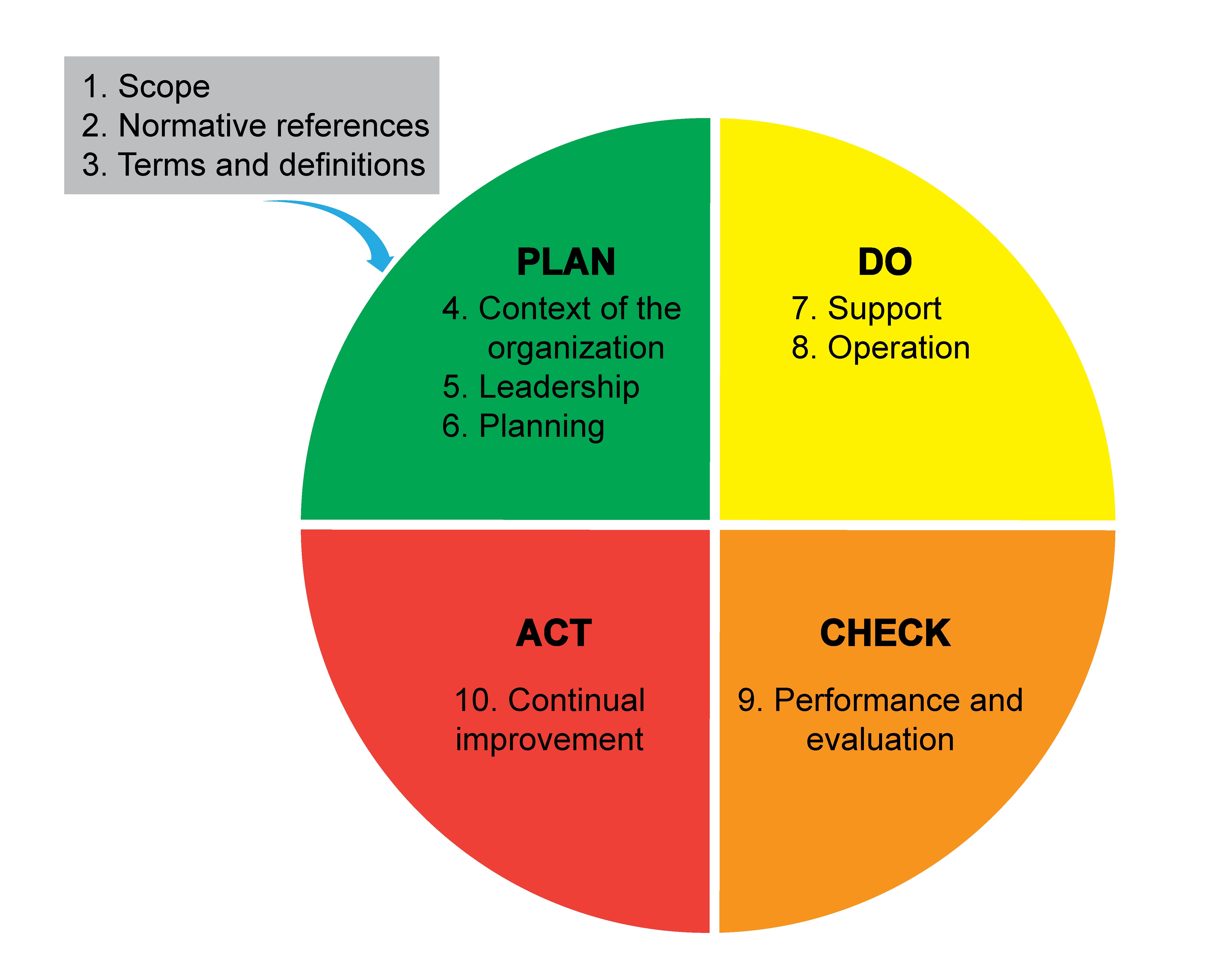
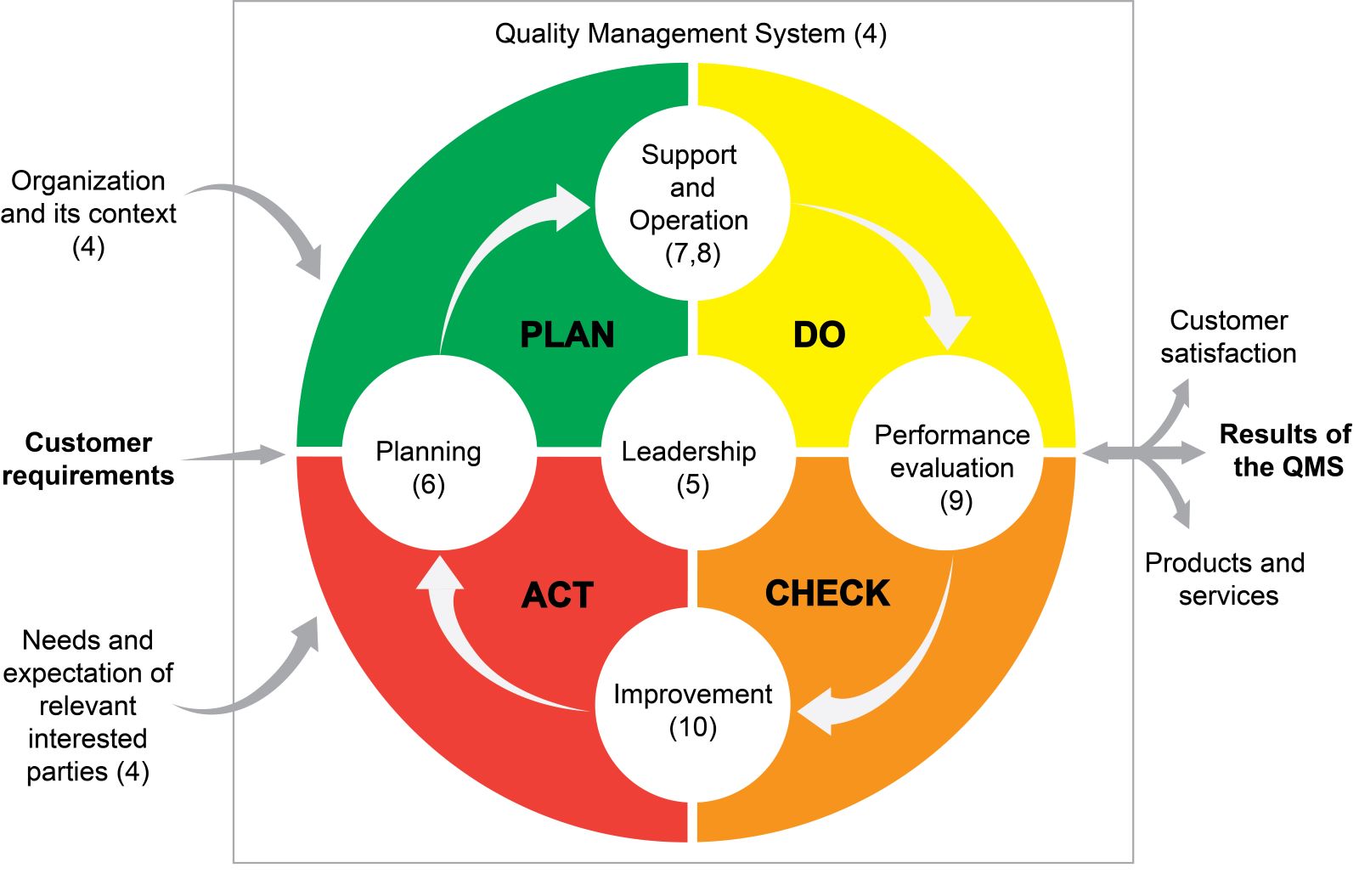
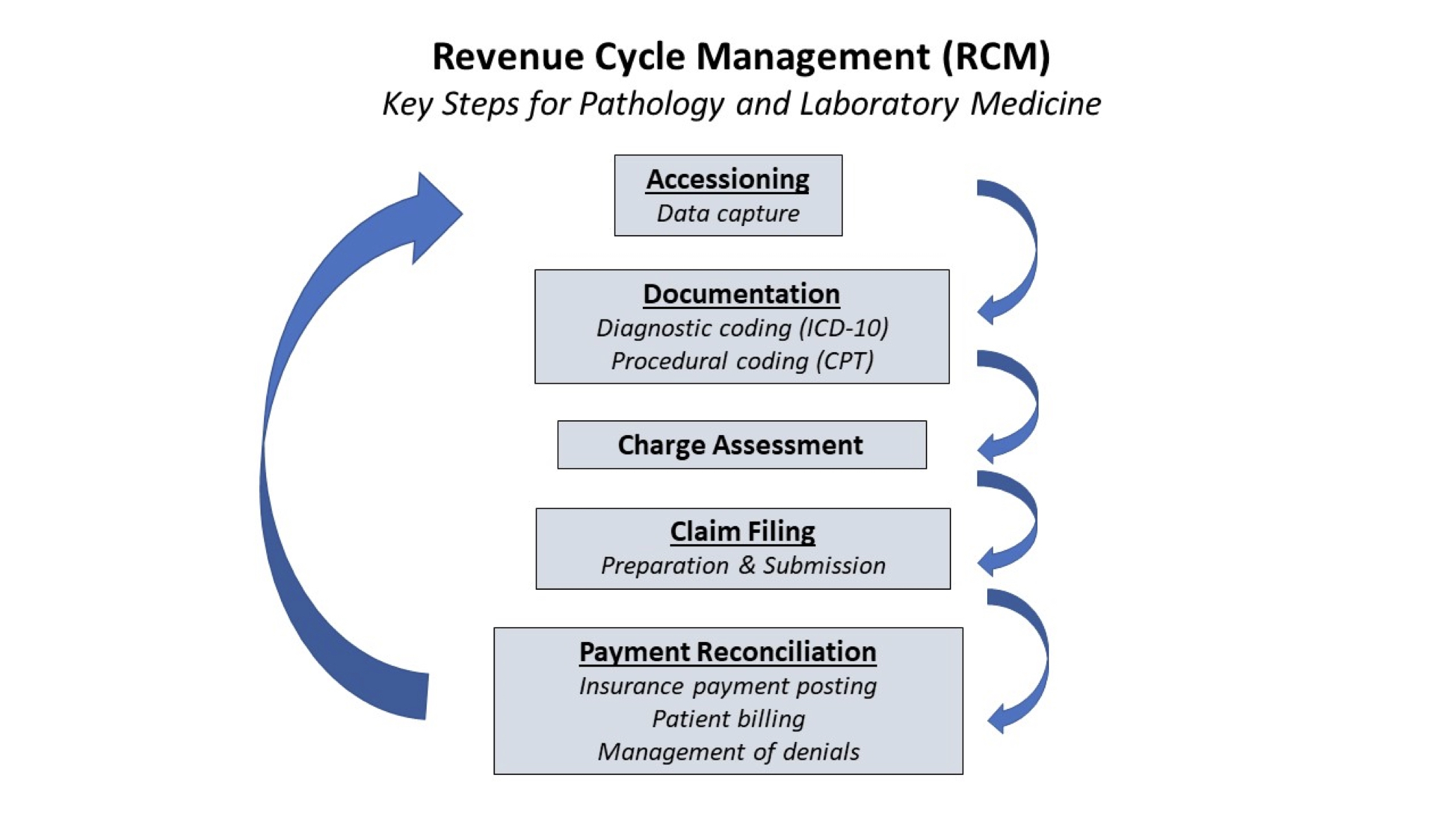
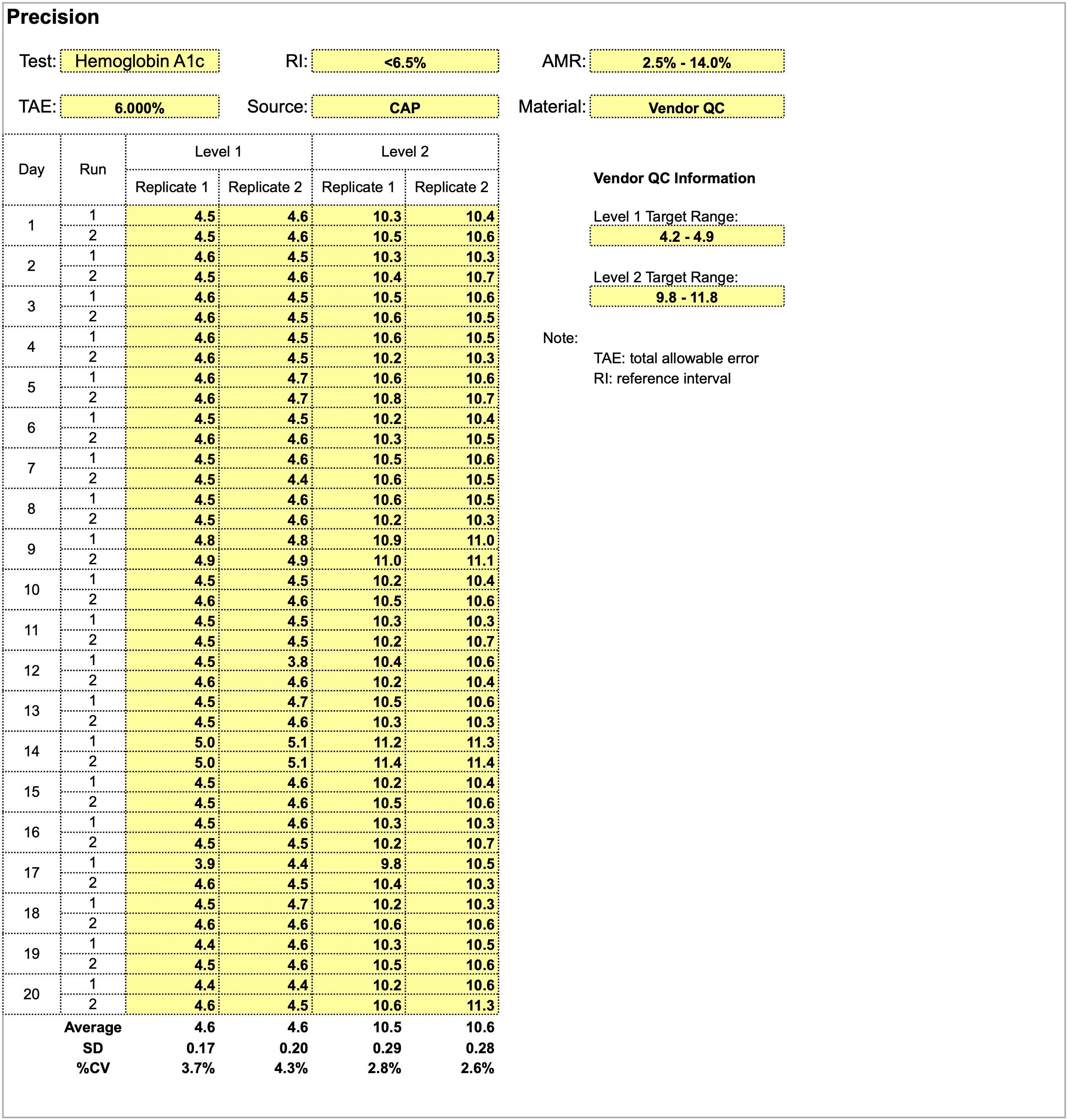
- Background (Mil Med 2000;165:48):
- Federal regulations for U.S. based clinical laboratories were enacted in 1988, in response to crisis, primarily in the Pap testing industry
- Provide industry standards for testing of human samples for diagnostic purposes
- FDA, CDC, CMS are responsible for ensuring compliance
- Aim:
- Assure accuracy and reliability of test results
- Give certificate of accreditation (COA) to a laboratory that performs moderate or high complexity testing, and attains accreditation by an organization approved by CMS
- Give certificate for provider performed microscopy procedures to the laboratory in which staff performs specific microscopy procedures during a patient's visit
- Give certificate of waiver (COW) to a laboratory that performs only waived tests
- Give certificate of compliance (COC) to a laboratory that performs moderate or high complexity testing; CMS designee performs inspection to determine that the laboratory is compliant with all applicable CLIA requirements
- Inspections: conducted by state departments of health or less commonly, by CMS or FDA (unannounced inspections)
- Cost (CMS: CLIA Certificate Fee Schedule [Accessed 6 June 2022])
- Ensuring that testing and billing is in accordance with applicable law
- Medicare has two compliance rules for teaching physicians: the CPT code must have a specific modifier for Medicare claims and physicians must indicate in every medical report that the teaching physician compliance rules were met
- Five digit codes and descriptive terms for medical services and procedures that physicians perform for payment CPT codes are owned by the AMA (American Medical Association: CPT® (Current Procedural Terminology) [Accessed 17 November 2017])
- Based on the official version of the World Health Organization's international classification of diseases
- Required by law for Medicare and for all practical purposes by all medical insurance companies for payment of a claim
- Clinician provides a code, as does the pathologist; they may be different (example: clinician - hematuria, pathologist - urothelial carcinoma)
- See CDC: ICD-9-CM [Accessed 17 November 2017], Online ICD9 / ICD9CM Codes: Diseases and Injuries Tabular Index [Accessed 17 November 2017]
- Payments for clinical pathology services rendered by pathologists for services generally, not related to a specific patient specimen
- Includes oversight of laboratory, supervising laboratory personnel, reviewing abnormal results, discussion with clinicians
- Paid to hospitals by Medicare; hospitals are supposed to pay reasonable compensation to pathologists
- See Vachette article for details
- Billing a patient for each clinical pathology test / procedure performed for that patient, regardless of whether pathologist performs or reviews the test
- Theory is that pathologist does oversight of the laboratory (see Medicare part A services above) and is entitled to bill patient for these efforts
- Standardized physician payment schedule implemented by Medicare in 1992
- Payment for a procedure code is calculated as the relative value unit (RVU) multiplied by the conversion factor
- RVU is calculated based on physician work, physician practice expense and the professional liability cost for that procedure code and each component is adjusted by geographic practice costs
- All figures are computed by the federal government
- Allows clinicians to charge for pathologist's services, pay pathologists a discount and keep the difference
- Appears to violate Medicare regulations and AMA policy but still is embraced by many clinicians
- Unclear if pod labs or other schemes by nonpathologist to bill for pathology services are legal
- May not appear obvious for pathology practices but considered necessary by many to (Arch Pathol Lab Med 1995;119:655):
- Keep current clients from switching to other groups
- Get new clients, either for expansion or to replace clients that go out of business, get smaller or switch to other groups
- Biosafety refers to the protection of workers, the community and the environment from accidental exposure to infectious agents, toxins and other biological hazards
- To ensure protection, a set of policies, rules and procedures, known as biosafety guidelines, have to be followed by personnel working in lab facilities while handling and disposing of materials containing biological hazards (StatPearls: Biosafety Guidelines [Accessed 11 October 2023])
- Biosafety includes implementation of training in practices to protect lab personnel, the community and the environment
- All lab workers should have or may be required to complete biosafety training annually and implement it in the practice
- Laboratory director must monitor compliance with biosafety
- Based on laboratory practices, safety equipment and facility construction, the U.S. Centers for Disease Control and Prevention (CDC) has executed 4 levels of biosafety
- Biosafety guidelines have a crucial role in surgical pathology labs and autopsy as well
- Biohazard: biological substances that pose a threat to the health or environment; also called biological hazards and biohazardous agents
- Laboratory biosecurity: the protection, control and accountability for valuable biological material agents and toxins within laboratories, in order to prevent their loss, theft, misuse, diversion, unauthorized access or intentional unauthorized release (Front Bioeng Biotechnol 2020;8:650)
- Biosafety level (BSL): a series of safety precautions based on the potential for harm and spread, intended to minimize the risk of exposure of laboratory personnel and the surrounding environment to potentially infectious biohazardous agents (Methods Mol Biol 2019;1897:213)
- Occupational Safety and Health Administration (OSHA): a part of the United States Department of Labor, established to ensure safe and healthful working conditions for workers by setting and enforcing standards and providing training, outreach, education and compliance assistance
- Safety data sheets (formerly material safety data sheets [MSDSs]): documents that list information relating to occupational safety and health for the use of various substances and products
- Blood borne pathogen: microorganisms such as viruses or bacteria that are carried in blood and can cause disease in people (CDC: Bloodborne Diseases [Accessed 11 October 2023])
- Universal precautions: practice standards for treating all potentially infectious bodily fluids or tissues as infectious, including the use of impermeable items such as medical gloves, goggles and face shields every time one handles such materials
- Personal protective equipment (PPE): equipment worn to minimize exposure to hazards that cause serious workplace injuries and illnesses; includes items such as gloves, safety glasses and shoes, earplugs or muffs, respirators, vests and full body suits (OSHA: Personal Protective Equipment [Accessed 11 October 2023])
- ICD-10: Z91.89 - other specified personal risk factors, not elsewhere classified
- Concept of biosafety came into existence at the time of Pasteur and Koch (around 1890)
- In 1910, while studying the disease, Dr. Howard Taylor Ricketts acquired typhus and died; this was followed by many reports of laboratory acquired infection (Curr Protoc Microbiol 2009;13:111)
- Sulkin and Pike (1949 and 1951) and Collins (1990) contributed to the implementation of protective measures against biological agents
- Biosafety measures were first implemented in North America and the United Kingdom in 1970 and accelerated as awareness of risks associated with hepatitis and human immunodeficiency virus (HIV) increased
- Working practices, personnel protection measures and physical containment measures have been designed to restrict the transmission of biological agents (Belgian Biosafety Server: Biosafety Worldwide - Historical Background [Accessed 11 October 2023])
- 4 levels as implemented by CDC based on laboratory practices, safety equipment and facility construction
- Dictates the type of work practices that are allowed to occur in a lab setting and plays a major role in the design of the facility
- BSL 1
- Laboratory settings that involve working with low risk microbes that pose little to no threat of infection in humans (e.g., nonpathogenic strain of E. coli)
- Follow basic safety procedures
- No special equipment or design features are required
- BSL 2
- Laboratories that work with agents associated with pathogenic human diseases and hence pose a moderate health hazard (e.g., HIV, S. aureus)
- Safety measures include using gloves and eyewear, handwashing sinks, waste decontamination facilities and self closing and locking doors
- BSL 3
- Laboratories that work with microbes that are either indigenous or exotic and can cause serious or potentially lethal diseases, which may be spread via inhalation (e.g., West Nile virus, tuberculosis)
- Personnel wear respirators and perform lab manipulations in a gas tight enclosure; other safety features include clothing decontamination, sealed windows and specialized ventilation systems
- BSL 4
- Laboratories that work with highly dangerous and exotic microbes that are often lethal and come without treatment or vaccines (e.g., Ebola, Marburg viruses)
- Lab personnel must wear full body, air supplied suits and shower when exiting the facility; along with BSL 3 features, the lab must be located in a separate building or an isolated and restricted zone of the building (StatPearls: Biohazard Levels [Accessed 11 October 2023])
- Aims to protect lab personnel, clients and the environment by following biosafety guidelines
- Environment health and safety (EHS) division of the institution develops a comprehensive framework that all labs must follow (National Research Council: Prudent Practices in the Laboratory - Handling and Management of Chemical Hazards, Updated Version, 2011)
- Laboratory safety program includes
- Responsibility: chief executive officer, along with all immediate associates, should have a continuing, explicit commitment to the safety program
- Safety plan: clearly defined safety rules that are available in written form and have means of monitoring compliance; should be coordinated with institutional and local community emergency services
- Safety meetings and safety committees: everyone involved in the laboratory should participate
- Safety communications: to alert people to newly recognized hazards, remind them of basic safety principles and instill good attitudes toward safety; safety newsletters containing practical safety advice, safety posters and reference books on laboratory hazards, occupational health and good laboratory practices
- Monitoring safety: observations of individual safety practices, operability of safety equipment and compliance with safety rules should be part of a regular audit (National Research Council: Biosafety In The Laboratory - Prudent Practices for the Handling and Disposal of Infectious Materials, 1989)
- Safety data sheets (formerly material safety data sheets [MSDSs]): a system for cataloging information about a particular chemical product, instructions for its safe use and associated potential hazards (Int J Pharm Compd 2017;21:118)
- OSHA's Health Communication Standard, revised in 2012, requires that the chemical manufacturer, distributor or importer provide safety data sheets (SDSs) for each hazardous chemical to communicate information on these hazards to users
- There are 16 sections in SDSs
- Section 1: identification
- Section 2: hazard(s) identification
- Section 3: composition / information on ingredients
- Section 4: first aid measures
- Section 5: firefighting measures
- Section 6: accidental release measures
- Section 7: handling and storage
- Section 8: exposure controls / personal protection
- Section 9: physical and chemical properties
- Section 10: stability and reactivity
- Section 11: toxicological Information
- Section 12: ecological information (not mandatory)
- Section 13: disposal considerations (not mandatory)
- Section 14: transport information (not mandatory)
- Section 15: regulatory information (not mandatory)
- Section 16: other information (OSHA: Hazard Communication Standard - Safety Data Sheets [Accessed 11 October 2023])
- Standard set of guidelines to prevent the transmission of blood borne pathogens (such as hepatitis A, hepatitis B, HIV and brucellosis) from exposure to blood and other potentially infectious materials (OPIM)
- Implemented by the CDC in 1985, primarily in response to the HIV epidemic
- OPIM
- Semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures and body fluid that is visibly contaminated with blood
- Any unfixed tissue or organ from human (living or dead)
- Cell, tissue or organ cultures containing HIV
- HIV or hepatitis B virus (HBV) containing culture medium or other solutions
- Blood, organs or other tissues from experimental animals infected with HIV or HBV
- Universal precautions practice includes
- Hand hygiene by hand rubbing with alcohol or hand washing with soap and water
- Use of PPE such as gowns, gloves, masks and goggles
- No mouth pipetting
- No food, drink or lip balm in the lab
- Proper disposal of biohazardous / medical waste
- Use of engineering controls (StatPearls: Universal Precautions [Accessed 11 October 2023])
- Universal precautions must be effectively implemented during autopsies due to documented instances of infection transmission, including but not limited to tuberculosis, AIDS, HBV and SARS-CoV-2, occurring during the procedure (Am J Pathol 2020;190:2180)
- Set up a hands free sink that includes either an automated faucet with motion detection capabilities or controls operated by your knee or foot
- Automatic door locking system so the door remains closed in the lab
- Having negative airflow into the laboratory or a work cabinet can prevent exposure to splashes and sprays or spread to the external environment
- Using a biosafety cabinet (BSC) to handle fresh tissue (CDC: Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories [Accessed 11 October 2023])
- Spill response is a crucial emergency management process that needs to have its own standard operating procedure (SOP)
- Regular training is needed to ensure lab personnel are well trained in spill management
- Conventional method involves 2 trained staff, one working in the contaminated area and the second providing support from the noncontaminated area
- Steps
- Take appropriate measures to reduce exposure for other lab workers or patients
- Isolate the spill area and vacate it for 30 minutes
- Prepare disinfectant and spill cleanup equipment
- Cover the area with a paper towel and then pour disinfectant
- Collect the paper towel and mop the area with disinfectant (Appl Biosaf 2019;24:141)
- Procedures may vary with larger volume spills
- Identify and define all categories of waste generated
- Waste needs to be decontaminated before disposal
- Most clinical laboratories can operate satellite accumulation points and accumulate, store, transport and dispose of waste in accordance with Environmental Protection Agency (EPA) and the Department of Transportation (DOT) regulations (Clin Lab Manage Rev 1990;4:160)
- Laboratory director must ensure that a laboratory specific biosafety manual is developed, adopted, annually reviewed and is accessible to all laboratory personnel
- Manual or comparable training should be reviewed annually and whenever procedures or policies change
- All lab personnel should take biosafety practice training annually; training must cover the following topics
- Institutional and laboratory safety policies
- Management, supervisor and personnel responsibilities
- Regulations and recommended guidelines
- Routes of exposure in the laboratory
- Risk assessment and reporting of exposures
- Biosafety principles and practices
- Standard precautions for safe handling of infectious materials
- Standard operating procedures
- Hazard communication and biohazard signs
- Engineering controls
- Administrative and work practice controls
- Personal protective equipment (PPE)
- When and how to work in a BSC (biosafety cabinet)
- Transport of biohazardous materials
- Emergency procedures
- Decontamination and disposal of biohazardous waste
- Training program and documentation
- Medical surveillance and exposure evaluation procedures (CDC: Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories [Accessed 11 October 2023])
- Surgical pathology laboratories receive specimens with the same potential exposures as those of other labs
- Needle stick, cut and formalin exposure by splash or prolonged skin exposure are common hazards; hence, the training must include education on needle stick injuries, PPE and the hazards of chemical exposures
- Incident reporting and subsequent follow up training have been reported to be more effective than didactic training alone
- Pathology departments should consider additional biosafety components when initiating and training new residents
- Discomfort caused by certain PPE, such as masks pressing on the skin, face shields obstructing dictation microphones and uncomfortable ventilators and respirators, leads to people not using them correctly or at all; hence, these items should be customized as per the pathologist's requirement (Hum Pathol 2013;44:951)
- Use of freeze spray in the frozen section should be discouraged (MMWR Suppl 2012;61:1)
- Exposure to aerosols generated during frozen sections should be minimized, especially in potentially infectious cases
- Cryostat decontamination procedures should be sufficient for viral, mycobacterial and other pathogens
- Autopsy can lead to exposure to infectious agents, toxic chemicals (e.g., formalin, cyanide and organophosphates) and radiation from radionuclides used for patient therapy and diagnosis
- Autopsy generated droplets (> 5 mm in diameter) also potentially transmit infections if inhaled or ingested
- Implantable cardioverter defibrillator can sustain an electrical discharge of 25 - 40 J if manipulated; hence, it needs to be deactivated and manufacturers must have service representatives available to assist with deactivation (Am J Forensic Med Pathol 2002;23:107)
- Autopsy biosafety program must be provided to autopsy staff and visiting personnel with an environment free from hazardous exposure risk (Finkbeiner: Autopsy Pathology, 2nd Edition, 2009)
Lab safety: biosafety
Biohazard spill management
Biosafety levels
Comment Here
Reference: Biosafety for lab and pathology
- Biosafety level 1
- Biosafety level 2
- Biosafety level 3
- Biosafety level 4
Comment Here
Reference: Biosafety for lab and pathology
- The work performed in clinical and anatomical pathology laboratories is often performed while handling infectious patient samples of many types
- The hazards posed by these samples vary, and proper handling and use of protective equipment is imperative to ensure the safety of laboratory personnel
- OSHA's Bloodborne Pathogens standard oversees the proper transport, storage and handling of bio-hazardous substances in the laboratory
- Bloodborne pathogens regulatory agencies:
- Occupational Safety and Health Administration (OSHA)
- 29 CFR 1910.1030: Bloodborne Pathogens - 1991
- U.S. Centers for Disease Control (CDC) / The National Institute for Occupational Safety and Health (NIOSH)
- Information for Employers Complying with OSHA's Bloodborne Pathogens Standard - 2009 (CDC)
- College of American Pathologists (CAP)
- Laboratory general inspection checklist
- Occupational Safety and Health Administration (OSHA)
- Laboratory requirements:
- Written exposure control plan (ECP)
- Updated annually
- Standard operating procedures
- Six major components:
- (1) Administration and exposure risk
- Define responsibilities of employees, supervisors and managers
- State who is responsible for the implementation and maintenance of the ECP
- State who is responsible for provision and maintenance of PPE, employee health administration and training
- Perform risk assessments to determine exposure risk for each employee category (technologist, phlebotomist, pathologist, etc.)
- Perform task assessments to determine exposure risk for laboratory procedures
- (2) Exposure control methods
- Describe the various methods the laboratory will use to prevent occupational exposure
- Mitigation of risk occurs through a "Hierarchy of Controls"
- Elimination of risk
- Preferred, but not usually feasible in the laboratory setting
- Engineering controls
- Physical change or application to the workplace to improve safety
- Ventilation hoods
- Safety shields
- Biological safety cabinets
- Administrative controls
- Require the employee to take an action to minimize the risk
- Include work practice controls
- No eating, drinking, smoking, applying cosmetics or lip balm and handling contact lenses in work areas
- Do not pick up broken glassware directly with hands
- Wash hands immediately or as soon as feasible after removal of gloves or other personal protective equipment
- Personal Protective Equipment (PPE)
- Considered least effective control in the hierarchy
- Requires the employee to wear something in order to reduce exposure risk
- Type of PPE used chosen based on the risk and task assessments
- Lab coats, gloves, face shields, aprons, respirators, etc.
- Elimination of risk
- (3) Vaccinations
- Hepatitis B vaccine must be offered to all potentially exposed employees
- Employees who refuse should fill out a vaccine declination form
- Employees who decline may reconsider later
- (4) Post-exposure follow-up
- ECP requires a complete exposure follow-up program
- First aid
- Clean the wound, flush eyes or other mucous membrane, etc.
- Incident evaluation
- Obtain information about how the exposure occurred and the route of entry
- Identify the source individual, if possible
- Obtain consent and make arrangements to have the source individual tested to determine HIV, HCV and HBV infectivity
- Ensure test results are given to the exposed employee
- Treat high-risk exposures as emergent in order to expedite the treatment or prophylaxis process
- Medical follow up
- Provided by licensed medical practitioner
- Provide employee with written evaluation of exposure and any further necessary treatment options within 15 days of the incident evaluation
- All involved parties document complete exposure incident evaluation
- Include review of engineering controls in place, work practices used, employee training, location and procedure being performed and PPE used
- First aid
- ECP requires a complete exposure follow-up program
- (5) Communication
- Provide information to laboratory employees about hazards in the workplace through labels and signage
- Use biohazard symbol for labeling (see figure 1)
- Label items that are used to contain or transport blood or OPIM
- Refrigerators / freezers
- Incubators
- Transport coolers
- regulated waste containers
- Biohazard symbol at the entryway to HIV and HBV Research Laboratory and Production Facilities required by OSHA
- CDC requires this entryway signage for all biological laboratories (CDC)
- (6) Training and records
- All employees need access to Bloodborne Pathogens standard
- All employees must be trained on all aspects of the ECP
- Training should occur upon employment, annually and whenever there is a change made to procedures which involve potential exposure
- Keep all training records for at least three years
- Employee medical records are maintained for the duration of employment plus 30 years
- Maintain a sharps injury log for any percutaneous injury acquired from a contaminated needle or other such device
Images hosted on other servers:
- Written exposure control plan (ECP)
- Biohazard spill handling:
- Provide spill response training for all staff that handles blood or body fluids
- Ensure use of PPE
- Confine or contain the spill
- Inspect for broken glass
- Absorb with towels or spill pillows
- Use blood / body fluid absorbent if available (see figure 2)
- Mop or sweep up the spill
- Dispose of waste in proper receptacles
- Disinfect spill area with 10% bleach solution
- Annual review of exposure control plan:
- Analyze effectiveness of your ECP
- Review exposure incidents
- Review training
- Review blood / body fluid spills
- Update employee risk exposure documentation
- Review / update task assessments
- Provide annual bloodborne pathogens training
Contributed by Dan Scungio, MT (ASCP), SLS, CQA (ASQ)
- Analyze effectiveness of your ECP
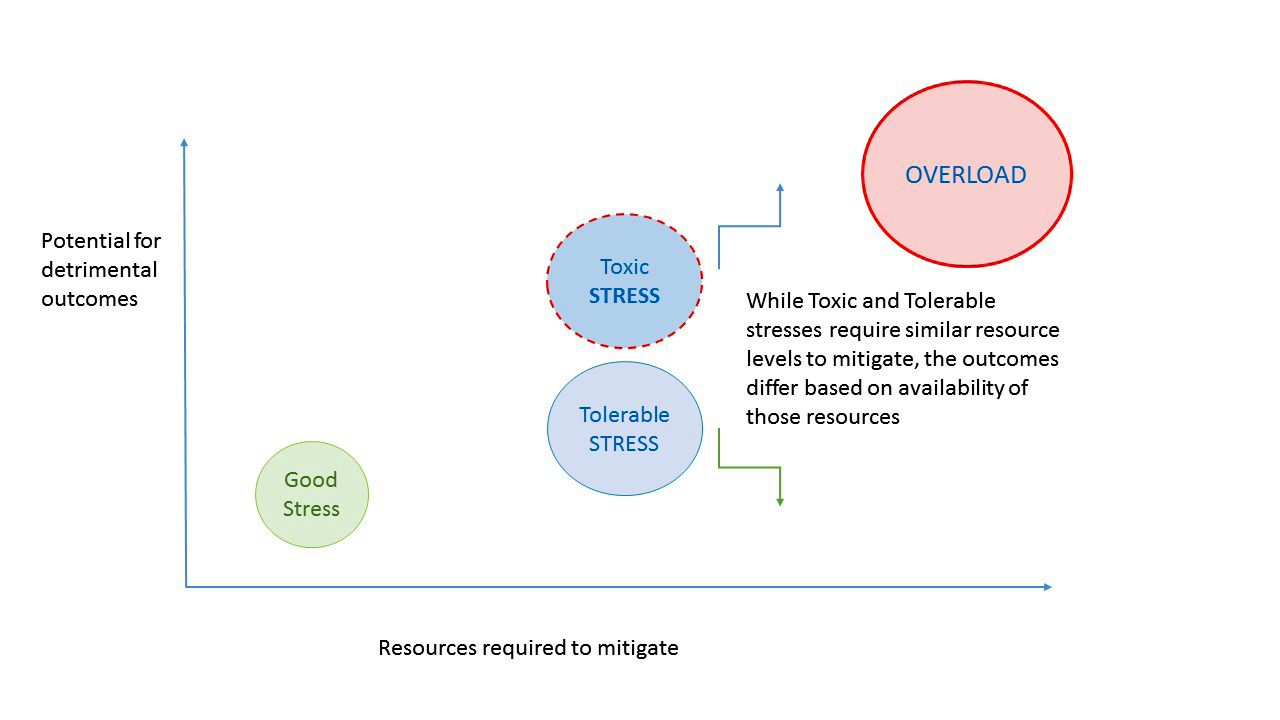
- Resilient work environment and culture is one that is capable of responding to a variety of stresses without compromising effectiveness
- Organizational resilience is "the ability of an organization to anticipate, prepare for, respond and adapt to incremental change and sudden disruptions in order to survive and prosper" (BSI: What is Organizational Resilience? [Accessed 18 January 2021])
- Proactive, regular assessment of organizational strengths, weaknesses, opportunities and threats (MindTools: SWOT Analysis [Accessed 13 January 2021] )
- Regular review of risks or threats to assess readiness, as well as conducting drills to identify and close performance gaps (MindTools: Risk Analysis and Risk Management [Accessed 13 January 2021], Chron: How to Conduct a Hypothetical Disaster Drill [Accessed 13 January 2021])
- Right people in the right jobs
- Individual resilience contributes greatly to organizational resilience
- Financial sustainability with reserve capacity
- Healthy external or outward focused relationships
- An ability to create, invent and discover unknown markets
- Competitive in the marketplace through being both progressive and flexible
- Stress management
- Employee engagement and commitment
- Strategic planning and disaster preparedness
- SWOT (strengths, weaknesses, opportunities, threats) analysis
- Healthy organizational culture
- Transparency of motives and goals, openness and humility throughout organization
- Alignment of interests of stakeholders, employees, owners, clients
- Environment of accountability and personal responsibility
- Freedom for risk taking, decision making within established bounds
- Fierce organizational commitment to do it right
- Uncompromising integrity in all things
- Willingness, even eagerness, to learn from mistakes
- Pursuit of collaboration, integration, holistic thinking and activity
- Courage in the face of setbacks or difficulties
- Reference: Ethix: Eight Traits of a Healthy Organizational Culture [Accessed 13 January 2021]
- Promote and offer stress reduction strategies, such as wellness, exercise or other healthy living tools
- Offer or provide direction to resources for managing or resolving individual stresses that impact the working environment (childcare options, counseling services, etc.)
- Provide growth and career development pathways
- Maintain positive work environment amid stressful circumstances
- Engage employees with larger picture of organizational goals and mission and help align their daily activities with that mission
- Implement and follow a plan to increase communication during times of stress or disaster (SHRM: Communicating with Employees During a Crisis [Accessed 13 January 2021])
- Celebrate successes and milestones, both individual and organizational
- Hiring and onboarding are 2 of the most important actions of organizational leaders
- Assess stress / risk levels of each position in organization to ensure healthy match of individual talents and affinities
- Cross training and backup structures critical in key positions
- Sound accounting and budgeting principles are followed
- Budgets are reasonable for operations and include allowances for reserves, replacement costs and other long term plans
- Financial reports are prepared and reviewed regularly by those with fiduciary responsibility (board, officers, etc.)
- Reference: MGMA: Principal Principles - Critical Accounting and Financial Concepts for Healthcare Leaders [Accessed 13 January 2021]
- Achieving and maintaining organizational trust via consistent performance, alignment of goals and methods with public and partners
- Accountability for decisions and outcomes
- Responsive to criticism and other feedback
- Resilient organizations prioritize reliability and are sensitive to possible threats; focus on the possibility that something could go wrong (Weick: Managing the Unexpected - Sustained Performance in a Complex World, 2007)
- Resilient organizations make a deliberate effort to create a complete picture of the work environment despite complexity; encourage diversity in perceptions so that assumptions can be challenged
- Leadership, senior management and staff are aware that their decision making can affect the entire organization (Weick: Managing the Unexpected - Sustained Performance in a Complex World, 2007)
- Resilient organizations show a commitment to a resiliency mindset that recognizes things can go wrong but challenges can be overcome
- Resolution focuses on minimizing harm
- Setbacks may be learning opportunities and a motivating force for improvement
- Resilient organizations value their staff by empowering them to make critical decisions during a crisis; senior leaders are present to reinforce behaviors and to help fix critical issues
- Regular SWOT analysis is a useful means to assess overall capacity and vulnerabilities, risks and challenges
- Strengths are those things where your organization and people excel
- Weaknesses are your organizational vulnerabilities, points where you lack depth, readiness or awareness
- Opportunities are existing or future events, circumstances or technologies that enable you to improve, grow or otherwise fulfill organizational goals
- Threats are new technologies, competitors, social shifts or other factors that could foil your progress towards organizational success
- Reference: CQ Net: Organizational resilience - What Is It and Why Does It Matter During a Crisis? [Accessed 13 January 2021]
Resilience in the workplace
How to give an effective performance review
How to use SWOT analysis
Value and range of risk assessment
Employee selection to drive organizational culture
How trust drives everything in an organization
Resilience, well being and performance in the workplace by Derek Mowbray
- Forbes: How To Build A Positive Company Culture [Accessed 13 January 2021], Ray Williams: Building Resilience for Individuals and Organizations During a Crisis [Accessed 13 January 2021], BSI: Organizational Resilience Index [Accessed 13 January 2021], BSI: Organizational Resilience [Accessed 13 January 2021], Dahl: Optimize Your Life! The One-page Strategic Planner, 2003, Collins: Good to Great - Why Some Companies Make the Leap and Others Don't, 2001, Covey: Speed of Trust - The One Thing That Changes Everything, 2008, Engemann: Business Continuity and Risk Management - Essentials of Organizational Resilience, First Edition, 2011, Weick: Managing the Unexpected - Sustained Performance in a Complex World, Third Edition, 2015
- Arranging affiliation agreements with child care services and personal fitness coaches that employees can access at reduced rates
- Providing content rich feedback to employees in performance evaluations that cover areas for improvement as well as kudos for progress and successes
- Holding occasional disaster drills that cover scenarios likely to be experienced in the region of the lab's operations
- Leaders who walk around and regularly correct personnel handling or processing specimens that are not covered in standard operating procedures
- Cross training key personnel involved in IT and data processing
Comment Here
Reference: Building resilience in the workplace
- S
- W
- O
- T
Comment Here
Reference: Building resilience in the workplace
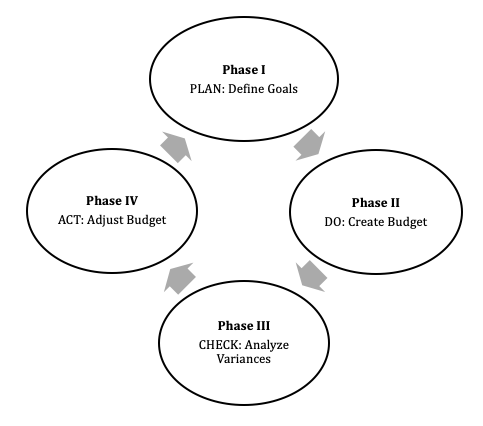
- Root cause analysis (RCA): a problem solving approach used to identify the principal underlying, originating (root) cause of an undesired or unintended outcome
- This topic is focused on applications in pathology and laboratory medicine
- RCA involves structured methods to study near misses and negative events
- There are many methods of RCA analysis
- Findings are then applied to systems and processes to implement quality improvements
- While typically used retrospectively, the structured methods may be most effective when applied to near misses and used for prospective risk mitigation and process improvement
- Objective / goals of RCA: to study causes of undesired pathology and laboratory outcomes and prevent future occurrences
- An investigative tool to address a specific event, as opposed to a surveillance tool that monitors baseline activity
- Recognizes that negative outcomes are usually reflective of underlying system and process design flaws, not the actions of an individual or random event
- Allows for design and implementation of a quality improvement solution that aims to address the failure at its source
- Improves safety by reducing the risk of harm from similar future events
- Common themes for RCA in pathology and laboratory medicine:
- RCA can be performed after an unintended outcome (with harm or potential harm) and include all phases of laboratory testing
- Undesired outcomes are often a result of simultaneous failures in complex laboratory processes and have multiple root causes
- Laboratory errors may affect many patients
- RCA can be used to supplement risk assessment, quality control plans and quality control tools, in developing individualized quality control plans (IQCP) per CMS and CDC guidelines
- Roles of the pathologist in RCA:
- Investigative leader: the lead investigator must have working knowledge of laboratory testing and be able to facilitate the formulation of relevant questions, delegate responsibilities and ensure timely investigation
- Quality leader: the professional responsible for quality must have specific knowledge about critical aspects of the root cause(s) that are uncovered
- Collaborators: the lead investigator must gather information from all parties, both inside and outside of the laboratory, who may have been involved in the event which led to the incident
- References: Proc (Bayl Univ Med Cent) 2001;14:154, Appl Immunohistochem Mol Morphol 2019;27:329
- Define the adverse event or undesired outcome
- Adverse event example: laboratory test resulted to incorrect patient
- Near miss example: labeling error identified and corrected by pathologist before test resulted to patient
- Identify all involved or knowledgeable personnel
- In the laboratory: frontline staff, support personnel, technologist, resident trainees, supervisors, managers and pathologists
- Nonlaboratory stakeholders: physicians or service lines involved (e.g., radiology, surgery), operating room (OR) staff, transport / courier staff, nurses
- Identify equipment, processes and policies that may be linked to the incident
- Gather information:
- Document objective information (who, what, where, when); question of "why" reserved for next step
- Interview and gather data from all personnel involved
- Create tools to help visually describe the components of the event (process map)
- References: Proc (Bayl Univ Med Cent) 2001;14:154, Appl Immunohistochem Mol Morphol 2019;27:329
5 "why's" method
- Iterative technique used to discover a problem source: ask "why" 5 consecutive times, each time using the previous answer as the starting point for the next question
- By the fifth "why", the root cause is often revealed
- Effective method to use when the root cause is likely internal to the laboratory (or organization)
- Example 1:
- I was late for work → why?
