (Arch Pathol Lab Med 2005;129:1317)
- Carcinomas usually have cohesive tumor cells, sinus involvement, clearly defined margins with lymphoid tissue; may resemble Hodgkin lymphoma due to prominent nucleoli and mixed inflammatory infiltrate
- Tumors misinterpreted as lymphoma: nasopharyngeal carcinoma - undifferentiated (tumor cells mix with lymphocytes and tumor does not appear cohesive), melanoma (tumor cells separate from each other within clusters), breast lobular carcinoma (may appear noncohesive), seminoma (inguinal and abdominal nodes)
- Recommended stains (by Rosai) - first round: CD45 / LCA, pankeratin, S100; second round: (as necessary) CD3 (T cells), CD20 (B cell), EMA, CEA, vimentin, possibly GCDFP15 or lactalbumin (for breast), chromogranin (for neuroendocrine), PSA / PAP (for prostate)
- Note: TdT+ lymphoid precursors are present in benign pediatric lymph nodes (Am J Clin Pathol 2002;118:248) and tonsils (Am J Clin Pathol 2001;116:12), causing possible confusion in ALL patients
- Site of metastases:
- Upper cervical nodes: associated with upper aerodigestive tract
- Midcervical nodes: thyroid carcinoma, salivary gland, upper aerodigestive tract; also thymus or ovary
- Supraclavicular nodes: breast or lung, also stomach, pancreas, prostate, testis
- Axillary nodes: breast (women), melanoma, lung
- Inguinal nodes: external genitalia, melanoma
- Undetectable primaries with nodal metastases: nasopharynx, retrotonsillar pillars
- Posttreatment changes: foamy histiocytes, fibrosis, mucin, atypical residual tumor cells
Microscopic (histologic) images
Adenocarcinoma
Definition / general
Gross images
Contributed by Dr. Mark R. Wick
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick, Marino Leon, M.D. and AFIP images
Cytology images
AFIP images
- Signet ring cells may actual be muciphages (Am J Surg Pathol 1998;22:545)
Gross images
Contributed by Dr. Mark R. Wick
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick, Marino Leon, M.D. and AFIP images
Cytology images
AFIP images
Breast adenocarcinoma
Definition / general
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick, Case #4 and AFIP images
- Lobular carcinoma may resemble lymphoma, particularly if signet ring cells are present
Microscopic (histologic) description
- Ductal: large apocrine-like pleomorphic cells with pink, granular cytoplasm, large nuclei and prominent nucleoli
- Often cytoplasmic mucin
- Comedo, trabecular and papillary patterns
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick, Case #4 and AFIP images
Images hosted on other servers:
Hepatocellular carcinoma
Melanoma
Mesothelioma
Definition / general
Microscopic (histologic) description
Cytology description
- Nodal involvement may be initial presentation (Am J Surg Pathol 1990;14:819)
Microscopic (histologic) description
- May expand sinuses
- Cuboidal cells with eosinophilic cytoplasm and central nucleus
Cytology description
- Large dyscohesive cells with abundant pale cytoplasm, ruffled cytoplasmic borders, prominent central nucleoli (Am J Clin Pathol 1992;97:493)
Nasopharyngeal carcinoma
Pancreatic adenocarcinoma
Papillary thyroid carcinoma
Paraganglia
Plexiform fibrohistiocytic tumor
Definition / general
Microscopic (histologic) description
- Uncommonly metastasizes to lymph nodes (Am J Surg Pathol 1999;23:662)
Microscopic (histologic) description
- Multiple plexiform nodules within and external to node
- Nodules composed of plump fibrohistiocytic cells with multinucleated giant cells
Rhabdomyosarcoma
Definition / general
Microscopic (histologic) description
Microscopic (histologic) images
AFIP images
- Alveolar subtype may first present with nodal involvement
Microscopic (histologic) description
- Alveolar subtype: large packets of cells with central dehiscence, cells have moderate eosinophilic cytoplasm, with some rhabdomyoblasts present
Microscopic (histologic) images
AFIP images
Serous borderline tumor
Case reports
- Nodal metastases may be metastatic ovarian serous borderline tumor (Am J Surg Pathol 2006;30:739)
- 58 year old woman with high grade urothelial carcinoma of bladder and unknown ovary tumor 33 years prior (Arch Pathol Lab Med 2005;129:537)
Small cell neuroendocrine carcinoma
Definition / general
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick and AFIP images
- May resemble lymphoma
Microscopic (histologic) description
- Minimal cytoplasm, dense chromatin with nuclear molding
- Often focal necrosis and crush artifact
- Azzopardi phenomena (dense hematoxylin staining of vessel walls)
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick and AFIP images
Squamous cell carcinoma
Definition / general
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick and AFIP images
- Squamous cyst within cervical lymph node should be considered metastatic carcinoma until proven otherwise
Microscopic (histologic) description
- Cystic change is common, particularly from head and neck region
- Often bland looking squamous cells with only focal atypia
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick and AFIP images
Metastases
Table of Contents
Definition / general | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
- Splenic metastasis is usually associated with extensive metastases - isolated splenic metastasis is rare
Sites
- Usually from lung, breast, melanoma, colon, ovary (Arch Pathol Lab Med 2000;124:526)
- Rarely from endometrium, brain, liver or salivary glands
Pathophysiology
- Metastasis may be rare due to spleen's immunological function
- Metastases developed by hematogenous, lymphatic or abdominal cavity implantation routes; also direct invasion from lesions in adjacent organs
- Hematogenous or direct seeding is more common
Clinical features
- No specific clinical presentation
- Rarely present with splenic rupture
Diagnosis
- By radiological examination followed by histopathological confirmation
Radiology description
- In ultrasonographic examination, the metastatic foci in the spleen may be hyperechoic, hypoechoic or nonechoic
- In CT, hypodense lesions are seen
Radiology images
Case reports
- 47 year old woman with metastatic glioblastoma (Neurol Med Chir (Tokyo) 2003;43:452)
- 54 year old woman with solitary spleen metastasis of endometrial carcinoma (Chin J Cancer 2010;29:30)
- 61 year old man with atraumatic splenic rupture secondary to metastatic non small cell lung cancer (J Surg Case Rep 2013;2013:rjt051)
- 66 year old man with splenic metastasis from carcinoma ex pleomorphic adenoma of parotid gland (World J Surg Oncol 2014;12:18)
Treatment
- Splenectomy and chemotherapy depending on the primary tumor
Gross description
- Splenic metastasis can be seen as solitary nodule, multiple nodules or diffuse infiltration or cyst
- Rarely resembles follicular lymphoma grossly with multiple nodules
Microscopic (histologic) description
- Histology is similar to primary tumor
- Red pulp may show nodular transformation similar to sclerosing angiomatoid nodular transformation
Microscopic (histologic) images
Mycobacteria-atypical / other than TB or leprosy
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Common cause of granulomatous lymphadenitis in immunocompetent children (1 - 5 years) and immunocompromised adults (Pediatr Int 2018;60:1062)
- Clinical definite diagnosis of nontuberculous (non-TB) mycobacterial lymphadenitis requires a positive mycobacterial culture or positive PCR test of the purulent discharge or fine needle aspirate (FNA; based on the International Pediatric Otolaryngology Group [IPOG] 2023 Consensus guidelines) (Int J Pediatr Otorhinolaryngol 2023;166:111469)
Essential features
- Unilateral anterior neck lymph nodes in children (1 - 5 years) and immunocompromised adults (e.g. HIV+)
- Granulomatous inflammation with or without necrosis or partial or total nodal effacement by sheets of foamy histiocytes
- Diagnosis is by positive culture for atypical mycobacteria or by excluding Mycobacterium tuberculosis in the presence of suggestive histology (Adv Exp Med Biol 2017;944:19)
- Diagnosis can be established by positive PCR on excisional tissue biopsy (FNA is controversial due to possible complications, i.e., fistula) (J Clin Tuberc Other Mycobact Dis 2021;24:100244)
Terminology
- Atypical mycobacterial lymphadenitis
- Non-TB mycobacterial lymphadenitis (NTML)
ICD coding
- ICD-10: A31.8 - other mycobacterial infections
Epidemiology
- Children ages 1 - 5 are most susceptible
- Immunocompromised adults, HIV+
Sites
- Head and neck, usually unilateral anterior cervical chain lymph nodes
Pathophysiology
- Mycobacterial, intracellular organisms, replicate within macrophages
- Macrophages antagonize bacterial growth via TNF dependent mechanisms
- Mycobacteria induce infected macrophage apoptosis
- Newly recruited macrophages engulf cellular debris, contributing to granuloma expansion
- Newly infected macrophages can exit the primary granuloma and establish secondary granuloma in distal tissue (Cold Spring Harb Perspect Med 2014;5:a018499)
Etiology
- Most common causes are M. avium intracellulare complex, M. marinum, M. fortuitum, M. scrofulaceum, M. kansasii (Clin Infect Dis 1995;20:954)
Diagrams / tables
N/A
Clinical features
- Chronic, slow growing, painless, lymphadenopathy
- Head and neck, unilateral
Diagnosis
- Culture: sensitivity 41%, specificity 100% (gold standard) (Int J Pediatr Otorhinolaryngol 2018;112:48)
- PCR: sensitivity 71.6%, specificity 100%
- Immunoassay: sensitivity of 87.5 - 100%, specificity of 81 - 100%
- Skin tests (PPD-S): sensitivity of 70%, specificity of 94%
- Higher sensitivity of skin test by using multiple species coverage (e.g., PPD-A and PPD-K) (Eur J Pediatr 2021;180:1279)
Laboratory
- PCR can identify Mycobacterium tuberculosis and 6 dominant nontuberculous mycobacterial species with a sensitivity of ~40% and 98% specificity (Microbiol Spectr 2023;11:e0160623)
- Nucleic acid amplification (NAAT) only for Mycobacterium tuberculosis complex; M. tuberculosis and M. bovis: 90% sensitivity
- Good for diagnosis but not follow up; could detect RNA 6 months after starting therapy
Radiology description
- Ultrasound: markedly decreased echogenicity, intranodal liquefactive / cystic necrosis, nodal matting and adjacent soft tissue edema (Pediatr Radiol 2006;36:1063)
Radiology images
N/A
Prognostic factors
N/A
Case reports
- 19 month old girl with the first reported case of human infection with Mycobacterium stomatepiae (JMM Case Rep 2018;5:e005146)
- 7 year old healthy girl with a rare case of Mycobacterium simiae cervical lymphadenitis (Aust J Otolaryngol 2022;5:31)
- 44 year old HIV+ man with diffuse large B cell lymphoma stage IV and on chemotherapy treatment presented with mycobacterial pseudotumor showing as an FDG avid mass on interim PET / CT (IDCases 2023;32:e01757)
- 46 year old man with necrotizing lymphadenitis during therapy for acute myeloid leukemia (Infect Chemother 2017;49:78)
- 67 year old man without immunodeficiency with right axillary lymphadenitis and lung right upper lobe nodule (Respir Med Case Rep 2019;28:100947)
Treatment
- Complete excision: highest cure rate and highest risk of facial nerve palsy
- Macrolides and rifampin for 6 months is the most commonly used regimen (Int J Pediatr Otorhinolaryngol 2023;166:111469)
- Decision on excision versus longterm antibiotics versus no treatment should be based on location and number of lymph nodes (J Infect 2015;71:9)
- Spontaneous regression may occur after 4 - 6 months
Gross description
- Enlarged rubbery lymph node, tan glistening surface with multifocal irregular necrotic soft tissue
Gross images
Frozen section description
- Identical to microscopic
Frozen section images
N/A
Microscopic (histologic) description
- Granulomatous inflammation with or without necrosis, the presence of microabscesses, ill defined granulomas, noncaseating granulomas and a small number of giant cells favor nontuberculous mycobacteria over tuberculosis (Histopathology 1999;35:534)
- Can present as bland appearing foamy histiocytes in sinuses or diffuse sheets occupying part or entire lymph node (mycobacterial pseudotumor) (IDCases 2023;32:e01757)
Microscopic (histologic) images
Virtual slides
N/A
Cytology description
- Suppurative granulomas
- Necrotizing granulomas are typical in M. tuberculosis infection but are also seen in atypical mycobacterial infection
- Granulomas without necrosis can be suggestive of sarcoidosis
- Multiple passes for cultures or PCR testing are recommended
- Reference: BMC Infect Dis 2020;20:224
Cytology images
Peripheral smear description
N/A
Peripheral smear images
N/A
Positive stains
- Ziehl-Neelsen
- Acid fast bacteria are red
- Growth only (hot stain)
- Identifies TB and all non-TB mycobacteria
- Kinyoun stain
- Acid fast bacteria are red
- Growth and susceptibility (cold stain)
- Limited non-TB identification
- Fite stain
- Acid fast bacteria are red
- Auramine O
- Bright yellow luminous rods against a dark background with fluorescent microscope
- Identifies TB and all non-TB mycobacteria
- Increased sensitivity and speed
- References: Ann Clin Lab Sci Spring 2014;44:131, J Clin Tuberc Other Mycobact Dis 2021;24:100244
Negative stains
Flow cytometry description
N/A
Flow cytometry images
N/A
Electron microscopy description
N/A
Electron microscopy images
N/A
Molecular / cytogenetics description
- 16S rRNA sequencing (New Microbes New Infect 2017;22:24, J Clin Microbiol 2000;38:246)
- PCR restriction fragment length polymorphism (PCR-RFLP) procedure capable of rapidly identifying 28 species of clinically encountered mycobacteria (J Clin Microbiol 1997;35:79)
Videos
N/A
Sample pathology report
- Lymph node, left neck, excision:
- Nonnecrotizing granulomatous lymphadenitis (see comment)
- Comment: The lymph node architecture is partially affected by sheets of foamy histiocytes and multifocal granulomas. The granulomas are ill defined and composed of epithelioid histiocytes, lymphocytes and occasional plasma cells. No evidence of necrosis is noted. Acid fast bacteria (AFB) Fite special stain is positive for mycobacterial organisms. Paraffin embedded block was sent for PCR analysis for strain identification and subspeciation and the results will be reported in an addendum.
Differential diagnosis
- Tuberculous mycobacterial infection:
- Usually necrotizing granulomas
- Few organisms seen by AFB stain
- Nucleic acid amplification and culture are positive for Mycobacterium tuberculosis
- Fungal infection:
- Foreign body reaction:
- Polarizable material may be present in tissue
- Sarcoidosis:
- Diagnosis of exclusion by definition
- Negative for cultures and stains
- Naked type granuloma with very few lymphocytes
Additional references
N/A
Board review style question #1
What is the most common histologic finding in immunocompromised patients with nontuberculous mycobacterial lymphadenitis caused by Mycobacterium avium complex (MAC) infection?
- Follicular hyperplasia
- Necrotizing granulomatous inflammation
- Nonnecrotizing granulomatous inflammation with sheets of foamy histiocytes
- Parafollicular hyperplasia
- Suppurative inflammation
Board review style answer #1
C. Nonnecrotizing granulomatous inflammation with sheets of foamy histiocytes is the correct answer because atypical mycobacterial infection typically shows nonnecrotizing granuloma. Sheets of foamy histocytes morphology is also not uncommon, especially in MAC infection. Answer A is incorrect because this morphology is typical of other bacterial and some viral infections. Answer B is incorrect because necrotizing granuloma is seen in typical mycobacterial tuberculosis. Answer D is incorrect because parafollicular hyperplasia is associated with viral lymphadenitis. Answer E is incorrect because suppurative lymphadenitis is associated with bacterial infection.
Comment Here
Reference: Mycobacteria - atypical / other than TB or leprosy
Comment Here
Reference: Mycobacteria - atypical / other than TB or leprosy
Board review style question #2
An HIV positive 34 year old man has an enlarged painless lymph node. Nucleic acid amplification and culture are negative for M. tuberculosis organisms. What is the most common mycobacterial infection in immunocompromised patients such as this one?
- M. aortuitum
- M. avium intracellulare
- M. gordonae
- M. scrufulaceum
Board review style answer #2
B. M. avium intracellulare is the most common nontuberculous mycobacterial infection in immunocompromised patients. Answers A, C and D are incorrect because they are isolated less frequently compared to M. avium intracellulare. Those organisms can be seen in immunocompetent children.
Comment Here
Reference: Mycobacteria - atypical / other than TB or leprosy
Comment Here
Reference: Mycobacteria - atypical / other than TB or leprosy
Mycobacteria-atypical / other than TB or leprosy
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Common cause of granulomatous lymphadenitis in immunocompetent children and immunocompromised adults (Pediatr Int 2018;60:1062)
Essential features
- Diagnosis by excluding Mycobacterium tuberculosis and positive culture for atypical Mycobacterium or suggestive histology (Adv Exp Med Biol 2017;944:19)
- Lymph node partially or totally affected by sheets of foamy histiocytes
- Nonnecrotizing granulomas not needed for diagnosis
- Unilateral anterior neck lymph nodes in children (2 - 5 years) and immunocompromised adults
- Spontaneous regression may occur after 4 - 6 months
Terminology
- Atypical mycobacterial lymphadenitis
ICD coding
- ICD-10: A31.8 - Other mycobacterial infections
Epidemiology
- Children ages 2 - 5 are most susceptible
- Immunocompromised adults, HIV+
Sites
- Head and neck, usually unilateral anterior neck chain
Pathophysiology
- Mycobacterial, intracellular organisms, replicate within macrophages
- Macrophages antagonize bacterial growth via TNF dependent mechanisms
- Mycobacteria induce infected macrophage apoptosis
- Newly recruited macrophages engulf cell debris, contributing to granuloma expansion
- Newly infected macrophages can exit the primary granuloma and establish secondary granuloma in distal tissue (Cold Spring Harb Perspect Med 2014;5:a018499)
Etiology
- Most common causes are M. avium intracellulare complex, M. marinum, M. fortuitum, M. scrofulaceum, M. kansasii (Clin Infect Dis 1995;20:9549)
Clinical features
- Chronic, painless, lymphadenopathy
- Head and neck, unilateral
Diagnosis
- Culture: sensitivity 41%, specificity 100% (gold standard) (Int J Pediatr Otorhinolaryngol 2018;112:48)
- PCR: sensitivity 71.6%, specificity 100%
- Sensitivity of immunoassay: sensitivity 87.5 - 100%, specificity 81 - 100%
- Skin tests (PPD-S): sensitivity 70%, specificity of 94%
Laboratory
- Nucleic acid amplification (NAAT) only for Mycobacterium tuberculosis complex; tuberculosis and M. bovis: 90% sensitivity
- Good for diagnosis not follow up; could detect RNA 6 months after starting therapy
Radiology description
- Ultrasound: markedly decreased echogenicity, intranodal liquefactive / cystic necrosis, nodal matting and adjacent soft tissue edema (Pediatr Radiol 2006;36:1063)
Case reports
- 19 month old girl with the first reported case of human infection with Mycobacterium stomatepiae (JMM Case Rep 2018;5:e005146)
- 2 year old African American girl presented to the clinic with anterior ear lobe and submandibular lymphadenitis (Cureus 2016;8:e846)
- 46 year old man with necrotizing lymphadenitis during therapy for AML (Infect Chemother 2017;49:78)
- 67 year old man without immunodeficiency with right axillary lymphadenitis and lung right upper lobe nodule (Respir Med Case Rep 2019;28:100947)
Treatment
- Complete excision: highest cure rate and highest risk of facial nerve palsy
- Decision on excision versus long term antibiotics versus no treatment should be based on location and number of lymph nodes (J Infect 2015;71:9)
Gross description
- Enlarged rubbery lymph node, tan glistening surface with multifocal irregular necrotic soft tissue
Gross images
Frozen section description
- Identical to microscopic
Microscopic (histologic) description
- Granulomatous inflammation with or without necrosis, the presence of microabscesses, ill defined granulomas, noncaseating granulomas and a small number of giant cells favors nontuberculous mycobacteria over tuberculosis (Histopathology 1999;35:534)
Microscopic (histologic) images
Cytology description
- Suppurative granulomas
- Necrotizing granulomas typical for tuberculosis infection; also seen in atypical mycobacterial infection
- Granulomas without necrosis can be suggestive of sarcoidosis
- Multiple passes for cultures or PCR testing are recommended
- Reference: BMC Infect Dis 2020;20:224
Cytology images
Positive stains
- Ziehl-Neelsen:
- Acid fast bacteria are red
- Growth only (hot stain)
- Kinyoun stain:
- Acid fast bacteria are red
- Growth and susceptibility (cold stain)
- Fite stain:
- Acid fast bacteria are red
- Modified Ziehl-Neelsen specifically for M. leprae
- Auramine O:
- Bright yellow luminous rods against a dark background with fluorescent microscope
- Increased sensitivity and speed
- Reference: Ann Clin Lab Sci Spring 2014;44:131
Negative stains
Molecular / cytogenetics description
- 16S rRNA sequencing (New Microbes New Infect 2017;22:24, J Clin Microbiol 2000;38:246)
- PCR restriction fragment length polymorphism (PCR-RFLP) procedure capable of rapidly identifying 28 species of clinically encountered mycobacteria (J Clin Microbiol 1997;35:79)
Sample pathology report
- Lymph node, left neck, excision:
- Nonnecrotizing granulomatous lymphadenitis (see comment)
- Comment: The lymph node architecture is partially affected by sheets of foamy histiocytes and multifocal granulomas. The granulomas are ill defined and composed of epithelioid histiocytes, lymphocytes and occasional plasma cells. No evidence of necrosis is noted. AFB Fite special stain is positive for mycobacterial organisms. Paraffin embedded block was sent for PCR analysis for strain identification and subspeciation and the results will be reported in an addendum.
