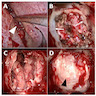
- pTX: Primary tumor cannot be assessed
- pTis: Carcinoma in situ
- pT1: Tumor limited to maxillary sinus mucosa with no erosion or destruction of bone
- pT2: Tumor causing bone erosion or destruction including extension into the hard palate or middle nasal meatus, except extension to posterior wall of maxillary sinus and pterygoid plates
- pT3: Tumor invades any of the following:
- Bone of the posterior wall of maxillary sinus
- Subcutaneous tissues
- Floor or medial wall of orbit, pterygoid fossa, ethmoid sinuses
- pT4: Moderately advanced or very advanced local disease
- pT4a: Moderately advanced local disease; tumor invades any of the following:
- Anterior orbital contents, skin of nose or cheek, minimal extension to anterior cranial fossa, pterygoid plates, sphenoid or frontal sinuses
- pT4b: Very advanced local disease; tumor invades any of the following:
- Orbital apex, dura, brain, middle cranial fossa, cranial nerves other than maxillary division of trigeminal nerve (V2), nasopharynx or clivus
- pT4a: Moderately advanced local disease; tumor invades any of the following:
Superpage
Superpage Topics
Adenocarcinoma-general
Allergic fungal sinusitis
Allergic rhinosinusitis
Anatomy
Antrochoanal polyps
Biphenotypic sinonasal sarcoma
Chordoma
Chronic rhinosinusitis
Fungal ball
Glial heterotopia
Grossing & features to report
Hairy polyp
High grade neuroendocrine carcinoma
Histology
HPV related multiphenotypic sinonasal carcinoma
Inflammatory sinonasal polyp
Intestinal type
Invasive fungal rhinosinusitis
Low grade nasopharyngeal papillary adenocarcinoma
Nasal chondromesenchymal hamartoma
Nasopharyngeal angiofibroma
Nasopharyngeal carcinoma
Nonintestinal type
NUT carcinoma
Olfactory neuroblastoma
Respiratory epithelial adenomatoid hamartoma
Rhinosclerosis
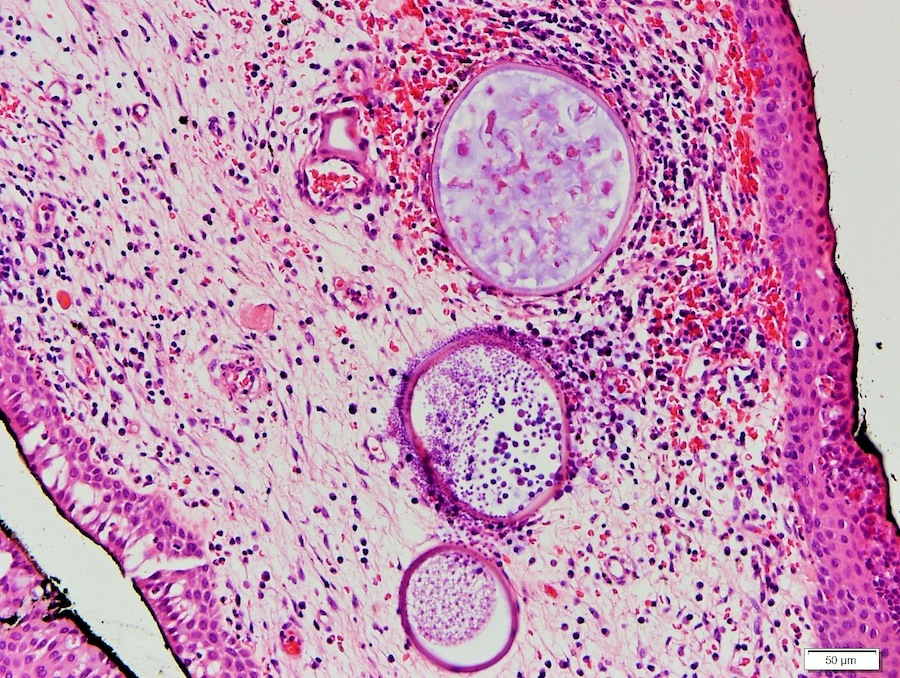