- Because I did not know about the accident on the highway → why?
- Because I did not turn on the radio while getting dressed → why?
- Because I woke up late and was rushing → why?
- Because I did not hear the alarm go off → why?
- Because I was out too late last night
- I was late for work → why?
- Example 2:
- The H&E stain has been suboptimal lately → why?
- Because the reagents have not been changed daily over the past week → why?
- Because the histology lab staff did not know daily routines → why?
- Because new histology staff did not receive adequate onboarding or supervision → why?
- Because there was no new staff orientation → why?
- Because the histology supervisor was absent and current operational policies and procedures do not specify who should supervise staff onboarding and orientation when the histology supervisor is absent
- The H&E stain has been suboptimal lately → why?
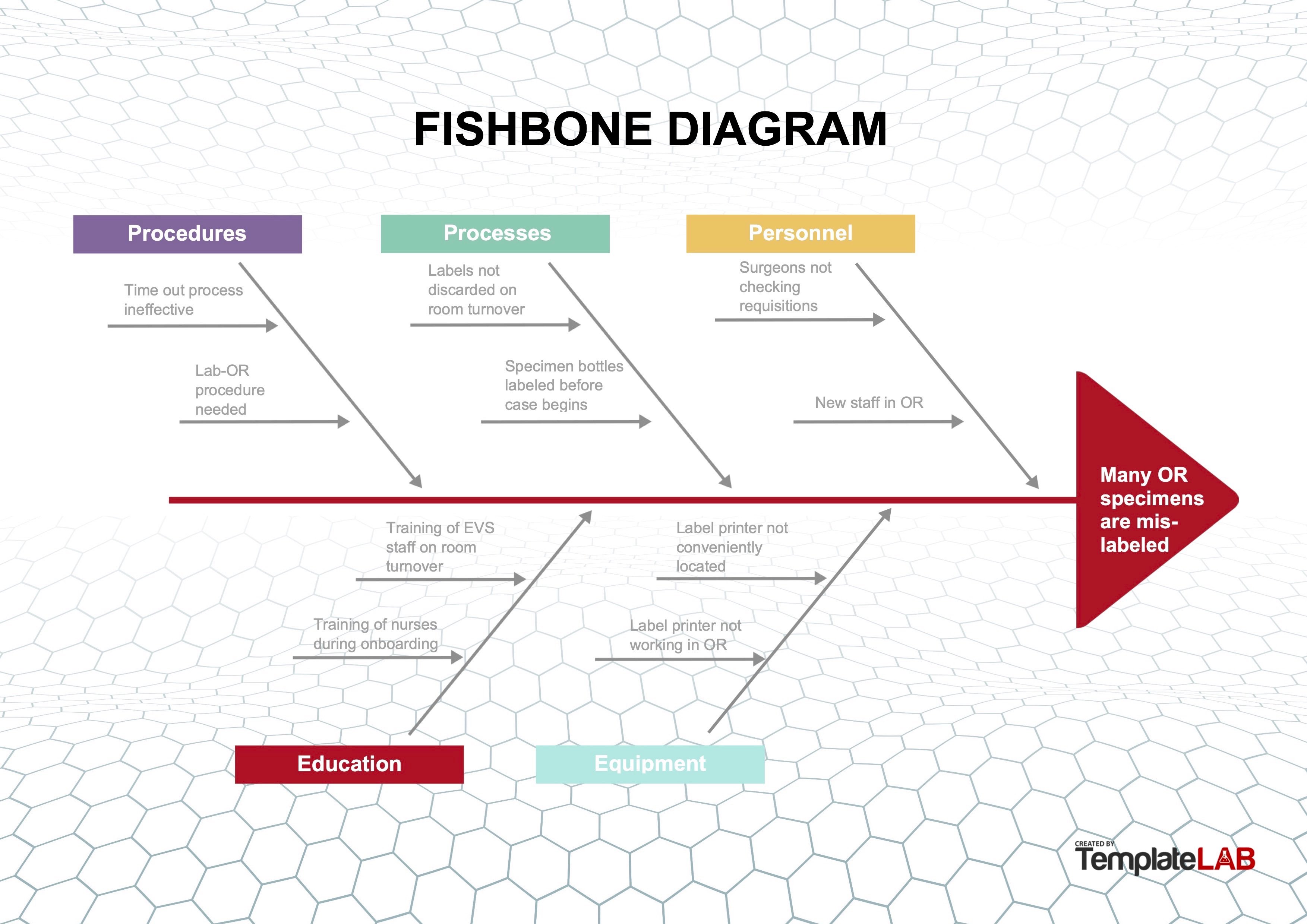
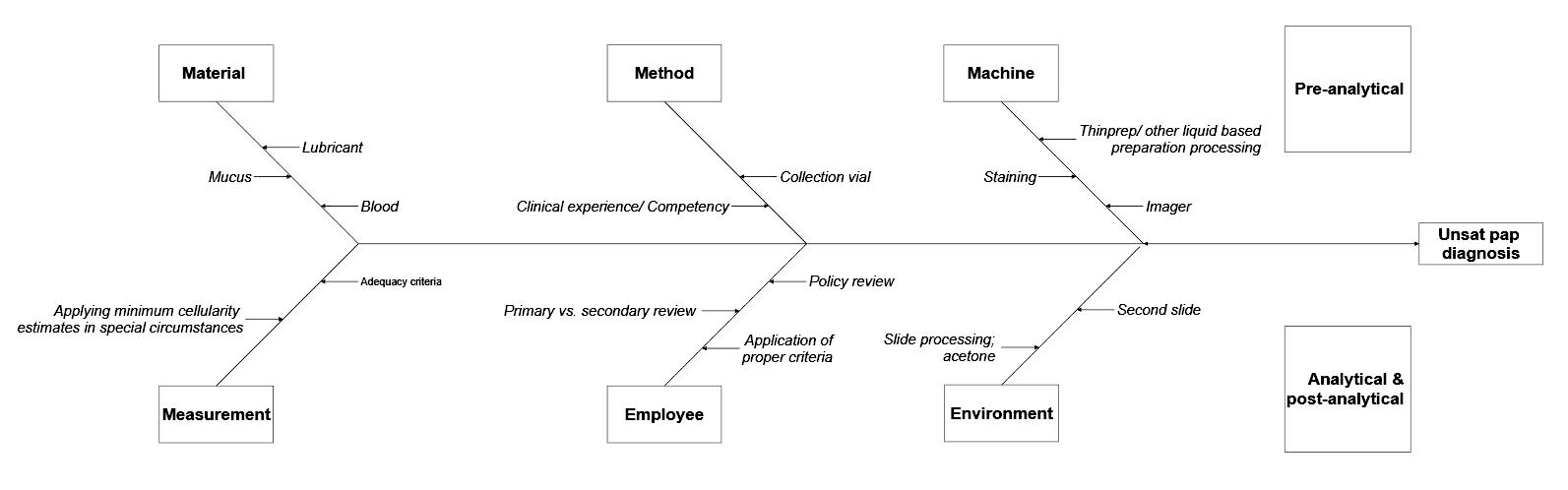
Fishbone or Ishikawa diagram
- Diagram used to identify possible causes of a problem and sort them into useful categories for investigation
- Problem to be addressed is the "head" of the fish and the categories of possible causes form the "bones," with various specific details along each of the bones
- Effective method to use when the root cause(s) is (are) deemed multifactorial or originating outside of the laboratory or department
- Example:
- During review of quality metrics, it was noted that the number of mislabeled specimens being received from the OR was above the metric threshold
- Because these specimens are critical, the standard re-education of OR staff regarding labeling was considered an insufficient measure to correct the problem
- Full RCA was undertaken
- Working group of stakeholders was convened to review the current processes; this included a pathologist, histotechnologist, specimen accessioning tech, nurse educator for the OR, circulating nurse and general surgeon
- Group implemented a fishbone diagram to review the processes and 5 areas of importance were investigated:
- Procedures: procedures for both the OR and the anatomic pathology department were reviewed and 2 major areas of potential weakness were identified
- Time out procedures for the OR required identification of the patient and procedure (operation) but did not include identification and confirmation of specimens for pathology
- Laboratory OR procedures did not detail the labeling and handoff process at each stage in relocating specimens from the OR, to the specimen drop off site, to collection of specimens, to accessioning
- Processes: a review of the OR processes showed that some staff were using shortcuts to "make things run more smoothly" or were not completing room turnover per protocol
- Specimen bottles were labeled with patient identifiers at the start of the surgery, rather than when specimens were acquired
- Prelabeled and unused bottles were not always discarded after patient surgery
- Therefore, these prelabeled bottles were at risk of being used incorrectly for a subsequent patient case
- Specimen label sheets were printed out for use at the start of a case and were not always discarded after the surgery
- Therefore, unused prelabeled specimen sheets were at risk of being used incorrectly for a subsequent patient case
- Specimen bottles were labeled with patient identifiers at the start of the surgery, rather than when specimens were acquired
- Personnel:
- Surgeons did not always perform a complete specimen count after case completion and did not sign the anatomic pathology requisition per protocol
- New personnel in the OR were not trained with standard procedures and practices
- Education:
- Onboarding of new staff in the OR and pathology required to understand the labeling and logging process for patient safety was incomplete
- Training OR staff and environmental care staff to identify material to be discarded during room turnover did not include empty labeled specimen containers or patient labels
- Equipment:
- Equipment limitations were encouraging staff to "prelabel" requisitions and containers as "shortcuts"
- Many specimen label printers were either inconveniently located outside the OR or not in working order
- Procedures: procedures for both the OR and the anatomic pathology department were reviewed and 2 major areas of potential weakness were identified
- All the above factors discovered during RCA contributed to specimen mislabeling; as a result, the following actions were taken:
- Procedures for specimen labeling, handoff and transfer were revised and clarified; all staff was retrained
- Procedures for room turnover were rewritten to emphasize items which must be discarded after case completion
- Unused patient labels and labeled specimen containers must be discarded at case completion
- Surgeons were mandated to review and sign laboratory specimen sheets at the end of a case
- IT was engaged to routinely round in the OR to check printer functionality
Contributed by Moira P. Larsen, M.D., M.B.A.
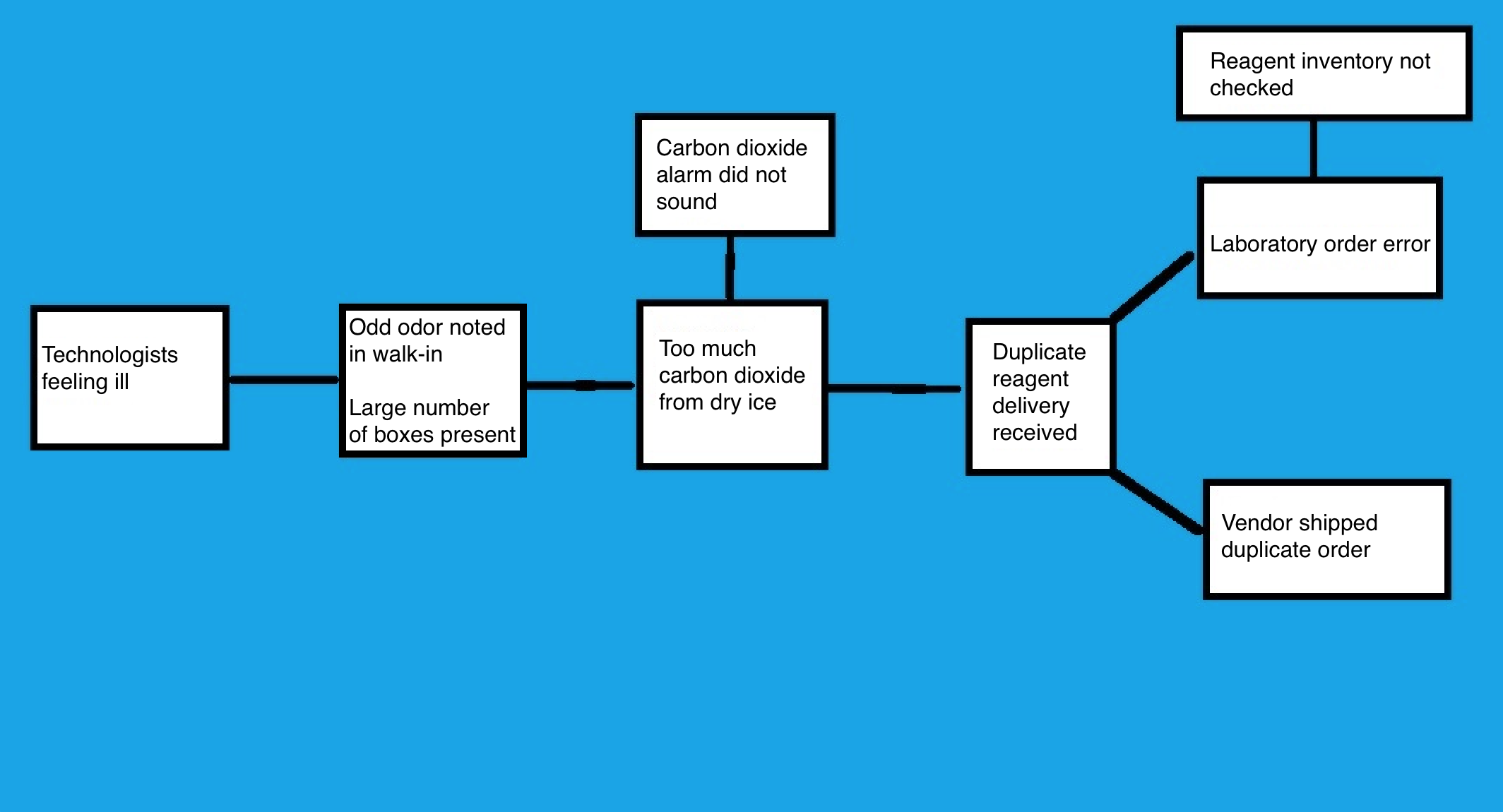
Cause map analysis
- Cause and effect diagram, similar to a fishbone diagram but organized from left to right on the page
- Start with the problem, then draw an arrow to the next box that answers the question of "why" or "was caused by"
- Continue with iterations until the root cause is revealed
- Example:
- Multiple medical technologists in the core laboratory reported to occupational health over the course of 2 days with symptoms including headaches and nausea
- Symptoms, which developed at work, were reported to laboratory leadership
- Investigation regarding the illnesses began and was conducted by the occupational health nurse, chemistry supervisor and laboratory administrative director
- Medical technologists were interviewed and all reported an odd odor in the walk-in freezer
- As the investigating team checked the freezer, they also noted the odor and began developing symptoms
- Chemistry supervisor discovered that there were twice as many reagent supply boxes in the walk-in as usual and when opened and inspected, all of the boxes contained dry ice
- Team suspected that excessive dry ice was leading to high levels of CO2 in the enclosed space and producing the symptoms
- Dry ice was removed and an RCA investigation was conducted
- Facilities were asked to check and confirm the high levels of CO2, then address the CO2 alarm
- Functional CO2 alarm should sound when CO2 levels are dangerously elevated
- Investigation revealed that the alarm had not sounded and was not functioning
- Alarm was repaired
- Chemistry supervisor contacted the vendor to determine why the standing reagent order had been doubled
- Investigation found an error in lab ordering
- Lab ordered the supplies, as inventory had not been appropriately checked
- To prevent recurrence of this error, additional logs and inventory management were implemented
Contributed by Moira P. Larsen, M.D., M.B.A.
- Multiple medical technologists in the core laboratory reported to occupational health over the course of 2 days with symptoms including headaches and nausea
- Identification and resolution of the underlying cause of sentinel event
- Framework for working with other departments to evaluate events as process issues, avoid personal accusations and allow the development of robust solutions
- Pathology example: RCA investigation of mislabeled specimens from the OR revealed → incomplete room turnover, resulting in → leaving patient labels from the previous case in the room to potentially be used in error, leading to → the addition of label disposal process to room turnover checklist
- Intradepartmental studies place emphasis on process rather than the individual facilitating sharing of details
- Laboratory medicine example: RCA investigation of the release of incorrect type blood revealed → safety risks in test tube handling and manual computer data entry when working on multiple first time patient specimens in the same test tube rack, leading to → implementation of single patient flow process for new patients with no transfusion history
- Framework for working with other departments to evaluate events as process issues, avoid personal accusations and allow the development of robust solutions
- Identification of underlying systemic problems and issues
- Focus on the process allows for development of "just culture" and self reporting of events before they become sentinel events
- Routine review of reported internal events at quality meetings to look for patterns, for example:
- Standard operating procedure violations
- Preanalytic errors: mislabeled specimens, label printer malfunctions, incorrect specimen tube selection
- Technical interpretation errors: Gram stain culture mismatch rate, identification of crystals in synovial fluid
- Manual data entry errors for noninterfaced tests: pregnancy tests, COVID-19 assays, point of care test results
- Prevent future new and recurrent adverse events
- Develop acute sense of what could go wrong to allow for proactive process improvement
- Constant assessment of preanalytic, analytic and postanalytic processes to allow for the avoidance of human error
- Pathology example: assuring that when accessioning and grossing specimens, the same specimen types (i.e., colon biopsy, tonsils, placenta) are separated by different specimen types to reduce risk of mix up
- Laboratory medicine example: require second technologist review and sign off on manual entry of COVID-19 test results that are not interfaced to avoid errors
- Risk mitigation
- Increased attention to potential errors with proactive process improvement efforts reduces risk
- Rapid cycle changes to evaluate possible solutions and monitor impact thus generating the best solutions (Kaizen events)
- Culture shift from personal blame to process improvement leads to development of "just culture"
- Breaks down hierarchy
- Makes staff comfortable speaking up for safety
- Includes self reporting of errors made
- Optimization of patient safety, compliance and operational efficacy
- Ultimately, culture of continuous process improvement reduces serious safety events
- Allows for more attention to be paid to the work at hand
- More efficient operations with fewer interruptions and corrections
- High quality and compliant work and test resulting
- Right test at the right time for the right patient allows safer patient care and more efficient flow through the healthcare system
- References: Proc (Bayl Univ Med Cent) 2001;14:154, Appl Immunohistochem Mol Morphol 2019;27:329
5 why's technique
Fishbone diagram
RCA2
- TemplateLAB: 25 Great Fishbone Diagram Templates & Examples [Accessed 4 February 2022], PSNet: Root Cause Analysis [Accessed 4 February 2022], Cancer Cytopathol 2017;125:79, Institute for Healthcare Improvement: RCA2 - Improving Root Cause Analyses and Actions to Prevent Harm [Accessed 4 February 2022], Institute for Healthcare Improvement: 5 Whys - Finding the Root Cause [Accessed 7 July 2022], Institute for Healthcare Improvement: Cause and Effect Diagram [Accessed 7 July 2022], ThinkReliability: Cause Mapping [Accessed 7 July 2022]
- Addressing the cause of blood product administration to the wrong patient
- Daily monitoring of personnel punctuality to ensure adequacy of shift coverage
- Explaining the reason for difficulties in personnel recruitment and retention
- Increased turnaround time for surgical pathology reports
- Investigating a complaint regarding frequent specimen mislabeling
Comment Here
Reference: Causal analysis
- The work performed in clinical and anatomical pathology laboratories is made possible through the use of several different types of hazardous chemicals
- The hazards posed by these chemicals vary, and proper chemical management is imperative to ensure the safety of laboratory personnel
-
OSHA and the EPA act as chemical management regulatory agencies to oversee the proper shipment, storage and handling of hazardous chemicals in the laboratory
- Occupational Safety and Health Administration (OSHA)
- 29 CFR 1910.1200 - Hazard Communication - 1987
- 29 CFR 1910.1450 - Chemical Hygiene Standard - 1990
- Supersedes Hazard Communication of 1987 - for laboratories only
- The Hazard Communication Standard was revised in 2012 in order to adopt the Globally Harmonized System for the classification and labeling of chemicals (GHS), a worldwide effort to standardize chemical labels and Safety Data Sheets (SDS)
- Environmental Protection Agency (EPA)
- Regulations focus on chemical waste handling / disposal
- Laboratories that generate waste must follow specific procedures (US Environmental Protection Agency-Hazardous Waste Generators)
- College of American Pathologists (CAP)
- Laboratory general inspection checklist
- Anatomic pathology inspection checklist
-
Laboratory requirements:
- Written chemical hygiene plan
- Updated annually
- Standard operating procedures
- Environmental monitoring
- Perform on chemicals such as xylene and formaldehyde
- Employee education
- Train all staff on chemical hazards, proper chemical management
- Engineering controls
- Remove the hazard from the workplace
- Example: chemical fume hoods with proper ventilation
- Work practice controls
- Provides a procedure to protect the employee from the hazard
- Example: when pouring chemicals, work under a hood and use goggles, a cover gown and gloves
- Personal protective equipment
- Provide lab coats, chemical-resistant gloves, face protection
- Chemical storage
- Do NOT store alphabetically by name
- Use a chemical incompatibility chart to prevent storage errors
- Chemical inventory
- Updated annually
- Electronic format is preferred
- Exempt chemicals: those in kit form or that contain less than 1% of a hazardous chemical
- Note carcinogens
- Note acutely and reproductively toxic chemicals
- Safety Data Sheets (SDS) for each hazardous chemical should be available
- Proper labeling of chemicals
- All primary containers need GHS-complaint labeling as of 6/1/16 (Occupational Safety & Health Administration)
- Transfer containers: may use NFPA / HMIS labeling
- Chemical waste
- Check local regulations and sewage treatment plant for capacity before disposing of anything down the drain
- Consider other waste handling options:
- Removal by outside firm
- Neutralizing
- Recycling
- Medical exam for overexposure
- Follow up for any high vapor badge monitor readings or accidental chemical exposures
- Documentation
- Safety Data Sheets (SDS):
- Needed for each hazardous chemical in your inventory
- Access quickly for exposures and spills
- GHS format has 16 standard sections
- Keep for 30 years if chemical has been involved in an exposure incident
-
Chemical labeling:
- Primary chemical label must contain:
- Product name
- Signal word (either warning or danger)
- Any needed hazard statements
- Precautionary statements
- Pictograms
- Manufacturer name, address and telephone number
- See figure 1
- Secondary chemical label must contain:
- Option 1:
- Name of the chemical
- Concentration
- Route of entry
- Health hazard
- Physical hazard
- Target organs affected
- Lot number and expiration date
- Option 2:
- Name of the chemical
- NFPA or HMIS label that is filled out to indicate the potential hazards
- Option 3:
- Name of the chemical
- GHS signal word
- Appropriate pictograms
- Option 1:
Contributed by OSHA
-
Administrative requirements:
- Name a Chemical Hygiene Officer (CHO) for the laboratory
- Responsible for chemical management administrative duties
- CHO should be in job description
- Perform task assessments for processed involving chemicals
- Assemble a safety committee
- Perform safety audits
- Discuss safety issues / incidents
- Chemical spill handling:
- Provide spill response training for all staff that handles chemicals
- Confine or contain the spill
- Small quantities of inorganic acids or bases - use a neutralizing agent
- Small quantities of other materials - absorb with towels or spill pillows
- Large quantities of inorganic acids or basis - flush with large amounts of water
- Mop or sweep up the spill (see figure 2)
- Dispose of waste in proper receptacles
- If spilled chemical is volatile, let it evaporate
- Annual review of chemical management:
- Analyze effectiveness of your chemical hygiene plan
- Review chemical inventory
- Review training
- Review chemical incidents
- Use substitutes for hazardous chemicals where possible
- Reduce hazardous waste volumes
- Remove outdated chemicals
- Analyze effectiveness of your chemical hygiene plan
Contributed by Dan Scungio, MT (ASCP), SLS, CQA (ASQ)
- Definition
- Narrative blueprint describing the nature of an intended business
- Road map describing the implementation of an intended business
- Deliverable value
- Defines activities by which to implement a business
- Exposes financial pitfalls
- Provides documentation for lenders, investors
- Applicability
- Business plans are generic templates applicable to any industry: manufacturing, technical, service, health care, etc.
- See Appendix: special considerations for pathology and laboratory services
- Not all elements listed in business plan below will be applicable to every business
- Elements described below may not be all encompassing for every business
- It can clarify what you are getting yourself into (i.e. tell you whether you are doing the right thing or the wrong thing)
- If you need capital or risk-sharing partners, reputable lenders and risk seekers will demand that you present them with a business plan
- It will help you determine whether or not your venture can achieve your goals, especially profitability
- Know why businesses fail (Entrepreneur: 10 Reasons Why 7 Out of 10 Businesses Fail Within 10 Years [Accessed 4 May 2020])
- Familiarize yourself with standard business metrics (process.st: 53 Essential Business Metrics You Need to Be Tracking in 2018 [Accessed 4 May 2020])
- Understand why a business plan is necessary (U.S. Small Business Administration: 5 Reasons You Need a Business Plan [Accessed 4 May 2020])
- Know what resources are available to business startups (Score: List of Startup Resources [Accessed 4 May 2020], Iowa State University: Business Development - Starting a Business [Accessed 4 May 2020])
- Discuss your concept with key advisors:
- Spouse, friends, colleagues, attorneys, business advisors, stakeholders, potential partners
- All principals in the proposed venture should answer the following questions:
- Why exactly am I doing this? Are you doing this because you have a need to be better tomorrow than you are today? Because you are worried about your future? Because you require greater income? Because it just seems like the right thing to do? Because you wish to seek new challenges? Answering this question honestly will keep you focused on achieving your goal.
- What are my goals - exactly what do I hope to accomplish? Greater income? Greater peace of mind? Greater respect in the community?
- Can I accomplish the same goal another way? What ways other than launching a new business might you be able to achieve your goals? Rethink your end point and whether other paths to it might be easier, more economical and more rewarding.
- What sorts of data will I need to make decisions? We don’t know what we don’t know. What sorts of information are you missing to arrive at your business decisions?
- How will I obtain that data? Will you be able to do your own research? Will you need help? Who will be your go to advisors and how will you engage them?
- Where will I procure capital? Will you need to put your personal assets on the line to secure a bank loan? Will you be comfortable engaging venture capitalists? Will your family be able to provide capital and are you comfortable in asking them?
- How do I know if I will have enough capital? The time will come when your business plan includes the capital you will need. How will you determine the accuracy of that figure?
- What is my comfort level for risk? Will you be willing to list your home as collateral on a bank loan? Can you tolerate bankruptcy?
- What are my key metrics for measuring success and failure? Given the goals you have stated above, how will you determine whether or not you are succeeding or failing?
- At what milestones of time will I assess success and failure? How long will you wait until you determine whether or not you are achieving your goals; whether you should continue or cease pursuing your goal?
- What are my contingencies for failure? If it becomes clear that you will not reach your goal, what will you do?
- Where are the weak spots of my proposed goal? Are there unintended negative consequences in achieving the goal you have laid out?
- Have I successfully and honestly neutralized skeptical arguments to the goal I would like to attain? Have others voiced skepticism to the goal you hope to attain and if so, are you being honest and realistic in your reaction to that skepticism?
- How will I eliminate bias in my assumptions and projections? Have you examined your bias in formulating your goal? Are you overly optimistic?
- Do I really know my market? Do you understand that you are not the market? Are you contemplating launching a service or a product that you would use but for which there is not widespread consumer interest?
- What is the intended lifespan of the business? Do you plan to grow your business over your lifetime or to exit early based on a finite timeline?
- What is my exit strategy? Regardless of the lifespan, how do you plan to terminate your business? Sell it? Retire?
- What is it:
- Study to:
- Determine the viability of an idea
- Answer the questions above
- Not a business plan but comprises many elements that will become part of a business plan:
- Feasibility - evaluates several options to accomplish business goals
- Business plan - provides specifications (i.e. roadmap, blueprint) for single final selected option
- Study to:
- Feasibility study elements
- Description of product / service: the product or service you would like to provide, e.g. an outreach laboratory performing molecular pathology for other pathology groups and billing patients directly.
- Projected income statement (e.g. revenue minus investment to achieve goal): the revenue will comprise volume times net reimbursement. The investment will include all the costs (e.g. labor, equipment, supplies, etc.) required to generate that revenue. This figure will determine in a nutshell whether or not the venture may be worthwhile. For your molecular lab, the investment in building the lab may be dependent on the volume. An unfavorable patient mix or less demand than you anticipated may terminate your proposal and keep you from getting in over your head.
- Market survey / research: this will determine the number of tests you will do and the revenue you will derive from them. It must be performed meticulously and consider the worst-case scenarios.
- Marketing and sales strategy: this strategy will determine how you obtain customers. Your laboratory will likely be competing against labs that have been doing this for a while. Be realistic as to how customers will prefer your service to that of others.
- Organization / management: the wrong people running your lab will sink it. Determine how you will choose your management wisely.
- Operations: know how you plan to run your lab—how will laboratory operations meet the demands of your customer?
- Staffing: this will be your single largest operational expense. To avoid unrealistic optimism, estimate on the high side.
- Scheduling: plan and budget laboratory scheduling to meet not your convenience but rather the needs of the customer.
- Opening day balance sheet: this is a summary of your assets and liabilities on your first day of business. It will tell you if your lab has the legs to walk to the break-even point.
- Technical, economic, regulatory, legal, operational, temporal and other considerations: this will be a compendium of the important considerations that may impact your laboratory. For example, are your molecular reagents verified for the application you are using them for or will you need to engage in costly and time consuming validation studies? Will that delay opening your laboratory?
- Findings / recommendations: this should be a summary of your feasibility study. It may contain recommendations for modifications of your plan if you decide to move forward with the lab. For instance, transporting specimens from the airport to your downtown location may be problematic and hence recommendations might include setting up your lab in an airport industrial park.
- Technical, economic, regulatory, legal, operational, temporal
- "Go or no go" to move onto a business plan: this is the self-explanatory endpoint of the feasibility study.
- Business name, address and contact information
- Legal form of ownership and reason for selection:
- Sole proprietor
- Partnership, corporation
- Limited liability corporation (LLC)
- Other
- Nature of business / service or product provided
- Mission statement
- Vision, goal, objectives (what the successful end point looks like)
- Basic strategies to:
- Achieve goal
- Take advantage of business environment / industry viability
- Neutralize competition
- Business culture or philosophy
- Markets: primary and secondary
- End game
- Continuous growth / maintenance to retirement
- Sale, acquisition
- Other
- Business environment
- Favorable
- Unfavorable
- In flux
- Industry viability
- Long term
- Short term
- Key company strengths, assets, competencies, expertise
- Key competitive strengths, assets, competencies, expertise
- Description of products and services
- Your view - defining features
- Customers’ view - defining benefits
- Competitive advantages or disadvantages
- Proprietary elements
- Patents, intellectual property
- Special features:
- Delivery
- Warranty
- Refund policy
- Satisfaction guarantees
- Service and support
- Associated with sale
- Ongoing
- Other
- Location (include drawings)
- Home
- Brick and mortar
- Location strategy (relationship to competition)
- Physical requirements
- Amount of space
- Type of building and zoning
- Power and other utilities
- Building costs with debt service
- Access
- Customers
- Employees
- Deliveries
- Shipping
- Lease / rent
- Maintenance
- Elements of importance to customers and employees
- Convenience
- Parking
- Interior space
- Image
- Hours of operations
- Production model
- Equipment
- Service delivery
- Personnel
- Organizational chart
- Type and number each:
- Skilled
- Nonskilled
- Professional
- Recruiting
- Onboarding
- Skill assessment and ongoing competency testing requirements
- Productivity assessment
- Retention methods / policies
- Pay structure
- Training methods and requirements
- Task assignment
- Schedules
- Policies and procedures
- Inventory
- Amount
- In stock
- Turnover
- Seasonal
- Value
- Cost
- Ordering
- Lead time (scheduling)
- Contingencies for delays / failures
- Suppliers
- Names, address
- Type and amount of supplies
- Credit
- Delivery policies
- History / reliability
- Quality / quality control
- Customer service
- Inventory control and reagent shelf life
- Research and development
- Amount
- Subcontracting
- Board of directors: a board of directors provides the governance that guides you to achieving your corporate goals. Often the boards of medical practices comprise doctors only. In doing so, they are unable to benefit from the objective advice of individuals who possess experience and expertise that the physician directors may not. For instance, an outreach molecular laboratory might include directors who are in the medical insurance, medical product retail and financial industries. Because board members have fiduciary responsibilities, unlike consultants they are legally responsible for the advice they bestow.
- Management advisory board: similar to a board of directors but without fiduciary responsibilities, a management advisory board can provide valuable critique of daily operations, sales, marketing, etc.
- Financial, banker
- Legal
- Licensing and bonding requirements
- Permits
- Patents, patent protection
- Trademarks, copyrights or patents (pending, existing or purchased)
- Contracts
- Sales agreements
- Regulatory (federal, state, municipal)
- Health
- Proficiency survey subscription
- Workplace (e.g. OSHA)
- Environmental (e.g. waste disposal)
- Zoning or building code requirements
- Accounting and bookkeeping
- Insurance coverage
- Consultant(s)
- Other mentors and key advisors
- Market
- Size
- Your proposed share
- Demand for product / service
- Trends
- Customer preference
- Product development
- Regulatory
- Growth potential and opportunity
- Barriers with mitigating plans
- Costs: plan on cost overruns in the acquisition and cost of capital, operations, sales and marketing, shipping, supplies, etc.; have access to additional capital or plans to cut costs without affecting quality or service
- Capital
- Production
- Marketing
- Shipping
- Consumer acceptance: be prepared to alter you product or services if your customers do not like them; for instance, if your drawing station closes at 4 p.m. but patients get off work at 5 p.m., be prepared to extend your hours
- Brand recognition: if customers recognize the brand of a competing laboratory but not yours, be prepared to boost your marketing.
- Training: if medical demand requires that you add a new test, consider how you will train your staff to perform it.
- Labor availability: it is often difficult to find experienced technologists. Have a plan and incentives to attract high quality labor if labor is in short supply.
- Technology: at one time, radioimmunoassay (RIA) seemed to be the future of chemical analysis in clinical laboratories. When other techniques replaced RIA, labs were stuck with expensive gamma and beta counters. Be flexible and have contingencies for methods that outdate unexpectedly.
- Patents: may prevent your laboratory from performing a test; do you find another test to replace it or just not do the test?
- Unions: union agendas might not advance your agenda. Know how you will interact with them.
- Tariffs and quotas: your laboratory instruments may be manufactured abroad; a sudden tariff can erode your bottom line.
- Costs: plan on cost overruns in the acquisition and cost of capital, operations, sales and marketing, shipping, supplies, etc.; have access to additional capital or plans to cut costs without affecting quality or service
- Competitors: list for each competitor
- Name and location
- Products and selection
- Scope of competition for products / services
- All
- Some
- Contingent: location, demographics, etc.
- Direct / indirect competitor
- Price and credit policies
- Quality and service
- Selection
- Service
- Reliability
- Stability
- Expertise
- Reputation, image and advertising
- Location
- Appearance
- Sales method
- Credit policies
- Other
- For each element, list company's:
- Strengths
- Weaknesses
- Importance to customer
- Importance to you
- Your relative position scaled according to your preference:
- Among competitors
- Among importance to customers
- Your niche
- Market research reports
- Primary
- Professional outsource
- Self
- Secondary
- Published information:
- Industry profiles
- Trade publications
- Trade association data
- Business association, chamber of commerce data
- Internet and media data
- Census, demographic data
- Published information:
- Primary
- Marketing strategy
- Promotion
- Budget, one time and ongoing
- Advertising
- Media
- Visual
- Live
- Frequency
- Cost
- Media
- Branding
- Logo
- Ancillary material
- Cards, letterhead, brochures, signs, other
- Customer identification methods
- Promotion
- Sales
- Model
- Retail, wholesale or both
- Distribution
- Direct
- Indirect (wholesale)
- Lease
- Franchise
- Venues
- Face to face
- Web / mail order
- Catalog
- Brick and mortar
- Wholesale
- Internal sales force
- Agents
- Independent representatives
- Contract bid
- Pricing and fees
- Strategy
- Actual numbers
- Discounts
- Credit
- Credit policies: check credit worthiness and terms
- Cost of credit
- Policies for slow paying / defaulting customers
- Model
- Startup expenses, capitalization
- Personal financial statement(s)
- Detailed itemization
- Sources of capital
- Loan
- Amount
- What for / how used
- Repayment terms
- Interest, debt service
- Use of funds
- Collateral and liens
- Equity
- Short term needs
- Long term needs (2 - 5 years)
- What for / how used
- Estimated return on investment
- Exit strategy
- Buyback, sale, IPO, other
- Percentage of ownership
- Conditions
- Financial reporting to be provided
- Involvement on board or in management
- Loan
- Projections
- 12 month profit and loss projection
- Sales and volume forecasts
- Best guess
- Worst case
- Cost of goods sold
- Expenses
- Monthly profit / loss for 1 year
- Sales and volume forecasts
- 4 year profit and loss projection
- Monthly cash flow (worksheet) with assumptions
- Sales
- Inventory purchases
- Equipment purchases
- Payroll
- Cash outlays
- Taxes
- Regulatory fees, licenses, etc.
- Maintenance, repairs
- Loans
- Other expenses
- Balance sheet (assets and liabilities)
- Opening day
- End of year
- 12 month profit and loss projection
- Break even (BE) calculation: break even point (units) = fixed costs ÷ (revenue per unit - variable cost per unit)
- Brochures and advertising materials
- Industry studies
- Blueprints and plans
- Maps and photos of location
- Magazine or other articles
- Detailed lists of equipment owned or to be purchased
- Copies of leases and contracts
- Letters of support from future customers
- Market research studies
- Other
- Last document to write
- 5 minute presentation: watch Shark Tank. Summarize the most important points in a 5 minute “elevator” presentation. Coring down to 5 minutes should allow you to present most efficiently, your concept to those who can help you with funding and resources. Don’t worry about including everything. Investors and stakeholders will fill the gaps with their questions.
- Before you launch the business plan, revisit questions from above and confirm / reject / modify initial answers
- Definition
- Narrative blueprint describing the nature of an intended new service
- Road map describing the implementation of an intended new service
- Deliverable value
- Defines activities by which to implement new service
- Exposes financial and institutional budgetary pitfalls
- Provides documentation for lenders, investors, administrators, other stakeholders
- Applicability
- Generic template that must be modified for specific service (e.g. outreach laboratory, molecular and genetics laboratories, point of care testing, etc.)
- Not all elements listed below apply to every service
- Elements listed below may not be all encompassing
- Extrapolate knowledge of why businesses fail in relation to pathology and laboratory services (Entrepreneur: 10 Reasons Why 7 Out of 10 Businesses Fail Within 10 Years [Accessed 4 May 2020])
- Know what consulting resources are available to laboratory startups
- Discuss your concept with key stakeholders as necessary and appropriate:
- Administration, hospital trustees, physicians, nursing, other health care providers, patients and other uses of the service, others
- Understand that patients and healthcare providers are the market, not the laboratory or pathology service providers
- Ask yourself the following questions:
- Why do we / the institutional leaders want to do this?
- What are our goals - exactly what do we hope to accomplish?
- Can we accomplish the same goal another way?
- What sorts of data will we need to make decisions?
- How will we obtain that data?
- Who will be funding this project?
- What are the key metrics for measuring success and failure?
- At what milestones of time will we assess success and failure?
- What are our contingencies for failure?
- Where are the weak spots of our proposed service?
- Have we successfully and honestly neutralized skeptical arguments to our service?
- How will we eliminate bias in our assumptions and projections?
- Do I really know our market?
- What is our exit strategy?
- Same as above
- Nature of service provided
- Key service strengths, assets, competencies, expertise
- Description of service
- Providers’ view - defining features
- Service users’ view - defining benefits and expectations
- Patients
- Patient safety and error reporting protocol
- Direct to consumer testing
- Specimen acquisition, phlebotomy
- Hours of service
- Pricing tolerance
- Billing policies (e.g. network provider, insurance acceptance, "surprise bills")
- Health care providers
- Pathologist consultation
- Critical value notification
- Teaching and conferences (e.g. tumor board, specialty, other)
- Delivery schedules
- Billing policies (same as for patients)
- Quality and satisfaction
- Access: order entry and result retrieval (e.g. LIS, electronic order entry, electronic result, office interface)
- Proprietary elements or subspecialty expertise
- Special features if different from existing (main) laboratory
- Patients
- Patients
- Ethnic breakdown that can influence:
- Disease prevalence
- Service patronage
- Insurance / payer mix
- Other, special
- Ethnic breakdown that can influence:
- Referring providers
- Scope
- Specialists
- Employed and private physicians
- Nonphysicians
- Location
- Main health system campus
- Peripheral / non main health system facility
- Free standing facility
- Other facility (e.g. physicians’ offices, mobile units, etc.)
- Scope
- Location (include drawings)
- Personnel
- Organizational chart
- Type (as per CLIA designation) and number each:
- Skilled (e.g. medical technologists)
- Less skilled (e.g. laboratory assistants and clerical staff)
- Unskilled (e.g. loading dock, maintenance, etc.)
