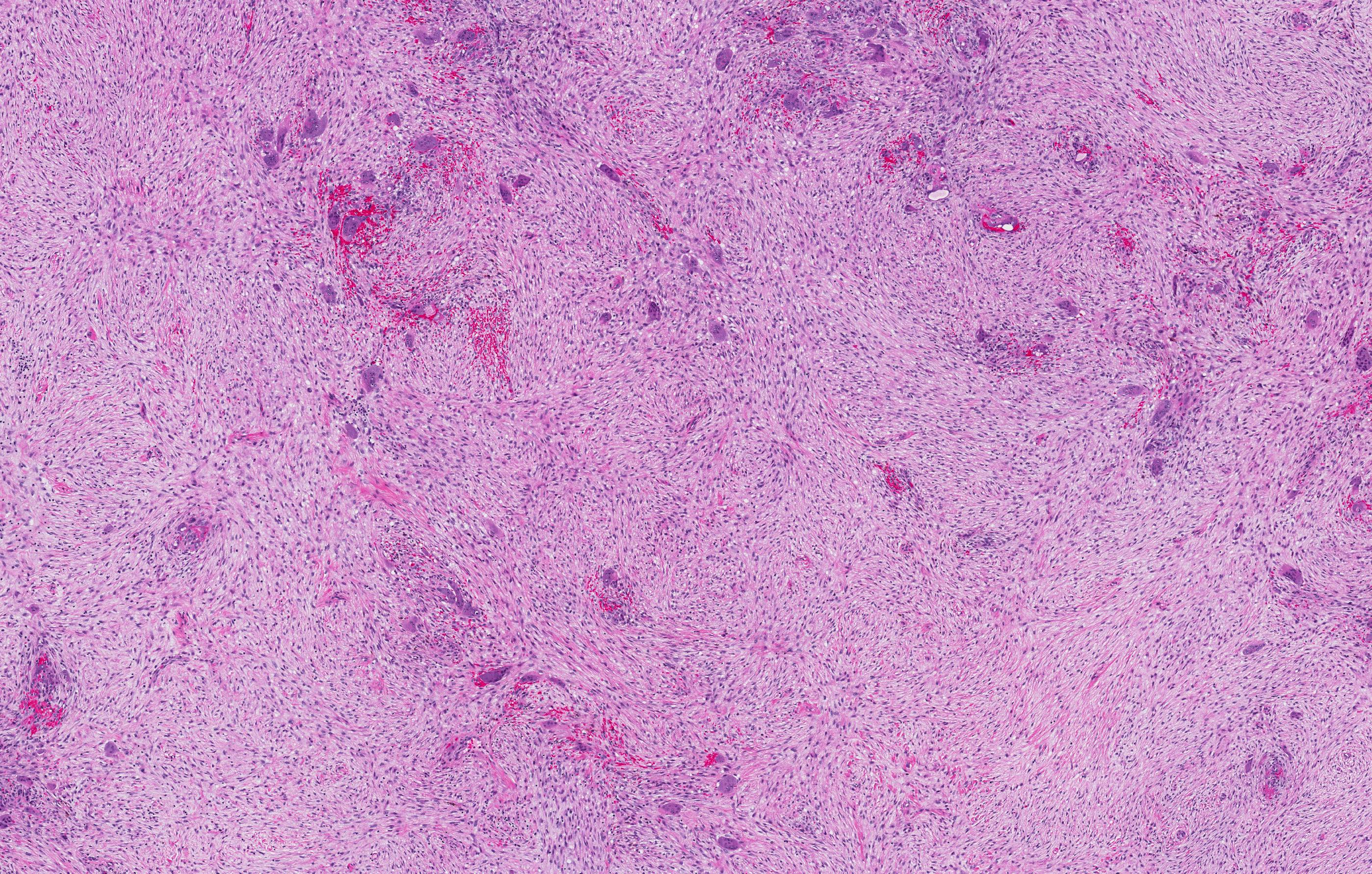
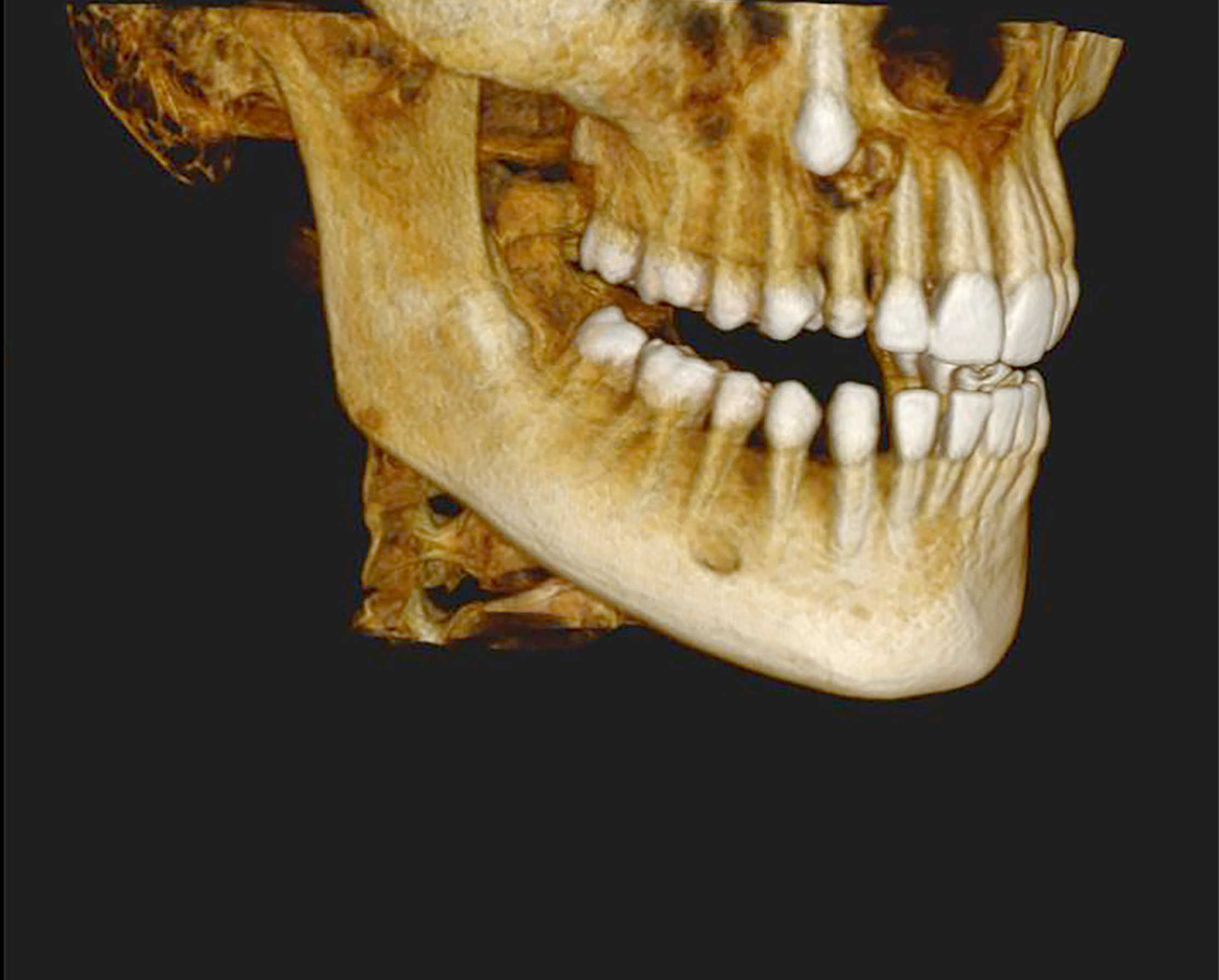
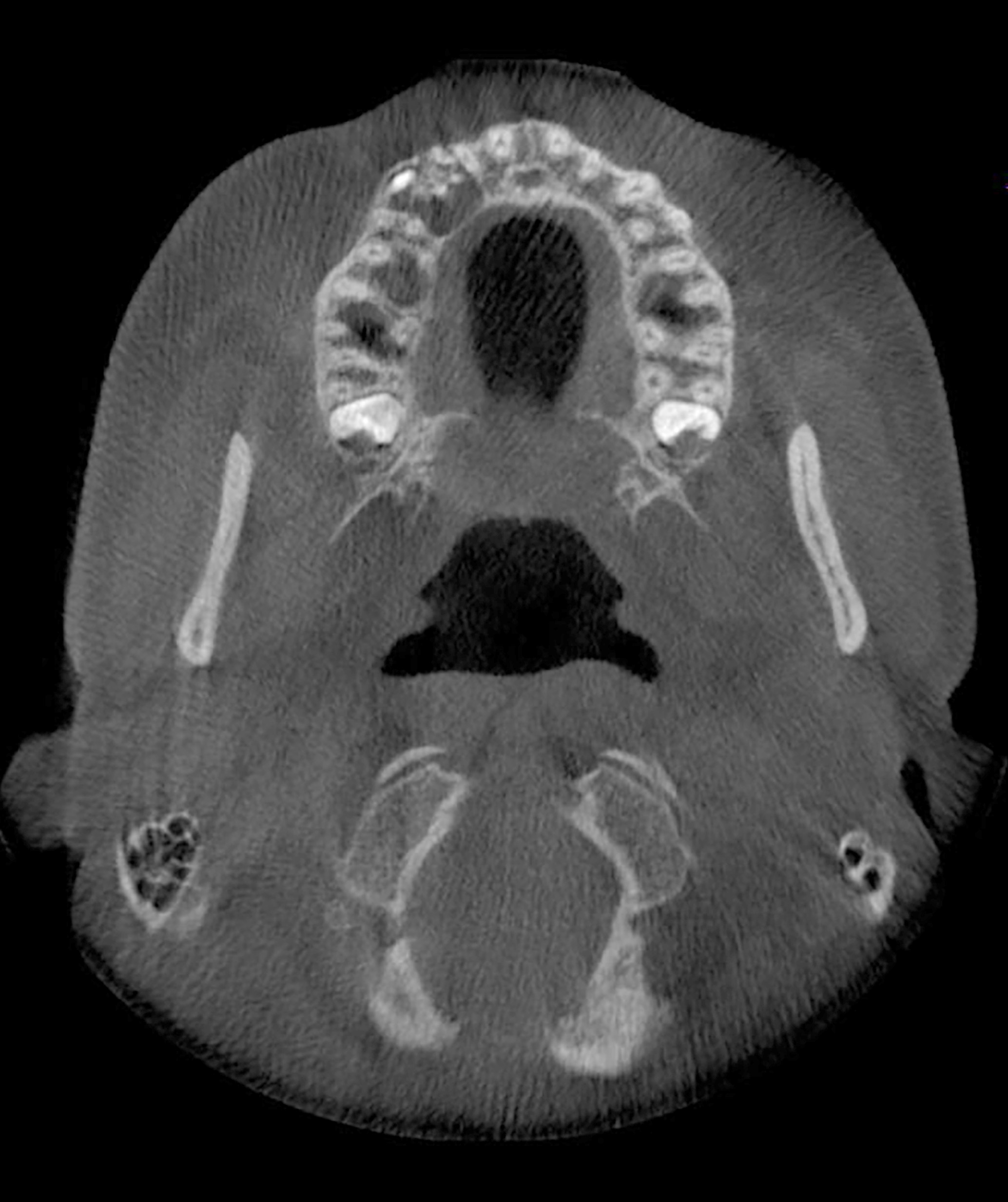
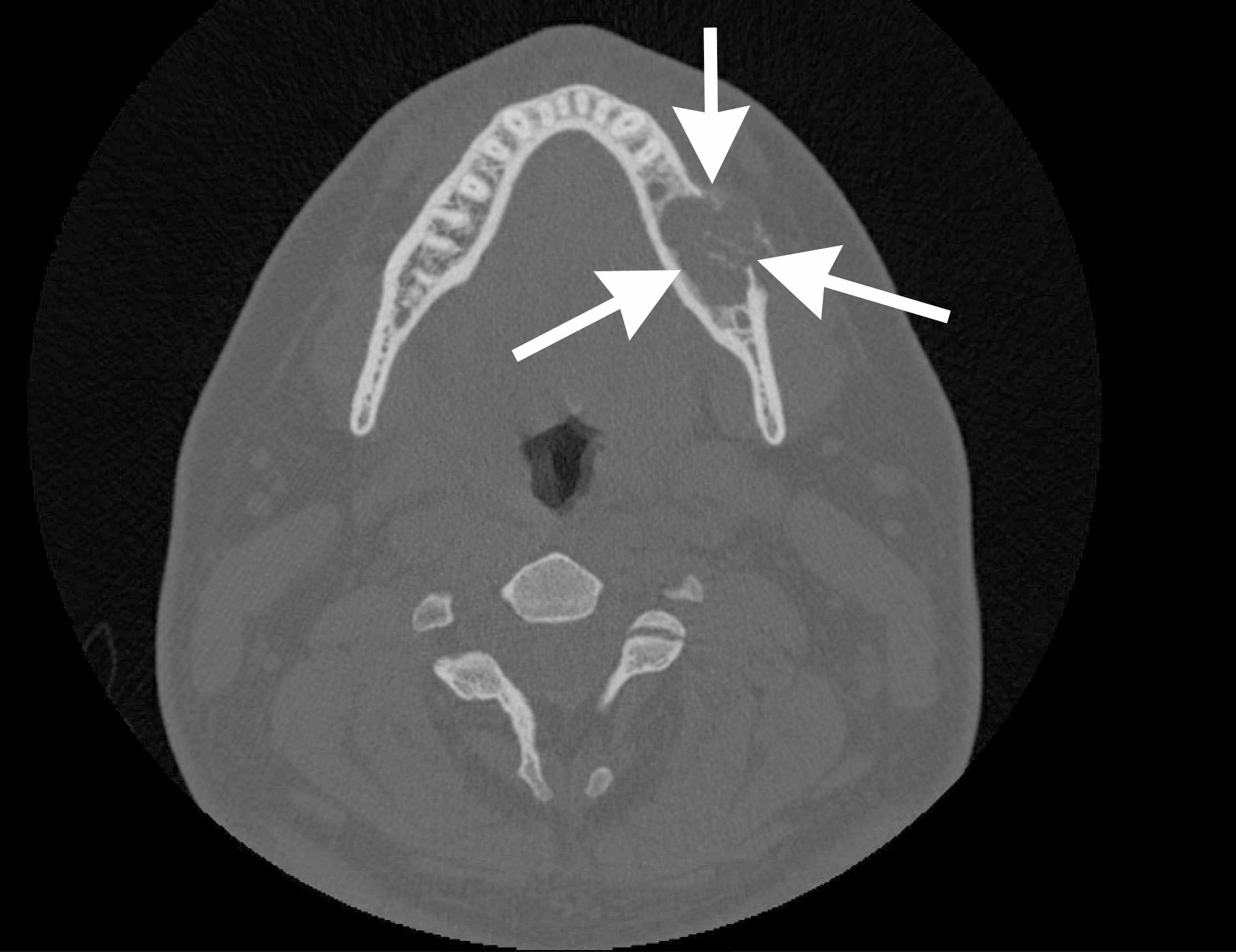
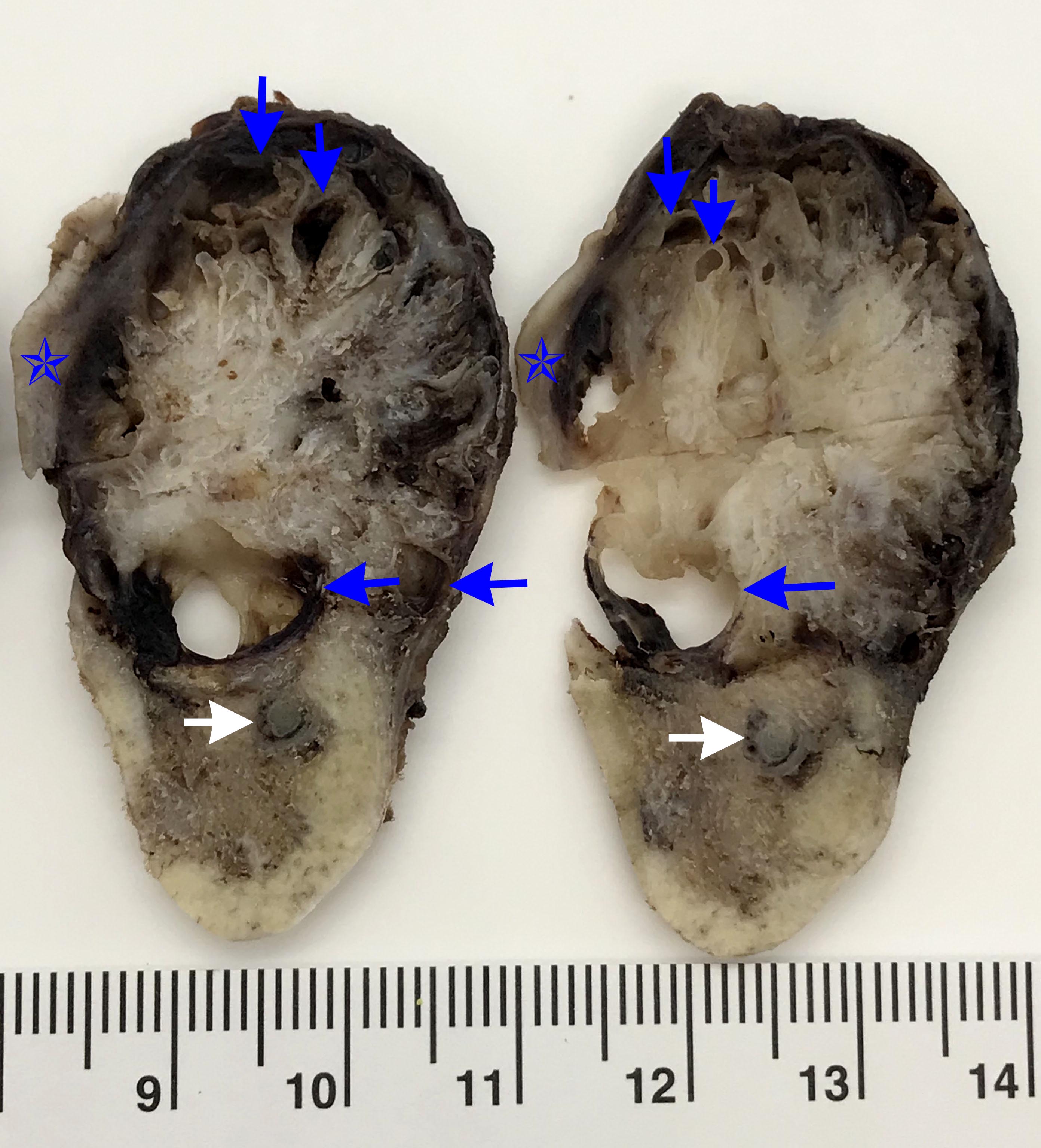
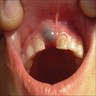
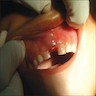
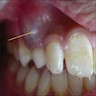
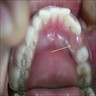
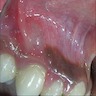
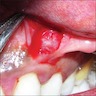
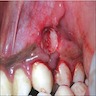
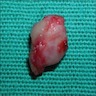
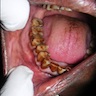
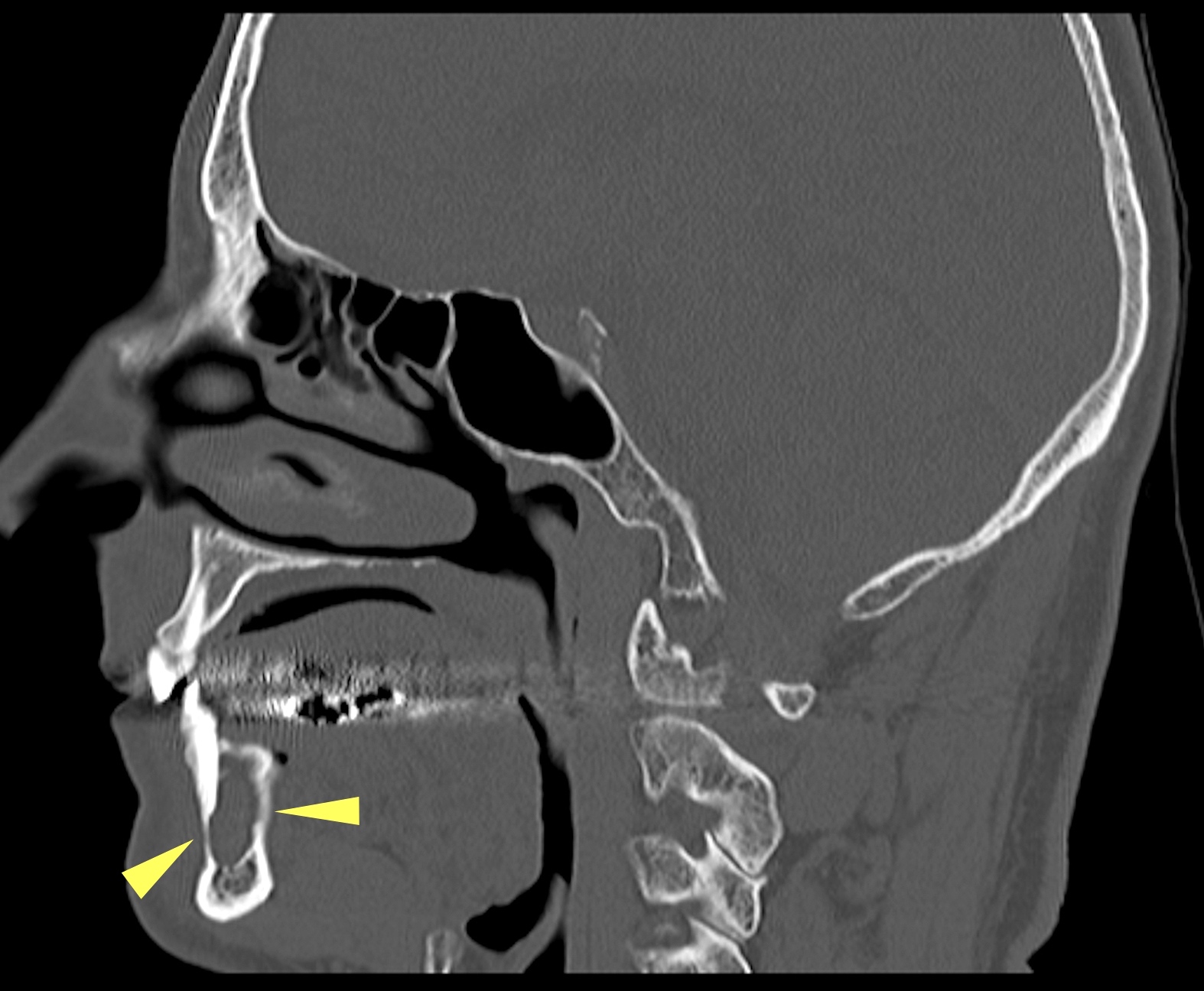
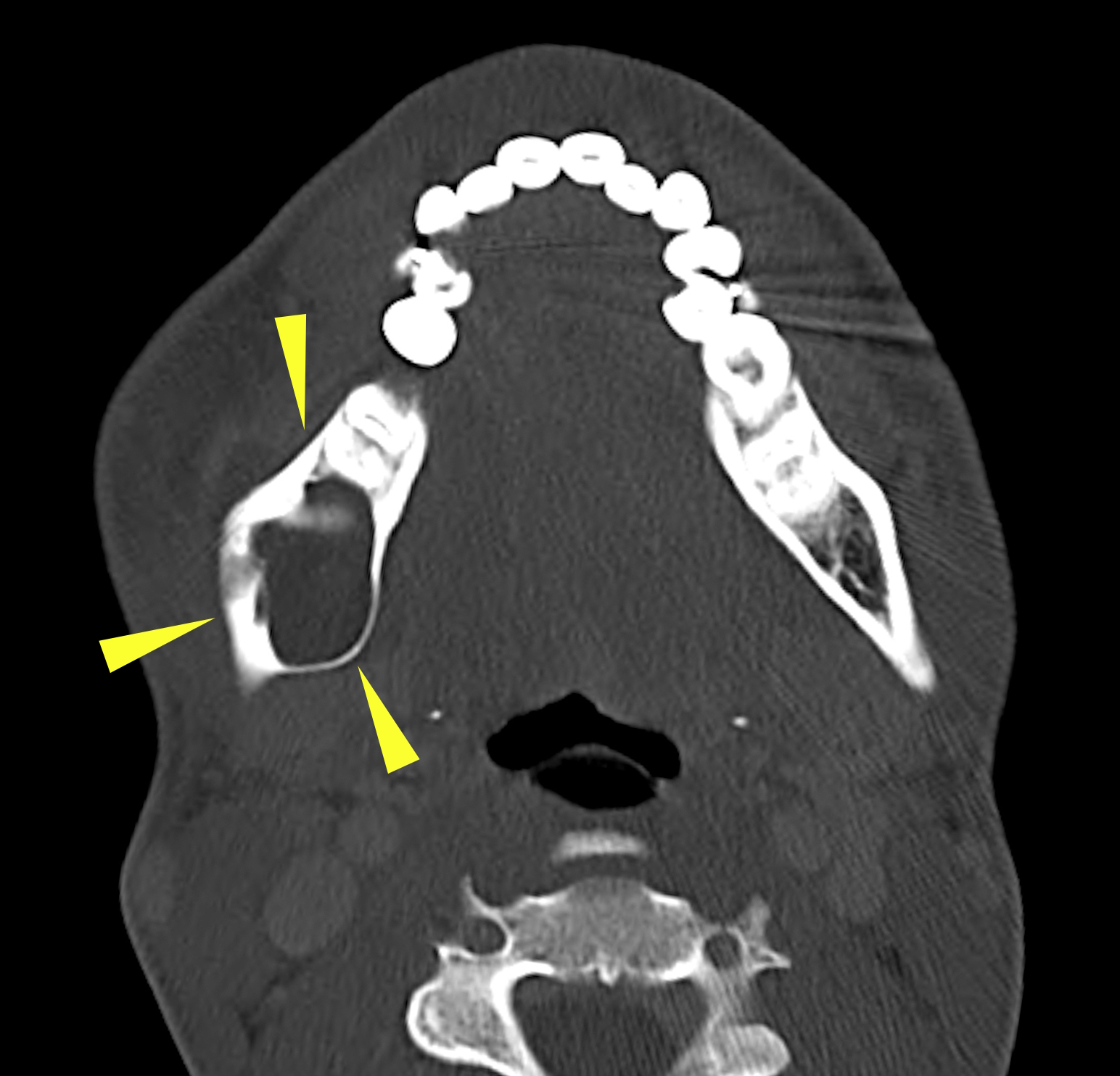
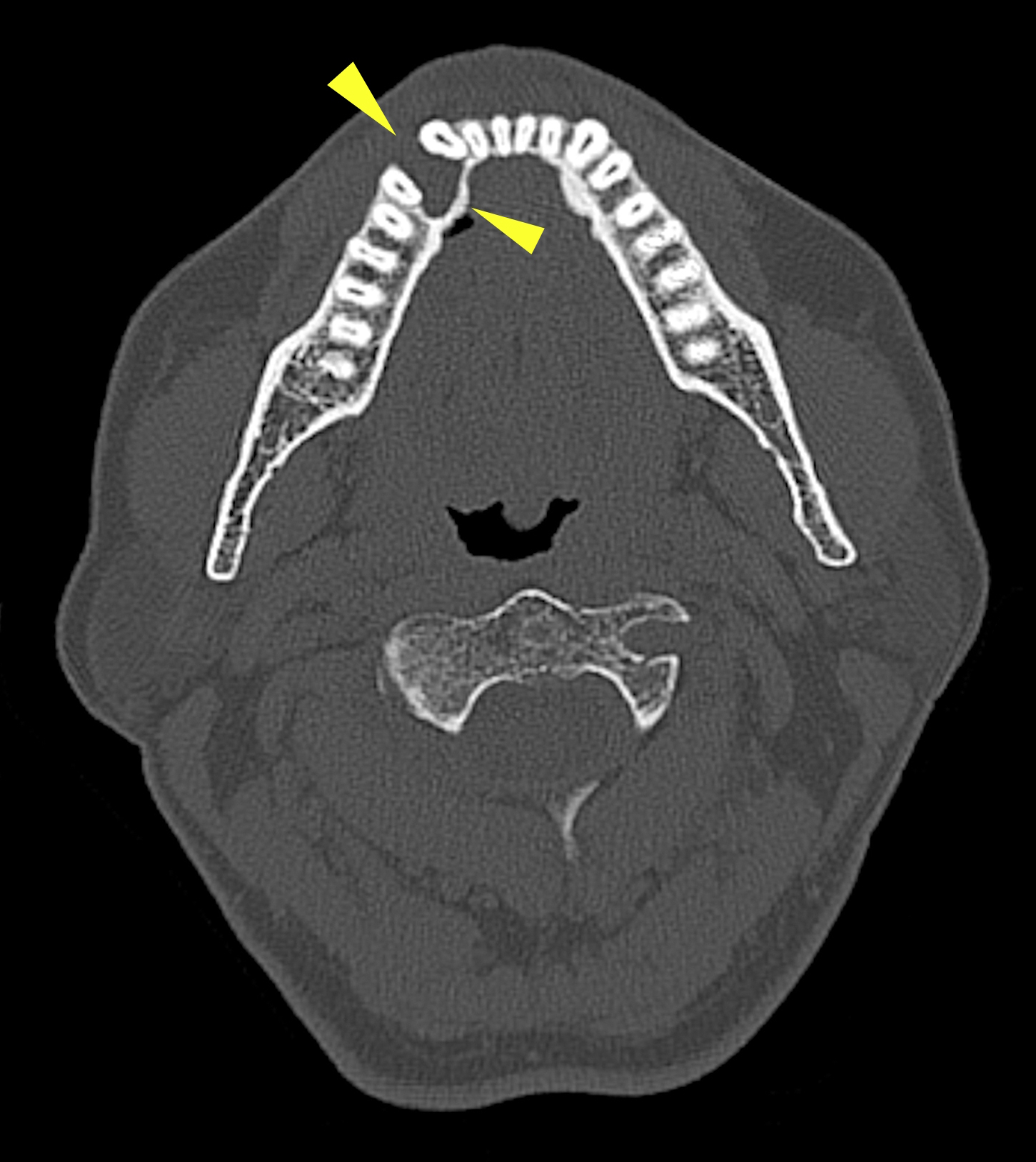
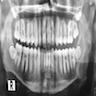
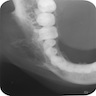
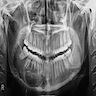
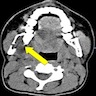
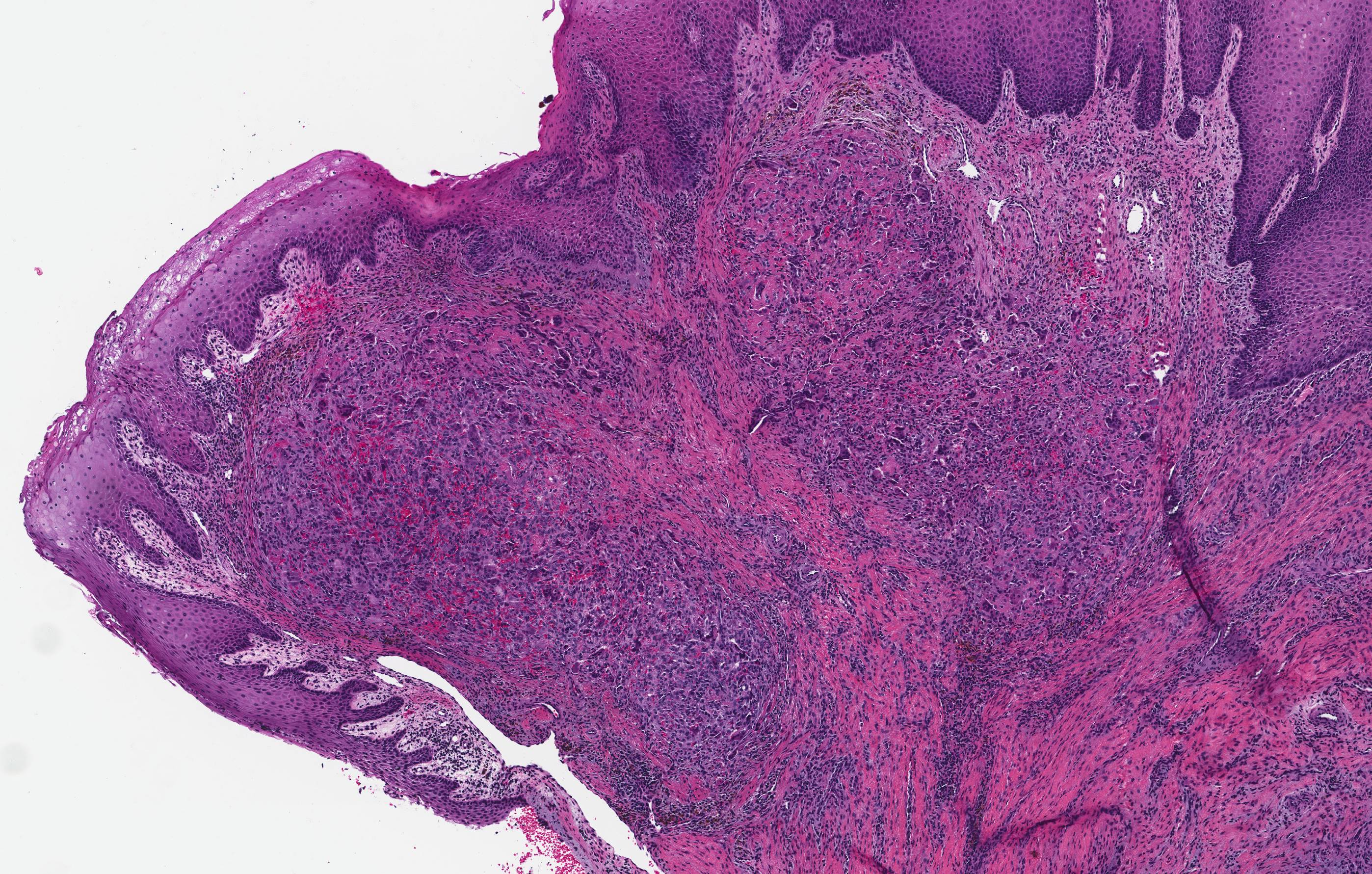
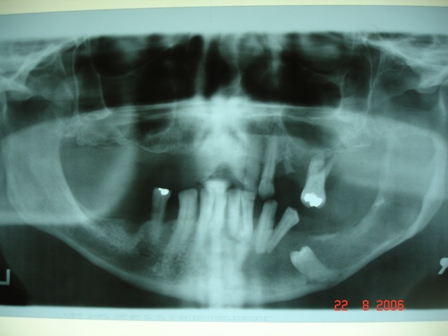
Superpage
Superpage Topics
Acute suppurative
Adenoid ameloblastoma
Adenomatoid odontogenic tumor
Ameloblastic carcinoma
Ameloblastic fibroma
Ameloblastoma
Anatomy & histology
Calcifying epithelial odontogenic tumor
Calcifying odontogenic cyst
Cemento-osseous dysplasia
Cemento-ossifying fibroma / ossifying fibroma
Cementoblastoma
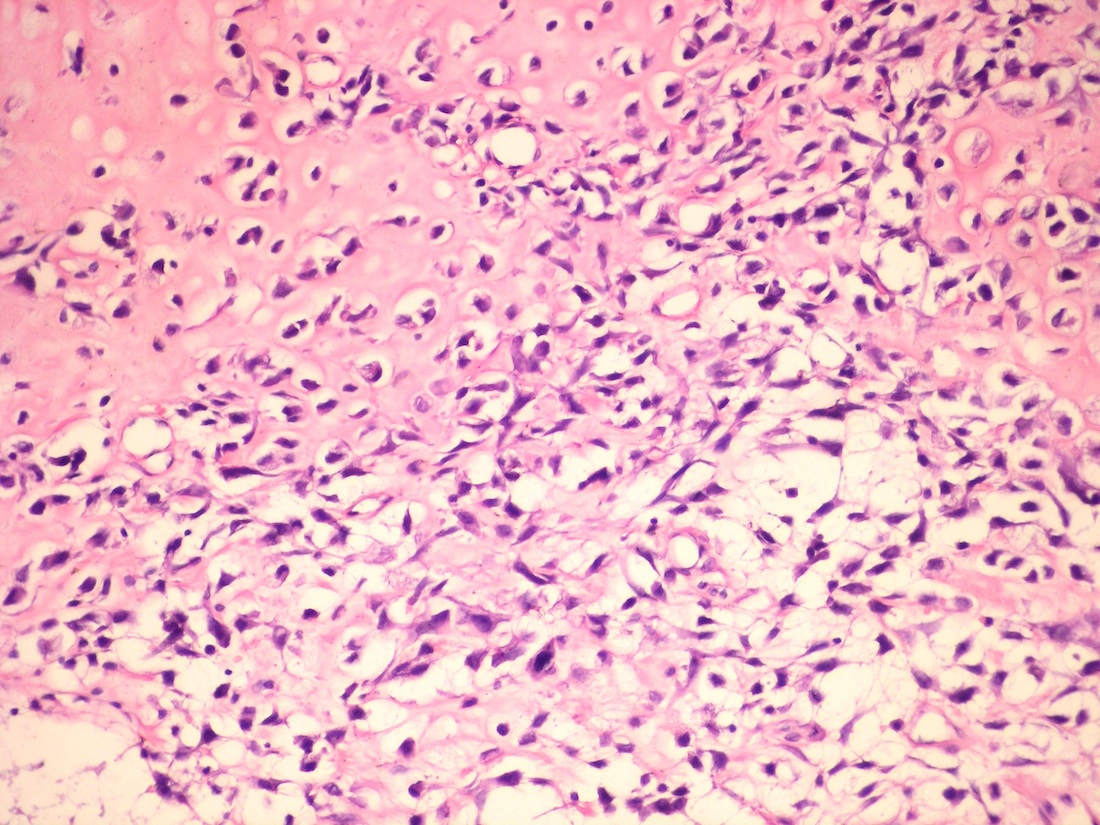
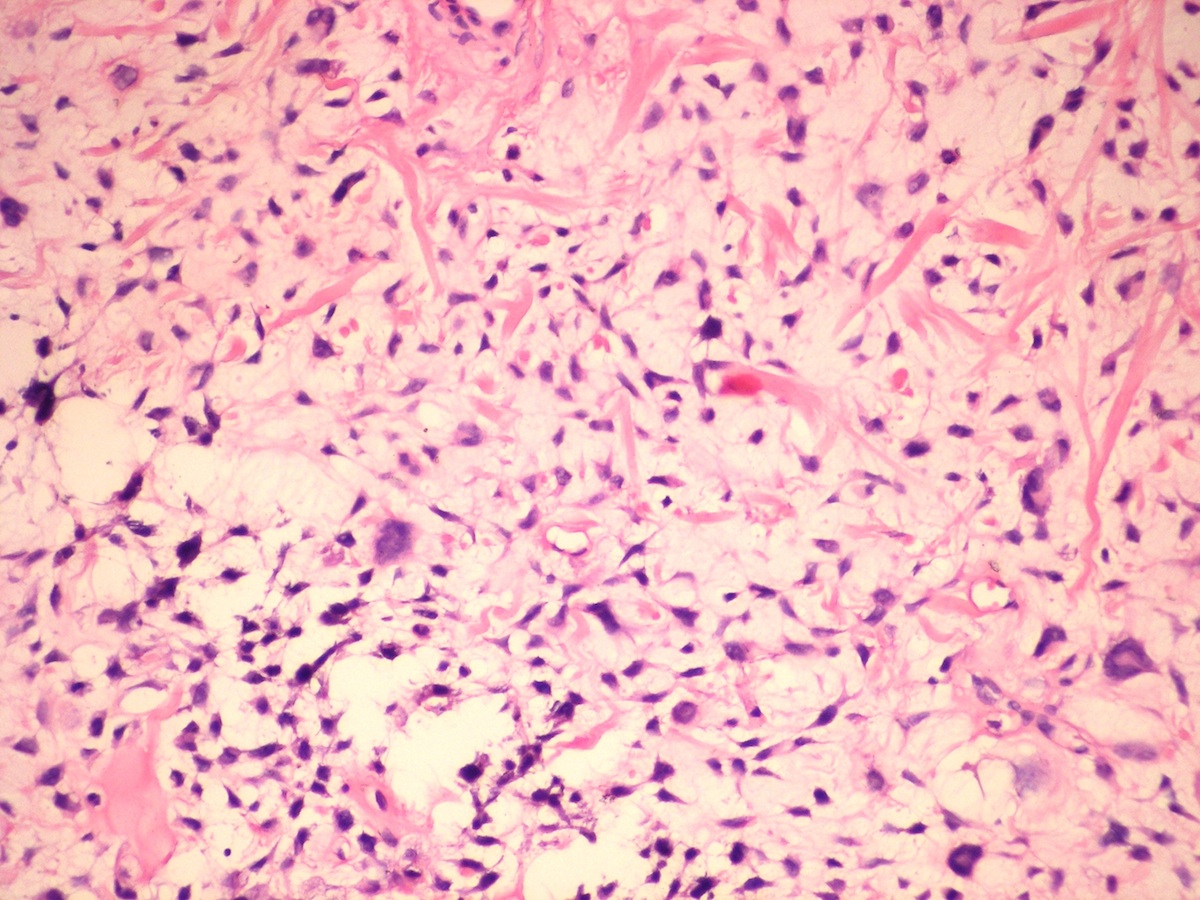
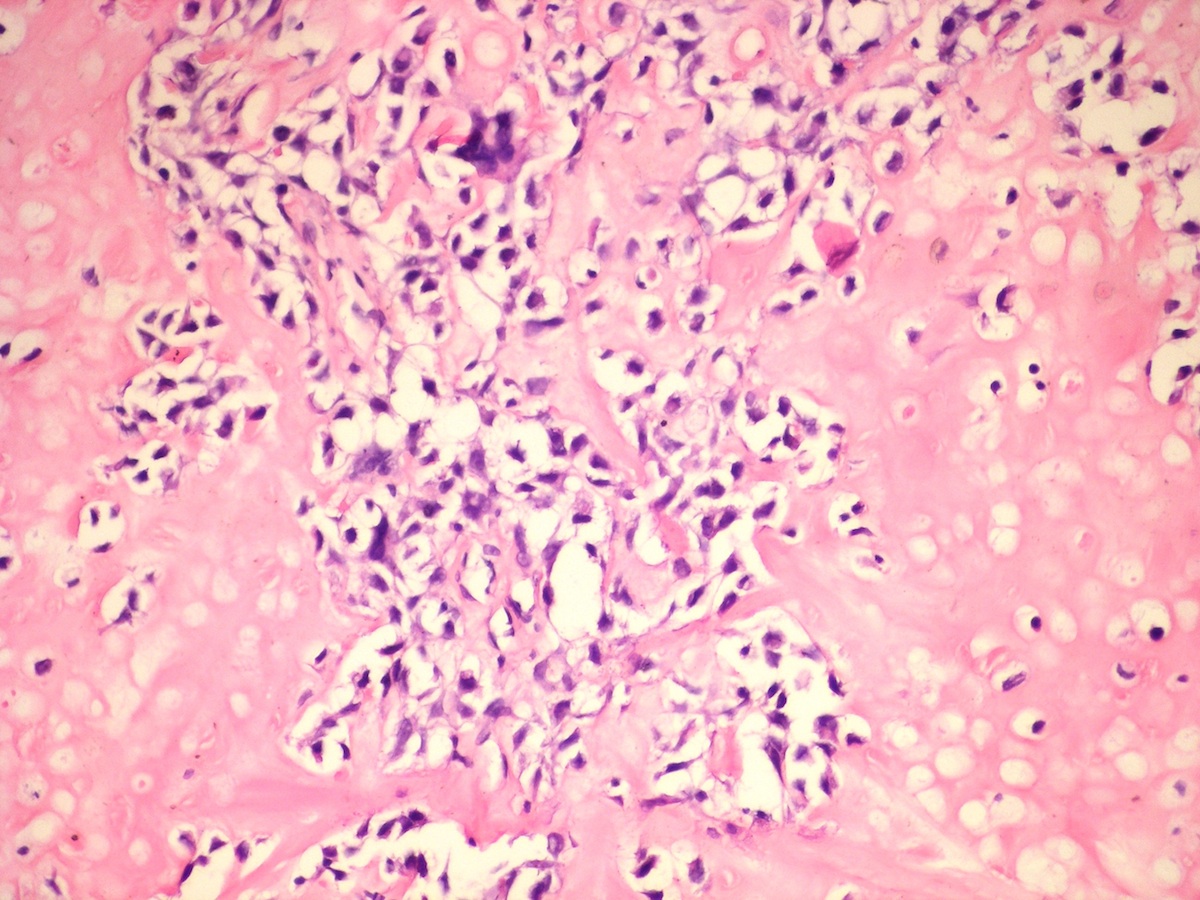
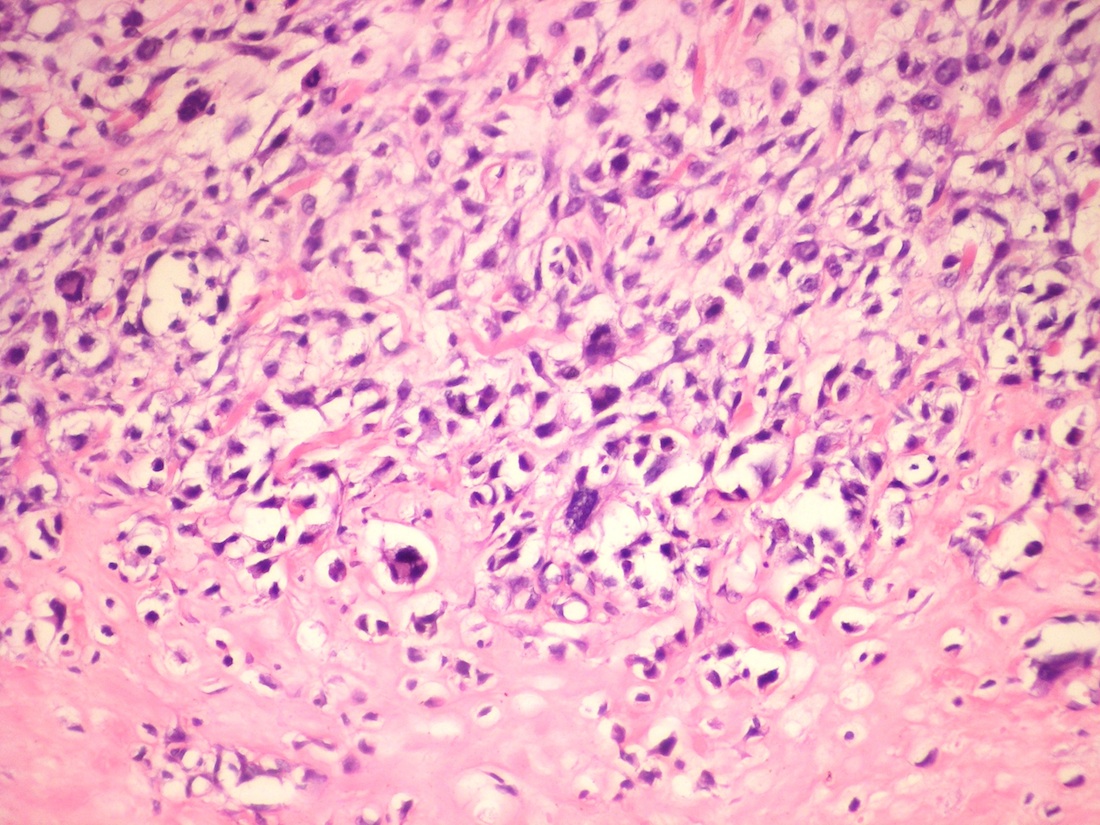
Central giant cell granuloma
Cherubism
Chronic recurrent multifocal
Clear cell carcinoma of salivary gland
Clear cell odontogenic carcinoma
Condensing osteitis
Dentigerous cyst
Dentinogenic ghost cell tumor
Diffuse sclerosing
Eruption cyst
Ghost cell odontogenic carcinoma
Gingival cyst (adult)
Gingival cyst (newborn)
Glandular odontogenic cyst
Grossing (pending)
Inflammatory collateral cyst
Juvenile trabecular ossifying fibroma and psammomatoid ossifying fibroma
Langerhans cell histiocytosis
Lateral periodontal cyst and botryoid odontogenic cyst
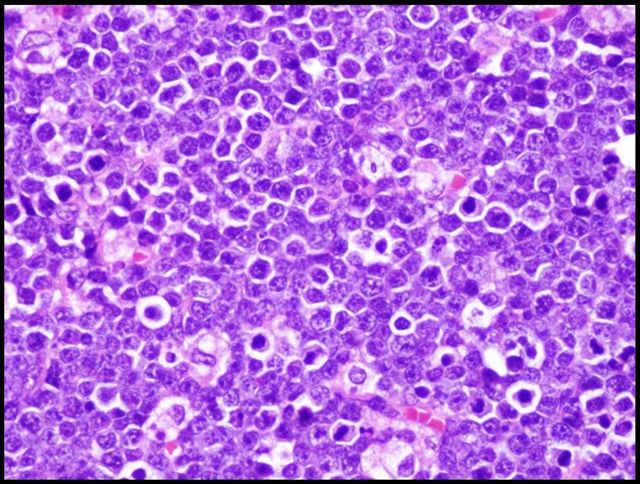
Lymphoma
Medication related osteonecrosis of jaw
Melanotic neuroectodermal tumor of infancy
Metastases
Nasopalatine duct cyst
Odontoameloblastoma
Odontogenic / jaw cysts overview
Odontogenic carcinosarcoma (pending)
Odontogenic fibroma
Odontogenic keratocyst
Odontogenic myxoma / fibromyxoma
Odontogenic sarcoma / ameloblastic fibrosarcoma
Odontoma
Orthokeratinized odontogenic cyst
Osteomyelitis overview
Osteoradionecrosis
Osteosarcoma
Periapical (dental) granuloma
Peripheral giant cell granuloma
Primary intraosseous carcinoma, NOS
Primordial odontogenic tumor
Radicular (periapical) cyst
Radicular (periapical) cyst
Residual cyst
Rhabdomyosarcoma with TFCP2 rearrangement (pending)
Sclerosing odontogenic carcinoma
Secondary chronic
Segmental odontomaxillary dysplasia (pending)
Simple bone cyst
Squamous odontogenic tumor
Surgical ciliated cyst
WHO classificationAcute suppurative
Table of Contents
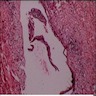
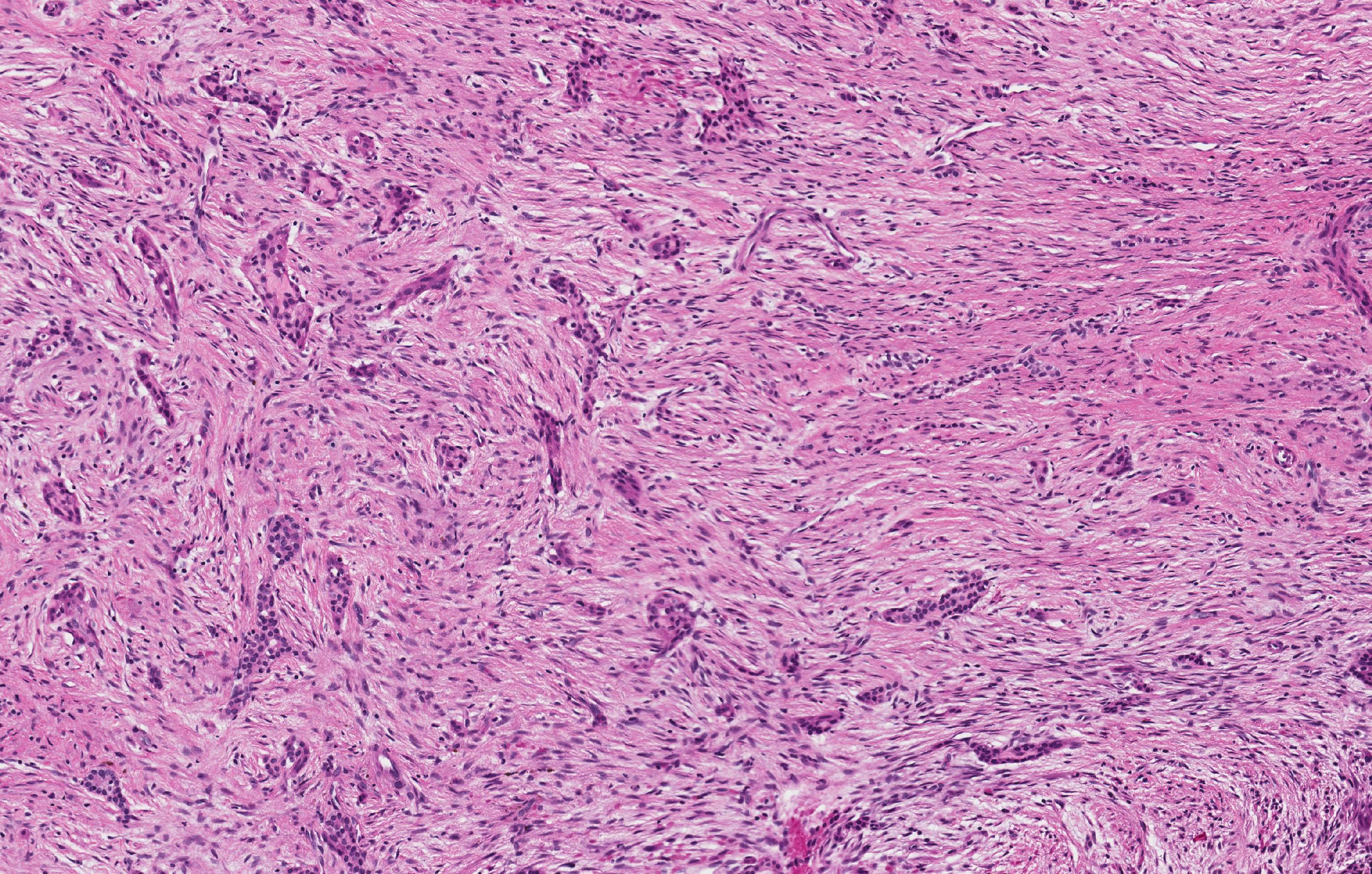
Definition / general | Terminology | Sites | Pathophysiology | Etiology and Pathogenesis, Subclassification Groups | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
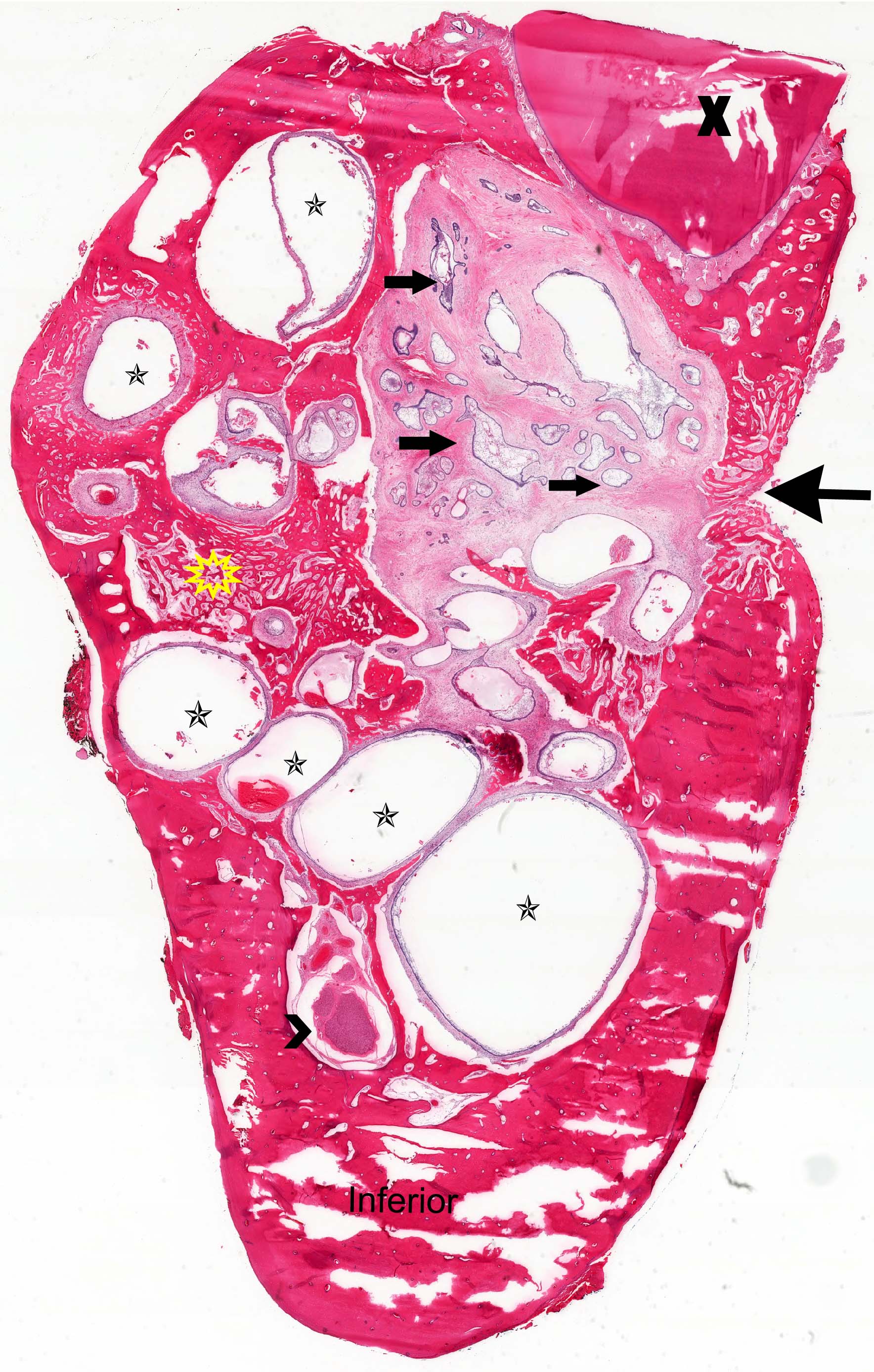
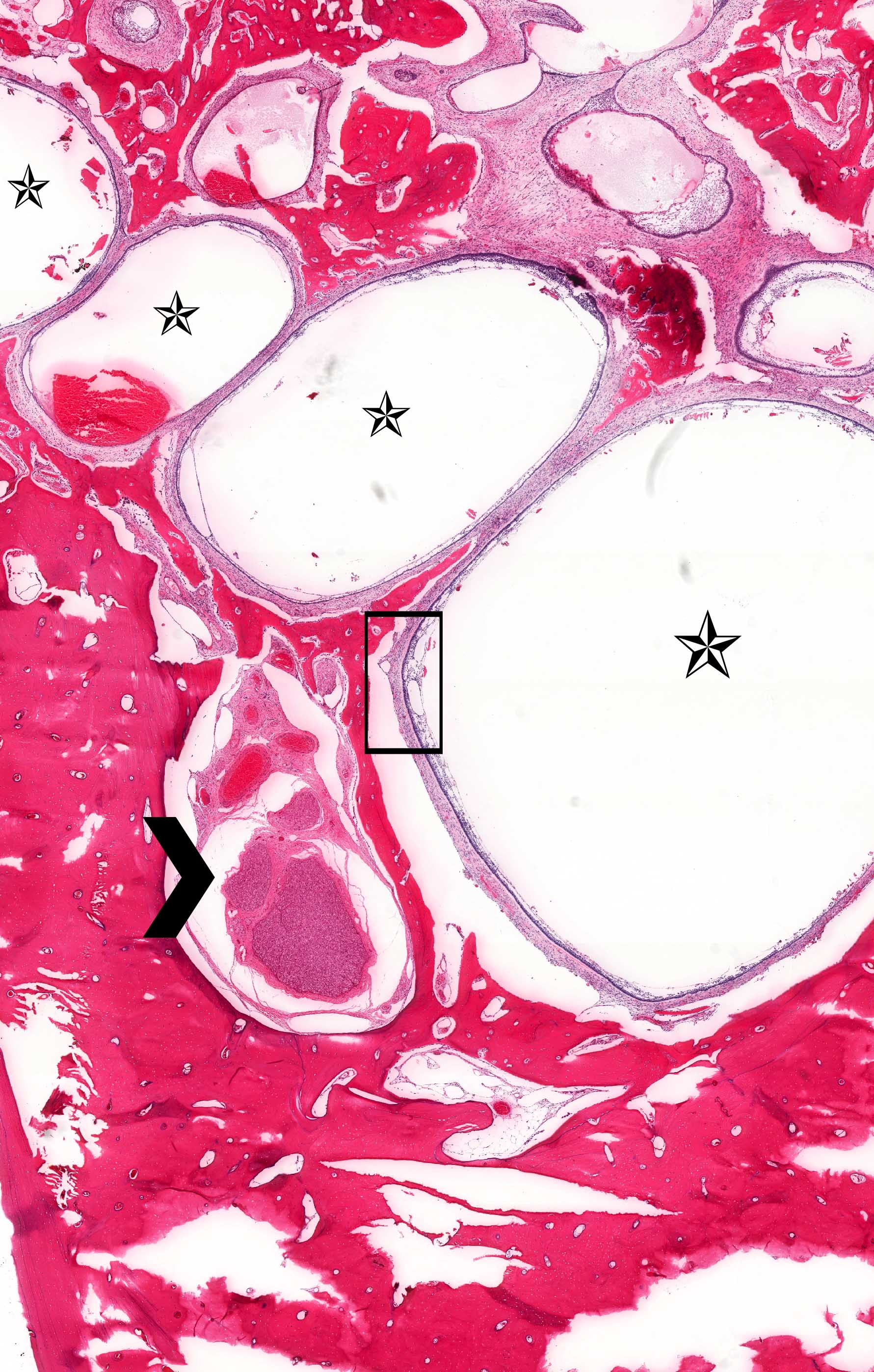
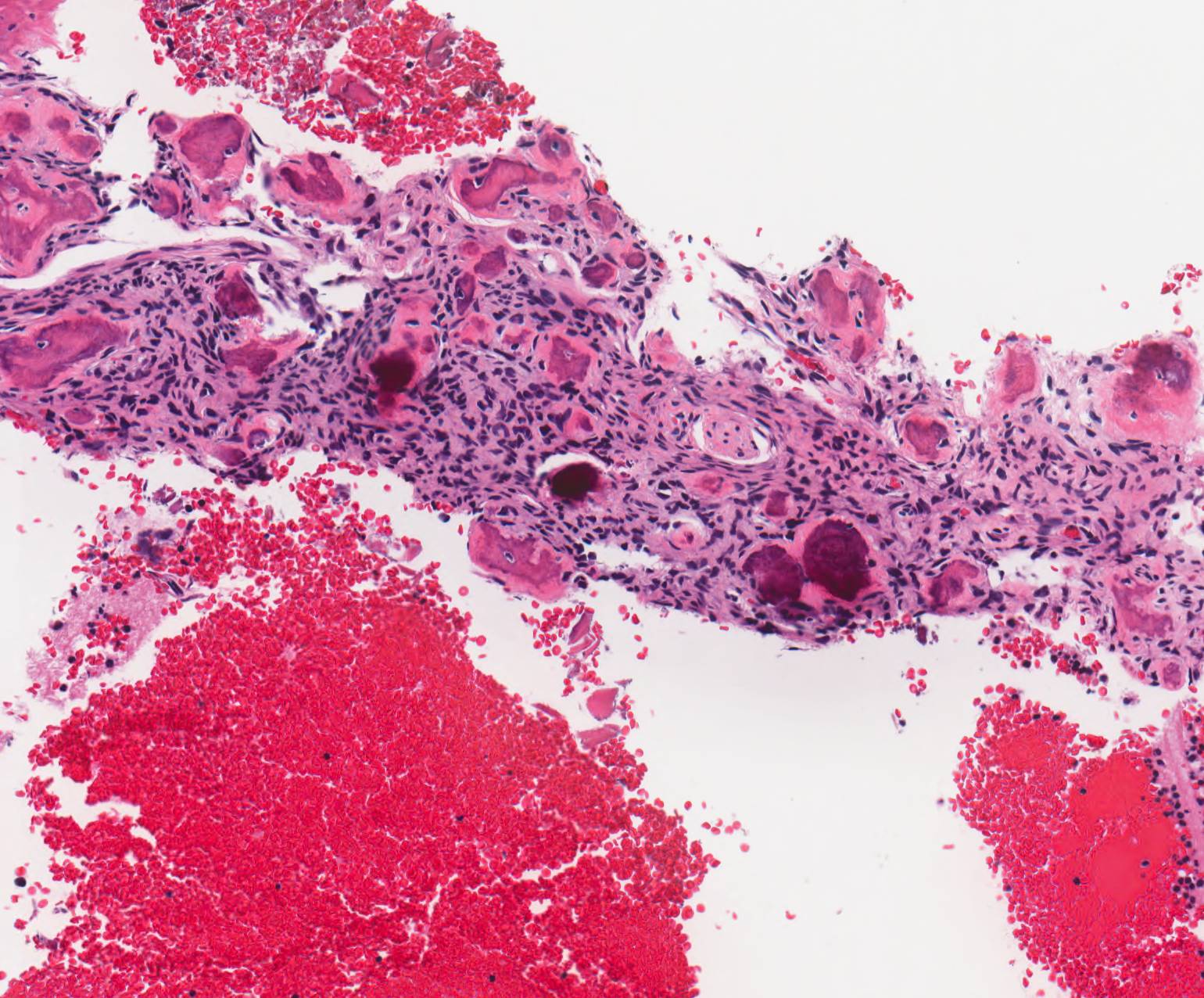
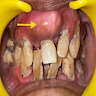
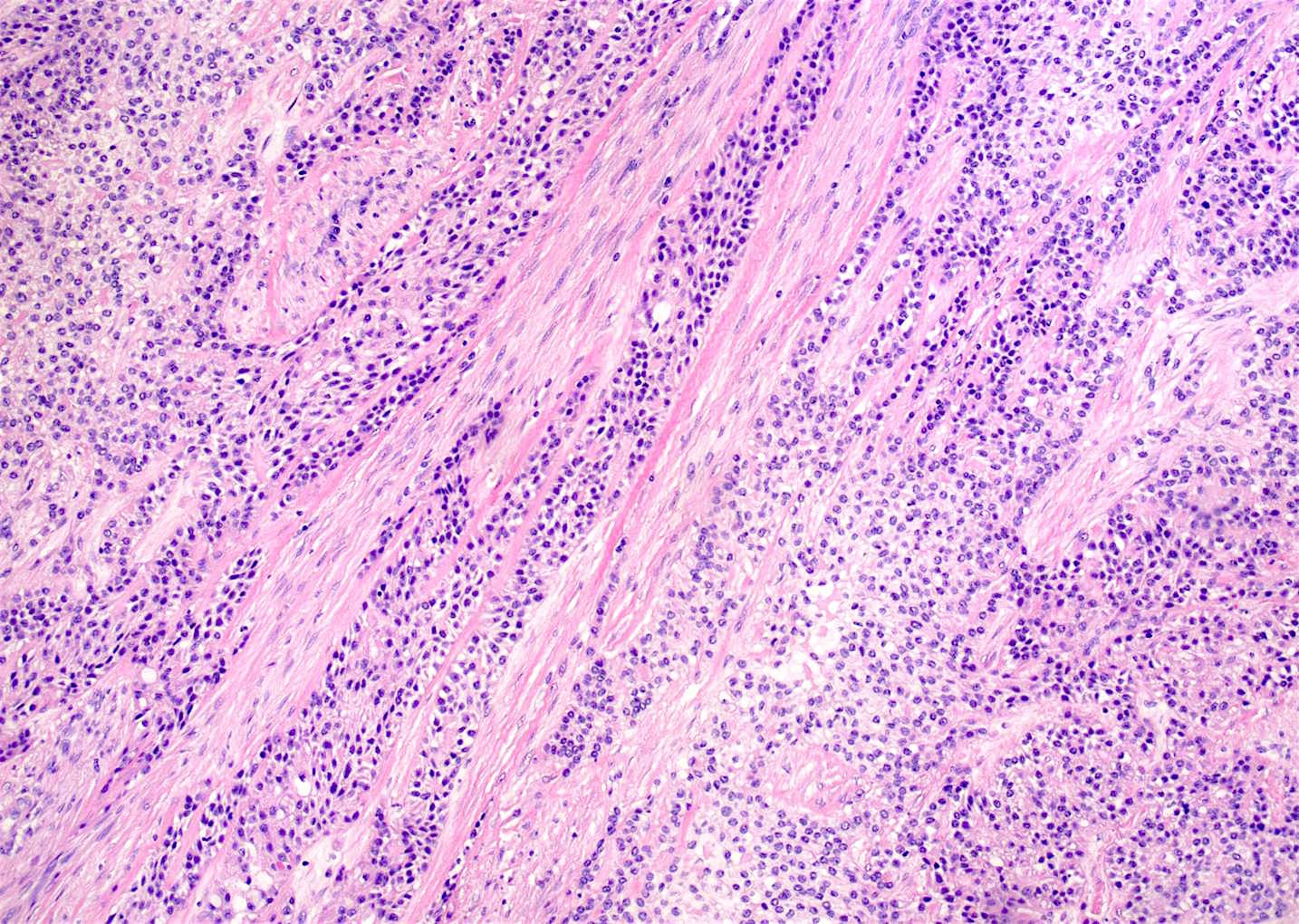
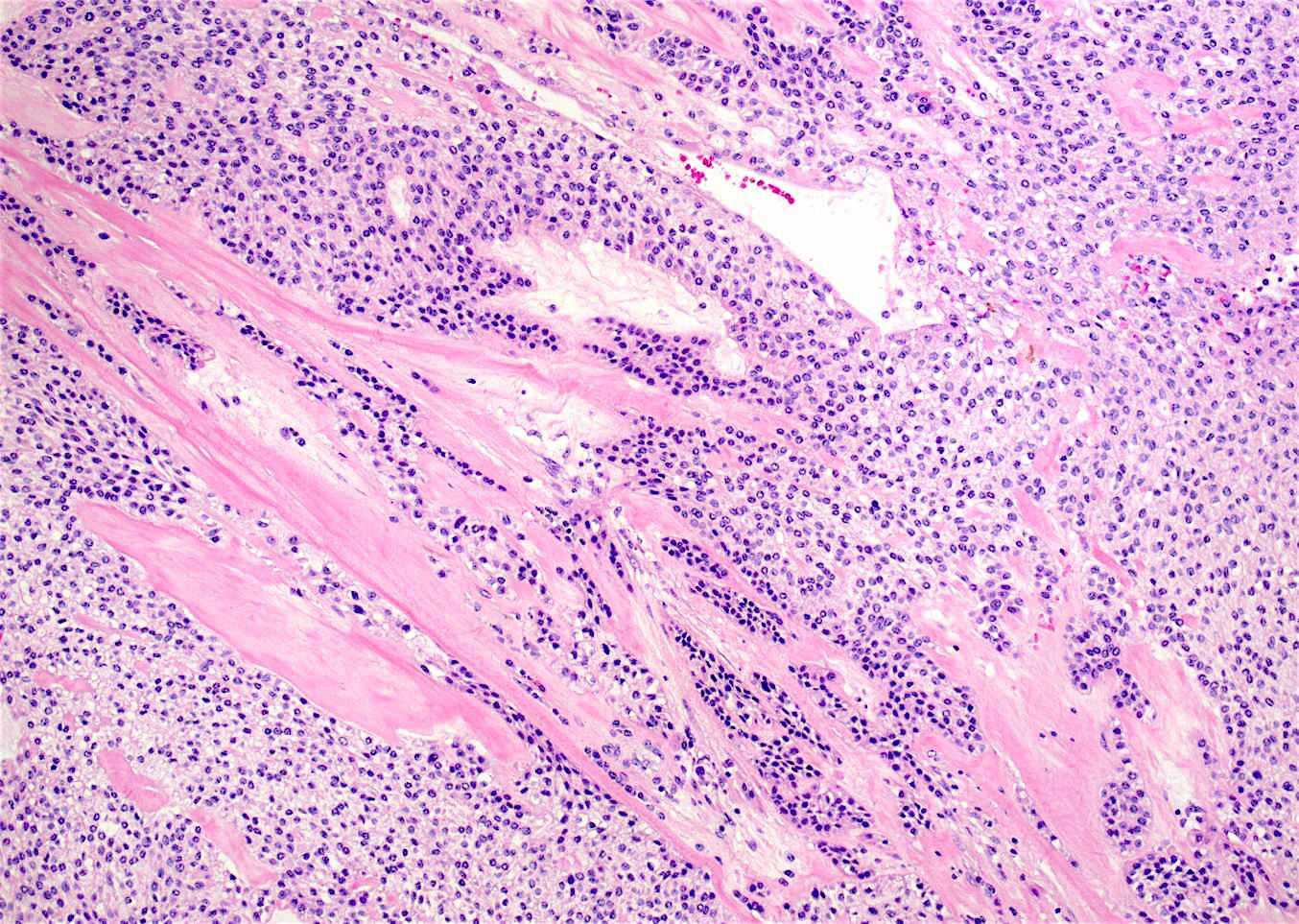
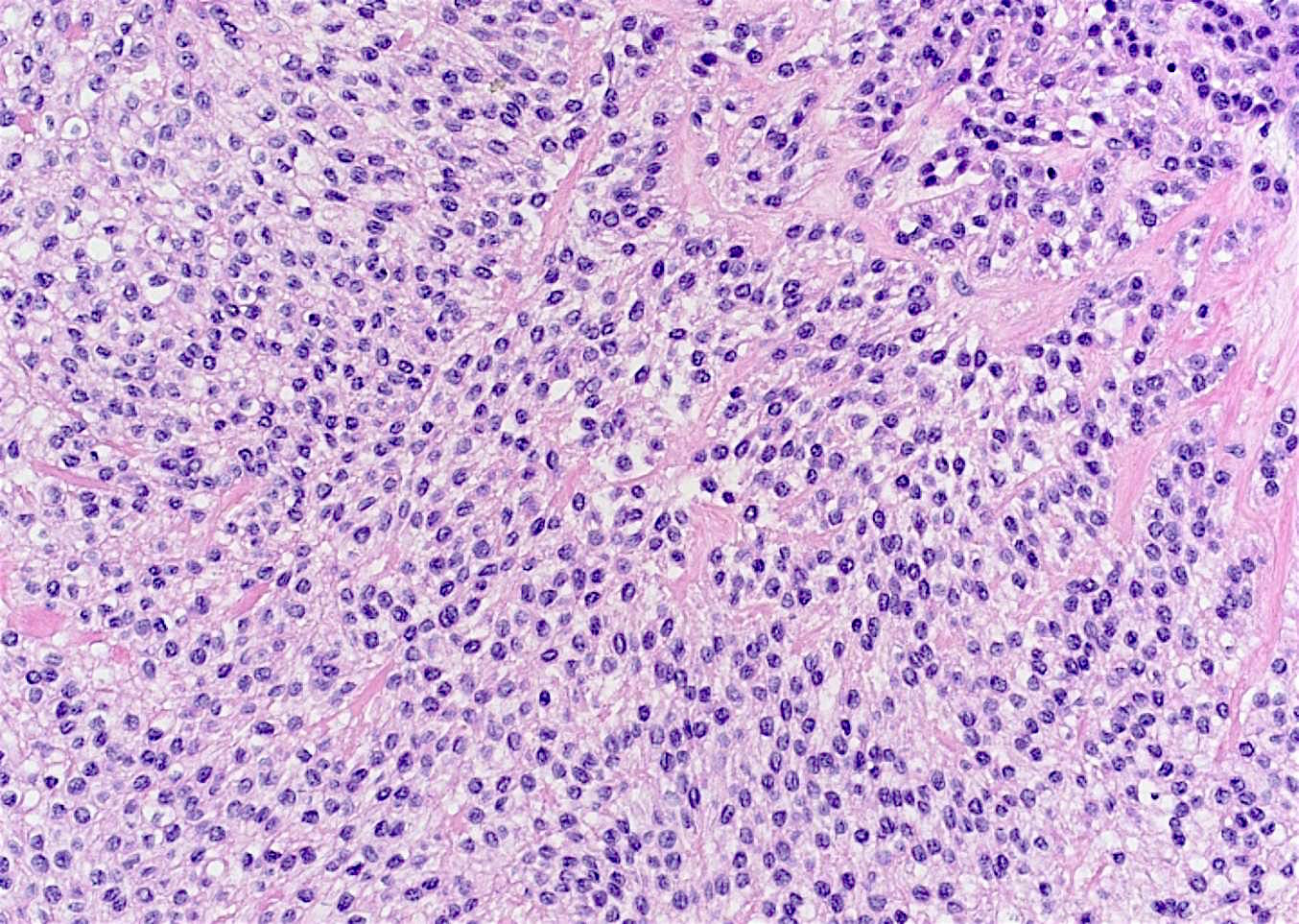
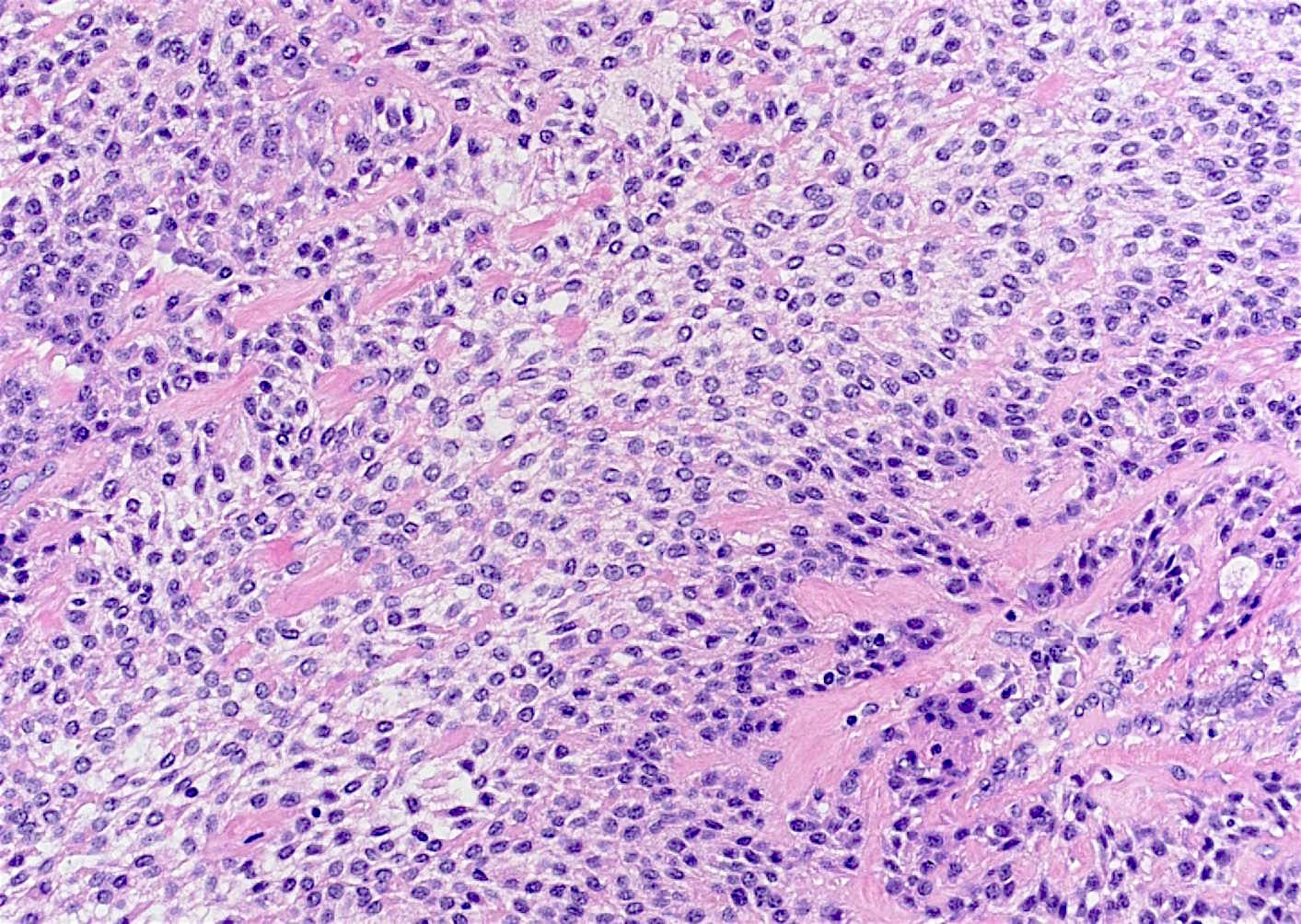
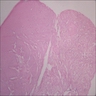
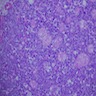
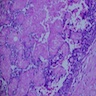
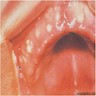
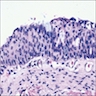
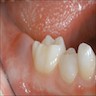
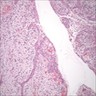
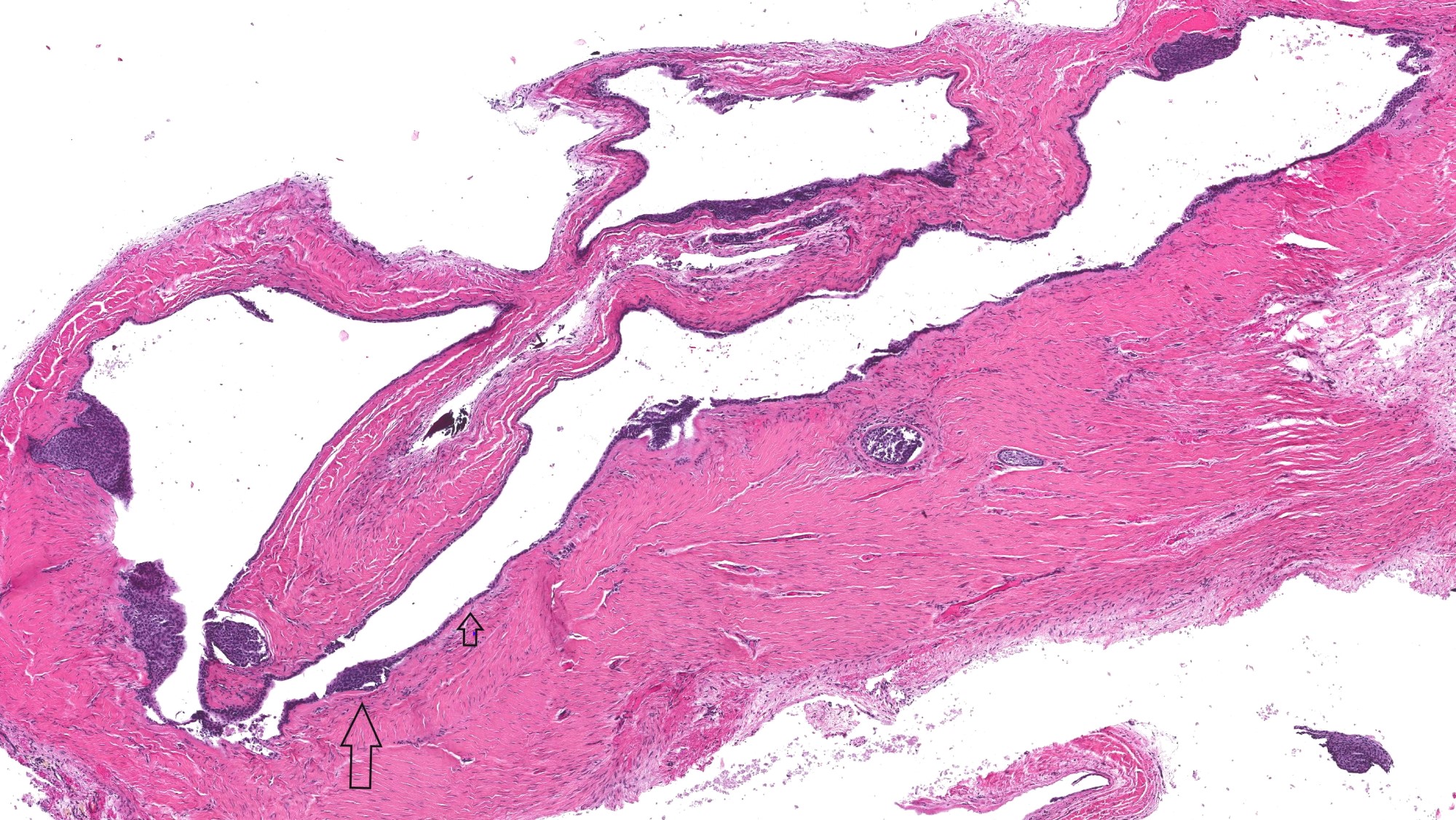
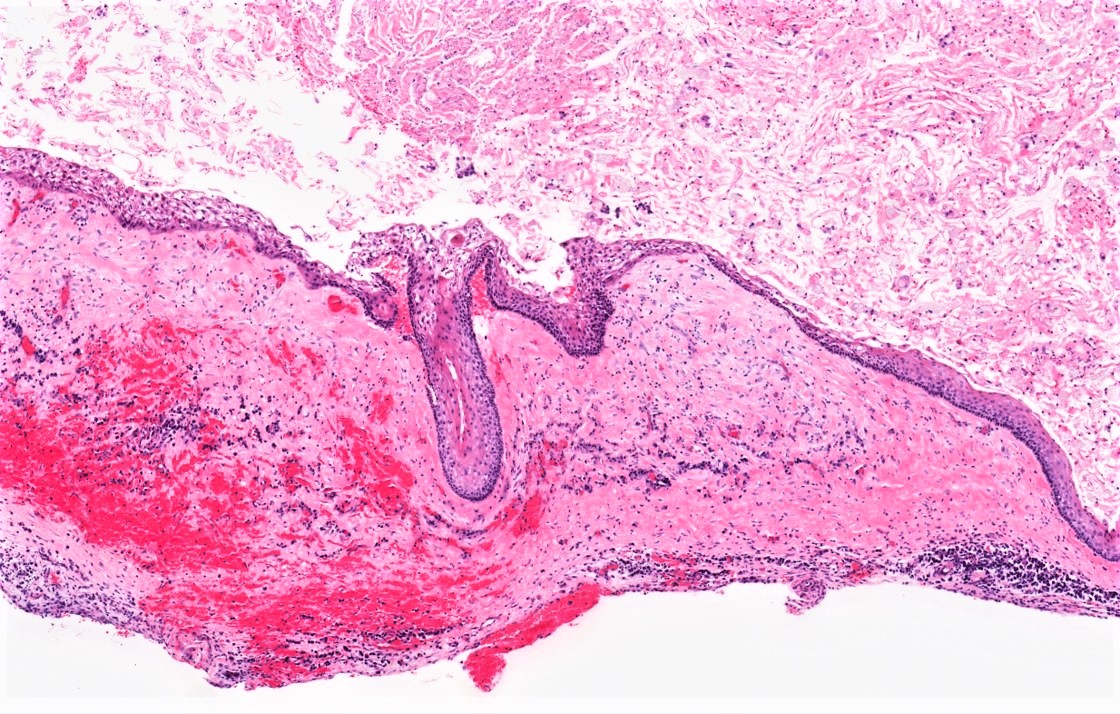
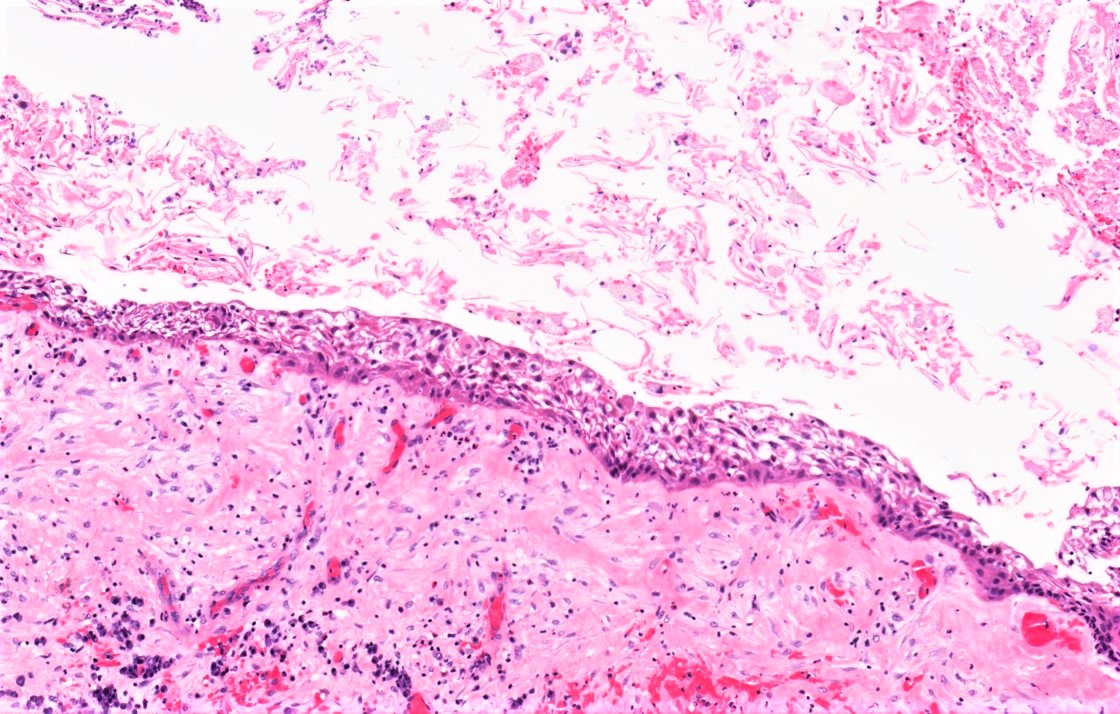
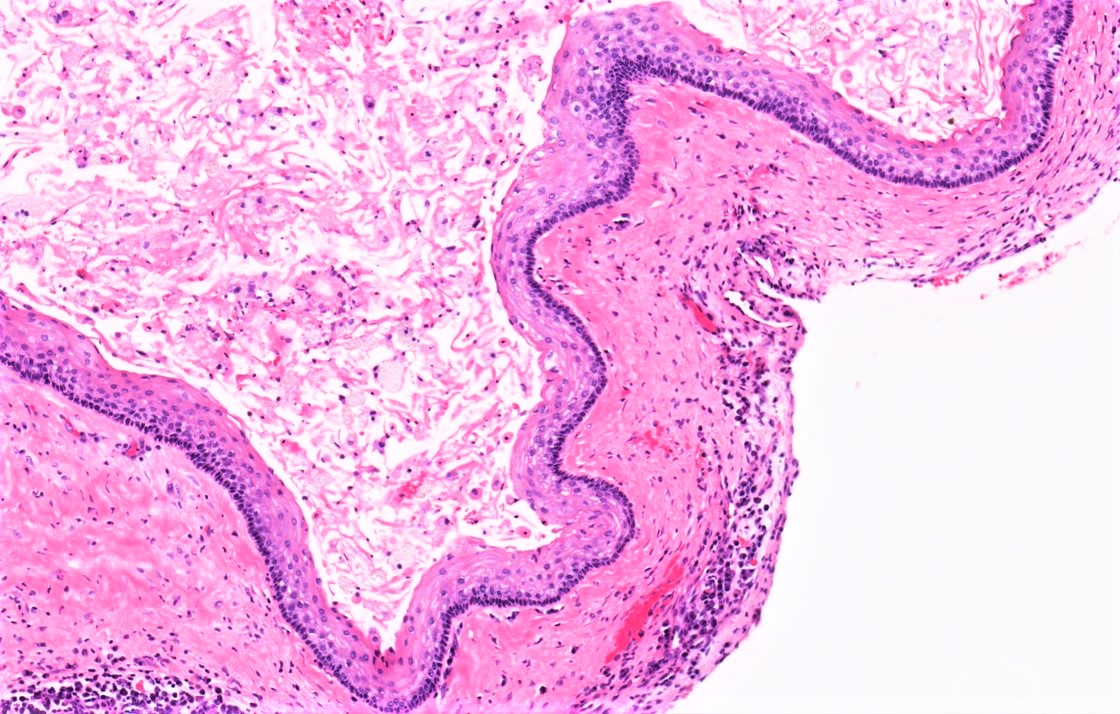
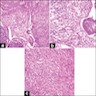
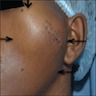
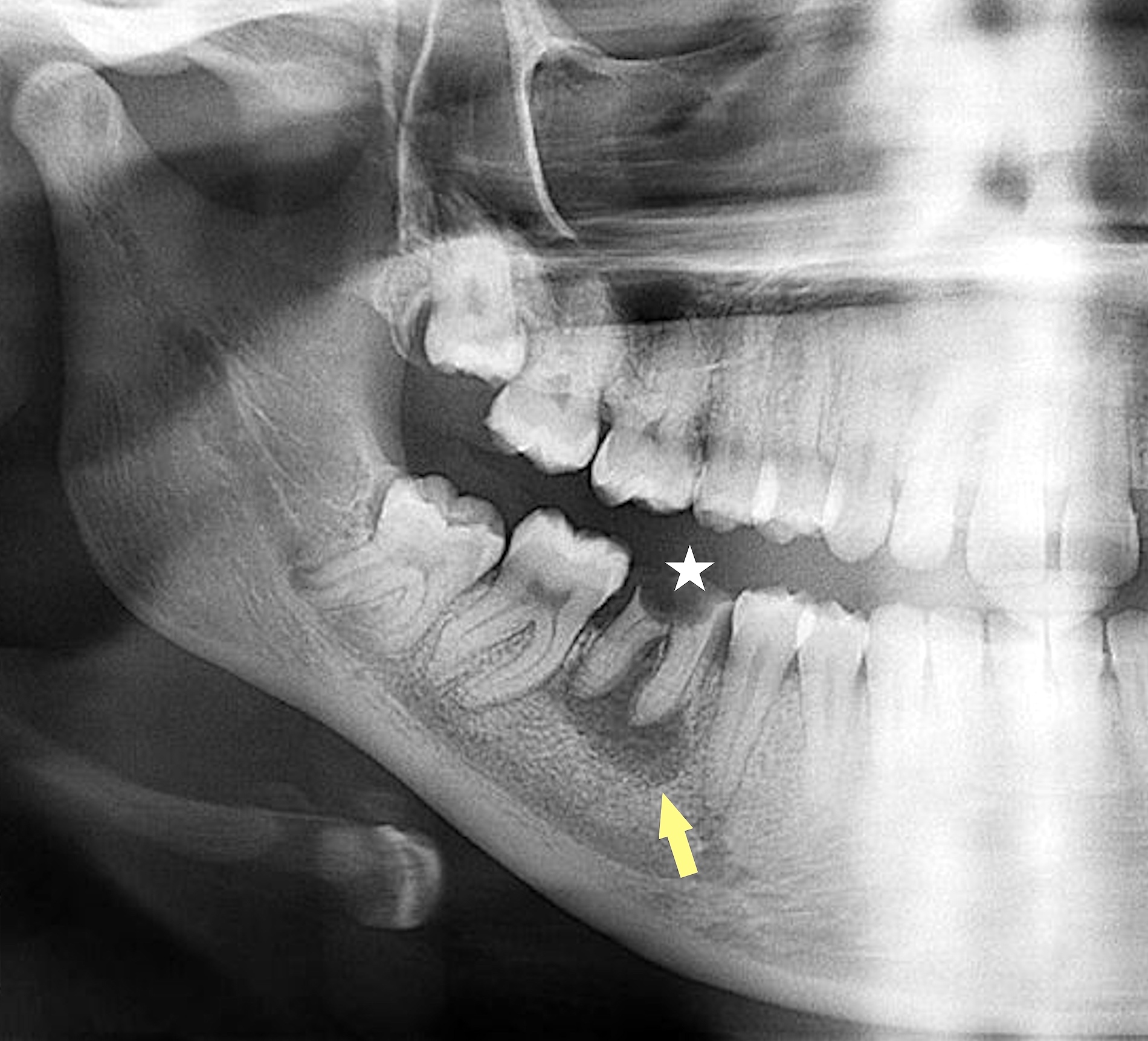
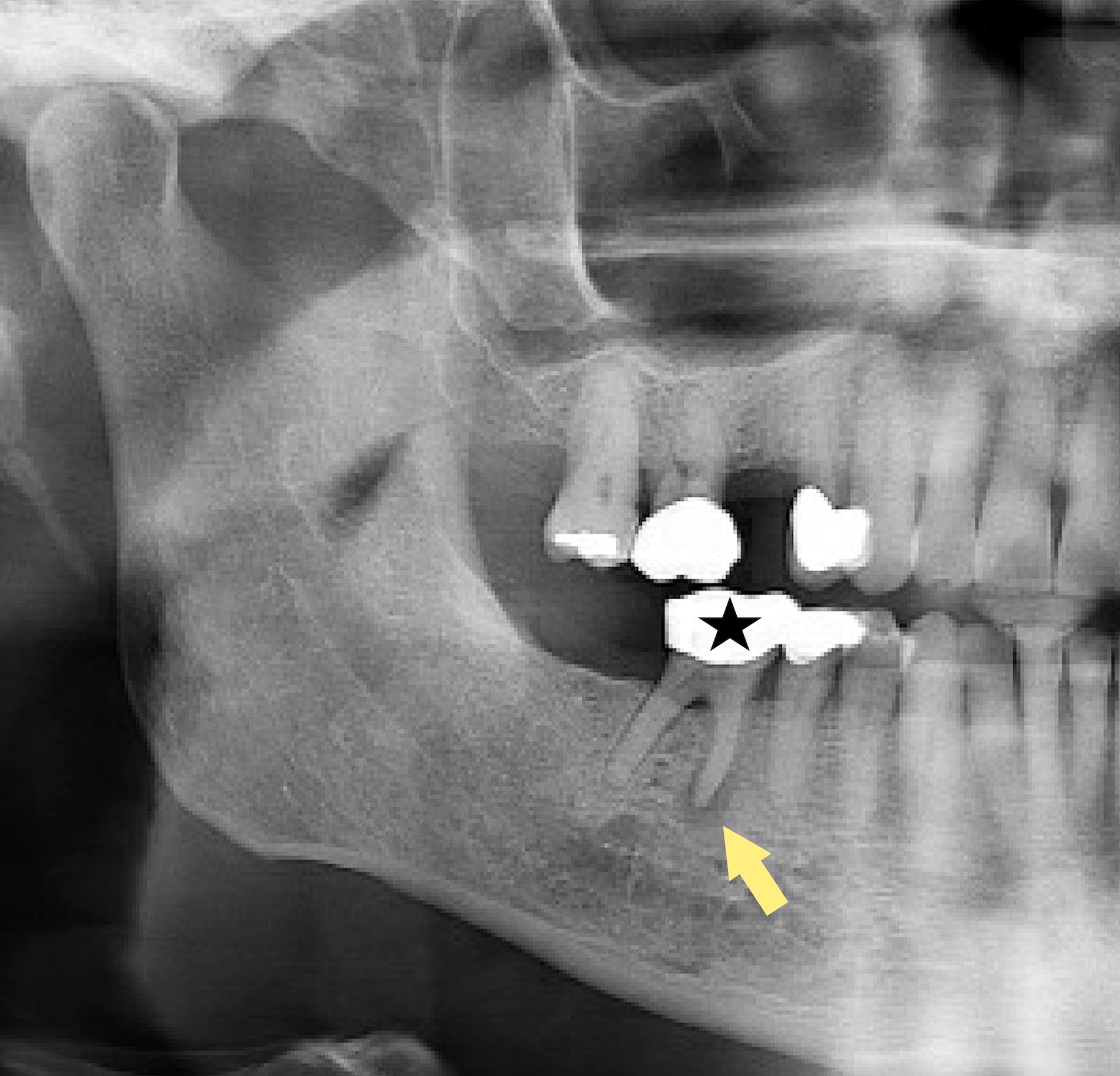
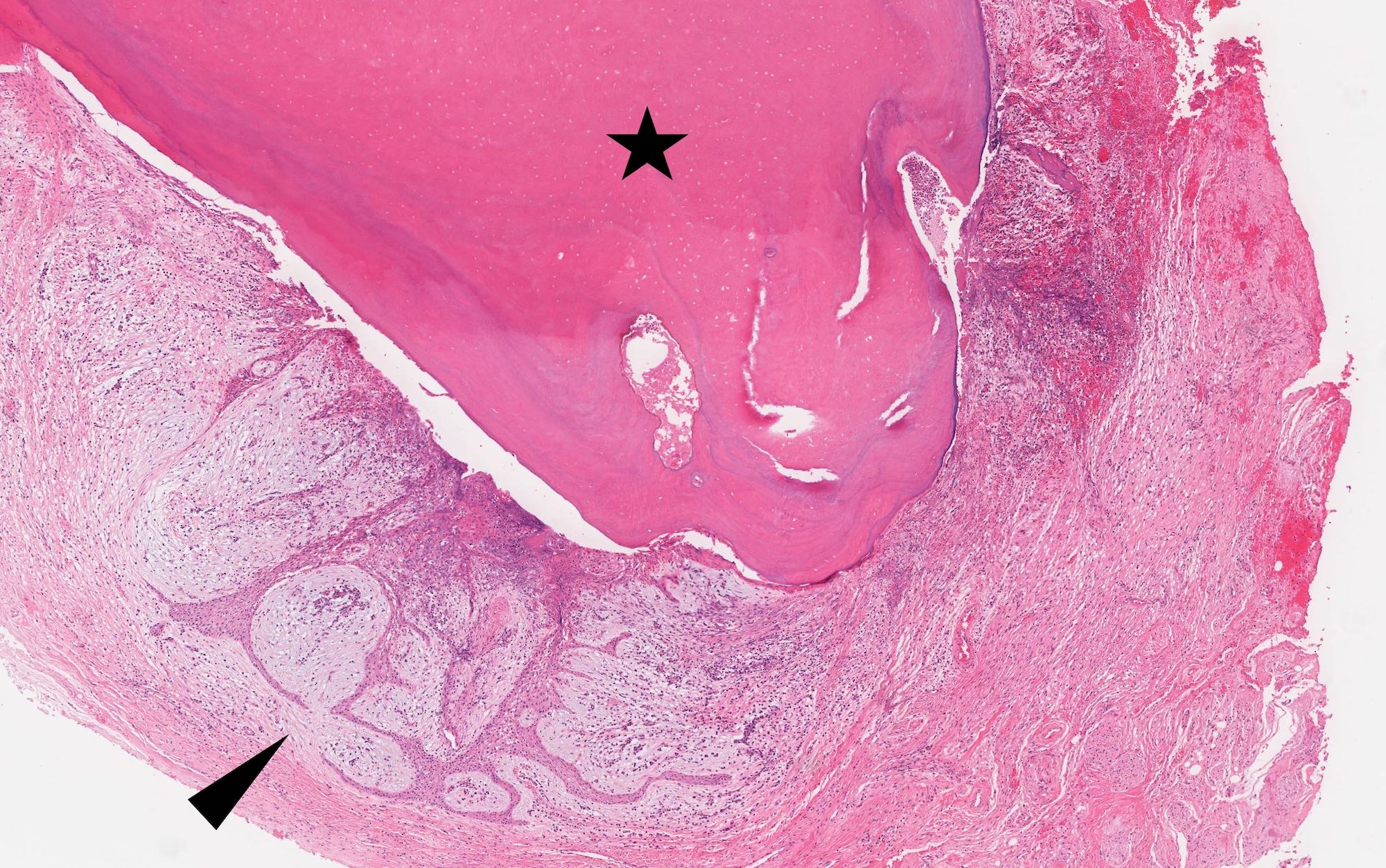
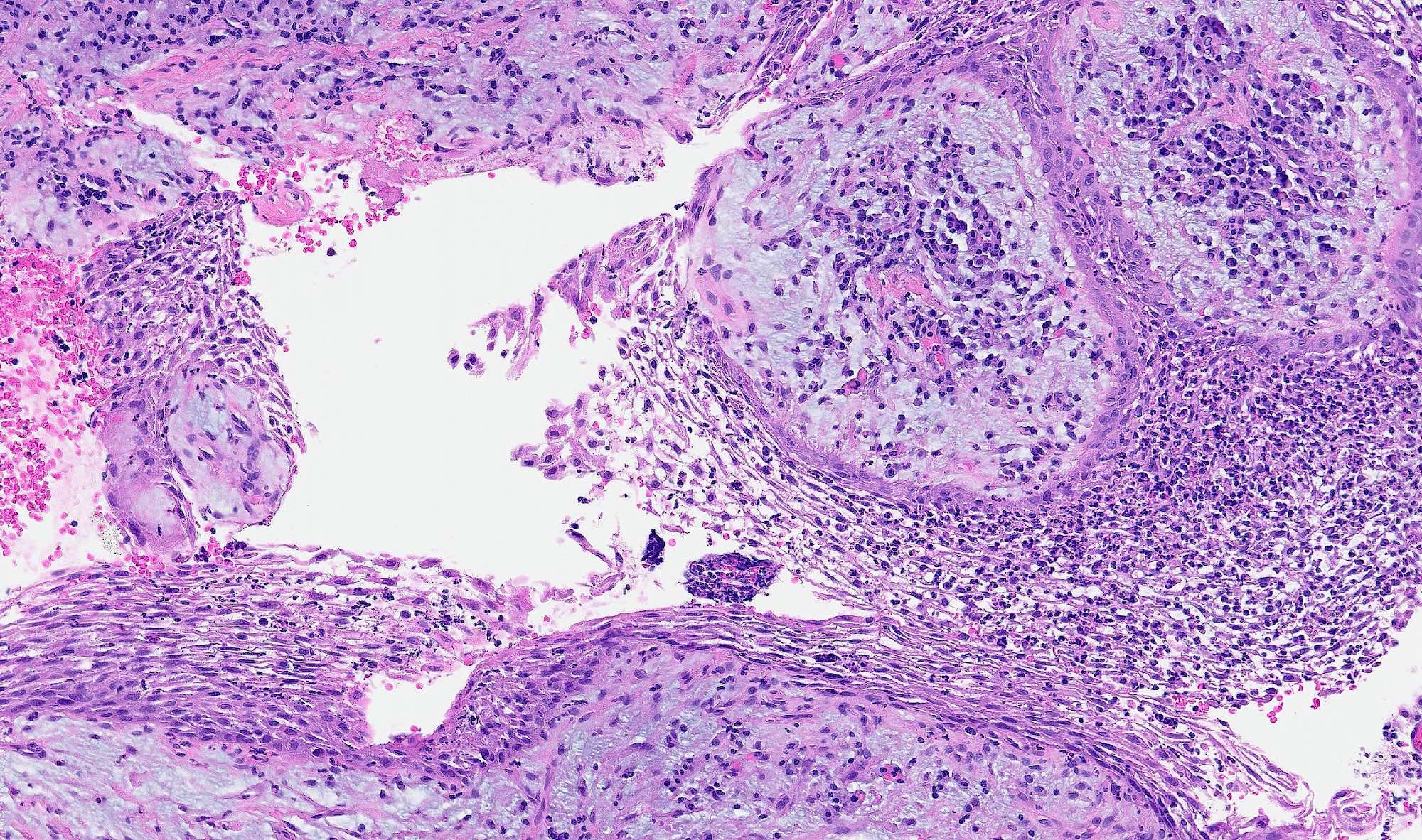
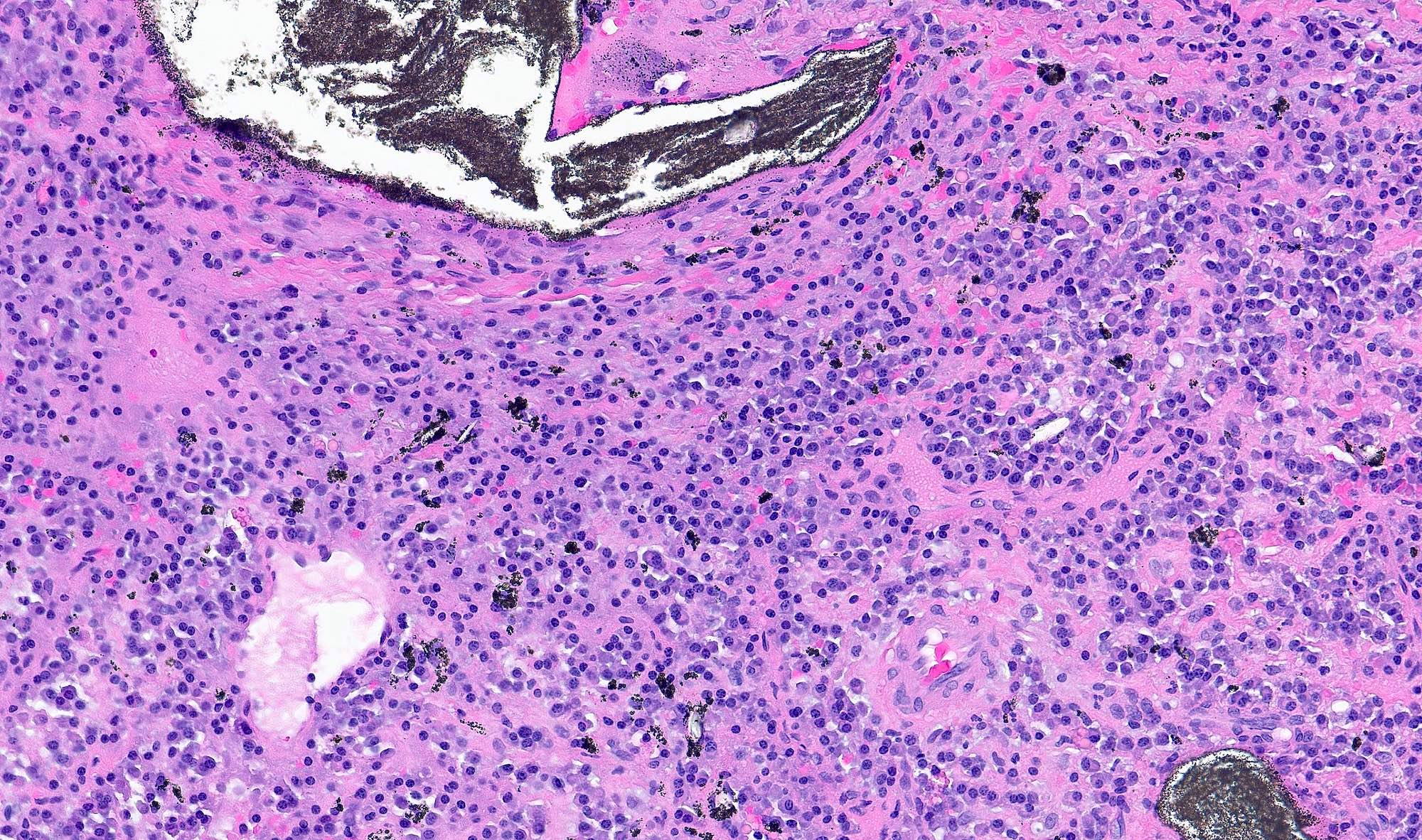
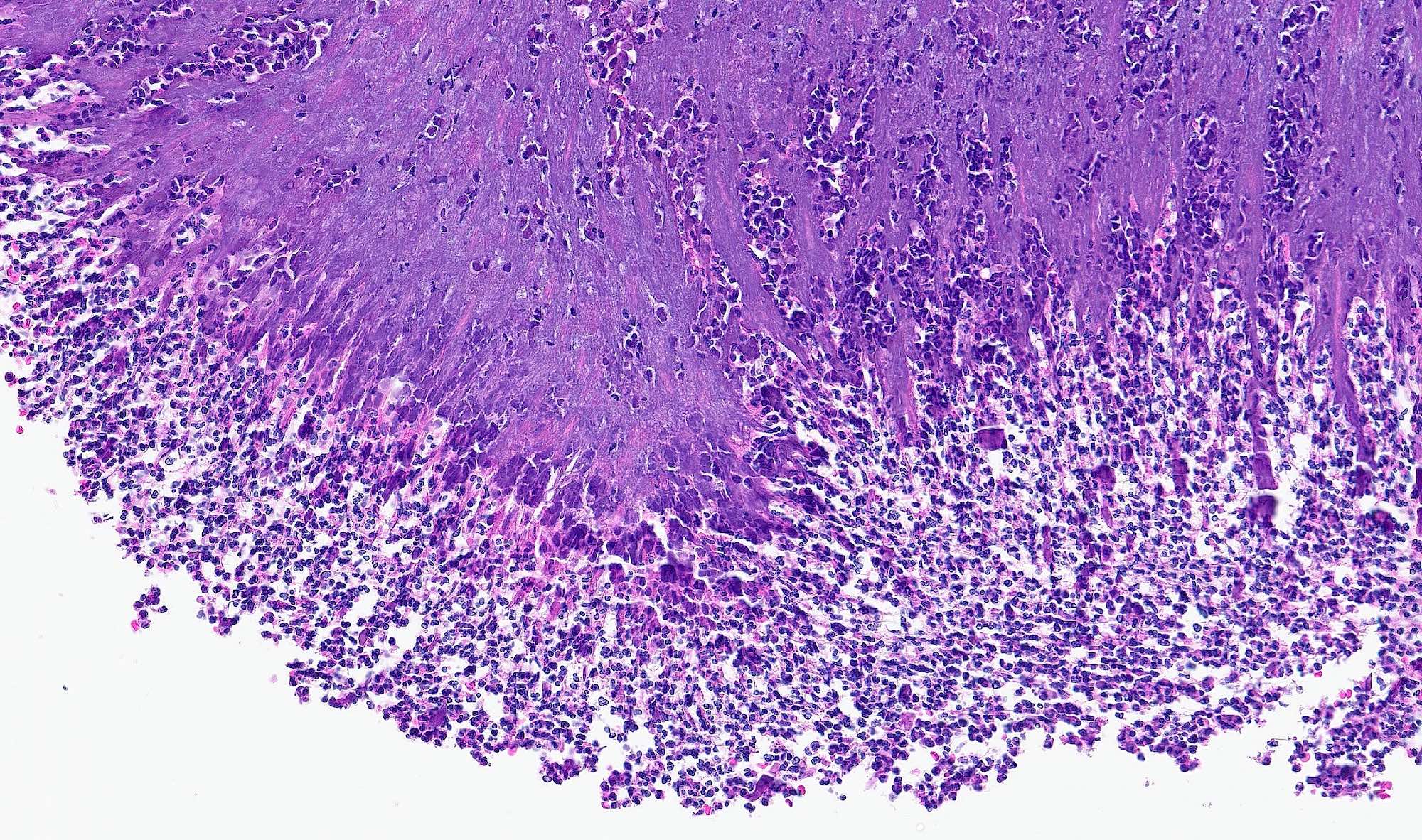
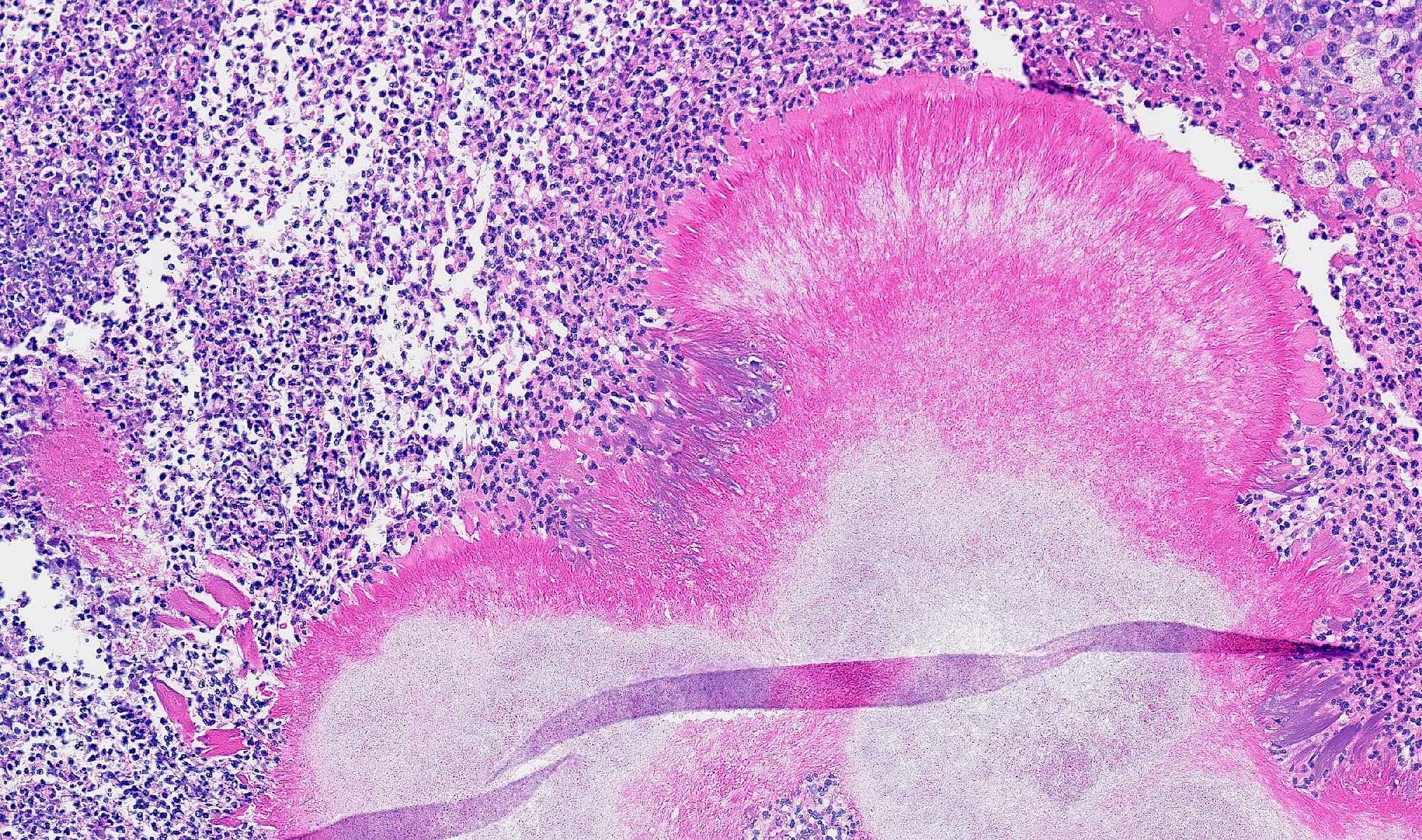
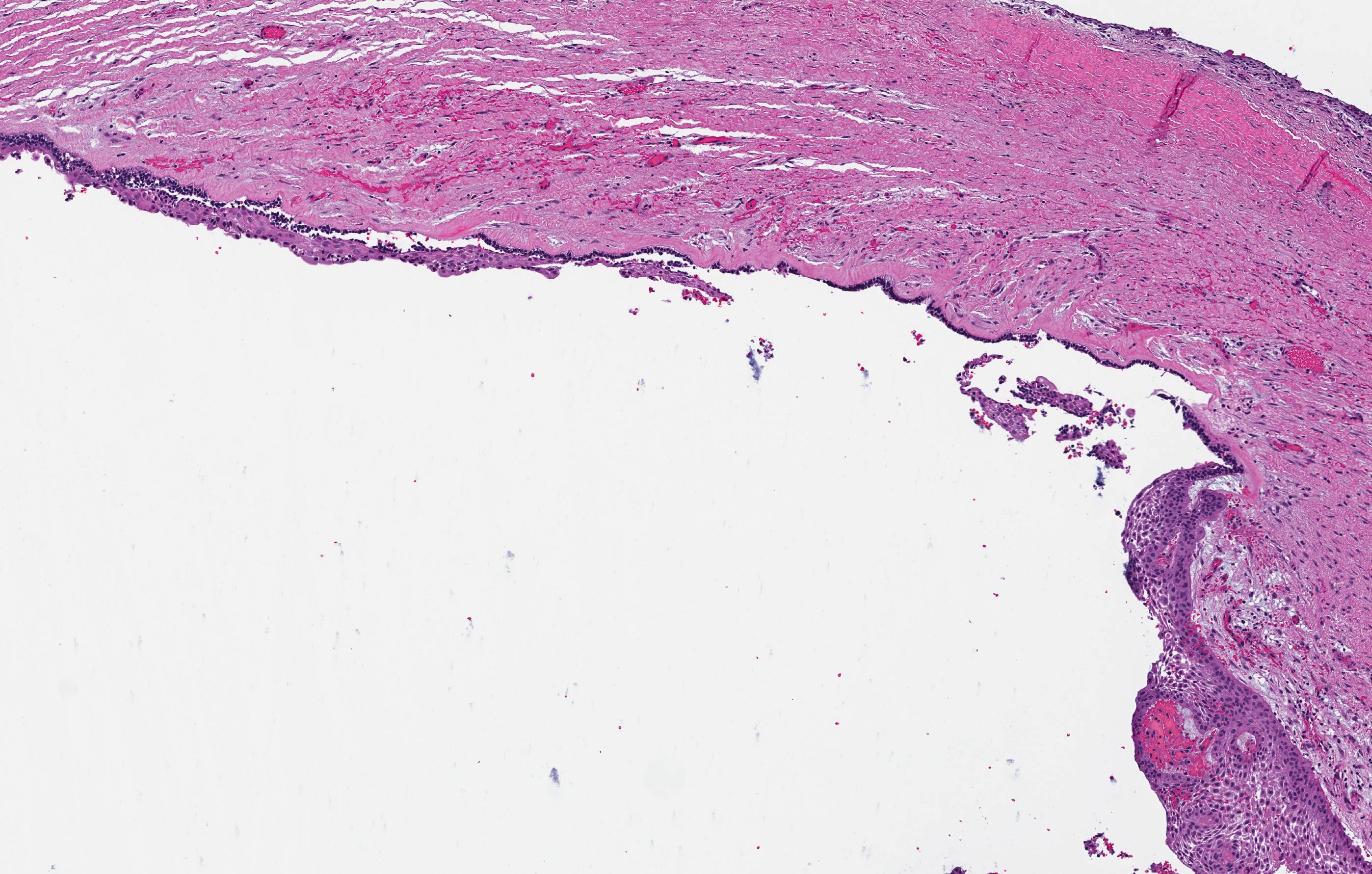
- Early phase of osteomyelitis, usually a suppurative (purulent) condition that exists when an acute inflammatory process moves away from the site of initial infection and spreads through the medullary space of the bone
- In most cases, insufficient time has passed for the body to react to the presence of the inflammatory infiltrate
Terminology
- Cortical bone
- Also called compact bone; one of two types of osseous tissue that form bones; forms the cortex, or outer shell, of most bones; denser, harder, stronger and stiffer than cancellous bone
- Cancellous bone
- Also called spongy bone; comprises walls of medullary cavity; lined by osteoprogenitor cells (endosteum)
- Medullary bone / medullary cavity / marrow cavity
- The medullary cavity (medulla, innermost part) of bone is the central cavity where red bone marrow or yellow bone marrow (adipose tissue) is stored; hence, the medullary cavity is also known as the marrow cavity
- Periosteum
- Connective tissue membrane lining the outer / external surface bones, except at the joints of long bones
- Composed of an outer fibrous and an inner cambium / osteogenic layer
- Endosteum
- Thin layer of connective tissues lining the medullary cavity within bones
- Contains osteoprogenitor cells including osteoblasts (build new bone) and osteoclasts (resorb bone to maintain appropriate bone thickness)
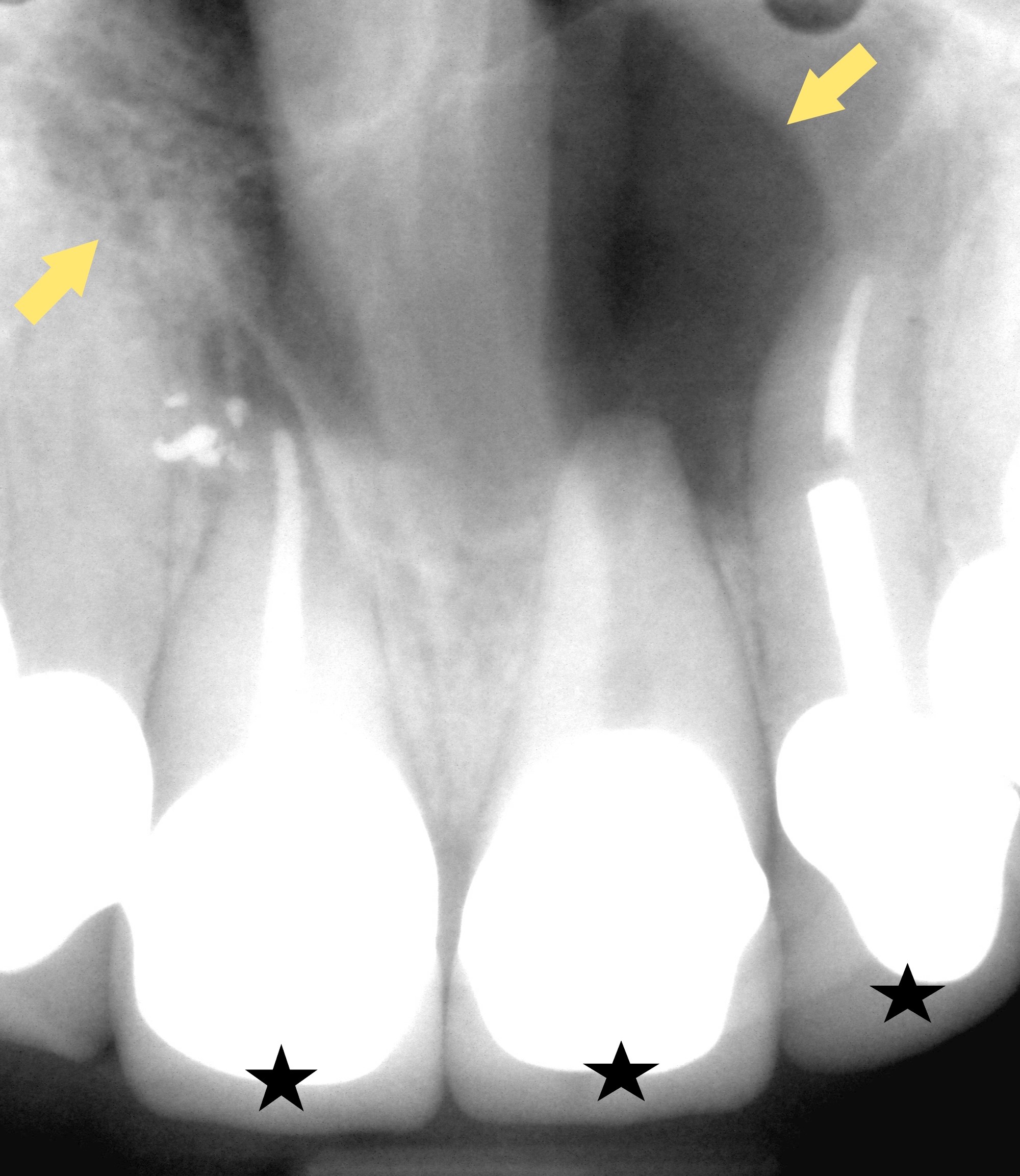
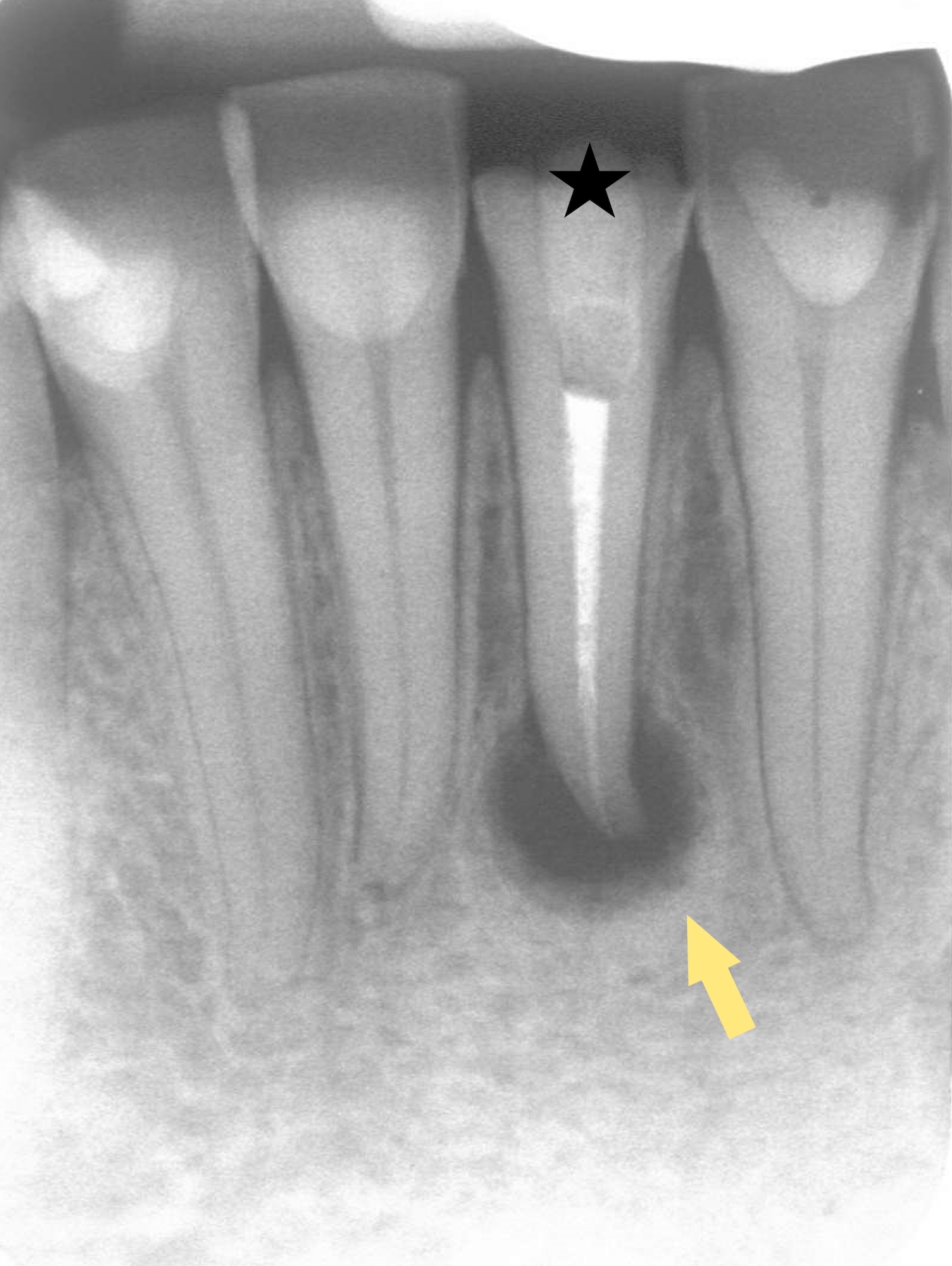
- Periapical granuloma
- Acute or chronic inflammation admixed with fibrous or granulation tissue locally at the apical or periapical region of a necrotic or partially necrotic tooth
- Devoid of epithelium (i.e. no cyst lining)
Sites
- Predominately involves the mandible
- The maxilla has higher vascularity and thin cortical plates making it less frequently involved by osteomyelitis
Pathophysiology
- Infection (example: dental granuloma) becomes established and spreads though bone, usually through medullary space
- Purulent / cellular debris or edema in the medullary cavity and beneath the periosteum compromise the local blood supply; ischemia can result in necrosis and sequestration, a classical sign of osteomyelitis
Etiology and Pathogenesis, Subclassification Groups
- Traumatic injuries, radiation and certain chemical substances may cause inflammation in the bone medullary space
- The oral cavity harbors a large number of bacteria which may cause infection of the jawbone
- Considering the high frequency and sometimes severity of odontogenic infections in daily dental and oral surgery practice, and the intimate relationship of dental root apices with the medullary cavity of the jawbone, it is remarkable that osteomyelitis cases are not more frequently observed
- The low incidence of osteomyelitis of the jawbones can be explained by these primary factors which are responsible for deep bacterial invasion into the medullary cavity and cortical bone and hence establishment of the infection:
- Number of pathogens and virulence of pathogens
- Although S. aureus, S. epidermidis, and Actinomyces were recently discussed as major pathogens, more recent studies favor the concept of a polymicrobic infection with several responsible pathogens
- This shift is explained mainly by modern, sophisticated culture methods, especially involving anaerobic media, which enable identification of possible pathogens more accurately
- Many pathogens, often found in the healthy oral flora, have been associated with jawbone osteomyelitis; however, prolonged antibiotic therapy prior to harvesting of the specimen and possible oral contamination complicate the interpretation of each result
- Local and systemic host immunity
- The oral cavity, like no other part of the human body, is constantly exposed to various potential aggressors
- Many of these bacteria, given the chance, may cause severe infection and tissue damage
- Due to its unique environment, many potent strategies have been developed to prevent deep tissue invasion of bacteria
- It is important for the treating physician to consider host compromise and treat any compromising condition:
- Alcohol and tobacco, autoimmune disorders, AIDS, agranulocytosis, anemia (especially sickle cell), chemotherapy, corticosteroids and other immunosuppressive therapy, cytomegalovirus infection, diabetes, drug abuse, Herpes simplex virus (Zoster), leukemia, major surgery, malnutrition, significant periodontal disease (found in 51% in one study), syphilis
- Local tissue perfusion
- Compromise of local blood supply is a critical factor in the establishment of osteomyelitis
- Systemic and local conditions which alter the vascularity of bone predispose to osteomyelitis, and include: bisphosphonate induced osteochemonecrosis, bone malignancy (primary or metastatic), diabetes mellitus, fibrous dysplasia, florid osseous dysplasia, osteopetrosis (Albers–Schonberg Disease), osteoporosis, osteoradionecrosis, other osteonecrosis (mercury, bismuth, arsenic), Paget disease, radiation therapy, tobacco
- In these conditions, immune cells and oxygen cannot reach the target area in an adequate manner
- This facilitates the growth and spread of microorganisms, especially anaerobes, leading to establishment and progression of osteomyelitis
- In many cases of acute and secondary chronic osteomyelitis, none of these factors may be detected, but they must always be considered, looked for and ultimately treated (Baltensperger 2003)
- Compromise of local blood supply is a critical factor in the establishment of osteomyelitis
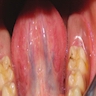
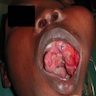
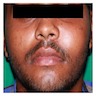
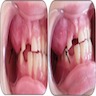
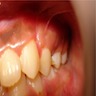
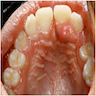
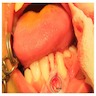
Clinical features
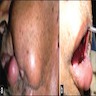
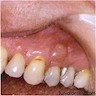
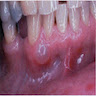
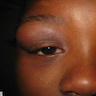
- Localized pain, swelling, decreased range of motion
- Trismus (difficulty opening mouth), dysphagia (difficulty swallowing)
- Anesthesia or parasthesias (changes or decreased sensation) in mental nerve distribution (anterior chin, lower lip)
- Systemic symptoms: fevers, chills, lymphadenopathy, fatigue, nausea
Diagnosis
- Dependent on clinical and radiographic findings with confirmation by histopathology and microbiologic evaluation
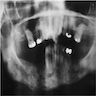
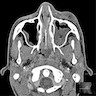
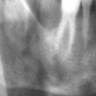
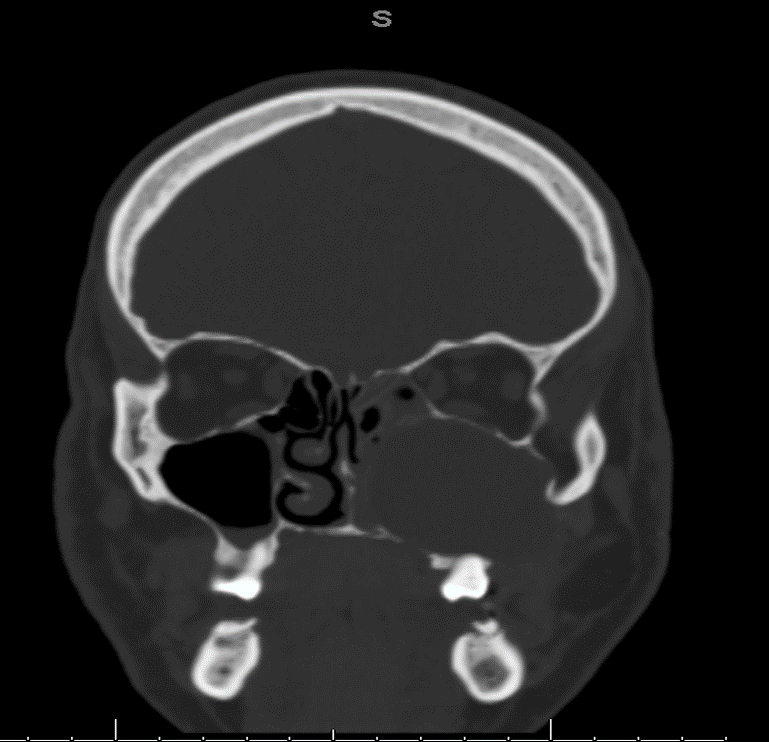
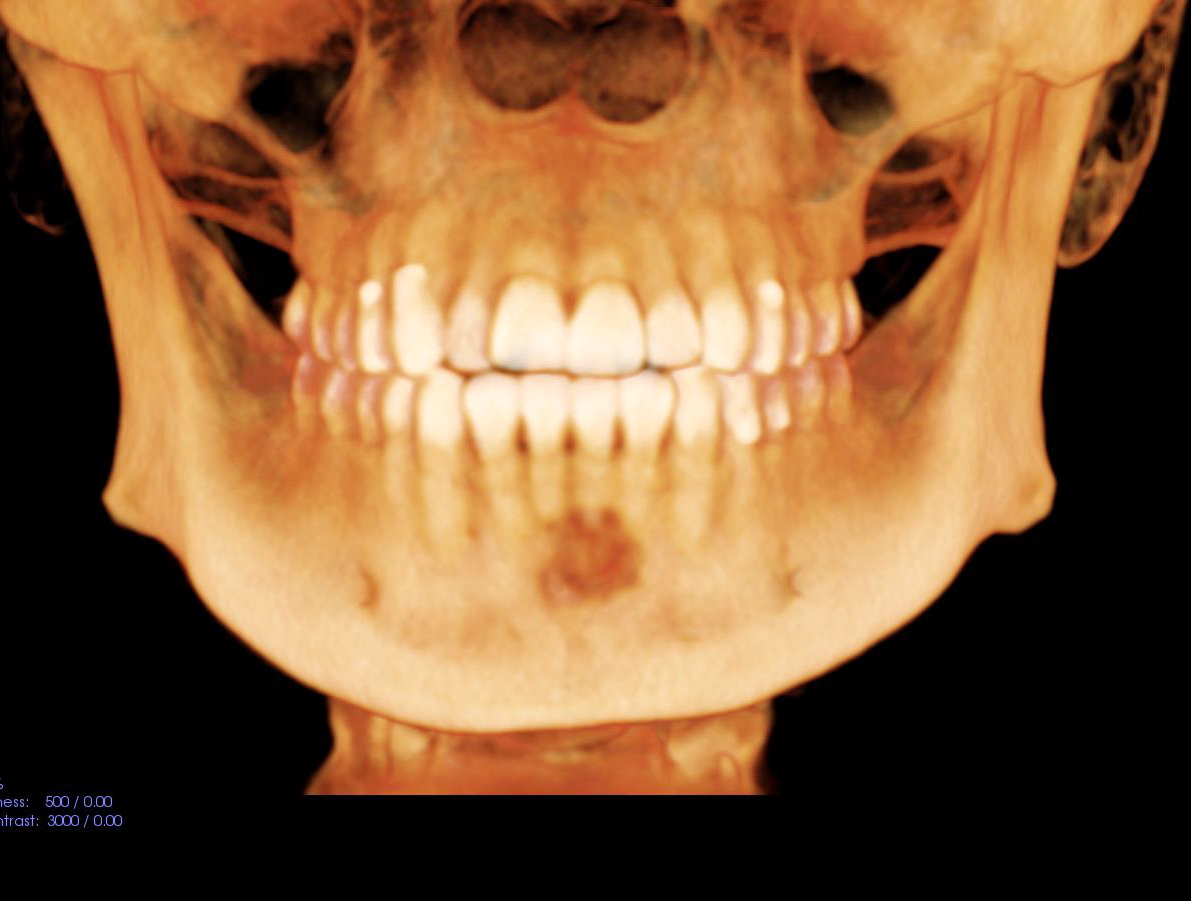
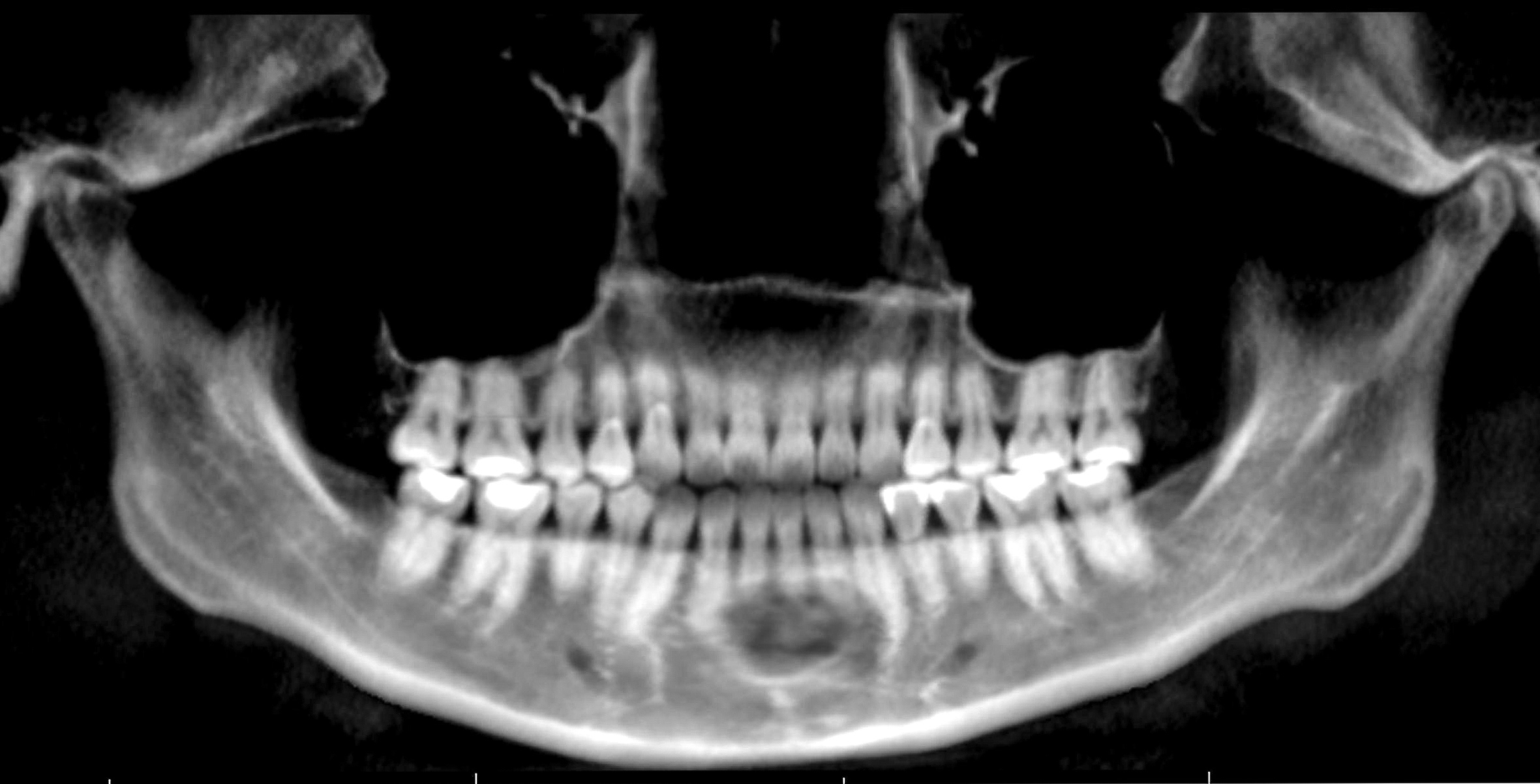
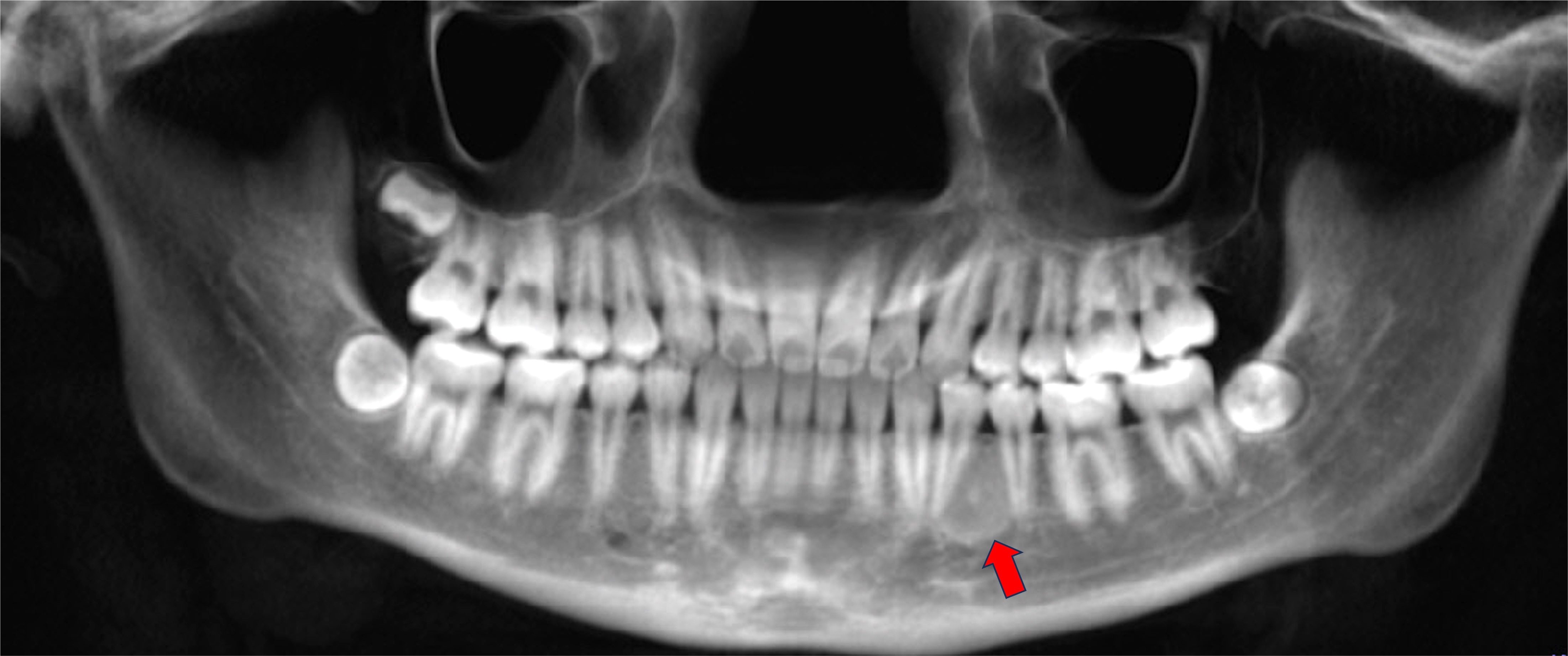
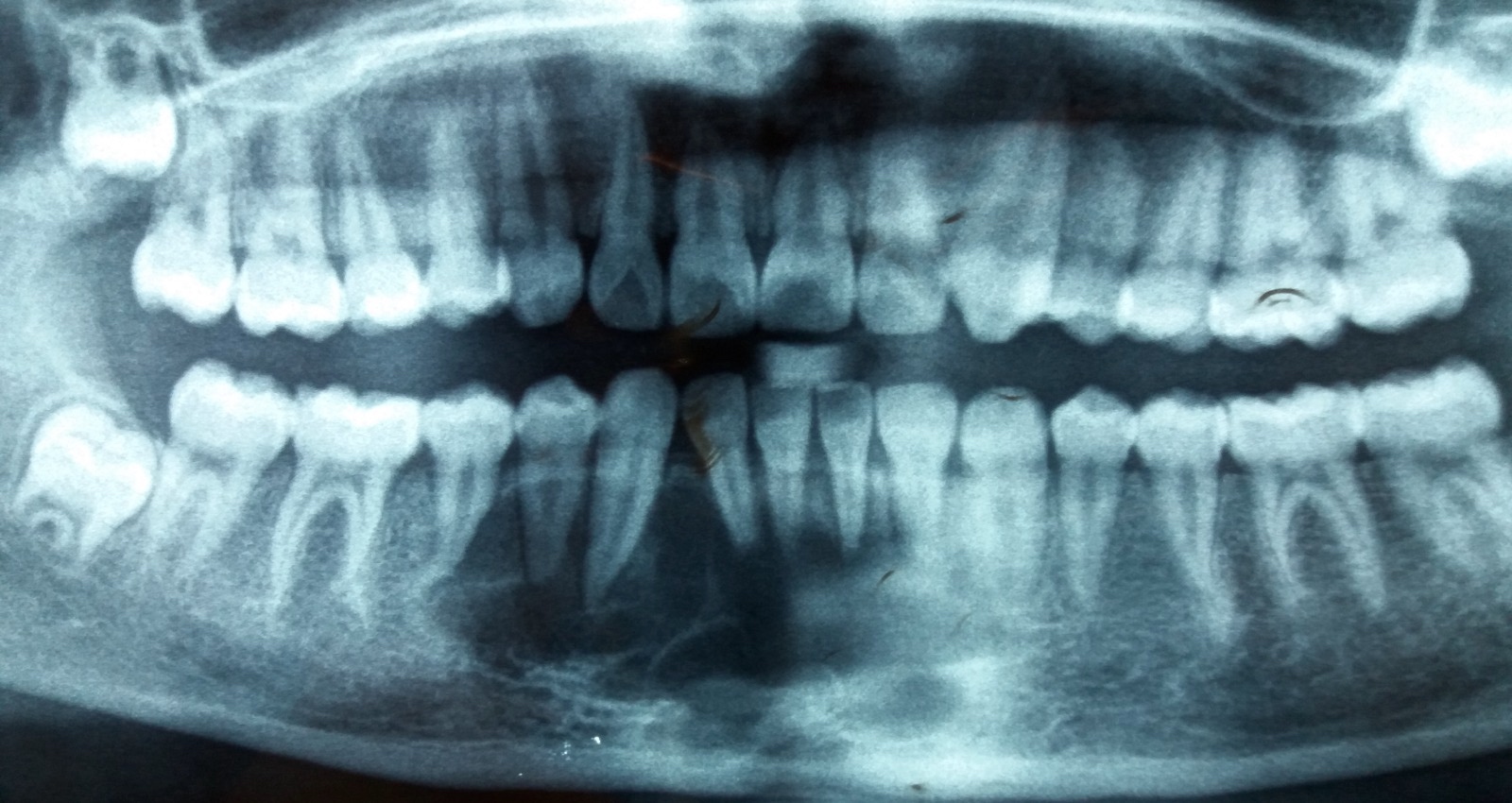
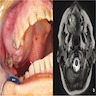
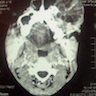
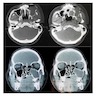
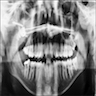
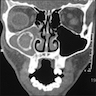
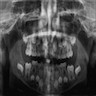
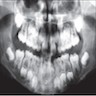
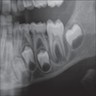
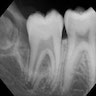
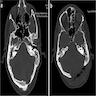
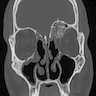
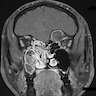
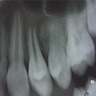
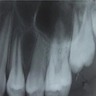
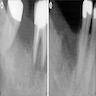
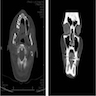
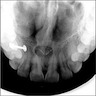
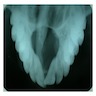
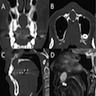
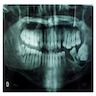
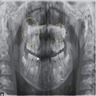
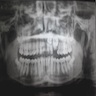
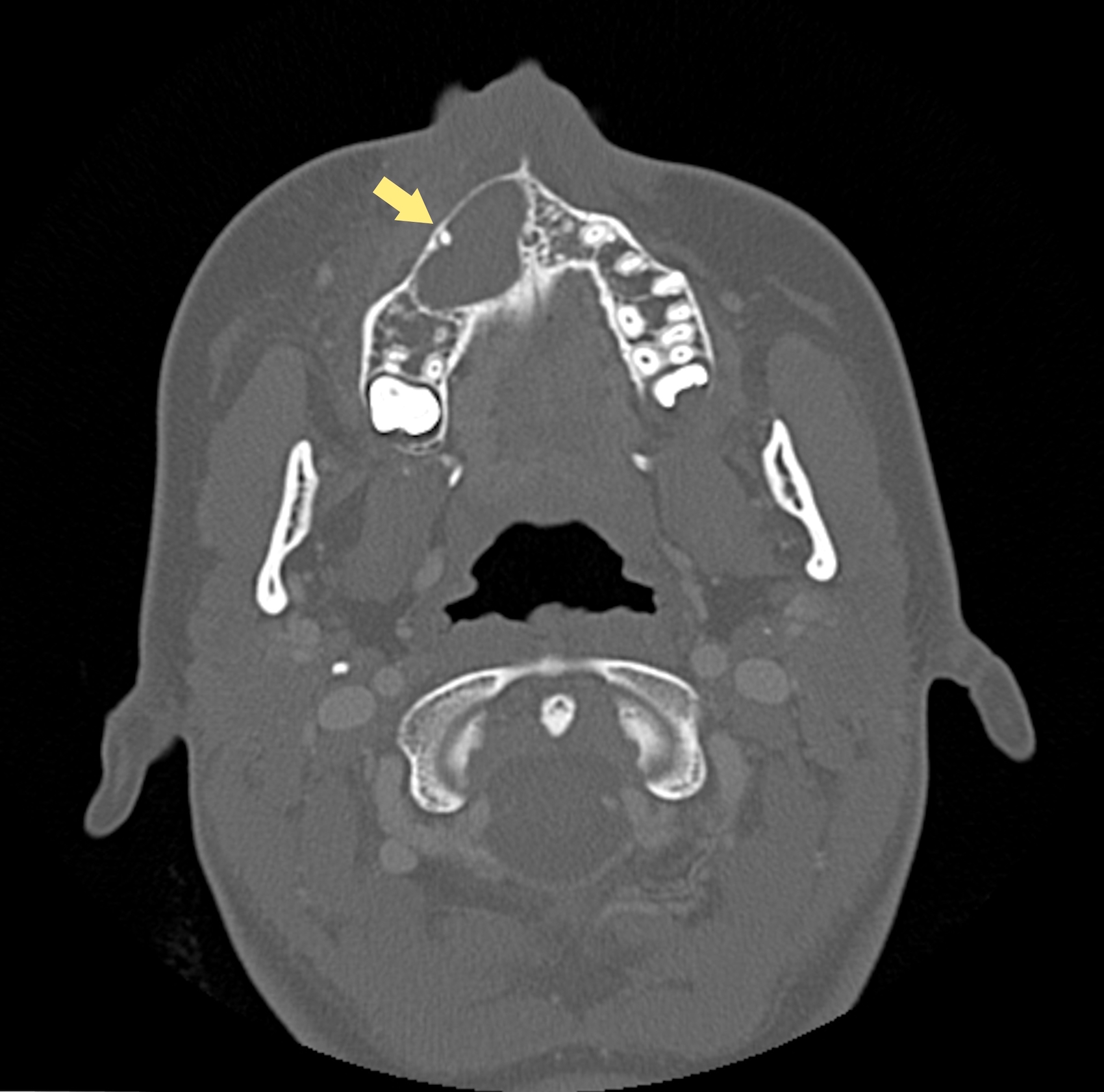