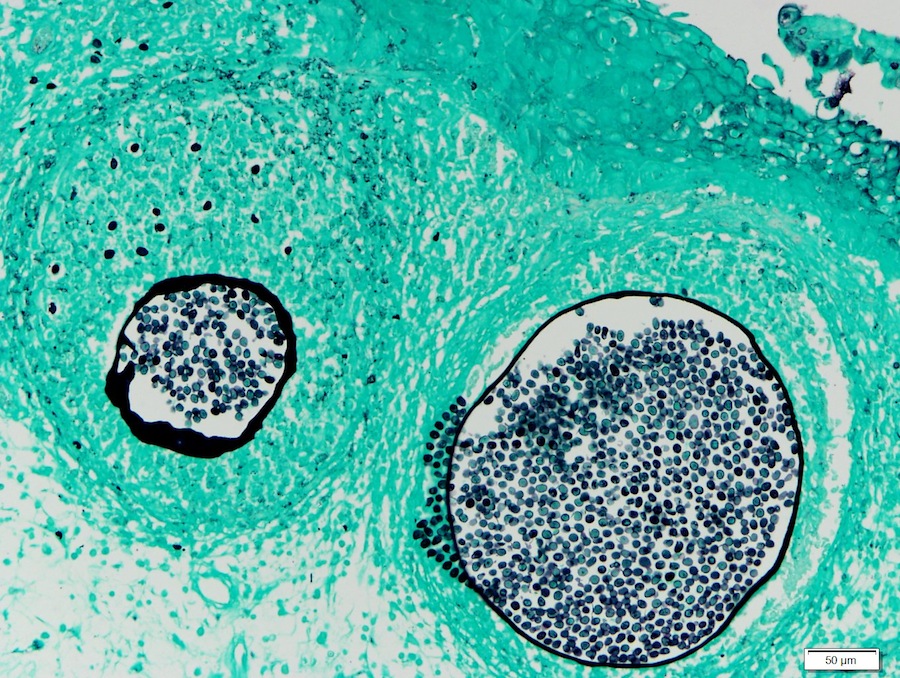
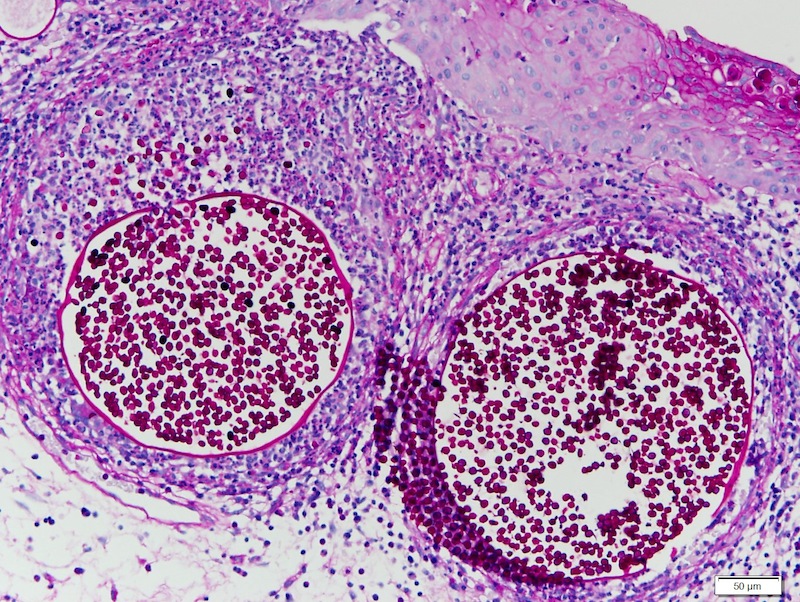
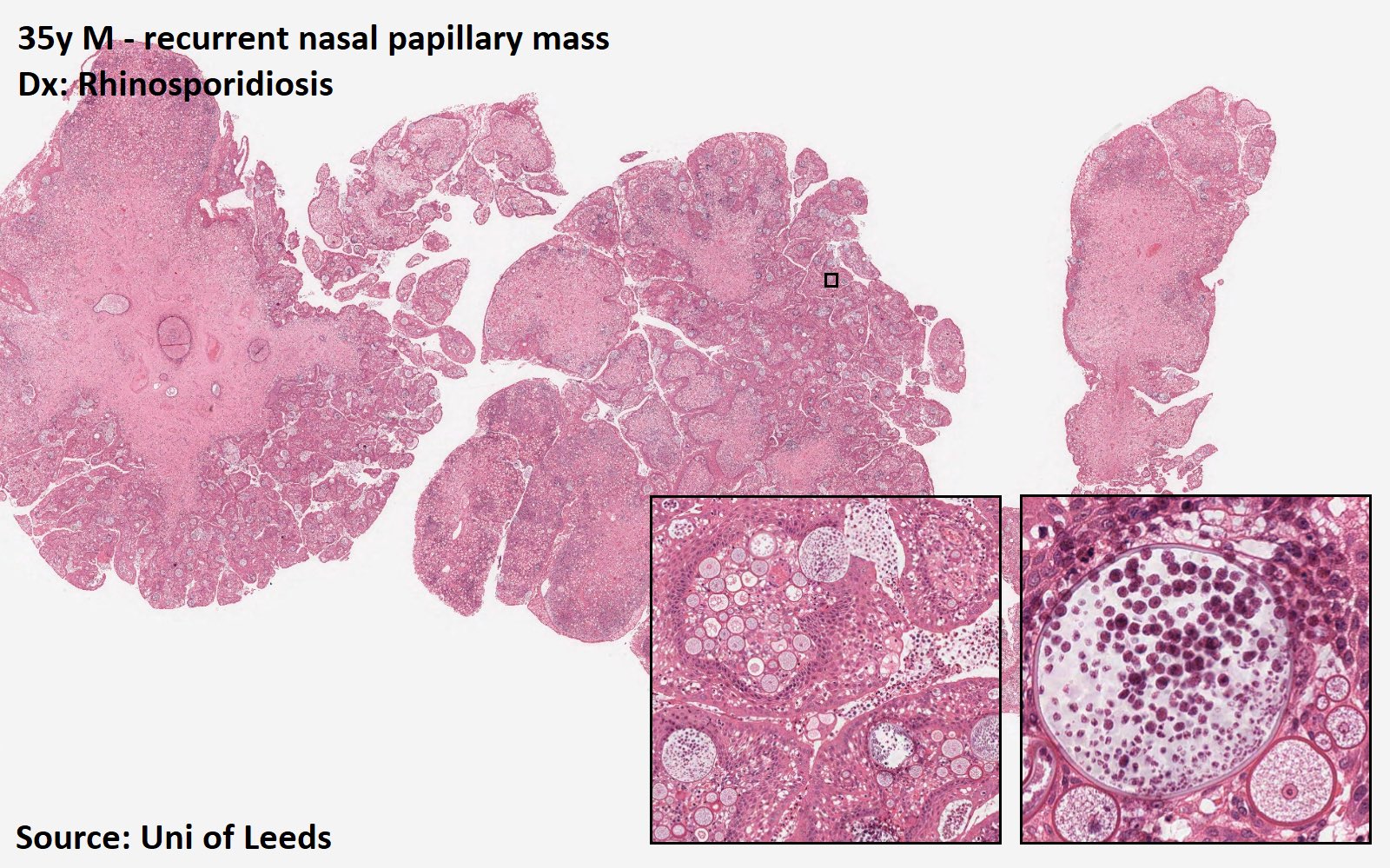
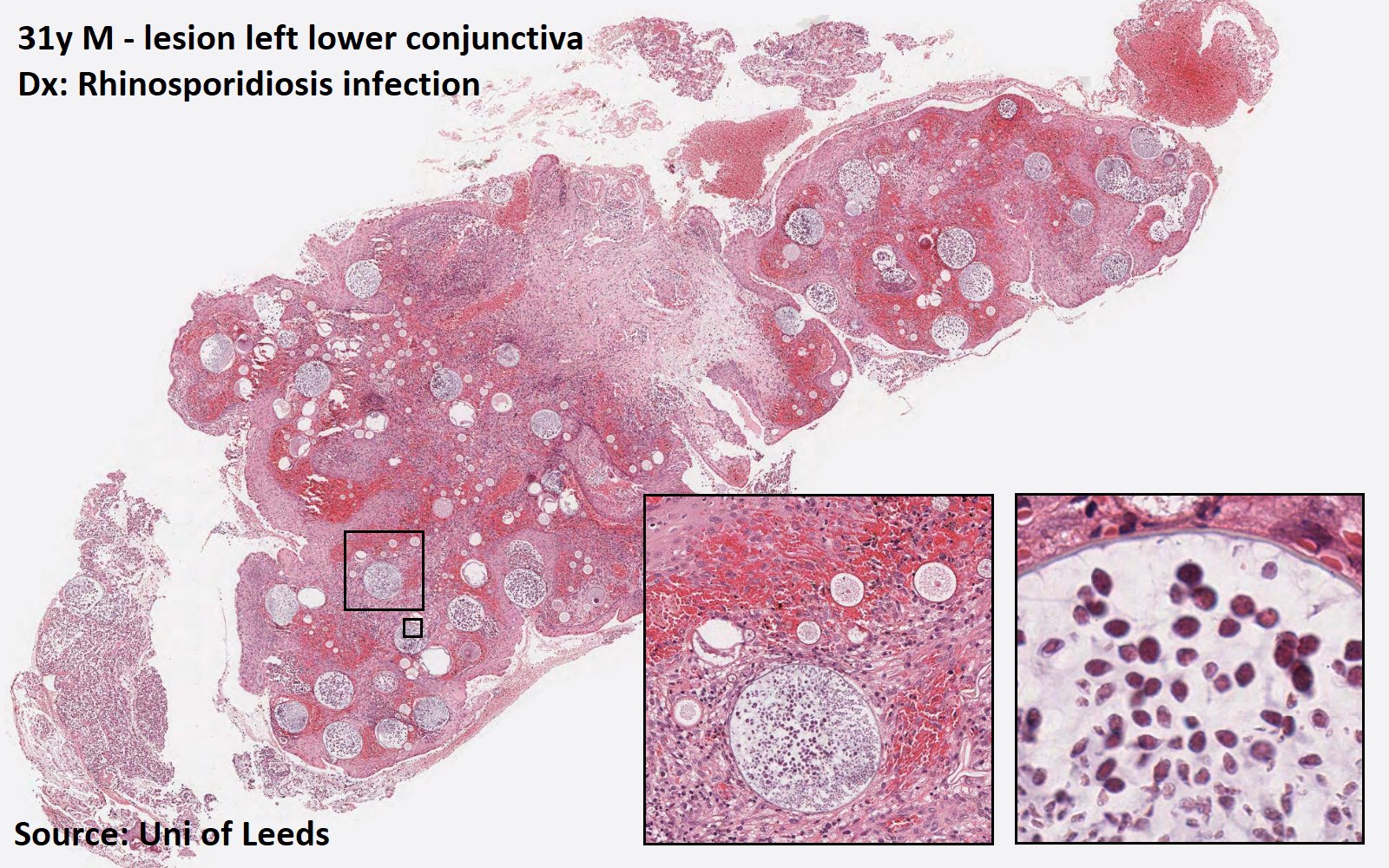
Rhinosporidiosis
Salivary gland anlage tumor
Seromucinous hamartoma
Sinonasal carcinoma-general
Sinonasal glomangiopericytoma
Sinonasal lymphoepithelial carcinoma (pending)
Sinonasal papilloma
Sinonasal undifferentiated carcinoma
Squamous cell carcinoma
Staging terminology
Staging-nasal cavity & sinuses
Staging-nasopharynx
SWI / SNF complex deficient sinonasal carcinoma
Teratocarcinosarcoma
WHO classificationAdenocarcinoma-general
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
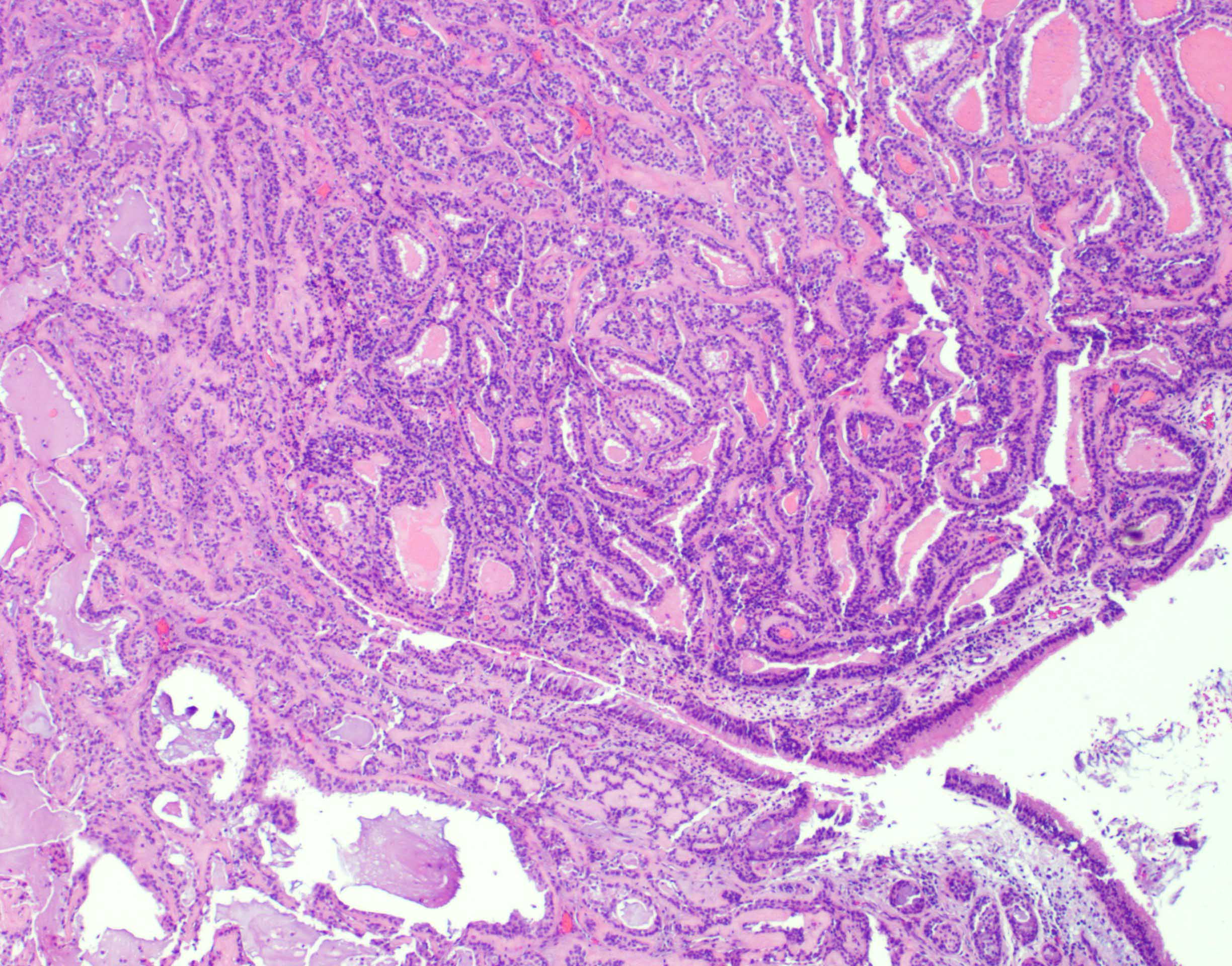
- Sinonasal adenocarcinoma (SNAC) is a relatively rare and heterogenous type of carcinoma of the sinonasal region (i.e., the nasal cavity and paranasal sinuses) with glandular differentiation
- May originate from respiratory surface epithelium or the underlying seromucinous glands (Head Neck Pathol 2016;10:68)
Essential features
- Rare type of carcinoma originating from the sinonasal area (nasal cavity and paranasal sinuses)
- On histology, the tumor resembles adenocarcinoma that arises at other body locations
- Several histologic tumor subtypes are described
- Adequate surgical resection is the usual treatment but has poor prognosis
ICD coding
- ICD-O:
- ICD-11:
- 2C20.0 & XH0349 - adenocarcinoma of nasal cavity & adenocarcinoma, intestinal type
- 2C22.0 & XH0349 - adenocarcinoma of accessory sinuses & adenocarcinoma, intestinal type
- 2C20.0 & XH74S1 - adenocarcinoma of nasal cavity & adenocarcinoma, NOS
- 2C22.0 & XH74S1 - adenocarcinoma of paranasal sinuses & adenocarcinoma, NOS
Epidemiology
- Second most common malignancy of the sinonasal region, after squamous cell carcinoma, comprising 10 - 20% of primary sinonasal malignancies (Acta Otolaryngol 2018;138:415)