- Professional (CLIA directorship, board certification, subspecialty training)
- Verification
- CLIA mandated credentials
- Onboarding
- Ongoing competency testing (technologists, pathologists)
- Specimen access
- Phlebotomy service
- Courier
- Specimen receiving and accessioning
- Specimen processing
- Test menu, scope of services
- Instruments and platforms
- Specimen and reagent storage
- Result reporting, critical value notification
- Information technology, LIS
- Quality control and performance improvement
- Safety
- Handicapped access
- Hazardous material / waste handling and disposal
- Laboratory plant, policies, procedures
- Client services / call center
- Inventory
- Inventory control and reagent shelf life
- Supply chain
- Hazardous materials
- Regulatory (federal, state, municipal)
- CLIA license as per laboratory complexity
- Laboratory accreditation (e.g. CAP, Joint Commission, ISO, AABB)
- Mock inspections
- Proficiency testing
- HIPPA
- Fraud and abuse compliance
- Workplace (e.g. OSHA)
- Environmental (e.g. waste disposal)
- Occupancy codes and building safety
- Pathologist partnership (policies and financial issues)
- Health system administration
- Laboratory advisory board
- Billing and insurance reimbursement
- Technology
- IT, LIS electronic interface
- Testing platforms
- Instrumentation
- Health care trends
- Legislation
- Regulatory
- Same as above
- Same as above
- Source of capital
- If self funded (e.g. private lab) - as above
- If institutionally funded (e.g. hospital outreach lab or new service):
- Parent corporation, if applicable
- Charitable foundations and grants
- Projections
- 12 month profit and loss projection
- Specimen volume forecast
- Revenue projection: by payer reimbursement / self pay per CPT codes
- Best guess
- Worst case
- 12 month profit and loss projection
- Last document to write
- 5 minute presentation: watch Shark Tank. Summarize the most important points in a 5 minute “elevator” presentation. Coring down to 5 minutes should allow you to present your concept most efficiently to those who can help you with funding and resources. Don’t worry about including everything. Investors and stakeholders will fill the gaps with their questions.
- Before you launch the business plan, revisit initial questions above
- It is a narrative blueprint describing the nature of an intended business
- It does not include details regarding how the business is to be implemented
- It never exposes financial pitfalls
- It provides the necessary documentation for the Internal Revenue Service
- It is not generic but differs substantially for every industry
Comment Here
Reference: Creating a business plan
- Competitors’ 12 month profit and loss projections
- 3 year inflationary trends
- Monthly cash flow worksheet
- National gross domestic product (GDP) projections
- Only best case scenarios
- Critical values (CVs): laboratory results that require prompt, appropriate intervention to avert potential serious outcomes
- The process of establishing CVs that merit immediate notification should involve not only laboratory personnel but also clinical providers, clinical informaticists and hospital management
- CV communication should be done promptly, ideally within 30 minutes, for all chemistry, toxicology, hematology, therapeutic drugs, microbiology, anatomical pathology and cytopathology
- The advisable means of CV communication is internal phone calls; significant / unexpected diagnosis in anatomical pathology can be communicated by a secured messaging system if there is failure to initiate a phone call
- Critical values
- Urgent actionable results
- Unexpected findings with potential urgent implications
- Rapid results
- Frozen section reporting
- The laboratory medical director develops the list of critical values based on CAP guidelines and the institution's patient population
- Establishes critical report procedure; lab technicians, clinical personnel, clinical informaticists and hospital management must be involved
- Categorizes the urgency level of critical values and expected turnaround time for each group (Jt Comm J Qual Patient Saf 2005;31:68):
- Red:
- Life threatening values
- Requires an immediate call, ideally within 30 minutes (Arch Pathol Lab Med 2012;136:148)
- Prompt clinical decision warranted
- Orange:
- Significantly abnormal results
- Requires quick calls but not immediate
- Clinical decision needed within hours
- Yellow:
- Abnormal results but not life threatening
- Can be sent passively
- Additional communication is necessary
- Clinical decisions are needed within days
- Red:
- Separate CVs list for anatomic pathology (including surgical pathology and cytopathology) with 2 categories (J Am Soc Cytopathol 2021;10:341):
- Red: time critical diagnoses that are medical emergencies or have infectious risks
- Orange: significant / unexpected diagnoses that necessitate additional communication
- Often use a middleware flagging system to raise an alerting pop up when CVs emerge
- Design a pop up with dropdowns on the electronic medical record to document when CVs emerge (example shown below); or a paper template to document the CVs calls log
- Include an on call roster so the lab staff can navigate to whom to report CVs, with a decision tree in the event of nonresponse
- Create an escalation policy, defining the order of receivers that the reporter should call when the ordering provider is not approachable
- Review / revise the critical value list periodically in consultation with medical providers
- Assess the effectiveness of the report critical system regularly
- Chemistry or microbiology critical values:
- Identify the reporter, usually the performing technologist
- Verify and repeat the test if needed; the maximum is 30 minutes
- Identify the receiver using the provider entry, the patient location and the on call roster
- Determine means of notification:
- Step 1: call the ordering physician (CAP recommended); call other extensions, including the nurse station, in the same department if there is no answer
- Verify the patient’s identification using 2 identifiers
- Test name
- Critical values (including unit and normal range), their category
- Read back for confirmation
- Verbally correct any errors and repeat the request for a read back if needed
- Document the notification process
- Step 2: page the clinical personnel if there’s no answer to the phone call; if the provider calls back in 10 minutes, conduct actions in Step 1
- Step 3: if there is no response after paging, call the chemistry / unit supervisor to take on the follow up
- That person initiates the escalation policy
- First task is to call the hospital operator for a medical alert call
- If there is still no response, call the department chairperson of ordering provider
- Step 1: call the ordering physician (CAP recommended); call other extensions, including the nurse station, in the same department if there is no answer
- The reporter must document all the calling attempts and notification details, including the reporter’s identification, time, date, critical values, category, receiver’s identification and whether a confirmation was provided (by a read back)
- Anatomical critical values:
- If needed, confirm the result with the additional tests, IHC staining and peer reviews
- Identify the reporter (usually the pathologist) who established the diagnosis
- Identify the receiver, usually the ordering provider or the physician who performed the biopsy / FNA / surgery
- Determine the category for the result
- If the result is in the red category, it needs immediate intervention; carry out the same procedure as in chemistry / microbiology CVs
- If the result falls in the orange category, carry out the following steps:
- Step 1: call the ordering physician (CAP recommended); call other extensions, including the nurse station, in the same department if there is no answer
- Verify the patient’s identification using 2 identifiers
- Specimen type
- Critical values, their category
- Request read back for confirmation
- Verbally correct any errors and repeat the request for a read back if needed
- Document the notification process
- Step 2: if there’s no answer to the phone call, send the critical values through a secure messaging system (i.e., EPIC) to the ordering physician and the patient’s provider; a confirmation of receipt should be requested (Lab Med 2020;51:e6)
- Step 3: check back within 1 - 2 hours; if there is no confirmation response, carry out the escalation policy; call the hospital operator for support; if there is still no response, call the department chairperson of ordering provider
- Step 1: call the ordering physician (CAP recommended); call other extensions, including the nurse station, in the same department if there is no answer
- The reporter must document all the call attempts and notification details, including the reporter’s identification, time, date, critical values, category, receiver’s identification and whether a confirmation is provided (by a read back)
- Critical values in outpatient settings:
- Step 1: verify the result and send the result to the ordering provider; call the patient’s primary care provider (PCP) clinic and report the result to the nurse / medical assistant / front desk personnel; carry out the same procedure as an inpatient critical values call:
- Verify the patient’s identification using 2 identifiers
- Test name
- Critical values (including unit and normal range), their category
- Read back for confirmation
- Verbally correct any errors and repeat the request for a read back if needed
- Document the notification process
- Step 2: if there is no answer from the primary care provider's clinic, call the lab supervisor / attending pathologist to take on the next steps
- Step 3: the lab supervisor / attending pathologist attempts to call the patient’s home or mobile phone number; explain to the patient that they have a critical test result and need to contact their primary care provider and come to the nearest emergency department for immediate treatment
- Step 4: if the patient is unable to comprehend the results or there is no call answer, call 911 for support
- The reporter must document all call attempts and notification details, including the reporter’s identification, time, date, critical values, category, receiver’s identification and whether a confirmation is provided (by a read back)
- Step 1: verify the result and send the result to the ordering provider; call the patient’s primary care provider (PCP) clinic and report the result to the nurse / medical assistant / front desk personnel; carry out the same procedure as an inpatient critical values call:
A. Chemistry critical values:
| Test | Less than or equal to | Greater than or equal to |
| ALT neonate < 12 months | 100 U/L (UC Irvine: Critical Values for Infants (<12 Months) [Accessed 2 September 2022]) | |
| Bilirubin neonate 1D | ≥ 6 µg/mL (UC Irvine: Critical Values for Infants (<12 Months) [Accessed 2 September 2022]) | |
| Bilirubin neonate 2D | ≥ 10 µg/mL (UC Irvine: Critical Values for Infants (<12 Months) [Accessed 2 September 2022]) | |
| Bilirubin neonate 3D - 4D | ≥ 12 µg/mL | |
| Bilirubin neonate 5D - 12M | ≥ 15 µg/mL (UC Irvine: Critical Values for Infants (<12 Months) [Accessed 2 September 2022]) | |
| Calcium adults | 6 mg/dL, adult 6.5 mg/dL, pediatric (UC Irvine: Critical Values for Infants (<12 Months) [Accessed 2 September 2022]) | 13 mg/dL, adult 12 mg/dL, pediatric (UC Irvine: Critical Values for Infants (<12 Months) [Accessed 2 September 2022]) |
| CO2 | 10 mmol/L | 50 mmol/L |
| Ethanol | 300 mg/dL, adult 10 mg/dL, pediatric | |
| Ethanol glycol | 20 mg/dL | |
| Glucose adults | 450 mg/dL 300 mg/dL | |
| Isopropanol | 10 mg/dL | |
| Magnesium | 1.0 mg/dL | 4.7 mg/dL |
| Methanol | 10 mg/dL | |
| Potassium | 2.8 mmol/L, adult 3.0 mmol/L, pediatric | ≥ 6.2 mmol/L, adult 6.5 mmol/L, pediatric |
| Propylene glycol | 100 mg/dL | |
| Sodium | 120 mmol/L | 160 mmol/L |
| Troponin T | > 0.01 ng/mL | |
| Test | Greater than or equal to |
| Acetaminophen | 150 µg/mL: 4 hours post ingestion 40 µg/mL: 12 hours post ingestion (ARUP Lab: Critical Values List [Accessed 2 September 2022]) |
| Amikacin | 35 µg/mL |
| Carbamazepine | 12 µg/mL |
| Digoxin | 2.0 ng/mL |
| Everolimus | 30 ng/mL (University of Michigan: Everolimus [Accessed 2 September 2022]) |
| Gentamicin | 15 µg/mL |
| Lead | 10 µg/dL (for < 16 years old) 20 µg/dL (for ≥ 16 years old) |
| Lidocaine | 5 µg/mL |
| Lithium | 1.5 mEq/L (University of Michigan: Lithium [Accessed 2 September 2022]) |
| Phenobarbital | 60 µg/mL, adult 50 µg/mL, neonate < 12 months (UC Irvine: Critical Values for Infants (<12 Months) [Accessed 2 September 2022]) |
| Phenytoin | 30 µg/mL |
| Procainamide | 16 µg/mL |
| Salicylate | 30 mg/dL (University of Michigan: Salicylate [Accessed 2 September 2022]) |
| Sirolimus | 25 ng/mL: liver transplantation 15 ng/mL: renal transplantation (UC Irvine: Critical Values for Adults and Pediatrics (≥ 12 Month) [Accessed 2 September 2022]) |
| Tacrolimus | 20 ng/mL (UC Irvine: Critical Values for Adults and Pediatrics (≥ 12 Month) [Accessed 2 September 2022]) |
| Theophylline | 20 µg/mL |
| Tobramycin | 10.5 µg/mL (University of Michigan: Tobramycin [Accessed 2 September 2022]) |
| Valproic acid | 150 µg/mL |
| Vancomycin peak / random | 60 µg/mL (University of Michigan: Vancomycin [Accessed 2 September 2022]) |
| Test | Less than or equal to | Greater than or equal to |
| Fibrinogen | 80 mg/dL | |
| Hematocrit, adult | 18% | 55% |
| Hemoglobin, adult | 6 g/dL (< 7 outpatient) | 22 g/dL |
| HIT (heparin dependent antibody) | Positive | |
| INR (venous) | 5.0 (University of Michigan: PT (Prothrombin Time) and INR [Accessed 2 September 2022]) | |
| Platelet count | 10.0 k/mm3 | 1,000 k/mm3 |
| PTT (outpatient only) | 50 seconds | |
| White blood count | 1.0 k/mm3 | 50.0 k/mm3 |
B. Microbiology critical values:
|
C. Anatomical pathology critical values:
or "orange" category |
|
or "red" category | ||
Site specific CVs | Pap smear |
|
| CSF |
| |
| BAL |
| |
Site agnostic CVs |
| |
or "red" category |
|
- Changes in methodology, reference ranges, availability, specimen requirements or other matters should be communicated to laboratory users in a timely manner
- Laboratory user manuals (specimen requirements, ordering, expected turn around times, etc.) should be available to all lab users
- Quality improvement effort results and feedback should be regularly provided to lab users as part of the laboratory quality plan
- Communication channels could include email, posters, newsletters, texts
- Incentive measures can improve user's awareness of laboratory policies and issues and improve quality; examples:
- Small gifts (lab trinkets, etc.)
- Recognition ("stars of the month" bulletin boards, newsletter mentions, etc.)
- Discounts on other services
- Contact information, for questions and interpretation, should be provided along with reported results
- Training should be provided to lab personnel to improve external communications
- To inform lab users of pertinent changes or issues, information flow within the laboratory should include appropriate branch points
Critical values in clinical chemistry
Critical communications in healthcare
Communication in customer service
A 56 year old woman was hospitalized due to a closed hip fracture. The patient has a 15 year history of diabetes mellitus type II and atrial fibrillation. She has been on insulin and warfarin to manage her conditions. Partial thromboplastin time (PTT) is in the normal range but prothrombin time (PT) and international normalized ration (INR) are increased at 43 seconds and 5.2, respectively. What is the best next step the performing lab technician should take?
- Call the lab director
- Call the ordering physician (or nurse)
- Call the pathology resident / attending
- Send the results to the ordering physician (or nurse)
- Verify or repeat the test
Comment Here
Reference: Critical reporting systems and laboratory communications
A basic metabolic panel (BMP) from a 40 year old outpatient male with unclear medical history includes a serum sodium of 119 mmol/L. After verifying the result, the lab technician attempted to call the clinic from which the sample was sent but there was no answer. What is the next step that the technician should take?
- Call 911
- Call the lab supervisor / attending pathologist
- Call the patient’s emergency contact
- Call the patient’s family phone number
- Call the patient’s phone number
Comment Here
Reference: Critical reporting systems and laboratory communications
- Currently, neither practice accreditation nor personal certifications are required for reimbursement of USFNA procedures or for US examinations (except for noninvasive vascular US studies, which pathologists do not perform, as some states require certification for reimbursement)
- To ensure proper documentation for reimbursement for an USFNA procedure or US exam, the practitioner must document these elements in the medical record:
- Demographics including facility name, patient name, date and time of exam, etc.
- Relevant patient clinical information
- Medical necessity for the examination (including ICD-9 code)
- Who performed the US examination
- Scope of the examination (i.e. limited versus complete US exam):
- Pathologist will almost always perform a limited US exam
- Limited US exam: evaluation of a limited number of organs or limited portion of region evaluated
- Complete US exam: one that attempts to visualize and diagnostically evaluate all of the major structures within the anatomic region
- US report with description of study, findings and impression, or limitations, etc.
- US image retention with permanent storage and availability for future review
10022
Fine needle aspiration; with imaging guidance
76942
Ultrasound guidance of needle placement (e.g. biopsy, aspiration, imaging supervision and interpretation)
Pathology professional and technical codes
88172
Cytopathology, evaluation of fine needle aspirate; immediate evaluation for adequacy of specimen(s)
88173
Cytopathology, evaluation of fine needle aspirate; final interpretation and report
88305
CELL BLOCK: level IV - surgical pathology, gross and microscopic examination
88104
Cytopathology, fluids; smears with interpretation
Limited ultrasound examination CPT codes
- The pathologist will most always perform a focused or goal directed US that should be billed as a limited US examination
- A limited examination may be defined by the CPT code
- In addition, CPT modifiers might need to be added to the examination
76857
Ultrasound, BUTTOCK and PERINEUM, real time with image documentation
76705
Ultrasound, ABDOMINAL WALL and LOWER BACK, real time with image documentation
76536
Ultrasound, HEAD and NECK (e.g. thyroid, parathyroid, parotid), real time with image documentation
76604
Ultrasound, CHEST WALL and UPPER BACK, real time with image documentation
76645
Ultrasound, BREAST(s) (unilateral or bilateral), real time with image documentation
76882
Ultrasound, AXILLA, GROIN, UPPER and LOWER EXTREMITY, nonvascular, real time with image documentation
- Evaluation and Management E/M codes are billed for the history, physical examination and medical decision making process documented by the pathologist who sees patients in an outpatient setting for evaluation and performance of a fine needle aspiration
NEW patient E/M codes
- Defined as a patient in which:
- No physician from same specialty / group has seen the patient for services before
- No professional services were rendered in the last 3 years for the patient
- Related E/M Codes 99201, 99202 etc.
99201
Office or other outpatient visit (10 min) for the evaluation and management of a new patient which have these components or criteria for documentation and billing purposes
- Requires 3 key components:
- Focused history
- Focused exam
- Straightforward medical decision
- Patient has a self limited or minor problem:
- Minimal number of diagnoses or management options
- Minimal or no data to be reviewed
- Minimal risk of complications, morbidity, mortality
- Physician time: 10 minutes
99202
Office or other outpatient visit (20 min) for the evaluation and management of a new patient which have these components or criteria for documentation and billing purposes
- Requires 3 key components:
- Expanded problem focused history
- Expanded problem focused exam
- Straightforward medical decision
- Patient has a low severity or moderate severity problem:
- Limited number of diagnoses or management options
- Limited amount or complexity of data to be reviewed
- Low risk of complications, morbidity, mortality
- Physician time: 20 minutes
Established patient E/M codes
- Established Patient E/M Codes are for patients that have been previously seen or in which FNA services have been rendered by the group / specialty within the last 3 years (e.g. repeat FNA biopsy or repeat cyst drainage)
- Related E/M Codes 99211, 99212, etc.
- The practice of pathology is uniquely dependent upon complex and sophisticated laboratory equipment, which is continuously upgraded, augmented or rendered obsolete
- Successful laboratory administration requires knowledge, experience, and judgment in the selection and maintenance of multiple technologies which are integral to our practice
- Capable equipment management also requires prudent interaction and communication with a variety of non-laboratory experts including biomedical engineers, service personnel, purchasing agents, administrators, contract attorneys, and even vendor representatives
- Equipment acquisition consists of establishing a need (from the strategic plan), technology assessment, justification, a Request For Information (RFI), a Request For Proposal (RFP), one or more site visits, selection and finally a contract
- The continuing quality of laboratory operations is, to a large degree, dependent on equipment maintenance
- Clinical Utility
- Does the technology make a difference in patient management or outcome?
- Do the clinicians want or need the new technology?
- Is the technology accurate, sensitive and reproducible?
- What are the operating characteristics of the new technology?
- What are the costs? (to be expanded in subsequent chapter on Budgeting)
- Necessary to meet governmental requirements
- Necessary for patient or employee safety
- Replace old equipment in order to continue operation
- Provide marked improvement in patient care
- Enhance productivity or reduce costs
- Improve patient or employee satisfaction
- Improve operating efficiency
- Improve quality
- Bottom Line Essentials
- Improved patient Care - obtain clinicians' corroboration
- Increased productivity, decreased cost
- Cost savings in the system, e.g. decreased length of stay
- Sent to potential suppliers to:
- Define your needs
- See what is available
- Specify rules and timeframes for the acquisition process and determine who receives an RFP
- RFI should answer the following:
- What equipment is available?
- What are the operating characteristics?
- How much does it cost?
- What training is provided?
- Installation requirements?
- Service and Maintenance
- Guarantees and warranty
- History of Vendor
- Number and duration of installations
- References
- Should include a pathologist, laboratory supervisor, administrator and a technologist who is slated to run the equipment
- Questions for pathologists and particularly for the technologists using the proposed equipment:
- Problems with delivery or installation?
- Availability of training?
- Reliability, unexpected downtime?
- Verify operating characteristics
- Vendor service and support?
- Do the technologists like it?
- Do the Pathologists like it?
- Availability of service, parts and reagents?
- Problems with computer interface?
- Would you buy this equipment again?
- Has it been on the market for at least 5 years?
- Is it simple with few moving parts?
- Is there a local parts warehouse and repairman?
- Are reagents cheap?
- Is it compatible with existing equipment?
- Is it inexpensive enough to buy two - so if one breaks down there is an immediately available back-up?
- A publication of detailed requirements by a prospective buyer in order to receive vendor proposal
- RFP is also known as request for bids or request to tender
- Sent to top vendors (from the RFI and site visits) for competitive bidding
- Key Sections of RFP:
- Statement of Need
- What is expected of Vendor
- Specific performance & operating characteristics
- Detailed deliverables: hardware, software, training
- Contractual requirements
- Payment requirements, incentives, penalties
- Proposal format
- Evaluation criteria and award process
- Schedule
- Selection of the winning proposal made on the basis of site visits and review of the submitted RFPs
- Selection made by the site visit team with administration
- Written by Pathologist with Purchasing Agent and Attorney
- Includes complete specifications:
- Equipment description
- Functionality
- Performance and operating characteristics and standards
- Incorporates vendor's RFP response
- Requirements for Installation:
- Space
- Utilities
- Code requirements
- Computer compatibility
- Cost to purchase or lease or reagent rental
- Delivery, liability, replacement
- Acceptance testing
- Penalty and lemon clauses
- Warranty and Maintenance contract
- Definitions
- Maintenance is scheduled and preventive
- Repair is unscheduled after failure
- First Line Maintenance is performed by machine operator and consists of frequent inspection, cleaning, disinfecting, lubricating, simple replacement, calibrating and adjusting
- Usually done by Lab Personnel
- May be done by Biomedical Engineering
- Must be recorded in a Maintenance Log
- Second Line Maintenance is performed by manufacturer's field service representative and consists of more complex replacements, alignments and adjustments
- Third Line Maintenance is major overhaul usually performed in the factory
- Maintenance Contracts should include:
- Detail of scope, terms, equipment covered
- Type of service: routine and emergency
- Availability of service technicians and parts
- Availability of loaners
- Response time: Hierarchy of response
- Costs: Parts, labor, travel out of pocket
- Average cost: 10% of purchase price per year
- Maintenance Records
- Documentation required by CAP, JCAHO, CLIA
- Need manuals and records of maintenance of each piece of equipment in the laboratory
- Generally maintained in each section or sub-section of the laboratory
- External quality assessment (EQA) is a system for objectively checking laboratory performance using an external agency or facility
- Proficiency testing (PT) is one method for achieving this
- PT is educational and promotes continuous learning; it supports laboratories in implementing new biomarkers into clinical testing as well as maintaining quality for existing biomarkers
- PT fulfills a different function from laboratory accreditation although the two are complementary; participation in PT is usually required for laboratory accreditation
- Clinical implementation of precision medicine means that biomarkers and new technologies for their detection are increasingly used in pathology
- Biomarkers assist in diagnosis and inform patient treatment as modern targeted therapies require highly accurate biomarkers to identify which patients should receive which treatment
- Methods may include:
- Rigorous quality management using internationally recognized standards is mandatory
- In addition to the potential impact on patients, errors can be costly to the healthcare system, leading to the retesting and incorrect use of medical resources
- For example, several studies have examined the economic burden of inaccurate estrogen receptor (ER) testing; data suggests that errors in ER status occurred in approximately 12,025 patients in the U.S. during 2012, costing nearly $1 billion (Value Health 2015;18:541)
- Adopting new biomarkers represents a key hurdle in the translation of innovations from research to clinical implementation
- Whether biomarkers are diagnostic, prognostic or predictive, proper validation needs to be done for their specific use (Appl Immunohistochem Mol Morphol 2017;25:4, Appl Immunohistochem Mol Morphol 2017;25:79, Appl Immunohistochem Mol Morphol 2017;25:151, Appl Immunohistochem Mol Morphol 2016 Dec 9 [Epub ahead of print]); laboratories require support to keep pace with developments and consistently deliver high quality test results
- With the increasing need for adopting new diagnostic methods and biomarkers in the clinic, quality assurance is becoming ever more important for both test introduction as well as test performance monitoring
- External quality assessment (EQA) is a practical tool for reviewing the performance of biomarkers and peer comparison of biomarker testing in diagnostic laboratories
- It allows laboratories to obtain an independent external assessment of their current process
- EQA is a way for laboratories to compare their results to an external source
- Another name, often used interchangeably, is proficiency testing (PT); however, PT is only one method of EQA
- World Health Organisation (WHO) defines EQA as a system for objectively checking the laboratory's performance, using an external agency or facility; they identify three common processes:
- Proficiency testing (PT): an external provider sends unknown samples for testing to a set of laboratories and the results of all laboratories are analyzed, compared and reported to the laboratories
- Rechecking or retesting: slides that have been read are rechecked by a reference laboratory; samples that have been analyzed are retested, allowing for interlaboratory comparison
- On site evaluation: usually done when it is difficult to conduct traditional proficiency testing or to use the rechecking / retesting method
- PT can be arranged in different ways and can differ on how the test results are compared and scored
- For most of the biomarkers, laboratories report the results to the organization that provides PT challenges
- Results are compared to either designated "true value" or "consensus score / results"
- Reasons for using one or the other method in evaluation of laboratory performance may be complex and often depend on the type of the assay used for the biomarker
- Different approaches may be used for qualitative markers for which reference standards are well defined and readily available (e.g. blood glucose testing)
- Descriptive methodologies would require designated descriptive true value for which no reference standards could be developed and do not exist (e.g. ER and PR testing in breast cancer)
- Reasons for using one or the other method in evaluation of laboratory performance may be complex and often depend on the type of the assay used for the biomarker
- Although not possible for all biomarkers, it is highly desirable for PT organizations to use a known, qualified reference sample that is very similar to clinical samples
- This is provided by the external third party for their challenges
- Since this is often not available, a second best option is artificial samples ; for example ctDNA mutation testing using synthetically prepared DNA spiked into plasma
- These reference samples are first qualified by the PT provider to confirm their biomarker status, then are sent to the participating laboratory for testing
- Laboratories run these samples through their normal diagnostic process, as they would standard clinical samples
- Organizations providing PT usually report to the participant whether the laboratory passed or failed the challenge
- They may or may not provide feedback to the laboratory on possible technical issues that could have influenced the suboptimal or poor results or suggestions for improvements
- This is provided by the external third party for their challenges
- If the readout process is automated (e.g. glucose testing), there will be no need and no possibility for central review
- Participating laboratories will send their results to the PT provider
- If the readout is interpretive, for example done by pathologists (e.g. ER and PR for breast cancer), the IHC results include both elements of the slide staining and the pathologist's readout
- Slides may be sent to the PT provider without the local pathologist's readout thereby only assessing the slide staining component
- Laboratories could also report the pathologist's readout results only without sending the slides
- They also could send the slides back and provide the local pathologist's readout
- Therefore, both protocol performance (the quality of IHC slide staining) and the readout performance (the quality of pathologist's readout) impact final results of IHC testing or both could be the subject of PT evaluation
- PT with central sample review
- IHC slides are reviewed in the participating laboratory and the results are reported to the PT provider
- When the IHC slides are sent back to PT provider, the PT provider performs a central review of the slides
- This central review is usually done by experienced pathologists and technologists who reach a consensus “true” result
- Independent providers verify the results from the laboratories by comparison to the validated “true” central review result and feed back discrepancies to the laboratory
- PT with consensus scoring
- Similar to above, except the PT provider does not perform a central review
- Laboratories run the test slides and perform the readout locally
- Laboratories do not need to return the IHC slides to the PT provider since no central review will be performed
- PT providers use a process to determine the consensus score and designate this as the designated correct result or truth set
- This method is less objective and can occasionally be problematic, especially when many laboratories return an incorrect result
- In this approach, the laboratories gain insight on how similar they are to other participants: designated as "concordance"
- PT programs support laboratories by providing valuable information (WHO: Overview of External Quality Assessment (EQA) [Accessed 5 November 2017]) which:
- Allows laboratory self checking and the chance to take appropriate corrective action, if needed
- Comparison of performance to other laboratories
- Provides early warning system for possible problems with tests, processes or operations
- Provides insights into test results among different test sites
- Facilitates continuous improvement and can highlight areas needing attention
- Helps identify training needs
- PT helps to assure the recipients of the test results (such as doctors, patients and health authorities) that the laboratory can produce reliable test results
- Data collected from PT could be very helpful in evaluation of:
- Performance of protocol methods
- Materials and equipment
- Where applicable (e.g. IHC and FISH), performance of pathologists' or technologists' readouts
- Impact of training
- Additionally, PT participation creates:
- Network for communication
- Forum for discussion of quality issues
- Source for conducting continuing education activities
- Organizations that provide PT can also:
- Engage in providing their participating laboratories more clarity
- Provide support during a time of new assay validation
- Offer verification prior to offering results in a clinical setting
- Quality assessment is a critical aspect of laboratory quality management
- Laboratories which hold an accreditation such as CAP or ISO 15189 are required to participate in an appropriate PT, where available
- Passing PT is not a replacement for laboratory accreditation as PT and accreditation fulfill different functions
- Laboratories test thousands of samples per week, every week of the year
- Continuous followup of laboratory performance over time is needed as many factors can influence laboratory operations:
- New reagents
- New methods
- Change in staff etc.
- In this regard, PT is different from accreditation because it is a continuous process, whereas accreditation reflects an assessment of standards in laboratories at a specific time
- PT is mandatory in some countries and not in others
- This may change as there is an increasing awareness of the benefits of participating in PT
- Even in jurisdictions that do not mandate PT, laboratories should consider voluntary participation and join PT programs offered locally, nationally or internationally for the biomarker tests they offer because of the many benefits gained from the participation
- Different PT providers offer a range of programs and laboratories can now easily participate in PT programs from other countries if local providers do not cover the biomarkers of interest
- To easily find information about different PT providers, visit the IQN Path webpage
- PT providers often need expert pathologists, laboratory technologists and scientists to participate as PT contributors
- Ways to contribute:
- Provide samples for testing
- Participate in expert readout assessments (e.g. IHC biomarkers)
- Provide feedback to PT provider or inquire about development of challenges for new biomarkers
- Help analyze reported results or help prepare summary reports of different challenges
- Many important discussions occur at the results assessment stage (the definition of what best practice should be when faced with the range of practicalities), providing learning opportunities for volunteers
- PT providers are positioned to review the results of different laboratories and compare the results to employed methodologies; this enables PT providers to learn about possible technical issues not previously recognized
- Not for profit PT providers amass a large amount of expertise
- Several PT providers aim to publish results of PT challenges when results provide new evidence that can help improve patient safety and provide scientific basis for future developments
- EQA provides a mechanism for laboratory self checking
- EQA is an opportunity for continuous learning by testing laboratories
- EQA provides a substitute for accreditation
- EQA provides a substitute to establishing a laboratory quality management system
- EQA provides support during a time of new assay validation and verification prior to offering a test in a clinical setting
Comment Here
Reference: External quality assessment and proficiency testing
- A frozen section (cryosection) is a pathological laboratory technique used for rapid microscopic analysis / diagnosis of a specimen / disease
- Usually used with oncologic surgery
- Rapid diagnosis can guide intraoperative patient management
- Why perform a frozen section:
- Provide rapid gross or microscopic diagnosis to identify an unknown pathologic process, identify extent of disease / evaluate margins, identify metastases or simply identify a tissue
- Process tissue to provide appropriate and accurate diagnosis, prognosis and to adhere to research and special study protocols
- Confirm that pathological tissue is present for diagnosis on permanent sections
- Why not to perform a frozen section:
- Frozen section diagnosis has no immediate implications for decision making
- Tissue is needed for permanent processing (is unique or small or requires extensive study for diagnosis)
- Frozen section is known to produce severe artifacts that hinder proper interpretation
- Tissue is heavily ossified / calcified
- Risk of serious infection (HIV, TB, hepatitis B or C)
- Tissue should be received fresh, otherwise it will not stay on slide
- At time of receipt of tissue, decide whether to obtain smears or touch preps and whether to freeze all or part of it
- Touch preps and smears are often performed on lymph nodes suspicious for lymphoma
- Some primary small lesions should not be entirely submitted for frozen section
- There is debate on whether sentinel nodes should be entirely or representatively submitted for frozen section
- Fixed tissue: there are special slides to keep tissue affixed to slide
- To freeze fixed tissue, make sure it has been preserved in formalin and not alcoholic fixatives like Carnoy's, because tissue fixed in alcohol is harder to freeze
- Avoid freezing tissue fixed with heavy metal salts such as B5 and Helly's (Zenker’s formal solution), which can denature proteins and shrink the tissue
- Freezing methods:
- Note: most are used in conjunction with heat sinks
- Histobath: being phased out
- Cryowells: useful in keeping all tissue on an even plane; also helpful in eliminating loss of smaller tissues that are frozen with larger ones, although recommended to not freeze different sizes together
- Aerosol sprays: often canned CO2 (but may aerosolize infectious diseases)
- Liquid nitrogen
- Isopentane based workflow (Virchows Arch 2008;452:305)
- Procedure:
- OCT (optimal cutting temperature) or similar embedding media like TBS or Cryogel should be placed on an appropriate sized chuck that has been precooled in a cryostat
- The chuck should be clean
- A toothbrush is useful to remove tissue and OCT
- Dipping the chuck in methanol removes ice crystals
- Place the chuck into a -20 to -15 degree (optimal) cryostat; note that the OCT media should not be frozen completely
- It is better to have a semisolid consistency; this will alleviate tissue artifact
- Tissue size should be no greater than 3 - 5mm in greatest dimension (thinner specimens have shorter freezing time and minimal ice crystal artifact formation)
- The smaller the tissue, the more even and thorough the freeze
- Place the tissue on the semisolid chuck and add more media rapidly over the tissue, covering it entirely but avoiding overflow
- Place chuck quickly back into the cryostat
- Apply heat sink or CO2 aerosol (optional) to rapidly freeze or use quick freeze option on cryostat
- Once the chuck is in position, there should be a manual or an automatic advance option to move the block close to the cutting blade
- Fully face the tissue by using a trim setting on your cryostat; if you do not have this setting, then an advance button should be available, which should be pressed each time before one full revolution of the instrument's wheel
- If wells are used to freeze the blocks, then the tissue should be on an even plane and the tissue will be faced faster
- To polish the tissue, avoid advancing the cryostat or deselect the trim setting on the cryostat and turn 10 - 15 times
- As you cut the tissue, anchor the tissue to prevent folding or curling; this can be done with an anti roll bar (a plastic plate attached to cryostat) or by using a precooled paintbrush with stiff bristles and a wide gripping surface
- The brush should be held like a pen with your left hand at an angle
- You can rest your fifth finger on the stage for stabilization
- Cutting the brushes' bristles at an angle can aid in the brush meeting the tissue flat over its length because you will hold it at an angle
- Turn the wheel with your right hand in a continuous motion without stopping; avoid speeding up or slowing down
- Avoid stopping the wheel at the beginning of the section, slowly grabbing the tissue and then resuming wheel revolutions; this can cause artifacts such as variation in section thickness and tissue folding
- Move the brush as the chuck moves towards the blade; your brush should move down in pace with the chuck
- You can rest your brush softly on the very bottom of your chuck avoiding tissue contact
- Pull the brush away easily as the chuck meets the blade
- The downward motion of the brush allows you to keep a continuous motion as you take your section
- A glass slide is gently laid upon the tissue section



Gently touch the section to the slide; avoid stretching or folding the section by keeping a steady hand, and keep the transverse axis of the slide parallel to the section
- The tissue section should melt onto the slide
- Prepared slides should immediately go into formal alcohol, 95% alcohol (methanol / ethanol) or formalin while awaiting the stain line; if you delay this step, drying artifact will occur
- You can take a deeper level after approximately 20 turns (multiple levels may be needed for breast or prostate biopsies)
- Optimal cutting thickness is 4 - 7 microns for sectioning and 20 - 40 microns for trimming
- Keep all stains and solutions fresh and well maintained
- Dip slide in reagents in this order for H&E staining:
- After obtaining frozen section, immediately fix in 95% ethanol (even 15 seconds of delay can cause significant artifact)
- Formal alcohol, formalin or 95% alcohol: 45 - 60 seconds
- Water: 5 - 7 seconds
- Hematoxylin: 60 seconds
- Lithium carbonate or 0.2 % aqueous ammonia (Bluing): 15 - 20 seconds
- Eosin: 20 - 60 seconds
- 95% alcohol: 10 seconds
- 100% alcohol: 10 seconds
- Xylene, toluene, limonene derivatives and Clearite: 10 seconds
- Then add mounting media for cover slipping
- Ice crystal artifacts:
- Due to slow freezing of tissue
- Solution: Freeze fast (flash / snap); the faster the freeze, the smaller the ice crystals, the less tissue damage (best freezing method is arguably liquid nitrogen)
- Smaller tissues yield less artifact - optimally tissue should be 0.5 x 0.5 x 0.3 cm or less
- Never freeze fragments larger than the diameter of the chuck
- Avoid freezing fat around tissue
- Blot the outer surface of the tissue dry with gauze before making your block
- Knife artifact:
- A nicked cutting blade will produce a split / tear in your section
- Solution: change your blade every few cases; some institutions use a new blade for each case
- Over / underfreezing:
- Overfreezing can cause section to have holes
- Solution: polish block with a couple extra turns of the blade to create friction and warm up block by pressing on it with your finger (5 - 10 seconds)
- Underfreezing can be troublesome for fatty tissue
- Solution: add heat sink to block or select rapid freeze setting on your cryostat (if available)
- Staining issues:
- Dirty stain line can cause floaters (extraneous foreign tissue) to adhere to slides; overly diluted stains and alcohols can diminish slide quality
- Poor staining hinders frozen section diagnoses, as nuclear detail is compromised
- Solutions: (a) maintain a clean stain line by frequent solution changes; (b) follow recommended staining times; (c) don't rush
- Note: brain tissue may stain best in eosin for 60+ seconds
- Water: should be changed after each frozen section
- Alcohols and stains: change at least weekly, alcohols may need to be changed more frequently depending on work load
- Fatty tissue:
- Includes lymph nodes, breast, skin; may be too soft to cut
- Solution: maintain an extremely cold cutting temperature (-20C)
- Firm lymph nodes, spleen, brain and liver cut better at -10C; tissue may shatter if sectioning is performed at lower temps
- Air bubbles:
- May be trapped under cover slips, which can cause the underlying tissue to dry out
- Solution: make sure an appropriate amount of resin (2 drops) is applied; gently move air bubbles off the slide with finger or tweezers; do not press on the slide too hard or it will break
- Overly thick sections:
- May cause tissue to fall off slide
- Solution: reduce the cryostat's sectioning thickness
Embedding small specimens
Speed embedding
Brush technique
- To administer or manage means doing four things: Plan, Lead, Organize and Control
- In previous chapter, we outlined the Essence of Planning, using a Template for Strategic Planning that culminates in specific goals for each section of the laboratory with detailed objectives and a business plan with financial projections and budgets
- This chapter outlines the general and specific leadership responsibilities and interfacing requirements of pathologists, the essential communication and interpersonal skills (Emotional Intelligence) that are pre requisites and "How to" connect and lead in the pathology group, in the medical staff, in the laboratory, in the hospital and beyond
- Pathologists are expected to be brilliant physicians and make accurate diagnoses but they are also responsible for the overall performance of their laboratory
- They must establish goals and objectives and determine the organizational structure
- They are responsible for employees, equipment and supplies
- They must assure quality and comply with laws and regulations and show a positive bottom line
- In other words, pathologists, in addition to being doctors, have to be managers; they are expected to plan, to lead, to organize and to control the laboratory
- Pathologists must be leaders in their hospitals or health systems, leaders in their professional organizations and in their community
- The prime competencies required for leadership are professional and technical expertise and interpersonal and communication skills
- A leader must also be able to lead and motivate, be decisive, be able to delegate and yet be humble and always ethical
- The toughest of these tasks is leading, all pathologists need to be leaders, leaders in their practice group or academic department, in their laboratory - even young pathologists just out of residency are given responsibilities for leading laboratory sections
- Most important, a leader must have emotional intelligence, or simply another way of defining interpersonal skills; emotional intelligence is what effective leaders have
- Dr. Daniel Goleman, the psychologist who articulated the concept, contends that intelligence and technical knowledge are important, but emotional intelligence is the sine qua non of leadership (Working with Emotional Intelligence; 2000, Emotional Intelligence; 2006)
- He studied nearly 200 large companies and found that effective leaders have a high degree of emotional intelligence while individuals without it, even though they may have a first class education, exceptional training and have good ideas, are not effective leaders
- According to Dr. Goleman there are five components: Self Awareness, Self Regulation, Motivation, Empathy and Social Skill:
- Self awareness means recognizing and understanding your own values, moods, emotions and drives and their effect on others; leaders with high self awareness are self confident and realistically assess themselves and others
- Self regulation is the ability to control or redirect disruptive impulses or moods, to temporarily suspend judgment - to think before acting; the self regulated leader is never impulsive and is seen as trustworthy and open to change
- Motivation is the third essential of emotional intelligence; it is a passion for work for reasons that go beyond money or status or the usual rewards
- The motivated leader pursues goals with energy and persistence, not for what it will get them but for achievement's sake alone
- The first three components of emotional intelligence, self awareness, self regulation and motivation are skills about managing the self; the last two, Empathy and Social Skill, concern a person's ability to manage relationships with others:
- Empathy means considering your associates and employees' feelings when making decisions; empathy requires the ability to understand the emotional make up of others, to care about it and treat people with consideration
- Social skill is not simple; it's more than friendliness, although people with high social skill are rarely mean spirited; social skill means proficiency in managing relationships and building networks; an ability to find common ground and build rapport
- Empathy means considering your associates and employees' feelings when making decisions; empathy requires the ability to understand the emotional make up of others, to care about it and treat people with consideration
- Developing or enhancing emotional intelligence and thus leadership effectiveness is not simple; it cannot happen without a sincere desire and a concerted effort
- Achieving excellence, renown and a reputation outside the hospital or healthcare system inevitably makes the pathologist more valuable inside the system
- Prerequisites
- Maintain an academic and intellectual attitude
- Recognize the value of organized medicine
- Understand your role in your community
- Develop a philanthropic agenda
- Some Things to Do
- Obtain an appointment at your local Medical Schools - teach, attend conferences, bring students or residents to your hospital for rotations and participate in research studies
- Become active in professional societies, including the local Pathology Society, County Medical Association, AMA, ASCP and CAP - not only as a member, but as a contributing member of committees and eventually as one of the leaders
- Become active in your community - have a civic and political agenda - serve on the school board, join service organizations
- Participate as a contributor and also as a leader in community and hospital fund raising campaigns
- Pathologists are "Information Specialists"; they do not perform surgery, prescribe or administer medications; they provide information that other physicians use to diagnose and treat
- The effective transmittal of that information demands interpersonal and communication skills and requires interfacing with the pathologists’ prime information recipient - the clinicians
- The pathologist's relationship to other physicians is that of colleague, consultant, friend and educator but requires diplomacy, empathy and humility
- Prerequisites
- Remember you are a physician first and a pathologist second
- Comport yourself and dress like the clinicians
- Have knowledge and experience in clinical medicine and patient care
- Know how to use the laboratory to solve clinical problems
- Be sensitive to the unique problems of clinicians
- Be informed about key and critical patients
- Some Things to Do
- Obtain a locker in the Surgeon's dressing room and change into scrubs there - like the other docs do
- Make "rounds" in the Doctor's Dining Room twice a day - at morning "break" time and at lunch; never eat lunch by yourself, behind your desk in the basement
- Make your office a welcoming place for clinicians to come and look at the slides of their patients' biopsies or just to come and chat
- Personally call all critical surgical pathology diagnoses - but don't only call the surgeon, also call the primary care physician who referred to patient for surgery in the first place
- Establish a computerized system of critical values in clinical pathology and pro-actively call the attending physician with the results, but be aware of the sensitivity, specificity, predictive value and interfering substances of the test before calling
- Establish a system for complaint management and conflict resolution; handle complaints yourself - do not delegate; when a problem arises, always thank the individual who brings it to your attention
- Develop social connections with clinical colleagues
- Because the pathologist laboratory director is responsible for the overall operation of the laboratory, including assurance of quality and budgetary prudence, there must be a good working relationship with the administration of the hospital or healthcare system
- The pathologist will necessarily interact with many department, including nursing, human resources, finance, purchasing and information technology
- The pathologist (and the laboratory) will report to, and be accountable to the hospital administration is some way
- Thus the pathologist must be able to deal with these non-physicians, be familiar with their nomenclature and know how to use their services to help the laboratory achieve excellence
- Prerequisites
- Knowledge of health care economics
- Empathy toward the problems faced by hospital administration
- Knowledge of hospital organization
- Regularly scheduled, monthly, formal meeting with the administrator in charge of the Laboratory with an agenda that includes: review of workload, revenue, budget variance and productivity; there should be discussion of needs, problems, incidents, any awards received or papers published
- The pathologist should establish rapport (professional and social) with the COO, CEO and CFO and with key people in the sponsoring organization, e.g., religious order, municipal or county boards and governing bodies
- Some Things to Do
- Conduct daily "administrative" rounds - walk by the offices of the various administrators, just to say "hello" or to see if they want to get a cup of coffee - or at least say "hello" to their secretaries
- Maintain a high level of visibility; continually remind administration how critical you are to the operation of one of their largest departments in terms of overall management responsibility, test selection, assurance of quality, result interpretation, adherence to regulations, medical-legal responsibility and cost control
- Prepare an Annual Report of the laboratory's activities, demonstrating that the pathologist is indispensable
- Participate in the laboratory and hospital budgeting process
- Participate in hospital committees such as Utilization Review, Joint Commission Inspections, Purchasing, Information Technology
- Participate in hospital events, e.g. Christmas parties for employees
- Volunteer to be active in, and contribute to fund raising campaigns
- Conduct nursing "rounds" - visit each nursing station in the hospital at least once a month to find out if the laboratory services are satisfactory; this establishes a relationship between the pathologist and the rest of the hospital; take the chief tech with you - the nurses and the doctors you encounter will see who is in charge and the chief tech can implement needed changes; report both problems and compliments back to the laboratory personnel
- Volunteer for in-service educational programs for nurses and other hospital personnel, e.g. seminar for chaplains regarding autopsies
- Volunteer to be the medical information officer for the hospitals information system - no physician is better suited than the pathologist to fill this role since the pathologist is an expert in computers, automation, QC and QA as well as disease and practice management
- Pathologists, unlike other physicians, are responsible for the quality of work of large numbers of others, including phlebotomists, technicians, technologists, doctoral level scientists as well as transcriptionists and clerical personnel
- This staff produces much of the work output of the laboratory without direct, personal oversight of each procedure or test by the pathologist
- Thus the pathologist, who has both the operational and legal responsibility for the laboratory's work, has to be a true leader who inspires, serves as a role model, and motivates the staff to attain the highest levels of ethical and conscientious performance
- Prerequisites
- Know the basic principles of management & administration: planning, leading, organizing and controlling
- Assure productive work and worker achievement
- Be competent in the technical aspects of anatomic and clinical pathology
- Know employees by name, know something about their personal lives and respect their worth
- Be able to communicate the clinical significance of test results to laboratory staff and to demonstrate the importance of their work in patient care
- Some Things to Do
- Construct and display an organizational chart that clarifies authority and reporting relationships
- Make work relevant and meaningful by involving employees in decision making when appropriate
- Make daily "rounds" in each section of the laboratory, greeting each employee by name, getting a sense of the workload, staffing, any problems or interesting cases; don't neglect the evening, graveyard or weekend shifts, surprise them with occasional donuts or pizzas
- Participate in the special activities of the lab staff, including social events
- The medical staff of a hospital or health care institution is responsible for establishing, monitoring, assessing and ensuring compliance with standards for professional quality and performance
- The medical staff organization is responsible for appointing and re-appointing the institution's professional staff and for delineating the privileges of each appointee
- In many instance, the medical staff is also responsible for establishing and maintaining an educational program for its members
- Much of the work of the medical staff is performed through committees such as Infection Control, Transfusion, Tissue and the Executive Committee
- It is essential for pathologists to become active and to assume leadership positions in the medical staff organization
- Prerequisites
- Communication skills
- Read the medical staff By-Laws and Policies; be aware of the structure and hierarchies of the medical staff organization
- Volunteer for service on committees, especially those in your area of expertise
- Volunteer to chair committees, but first know how to conduct and chair a meeting
- Volunteer to present educational programs, but first know how to give a talk
- Some Things to Do
- Cheerfully accept any committee assignment
- Request assignment to specific committees, e.g., tumor board, infection control, transfusion, tissue committee and volunteer to chair the committee
- Participate in Medical Staff oversight functions such as Utilization review, peer review, performance measurement and outcomes research
- Present educational programs, such as CPC's and organ recitals
- Contribute to, or edit, the medical staff newsletter
- Establish periodic conferences with clinicians regarding appropriate use of the laboratory
- Participate in Medical Staff social events, e.g., dances, golf tournaments
- The organizational structure of pathology groups varies widely
- Some groups are part of a larger entity, such as Permanente or an academic medical practice group
- Sometimes, a single pathologist has a contract with a hospital and employs other pathologists
- In other cases, there is a partnership of professional corporations
- Other models include simple employment by a commercial laboratory or by a government entity, such as the VA
- Regardless of the structure, pathologists will probably spend more time with the practice group than with their families, and thus both professional and personal satisfaction depend upon a collegial and supportive practice environment
- Prerequisites
- A written organizational chart showing the lines of authority and reporting relationships of all the members
- A written mission and vision statements for the group, separate from the mission or vision of the hospital, as well as a budget for the operations of the group
- Accepted policies which define each member’s role in the group and procedures for scheduling and vacation planning
- Written position charters (job descriptions) for the director and for each of the pathologists and other professionals, e.g., PhD chemists, detailing the authority, responsibilities and domains of each
- Regularly scheduled, formal meetings of the group
- Some Things to Do
- Communicate informally on a daily basis; maintain an "open door" policy
- Prepare an assignment calendar and vacation schedules
- Establish a peer-review and internal consultation methodology that is non accusatory and non punitive
- Have bi weekly or monthly formal group meeting, with an agenda addressing recurrent items such as fiscal review, operations review, discussion of incidents, schedules, etc., as well as an open agenda with subjects that can be brought up by any member of the group
- Encourage social interaction among the group members and their families
- Budget is a detailed plan outlining expenses (costs) and revenues (incomes); it is also called a financial plan, a map to get where we intend to go
- Budgeting is the process of planning, forecasting, controlling and monitoring the financial resources of an organization (Garcia: Clinical Laboratory Management, 1st Edition, 2004)
- Budgeting for the laboratory should include operating costs based on past experience and projected changes in both fixed and variable costs, and be monitored regularly (usually monthly)
- Capital budgets should be managed separately from operating budgets
- Where possible, profitability should be monitored as a measure of laboratory efficiency; in the absence of accurate revenue numbers, other metrics of efficiency can be used
- Expense (cost) is the money spent on supply, labor, overhead or on a product or service (Travers: Clinical Laboratory Management, 1st Edition, 1997)
- Capital costs are investments on building, land, new equipment and innovations
- Operating costs are the expenses to produce products or services, such as reagents, supplies, maintenance, transportation or equipment rental
- Personnel costs include employee salary, raises, benefits and training fees
- Overhead costs refer to expenses that are not directly related to billable tests; these could be management, utilities, advertising and rent
- Revenue (income) is the money a lab receives for its services or products
- Income is categorized by the source of the activity; for the laboratories, this could be inpatient, outpatient, research or outreach testing (Lab Med 2003;34;515)
- (Net/gross) margins = [(net/gross) revenues - (net/gross) expenses]/revenue
- Variance is the difference between the predicted / planned budget and actual revenues and expenses
- Costs can be classified in different ways as shown in the following tables (McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2016):
Direct Indirect (overhead) Variable Fixed Salary Nonsalary Operating Capital Reagents ~ ~ ~ ~ Proficiency testing ~ ~ ~ ~ Analyzer service ~ ~ ~ ~ Analyzer ~ ~ ~ ~ Testing staff ~ ~ ~ ~ Management staff ~ ~ ~ ~ Rent ~ ~ ~ ~
Sample laboratory budget
Reference: Lab Med 2003;34;515Description Current actual Current budget Variance % Variance Inpatient charges
Outpatient charges
Total revenues$246,958
$1,574,862
$1,821,820$219,370
$1,476,560
$1,695,930$27,588
$98,302
$125,89012.6%
6.7%
7.4%Salary - professional
Salary - technical regular
Salary - technical overtime
Total salary$36,484
$50,548
$9,438
$96,470$35,213
$53,030
$2,610
$90,853$(1,271)
$2,483
$(6,828)
$(5,617)-3.6%
4.7%
-261.6%
-6.2%General lab supplies
Reagents
Lease, rentals
Total supplies$24,546
$130,356
$2,070
$156,972$19,476
$75,464
$2,070
$97,010$(5,070)
$(54,892)
$0
$(59,962)-26.0%
-72.7%
0.0%
-61.8%Total expenses $253,422 $187,863 $(65,579) -34.9%
- Operational budget provides a plan of day to day expenses and revenues
- Capital budget provides a plan for long term upgrades and improvements, such as buying a new building or equipment (Chron: Differences and Similarities of Capital and Operational Budgeting [Accessed 23 May 2022])
- There are several ways of budgeting; these include (but are not limited to) the following: pro forma budgeting, zero based budgeting, priority based budgeting, activity based budgeting and flexible budgeting (CFI: The Four Main Types of Budgets and Budgeting Methods [Accessed 23 May 2022])
- The following table compares 2 commonly used budgeting strategies: pro forma versus zero based budgeting
Pro forma budget (incremental) Zero based budget - Build a new budget plan based on the previous or historical budget data
- Simple to prepare
- More commonly used
- Does not encourage cost effectiveness because it is based on assumptions that preexisting operations are essential
- Build a new budget plan from scratch
- More complex to prepare
- Less commonly used; typically used to propose a new service, laboratory section or test
- Encourages cost effectiveness
- We recommend using the Plan, Do, Check, Act cycle for budget process and management
Contributed by Duy K. Doan, M.D.