Differential diagnosis
- Tuberculous mycobacterial infection:
- Usually necrotizing granuloma
- Few organisms are AFB stain positive
- Nucleic acid amplification and culture are positive for Mycobacterium tuberculosis
- Fungal infection:
- Foreign body reaction:
- Polarizable material may be present on tissue
- Sarcoidosis:
- Diagnosis of exclusion by definition
- Negative for cultures and stains
- Naked type granuloma with very few lymphocytes
Board review style question #1
What is the most common histologic finding in non-TB mycobacterial lymphadenitis caused by MAC infection in an immunocompromised patient?
- Necrotizing granulomatous inflammation
- Suppurative inflammation
- Follicular hyperplasia
- Nonnecrotizing granulomatous inflammation with sheets of foamy histiocytes
- Parafollicular hyperplasia
Board review style answer #1
D. Nonnecrotizing granulomatous inflammation with sheets of foamy histiocytes
Comment Here
Reference: Mycobacteria - atypical / other than TB or leprosy
Comment Here
Reference: Mycobacteria - atypical / other than TB or leprosy
Board review style question #2
An HIV positive 34 year old man has an enlarged painless lymph node. Nucleic acid amplification and culture are negative for mycobacterial tuberculosis organisms. What is the most common mycobacterial infection in immunocompromised patients such as this one?
- M. aortuitum
- M. avium intracellulare
- M. gordonae
- M. scrufulaceum
Board review style answer #2
B. M. avium intracellulare is the most common nontuberculous mycobacterial infection in immunocompromised patients.
Comment Here
Reference: Mycobacteria - atypical / other than TB or leprosy
Comment Here
Reference: Mycobacteria - atypical / other than TB or leprosy
Other ectopic tissue / inclusions
Table of Contents
Extramedullary hematopoiesis | Megakaryocytes | Müllerian inclusions | Nevus cells | Salivary gland | Smooth muscle | Squamous epithelium | ThymusExtramedullary hematopoiesis
Definition / general
Case reports
Microscopic (histologic) images
Contributed by Nikhil Sangle, M.D., Sherif Shabaan, M.D. and Julie M. Jorns, M.D. (Case #537)
Differential diagnosis
- Rarely associated with myeloproliferative disorders but also in healthy patients (Diagn Cytopathol 1993;9:522, Arch Otolaryngol 1982;108:523)
Case reports
- 26 year old woman presented with modified Bloom Richardson grade 2 invasive ductal carcinoma and ipsilateral axillary lymph node metastasis at diagnosis; her cancer was hormone receptor positive, HER2 equivocal (2+) on IHC (Case of the month #537)
Microscopic (histologic) images
Contributed by Nikhil Sangle, M.D., Sherif Shabaan, M.D. and Julie M. Jorns, M.D. (Case #537)
Differential diagnosis
Megakaryocytes
Definition / general
Case reports
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Negative stains
Differential diagnosis
- Often associated with extramedullary hematopoiesis when present in lymph nodes
Case reports
- 35 year old woman with megakaryocytes mimicking metastatic breast carcinoma (Arch Pathol Lab Med 2002;126:618)
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Negative stains
Differential diagnosis
- Multinucleated histiocytes:
- Larger, more cytoplasm, vesicular nuclei that are not multilobated
- CD68+
Müllerian inclusions
Definition / general
Terminology
Sites
Pathophysiology
Clinical features
Case reports
Treatment
Gross description
Microscopic (histologic) description
Microscopic (histologic) images
AFIP images
Positive stains
Differential diagnosis
Additional references
- Benign glandular inclusions lined by salpingeal or ovarian epithelium with no endometrial stroma or hemosiderophages
Terminology
- Also called endosalpingiosis
Sites
- Predominantly pelvic and paraaortic lymph nodes
- Present in 5 - 41% of intra-abdominal nodes of women, 20% of women with gynecologic malignancies in paraaortic / pelvic nodes (Am J Obstet Gynecol 1978;130:813, Gynecol Oncol 2000;78:242)
Pathophysiology
- Theories include:
- Passage of epithelial cells through the tubal ostium for implantation in the peritoneum following peeling, followed by transportation by the lymphatic route to reach the pelvic lymph node
- Metaplastic origin from the peritoneum
- Foci of endometriosis
- Vestiges of paramesonephricus ducts
- Metastases from ovarian serous borderline tumors (Am J Surg Pathol 2000;24:710)
Clinical features
- Incidental finding / enlarged lymph nodes
Case reports
- 64 year old woman with endosalpingiosis with psammoma bodies within an intramammary lymph node presented as microcalcifications on mammography (Arch Pathol Lab Med 1995;119:841)
- Coexisting with breast metastatic carcinoma in an axillary lymph node (Virchows Arch 2005;446:467)
Treatment
- Benign process, with no clinical significance
Gross description
- Enlarged lymph nodes
Microscopic (histologic) description
- Glandular / cystic inclusions lined by cuboidal / columnar cells with a Müllerian or coelomic appearance commonly found in the capsule
- Psammoma bodies are common
- May undergo squamous metaplasia
- No / rare mitotic figures, no / mild atypia, no desmoplastic stroma, no endometrial stroma
Microscopic (histologic) images
AFIP images
Positive stains
Differential diagnosis
- Metastasis from borderline / malignant ovarian tumors or uterine carcinomas
Additional references
- J Clin Oncol 2006;24:2013, Ioachim: Ioachim's Lymph Node Pathology, 4th Edition, 2008, Rosai: Rosai and Ackerman's Surgical Pathology, 10th Edition, 2011, Robboy: Robboy's Pathology of the Female Reproductive Tract, 2nd Edition, 2008, Dunphy: Frozen Section Library - Lymph Nodes, 2012, Weiss: Lymph Nodes, 1st Edition, 2008, University of Pittsburgh: Endometriosis and Bland Glandular Inclusions [Accessed 12 April 2023]
Nevus cells
Definition / general
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Debra L. Zynger, M.D.
Positive stains
Negative stains
Differential diagnosis
- Incidence in axillary nodes is 7% per patient and 0.5% per node in one study (Am J Clin Pathol 1994;102:102)
- Presence in sentinel nodes in melanoma patients is associated with
- Cutaneous nevi (Am J Clin Pathol 2004;121:58)
- Congenital cutaneous nevi (Am J Dermatopathol 2002;24:1)
- May represent benign metastases from intradermal nevus in area of lymphatic drainage (Am J Clin Pathol 1985;84:220)
Microscopic (histologic) description
- Single cells, linear arrangements or aggregates of small, round / oval nevus cells with moderate pale / clear cytoplasm, round nuclei with fine chromatin, no prominent nucleoli or pleomorphism, usually within fibrous capsule and trabeculae but also within nodal parenchyma or surrounding a small vessel (Am J Surg Pathol 2003;27:673, Am J Surg Pathol 1996;20:834)
Microscopic (histologic) images
Contributed by Debra L. Zynger, M.D.
Positive stains
Negative stains
- HMB45 (occasionally is very focal), Ki-67 (< 1%, Am J Surg Pathol 2002;26:1351)
Differential diagnosis
- Blue nevus:
- Also spindled / dendritic cells with heavy pigmentation
- Metastatic carcinoma or melanoma:
- Usually not confined to capsule, atypia, mitotic figures, different immunostaining
- Spitz nevus:
- Larger cells with abundant eosinophilic cytoplasm
- Vesicular nuclei with prominent nucleoli
Salivary gland
Definition / general
Terminology
Epidemiology
Sites
Pathophysiology
Clinical features
Prognostic factors
Case reports
Treatment
Clinical images
Images hosted on other servers:
Gross description
Microscopic (histologic) description
Microscopic (histologic) images
AFIP images
Images hosted on other servers:
Differential diagnosis
Additional references
- Benign salivary gland structures in lymph nodes
- Includes ducts and acini
Terminology
- Salivary gland inclusions
- Ectopic salivary tissue
- Heterotropic salivary gland tissue
Epidemiology
- Common incidental finding
Sites
- Most common in upper cervical lymph nodes
- Rarely - thoracic, mediastinal lymph nodes
Pathophysiology
- Considered a normal event related to the embryology of the region
Clinical features
- None - incidental finding
- Enlarged lymph nodes
Prognostic factors
- Benign with no significance
- May undergo diseases of salivary glands including neoplastic processes - Warthin tumor is most common tumor
Case reports
- 55 year old woman with sialometaplasia arising in ectopic salivary gland ductal inclusions (J Clin Pathol 2004;57:1335)
- 60 year old man with salivary gland tissue in cervical lymph nodes (Laryngorhinootologie 2007;86:44)
- 68 year old woman with benign salivary gland tissue inclusion in a pulmonary hilar lymph node (Int J Surg Pathol 2011;19:382)
- Proliferative sialometaplasia arising in an intraparotid lymph node (Am J Clin Pathol 1986;86:116)
Treatment
- Usually none but may be indicated based on clinical situation
Clinical images
Images hosted on other servers:
Gross description
- Normal / enlarged lymph nodes
Microscopic (histologic) description
- Both ducts and acini are usually present
- Benign ducts are lined by double layer of epithelium and myoepithelium
- Benign acini have zymogen granules
Microscopic (histologic) images
AFIP images
Images hosted on other servers:
Differential diagnosis
Additional references
Smooth muscle
Definition / general
Sites
Etiology
Clinical features
Diagnosis
Case reports
Gross description
Microscopic (histologic) description
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Negative stains
Differential diagnosis
Additional references
- Topic discusses the presence of smooth muscle within the capsule and hilum of normal lymph nodes
- Common, incidental finding in lymph nodes of hilar smooth muscle proliferation, more commonly in males than females (Virchows Arch A Pathol Anat Histopathol 1985;406:261)
Sites
- Superficial, inguinal and axillary lymph nodes are most commonly involved
Etiology
- Suggested etiological factors include back pressure in efferent lymphatics, local inflammation
Clinical features
- Asymptomatic, with no apparent clinical significance, but may have enlarged lymph nodes
Diagnosis
- Biopsy
- Immunohistochemistry for smooth muscle to confirm
Case reports
- 6 year old boy with intranodal leiomyoma (Pediatr Dev Pathol 2014;17:118)
Gross description
- Enlarged lymph nodes with firm, tan cut surface
Microscopic (histologic) description
- Nodal architecture is well preserved
- Muscle fibers run parallel to capsule or hilar blood vessels
- Varied patterns of hilar changes:
- Smooth muscle fibers may be densely interwoven within fibrous tissue and hug the hilar nodal capsule
- Smooth muscle fibers may form a loose network extending in all directions within hilar adipose tissue
- Smooth muscle may surround individual islands of fat cells
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Negative stains
Differential diagnosis
- Angiomyomatous hamartoma (Korean J Path 2011;45:S58)
- Benign metastasizing leiomyoma
- Inflammatory myofibroblastic tumor
- Intranodal leiomyoma
- Lymphangioleiomyomatosis
- Pallisading myofibroblastoma
- Vascular transformation of lymph node sinuses
Additional references
Squamous epithelium
Definition / general
Case reports
Microscopic (histologic) description
Differential diagnosis
- Also called lymphoepithelial cyst
- Most common in upper cervical nodes
- Depending on location, may be a branchial pouch derivative or derive from metaplastic calyceal urothelium (Hum Pathol 1990;21:1239)
Case reports
- 33 and 64 year old women with benign heterotopic epithelial inclusions in axillary lymph nodes (Arch Pathol Lab Med 2003;127:e25)
- 48 year old woman with axillary intranodal cysts associated with breast malignancy (Arch Pathol Lab Med 2004;128:361)
- 57 and 75 year old women with cystic lymphoepithelial lesions of peripancreatic nodes (Surg Today 1999;29:467)
- Epithelial inclusion in axillary lymph node associated with a breast carcinoma (Pathol Res Pract 1999;195:263)
Microscopic (histologic) description
- Small cystic structures lined by well differentiated squamous epithelium
Differential diagnosis
- Metastatic well differentiated squamous cell carcinoma:
- Often undergoes cystic change
Thymus
Other infections
Table of Contents
Actinomyces | Bacillary angiomatosis | bCG | Brucellosis | Coccidioides | Herpes simplex | Histoplasmosis | Leishmania | Leprosy | Lymphogranuloma venereum | Measles | Microfilaria | Mycobacterial spindle cell pseudotumor | Parvovirus B19 | Tuberculosis | Tularemia | Whipple disease | Yersinia pestis | Yersinia pseudotuberculosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Actinomyces
Definition / general
- Rare
- Associated with poor dental hygiene (is a normal inhabitant of the oral cavity)
- Causes lymphadenopathy
Gram stain
- Gram positive, filamentous branching bacteria
Treatment
- Appropriate dental care and antibiotics
Case reports
- 12 year old girl with retroperitoneal tumor with rib involvement (Eur J Pediatr Surg 2005;15:38)
Gross description
- Tan-white cut surface, multiple pinpoint white spots
Microscopic (histologic) description
- Marked capsular fibrotic thickening with thick fibrous bands dividing nodes into nodules
- Also follicular hyperplasia, marked interfollicular fibrosis and multiple interfollicular microabscesses containing focal Actinomyces colonies with classic sulfur granules (may require deeper levels to identify)
- No palisading of histiocytes around the abscesses
- Numerous macrophages are present in germinal centers
Cytology images
Images hosted on other servers:
Differential diagnosis
- Cat-scratch disease
- Lymphogranuloma venereum:
- Palisading histiocytes present
- Syphilis:
- Fibrotic capsule but also prominent plasma cells
Additional references
Bacillary angiomatosis
Definition / general
- Caused by Bartonella (formerly Rochalimaea) henselae, which also causes cat-scratch disease
- May also be caused by Bartonella quintana (Ann N Y Acad Sci 2005;1063:302)
- Almost all patients are HIV+ or otherwise immunosuppressed
- May have multiple red / violet skin lesions resembling Kaposi sarcoma but also involves lymph nodes and spleen
- Bacterial reservoir is domestic cats (transmit to humans) and cat fleas (transmit to other cats)
- May appear neoplastic but is reactive
Gram stain
- Small, curved, motile, gram negative rod that is difficult to culture
Diagnosis
- PCR
Case reports
- 17 year old renal transplant patient with persistent fever, pancytopenia and axillary lymphadenopathy (Arch Pathol Lab Med 2004;128:e12)
Treatment
- Erythromycin, other macrolides or doxycycline are very effective
Microscopic (histologic) description
- Focal nodal effacement by multiple coalescing intranodal clusters of small blood vessels, lined by epithelioid endothelial cells with pale cytoplasm
- May have focal nuclear atypia
- Interstitium contains abundant eosinophilic to amphophilic, amorphous or granular material containing aggregates of bacteria (highlighted by Warthin-Starry stain)
- Also neutrophils
Microscopic (histologic) images
AFIP images
Differential diagnosis
- Epithelioid hemangioma or hemangioendothelioma:
- Eosinophilic and vacuolated cytoplasm
- No amorphous / granular material
- Kaposi sarcoma:
- Cleft-like vessels, spindled endothelial cells, no bacteria
Additional references
bCG
Definition / general
- Post vaccination bacille Calmette-Guerin infection occurs in 1% of infants, although swelling usually subsides (Braz J Med Biol Res 2004;37:697)BACILLARY ANGIOMATOSIS Left: Proliferated blood vessels are separated by abundant eosinophilic, vaguely fibrillary material. Some neutrophils are also seen. Right: Barely canalized blood vessels separated by eosinophilic interstitial materials in the absence of neutrophils.
- Patients with normal immunity have complete recovery after postvaccination bCG infection after excision of infected lymph node; immunosuppressed patients may require antiTB therapy to avoid fatal disseminated infection (Am J Clin Pathol 2000;113:703)
Case reports
- 3 month old Chinese baby with post bcg vaccination lymphadenitis (Arch Dis Child 2004;89:812)
- 68 year old man with cervical lymphadenitis post-bCG for bladder cancer (Respiration 2001;68:420)
Treatment
- Surgery for large (3 cm) nodes, anti-TB therapy for small (1 cm) nodes (J Ayub Med Coll Abbottabad 2005;17:16)
Microscopic (histologic) description
- Patients with normal immunity:
- Multiple epithelioid granulomas and Langhans giant cells
- Variable suppuration, minimal caseous necrosis
- Few acid fast bacteria identified with Ziehl-Neelsen stain
- Immunosuppressed patients:
- Histiocytes with abundant gray cytoplasm containing numerous acid fast bacilli, plump nuclei
Cytology description
- Poorly to moderately cellular smears of epithelioid histiocytes in a granuloma pattern with occasional multinucleated Langerhans type giant cells, lymphocytes and neutrophils
- Background is finely granular with necrotic debris
- Isolated calcified spherules (Diagn Cytopathol 2001;25:134)
- May be heavy involvement of acid fast bacilli (World J Surg Oncol 2003;1:25)
Additional references
Brucellosis
Definition / general
- Due to Brucella abortus, melitensis or suis
- Either occupational or food related (milk and cheese)
- Fever, hepatosplenomegaly, rarely lymphadenopathy
Case reports
- 22 year old woman with Kikuchi-Fujimoto necrotizing lymphadenitis associated with brucellosis (Sangre (Barc) 1992;37:201)
- 43 year old man with B. melitensis and subsequent Kikuchi-Fujimoto disease (In Vivo 2003;17:51)
Diagnosis
- Culture, PCR, serology
Microscopic (histologic) description
- Follicular hyperplasia, clusters of epithelioid histiocytes that may form large noncaseating granulomas
- Eosinophils, plasma cells, immunoblasts
Differential diagnosis
Coccidioides
Definition / general
- Also called San Jaquin Valley Fever
- Dimorphic fungus with distinct yeast and mold stages
- Endemic to Lower Sonoran Desert (southwest US, Central and South America)
- Usually self limiting but more severe disease in immunocompromised hosts
Case reports
- 27 year old man on steroids for clinical pneumonia with enlarged paratracheal lymph node (Arch Pathol Lab Med 2005;129:699)
Diagnosis
- Culture (grows on all media)
- Has enteroarthric conidia (alternating segments of hyphae undergo autolysis, while surviving segments form infective barrel shaped multinucleate arthroconidia)
Microscopic (histologic) description
- Thick walled spherules 20 - 150 microns containing 3 - 5 micron endospores, surrounded by a giant cell granulomatous reaction
Additional references
Herpes simplex
Definition / general
- HSV lymphadenitis is very uncommon
- Usually inguinal nodes (Am J Clin Pathol 1991;95:709)
- Often associated with hematopoietic malignancies
Case reports
- 23 year old woman with lymphadenitis before appearance of cutaneous vulvar lesions (Arch Pathol Lab Med 1985;109:1043)
- 43 year old man with immunodeficiency (Clin Infect Dis 2002;34:1)
- 43 year old woman with mantle cell lymphoma (Arch Pathol Lab Med 2006;130:536)
- 59 year old man with HSV infection resembling Richter transformation (Am J Hematol 2001;68:287)
Microscopic (histologic) description
- Well circumscribed necrosis, follicular and paracortical hyperplasia (Histopathology 1991;19:355)
- Cells have intranuclear inclusions, particularly at edge of necrotic areas
- Also marked immunoblasts, T cell hyperplasia
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Positive stains
- HSV
Electron microscopy description
- Intranuclear and cytoplasmic virus particles (Am J Surg Pathol 1990;14:571)
Histoplasmosis
Definition / general
- May clinically mimic tuberculosis
Microscopic (histologic) description
- Chronic suppurative lesion
- Granulomatous process or widespread nodal necrosis with diffuse sinus histiocytosis (Centers for Disease Control and Prevention: Histoplasmosis [Accessed 3 July 2018])
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Positive stains
Leishmania
Definition / general
- Parasites may continue in lymph nodes after clinical cure (J Infect 2003;47:77)
Case reports
- 8 year old boy with atypical presentation with hepatitis and adenopathy in disseminated disease (Trans R Soc Trop Med Hyg 2006;100:79)
Microscopic (histologic) description
- Granulomas with abundant plasma cells, focal fibrosis, variable necrosis, leishmanian amastigotes (by immunostain, Am J Clin Pathol 1994;102:11)
Cytology description
- Polymorphous lymphocytes, histiocytes, plasma cells, giant cells and tingible body macrophages
- Amastigote forms within histiocytes and multinucleated giant cells and extracellularly (Acta Cytol 2005;49:286)
Leprosy
Diagnosis
- PCR, immunofluorescence
Case reports
- 25 year old man with coexisting tuberculosis (Lepr Rev 1999;70:345)
Microscopic (histologic) description
- Lepromatous leprosy exhibits large, pale, round histiocytes without granulomas and with no / rare necrosis
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
- Acid fast (modified Ziehl-Neelsen)
Differential diagnosis
- Lymphoma:
- Clinically (Lepr Rev 2005;76:87)
Lymphogranuloma venereum
Definition / general
- Sexually transmitted disease caused by Chlamydia trachomatis serotypes L1, L2 and L3 (Mod Pathol 1995;8:924, MedlinePlus: Lymphogranuloma venereum [Accessed 3 July 2018], eMedicine: Lymphogranuloma Venereum (LGV) in Emergency Medicine [Accessed 3 July 2018], Wikipedia: Lymphogranuloma venereum [Accessed 3 July 2018])
- Endemic in tropical areas, rare in industrialized countries
- Outbreaks in Western Europe
- Among male homosexuals (Rev Prat 2005;55:1747)
- In Bahamas associated with crack cocaine and HIV (Sex Transm Dis 2002;29:253)
- Three clinical stages
Diagnosis
- PCR
- Previously Frei test (delayed hypersensitivity skin test using "lygranum" chlamydial antigen)
Treatment
- Doxycycline for 21 days
Microscopic (histologic) description
- Early: tiny necrotic foci with neutrophils
- Late: stellate abscesses surrounded by pale epithelioid cells
- Abscesses may merge and sinus tracts may develop
- Macrophages may have organisms within vacuoles
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Electron microscopy description
- Elementary and reticulate bodies
Differential diagnosis
Measles
Definition / general
- Live attenuated vaccine may cause regional lymphadenopathy
- Measles associated lymphopenia may be due to apoptosis of uninfected lymphocytes (Arch Virol 2000;145:905)
- Fatal measles cases in South African in 1976-1982 were associated with malnutrition; caused depletion of T cell zones (S Afr Med J 1985;68:858)
Case reports
- 14 month old boy with familial immunodeficiency and necrotizing lymphadenitis with Warthin-Finkeldey type giant cells after measles vaccination (Ultrastruct Pathol 1980;1:243)
Microscopic (histologic) description
- Polykaryocytes
- In germinal centers, Warthin-Finkeldey giant cells have large nuclei and B cell markers
- In interfollicular areas they have small hyperchromatic nuclei and T cell markers (Pathol Int 1994;44:442)
Microscopic (histologic) images
Images hosted on other servers:
Microfilaria
See also related Breast topic
Definition / general
Essential features
Epidemiology
Pathophysiology
Clinical features
Diagnosis
Treatment
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Sajna V.M. Kutty, M.D.