- Average age of presentation is between 50 and 60 years (Nat Rev Clin Oncol 2014;11:460)
- Higher incidence in Europe compared to North America, while less is known about the incidence in Asia, Africa, Oceania and South America (J Neurooncol 2020;150:405)
- Higher incidence in men compared to women (up to 6:1 in ITAC), probably due to occupational hazards (Nat Rev Clin Oncol 2014;11:460)
Sites
- Intestinal type adenocarcinoma (ITAC) is most often localized in the ethmoid sinus (40%), followed by the nasal cavity (25%) and the maxillary antrum (20%)
- ITAC associated with wood dust exposure occurs predominantly in the ethmoid sinus, while sporadic ITAC often arises in the maxillary sinus
- Due to their aggressive nature, ITAC may spread to adjacent structures, including the orbit, the pterygopalatine fossa, the infratemporal fossa and the cranial cavity
- Low grade nonintestinal type adenocarcinomas are uncommon and occur mainly in the ethmoid sinus, the nasal cavity and the maxillary sinuses (Head Neck Pathol 2016;10:68)
Etiology
- Occupational exposure to wood dust (furniture), leather (shoe production), chromium and nickel
- Large particle dust from certain hardwoods (ebony, oak and beech) is thought to provide a 900 fold risk of developing adenocarcinoma (Am J Rhinol Allergy 2013;27:S35)
- Cumulative exposure time to wood dust in patients with ITAC has been 40 - 43 years (Head Neck Pathol 2016;10:68)
- < 5 years of exposure is still considered critical and the latency to tumor development is delayed (~40 years)
- Other significant etiologic associations include
- Alcohol and cigarette smoking (Head Neck 2014;36:1490)
- Formaldehyde (Cancer Causes Control 2002;13:147)
- Leather dust (Acta Otorhinolaryngol Ital 2004;24:199)
Clinical features