- Phase I - Plan: define goals
- Clear goals and objectives guide resource allocation
- Senior management usually sets these, although departments can get involved
- Phase II - Do: create budget
- Review the past
- Collect statistical data of past revenue, expenses, margins and volumes (for pro forma budget)
- Evaluate the present
- Assess new programs, expanded programs, reduced or discontinued programs
- Review key influential factors, such as politics, economy and technology
- Predict the future
- Predict new revenues, expenses and margins
- Predicting future = past performance + estimated changes
- Predicting revenues can be hard; you need to estimate the lab growth rate
- Lab growth rate = existing client growth + new clients - lost clients
- Predicting expenses may also be challenging; this requires both a prediction of lab growth rate and careful justifications
- Capital expenses:
- Why do we need this new equipment?
- Is it the best?
- How much does it cost?
- What is the return on investment (ROI)?
- Defined as net income of an investment divided by its cost
- Personnel expenses: evaluation of current workload and productivity
- Are there any expected changes in workload, productivity, staff scheduling and new or expanded programs in the future?
- Operating expenses change proportionately with the number of tests: expected costs = current spending + lab growth rate
- Review the past
- Phase III - Check: analyze variances
- Review monthly for variances (Garcia: Clinical Laboratory Management, 2nd Edition, 2013)
- Favorable budget variance refers to positive variances, showing gains; unfavorable budget describes negative variance, indicating losses
- Try to explain any significant variances from budget
- Phase IV - Act: adjust budget
- Make adjustments in order to meet the goals
- Budget, as discussed earlier, is the internal reporting tool that management uses to control expenses and makes decisions; the budget variance analysis is performed periodically, at least monthly
- Labs also need to report their business information to external entities, such as government agencies, stakeholders and banks; these external reports are called financial statements
- There are 3 major financial statements that reflect a lab's business activities and profitability for each accounting period; these statements are balance sheet, income statement and statement of cash flows
- These statements are reported yearly (Gitman: Principles of Managerial Finance, 10th Edition, 2002)
- Balance sheet: shows what a lab owns (assets), what a lab owes (liabilities) and its net worth (equity); the balance sheet carries over from year to year
- Investors analyze the balance sheet for indication of management's effectiveness in using debt and assets to generate revenue
- Income statement: also called the profit and loss statement; shows revenues and expenses over a period, which is typically a fiscal year
- Income statement starts from zero at the beginning of the new fiscal year
- Investors use the income statement to assess whether the lab is generating profits or losses
- Cash flow statement: summarizes the movement of cash that comes in and goes out of a lab in a period; the cash flow statement highlights how well a lab generates cash
- Productivity is the ratio of what is produced (product or service) to what is required to produce it (labor and supplies)
- Important terminology in productivity calculation is full time equivalent (FTE):
- FTE is a metric that measures the total number of full time employees you have based on hours worked, rather than the exact number of employees (e.g., an employee who works full time is equal to 1 FTE, while an employee works half time would be equivalent to 0.5 FTE)
- Common productivity measures for clinical laboratories are billable tests / paid FTE, billable tests / worked FTE, worked FTE / paid FTE, labor cost / billable test, supply cost / billable test, overtime / worked straight time
- Benchmarking is a practice to compare an organization's performance / productivity to a standard; benchmarking helps management to:
- Identify problem areas in its own organization
- Learn better practice from other laboratories (Lab Med 2008;39;108)
- Benchmarking can be internal or external:
- Internal benchmarking compares productivity within a laboratory
- External benchmarking compares a laboratory's productivity to that of other laboratories in the market
Financial management:
effective budgeting in the laboratory
- Ian McNeal, CPA
Your lab medical director decides that it is time to purchase this new piece of equipment in order to change the way the lab handles certain samples. The manufacturer quotes you a price tag for the system of $140,000, with an associated maintenance contract of $7,000 to cover service during the first year of ownership. Which of the following statements is true?
- Capital budget should include the instrument cost plus the first year of maintenance
- If savings from the new method matched the added operating cost, net lab profit margins would not change
- Operating budget would increase by $7,000, less any savings in personnel costs gained by shifting to the new methodology
- Your operating budget would include an amortized portion of the $140,000 purchase cost
Comment Here
Reference: Laboratory budgeting
- Employee search and recruitment expenses for a new lab director
- Fuel surcharges associated with incoming shipments of reagents
- Housekeeping expenses associated with needing a clean room for PCR
- Lab information system upgrades
- Lab website and order entry system upgrade
Comment Here
Reference: Laboratory budgeting
- On September 29, 2023, the U.S. Food and Drug Administration (FDA) announced a new proposed rule on laboratory developed tests (LDTs) to increase oversight for patient safety
- It is important to understand the historical regulatory background and basis for this proposed change to assess its implications for pathology practice
- Laboratory developed tests extend testing of specimens and populations outside of the manufacturer's instructions or offer unique tests
- Applications include testing rare diseases and complicated conditions for which FDA approved testing is not available, thus promoting testing innovation
- The FDA has exercised general enforcement discretion of LDTs, meaning that the Centers for Medicare & Medicaid Services (CMS) through the Clinical Laboratory Improvement Act (CLIA) '88 has been evaluating these assays during laboratory inspections
- Due to changes in the LDT landscape since it was defined in 1976, the FDA proposes to increase oversight and regulation of these tests to promote patient safety
- The pathology community believes adequate oversight and regulation already exist and is concerned that the proposed additional FDA oversight will
- Limit availability of testing to patients and populations currently benefiting from access to LDTs
- Suppress innovation
- Delay diagnosis of seriously ill patients
- Add significant costs to produce and administer LDTs
- Further healthcare inequity
- Laboratory developed test: in vitro diagnostic (IVD) test intended for clinical use and is designed, manufactured and used within a single CLIA certified laboratory that meets regulatory requirements for high complexity testing (Federal Register: Framework for Regulatory Oversight of Laboratory Developed Tests; Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories; Availability [Accessed 4 March 2024])
- In vitro diagnostics: tests performed on blood or tissue taken from the human body that can detect disease, conditions and infections that are conducted in test tubes using laboratory equipment
- Verifying Accurate Leading Edge In Vitro Clinical Tests (IVCT) Development Act (VALID Act): an earlier attempt by the FDA to regulate laboratory testing
- Clinical Laboratory Improvement Act (CLIA) of 1967: first CMS regulation of clinical laboratories, outlining quality standards and federal oversight
- CLIA '88: improved CMS oversight of all clinical laboratories, introducing the categories of test complexity and metrics to assure quality performance
- Additional amendments were passed in 1997, 2003 and 2012 but the law continues to be referred to as CLIA '88
- 2003 revision described LDT as not subject to FDA regulation or approval
- Federal Food, Drug and Cosmetic Act (FFDCA, FDCA or FD&C): laws passed by U.S. Congress in 1938 granting the FDA authority to oversee the safety of food, drugs, medical devices and cosmetics
- 510(k): the section that allows for clearance of class II medical devices
- 515: the description of class III device approval
Images hosted on other servers:
Comparison: FDA versus CLIA / CMS oversight of testing (FDA: Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories - Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs) [Accessed 4 March 2024])
| U.S. FDA oversight | CLIA '88 / CMS oversight |
| Focus on devices themselves and how they perform | Focus on laboratory processes to use devices, not device quality |
| Review of analytic validity performed before test may be used on patients | Review of analytic validity performed during a 2 year inspection cycle; test may be in use for 2 years before assessment of data and test use |
| Analytic validity large in scope with thousands to tens of thousands of data points | Analytic validity may be performed on the smallest number of patients required for statistical significance |
| Requires assessment of clinical validity / utility of testing | Does not require clinical validity / utility |
| Review requires assessment of patient safety | Review does not require assessment of patient safety |
| Required demonstration of effectiveness in determining presence / absence of condition being assessed | No required demonstration of effectiveness in determining presence / absence of condition being assessed |
| Requires adverse event reporting to identify inaccurate, unsafe and ineffective devices | Does not require adverse event reporting |
| Requires removal of unsafe devices from market | Does not remove devices from the market |
- In vitro diagnostics
- Commercially manufactured
- Majority of all laboratory testing
- Examples: comprehensive metabolic panel, complete blood count
- Laboratory developed tests
- Developed by high complexity CLIA laboratories overseen by trained medical directors and scientists
- Developed to fill unmet diagnostic or patient needs
- Examples
- Panels to monitor immunosuppressants after transplantation
- Comprehensive toxicology panels
- Newborn screening for early diagnosis of serious treatable conditions
- Genetic tests (e.g., next generation sequencing) to detect mutations in cancer (excluding companion diagnostics)
- Syndromic microbiology PCR testing
- Most immunohistochemical stains used in anatomic pathology
- Flow cytometry markers for lymphoma / leukemia
- Reference: J Mass Spectrom Adv Clin Lab 2023;28:67
- 1976 Medical Device Amendments Act: rule established by Congress, amended Food, Drug and Cosmetic (FD&C) Act to create a system for FDA regulation of medical devices used in humans, specifically including in vitro reagents (FDA: Laboratory Developed Tests [Accessed 7 March 2024])
- FDA established a specific definition for LDT and claimed regulatory authority
- Manufactured in small volume
- Used locally, at single CLIA certified lab
- Diagnose rare diseases
- Manual methods and skilled laboratory personnel
- FDA utilized general enforcement discretion approach for LDT
- Deferred oversight and regulation
- Did not strictly apply FDA standard oversight / approval process to LDT
- 510K approvals and data submissions not required
- FDA established a specific definition for LDT and claimed regulatory authority
- October 2014: FDA guidance document issued suggests that labs performing LDTs should comply with same FDA requirements as manufacturers
- Data submission for premarket review
- Continuous postmarket safety surveillance
- November 2016: FDA guidance document withdrawn
- Strong opposition from the laboratory community
- Concern that regulation would prevent laboratories from providing important testing in a timely manner
- VALID Act, 2018 - 2021: multiple versions proposed in an attempt to find a compromise between the FDA and laboratory providers
- Proposed risk based framework for new category, in vitro clinical tests (IVCT), to include both IVD and LDT
- High risk tests require premarket review
- Low risk tests exempt from premarket review
- Grandfathering of existing LDTs if certain criteria are met
- Opposition from the laboratory community
- Laboratory is already heavily regulated
- Restriction of patient access to necessary care
- Suppression of innovation
- Duplicates CLIA defined regulations imposed by CMS through the College of American Pathologists (CAP) / The Joint Commission (TJC) inspection processes and specific state (e.g., New York) requirements
- Definitions unclear
- Discretion left to the Secretary of State would produce an unpredictable future
- Subject matter experts not involved in accreditation
- FDA did not have resources to meet this obligation
- Demonstrated by challenges of EUA (emergency use authorization) during COVID-19 for testing
- Lack of review of non-COVID FDA submissions during Public Health Emergency
- Was proposed as part of the Consolidated Appropriations Act of 2023 but was ultimately withdrawn from that bill, which was passed on December 20, 2022
- Proposed risk based framework for new category, in vitro clinical tests (IVCT), to include both IVD and LDT
- FDA's new proposed rule on LDTs, September 29, 2023 (Regulations.gov: Medical Devices; Laboratory Developed Tests [Accessed 4 March 2024])
- Above website includes the rule itself and supporting documents
- Comments / responses from individuals, professional associations and manufacturers
- Update the definition of in vitro diagnostic products to make explicit
- IVDs are devices under the Food, Drug and Cosmetics Act
- Clinical laboratory is considered the manufacturer of the IVD / LDT
- Intention to phase out general enforcement discretion approach to LDT
- IVDs manufactured by a laboratory to fall under the same enforcement approach as other IVDs
- Require preapproval data submission
- Analytic, effectiveness and safety assessment submission prior to use
- Continuous data submission for monitoring of test performance and safety
- Better protect the public by assuring safety and effectiveness
- Nuts and bolts of proposal
- Will require establishment of a new division of FDA
- Will require substantial financial investment in FDA
- Phase out of general enforcement discretion to take at least 4 years
- Stratify LDT to evaluate high risk tests first
- Includes both modifications of approved IVD (for patient age, matrix, etc.) and new tests
- LDT to be excluded from proposal
- Those that follow the original 1976 definition
- HLA tests for transplantation and histocompatibility
- Forensic testing
- Public health surveillance testing (not for individual patient diagnosis)
- Public comment period ended December 4, 2023
- Over 6,000 comments received
- Current LDT landscape
- Today's tests more complex scientifically and technically than in 1976
- Little difference from other manufactured IVD under FDA oversight
- Being used outside local community
- Marketed nationwide
- Widely screen for disease or diagnose serious medical conditions
- Avoid FDA submission / approval / renewal
- A new assay can then be launched faster and at a lower cost
- Need to demonstrate clinical utility / efficacy
- Tests significantly impact larger groups of patients
- Growing sector of testing market
- Used in complicated areas of medicine: precision medicine, cancer diagnostics and prognostics
- Some algorithms and scores for interpretation are not transparent about the basis for scoring
- Directly influence drug treatment and choice
- Articles cited by the FDA indicating that current LDTs may be causing patient harm
- ProPublica: They Trusted Their Prenatal Test. They Didn't Know the Industry Is an Unregulated "Wild West." [Accessed 4 March 2024]
- ProPublica: The COVID Testing Company That Missed 96% of Cases [Accessed 4 March 2024]
- MedScape: Blood Test Positive for Cancer, But Is There Really a Tumor? [Accessed 4 March 2024]
- Nat Commun 2019;10:3328
- Today's tests more complex scientifically and technically than in 1976
- Public comment period has ended, awaiting final rule and anticipate FDA will proceed
- FDA released a statement jointly with CMS in January 2024, signaling its firm stance on increased LDT oversight (FDA: FDA and CMS - Americans Deserve Accurate and Reliable Diagnostic Tests, Wherever They Are Made [Accessed 4 March 2024])
- FDA intends to release the final rule in April 2024 under an expedited timeline (OIRA: Medical Devices; Laboratory Developed Tests [Accessed 4 March 2024])
- Comments and concerns voiced by laboratory community similar to those of VALID Act
- Laboratory is already heavily regulated
- Restriction of patient access to necessary care
- Suppression of innovation
- Definitions unclear
- Subject matter experts not involved in accreditation
- FDA does not have resources to meet this obligation
- Demonstrated by challenges of EUA (emergency use authorization) approvals during COVID-19
- Lack of progress of non-COVID-19 testing applications during Public Health Emergency
- Cost of setting up a new department of FDA for this oversight has been underestimated
- Cost of this oversight to the performing laboratories has been underestimated
- Costs include administrative time, data collection and submission of data to FDA
- Will prevent smaller hospitals and systems from offering LDT
- Limitations in testing will adversely affect already disadvantaged populations and some patients with rare serious disease
- Examples of specific pathology professional society responses
- College of American Pathologists response (CAP: Re: FDA Proposed Rule, "Medical Devices; Laboratory Developed Tests" - Docket No. FDA-2023-N-2177 [Accessed 4 March 2024])
- LDTs play a critical role in patient care
- Innovative tests that may be the only tests of their kind
- Promote innovation and patient safety
- Propose an oversight framework with a stratified approach to balance regulation by FDA and CMS without stifling innovation or patient access
- Some LDTs represent modified manufacturers' tests because the population is too small (pediatrics, rare diseases) or because an FDA approved test is not available
- Some LDTs exist because manufacturers do not have a product for an older instrument or new scientific data, such as antimicrobial breakpoints
- American Society of Clinical Pathology response (ASCP: RE: "Medical Devices; Laboratory Developed Tests" [Accessed 4 March 2024])
- Majority of LDTs provide good results and strongly support patient care
- The limited number of poorly performing LDTs do not require an extraordinary level of regulatory oversight proposed
- Challenges for the laboratory community include
- Financial and administrative burden too high for most laboratories to bear
- Will undermine patient access to testing needed for optimum diagnosis and treatment
- Stifles the innovation that laboratories should be encouraged to develop
- LDTs are often the first diagnostics for infectious diseases, public health emergencies and cancers
- LDTs that are modified under CLIA standards allow testing on alternative specimen types when supplies are not available and are customized to patient populations
- Only about 1% of laboratory testing is done with LDTs
- Approximately 120 million tests per year
- Current evidence of poor performance is limited and sporadic, suggesting an absence of systematic issues with current oversight
- FDA has limited ability to review submissions in a timely manner
- Could delay standard test approvals while getting LDTs evaluated
- Some LDTs diagnose patients with medical emergencies that need prompt diagnosis and could cause harm if not available
- Digital Pathology Association response (Regulations.gov: Comment from Digital Pathology Association [Accessed 4 March 2024])
- Digital pathology relies on robustly validated LDTs to support adoption and use for safe patient care, thus can contribute meaningful experience
- Not every result with an LDT will be accurate but
- Results of IVD are not always accurate
- Sensitivity and specificity of IVD are often less than 100%
- Consider the benefit of LDT: remote use of LDT for assessment of thyroid fine needle aspiration quality in over 600 procedures
- Saved 236 hours of procedure time per year
- Reduced the number of needlesticks needed for diagnosis by 33%, 1 stick per patient
- Substantial cost and administrative time required to comply with device requirements
- Will reduce testing compendiums significantly
- FDA has underestimated the number of tests to be withdrawn
- Evidence of risks of LDT is often published in newspapers, patent issues, lawsuits and anecdotes rather than peer reviewed and systematic research reviews
- EUA situation during COVID-19 cannot be used to justify oversight of LDT as this was a unique experience
- Oversight rule will overburden the FDA
- Current rule does not clearly address the anatomic pathology arena incorporating different and complex methods that will require a different set of experts to evaluate (artificial intelligence / machine learning [AI / ML], liquid biopsy, multiplex assays, multianalyte tests incorporating complex algorithms and whole genome sequencing)
- Among the recommendations made
- Harmonizing regulations for LDT and IVD in which clinical validation is considered with the laboratory regulation setting
- Convene a special advisory panel on digital pathology, which is not just a device but is transforming practice
- College of American Pathologists response (CAP: Re: FDA Proposed Rule, "Medical Devices; Laboratory Developed Tests" - Docket No. FDA-2023-N-2177 [Accessed 4 March 2024])
- Regulation on in vitro diagnostic medical devices (IVD regulation)
- Entered into force May 2017 and applicable on May 26, 2022 with staggered grace periods extending to May 26, 2025 for high risk IVDs and May 26, 2027 for lower risk IVDs
- Updates first EU level rules for placing IVDs on the market in 1998
- Response to substantial technological and scientific progress over the last 20 years
- To improve the safety of IVDs
- To modernize regulations and the sector
- Ensure a high level of public health and safety
- Ensure smooth operation of a single market for these devices
- IVD Regulation (EU-IVDR) - EU Regulation 2017/746 on in vitro diagnostic medical devices
- Clear obligations for economic operators
- Risk based classification system with 4 risk classes of IVDs
- Class A: low individual risk and low public health risk (e.g., buffer solutions)
- Class B: moderate individual risk or low public health risk (e.g., pregnancy home tests)
- Class C: high individual risk or moderate public health risk (e.g., cancer tests)
- Class D: high individual risk and high public health risk (e.g., hepatitis and HIV tests)
- Stricter control of high risk IVD with new premarket scrutiny mechanism
- Oversight by independent third party conformity assessment bodies (notified bodies)
- Improved transparency through comprehensive EU database
- Traceability system based on unique device identifier
- Reinforced rules on clinical evidence and performance evaluation
- Strengthened postmarket surveillance requirements
- Improved coordination mechanisms among EU countries on vigilance and surveillance
- Specific regime for devices manufactured and used in the same health institution (in house devices or LDT)
- Not marketed or transferred to other legal entities
- Not CE marked
- General safety requirements must be met
- Requirements of health institution using an LDT
- Must have an appropriate quality management system
- Must comply with international standards setting out the quality and competency requirements of the laboratory (EN ISO 15189) or other national provisions
- Must justify that the target patient group's specific needs cannot be met by an equivalent IVD available on the market
Canada (CMAJ 2020;192:E1166, CMAJ 2019;191:E1067)
- Health Canada, a federal institution, is responsible for
- Helping Canadians maintain and improve health
- Ensuring high quality health services are accessible
- Reducing health risks
- Federal Food and Drugs Act
- Oversees IVD devices under Medical Devices Regulation
- Has oversight of diagnostics developed as test kits only
- Does not address LDT
- Other regulation of laboratory testing is overseen by public and private entities with oversight from provincial governments, nongovernmental organizations and professional societies
- No single entity is formally responsible across Canada for independent evaluation of LDT or assessment of development, validity and adverse effects
- Standards Council of Canada, Canadian Standards Association Technical Committee Z252.11 for Lab Developed Tests, April 2018
- Voluntary standard
- In concert with Medical Devices Bureau of Health Canada and laboratory partners Siemens and Roche
- Provides high level guidelines for test validation
- Distinguishes testing performed for clinical use versus research
- Demonstration of diagnostic effectiveness
- Demonstration of patient safety
- Requirement for analytic validity
- Requirement for clinical validity
Comment Here
Reference: Laboratory developed tests
- Are based on manual methods requiring scientific expertise
- Are used by reference laboratories across the U.S.
- Require complex and proprietary algorithms for interpretation
- Widely screen for disease
Comment Here
Reference: Laboratory developed tests
- Responsible for:
- The overall operation and administration of the laboratory
- Employment of personnel who are competent to perform test procedures
- Recording and reporting test results promptly, accurately, and proficiently
- Assuming compliance with the regulations
- Moderate complexity
- Technical consultant must meet one of these requirements:
- MD, DO with current medical license in state of lab’s location AND certified in anatomic and/or clinical pathology by ABP or AOBP or equivalent qualifications
- MD, DO, or DPM with current medical license in state of lab’s location AND 1 year laboratory training/experience in the designated specialty/subspecialty or responsibility
- PhD in chemical, physical, biological or clinical laboratory science or medical technology AND 1 year laboratory training/experience in the designated specialty/subspecialty of responsibility
- Masters in chemical, physical, biological or clinical laboratory science or medical technology AND 1 year laboratory training/experience in the designated specialty/subspecialty of responsibility
- Bachelors in chemical physical, biological or clinical laboratory science or medical technology AND 2 years laboratory training/experience in the designated specialty/subspecialty of responsibility
- Clinical consultant must meet one of these requirements:
- MD, DO with current medical license in state of lab’s location AND certified in anatomic and/or clinical pathology by ABP or AOBP or equivalent qualifications
- MD, DO, DPM with current medical license in state of lab’s location AND laboratory training/experience consisting of (only one):
- 1 year directing or supervising non-waived tests
- 20 CME credit hours in laboratory practice commensurate with director responsibilities (effective 08/02/1993)
- Equivalent laboratory training (20 CMEs) obtained during medical residency
- PhD in chemical, physical, biological or clinical laboratory science AND certification by ABMM, ABCC, ABB, ABMLI
- MD, DO, DPM with current medical license in state of lab’s location
- Technical consultant must meet one of these requirements:
- High complexity
- General supervisor must meet one of these requirements:
- Qualify as laboratory director of high complexity testing
- Qualify as technical supervisor of high complexity testing
- MD, DO, DPM with current medical license in state of lab’s location AND 1 year training in high complexity testing
- PhD in clinical laboratory science or chemical, physical, biological science AND 1 year training/experience in high complexity testing
- Masters in clinical laboratory science, medical technology, or chemical, physical, biological science AND 1 year training/experience in high complexity testing
- Bachelors in medical technology or chemical, physical, biological science AND 1 year training/experience in high complexity testing
- Associate Degree in laboratory science or medical laboratory technology AND have at least 2 years of laboratory training or experience, or both, in high complexity testing
- Education and training equivalent to an associate degree in laboratory science or medical laboratory technology AND have at least 2 years laboratory training or experience, or both, in high complexity testing
- Education
- 60 semester hours including either 24 semester hours of medical laboratory courses or 24 semester hours of science courses (6 hours chemistry; 6 hours biology)
- 12 hours in chemistry, biology, or medical laboratory technology, or any combination
- Training
- Either completion of an approved/accredited clinical laboratory or medical laboratory training program
- May be included in the 60 semester hours specified above or three months of documented laboratory training in each specialty in which the individual performs high complexity testing
- For blood gas analysis, earned an associate degree related to pulmonary function AND have at least 2 years of laboratory training or experience, or both, in blood gas analysis
- Technical supervisor must meet one of these requirements:
- MD, DO with current medical license in state of lab’s location AND certified in anatomic and/or clinical pathology by ABP, AOBP, or equivalent qualifications
- MD, DO, DPM with current medical license in state of lab’s location AND certified in clinical pathology by ABP, AOBP, or equivalent
- MD, DO, DPM with current medical license in state of lab’s location AND 1 year training/experience in high complexity testing in the respective specialty
- PhD in clinical laboratory science, chemical, physical, biological science AND 1 year training/experience in high complexity testing in the respective specialty
- Masters in medical technology, clinical laboratory science, chemical, physical, or biological science AND 2 years training/experience in high complexity testing in the respective specialty
- Bachelors in medical technology, chemical, physical, or biological science AND 4 years training/experience in high complexity testing in the respective specialty
- Clinical consultant must meet one of these requirements:
- MD, DO with current medical license in state of lab’s location AND certified in anatomic and/or clinical pathology by ABP or AOBP or equivalent qualifications
- MD, DO, or DPM with current medical license in state of lab’s location AND 1 year laboratory training during medical residency
- MD, DO, DPM with current medical license in state of lab’s location AND 2 years experience in directing/supervising high complexity testing
- PhD in chemical, physical, biological or clinical laboratory science AND certification by ABMM, ABCC, ABB, ABMLI, or other board deemed comparable by HHS
- MD, DO, DPM with current medical license in state of lab’s location
- General supervisor must meet one of these requirements:
- Testing systems provide quality laboratory services, both existing and new
- Physical plant and environment conditions are appropriate size, contain required components and are safe
- Test methodologies provide the quality of results required for patient care
- Verification procedures are adequate to determine accuracy, precision, and other pertinent performance characteristics of the method
- QC and Quality Assurance programs are established, documented and maintained
- Establish and maintain acceptable levels of analytic performance for each test
- All remedial actions are taken and documented
- Test reports include pertinent information required for interpretation
- Consultation is available
- Current, approved procedure manual is available
- Specify in writing the responsibilities of each consultant, supervisor, and person engaged in the performance of the pre-analytic, analytic, and post analytic phases of testing
- Identifies which procedures they are authorized to perform, including competency assessment
- Whether supervision is required for processing, test performance, or result reporting
- Whether consultant, supervisor, or director review is required prior to reporting patient test results
- General Supervisor provides on-site supervision of high complexity test performance by qualified testing personnel as required by CLIA
- Enrolled in a CLIA approved PT program for all regulated analytes
- PT samples are tested as required under CLIA regulations Part 493, subpart H
- Results are returned within the timeframe established by the PT provider
- Reports are reviewed by appropriate staff to evaluate performance and to identify any problems that require corrective action
- The approved corrective action plan is followed when results are unacceptable or unsatisfactory
- Includes educational challenges and ungraded results
- Perform test methods as required for accurate and reliable results
- Provides a sufficient number of lab personnel with appropriate education and experience or training
- Prior to testing patient specimens, all personnel have the following:
- Education and experience
- Appropriate training
- Demonstrated proficiency of all testing operations
- Test methods performed as required for accurate and reliable results
- If qualifications are met per regulations, the same person can be the Technical Supervisor, General Supervisor, Technical Consultant, Clinical Consultant, and Testing Personnel
- No limit to number of roles
- Recommended to designate qualified personnel in above categories regardless of title to ensure redundancy
- Must be accessible to the lab to provide onsite, telephone, or electronic consultation as needed or required
- If the testing is being performed under your CLIA certificate, then you are responsible (includes POCT, Blood Gases)
- May direct no more than 5 labs per regulations
- The Laboratory Director still remains responsible for ensuring all duties are properly performed even when delegated
- Duties must be delegated to qualified individuals:
- Technical Consultant
- Technical or General Supervisor
- Clinical Consultant
- These can also be delegated:
- New Hires - Completed orientation and have demonstrated competency
- Assessing competency of existing employees
- Review of PT report
- Quality Control review and Quality Assurance Programs
- Process Improvement
- Method Validations
- Correlations
- Calibration Verification
- Once every two year policy and procedure review
- CANNOT delegate approval of new and revised procedures
- Document delegation in writing
- Complete a laboratory-wide organization chart and show qualified roles on chart
- Use the resources provided by your accrediting organization to document personnel
- Leading Practice Library
- Procedures shared by other laboratories and hospitals
- Standards FAQS on website
- Online Question options for Standards (see below)
- Lab Central Connect™
- Portal for test menu, personnel, and other compliance information
- Standards Online Question Form, Standards Interpretation Group at 630-792-5900, 8:30 a.m. - 5:00 p.m. Central Time
- Laboratory accreditation refers to a stamp of approval granted to a laboratory by an external entity, intended to assure quality of processes and personnel; it generally indicates that the lab meets the standards established by the accrediting agency
- Laboratory inspection refers to the processes by which an accrediting entity verifies that the laboratory has met their requirements
- Standards of performance and operation are specified by the accrediting agency
- Transparency implies that the agency and the laboratory engage in good faith, without collusion or secondary motive, with the goal to improve the quality of patient care offered
- Time frames for application, inspection and accreditation are defined and understood
- LAP: laboratory accreditation program
- Driven by standards of established processes and personnel; provides an externally based affirmation of laboratory performance and quality
- Verified by inspection and performance comparison with other labs
- Accrediting entity may be local, regional, national or international
- ISO: International Organization for Standardization, a federation of national standards bodies including more than 160 countries, providing standards and specifications that cover a wide array of fields, from healthcare to manufacturing, security and the environment
- CAP: College of American Pathologists, a professional association of pathologists which also provides lab accreditation services, proficiency materials and other activities that aim to improve and assure quality laboratory services to patients
- CLIA: Clinical Laboratory Improvement Amendments, a section of U.S. law governing laboratory activities in the U.S., the purpose of which is to ensure quality laboratory testing (Mil Med 2000;165:48)
- A CLIA certificate is required to perform testing on patients covered under the U.S. Medicare or Medicaid programs
- CLIA certificates are proof of registration and require regular inspection and accreditation to be recognized
- JC: Joint Commission, an accrediting agency for hospitals and healthcare organizations with deemed status for inspection of laboratories within such entities
- CDC: Centers for Disease Control and Prevention
- FDA: Food and Drug Administration, the U.S. agency with regulatory oversight of food, drugs and medical devices sold in the U.S.; it thus has oversight of many laboratory devices or testing platforms or processes used in the U.S. and sets the provisions under which they can be marketed
- CMS: Center for Medicare and Medicaid Services, the payer for a large portion of the U.S. populace's health expenses
- COLA: Commission on Office Laboratory Accreditation
- UKAS: United Kingdom Accreditation Service
- Deemed status: in U.S. laboratory accreditation, an inspection and accreditation entity has deemed status when their inspections are deemed to be equivalent to those of a primary regulatory body (such as CLIA or FDA), and therefore the lab need not be dually inspected by the primary regulatory agency
- Deficiencies: items identified during the inspection process that need the attention of the laboratory, as they do not meet the standards of the accrediting agency
- Phase 1 deficiencies are items that need to be addressed but may require long term improvements and do not pose an imminent risk to patient care or quality
- Phase 2 deficiencies are items that must be corrected prior to receiving accreditation
Contributed by Mai Thy Tran, M.D.