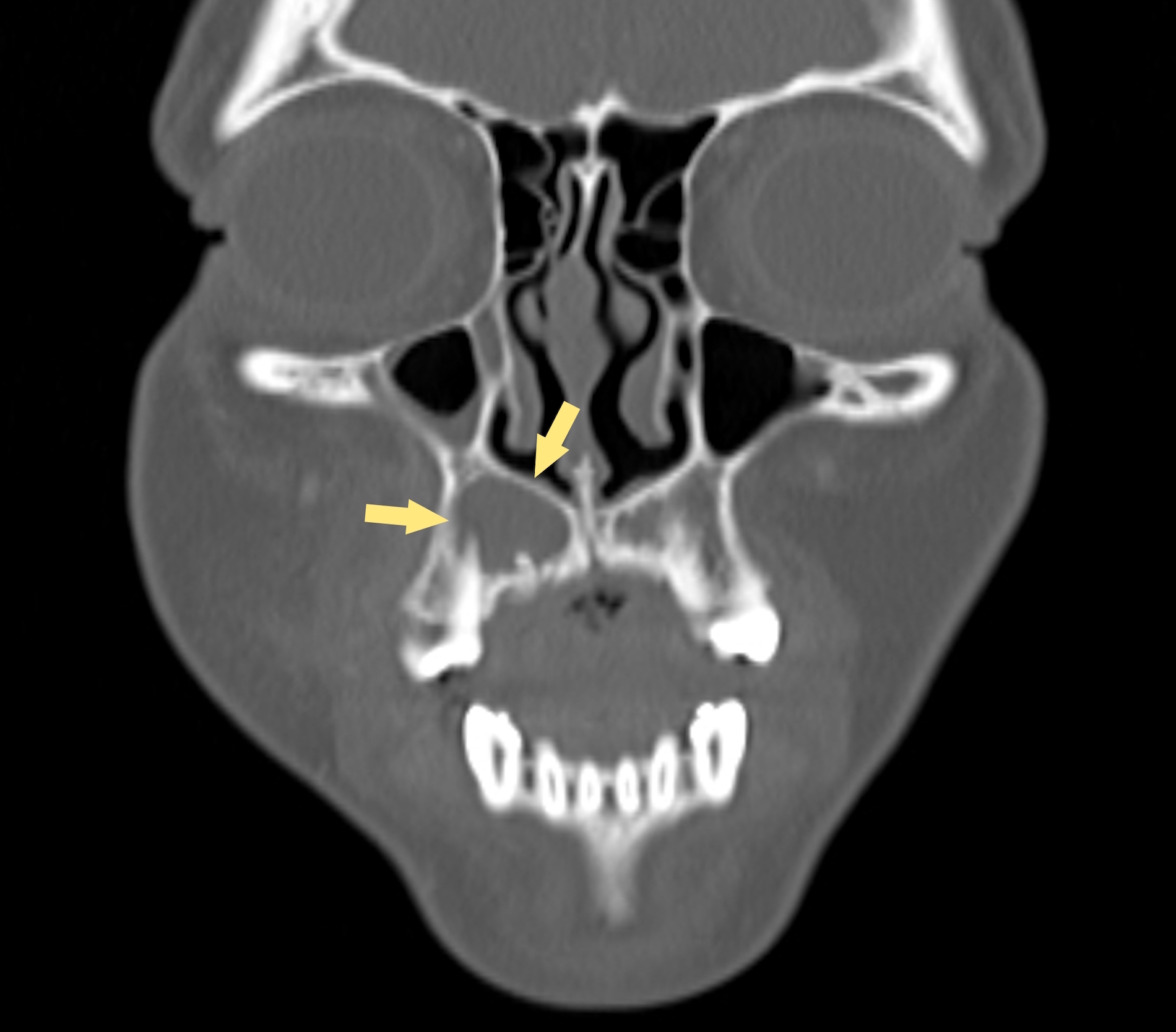
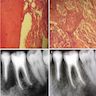
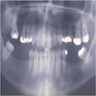
Radiology description
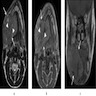
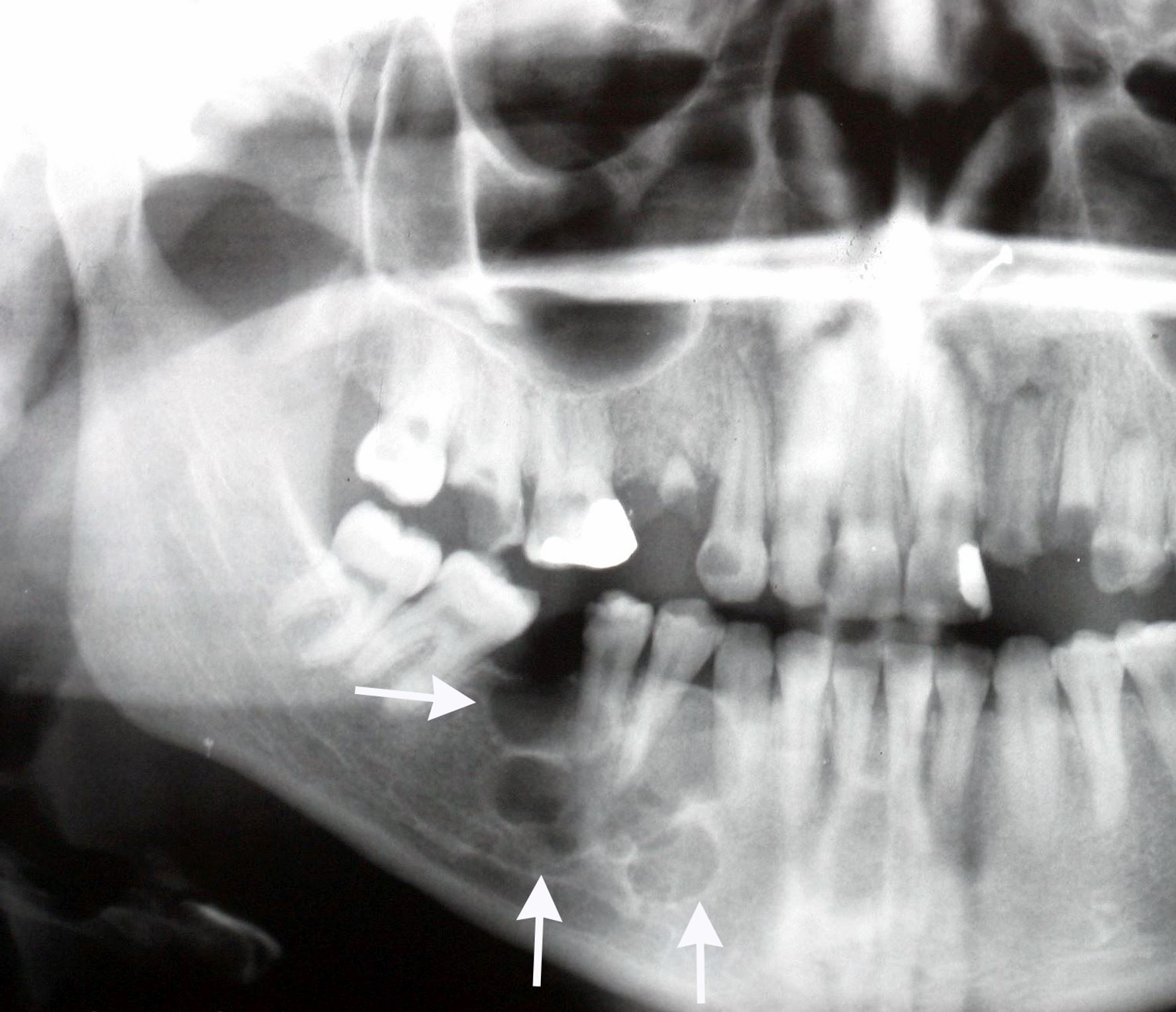
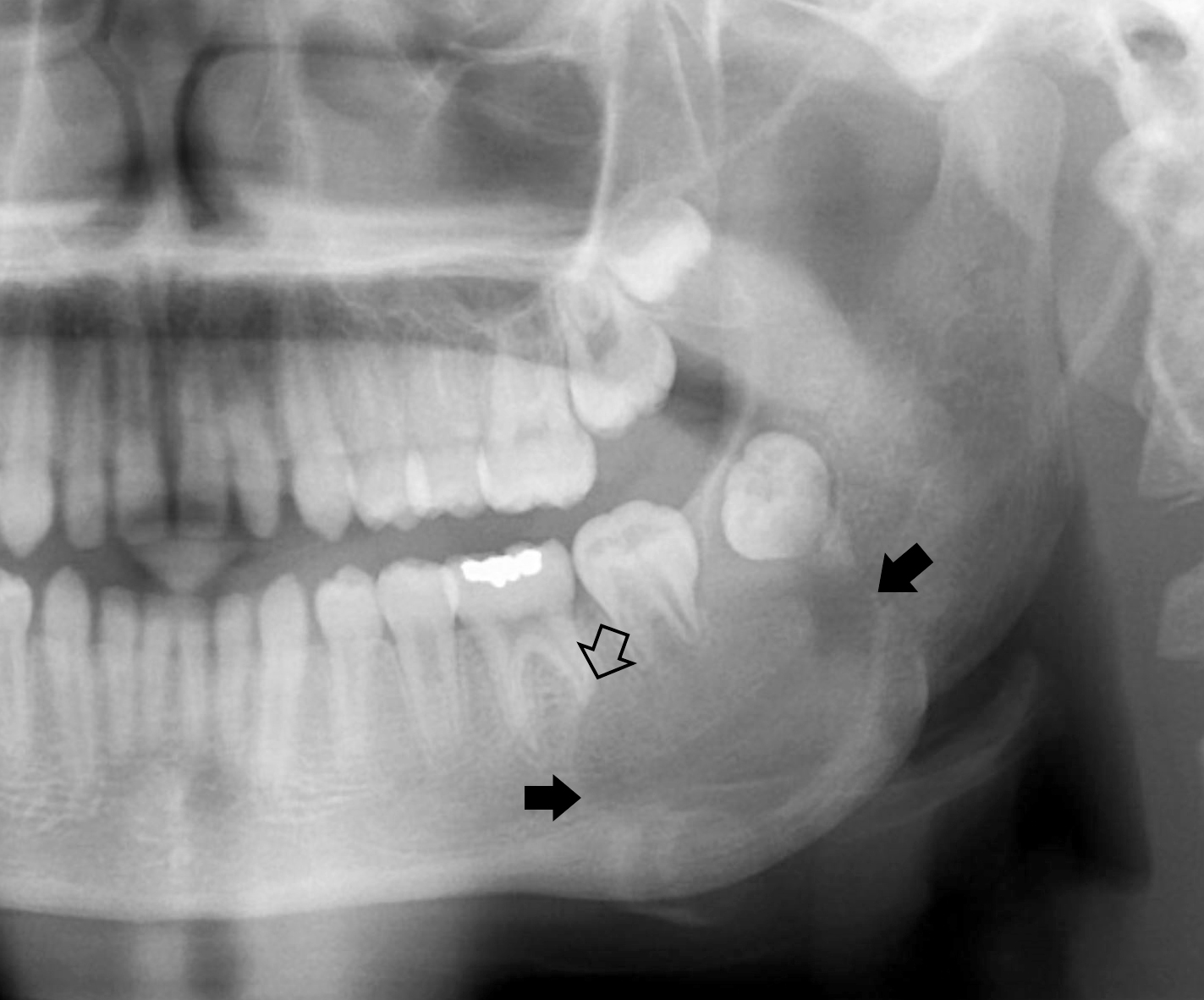
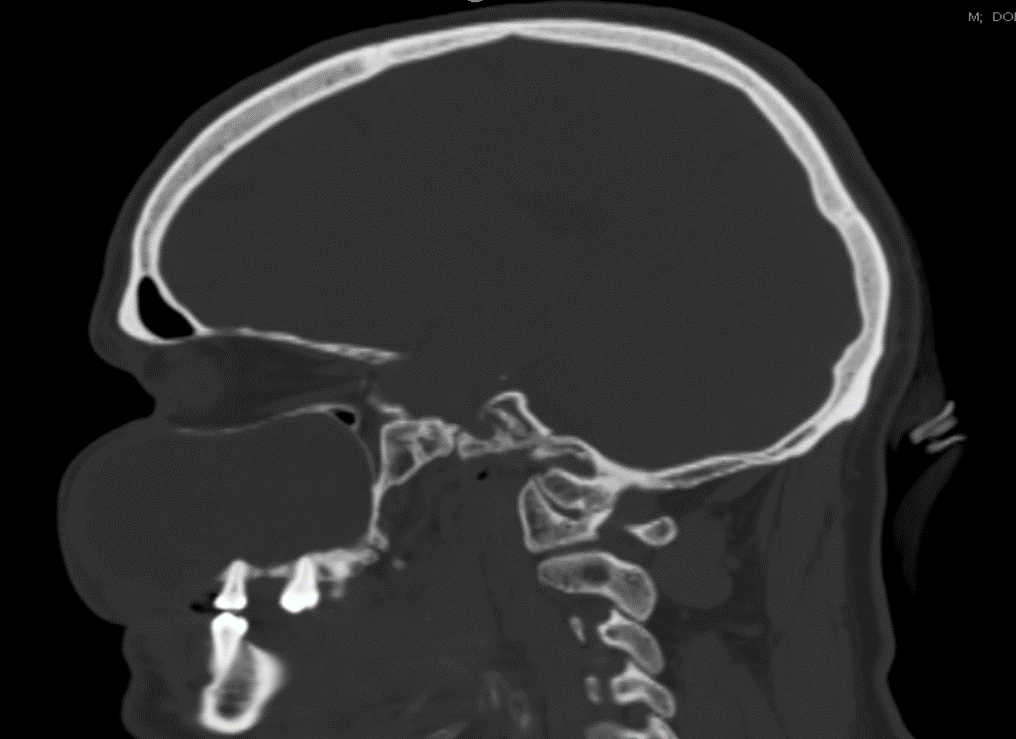
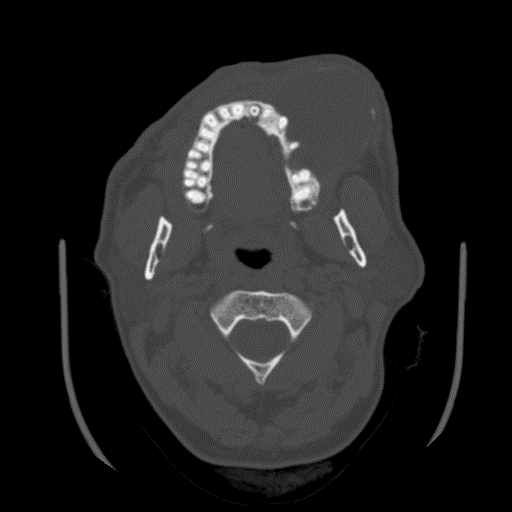
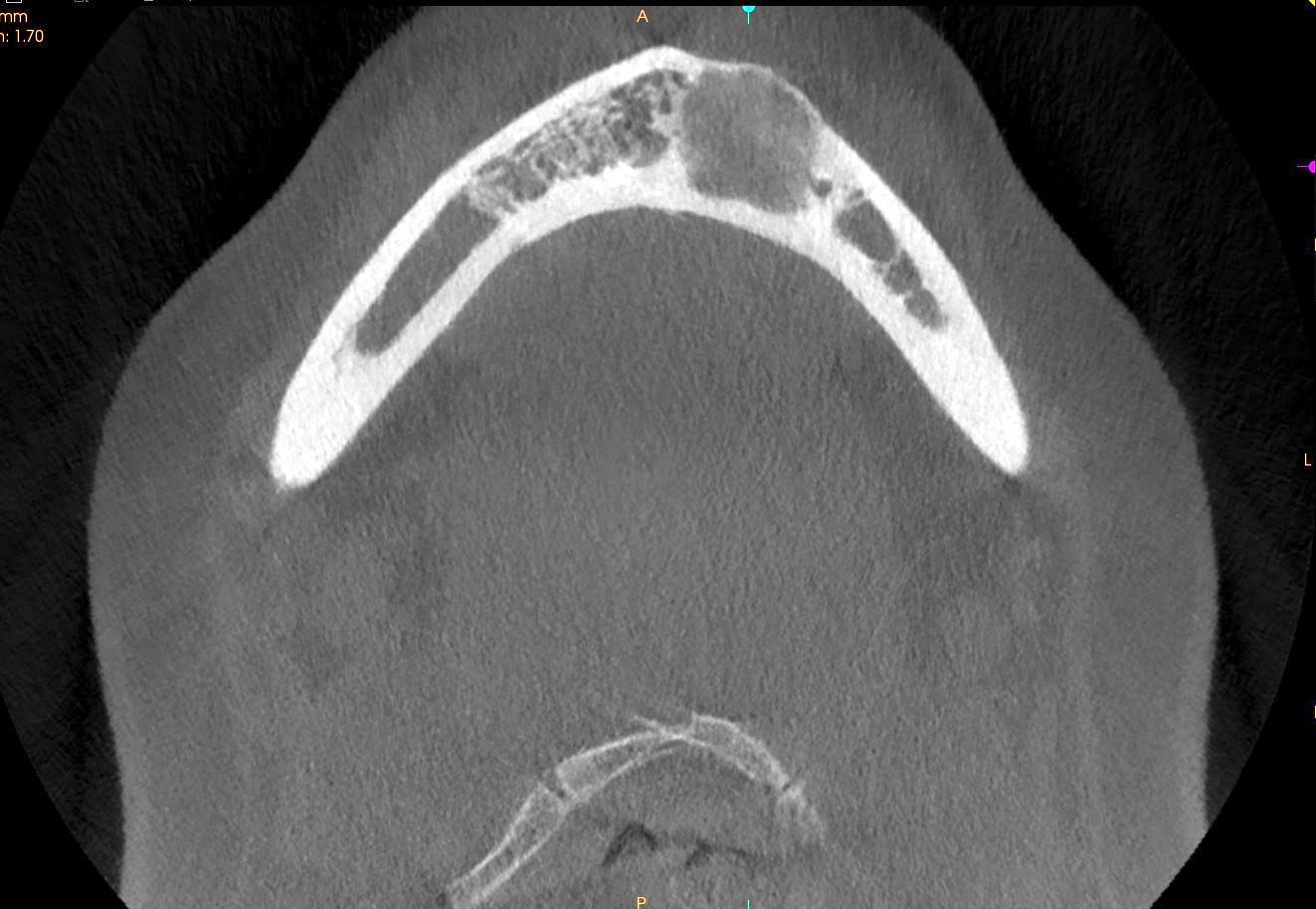
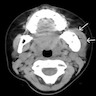
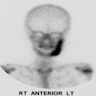
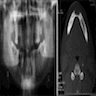
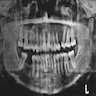
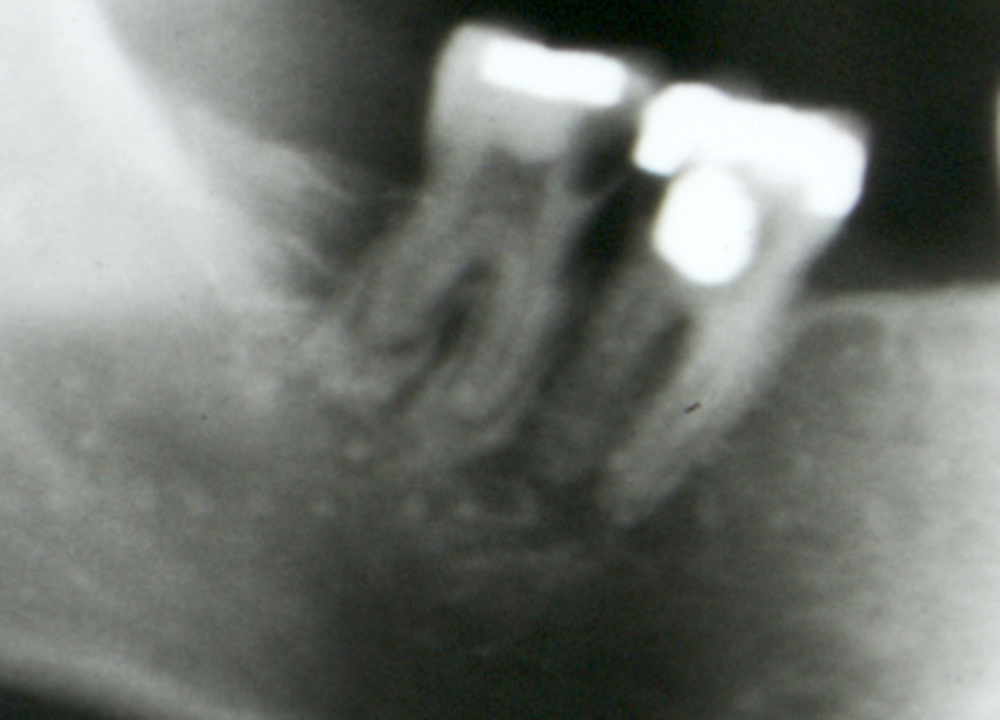
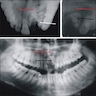
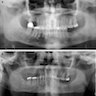
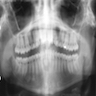
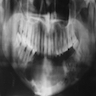
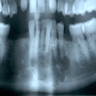
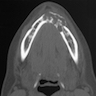
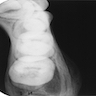
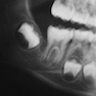
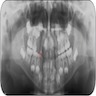
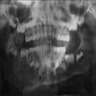
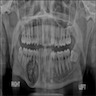
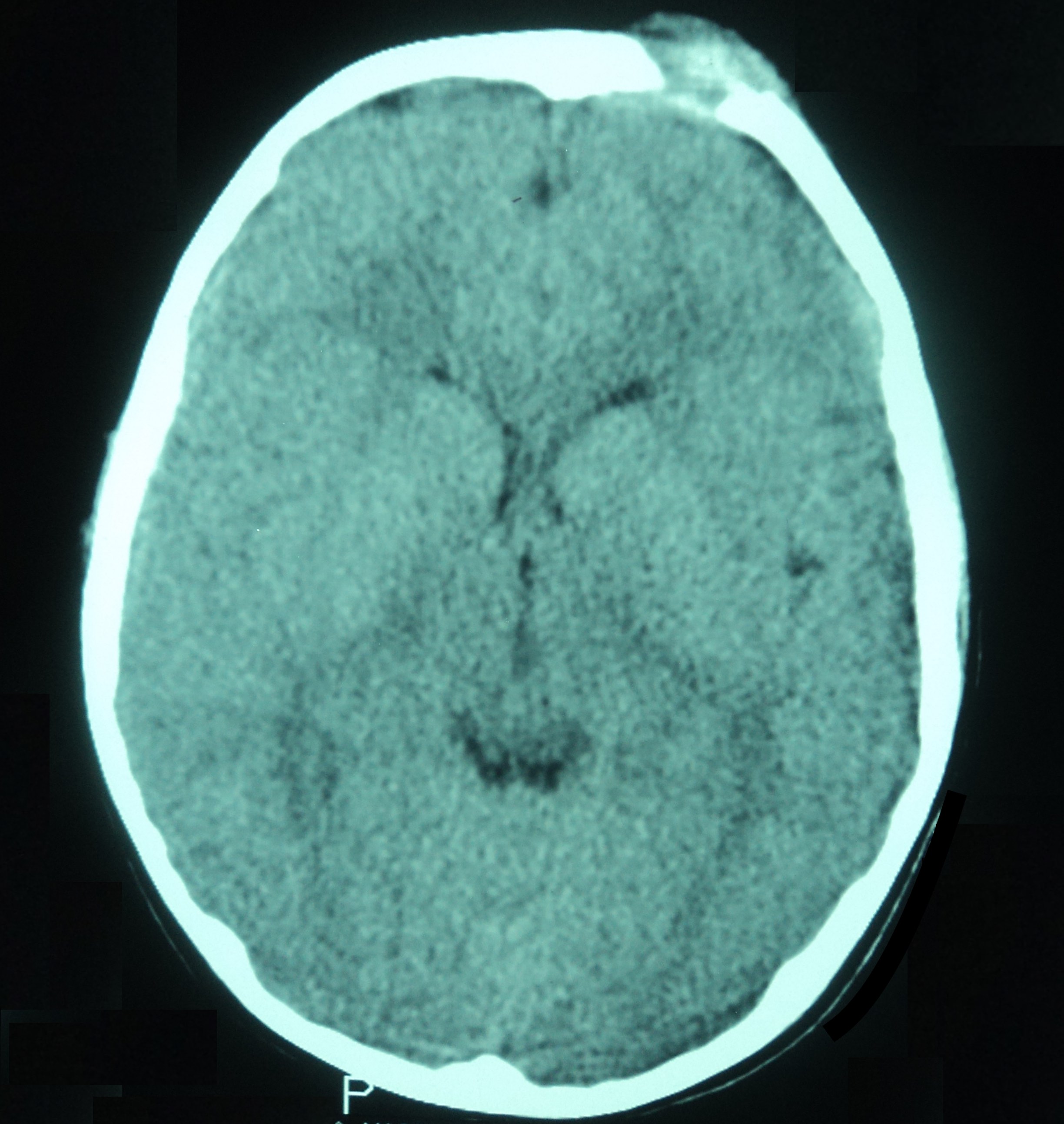
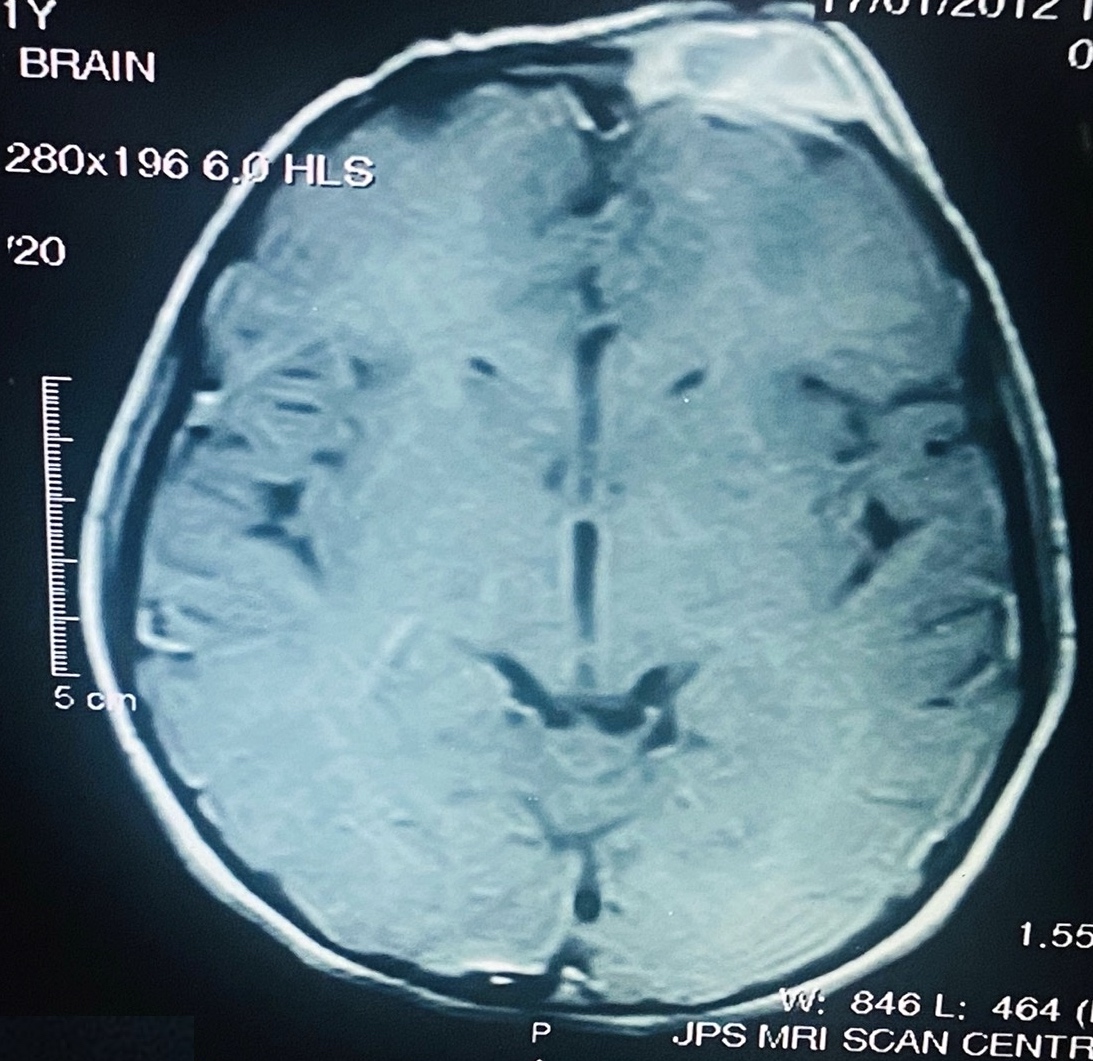
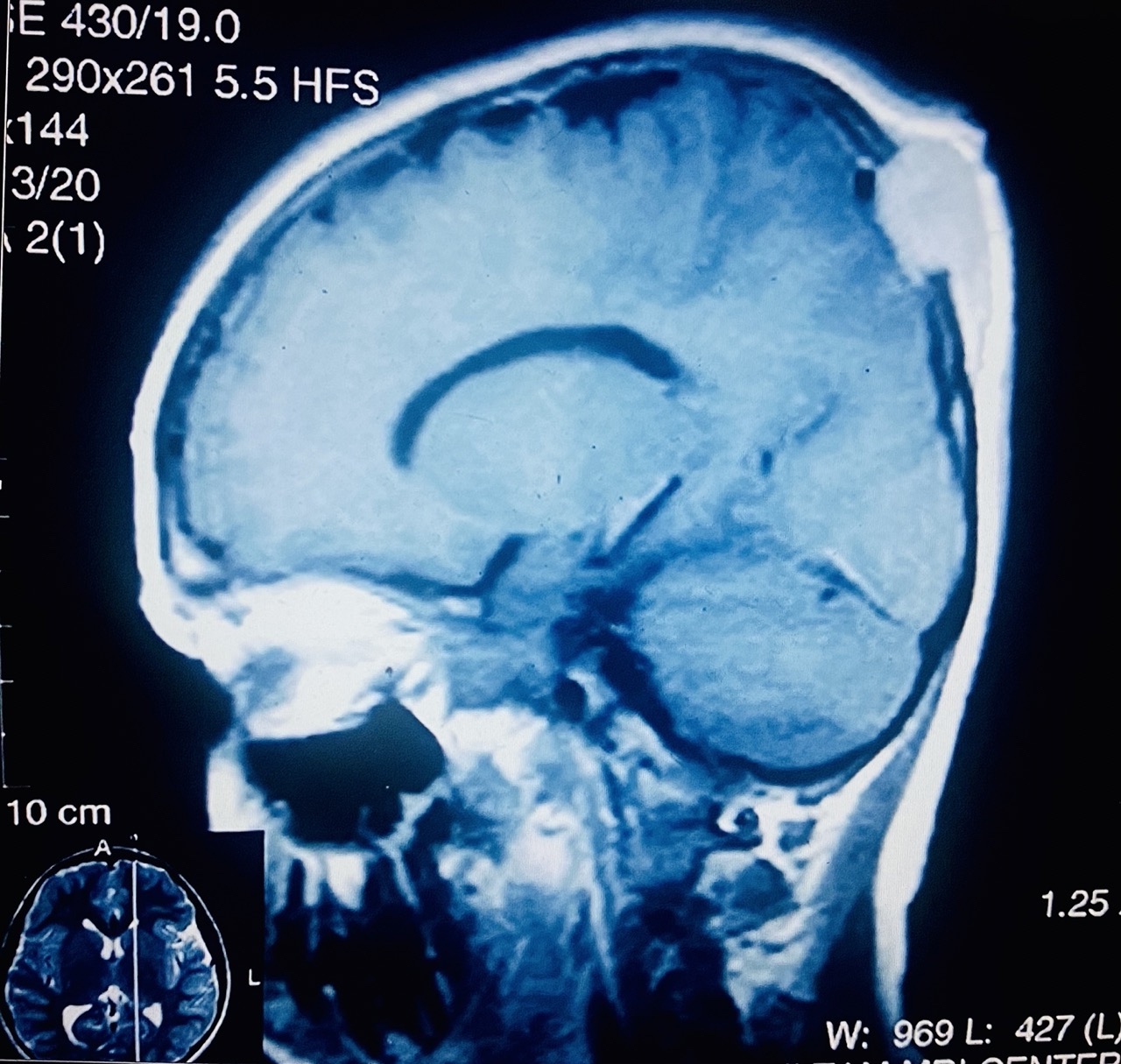
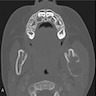
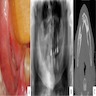
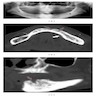
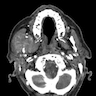
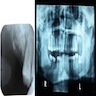
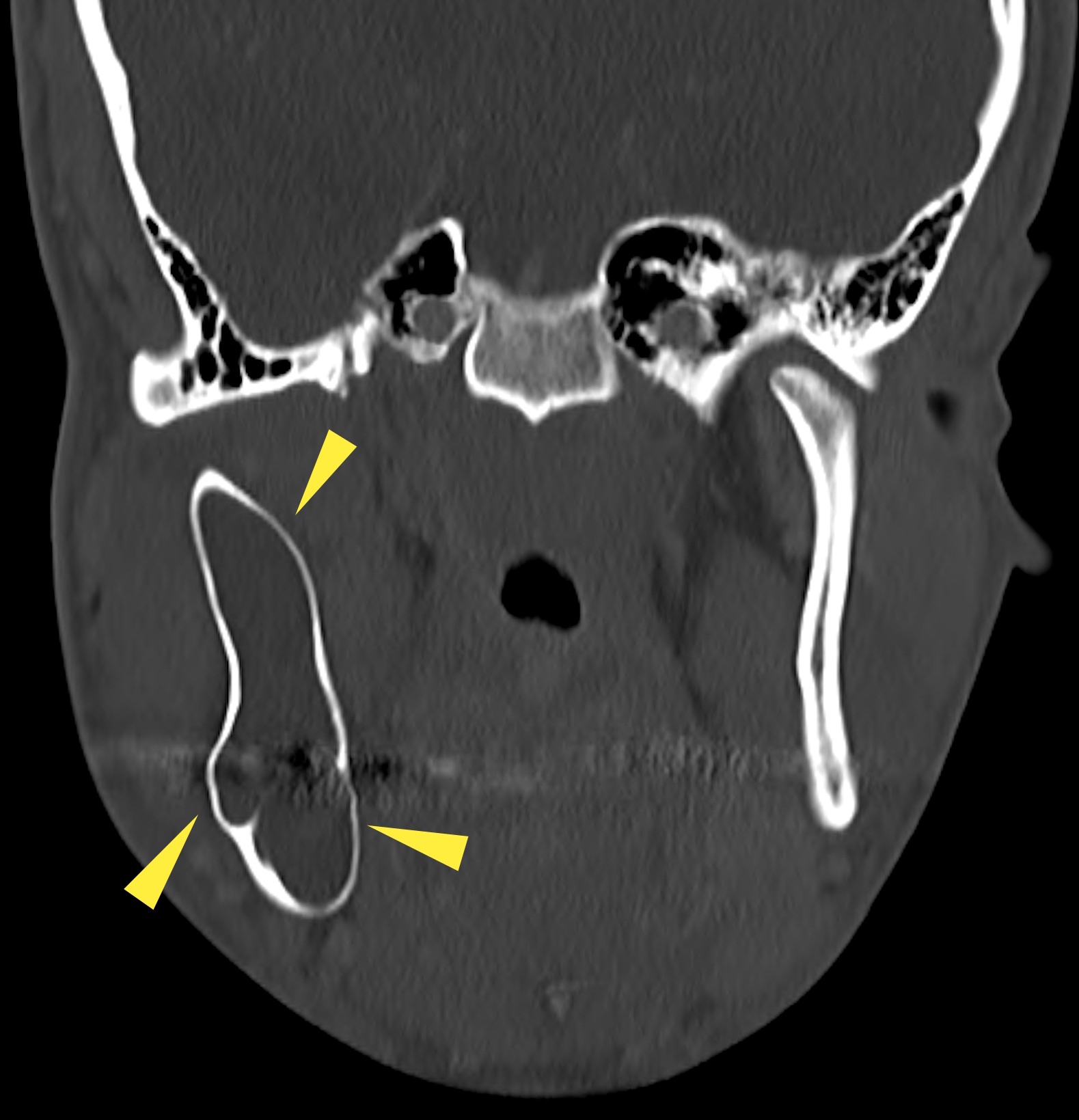
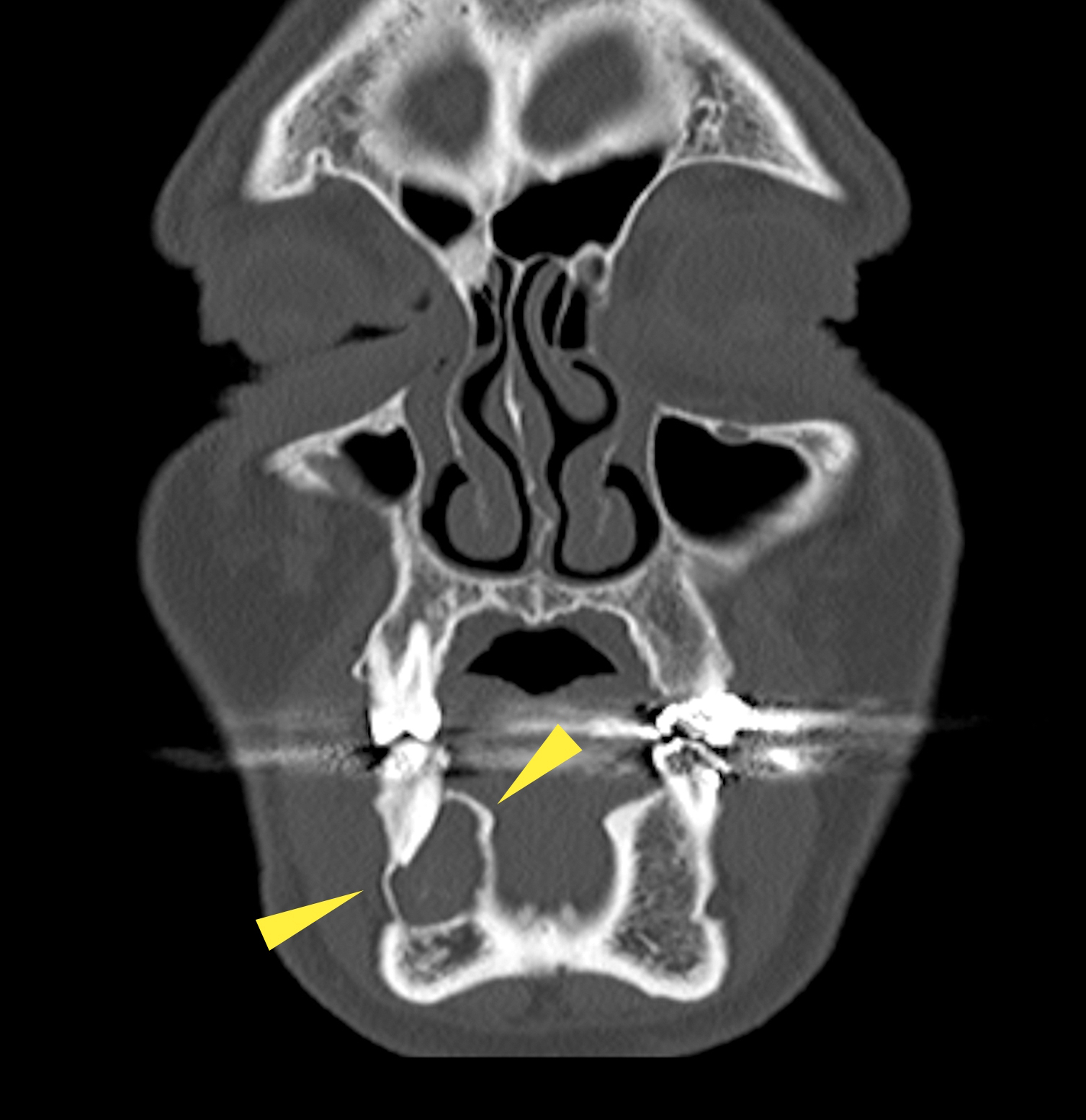
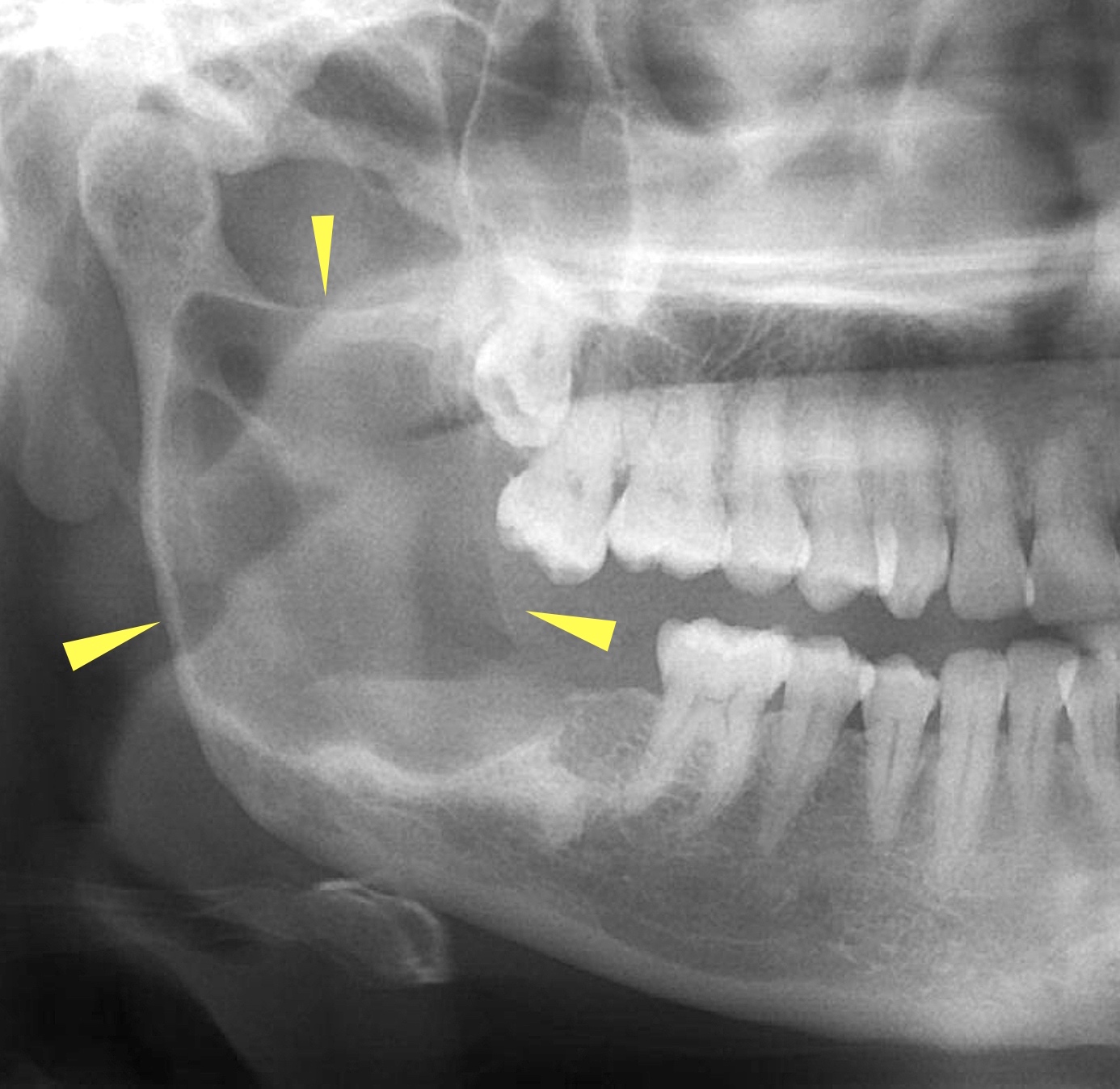
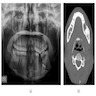
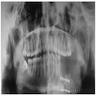
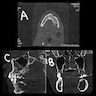
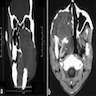
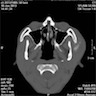
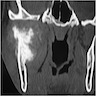
- Plain films: irregular radioleucency, adjacent fracture site (if present), osteomyelitis
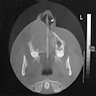
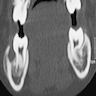
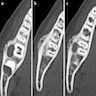
- CT: lytic permeative changes with soft tissue inflammation
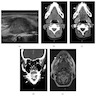
- Magnetic Resonance Imaging: less often affected than CT by dental amalgam artifact; if amalgam obscures, can perform MR to help detect abscess
- Bone scintigraphy: sensitive for osteomyelitis; radiolabeled white blood cell scintigraphy has proven to be useful for detecting septic activity in the jaw bone
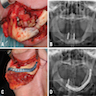
Radiology images
Prognostic factors
- Pathologic fracture associated with more protracted / complicated recovery
- Requires antibiotics
- Often requires surgical debridement
- Risk of persisting into a chronic osteomyelitis if delay in diagnosis / treatment, extensive bone necrosis, inadequate duration of antibiotic therapy, inadequate surgical debridement, decreased host ability to fight infection
- Recurrences reduced with treatment of contributing risk factors (ex: diabetes, immunocompromise, poor oral hygiene)
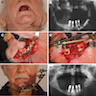
Case reports
- 19 year old man with Aspergillus tubingensis in maxillary bone (BMC Infect Dis 2013;13:59)
- 73 year old woman with an implant related periapical lesion leading to acute osteomyelitis (Br Dent J 2008;205:489)
- 77 year old man with rapidly progressing osteomyelitis of mandible (Case Rep Dent 2013;2013:249615)
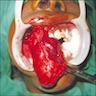
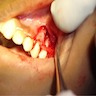
Treatment
- Surgical debridement
- Cultures and antibiotic sensitivity
- Infectious disease consultation if considering an extended antibiotic regimen; may require augmentation if antibiotic resistance develops (common)
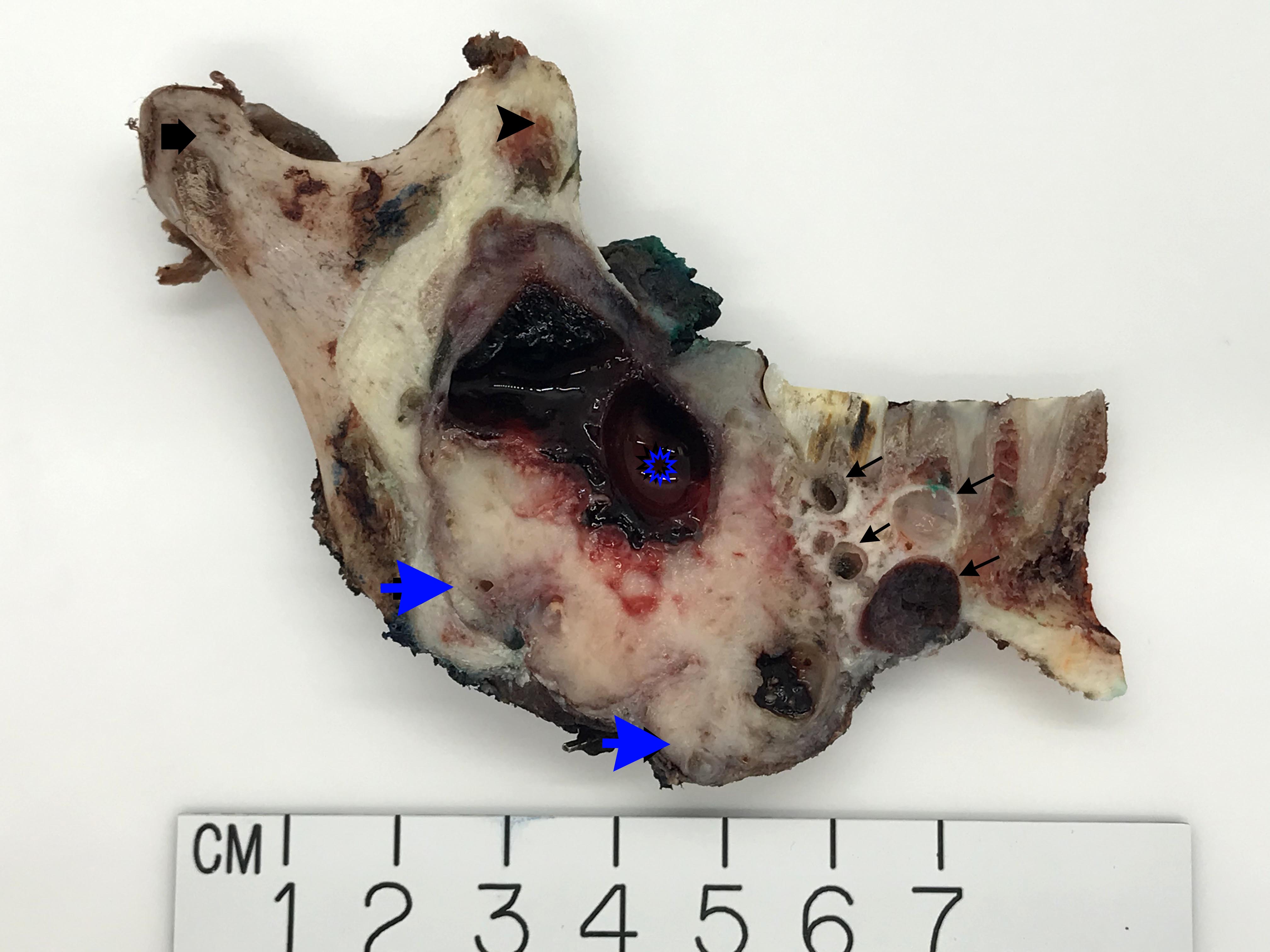
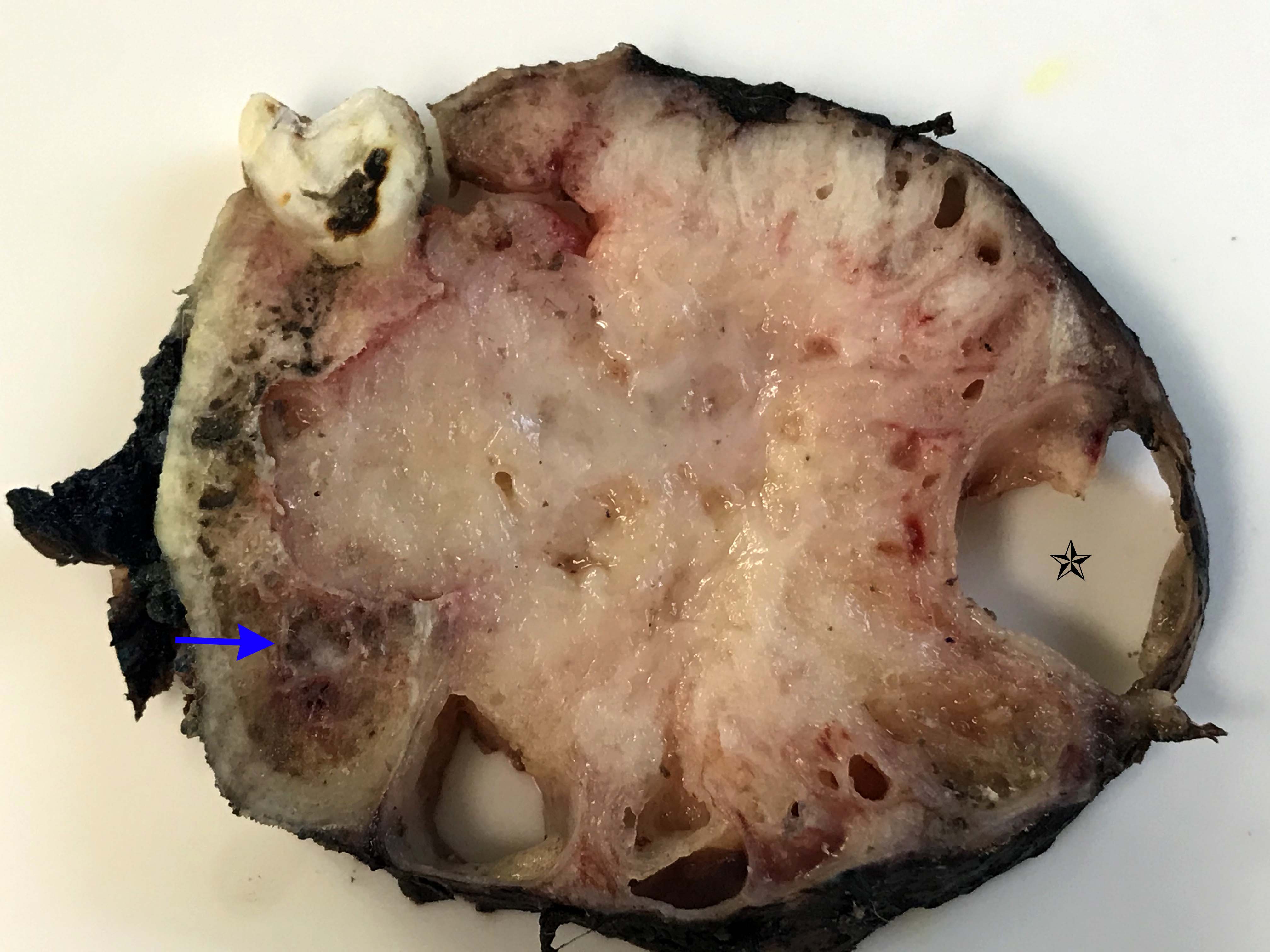
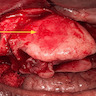
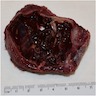
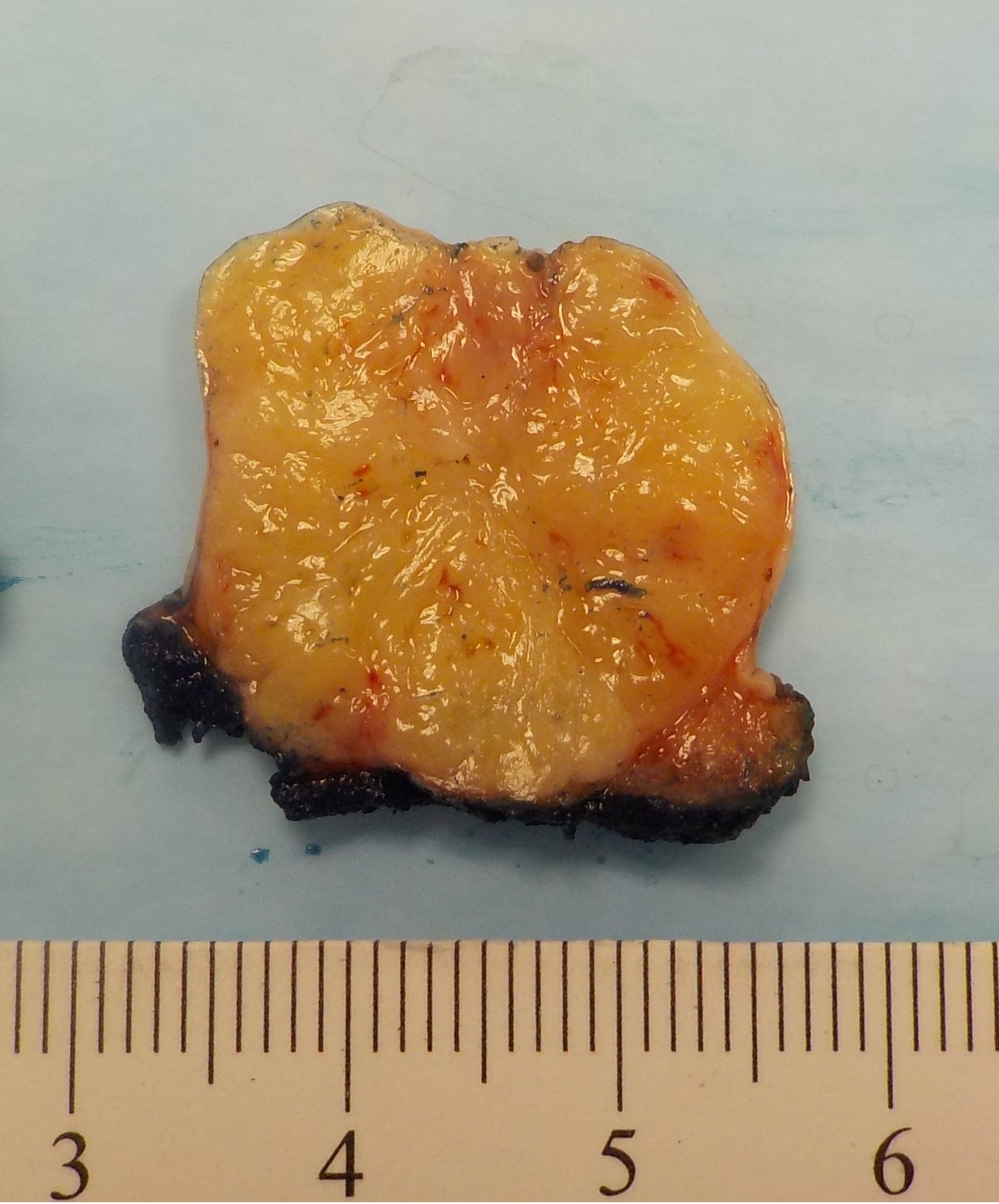
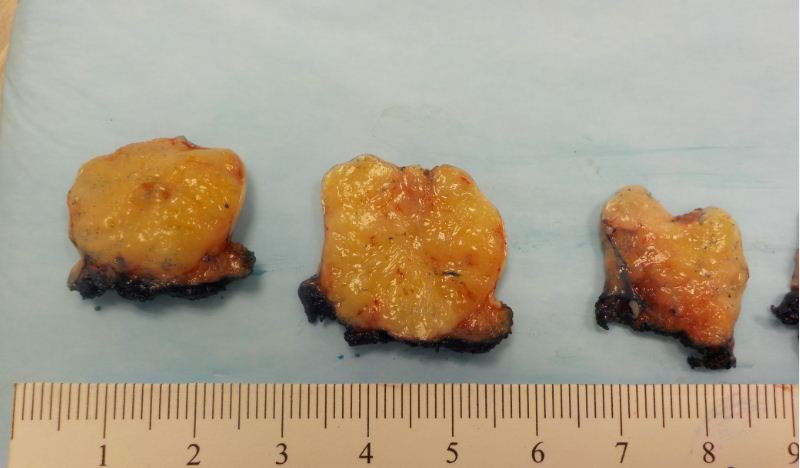
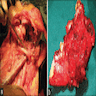
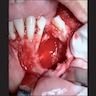
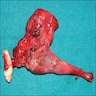
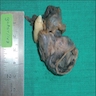
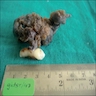
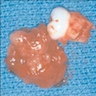
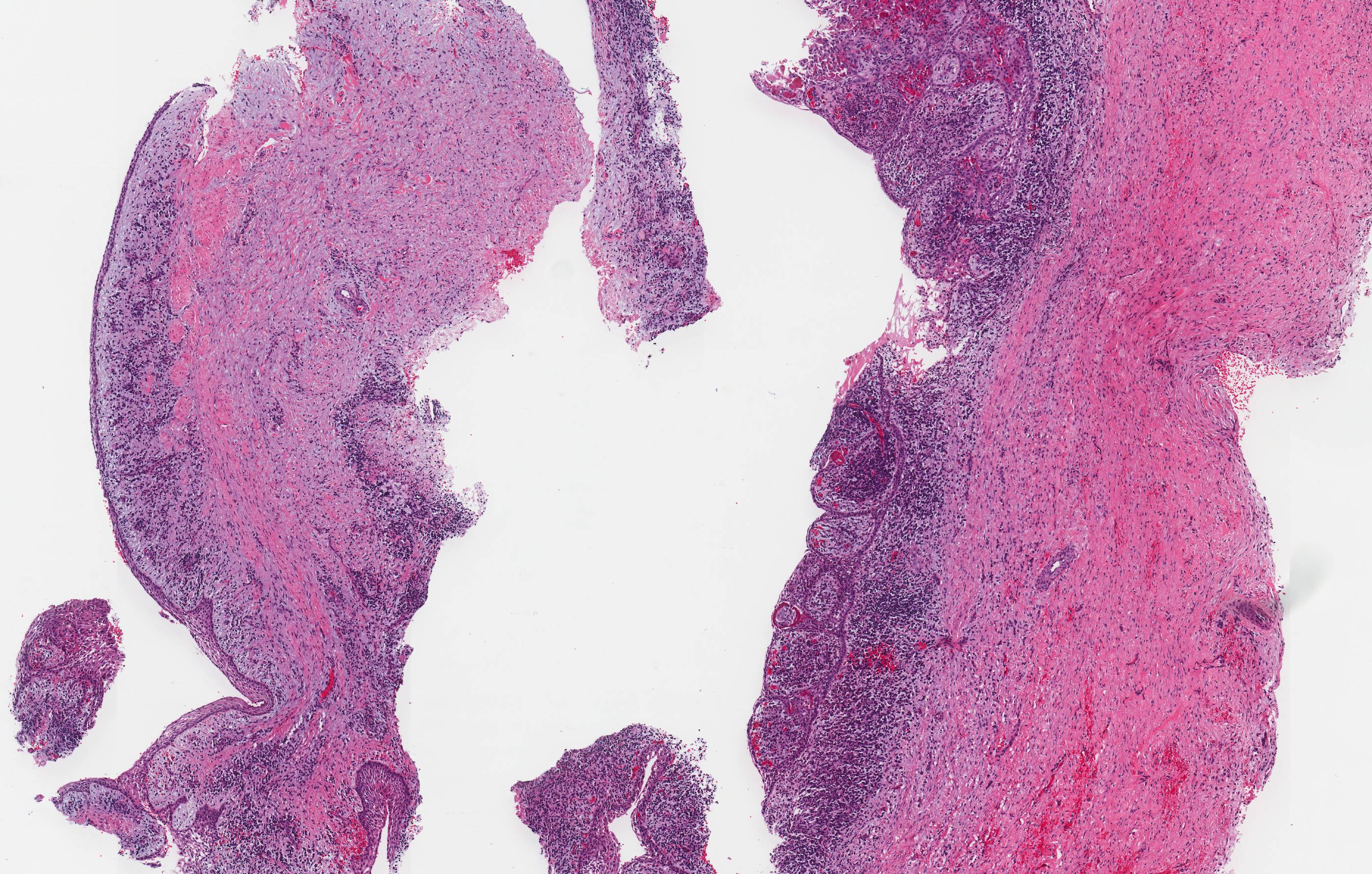
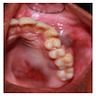
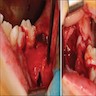
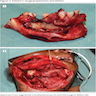
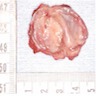
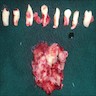
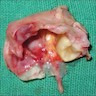
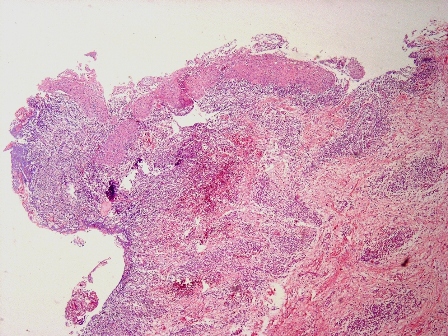
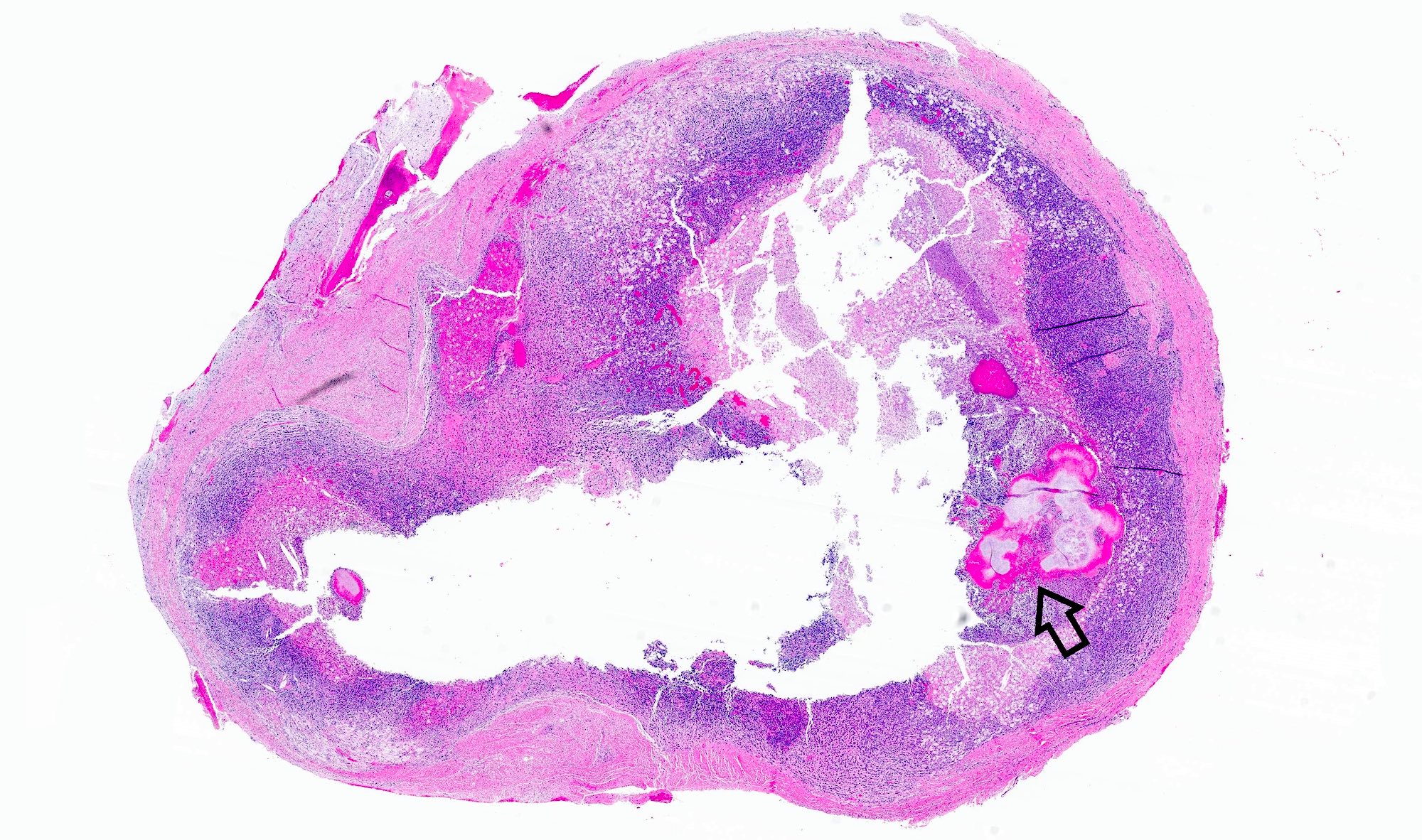
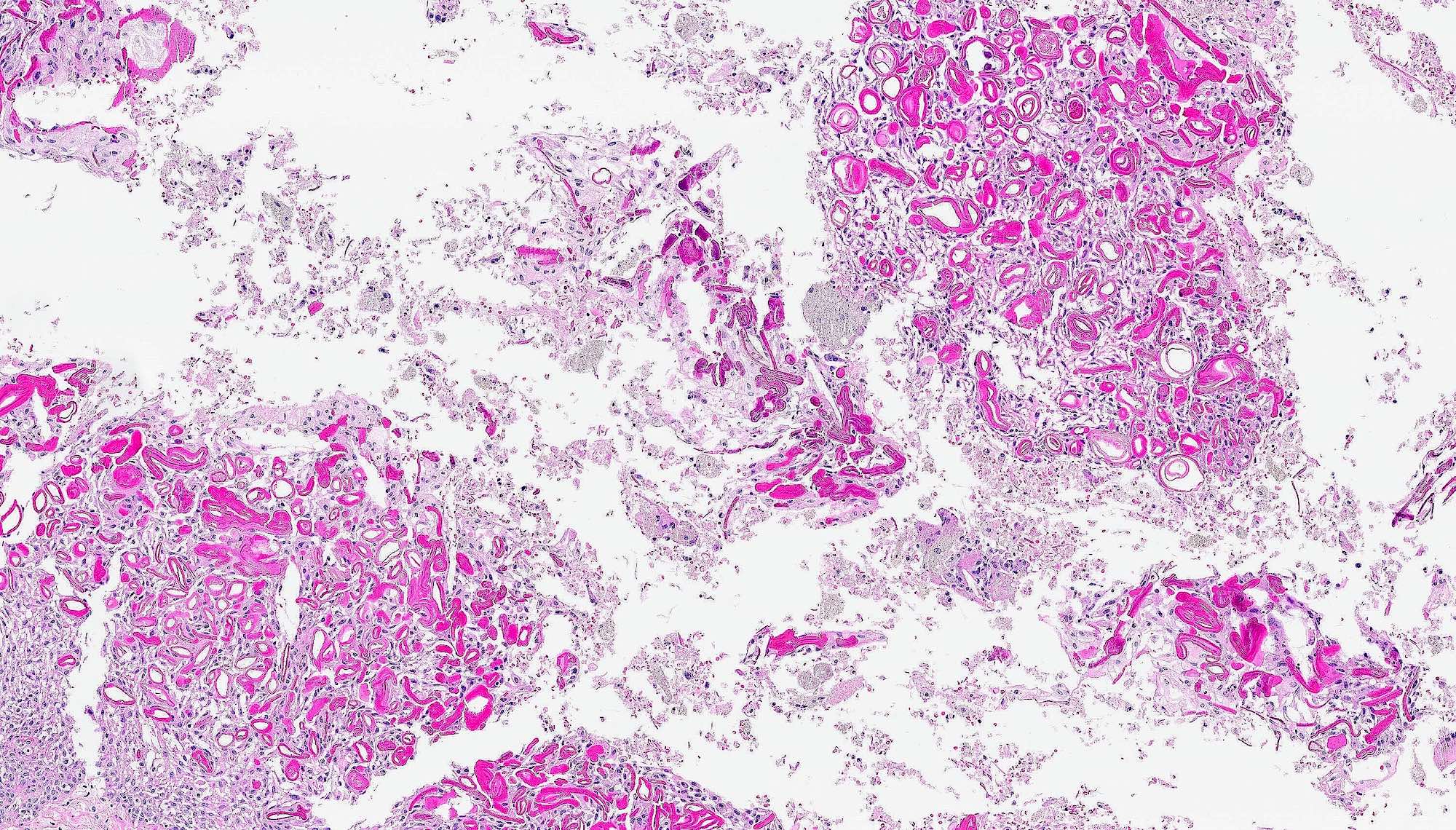
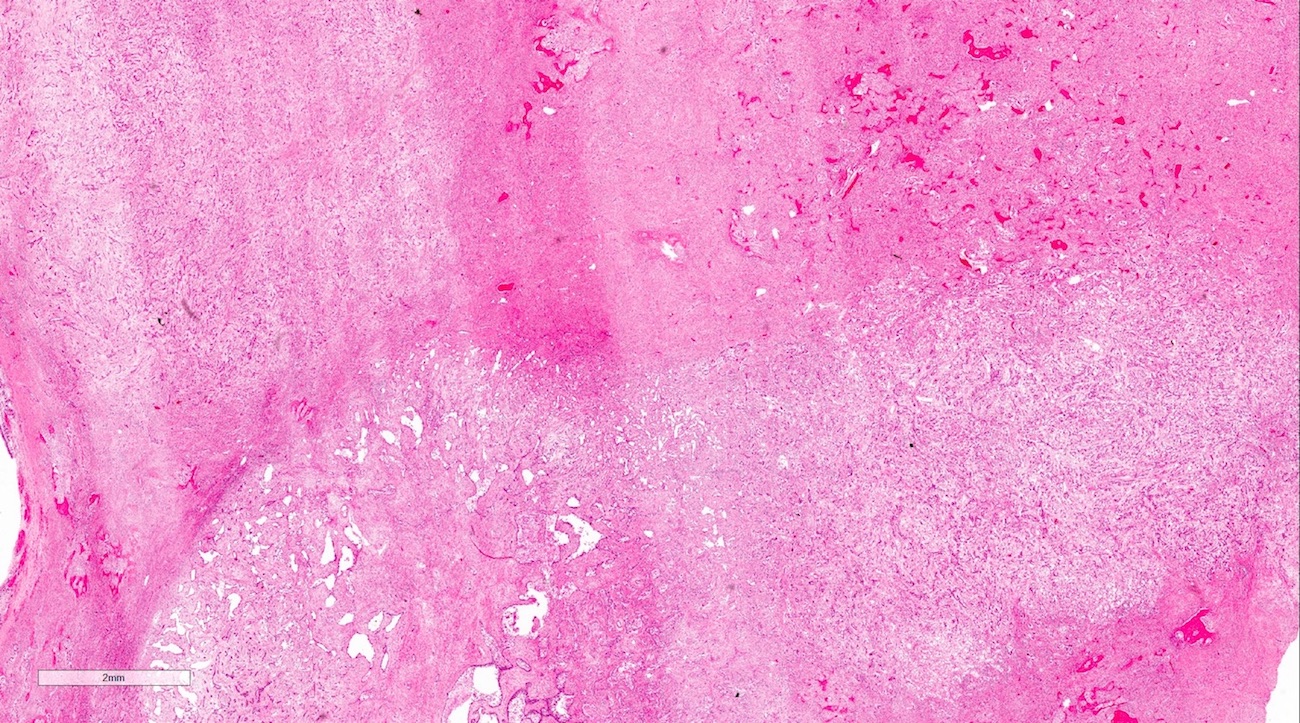
Gross description
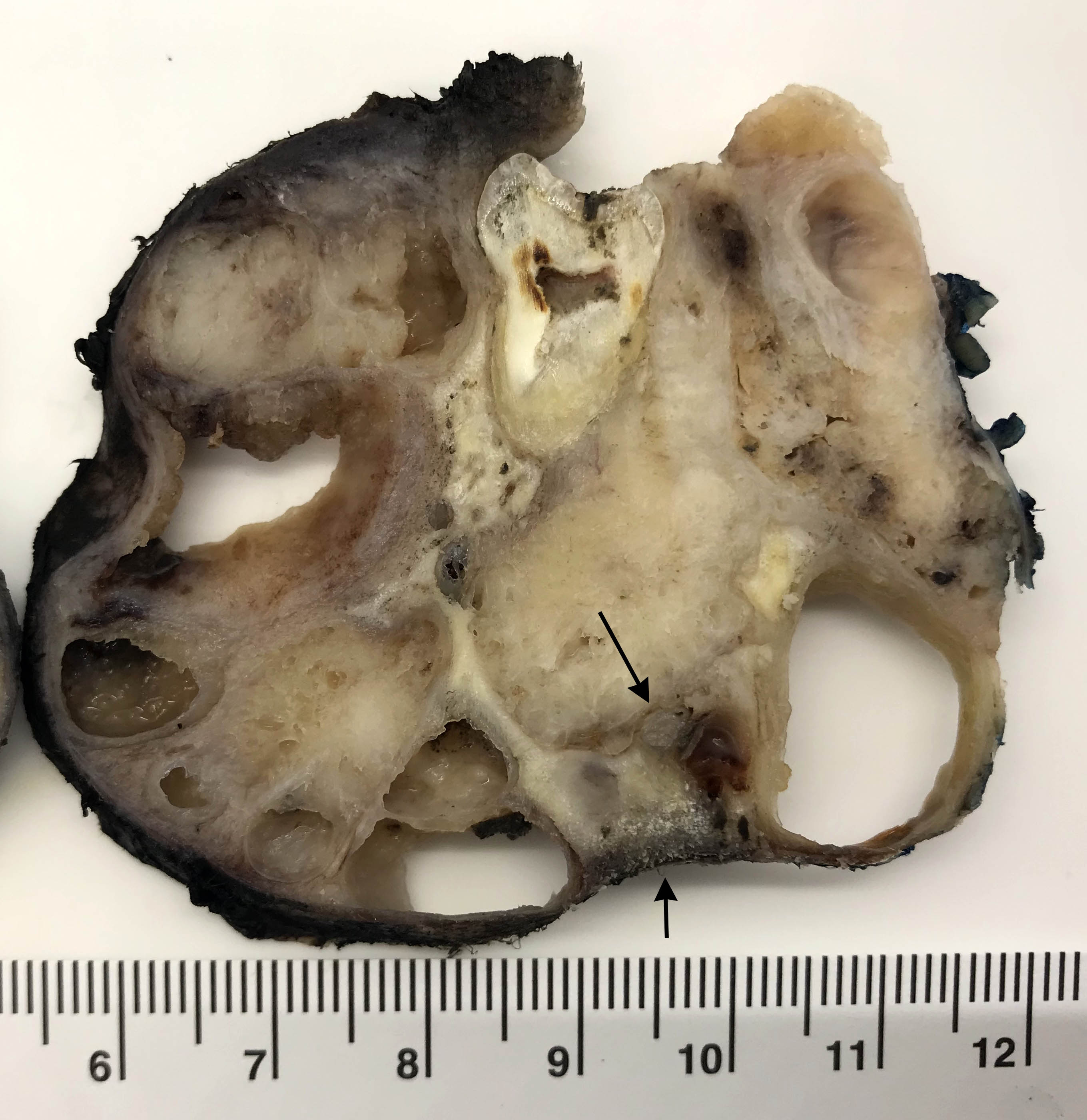
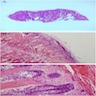
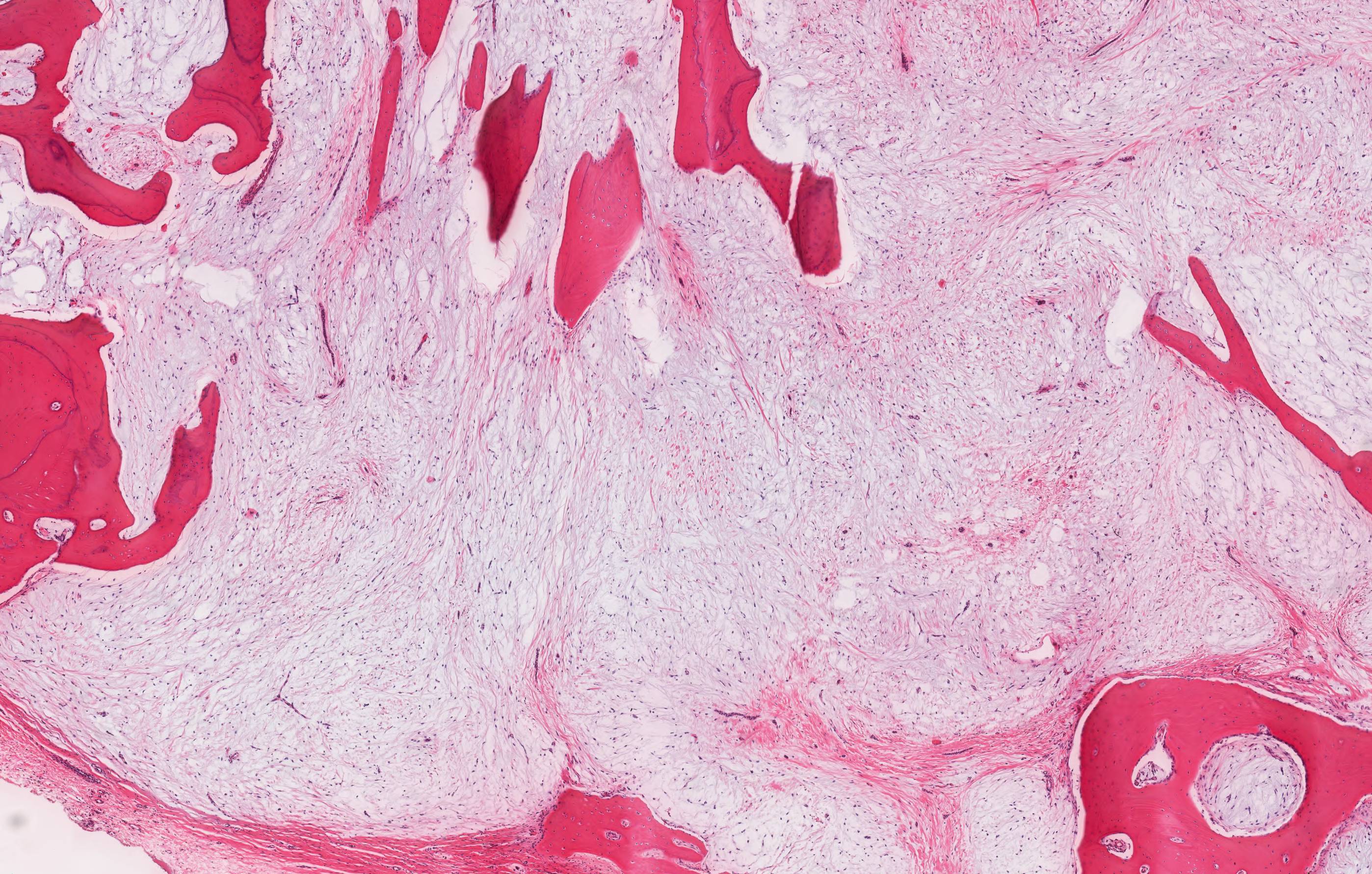
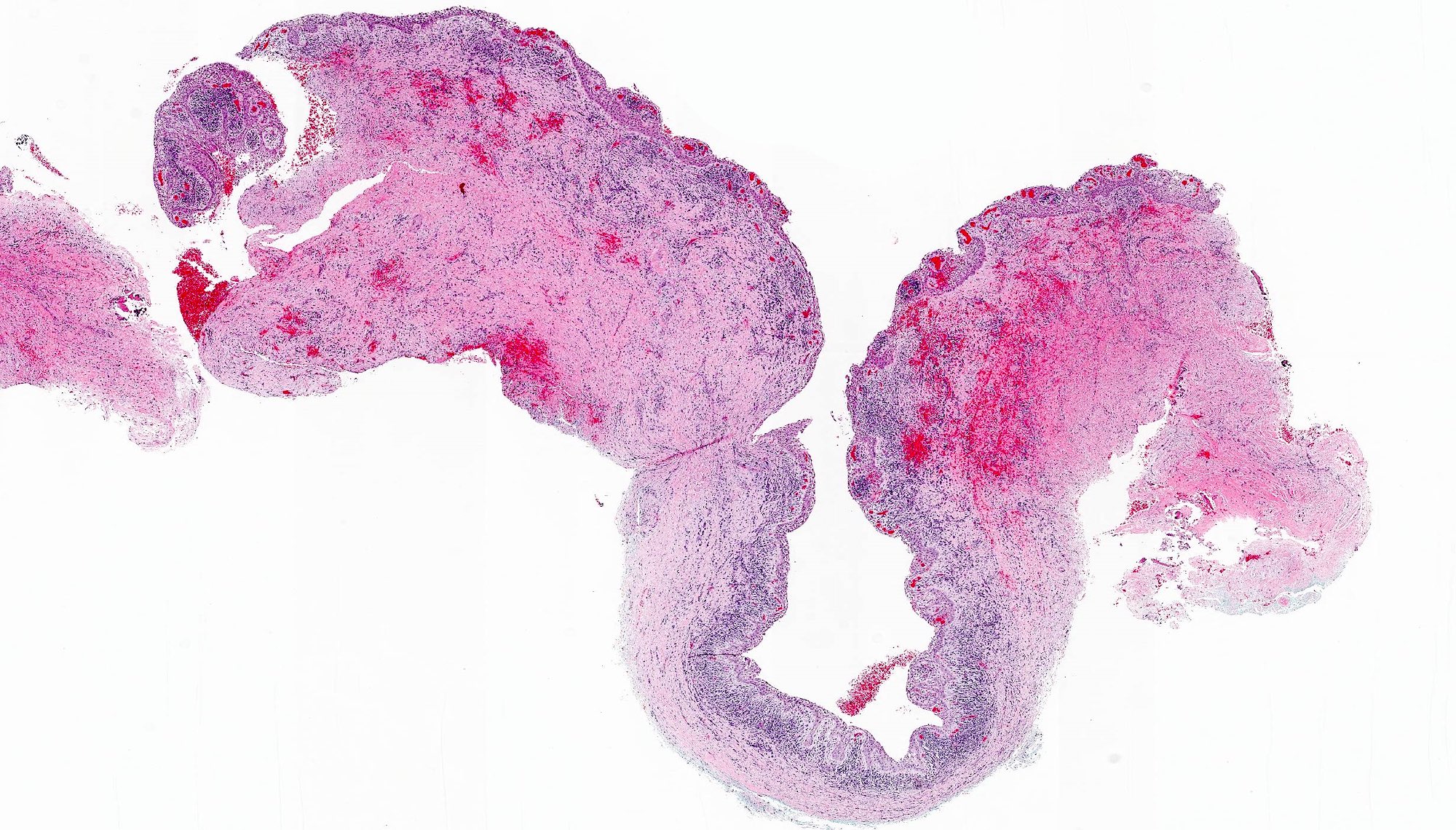
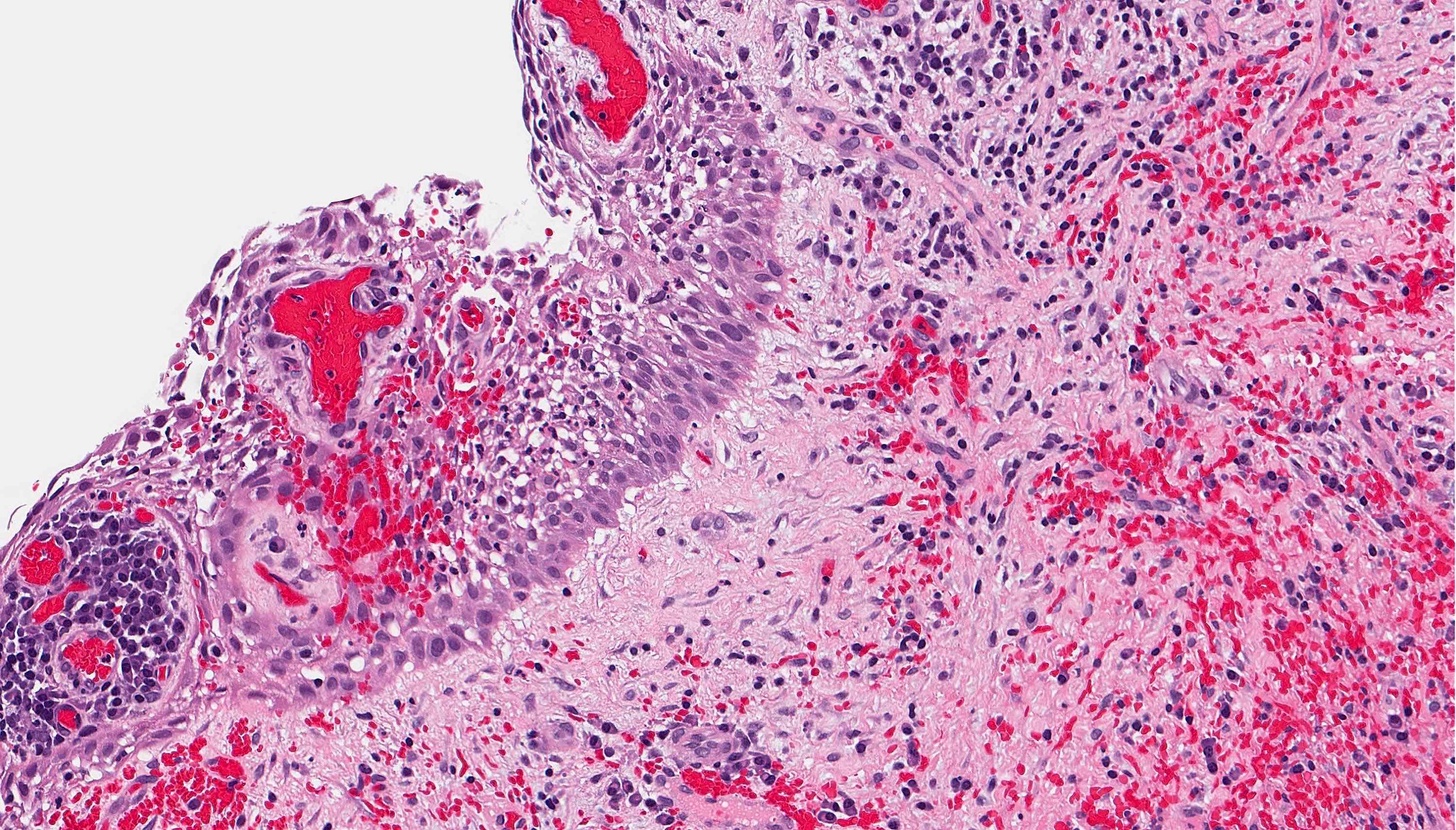
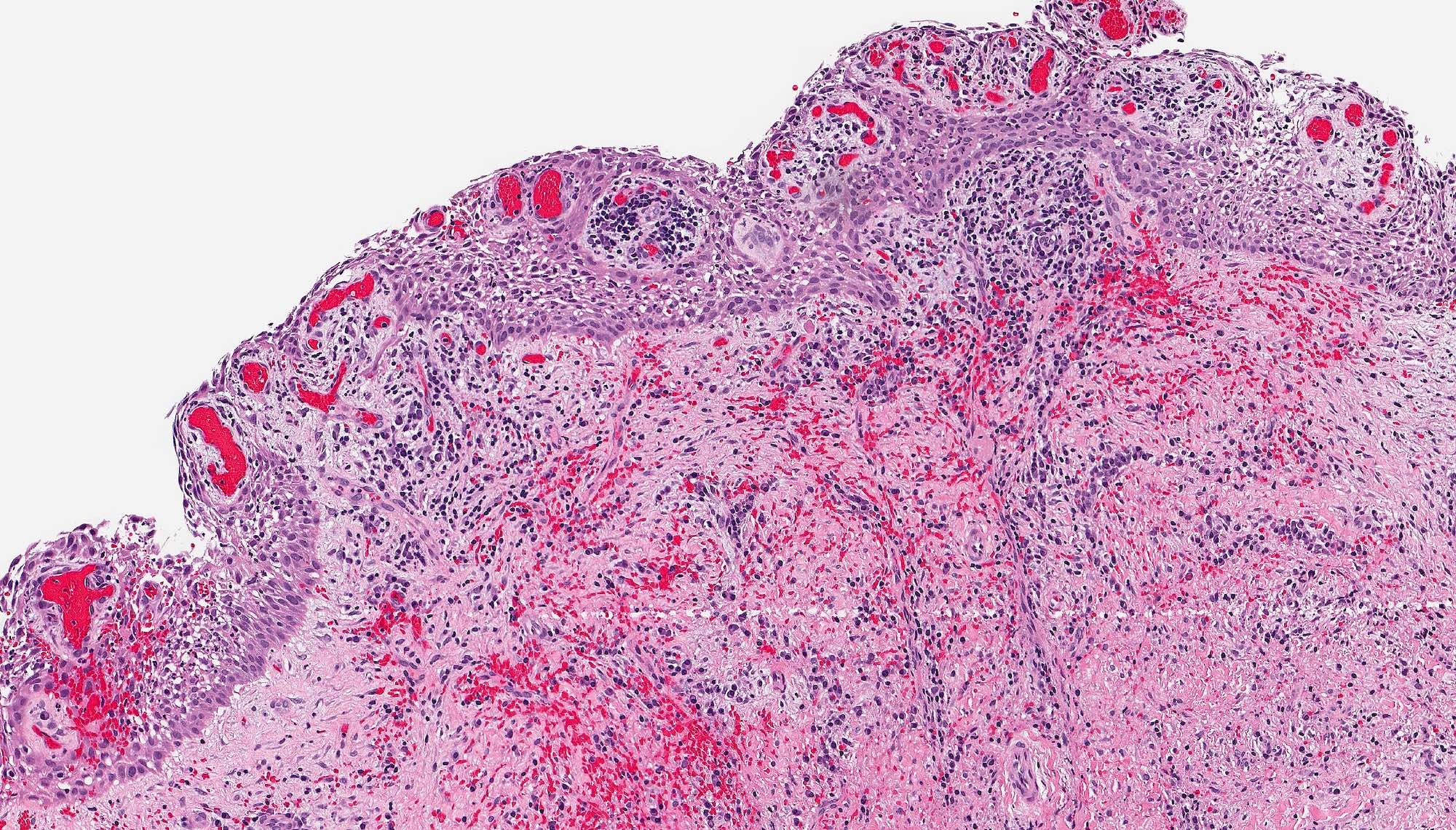
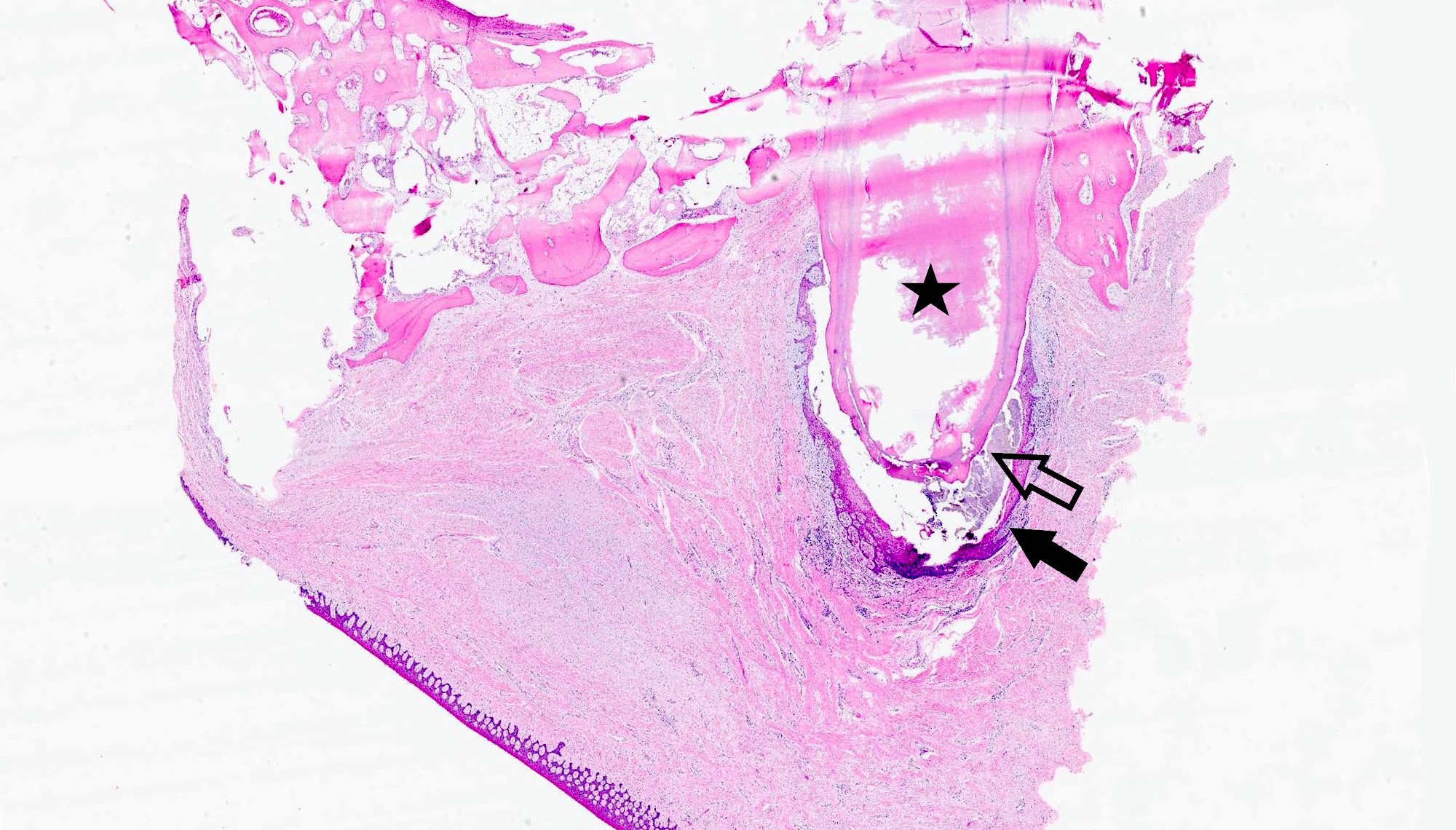
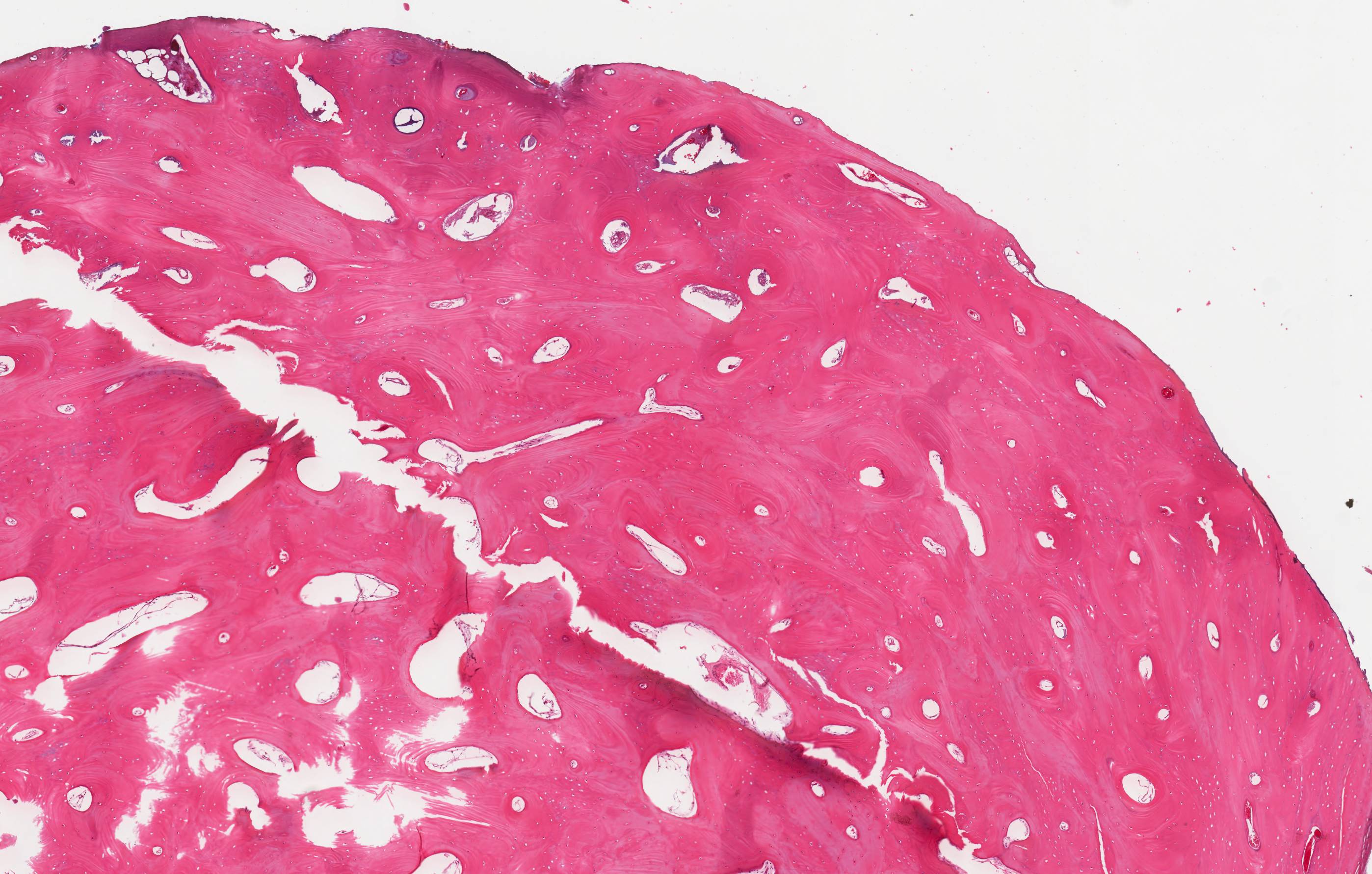
- Fragments of irregular bone (+/- teeth) with purulent to necrotic marrow
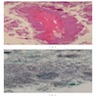
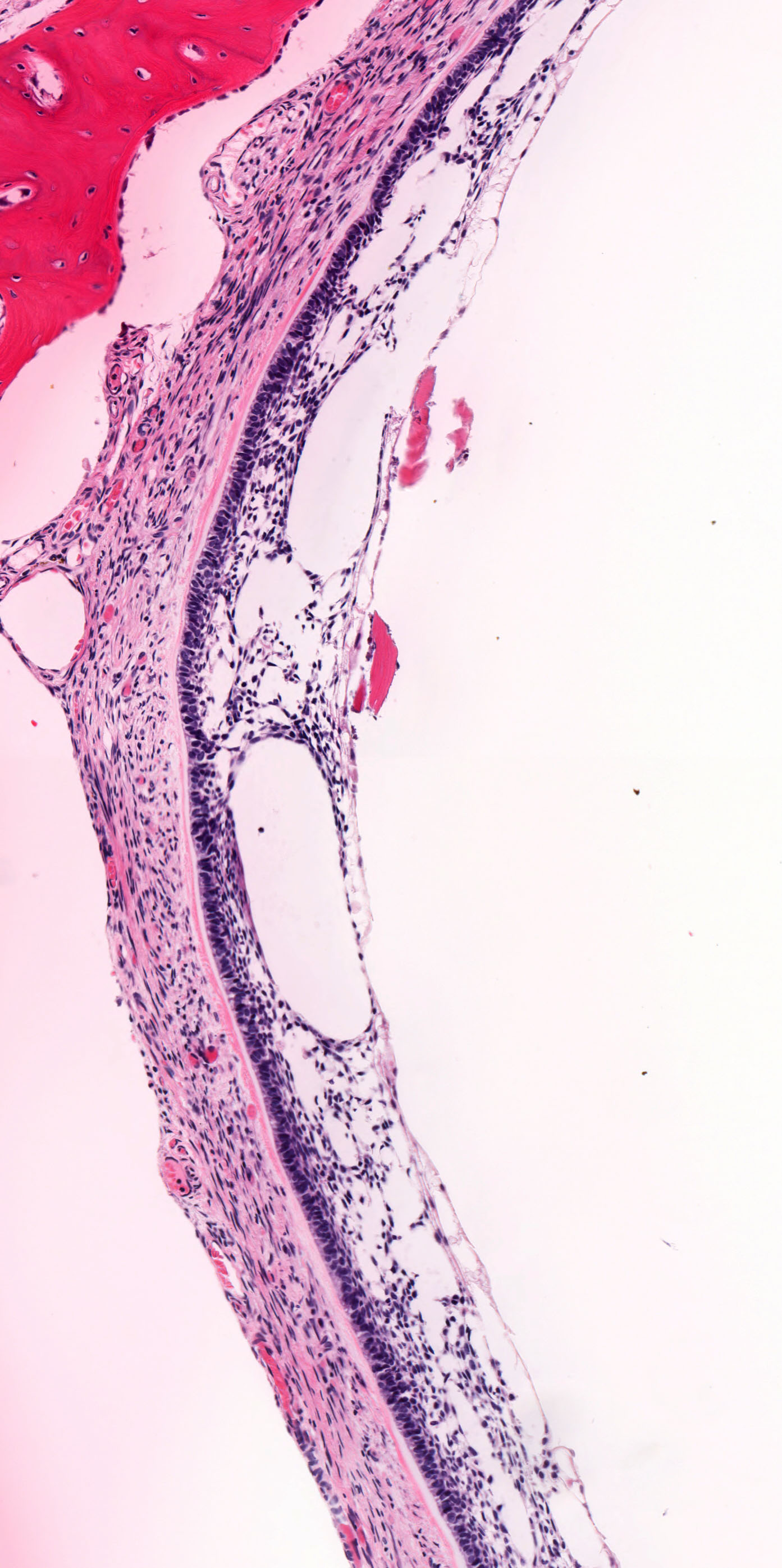
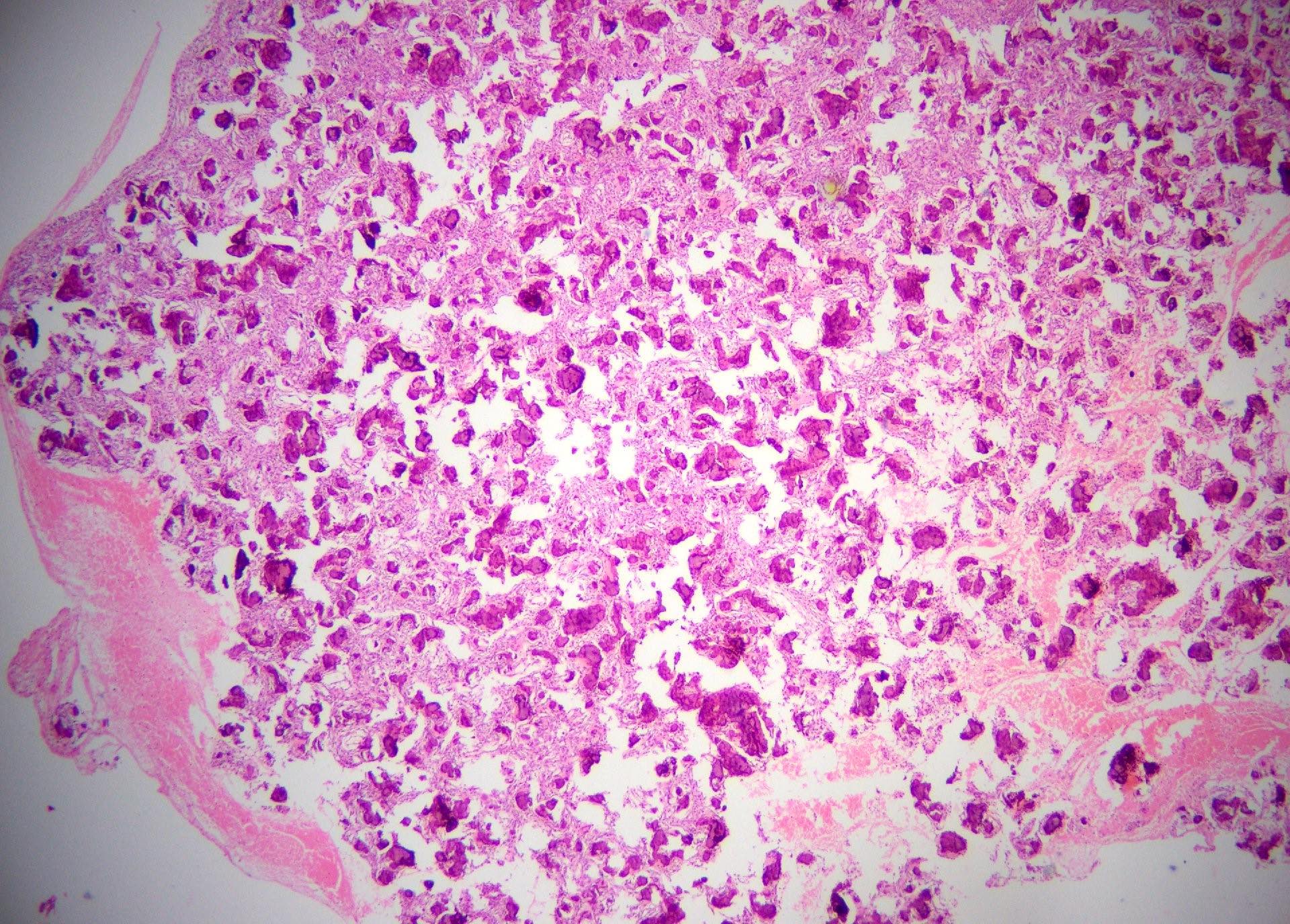
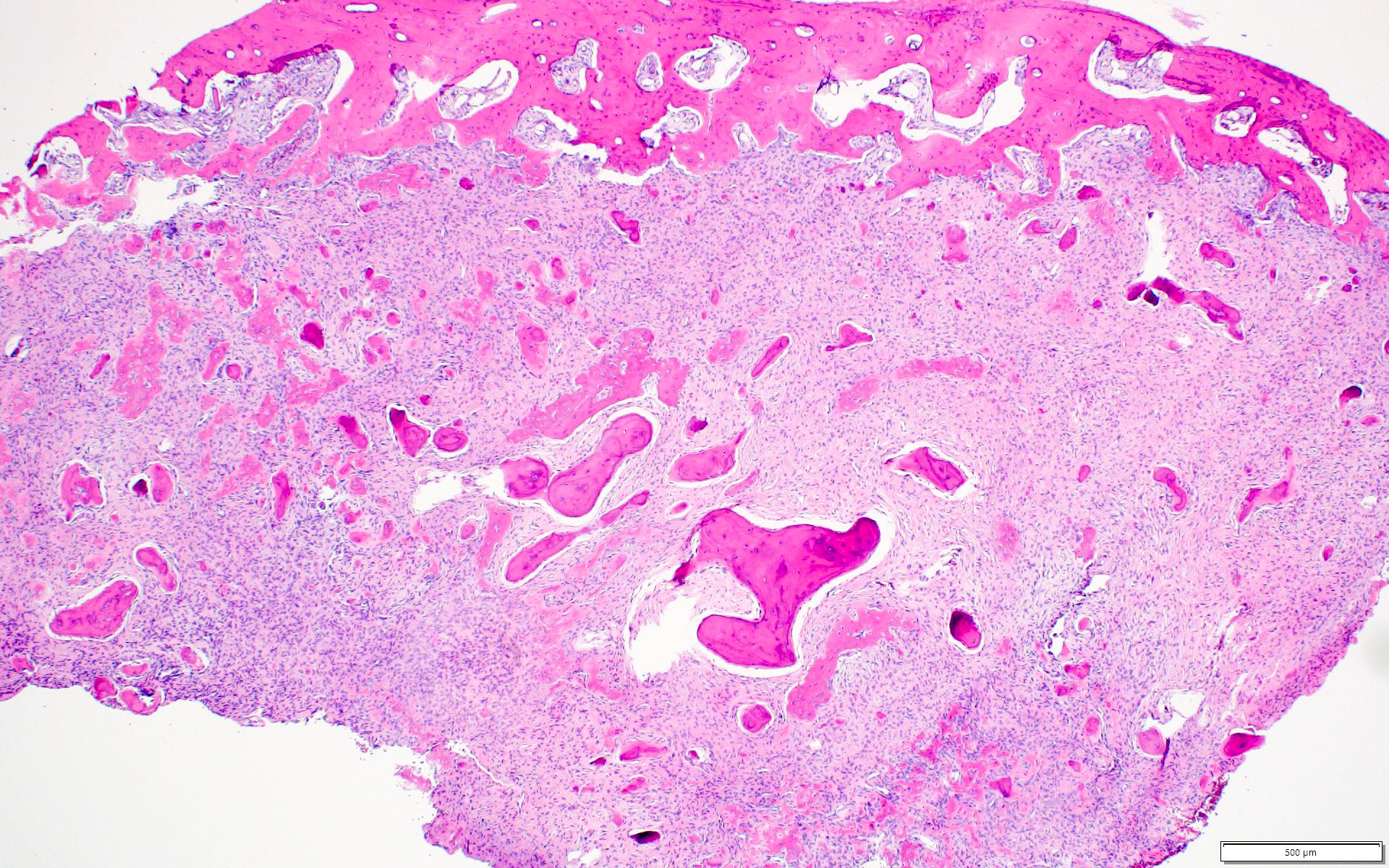
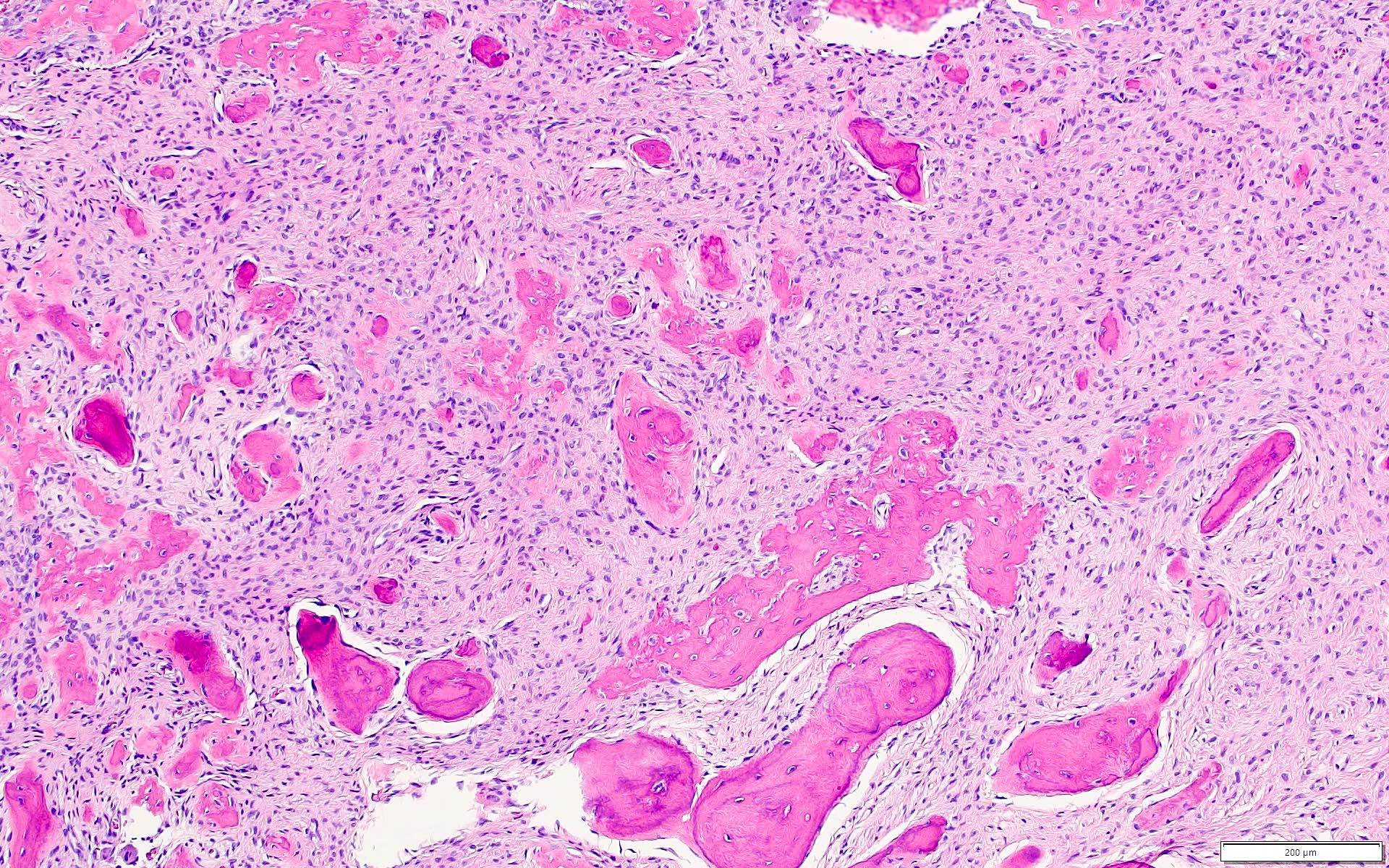
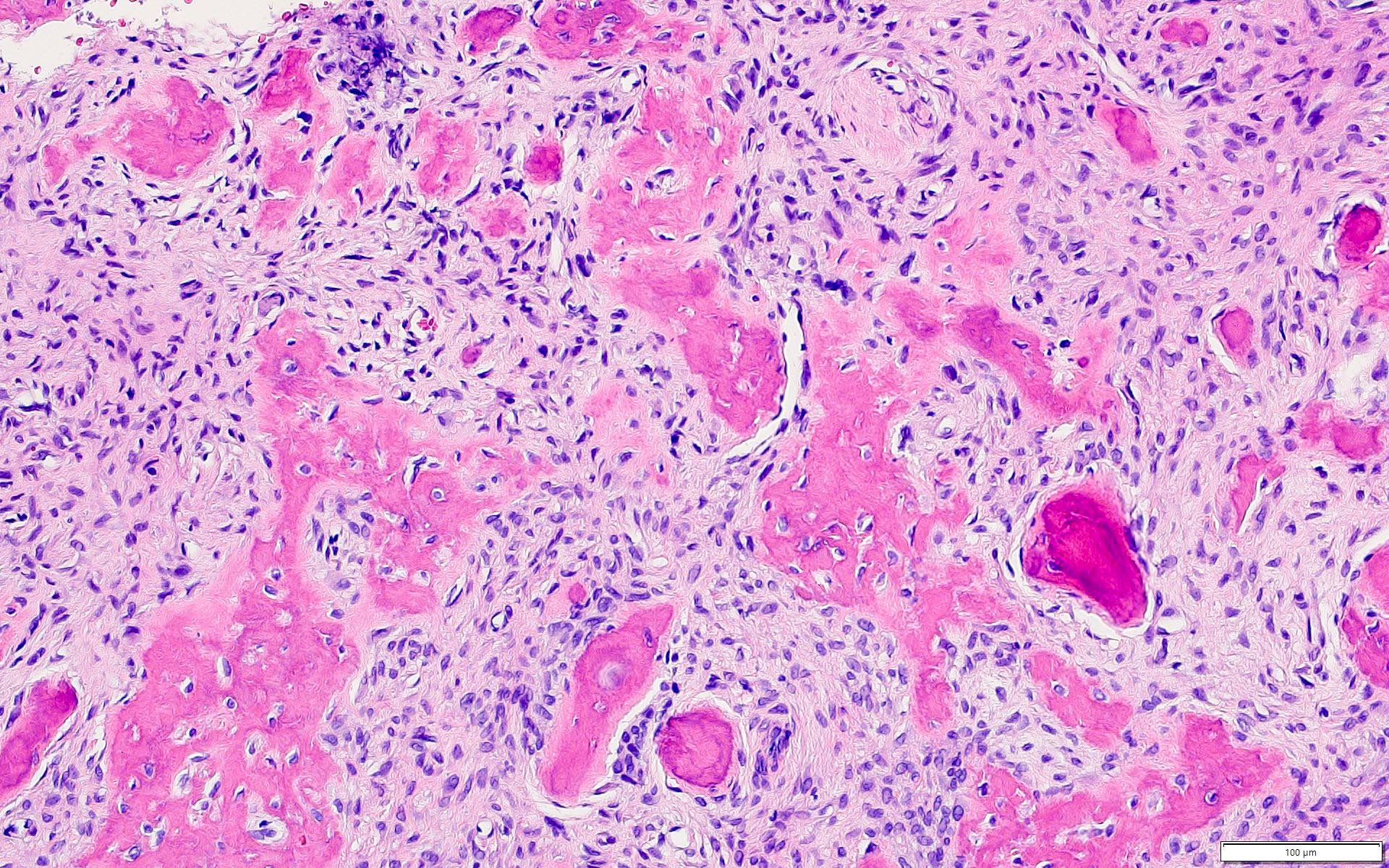
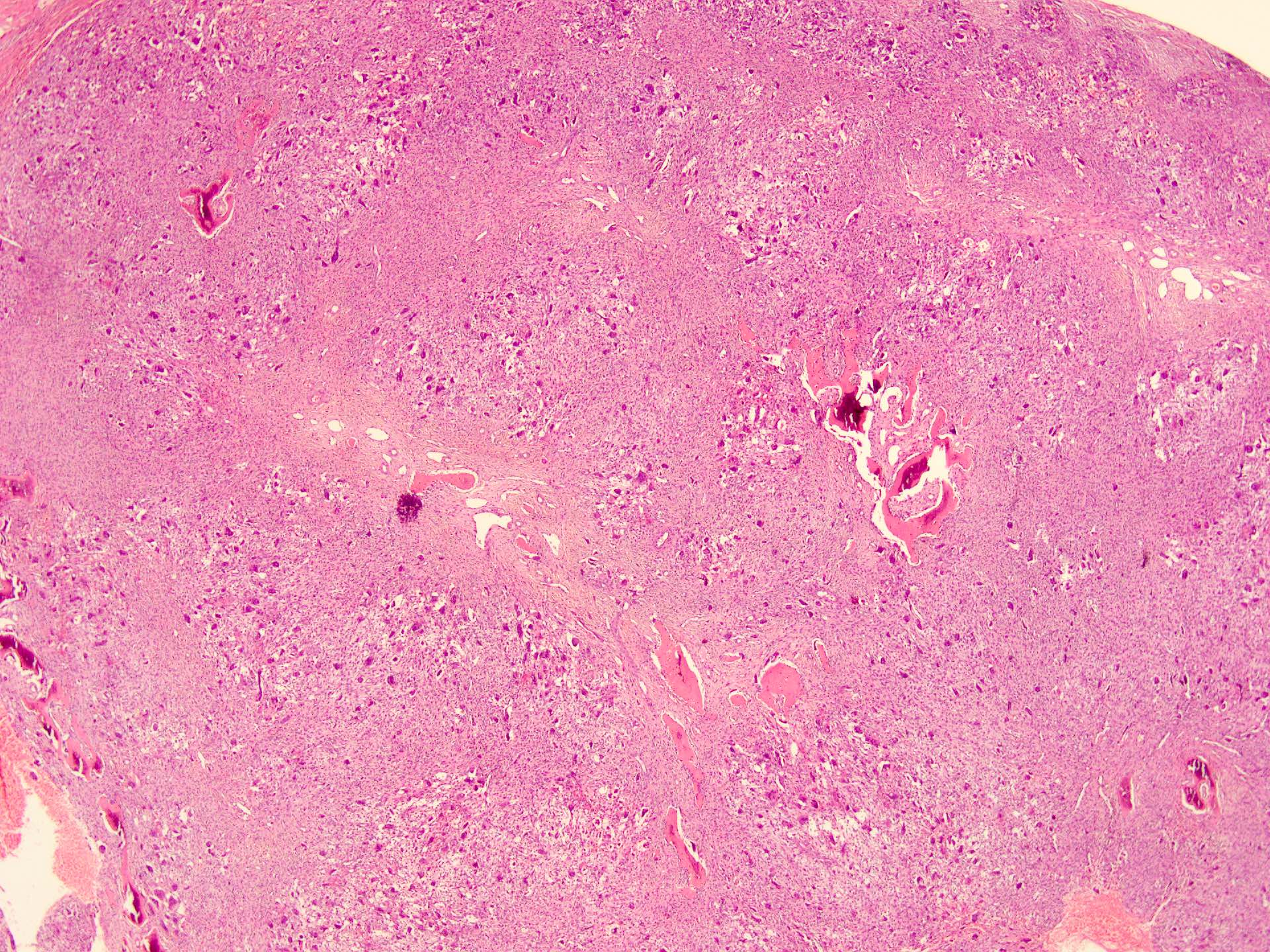
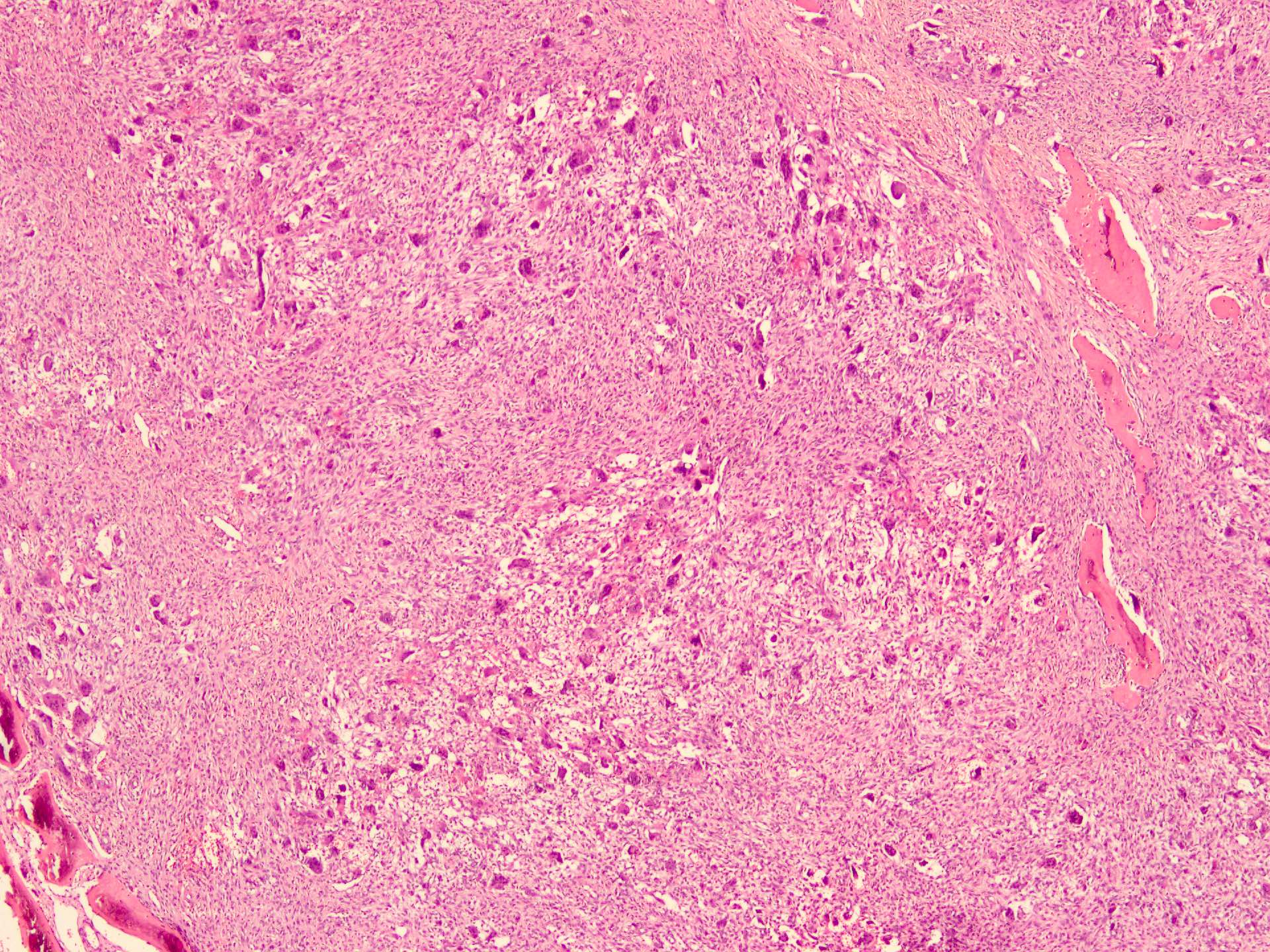
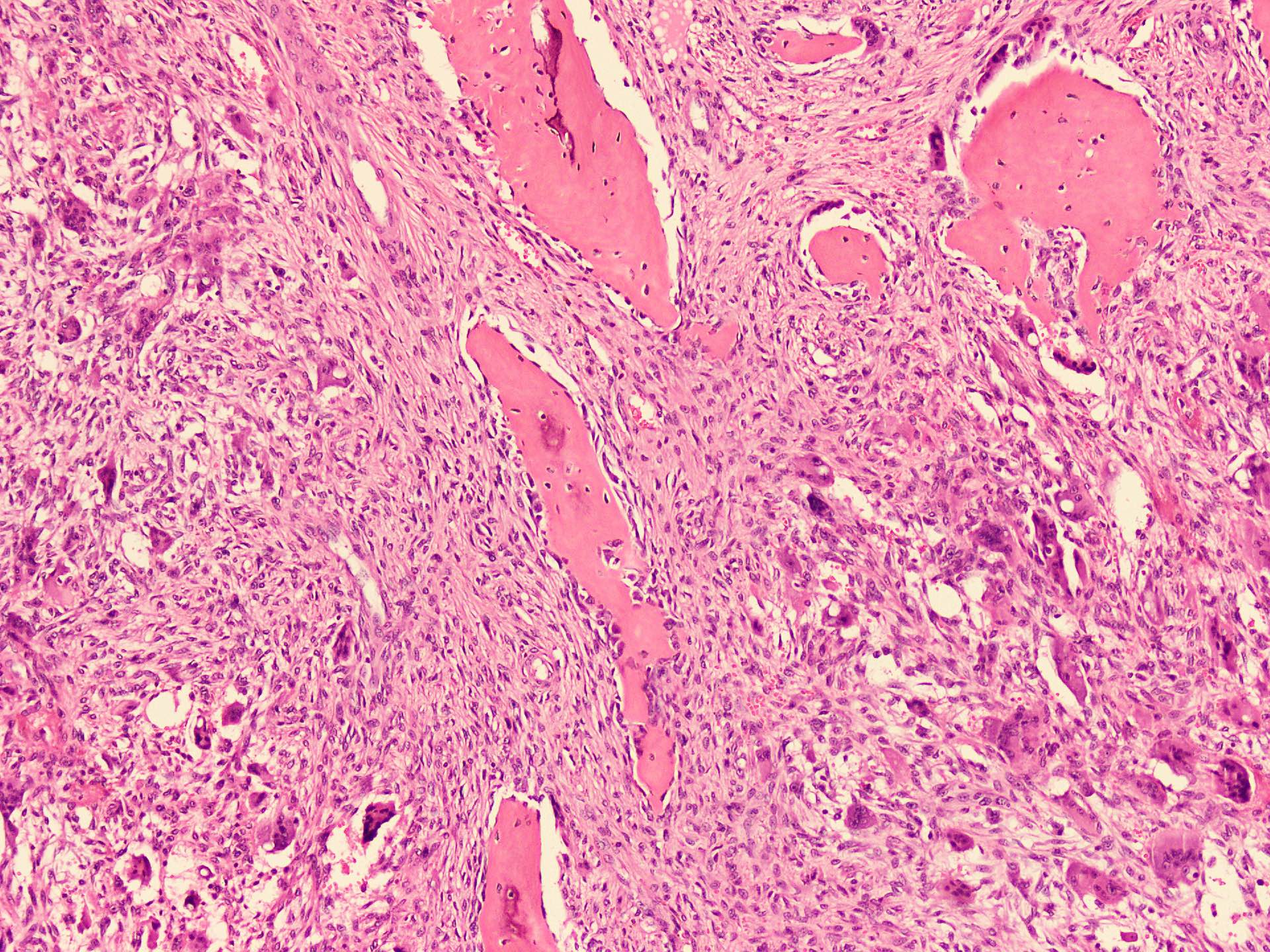
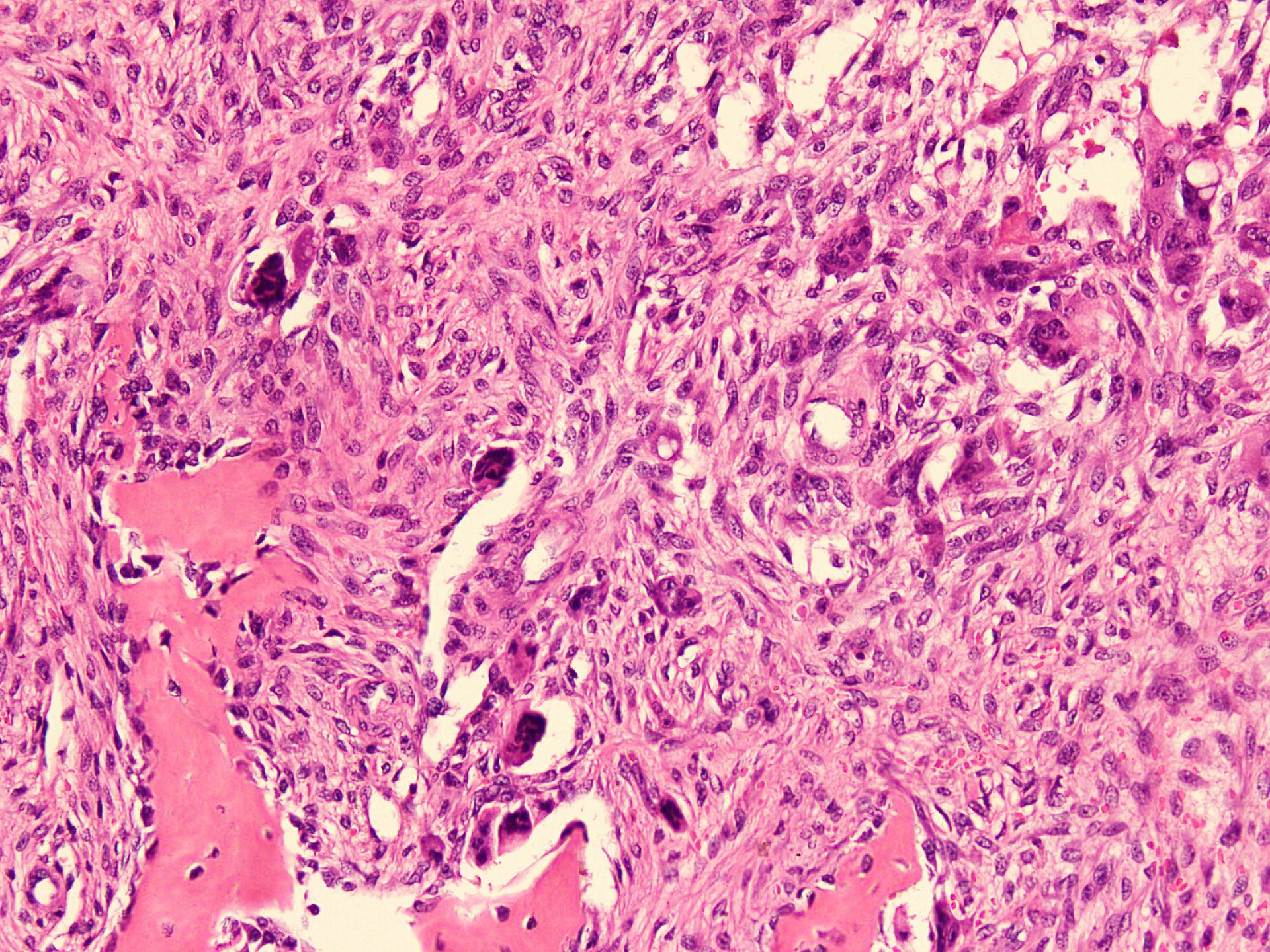
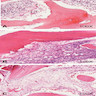
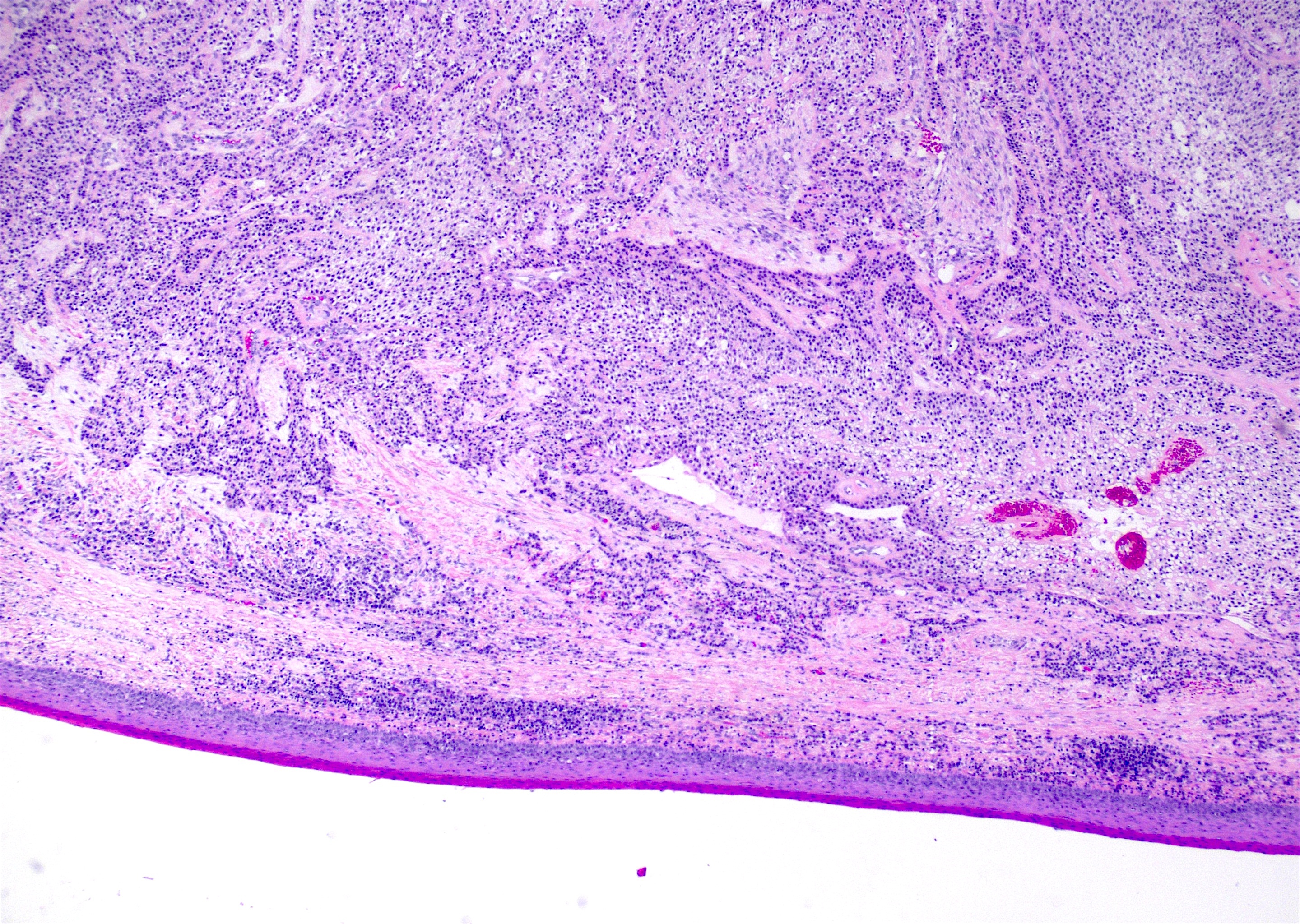
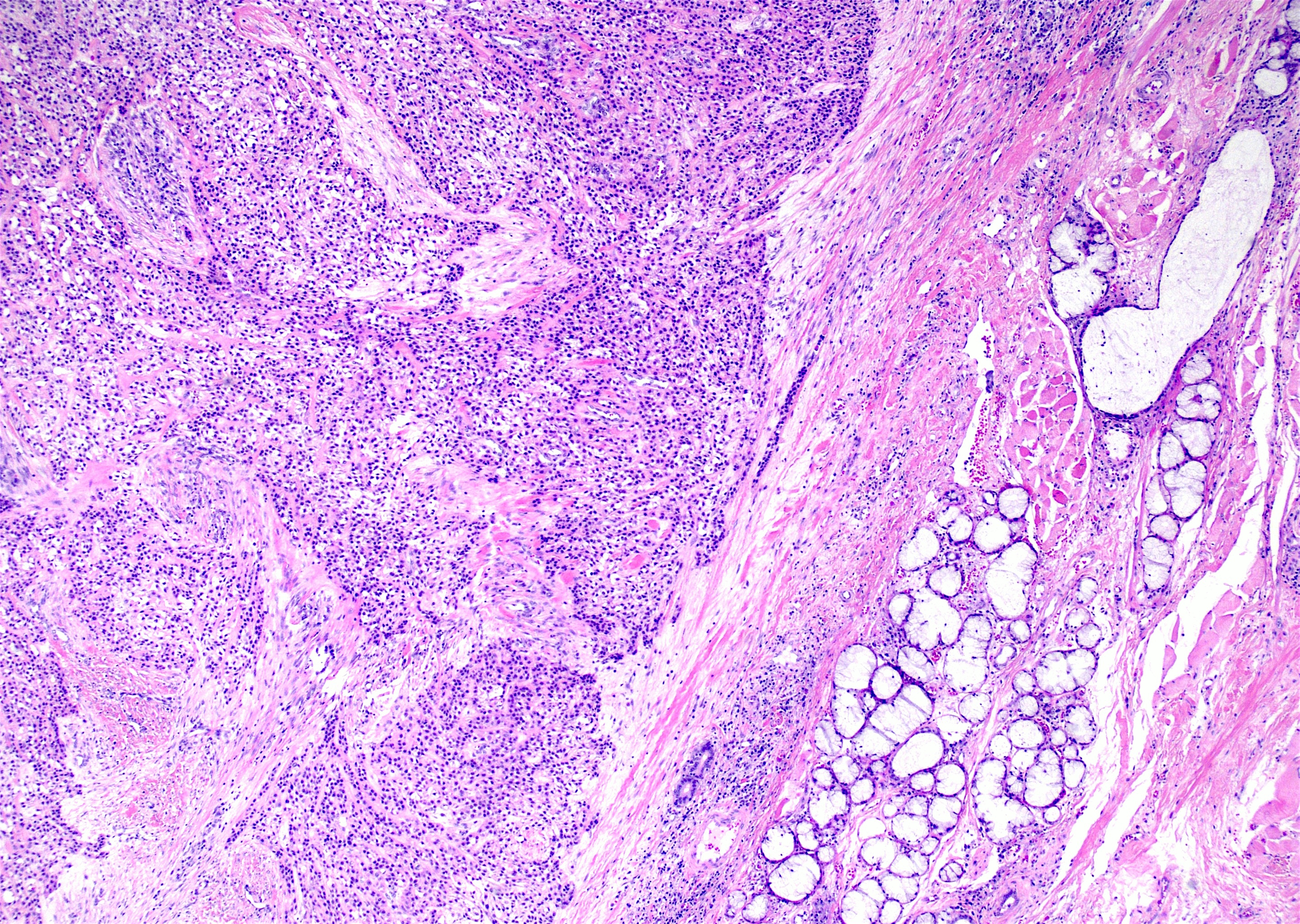
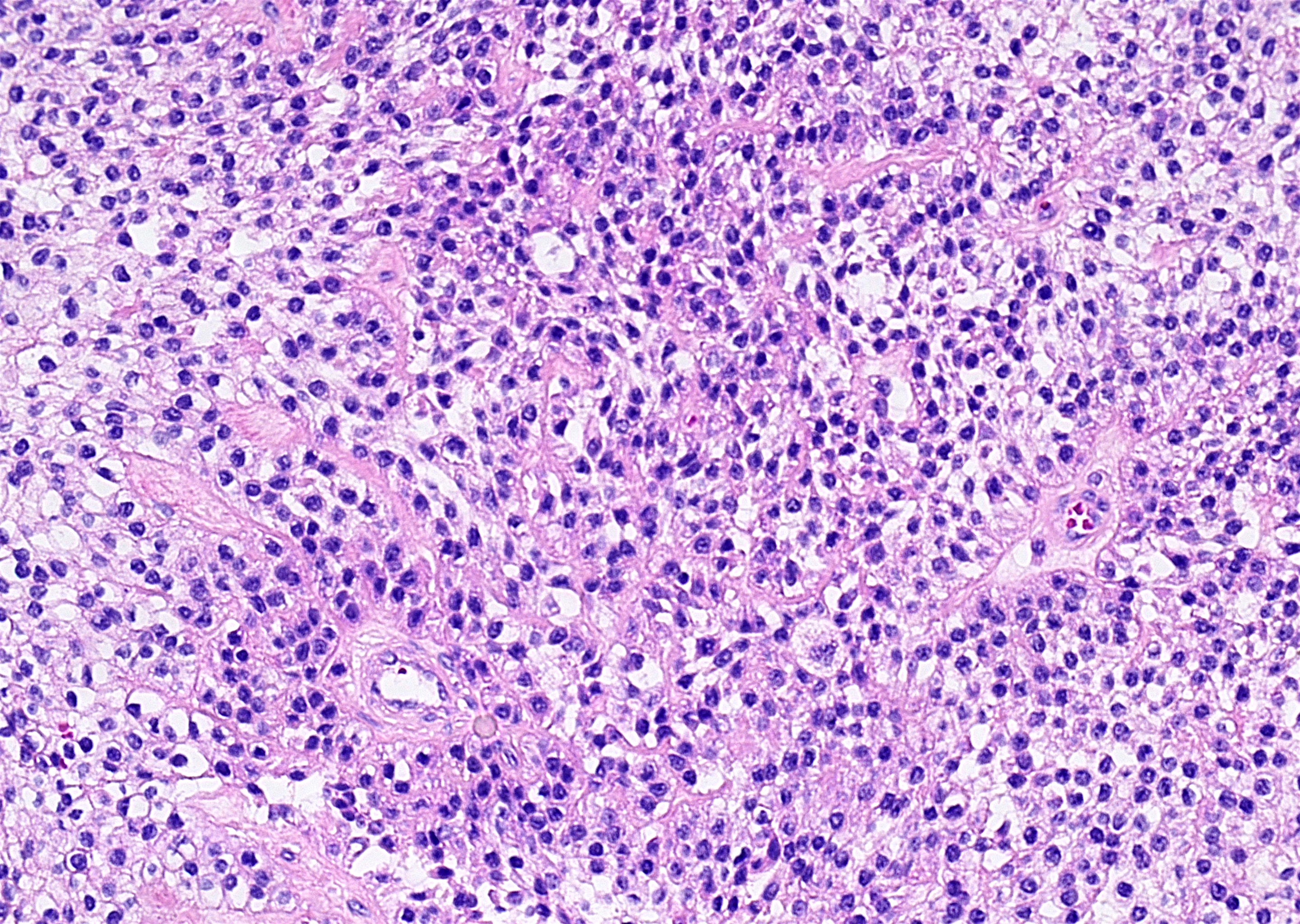
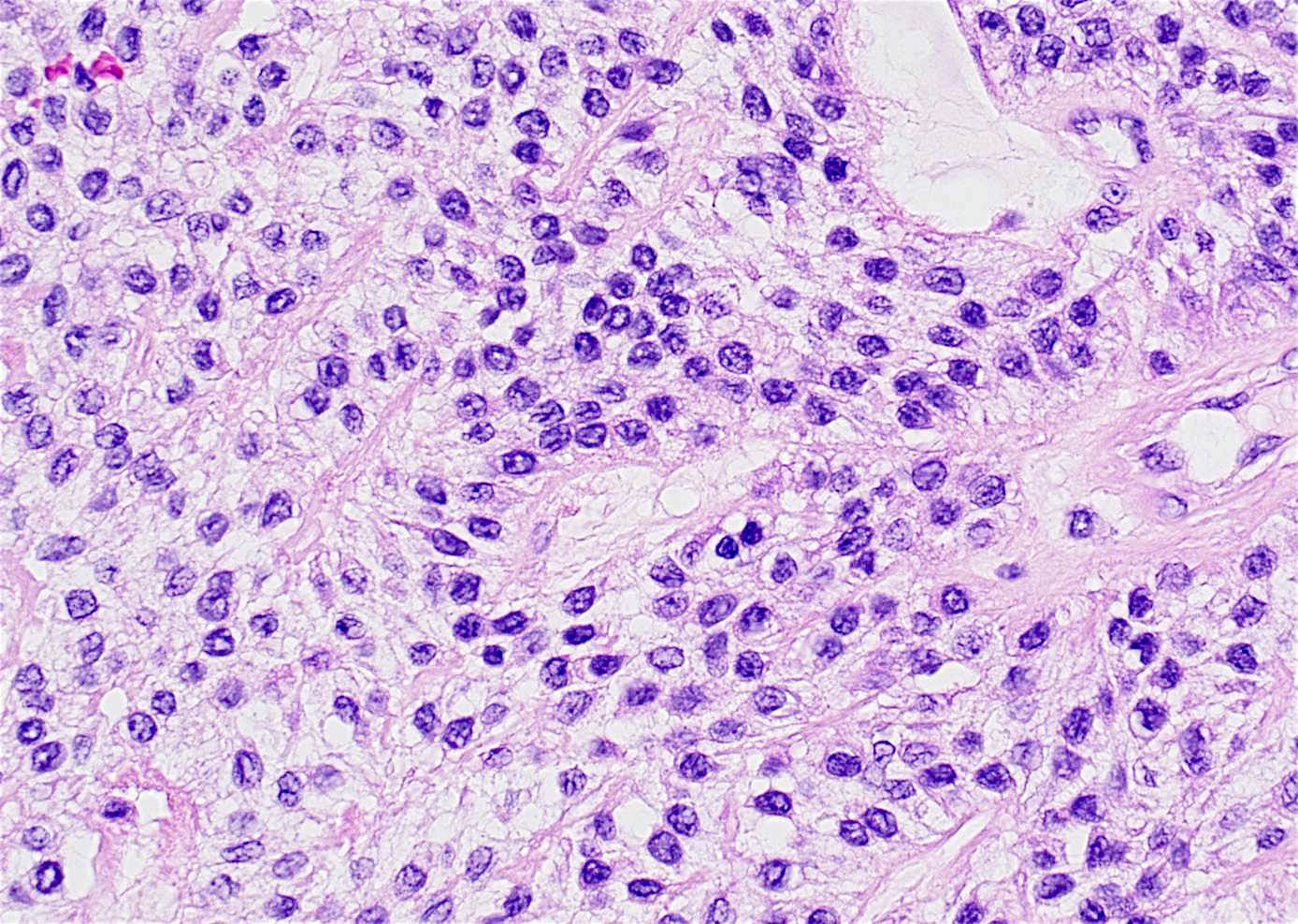
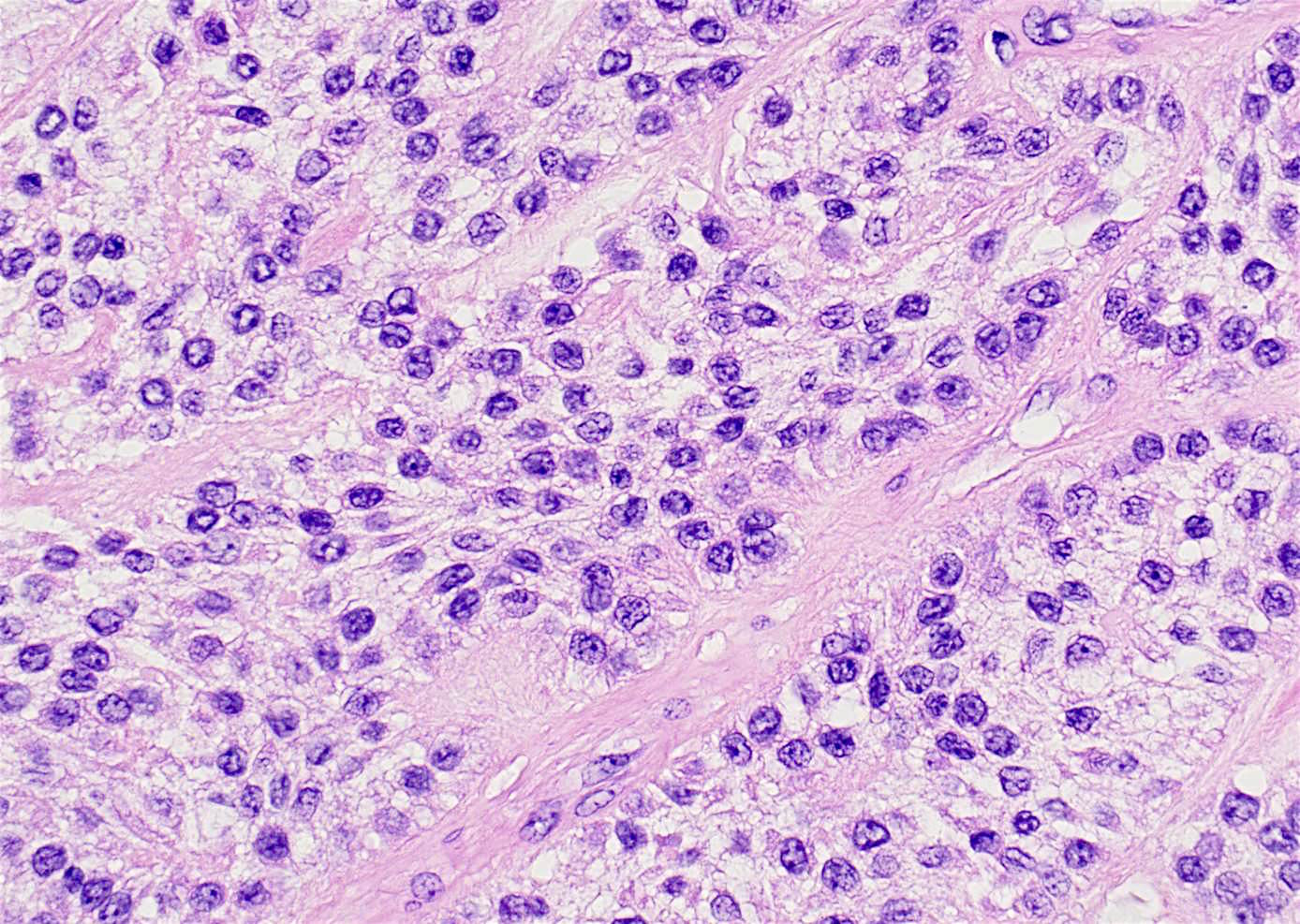
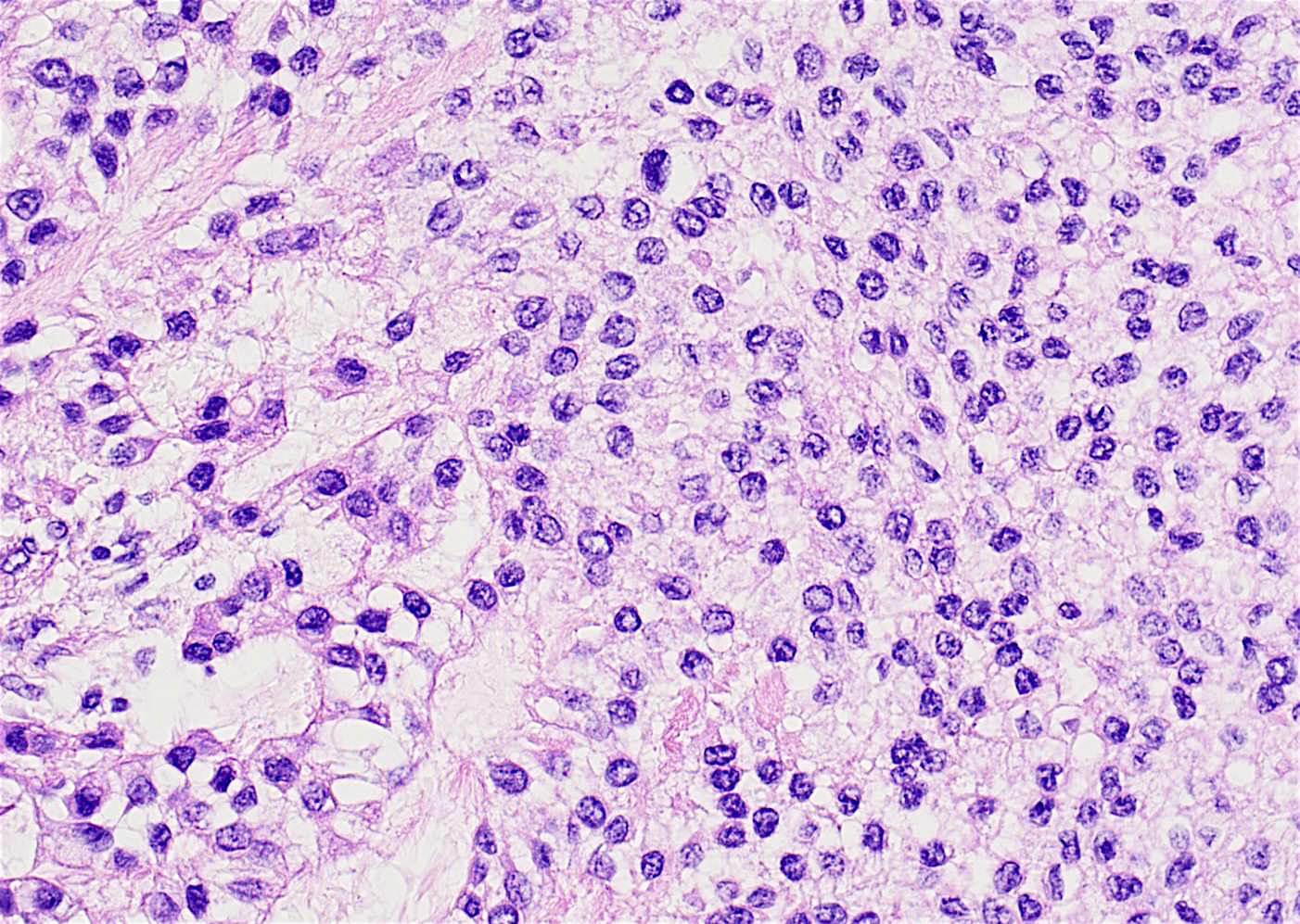
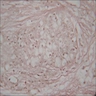
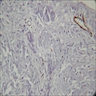
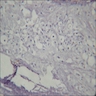
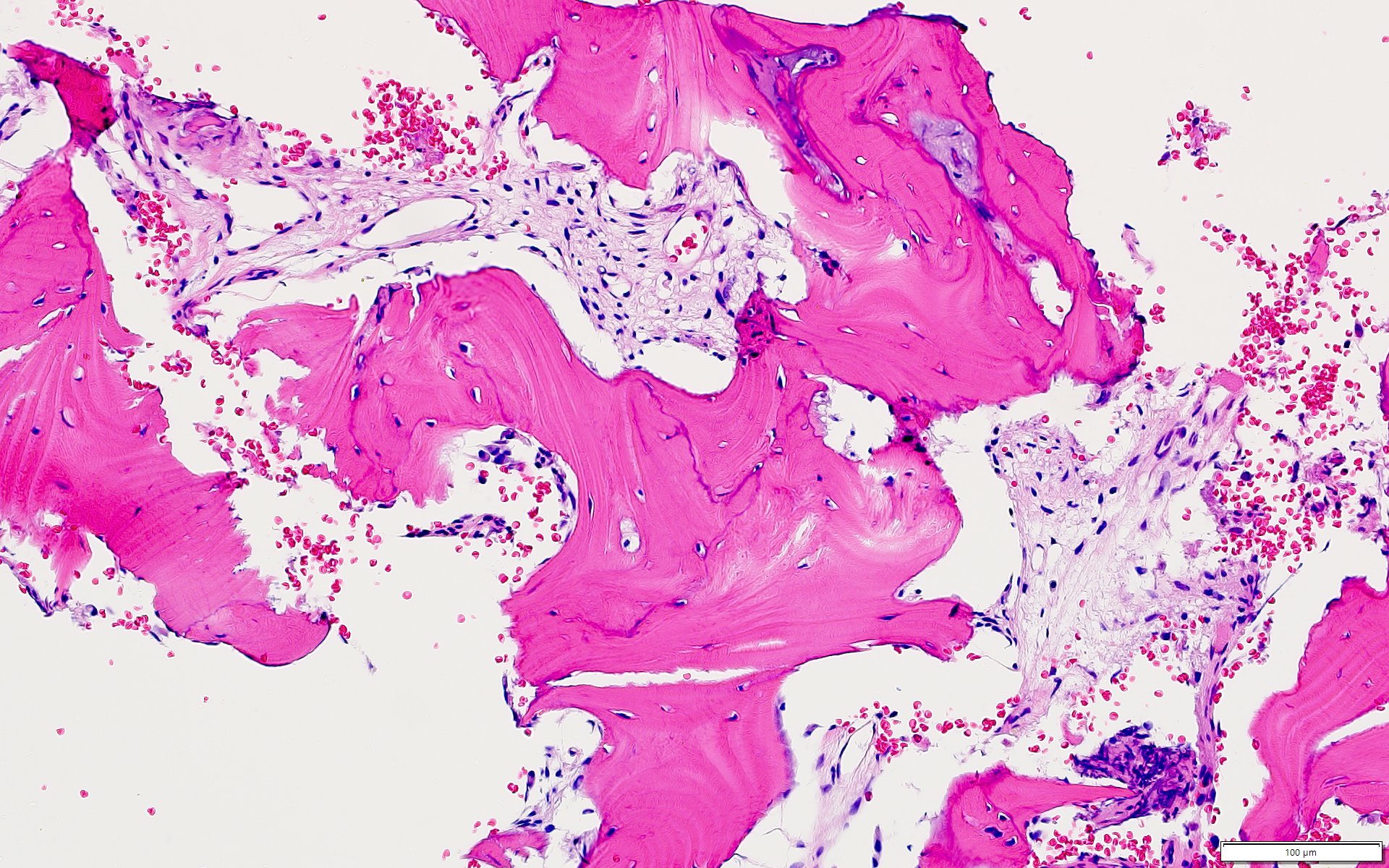
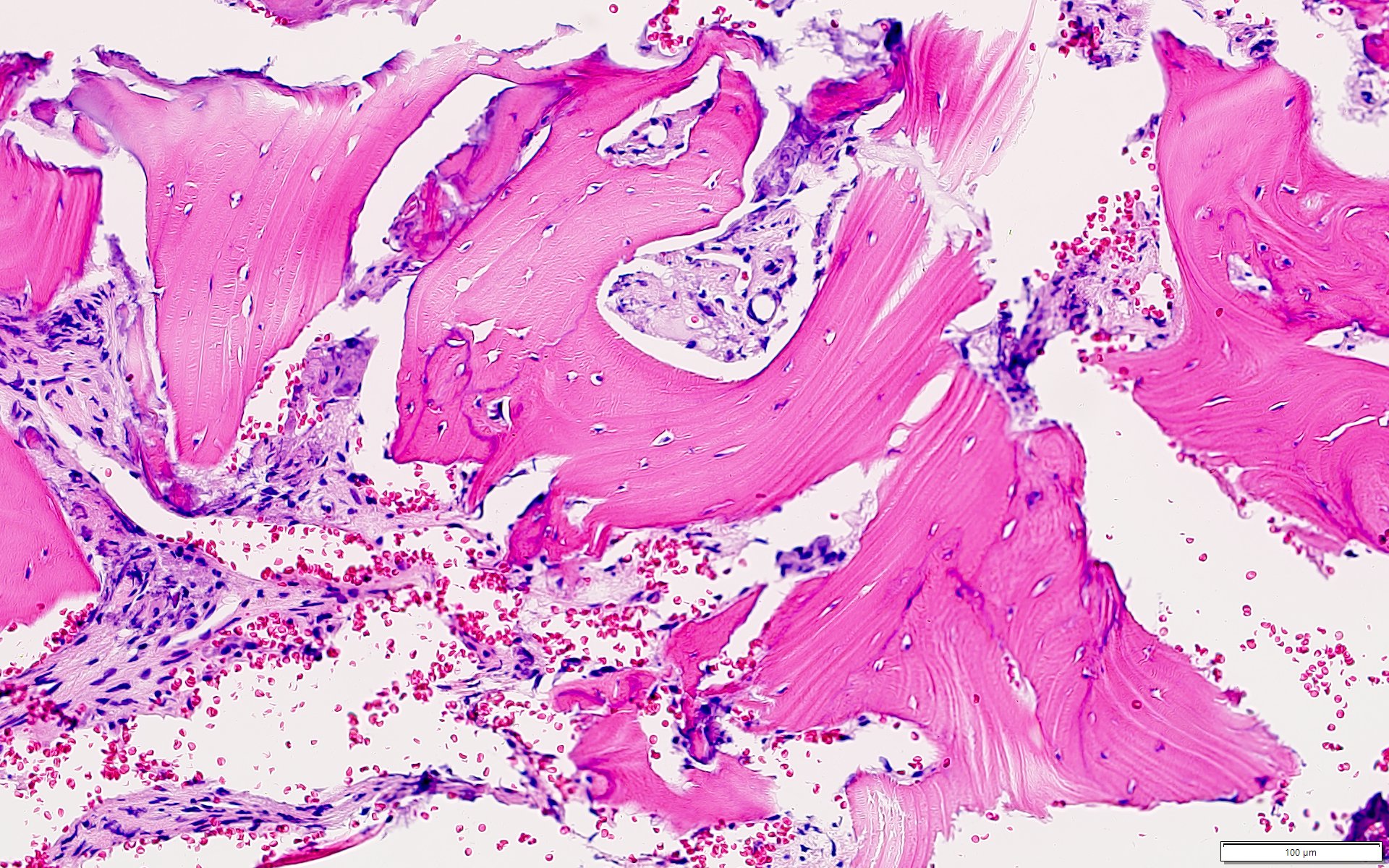
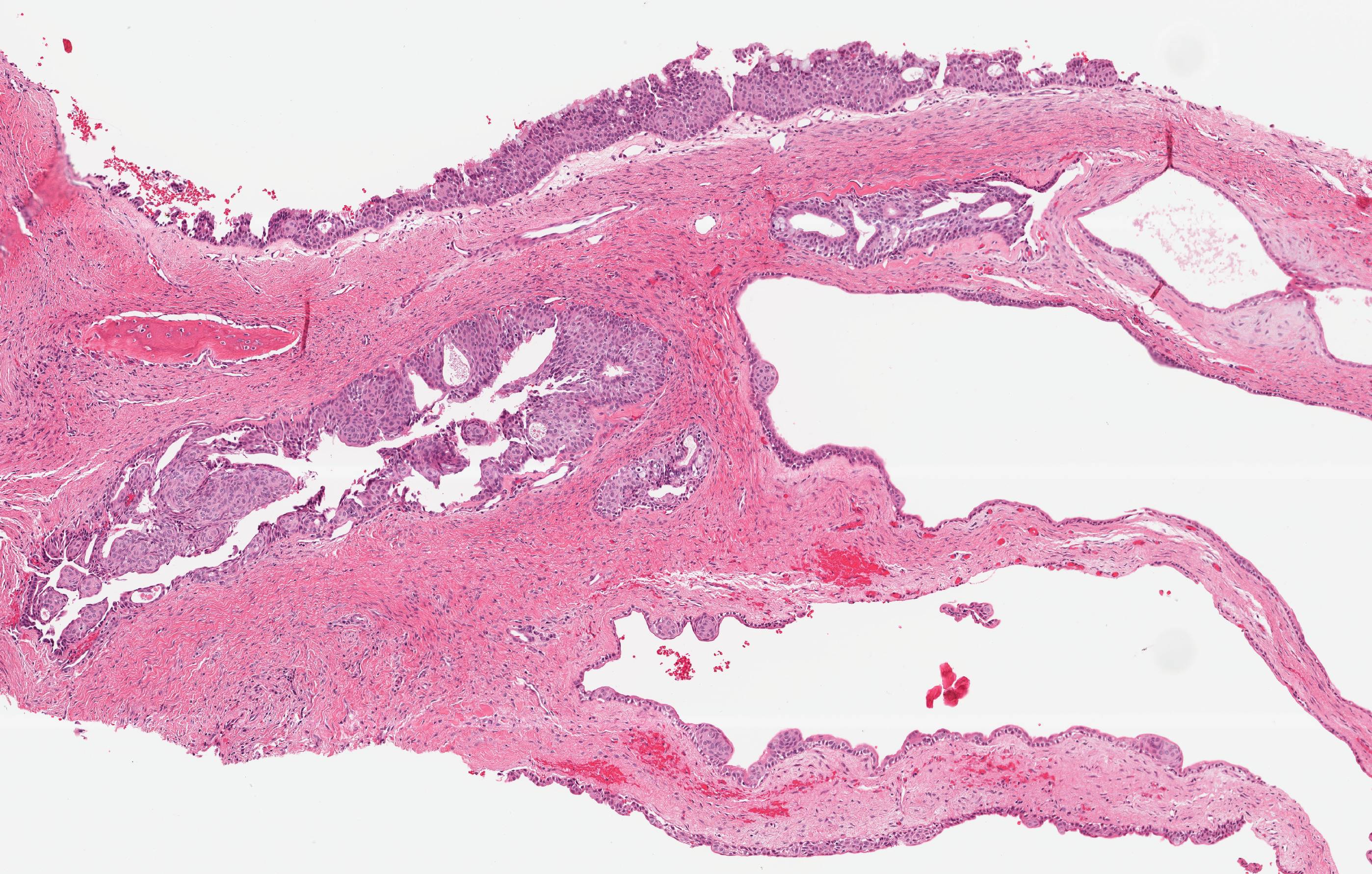
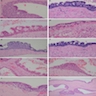
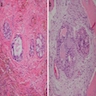
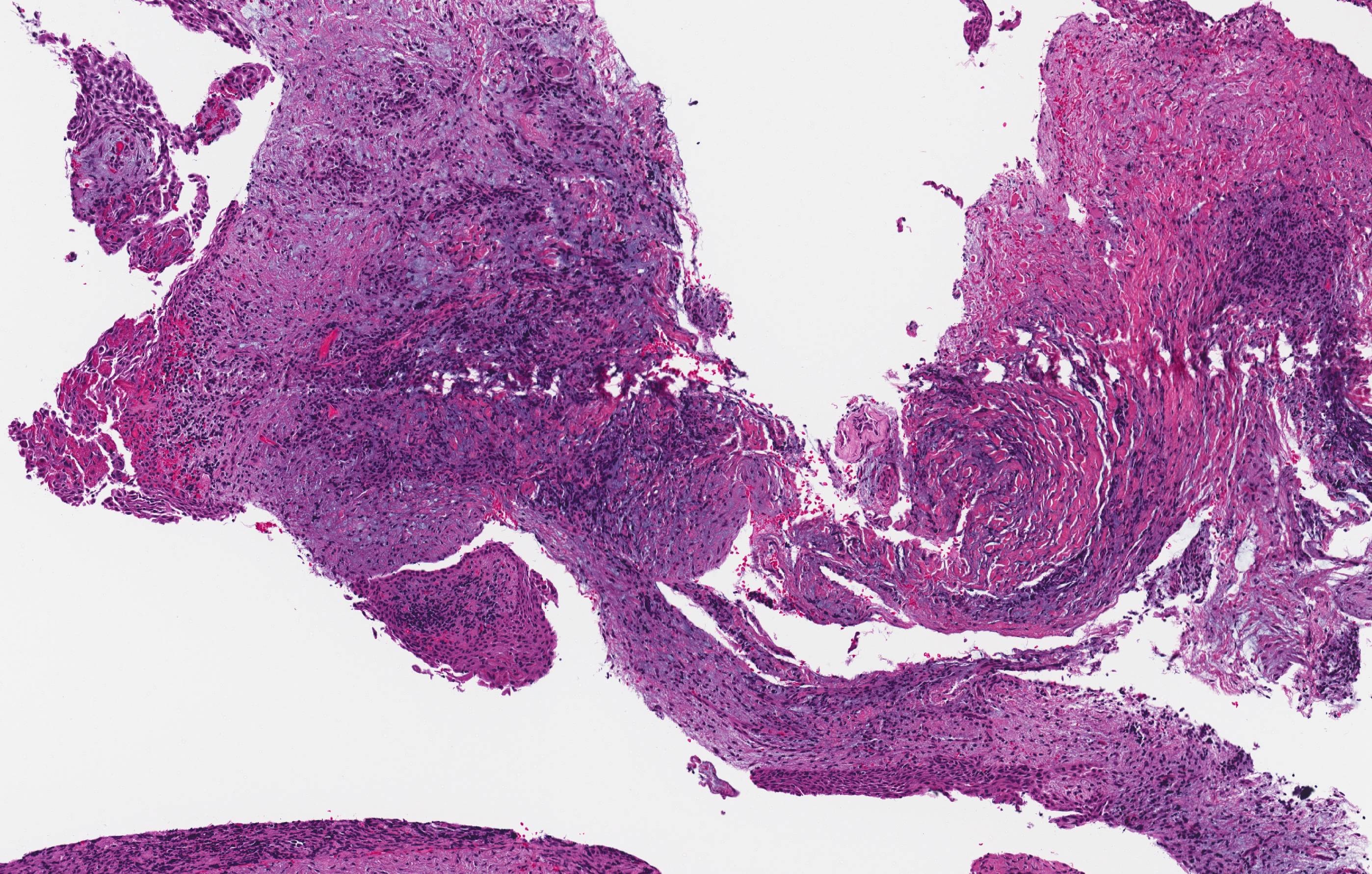
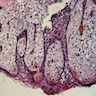
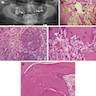
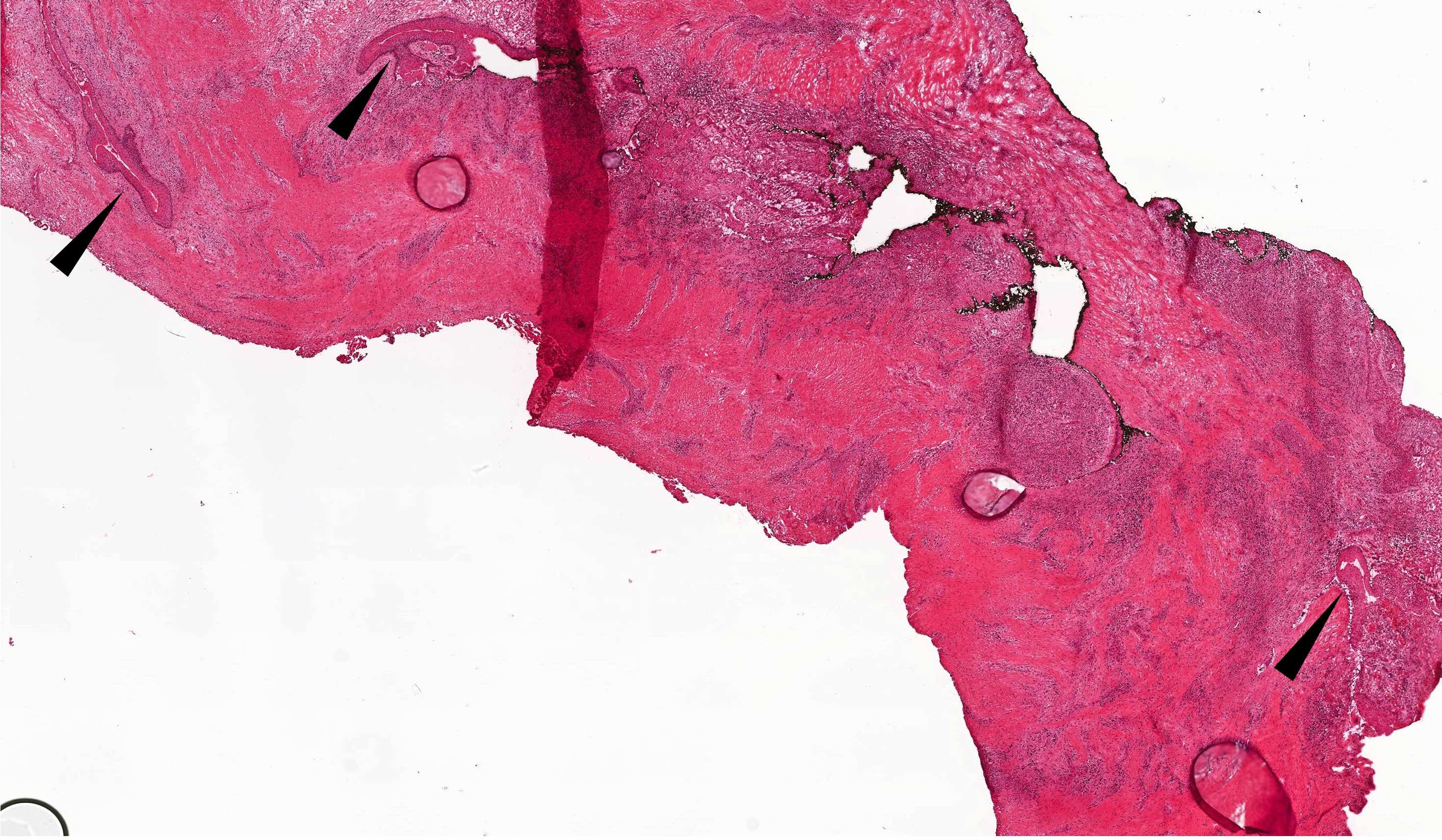
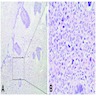
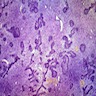
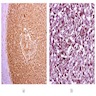
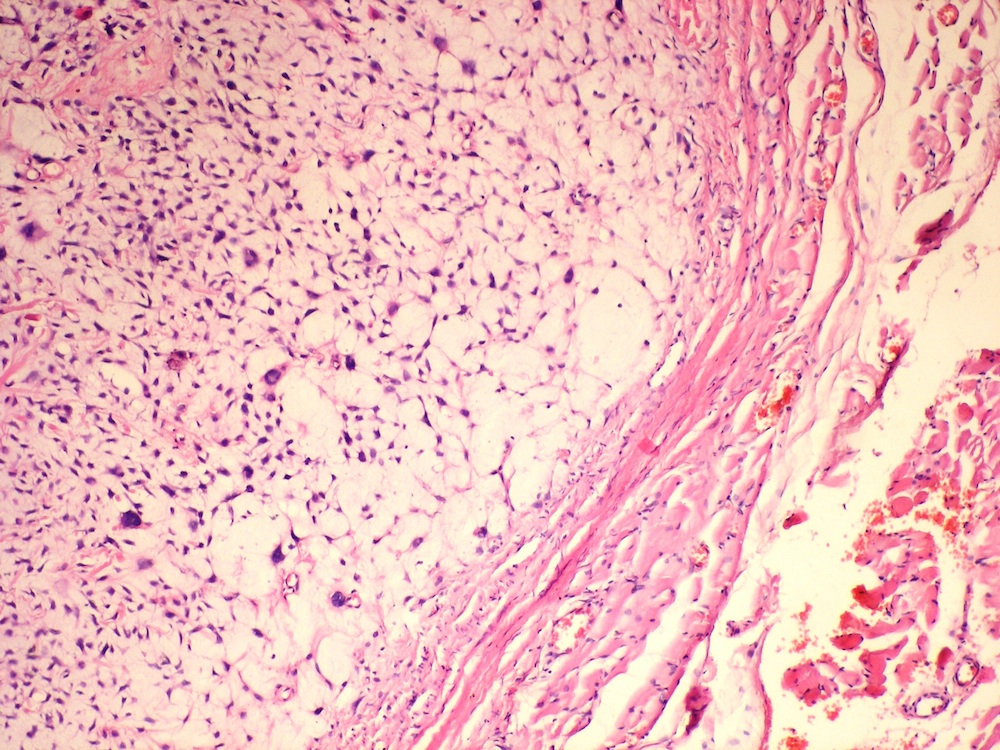
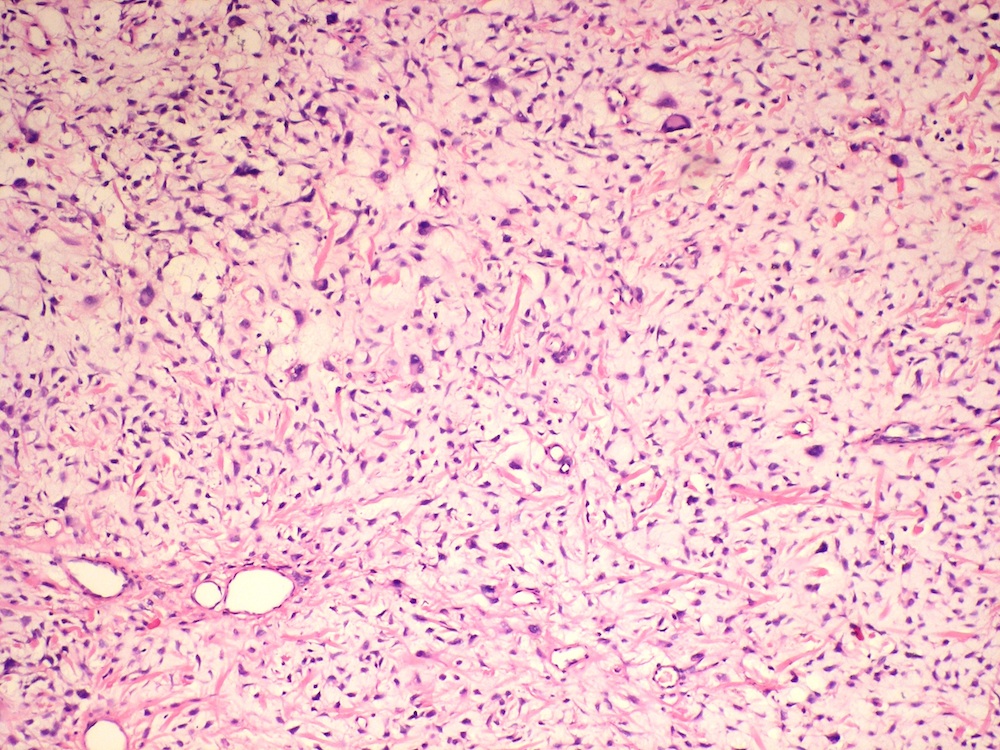
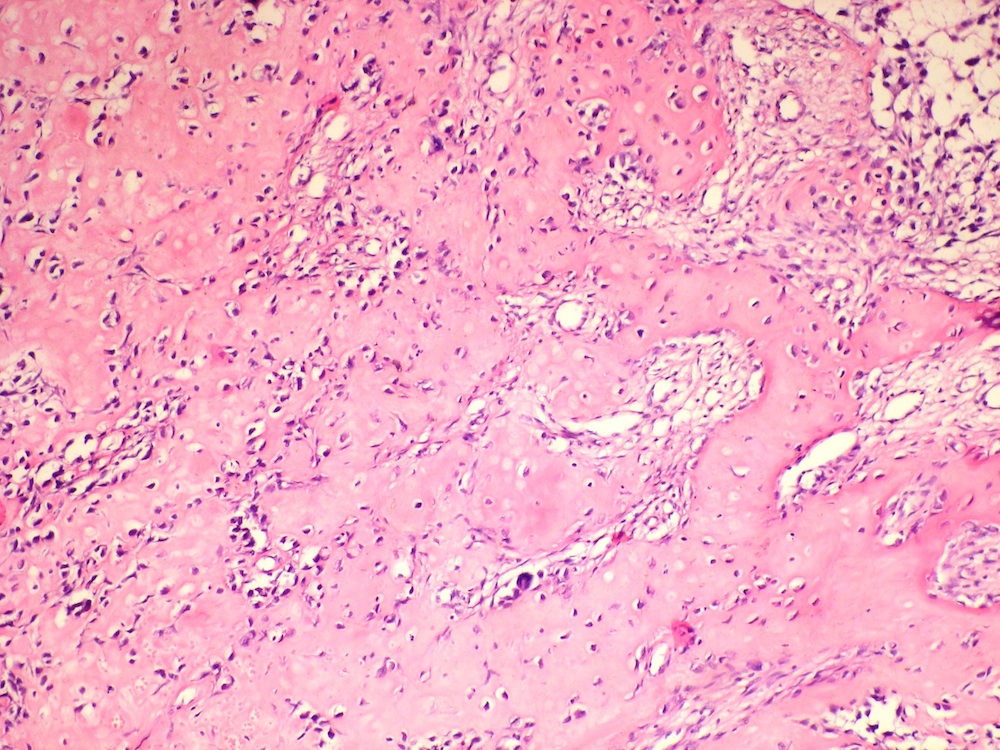
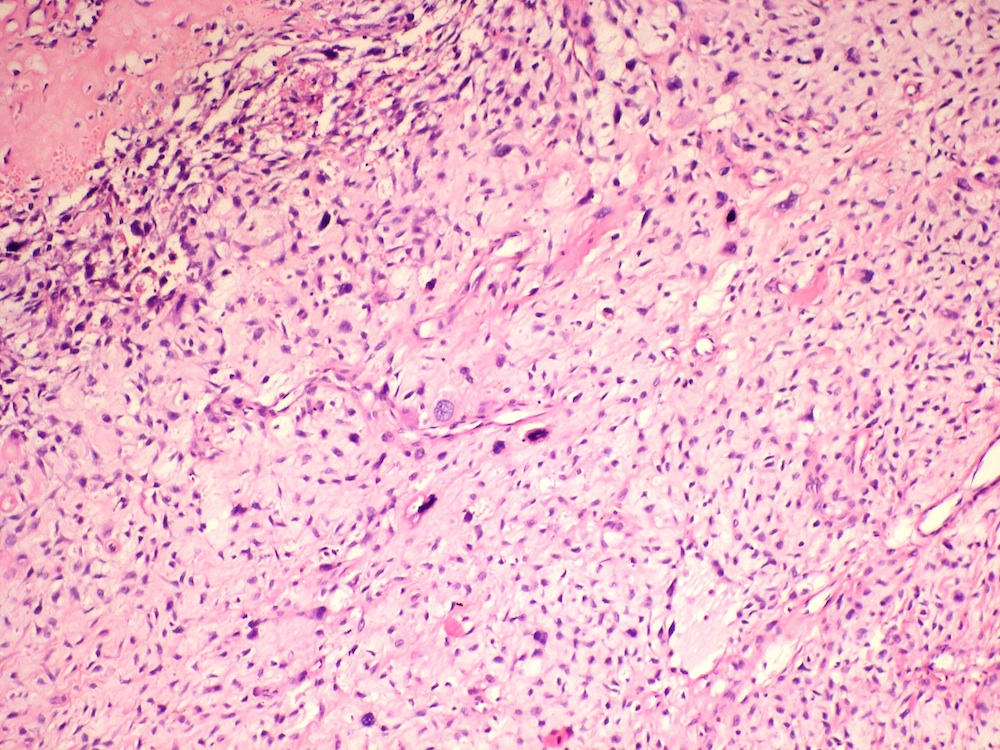
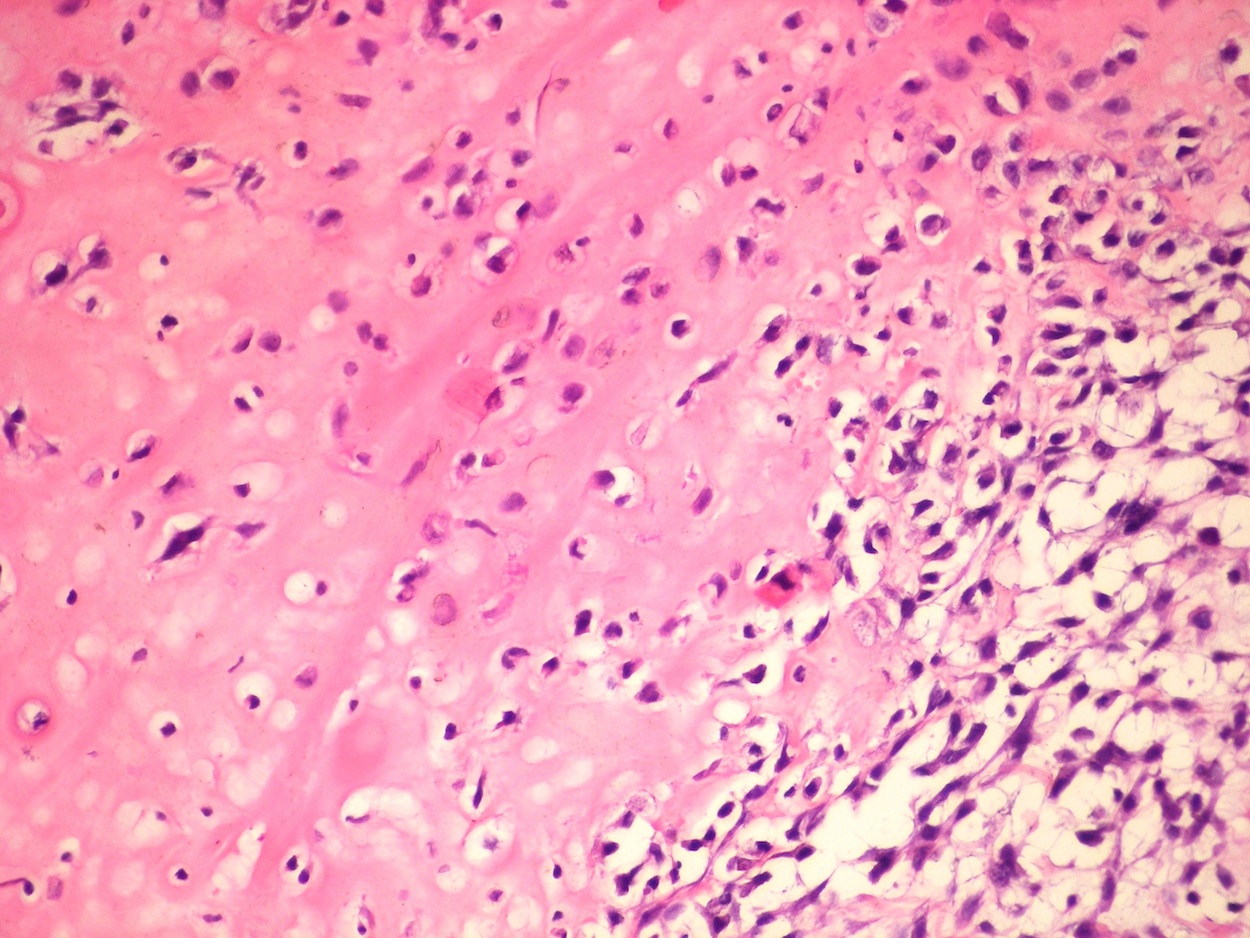
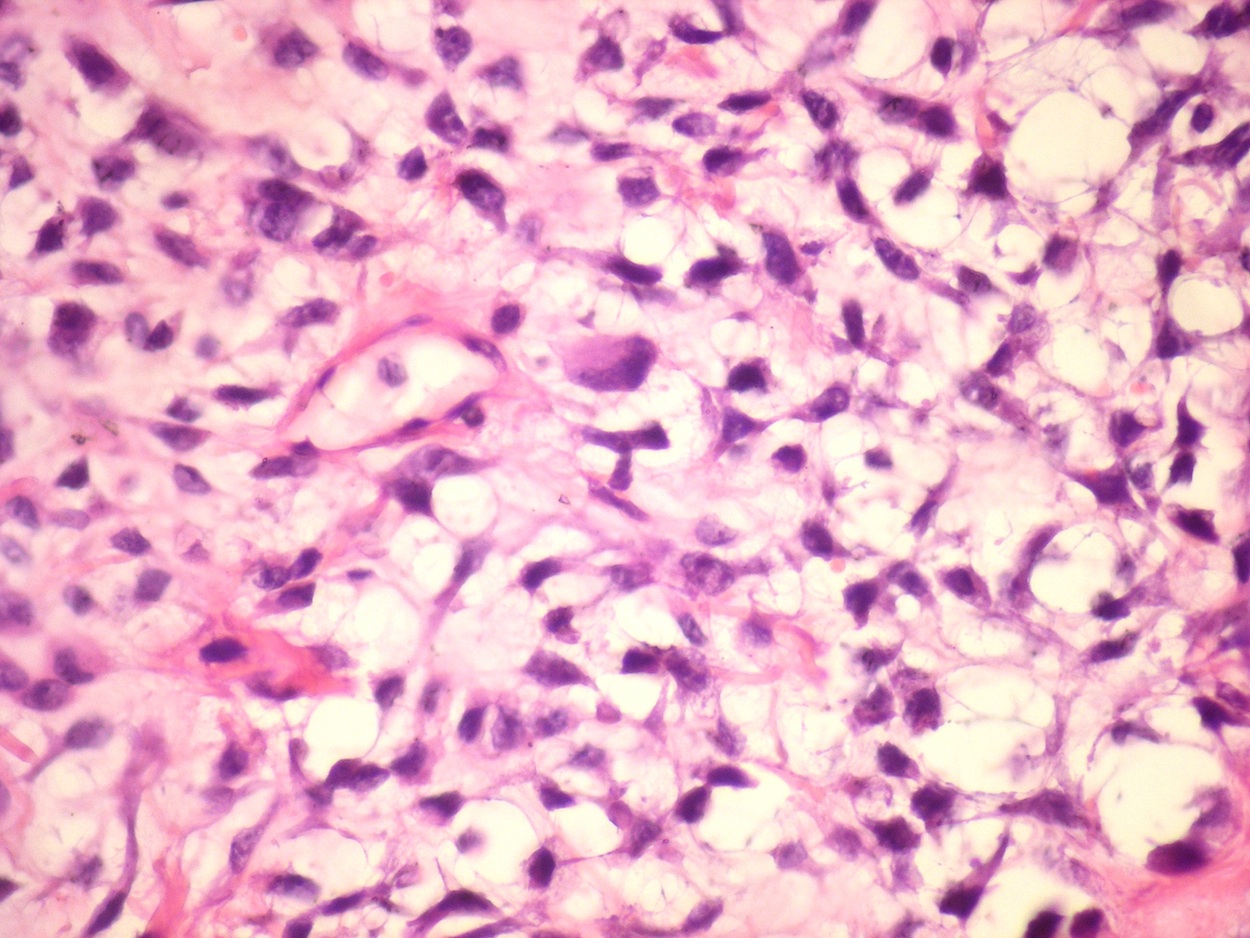
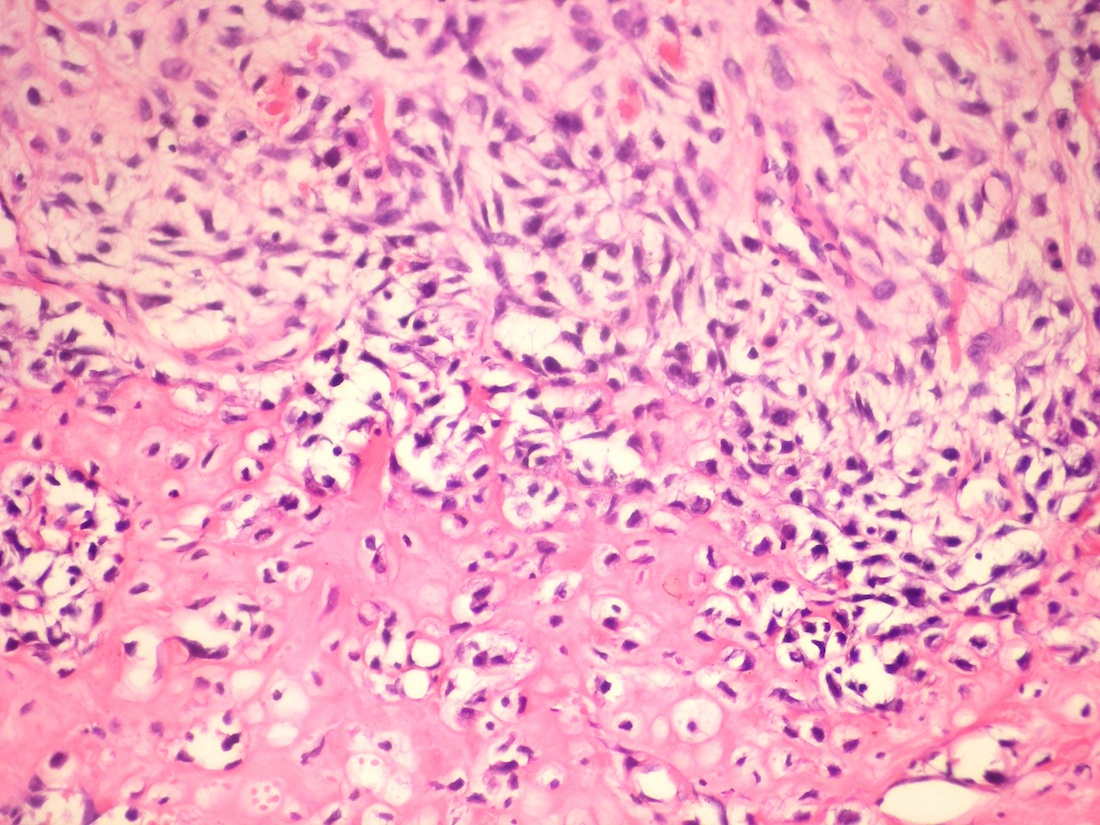
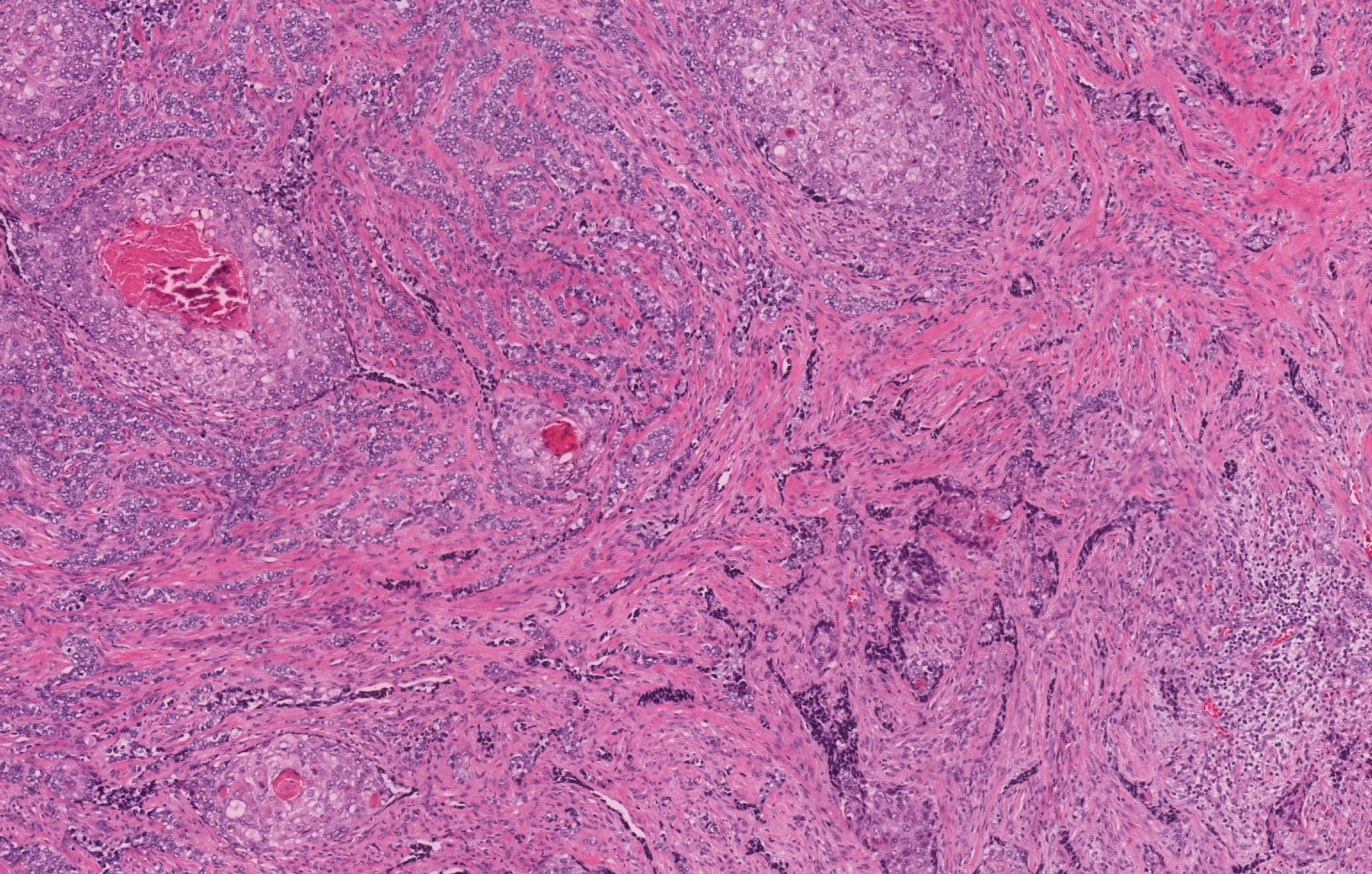
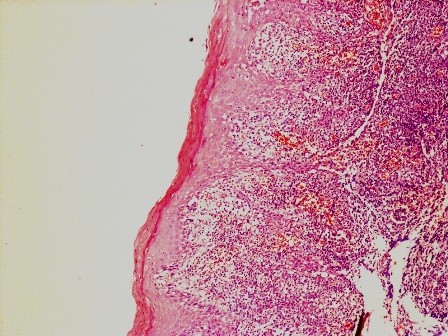
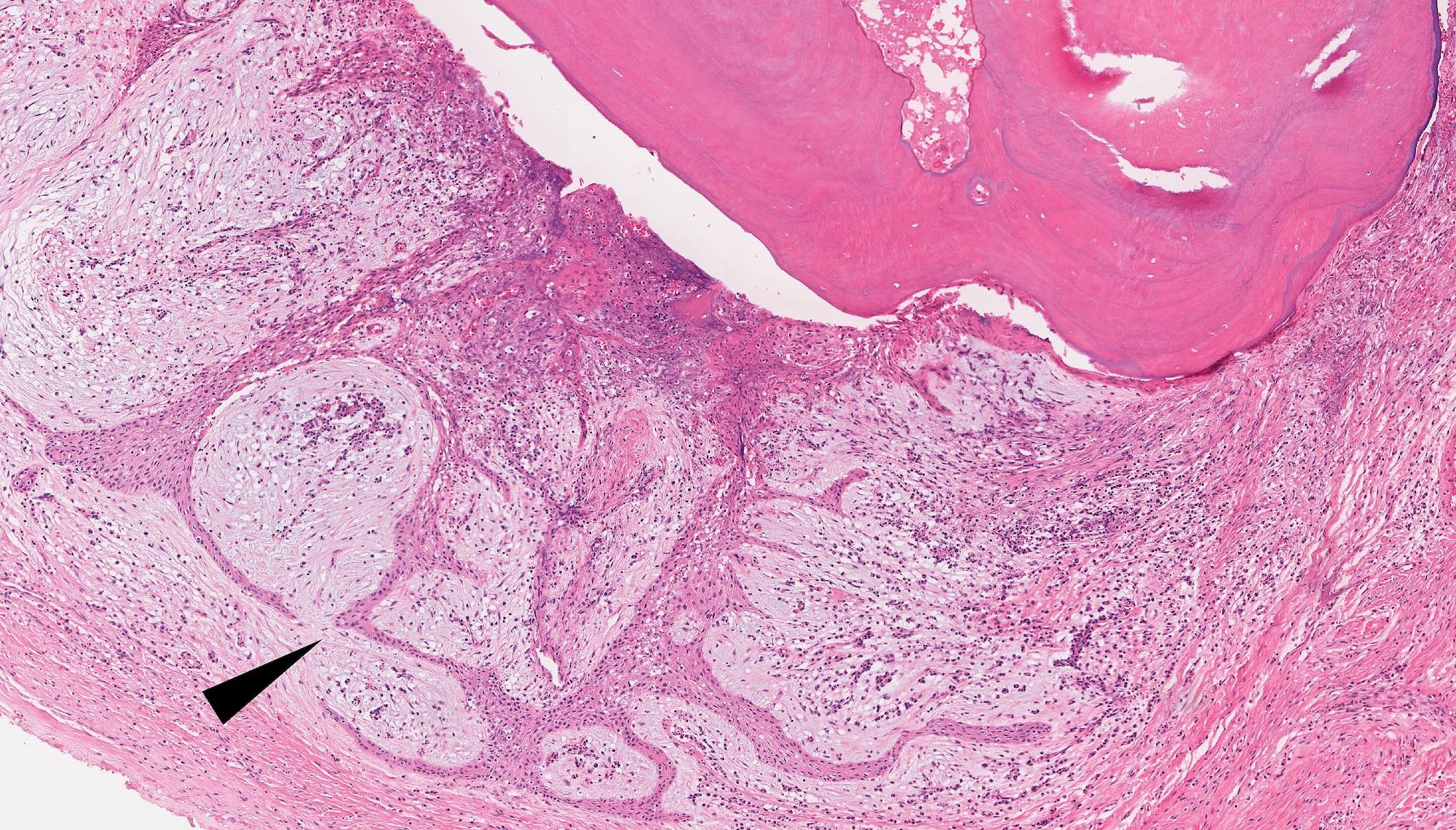
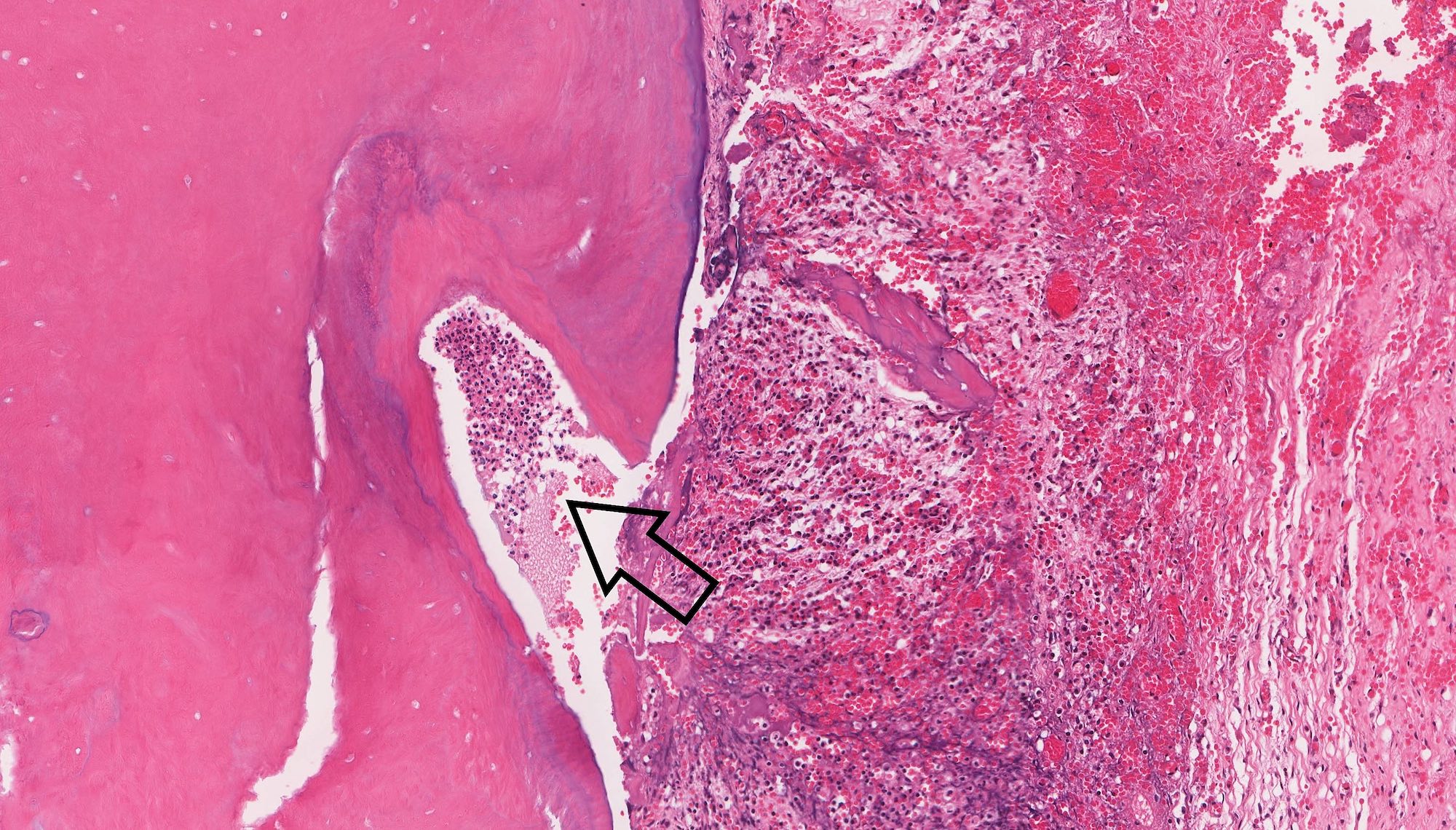
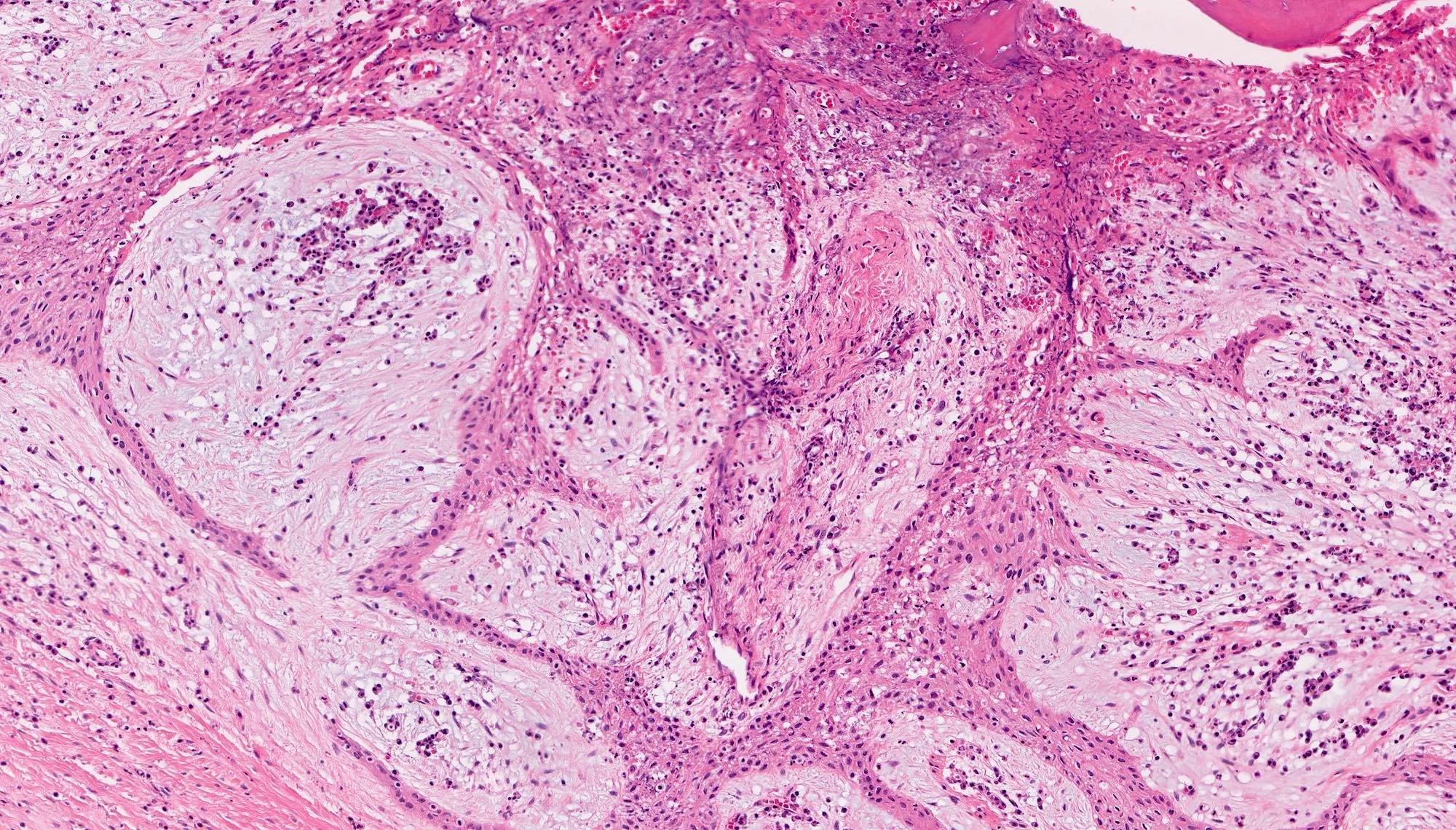
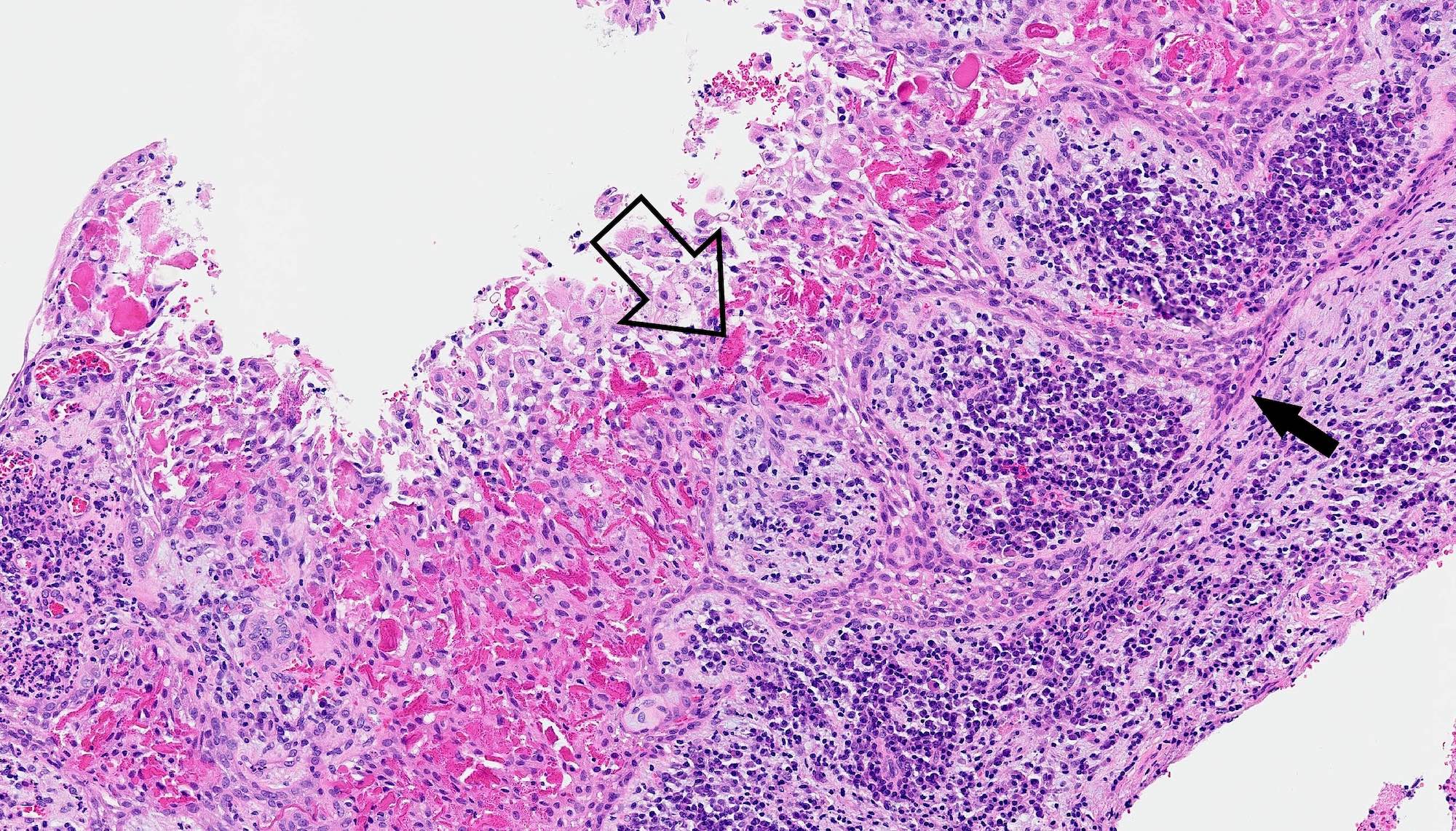
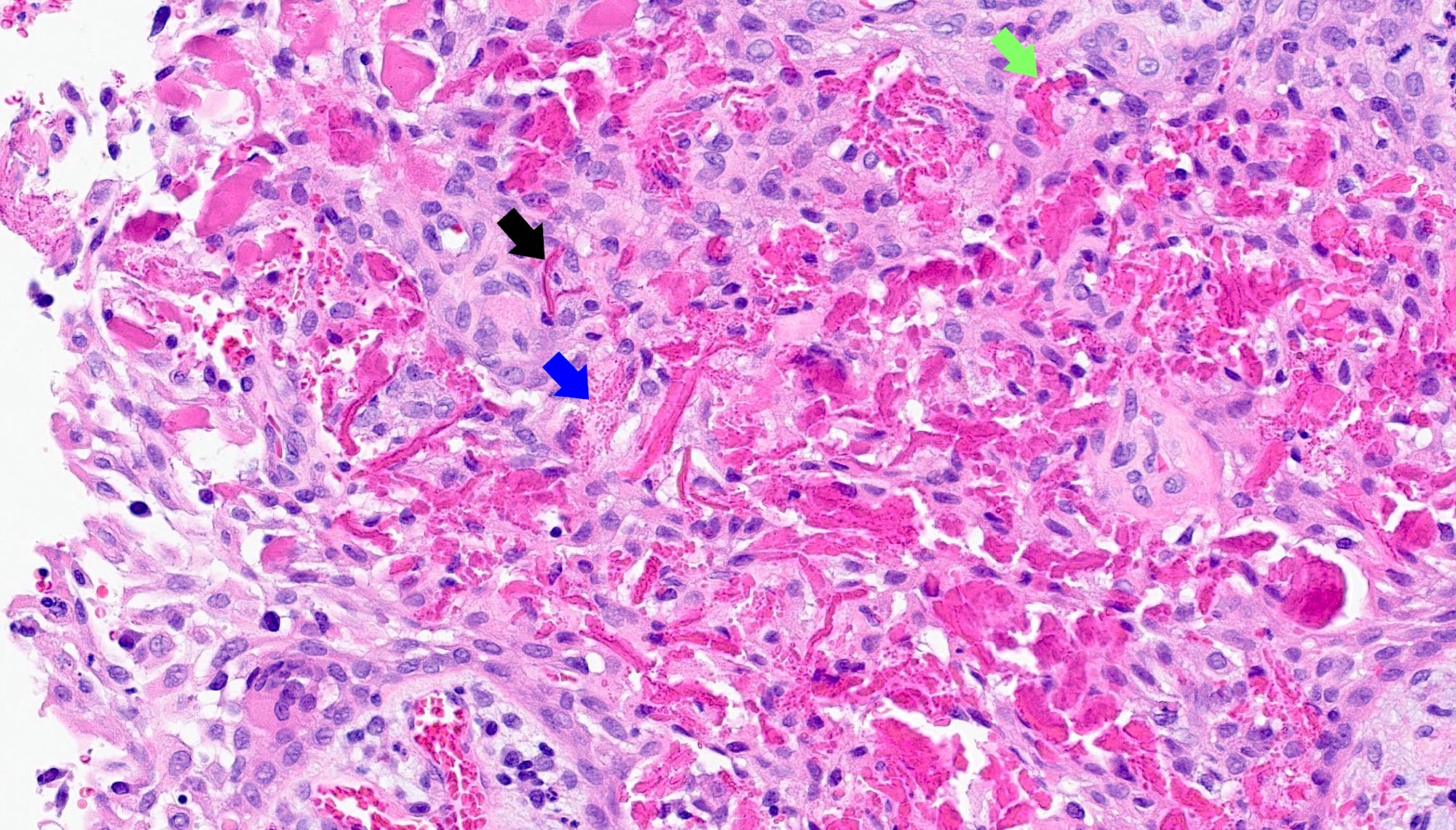
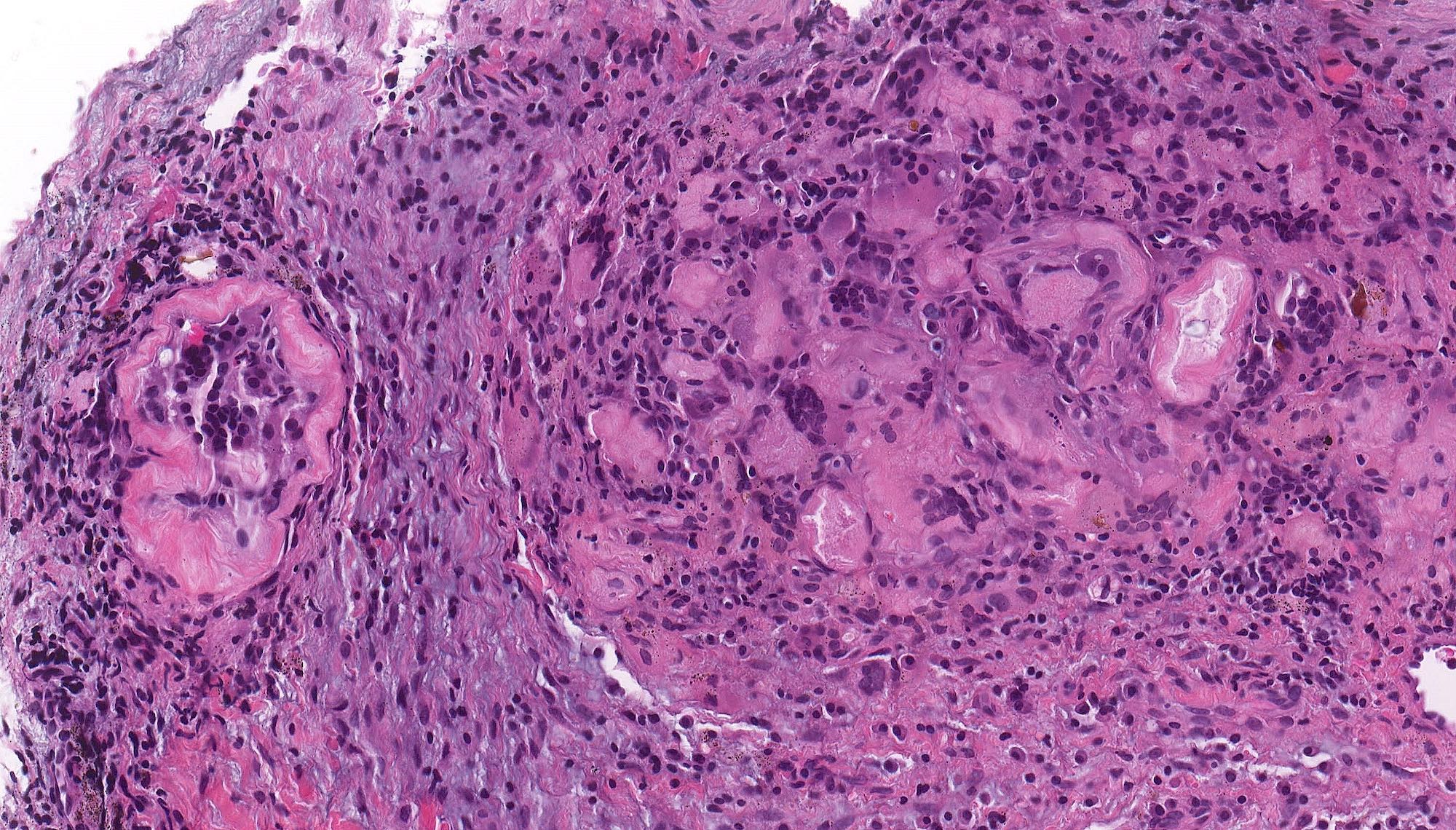
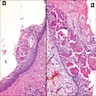
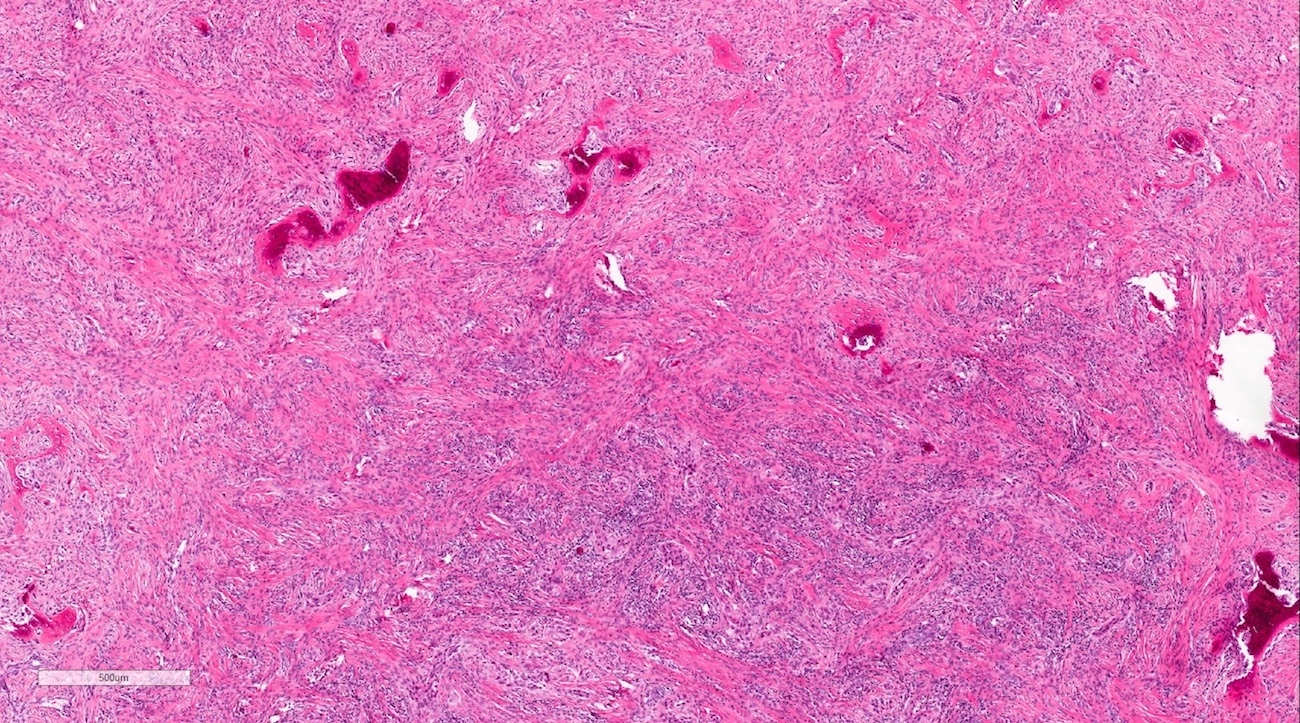
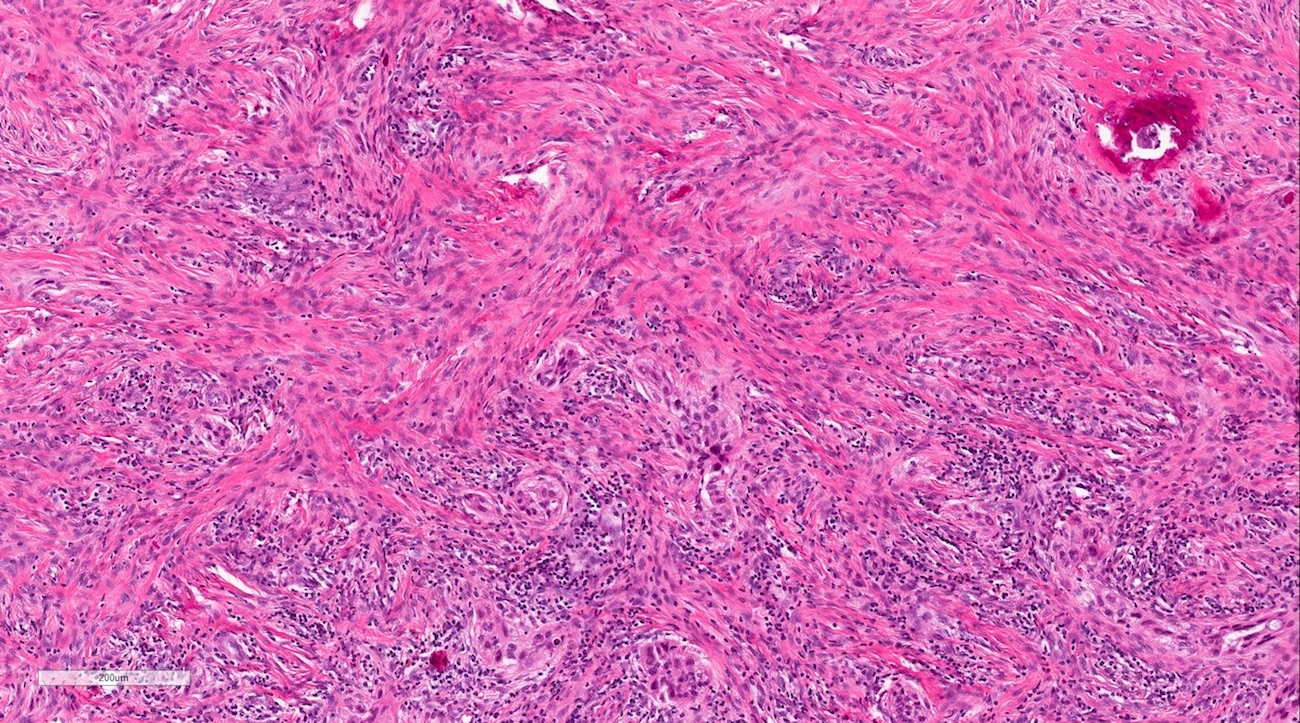
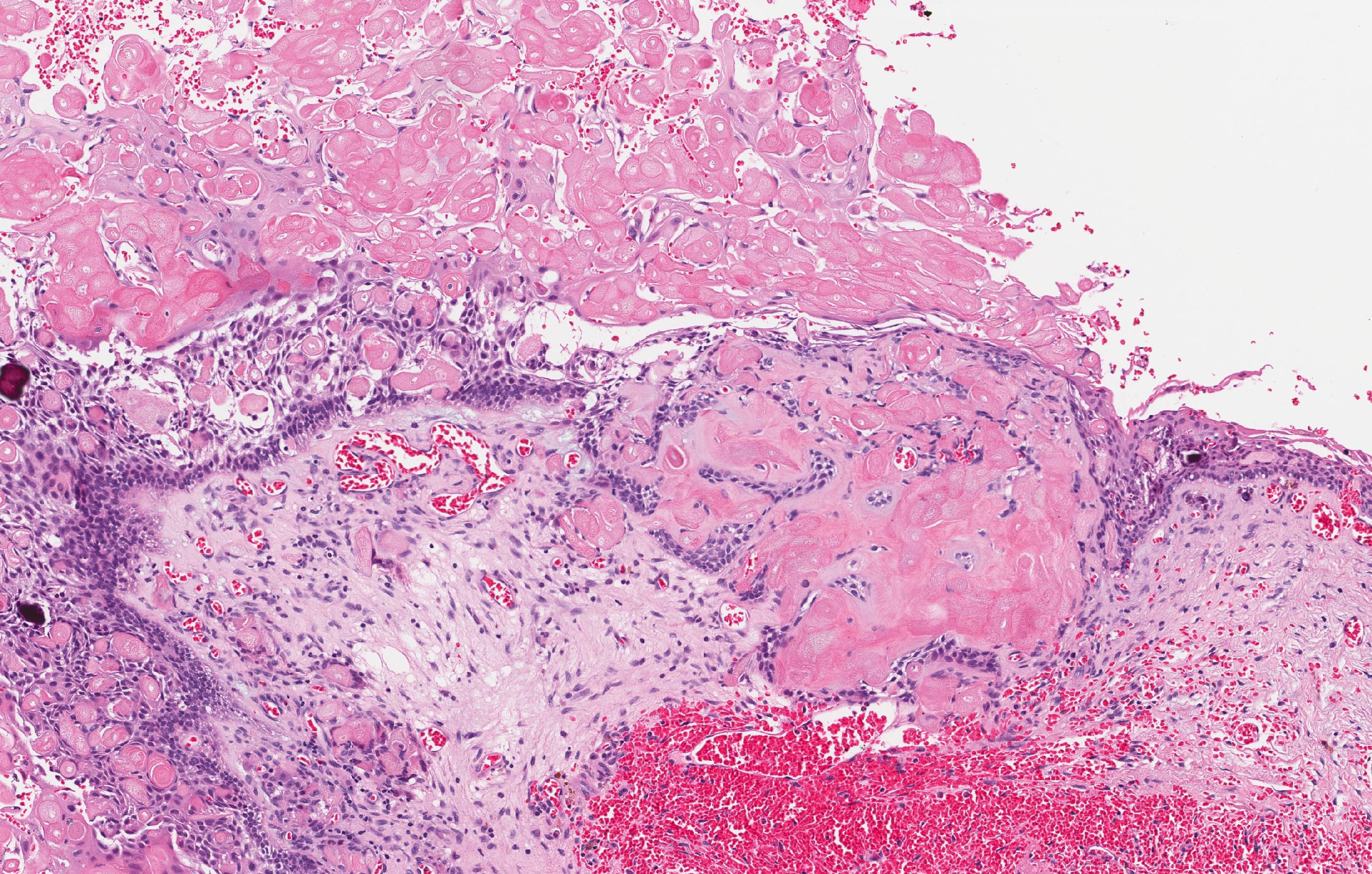
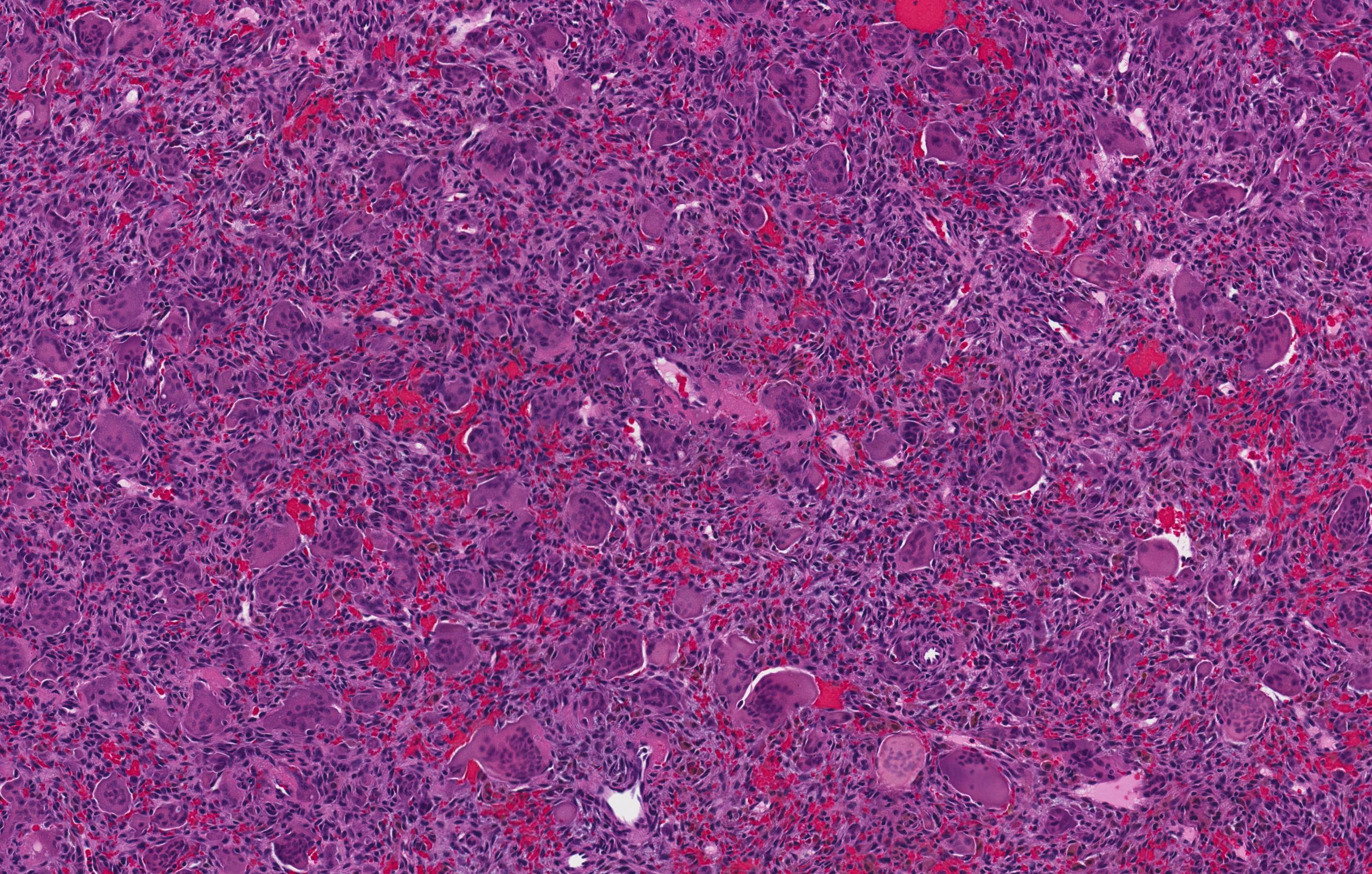
Microscopic (histologic) description
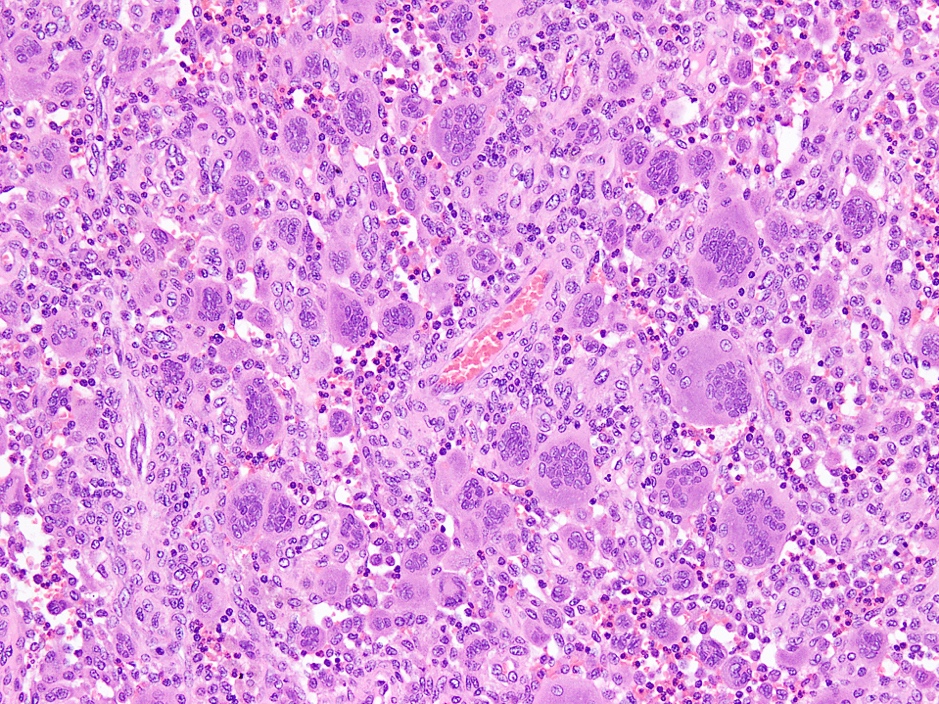
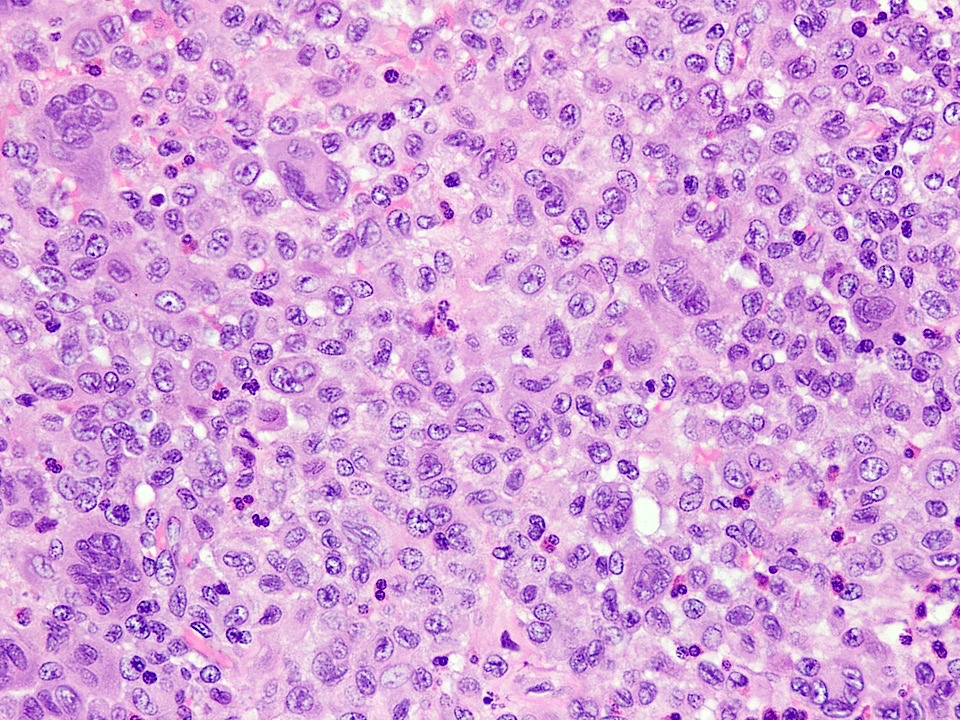
- According to the Zurich classification on osteomyelitis of the jaws, pathology is considered a secondary classification criterion - pathology confirms the diagnosis of osteomyelitis if clinical judgment and diagnostic imaging are not conclusive
- Histology of jaw osteomyelitis should always be complemented and interpreted in conjunction with clinical and radiological findings and should not be used independently
- Importantly, histology is an essential tool to exclude differential diagnoses
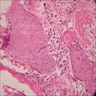
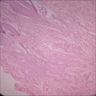
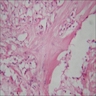
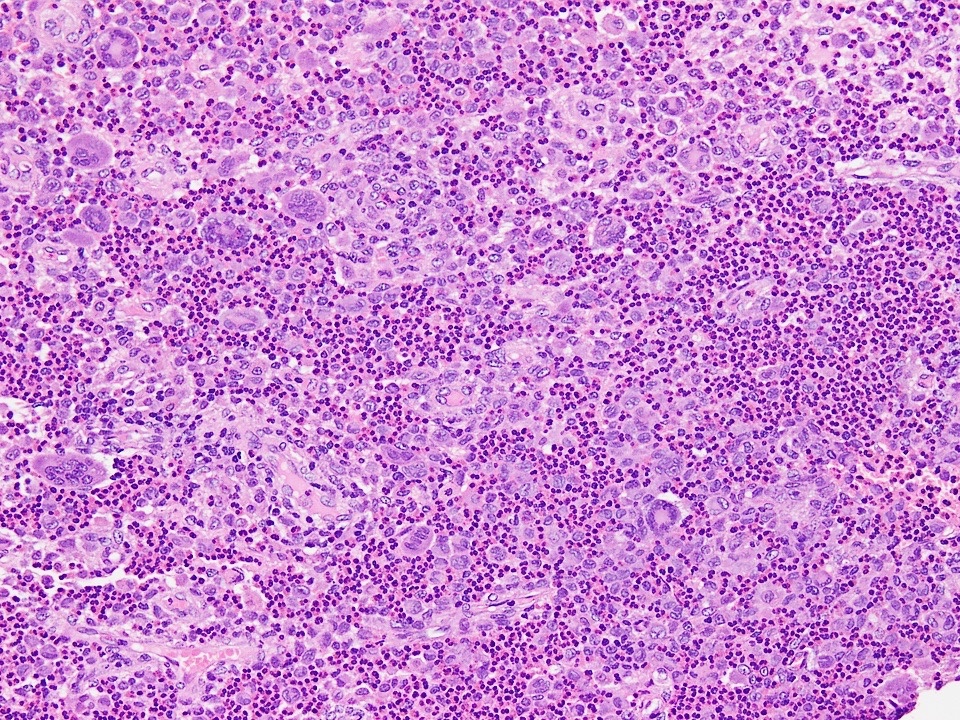
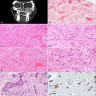
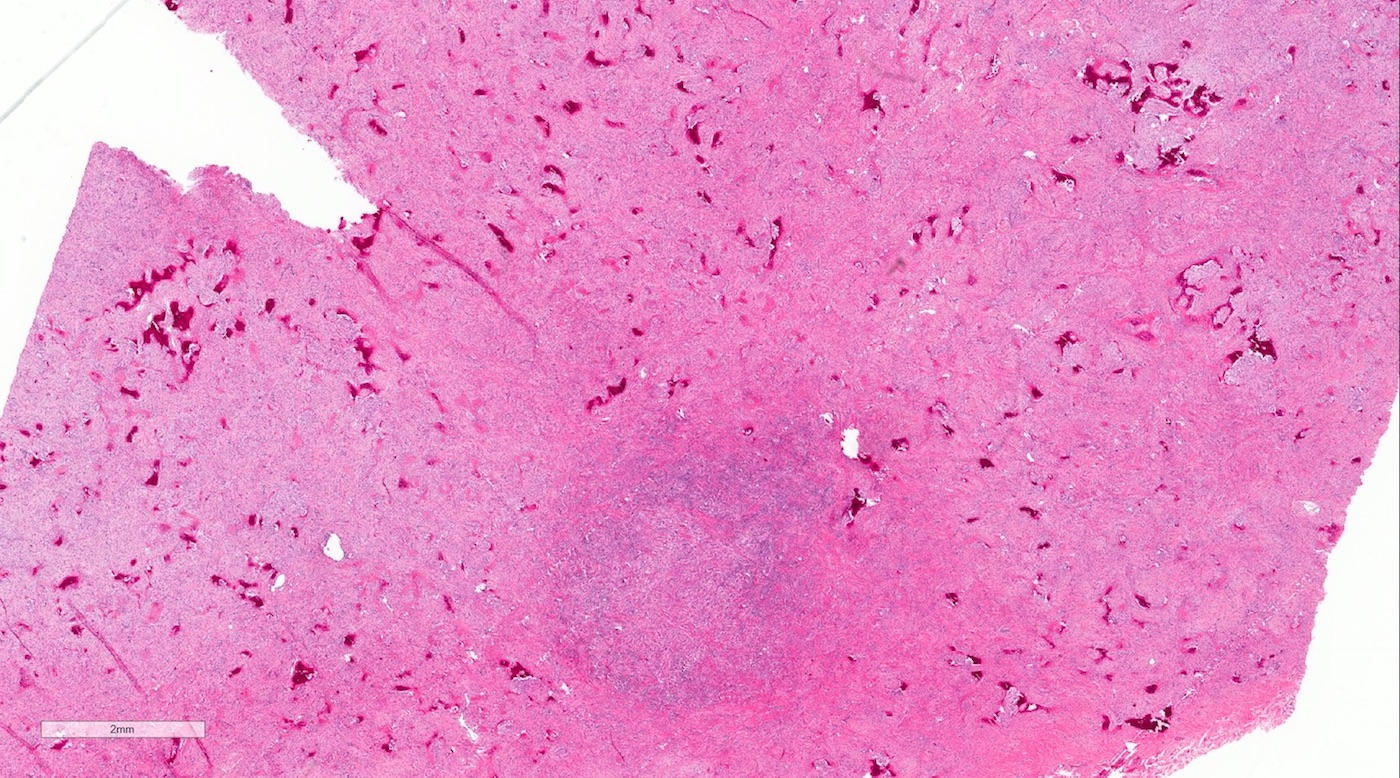
- Predominantly acute inflammation and fibrin are common but may have a variable degree of plasma cell infiltration with marrow fibrosis
- A distinction between acute and chronic solely based on histopathology is not always possible
- Utilizing the context with clinical presentation and imaging studies will provide a more specific diagnosis
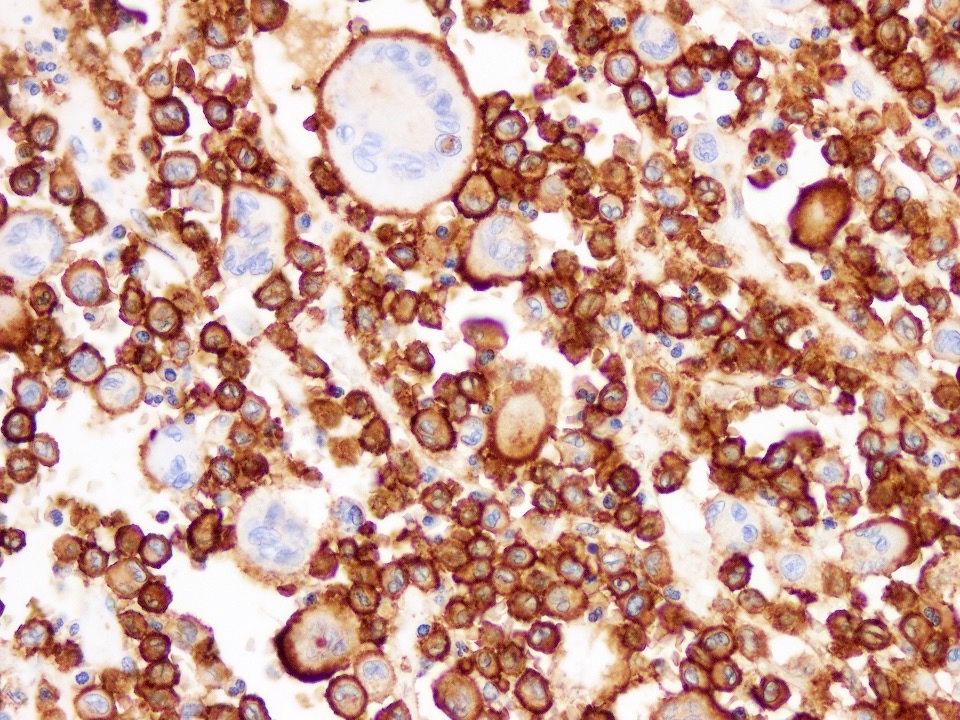
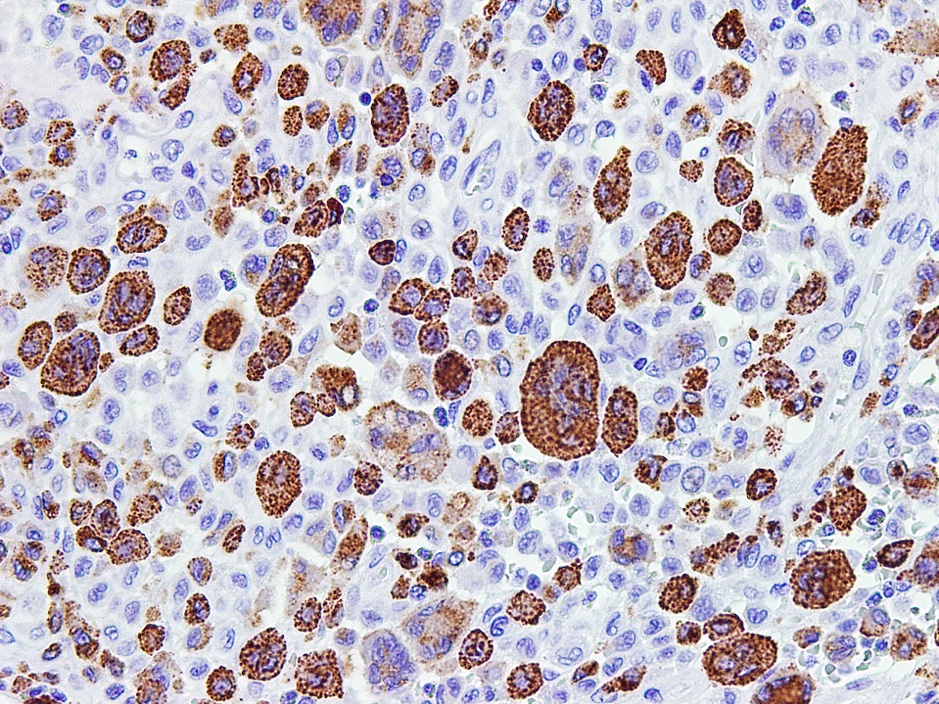
Differential diagnosis
- Acute exacerbation of bisphosphonate osteonecrosis
- Infected cemento-osseous dysplasia
- Langerhan cell histiocytosis
- Metastatic disease to jaws, with abscess
- Periapical granuloma with abscess
Additional references
- Wikipedia: Periosteum [Accessed 1 June 2018], Oral Surg Oral Med Oral Pathol 1970;29:641, Oral Surg Oral Med Oral Pathol 1970;30:396, Oral Maxillofac Surg Clin North Am 2011;23:401
- J Oral Maxillofac Surg 1993;51:1294, J Can Dent Assoc 1995;61:441, J Craniomaxillofac Surg 2004;32:43
- Topazian: Oral and Maxillofacial Infections, 4th Edition, 2002 (pg. 251 - 88), Baltensperger: Osteomyelitis of the Jaws, 2008 (pg. 55 - 56)
- Oral Maxillofac Clin North Am 1991;3:367, Oral Maxillofac Surg Clin North Am 1991;3:355
Adenoid ameloblastoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Benign, rare tumor of odontogenic origin
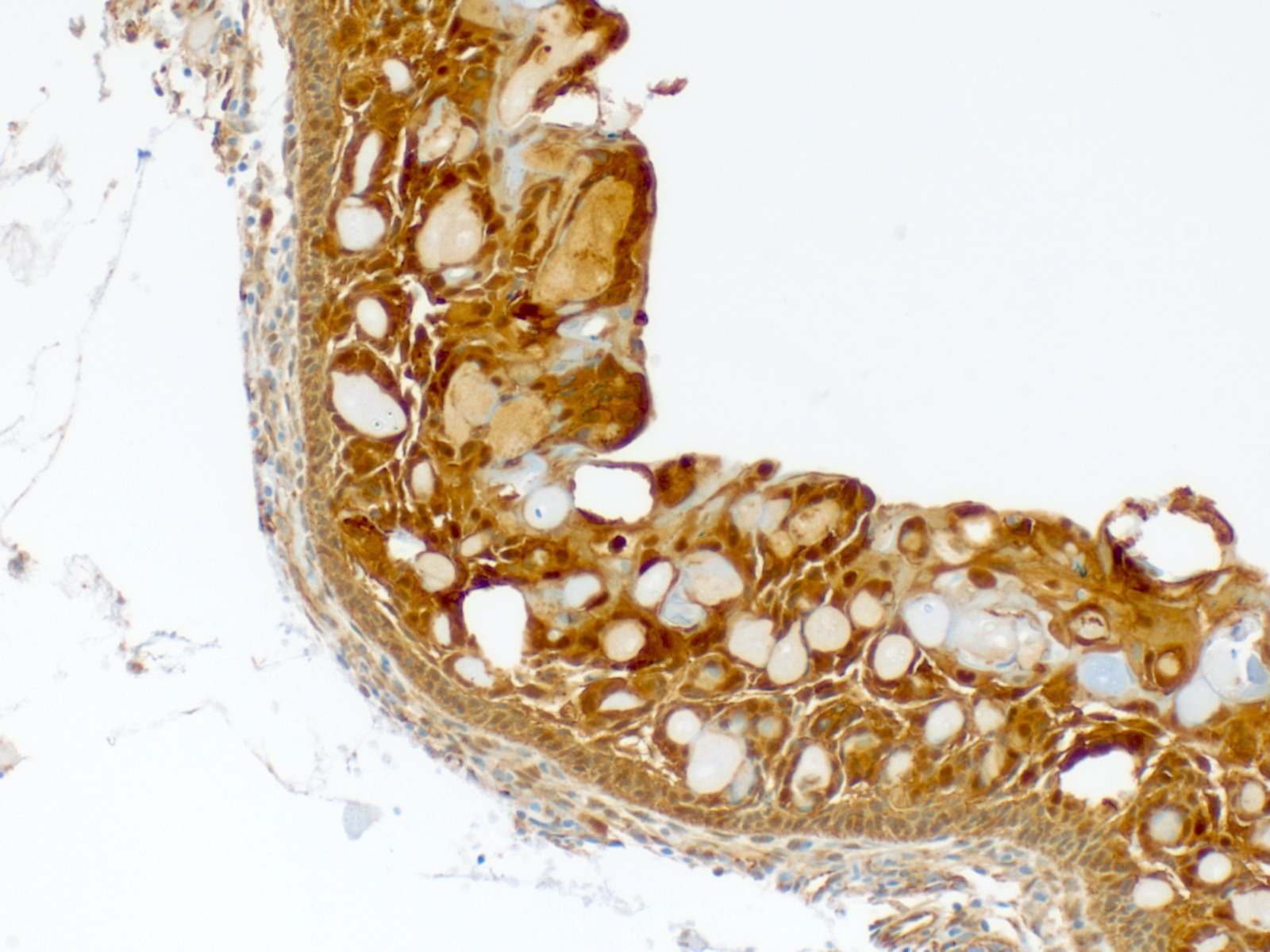
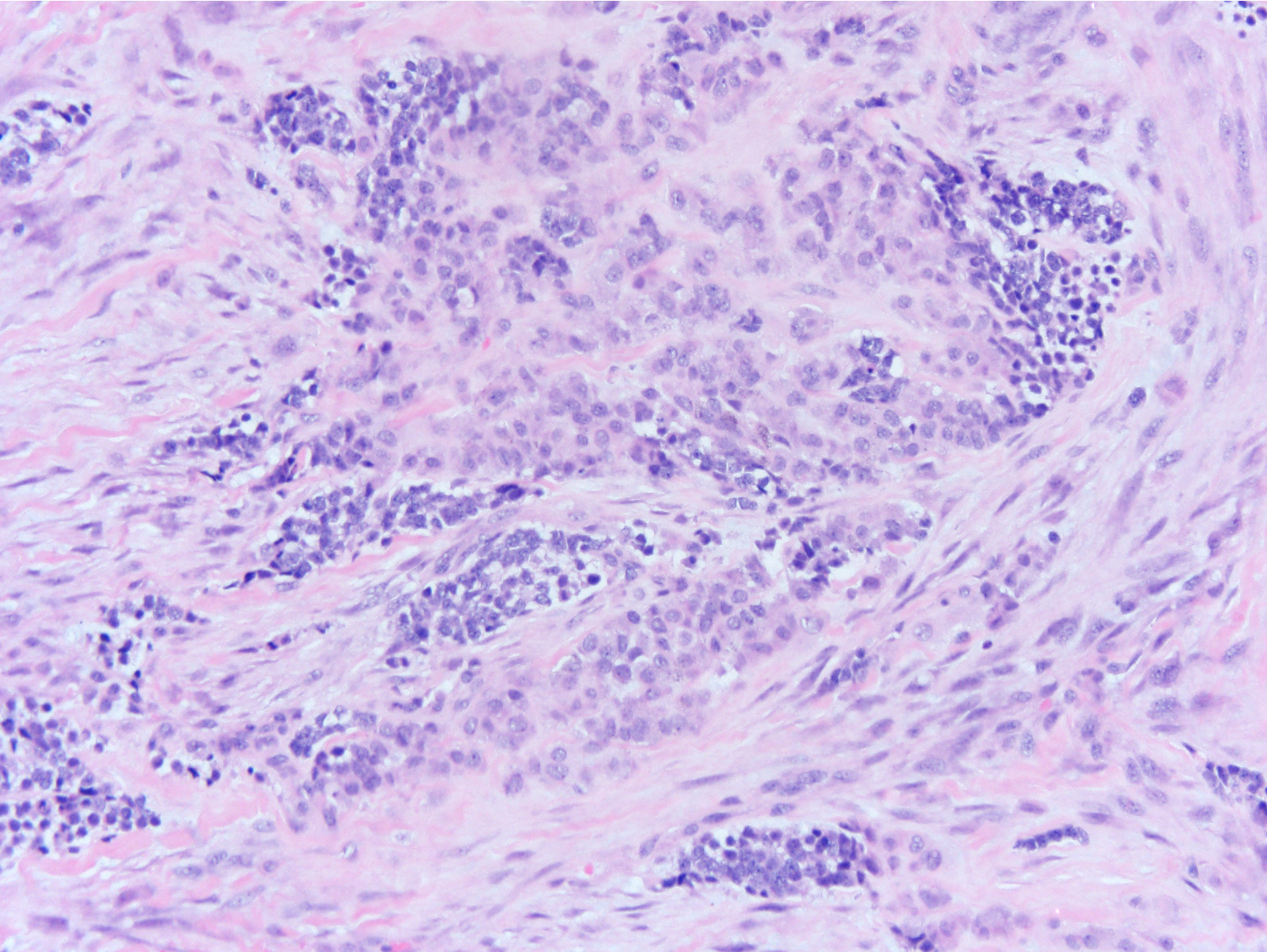
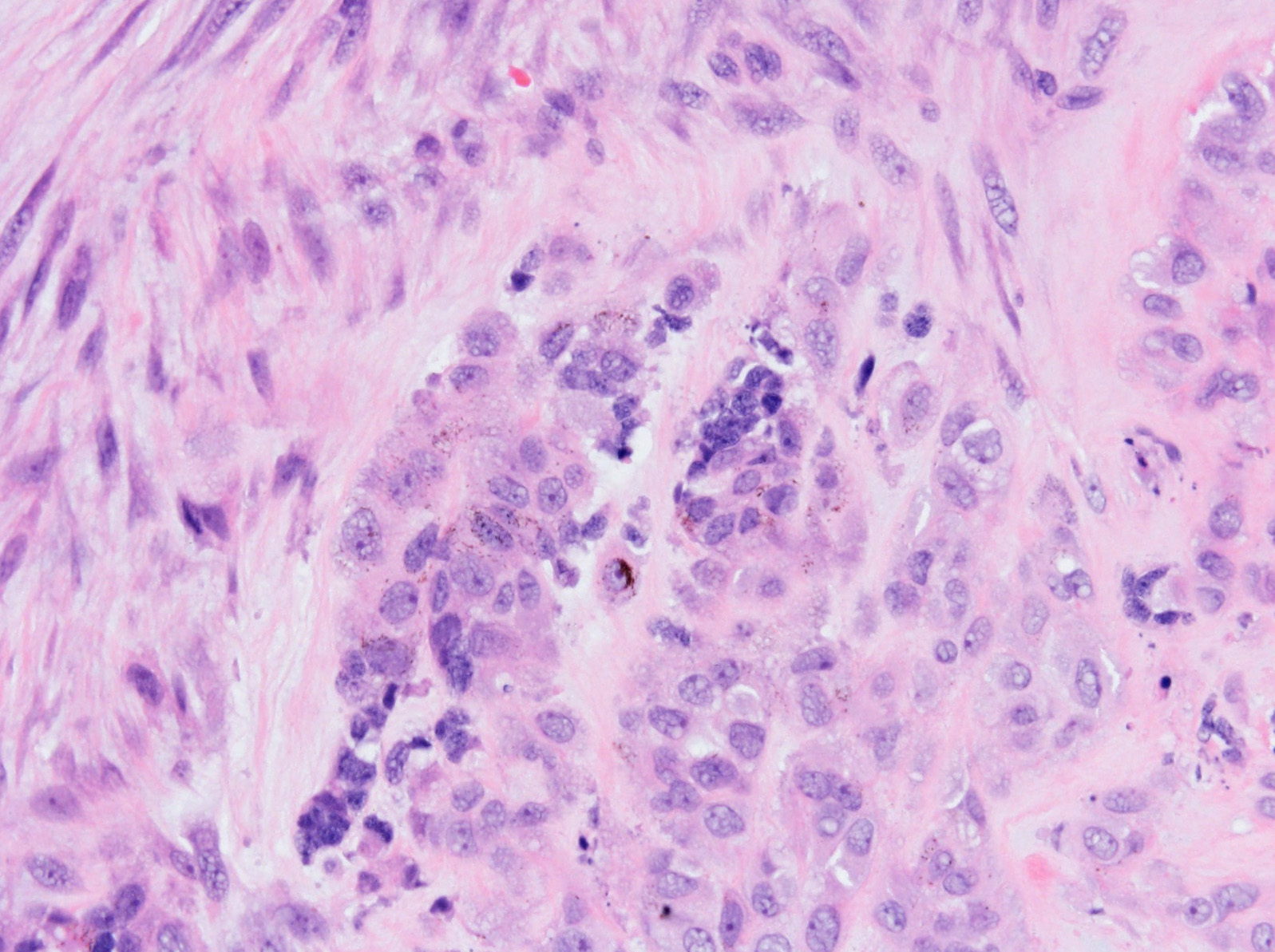
- Cuboidal to columnar ameloblastic epithelium that forms duct-like structures, epithelial whorls and cribriform architecture; dentinoid deposits, clusters of clear cells and ghost cell keratinization may also be present (J Oral Maxillofac Pathol 2012;16:272)
Essential features
- Adenoid ameloblastomas have a predilection for the posterior mandible (Head Neck Pathol 2022;16:344)
- Microscopically, adenoid ameloblastomas consist of epithelium resembling conventional ameloblastoma in addition to duct-like structures, epithelial whorls and cribriform architecture
- Dentinoid deposits, clusters of clear cells and ghost cell keratinization may also be observed (J Oral Maxillofac Pathol 2012;16:272)
- Adenoid ameloblastoma can be a challenging and controversial diagnosis as this tumor has features seen in numerous odontogenic tumors (i.e., ameloblastoma, adenomatoid odontogenic tumor, calcifying odontogenic cyst / dentinogenic ghost cell tumor and odontogenic carcinoma with dentinoid)
Terminology
- Adenoid ameloblastoma with dentinoid was proposed by Brannon of the Armed Forces Institute of Pathology in 1994; this terminology has been deemed acceptable by the World Health Organization (WHO) (Brannon: Adenoid Ameloblastoma With Dentinoid, 1994)
- Described as dentinoameloblastoma by Slabbert et al. in 1992 (J Oral Pathol Med 1992;21:46)
ICD coding
- ICD-O: 9300/0 - adenoid ameloblastoma
- ICD-10
- ICD-11
- 2E83.0 & XH1SV4 - benign osteogenic tumors of bone or articular cartilage of skull or face & ameloblastoma, NOS
- 2E83.1 & XH1SV4 - benign osteogenic tumors of bone or articular cartilage of lower jaw & ameloblastoma, NOS
Epidemiology
- Occurrence peaks in the fourth decade, with a slight male predilection (M:F = 1.3:1) and a wide age range of 15 - 82 years (Head Neck Pathol 2022;16:344)
Sites
- ~64.7% of adenoid ameloblastomas occur in the mandible (Head Neck Pathol 2022;16:344)
- Posterior gnathic bones are most commonly affected (Head Neck Pathol 2023;17:688)
Etiology
- Unknown
Clinical features
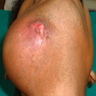
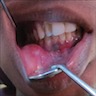
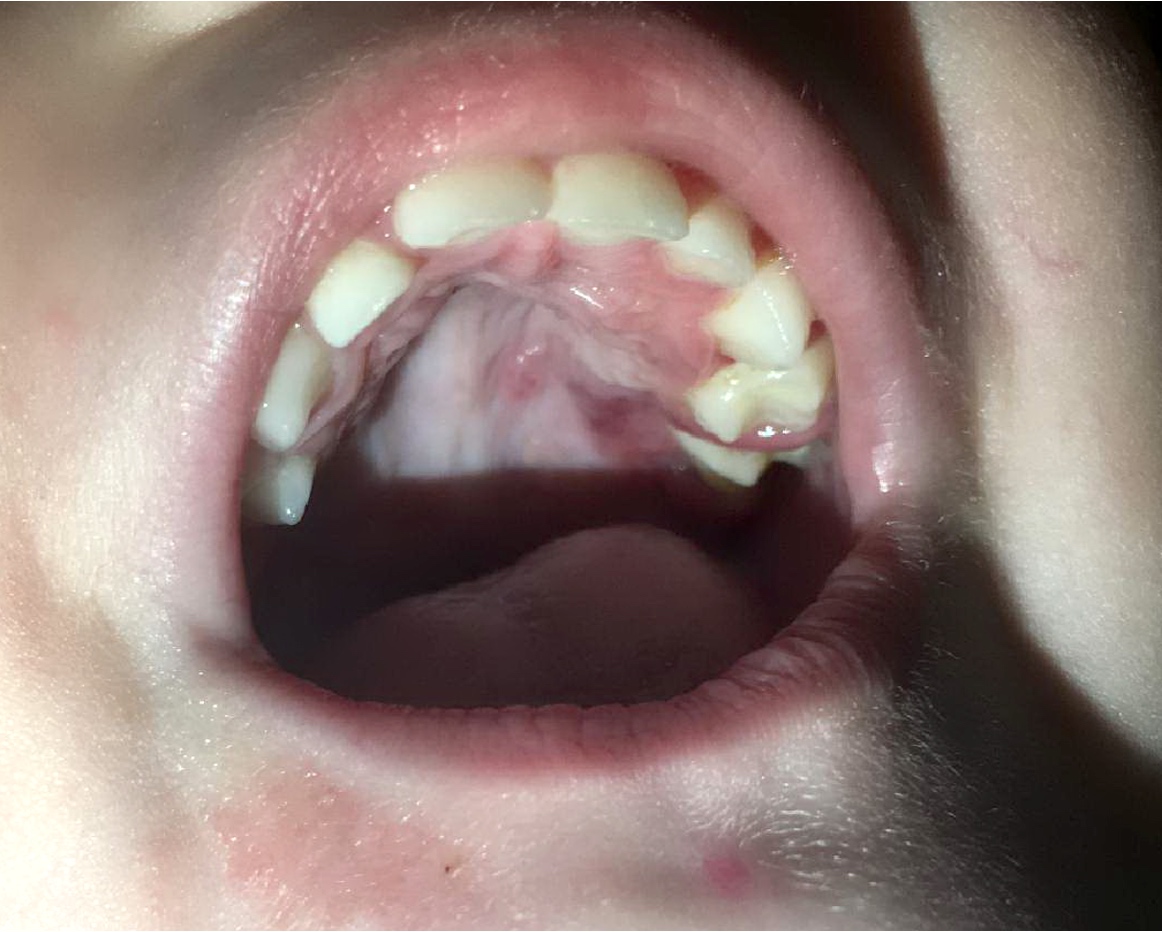
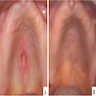
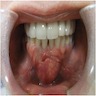
- Usually an asymptomatic swelling (unless secondarily infected)
- Tooth displacement may be present
Diagnosis
- Adenoid ameloblastoma may be detected radiographically as an incidental finding or present as a clinical swelling
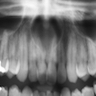
Radiology description
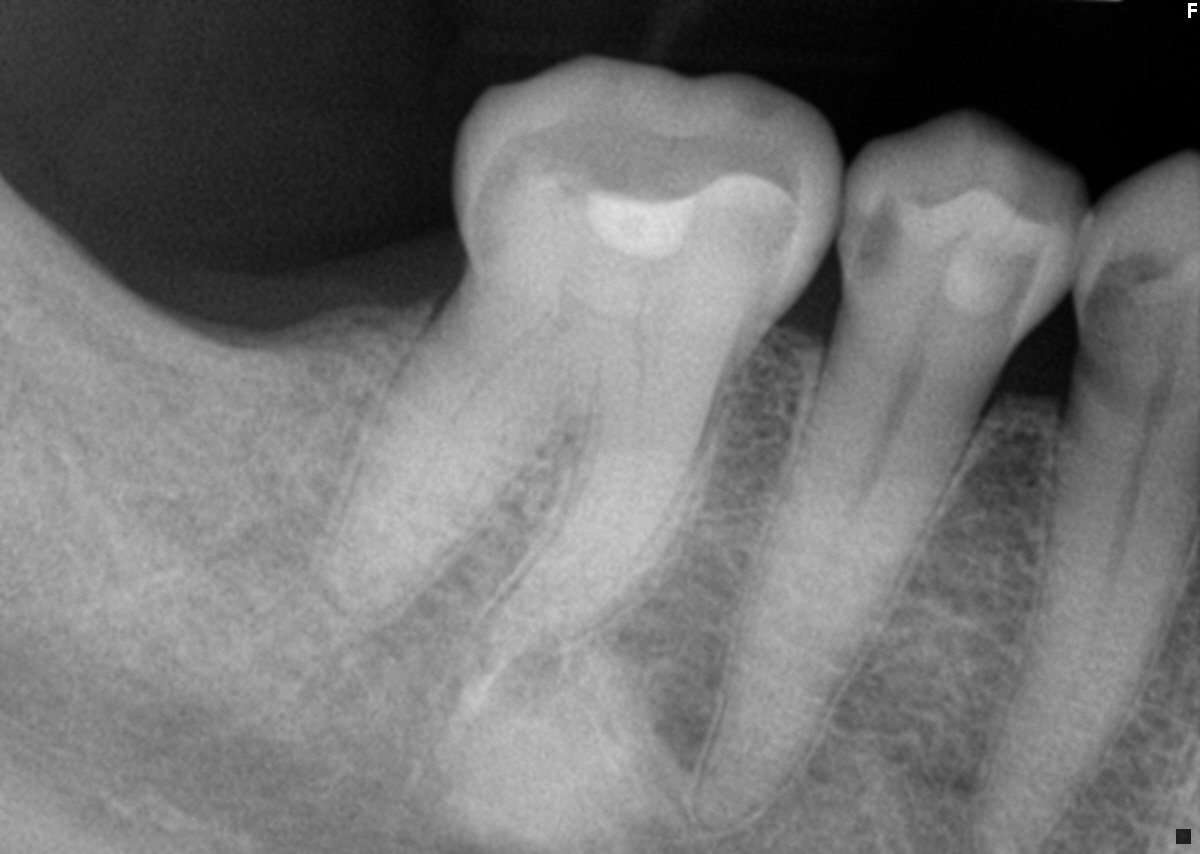
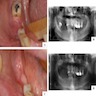
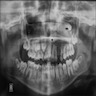
- 82.4% of adenoid ameloblastomas present exclusively as radiolucent lesions and may be ill defined (Head Neck Pathol 2022;16:344)