- Usually nonspecific clinical findings that mimic benign conditions, including rhinorrhea, nasal obstruction, epistaxis and hyposmia, highlight the importance of initial clinical suspicion
- Regional symptoms such as neck lumps, orbital changes (proptosis), diplopia, epiphora and cranial nerve dysfunction are relatively uncommon for most subtypes and are seen in advanced stage tumor (Nat Rev Clin Oncol 2014;11:460)
- Visible morphologic changes to hard palate mucosa or overlying skin are rarely seen (Am J Rhinol Allergy 2013;27:S35)
Diagnosis
- Often delayed due to location and nonspecific symptoms (Otolaryngol Clin North Am 2004;37:473)
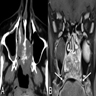
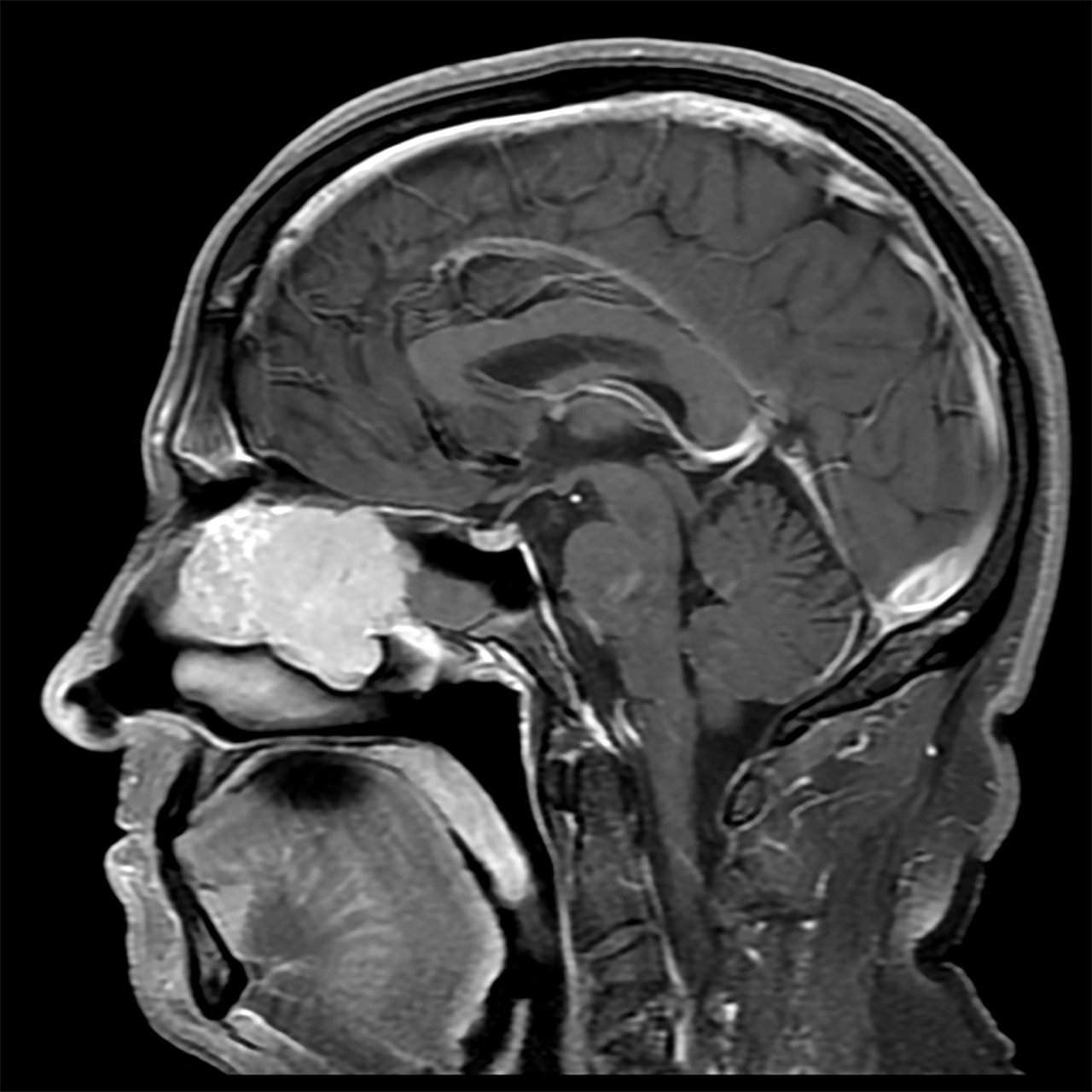
- Most patients will undergo both computed tomography (CT) and magnetic resonance imaging (MRI) to obtain precise anatomical details regarding the tumor localization and extension (staging), which are critical in determining operability or in planning radiotherapy (Nat Rev Clin Oncol 2014;11:460)
- Endoscopic guided biopsy under local anesthesia for histologic confirmation
- If a deep biopsy is required or if profuse bleeding is anticipated, the procedure is performed in an operating room under general anesthesia
- Nonintestinal type adenocarcinoma (non-ITAC) is a diagnosis of exclusion (J Neurooncol 2020;150:405)
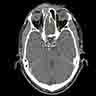
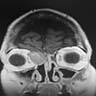
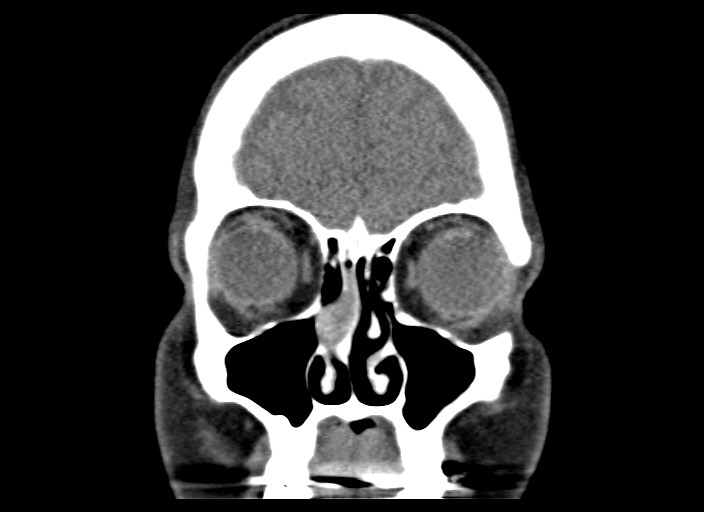
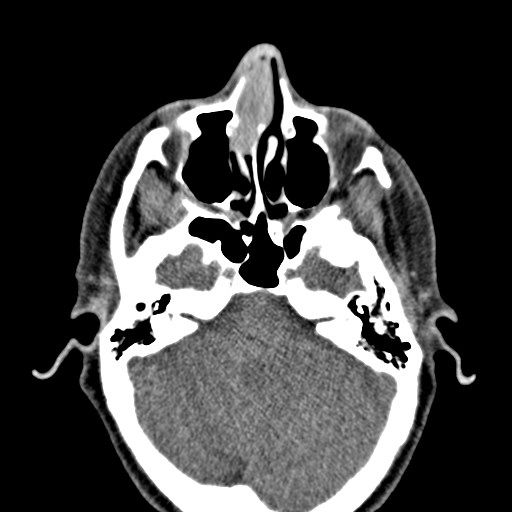
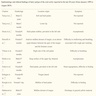
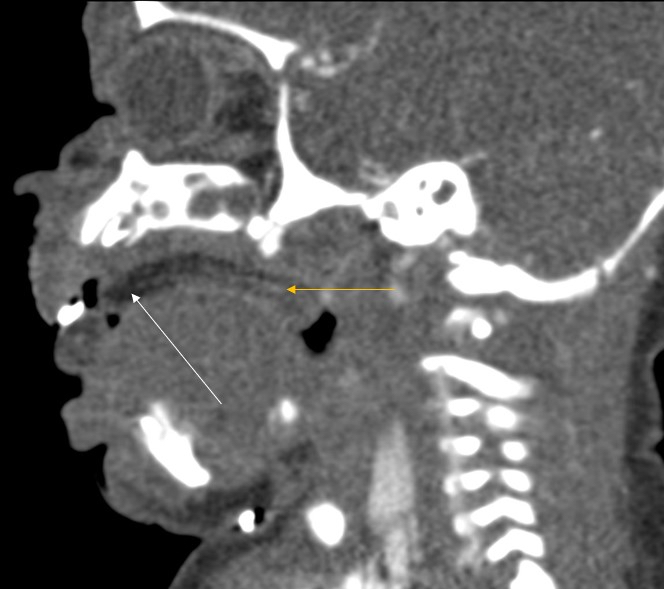
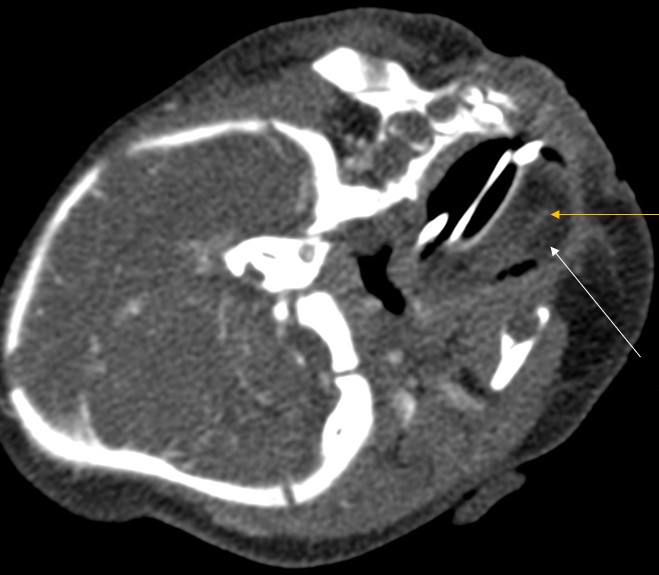
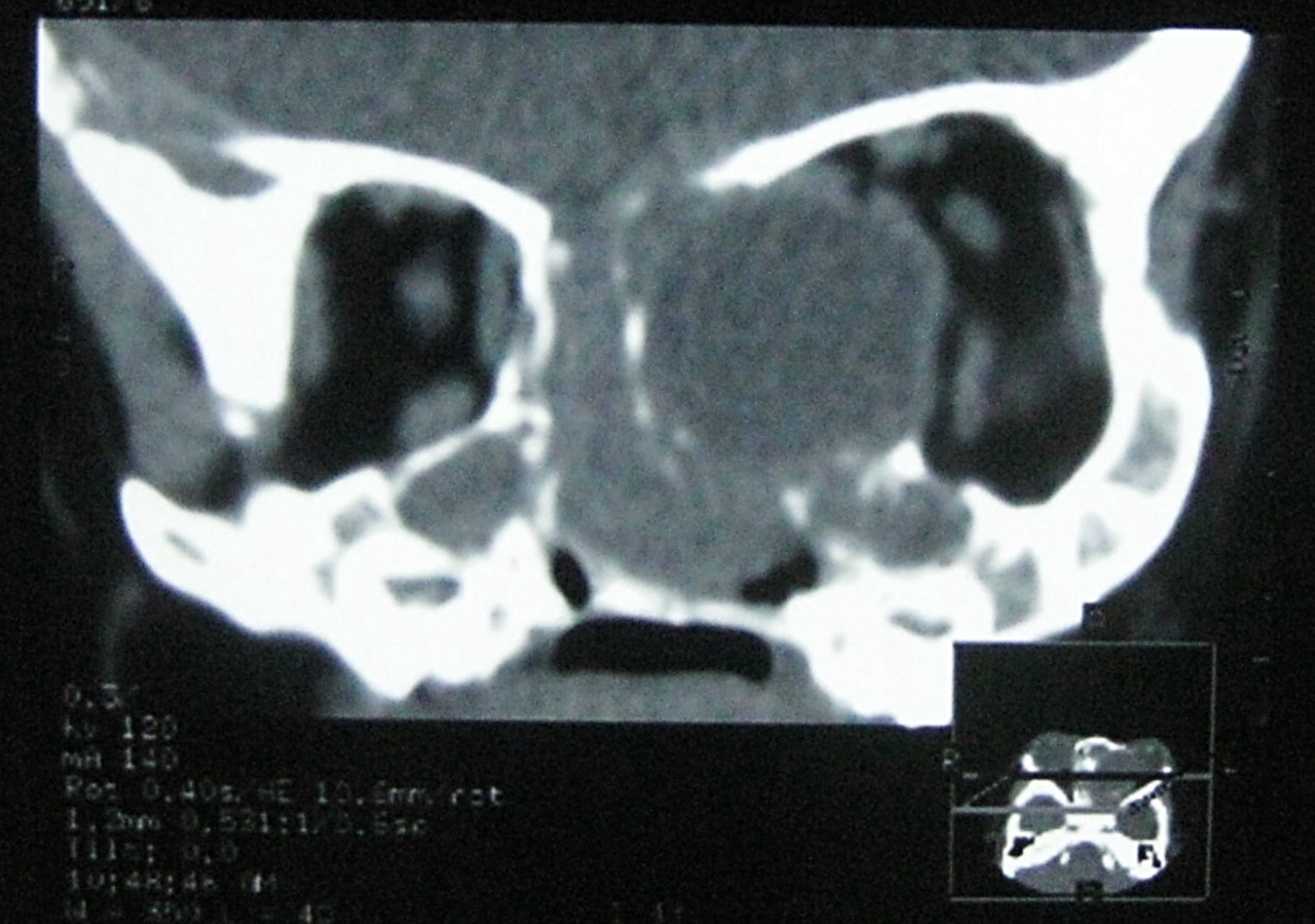
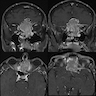
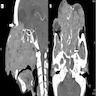
Radiology description
- Presents as an ill defined and heterogeneously enhancing mass in the sinonasal cavity or area in the base of the skull