Images hosted on other servers:
Options for lab accreditation
| CLIA | CAP | ISO 15189 | COLA | JC | |
| Purpose |
|
|
|
|
|
| Inspector | CLIA inspectors (usually state department of health personnel) | Peer inspections by CAP inspectors and self inspections by staff | Certified inspectors | COLA surveyor (most often medical technologists) | JC surveyors |
| Accreditation cycle | 2 years | 2 years | 3 years | 2 years | 2 years |
| Frequency of inspection | Every 2 years | Peer inspections every 2 years and annual self inspection | Annual internal audit | Every 2 years | Every 2 years |
| Cost | One time registration fee of $100; certification fee based on the annual testing volume and number of laboratory specialties performed ($180 - $9,500 / year) | Nonrefundable one time application fee of $1,200 (domestic) or $1,500 (international); CAP's annual accreditation fee is determined based on the laboratory's size and complexity | The fee schedule will include an annual base fee and fees for assessments; cost will vary depending on the size and scope of the lab | Enrollment fee and certification fee | Onsite survey fee; annual fee based on the number of specialties the lab provides and the number of locations |
Example for lab accreditation check list
| Lab accreditation - general check list | Yes | No | N/A |
| Specimen collection, handling and reporting | |||
| Proficiency testing | |||
| Quality management | |||
| Result reporting and referral of testing | |||
| Quality of water and glassware washing | |||
| Reagents (storage, handling, labeling) | |||
| Instrument and equipment maintenance / function check | |||
| Personnel | |||
| Laboratory computer services | |||
| Physical facility | |||
| Laboratory safety |
- Benefits of laboratory accreditation (J Med Biochem 2017;36:231):
- Benefits to the laboratory:
- Demonstrates competence of the laboratory
- Assists lab employees in learning, developing a sense of pride in their work and in maintaining high standards
- A tool to recognize laboratories worldwide
- Ensure accurate patient diagnostics and efficiency of treatment
- Better documentation of processes and responsibilities
- Required for participation in payments from Center for Medicare / Medicaid Services
- Benefit for patient:
- Delivers a high quality of lab service
- Benefits to the public:
- Provides assurance of quality of results and related treatment choices
- Assures public payments are used for quality testing
- Benefits to the laboratory:
- Costs or drawbacks to accreditation:
- Administrative costs and effort to achieve compliance
- Proficiency testing expenses
- Inspection expenses and disruption of operations during inspection
- Remediation of deficiencies may require added expense and time
- Background (Arch Pathol Lab Med 1997;121:745):
- Founded by a group of pathologists in 1946
- Fosters and advocates for excellence in pathology laboratories
- Accredits approximately 8,000 laboratories in over 50 countries
- Revolves around 4 standards: evaluation of the laboratory director, physical facility and safety, quality control and performance improvement, inspection requirements
- Becoming CAP accredited means that the lab meets all requirements of CLIA (deemed status)
- Aim:
- Maintain accuracy of test results
- Offer professional development and learning opportunities for laboratory staff
- Quality process improvements through (required) PT / EQA programs
- Support the laboratory management to improve laboratory services
- Risk reduction
- Lab becomes more marketable in the industry
- Inspections:
- Conducted (biannually) by volunteer teams of peers who also work in and operate laboratories and by self inspection using standard checklists adapted to the individual laboratory's activity menu
- American Association of Blood Banks (AABB) serves blood collection and transfusion services of all sizes; it provides peer inspectors and standards unique to the blood collection, processing and administration process
- Blood bank services are also subject to FDA inspections since they deal with a product administered to patients (Arch Pathol Lab Med 1999;123:468)
- American Society for Histocompatibility and Immunogenetics (ASHI) offers inspection and accreditation services to laboratories performing HLA serotyping, DNA typing, next generation sequencing, flow cytometry, engraftment monitoring and some crossmatch, antibody identification and ABO / Rh typing services in support of organ transplantation and related processes
- International Organization for Standardization 15189 (ISO 15189):
- Background:
- First published in 2003 and revised in 2012
- Originated from the requirements of the International Organization for Standardization / International Electrotechnical Commission (ISO / IEC) 170254 and the quality management system requirements of ISO 9001 (Arch Pathol Lab Med 2018;142:1047)
- Verifies that medical laboratories meet both the technical competencies and management system requirements
- By 2015, approximately 60 countries have made ISO 15189 a part of their mandatory medical laboratory accreditation requirements
- U.S. laboratories must first be accredited in the CAP LAP before seeking accreditation to the ISO 15189 standard with CAP 15189
- CAP 15189 is a quality management program the CAP designed for accreditation to ISO 15189
- Purpose:
- Create systems aimed at preventing problems and reducing errors
- Identify opportunities for improvement
- Train staff by involving them in the problem solving process (Ann Lab Med 2017;37:365)
- Background:
- Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA):
- Background:
- WHO in African regions and U.S. CDC established SLIPTA (WHO: Guide for the Stepwise Laboratory Improvement Process Towards Accreditation in the African Region (SLIPTA) [Accessed 6 June 2022])
- Created because of the urgent need to strengthen laboratory systems and services in Africa
- Aim:
- Framework for improving quality of laboratories in developing countries to meet the requirements of the ISO 15189 standard
- Enables laboratories to develop and document their ability to detect, identify and promptly report all diseases of public health significance
- Background:
- The Joint Commission (StatPearls: The Joint Commission [Accessed 23 June 2022]):
- Background:
- Nonprofit, global entity, with various accreditation / certification programs, that is recognized and relied upon by many states
- JC collaborated with ASCP to develop the Leading Laboratories, a unique designation exclusively for Joint Commission accredited laboratories
- Accrediting hospital laboratory services since 1979 and freestanding laboratories since 1995
- 22,000 accredited healthcare systems in the U.S. and 1,000 healthcare organizations in over 70 countries have achieved the gold seal of approval as JCI accredited organizations; over 1,500 laboratories are accredited by the JC
- Satisfies requirements of systems participating in providing care for Medicare and Medicaid patients
- Aim:
- Process of accreditation starts with the onsite survey with JC standards
- Provides advisory services, education and training programs which are focused on leadership, quality improvement, patient safety
- 2 year accreditation cycle with unannounced inspections by nonvolunteer JC surveyors (not peer inspectors)
- Cost:
- Onsite survey fee
- Annual fee, based on the number of specialties that the lab provides and the number of locations
- Background:
- The United Kingdom Accreditation Service (UKAS):
- Background:
- Not for profit organization; sole national accreditation body for the United Kingdom
- Recognized and appointed by the government to assess against agreed standards for organizations and provide services for certification, testing, inspection and calibration; UKAS accreditation confirms the competence and performance capacity of these organizations
- UKAS is a signatory, along with other recognized accreditation bodies from around the world, to multilateral agreements for the purposes of mutual recognition through the European cooperation for Accreditation (EA), the International Accreditation Forum (IAF) and the International Laboratory Accreditation Cooperation (ILAC)
- Aim:
- UKAS accredits medical laboratories with the ISO 15189 standards
- Quality standard for imaging (QSI), medical physics and clinical engineering (MPACE) are addressed to BS 70000, point of care testing to ISO 22870, proficiency testing against the requirement of ISO / IEC 17043
- Provides reassurance of the quality of a diagnostic service to patients, commissioners and healthcare providers
- Background:
- Select accrediting agency
- Review laboratory operations in light of accreditation standards and published checklists
- Ensure that the laboratory quality management and procedures are effective (PT testing, quality systems, error detection systems, etc.) (J Med Biochem 2017;36:231)
- Perform a mock inspection (self or otherwise)
- Submit application and await inspection
- After inspection, inspectors will review areas of weakness, identify problems and further evaluate lab's corrective action and resolution
- A review or audit of procedures and records, observations of operations, interviews with workers and evaluation of work product that may be:
- Announced inspections (depending on agency): initial and biennial recertifications
- Unannounced inspections: routine process or as a result of complaints and follow up inspections (CLIA will generally conduct a number of validation inspections following inspection / accreditation by a deemed agency)
- Types of lab inspection:
- Self inspection:
- Purpose: ongoing compliance with the checklist requirement
- Lab improvement and better patient care
- Prepare for the next unannounced onsite inspection
- Provides benefits to employees and trainees, including pathology residents
- Process exposes the residents to operational issues and corrective actions
- Provides them the opportunity to take a more active role in laboratory management (Acad Pathol 2017;4:2374289517699230)
- Increases knowledge in laboratory QA management, resulting in better performance on the resident in service examination (RISE)
- Helps residents prepare for board exams and future career as a lab director
- Helps the department identify ongoing problems (Pract Lab Med 2019;16:e00123)
- Peer inspection:
- Benefits both the inspected laboratories and the laboratories providing the inspection teams
- Benefits for pathologist / director led inspection teams:
- All CAP inspectors are required to successfully complete training on the CAP website
- Training provides in depth instruction on how inspections should be conducted
- Allows laboratorians to interact with their coworkers and industry colleagues in different labs
- Allows laboratorians to observe and learn from a variety of laboratory environments, while exchanging best practices to ensure high quality patient safety and care
- Benefits for pathologist / director led inspection teams:
- Benefits both the inspected laboratories and the laboratories providing the inspection teams
- Virtual and onsite inspection (see Diagrams)
- Self inspection:
CAP15189
UKAS accreditation
CAP accreditation
What is CLIA?
What is the Joint Commission?
Who is FDA?
CDC
CMS
- All laboratories in the U.S. are required to be accredited first by CAP, followed by CLIA
- CAP has deemed status, which means that CAP can inspect on behalf of CLIA
- CLIA standards are more stringent than those of CAP, so most laboratories choose to be CAP accredited
- They are 2 entirely independent accreditation organizations
Comment Here
Reference: Laboratory inspection and accreditation
- A corrective action plan must be included, along with the interim inspection results submitted to the accrediting agency
- Employees responsible for PT evaluation in that lab section should be disciplined or terminated
- Reporting this will automatically trigger an unannounced CLIA inspection
- The lab's accreditation status will be revoked
- The lack of PT results for the period means all analyte results must be invalidated
Comment Here
Reference: Laboratory inspection and accreditation
- Lean Six Sigma is a customer focused, team oriented and data driven performance improvement and problem solving technique directed at breakthrough improvement to essential business processes
- Lean Six Sigma methods improve quality and satisfaction of workers and customers by delivering consistent, defect free results, services or products at low cost by eliminating waste
- Lean Six Sigma is a manner of thinking and approaching problems or design, as well as a methodology with a robust set of special tools
- Lean Six Sigma has a proven record of breakthrough improvements in laboratory and healthcare services, significantly improving quality and safety outcomes
- Lean: set of methods intended to drive out waste and improve process flow
- 5S: Sort, Set in order, Shine, Standardize, Sustain (or in Japanese: Seiri, Seiton, Seiso, Seiketsu, Shitsuke); for maintaining an orderly workplace and using visual cues to achieve operational consistency
- Value stream map: defining the entire value stream for a process and measuring performance in time, work in progress (WIP) and resource utilization
- Single piece flow: workflow with no or minimal batches, no queues or waiting and no wasted motion
- Cycle and lead time: measurements of process time used to identify workflow improvements
- Pull systems: activities driven by customer needs in terms of quantity, specifications and delivery time
- Kanban: pull system of material and production control; an inventory control system for just in time manufacturing
- 7(+1) wastes: also known as muda (Japanese term for waste) or non value adding activities; defined as overproduction, waiting, transport, poor process design, inventories, motion, defective parts and not meeting external customer requirements
- Kaizen: defined as change for the better or the desire for continuous improvement
- Henry Ford production system: concept of a continuously moving production line, producing a high quality, low cost product (J Mol Diagn 2009;11:390)
- Toyota production system: a system of continuous flow, rapid changeover between tasks and minimal waste (Am J Clin Pathol 2006;125:16)
- Six Sigma: a quality improvement methodology oriented around reduction of defects or errors to less than 3.4 defects per million opportunities; it is often linked to Lean, as both together have a very salutary effect on customer satisfaction, employee satisfaction and company performance
- Black Belt: a certification that an individual is thoroughly familiar with Six Sigma methodologies and can successfully lead quality improvement projects
- Green Belt, Yellow Belt: lesser certifications of individuals engaged in or supervising Six Sigma quality improvement projects
- Define, Measure, Analyze, Innovative Improvement, Control (DMAIIC): the Six Sigma structured, disciplined and rigorous approach to problem solving
- Voice of the Customer (VoC): refers to identifying customer needs and perceptions (CallMiner: Voice of the Customer Tools and Best Practices [Accessed 1 April 2021])
- Critical to Quality (CTQ): translates customer needs into specifications
- Design of experiments (DOE): an effective and efficient approach for determining the relationship between process variables
- Plan, Do, Study, Act (PDSA): an analytical approach that considers the process as is, analyzes it further, revises it as appropriate and then repeats the cycle for continuous improvement
- Failure mode and effect analysis (FMEA): a tool to help focus on process failures (Clin Leadersh Manag Rev 2004;18:37)
- Process map: using flowcharts to better understand and document workflow processes
- Descriptive statistics: use of carefully selected sampling techniques to predict characteristics of a population (Anesth Analg 2017;125:1797)
- Pareto chart: combination of bar graph and line graph that can used to highlight the frequency of defects and their cumulative impact; a funneling tool to understand various parts of a problem and decide where to focus problem solving efforts
- Gage R&R: set of trials to assess the repeatability and reproducibility of a measurement system
- Box plot: data visualization tool that provides pictorial representation of the median, first and third quartile that can be quickly understood by others
- Multi vari chart: data visualization tool that identifies the component that affects variability the most
- Hypothesis testing: procedure that summarizes data in order to detect differences among 2 or more groups
- Upper level administrative leadership and support required to clear the path for difficult changes
- Leadership must be customer focused, challenge the status quo, be visible and available, champion excellence, promote tough standards, act with integrity and facilitate teamwork
- Well trained project team leadership (Black Belts, Green Belts or Yellow Belts) and carefully selected team members with process content knowledge
- Structured regular meetings with agenda topics focused on assigning activities, reporting and documenting findings to promote both team efficiency and effectiveness
- Clinical and anatomic pathology project teams best include carefully selected administrators, pathologists, managers and staff members with process content knowledge
- Projects often span multiple departments and may be data intensive
- Project leaders (Black, Green or Yellow Belts) manage the projects and select the proper Lean and Six Sigma tools
- Pathologists and administrators support the project, clear barriers, identify CTQs (J Clin Lab Anal 2018;32:e22180)
- Team members with content knowledge are necessary to collect and process data and implement solutions
- Both Lean and Six Sigma focus on meeting customer requirements
- Lean emphasizes the elimination of waste and time
- Six Sigma focuses on quality improvement through reducing defects
- Lean and Six Sigma have complementary tools to help develop a shared understanding of the workflow process by identifying defects, areas of waste and process delay, workstation design and organization
- Lean Six Sigma applications include improving turnaround times, reducing data entry defects, improving insurance claim performance or improving workflow across multiple departments, such as with lab and pathology specimens (see Principles of laboratory quality improvement topic)
- Project team that includes both the emergency department and laboratory staff is more effective when working to improve emergency department laboratory test turnaround time
- Project team with gross room staff, histology staff and pathologists is more effective when working to improve specimen processing and slide distribution
- Workflow process that is designed based on Lean and Six Sigma principles will be more efficient and effective than a workflow process that has been allowed to evolve naturally over time
- Clear and concise project description (problem statement)
- Clear scope with a starting and ending point
- Tied to key Critical to Quality (CTQ) measures
- Defined links to company goals
- Quantified analysis of the financial benefit
- Identified team members and other resources (time)
- Identified customers and link to their needs
- Sign off that everyone is clear and committed to success
- Select a problem to solve or process to improve
- Start the Define phase with the project charter, stakeholder analysis, process maps, Voice of the Customer and Critical to Quality measures to clarify problem, scope, customer needs and benefits
- Continue with the Measure phase using a data collection plan, Gage R&R, control charts, frequency plots, Pareto charts, FMEA and process capability studies to measure baseline performance
- Continue with the Analyze phase using cause and effect diagrams, affinity diagrams, flow diagrams, DOE, hypothesis tests, regression analysis, scatter plots and brainstorming to identify root causes
- Continue with the Innovative Improvement phase using the same analysis tools testing new solutions
- Finish with the Control phase using control charts, standards operating procedures and monitor performance
- All project activities must be completely and clearly documented
- Keep stakeholders and other interested parties well informed
- Detailed capture of steps, times, capabilities or capacity to identify areas of batch processing versus single piece flow, signals of when work is available to be done and other process details
- Identification of bottlenecks, inventory, delays, rework due to defects and other sources of waste
- Graphical presentation allows grasp of the full scope of processes
- Spaghetti maps allow analysis of physical movements to complete work to detect movement waste
- Delivery times for critical results to emergency department (J Med Biochem 2021;40:26)
- Elimination of error prone data entry or other steps (J Clin Lab Anal 2018;32:e22180)
- Use of Sigma metrics in coagulation laboratory (Int J Health Care Qual Assur 2018;31:600)
- Application to histology workflow (BMC Clin Pathol 2010;10:2)
- Reduction in total turnaround time in surgical pathology reporting (BMJ Qual Improv Rep 2015;4:u209223.w3773)
- Improving services from a molecular pathology lab (J Mol Diagn 2009;11:390)
- Hospital system laboratory (Lab Quality Confab: Listening to the Voice of the Customer at North Shore LIJ Laboratories [Accessed 31 March 2021])
- Improvement in phlebotomy experience and workflow (Am J Clin Pathol 2009;132:914)
- Pap test value stream assessment and improvement (Am J Clin Pathol 2013;139:574)
What is Lean?
What is Six Sigma?
5 whys
Lean / Six Sigma - waste, DMAIIC, etc.
Managers, steps and duties
Descriptive statistics
Five S
Value stream mapping basics
What is Kanban?
What is DMAIIC
- Am J Clin Pathol 2007;128:423, Clin Lab Med 2004;24:865, Froehling: Measuring the "Voice of the Customer" in the Medical Laboratory Industry Using Surveys - A Case Study, 1st Edition, 2011, Pathol Lab Med Open J 2016;1:11, Six Sigma Material: Spaghetti Diagram [Accessed 31 March 2021], George: Lean Six Sigma for Service, 1st Edition, 2003, Voehl: The Lean Six Sigma Black Belt Handbook, 1st Edition, 2013, Eckes: Six Sigma Team Dynamicss, 1st Edition, 2002, Productivity Press Development Team: Kanban for the Shopfloor, 1st Edition, 2002
- Failure modes effect analysis (FMEA)
- Five S
- Five whys
- Root cause analysis (RCA)
- Value stream mapping (VSM)
Comment Here
Reference: Lean workflow / Six Sigma
Comment Here
Reference: Lean workflow / Six Sigma
- The organization or systematization of the laboratory is enshrined in several documents: The Organizational Chart, The Policy Manual(s) and The Procedure Manuals
- These documents describe the integration of personnel, equipment, supplies and facilities for efficient and effective laboratory operation
- Clear designation of authority and responsibility
- Precise identification of operations to be performed and goals to be achieved
- Accurate assessment of available personnel, skills, space, equipment and supplies needed to perform operations and implement the Strategic Plan
- The Organizational Chart:
- Establishes core authority and responsibility (JC Requirements)
- Clarifies different authorities and responsibilities for the Laboratory Director, the Pathologists, the Laboratory Manager and the Technical Supervisors (CLIA requirements)
- Identifies hierarchies and relationships
- Clarifies reporting lines
- Clarifies expectations
- Types include Hierarchical, Horizontal and Matrix
- To be of value, the organizational chart must be prominently displayed
- See example of hierarchical chart below
- The Policy Manual(s):
- Policies are the laws and rules of the organization and its units
- Employee Handbook is published by human resource department
- Safety Manual generally comes from the risk management department
- IT and Purchasing Manuals originate in these departments
- The Laboratory (User) Manual is the responsibility of the laboratory and contains information for the laboratory's users:
- Laboratory Staff - names, phones, expertise
- Accreditation Information - CAP, AABB, CLIA
- Location, hours of operation
- Test Ordering Information, STAT's
- Specimen requirements, collection information
- Result reporting systems
- How to use computer
- Listing of individual tests including test name, synonyms, when to order test, patient preparation, what specimen to collect, availability, TAT, methodology, result interpretation, reference ranges, sensitivity, specificity, predictive value, diagnostic efficiency
- Other important information, e.g. POC testing
- Procedure Manuals contain the instructions for performing specific tests or other laboratory specific tasks such as Equipment Maintenance Manuals
- The fundamental product of pathology and laboratory medicine is information based on the performance of tests
- In order to plan, organize and control lab operations the "test" must be the central element of concern
- George Lundberg, MD expanded the concept of a "test" from merely an analysis to the "brain-to-brain loop" wherein a "test" really commences when a clinician has a problem and is not completed until the problem is solved
- The terms "pre-analytic", "analytic" and "post-analytic" have been used to separate the phases of testing and to identify those phases which are clearly the responsibility of the laboratory and those that occur outside of the laboratory and are outside its control
- Regardless of where in the testing cycle a defect exists, or an error occurs, if the clinician's problem is not solved, it is considered a "lab error"
- Therefore, when designing (or assessing) laboratory operations, it is necessary to scrutinize all components of the "loop"
- In general, the analytic phase which is clearly under the control of the laboratory is well controlled by the laboratory's quality assurance and performance improvement systems and causes few slip-ups; the other phases of the "loop" are where errors most often occur and where the laboratory must take ownership and responsibility
- In this section the various sub-systems, or phases, which comprise the brain-to-brain loop are described
- The Brain-to-Brain Loop (JAMA 1981;245:1762, JAMA 1998;280:565, Am J Clin Pathol 2011;136:829)
- A physician has a problem
- The physician thinks of using a test to help solve the problem
- A test is ordered, verbally or in writing
- The test request is entered into an information system (HIS or Web)
- The requisition is transferred to the laboratory computer
- Laboratory computer generates pick-up lists, work lists, billing data, checks for duplicate test orders, duplicate names and checks for appropriateness of order (in terms of Admitting Diagnosis or ICD codes)
- Phlebotomist or nurse obtains specimen and sends it to the laboratory
- Specimen is triaged in laboratory central receiving area
- Test is performed and verified (the old concept of what a test is)
- Pathologist interprets test as needed
- Test results are transferred to the originating information system
- Test results are available at the nursing station (paper or CRT)
- Test results are placed in patient chart and sent to physician's office
- Physician uses the test results to help solve the problem
- Requirements of the Specimen Acquisition Phase
- This is the next phase; the LIS generates pick-up lists, work lists, billing data, checks for duplicate test orders, duplicate names and checks for appropriateness of order based on Admitting Diagnosis or ICD codes
- A phlebotomist or nurse obtains specimen and sends it to the laboratory and the specimen is triaged in laboratory central receiving area
- This phase of the "TEST" has many requirements, most not in, or related to, the laboratory; these include:
- Patient and specimen identification system (bar code, RFID)
- Hospital computer system that has the capability for duplicate name check
- Laboratory computer system that has capability for duplicate test order checking
- Personnel training for proper specimen acquisition; phlebotomists, nurses, couriers - all need training - particularly nurses and orderlies who are responsible for specimen acquisition, e.g., sputum or urine or ICU specimens from IV lines
- Special training for personnel outside the hospital, particularly doctor's office personnel, surgicenter personnel, remote outpatient phlebotomy sites, etc.
- Equipment: computer, cars, pneumatic tubes, bar code printers, phlebotomy carts, robots for specimen transport must all be checked for proper functioning; for example, does the car used by the courier service have appropriate refrigeration for specimens? Does the pneumatic tube system cause hemolysis?
- Specimen triage in the laboratory's Central Receiving and Processing area is often the source of errors, it requires fail-safe systems and protocols for:
- Test ordered lists (on paper or on computer)
- Specimen receipt list
- Specimen splitting and distribution
- Work lists
- Send-out list
- Overdue test lists
- Re-draw lists
- Requirements of the Testing Phase
- This is the next phase of the "Test Loop" and the one usually thought of by laboratorians as constituting the "test"
- Some general requirements are listed below - these obviously differ for the various tests done in the laboratory, but must be kept in mind at all times:
- Test development and selection requires assessment of clinical utility, cost analysis and comparison, technology assessment, impact on workload, need for information management and employee education and test validation
- Test performance is dependent on: competent personnel, functioning equipment, adequate space and proper reagents
- A Standard Operating Procedure (SOP) for every test needs detailed descriptions of:
- Purpose and principles of the test
- Specimen requirements
- Reagents and equipment
- Testing procedure
- Calibration
- Quality control
- Calculations
- Interpretation
- References
- History of the test in the laboratory
- Quality assurance (encompassing QA, QC, quality management, proficiency testing, performance measurement, process improvement, outcomes management, etc.) is an essential component of the Testing Phase
- Point of care and ancillary testing is generally the responsibility of the laboratory and needs special attention with regard to:
- Justifying the point of care testing
- Developing simple methods for non-lab personnel
- Acquiring fail-safe instruments
- Selecting methods and equipment
- Training personnel
- Quality management and proficiency testing
- Billing
- Transfer of result data from POC to LIS
- Pathologist's interpretation and verification is required on all anatomic and selected clinical pathology tests
- Requirements of the Reporting Phase
- This is the final phase of the "Loop" and consists of: results transfer to the originating information system, to the nursing station, to the patient chart, to the ordering physician's office and to the physician's brain so he can use the information to solve his original problem
- See Arch Pathol Lab Med 2008;132:1608, Arch Pathol Lab Med 2008;132:84, Arch Pathol Lab Med 2009;133:942
- This phase requires a report which may be paper, computer screen, verbal or other
- Pathology is an information business; our product is information in the form of reports, numbers, graphs, pictures, descriptions and diagnoses
- Our various report formats are the "gift wrap" for our product; this includes the classical paper report, the telephone report and the report appearing on the computer screen
- The design of the various report formats is the responsibility of the laboratory working with the clinicians and other customers, e.g., nurses, ward clerks
- Essentials of a good form
- Visual appeal - include logo - get design help
- Easy data entry and readability
- Customized report formats for different customers
- Zoning, spacing, sequencing, emphasizing and elimination of unnecessary data
- Establish a system of forms control
- If there are too many forms, too much paper, poor readability, filing problems
- Collect all forms, index and classify them, analyze the need for each form, eliminate, consolidate and redesign the essential forms
- Pathologists' special reporting functions - the TELEPHONE
- Call the surgeon and attending physician on all malignant, interesting or strange surgical diagnoses
- Call attending physician about all new panic values or unusual clinical laboratory test results
- STAT and critical values reporting
- Criteria for inclusion and ranges by clinicians
- Reporting methods: phone, computer, text message
- Hierarchical escalation of reporting
- HIPAA
- Privacy protection standards
- Billing Subsystem
- Although not included in the original Lundberg Loop, billing is an essential component of laboratory operations and has many requirements that will be discussed in subsequent chapters on financial management
- References: JAMA 1981;245:1762, JAMA 1998;280:565, Am J Clin Pathol 2011;136:829
- Work Flow Analysis
- Synonyms: process analysis, flow charting, time motion study, function sequencing; this is the best way to learn how your laboratory operates
- How to do work flow analysis:
- Direct observation by Laboratory Director and/or Chief Tech of one work station at a time, for a representative time span
- Include every step in the testing process
- Assess all involved personnel, equipment, functions including machine set up time, reagent storage, batching, when work arrives, how work is done, how results are reported, breaks, backup personnel and equipment, interactions with others in the laboratory, distance to storeroom, to computer terminal, availability of terminals, etc.
- Simple observation and charting of above and reviewing it with the involved personnel can lead to remarkable efficiencies
- It is essential to perform a work flow analysis for the entire laboratory before embarking on a major new program like installation of a laboratory computer, a collaborative venture or before designing a new laboratory or when assigned to a new laboratory
- The critical evaluation of the work flow analysis will result in improved efficiency by eliminating duplication, improving staffing and scheduling of personnel, better space and equipment utilization, better computer interfaces, improved reagent and supply management
- Other project management techniques
- Gannt Charts - a simple bar chart showing tasks, projects, startup and completion dates; overlapping bars show interrelationship of the various tasks
- PERT Charts - Program Evaluation and Review Technique: a method for setting time goals, particularly for research and development projects
- CPM - Critical Path Method: defines the tasks that need to be done, analyzes the sequence in which the tasks must be completed and estimates the time needed for completion; the method uses a diagram which consist of "nodes" representing activities connected by arrows showing the relationships among the activities
- How to Assess a Laboratory
- Is management system evident
- Is an organizational chart visible?
- Are policy and procedure manuals evident?
- Safety, environment, cleanliness, order
- Good lighting, air quality, noise levels
- Scheduling system
- Teamwork and motivation
- Are performance goals and achievements posted and visible?
- Space utilization, movement of materials, products
- Well labeled storage
- Crowding, use of hallways
- Maintenance of equipment and tools
- Are preventive maintenance schedules posted and visible?
- Levels of inventory?
- Has there been a work flow analysis?
- Commitment to quality and customer satisfaction
- Are customer surveys and QA data displayed?
- Is there a complaint management program?
- Is management system evident
- Signs and Symptoms of Poor Laboratory Operations
- See Wagar, Horowitz, and Siegal: Laboratory Administration for Pathologists (chapter 3)
- Recurrent overload crises
- Supervisors are unable to handle their sections
- Excessive overtime
- Prolonged turnaround time
- Delays due to low supplies
- Skilled workers doing menial tasks
- Supervisors doing bench work
- Excessive traffic, noise, crowding, talking
- Too many phone calls
- Too many STATs
- Complaints, external
- Complaints, internal
- Too many notices or rules posted in the laboratory
- Too many forms
- Frequent equipment failure
- Excess employee turnover
- Decreasing productivity (Productivity = Output/Input)
- Total Tests/FTE or Billable Tests/FTE or WLU/FTE or Billable Tests /Total Labor Expense
- Decreasing efficiency
- Total Revenue $/Test or /Admission or /Month
- Total Expense $/Test or /Admission or /Month
- How to Change a Laboratory
- See Wagar, Horowitz, and Siegal: Laboratory Administration for Pathologists (chapter 3)
- Identify current dissatisfactions or perceptions
- Independently verify and validate those perceptions and dissatisfactions and confirm that change is necessary
- Examine the various possible changes and choose the ideal one
- Define the IDEAL: What are the components? What space, equipment, personnel, supplies, etc., are needed to achieve the IDEAL?
- What are the implications and consequences of the IDEAL (on the laboratories personnel, on other programs and activities)? What are the costs? Where are the areas of resistance to change? Could the IDEAL be modified and still achieve the desired result?
- How can IDEAL be implemented? Who will communicate and direct the changes? What needs to be done? What is the time frame?
- How will the change be evaluated? By whom? According to what standards? Over what period of time?
- Note: to minimize the need to change, there should be an ongoing, monthly evaluation of operations; this is the equivalent of QC in chemistry - it is QC of the management of the laboratory; it is the sine qua non of operational success and needs to be incorporated into the monthly staff meeting with review of marketing, finance, space and equipment, human resources, productivity, efficiency, and bottom line (achievement of goals)
- How to Change People
- In order to change a laboratory, people must change - very difficult
- Any suggestion or request to change implies that what is currently being done is inadequate or "bad"
- People generally think of themselves as "whole" or 100%
- Change takes a hunk out of that self perception, perhaps 20%
- Leadership must fill in the gap with 20% of something new
- Resistance to change can be reduced by:
- Leadership support
- Getting everyone involved in decision making
- Sell change as decreasing difficulties
- Sell change as increasing opportunities and experience
- Other
- Obsolete: TQM, CQI, Quality Circles, Benchmarking
- Currently in Vogue: PDSA, DMAIC, Lean, Six Sigma and RCA
- PDSA is used by Systems Engineers "Plan-Do-Study-Act"
- DMAIC is "Define, Measure, Analyze, Improve, Control"
- Lean was first implemented at Toyota Motors in Japan as a means for creating more value for customers by eliminating waste; the goal is to create processes that need less human effort, less space, less capital, and less time to make products and services at far less costs and with fewer defects; Lean empowers employees to improve the processes
- Six Sigma is a business management strategy, initially implemented at Motorola, that seeks to improve quality by identifying and removing the causes of defects (errors) and variability in manufacturing and business processes (six sigma refers to six standard deviations or 3.4 errors/million)
- Root Cause Analysis (RCA) is a problem solving method which identifies the root causes of problems or events; the practice of RCA is predicated on the belief that problems are best solved by attempting to correct or eliminate multiple root causes, as opposed to merely addressing the immediately obvious symptoms; there is usually more than one potential contributing factor that causes any given problem
- References: Gaithersburg: Medical Laboratory Management: Forms, Checklists & Guidelines
- Consultation service in which the pathologist meets with patient to explain diagnosis and pathology report
- Does not include discussions regarding therapeutic treatment options or decisions
- Up to 30% of surgeons misunderstand their patients' pathology reports (Arch Pathol Lab Med 2000;124:1040)
- Patients that were invited to view their doctors' visit notes through a fully transparent electronic medical record reported improved understanding of health information, enhanced relationships with health care providers and improved self care (therapy compliance and empowerment) (BMJ Open 2016;6:e010034)
- Personalized, focused clinical encounters with radiologists improves patient satisfaction with the health care system (ACR: Patient Engagement [Accessed 7 February 2023])
- Pathologist led consultations could enhance the accurate communication of pathology reports and results to patients, while offloading this task from clinical colleagues
- Anecdotal evidence suggests patient centered pathology consultations have the potential to increase treatment adherence (Arch Pathol Lab Med 2019;143:852)
- Looking at tumor cells or diseased tissue can enable patients that wish to use guided imagery in their treatment strategy
- Randomized controlled trials in cohorts of breast and prostate cancer patients revealed that beneficial immunomodulatory effects can arise from relaxation training and guided imagery, including upregulation of natural killer cells and lymphokine activated killer cells (Breast 2009;18:17, Psychosom Med 2011;73:218)
- Many patients do not understand the vital role pathologists play in their health care
- Patient centered pathology is a means of cultivating relationships with patients, fellow clinicians, health system leaders and lawmakers
- Patient testimonials are powerful political activism tools that can highlight the positive effects that pathologists have on personalized care (Arch Pathol Lab Med 2015;139:441, CAP Today: 50 Ways to Leave the Basement [Accessed 7 February 2023])
- References: PathPod Podcast: Beyond The Scope - Patient Consultation with Drs. Lija Joseph, Thomas J. Cummings, and Rachel Jug [Accessed 7 February 2023], CAPcast: Pathologist-Patient Consults - Insight into a Unique Care Model [Accessed 7 February 2023], Am J Clin Pathol 2021;155:887, Am J Clin Pathol 2021;156:939
- Establishment and patient recruitment
- Interest and feasibility for a patient pathology consult program needs to be evaluated by hospital administration, risk management, marketing and practice managers
- Gaining support from pathologists and clinical colleagues is critical for long term success
- Current consultation services have distributed flyers institutionally in the clinic, hospital and cancer center to promote their program
- Posting information about the program on the institution website has also been well received
- In addition, the program can be promoted at venues, such as tumor board, cancer committee and medical executive committee meetings
- The patient, oncologist or surgeon can initiate the consultation process by contacting the pathology department or the pathologist can insert an invitation in the pathology report or send a text message in the electronic medical record; this allows for an appointment to be made like any other doctor's visit
- Example of text message in electronic medical record from Thomas J. Cummings, M.D., at Duke University Medical Center: "Pathologists are physicians who are part of your health care team and who specialize in diagnosing disease by examining your cells and / or tissues under a microscope. If you would like to see your pathology under the microscope, please send me a message in Duke MyChart and I will arrange this with you."
- Interest and feasibility for a patient pathology consult program needs to be evaluated by hospital administration, risk management, marketing and practice managers
- Preconsultation
- Staff uses a standard script to schedule the patient's appointment, with a preference given to times closest to existing appointments
- Preparation time is ~15 minutes, which includes gathering slides, reports and consent forms
- Additional care is taken to coordinate a HIPPA compliant space for the consult to take place (i.e., pathologist's office or conference room) that contains a computer or multiheaded microscope
- Pathologist spends ~10 minutes for slide and chart review prior to the appointment
- When the patient arrives, routine clinic check in procedures are followed, including confirmation of the patient's identity and consent form signatures
- Importantly, the slides and pathology report are considered the patient's medical record
- If the patient is accompanied by a family member, they are required to provide identification and the patient is required to provide consent for the family member to be in the room
- Staff uses a standard script to schedule the patient's appointment, with a preference given to times closest to existing appointments
- During consultation
- Agenda for the appointment is set by inquiring if the patient has any specific questions and establishing the patient's level of understanding of the disease
- Pathologist then shows the patient their name on the slide and briefly describes the tissue processing procedure, including slide preparation
- Patient is shown normal tissue in order to identify significant structures using basic terminology (with care to avoid unnecessary medical jargon); for example, "The nucleus is the brain of the cell and the cytoplasm is the body of the cell"
- Once a foundation of normal is established, the patient is shown diseased tissue, which often leads to additional questions
- Pathologist defers specific questions regarding treatment to the referring physician
- Appointment lasts ~30 minutes
- Patient is free to take notes; however, no audio or video recording is permitted
- At the conclusion, the patient is offered a copy of their pathology report and asked if they have any further questions
- Following this, the patient is escorted to the hospital exit
- Signed consent forms are filed in accordance with hospital protocol
- Pathologist may give the patient a business card and hospital administration contact information, should the patient choose to write a letter regarding the experience
- Thank you card is sent to the patient for choosing to obtain his or her care at the hospital (Arch Pathol Lab Med 2019;143:852)
- Postconsultation
- Issue addendum to the pathology report or clinical note documenting the visit stating that slides were reviewed with the patient, including date / time / duration and send an email to the referring clinician
- Patient pathologist consultation service meets the criteria for time based evaluation and management (E / M) services when counseling dominates the encounter
- Evaluation and management CPT codes such as 99203 (~$105 per Medicare 2019 billing) may be applied pending institutional approval (Clin Colon Rectal Surg 2005;18:279)
- Counseling in this setting would be discussion of diagnostic results with patient and patient / family education
- University of Michigan offers face to face meetings between pathologists, patients and family members (The Dark Report: University of Michigan Pathologists Bet on Patient-Centered Care [Accessed 7 February 2023])
- At Lowell General Hospital (Lowell, MA), pathologist Lija Joseph, M.D., has launched this program since 2017 with over 150 patients utilizing this program so far (CAP Today: New Pathology Patient Consult Program Takes Off [Accessed 7 February 2023], WCVB: "See the Dragon that I'm Slaying:" Hospital Opens Lab to Cancer Patients [Accessed 7 February 2023], CAP: Dr. Lija Joseph [Accessed 7 February 2023])
- Contacting competitors to see what they are doing
- Contacting personnel of competitors to see how they are doing it
- Reading laboratory marketing textbooks and journal articles
- Reviewing market research documents that are commercially available
- Sending a questionnaire to active or potential customers (patients, clinicians and hospital administrative staff)
Comment Here
Reference: Patient centered pathology
- CPT coding for consultation does not apply to pathologists
- Evaluation and management code exists and pathologists can be reimbursed through a time based qualifier
- Evaluation and management code exists but requires consultants to counsel on therapeutic options
- They are correct and cannot be reimbursed
Comment Here
Reference: Patient centered pathology
- Per WHO: “protection of patients by preventing and reducing errors and adverse effects during health care provision” (WHO: Patient Safety [Accessed 27 April 2022])
- Promoting a just culture; preventing / reducing pre-analytic, analytic and postanalytic errors; using tools and technology to evaluate and improve patient safety
- Building a highly reliable team in the laboratory; encouraging laboratory staff and healthcare professionals to prioritize patient safety, learn from errors and prevent them in the future
- It is imperative to deliver quality health care, which is safe, effective, timely and people centered, to patients
- Prevention of errors and adverse effects
- Promoting a safe and just culture
- Classifying and monitoring errors in the laboratory, in addition to proper error reporting and follow up
- Addressing human factors to enhance patient safety in the laboratory
- Building high reliability teams in the laboratory
- Using automation and technology to prevent human error
- Promoting fail safe communication practices
- Patient safety culture in the laboratory: preventing errors and reducing adverse effects in the laboratory in order to improve quality and patient safety
- Just culture: foundation for promoting a laboratory culture of patient safety
- Just culture is a learning culture where an individual is not blamed for an error or adverse event; instead, the whole system is analyzed and errors are perceived as an opportunity to learn from the mistake and improve
- Patient safety initiatives: include a patient centered care system, increase accountability, create a just culture, evaluate system failures and focus on proper communication and teamwork
- References: Arch Pathol Lab Med 2005;129:1252, Ergonomics 2015;58:33, Nakhleh: Error Reduction and Prevention in Surgical Pathology, 2nd Edition, 2019
- Spaghetti diagrams or workflow charts can be created in order to maximize the utility and efficiency of work space (see Lean workflow)
- Root cause analysis (RCA): problem → ask why (and then why again and again and again) → root cause → corrective action
Images hosted on other servers:
Workflow examples
- Developing a patient safety focused culture:
- Taking proper initiatives to prevent errors and adverse effects
- Classifying errors in order to monitor and prevent them
- Reporting errors in an appropriate and timely fashion
- Taking appropriate actions; for instance, performing a root cause analysis (RCA) or implementing a Plan, Do, Study, Act (PDSA) or failure mode and effects analysis (FMEA) to address and prevent those mistakes in the future
- RCA is a process of identifying the root cause of an error or an adverse event in order to discover potential solutions and prevent the same error from happening in the future; RCA tends to be a systematic approach where the processes are examined and analyzed and then proper techniques and protocols are implemented for future patient care
- PDSA is a process of testing a change that was implemented and analyzing its efficacy in order to improve and repeat the process again, if needed
- FMEA is a process where all the possible components of system failure are identified in order to remove the causes behind them; FMEA can be both qualitative and quantitative and is intended to identify areas that need improvement and change
- Ideal for preimplementation evaluation
- References: Health Serv Res 2006;41:1690, JAMA 2005;293:1359, Sesok-Pizzini: Patient Safety in Anatomic and Clinical Pathology Laboratories, 1st Edition, 2017
- Laboratory errors are divided into pre-analytic, analytic and postanalytic phases, depending on the phase during which the error / adverse event occurs:
- Pre-analytic errors in anatomic pathology (Arch Pathol Lab Med 2013;137:1798, Am J Clin Pathol 2006;126:833):
- Errors in specimen identification (which may be related to unintended workaround of barcoded tracking system), patient misidentification and specimen mix up
- Wrong / unreliable clinical information
- Delayed requisitions
- Amended reports in the electronic medical record by physicians / clinicians
- Analytic errors in anatomic pathology (Arch Pathol Lab Med 2008;132:181):
- Mistakes in gross examination of specimens
- Inappropriate dissection and sectioning of the specimen
- Problems in histologic processing and staining of slides
- Mistakes in interpretation by pathologists under the microscope
- Tissue contamination
- Failure to obtain expert opinion when needed
- Postanalytic errors in anatomic pathology:
- Incomplete reports / mistakes in reports, wrong diagnosis, misdirected reports, failure to report critical values
- Pre-analytic errors in clinical pathology (Clin Chim Acta 2009;404:28):
- Wrong physician orders (inappropriate selection of test, duplicate order, wrong test ordered)
- Mistakes in patient preparation sites
- Mislabeled specimens, inaccurate wrist bands, specimens collected from site of IV infusion
- Certain patient conditions that affect specimen quality (hemolysis, icterus, lipemia, clotting, etc.)
- Requisition not matching the label
- Specimen collected in the wrong tube
- Specimen contamination (e.g., skin flora in blood culture)
- Inappropriate specimen transport medium and temperature
- Analytic errors in clinical pathology (Clin Chem 2000;46:746):
- Instrument malfunction
- Reagent carryover, especially in random access instruments that use dedicated probes
- Instrument validation errors and quality control failure
- Random errors caused by the instrument
- Frozen specimen thawing and coagulation errors
- Postanalytic errors in clinical pathology (Arch Pathol Lab Med 2005;129:1252):
- Errors in reporting of results
- Wrong data entered into the system
- Results reported to wrong provider or delayed results
- Incorrect reference ranges
- Delay in receiving results from the clinical teams
- Data integrity failures in the electronic health record
- Monitoring errors in clinical and anatomic pathology (Arch Pathol Lab Med 2005;129:1252):
- Pneumatic tube systems need to be regularly checked and validated
- Proper identification, accurate accessioning of the specimen and using high quality barcoded labels before sending specimens to the automated machine
- Certain analytic errors can be easily avoided by preventing reagent carryover, specimen carryover, proper calibration and verification of the instruments and appropriate quality control
- There should be a backup plan for reporting critical values, etc., in case the system fails (downtime procedure)
- Reference ranges need to be periodically monitored and updated
- Lower limit of detection for certain molecular assays needs to be reviewed regularly, for accuracy
- There should be accountability at both ends (the laboratory and the clinical team)
- Ordering physician should be vigilant enough to follow unusual trends (e.g., gradually increasing cell counts and critical values, etc.)