- May also appear as mixed density due to the presence of dentinoid
- May be unilocular or multilocular
Prognostic factors
- 45.4% of adenoid ameloblastomas developed at least 1 recurrence following surgical excision (Head Neck Pathol 2022;16:344)
Case reports
- 39 year old man with a chief concern of painful, mobile teeth in the right posterior maxilla (Oral Surg Oral Med Oral Pathol Oral Radiol 2019;128:E53)
- 54 year old woman with asymptomatic radiolucency of the left posterior maxilla (Oral Maxillofac Surg 2020;24:243)
- 57 year old woman with a well defined, unilocular radiolucency between the roots of the right mandibular premolars without intraoral changes (Oral Surg Oral Med Oral Pathol Oral Radiol 2022;134:E162)
Treatment
- Conventional treatment consists of surgical resection as primary therapy (Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:368)
- Patients may also receive radiotherapy as adjuvant to prevent recurrence (Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:368)
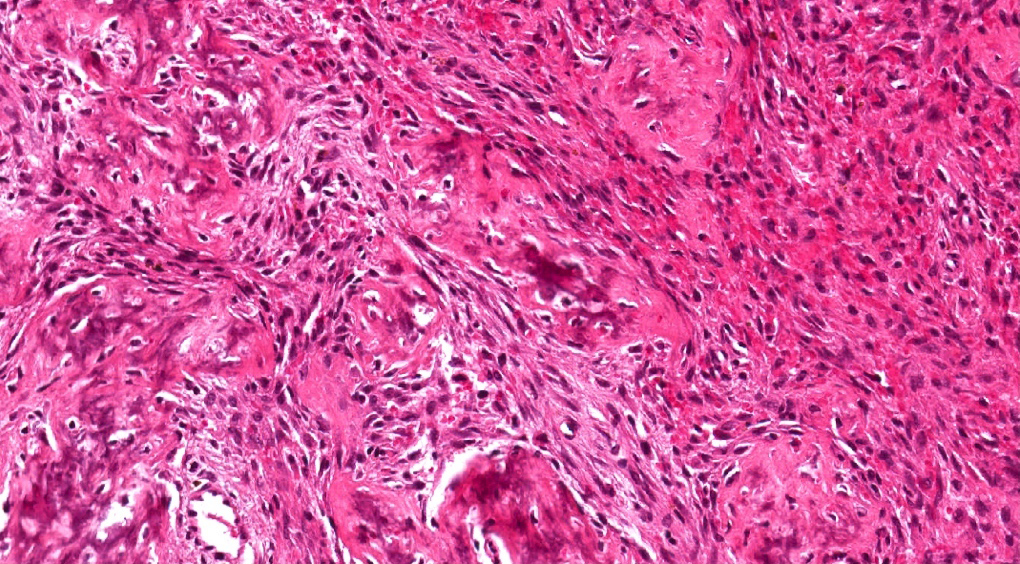
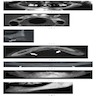
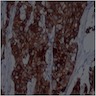
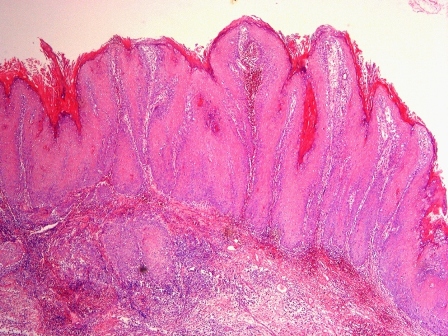
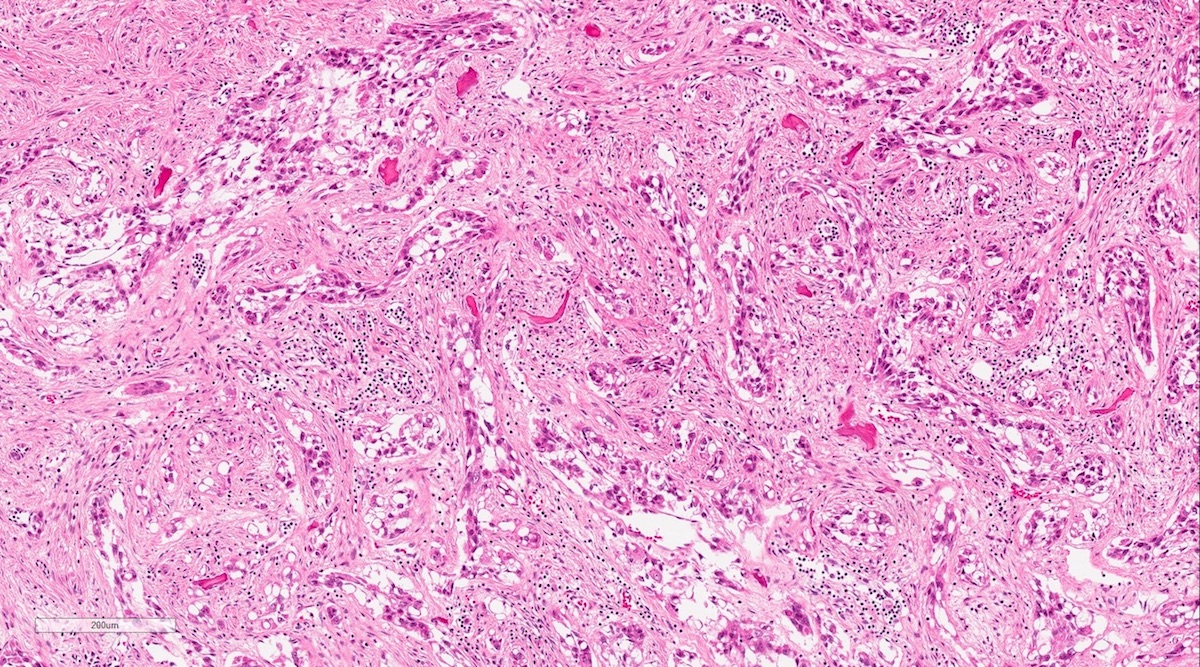
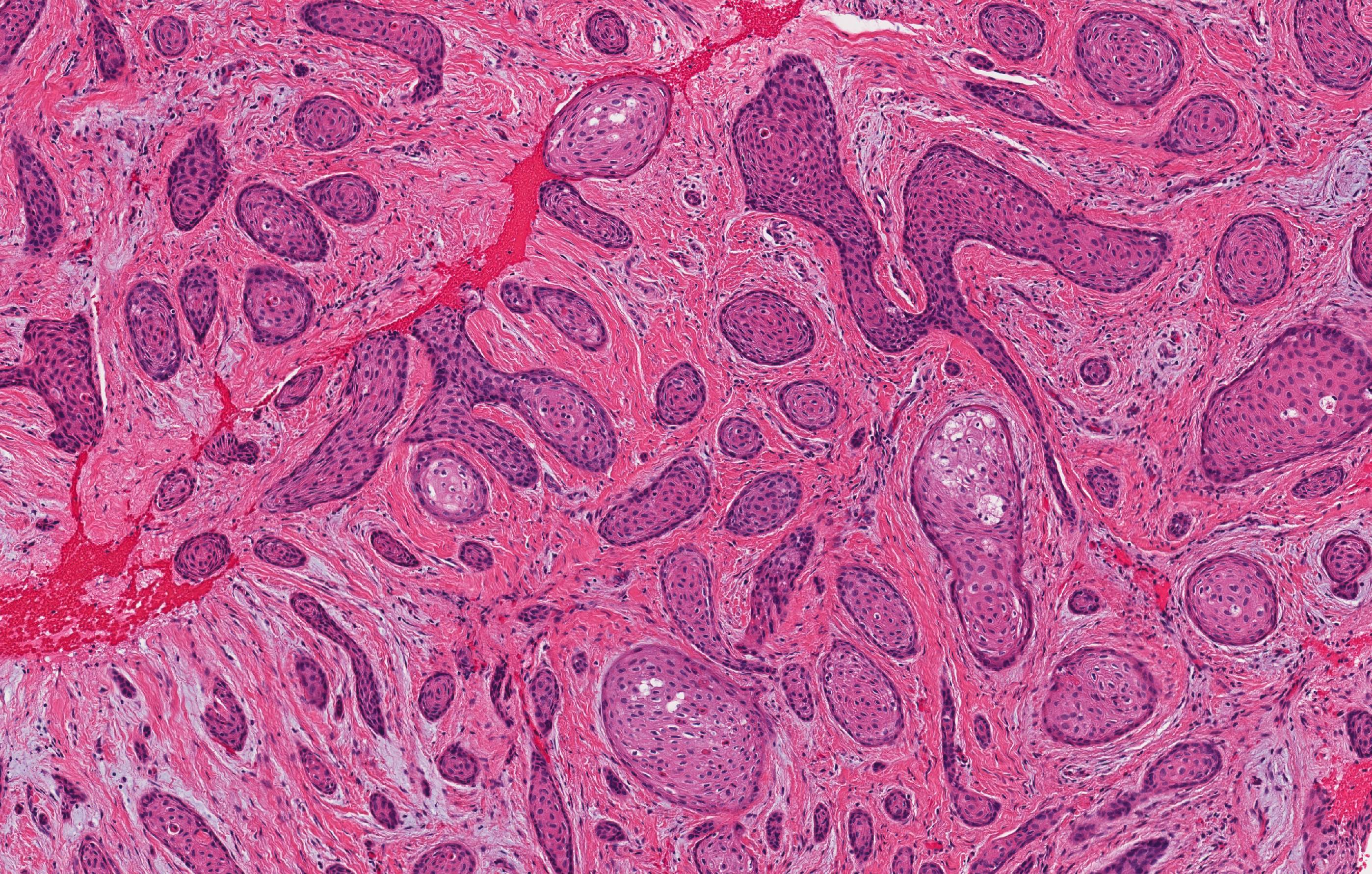
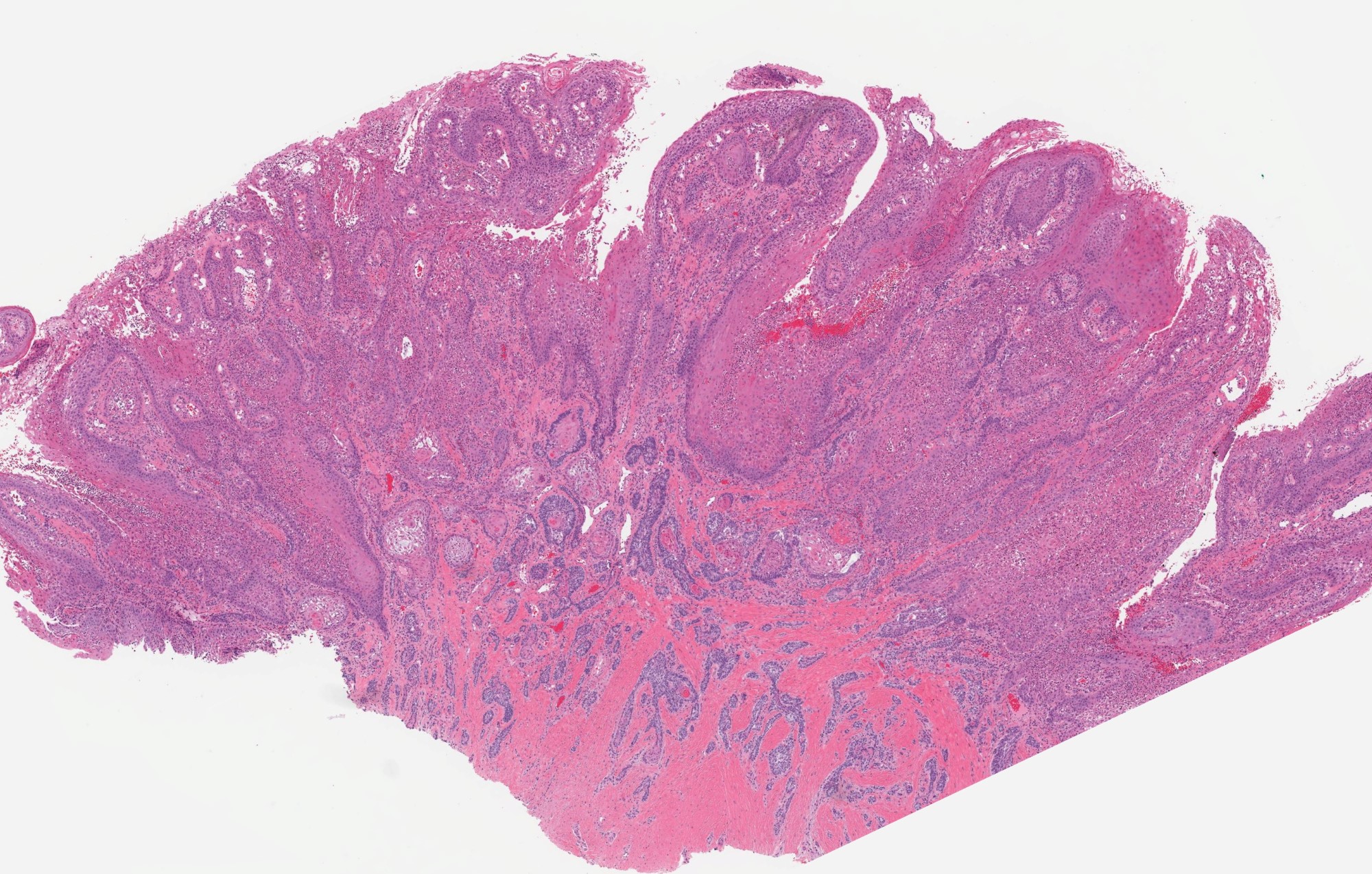
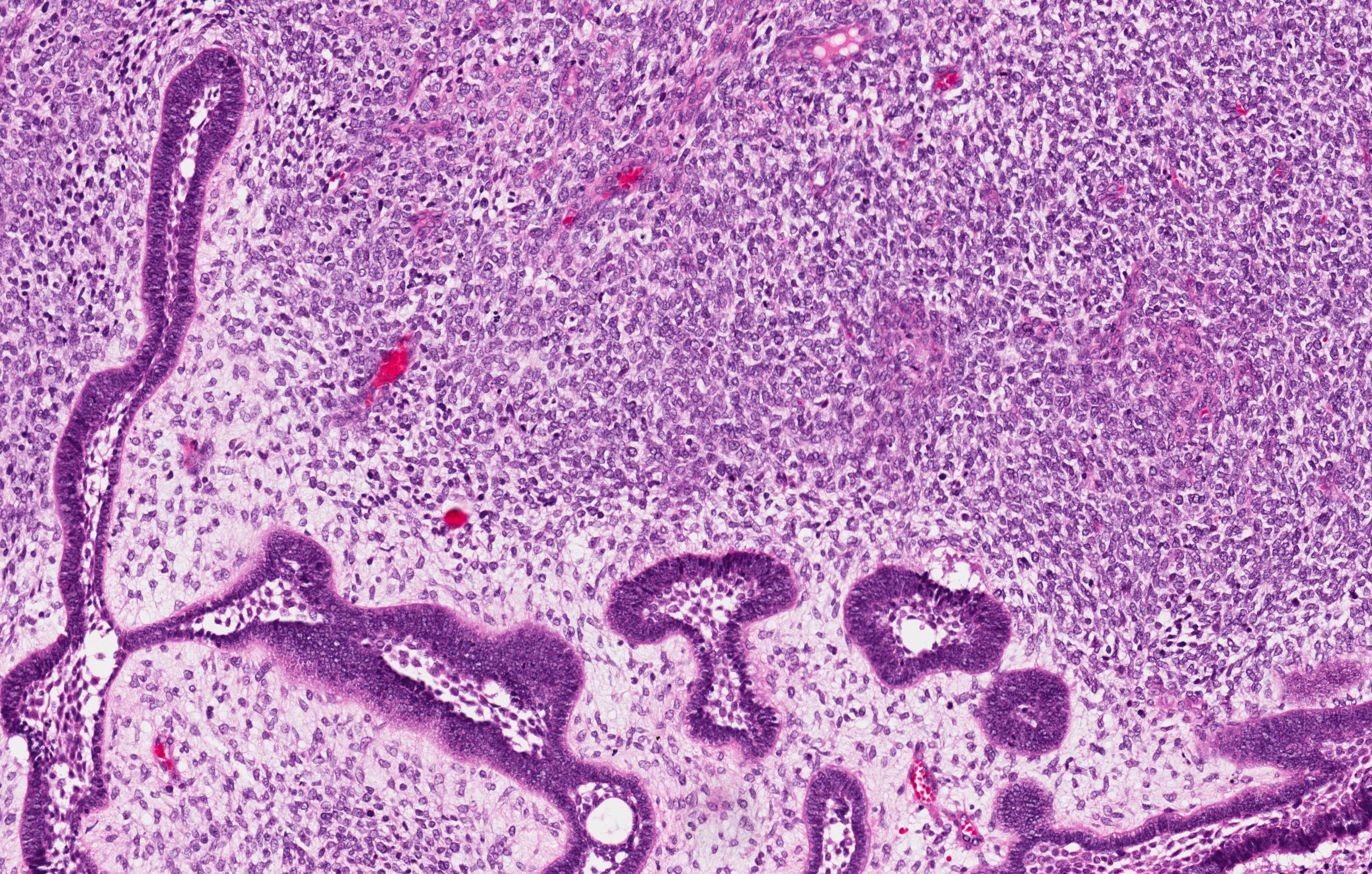
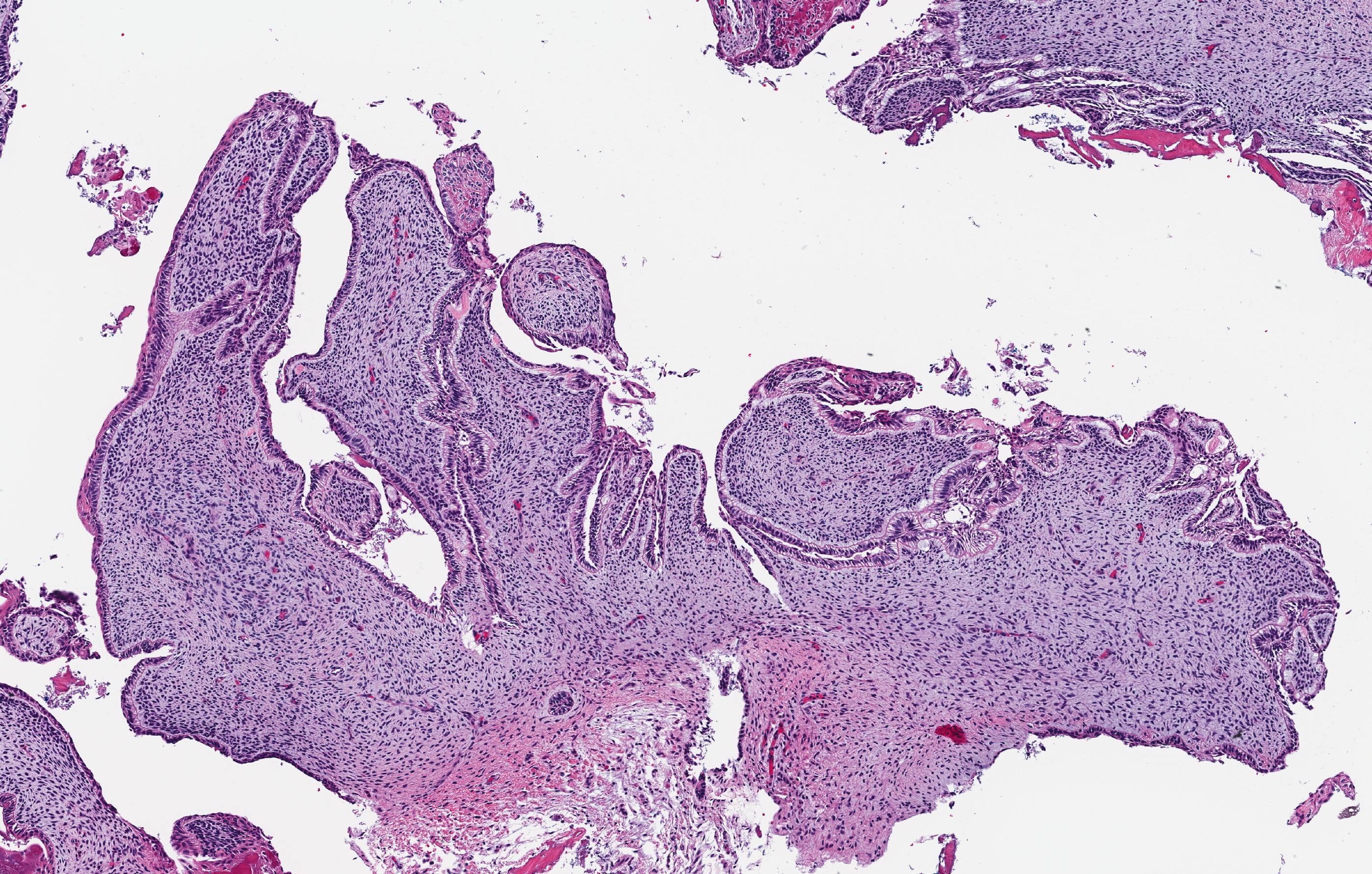
Microscopic (histologic) description
- Epithelium resembling conventional ameloblastoma, particularly palisading of cells at peripheries of the tumor nests, with additional duct-like structures and cribriform architecture
- Basophilic areas of cellular condensation or morules may be present
- Dentinoid deposits, clusters of clear cells and ghost cell keratinization may also be observed (J Oral Maxillofac Pathol 2012;16:272)
- Mitotic activity may be increased (Oral Surg Oral Med Oral Pathol Oral Radiol 2019;128:e78, Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:368)
Microscopic (histologic) images
Positive stains
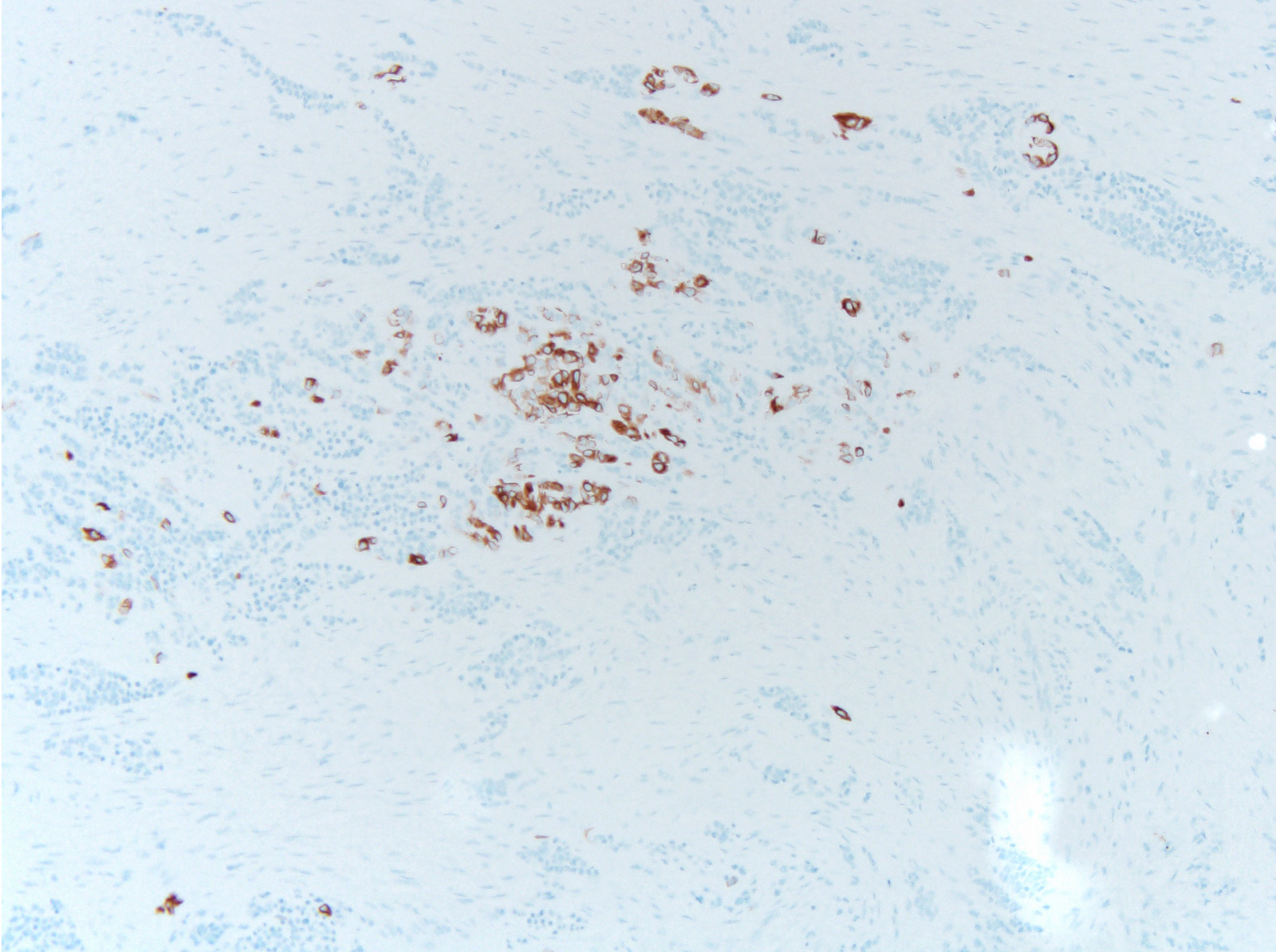
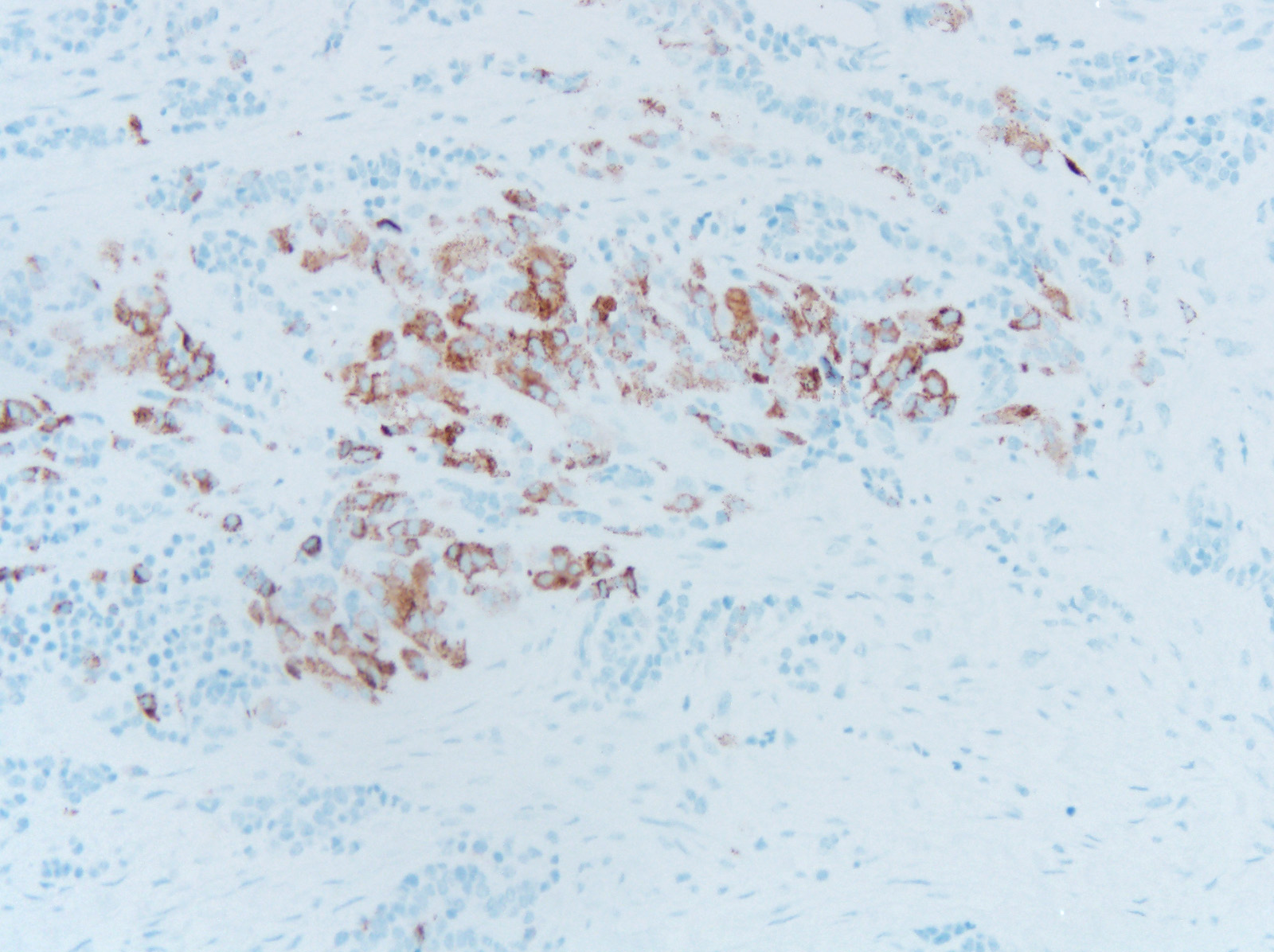
- AE1 / AE3 (Oral Surg Oral Med Oral Pathol Oral Radiol 2019;128:e78)
- Beta catenin within morules (Oral Surg Oral Med Oral Pathol Oral Radiol 2019;128:e78)
- CK5/6 (Oral Surg Oral Med Oral Pathol Oral Radiol 2019;128:e78)
- Ki67 may have variable expression, with the upper limit being 72.4 plus or minus 24.9 positive cells per high power field (Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:368)
- p63 and p40 are positive (Oral Surg Oral Med Oral Pathol Oral Radiol 2019;128:e78)
- p16 is variable (Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:368, Oral Surg Oral Med Oral Pathol Oral Radiol 2019;128:e78)
Negative stains
Molecular / cytogenetics description
- BRAF p.V600E mutation (which is observed in other ameloblastomas) in addition to KRAS p.G12V and KRAS p.G12R mutations (both of which are typical of adenomatoid odontogenic tumors) are absent in adenoid ameloblastomas (J Oral Pathol Med 2021;50:1067)
- Based on limited data, beta catenin mutations, particularly p.Ser33Cys, p.Gly34Arg and p.Ser37Phe, were observed in 4 of 9 patients (Mod Pathol 2022;35:1562)
Sample pathology report
- Posterior mandible, right, incisional biopsy:
- Adenoid ameloblastoma, 1.5 cm
Differential diagnosis
- Ameloblastoma:
- Columnar cells with palisading, hyperchromatic nuclei of basal cells
- No hard tissue formation is present
- Adenomatoid odontogenic tumor:
- Epithelium may appear nodular, trabecular or cribriform with duct-like structures
- Amyloid deposits may be present
- Enclosed in thick capsule; radiographically often well defined and associated with impacted maxillary canines
- Calcifying cystic odontogenic tumor:
- Ameloblastic epithelium and ghost cells
- Lacks whorls, duct-like structures and cribriform architecture
- Dentinogenic ghost cell tumor:
- Ameloblastic-like areas with palisading of basaloid cells
- Admixed ghost cells: anucleate epithelial cells with pale, cleared cytoplasm
- Lacks whorls, duct-like structures and cribriform architecture
Additional references
Board review style question #1
Board review style answer #1
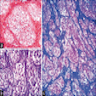
A. Adenoid ameloblastoma. The photomicrograph depicts cribriform areas with duct-like structures and interspersed dentinoid deposits that are associated with clear cells. Answer D is incorrect because calcifying cystic odontogenic tumors lack duct-like structures and cribriform architecture. Answer B is incorrect because adenomatoid odontogenic tumors are well encapsulated and lack mature dentinoid. Answer C is incorrect because conventional solid / multicystic ameloblastomas lack hard tissue formation.
Comment Here
Reference: Adenoid ameloblastoma
Comment Here
Reference: Adenoid ameloblastoma
Adenomatoid odontogenic tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
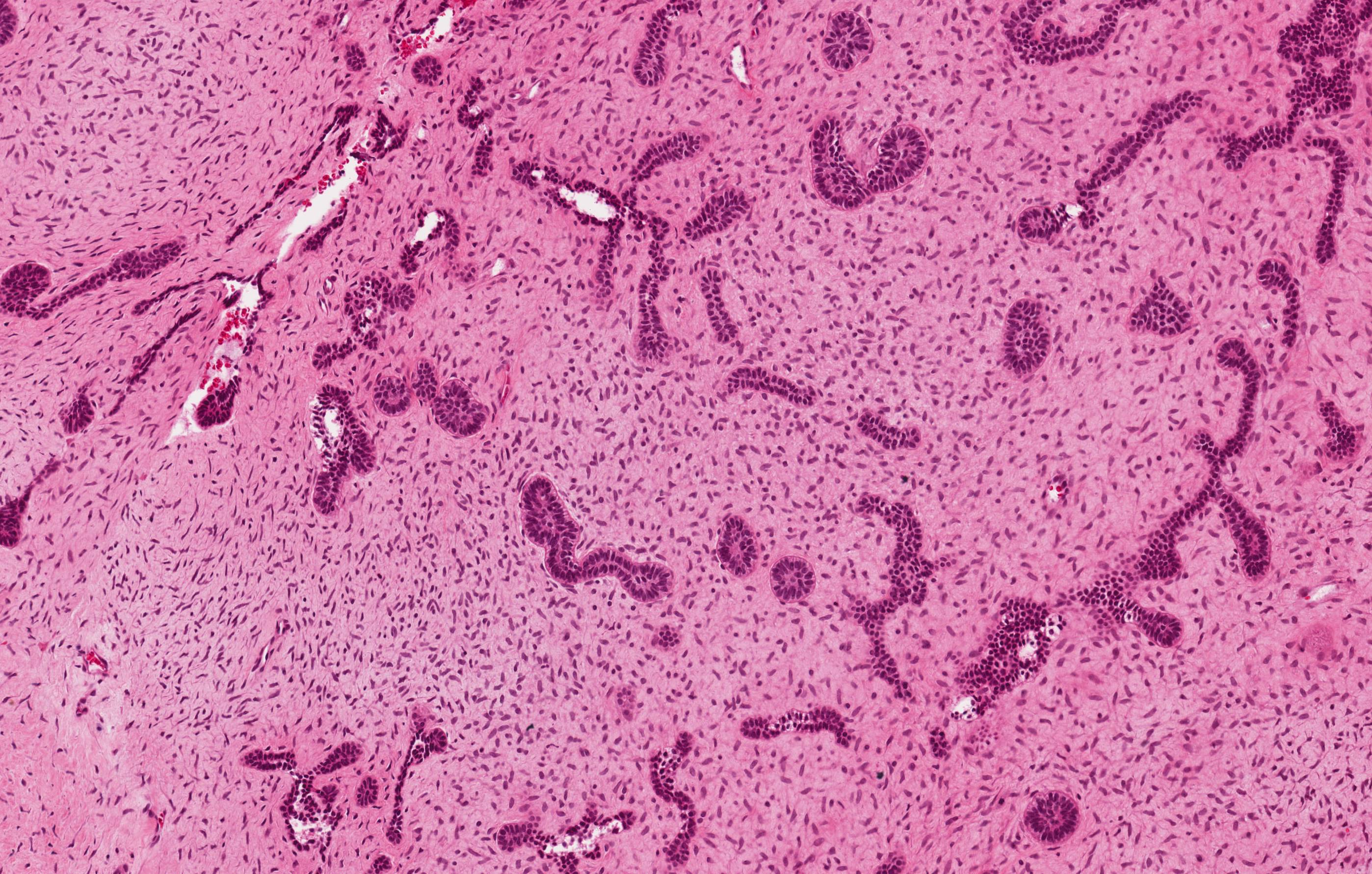
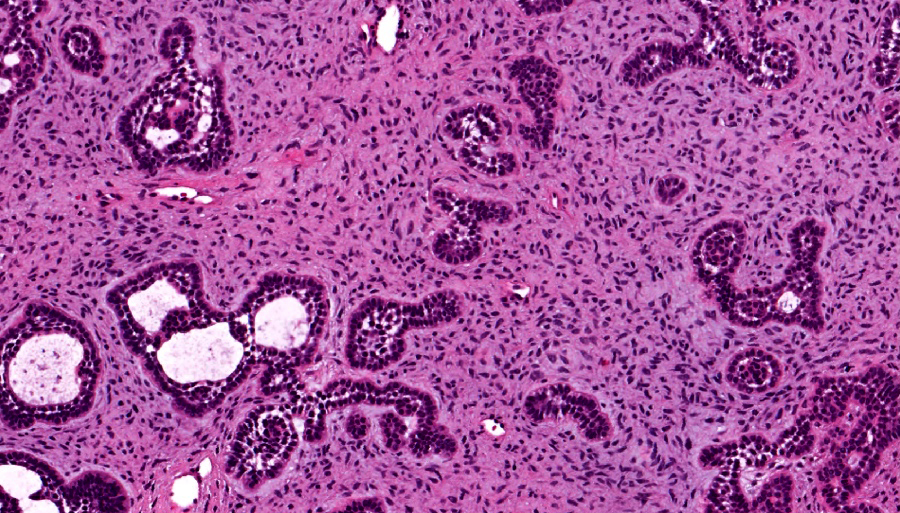
- Benign, rare tumor of odontogenic origin
- Encapsulated, characterized by spindled or cuboidal epithelium forming a nodular pattern, with duct-like structures resulting in cribriform areas; eosinophilic amyloid and calcifications may be seen (Head Neck Pathol 2021;15:71)
Essential features
- Adenomatoid odontogenic tumors (AOTs) are most common in the anterior region of the jaws in young female patients associated with impacted teeth (J Nat Sci Biol Med 2013;4:457)
- Microscopically, AOTs contain spindled, cuboidal, polygonal epithelium, forming duct-like structures, whorls and cribriform architecture surrounded by a thick capsule
- Eosinophilic matrix may be seen, which can calcify (Oral Surg Oral Med Oral Pathol Oral Radiol 2022;133:675)
- Also called the tumor of two - thirds as two - thirds of cases occur in female patients in the maxilla, surrounding an impacted tooth (Oral Oncol 1999;35:125, Surg Pathol Clin 2017;10:177)
Terminology
- Historic terminology (no longer appropriate) includes adenoameloblastoma, adenoameloblastic odontoma, adenomatoid ameloblastoma, pseudoadenomatous ameloblastoma (J Oral Pathol Med 1991;20:149)
- In 1971, the World Health Organization (WHO) adopted terminology proposed by Philipsen and Birn: adenomatoid odontogenic tumor (Acta Pathol Microbiol Scand 1969;75:375)
ICD coding
Epidemiology
- 2.2 - 7.1% of all odontogenic tumors (J Oral Med Oral Surg 2021;27:19, J Oral Pathol Med 2007;36:383)
- 87.2% occur in the second or third decade of life but may occur across wide age range
- ~2:1 female predilection
- 3 variants of AOT are follicular (pericoronal), extrafollicular and peripheral (J Oral Pathol Med 2007;36:383)
- Follicular variant accounts for ~71% of all cases
- Extrafollicular (extracoronal) variant accounts for ~27% of cases
- Peripheral (extraosseous) variant accounts for 2% of all cases
Sites
- Anterior maxilla is the most affected (66.6%) (Head Neck Pathol 2012;6:430)
- ~42% of cases are associated with impacted maxillary canines (J Oral Pathol Med 1991;20:149)
- There is a significant predilection for the anterior region (93.3%), with only 6.7% of cases affecting the posterior area, exclusively in the mandible (Head Neck Pathol 2012;6:430)
Pathophysiology
- KRAS mutations and MAPK pathway activation are commonly implicated in the pathogenesis in AOT
- In one series, 71% (27/38) of AOT cases expressed KRAS codon 12 mutations (Mod Pathol 2019;32:799)
Clinical features
- Usually an asymptomatic swelling (unless secondarily infected)
- Tooth displacement may be present
Diagnosis
- May be detected radiographically as an incidental finding or present as clinical swelling
Radiology description
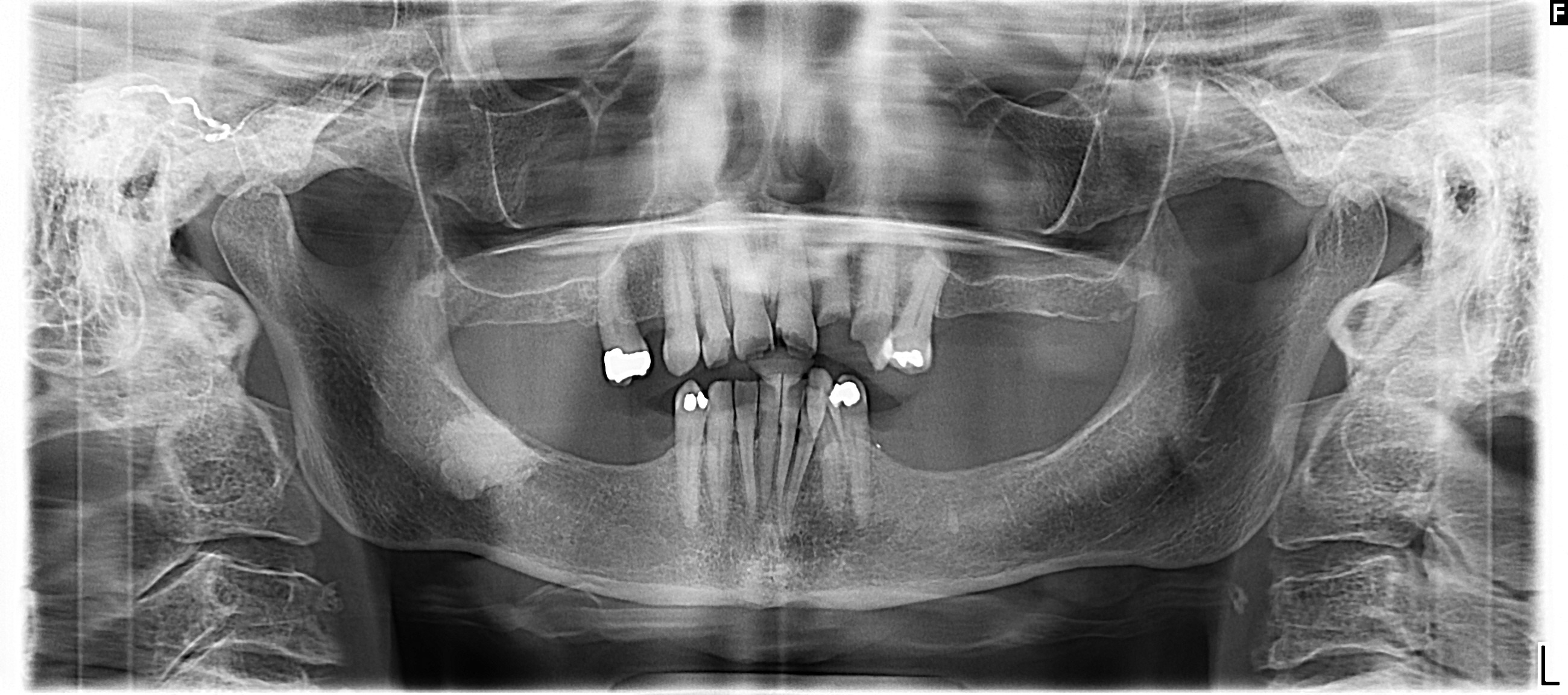
- Most commonly (~75%) appear as well defined, unilocular radiolucencies (J Oral Pathol Med 2007;36:383)
- May also appear as mixed density or opaque lesions
- Encompass impacted teeth and extend beyond the cementoenamel junction and sometimes continue to the apex (Quintessence Int 2022;53:260)
Radiology images
Prognostic factors
- Prognosis excellent, few recurrences (< 5% documented)
Case reports
- 17 year old girl with a 2 cm maxillary lesion surrounding an impacted canine tooth (Case #490)
- 18 year old woman with nonpainful lesion of right anterior maxilla (Anticancer Res 2013;33:2673)
- 28 year old man with impacted tooth (Arch Pathol Lab Med 2003;127:e173)
- 31 year old man with cystic radiolucent lesion of the right mandible (J Oral Med Oral Surg 2021;27:19)
Treatment
- Generally treated by surgical enucleation
- Cases of large lesions that have caused significant bone loss and thinning are often managed by insertion of a drain or via marsupialization (Maxillofac Plast Reconstr Surg 2014;36:173)
- Both surgical and orthodontic modalities can be used to preserve the associated impacted tooth (Maxillofac Plast Reconstr Surg 2014;36:173)
Gross description
- 1 - 3 cm in size, unicystic with a thick capsule and soft tissue filling most of the cystic space
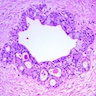
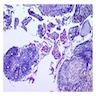
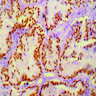
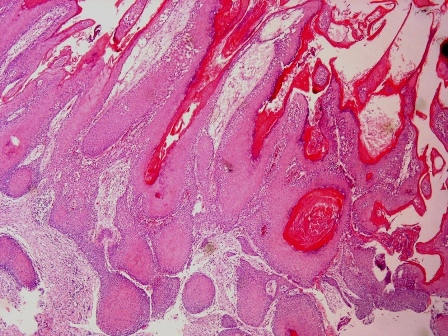
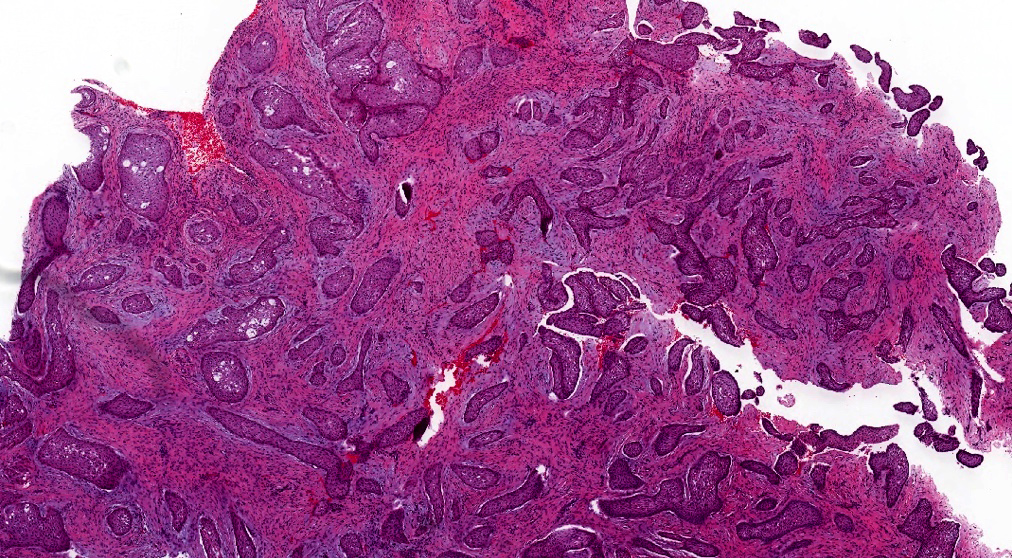
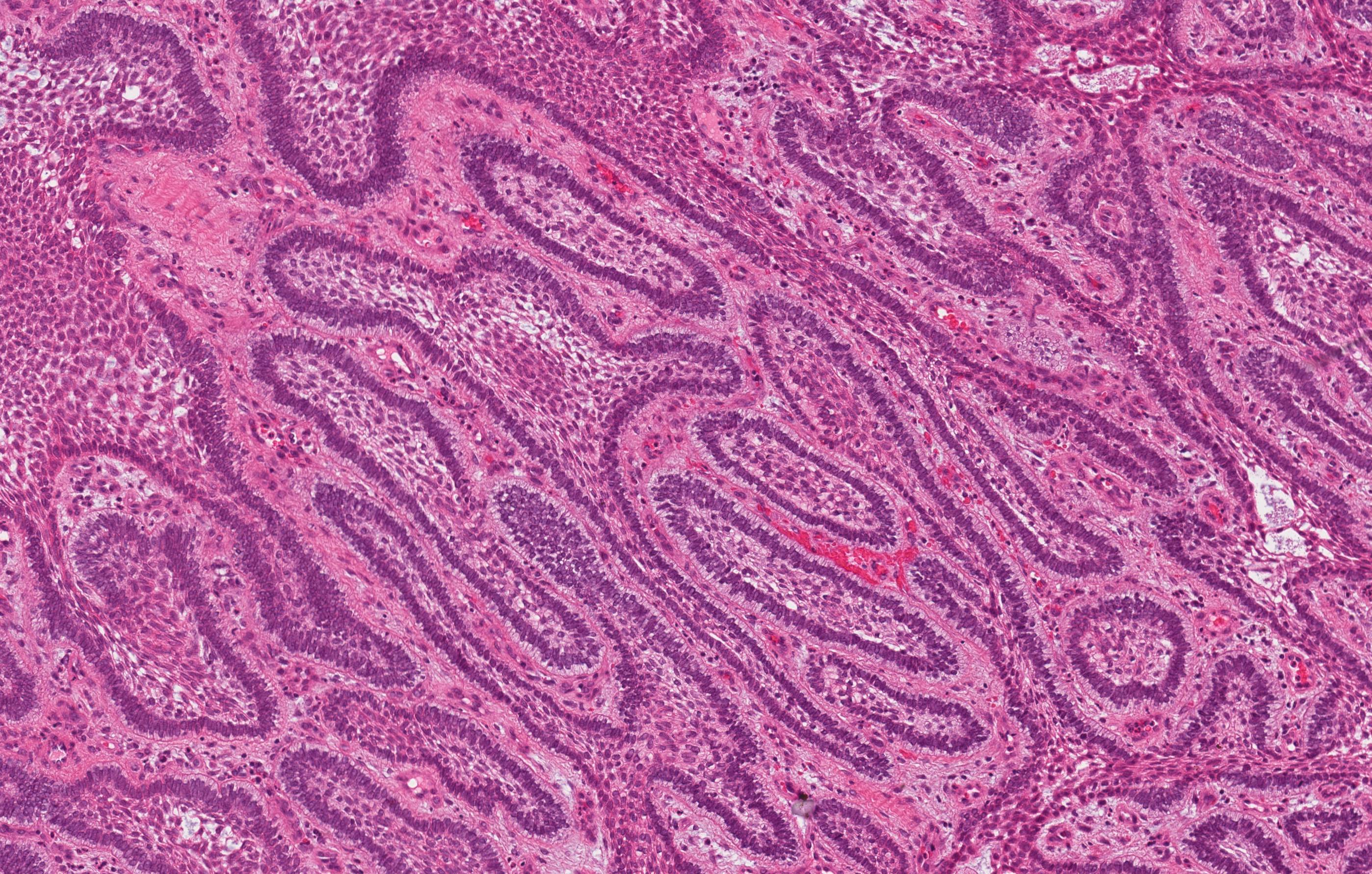
Microscopic (histologic) description
- Epithelium may appear nodular, trabecular, cribriform and form duct-like structures
- Cells may be spindly, cuboidal or columnar with the nuclei palisading away from the lumen
- Lesion is enclosed in a thick capsule
- Amyloid deposits may be present (Quintessence Int 2022;53:260)
- Calcifications may be seen
- Calcifying epithelial odontogenic tumor (CEOT)-like areas may be seen
- AOT - CEOT hybrid lesions have been described as distinct entities; these are customarily considered to be an AOT variant, with the clinical behavior of AOT
- References: Head Neck Pathol 2017;11:519, Oral Oncol 2005;41:835
Microscopic (histologic) images
Contributed by Elizabeth Ann Bilodeau, D.M.D., M.D., M.S.Ed. and Kelly Magliocca, D.D.S., M.P.H. (Case #490)
Positive stains
- AE1 / AE3 (Oral Surg Oral Med Oral Pathol Oral Radiol 2022;133:675)
- CK5, CK17 and CK19 (J Oral Pathol Med 2003;32:55)
- Congo red may highlight focal amyloid deposits, especially in CEOT-like areas (Oral Oncol 2005;41:835)
Molecular / cytogenetics description
- KRAS mutations and MAPK pathway activation are commonly implicated in the pathogenesis of AOT (Mod Pathol 2019;32:799)
Sample pathology report
- Anterior maxilla, left, excisional biopsy:
- Adenomatoid odontogenic tumor, 1.8 cm
Differential diagnosis
- Adenoid ameloblastoma:
- May have extensive dentinoid clear cells and occasional ghost cells
- Morules / whorls, duct-like structures may be seen
- Lacks thick capsule, radiographically often not well defined
- Calcifying cystic odontogenic tumor:
- Ameloblastic epithelium and ghost cells
- Lacks whorls, duct-like structures and cribriform architecture
- Calcifying epithelial odontogenic tumor:
- Sheets of polygonal cells with pleomorphism and prominent intercellular bridging
- Lacks architectural pleomorphism (whorls, cribriform, duct-like pattern)
Additional references
Board review style question #1
Board review style answer #1
A. Adenomatoid odontogenic tumor. The photomicrograph depicts nodular islands and duct-like structures with interspersed calcifications surrounded by a thick fibrous capsule. Answer B is incorrect because ameloblastomas are characterized by ameloblastic epithelium with reverse polarity away from the basement membrane but nodular architecture, encapsulation, calcifications and duct formation are not seen. Answer C is incorrect because calcifying cystic odontogenic tumors / calcifying odontogenic cysts have ameloblastic epithelium, varying numbers of ghost cells and calcifications, lacking the nodular architecture seen. Answer D is incorrect because calcifying epithelial odontogenic tumors lack encapsulation and have sheets, nest and cords of polygonal, pleomorphic cells that exhibit prominent intercellular bridging and amyloid with concentric calcifications.