Board review style question
Definition / general
- Filariasis is due to infection by threadlike nematodes of the family Filarioidea
- Filarial infection can cause lymphedema of the limbs (elephantiasis), genital disease (hydrocele, chylocele and swelling of the scrotum and penis) and recurrent painful acute attacks (WHO: Lymphatic filariasis [Accessed 29 March 2021])
Essential features
- Wuchereria bancrofti accounts for up to 90% of cases
- Diagnosis is typically made by identifying microfilariae in peripheral blood smears
- Rarely, microfilariae are coincidentally detected in FNAC in association with various inflammatory and neoplastic lesions
- Wuchereria bancrofti can be identified by its sheath and multiple, coarse, discrete nuclei extending from head to tail except in the small terminal portion of the caudal end
Epidemiology
- Major public health problem in India, China, Indonesia, Africa and the Far East
Pathophysiology
- Wuchereria bancrofti accounts for up to 90% of cases
- Adult worms lodge in the lymphatics, where females release larvae (microfilaria) which periodically circulate in the blood and are occasionally ingested by feeding mosquitoes
- Microfilaria mature in the mosquitoes before becoming infective and are spread to new humans during mosquito feeding
Clinical features
- Humans are the exclusive host of infection with W. bancrofti
- Many infected individuals remain asymptomatic and serve as potential sources of infection
- Virtually all infected people have subclinical lymphatic damage and up to 40% have kidney damage with proteinuria and hematuria
Diagnosis
- Diagnosis is typically made by identifying microfilariae in peripheral blood smears
- Rarely, microfilariae are coincidentally detected in FNAC in association with various inflammatory and neoplastic lesions (J Cytol 2010;27:78, J Cytol 2017;34:43)
- Finding microfilaria in cytosmears is rare
Treatment
- Treatment consists of multiple doses of albendazole and ivermectin to the entire at risk population, in addition to mosquito control
Microscopic (histologic) description
- Wuchereria bancrofti can be identified by its sheath and multiple, coarse, discrete nuclei extending from head to tail except in the small terminal portion of the caudal end
Microscopic (histologic) images
Contributed by Sajna V.M. Kutty, M.D.
Board review style question
Mycobacterial spindle cell pseudotumor
Definition / general
- HIV+ patients, often involvement of many sites
- Also infants after
- bCG vaccination (Zhonghua Bing Li Xue Za Zhi 2001;30:89)
- Posttransplant (Am J Clin Pathol 1985;83:524)
- Spindle cells are macrophages with large amounts of mycobacteria (Am J Surg Pathol 1992;16:276)
- Intraoperative touch imprints may demonstrate numerous intracellular organisms (Arch Pathol Lab Med 1995;119:811)
Radiology images
Contributed by Chunyu Cai, M.D., Ph.D. (Case #532)
Case reports
- 35 year old HIV+ man with diffuse lymphadenopathy (Case of the Week #218)
- 52 year old man with a history of HIV infection and Kaposi sarcoma 5 years prior, presented with lower extremity weakness (Case of the month #532)
Treatment
- Antiretroviral therapy for HIV
- Antibiotics
Microscopic (histologic) description
- Nodes show partial / complete effacement by storiform pattern of bland spindle cells, some with vacuoles
- Numerous vessels lined by plump endothelial cells, plasma cells and lymphocytes
- No multinucleated tumor cells, no foamy histiocytes
Microscopic (histologic) images
Contributed by AFIP and Chunyu Cai, M.D., Ph.D. (Case #532)
Cytology description
- Spindle cell proliferation resembling Kaposi sarcoma; no foamy histiocytes (Acta Cytol 1995;39:125)
Positive stains
Differential diagnosis
- Kaposi sarcoma:
- Fascicular spindle cells, slit-like spaces, mitotic figures, no granular or acidophilic cytoplasm
- Spindle cells are CD31+ and CD34+
- S100- and CD68- (Am J Surg Pathol 1999;23:656)
- Smooth muscle tumor
Board review style question
Parvovirus B19
Definition / general
- In adults, parvovirus B19 infection is associated with fever (81%), arthralgia / myalgia (62%), skin rash (48%), general fatigue (43%), lymphadenopathy (38%) and edema (38%) (Intern Med 2002;41:295)
- May be associated with hemophagocytic syndrome (Br J Haematol 1997;96:868)
Diagnosis
- PCR
- Immunohistochemistry
Case reports
- 16 year old girl with cervical lymphadenopathy, fever and fatigue (J Clin Pathol 2005;58:872)
Microscopic (histologic) description
- Massive nodular histiocytic proliferation resembling Kikuchi disease with prominent apoptosis but no necrosis
- One case showed florid reactive hyperplasia with paracortical expansion, neutrophils and hemophagocytosis (Pathol Int 1998;48:829)
Microscopic (histologic) images
Images hosted on other servers:
Tuberculosis
See also: Lung - nontumor chapter
Definition / general
Diagnosis
Case reports
Gross description
Gross images
Contributed by Mark R. Wick, M.D.
Images hosted on other servers:
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Images hosted on other servers:
Definition / general
- Usually nodal involvement of cervical region (scrofula), often with draining sinus to skin
- Generalized TB in AIDS cases at autopsy show thoracic or abdominal nodal involvement in almost all cases, although TB often not diagnosed prior to death (Arch Pathol Lab Med 2000;124:1267)
- Needle biopsy of enlarged nodes may be helpful in smear negative, HIV+ patients with suspected TB (Int J Tuberc Lung Dis 2005;9:220)
Diagnosis
- PCR (preferred, J Clin Pathol 2000;53:355)
- Ligase chain reaction (Scand J Infect Dis 2004;36:724)
- Immunostains
- Culture
Case reports
- 8 year old boy with chronic renal failure and mediastinal nodal TB (Clin Exp Nephrol 2006;10:152)
- 52 year old woman with TB and metastatic breast carcinoma in axillary node (World J Surg Oncol 2003;1:3)
- 63 year old woman with Hodgkin lymphoma (Med Klin (Munich) 2006;101:500)
- 82 year old woman with coinfection with Trichomonad tenax (Hum Pathol 2000;31:1317)
Gross description
- Large multinodular mass that resembles carcinoma with multiple foci of caseous necrosis
Gross images
Contributed by Mark R. Wick, M.D.
Images hosted on other servers:
Microscopic (histologic) description
- Either multiple small epithelioid granulomas or huge epithelioid granulomas with prominent Langhans giant cells and central necrosis (J Clin Pathol 1988;41:93)
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Images hosted on other servers:
Tularemia
Definition / general
- Caused by Francisella tularensis, a gram negative coccobacilli found in rodents, rabbits and hares, transmitted by ticks and deer flies
- Commonly due to food and water contamination by rodents, hunting hares and mosquito bites (Med Clin (Barc) 2002;119:455, Emerg Infect Dis 2002;8:956)
- Also a virulent, potential biowarfare agent (Centers for Disease Control and Prevention: Tularemia [Accessed 2 July 2018], eMedicine: Tularemia [Accessed 2 July 2018])
- Symptoms: sudden fever, chills, headaches, diarrhea, muscle aches, joint pain, dry cough, progressive weakness; also pneumonia, skin / mouth ulcers, lymphadenopathy, eye involvement
- Ulceroglandular form: prominent lymphadenopathy of either axilla (mammalian vectors, such as handling rabbits) or cervical / inguinal regions (arthropod vectors)
Diagnosis
- Rise in titers
- PCR (Mod Pathol 2004;17:489)
- Immunofluorescence (Scand J Infect Dis 2004;36:785)
Microscopic (histologic) description
- Early: reactive changes
- Second week: abscess with variable epithelioid cell reaction
- Fourth week: caseous necrosis, diffuse lymphadenitis
- Late: granulomatous reactions that may resemble TB
- Often extracapsular inflammation (Arch Pathol Lab Med 1986;110:42)
Differential diagnosis
Whipple disease
Definition / general
- Rare; due to infection by Tropheryma whipplei, present in soil and sewage but not animals
- Typically affects farmers and outdoor workers
- Symptoms include diarrhea, malabsorption, weight loss, fever, arthralgias; also occasional CNS and cardiac involvement
- May cause marked enlargement of mesenteric and periaortic lymph nodes; enlargement of peripheral lymph nodes may occur early
- Diagnosis requires massive involvement of node plus intense PAS+ staining (small aggregates of PAS+ macrophages are nonspecific) or PCR
- Gram stain: gram+ bacteria
Case reports
- 55 year old woman with mesenteric lymphadenopathy and a monoclonal B cell proliferation (Arch Pathol Lab Med 2003;127:1619)
Treatment
- IV penicillin and streptomycin or third generalization cephalosporin for 10 - 14 days, plus cotrimoxazone for 1 year
Microscopic (histologic) description
- Nodal architecture obscured by ill defined lipogranulomas
- Involvement of sinuses by macrophages with foamy cytoplasm
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Positive stains
- PAS+ diastase resistant bacilli within histiocytes
- Immunostains for bacteria (Am J Clin Pathol 2002;118:742)
Negative stains
Electron microscopy description
- Rod-like organisms
Electron microscopy images
Images hosted on other servers:
Molecular / cytogenetics description
- PCR to confirm diagnosis; difficult to culture (Am J Clin Pathol 2001;116:898)
Differential diagnosis
Yersinia pestis
Definition / general
- Similar to Y. pseudotuberculosis
Clinical features
- Infection is usually limited to lymph nodes ("bubo" as in bubonic plague) and is self limited
- Clinical picture may resemble acute appendicitis but laparotomy often shows mesenteric lymphadenitis (Pediatr Surg Int 1998;13:2)
- Pneumonic plague occurs if bacterial are aerosolized (eMedicine: Plague [Accessed 2 July 2018], Wikipedia: Bubonic Plague [Accessed 2 July 2018])
Diagnosis
- Gram stain: gram negative, polymorphic, coccoid or ovoid mobile bacteria
- PCR
- Cultures
Case reports
- 46 year old woman with weight loss and abdominal lymphadenopathy (Radiologe 1998;38:37)
Microscopic (histologic) description
- Capsular thickening and edema
- Immunoblasts and plasma cells in cortical and paracortical region
- Large lymphocytes within sinuses, germinal center hyperplasia
- No granulomas (unlike Y. pseudotuberculosis)
- Depletion of lymphocytes, edema, necrosis, foamy macrophages
- Bacteria may involve blood vessels
Positive stains
- Yersinia immunostain (Am J Clin Pathol 2002;117:205)
Yersinia pseudotuberculosis
Definition / general
- Clinical picture usually resembles acute appendicitis with possible abdominal mass but laparotomy often shows mesenteric lymphadenitis (Eur J Pediatr Surg 1997;7:180)
- Usually self limited but may be associated with Kawasaki disease (Kansenshogaku Zasshi 2005;79:895, Acta Paediatr 1997;86:661)
- Ampicillin reduces fetal excretion of bacteria but provides no clinical benefit (Pediatr Infect Dis J 1988;7:686)
- Gram stain: gram negative, polymorphic, coccoid or ovoid mobile bacteria
Diagnosis
- PCR
- Cultures
Gross description
- Inflammation of terminal ileum and cecum
Microscopic (histologic) description
- Capsular thickening and edema
- Granulomas with central necrosis and microabscesses
- Immunoblasts and plasma cells in cortical and paracortical region, large lymphocytes within sinuses, germinal center hyperplasia
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
A 52 year old AIDS patient with low CD4 count presented with multiple enhancing brain nodules. Infectious workup revealed disseminated mycobacterium avium intracellulare complex. Biopsy of the brain nodule showed a tumor with mixed spindle and epithelioid cells. What is the mostly likely immunohistochemical profile of this tumor?
- CD31+, CD68-, SMA-,EBER-, HHV8+
- CD31-, CD68+, SMA-, EBER-, HHV8-
- CD31-, CD68-, SMA+, EBER+, HHV8-
- CD31-, CD68-, S100+, EBER-, HHV8-
Board review style answer #2
B. CD31-, CD68+, SMA-, EBER-, HHV8-. This describes mycobacteria pseudotumor. Answer A is incorrect because it describes Kaposi sarcoma. Answer C is incorrect because it describes leiomyosarcoma. Answer D is incorrect because it describes schwannoma. See Case #532 for more information.
Comment Here
Reference: Other infections
Comment Here
Reference: Other infections
Other nonspecific findings
Table of Contents
Clofazimine induced changes | Hyaline deposits | Infarction | Lymphedema | Mantle cell / marginal zone hyperplasia | Monocytoid B cell hyperplasia | Plasmacytosis | Polykaryocytes | Sinus histiocytosis | Board review style question #1 | Board review style answer #1Clofazimine induced changes
Definition / general
- Clofazimine induced crystal storing histiocytosis
ICD coding
- ICD-10: R59.9 - enlarged lymph nodes, unspecified
Epidemiology
- Rare condition in patients taking clofazimine, an antileprosy medication
Sites
- Most commonly subcutaneous tissue, spleen, lymph nodes, liver, lung and gastrointestinal tract
Clinical features
- Red discoloration of skin and other tissues, mass formation, lymphadenopathy
Diagnosis
- History of clofazimine intake, biopsy
Radiology description
- Enlarged lymph nodes, particularly intra-abdominal lymph nodes
Prognostic factors
- Extent of involvement
Case reports
- 32 year old man with chronic abdominal pain (Am J Surg Pathol 2000;24:129)
- 41 year old leprosy patient with abdominal pain (Pathology 1993;25:24)
- 44 year old woman with adenocarcinoma of colon and crystal storing histiocytosis (Lepr Rev 2004;75:171)
Treatment
- Excision of affected lymph node
- Cessation of treatment apparently does not reverse the process
Gross description
- Enlarged lymph node with light brown to dark red discoloration
- Fixative and solutions used for processing the specimen turn red with the crystals dissolving in them
Microscopic (histologic) description
- Marked interfollicular plasmacytosis and histiocytes containing crystals in their cytoplasm, which are elongated with irregular edges, some forming bundles
- Crystals are typically deep red, but depending on the fixative and processing can be clear and colorless (crystals dissolve in alcohol)
- Crystals show bright red birefringence if polarized
- They can be seen in extra and intracellular locations
Positive stains
- Plasma cells are highlighted by CD138 and are polyclonal by kappa and lambda light chain stains
- Lymphocytes are mixed B and T cells and histiocytes show positive staining with CD68 and CD163
Negative stains
- Crystals are negative for PAS and immunoglobulin heavy and light chains
Electron microscopy description
- Transmission electron microscopy shows the empty spaces which crystals have been occupying
- Crystals show some osmiophilic material which appear to be granular or multivesicular bodies
- Upon higher magnification, these crystals show a multilamellar core or a lattice with elements spaced periodically
Electron microscopy images
Images hosted on other servers:
Molecular / cytogenetics description
- No clonal populations of cells
Differential diagnosis
- Crystal storing histiocytosis with immunoglobulin crystals:
- Usually associated with a lymphoid or plasma cell neoplasm
- Fungi
- Parasites
Additional references
- PLoS One 2012;7:e47494, Head Neck Pathol 2012;6:111, Antimicrob Agents Chemother 2016;60:3470, Antimicrob Agents Chemother 2011;55;4000, J Pharmac Sciences 2017;106:1162
Board review style question #1
Hyaline deposits
Definition / general
- Also called proteinaceous lymphadenopathy
- Often present in stroma of pelvic or abdominal nodes
- Nonspecific finding
- Increases with age (Am J Pathol 1975;78:7, Histol Histopathol 2003;18:1169)
- May reduce nodal function
- Associated with
- Rheumatoid arthritis and systemic sclerosis (J Clin Pathol 1994;47:138, Br J Rheumatol 1995;34:1087)
- Hypergammaglobulinemia (Arch Pathol Lab Med 1990;114:34, Am J Surg Pathol 1979;3:137)
- Posttreatment changes for carcinoma (Histopathology 1996;29:63)
- May calcify
Microscopic (histologic) description
- Extracellular eosinophilic material resembling amyloid but Congo Red negative
- Hyaline sclerosis of small and midsized vessels of lymph nodes and organs (Blood 1995;86:1159)
Positive stains
- Immunoglobulins
Negative stains
Differential diagnosis
Infarction
Definition / general
- Uncommon
- Associated with fever, pain and lymphadenopathy (Am J Clin Pathol 1980;74:687)
- Causes:
- Lymphoma
- Melanoma or granulocytic sarcoma (APMIS 2003;111:1133)
- Venous thrombosis
- Arterial occlusion in vasculitis (polyarteritis nodosa)
- Emboli (cholesterol) (Hum Pathol 1978;9:597)
- Heart lung transplantation (J Thorac Cardiovasc Surg 1990;99:861)
- Fine needle aspiration (J Clin Pathol 1982;35:855, Diagn Cytopathol 2001;25:104)
- Mediastinoscopy (Ann Thorac Surg 1989;48:247)
- Gold injection (J Clin Pathol 2001;54:562)
- Dengue fever with DIC (BMC Clin Pathol 2005;5:11)
- Infectious mononucleosis (Int J Surg Pathol 2002;10:223)
- Parvovirus B19
- Intestinal volvulus
- Examine node carefully (possibly with levels or immunohistochemistry) and follow patient to rule out lymphoma or other malignancies (Histopathology 1986;10:571, Indian J Pathol Microbiol 2005;48:510)
Microscopic (histologic) description
- Extensive necrosis with perinodal inflammation and granulation tissue
- May retain a rim of viable lymphatic tissue
- Reticulin architecture is retained
Microscopic (histologic) images
Images hosted on other servers:
Superficial lymph nodes:
Immunohistochemistry
- Tumor cells may stain with traditional markers (keratin, S100), even if remaining node is necrotic but be cautious (Arch Pathol Lab Med 2003;127:60, Arch Pathol Lab Med 2003;127:922)
Differential diagnosis
- Granulomatous inflammation
- Infectious mononucleosis
- Kikuchi disease
- Malignancy: lymphoma or melanoma
- Mucocutaneous lymph node syndrome
Additional references
Lymphedema
Definition / general
- Either congenital, infectious, obstructive, traumatic or idiopathic
- Congenital: also called Milroy disease (primary hereditary lymphedema), autosomal dominant (OMIM: Lymphedema, Hereditary, IA; LMPH1A [Accessed 28 June 2018], OMIM: Lymphedema, Hereditary, II; LMPH2 [Accessed 28 June 2018], OMIM: Yellow Nail Syndrome [Accessed 28 June 2018], OMIM: Lymphedema-Distichiasis Syndrome [Accessed 28 June 2018])
- Infectious: due to schistosomiasis in endemic areas; occasionally due to minor infection such as furuncle
- Obstructive: associated with malignancy or nodal dissection (axilla or groin most common)
- Traumatic: may be as minor as sprained ankle
- Idiopathic: called lymphedema praecox (if before age 35 years) or tarda
- All causes are associated with lymphangitis, cellulitis and recurrent infections
- Postmastectomy lymphedema may lead to angiosarcoma
Treatment
- Elevation of extremity, compression and massage; if necessary, excise thickened skin and replace with skin grafts
Microscopic (histologic) description
- Markedly dilated dermal lymphatics, including in lymph nodes
- Fibrous tissue deposition in overlying skin, subcutaneous tissue and fascia
Microscopic (histologic) images
Images hosted on other servers:
Additional references
Mantle cell / marginal zone hyperplasia
Definition / general
- Gene rearrangement studies and followup recommended to rule out occult lymphoma in cases with clear cells (Am J Clin Pathol 2001;116:550)
- Marginal zone hyperplasia is rare (except in mesenteric nodes) but does occur in reactive nodes (APMIS 2002;110:325)
Microscopic (histologic) description
- Monomorphic proliferation of small lymphocytes with clear cytoplasm and round nuclei
- No pericapsular infiltration, sinuses are identifiable, scattered reactive follicles
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Positive stains
- BCL2 (by marginal zone B cells) (Am J Surg Pathol 2003;27:888)
Negative stains
- No light chain restriction
Molecular / cytogenetics description
- Usually negative
Monocytoid B cell hyperplasia
Definition / general
- Benign disorders are toxoplasmosis, cat scratch disease, EBV, HIV, autoimmune disorders, zoonotic Brugia (Am J Surg Pathol 2005;29:595, Hum Pathol 1985;16:979, Am J Clin Pathol 1996;105:384)
- Also associated with mantle cell / marginal zone hyperplasia
Microscopic (histologic) description
- Sinuses distended by small lymphoid cells with clear cytoplasm and central, round / oval nuclei
- Variable neutrophils
Differential diagnosis
Additional references
Plasmacytosis
Definition / general
- Common in paracolic lymph nodes draining tumor (Virchows Arch A Pathol Anat Histopathol 1987;411:239)
- Associated with idiopathic plasmacytic lymphadenopathy, which resembles Castleman disease (Int J Surg Pathol 2004;12:25)
Case reports
- 72 year old woman with Sjögren syndrome and plasmacytosis resembling myeloma (J Korean Med Sci 2005;20:506)
Microscopic (histologic) description
- Well preserved nodal architecture with plasma cells and plasma cell precursors in medullary cords
- Sinuses are patent
Negative stains
- No light chain restriction
Molecular / cytogenetics description
- Not clonal
Differential diagnosis
- Plasmacytic SLL / CLL:
- Diffuse, not just involvement of medullary cords
Polykaryocytes
Definition / general
- Warthin-Finkeldey polykaryocytes are associated with various benign and malignant lymphoid conditions
- Initially identified in tonsils of patients with measles
- Also present in HIV patients
Case reports
- 65 year old woman with systemic lupus erythematosus (Hum Pathol 1988;19:1358)
Microscopic (histologic) description
- Multinucleated giant cells from 25 - 150 microns, with minimal cytoplasm and up to 60 nuclei
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Additional references
Sinus histiocytosis
Definition / general
- Also called sinus hyperplasia
- Nonspecific finding associated with various etiologies (benign or malignant), including postprosthesis
- May represent pregranulomatous phase of sarcoidosis (Am J Surg Pathol Acta Histochem 2006;107:473)
- Rarely occurs in pelvic nodes of prostate cancer and axillary nodes of breast cancer patients (Cancer 1997;80:277)
Case reports
- 16 year old girl with parvovirus (J Clin Pathol 2005;58:872)
- 68 year old woman with postarthroplasty histiocytic lymphadenopathy (Arch Gynecol Obstet 2004;269:217, J Urol 1997;158:128)
- 70 year old diabetic patient with signet ring cell sinus histiocytosis resembling carcinoma (Am J Clin Pathol 1989;92:509, Int J Clin Oncol 2003;8:184)
Microscopic (histologic) description
- Dilated and prominent sinuses, often containing increased macrophages or sinus lining cells
- May resemble signet ring cell carcinoma cells but lack atypia and are mucin negative
Microscopic (histologic) images
Images hosted on other servers:
Cytology description
- Polygonal histiocytes with rounded nuclei (Acta Cytol 1998;42:1347)
Board review style question #1
Board review style answer #1
A. The crystals in CSH in tissues get phagocytosed by histiocytes where they form collections. When these cells accumulate, they form a mass lesion.