- Bone involvement is shown by CT scan as an aggressive pattern of bone erosion invading surrounding structures
- Soft tissue details are seen in MRI findings
- PET CT scan defines the regional and distant metastasis; an efficient tool for tumor staging (Am J Rhinol Allergy 2013;27:S35)
Radiology images
Prognostic factors
- ITAC: usually advanced disease at presentation, poor prognosis with a 5 year survival rate of ~60% depending upon disease extent (local extension and metastasis) and histologic type
- Low grade non-ITAC: overall favorable outcome; usually localized but the possibility of localized recurrence, metastasis is unusual, death is rare
- High grade non-ITAC: poor prognosis (Head Neck Pathol 2016;10:68)
- High grade, signet ring cell morphology and local invasion have a worse prognosis
Case reports
- 26 year old man presented with 3 - 4 months of epistaxis, bilateral nasal obstruction, hyposmia and headaches (Cureus 2021;13:e14285)
- 29 year old woman was referred for a posterior choanal mass (Ear Nose Throat J 2022 Aug 10 [Epub ahead of print])
- 44 year old man, without exposure to wood dust, presented with left nasal discharge and obstruction and left sided headache (South Asian J Cancer 2020;9:183)
- 45 year old man who was a chronic smoker presented with an isolated retro-orbital headache, resistant to analgesics (Pan Afr Med J 2014;18:284)
- 81 year old man who was formerly a carpenter presented with unilateral deteriorating vision (Ugeskr Laeger 2018;180:V12170942)
- 84 year old male nonsmoker and nondrinker complained of nasal fullness (Ann Ital Chir 2016;87:S2239253X16025019)
Treatment
- Adequate surgical resection is the usual treatment
- Surgery followed by radiotherapy for advanced stage disease or if there is a possibility of recurrence
- Radiotherapy or combined chemoradiotherapy may be an alternative to definitive surgery for a nonsurgical candidate