- Point of care testing should be carefully implemented in critically ill patients, as this patient population often requires urgent laboratory support and can experience wide fluctuations in blood levels of different gases and electrolytes
- Pre-analytic errors in anatomic pathology (Arch Pathol Lab Med 2013;137:1798, Am J Clin Pathol 2006;126:833):
- A quality indicator portfolio has been offered by College of American Pathologists (CAP), which is called the Q probes program, including the Q track program
- Created in 1999 as a subscription service for quality monitoring; the main objectives were the establishment of quality benchmarks, monitoring performance over time and identification of practices associated with better performance
- References: Arch Pathol Lab Med 2014;138:1003, Arch Pathol Lab Med 2014;138:1139
- Identification of risks and implementation of certain interventions to prevent and decrease those risks
- Medical errors should be disclosed directly to the patient, in order to gain patient trust and avoid lawsuits
- Information should be compiled from resources, such as incident reports, chart reviews and patient complaints, in order to have a comprehensive overview of errors
- Reporting should be followed by an RCA to investigate the incident, define risks and prevent the same incident from happening in the future
- Local system hazards should be identified and worked on as well
- References: Arch Pathol Lab Med 1998;122:231, JAMA 2003;289:1001, JAMA 2005;293:1359
- Transparency and a team approach should be encouraged whenever an error occurs
- Just culture promotes the concept of holding the system (instead of an individual) responsible for an incident and emphasizes the importance of evaluating system design as opposed to blaming an employee
- Addressing stress, fatigue, call schedule, communication, handoffs and transitions in the laboratory staff and healthcare professionals is extremely important to avoid blunders that are due to burnout or lack of communication
- Effective communication, written documentation and proper hand off between pathology and clinical teams, during daytime and on call
- Implementation of read back culture after each result is called out (frozen section results, critical values and during patient handoffs)
- Phonetic spelling and clarification of questions whenever a result is called out
- Implementation and incorporation of a patient safety curriculum for residents / fellows
- Patient safety education should be mandatory for accreditation purposes before resident physicians enter independent practice
- Quality assurance (QA) and quality improvement (QI) activities should be incorporated in resident and fellow curriculum, to minimize the chances of human error by increasing awareness and understanding the importance of lab safety culture
- References: Health Serv Res 2006;41:1690, Acad Pathol 2021;8:2374289521998046
- Rigorous staff orientation / training / competency assessment programs to elevate skills of laboratory staff
- Use customized charts and graphs in the work area
- Use robotics for hazardous and repetitive activities to minimize human error
- Deploy automation and information technology, such as laboratory information system (LIS), to the individual benches and workstations for laboratory personnel
- Lab automation decreases the risk of human errors, at the cost of its own errors and shortcomings
- Flagging of critical values / results by automated systems
- Computerized provider order entry (CPOE) improves order entry lab orders and avoids downstream human errors at multiple levels
- Many laboratory functions such as quality control, inventory, data verification and reporting can be automated and help to decrease the workload and fatigue among the laboratory staff
- Lab automation increases reliability by standardizing processes
- References: Clin Leadersh Manag Rev 2007;21:E3, Ergonomics 2015;58:33
Root cause analysis (RCA)
Plan, Do, Study, Act (PDSA) cycle
Failure mode and effects analysis (FMEA)
Root cause analysis techniques
- Assign a lab safety officer that is responsible for all things safety in the lab
- Collect data on quality practices without ever reporting or reviewing the collected data
- Focus error reduction efforts on all the pre-analytic problems identified by clinical colleagues
- Quickly identify the individual responsible for an error or defect and release or retrain them
- Seek to learn from errors or mishaps through efforts such as open reporting, root cause analysis and failure mode effects analyses, so that errors are not repeated
Comment Here
Reference: Patient safety
- All patients with prostate biopsies accessioned in your lab on that day (pre-analytic within the lab)
- All prostate biopsies dictated by the GU pathologist the day patient J was reported (analytic)
- All prostate biopsy reports distributed the day patient J was reported (postanalytic)
- All prostate biopsy reports sent to the same clinic the day patient J was reported (postanalytic, outside the lab)
- Patients seen in the same clinic as Patient J on the day of his biopsies (pre-analytic outside the lab)
Comment Here
Reference: Patient safety
- Every year, 1.6 million Americans are diagnosed with cancer (CA Cancer J Clin 2010;60:139)
- Getting the diagnosis right the first time is critical to designing the appropriate treatment plan and therapy
- Pathology and radiology play significant roles in the diagnostic process
- When things go wrong, 46% of the error in diagnosis comes from pathology and radiology (Arch Intern Med 2009;169:1881), while 97% of the cancer diagnosis is based on the pathology specimen (Silverman: The Anatomic Pathologist's Role in Error Reduction and Patient Safety, 2011)
- Over the past 20 years, multiple studies have shown that the rate of major discrepancies identified for cancer patients referred to another institution ranges from 0.6 to 15 - 18%; yet published data indicates the current intralab quality assurance (QA) ability to detect these discrepancies is only 0.8 - 1.7% (CA Cancer J Clin 2010;60:139, Patient Safety & Quality Healthcare: Quality, Assurance, Diagnosis, Treatment and Patient Care [Accessed 17 November 2017])
- This clearly identifies a gap in our current quality practice and an opportunity to improve quality assurance initiatives
- This topic reviews the formal quality assurance (QA) programs focused on peer or case review and how they can be utilized to provide continuous improvement guidance for your laboratory
- For most laboratories, the quality strategy is made up of multiple QA / QC programs that best fit the institution's patient mix, staff experience and specialty status
- QA programs can be:
- Formal: those that are scheduled, (volume and time) predictable and under your control
- Informal: having programs that apply as QA but do not have a formal schedule, frequency or under your control (table 1)
- For this discussion we will focus on the formal QA programs, although the informal programs can offer a wealth of quality information and should be tracked and documented as part of your overall quality program
- In a CAP Q-Probe (Arch Pathol Lab Med 2014;138:602) with 73 labs responding, of those reporting (56), 45% of the laboratories reported using post (retrospective) sign out case review as the means to help detect defects, followed by don't know 29%, clinician request 21% and tumor conference of 5%; table 2 breaks down the current formal QA programs
| Formal quality assurance programs | Informal quality assurance programs |
|---|---|
| Retrospective case review | Autopsy |
| Proficiency testing | Diagnostic consult |
| Prospective case review | Patient referral |
| Characteristic | Proficiency testing | Internal case review (retrospective) | Internal case review (prospective) | External peer case review by subspecialist (retrospective) |
|---|---|---|---|---|
| Adds to the pathologist workload | ✔ | ✔ | ✔ | |
| Peer reviewed | ✔ | ? | ? | ✔ |
| Standardized | ✔ | ✔ | ||
| False negative & positive cases | ✔ | ✔ | ||
| QA total process | ✔ | ✔ | ✔ | |
| Benchmarking | ✔ | ✔ | ||
| Ability to influence the diagnosis in real / near real time | ✔ | |||
| Key positive feature(s) | Established minimum quality tool | Most common QA practice | Real time | External subspecialist review, does not use pathologist time |
| Negative consideration | Does not QA the full case detail from gross to report | Demanding on pathologist and technologist time | Most demanding on pathologist and technologist time | Program needs to be double blinded for confidentiality |
| Best demonstrated practice | CAP and ASCP proficiency programs | ADASP guidelines on QC & QA in AP quality assurance | UPMC | QualityStar™ external QA case review by subspecialist |
- This compares a laboratory's test results using unknown specimens (usually digital images) to results from other laboratories
- It is the most established QA program and should be considered the minimum requirement for AP laboratory quality assurance
- Clinical feedback and reference to subspecialists are provided and standardization allows for national benchmarking capabilities
- PT programs from CAP, ASCP and others are approved by the American Board of Pathology and meet level IV requirements for Maintenance of Certification (MOC) (see American Board of Pathology website for a complete listing of PT programs that are level IV compliant)
- Drawbacks: adds to pathologist workload, does not offer full case review from gross to clinical report and is not representative of pathologist caseload
- Random selection of 1 - 10% of cases or more, for secondary QA case review also referred to as peer review
- This is the most common practice today for QA case review, allows for complete case review and represents the pathologist's workload
- However, it is also subjected to onsite biases and personnel conflicts
- It is not standardized, so benchmarking is difficult between institutions and it adds to the pathologist's workload
- Most laboratories also lack true peer review from specialists and subspecialist in all tissue types
- Case reviews like above but performed prior to sign out in real time to allow findings to influence the final diagnosis and add additional comments that may contribute to enhanced patient care
- Elegant example was presented by University of Pittsburgh Medical Center UPMC, Professional QA / QC Surgical Pathology Program - Yousem 2010
- Presentation demonstrated similar error rates pre and postsignout with no effect on case turnaround time
- Program does require a significant depth of pathology, software (AP / LIS) and development support that is not found in most AP laboratories
- As the program is not standardized, it is difficult to receive the benefits of benchmarking with similar programs nationally
- This is a new AP / QA program that is built around case review outside the institution (interlab) as a new level of granularity with error detection of 1 to 45% (Patient Safety & Quality Healthcare: Quality, Assurance, Diagnosis, Treatment and Patient Care [Accessed 17 November 2017])
- It offers a significant enhancement in the ability to provide quality feedback for guidance and continuous improvement
- Two characteristics stand out when comparing the sensitivity of error detection between intra and interlaboratory case review:
- Difference in the ability to gain incremental case scrutiny by using subspecialists for review (when compared to using generalist pathologists)
- Difference in moving the review outside the institution to reduce onsite bias and feedback confrontation
- It is very difficult for a general pathologist to stay current in all organ systems and cancer types
- As with all disciplines, frequency of interactions builds confidence and skills and helps keep practitioners current with evolving diagnostic tools such as molecular assays and immunohistochemical stains
- With the current scarcity of pathologists and expected continued demand for pathology services resulting from an aging population, having subspecialists onsite is rare in the average hospital setting (3 - 4 pathologists) and having multiple subspecialists to provide quality assurance peer review is extremely rare
- Cases can be submitted via glass slides or digital images (cases are de-identified prior to submission and cases with digital images are uploaded to a secure cloud)
- Academic medical centers provide blinded subspecialist case review
- Benefit is a standardized program that allows benchmarking at an increased level of granularity without adding to pathologist's workload
- Program is also ABP approved for MOC level IV
- However it does require additional efforts to blind each case prior to submission and uploading of multiple WSI images takes time and may need to be coordinated within the lab
- As professionals in the healthcare system, we realize that focusing on quality is imperative
- For the first time in 2014, the National Patient Safety Foundation placed "diagnostic errors" as a top patient safety challenge
- That ranks it with initiatives such as healthcare acquired infections and readmissions, proving that additional focus on this area will build over the coming months and years
- Case (peer) review is just one component of a good quality program but it plays a major role in continuous improvement and is the closest to patient outcomes
- When thinking about the programs reviewed in this article, each have a contribution to your quality initiatives and goals
- It is not about choosing which one to implement but more about mastering one and moving to the next
- Goal is to build your quality tool set to close the gap on diagnostic errors in anatomic pathology
- Quality improvement (QI) is the framework to systematically improve patient care (Agency for Healthcare Research and Quality: Approaches to Quality Improvement [Accessed 11 September 2020])
- Multiple quality improvement models and tools are available but the one that you can remember and use is the best one
- Quality assurance ≠ quality improvement (Agency for Healthcare Research and Quality: Table 4.1. Quality Assurance vs. Quality Improvement [Accessed 6 October 2020]
- Principles of quality improvement:
- Good intentions are not good enough
- Set worthwhile goals
- Understand processes and systems
- Go for the high yield
- It doesn't count unless you can measure it
- Your job is never done
- EPIDEM
- Definition: EPIDEMic of healthcare professionals improving patient care
- Exploration, promotion, implementation, documentation, evaluation, modification
- Other quality improvement terms to be familiar with
- Total quality management (TQM): organizational / management approach
- Root cause analysis (RCA): tool used in engineering and required for healthcare sentinel events
- Failure modes and effects analysis (FMEA): used by U.S. military and NASA
- Health failure modes and effects analysis (HFMEA): used by VA
- A3 template: named after the 11 x 17 inch sheet of paper that can be used to lay out the various elements of process improvement (see Diagram 1)
- Kaizen: continuous process improvement
- Gemba: create value
- Poka yoke: mistake proof
- 5 S: sorting, set in order, shine, standardize, sustain
- DMADV: define, measure, analyze, design, verify
- 6 domains of healthcare quality according to the Institute of Medicine (Institute of Medicine: Crossing the Quality Chasm, 2001)
- Safe
- Effective
- Patient centered
- Timely
- Efficient
- Equitable
- Total Quality Management (TQM) (BMC Exchange: What is TQM? Total Quality Management Explained [Accessed 6 October 2020], ASQ: What is Total Quality Management (TQM)? [Accessed 6 October 2020])
- Management approach focused on improving quality and customer service
- Includes 8 key principles
- Customer focused
- Employee ownership
- Process based
- System integration
- Strategic and systematic approach
- Continuous improvement
- Data driven
- Communication
- Model for improvement: used by Institute for Healthcare Improvement (IHI) (Agency for Healthcare Research and Quality: Approaches to Quality Improvement [Accessed 11 September 2020])
- Combines TQM + rapid cycle improvement (RCI)
- Aim, measures, changes
- RCI: repetitive Plan, Do, Study, Act (PDSA) cycles (see Diagram 2)
- Combines TQM + rapid cycle improvement (RCI)
- Lean Six Sigma: increasingly used in clinical labaoratory
- Combines Lean + Six Sigma (ASQ: What is Six Sigma? [Accessed 11 September 2020])
- Lean: management philosophy on reducing waste
- 8 sources of waste (DOWNTIME)
- Defects
- Overproduction
- Waiting
- Neglected talents
- Transporting
- Inventory
- Motion
- Excess processing
- Lean in clinical laboratory: evaluate workflow and eliminate steps that don't add value
- Six Sigma: statistical control
- Problem solving method to eliminate defects due to process variation
- Sigma describes variability (defects per unit)
- Six Sigma: 99.99966% effective, with only 3.4 defects per million opportunities
- DMAIC: Define, Measure, Analyze, Improve, Control
- Six Sigma in clinical laboratory: focus on error reduction and eliminating variation
- EPIDEM: exploration, promotion, implementation, documentation, evaluation, modification (Lab Med 2019;50:e9)
- Goal = EPIDEMic of healthcare providers working to improve patient care
- 6 principles of quality improvement
- Easy way to remember the steps of quality improvement
- Compatible with other models and tools
- Good intentions are not good enough
- Understand culture, context and resources
- Be self aware and mindful of your surroundings
- Set worthwhile goals
- Establish meaningful goals and measures
- Make sure your goals actually improve patient care in a meaningful way
- Understand processes and systems
- Evaluate systems and simplify processes
- Apply substitution test: would anybody else make a similar mistake?
- Go for the high yield
- Prioritize problem players and interventions
- 20% of the people / mistakes account for 80% of the problems (Pareto principle)
- It doesn't count unless you can measure it
- Measure at baseline and with any changes
- Collect data and document numbers
- Your job is never done
- Adopt a culture of continuous improvement
- Apply lessons learned to other situations
- Reference: Lab Med 2019;50:e9
- Exploration
- Clarifying problem, scope of problem and potential solution
- Understanding local culture, context, resources
- Benchmarking against peers
- Promotion
- Developing a strategy to fix the problem
- Establishing shared goals and QI team
- Promoting to stakeholders and leaders at your institution
- Identifying events and opportunities to fix problem
- Implementation
- Mistake proof (poka yoke) processes
- Performing pilot studies
- Optimizing timing of intervention
- Documentation
- Document at baseline and throughout the process
- Save written correspondence, meeting minutes, important dates
- Document success, challenges and impact
- Evaluation
- Evaluate measures and quantify them
- Solicit feedback
- Modification
- Review for workarounds and outliers
- Seek to expand or improve intervention further
- Reference: Lab Med 2019;50:e9
- Reducing BCR-ABL ordering errors (Am J Clin Pathol 2019;151:68)
- Exploration: ordering errors increased steadily from 2011 - 2015 in molecular pathology laboratory
- Promotion: confusion regarding naming of BCR-ABL tests was problematic; obtained buy-in from oncology and hemepath re: new workflow
- Implementation: validated and implemented a BCR-ABL qualitative assay that reflexes to quantitative major, went live in electronic medical record
- Documentation: documented errors before and after intervention
- Evaluation: intervention resulted in a sharp reduction in ordering errors, especially for BCR-ABL minor (acute lymphoblastic leukemia) patients
- Modification: continue to improve electronic medical record ordering to decrease incorrect orders
- Improving molecular testing workflow for surgical pathology specimens (Mod Pathol 2020;33:2025, Mod Pathol 2018;31:764)
- Exploration: surgical pathologist marked areas appeared suboptimal
- Promotion: promoted a research study and educated department residents / faculty on preanalytics of molecular testing (i.e. tumor cellularity)
- Implementation: prior to implementing new workflow, conducted a research study which helped garner buy-in
- Documentation: documented tumor cellularity assessments of surgical pathologists versus molecular pathologists
- Evaluation: compared accuracy of the tumor estimates; measured provider satisfaction and turnaround time
- Modifications: eliminated step of requiring surgical pathologists to review slides
- Improving pregnancy screening and use of urine pregnancy test (J Pediatr Adolesc Gynecol 2020 Jun 11 [Epub ahead of print])
- Exploration: no standardized pregnancy risk assessment in family planning teen clinic
- Promotion: worked with stakeholders to develop new protocol
- Implement: used feedback to train and develop changes
- Document: recorded feedback, documented changes to job aid
- Evaluate: percent uptake increased and urine pregnancy tests decreased
- Modifications: changed design of job aid, modified checklist and adjusted process throughout
- Institute for Healthcare Improvement (IHI) has online courses free for individual students, residents and faculty as well as members of the least developed countries (by the United Nations) (Institute for Healthcare Improvement: IHI Open School Online Courses [Accessed 11 September 2020])
- We often default to the idea that individuals lack personal motivation to change but author and social scientist Joseph Grenny describes 6 sources of influence that need to be addressed to make change inevitable; the key sources are classified along the dimensions of motivation versus ability and personal versus social versus structural (Grenny: Influencer - The New Science of Leading Change, 2nd Edition, 2013)
- Ask your fellow to manually review every send out test above $1,000
- Gather data from the last 6 months of send out tests to clarify the issue
- Meet with the clinicians and educate them about these unnecessary orders
- Work with the information technology staff to eliminate the ability to order these tests through the electronic medical record
Comment Here
Reference: Principles of laboratory quality improvement
- Exploration
- Promotion
- Implementation
- Evaluation
- Cytopathology is an anatomic pathology specialty that is highly regulated; the quality assurance (QA) program in cytopathology is mandated by CLIA '88 and implemented and constantly updated by the College of American Pathologists (CAP) (CMS: Clinical Laboratory Improvement Amendments (CLIA) [Accessed 7 September 2023])
- Such QA measures must be systematically implemented for all test phases (preanalytic, analytic, postanalytic)
- Preanalytical QA measures include review of stain quality, cytologic preparation technical quality and cross contamination checks, among others (CYP.04300 and CYP.04150, respectively)
- Analytical QA measures include prospective 10% QA rescreening (CYP.07478)
- Postanalytical QA measures include retrospective review of all negative gynecologic Paps from the index patient when a new high grade dysplasia is detected (CYP.07517), surgical pathology correlation of nongynecologic cases (CYP.07675) and all gynecologic cases diagnosed as high grade dysplasia (CYP.07543)
- Reference: CAP: Accreditation Checklists [Accessed 7 September 2023]
- Cytopathology, especially gynecologic cytology, is considered a prototype screening test; therefore, rigorous regulations revolve around this specialty to reduce (if not eliminate) significant false negative test results, which can have a momentous impact on patient safety and the laboratory's credibility
- There must be a multilayered approach to QA, resulting in a robust quality management plan that conforms to CLIA '88 / CAP laboratory accreditation program
- Therefore, the QA program in cytopathology must integrate prospective, retrospective and correlational QA in gynecologic cytology, retrospective and correlational QA in nongynecologic cytology and fine needle aspiration (FNA) cytology (Cancer Cytopathol 2014;122:3)
- Other approaches such as rapid random rescreening and targeted rescreening (negative Pap test with positive human papillomavirus [HPV] cotesting and including certain high risk cases into the 10% prospective rescreening pool) can be used as additional measures to enhance the QA program (Roum Arch Microbiol Immunol 2013;72:93)
- Creative adaptation of Six Sigma principles such as root cause analysis (RCA), define, measure, analyze, improve, control (DMAIC) and define, measure, analyze, design, verify (DMADV) will offer unique advantage to the laboratory in optimizing workflow, efficiency or accuracy
- Quality assurance: the process of ensuring quality
- Quality control: the way the laboratories assure that their results are valid and accurate by evaluating the quality (Clin Lab Med 1983;3:541)
- Quality improvement: improving patient care, system performance, outcomes and cost by systematic change methods and strategies
- CLIA '88: Clinical Laboratory Improvement Amendments of 1988
- Prospective QA: a minimum of 10% rescreening of all slides with negative gynecologic Pap test diagnosis get rescreened before the reports are released
- Retrospective QA: rescreening of all prior negative gynecologic Pap tests from the past 5 years on an index patient when a new high grade dysplasia is detected
- Correlational QA: surgical pathology correlation of all high grade dysplasia in gynecologic cytology, surgical pathology correlation of nongynecologic and FNA cytology cases
- Rapid random rescreening: rescreening of negative Pap tests for a limited duration and at low magnification (Cancer Cytopathol 2011;119:357)
- Targeted rescreening: e.g., rescreening of cases with negative Pap test with positive HPV cotesting
- Cytohistologic correlation (CHC): correlation of cytology diagnoses with review of follow up or concurrent surgical pathology
- Root cause analysis (RCA): a standardized approach to understanding the causes of an adverse event or identifying system flaws for correction
- DMAIC (define, measure, analyze, improve, control): refers to a data driven improvement cycle used for improving, optimizing and stabilizing business processes and designs
- DMADV (define, measure, analyze, design, verify): a Six Sigma framework that focuses primarily on the development of a new service, product or process as opposed to improving a previously existing one
- Six Sigma: a management strategy to improve quality of business processes, which can be applied in the laboratories (Clin Chem Lab Med 2007;45:789)
- Ongoing QA programs facilitate monitoring the quality metrics and benchmark standards mandated by CLIA '88 and assure quality (CMS: Clinical Laboratory Improvement Amendments (CLIA) [Accessed 7 September 2023])
- Familiarity with the robust QA and quality management program of the cytology laboratory enhances knowledge of the pathologist to be an efficient medical director and prudent CAP inspector for laboratory accreditation
- RCA is a valuable QA tool that identifies the steps that have led to a sentinel event, capture early trends and identify prevention strategies for process improvement by reducing variability in the process (Diagn Cytopathol 2021;49:633)
- This can be initiated by setting internal triggers based upon CAP benchmark standards or the voice of the customer
- Real life example
- One of the clinicians reached out to a cytopathology lab for the high number of unsatisfactory Pap smear diagnoses
- At the same time, that lab also noticed that the overall unsatisfactory rate doubled in that month
- That was a trigger to start RCA
- Fishbone diagram (see Diagram 1) shows the preanalytical, analytical and postanalytical factors to consider when investigating for the previous example
- Plan of action should be developed based on the findings
- Familiarity with Six Sigma principle (DMAIC) and creative adaptation offer innovative approaches to improve an existing process (DMAIC) in optimizing workflow, efficiency or accuracy
- Familiarity with Six Sigma principle (DMADV) aids in developing a new process and integration of new technology such as telecytology, automation and digital transformation
- Prospective QA (10% rescreening)
- CAP checklist, requirement CYP.07478: at least 10% of each cytotechnologist's gynecologic cases that have been interpreted to be negative are rescreened
- A minimum 10% rescreening of all slides with negative gynecologic Pap test diagnosis get rescreened by a qualified cytotechnologist before the cases are signed out (prospective rescreen) (CAP: Accreditation Checklists [Accessed 7 September 2023])
- If an epithelial cell abnormality (ECA) is detected, then the case is reflexed for pathologist's review
- This 10% rescreen error rate is an effective tool to monitor the quality on an ongoing basis
- Laboratory can utilize the 10% rescreen error rate towards cytotechnologist diagnostic accuracy calculation, especially when 2 step discrepancy is detected; the laboratory can also utilize as one of the triggers for targeted rapid screening, in the rare instance when a low grade dysplasia is detected upon 10% prospective rescreen (CAP: Accreditation Checklists [Accessed 7 September 2023])
- Retrospective QA (5 year retrospective review of cases diagnosed as high grade squamous intraepithelial lesion [HSIL] or higher)
- CAP checklist, requirement CYP.07517: all available (either onsite or in storage) previously negative slides received within the past 5 years are reviewed whenever a new HSIL (moderate or severe dysplasia, carcinoma in situ, cervical intraepithelial neoplasia [CIN] II or III) or malignant cervical / vaginal cytology is reported
- Any previously negative Pap smears from the patient need to be reviewed by the cytology supervisor or a qualified cytotechnologist
- CAP checklist, requirement CYP.07530: amended report needs to be issued if a discrepancy that could affect the current patient care is identified (CAP: Accreditation Checklists [Accessed 7 September 2023])
- Correlational QA (cytohistological correlation [CHC])
- CAP checklist, requirement CYP.07543: when the histologic diagnosis is available, correlation to the cytologic findings must be recorded and these records must be readily accessible; the number of cases that have histologic correlation must be recorded
- Review of follow up surgical pathology on all cases diagnosed as high grade dysplasia or higher by cytology
- Any discrepancy between cytology and surgical pathology is determined as cytology sampling, surgical pathology sampling, acceptable minor deviation in interpretation in both cytology and surgical pathology and major interpretation errors in both cytology and surgical pathology
- The major cause of discordant pairs in CHC remains sampling error (Arch Pathol Lab Med 2013;137:199)
- Reason for discordance gets documented in QA report and communicated with the clinician as appropriate
- CAP checklist, requirement CYP.07556: while looking for follow up surgical pathology on all cases diagnosed as high grade cytology, if no follow up surgical pathology is available, there should be records of attempts to obtain follow up histological information for correlative review (e.g., follow up correspondence, etc.) (CAP: Accreditation Checklists [Accessed 7 September 2023])
Atypical squamous cells (ASC)/squamous intraepithelial lesions (SIL) ratio
- ASC/SIL ratio is number / percentage of atypical squamous cells of uncertain significance (ASCUS) and atypical squamous cells - cannot exclude high grade dysplasia (ASC-H) diagnosed cases divided by the number / percentage of low (LSIL) and high (HSIL) grade squamous intraepithelial lesions and malignant cases (Am J Clin Pathol 2007;128:653)
- For example, if the percentage of ASCUS and ASC-H is 5% and the percentage of LSIL, HSIL and malignant cases is 3%, the ASC/SIL ratio will be 1.6
- Investigation needs to be initiated if the ASC/SIL ratio for gynecologic cases falls outside of the 5th or 95th percentile
- CAP checklist requirement CYP.07650: if the laboratory's annual ASC/SIL ratio for gynecologic cases falls outside of the 5th or 95th percentiles, the laboratory determines and records the reason(s) (CAP: Accreditation Checklists [Accessed 7 September 2023])
- See tables from cytopathology checklist CAP accreditation program (CAP: Accreditation Checklists [Accessed 7 September 2023])
External quality assurance
- Gynecologic Pap proficiency testing (PT) participation
- Laboratory and all individuals who examine gynecologic preparations participate in the CAP Gynecologic Cytology PT Program (PAP PT) or another proficiency testing program in gynecologic cytopathology approved by the Centers for Medicare and Medicaid Services (CMS) (CMS: Cytology Proficiency Testing [Accessed 7 September 2023])
- Annual proficiency testing is mandated for all cytotechnologists and pathologists who sign out Pap tests
- A score of < 90% is failure; an initial test failure requires reexamination (CAP TODAY: Cytopathology in Focus - Pap Proficiency Testing - What's Permitted, What's Not [Accessed 7 September 2023], CAP: Accreditation Checklists [Accessed 7 September 2023])
- Correlational QA in FNA and nongynecologic cytology (cytohistological correlation [CHC])
- CAP checklist, requirement CYP.07675: correlation of all or a subset of nongynecologic cytology specimens should be performed
- Methods of correlation should be recorded in the laboratory procedure manual and selected reports can be reviewed to confirm practice
- Possible mechanisms for correlation of histology include correlation of current specimens, focused review of specific specimen / organ types or follow up of suspicious / positive specimens, possible clinical correlation (CAP: Accreditation Checklists [Accessed 7 September 2023])
- Any discrepancy between cytology and surgical pathology is determined as cytology sampling, surgical pathology sampling, acceptable minor deviation in interpretation in both cytology and surgical pathology and major interpretation errors in both cytology and surgical pathology; the same documented in QA report and communicated with the clinician as appropriate
- Laboratory determines and records the reasons
- Monitoring of unsatisfactory diagnoses rate on a monthly basis to capture early trends, shifts and drifts, set triggers for when to start RCA
- Monitoring indeterminate diagnosis rate (e.g., atypical FNA diagnosis on thyroids, pancreas, etc.) (Cancer Cytopathol 2017;125:502)
- Monitoring of HPV negative ASCUS cases and set triggers for when to start RCA
- Automated image assisted screening failure rate to assess efficiency of image assisted screening and subsequent full manual review as an indirect measure for laboratory's productivity
- DMAIC (Rev Environ Health 2019;34:427)
- This approach (define, measure, analyze, improve, control) can be creatively adapted to improve existing processes in cytology laboratory and can be applied to process improvement (PI) projects
- Key phase in DMAIC methodology is the control phase, which needs to be strictly followed to ascertain consistency and keeping the PI project on track
- Control phase is a vital step once the PI project goes in to effect; in the healthcare and laboratory setting, the following steps will keep the QI plan on track
- Periodic monitoring of the plan
- Review of data for any shift or drift
- Regular in service to all involved parties
- Orienting new staff appropriately
- DMADV (Int J Health Care Qual Assur 2012;25:254)
- This approach (define, measure, analyze, design, verify) is especially useful when implementing new strategies and initiatives because of its basis in data, early identification of success and thorough analysis
- Define: define requirements for the project
- Measure: collect data and requirement for the new process that are critical to quality (CTQ)
- Analyze: identify bottlenecks or areas where problems are likely to happen
- Design: work towards finished product
- Verify: tie loose ends and transition the process to go live
- DMADV can be creatively adapted in the integration of new technology such as telecytology, automation and digital transformation
- RCA (StatPearls: Root Cause Analysis and Medical Error Prevention [Accessed 7 September 2023])
- It is one of the main components of the continuous improvement process
- It can be requested in response to an identified problem and may include 1 or more of the following: events that led to a significant or sentinel event, failure to achieve performance goals, variation in a process contributing to one of the above or identify prevention strategies for process improvement
- Facilitates identifying early trends, reducing variability in the process and promoting interdepartmental dialogue (Diagn Cytopathol 2021;49:633)
- See Advantages section for an example and Diagrams / tables for a fishbone diagram
- Factoring in image assisted screening as opposed to full manual screening to conform to workload limits for cytotechnologists mandated by CLIA '88
- CAP checklist, requirement CYP.08450: each individual screening cytology slides by manual microscopic technique examines no more than 100 gynecologic slides per 24 hours
- When automated image assisted Pap screening methods (e.g., ThinPrep imaging system) are used by the laboratory, then the following will be applicable
- Cytotechnologist review of imaged slide (only looking at the field of view [FOV] marked by the imager) is counted as 0.5 slide
- Cytotechnologist review of imaged slide + full manual review (FMR) (upon detection of abnormality) is counted as 1.5 slides
- Manual cytotechnologist review of slide without prior imaging (full manual review) is counted as 1 slide
- Turnaround time standards must be established prudently factoring in laboratory's epithelial cell abnormality (ECA) rate, manual screening of Pap tests or use of automated image assisted Pap screening methods, while abiding by stringent quality standards mandated by CLIA '88 (ASC: ASC's Workload Recommendations for Automated Pap Test Screening [Accessed 7 September 2023])
Mandatory internal quality assurance (QA) mechanisms in the cytology laboratory include which one of the following procedures?
- Correlational QA and review of follow up surgical pathology on all cases diagnosed as low grade dysplasia (LSIL) by cytology
- Gynecologic Pap proficiency testing (PT)
- Retrospective review of previously negative Pap slides within the past 5 years on an index patient whenever a new high grade squamous intraepithelial lesion (HSIL) or malignant cervical / vaginal cytology is reported
- Thorough rescreening of all (100%) negative Pap cases by a qualified cytotechnologist prior to sign out
Comment Here
Reference: Quality assurance for cytopathology
| AGC (%) | 0.2% |
| ASCUS (%) | 10.2% |
| ASC-H (%) | 0.4% |
| LSIL (%) | 4.4% |
| HSIL (%) | 0.4% |
| Malignant (%) | 0.1% |
- 1
- 2.1
- 2.3
- 25.5
Comment Here
Reference: Quality assurance for cytopathology
- A quality management system (QMS) can be defined as coordinated activities to direct and control an organization with regard to quality (WHO: Laboratory Quality Management System - Handbook [Accessed 10 July 2023])
- QMS helps coordinate and direct an organization's activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous basis
- Concept of the path of workflow is key to the QMS
- 7 quality management principles form the basis for the QMS standards
- 12 quality system essentials (QSEs) represent the most fundamental elements of the QMS model
- Implementing the QMS in all laboratory processes based on
- 12 QSEs
- 10 clauses of the ISO 9001:2015 standard
- Plan, do, check, act (PDCA) cycle
- Several tools, techniques and methods can be used individually or in combination to ensure consistent quality management and continual improvement
- QMS is designed to meet specific goals and offer numerous benefits to the laboratory
- Primary objective of QMS is to improve patient safety, satisfaction and outcome
- Laboratory is always prepared to respond effectively to a disaster
- International Organization for Standardization (ISO): a widely recognized and respected international standard setting organization that develops and publishes standards in quality management
- Laboratory QMS is applicable to 2 ISO standards
- ISO 15189: medical laboratories - requirements of quality and compliance (Clin Biochem 2009;42:274)
- ISO 17025: testing and calibration laboratories (J AOAC Int 2003;86:1038)
- Laboratory QMS is applicable to 2 ISO standards
- Quality management (QM): the assembly and management of all activities aimed at the production of quality by organizations of various kinds
- Quality control (QC): the process of monitoring and verifying that a product or service meets the specified quality requirements
- Quality improvement (QI): the framework used to systematically improve processes and systems
- Quality assurance: a systematic approach to ensuring that a product or service meets specified quality requirements
- Customer satisfaction: the degree to which a product or service meets or exceeds a customer's expectations
- Process improvement: a process of making changes to a process or system to increase its effectiveness, efficiency or quality (Clin Microbiol Rev 2018;31:e00062)
- Plan, do, check, act (PDCA): a cyclical model for continual quality improvement
- Audit: a systematic and independent examination of an organization to determine whether it conforms to specified requirements
- Quality toolbox: a collection of tools and techniques used in quality management
- Root cause analysis (RCA): a set of problem solving tools targeted at identifying the true root cause for a nonconformity
- Failure mode and effects analysis (FMEA): the prospective risk analysis of high risk processes to identify needed improvements that will reduce the chance of an unintended adverse event
- Lean management: a methodology that aims to optimize processes and reduce waste by eliminating nonvalue added activities
- Six Sigma: a quality management approach that focuses on identifying and eliminating defects in a process, resulting in improved quality and efficiency
- Define, measure, analyze, improve, control (DMAIC): Six Sigma model for continual quality improvement
- External quality assessment (EQA) or proficiency testing: a method used to evaluate the performance of a laboratory's testing processes by comparing its results to those of other laboratories
- External quality assessment program: a provider sends a set of blinded samples to participating laboratories, which are then analyzed and the results are reported back to the provider for analysis
- Quality dashboards: a type of health information technology that uses data visualization techniques to support clinicians and managers in viewing and exploring data on processes and outcomes of care
- 5 whys: a simple technique that involves asking why multiple times in order to identify the root cause of a problem
- Fault tree analysis (FTA): a graphical tool used to identify the various events or conditions that can lead to a particular problem
- Brainstorming: a group technique used to generate a large number of ideas and potential causes for a problem
- Design of experiments (DOE): a statistical technique used to determine the relationship between multiple variables in a process and the output of that process
- Value stream mapping: a lean management technique to identify and eliminate waste in laboratory processes, reduce turnaround times and enhance the quality of test results
- 5S: a lean management technique that involves sorting, simplifying, sweeping, standardizing and sustaining work areas to reduce waste and improve efficiency
- Kaizen: a continuous improvement approach to improve laboratory processes, reduce errors and enhance the quality of test results
- Standardization: the process of establishing a set of guidelines, procedures or best practices to improve efficiency, reduce errors and ensure consistency in processes and outcomes
- Visual management: a lean management technique that uses visual aids, such as signs, symbols, charts and graphs, to communicate information and improve the efficiency of processes
- Clinical and Laboratory Standards Institute (CLSI): a nonprofit organization whose goal is to develop quality assurance standards for the operation of various types of laboratories
- World Health Organization (WHO): a specialized agency of the United Nations responsible for international public health
- There are 7 QM principles based on ISO 9001:2015 (Cell Tissue Bank 2020;21:563)
- Customer focus
- Leadership
- Engagement of people
- Process approach
- Improvement
- Evidence based decision making
- Relationship management
- These 7 quality management principles form the basis for the QMS standards
(ISO: Quality Management Principles [Accessed 10 July 2023])
- Used by top management in order to lead the organization toward improved performance
- The QMS model was developed by CLSI and is fully compatible with ISO standards
- Concept of the path of workflow is key to the QMS; it begins with the patient and ends with reporting and result interpretation (see Diagram 3)
- QMS model used here organizes all of the laboratory activities into 12 QSEs
- Organization
- Personnel
- Equipment
- Purchasing and inventory
- Process control
- Information management
- Documents and records
- Occurrence management
- Assessment
- Process improvement
- Customer service
- Facilities and safety
- 12 QSEs represent the most fundamental elements for maintaining quality, safety and efficiency throughout the laboratory's path of workflow (see Diagram 4)
- Diagram 1 provides a comprehensive framework for the development and implementation of an effective QMS in healthcare organizations
- 12 QSEs provide specific guidance on how to achieve the principles in practice
- 7 QM principles provide a broad framework for the development and implementation of a QMS
- Table below describes brief instructions for all aspects of a QMS; for detailed and specific instructions, please see the laboratory quality management system handbook by WHO (WHO: Laboratory Quality Management System - Handbook [Accessed 10 July 2023])
| 12 QSEs | Brief instructions |
| Documents and records |
|
| Organizational structure |
|
| Personnel |
|
| Equipment |
|
| Purchasing and inventory |
|
| Process control |
|
| Information management |
|
| Occurrence management |
|
| Assessment |
|
| Process improvement |
|
| Customer service |
|
| Facilities and safety |
|
| Reference: WHO: Laboratory Quality Management System - Handbook [Accessed 10 July 2023] | |
- 12 QSEs provide a framework for implementing a laboratory QMS
- 10 clauses of the ISO 9001:2015 standard provide a comprehensive framework for QM (Ann Ig 2022;34:627)
- Reflecting on the PDCA cycle and covering all aspects of a QMS
- Diagrams 5 and 6 illustrate the PDCA cycle in the ISO 9001:2015 structure
- Diagram 2 shows the contents of the PDCA division according to the ISO 9001:2015 structure
- Helping organizations implement and maintain effective QMS
- Providing guidance on how to establish, implement, maintain and continually improve a QMS
- Reflecting on the PDCA cycle and covering all aspects of a QMS
- Some key steps to follow (Indian J Med Microbiol 2020;38:243, Afr J Lab Med 2017;6:490)
- Developing a quality policy
- Defining laboratory processes
- Conducting a gap analysis
- Developing a quality manual
- Establishing document control procedures
- Implementing quality control procedures
- Developing a training program
- Conducting competency assessments
- Establishing management review processes
- Seeking accreditation
- PDCA cycle can be briefly described as follows
- Plan: establish the objectives of the system and its processes
- Do: implement the processes defined in the plan stage
- Check: monitor and measure the effectiveness of the implemented processes
- Act: take actions to continually improve the QMS
- Continual improvement: start the cycle again by planning further improvements based on the results achieved
- Statistical process control (SPC) chart (Qual Saf Health Care 2007;16:387)
- Graphical tools used to monitor, control and improve processes by displaying process data over time
- There are several types of SPC charts but the most common ones are
- Control charts for variables: measure continuous variables
- Control charts for attributes: measure categorical or binary data
- Control charts for count data: measure count data
- Identifying a process that is out of control or is trending towards an out of control condition
- Moving average quality control (Biochem Med (Zagreb) 2022;32:010705)
- Detecting changes in the central tendency of the process over time
- 5 steps
- Determining the sample size and time period
- Collecting data
- Calculating the moving average
- Plotting the moving average on a control chart
- Monitoring the control chart
- Identifying shifts or trends that may indicate a need for corrective action
- Flow chart or process flow diagram (BMC Health Serv Res 2021;21:342)
- A useful tool for documenting, analyzing and improving laboratory processes in quality control
- Documenting laboratory procedures
- Identifying potential sources of error
- Standardizing laboratory processes
- Communicating laboratory processes to stakeholders
- Facilitating continuous improvement
- A useful tool for documenting, analyzing and improving laboratory processes in quality control
- Root cause analysis (J Clin Diagn Res 2014;8:FC05, StatPearls: Root Cause Analysis and Medical Error Prevention [Accessed 10 July 2023])
- A problem solving method to investigate and resolve issues that may arise in laboratory processes, procedures or test results
- Some key steps in the root cause analysis process
- Defining the problem
- Identifying possible causes
- Determining the root cause
- Developing corrective actions
- Implementing corrective actions
- Verifying the solution
- Some common tools and techniques used in root cause analysis include fishbone diagrams, Pareto charts, process flow diagrams, 5 whys, fault tree analysis, failure mode and effects analysis, brainstorming and statistical analysis
- Failure mode and effects analysis (J Am Coll Radiol 2014;11:572)
- A systematic approach to identifying and preventing potential failures in laboratory processes
- Typically conducted in 3 stages
- Identification of potential failures
- Assessment of potential failures
- Mitigation of potential failures
- Design of experiments (Eur J Pharm Biopharm 2021;166:144)
- Optimizing laboratory processes, improving the accuracy and precision of laboratory test results and reducing the time required to perform tests
- Involves several steps, including
- Identifying the process to be improved
- Identifying the factors that may affect the process output
- Designing the experiment to test the effects of these factors on the process output
- Conducting the experiment and collecting data
- Analyzing the data to determine the effects of the factors on the process output
- Optimizing the process by identifying the optimal settings for the factors that have the most significant impact on the process output
- Lean management (J Clin Lab Anal 2018;32:e22180)
- Principles of Lean are applied to laboratory quality management to optimize processes, reduce errors and enhance patient care
- Some of the ways Lean principles are applied to laboratory quality management include
- Value stream mapping
- Kaizen
- Standardization
- 5S
- Visual management
- Six Sigma (J Clin Lab Anal 2018;32:e22180, J Clin Lab Anal 2021;35:e24041, J Clin Lab Anal 2020;34:e23126)
- A data driven approach to quality management that seeks to minimize defects and variability in processes
- A set of tools and techniques for improving process efficiency and effectiveness by identifying and removing causes of defects and minimizing variability
- Some of the tools and techniques used in Six Sigma include
- DMAIC methodology for process improvement
- Statistical process control charts to monitor and control process variation
- Design of experiments to optimize process settings
- Root cause analysis to identify and eliminate causes of defects and errors
- Failure mode and effects analysis to identify and eliminate potential failure modes in processes
- External quality assessment / proficiency testing (Clin Chem Lab Med 2006;44:740, Cancers (Basel) 2022;14:3686, Biochem Med (Zagreb) 2017;27:19, Clin Chem 2011;57:1670)
- Comparing laboratory results to those of other laboratories
- Proficiency testing programs can help identify errors and areas for improvement, ultimately leading to improved patient care and outcomes
- External quality assessment process typically involves the following steps
- Sample preparation
- Sample distribution
- Sample analysis
- Result reports
- Data analysis
- Feedback and improvement
- Quality dashboards (J Pathol Inform 2016;7:24, AMIA Annu Symp Proc 2020;2019:735)
- A visual representation of key performance indicators (KPIs) and metrics
- Some of the key performance indicators and metrics
- Turnaround time
- Error rates
- Quality control results
- Test volumes
- Customer satisfaction
- Allowing laboratory managers to identify areas for improvement and take corrective action
- Improving patient safety by ensuring accurate and reliable test results
- Increasing efficiency and productivity in laboratory processes
- Meeting regulatory requirements and accreditation standards
- Minimizing errors and reducing the risk of testing related incidents
- Enhancing customer satisfaction by meeting or exceeding their expectations for quality
- Providing a framework for continuous improvement and optimization of laboratory operations
- Reducing costs associated with rework, repeat testing and equipment downtime
- Enhancing communication and collaboration among laboratory staff and stakeholders
- Ensuring compliance with ethical and legal requirements for laboratory operations
- Improving data management and ensuring the integrity and confidentiality of laboratory records
- Enhancing the reputation and credibility of the laboratory and its services
- Facilitating risk management and mitigation in laboratory operations
- Enhancing staff training and professional development
- Promoting a culture of quality, safety and excellence in laboratory operations
- Supporting evidence based decision making and strategic planning for the laboratory
- References: Comput Med Imaging Graph 2001;25:217, J Oral Biol Craniofac Res 2019;9:180
- Laboratory operations can continue during and after a disaster (Gac Sanit 2021;35:S180, Lab Anim (NY) 2013;42:F18)
- Some steps to prepare for disasters (StatPearls: Disaster Planning [Accessed 10 July 2023])
- Developing a disaster preparedness plan
- Establishing communication protocols
- Identifying and securing critical equipment and supplies
- Training staff on disaster response
- Participating in disaster drills and exercises
- Some of the key elements of a disaster preparedness plan (Int J Environ Res Public Health 2019;16:1046)
- Conducting a risk assessment
- Establishing emergency response procedures
- Creating an evacuation plan
- Ensuring adequate emergency response equipment and supplies
- Reviewing and updating the plan
- Disaster or emergency drills and exercises (Am J Pharm Educ 2016;80:50)
- Testing the effectiveness of emergency response procedures and identifying areas for improvement
- Identifying strengths and weaknesses
- Testing equipment and supplies
- Improving teamwork and communication
- Increasing preparedness
- Laboratory personnel are prepared to respond effectively to emergencies
- Testing the effectiveness of emergency response procedures and identifying areas for improvement
- Evacuation plans (Heliyon 2023;9:e14277)
- Ensuring the safety of pathologists, staff members and patients during a disaster or emergency
- Some steps to developing an effective evacuation plan
- Identifying evacuation routes and procedures
- Assigning responsibilities
- Establishing a communication plan
- Considering special needs
- Practicing drills
- Well designed disaster preparedness plan can help to minimize the impact of a disaster on laboratory operations, staff and patients (J Emerg Manag 2022;20:351)
WHO: LQSI series - implementing a laboratory quality management system
Laboratory quality management and standards
CAP: quality management
- Expand laboratory services
- Improve patient outcomes
- Increase laboratory efficiency
- Reduce laboratory costs
- Support evidence based decision making
Comment Here
Reference: Quality management systems
- Failure mode and effects analysis
- Flow chart
- Pareto chart
- Root cause analysis
- Statistical process control chart
Comment Here
Reference: Quality management systems
- Describes the mechanics of the payment process:
- Administrative activities involved in the documentation of patient care
- Collection of revenue associated with patient care
- Complete charge reconciliation
- Applicable to any medical specialty and practice environment, with some unique attributes specific to pathology and laboratory medicine
- Compliance with regulatory standards, payer requirements and generally accepted accounting principles (GAAP) are critical
- Revenue cycle management (RCM) assures sufficient cash flow to maintain the practice, physicians and staff, building and supplies
- Basic elements are critical for all pathologists to know regardless of employment situation, as there are key compliance issues, such as accurate coding, billing and documentation, that are the direct responsibilities of the pathologist
- Begins with patient data collection from a specimen requisition and ends when payments and adjustments from all collectible sources have been received, posted and reconciled
- Accurate diagnostic coding (with ICD codes) and procedural coding (with CPT codes) in compliance with rules and regulations
- Clean claims, without errors, are key to achieving timely payments
- Continual evaluation and optimization of RCM processes
- Pathologists play critical roles in RCM
- Ensuring accurate diagnostic and procedural coding
- Management of denials
- Justifications for billing resubmissions
- Ensure maximum, timely cash flow into the practice group, department or institution
- Permit financial projections critical to sustainability, including:
- Pathologist and staff recruitment and retention
- Evaluation of the scope and menu testing offered
- Need for process improvement and investments in future personnel and technology
- Entry point to RCM; preanalytic phase of laboratory testing includes:
- Capture of all patient demographic and insurance data
- Capture of referring physician information
- Capture of relevant medical history and current illness
- Verification of patient's insurance or payer information
- Case accessioning: entry of the above information to generate a unique record for the specimen received
Documentation, diagnostic and procedural coding
- To ensure appropriate payment for rendered patient services, care charge codes for subsequent billing must be generated with attention to the following:
- Verification of why a patient came for care (history of current illness) provided by the appropriate ICD-10 code(s)
- ICD-10 codes document medical necessity and justify the reason the laboratory tests were performed
- Documentation of a final diagnosis in the pathology report
- Entry and verification of the appropriate procedural (CPT) code(s) for the service(s) performed
- Common procedural terminology (CPT) codes communicate what services were provided to the patient
- In laboratory medicine, the CPT code reflects the specimen source, specimen type and complexity and the number of specimens
- Front end coding refers to the assignment of CPT codes during initial case accessioning
- Back end coding refers to the assignment of CPT codes after diagnostic work is complete, ensuring coding accuracy
- Final diagnosis must include justification for every CPT code that is assigned
- Basic CPT code must match the specimen type, i.e. 88305 for breast excision without margin assessment versus 88307 for breast excision with margin assessment
- CPT code for special stains, infection or other, used only when the specific stain is needed for diagnosis AND mentioned in the final diagnosis
- Coding verification is a critical compliance step as it prevents upcoding (overcharging) or undercoding (undercharging)
- Pathologist of record is ultimately responsible for ensuring coding accuracy and compliance, regardless of who assigned the billing codes
- Verification of why a patient came for care (history of current illness) provided by the appropriate ICD-10 code(s)
Charge assessment
- To ensure that payment received for services rendered is correct and complete, standard terminology is used as follows:
- Gross charge
- Amount charged for a service, determined by the practice's fee schedule
- Gross charge should exceed the maximum amount that private insurers pay, to ensure maximum allowable collection from any payer source
- Example: gross charge for CPT 88305 = $175.00
- Contractual amount
- Dollar amount (discount) a provider has agreed to accept from a payer for a service, as defined by contract
- Contractual amount should not be less than the cost to provide the test
- Example: payer and provider have agreed by contract that CPT 88305 will be paid $100.00
- Adjustment or contractual adjustment
- Difference between gross charge and the contractual amount
- May be expressed as a percent of gross charge
- Effectively reflects a contracted discount off of the gross charge
- Example: $175.00 (gross charge) - $100.00 (contractual amount) = $75.00 (adjustment), which is 43% of gross charge
- Net charge
- Total amount to be collected from the payer after adjustments
- Typically equals the contractual amount
- Example: $175.00 (gross charge) - $75.00 (adjustment) = $100.00 (net charge)
- Payment amount (from payer)
- Percentage of the contracted amount to be paid directly by insurance (the remaining amount will be paid by the patient, see copayment below)
- Example: payer A's contract agrees to pay 80% of contractual amount; $100.00 (net charge) x 0.80 = $80.00 (amount insurance will pay)
- Copayment
- Difference between contractual amount and payment insurance covers
- Copayment will be paid by the patient
- Example: $100.00 (contractual amount) - $80.00 (insurance pays) = $20.00 (patient pays)
- Gross charge
Claim preparation and submission
- Clean claims, without missing information or error, are critical for quick payment; a clean claim has the following:
- Identifies health care provider(s) and entities providing service with any affiliations and identifying numbers (i.e. national provider identifier)
- Identifies patient and individual holding the health insurance
- Lists correct date and place of service
- Bills for a covered service
- Identifies service provided using appropriate codes (CPT)
- Establishes medical necessity for service using appropriate codes (ICD-10)
- If necessary, includes preauthorization codes
- Submission of claims is usually electronic
- Time allowed between rendering service and issuing a bill for service may be stipulated in payer contracts
Payment posting, balance billing and management of denials
- Payment posting involves logging payment received for services into the practice management or billing software; once all insurance payments have been received and account adjustment made, the remaining patient responsibility can be billed
- Payment posting must include:
- Electronic remittance advice (ERA) payments; insurance payers often pay the practice electronically with a lump payment for multiple claims in one check, which is explained on the ERA
- Any direct payments from patients (checks, credit, cash) to the patient accounts
- Data from the explanation of benefits (EOB) from the insurance company to the patient accounts
- Information received from the EOB must also be assessed for:
- Denials of payment - review the reasons for denial
- Copays (difference between payment amount and allowed contractual amount) that should be billed to the patient
- Amounts for write offs, the difference between the claim submitted and the claim defined in the EOB
- Coinsurance / copay billing
- This is the billing of patients for the difference between the contractually allowed charges and the amount paid by insurance
- A copay is a permissible form of balance billing, representing the dollar amount the insurance company expects the patient to contribute to care, thus reflecting the patient's financial responsibility
- For special circumstances where the pathologist or laboratory performing diagnostic services are not within the insurance's provider network, specific jurisdictional laws should indicate if additional balance billing is legal
- Management of denials
- Insurance companies will indicate reasons for payment denials
- Common reasons for denials include:
- Demographic errors
- Wrong day of service
- Uncovered service
- Lack of medical necessity
- Claims can be reworked and resubmitted with corrected information within time limit specified by the payer
- With Medicare, for example, a practice has 120 days from the date of receipt of the initial claim determination to file an appeal (redetermination) request
- There is a cost to the practice associated with resubmission, estimated by some to be up to $35.00 per reworked claim
- It is important to explore reasons for denials and adjust internal processes to reduce denials (i.e. producing an initial clean claim)
- Continuous evaluation and optimization of practice inefficiency can improve both practice effectiveness and revenue
- Personnel: the largest practice expense is employee salary
- Maximize staffing efficiency by:
- Understanding peak and minimum workflows and coordinating the scheduling of personnel accordingly
- Matching employee expertise to tasks; avoid having highly trained staff performing entry level work
- Instituting robust succession planning: internal training programs may avoid rapid employee turnover by institutionalizing upward mobility; recruiting and training new staff is costly
- Maximize staffing efficiency by:
- Workflow and technology
- Most efficient way to manage your revenue cycle is through electronic billing and claims submission
- Continuously review and assess for performance improvement opportunities
- Streamline the RCM process
- Establish checks and balances to assure claims are clean and payment posting is efficient
- Evaluate reasons for claims denials and implement procedures to reduce such denials
- Use appropriate financial reports and metrics to assess performance
- Examples of metrics to consider include:
- Net collection rate: total payment received divided by total net charges
- Bad debt rate: total uncollectable charges divided by total net charges
- Denial rate: total denied charges divided by total gross charges
- Examples of metrics to consider include:
- Review payer contracts on a regular basis in order to optimize the following:
- Assure time requirements for claims submission and payment posting
- Assure contractual, agreed upon payment rates are being met and do not fall below the cost of production
- Catch any changes to contractual terms or need for renewal
- Verifying clinical information / history on pathology and laboratory orders
- Critical step that establishes medical necessity, justifying the procedure
- Example: Flow cytometry is ordered on a peripheral blood specimen without any history provided. The pathologist must investigate the clinical indication for this laboratory order.