Comment Here
Reference: Other nonspecific findings
Comment Here
Reference: Other nonspecific findings
Other pigment / foreign material
Asbestos
Terminology
Epidemiology
Sites
Clinical features
Diagnosis
Radiology description
Prognostic factors
Case reports
Clinical images
Images hosted on other servers:
Asbestos with mesothelioma:
Gross description
Microscopic (histologic) description
Differential diagnosis
Additional references
- Also called Ferruginous bodies
Epidemiology
- Usually due to industrial / occupational exposure
Sites
- Most common in thoracic / hilar lymph nodes
- Concentration of asbestos fibers in lymph nodes is 2 - 3x higher than in lung
Clinical features
- Inhaled asbestos fibers have iron protein mucopolysaccharide coating
- Enlarged lymph nodes are common
- Associated symptoms / signs of pulmonary asbestosis
Diagnosis
- Biopsy of affected lymph node
- Bleach digestion for confirmation
Radiology description
- Mediastinal / hilar lymphadenopathy
Prognostic factors
- Pulmonary asbestosis is a risk factor for lung carcinoma and mesothelioma
Case reports
- 26 year old man with mesothelioma due to asbestos exposure (Case Rep Med 2011;2011:951732)
Clinical images
Images hosted on other servers:
Asbestos with mesothelioma:
Gross description
- Lymph nodes may be enlarged but show no significant abnormalities on cut surface
Microscopic (histologic) description
- Asbestos bodies are golden-brown, beaded or dumbbell shaped structures with a thin, translucent core
Differential diagnosis
- Pseudoasbestos bodies
- Other ferruginous bodies
Additional references
Gold
Definition / general
Sites
Clinical features
Diagnosis
Laboratory
Radiology description
Case reports
Gross description
Microscopic (histologic) description
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Differential diagnosis
Additional references
- Colloidal gold is used for anti-tumor therapy, to treat autoimmune diseases and for drug delivery (Wikipedia: Colloidal gold [Accessed 22 June 2018])
- Lymphadenopathy and lymph node infarction are uncommon complications of gold injections; occur via accumulation of gold particles in macrophages
Sites
- Cervical, axillary, mesenteric lymph nodes, depending on route of administration
Clinical features
- Tender enlarged lymph nodes
- Benign process with resolution of symptoms on withdrawal of gold treatment
Diagnosis
- Biopsy
- Darkfield microscopy and autometallography methods demonstrate gold nanoparticles 15 to 50 nm
Laboratory
- Polymorphonuclear neutrophilia, leukopenia, thrombocytopenia
- Hepatic toxicity
Radiology description
- Gold deposits can mimic intranodal axillary calcific deposits on mammography in patients with rheumatoid arthritis (Proc (Bayl Univ Med Cent) 2013;26:28)
Case reports
- 34 year old woman with lymphadenopathy and lymph node infarction (J Clin Pathol 2001;54:562)
- Intramammary lymph node gold deposits simulating microcalcifications on mammogram (Hum Pathol 1988;19:992)
- Lymphadenopathy and lymph node infarction (Am J Med 1986;80:537)
Gross description
- Extensive necrosis of lymph node
Microscopic (histologic) description
- Subtotal or complete infarction with peripheral rim of organizing granulation tissue in region of subcapsular sinus
- Small focus of residual viable lymphoid tissue has features of follicular hyperplasia
- Center of node has ghost outlines of necrotic cells
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
- Immunohistochemical stains confirm the reactive nature of the process
Differential diagnosis
- Lymphoma
- Vascular thrombosis, infections and mechanical pressure can also cause infarction of lymph nodes
Additional references
Iron
Definition / general
Sites
Pathophysiology
Clinical features
Diagnosis
Radiology description
Prognostic factors
Case reports
Treatment
Gross description
Gross images
Images hosted on other servers:
Microscopic (histologic) description
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Positive stains
Negative stains
Electron microscopy description
Differential diagnosis
Additional references
- Iron deposition in lymph nodes is rare
- Also called hemosiderosis
- May be associated with hemochromatosis
Sites
- Portal, splenic, mesenteric or axillary, depending on the primary cause
Pathophysiology
- Parenteral iron administration
- Multiple transfusions: red blood cells are lysed and iron is deposited in macrophages in the liver and spleen followed by drainage to adjacent lymph nodes
- Hereditary hemosiderosis: abnormalities in iron transport - most common is HFE1 gene mutation; also mutations in TfR2, HJV, HAMP, ferroportin-V162del (Hepatology 2005;42:466)
- Anemia of inflammation: associated with rheumatoid arthritis, gout (Ann Rheum Dis 1970;29:81)
Clinical features
- Depends on the primary cause
- Enlarged lymph nodes
Diagnosis
- Biopsy with Perls Prussian blue stain
Radiology description
- CT: enlarged and hyperdense lymph nodes (AJR Am J Roentgenol 1981;136:1191)
Prognostic factors
- Depends on the primary cause, severity and extent of the disease
Case reports
- Women, 19 to 49 years, with lymphadenopathy after single infusion of iron dextran (J Clin Pathol 1968;21:492)
Treatment
- Reduce iron overload, treat the primary cause
Gross description
- Enlarged lymph nodes
Gross images
Images hosted on other servers:
Microscopic (histologic) description
- Follicular hyperplasia, sinus histiocytosis, golden brown pigment deposition
Microscopic (histologic) images
Contributed by Mark R. Wick, M.D.
Positive stains
Negative stains
Electron microscopy description
- Prominent phagocytic reticulum cells containing vacuoles, myelin figures, lipid droplets and very large lysosomal bodies with fine electron dense granules concentrated in these lysosomal bodies and in the cytoplasm, which show molecular structure of ferritin
Differential diagnosis
- Charcoal laden macrophages
- Hemazoin pigment: seen in malaria
- Melanin pigment deposition: melanoma, dermatopathic lymphadenitis
- Tattoo pigment
Additional references
Lipogranuloma
Definition / general
Case reports
Microscopic (histologic) description
- Also called lipophagic granuloma
- Secondary to various inflammatory and neoplastic conditions or primary lesion of lymph node
- In West, commonly due to mineral oil ingestion or total parenteral nutrition
Case reports
- 27 year old woman with prior giant cell tumor (Acta Cytol 2002;46:772)
Microscopic (histologic) description
- Giant cells (mono or multinuclear) with foamy and vacuolated cytoplasm
Melanosis
Definition / general
Terminology
Epidemiology
Sites
Pathophysiology
Clinical features
Case reports
Gross description
Microscopic (histologic) description
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Negative stains
Differential diagnosis
Additional references
- A rare manifestation of a completely regressed melanoma
Terminology
- Tumoral melanosis
- Nodular melanosis
- Nodal melanosis
Epidemiology
- Very rare in lymph nodes
Sites
- Sentinel lymph nodes of regressed melanoma
- Mesenteric lymph nodes
Pathophysiology
- Possibilities include:
- A regressed focus of metastatic melanoma
- Melanophages migrating to the lymph node from the primary lesion
Clinical features
- Enlarged lymph nodes
- Yellow brown spindle bodies in mesenteric nodes may be due to melanosis coli (Histopathology 1978;2:47)
- The paucity of cases precludes adequate evaluation of long term implications and treatment outcomes
Case reports
- 36 year old man with lymph node melanosis and metastatic melanoma of unknown primary (Arch Pathol Lab Med 2009;133:1332)
- 83 year old man with melanosis coli involving pericolonic lymph nodes associated with the herbal laxative Swiss Kriss (Arch Pathol Lab Med 2004;128:565)
- Tumoral melanosis involving the sentinel lymph nodes (J Cutan Pathol 2007;34:284)
- Lymph node melanosis from a primary cutaneous lesion combining a nodular (tumoral) melanosis and a congenital dermal melanocytic nevus (Am J Dermatopathol 2012;34:653)
Gross description
- Enlarged lymph nodes with or without brown pigment deposition
Microscopic (histologic) description
- Subcapsular and sinusoidal accumulation of pigmented cells with vesicular chromatin and inconspicuous nucleoli
- Cells may be spindle shaped or epithelioid
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Negative stains
Differential diagnosis
Additional references
Peliosis
Table of Contents
Definition / general | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Also called peliosis lienis
- Multiple blood filled cystic spaces
- Usually associated with peliosis hepatis but may be independent
- Multiple pathogenetic mechanisms possible - may be caused by infection with Bartonella henselae in HIV patients with bacillary angiomatosis
- May cause splenic rupture and death, particularly in patients with carcinomatosis, chronic leukemia, post liver transplantation, recipients of anabolic androgenic steroids, tuberculosis
Case reports
- 56 year old woman with abdominal pain and anorexia (Br J Radiol 2010;83:e126)
- 62 year old man with peliosis and splenic rupture secondary to untreated chronic myelomonocytic leukemia (Am J Surg Pathol 1983;7:197)
Microscopic (histologic) description
- Blood filled sinuses with reduced lining cells, similar to hairy cell leukemia
Differential diagnosis
Additional references
Plasmacytoma
Table of Contents
Definition / general | Case reports | Microscopic (histologic) images | Differential diagnosisDefinition / general
- This topic only discusses features of plasmacytoma in lymph nodes different from plasmacytoma and myeloma
- Very rare (< 50 reported cases)
- Diagnosis of primary plasmacytoma of lymph node requires exclusion of extramedullary plasmacytoma (15% of upper respiratory tract plasmacytomas metastasize to cervical nodes) and myeloma (40% of high stage myelomas metastasize to nodes)
- 2/3 male; median age 59 years (range 39 - 76 years)
- Often involves cervical nodes
- Similar survival to other extramedullary plasmacytomas, although does not progress to myeloma (Am J Clin Pathol 2001;115:119, Hum Pathol 1997;28:1083)
Case reports
- 65 year old man with cervical and submandibular node involvement (Korean J Intern Med 2005;20:183)
- 71 year old man with diffuse hypermetabolic lymphadenopathy of chest, abdomen and pelvis (J Med Case Rep 2019;13:153)
- 81 year old man with Sjögren syndrome (Pathol Int 1999;49:577)
- Patient with Castleman disease (Arch Pathol Lab Med 1986;110:157, Am J Clin Pathol 1982;78:541)
Microscopic (histologic) images
Differential diagnosis
- Castleman disease, plasma cell variant
- Lymphoplasmacytic lymphoma:
- Also has neoplastic small lymphocytes
- Often shows IgM restriction and MYD88 gene mutation
- Marginal zone lymphoma:
- May have plasmacytoid features but more extensive sampling may reveal B cell component
- CD20 expression by neoplastic B cells and plasmacytoid cells
- Clonal B cell population by flow; molecular or cytogenetic features of marginal zone lymphoma
- Large cell lymphoma:
- May have immunoblastic or plasmablastic features
- Plasma cell myeloma with nodal involvement:
- Must be excluded by radiographs, bone marrow biopsy
- Plasmablastic lymphoma:
- Mostly extranodal; less frequent nodal involvement
- Associated with HIV or other immunosuppressive states and EBV
- Reactive plasmacytosis:
- Often follicular hyperplasia
- No light chain restriction
Progressive transformation of germinal centers
Table of Contents
Definition / general | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- 75% males
- Usually presents with solitary asymptomatic lymphadenopathy of head and neck; more frequently in elderly in Japan (Int J Surg Pathol 2003;11:101)
- May be part of evolutionary spectrum with follicular hyperplasia and follicular lysis, in the resolution of lymphoid hyperplasia by sequential ingression of T cells followed by mantle B cells (Am J Clin Pathol 2003;120:322)
- Either self limited (particularly in young men, Am J Surg Pathol 1992;16:252) or associated with lymphocyte predominant Hodgkin lymphoma (Am J Clin Pathol 1990;93:219)
Microscopic (histologic) description
- Germinal centers are markedly (3 - 5x) larger than normal, with indistinct margins; are composed of follicular mantle lymphocytes and extensive follicular dendritic cells
- Tingible body macrophages are also present
- Associated with more typical germinal centers
- Large numbers of T cells are present
- May have a few small hyaline vascular type germinal centers
- Rarely have T cell rosettes
- May have BCL2 expression in mantle B cells
Differential diagnosis
Progressive transformation of germinal centers
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Progressive transformation of germinal centers (PTGC) is a clinicopathologic entity characterized by chronic lymphadenopathy with a wide age distribution
- Characterized by enlarged nodal lymphoid follicles with ingress of mantle zone B cells into the germinal centers (Arch Otolaryngol Head Neck Surg 2006;132:797)
- Nonspecific manifestation of a variety of associated conditions, including immune and autoimmune disorders
- Associated with previous, concurrent or subsequent lymphoma, particularly nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), in a small proportion of patients; less frequently with classic Hodgkin lymphoma (J Clin Exp Hematop 2014;54:205)
Essential features
- Common differential diagnosis of persistent reactive lymphadenopathy, especially in adolescent boys (Pediatr Blood Cancer 2022;69:e29638)
- Expansile lymphoid follicles with mantle zone B cells expanding into and disrupting the germinal centers in a hyperplastic lymph node (Pediatr Blood Cancer 2022;69:e29638)
- Not a premalignant condition; may occur prior to, concurrent with or following Hodgkin lymphoma, most commonly nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) (Int J Pediatr Otorhinolaryngol 2002;65:195)
- Associated with immune and autoimmune conditions in some patients
Terminology
- Progressive transformation of germinal centers
- Follicle lysis: follicle centers fractured by ingrowth of mantle cells
ICD coding
- ICD-10: R59.9 - enlarged lymph nodes, unspecified
Epidemiology
- M:F = 3:1 (Int J Pediatr Otorhinolaryngol 2002;65:195)
- Occurs in both adult and pediatric populations (Int J Pediatr Otorhinolaryngol 2002;65:195)
Sites
- Cervical lymph nodes: most commonly affected site (50%) (Pediatr Blood Cancer 2022;69:e29638)
- Inguinal (25%) and axillary (22%) lymph nodes also common (Pediatr Blood Cancer 2022;69:e29638)
- Abdominal lymph nodes infrequent (Int J Pediatr Otorhinolaryngol 2002;65:195)
Pathophysiology
- Nonspecific, likely multifactorial, representing a state of follicular hyperplasia following reactive stimuli (Case Rep Otolaryngol 2017:2017:5982168)
- Does not follow the typical progression of lymphoid follicular reaction (whereby germinal center reaction comes to an end, follicle regresses, reaction dissipates)
- However, there is somatic hypermutation, clonal expansion and selection, similar to usual hyperplastic germinal centers (Blood 2001;97:714)
- Instead, germinal center proceeds through morphologically distinct stages
- Germinal center becomes enlarged and mantle zone thickens
- Mantle zone B cells migrate into the germinal center, disrupting the follicular dendritic meshwork and fracturing the germinal center into irregular clusters of centrocytes and centroblasts (follicle lysis)
- Stromal microenvironment is distorted and the follicle becomes entirely overrun by mantle cells (Ann Diagn Pathol 2020;44:151421)
Etiology
- Unknown etiology; probably a nonspecific lymphoid reaction
- May represent an abnormal or unusual immune response to a variety of stimuli (Am J Surg Pathol 1992;16:252)
Diagrams / tables
No information provided
Clinical features
- Can present as an isolated lesion or as part of the presentation of systemic conditions (Pediatr Blood Cancer 2022;69:e29638)
- More commonly presents as slow growing lymphadenopathy with no constitutional symptoms (Semin Diagn Pathol 2023;40:371)
- May involve numerous lymph nodes in one region (Pediatr Blood Cancer 2022;69:e29638)
- Lymphadenopathy with constitutional symptoms should raise the suspicion of concurrent malignancy or other systemic disorders (Pediatr Blood Cancer 2022;69:e29638)
- Associated noncausally, most commonly with NLPHL, which may precede, follow or coincide with the diagnosis of PTGC (Leuk Lymphoma 2011;52:2041)
- May also be associated with immune and autoimmune disorders, such as Castleman disease and lupus (Pediatr Blood Cancer 2013;60:26)
- Rare case associated with papillary thyroid carcinoma (Case Rep Otolaryngol 2016;2016:6469073)
Diagnosis
- Excisional biopsy is needed to establish the diagnosis, usually evident at low magnification
- Careful microscopic examination for the possibility of potential concurrent Hodgkin lymphoma
- Evaluation for possible associated conditions should be tailored for patients with constitutional symptoms (Pediatr Blood Cancer 2022;69:e29638)
Laboratory
- Not usually associated with elevated lactate dehydrogenase (LDH), as seen in lymphoma
Radiology description
- Hypoechoic lymph node on ultrasound (Ann Hematol 2018;97:1081)
Radiology images
No information provided
Prognostic factors
- PTGC follows a clinically indolent course (Semin Diagn Pathol 2023;40:371)
- Increased risk of recurrence seen in pediatric patients (Pediatr Blood Cancer 2022;69:e29638)
- Not a premalignant condition but there is a noncausal association with NLPHL in a small number of patients (Leuk Lymphoma 2011;52:2041)
- May also be seen in association with immune and autoimmune conditions
Case reports
- 2 year old boy with a lump in the right axilla (Children (Basel) 2022;9:214)
- 32 year old woman with palpable lymph nodes in the left axilla (Taehan Yongsang Uihakhoe Chi 2021;82:423)
- 39 year old woman with right neck lymphadenopathy (Case Rep Otolaryngol 2017:2017:5982168)
Treatment
- Standard of care: active surveillance (Leuk Lymphoma 2011;52:2041)
- Ongoing follow up and annual examination, including biopsy of any subsequent enlarged lymph nodes to assess for possibility of reactive follicular hyperplasia, PTGC or Hodgkin lymphoma (Int J Pediatr Otorhinolaryngol 2002;65:195)
Clinical images
No information provided
Gross description
- Well encapsulated, enlarged lymph node
- Pale gray to tan lobulated cut surface; typically not fish flesh as seen in lymphoma
Gross images
No information provided
Frozen section description
No information provided
Frozen section images
No information provided
Microscopic (histologic) description
- Occurs in a background of follicular hyperplasia of lymph node
- Preserved nodal architecture with PTGC follicles diameter being 3 - 5 times larger than usual reactive follicles (Am J Clin Pathol 1990;93:219)
- Background lymphoid follicles appear as primary follicles when PTGC is associated with NLPHL (Hum Pathol 2015;46:1655)
- PTGC nodules may appear in different morphologic stages
- Multifocal migration of small lymphocytes from the thickened mantle zone into the germinal center, disrupting the germinal center and breaking up the germinal center into variegated clusters of follicle center cells (follicle lysis)
- Loss of demarcation between germinal center and mantle zone
- Normal germinal center cells (centrocytes, centroblasts, follicular dendritic cells) may be lost (Acta Haematol 2002;108:33)
Microscopic (histologic) images
Contributed by Roaa Aljuaid, M.B.B.S.