- Reference: J Neurol Surg B Skull Base 2020;81:627
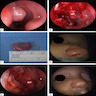
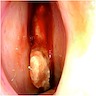
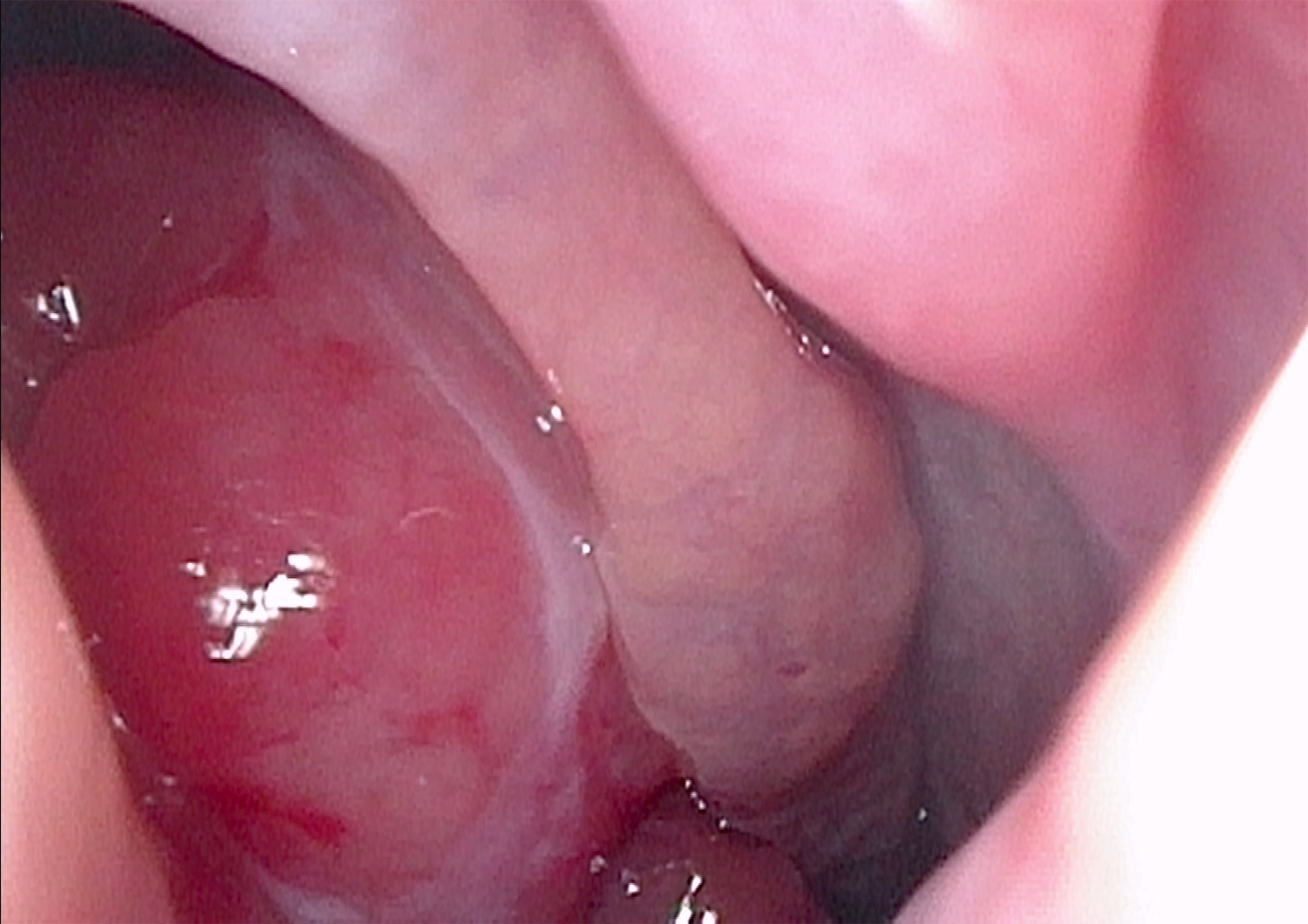
Clinical images
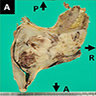
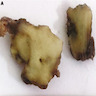
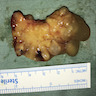
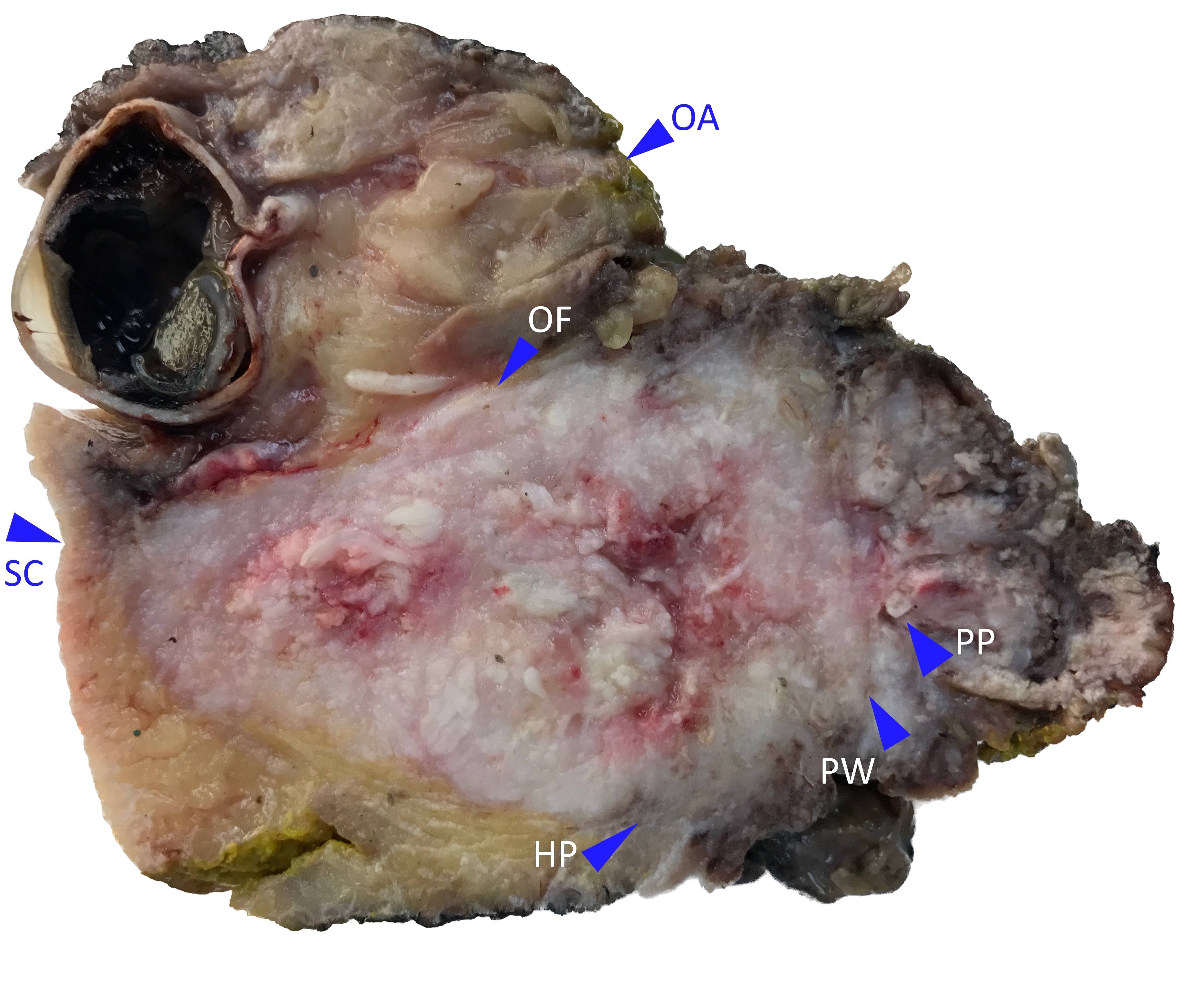
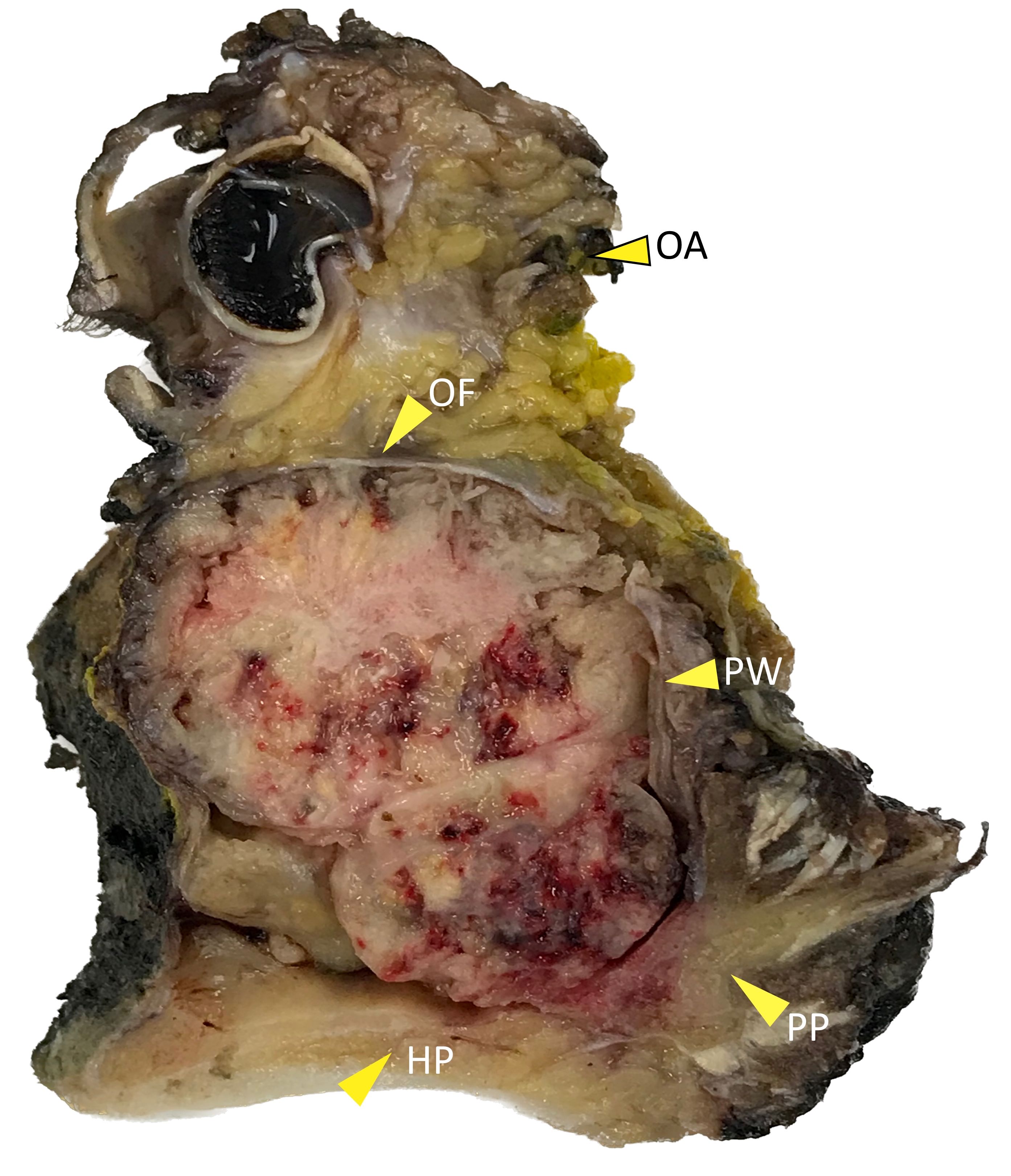
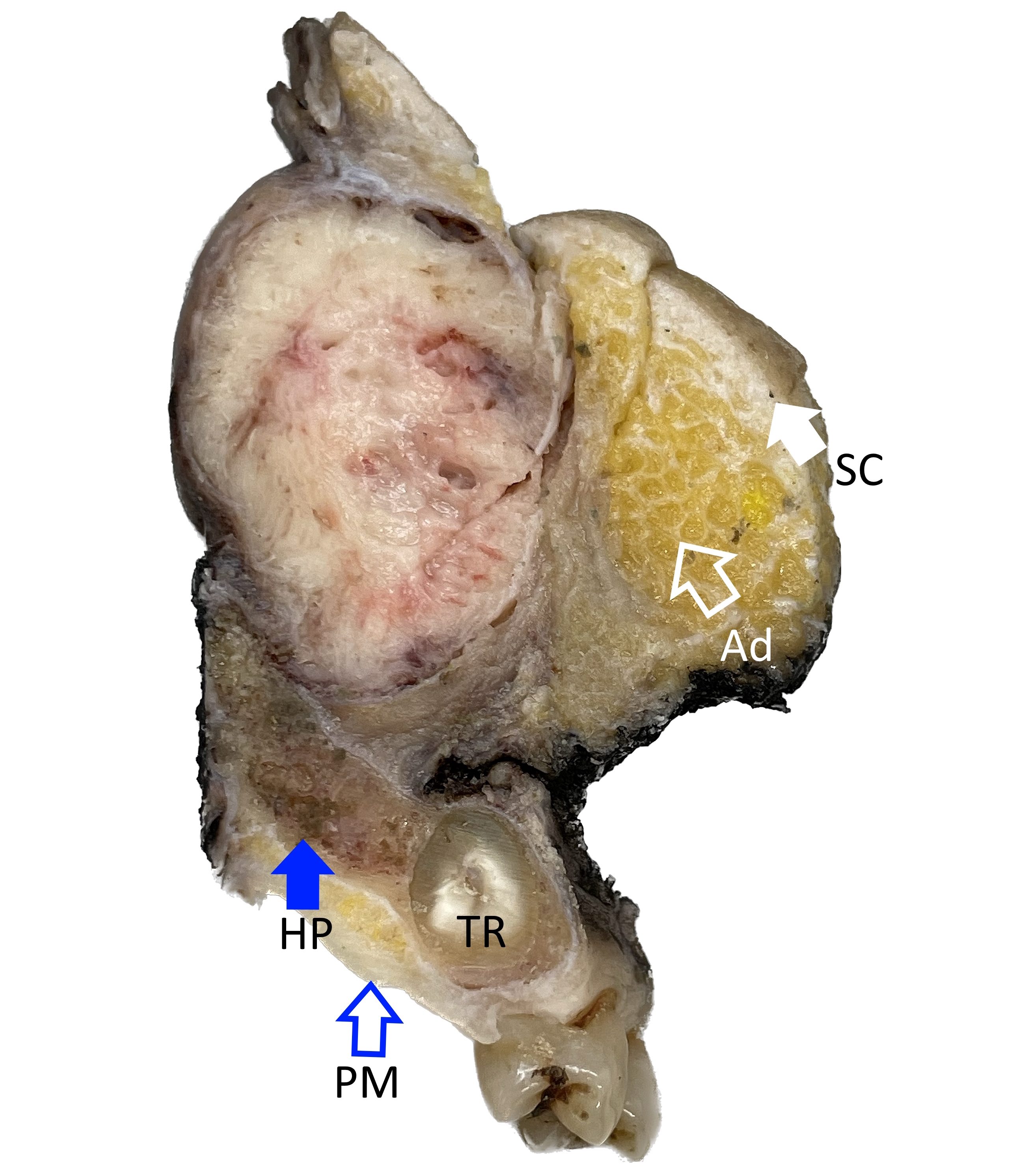
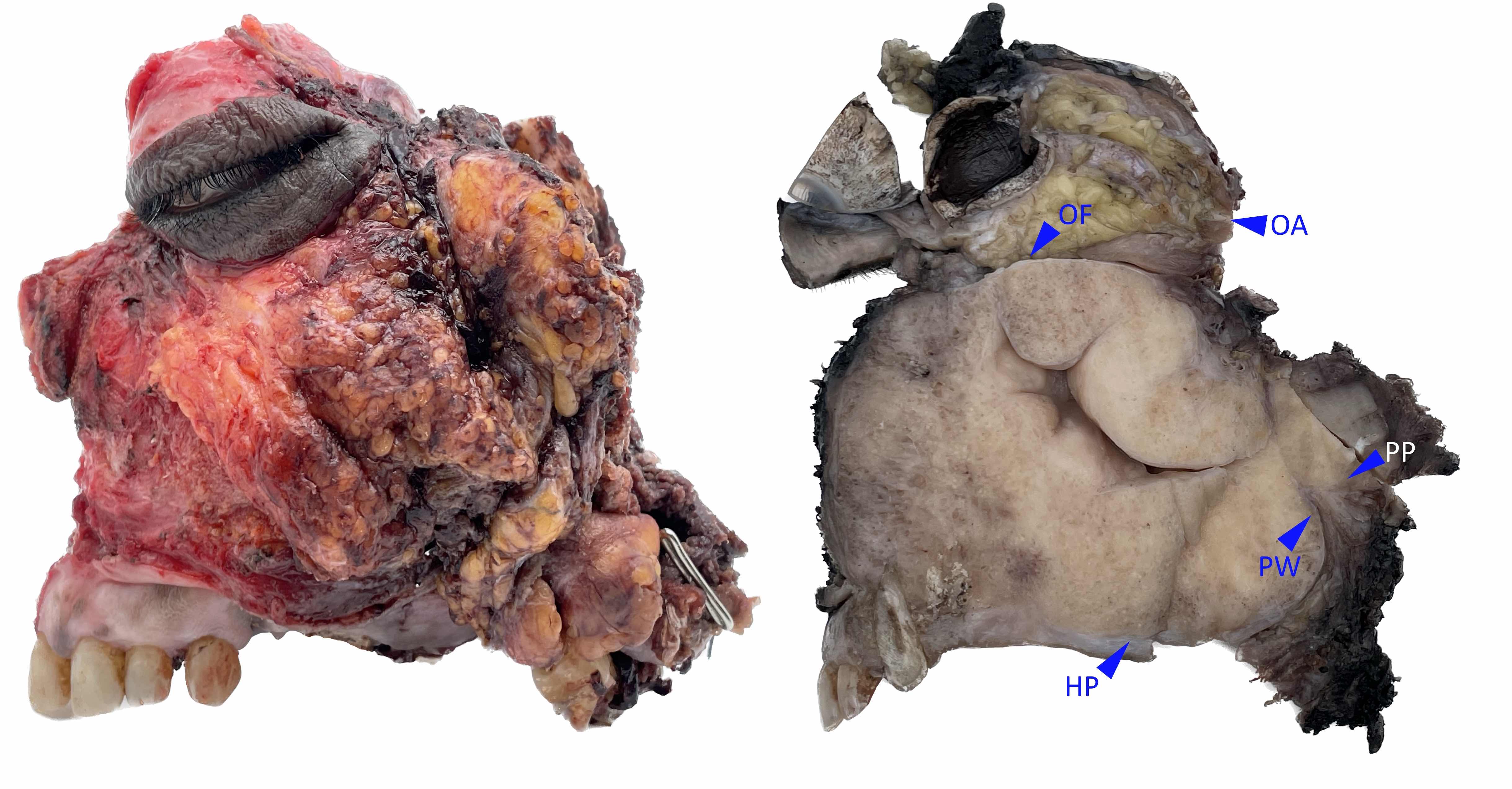
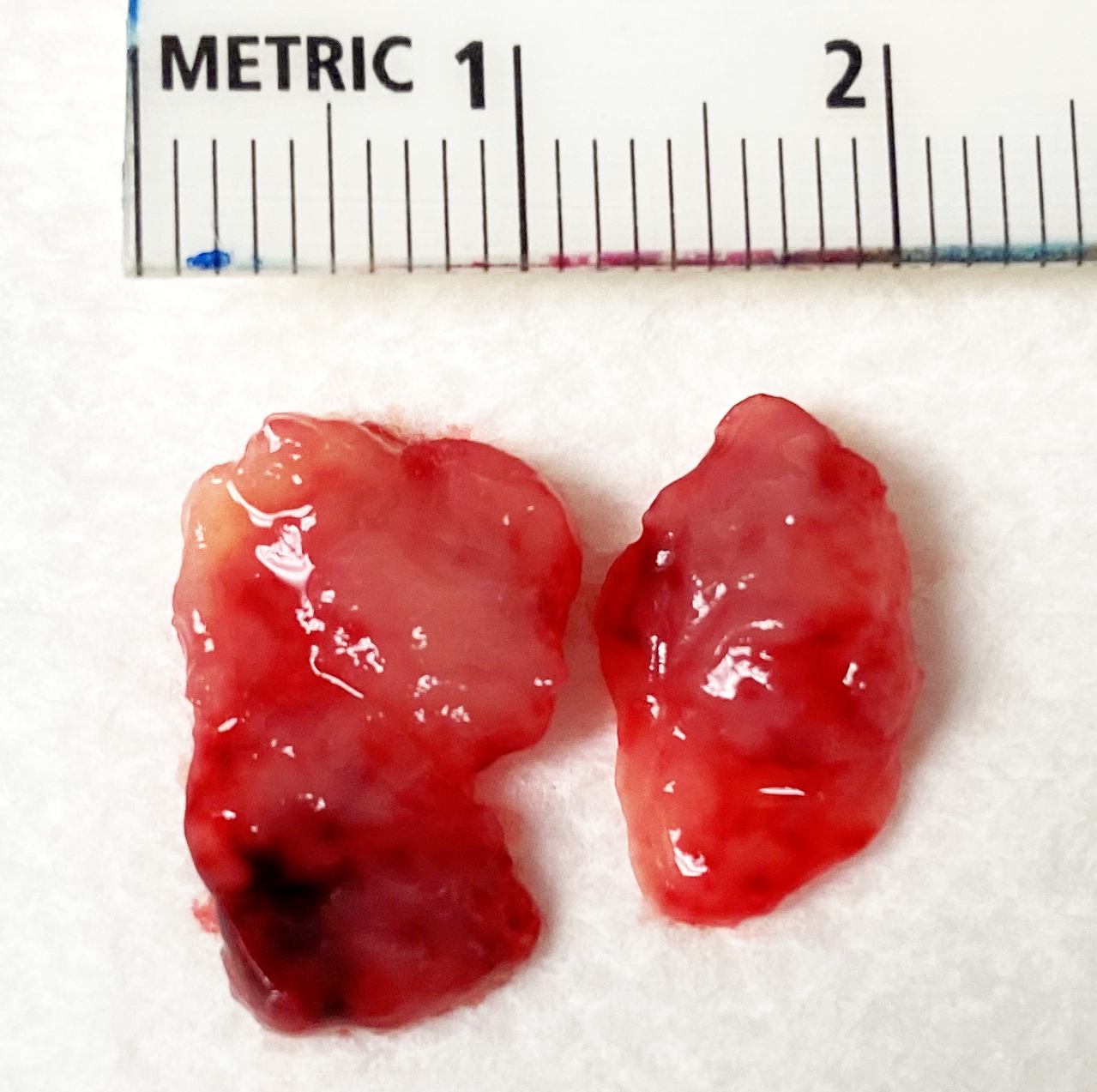
Gross description
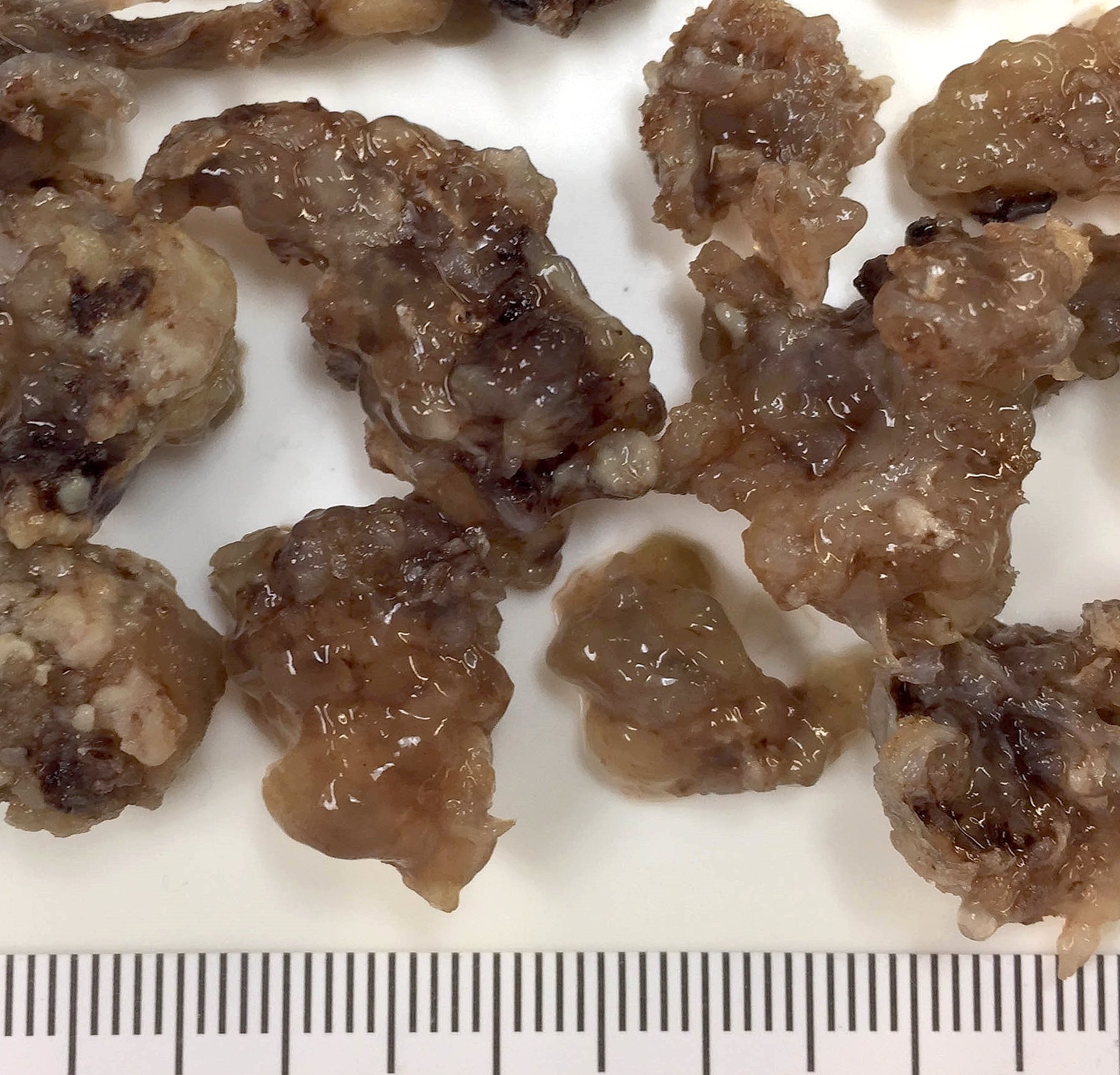
- Polypoidal, fungating, nodular or papillary mass
- Usually friable, with ulceration and hemorrhage
- Rarely gelatinous or mucoid
- Reference: West Indian Med J 2014;63:678
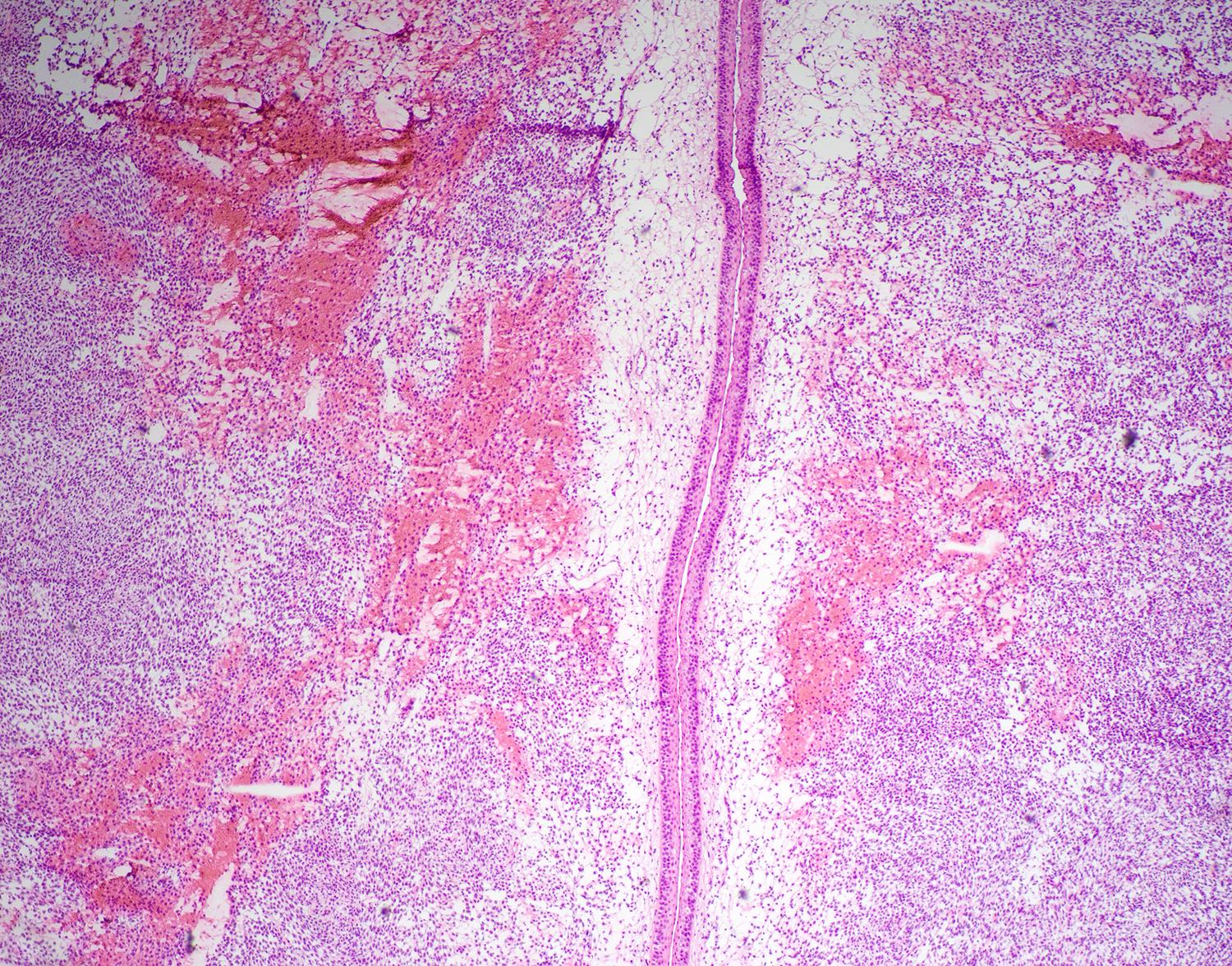
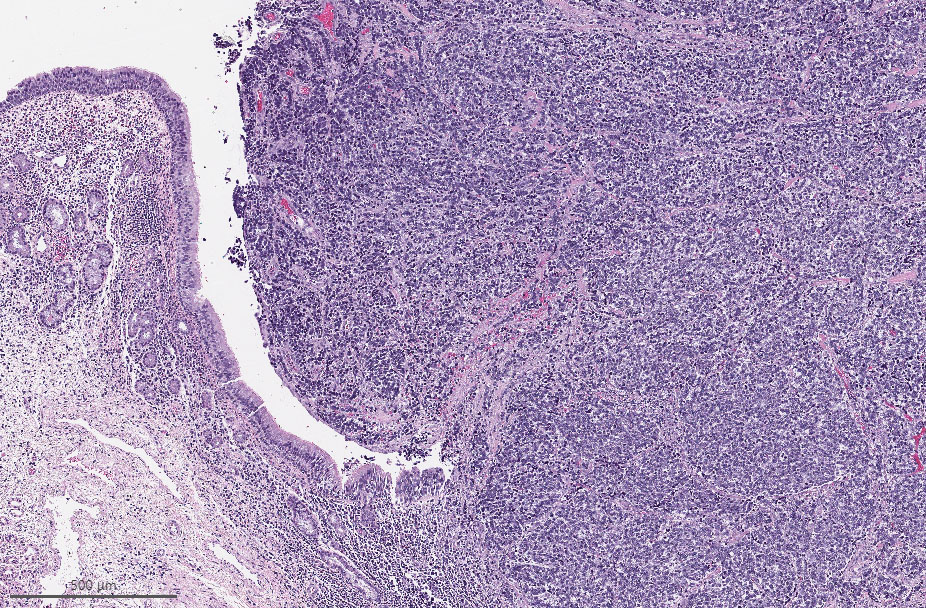
Microscopic (histologic) description
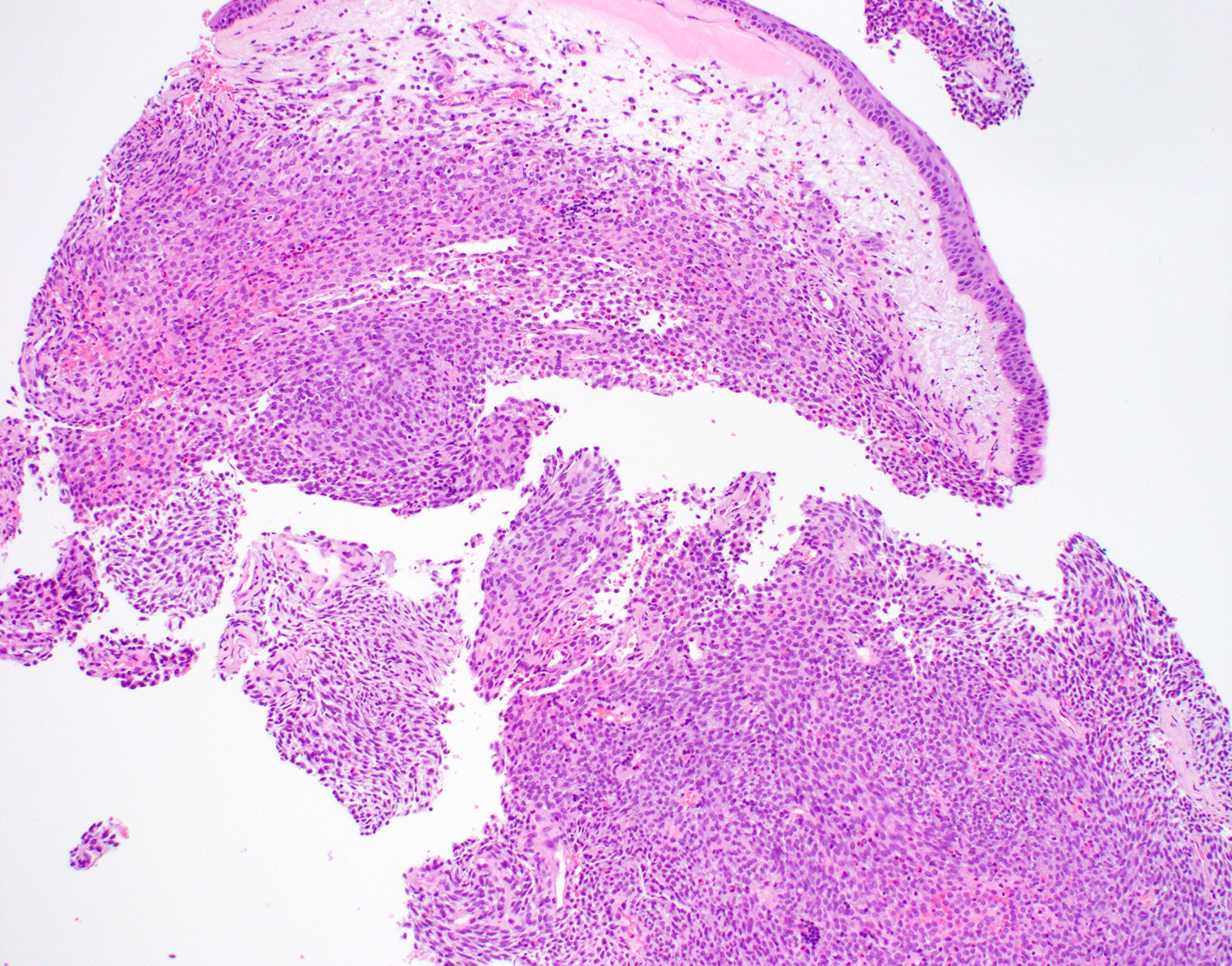
- Per the WHO classification, based on the histologic characteristics, sinonasal adenocarcinoma is divided into
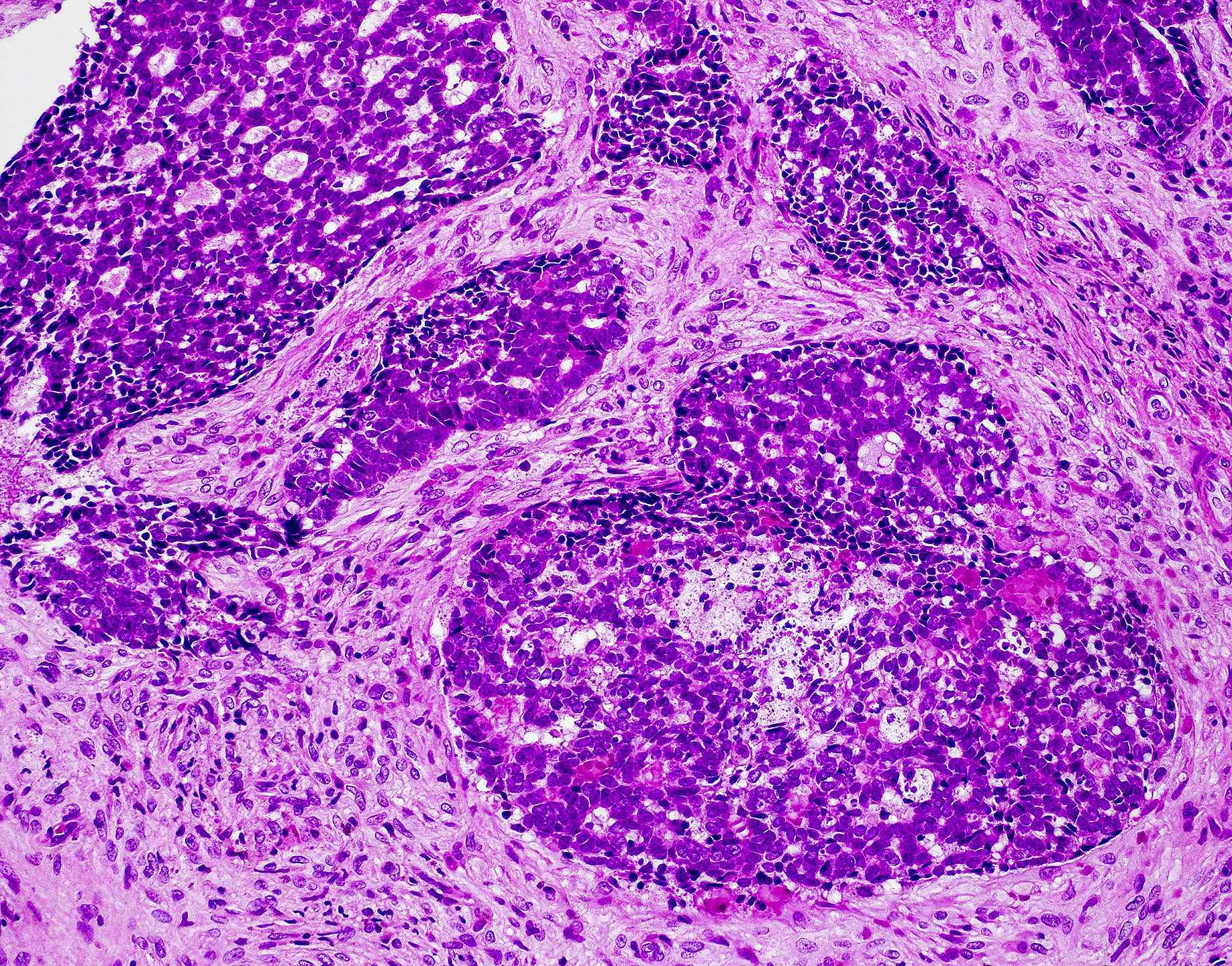
- Intestinal adenocarcinoma (ITAC)
- Nonintestinal adenocarcinoma (non-ITAC)
- Non-ITAC is subsequently classified into
- Low grade
- High grade (Acta Otolaryngol 2018;138:415)
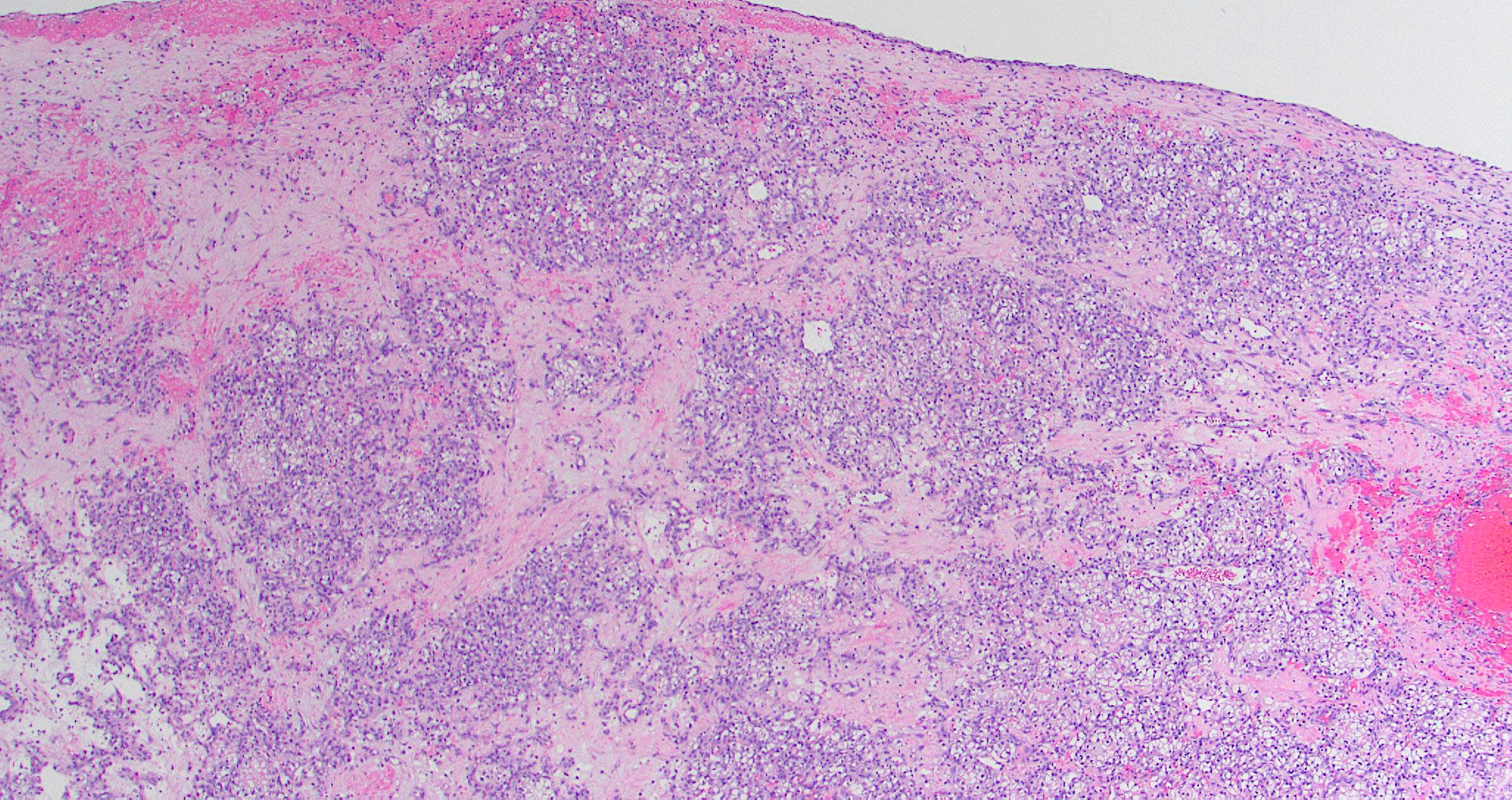
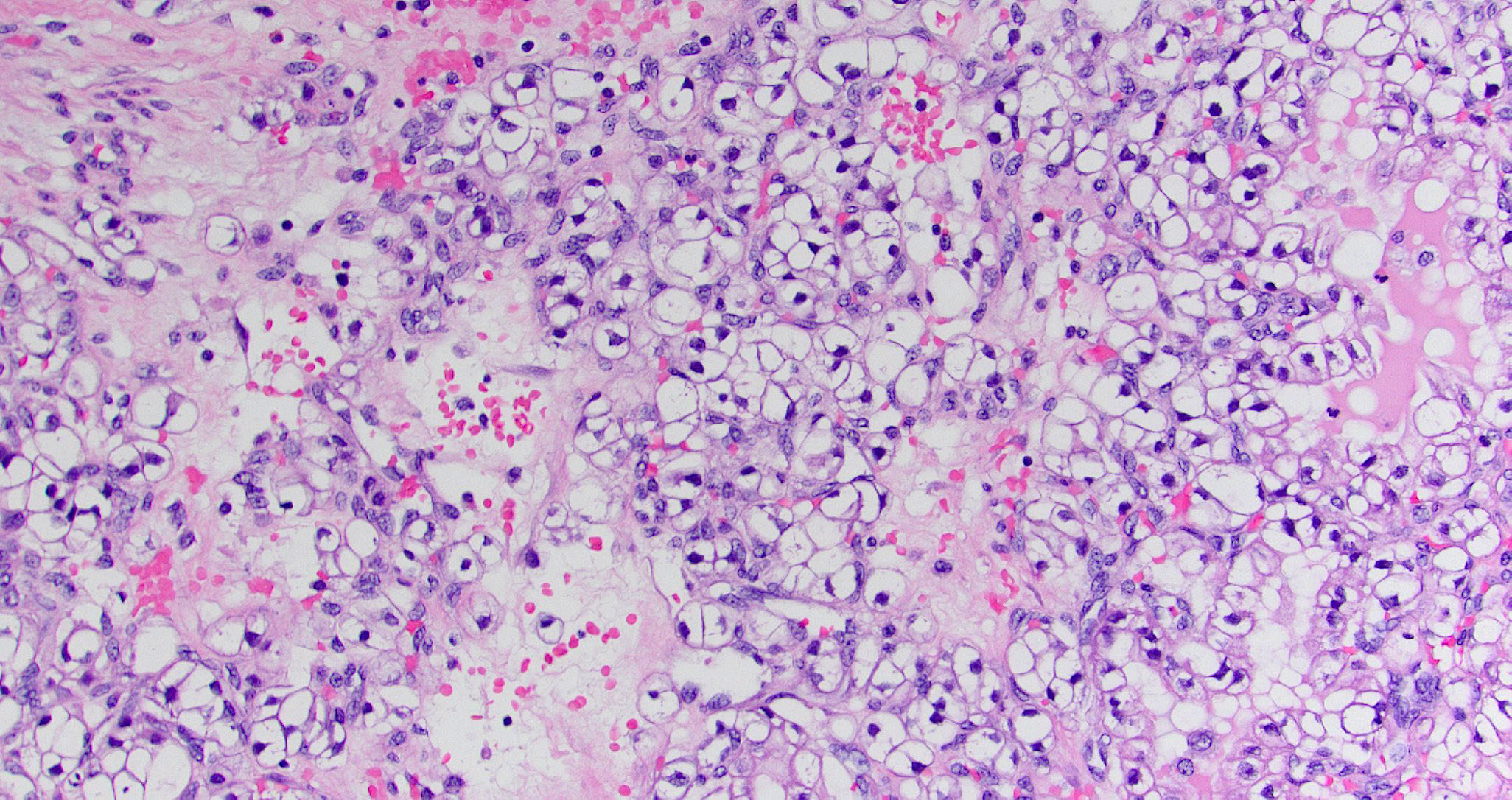
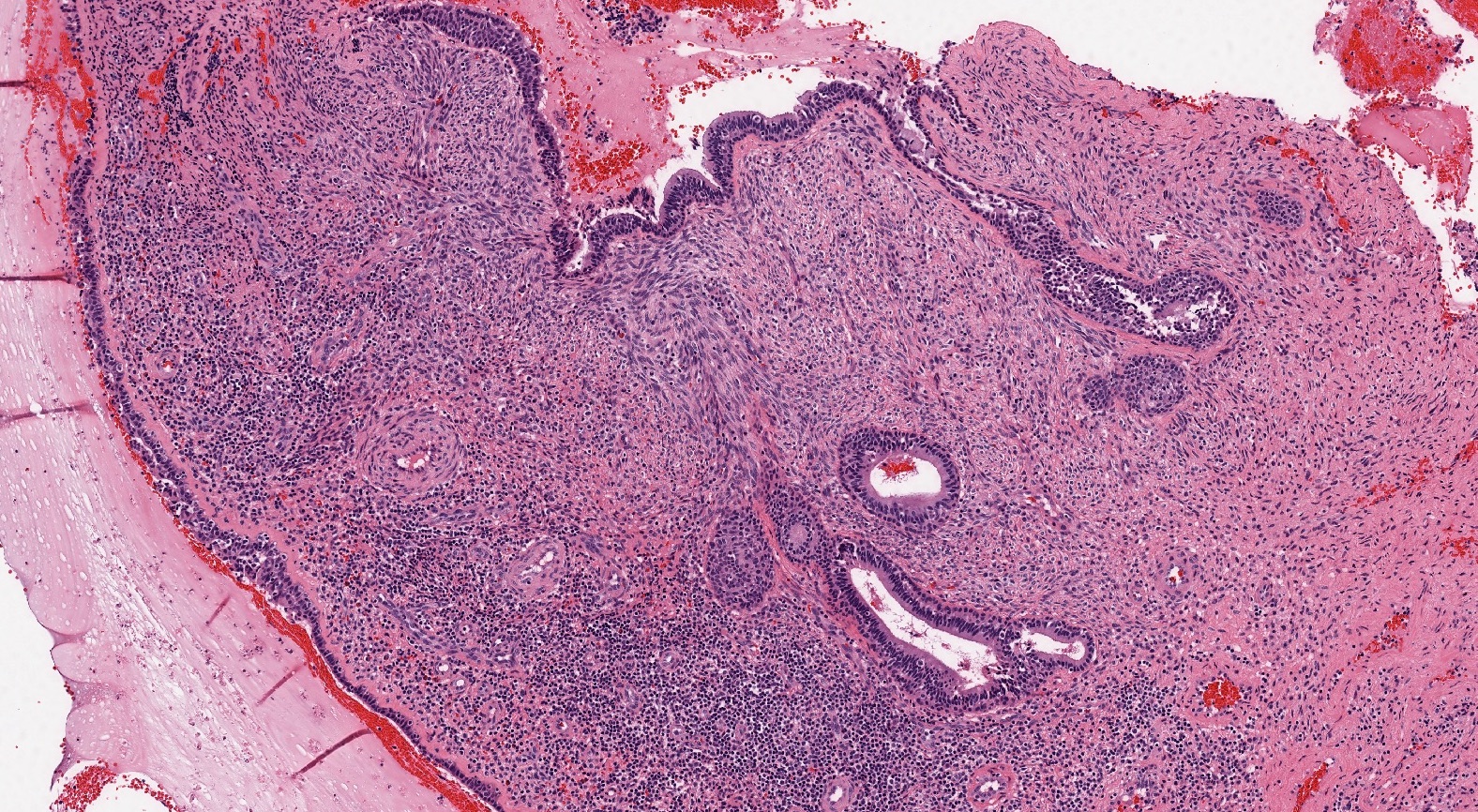
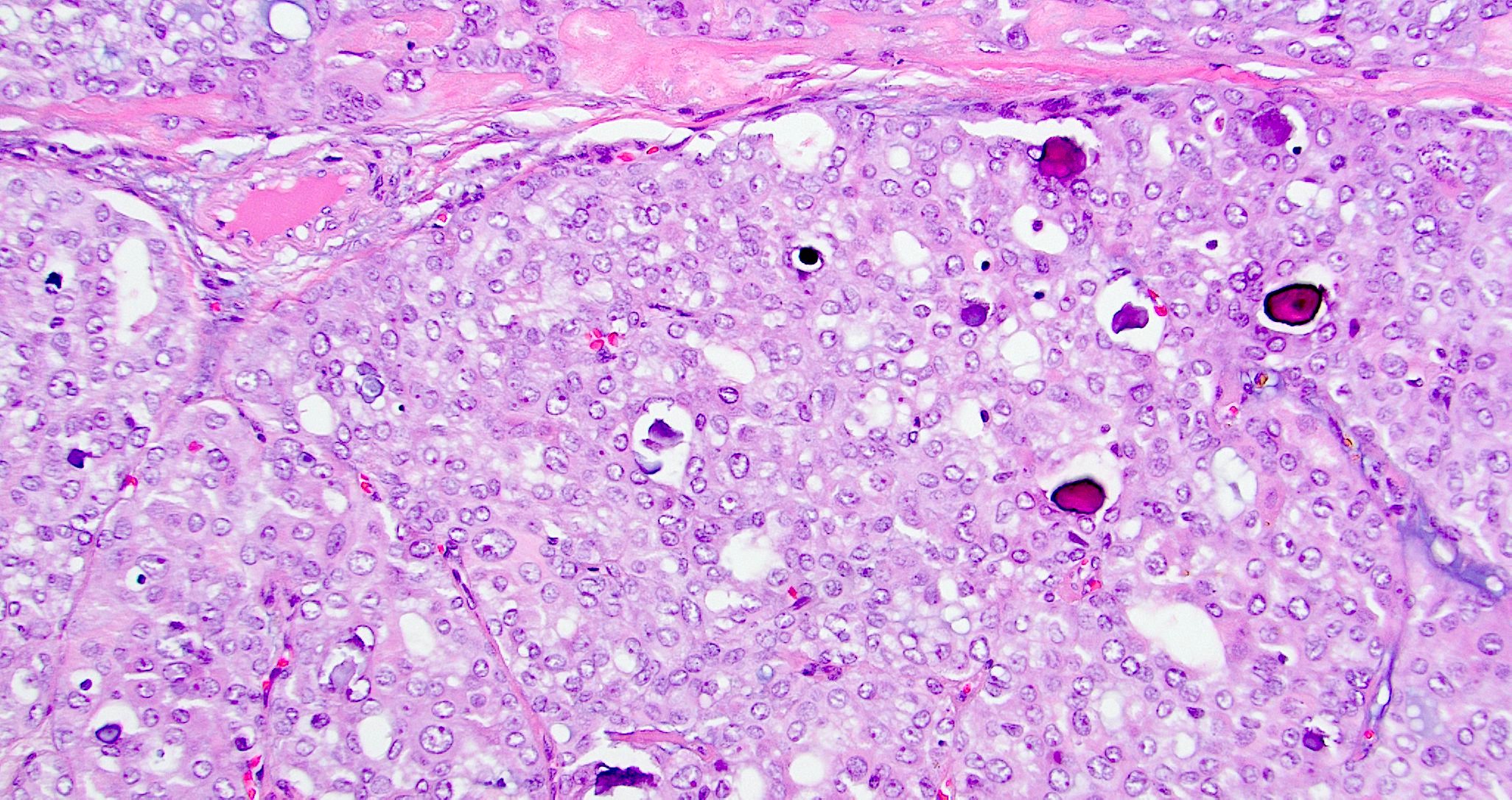
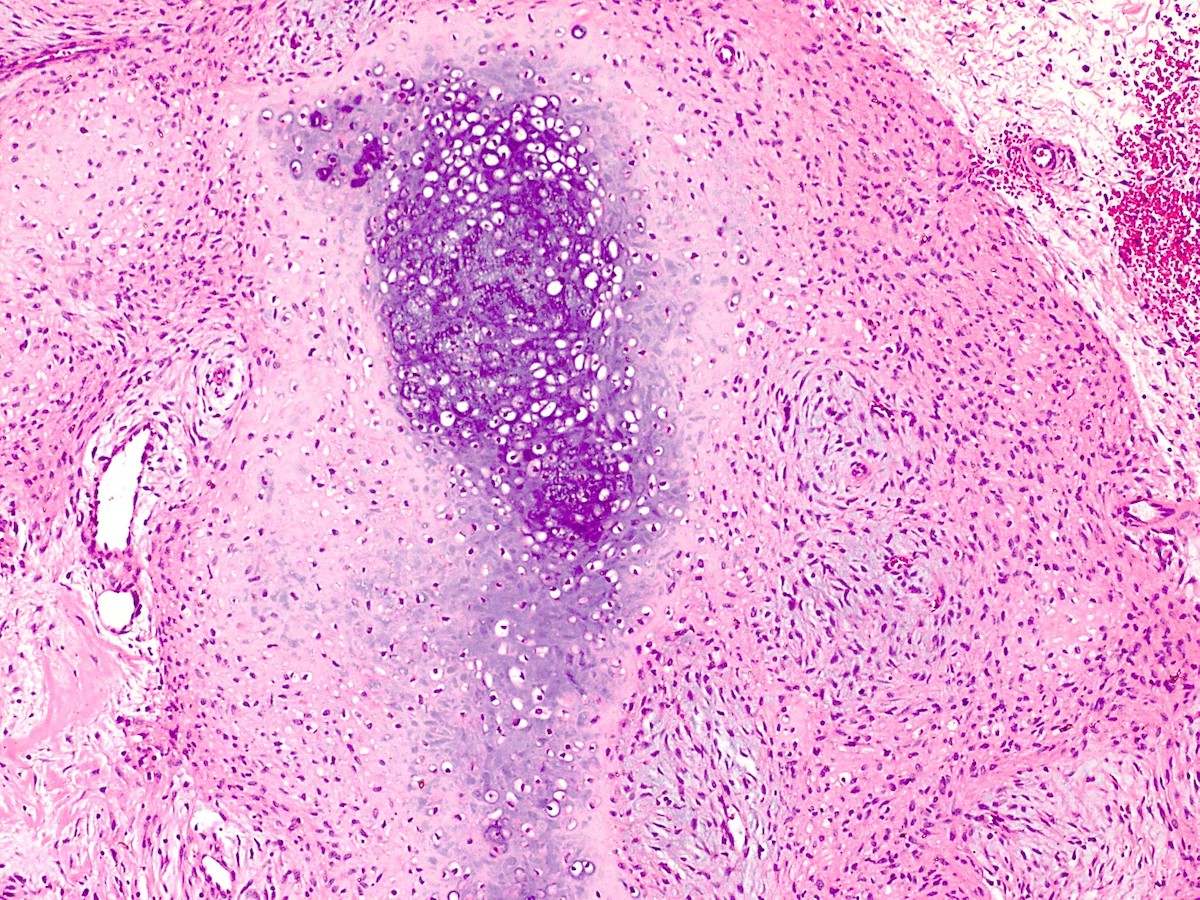
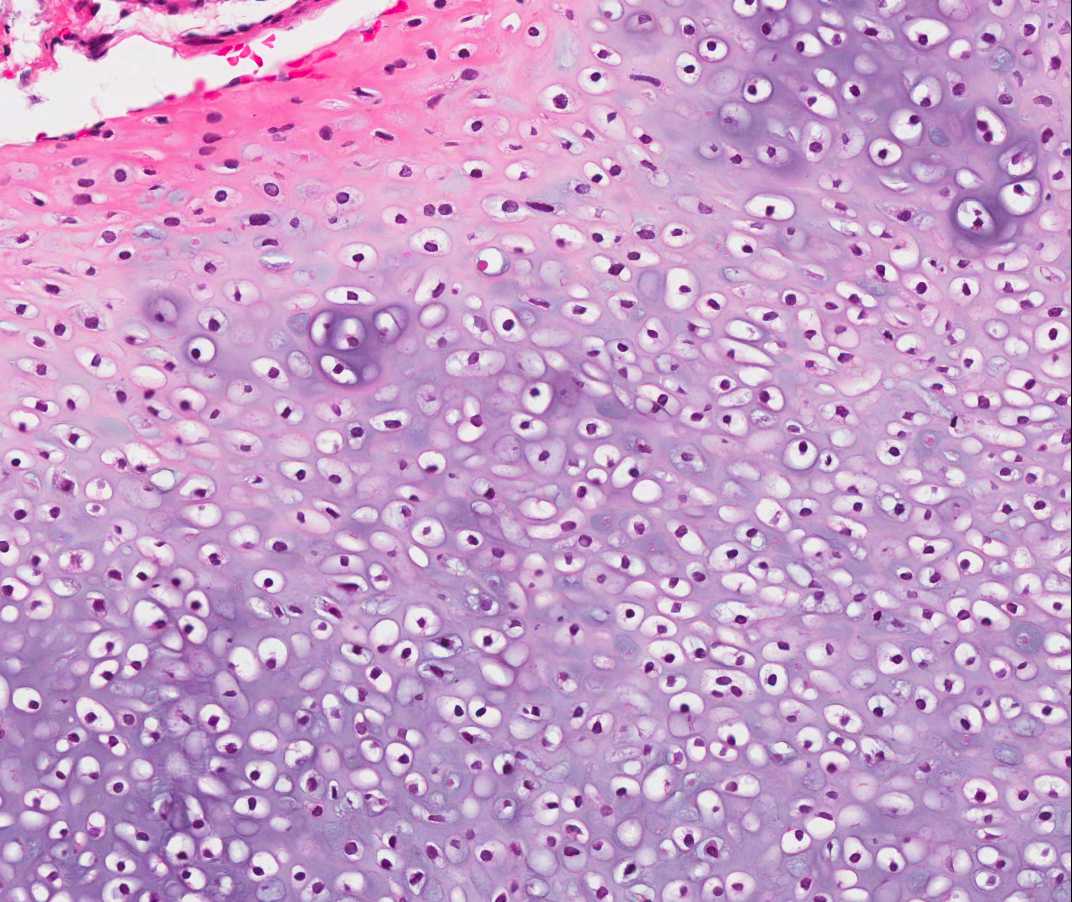
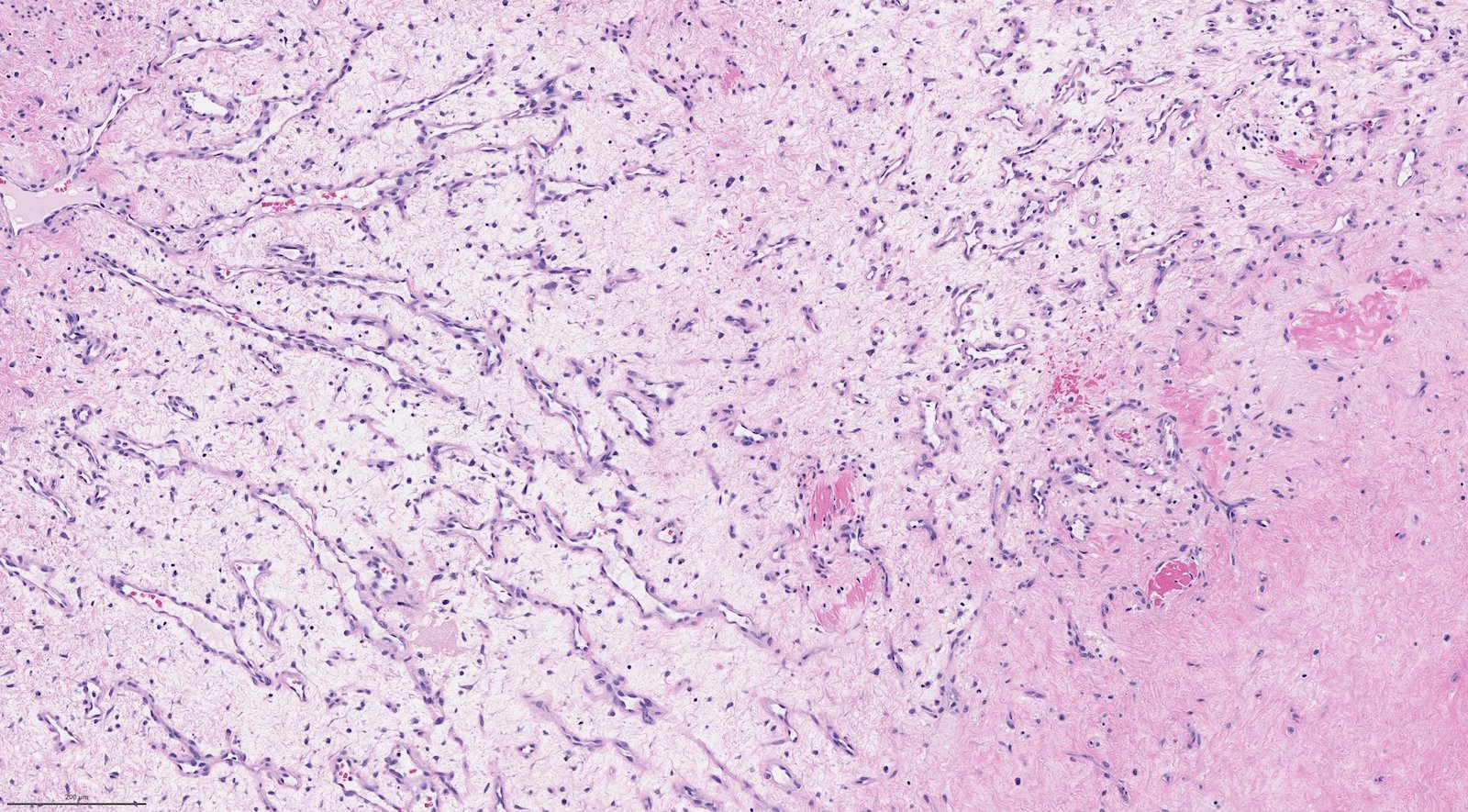
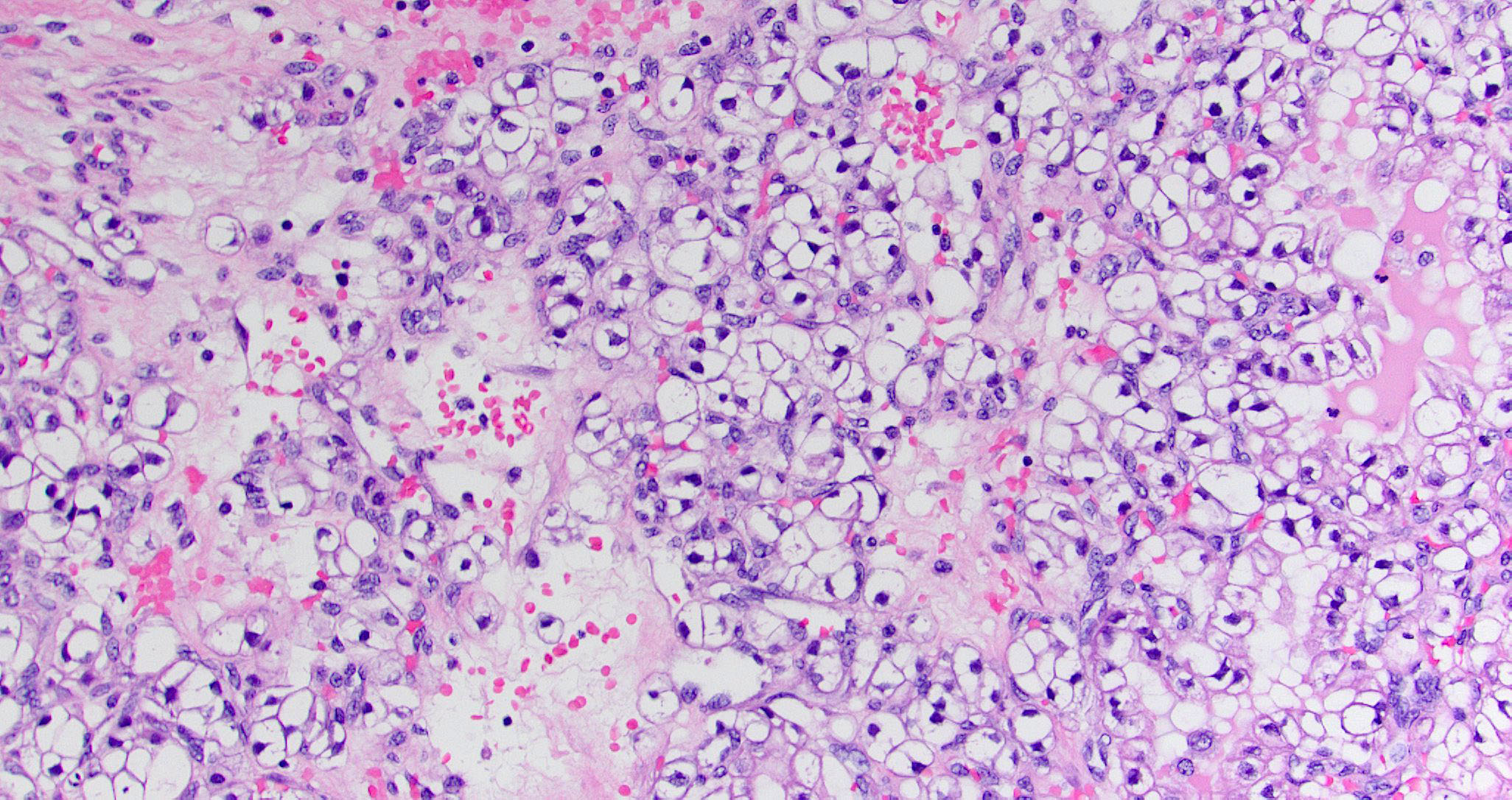
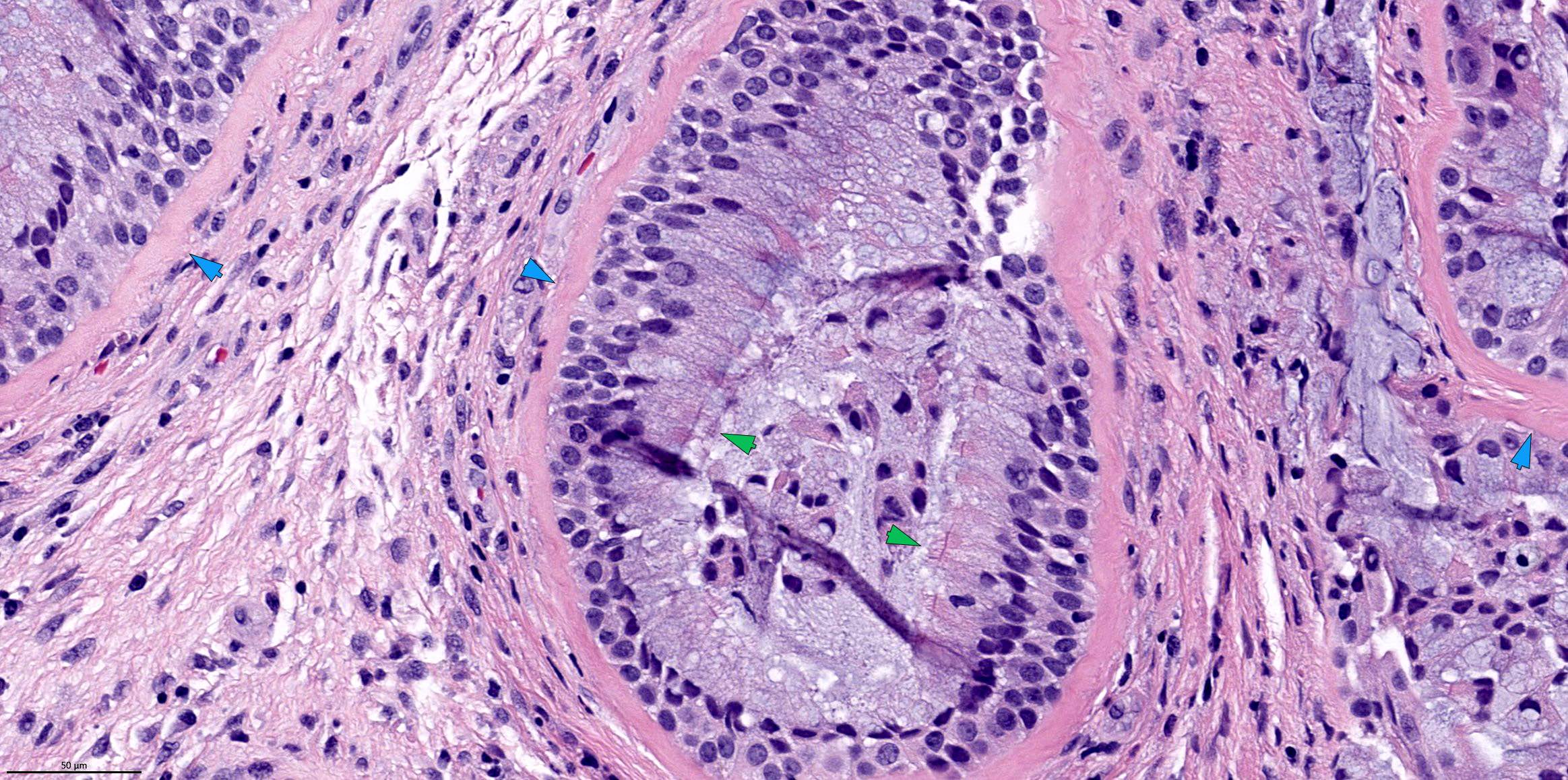
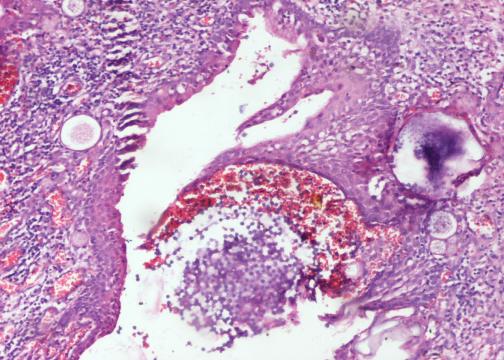
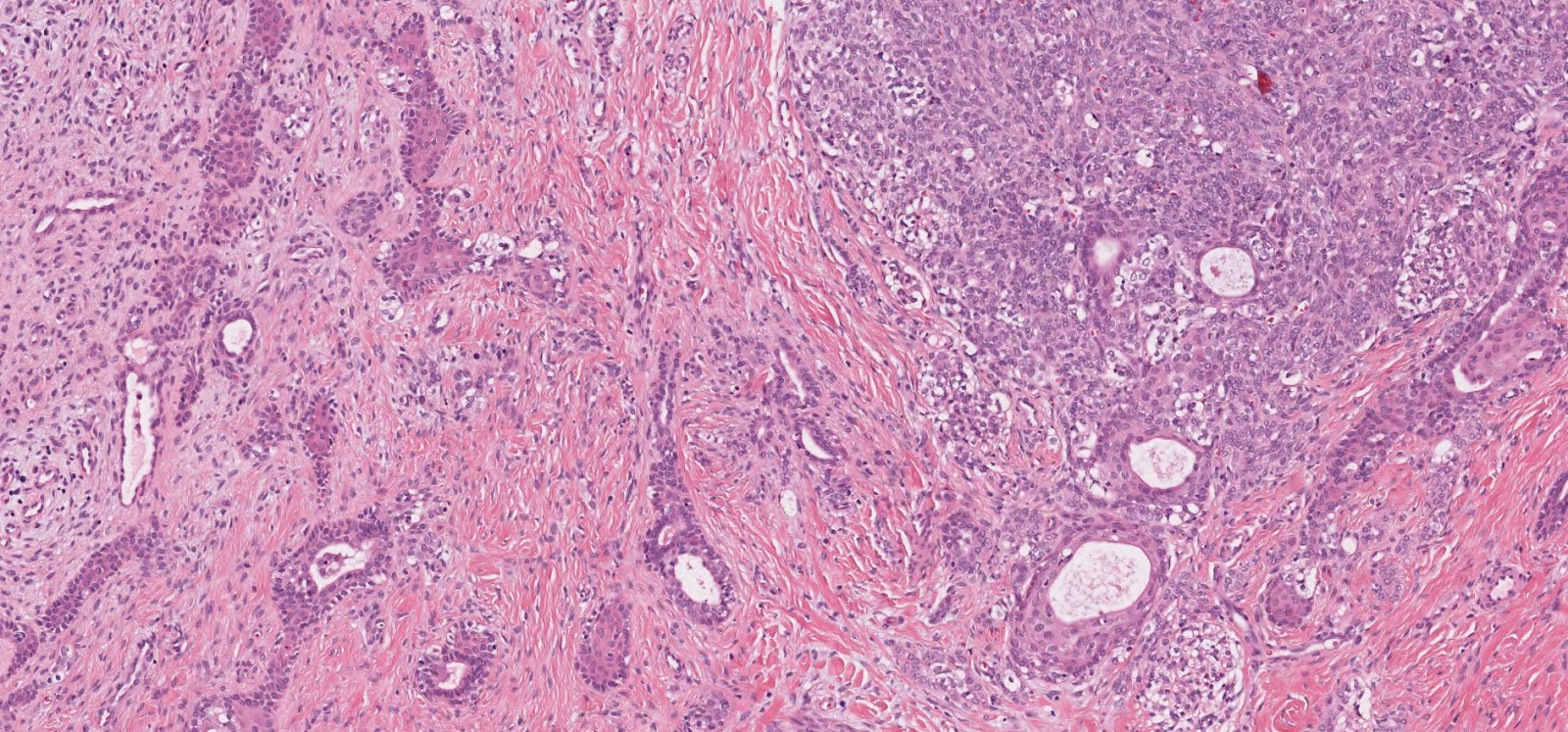
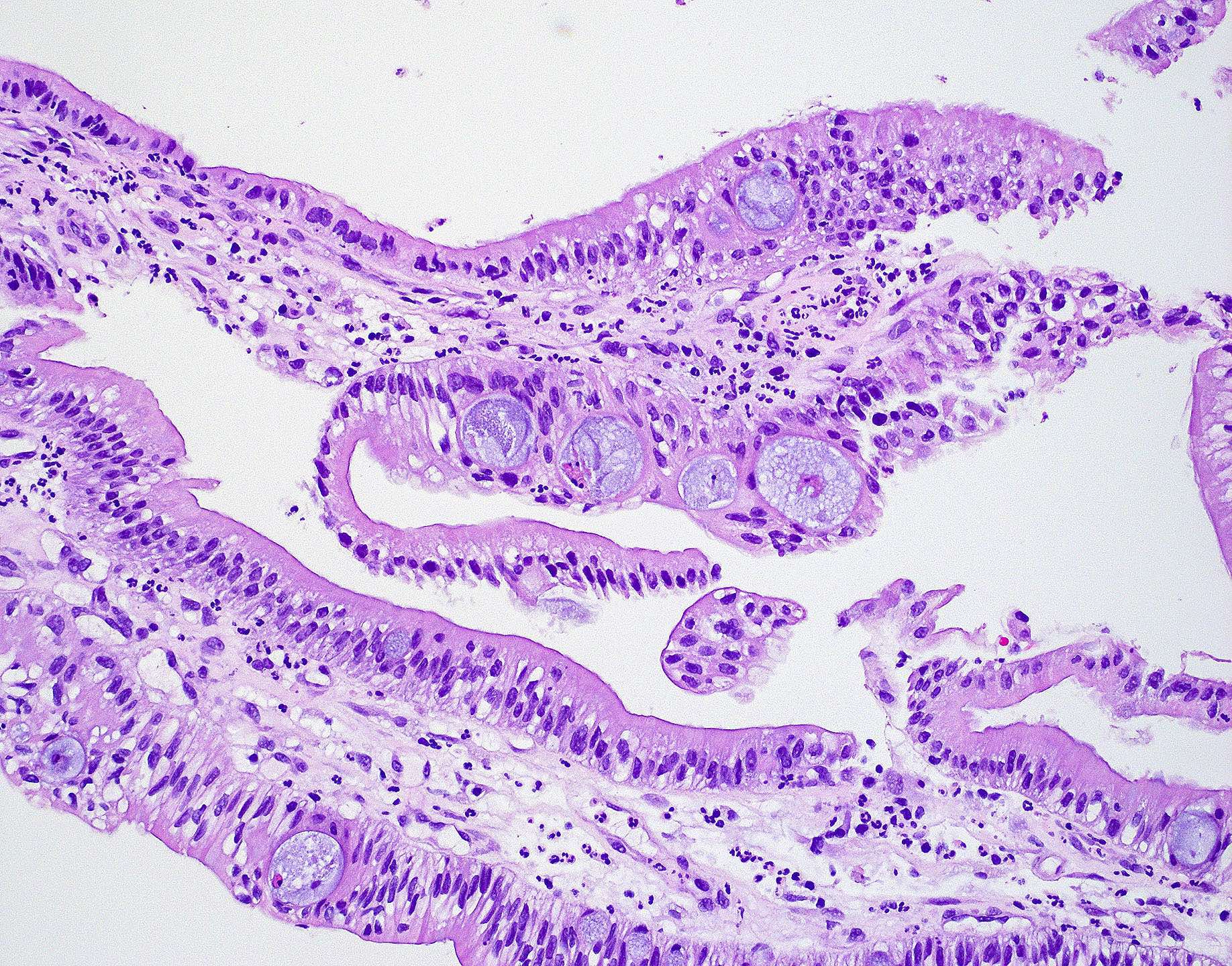
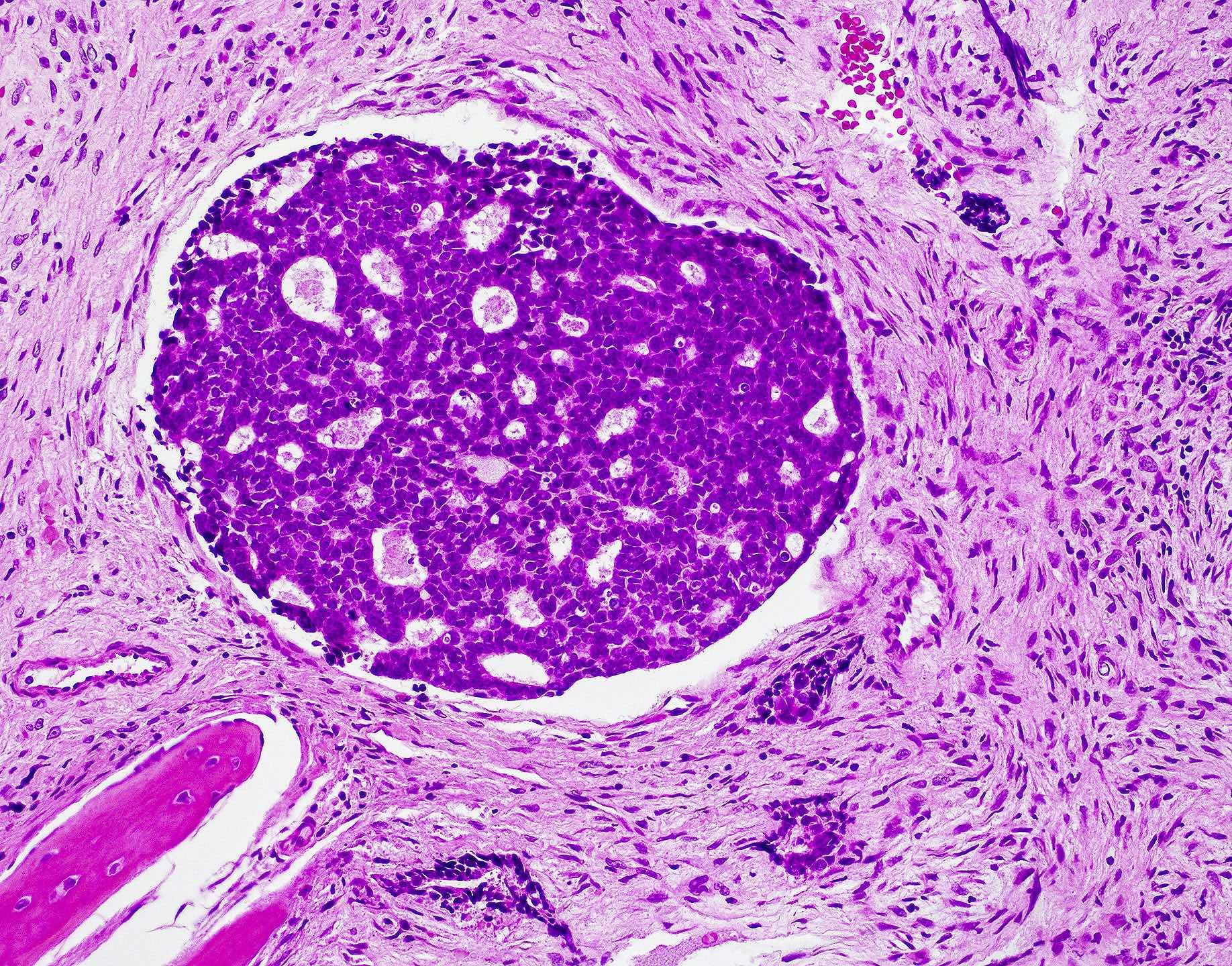
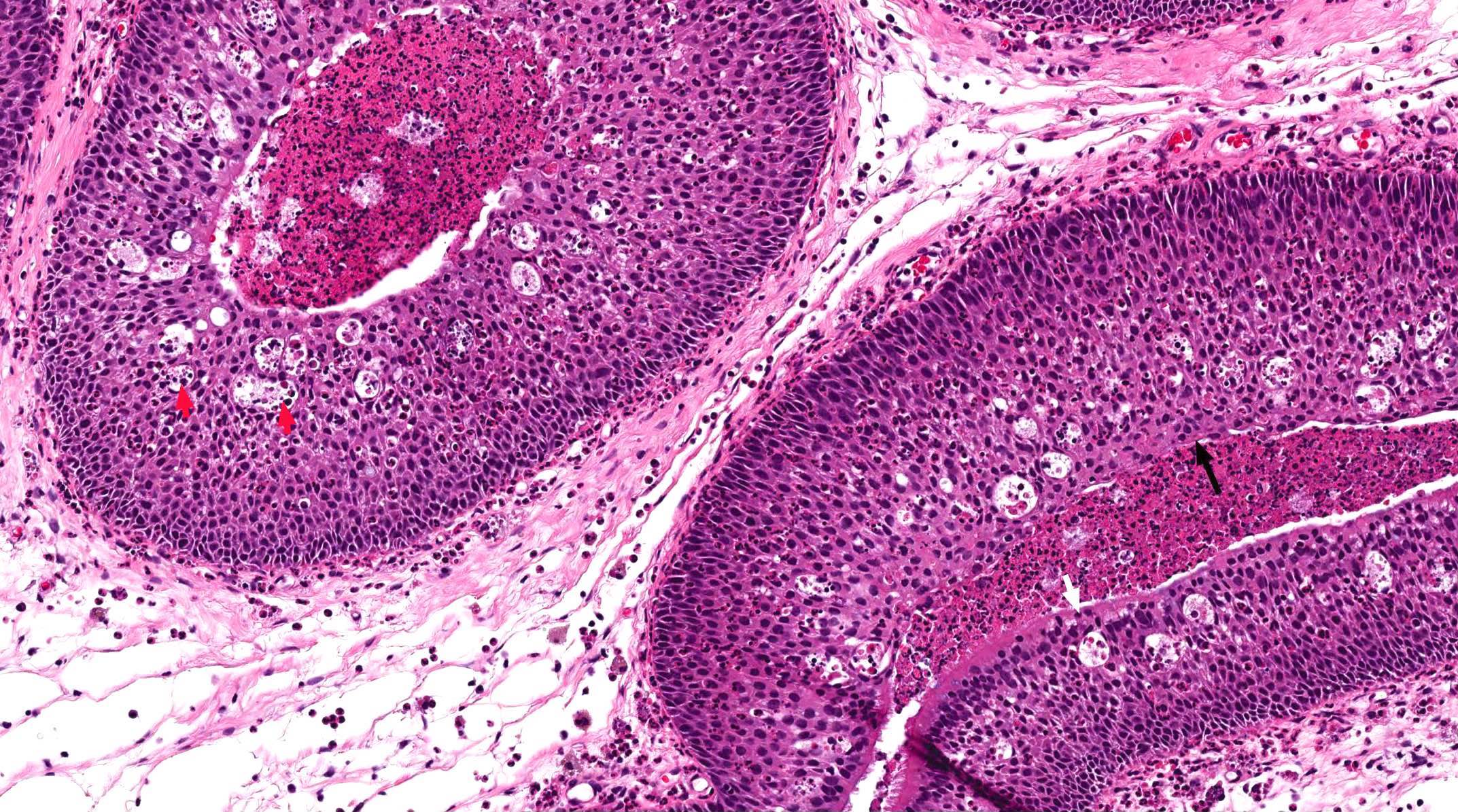
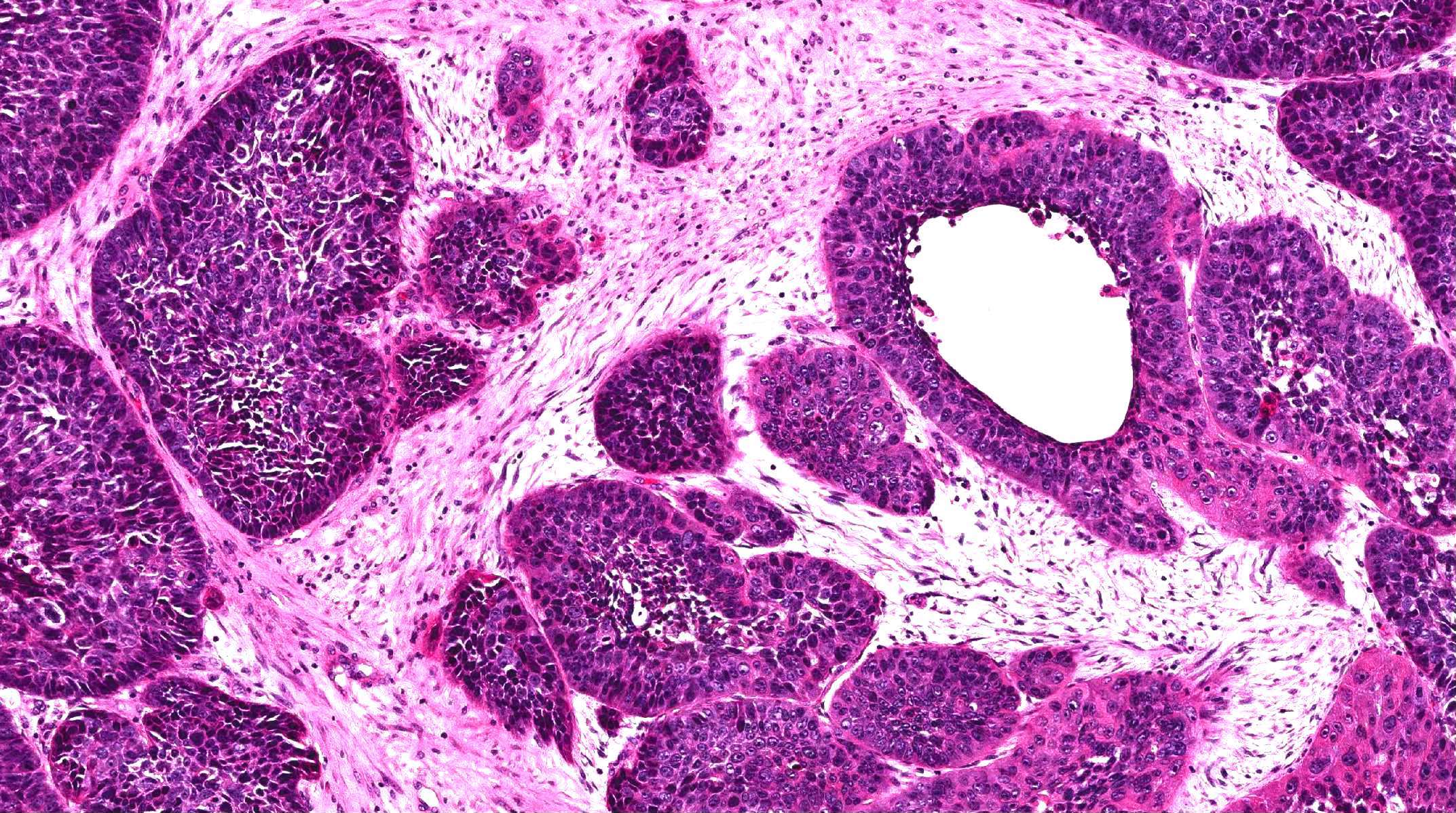