Comment Here
Reference: Adenomatoid odontogenic tumor
Comment Here
Reference: Adenomatoid odontogenic tumor
Ameloblastic carcinoma
Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Radiology description | Radiology images | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
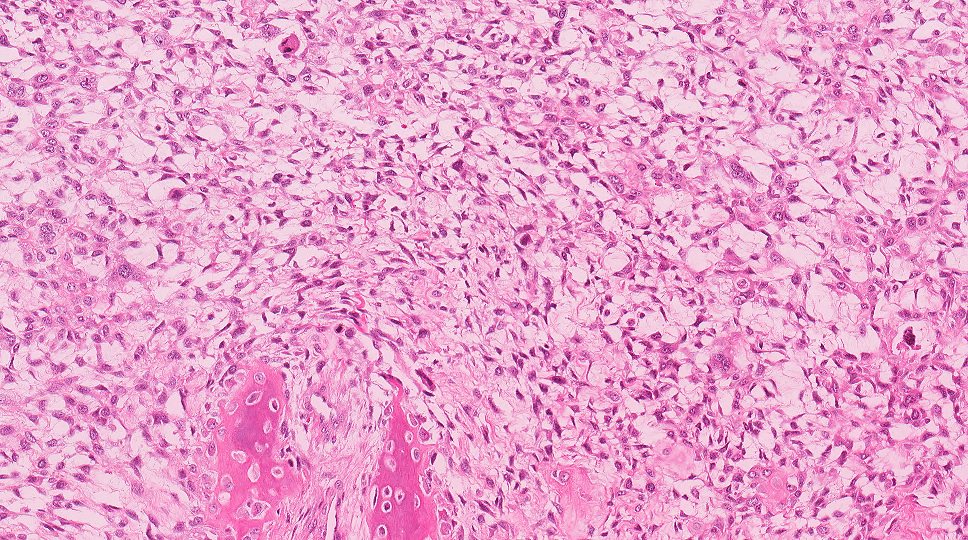
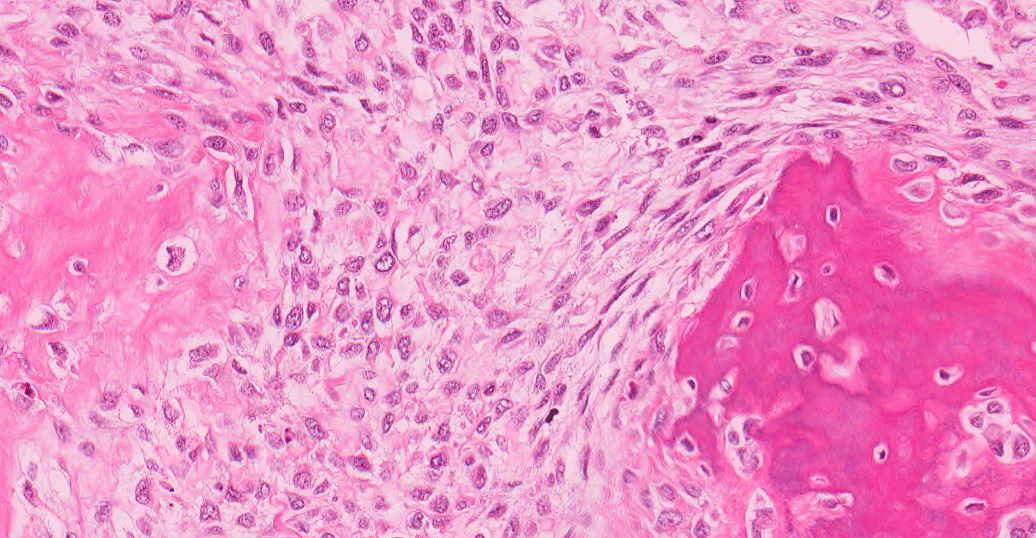
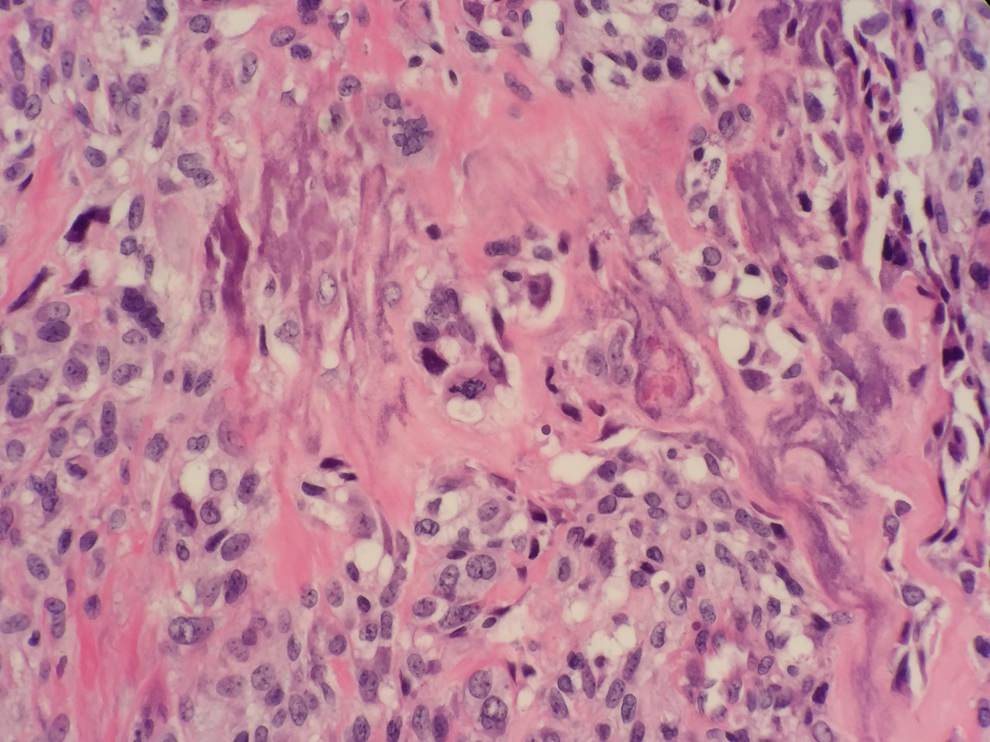
- Rare epithelial odontogenic tumor with histologic features of an ameloblastoma with marked cytologic atypia
Epidemiology
- Rare, < 100 case reports in English literature
- Represent < 1% of all odontogenic tumors
Sites
- More common in mandible (~80%), particularly posterior mandible
- Rare reports of maxillary involvement
Etiology
- Majority arise de novo
- Less commonly arise from pre-existing ameloblastoma, and are considered secondary or dedifferentiated lesions
Clinical features
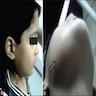
- May be painful as they cause expansion of the jaw, grow rapidly and perforate the cortex
- "Expansion" or "hard mass" is the most common chief complaint
- Other complaints include toothache, ulceration, trismus and facial asymmetry
Diagnosis
- Requires clinical, radiologic and pathologic correlation
Prognostic factors
- Malignant lesions have 30% recurrence rate, 22% metastasis rate
- 5 year survival is 70% without metastases, 20% with metastases
Radiology description
- On radiograph, well-defined unilocular or multilocular radiolucent lesion
- Often shows cortical expansion with perforation
Radiology images
Case reports
- 16 year old boy with metastasis to skull and lung (Dentomaxillofac Radiol 2010;39:449)
- 24 and 30 year old men (J Oral Maxillofac Pathol 2014;18:S96, J Clin Diagn Res 2015;9:ZD27)
- 66 year old man and 67 year old woman (Am J Case Rep 2015;16:415, J Maxillofac Oral Surg 2010;9:198)
Treatment
- Composite surgical resection, with adjuvant radiation and chemotherapy as appropriate
Microscopic (histologic) description
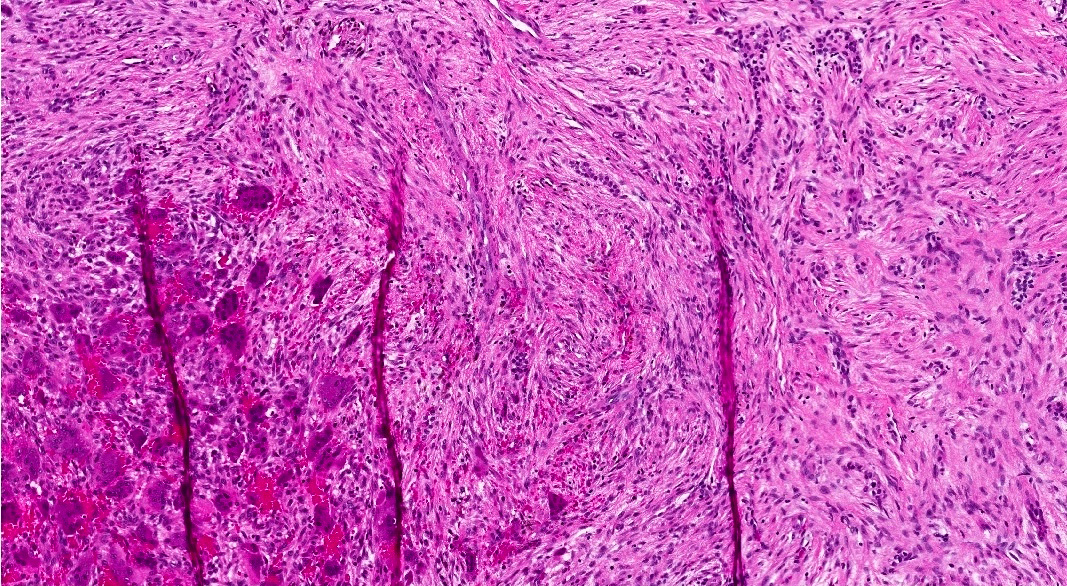
- Variable features of amelobastoma: peripheral palisading, reverse polarization, stellate reticulum
- Features of malignancy include cytological atypia, high N:C ratio, increased mitoses with atypical forms, necrosis
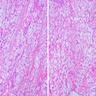
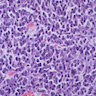
Microscopic (histologic) images
Differential diagnosis
- Ameloblastoma
- Histologically, may share some of same features such as peripheral palisading, reverse polarization and stellate reticulum, but should not show features of malignancy (pleomorphism with hyperchromasia, atypical mitoses)
- Clear cell odontogenic carcinoma
- Malignant epithelial odontogenic tumor composed primarily of nests and islands of clear cells
- May have focal peripheral palisading similar to ameloblastoma, but not as much cytologic atypia as ameloblastic carcinoma
- More common in anterior mandible
- EWSR mutation
- Malignant ameloblastoma
- Like amelobastoma histologically but termed "malignant" after discovery of metastases
- Should not show any cytologic features of malignancy
- Metastatic disease
- Primary intraosseous squamous cell carcinoma
- Carcinoma composed of moderately to poorly differentiated squamous epithelial cells with variable keratinization
- Also derived from odontogenic epithelium
Additional references
Ameloblastic fibroma
Table of Contents
Definition / general | Radiology description | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Rare
- Usually age 20 or less
- Benign, rarely recurs
Radiology description
- Variable radiolucent - radiopaque appearance
Treatment
- Simple curettage
Gross description
- Usually solid
Microscopic (histologic) description
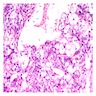
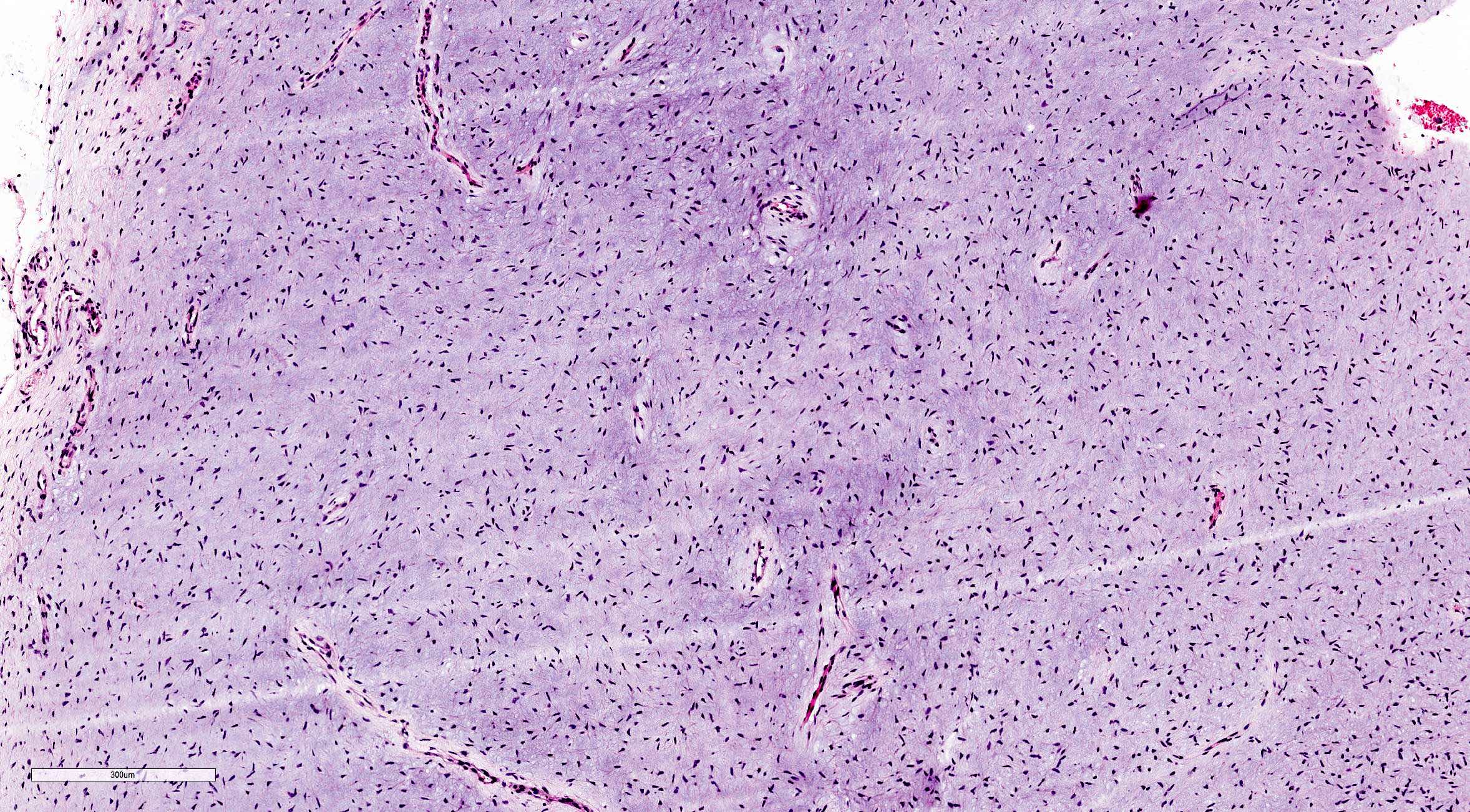
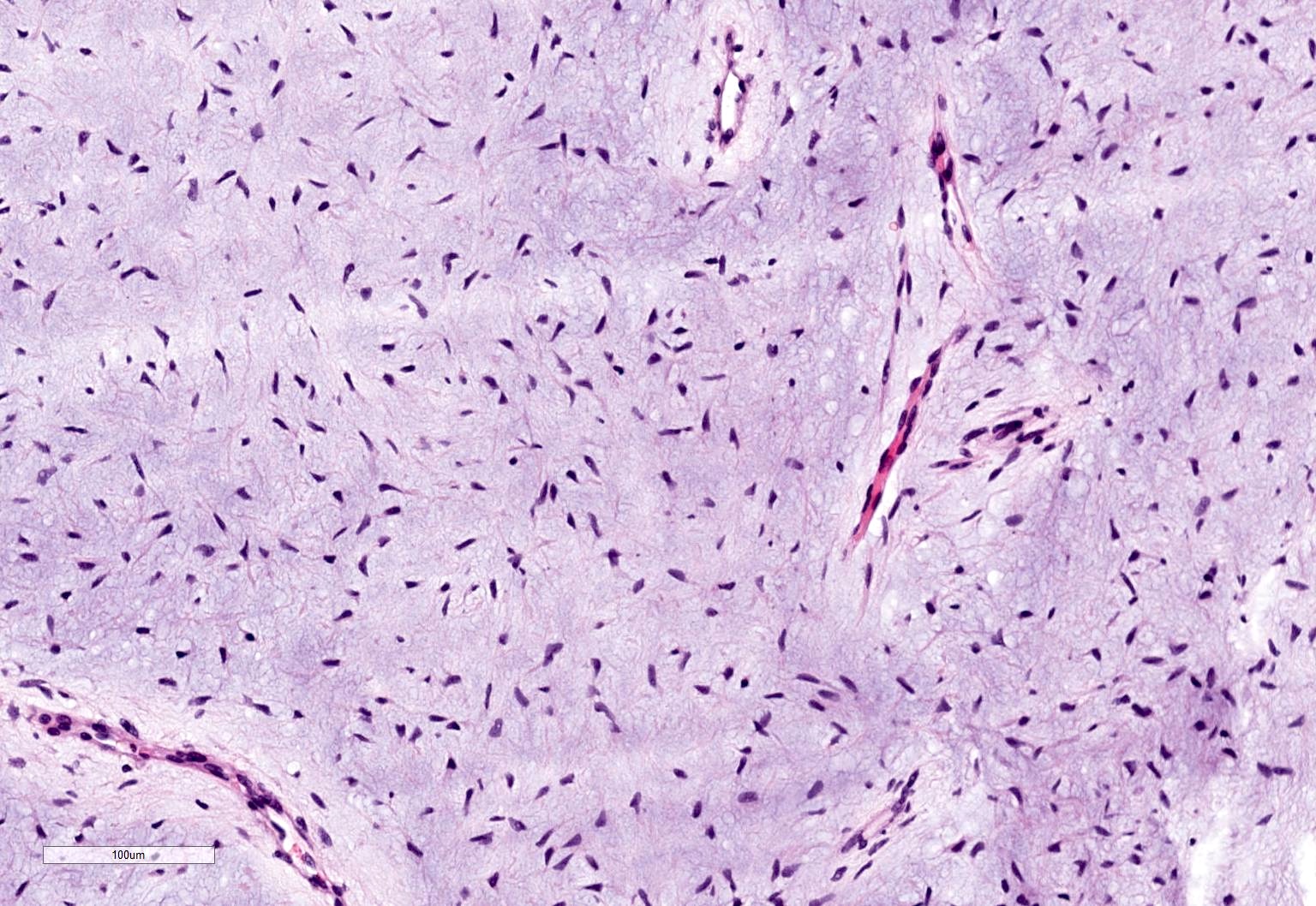
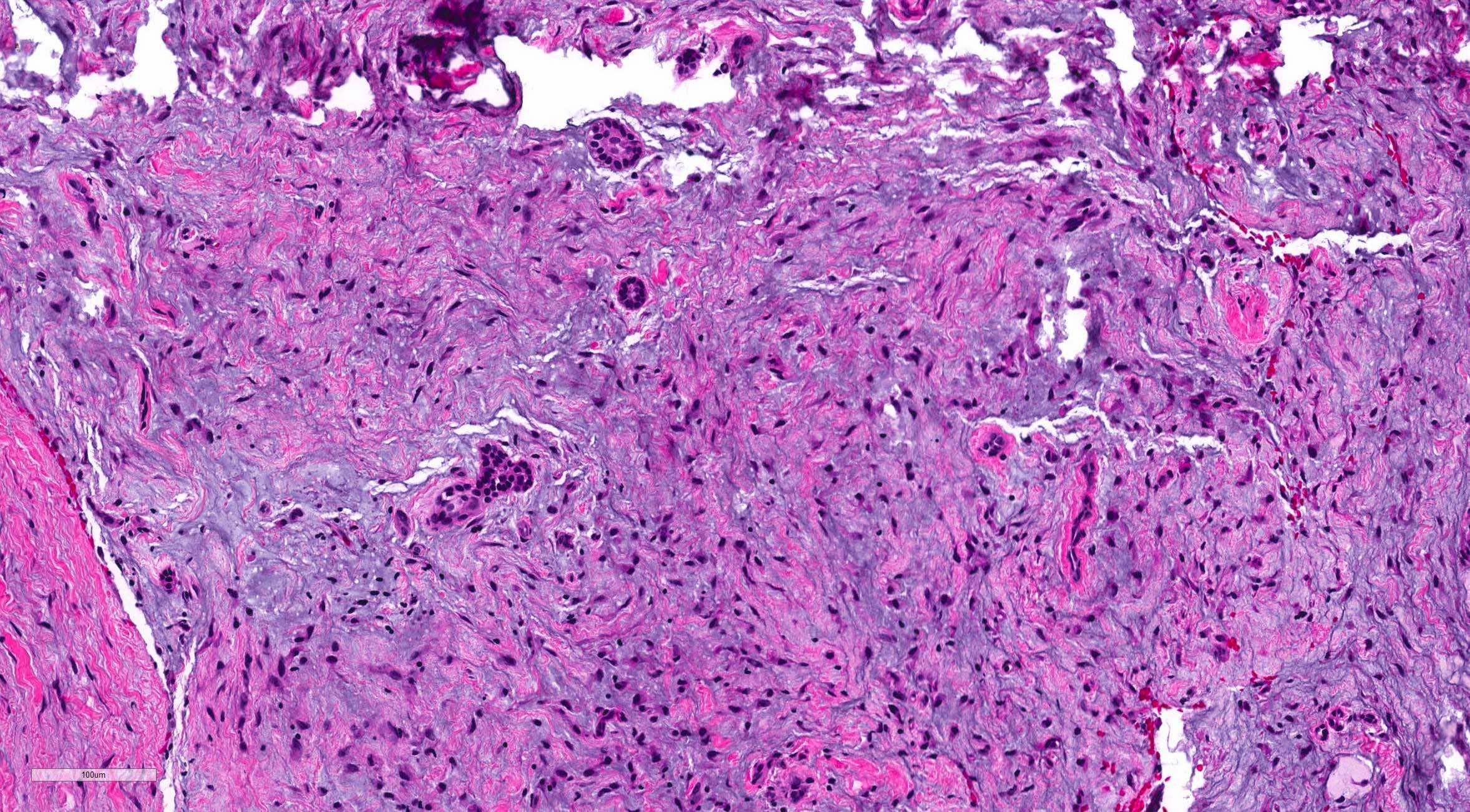
- Small islands and cords of markedly attenuated ameloblastic epithelium two cells thick within dense collagenous stroma that is often immature
- Occasional dentin or cementum production and stellate reticulum
- Also granular cell variant
Microscopic (histologic) images
Differential diagnosis
- Ameloblastoma: no connective tissue stroma, has dentin and enamel
Ameloblastoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Odontogenic epithelial neoplasm characterized by slow, expansile growth
- Classified as a benign neoplasm; ameloblastoma behaves in a locally aggressive manner with a tendency to recur
Essential features
- Slow growing, locally aggressive odontogenic epithelial neoplasm
- Most commonly occurs in mandible
- Multiple microscopic variants
- Treatment most often involves loss of bone and teeth
Terminology
- Adamantinoma (previous name): proposed early on by Malassez in 1885; subsequently abandoned as the term largely implies formation of hard tissues, which would be unexpected in ameloblastoma
- Ameloblastoma, conventional: name changed from solid / multicystic ameloblastoma to conventional ameloblastoma in WHO 2017, 4th edition, to avoid confusion with unicystic ameloblastoma
- Cystic ameloblastoma: conventional ameloblastoma with macrocystic change variably referred to as cystic ameloblastoma in the past; term is discouraged as it may be confused with unicystic ameloblastoma
- Desmoplastic ameloblastoma: previously classified as a separate and distinct subtype of ameloblastoma in WHO 2005, 3rd edition; no longer classified as distinct, now considered a microscopic variant of conventional ameloblastoma within WHO 2017, 4th edition
- Metastasizing ameloblastoma: previously classified as metastasizing (malignant) ameloblastoma, a malignant odontogenic tumor in WHO 2005, 3rd edition; removed from classification of malignant odontogenic tumors and reclassified in the spectrum of benign odontogenic tumors in WHO 2017, 4th edition
ICD coding
Epidemiology
- Represents 1% of all jaw cysts and tumors
- Most common odontogenic neoplasm
- Incidence: 0.5 cases per million population
- No sex predilection
- Majority of ameloblastomas are conventional type, followed by unicystic, peripheral
- Broad age range, narrowed somewhat by tumor type:
- Ameloblastoma, conventional type: peak incidence in fourth to fifth decades of life
- Ameloblastoma, unicystic type: peak incidence in second to third decades of life
- Ameloblastoma, extraosseous / peripheral type: peak incidence in fifth to seventh decades of life
Sites
- Intraosseous gnathic ameloblastoma (conventional and unicystic)
- ~80% cases occur in mandible
- ~20% cases occur in maxilla
- Desmoplastic ameloblastoma has predilection for anterior jaws, especially anterior maxilla
- Ameloblastoma, extraosseous / peripheral type (Head Neck Pathol 2010;4:192)
- Found in soft tissue of posterior gingiva and retromolar area
- Predilection for lingual side of mandible
- Other head and neck sites reported (all very rare):
- Sinonasal tract (Cancer 1998;82:667)
- Middle ear (J Int Adv Otol 2019;15:173)
- Temporal bone (J Oral Maxillofac Surg 2017;75:1300.e1)
- Infratemporal fossa; most commonly occurs in this location in the form of recurrent disease (Dentomaxillofac Radiol 2007;36:416)
Etiology
- Intraosseous gnathic ameloblastoma originates from:
- Remnants of the enamel organ or dental lamina
- Reported to rarely arise within odontogenic cyst
- Ameloblastoma, extraosseous / peripheral type: arises from rests of Serres (dental lamina remnants in gingiva)
Clinical features
- Ameloblastoma, conventional:
- Most commonly grossly solid / multicystic, expansile, locally aggressive, requiring resection with uninvolved margins
- May show macrocystic change grossly
- Microscopic variants include follicular, plexiform, basal, acanthomatous, granular and desmoplastic
- Usually asymptomatic; may be found incidentally on radiographic exam
- When symptoms present, they are usually limited or nonspecific
- Slow growing, painless expansion of the involved jaw
- Increased size often related to more clinically significant symptoms
- Malocclusion
- Loose teeth
- Pain possible if infected or hemorrhaging into tissues
- Very large tumors may cause facial deformity, restrict mouth opening and eventually lead to airway obstruction
- Ameloblastoma, unicystic type:
- Occurs as single cystic cavity with or without intramural growth
- Microscopic variants include luminal, intraluminal and mural; luminal and intraluminal considered a less aggressive variant of ameloblastoma, amenable to more conservative treatment
- Natural history and treatment of mural unicystic ameloblastoma remains controversial
- Usually asymptomatic; may be found incidentally on radiographic exam
- When symptoms present, they are usually limited or nonspecific
- Slow growing, painless expansion of the involved jaw
- Increased size often related to more clinically significant symptoms
- Malocclusion
- Loose teeth
- Pain possible if infected or hemorrhaging into tissues
- Very large tumors may cause facial deformity, restrict mouth opening and eventually lead to airway obstruction
- Possible rare association with nevoid basal cell carcinoma (Gorlin) syndrome, among other inherited conditions (Fam Cancer 2012;11:411, J Stomatol Oral Maxillofac Surg 2020;121:146)
- Ameloblastoma, extraosseous / peripheral type:
- Most commonly occurs within gingiva
- Peripheral ameloblastoma accounts for 1% of ameloblastoma cases and is amenable to conservative surgical treatment and show low recurrence rates (J Oral Maxillofac Pathol 2018;22:396)
- Usually asymptomatic
- Found in soft tissue of posterior gingiva and retromolar area
- Predilection for lingual side of mandible
- Gingival lesions showing radiographic cupping of bone may represent a peripherally placed intraosseous ameloblastoma that has perforated cortical bone (Head Neck Pathol 2010;4:192)
- Cross sectional imaging essential to exclude small intraosseous ameloblastoma with a prominent extraosseous component
- Metastasizing ameloblastoma:
- Rare
- Usually metastasizes to lymph nodes or lung, other sites possible with organ associated symptoms
- Associated with longstanding tumors, multiple surgical procedures, radiation therapy
Diagnosis
- Based on clinical, radiologic and pathologic correlation
- Ameloblastoma, extraosseous / peripheral type: requires exclusion of an intraosseous tumor with extraosseous extension mimicking a gingival lesion (Head Neck Pathol 2010;4:192)
- Metastasizing ameloblastoma can only be diagnosed retrospectively
- Ameloblastoma reported very rarely to occur in nongnathic sites (sinonasal, ear / temporal bone) and diagnosed only after:
- Sinonasal: requires exclusion of tumor extension into sinonasal tract from a maxillary alveolar bone location
- Ear / temporal bone: requires exclusion of competing differential diagnoses such as:
- Nonkeratinizing carcinoma (Am J Surg Pathol 2020;44:1244)
- Primary basal cell carcinoma of middle ear (J Int Adv Otol 2020;16:291)
Laboratory
- Hypercalcemia, rarely documented and is thought to be related to 1 or both of the following mechanisms:
- Increased parathyroid hormone related peptide (PTHrP)
- Osteolytic hypercalcemia: tumor invades bone and in combination with local inflammatory response, results in excessive calcium release from bone
Radiology description
- Ameloblastoma, conventional
- Expansile multilocular radiolucency, well defined, corticated border
- Some cases exhibit classic soap bubble appearance
- May or may not be associated with impacted tooth / teeth
- Resorption or displacement of tooth roots
- Reactive bone formation may occur, most commonly in desmoplastic ameloblastoma, which may resemble a fibro-osseous lesion radiographically due to the presence of osteoplasia
- May have a unicystic radiographic appearance on plain images; requires microscopic examination for distinction from ameloblastoma, unicystic type (Dentomaxillofac Radiol 2018;47:20170288)
- Ameloblastoma, unicystic type
- Unilocular radiolucency, well defined, corticated border
- Often associated with an impacted tooth, specifically mandibular third molar
- Root resorption may occur
- Cortical perforation in 33% of cases
- Ameloblastoma, extraosseous / peripheral type
- Radiographic cupping of bone described but may represent a peripherally placed intraosseous ameloblastoma that has expanded and subsequently perforated through cortical bone to mimic a peripheral gingival based lesion; requires close clinicoradiographic correlation
- Cross sectional imaging needed to exclude intraosseous ameloblastoma with a prominent extraosseous component
Radiology images
Prognostic factors
- Ameloblastoma, conventional type (Oral Dis 2019;25:1683)
- Surgery type:
- Curettage associated with high recurrence rate (60 - 80%)
- Marginal or segmental resection in mandible with planned 1 cm margins reduces rate of recurrence
- Histologic type: histologic type does not affect prognosis
- Tumor anatomic location: maxilla, higher recurrence rate
- BRAF tumor status:
- Some studies note that ameloblastoma lacking BRAF V600E mutations associated with an increased rate of local recurrence (P = 0.0003)
- Surgery type:
- Ameloblastoma, unicystic type
- Treatment and recurrence depend on pattern and extent of tumor proliferation
- When mural involvement is present, the tumor may behave like conventional ameloblastoma; clarification needed
- Overall recurrence rate of 5 - 10%
- Ameloblastoma, extraosseous / peripheral type
- If tumor represents a peripherally placed intraosseous ameloblastoma that has perforated cortical bone and mimics a gingival based lesion, then tumor will recur if intraosseous component not removed (Head Neck Pathol 2010;4:192)
Case reports
- 14 year old boy with Gardner syndrome has unusual case of a unicystic ameloblastoma mimicking a dentigerous cyst (Dentomaxillofac Radiol 2018;47:20170288)
- 15 year old girl with mandibular unicystic ameloblastoma presenting in Gardner syndrome (Head Neck Pathol 2014;8:239)
- 20 year old woman with large mandibular ameloblastoma and hypercalcemia (BMJ Case Rep 2014;2014:bcr2014205491)
- 52 year old man with multiply recurrent ameloblastoma and delayed pulmonary metastases (Respirology 2009;14:1208)
- 72 year old woman with large mandibular ameloblastoma and hypercalcemia (Consultant 2018;58:124 )
Treatment
- Ameloblastoma, conventional type:
- Mandible: usually treated with a segmental resection (marginal if small), which includes at least 1 cm bone margins and at least 1 adjacent uninvolved anatomic barrier
- Maxilla: resected en bloc via various midface approaches, at least 1 cm margins and at least 1 adjacent uninvolved anatomic barrier
- Curettage associated with high recurrence rate (60 - 80%)
- Nonsurgical therapy: although BRAF inhibitors have been used, no advantage over traditional surgical treatment for a previously untreated, intraosseous gnathic ameloblastoma has been documented
- Ameloblastoma, unicystic type: enucleation or resection
- Ameloblastoma, extraosseous / peripheral type: conservative excision
- Metastasizing ameloblastoma: extremely rare and there is currently no established treatment protocol; to be distinguished from ameloblastic carcinoma, an overtly malignant odontogenic neoplasm
- Longterm follow up is recommended for all types of ameloblastoma due to the potential for delayed presentation of recurrent disease; recurrent ameloblastoma may be difficult to treat (Oral Dis 2019;25:1683)
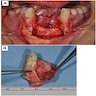
Gross description
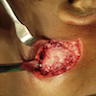
- General grossing tips for intraosseous ameloblastoma:
- Prior to sectioning bone, review preoperative radiograph to evaluate for presence and location of any pre-existing metal hardware, titanium dental implants or impacted teeth that could interfere with bone sectioning, could increase difficulty with bone sectioning and be potentially hazardous if unexpectedly encountered
- If possible, recommend submitting at least 1 block as nondecalcified tumor specimen for immunohistochemistry or molecular studies
- Grossly exposed tumor or tumor fragmentation should be documented
- Shaving bone margins for odontogenic neoplasms discouraged:
- Difficulty in measuring extent of tumor from bone margins
- Incidental histologic findings can confound bone margin assessment (Int J Surg Pathol 2020;28:507)
- Ameloblastoma, conventional type:
- Viscous mucoid fluid present on cut surface
- Macrocystic degeneration: larger cystic spaces, may contain clear or red-brown fluid
- Solid, multiple cystic spaces or a combination thereof
- Resorbs tooth roots
- Note expansion or extension beyond bone
- May be associated with impacted tooth
- In mandible, note relation to inferior alveolar nerve if applicable
- Ameloblastoma, unicystic type:
- If surgically removed by enucleation, very little bone may present
- Evaluation of margins precluded by enucleation or curettage type of treatment
- When resected for margins with surrounding bone, unicystic gross appearance is conspicuous and may contain red-brown fluid
- If no mural growth identified on representative sections, consider submitting entire lesion
Gross images
Frozen section description
- Architectural organization preserved
- Subnuclear vacuolization difficult to appreciate
Frozen section images
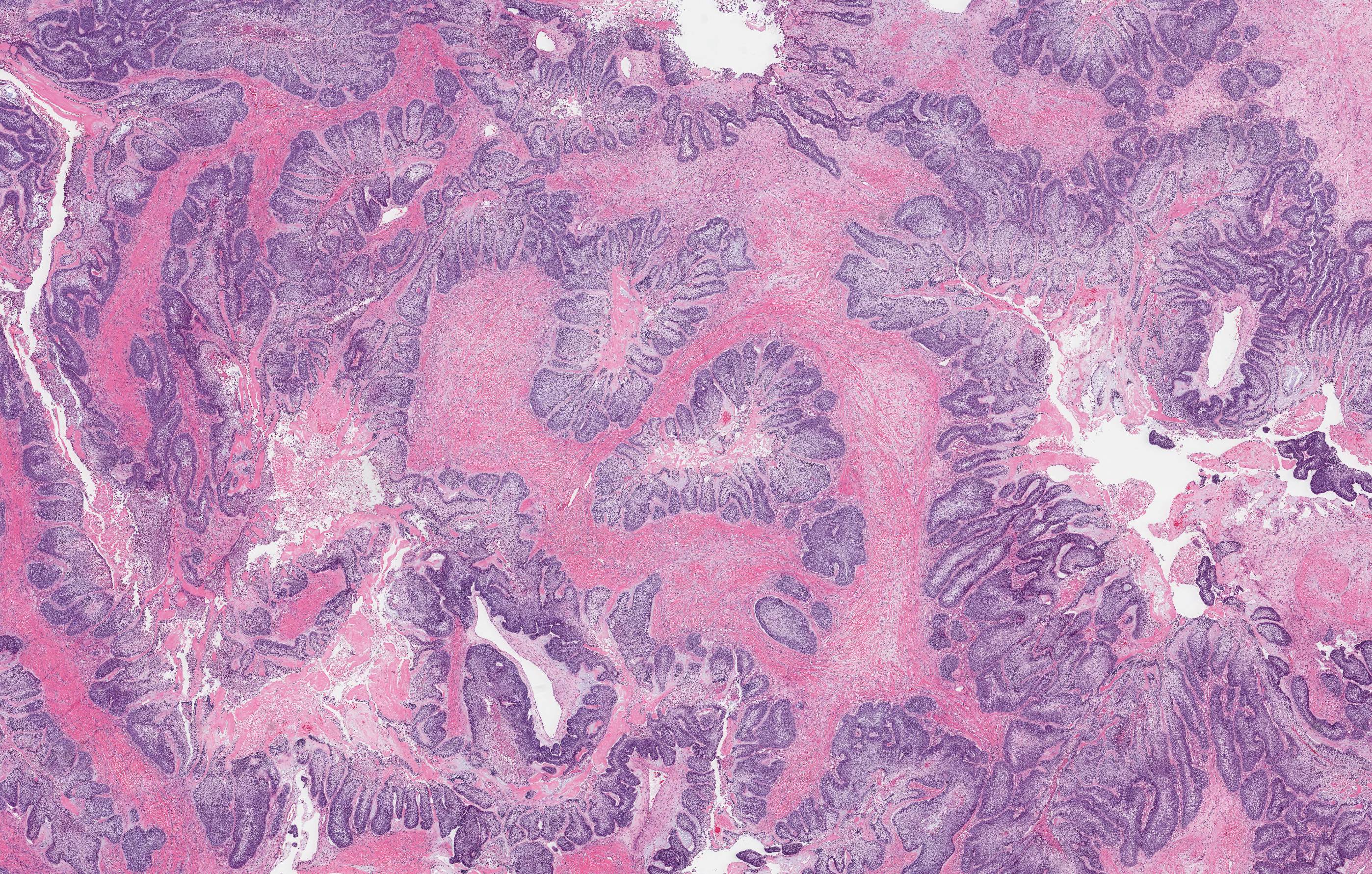
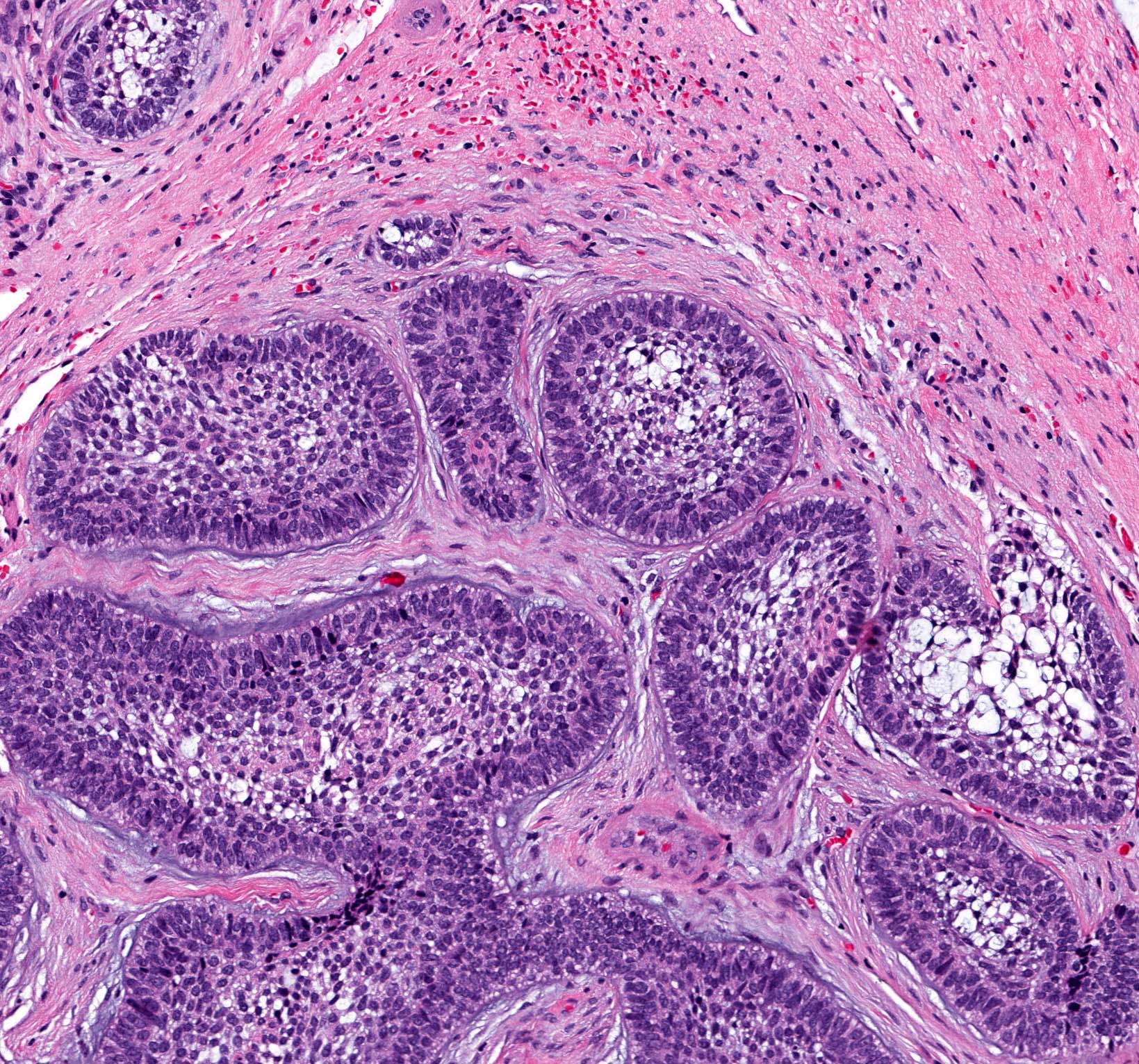
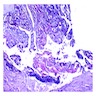
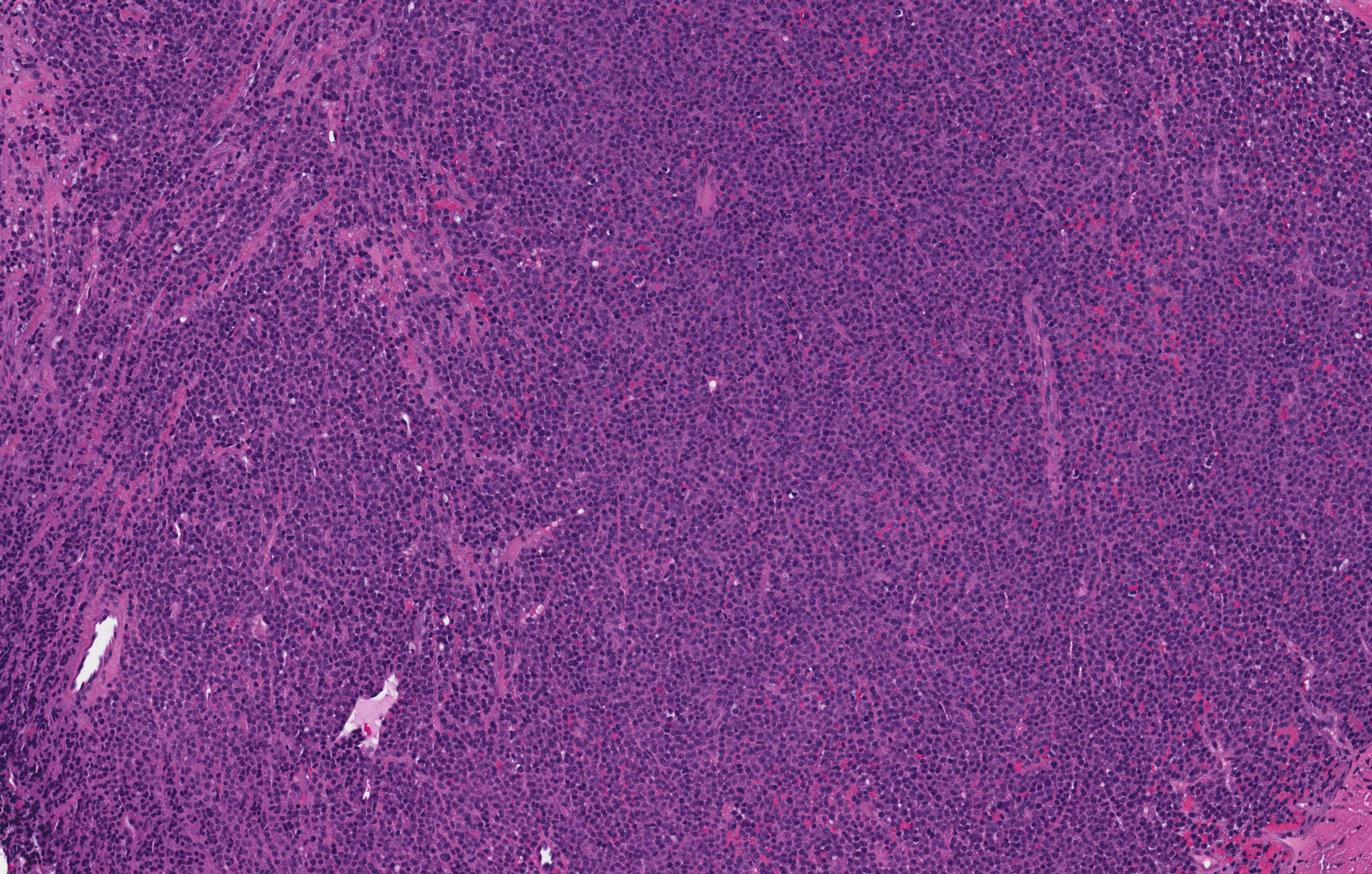
Microscopic (histologic) description
- Within the epithelial islands and cords of conventional ameloblastoma and the cystic epithelial lining of unicystic ameloblastoma, the odontogenic epithelium shows similar changes:
- Columnar cells with hyperchromatic nuclei at basal layer, exhibiting peripheral palisading
- Cells show reverse polarization away from basement membrane (Vickers-Gorlin change)
- Subnuclear vacuolization
- Suprabasal cells with a loose, network-like arrangement, recapitulating stellate reticulum formation seen in normal odontogenesis
- No dentin or enamel formation
- Ameloblastoma, conventional type may show macrocystic change of the tumor islands; when a limited portion of this phenomenon is sampled at initial biopsy, the appearance may suggest an odontogenic cyst, NOS or ameloblastoma, unicystic type
- Rarely, involvement of the inferior alveolar nerve has been reported in benign, conventional ameloblastoma (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:557, Br J Oral Maxillofac Surg 2013;51:757)
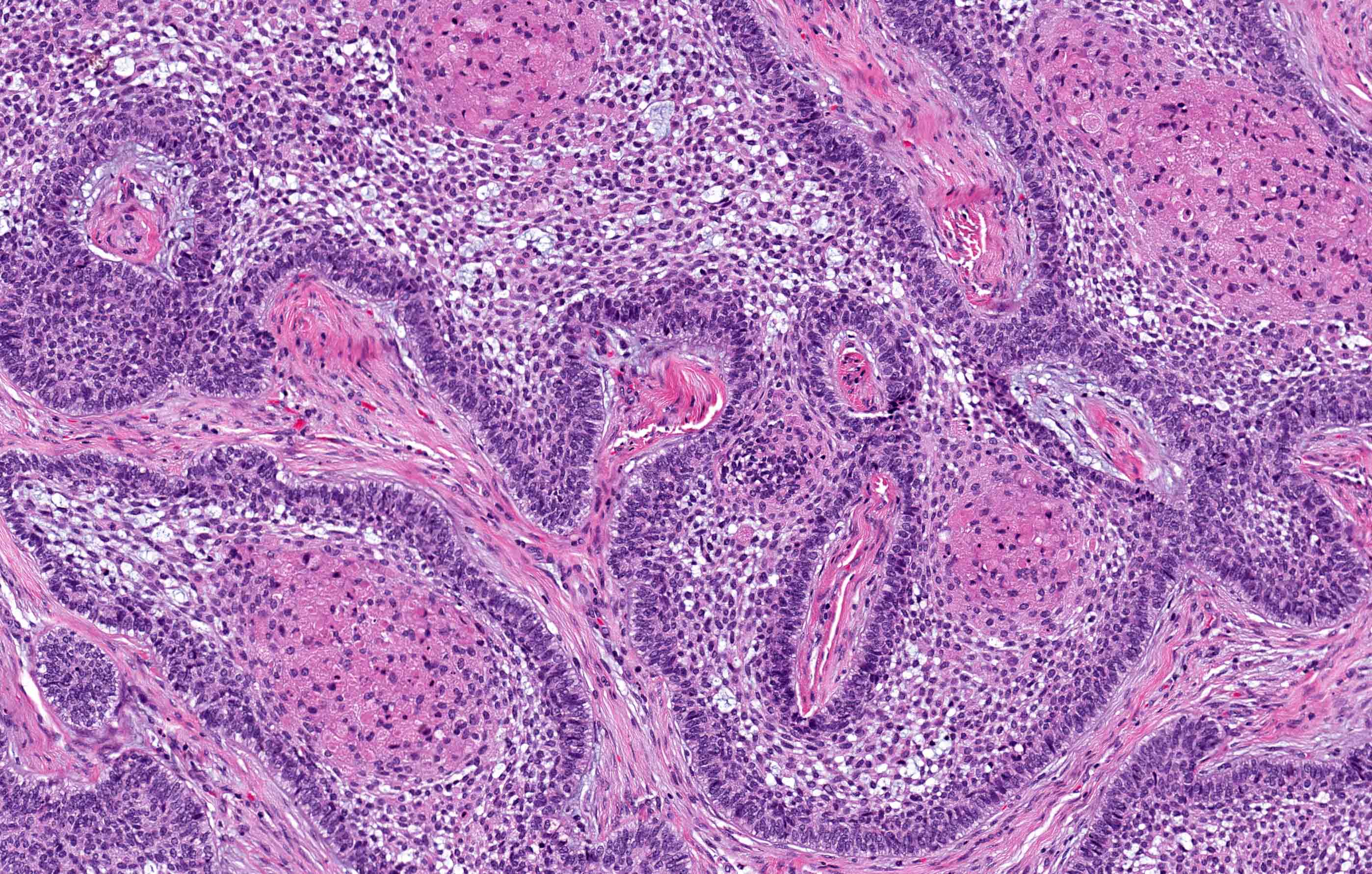
- Ameloblastoma, conventional type has at least 6 histopathological patterns
- Single patterns may predominate within a given lesion, often mixed with 1 or more patterns
- Microscopic pattern has no documented prognostic significance
- Follicular: most common subtype; islands of odontogenic epithelium in fibrous connective tissue; may be cystic; classic peripheral palisading and stellate reticulum-like areas
- Plexiform: cords and sheets of anastomosing odontogenic epithelial cells; classic peripheral palisading and reverse polarity not always obvious