Cytology description
No information provided
Cytology images
No information provided
Peripheral smear description
No information provided
Peripheral smear images
No information provided
Positive stains
- Mantle zone B cells: express pan B cell markers (CD19, CD20, PAX5 and CD79a) and IgD
- Germinal center cells: express pan B cell markers as well as germinal center markers, BCL6, CD10, HGAL and GCET1
- CD21 shows disruption of the follicular dendritic cell meshwork
- Reference: Pediatr Blood Cancer 2022;69:e29638
Negative stains
- All cells are negative for EBV (EBER, LMP), EMA, CD15 and except few CD30+ small cells
- Mantle zone B cells: negative for BCL1, SOX11, CD5 and CD43
- Germinal center B cells: negative for BCL2
- Reference: Int J Pediatr Otorhinolaryngol 2002;65:195
Flow cytometry description
- Polytypic B cells
- No T cell immunophenotypic aberrancy
Flow cytometry images
No information provided
Electron microscopy description
No information provided
Electron microscopy images
No information provided
Molecular / cytogenetics description
- No evidence of B cell (IGH and IGK) or T cell receptor (TRB and TRG) gene rearrangement
- No BCL2 or BCL6 gene alterations
Molecular / cytogenetics images
No information provided
Videos
No information provided
Sample pathology report
- Right cervical lymph node, excisional biopsy:
- Reactive lymphoid hyperplasia with progressive transformation of germinal centers (PTGC) (see comment)
- Negative for malignancy
- Comment: PTGC may precede, follow or coincide with nodular lymphocyte predominant Hodgkin lymphoma in certain patients.
Differential diagnosis
- Nodular lymphocyte predominant Hodgkin lymphoma:
- Nodular lymphocyte predominant Hodgkin lymphoma (WHO HAEM5), also known as nodular lymphocyte predominant B cell lymphoma (Blood 2022;140:1229)
- Lymph node architecture is partially or completely effaced
- Nodules contain lymphocytic and histiocytic (popcorn) neoplastic B cells
- Large lymphocytes express pan B cell markers, BCL6, EMA (variable) and strong OCT2
- T cells rosette around lymphocytic and histiocytic cells and often express CD3, PD-1 and CD57
- Follicular lymphoma, floral variant:
- Partial or complete effacement of the lymph node architecture
- Follicles are fused, fragmented and back to back
- Neoplastic germinal centers may appear monotonous with various proportions of centrocytes and centroblasts and diminished tingible body macrophages and mitoses
- Monoclonal B cells typically express BCL6, CD10, HGAL, GCET1 and BCL2
- Lymphocyte rich Hodgkin lymphoma, nodular variant:
- Human immunodeficiency virus (HIV) associated lymphadenopathy:
- Affected lymph node in acute HIV infection can resemble PTGC
- Follicle lysis: small mantle zone B cells penetrate and disrupt the germinal centers
- Affected follicles are not as large as the PTGC follicles
- Hemorrhage with large monocytoid cell proliferation is common
- IgG4 related lymphadenopathy (Semin Diagn Pathol 2024;41:108, Am J Surg Pathol 2018;42:977)
- Pediatric nodal marginal zone lymphoma (Virchows Arch 2023;483:317)
Additional references
No information provided
Board review style question #1
Board review style answer #1
E CD79a. The mantle cells express pan B cell antigens, including CD79a. Answers A, C and D are incorrect because these markers are characteristically positive in mantle cell lymphoma but not in the nonneoplastic mantle cells of PTGC. Answer B is incorrect because BCL6 is immunoreactive on follicle center cells and not on mantle cells.
Comment Here
Reference: Progressive transformation of germinal centers
Comment Here
Reference: Progressive transformation of germinal centers
Board review style question #2
Which of the following lymphoid neoplasms is more commonly associated with progressive transformation of germinal centers (PTGC)?
- Follicular lymphoma
- Mantle cell lymphoma
- Mycosis fungoides
- Nodular lymphocyte predominant Hodgkin lymphoma
- Plasma cell neoplasm
Board review style answer #2
D. Nodular lymphocyte predominant Hodgkin lymphoma has been found to have the highest risk of being seen in association with PTGC. Answer C is incorrect because mycosis fungoides is associated with dermatopathic lymphadenopathy and not PTGC. Answers A, B and E are incorrect because these hematopoietic neoplasms are not known to occur with increased incidence in patients with PTGC.
Comment Here
Reference: Progressive transformation of germinal centers
Comment Here
Reference: Progressive transformation of germinal centers
Pseudocyst
Table of Contents
Definition / generalDefinition / general
- 75% of nonparasitic splenic cysts
- Usually due to trauma; some may be epithelial cysts with denuded epithelial lining
- Usually solitary, asymptomatic
- Wall composed of dense fibrous tissue without an epithelial lining, often calcified
- Often contains blood and necrotic debris
- Rupture may cause massive hemoperitoneum
Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Table of Contents
Definition / general | Essential features | Nodal follicular / nodular proliferations | Nodal immunoblastic proliferations | Extranodal follicular proliferations | Extranodal immunoblastic proliferations | Case reports | Microscopic (histologic) images | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Heterogeneous group of nonneoplastic proliferations that mimic B cell lymphomas and affect lymphoid tissue in nodal or extranodal sites
Essential features
- Heterogeneous group of nonneoplastic proliferations that mimic B cell lymphomas and affect lymphoid tissue in nodal or extranodal sites
- 3 different main patterns
- Nodal and extranodal follicular proliferations
- Nodal and extranodal nodular proliferations
- Nodal and extranodal immunoblastic proliferations
Nodal follicular / nodular proliferations
Florid follicular hyperplasia (Hematol Oncol Clin North Am 2009;23:729)
Progressive transformation of germinal centers (PTGC) (Virchows Arch 2019;475:771)
Hyaline vascular Castleman disease (HVCD)
- Increased number of widely spaced primary and secondary follicles
- Florid cases usually involve medulla
- Follicles show uneven size and shape
- Hyperplastic germinal centers (GC) comprise a mixture of centroblasts and centrocytes with brisk mitoses, reactive T cells, follicular dendritic cells (FDCs) and tingible body macrophages (Ann Diagn Pathol 2020;44:151421)
- B lymphocytes
- Centroblasts
- Mainly in the dark zone of GC
- 3 - 4x size of small lymphocytes
- Large vesicular nuclei, 1 - 3 peripheral nucleoli
- Centrocytes
- Mainly in the light zone of GC
- Small to intermediate sized cells with cleaved, hyperchromatic nuclei and small or absent nucleolus
- Both centroblasts and centrocytes are BCL6+, CD10+, LMO2+, HGAL / GCET+, OCT2+
- Centroblasts
- T lymphocytes
- Small and round
- CD3+ and BCL2+
- Subset of T helper cells (TFH): polarized CD4+, PD-1 / CD279+, BCL6+, CD10+, CXCL13+, ICOS+
- Follicular dendritic cells (FDCs)
- Few (usually ~1%)
- 2 square shaped and adjacent nuclei (kissing cells) with vesicular chromatin
- May display small nucleolus
- Long cytoplasmic processes
- CD21+, CD23+ or CD35+
- Tingible body macrophages
- Display oval or twisted vesicular nuclei
- Abundant pale cytoplasm containing karyorrhectic nuclei
- Impart a starry sky pattern when prominent
- CD4+, CD68+ or CD163+
- B lymphocytes
- Well developed mantle zones
- Distinction from germinal centers
- Composed predominantly of small lymphocytes and IgD+
- No or limited extension outside the capsule into perinodal soft tissue
- Location can suggest underlying disease and additional workup is necessary to confirm the diagnosis (Pediatr Clin North Am 2002;49:1009)
- Cervical: infectious mononucleosis
- Posterior cervical: toxoplasmosis
- Parotid, submaxillary, epitrochlear: HIV infection
- Cervical and axillary: cat scratch disease, dermatopathic lymphadenitis
- Inguinal: sexually transmitted diseases
- Immunoarchitecture (Ann Diagn Pathol 2020;44:151421, Clin Lab Med 2021;41:433)
- Lymphoid follicles express B cell antigens
- Primary follicles
- Small lymphocytes
- BCL2+, Ki67 (usually < 10%), BCL6- and CD10-
- Secondary follicles
- GCs are BCL2-
- TFH cells: subset of T cells in germinal centers are BCL2 positive and occasionally are increased
- May mimic follicular lymphoma
- Pediatric type follicular lymphoma (FL) is BCL2 negative
- FOXP1 is positive in GC cells of pediatric FL (Virchows Arch 2019;475:771)
- TFH cells: subset of T cells in germinal centers are BCL2 positive and occasionally are increased
- High proliferation Ki67
- Polarization (centroblast rich dark zone and a centrocyte rich pale zone)
- Low Ki67 proliferation rate within a germinal center should always be considered atypical and suspicious for follicular lymphoma
- BCL6+ and CD10+ are restricted to GCs, minimal in interfollicular areas
- GCs are BCL2-
- Flow cytometry
- Polytypic B cells; CD10+, T cell antigens-
- Polymerase chain reaction (PCR)
- Polyclonal immunoglobulin heavy chain gene rearrangement
- No evidence of t(14;18)(q32;q21) or IGH::BCL2 fusion
Progressive transformation of germinal centers (PTGC) (Virchows Arch 2019;475:771)
- Single or few large (4 - 5x normal) lymphoid follicles with mantle cells extensively indenting into GCs
- Background lymph node exhibits reactive follicular hyperplasia (RFH)
- Negative for rosetting by PD-1+ cells (TFH) around large B cells
- GCs remnants rarely show tingible body macrophages
- Morphological changes seem to proceed gradually
- Early stages show hyperplastic GCs
- Fusion of GCs within one single follicle (usually 2 - 3x size)
- Inward migration of mantle zone small B cells into the germinal center
- Later stages: dissolution of GCs results in islands or scattered centrocytes and centroblasts with follicular dendritic cells among small mantle zone B cells
- Immunoarchitecture
- Preserved B and T cell compartments of lymph node and prominent follicular pattern
- Both GC and mantle zone cells express pan-B cell antigens
- Mantle cells: BCL2+, IgD+, BCL6- and CD10-
- Disrupted GCs: BCL6+, CD10+, BCL2- and IgD-
- CD21, CD23 or CD35 show disruption of FDCs meshwork
- IgG4+ plasma cells: ~40 - 50% of cases
- Flow cytometry
- Polytypic B cells
- Genetic testing
- Polyclonal IGH rearrangements
Hyaline vascular Castleman disease (HVCD)
- Young adults (Blood 2017;129:1658)
- Usually < 30 years old
- Can also affect children
- Numerous follicles in cortex and medulla of lymph node or extramedullary sites
- Obliteration of subcapsular and interfollicular sinuses
- ≥ 2 germinal centers in follicle (also known as twinning)
- Follicles typically large with regressed (or involuted) germinal centers
- Mostly composed of FDCs with few lymphocytes
- Mantle zones prominent
- FDCs often hyperplastic and can show dysplasia
- Many follicles show so called lollipop features
- Concentric rings of mantle zone lymphocytes (onion skin appearance)
- Sclerotic blood vessels radially traversing into germinal center
- Interfollicular or stromal component is also important (J Clin Exp Hematop 2022;62:60)
- Increased number of high endothelial venules with hyalinized walls
- Stromal component can predominate with only a few hyaline vascular follicles
- Clusters of plasmacytoid dendritic cells can occur
- Plasma cells and immunoblasts are not abundant in HVCD
- More common and abundant in plasma cell Castleman disease (PCCD)
- Immunohistochemistry
- HHV8 is absent
- Polytypic B, T cells and plasma cells
- Increased FDCs in involuted germinal centers
- CD21+, CD23+, CD35+ or EGFR+
- Dysplastic FDCs often stain variably for FDC markers
- Differential diagnosis
- Classic Hodgkin lymphoma concurrent with Castleman disease (Virchows Arch 2020;477:437)
- Diagnosis is established upon identification of Hodgkin Reed-Sternberg (HRS) cells with usual phenotype
- Castleman-like follicles in infectious lymphadenopathies
- Changes are focal and sinuses are open
- Classic Hodgkin lymphoma concurrent with Castleman disease (Virchows Arch 2020;477:437)
| Table 1. Differential diagnosis between florid follicular hyperplasia and B cell lymphomas with follicular formation | ||||
| Feature | Florid follicular hyperplasia | Follicular lymphoma | Marginal zone lymphoma | Mantle cell lymphoma, mantle zone pattern |
| Follicles | Enlarged, with prominent GCs and distinct mantle zones | Variably sized surrounded by faint mantle zones | Nodules with remnants of GCs surrounded by monocytoid cells | Thickened mantle zones |
| Density | Low, widely spaced | Back to back | Variable; may be confluent | Variable |
| Size | Uneven | Uniform | Variable | Uniform |
| Borders of GC | Sharp, well defined | Fainted, crack artifact | Blurry | Sharp, well defined |
| Distribution | Cortical predominance; florid cases involve medulla | Cortex and medulla | Cortex and medulla | Even distribution throughout cortex and medulla |
| Extension to perinodal fat | Absent or unusual | Often present | Often present | Uncommon |
| Mantle zone | Present, well developed | Attenuated to absent | Generally absent | Expanded in mantle zone pattern |
| Marginal zone | Can be hyperplastic | Absent | Expanded, may coalesce | Absent |
| Germinal center cells | Colonized by monocytoid cells | Absent | ||
| Tingible body macrophages | Present and common | Decreased or absent | Decreased or absent | Variable |
| Polarization | Present | Absent | Absent | Variable |
| Cytologic features | Centroblasts, centrocytes and macrophages | Centroblasts and centrocytes in various proportions | Monocytoid and plasmacytoid; scattered large cells | Small and intermediate centrocyte-like cells; few large cells |
| Immunoarchitecture | ||||
| BCL2 | Negative in GCs | Positive in germinal centers of FL grades 1 - 2; 50% in FL grade 3 | Positive in neoplastic cells; GC remnants are negative | Positive in mantle zones |
| BCL6, CD10 | Positive, restricted to GCs | Positive in GCs and interfollicular areas | Negative, GC remnants positive | Positive, restricted to GCs |
| Ki67 | High proliferation rate; polarized in GCs | Low, nonpolarized | Low, nonpolarized | Variable in mantle zones |
| CD21, CD23, CD35 | FDC meshworks preserved | FDC meshworks preserved | Distorted FDC meshworks | FDC meshworks preserved |
| Common positive markers | BCL6, LMO2, OCT2, HGAL | CD10, BCL2, BCL6, LMO2 | CD43, MNDA, CD5-/+ | Cyclin D1, SOX11, CD5 |
| Common negative markers | CD3, BCL2 | CD5, cyclin D1 | CD10, cyclin D1, SOX11 | CD10-, CD23- in mantle zones |
| Flow cytometry | Polytypic | Monotypic surface Ig; CD10 positive | Monotypic surface Ig; CD10 neg; CD5 neg or weak | Monotypic surface Ig; CD5 positive |
| Table 2. Differential diagnosis between progressive transformation of germinal centers and lymphomas with large nodules | |||
| Feature | Progressive transformation of germinal center | Nodular predominant Hodgkin lymphoma (patterns A / B) | Lymphocyte rich classic Hodgkin lymphoma, nodular variant |
| Follicles | |||
| Size | Scattered large nodules | Nodules larger than PTGC | Moderately enlarged |
| Germinal centers | Variably disrupted or involuted | Absent | Present within neoplastic nodules |
| Centrocytes and centroblasts | Decreased; scattered, BCL2- | Absent | In residual GCs |
| Mantle zone cells | Inward growth into GCs, BCL2+ and IgD+ | Absent | Expanded |
| Reactive follicular hyperplasia | Present at background | Absent or focal | Absent or focal |
| Immunoarchitecture | |||
| T follicular helper cells rosettes (PD-1+) | Absent | Present | May be present |
| IgG4+ plasma cells | In 40 - 50% of cases | Absent | Absent |
| Large B cells | Rare immunoblasts | LP cells (popcorn cells) | Present, HRS cells |
| Immunophenotype | IgG+ | CD20+, CD45+, OCT2+, EMA+/-, PAX5+ strong, IgD-/+ | CD30+, CD45-, CD15+, PAX5+ dim, OCT2-, EMA-, CD20- |
Nodal immunoblastic proliferations
Infectious mononucleosis (Semin Diagn Pathol 2018;35:54, Virchows Arch 2019;475:771)
Herpesvirus lymphadenitis (Virchows Arch 2019;475:771)
Cytomegalovirus lymphadenitis (Virchows Arch 2019;475:771)
- Caused by acute Epstein-Barr virus (EBV) infection
- Histologic changes are variable and depend on disease duration
- Early stage: reactive follicles, with mild paracortical expansion
- Progression: follicles sparse due to interfollicular expansion
- Advanced stage: follicles may become effaced, with interfollicular expansion
- Interfollicular areas with numerous immunoblasts or mixed infiltrate
- Immunoblasts can be dispersed or abundant
- Lymphocytes range from small to intermediate and large in size
- Plasma cells, plasmacytoid cells and histiocytes are variably present, less frequently accompanied by eosinophils
- Sinuses are regularly occupied by immunoblasts, monocytoid B cells and histiocytes
- Immunoblasts can be binucleated, mimicking HRS
- Intact nodal architecture admixed with B and T immunoblasts are indicative of a reactive process
- Focal necrosis may be present
- Immunohistochemistry
- HRS-like cells are CD45+, CD15-, CD30+ (weak) admixed with B and T immunoblasts
- EBV latency type 3 (Semin Diagn Pathol 2018;35:54)
- EBV encoded RNA (EBER) present in numerous infected cells, ranging from small and intermediate lymphocytes to HRS-like immunoblasts
- EBNA1+, EBNA2 variable and EBNA3+
- LMP1+ and LMP2+
- Most lymphocytes in the background are CD8+, TIA1+
Herpesvirus lymphadenitis (Virchows Arch 2019;475:771)
- Uncommon complication from either primary exposure or reactivation (more common)
- Prominent immunoblastic paracortical hyperplasia
- Similar to infectious mononucleosis
- Sharply circumscribed areas of necrosis and sinus histiocytosis
- Adjacent to areas of necrosis
- Multinucleated cells with ground glass chromatin and intranuclear viral inclusions (halo)
- Herpes simplex virus (HSV) immunohistochemistry confirms the diagnosis
Cytomegalovirus lymphadenitis (Virchows Arch 2019;475:771)
- Morphologic features overlapping with those described in other viral infections
- Monocytoid B cell hyperplasia is frequent
- Characteristic owl eye central intranuclear inclusions with a halo
- Cells infected with cytomegalovirus (CMV) often are T cells or endothelial cells
- Diagnosis can be established utilizing CMV immunohistochemical stain
| Table 3. Differential diagnosis between nodal B cell immunoblastic proliferation and lymphomas | |||
| Feature | Infectious mononucleosis | Classic Hodgkin lymphoma | Diffuse large B cell lymphoma, EBV+ |
| Nodal compartment | Paracortical | Diffuse effacement | Diffuse effacement |
| Neoplastic cells | Not present | Scattered large cells; confluent in nodular sclerosis syncytial variant | Abundant large cells; diffuse pattern |
| Background lymphocytes | |||
| B cells | Small to intermediate | Small | Small |
| T cells | CD8+, small to intermediate | CD4+, small | CD8+, small |
| Monocytoid B cells hyperplasia | Often present | Absent | Absent |
| Plasma cells | Variably present | Few | Not frequent |
| Histiocytes | Variably present | Variable | Variable |
| Eosinophils | Rare | Present, often abundant | May be present |
| Necrosis | Focally present | Often present in nodular sclerosis subtype | May be present |
| Immunoarchitecture | |||
| EBV latency type | Type III | Type II | Type I |
| EBER | Positive in most cells | Positive in HRS cells | Positive in large cells |
| LMP1 | Positive, fewer small and large cells (less sensitive) | Restricted to HRS cells in positive cases | If positive, restricted to large neoplastic cells |
| EBNA2 | Mostly positive | Negative | Negative |
| EBNA3 | Positive | Negative | Negative |
| CD20 | Positive in large cells | Faint reactivity in HRS cells in ~20% of cases | Positive in large cells |
| CD3 | Positive in small lymphocytes | Negative in HRS cells | Negative in large cells |
| CD30 | Positive in HRS-like cells, usually dim | Positive in HRS cells, strong | May be positive in neoplastic cells |
| CD45 | Positive in most cells | Negative in HRS cells | Positive in neoplastic cells |
| CD4, PD-1, ICOS, CXCL13 | Negative in immunoblasts | Negative in HRS cells | Negative in neoplastic cells |
| CD21 | Present only in GCs | Present only in residual GCs | Absent |
| Diagnostic molecular testing | Polyclonal B and T cells | Polyclonal B and T cells | Monoclonal IGH gene rearrangements |
Extranodal follicular proliferations
Florid reactive lymphoid hyperplasia
- Most cases represent reactive tertiary lymphoid tissue and may be associated either with an infectious agent, autoimmune phenomena or chronic repetitive trauma; these processes may lead to extranodal marginal zone lymphoma (Pathology 2020;52:15)
- Stomach: Helicobacter pylori