- Sinonasal renal cell-like adenocarcinoma (SRCLA) is a recently described (first case in 2002) subtype of low grade non-ITAC
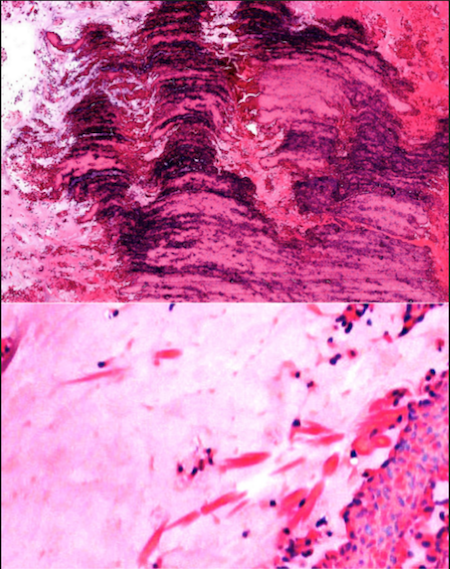
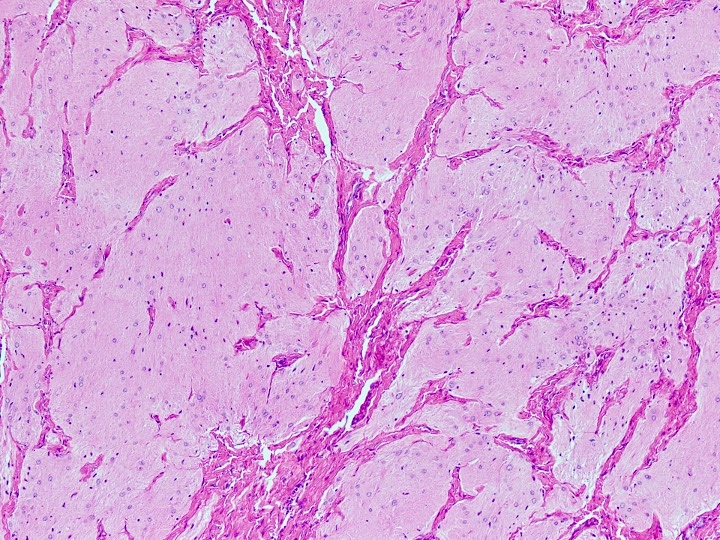
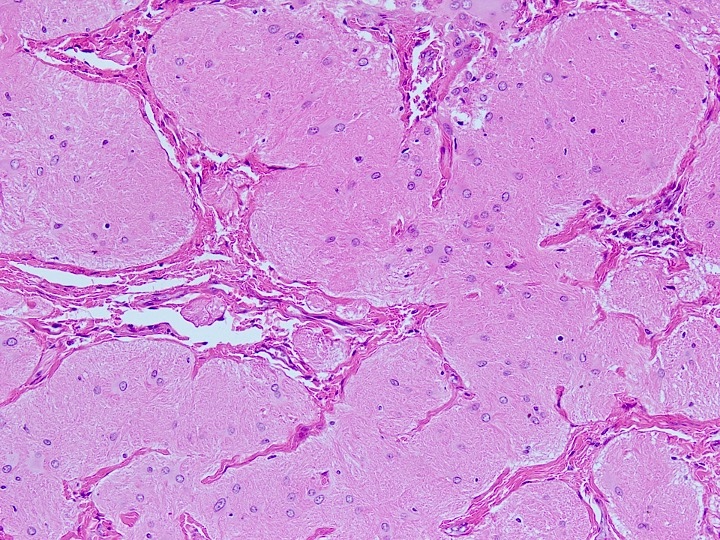
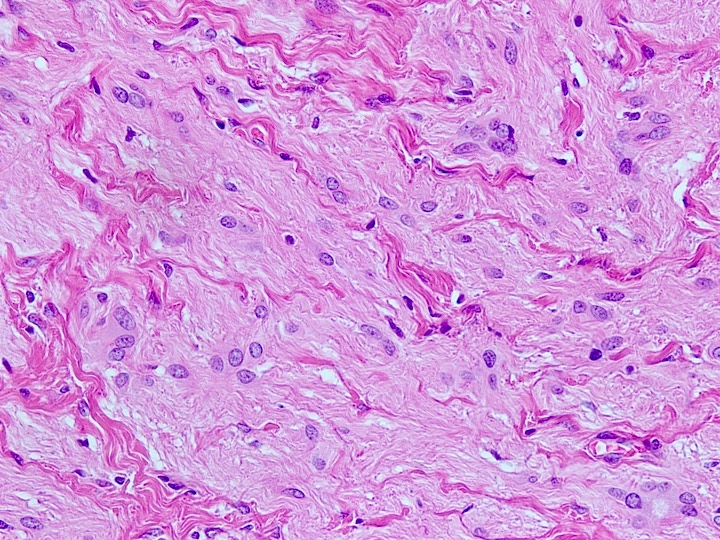
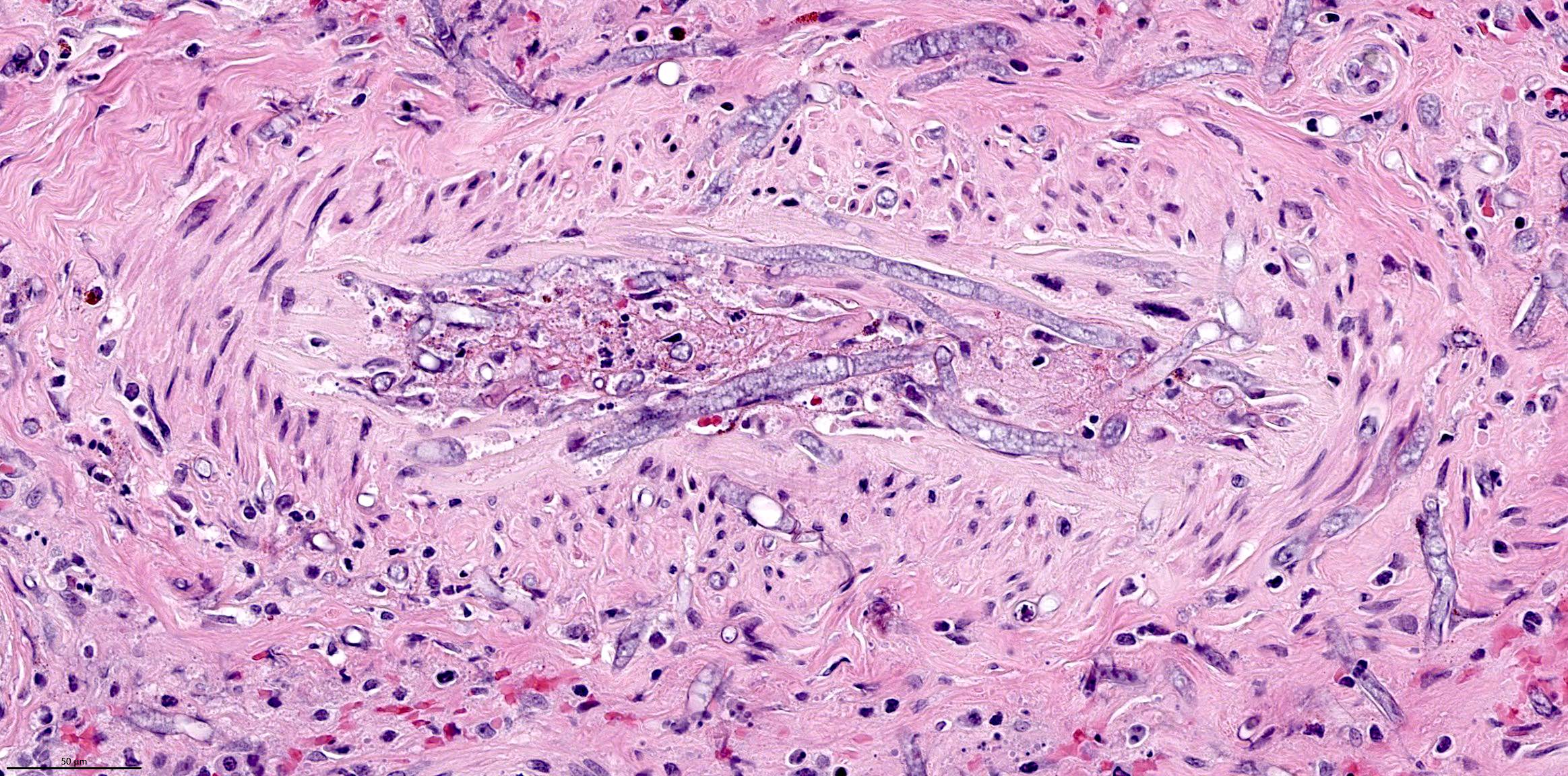
- SRCLA exhibits uniform, cuboidal to polyhedral glycogen rich cells with clear cytoplasm that lacks mucin production (Head Neck Pathol 2016;10:68)
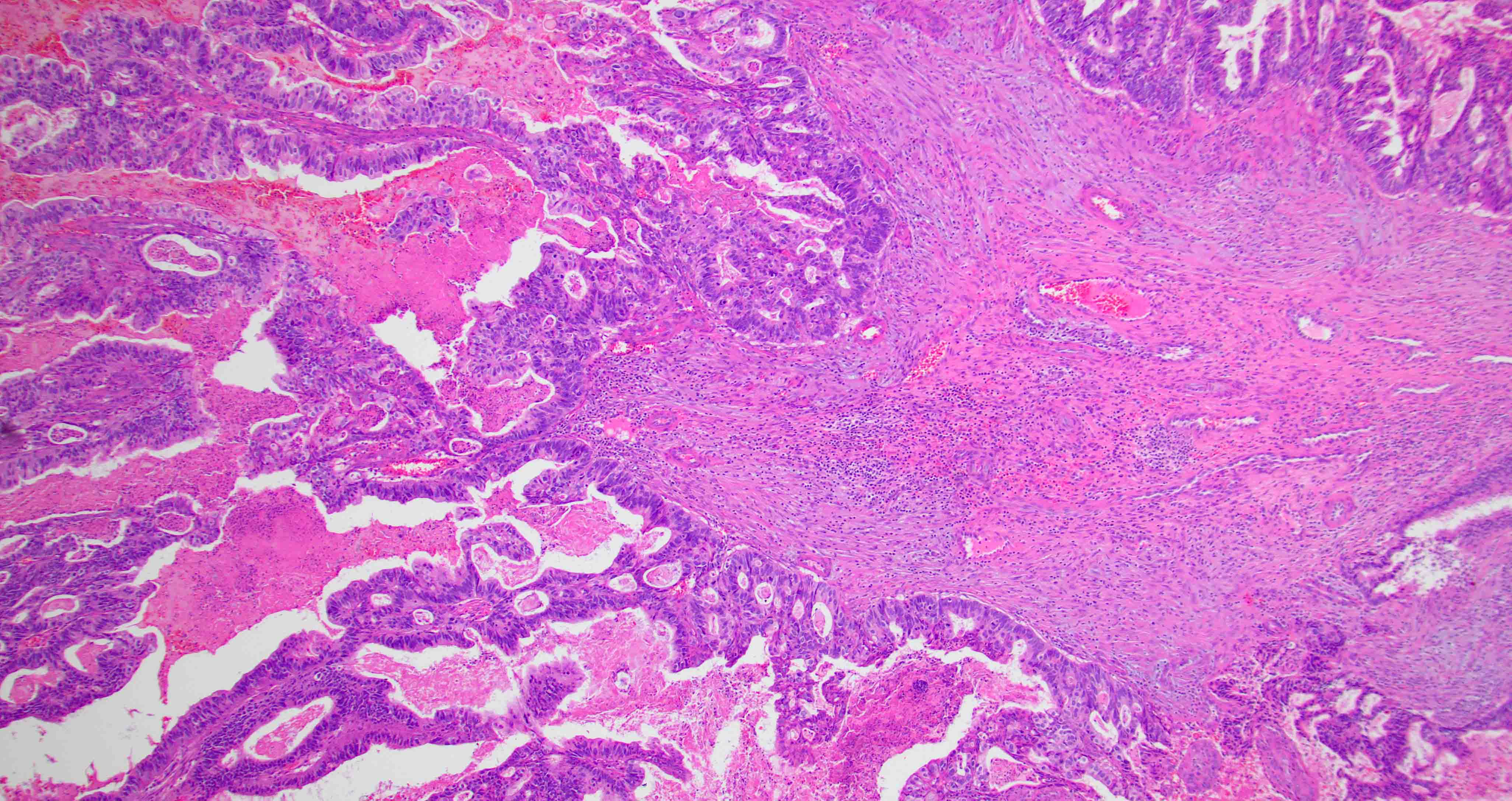
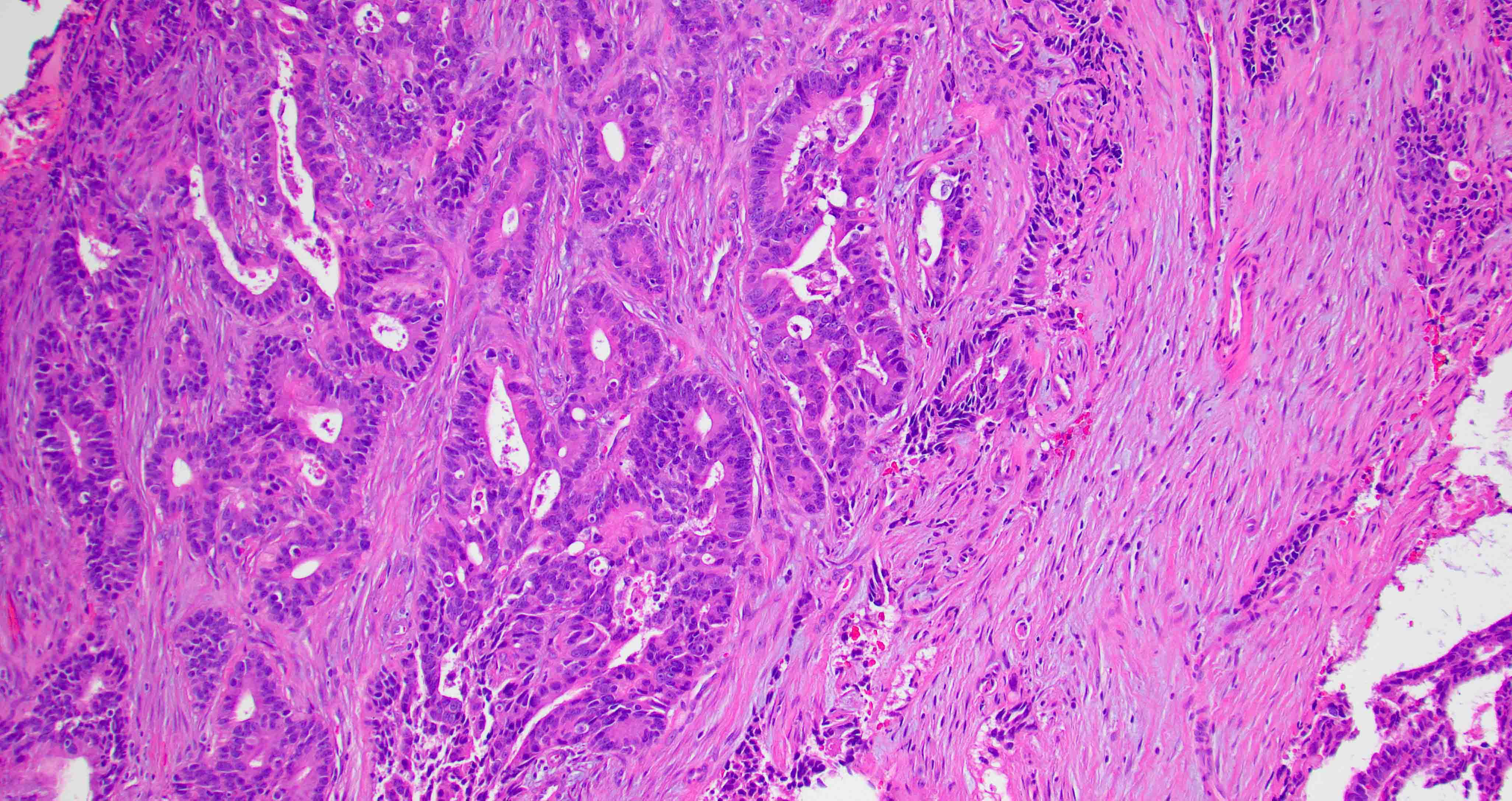
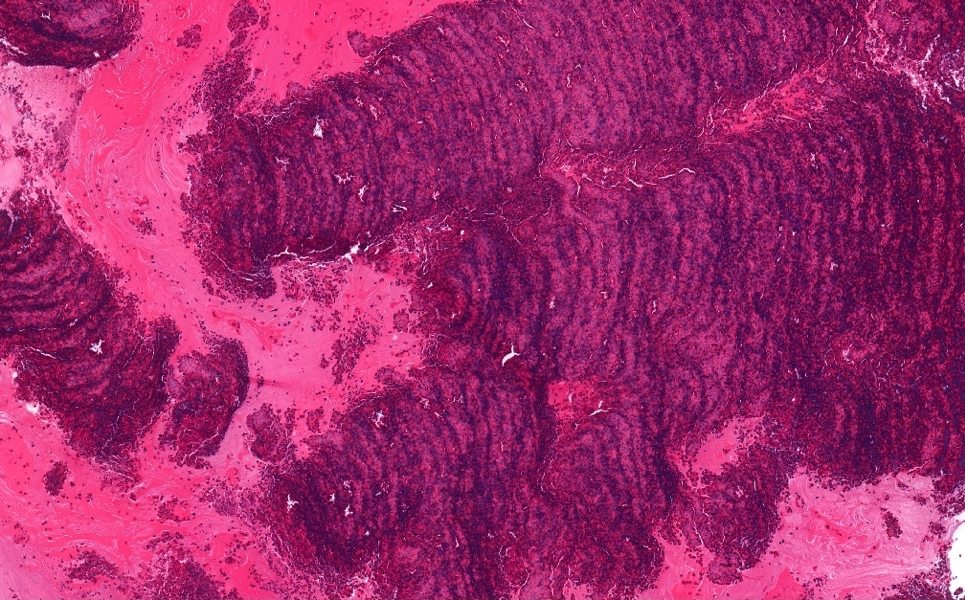
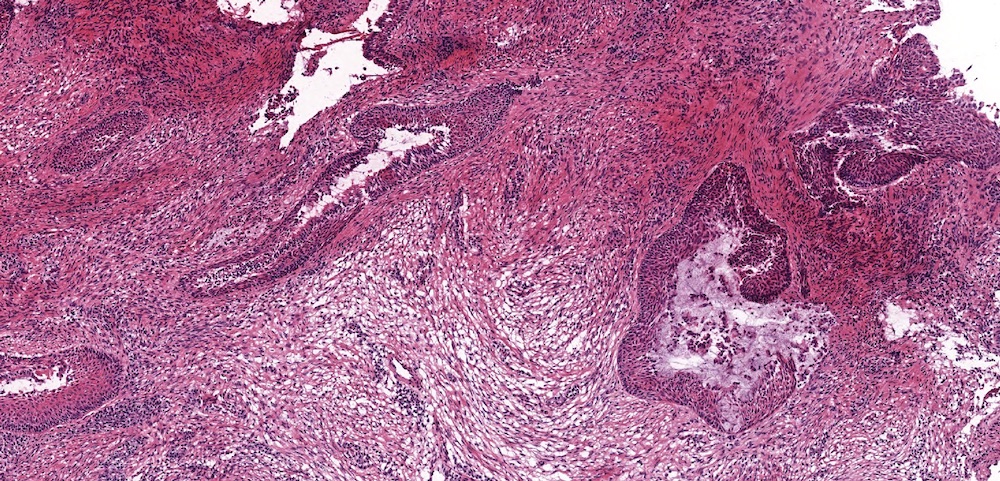
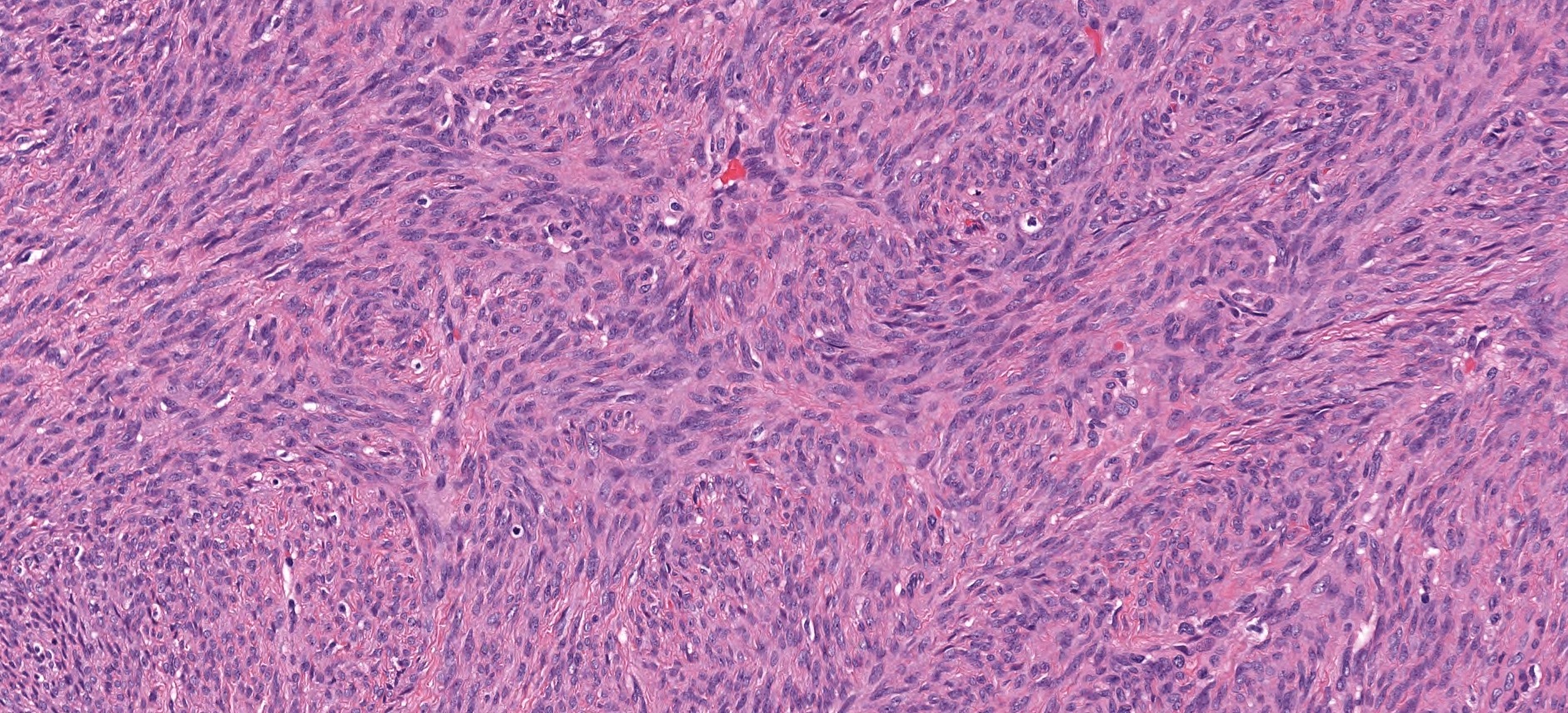
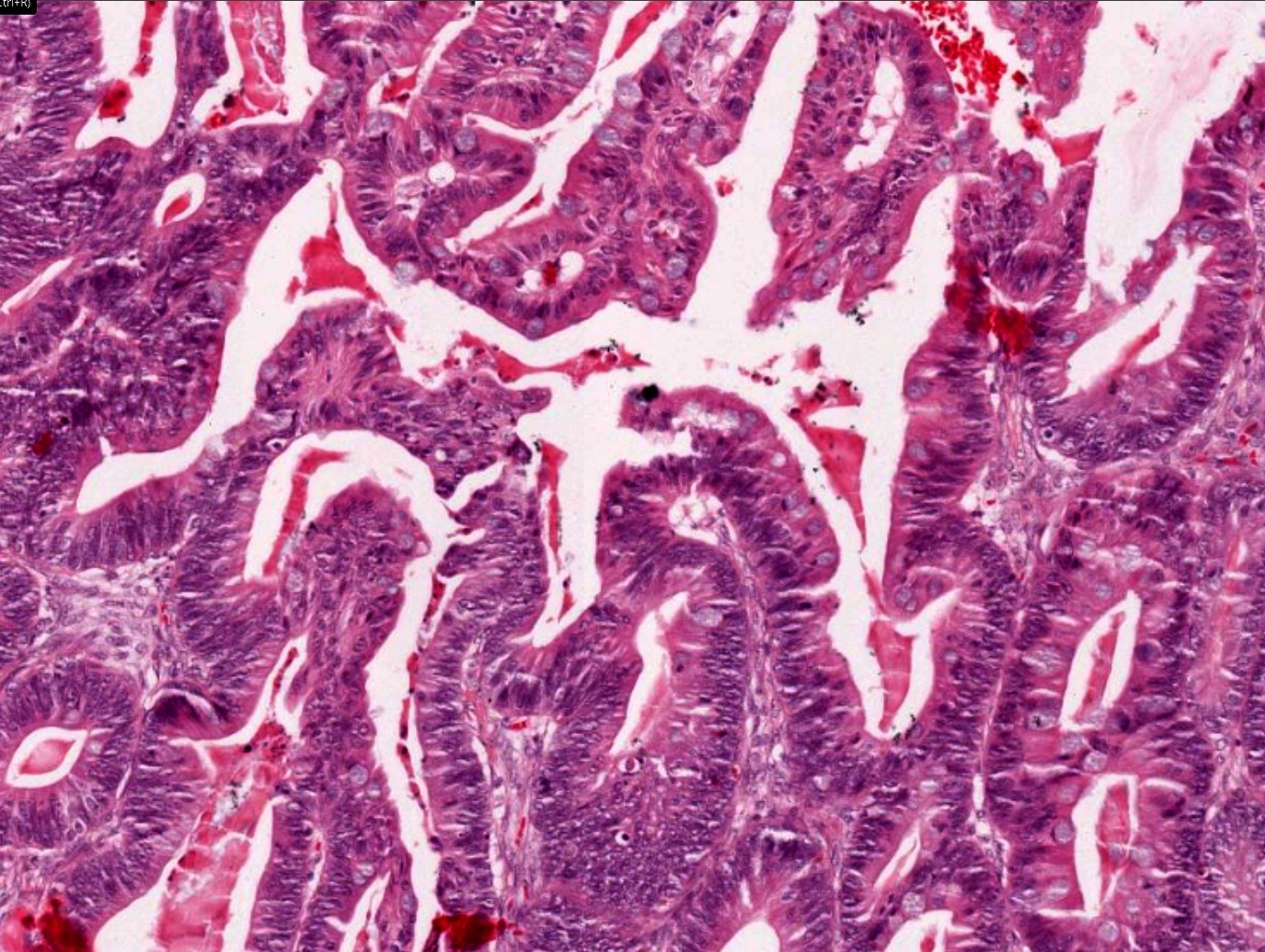
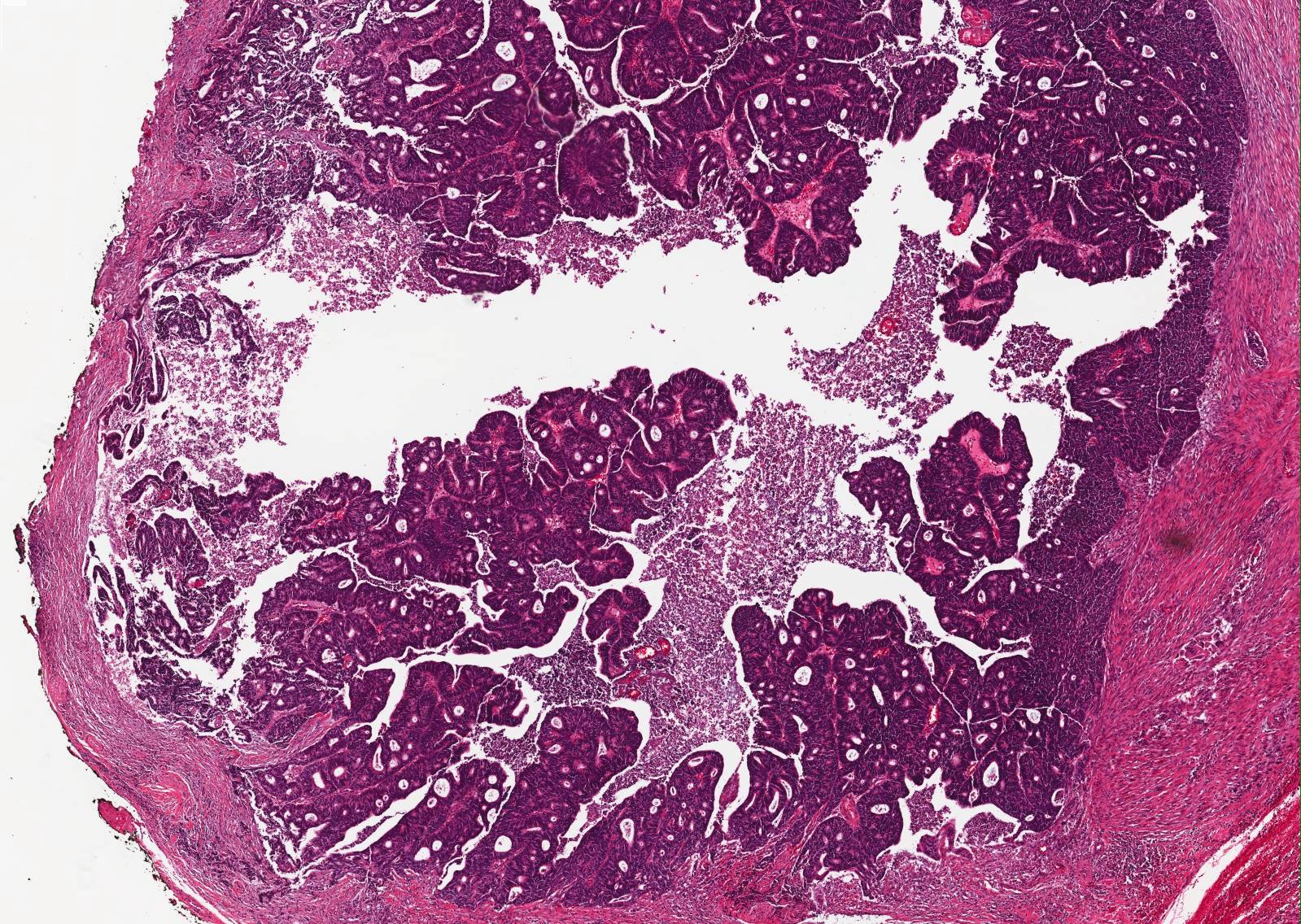
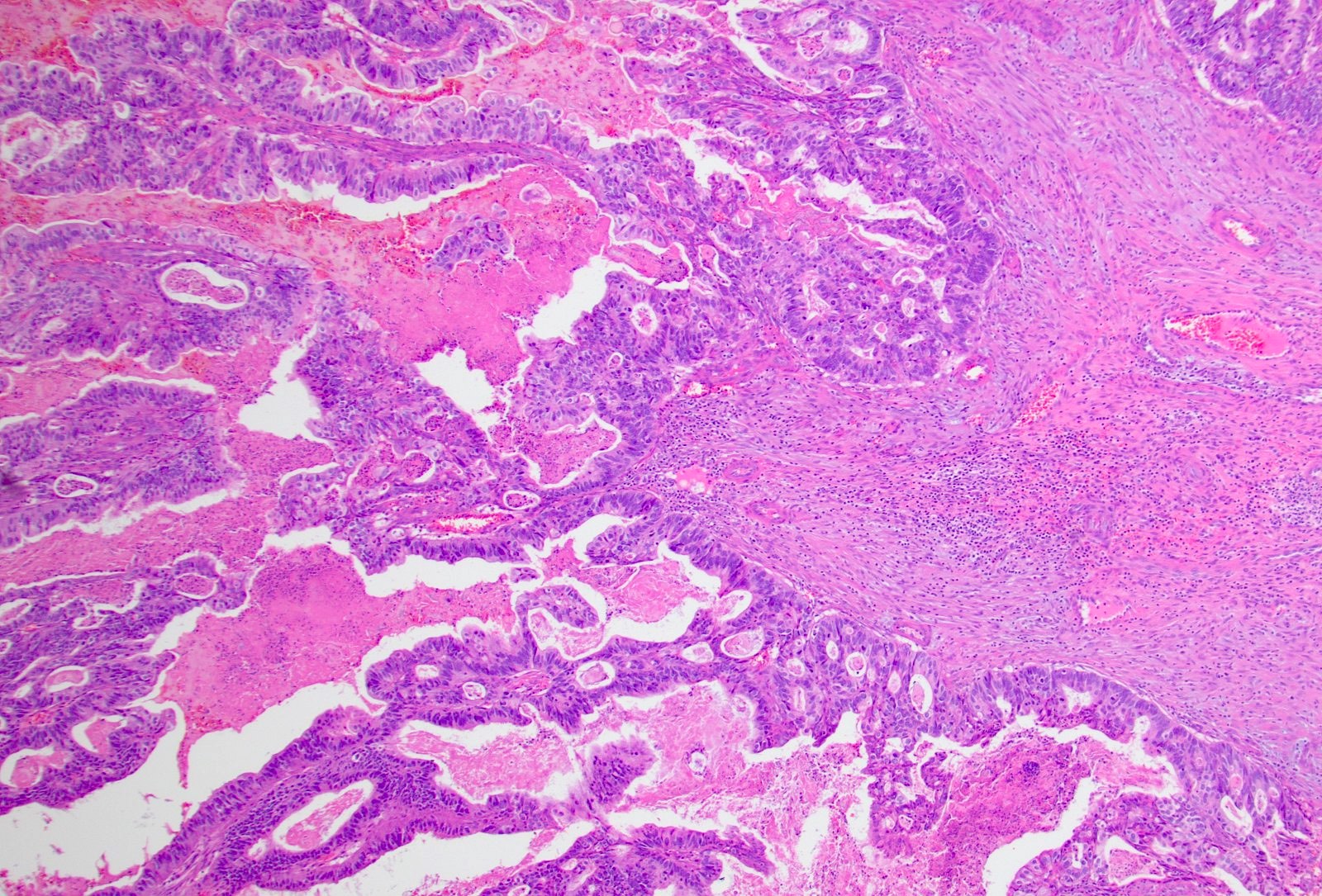
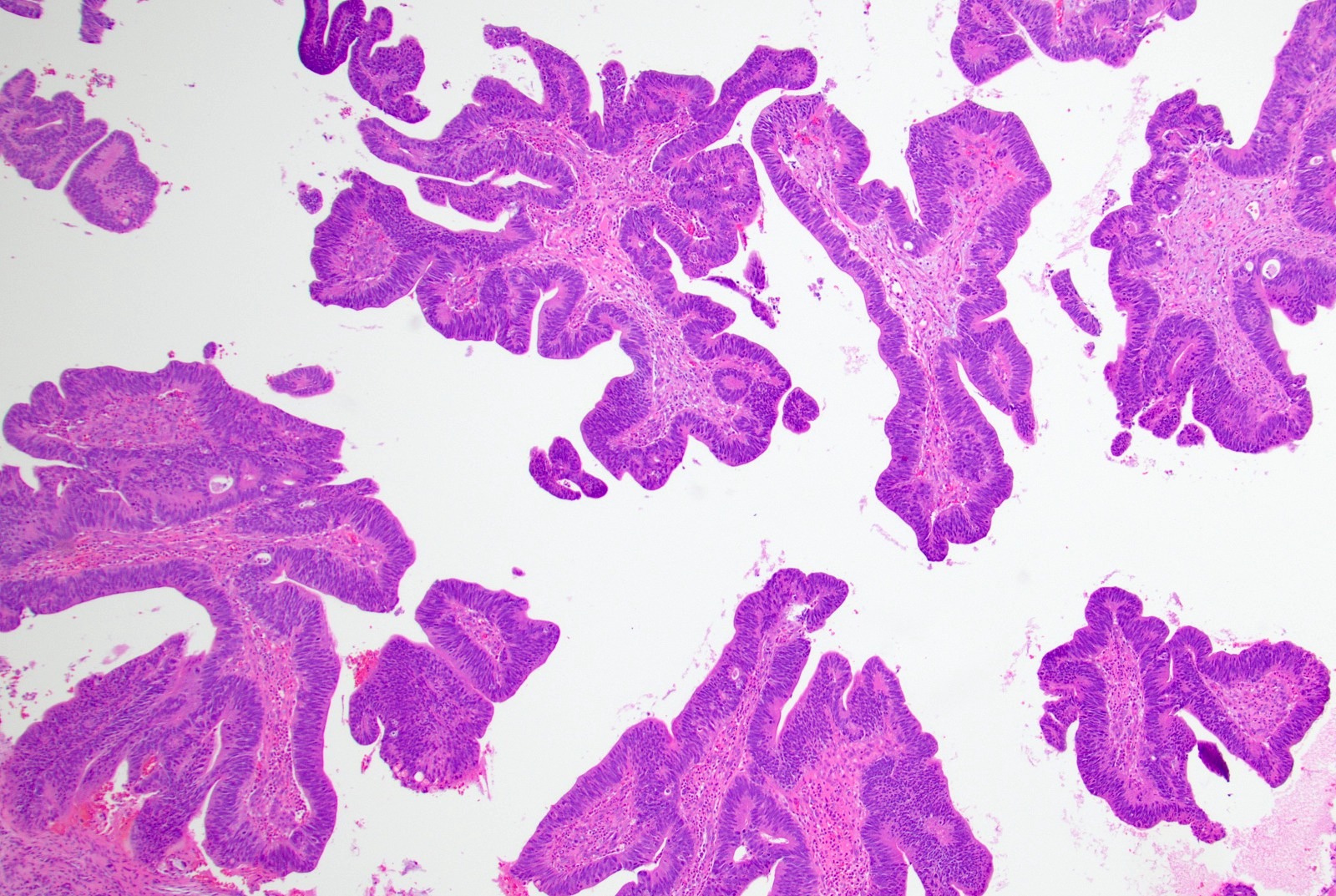
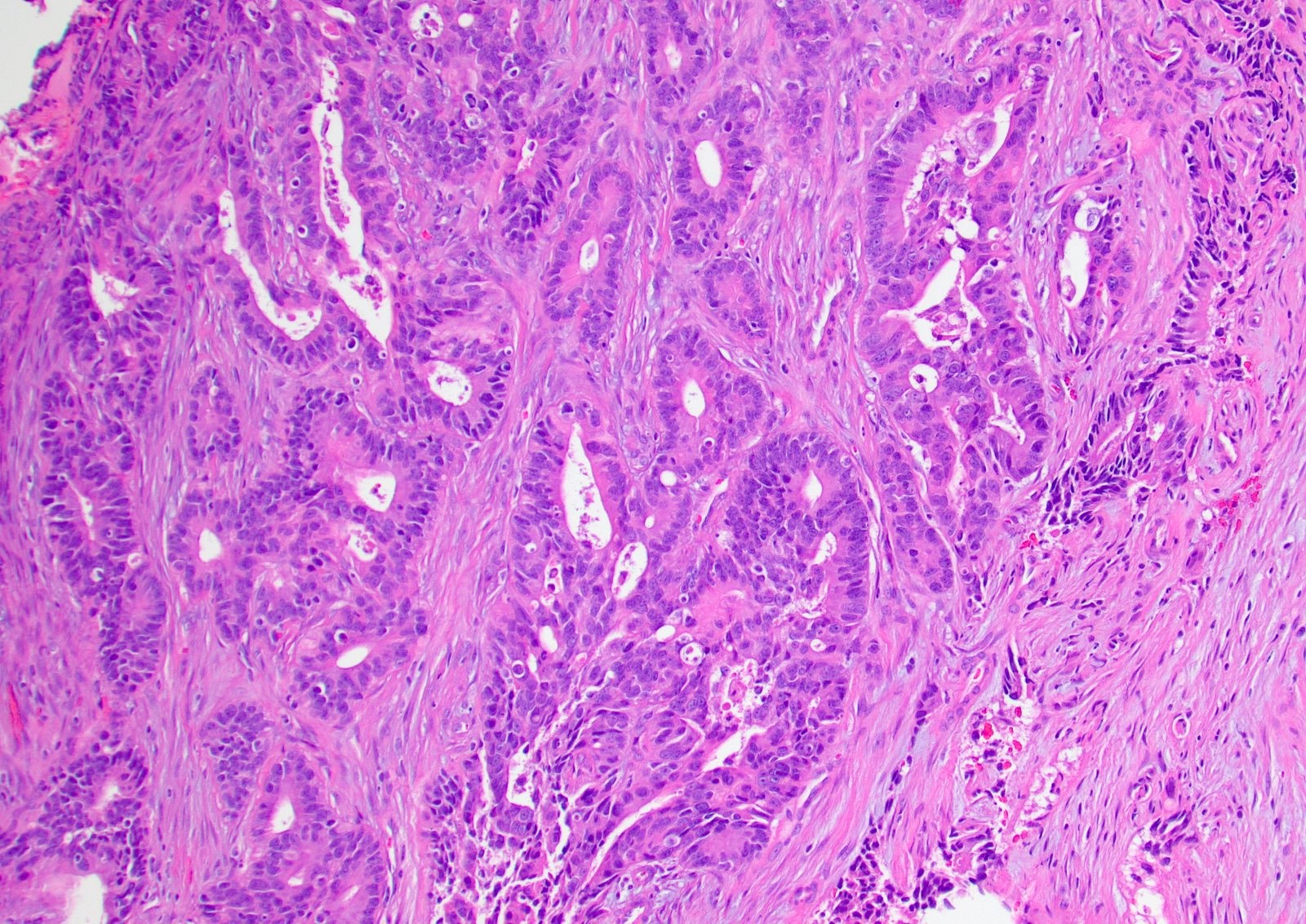
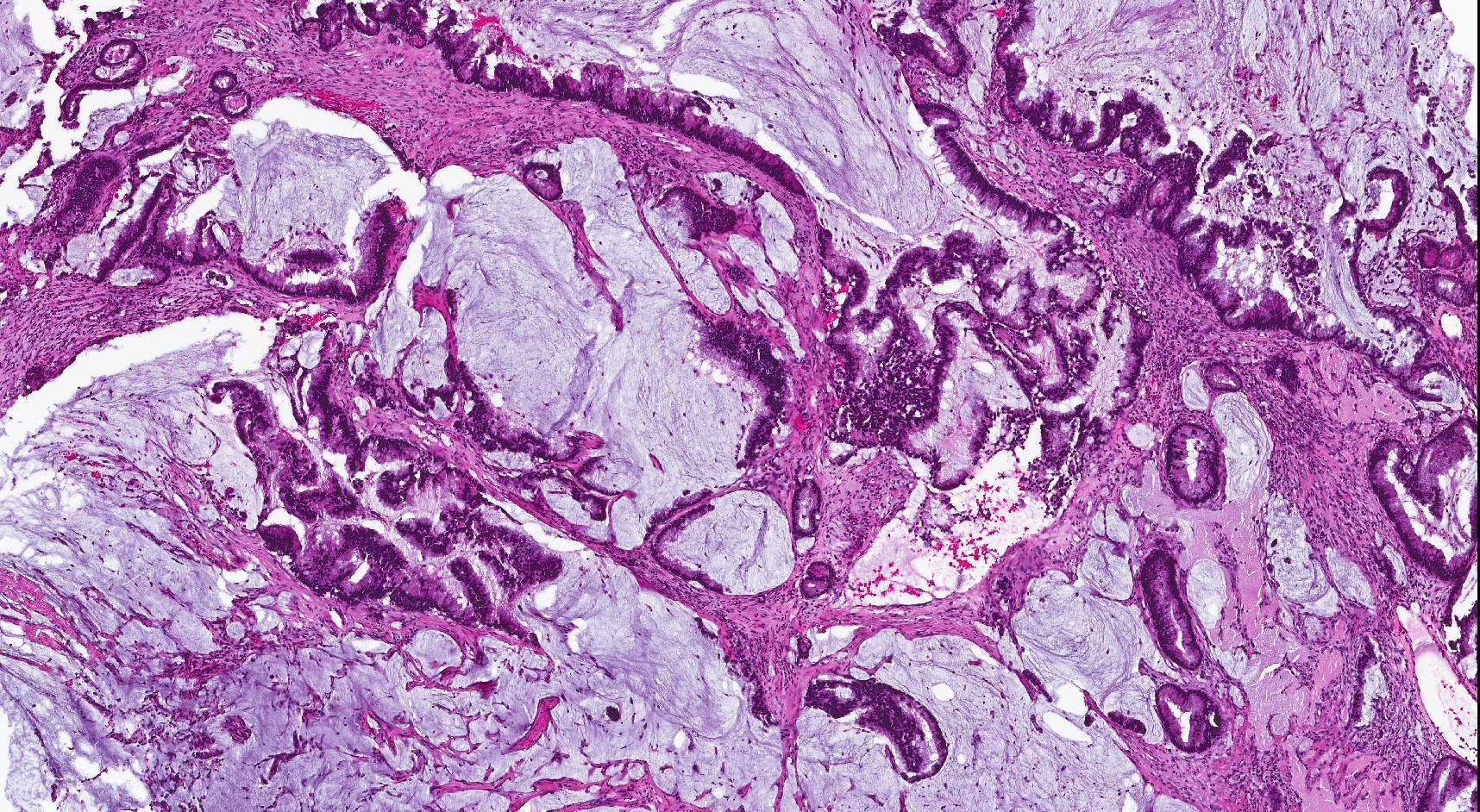
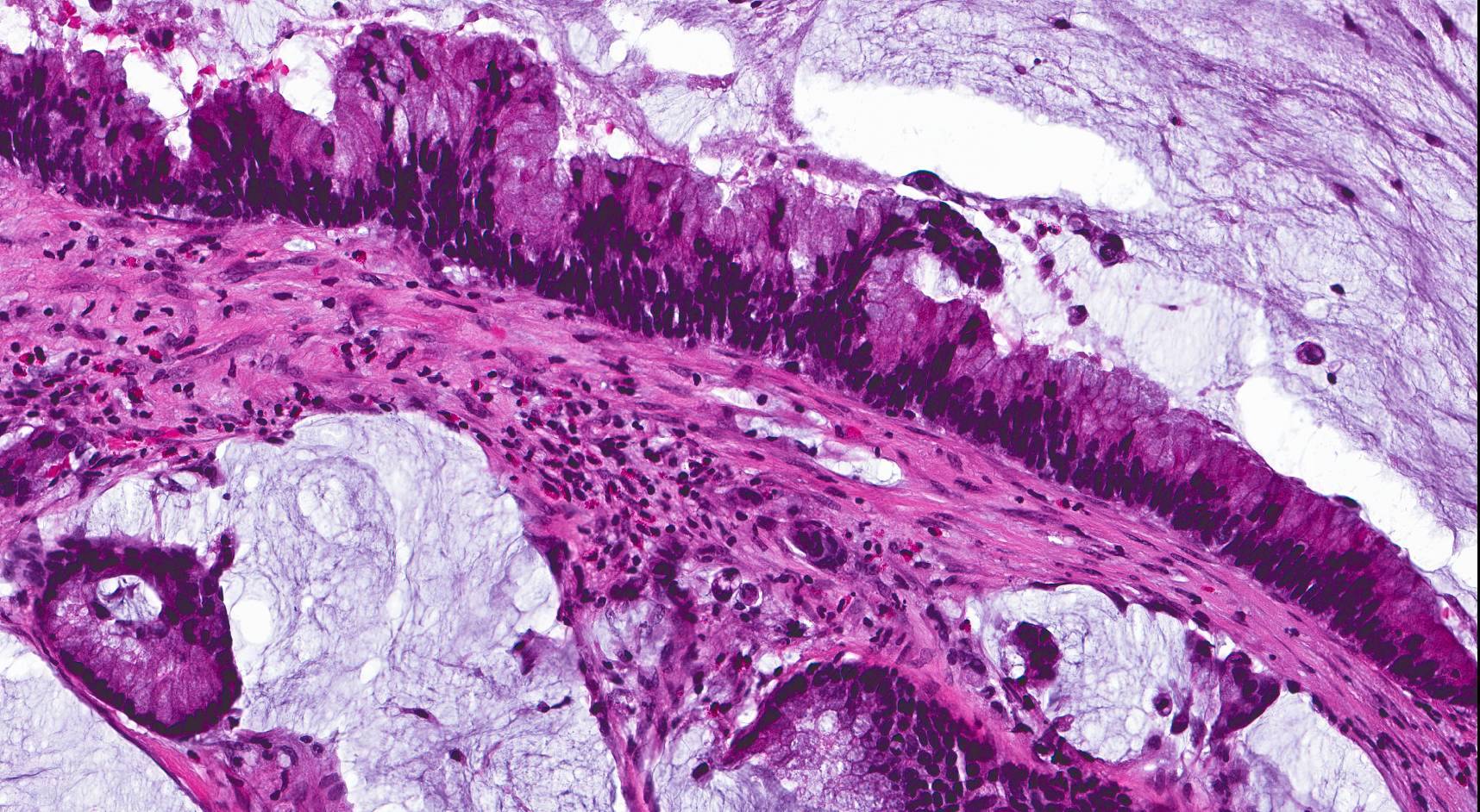
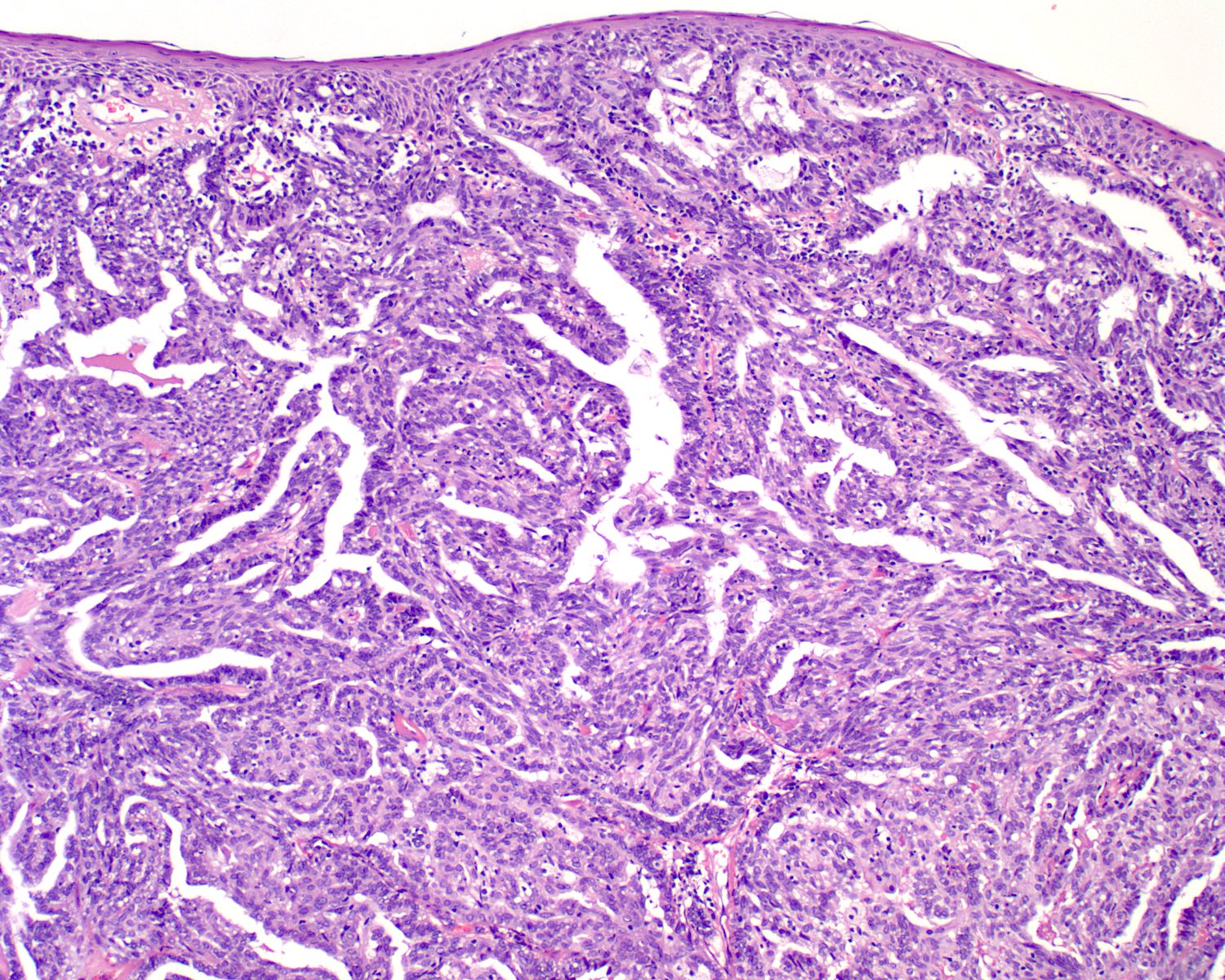
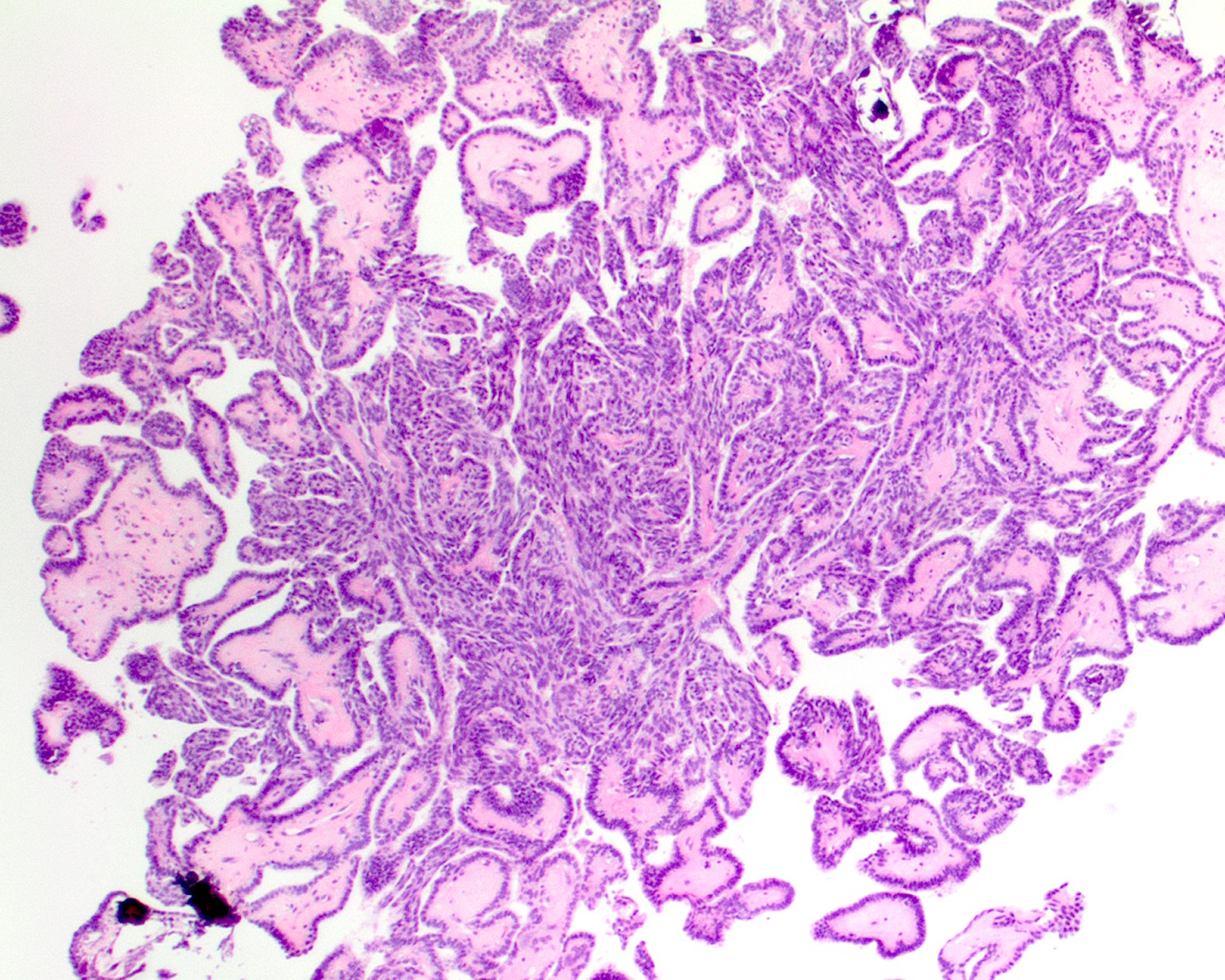
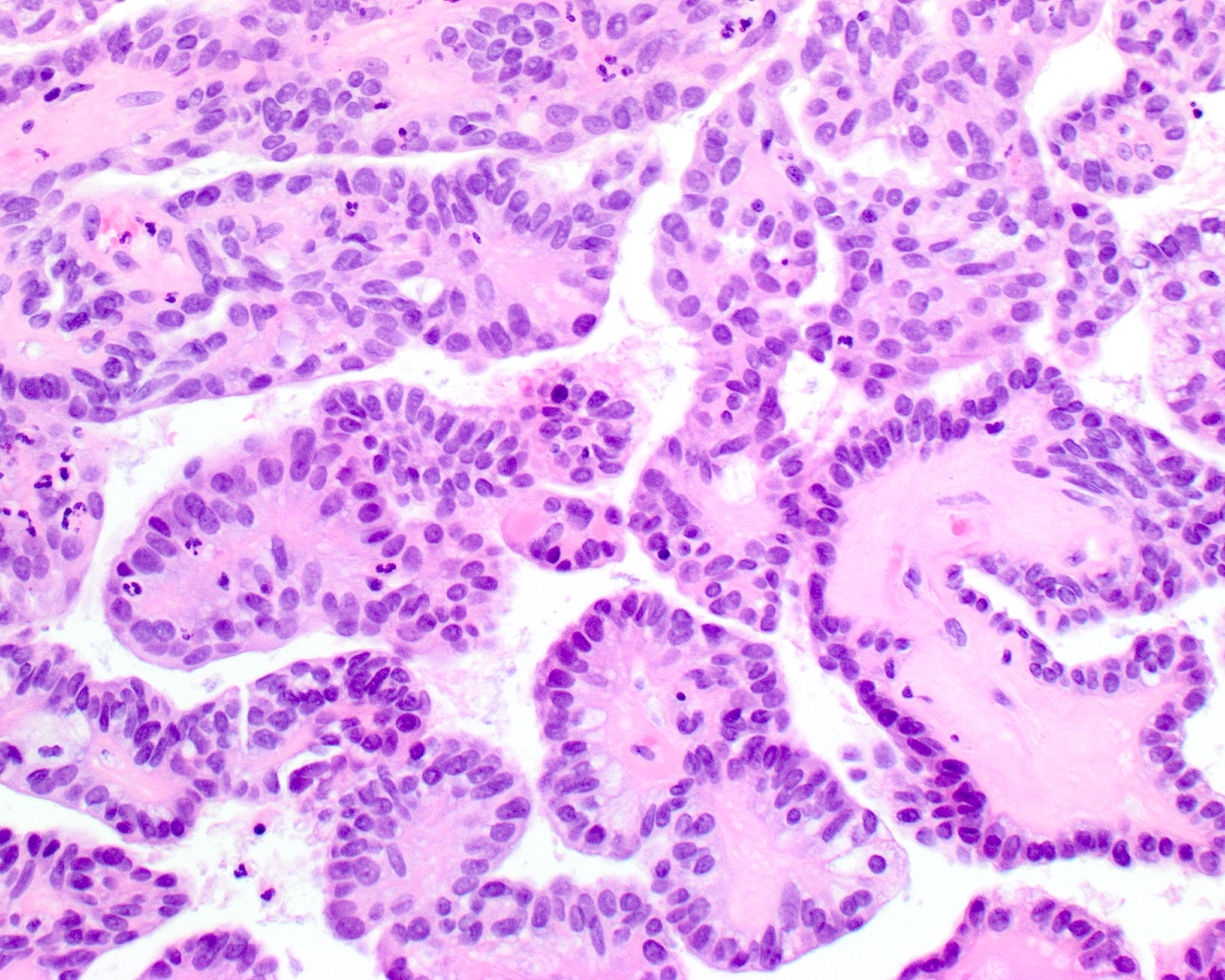
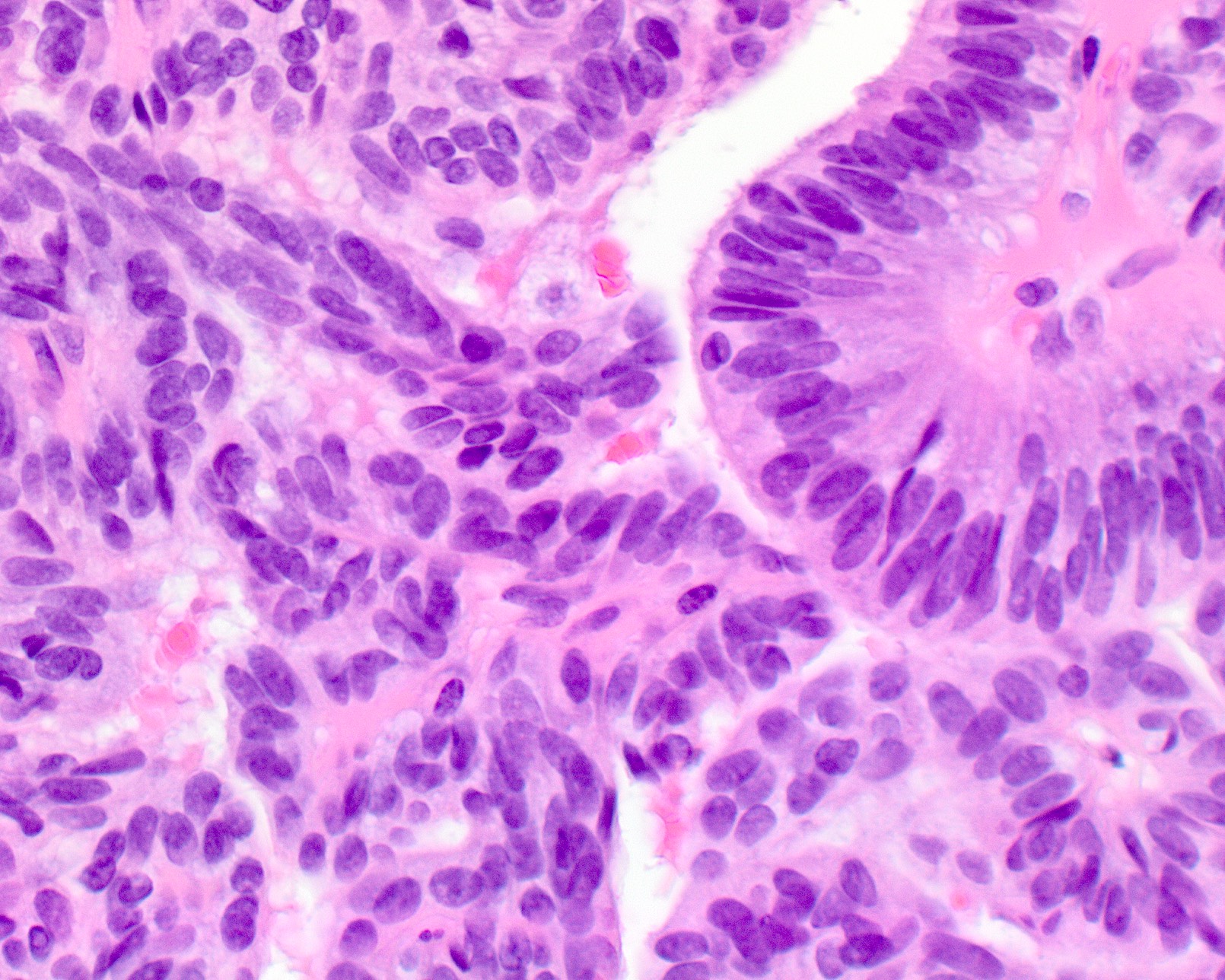
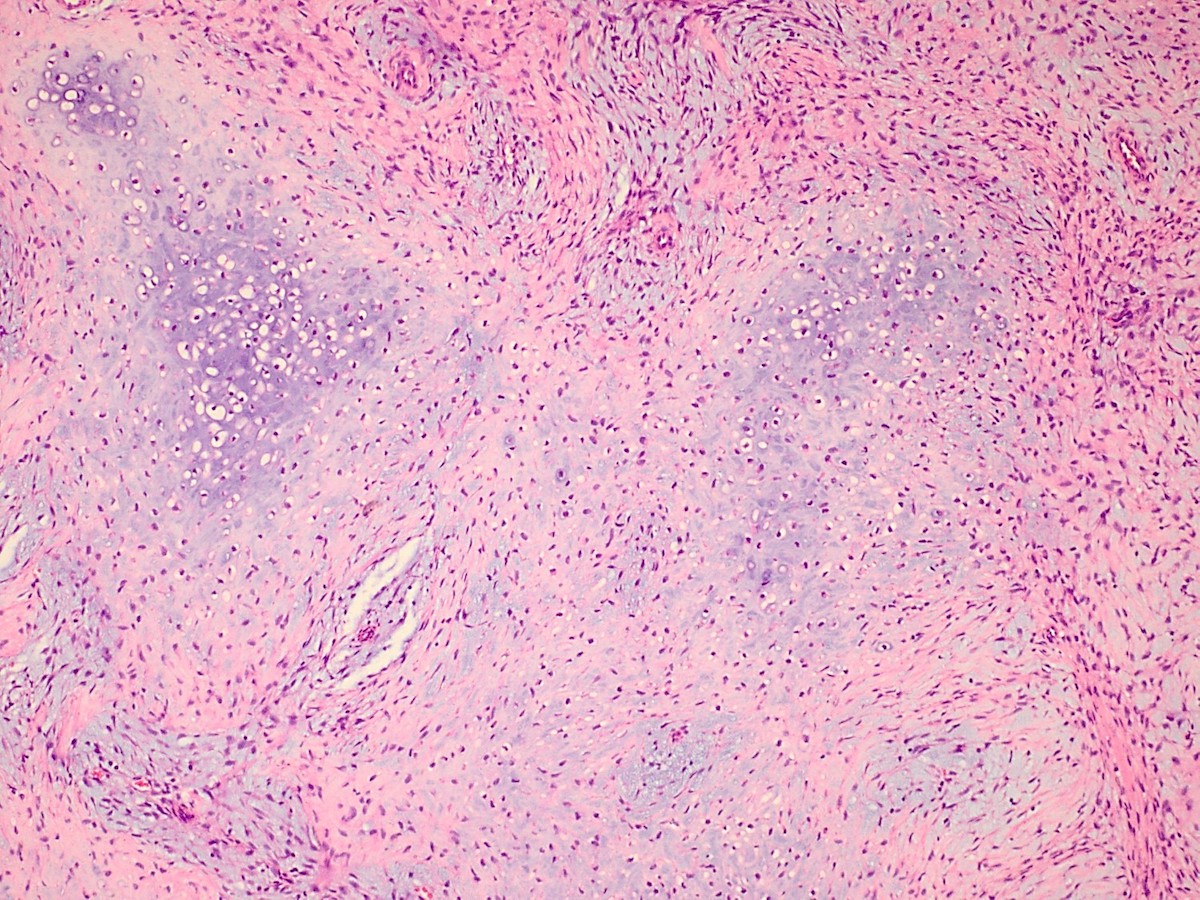
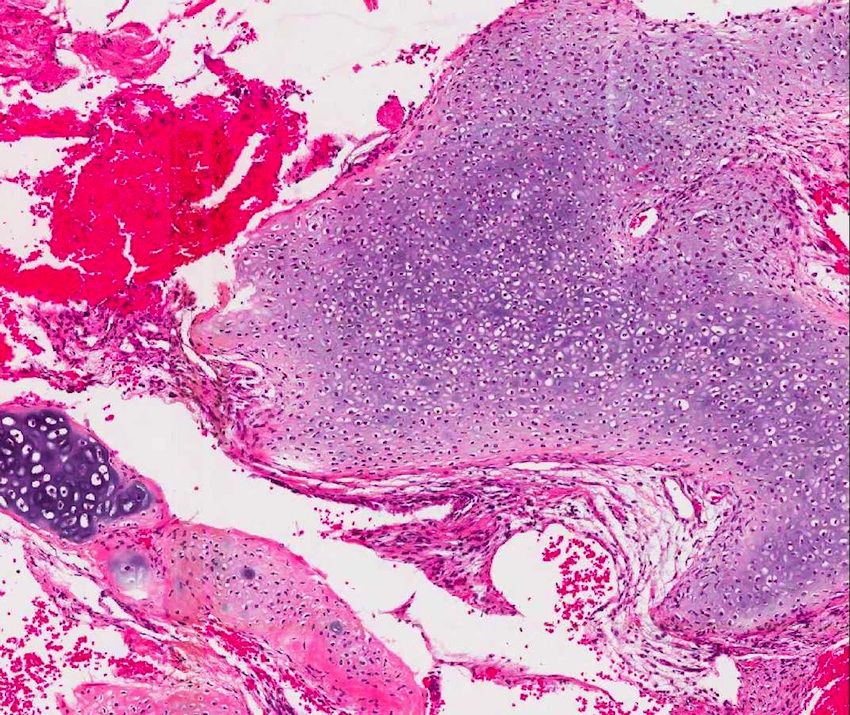
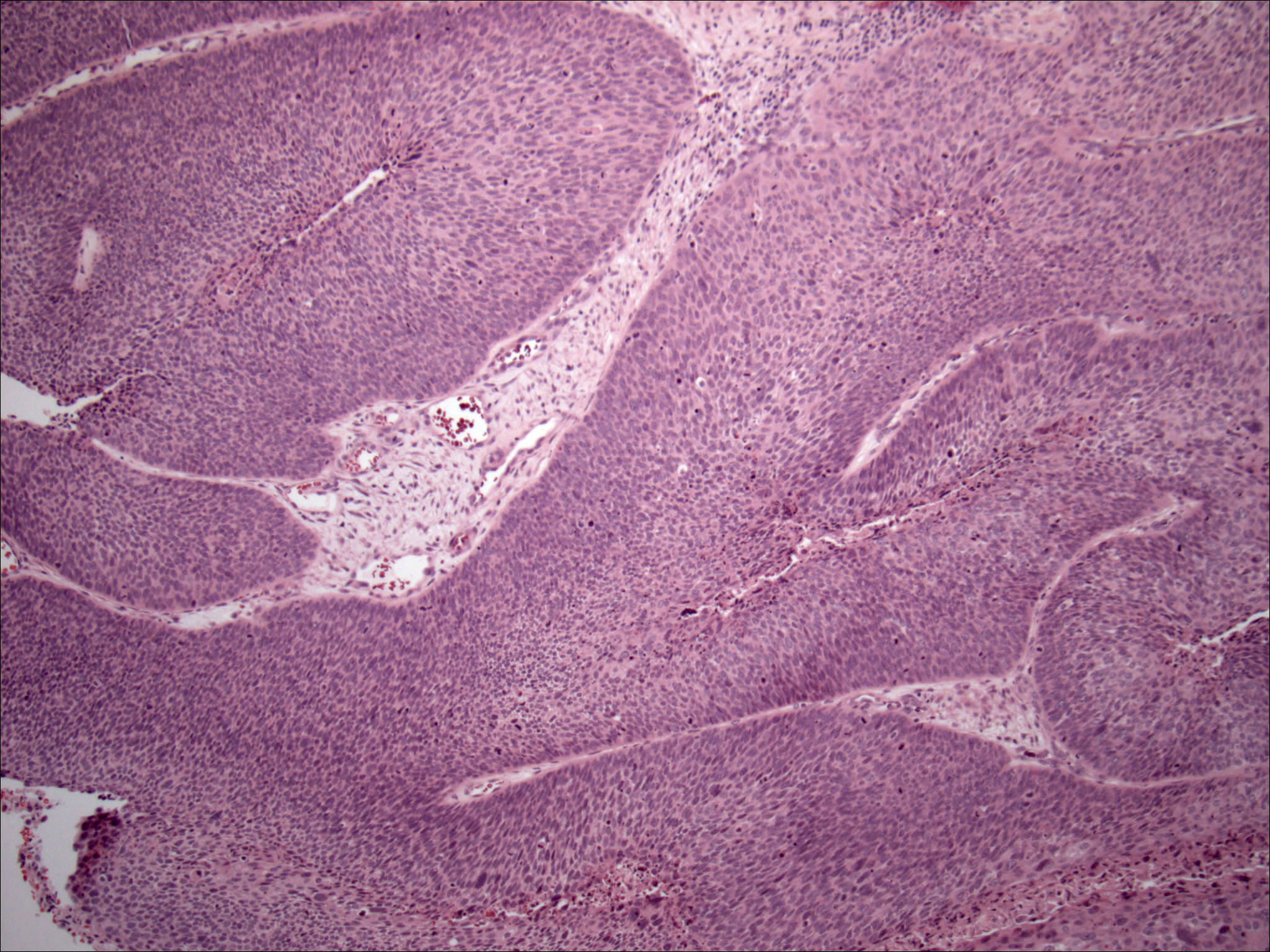
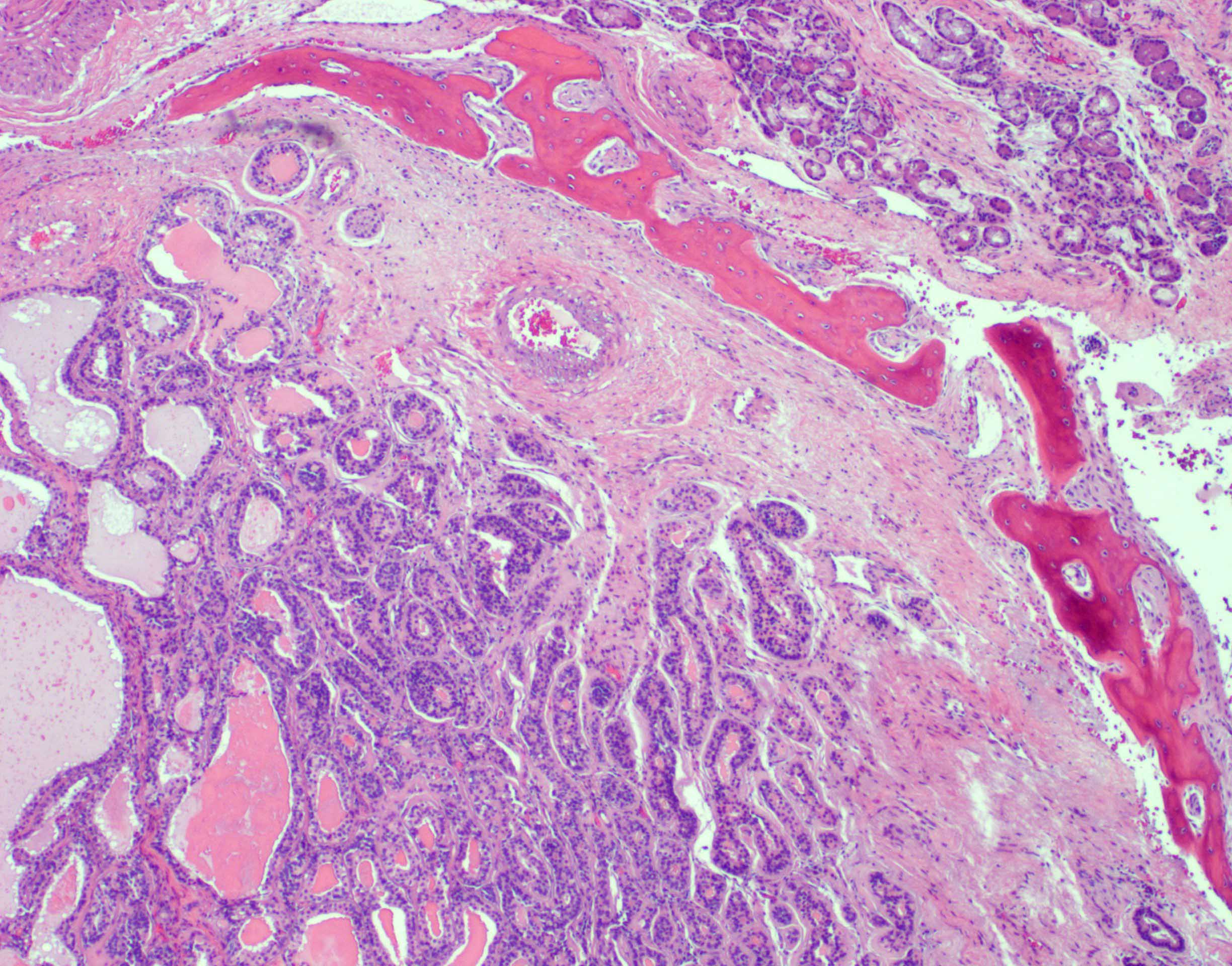
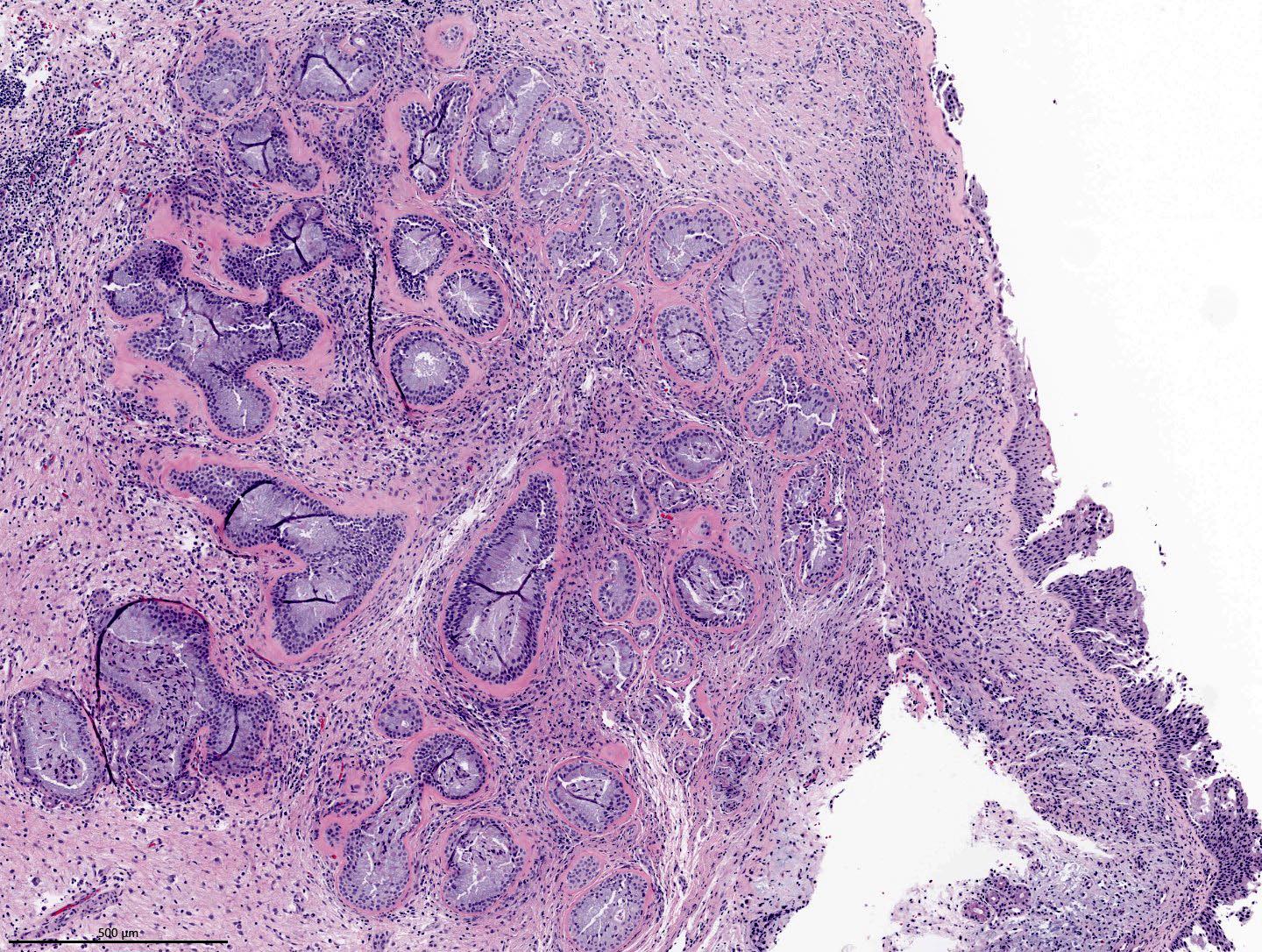
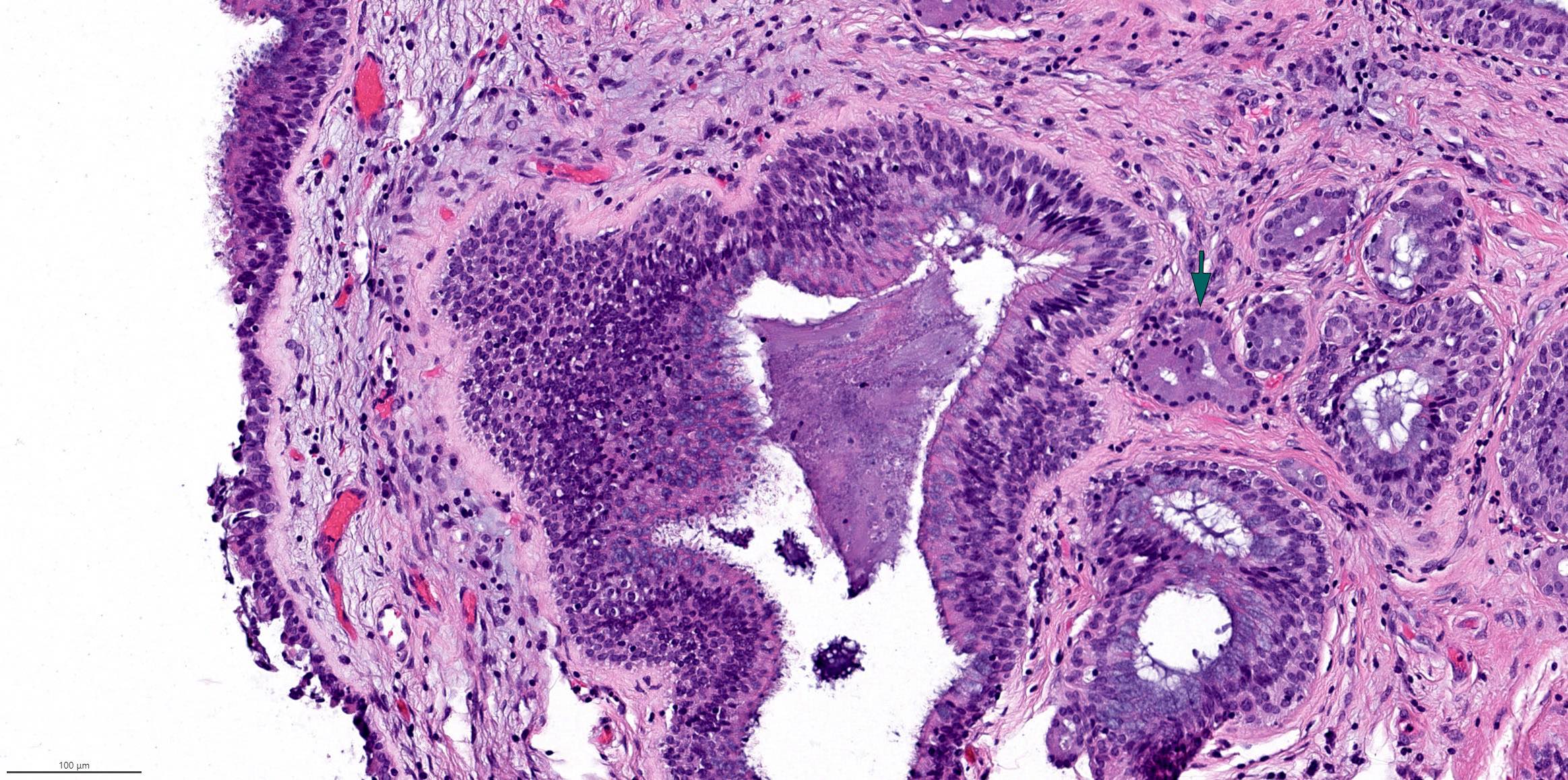
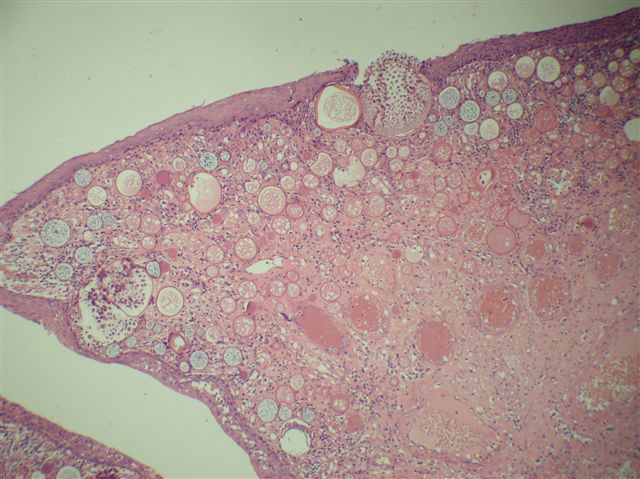
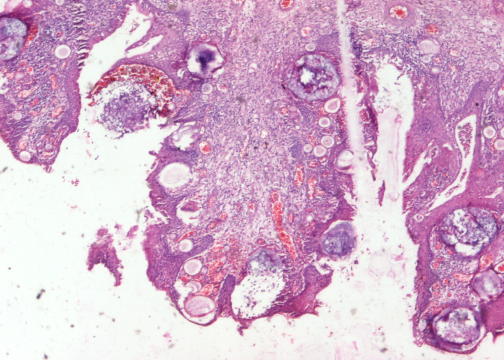
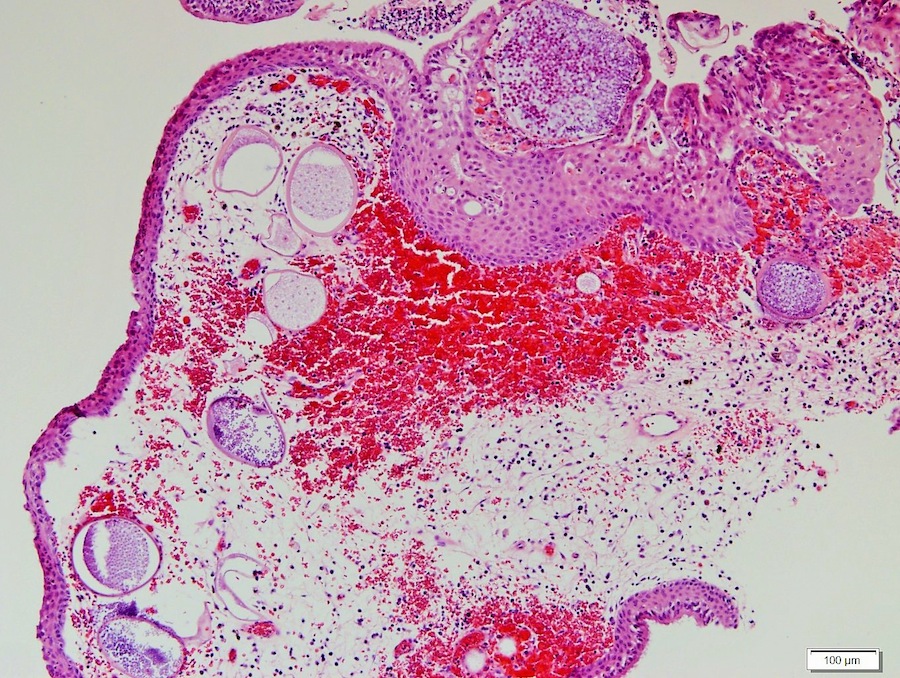
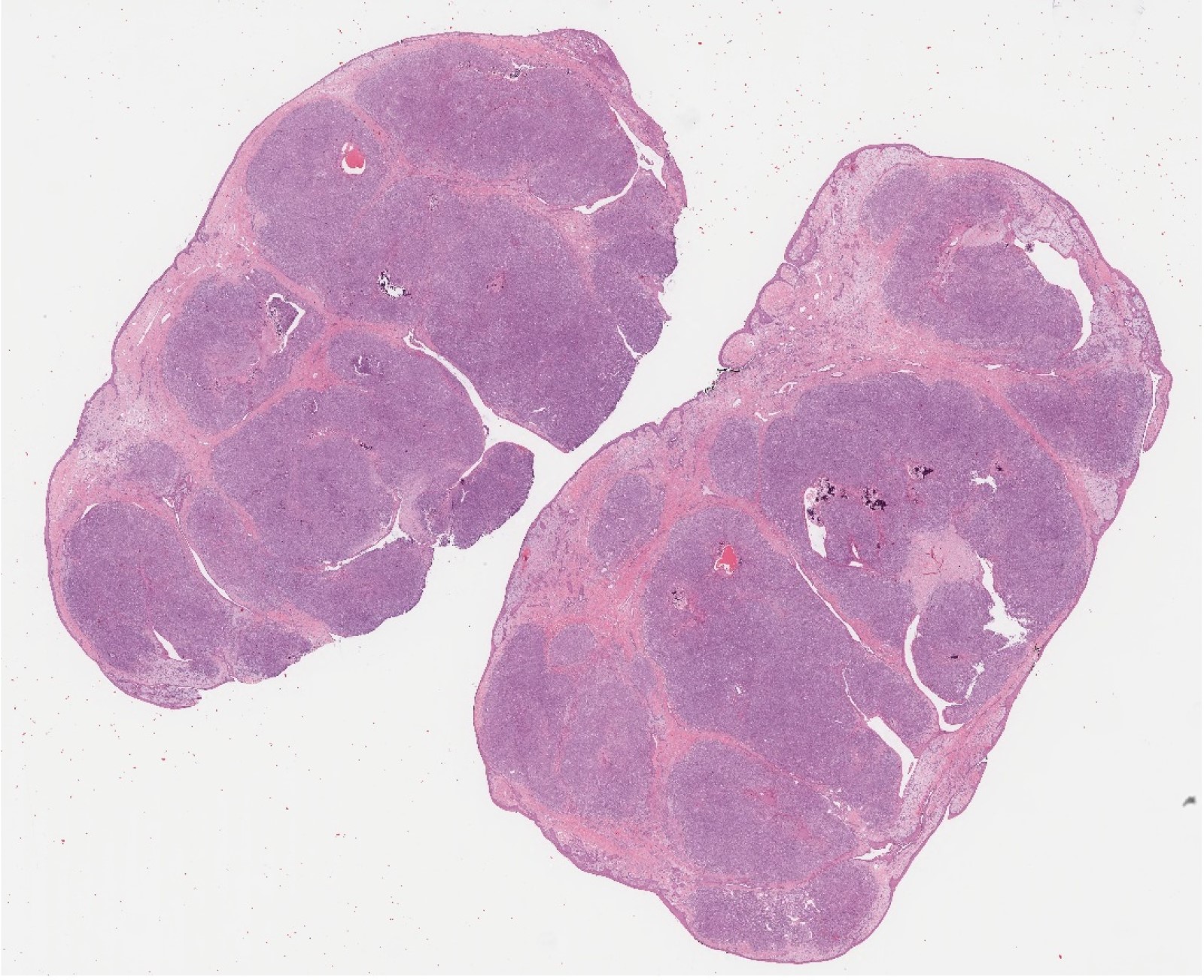
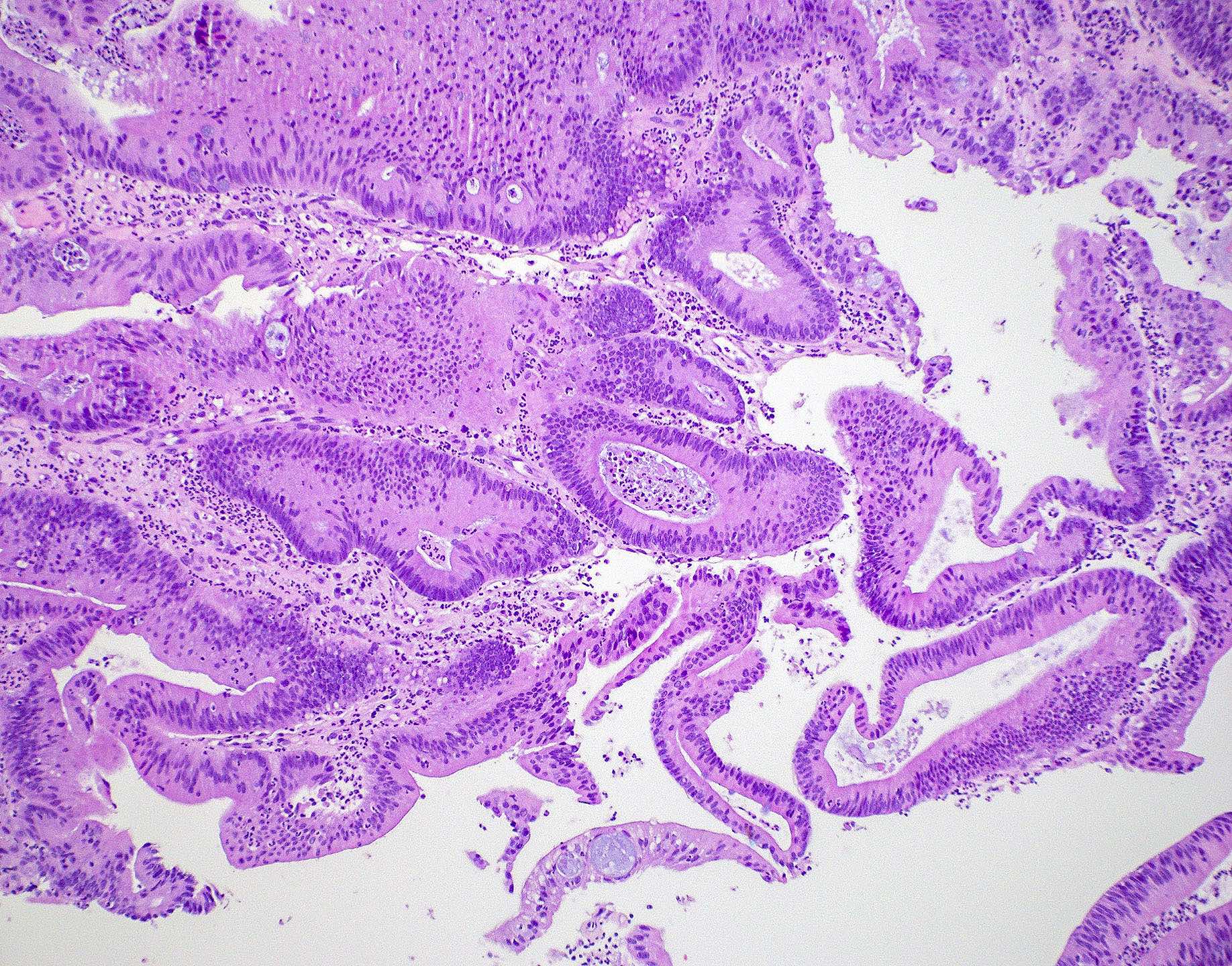
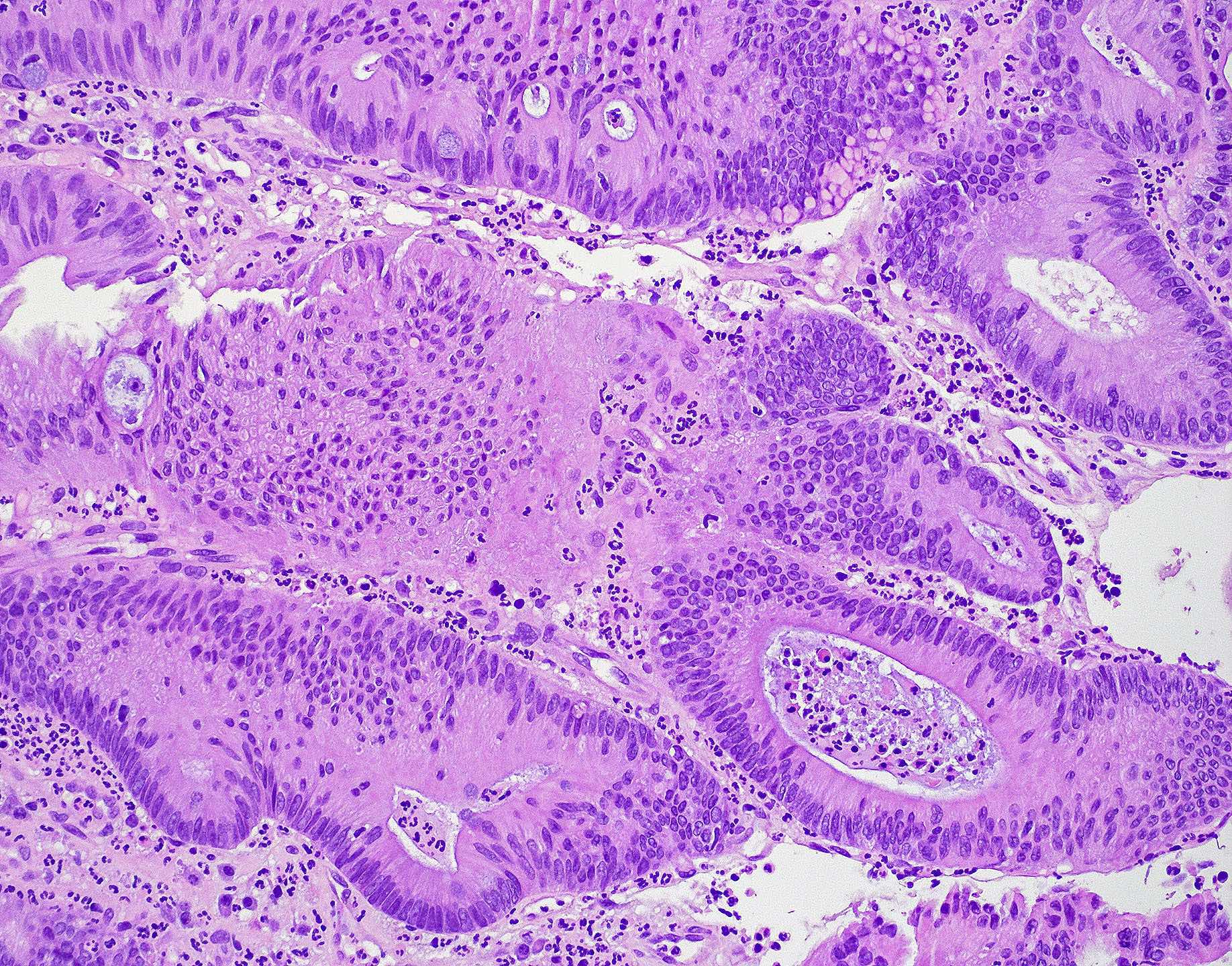
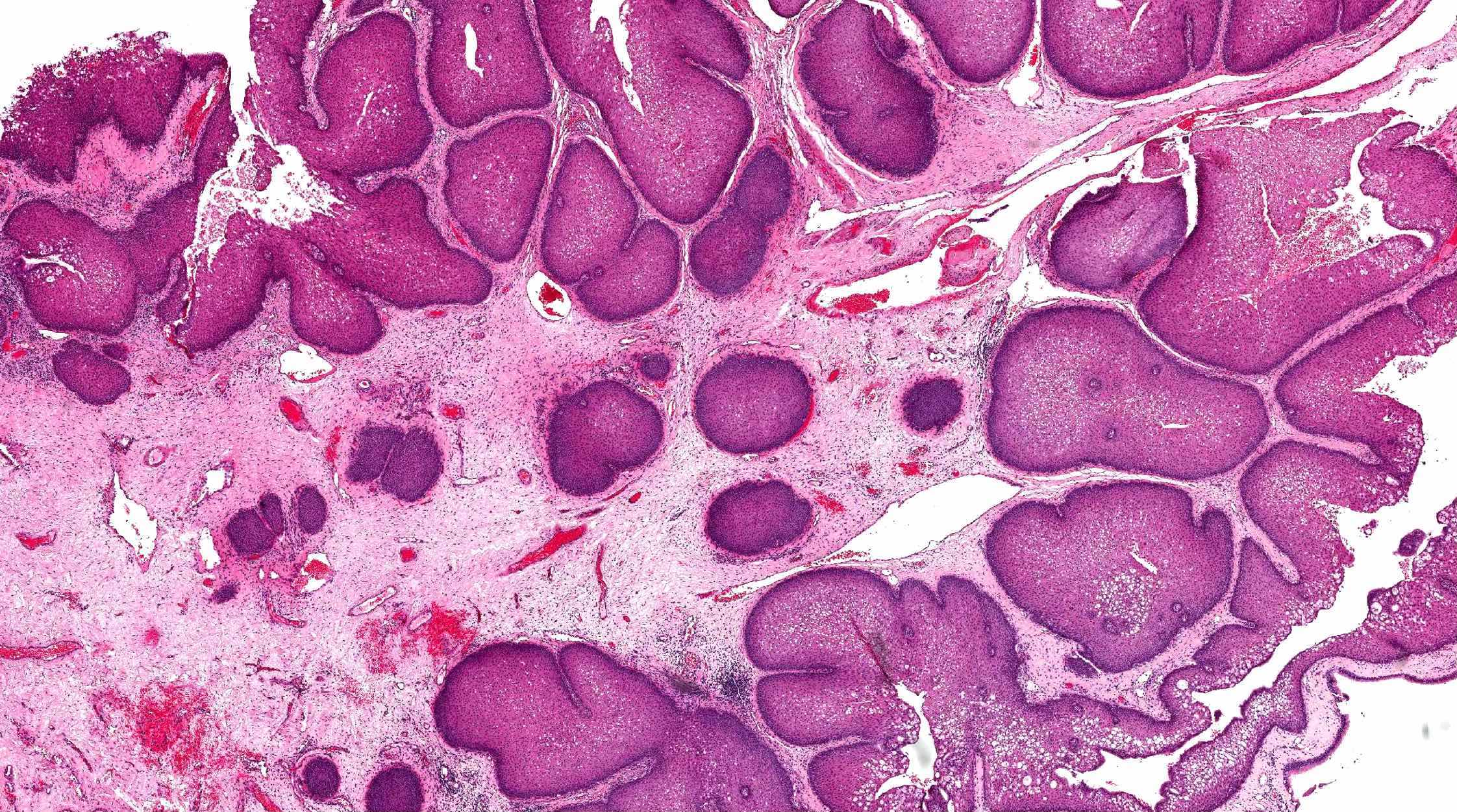
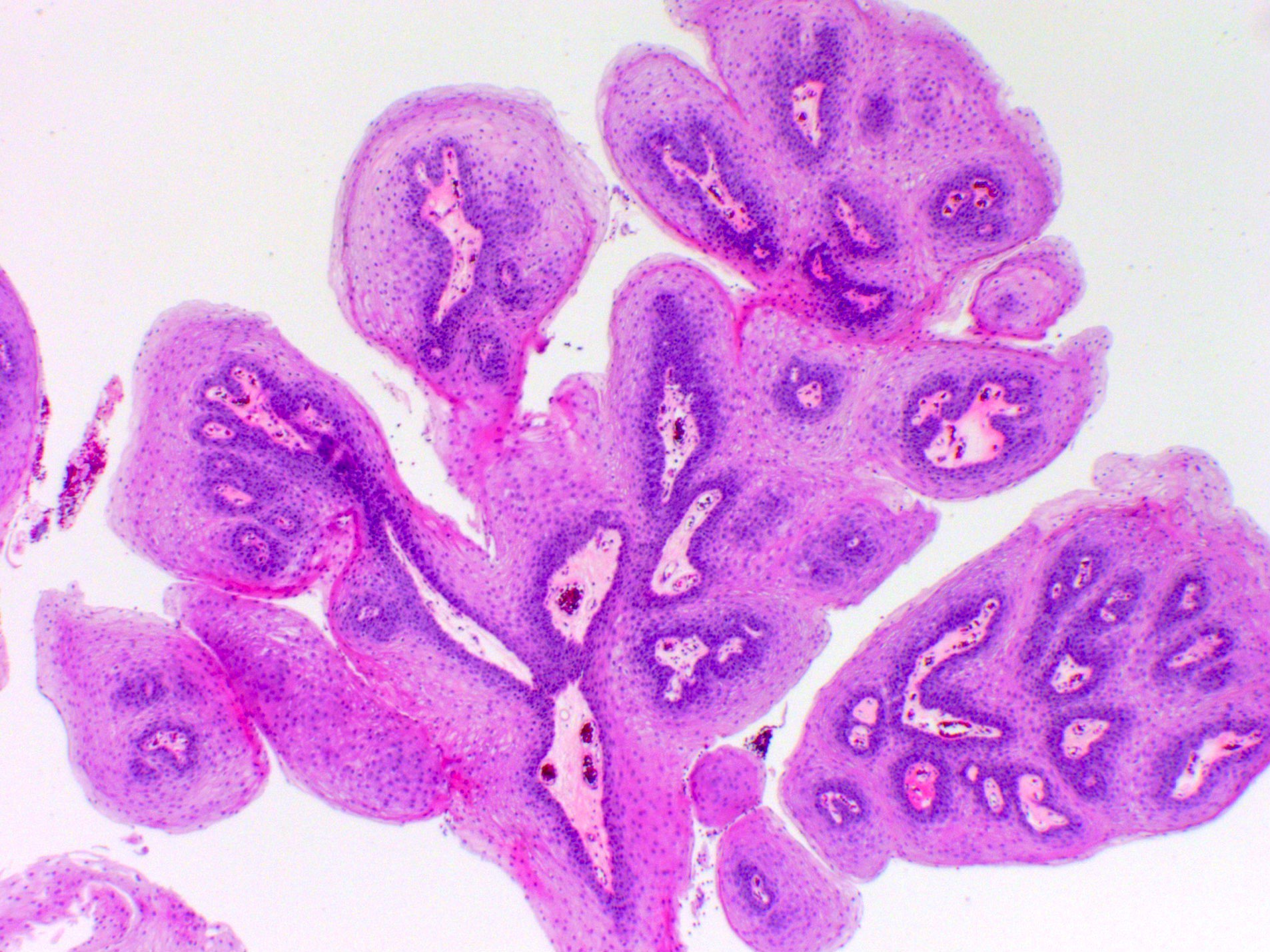
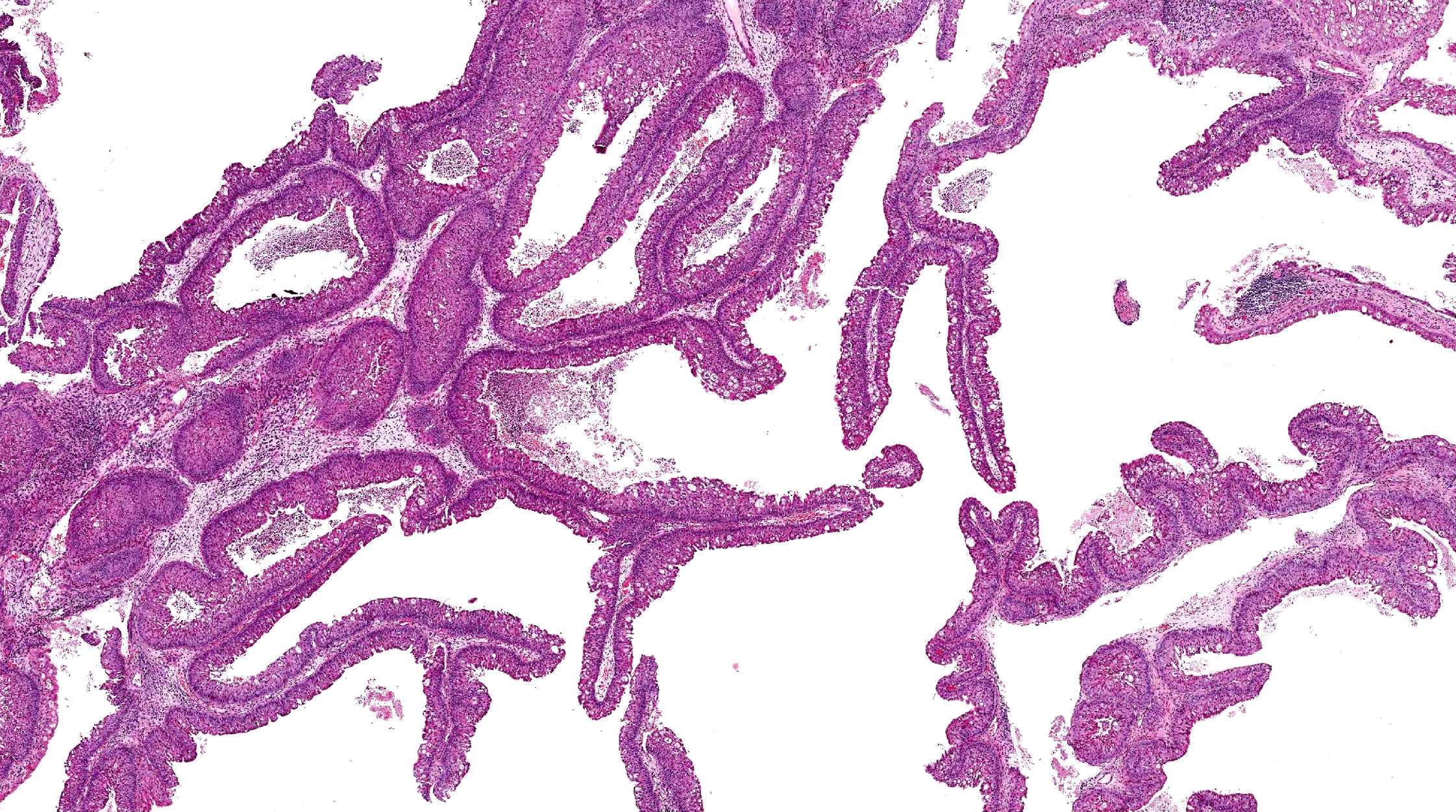
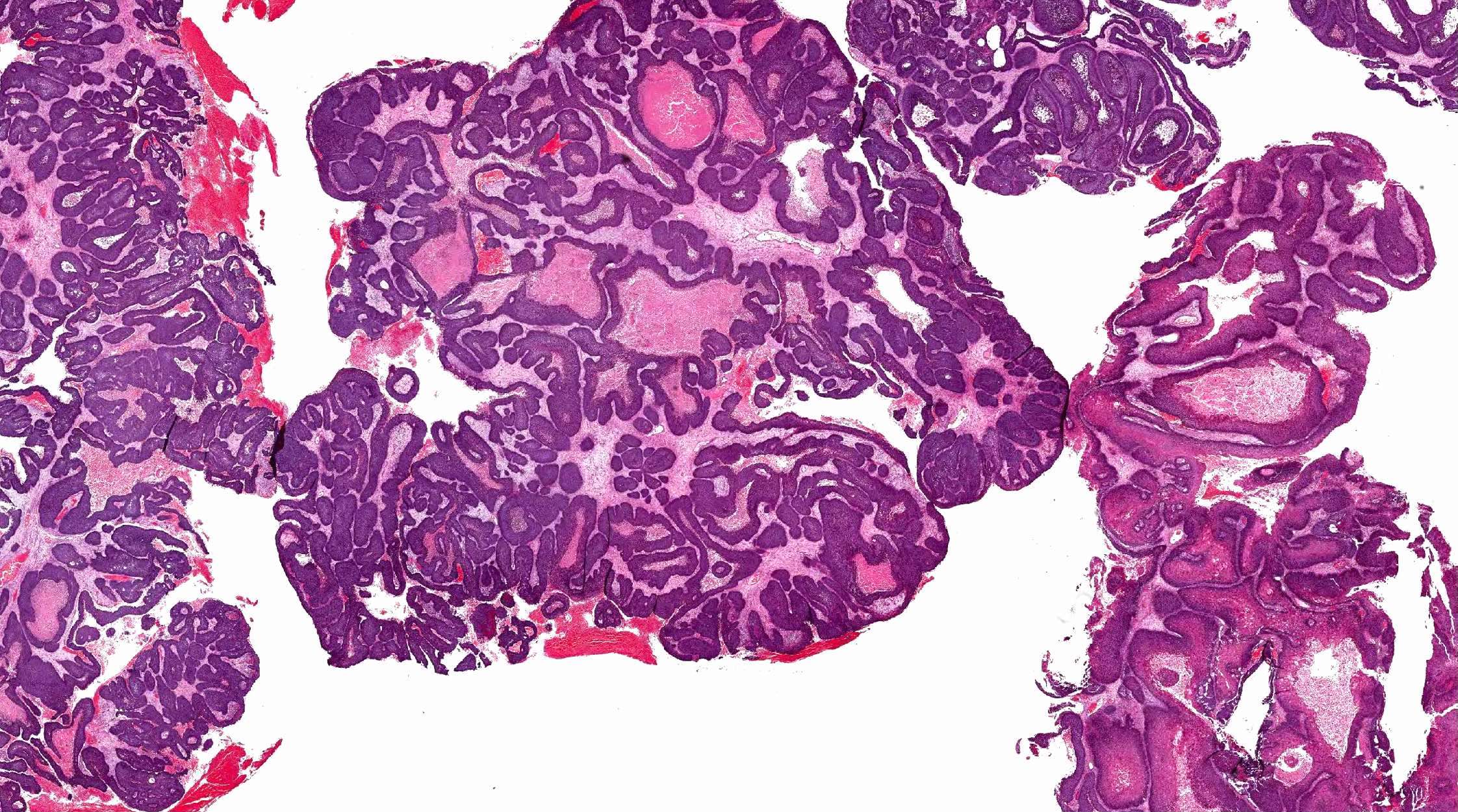
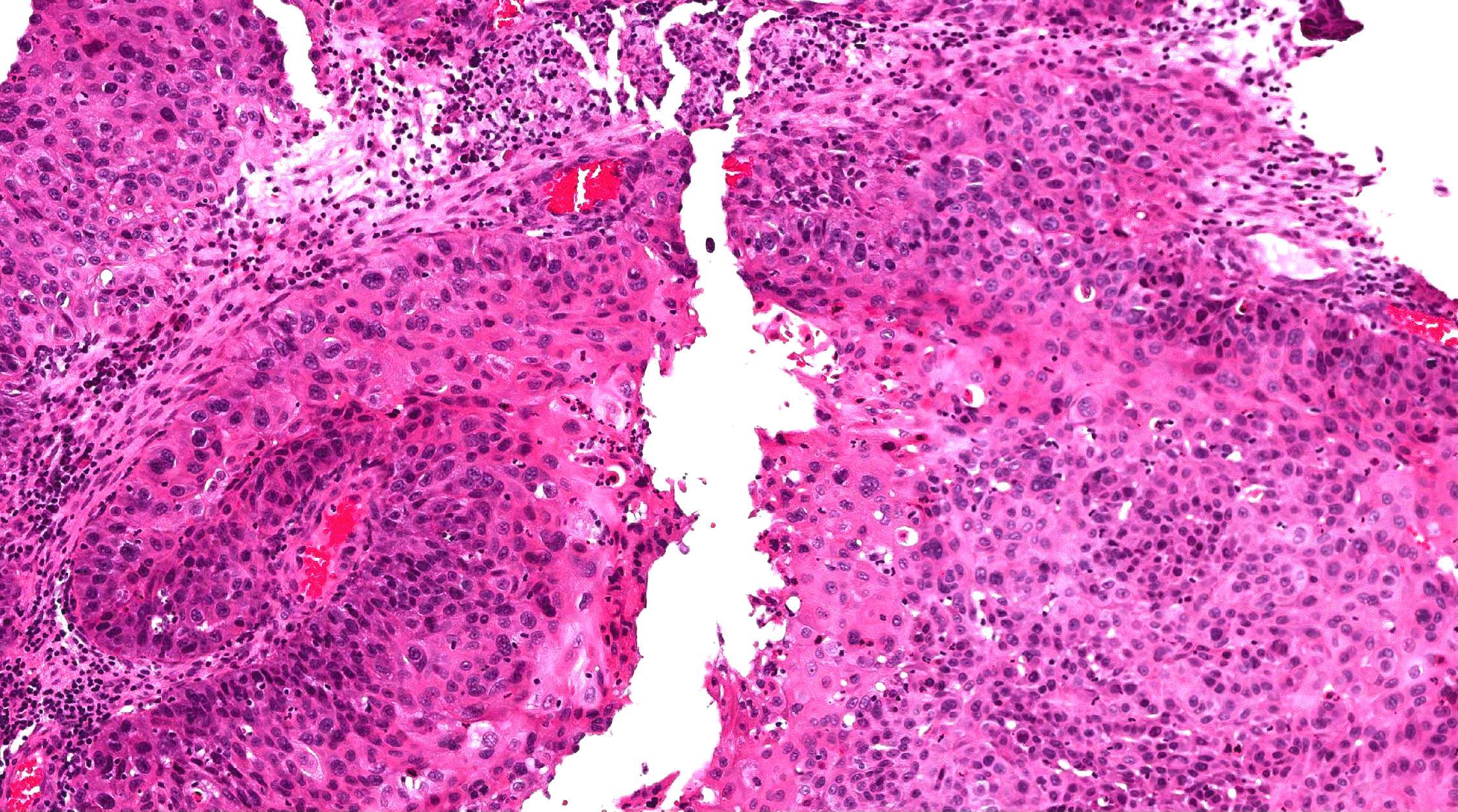
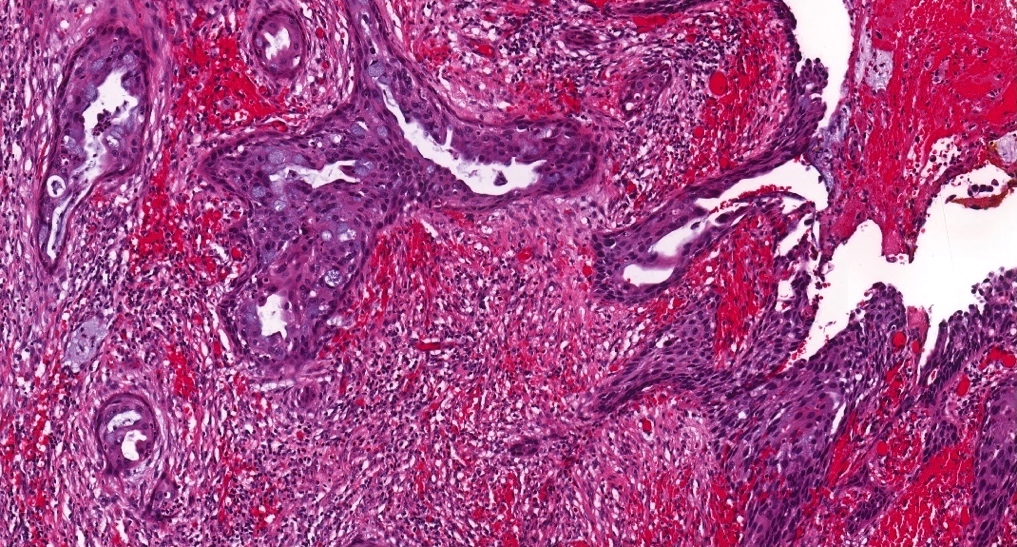