- Acanthomatous: squamous metaplasia and variable keratinization of stellate reticulum-like cells
- Granular cell: stellate reticulum-like cells have granular eosinophilic cytoplasm; less commonly involves cells at periphery of nests
- Basal cell / basaloid: least common histologic subtype; islands of hyperchromatic basal cells without stellate reticulum-like areas
- Desmoplastic: compressed and angular islands of epithelial tumor cells with dense moderately cellular fibrous connective tissue or collagenous stroma; the formation of metaplastic bone trabeculae is also described
- Ameloblastoma, unicystic type has 3 histopathological patterns
- Single cystic lesion lined by ameloblastic epithelium that shows typical features of ameloblastoma in some areas, including columnar basal cells in palisading arrangement with vacuolated cytoplasm, hyperchromatic nuclei polarized away from basement membrane
- Suprabasal cells loosely textured and noncohesive resembling stellate reticulum, epithelial invagination, epithelial edema and separation
- Microscopic variants (may result in treatment differences - controversial)
- Luminal: cystic odontgenic epithelium with characteristic features (above) lining fibrous connective tissue wall
- Intraluminal: cystic odontgenic epithelium with characteristic features (above) lining fibrous connective tissue wall, with tumor extending into the cystic luminal space; may have intraluminal plexiform patterns
- Mural: cystic odontgenic epithelium with characteristic features (above) lining fibrous connective tissue wall but with the additional finding of definite ameloblastoma tumor islands within the fibrous connective tissue wall
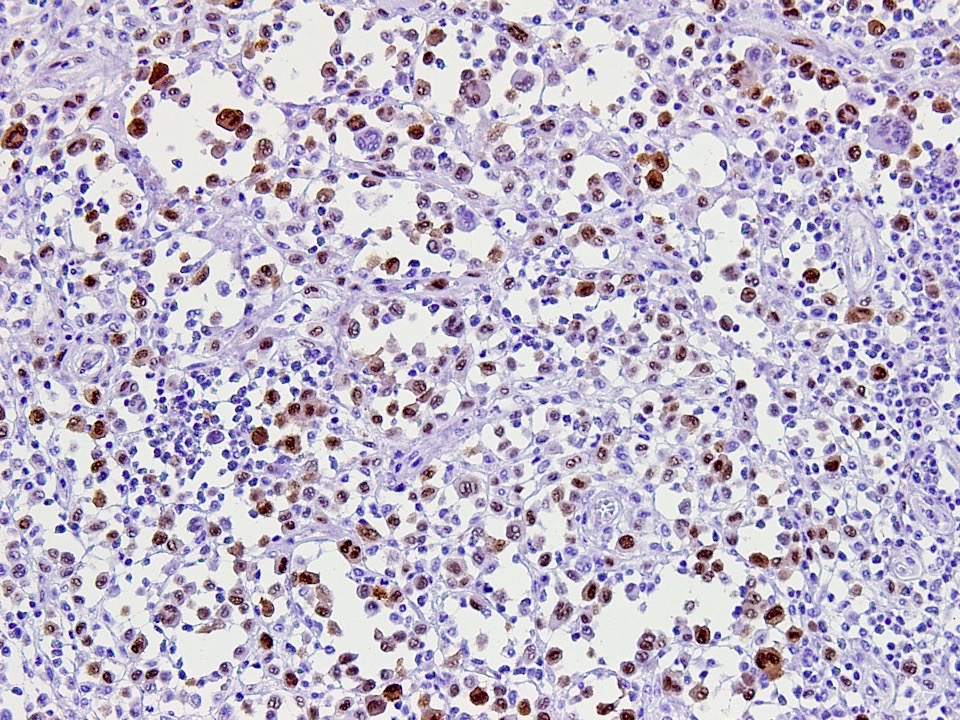
Microscopic (histologic) images
Contributed by Kelly Magliocca, D.D.S., M.P.H. and Anne C. McLean-Holden, D.M.D., M.S.
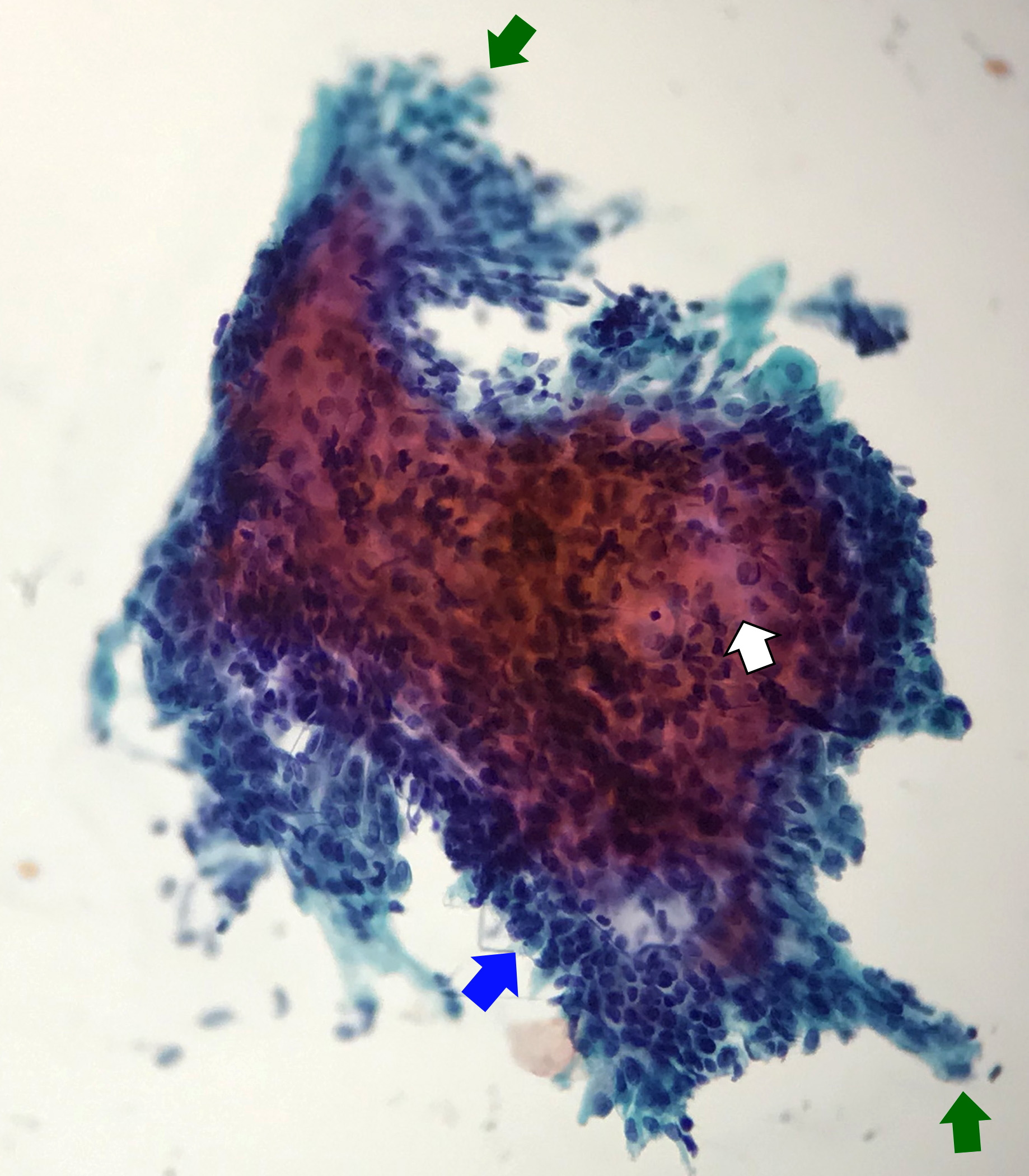
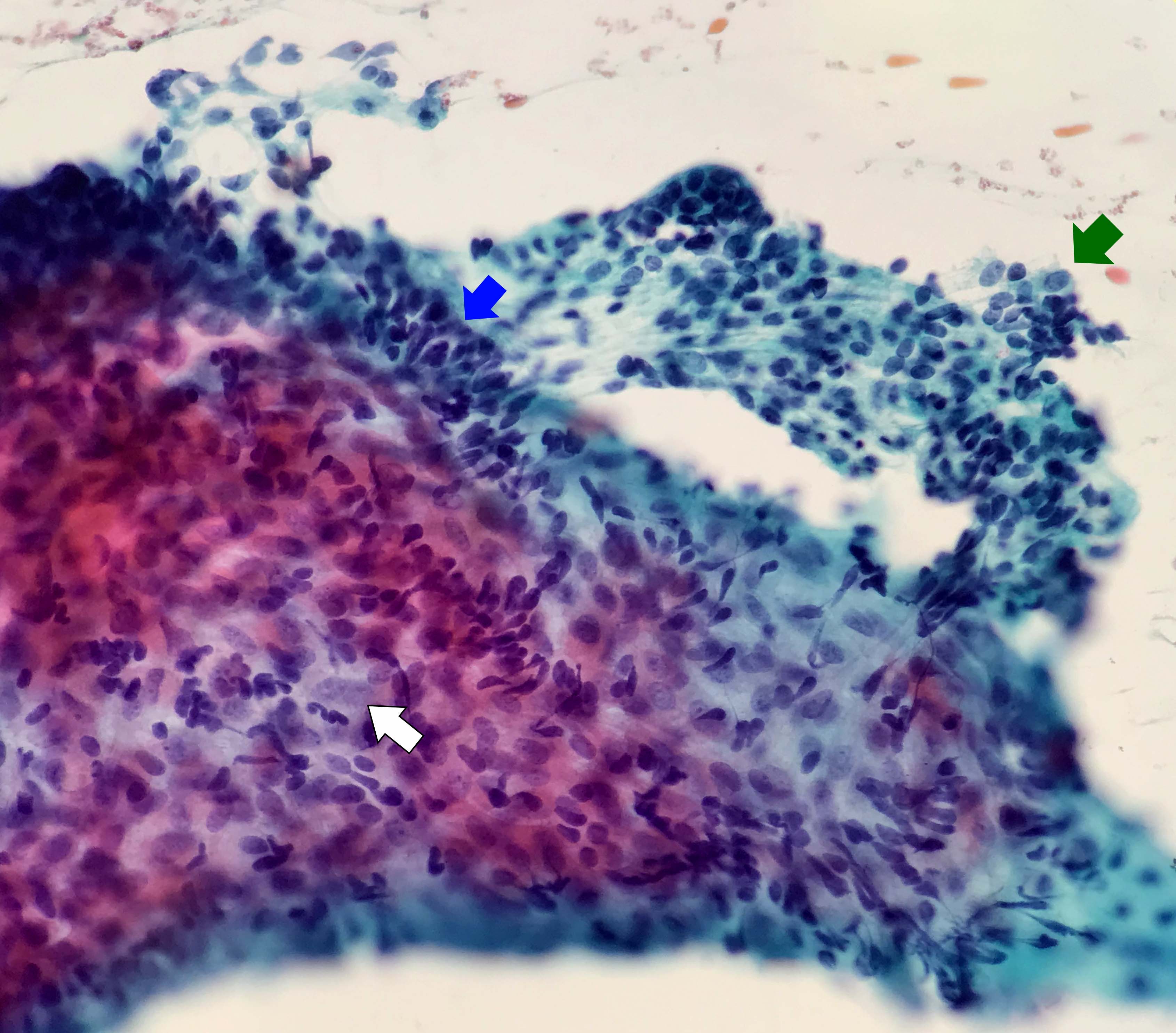
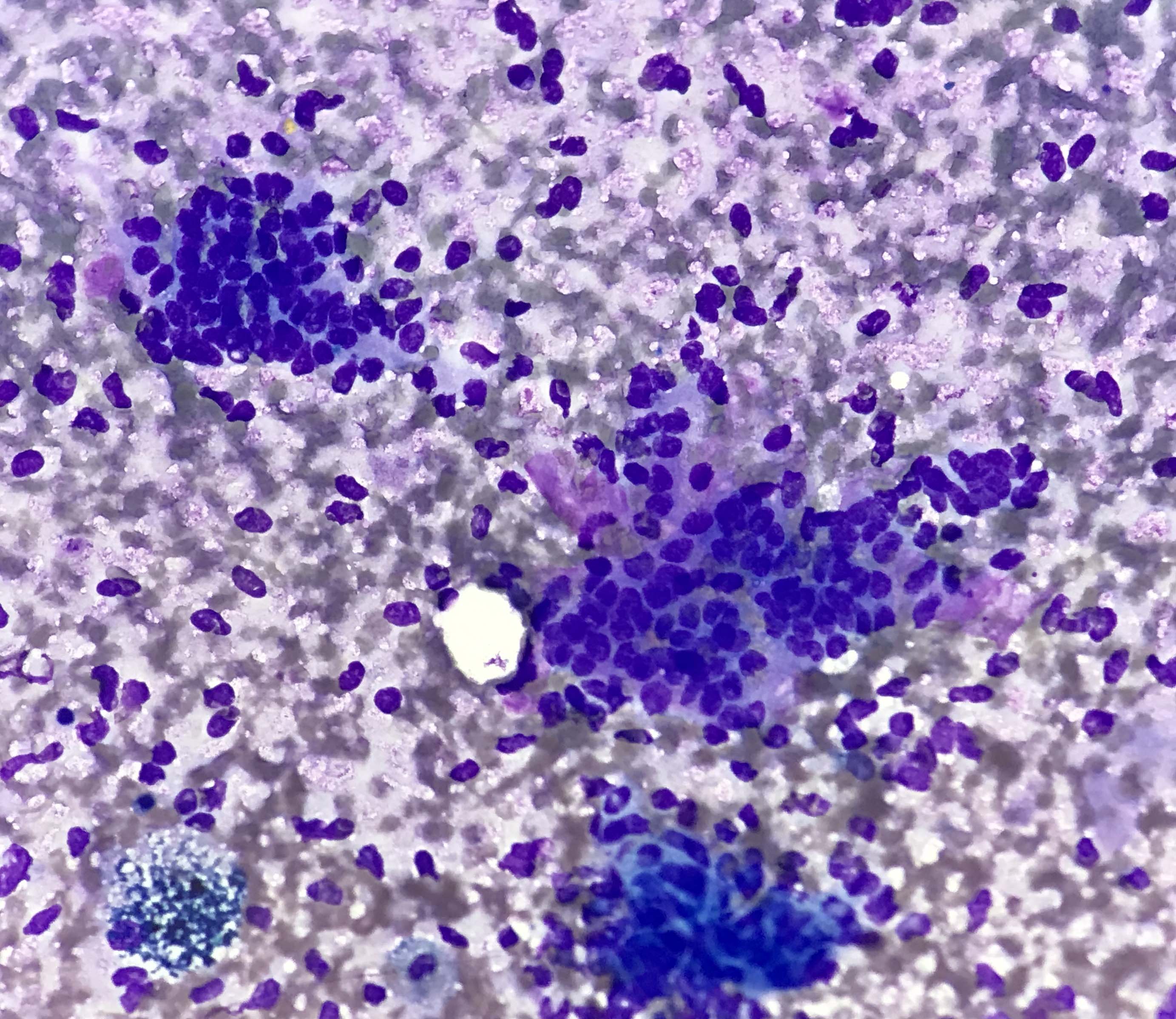
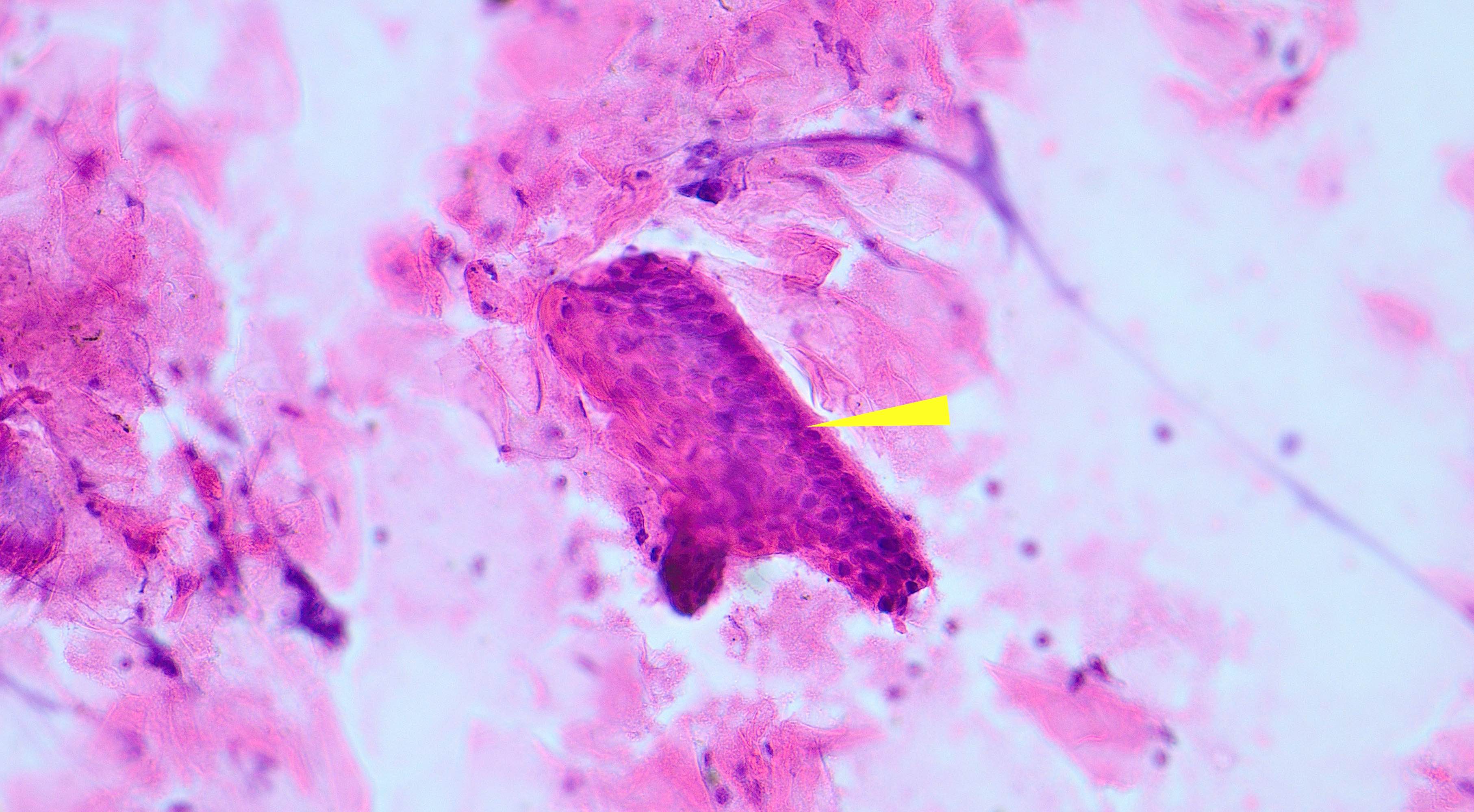
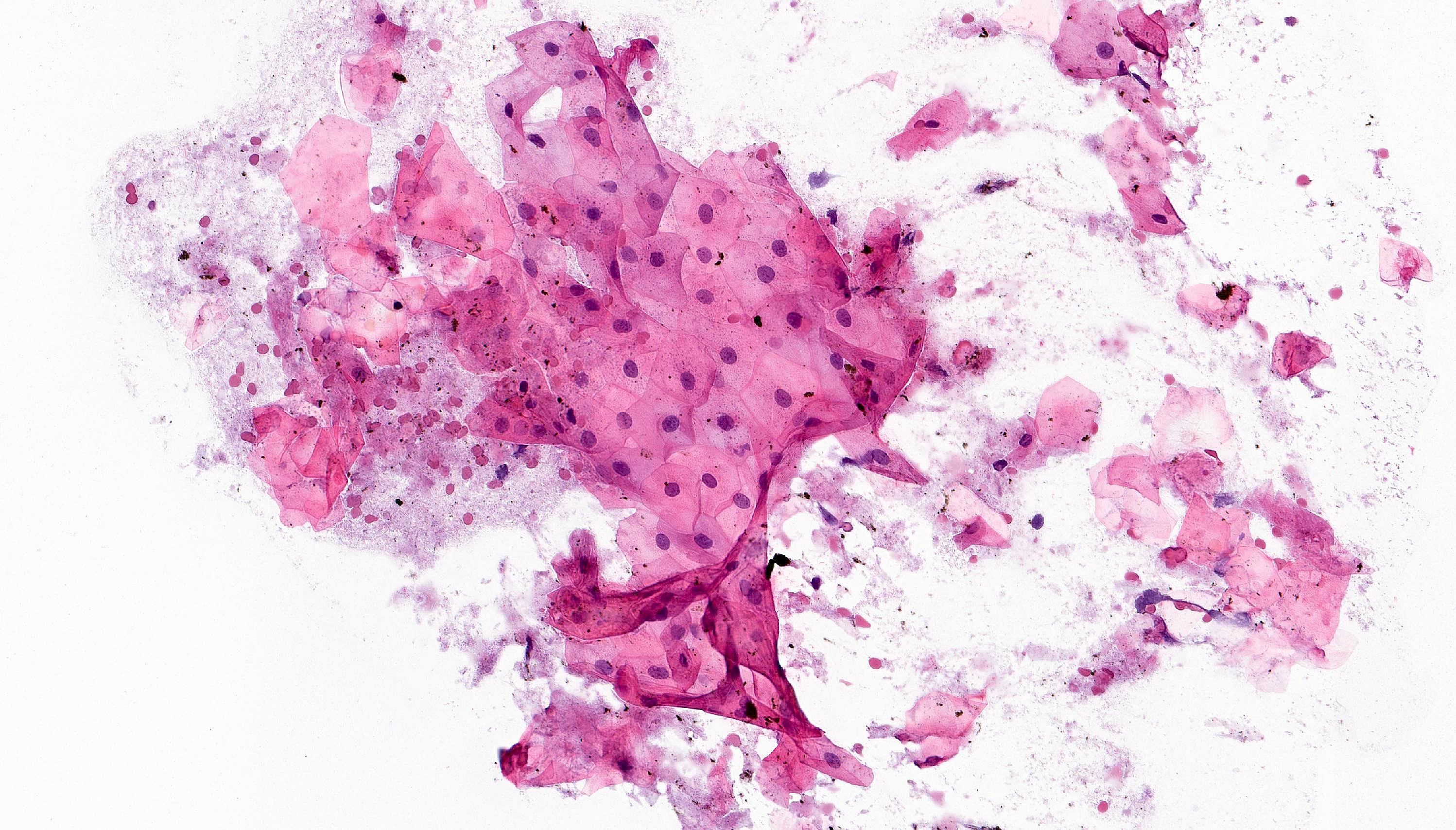
Cytology description
- Compact and cohesive cell clusters with areas of short branching of tumor cells along the periphery (Diagn Cytopathol 2013;41:206)
- Oval to round basaloid cells with high nuclear to cytoplasmic ratios, fine powdery chromatin, nucleoli with small peripheral nucleoli to inconspicuous nucleoli
- Squamous cells centrally with more abundant cytoplasm (particularly in acanthomatous variant)
- Possible limitations to FNA
- Inadequate sampling due to extensive cyst formation within the tumor
- Inability to distinguish ameloblastoma with macrocystic degeneration from ameloblastoma, unicystic type
- Inability to distinguish ameloblastoma from metastatic ameloblastoma without prior knowledge of metastatic disease
- Inability to distinguish ameloblastoma from other benign odontogenic tumors that are managed with curettage (e.g. adenomatoid odontogenic tumor, ameloblastic fibroma) (Diagn Cytopathol 2013;41:206)
Positive stains
- CK19 and CK14 (J Oral Pathol Med 2016;45:704)
- CK5 (J Oral Pathol Med 2008;37:177)
- CD56 (Histopathology 2010;57:544)
- Calretinin (Histopathology 2001;38:312, Histopathology 2000;37:27)
- BRAF V600E (VE1) (Clin Oral Investig 2019;23:779)
- Beta catenin nuclear positive exceptionally rare (PLoS One 2017;12:e0180224)
- p63 and p40 (Oral Surg Oral Med Oral Pathol Oral Radiol 2018;126:506)
- FOXP1 (Am J Surg Pathol 2020;44:665)
Negative stains
- Uniformly SOX10 negative (Am J Surg Pathol 2020;44:665)
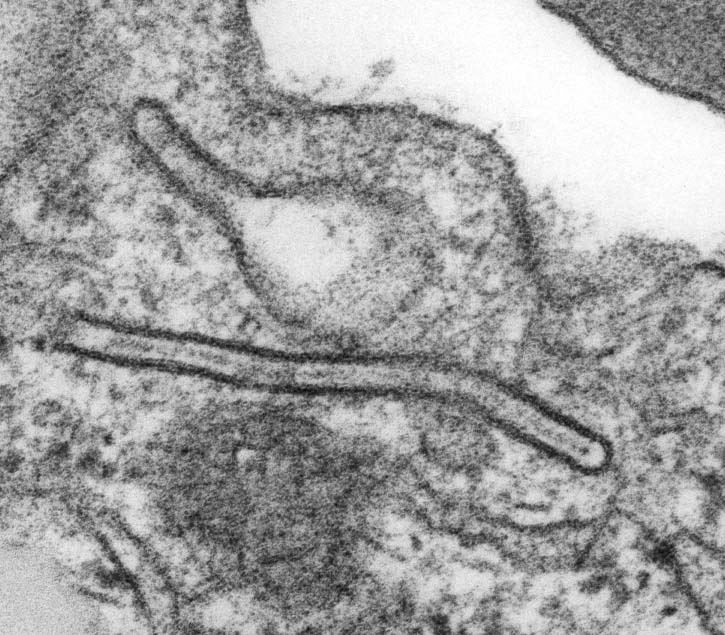
Electron microscopy description
- Epithelial differentiation (tonofilaments, complex desmosomes)
- Ultrastructural analyses have previously shown the presence of lysosomal aggregates in the cytoplasm of granular cells and apoptotic cell fragments were detected between these cells within the neoplastic clusters, corroborating this hypothesis (J Oral Pathol Med 2001;30:245)
Molecular / cytogenetics description
- Recurrent somatic and activating mutations in mitogen activated protein kinase (MAPK) pathway present in ~80% ameloblastomas identified in 2014 by 3 separate groups
- BRAF V600E (most common mutation; at least 60%)
- RAS and FGFR2
- Hedgehog signaling pathway; appear mutually exclusive from MAPK
- In a recent study, 94% of the mandibular unicystic ameloblastomas revealed BRAF V600E mutations; in 1 BRAF wild type mandibular tumor, an SMO p.L412F mutation was identified
Videos
Overview of odontogenic tumors by Dr. Khurram
Sample pathology report
- Mandible, left segmental mandibulectomy:
- Ameloblastoma (2.3 cm), confined to bone (see comment)
- Comment: Tumor measures 0.9 cm from anterior bone margin and 0.6 cm from posterior bone margin.
Differential diagnosis
- Differential diagnosis of ameloblastoma, conventional type
- Odontogenic fibroma:
- Short cords of odontogenic epithelium embedded in a moderately cellular fibrous / fibroblastic stroma
- No perineural or skeletal muscle invasion is seen
- Ameloblastic fibroma:
- Odontogenic epithelial component may exhibit peripheral palisading, reverse polarization and stellate reticulum-like areas
- Primitive appearance to stroma is essential to the diagnosis; evenly dispersed delicate spindled cells in an amphophilic background
- BRAF pV600E mutations described (Oral Oncol 2015;51:e77)
- Most common in children
- Adenomatoid odontogenic tumor:
- Benign odontogenic epithelial tumor with basaloid duct-like structures, lined by cuboidal or columnar cells
- May show focal reverse polarity
- Duct-like spaces contain eosinophilic secretions
- Amyloid-like material may be present
- Predilection for anterior maxilla, often associated with unerupted tooth
- 67% of patients are female
- Adenomatoid odontogenic tumor usually occurs in second decade
- See Case #490
- Squamous odontogenic tumor:
- Benign odontogenic epithelial tumor with squamous differentiation
- Jigsaw puzzle-like proliferation of squamous epithelial islands without atypia
- Lacks peripheral palisading or stellate reticulum-like areas
- Very rare; thought to arise from rests of Malassez in periodontal ligament
- Dentinogenic ghost cell tumor:
- Can have histologic features overlapping with ameloblastoma; is a solid proliferation of odontogenic epithelium with peripheral columnar or cuboidal basal cells with stellate reticulum-like central areas
- Will have ghost cells or anucleate epithelial cells
- Dentinoid present
- Beta catenin immunohistochemical stain positive
- BRAF pV600E has not been detected
- Sclerosing odontogenic carcinoma:
- Short epithelial nests and thin epithelial cords consisting of bland cuboidal or polygonal epithelial cells with cytoplasmic clearing within sclerotic stroma
- Mitoses are difficult to find; however, infiltration of skeletal muscle and perineural invasion is conspicuous
- Clear cell odontogenic carcinoma:
- 3 histopathologic patterns have been described for clear cell odontogenic carcinoma: clear cell, squamoid appearing and ameloblastoma-like
- Classically, a monophasic, clear cell population of epithelial cells are arranged in nests and cords
- Hyalinized connective tissue often separate the clear cell nests
- Eosinophilic cells represent the dominant cell type within the squamoid variant
- Third pattern has a resemblance to ameloblastoma, since peripheral cells within tumor islands may demonstrate palisading
- EWSR1 mutated in majority of cases
- 3 histopathologic patterns have been described for clear cell odontogenic carcinoma: clear cell, squamoid appearing and ameloblastoma-like
- Ameloblastic carcinoma:
- May show features of amelobastoma, such as peripheral palisading, reverse polarity of basal cells, stellate reticulum-like areas
- Features of malignancy include cytologic atypia, high N/C ratio, increased mitotic figures with atypical forms or necrosis
- Odontogenic fibroma:
- Differential diagnosis of ameloblastoma, unicystic type
- Dentigerous cyst:
- Benign odontogenic epithelial cyst
- Inflamed, stratified squamous epithelial lining, versus 2 - 4 layers of cuboidal epithelium, devoid of superficial keratinization (uninflamed dentigerous cyst)
- No rete ridges, flat interface
- Fibrous to fibromyxoid connective tissue
- BRAF negative (J Oral Pathol Med 2016;45:780)
- Reference: J Investig Clin Dent 2014;5:220
- Odontogenic keratocyst (OKC):
- Benign odontogenic epithelial cyst
- Stratified squamous epithelial lining characterized by palisaded hyperchromatic basal cell layer comprised of cuboidal cells
- Uniform epithelial lining 6 - 8 cells thick lacking rete ridges
- May have areas of budding growth from the basal cells
- Luminal surface has wavy ("corrugated") parakeratotic epithelial cells
- Lumen may contain keratinaceous debris
- May have artifactual clefting between epithelium and underlying fibroconnective tissue
- In contrast to unicystic ameloblastoma, BRAF pV600E mutations would be considered rare, if ever, in bona fide OKC
- Calcifying odontogenic cyst:
- Benign odontogenic epithelial cyst
- Well defined layer of palisading basal cells, loosely arranged suprabasal epithelial cells, resembling stellate reticulum similar to ameloblastoma
- Unlike ameloblastoma, variable numbers of lesional epithelial cells undergo ghost cell change in suprabasilar epithelium
- Epithelium also contains pale, eosinophilic ghost cells that may keratinize or calcify; variable foreign body reaction
- Ghost cells: altered epithelial cells with preservation of basic cell outline, eosinophilic cytoplasm but loss of nucleus
- Dentinoid present to a variable degree; paucicellular, eosinophilic calcified material considered to represent dysplastic dentin
- Beta catenin nuclear positive
- BRAF negative (immunohistochemistry and molecular)
- Limited biopsy sampling of the macrocystic degeneration component of ameloblastoma, conventional type:
- Basal palisading with reverse polarity, hyperchromatic basal layer
- Stellate reticulum-like layer
- Larger sample can be helpful in distinguishing type
- Dentigerous cyst:
Additional references
Board review style question #1
Which of the following is true about microscopic variants of ameloblastoma?
- Multiple microscopic variants possible in the same tumor
- Multiple microscopic variants unlikely to occur in the same tumor
- Multiple microscopic variants within the same tumor is associated with hypercalcemia
- Multiple microscopic variants within same tumor suggests high grade transformation has occurred
Board review style answer #1
A. Multiple microscopic variants possible in the same tumor, as described in WHO 4th edition
Comment Here
Reference: Ameloblastoma
Comment Here
Reference: Ameloblastoma
Board review style question #2
Which of the following histologic patterns of ameloblastoma is most likely to be misidentified as an odontogenic cyst?
- Ameloblastoma, extraosseous peripheral type with follicular growth
- Ameloblastoma, granular cell variant histology
- Ameloblastoma, unicystic type with luminal growth
- Ameloblastoma with acanthomatous variant histology
Board review style answer #2
C. Ameloblastoma, unicystic type with luminal growth. Ameloblastoma, unicystic type lacks mural island formation, thus mimicking a cyst.
Comment Here
Reference: Ameloblastoma
Comment Here
Reference: Ameloblastoma
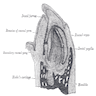
Anatomy & histology
Table of Contents
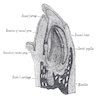
Anatomy | Development | Three phases of activity | Teeth | Ameloblasts | Cementicles | Cementum | Dental lamina | Dental pulp | Dentin | Enamel | Nests of Serres | Odontoblasts | Rests of Malassez | Drawings | Microscopic (histologic) description | Microscopic (histologic) imagesAnatomy
- Contain primitive embryonic structures from early fetal development to 25 years of age
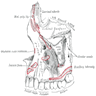
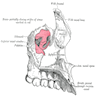
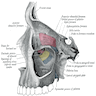
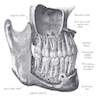
- Divided into alveolar bone (supports teeth) and body with variable thickness ranging from paper thin overlying roots of cuspid (canines) and bicuspid (premolar) teeth to thick at apex of chin
- Mandible
- Receptor for lower teeth
- Consists of curved horizontal body and two perpendicular rami
- Maxillae
- Forms upper jaw, boundaries of roof of mouth, floor and lateral wall of nose, floor of orbit
- Consists of zygomatic, frontal, alveolar and palatine processes
Development
- Primitive oral cavity (dental lamina, derived from ectoderm) invaginates, acquires a bell shape, and ameloblasts develop along inner (concave) surface; called "enamel organ" at this stage
- Dental papilla, derived from mesoderm, forms within "enamel organ", is loose stellate reticulum; its outer layer matures to become odontoblasts
- Dental sac or follicle forms from outer layer of dental papillae / odontoblasts, which form periodontium, a fibrous sheath enveloping the tooth
- Inner layer of dental sac becomes cementoblasts and deposit cement over newly formed dentin, and outer layer of dental sac becomes osteoblasts and produces alveolar bone
Three phases of activity
- Initiation of entire deciduous dentition (first set of teeth) during second month of gestation (above)
- Initiation of permanent dentition (second set of teeth) and extension of dental lamina distal to dental organ of second deciduous molar
- After 5 - 6 years of activity, dental lamina breaks up into epithelial rests or islands that may develop into odontogenic cysts or tumors
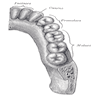
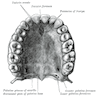
Teeth
- From medial to lateral:
- Incisors
- Canines (cuspids)
- Premolars (bicuspids)
- Molars
Ameloblasts
- Also called adamantoblasts
- Secrete enamel matrix
Cementicles
- Rounded, strongly basophilic structures normally within periodontal ligament
Cementum
- Similar to bone, cellular or acellular, intensely basophilic
- Also present in other parts of skeletal system
Dental lamina
- Epithelium with potential to develop teeth
- Derived from primitive oral epithelium along free margin of arches of jaws
Dental pulp
- Hypocellular, myxoid
- Resembles myxoma but is compact and has peripheral odontoblasts
Dentin
- Radially striated due to numerous minute canals (dentinal tubules) containing cytoplasmic processes from odontoblasts
- Resembles osteoid if tubules / canals are absent
Enamel
- Thin rods or prisms separated on cross section by concentric lines of Retzius
Nests of Serres
- Nests of ameloblasts, cementoblasts and odontoblasts located in alveolar mucosa, due to breakup of dental lamina
Odontoblasts
- Form dentinal matrix
Rests of Malassez
- Nests of ameloblasts, cementoblasts and odontoblasts embedded within periodontium
Drawings
Images hosted on other servers:
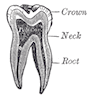
Microscopic (histologic) description
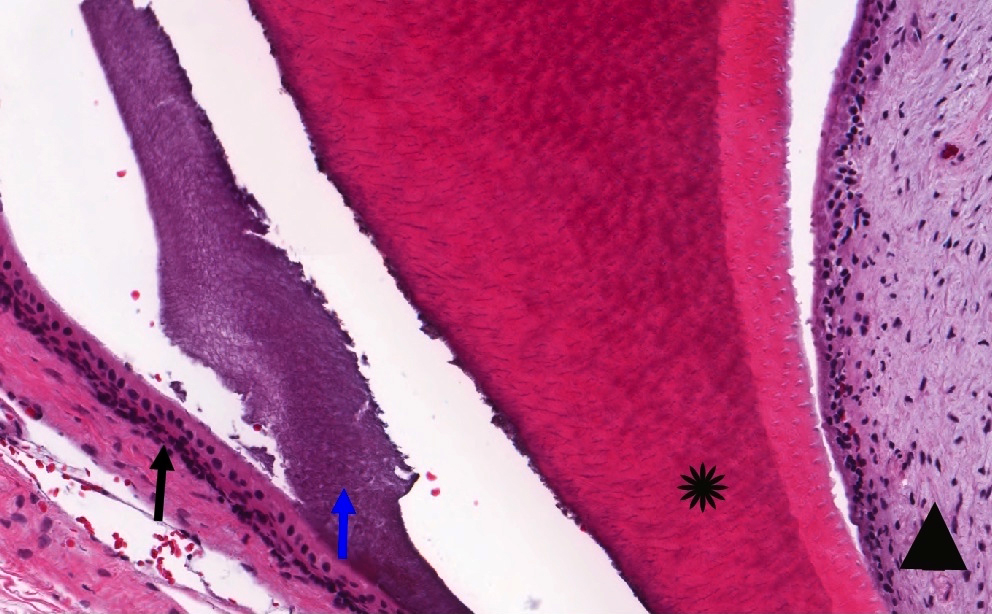
- Composed of outer enamel adjacent to dentin and inner pulp
- Surrounded by periodontal membrane and attached to bone by cementum
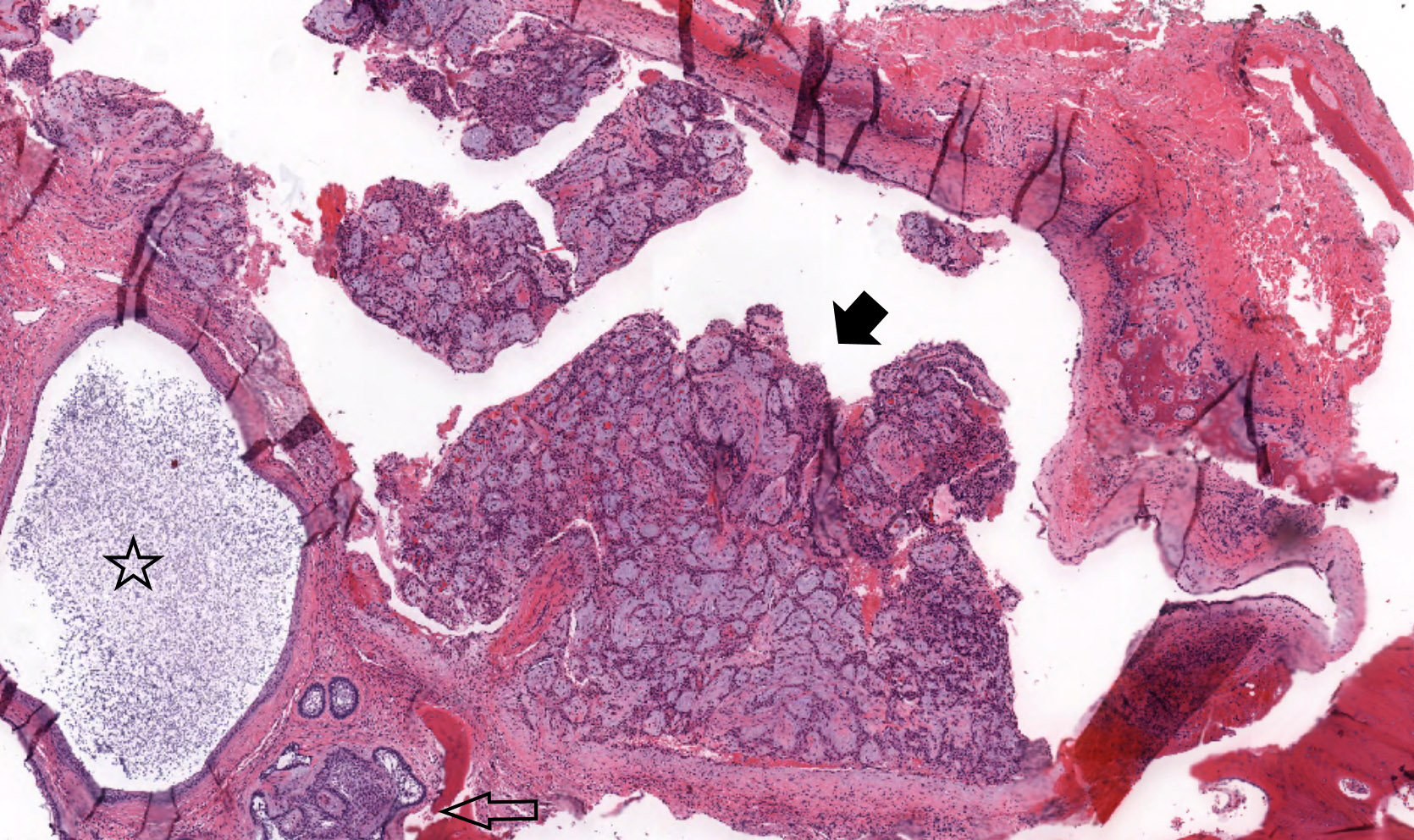
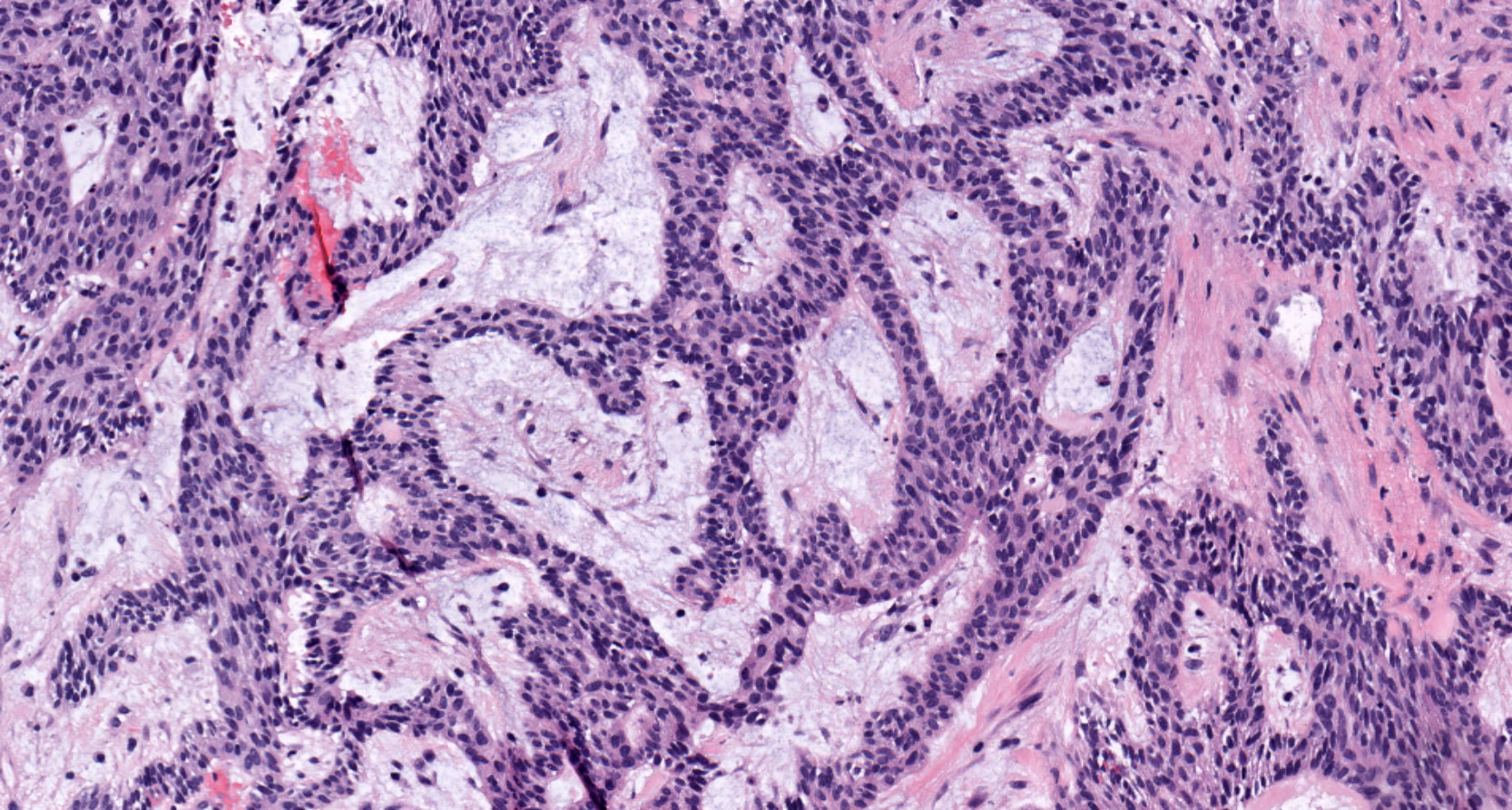
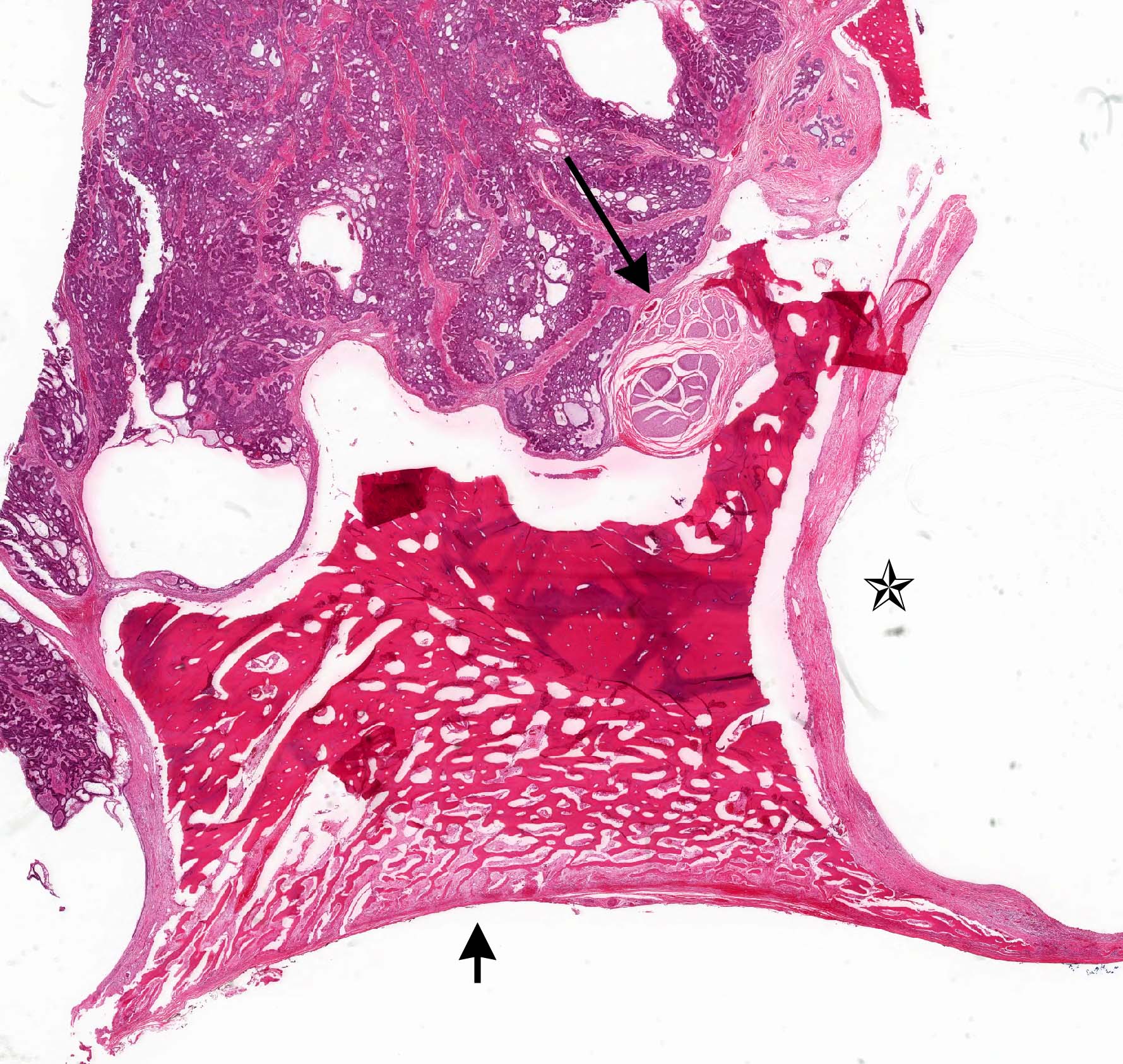
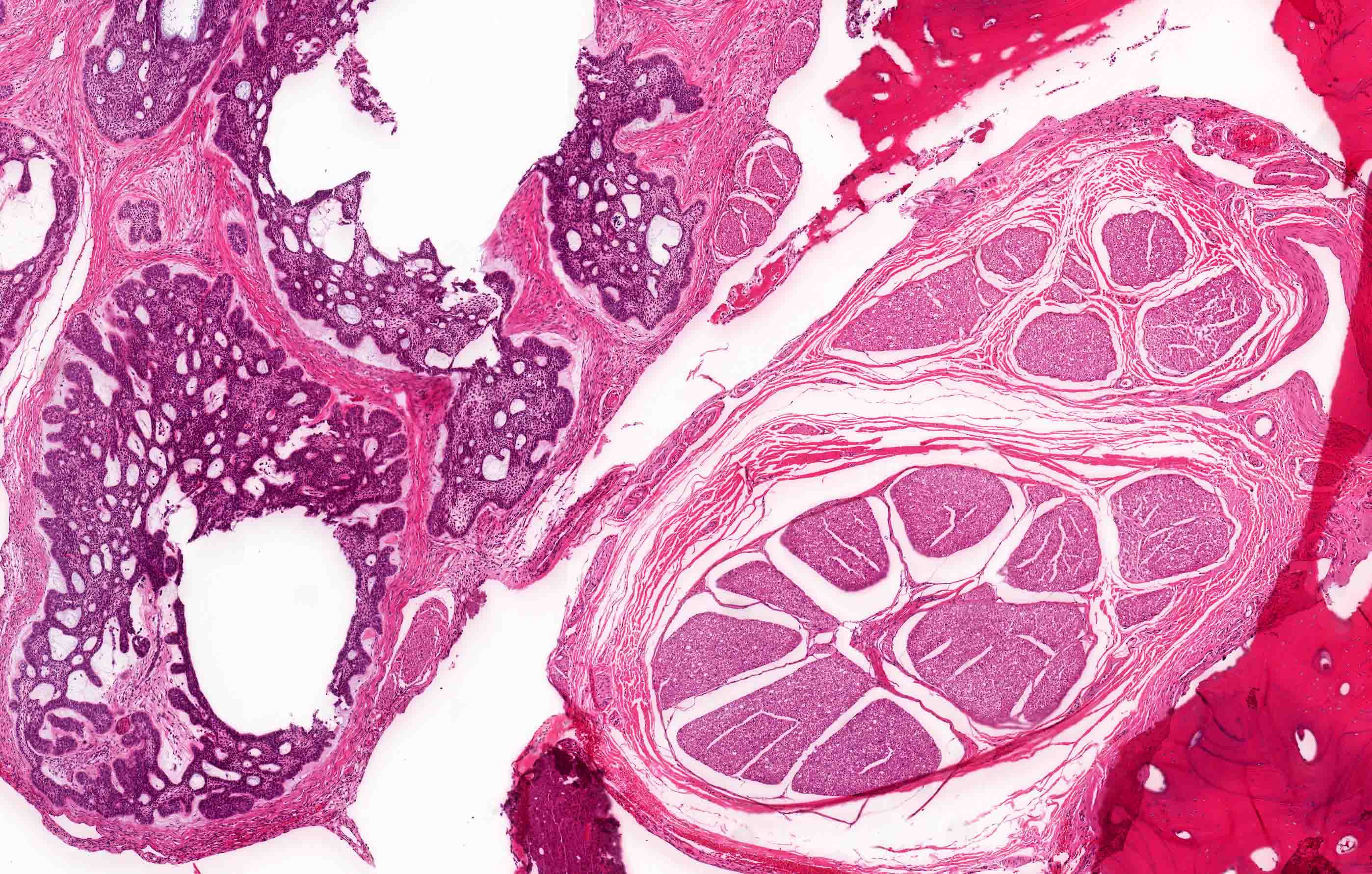
Calcifying epithelial odontogenic tumor
Table of Contents
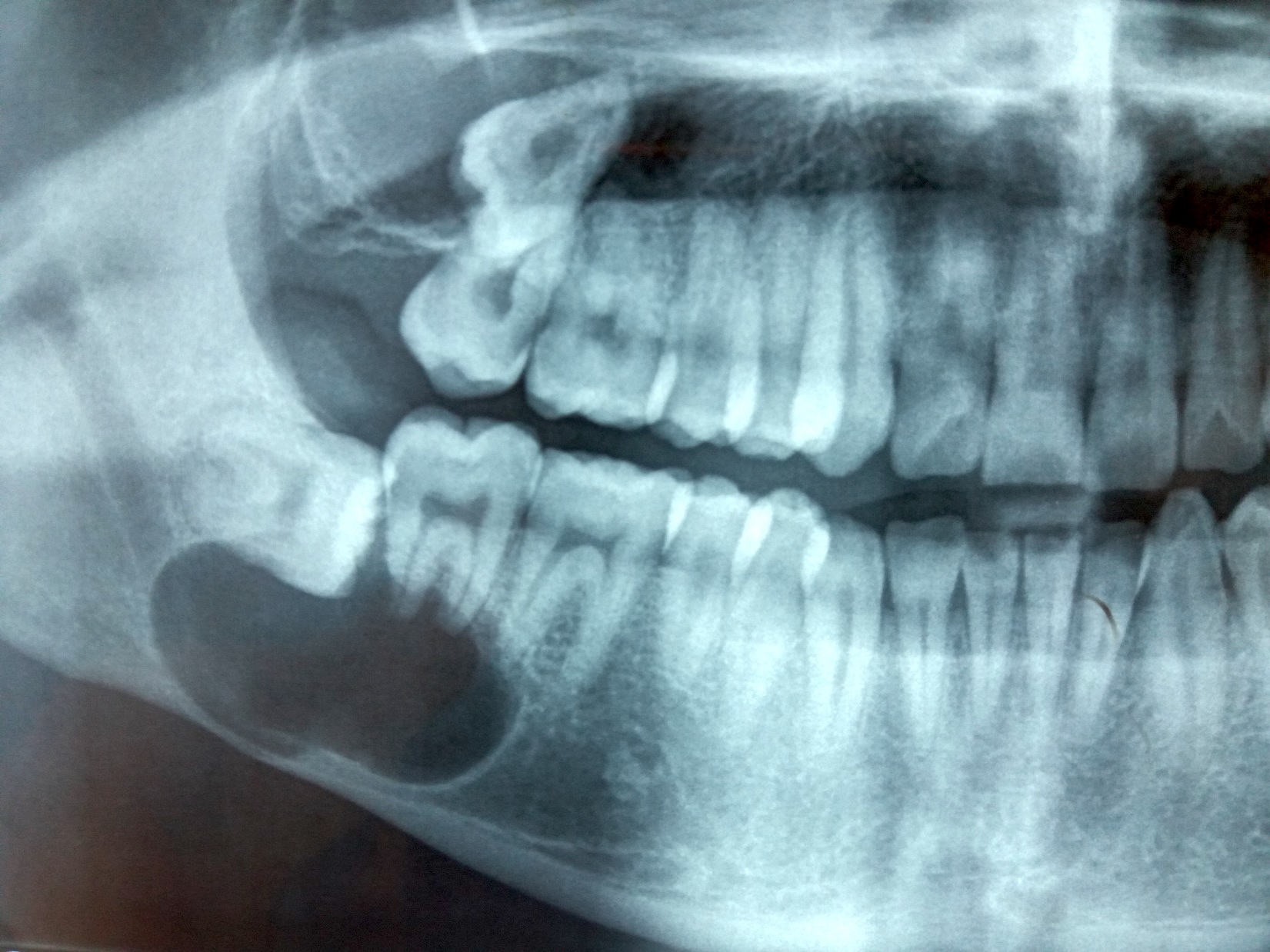
Definition / general | Sites | Radiology description | Treatment | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Positive stainsDefinition / general
- Also called Pindborg tumor
- Rare
- Ages 30 - 49 years
- May also occur within gingiva (peripheral tumor)
- May be invasive and recur locally, but less aggressive than ameloblastoma
Sites
- Premolar mandible
- Often associated with embedded tooth
Radiology description
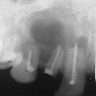
- Resembles dentigerous cyst with occasional small radiopacities within large radiolucent area
Treatment
- Less aggressive than ameloblastoma
Case reports
- 62 year old woman with amyloid deposit in calcifying epithelial odontogenic tumor that was immunoreactive for cytokeratins (Arch Pathol Lab Med 2000;124:872)
- 75 year old man with calcifying epithelial odontogenic tumor (J Oral Pathol 1984;13:310)
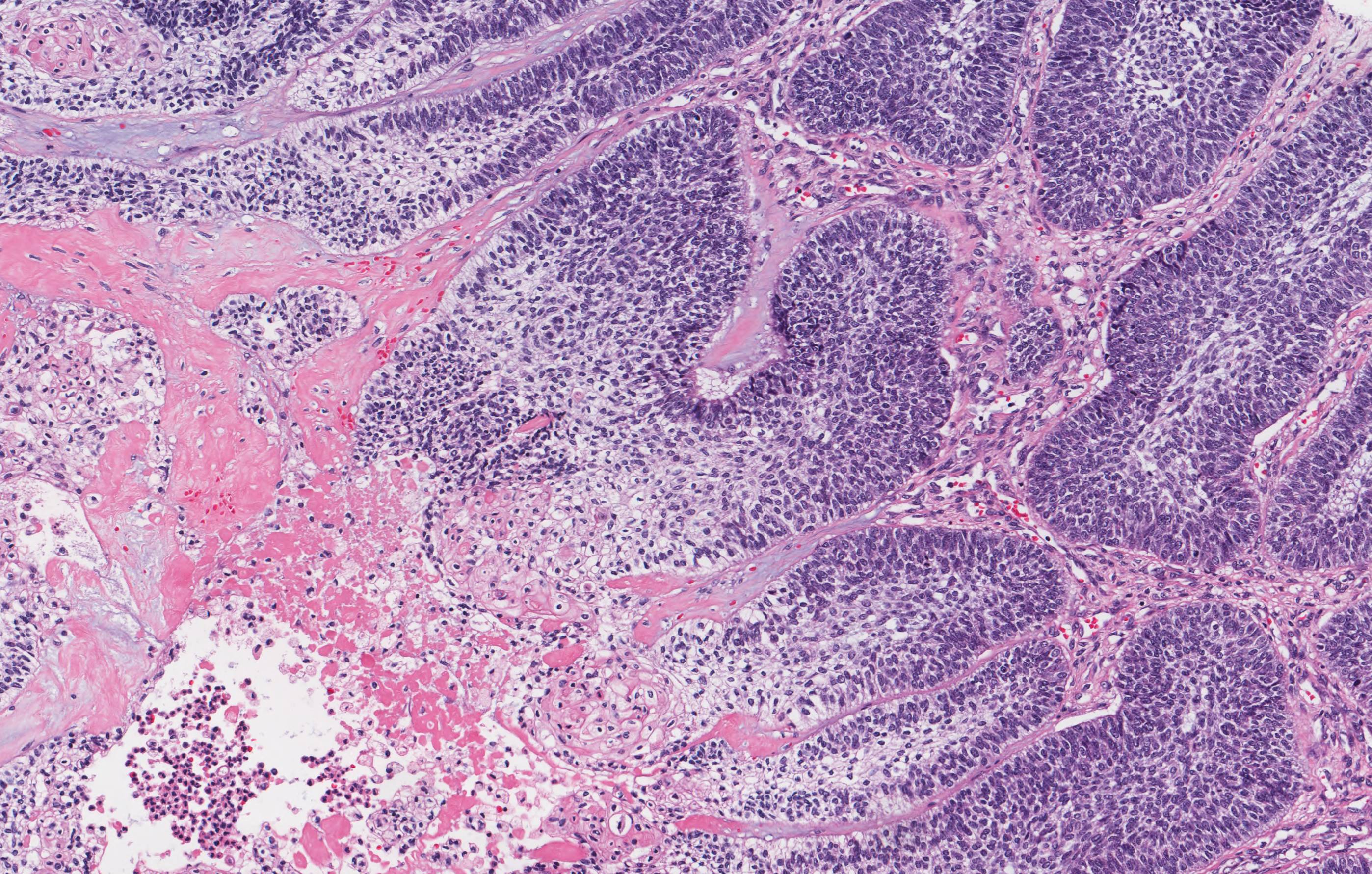
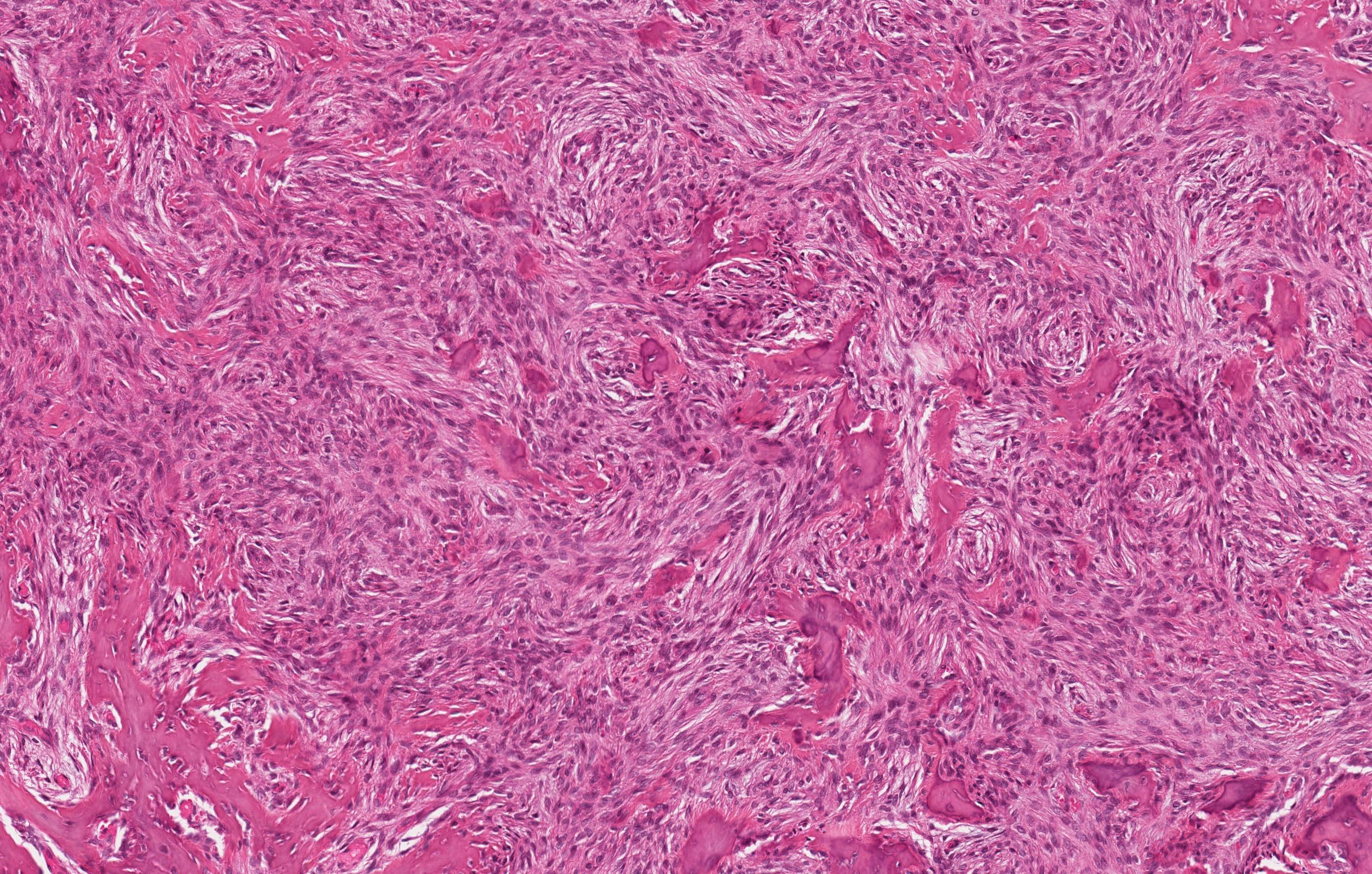
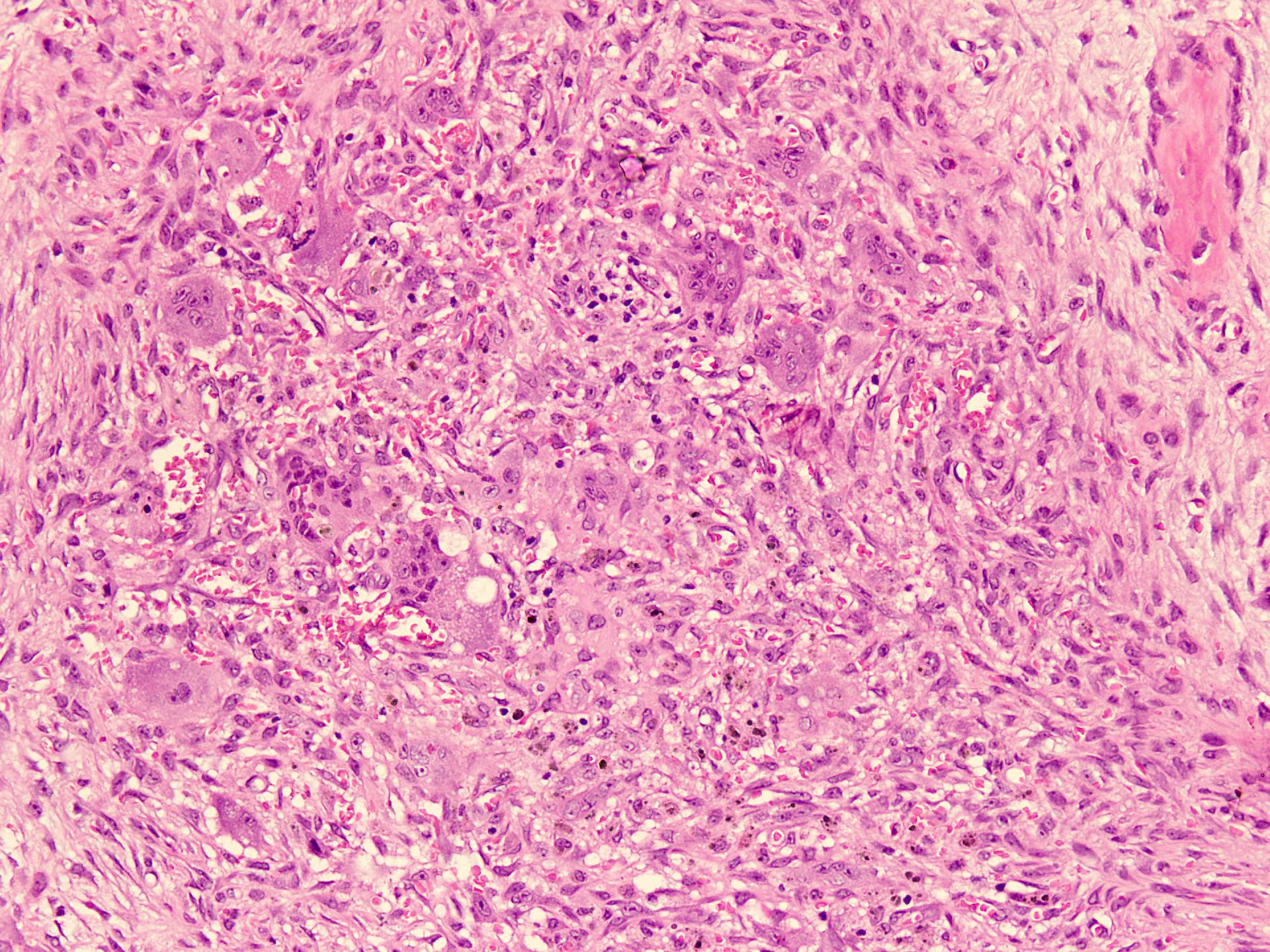
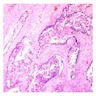
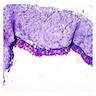
Microscopic (histologic) description
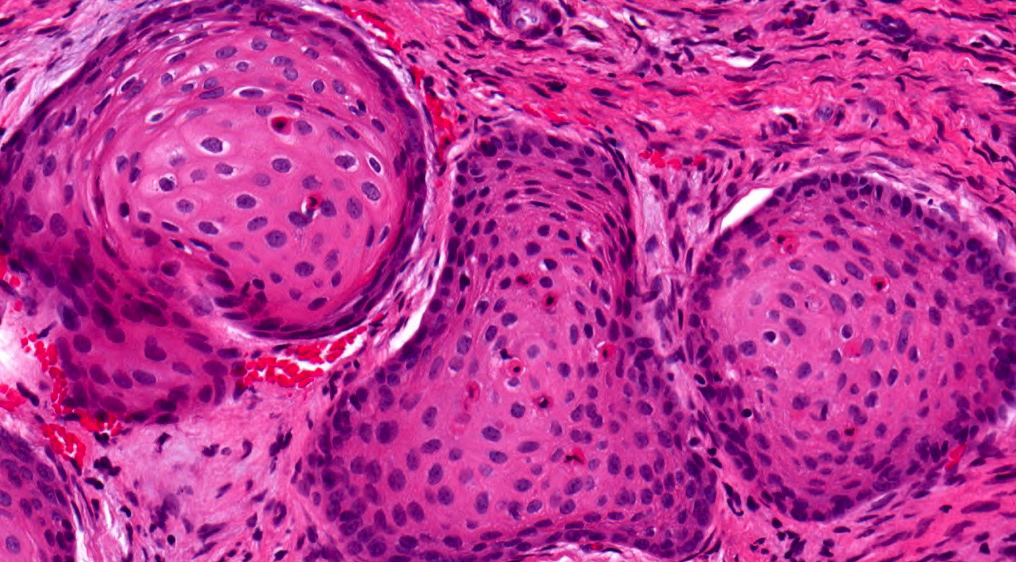
- Sheets of polyhedral, eosinophilic squamous epithelial cells with focal psammoma bodies
- Variable markedly pleomorphic cells with 2 - 3 nuclei
- Amyloid bodies (containing degenerated keratin filaments)
- Often scanty stroma, although epithelium / stromal ratio is variable between tumors
- Clear cell variant has clear vacuolated cytoplasm
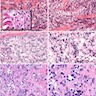
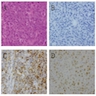
Microscopic (histologic) images
Calcifying odontogenic cyst
Table of Contents
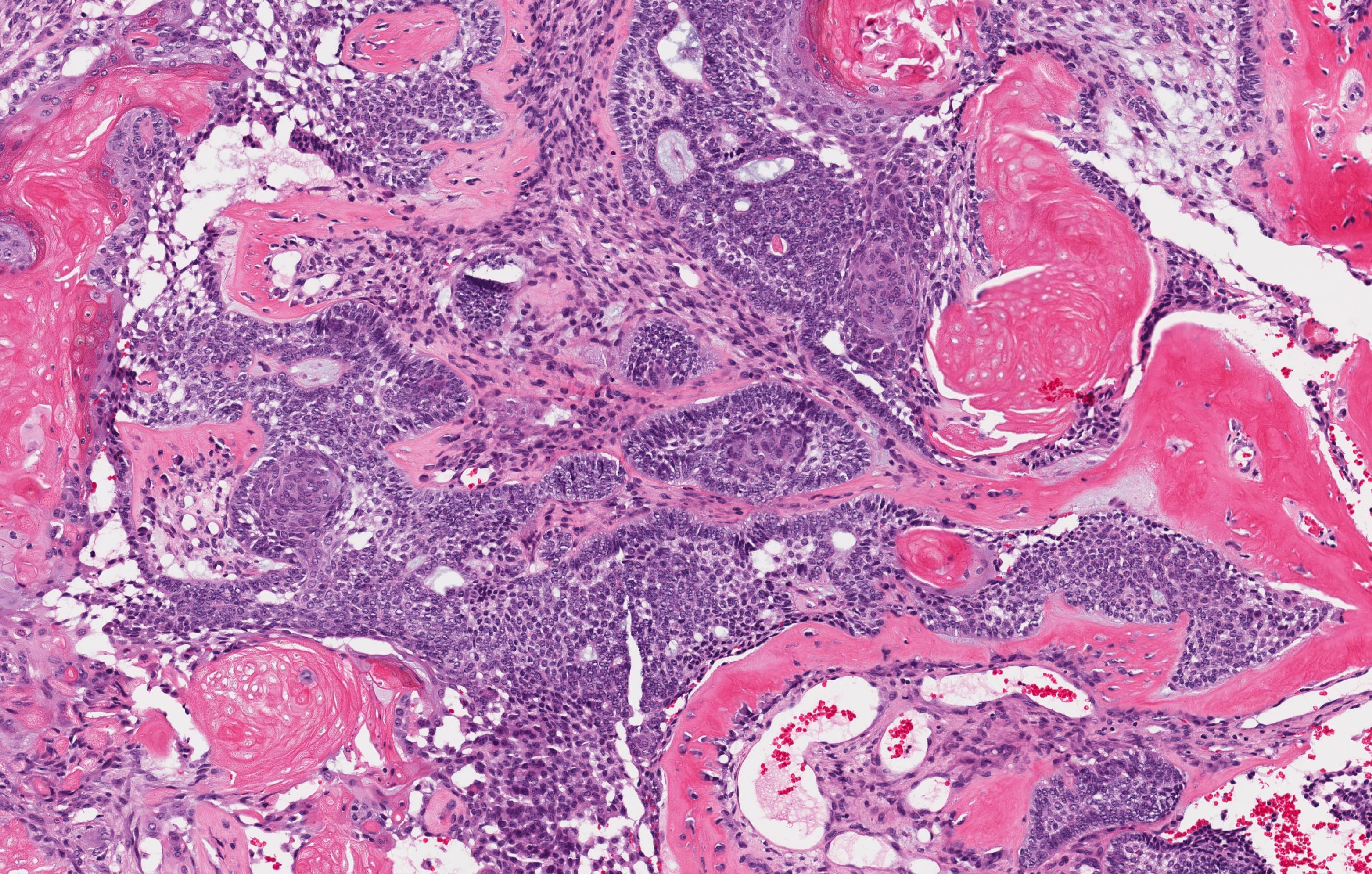
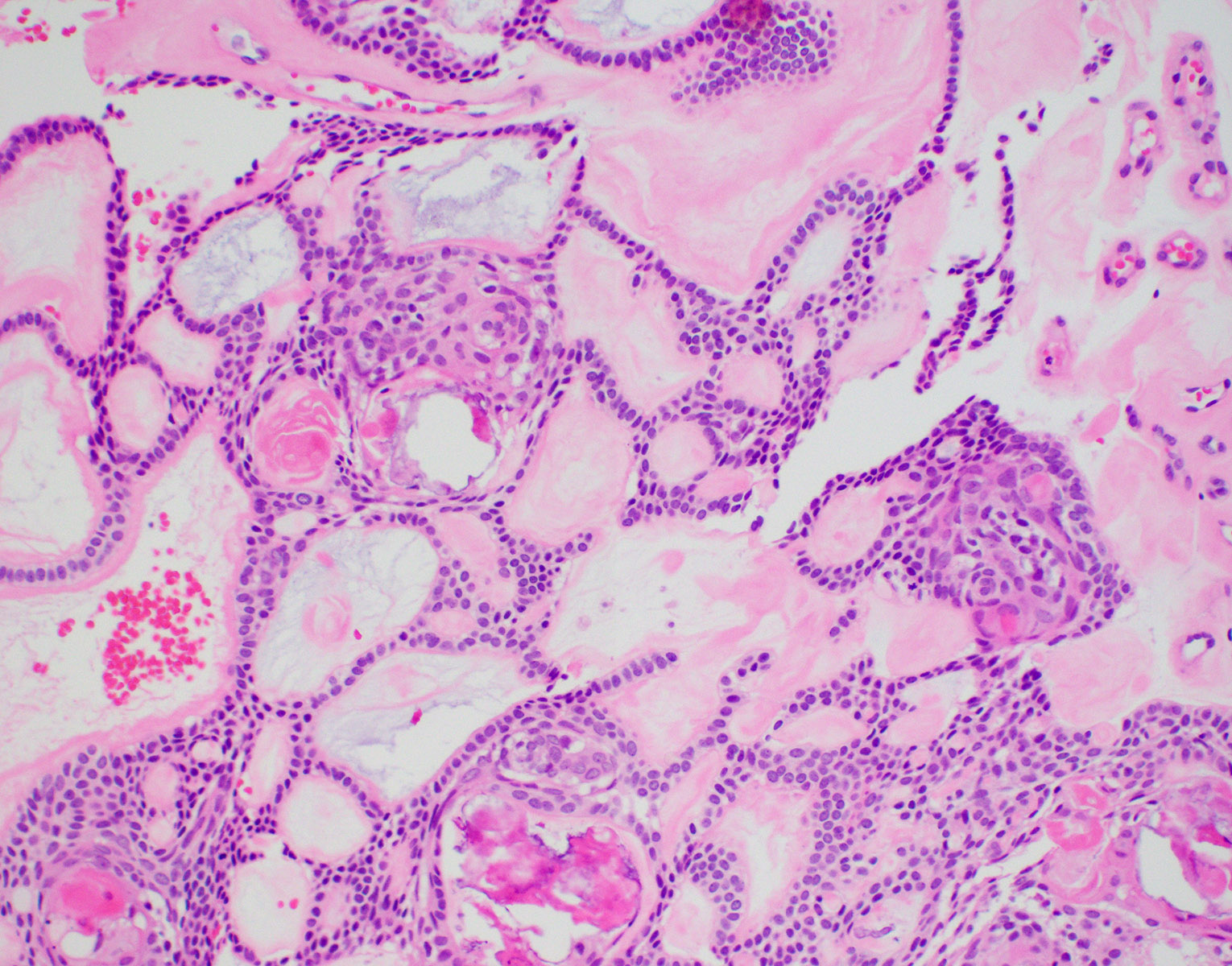
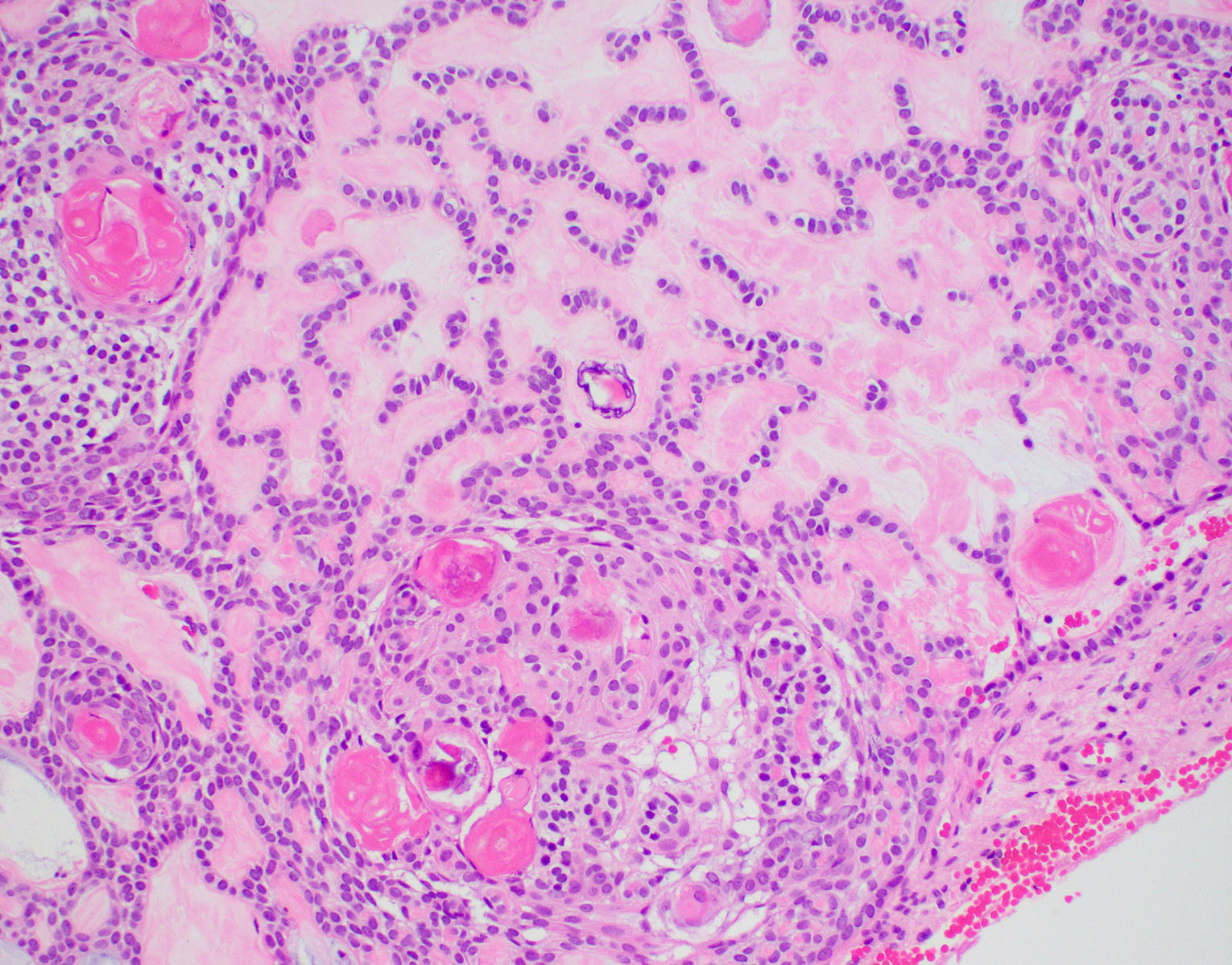
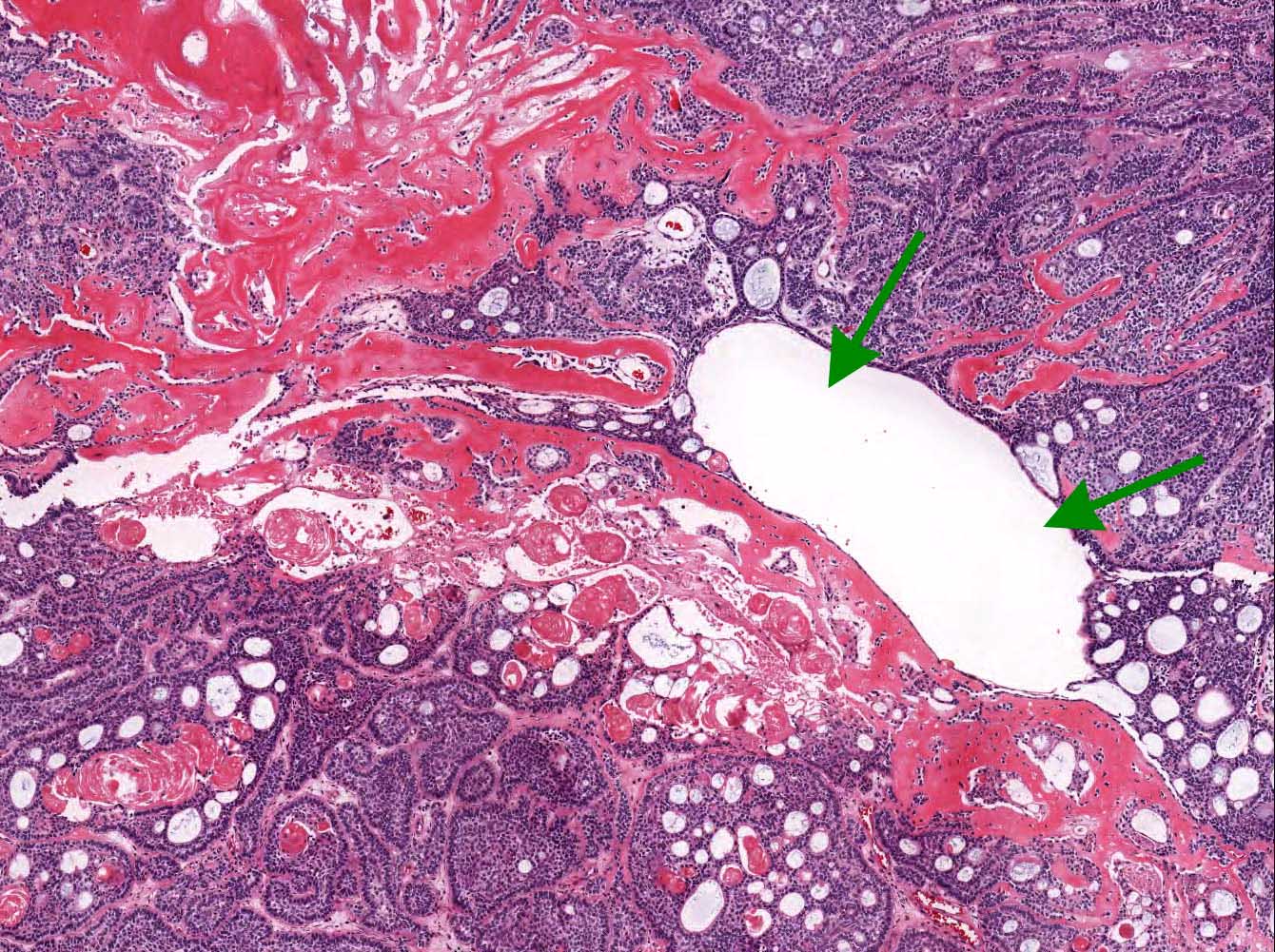
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
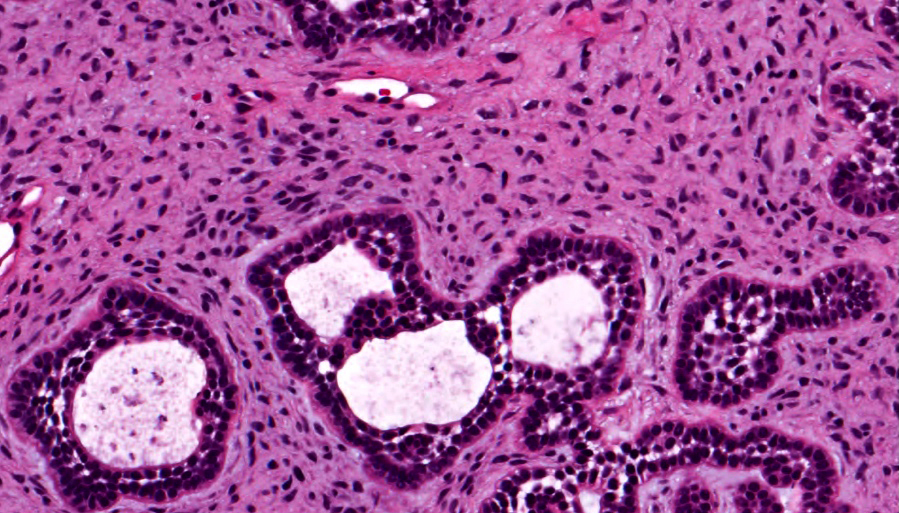
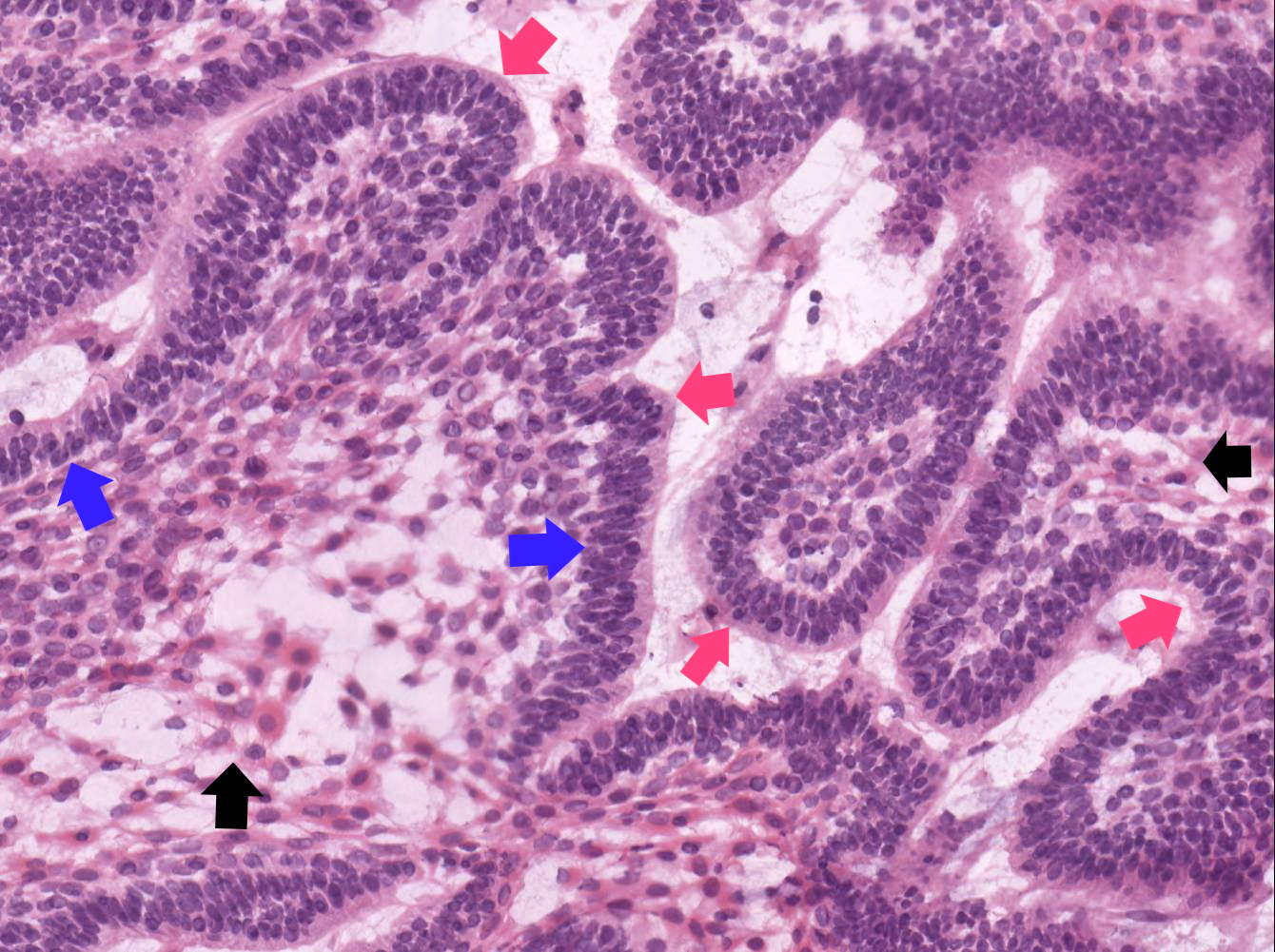
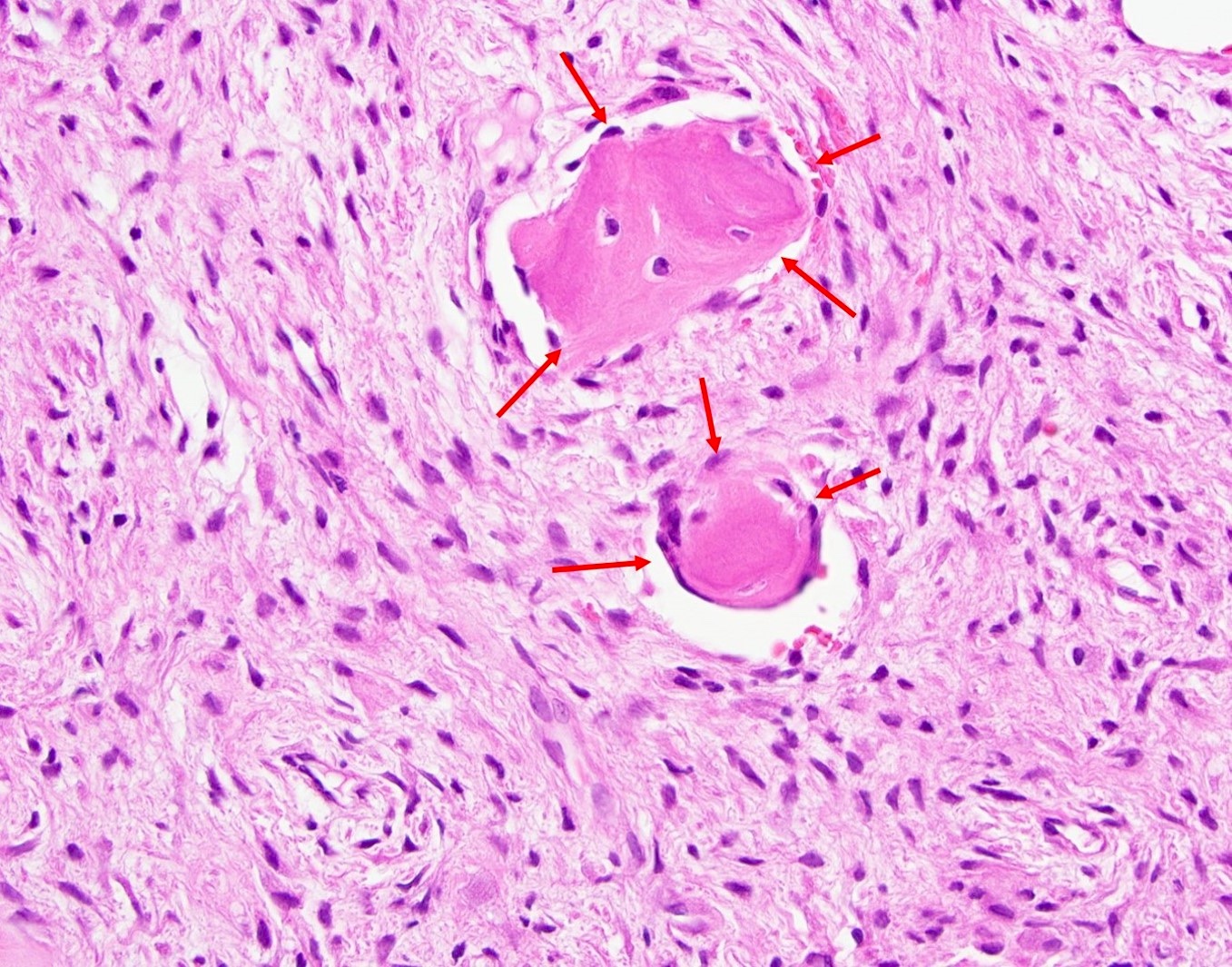
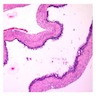
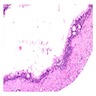
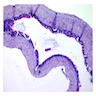
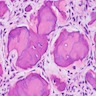
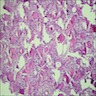
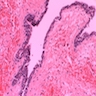
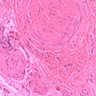
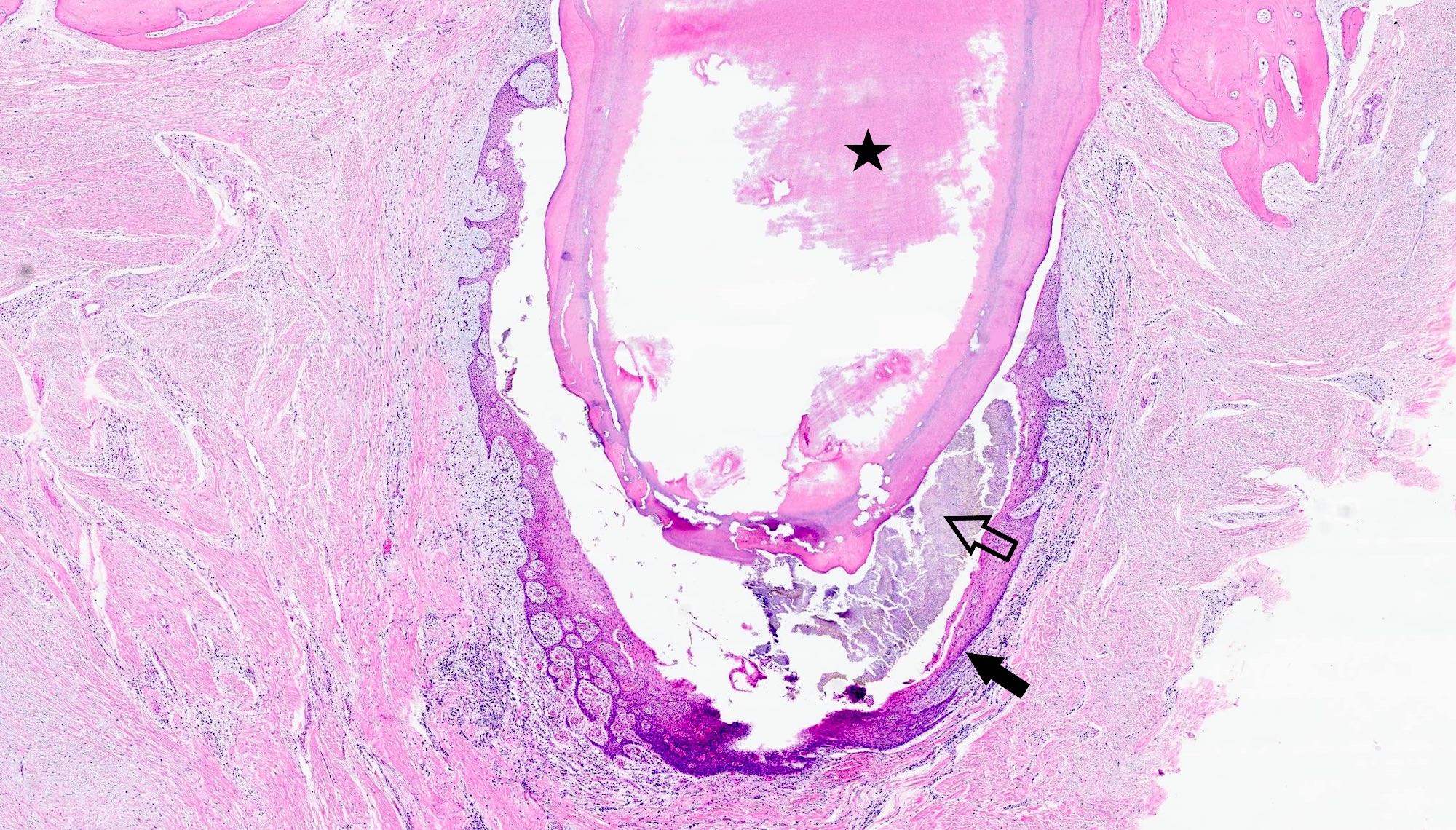
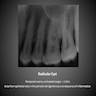
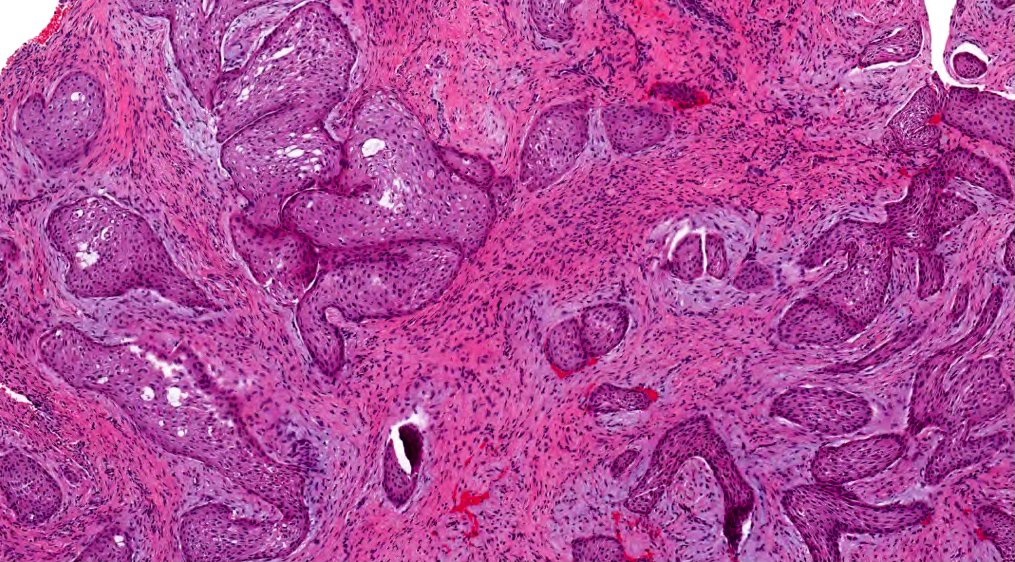
- Benign cyst of odontogenic origin, characterized by an ameloblastoma-like epithelial lining containing ghost cells that may calcify
- Originally described by Gorlin and colleagues in 1962 as a possible oral analogue to pilomatrixoma of skin, owing to the presence of ghost cell keratinization in both lesions
Essential features
- Calcifying odontogenic cysts are located most commonly in the anterior regions of jaws
- Microscopically calcifying odontogenic cysts contain an ameloblastoma-like epithelial lining containing ghost cells that may calcify
- Calcifying odontogenic cysts are associated with β catenin (CTNNB1) mutations
Terminology
- Calcifying odontogenic cyst (COC) is the preferred terminology in the 2017 WHO classification
- Nomenclature has been continuously changing, due to debate as to whether COC is a neoplasm or a developmental cyst
- In 1992, WHO classified this lesion as an odontogenic tumor but continued to use the term calcifying odontogenic cyst
- In 2005, WHO redesignated the lesion as calcifying cystic odontogenic tumor (CCOT)
- In 2017, the term calcifying odontogenic cyst was reapplied to this lesion and it was reclassified as a benign odontogenic cyst
- Other archaic names include Gorlin cyst and keratinizing and calcifying odontogenic cyst