- Skin: Borrelia burgdorferi
- Conjunctiva: Chlamydia psittaci
- Cervix: Chlamydia trachomatis
- Small intestine: Campylobacter jejuni
- Lung: Achromobacter xylosoxidans
- Gallbladder: extrahepatic biliary obstruction
- Thyroid: Hashimoto thyroiditis
- Salivary gland: Sjögren syndrome
- Differential diagnosis with marginal zone lymphoma can be challenging in some cases
- Cutaneous reactive lymphoid hyperplasia must be also distinguished from primary cutaneous follicular center lymphoma (see table) (Arch Pathol Lab Med 2018;142:1313)
- Florid reactive lymphoid hyperplasia of the female reproductive tract
- Cervix is the most frequent affected site, followed by endometrium and vulva (Int J Gynecol Pathol 2022;41:459)
- Most cases show lymphoid infiltrate with superficial distribution, with or without ulceration
- Architecture varies from patchy and nodular to diffuse, with secondary follicles in most cases
- Tends to imitate lymph nodes organization, with a B follicular and T interfollicular compartments
- Cytologically composed by small lymphocytes and polytypic plasma cells, with variable number of granulocytes and histiocytes in the background
- Scattered large CD30+ immunoblasts are present in the majority of cases
- Clonal IGH rearrangement is not uncommon, although patients are free of lymphoma on follow up (Arch Pathol Lab Med 2018;142:1313)
| Table 4. Differential diagnosis between cutaneous follicular hyperplasia and B cell lymphomas | |||
| Feature | Reactive follicular hyperplasia | Primary cutaneous follicular center lymphoma | Primary cutaneous marginal zone lymphoma |
| Infiltrate | Denser in superficial dermis, wedge shaped | Denser in deep dermis, Grenz zone | Vertical orientation around follicles |
| Follicular architecture | Present, uneven shapes and size | Follicular or diffuse pattern | Pale nodules, some residual germinal centers |
| Mantle zone | Present, well developed | Attenuated to absent | Absent |
| Marginal zone | Generally absent | Absent | Expanded, may coalesce |
| Germinal center cells | Colonized by monocytoid cells | ||
| Tingible body macrophages | Present | Absent | Present in GC remnants |
| Polarization | Present | Absent | Absent |
| Cytologic features | Centrocytes and centroblasts | Centroblasts and centrocytes | Monocytoid cells, few large cells; plasma cells |
| Immunoarchitecture | B and T compartments preserved | Effaced, B cell predominant | Effaced, B cell predominant |
| BCL2 | Negative in germinal centers | Negative in germinal centers | Positive in neoplastic cells; GC remnants are negative |
| BCL6 | Positive, restricted to GCs | Positive in both GCs and interfollicular areas | Negative, GC remnants positive |
| Ki67 | High and polarized in GCs | Low, nonpolarized | Low, nonpolarized |
| CD21, CD23, CD35 | FDC meshworks preserved | FDC meshworks preserved | Distorted FDC meshworks |
| Light chain restriction | Absent | May be present | Present in plasmacytoid lymphocytes or mature plasma cells |
| Positive IgH / IgK clonality assays | No | Yes | Yes |
Extranodal immunoblastic proliferations
EBV positive mucocutaneous ulcer (Surg Pathol Clin 2023;16:213)
- Lymphoproliferative disorder characterized by EBV+ large B cells / Reed-Sternberg-like cells (RS-like cells) cells
- Oral mucosa is most common site, followed by skin
- Multiple small lesions within same anatomic region can occur
- No systemic symptoms, lymphadenopathy, hepatosplenomegaly or bone marrow involvement
- Well circumscribed superficial ulcer with a subjacent polymorphous infiltrate
- Deep base shows a sharp demarcation by a dense rim of small lymphocytes
- Angioinvasion and high proliferation index may be present
- Monoclonal IGH or TCR gene rearrangements can occur in up to a third of cases
- Immunohistochemistry
- HRS-like cells: CD45+, CD15- and CD30+ (usually weak)
- Background: B and T cells
- EBV latency type 3 (Semin Diagn Pathol 2018;35:54)
- EBV encoded RNA (EBER) present in numerous infected cells, ranging from small and intermediate lymphocytes to HRS-like immunoblasts
- LMP1+, EBNA2+
- Basal rim of T cells are CD8+
Case reports
- 24 year old man with follicular lymphoid hyperplasia with aggressive behavior in maxilla (Oral Maxillofac Surg 2017;21:475)
- 40 year old man with infectious mononucleosis that presented as a lymphadenopathy with geographic necrosis (Pathol Res Pract 2004;200:53)
- 57 year old woman with idiopathic multicentric Castleman disease (TAFRO subtype) (Br J Haematol 2022;196:461)
- 70 year old woman with atypical follicular hyperplasia after COVID-19 booster (Virchows Arch 2023;482:905)
Microscopic (histologic) images
Contributed by Roberto N. Miranda, M.D. and Roman Segura-Rivera, M.D.
Board review style question #1
Which of the following statements is true about progressive transformation of germinal center (PTGC)?
- Centrocytes are increased and have a BCL2+ phenotype
- IgG4+ plasma cells are usually absent
- Reactive follicular hyperplasia is usually absent in the background
- Rosettes of T follicular helper (TFH) cells are usually absent
Board review style answer #1
D. Rosettes of TFH cells are usually absent. Answer B is incorrect because PTGC cases usually present with IgG4+ plasma cells in up to 50% of cases. Answers A and C are incorrect because there is a decrease of centrocytes and reactive follicular hyperplasia in the background.
Comment Here
Reference: Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Comment Here
Reference: Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Board review style question #2
Board review style answer #2
D. Type III: EBER+, LMP1+, EBNA2+. Answers A, B and C are incorrect because the most common latency pattern in infectious mononucleosis is the pattern III, which is characterized by the positivity of EBER, LMP1 and EBNA2.
Comment Here
Reference: Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Comment Here
Reference: Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Reactive lymphadenopathy
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Lymph node enlargement due to hyperplasia of cellular components reflecting antigenic stimulation
- Benign and reversible process.
Essential features
- Clinically manifests as lymph node enlargement
- No clonal process
- No cytologic or architectural atypia
Terminology
- Reactive lymphoid hyperplasia
- Reactive follicular hyperplasia
- Diffuse paracortical hyperplasia
- Sinus histiocytosis
ICD coding
Epidemiology
- Represents the reaction of lymphoid tissue to intrinsic or environmental antigens
- Most lymph node enlargements are reactive
- In children, most lymphadenopathies are benign; in adults, chance of malignancy increases with age
Sites
- Any lymph node group can be affected depending on the stimulation
Pathophysiology
- Lymph nodes filter lymph drained from tributary regions
- Substances carried by lymph reach the nodes; these may be antigenic and cause an immune reaction
- Bacteria and fungi cause predominantly inflammatory reactions; viruses and drugs cause predominantly immune reactions
- 4 patterns of reactive hyperplasia have been described, depending on the etiology: follicular, paracortical / diffuse, sinus and mixed
Etiology
- Multiple etiologic factors, including:
- Bacteria
- Fungi
- Viruses
- Chemicals
- Environmental pollutants
- Drugs: phenytoin, allopurinol, atenolol, gold, penicillin, quinidine
- Altered tissue components
- Other antigens or allergens
- Definitive identification of etiologic agent is possible only in a small subset of cases
Clinical features
- Clinical syndrome usually reflects the underlying disorder
- Generally, of short duration but may be prolonged
- Enlargement of lymph node(s) may be painful or tender
- Associated symptoms include fever, weight loss, malaise, loss of appetite
- Nodes are soft or fluctuant in inflammation and suppuration
Diagnosis
- Histopathology
- Exclude specific causes by clinical, laboratory and imaging studies
Laboratory
- Depends on the etiologic agent
Radiology description
- Enlarged lymph node(s)
Prognostic factors
- Benign, self limiting process
- Prognosis depends on the etiology
Treatment
- Treatment of the underlying disorder
Gross description
- Enlarged, soft, lymph node with tan homogenous cut surfaces
Microscopic (histologic) description
- Reactive follicular hyperplasia
- B cell response pattern
- Enlarged follicles, varying in size and shape, may coalesce and display different configurations
- Prominent germinal center and mantle zone
- Germinal centers show mixed small and large lymphocytes - centrocytes and centroblasts
- Centroblasts polarize to the medial pole forming the darker zone and centrocytes accumulate at the peripheral pole forming the lighter zone
- Centroblasts are 3 - 4 times larger than the inactivated lymphocytes and show narrow rim of basophilic cytoplasm and large, round to oval vesicular nuclei with 1 - 3 prominent peripheral nucleoli
- Mitotic figures are frequent
- Centrocytes are smaller lymphocytes with scant cytoplasm, cleaved nuclei, clumped chromatin and small or absent nucleoli
- Numerous tingible body macrophages are a characteristic feature of follicular hyperplasia
- Diffuse paracortical hyperplasia
- T cell response pattern
- T cell zones, paracortical or interfollicular are expanded with a heterogeneous population of cells, including numerous small lymphocytes and admixed immunoblasts resulting in a starry sky or moth eaten appearance
- Immunoblasts in some cases may resemble Reed-Sternberg cells
- Proliferation of high endothelial venules is another characteristic finding
- Sinus histiocytosis
- Sinuses are prominent and are lined by hyperplastic sinus histiocytes
- Mixed
- Follicular, diffuse and sinus patterns coexist in one lymph node
Cytology description
- Cellular smears with mixed small and large lymphocytes and numerous tingible body macrophages
- No cytologic atypia
Positive stains
Negative stains
- BCL2 is negative in reactive follicular centers (in contrast to follicular lymphoma, in which neoplastic follicles are BCL2+)
Flow cytometry description
- No aberrant immunophenotype
Molecular / cytogenetics description
- Gene rearrangement - polyclonal pattern
Differential diagnosis
- Atypical lymphoid hyperplasia: cellular atypia
- Follicular lymphoma: effaced architecture, back to back nodules which invade surrounding tissues and capsule; no tingible body macrophages, no mantle zones; germinal center is BCL2+; follicular cells are monoclonal, t(14;18) present
- Hodgkin lymphoma: Reed-Sternberg cells and variants are present
- Other non-Hodgkin lymphomas: monotonous population of atypical lymphoid cells, invasion of capsule and surrounding tissues, monoclonal nature and specific surface markers
Additional references
Rheumatoid arthritis
Table of Contents
Definition / general | Microscopic (histologic) description | Microscopic (histologic) images | Negative stainsDefinition / general
- Associated with lymphadenopathy during course of disease in 82%, usually axillary (J Int Med Res 2003;31:345)
- Lymphadenopathy usually disappears during disease remission
- May also have fever, weight loss, anemia
- Modestly increased risk of lymphoma, may be due to methotrexate treatment
Microscopic (histologic) description
- Follicular hyperplasia with sparse (J Clin Pathol 1990;43:106) vs. active (Acta Pathol Jpn 1990;40:249) proliferative activity, interfollicular plasma cells with Russell bodies, vascular proliferation
- Capsular lymphocytic infiltrate
- May resemble plasma cell variant of Castleman disease
- Often PAS+ extracellular hyaline material
- Occasionally focal necrosis and microabscesses
- May have sarcoid-like granulomas
Microscopic (histologic) images
Negative stains
- Polyclonal plasma cells (no light chain restriction)
Rosai-Dorfman disease (RDD)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, non-Langerhans cell histiocytosis with heterogeneous clinical features
- Described in 1960s as a self limiting condition involving the lymph nodes (Arch Pathol 1969;87:63)
- Extranodal involvement in approximately 40% of cases; may be limited or disseminated disease (Blood 2018;131:2877)
- May be familial or associated with neoplasia or immune disease (Blood 2016;127:2672)
Essential features
- Clinical features depend on the site(s) involved and disease associations
- Classic (nodal) Rosai-Dorfman disease (RDD) and cutaneous RDD often self limited and benign
- Disseminated RDD may have an aggressive clinical course necessitating systemic therapies (Haematologica 2020;105:348, Blood 2018;131:2877)
- Approximately 33% of cases harbor clonal mitogen activated protein kinase / extracellular signal regulated kinase (MAPK / ERK) pathway alterations (Mod Pathol 2017;30:1367, Haematologica 2020;105:e5)
Terminology
- Initially described by Destombes as adenitis with lipid excess (Bull Soc Pathol Exot Filiales 1965;58:1169)
- Rosai and Dorfman described as sinus histiocytosis with massive lymphadenopathy (Arch Pathol 1969;87:63)
- Classified in the R group (nodal and extranodal disease) and the C group (cutaneous disease only) under the revised classification of histiocytic disorders (Blood 2016;127:2672)
ICD coding
- ICD-10: D76.3 - Rosai-Dorfman disease
Epidemiology
- Mean age at diagnosis: 50 years (range 2 - 79) (Haematologica 2020;105:348)
- F:M = 1.5:1
Sites
- Lymph node involvement common (30 - 50%) (Am J Surg Pathol 2021;45:35, Blood 2018;131:2877)
- Skin is the most common extranodal site (50%)
- Other extranodal sites include bone, upper respiratory tract (sinus), central nervous system including dura and parenchymal lesions, orbit, retroperitoneum (Mayo Clin Proc 2019;94:2054)
Etiology
- Pathogenesis unclear and likely multifactorial
- Association with immune disease suggests that immune dysregulation may play a role in pathogenesis
- Subset harbor MAPK / ERK pathway alterations, suggesting a pathogenesis similar to Langerhans cell histiocytosis and Erdheim-Chester disease (Mod Pathol 2017;30:1367, Cancer Discov 2016;6:154)
Clinical features
- Heterogeneous clinical course, depending on the site of involvement
- Associated conditions include autoimmune disorders (systemic lupus erythematosus, idiopathic juvenile arthritis, autoimmune hemolytic anemia), neoplasia such as lymphomas and myeloid neoplasms and IgG4 related RDD characterized by increased IgG4 positive plasma cells (Blood 2018;131:2877, Haematologica 2020;105:348)
- Classic (nodal) RDD presents as enlarged lymph nodes, most commonly involving bilateral cervical nodes in young males
- Extranodal RDD manifests with diverse clinical presentations (Blood 2018;131:2877)
- Cutaneous lesions present as slow growing, painless nodules, papules or plaques
- Neurologic involvement may present with headache, seizures, motor / sensory abnormalities and gait disturbances
- Orbital involvement may present with exophthalmos or visual disturbance
- Sinus involvement may display nasal congestion or obstruction
- Pulmonary RDD can present with dry cough, dyspnea and may mimic primary lung cancer, interstitial lung disease and other inflammatory / infectious conditions
- Retroperitoneal involvement may present as diffuse infiltration and less commonly a discrete mass with or without hydronephrosis and ureteral obstruction
- Multisystem involvement in approximately 20% cases and prognosis related to the number of extranodal sites
- Rosai-Dorfman disease may be a component of mixed histiocytosis with Langerhans cell histiocytosis or Erdheim-Chester disease (Mod Pathol 2010;23:1616, Haematologica 2020;105:e5)
Diagnosis
- Comprehensive evaluation, including clinical history, physical examination and imaging studies in conjunction with morphologic features
- RDD may be associated with other histiocytic neoplasms, including Langerhans cell histiocytosis and Erdheim-Chester disease, which should be confirmed by biopsy in the setting of atypical clinical manifestations (Mod Pathol 2010;23:1616, Haematologica 2020;105:e5)
Radiology description
- Nodal involvement on PET CT harboring RDD shows increased central intake with a photopenic halo (Mayo Clin Proc 2019;94:2054)
- Extranodal involvement may show the following findings:
- CNS involvement often presents as hyperattenuating meningeal based mass showing contrast enhancement on CT (Asian J Neurosurg 2010;5:19)
- Skeletal RDD lesions are usually lytic and centered in medullary space (Mayo Clin Proc 2019;94:2054)
- Pulmonary involvement may present as cystic change, interstitial lung disease, nodules, focal pulmonary infiltrate or airway disease
- Sinus involvement may present with maxillary wall thickening
- Retroperitoneum involvement may present as renal hilar masses, subcapsular hypodense infiltration or renal cortical hypodense nodules
- CNS involvement often presents as hyperattenuating meningeal based mass showing contrast enhancement on CT (Asian J Neurosurg 2010;5:19)
Prognostic factors
- Optimal duration of treatment with systemic therapy is individualized, depending on the extent of involvement
- Usually favorable outcomes in cases with nodal and cutaneous involvement
- Subset may demonstrate aggressive clinical course usually associated with multifocal involvement (Haematologica 2020;105:348)
Case reports
- 10 year old boy with polyuria and polydipsia (Front Med (Lausanne) 2020;7:613756)
- 12 year old boy with dyspnea and left sided chest pain presented with interatrial septal mass (J Radiol Case Rep 2012;6:1)
- 29 year old woman with arthralgias in the hands and knees and lytic lesions of the left distal radius (Curr Rheumatol Rep 2017;19:29)
- 41 year old man with paravertebral mass (J Int Med Res 2017;45:875)
- 53 year old man with diminished vision of right eye associated with a palpable mass (J Microsc Ultrastruct 2019;7:50)
- 57 year old woman with progressive vision loss (Head Neck Pathol 2016;10:414)
Treatment
- Management varies, depending on the site of involvement and the presence / absence of symptoms (Blood 2018;131:2877)
- Nodal / cutaneous involvement usually self limited and requires only observation
- Surgical resection may be considered in symptomatic cases with single site of involvement
- Multifocal and refractory cases may require systemic therapy (first line therapy with cladribine, cytarabine, methotrexate or prednisone)
- Targeted therapy with MEK inhibitors is under investigation in those with MAPK / ERK pathway alterations (Haematologica 2020;105:348)
Microscopic (histologic) description
- Characterized by the accumulation of histiocytes with enlarged, round to oval hypochromatic nuclei and abundant eosinophilic cytoplasm, often containing engulfed intact inflammatory cells known as emperipolesis (Am J Surg Pathol 2021;45:35)
- Lesional histiocytes usually associated with fibrosis and prominent inflammatory infiltrate comprised of plasma cells and lymphocytes
- Occasional neutrophilic infiltrates are present
- Nodal involvement is characterized by distended sinuses with lesional histiocytes
- Rosai-Dorfman disease is rarely detected as an incidental finding concomitantly with lymphoma in the same biopsy specimen (Mod Pathol 2019;32:16)
Microscopic (histologic) images
Positive stains
- Monocyte / macrophage markers: CD68, CD163, OCT2
- S100, cyclin D1 / BCL1
- Reference: Am J Surg Pathol 2021;45:35
Negative stains
- CD1a, langerin
- BRAF V600E
- Reference: Am J Surg Pathol 2021;45:35
Molecular / cytogenetics description
- Approximately 33% of cases harbor mutually exclusive recurrent KRAS and MAP2K1 mutations (Mod Pathol 2017;30:1367)
- Germline mutations in SLC29A3 gene may be present in familial cases of RDD (Blood 2016;127:2672)
Sample pathology report
- Lymph node, right axillary, excisional biopsy:
- Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy) (see comment)
- Comment: Histologic examination shows distended lymph node sinuses containing a prominent population of histiocytes characterized by large, round nuclei and voluminous cytoplasm showing emperipolesis with engulfment of lymphocytes. The lymphoplasmacytic infiltrate within the lymph node parenchyma is cytologically unremarkable. Immunohistochemistry shows that the atypical histiocytes within the lymph node sinuses are positive for CD68, CD163 with coexpression of S100, OCT2 and cyclin D1. They are negative for BRAF V600E by immunohistochemistry. There is no increase in the number of IgG4 positive plasma cells or an abnormal IgG4/IgG ratio. CD3 and CD20 reveal an appropriate mixture of T cells and B cells, respectively. Kappa and lambda immunoglobulin light chains reveal polytypic plasma cells. CD1a and langerin are negative.