- Verifying appropriate diagnostic ICD-10 codes to justify the laboratory procedure performed, based on local coverage determination (LCD)
- Example: Flow cytometry is ordered on a peripheral blood specimen. Following chart review, the pathologist concludes that the appropriate clinical diagnostic indication is lymphocytosis (ICD-10 code D72.820). Under certain local coverage determination, this ICD-10 code would justify the flow cytometry ordered. A clinical history of chronic lymphocytic leukemia (ICD-10 code C91) would not justify the test ordered and would likely result in claim denial.
- Entering and verifying correct procedural (CPT) codes to reflect appropriate and permissible units of service rendered
- Example: A pathologist performed pancytokeratin immunohistochemistry to look for metastasis on 2 blocks originating from the same lymph node. The laboratory system automatically codes for 2 units of CPT 88342. The pathologist is obligated to adjust the changes to reflect only 1 unit of CPT 88342, as pancytokeratin can only be charged once per specimen.
- Technical and professional coding modifier:
- Charges for the technical component of testing only would be appended by modifier "TC"
- Example: A laboratory performing only tissue processing for a colon biopsy would bill 88305-TC.
- Charges solely for the profession component of testing would be appended by modifier "26"
- Example: A pathology practice interpreting cytokeratin immunohistochemistry from a slide that was prepared by an outside reference laboratory would bill 88342-26.
- Global charges that encompass both technical and professional components are NOT appended by a technical or professional modifier
- Example: A laboratory that performs the technical component of immunohistochemistry and where its pathologist also interprets the resultant slide would globally bill 88342.
- Charges for the technical component of testing only would be appended by modifier "TC"
- Review of claim denials and providing justification to substantiate charge submission and resubmission
- Example: An insurance payer denies payments for acid fast bacillus and GMS stains performed on a gastric biopsy, claiming that medical necessity was not established. The pathologist, in review of the slides and pathology report, confirms the finding of granulomatous inflammation. A written appeal can be compiled by the pathologist, with explanation that granulomatous inflammation justifies special stains to evaluate for possible AFB and fungus before entertaining the possibilities of inflammatory bowel disease or sarcoidosis.
- Contractual adjustment
- Contractual amount
- Fee schedule charge
- Gross charge
- Payment received from insurance payer
The gross charge, which is also the fee schedule charge, is typically set high to ensure maximum collection from any payer and does not reflect what will ultimately be received. Payment from the insurance payer may reflect a significant portion of the contractual amount; however, the patient must be billed for the difference. Contractual adjustment is the difference between the gross charge and the contractual amount, reflecting effectively a discount off of the gross charge.
Comment Here
Reference: Revenue cycle management
- Definitions of strategy and approaches to strategic planning comprise a sizable, diverse body of literature
- There is no single right way to go about strategic planning, no perfect plan; they all have something to offer
- The process of establishing a direction that a business will pursue
- Strategic planning involves answering 3 basic questions about a business:
- Where do we want it to go?
- Why do we want it to go there?
- What will it all look like when we arrive?
- Reasons to develop a strategic plan include:
- Start or grow a business
- Improve existing services
- Prepare for the advent of a new service or venture
- Prepare for the fiscal year
- Realize a corporate mission
- Mitigate a threatening issue
- Solve internal problems
- Galvanize the morale and focus the work effort of employees
- Improve efficiency
- Developing a strategic plan is a governance function, the responsible individuals of which comprise:
- Business owners:
- Practice owners, e.g. equity holders, partners
- Laboratory owners, e.g. equity holders, hospitals, etc.
- Board of directors or individuals who serve in that capacity and hold fiduciary positions in the practice, laboratory or hospital
- Chief operating officer or individuals who serve in that capacity
- Business owners:
- Individuals responsible and accountable for governance functions (i.e. strategic planning) do not include nonfiduciaries:
- Vice presidents
- Directors
- Staff
- Nonstaff employees and contractors
- Implementing a strategic plan is a management / administrative function, the responsible individuals of which may comprise:
- Professional and nonprofessional staff / executive and nonexecutive staff
- Subcontractors and vendors
- Consultants
- Classically, strategic planning proceeds in 3 basic steps:
- Discussion: a series of sessions to determine the preferences of the practice partners, strategic options, feasibilities of those options and crafting of a strategic plan
- Implementation: executing the strategic plan
- Evaluation: following up on the metrics that determine the plan's success or failure and acting on contingencies designed to mitigate failure
- Members of strategic planning teams commonly include:
- CEO, chairman of the board of directors or whoever serves in these capacities
- Pathology practices may have a group president, chair or chief pathologist who serves in these roles
- Board of directors: pathology practices may have partners or an executive committee serving in this role but if they intend on growing substantially, must also consider having independent (nonpathologist) board members with fiduciary responsibilities
- Staff who will be implementing the plan
- Administrators to record and oversee planning activities
- Content providers and consultants, if necessary
- Consultants may provide data, content and opinion but they have no fiduciary responsibility and should make no decisions for the team
- Mediator or facilitator if the group is engaging in strategic planning for the first time, previous planning has not been successful, consensus cannot be reached or the group believes that they require a greater degree of impartiality than is available locally
- CEO, chairman of the board of directors or whoever serves in these capacities
- Items to consider in a strategic planning protocol may include:
- Policies and procedures for decision making and conflict resolution
- Designations for accountability
- Planning team responsibilities, job descriptions, roles, responsibilities and timelines
- Designations of individuals with ultimate responsibilities for planning team organization, planning and conducting meetings, assigning responsibilities, gathering data, following up of assigned tasks, communication to team members, etc.
- Schedule to obtain regular status updates on implementation
- Gather intelligence about what options the plan might pursue: SWOT (strengths / weaknesses / opportunities / threats) analysis
- May benefit from engaging marketing consultants
- In pathology practices, partners and staff members who have been in practice a while and who are connected with the industry may be able to fulfill this function
- Examples of SWOT elements include:
- Strengths: what are they and how will you leverage them?
- What are the organization's assets and unique resources?
- What differentiates the organization from its competitors? What is its competitive advantage?
- How broad are the current and potential customer bases?
- Weaknesses: what are they and how will the organization overcome them?
- What must improve? What expertise / resources / staff does the organization lack?
- What capital or access to capital does the organization lack?
- Does the organization rely on one or only a few customers?
- Opportunities: what are they and how will the organization maximize / capitalize on them?
- What emerging trends can the organization service profitably?
- Is there talent that the organization needs and can acquire readily?
- Are competitors failing to service their customers adequately? Is there an unmet need that the organization can fulfill?
- Is there a new technology that the organization can embrace?
- Threats: what are they and how will the organization mitigate them?
- Is there a better equipped (funding, talent, mobility, etc.) competitor in the proposed market?
- Are new competitors on the horizon?
- Is there a new technology that will replace the organization's current services?
- Is the organization overly dependent on third parties, suppliers or key customers?
- Strengths: what are they and how will you leverage them?
- With SWOT analyses in hand, organizations should be ready to brainstorm strategic options
- Pathology practices will also need to have their stakeholder analysis in hand
- Laboratory owners / institutions will have performed their own stakeholder analysis, which will likely be confidential
- Determining whether or not strategies and strategic plans are successful requires tracking:
- Hard metrics of success
- Threshold measurements that establish success or failure of the plan
- Timetables by which to record those measurements
- Contingency plans if desired thresholds are not met
- Hard versus soft metrics
- Soft metrics focus on process, e.g.
- Number of customer contacts
- Published articles
- Hard metrics focus on definitive outcomes, e.g
- Quarterly revenue
- Number of customers
- Soft metrics focus on process, e.g.
- The chart below provides some examples of using metrics to evaluate a strategic plan:
Goals of strategic plan Metric Threshold Measurement timetable Contingency if thresholds not met Grow revenue Revenue 10K Quarterly Cease growth; terminate subcontractors Grow volume / customers Accessions 1K/quarter, 4K/year Build relationships: laboratory Satisfaction scores > 4.8/5 Monthly Switch to point of service satisfaction surveys, address all dissatisfaction immediately Build relationships: pathology practice Committee appointments / trustee seat 3/1 Within 2 years / within 3 years - Commence political activities
- Increase volunteerism
- Increase donations
- See Business plan
- Failure to link in a single clear trajectory, mission, vision, strategy, goals and outcomes
- All stakeholders in the plan are not in complete agreement with, aligned or vested in the plan or its outcomes
- Goals and objectives are unrealistic or overambitious
- Strengths overestimated or not important to customers
- Opportunities and trends poorly researched and hence overestimated or nonexistent
- Adhering to unsupported or fault assumptions
- Organization does not provide adequate leadership
- Plan, its assignments and accountabilities not communicated adequately
- Plan focuses on soft or the wrong outcome measurements
- Not performing timely, meticulous scrutiny, follow up and challenging of outcome metrics
- Inertia: waiting to come up with the mythical perfect plan before embarking on a plan
- Devising and then ignoring implementation of the plan
- Waiting for a disaster before beginning to plan
- Thinking the plan is a one time event, i.e. a weekend retreat rather than an ongoing process that often entails trial and error
- Putting tactics before strategy: starting with a tactical approach and working backwards into strategy; diluting strategy to accommodate preconceived tactics
- Stubbornly adhering to old principles, policies and procedures / failing to consider new ideas and innovations
- Stakeholder analysis engages in honest, sometimes uncomfortable discussions among decision makers / pathology group partners to lay all cards face up on the table describing:
- Their intimate business and life needs
- Their levels of comfort with the risk that launching new business ventures entails
- Unless the practice comprises more than 1 or only a few owners, all of whom are of the same mind, this group discussion may be the most difficult yet most important activity that assembling a strategic plan requires
- Obstacles that may lead to decisional inertia or fiscal misadventure:
- Lack of corporate mentality results in unaligned agendas
- Decision makers are physician practice partners / owners / service providers who may see their practices existing primarily to serve their own needs rather than the other way around
- It is not realistic to expect everyone to share the same aspirations, lifestyles and professional requirements, all of which are likely to change during progressive stages of their careers
- Consequently, everyone may not agree on the same corporate direction
- Lack of business acumen
- Partners / associates are generally hired for their medical expertise, not for their business acumen
- Some partners may have MBAs but not necessarily any experience in building, owning or managing businesses
- Partners may be ill equipped and perhaps reluctant to admit to their difficulties in making the business decisions that the privilege of partnership bestows upon them
- Differences in risk tolerance
- Not everyone may be comfortable with leveraging their personal assets to secure investment capital or accepting the level of accountability that strategic decisions require
- Differences in culture, social skills, self awareness, self efficacy, emotional IQ and ability to compromise
- Partners may not possess equal willingness to understand and appreciate each other's needs and personal goals, to work as a team, to accept criticism or to be challenged
- It may be impossible to reach consensus unless these differences are resolved
- Differences in processing information
- Some partners may base their decisions on clear, tangible, objective analytical data that focus on outcomes
- Others may base their decisions on abstract, visceral, value oriented perceptions that focus on the impact those decisions have on other people
- Explaining the efficacies of different points of view will require the partners to know how they must present their positions to each other
- Lack of corporate mentality results in unaligned agendas
- Examples of partners' diverse agendas:
Where do you want to go? Why do you want to go there? What must it look like when you arrive? Increase practice worth Posture practice for sale Sell practice for 1.5x annual revenue Increase income Pay college tuitions Graduate kids with no debt to myself or them Increase time off Love of travel 4 months off to travel the globe / do international volunteer work Exit practice Aging, tired of working Retire comfortably with no debt Make a career change Enjoy a particular specialty Become an academic specialist Preserve job Hospital consolidation may put me out of work Become a hospital employee with a long term contract
- Sample questions that might:
- Allow partners to better understand each other
- Lead practice members to arrive at a uniformly acceptable plan, eliminate certain plan options or cease planning altogether
- If required to implement our plan:
- What magnitude of pay cut are you willing to take?
- How many more hours a day are you willing to work?
- How much less time are you willing to take off?
- Are you willing to terminate nonpartner professional staff?
- Are you willing to terminate nonprofessional staff?
- Are you willing to sell the practice for the right price?
- Are you willing to become an employee of the hospital?
- On what magnitude of loan note are you willing to place your personal signature?
- What magnitude of differential pay levels or work requirements among partners and other group members will you tolerate?
- How much time and resources are you willing to devote to learning new skills?
- With stakeholder and SWOT analyses in hand, pathology practices should be ready to brainstorm strategic options
- 2 examples of strategic options:
- Majority of partners may want to grow revenue in preparation for selling the practice in the distant future
- SWOT analysis may show that the market provides a growth opportunity in available customers or need for new services
- Pursuing this opportunity may require capital (debt), temporarily adding work hours and decreasing salaries
- Stakeholder analysis may reveal that several members have conflicting personal agendas and are not on board with this strategic direction
- Implementing this strategy might require rewriting corporate bylaws
- Implementing this strategy might require stratifying salaries based on an inverse relationship between income and risk: for instance, providing higher current salaries but less interest in future profits for those who desire less risk and choose not to invest in a corporate venture versus lower current salaries and greater interest in future profits for those who are willing to take more risk and invest their own capital
- Majority of partners want to preserve their livelihood in the face of cuts to reimbursement and hospital mergers
- SWOT analysis may show that local clinicians desire additional services and attention and several hospital projects may benefit from additional effort from the pathology group
- Majority of partners want to adopt a strategy of deepening their relationships with the local customers and administrators
- Doing so involves attending more committee meetings, assuming hospital leadership roles and interacting more frequently with customers
- Stakeholder analysis reveals that several partners are uncomfortable with these activities
- Implementing this strategy might require those dissenting partners to assume more service responsibilities that free up other partners to engage in relationship building activities
- Majority of partners may want to grow revenue in preparation for selling the practice in the distant future
- Governing body and fiduciaries
- Laboratory owners: e.g. equity holders, hospital, etc.
- Board of trustees
- Chief operating officer
- Individuals responsible and accountable for governance functions (i.e. strategic planning) do not include nonfiduciaries such as:
- Vice presidents
- Director level employees and subcontractors
- Laboratory director, staff pathologists, department chairs
- Clinical Laboratory Improvement Amendments (CLIA) does not specifically list strategic planning as a required duty and responsibility of laboratory directors
- CAP Accreditation Laboratory Director Assessment checklist (DRA.11200) Director Responsibility Education / R&D Phase II requirement (CAP: Director Assessment Checklist [Accessed 3 February 2021])
- Laboratory director must ensure provision of strategic planning
- Evidence of compliance: "Records or minutes from strategic planning sessions demonstrating participation and role of laboratory director"
- Some reasons why laboratory directors become involved in strategic planning activities
- Ethical and professional motivation
- Business and posturing
- Career advancement
- CAP accreditation requirement
- Laboratory director / pathologist roles that provide value to institutional / laboratory strategic planners
- Determining the extent and type of support the institutional strategic planners require of the laboratory and its director
- Ensuring that laboratory operations align with and advance those of the institution / laboratory owners
- Keeping strategic planners abreast of laboratory trends
- Providing content to institutional strategic planners
- Supporting decisions of institutional strategic planners
- Ensuring that laboratory operations advance institutional strategic agendas
- Ensuring biases and preferences among laboratory professional and nonprofessional staff do not undermine institutional strategic agendas
- Providing leadership in implementing institutional strategies
- Providing insight and suggestions for outcome metrics, thresholds, milepost measurements and contingency plans
- Engaging the laboratory director in strategic planning
- Solicit CEO's / owner's expectations, extent and parameters of laboratory director's involvement
- Consider having expectations of involvement incorporated into laboratory director's job description, if not already incorporated
- Document all conversations and activities involving strategic planning
- Review organization's SWOT analysis and strategic plan
- Secure support among professional and nonprofessional laboratory staff for institutional / laboratory strategic planning
- Neutralize / mitigate dissent among professional and nonprofessional laboratory staff for institutional / laboratory strategic planning
- Unintended consequences of and mistakes in engaging in laboratory strategic planning
- Overestimating laboratory director's responsibility and authority in strategic planning
- Placing the agenda of the laboratory / laboratory staff / colleagues over that of the institution / owner
- Engaging in confrontations with institutional planners / owners over design and implementation of plan (other than those regarding ethical issues)
- Frustration over strategic planning decisions that run counter to the opinions and desires of the laboratory director and laboratory staff
- Communicating dissent with plan to nonengaged outsiders
- Professional and nonprofessional staff who will be implementing the plan
- Administrators to record and oversee planning activities
- Content providers and consultants, if necessary
- Liaison to institutional / owner strategic planners, e.g. COO, director of ancillary services, etc.
- Liaisons to other physician and operational departments, e.g. hospital medical director, nursing director, pharmacy director, etc.
- Board of directors and owners
- Chief financial officer
- Chief operating officer
- Medical staff
- Vice president for business development
Comment Here
Reference: Strategic planning for pathology practices and laboratories
- Differences in processing information
- Differences in risk tolerance
- Lack of business acumen
- Lack of understanding of basic pathology principles
- Unaligned agendas
Comment Here
Reference: Strategic planning for pathology practices and laboratories
- Jump to: General PathOut format, Capitalization, Spelling / specific words, Punctuation, Italics, Bolding, Numbers, Bullets, References, Internal links, Templates, Editing checklist, Publish topic checklist, Completed topic checklist
General PathOut format
- Do not add extra formatting unless necessary / try to avoid bold font, caps, etc.
- Use as few words as possible to express an idea
- Add spaces around symbols such as / > < but do not add spaces if it's a fraction or ratio
- Remove articles (the, a) from the beginning of a bullet when possible
- When using a verb without a noun to describe a disease, use the singular form (e.g., "displays" rather than "display")
- If an author omits a section, keep the section and write "No information provided" to help editors see which sections are missing
Capitalization
- Capitalize the first letter of the bullet (or subbullet, title, section, caption, legend, etc.)
- Use lowercase for the rest of the text and after colons
- Exceptions
- These should always be capitalized, no matter where they are in the text
- Proper nouns and medical terms requiring capitalization, such as genus (Candida), genes (BRAF), abbreviations (FISH), diseases named after a person (Epstein-Barr virus)
- References (PubMed, other articles, books)
- These should always be lowercase (even as the first word)
- Pathology terms that require lowercase formatting, such as certain stains (p16, p21, p27, p40, p53, p57, p63) and molecular terminology, like translocations: t(11;14)
- For Gram stain, "Gram" should be capitalized and we now also capitalize "Gram positive" and "Gram negative"
- These should always be capitalized, no matter where they are in the text
Spelling / specific words
- Use American, not British spellings (tumor, not tumour; hematology, not haematology)
- Spell out abbreviations, except for common ones such as CT, MRI, DNA, FISH, HIV, EBV, IHC, RT-PCR, RNA, PSA, PET, N:C ratio
- Abbreviate units when used with numbers but spell out in text, e.g., "7 cm" but "measured in centimeters"
- Use "mL" rather than "ml"
- Change "and/or" to "or"
- Change "+/-" or "±" to "variable" (in stains) or "with or without" (in cases where variable doesn't make sense)
- Change "vs" to "versus"
- Change "more than" or "greater than" to ">"
- Change "less than" to "<"
- Change "greater than or equal to" to "≥"
- Change "less than or equal to" to "≤"
- For symbols, use the HTML entity code, e.g., use ≥ for ≥, use ≤ for ≤, etc. (see Entities for Symbols and Greek Letters)
- Change the following terms named after people with Nazi ties (can include "(formerly + outdated term)" after the new term for clarity)
- Wegener granulomatosis → granulomatosis with polyangiitis
- Clara cell → club cell
- Reiter syndrome → reactive arthritis
- For race, use Black and White (singular); for plural, you can use Black and White population, people, patients, etc. (Medical Copy Editor can update)
- Do not use Blacks or Whites (it's offensive)
- Let Medical Copy Editor know if your topic contains terms such as "African American" or "Caucasian" to check references to verify correct usage of these terms
- For magnification, use a lowercase x after the number, e.g., 40x rather than X40
- For grade in CNS tumor topics, use Arabic numerals (1, 2, 3, 4) instead of Roman numerals (I, II, III, IV)
Punctuation
- Commas
- Do not use oxford commas
- Do not use commas before or after "but" "and" "or"
- Include a comma after e.g. and i.e.
- Periods
- Do not end sentences with periods
- Use periods for degrees such as M.D., Ph.D.
- Use periods when abbreviating a country, e.g., U.S.
- Use periods in terms that require them, e.g., et al., etc., spp., var.
- Hyphens
- Always use the hyphen (-) rather than the en dash (–) or em dash (—)
- Remove hyphen from compound words and prefixes and format according to the following rules
- Add prefix to the root without any spaces, e.g., change "post-operative" to "postoperative"
- Exception: keep hyphen with no spaces to combine the prefix with a compound word, e.g., non-small cell carcinoma
- Common prefixes include pre, post, non, pan, hypo, hyper, anti, etc. (these are not standalone words)
- For compound words, keep a space when removing the hyphen, e.g., change "year-old" to "year old" and "wild-type" to "wild type"
- For compound words that are included in the dictionary as a single word, no space is needed, e.g., change "long-standing" to "longstanding"
- For acronyms, remove the hyphen and use a space instead, e.g., change "B-ALL" to "B ALL"
- For words where hyphens are required, add spaces around the hyphen, e.g., change "two-thirds" to "two - thirds"
- To show a range of numbers, add spaces around the hyphen, e.g., 1 - 5 cm
- Add prefix to the root without any spaces, e.g., change "post-operative" to "postoperative"
- Keep hyphen with no spaces added in the following cases
- To combine 2 vowels, e.g., salpingo-oophorectomy
- To add "-like" to the end of a word, e.g., osteoclast-like
- To join 2 names, e.g., McCune-Albright syndrome
- To join 2 colors, e.g., blue-green
- Exception: no hyphen when a color ends in -ish, e.g., blueish green
- For fusion genes and proteins, we no longer use a hyphen, e.g., FUS-CREB3L2, but instead use a double colon, e.g., FUS::CREB3L2
- When root word is capital, e.g., non-Hodgkin
- As a negative symbol, e.g., p53-
- Slashes
- Add a space on both sides of slash but not for fractions or ratios, e.g., "lesion / polyp" but "100 g/mL"
- Quotation marks
- Remove when possible (e.g., change "block-like" to block-like)
- Apostrophes
- For diseases named after a person, do not end with 's, e.g., change "Hashimoto's thyroiditis" to "Hashimoto thyroiditis"
- Exception: include the apostrophe in Crohn's disease
- Parentheses
- For nested parentheses, replace inner parentheses with brackets, e.g., (transurethral resection of the prostate [TURP])
- Colons
- Remove colon before a subbulleted list (unless template indicates to use a colon)
Italics
- Italicize genus and species and capitalize the genus, e.g., Candida albicans
- Do not italicize the abbreviations spp. and var., e.g., Plasmodium spp. and H. capsulatum var. duboisii
- Italicize genes, e.g., BRAF
- Do not italicize mutations, e.g., BRAF V600E (V600E is the mutation, BRAF is the gene)
- Italicize genes in tumor names as well, e.g., glioblastoma, IDH mutant
- Do not italicize proteins, e.g., p16
- Many genes and proteins have the same name, so italics are determined by context
- Gene keywords: mutated, translocation, deleted, amplification, rearrangement, FISH
- Protein keywords: immunohistochemistry, positive, negative, binds to
- Do not italicize latin words, e.g., in situ
Bolding
- Bold stains that do not have a link in stains and differential diagnosis sections, e.g., LEF1
- Bold differential diagnoses that do not have a link, e.g., Benign reactive endocervical glands
- Only bold the diagnosis and not any additional words or phrases
- Bold the colon when it follows a bolded word, e.g., Vasculitis:
- Try to avoid bolding other words throughout the text, e.g., Which of the following is not ("not" rather than "not") associated with...
Numbers
- Spell out ordinal numbers, e.g., first, second, third
- Exception: use numerals for book editions, e.g., 1st Edition
- Use numerals for cardinal numbers, e.g., 3 tiered classification
- Exception: spell out number if it is not countable / measurable or if it could be replaced by "a / an", e.g., one side of the cell
- For approximations, use a tilde with no space before the number, e.g., ~5 cm
- Use roman numerals for staging, e.g., stage IV
- Change simple fractions to percentages when possible, e.g., change "one - third" to 33%
- When needed, spell out fractions and include a hyphen with spaces around it, e.g., two - thirds
Bullets
- Every section should be formatted with bullets, except for images, virtual slides, videos and board review questions / answers
- Occasionally, there will be > 1 sentence for a bullet; combine them with a semicolon (do not use periods)
- Or use subbullets
- Or subsubbullets, if necessary
- Or use subbullets
- To format an unordered list, start with
- , add bullets as
- and end the list with
- For ordered lists, a class must be added to specify which type, then bullets are added as
- and the list ends with
- - numbers - 1.
- - uppercase letters - A.
- - lowercase letters - a.
- - uppercase roman numbers - I.
- - lowercase roman numbers - i.
- "liststyle2" is used with Board review questions
References
- References should be added at the end of the bullet, not in the middle of a sentence
- For general references for the section, add as the last bullet using the text "References:" (or "Reference:" if only 1 is included)
- To format a PubMed or journal reference, use this code: (Journal year;volume:page, e.g., Clin Exp Dermatol 2006;31:807)
- Use class="bl" for all articles
- We no longer use class="gr" for free full text articles
- Use this format for Epub ahead of print articles: Gynecol Oncol 2020 Jan 30 [Epub ahead of print]
- Use this format for preprint articles: medRxiv 2023 May 16 [Preprint]
- Use this format for StatPearls articles: StatPearls: Breast Fat Necrosis [Accessed 1 May 2020]
- To include a reference link in an image legend, use this code (Journal Year;Volume:Page)
- To format an Amazon or PubMed book reference, use this code: (Author's last name: Book Title, Edition, Year, e.g., Louis: WHO Classification of Tumours of the Central Nervous System, 4th Edition, 2016, Baron: Medical Microbiology, 4th Edition, 1996)
- Use class="bl" for Amazon books or books on PubMed
- Capitalize the first letter of each word
- Link to the Amazon page with the PathOut code (/pathologyoutl-20 at the end)
- Link to the PMID for a PubMed book
- Try to avoid WHO books as a reference throughout the topic; list in Additional references instead
- Use this format for 5th edition WHO books: WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019
- To link to a PathOut topic, use this code: topic name, e.g., acute fibrinous and organizing pneumonia
- Do not include a class, simply add the href
- Be sure to use the https address
- To add a non-PubMed reference, use this code: (Website Name: Article Title [Accessed XX Month 2020], e.g., eMedicine: Emergent Management of Acute Schistosomiasis [Accessed 7 February 2020])
- Use class="bl" for all articles
- We no longer use class="gr" for free full text articles
- For the accessed date, use today's date; update this date when doing touch ups
- Capitalize the first letter of each word
- Abbreviate the names of organizations when possible, e.g., change "College of American Pathologists" to "CAP"
- To add a reference that does not have a link, use this code: Reference without link, e.g., Pathol Annu 1968;3:145
Internal links
- When another section is mentioned in the same topic (e.g., see Gross description), add an internal link
- To do this, add an id in the section that will be linked to:
- We used to use the format "chaptertopicsection" but now abbreviate it as just the topic and section
- Add the link to the text by adding a # to the id (which is used as the href) and using the "scrollto" class
Gross description - Add the URL to the link so that it also works in a new tab
Gross description - Test the link to make sure it works (e.g., see Gross description)
- Don't use the code generated by the admin as these are unstable (e.g., #definitiongeneral168172 will change the next time a draft is saved)
- To link to a specific section on a different page, use the id code and URL but no "scrollto" class is needed, e.g., to link to the molecular section on Ovary - fibroma: Molecular / cytogenetics description
Templates
- Surgical pathology topic template
- Use for all chapters except for the ones noted below
- Chemistry, toxicology & urinalysis topic template
- Use for Chemistry, toxicology & urinalysis chapter
- Cytology topic template
- Use for cytology topics in any chapter
- Grossing topic template
- Use for grossing topics in any chapter
- Hematopathology topic template
- Histology topic template
- Use for histology topics in any chapter
- Informatics, digital & computational pathology topic template
- Use for informatics, digital & computational pathology chapter
- Medical renal topic template
- Use for kidney nontumor chapter
- Microbiology & infectious diseases topic template
- Use for microbiology & infectious diseases chapter
- Stains, CD markers & molecular markers topic template
- Use for stains & CD markers and molecular markers chapters
- Staging topic template
- Use for staging chapter and for staging topics in other chapters
- Transfusion medicine topic template
- Use for transfusion medicine chapter
- WHO classification topic template
- Use for WHO classification topics in any chapter
- Other topic template
- Use for chapters that don't follow our normal format, such as forensics, lab administration, coagulation
Editing checklist
- Top of page
- Author information includes headshot (900 x 1,200 px), rank, title, university (or hospital), city, state, country, email and directory link
- Copyright date is in correct format
- PubMed search generates 20 - 100 results
- Cite author is in correct format
- Grammar / text
- First letter of bullet is capitalized, other words are lowercase (unless capitals are required)
- Articles (the, a, an) have been removed from the beginning of bullets whenever possible
- Periods at end of sentences have been removed
- Oxford commas have been removed
- Commas before and after "but" "and" "or" have been removed
- Genes have been italicized
- Abbreviations have been expanded
- Hyphens have been removed according to our rules
- 's has been removed from diseases named after a person (e.g., Barrett's > Barrett)
- Links
- PubMed links have been cited as Journal Year;Volume:Page
- Accessed date has been updated for non-PubMed links
- Images
- "Contributed by" heading is in font size 11 bold italics () with one
before and after the heading - There is a short caption under each image (< 4 lines of text)
- Legends are included in the lightbox and follow our grammar / text rules
- Images have been resized to 400 - 600 KB
- Images have been edited using "autolevels"
- "Contributed by" heading is in font size 11 bold italics () with one
- DDx
- Entity is linked to corresponding PathOut page or bolded if no page available
- Colon is included after entity
- All text after colon is formatted as subbullets
- All stains are linked to corresponding PathOut stain page
- Admin
- Topic progress has been changed from 4 to 5 when draft is complete
- Topic follow up date has been changed to 1 month from now
- Comment has been added indicating that draft is complete
- Page keywords and description have been added
- Other
- Each author has comments on their entry in the admin
- Missing sections from template have been included with notes from author (doesn't need bullets)
- On Pending Topics list, topic has green "FE" after it when sent for formatting (or blue "MC" when assigned to medical copy editor)
- Deficiencies / questions have been listed / sent back
Publish topic checklist (after EIC / DEIC and Copy Editor review)
- Save the original page as an "Old" topic
- Remove the "0" from the url of the draft version
- Keep the Topic Complete status as "In Progress"
- No Topic Completion Date is added yet
- Change Topic Progress to "7 - to second editorial review"
- Change Topic Follow Up Date to 1 month from now
- Add a comment indicating changes made
- Publish the draft version live
- Add the updated topic to the chapter TOC
- Add topic link to the Published topics list
Completed topic checklist (after Editorial Board review)
- Change Topic Complete status to "Complete"
- Change Topic Completion Date to today’s date
- Change the Topic Priority to "None"
- Change Topic Progress to "--Select topic progress--"
- Remove Topic Follow Up Date and Assigned Date
- Remove Topic Progress Comment
- Keep Touch Up designation as "Yes" or "No"
- Add a comment indicating changes made
- Publish the topic
- 1 - 2 sentence summary of the topic
- Sections included on this page are from the Surgical pathology template; see list of templates with corresponding chapters above
- 3 - 5 most important points, typically from clinical features, microscopic description or positive stains
- Other names used
Notes:
- For diseases named after a person, do not end with 's, e.g., change "Hashimoto's thyroiditis" to "Hashimoto thyroiditis"
- Exception: include the apostrophe in Crohn's disease
For multiple codes of the same type, use this format (no colon on main bullet, codes indented as subbullets)
- ICD-O
- ICD-10
- ICD-11
Notes:
- For the ICD-O PDF link, replace #page=94 with the page number the code is on, e.g., 9390/3
- Page number is found at the top of the document; do not use the number in the lower right or left corner
- If the ICD-O code is not found in the PDF file, search for a different link to use; if no link is found, bold the ICD-O code
- Do not change the text of the ICD-O code to match the text in the PDF since the PDF is outdated (from 2013)
- For the ICD-10, link to the appropriate code on the icd10data site, e.g., C4A
- For the ICD-11, link to the appropriate code on the WHO site, e.g., FB80.0
- Add postcoordination code to same link (if provided by author), e.g., FB80.0 & XK9J
- Note: ICD-11 recently changed their URLs; be sure to use the updated URL
- Be sure to add in the disease name associated with the code if the author didn't include it
- Between the ICD code and disease name, be sure to use the hyphen (-) rather than the en dash (–) or em dash (—)
- If an author includes a CPT code, please link here
- If an author includes an HCPCS code, please link here
- Who gets the lesion
- For male to female ratio, use M:F = #:# (fill in numbers, keep spaces around equal sign, e.g., M:F = 2:1)
- For predominance, use M > F
- For equal incidence, use M = F
- For decade of onset, spell out ordinal number, e.g., seventh decade
- For number of people affected per number of population, use numerals and commas with thousands, e.g., 1 per 1,000,000
- For % of population, use numerals, e.g., 10%
- For range, use a dash with spaces around it and only include percent sign or unit at end, e.g., 10 - 20% or 50 - 80 years
- Sites of body affected
- How the lesion arises; be sure to italicize genes if they are included, e.g., PTEN
- Causes of the lesion; be sure to italicize genes if they are included, e.g., PTEN
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Can include images on our server or other servers
- Add a
between the table and section header - If adding a table that does not have a thumbnail available, use the PathOut table thumbnail: https://www.pathologyoutlines.com/thumb/table.jpg
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
- If adding in a table from an author's word doc, use the following table format
- Start the table using
- Add rows using
- Add cells using
- Double borders will be added automatically
- Width automatically adjusts as more text is added
- To include a specific width in a cell, add width="#px" or width="#%", e.g.,
or - To center text horizontally, use
- To center text vertically, use
- To center text both horizontally and vertically, use
tags are obsolete, use style="text-align:center" instead - End the table using
- To center text horizontally, use
Table title in bold (no italics)Heading Heading Heading Text text text text text Text text text text text Text text text text text Text Text Text - Add rows using
- Start the table using
- Anything clinical such as associations with other conditions
- If associated with another topic on our site, link to the topic using this format: topic name, e.g.,
fibrous dysplasia
- You can find associated topics by doing a site search
- How a diagnosis is made including imaging modalities and procedures utilized but not microscopic findings
- Typical findings
- Remember to expand abbreviations
- For numbers, use numerals and commas with thousands, e.g., 15,000 cells/mm3
- Use this superscript code so that it doesn't change the spacing of the line: text
- Use this subscript code so that it doesn't change the spacing of the line: text
- Leave a space between the number and unit, e.g., 100 ng/mL
- If the unit is a fraction, do not put spaces around the slash, e.g., 46 U/mL
- Use "mL" rather than "ml"
- Abbreviate units when used with numbers but spell out in text, e.g., "7 cm" but "measured in centimeters"
- Typical findings, each on a separate bullet
- Common abbreviations are ok to use, such as CT, MRI and PET
- Xray should be formatted with capital X and no space before ray
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Common abbreviations are ok to use, such as CT, MRI and PET
- Xray should be formatted with capital X and no space before ray
- Can include images on our server or other servers
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
- Favorable or unfavorable prognostic factors
- Section name is "Prognostic factors," not "Prognosis"
- 3 - 5 quality case reports, preferably recent cases
- __ year old (man / woman / girl / boy) with ____ (reference)
- Use boy or girl if < 18 years old
- Use man or woman if ≥ 18 years old
- Use male or female as an adjective before a noun, e.g., 42 year old male gardener
- No dashes in "year old"
- Reference at end of bullet
- If the author doesn't include the age and gender, find it in the article and add it
- Use "presented with" in past tense rather than "presenting with"
- Examples:
- 1 year old girl with pigmented plexiform neurofibroma (J Am Acad Dermatol 2007;56:862)
- 15 year old boy with a 3 month history of a left lower labial mucosa swelling (J Oral Maxillofac Pathol 2014;18:S151)
- 18 year old woman with advanced tumor showing rare pseudopapillary and macrofollicular growth (Iran J Pathol 2018;13:94)
- 51 year old man with lower limb rash, fatigue and bulky abdominopelvic lymphadenopathy (Case Rep Hematol 2018;2018:4312594)
- Sort case reports in order of age, with youngest first
- Put cases without specific age at end of list
- When listing a PathOut Case, use this reference format (class="bl"): (Case #222)
- For rare entities, it is acceptable to include case series; change the section heading to "Case reports & case series"
- General modes of treatment
- If drug names are included, capitalize brand names but not general types, e.g., rituximab is lowercase but Rituxan is capitalized (if you aren't sure, google it)
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Includes intraoperative images or images of the patient
- Can include images on our server or other servers
- Does not include radiology images, which belong in the Radiology images section
- Does not include gross images, which belong in the Gross images section
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
- Description of the excised specimen
- For combined colors, use a hyphen with no space, e.g., yellow-green
- For colors ending in -ish, do not hyphenate, e.g., grayish white
- For size, use numerals with space between number and unit, e.g., 50 mm
- To show a range, use a dash with spaces around it and include the unit after the last number, e.g., 1 - 13 cm
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Images of excised specimen
- Can include images on our server or other servers
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
- Description of features
Contributed by Name, Degrees
Images hosted on other servers:
Notes:
- Can include images on our server or other servers
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
- For CNS tumor topics only, cytology and frozen section images should be grouped together in frozen section rather than separated (use heading "Intraoperative frozen / smear cytology images" for these)
- Diagnostic criteria, histologic features, etc.