- Given the diversity of the histopathologic features seen in COC and its tendency to coexist with other odontogenic lesions, complex classification systems have been proposed (Am J Surg Pathol 2003;27:372, J Oral Pathol Med 2008;37:302, Reichart: Odontogenic Tumors and Allied Lesions, Illustrated Edition, 2004)
- COC belongs to odontogenic ghost cell family of lesions (J Oral Pathol Med 2008;37:302)
- From a practical standpoint, a spectrum of histopathologic patterns exists, ranging from a benign lesion that is primarily cystic, a benign lesion with a solid pattern of growth to a rare tumor with features of carcinoma
- Solid lesions are designated dentinogenic ghost cell tumors in the 2017 WHO classification
- Malignant counterpart is ghost cell odontogenic carcinoma
- From a practical standpoint, a spectrum of histopathologic patterns exists, ranging from a benign lesion that is primarily cystic, a benign lesion with a solid pattern of growth to a rare tumor with features of carcinoma
ICD coding
Epidemiology
- 0.3% of odontogenic cysts (J Oral Pathol Med 2006;35:500)
- Mean age: 31 years (standard deviation: 21 years) range 5 - 92 years; however, this varies geographically (J Oral Pathol Med 2018;47:721)
- No consistent gender predominance
- Around 33% of cases are associated with odontogenic lesions, most commonly odontoma; odontoma associated COC occurs in younger patients on average (20.4 years) (J Oral Pathol Med 2018;47:721)
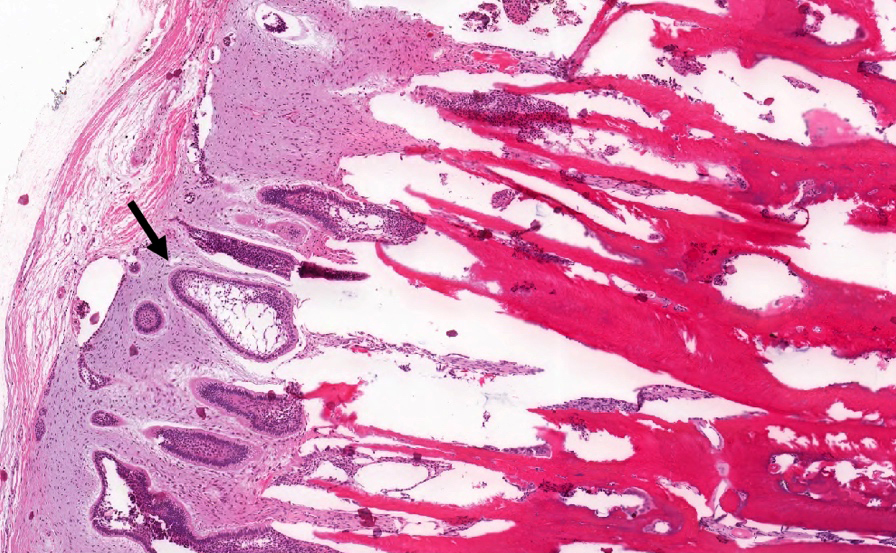
Sites
- Vast majority of lesions are central COC (within bone of jaw, intraosseous) (Reichart: Odontogenic Tumors and Allied Lesions, Illustrated Edition, 2004)
- Less commonly, peripheral lesions may be seen (located on gingival tissues, extraosseous) most of which can be classified as dentinogenic ghost cell tumors
- Usually anterior regions of jaws, incisor / cuspid region
- Lesions associated with odontoma are most commonly found in the anterior maxilla (J Oral Pathol Med 2008;37:302)
Pathophysiology
- Cystic neoplasms with β catenin (CTNNB1) mutations in the Wnt signaling pathway
- In one series, 91% (10/11) cases of CCOT / COC demonstrated CTNNB1 point mutations COC (PLoS One 2017;12:e0180224)
- Immunohistochemistry for LEF1 (a transcription factor of the Wnt pathway) is reported to be positive in 64% (7/11) of CCOT (Hum Pathol 2015;46:255)
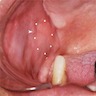
Clinical features
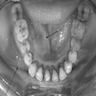
- Either asymptomatic (incidental radiographic finding) or a painless swelling
- Extraosseous lesions present as a gingival swelling, which may be painful
- May be associated with other odontogenic pathology, most commonly odontoma (J Oral Pathol Med 2018;47:721)
Diagnosis
- Diagnosis can be made on an incisional biopsy or enucleation specimen
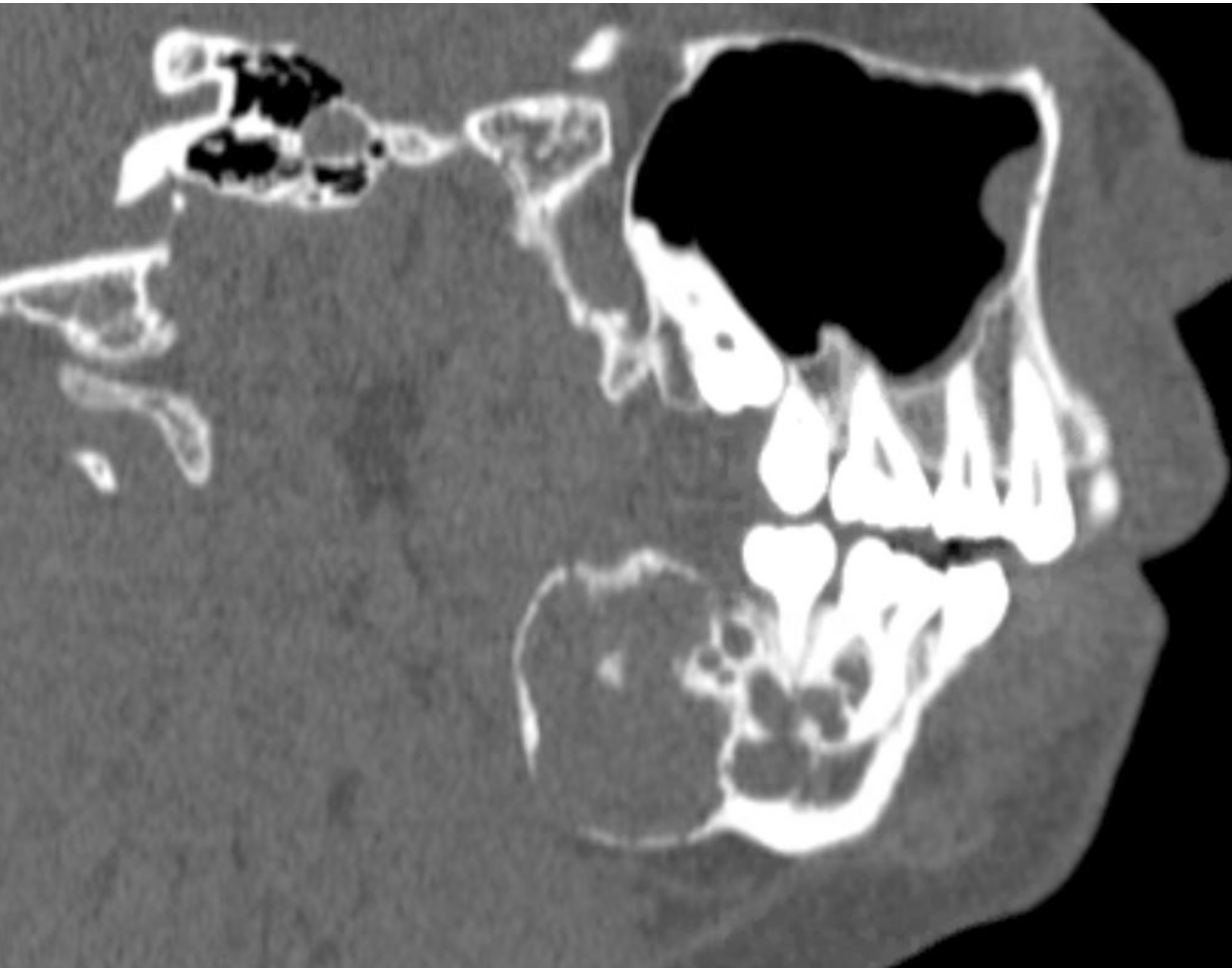
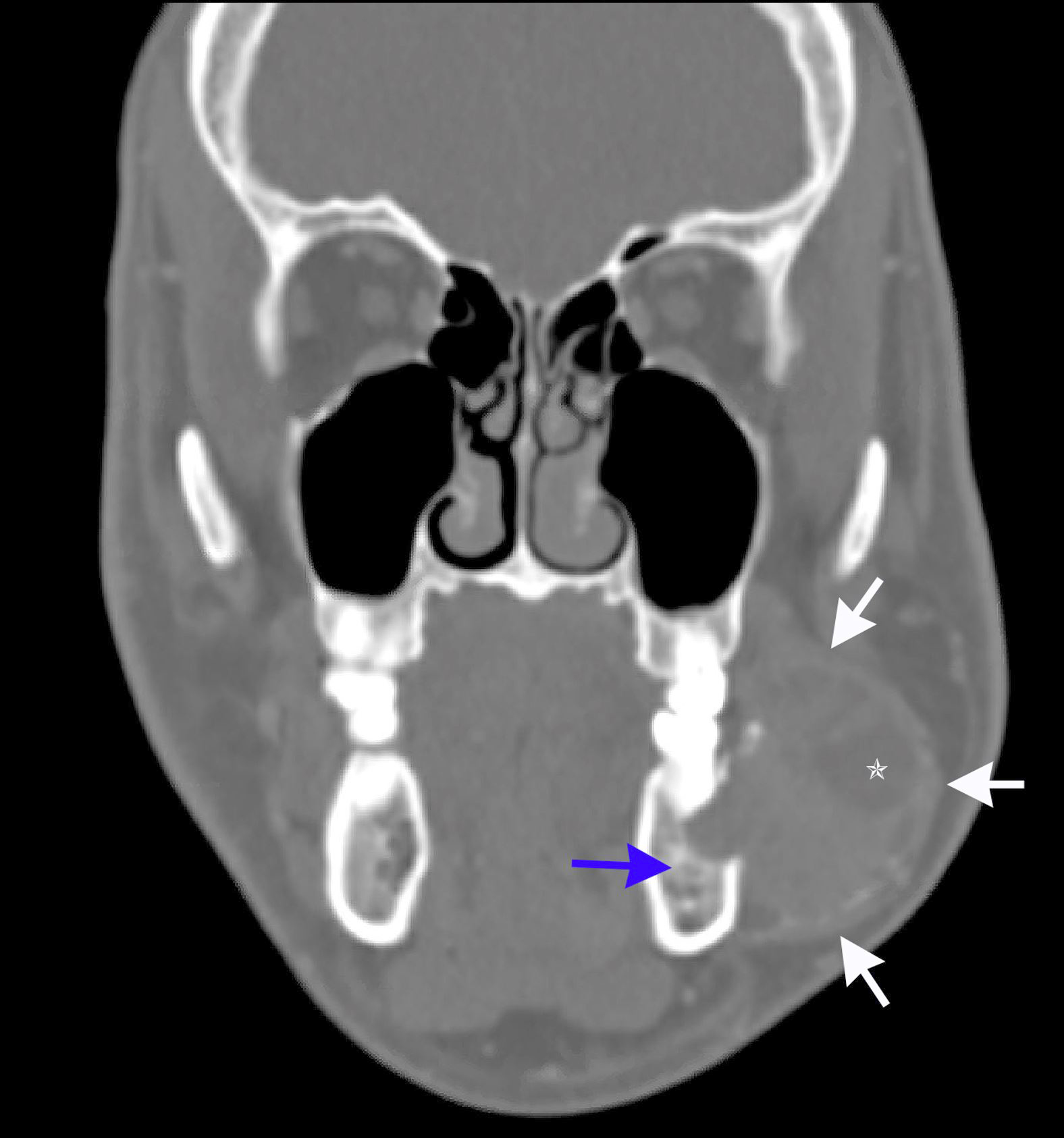
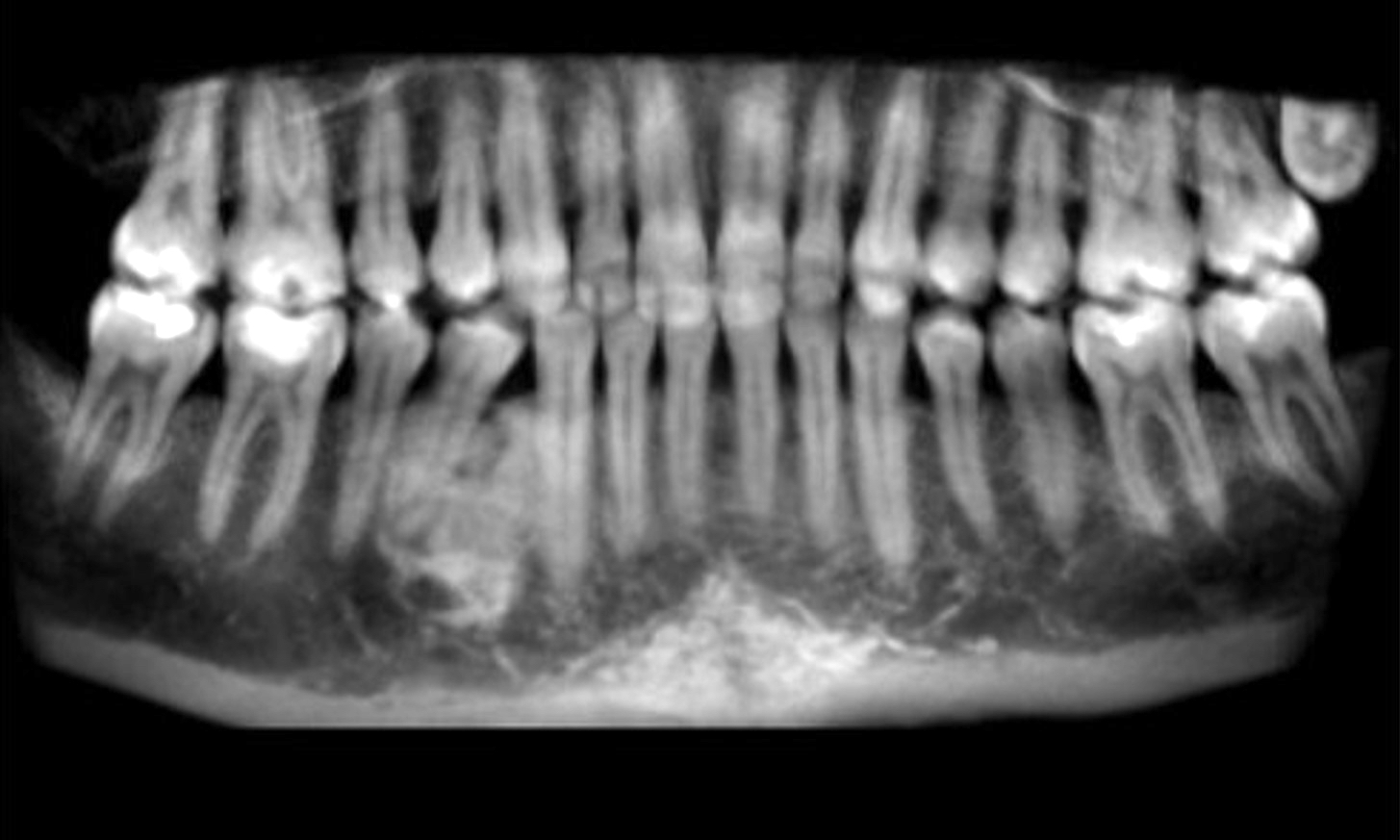
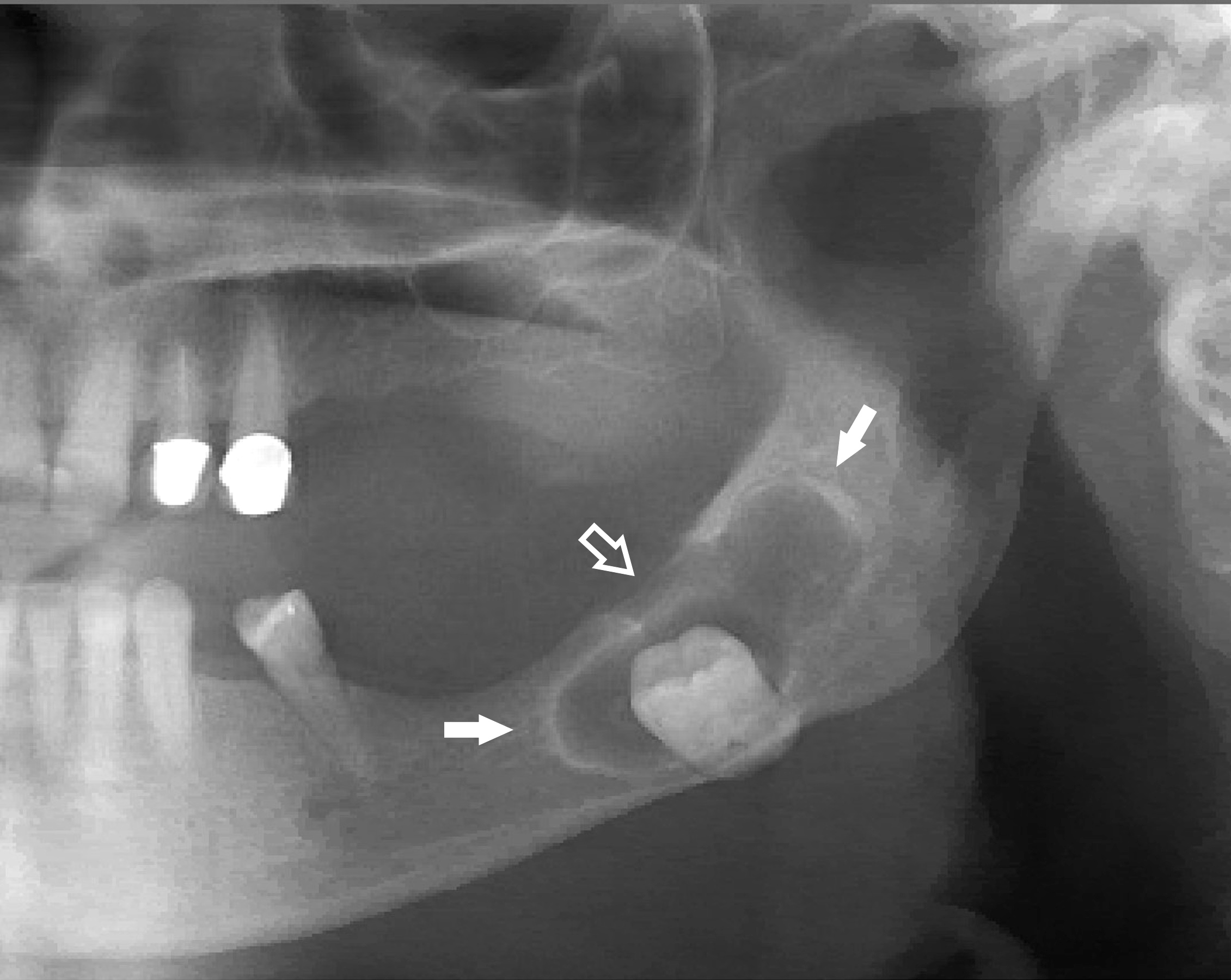
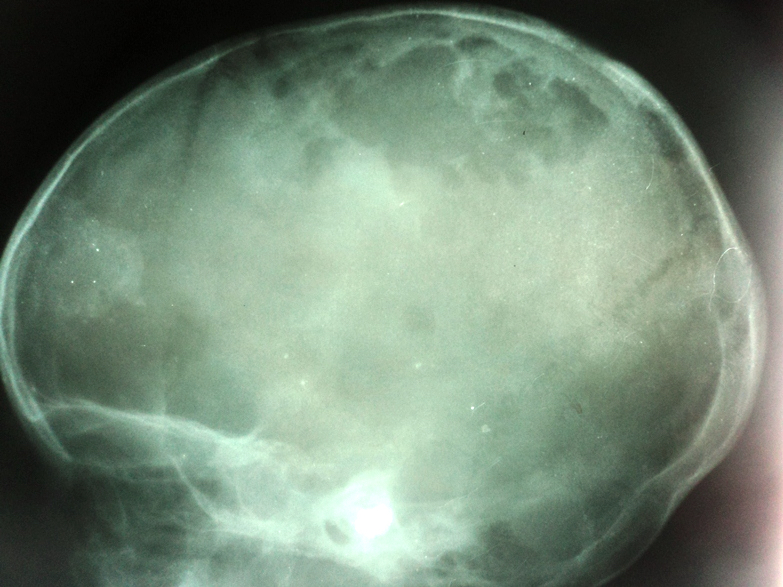
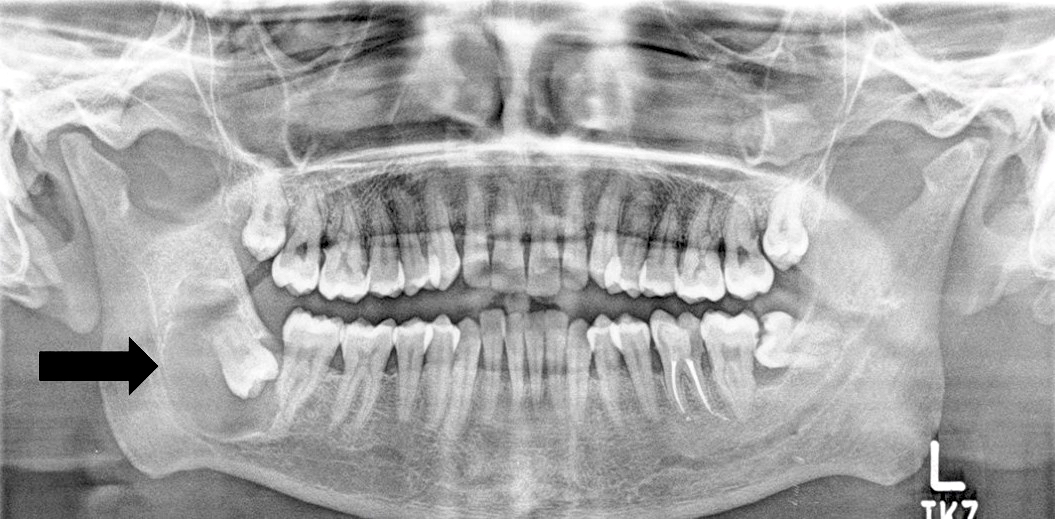
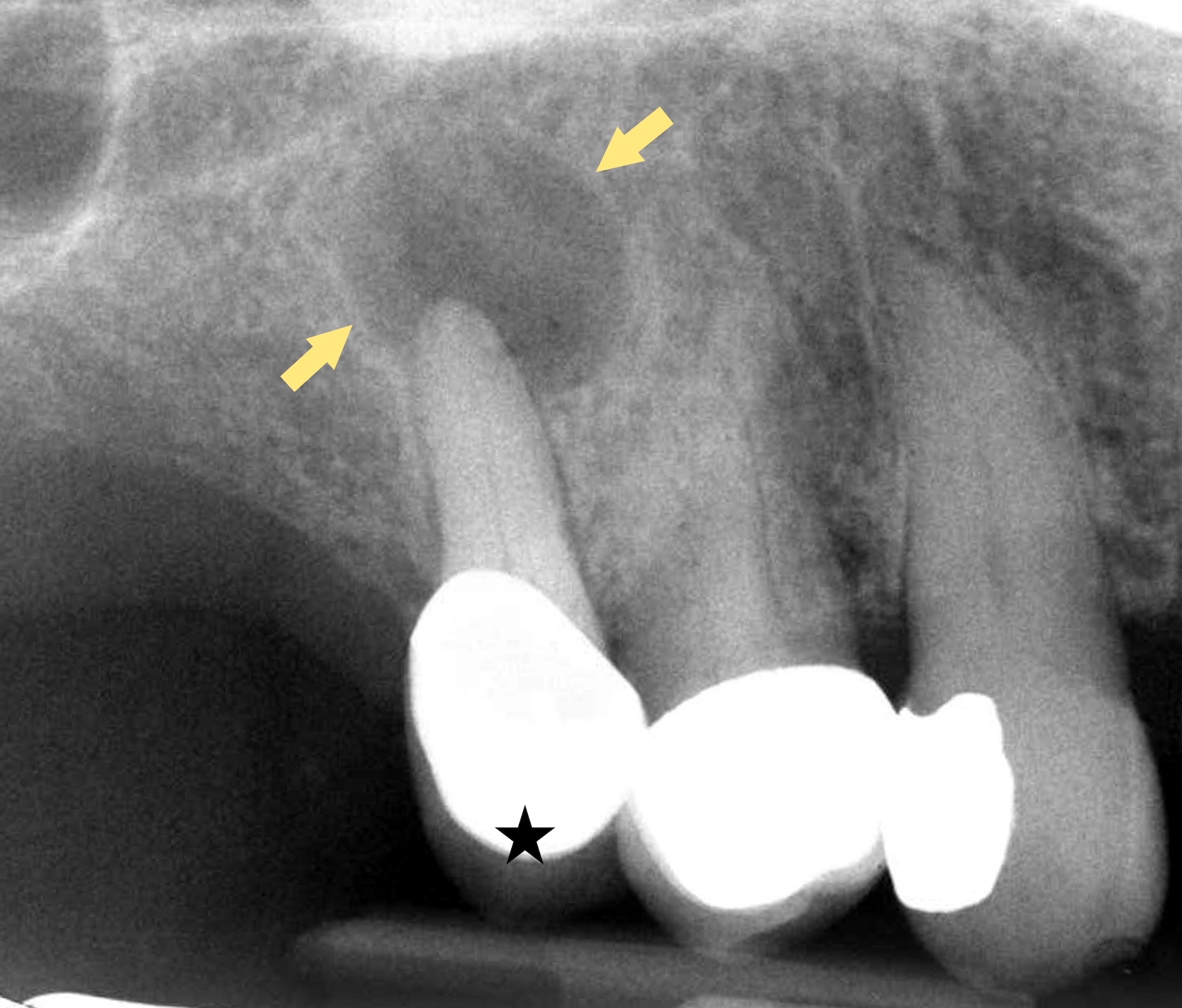
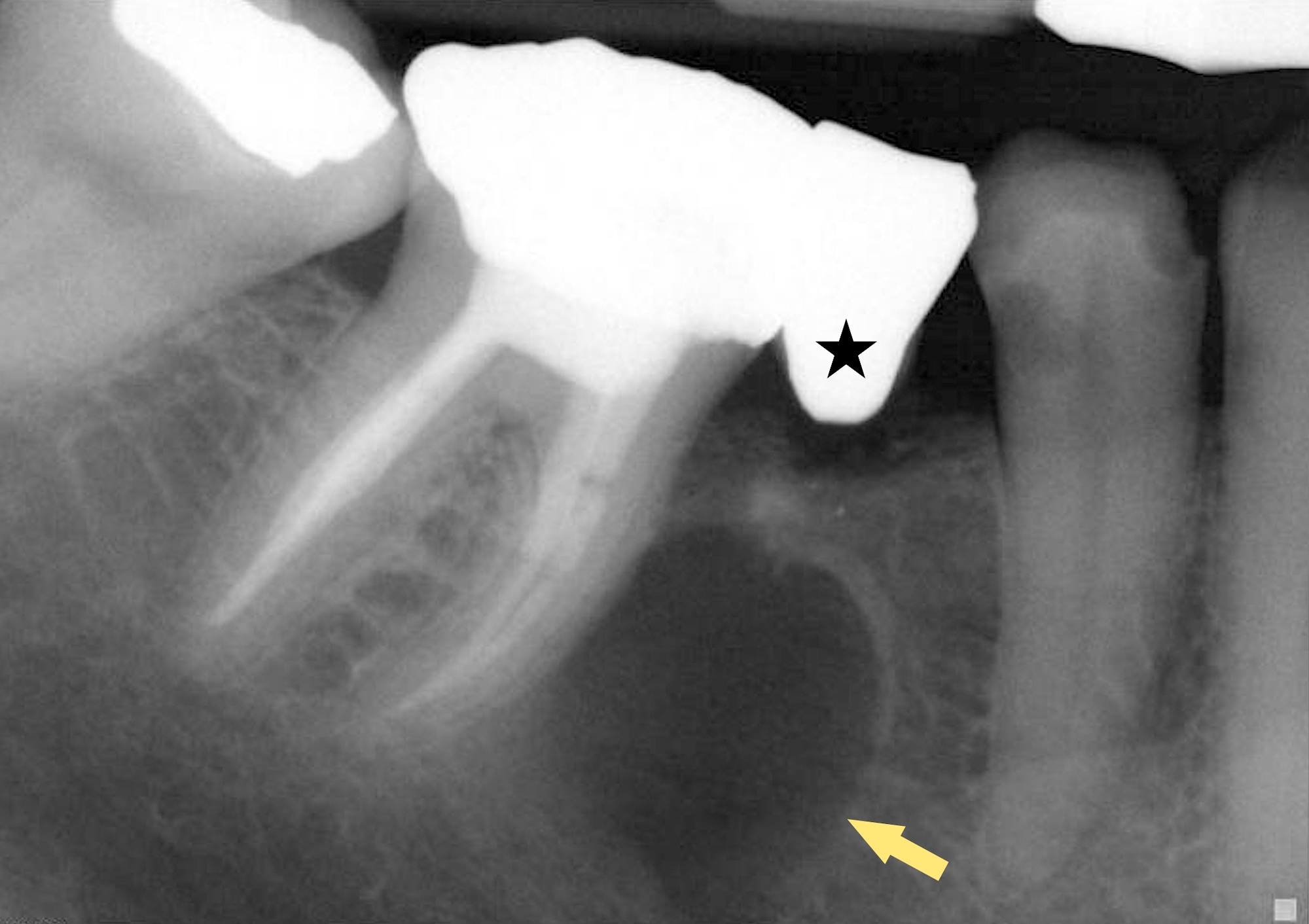
Radiology description
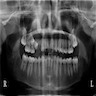
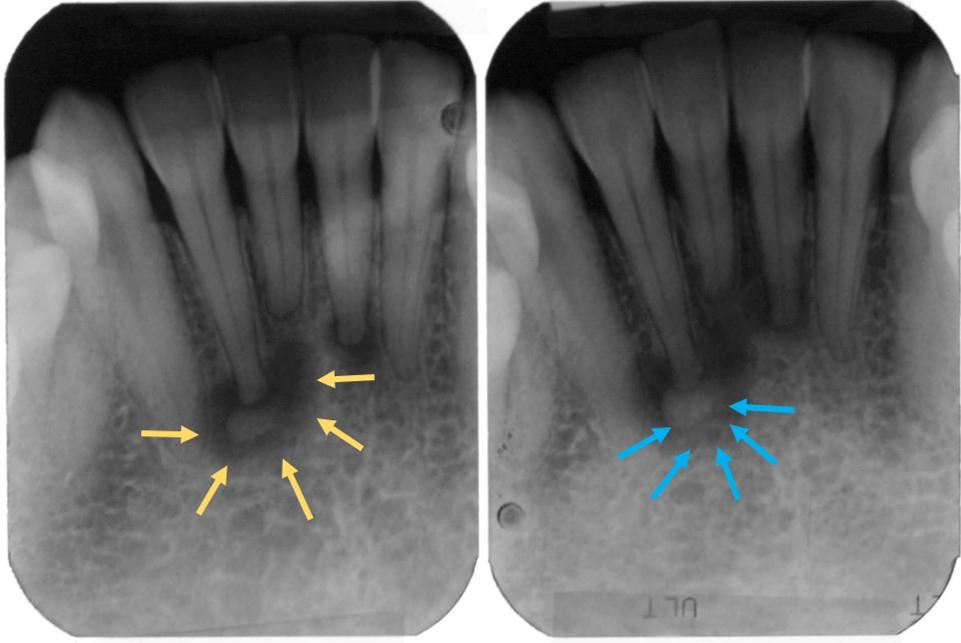
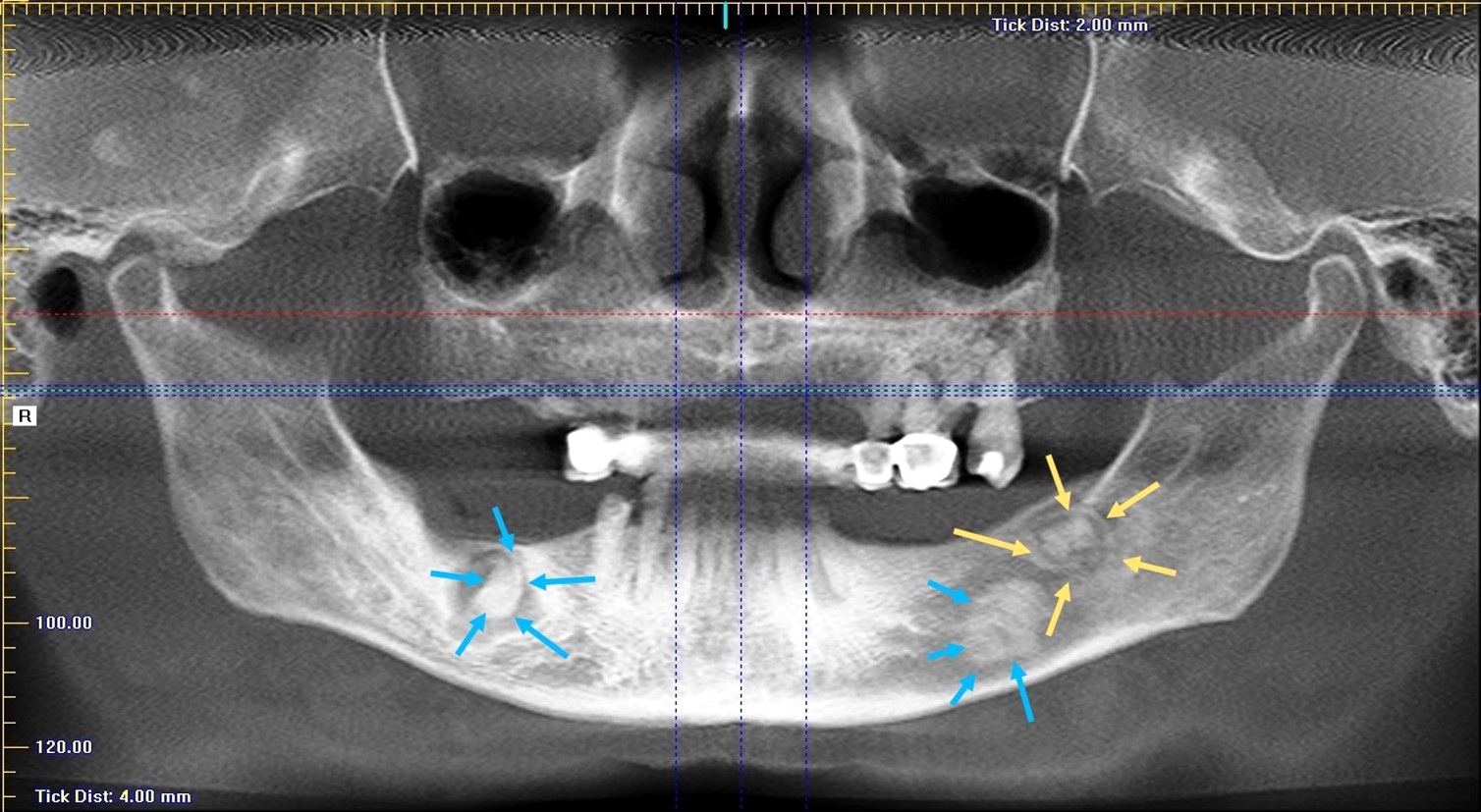
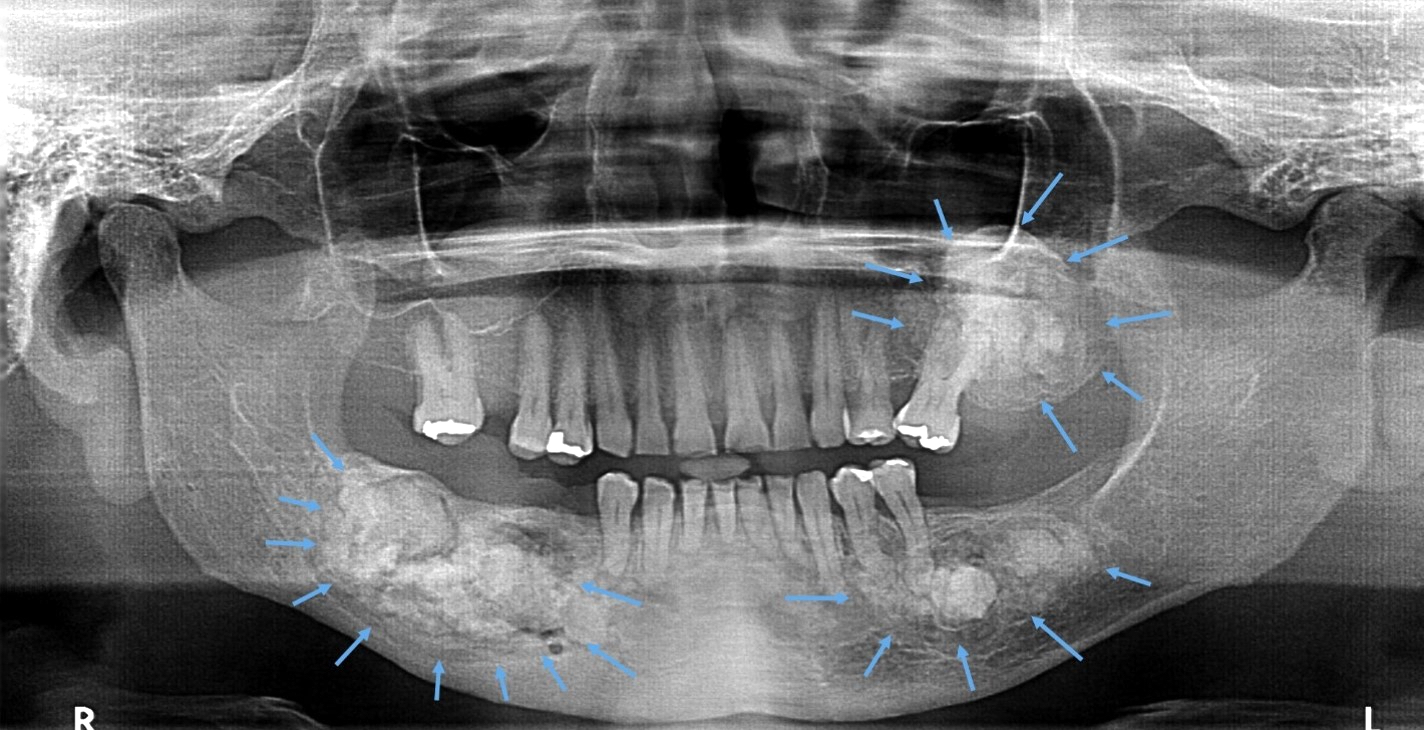
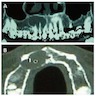
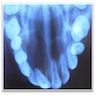
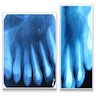
- Usually unilocular, well defined radiolucency with focal opacification but may rarely be multilocular (Br J Radiol 2012;85:548)
- Scattered radiopacities in 33 - 50%
- 33% associated with impacted tooth
- Usually 2 - 4 cm
- Adjacent tooth root displacement, resorption or root divergence may occur (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:356)
Radiology images
Prognostic factors
- Prognosis excellent, few recurrences (< 5% documented)
- Rarely, malignant transformation has been reported (Case Rep Pathol 2013;2013:853095, Korean J Pathol 2012;46:478, Ann Diagn Pathol 2009;13:394)
Case reports
- 15 year old Japanese boy with pigmented calcifying cystic odontogenic tumor associated with compound odontoma (Head Face Med 2007;3:35)
- 23 year old man with ameloblastic fibro-odontoma arising from a calcifying odontogenic cyst (Bull Tokyo Dent Coll 2001;42:51)
- 24 year old woman with calcifying odontogenic cyst associated with an orthokeratinized odontogenic cyst (Head Neck Pathol 2008;2:324)
- 36 year old man with a 5.9 cm calcifying odontogenic cyst treated with 2 stage decompression (J Oral Maxillofac Surg 2017;75:1915)
- Hybrid odontogenic tumor with features of ameloblastic fibro-odontoma, calcifying odontogenic cyst and adenomatoid odontogenic tumor (J Oral Maxillofac Surg 2010;68:470)
Treatment
- Treated by enucleation and curettage
- For combined lesions, treat according to characteristics of more aggressive lesion
- Large cysts may be decompressed prior to surgical management in a 2 stage approach (J Oral Maxillofac Surg 2017;75:1915)
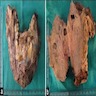
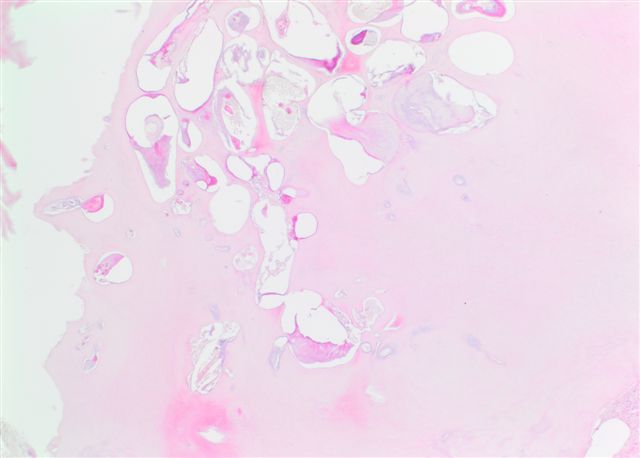
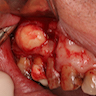
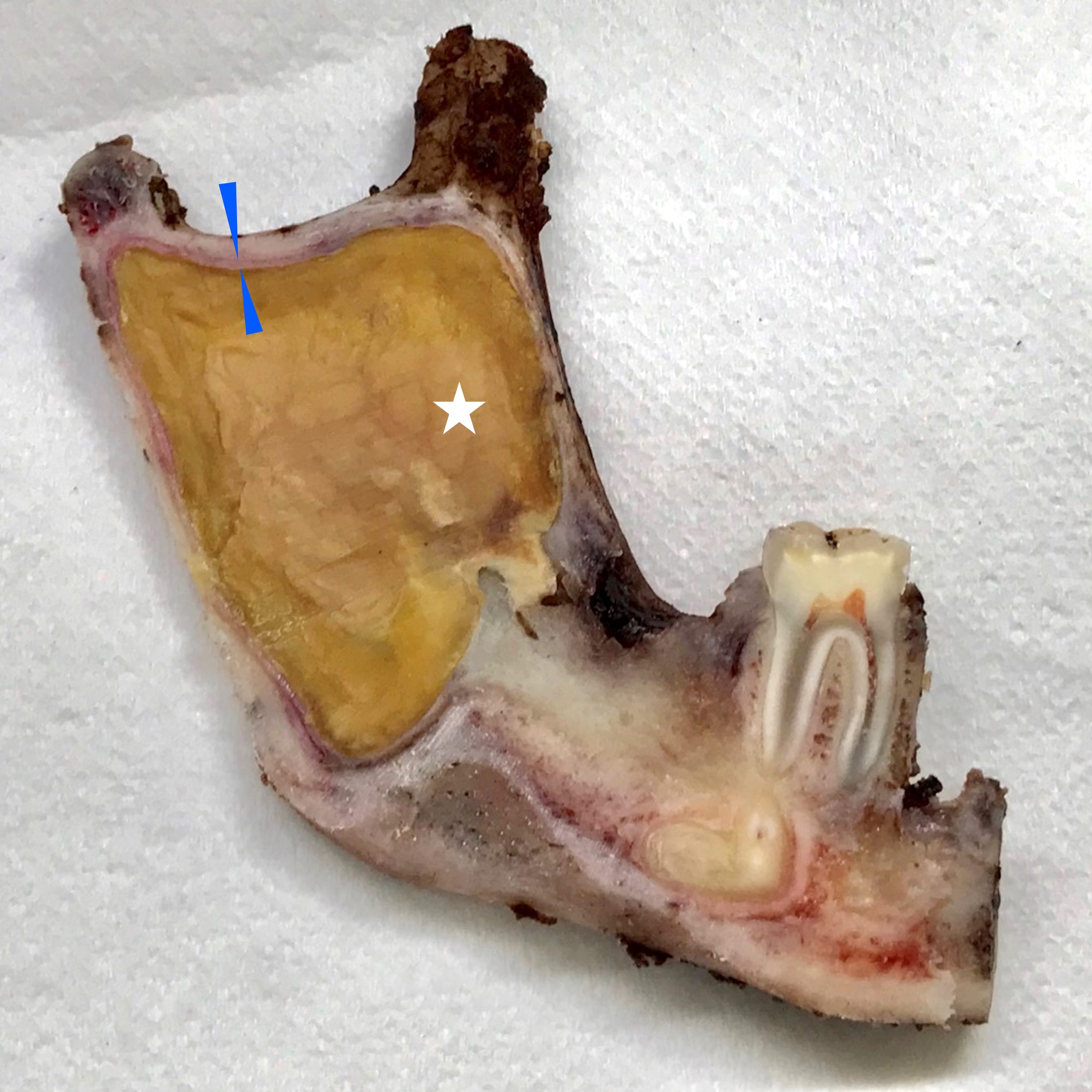
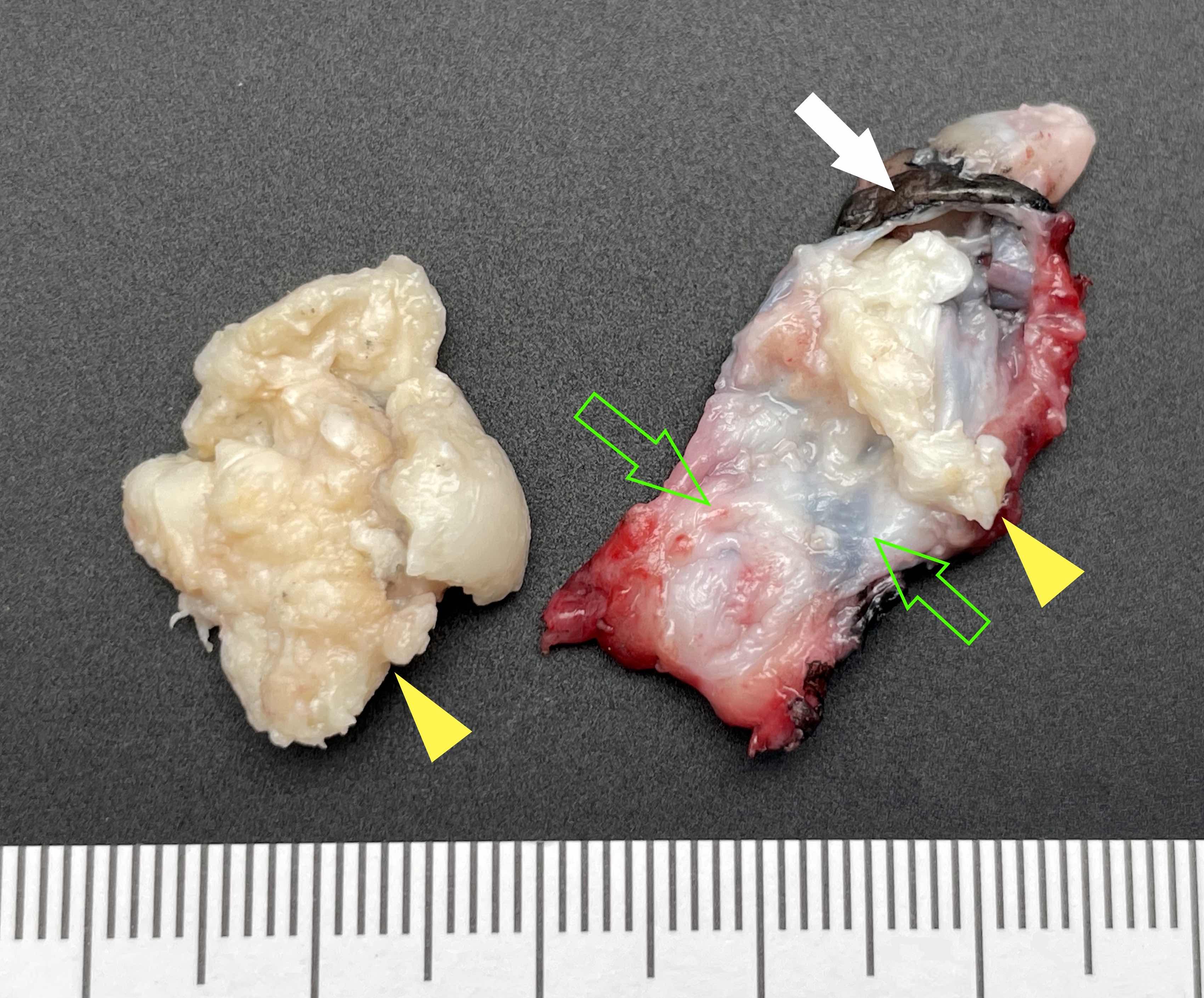
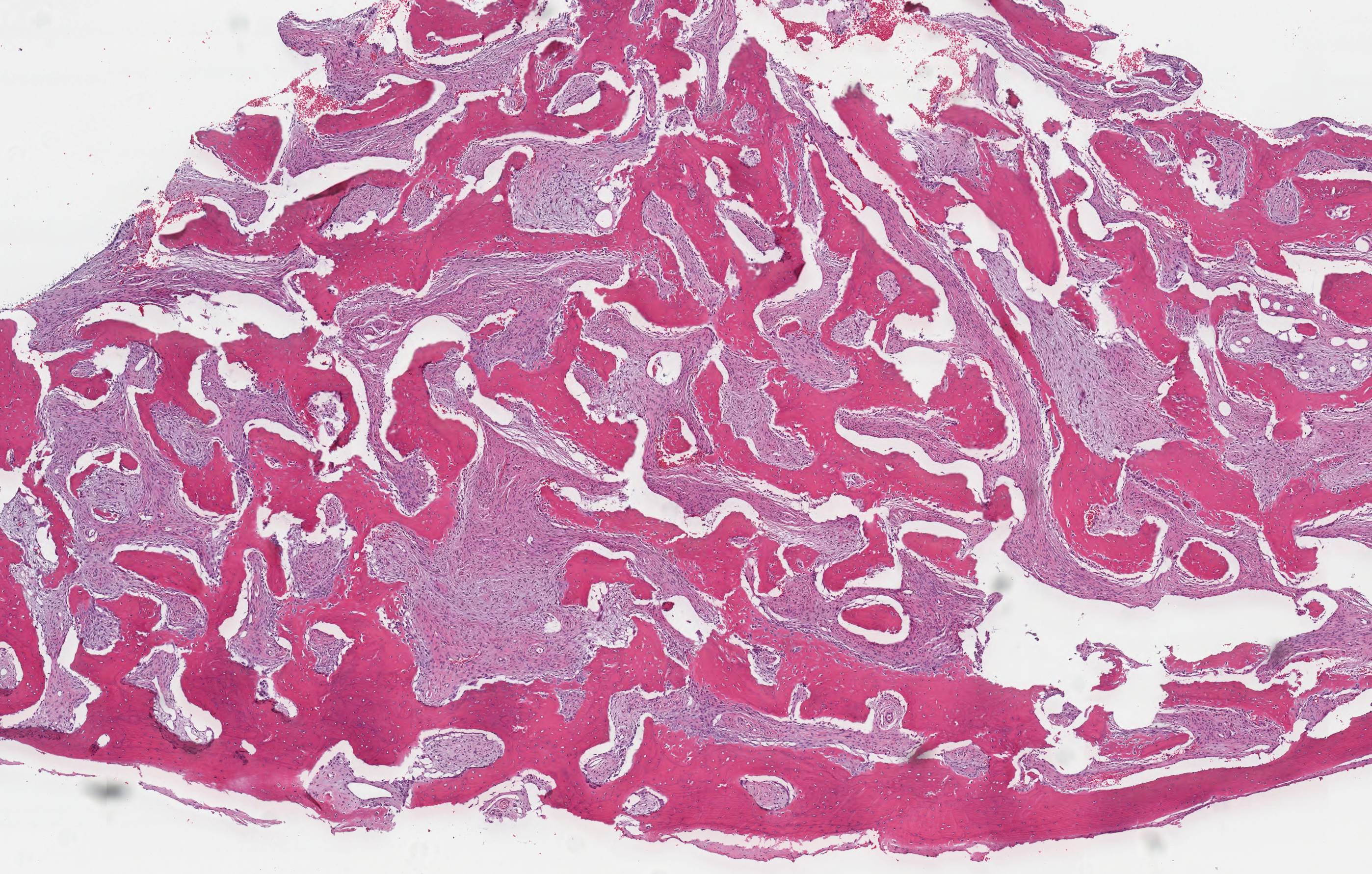
Gross description
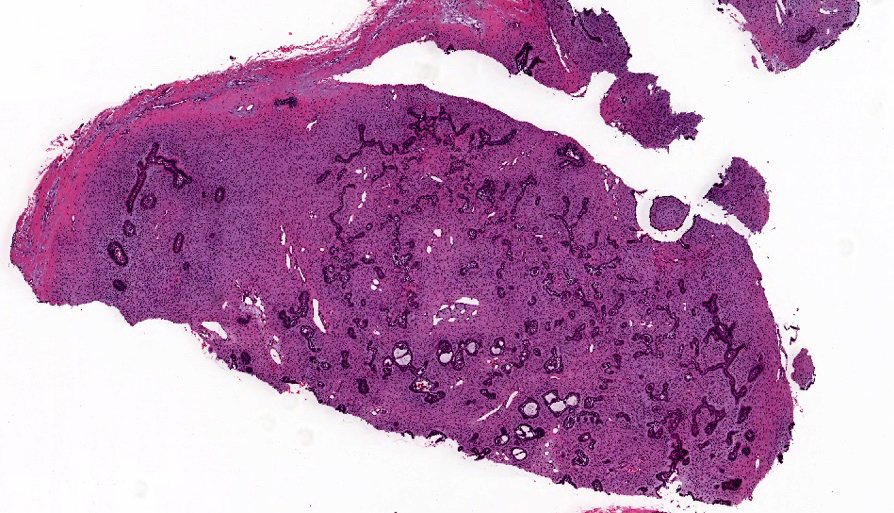
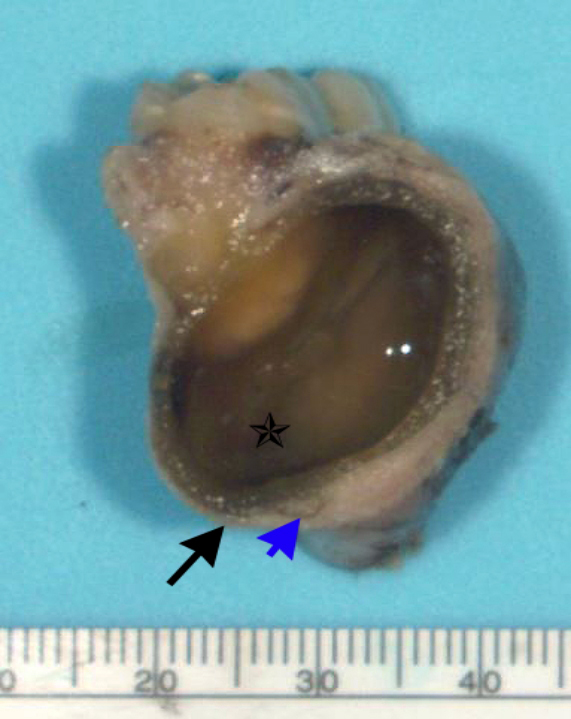
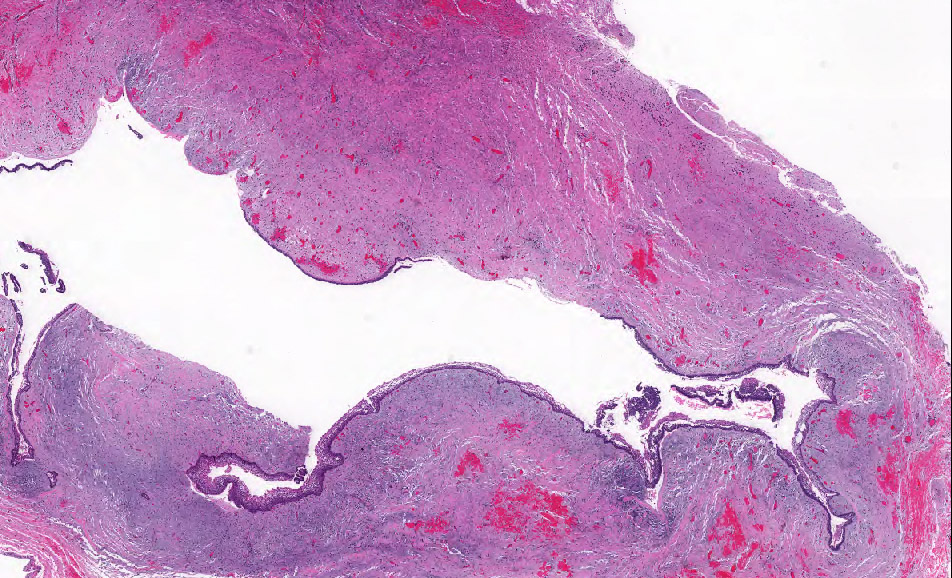
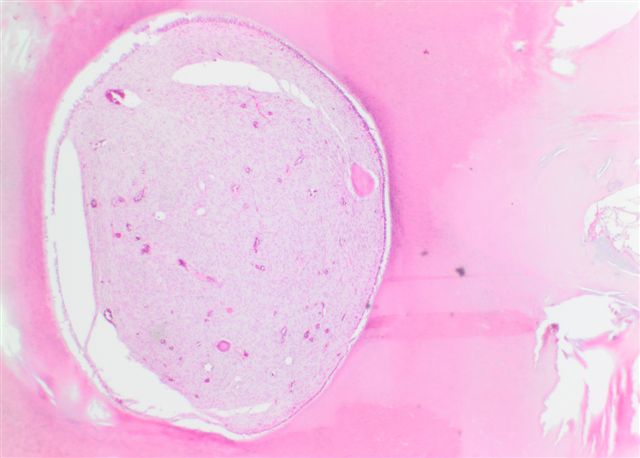
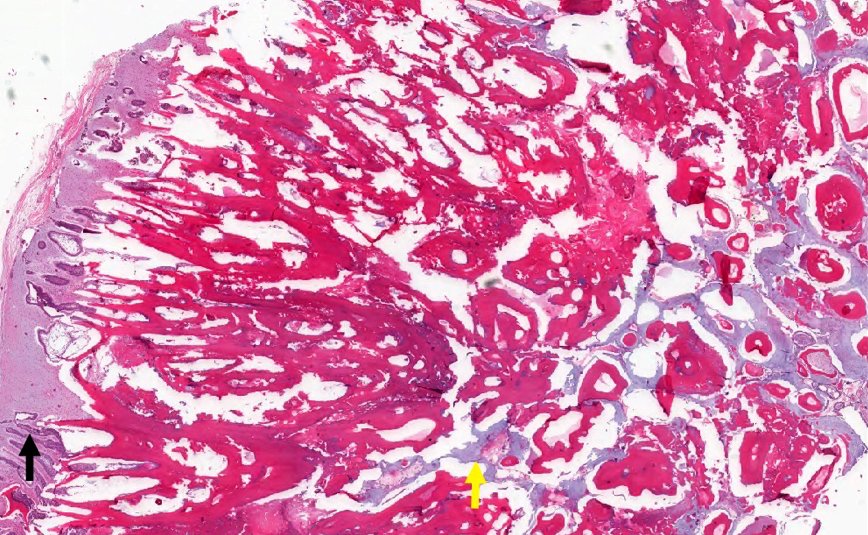
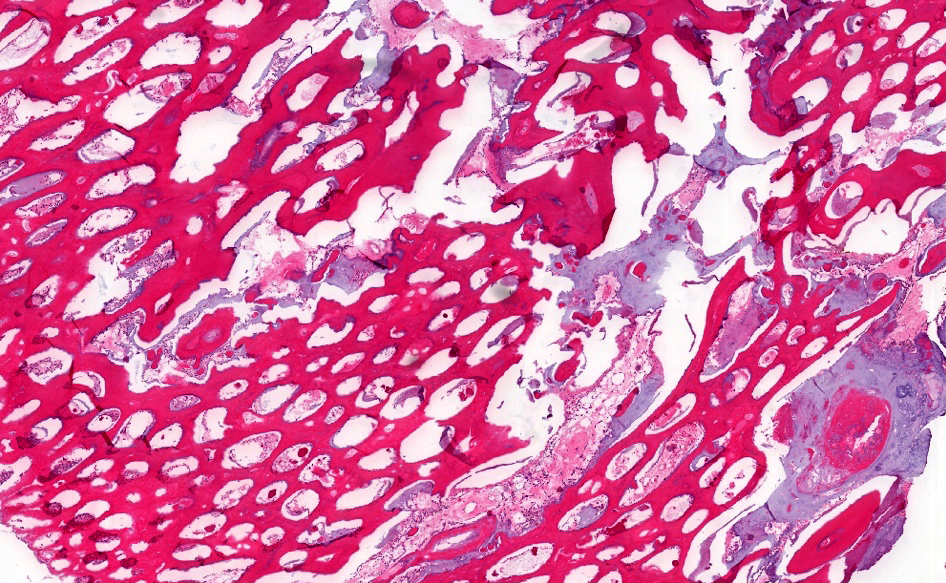
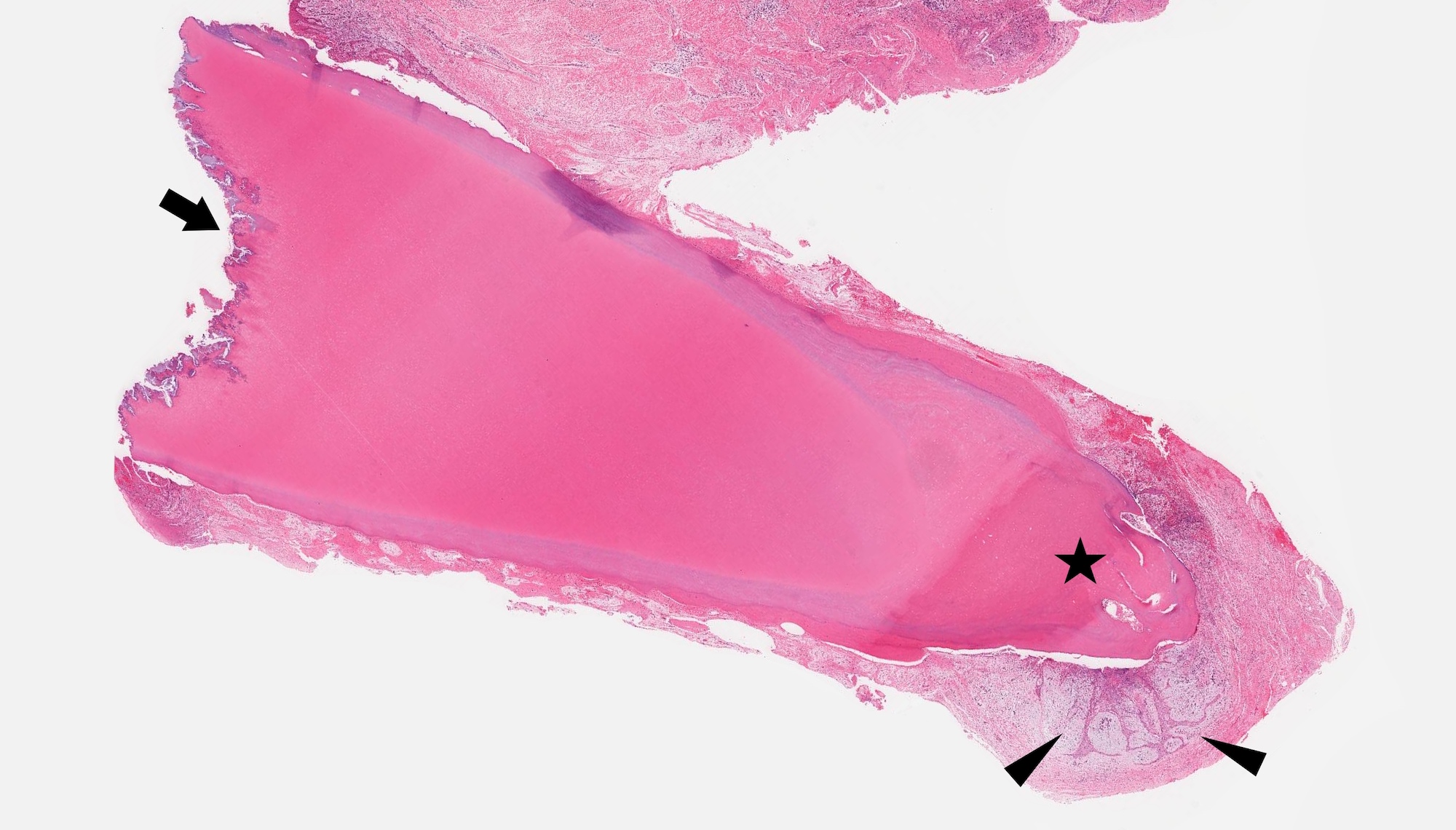
- Unicystic, often 2 cm or less; no solid areas
- Grossly cystic with focal luminal thickenings
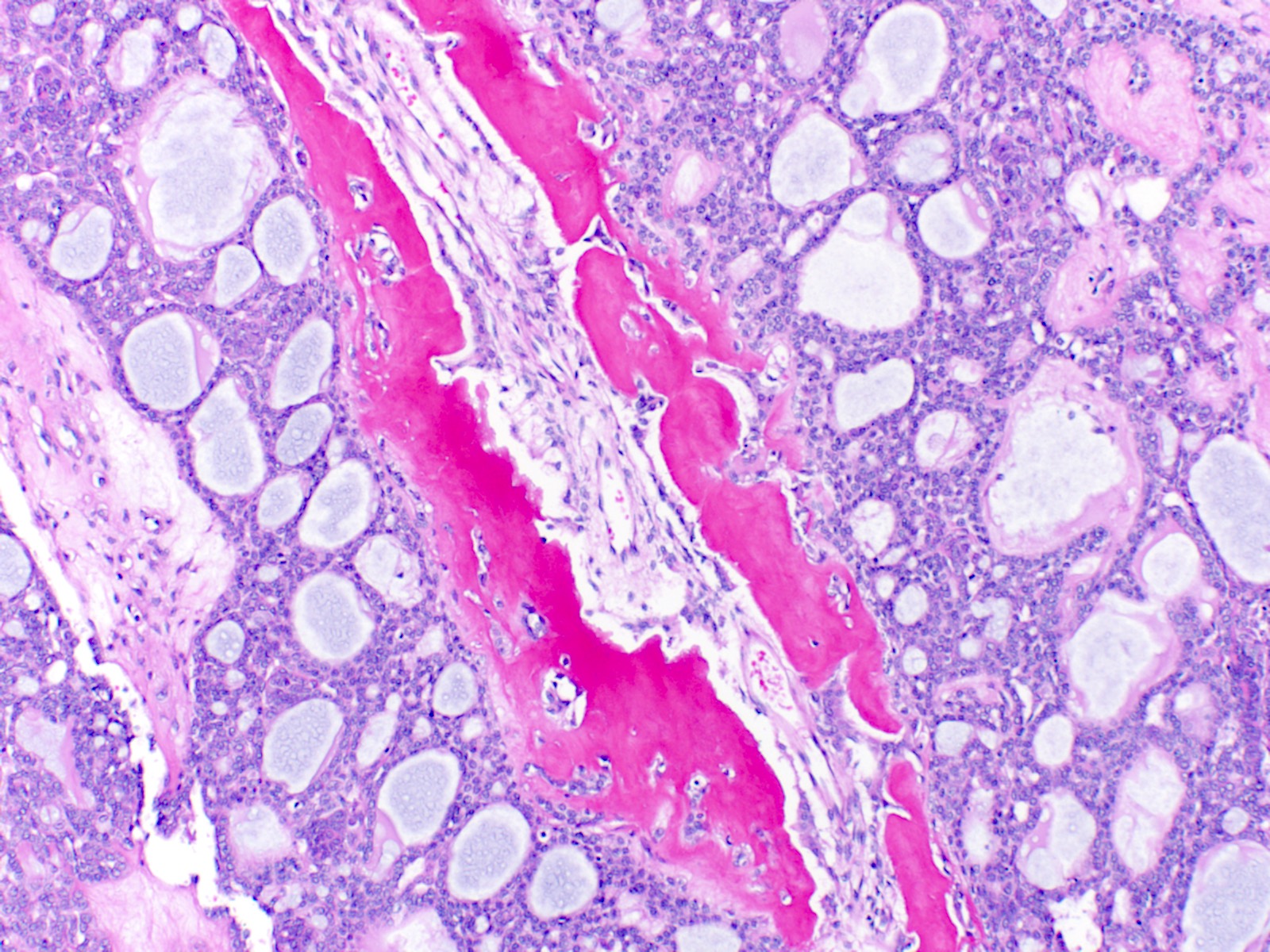
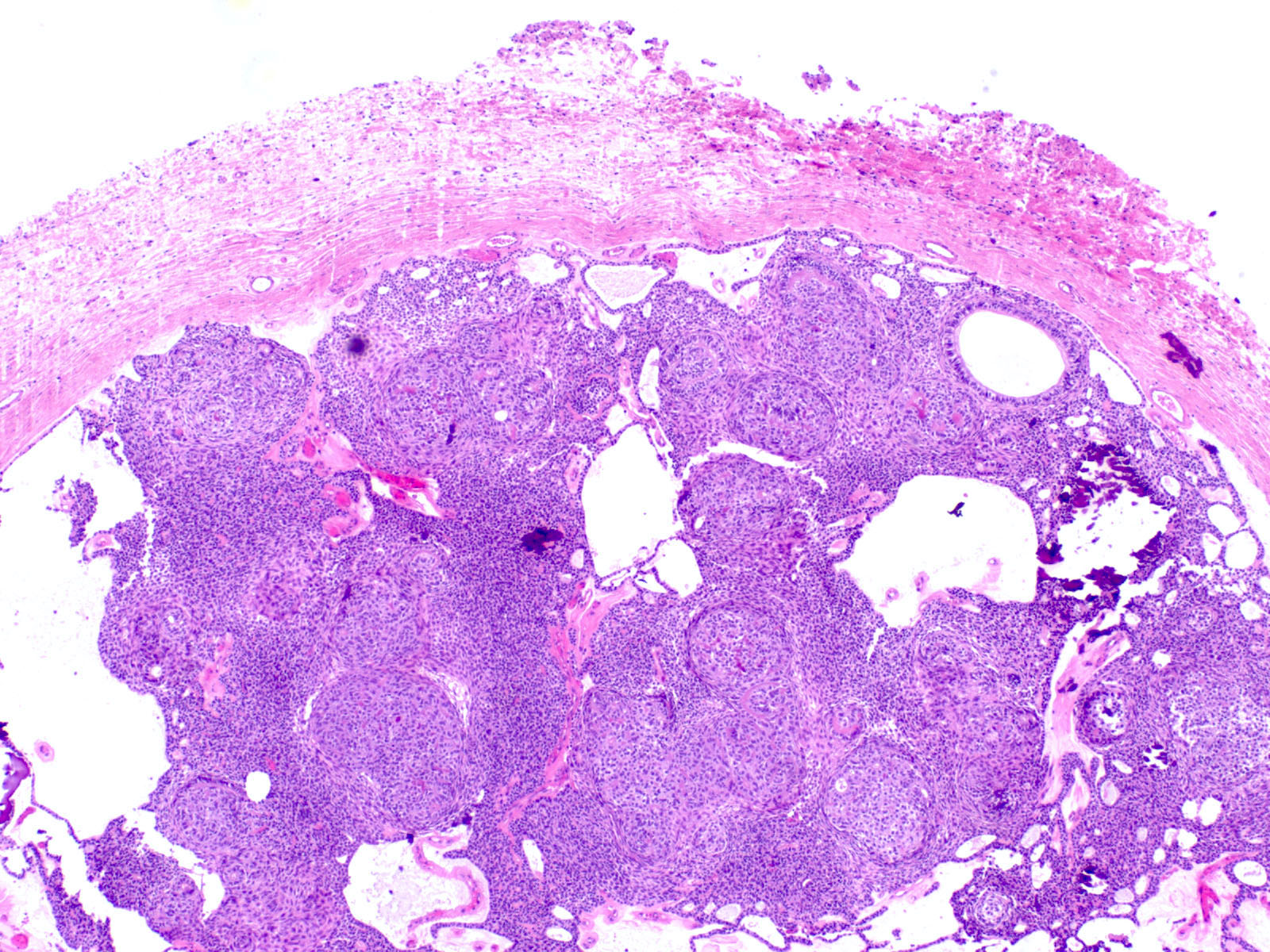
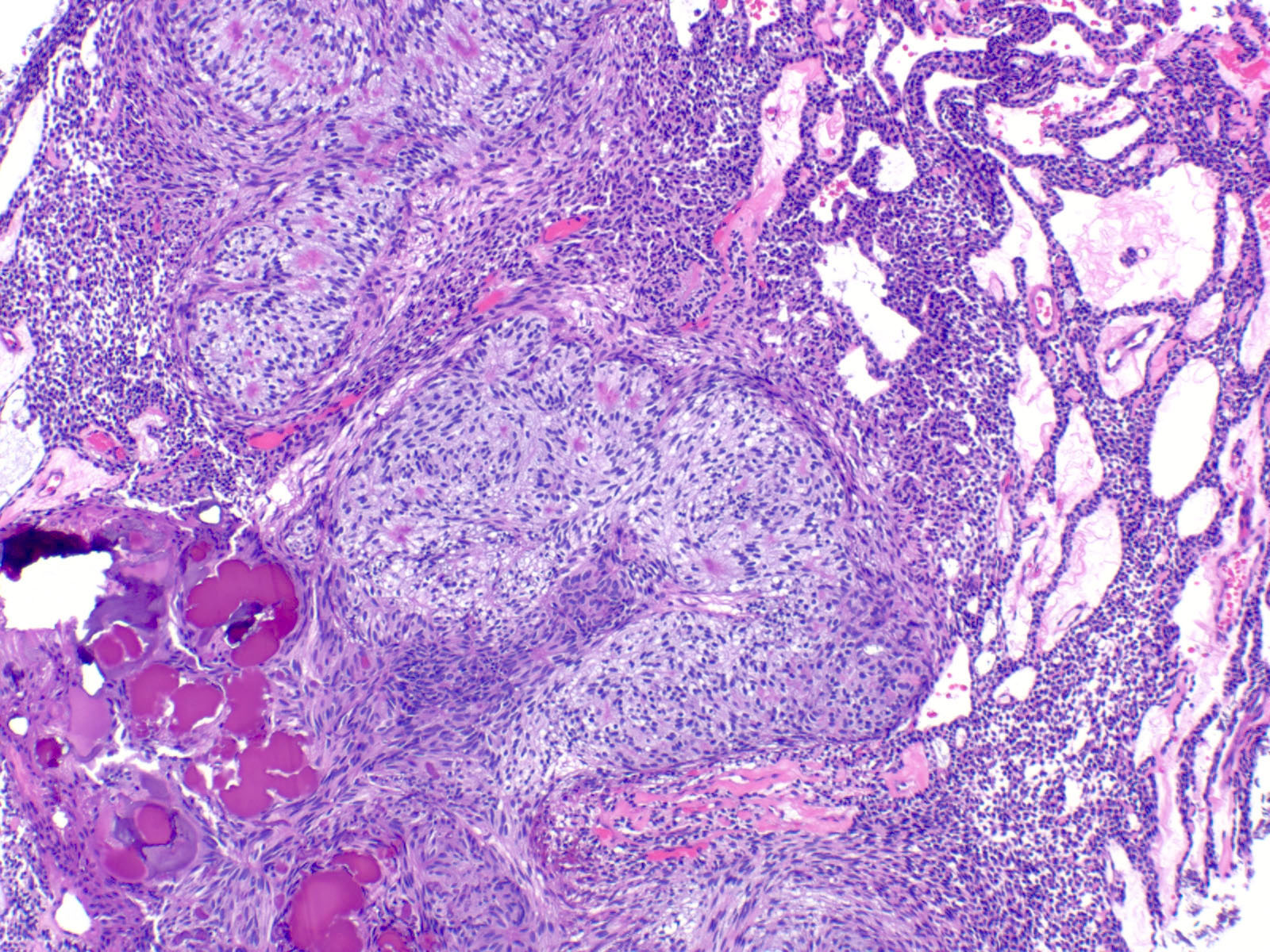
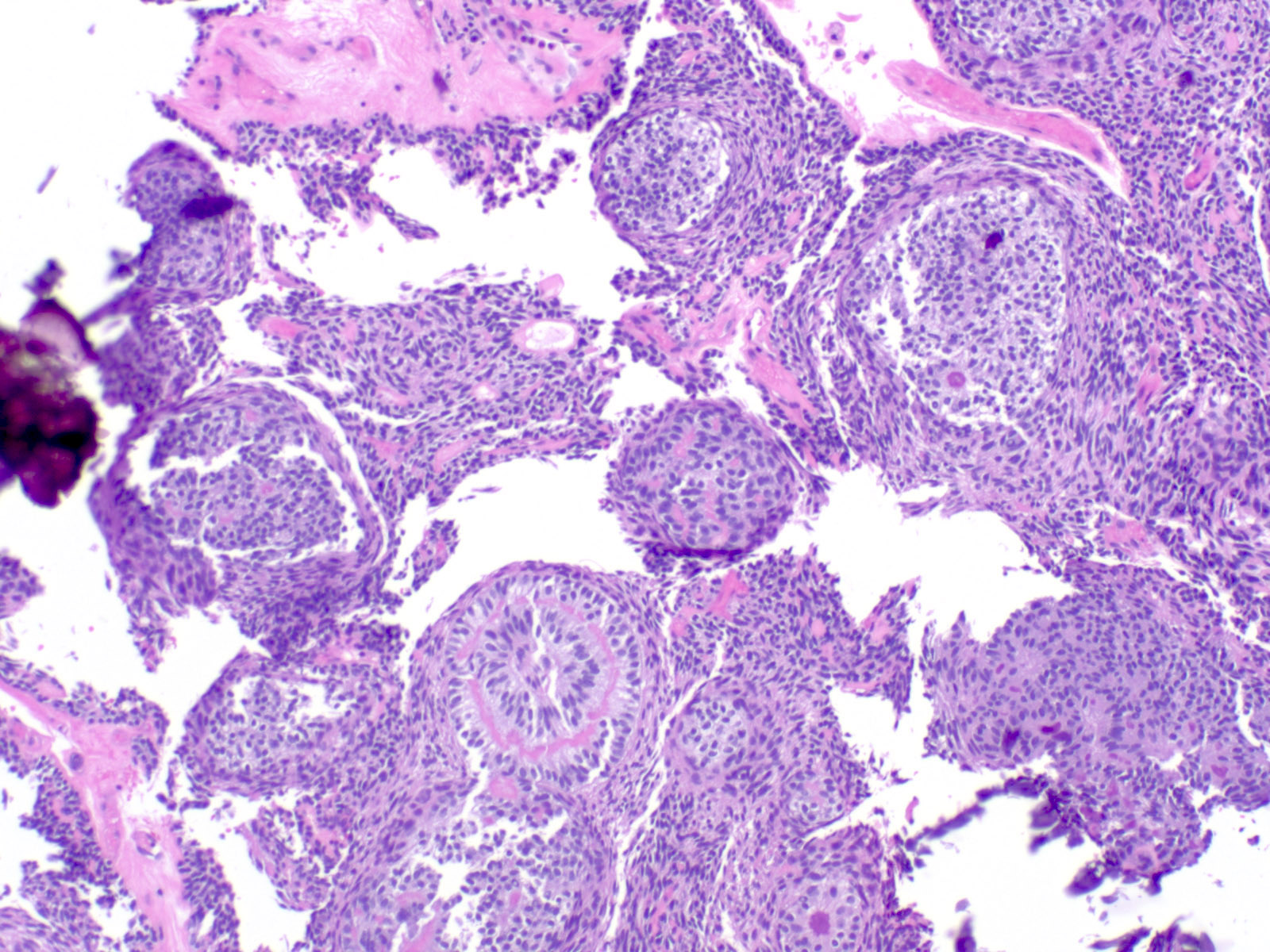
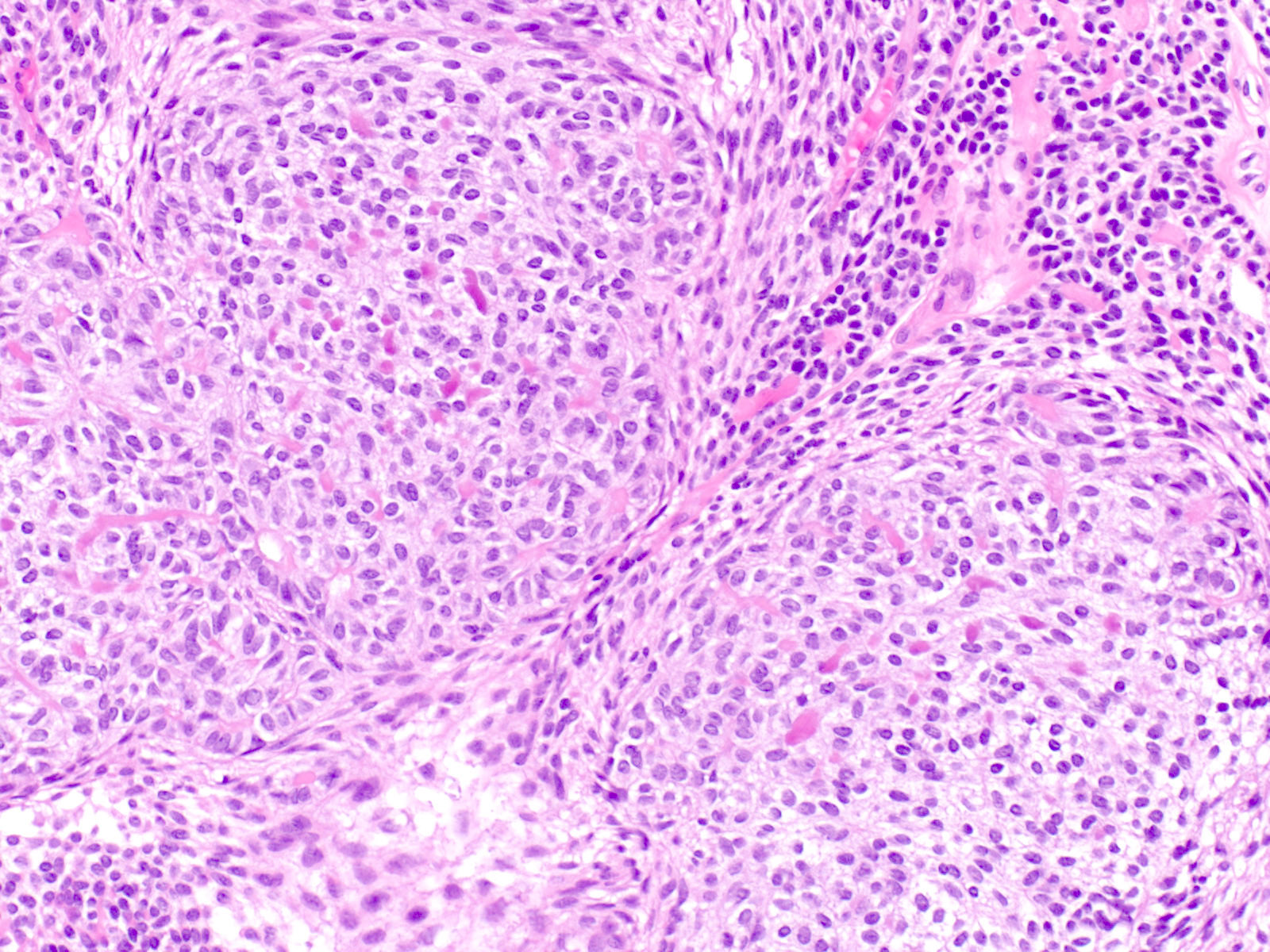
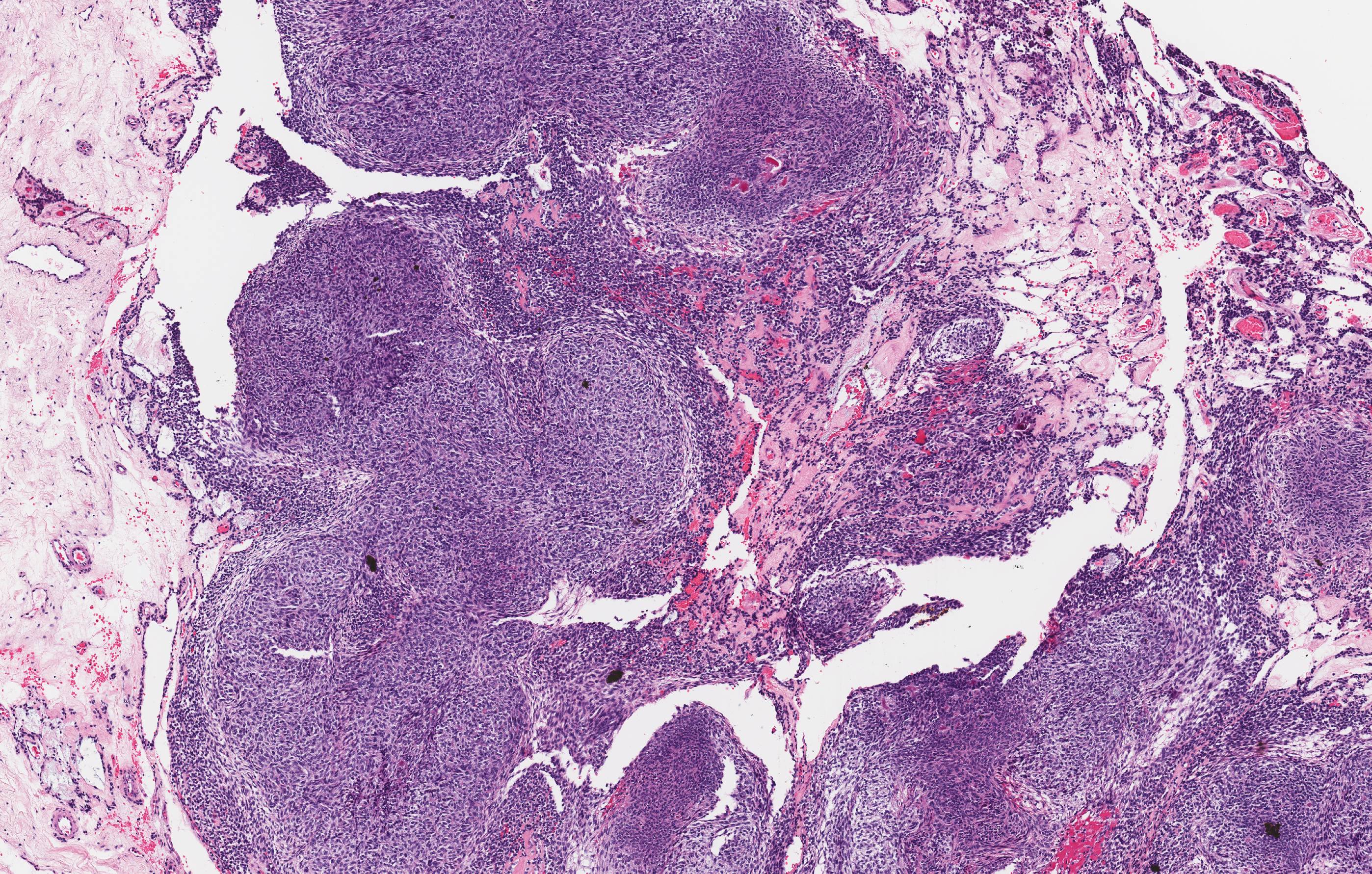
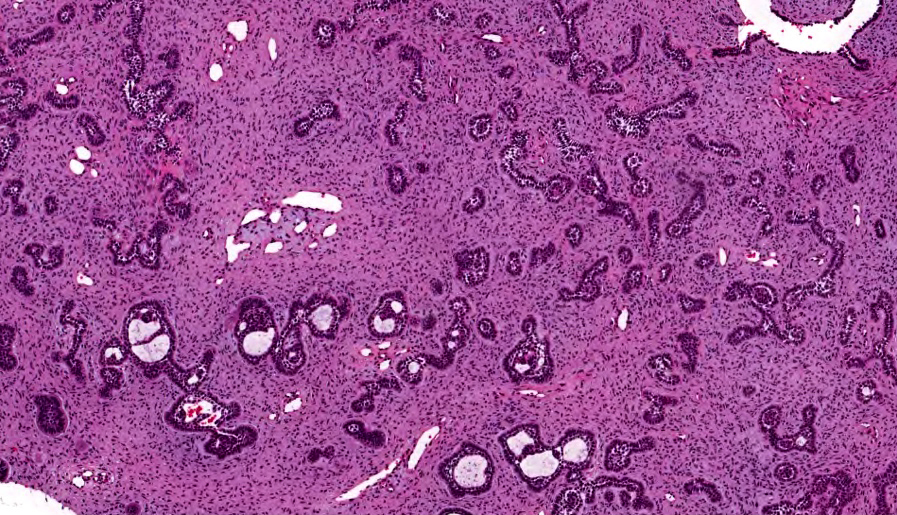
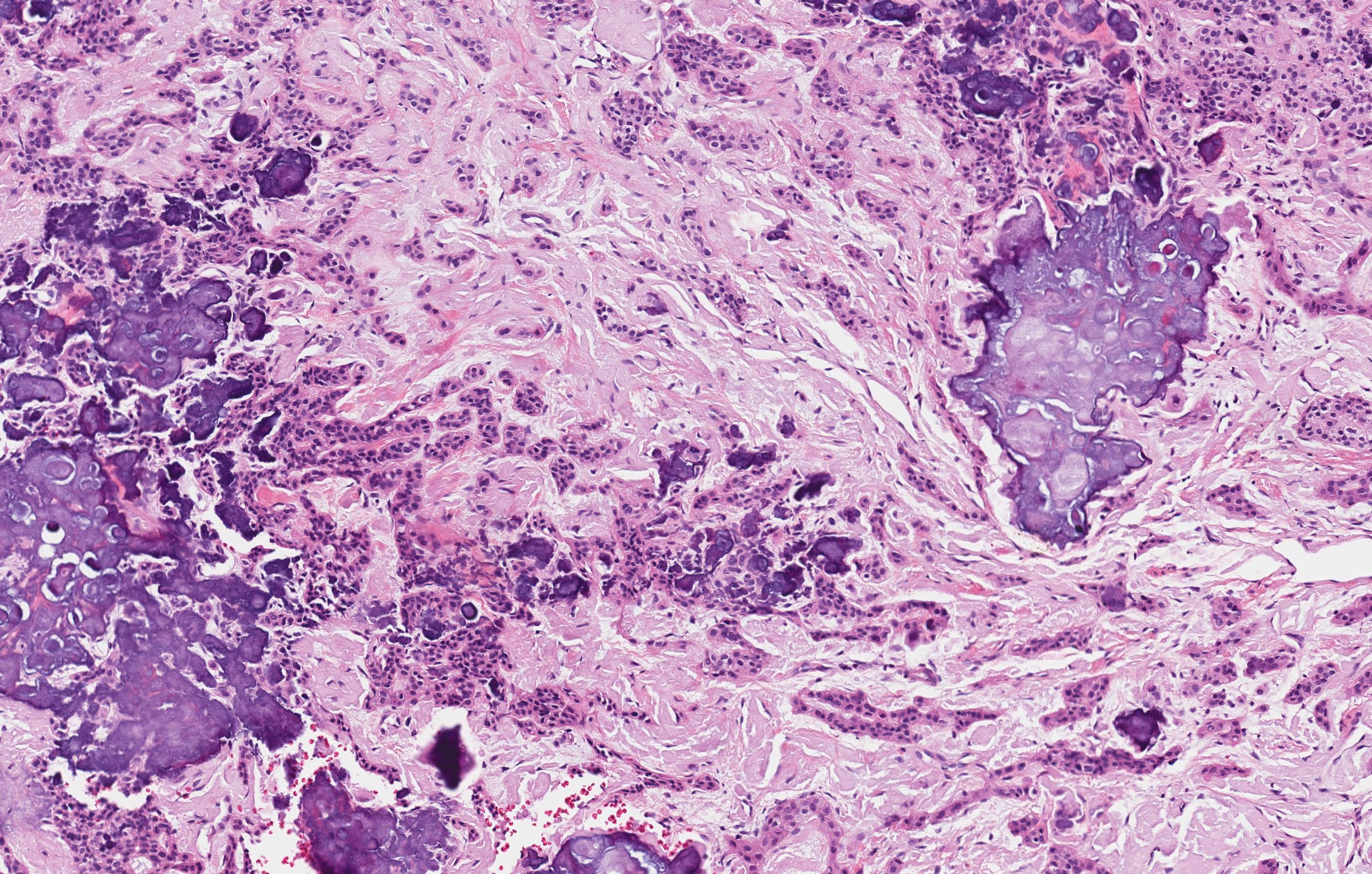
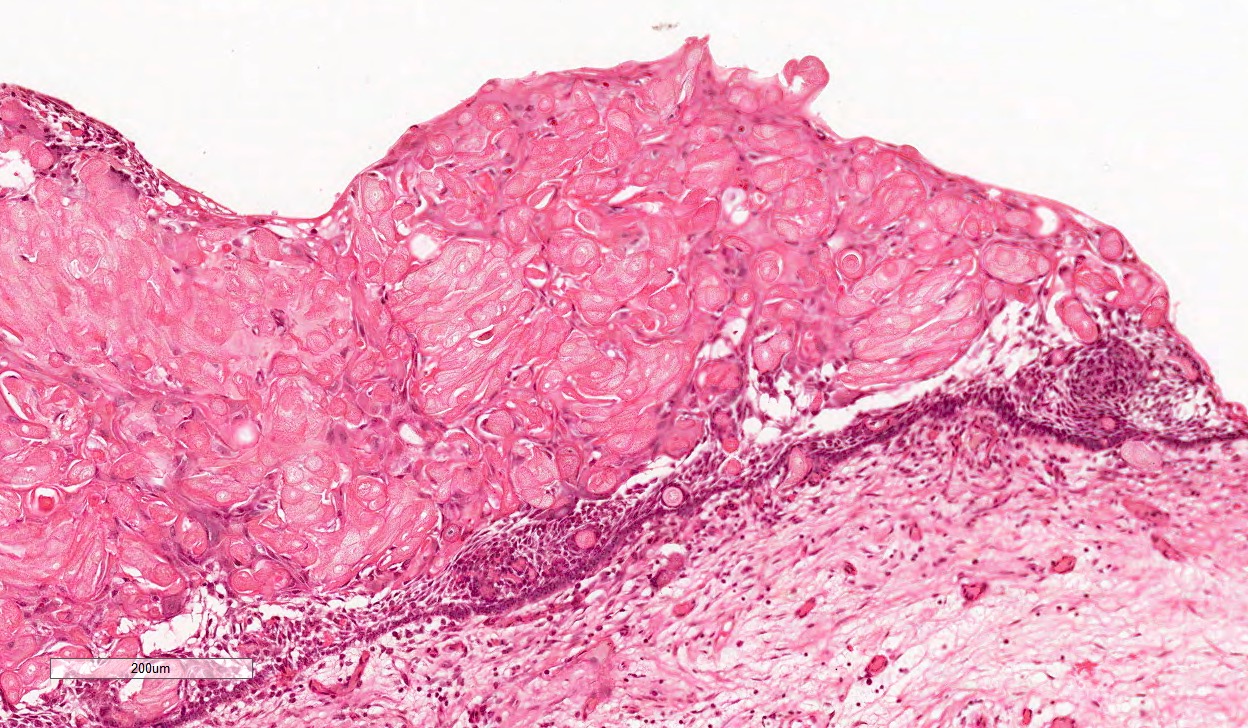
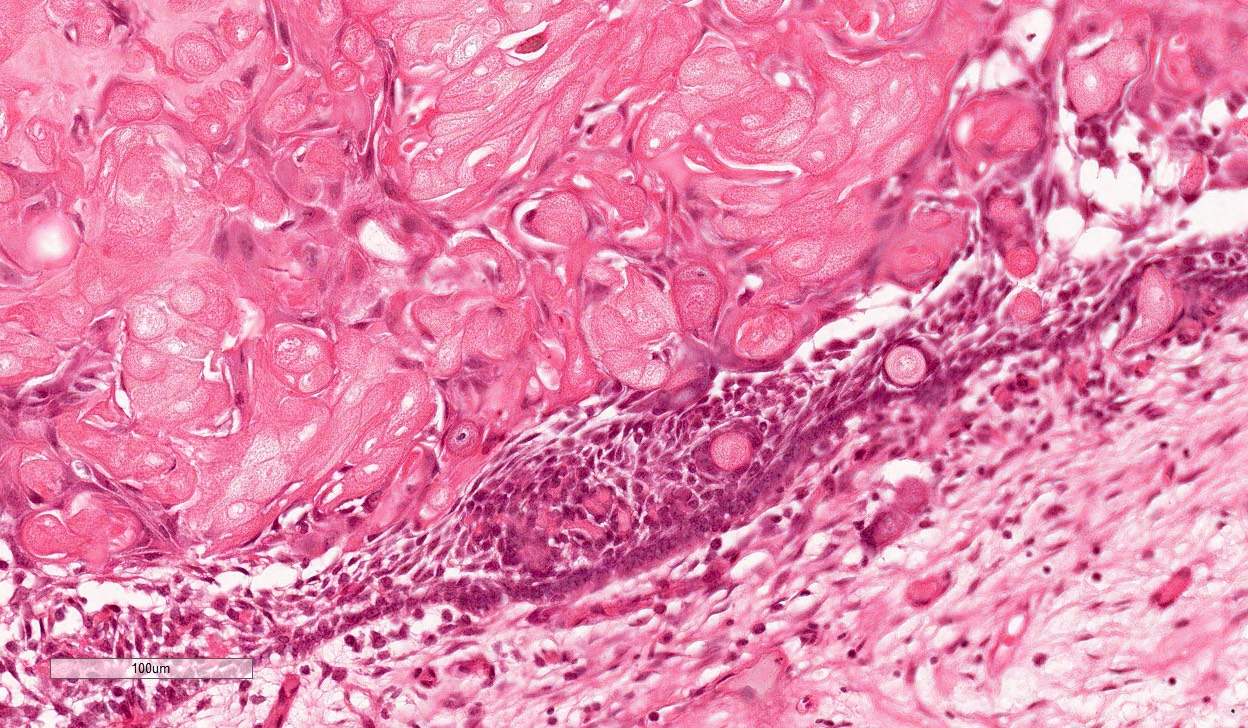
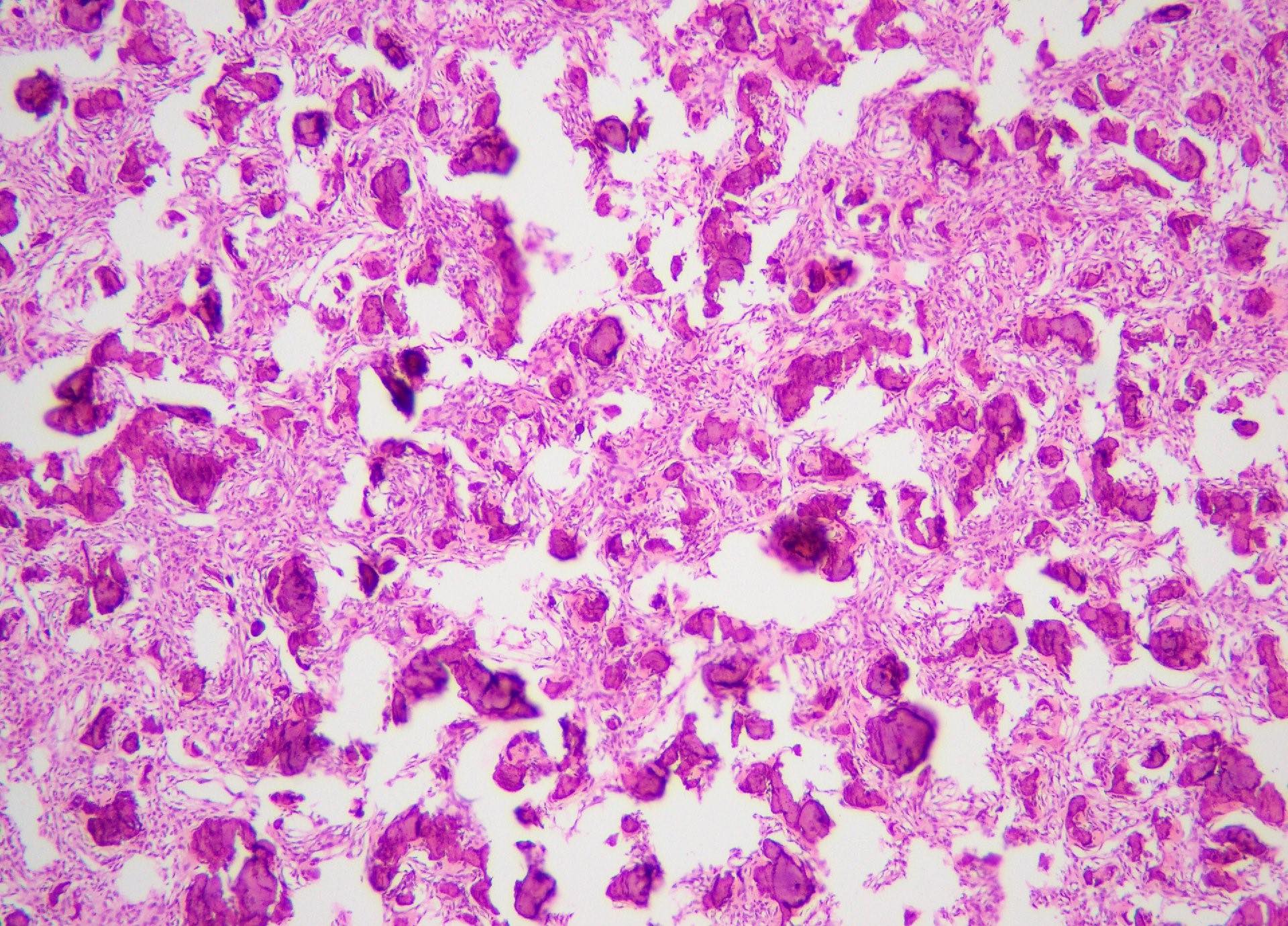
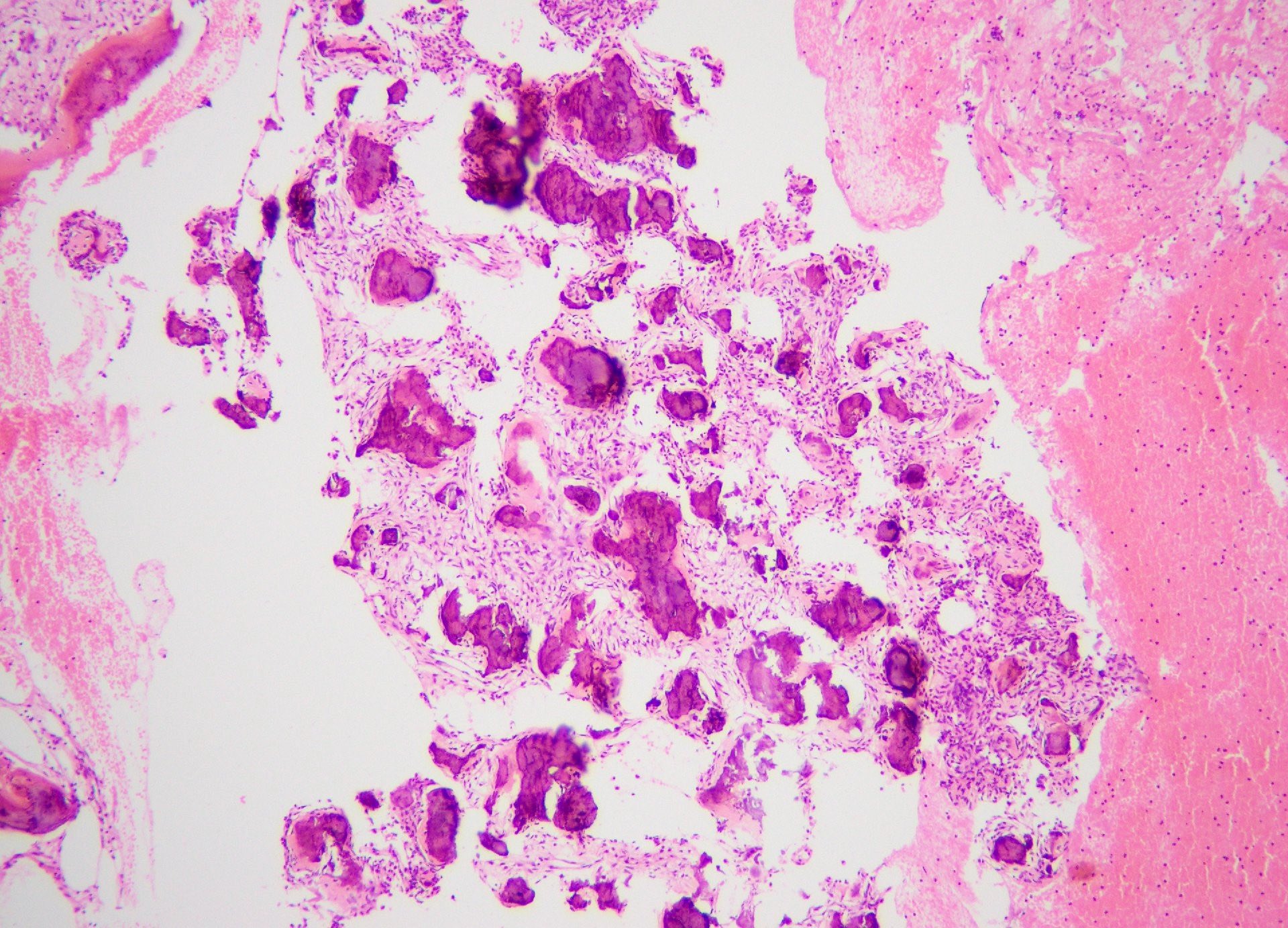
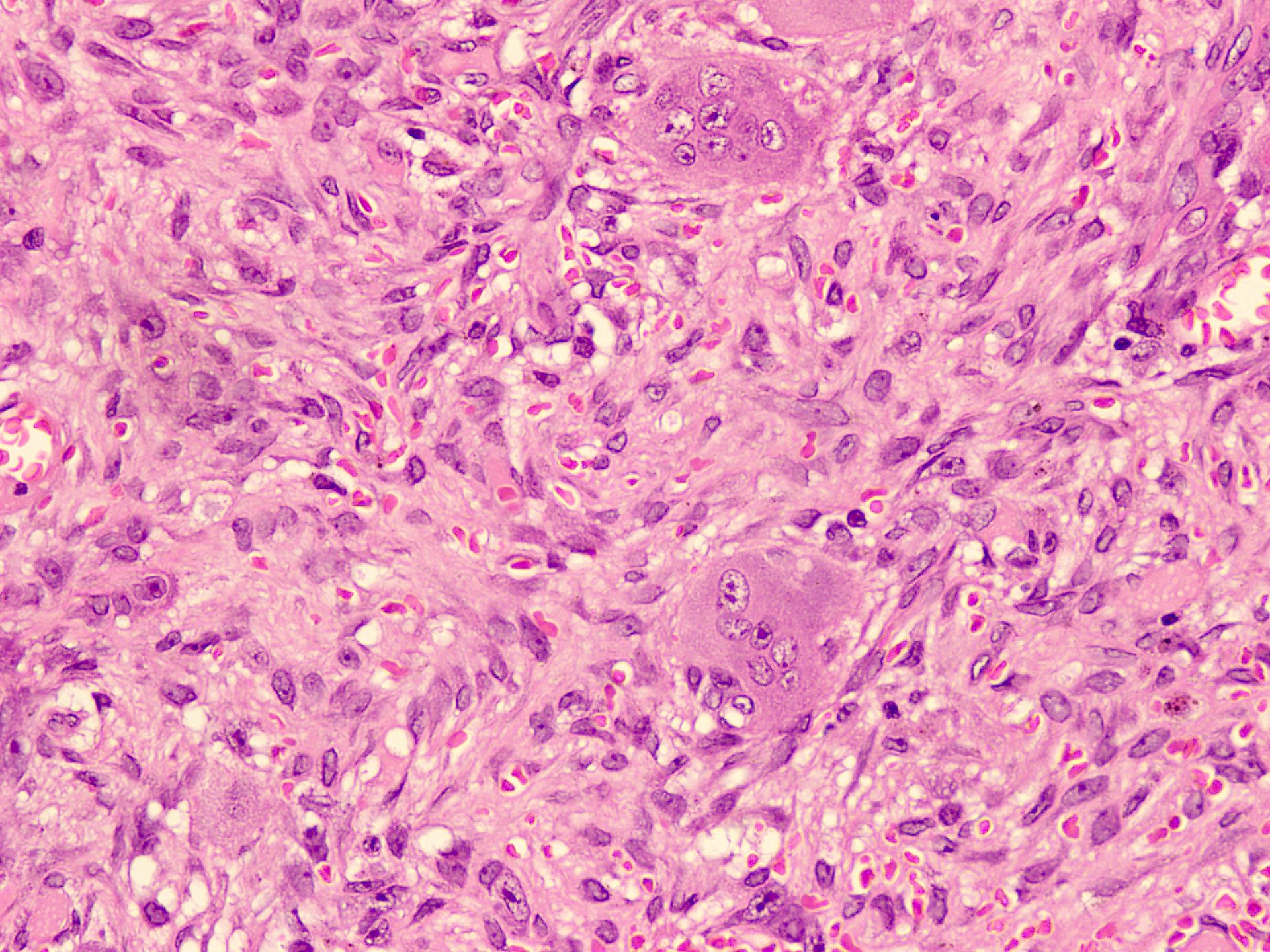
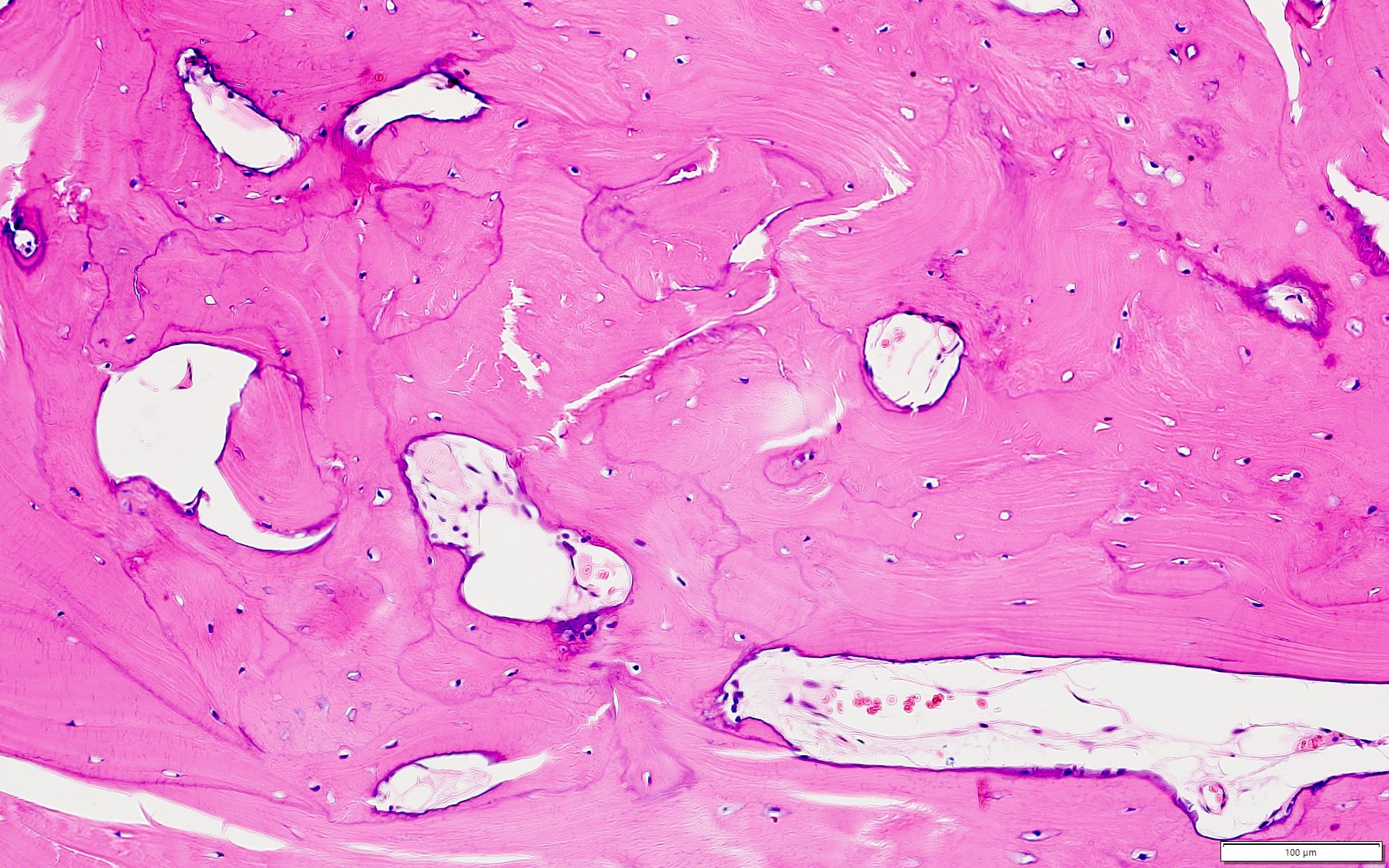
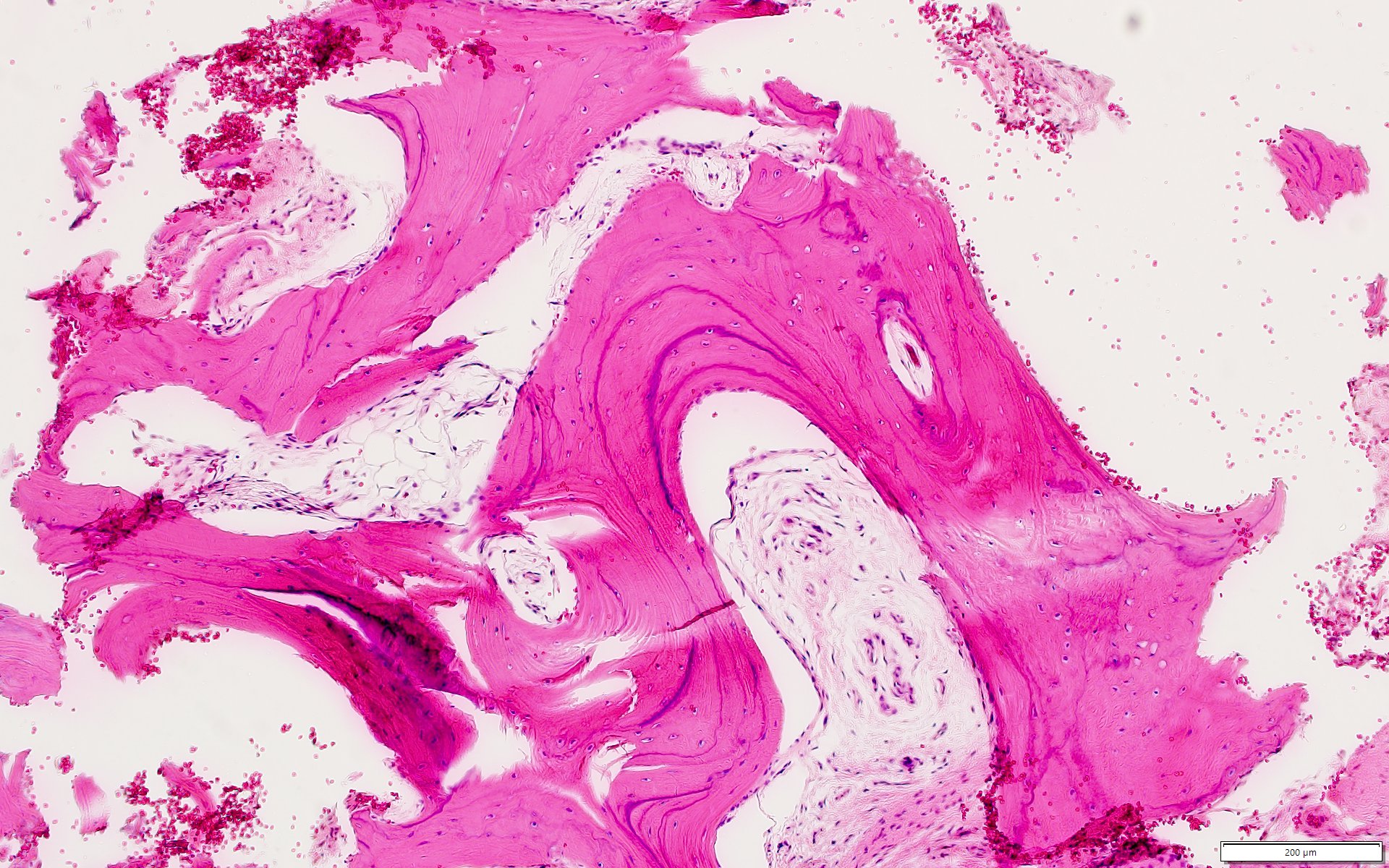
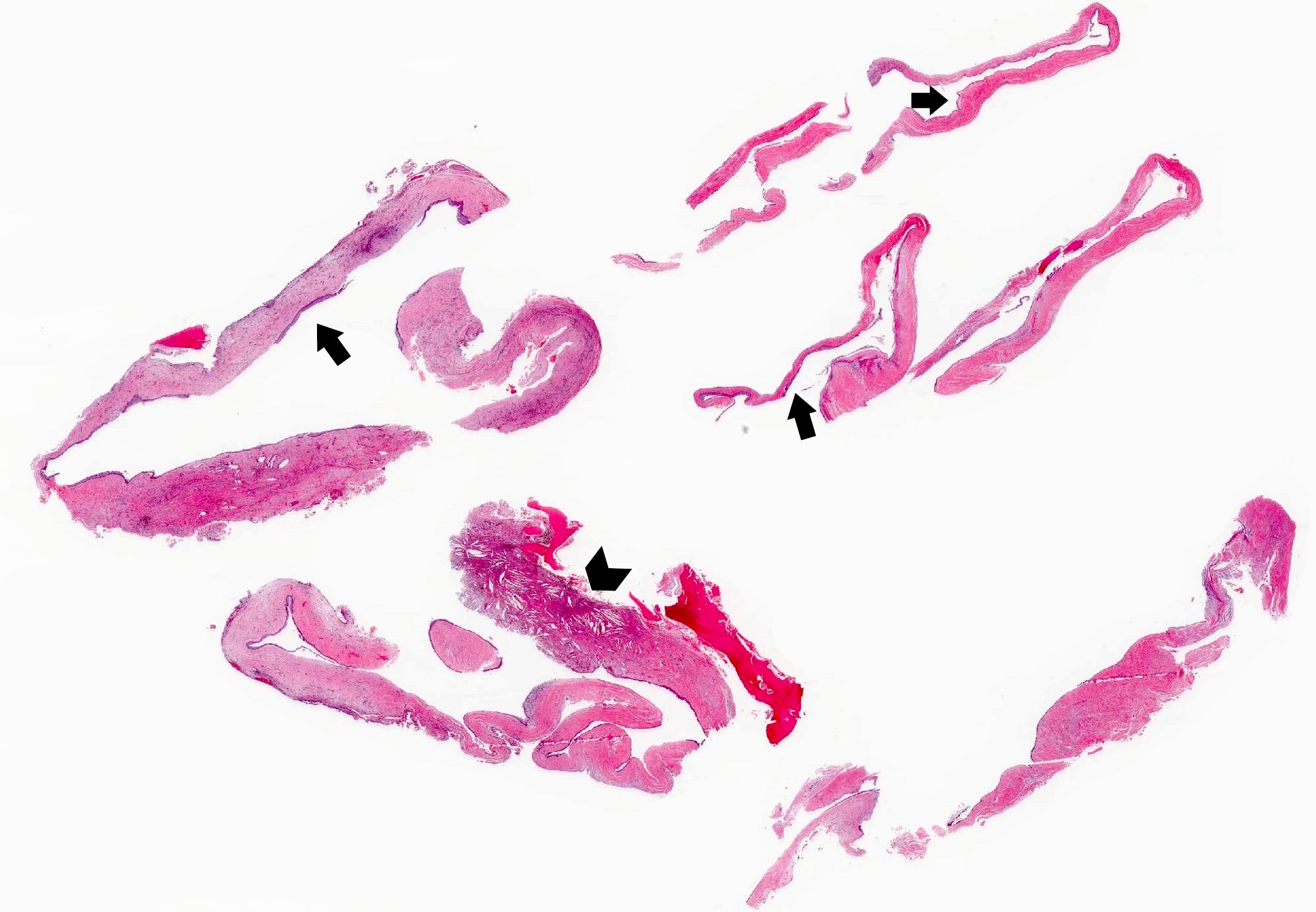
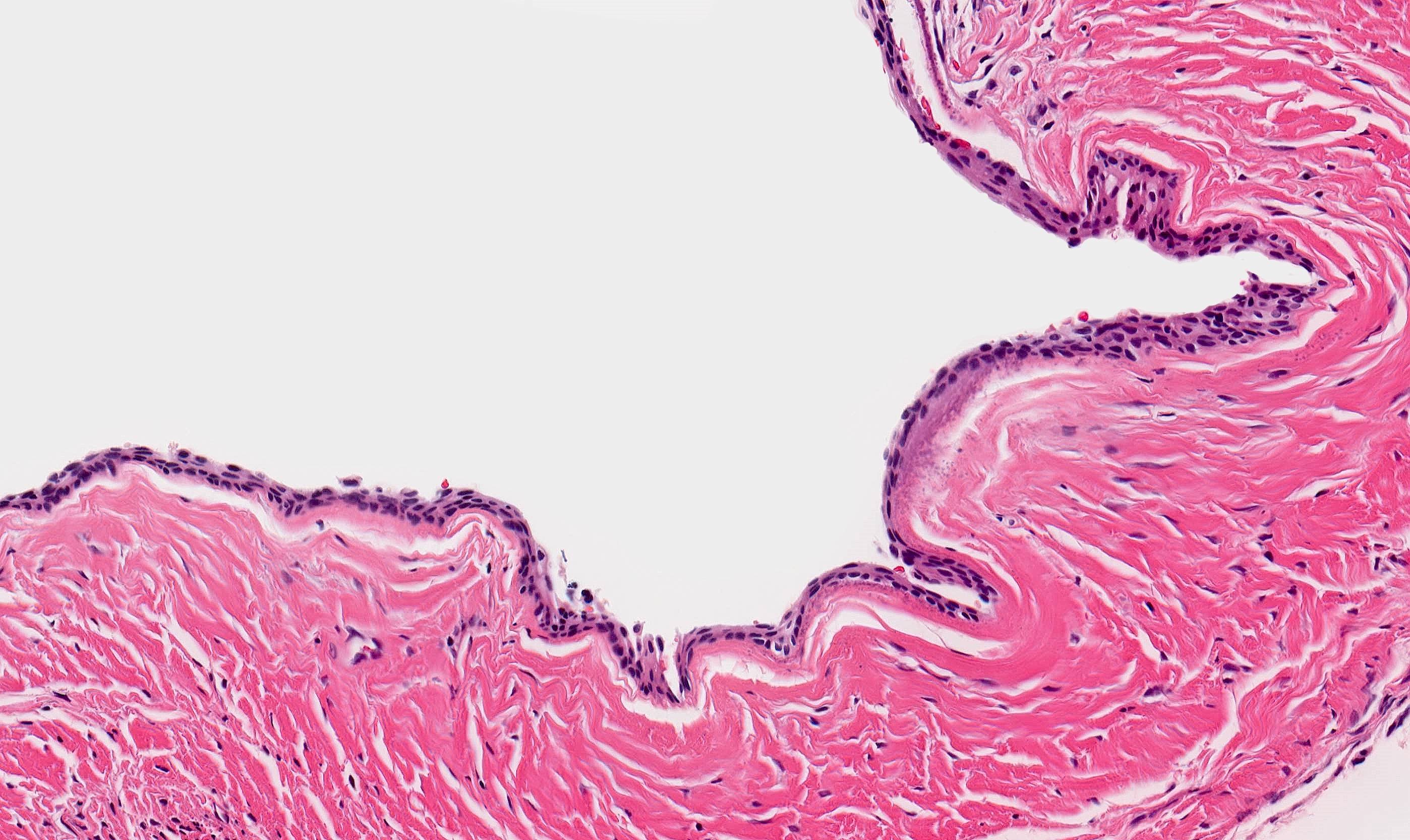
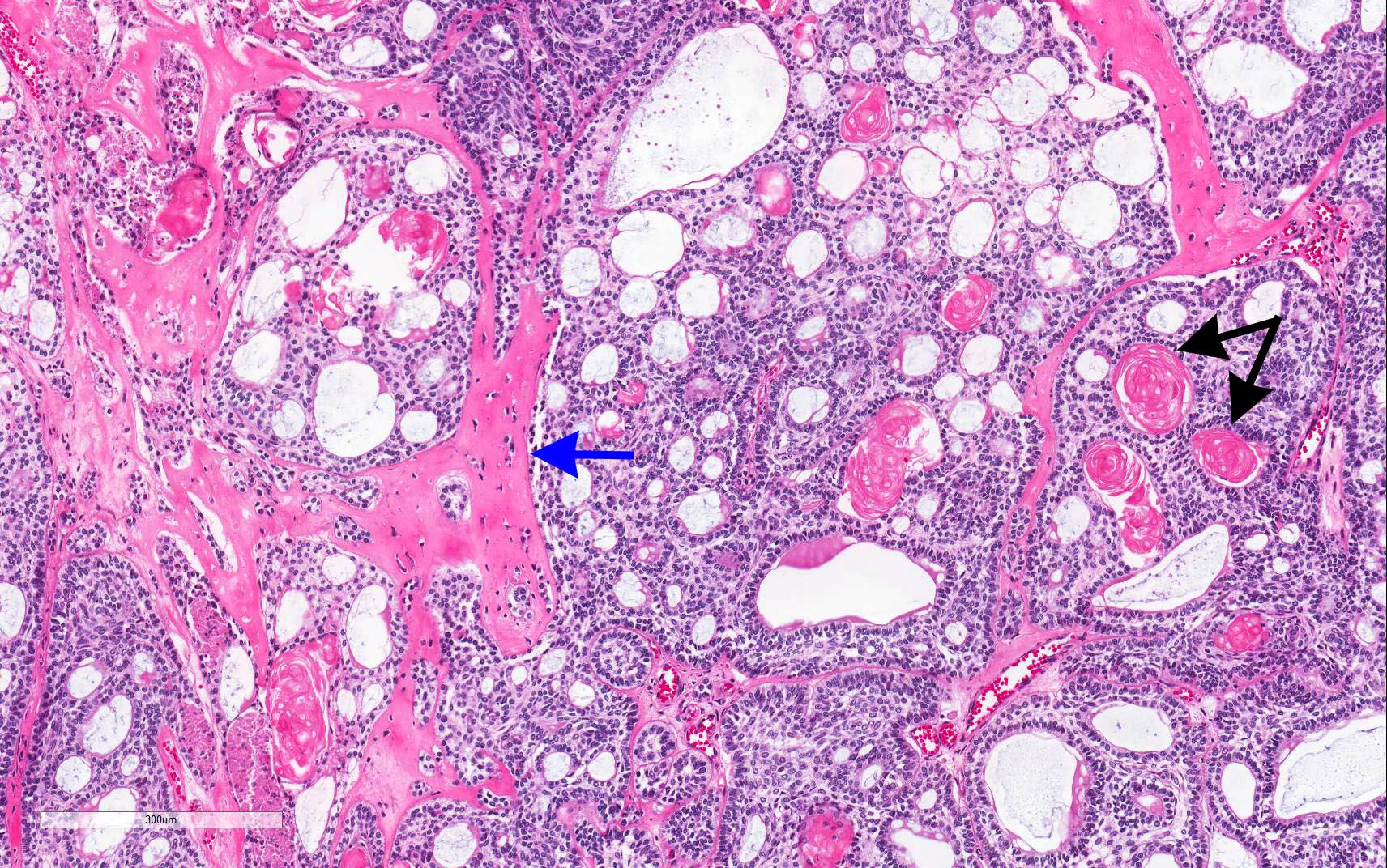
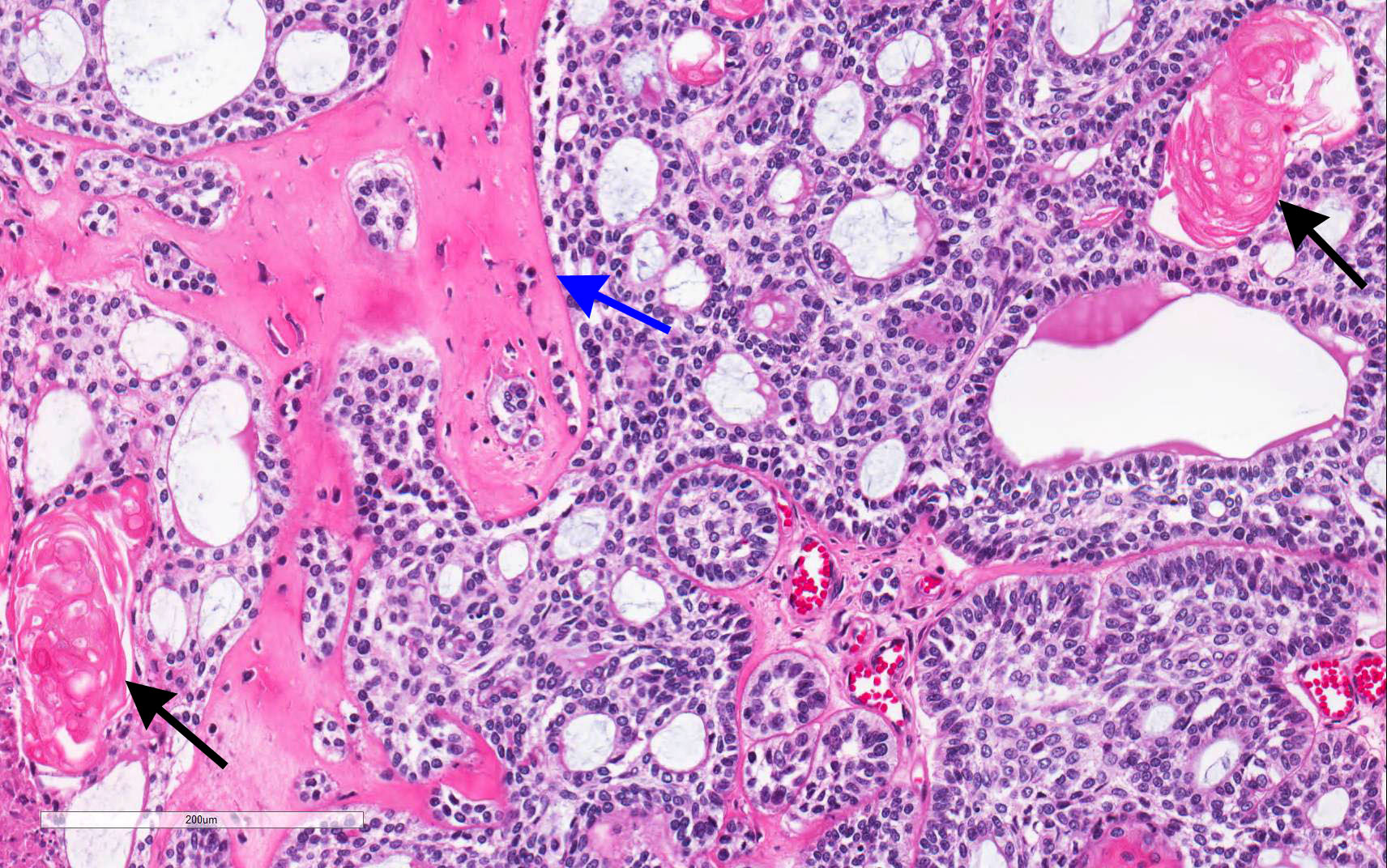
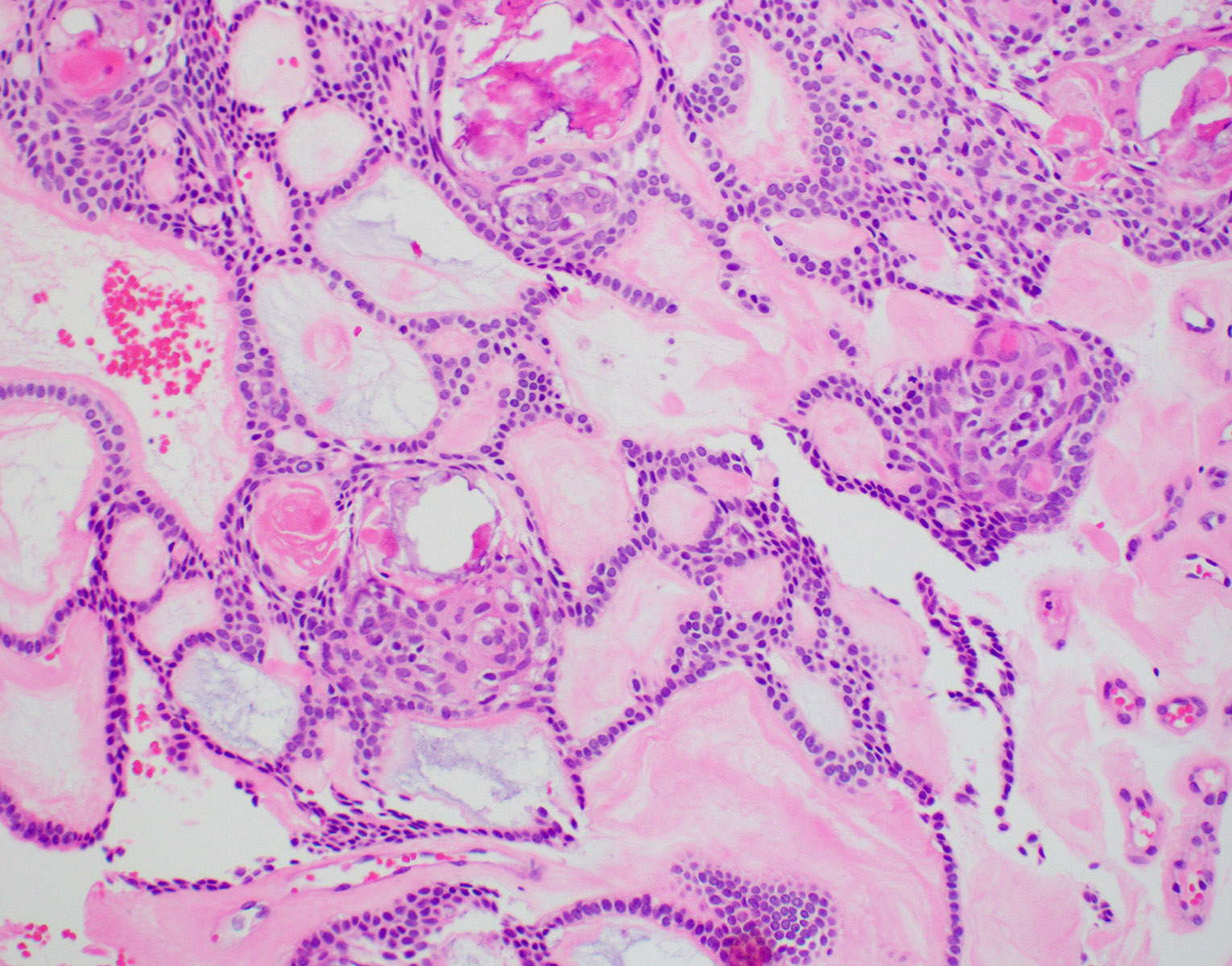
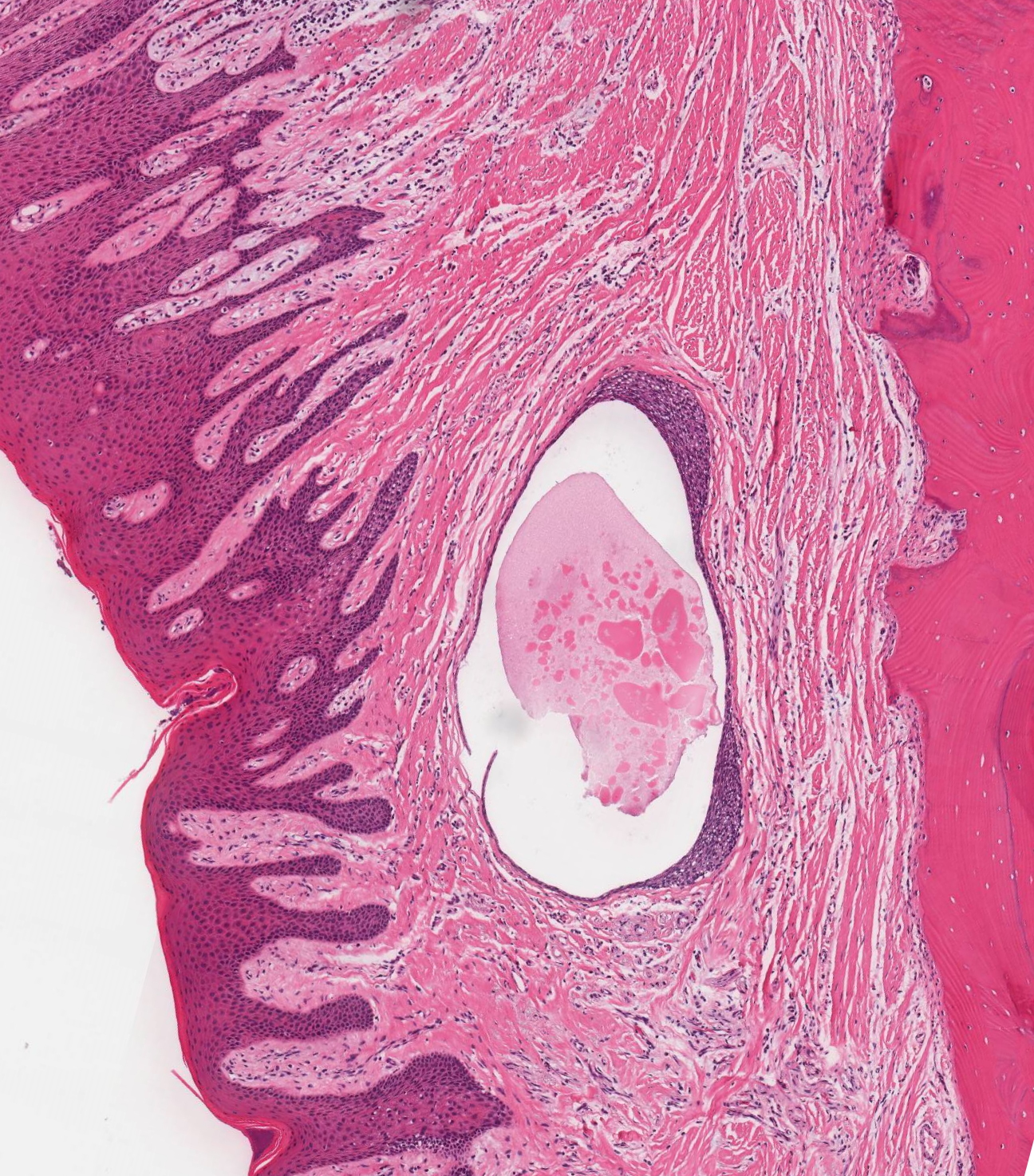
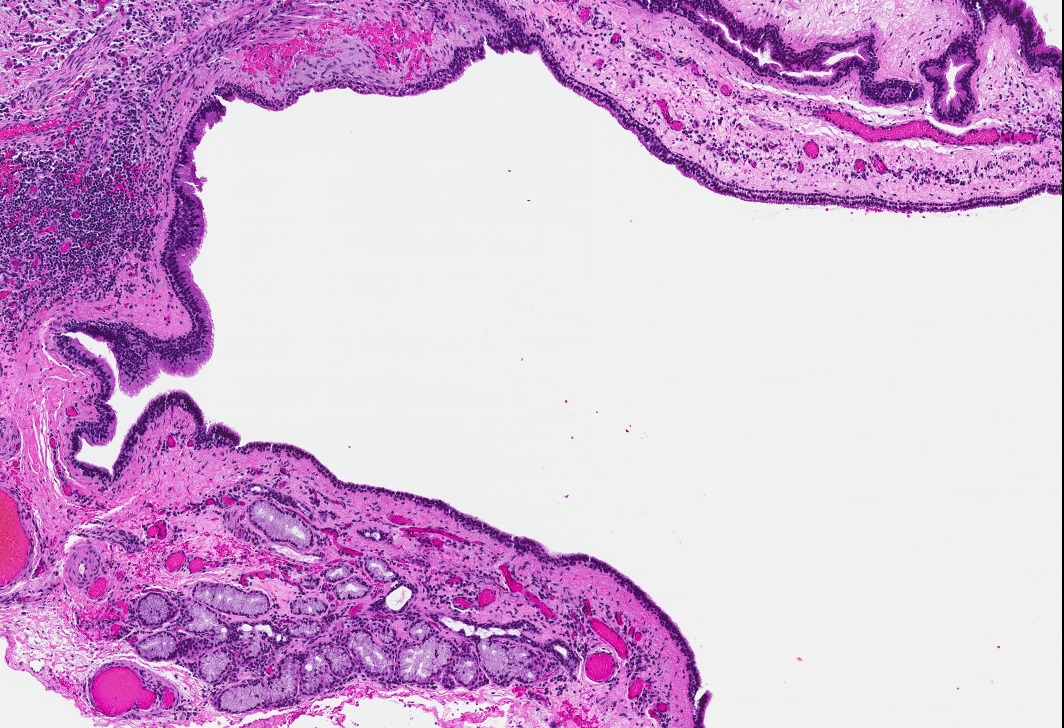
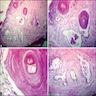
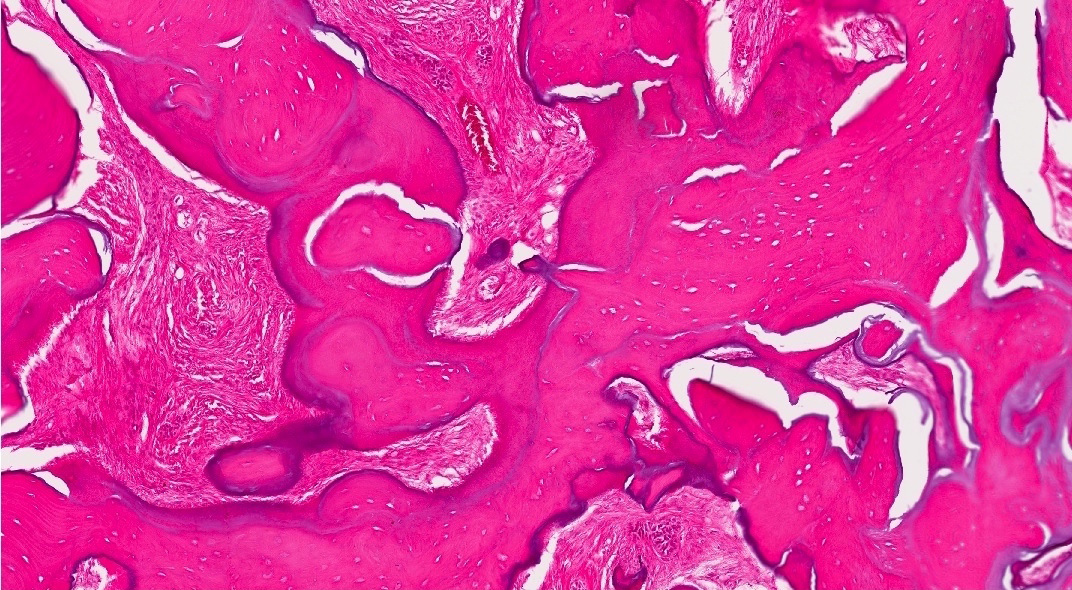
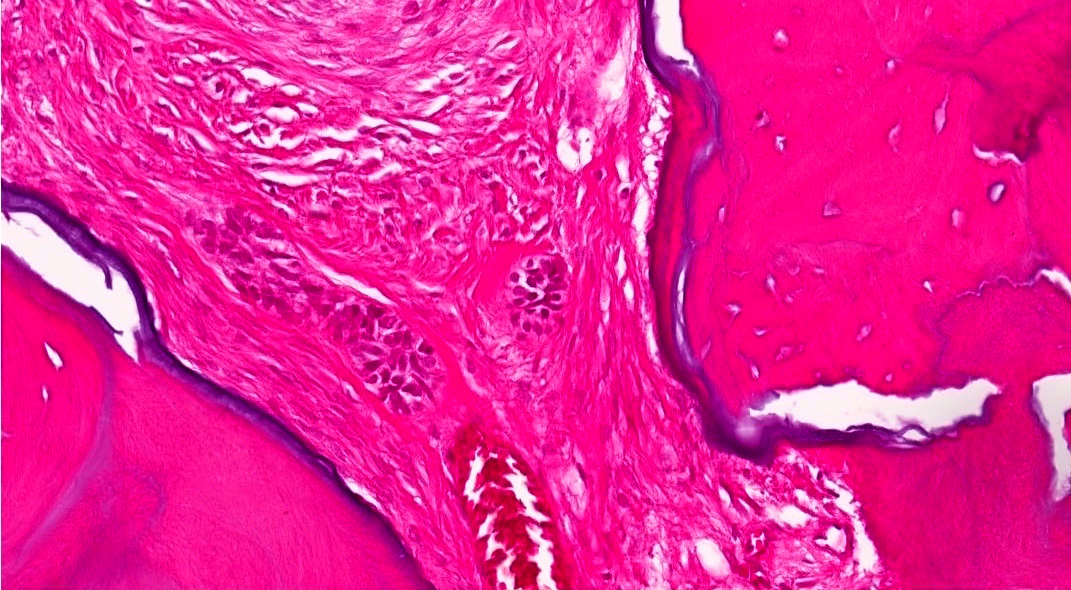
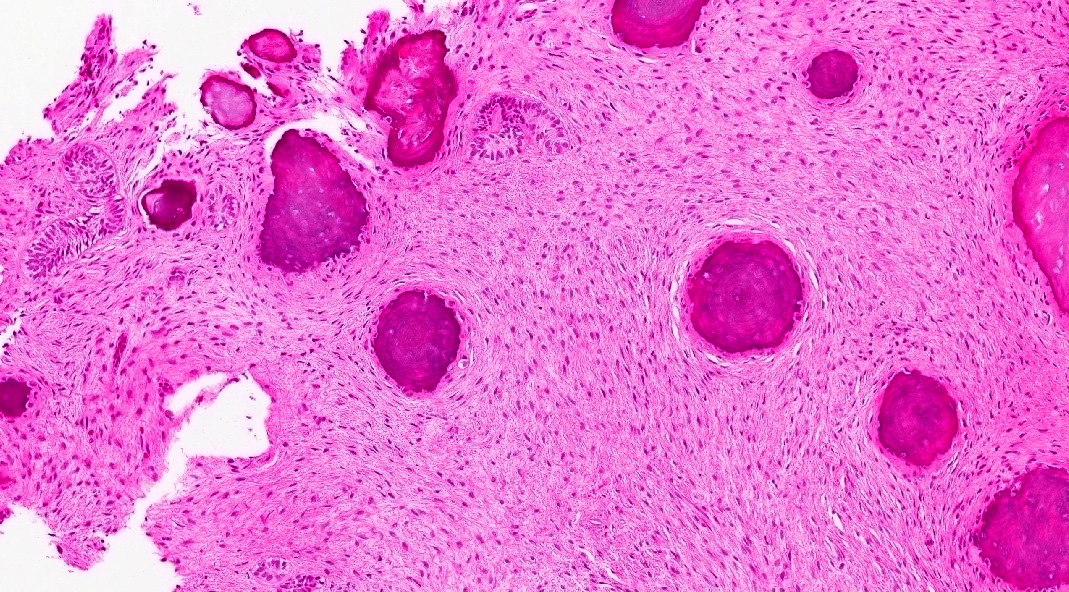
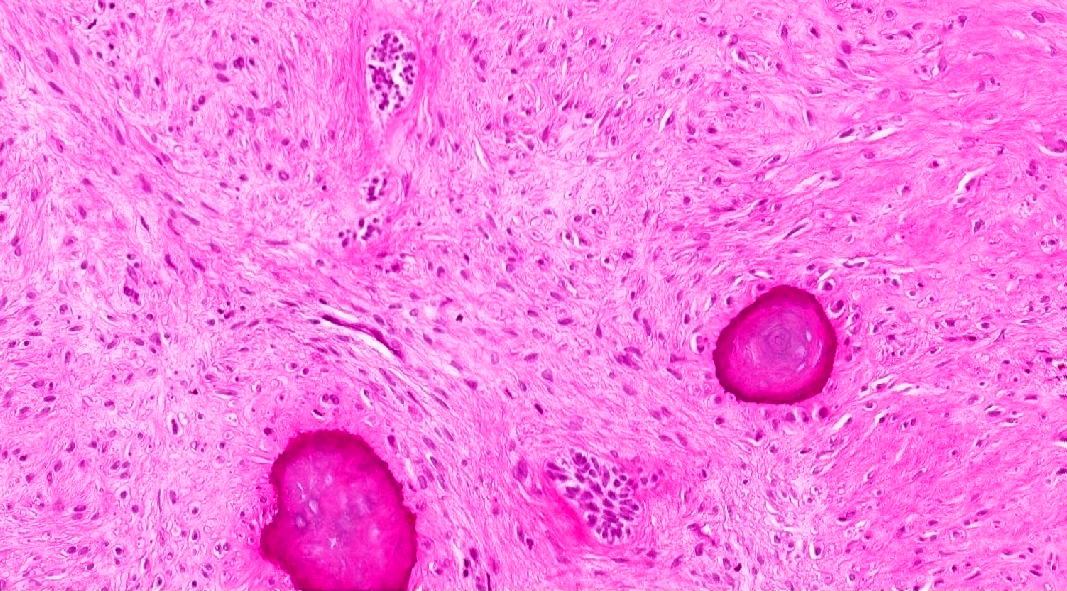
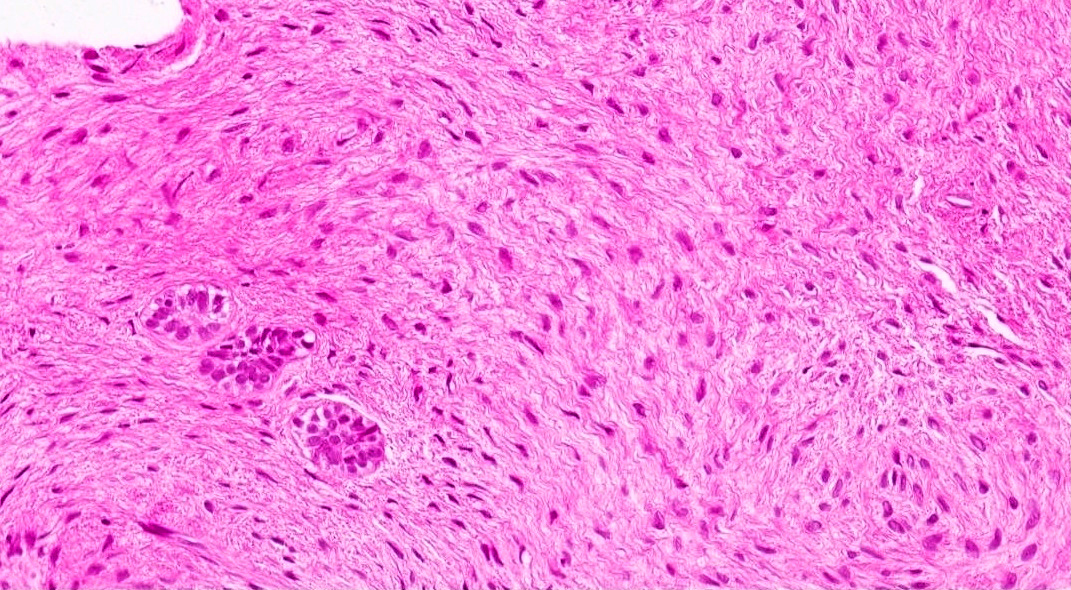
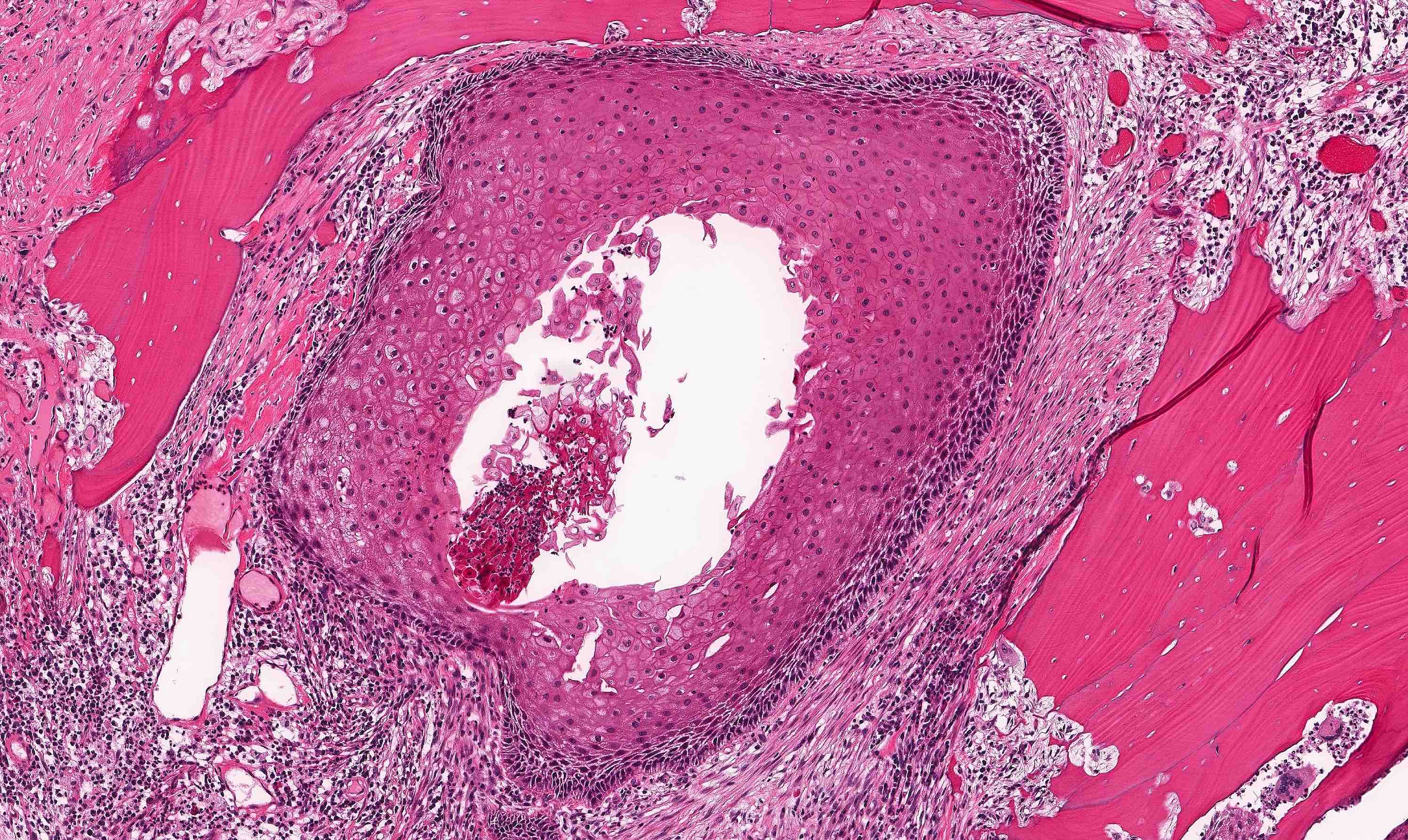
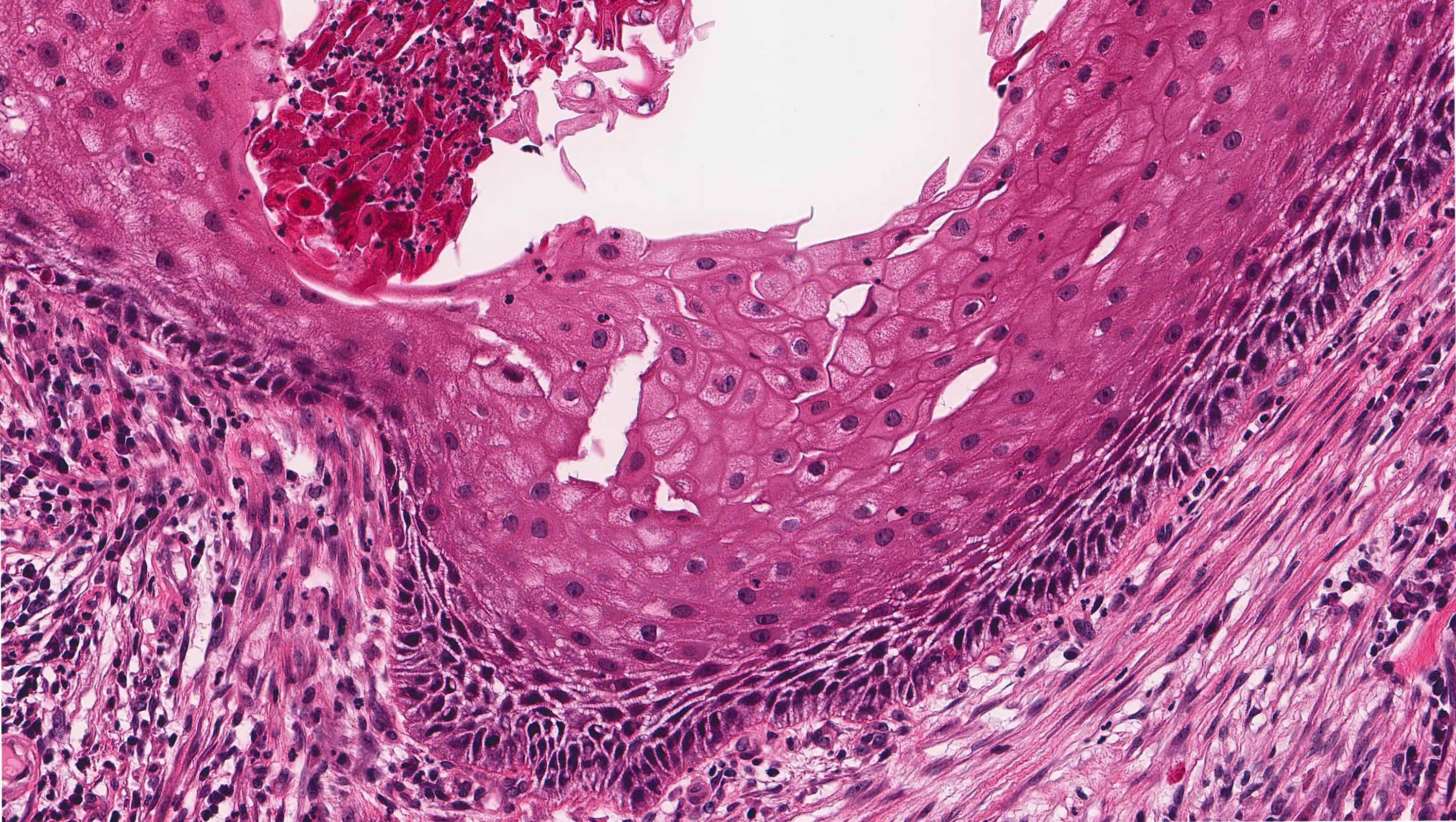
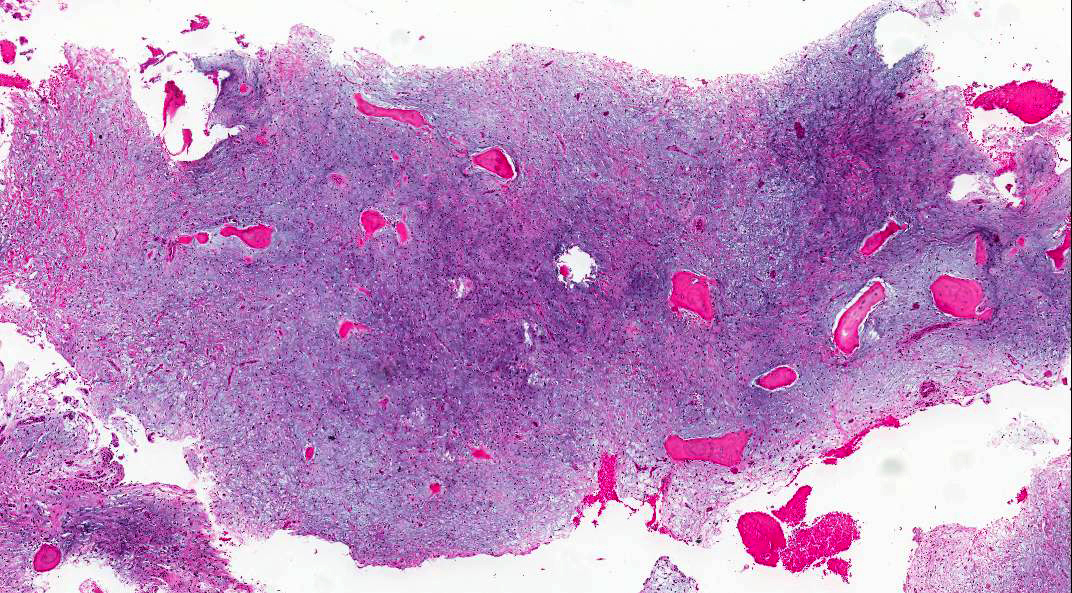
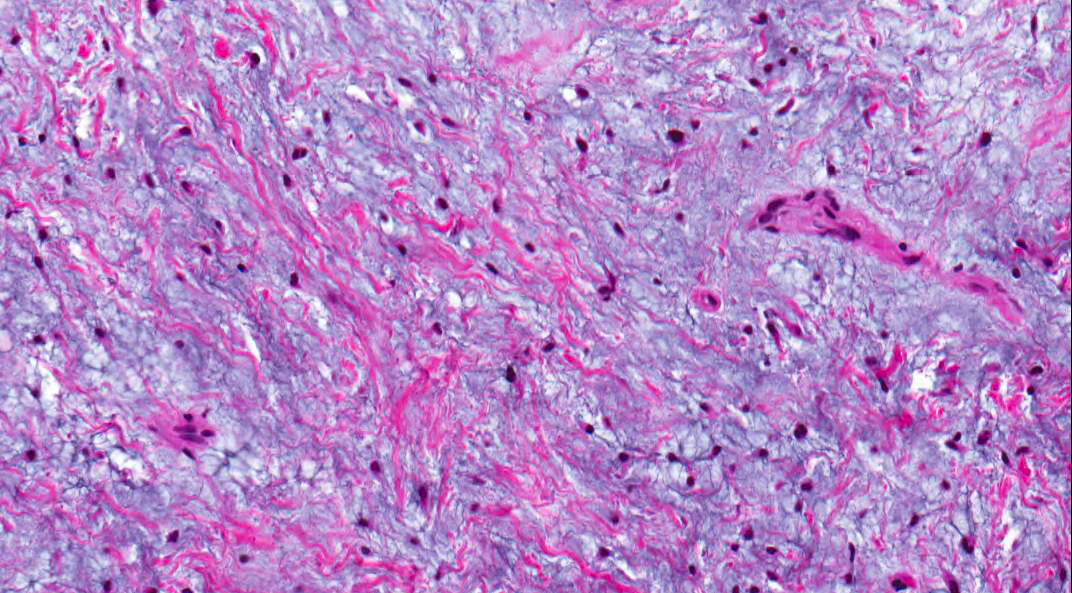
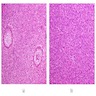
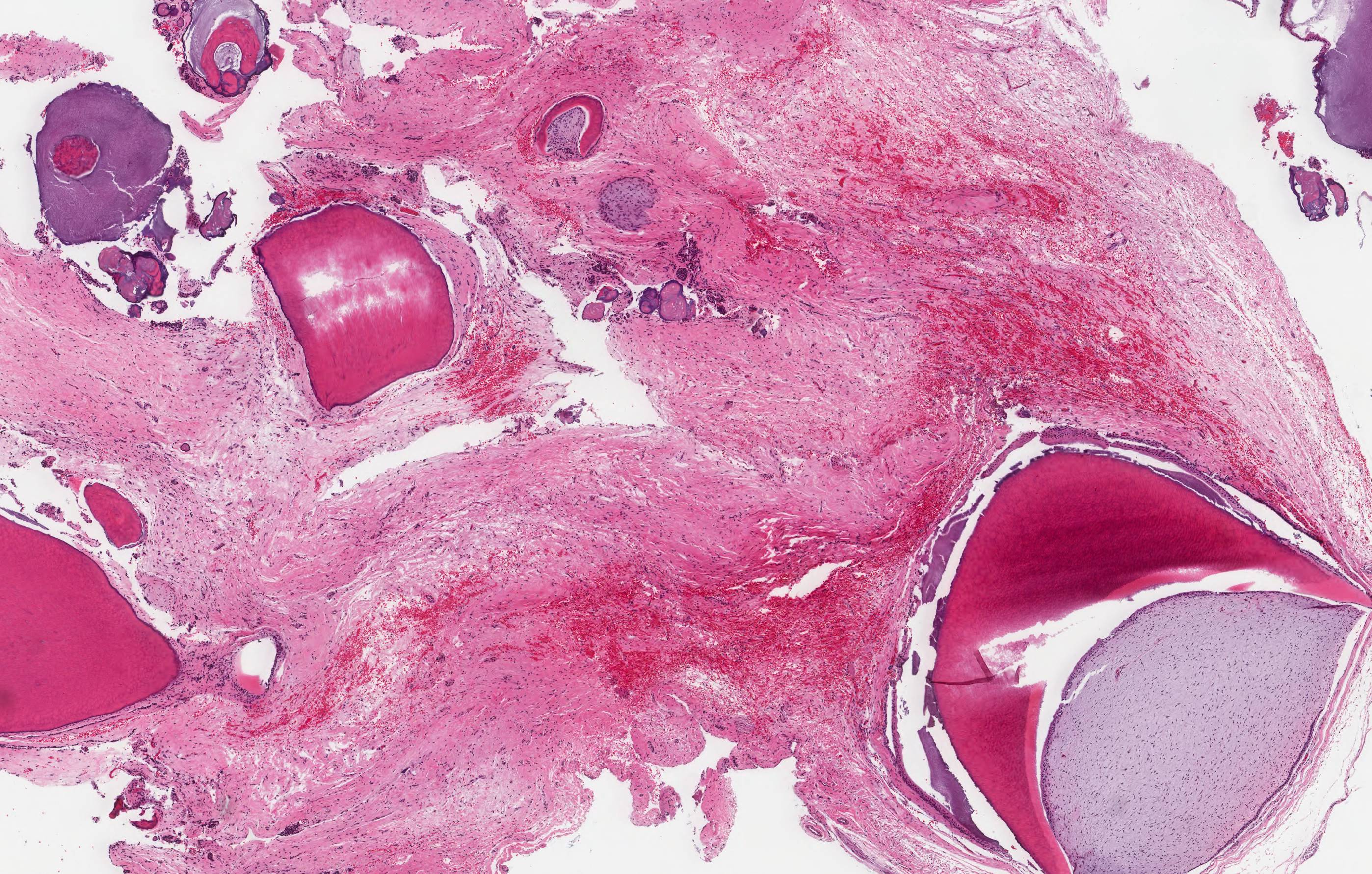
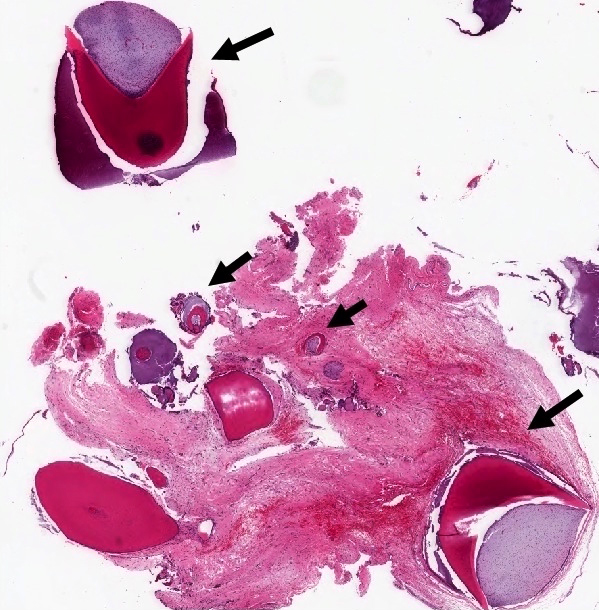
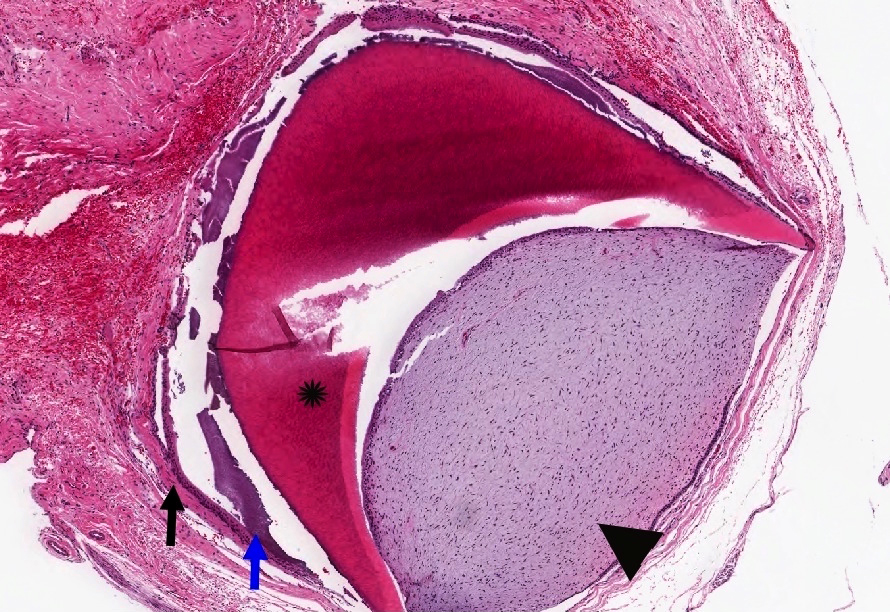
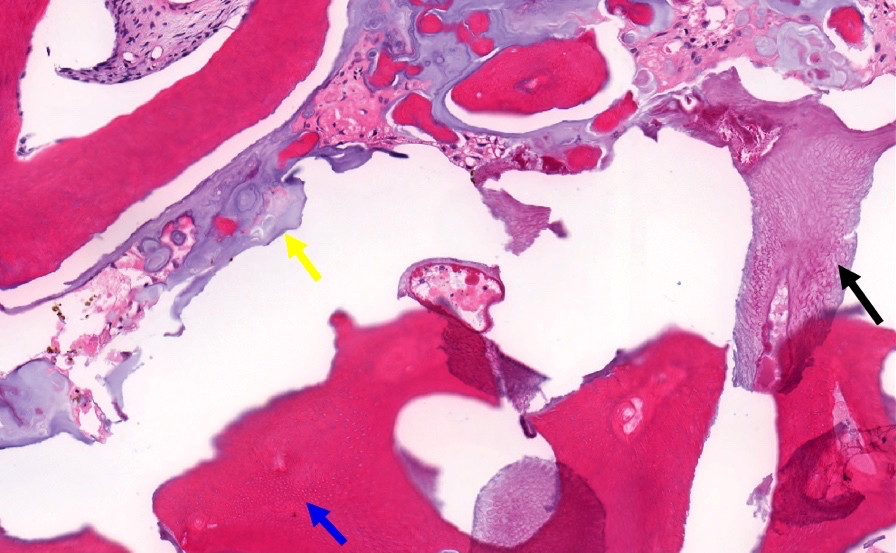
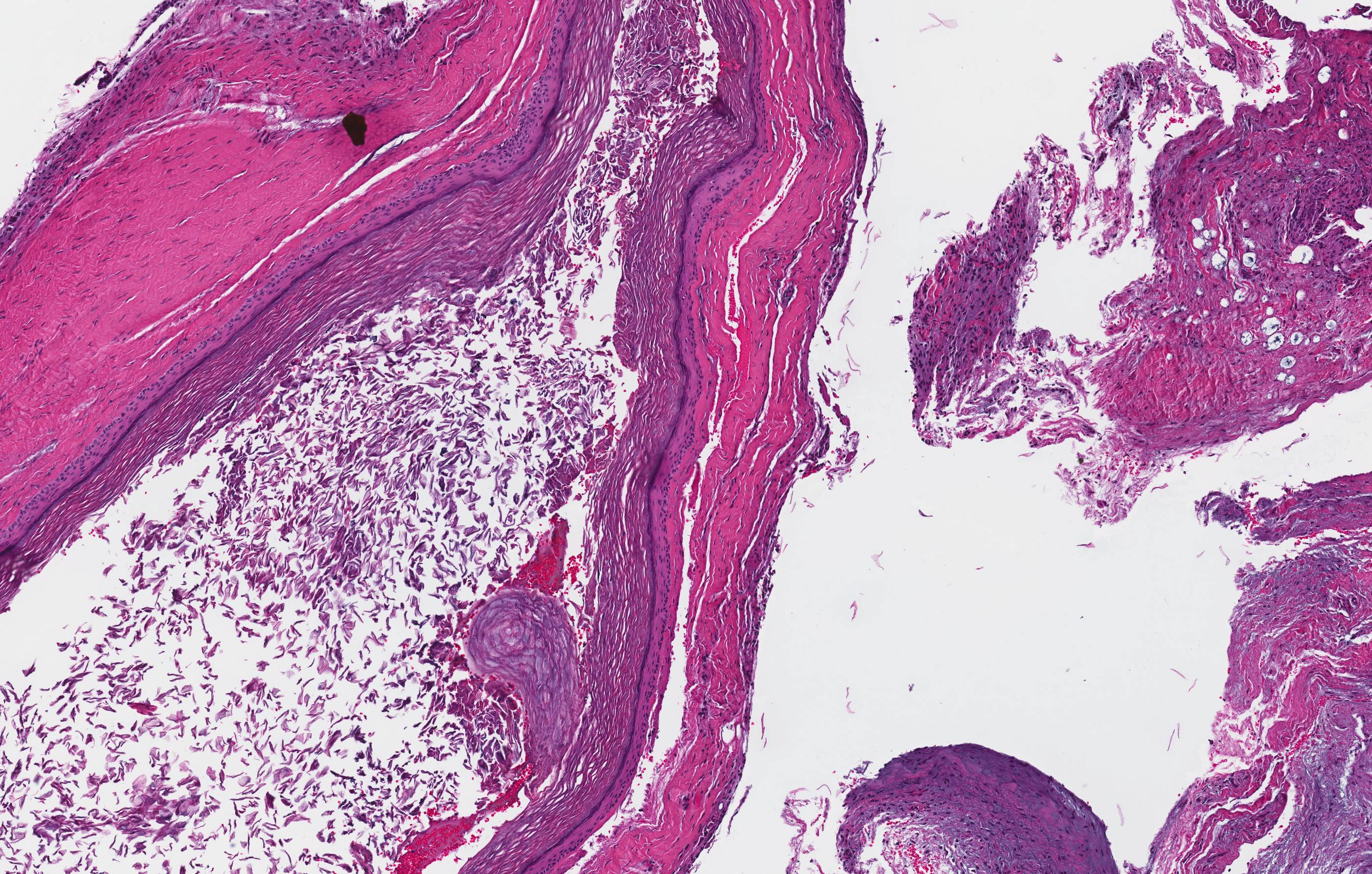
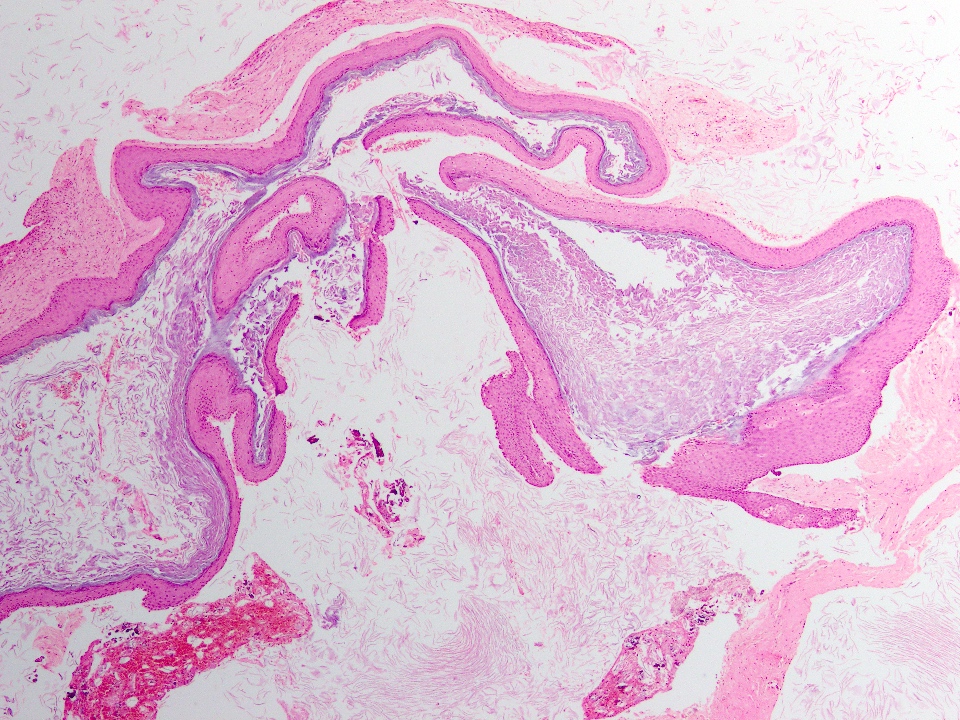
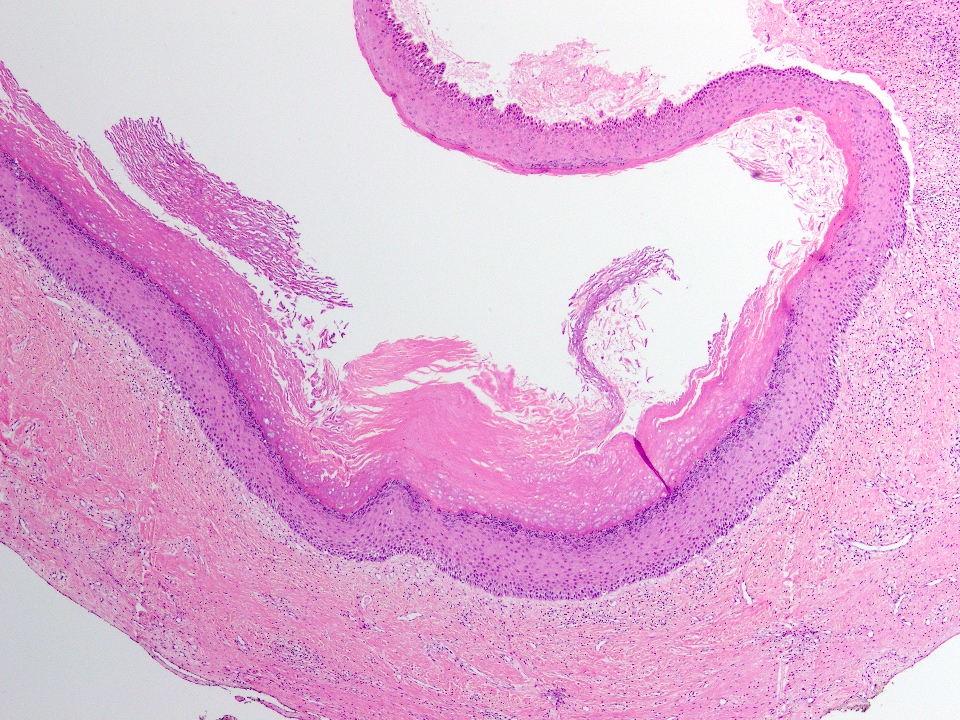
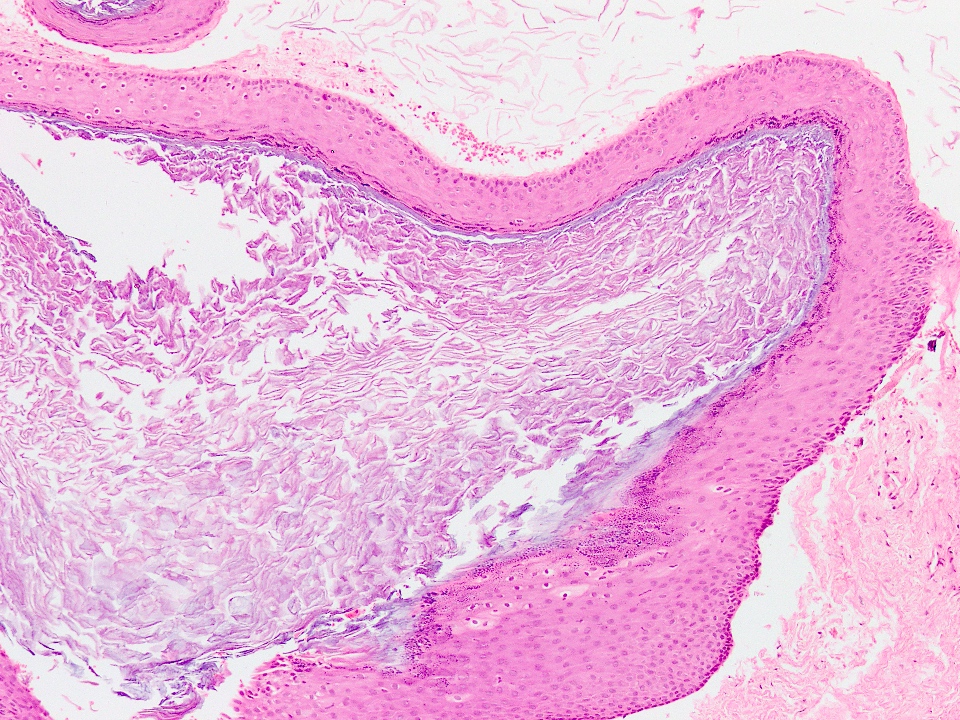
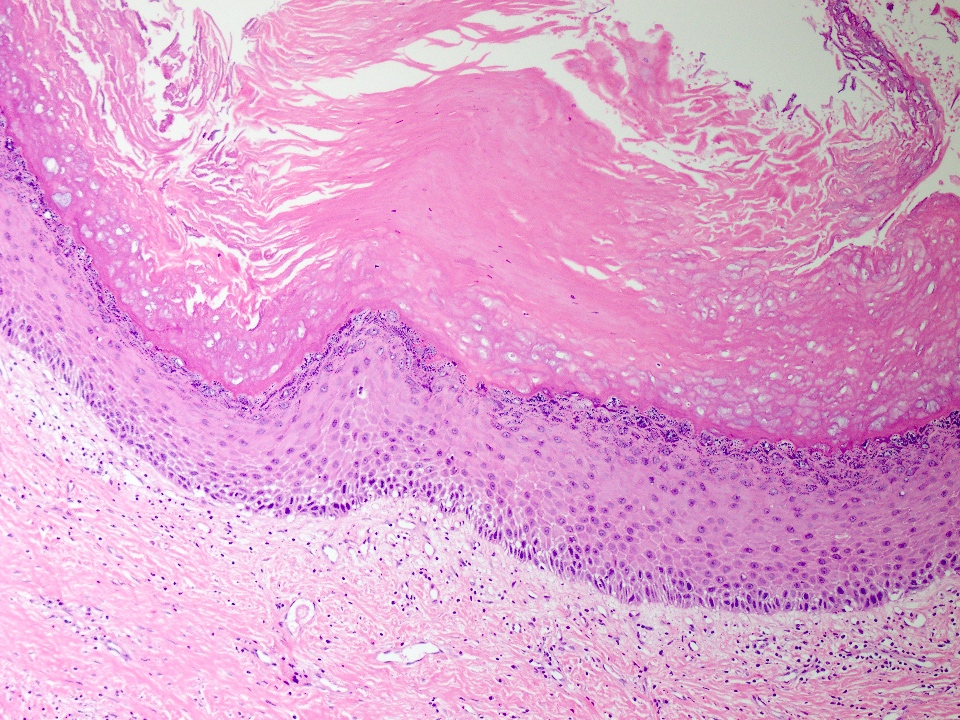
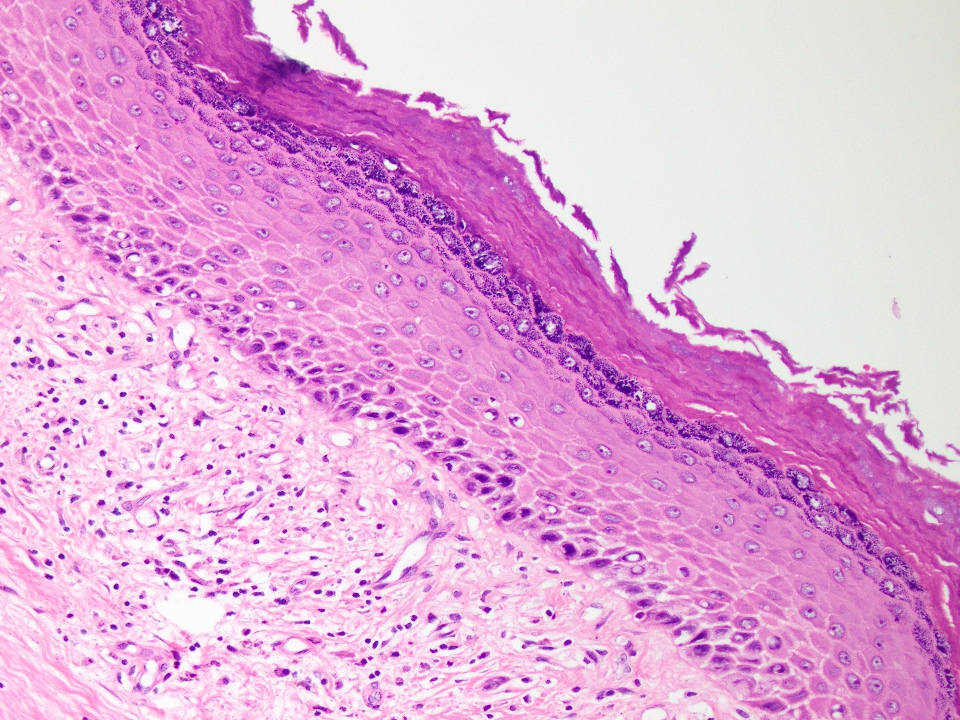
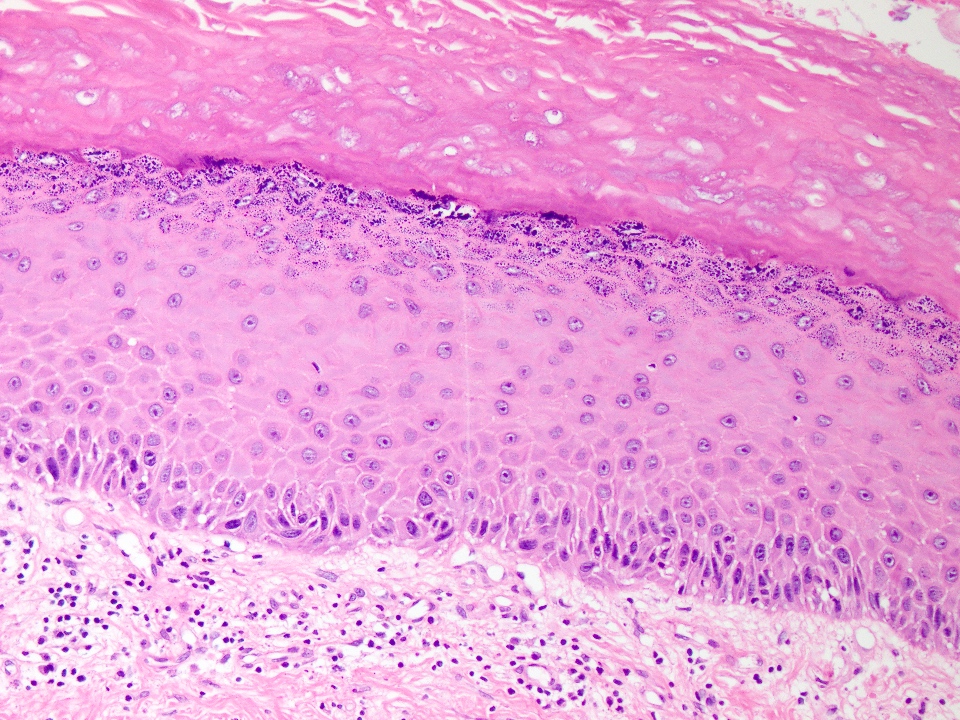
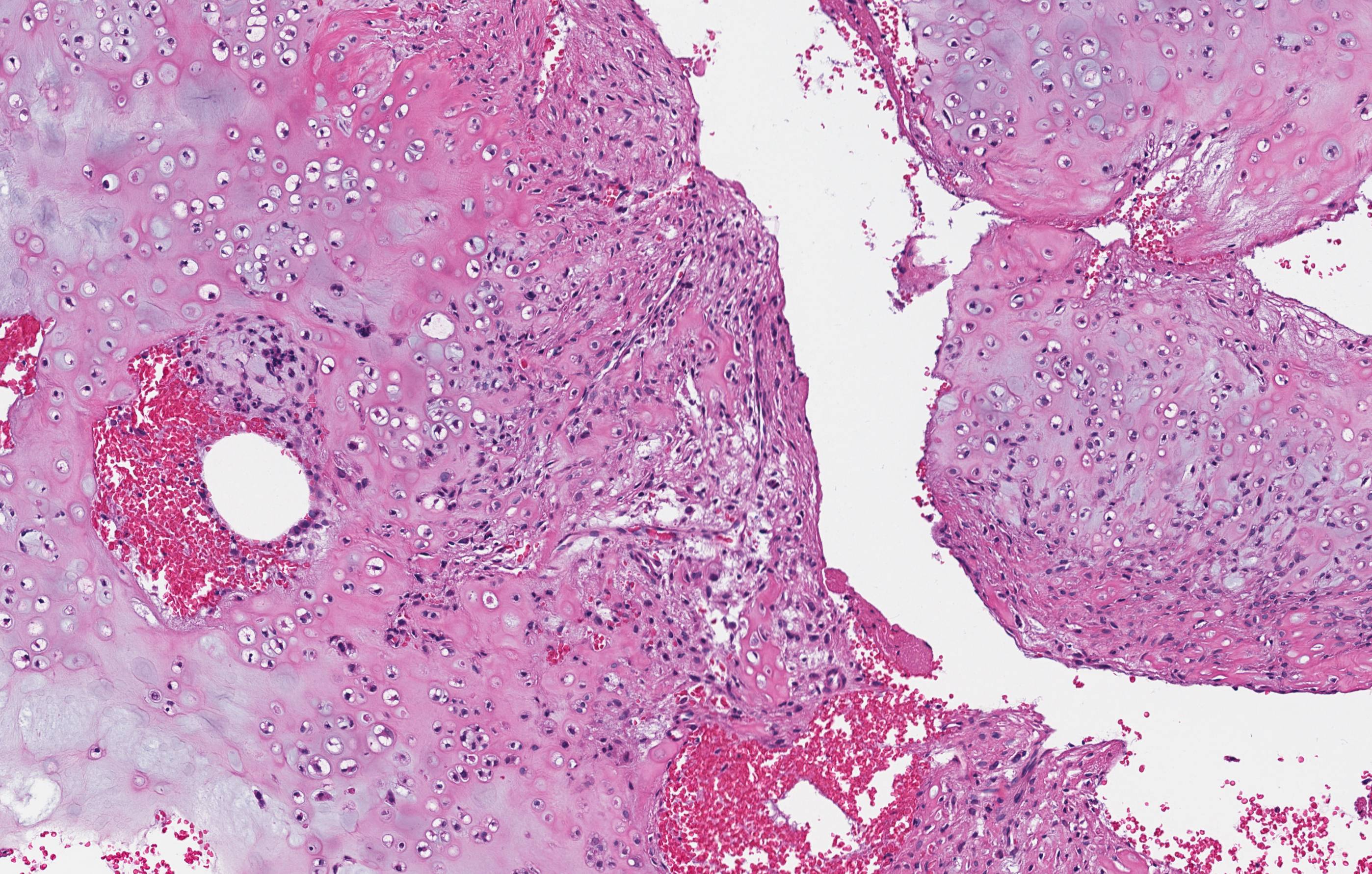
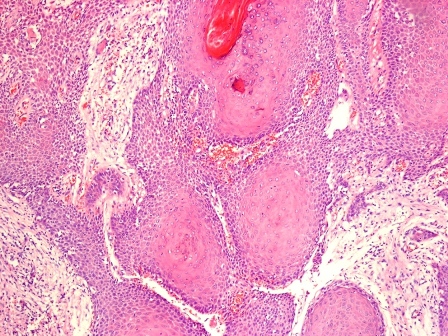
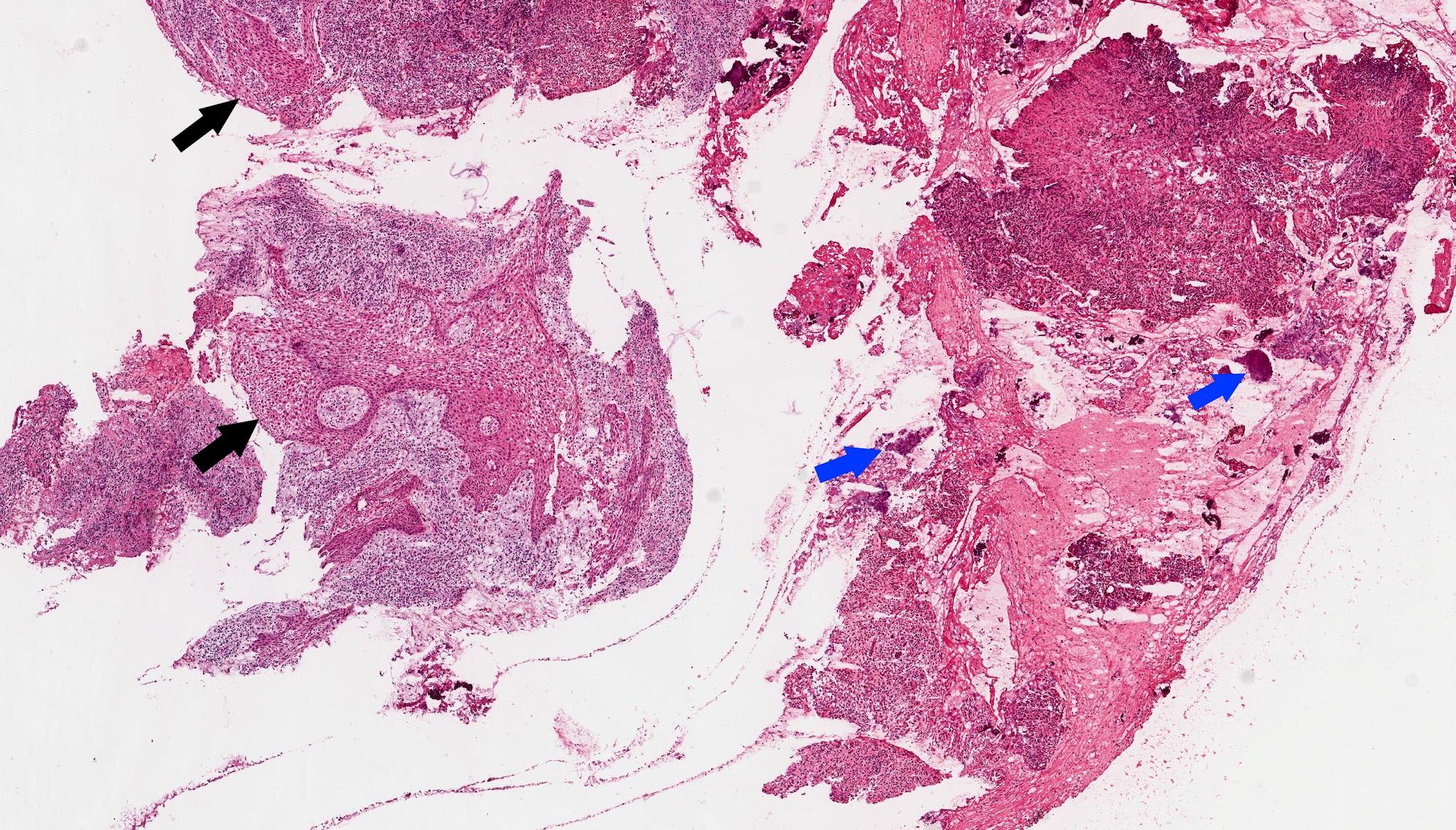
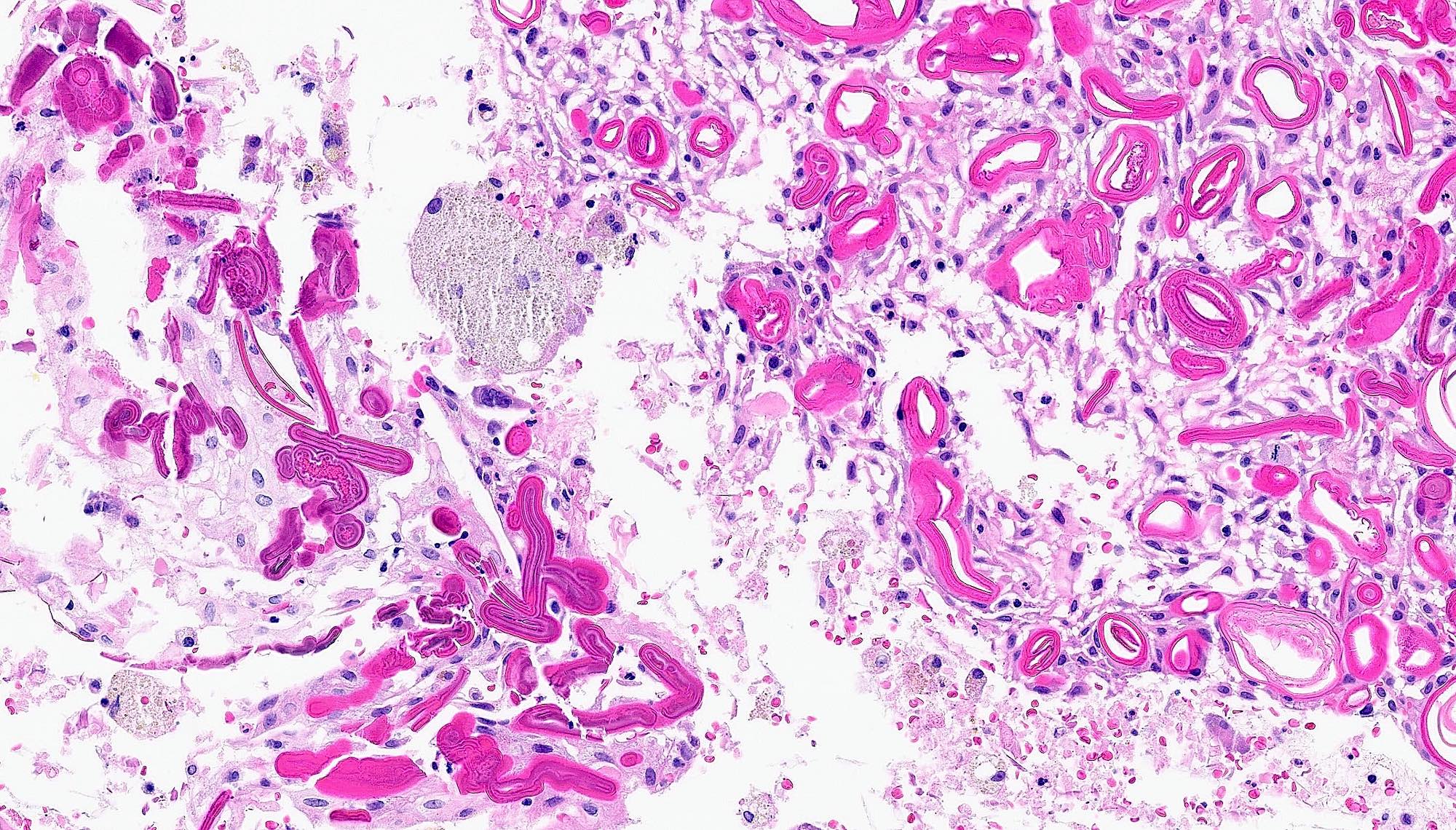
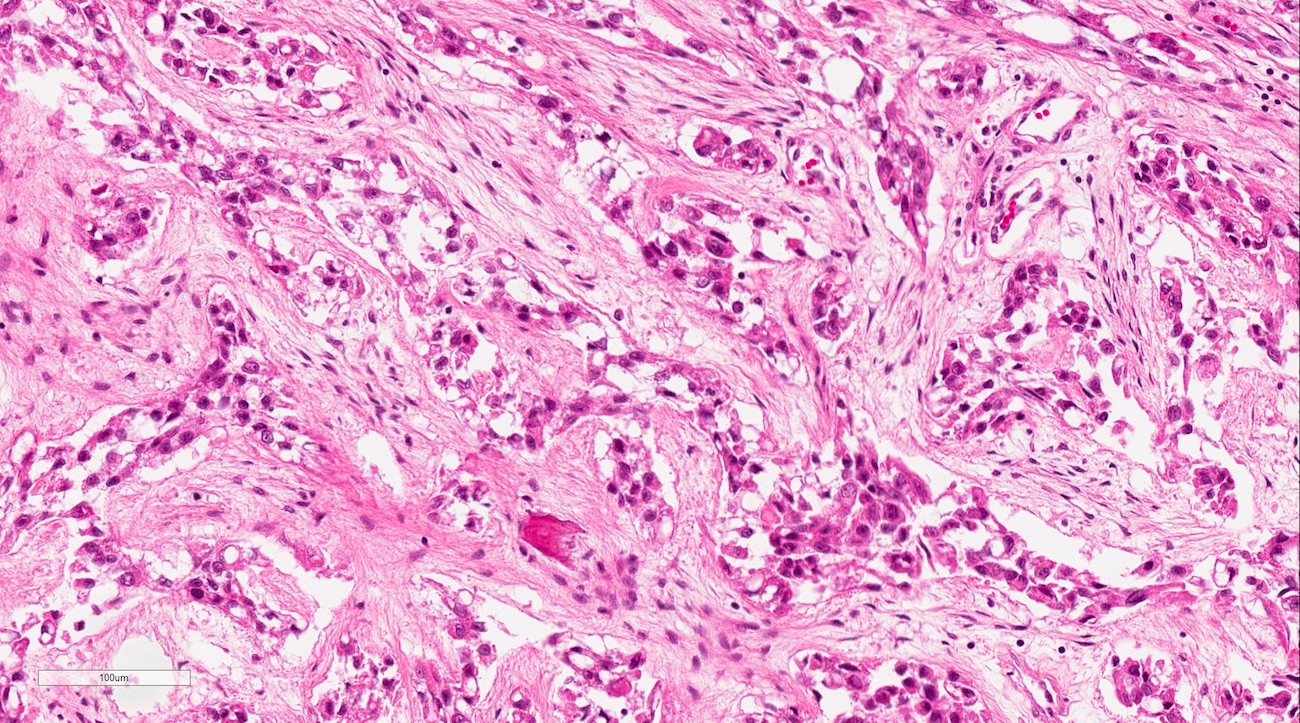
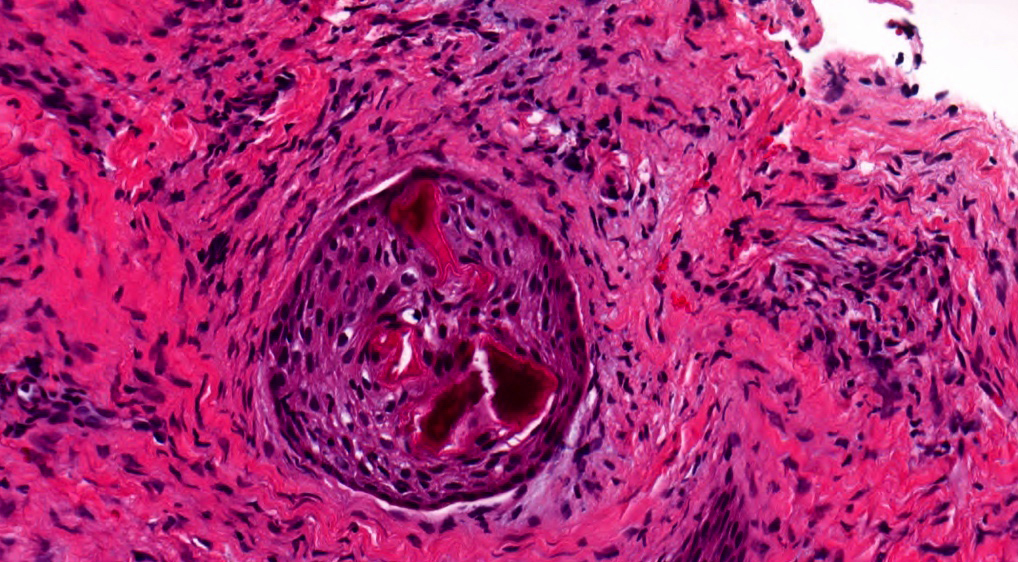
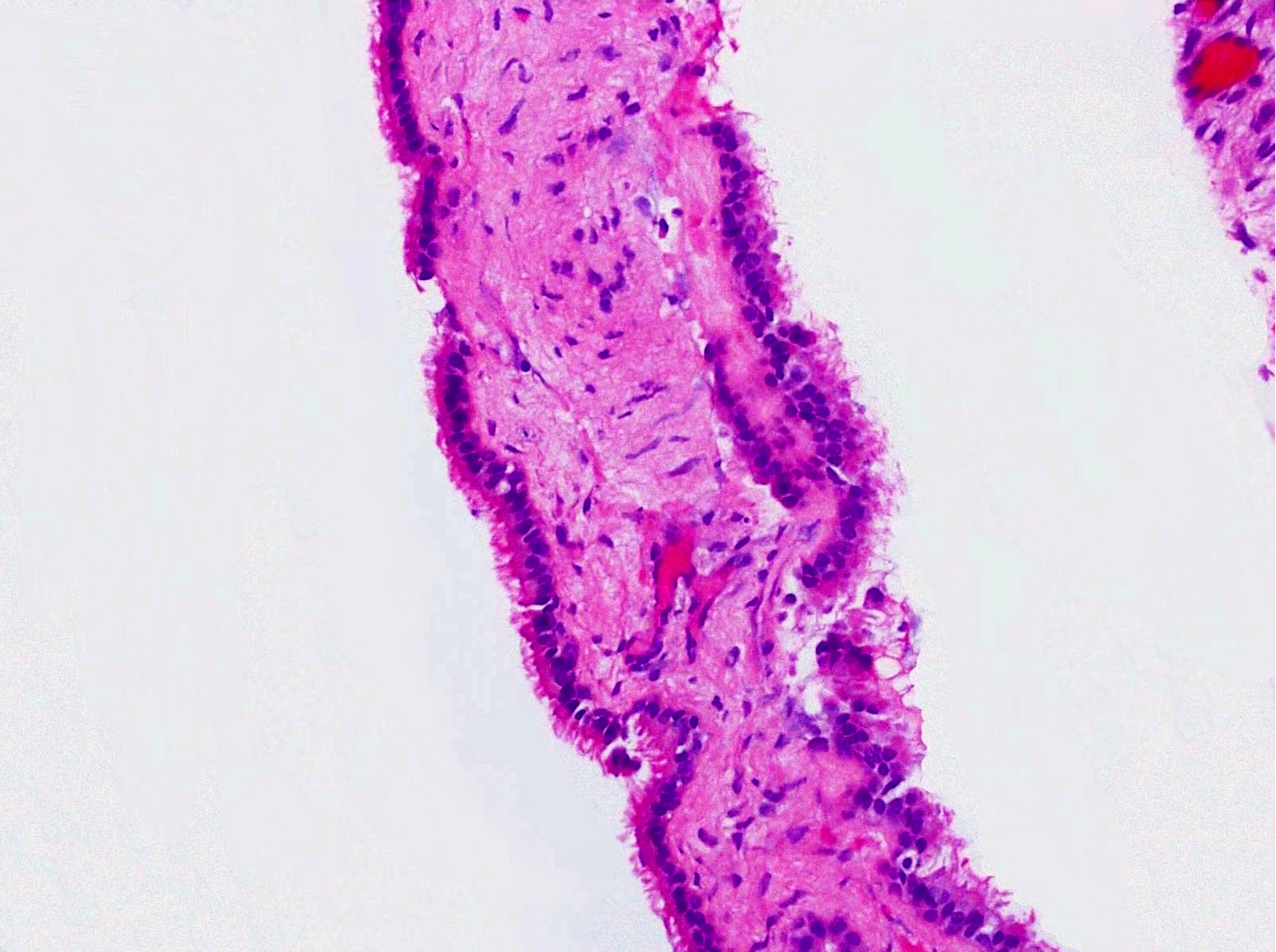
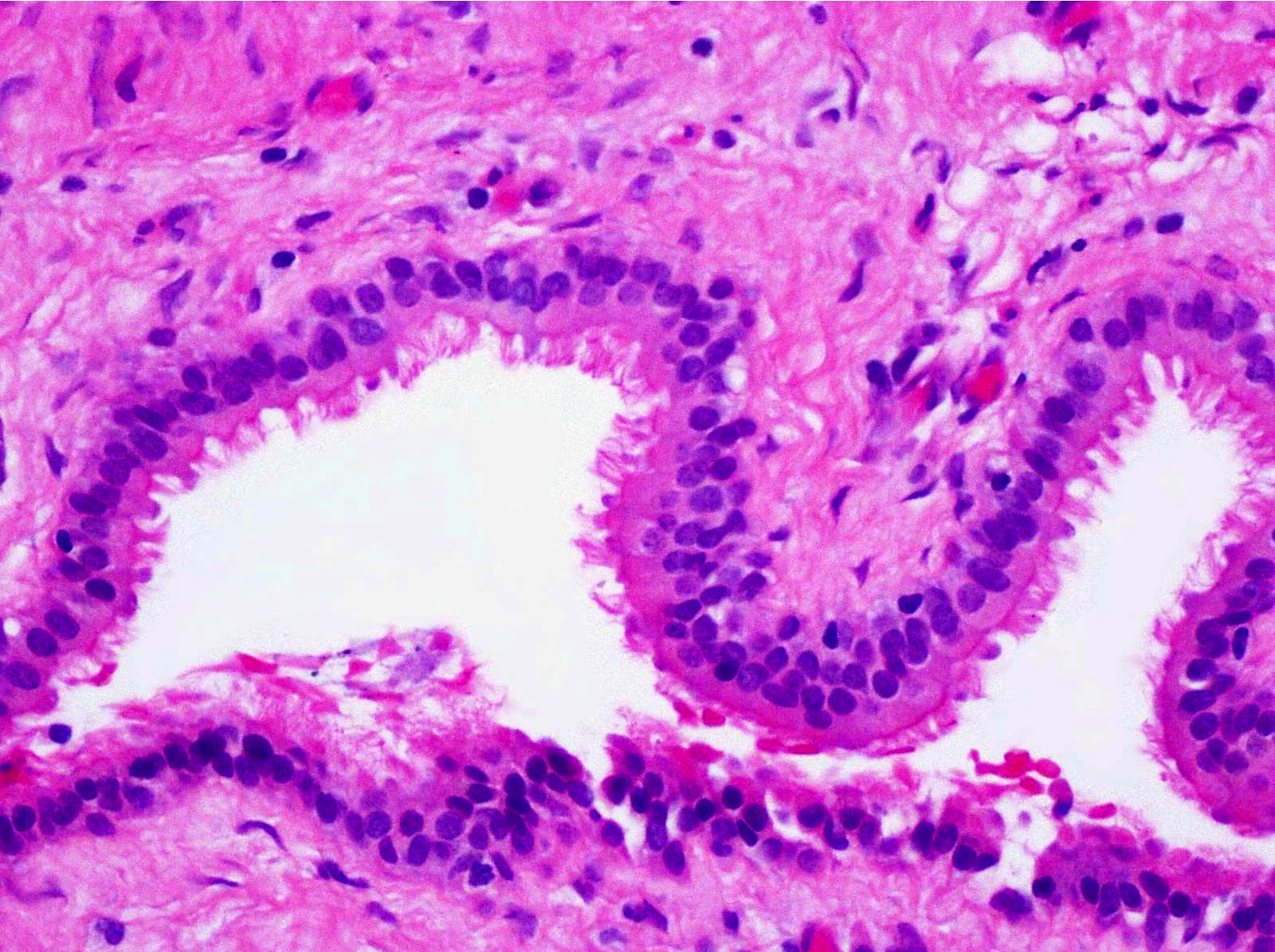
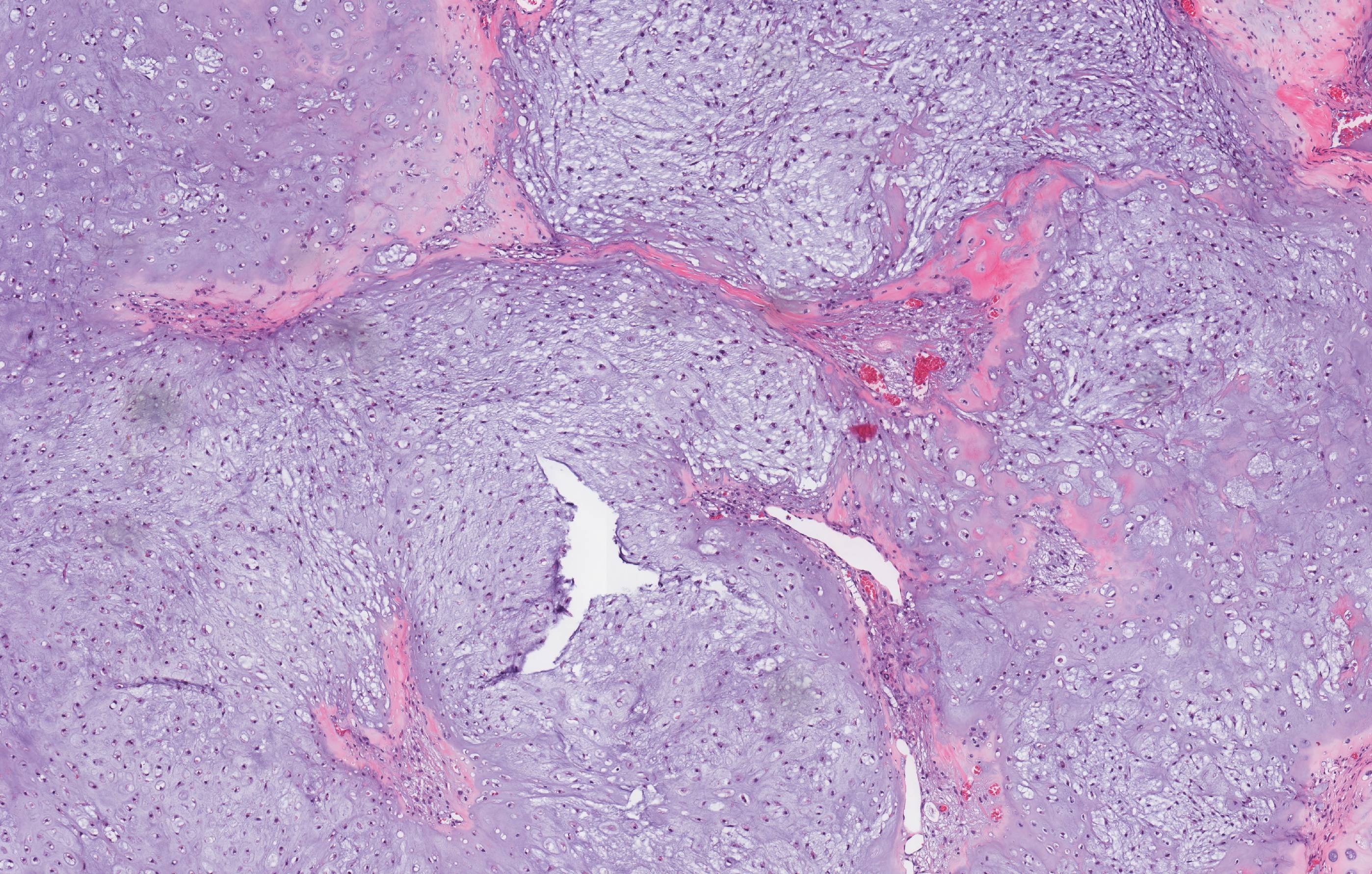
Microscopic (histologic) description
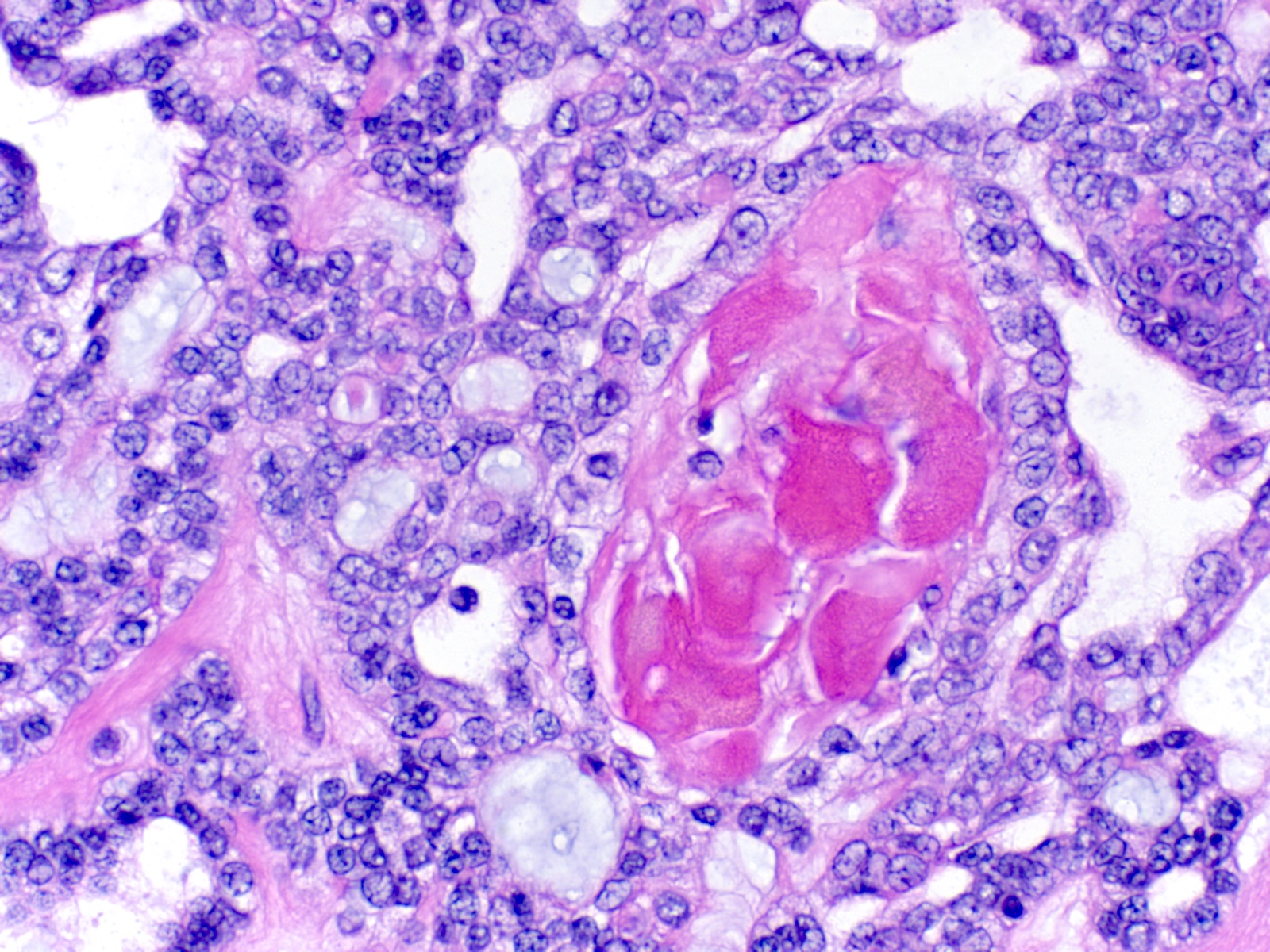
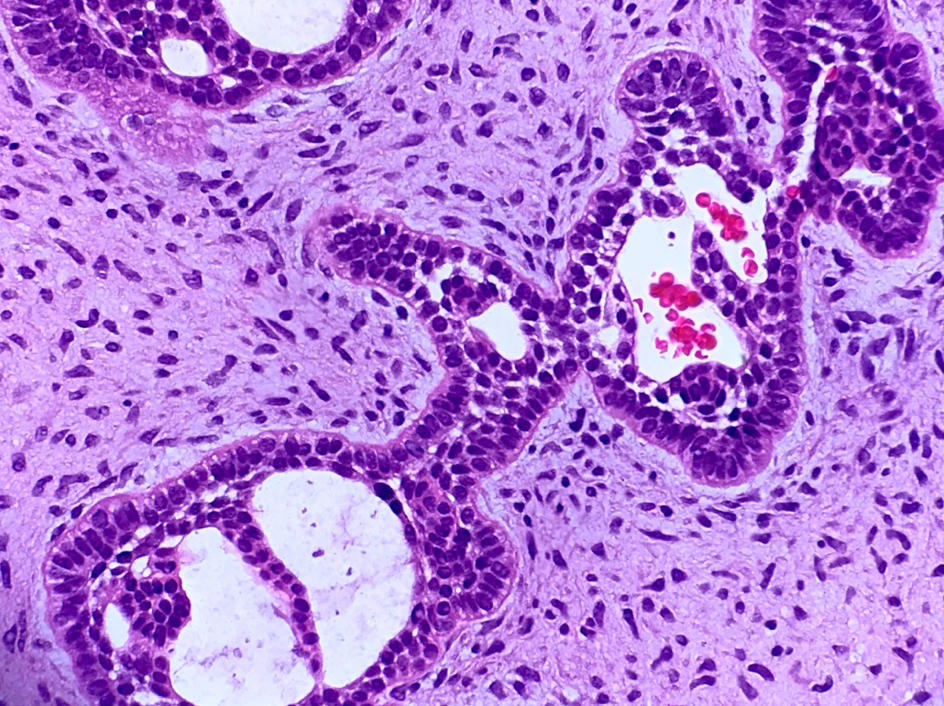
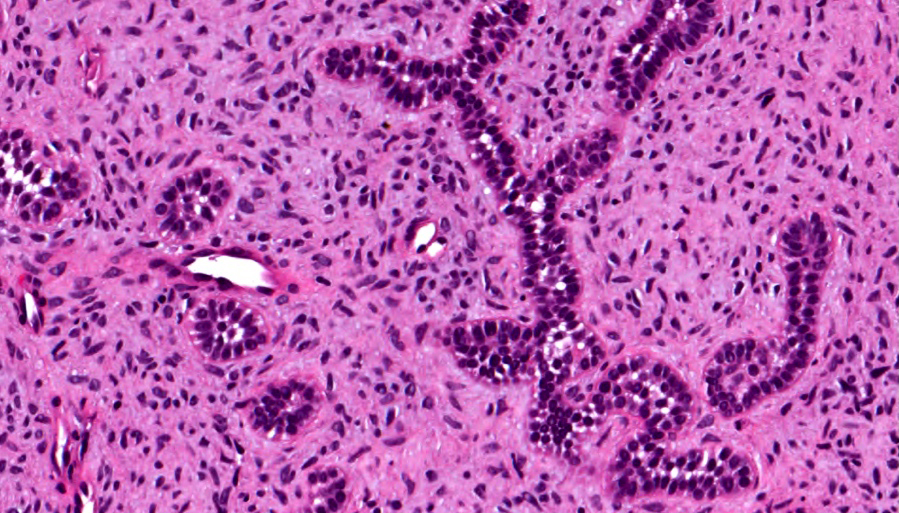
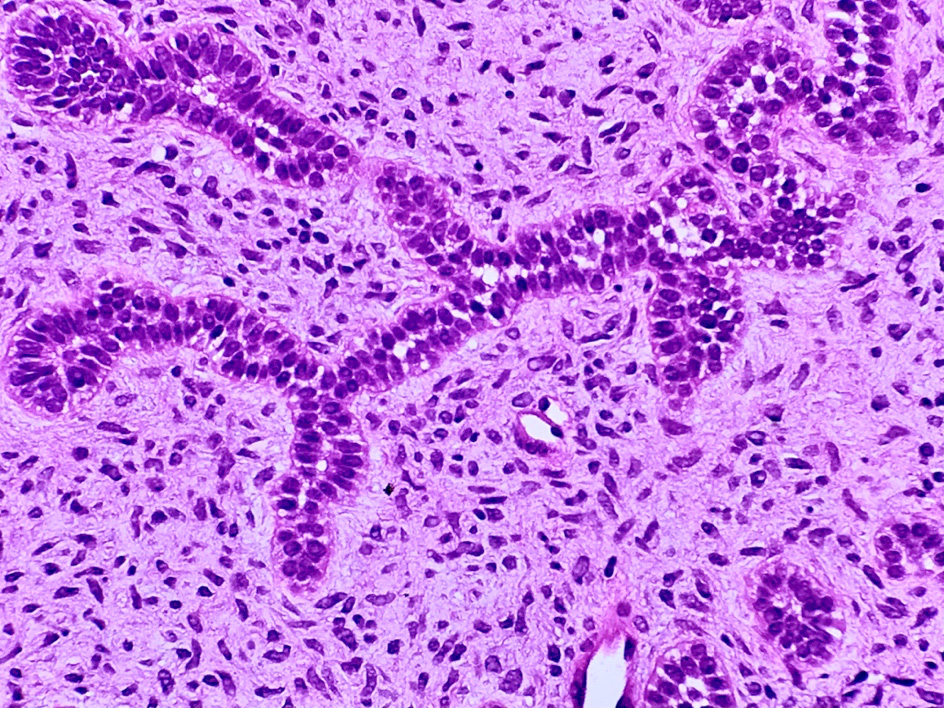
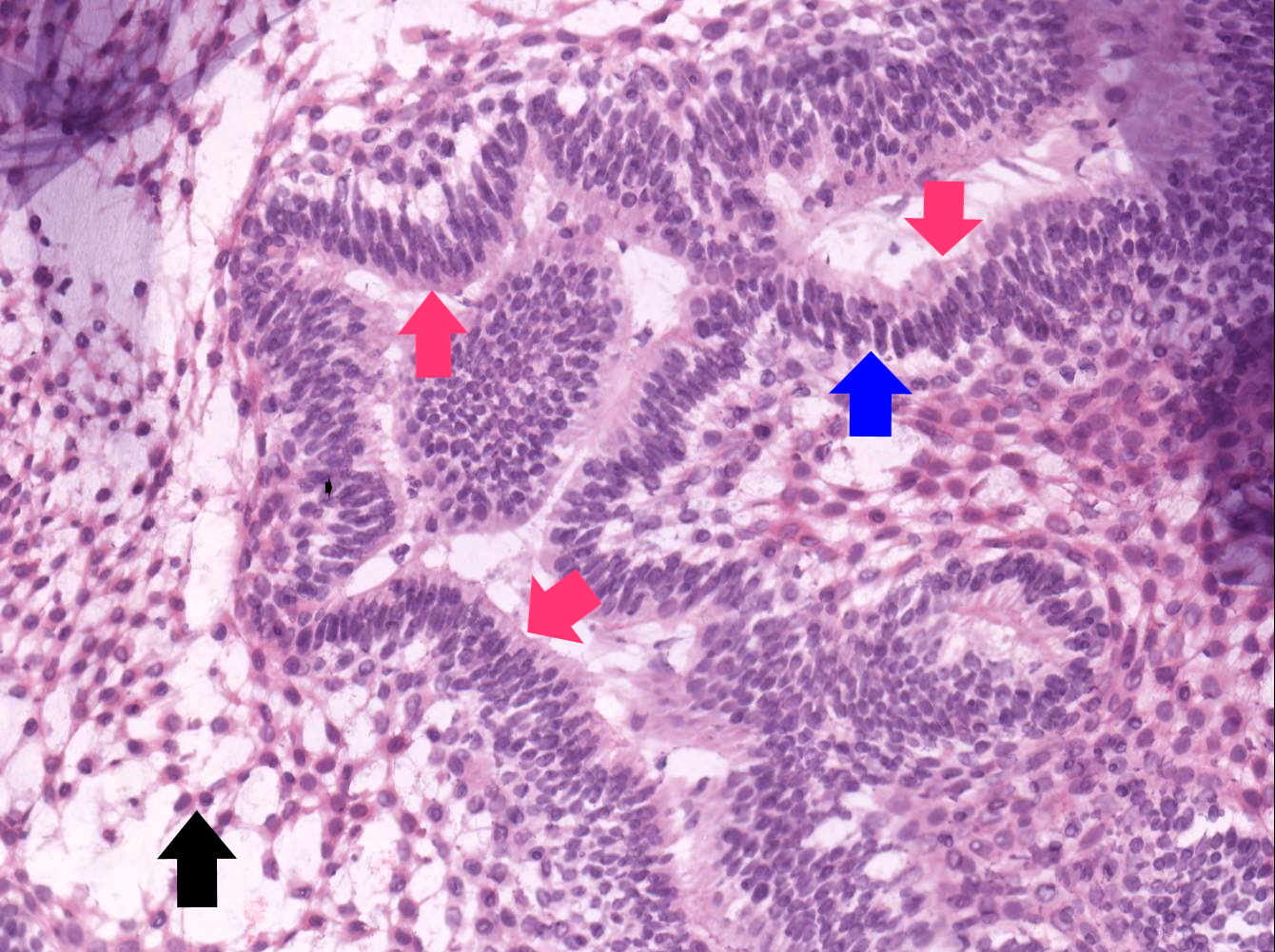
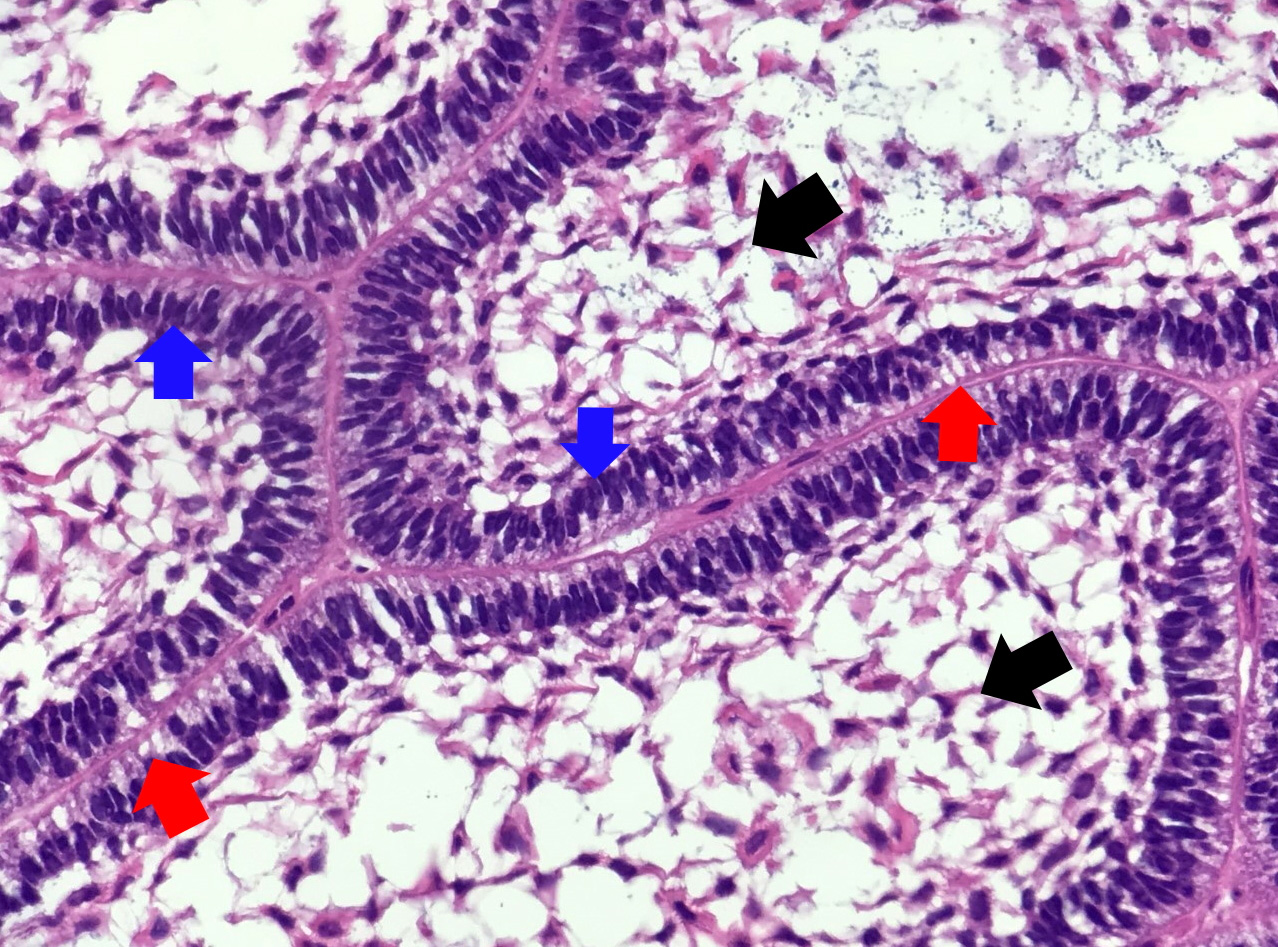
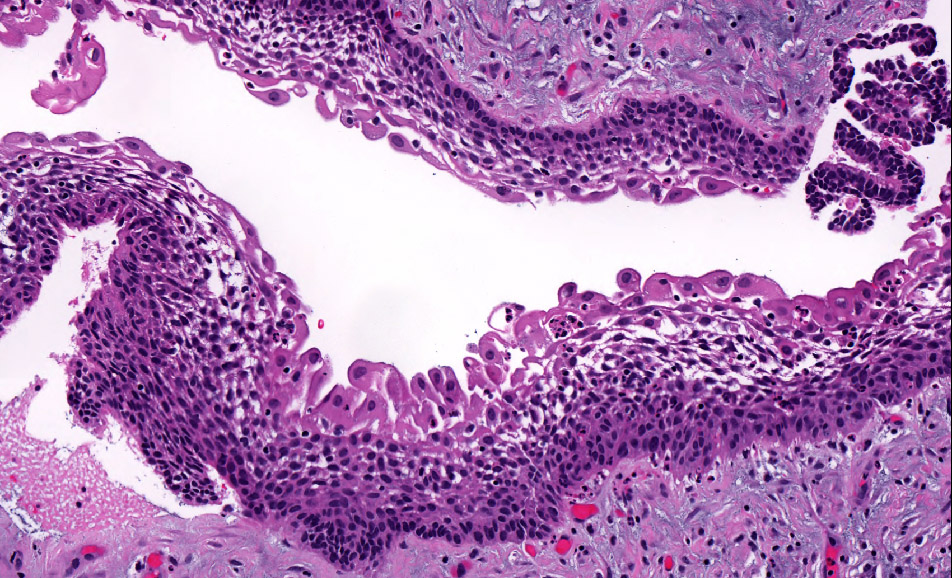
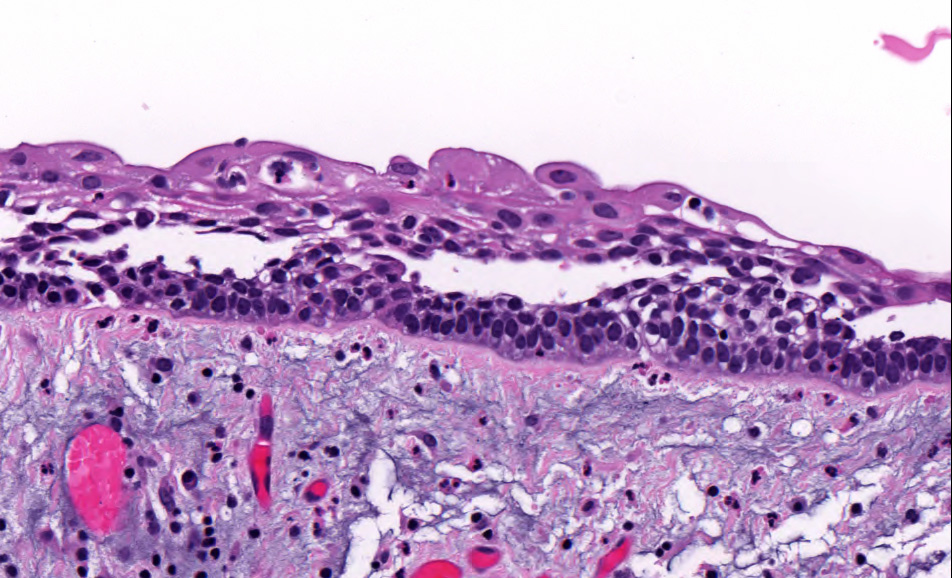
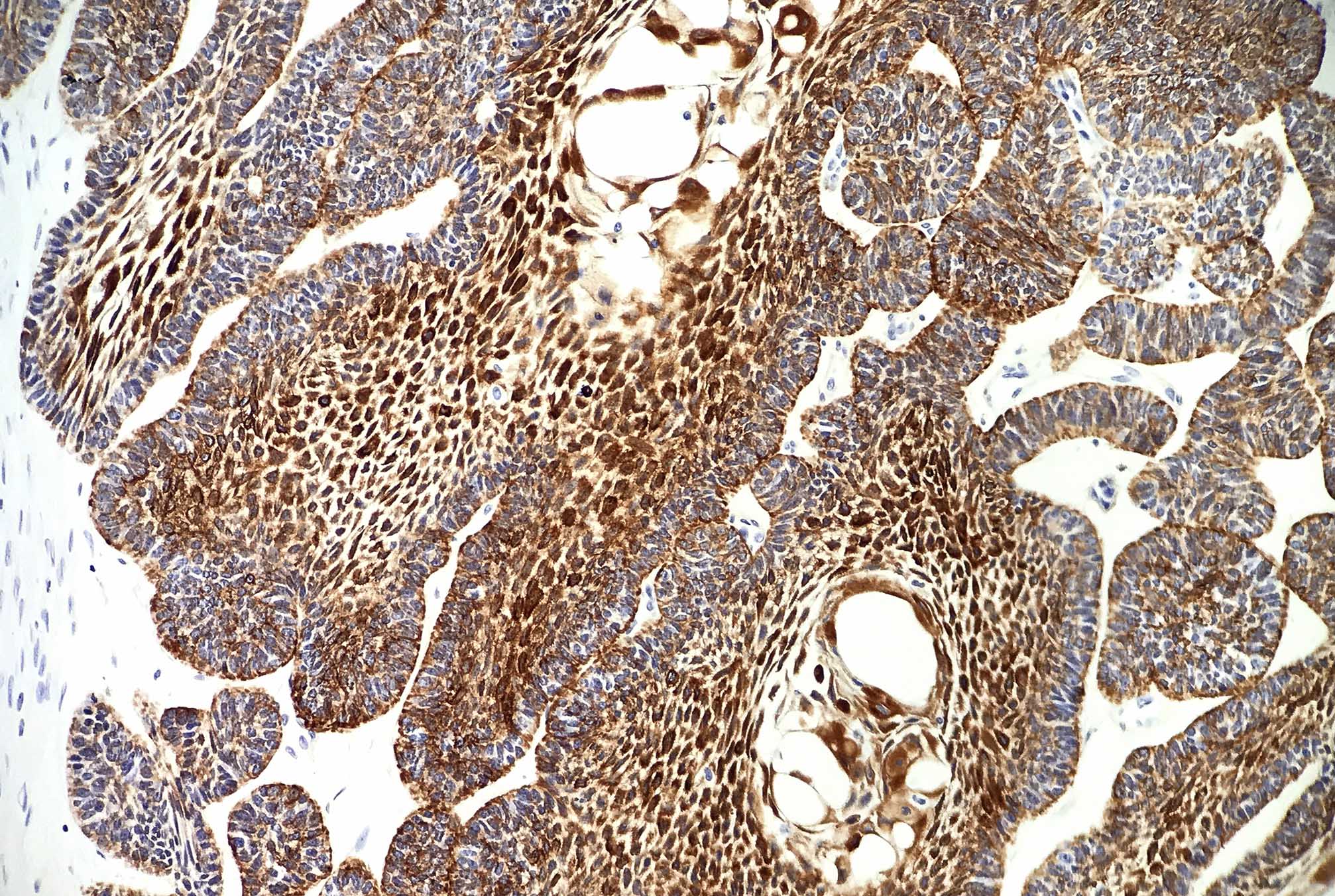
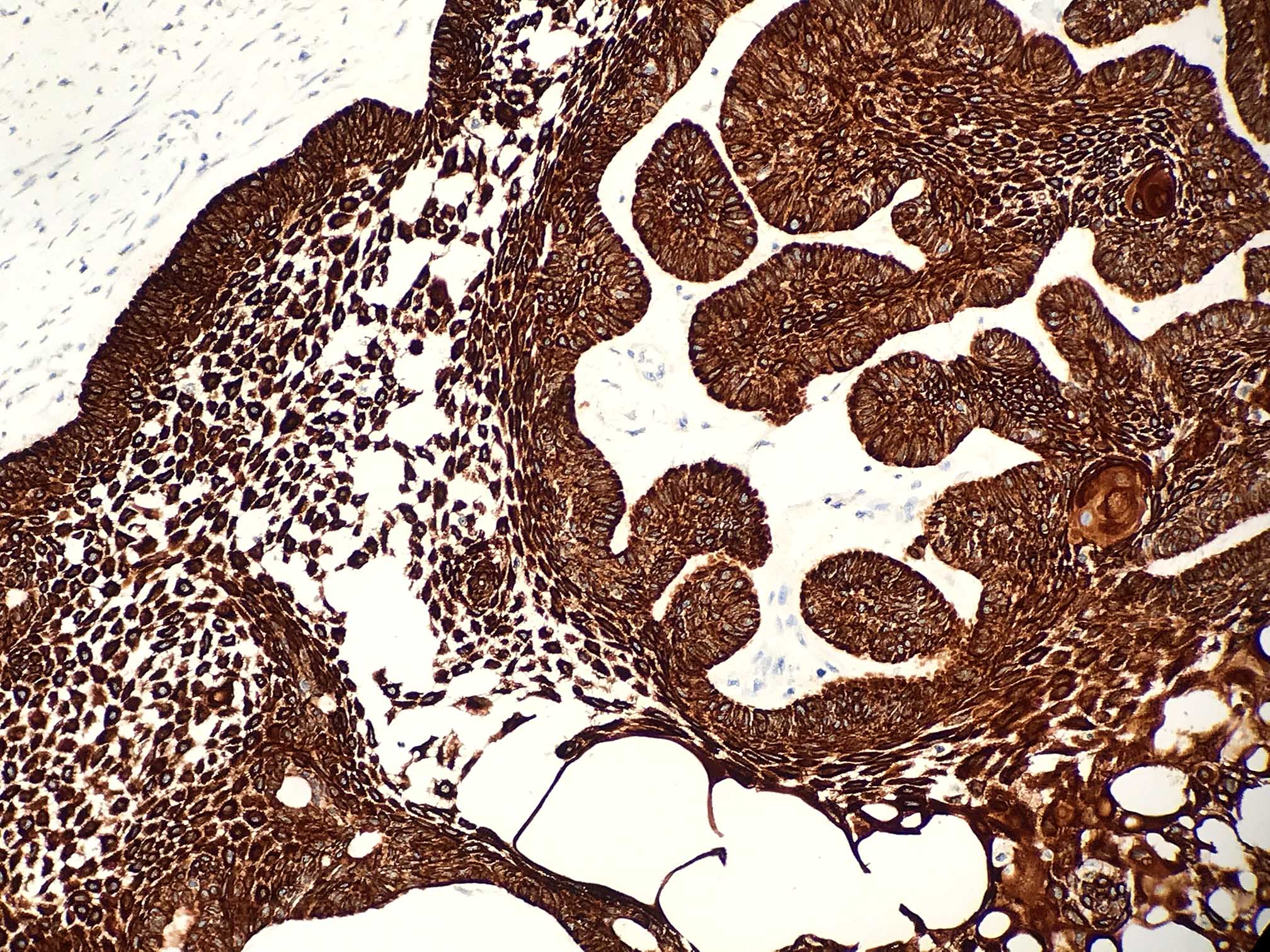
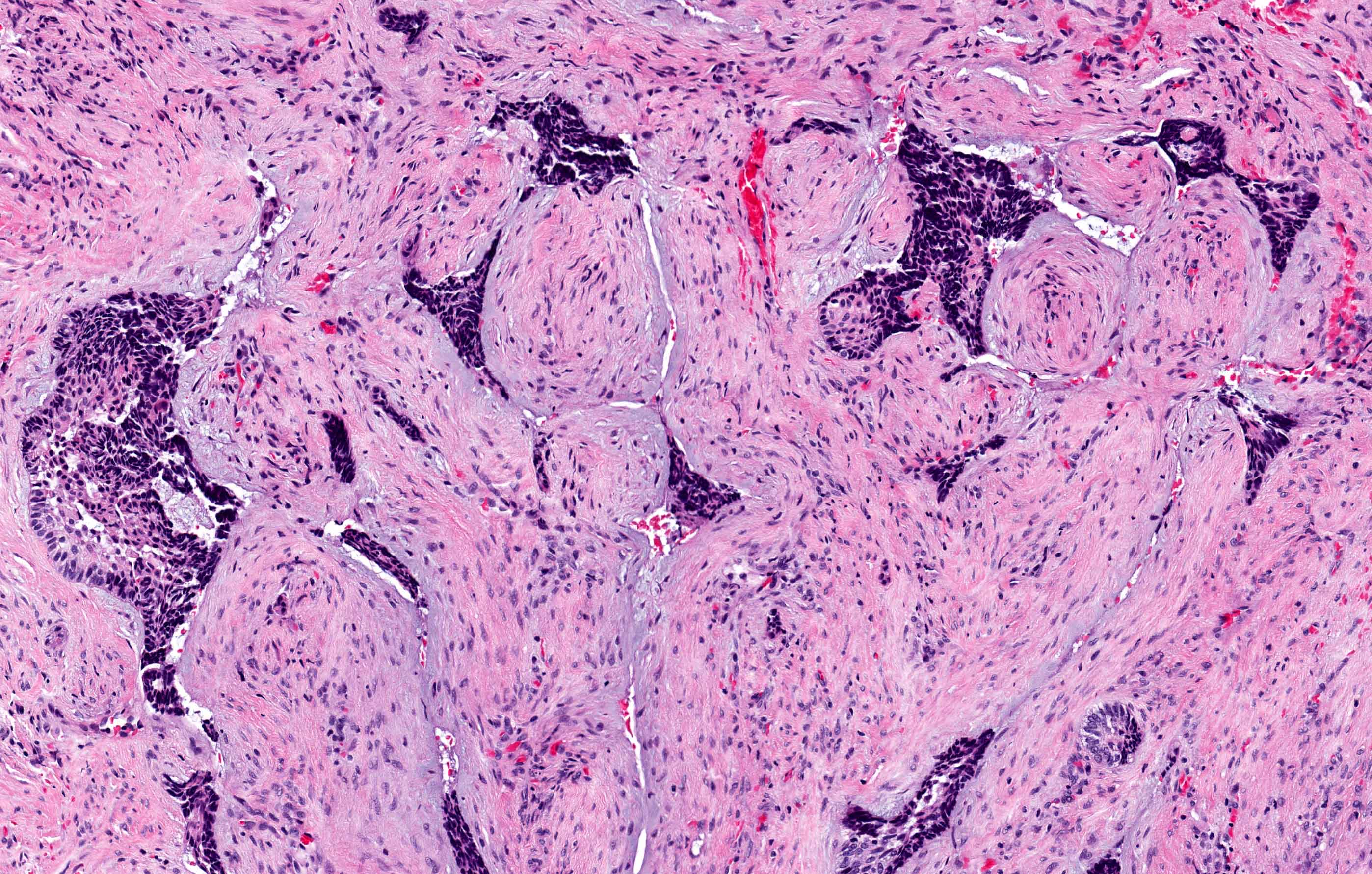
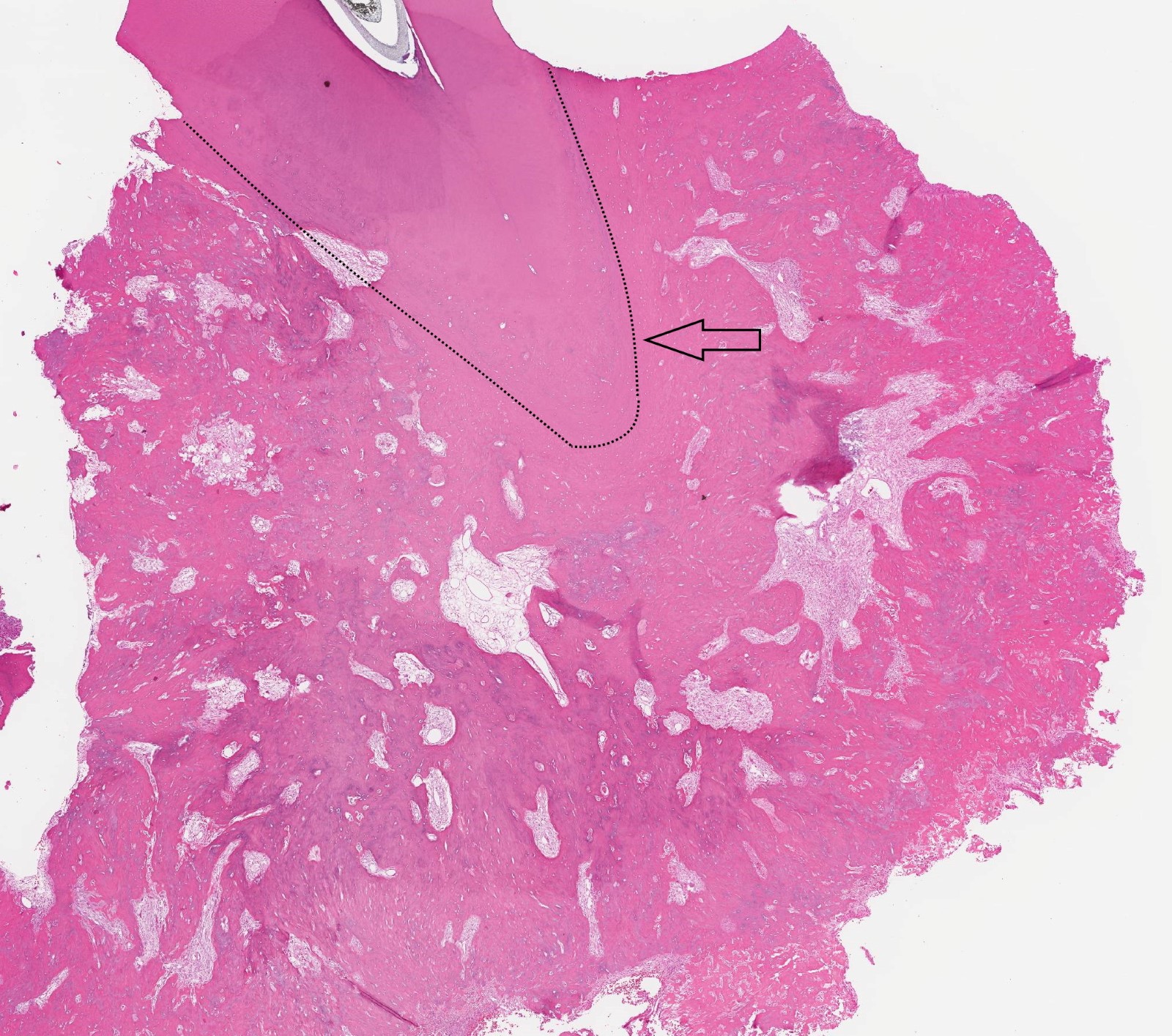
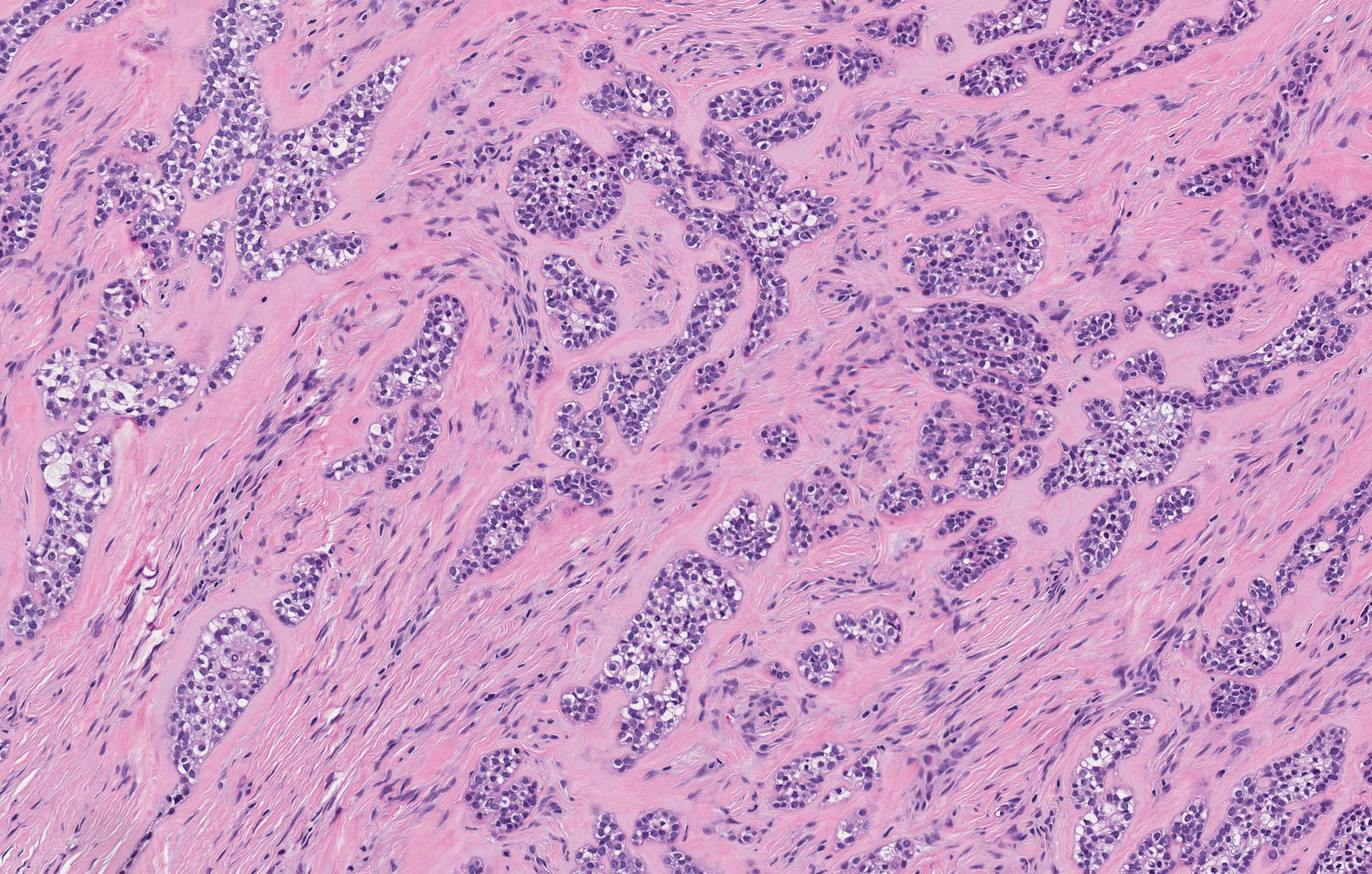
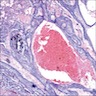
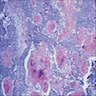
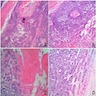
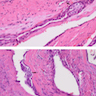
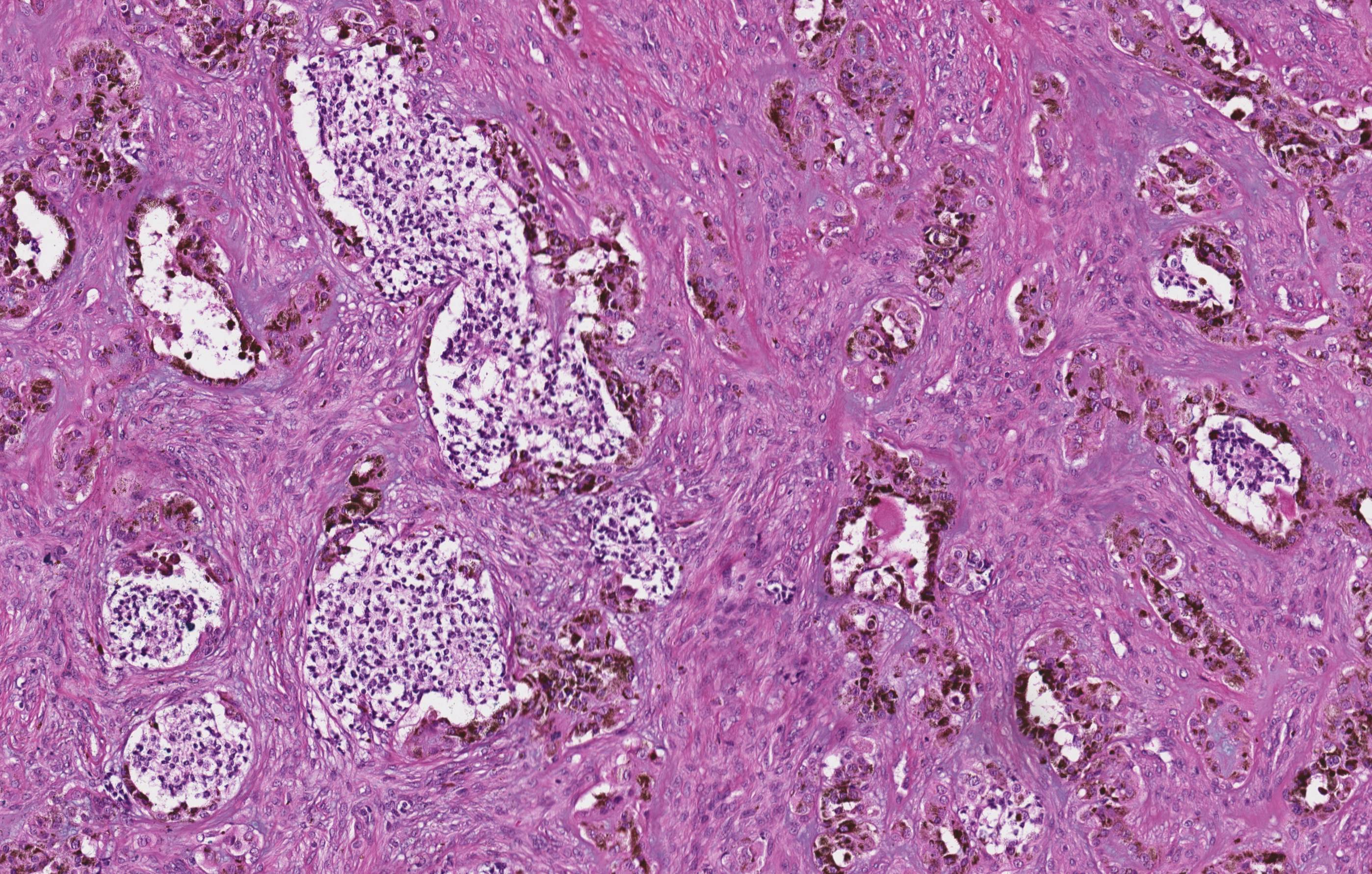
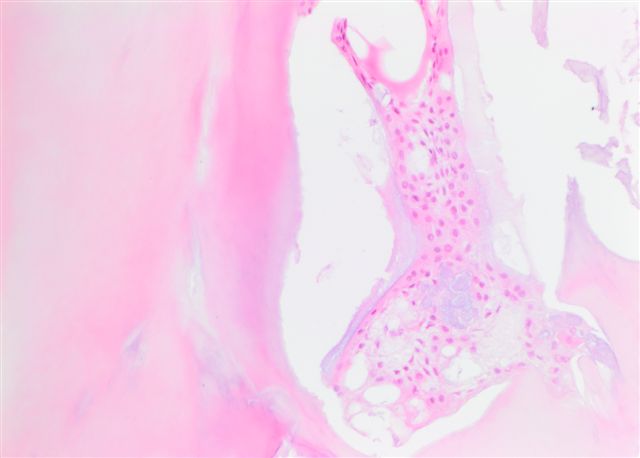
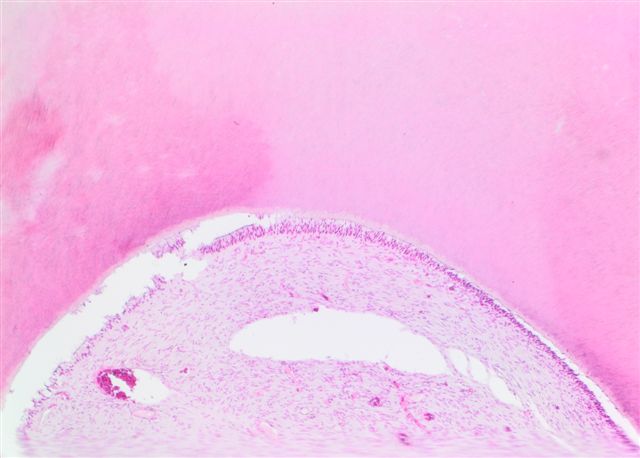
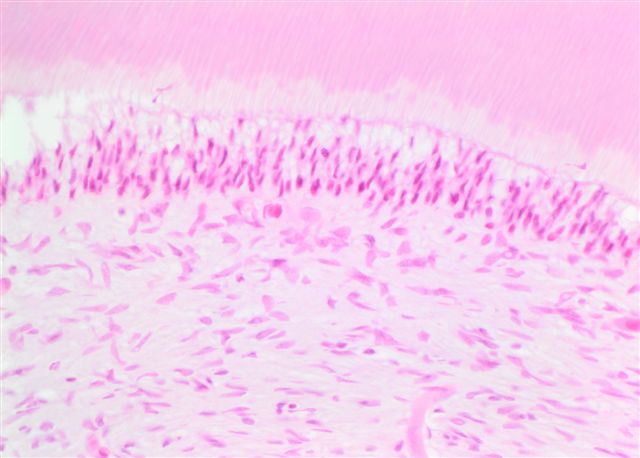
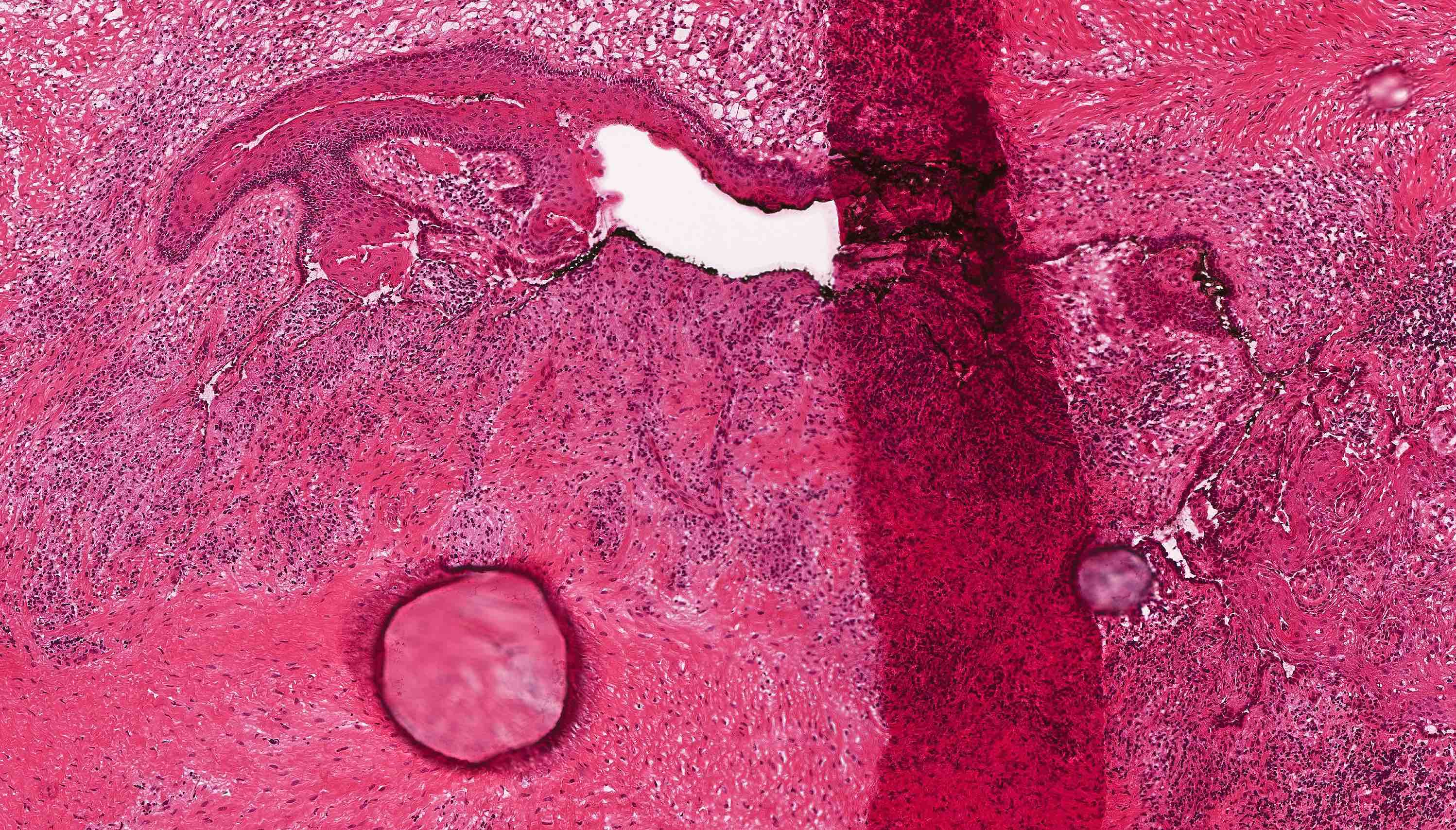
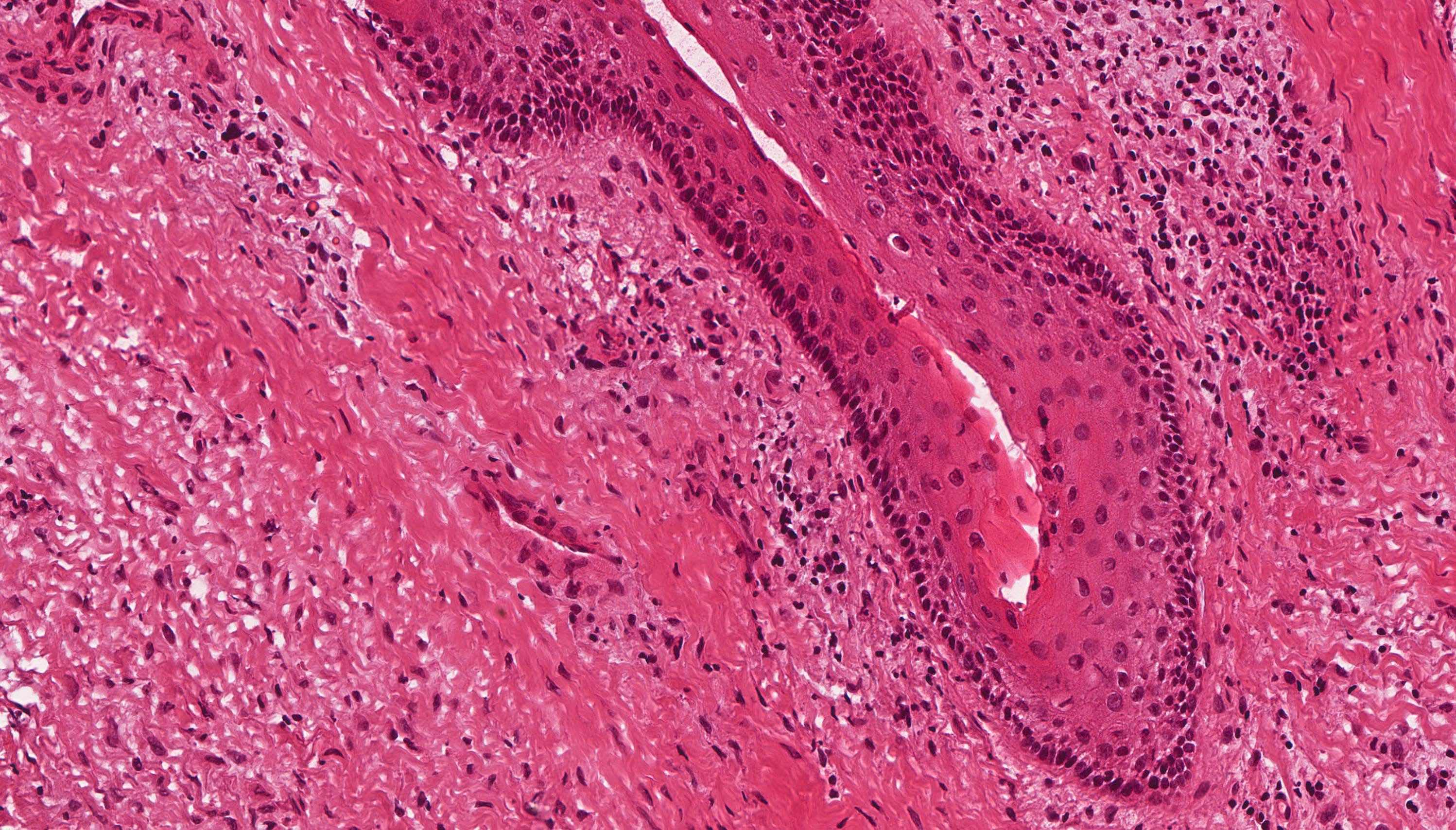
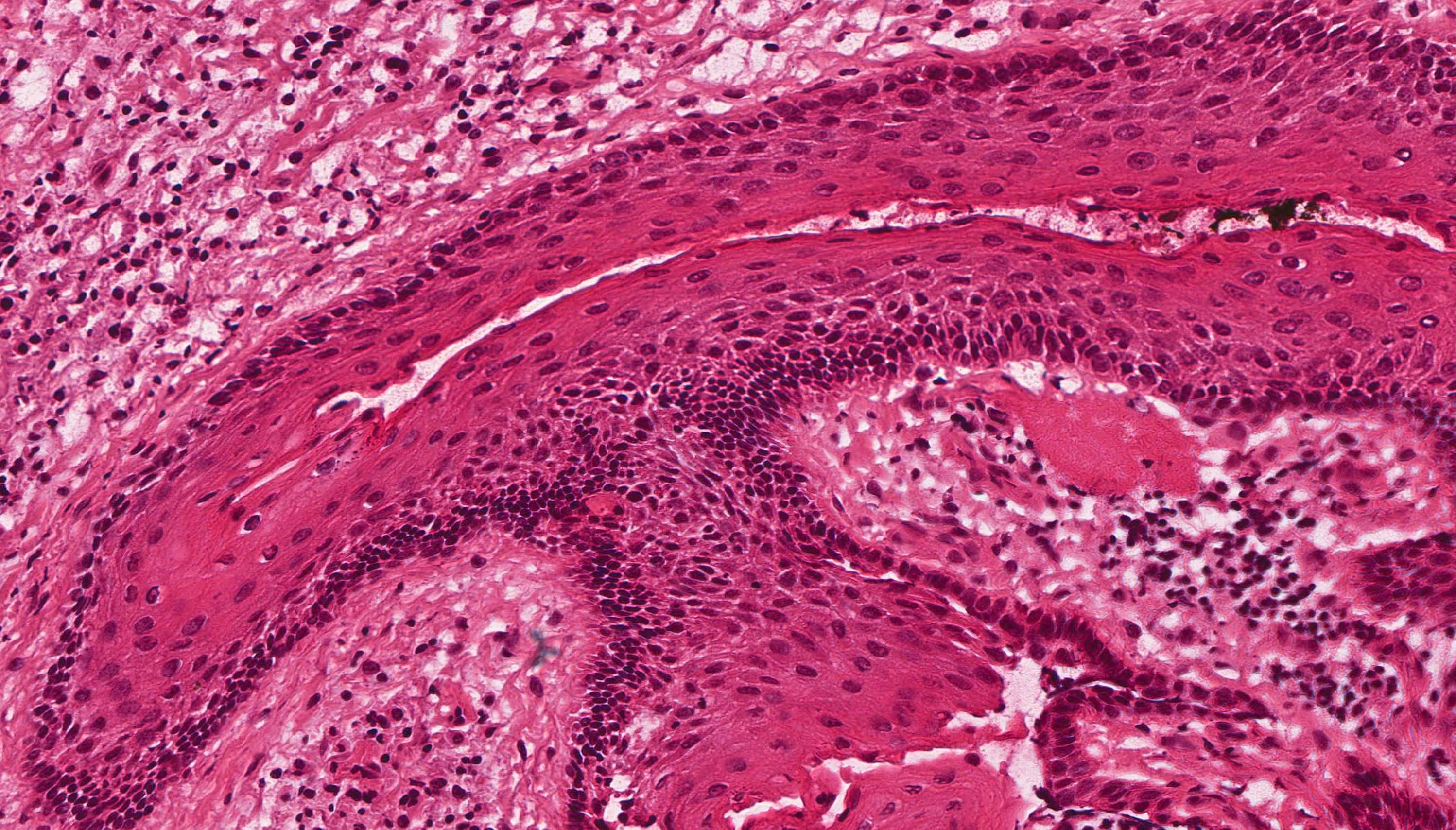
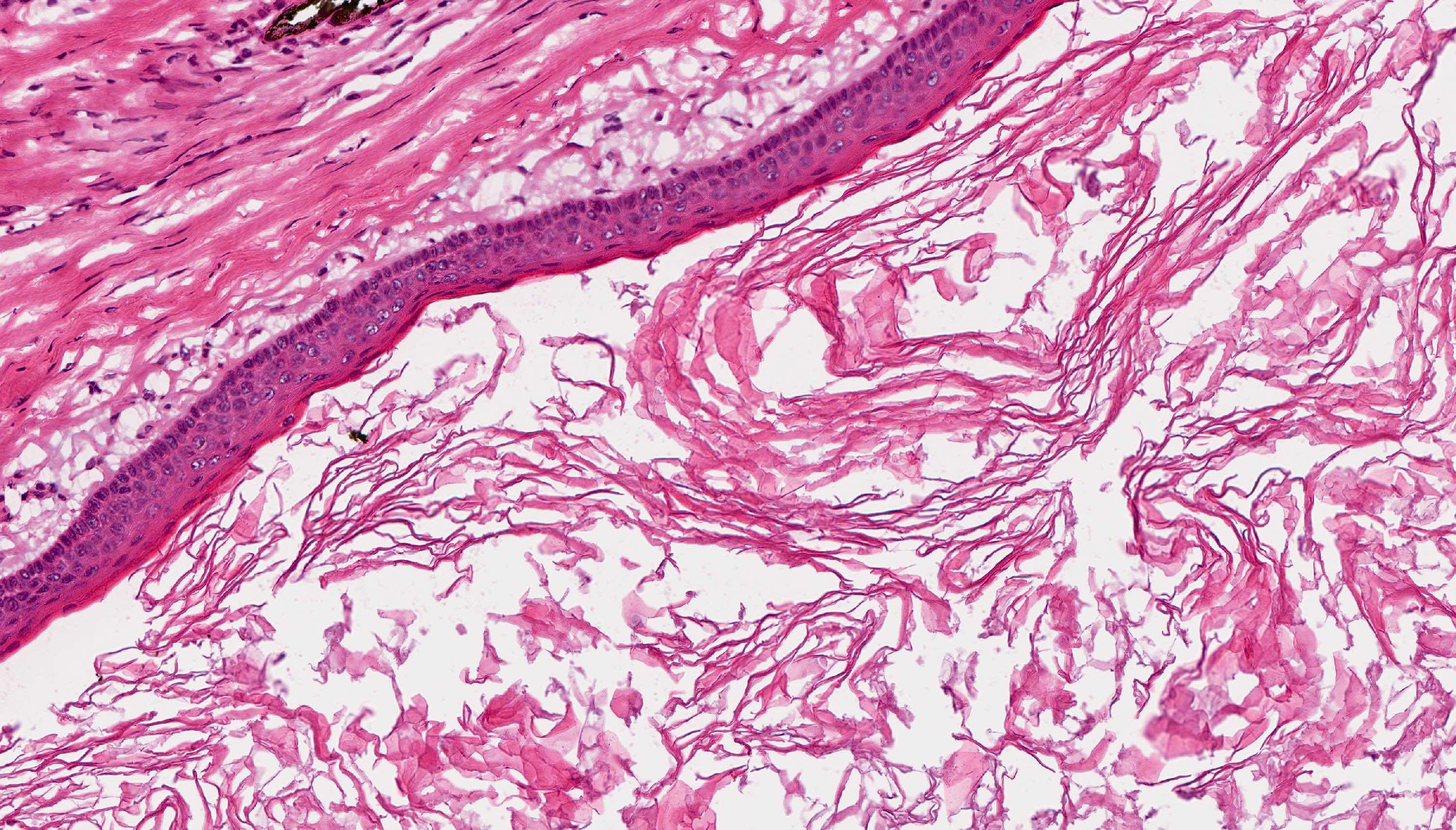
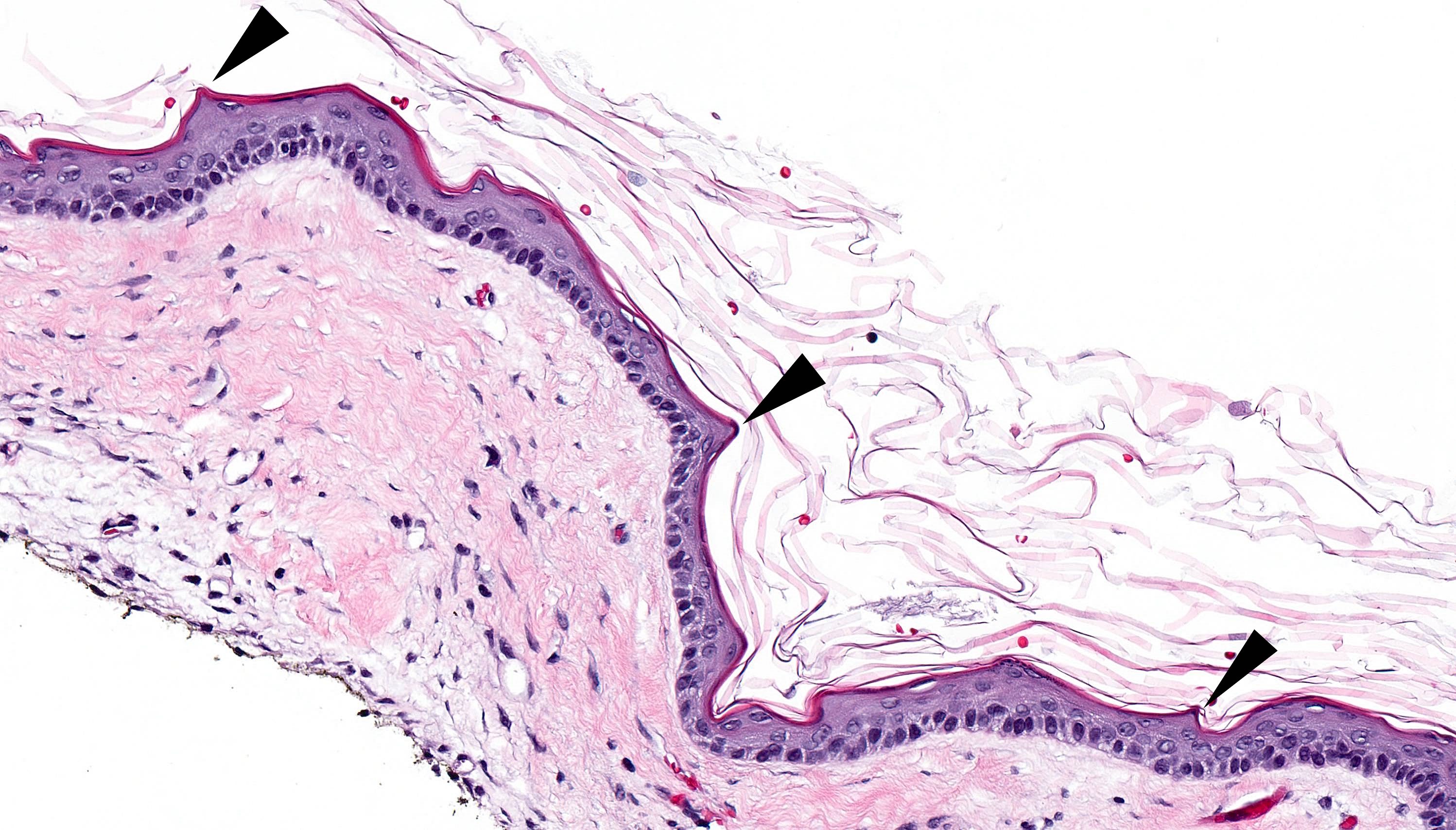
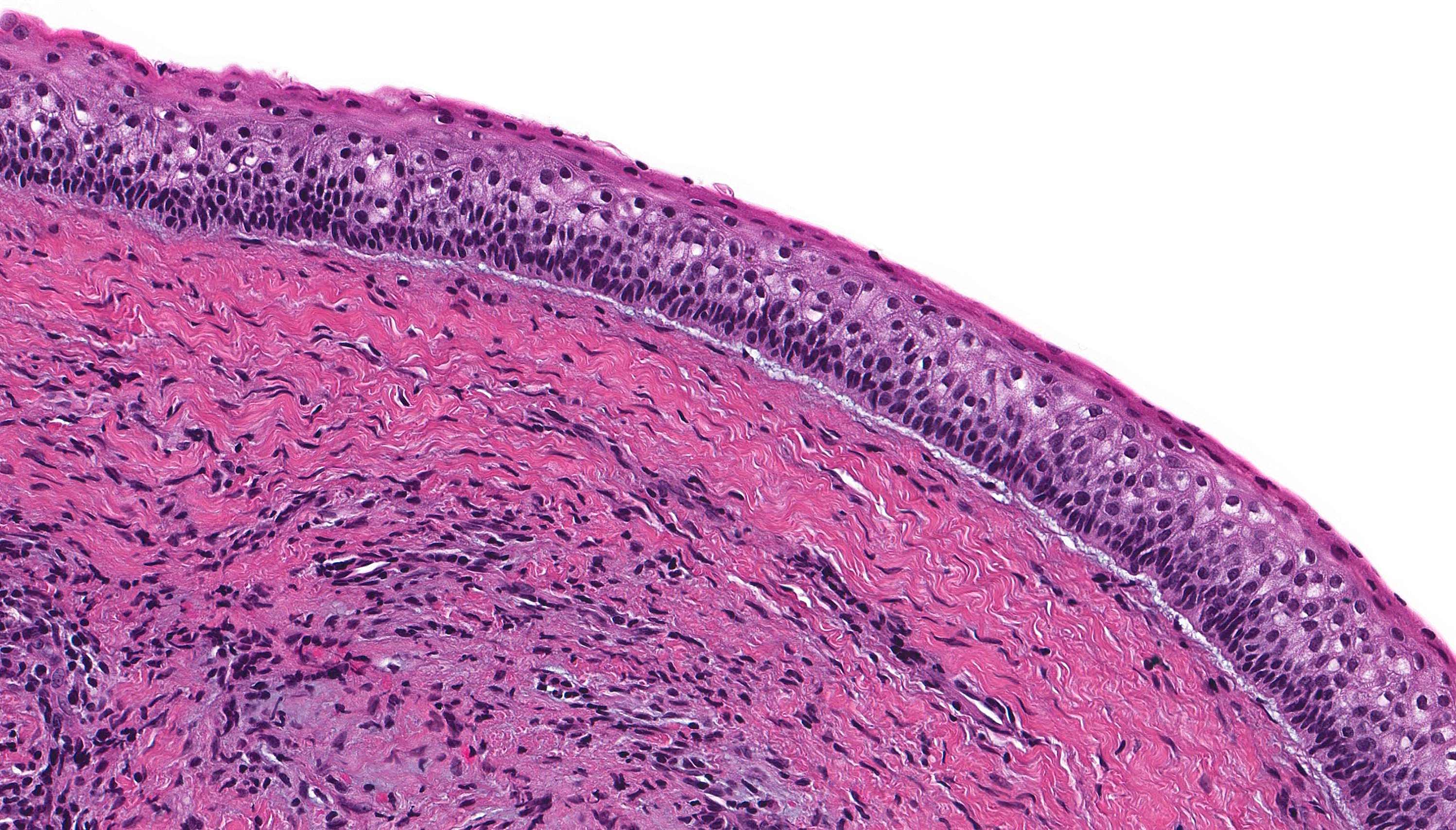
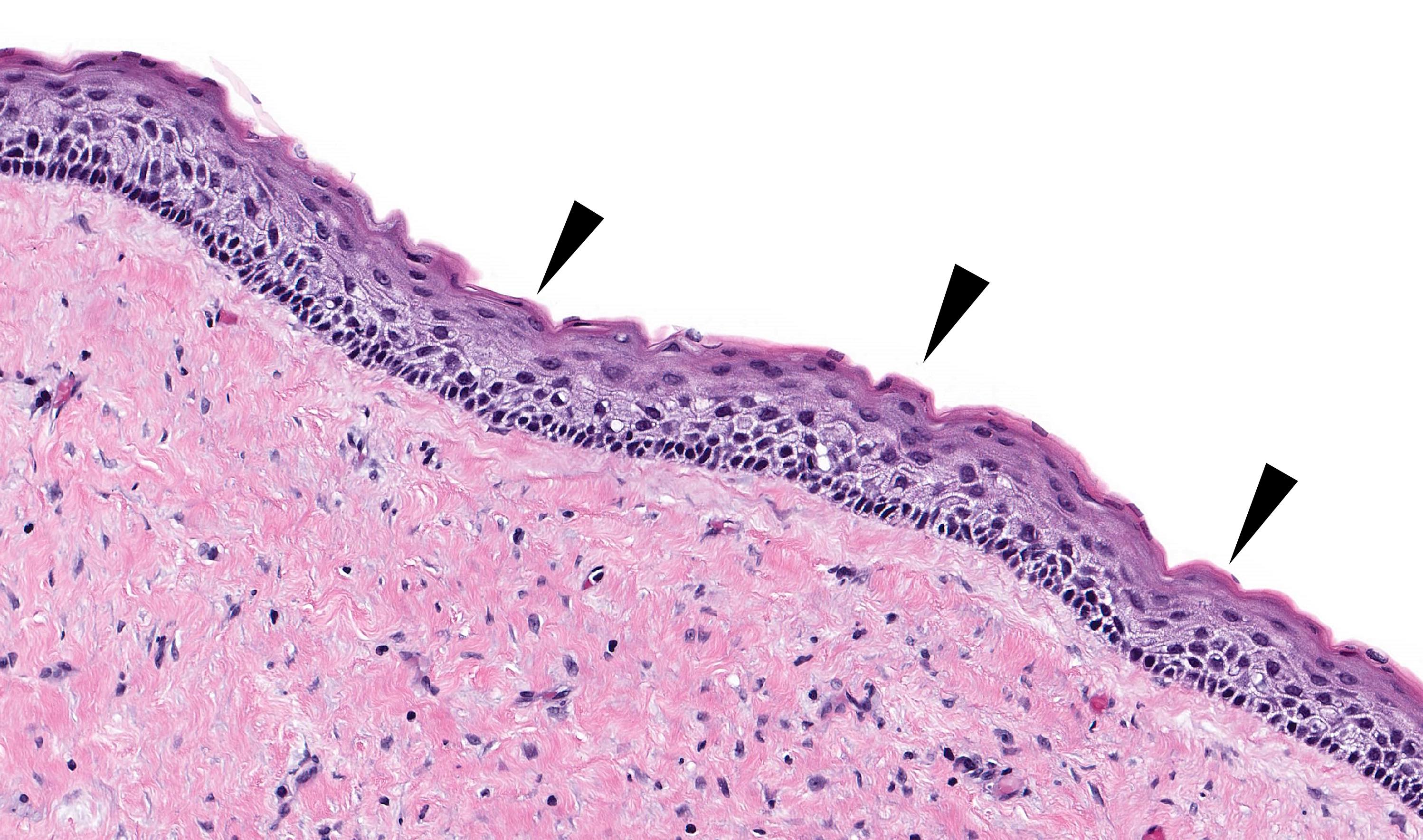
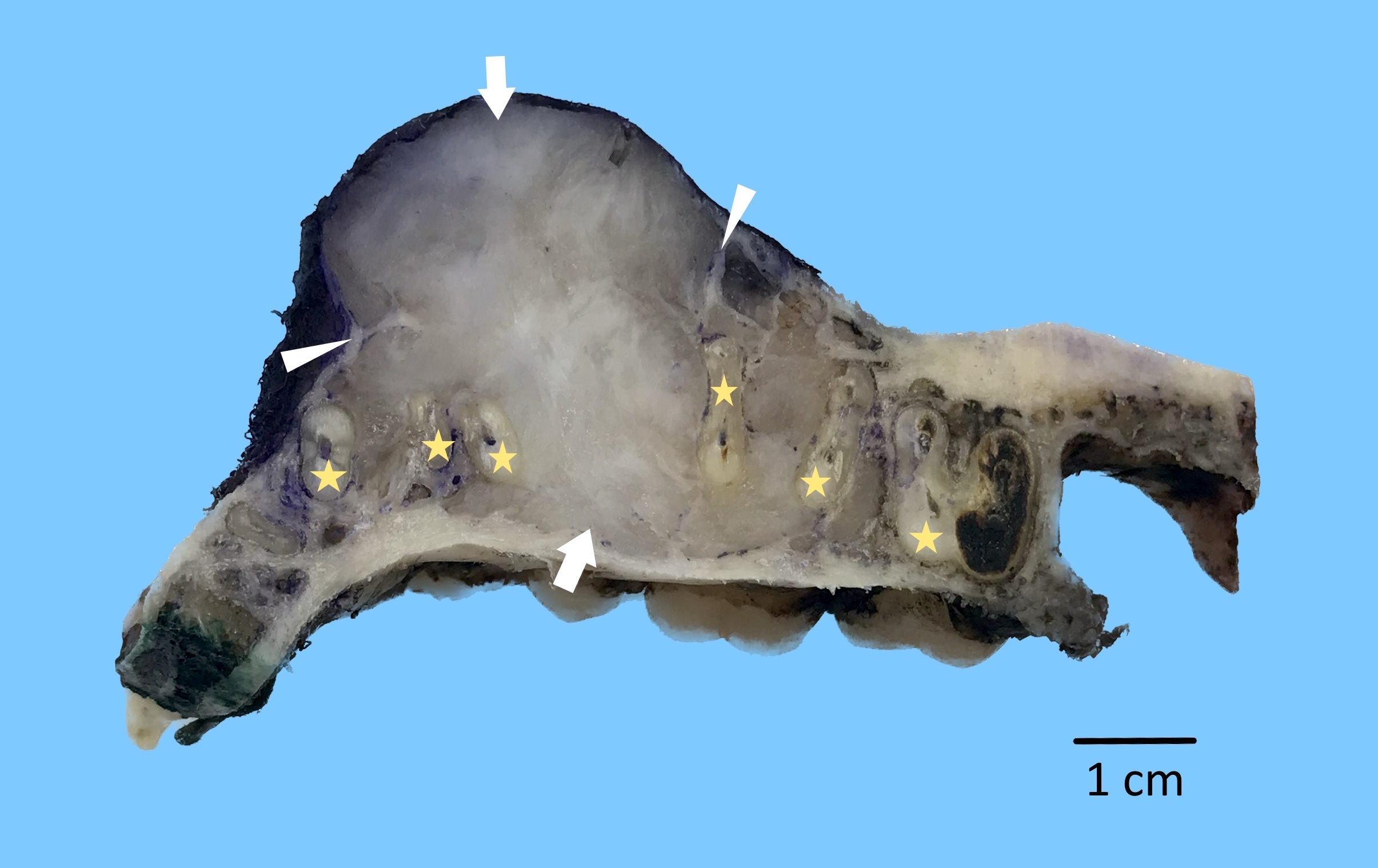
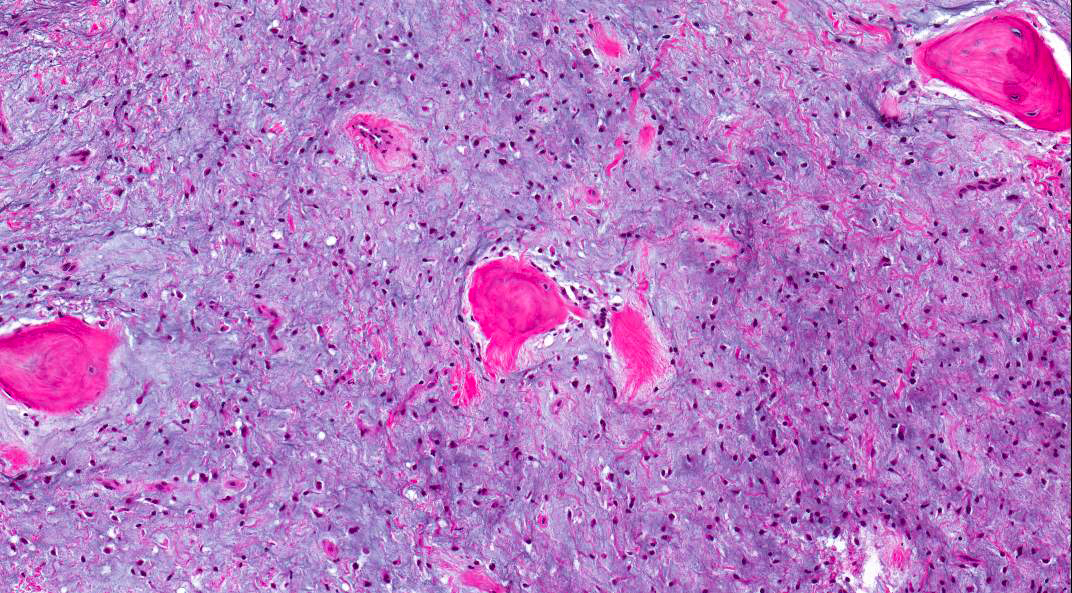
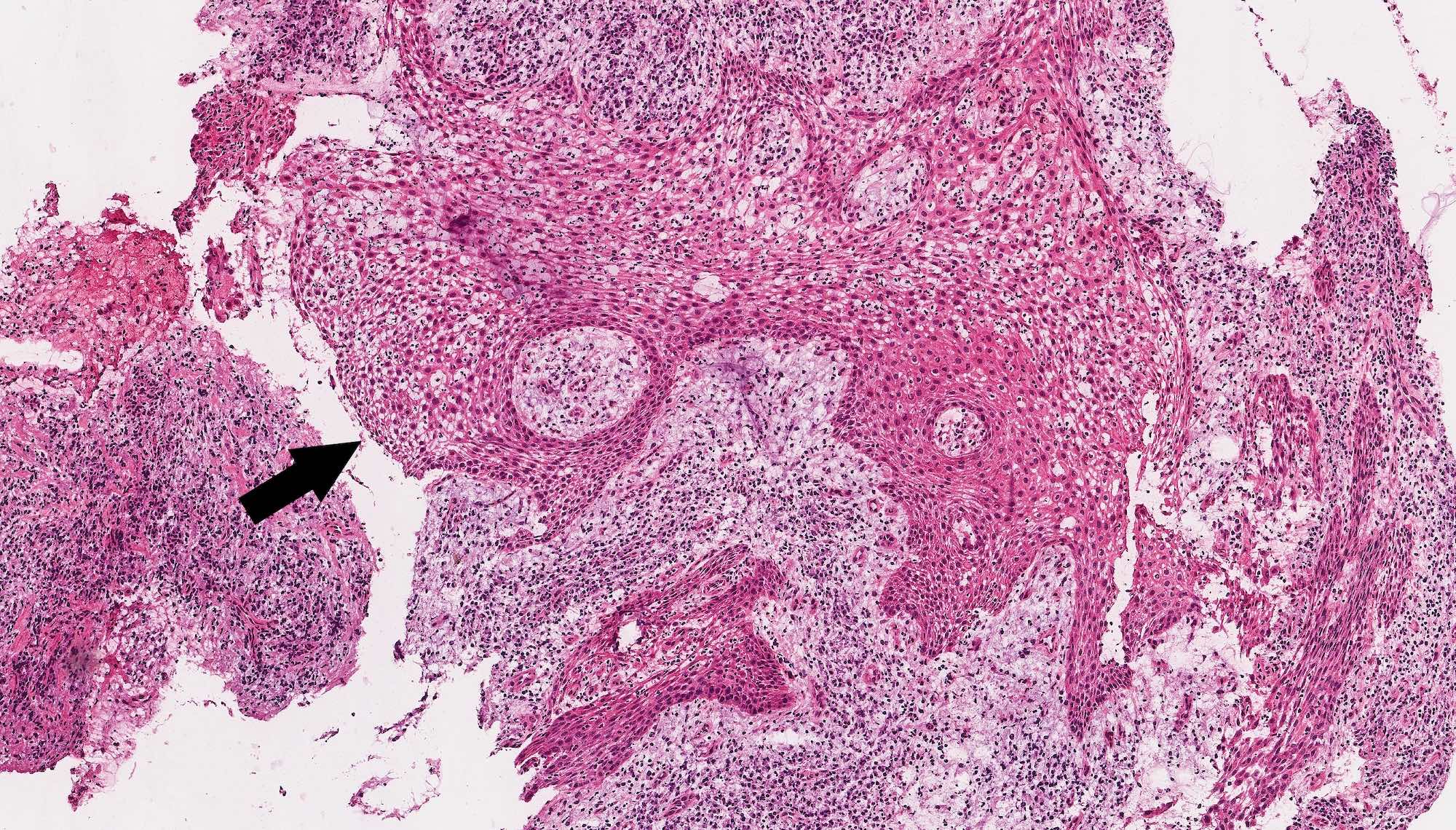
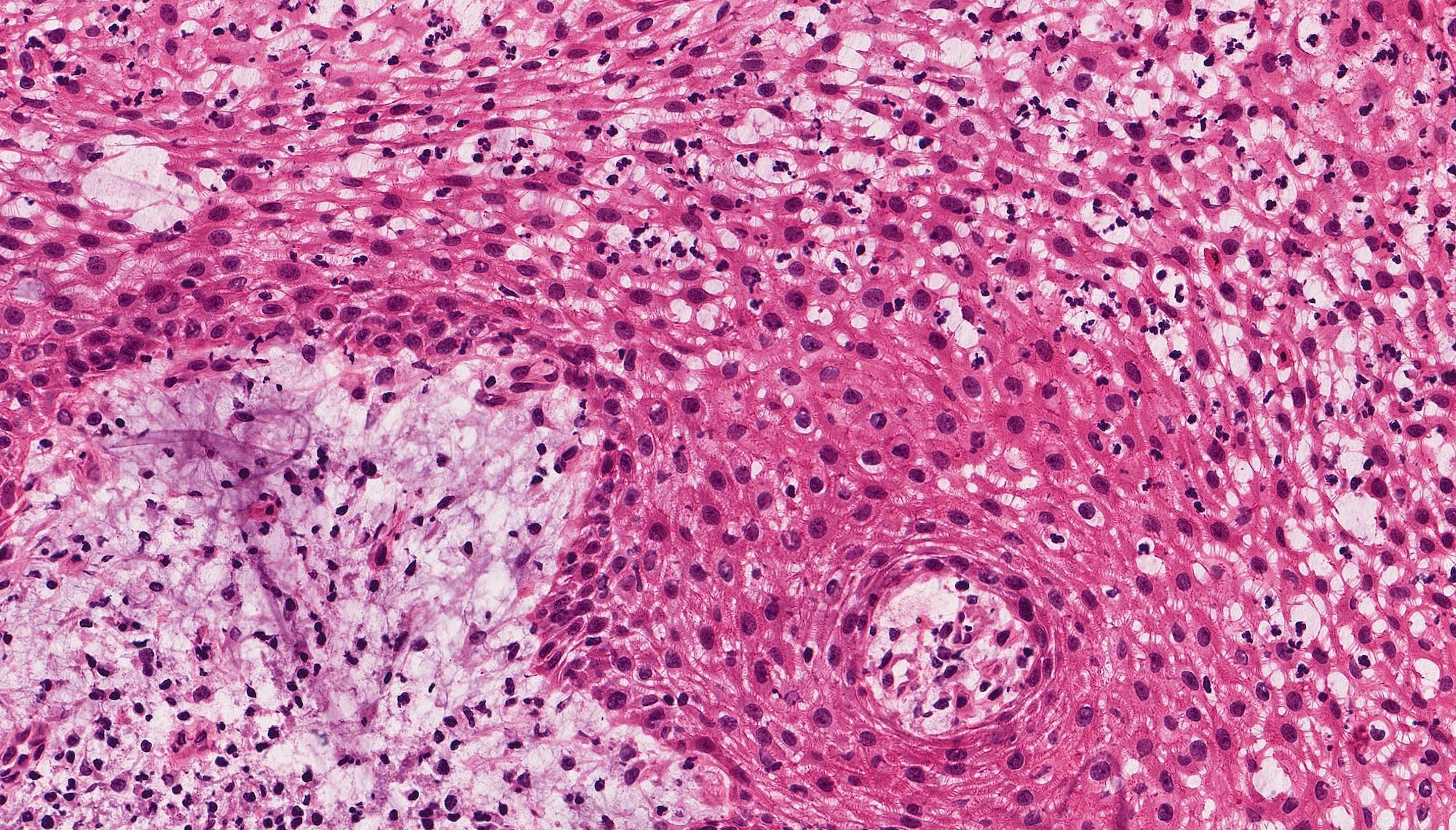
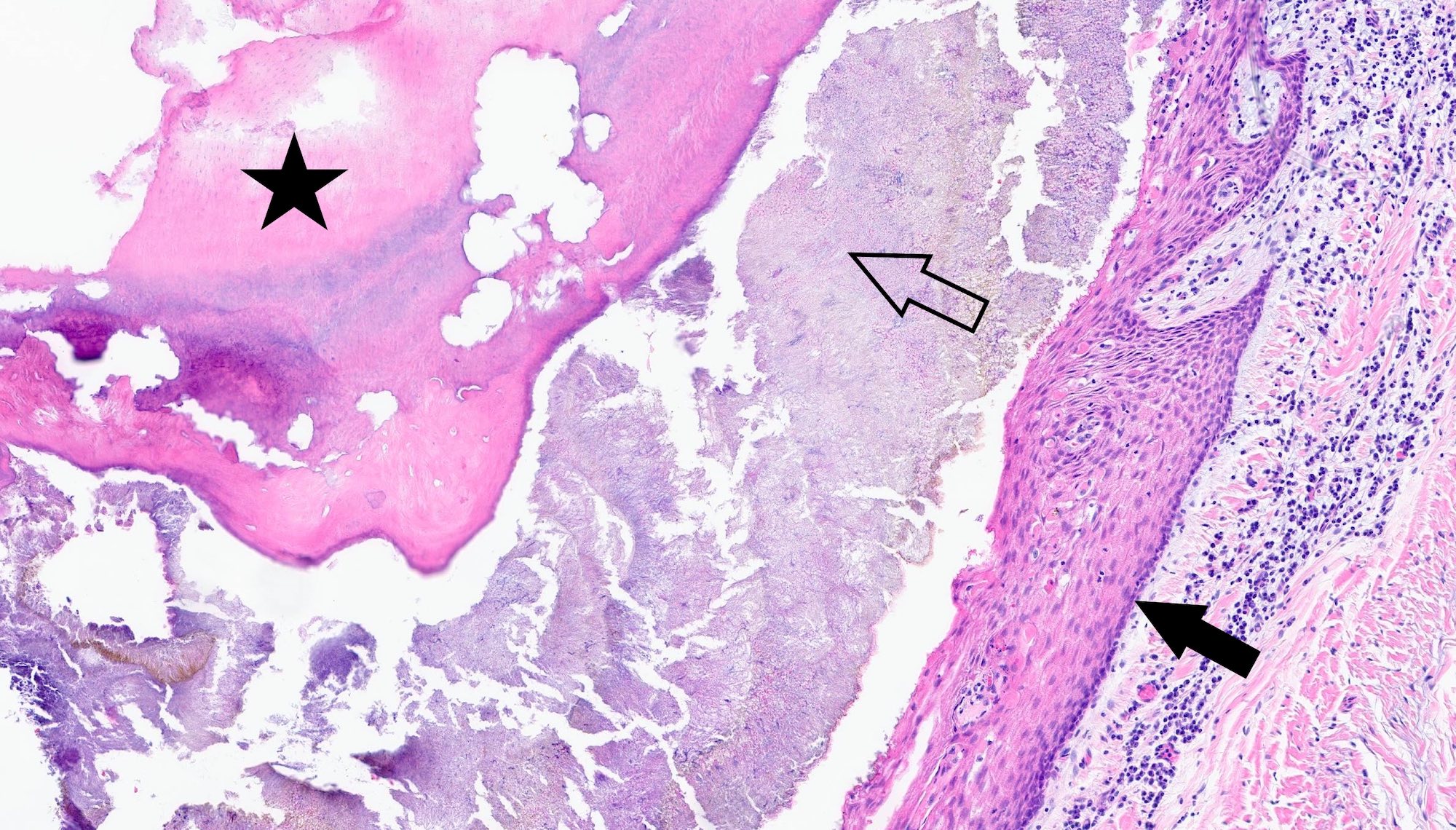
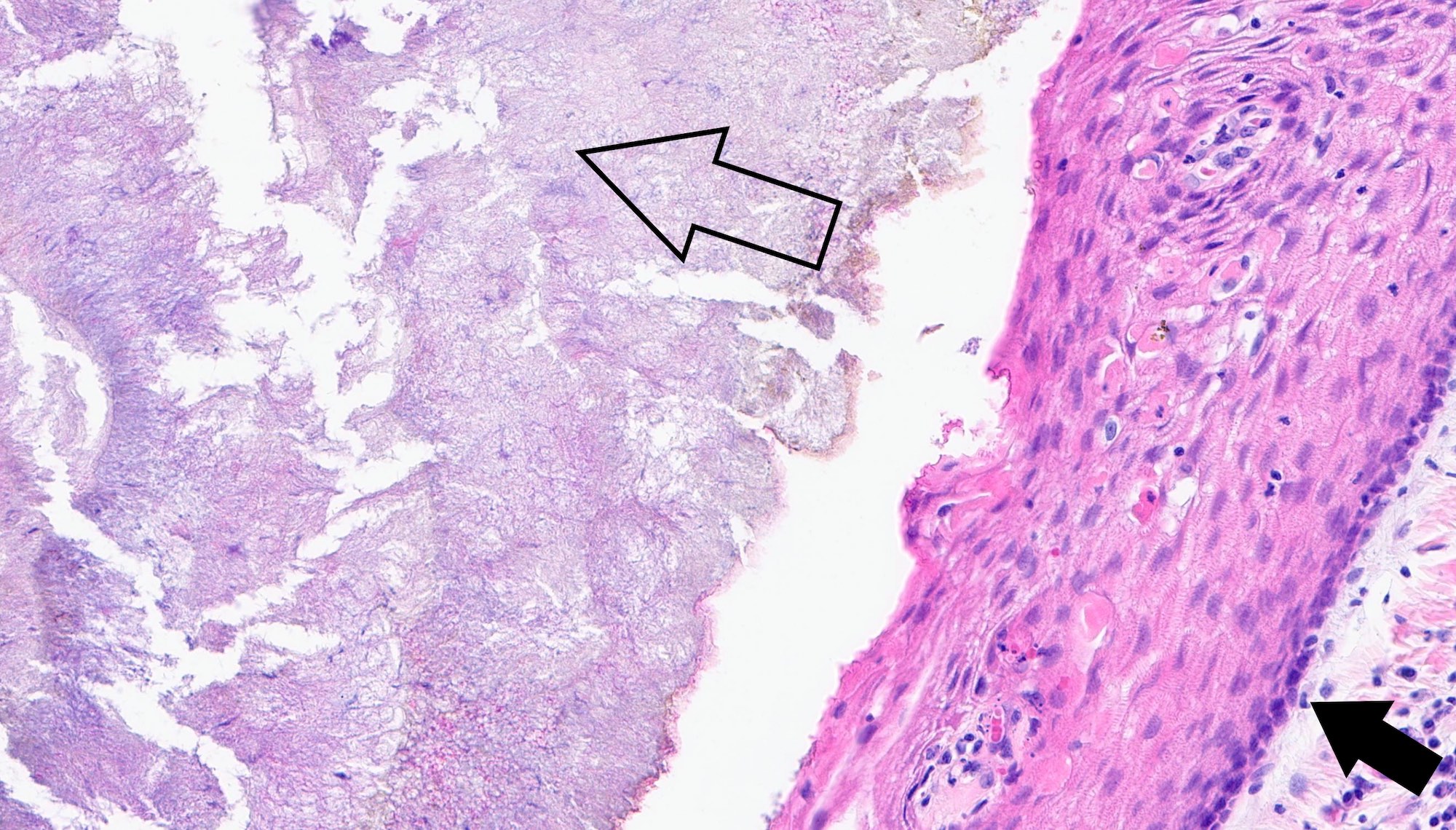
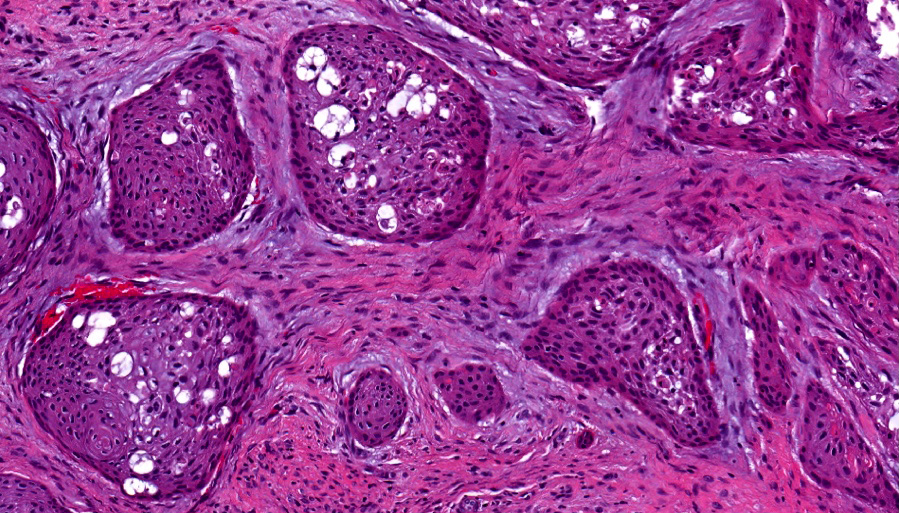
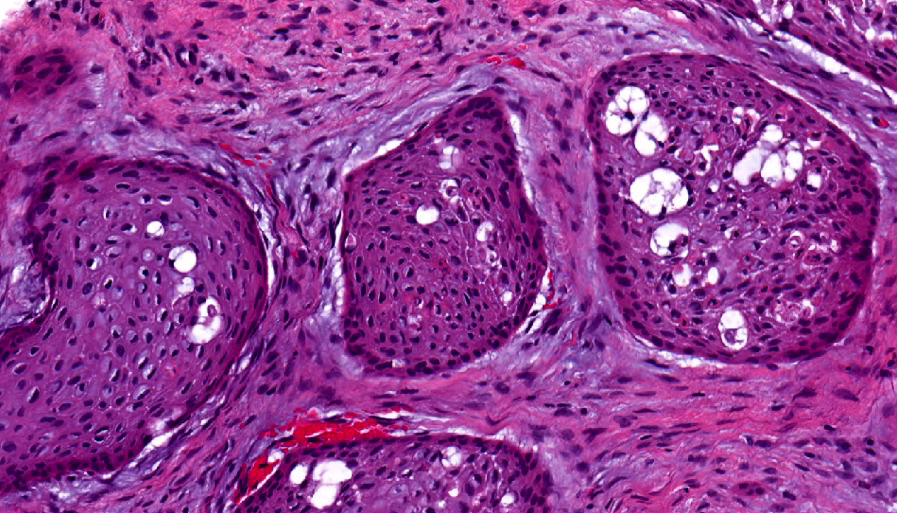
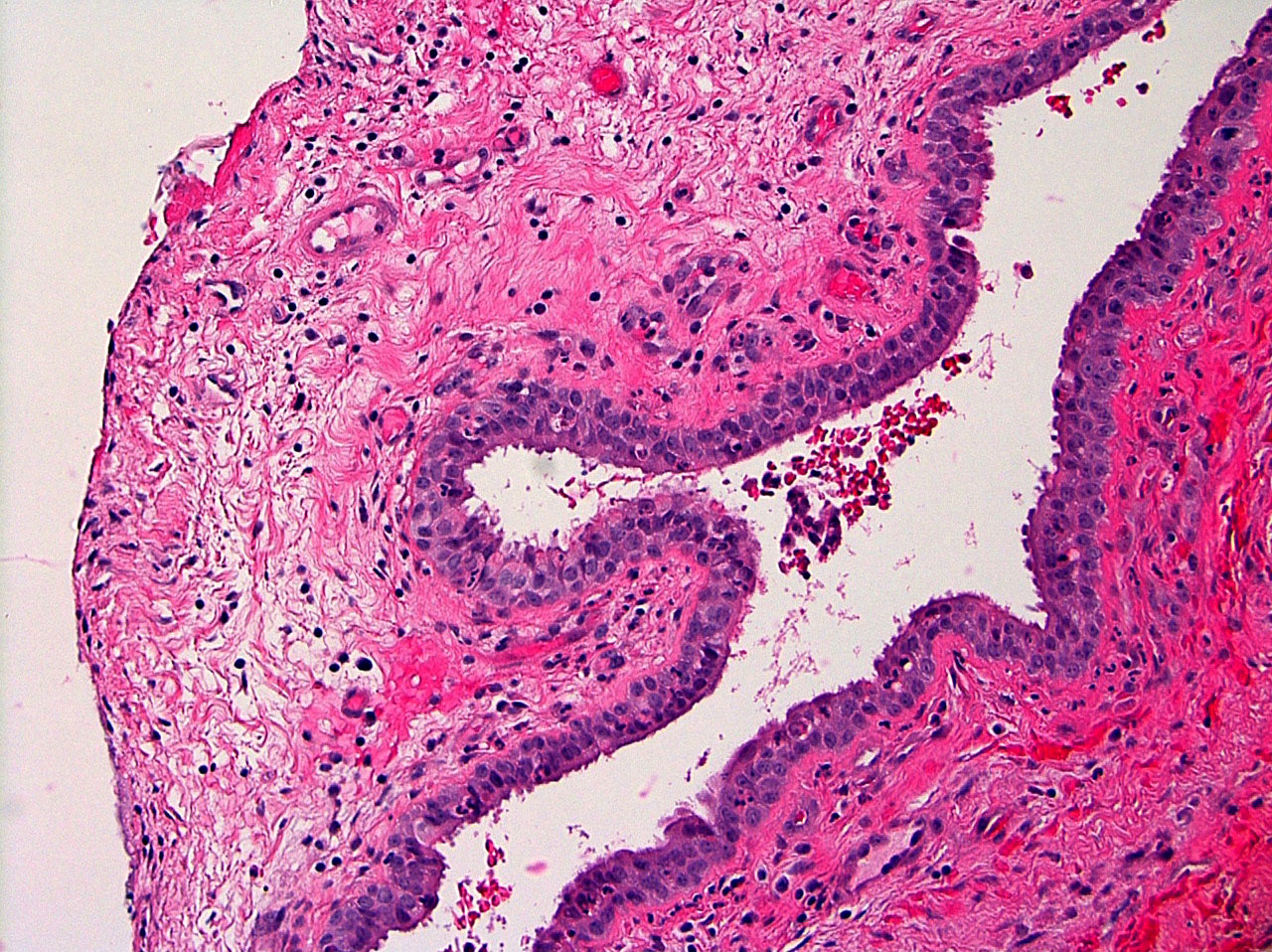
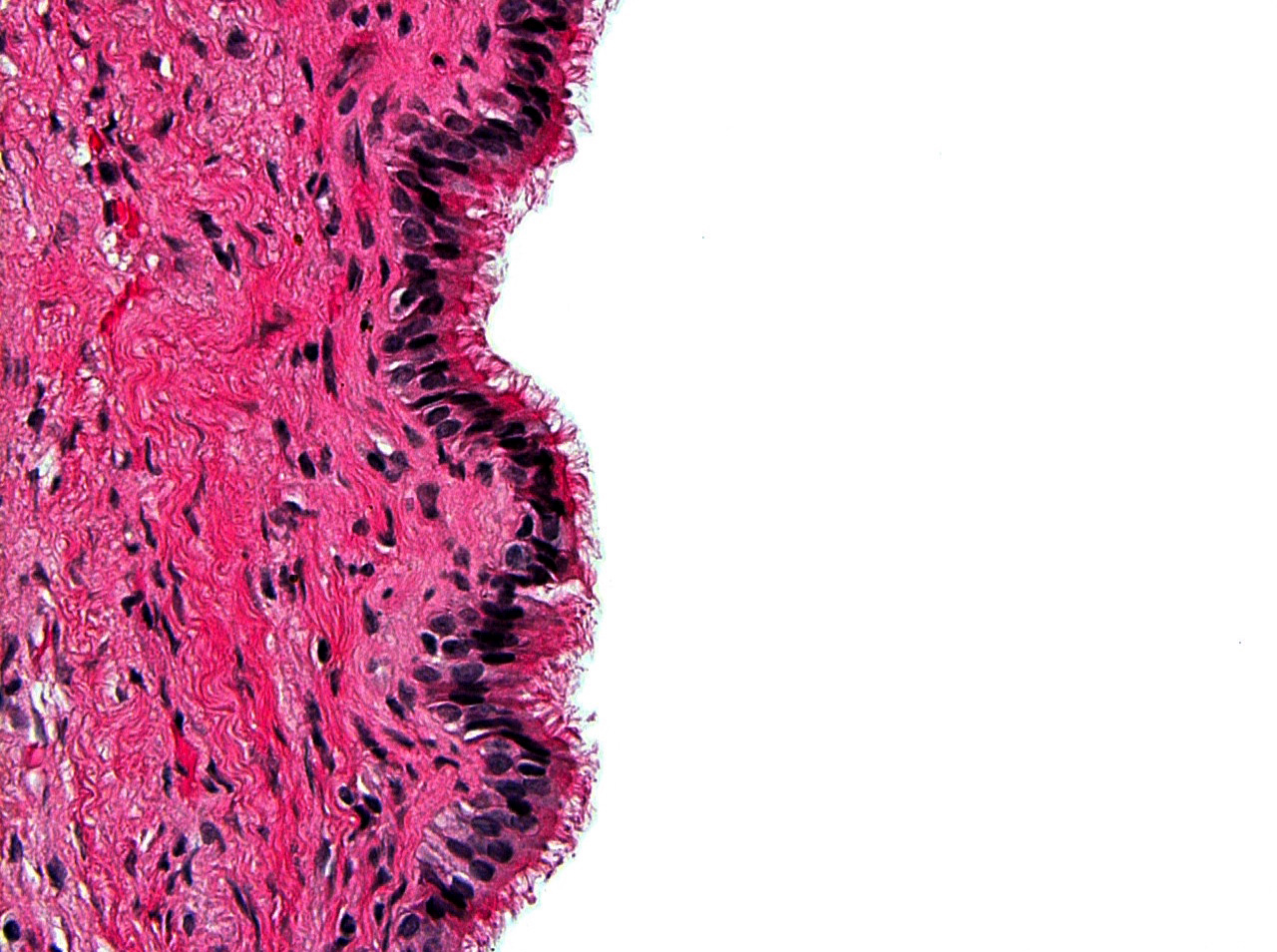
- Cyst lining is of odontogenic epithelium with a well defined layer of palisading basal cells, loosely arranged suprabasal epithelial cells resembling stellate reticulum, similar to ameloblastoma (microscopic image #1)
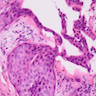
- Unlike ameloblastoma, variable numbers of cells undergo ghost cell change in suprabasilar epithelium (microscopic image #1)
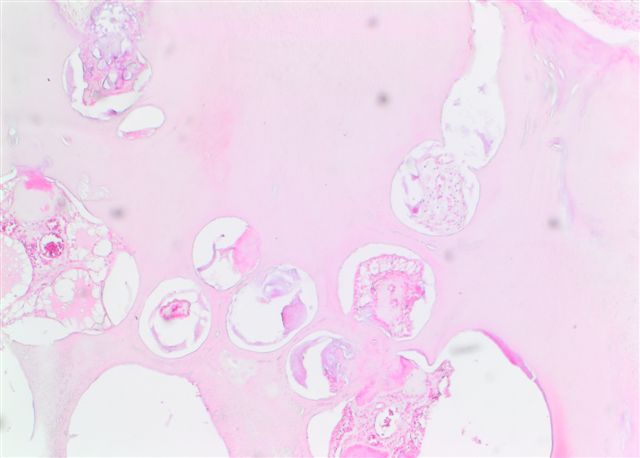
- Pale, eosinophilic ghost cells are altered epithelial cells with preservation of basic cell outline and eosinophilic cytoplasm but loss of the nucleus; ghost cell change may be due to coagulative necrosis, accumulation of enamel protein, aberrant keratinization of odontogenic epithelium and these cells may calcify (microscopic images #2 - 3)
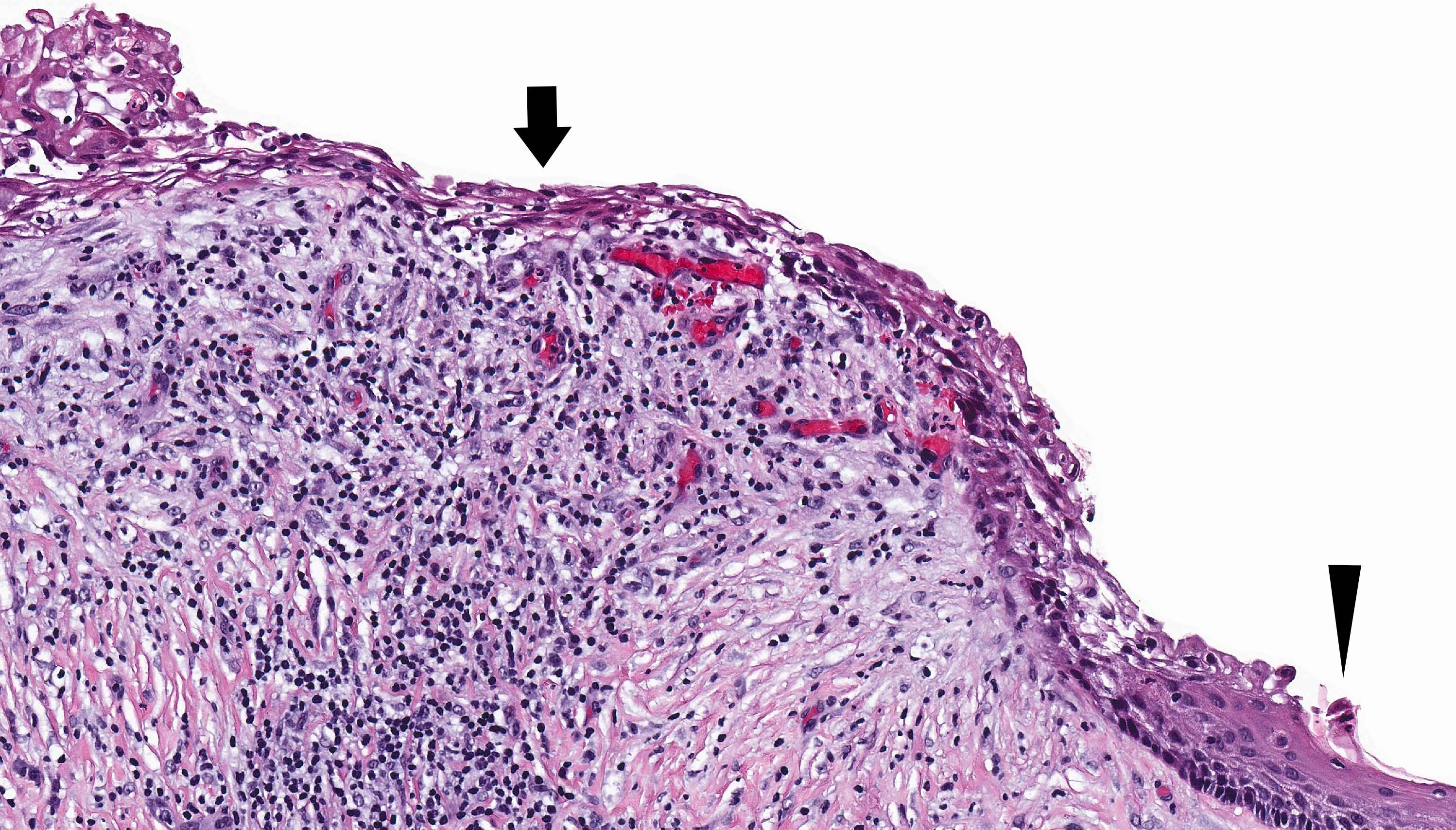
- Other variable findings may include:
- Foreign body giant cell reaction
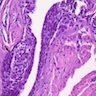
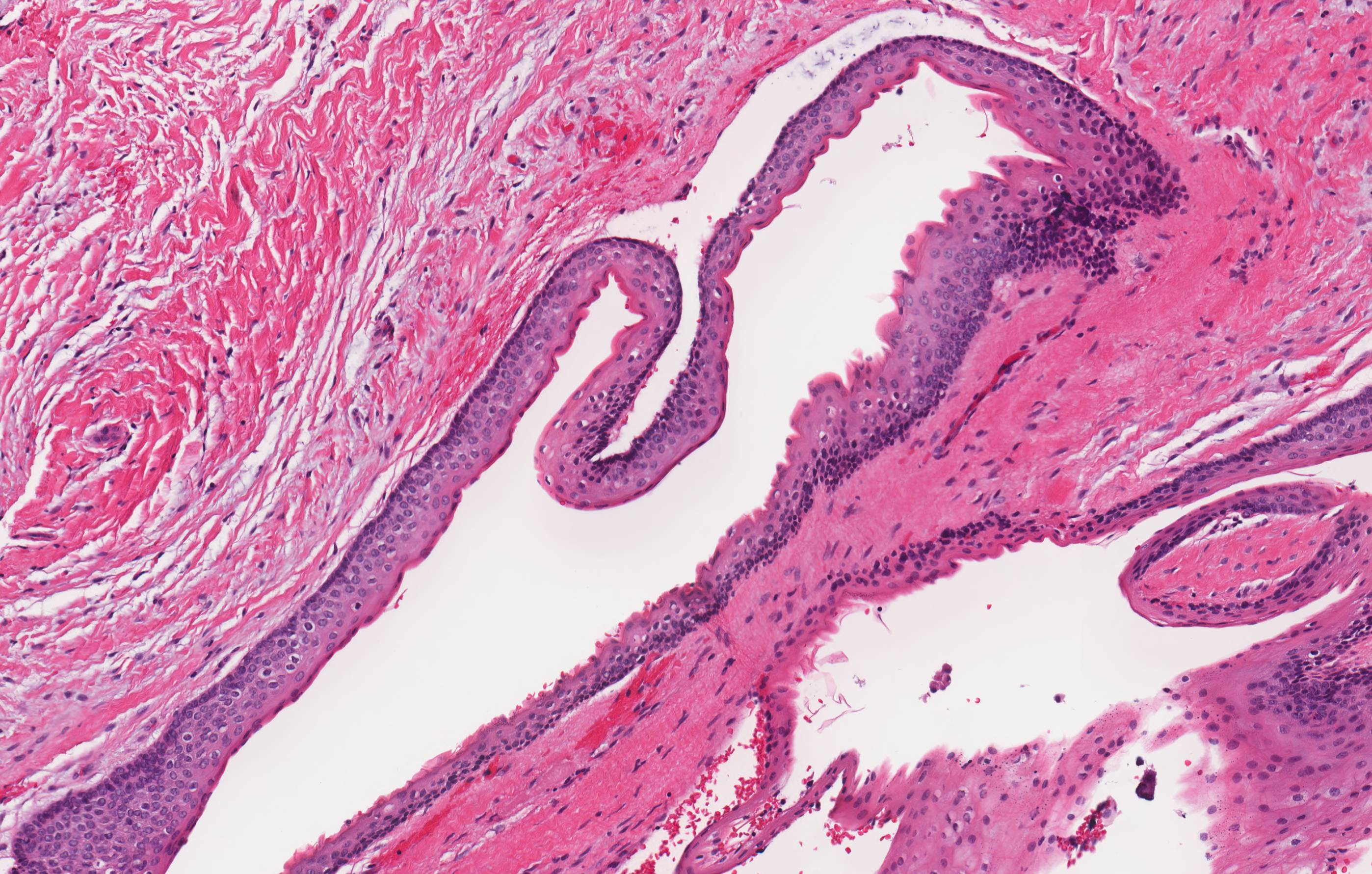
- Proliferation of odontogenic epithelium into the cyst wall which can resemble strands of dental lamina (microscopic image #4)
- Dystrophic calcifications
- Dentinoid may be laid down next to basal cells:
- Paucicellular, eosinophilic calcified material considered to represent dysplastic dentin (microscopic image #4)
- May be present adjacent to epithelial component
- Most likely formed due to an inductive effect of odontogenic epithelium on adjacent mesenchymal tissue
- Cyst wall consists of mature fibrous connective tissue containing scattered inflammatory cells (unless secondarily infected)
- Reference: Reichart: Odontogenic Tumors and Allied Lesions, Illustrated Edition, 2004
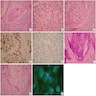
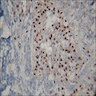
Microscopic (histologic) images
Cytology description
- Numerous polyhedral epithelial cells and occasional columnar cells with calcification and Congo red negative extracellular homogenous material in background (Acta Cytol 2009;53:460)
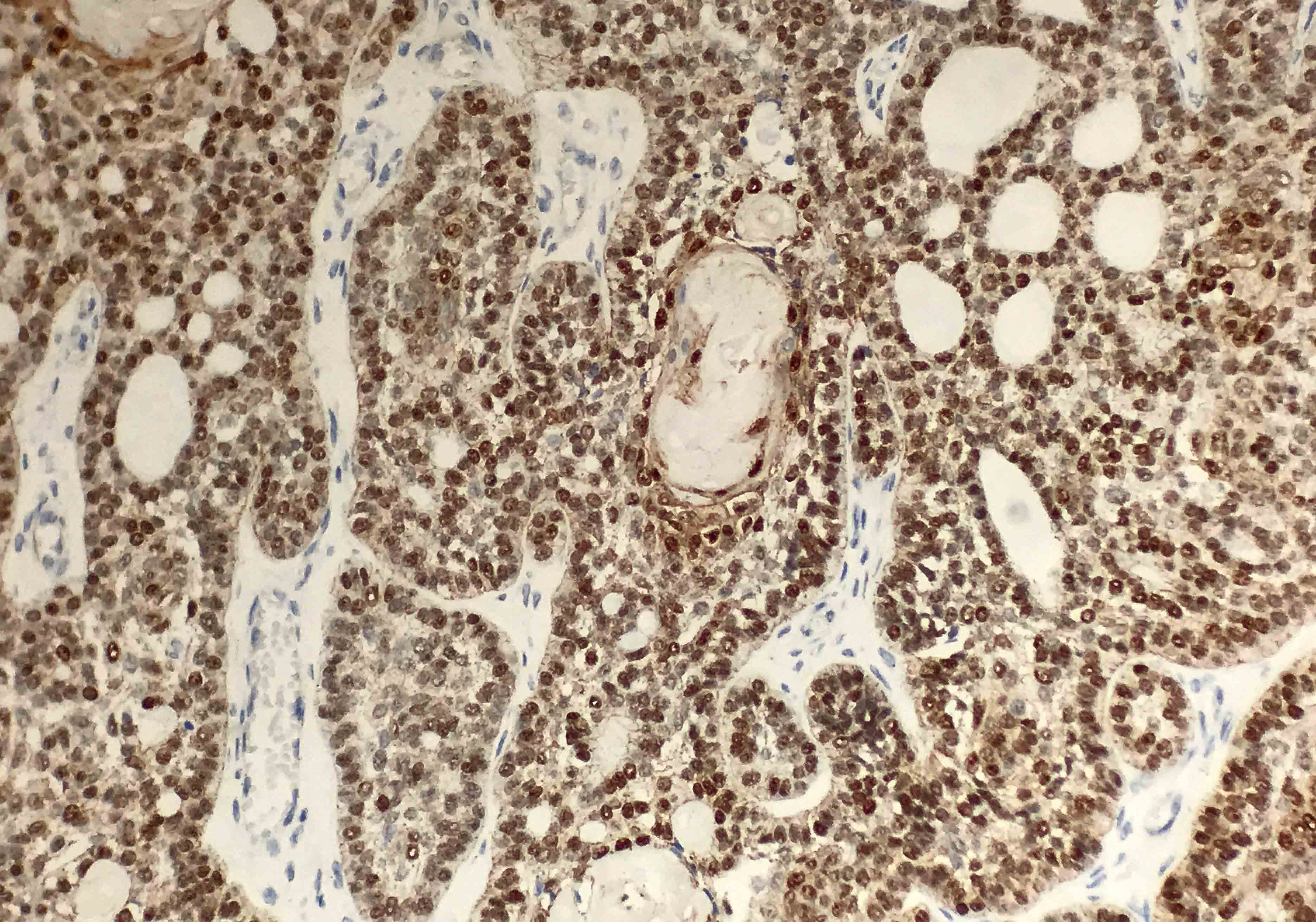
Positive stains
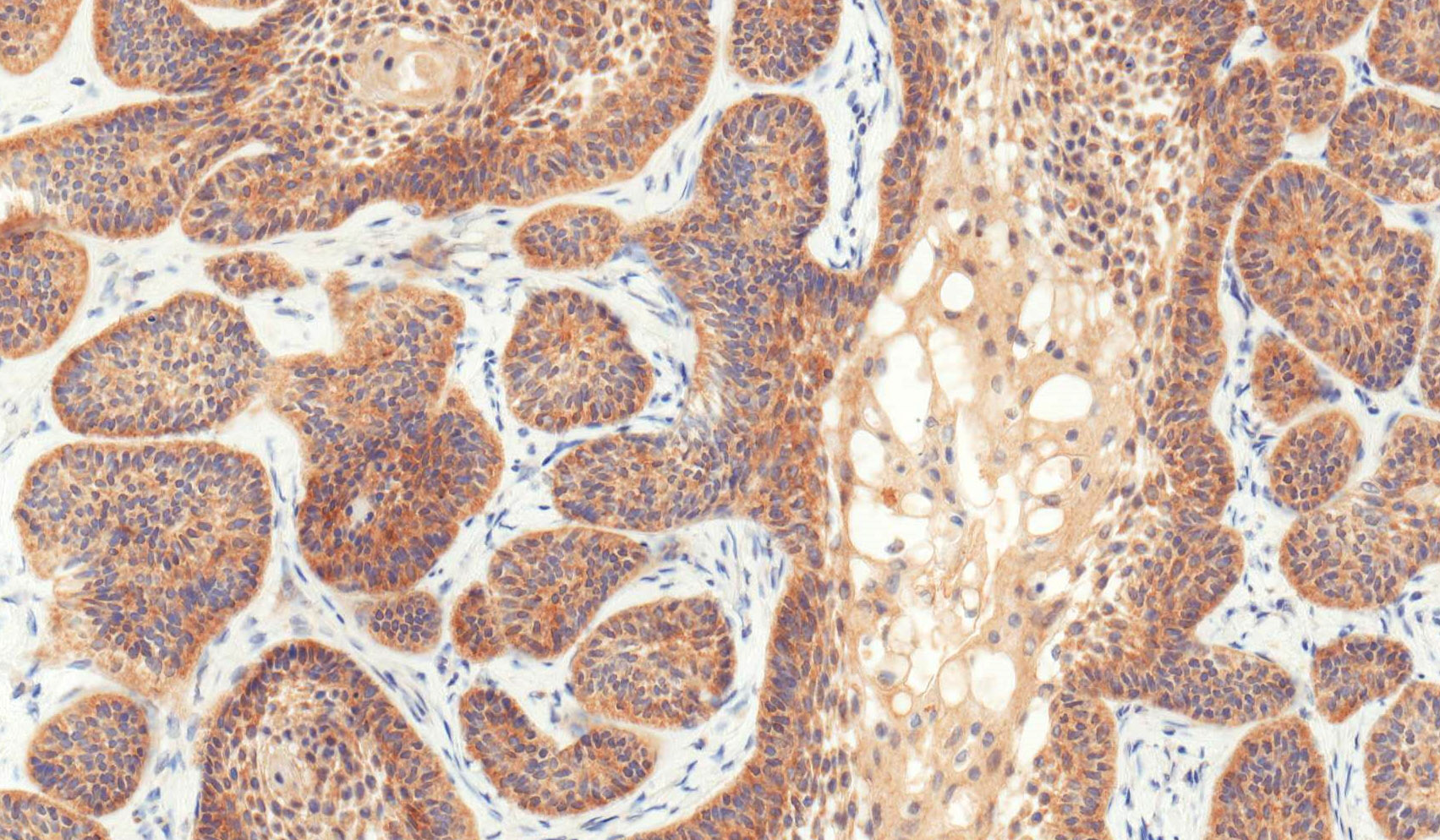
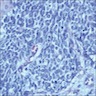
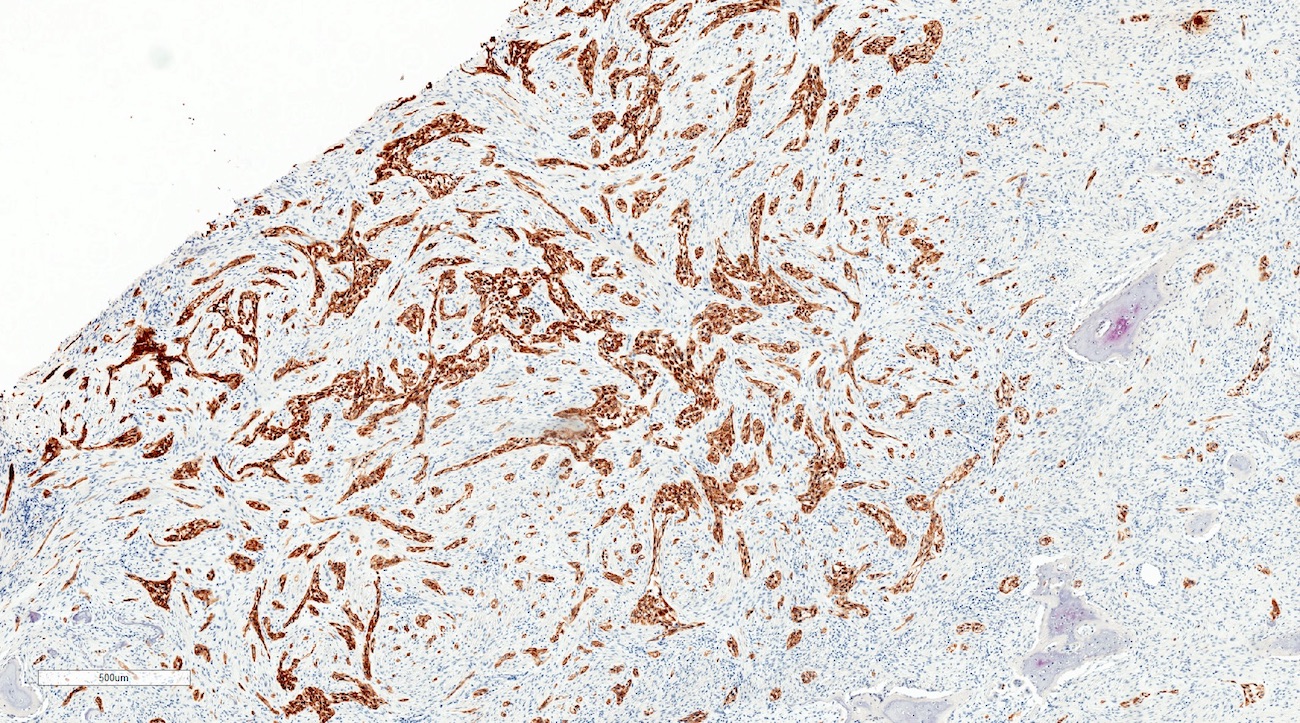
- β catenin (microscopic image #5) (Hum Pathol 2015;46:255, APMIS 2008;116:206, Pathol Int 2006;56:732, Am J Clin Pathol 2003;120:732, Am J Pathol 2003;163:1707)
- LEF1 (microscopic image #6) (Hum Pathol 2015;46:255)
- Cytokeratins 7, 8, 14 and 19 (J Oral Pathol Med 2003;32:163)
- Amelogenesis related proteins (J Oral Pathol Med 2012;41:272)
- MMPs (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112:609)
- Hard α keratins (Am J Clin Pathol 2005;123:376)
- Podoplanin (J Oral Sci 2012;54:165)
- Ki67 lower in COC than in other odontogenic tumors with ghost cells and solid growth pattern (Oral Oncol 2009;45:515)
Negative stains
- Nothing useful or significant
Electron microscopy description
- Basal and suprabasilar cells contain tonofilaments and organelles
- Ghost cells contain coarse bundles of tonofilaments intermingled with dilated membranous organelles (Med Electron Microsc 2002;35:109, Oral Surg Oral Med Oral Pathol 1975;39:769)
Molecular / cytogenetics description
- Aberrations of Wnt signaling pathway with β catenin overexpression (APMIS 2008;116:206)
- Mutations in CTNNB1 but not BRAF (PLoS One 2017;12:e0180224)
Sample pathology report
- Anterior maxilla, right, excisional biopsy:
- Calcifying odontogenic cyst (calcifying cystic odontogenic tumor) 1.2 cm
Differential diagnosis
- Ameloblastoma (conventional or unicystic):
- Usually lacks ghost cells and calcifications
- Dentinogenic ghost cell tumor / carcinoma
- Solid counterpart of COC
- Ghost cell odontogenic carcinoma:
- Infiltrative
- Exhibits mitotic activity, nuclear atypia or necrosis
- Odontoma:
- Lacks the ameloblastic epithelium of COC
- Ameloblastic fibro-odontoma:
- Exhibits primitive mesenchymal stroma
Additional references
Board review style question #1
What is the most common mutation in calcifying odontogenic cyst?
- β catenin
- BRAF
- PPARγ
- PTCH
- RAS
Board review style answer #1
Board review style question #2
What odontogenic tumor most commonly is associated with a calcifying odontogenic cyst?
- Adamantinomatous craniopharyngioma
- Adenomatoid odontogenic tumor
- Ameloblastoma
- Basal cell adenocarcinoma
- Odontoma
Board review style answer #2
Board review style question #3
Board review style answer #3
A. Cyst lining contains a well defined layer of palisading basal cells and a stellate reticulum-like suprabasal layer
Comment Here
Reference: Calcifying odontogenic cyst
Comment Here
Reference: Calcifying odontogenic cyst
Cemento-osseous dysplasia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
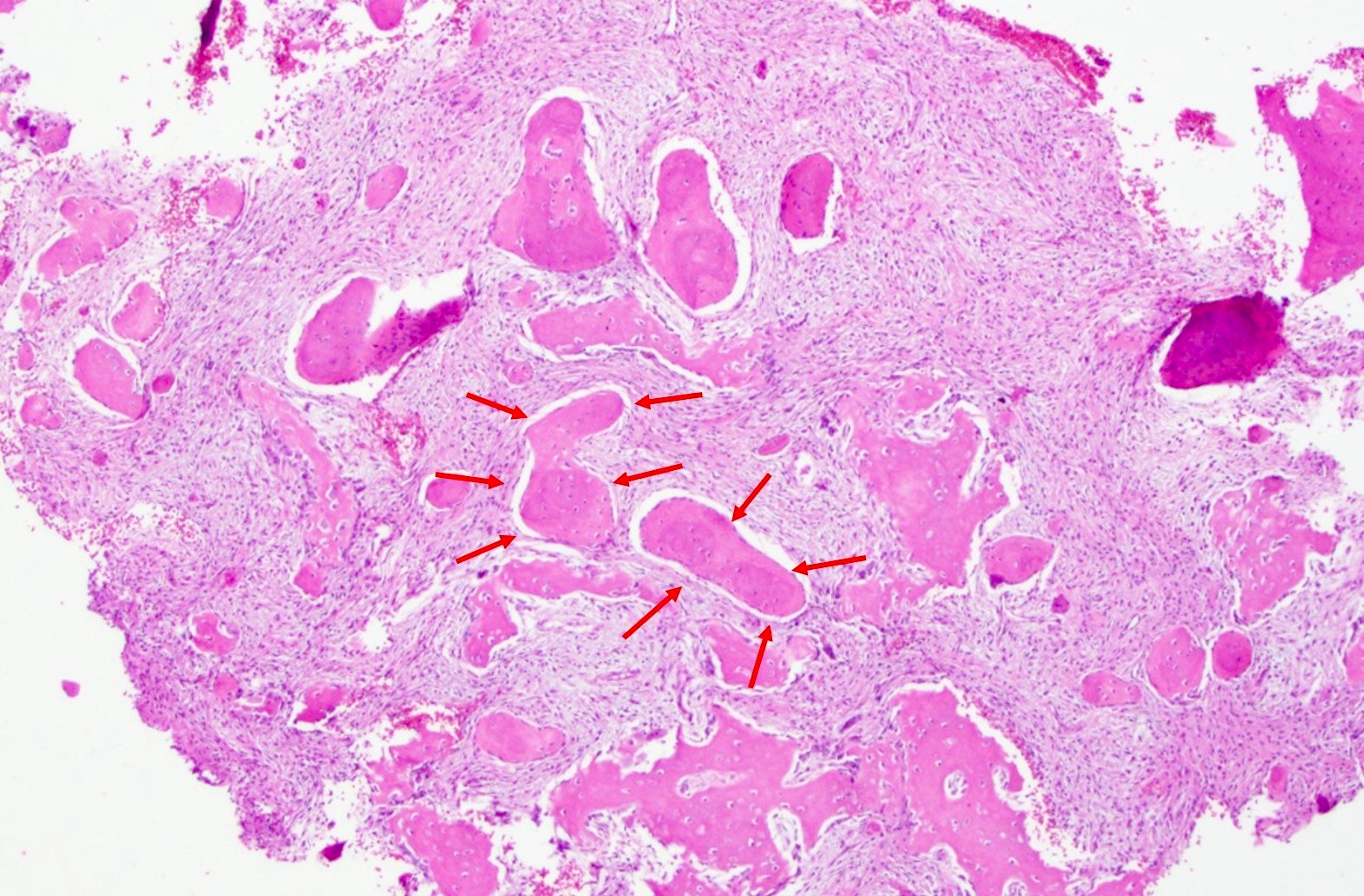
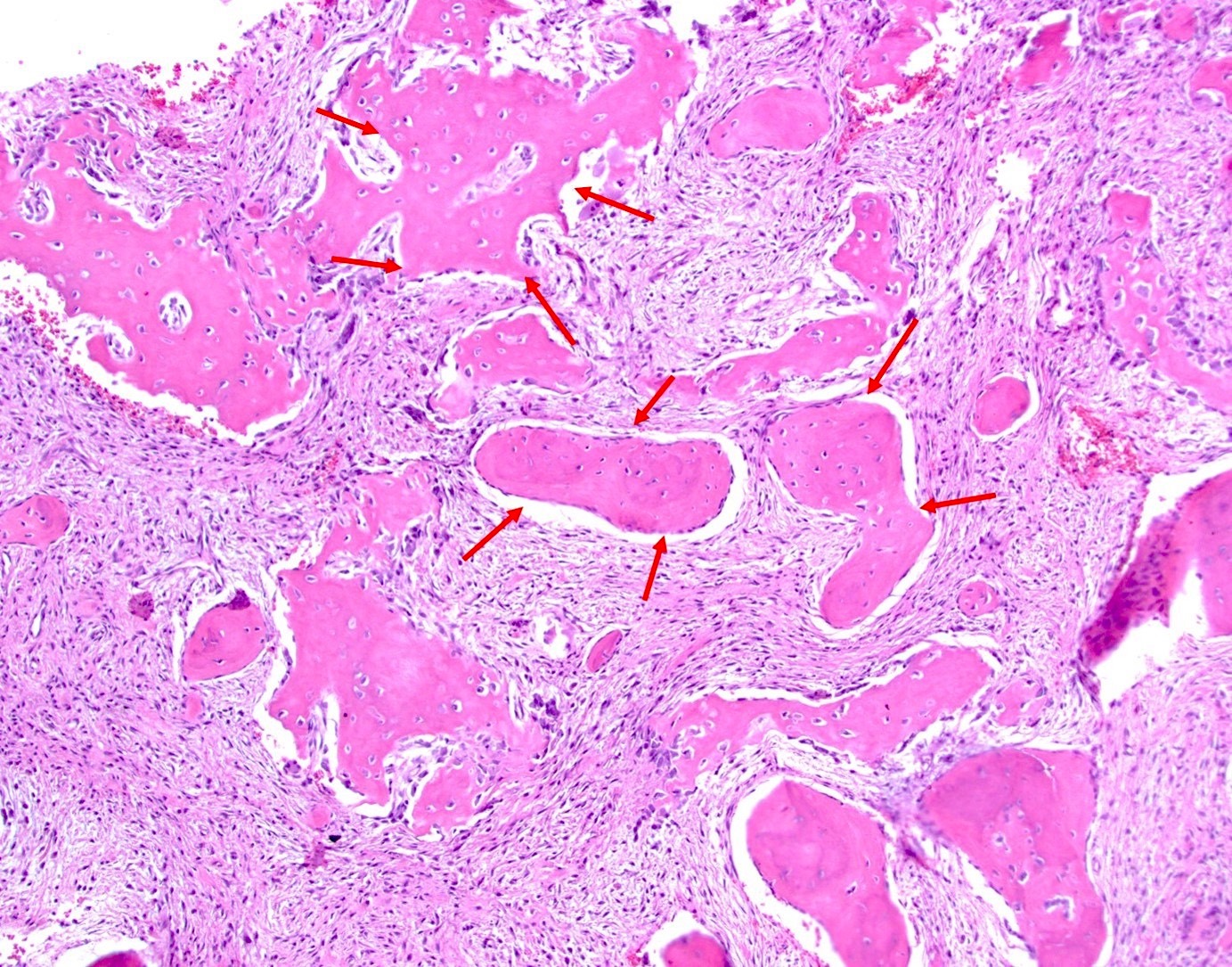
- Cemento-osseous dysplasia is a benign fibro-osseous lesion of the jaws
- Exhibits replacement of mature bone with cementum or immature woven bone surrounded by moderately cellular fibrous connective stroma
- 3 clinicoradiographic subtypes accepted:
- Focal cemento-osseous dysplasia
- Periapical cemento-osseous dysplasia
- Florid cemento-osseous dysplasia
Essential features
- Diagnosis is dependent on clinical, gross description, radiologic and pathologic correlation
- Cemento-osseous dysplasia, ossifying fibroma, fibrous dysplasia and osteoblastoma may exhibit marked histologic, clinical and radiographic overlap
Terminology
- Cemento-osseous dysplasia
- Osseous dysplasia
ICD coding
Epidemiology
- Periapical cemento-osseous dysplasia and florid cemento-osseous dysplasia: middle aged females of African descent
- Focal cemento-osseous dysplasia: middle aged white females
Sites
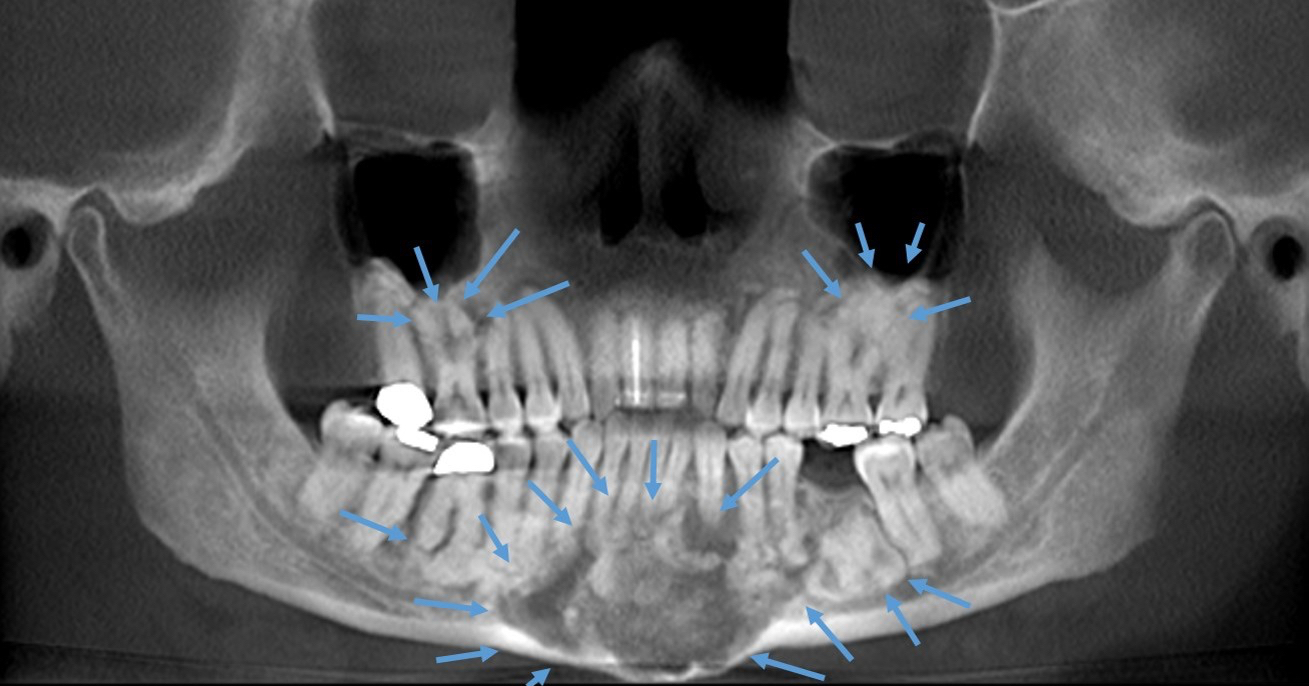
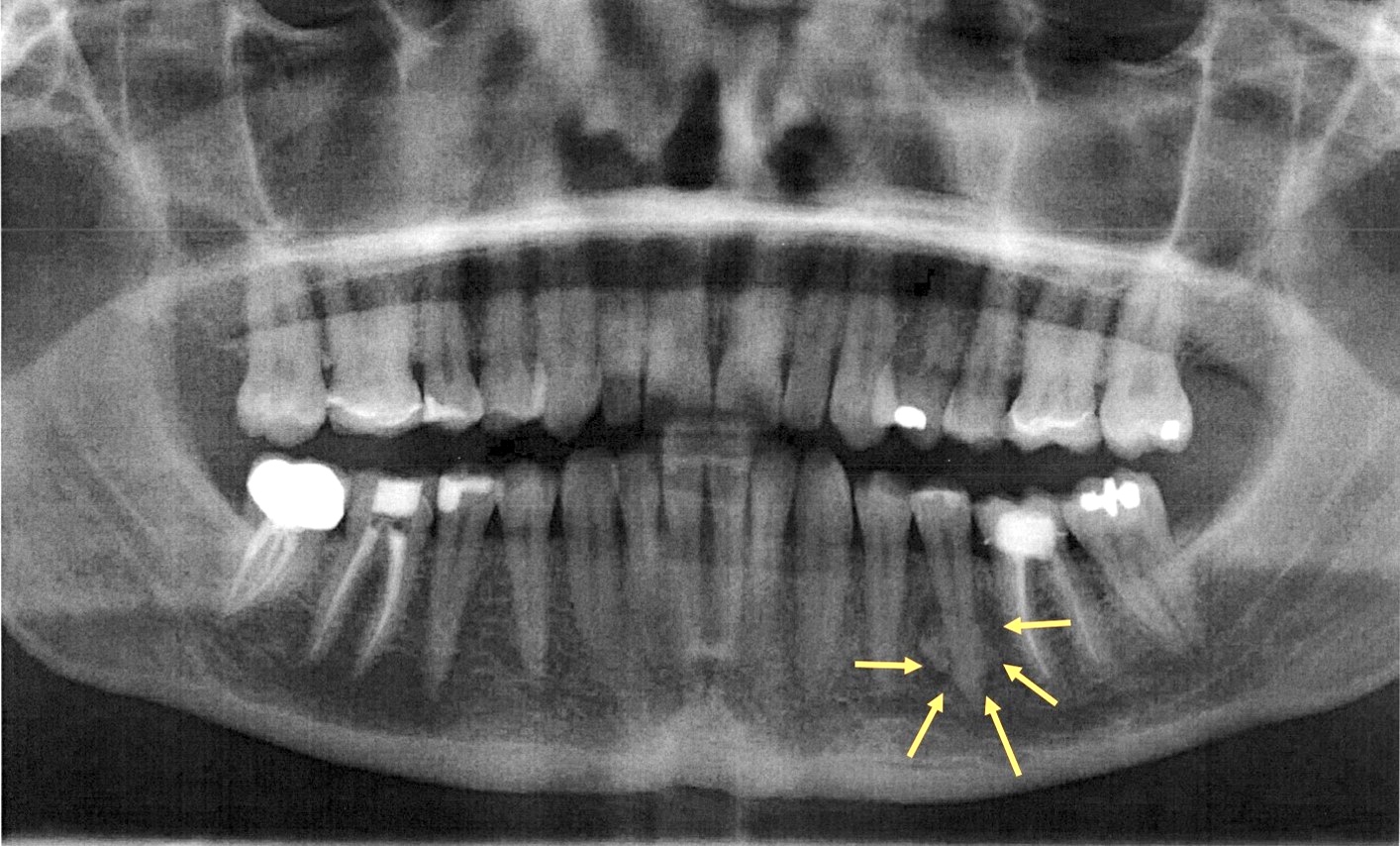
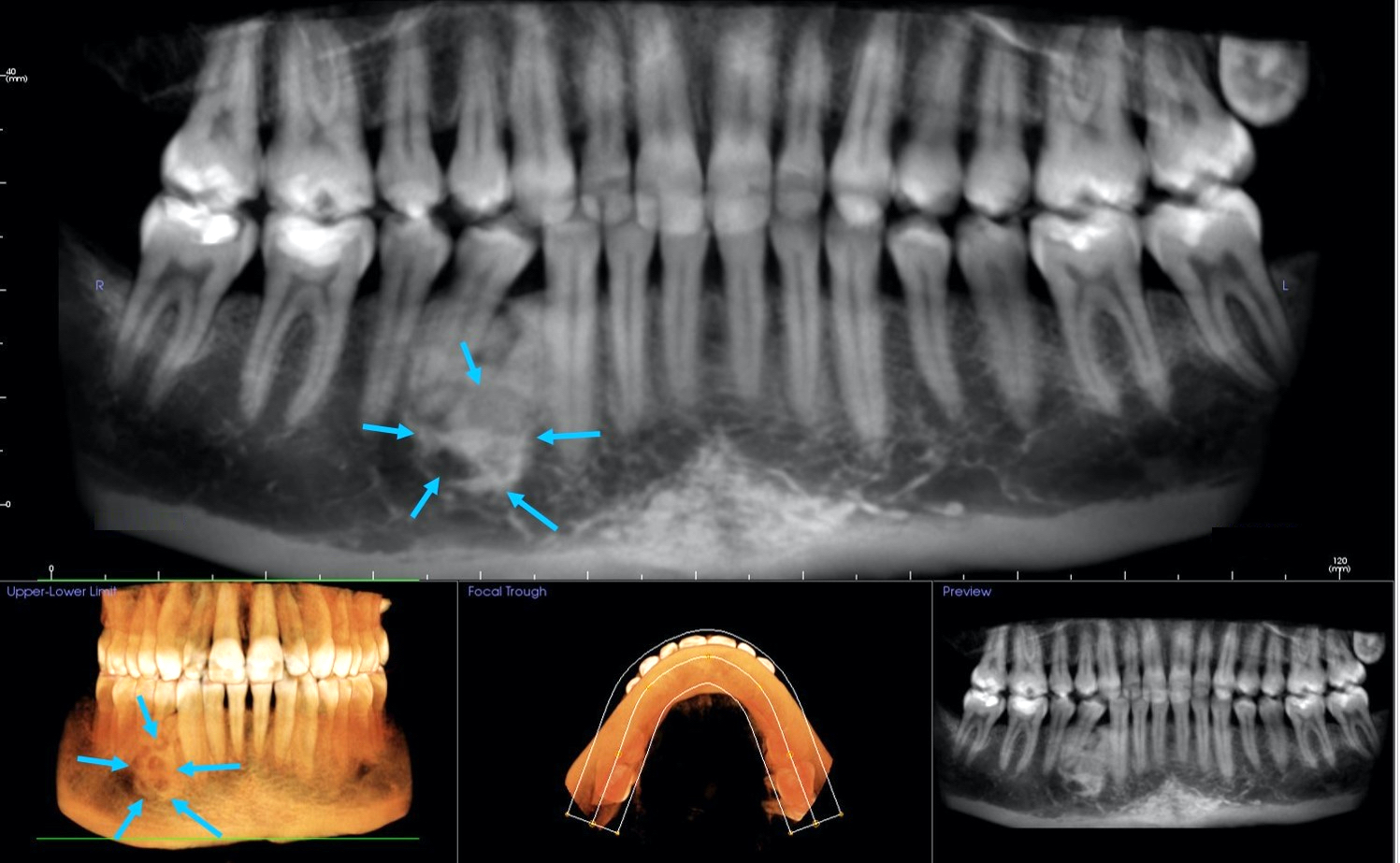
- Periapical cemento-osseous dysplasia: apices of anterior mandibular teeth
- Focal cemento-osseous dysplasia: apices of posterior mandibular teeth, #19 and #30
- Florid cemento-osseous dysplasia: multiquadrant involvement with more in posterior jaws
Etiology
- Believed to arise from cells of the periodontal ligament
Clinical features
- Most common form of nonexpansile, benign fibro-osseous lesion of the jaws
- Usually asymptomatic, noted as an incidental radiographic finding
- Associated teeth usually test vital, unlike with periapical infections that render teeth nonvital
- Periapical cemento-osseous dysplasia is associated with apices of mandibular anterior teeth
- Focal cemento-osseous dysplasia is a solitary lesion usually associated with apices of teeth #19 and #30
- Florid cemento-osseous dysplasia exhibits multiquadrant involvement of jaws
- Clinical or radiographic expansion does not preclude diagnosis; bone expansion can occur in the setting of a second lesion, for example, inflammation or simple bone cyst formation
- Reference: Dentomaxillofac Radiol 2011;40:230
Diagnosis
- Diagnosis is dependent on clinical, radiologic and pathologic correlation in association with the clinical description and gross findings
Radiology description
- Depends on subtype
- Mixed radiolucent / radiopaque, sclerotic lesion presenting with a lucent halo or rim at apices of teeth
- Mature cemento-osseous dysplasia may be sclerotic, fuse and become completely radiopaque with involvement of apices of anterior mandibular vital teeth, adjacent to the mandibular posterior teeth or may even be multifocal, multiquadrant
- Small isolated lesions often nonexpansile
- Reference: Head Neck Pathol 2020;14:70
Radiology images
Prognostic factors
- In some cases, instrumentation may be associated with local complications
- In cases in which secondary infection occurs, antibiotics and sequestrectomy may be required
- Reference: Regezi: Oral Pathology - Clinical Pathologic Correlations, 7th Edition, 2016
Case reports
- 27 year old man with lower left posterior mandibular lesion (J Oral Maxillofac Pathol 2020;24:S15)
- 37 year old woman with apical radiopaque lesion surrounded by a radiolucent halo and 42 year old woman with mixed radiolucent / opaque lesion of teeth (J Clin Exp Dent 2018;10:e1145)
- 49 year old white woman with several jaw lesions on the apical aspect of teeth (J Clin Exp Dent 2018;10:e291)
Treatment
- Requires no treatment, especially for asymptomatic lesions
- Reference: Regezi: Oral Pathology - Clinical Pathologic Correlations, 7th Edition, 2016
Gross description
- Aggregates of tan-brown, sandy, gritty or granular hard tissue
Microscopic (histologic) description
- Fragment of calcified material exhibiting viable cells with numerous smaller calcified masses distributed in a fibrous stroma
- Calcified fragment exhibits a haphazard woven pattern of mineralization with occasional resting and reversal lines with droplets or spherules of cementum-like material and some mature areas exhibiting fused calcified masses resembling ginger root-like formation
- Reference: Head Neck Pathol 2020;14:70
Microscopic (histologic) images
Sample pathology report
- Right mandible, area of tooth #28, excision:
- Benign fibro-osseous lesion consistent with focal cemento-osseous dysplasia
- Comment: Examination of the decalcified specimen revealed cellular fibrous connective with variably mineralized and calcified bone and cementum-like material. The bony material is variably sized and shaped, ranging from ginger root-like configurations to smaller spherical droplets of material. Dense cortical bone being replaced by cellular fibro-osseous material is also seen. The fibrous tissue exhibits spindle shaped cells that produce osteoid and spherical collections of cementum. The histomorphology combined with the clinical description and radiographic presentation is consistent with focal cemento-osseous dysplasia.