Differential diagnosis
- Sinus histiocytosis:
- Langerhans cell histiocytosis:
- Langerhans cells are characterized by their irregular, grooved, folded or indented nuclei with fine chromatin, inconspicuous nucleoli and abundant pale eosinophilic cytoplasm
- Positive for CD1a and langerin
- Erdheim-Chester disease:
- Bland appearing histiocytes characterized by abundant foamy (xanthomatous) cytoplasm with surrounding fibrosis (Mod Pathol 2018;31:581)
- Touton giant cells are frequently present
- Positive for CD68, CD163, factor XIIIa and negative for OCT2 expression by immunohistochemistry (Am J Surg Pathol 2021;45:35)
- Clinical, radiologic and morphologic correlation required (Mayo Clin Proc 2019;94:2054)
- Juvenile xanthogranuloma:
- Usually in infants and young children
- Histologically and phenotypically similar to Erdheim-Chester disease
- Low grade B cell lymphoma:
Board review style question #1
A 47 year old woman presented with multiple skin nodules on bilateral arms that were gradually increasing in size over 6 months. Based on the morphology, which of the following is the most likely diagnosis?
- Follicular dendritic cell sarcoma
- Juvenile xanthogranuloma
- Langerhans cell histiocytosis
- Reactive process
- Rosai-Dorfman disease
Board review style answer #1
Board review style question #2
Lesional cells in sinus histiocytosis with massive lymphadenopathy show positive expression of which of the following immunostains?
- AE1 / AE3, CAM5.2, CK34βE12
- CD117, DOG1, muscle specific actin
- Cyclin D1, S100 and OCT2
- Desmin, MyoD1, myogenin
- SMA, p63, p40
Board review style answer #2
C. Cyclin D1, S100 and OCT2 (Am J Surg Pathol 2021;45:35)
Comment Here
Reference: Rosai-Dorfman disease (RDD)
Comment Here
Reference: Rosai-Dorfman disease (RDD)
Sclerosing angiomatoid nodular transformation
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, benign splenic lesion of unknown etiology, characterized by confluent angiomatoid nodules surrounded by concentric collagen fibers
Essential features
- Nonneoplastic condition that affects the spleen only; not described in other sites
- Microscopically characterized by multiple angiomatoid nodules in a fibrosclerotic stroma
- Surgery is curative in all the cases and recurrence has not been reported
Terminology
- Multinodular hemangioma (obsolete)
ICD coding
- ICD-10: D18.03 - hemangioma of intra-abdominal structures
Epidemiology
- Female predilection (F:M = 2:1)
- Mean age of 48 years
- Range is 30 - 60 years (Int J Surg Case Rep 2012;3:492)
Sites
- Only described in the spleen
- Confined to the splenic parenchyma, with no extrasplenic involvement
Etiology
- Etiology and pathogenesis unknown
- Exaggerated sclerotic and neoangiogenic splenic reaction could be secondary to different pre-existing conditions, including vascular lesions undergoing thrombosis, infarcts and even hamartoma (Int J Surg Case Rep 2012;3:492, Am J Surg Pathol 2004;28:1268)
Clinical features
- Asymptomatic in the majority of the cases; incidentally discovered by imaging studies or at time of surgery for unrelated processes (Am J Surg Pathol 2004;28:1268)
- Symptomatic patients may experience nonspecific symptoms, including abdominal discomfort, abdominal fullness, nausea, vomiting and undesired weight loss
Diagnosis
- Clinically and radiologically indistinguishable from other lesions of the spleen, including hemangioma, hamartoma and rarely, malignancies
- Final diagnosis is achieved only with histopathologic examination and immunohistochemistry of resected spleen specimens
- Splenectomy serves both diagnostic and treatment functions
- References: Am J Surg Pathol 2004;28:1268, Semin Diagn Pathol 2021;38:159
Laboratory
- Laboratory values are mostly unaltered
- Occasional reports of nonspecific findings, including raised erythrocyte sedimentation rate, leukocytosis and polyclonal hypergammaglobulinemia (Am J Surg Pathol 2004;28:1268)
Radiology description
- No characteristic and unique radiologic findings of this entity
- Abdominal ultrasound, CT scan and MRI studies usually reveal an isolated, multinodular solid splenic mass
- References: J Comput Assist Tomogr 2019;43:863, Diagn Interv Imaging 2021;102:389
Radiology images
Prognostic factors
- Benign clinical behavior with favorable prognosis and no reported recurrences after splenectomy (Am J Surg Pathol 2004;28:1268)
Case reports
- 21 year old woman with mild thrombocytopenia and a hypervascular splenic mass (Radiol Case Rep 2018;14:521)
- 26 year old man and 65 year old woman presenting with undesired weight loss and a lasting feeling of abdominal fullness (World J Clin Cases 2020;8:103)
- 37 year old woman with a 5 cm hypoechoic mass in the spleen with low standardized uptake value (SUV) at FDG PET / CT scan (Acta Radiol Open 2016;5:2058460116649799)
- 49 year old woman with a newly diagnosed invasive clear cell carcinoma of the uterus and a 4 cm isolated splenic nodule suspicious for metastatic involvement (J Med Case Rep 2018;12:377)
- 67 year old man with an isolated 55 mm splenic lesion suspicious for an abscess (Int J Surg Case Rep 2019;56:1)
Treatment
- Splenectomy performed at discovery of the splenic mass is curative in all the cases
Gross description
- Single unencapsulated, well circumscribed solid mass with a striking multinodular quality and variable fibrotic strands (Semin Diagn Pathol 2021;38:159)
Gross images
Contributed by Shafinaz Hussein, M.D. and Huifei Liu, M.D., Ph.D. (Case #364)
Images hosted on other servers:
Microscopic (histologic) description
- Multiple, well circumscribed and confluent angiomatoid nodules surrounded by a variable fibrosclerotic stroma and occasionally, by a fibrinoid rim imparting a granuloma-like appearance at low magnification (Arch Pathol Lab Med 2007;131:974, Semin Diagn Pathol 2021;38:159)
- Nodules contain a mixture of slit-like, round or irregular vascular spaces lined by cytologically bland, sometimes plump endothelial cells; lacking atypia, necrosis and mitotic figures
- 3 different types of vessels within the nodules can be highlighted by immunohistochemistry (see Positive stains), which recapitulate the normal red pulp composition: capillaries, sinusoids and small veins
- Stroma separating the nodules is fibrous to fibromyxoid with bland fibroblasts and myofibroblasts; contains abundant inflammatory cells, including lymphocytes, hemosiderin laden macrophages and polytypic plasma cells (Semin Diagn Pathol 2021;38:159)
- IgG4 positive plasma cells occasionally numerous with IgG4:IgG ratio increased; however, serum IgG4 concentration not elevated (Pathol Int 2009;59:844)
- Adjacent splenic tissue is unremarkable
Microscopic (histologic) images
Contributed by Valentina Sangiorgio, M.D., Shafinaz Hussein, M.D. and Huifei Liu, M.D., Ph.D. (Case #364)
Positive stains
- 3 types of vessels (as seen in the normal splenic red pulp):
- SMA positive in myofibroblasts in the nodules
- CD68 positive in histiocytes / macrophages
- Polytypic expression of kappa and lambda light chains within plasma cells
- References: Am J Surg Pathol 2004;28:1268, Arch Pathol Lab Med 2013;137:1309
Negative stains
- D2-40
- Epstein Barr virus (EBV) in situ hybridization
Electron microscopy description
- Small vascular spaces lined by endothelial cells with pinocytotic vesicles but no Weibel-Palade bodies (Am J Surg Pathol 2004;28:1268)
Sample pathology report
- Spleen, splenectomy:
- Sclerosing angiomatoid nodular transformation (SANT) of the spleen
Differential diagnosis
- Hemangioma:
- Often of cavernous type
- Cystically dilated, blood filled spaces lined by a layer of bland endothelial cells
- Vascular spaces separated by thin fibrous septa, without the striking angiomatoid nodular appearance of sclerosing angiomatoid nodular transformation (Semin Diagn Pathol 2021;38:154)
- Littoral cell angioma:
- Macroscopically enlarged spleen overrun by multiple hemorrhagic nodules with a vague lobular configuration and a spongy appearance; uncommonly solitary nodules
- Nodules composed of pleomorphic vascular spaces filled with papillary projections
- Papillary stalks lined by plump endothelial cells
- Characteristic immunophenotype of splenic littoral cells with dual histiocytic and endothelial differentiation lacking CD34
- Hemangioendothelioma and angiosarcoma:
- Ill defined vascular spaces lined by variably atypical, malignant endothelial cells, with necrosis and increased mitotic activity (Semin Diagn Pathol 2021;38:154)
- Hamartoma:
- Structurally disorganized red pulp tissue, with no Malpighian follicles and only occasional fibrous trabeculae
- Compared with sclerosing angiomatoid nodular transformation, the borders are poorly defined and it lacks the angiomatoid nodular appearance (Semin Diagn Pathol 2021;38:154)
Board review style question #1
What does this image from a sclerosing angiomatoid nodular transformation of the spleen show?
- Areas of geographic necrosis
- Atypical endothelial cells with prominent nucleoli
- Easily identifiable mitotic figures (≥ 1 per 10 high power fields)
- Small capillaries admixed with scattered inflammatory cells, including small lymphocytes, histiocytes and plasma cells
Board review style answer #1
D. Small capillaries admixed with scattered inflammatory cells, including small lymphocytes, histiocytes and plasma cells. Necrosis, atypia and increased mitotic activity (≥ 1 per 10 high power fields) are not seen in this image. They are not seen in otherwise conventional sclerosing angiomatoid nodular transformation and their identification should prompt alternative diagnoses, including hemangioendothelioma or angiosarcoma.
Comment Here
Reference: Sclerosing angiomatoid nodular transformation
Comment Here
Reference: Sclerosing angiomatoid nodular transformation
Board review style question #2
Which of the following statements best describes sclerosing angiomatoid nodular transformation of the spleen?
- Benign condition that affects the spleen only, is cured with splenectomy and does not manifest any metastatic potential
- Benign neoplasm that can affect parenchymatous organs (mostly spleen, liver and lung) and does not manifest any metastatic potential
- Neoplasm of uncertain malignant potential as it can recur after resection in sites other than spleen
- Neoplasm of uncertain malignant potential as occasional cases transform into overt angiosarcoma
Board review style answer #2
A. Benign condition that affects the spleen only, is cured with splenectomy and does not manifest any metastatic potential. Sclerosing angiomatoid nodular transformation is a nonneoplastic lesion which affects the spleen only, with no description in other anatomic sites. It is cured by splenectomy and never recurs thereafter.
Comment Here
Reference: Sclerosing angiomatoid nodular transformation
Comment Here
Reference: Sclerosing angiomatoid nodular transformation
Silicone
Table of Contents
Definition / general | Terminology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
- Rare enlargement of regional lymph nodes caused by the presence of silicone carried from tributary organs
- Either an incidental finding or causes painful / enlarged lymph node
- May be associated with granulomatous inflammation (Histol Histopathol 1997;12:1003)
Terminology
- Silicone lymphadenopathy
Sites
- Draining lymph nodes of the implant site, commonly axillary lymph nodes (Respiratory Medicine CME 2011;4:126)
Pathophysiology
- Silicone is widely used in implants, especially augmentation mammoplasty and joint prostheses (Hum Pathol 1980;11:240)
- Once released into tissue, silicone migrates to distant sites through lymphatic channels and bloodstream
- Once it reaches lymph nodes, it elicits a reaction and cause silicone lymphadenopathy
Etiology
- Rupture / leak of the implant, or implant 'bleeds', or releases microparticles into blood / lymphatics
Clinical features
- Enlarged lymph nodes
- Asymptomatic or with pain
Diagnosis
- Biopsy
Radiology description
- Ultrasonogram: hyperechoeic (increased echogenicity of the lymph node mediastinum with dirty acoustic shadowing), beginning in the hilum and progressing outward through the cortex with time and amount of silicone
- In severe cases, snowstorm appearance
Prognostic factors
- Depends upon the amount of silicone and severity of the reaction
Case reports
- 35 year old woman with axillary silicone lymphadenopathy (J Med Case Rep 2009;3:6442)
- 40 year old woman with silicone lymphadenopathy involving intramammary lymph nodes (AJR Am J Roentgenol 1994;162:1089)
- 45 year old woman with axillary silicone lymphadenopathy secondary to augmentation mammaplasty (Indian J Plast Surg 2010;43:206)
- 47 year old woman with silicone lymphadenopathy mimicking lymphoma (J Clin Pathol 2000;53:549)
- 49 year old woman with lymphadenopathy associated with total joint prostheses (J Bone Joint Surg Am 1996;78:588)
- 59 year old woman with silicone migration to the contralateral axillary lymph nodes and breast after highly cohesive silicone gel implant failure (Cases J 2009;2:6420)
- 71 year old woman with siliconoma in internal mammary lymph node (Radiology Case Reports 2011;6(4))
Treatment
- Removal of lymph nodes
Gross description
- No specific gross features
- Enlarged and firmer than normal
- Extreme cases show distorted architecture and fibrosis
Microscopic (histologic) description
- Diffuse follicular hyperplasia
- Histiocytes with vacuolated cytoplasm especially inside the sinusoids
- Histiocytes cause foreign body granulomatous reaction, giant cells and empty vacuoles
- Giant cells have refractile and non-birefringent particles
- Asteroid bodies may be seen
- Silicone from orthopedic devices: prominent granulomatous reaction with clumps of granular yellowish refractile material
- Silicone from mammary prostheses: finer vacuolated deposits
Microscopic (histologic) images
Contributed by Dr. Mark R. Wick
Images hosted on other servers:
Cytology description
- Numerous multi-vacuolated histiocytic cells both scattered individually as well as in aggregates
- Contain clear, refractile but non-polarizable material
- Background may show scattered lymphocytes
Electron microscopy description
- Electron opaque fragmented spicules or flakes
Differential diagnosis
- Fat necrosis
- Lipogranuloma
- Metastatic lobular carcinoma
- Metastatic renal cell carcinoma
- Metastatic signet ring cell carcinoma
- Signet ring cell type lymphoma
- Sinus histiocytosis with massive lymphadenopathy
Splendore-Hoeppli phenomenon
Definition / general
- Abscess containing brightly eosinophilic, pseudomycotic structures composed of necrotic debris and immunoglobulin in starburst pattern
- In U.S., usually due to Staphylococcus aureus or Pseudomonas aeruginosa; in tropics, due to schistosomiasis, microfilariae, various fungi
Case reports
- 61 year old woman with CLL for 10 years with Nocardia asteroides in spleen (Arch Pathol Lab Med 2001;125:1515)
Splenectomy, rupture & splenosis
Splenectomy
Definition / general
- Usually performed for traumatic rupture
- Usually no clinical consequence in adults but elevated risk of infection is present, especially in elderly and those with malignancy (Med J Aust 2012;196:582)
- In children, associated with increased incidence and severity of infections, particularly encapsulated bacteria such as Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae; overwhelming infections (OPSI: overwhelming postsplenectomy infection) may begin days to years after splenectomy, without an identifiable focus and have 50 - 80% mortality despite antibiotics
- Increased susceptibility to severe infections with intraerythrocytic parasites Babesia and Plasmodium (Clin Microbiol Rev 2010;23:740)
- In children, splenectomy is avoided in favor of splenic repair, partial splenectomy or splenic autotransplant
- Must consider possibility of accessory spleen(s) if splenectomy is performed for hematologic disorders
Laboratory
- Howell-Jolly bodies are evidence of no splenic function
Case reports
- 2 cases of postsplenectomy pneumococcemia (Arch Pathol Lab Med 1980;104:258)
Peripheral smear images
Images hosted on other servers:
Rupture
Definition / general
- Due to blunt trauma or abdominal surgery, causing hemoperitoneum and emergency splenectomy
- Only rarely ruptures spontaneously (associated with acute splenitis, amyloidosis [rare], infectious mononucleosis, inflammatory disorders, leukemia / lymphoma, malaria, other tumors, peliosis lienis, pregnancy, subacute bacterial endocarditis, typhoid fever)
- Splenic rupture may be delayed after trivial / minor trauma
Case reports
- 16 year old girl with infectious mononucleosis (Int J Surg Case Rep 2012;3:97)
- 29 year old woman with splenic pregnancy (Arch Pathol Lab Med 2004;128:e146)
- 54 year old woman with systemic lupus erythematosus (Clin Rheumatol 2012;31:1019)
- 2 patients with pancreatic cancer presenting with splenic rupture (Arch Pathol Lab Med 2004;128:1146)
Clinical images
Images hosted on other servers:
Gross description
- Rupture may be a very small capsular tear, often in superior pole or hilum
Microscopic (histologic) description
- Neutrophils below capsular tear with intraparenchymal hemorrhage; also lymphoid hyperplasia with prominent marginal zone
Additional references
Splenosis
Definition / general
- Autotransplantation of splenic tissue on peritoneal surface, abdominal wall or elsewhere after rupture or splenectomy
- Usually affects young men
- Common; may affect 67% of those with trauma to spleen
- May also implant within pleural cavity, lung parenchyma or brain
Case reports
- 20 year old man with cerebral splenosis 15 years after posttraumatic splenectomy (Am J Surg Pathol 1998;22:894)
- 49 year old woman with abdominal splenosis mimicking colon and liver tumors (Int J Med Sci 2012;9:174)
Clinical images
Images hosted on other servers:
Microscopic (histologic) description
- Red and white pulp, resembling accessory spleen
Differential diagnosis
Splenic malformations
Table of Contents
Asplenia | Hepatolienal fusion | Hyposplenism | Polysplenia | Splenic gonadal fusion | Splenorenal fusion | Wandering spleen | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) imagesAsplenia
- Rare, associated with cardiac malformations (80%, usually involving atrioventricular endocardial cushion and ventricular outflow tract), situs inversus, anomalies of blood vessels, lung, abdominal viscera
- Can present with pneumococcal sepsis (OPSI)
Hepatolienal fusion
- Fusion of liver and spleen (Hum Pathol 1978;9:234)
- Very rare
Hyposplenism
- Atrophy of spleen with concomitant compromised splenic function (Lancet 2011;378:86)
- Usually acquired but rare congenital forms exist (isolated congenital hyposplenia, Ivemark syndrome, hypoparathyroidism syndrome, autoimmune polyendocrinopathy candidiasis ectodermal dystrophy [APECED] syndrome, Stormorken syndrome)
Polysplenia
- Multiple spleens, found as part of heterotaxy syndromes (malpositioning of organs on opposite side of body, e.g. situs inversus)
- Associated with extrahepatic biliary atresia (Arch Pathol Lab Med 1980;104:212), cardiac abnormalities and developmental anomalies of pancreas, portal vein, inferior vena cava
Splenic gonadal fusion
- Rare congenital anomaly in which ectopic splenic tissue unites with a gonad (< 200 cases reported)
- Continuous: spleen connected to ectopic splenic mass by cord of splenic and fibrous tissue
- Discontinuous: no connection between spleen and ectopic splenic mass
- 20% of continuous types associated with other congenital defects, including peromelus (fetus with malformed limbs) and micrognathia; also testicular ectopia, inguinal hernia
- 90% in males; usually left sided; usually less than 20 years old
- Diagnosis: Technetium Tc 99m sulfur colloid scan
- Treatment: Surgical excision of ectopic splenic tissue to prevent testicular atrophy, torsion or infarction and preserve fertility
Splenorenal fusion
- May be due to splenosis after splenic trauma or splenectomy; less commonly is developmental anomaly resulting in fusion of splenic and renal tissue
- May present as a renal mass or with symptoms of hypersplenism
Wandering spleen
- Due to congenital loss, weakness of splenic ligaments or abnormal length of splenic vessels
- Susceptible to torsion
- May rarely be associated with polysplenia
Case reports
- 16 year old boy with chronic, painless testicular mass (Case #309)
- 22 year old woman with intermittent hypochondrial pain for over a year (Int J Surg Case Rep 2012;3:151)
- 27 year old man with bilateral cryptorchidism and nonseminomatous germ cell tumor in intra-abdominal splenic gonadal mass (Arch Pathol Lab Med 2002;126:1222)
- 51 year old woman with renal mass (Arch Pathol Lab Med 2003;127:e1)
- 56 year old man with asymptomatic testicular mass (Arch Pathol Lab Med 2003;127:e277)
- 62 year old woman with polysplenia with agenesis of dorsal pancreas and preduodenal portal vein presenting with obstructive jaundice (Br J Radiol 2011;84:e217)
- Familial congenital asplenia (Eur J Pediatr 2010;169:315)
- Woman with discontinuous type (Hum Pathol 1989;20:486)
Gross description
- Splenic gonadal fusion: ectopic splenic tissue is well demarcated from gonad, only rarely is intermingling of tissue
Microscopic (histologic) description
- Splenic gonadal fusion:
- Normal splenic parenchyma but may have thrombosis, calcification, fibrosis, fat degeneration, hemosiderin deposits
- Testicular tissue may be normal but may have atrophy or fibrosis of seminiferous tubules, increased Leydig cells, thrombosis of spermatic vessels or testicular tumors
Systemic lupus erythematosus
Table of Contents
Definition / general | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Differential diagnosisDefinition / general
- Most SLE patients have cervical lymphadenopathy
Case reports
- 32 year old woman with coexisting Kukuchi disease (Int J Dermatol 2006;45:454)
- Patient with coexisting Castleman disease (J Rheumatol 1999;26:1400)
Microscopic (histologic) description
- Architectural preservation but follicular hyperplasia with variable sized follicles, increased vascularity, interfollicular immunoblasts and plasma cells
- Often well circumscribed areas of paracortical necrosis with necrosis of small vessels (APMIS 2001;109:141)
- Occasionally DNA deposition / hematoxylin bodies (hematoxyphilic material) in stroma, sinuses and blood vessel walls
- May have giant follicles (APMIS 2005;113:558), often disarray of follicular dendritic cell network (Pathol Int 2000;50:304), no / rare granulomas, no / rare neutrophils
Cytology description
- Typical and atypical immunoblasts, plasma cells, occasional Reed-Sternberg-like cells and dispersed hematoxylin bodies (Acta Cytol 2000;44:67)
Positive stains
- CD11b+ and CD15+ histiocytes
- CD8+ T cells, EBV (up to 20%, Int J Surg Pathol 2005;13:273)
Differential diagnosis
- Castleman disease (Pathol Res Pract 1997;193:565)
- Cat scratch disease: has neutrophils
- Kikuchi disease: less prominent follicular hyperplasia and interfollicular plasma cells, more prominent immunoblasts resembling lymphoma
- Lymphogranuloma venereum: has granulomas
- Polykaryocytes
Toxoplasmosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Lymphadenitis caused by infection with the protozoan Toxoplasma gondii
Essential features
- Worldwide distribution:
- Toxoplasma infection is seroprevalent in approximately 33% of the world's population
- 10 - 20% of patients with acute infection may develop cervical lymphadenopathy (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022])
- Most common parasite infection in the U.S.