- If associated with another topic on our site, link to the topic using this format: topic name, e.g.,
fibrous dysplasia
- You can find associated topics by doing a site search
- Remove quotation marks when possible (e.g., change "block-like" to block-like)
- To show a range of numbers, add spaces around the hyphen, e.g., 1 - 5%
- Add spaces around symbols such as / > < but do not add spaces if it's a fraction or ratio
Notes:
- Images on our server
- Format heading
- Use the "Contributed by" heading (class="f11bi"), no colon at end
- Heading should have one
before and one
after - Use periods for degrees such as M.D., Ph.D.
- Include only the contributor's name and degrees, not their university or country
- For multiple contributors, list their names separated by a comma in the same heading (the lightbox will indicate whose image it is)
- If images are from an old PathOut Case, use the heading "Case #__" and link to the Case, e.g., Case #300
- For new Case images, use the heading "Contributed by __ (Case #__)"
- If images are from AFIP, use the heading "AFIP images" (and also use this in the legend)
- Exception: if there is also an author in the heading, use this format: Contributed by Author Name and AFIP
- If images are taken from virtual slides, use the heading "Contributed by Author name (source: virtual slide source)"
- Subheadings should use format class="f10bi" (both bolded and italicized)
- Load images to our server
- Edit the files using "auto levels" to even out the colors
- Be sure to resize images so that the files are 400 - 600 KB
- Load to the imgau directory or the imgau/chapter directory
- We are no longer adding legends to the image files
- Save the image file as chaptertopicauthor#.jpg, e.g., lymphomanonBanaplasticbalakrishna01.jpg
- Add lightbox
- Each section should have a unique lightbox code in the format data-lightbox="chaptertopicsection"
- Add in depth description as a legend in the data-title section
- The legend should be formatted in complete sentences with periods
- Ok to include magnification in image legend (2x, 4x, 10x, 20x, 40x, etc.)
- Stain and magnification should be at the end of the image legend and formatted as (stain, magnification).
- To include a reference link in the legend, use this code (Journal Year;Volume:Page)
- After the legend, include a
and the Contributed by text (only include the contributor of that specific image if there are multiple contributors)
- Add captions
- Include a few words as a caption in the html p tag
- Should not include magnification, H&E or figure #
- To make images share a caption, add the appropriate div class with between each image (each image still needs its own legend)
- (for special formatting, can add style="text-align:center" to the div and p tags to center the caption, be sure to add in
s)(for 1 image, 1 caption)(for 2 images, 1 caption)(for 3 images, 1 caption)(for 4 images, 1 caption)(for 5 images, 1 caption)(for 6 images, 1 caption)- Captions should include no more than 4 lines of text; if the caption cannot be trimmed down, use
and add
s to divide the words onto 4 lines- Add style="text-align:center" into the div and p tags to center the caption: and
- Add alt text
- Copy and paste the text from the caption into the alt text (after width and height, add alt="caption")
- Images on other servers (for author topics, delete all micro images from other servers)
- Use the other servers heading (class="f11bi"), colon at end, one
before heading (two
if after other row of images), one
after heading - Fill in the href
- Link to the image on the page by finding the id in the source code
- To link to an image in a PDF, add #page=NUMBER to the end of the URL and fill in "NUMBER" with the page number, e.g., https://www.gastrojournal.org/action/showPdf?pii=S0016-5085%2897%2900366-1#page=5
- For the src, add the thumbnail to our server (see thumbnail procedure on page 60 of Procedure manual)
- If it is a composite image, first crop the image so that it only shows the relevant image
- Resize to 96 x 96
- Load to thumb directory
- For composite images in multiple sections, crop the image for each section and link to same image
- For composite images in the same section, only include one link / thumbnail (showing all relevant composite images)
- Include a few words as a caption in the html p tag
- Copy and paste the text from the caption into the alt text (after width and height, add alt="caption")
- Link to images that include a description; do not link to images on other servers if they don't have a description (unless there are no other images)
- Remove images from sites that are not stable, such as Archives of Pathology and WebPathology
- Remove images from sites that bypass paywalls, such as Sci-Hub
- General notes
- We now require at least 6 micro images per topic
- Include up to 6 images per row, then
onto next line - Include up to 6 rows (we no longer use the scroll bar)
- If images are on the PathOut Flickr account, add them to our server
- Also add Flickr images from the following pathologists who have given us permission: Yale Rosen, Nicole Andeen, Maria Tretiakova, Debra Zynger, Jian-Hua Qiao or the author of the topic
- We prefer images on our server but we allow images on other servers in every section except for micro images
- Click here to view the different types of images
- Add alt text
Virtual slides
Images hosted on other servers:
Notes:- Most virtual slides use the "other servers" heading but we have begun hosting slides on PathPresenter - these should use the "Contributed by" heading
- For slides from the University of Toronto, link to the page that includes the text rather than directly to the slide
- See detailed image instructions in Microscopic (histologic) images
Cytology description- Cytologic features
- Format Diff-Quik with hyphen
- Always capitalize Pap
Cytology images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:- Can include images on our server or other servers
- Format Diff-Quik with hyphen
- Always capitalize Pap
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
- For CNS tumor topics only, cytology and frozen section images should be grouped together in frozen section rather than separated (use heading "Intraoperative frozen / smear cytology images" for these)
Immunofluorescence description- Immunofluorescence diagnostic criteria, histologic features, etc.
Immunofluorescence images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:- Can include images on our server or other servers
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
Positive stains- List relevant IHC and special stains that are positive at least 50% of the time
- Link each stain to the corresponding Stains & CD markers topic
- Format the name of the stain according to the Stains & CD markers table of contents
- For example, if an author includes c-myc, change to MYC based on Stains & CD markers page
- For example, if an author includes LCA, change to CD45
- Authors often use different names for stains; to find a stain, use the site search at the top of the page
- For stains that do not have a link on the table of contents, include them in bold font, e.g., TRAF1
- Change "+/-" or "±" to "variable," e.g., +/- CD57 → variable CD57
- Do not include "positive" or "+" after a stain when listed in the positive stains section, as this is implied
- When adding positive or negative (in other sections), use the same format in the same section
- Use positive and negative (written out) or + and - (symbols)
- Don't use both positive and + in the same section
- Abbreviations are ok to use in this section if the Stains & CD markers table of contents uses the abbreviation, e.g., use CK7 rather than cytokeratin 7
- For Gram stain, "Gram" should be capitalized and we now also capitalize "Gram positive" and "Gram negative"
Negative stains- See instructions in Positive stains
- Do not include "negative" or "-" after a stain when listed in the negative stains section, as this is implied
Flow cytometry descriptionFlow cytometry images- Composite images are allowed for this section only
Electron microscopy description- Electron microscopy diagnostic findings
Electron microscopy images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:- Can include images on our server or other servers
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
Molecular / cytogenetics description- Description of DNA / RNA findings based on testing via sequencing, PCR, FISH, CISH, ISH, cytogenetics, etc.
- All of the above abbreviations are ok to use
- Italicize all genes, e.g., TFE3
- Format fusion genes and proteins with a double colon rather than a hyphen, e.g., FUS::CREB3L2
Molecular / cytogenetics images
Contributed by Name, Degrees
Images hosted on other servers:
Notes:- Can include images on our server or other servers
- Common abbreviations are ok to use, e.g., DNA, RNA, PCR, FISH, CISH, ISH
- Italicize all genes, e.g., TFE3
- See detailed image instructions in Microscopic (histologic) images
- Click here to view the different types of images
Videos
Short caption
Shared caption
Notes:- For the src, be sure to use the link with "embed" in it (e.g., https://www.youtube.com/embed/v80kA_dt6EE)
- To link to a specific portion of a video, add the start time in seconds (?start=XXXs), e.g., https://www.youtube.com/embed/AO9LKJdCpFQ?start=960
- Add a
between the video and section header
Sample pathology report- Organ, procedure:
- Diagnosis (see comment)
- Comment: Write in complete sentences with periods.
Differential diagnosis- Linked PathOut topic:
- Use the https address for the linked PathOut topic
- Bold the diagnosis if a link isn't available; only bold the portion that would be linked and not any additional words or phrases
- Add a colon after the linked topic
- Include additional info as subbullets
- Be sure to link all stains to the stains page, e.g., PAX5
- Next linked PathOut topic:
- List in order that author provides; should be from most to least important (we don't list alphabetically anymore)
Image format for DDx (see example here):
- Linked PathOut topic:
- DDx bullets
- DDx bullets
- Next linked PathOut topic:
- DDx bullets
- DDx bullets
Additional references- List references not included elsewhere in topic, separated by commas
- To format a PubMed or journal reference, use this code: (Journal year;volume:page, e.g., Clin Exp Dermatol 2006;31:807)
- Use class="bl" for all articles
- We no longer use class="gr" for free full text articles
- Use this format for Epub ahead of print articles: Gynecol Oncol 2020 Jan 30 [Epub ahead of print]
- Use this format for preprint articles: medRxiv 2023 May 16 [Preprint]
- Use this format for StatPearls articles: StatPearls: Breast Fat Necrosis [Accessed 1 May 2020]
- To format an Amazon or PubMed book reference, use this code: (Author's last name: Book Title, Edition, Year, e.g., Louis: WHO Classification of Tumours of the Central Nervous System, 4th Edition, 2016, Baron: Medical Microbiology, 4th Edition, 1996)
- Use class="bl" for Amazon books or books on PubMed
- Capitalize the first letter of each word
- Link to the Amazon page with the PathOut code (/pathologyoutl-20 at the end)
- Link to the PMID for a PubMed book
- Try to avoid WHO books as a reference throughout the topic; list in Additional references instead
- Use this format for 5th edition WHO books: WHO Classification of Tumours Editorial Board: Digestive System Tumours, 5th Edition, 2019
- To add a non-PubMed reference, use this code: (Website Name: Article Title [Accessed XX Month 2020], e.g., eMedicine: Emergent Management of Acute Schistosomiasis [Accessed 7 February 2020])
- Use class="bl" for all articles
- We no longer use class="gr" for free full text articles
- For the accessed date, use today's date; update this date when doing touch ups
- Capitalize the first letter of each word
- Abbreviate the names of organizations when possible, e.g., change "College of American Pathologists" to "CAP"
- To add a reference that does not have a link, use this code: Reference without link, e.g., Pathol Annu 1968;3:145
Board review style question #1
Write in complete sentences. If the last sentence is worded as a question, include a question mark at the end. If it is not worded as a question, then no punctuation at the end
- Answer option 1 (listed in alphabetical order)
- Answer option 2 (listed in alphabetical order)
- Answer option 3 (listed in alphabetical order)
- Answer option 4 (listed in alphabetical order)
- Answer option 5 (listed in alphabetical order)
Notes:- For answer options, use
- - uppercase letters - A.
- For answer options, use consistent format with acronyms, i.e., either all expanded or all abbreviated
- At least one BRQ should include an image
- Don't include the caption / legend with the image if it gives away the answer
- Always include the image contributor's name in the lightbox
- Question should test basic, essential concepts rather than minor details
- We don't accept the question format: "Which of the following is false?"
- We don't accept multiple answers, such as "All of the above" or "A and C"
- Answers should be ordered alphabetically; if changing the order, make sure to update the letter of the correct answer
- Do not include punctuation at the end of the question unless it is a question mark
- Do not use periods at the end of the answer options
Board review style answer #1Letter. Answer. Answer explanation. Answer __ is incorrect because ___________________. Answer __ is incorrect because ___________________. Answer __ is incorrect because ___________________.
Comment Here
Reference: Topic name
Notes:- Use periods at end of sentences in this section
- Do not change the answer explanation order to alphabetical; keep in the order provided by the author
- Make sure the letters match the answer options
- For the reference, link to the topic that the question is on
- For references in the explanation, include them at the end of the relevant sentence
Board review style question #2
Write in complete sentences. If the last sentence is worded as a question, include a question mark at the end. If it is not worded as a question, then no punctuation at the end
- Answer option 1 (listed in alphabetical order)
- Answer option 2 (listed in alphabetical order)
- Answer option 3 (listed in alphabetical order)
- Answer option 4 (listed in alphabetical order)
- Answer option 5 (listed in alphabetical order)
Notes:- See Board review question #1 for detailed instructions
Board review style answer #2Letter. Answer. Answer explanation. Answer __ is incorrect because ___________________. Answer __ is incorrect because ___________________. Answer __ is incorrect because ___________________.
Comment Here
Reference: Topic nameValidating a new quantitative assayTable of ContentsDefinition / general | Essential features | Terminology | Overview | Precision | Bias and accuracy | Linearity | Reference interval | Other studies | Analytical validation tools | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general- Proper analytical validation of a new assay is essential prior to patient testing
- Using Clinical Laboratory Standards Institute (CLSI) guidelines as a framework for designing new assay validations is highly recommended
Essential features- Minimum requirements for analytical validation of a quantitative assay include studies for precision, bias, accuracy, linearity and reference interval
- Required validation studies each have an associated CLSI standard which outlines how to design, carry out and evaluate the specific analytical aspect (CLSI: Method Evaluation Standards [Accessed 1 December 2022])
- Statistical software, such as EP Evaluator and Analyse-it, are useful for analyzing the validation data
Terminology- Total allowable error (TAE): the amount of error that is allowable without invalidating the interpretation of a test result
- Precision: closeness of agreement between values obtained by replicate measurements
- Bias: estimate of a systematic measurement error
- Linearity: ability to provide results that are directly proportional to the concentration / amount of analyte in the test sample within a given range
Overview- Precision, bias, accuracy, linearity and reference interval studies are typically the minimum requirements
- Establish performance limits for validation studies a priori using appropriate total allowable error goals
- Laboratory medicine associations have defined total allowable error goals for clinical assays: EFLM Biological Variation, CLIA, CAP, RCPA, IQMH
- Factors to consider for each validation study:
- Sample: what type of samples to use?
- Target values: what concentrations are relevant?
- N (number): how many samples / replicates are needed?
- Limits: what are the performance limits?
Precision- Precision studies establish variance within replicate measurements and the likelihood / magnitude of random errors
- Refer to CLSI EP05-A3 for designing precision studies:
- Sample: quality control (QC) material, optimally from a third party vendor
- Target values: at least 2 levels of QC near medical decision limit(s)
- N: 20 x 2 x 2 format consisting of 2 runs of duplicate measurements daily for 20 days for each level of QC
- Limits: total observed variance should not typically exceed 33% of the total allowable error
- For the 20 x 2 x 2 format, CLSI recommends calculating the coefficient of variation (%CV) via 2 way nested analysis of variance (ANOVA) (see Analytical validation tools below)
- Reference: CLSI: EP05-A3 - Evaluation of Precision of Quantitative Measurement Procedures [Accessed 1 December 2022]
Bias and accuracy- Bias studies establish agreement to a predicate assay and the magnitude of systematic error
- Refer to CLSI EP09-A3 for designing bias studies:
- Sample: patient samples with known values as reference standard for comparison
- Target values: preferably an even distribution across the analytical measuring range (AMR)
- N: minimum of 40 patient sample singleton measurements, preferably tested over several days
- Limits: mean bias (absolute or relative) should not typically exceed half of the total allowable error
- Additional analyses to perform for bias studies:
- Regression analyses are typically performed to assess absolute or proportional bias in the form of a regression line
- Passing-Bablock or Deming are the recommended regression methods (see Analytical validation tools below)
- Differences (absolute or relative) are typically plotted on Bland-Altman plots to assess bias trends across the analytical measuring range
- Consider inclusion of higher metrological order samples such as proficiency testing materials or certified reference material to establish bias against a reference method
- Regression analyses are typically performed to assess absolute or proportional bias in the form of a regression line
- Reference: CLSI: EP09-A3 - Measurement Procedure Comparison and Bias Estimation Using Patient Samples [Accessed 1 December 2022]
Linearity- Linearity studies verify vendor quoted analytical measuring range and reportable limits to be implemented with the assay
- Refer to CLSI EP06 for designing linearity studies:
- Sample: patient derived sample pools preferable (see below for preparation steps)
- Target values: preferably equally spaced concentrations across the analytical measuring range bounded by sample pools at or near the low and high limits
- N: minimum of 5 sample pools, each tested in duplicate
- Limits: various schema are used to evaluate linearity, though CLSI recommends bias limits be used for linearity
- Linearity samples are ideally matrix matched, patient derived sample pools
- Typical preparation involves serial dilutions of a high sample pool with a low sample pool
- If patient derived sample pools are not attainable, consider alternatives:
- Vendor provided material (linearity, calibrator, quality control, etc.)
- High sample pool diluted with vendor diluent
- Reference: CLSI: EP06 - Evaluation of Linearity of Quantitative Measurement Procedures [Accessed 1 December 2022]
Reference interval- Reference interval (RI) studies verify vendor quoted reference intervals, which represent the normal range for the assay
- Refer to CLSI EP28 for designing reference interval studies:
- Sample: samples from apparently healthy volunteers
- Target values: not applicable
- N: minimum of 20 samples per appropriate stratification (i.e., an assay with reference intervals for males and females would require 40 samples)
- Limits: ≥ 95% of samples fall within vendor quoted reference interval(s)
- Reference: CLSI: EP28 - Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory [Accessed 1 December 2022]
Other studies- Additional studies (stability, interferences, carryover, limit of quantification / detection, dilutional recovery) should be considered if appropriate for the assay in question
- Stability studies to establish the viability of samples for testing across time and storage conditions (i.e., temperature) after collection, as well as any specific sample processing or transportation needs for labile analytes
- Consider adopting CLSI based continuous study design (Clin Chem Lab Med 2020;58:188)
- Interference studies to evaluate interfering substances that can cause a clinically significant difference in assay results; assays on automated analyzers are typically evaluated for interference effects from hemolysis, icterus and lipemia (CLSI: EP07 - Interference Testing in Clinical Chemistry [Accessed 1 December 2022])
- Carryover studies to ensure that instrumentation prevents samples being analyzed from contaminating subsequent samples; especially relevant for assays with a wide expected range of values (i.e., beta hCG, serum free light chains) (CLSI: EP10A3AMD - Preliminary Evaluation of Quantitative Clinical Laboratory Measurement Procedures [Accessed 1 December 2022])
- Limit of quantification / detection (analytical sensitivity) studies to establish assay performance at the lower limit of the analytical measuring range; especially relevant for assays with laboratory or clinical requirements near the lower limit of measurement (i.e., thyroglobulin, high sensitivity cardiac troponins) (CLSI: EP17 - Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures [Accessed 1 December 2022])
- Dilutional recovery studies to establish appropriate dilution protocol (i.e., dilution factor, diluent) for high concentration samples beyond the upper limit of the analytical measuring range for undiluted sample analysis (CLSI: EP34 - Establishing and Verifying an Extended Measuring Interval Through Specimen Dilution and Spiking [Accessed 1 December 2022])
Analytical validation tools- Statistical programs / software are useful tools to aid the analyses and calculations required for analytical validation studies based on CLSI standards
- EP Evaluator (Data Innovations LLC) is a standalone software
- Analyse-it (Analyse-it Software, Ltd.) is an Excel add on software
Board review style question #1
Using the provided dataset for precision studies for a hemoglobin A1c assay, which of the following statements is true?
- A positive shift in both levels is observed on day 14
- Level 1 quality control shows more consistency and stable performance compared to level 2
- Quality control material is inappropriate for precision studies
- The total allowable error of 6% is too stringent and should be widened
Board review style answer #1A. A positive shift in both levels is observed on day 14. Overall, precision studies show adequate performance for both levels of quality control material in the 20 x 2 x 2 format and are acceptable. Level 2 quality control shows a smaller CV and more consistency than level 1. A total allowable error of 6% is too wide as a precision goal for hemoglobin a1c, which has relatively small absolute values.
Comment Here
Reference: Validating a new quantitative assayBoard review style question #2
Using the provided dataset for method comparison studies for a hemoglobin A1c assay, which of the following statements is true?
- The data shows acceptable linearity
- The result suggests analytical errors due to carryover
- There is an increasing negative bias of the new method with increasing HbA1c concentrations
- There is an insufficient number of data points for the analysis to be conclusive
Board review style answer #2C. While the average bias of -1.5% is within the total allowable error, it is clear that there is an increasing negative bias of the new method with increasing HbA1c concentrations (current method is negatively biased against new method). The bias hovers around 6% starting around 6.0% A1c units and greater, which encompasses the medical decision limit of 6.5% A1c units. This bias renders the method comparison result not acceptable for patient testing.
Comment Here
Reference: Validating a new quantitative assayValidation of reference intervals and reportable rangeTable of ContentsDefinition / general | Essential features | Terminology | Diagrams / tables | Establishing reference intervals | Transference of reference intervals | Validation of reference intervals | Validation of reportable range | Videos | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general- Reference intervals (RI) are defined as the central 95% of laboratory test results obtained from a healthy reference population (McPherson: Henry's Clinical Diagnosis and Management by Laboratory Methods, 23rd Edition, 2016)
- 5% of all results from healthy people will fall outside of the reported RI and as such, will be flagged as being abnormal (Am J Clin Pathol 2010;133:180)
- Reportable range (analytical measurement range) is the span of test result values over which the laboratory can establish or verify the accuracy of the instrument or test system measurement response (Legal Information Institute: Definitions [Accessed 26 July 2022])
Essential features- Nearly 80% of physicians' medical decisions are based on information provided by laboratory reports (Am J Clin Pathol 2010;133:180)
- RIs given in laboratory reports have an important role in aiding the clinician in the interpretation of test results as compared to values for healthy populations (Biochem Med (Zagreb) 2016;26:5)
- Reference ranges are population specific and are also influenced by instrumentation and testing methods
- Hence reference ranges used within a given lab may vary from those of another lab using different testing systems and serving different patient populations
Terminology- Reference population is a group of healthy individuals that is served by the laboratory
- Outlier is an observation that lies an abnormal distance from other values, in a random sample from a population
- Normal distribution, also called Gaussian distribution, is a probability distribution that is symmetric about the mean, showing that data near the mean are more frequent in occurrence than data far from the mean; the normal distribution data show a bell shaped curve
- Parametric method is a statistical test that assumes that observed values follow the normal distribution
- Nonparametric method is a statistical test that makes no specific assumption about the distribution of the data (Bishop: Clinical Chemistry - Principles, Techniques, and Correlations, 9th Edition, 2022)
- Box-Cox transformation is a mathematical method used to convert nonnormal distribution data into normal distribution data
- Systematic error is a consistent or proportional difference between the observed and true values
- Random error is a chance difference between the observed and true values
- Validation of reference interval is a study to establish that an assay works as intended
- This applies to non-FDA cleared tests (e.g., laboratory developed methods) and modified FDA approved tests
- Modifications refer to changes to the assay that are not specified as acceptable by the manufacturer
- Verification of reference interval is a one time study meant to demonstrate that an assay performs in line with previously established performance characteristics; this applies to unmodified FDA approved or cleared tests
- Transference refers to adoption of previously established RI by a laboratory
- Reportable range refers to the span of results within which the method performs acceptably in terms of sensitivity, linearity and reproducibility
Diagrams / tables
Contributed by Duy K. Doan, M.D. and Lewis A. Hassell, M.D.
Validation of reportable rangesStandard values Observed values Means Replicate 1 Replicate 2 Replicate 3 0 2 0 4 3 200 220 210 200 210 400 400 400 400 400 600 620 630 610 620 800 760 760 790 770 1,000 920 900 880 900
- In this example:
- Expected reportable ranges: 0 - 1,000 mg/dL
- Number of concentration levels: 5
- Number of replicates: 3
Establishing reference intervals- Direct approach: subjects representing the reference population are selected and sampled and the specimens are analyzed to determine the reference intervals
- Indirect approach: results from specimens collected for routine purposes, such as for screening, diagnostic or monitoring purposes, are used to determine the reference intervals
- Reference: Clin Chem Lab Med 2018;57:20
Direct approach Indirect approach - New data are generated from the population
- Higher cost to perform
- Preanalytical and analytical conditions may not match the routine conditions
- Hard to repeat in different locations
- Ethical issues
- Requires basic statistical knowledge and expertise
- Use the data that are already generated
- Lower cost to perform
- Preanalytical and analytical conditions match the routine conditions
- Easy to repeat in different locations
- No ethical issues
- Requires significant statistical knowledge and expertise
Direct approach1. Selection of reference individuals - Determine healthy candidates by medical history, physical examination and laboratory investigations
- Implement inclusion, exclusion and partitioning criteria
- Obtain informed consent from participants
- Take samples from reference individuals; 2 sampling approaches:
- A priori approach: inclusion of reference individuals is determined before samples are collected
- A posteriori approach: the definition of reference individuals includes information applied after the samples have been collected
- Reference: Turk J Biochem 2020;45:1
2. Analysis of the samples - Preanalytical considerations:
- Biological factors: circadian rhythms, fasting status, physical activities
- Methodological factors: collection techniques, types of additives, specimen handling, transportation, storage
- Analytical aspects: methods of measurement, equipment, reagents, calibration standards and calculation methods
3. Evaluation - Outlier removal: very important step to make RIs reliable
- Dixton's Q test: simple and effective
- Q = D/R (D is the absolute difference between the outlier in question and the closest number to it; R is the entire range of the observation)
- If D/R > 1/3, the outlier should be discarded
- Drawback of Dixton's Q test: if there is more than one outlier, the test become less sensitive
- Tukey fence method: more sophisticated
- Q1 = lower quartile, Q3 = higher quartile; IQR (interquartile range) = Q3 - Q1
- If number < Q1 – 1.5 IQR or > Q3 + 1.5 IQR, it is an outlier and should be removed
- RIs calculation: parametric and nonparametric methods
- Parametric method: only apply to Gaussian distribution data
- Reference values need to be a normal distribution or transformed to a normal distribution
- Box-Cox power transformation is often used
- RIs = mean ± 1.96 SD
- Nonparametric method: applied to data irrespective of distribution characteristics
- IFCC recommended method for estimating reference intervals is a nonparametric method that essentially involves simply excluding the lowest and highest 2.5% of reference values
- Parametric method: only apply to Gaussian distribution data
- Partitioning: stratification by age and gender is important for establishing more accurate RIs
- Calculate the confidence intervals of the lower and upper RIs; at least 120 samples are needed for analysis
- Dixton's Q test: simple and effective
- References: Biochem Med (Zagreb) 2016;26:5, Am J Clin Pathol 2010;133:180, CLSI: EP28 - Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory, 3rd Edition, 2010
Indirect approach1. Select the population - Data from outpatient settings are preferred; pathological changes in admitted patient populations make the laboratory results less reliable
- Number of subjects: more is better; 1,000 may be small number, 10,000 can be seen as large number
- Stability: it is imperative to ensure that the analytical method and the population are stable during the time data are collected
- Partitioning: all data should be evaluated for cofactors such as age and gender; if standard deviation ratio between subgroups exceeds 1.5, a separated reference interval is highly recommended (Clin Chem 1990;36:265)
- Exclusions: eliminate results from subjects that are more likely to have diseases based on hospital settings, clinical information and other laboratory results
2. Statistical evaluation - Standard parametric and nonparametric statistics can be used in indirect RI studies; outlier removal is very important because standard statistic techniques are significantly affected by these extreme results
- Hoffmann method is a simple graphical method for estimating reference intervals from routine laboratory data
- Distribution of results could be represented by a mixture of 2 underlying Gaussian distributions, corresponding to the healthy and diseased subpopulations, with the healthy subpopulation dominating the sample
- Drawback of the Hoffmann procedure is that it is influenced by the size of the diseased subpopulation (Am J Clin Pathol 2019;151:328)
- Bhattacharya method is also a graphical method for estimating reference intervals
- Bhattacharya method has been shown to be less influenced by data not included in the Gaussian distribution compared with the Hoffmann method (Am J Clin Pathol 2019;151:328)
- Special programs: Arzideh developed a method that is more sophisticated than Hoffman's and Bhattacharya's
- First, kernel density estimation is used to generate the distribution of the combined data of the nondiseased and diseased subjects
- Central part of the distribution of all data should represent the nondiseased population
- Then, Box-Cox transformation is used to produce a truncated Gaussian distribution of the nondiseased values
- Finally, a goodness-of-fit statistic is applied to find the main part of the data
- Reference limits are calculated as the 2.5 and 97.5 percentiles of the estimated power normal distribution for the nondiseased values (Clin Chem Lab Med 2018;57:20)
Transference of reference intervals- Laboratories are not required to establish their own RIs; most laboratories use previously established RIs
- If the patient population and methodology are comparable, the RIs can be transferred
- Like establishing RIs, parametric and nonparametric methods are also used for comparing data sets in transference
- Nonparametric methods such as Mann-Whitney, Run and Monte Carlo tests are preferred because they can be applied to data irrespective of distribution characteristics (Laboratory Medicine 2006;37:306)
Validation of reference intervals- CLSI document C28-A recommends 3 methods for meeting the CLIA specified requirement:
- Inspection method
- This is a not a statistical approach
- If there is no evidence to show that the patient population served by the laboratory differs from the reference population, the use of reference intervals is acceptable
- Decision can be made by the laboratory medical director
- This method can be used only if the local population data are unavailable
- Limited validation
- 20 samples are obtained from the healthy subjects served by the laboratory
- If no more than 2 values (10%) fall outside the reference interval, the range is validated
- If ≥ 3 reference specimens are outside of the reference range, 20 additional reference samples can be obtained; if ≥ 3 of the second reference sample are again out of the reference interval, the laboratory should consider establishing its own reference range (Laboratory Medicine 2006;37:306)
- Extended validation
- 60 samples are obtained from healthy subjects served by the laboratory
- Reference interval of the local population is generated (parametric method, Gaussian distribution)
- Another 60 samples are randomly collected from the same population; if the reference interval calculated in this study is similar to that of the above, the range is validated
- This method is not recommended because this is nearly the same as the sample size required for an RI study (Clin Chem Lab Med 2018;57:30)
- Inspection method
Validation of reportable range- Procedure to confirm the linear relationship between the analytical results of a method and the concentration of the analyte over the range of measurement
- Materials: patient specimens, commercial kits, standard reference materials, calibrators
- Number of concentration levels: CLSI recommends at least 4, preferably 5 different concentration levels; 1 minimum value, 1 midpoint value and 1 maximum value should be included
- Analyze 3 samples at each value in replicate
- Data analysis: plot observed values against standard values
- Check visually for linearity
- Calculate the slope and intercept
- Coefficient of variance (CV) = SD/mean; CV < 10 is very good, 10 - 20 is good, 20 - 30 is acceptable and CV > 30 is not acceptable
- Reference: Arch Pathol Lab Med 2014;138:1173
Videos
Reference intervals, the basics
Additional referencesBoard review style question #1
Reference interval Sample size Ceruloplasmin (mg/L) 159 - 414 603
A group of investigators from Saudi Arabia was studying the reference intervals of serum ceruloplasmin in adult healthy individuals. The results of their study are shown above. How many of the 603 subjects are expected to have a ceruloplasmin concentration of greater than 454 mg/L?
- 3
- 6
- 9
- 12
Board review style answer #1A. 3. The data show a normal distribution; mean: 286.5. By definition, reference interval = mean ± 1.96 SD → 1 SD = 65. Ceruloplasmin levels of more than 454 will be approximately 2.58 SD from the mean / median (454 - 286.5 = 167.5 = 2.58 x 65 = 2.58 SD). 99% of values will be between (mean - 2.58 SD) and (mean + 2.58 SD), 0.5% values will be < (mean - 2.58 SD) and 0.5% values will be > (mean + 2.58 SD). Number of values > 454 will be 0.5% x 603 (n) = 3.
Comment Here
Reference: Validation of reference intervals and reportable rangeBoard review style question #2Which of the following is a disadvantage of using the indirect approach to establish reference intervals?
- Calculation method is complicated
- More expensive to perform
- Need informed consent to use samples
- Repeating the evaluation is technically challenging
Board review style answer #2A. Calculation method is complicated
Comment Here
Reference: Validation of reference intervals and reportable rangeWho does what?Table of ContentsDefinition / general | Hospital or System Administration | Human Resources Department | Unions | Laboratory Section Supervisors | Laboratory Director and Laboratory Manager | The job description | Recruitment and selection | Compensation | Orientation and training | Employee retention and engagement strategies | Performance evaluation | Counseling, discipline and termination | Labor lawsDefinition / general- The goal of Personnel Management is to work with people, to motivate them, allow them to contribute, develop and achieve
- Many in a hospital or healthcare system are responsible for the various aspects of personnel management
Hospital or System Administration- Responsible for:
- Strategic planning
- Approval of personnel budget
Human Resources Department- Responsible for:
- Developing personnel policies
- Producing an employee handbook
- Approving job descriptions
- Developing job classifications
- Recruitment and advertising for vacancies
- Preliminary interviews and reference checks
- Assistance with disciplinary actions
- Implementation of labor laws and regulations
Unions- Function as employee advocates
- May be involved in:
- Organizing
- Bargaining
- Working conditions
- Grievances
Laboratory Section Supervisors- Responsible for:
- Defining the job description(s) with the Lab Manager
- Developing performance standards for each job
- Interviewing and selecting candidate best qualified to fill the job
- Providing new employees orientation
- Assuring employee retention
- Assessing competencies
- Staffing and scheduling
Laboratory Director and Laboratory Manager- Responsible for:
- Approving job descriptions and performance standards
- Approving employee selection
- Defining job descriptions and standards for higher level positions
- Interviewing and selecting higher level positions
- Developing personnel budget for the department
- Implementing and enforcing departmental and institutional personnel policies
- Assuring worker achievement, worker safety and esprit de corps
The job description- The job description is critical to personnel management
- It is essential for recruiting and advertising because it defines specific prerequisites, requirements and expectations
- It is indispensable during job interviews to determine whether a candidate is suitable for a specific position
- The job description also tells new employees what is expected of them
- Is also used to evaluate employees' performance and determine whether merit pay, promotion, or other action is warranted
- The essential components of the job description are indicated below
- The Heading
- Includes basic employment information such as:
- Job title
- Job classification
- Level and step
- Exempt status
- Salary/benefits
- Job location
- Schedule
- i.e. Shift/PT/FT
- Includes basic employment information such as:
- Position Description
- Job summary
- Major (general) duties
- Reporting/supervisorial relationships
- Basic Requirements
- Citizenship
- Education
- Licensure, certification and re-certification
- Experience
- Organizational requirements
- English language proficiency
- Interpersonal skill
- Time, attendance and dress code standards
- Compliance with organizational rules and regulations
- Compliance with CLIA personnel standards
- Specific Requirements and Competencies / Duties
- General or core tasks
- Specific knowledge and skills required
- Specific detailed duties and tasks
- Specific performance expectations
- Physical requirements/working conditions
- Other requirements for specific jobs
- Research and teaching responsibilities
- Administrative or management responsibilities
- Final comments
- Writing the job description cannot be delegated to the personnel department
- Only the lab supervisors, lab managers and pathologists know both the general and the specific knowledge, skills and experience any particular job requires
- In order to recruit, advertise and interview successfully, those specifics must be included
- In addition, employees deserve to know precisely what is expected of them and on what basis they will be evaluated
- The thorough job description provides that essential information
- The Heading
Recruitment and selection- Efficient laboratory operation depends on a stable workforce and a low turnover rate
- The more effective the recruitment and selection process, the greater degree of job satisfaction and the lower the turnover rate
- A new employee needs three to six months to become fully productive, so the lower the turnover rate, the more effective the laboratory
- The job description is the main document in this process
- It determines the content of advertisements, forms the basis of the interview and informs the applicant of the employer's expectations
- Attracting applicants
- Newspaper and professional Journal advertisement
- Internet (e.g., PathologyOutlines) job sites
- Professional Society (e.g., ASCP) job sites
- Employment agencies
- In-house posting, especially for higher level positions
- Hiring from within establishes and maintains a “Promotional Ladder”
- Search committees
- Screening applicants
- Preliminary by Personnel Department - does applicant meet the requirements
- Review of application and resume
- Screening interview
- Review of letters of reference
- By laboratory supervisorial personnel
- Interview - questions should be based on job requirements
- Skill or competency assessment (New CMS/CLIA Guidelines)
- Avoid “illegal” questions regarding age, religion, sexual orientation, etc.
- Psychological and drug testing
- The job offer
- Reiterates the job description and its expectations
- Specifies criteria and timing of performance evaluations
- Specifies terms of employment
- Compensation, benefits and working conditions
- Retain documentation of all conversation and of any offers
- Preliminary by Personnel Department - does applicant meet the requirements
Compensation- Compensation is regulated by both Federal and State laws which address issues such as:
- Minimum wage
- Overtime
- Required meal and rest periods
- Permissible vacation
- Holidays
- Parental leave
- Assorted benefits including worker's compensation and other insurance
- An institution may have additional policies governing such things as shift differential and on-call pay
- The Human Resources department should assist the laboratory in assuring compliance with the Federal Fair Labor Standards Act and other pertinent laws
- Laboratory management must also be knowledgeable about compensation of the laboratory staff
- Salaries
- Determined by Administration
- May utilize community standards for rate setting
- Should maintain internal equity with comparable salaries for comparable jobs
- Benefits
- Often more important than salary to employees
- Insurance
- Health, Life, Disability
- Continuing Education
- Tuition reimbursement
- Uniforms
- Hiring bonus
- Child care
- Free parking
- Retirement and pension plans
- Changing compensation
- Employees often feel they are not compensated adequately, but institutional salary structures are usually quite rigid
- When there is justification to increase an employee's salary the following are available:
- Annual cost of living increase
- Merit increases
- Promotion to a higher grade or step
- Reclassification to a new position
- Inequity adjustments
- e.g. is MT paid less than a RN?
- Incentive and profit sharing systems
- Spot bonuses
Orientation and training- New employees need to be oriented to the laboratory and to the institution
- The immediate supervisor should first review the job description and the performance expectations with the new employee and inform them of any competency assessments which will be expected
- Supervisor responsibility
- Must provide and arrange for:
- Personal Items
- Badge
- Keys
- Locker
- Uniform
- Parking space
- Computer access
- Another seasoned employee to serve as “buddy” to introduce the new employee to the department and to the rest of the institution
- Institutional training, such as HIPAA, fire, safety, sexual harassment, etc.
- An Employee Handbook with institutional rules and regulations
- Personal Items
- Must provide and arrange for:
Employee retention and engagement strategies- An important measure of laboratory administration's competence is employee turnover
- Every time an employee is replaced, there is a 3 to 6 month period of decreased productivity while the new employee "gets up to speed"
- More than 20% annual turnover in technologists and more than 30% annual turnover in lower job categories is excessive
- The laboratory needs a formal program to assure employee retention and this should include:
- Emphasizing worker relevance and achievement
- Recognizing productive work
- Establishing a recognition and reward mechanism
- Maintaining a career ladder - promotional opportunities
- Providing continuing education, in house and external
- Providing adequate compensation
- Providing unique benefits, e.g., Child Care
- Providing a pleasant work environment
Performance evaluation- An annual performance evaluation or appraisal and/or competency assessment is a standard procedure which evaluates:
- An employee's achievements or deficiencies
- Determines whether the job expectations have been met
- How performance can be rewarded or improved
- Evaluation basis
- The job description, with its performance standards and specific competencies, is the basis of the evaluation
- The immediate supervisor must gather performance data and conduct the evaluation based on the job description
- Specific examples of an employee’s meeting, exceeding, or failing to meet expectations should be documented
- Success should be recognized with compensation and/or promotion
- When there are deficiencies, they should be analyzed:
- Were performance standards known?
- Is it a training issue?
- Is it a motivation issue?
- Is it a competency issue?
- Obstacles to successful performance?
- Is it just a bad fit between person and job?
- The evaluation should include a plan for improvement when necessary
- Documentation of the evaluation must be thorough and acknowledged, in writing, by the employee
Counseling, discipline and termination- In addition to the formal annual performance evaluation there may be ongoing evaluations acknowledging exceptional effort or achievement and these should be recognized in public
- There may also be ad hoc evaluations because of failure to meet standards - these should be timely, specific, held in private and include counseling with a plan for improvement
- When counseling fails to correct unsatisfactory performance, disciplinary actions need to be implemented
- Progressive disciplinary actions
- They include:
- First a verbal warning
- Next, verbal warning with consequences
- Next, written counseling memo
- Next, written memo with penalties, e.g., fine or demotion
- Finally, dismissal / termination
- They include:
- Components of a disciplinary warning / report
- Essential components are:
- Description of the problem
- Record and/or copies of previous warnings
- Employee’s response
- Penalties or remediation being instituted
- Description of future performance expectation with time line
- Signature of rater, employee and witness
- Essential components are:
- Unsuccessful disciplinary actions
- When disciplinary actions are unsuccessful, involuntary termination “for cause” is the consequence and the specific causes must be documented, such as:
- Incompetence
- Insubordination
- Excess absence or late arrival
- Repeated violation of employee rules
- Verbal or physical abuse of patients, co-workers
- Breach of HIPAA confidentiality regulation
- Falsification of records or tests
- Criminal activity
- When disciplinary actions are unsuccessful, involuntary termination “for cause” is the consequence and the specific causes must be documented, such as:
- Other terminations
- Voluntary retirement
- Voluntary resignation
- Involuntary due to downsizing
- Other actions
- When termination is other than “for cause”, the employee's contribution to the institution should be formally recognized
- An exit interview should be conducted any time an employee is terminated, whether voluntarily or “for cause”
- Vital information about a department, the staff and procedures often surfaces during exit interviews
- However, exit interviews must be evaluated judiciously and in context
Back to topLabor laws- Federal and State laws and regulations govern many aspects of personnel management
- Although most are implemented by the hospital Personnel or Human Resources department, whenever there is an alleged violation, the laboratory directors, managers and supervisors are frequently held responsible
- It is important to be conversant with the most important laws and regulations
- National Labor Relations Act (1935)
- Established the National Labor Relations Board (NLRB) which:
- Guarantees the right of employees to organize into unions and to bargain collectively
- Protects employers from certain unlawful union activities
- Provides bargaining equality between employers and employees
- Established the National Labor Relations Board (NLRB) which:
- Fair Labor Standards Act (1938)
- Defines minimum wage, overtime and other salary items
- Differentiates between exempt and non-exempt employees
- U.S. Equal Employment Opportunity Commission (EEOC)
- Enforces the following laws and provides oversight and coordination of all federal equal employment regulations, practices, and policies:
- Title VII of the Civil Rights Act of 1964 prohibits employment discrimination based on race, color, religion, sex, or national origin
- The Equal Pay Act of 1963 (EPA), protects men and women who perform substantially equal work in the same establishment from sex-based wage discrimination
- The Age Discrimination in Employment Act of 1967 (ADEA), which protects individuals who are 40 years of age or older
- Title I and Title V of the Americans with Disabilities Act of 1990 (ADA), prohibits employment discrimination against qualified individuals with disabilities
- The Civil Rights Act of 1991, provides monetary damages in cases of intentional employment discrimination
- Enforces the following laws and provides oversight and coordination of all federal equal employment regulations, practices, and policies:
Recent Laboratory Administration & Management of Pathology Practices Pathology books
Find related Pathology books: general surgical pathology, lab medicine, management, frozen section
- Format heading