Differential diagnosis
- Ossifying fibroma:
- Cellular stroma, cemento-osseous: lace-like, droplets or spherule with intimate brush borders with the stroma
- Fibrous dysplasia:
- Curvilinear, mature bone dispersed in acellular, dense scar-like fibrous stroma, retraction artifact with absence of osteoblastic rimming and lack of cementum
- Osteoblastoma:
- Highly cellular fibrovascular stroma with reactive boney trabeculae dispersed within vascular, sinusoidal channels, hemorrhage, numerous multinucleated osteoclastic giant cells
Additional references
Board review style question #1
A 39 year old woman presented with an asymptomatic mixed radiolucent / radiopaque lesion at the apical area of vital tooth #28 consistent with cemento-osseous dysplasia. Which of the following is true?
- Antibiotics are often indicated
- Higher recurrence potential
- Lesion best treated by local excision or curettage
- Surgical intervention is required every 2 years
- Treatment is not required but should be observed periodically
Board review style answer #1
E. Treatment is not required but should be observed periodically
Comment Here
Reference: Cemento-osseous dysplasia
Comment Here
Reference: Cemento-osseous dysplasia
Board review style question #2
A 49 year old woman was biopsied for mixed asymptomatic mixed radiolucent / radiopaque lesions at the apices of anterior mandibular teeth. If microscopic features revealed ginger root-like formations as shown in the image, what is your diagnosis?
- Central ossifying fibroma
- Florid cemento-osseous dysplasia
- Focal cemento-osseous dysplasia
- Osteoblastoma
- Periapical cemento-osseous dysplasia
Board review style answer #2
Board review style question #3
Upon diagnosis, cemento-osseous dysplasia is best left untreated with a watchful waiting because
- High risk of severe bleeding during surgical manipulation
- The lesion is an asymptomatic, self limited process
- Teeth in the area must be extracted
- These are difficult to resect and therefore, difficult to treat
- These lesions tend to have a high recurrence rate
Board review style answer #3
B. The lesion is an asymptomatic, self limited process
Comment Here
Reference: Cemento-osseous dysplasia
Comment Here
Reference: Cemento-osseous dysplasia
Cemento-ossifying fibroma / ossifying fibroma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign fibro-osseous neoplasm (J Maxillofac Oral Surg 2021;20:240, Oral Dis 2017;23:440, Head Neck Pathol 2020;14:70, J Stomatol Oral Maxillofac Surg 2022;123:364)
- Origin is controversial but is mostly odontogenic in origin and usually arises in jaw (J Maxillofac Oral Surg 2021;20:240, Head Neck Pathol 2022;16:248)
- Characterized by production of bone and cementum-like calcifications in a fibrous stroma
- Usually located in the mandible during third to fourth decade of life (Oral Dis 2017;23:440, Head Neck Pathol 2020;14:70, J Stomatol Oral Maxillofac Surg 2022;123:364)
Essential features
- Cemento-ossifying fibroma (COF) is a fibro-osseous lesion characterized by varied patterns of bone formation in a fibroblastic stroma (Head Neck Pathol 2022;16:991)
- Putatively benign lesion with very rare malignant transformation or metastasis (Head Neck Pathol 2022;16:991, Ear Nose Throat J 2023;102:24)
- Cemento-ossifying fibroma is named because of its usual location in the tooth bearing region of the jaw
Terminology
- Ossifying fibroma, conventional type; cemento-ossifying fibroma; ossifying fibroma; conventional ossifying fibroma; cementifying fibroma
ICD coding
- ICD-O: 9274/0 - cemento-ossifying fibroma
Epidemiology
- Rare lesion that occurs over a broad age range, with peak incidence in third to fourth decade of life (Oral Dis 2017;23:440, Head Neck Pathol 2020;14:70, J Stomatol Oral Maxillofac Surg 2022;123:364)
- Has female predilection (F:M = 5:1)
- Tumor is more prevalent in White people than Black people (Oral Dis 2017;23:440)
Sites
- Mandible is most common, followed by the maxilla
- Mandibular premolar and molar area have more predilection (Head Neck Pathol 2020;14:70)
- Maxillary lesions tend to involve antrum and canine fossa
- Cemento-ossifying fibroma can be classified according to site of origin as (Int J Surg Case Rep 2020;68:257)
- Soft tissue counterpart is referred to as peripheral cemento-ossifying fibroma (PCOF) (Int J Surg Case Rep 2020;68:257)
- Central cemento-ossifying fibroma (CCOF) arises from cells of periodontal ligament in the apical area (Int J Surg Case Rep 2020;68:257)
Pathophysiology
- Multiple cemento-ossifying fibromas rarely show syndromic / inherited association and usually present at a younger age (Case Rep Dent 2023;2023:4664619)
- Hyperparathyroidism jaw tumor syndrome (HPT JT): rare autosomal dominant disease caused by mutation of the tumor suppressor gene CDC73 that encodes for parafibromin resulting in premature truncation of parafibromin protein product; the syndrome predisposes to a triad occurrence: multiple maxillary or mandibular cemento-ossifying fibroma, parathyroid adenoma or carcinoma and renal and uterine tumors (Int J Surg Case Rep 2020;68:257)
Etiology
- Origin of cemento-ossifying fibroma has been suggested as odontogenic or from periodontal ligament progenitor cells (J Maxillofac Oral Surg 2021;20:240)
- Origin of nonodontogenic lesions of similar histology arising from craniofacial bone outside the jaw is unclear (Head Neck Pathol 2022;16:257)
- Periodontal membrane has multipotent cells capable of forming cementum, lamellar bone and fibrous tissue (J Maxillofac Oral Surg 2021;20:240)
- Lesion can present as a component of hyperparathyroidism jaw tumor syndrome; 25 - 50% of patients of this syndrome develop cemento-ossifying fibroma of the jaws with the mandible as a favored site (Case Rep Dent 2023;2023:4664619, J Dent Sci 2020;15:426)
Clinical features
- Smaller lesions are asymptomatic
- Larger / solitary lesions present as painless swelling that causes jaw expansion, facial asymmetry, tooth divergence (Heliyon 2021;7:e07594)
- Grows slowly but can reach a considerable size if left untreated (Head Neck Pathol 2020;14:70)
- Extension to the nasal septum, orbital floor and infraorbital foramen can be seen in larger lesions; rarely associated with the destruction of extraosseous soft tissue components (Heliyon 2021;7:e07594)
- Juvenile variant of lesion occurring at a young age is clinically aggressive and highly recurrent (Heliyon 2021;7:e07594, J Maxillofac Oral Surg 2021;20:240)
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation (J Maxillofac Oral Surg 2021;20:240)
Laboratory
- No specific laboratory tests are available
Radiology description
- Radiological findings vary according to maturity of lesion; early lesions show well defined corticated radiolucency and over time, lesion becomes progressively more radiopaque (Heliyon 2021;7:e07594, BMJ Case Rep 2020;13:e239286)
- Well circumscribed, unilocular lesion with centrifugal pattern of growth (Heliyon 2021;7:e07594)
- Can present as lesion with well defined sclerotic borders and is predominantly unilocular (BMJ Case Rep 2020;13:e239286)
- Larger lesions show expansion, thinning and perforation of buccal and lingual cortex and involvement of the lower border of mandible (BMJ Case Rep 2020;13:e239286)
Radiology images
Prognostic factors
- Slow growing, benign neoplasm with no recurrence in majority of cases
- Malignant transformation has been reported in a single case (Ear Nose Throat J 2023;102:24)
Case reports
- 19 year old woman with a large maxillary cemento-ossifying fibroma superimposed with solitary bone cyst (Int J Surg Case Rep 2020;68:257)
- 30 year old man with multiple cemento-ossifying fibromas (Case Rep Dent 2023;2023:4664619)
- 45 year old man with large cemento-ossifying fibroma of the mandible involving the infratemporal and parapharyngeal spaces (Heliyon 2021;7:e07594)
Treatment
- Treated by enucleation and curettage as it shells out easily and recurs rarely (J Maxillofac Oral Surg 2021;20:240)
- Untreated tumors can attain a massive size and rarely require en bloc resection (J Maxillofac Oral Surg 2021;20:240)
Clinical images
Gross description
- Well circumscribed tumor that can be shelled out easily in one piece
Microscopic (histologic) description
- Well defined lesion; may have thin fibrous capsule
- Well demarcated margin from surrounding normal bone
- Lesion consists of variable proportion of fibrous and mineralized tissue, more heavily mineralized centrally; it shows variation in the amount and type of mineralization, even within a single lesion
- Osteoblastic rimming of bone trabeculae is frequent (J Stomatol Oral Maxillofac Surg 2022;123:364)
- Stroma is fibroblastic with areas of hypercellularity and nuclear hyperchromasia
- No significant atypia and mitoses are infrequent (Head Neck Pathol 2020;14:70)
- Woven to lamellar bone, osteoid and dense acellular or paucicellular basophilic rounded cementum-like calcifications may all be present (Head Neck Pathol 2020;14:70)
- Bony trabeculae may form thick anastomosing strands or fuse into large sheets centrally
- Hemorrhagic cystic degeneration, resembling aneurysmal bone cyst formation, is more often found in juvenile active ossifying fibromas but has been reported in rare COFs (J Stomatol Oral Maxillofac Surg 2022;123:364, Head Neck Pathol 2022;16:248)
- Areas resembling cemento-ossifying fibroma may be seen in psammomatoid and trabecular ossifying fibroma; cemento-ossifying fibroma may contain ossicles like psammomatoid ossifying fibroma
- Cemento-ossifying fibroma with syndromic association has histology similar to that of nonsyndromic cemento-ossifying fibroma
- Cemento-ossifying fibroma in gnathodiaphyseal dysplasia is at the more fibrous end of spectrum with predominantly basophilic rounded islands and droplets of acellular bone (Head Neck Pathol 2014;8:432)
Microscopic (histologic) images
Contributed by Saira Javeed, M.B.B.S., M.Phil., Kelly Magliocca, D.D.S., M.P.H. and Molly Housley Smith, D.M.D.
Positive stains
- Cemento-ossifying fibromas showed significant immune reactivity for keratan sulphate (IHC is not necessary for diagnosis) (J Maxillofac Oral Surg 2021;20:240)
Negative stains
- p53 (J Clin Exp Dent 2022;14:e27)
- MDM2 (25%) and CDK4 (2%) can be seen in a subset of cases but is typically focal (≤ 10% of nuclear staining) (Head Neck Pathol 2022;16:991)
Molecular / cytogenetics description
- None for sporadic cemento-ossifying fibroma
- Syndromic cemento-ossifying fibroma can be identified clinically or by genetic mutations in CDC73 (also known as HRPT2) (J Dent Sci 2020;15:426, Case Rep Dent 2023;2023:4664619)
- Multiple ossifying fibromas can be associated with hyperparathyroidism jaw tumor syndrome (Case Rep Dent 2023;2023:4664619, J Dent Sci 2020;15:426)
- Amplification of MDM2 gene by fluorescence in situ hybridization is rarely (3%) seen in ossifying fibroma (Head Neck Pathol 2022;16:991)
- Positive MDM2 amplification in craniofacial fibro-osseous lesion does not completely exclude ossifying fibroma; however, it should still raise concern for low grade osteosarcoma because this genetic event is much more common in the latter (Head Neck Pathol 2022;16:991)
Molecular / cytogenetics images
Sample pathology report
- Mandible, enucleation and curettage:
- Benign fibro-osseous neoplasm consistent with cemento-ossifying fibroma / ossifying fibroma (see comment)
- Comment: This is a benign fibro-osseous neoplasm that requires clinical and radiological correlation.
Differential diagnosis
- Peripheral ossifying fibroma (Int J Health Sci (Qassim) 2019;13:63, Med Oral Patol Oral Cir Bucal 2022;27:e460):
- Reactive, inflammatory, hyperplastic growth of the gingiva
- It is the soft tissue counterpart to COF; microscopically, it is lined by mucosa and can have identical microscopic appearance
- Cemento-osseous dysplasia:
- Nonneoplastic fibro-osseous lesion of tooth bearing regions
- Most common benign fibro-osseous lesion of jaws, affects middle aged women
- Patients can be managed with follow up and symptomatic treatment
- Radiology: radiolucent, radiodense or mixed, as the lesion matures becomes more radiodense and calcified
- Grossly usually received in multiple gritty fragments with tan brown color
- Microscopically identical to COF
- Unencapsulated with variable cellular fibrous stroma and areas of loose collagen
- Stroma can display osteoid, bone and cementum-like material
- Mature lesion shows more calcification
- Clinical and radiographic correlation is essential to differentiate the two, especially on incisional biopsies
- An expansile variant of cemento-osseous dysplasia exists and can mimic COFs
- Chronic osteomyelitis (Head Neck Pathol 2020;14:842):
- Inflammatory process with a history of trauma and sinus formation
- No age or gender preference
- Treatment includes surgery to remove portions of bone that are infected or dead, followed by intravenous antibiotics given in the hospital
- Microscopically: findings are nonspecific, such as chronic inflammatory cell infiltration, bone marrow fibrosis and sclerotic bone formation
- Desmoplastic fibroma:
- Locally aggressive fibroblastic lesion of bone
- Mostly affects mandible in people younger than 30 (mean: 16 years), no gender predilection
- Excision, recurrence is common
- Radiology: well defined without mineralization
- Grossly: firm, tan-white with rough cut surface, myxoid areas can be seen
- Microscopically
- Infiltrative lesion comprising fascicles of uniform myofibroblasts / fibroblasts having tapering nuclei in a collagenous to myxoid stroma
- Perivascular edema and mitotic figures are present
- Overt bony trabeculae or droplets absent
- IHC: alpha smooth muscle actin (ASMA) and beta catenin positive in some cases
- Associated with activating CTNNB1 gene / APC mutation
- Fibrous dysplasia:
- Skeletal anomaly, a disorder of growing bones
- Monostotic or polyostotic lesion, affects craniofacial bones and femur in children and adolescents, no gender predilection
- Lesions stabilize with skeletal maturation, surgical intervention in younger patients is delayed
- Radiology: radiolucent to sclerotic, ground glass appearance with indistinct borders
- Grossly: affected bone is rubbery, compressible with gritty texture
- Microscopically
- Has bland hypocellular appearance if the lesion is mature; can be hypercellular when the lesion is in actively growing phase
- Lesion fuses with adjacent normal bone
- Irregularly shaped trabeculae of bone in a background fibrous stroma
- Trabeculae of woven bone are elongated and curvilinear, described as Chinese characters or letters in alphabet soup
- Osteoblastic rimming absent or minimal
- Retraction artifact / peritrabecular clefting often present
- Associated with GNAS gene mutation and McCune-Albright syndrome
- Intraosseous meningioma (Surg Neurol Int 2021;12:485):
- Most common benign brain / intracranial tumors, however, intraosseous meningiomas are rare lesions accounting for < 1% of intracranial meningiomas
- Common locations are frontoparietal and orbital regions
- Surgical removal though challenging is curative
- Radiology: depending upon location, usually circumscribed with surrounding bone shell
- Microscopically: morphology is similar to dura based lesions
- Osteoblastoma:
- Benign bone forming tumor
- Rare lesion, mostly affects craniofacial bones specially mandible in people in second to third decade with slight male predominance
- Can be excised however recurrences are reported after incomplete removal
- Radiology: circumscribed rounded of usually > 20 mm in size, radiolucent to radiopaque, sclerotic border; can mimic malignancy
- Grossly: multiple firm tan-white fragments
- Microscopically
- Lesion is composed of haphazard mineralized trabeculae of bone showing prominent osteoblastic rimming, lying in cellular fibrous stroma
- Rare mitotic figures
- Osteosarcoma (J Cancer Res Ther 2018;14:471):
- Malignant bone tumor
- Greatest predilection for the metaphysis (femur and tibia), osteosarcoma of craniofacial bones is infrequent
- Osteosarcoma of the jaws are rare and comprise ~7% of all osteosarcoma cases
- Radiology: irregular mixed radiolucent radiopaque pattern with infiltrative margins
- Microscopically: displays features commonly found in various types of malignant neoplasms
- Reference: J Maxillofac Oral Surg 2021;20:240
Additional references
Board review style question #1
The histologic pattern shown above is from a 32 year old woman who has had small swelling in the mandible for the last 2 weeks. On radiology, the lesion was found to be well circumscribed. What is the most expected behavior of this lesion?
- Benign
- Intermediate, locally aggressive
- Intermediate, rarely metastasizing
- Malignant
Board review style answer #1
A. Benign. This is a slow growing, benign neoplasm with no recurrence in most cases (J Maxillofac Oral Surg 2021;20:240, Oral Dis 2017;23:440, Head Neck Pathol 2020;14:70, J Stomatol Oral Maxillofac Surg 2022;123:364). Answers B, C and D are incorrect because this neoplasm rarely demonstrates malignant transformation or metastasis (Head Neck Pathol 2022;16:991, Ear Nose Throat J 2023;102:24).
Comment Here
Reference: Cemento-ossifying fibroma / ossifying fibroma
Comment Here
Reference: Cemento-ossifying fibroma / ossifying fibroma
Board review style question #2
Which of the following conditions is typically associated with hyperparathyroidism jaw tumor syndrome (HPT JT)?
- Cemento-ossifying fibroma
- Odontogenic keratocyst of jaw
- Ovarian fibromas
- Pits in palms and soles
Board review style answer #2
A. Cemento-ossifying fibroma is associated with hyperparathyroidism jaw tumor syndrome. The syndrome predisposes to a triad occurrence: multiple maxillary or mandibular cemento-ossifying fibroma, parathyroid adenoma or carcinoma and renal and uterine tumors (Int J Surg Case Rep 2020;68:257). Answers B, C and D are incorrect because they are usually associated with basal cell nevus syndrome.
Comment Here
Reference: Cemento-ossifying fibroma / ossifying fibroma
Comment Here
Reference: Cemento-ossifying fibroma / ossifying fibroma
Cementoblastoma
Table of Contents
Definition / general | Radiology description | Gross description | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
- Also called true cementoma, benign osteoblastoma
- Men and women less than 25 years old
- Benign, may stop growing as a heavily calcified jaw nodule, may recur if excised
Radiology description
- Dense homogenous mass continuous with tooth root
Gross description
- Large mass of cementum on root of mandibular premolar or molar
- Tooth viable
Microscopic (histologic) description
- Large cementicles (globules) fused to form a mass within proliferative fibrovascular stroma
- Trabeculae lined by plump osteoblasts
Central giant cell granuloma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign, nonodontogenic lesion of the jaw, osteoclastic in origin, with unknown etiology (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024], Indian J Otolaryngol Head Neck Surg 2013;65:192)
- Can be locally aggressive and destructive (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
Essential features
- Sharply demarcated radiolucency with sclerotic margins, sometimes extends between the displaced tooth roots
- 80% of cases involve the region anterior to the first premolar
- Localized and lobulated but sometimes lytic and aggressive
- Vascular connective tissue is observed, with a patchy distribution of multinucleated giant cells in the surrounding stroma
Terminology
- Giant cell granuloma, central (CGCG)
- Not recommended: reparative giant cell granuloma, central giant cell lesion
ICD coding
Epidemiology
- Population incidence is thought to be 0.0001% (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Peak age is between 5 and 15 years; rarely develops after 30 years old (J Oral Maxillofac Pathol 2020;24:413, StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Most common in female patients (2:1) (J Oral Maxillofac Pathol 2020;24:413)
- Both jaw bones can be affected; 80% of cases involve the region anterior to the first premolar (J Oral Maxillofac Pathol 2020;24:413)
- Multiple cases revealed association with the RAS / MAPK pathway (J Oral Maxillofac Pathol 2020;24:413)
Sites
- Comprises close to 7% of all intraosseous lesions in the jaw (J Clin Med 2022;11:4239)
- Both jaws are affected (J Oral Maxillofac Pathol 2020;24:413)
- 80% of cases involve the region anterior to the first premolar (J Oral Maxillofac Pathol 2020;24:413)
- Mandible involvement is 2x more common than maxilla (Imaging Sci Dent 2022;52:123)
Pathophysiology
- Altered bone microenvironment can be a possible reason for deregulation of the cell cycle (J Oral Maxillofac Pathol 2020;24:413, StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- CGCG arises from osteoclast precursors in the mononuclear stroma, induced by RANKL, VEGF and b-FGF; the last amplifies the resorptive action of parathyroid hormone
- Sporadic CGCG has low tumor mutation burden, lacks fusions and is driven by mutually exclusive somatic mutations in KRAS (more often affecting codon 12), FGFR1 (either p.C381R or p.N330I) and TRPV4 (p.M713V/I) that occur in 70% of cases, leading to MAPK pathway activation (Nat Commun 2018;9:4572)
- TRPV4 codes a polymodal Ca2+ permeable channel and is mutated in hereditary channelopathies characterized by peripheral nervous system and skeletal changes
- Recently, CGCG has been described as one feature of a polysystemic syndrome due to germline TRPV4 mutation, expanding the spectrum of TRPV4 channelopathies (J Med Genet 2022;59:305)
Etiology
- Precise predisposing factor is yet unidentified (J Oral Maxillofac Pathol 2020;24:413)
- Altered bone microenvironment can be a possible cause (J Oral Maxillofac Pathol 2020;24:413)
Clinical features
- In general, CGCG is asymptomatic (painless swelling) in the beginning but later becomes expansile (J Oral Maxillofac Pathol 2020;24:413)
- When aggressive, it causes a rapidly enlarging painful swelling, displacing teeth, resorbing roots and the cortical plate of the alveolar ridge (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Intraorally, a blue-brown discoloration may be seen due to thinning of the cortical plate and overlying mucosa (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Clinical expansion occurs over a period of weeks to a couple of months (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
Diagnosis
- Diagnosis of central giant cell granuloma is normally made histologically from an incisional / excisional biopsy (Ann Maxillofac Surg 2012;2:102)
- Combination of clinical, radiological and histopathological evaluations is recommended for definite diagnosis and confirmation (Ann Maxillofac Surg 2012;2:102)
- Radiologic findings alone are not sufficient for diagnosis (Ann Maxillofac Surg 2012;2:102)
Laboratory
- No specific laboratory test is available
Radiology description
- Sharply demarcated radiolucency sometimes extends between the displaced tooth roots (J Oral Maxillofac Pathol 2020;24:413)
- Sclerotic margin denoting the slow expansile nature (J Oral Maxillofac Pathol 2020;24:413)
- Faint calcifications are noticed, depicting the poorly mineralized osteoid trabeculae within the lesion, which helps to differentiate from giant cell tumors (J Oral Maxillofac Pathol 2020;24:413)
- Larger lesions may exhibit multilocularity (J Oral Maxillofac Pathol 2020;24:413)
- Cortex generally is intact but in more aggressive lesions, it may be breached (J Oral Maxillofac Pathol 2020;24:413)
Radiology images
Prognostic factors
- Central giant cell granuloma can have aggressive, locally destructive behavior or may be indolent and respond to nonsurgical therapies (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Recurrence rate is near 15 - 20% and is higher in aggressive forms, particularly if incompletely removed (40 - 70%) (Ann Maxillofac Surg 2012;2:102)
- Recurrence normally occurs within the first 12 - 18 months (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
Case reports
- 9 year old girl with hybrid CGCG within ossifying fibroma (F1000Res 2019;8:1218)
- 11 year old girl with CGCG of mandibular condyle (J Clin Med 2022;11:4239)
- 17 year old girl with coexistent CGCG and aneurysmal bone cyst (ABC) (Iran J Otorhinolaryngol 2021;33:319)
- 33 year old woman with CGCG at posterior portion of mandible (Int J Surg Case Rep 2023;112:108971)
Treatment
- Can be treated via surgical and nonsurgical means (Ann Maxillofac Surg 2012;2:102)
- Surgery varies depending on the location, size and aggressiveness of the lesion and can range from curettage to large scale resection and reconstruction (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Nonsurgical treatment is often considered for smaller, less aggressive lesions; however, some have suggested its use as neoadjuvant therapy for large lesions (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Intralesional steroids, daily subcutaneous calcitonin and interferon alpha for its antiangiogenic effects have all been used with some success (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
Clinical images
Gross description
- Fleshy, reddish brown, hemorrhagic appearance
Microscopic (histologic) description
- Unencapsulated, localized, lobulated and can have osteolytic proliferations (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Primary cells of the CGCG are fibroblasts and secondary cells are multinucleated giant cells; accessory cells are macrophages, dendrocytes and endothelial cells (J Oral Maxillofac Pathol 2020;24:413)
- Vascular connective tissue is observed, with a patchy distribution of multinucleated giant cells in the surrounding stroma (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Multinucleated giant cells are unevenly distributed and form clearly recognizable clusters separated by scar-like stromal tissue (J Oral Maxillofac Pathol 2020;24:413)
- Clustering is particularly evident in areas of hemorrhage (J Oral Maxillofac Pathol 2020;24:413)
- Extravasated erythrocytes and hemosiderin deposits are frequently seen, highlighting multifocal hemorrhage (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- More aggressive versions have been shown to have more numerous, larger giant cells with a greater surface area density (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
Microscopic (histologic) images
Cytology description
- Cohesive monolayered clusters of round to oval mononuclear cells with dispersed single cells and randomly distributed multinucleated giant cells in the background (Acta Cytol 1994;38:475)
- Round to ovoid nuclei with fine chromatin and inconspicuous nucleoli (Acta Cytol 1994;38:475)
- Hemosiderin laden macrophages and inflammatory cells can also be present (Acta Cytol 1994;38:475)
- Absence of mitotic figures and cellular pleomorphism (Acta Cytol 1994;38:475)
Cytology images
Positive stains
Molecular / cytogenetics description
- KRAS, TRPV4 and FGFRI can be seen in CGCG, which is histologically similar to nonossifying fibroma of long bones (J Oral Maxillofac Pathol 2020;24:413)
- Multiple giant cell lesions occur in syndromes, including neurofibromatosis type 1, osteoglophonic dysplasia, Noonan, cardiofaciocutaneous, oculoectodermal, LEOPARD, Schimmelpenning-Feuerstein-Mims and Jaffe-Campanacci, most of which are caused by MAPK pathway gene mutations (J Pathol 2020;250:126)
Videos
Central giant cell granuloma
Sample pathology report
- Mandible, anterior portion lytic lesion, excisional biopsy:
- Features suggest central giant cell granuloma (CGCG) (see comment)
- Comment: Histologically an unencapsulated spindle cell lesion with unevenly distributed clusters of osteoclast type giant cells in a hemorrhagic background along with bland fibroblasts and peripheral woven bone formation. Immunohistochemically the multinucleated giant cells are positive for CD68 and negative for H3 G34W. This constellation of morphological and immunohistochemical features strongly supports the diagnosis of central giant cell granuloma.
- Serological correlation is recommended to rule out the possibility of Brown tumor.
Differential diagnosis
- Brown tumor of hyperparathyroidism:
- Histologically indistinguishable but usually present in multiple bones and with deranged bone profile results (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Hyperparathyroidism can be differentiated on the basis of biochemical tests, where hypercalcemia, hypophosphatasia and increased parathyroid hormone (PTH) will point toward hyperparathyroidism (J Oral Maxillofac Pathol 2020;24:413)
- Bone tunneling is a useful clue for Brown tumor
- Peripheral giant cell granuloma:
- Reactive condition involves gingiva as a nodular mass while CGCG involves bones (Indian J Med Paediatr Oncol 2017;38:440)
- More common in the fifth and sixth decades (J Oral Maxillofac Res 2019;10:e5)
- Rarely bone or osteoid formation is seen (J Oral Maxillofac Res 2019;10:e5)
- Giant cell tumor:
- Difficult to differentiate, especially when the CGCG is of the aggressive type (J Oral Maxillofac Pathol 2020;24:413)
- Giant cell tumors have larger giant cells, greater numbers of nuclei and generalized distribution of giant cells and absence of osteoid formation (J Oral Maxillofac Pathol 2020;24:413)
- Practically, the occurrence of giant cell tumor in the jawbones is very rare and cases have been reported where patients are already suffering from Paget disease (J Oral Maxillofac Pathol 2020;24:413)
- Positive H3 G34W and p63 staining in mononuclear cells (Cancer Cytopathol 2018;126:552)
- Cherubism:
- Autosomal dominant disorder, most commonly caused by a mutation in SH3P2
- Widespread and multiple osteolytic lesions of primarily the posterior mandible (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Onset is in childhood, producing a classical, symmetric full cheek appearance (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024])
- Nonossifying fibroma:
- Fibrohistiocytic stroma with a storiform pattern and prominent xanthogranulomatous reaction (J Oral Maxillofac Pathol 2020;24:413)
- Maxillofacial involvement is very rare
- Aneurysmal bone cysts (ABC) (StatPearls: Non-Odontogenic Tumors of the Jaws [Accessed 28 February 2024]):
- ABCs show blood filled cystic spaces (J Pathol Transl Med 2023;57:81)
- Common in the metadiaphysis of long bones and vertebral bones (J Pathol Transl Med 2023;57:81)
- ABCs have a rearrangement of the USP6 gene (J Pathol Transl Med 2023;57:81)
Additional references
Board review style question #1
A 20 year old woman presents with a single large painless mass at the anterior portion of the mandibular bone. Excisional biopsy shows a fleshy hemorrhagic mass. Microscopically it shows a lesion composed of mononuclear spindle cells and clusters of osteoclast type multinucleated giant cells set in a vascular and hemorrhagic background. The multinucleated giant cells are positive for CD68. The mononuclear cells are negative for p63 and H3 G34W. No hypercalcemia or hypophosphatasia noted and parathyroid hormone (PTH) level is in normal range.
Which of the following is true regarding the above scenario?
- Features favor Brown tumor
- Findings are compatible with giant cell tumor of jaw
- Findings are in favor of central giant cell granuloma
- Findings are in favor of peripheral giant cell granuloma
- The neoplasm rarely involves the anterior portion of the mandible
Board review style answer #1
C. Findings are in favor of central giant cell granuloma. The histologic image reveals an unencapsulated spindle cell lesion with unevenly distributed clusters of osteoclast type giant cells in a hemorrhagic background along with bland fibroblasts and peripheral woven bone formation. Answer B is incorrect because p63 and H3 G34W are positive in giant cell tumor. Answer D is incorrect because the lesion is in between the bone. Answer A is incorrect because no hypercalcemia, hypophosphatasia or increased PTH were noted. Answer E is incorrect because CGCG mostly involves the anterior portion of the mandible.
Comment Here
Reference: Central giant cell granuloma
Comment Here
Reference: Central giant cell granuloma
Board review style question #2
Which of the following is true about central giant cell granuloma (CGCG)?
- Benign lesion that can be locally aggressive
- It is common in males and complete excision is the only treatment
- Multinucleated giant cells are evenly distributed in CGCG
- Primary cells of CGCG are giant cells
- Spindle cell component is positive for CD68
Board review style answer #2
A. Benign lesion that can be locally aggressive. Answer D is incorrect because primary cells of the CGCG are fibroblasts and secondary cells are multinucleated giant cells. Answer C is incorrect because in CGCG, one of the important histologic findings is the uneven distribution of multinucleated giant cells. Answer E is incorrect because the giant cells are positive for CD68, not the spindle cells, which are fibroblastic in nature. Answer B is incorrect because CGCG is more common in females and can have other treatment methods (nonsurgical treatment is often considered for smaller, less aggressive lesions; however, some have suggested its use as neoadjuvant therapy for large lesions).
Comment Here
Reference: Central giant cell granuloma
Comment Here
Reference: Central giant cell granuloma
Cherubism
Table of Contents
Definition / general | Treatment | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
- Hereditary and intraosseous fibrous swellings of jaws
- Autosomal dominant, bilateral involvement of mandible and maxilla in young individuals
Treatment
- Surgery may not be indicated
Microscopic (histologic) description
- Similar to central giant cell granuloma
- Also more delicate fibrovascular stroma without bone formation
Chronic recurrent multifocal
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Diagrams / tables | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Chronic recurrent multifocal osteomyelitis (CRMO) is a relapsing inflammatory disease and is considered a type of seronegative spondyloarthropathy characterized by periods of exacerbations and remissions over many years
- Characterized by an inflammatory process presenting with findings similar to infectious osteomyelitis; however, no infectious source is identifiable
- Most often characterized by the insidious onset of low grade fever, local swelling and bone pain
- Radiologic findings may suggest osteomyelitis, and bone seeking isotopes reveal other asymptomatic sites of involvement
- The lesions occur mainly in the metaphyses of long tubular bones, as well as the clavicles, spine, and pelvis; the lesions are sometimes symmetrically distributed
- The precise relationship between primary chronic osteomyelitis, diffuse sclerosing osteomyelitis, CRMO and SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) is highly debated and not clear at this time
- Some consider CRMO and SAPHO as primary chronic osteomyelitis with additional extragnathic manifestations
- Whether CRMO in juvenile patients and SAPHO syndrome are the same disorder in different age groups remains to be determined
Terminology
- Acute suppurative osteomyelitis:
- Early phase of osteomyelitis and usually a suppurative (pus forming) condition
- Exists when an acute inflammatory process moves away from the site of initial infection and spreads through the medullary space of the bone and, in most cases, insufficient time has passed for the body to react to the presence of the inflammatory infiltrate
- Acute phase may lead to the chronic phase which has been arbitrarily defined as an osseous infection lasting at least 1 month
- Alveolar osteitis / fibrinolytic alveolitis:
- After extraction of a tooth, a blood clot is formed at the extraction site with eventual organization of this clot by granulation tissue and gradual replacement by bone
- Destruction of the initial clot is thought to delay the aforementioned additional series of steps required for uneventful extraction site healing and leads to clinical condition known as alveolar osteitis
- Clot is lost secondary to transformation of plasminogen to plasmin with subsequent lysis of fibrin and formation of kinins which are potent pain mediators
- Biofilm:
- Collection of microorganisms often embedded in a self produced extracellular polymeric matrix which allows them to adhere to or coat the surface of a living or inanimate structure
- Chronic osteomyelitis:
- May be classified as primary or secondary and as suppurative or non-suppurative
- Secondary chronic osteomyelitis (SCO)
- Primary chronic osteomyelitis (PCO)
- Chronic tendoperiostitis
- Initially thought to be an obscure infectious process, the clinical presentation is similar to that of primary chronic osteomyelitis
- May represent a reactive alteration of bone initiated and exacerbated by chronic overuse of the masticatory muscles, predominantly the masseter and digastric
- Patients usually present with parafunctional complaints
- Palpation of the temporal, masseter, medial pterygoid and the digastric muscles reveals tenderness
- Radiographically, may be very similar to primary chronic osteomyelitis with diffuse sclerosing of the marrow and the cortical plate in the absence of pus formation or sequestration
- May be classified as primary or secondary and as suppurative or non-suppurative
- Chronic sclerosing osteomyelitis / diffuse sclerosing osteomyelitis:
- Although the term is usually used synonymously with primary chronic osteomyelitis, it represents a description of a strictly radiological appearance that can be caused by several similar processes including:
- Primary and secondary chronic osteomyelitis
- Chronic tendoperiostitis
- Ossifying periostitis or Garrè's osteomyelitis
- Medullary osseous infection, probably bacterial, which induces the complete sclerosis
- Favors young women, is painful and appears radiographically as medullary sclerosis
- Therapy includes antibiotics, and surgical debridement and hyperbaric oxygen therapy in refractory cases
- Although the term is usually used synonymously with primary chronic osteomyelitis, it represents a description of a strictly radiological appearance that can be caused by several similar processes including:
- Condensing osteitis:
- Localized areas of radiographic bone sclerosis associated with the apices of inflamed dead or dying teeth (pulpitis or pulpal necrosis) are termed condensing osteitis
- The association with an area of inflammation, usually a neighboring tooth, is critical because these lesions can resemble several other intrabony processes that produce a somewhat similar pattern
- Is not considered a true osteomyelitis in the classical sense
- Cortical bone:
- Cortical bone, synonymous with compact bone, is one of the two types of osseous tissue that forms bone
- As its name implies, cortical bone forms the cortex, or outer shell, of most bones
- Compact bone, as its name implies, is much denser than cancellous bone which is the other type of osseous tissue
- Furthermore, it is harder, stronger and stiffer than cancellous bone
- Garrè's sclerosing osteomyelitis:
- In 1893, a Swiss physician, Carl Garrè, reported on patterns of acute osteomyelitis
- Garrè did not have any pathologic specimens for microscopic examination nor radiographs to augment his descriptions
- Nonetheless, his name is often associated with the condition in which periosteal duplication, or an "onion-skinning" pattern of periostitis, leads to enlargement of the jaw, usually mandible
- Involucrum:
- A sheath of new bone surrounding the sequestrum (N Engl J Med 2007; 356:e7)
- Medullary bone / medullary cavity / marrow cavity:
- The medullary cavity (medulla, innermost part) of bone is the central cavity where red bone marrow or yellow bone marrow (adipose tissue) is stored; hence, the medullary cavity is also known as the marrow cavity
- The medullary cavity has walls composed of spongy bone (cancellous bone) and is lined by endosteum, which are osteoprogenitor cells
- Osteochemonecrosis of Jaws:
- Synonyms: bisphosphonate related osteonecrosis of the jaws, bisphosphonate induced osteonecrosis of the jaw, osteonecrosis of jaw, bisphosphonate osteonecrosis, bis-phossy jaw
- Necrosis of bone related to long term use of antiresorptive medications such as bisphosphonate medications and altered bone metabolism
- Osteoclasts are thought to be qualitatively impaired, particularly with intravenous forms of bisphosphonate medication which leads to inadequate remodeling of bone, necrosis
- Osteoradionecrosis (ORN):
- Clinically, a chronic non-healing wound of the affected jaw (most commonly mandible), typically with exposure of bone, in a patient with a history of radiation therapy to the head and neck region
- Radiation injury to jaw that leads to ORN results from chronic hypovascularity, hypocellularity of marrow space and ultimately hypoxemia
- Is more similar to avascular necrosis of bone than primary infection of bone, although infection or bacterial colonization can occur
- Periapical granuloma:
- Acute or chronic inflammation admixed with fibrous or granulation tissue locally at the apical or periapical region of a tooth
- Is devoid of epithelium (i.e. no cyst lining) which distinguishes it from a periapical cyst
- Periapical granuloma is located at the apex of a necrotic or partially necrotic tooth root
- Periosteum:
- Connective tissue membrane lining the outer / external surface bones, except at the joints of long bones
- Is composed of an outer fibrous layer and an inner cambium / osteogenic layer (Wikipedia: Periosteum [Accessed 1 June 2018] )
- Proliferative periostitis:
- Represents a periosteal reaction to the presence of inflammation
- The affected periosteum forms several rows of reactive vital bone that parallel each other and expand the surface of the altered bone
- SAPHO syndrome:
- SAPHO syndrome is an acronym for a complex clinical presentation that includes synovitis, acne, pustulosis, hyperostosis and osteitis
- The osseous lesions mirror primary chronic osteomyelitis and CRMO
- Unknown cause but thought to arise in genetically predisposed individuals who develop an autoimmune disturbance secondary to exposure to dermatologic bacteria
- Increased prevalence of HLA-27
- SAPHO and CRMO are distinguished from other forms of osteitis / osteomyelitis as these two diseases may have extragnathic skeletal involvement
- Sequestrum:
- Predominantly non-viable bone that has become separated during the process of necrosis from the original surrounding native bone (Medical Dictionary: sequestrum [Accessed 1 June 2018])
- Zurich Classification of Osteomyelitis:
- The Zurich classification system of osteomyelitis is primarily based on clinical course and features of imaging of the disease
- Histopathology is considered a secondary classification criterion, taking into account that findings are mostly nonspecific and inconclusive by themselves; however, tissue examinations of biopsies are irreplaceable for confirmation of the diagnosis if the clinical and radiological appearance is unclear or atypical and to exclude possible differential diagnoses
- The three major classifications are: (Baltensperger, Osteomyelitis of the Jaws: Definition and Classification (Figure 2.2))
- Acute Osteomyelitis (AO)
- Secondary Chronic Osteomyelitis (SCO)
- Primary Chronic Osteomyelitis (PCO)
- Further subclassification of these major osteomyelitis groups is based on presumed etiology and pathogenesis of disease
- These criteria are therefore considered a tertiary classification criteria
- These tertiary criteria help determine the necessary therapeutic strategies which may differ somewhat among the subgroups
Epidemiology
- Rare, estimated frequency of 1:1,000,000
- 2 - 5% of all osteomyelitis cases
- Primarily affects children, median age 10 years, with female / male ratio of 5:1
Sites
- Mainly affects the metaphyses of the long bones
- The spine, pelvis, and shoulder girdle may also be affected
- When involves the jaws, predominantly involves the mandible and the prevalence of mandibular involvement is 2 – 8%
- The neurocranium is thought to be spared
Etiology
- A number of causes have been proposed, such as dysregulated innate immune or other altered immune response, but no single theory has received widespread acceptance
Clinical features
- Characterized by periods of intermittent exacerbations and improvements over a period of years
- Some patients have associated recurrent skin lesions (pustulosis palmoplantaris) that closely parallel the exacerbations of the bone lesions and makes distinction from SAPHO challenging
Diagnosis
- Clinical diagnosis may be challenging because the clinical picture and course of the disease may vary significantly
- Therefore, the diagnosis is collectively based on clinical, radiologic, pathological and longitudinal findings
- When attempting to provide a specimen for culture material, it is of the utmost importance for the surgeon to avoid contamination when harvesting a bone biopsy from a lesion for microbiological analysis
- While lesions of CRMO in the mandible may be easier to access, a transoral approach may induce microbial contamination and a specimen from another part of the skeleton may therefore be of more diagnostic value for cultures
- CRMO is a diagnosis of exclusion since the clinical presentation and laboratory findings are not disease specific and vary between affected individuals
Radiology description
- Inflammatory bone lesions may be detected in plain radiographs presenting as radiolucent, osteolytic or sclerotic lesions, depending on the disease stage
- In early phases of CRMO, radiographs may remain normal
- Diffuse bone radiopacity is the prominent finding in patients with mandibular manifestations
- While radiopacity governs the quiet phase of CRMO, areas of mottled cortical and cancellous bone osteolysis prevail during recurrence
- Small osteolytic lacunae that contain lymphocytes and plasma cells are potential sites of exacerbation leading to cortical erosion and periosteal reaction
- Scintigraphy aids in detecting synchronous or metachronous involvement of multiple bones such as the clavicle, humerus, radius, femur or tibia
- Whole body imaging techniques, including MRI and Tc-99 m labeled methylene bone scintigraphy, represent novel and valuable diagnostic tools
- CRMO represents a systemic disease with potentially multiple inflammatory lesions, therefore whole body imaging techniques should be performed at least at the time of diagnosis to exclude asymptomatic lesions, such as in the vertebral column
- MRI is especially sensitive during early stages since it can detect bone edema even before erosions or sclerosis become apparent
- At diagnosis, gadolinium enhanced T1 or strongly T2 weighted sequences with fat saturation may be superior to conventional radiographs or scintigraphy
- Radiographic Differential Diagnosis (will be influenced by age of patient, clinical history):
- Acute osteomyelitis
- Cases of secondary chronic osteomyelitis with a predominant sclerosing character are particularly difficult, if not impossible, to distinguish from primary chronic osteomyelitis, if the disease is only viewed at a certain point in time and the whole course of the disease is neglected
- Theoretically, a coincidence of primary chronic osteomyelitis with secondary chronic osteomyelitis may occur when a "superimposed" putrid bacterial infection is manifested in the impaired sclerotic bone
- SAPHO syndrome
- Tendoperiostitis
- Chronic sclerosing osteomyelitis / diffuse sclerosing osteomyelitis / Garrè's sclerosing osteomyelitis
- These terms merely describe radiological features which may be demonstrated in primary and secondary chronic osteomyelitis, with extensive sclerosis, variable osteolysis and periosteal reaction on diagnostic imaging
- Osteochemonecrosis of the Jaws
- Osteoradionecrosis
- Cemento osseous dyplasia (COD)
- Has often been confused with primary chronic osteomyelitis in the literature
- It may be seen as a predisposing factor for bone infection
- Florid cemento osseous dysplasia appears on radiographs as sclerosing opaque and dense masses
- The bone changes are often adjacent to the apices of the teeth and are restricted to the alveolar process in either or both jaws
- Malignancy or benign aggressive intraosseous neoplasms inducing proliferative periostitis
- Osteosarcoma
- Ewing sarcoma
- Leukemia / lymphoma
- Metastatic disease to the jaws
- Langerhans cell histiocytosis
- Paget disease (deforming ostitis)
- Disease of disturbed bone metabolism of unknown origin
- Usually occurs in advanced age and affects the spine, pelvic skeleton, cranial vault
- Long bones and the facial skeleton are rarely involved
- If the jaws are affected, the maxilla is somewhat more often involved than the mandible
- Osteopetrosis (Albers-Schonberg disease)
- Osteopetrosis is a genetically inherited disease which involves all bones
- Two major types are differentiated: (a) autosomal recessive malignant form with anemia which manifests at birth; (b) autosomal dominant benign form
- Malignant form is dramatic, with severe impairment and systemic complications, patients die by age 10; benign form is much more common
- The jawbone in osteopetrosis cases shows massive sclerosis (Figure 2.32)
- Disturbed physiology of bone due to reduced osteoclast function causes osteopetrotic bone to be resorbed inadequately and sclerosis increases with time jeopardizing bone perfusion
- Secondary infection of the bone is common and the clinical picture resembles primary or secondary chronic osteomyelitis with predominant sclerosis
- Because bone physiology is generally disturbed, both jaws may be completely affected by the pathology, contrary to primary chronic osteomyelitis which generally just affects the mandible
- Acute osteomyelitis
Prognostic factors
- Often a prolonged course and difficult to predict
Case reports
- 8 year old girl with chronic recurrent multifocal osteomyelitis involving the mandible (Dentomaxillofac Radiol 2010;39:184)
- 8 year old girl with vertebral collapse (J Paediatr Child Health 2006;42:212)
- 10 year old boy with chronic recurrent multifocal osteomyelitis (J Nucl Med Technol 2014;42:299)
Treatment
- NSAID are commonly used as first line treatment of CNO/CRMO in less to moderately severe cases
- NSAIDs provide pain control in most cases and most likely alter the disease course since prostaglandins are centrally involved in osteoclast activation and bone remodeling
- Other reported nonsurgical treatments include: corticosteroids, sulfasalazine, methotrexate (MTX), anti-TNF agents (usually etanercept or infliximab), or bisphosphonates (usually pamidronate) but consistent data regarding success is not available
- Surgical decortication has been reported for refractory cases but in young, growing patients the associated risks must be considered since the results from surgical intervention have been inconsistent (Oral Surg Oral Med Oral Pathol 1970;29:641)
- Obtain cultures for organism identification and antibiotic sensitivity to exclude a primary or superimposed secondary treatable infectious process
Clinical images
Gross description
- If a biopsy is performed to confirm a diagnosis of osteomyelitis and to rule out neoplasia, often only a minute sample is received
Microscopic (histologic) description
- While histological analysis of bone lesions may help to exclude malignant processes, the histologic appearance of CRMO may mimic acute and secondary chronic osteomyelitis caused by microbiological infection
- Therefore, an extensive microbiological workup of the biopsy, including culture (see Diagnosis section) and PCR techniques, may be considered
- Cellular infiltrates are highly dependent on the disease stage
- Early phases: neutrophils are the predominant cell subset
- Later phases: monocytes, macrophages, lymphocytes, and plasma cells; also osteolysis and concomitant sclerosis or fibrosis
- The cellular distribution in inflammatory lesions reflects the immunological reaction at the time
- Unfortunately, very little is known about the kinetics of inflammatory responses
- Histologic examination does not allow distinction of CRMO from acute or subacute bacterial osteomyelitis
Differential diagnosis
- Acute exacerbation of bisphosphonate osteonecrosis
- Diffuse sclerosing osteomyelitis
- Infected cemento-osseous dysplasia
- Primary chronic osteomyelitis
- SAPHO syndrome
- Secondary chronic osteomyelitis
Additional references
- Oral Surg Oral Med Oral Pathol 1970;30:396, Oral Maxillofac Surg Clin North Am 2011;23:401, Oral Maxillofac Surg Clin North Am 1991;3:367, Oral Maxillofac Surg Clin North Am 1991;3:355
- J Oral Maxillofac Surg 1993;51:1294, J Can Dent Assoc 1995;61:441, J Craniomaxillofac Surg 2004;32:43, Topazian: Oral and Maxillofacial Infections, 4th Edition, 2002
- Baltensperger: Osteomyelitis of the Jaws, 2008, pages 5-56, 57, 95, 121-133, 145-78, N Engl J Med 2007;356:e7, Pediatr Rheumatol Online J 2013;11:47, Pediatr Radiol 2013;43:355
- Ann Rheum Dis 2005;64:279, Bullough: Orthopaedic Pathology, 5th Edition, 2009, Chapter 5, p. 115-116
Clear cell carcinoma of salivary gland
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Rare, malignant, translocation associated epithelial salivary gland tumor characterized by nests and cords of clear cells surrounded by hyalinizing stroma
- EWSR1 rearrangements in >80% of cases
Terminology
- AFIP uses terminology - clear cell adenocarcinoma
- WHO uses terminology - clear cell carcinoma, not otherwise specified (NOS)
- Also called hyalinizing clear cell carcinoma (HCCC)
Epidemiology
- Rare, < 1% of all salivary gland tumors
- Mean age: 6th decade
- Slightly more common in females (1.2:1)
Sites
- More common in minor salivary glands (~80%), particularly base of tongue, palate, floor of mouth, tongue and buccal mucosa in oral cavity / oropharynx (Head Neck 2016;38:426)
- Less common in major salivary glands; parotid more common than submandibular gland
Etiology
- As with many translocation tumors, lesion tends to pursue a line of differentiation rather than originate from a particular line of derivation
- Currently, there is evidence that HCCC pursues a squamous line of differentiation based on:
- Ultrastructurally, the tumors have tonofilaments, desmosomes and glycogen
- Frequent connection to the surface mucosal epithelium
- Electron microscopy findings including some basal lamina reduplication
Clinical features
- Clinically, patients present with swelling and mass lesion
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Prognostic factors
- Generally, has an indolent clinical course with a good prognosis
- Recurrence rate ~11%
- The presence of necrosis, local / regional disease or positive margins is associated with recurrence
- Some sources say ~25% have regional nodal metastases at presentation (Cancer 2009;115:75) but recent studies have challenged that (Genes Chromosomes Cancer 2011;50:559)
Case reports
- 36 year old woman with painless swelling of upper right back tooth region (J Oral Maxillofac Pathol 2011;15:335)
- 37 year old woman with painless swelling on ventral tongue (J Pathol Transl Med 2015;49:351)
- 52 year old woman with gradually growing and indolent mass in right buccal mucosa (Case Rep Otolaryngol 2015;2015:471693)
- 60 year old man with a swelling in the base of the tongue (Case of the Month #483)
Treatment
- Primary resection with negative margins
Clinical images
Gross images
Microscopic (histologic) description
- Nests and cords of clear cells with distinct borders and round to oval nuclei
- As with many translocation tumors, many areas of the lesion have a monotonous appearance
- A minority of the cells can contain eosinophilic as opposed to clear cytoplasm
- The cells are surrounded by variably hyalinized stroma
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Molecular / cytogenetics description
- > 80% show EWSR1 rearrangement by FISH
Differential diagnosis
- Clear cell odontogenic carcinoma (CCOC)
- Malignant epithelial odontogenic neoplasm also composed primarily of clear cells, which usually occurs in anterior mandible
- Unlike HCCC, the nests or cords may show focal palisading of basal cells (“ameloblastic”)
- Both tumors have EWSR1 rearrangements
- May represent "odontogenic analogue"
- Clear cell variant of calcifying epithelial odontogenic tumor (CEOT)
- Benign epithelial odontogenic neoplasm, clear cell variant may be composed primarily of clear cells
- Occurs in posterior mandible, intra-osseous location
- Variably sized polyhedral eosinophilic epithelial cells with distinct cell borders are arranged in small clusters, trabeculae, islands or a sheet-like pattern
- Nuclear pleomorphism is expected, but without appreciable mitotic activity
- Eosinophilic amyloid-like matrix material is haphazardly deposited in association with the tumor islands and calcified concentric profiles (Liesegang rings) are often identified.
- Ancillary studies show the epithelial cells of CEOT highlight with cytokeratin AE1/3, CK5/6 and p63, and amyloid-like material exhibits apple green birefringence when stained with Congo red and viewed with polarized light
- Clear cell renal cell carcinoma (CCRCC)
- Usually a known history of renal cell carcinoma
- CCRCCs are positive for PAX8
- Epithelial-myoepithelial carcinoma
- Malignant bisphasic tumor with an inner duct-like epithelial component and an S100+ outer myoepithelial component
- Occurs more commonly in the major salivary glands
- Hyalinizing clear cell carcinoma has only the clear cell epithelial component, and is S100-
- Mucoepidermoid carcinoma, clear cell variant
- Malignant epithelial tumor with variable amounts of mucous, epidermoid and intermediate cells
- Parotid gland most common location
- Mucicarmine will be positive in mucocytes
- Can be associatied with MAML2 rearrangement and NOT EWSR1 like HCCC
- Sinonasal renal cell-like adenocarcinoma (SRCLA)
- May be difficult to differentiate, but clear cell tumors involving the maxillary or palatal structures require consideration of a sinonasal neoplasm with secondary involvement of the oral region (Int J Clin Exp Med 2014;7:5469)
- Rare tumor characterized by a clear cell glandular proliferation, most often involving the nasal cavity, associated with a favorable clinical course
- Has round cells with clear cytoplasm and a prominent nucleolus arranged in a follicular pattern
- A tubular arrangement and papillary architecture of the clear cell proliferation have also been described
- No mucinous or myoepithelial differentiation, no necrosis, no hyalinization of stroma
- SRCLA vs. HCCC: no stromal hyalinization, no stromal vascularity; often has larger clear cells than HCCC and CCOC; has robust CA-IX immunostaining vs. focal positive in HCCC; negative for EWSR1 rearrangement
Additional references
Board review style question #1
Clear cell carcinoma of salivary gland is most commonly associated with the following gene
fusion:
A. HTN3-MSANTD3
B. EWSR1-ATF1
C. ETV6-NTRK3
D. MYB-NFIB
A. HTN3-MSANTD3
B. EWSR1-ATF1
C. ETV6-NTRK3
D. MYB-NFIB
Board review style answer #1
Clear cell odontogenic carcinoma
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Differential diagnosis | Additional referencesDefinition / general
- Rare, malignant, translocation associated odontogenic epithelial neoplasm composed of nest of clear cells and fibrous, hyalinized stroma
- EWSR1 rearrangements found in > 80% of cases (Am J Surg Pathol 2013;37:1001)
Terminology
- Clear cell odontogenic carcinoma (CCOC), clear cell odontogenic tumor
- Previously called clear cell ameloblastoma
- Considered benign by WHO of 1992, but due to its high potential for regional spread and distant metastases, it was reclassified as malignant in 2005
Epidemiology
- Rare, < 100 cases reported
- Most common in 5th to 6th decades
- Mean age ~ 60 years
- More common in females (1.5:1 to 2:1)
- First described in 1980's as jaw tumors that resembled metastatic clear cell renal carcinoma (Head Neck Surg 1985;8:115)
Sites
- Mandible most common site (75%)
- Soft tissue involvement common as lesion often perforates bone
Etiology
- Unknown, as with many translocation tumors, lesion tends to pursue a line of differentiation rather than originate from a particular line of derivation
- However, the tumor cells resemble clear cell rests of primitive dental lamina that are frequently in the same locations
Clinical features
- Often presents as jaw swelling with loosening of the teeth
- Can be painful, asymptomatic or associated with paresthesias
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Radiology description
- Poorly defined radiolucency
- May show cortical destruction of bone
Prognostic factors
- Recurrence rate of 30% of resected and 87% of curetted/enucleated lesions
- Metastases to lymph nodes, lung and bone
- Up to 25% die of disease
Case reports
- 46 year old with swelling in lower front tooth region for 4–5 months (Contemp Clin Dent 2015; 6: 559)
- 50 year old woman with involvement of mandible and temporomandibuar joint and cervical nodal metastases (Natl J Maxillofac Surg 2014;5:221)
- 55 year old woman with tumor extending from canine to molar region (J Oral Maxillofac Pathol 2013;17:89)
- 64 year old man with CCOC in anterior mandibular region (J Oral Maxillofac Pathol 2014;18:442)
Treatment
- Must tailor surgical treatment to overall clinical and image findings, but often involves a composite / en bloc resection
Clinical images
Microscopic (histologic) images
Positive stains
Negative stains
- S100, SOX10, HMB45, SMA, Mucicarmine, Alcian blue, Congo red
Molecular / cytogenetics description
- > 80% show EWSR1-ATF1 translocations (Am J Surg Pathol 2013;37:1001)
Differential diagnosis
- May vary based on biopsy sample size and whether histology is monophasic (predominantly clear cell), biphasic or ameloblastomatous:
Monophasic (predominantly clear cell) or focal biphasic appearance:
Ameloblastomatous appearance:
- Clear cell carcinoma of salivary gland
- More common in minor salivary glands (~80%), particularly base of tongue, palate, floor of mouth, tongue and buccal mucosa in oral cavity/oropharynx
- The nests or cords lack focal palisading of basal cells ("ameloblastic") seen in CCOC
- Both lesions show EWSR1 rearrangements
- May represent "salivary gland analogue"
- Clear cell variant of calcifying epithelial odontogenic tumor (CEOT)
- Benign epithelial odontogenic neoplasm, clear cell variant may be composed primarily of clear cells
- Occurs in posterior mandible, intra-osseous location
- Variably sized polyhedral eosinophilic epithelial cells with distinct cell borders are arranged in small clusters, trabeculae, islands or a sheet like pattern
- Nuclear pleomorphism is expected, but without appreciable mitotic activity
- Eosinophilic amyloid-like matrix material is haphazardly deposited in association with the tumor islands, and calcified concentric profiles (Liesegang rings) are often identified
- Ancillary studies show the epithelial cells of CEOT highlight with cytokeratin AE1/3, CK5/6 and p63, and amyloid-like material exhibits apple green birefringence when stained with Congo red and viewed with polarized light
- Sclerosing odontogenic carcinoma
- Rare and controversial entity, described in 2008
- Low grade odontogenic carcinoma, locally aggressive
- Infiltrating single file strands, cords and nests of cuboidal or polygonal epithelial cells with cytoplasmic clearing, similar to signet ring change
- No prominent pleomorphism or mitotic figures; no necrosis
- Skeletal muscle and perineural infiltration with stromal sclerosis are characteristic
- Clear cell renal cell carcinoma (metastatic)
- Usually a known history of renal cell carcinoma, which is PAX8+
- Epithelial-myoepithelial carcinoma
- Malignant biphasic tumor with an inner duct-like epithelial component and an outer S100+ myoepithelial component
- Usually occurs in major salivary glands
- CCOC has only a clear cell epithelial component, and is S100 negative
- Mucoepidermoid carcinoma, clear cell variant
- Malignant epithelial tumor with variable amounts of mucous, epidermoid and intermediate cells
- Mucocytes are mucicarmine+
- Can be associatied with MAML2 rearrangement and NOT EWSR1
- Sinonasal renal cell-like adenocarcinoma (SRCLA)
- May be difficult to differentiate, but clear cell tumors involving the maxillary or palatal structures require consideration of a sinonasal neoplasm with secondary involvement of the oral region (Int J Clin Exp Med 2014;7:5469)
- Rare tumor characterized by a clear cell glandular proliferation, most often involving the nasal cavity, associated with a favorable clinical course
- Has round cells with clear cytoplasm and a prominent nucleolus arranged in a follicular pattern
- A tubular arrangement and papillary architecture of the clear cell proliferation have also been described
- No mucinous or myoepithelial differentiation, no necrosis, no hyalinization of stroma
- SRCLA vs. hyalinizing clear cell carcinoma of salivary gland (HCCC): no stromal hyalinization, no stromal vascularity; often has larger clear cells than HCCC and CCOC; has robust CAIX immunostaining vs. focal positive in HCCC; negative for EWSR1 rearrangement
Ameloblastomatous appearance:
- Desmoplastic ameloblastoma
- Dense collagenous stroma with compressed, angular islands of basaloid odontogenic epithelium
- Squamous odontogenic tumor
- Benign tumor of odontogenic squamous epithelium
- Very rare; thought to arise from rests of Malassez in periodontal ligament
- No peripheral palisading or stellate reticulum
- Odontogenic fibroma
- Rare tumor, and poorly described in literature
- Loose to dense collagenous stroma with small, rounded or elongated islands of bland odontogenic epithelial islands
- Islands not elongated, interconnected or arborizing
- Minimal clear cell change
Additional references
Condensing osteitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- An area of sclerotic bone related to chronic osteomyelitis that is present at root of tooth (J Mass Dent Soc 2003;52:52)
- Tooth often demonstrates pulpitis or pulpal necrosis or previous restoration (J Mass Dent Soc 2003;52:52, J Endod 2013;39:977)
Essential features
- Dense sclerotic bone, seen radiographically, in area of a tooth with pulpitis or previous restoration
- Lesion resolves with treatment of affected tooth
Terminology
- Focal sclerosing osteomyelitis (J Mass Dent Soc 2003;52:52)
ICD coding
- ICD-10: M27.2 - inflammatory conditions of jaws
Epidemiology
- 4 - 7% of population (J Endod 2013;39:977)
- Early adolescence / young adults but can be any age (J Mass Dent Soc 2003;52:52)
- Mandible more frequently than maxilla (J Mass Dent Soc 2003;52:52)
Sites
- Oral cavity, mandible or maxilla, root of tooth (J Endod 2013;39:977)
Pathophysiology
- Related to chronic inflammation of pulp of tooth causing sclerotic bone formation (J Mass Dent Soc 2003;52:52)
Etiology
- Longstanding or low intensity infection with chronic inflammation and trabecular bone formation (J Mass Dent Soc 2003;52:52, J Endod 2013;39:977)
Clinical features
- Only clinical finding may be degenerative pulp disease (J Endod 2013;39:977)
Diagnosis
- Diagnosis made by imaging of jaw
Radiology description
- Diffuse radiopaque borders concentrically arranged around root apex (J Endod 2013;39:977)
Radiology images
Prognostic factors
- Most regress after treatment of affected tooth
Case reports
- 23 year old woman, 27 year old man and 32 year old woman with radiopaque apical lesions (Bratisl Lek Listy 2009;110:713)
- 28 year old man with radiopaque lesion on routine imaging (Tex Dent J 2003;120:178)
- 57 year old woman with pain in right maxillary anterior region after extraction of upper left incisor (J Mass Dent Soc 2003;52:52)
Treatment
- Treatment of infection in affected tooth with hopeful regression after root canal therapy or extraction (J Mass Dent Soc 2003;52:52)
Gross description
- Bone, often fragmented
Frozen section description
- Frozen section not performed
Microscopic (histologic) description
- Replacement of cancellous bone with compact bone (J Endod 2013;39:977)
- Scant fibrous tissue (J Mass Dent Soc 2003;52:52)
- Often no obvious inflammation (J Endod 2013;39:977, J Mass Dent Soc 2003;52:52)
Microscopic (histologic) images
Positive stains
- Morphologic and clinical diagnosis, no stains needed
Negative stains
- Morphologic and clinical diagnosis, no stains needed
Sample pathology report
- Mandible, partial mandibulectomy:
- Sclerotic bone consistent with condensing osteitis
Differential diagnosis
- Idiopathic sclerosis:
- Not inflammatory or neoplastic
- Intraosseous radiopacity of noninflammatory trabecular bone
- Cemento-osseous dysplasia:
- May demonstrate multiple foci
- Cellular fibrovascular connective tissue with mixture of bone and cementum-like particles
- Radiographically characterized by radiolucency or radiopacity surrounded by radiolucency
- Osteoma:
- May be associated with Gardner syndrome, particularly when multiple
- Demonstrates expansion / growth over time with possible displacement of teeth
- No association with inflammation
Additional references
Board review style question #1
Board review style answer #1
D. Radiopaque lesion. Condensing osteitis is most often seen on panoramic Xray as a radiopaque lesion. There is always a lesion identified as it is how the diagnosis is made. Radiolucent lesions include other cysts such as odontogenic keratocyst, dentigerous cyst or periapical cyst.
Comment Here
Reference: Condensing osteitis
Comment Here
Reference: Condensing osteitis
Board review style question #2
What is the most common histologic finding in condensing osteitis?
- Acute osteomyelitis
- Compact / sclerotic bone
- Marked chronic inflammatory infiltrate
- Replacement of bone by fibrous tissue
Board review style answer #2
B. Compact / sclerotic bone. Condensing osteitis is related to chronic inflammation causing sclerotic bone formation. Acute osteomyelitis will not be seen as this is a chronic process. There also will not be a chronic inflammatory infiltrate given the chronicity of the process. There is scant fibrous tissue in this process; it is a reactive boney process.
Comment Here
Reference: Condensing osteitis
Comment Here
Reference: Condensing osteitis
Dentigerous cyst
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Dentigerous cyst is a developmental odontogenic cyst that originates from the separation of the dental follicle from around the crown of an unerupted tooth, thus showing an epithelial lining attached to the cementoenamel junction
Essential features
- Intraosseous location, maxilla or mandible
- Cystic strips of nonneoplastic, nondysplastic epithelium with a fibrous or fibromyxoid cyst wall
- Epithelial lining is composed of stratified squamous epithelium
- Radiographic findings show cyst associated with crown of unerupted tooth
- Treatment is surgical, unlikely to recur once excised
- Diagnosis requires correlation with radiographs or knowledge of radiographic findings
Terminology
- Follicular cyst is synonymous
- Extraosseous counterpart: eruption cyst, involves an erupting tooth, usually a deciduous first molar tooth (Med Oral Patol Oral Cir Bucal 2017;22:e228)
- Ameloblasts: specialized epithelial cells that form tooth enamel
- Reduced enamel epithelium
- Enamel is normally composed of 2 cell layers: inner layer of reduced or atrophied ameloblasts and external layer, probably stratum intermedium cells
- Reduced enamel epithelium is normally found overlying an unerupted, otherwise developed tooth
- Deciduous tooth (also called baby tooth or primary tooth): exfoliated during childhood
- Succedaneous tooth (also called adult tooth): permanent tooth; replaces deciduous teeth
ICD coding
- ICD-11: DA05.0 - developmental odontogenic cysts
Epidemiology
- Second most common odontogenic cyst (Braz Oral Res 2021;35:e129)
- Most common developmental odontogenic cyst (Braz Oral Res 2021;35:e129)
- Multiple simultaneous dentigerous cysts are uncommon
- Broad age range (5 - 83 years), with peak incidence in second to third decade and a slight male predilection (J Oral Pathol Med 2013;42:462, Surg Pathol Clin 2017;10:177, J Oral Pathol Med 2019;48:74, Oral Maxillofac Surg 2020;24:73)
Sites
- By definition, a dentigerous cyst occurs in association with an unerupted tooth
- Most common around permanent mandibular third molars (wisdom teeth)
- Somewhat less common around permanent maxillary third molars, maxillary cuspids and mandibular second premolars but any tooth may be involved (J Oral Pathol Med 2013;42:462)
- Rarely involves supernumerary teeth and odontomas
- Distinctly rare to occur around unerupted primary teeth
Pathophysiology
- Vast majority are developmental odontogenic cysts with unknown pathogenesis; subset may have inflammatory pathogenesis
- Inflammation progressing from root apex of carious or necrotic deciduous tooth brings about development of dentigerous cyst around underlying, unerupted permanent tooth (J Korean Assoc Oral Maxillofac Surg 2022;48:342, Exp Ther Med 2021;22:750)
- Difficult to histologically distinguish inflamed developmental odontogenic dentigerous cyst from those induced by inflammation
Etiology
- In normal tooth development, tooth enamel is produced by the enamel organ, an ectodermally derived specialized epithelium
- After enamel formation is complete, the enamel organ epithelium atrophies
- This reduced enamel epithelium eventually merges with the overlying mucosal epithelium to form the initial gingival crevicular epithelium of the newly erupted tooth
- Develops from accumulation of fluid (including glycosaminoglycans) between reduced enamel epithelium of dental follicle and crown of unerupted tooth (Mod Pathol 2017;30:S96, Surg Pathol Clin 2017;10:177, J Oral Pathol Med 2019;48:74)
Clinical features
- May be small / asymptomatic; then may be incidentally detected on radiographs taken for unrelated reasons or for imaging to investigate delayed tooth eruption (Oral Maxillofac Surg 2020;24:73)
- Large lesions may produce a painless bony expansion; can displace the involved tooth or cause resorption of adjacent teeth
- If secondarily infected, may be associated with pain
Diagnosis
- Diagnosis incorporates elements such as clinical examination, patient history, symptoms, clinical examination, imaging and supportive histopathology
- Patient symptoms (see Clinical features)
- Clinical examination findings: dentigerous cyst is not clinically visible on examination of oral cavity
- Surgical findings during operative exploration
- Cyst fluid may be released during extirpation
- Cyst cavity may be appreciated during extirpation
- Lesion classically surrounds crown of unerupted tooth
- Lesion is intraosseous and will require surgical exposure through mucosa and bone
- Imaging findings of lesion relative to unerupted tooth (Diagnostics (Basel) 2022;12:2006)
- Histopathology additionally supports the diagnosis
Radiology description
- Radiology is essential to identify landmarks and the extent of disease in the preoperative and follow up setting
- Panoramic radiograph (panorex), intraoral film or cross sectional imaging can detect most cases of dentigerous cyst
- Most commonly a well defined, unilocular radiolucency on Xray
- Often has sclerotic border
- Can cause resorption of adjacent teeth
- 3 different radiographic relationships between unerupted tooth and cyst are described (Imaging Sci Dent 2021;51:291)
- Central
- Most common radiographic relationship
- Cyst develops around and surrounds the entire crown of tooth; thus, the tooth appears to be erupting into the cyst (see Clinical images)
- Lateral
- Cyst develops at lateral tooth root and only partially surrounds the crown (see Clinical images)
- Circumferential
- Cyst develops around the crown and extends down the root(s); thus, roots also appear within the cyst
- Central
- Radiographic differential diagnosis of dentigerous cyst: odontogenic keratocyst, orthokeratinized odontogenic cyst and ameloblastoma among other odontogenic tumors
- Detection of bilateral dentigerous cysts is uncommon
- Radiographic distinction between an enlarged dental follicle and a small dentigerous cyst can be arbitrary
- Generally, a pericoronal radiolucency that is > 3 - 4 mm in diameter is considered suggestive of cyst formation
Radiology images
Prognostic factors
- Excellent prognosis
- Almost never recurs with complete enucleation
- Recurrence may indicate incomplete excision or possibly incorrect original diagnosis (Head Neck Pathol 2021;15:107)
Case reports
- 12 year old boy with bilateral inflammatory dentigerous cysts (Int J Clin Pediatr Dent 2020;13:429)
- 20 year old woman with florid cemento-osseous dysplasia in association with dentigerous cyst (J Oral Maxillofac Pathol 2010;14:63)
- 44 year old man with bilateral dentigerous cysts (Am J Case Rep 2019;20:1148)
- 65 year old woman with dentigerous cyst in association with odontoma (Case Rep Dent 2022;2022:6210289)
Treatment
- Varies based on age, maturity, anatomic position and relative importance of tooth involved, size of cyst, presence of additional neoplasms; also patient preference, including cosmetic and functional considerations
- Enucleation of entire cyst with extraction of the associated tooth is the most common approach
- Marsupialization
- Removal of the cyst sparing the permanent tooth (J Appl Oral Sci 2012;20:282)
- Requires close follow up to monitor for recurrence
Clinical images
Gross description
- Relationship of tooth and cyst is usually disrupted during surgery; however, examination of associated tooth may reveal tags of adherent tissue at cementoenamel junction (Surg Pathol Clin 2017;10:177)
- Surgical specimens usually consist of multiple, irregular fragments
- Possible minute bone fragments
- All mucosal, soft tissue and bone fragments should be submitted for histologic examination to definitively confirm the diagnosis
- Tooth received as specimen can be photographed and grossly described unless cyst develops in association with odontoma
- Odontoma requires microscopic examination, further underscoring the importance of radiographic correlation
Gross images
Frozen section description
- Variability depending on the degree and extent of inflammation
- Inflamed cysts may show multilayered stratified squamous epithelium making it difficult to distinguish from unicystic ameloblastoma, luminal subtype or inflamed odontogenic keratocyst
- Uninflamed cysts may show low cuboidal or bilaminar epithelial lining
- Frozen section limitations include sampling bias, frozen section artifact and inability to perform immunohistochemistry, such as BRAF (J Oral Maxillofac Surg 2014;72:914)
Frozen section images
Microscopic (histologic) description
- Microscopic features are influenced by presence of inflammation
- Inflamed dentigerous cyst
- Fibrous connective tissue
- Hyperplastic nonkeratinized epithelium, sometimes elongated interconnecting rete ridges
- Acute and chronic inflammatory cells
- Cholesterol clefts, possibly formation of cholesterol granuloma
- Rushton bodies
- Scattered mucous, ciliated or sebaceous cells are uncommon but possible (J Oral Sci 2005;47:77)
- Occasional dystrophic calcifications
- Odontogenic epithelial rests, small, inactive appearing within fibrous wall
- Noninflamed dentigerous cyst (J Oral Pathol Med 2013;42:462)
- Fibrous to fibromyxoid connective tissue
- No rete ridges, flat interface
- Lining epithelium, 2 - 4 layers of cuboidal epithelium, devoid of superficial keratinization
- Occasional mucous cells; rare ciliated cells
- Occasional dystrophic calcifications
- Odontogenic epithelial rests, small, inactive appearing within fibrous wall
- Some lesions submitted as dentigerous cysts are partially lined with a thin, fragmented layer of eosinophilic columnar cells / low cuboidal epithelium representing the postfunctional ameloblastic layer of the reduced enamel epithelium
- Classification of these lesions as cysts versus dental follicles shows interobserver variability
Microscopic (histologic) images
Positive stains
- No specific immunohistochemistry required for dentigerous cyst
- Squamous epithelium expected to be immunoreactive for cytokeratin AE1 / AE3, p63 and p40
Negative stains
- BRAF immunostain
- Beta catenin immunostain negative for nuclear staining
Sample pathology report
- Left mandible lesion, excision:
- Stratified squamous epithelial lined cyst consistent with dentigerous cyst
Differential diagnosis
- Unicystic ameloblastoma:
- Columnar basal cells with hyperchromatic nuclei
- Usually but not always exhibit reverse polarization of nuclei (away from basement membrane)
- Dentigerous cysts do not harbor the BRAF p.V600E mutations found in ameloblastomas (J Oral Pathol Med 2016;45:780, Appl Immunohistochem Mol Morphol 2021;29:390)
- Odontogenic keratocyst:
- Uniform epithelium
- 4 - 8 cell layers in thickness
- Hyperchromatic basilar palisading of cuboidal to columnar cells
- Characteristic wavy / corrugated surface parakeratosis
- With or without keratin flakes within cyst lumen
- Radicular / periapical cyst:
- Radicular cyst (RC) is an inflammatory odontogenic cyst associated with the root of a nonvital tooth
- Surfaced by nonkeratinized stratified squamous epithelium
- Often characterized by an arcading pattern
- Cyst wall is composed of inflamed fibrous tissue; may have foamy histiocytes
- Inflammatory collateral cysts:
- Inflammatory cysts occur on the buccal or distobuccal aspect of the roots of partially or recently erupted teeth
- Microscopically can be almost indistinguishable from inflamed radicular cyst or inflamed dentigerous cyst
- Further divided into 2 subtypes
- Mandibular buccal bifurcation cyst occurs on the lateral aspect of partially or recently erupted tooth
- Paradental cyst occurs on the distobuccal aspect of partially or recently erupted tooth
Additional references
Board review style question #1
Board review style answer #1
B. Mandibular buccal bifurcation cyst. Mandibular buccal bifurcation cysts are lined by an inflamed, nonkeratinizing stratified squamous epithelial lining, similar to an inflamed dentigerous cyst. Buccal bifurcation cysts occur lateral to an erupting or recently erupted mandibular tooth whereas dentigerous cyst occurs in relation to an impacted tooth.
Answer A is incorrect because ameloblastic fibroma is an odontogenic neoplasm composed of epithelial and mesenchymal components. It is not cystic. Answer C is incorrect because odontogenic myxoma is a mesenchymal neoplasm without cystic change. Answer D is incorrect because the epithelial lining of orthokeratinized odontogenic cyst is heavily keratinized.
Comment Here
Reference: Dentigerous cyst
Answer A is incorrect because ameloblastic fibroma is an odontogenic neoplasm composed of epithelial and mesenchymal components. It is not cystic. Answer C is incorrect because odontogenic myxoma is a mesenchymal neoplasm without cystic change. Answer D is incorrect because the epithelial lining of orthokeratinized odontogenic cyst is heavily keratinized.
Comment Here
Reference: Dentigerous cyst
Board review style question #2
What is the most common locational relationship for a dentigerous cyst to have with a tooth?
- Surrounds the apex of erupted tooth
- Surrounds the crown of erupted tooth
- Surrounds the crown of unerupted tooth
- Surrounds the lateral aspect of erupted tooth
Board review style answer #2
C. Surrounds the crown of unerupted tooth. Dentigerous cysts most commonly surround the crown of an unerupted tooth. While the circumferential and lateral radiographic appearance have been described, the classically described dentigerous cyst shows the crown of the tooth projecting into the cyst.
Answer A is incorrect because a dentigerous cyst surrounding only the apex of an unerupted tooth has not been described. Answer B is incorrect because a dentigerous cyst involves an unerupted tooth, not an erupted tooth. Answer D is incorrect because a dentigerous cyst involves an unerupted tooth, not an erupted tooth.
Comment Here
Reference: Dentigerous cyst
Answer A is incorrect because a dentigerous cyst surrounding only the apex of an unerupted tooth has not been described. Answer B is incorrect because a dentigerous cyst involves an unerupted tooth, not an erupted tooth. Answer D is incorrect because a dentigerous cyst involves an unerupted tooth, not an erupted tooth.
Comment Here
Reference: Dentigerous cyst
Dentinogenic ghost cell tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign, locally aggressive odontogenic neoplasm of maxilla and mandible with a predominantly solid pattern of growth
Essential features
- Neoplasm with predominantly solid growth of islands of odontogenic and ameloblastoma-like epithelium
- Ghost cells composed of anucleate epithelial cells with pale cytoplasm
- Focal stellate reticulum-like epithelium
- Varying levels of calcified material to include products of odontogenesis and calcification of the ghost cell
- Locally aggressive with high rates of recurrence
- Histopathologic overlap with calcifying odontogenic cysts
Terminology
- Epithelial odontogenic ghost cell tumor
- Calcifying ghost cell odontogenic tumor
- Previously classified along with calcifying odontogenic cyst (J Periodontol 1985;56:340)
ICD coding
- ICD-10: D16.4 - benign neoplasm of bones of skull and face
Epidemiology
- Broad age range (11 - 79 years old); peak 40 - 60 years old
- M:F = 1.8:1 (J Oral Pathol Med 2018;47:721)
- Rarity may affect demographics
Sites
- Intraosseous sites: posterior maxilla and mandible
- Rarely reported as gingiva or alveolar mucosal tumor
Pathophysiology
- Wnt signaling pathway may have a role in the development similar to calcifying odontogenic cyst since beta catenin gene mutations and beta catenin overexpression are also identified in DGCT (PLoS One 2017;12:e0180224, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103:97)
Etiology
- Unknown
Clinical features
- Patients frequently present with asymptomatic swelling of the jaw
Diagnosis
- Radiologic and histopathologic correlation required for diagnosis
Radiology description
- Majority present as radiopaque lesions with well defined borders and a mixed radiodensity due to varying levels of calcification
- Rarely associated with odontomas, similar to calcifying odontogenic cyst (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:356)
Radiology images
Prognostic factors
- High rates of recurrence with simple enucleation (up to 73%) (J Oral Maxillofac Surg 2016;74:307)
- Lower but still significant rates of recurrence with more extensive procedures (up to 33%) (J Oral Maxillofac Surg 2016;74:307)
- Limited reported cases may affect knowledge of true prognosis
Case reports
- 18 year old man reported with recurrent swelling and pain in upper jaw (World J Clin Oncol 2019;10:192)
- 26 year old man with a growth in the upper left front part of the jaw (J Oral Maxillofac Pathol 2018;22:150)
- 40 year old man with swelling on the right side of the face (J Oral Maxillofac Pathol 2019;23:478)
- 40 year old woman with swelling in the front region of the lower jaw for 2 years (J Oral Maxillofac Pathol 2019;23:66)
- 68 year old man with a soft tissue growth in lower anterior region of the jaw for 3 years (J Oral Maxillofac Pathol 2016;20:163)
Treatment
- Rarity of this tumor limits study of the optimal form of treatment
- Simple enucleation, curettage: recurrence rate of up to 73% after a follow up period of 1 - 20 years
- Recurrence rates vary by surgical approach; recent recommendation includes wide local resection
Gross description
- Predominantly solid tumor; limited macrocystic change
Microscopic (histologic) description
- Predominantly solid mass consisting of sheets of anastomosing cords and strands of odontogenic epithelium; microcystic development possible
- Admixed ghost cells: anucleate epithelial cells with pale cytoplasm containing cytoplasmic clearings representing the location of a previously resorbed nucleus or organelles
- Interspersed with islands of swirling cells with squamous differentiation
- Ameloblastic-like areas with palisading of basaloid cells
- Odontogenic epithelial cells demonstrate round uniform basophilic nuclei and pale eosinophilic to clear cytoplasm
- Background stellate reticulum-like proliferation
- Varying levels of dentinoid and cementum-like calcified collagenous matrix
- Mitosis rare
- Reference: J Oral Maxillofac Surg 2016;74:307
Microscopic (histologic) images
Positive stains
- Positive but nonspecific:
- Beta catenin (nuclear and cytoplasmic)
- LEF1 (nuclear)
Negative stains
- Negative but nonspecific:
Sample pathology report
- Posterior mandible, right, segmental mandibulectomy:
- Dentinogenic ghost cell tumor (3.2 cm) (see comment)
- Comment: Tumor confined to bone and measures 0.5 cm from anterior and posterior bone margins
- Posterior mandible, right, excision / curettage:
- Dentinogenic ghost cell tumor, in fragments
Differential diagnosis
- Ameloblastoma:
- Second most common odontogenic tumor; however, most clinically significant odontogenic tumor after odontoma
- May have similar basaloid epithelial cells, reverse polarity, stellate reticulum but no ghost cells
- Typically does not have calcifications
- Conventional (nonunicystic), commonly solid and multilocular
- Calcifying odontogenic cyst:
- Similar histology, ameloblast-like epithelium, stellate reticulum-like proliferation, ghost cells, dentinoid and calcifications
- Grossly, predominantly cystic; small satellite cysts, islands of epithelium or ghost cells may be seen in the fibrous capsule
- Likely on a spectrum with dentinogenic ghost cell tumor
- Craniopharyngioma:
- Similar histology, ghost cells and islands of squamous cells present but originates in the sella turcica
- Ghost cell odontogenic carcinoma:
- Extremely rare with only isolated case reports
- Demonstrates pleomorphism and malignant cytology with invasive features
Additional references
Board review style question #1
Board review style answer #1
D. Predominantly solid pattern of growth. Dentinogenic ghost cell tumor and calcifying odontogenic cyst have significant histopathologic overlap. Both demonstrate amelobastic-like epithelium, ghost cells, stellate reticulum, squamous differentiation and varying levels of dentin and calcification; however, dentinogenic ghost cell tumor is a predominantly solid neoplasm and calcifying odontogenic cyst is a single chamber, unilocular cyst.
Comment Here
Reference: Dentinogenic ghost cell tumor
Comment Here
Reference: Dentinogenic ghost cell tumor
Board review style question #2
Which radiographic features distinguishes an ameloblastoma from a dentinogenic ghost cell tumor?
- Air fluid levels
- Calcifications
- Cystic spaces
- Invasive features
Board review style answer #2
B. Calcifications. Ameloblastoma and dentinogenic ghost cell tumor have significant radiographic and histopathologic overlap; however, radiographically ameloblastoma generally does not have calcifications. Radiographically, ameloblastoma typically demonstrates a multilocular appearance. Ameloblastoma is the most common clinically significant odontogenic tumor and by far more common than dentinogenic ghost cell tumor.
Comment Here
Reference: Dentinogenic ghost cell tumor
Comment Here
Reference: Dentinogenic ghost cell tumor
Diffuse sclerosing
Table of Contents
Definition / general | Terminology | Sites | Clinical features | Diagnosis | Radiology description | Case reports | Treatment | Gross description | Microscopic (histologic) description | Differential diagnosis | Additional referencesDefinition / general
- Sclerosing osteomyelitis has traditionally been divided into focal and diffuse types:
- The focal type, also known as condensing osteitis, describes a relatively common, limited, well circumscribed radiopaque alteration of bone surrounding the apex of a root of a tooth, the latter often with an inflamed or necrotic dental pulp
- Diffuse sclerosing osteomyelitis, on the other hand, is an uncommon condition affecting a large portion of the bone, usually mandible, and is associated with mandibular expansile remodeling and clinical symptoms
- Many views of DSO exist in the literature:
- DSO is a form of primary chronic osteomyelitis (PCO) - the remainder of this topic follows this view
- DSO is synonymous to juvenile chronic osteomyelitis and an early onset form of PCO
- DSO is a localized form of SAPHO
- DSO is distinct from PCO and the term DSO is only used when an obvious infectious process is responsible for sclerosis of bone (i.e. with a chronic intraosseous bacterial infection which creates chronically inflamed granulation tissue that incites sclerosis of the surrounding bone)
- DSO is used only as a description of radiological features
- Both primary and secondary chronic osteomyelitis may demonstrate radiographic changes of extensive sclerosis with periosteal reaction (proliferative periostitis) and variable osteolysis on diagnostic imaging
Terminology
- Sequestrum:
- Predominantly nonviable bone that has become separated during the process of necrosis from the original surrounding native bone (Medical Dictionary: sequestrum [Accessed 4 June 2018] )
- Periosteum
- Connective tissue membrane lining the outer / external surface bones, except at the joints of long bones
- Is composed of an outer fibrous layer and an inner cambium / osteogenic layer
- Chronic recurrent multifocal osteomyelitis (CRMO)
- Chronic recurrent multifocal osteomyelitis (CRMO) is a relapsing inflammatory disease and is considered a type of seronegative spondyloarthropathy characterized by periods of exacerbations and remissions over many years
- Characterized by an inflammatory process presenting with findings similar to infectious osteomyelitis; however, no infectious source is identifiable
- Most often characterized by the insidious onset of low grade fever, local swelling and bone pain
- Radiologic findings may suggest osteomyelitis, and bone seeking isotopes reveal other asymptomatic sites of involvement
- The lesions occur mainly in the metaphyses of long tubular bones, as well as the clavicles, spine, and pelvis; the lesions are sometimes symmetrically distributed
- The precise relationship between primary chronic osteomyelitis, diffuse sclerosing osteomyelitis, CRMO and SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) is highly debated and not clear at this time
- Some consider CRMO and SAPHO as primary chronic osteomyelitis with additional extragnathic manifestations
- Whether CRMO in juvenile patients and SAPHO syndrome are the same disorder in different age groups remains to be determined
- Synovitis, Acne, Pustulosis, Hyperostosis and Osteitis (SAPHO)
- Acronym for a complex clinical presentation that includes Synovitis, Acne, Pustulosis, Hyperostosis and Osteitis
- Unknown cause but may arise in genetically predisposed individuals who develop an autoimmune disturbance due to exposure to dermatologic bacteria
- Increased prevalence of HLA-27 (10 - 15% cases of SAPHO)
- SAPHO and Chronic recurrent multifocal osteomyelitis / CRMO are distinguished from other forms of osteitis / osteomyelitis as these two diseases may have extragnathic skeletal involvement
- The precise relationship between primary chronic osteomyelitis, diffuse sclerosing osteomyelitis, CRMO and SAPHO is controversial - some consider CRMO and SAPHO as primary chronic osteomyelitis with additional extragnathic manifestations
- Proliferative periostitis
- Represents a periosteal reaction to the presence of inflammation
- The affected periosteum forms several rows of reactive vital bone that parallel each other and expand the surface of the altered bone
- Garrè sclerosing osteomyelitis
- In 1893, Swiss physician Carl Garrè reported on patterns of and complications of acute osteomyelitis
- Garrè did not have any pathologic specimens for microscopic examination nor radiographs to augment his descriptions
- Nonetheless, his name is often associated with the condition in which periosteal duplication or "onion-skinning" pattern of periostitis leads to enlargement of the jaw, usually mandible
- Cases of osteomyelitis with a predominant periosteal reaction are in some instances falsely labeled as Garrè osteomyelitis
- Garrè osteomyelitis and Garrè sclerosing osteomyelitis are often used synonymously and should be disassociated with this clinical presentation
- Note that Garrè name was and is often misspelled as Garré with an improper accent
- Numerous synonyms for Garrè sclerosing osteomyelitis have been used:
- Chronic osteomyelitis with proliferative periostitis
- Chronic sclerosing osteomyelitis of Garrè
- Chronic non-suppurative osteomyelitis with proliferative periostitis
- Garrè chronic sclerosing osteomyelitis
- Garrè chronic nonsuppurative sclerosing osteitis
- Garrè proliferative periostitis
- Garrè sclerosing osteomyelitis
- Ossifying periostitis
- Osteomyelitis of Garrè
- Osteomyelitis sicca
- Osteomyelitis with proliferative periostitis
- Periostitis ossificans
- Sclerosing osteomyelitis of Garrè
- Condensing osteitis
- Localized areas of radiographic bone sclerosis associated with apices of inflamed, dead or dying teeth (pulpitis or pulpal necrosis)
- The association with an area of inflammation, usually the apex of an associated tooth, is critical, because these lesions can resemble several other intrabony processes that produce a somewhat similar radiographic pattern
- This secondary sclerosis of bone is not considered a true / classical osteomyelitis
- Zurich Classification of Osteomyelitis:
- Primarily based on clinical course and imaging features
- Histopathology is considered a secondary classification criterion because findings are mostly unspecific and inconclusive by themselves; however, tissue examination is irreplaceable to confirm diagnosis, when clinical and radiological appearance are unclear / atypical, and to exclude possible differential diagnoses
- The three major classifications are:
- Acute Osteomyelitis (AO)
- Secondary Chronic Osteomyelitis (SCO)
- Primary Chronic Osteomyelitis (PCO)
- Further subclassification is based on presumed etiology and pathogenesis of disease
- These criteria are considered tertiary classification criteria, and are helpful to determine the necessary therapeutic strategies which may differ somewhat among the subgroups
Sites
- When involves the jaws, predominantly involves the mandible
Clinical features
- Insidious onset, lacking an obvious acute state
- Often affects children and young adults
- Pain (days to weeks, often with symptom free intervals), swelling and limitation of mouth opening
- Jaw expansion, usually mandible, in the absence of pus, fistula and sequestration
Diagnosis
- Clinical diagnosis challenging because the clinical picture and course of the disease may vary significantly
- Whether DSO represents variations of a single disorder or different pathologic processes is highly debated with no clear answer at this time
- Clinicians should consider all possibilities to ensure appropriate care
Radiology description
- Expansile remodeling of jaw, usually mandible
- Sclerosis, punctuated by areas of lytic change
- Periosteal duplication
Case reports
- Diffuse sclerosing osteomyelitis (DSO) of the mandible in SAPHO syndrome (J Craniomaxillofac Surg 2014;42:1990)
Treatment
- Treatment is influenced by stage, extent and symptoms of disease
- May include NSAIDs, TNF inhibitors, bisphosphonates
Gross description
- Biopsies to rule out neoplasia may be minute
Microscopic (histologic) description
- Histology may be nonspecific and may mimic acute and secondary chronic osteomyelitis due to microbiological infection
- An extensive microbiological workup including culture and PCR may be indicated
- Bone biopsy is usually performed to exclude malignancy and competing radiographic and clinical differential diagnostic entities
- Microscopic findings vary with area sampled (as do radiographics)
- Chronic phase: paucity of inflammation with predominant sclerosis and fibrosis
- Prior biopsy or sample site: organizing fibrous and granulation tissue with mixed inflammation
- Reactive and remodeling bone, particularly in a region of periosteal reaction
- Unerupted tooth buds may be disturbed or inadvertently removed during mandibular biopsy
Differential diagnosis
Eruption cyst
Table of Contents
Definition / general | Terminology: normal cells / structures | Terminology: cysts | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Differential diagnosisDefinition / general
- Develops in soft tissues overlying alveolar bone due to separation of dental follicle from crown of erupting tooth
Terminology: normal cells / structures
- Ameloblasts:
- Specialized epithelial cells that form tooth enamel
- Deciduous tooth:
- Also called baby tooth or primary tooth
- Falls out during childhood
- Succedaneous tooth:
- Also called permanent tooth or adult tooth
- Replaces deciduous teeth
- Ideally lasts throughout life under most normal circumstances
- Natal tooth:
- Tooth or teeth present at birth
- Neonatal tooth:
- Tooth or teeth present during the first 30 days of life
Terminology: cysts
- Dentigerous cyst:
- Developmental odontogenic cyst occurring within the jawbone, originates by separation of dental follicle from around the crown of an unerupted tooth
- Follicular cyst:
- Synonym for dentigerous cyst
- Eruption cyst:
- Soft tissue counterpart to dentigerous cyst involving an erupting tooth
- Whereas dentigerous cyst develops around crown of an unerupted tooth lying in the bone, eruption cyst occurs when a tooth is impeded in its eruption within the soft tissues
- The epithelium lining the dilated cystic space is reduced enamel epithelium
- Eruption hematoma:
- Surface trauma may result in a considerable amount of blood in the fluid of the eruption cyst, which imparts a blue to purple-brown color
- Reduced enamel epithelium:
- Tooth enamel is normally composed of two cell layers: inner layer of reduced or atrophied ameloblasts and external layer, probably stratum intermedium cells
- Reduced enamel epithelium is normally found overlying an unerupted, otherwise developed tooth
Epidemiology
- 1 - 10% of submitted jaw cysts, but may be underrepresented since cysts that rupture spontaneously are not submitted for examination
- Age: usually < age of 10, occasionally older patients with delayed tooth eruption, rarely congenital
- Gender: male to female ratio 2:1
Sites
- Mandibular central incisors and permanent first molars most common sites affected
Pathophysiology
- Accumulation of fluid or hemorrhage between crown of erupting tooth and the reduced enamel epithelium of the surrounding dental follicle
- In eruption cyst, tooth is within oral soft tissues, not bone, as occurs in dentigerous cyst
- Factors actually impeding eruption through soft tissues are unknown, possibly dense fibrous tissue
Etiology
- Develops secondary to accumulation of blood or fluid between tooth crown and an erupting primary or permanent tooth and the overlying mucosa
Clinical features
- Raised, bluish or mucosal colored dome shaped gingival mass on alveolar ridge
- Variable size, dependent on size and number of associated teeth, but usually < 1.5 cm
- Usually asymptomatic but, if inflamed, may be painful and tender
- Occasionally multiple cysts
Diagnosis
- Radiographic findings, in combination with clinical information, can support a histomorphologic diagnosis
Radiology description
- Usually no bone involvement except the dilated erupting tooth crypt
- Soft tissue "shadow" of cyst may be seen on radiograph
Case reports
- 8 year old boy with bilateral mandibular cysts associated with cyclosporine use (Pediatr Nephrol 2001;16:993)
- Boy with eruption cyst associated with cyclosporin A (J Clin Periodontol 2003;30:462)
- Child with hepatoblastoma and oral eruption cysts (J Pediatr Hematol Oncol 2009;31:509)
- Child with Lowe syndrome and multiple eruption cysts and hematomas (J Clin Pediatr Dent 1999;23:357)
- Kinky hair disease with multiple eruption cysts (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;82:537)
Treatment
- None:
- Treatment not likely required if the cyst ruptures spontaneously, permitting the tooth to erupt
- Marsupialization:
- If tooth eruption appears to be impeded by the lesion, the cyst can be unroofed, which usually allows the tooth to erupt
- The dome of the cyst is excised, exposing the crown of the tooth, which is allowed to erupt
- Submission of the cyst roof is recommended to confirm the clinical impression and exclude other pathology
Clinical images
Microscopic (histologic) description
Epithelial surface facing oral cavity:
- Keratinized squamous oral epithelium on superior / external or oral aspect of specimen corresponding to gingiva or alveolar ridge tissue
- Lamina propria underlying the oral epithelium and overlying the roof of the cyst shows a variable inflammatory cell infiltrate
- May be possible to distinguish a line of demarcation between gingival and follicular connective tissues: the gingival connective tissue is relatively acellular and densely collagenous and so has an eosinophilic hue; the follicular connective tissue is more densely cellular, less collagenous and has a more basophilic hue, presumably because of a higher content of sulphated glycosaminoglycans in the ground substance
- Odontogenic epithelial cell rests may be present in the connective tissue
- The deep portion of the specimen, which represents the roof of the cyst, shows a thin layer of nonkeratinizing squamous epithelium
- In non-inflamed areas, the epithelial lining of the cyst is characteristically of reduced enamel epithelial origin, consisting of 2 - 3 cell layers of squamous epithelium with a few foci where may be thicker
- If the epithelial lining is inflamed, acute inflammatory cells are found in the epithelium which proliferates and shows reactive spongiosis
Differential diagnosis
- Correlation with patient age, specific anatomic location at tooth bearing area of jaws, and radiographic findings is essential
- Congenital, neonates, infants:
- Epstein pearls
- Bohn nodules
- Gingival cyst of newborn
- Children (less than 14 - 16 years of age):
- Buccal bifurcation cyst
- Dentigerous cyst
- Dental follicle
- Unicystic ameloblastoma
- Soft tissue keratocystic odontogenic tumor
- Adults:
- Gingival cyst
- Dentigerous cyst
- Dental follicle
- Paradental cyst
- Unicystic ameloblastoma
- Soft tissue keratocystic odontogenic tumor
Ghost cell odontogenic carcinoma
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Immunohistochemistry & special stains | Molecular / cytogenetics description | Differential diagnosisDefinition / general
- According to WHO 2005, ghost cell odontogenic carcinoma is a malignant epithelial tumor with features of calcifying cystic odontogenic tumor or dentinogenic ghost cell tumor
- Classified as an odontogenic carcinoma, which includes:
- Metastasizing (malignant) ameloblastoma
- Ameloblastic carcinoma
- Primary intraosseous squamous cell carcinoma
- Solid type
- Derived from keratocystic odontogenic tumor
- Derived from odontogenic cysts
- Clear cell odontogenic carcinoma
- Ghost cell odontogenic carcinoma
Terminology
- Odontogenic ghost cell tumor
- Calcifying ghost cell odontogenic carcinoma
- Malignant epithelial odontogenic ghost cell tumor
- Aggressive epithelial ghost cell odontogenic tumor
- Carcinoma arising in a calcifying odontogenic cyst
- Malignant calcifying ghost cell odontogenic tumor
- Malignant calcifying odontogenic cyst
Epidemiology
- Rare, < 40 cases reported in literature and more than half from Asia
- More common in males
Sites
- Maxilla more common than mandible
Etiology
- Malignancy thought to arise from calcifying ondontogenic cysts (COCs) with features of either calcifying cystic odontogenic tumor or dentinogenic ghost cell tumor
Clinical features
- Can present as painful, hard swelling in the maxilla or mandible
- Can also have paresthesia associated with root resorption or tooth displacement
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Radiology description
- Most commonly seen as a mixed radiolucency and radio opacity with ill defined margins
Prognostic factors
- Overall 5 year survival rate is 73% and recurrence is common
Case reports
- 21 year old man with ghost cell odontogenic carcinoma (GCOC) (Pan Afr Med J 2015;21:260)
- 54 year old man with tender swelling in the malar region (J Oral Maxillofac Pathol 2015;19:371)
- 64 year old woman with metastatic odontogenic carcinoma arising from dentinogenic ghost cell tumor (Rare Tumors 2015;7:5813)
- 70 year old woman with rapid onset of painful swelling right maxillary tumor (J Clin Exp Dent 2014;6:e602)
Treatment
- Composite surgical excision, may be followed by radiation
Clinical images
Microscopic (histologic) description
- Ameloblastomatous areas: peripheral palisading, reverse polarization, stellate reticulum
- Ghost cells (polygonal epithelial cells with eosinophilic cytoplasm that have lost their nuclei but maintain a faint outline of cellular and nuclear membrane)
- Ghost cells may be calcified
- Atypia with changes such as increased cellularity, pleomorphism, mitosis, necrosis and infiltrative growth
Microscopic (histologic) images
Immunohistochemistry & special stains
- Positive for pan-cytokeratin, p63
- Often have an increased Ki67 proliferative index
- Can also show p53 expression
Molecular / cytogenetics description
- Limited literature, but aberrations of Wnt signaling pathway with beta catenin overexpression have been shown in calcifying cystic odontogenic tumors (APMIS 2008;116:206)
Differential diagnosis
- Ameloblastic carcinoma
- Variable features of ameloblastoma: peripheral palisading, reverse polarization, stellate reticulum-like cells
- Features of malignancy include cytological atypia, high N:C ratio, increased mitoses with atypical forms, necrosis
- Can also metastasize
- No ghost cells
- Benign epithelial odontogenic neoplasm
- Occurs in posterior mandible, intraosseous location
- Variably sized polyhedral eosinophilic epithelial cells with distinct cell borders are arranged in small clusters, trabeculae, islands or a sheet-like pattern
- Nuclear pleomorphism is expected, but without appreciable mitotic activity
- Eosinophilic amyloid-like matrix material is haphazardly deposited in association with the tumor islands, and calcified concentric profiles (Liesegang rings) are often identified
- Calcifying cystic odontogenic tumor (CCOT)
- Benign cystic tumor of odontogenic origin, aka “Gorlin cyst” or “Calcifying odontogenic cyst”
- Can have “ameloblastic” features: columnar or cuboidal basal cells with lumen lined by tissue resembling stellate reticulum
- Will have ghost cells or anucleate epithelial cells
- Should not have cytologic atypia, increased mitotic activity, necrosis
- Calcifying epithelial odontogenic tumor (CEOT)
Gingival cyst (adult)
Table of Contents
Definition / general | Epidemiology | Sites | Pathophysiology | Clinical features | Radiology description | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Gingival cysts of adults occur along gingiva of older adults, arise from dental lamina epithelial rests
Epidemiology
- Rare, approximately 0.5% of all odontogenic cysts
- Arise in middle aged or elderly adults, peak incidence in fifth to sixth decade of life
- More common in females
Sites
- Most common location is facial gingiva of mandibular canines and premolars
- Maxillary gingival cysts usually found in facial gingival incisor, canine, premolar region
Pathophysiology
- Arise from epithelial rests of dental lamina epithelium (rests of Serres) within soft tissue
- Cause of cystic degeneration of this epithelium is unknown
- Is considered the soft tissue counterpart of lateral periodontal cyst
Clinical features
- Solitary, well circumscribed, usually less than 0.5 cm
- Rarely bilateral
- Bluish, smooth surfaced, dome shaped swelling on attached gingiva or unattached alveolar mucosa
- May cause superficial pressure resorption of underlying alveolar bone (See "Bone defect" in Clinical images)
Radiology description
- Cyst may cause a superficial "cupping out" of alveolar bone, usually not detected on a radiograph but apparent when cyst is excised
- If more bone is missing or the pre-operative lesion is visible on a radiograph, one could argue that the lesion may be a lateral periodontal cyst that has eroded the cortical bone rather than a gingival cyst that originated in the mucosa
Case reports
- 16 year old boy with gingival cyst (J Indian Soc Periodontol 2012;16:465)
- Gingival "surgical cyst" developing post-subepithelial connective tissue graft (J Periodontol 1997;68:392)
- Bilateral gingival swellings in mandibular canine-premolar areas (J Am Dent Assoc 1990;120:71)
- Gingival cyst of adult with bilateral presentation (J Periodontol 1987;58:796)
Treatment
- Local surgical excision, typically do not recur
Clinical images
Microscopic (histologic) description
- Unicystic structure lined with attenuated, non-keratinized low cuboidal or stratified squamous epithelium
- Cyst lining may be with or without contiguous epithelial plaques (focal thickened areas of epithelium) which may have clear cells
- Surrounding fibrovascular stroma is relatively uninflamed, except biopsy includes a portion of junctional epithelium
- Variable inflammation
Microscopic (histologic) images
Differential diagnosis
- Cyst of incisive papilla: only occurs in soft tissues of midline anterior hard palate between central incisor teeth
- Epidermoid cyst: usually floor of mouth, large size
- Epithelial inclusion cyst: after gingival graft
- Lateral periodontal cyst: originates within bone
- Odontogenic keratocyst: occasionally reported in soft tissues
- Salivary duct cyst: occurs in non-gingival, salivary gland bearing tissues
Gingival cyst (newborn)
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Differential diagnosis | Additional referencesDefinition / general
- Occur on palate (interchangeably referred to as palatal cysts of newborn) and along alveolar ridge
Terminology
- Epstein Pearls:
- Originally descripted cysts along medial raphe of palate
- Now used interchangeably for palatal and gingival cysts of newborns
- Bohn Nodules:
- Cysts originating from palatal glands, scattered across hard and soft palate
- Now used interchangeably for palatal and gingival cysts of newborns
- Alveolar Cyst:
- Gingival cysts of newborn occurring along alveolar ridge
Epidemiology
- Gingival cysts of newborn arise after fourth month in utero, during tooth development
- Common, some estimate at least 50% of newborns have gingival cysts that spontaneously resolve
Sites
- Occur along alveolar ridge, while palatal cysts occur along midline of posterior hard palate or anterior soft palate
Pathophysiology
- Arise from epithelial remnants of deeply budding dental lamina after fourth month in utero and are present at birth
Clinical features
- Multiple (usually fewer than six), yellow-white sessile to pearl-like papules, 1 - 4 mm
- Superficial (rupture easily)
- Some resemblance to milia
Case reports
- 7 day old boy with Bohn nodules mistaken for natal teeth (Eur J Pediatr 2014;173:403)
Treatment
- None; spontaneously rupture with cyst lining incorporated into oral mucosa
Microscopic (histologic) description
- Small cysts lined with attenuated stratified squamous epithelium, fibrovascular stroma, without inflammatory cells
- Cysts are filled with keratin debris and concentric layers of keratin
Differential diagnosis
- Epidermoid cyst: most common location is floor of mouth, large size
- Congenital eruption cysts around natal teeth
Additional references
Glandular odontogenic cyst
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Locally aggressive developmental cyst occurring within the jaws, recognized by WHO in 1992 as odontogenic in origin
- Noteworthy for locally aggressive growth, potential for recurrence and differential diagnostic considerations
- While generally accepted as odontogenic in origin, lesion demonstrates glandular features including presence of cuboidal / columnar cells, mucin production, and cilia
- Highlights pluripotentiality of odontogenic epithelium
Terminology
- Glandular odontogenic cyst (GOC), accepted by WHO 1992
- Sialo-odontogenic cyst
- Polymorphous Odontogenic Cyst
- Mucoepidermoid Odontogenic Cyst
Epidemiology
- Rare lesion, 0.2% of all odontogenic cysts
- Average age at diagnosis is 51 years
- Peak occurrence in 5th to 7th decades
- No gender predilection
- Distinct peak frequency in sixth decade, particularly in men
Sites
- Mandible is most common location, 80%
- 55% occur in anterior mandible
- Maxilla affected in 20% cases
- 88% in anterior maxilla, usually canine area
Etiology
- Probable origin is rests of dental lamina
Clinical features
- Can present with painful swelling (most common symptom is swelling) or paresthesia
Diagnosis
- Diagnosis made by correlating microscopic findings, location of cyst and jaw imaging studies
Radiology description
- Variable: unilocular or multilocular radiolucent lesions with well defined borders on plain radiography
- Scalloped borders possible, sclerotic borders less common
- Tooth displacement 50%, tooth root resorption 30%, association with unerupted tooth or teeth 11%
- Can mimic other 'classic' developmental jaw cysts
- Dentigerous cyst: radiolucent lesion associated with unerupted tooth
- Lateral periodontal cyst: radiolucent lesion located between roots of mandibular premolar-canine area
- So called "globulomaxillary" position: inverted pear shaped or inverted tear drop shaped radiolucent lesion in maxilla located between roots of canine-premolar area
Radiology images
Images hosted on other servers:
Prognostic factors
- In some series, 20 - 50% recurrence rate
- Multiple recurrences possible
- Time to recurrence variable: may depend on initial size of lesion, method of treatment, method of postoperative surveillance (plain radiography vs cross sectional imaging)
- Interval in literature ranges 2 to 13 years
Treatment
- Range of interventions described: enucleation, curettage, +/- peripheral ostectomy, marsupialization segmental mandibulectomy, marginal mandibulectomy
- More aggressive initial surgery may reduce rate of recurrence, although no definitive studies
- Treatment influenced by size of lesion, involvement of teeth, bone compromise present, cortical bone perforation, history of recurrence, proximity to vital anatomic structures, patient desires and other patient related variables
Clinical images
Microscopic (histologic) description
- Nonkeratinized or slightly basaloid epithelial cyst lining which may exhibit a number of microscopic parameters
- The combination of specific microscopic parameters is important in making an accurate diagnosis
- Presence of 7 or more microscopic parameters predictive of diagnosis; see Table 1 - Head Neck Pathol 2011;5:364
- Most helpful microscopic parameters: Table 2 - Head Neck Pathol 2011;5:364
- Intraepithelial microcysts, crypts or duct-like spaces:
- Most commonly lined by a single layer of cuboidal to columnar cells similar to surface cells, less commonly lined by mucous goblet cells
- Microcysts:
- May contain mucous pools, eosinophilic material, or appear empty
- May open onto surface of lining epithelium
- May give glandular or pseudoglandular structure appearance
- Epithelial spheres or plaque-like thickenings:
- Epithelium in these plaques exhibits swirling or spherule formation
- May protrude into cyst lumen or extend into underlying connective tissue wall
- Identical to structures present in lateral periodontal cysts or botryoid odontogenic cysts or soft tissue odontogenic cyst, gingival cyst of adult
- Multiple cyst compartments:
- Multiple cystic spaces similar to those seen in botryoid odontogenic cysts
- Variable thickness of the cyst lining
- Papillary projections or "tufting" of epithelium into cyst lumen:
- Papillary projections may be formed by several microcysts opening onto luminal surface of the cyst lining or be formed independent of microcysts
- Clear or vacuolated cells:
- Cells with clear cytoplasm which may be present in basal or parabasal layers
- In areas of attenuated cyst lining, clear basal cells may be directly subjacent to the surface eosinophilic cuboidal cells
- Surface eosinophilic cuboidal cells (hobnail cells):
- Present on luminal surface of cyst lining; resemble cuboidal cells of the reduced enamel epithelium that lines dental follicles and dentigerous cysts
- May demonstrate cilia
- Not specific for GOC diagnosis
- Apocrine snouting of hobnail cells:
- Hobnail cells may demonstrate "pinching off" of surface similar to decapitation secretion seen in cells that line apocrine gland ducts
- Mucous goblet cells:
- Present individually or in small clusters on cyst lumen surface or within cyst lining
- May also line microcysts
- Not specific for GOC diagnosis
- Ciliated cells:
- True cilia may be present on surface of eosinophilic cuboidal cells
- Are distinct from apocrine snouting
- Solid islands of odontogenic epithelium in connective tissue wall:
- May have microcyst formation present
- Intraepithelial microcysts, crypts or duct-like spaces:
Microscopic (histologic) images
Contributed by Kelly Magliocca, D.D.S., M.P.H.
Images hosted on other servers:
Differential diagnosis
- Helpful to consider microscopic parameters in combination with radiographic and clinical / historical data:
- Associated with unerupted tooth / teeth, no prior history of cyst removed from same area, no prior history of major oral surgery in same anatomic area:
- Associated with the root of erupted tooth / teeth, no prior history of odontogenic cyst removed from same area:
- Radicular cysts: can have mucous cell metaplasia
- Odontogenic keratocyst/KOT
- Central mucoepidermoid carcinoma
- Botryoid cyst/lateral periodontal cyst
- Surgical ciliated cyst
- Edentulous area or associated with the root of erupted tooth / teeth, positive history of odontogenic cyst removed from same area:
- Odontogenic keratocyst/KOT
- Central mucoepidermoid carcinoma
- Botryoid cyst
- Recurrent glandular odontogenic cyst
Inflammatory collateral cyst
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Inflammatory odontogenic cyst occurring at the crown or root of a partially or fully erupted tooth
- Includes paradental cyst and buccal bifurcation cyst
- Is a term applied to a spectrum of clinicoradiographic entities - see terminology
Terminology
- Reduced enamel epithelium (REE): ameloblastic and epithelial cells from the outer enamel that overly an unerrupted tooth, as the REE degenerates the underlying tooth is exposed
- Crevicular epithelium: epithelium lining the inner aspect of the gingival sulcus
- Epithelial rests of Malassez: discrete clusters of residual cells from Hertwig epithelial root sheath
- Inflammatory odontogenic cysts: distinguished from developmental odontogenic cysts by their association with inflammation
- Clinicoradiographic variants include:
- Apical radicular cyst
- Lateral radicular cyst
- Residual cyst
- Paradental cyst
- Clinicoradiographic variants include:
- Buccal bifurcation cyst (BBC):
- Aka paradental cyst, juvenile paradental cyst or mandibular infected buccal cyst
- Occurs along the buccal root surface of partially erupted mandibular molar of children and young adults
- Affected tooth is vital
- Paradental cyst:
- After excluding cysts occurring along the lateral / buccal surface of a partially impacted mandibular molar of a young individual, the term paradental cyst also refers to a variant of the dentigerous cyst with an inflammatory, rather than developmental pathogenesis
- The two most common scenarios include vital (non-necrotic) teeth:
- A dentigerous cyst that develops around the crown of an unerupted permanent tooth as a result of periapical inflammation from an overlying primary tooth
- A partially erupted mandibular third molar that develops an inflamed cystlike lesion along the distal aspect associated with inflammation or recurrent pericoronitis
- These are often called dentigerous cyst, as it is impossible to determine histopathologically whether the inflammatory component is primary or secondary in nature
Epidemiology
- 1 - 5% of odontogenic cysts
Sites
- Buccal >> Mesial surface
- Erupted or partially erupted teeth
- Molars >> premolars > cuspids
- 3rd molar (wisdom teeth) >> 1st / 2nd molars
- Mandibular teeth >> Maxillary teeth
- ~24% of paradental cysts in 1st or 2nd molars are bilateral
Pathophysiology
- Theorized to arise from one of the following, however, each can be debatable given the specified location of paradental cysts:
- Reduced enamel epithelium
- Epithelial rests of Malassez
- Crevicular epithelium
- Epithelial remnants of dental lamina
Clinical features
- Recurring periodontal inflammatory process (pericoronitis)
- Symptoms: discomfort, swelling, tenderness, pain
- Often Asymptomatic
Diagnosis
- Based on location, radiography, pathology, root vitality studies
Radiology description
- Periosteal reaction common
- Onion skin deposition of bone appears as parallel opaque layers
Radiology images
Images hosted on other servers:
Prognostic factors
- Good prognosis
- No reports of recurrence to date
Case reports
- 8 year old boy with paradental cyst of first molar (J Indian Soc Pedod Prev Dent 2012;30:343)
- Two cases involving paradental cysts (J Periodontol 2006;77:1602)
- Paradental cyst mimicking a radicular cyst on the adjacent tooth (J Endod 2003;29:73)
Treatment
- Curettage
Clinical images
Gross description
- A cyst-like soft tissue (may or may not be intact) attached to the cementoenamel junction, or along the root of the tooth
Microscopic (histologic) description
- The microscopic findings are not specific and cannot distinguish between the variants of inflammatory odontogenic cysts, or a markedly inflamed developmental dentigerous cyst
- Clinical and radiographic correlation essential
- Connective tissue wall
- Heavy mononuclear inflammatory cell infiltrate
- Hyperplastic nonkeratinizing stratified squamous epithelium
- Often with hemosiderin pigment or cholesterol clefts
Microscopic (histologic) images
Differential diagnosis
- Apical radicular cyst
- Dentigerous cyst, markedly inflamed
- Inflamed periodontal pocket / periodontal tissues
- Lateral radicular cyst
- Residual cyst
Additional references
Juvenile trabecular ossifying fibroma and psammomatoid ossifying fibroma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Immunohistochemistry & special stains | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Juvenile ossifying fibroma (JOF) is a rare variant of ossifying fibroma (OF), a benign fibro-osseous lesion
- It has aggressive behavior
- It has two histologic subtypes:
- Juvenile psammomatoid ossifying fibroma
- Juvenile trabecular ossifying fibroma
Essential features
- Diagnosis of JOF is dependent on clinical, radiologic and pathologic correlation
- JOF, OF, fibrous dysplasia (FD) and osseous dysplasia may show significant histologic overlap particularly on small biopsies, however, clinical / surgical management of these entities is markedly different
- IHC is typically not useful to distinguish the entities
- Identifying fibro-osseous lesions which occur in association with hyperparathyroidism-jaw tumor (HJT) syndrome is important, as HJT is associated with development of other tumors, including carcinoma
Terminology
- Juvenile ossifying fibroma (JOF)
- Active ossifying fibroma (AOF)
- Juvenile active ossifying fibroma (JAOF)
- Juvenile trabecular ossifying fibroma (JTOF)
- Juvenile psammomatoid ossifying fibroma (JPOF)
Epidemiology
- The average age of JPOF is younger than conventional OF, with a slight male predominance
- Most cases occur in patients < 12 years old, though a wide age range has been reported (3 months to 72 years)
- JTOF primarily affects males, ages 8 - 12
Sites
- JPOF mainly occurs in the bony walls of the paranasal sinuses
- Paranasal sinus involvement is overall the most common location for JOF
- JTOF occurs more often in the maxilla
Etiology
- Ossifying fibromas are thought to originate from the periodontal ligament
Clinical features
- Can demonstrate rapid growth, causing local destruction and facial asymmetry
- Paranasal sinus or orbital bone involvement can cause nasal obstruction, proptosis, exophthalmia and visual changes
- Some lesions are found incidentally on routine radiographic exams
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Radiology description
- Well circumscribed lesion that can be radiolucent, mixed or radiopaque depending on the degree of calcification and presence of cystic areas
- On CT, well circumscribed lesion with mixed soft tissue and bone density
- May have thin "egg shell" periphery of bone
- Usually unilocular
Radiology images
Prognostic factors
- Excellent prognosis following complete excision
- 30 - 58% recurrence rate for incompletely excised tumors
Case reports
- 7 year old girl with firm swelling localized at the upper right maxillary region (Med Arch 2016;70:470)
- 10 year old boy with swelling of maxilla (Arch Pathol Lab Med 2003;127:e359)
- 15 year old boy with rapidly growing expansile lesion of jaw (Arch Pathol Lab Med 2001;125:1117)
- 18 year old man with 6 weeks of headache and blurred vision (Springerplus 2016;5:1089)
- 20 year old man with swelling in the right lower jaw (Contemp Clin Dent 2015;6:581)
- 27 year old man with long standing nasal congestion and recurrent sinus infections (Head Neck Pathol 2015;9:384)
Treatment
- Surgical excision
Clinical images
Microscopic (histologic) description
- Juvenile trabecular ossifying fibroma
- Cellular fibrous stroma composed of spindled to stellate fibroblastic cells with bands of osteoid without osteoblastic rimming together with immature bony trabeculae surrounded by plump osteoblasts
- Trabeculae can show an anastomosing or "lattice" pattern
- Mitoses can be present but without cytologic atypia
- Juvenile psammomatoid ossifying fibroma
- Fibroblastic stroma containing ossicles resembling psammoma bodies
- Ossicles may fuse to form trabeculae showing reversal lines
- Stroma can be cellular or loose / myxoid
- Fibroblastic stroma containing ossicles resembling psammoma bodies
- Both can have aneurysmal bone cyst type changes
- Pseudocystic stromal degeneration and hemorrhages
Microscopic (histologic) images
Contributed by Kelly R. Magliocca, D.D.S., M.P.H.
Images hosted on other servers:
Immunohistochemistry & special stains
- Stromal cells positive for RUNX2, an important transcription factor for the osteogenic lineage
- IHC may not be useful as other entities in differential, such as fibrous dysplasia, are RUNX2 positive
Molecular / cytogenetics description
- HPRT2 mutations have been found in OF (25 - 50%) associated with hyperparathyroidism-jaw tumor syndrome
Differential diagnosis
- Ossifying fibroma
- Conventional OF is more common than JOF
- May have similar radiographic appearance although conventional ossifying fibroma typically has a thin capsule
- More commonly occurs in the mandible
- Fibrous dysplasia
- Radiographic appearance is usually radiolucent, asymmetric with a "ground glass" appearance that blends into normal bone
- Histologically, has immature woven bone characterized by "Chinese letters" or "alphabet letters"
- Bony trabeculae usually without osteoblastic rimming
- Fibrous dysplasia tends to blend with surrounding bone and this continuity of normal and pathologic bone is not a typical feature of JOF
- Associated with GNAS mutations
- Osseous dysplasia
- Idiopathic process involving the periapical areas of teeth
- Many different types depending on location and extent of involvement
- Characterized by replacement of normal bone by metaplastic bone and fibrous tissue
- Cementoblastoma
- Neoplasm of cementum intimately involved with tooth root
- Radiographically is a radiopaque mass with a narrow radiolucent rim (pathognomonic)
- Histologically, consists of dense cementum-like tissue with reversal lines and fibroblastic stroma
- Most are in mandible
- Extra cranial (extra dural) meningioma
- Psamommatous meningioma variant most likely to create confusion
- Will be Somatostatin receptor subtype 2A (SSTR2A), EMA and variably PR positive
Additional references
Langerhans cell histiocytosis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Most common histiocytic disorder characterized by aberrant function and differentiation or proliferation of mononuclear phagocyte system cells
- Langerhans cell histiocytosis (LCH) (clonal neoplasm of myeloid dendritic cells) expresses CD1a and CD207 (Langerhans cell phenotype)
Essential features
- LCH is particularly a disease of childhood (in adults it is less common)
- Most commonly presents as an incidental lytic bone lesion and a skin lesion
- Characteristic LCH cell (large cell with nuclear grooves that are CD1a and CD207 positive) in an eosinophil rich mixed inflammatory background
- Cytoplasmic Birbeck granules (tennis racket shaped structures) on electron microscopy (pathognomonic)
- BRAF V600E mutation: therapeutic and prognostic significance (Blood 2022;139:2601)
- Stage of the disease (including high risk organ involvement) determines the prognosis
Terminology
- Langerhans cell histiocytosis (current WHO 5th edition; recommended)
- Histiocytosis X and eosinophilic granuloma (not recommended)
- Hand-Schüller-Christian disease (lytic bone lesions, diabetes insipidus [DI] and exophthalmos)
- Letterer-Siwe disease (systemic disease with spleen, liver and bone marrow involvement)
- Hashimoto-Pritzker disease (congenital, self resolving skin lesions; benign variant of LCH)
ICD coding
Epidemiology
- Annual incidence rate in the pediatric population (below 2 years) is 5 - 9 new cases per 1 million population; in adults, 1 - 2 new cases per 1 million population (Pediatr Blood Cancer 2008;51:71, Br J Haematol 2022;199:728)
- Slight male predominance
- Pulmonary Langerhans cell histiocytosis (PLCH) is more common during the third and fourth decades of life (Immunol Rev 2010;234:213)
- More commonly found in Hispanics and individuals of European descent (Blood 2016;127:2672)
Sites
- Can arise in virtually any organ system but has a particular affinity for bone (79%), skin (36%) and pituitary gland (25%)
- Among bones, skull is the most common site; less common sites include mandible and orbit (World J Pediatr 2019;15:536)
- Lung is a common site in young men with smoking history
- Other organs include liver, spleen, lymph node, gastrointestinal tract and parotid gland
Pathophysiology
- Constitutive activation of MAPK gene pathway with universal phosphorylated ERK (p-ERK) expression leading to disrupted cell migration, apoptosis inhibition and oncogene induced senescence (N Engl J Med 2018;379:856)
- Majority harbor somatic BRAF V600E mutation (most common) and MAP2K1 (second common)
- Misguided myeloid differentiation model: stage of differentiation in which the myeloid cell acquires activating MAPK mutations determines the extent of LCH
- High risk, multisystem LCH: self renewing stem or progenitor cells from bone marrow (J Exp Med 2015;212:281)
- Low risk, multisystem LCH: MAPK activation of committed dendritic cell (DC) precursors or monocytes
- Low risk, single lesion: arises from a regional dendritic cell precursor
Etiology
- Etiology of LCH remains unknown
- Cigarette smoking (chronic irritant) plays role in PLCH (N Engl J Med 2022;387:2449)
Clinical features
- Facial swelling and preauricular pain (most common) due to tooth mobility and involvement of the posterior mandibular region, indicating alveolar process damage (Medicine (Baltimore) 2019;98:e16331)
- Skin manifestations (particularly scalp): seborrheic dermatitis-like eruptions, erythematous, crusted, scaly papules and eczematous lesions (Head Neck Pathol 2021;15:41)
- Pituitary gland involvement: central diabetes insipidus (most common initial sign of central nervous system [CNS] LCH), growth hormone deficiency and thyroid dysfunction (Pediatr Blood Cancer 2018;65:e26784)
- LCH associated neuroinflammation and neurodegeneration: neurological deficit, cognitive impairment and behavioral changes
Diagnosis
- Lesional tissue biopsy for confirmation (even if the case is highly suggestive of LCH through clinical and imaging findings with a reasonable exception for single system PLCH with classic radiologic and clinical findings) (Blood 2022;139:2601)
- Baseline full body (vertex to toes) FDG PET / CT (including distal extremities) to define the extent of the disease
- Magnetic resonance imaging (MRI) of the brain with gadolinium, with a dedicated examination of the sella turcica for pituitary dysfunction and neurological symptoms
- All patients should undergo BRAF V600E mutational analysis for the diagnosis and treatment
- For wild type BRAF V600E LCH cases, next generation sequencing (NGS) can be performed to assess MAPK-ERK pathway mutations for questionable diagnosis or second line treatment
Laboratory
- Liver function tests (LFTs) to assess liver function
- Complete blood count with differential (CBC) for cytopenia
- Inflammatory markers (C reactive protein)
- Morning urine and serum osmolality and water deprivation test (to evaluate DI)
- Morning serum cortisol, thyroid stimulating hormone (TSH) and free T4, follicle stimulating hormone (FSH) / luteinizing hormone (LH), prolactin, with testosterone (men) and estradiol (women) in case of pituitary dysfunction or involvement on MRI (Blood 2022;139:2601)
- Low insulin-like growth factor 1 levels due to suspected growth hormone deficiency
- Cell free DNA analysis on peripheral blood for BRAF V600E mutation in cases of absent or insufficient tissue (variable sensitivity but category A consensus recommendation)
Radiology description
- Punched out, lytic osseous lesions, often involving flat bones (skull, sternum, ribs, pelvis)
- In jaw: expansile soft tissue mass with mixed attenuation or nonenhancing, ill defined, infiltrating lesion with hyperintense T2 signal intensity and radiolucent lesion with thinning of the cortex (mandible) on plain orthopantomogram (OPG) (Medicine (Baltimore) 2019;98:e16331)
- Upper lobe predominant nodular and cystic lung lesions in a smoker (J Thorac Imaging 2019;34:W144)
Radiology images
Prognostic factors
- Largely dependent on the extent of the disease, high risk organ involvement (brain, lung and liver) and response to therapy (Blood 2022;139:2601)
- Unifocal LCH has an excellent prognosis with a 5 year survival of > 90%
- Single system (SS) LCH (lung) has variable course depending upon pulmonary function and development of pulmonary hypertension (HTN) (death occurs due to respiratory failure)
- Multisystem (MS) LCH / disseminated LCH has improved overall 5 year survival by current therapy regimes
- Permanent consequences of diabetes insipidus and neurodegeneration have worse clinical outcome
Case reports
- 4 year old boy presented with painful swelling left cheek (Clin Case Rep 2021;9:e04321)
- 10 year old boy presented with right temporoparietal region swelling after a fall from bicycle (Int J Surg Case Rep 2021;85:106179)
- 46 year old male nonsmoker with a history of fever and dry cough, longstanding hypothyroidism and diabetes insipidus (World J Clin Cases 2021;9:11029)
- 47 year old woman presented with headache, weakness in lower extremities and urinary retention (Radiol Case Rep 2022;17:1407)
- 65 year old man with intermittent pruritis, fever and abnormal liver function tests (Cureus 2020;12:e8591)
Treatment
- Unifocal LCH: for bone, limited curettage followed by intralesional corticosteroid therapy / radiation therapy (10 - 20 Gy) in case of soft tissue component
- Single system PLCH: smoking cessation, corticosteroid and cladribine based systemic therapy (Blood 2022;139:2601)
- Multifocal multisystem LCH: cladribine and methotrexate combination
- BRAF and MEK inhibitors under trial
Clinical images
Microscopic (histologic) description
- Active lesions
- Sheets of large, round to oval cells with nuclear grooves (coffee bean appearance), abundant eosinophilic cytoplasm, minimal nuclear atypia and variable mitotic count (no atypical forms)
- Background predominantly eosinophil rich with scattered multinucleated histiocytes (LCH type and osteoclast-like); neutrophils and plasma cells are rare
- Central necrosis is common but of no prognostic significance (Int J Surg Case Rep 2021;85:106179)
- Late lesions
- Decreased number of lesional cells, accompanied by fibrosis
- Neurodegenerative lesions: collection of foamy macrophages with CD1a positive cells or perivascular infiltrate of mononuclear cells
- Biliary type lesions: portal noncaseating granuloma with eosinophil rich infiltrate and multinucleated giant cells encasing the duct
- PLCH: focal bronchiolocentric eosinophil rich inflammatory infiltrate with clusters of characteristic lesional cells, noncaseating granuloma and multinucleated giant cells
Microscopic (histologic) images
Cytology description
- Typical LCH cells have longitudinal grooves, folded nuclei and abundant pale cytoplasm, which are best appreciated on Pap stain
- Background is eosinophil rich with scattered multinucleated cells
Positive stains
- CD1a: membranous staining
- CD207 (langerin): granular cytoplasmic staining
- CD68: Golgi dot-like staining
- Cyclin D1
- Reference: Blood 2022;139:2601
Negative stains
- CD30
- CD20
- HMB45
- CD21
- Reference: Blood 2022;139:2601
Electron microscopy description
- Indented nucleus with cytoplasmic vacuoles, lamellated dense bodies and Birbeck granules (tennis racket type)
Molecular / cytogenetics description
- BRAF V600E mutation (most common)
- MAPK21 (second most common)
Videos
Radiology and histopathology descriptions
Sample pathology report
- Lytic lesion scalp, biopsy:
- Langerhans cell histiocytosis, not otherwise specified (see comment)
- Comment: The biopsy shows a histiocytic lesion with dense eosinophilic infiltrate, admixed histiocytes with folded nuclei and longitudinal grooves and scattered multinucleated osteoclast-like giant cells. The histiocytes are positive for CD1a and cyclin D1 and negative for CD68, features consistent with Langerhans cell histiocytosis. Radiological skeletal survey, 99mTc bone scan and MRI brain imaging with gadolinium / FDG PET / CT guided imaging is recommended for diagnostic staging.
Differential diagnosis
- Skin
- Angiolymphoid hyperplasia with eosinophilia (ALHE) / epithelioid hemangioma:
- Solitary / multiple skin nodules or papules (J Int Med Res 2021;49:3000605211040976)
- Head and neck region is most common (particularly scalp)
- Female predilection
- Age range: 30 - 50 years
- Microscopy: presence of abundant eosinophils, reactive lymphoid follicles and vascular proliferation (lined by plump endothelial cells)
- Kimura disease:
- Chronic inflammatory disorder (allergic disease)
- More commonly found in Asians with slight male predominance
- Usually deep seated lesion is associated with lymphadenopathy
- Elevated eosinophil count and serum IgE levels (J Int Med Res 2021;49:3000605211040976)
- Abundant lymphocytes, eosinophils (including abscesses) and plasma cells, always with lymphoid follicles
- Juvenile xanthogranuloma:
- Histiocytic (non-Langerhans cell) disorder
- Usually first year of life but can be seen in adults
- Male predominance
- Head and neck predilection (especially orbit) followed by trunk and extremities (BMC Pediatr 2022;22:87)
- Solitary / multiple yellowish papules and nodules
- Touton-like multinucleated giant cells and mononuclear cells (CD68 and CD163 positive)
- Angiolymphoid hyperplasia with eosinophilia (ALHE) / epithelioid hemangioma:
- Head and neck
- Central giant cell granuloma:
- Locally invasive, intraosseous, benign lesion
- Associated with local trauma, repair process or developmental disorder
- Mandible is most commonly affected bone followed by temporal and sphenoid bone (sella turcica)
- Clinical findings: painful jaw swelling, proptosis or headache
- Imaging: well circumscribed, radiolucent and expansile lytic bone lesion with cortical thinning (Dentomaxillofac Radiol 2021;50:20200429)
- Microscopy: numerous multinucleated osteoclast-like giant cells and mononuclear stromal cells, remote hemorrhage and hemosiderin laden macrophages (Histol Histopathol 2008;23:1151)
- Immunostains: CD68 positive and CD1a negative
- Extranodal Rosai-Dorfman disease:
- Benign histiocytic disorder
- Paranasal sinuses (particularly maxilla) and mandible are most common extranodal sites
- Clinical features: presents as nasal obstruction, proptosis or jaw swelling
- Imaging: expansile osteolytic lesion
- More commonly found in individuals who are 22 - 55 years of age with no gender predilection
- Microscopy: sheets of large histiocytes with abundant eosinophilic cytoplasm, mixed inflammatory infiltrate and emperipolesis (engulfment of eosinophils, plasma cells, lymphocytes or neutrophils by large histiocytes) (Head Neck Pathol 2020;14:442)
- Immunostains: S100, CD68 and CD163 positive and CD1a negative
- Erdheim-Chester disease (ECD):
- Rare multisystem histiocytic (non-Langerhans cell type) disorder
- Middle aged adults (mean age: 46 years); pediatric cases are exceedingly rare
- Male preponderance
- Clinical findings: retro-orbital pain and exophthalmos, orbital masses, polydipsia and polyuria (central DI), long bone pains (most common), renal insufficiency and lung function compromise (multisystem disease)
- Imaging: osteosclerotic lesions rather than punched out (as in LCH), abnormal signals in pituitary stalk on MRI
- Microscopy: foamy histiocytes with small nuclei, Touton-like and multinucleated giant cells; in some cases, infiltration of lymphocytes and plasma cells (Blood 2020;135:1929)
- Immunostains: CD68 (cytoplasmic positivity), CD163 (membranous positivity), ALK (cytoplasmic positivity) and CD1a negative
- Central giant cell granuloma:
Additional references
Board review style question #1
A 17 year old girl presented with a lesion in the pituitary fossa. Biopsy shows dense mixed inflammation (containing eosinophils) and large cells with pale cytoplasm, containing vesicular nuclei with grooves and membrane folding. Which immunohistochemical stain will be confirmatory for the diagnosis?
- CD1a
- CD30
- Chromogranin
- CK
- SALL4
Board review style answer #1
A. CD1a. The histologic findings described above are characteristic of Langerhans cell histiocytosis. CD1a is a specific marker of Langerhans cell histiocyte. Answer B is incorrect because large multinucleated cells (simulating Reed-Sternberg cells in a mixed inflammatory background) do not express CD30 stain. Answers D and C are also incorrect because the morphology shown in the images lacks features of pituitary adenoma (stained by chromogranin) or metastatic carcinoma (stained by CK). Answer E is incorrect because SALL4 is a marker of germ cell tumors and is negative in histiocytic lesions.
Comment Here
Reference: Langerhans cell histiocytosis
Comment Here
Reference: Langerhans cell histiocytosis
Board review style question #2
A 35 year old male smoker presents with a history of mild exertional dyspnea. High resolution computed tomography shows reticulonodular opacities and small cysts. The lung biopsy shows CD1a positive histiocytes, eosinophils and admixed plasma cells. The electron microscopy shows tennis racket shaped granules. Which of the following is a surrogate marker of these cytoplasmic granules?
- CD1a
- CD163
- CD207
- CD68
- S100
Board review style answer #2
C. CD207. CD207, also called langerin, is a part of type II lectin membrane protein and is responsible for the formation of Birbeck granules. Answer A is incorrect because CD1a stains membrane bound proteins rather than cytoplasmic. Similarly, answers B, D and E are incorrect because these are markers of non-Langerhans cell histiocytes.
Comment Here
Reference: Langerhans cell histiocytosis
Comment Here
Reference: Langerhans cell histiocytosis
Lateral periodontal cyst and botryoid odontogenic cyst
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Non-keratinizing, developmental odontogenic cyst which occurs along the lateral tooth root surface within bone
Terminology
- Epithelial rest of Malassez:
- Derived from Hertwig epithelial root sheath
- Small spherules of 6 - 8 epithelial cells with high nuclear to cytoplasmic ratio; little or no reverse polarity of cells
- Reduced enamel epithelium:
- Enamel is normally composed of two cell layers: inner layer of reduced or atrophied ameloblasts and external layer, probably stratum intermedium cells
- Reduced enamel epithelium is normally found overlying an unerupted, otherwise developed tooth
- Dental lamina:
- Band of epithelial tissue derived from ectoderm that initiates tooth development in utero
- After tooth development, the dental lamina disintegrates into small nests, most of which are reabsorbed, but some persist as dental lamina rests (rests of Serres)
- Gingival cyst:
- Lateral periodontal cyst (LPC) has been pathogenetically linked to the gingival cyst of the adult
- The former is believed to arise from dental lamina remnants within bone and the latter from dental lamina remnants in soft tissue between the oral epithelium and the periosteum (rests of Serres)
- The close relationship between the two entities is further supported by their similar distribution in sites containing a higher concentration of dental lamina rests and their identical histology
- Botryoid odontogenic cyst:
- Multicystic variant of lateral periodontal cyst (LPC), generally larger (5 - 45 mm) with higher recurrence rates (up to 33%) than LPC
- Controversial: some regard the botryoid odontogenic cyst (BOC) as a variant of LPC, others view BOC as distinct entity because it can be larger in size, has a polycystic radiographic appearance (occasionally can be unilocular) and has higher rate of recurrence
Epidemiology
- Usually 5th to 7th decade of life
- Rare in patients less than 30 years of age
- Favor males 2:1
- Accounts for less than 2% of all jaw cysts
- Very rarely multifocal
Sites
- Most common adjacent to roots of cuspid or bicuspid teeth
- 60 - 80% occur in mandibular premolar-canine-lateral incisor area, but favors premolar-canine region
- When occur in maxilla, usually involve this same tooth region but favors incisor area
Pathophysiology
- Origin of LPC still debated with histologic and developmental evidence from various researchers providing support for the lesion developing from either dental lamina, reduced enamel epithelium or epithelial rests of Malassez
- Most sources regard the cyst as originating from rests of dental lamina
Clinical features
- Usually asymptomatic
- Can cause painless expansion of bone
- Rarely perforates bone to communicate with gingival surface
- Associated with vital teeth, however prior endodontic treatment or tooth extraction can confound the diagnosis
Radiology description
- Radiographic appearance is not specific for LPC
- Well demarcated, round to elongated tear drop shaped radiolucent lesion associated with lateral aspect of tooth root
- Rare to exceed 1.0 cm in size
- Root divergence uncommon
- Most commonly is a solitary lesion, rarely affects multiple sites (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;111:225)
- Most cysts are unilocular but occasionally appear polycystic
- When polycystic, have been termed botryoid odontogenic cysts
Radiology images
Prognostic factors
- Good prognosis with rare recurrence
- Recurrence reported with botryoid odontogenic cyst
Case reports
- 13 year old girl with unusual clinicoradiographic presentation (J Dent (Tehran) 2012;9:265)
- 14 year old boy with bilateral lateral periodontal cyst (BMJ Case Rep 2013 May 10;2013)
- 38 year old man with pigmented lateral periodontal cyst and other pigmented odontogenic lesions (Oral Dis 1996;2:299)
Treatment
- Enucleation, curettage or excision with preservation of adjacent teeth is adequate for conventional LPC
Clinical images
Gross description
- Thin walled cyst with nodular excrescences within cyst wall
Microscopic (histologic) description
- Thin, generally non-inflamed fibrous connective tissue wall
- Non-keratinized epithelial lining of cuboidal to stratified squamous cells
- Epithelium is 2 - 5 cells thick in most areas
- Foci of PAS+ glycogen rich clear cells interspersed among lining epithelial cells
- Focal nodular areas of epithelial thickening that may have a whorled, swirling architecture and appear in continuity with the epithelial lining
- These mural epithelial plaques extend into the fibrous connective cyst wall or may protrude into cyst lumen
- Epithelial rests (may or may not be clear cells) can be seen in fibrous wall
- LPC may be histologically unicystic or multicystic; microscopic presence of multiple cystic spaces may not correlate with the typical radiographic finding of a unilocular radiolucency
- Botryoid odontogenic cyst has more pronounced mural thickenings / protrusions, comprised of multilocular cysts with thin fibrous septations and typically has a multilocular, often larger radiographic appearance
Microscopic (histologic) images
Differential diagnosis
- Cystic degeneration of, or unicystic ameloblastoma
- Gingival cyst: represents the gingival soft tissue counterpart of LPC
- Glandular odontogenic cyst
- Odontogenic keratocyst (keratocystic odontogenic tumor)
Lymphoma
Table of Contents
See also | Definition / general | Terminology | Epidemiology | Sites | Clinical features | Diagnosis | Prognostic factors | Radiology images | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Flow cytometry description | Differential diagnosis | Additional referencesSee also
Definition / general
- More commonly non-Hodgkin lymphoma (NHL) (B or T cell); also Hodgkin lymphoma
- Primary extranodal lymphomas of the mandible and maxilla are rare
Terminology
- Also called intrabony lymphoma or primary osseous lymphoma at this site
Epidemiology
- #2 most common malignant tumor of head and neck after squamous cell carcinoma (3.5% of intra-oral malignancies)
- Mean age for extranodal lymphoma: 5th decade
- Male to female ratio for lymphoma is 1.4:1
- Approximately 16% of NHLs involve the jaw bones
- 2/3 are diffuse large B cell lymphoma (DLBCL)
- Plasmablastic lymphoma, a subtype of DLBCL, more common in the oral cavity of immunocompromised / HIV patients, can also involve the jaw, and can occur in elderly immunocompetent patients
Sites
- Most head and neck lymphomas involve Waldeyer's ring
- ~1/3 of NHLs involve Waldeyer's ring
- The mandible is the most common site for osseous lymphomas followed by the maxilla
Clinical features
- Clinically, patients can present with tooth mobility, swelling, pain or a mass
- Lymphadenopathy and B symptoms (fever, night sweats, weight loss, anorexia, fatigue, etc) can also be present
- May also present as numb chin syndrome (NCS): sensory neuropathy characterized by unilateral hypoesthesia or paresthesia over the lower lip, chin and occasionally gingival mucosa
- Most common non-hematologic cause is breast cancer
- Most common hematologic cause is NHL
Diagnosis
- May be challenging because there is usually a low index of suspicion for lymphoma
- Usually diagnosed at biopsy with flow cytometry, immunohistochemistry and molecular studies to help subclassify the lesion and to help risk stratify the patient
Prognostic factors
-
Diffuse large B cell lymphoma
- Favorable: limited disease (no or <10% marrow involvement), favorable international prognostic index
- Favorable: germinal center gene expression (bcl6+, CD10+, negative for MUM1/IRF4, negative for CD138) versus activated B cell-like profile (Am J Surg Pathol 2004;28:464)
- Unfavorable: p53 mutations present
- Unfavorable: CD10+ and bcl2+ coexpression (Am J Clin Path 2001;116:183)
- Clinical course is very aggressive with most patients dying in the first year after diagnosis
- Outcome may have improved recently due to better management of HIV
- In endemic and sporadic cases, the tumor is highly aggressive but potentially curable
- Poor prognosis if bone marrow involvement, unresected tumor >10 cm and high serum LDH
- Poor prognosis - 1 year survival if multiple lesions and no treatment, but many years if indolent
Plasmablastic lymphoma
Burkitt lymphoma
Multiple Myeloma (MM)
Radiology images
Case reports
- 10 year old boy with T cell lymphoblastic lymphoma (J Clin Diagn Res 2015;9:ZD22)
- 30 year old woman (Natl J Maxillofac Surg 2011;2:210)
- 48 year old woman with involvement of mandible by non-Hodgkin lymphoma (J Postgrad Med 1995;41:90)
- 51 year old woman with non-Hodgkin lymphoma presenting with numb chin syndrome (BMJ Case Rep 2011;2011:bcr0120113712)
- 53 year old man with diffuse large B cell lymphoma of mandible (Case Rep Oncol 2015;8:451)
Treatment
- Managed by chemotherapy, radiotherapy and surgery in various combinations
Microscopic (histologic) description
-
Diffuse large B cell lymphoma
- Monotonous population of large to medium sized lymphoid cells with the morphologic features of centroblasts (large noncleaved cells), immunoblasts (round nuclei with vesicular chromatin and single large nucleolus) or cells with intermediate features
- Subtype of DLBCL most commonly associated with HIV that has predilection for oral cavity
- Characterized by diffuse infiltrate of large lymphoma cells that can also have a “starry sky” pattern like Burkitt lymphoma
- The cells can be plasmacytoid with eccentric nuclei, a single, centrally located prominent nucleoli, abundant basophilic cytoplasm and a paranuclear hof
- "Starry sky" appearance composed of monotonous, medium sized lymphocytes
- The cell size approximates the nuclear size of macrophages
- A so called "squaring off" of the cytoplasm may be encountered, as the cell borders appear to abut one another
- Sheets and aggregates of plasma cells that have abundant basophilic cytoplasm, low N/C ratio, eccentrically placed nucleus with clumped "spoke wheel" or "clock face" chromatin
- The less mature plasma cells show loose reticular chromatin, prominent nucleoli, high N/C ratio
- Mott cells (blue grapelike cytoplasmic inclusions), Russell bodies (cytoplasmic, cherry red, refractive round bodies), Dutcher bodies (intranuclear crystalline rods) can also be present
Plasmablastic lymphoma
Burkitt lymphoma (BL)
Multiple myeloma
Microscopic (histologic) images
Positive stains
-
Diffuse large B cell lymphoma: CD20, CD22, CD79a, PAX5, BCL6 (~60%), CD10 (~40%), CD5 (~10%), CD30 (~10%), BCL2 (~50%)
Plasmablastic lymphoma: CD38, CD138, IRF4/MUM1, VS38c, EBV
Burkitt Lymphoma: CD10, CD19, CD20, CD22, BCL6 and CD79a
Myeloma: Kappa or lambda light chains, CD38, CD79a, CD138, CD56+/-
Negative stains
Flow cytometry description
-
Diffuse large B cell lymphoma: clonally rearranged immunoglobulin genes, BCL2 rearranged in ~20%, BCL6 rearranged in ~30%, BCL6 mutated in ~70%, MYC rearranged in <10%
- del 17p, del 13 or 13q; t(4;14)(p16.3;q32) in 25% of cases, causing increased expression of FGFR3 (fibroblast growth factor receptor 3) and IgH
- t(11;14)(q13;q32) [cyclin D1] is usually part of complex karyotype
- May be missed by routine cytogenetics, particularly if the proliferative rate is low (Am J Clin Pathol 2000;113:831)
- Hyperdiploidy and hypoploidy
Plasmablastic lymphoma: clonal IgH chain gene rearrangement
Burkitt lymphoma: IG-MYC fusions of either t(8;14)(q24;q32), t(2;8)(p12;q24), or t(8;22)(q24;q11); t(8;14) most common
Myeloma
Differential diagnosis
- Because there is usually a low index of suspicion, the differential is broad, mainly based on radiologic findings, and includes:
- Odontogenic cysts
- Benign and malignant odontogenic tumors
- Reactive / inflammatory processes related to odontogenic infection or inflammation
- Other metastases
Additional references
Medication related osteonecrosis of jaw
Table of Contents
Definition / general | Terminology | Sites | Pathophysiology | Etiology | Clinical features | Clinical images | Diagnosis | Radiology description | Case reports | Treatment | Gross description | Gross images | Micro description of bone lesions | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Medication related osteonecrosis of the jaw (MRONJ) is an adverse drug reaction consisting of progressive bone destruction in the maxillofacial region of patients under current or previous treatment with antiresorptive and antiangiogenic medications
- In 2014, the American Association of Oral and Maxillofacial Surgeons suggested a nomenclature change from Bisphosphonate related osteonecrosis of the jaw (BRONJ) to MRONJ to accommodate the growing number of gnathic osteonecrosis cases associated with other antiresorptive (denosumab) and antiangiogenic therapies
Terminology
- Synonyms, some still in use:
- Bisphosphonate related osteonecrosis of the jaw (BRONJ)
- Bisphosphonate induced osteonecrosis of the jaws (BIONJ)
- Osteochemonecrosis (OCN)
- Osteonecrosis of the jaw (ONJ)
- Antiresorptive agent induced osteonecrosis of the jaw (ARONJ)
- Bisphosphonate associated osteonecrosis (BON)
- Acute suppurative osteomyelitis:
- Early phase of osteomyelitis and usually a suppurative (pus forming) condition
- Exists when an acute inflammatory process moves away from the site of initial infection and spreads through the medullary space of the bone and, in most cases, insufficient time has passed for the body to react to the presence of the inflammatory infiltrate
- Acute phase may lead to the chronic phase which has been arbitrarily defined as an osseous infection lasting at least 1 month
- Alveolar osteitis / Fibrinolytic alveolitis
- After extraction of a tooth, a blood clot is formed at the extraction site with eventual organization of this clot by granulation tissue and gradual replacement by bone
- Destruction of the initial clot is thought to delay the aforementioned additional series of steps required for uneventful extraction site healing and leads to clinical condition known as alveolar osteitis
- Clot is lost secondary to transformation of plasminogen to plasmin with subsequent lysis of fibrin and formation of kinins which are potent pain mediators
- Biofilm
- Collection of microorganisms often embedded in a self produced extracellular polymeric matrix which allows them to adhere to or coat to the surface of a living or inanimate structure
- Chronic osteomyelitis:
- May be classified as primary or secondary and as suppurative or non-suppurative
- Secondary chronic osteomyelitis (SCO)
- Primary chronic osteomyelitis (PCO)
- Chronic tendoperiostitis
- Although initially thought to be an obscure infectious process, the clinical presentation is similar to primary chronic osteomyelitis
- May represent a reactive alteration of bone initiated and exacerbated by chronic overuse of the masticatory muscles, predominantly the masseter and digastric
- Patients usually present with a history of parafunctional complaints
- Palpation of the temporal, masseter, medial pterygoid and the digastric muscles reveals tenderness in one or more of locations
- Radiographically, may be very similar to primary chronic osteomyelitis with diffuse sclerosing of the marrow and the cortical plate in the absence of pus formation or sequestration
- May be classified as primary or secondary and as suppurative or non-suppurative
- Chronic recurrent multifocal osteomyelitis (CRMO)
- CRMO is a relapsing inflammatory disease and is considered a type of seronegative spondyloarthropathy characterized by periods of exacerbations and remissions over many years
- Characterized by an inflammatory process presenting with findings similar to infectious osteomyelitis; however, no infectious source is identifiable
- Characterized by insidious onset of low grade fever, local swelling and bone pain
- Radiologic findings may suggest osteomyelitis, and bone seeking isotopes reveal other asymptomatic sites of involvement
- Lesions occur mainly in metaphyses of long tubular bones, as well as clavicles, spine, and pelvis; lesions are sometimes symmetrically distributed
- Precise relationship between primary chronic osteomyelitis, diffuse sclerosing osteomyelitis, CRMO and SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) is highly debated and not clear at this time
- Some consider CRMO and SAPHO as primary chronic osteomyelitis with additional extragnathic manifestations
- Chronic sclerosing osteomyelitis / diffuse sclerosing osteomyelitis:
- Although the term is usually used synonymously with primary chronic osteomyelitis, it represents a description of a strictly radiological appearance that can be caused by several similar processes, including:
- Primary and secondary chronic osteomyelitis
- Chronic tendoperiostitis
- Ossifying periostitis or Garrè osteomyelitis
- Medullary osseous infection, probably bacterial, which induces the complete sclerosis
- Favors young women, is painful and appears radiographically as medullary sclerosis
- Therapy includes antibiotics and surgical debridement and hyperbaric oxygen therapy in refractory cases
- Although the term is usually used synonymously with primary chronic osteomyelitis, it represents a description of a strictly radiological appearance that can be caused by several similar processes, including:
- Cortical bone:
- Cortical bone, synonymous with compact bone, is one of the two types of osseous tissue that forms bone
- As its name implies, cortical bone forms the cortex, or outer shell, of most bones
- Compact bone, as its name implies, is much denser than cancellous bone which is the other type of osseous tissue
- Furthermore, it is harder, stronger and stiffer than cancellous bone
- Garrè sclerosing osteomyelitis:
- In 1893, a Swiss physician, Carl Garrè, reported on patterns of acute osteomyelitis
- Garrè did not have any pathologic specimens for microscopic examination nor radiographs to augment his descriptions
- Nonetheless, his name is often associated with the condition in which periosteal duplication, or an "onion-skinning" pattern of periostitis, leads to enlargement of the jaw, usually mandible
- Involucrum:
- A sheath of new bone surrounding the sequestrum (N Engl J Med 2007; 356:e7)
- Medullary bone / medullary cavity / marrow cavity:
- The medullary cavity (medulla, innermost part) of bone is the central cavity where red bone marrow or yellow bone marrow (adipose tissue) is stored; hence, the medullary cavity is also known as the marrow cavity
- The medullary cavity has walls composed of spongy bone (cancellous bone) and is lined by endosteum, which are osteoprogenitor cells
- Osteoradionecrosis (ORN)
- Exposed, devitalized bone in radiated field with no healing for 3 months and no evidence of tumor recurrence
- Osteoradionecrosis (ORN) is considered a late effect of a radiation induced fibroatrophic process but is not considered as primary osteomyelitis
- Is more similar to aseptic / avascular necrosis of bone than primary infection of bone, although infection or bacterial colonization can occur
- Periapical granuloma
- Acute and / or chronic inflammation admixed with fibrous or granulation tissue locally at the apical or periapical region of a tooth
- Is devoid of epithelium (i.e. no cyst lining), which distinguishes it from a periapical cyst
- Periapical granuloma is located at the apex of a necrotic or partially necrotic tooth root
- SAPHO syndrome:
- SAPHO syndrome is an acronym for a complex clinical presentation that includes synovitis, acne, pustulosis, hyperostosis and osteitis
- The osseous lesions mirror primary chronic osteomyelitis and CRMO
- Unknown cause but thought to arise in genetically predisposed individuals who develop an autoimmune disturbance secondary to exposure to dermatologic bacteria
- Increased prevalence of HLA-27
- SAPHO and CRMO are distinguished from other forms of osteitis / osteomyelitis as these two diseases may have extragnathic skeletal involvement
- Sequestrum:
- Predominantly nonviable bone that has become separated during the process of necrosis from the original surrounding native bone (Medical Dictionary: sequestrum [Accessed 4 June 2018])
- Zurich Classification of Osteomyelitis:
- The Zurich classification system of osteomyelitis is primarily based on clinical course and features of imaging of the disease
- Histopathology is considered a secondary classification criterion, taking into account that findings are mostly unspecific and inconclusive when considered by themselves; however, tissue examinations of biopsies are irreplaceable for confirmation of the diagnosis, in cases of unclear and atypical clinical and radiological appearance, and moreover in excluding possible differential diagnoses
- The three major classifications are: (Baltensperger, Osteomyelitis of the Jaws: Definition and Classification (Figure 2.2))
- Acute Osteomyelitis (AO)
- Secondary Chronic Osteomyelitis (SCO)
- Primary Chronic Osteomyelitis (PCO)
- Acute Osteomyelitis (AO)
- Further subclassification of these major osteomyelitis groups is based on presumed etiology and pathogenesis of disease
- These criteria are therefore considered tertiary classification criteria
- These tertiary criteria are helpful in determining the necessary therapeutic strategies which may differ somewhat among the subgroups
Sites
- Tooth bearing sites of maxilla and mandible
- Rarely described in other locations
Pathophysiology
- Pathophysiology of the disease has not been fully elucidated but theories include:
- Inhibition of osteoclastic bone resorption and remodeling:
- Antiresorptive drugs inhibit osteoclast differentiation and function and increase apoptosis, all leading to decreased bone resorption and remodeling
- Inflammation and infection:
- Early studies identified bacteria, especially Actinomyces species, in biopsied specimens of necrotic bone removed from patients with ONJ
- The presence of bacteria has prompted studies to evaluate the possibility of a complex biofilm on exposed bone
- Inhibition of angiogenesis:
- Osteonecrosis is classically considered an interruption in vascular supply or avascular necrosis; it is the leading hypothesis in ONJ pathophysiology
- Soft Tissue Toxicity:
- Multiple cell types have exhibited increased apoptosis or decreased proliferation after exposure to bisphosphonate medications in vitro
- Immune Dysfunction
- Inhibition of osteoclastic bone resorption and remodeling:
Etiology
- Associated with multiple groups and classes of medications, primarily those with anti-resorptive and those with anti-angiogenic properties
Clinical features
- Variable depending on the underlying reason associated with the putative medication / drug
- In general, older adults, female > male
- Many with antecedent dental trauma (oral surgery, tooth extraction)
- Clinical features: pain, swelling, oral bone exposure, orocutaneous fistula, malocclusion
Clinical images
Diagnosis
- Patients may be considered to have MRONJ if ALL these characteristics are present:
- Current or previous treatment with antiresorptive or antiangiogenic agents
- Exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region that has persisted for > 8 weeks
- No history of radiation therapy or obvious metastatic disease to the jaws
- Staging of disease: (J Oral Maxillofac Surg 2014;72:1938)
- Use of these criteria helps to distinguish MRONJ from other clinical mimics (dental disease such as periapical granuloma, osteomyelitis, etc.), treatment complications (ex: osteoradionecrosis) and forms of delayed healing such as alveolar osteitis
- Exposed bone or sequestra can occur in patients not treated with antiresorptive or antiangiogenic agents; thus all the above criteria are important
Radiology description
- Plain films such as intraoral dental films or extraoral Panoramic radiography may be utilized to evaluate relation to dentition
- Depending on disease stage, computed tomography (CT) or magnetic resonance imaging (MRI) may show cancellous or cortical bone destruction, sequestration and osteosclerosis
- Periosteal new bone formation tends to favor higher stage disease
Case reports
- 51 year old woman receiving cabozantinib (Aust Dent J 2015; 60:528)
- 90 year old woman with acute nontraumatic clavicle fracture associated with long term bisphosphonate therapy (Case Rep Orthop 2014;2014:986718)
Treatment
- Management remains controversial and may be variable based on these clinical scenarios:
- Patients about to initiate antiresorptive or antiangiogenic treatment for cancer therapy or osteoporosis
- Asymptomatic patients receiving antiresorptive or antiangiogenic treatment for cancer therapy or osteoporosis
- Patients with established MRONJ
- Since universally accepted guidelines are unavailable, position papers based on expert opinion and existing evidence are often cited (Oral Oncol 2014;50:1049)
- Staging of disease: J Oral Maxillofac Surg 2014;72:1938
Gross description
- Biopsies to confirm diagnosis of osteomyelitis are often minute
- Evaluate bone margins with mandibular (see gross image below) or maxillary resection because malignancy may later be noted
Micro description of bone lesions
- In patients taking medications associated with MRONJ for malignancy, metastatic foci admixed with MRONJ may represent a diagnostic pitfall
- Histological features of MRONJ may overlap with osteomyelitis and osteoradionecrosis
- Histologic findings are variable and likely influenced by specimen presentation (segmental resection of jaw will have a spectrum of changes vs. debridement of long-standing exposed bone will represent non-viable tissue)
- Bone necrosis, empty Haversian systems, sequestrum formation, possible areas of viable bone
- May observe osteoclastic detachment, thought to be related to medication effect
- Empty Howship lacunae and viable osteoblasts possible
- Also variable increased trabecular thickness, pseudoepitheliomatous hyperplasia, acute or chronic inflammation
Microscopic (histologic) images
Differential diagnosis
- Acute osteomyelitis
- Chronic osteomyelitis
- Diffuse sclerosing osteomyelitis
- Infected cemento-osseous dysplasia
- Osteoradionecrosis
- Reactive bone due to metastatic disease foci
Melanotic neuroectodermal tumor of infancy
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- A rare, locally aggressive biphasic tumor composed of a small round blue cell (neuroblast-like) component along with larger, melanin producing epithelioid cells
Essential features
- A rapidly growing lesion in infants (usually in the first year of life) that can be associated with elevated VMA
- Histologically is a biphasic tumor composed of a small round blue cell neuroblast-like component and larger, melanin producing epithelioid cells
Terminology
- Melanotic neuroectodermal tumor of infancy (MNTI)
- Other synonyms the current WHO no longer recommends:
- Melanotic progonoma
- Retinal anlage tumor
Epidemiology
- Rare, > 90% are in infants
- Median age 5 months
- Slightly more common in males (J Oral Maxillofac Surg 2015 Oct;73:1946)
Sites
- Most cases occur in the craniofacial region, most commonly the maxilla ( > 60% of cases)
Etiology
- Thought to be neural crest origin, based on:
- Secretion of vanillylmandelic acid (VMA), characteristic of other neural crest tumors such as pheochromocytoma and neuroblastoma
- VMA levels generally return to normal when excised
Clinical features
- Usually presents as a pigmented, rapidly expansile mass
Diagnosis
- Based on clinical, radiographic and pathologic features, but most important is biphasic components with histology
Laboratory
- Can show elevated vanillylmandelic acid (VMA) levels
Radiology images
Prognostic factors
- Wide range of recurrence rates:
- One large study found local recurrence rates after resection of 10 - 15% (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:204)
- Others indicate recurrence rate is > 35% (Fetal Pediatr Pathol 2006;25:59)
- A systematic review found a metastatic rate of 6.5% (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:204)
Case reports
- 2 month old female with mass arising in fibula (BMC Cancer 2016;16:629)
- 3 month old girl with swelling of the upper gums (J Clin Diagn Res 2016;10:ZJ07)
- 3 month old with MNTI involving anterior maxilla (Ann Maxillofac Surg 2015;5:234)
- 10 month old with soft tissue mass near right elbow (Int J Clin Exp Pathol 2015;8:13584)
Treatment
- Complete local excision with clear margins
- Adjuvant therapy for recurrent or residual tumor
Clinical images
Gross description
- Lesions are usually gray to blue, firm and lobulated
Microscopic (histologic) description
- Biphasic population of cells composed of:
- Nodules of round blue cells that are small and hyperchromatic with scant cytoplasm, often termed “neuroblastoma-like”
- A second population of larger epithelioid cells arranged in
cords and nests composed of abundant pale cytoplasm and round nuclei with vesicular
chromatin
- Within the cytoplasm is melanin pigment, although it can be focal and difficult to identify
- The background consists of dense fibrosis creating the appearance of third component.