- Cats are the only known definitive host (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022])
- Humans infected through contaminated soil, pet litter boxes and ingesting undercooked meat
- Primary infection is subclinical
- Unilateral posterior cervical lymphadenopathy is classic presentation
- Infection during pregnancy can be detrimental to the fetus: congenital toxoplasmosis
- Histologic triad on microscopy (Ioachim: Ioachim’s Lymph Node Pathology, 4th Edition, 2008):
- Reactive germinal centers
- Perifollicular / interfollicular epithelioid histiocytes
- Patches of monocytoid B cells
Terminology
- Toxoplasma lymphadenitis
- Glandular toxoplasmosis
- Piringer-Kuchinka lymphadenopathy
ICD coding
Epidemiology
- Common parasite worldwide (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022])
- Prevalent in warm and humid climates
- Affects children and young adults most commonly
- No gender predilection
- 1.9% IgM and 32.9% IgG seroprevalence globally (Sci Rep 2020;10:12102)
- Most common parasitic infection in the U.S.
- 11% of U.S. population greater than 6 years have been infected with T. gondii (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022])
- Humans can become infected by (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022]):
- Eating undercooked meat of animals harboring tissue cysts
- Consuming food or water contaminated with cat feces
- Exposure to environmental samples
- Fecal contaminated soil
- Changing pet cat litter box
- Blood transfusion or organ transplantation
- Transplacental transmission from mother to fetus
- Greatest risk of infection in first trimester
- Risk of stillbirth in seropositive mothers
- Every year about 400 - 4,000 children are infected at birth in the U.S. (Am Fam Physician 2003;67:2131)
- Often an opportunistic infection of immunocompromised patients
- Infection may result from reactivation in patients with cancer or diabetes
- Asymptomatic infection of immunocompetent individuals
Sites
- Toxoplasma gondii is a protozoan which can invade many cell types (Ioachim: Ioachim’s Lymph Node Pathology, 4th Edition, 2008)
- Lymph nodes are commonly affected: unilateral posterior cervical node is characteristic
- Other lymph nodes can often be involved
- Generalized lymphadenopathy or hepatosplenomegaly unusually occurs
- In the human host, the parasites form tissue cysts in skeletal muscle, myocardium, brain and eyes; these cysts may remain throughout the life of the host
Pathophysiology
- Cats are the only known definitive host for sexual stage of reproduction (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022])
- Cats become infected after consuming intermediate hosts harboring tissue cysts or by ingestion of sporulated oocysts
- Trophozoites multiply in intestine and produce oocytes
- Unsporulated oocysts are shed in the cat's feces
- Oocysts are usually shed for 1 - 3 weeks and in large numbers, up to several million (Lancet 2004;363:1965)
- Oocysts take 1 - 5 days to sporulate in the environment and become infective
- Intermediate hosts in nature (including humans, birds and rodents) become infected after ingesting soil, water or plant material contaminated with oocysts
- Through ingestion of contaminated soil or water
- Ingestion of infected, undercooked meat
- Oocysts are degraded by digestive enzymes and transform into trophozoites
- Tachyzoites are the crescentic / oval shaped, rapidly multiplying stage of the parasite (Lancet 2004;363:1965)
- Tachyzoites are released into the intestine
- Enter all nucleated cells, form cytoplasmic vacuoles, replicate, where finally host cells are disrupted, releasing tachyzoites (Lancet 2004;363:1965)
- Carried by macrophages and spread by lymphatics and blood vessels to infect tissues
- Tachyzoite form leads to the strong inflammatory response and tissue destruction, causing the clinical manifestations of the infection (Lancet 2004;363:1965)
- Tachyzoites are transformed into bradyzoites under the pressure of the immune response to form cysts (thousands of bradyzoites per cyst) and survive the lifetime of the host
Etiology
- Toxoplasmosis gondii infection
Clinical features
- Immunocompetent individuals:
- Asymptomatic infection with or without nontender, nonsuppurative lymphadenopathy (Lancet 2004;363:1965)
- Mild illness in the form of malaise, fever, myalgia (10 - 20%) (Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017)
- Disease is usually benign and self limited with resolution in weeks or months
- Immunocompromised patients (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022]):
- Caused by reactivation of chronic infection
- Often have central nervous system (CNS) disease
- Systemic disease occurs in severe immunodeficiency
- Myocarditis, pneumonitis, chorioretinitis, encephalitis
- In AIDS, toxoplasmic encephalitis is the most common cause of intracerebral mass lesions
- Congenital toxoplasmosis:
- In the U.S., the age adjusted seroprevalence among women of childbearing age (15 - 44 years) was 9% in 2009 to 2010 (Am J Trop Med Hyg 2014;90:1135)
- Third trimester has the highest risk of transmission
- First trimester poses greatest damage to fetus
- Neonatal clinical manifestations vary widely:
- Hydrocephalus, microcephaly, intracranial calcifications, chorioretinitis, strabismus, blindness, epilepsy, psychomotor or mental retardation, petechia due to thrombocytopenia and anemia (Lancet 2004;363:1965)
- In congenital infection, patients are often asymptomatic until the second or third decade of life, when lesions develop in the eye
- Rarely, ocular infection may lead to visual loss
- Ocular Toxoplasma infection, an important cause of retinochoroiditis in the U.S., can be the result of congenital infection or infection after birth
- T. gondii chorioretinitis develops in ~21,000 persons each year in the U.S. (Am J Trop Med Hyg 2014;90:794)
Diagnosis
- Diagnosis is usually achieved by serology, although tissue cysts may be observed in stained biopsy specimens
- Diagnosis of congenital infections can be achieved by detecting T. gondii DNA in amniotic fluid using molecular methods such as PCR (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022])
Laboratory
- Sabin-Feldman dye test
- Highly sensitive and specific
- Requires live organisms so has been replaced with indirect immunofluorescence (IF)
- IF and enzyme immunoassays measure antibodies to T. gondii
- Can measure immunoglobulin M (IgM) and IgG antibodies to cell wall antigens
- IgM antibodies detected within a few days after infection
- IgG antibodies detected at 6 - 8 weeks
- Latex agglutination test and enzyme linked immunosorbent assay (ELISA)
- PCR can be used to amplify T. gondii DNA
- References: Ioachim: Ioachim’s Lymph Node Pathology, 4th Edition, 2008, Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017
Prognostic factors
- Immunocompetent individuals (Lancet 2004;363:1965):
- Self limiting infection
- Immunodeficient patients:
- Risk of disseminating infection
- Almost always due to reactivation of chronic infection (Lancet 2004;363:1965)
- Encephalitis, chorioretinitis, pneumonia, multiple organ involvement or death
Case reports
- 11 month old HIV / AIDS girl with cerebellar toxoplasmosis (Neurol Sci 2012;33:1423)
- 29 year old man with disseminated toxoplasmosis associated with hemophagocytic lymphohistiocystosis after kidney transplant (Transpl Infect Dis 2019;21:e13154)
- 33 year old pregnant woman with axillary lymphadenopathy (Ann R Coll Surg Engl 2020;102:e167)
- Squash preparation of a brain biopsy (Creepy Dreadful Wonderful Parasites: Case of the Week 519 [Accessed 6 January 2022])
Treatment
- Combination therapy of pyrimethamine and sulfadiazime with folinic acid (Centers for Disease Control DPDx: Toxoplasmosis [Accessed 6 January 2022], Lancet 2004;363:1965)
Microscopic (histologic) description
- Lymph node
- Preserved architecture
- Capsule / pericapsule: with minimal involvement
- Sinuses with distended monocytoid B cells
- Large cells with sharp cell boarders, clear cytoplasm and darkly stained nuclei
- Follicles with florid reactive follicular hyperplasia
- Numerous tingible body macrophages
- Germinal centers with ragged, indistinct margins
- Interfollicular and paracortical areas with epithelioid histiocytes
- Invade germinal centers
- Form collections of < 25 histiocytes (microgranuloma)
- Medullary cords with plasma cells and immunoblasts
- Toxoplasma cysts and bradyzoites are rare (1% of cases)
- References: Ioachim: Ioachim’s Lymph Node Pathology, 4th Edition, 2008, Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017
Microscopic (histologic) images
Cytology description
- Cytologic features based on fine needle aspiration biopsy:
- Polymorphous cell population
- Small and large lymphocytes
- Clusters of epithelioid histiocytes (microgranulomas)
- Parasitic cysts can be detected, rarely
- Polymorphous cell population
- References: Ioachim: Ioachim’s Lymph Node Pathology, 4th Edition, 2008, Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017
Positive stains
- Anti-T. gondii antibodies can detect parasites in tissue
Negative stains
Electron microscopy description
- Distinctive features on electron microscopy:
- Paired organelles, dense bodies
- Concoid nuclei at rounded posterior end
- Double layered pellicles
- References: Ioachim: Ioachim’s Lymph Node Pathology, 4th Edition, 2008, Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017
Electron microscopy images
Molecular / cytogenetics description
- Polymerase chain reaction (PCR)
- Toxoplasma genomes are detected by conventional and nested PCR
Videos
Classic histomorphology of toxoplasma lymphadenitis
Great teaching case of cerebral toxoplasmosis
Sample pathology report
- Lymph node, right inguinal, excisional biopsy:
- Reactive lymphoid follicular hyperplasia, monocytoid B cell hyperplasia and aggregates of epithelioid histiocytes, suggestive of toxoplasma lymphadenitis (see comment)
- Comment: The classic triad present in this case (follicular hyperplasia, intrafollicular clusters of epithelioid cells and aggregates of monocytes) is reported to have a 91% specificity for toxoplasma lymphadenitis. There is no evidence of lymphoma. The morphologic findings are very suggestive of toxoplasmosis. Serologic studies are recommended to confirm the diagnosis.
- Microscopic description:
- Right cervical lymph node: The lymph node is enlarged and has a preserved architecture with a thin capsule and open sinuses. There is evidence of reactive lymphoid follicular hyperplasia. In addition, numerous small, well defined aggregates of epithelioid histiocytes are noted, including within the germinal centers. Focally, monocytoid B cell hyperplasia is present. There is no morphologic evidence of lymphoma.
- Special stains for AFB and GMS are negative for microorganisms.
- Immunohistochemical staining performed on block A1 shows that the CD20+ B cells are confined to the germinal centers, while the CD3+ T cells are interfollicular. Monocytoid B cells also express CD20. Immunohistochemical stain for toxoplasma is positive. There is no staining for cytomegalovirus. In situ hybridization for EBV encoded RNA (EBER) is negative.
- Note: Control slides show appropriate reactivity.
- Left inguinal lymph node for flow cytometry:
- The following analytes were tested: Kappa, Lambda, CD5, CD23, CD10, CD20, CD19, CD45, sCD3, CD57, CD4, CD7, CD8, CD2 (total of 14)
- Flow cytometry interpretation:
- Cytospin: Morphologic evaluation of Diff-Quik stained cytospin shows no increase in atypical lymphocytes.
- Flow cytometry: Analysis was performed with the above antigens. The T cells show no loss of pan T cell antigens. The B cells are polytypic. The findings provide no evidence of a non-Hodgkin lymphoma.
Differential diagnosis
- Sarcoid lymphadenopathy:
- Well formed granulomas with multinucleated giant cells
- Usually lacks monocytoid B cells (Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017)
- HIV lymphadenitis:
- Early stages of HIV lymphadenitis may mimic toxoplasmosis
- Pattern A: aggregates of monocytoid cells and florid follicular hyperplasia
- May lack clusters of epithelioid cells or may be present
- HIV p24 stains positive
- Leishmaniasis lymphadenitis:
- Histomorphologically very similar
- Multinucleated giant cells present
- Necrosis and fibrosis present with Leishmania infection
- Histiocytes contain Leishman-Donovan bodies within the cytoplasm (Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017)
- Electron microscopy demonstrates presence of rod shaped kinetoplast and target shaped basal body
- Dermatopathic lymphadenopathy:
- Paracortical distribution of of histiocyte collections, pigment and lipids
- Histiocytes with twisted nuclei
- S100+, CD1a (+ in a subset) (Medeiros: Diagnostic Pathology - Lymph Nodes and Extranodal Lymphomas, 2nd Edition, 2017)
Board review style question #1
Which feature is included in the the classic histologic pattern of toxoplasmosis lymphadenitis?
- Fibrosis with necrosis
- Florid follicular hyperplasia, perifollicular epithelioid histiocytes and patches of monocytoid B cells
- Necrosis without neutrophils
- Reactive follicular hyperplasia, T cells and a lack of histiocytes
- Well formed granulomas with multinucleated giant cells
Board review style answer #1
B. The histologic triad of Toxoplasma lymphadenitis includes reactive follicular hyperplasia, perifollicular / interfollicular epithelioid histiocytes and aggregates of monocytoid B cells.
Comment Here
Reference: Toxoplasmosis
Comment Here
Reference: Toxoplasmosis
Board review style question #2
Which of the following statements is correct regarding the life cycle of Toxoplasma gondii?
- Although prevalent worldwide, T. gondii is relatively uncommon in the U.S.
- Cysts containing tachyzoites localize in tissues (skeletal muscle, brain and eyes)
- Oocysts are shed, unsporulated, in the feces of cats and sporulate in the environment to become infective
- Rodents, cats and birds are definitive hosts, while humans are intermediate hosts
Board review style answer #2
C. Oocysts are shed, unsporulated, in the feces of cats and sporulate in the environment to become infective. Cats are the only definitive host, while birds, rodents and humans are intermediate hosts. T. gondii is the most common parasite in the U.S. Oocysts transform into tachyzoites after ingestion. Tachyzoites localize in tissues and develop into tissue cysts containing bradyzoites.
Comment Here
Reference: Toxoplasmosis
Comment Here
Reference: Toxoplasmosis
Uncommon infections
AIDS
- Prior to highly active antiretroviral therapy (HAART), typical findings were white pulp depletion, hemosiderin deposition, spindle cell proliferation and perivascular hyalinization; also infectious and malignant infiltrates (Mod Pathol 2002;15:406)
- Post-HAART findings include less frequent white pulp depletion but similar rates of splenic involvement by atypical mycobacteria and CMV in those with systemic disease
Hantavirus
- Rare but deadly disease transmitted to humans through aerosolized virus from rodent urine, droppings or saliva
- Usually causes pulmonary syndrome with acute respiratory distress syndrome
- Gross description: dense, rubbery and heavy lungs floating within yellow serous fluid within pleural cavity; no specific splenic findings
- Micro description: generalized capillary dilation and edema, immunoblasts in red pulp and periarteriolar sheaths of spleen, occasional prominent and swollen endothelial cells
Mycobacteria
- Small, weakly gram positive bacteria that are acid fast (due to mycolic acid in cell wall) and slow growers (3 - 4 weeks to develop a visible colony)
- Mycobacterium avium complex (MAC) consists of M. avium and M. intracellulare, which cause disseminated disease associated with advanced HIV; cause pulmonary disease and cervical lymphadenitis in immunocompetent individuals
- Disseminated MAC is most common systemic bacterial infection in HIV+ patients
- Spleen may show presence of pseudo-Gaucher cells (J Clin Pathol 2005;58:1113)
- Treatment: clarithromycin or azithromycin plus ethambutol, for life
Parvovirus
- Causes "fifth" disease in children, a mild illness with a "slapped cheek" facial rash
- Pregnant women may pass virus to fetus, where it causes marked fetal anemia and hydrops
- Attacks erythroblasts, affecting those with minimal reserve (sickle cell patients, fetuses)
Typhoid fever
Case reports
- 37 year old white man with advanced HIV and splenomegaly (Arch Pathol Lab Med 2001;125:697)
Vascular transformation of sinuses
Table of Contents
Definition / general | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Also called nodal angiomatosis
- Usually found incidentally after resection of a nearby tumor
- Benign; all ages
- Due to obstruction of lymphatic efferent vessels or venous obstruction (Arch Pathol Lab Med 1990;114:656) or perhaps other angiogenic factors
Case reports
- 57 year old man with gastric cancer; lymph nodes removed during surgery (Case of the Week #405)
- Man with congestive heart failure (G Ital Nefrol 2002;19:60)
Microscopic (histologic) description
- Subcapsular and medullary sinuses contain complex network of anastomosing blood vessels of variable sizes with fibrosis
- May have irregularly branching vascular slits accompanied by pericytes with maturation towards well formed vascular channels, may have extravasated red blood cells and interstitial fibrin deposits
- Reactive, not neoplastic
- May also have spindle cell nodules confined to sinus (associated with retroperitoneal nodes removed for renal cell carcinoma)
- Lymphoid parenchyma may show atrophy
- No atypia, no PAS+ hyaline globules, no capsular involvement
Microscopic (histologic) images
Differential diagnosis
- Kaposi sarcoma: involvement throughout lymph node including capsule, not just sinuses; well formed and curved spindle cell fascicles, vascular slits are nonbranching; atypia and PAS+ hyaline globules, no fibrosis, patients are HIV+ or African children (Am J Surg Pathol 1991;15:732)
WHO system for reporting lymph node cytopathology (pending)
[Pending]
Recent Lymph nodes & spleen, nonlymphoma Pathology books
Find related Pathology books: cytopathology, hematopathology