Microscopic (histologic) images
Positive stains
Negative stains
- Usually negative for S100, chromogranin
Molecular / cytogenetics description
- Limited data but occasional mutations identified:
- Germline mutation of CDKN2A and a novel RPLP1- C19MC fusion identified in 1 case (BMC Cancer 2016;16:629)
- A BRAFV600E mutation was found in 1 case (Pediatrics 2015;136:e267)
Differential diagnosis
- Ewing sarcoma
- Only 2 - 10% develop in the head and neck
- Small round blue cell tumor that grows in sheets and nests
- Can have similar appearance to “neuroblast-like” component of MNTI, but lacks the epithelioid component
- Should have rearrangements of EWSR1, unlike MNTI
- Olfactory neuroblastoma
- Most common during fifth and sixth decades
- Typically involves the cribriform plate, nasal concha and septum
- Also composed of small round blue cells
- Can have Homer-Wright rosettes imparting biphasic
morphology, but they are all the same population of cells
- These will also stain with NSE and synaptophysin like the primitive component of MNTI
- No epithelioid component with melanin pigment
- Can have Homer-Wright rosettes imparting biphasic
morphology, but they are all the same population of cells
- Desmoplastic small round cell tumor
- Rare, aggressive tumor, usually in abdomen of adolescents
and young adults
- More commonly affects men
- Composed of nests of round blue cells with variable amounts of cytoplasm and hyperchromatic nuclei surrounded by desmoplastic stroma
- Should not have the epithelioid component with melanin pigment
- Also shows EWSR1 rearrangement as in Ewing sarcoma
- Rare, aggressive tumor, usually in abdomen of adolescents
and young adults
- Rhabdomyosarcoma
- Individuals tend to be older at presentation than MNTI
- More commonly involves sinonasal tract
- Also composed of small primitive round blue cells but with scattered rhabdomyoblasts
- Embryonal most common subtype in younger children
- Should be positive for myogenic markers such as myogenin and myoD1
- Should not have the epithelioid component with melanin pigment of MNTI
- Lymphoma
- Approximately 16% of NHLs involve the jawbones
- 2/3 are diffuse large B cell lymphoma (DLBCL) which usually occur in an older age group
- Main lymphoma mimics for MNTI are lymphoblastic lymphoma or Burkitt lymphoma, which feature monotonous, small to medium size round cells, without a biphasic component
- Flow cytometry, immunohistochemistry (such as Tdt) and molecular studies would help classify and risk stratify the patient
- Approximately 16% of NHLs involve the jawbones
Board review style question #1
What rearrangement has been described in melanotic neuroectodermal
tumor of infancy?
A. EWRS1-FLI1
B. EWSR1-WT1
C. PAX3-FOXO1
D. RPLP1-C19MC
A. EWRS1-FLI1
B. EWSR1-WT1
C. PAX3-FOXO1
D. RPLP1-C19MC
Board review style answer #1
D. RPLP1-C19MC has been described in one case of melanotic neuroectodermal tumor of infancy. EWRS1-FLI1 is seen in Ewing sarcoma, EWSR1-WT1 is seen in desmoplastic small round cell tumor and PAX3-FOXO1 is seen in alveolar rhabdomyosarcoma
Comment Here
Reference: Melanotic neuroectodermal tumor of infancy
Comment Here
Reference: Melanotic neuroectodermal tumor of infancy
Metastases
Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) images | Positive stains | Differential diagnosis | Additional referencesDefinition / general
- Metastases to the jaw and oral cavity are uncommon, ~ 1% of oral malignancies
- However, up to 25% of oral cavity metastases represent the first manifestation of disease
- Metastases to the jaw often indicate end stage disease, and are associated with poor survival
Epidemiology
- Uncommon, ~ 1% of all oral malignancies
- Most common in 5th to 7th decades; mean age ~ 54 years
- Common primary sites of origin:
- Males: lung, kidney, liver, prostate
- Females: breast, gynecologic, kidney, colorectum
Images hosted on other servers:
Sites
- Jaw bones are more frequently affected than oral soft tissue
- 2 : 1 ratio
- Mandible more common than maxilla
- The gingiva is the most common site of oral soft tissue metastases
Etiology
- Pathogenesis of oral metastases is not fully understood but current hypotheses include:
- A multistage process in which cells detach themselves from the primary tumor and are transported by lymphatic or blood vessels
- In the oral soft tissues, chronically inflamed mucosa, especially gingiva, has a rich capillary network, which can trap the malignant cells and cause metastases
- Possible role of Batson’s plexus, a valveless vertebral venous network that permits the retrograde movement of tumor cells from lungs towards the face
Clinical features
- Variable; symptoms include jaw pain, swelling, paresthesias, toothache, tooth abscess, non-healing tooth infection, tooth exfoliation
- May present with mental nerve neuropathy, also known as “numb chin syndrome”
- Characterized by facial numbness along distribution of mental branch of trigeminal nerve
- Most cases that are not dental in origin are associated with malignant tumors or diffuse metastatic disease
Diagnosis
- Dependent on clinical, radiologic and pathologic correlation
Radiology description
- Varies from well circumscribed to poorly circumscribed radiolucencies
- Most metastases to jaw tend to be expansile, lytic lesions with ill defined margins and cortical destruction
Prognostic factors
- Metastases to the jaw often indicate end stage disease, and are associated with poor survival
- Mean time to death from diagnosis of jaw metastasis was 12 months versus 8 months for oral soft tissue metastasis (J Craniomaxillofac Surg 2016;44:1047)
Case reports
- 45 year old woman with pain and swelling in jaw for 3 months (J Clin Diagn Res 2015;9:ZD25)
- 58 year old man with three month history of unilateral anesthesia of chin and lip (Br Dent J 2010;208:283)
- 60 year old man with lung metastasis to mandible (J Oral Maxillofac Pathol 2015;19:385)
- 62 year old man with lung metastasis to gingiva (Iran J Med Sci 2015;40:287)
- 69 year old man with progressive hypoesthesia of corner of mouth and chin (Anticancer Res 2010;30:1819)
- 75 year old man with 3 week history of numbness over lower lip (BMJ Case Rep 2011 Apr 15;2011)
Treatment
- Depending on type of metastasis, treatment includes surgical resection, sometimes combined with radiation therapy or chemotherapy
Clinical images
Microscopic (histologic) images
Positive stains
Differential diagnosis
- The differential is broad, mainly based on radiologic findings, and includes:
- Odontogenic cysts
- Benign and malignant odontogenic tumors
- Reactive / inflammatory processes related to odontogenic infection or inflammation
- Salivary gland neoplasms
- Other metastases such as lymphoma, melanoma, etc.
Additional references
Nasopalatine duct cyst
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Prognostic factors | Radiology description | Radiology images | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Most common intraosseous, nonodontogenic cyst of jaw (maxilla)
Terminology
- Median anterior cyst
- Midline maxillary cyst
- Anterior median palatine cyst
- Incisive canal cyst
- Incisor duct cyst
Epidemiology
- Occurs in ~1% of population
- Represents 1.7 - 11.9% of all jaw cysts
- Usually adults, peak prevalence in fourth and fifth decades
- More common in males (ranges in literature from slightly more common to up to 3x more common in males than females)
Sites
- Exclusively in maxilla, located in anterior midline of hard palate
- Occasionally can produce a midline anterior maxillary swelling if cyst erodes bone of anterior maxilla
- Cysts can form within the incisive canal located in palatine bone behind alveolar process of maxillary central incisors
- Some doubt the existence of median palatine cyst as a distinct entity and characterize all nonodontogenic cysts of the midline maxilla regardless of anterior or midline location, as nasopalatine duct cysts
- Rarely, may develop within incisive papilla, the anterior soft tissue protruberance that overlies the incisive foramen
- In this instance, is termed cyst of incisive papilla, or cyst of palatine papilla
Etiology
- Two main theories:
- First: originates from spontaneous proliferation of remnants of nasopalatine duct within incisive canal
- Exact trigger that stimulates development is unknown, but factors proposed include trauma and infection
- Second: theory now out of favor; originates from trapping of epithelial remnants during embryologic fusion between nasal cavity and anterior maxilla
- First: originates from spontaneous proliferation of remnants of nasopalatine duct within incisive canal
Clinical features
- Usually asymptomatic, may have swelling of palate in relation to maxillary central incisors
- Occasionally produces a midline anterior maxillary swelling if cyst erodes bone of the anterior maxilla
- Can present with painful swelling or drainage, or tooth root displacement
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Prognostic factors
- Although extremely rare, malignant transformation (squamous cell carcinoma) has been reported
- Relapse rate varies but usually from 0 - 11%
- Hyperkeratotic features associated with higher relapse rate (closer to 30%)
Radiology description
- Differential diagnosis:
- Enlarged incisive fossa
- The incisive foramen by convention is not expected to exceed 6 mm
- A radiolucency in this region with ill defined borders is regarded as a large incisive fossa
- Distinction from a nasopalatine duct cyst can be made clinically by aspiration
- Central giant cell granuloma
- Can have similar radiologic findings
- Histologic features of central giant cell granuloma consist of a proliferation of fibrous tissue, hemorrhagic focuses, hemosiderin deposits, osteoclast-like giant cells and reactive bone formation
- Ameloblastoma
- Keratocystic Odontogenic Tumor
- Periapical (radicular) cyst
- Enlarged incisive fossa
- On radiograph and CT, is well circumscribed, rounded or heart shaped radiolucency of anterior maxilla
Radiology images
Case reports
- 26 year old woman with unusually large destructive nasopalatine duct cyst (J Maxillofac Oral Surg 2013;12:100)
- 29 year old man treated with with bilateral endoscopic endonasal marsupialization of nasopalatine duct cyst (Clin Pract 2015;5:748)
- 35 year old man with nasopalatine duct cyst (Case Rep Dent 2013;2013:869516)
- 35 year old man with nasopalatine duct cyst mistaken for clinical radicular cyst (BMJ Case Rep 2014 Mar 18;2014)
- 36 year old woman with nasopalatine duct cyst ( Indian J Otolaryngol Head Neck Surg 2013;65:385)
Treatment
- Surgical excision is most common, but marsupialization has also been performed
Gross description
- Variable size, mean diameter ~1.5 cm
- Sectioning reveals cystic and fibrous areas
Microscopic (histologic) description
- Lined by stratified squamous epithelium alone or with pseudostratified columnar epithelium (variable cilia and goblet cells), simple columnar epithelium or simple cuboidal epithelium
- Cyst wall is composed of fibrous tissue with nerves, cartilaginous rests, arteries and veins
- The nasopalatine duct contains the nasopalatine nerve and the terminal branch of the descending palatine artery
Microscopic (histologic) images
Differential diagnosis
- Glandular odontogenic cyst:
- Intraosseous developmental odontogenic cyst, may have ciliated or mucous cells within cystic lining
- Should NOT have contents of incisive foramen (peripheral nerve, cartilaginous rests, muscular vascular channels)
- Nasolabial (nasoalveolar) cyst:
- Soft tissue (nonintraosseous) cyst with histologic features similar to nasopalatine cyst
- Occurs in soft tissues of upper lip lateral to midline
- Should not have contents of incisive foramen (peripheral nerve, cartilaginous rests, muscular vascular channels)
- Periapical (radicular) cyst:
- Most common inflammatory odontogenic cyst
- Lined by stratified squamous epithelium of variable thickness, often with scattered ciliated cells
- Derived from rests of Malassez
- In nasopalatine cysts, the lamina dura is intact and the pulp is usually vital, but radicular cysts are associated with a pulpless tooth and involve a portion of the root, usually with loss of continuity of the lamina dura
- Surgical ciliated cyst:
- Postoperative "complication" with cystic expansion of respiratory epithelium within maxilla, may have ciliated or mucous cells within cystic lining
- Usually located in posterior maxilla and lacks contents of incisive foramen (peripheral nerve, cartilaginous rests, muscular vascular channels)
Additional references
Odontoameloblastoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Odontoameloblastoma (OA) is / was viewed as an extremely rare mixed odontogenic tumor with both epithelial and mesenchymal components
- Epithelial component represented by ameloblastoma
- Mesenchymal component - a compound or complex odontoma
- Not considered an entity in the current 4th edition WHO (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
Essential features
- Odontoameloblastoma (OA) is no longer considered an entity in the current 4th edition WHO (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
Terminology
- Odontoblastoma
- Adamant-odontoma
- Calcified mixed odontogenic tumor
- Soft and calcified odontoma
- Ameloblastic odontoma
Epidemiology
- Wide age range (2 - 50 years) with mean age of ~20 years (Oral Oncol 2002;38:800)
- Slight male predilection
Sites
- Molar / premolar region of maxilla or mandible
Clinical features
- Swelling, dull pain, delayed eruption of teeth, bone expansion
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Radiology description
- Radiolucent lesion that can be destructive
- Contains calcified structures resembling mature dental tissue
Prognostic factors
- Known to recur after curettage
- No long term prognostic data, given so few cases
Case reports
- 11 year old Japanese boy with a painless and large mass of the right maxillary region (J Med Case Rep 2015;9:278)
- 12 year old Caucasian girl referred for occasional pain and gingival swelling (Med Oral 2004;9:340)
- 12 year old boy with mass in upper jaw (Case of the Week #367)
- 17 year old girl with odontoameloblastoma resembling a fibro-osseous lesion (J Oral Maxillofac Pathol 2015;19:251)
- 47 year old man with a chief complaint of rapidly growing swelling on the back side of the anterior lower jaw for 3 months (J Oral Maxillofac Pathol 2011;15:60)
Treatment
- Suggested to treat similarly to ameloblastoma
Microscopic (histologic) description
- Proliferating odontogenic epithelium portion identical to that of an ameloblastoma with peripheral palisading, reverse polarization and stellate reticulum
- Generally presenting as plexiform or follicular pattern
- This epithelial portion appears intermingled with dental tissues of variable degrees of maturity
- Can show resemblance to the developing rudimentary teeth, as in a compound odontoma
- Can also show conglomerate masses of enamel, dentin and cementum, as seen in complex odontoma
Microscopic (histologic) images
Differential diagnosis
- Ameloblastic fibroma
- Composed of odontogenic epithelial component strands, cords and islands that may exhibit peripheral palisading, reverse polarization and stellate reticulum
- Primitive appearing stroma that is delicate and lobular in appearance
- Should not have the mesenchymal (odontoma) component
- Ameloblastic fibro-odontoma
- Has soft tissue component that is similar to ameloblastic fibroma
- Also contains a calcifying component composed of enamel and dentin structures
- Ameloblastoma
- Same histologic epithelial features with peripheral palisading, reverse polarization and stellate reticulum
- Should not have mixed mesenchymal component (odontoma)
- Calcifying cystic odontogenic tumor (CCOT)
- Benign cystic tumor of odontogenic origin, also known as Gorlin cyst or calcifying odontogenic cyst
- Can have ameloblastic features: columnar or cuboidal basal cells with lumen lined by tissue resembling stellate reticulum
- Will have ghost cells or anucleate epithelial cells
- Can also have dentinoid materal resembling odontoma in around 20% of cases
- Odontoma
- Compound odontoma: cluster of denticles of varying sizes
- Complex odontoma: conglomerate of dentin admixed with enamel matrix
Odontogenic / jaw cysts overview
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Microscopic (histologic) description | Positive stains | Negative stains | Electron microscopy description | Videos | Differential diagnosis | Additional referencesDefinition / general
- Oral cavity cysts exist in jaw bones and soft tissue, including gingiva
- Odontogenic: arise from tissues involved in tooth formation
- In jaw, cysts are either odontogenic or non-odontogenic in origin
Terminology
- Jaw / gnathic bones are maxilla and mandible; pathology is termed intra-osseous / central
- Pathology within oral soft tissue is termed extra-osseous / peripheral
- Odontogenic cyst: heterogenous group of lesions, classified into three groups:
- Inflammatory, such as periapical / radicular cyst
- Developmental, such as lateral periodontal cyst
- Neoplastic, such as keratocystic odontogenic tumor
- Non-odontogenic cyst: also heterogenous group of lesions, such as nasopalatine duct cyst
Epidemiology
- Most common odontogenic cyst is periapical / radicular cyst (inflammatory cyst), followed by dentigerous cyst, which is usually considered developmental but can arise from inflammation (J Investig Clin Dent 2014;5:9)
Sites
- Odontogenic cyst, inflammatory: originate in tooth bearing areas of maxilla and mandible; precise location depends on cyst type
- Odontogenic cyst, developmental or neoplastic: variable location within maxilla or mandible depending on cyst type
Etiology
- Teeth develop from bud-like invagination of ectodermal lining of primitive oral cavity epithelium
- Through various complex interactions, dental lamina guides tooth bud formation for primary (deciduous / baby) teeth and permanent (succedaneous / adult) teeth
- Each tooth bud consists of an enamel organ which has several layers, both epithelial and mesenchymal
- Crown of tooth is formed first, with root formation completed afterwards
- A specialized form of epithelium (Hertwig epithelial root sheath) directs root formation, and once complete, it degenerates, leaving epithelial rests of Malassez
- These rests are not isolated but form vague net-like structures around root of tooth within periodontal ligament (fibrous tissue connection between tooth root and bone of jaw)
- Residual dental lamina, from bud-like invagination process of tooth formation, is source of epithelial rests of Serres, located primarily in gingival soft tissues
- A third source of epithelium is reduced enamel epithelium, which arises from enamel organ (www.Embryology.ch., chapter 19, J Endod 2007;33:908)
Microscopic (histologic) description
- Odontogenic cysts are variable, depends on cyst type
- Rests of Malassez (from Hertwig epithelial root sheath): small spherules of 6 - 8 epithelial cells with high nuclear to cytoplasmic ratio; little or no reverse polarity of cells
Positive stains
- Keratogenic odontogenic cysts: CK14 (J Mol Histol 2009;40:269)
Negative stains
- Vimentin, amelogenin (J Oral Pathol Med 2012;41:272)
Electron microscopy description
Rests of Malassez:
- High nuclear - cytoplasmic ratio of epithelial cells
- Nucleus with condensed heterochromatin and 1 - 2 poorly developed nucleoli; nuclear contour is irregular with occasional deep infoldings
- Tonofilaments and relatively abundant mitochondria in most cells
- Varying amounts of glycogen granules and lipid droplets in cytoplasm
- Rough endoplasmic reticulum is poorly developed and Golgi complex is close to nucleus (Arch Oral Biol 1989;34:179)
- Variable, depends on cyst type
Videos
Eruption of teeth
Tooth development
Tooth development
Differential diagnosis
- In general, 'epithelium' within an intraosseous, gnathic location may have a broad differential diagnosis depending on the amount of epithelium present for evaluation, type of epithelium, anatomic location of lesion in gnathic bones, radiographic appearance, clinical scenario and patient demographics
- Odontogenic, inflammatory cysts: apical cyst, buccal bifurcation cyst, lateral cyst, residual cyst
- Odontogenic, developmental cysts: botryoid odontogenic cyst, dentigerous cyst, glandular odontogenic cyst, lateral periodontal cyst, orthokeratinized odontogenic cyst, primordial cyst
- Odontogenic, neoplastic cysts: calcifying odontogenic cyst, carcinoma ex-cyst, keratocystic odontogenic tumor (often referred to as odontogenic keratocyst), unicystic ameloblastoma
- Non-odontogenic cysts: dermoid cyst, epidermoid cyst, median palatal cyst, nasopalatine duct cyst, surgical ciliated cyst
- Benign odontogenic hamartomas and odontogenic tumors that may contain epithelium:
- Adenomatoid odontogenic tumor
- Ameloblastic fibro-odontoma
- Ameloblastic fibroma
- Ameloblastic odontoma
- Ameloblastoma
- Calcifying epithelial odontogenic tumor
- Central odontogenic fibroma
- Dentinogenic ghost cell tumor
- Granular cell odontogenic tumor
- Odontogenic myxoma
- Odontoma (may have secondary dentigerous cyst formation)
- Squamous odontogenic tumor
- Primary intraosseous carcinoma:
- Ameloblastic carcinoma
- Ameloblastic fibrosarcoma
- Clear cell odontogenic carcinoma
- Ghost cell odontogenic carcinoma
- Intraosseous squamous cell carcinoma
- Mucoepidermoid carcinoma
- Primary intraosseous carcinoma ex-cyst (some consider it in this category)
- Sclerosing odontogenic carcinoma
- Malignant epithelial lesions: metastatic or invading by extension into jaw bones: (Oral Oncol 2008;44:743)
- Basal cell carcinoma, by extension / perineural invasion from overlying skin, not metastasis
- Breast
- Lung
- Prostate
- Squamous cell carcinoma
- Squamous cell carcinoma, by extension from oral cavity
-
Odontogenic rests or benign regional anatomy:
- Inflamed gingival crevicular epithelium in periodontal disease; may be fragmented and appear intraosseous
- Normal dental follicle epithelium around unerupted teeth
- Rests of Malassez
Additional references
Odontogenic carcinosarcoma (pending)
[Pending]
Odontogenic fibroma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Rare, benign mesenchymal odontogenic tumor composed of mature fibrous tissue with variable amounts of inactive appearing odontogenic epithelium
- Calcification may be present
- Two common variants:
- Intraosseous or central odontogenic fibroma
- Extraosseous or peripheral odontogenic fibroma, centered in gingival tissues associated with tooth bearing regions of jaws
- Rare variants (see Microscopic (histologic) description)
Essential features
- Strands or nests of odontogenic epithelium, with or without calcification, set in a variably cellular fibrocollagenous stroma
Terminology
- Odontogenic fibroma
- Central odontogenic fibroma
- Peripheral odontogenic fibroma
Epidemiology
- Peak age is 20 - 40 years
- Peripheral odontogenic fibroma is more common than central odontogenic fibroma
- Peripheral odontogenic fibroma: occurs 2x as often in females (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Odontogenic fibroma: slight female predilection
Sites
- Mandible and maxilla affected equally
- Peripheral odontogenic fibroma
- More common along anterior gingival region
- Central odontogenic fibroma
- Most involving mandible are posterior to first molar
- Most involving maxilla are anterior to first molar
Clinical features
- Can be asymptomatic
- If symptomatic, can present with swelling and pain
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Radiology description
- Can present as a unilocular or multilocular radiolucency with distinct borders
- Root resorption and displacement of teeth have been reported
- ~10% exhibit radiopaque flakes that correlate with calcification
Radiology images
Prognostic factors
- Peripheral odontogenic fibromas
- High recurrence rate, up to 50% (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Central odontogenic fibromas
- Recurrence uncommon
Case reports
- 12 year old boy with a slow growing, asymptomatic gingival mass distal to the mandibular right first molar (Ann Med Surg (Lond) 2015;5:14)
- 14 year old boy with an asymptomatic firm and sessile gingival mass (Int J Clin Pediatr Dent 2017;10:103)
- 14 year old boy with concurrent central odontogenic fibroma and dentigerous cyst in the maxilla (J Oral Maxillofac Pathol 2017;21:149)
- 18 year old man with odontogenic fibroma of the posterior maxilla (Iran J Pathol 2016;11:435)
Treatment
- Peripheral odontogenic fibromas are usually treated by excision
- Central odontogenic fibromas are usually treated by enucleation and curettage
Microscopic (histologic) description
- Calcification can be seen in association with odontogenic epithelium and when extensive could consider classification as ossifying variant of odontogenic fibroma
- Ossifying variant of odontogenic fibroma: epithelial odontogenic islands intimately admixed with bone trabeculae (see Fig. 8 below)
- Granular cell odontogenic fibroma: variant characterized by granular cell appearance within the odontogenic epithelium or stromal granular cells
- Central odontogenic fibroma (COF) with amyloid-like protein deposition
- Characteristic COF with ovoid or globular acellular hyalinized structures and possible areas of periepithelial amyloid deposition (cuffing)
- Deposits positive for Congo red and demonstrated green birefringence under polarized light examination
- A positive immunohistochemical reaction pattern employing antibodies to odontogenic ameloblast associated protein (ODAM) confirmed that this protein is, in fact, odontogenic amyloid, as found in developing tooth germs (see Fig. 9 below)
- Central odontogenic fibroma associated with central giant cell granuloma (CGCG)
- In some cases, the COF and CGCG histologic components were not intimately admixed; rather, they were arranged in a juxtaposed yet separate dimorphic pattern
- In other cases, the two patterns comingle (see Fig. 7 below, Head Neck Pathol 2017 Aug 7 [Epub ahead of print])
Microscopic (histologic) images
Contributed by Kelly Magliocca, D.D.S., M.P.H.
Images hosted on other servers:
Differential diagnosis
-
Central odontogenic fibroma
- Ameloblastic fibroma
- Composed of odontogenic epithelial component strands, cords and islands that may exhibit peripheral palisading, reverse polarization and stellate reticulum
- Primitive appearing stroma that is delicate and lobular in appearance
- Desmoplastic fibroma
- Composed of fascicles of variably SMA positive myofibroblasts
- Consider desmoid tumor of bone
- Hyperplastic dental follicles with odontogenic fibroma-like islands
- Clinicoradiographically enlarged follicle surrounding impacted tooth
- Collections of odontogenic islands, lack dispersed nature of odontogenic fibroma (Oral Surg Oral Med Oral Pathol 1988;66:78)
- Odontogenic myxoma
- Spindled to stellate cells with eosinophilic cytoplasm set in myxoid matrix
- Binucleated "heart shaped" cells, mitoses and minimal atypia can be seen
- If abundant collagen is present, the term myxofibroma or fibromyxoma has been used
- Occasional scattered epithelial rests can be seen
- Sclerosing odontogenic carcinoma
- Rare, primary intraosseous carcinoma of the jaws with bland cytology, markedly sclerotic stroma and aggressive pattern of infiltration
- Single file thin cords, nests and strands of epithelium in a densely sclerotic stroma
- Epithelium or stroma may dominate in different areas
- Epithelium may be so compressed so as to be best seen with immunohistochemistry (such as cytokeratin AE1 / 3)
- Cytologically, individual epithelial cells are bland with uncommon mitoses
- Cytoplasm may show vacuolation or partial clearing
- Despite the bland cytological appearance, there is invasion of skeletal muscle and commonly, perineural invasion
Peripheral odontogenic fibroma
- Irritation fibroma
- Nodular lesion with a dermal proliferation of haphazard collagen bundles in response to local irritation
- Peripheral giant cell granuloma
- Proliferation of multinucleated giant cells, plump oval mononuclear cells and hemorrhage
- Has similar appearance to solid aneurysmal bone cyst but without USP6 rearrangements
- Pyogenic granuloma (lobular capillary hemangioma)
- Lobular proliferation of well formed, small capillary vascular channels with larger, feeder vessels
- Can show ulceration
Board review style question #1
- A mandibular mass that is radiolucent by plain radiograph, is characterized by strands or nests of inactive appearing odontogenic epithelium, with or without calcification, set in a variably cellular fibrocollagenous stroma.
- Central odontogenic fibroma
- Desmoplastic fibroma
- Odontogenic myxoma
- None of the above
Which of following is the best diagnosis?
Board review style answer #1
Odontogenic keratocyst
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Odontogenic keratocyst (OKC) is a developmental odontogenic cyst that is thought to arise from dental lamina and is characterized histologically by a thin, uniform parakeratinized stratified squamous epithelial lining with a hyperchromatic basal layer of cells
Essential features
- Intraosseous origin, maxilla or mandible
- Cystic strips of nonneoplastic, nondysplastic epithelium with a fibrous cyst wall
- Epithelial lining composed of parakeratinized stratified squamous epithelium with a hyperchromatic basal cell layer
- Treatment is surgical
- With surgical enucleation, risk of recurrence is 20 - 30% (Oral Surg Oral Med Oral Pathol Oral Radiol 2019;127:282, J Maxillofac Oral Surg 2024;23:145)
Terminology
- Keratocystic odontogenic tumor (KCOT)
- Orthokeratinizing odontogenic cyst: similar name, however, this cyst is unrelated to odontogenic keratocyst
ICD coding
- ICD-11: DA05.0 - developmental odontogenic cysts
Epidemiology
- Third most common odontogenic cyst (Dentomaxillofac Radiol 2011;40:1)
- Broad age range, with peak incidence in second to third decade and second smaller peak in older age (J Craniomaxillofac Surg 2022;50:1)
- Slight male predilection (J Craniomaxillofac Surg 2022;50:1)
- Multiple simultaneous odontogenic keratocysts in the first decade of life raise concern for inherited predisposition (basal cell nevus syndrome [Gorlin syndrome and nevoid basal cell carcinoma syndrome]) (Am J Med Genet A 2011;155A:2091)
Sites
- Mandible is affected more commonly than maxilla (J Craniomaxillofac Surg 2022;50:1)
- Posterior mandible is the most common site of occurrence
Pathophysiology
- Thought to arise from dental lamina
- Involvement of the sonic hedgehog signaling pathway plays a role in the majority of cases (Am J Surg Pathol 2020;44:553)
- Mutations of the tumor suppressor gene PTCH1 (9q22.3-q31) is common in syndrome related OKCs as well as sporadic OKCs (Nat Genet 1996;12:85, Oral Dis 2019;25:1600)
Etiology
- Unknown
Clinical features
- May be small or asymptomatic, incidentally detected on radiographs taken for unrelated reasons or for imaging to investigate delayed tooth eruption (Clin Oral Investig 2023;27:6951)
- Large lesions may produce a painless bony expansion, can displace the involved tooth
- If secondarily infected, may be associated with pain and swelling (Maxillofac Plast Reconstr Surg 2014;36:73)
Diagnosis
- Histopathology supports the diagnosis
- Lesion is intraosseous (within maxilla or mandible but may extend beyond bone borders into maxillary sinus, nasal floor or submucosa to mimic a peripheral lesion)
Radiology description
- Radiology is essential to identify landmarks and the extent of disease in the preoperative and follow up setting
- Panoramic radiograph (panorex), intraoral film or cross sectional imaging is useful depending on cyst location and size
- Most commonly a well defined, unilocular radiolucency on Xray
- May have sclerotic border (Dentomaxillofac Radiol 2018;47:20170288)
Radiology images
Prognostic factors
- Overall, excellent prognosis
- Recurrence rate, 20 - 40% following enucleation (J Maxillofac Oral Surg 2024;23:145)
Case reports
- 25 year old man with Lowe syndrome and multiple odontogenic keratocysts (Oral Surg Oral Med Oral Pathol Oral Radiol 2023;136:e171)
- 27 year old man with recurrent odontogenic keratocyst eroding into the floor of the orbit (Orbit 2022;41:368)
- 32 year old woman with carcinoma arising in odontogenic keratocyst (Oral Surg Oral Med Oral Pathol Oral Radiol 2015;120:e204)
Treatment
- Surgical approach varies and factors for consideration include age, maturity, skeletal maturity, anatomic position, history of local recurrence, relative importance of tooth involved (if any), size of cyst, presence of additional pathology including but not limited to pathologic fracture, risk for fracture once removed, patient preference (risk versus benefits, acceptable risk of recurrence, cosmetic and functional considerations) (J Maxillofac Oral Surg 2024;23:145)
- In small lesions, enucleation and curettage of the entire cyst is most common
Clinical images
Gross description
- Surgical specimens usually consist of multiple, irregular fragments and may contain white or yellow keratinous debris (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:543)
- Possible minute bone fragments
- In cases of curettage type treatment, all mucosal, soft tissue and bone fragments should be submitted for histologic examination to definitively confirm the diagnosis
- In resection cases, sampling surgically sectioned bone margins and relationship of cyst to bone and bone margins
- Cyst lining is generally thin (0.1 - 0.2 cm in thickness), white and may have areas of folds and furrows but lacks papillary excrescences
- Associated tooth received as specimen can be described grossly and supplemented with photographs where equipment is available
Gross images
Frozen section description
- Uninflamed cysts show characteristic uniform epithelial lining, basal layer hyperchromatism and absent rete ridge formation
- Histologic distortion is possible in the presence of inflammation
- Inflamed cysts may show multilayered stratified squamous epithelium making it difficult to distinguish from unicystic ameloblastoma, luminal subtype or inflamed dentigerous cyst
- Frozen section limitations include sampling bias, frozen section artifact and inability to perform immunohistochemistry such as BRAF V600e (J Oral Maxillofac Surg 2014;72:914)
Frozen section images
Microscopic (histologic) description
- Uniform epithelial lining 6 - 8 cells thick lacking rete ridges
- May have artifactual clefting between epithelium and underlying fibroconnective tissue
- Epithelium is characterized by palisaded hyperchromatic basal cell layer composed of cuboidal to columnar cells
- May have areas of budding growth from the basal cells
- Luminal surface has wavy (corrugated) parakeratotic epithelial cells
- Lumen may contain keratinaceous debris
- Maxillary cysts can extend into maxillary sinus (Cureus 2022;14:e21002)
- May have satellite cysts (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:328)
- Rarely reported in association with psammomatoid calcifications (Oral Oncol 2023;147:106618)
Microscopic (histologic) images
Cytology description
- Isolated groups of keratinocytes with no nuclear atypia in a background of keratin debris (Cytopathology 2007;18:361)
- Full thickness epithelial strips may demonstrate basal layer palisading
Cytology images
Positive stains
- No specific immunohistochemistry required for odontogenic keratocyst
- Squamous epithelium is expected to be immunoreactive for cytokeratin AE1 / AE3, p63 and p40
Negative stains
- BRAF immunostain
- Beta catenin immunostain negative for nuclear staining
Molecular / cytogenetics description
- Sonic hedgehog pathway alterations are common in odontogenic keratocysts, specifically PTCH1 inactivating mutations (Am J Surg Pathol 2020;44:553)
Videos
Odontogenic keratocyst - histopathology
Sample pathology report
- Left mandible lesion, enucleation and curettage:
- Odontogenic keratocyst
Differential diagnosis
- Orthokeratinized odontogenic cyst:
- Similar name, however, this cyst is unrelated to odontogenic keratocyst with limited risk for recurrence and no association with basal cell nevus syndrome
- Uniform stratified squamous epithelium lining with prominent granular layer
- Cuboidal to flat basal layer, no basal palisading
- No surface corrugation
- Thick luminal lamellated keratin (onion skin-like)
- Calcifying odontogenic cyst:
- Cystic basaloid odontogenic epithelium with palisading basal cells, loosely arranged suprabasal epithelial cells resembling stellate reticulum, variable numbers of ghost cells in suprabasilar epithelium
- Dystrophic calcifications
- Paucicellular, eosinophilic partially calcified material (dentinoid)
- Beta catenin nuclear positive
- Unicystic ameloblastoma:
- Palisading of columnar basal cells with hyperchromatic nuclei
- Generally exhibits reverse polarization of nuclei (away from basement membrane)
- Stellate reticulum is often less distinct
- Mandibular lesions often have BRAF p.V600E mutations (Int J Oral Maxillofac Surg 2024;53:122)
- Dentigerous cyst:
- Fibrous to fibromyxoid connective tissue
- No rete ridges, flat interface
- Lining epithelium, 2 - 4 layers of cuboidal epithelium, devoid of superficial keratinization
- Occasional mucous cells; rare ciliated cells
- Occasional dystrophic calcifications
- Odontogenic epithelial rests, small, inactive appearing within fibrous wall
- Radicular / periapical cyst:
- Radicular cyst (RC) is an inflammatory odontogenic cyst associated with the root of a nonvital tooth
- Surfaced by nonkeratinized stratified squamous epithelium
- Often characterized by an arcading pattern
- Cyst wall is composed of inflamed fibrous tissue, may have foamy histiocytes
- Carcinoma cuniculatum:
- Sampling of the more deeply positioned cystic burrows of carcinoma cuniculatum bears resemblance to OKC in some cases
- Very well differentiated subtype of carcinoma
- Prominent intercellular bridges, minimal atypia and rare mitotic figures
- Can have keratin cysts
- May have superficial vacuolated clear cells
- Often associated with inflammatory infiltrate that may obscure tumor stroma boundary
- Intraepithelial abscess formation is common
Additional references
Board review style question #1
Board review style answer #1
D. Well defined layer of palisading hyperchromatic basal cells without reverse polarity. Odontogenic keratocyst has a well defined layer of palisading hyperchromatic basal cells without reverse polarity. Answer C is incorrect because the stellate reticulum-like suprabasal layer is common in ameloblastoma and can also be seen in calcifying odontogenic cyst. Answer B is incorrect because odontogenic keratocyst is a benign lesion; an infiltrative lesion with mitotic activity is concerning for malignancy. Answer A is incorrect because if a granular cell layer is present, consider the possibility of an orthokeratinized odontogenic cyst.
Comment Here
Reference: Odontogenic keratocyst
Comment Here
Reference: Odontogenic keratocyst
Board review style question #2
Which of the following sites is most commonly involved by odontogenic keratocyst?
- Anterior mandible
- Anterior maxilla
- Maxillary sinus
- Posterior mandible
Board review style answer #2
D. Posterior mandible. The posterior mandible is the most common site of involvement for odontogenic keratocyst. Answer B is incorrect because a cystic lesion in the anterior maxilla, specifically in the midline, could represent a nasopalatine duct cyst. Answer A is incorrect because odontogenic keratocyst occurs in the anterior mandible but this is not the most common site. Answer C is incorrect because odontogenic keratocyst occurs in the maxilla with extension into the maxillary sinus but this is not the most common site of occurrence.
Comment Here
Reference: Odontogenic keratocyst
Comment Here
Reference: Odontogenic keratocyst
Odontogenic myxoma / fibromyxoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare, benign odontogenic neoplasm resembling odontogenic ectomesenchyme
- Characterized by bland spindled to stellate cells in myxoid stroma
- Requires radiographic and clinical correlation to differentiate from a dental follicle / papilla
- References: Head Neck Pathol 2020;14:1021, J Oral Surg 1975;33:523, Eur J Clin Invest 2020;50:e13214, Oral Dis 2019;25:676
Essential features
- Stellate, spindle shaped, round cells arranged haphazardly in abundant, fibrillary myxoid / mucoid stroma with infiltration into surrounding bone
- Radiolucent lesion with thin, fine, coarse or wispy trabeculae of residual bone arranged perpendicular to one another (tennis racket or soap bubble appearance)
- Recurrence is common due to infiltrative nature of the neoplasm
Terminology
- Odontogenic myxoma
- Odontogenic fibromyxoma
ICD coding
- ICD-O: 9320/0 - odontogenic myxoma
- ICD-10
- ICD-11
- 2E83.0 & XH48L4 - benign osteogenic tumors of bone or articular cartilage of skull or face & odontogenic myxoma
- 2E83.1 & XH48L4 - benign osteogenic tumors of bone or articular cartilage of lower jaw & odontogenic myxoma
Epidemiology
- Incidence is 0.5 - 17.7% of all odontogenic tumors of the jaw (Head Neck Pathol 2020;14:1021, Eur J Clin Invest 2020;50:e13214, J Oral Pathol Med 2016;45:599)
- Wide age range but generally occurs in second and third decades (Head Neck Pathol 2020;14:1021, Eur J Clin Invest 2020;50:e13214)
- More common in women but may vary by population (Head Neck Pathol 2020;14:1021, Eur J Clin Invest 2020;50:e13214, Oral Dis 2019;25:676, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:101)
Sites
- More common in mandible (67%) than maxilla (33%) (Head Neck Pathol 2020;14:1021)
- In both the mandible and maxilla, more common posteriorly (Eur J Clin Invest 2020;50:e13214)
Pathophysiology
- Not well described
- May originate from the primitive mesenchymal portion of a developing tooth or periodontal membrane (J Oral Surg 1975;33:523, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:101)
- May result from myxomatous degeneration of odontogenic fibroma (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:101)
Etiology
- Unknown
Clinical features
- Often asymptomatic, incidentally discovered by imaging (Head Neck Pathol 2020;14:1021, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:101)
- Can present with slow growing swelling, rarely pain (Head Neck Pathol 2020;14:1021, Oral Dis 2019;25:676)
Diagnosis
- Diagnosis is often made by radiographical evaluation and correlated clinical and pathologic findings (Head Neck Pathol 2020;14:1021, Eur J Clin Invest 2020;50:e13214)
Radiology description
- Unilocular or multilocular radiolucency (Head Neck Pathol 2020;14:1021, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:101, Int J Dent 2021:2021:1093412)
- Variable margins from well defined and corticated to poorly defined scalloped (Head Neck Pathol 2020;14:1021)
- Characteristic radiographic finding of thin, fine, coarse or wispy trabeculae of residual bone arranged perpendicular to one another (tennis racket or soap bubble appearance) (Head Neck Pathol 2020;14:1021, Eur J Clin Invest 2020;50:e13214)
Radiology images
Prognostic factors
- Recurrence rates are variable, 10 - 43% and related to extent of excision (Head Neck Pathol 2020;14:1021, Eur J Clin Invest 2020;50:e13214)
Case reports
- 14 year old boy with maxillary mass (BMJ Case Rep 2020;13:e234933)
- 18 year old man with maxillary mass (J Clin Diagn Res 2015;9:ZD29)
- 22 year old woman with right mandibular mass (Head Neck Pathol 2018;12:44)
- 28 year old woman and 36 year old man both with maxillary masses (Contemp Clin Dent 2015;6:131)
Treatment
- Surgical excision is mainstay of treatment (Head Neck Pathol 2020;14:1021, Eur J Clin Invest 2020;50:e13214, Oral Dis 2019;25:676, J Clin Exp Dent 2021;13:e637)
- Varies from curettage to surgical resection with 1 cm wide margin (Oral Dis 2019;25:676)
Clinical images
Gross description
- Whitish gray gelatinous loose structure (Head Neck Pathol 2020;14:1021)
Gross images
Microscopic (histologic) description
- Stellate, spindle shaped, round cells arranged haphazardly in abundant, fibrillary myxoid / mucoid stroma (Head Neck Pathol 2020;14:1021)
- Infiltrates surrounding bone (Eur J Clin Invest 2020;50:e13214)
- Small islands of inactive odontogenic epithelial rests can be seen (Head Neck Pathol 2020;14:1021)
- If abundant collagen is present, the term fibromyxoma is used (Head Neck Pathol 2020;14:1021, Eur J Clin Invest 2020;50:e13214)
Microscopic (histologic) images
Positive stains
- SMA
- β catenin: nuclear positive staining is rarely reported (Am J Surg Pathol 2023;47:1301)
Negative stains
Molecular / cytogenetics description
- MAPK / ERK pathway activation (Oral Oncol 2011;47:325)
Sample pathology report
- Mandible, segmental resection:
- Odontogenic myxoma
- Margins of excision negative
Differential diagnosis
- Dental papilla or dental follicle:
- Immature dental pulp or follicle from a developing tooth
- Often requires clinical and radiographic correlation to differentiate from myxoma
- Dental papillae may show a rim of odontoblasts
- Myxoid neurofibroma:
- S100 positive
- Chondromyxoid fibroma:
- Presence of chondroid / mature cartilaginous component
- Odontogenic fibroma:
- More cellular and fibrocollagenous
- Strands or islands of odontogenic epithelium more prominent
- Infantile sinonasal myxoma (Am J Surg Pathol 2023;47:1301):
- Short, intersecting fascicles of bland, stellate to spindle cells
- Prominent stromal vessels and variably myxoid to collagenous stroma
- Neoplastic cells exhibited bipolar to stellate, fibroblastic cytomorphology, with pale pink cytoplasm, vesicular nuclei and small, distinct nucleoli
- Pushing border rather than infiltrative growth
- No dystrophic calcifications
- No rests of odontogenic epithelium
- Mitotic index low (median < 1 per 10 HPF)
- Strong and diffuse nuclear β catenin in majority of cases, rare SMA positive
- Negative for S100 protein, CD34 and desmin
- Molecular level: most tumors harbor CTNNB1 mutations (D32Y, G34E, G34R and I35S) on exon 3 or APC alterations consistent with biallelic inactivation
- Combination of patient age, tumor site and strong and diffuse nuclear β catenin expression generally distinguished infantile sinonasal myxomas from odontogenic myxoma
Board review style question #1
Board review style answer #1
D. Odontogenic myxoma. The image shows scant spindle cells in a myxoid background consistent with odontogenic myxoma. Answer B is incorrect because intraosseous mucoepidermoid carcinoma would have an epidermoid component and lack spindle cells. Answer A is incorrect because chondromyxoid fibroma contains cartilage (not seen in the image). Answer C is incorrect because odontogenic keratocyst is lined by a squamous type epithelium and lacks significant myxoid change.
Comment Here
Reference: Odontogenic myxoma / fibromyxoma
Comment Here
Reference: Odontogenic myxoma / fibromyxoma
Board review style question #2
Odontogenic myxomas have been shown to have which of the following molecular alterations?
- BRAF mutations
- GNAS mutations
- KRAS mutations
- MAPK / ERK pathway activation
Board review style answer #2
D. MAPK / ERK pathway activation. Odontogenic myxomas have been shown to have activation of the MAPK / ERK pathway. Answer A is incorrect because BRAF mutations can be seen in ameloblastomas. Answer B is incorrect because GNAS mutations are seen in fibrous dysplasia. Answer C is incorrect because KRAS mutations can be seen in adenomatoid odontogenic tumors.
Comment Here
Reference: Odontogenic myxoma / fibromyxoma
Comment Here
Reference: Odontogenic myxoma / fibromyxoma
Odontogenic sarcoma / ameloblastic fibrosarcoma
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Rare mixed odontogenic tumor that consists of a benign ameloblastic epithelium and a malignant mesenchymal stroma
- Thought to be malignant counterpart to ameloblastic fibroma
Terminology
- Ameloblastic fibrosarcoma
- Ameloblastic fibrodentinosarcoma: used when dentin is present within the malignant stroma
- Ameloblastic fibroodontosarcoma: used when enamel is present within the malignant stroma
Epidemiology
- Rare, < 100 case reports in English literature
- More common in males (1.5 - 1.6:1)
- Mean age: 3rd decade
Sites
- More common in mandible (80%); favors posterior mandible
- Can have maxillary involvement; may extend into sinus
Etiology
- Close to half (45%) arise from ameloblastic fibroma
- De novo tumors tend to occur in younger patients
Clinical features
- Patients often present with swelling and pain
- Occasionally painless facial mass with accompanying paresthesia
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Radiology description
- Appears as an expansive, multilocular radiolucent lesion
- Often shows cortical perforation
Radiology images
Prognostic factors
- Locally aggressive with high (~45%) recurrence
- Multiple recurrences are not uncommon
- Distant metastases are not as common
Case reports
- 22 year old woman and man (Case Rep Pathol 2015;2015:245026, Exp Ther Med 2014;8:1463)
- 26 year old man with ameloblastic fibrosarcoma arising from recurrent ameloblastic fibroma (Am J Case Rep 2015;16:548)
- 28 year old woman (J Oral Maxillofac Pathol 2013;17:424)
Treatment
- Surgical resection with a wide margin is the optimal treatment strategy
- Adjuvant chemotherapy and radiotherapy as needed; may reduce the recurrence rate and enhance the quality of life
Clinical images
Microscopic (histologic) description
- Biphasic with benign epithelium and malignant stroma:
- Benign odontogenic epithelium with “ameloblastic” appearance of basaloid odontogenic epithelium
- Strands, cords and nests of odontogenic epithelium
- Focally, larger tumor islands may show peripheral palisading, reverse polarization, stellate reticulum-like material
- Malignant stroma consisting of variably spindled cells with pleomorphism, hyperchromasia, increased mitoses
- Stromal cells can be arranged in a herringbone or storiform pattern
- Benign odontogenic epithelium with “ameloblastic” appearance of basaloid odontogenic epithelium
Microscopic (histologic) images
Differential diagnosis
- Ameloblastic fibroma
- Tends to occur at younger age (first two decades of life)
- Histologically, may share same features within the odontogenic epithelial component strands, cords and islands that may exhibit peripheral palisading, reverse polarization and stellate reticulum
- However, stroma is less cellular and more primitive, delicate and lobular in appearance
- Lacks sarcomatous features (pleomorphism, hyperchromasia, atypical mitoses)
- Some case reports indicate Ki-67 in the stroma is higher in ameloblastic fibrosarcoma when compared to ameloblastic fibroma (10x increase)
- Ameloblastoma
- Similar to ameloblastic fibrosarcoma and ameloblastic fibroma, may show some histological overlap of the basaloid odontogenic epithelial islands (peripheral palisading, reverse polarization, stellate reticulum)
- Does not have malignant stroma (spindle cells with pleomorphism, hyperchromasia, atypical mitoses)
- Fibrosarcoma
- Rare, malignant tumor of fibroblasts with herringbone architecture and variable collagen
- Usually deep soft tissue of lower extremities or trunk, only rarely in retroperitoneum or mediastinum
- Tends to occur in slightly older population (ages 40 - 55)
- Should have no odontogenic or ameloblastic epithelial component
Additional references
Odontoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Mixed epithelial and mesenchymal tumor-like malformation (hamartoma) composed of dental hard and soft tissues
Essential features
- Subdivided into compound odontoma and complex odontoma
- The most common odontogenic tumor
- Can obstruct the path of erupting teeth
- Most recent WHO Classification of Head and Neck Tumours regards majority of ameloblastic fibro-odontomas as developing odontomas rather than a distinct tumor (IARC: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
Terminology
- Compound odontoma
- Complex odontoma
- Ameloblastic fibro-odontoma
- Peripheral odontoma
ICD coding
Epidemiology
- Most common odontogenic tumor
- Usually diagnosed during the first two decades of life
- No sex predilection
Sites
- Can occur in any tooth bearing area
- Compound odontoma usually occurs in anterior maxilla
- Complex odontoma usually occurs in posterior mandible
Pathophysiology
- Unknown; trauma, local infection, inheritance have been proposed
Etiology
- Unknown
Clinical features
- Many asymptomatic; discovered upon exploring potential causes of
- Asymmetric tooth eruption
- Malposition of erupted teeth
- Occasional devitalization of adjacent teeth
- Can become symptomatic due to local infection (sinusitis, infected adjacent tooth, exposure of odontoma to oral environment, etc) and can present with swelling and pain
- Size: ranges from several millimeters to 6 cm, most between 1 - 2 cm
- Syndrome associations:
- Gardner syndrome (familial colorectal polyposis): multiple odontoma or supernumerary teeth
- Otodental syndrome
- Peripheral odontoma may present as a firm submucosal nodule, most commonly in the maxilla
Diagnosis
- Dependent on clinical, radiologic and pathologic correlation
Radiology description
- Frequently associated with an unerupted tooth
- Usually detected on routine radiographs
- Most commonly located between roots of erupted teeth or impeding the eruption of tooth
- Well marginated from surrounding bone
- May have a thin radiolucent region surrounding opaque mass
- Radiopaque matrix same density as components of adjacent teeth
- Early stage odontoma may have little calcified matrix, expanding the radiographic differential diagnosis
- Can occur as a component of combined odontogenic tumor, most commonly Calcifying cystic odontogenic tumor (CCOT)
- Multiple odontomas may occur as a component of:
- Gardner syndrome (familial colorectal polyposis)
- Otodental syndrome
- Multiple supernumerary teeth (similar radiographic appearance) can occur as a component of cleidocranial dysostosis or arise in non syndromic setting
Radiology images
Prognostic factors
- Recurrence uncommon for isolated sporadic odontoma
- Remaining teeth in region of odontoma may be affected by the presence of odontoma and require treatment
Case reports
- 4 year old girl with bilateral amorphic radio-opaque masses in the body of the mandible (J Craniofac Surg 2009;20:973)
- 7 year old boy with left mandibular body mass (Imaging Sci Dent 2016;46:267)
- 11 year old girl with painless nodular lesion on the palatal gingiva close to the incisive papilla (Autops Case Rep 2018;8:e2018009)
- 12 year old boy with painful swelling of the left cheek (Rom J Morphol Embryol 2013;54:1153)
Treatment
- Peripheral odontomas usually treated by excision
- Central (intraosseous) complex and compound odontomas usually treated by enucleation and curettage
- Remaining teeth in region of odontoma may be affected, requiring treatment
Clinical images
Microscopic (histologic) description
- General:
- Can have scant or occasional ghost cell formation, can cause confusion with COC (CCOT / Gorlin cyst) Calcifying cystic odontogenic tumor (CCOT)
- Odontoma can also occur as a component of COC (about 20% of COC associated with odontoma)
- Odontoma is composed of dental hard tissues; dentin and enamel
- Bone is not a dental hard tissue
- Can have scant or occasional ghost cell formation, can cause confusion with COC (CCOT / Gorlin cyst) Calcifying cystic odontogenic tumor (CCOT)
- Compound:
- Histologically similar to the layering of normal tooth in the relation of dentin, enamel matrix and pulp
- Complex:
- More disorganized or haphazard arrangement of pulpal tissues, enamel or dentin
Microscopic (histologic) images
Differential diagnosis
- Complex:
- Malformed tooth developing in area of prior trauma
- Could be histologically identical to complex odontoma
- Osteoma
- Benign neoplasm composed of mature lamellar bone (compact, trabecular or both)
- Can also affect craniofacial bones
- Should not have odontogenic epithelium
- Ameloblastic fibro-odontoma (AFO)
- Has biphasic (mesenchymal and odontogenic epithelium) "soft tissue" component that is identical to ameloblastic fibroma
- Also contains a calcifying component composed of enamel and dentin structures
- Most recent WHO Classification of Head and Neck Tumours regards most cases of AFO as developing odontomas rather than a distinct tumor (IARC: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Malformed tooth developing in area of prior trauma
- Compound:
- Supernumerary teeth
- Complete well formed teeth
- Usually in males
- Can rarely be associated with syndromes such as cleidocranial dysostosis
- Supernumerary teeth
- General:
- Calcifying cystic odontogenic tumor (CCOT)
- Benign cystic tumor of odontogenic origin, current terminology favors use of calcifying odontogenic cyst rather than CCOT (IARC: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Can have ameloblastic epithelium: columnar or cuboidal basal cells with lumen lined by tissue resembling stellate reticulum
- Will have ghost cells or anucleate epithelial cells
- Can also have dentinoid material admixed with odontogenic epithelial rests
- Separate discrete focus of odontoma may be present in approximately 20% of cases
- Calcifying cystic odontogenic tumor (CCOT)
Additional references
Board review style question #1
- A 15 year old boy is found to have multiple odontomas of the left mandibular body. Upon reviewing his history with the family, he is noted to have had other bone tumors in the past along with multiple epidermal inclusion cysts and GI polyps. What syndrome is he likely to have?
- Cowden syndrome
- Gardner syndrome
- Gorlin syndrome
- Peutz Jeghers syndrome
Board review style answer #1
B. Gardner syndrome, a variant of familial adenomatous polyposis (FAP), is an autosomal dominant disease characterized by GI polyps, multiple osteomas, skin and soft tissue tumors such as epidermal inclusion cysts and desmoid tumors.
Gorlin syndrome, also known as nevoid basal cell carcinoma syndrome, is associated with basal cell carcinomas, odontogenic keratocysts and fibromas (ovarian and heart). It is caused by PTCH1 mutations.
Peutz-Jeghers syndrome is characterized by the development of noncancerous growths called hamartomatous polyps in the GI and an increased risk of developing certain types of cancer. It is autosomal dominant and caused by STK11 mutations.
Cowden syndrome is an autosomal dominant multiple hamartoma syndrome with PTEN mutations. There is an increased risk of certain forms of cancer, including breast, thyroid, endometrial and kidney cancers.
Comment Here
Reference: Odontoma
Gorlin syndrome, also known as nevoid basal cell carcinoma syndrome, is associated with basal cell carcinomas, odontogenic keratocysts and fibromas (ovarian and heart). It is caused by PTCH1 mutations.
Peutz-Jeghers syndrome is characterized by the development of noncancerous growths called hamartomatous polyps in the GI and an increased risk of developing certain types of cancer. It is autosomal dominant and caused by STK11 mutations.
Cowden syndrome is an autosomal dominant multiple hamartoma syndrome with PTEN mutations. There is an increased risk of certain forms of cancer, including breast, thyroid, endometrial and kidney cancers.
Comment Here
Reference: Odontoma
Orthokeratinized odontogenic cyst
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- A benign developmental odontogenic cyst, mostly unilocular, with a fibrous tissue wall lined predominantly or entirely by orthokeratinized stratified squamous epithelium
Essential features
- Cystic bone lesion in mandible or maxilla with compatible radiological features
- Cyst has orthokeratinized stratified squamous epithelial lining
- Cyst wall is fibrous and uninflamed
Terminology
- Previously called orthokeratinized variant of odontogenic keratocyst (OKC) (no longer recommended to use by WHO)
Epidemiology
- Comprised 10.5% of cases in series of odontogenic keratocysts (Arch Pathol Lab Med 2010;134:271)
- Comprises probably 1% of all odontogenic cysts
- Peak incidence during third and fourth decades
- More common in males (3:1) (Arch Pathol Lab Med 2010;134:271)
Sites
- Most commonly (90.16%) involves mandible (mandibular molar and ramus) (Arch Pathol Lab Med 2010;134:271)
- Maxilla involvement in 9.84% of cases
- Multiple and bilateral cases have been reported
Pathophysiology
- Thought to originate from dental lamina and its remnants; developmental in nature
Etiology
- Unknown
Clinical features
- Most present as painless asymptomatic swelling (Arch Pathol Lab Med 2010;134:271)
- Others discovered incidentally in orthodontic radiographs
Diagnosis
- Requires correlation of orthopantomogram (OPG) findings with histological features
Radiology description
- Orthopantomogram
- Well demarcated unilocular radiolucency (93%) with a corticated margin (Dentomaxillofac Radiol 2010;39:455)
- Associated with unerupted tooth in 50 - 75% of cases (Arch Pathol Lab Med 2010;134:271, Dentomaxillofac Radiol 2010;39:455)
Prognostic factors
- Overall prognosis is good
- Recurrence seen in 2% (Dentomaxillofac Radiol 2010;39:455, Arch Pathol Lab Med 2010;134:271)
Case reports
- 18 year old woman with posterior mandible calcification (J Oral Maxillofac Pathol 2018;22:S20)
- 20 and 23 year old men with multiple tumors of mandible and maxilla (Head Neck Pathol 2020;14:381)
- 41 year old man with tumor in maxilla (Turk Patoloji Derg 2017;33:81)
- 41 year old man with tumor of condylar head (Rom J Morphol Embryol 2017;58:689)
- 50 year old man with tumor of maxilla masquerading as dentigerous cyst (Int J Appl Basic Med Res 2016;6:297)
- 52 year old man with progression into squamous cell carcinoma of left mandible (World J Clin Cases 2019;7:1686)
Treatment
- Enucleation
Gross description
- If an intact cyst is enucleated:
- Keratinous material filled cystic lesion
- Usually unilocular but may be multilocular
- Fibrous cyst membrane may envelope crown of molar tooth (Int J Appl Basic Med Res 2016;6:297)
- In curated specimens:
- Multiple flattened, irregular, soft to firm and pearl white to gray-white to tan-brown cystic tissue fragments
Frozen section description
- Frozen section is seldom done for odontogenic cysts
Microscopic (histologic) description
- Noninflamed fibrous cyst wall lined by thin uniform stratified squamous epithelium
- Prominent granular layer
- Thick luminal lamellated keratin (onion skin-like)
- Chevroning of lamellated keratin in some cases
- Cuboidal to flat basal layer
- No surface corrugation or basal palisading is seen
- Focal parakeratinized epithelium in case of inflammation
- Reference: Arch Pathol Lab Med 2010;134:271
Microscopic (histologic) images
Positive stains
- Immunohistochemical stains are not usually used for diagnostic purpose
- Decreased Ki67 index and p53 staining when compared with odontogenic keratocyst (Arch Pathol Lab Med 2010;134:271)
- Reduced expression of BCL2, bax, p53 and TGFα as compared with odontogenic keratocyst (Oral Oncol 2009;45:e41, Dent Res J (Isfahan) 2012;9:S39)
- Cytokeratin 10 positivity with variable expression of cytokeratin 13 and 14 (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:732)
- Expression of cytokeratin 1, 2, 10 and loricrin
- This profile suggests differentiation toward normal epidermis (Hum Pathol 2010;41:1718)
Sample pathology report
- Right posterior mandible, enucleation:
- Orthokeratinized odontogenic cyst (see comment)
- Comment: Histologic and radiologic features support the diagnosis.
Differential diagnosis
- Odontogenic keratocyst (OKC):
- Peak incidence in second and third decades
- Mostly solitary, unilocular, well demarcated radiolucency
- Multiple cysts in 10% of cases
- Much more common
- Associated with nevoid basal cell carcinoma syndrome
- Associated with aggressive behavior (28 - 30% recurrence)
- Thin uniform lining of parakeratinized squamous epithelium
- Corrugated layer of parakeratin on its luminal surface
- Palisaded layer of columnar basal cells (reverse polarity)
- No keratohyalin granules
- Lumen may contain keratinaceous debris
- Frequent separation of the epithelial lining from underlying connective tissue
- Dental lamina rests and microcysts occasionally present in capsule wall
- Dentigerous cyst (DC):
- Radiologically similar as both are associated with unerupted molar tooth of mandible
- Much more common
- Peak incidence in second to fourth decades of life
- Noninflamed lining is thin; 2 - 3 layers of regular nonkeratinizing stratified squamous epithelium
- Inflamed lining is hyperplastic with elongated rete ridges
- Mucus metaplasia and cilia are also seen
Additional references
Board review style question #1
The most important histological feature to differentiate orthokeratinized odontogenic cyst (see image) from odontogenic keratocyst is
- Absence of parakeratin
- Orthokeratinized squamous epithelium with prominent granular layer
- Presence of luminal lamellated keratin
- Thick stratified squamous epithelium
- Uninflamed fibrous cyst wall
Board review style answer #1
B. Orthokeratinized squamous epithelium with prominent granular layer
Comment Here
Reference: Orthokeratinized odontogenic cyst
Comment Here
Reference: Orthokeratinized odontogenic cyst
Board review style question #2
Which of the following cysts most closely mimics orthokeratinized odontogenic cyst radiologically?
- Calcifying epithelial odontogenic cyst
- Dentigerous cyst
- Lateral periodontal cyst
- Odontogenic keratocyst
- Radicular cyst
Board review style answer #2
Board review style question #3
The most important histological feature to differentiate orthokeratinized odontogenic cyst from dentigerous cyst is
- Absence of basal palisading
- Elongated rete ridges
- Orthokeratinzed granular squamous epithelium
- Thick stratified squamous epithelium
- Uninflamed fibrous wall
Board review style answer #3
C. Orthokeratinzed granular squamous epithelium
Comment Here
Reference: Orthokeratinized odontogenic cyst
Comment Here
Reference: Orthokeratinized odontogenic cyst
Osteomyelitis overview
Table of Contents
Definition / general | Terminology | Spectrum of Classification Systems Related to Gnathic Osteomyelitis | Additional referencesDefinition / general
- Originates from Greek words osteon (bone) and muelinos (marrow)
- While the original derivation emphasizes involvement of marrow, common medical literature extends the definition to an inflammation process of the entire bone including cortex and periosteum, recognizing that the pathological process is rarely confined to the medullary portion
- Osteomyelitis has been used to encompass a wide variety of pathoses / etiologies, such as traumatic injuries, radiation, and certain chemical substances, but the term is mostly often used to describe infection of the bone
- Osteomyelitis of the jaws is predominantly a disease of the mandible, because the maxilla is vascular with thin cortical plates and less frequently involved
- When infection becomes established, pus or edema in the medullary cavity and beneath the periosteum can compromise the local blood supply
- Ischemia can lead infected bone to become necrotic and sequester which is considered a classical sign of osteomyelitis
- Osteomyelitis of the jaws is still fairly common in maxillofacial clinics and offices despite the introduction of antibiotics and the improvement of dental and medical care
- Infection of the jaws, due to its unique feature bearing teeth and connecting to the oral cavity / oral environment with the periodontal membrane, differs in several important aspects from osteomyelitis of long bones
- The specific local immunological and microbiological aspects determine a major factor in the etiology and pathogenesis of this disease and hence also have a direct impact on its treatment; therefore, to extrapolate from long bone infections to disease of the jaws has limitations
- This is reflected by the longstanding recognition of osteomyelitis of jawbones as a clinical entity which differs in many important aspects from that in long bones; hence, a wide variety of classifications, specifically for the jawbones, have been established
- Classifications are based on different aspects such as clinical course, pathological–anatomical or radiological features, etiology and pathogenesis
- A mixture of these classification systems is often used, leading to confusion, hindering comparative studies and obscuring classification criteria
- The Zurich system of osteomyelitis is generally accepted as the most reliable classification for osteomyelitis
- Classification is primarily based on clinical course and imaging
Terminology
- Cortical bone
- Cortical bone, synonymous with compact bone, is one of the two types of osseous tissue that form bones
- Forms the cortex, or outer shell, of most bones
- Much denser than cancellous bone, the other type of osseous tissue; is also harder, stronger and stiffer than cancellous bone
- Medullary bone / medullary cavity / marrow cavity
- The medullary cavity (medulla, innermost part) of bone is the central cavity where red bone marrow or yellow bone marrow (adipose tissue) is stored; hence, the medullary cavity is also known as the marrow cavity
- The medullary cavity has walls composed of spongy bone (cancellous bone) and is lined by endosteum which are osteoprogenitor cells
- Periosteum
- Connective tissue membrane lining the outer / external surface bones except at the joints of long bones
- Is composed of an outer fibrous layer and an inner cambium / osteogenic layer
- Periapical granuloma
- Acute or chronic inflammation admixed with fibrous or granulation tissue locally at the apical or periapical region of a tooth
- Is devoid of epithelium (i.e. no cyst lining) which distinguishes it from a periapical cyst
- Periapical granuloma is located at the apex of a necrotic or partially necrotic tooth root
- Biofilm
- Collection of microorganisms often embedded in a self produced extracellular polymeric matrix which allows them to adhere to or coat the surface of a living or inanimate structure
- Acute suppurative osteomyelitis
- Early phase of osteomyelitis, usually suppurative (pus forming)
- Exists when an acute inflammatory process moves away from the site of initial infection and spreads through the medullary space of the bone and, in most cases, insufficient time has passed for the body to react to the presence of the inflammatory infiltrate
- Acute phase may lead to the chronic phase which has been arbitrarily defined as an osseous infection lasting at least 1 month
- Chronic osteomyelitis
- Classified as primary or secondary, suppurative or non-suppurative
- Chronic suppurative (or non suppurative) osteomyelitis: generally regarded as a secondary osteomyelitis characterized by a defensive response that leads to production of granulation tissue which subsequently forms dense scar tissue in an attempt to wall off the infected area
- The encircled dead space acts as a reservoir for bacteria and antibiotics have great difficulty reaching the site
- Acute and secondary chronic osteomyelitis: basically the same disease separated by the arbitrary time limit of 1 month after disease onset
- Suppuration, fistula formation and sequestration are characteristic features
- Depending on the intensity of the infection and the host bone response, the clinical presentation and course may vary significantly
- Acute and secondary chronic osteomyelitis of the jaws: caused mostly by a bacterial focus (e.g. odontogenic disease, pulpal and periodontal infection, extraction wounds, foreign bodies, infected fractures)
- Primary chronic osteomyelitis (PCO): often confused with, but must be distinguished from, chronic suppurative osteomyelitis (secondary chronic osteomyelitis)
- No obvious association with a bacterial infection
- No suppuration or sequestration characteristically
- May be due to altered immune response to an organism of low virulence but no single theory has received widespread acceptance
- Chronic suppurative (or non suppurative) osteomyelitis: generally regarded as a secondary osteomyelitis characterized by a defensive response that leads to production of granulation tissue which subsequently forms dense scar tissue in an attempt to wall off the infected area
- Classified as primary or secondary, suppurative or non-suppurative
- Chronic recurrent multifocal osteomyelitis (CRMO)
- Characterized by an inflammatory process presenting with findings similar to infectious osteomyelitis but no infectious source is identifiable
- Often affects preteen and teenage patients
- Often polyostotic and may affect the mandible
- May represent a widespread variant of primary chronic osteomyelitis
- CRMO and SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) are distinguished from other forms of osteitis / osteomyelitis as these two diseases may have extragnathic skeletal involvement
- Chronic tendoperiostitis
- Initially thought to be an obscure infectious process, the clinical presentation is similar to that of primary chronic osteomyelitis
- May represent a reactive alteration of bone initiated and exacerbated by chronic overuse of the masticatory muscles, predominantly the masseter and digastric
- Chronic sclerosing osteomyelitis / diffuse sclerosing osteomyelitis
- Medullary osseous infection, probably bacterial, induces the complete sclerosis
- More common in young women; painful; appears radiographically as medullary sclerosis
- Therapy includes antibiotics, surgical debridement and hyperbaric oxygen therapy in refractory cases
- SAPHO syndrome
- Acronym for a complex clinical presentation that includes synovitis, acne, pustulosis, hyperostosis and osteitis
- The osseous lesions mirror primary chronic osteomyelitis and CRMO
- Unknown cause but thought to arise in genetically predisposed individuals who develop an autoimmune disturbance secondary to exposure to dermatologic bacteria
- Increased prevalence of histocompatibility antigen 27 noted
- SAPHO and CRMO distinguished from other forms of osteitis / osteomyelitis as these two diseases may have extragnathic skeletal involvement
- Garrè's sclerosing osteomyelitis:
- In 1893, Swiss physician Carl Garrè reported on patterns of acute osteomyelitis
- Garrè did not have any pathologic specimens for microscopic examination nor radiographs to augment his descriptions
- Nonetheless, his name is often associated with the condition in which periosteal duplication, or "onion-skinning" pattern of periostitis leads to enlargement of the jaw, usually mandible
- Garrè's osteomyelitis and Garrè's sclerosing osteomyelitis are often used synonymously, and should be disassociated with this clinical presentation
- Note that Garrè's name was and is often misspelled as Garré with an improper accent
- Numerous synonyms for Garrè's sclerosing osteomyelitis have been used:
- Chronic osteomyelitis with proliferative periostitis
- Chronic sclerosing osteomyelitis of Garrè
- Chronic non-suppurative osteomyelitis with proliferative periostitis
- Garré's chronic sclerosing osteomyelitis
- Garré's chronic nonsuppurative sclerosing osteitis
- Garré's proliferative periostitis
- Garré's sclerosing osteomyelitis
- Ossifying periostitis
- Osteomyelitis of Garré
- Osteomyelitis sicca
- Osteomyelitis with proliferative periostitis
- Periostitis ossificans
- Sclerosing osteomyelitis of Garré
- Sclerosing osteomyelitis of Garrè
- Proliferative periostitis
- Periosteal reaction to inflammation
- The affected periosteum forms several rows of reactive vital bone that parallel each other and expand the surface of the altered bone
- Osteoradionecrosis (ORN)
- Clinically, a chronic non-healing wound of the affected jaw (most commonly mandible), typically with exposure of bone, in a patient with a history of radiation therapy to the head and neck region
- Radiation injury to jaw that leads to ORN results from chronic hypovascularity, hypocellularity of marrow space and ultimately hypoxemia
- Is more similar to avascular necrosis of bone than primary infection of bone, although infection or bacterial colonization can occur
- Osteochemonecrosis of the jaws
- Synonyms: bisphosphonate related osteonecrosis of the jaws, bisphosphonate induced osteonecrosis of the jaw, osteonecrosis of jaw, bisphosphonate osteonecrosis, bis-phossy jaw
- Necrosis of bone related to long term use of antiresorptive medications such as bisphosphonate medications and altered bone metabolism
- Osteoclasts are thought to be qualitatively impaired, particularly with intravenous forms of bisphosphonate medication which leads to inadequate remodeling of bone and necrosis
- Condensing osteitis
- Localized areas of radiographic bone sclerosis associated with the apices of inflamed dead or dying teeth (pulpitis or pulpal necrosis)
- The association with an area of inflammation, usually a neighboring tooth, is critical because these lesions can resemble other intrabony processes
- Is not considered a true osteomyelitis
- Alveolar osteitis / Fibrinolytic alveolitis
- After extraction of a tooth, a blood clot is formed at the extraction site with eventual organization by granulation tissue and gradual replacement by bone
- Destruction of the initial clot is thought to delay the above steps required for extraction site healing and leads to a clinical condition known as alveolar osteitis
- Clot is lost secondary to transformation of plasminogen to plasmin with subsequent lysis of fibrin and formation of kinins, which are potent pain mediators
Additional references
- Wikipedia: Periosteum [Accessed 31 May, 2018], Oral Surg Oral Med Oral Pathol 1970;29:641, Oral Surg Oral Med Oral Pathol 1970;30:396, Oral Maxillofac Surg Clin North Am 2011;23:401
- J Oral Maxillofac Surg 1993;51:1294, J Can Dent Assoc 1995;61:441, J Craniomaxillofac Surg 2004;32:43,
- Topazian: Oral and Maxillofacial Infections, 4th Edition, 2002 (pg. 251 - 88), Baltensperger: Osteomyelitis of the Jaws, 2008 (pg. 55 - 56)
- Oral Maxillofac Surg Clin North Am 1991;3:367, Oral Maxillofac Surg Clin North Am 1991:3:355
Osteoradionecrosis
Table of Contents
Definition / general | Terminology | Incidence | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Differential diagnosis | Additional referencesDefinition / general
- Exposed, devitalized bone in radiated field with no healing for 3 months and no evidence of tumor recurrence
- Osteoradionecrosis (ORN) is considered a late effect of a radiation induced fibroatrophic process, but is not considered as primary osteomyelitis
- Is more similar to aseptic / avascular necrosis of bone than primary infection of bone, although infection or bacterial colonization can occur
Terminology
- Synonyms: Radiation osteitis, radio-osteonecrosis, radiation osteomyelitis, osteomyelitis of irradiated bone, osteonecrosis, radio-osteomyelitis, postradiotherapy osteonecrosis
- Acute suppurative osteomyelitis:
- Early phase of osteomyelitis, and usually a suppurative (pus forming) condition
- Exists when an acute inflammatory process moves away from the site of initial infection and spreads through the medullary space of the bone and in most cases, insufficient time has passed for the body to react to the presence of the inflammatory infiltrate
- Acute phase may lead to the chronic phase which has been arbitrarily defined as an osseous infection lasting at least 1 month
- Chronic osteomyelitis:
- May be classified as primary or secondary, and as suppurative or non-suppurative
- Secondary chronic osteomyelitis (SCO)
- Primary chronic osteomyelitis (PCO)
- Chronic tendoperiostitis
- May be classified as primary or secondary, and as suppurative or non-suppurative
- Chronic recurrent multifocal osteomyelitis (CRMO)
- CRMO is a relapsing inflammatory disease and is considered a type of seronegative spondyloarthropathy characterized by periods of exacerbations and remissions over many years
- Characterized by an inflammatory process presenting with findings similar to infectious osteomyelitis; however, no infectious source is identifiable
- Characterized by insidious onset of low grade fever, local swelling and bone pain
- Radiologic findings may suggest osteomyelitis, and bone seeking isotopes reveal other asymptomatic sites of involvement
- Lesions occur mainly in metaphyses of long tubular bones, as well as clavicles, spine, and pelvis; lesions are sometimes symmetrically distributed
- Precise relationship between primary chronic osteomyelitis, diffuse sclerosing osteomyelitis, CRMO and SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) is highly debated and not clear at this time
- Some consider CRMO and SAPHO as primary chronic osteomyelitis with additional extragnathic manifestations
- Chronic sclerosing osteomyelitis / diffuse sclerosing osteomyelitis:
- Although the term is usually used synonymously with primary chronic osteomyelitis, it represents a description of a strictly radiological appearance that can be caused by several similar processes, including:
- Primary and secondary chronic osteomyelitis
- Chronic tendoperiostitis
- Ossifying periostitis or Garrè osteomyelitis
- Medullary osseous infection, probably bacterial, which induces the complete sclerosis
- Favors young women, is painful and appears radiographically as medullary sclerosis
- Therapy includes antibiotics and surgical debridement and hyperbaric oxygen therapy in refractory cases
- Although the term is usually used synonymously with primary chronic osteomyelitis, it represents a description of a strictly radiological appearance that can be caused by several similar processes, including:
- Cortical bone:
- Cortical bone, synonymous with compact bone, is one of the two types of osseous tissue that forms bone
- As its name implies, cortical bone forms the cortex, or outer shell, of most bones
- Compact bone, as its name implies, is much denser than cancellous bone, which is the other type of osseous tissue
- Furthermore, it is harder, stronger and stiffer than cancellous bone
- Garrè sclerosing osteomyelitis:
- In 1893, a Swiss physician, Carl Garrè, reported on patterns of acute osteomyelitis
- Garrè did not have any pathologic specimens for microscopic examination, nor radiographs to augment his descriptions
- Nonetheless, his name is often associated with the condition in which periosteal duplication, or an "onion-skinning" pattern of periostitis, leads to enlargement of the jaw, usually mandible
- Involucrum:
- A sheath of new bone surrounding the sequestrum (N Engl J Med 2007; 356:e7)
- Medullary bone / medullary cavity / marrow cavity:
- The medullary cavity (medulla, innermost part) of bone is the central cavity where red bone marrow or yellow bone marrow (adipose tissue) is stored; hence, the medullary cavity is also known as the marrow cavity
- The medullary cavity has walls composed of spongy bone (cancellous bone) and is lined by endosteum, which are osteoprogenitor cells
- Osteochemonecrosis of Jaws:
- Synonyms: bisphosphonate related osteonecrosis of the jaws, bisphosphonate induced osteonecrosis of the jaw, osteonecrosis of jaw, bisphosphonate osteonecrosis, bis-phossy jaw
- Necrosis of bone related to long term use of antiresorptive medications such as bisphosphonate medications and altered bone metabolism
- Osteoclasts are thought to be qualitatively impaired, particularly with intravenous forms of bisphosphonate medication, which leads to inadequate remodeling of bone, necrosis
- SAPHO syndrome:
- SAPHO syndrome is an acronym for a complex clinical presentation that includes synovitis, acne, pustulosis, hyperostosis and osteitis
- The osseous lesions mirror primary chronic osteomyelitis and CRMO
- Unknown cause but thought to arise in genetically predisposed individuals who develop an autoimmune disturbance secondary to exposure to dermatologic bacteria
- Increased prevalence of HLA-27
- SAPHO and CRMO are distinguished from other forms of osteitis / osteomyelitis, as these two diseases may have extragnathic skeletal involvement
- Sequestrum:
- Predominantly nonviable bone that has become separated, during the process of necrosis from the original surrounding native bone (Medical Dictionary: sequestrum [Accessed 4 June 2018])
Incidence
- Develops in ~5% (range 5 - 15%, depending on risk factors) treated by radiation therapy to jaws, usually for oral cavity squamous cell carcinoma
- Rare when exposed to radiation doses less than 60Gy or with use of intensity modulated radiotherapy
- Risk increases when chemotherapy or brachytherapy also used
- Onset usually within 2 years of radiotherapy
- May occur later if associated with a defined traumatic event
- If onset > 8 years after radiation, must exclude postradiation sarcoma
- Risk of ORN continues for life of patient
Sites
- In jaws, predominantly involves the posterior mandible
Pathophysiology
- Direct or indirect radiation damage to tissues impairs bone's ability to remodel and cope with insult
- Causes hypoxia, hypovascularity, and hypocellularity; also generation of free oxygen radicals and cytokine release, dysregulation of fibroblasts
Etiology
- In the setting of radiation therapy, bone has greater density than soft tissue, absorbs more photons with higher energy deposition
- Mandible bone is higher density than other bones within the facial radiation field
- Risk factors for the development of ORN:
- Site and size of primary tumor treated
- Dose of radiation
- Large size radiation field
- Short treatment time
- Trauma, including type of surgery on mandible
- Preoperative dental extractions
- Presence of infection
- Immune deficiencies
- Malnutrition
- Stage III / IV primary carcinoma
Clinical features
- Foul odor, exposed bone
- Cutaneous or mucosal wound
- Pain, which can be intractable
- Ulceration of mucosa
- Trismus
- Local infection with intraoral or extraoral fistula formation
- Pathologic fracture
- Food impaction into exposed wounds
- Nutritional deficiencies, loss of time from work, decreased quality of life
Diagnosis
- Diagnosis involves the detection of exposed, devitalized bone in a radiated field with no healing for 3 months and no evidence of tumor recurrence
- Diagnosis is both clinically and radiographically based but a universal staging system is lacking
- Biopsy may be necessary to differentiate recurrent malignant disease and ORN
- Cultures are important in cases with superimposed infection
- Initial correct diagnosis of secondary chronic osteomyelitis must be established prior to any successful treatment
- Staging Systems:
- Marx et al:
- Stage I: exposed bone greater than 6 months
- Stage II: failure of treatment of Stage I
- Stage III: poor prognostic factors present (pathologic fracture, orocutaneous fistula, osteolytic involvement of inferior border)
- Schwartz et al:
- Stage I:small area of limited exposed cortical bone
- Stage II: exposed cortical and underlying medullary bone necrotic
- Stage III: full thickness necrosis, +/- pathologic fracture
- Store and Boysen Staging:
- Stage 0: mucosal defects only
- Stage I: radiologic evidence of ORN + intact mucosa
- Stage II: radiologic evidence of ORN + denuded bone
- Stage III: exposed radionecrotic bone + orocutaneous fistulae
- Marx et al:
Radiology description
- Radiographic work-up often involves multiple imaging modalities:
- Plain films:
- Often panoramic radiographs
- Good for screening but tends to underestimate extent of disease
- Lytic areas, with ill defined, non-sclerotic borders
- May have alternating radiopaque areas
- Computed Tomography:
- Best diagnostic modality
- Cortical bone disruption with mixed lysis and sclerosis
- Loss of bone results in lytic appearance
- Pathologic fracture may be present
- PET/CT:
- Markedly FDG-avid lesions, may represent inflammation
- Magnetic resonance imaging:
- Helpful to look at bone marrow and surrounding soft tissue
- Low signal intensity on T1 weighted images, postcontrast shows enhancement
- High T2 signal and contrast enhancement present in setting of infection
Treatment
- Prevention most important
- Multiple approaches predicated on clinical presentation and symptoms, size of clinical lesion, response to prior therapies, staging systems
- Conservative management: oral care, local irrigation, antibiotics, gentle debridement
- Surgical resection for advanced disease: common indications are pathologic fracture, severe intractable pain
- Antibiotics
- Hyperbaric oxygen to promote angiogenesis
Gross description
- Biopsies to confirm diagnosis of osteomyelitis and rule out neoplasia are often minute
- If segmental mandibulectomy is performed, must sample margins of resection, soft tissue to exclude recurrent disease, pathologic fracture sites to exclude recurrent disease, multiple full thickness cross sections of mandible to document possible disease heterogeneity
Microscopic (histologic) description
- Microscopic features may overlap with osteomyelitis, particularly in cases with superinfection
- Three phases:
- Prefibrotic phase: chronic inflammation
- Organized fibrosis phase: abnormal fibroblastic activity predominates, poorly organized matrix
- These areas are adjacent to regions of aging fibroblasts in a poorly cellular, fibrotic, and dense sclerotic matrix
- Fibroatrophic phase: dense hyalinization and fibrosis with loss of marrow cells
- Reactive squamous mucosa may line a fistulous tract
- Secondary infection possible
- May have necrotic or sclerotic bone with empty osteocyte lacunae
- Marrow fibrosis, irregular bony trabeculae may lack osteocytes within lacuna
- Marrow necrosis may evolve through an edematous fibromyxoid nature in the earlier stage of disease
Differential diagnosis
- Acute exacerbation of bisphosphonate osteonecrosis
- CRMO
- Diffuse sclerosing osteomyelitis
- Infected cemento-osseous dysplasia
- Primary chronic osteomyelitis
- Recurrent squamous cell carcinoma
- Secondary chronic osteomyelitis
Osteosarcoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Classification | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Immunohistochemistry & special stains | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Osteosarcoma (OS) is a malignant, high grade tumor of bone in which the tumor cells produce osteoid (bone)
Essential features
- Craniofacial osteosarcomas represent about 6.5% - 7% of all osteosarcomas
- Patients with osteosarcomas of the jaw are generally 10 to 20 years older than those with osteosarcomas of long bones
- Chondroblastic OS may be difficult to distinguish from chondrosarcoma due to sampling bias or OS that have large cartilaginous component
- The cartilaginous component of a chondroblastic OS is usually high grade
- Chondroblastic OS should be accompanied by osteoid production from the sarcomatous stromal cells
- Many chondrosarcomas have IDH1 and IDH2 mutations, whereas chondroblastic OS do not
- Low grade central osteosarcoma may demonstrate histologic overlap with benign fibro-osseous lesions
- Complete surgical resection with wide margins has been reported as the most significant prognostic factor
Terminology
- Osteosarcoma (OS)
- Osteogenic sarcoma
- Osteosarcoma of the jawbones
- Conventional osteosarcoma
- Chondroblastic osteosarcoma
- Osteoblastic osteosarcoma
- Secondary osteosarcoma
Epidemiology
- Most common nonhematopoietic bone malignancy
- Second most common bone malignancy after myeloma
- Conventional OS has a bimodal age distribution
- Most (~60%) seen in second decade
- Second peak after 50 years
- Usually in setting of radiation or other secondary causes such as Paget disease
- Craniofacial osteosarcomas represent about 6.5% - 7% of all osteosarcomas
- Conventional osteosarcomas are most common
- Approximately 25% of craniofacial OS are of the chondroblastic subtype
- Patients with osteosarcomas of the jaw are generally 10 to 20 years older than those with osteosarcomas of long bones
- Males are affected more frequently than females
Classification
2013 WHO Classification for osteosarcoma is as follows:
- Low grade central osteosarcoma
- Conventional osteosarcoma
- Osteoblastic
- Chondroblastic
- Fibroblastic
- Secondary
- Small cell osteosarcoma
- Telangiectatic osteosarcoma
- Parosteal osteosarcoma
- Periosteal osteosarcoma
Sites
- Mandibular tumors are more frequent in the posterior body and ramus
- Maxillary lesions are more common along the alveolar ridge, sinus floor and palate than the zygoma or orbital rim
- For other sites, please see osteosarcoma of long bones
Etiology
- It usually occurs de novo
- Primary osteosarcoma is associated with genetic syndromes
- Li-Fraumeni
- Hereditary retinoblastoma
- Rothmund-Thomson
- May also be associated with radiation therapy, Paget disease of bone or fibrous dysplasia
Clinical features
- Variable but can include pain, swelling, paresthesia and ulceration
Diagnosis
- Diagnosis dependent on clinical, radiologic and pathologic correlation
Radiology description
- Can show radiopaque, radiolucent or mixed appearances on plain radiographs
- May be destructive with permeative margins
- May break through cortex and elevate periosteum
- Sunburst pattern due to new bone formation in soft tissue
Prognostic factors
- Complete surgical resection with wide margins has been reported as the most significant prognostic factor
- 70% overall 5 year survival with surgery alone
- Better than extragnathic OS
- 70% overall 5 year survival with surgery alone
- ~50% of mandibular osteosarcomas recur locally after resection
- Recurrence rate is even higher with maxillary and skull lesions (80% and 75%, respectively)
- Metastases are less frequent than local recurrence and occur in about 1/3 of patients with craniofacial osteosarcomas
- Typically occur within 2 years of initial treatment
- Radiotherapy in addition to surgery improves overall survival, disease specific survival and local control in craniofacial osteosarcoma with positive / uncertain resection margins (Cancer 2009;115:3262)
Case reports
- 18 year old woman with a slow growing asymptomatic swelling in the left upper buccal vestibule for 2 months (J Maxillofac Oral Surg 2009;8:290)
- 20 year old man with pain and swelling in the left zygomatic region (J Oral Maxillofac Pathol 2014;18:281)
- 27 year old woman with facial asymmetry caused by the a swelling in the premaxillary region with upper lip protrusion (J Oral Maxillofac Pathol 2014;18:464)
- 27 year old woman with maxillary mass (Case of Week #350)
- 59 year old woman with osteosarcoma developing from fibrous dysplasia of the sphenoid bone (Head Neck Pathol 2015;9:100)
Treatment
- Radical surgery with adjuvant chemotherapy
- Radiation can be added if tumor cannot be fully removed surgically
Clinical images
Images hosted on other servers:
Microscopic (histologic) description
- Osteosarcomas are composed of sarcomatous tumor cells that produces malignant bone or osteoid
- The tumor cells may have densely eosinophilic cytoplasm resembling osteoblasts but often are larger than normal osteoblasts and vary in size with nuclear atypia
- The osteoid may be thin, lace-like (some say it resembles fungal hyphae) or or it may consist of broad, irregular trabeculae
- The osteoid may be variable in amount
- For craniofacial OS, conventional OS are most common so morphology would include:
- Variably amounts of osteoblastic, fibroblastic (pure spindle cell growth with minimal matrix) or chondroblastic (malignant appearing cartilage with peripheral spindling and osteoid production)
Microscopic (histologic) images
Contributed by Kelly Magliocca, D.D.S., M.P.H.
Case of the Week #350: chondroblastic osteosarcoma of maxilla
Contributed by Dr. Kelly Magliocca and Dr. Anthony Martinez:
Images hosted on other servers:
a) Large osteosarcoma involv-
ing mandible ramus and body
b) Classic chondroblastic
osteosarcoma of the jaw
Immunohistochemistry & special stains
- IHC is of limited value in the diagnosis of osteosarcoma
- Can be positive for osteoblastic lineage biomarkers, including bone morphogenetic proteins, osteocalcin and osteopontin
- S100 protein can be seen in the areas that exhibit cartilaginous differentiation
- Occasional focal positivity for keratins, epithelial membrane antigen and muscle markers (smooth muscle actin and desmin)
Molecular / cytogenetics description
- Conventional osteosarcomas have complex karyotype with numerous aberrations
- Chromothripsis is a genetic mechanism where a single cataclysmic event results in tens to hundreds of genomic rearrangements
Differential diagnosis
- Chondrosarcoma
- Can usually be distinguished from osteosarcoma on clinical, radiologic and pathologic grounds
- More difficult to distinguish when osteosarcomas have large cartilaginous component such as chondroblastic OS
- The cartilaginous component of a chondroblastic OS is usually high grade
- Chondroblastic OS should be accompanied by osteoid production from the sarcomatous stromal cells
- Many chondrosarcomas have IDH1 and IDH2 mutations
- Fibrous dysplasia
- Benign fibro-osseous lesion that may involved one or more bones
- Curvilinear trabeculae (Chinese letters) of metaplastic woven bone (never matures) in hypocellular, fibroblastic stroma
- Fibrous dysplasia is caused by mutations in GNAS gene
- Malignant transformation rarely occurs
- Can be secondary osteosarcoma
- Ossifying fibroma
- Benign fibro-osseous neoplasm that is composed of fibrous tissue that contains a variable amount of bony trabeculae or cementum-like spherules
- Bony trabeculae may be lined by osteoblasts
- Osteoblasts should not be atypical like the sarcomatous cells of osteosarcoma that produce osteoid
- Some larger osteoblastomas may show prominent periosteal new bone formation and may mimic osteosarcoma radiographically
- May also involve the craniofacial bones
- Similar to osteoid osteoma with a nidus containing an interlacing network of bone trabeculae distributed in a loose fibroblastic stroma with a prominent vasculature
- Prominent rimming osteoblasts and multinucleated osteoclast-like giant cells can be seen
- The mitotic rate can be high, but atypical mitoses are not seen
Additional references
Periapical (dental) granuloma
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Inflamed fibrous or granulation tissue at the periapical region (or less commonly laterally along the tooth rooth) of a necrotic or infected tooth
Terminology
- Dental pulp:
- Unmineralized tissue composed of connective tissue, vascular, lymphatic and nervous elements
- Occupies the central cavity portion of each tooth
- Is a loose connective tissue (Wikipedia: Pulp (tooth)[Accessed 5 June 2018])
- Pulpitis: Inflammation of dental pulp tissue, may manifest as a toothache
- May be caused by trauma or bacteria accessing dental pulp
- Reversible pulpitis: may resolve spontaneously, with medication, by correcting / removing the cause
- Irreversible pulpitis: possibility of restoration to a healthy, disease free pulp is not possible
- Treatment may be limited to root canal therapy or removal of the tooth, depending on the clinical and radiographic presentation
- Apical foramen:
- Small opening at the apex (toward root tip) of the tooth root that allows passage of neural and vascular supply of tooth
- Periapical region:
- Also called periapex
- Localization around the apical foramen or region of root tip
- Periapical granuloma:
- Periapical granuloma (PG) is located at the apex / periapex or less commonly along the lateral surface of a necrotic or partially necrotic or infected tooth
- May be referred to as dental granuloma (DG) or apical periodontitis (AP)
- The terms PG and DG are both misnomers, as is not necessarily composed of granulomatous inflammation
- PG/DG devoid of epithelium (i.e. no cyst lining) which histologically distinguishes it from a periapical cyst
- Periapical granuloma (PG) is located at the apex / periapex or less commonly along the lateral surface of a necrotic or partially necrotic or infected tooth
- Granuloma:
- Collection of epitheliod histiocytes or macrophages (Wikipedia: Granuloma [Accessed 5 June 2018])
- Odontogenic cysts:
- All odontogenic cysts found within the jawbones are inflammatory, developmental or less commonly (and more controversially) neoplastic
- Source epithelium from which odontogenic cysts derive include:
- Rests of Malassez
- Dental lamina rests
- Reduced enamel epithelium
- Degenerated enamel organ
- Rarely crevicular epithelium or even surface epithelium
- In general, inflammatory odontogenic cysts have proliferative epithelium and developmental odontogenic cysts have a more uniform epithelium, although inflammation may lead to epithelial proliferation
- Inflammatory odontogenic cysts appear to arise in response to inflammation
- Clinicoradiographic variants include:
- Apical (or periapical cyst, or radicular cyst) radicular cyst: present at root apex
- Lateral radicular cyst: present at the opening of lateral accessory root canals
- Residual cyst remains even after extraction of offending tooth
- Buccal bifurcation cyst
- Epithelial rests of Malassez:
- Discrete clusters of residual cells derived from Hertwig's epithelial root sheath
- Small spherules of 6 - 8 epithelial cells with high nuclear to cytoplasmic ratio
- Little or no reverse polarity of cells
- Reduced enamel epithelium (REE):
- Ameloblastic and epithelial cells from the outer enamel that overly an unerrupted tooth, as the REE degenerates the underlying tooth is exposed
- Dental lamina:
- Band of epithelium that invades the underlying ectomesenchyme of the future dental arches at 6th week gestation
- Is major component contributing to future tooth formation
- Enamel organ:
- One recognizable step / stage in the formation of teeth
- Formed from dental lamina
- Crevicular epithelium:
- Epithelium lining the inner aspect of the gingival sulcus
Epidemiology
- ~75% of apical inflammatory jaw lesions
- 50% of apical inflammatory jaw lesions that failed to respond to root canal therapy
Sites
- Within tooth bearing portions of the maxilla and mandible
- Apical, periapical or rarely, lateral aspect of tooth
Pathophysiology
- Bacteria or trauma incites an inflammatory response, possibly necrosis, or permits bacteria to invade dental pulp and cause pulpitis
- Formation of apical inflammatory lesions represents a defensive reaction secondary to the presence of microbial infection in the root canal with spread of related toxic products into the apical zone
- Initially, the defense reaction eliminates noxious substances that exit the apical or lateral canals. With time, however, the host reaction becomes less effective with microbial invasion or spread of toxins into the apical area of tooth bearing areas of the jaws
- In the early stages of infection, neutrophils predominate and radiographic alterations of the tooth bearing areas of the jaws are not present; this phase of periapical inflammatory disease is termed acute apical periodontitis
- Over time, osteoclasts resorb gnathic bone, leading to a radiographically detectable periapical radiolucency
- Some believe that the bone destruction is an attempt to prevent the spread of the infection and provide space for the arrival of defense cells specialized against the infectious process
Etiology
- Trauma, carious lesion or bacterial colonization of developmental anomaly or diseased dental structures affecting tooth injures dental pulp
- Tooth pulp degenerates, inflammation ensues, and inflammatory products escape from tooth via apical foramen and access the surrounding / supporting periapical region of the jaw
Clinical features
- Variable; may have history of painful pulpitis or may be asymptomatic
- May have dental caries / large restoration, evidence of a traumatic tooth injury, periodontal compromise involving the affected tooth
- Non-vital reaction to electric pulp testing (may be vital reaction if dental granuloma involves one root of a multiroot tooth)
Diagnosis
- Cannot reliably differentiate dental granuloma from periapical cyst clinically / radiographically; need histological evaluation
- Diagnosis confirmed with removal of lesion and submission for microscopic examination
- Ideal to have radiographic evidence of a necrotic or carious tooth to correlate with histologic findings
Radiology description
- Radiolucent apical / periapical lesion, usually indistinguishable from periapical cyst
- Radiolucent lesions can range from small, barely perceptible lesions to lytic lesions > 2 cm in diameter and any lesion can be circumscribed or somewhat ill defined
- Affected teeth typically reveal loss of the apical lamina dura
- Root resorption is not uncommon
- Intraosseous fibrous scars are possible, especially when both cortical plates have been lost; this can give the appearance of a radiographic persistent radiolucent lesion
- Radiographically detectable lesions at the apical / periapex or lateral region in the setting of a root canal treated tooth may have failed to resolve for several reasons:
- Residual cyst formation
- Persistent pulpal infection
- Extraradicular infection (usually localized periapical actinomycotic colonization)
- Accumulation of endogenous debris (e.g., cholesterol crystals)
- Periapical foreign material
- Associated periodontal disease
- Penetration of the adjacent maxillary sinus
- Fibrous scar formation
- All soft tissue removed during periapical surgical procedures should be submitted for histopathologic examination as unexpected findings are not rare, including neoplasms
Prognostic factors
- Vast majority have excellent prognosis after treatment
- True periapical granulomas do not recur after appropriate treatment and are not premalignant
- If left untreated, may develop into a periapical cyst or become secondarily acutely infected and develop into a periapical abscess, which can extend through bone and soft tissues and, less commonly, skin
- Intraosseous fibrous scars are possible, especially when both cortical plates have been lost; this can give the radiographic appearance of a persistent radiolucent lesion
Case reports
- A residual granuloma in association with a dental implant (Implant Dent 2012;21:87)
- Differential diagnosis of apical periodontitis and nasopalatine duct cyst (J Endod 2011;37:403)
Treatment
- Dependent on clinical factors (including patient age, cost of treatment, patient wishes) and radiographic parameters, but may include:
- Dental extraction of involved tooth or
- Root canal therapy
- Teeth treated with root canal therapy should be re-evaluated to rule out possible lesional enlargement and to ensure appropriate healing (see radiology)
- Strong emphasis should be placed on the importance of the recall appointments
Gross description
- Soft tissue may be adherent to root apex of an extracted tooth
- May be firm (if fibrotic) or soft
- May be granular and hemorrhagic (with extensive vascular proliferation)
Microscopic (histologic) description
- Entirely variable, based on time lesion has been present, prior treatment, presence of superimposed abscess
- Dense fibrous or granulation tissue, often with an inflammatory infiltrate of lymphocytes variably intermixed with neutrophils, plasma cells, histiocytes, mast cells and eosinophils
- Occasionally scattered hyaline bodies (pulse granuloma giant cell hyaline angiopathy) which appear as small circumscribed pools of eosinophilic material that exhibit a corrugated periphery of condensed collagen, often surrounded by lymphocytes and multinucleated giant cells
- Spicules of remodeling bone or dystrophic calcifications
- Russell bodies or pyronine bodies (clusters of lightly basophilic particles) may be associated with the plasmacytic infiltrate; both are plasma cell products but are not specific for periapical granuloma
- Epithelial rests of Malassez may be identified within granulation tissue
- Cholesterol clefts with multinucleated giant cells, red blood cells and areas of hemosiderin pigmentation
- In histological sections, the cholesterol crystals are dissolved out and clefts are seen surrounded by dense aggregations of multinucleated giant cells
- The cholesterol may be due to disintegrating red blood cells in a form that readily crystallizes and incites a foreign body giant cell reaction
Microscopic (histologic) images
Differential diagnosis
- Tooth bearing region of maxilla and mandible
- Anterior maxillary tooth bearing
- Nasopalatine duct cyst or enlarged duct
Peripheral giant cell granuloma
Table of Contents
Definition / general | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Also called giant cell epulis
- Resembles pyogenic granuloma but may erode alveolar bone or involve periodontal membrane
- Usually women, mean age 30 years, although may involve children or elderly patients without teeth
- May be due to trauma, local irritation or chronic infection
- Recurs if not completely excised
Treatment
- Excision with curettage of base of lesion extending into adjacent periodontal membrane
Gross description
- Inflammatory lesion up to 1.5 cm that protrudes from gingiva at site of chronic inflammation
- Covered by gingival mucosa or ulcerated
Microscopic (histologic) description
- Nonencapsulated aggregates of foreign body giant cells and fibroangiomatous stroma with hemorrhage, hemosiderin, acute and chronic inflammatory cells
- Alveolar bone often expanded in edentulous patients leading to superficial bone loss with peripheral cuffing
Microscopic (histologic) images
Differential diagnosis
- Giant cell granulomas of maxilla / mandible
- Giant cell "brown tumors" of hyperparathyroidism
Primary intraosseous carcinoma, NOS
Table of Contents
Definition / general | Radiology description | Radiology images | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Additional referencesDefinition / general
- May arise from epithelial lining of an odontogenic cyst or de novo from intraosseous odontogenic rests
- Mean age is 52 years (range 4 to 76 years); 70% occur in men (Int J Oral Maxillofac Surg 2001;30:349); most tumors (92%) occur in mandible
- Progressive swelling of the jaw, pain and loosening of the teeth
- Cervical nodal metastases are common
- Aggressive behavior (4 year survival is 40%)
Radiology description
- Radiolucent cystic-like pattern of bone destruction but with well defined margins
Case reports
- 66 year old man with enlargement of mandible (Case of Week #73)
- Primary intraosseous verrucous carcinoma developing from a maxillary odontogenic cyst (Tumori 2001;87:444)
- Verrucous carcinoma arising in an odontogenic cyst (Oral Surg Oral Med Oral Pathol 1980;49:151)
Treatment
- Aggressive surgical excision (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103:e29)
- Possibly with postoperative radiotherapy
Microscopic (histologic) description
- Usually squamous cell carcinoma, high grade
Microscopic (histologic) images
Additional references
Radicular (periapical) cyst
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Classification | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Inflammatory type odontogenic cyst associated with the root of a nonviable (necrotic) tooth (PLoS One 2022;17:e0277387)
- Lined by nonkeratinized stratified squamous epithelium, derived from rests of Malassez
Essential features
- Located in the maxilla or mandible (Head Neck Pathol 2022;16:63)
- Must involve the root of a nonviable (necrotic) tooth
- Cystic strips of nondysplastic (para)keratinizing squamous epithelium
- Background stromal inflammation
Terminology
- Generally synonymous terms: radicular cyst, apical cyst, periapical cyst
- Radicular cyst: present in association with any portion of the tooth root
- Periapical cyst: present at the tooth root apex
- Lateral radicular cyst: present at the opening of lateral accessory root canals
- Residual cyst: radicular (periapical) cyst that persists after extraction of offending tooth (PLoS One 2020;15:e0244250)
- Dental implant associated cyst: likely best viewed as a subtype of residual cyst (PLoS One 2022;17:e0277387)
- Periapical granuloma: acute or chronic inflammation of radicular (root) area tissues without an epithelium lined cyst
ICD coding
- ICD-11: DA09.8 - radicular cyst
Epidemiology
- Most common jaw cyst (PLoS One 2022;17:e0277387)
- Accounts for ~60% of all odontogenic cysts
- Most common in fourth and fifth decades but occurs over a wide age range
Sites
- Radicular cyst is associated with the root of a nonviable (necrotic) tooth within the maxilla or mandible
- Most common location: anterior maxilla
- Second most common location: posterior mandible (J Oral Pathol Med 2006;35:500, Head Neck 2012;34:852)
Pathophysiology
- Inflammation (usually associated with dental caries extending into the dental pulp or trauma) stimulates cells in the rests of Malassez
- Epithelial cells proliferate to form a cystic cavity
- Cystic cavity can enlarge secondary to
- Osmotic pressure
- Secondary infection and inflammation related to foreign material or bacteria
- Ongoing inflammation and proinflammatory cytokines (J Endod 2007;33:908)
Etiology
- Radicular (periapical) cyst is caused by inflammation at the apex of a nonviable (necrotic) tooth root (WHO)
Diagrams / tables
N/A
Clinical features
- May be asymptomatic and incidentally discovered on radiographs taken for another purpose
- May have recent or remote history of tooth pain (WHO)
- Symptoms of pain and drainage if secondarily infected
- May be associated with parulis formation
- Reference: J Oral Pathol Med 2006;35:500
Classification
- See Terminology
Diagnosis
- Lesion is intraosseous within the maxilla or mandible
- Lesion is associated with necrotic tooth root (radiographically or grossly)
- Histopathology is stratified squamous epithelium (nonspecific but in the context of the above parameters is compatible with radicular cyst)
Laboratory
N/A
Radiology description
- Panoramic (panorex) radiograph, intraoral film or cross sectional imaging may be useful depending on cyst location, lesion size and the need to evaluate additional parameters (J Clin Med 2023;12:4647)
- Radiology is essential to identify landmarks and the extent of disease in the preoperative and follow up setting
- Round to oval radiolucency associated with the root of a nonviable (necrotic) tooth
- Can resorb or remodel the root of the tooth
Radiology images
Prognostic factors
- Overall prognosis is excellent once lesion is treated
- Recurrence rate low
Case reports
- 19 year old woman with bilateral radicular cysts (J Int Oral Health 2015;7:61)
- 47 year old woman with radicular cyst mimicking a nasopalatine duct cyst (J Endod 2020;46:1155)
- 49 year old woman with persistent pain in the left mandibular angle with limitations in mouth opening (Medicine (Baltimore) 2018;97:e13529)
Treatment
- Varies with age of patient, access to care and state or prognosis of the tooth
- In some patients, necrotic tooth pulp can be treated via root canal with the goal of inducing cyst involution (J Clin Med 2023;12:4647)
- In some patients with very large cysts or cysts that fail to involute after the above treatment, surgical management is likely required to treat the intraosseous cyst (J Clin Med 2023;12:4647)
- Any surgically removed material is submitted for pathologic examination (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:642)
Gross description
- Surgical specimens usually consist of multiple, irregular tissue fragments
- Possible minute bone fragments
- All (nontooth) tissue should be submitted for histologic examination to definitively confirm the diagnosis
- Associated tooth received as specimen can be described grossly and supplemented with photographs where equipment is available
Gross images
N/A
Frozen section description
- Multilayered stratified squamous epithelial lining
- Background fibrous and granulation tissue with acute or chronic inflammation
- Associated mineralized tissues (bone, tooth) may be present but technically difficult to section
- May have associated bacterial colonies present
Frozen section images
Microscopic (histologic) description
- Epithelial lining
- Lined by nonkeratinizing stratified squamous epithelium of variable thickness
- Architecturally creates an arcading pattern
- Can have limited areas of keratinization, goblet cells or scattered ciliated cells
- Rushton hyaline bodies can occur within the epithelium (not necessary for diagnosis, not specific to radicular cyst) (J Oral Maxillofac Pathol 2020;24:572)
- Fibrous capsule
- Varying thickness with acute or chronic inflammatory cells
- Plasma cells may be particularly prominent
- Foamy histiocytes may also be present
- Additional features possible (Kaohsiung J Med Sci 2018;34:249, Otolaryngol Head Neck Surg 2003;129:441)
- Cholesterol clefts
- Pulse granuloma
- Bacterial colonies
- Foreign material related to prior dental treatments
- Portions of bone, tooth or tooth root
- Regional odontogenic rests and neural tissue may be incorporated into biopsy tissue sample material by virtue of the curettage procedure
Microscopic (histologic) images
Contributed by Kelly Magliocca, D.D.S., M.P.H.
Cytology description
N/A
Cytology images
N/A
Immunofluorescence description
N/A
Immunofluorescence images
N/A
Positive stains
- No specific immunohistochemistry required for radicular cyst
- Squamous epithelium expected to be immunoreactive for cytokeratins, p63 and p40
Negative stains
- BRAF V600E
- Beta catenin nuclear staining
Electron microscopy description
N/A
Electron microscopy images
N/A
Molecular / cytogenetics description
N/A
Molecular / cytogenetics images
N/A
Videos
N/A
Sample pathology report
- Left mandible, biopsy:
- Intraosseous stratified squamous epithelial lined cyst, consistent with radicular cyst
Differential diagnosis
- Dentigerous cyst:
- Elongated clefts (spaces) in tissue remaining after cholesterol crystals dissolved in processing
- Clefts are associated with an adjacent or surrounding foreign body type giant cell reaction
- Rich granulation type inflammatory infiltrate, including hemosiderin laden macrophages
- Buccal bifurcation cyst:
- Polypoid chronic inflammation and granulation tissue only
- Lacking keratinizing squamous epithelial proliferation
- Lacking anucleate squames
- Microscopically some cases may be identical to cholesteatoma, though if middle ear is preserved, no middle ear mucosa will be seen microscopically
- Odontogenic keratocyst (inflamed):
- Uniform epithelial lining 6 - 8 cells thick, lacks rete ridges
- May have artifactual clefting between epithelium and underlying fibroconnective tissue
- Epithelium characterized by palisaded hyperchromatic basal cell layer comprised of cuboidal to columnar cells
- May have areas of budding growth from the basal cells
- Luminal surface has wavy (corrugated) parakeratotic epithelial cells
- Lumen may contain keratinaceous debris
- Squamous cell carcinoma:
- Lack of epithelial cell maturation or polarity, atypical mitosis, pleomorphism, dyskeratosis or individual cell keratinization
Additional references
Board review style question #1
Board review style answer #1
B. Association with nonviable (necrotic) tooth. Radicular cyst is seen in association with a nonviable (necrotic) tooth. Answer A is incorrect because association with an impacted tooth is a required element for dentigerous cyst. Answer C is incorrect because radicular cyst uncommonly occurs as a bilateral lesion. Answer D is incorrect because radicular cyst is located in association with teeth, within bone.
Comment Here
Reference: Radicular cyst
Comment Here
Reference: Radicular cyst
Board review style question #2
Which of the following sites is most commonly involved by radicular cyst?
- Anterior mandible
- Anterior maxilla
- Maxillary sinus
- Posterior mandible
Board review style answer #2
B. Anterior maxilla. Anterior maxilla is the most common site of involvement for radicular cyst. Answer A is incorrect because radicular cyst can involve the anterior mandible but is not the most common location. Answer C is incorrect because radicular cyst is unlikely to be centered in the maxillary sinus, unless the lesion secondarily extends into the sinus, which is uncommon. Answer D is incorrect because the posterior mandible is the second most common site for radicular cyst to occur.
Comment Here
Reference: Radicular cyst
Comment Here
Reference: Radicular cyst
Radicular (periapical) cyst
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Inflammatory odontogenic cyst
- Lined by epithelial cells derived from rests of Malassez
- Also called radicular cyst, apical periodontal cyst, root end cyst, or dental cyst
Terminology
- Epithelial rest of Malassez:
- Derived from Hertwig epithelial root sheath
- Small spherules of 6 - 8 epithelial cells with high nuclear to cytoplasmic ratio
- Little or no reverse polarity of cells
- Periapical cyst: present at root apex
- Lateral radicular cyst: present at the opening of lateral accessory root canals
- Residual cyst: remains even after extraction of offending tooth
- Periapical granuloma: chronic granulomatous inflammation of periapical tissues
Epidemiology
- Most common odontogenic cyst (52% of jaw cystic lesions)
- Most common in 4th & 5th decades, but occurs over wide age range
Sites
- 60% in maxilla (vs. mandible)
- Most commonly in apex of lateral incisors, but also along lateral accessory root canals
Pathophysiology
- Dental caries or trauma cause chronic inflammation which eventually forms a periapical inflammation; continued inflammation stimulates cells of the rests of Malassez, the epithelial cells undergo necrosis to form the cyst which may be sterile or become secondarily infected
- While most are lined by epithelium derived from rests of Malassez, epithelial lining may be respiratory type derived from the maxillary sinus, in the setting of a periapical lesion communicating with the sinus wall
- May be oral epithelium from a fistula or oral epithelium proliferating down a periodontal pocket
Clinical features
- May be asymptomatic and incidentally found with radiographs
- Possible swelling (occurs slowly)
- May be painful if infected
Radiology description
- Round to oval radiolucency, often with well defined cortical border (this border can be lost when infected)
- Can displace or reabsorb roots of adjacent teeth if large
Radiology images
Prognostic factors
- Dependent on tooth affected, size of cyst / extent of bone destruction and accessibility for treatment
- Rare complications:
- Squamous cell carcinoma and epidermoid carcinoma may arise from the epithelial lining of periapical cysts
- Pathologic bone fracture (occurs with large cysts that erode nearly completely through the jaw)
Case reports
- 8 year old boy with radicular cyst followed by incomplete pulp therapy in primary molar (J Indian Soc Pedod Prev Dent 2013;31:191)
- 8 year old boy with radicular cyst (Case Rep Dent 2013;2013:123148)
- 10 year old girl with nonsurgical management of a periapical cyst (J Int Oral Health 2013;5:79)
- 18 year old woman with radicular cyst with severe destruction of the buccal cortical plate secondary to endodontic failure (J Clin Diagn Res 2013;7:1816)
- 39 year old man with eosinophilic granuloma in the anterior mandible mimicking radicular cyst (Imaging Sci Dent 2013;43:117)
- 55 year old woman with squamous odontogenic tumor-like proliferation in a radicular cyst (J Clin Exp Dent 2013;5:e298)
Treatment
- In adult teeth, can treat necrotic pulp (infection source) via pulpectomy ("root canal") with sparing of the tooth; this induces involution of the cyst; can also extract tooth
- In some very large cysts, after above treatment, additional surgical management (enucleation or marsupialization) is required for the osseous cyst
Gross description
- Usually attached to tooth root, may be firm or have deflated capsule, lumen can contain thin serous or straw colored fluid, opaque yellow-white debris, muddy brown fluid from old hemorrhage or frank purulent debris
Microscopic (histologic) description
- Lined by stratified squamous epithelium of variable thickness, often with scattered ciliated cells
- Exception is when epithelium is derived from maxillary sinus and thus lined with respiratory epithelium (pseudostratified ciliated columnar epithelium), may have acute inflammatory cell infiltrate
- Rushton hyaline bodies: amorphic, eosinophilic, linear to crescent shaped bodies, found in epithelium of 10% of periapical cysts
- Fibrous capsule: varying thickness with chronic inflammatory cells, plasma cells may be particularly prominent
- Cholesterol clefts are common within cyst lining
Microscopic (histologic) images
Differential diagnosis
- Influenced by degree of inflammation, anatomic location of cyst, amount of cystic epithelium present (incisional biopsy vs complete enucleation)
- Inflamed developmental odontogenic cyst
- Periapical granuloma
- Surgical ciliated cyst
- Traumatic bone cyst
Additional references
Residual cyst
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Treatment | Microscopic (histologic) description | Differential diagnosis | Additional referencesDefinition / general
- Inflammatory fibrous and granulation tissue at the apex / periapical region of a tooth not removed / curetted at the time of dental extraction may give rise to residual cyst
Terminology
- Odontogenic cysts: All odontogenic cysts found within the jawbones are inflammatory, developmental or less commonly (and more controversially) neoplastic
- Source epithelium from which odontogenic cysts derive include:
- Rests of Malassez
- Dental lamina rests
- Reduced enamel epithelium
- Degenerated enamel organ
- Rarely crevicular epithelium, or even surface epithelium
- In general, inflammatory odontogenic cysts have proliferative epithelium, and developmental odontogenic cysts have a more uniform epithelium, although inflammation may lead to epithelial proliferation
- Inflammatory odontogenic cysts appear to arise in response to inflammation
- Clinicoradiographic variants include:
- Apical (or periapical cyst, or radicular cyst) radicular cyst: present at root apex
- Lateral radicular cyst: present at the opening of lateral accessory root canals
- Residual cyst remains even after extraction of offending tooth
- Buccal bifurcation cyst
- Clinicoradiographic variants include:
- Developmental odontogenic cysts: have unclear pathogenesis
- Inflammatory odontogenic cysts appear to arise in response to inflammation
- Dental pulp:
- Unmineralized tissue composed of connective tissue, vascular, lymphatic and nervous elements
- Occupies the central cavity of each tooth
- Is a loose connective tissue (Wikipedia: Pulp (tooth) [Accessed 11 June 2018])
- Apical foramen: small opening at the apex of the tooth root that allows passage of neural and vascular supply of tooth
- Periapical granuloma:
- Apical / periapical acute or chronic inflammation admixed with fibrous or granulation tissue
- Is devoid of epithelium (i.e. no cyst lining) which distinguishes it from a periapical cyst
- Periapical granuloma is located at the apex of a necrotic or partially necrotic tooth
- Epithelial rests of Malassez: discrete clusters of residual cells derived from Hertwig epithelial root sheath
- Small spherules of 6 - 8 epithelial cells with high nuclear to cytoplasmic ratio
- Little or no reverse polarity of cells
- Reduced enamel epithelium (REE): ameloblastic and epithelial cells from the outer enamel that overly an unerrupted tooth, as the REE degenerates the underlying tooth is exposed
- Dental lamina: band of epithelium that invades the underlying ectomesenchyme of the future dental arches at 6th week gestation
- Is major component contributing to future tooth formation
- Enamel organ: one recognizable step / stage in the formation of teeth
- Formed from dental lamina
- Crevicular epithelium: epithelium lining the inner aspect of the gingival sulcus
- Source epithelium from which odontogenic cysts derive include:
Epidemiology
- 8% of all jaw cysts
Sites
- Tooth bearing regions (or if dental extraction completed, former tooth bearing region) of maxilla and mandible
Pathophysiology
- Pathophysiology of apical cyst and residual cyst similar
- Activated T cells in periapical granulomas produce cytokines that act on rests of Malassez causing proliferation and altered differentiation leading to cyst formation
- The proliferating epithelial masses become edematous, accumulate fluid and coalesce, forming microcysts containing epithelial and inflammatory cells
- Cyst walls appear to have properties of a semi-permeable membrane, so osmosis contributes to increasing the size of cysts
- Lytic products of the epithelial and inflammatory cells in the cyst cavity provide the greater numbers of smaller molecules which raise the osmotic pressure of the cyst fluid
Etiology
- Trauma, carious lesion or bacterial colonization of developmental anomaly affecting tooth irreversibly injures dental pulp
- Tooth pulp degenerates, inflammation ensues, and inflammatory products escape from tooth via apical foramen and access the surrounding / supporting periapical region of the jaw
Clinical features
- Variable: range from asymptomatic and only incidentally detected on imaging, to expansion of affected jaw region, to pain and drainage
Diagnosis
- By definition, the offending tooth has been removed, therefore the radiographic and clinical differential diagnosis of a radiolucent lesion in the jaws without a documented association to a tooth is broad
- Diagnosis confirmed via removal of lesion and submission for microscopic examination
- Ideal if have radiographic evidence of a necrotic or carious tooth (prior to dental extraction) to correlate
Radiology description
- Round to oval radiolucency of variable size within the tooth bearing regions of jaws at the site of a previous tooth extraction
- As the cyst ages, degeneration of the cellular contents within the lumen occasionally leads to dystrophic calcifications and radiographic opacities
Prognostic factors
- Vast majority have excellent prognosis
- True residual cysts do not recur after appropriate treatment and are not premalignant - they have no increased risk of squamous cell carcinoma
- Occasionally a secondary pathology, such as squamous cell carcinoma, arises from the epithelial lining of radicular, or other (odontogenic) gnathic cysts; careful patient questioning and clinical examination are necessary to exclude a primary oral mucosal neoplasm or metastases
- Must also rule out an epithelial neoplasm which underwent secondary cystic change
- Histological evidence of cyst lining with transition to epithelial dysplasia and infiltrating squamous carcinoma provides acceptable proof
Treatment
- Any number of odontogenic and nonodontogenic cysts and tumors can mimic the appearance of a residual periapical cyst, therefore, these lesions should be excised surgically, even in the absence of symptoms
- Residual cysts do not recur after appropriate management
- Intraosseous fibrous scars are possible, especially when both cortical plates have been lost; this can give the appearance of a persistent radiolucent lesion
Microscopic (histologic) description
- Epithelial lining of cyst: stratified squamous epithelium which may demonstrate exocytosis, spongiosis, or hyperplasia
- Epithelium may be discontinuous in part and range in thickness from 1 to 50 cell layers
- The majority are 6 - 20 cell layers thick
- The nature of the lining may depend on the age or stage of development of the cyst, or on the intensity of the inflammation
- In early cysts, the epithelial lining may be proliferative and show arcading with an intense associated inflammatory process but, as the cyst enlarges, the lining becomes quiescent and fairly regular with a certain degree of differentiation to resemble a simple stratified squamous epithelium
- Rarely, scattered mucous cells or areas of ciliated pseudostratified columnar epithelium are noted
- Cyst epithelium may also demonstrate:
- Linear or arch shaped calcifications known as Rushton bodies
- The bodies measure up to about 0.1 mm and are linear, straight or curved or of hairpin shape and sometimes they are concentrically laminated
- Although the origin of hyaline bodies remains obscure, it is generally now thought that they represent a secretory product of odontogenic epithelium
- Dystrophic calcifications
- Linear or arch shaped calcifications known as Rushton bodies
- Cyst lumen may demonstrate fluid and cellular debris
- Cyst lumen or wall may demonstrate:
- Cholesterol clefts with multinucleated giant cells, red blood cells and areas of hemosiderin pigmentation
- In histological sections, the cholesterol crystals are dissolved out and clefts are seen surrounded by dense aggregations of multinucleate giant cells
- The cholesterol may be due to disintegrating red blood cells in a form that readily crystallizes and incites a foreign body giant cell reaction
- Cyst wall may demonstrate
- Dense fibrous connective tissue, often with an inflammatory infiltrate containing lymphocytes variably intermixed with neutrophils, plasma cells, histiocytes, and (rarely) mast cells and eosinophils
- Occasionally will contain scattered hyaline bodies (pulse granuloma giant cell hyaline angiopathy)
- These bodies appear as small circumscribed pools of eosinophilic material that exhibits a corrugated periphery of condensed collagen often surrounded by lymphocytes and multinucleated giant cells
- Spicules of remodeling bone
- Russell bodies are commonly seen
Differential diagnosis
- Parameters:
- Radiograph demonstrates radiolucent lesion in toothbearing region of jaws without a close association to the periapical region of tooth
- Histology shows benign hyperplastic squamous epithelium
- May or may not show spongiosis or acanthosis
- Acute or chronic inflammation may be present
- Unicystic ameloblastoma, inflamed
- Odontogenic keratocyst, inflamed
- Glandular odontogenic keratocyst, inflamed
- Lateral periodontal cyst, inflamed
- Maxillary surgical ciliated cyst, inflamed
Additional references
- Woo: Oral Pathology: A Comprehensive Atlas and Text, 1st Edition, 2012, Regezi: Oral Pathology: Clinical Pathologic Correlations, 6th Edition, 2011, Shear: Cysts of the Oral and Maxillofacial Regions, 4th Edition, 2007, Univ of Missouri - Kansas City, School of Dentistry: Cysts of the Jaws [Accessed 11 June 2018]
Rhabdomyosarcoma with TFCP2 rearrangement (pending)
[Pending]
Sclerosing odontogenic carcinoma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Relatively new entity recently defined in the 2017 WHO classification of head and neck tumors, 4th edition (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Very rare, fewer than 15 case reports have been described in the literature as of January 2020
- Currently regarded as diagnosis of exclusion due to clinical and histologic similarity to other head and neck entities (Head Neck Pathol 2019;13:371)
- Locally aggressive malignancy with low metastatic potential (Am J Surg Pathol 2008;32:1613, Oral Surg 2019;12:133)
Essential features
- Rare, newly defined odontogenic malignancy that is a diagnosis of exclusion
- Variable radiographic appearance but has the invasive and destructive features of a malignancy
- Markedly sclerotic stroma containing bland epithelial cords to nests but with infiltrative margins
ICD coding
Epidemiology
- M = F
- Affects adults with wide age range from 30 to > 70 years old (Am J Surg Pathol 2008;32:1613, Am J Surg Pathol 2008;32:1613)
Sites
- Mandible > maxilla (Int J Oral Maxillofac Surg 2017;46:1641)
- Typically premolar and molar regions in the mandible
- Typically anterior or molar regions in the maxilla
Pathophysiology
- Neoplastic but exact pathophysiology is not known at this time
Etiology
- Currently unclear
- Potential sources of origin include odontogenic epithelial rests (dental lamina Rests of Serres, Hertwig epithelial root sheath, rests of Malassez) and malignant transformation of odontogenic cyst epithelial lining (Am J Surg Pathol 2008;32:1613, Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:e204)
- Aggressive nature may be associated with a specific genetic etiology, which has not yet been identified (Int J Oral Maxillofac Surg 2017;46:1641, J Oral and Maxillofacial Surg Med Pathol 2018;30:428)
- Marked sclerosis in the surrounding stroma may play a role in the low rate of metastasis (Pathol Int 2010;60:694, J Oral and Maxillofacial Surg Med Pathol 2018;30:428)
Clinical features
- May have expansion of the surrounding jawbone and increased mobility of involved teeth (Am J Surg Pathol 2008;32:1613)
- May have paresthesia, especially if the tumor involves the inferior alveolar nerve or mental nerve (Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e283, J Oral and Maxillofacial Surg Med Pathol 2018;30:428)
Diagnosis
- Based on a combination of clinical examination and definitive diagnosis requires correlation between both radiographic and histologic findings
Radiology description
- Poorly defined radiolucency but there may be a mixed radiolucent-radiopaque character that radiographically mimics benign fibro-osseous lesions such as fibrous dysplasia or cemento-osseous dysplasia (Int J Oral Maxillofac Surg 2017;46:1641, Head Neck Pathol 2019;13:371, Pathol Int 2010;60:694)
- Tumor has been reported to extend beyond radiographic margins (J Oral and Maxillofacial Surg Med Pathol 2019 Nov 25 [Epub ahead of print])
- Frequently involves cortical bone causing thinning and may trigger root resorption and periapical bone loss of adjacent teeth (Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e283)
- Generally displays localized but aggressive growth and destruction, possibly extending into the surrounding soft tissues (Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:e204)
Prognostic factors
- Slow growing malignancy with good prognosis and rare metastasis (Am J Surg Pathol 2008;32:1613)
- Marginal status, specifically the peripherally infiltrative tumor cords and nests may be associated with recurrence (Am J Surg Pathol 2008;32:1613)
- Three cases of recurrence have been reported (Pathol Int 2010;60:694, Head Neck Pathol 2019;13:371, J Oral and Maxillofacial Surg Med Pathol 2019 Nov 25 [Epub ahead of print])
Case reports
- 31 year old woman with mass at previously extracted lower right first molar (Am J Surg Pathol 2008;32:1613)
- 43 year old woman with a soft tissue lesion at the anterior hard palate (Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:e204)
- 43 year old woman with radiolucency at the right mid anterior maxilla (Oral Surg 2019;12:133)
- 48 year old man with diffuse recurrent right mandibular expansion (J Oral and Maxillofacial Surg Med Pathol 2019 Nov 25 [Epub ahead of print])
- 54 year old man with radiolucency associated with vital upper right lateral incisor and canine (Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e283)
- 60 year old man with radiolucency 4 weeks after extraction of a left mandibular third molar (Int J Oral Maxillofac Surg 2017;46:1641)
- 62 year old man with hepatocellular carcinoma and 6 month progressive left maxillary swelling (Head Neck Pathol 2019;13:371)
- 67 year old man with left lower lip and mental region paresthesia (Pathol Int 2010;60:694)
- 68 year old woman with mandibular gingival mass around a vital central incisor (J Oral and Maxillofacial Surg Med Pathol 2018;30:428)
- 73 year old woman with enlargement of the right maxilla by a radiolucent lesion (Am J Surg Pathol 2008;32:1613)
Treatment
- Due to limited literature, there is no consensus treatment (Head Neck Pathol 2019;13:371, Int J Oral Maxillofac Surg 2017;46:1641, Oral Surg 2019;12:133)
- Surgery appears to be the main curative modality but there is no agreement on the need for neck dissection given the low grade histologic appearance but high prevalence for perineural invasion (Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e283)
- Adjuvant radiotherapy appears to be reserved for cases with positive surgical margins or recurrence; chemotherapy has also been used (Am J Surg Pathol 2008;32:1613, Head Neck Pathol 2019;13:371, Pathol Int 2010;60:694, J Oral and Maxillofacial Surg Med Pathol 2019 Nov 25 [Epub ahead of print])
Gross description
- Firm expansile mass with poorly defined pushing borders (Head Neck Pathol 2019;13:371)
- Erosion of surrounding bone and teeth, if present (Am J Surg Pathol 2008;32:1613)
- May have infiltration into adjacent structures such as muscle, fat and sinus membranes (Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:e204)
- Definitive delineation between resection margin and tumor may be difficult to distinguish
Microscopic (histologic) description
- Bland epithelial cells of odontogenic origin without keratin differentiation in a background of sclerotic connective tissue stroma (Am J Surg Pathol 2008;32:1613, Oral Surg 2019;12:133)
- Cytoplasm may appear clear (Am J Surg Pathol 2008;32:1613, J Oral and Maxillofacial Surg Med Pathol 2019 Nov 25 [Epub ahead of print])
- Tumor cells range from cords of single file epithelial cells to nests mimicking odontogenic rests (Oral Surg Oral Med Oral Pathol Oral Radiol 2016;122:e204)
- The abundant eosinophilic stroma may be so predominant that it compresses the tumor cells, making them difficult to identify on hematoxylin and eosin staining alone (Am J Surg Pathol 2008;32:1613)
- Mitoses and nuclear pleomorphism are rare (Am J Surg Pathol 2008;32:1613, J Oral and Maxillofacial Surg Med Pathol 2018;30:428)
- Invasion of adjacent cortical bone, tooth roots, musculature, as well as perineural and perivascular invasion are common despite the low grade histology (Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e283, Oral Surg 2019;12:133)
- Diagnosis is extremely difficult on small biopsies and the histology mimics other head and neck neoplasms (Int J Oral Maxillofac Surg 2017;46:1641, Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e283)
- Overall cytologically bland but architecturally malignant and invasive
Microscopic (histologic) images
Positive stains
- CK5/6
- p63
- E-cadherin
- CK19 (but two case reports were negative) (Head Neck Pathol 2019;13:371, J Oral and Maxillofacial Surg Med Pathol 2019 Nov 25 [Epub ahead of print])
Negative stains
- CK7 (may show focal positivity) (Head Neck Pathol 2019;13:371)
- CK20
- CK8 / CK18
- S100
- SMA
- Desmin
- CEA
- PAS-D / mucicarmine (Am J Surg Pathol 2008;32:1613, Pathol Int 2010;60:694)
Sample pathology report
- Left maxilla, resection:
- Sclerosing odontogenic carcinoma (see synoptic report)
Differential diagnosis
- Benign fibro-osseous lesion (fibrous dysplasia, cemento-osseous dysplasia):
- Radiologic margins should be neither poorly defined nor destructive
- Histology is predominantly osteoid / dentoid / cementoid material without sclerotic stroma or epithelial cells
- No recurrence of these lesions even on curettage
- Desmoplastic ameloblastoma:
- Tumor cells have reverse polarization of the nuclei along with peripheral palisading
- Stellate reticulum shows loose connective tissue
- Calcifying epithelial odontogenic tumor (CEOT):
- Has amyloid-like (odontogenic ameloblast associated protein) concentric deposits (Congo red apple green birefringence) and more scanty hyalinized stroma
- Squamous odontogenic tumor:
- Round to oval nests of squamous epithelium are much larger with occasional cystic degeneration
- Non-hyalinized stroma
- Central odontogenic fibroma, WHO type / epithelium rich variant (subtype removed in WHO Classification of Head and Neck Tumours, 4th Edition, 2017):
- Stroma has fibroblastic proliferation without sclerosis and has dystrophic calcification resembling dentin or cementum
- Central calcifying odontogenic cyst (CCOC):
- Tumor cells have vacuolated clear cytoplasm with eccentric dark nuclei with only a few eosinophilic cells
- EWSR1-ATF1 gene fusion
- Primary intraosseous odontogenic carcinoma:
- Tends to have much more cytologic atypia
- Metastatic disease:
- Most likely of epithelial origin, must be ruled out
Board review style question #1
A 62 year old male presented with a destructive and invasive odontogenic lesion involving the left maxilla and sinus. Which of the following immunohistochemical stain results would you expect from this sclerosing odontogenic carcinoma?
- CD117 positive
- CK20 positive
- Desmin positive
- p63 positive
- S100 positive
Board review style answer #1
Board review style question #2
A 62 year old male presented with a destructive and invasive lesion involving the midline maxilla and left sinus. Which of the following entities would not be on the differential diagnosis?
- Metastatic squamous cell carcinoma
- Olfactory neuroblastoma
- Primary intraosseous odontogenic carcinoma
- Sclerosing odontogenic carcinoma
- Squamous odontogenic carcinoma
Board review style answer #2
Secondary chronic
Table of Contents
Definition / general | Terminology | Zurich Classification of Osteomyelitis | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Differential diagnosis | Additional referencesDefinition / general
- Chronic suppurative osteomyelitis is generally regarded as a secondary, chronic osteomyelitis characterized by a defensive response that leads to production of granulation tissue which subsequently forms dense scar tissue in an attempt to wall off the infected area; the encircled dead space acts as a reservoir for bacteria and antibiotic medications have great difficulty reaching the site
- Acute and secondary chronic osteomyelitis of the jaws is caused mostly by a bacterial focus (e.g. odontogenic disease, pulpal and periodontal infection, extraction wounds, foreign bodies, infected fractures); they are essentially the same disease separated by the arbitrary time limit of 1 month after disease onset
- Chronic secondary osteomyelitis: infection is more advanced than in acute cases, by definition, therefore radiographic imaging is usually more conclusive; suppuration, fistula formation and sequestration are characteristic features but are not required for diagnosis
Terminology
- Acute suppurative osteomyelitis:
- Early phase of osteomyelitis, usually a suppurative (pus forming) condition
- Exists when an acute inflammatory process moves away from the site of initial infection and spreads through the medullary space; in most cases, insufficient time has passed for the body to react to the presence of the inflammatory infiltrate
- Acute phase may lead to the chronic phase, arbitrarily defined as an osseous infection lasting at least 1 month
- Alveolar osteitis / fibrinolytic alveolitis
- After tooth extraction, a blood clot is formed at extraction site with eventual organization by granulation tissue and gradual replacement by bone (Oral Maxillofac Surg Clin North Am 2011;23:401)
- Destruction of the initial clot may delay the steps required for uneventful extraction site healing and leads to alveolar osteitis
- Clot is lost secondary to transformation of plasminogen to plasmin with subsequent lysis of fibrin and formation of kinins, potent pain mediators
- Biofilm
- Collection of microorganisms often embedded in a self produced extracellular polymeric matrix which allows them to adhere to or coat the surface of a living or inanimate structure
- Chronic osteomyelitis:
- May be classified as primary chronic osteomyelitis (PCO) or secondary chronic osteomyelitis (SCO) and as suppurative or non-suppurative
- Chronic recurrent multifocal osteomyelitis (CRMO)
- Characterized by an inflammatory process presenting with findings similar to infectious osteomyelitis but with no identifiable infectious source
- Often preteen and teenage patients
- Disease is often polyostotic and may affect the mandible
- May represent a widespread variant of primary chronic osteomyelitis
- CRMO and SAPHO (see below) are distinguished from other forms of osteitis / osteomyelitis as these two diseases may have extragnathic skeletal involvement
- Chronic sclerosing osteomyelitis / diffuse sclerosing osteomyelitis
- Primarily descriptive of the radiological appearance of the pathological bone reaction; however, although usually used synonymously with primary chronic osteomyelitis, it represents a description of a strictly radiological appearance that can be caused by several similar processes, including:
- Primary and secondary chronic osteomyelitis
- Chronic tendoperiostitis
- Ossifying periostitis or Garrè osteomyelitis
- Medullary osseous infection, probably bacterial, which induces the complete sclerosis
- Favors young women, is painful and appears radiographically as medullary sclerosis
- Therapy includes antibiotics, surgical debridement and hyperbaric oxygen therapy in refractory cases
- Primarily descriptive of the radiological appearance of the pathological bone reaction; however, although usually used synonymously with primary chronic osteomyelitis, it represents a description of a strictly radiological appearance that can be caused by several similar processes, including:
- Chronic tendoperiostitis
- Initially thought to be an obscure infectious process, the clinical presentation is similar to primary chronic osteomyelitis
- May represent a reactive alteration of bone initiated and exacerbated by chronic overuse of the masticatory muscles, predominantly the masseter and digastric
- Patients usually present with a history of parafunctional complaints
- Palpation of the temporal, masseter, medial pterygoid and digastric muscles reveals tenderness in one or more of these locations
- Radiographically may be similar to primary chronic osteomyelitis with diffuse sclerosing of the marrow and the cortical plate but no pus formation or sequestration
- Condensing osteitis
- Localized areas of radiographic bone sclerosis associated with apices of inflamed dead or dying teeth (pulpitis or pulpal necrosis)
- The association with inflammation, usually a neighboring tooth, is critical, because these lesions can resemble other intrabony processes that produce a somewhat similar pattern
- Is not considered a true osteomyelitis in the classical sense
- Cortical bone:
- Synonymous with compact bone, one of the two types of osseous tissue that forms bone
- Forms the cortex, or outer shell, of most bones
- More dense, hard, strong and stiff than cancellous bone (the second type)
- Garrè sclerosing osteomyelitis
- In 1893, a Swiss physician, Carl Garrè, reported on patterns of acute osteomyelitis
- Garrè did not have any pathologic specimens for microscopic examination or radiographs to augment his descriptions
- Nonetheless, his name is often associated with the condition in which periosteal duplication, or "onion-skinning" pattern of periostitis leads to enlargement of the jaw, usually mandible
- Involucrum:
- Sheath of new bone surrounding the sequestrum (N Engl J Med 2007;356:e7)
- Medullary bone / medullary cavity / marrow cavity:
- Medullary cavity (medulla, innermost part) of bone is the central cavity where red bone marrow or yellow bone marrow (adipose tissue) is stored; hence it is also known as the marrow cavity
- Has walls composed of spongy bone (cancellous bone) and is lined by endosteum, which are osteoprogenitor cells (Wikipedia: Periosteum [Accessed 5 June 2018])
- Osteochemonecrosis of jaws
- Synonyms: bisphosphonate related osteonecrosis of jaws, bisphosphonate induced osteonecrosis of jaw, osteonecrosis of jaw, bisphosphonate osteonecrosis, bis-phossy jaw,
- Necrosis of bone related to long term use of antiresorptive medications such as bisphosphonate and altered bone metabolism
- Osteoclasts may be qualitatively impaired, particularly with intravenous forms of bisphosphonate, which leads to inadequate remodeling of bone and necrosis
- Osteoradionecrosis (ORN)
- Clinically, a chronic non-healing wound of the affected jaw (most commonly mandible), typically with exposure of bone, in a patient with a history of radiation therapy to the head and neck region
- Radiation injury to jaw that leads to ORN results from chronic hypovascularity, hypocellularity of marrow space and ultimately hypoxemia
- More similar to avascular necrosis of bone than primary infection of bone, although infection or bacterial colonization can occur
- Periapical granuloma:
- Acute or chronic inflammation admixed with fibrous or granulation tissue at the apical or periapical region of a necrotic or partially necrotic tooth root
- Is devoid of epithelium (i.e. no cyst lining) which distinguishes it from a periapical cyst
- Proliferative periostitis
- Represents a periosteal reaction to inflammation
- The affected periosteum forms several rows of reactive vital bone that parallel each other and expand the surface of altered bone
- SAPHO syndrome
- Acronym for a complex clinical presentation that includes synovitis, acne, pustulosis, hyperostosis, osteitis
- The osseous lesions mirror primary chronic osteomyelitis and CRMO
- Unknown cause but thought to arise in genetically predisposed individuals who develop an autoimmune disturbance secondary to exposure to dermatologic bacteria
- Increased prevalence of HLA 27
- SAPHO and CRMO are distinguished from other forms of osteitis / osteomyelitis as these two diseases may have extragnathic skeletal involvement
- Sequestrum:
- Predominantly nonviable bone that has become separated during the process of necrosis from the original surrounding native bone (Medical Dictionary: sequestrum [Accessed 5 June 2018])
Zurich Classification of Osteomyelitis
- Primarily based on clinical course and imaging features
- Histopathology is considered a secondary classification criterion because findings are mostly unspecific and inconclusive by themselves; however, tissue examination is irreplaceable to confirm diagnosis, when clinical and radiological appearance are unclear / atypical, and to exclude possible differential diagnoses
- The three major classifications are: (Baltensperger, Osteomyelitis of the Jaws: Definition and Classification (Figure 2.2))
- Acute Osteomyelitis (AO)
- Secondary Chronic Osteomyelitis (SCO)
- Primary Chronic Osteomyelitis (PCO)
- Further subclassification is based on presumed etiology and pathogenesis of disease
- These criteria are considered tertiary classification criteria and are helpful to determine the necessary therapeutic strategies which may differ somewhat among the subgroups
Sites
- Predominantly involves mandible
Pathophysiology
- Chronicity of disease reflects inability of host to eradicate the pathogen, may be due to lack of treatment or inadequate treatment
- After local blood supply is compromised by acute phase of infection, bone undergoes necrosis and nonviable fragments become sequestra
- Periosteum still contains osteogenic potential in most cases which contributes to formation of a bony shell (involucrum) covering the sequestra
- The involucrum may interfere with extrusion of the sequestrum, which may perpetuate the process, in combination with a compromised blood supply and the presence of organisms
- The role of biofilms in chronic osteomyelitis of the jaws is not well studied
Etiology
- Possible polymicrobial bacterial focus: odontogenic disease, pulpal and periodontal infection, extraction wounds, foreign bodies, and infected fractures
- Specific etiologies:
- Actinomyces: cervicofacial actinomycosis is a slowly progressive infection with granulomatous and suppurative features
- Candida albicans: extremely rare, despite being a known commensal of oral cavity
- Nocardiosis: chronic disease that may resemble actinomycotic infection; primary target is usually lung, then hematogenous spread to other organs, cervicofacial region, occasionally including bone
- Tuberculosis: osteomyelitis of jaws caused by Mycobacterium tuberculosis; uncommon, usually NOT confined to bone
- Underlying malignancy and associated osteomyelitis: clinical and radiographic signs of secondary chronic osteomyelitis may be similar to malignant neoplasm complicated by secondary bone infection
- Underlying additional pathology and associated osteomyelitis: clinical and radiographic signs of secondary chronic osteomyelitis may be difficult or impossible to distinguish from a pre-existing, presently infected pathologic disease in the same anatomic area (example: florid cemento-osseous dysplasia)
Clinical features
- Clinical presentation of secondary chronic osteomyelitis of jaws may be variable, based on intensity of disease, the host, the organisms and length of time the disease has been in place
- Clinically and radiographically, ranges from aggressive osteolytic putrefactive phase to a 'dry' osteosclerotic phase
- Deep pain frequently observed in acute phase, may be less intense or a dull pain in the chronic phase
- Firm tenderness caused by a periosteal reaction may be seen more often in chronic osteomyelitis rather than the exquisitely painful swelling due to abscess formation in the acute phase
- Sequestra and fistula formation are more commonly associated with secondary chronic osteomyelitis
Diagnosis
- Initial correct diagnosis of secondary chronic osteomyelitis must be established prior to any successful treatment
- Adequate diagnosis can usually be achieved based on history, clinical evaluation, imaging studies
- In special situations when an underlying malignancy is suspected, biopsy prior to the actual surgical intervention is advisable
Radiology description
- Features:
- Clinically and radiographically, a spectrum ranging from an aggressive osteolytic putrefactive phase to a 'dry' osteosclerotic phase
- Early: osteolysis may be predominant feature
- Advanced: osteolysis and sclerosis, may mimic fibro-osseous lesion of bone
- Radiographic differential diagnosis (broad since radiologic features are variable)
- Acute suppurative osteomyelitis
- Cemento osseous dyplasia (COD) often confused with primary chronic osteomyelitis in literature
- May be a predisposing factor for bone infection
- Florid cemento osseous dysplasia appears on radiographs as sclerosing opaque and dense masses
- The bone changes are often adjacent to apices of teeth and are restricted to alveolar process in either or both jaws
- Chronic sclerosing osteomyelitis / diffuse sclerosing osteomyelitis
- Ossifying periostitis and diffuse sclerosing osteomyelitis are descriptive radiological features
- Both primary and secondary chronic osteomyelitis may demonstrate these patterns with extensive sclerosis, variable amounts of osteolysis and periosteal reaction on diagnostic imaging
- CRMO
- Garrè sclerosing osteomyelitis
- Osteochemonecrosis
- Osteoradionecrosis
- Primary chronic osteomyelitis
- SAPHO syndrome
- Tendoperiostitis
- Malignancy or benign aggressive intraosseous neoplasms inducing proliferative periostitis:
- Ewing sarcoma
- Langerhans cell histiocytosis
- Leukemia / lymphoma
- Metastatic disease to jaws
- Osteosarcoma
- Paget disease (deforming ostitis)
- Due to disturbed bone metabolism, unknown origin
- Usually occurs in advanced age and affects spine, pelvic skeleton, cranial vault
- Long bones and facial skeleton are rarely involved
- If jaws are affected, maxilla is somewhat more often involved than mandible
- Osteopetrosis (Albers-Schonberg disease)
- Genetically inherited disease which involves all bones
- Two major types: autosomal recessive form is "malignant" with anemia, manifests at birth; autosomal dominant form is "benign"
- Malignant form is more dramatic, with severe impairment and systemic complications, patients rarely living beyond age 10 years
- Benign form is more likely to be seen in daily practice
- The jawbone shows massive sclerosis
- Because of the disturbed physiology of the bone due to insufficient osteoclasts, the osteopetrotic bone cannot be resorbed adequately and sclerosis increases with time jeopardizing bone perfusion; secondary bone infection is common and clinical picture resembles primary or secondary chronic osteomyelitis with predominant sclerosis
- Because bone physiology is generally disturbed, both jaws may be completely affected, contrary to primary chronic osteomyelitis which generally just affects the mandible
Prognostic factors
- Often a prolonged course, difficult to predict
- Risk factors for recurrence: ages 6 - 12 years or > 65 years, pre-admission antibiotic administration, a lesion at the mandibular ramus, concurrent maxillofacial space infection (MSI), and conservation of pathogenic teeth (BMC Infect Dis 2013;13:313)
- Risk factors for life threatening complications: age > 65 years, admission temperature > 39 degree C, admission white blood cell (WBC) count >15×109/L, pre-admission antibiotic administration, concurrent MSI, pre-existing diabetes, and respiratory difficulty
Treatment
- Treatment based on age of patient, presence of underlying additional pathology such as florid cemento osseous dysplasia, presence of systemic disease
- Surgical debridement: dictated by extent of infection, presence of foreign bodies and nonviable tissues included (J Oral Maxillofac Surg 1993;51:1294)
- Culture material: tissue samples are favored over surface swabs of necrotic bone
- Antibiotic therapy: ideally withhold until cultures obtained; initially empiric therapy, often broad spectrum; then Infectious Disease consultation, particularly if considering extended antibiotic regimen
- Hyperbaric oxygen therapy may be offered
Gross description
- In osteomyelitis, surgical therapy intends to remove necrotic bone by "debridement"
- Macroscopy of specimens removed for osteomyelitis is determined by the type of surgery and depends on the extent and duration of disease
- Curettage yields small tan to brown hemorrhagic bone fragments and beige inflammatory exudate
- Small curettage fragments are usually totally embedded in paraffin and normally do not require dissection
- Larger tissue specimens should be oriented and cut perpendicular to osseous surface
- Necrotic bone fragments are pale whitish-gray; sequestra usually show an irregular outline
- Occasionally, a jaw resection is required; on cross section, the bony cortex appears thickened and the cancellous bone sclerosed; osteolytic and osteosclerotic foci may alternate
- Biopsies to confirm a diagnosis of osteomyelitis and rule out neoplasia may be very small
Microscopic (histologic) description
- According to Zurich classification, pathology is a secondary classification criterion serving to confirm the diagnosis of osteomyelitis if clinical judgment and diagnostic imaging are inconclusive (J Craniomaxillofac Surg 2004;32:43)
- Histology of jaw osteomyelitis should always be complemented and interpreted in conjunction with clinical and radiological findings and should NOT be used independently
- Histology is also essential to exclude differential diagnoses
- A clear distinction of primary and secondary chronic osteomyelitis solely based on histopathology is not always possible but may be helpful in context with clinical presentation and imaging studies
- When few clinical signs of suppuration (pus, fistula, sequestra) are evident, histopathology resembles typical appearance of chronic osteomyelitis with no clear distinction of primary and secondary chronic forms
- An important purpose of histopathological investigation - beyond the confirmation of a clinically and radiologically suspected diagnosis of osteomyelitis - consists of typing and grading of the inflammatory activity; this may help distinguish secondary chronic osteomyelitis from primary chronic osteomyelitis in some cases when clinical course and imaging studies are inconclusive
- Histopathology in cases of secondary chronic osteomyelitis with significant suppuration demonstrates similar features as acute osteomyelitis with large amounts of polymorphonuclear leucocytes, macrophages, and plasma cells, accompanied by a variable degree of marrow fibrosis and reactive bone formation
- Cases of secondary chronic osteomyelitis with a less fulminant (e.g., more chronic) course have lymphocytic infiltrate, marrow fibrosis, reactive woven bone formation, minimal marrow adipose
- Periosteum still contains osteogenic potential in most cases, which contributes to formation of a bony shell (involucrum) covering the sequestra
- The involucrum may interfere with extrusion of the sequestrum, which may perpetuate the process, in combination with a compromised blood supply and the presence of organisms
Differential diagnosis
Additional references
Segmental odontomaxillary dysplasia (pending)
[Pending]
Simple bone cyst
Table of Contents
Definition / general | Radiology description | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Young patients
- 50% have history of trauma
- May be jaw counterpart of solitary bone cyst
Radiology description
- Sharply outlined radiolucent mass with thin shell of remaining bone
Treatment
- Thorough curettage
- Rarely recurs
Gross description
- Body or symphyseal area of mandible, large, intraosseous lesion
- Usually solitary
Microscopic (histologic) description
- Lined by fibrovascular tissue, no epithelial lining
- Occasional hemosiderin laden macrophages and osteoclast-like giant cells
Differential diagnosis
- Aneurysmal bone cyst (associated with benign fibro-osseous lesions)
- Latent bone cavity
Squamous odontogenic tumor
Table of Contents
Definition / general | Radiology description | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Uncommon
- Most common in 20s but occurs in all ages, 2/3 male
- Usually in anterior maxilla or posterior mandible in soft tissue or bone; 25% are multiple lesions
- Arises from rests of Malassez in periodontal ligament
- Low probability of recurrence, no malignant transformation reported
Radiology description
- Well circumscribed
- Semicircular radiolucency
- Sclerotic border
- Near teeth roots
Case reports
- 30 year old woman with an incidental finding during orthodontic treatment (Arch Pathol Lab Med 2001;125:297)
Treatment
- Excision with extraction of involved teeth or en bloc resection
Microscopic (histologic) description
- Anastomosing islands of benign, stratified squamous epithelium within fibrous stroma, often well defined nests with clear cells
- May contain keratin or psammoma bodies
- Often epithelial vacuolization and microcysts
- No atypia, no mitotic figures, no inflammation, no peripheral palisading
Microscopic (histologic) images
Differential diagnosis
- Acanthomatous ameloblastoma: peripheral palisading, stellate reticulum
- Mucoepidermoid carcinoma
- Well differentiated squamous cell carcinoma
Additional references
Surgical ciliated cyst
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Immunohistochemistry & special stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Surgical ciliated cysts develop mostly in maxilla at a site of previous surgery or trauma
- Rare occurrences at mandible have been reported (J Oral Maxillofac Surg 2014;72:1736, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:36)
- Surgeries may be Caldwell-Luc / Le Fort I osteotomy, orthognathic or for chronic sinus inflammatory disease (J Oral Maxillofac Surg 2014;72:1736, J Oral Maxillofac Surg 2012;70:e264)
Essential features
- Prior surgical procedures or trauma
- Cystic lining cells are respiratory type epithelium with cilia
Terminology
- Postoperative maxillary cyst
- Postoperative paranasal cyst
- Implantation cyst
ICD coding
- J34.1: cyst and mucocele of nose and nasal sinus
Epidemiology
- Frequently encountered bony cyst in Asian population, including Japan (~20%) (Oral Surg Oral Med Oral Pathol 1986;62:544)
- Rare in non Asian populations (J Oral Maxillofac Surg 2012;70:e264)
- No gender predilection (J Oral Maxillofac Surg 2014;72:1736)
- Patients often in 30's to 40's, but wide age range (J Oral Maxillofac Surg 2014;72:1736)
- Time between first surgery and development of cyst varies from six months to 50 years (Br J Oral Maxillofac Surg 1990;28:264)
Sites
- Most common at posterior maxilla (J Oral Maxillofac Surg 2014;72:1736)
- Mandibular locations have been reported (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:36, J Oral Maxillofac Surg 2014;72:1736)
Pathophysiology
- Entrapment of maxillary sinus mucosa in the wound by a surgical procedure (e.g. Caldwell-Luc procedure, Le Fort I osteotomy)
- Ensuing inflammatory process that induces cystic changes of the trapped respiratory mucosa
- Expansion of the cyst by the osmotic difference from the surrounding tissue (J Prosthet Dent 1990;64:466, J Oral Maxillofac Surg 2014;72:1736)
Clinical features
- Most commonly presenting with swelling or pain of the cheek and the mucogingival fold (J Oral Maxillofac Surg 1988;46:310)
Diagnosis
- Histomorphology is not entirely specific; a final diagnosis should only be rendered with, most importantly, a documented history of surgery, along with collaborating radiologic findings
- If the biopsy tissue is limited and does not appear cystic, one may need to consider the possibility of sampling of normal sinonasal mucosa
- In such case, a communication with the submitting surgeons may be necessary
Laboratory
- Fluid aspirated from the surgical ciliated cyst contains predominantly glycosaminoglycans (hyaluronic acid and heparan sulfate) (Oral Surg Oral Med Oral Pathol 1988;65:222)
Radiology description
- Well demarcated, unilocular radiolucency with a sclerotic or well corticated border (J Oral Maxillofac Surg 2014;72:1736, J Prosthet Dent 1990;64:466, J Oral Maxillofac Surg 2003;61:138)
Prognostic factors
- Recurrences are rare but could happen, if cystic lining incompletely removed (J Oral Maxillofac Surg 1982;40:487)
Case reports
- 22 year old man with gradual swelling and tenderness of the chin region (Br J Oral Maxillofac Surg 2015;53:1040)
- 22 year old man with history of Le Fort I maxillary advancement osteotomy (J Oral Maxillofac Surg 2003;61:138)
- 27 year old Caucasian woman with two radiolucencies in the mandible (Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;100:36)
- 45 year old woman with a slow growing swelling at the left posterior maxillary region (J Oral Maxillofac Surg 2012;70:e264)
- 72 year old Caucasian man with painless swelling in the anterior mandible and history of rhinoplasty and genioplasty at age 16 (J Oral Maxillofac Surg 2014;72:1736)
Treatment
- Surgical enucleation or curettage
- Marsupialization, if the lesion is extensive and involves the cortical bone (J Oral Maxillofac Surg 1982;40:487)
Gross description
- Cysts, often containing slimy cystic fluid
Microscopic (histologic) description
- Cystic lumen lined by pseudostratified ciliated columnar epithelium with occasional mucous goblet cells (characteristic of maxillary sinus lining)
- Occasional areas of squamous metaplasia and secondary inflammation
Microscopic (histologic) images
Immunohistochemistry & special stains
- Not often utilized for diagnosis
Molecular / cytogenetics description
- No molecular testing utilized
Sample pathology report
- Right maxillae, cyst, curettage:
- Respiratory lined cyst, consistent with surgical ciliated cyst (see comment)
- Comment: Imaging noted a 3 x 2 cm well demarcated unilocular cystic lesion at right maxilla. Microscopic examination reveals a cystic lesion with its cyst wall lined by ciliated respiratory type epithelium. The cyst wall is variably fibrotic with sparse chronic inflammation. No cytologic atypia is seen. In this patient with a documented history of Le Fort surgery, the findings are consistent with surgical ciliated cyst.
- Respiratory lined cyst, consistent with surgical ciliated cyst (see comment)
Differential diagnosis
- Glandular odontogenic cyst
- Microcysts or duct-like spaces (good features to separate from surgical ciliated cysts)
- Lined by stratified eosinophilic cuboidal cells
- Apocrine snouting of hobnail cells
- Epithelial spheres or plaque-like thickening
- May have cilia
- Mucous goblet cells may be present
- High recurrence rate (up to 50%)
- Dentigerous cyst
- Association with an impacted or unerupted tooth (good features to separate from surgical ciliated cysts)
- Lined by non keratinizing stratified squamous epithelium
- Primordial odontogenic cyst
- A developmental odontogenic cyst
- Lined by simple stratified squamous epithelium
- Mucous or ciliated cells may be present
Board review style question #1
Board review style answer #1
D. This image is a surgical ciliated cyst. This lesion occurs over a wide age range, although many patients are in their 30s to 40s. There is no sex predilection. Although surgical ciliated cysts are more common in Asian patients and relatively rarer in non-Asian populations, the surgical history is the most important consideration in establishing the diagnosis given its pathophysiology/etiology.
Comment Here
Reference: Surgical ciliated cyst
Comment Here
Reference: Surgical ciliated cyst
Board review style question #2
- Which is true when comparing surgical ciliated cyst and glandular odontogenic cyst?
- Both entities never recur
- Cilia is only seen in surgical ciliated cyst
- Mucous goblet cells can only be seen in glandular odontogenic cyst
- Cyst with microcystic / duct-like spaces is a differentiating feature
Board review style answer #2
D. While recurrence of surgical ciliated cyst is rare, it does occur, especially if the cyst is incompletely removed. Glandular odontogenic cyst has a recurrence rate of 20 - 50%. Cilia can sometimes be seen in glandular odontogenic cysts. Mucous goblet cells can sometimes be seen in surgical ciliated cysts. Microcystic / duct-like spaces in the cyst lining are characteristically seen in glandular odontogenic cysts but not in surgical ciliated cysts.
Comment Here
Reference: Surgical ciliated cyst
Comment Here
Reference: Surgical ciliated cyst
WHO classification
Table of Contents
Definition / general | Major updates | WHO (2017) | Microscopic (histologic) images | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- This classification (WHO 2017) is applicable to tumors that occur throughout the head and neck, including the nasal cavity, paranasal sinuses, skull base, nasopharynx, hypopharynx, larynx, trachea, parapharyngeal space, oral cavity, mobile tongue, oropharynx, salivary glands, neck (including lymph nodes), gnathic maxillofacial bones and ear
Major updates
- New entities:
- Sclerosing odontogenic carcinoma: cytologically bland epithelial malignancy with stromal sclerosis exhibiting aggressive infiltrative growth into muscle and nerve; the infiltrating epithelial component tends to be small single file cords and strands which can be difficult to appreciate on routine sections and is best demonstrated with cytokeratin immunohistochemistry
- Primordial odontogenic tumor: benign tumor composed of an immature variably cellular connective tissue resembling dental papilla or odontogenic ectomesenchyme; the tumor is surfaced by cuboidal to columnar epithelium resembling the inner enamel epithelium of the enamel organ
- Renamed / refined entities:
- Ameloblastic carcinoma: three subtypes of ameloblastic carcinoma listed in WHO 2005 collapsed into a single entity, ameloblastic carcinoma
- Primary intraosseous carcinoma: collapsed the complex 2005 WHO classification of primary intraosseous carcinoma into a single entity, primary intraosseous carcinoma
- Ameloblastoma: classification simplified to three types, ameloblastoma, unicystic ameloblastoma and extraosseous / peripheral types
- Desmoplastic ameloblastoma: reclassified as a histologic subtype of ameloblastoma and not a unique clinicopathologic entity
- Odontogenic fibromas: previously divided into a simple type (epithelium poor) and WHO type (epithelial rich); this distinction was eliminated and odontogenic fibroma is now defined as having variable amounts of inactive looking odontogenic epithelium with or without evidence of calcification
- Cemento-ossifying fibroma: included in the 2017 classification under odontogenic tumors to distinguish it from the juvenile categories of ossifying fibroma
- Keratocystic odontogenic tumor: renamed and reclassified as odontogenic keratocyst; the working group noted evidence currently lacking to justify the continuation of 2005 designation of keratocystic odontogenic tumor
- Calcifying cystic odontogenic tumor: renamed and reclassified as calcifying odontogenic cyst in the absence of justification for classifying the lesion as neoplastic
- Removed entities:
- Odontoameloblastoma: now part of ameloblastoma
- Ameloblastic fibro-odontoma and ameloblastic fibro-dentinoma: now recognized as falling within the spectrum of odontoma, therefore are now classified as odontomas
- Reinstated entities:
- Odontogenic carcinosarcoma included in the 2017 classification of malignant odontogenic tumors due to improved documentation confirming it as distinct
- Notable updates:
- Inclusion of a classification for gnathic epithelial lined cysts is notable; such a classification has not appeared in the WHO since 1992
- Primordial cyst is not used synonymously with odontogenic keratocyst
- Orthokeratinized odontogenic cyst is recognized as distinct from odontogenic keratocyst
- Refined criteria for glandular odontogenic cyst presented
- One nonodontogenic epithelial lined cyst included in the 2017 classification: nasopalatine duct cyst
WHO (2017)
- Odontogenic carcinomas ICD-O
- Ameloblastic carcinoma9270/3
- Primary intraosseous carcinoma, NOS9270/3
- Sclerosing odontogenic carcinoma9270/3
- Clear cell odontogenic carcinoma9341/3
- Ghost cell odontogenic carcinoma9302/3
- Odontogenic carcinosarcoma 8980/3
- Odontogenic sarcomas 9330/3
- Benign epithelial odontogenic tumors ICD-O
- Ameloblastoma9310/0
- Squamous odontogenic tumor9312/0
- Calcifying epithelial odontogenic tumor9340/0
- Adenomatoid odontogenic tumor9300/0
- Benign mixed epithelial and mesenchymal odontogenic tumors ICD-O
- Ameloblastic fibroma9330/0
- Primordial odontogenic tumor
- Odontoma9280/0
- Odontoma, compound type9281/0
- Odontoma, complex type9282/0
- Dentinogenic ghost cell tumor9302/0
- Benign mesenchymal odontogenic tumors ICD-O
- Odontogenic fibroma 9321/0
- Odontogenic myxoma / myxofibroma9320/0
- Cementoblastoma 9273/0
- Cemento-ossifying fibroma 9274/0
- Odontogenic cysts of inflammatory origin ICD-O
- Odontogenic and nonodontogenic developmental cysts ICD-O
- Dentigerous cyst
- Odontogenic keratocyst
- Lateral periodontal cyst and botyroid odontogenic cyst
- Gingival cyst
- Glandular odontogenic cyst
- Calcifying odontogenic cyst9301/0
- Orthokeratinized odontogenic cyst
- Nasopalatine duct cyst
- Malignant maxillofacial bone and cartilage tumors ICD-O
- Chondrosarcoma9220/3
- Chondrosarcoma, grade 19222/1
- Chondrosarcoma, grade 2 / 39220/3
- Mesenchymal chondrosarcoma9240/3
- Osteosarcoma, NOS9180/3
- Low grade central osteosarcoma9187/3
- Chondroblastic osteosarcoma9181/3
- Parosteal osteosarcoma9192/3
- Periosteal osteosarcoma9193/3
- Chondrosarcoma9220/3
- Benign maxillofacial bone and cartilage tumors ICD-O
- Chondroma 9220/0
- Osteoma 9180/0
- Melanotic neuroectodermal tumor of infancy9363/0
- Chondroblastoma 9230/1
- Chondromyxoid fibroma9241/0
- Osteoid osteoma9191/0
- Osteoblastoma9200/0
- Desmoplastic fibroma8823/1
- Fibro-osseous and osteochondromatous lesions ICD-O
- Ossifying fibroma9262/0
- Familial gigantiform cementoma
- Fibrous dysplasia
- Cemento-osseous dysplasia
- Osteochondroma 9210/0
- Giant cell lesions and bone cysts ICD-O
- Central giant cell granuloma
- Peripheral giant cell granuloma
- Cherubism
- Aneurysmal bone cyst 9260/0
- Simple bone cyst
- Hematolymphoid tumors ICD-O
- ICD-O note: The first four digits indicate the specific histological term. The fifth digit after the slash (/) is the behavior code, which indicates whether a tumor is malignant, benign, in situ or uncertain (whether benign or malignant) (WHO: International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) [Accessed 28 October 2020])
Microscopic (histologic) images
Contributed by Kelly Magliocca, D.D.S., M.P.H.
Additional references
Board review style question #1
Board review style answer #1
C. Primordial odontogenic tumor. Primordial odontogenic tumor is a recently recognized benign odontogenic tumor. Being exceptionally rare in the lung, it was introduced in the 2015 WHO classification of lung tumors. Adenomatoid odontogenic tumor is a benign odontogenic tumor in the WHO classification but is not a new entity in the 2017 edition. Sclerosing odontogenic carcinoma is a new entity in the 2017 WHO but is malignant. Ameloblastic carcinoma is an odontogenic malignancy and is not a new entity in the 2017 edition.
Comment Here
Reference: WHO Classification of Odontogenic and Maxillofacial Bone Tumors
Comment Here
Reference: WHO Classification of Odontogenic and Maxillofacial Bone Tumors
Recent Mandible & maxilla Pathology books
Find related Pathology books: head & neck/endocrine