- ITAC displays glandular, tubular and trabecular architecture and few papillae and resembles a conventional colorectal adenocarcinoma
- Exclusion of metastasis from primary gastrointestinal tract tumor is required
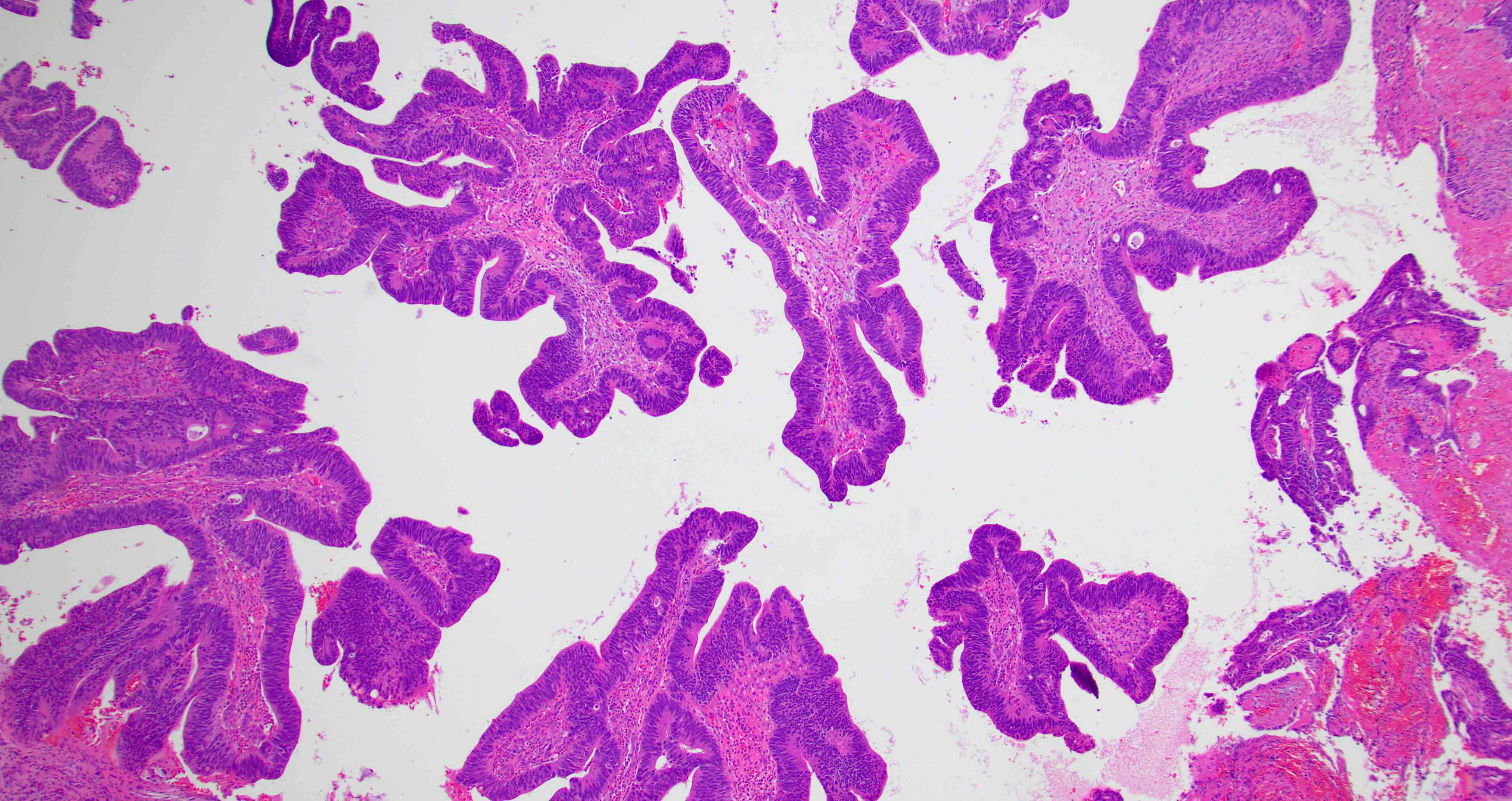
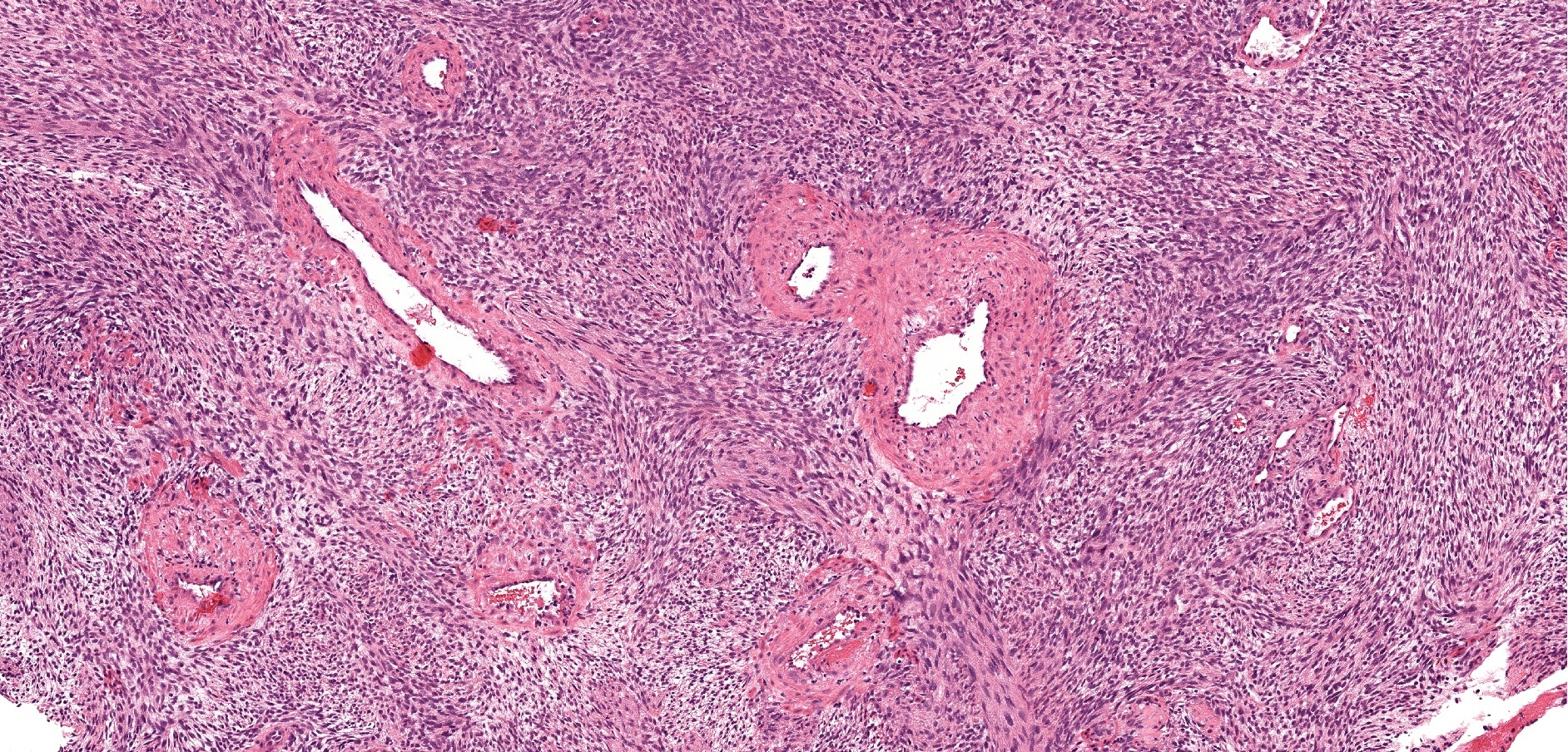
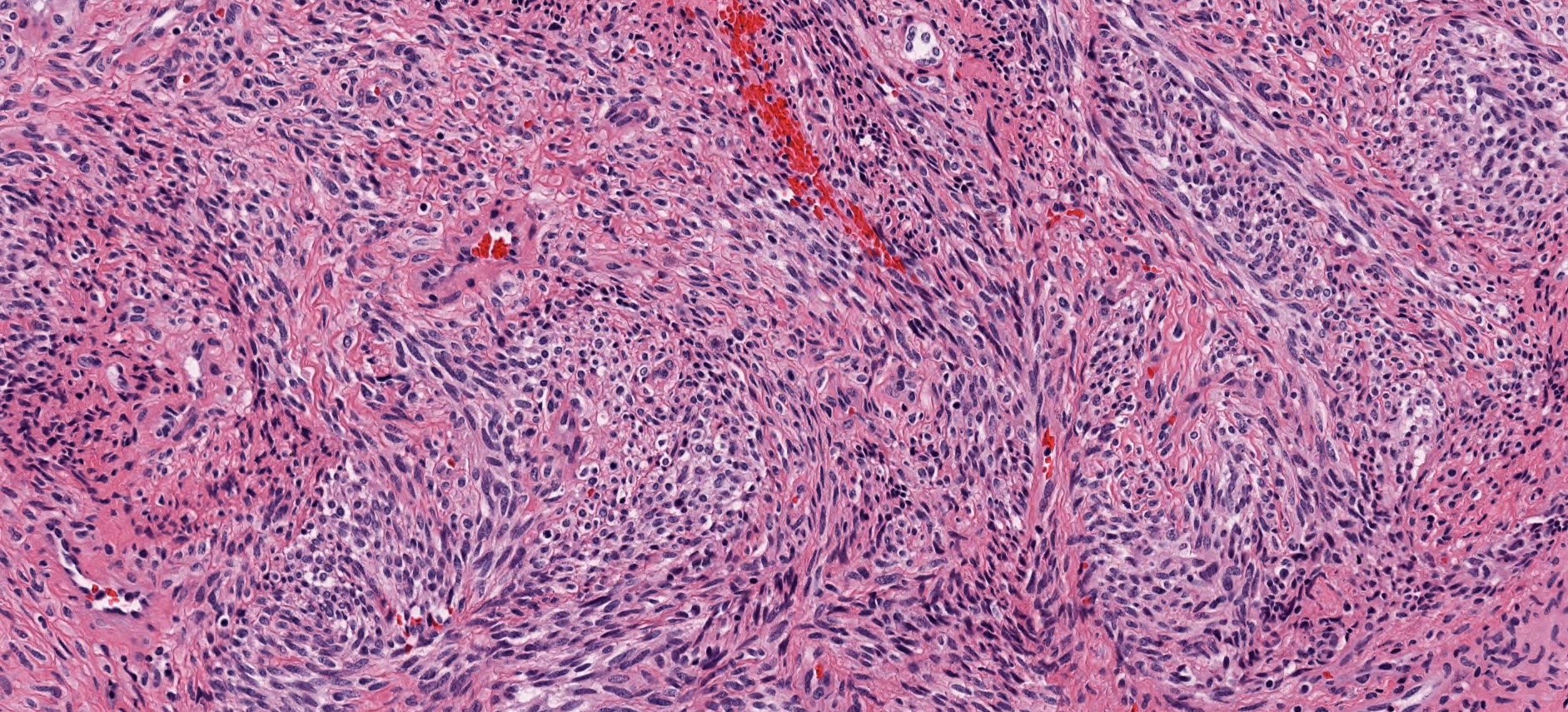
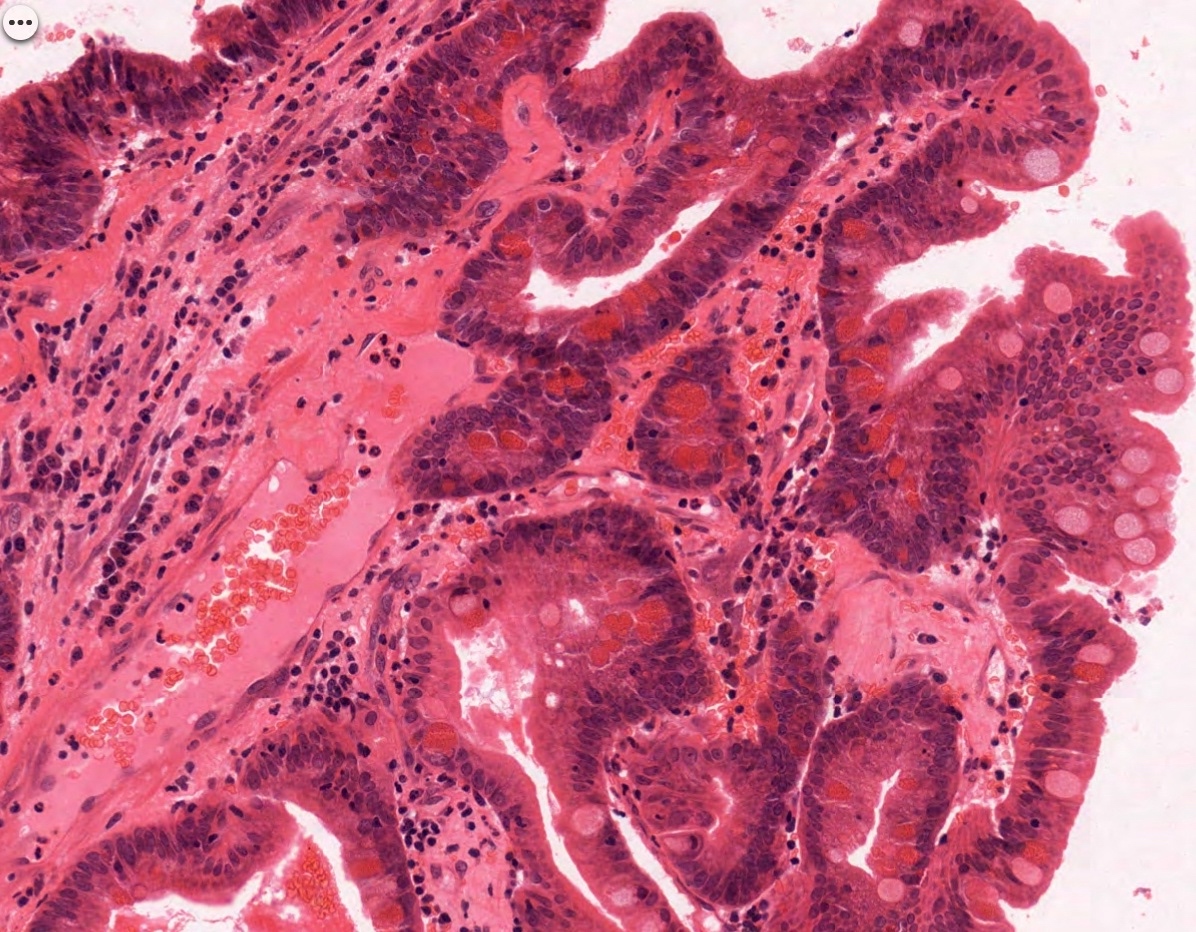
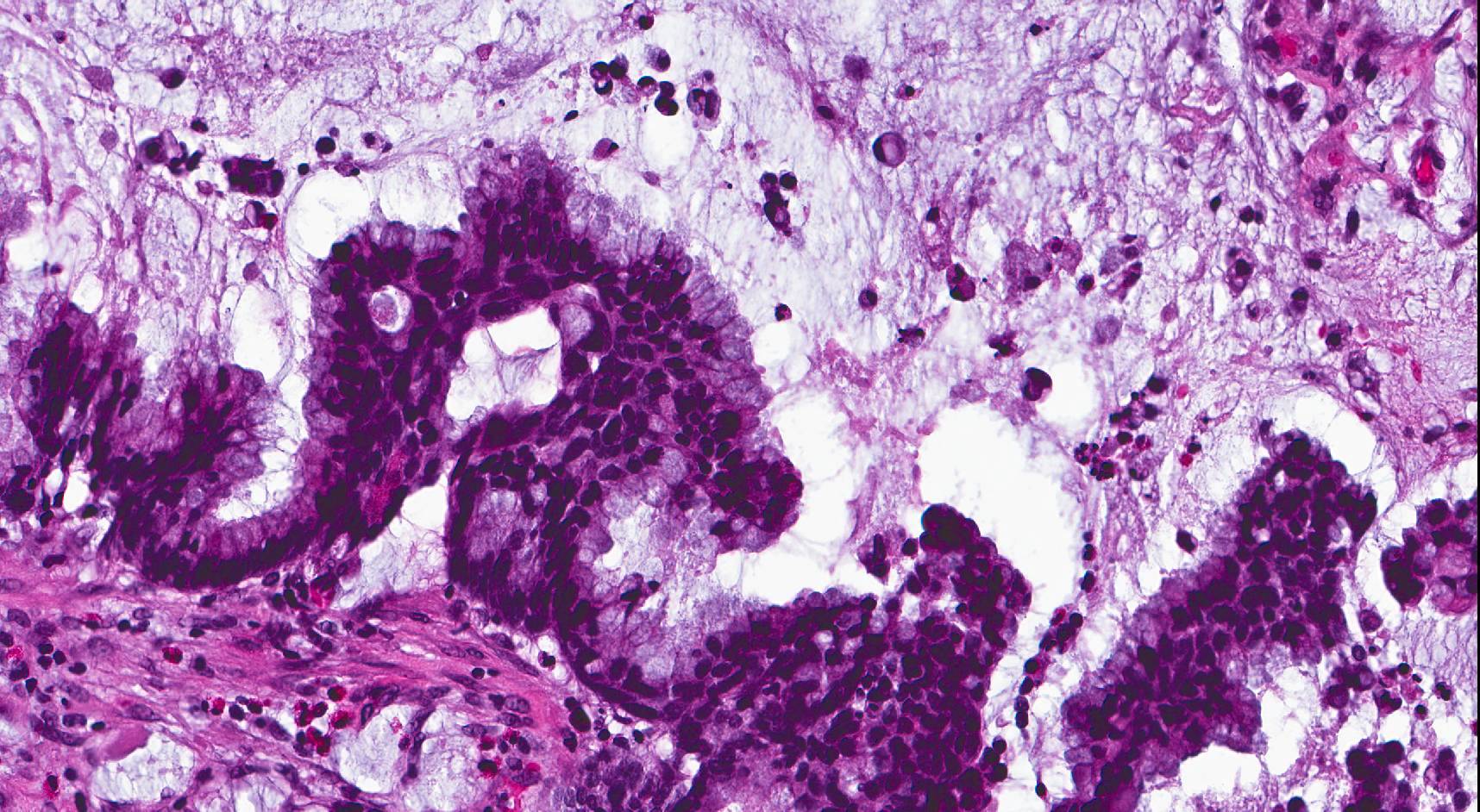
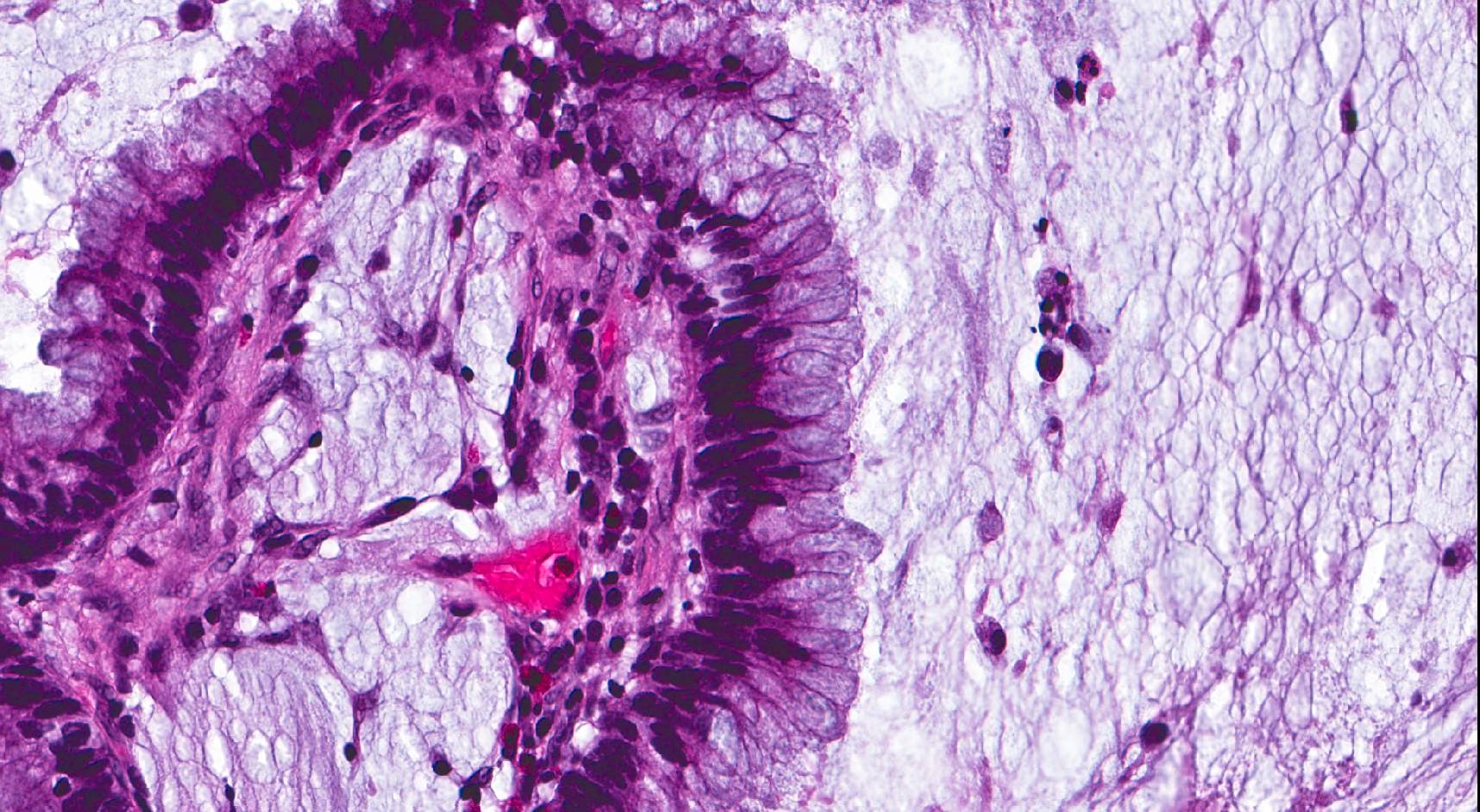
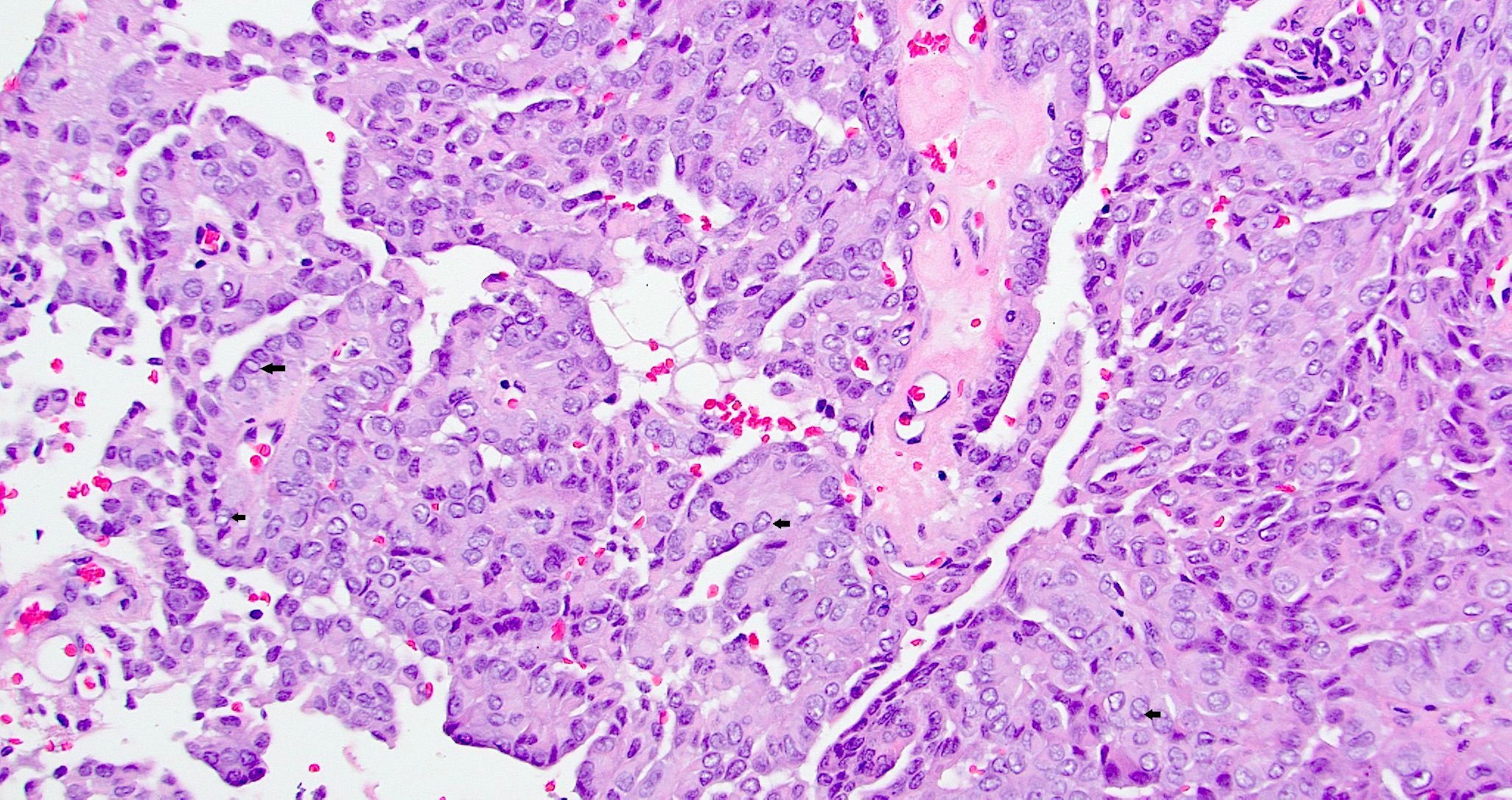
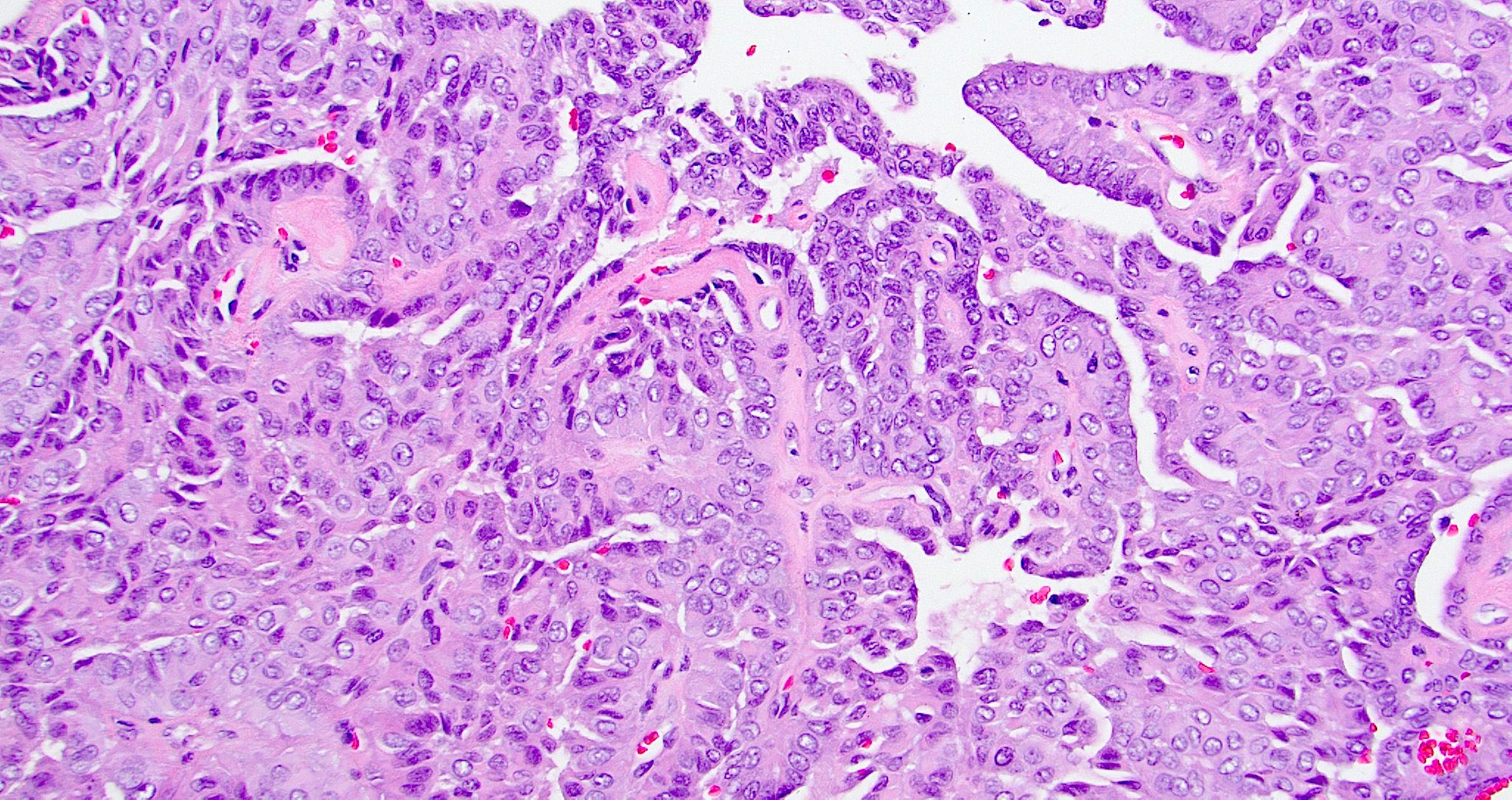
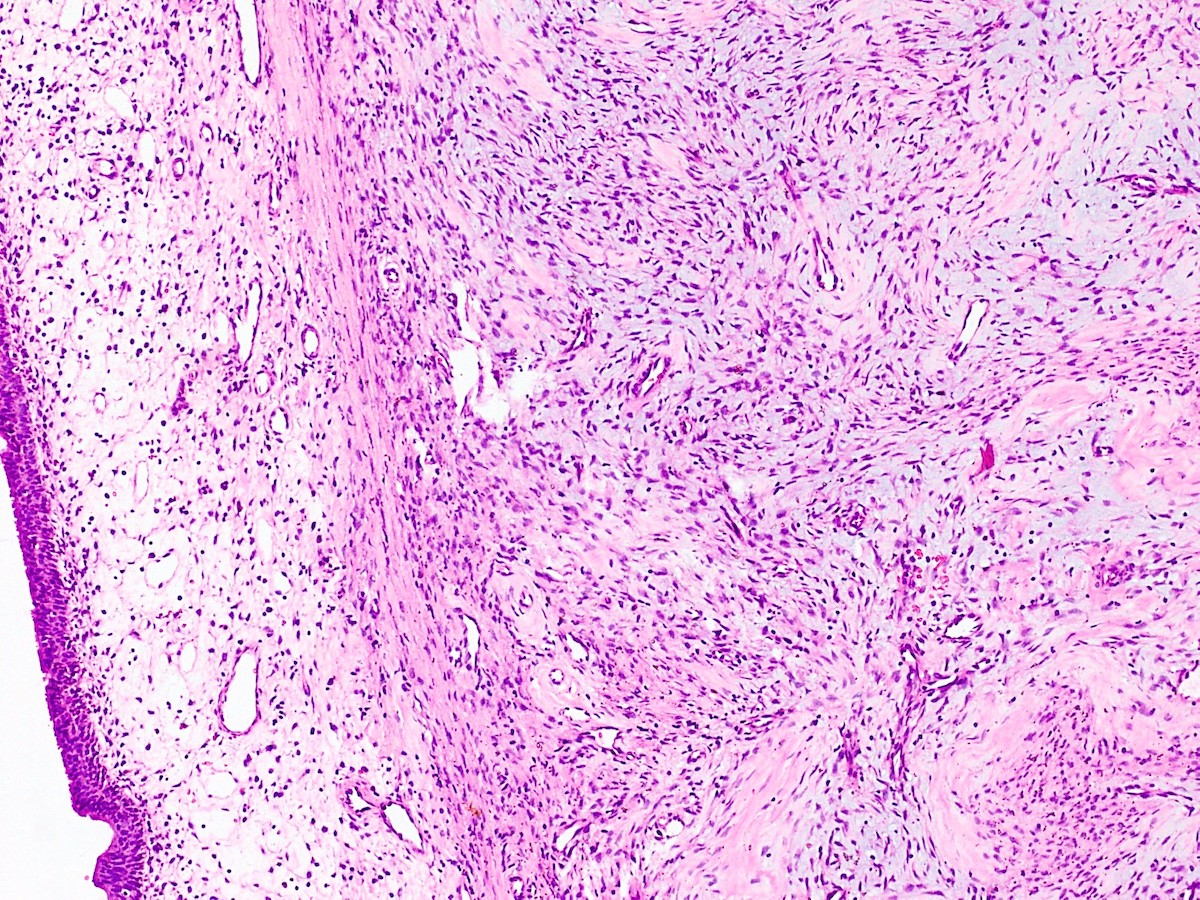
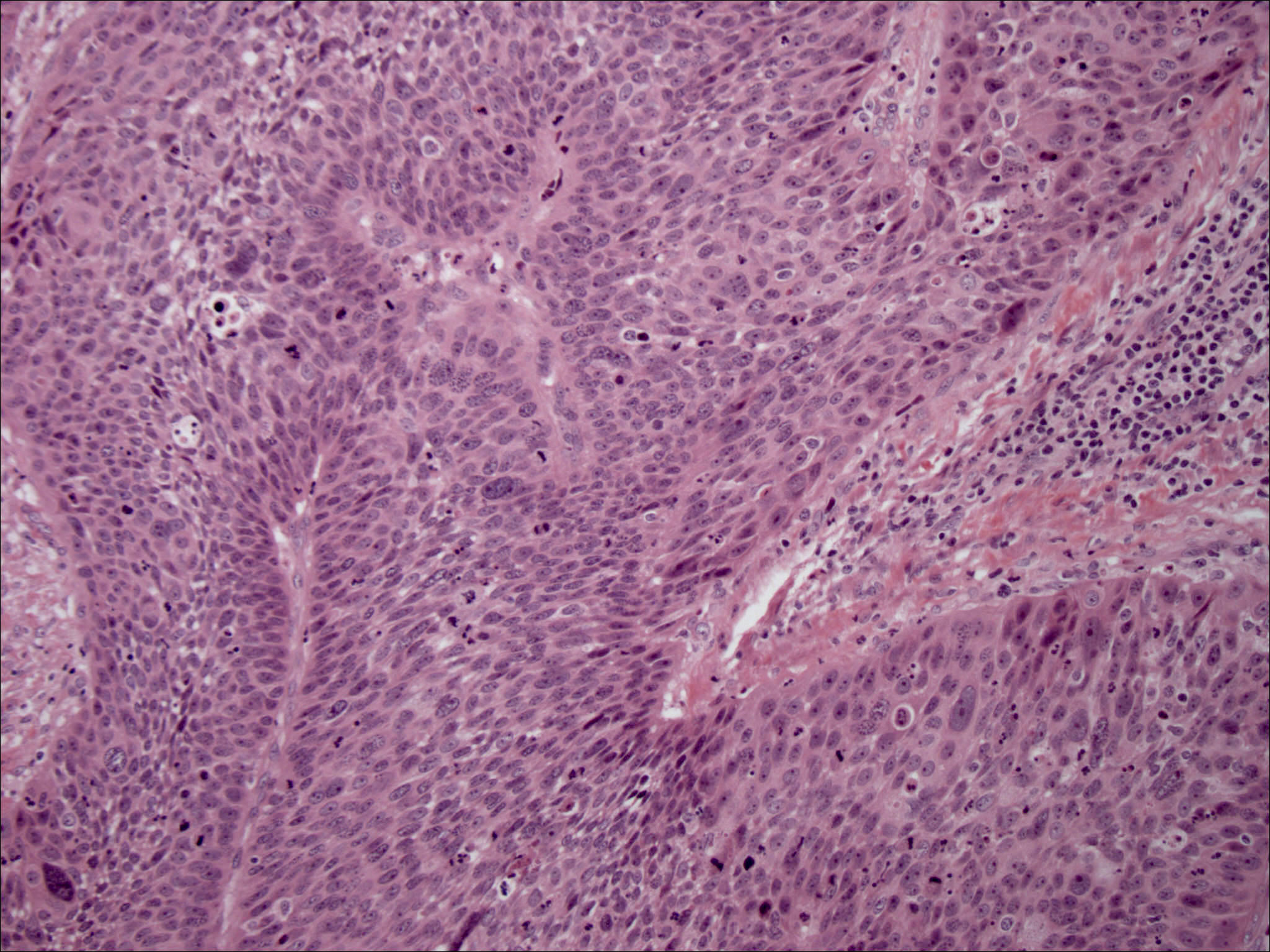
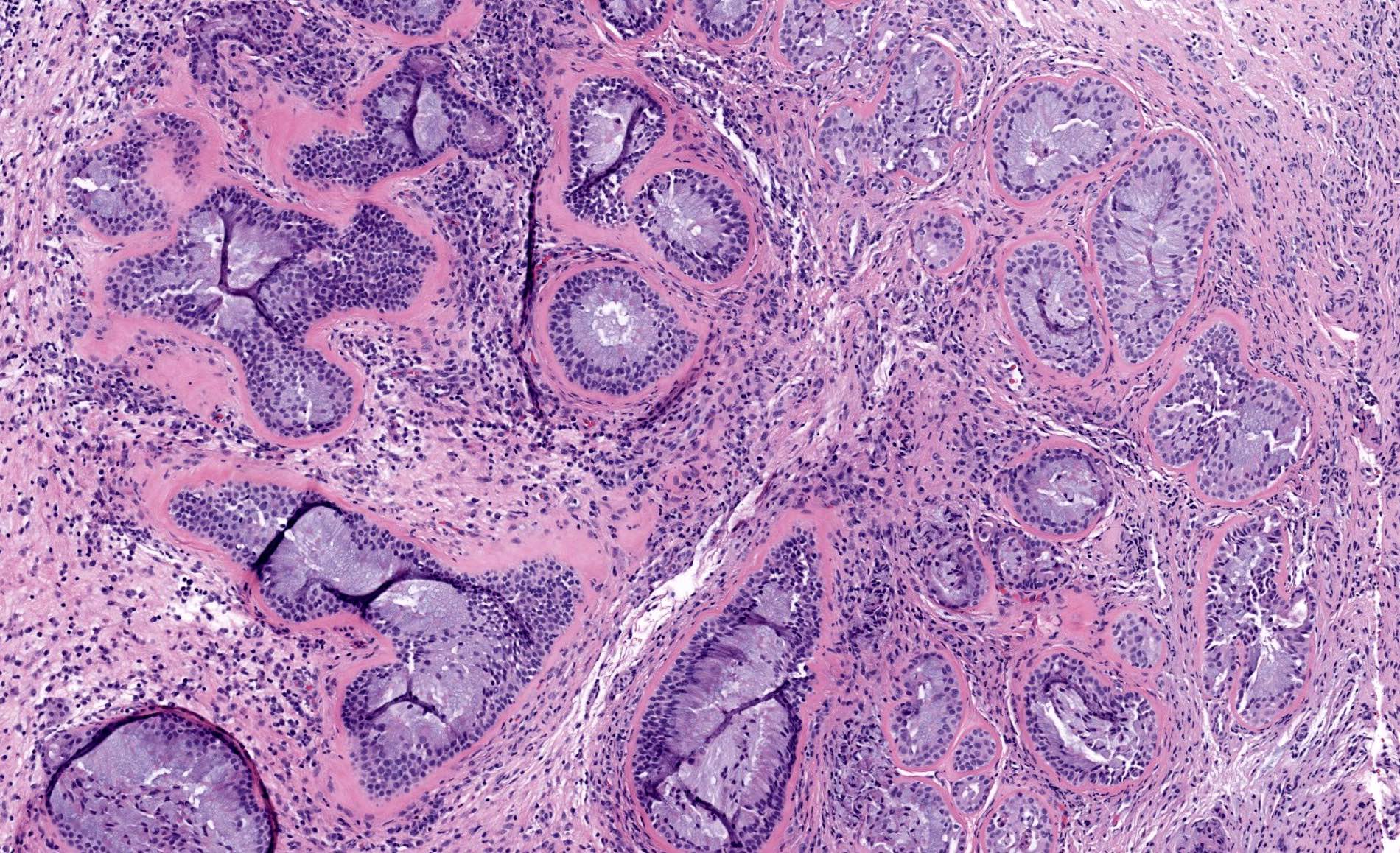
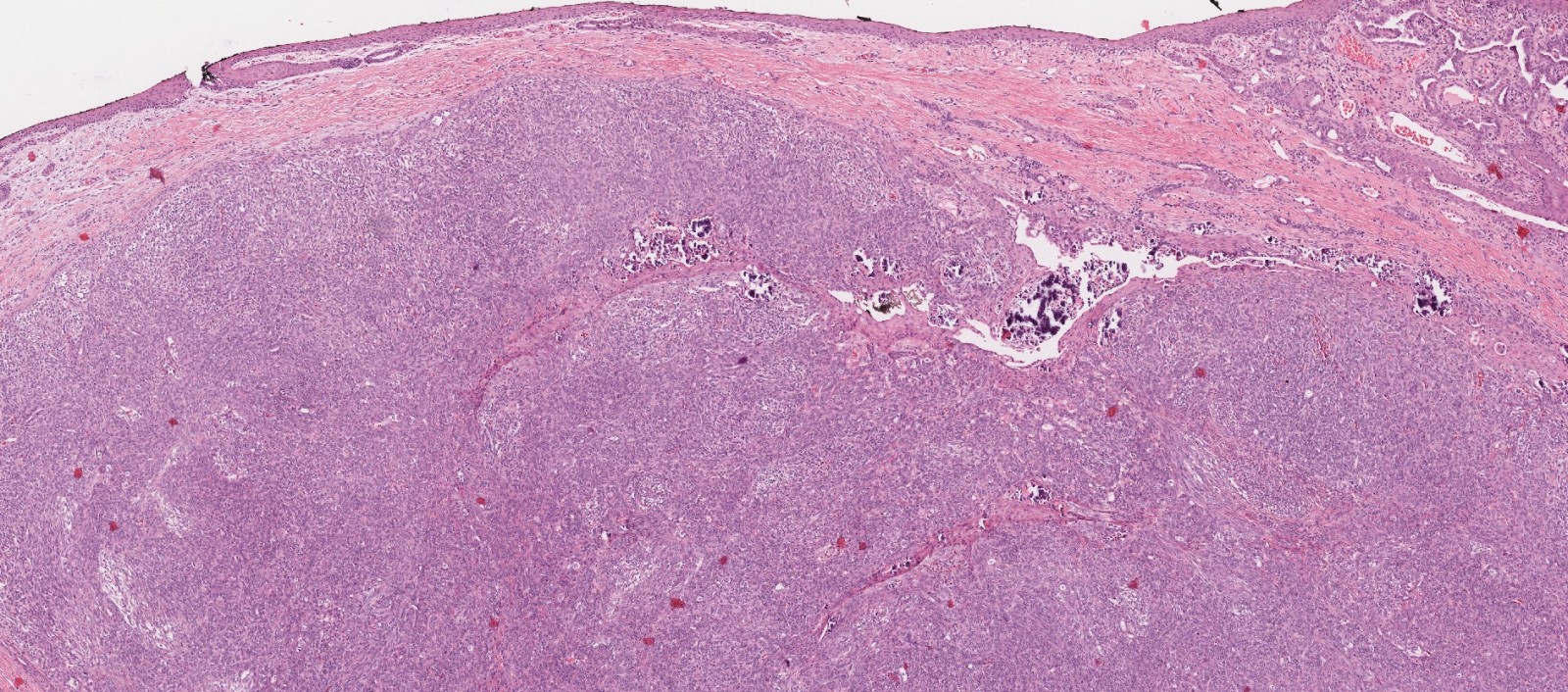
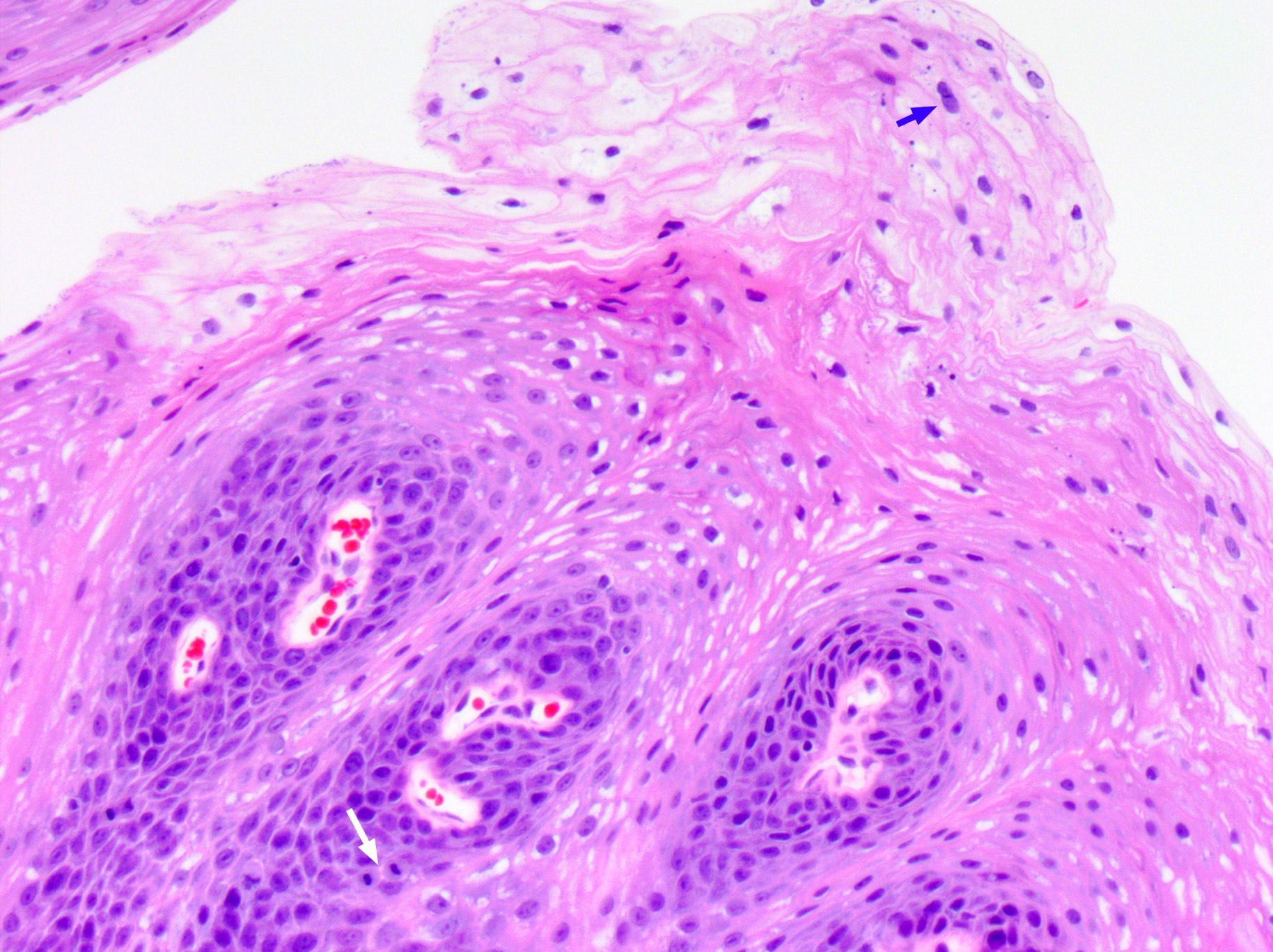
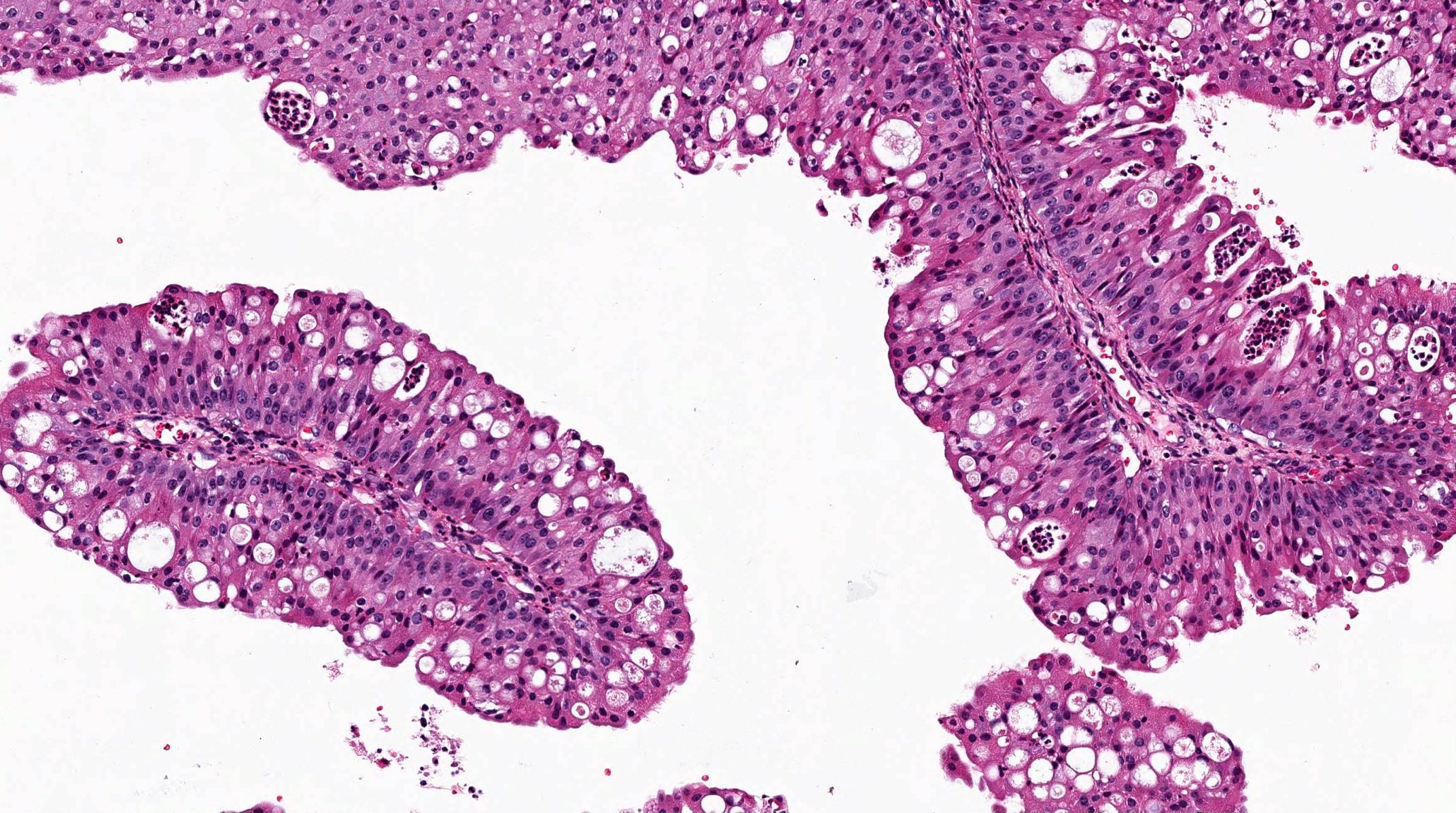
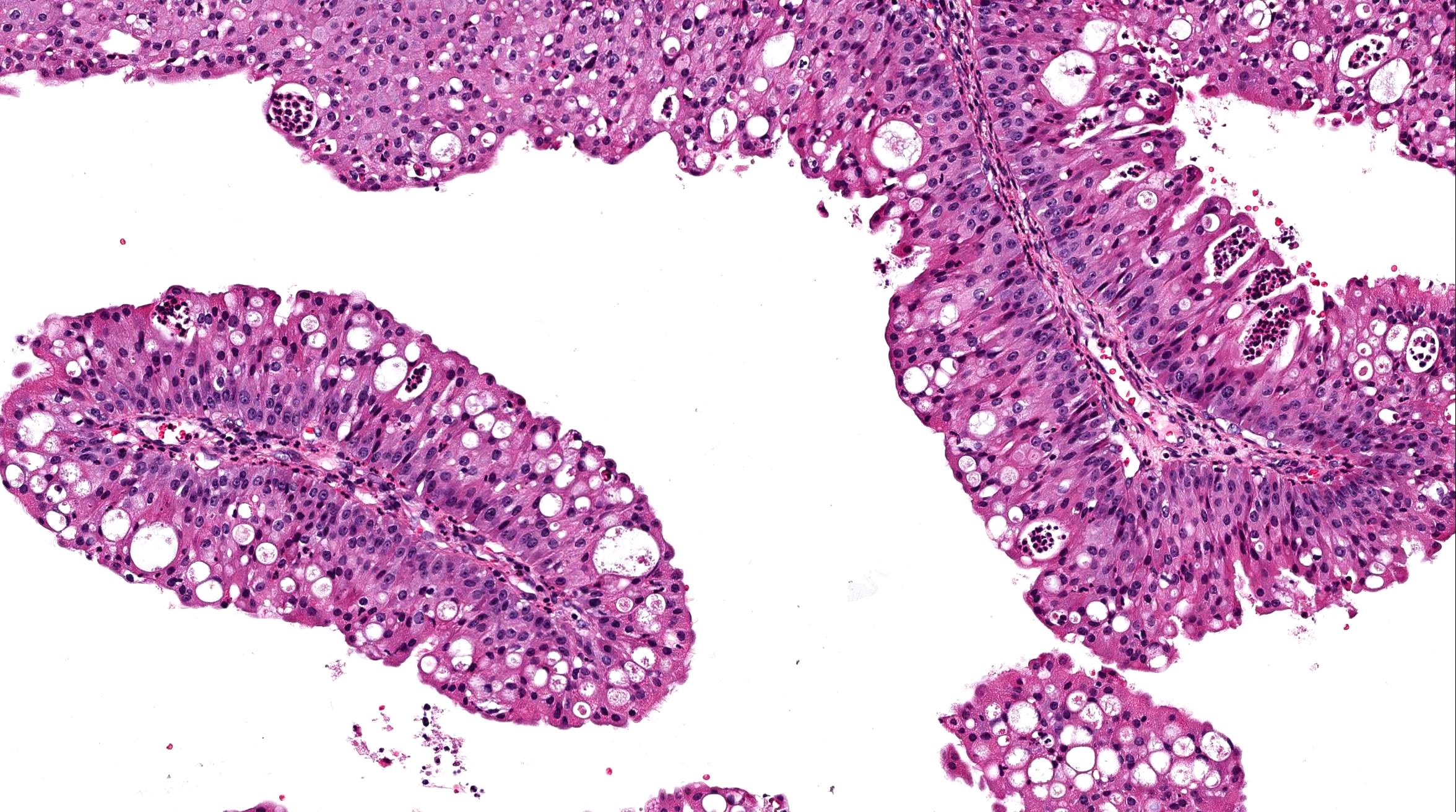
- Low grade non-ITAC can exhibit exophytic papillae, tubular or glandular, trabecular, cribriform, clear cell and mucinous patterns
- Papillae and glands are usually lined by a single layer of uniform columnar or cuboidal cells with eosinophilic cytoplasm and slight cytologic aberrations
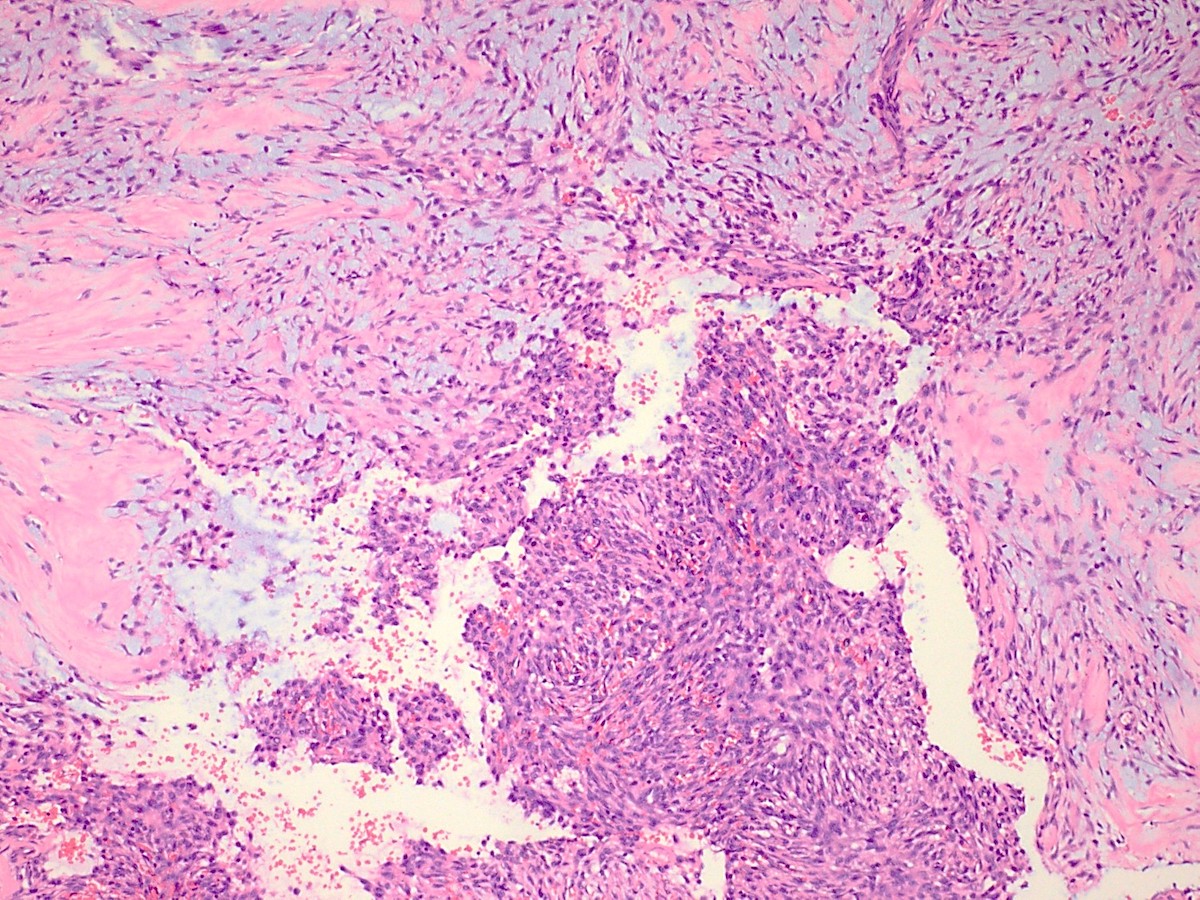
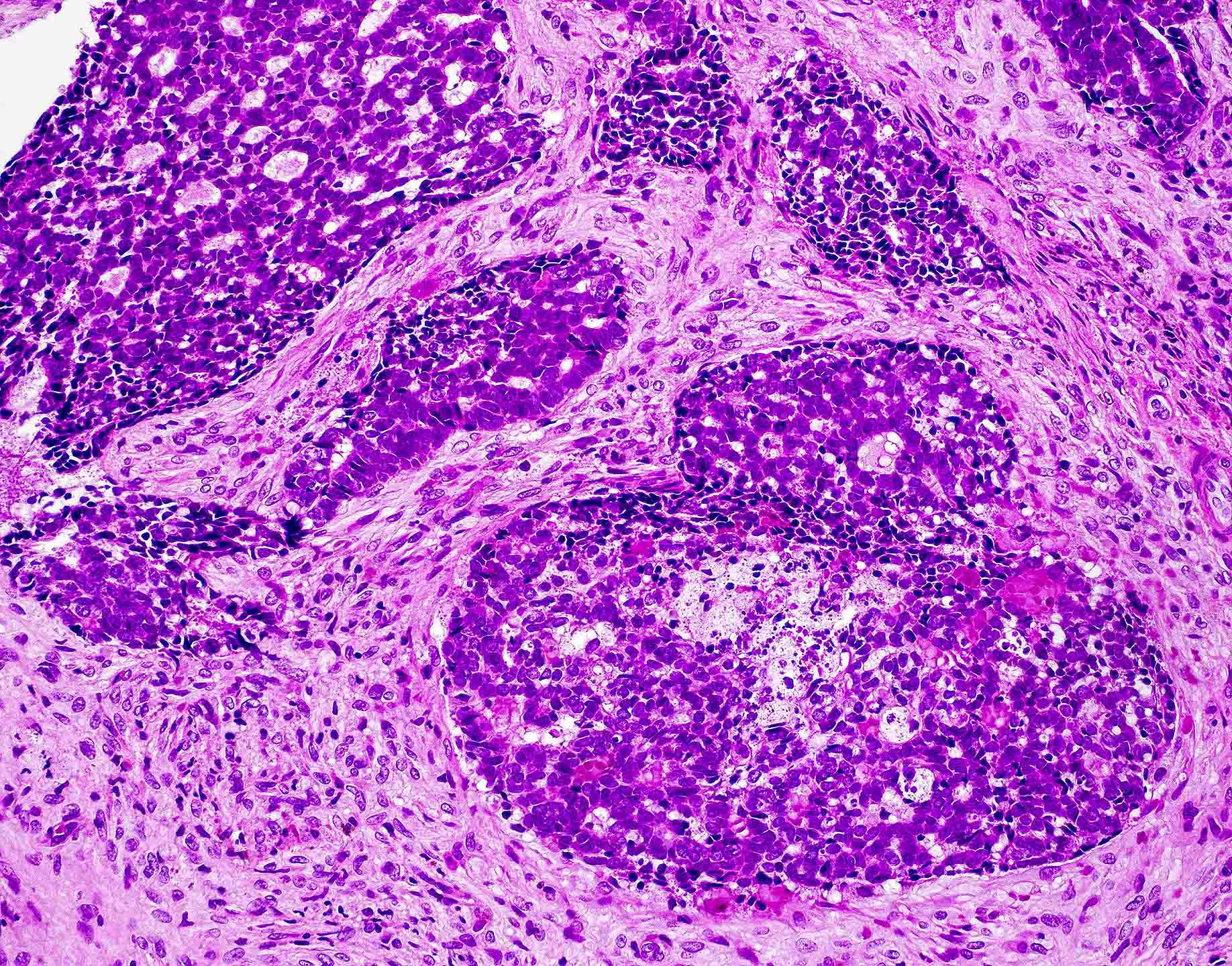
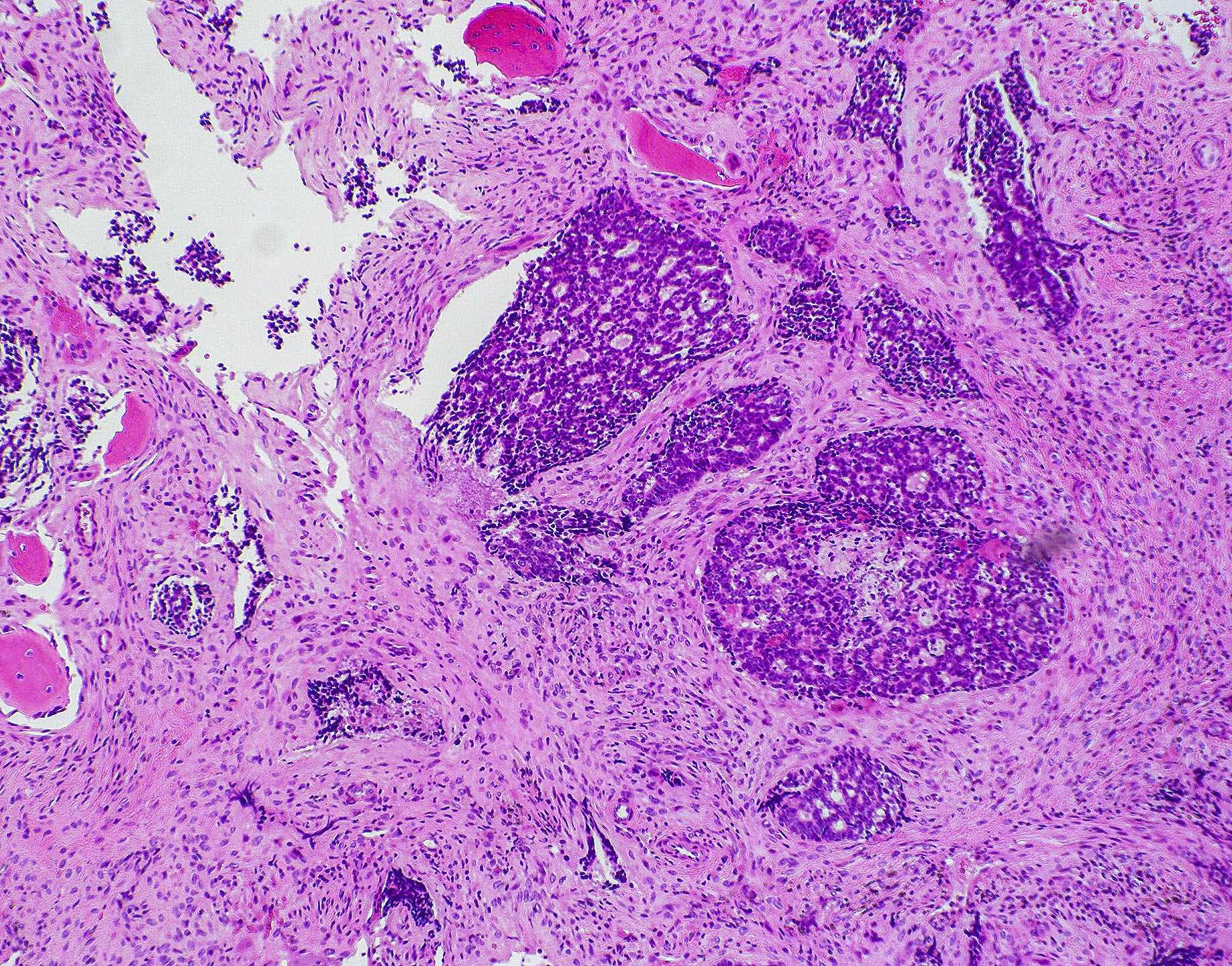
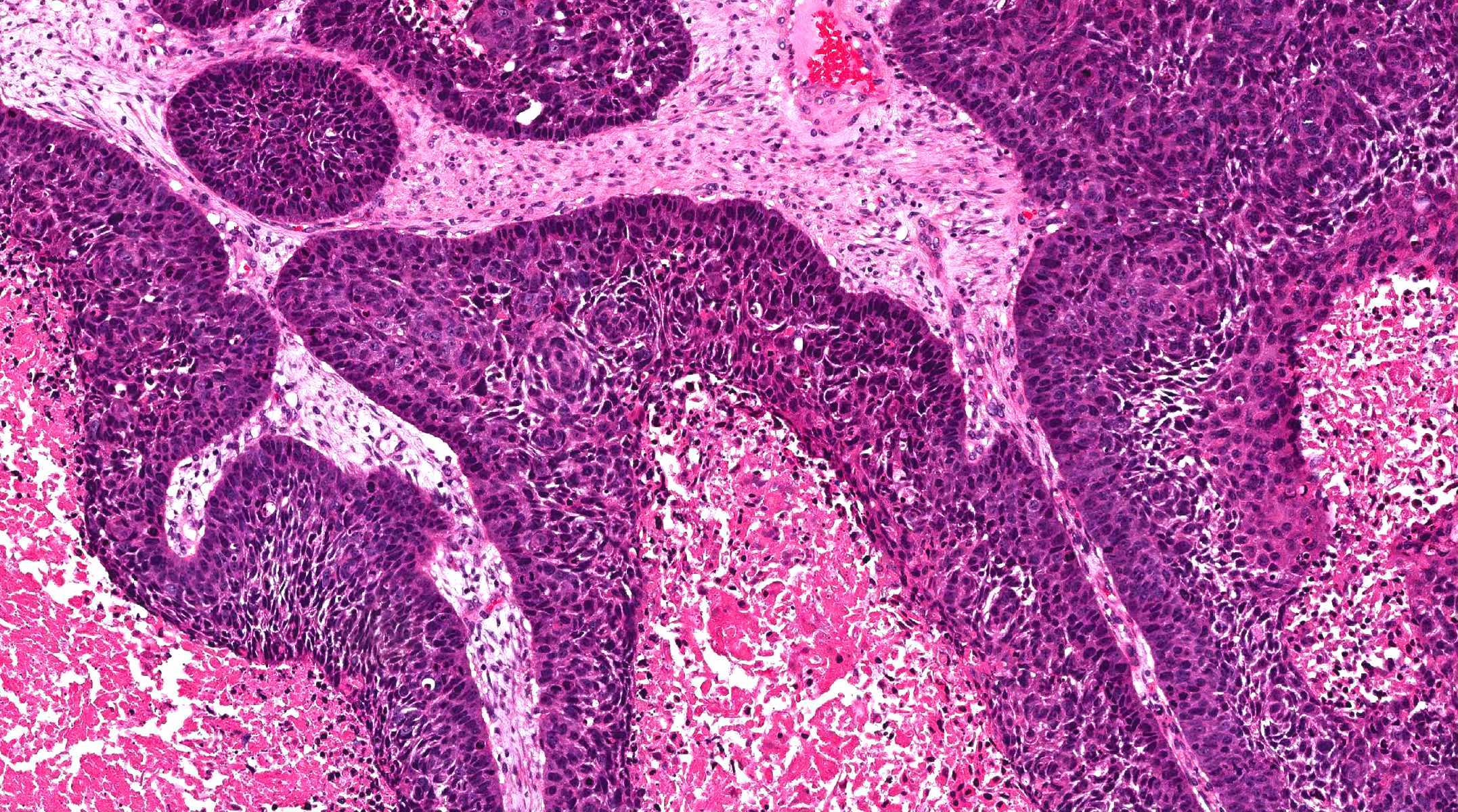
- High grade non-ITAC can display blastomatous, apocrine, mucinous, poorly differentiated patterns with pleomorphic nuclei with predominantly solid growth pattern
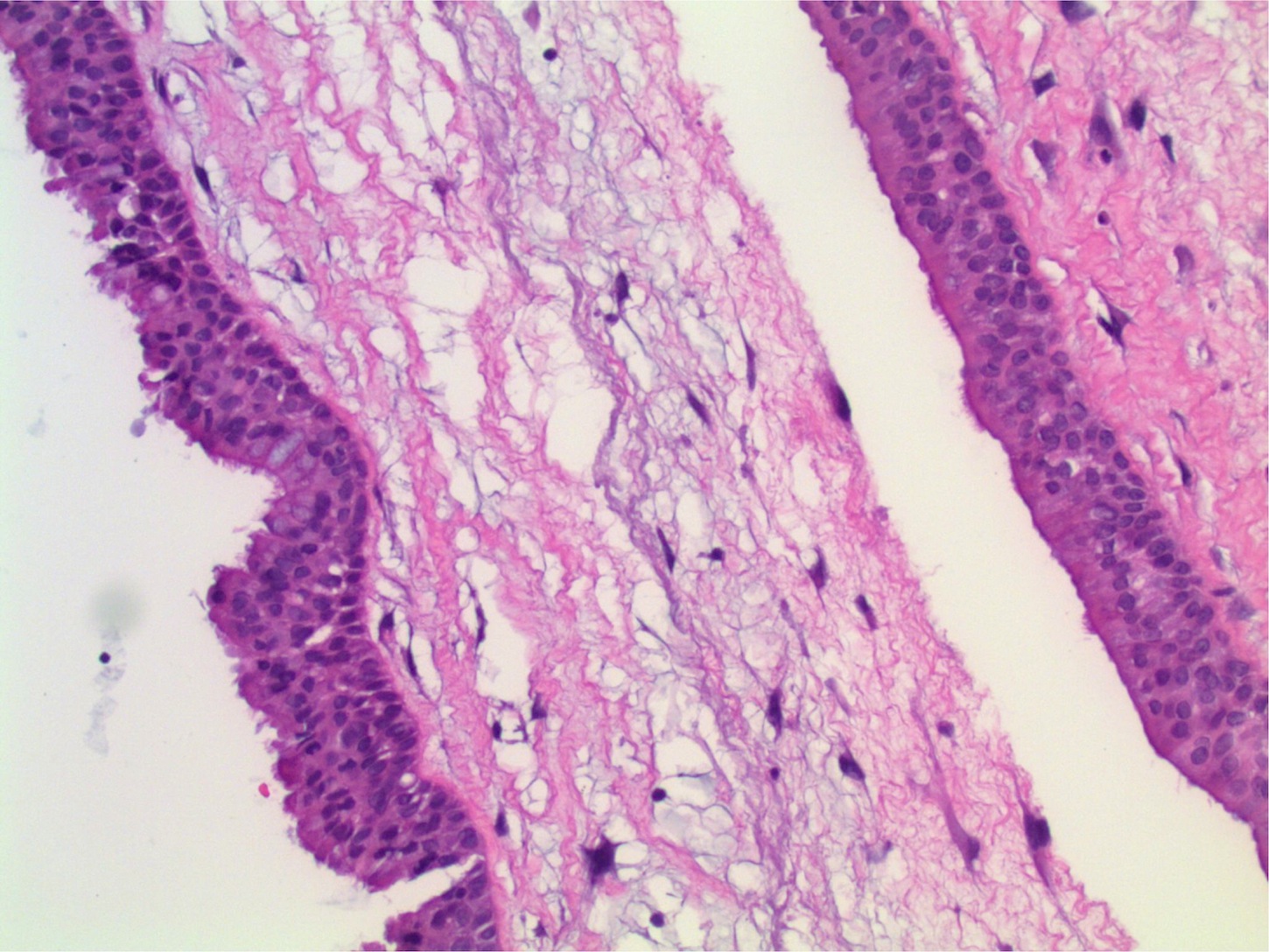
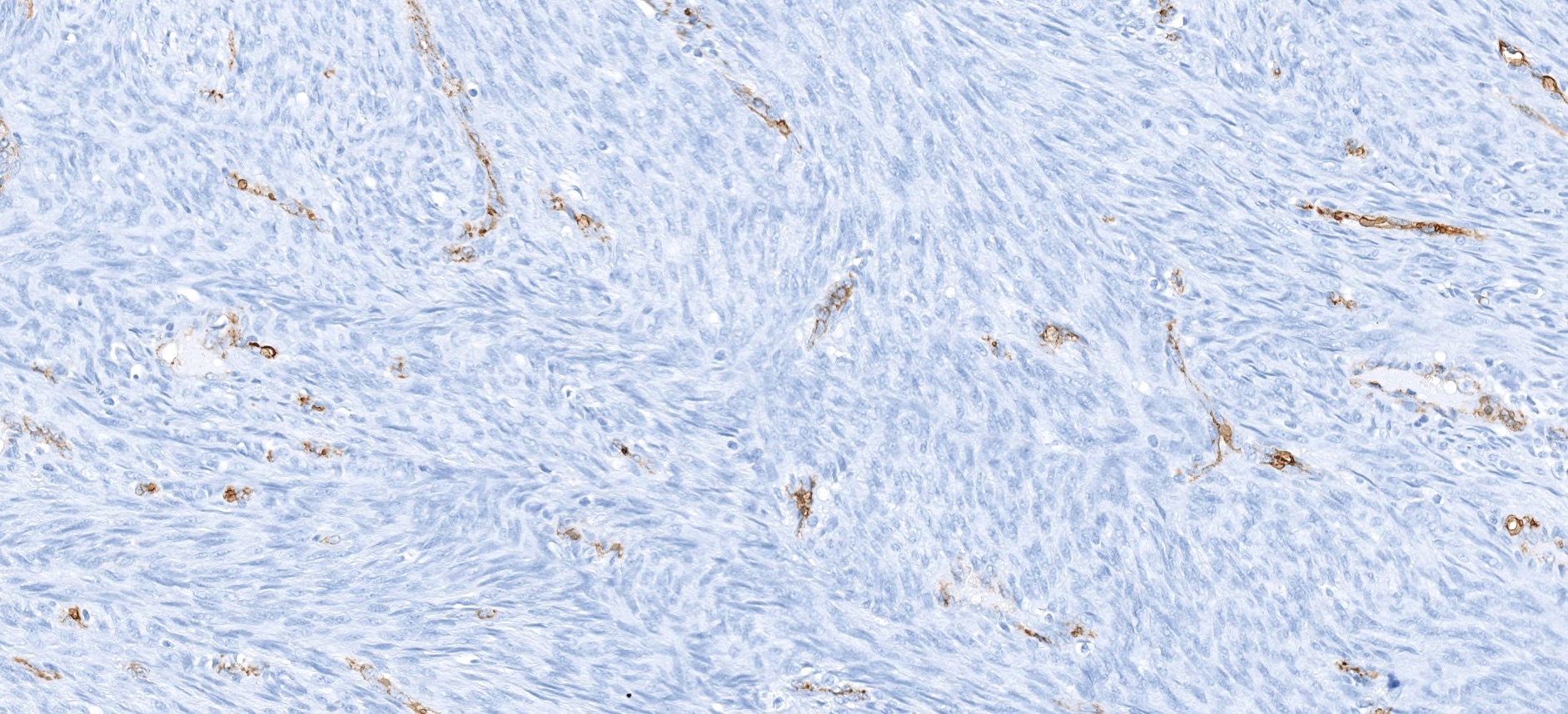
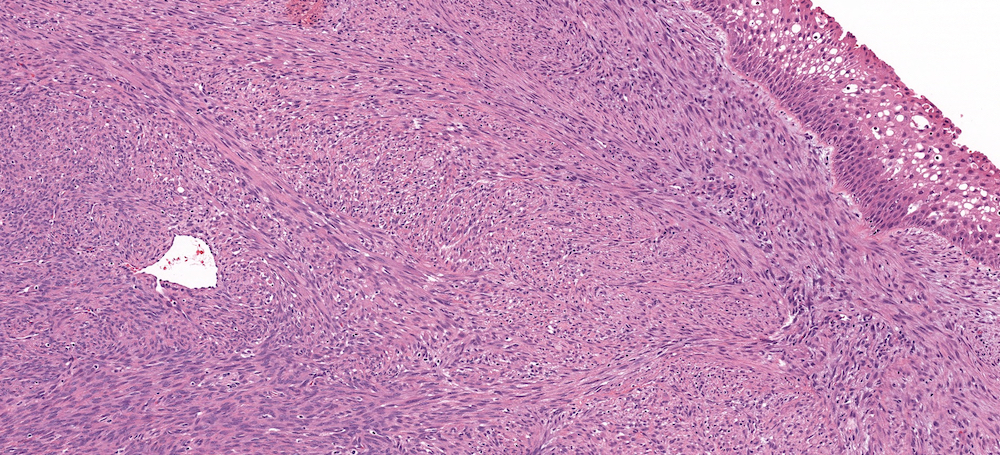
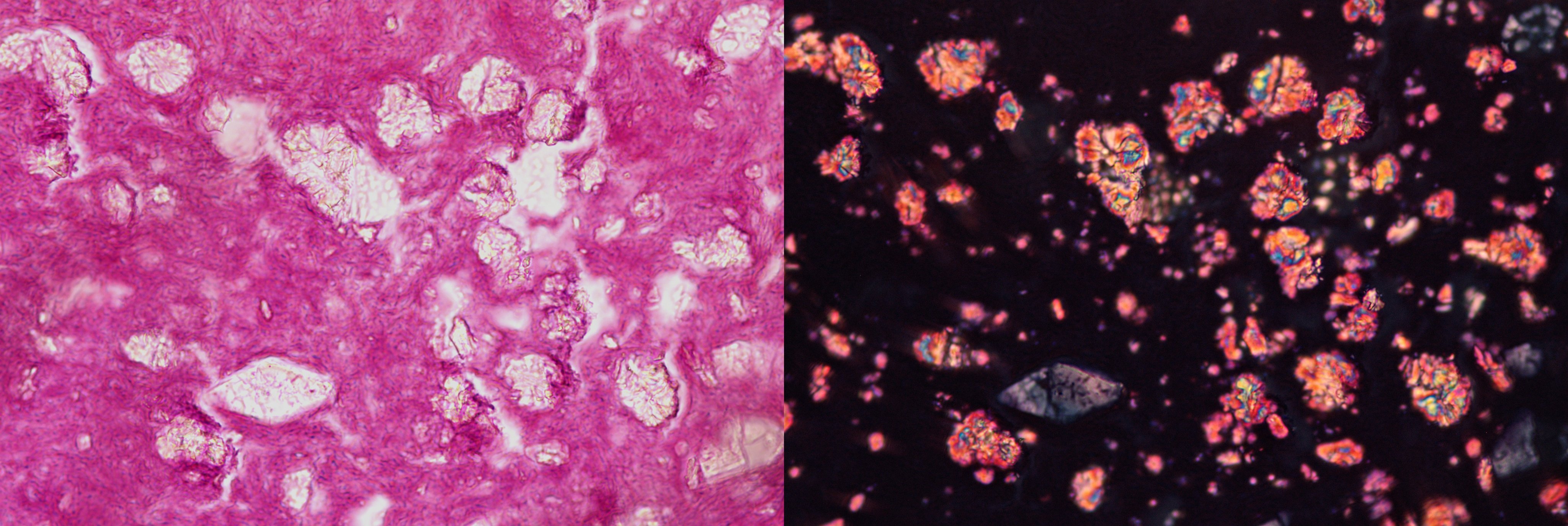
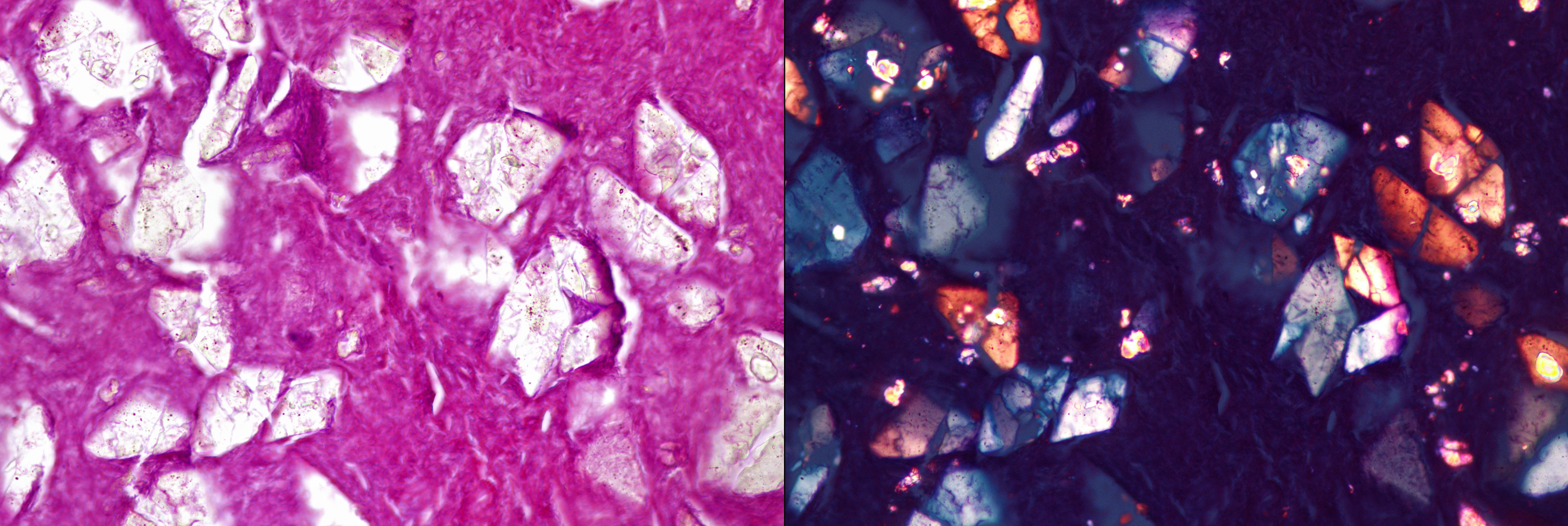
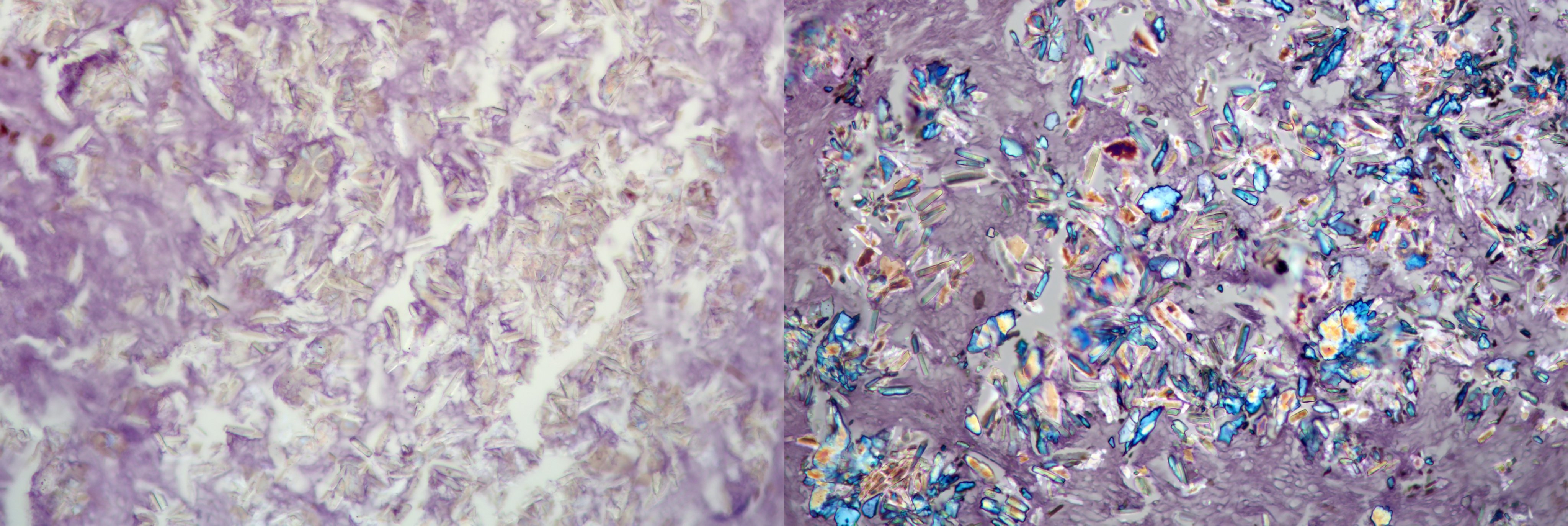
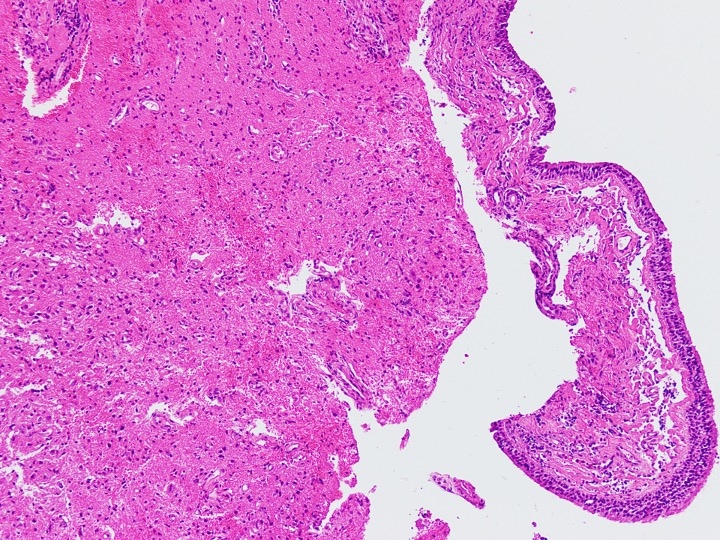
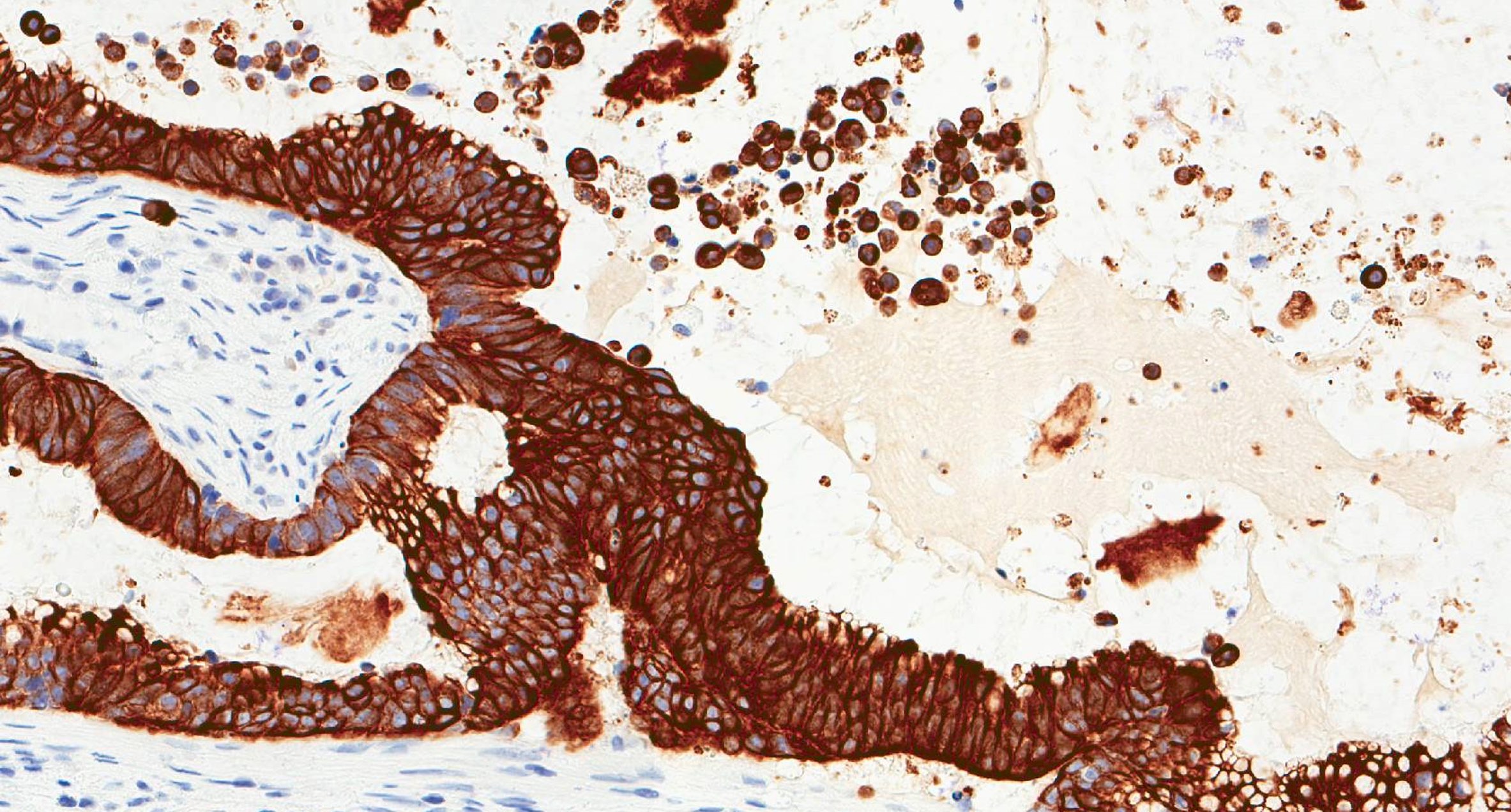
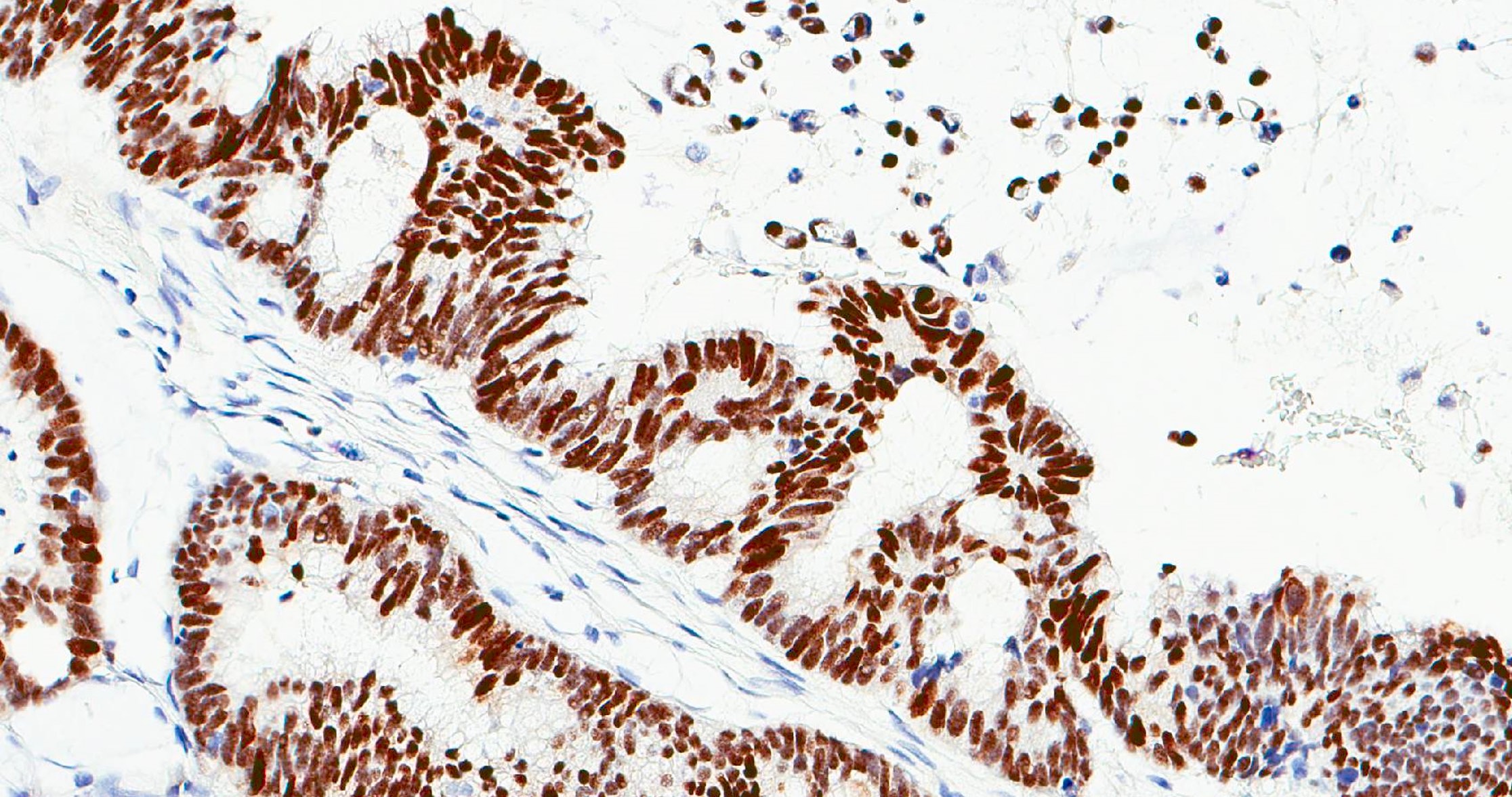
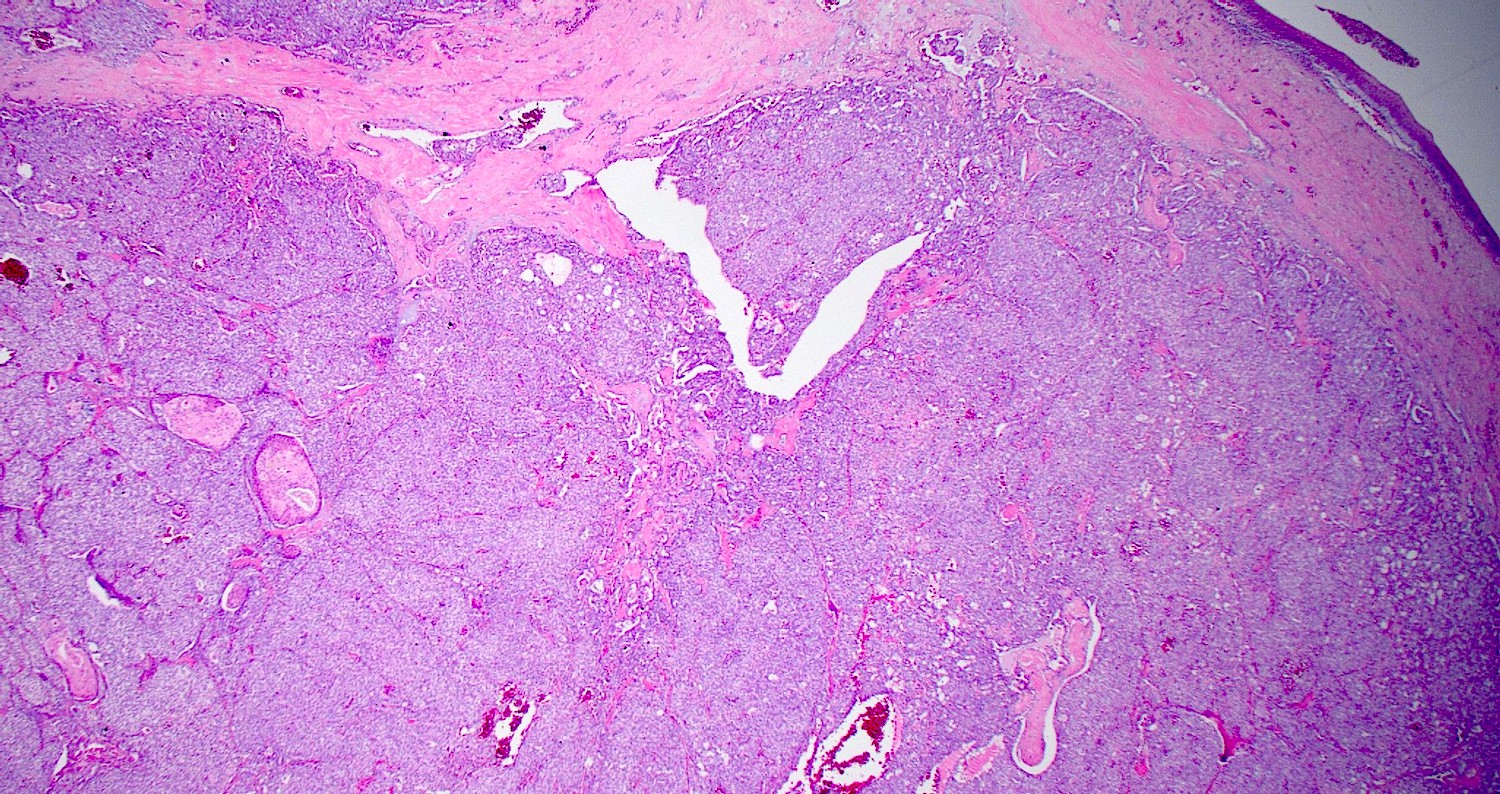
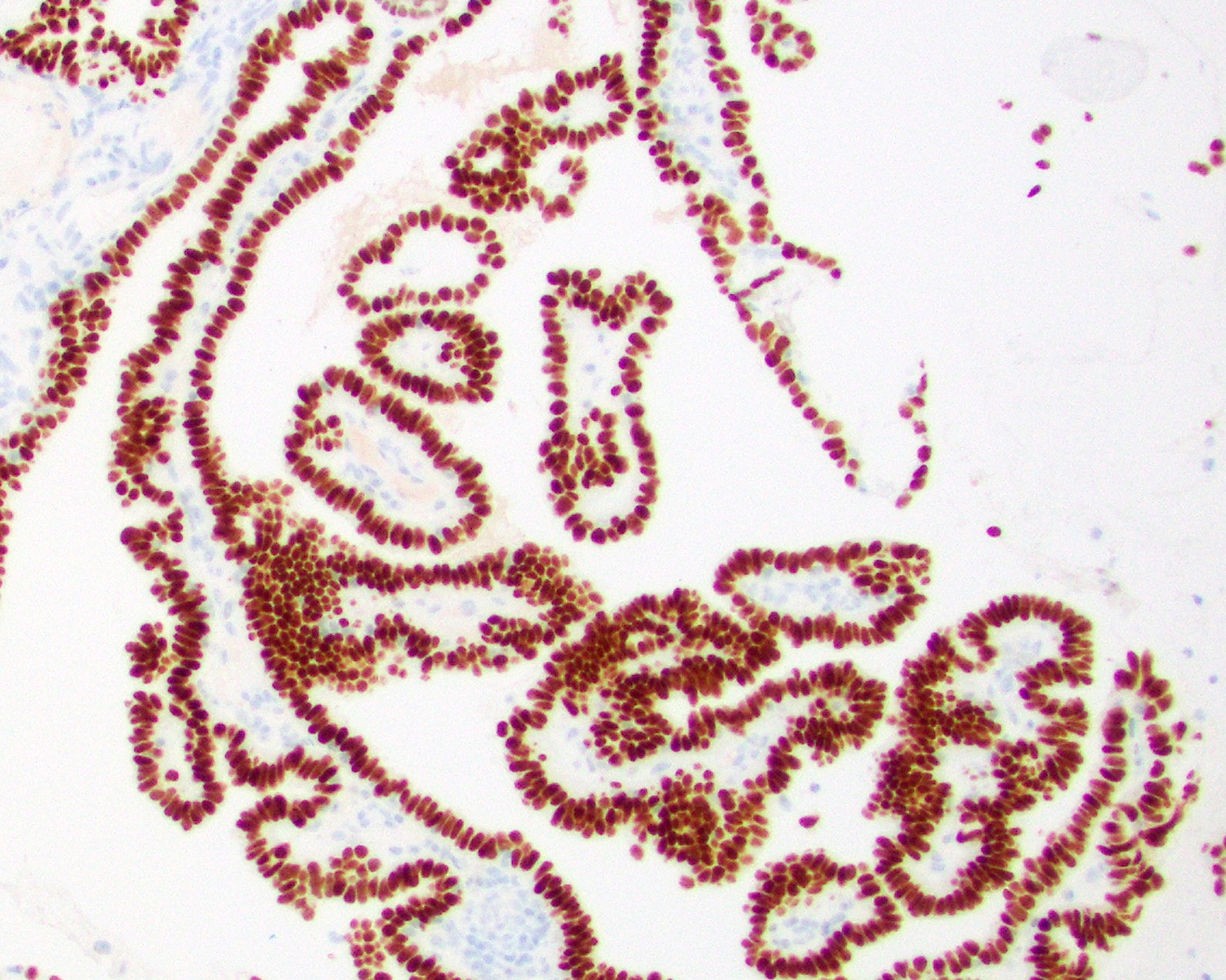
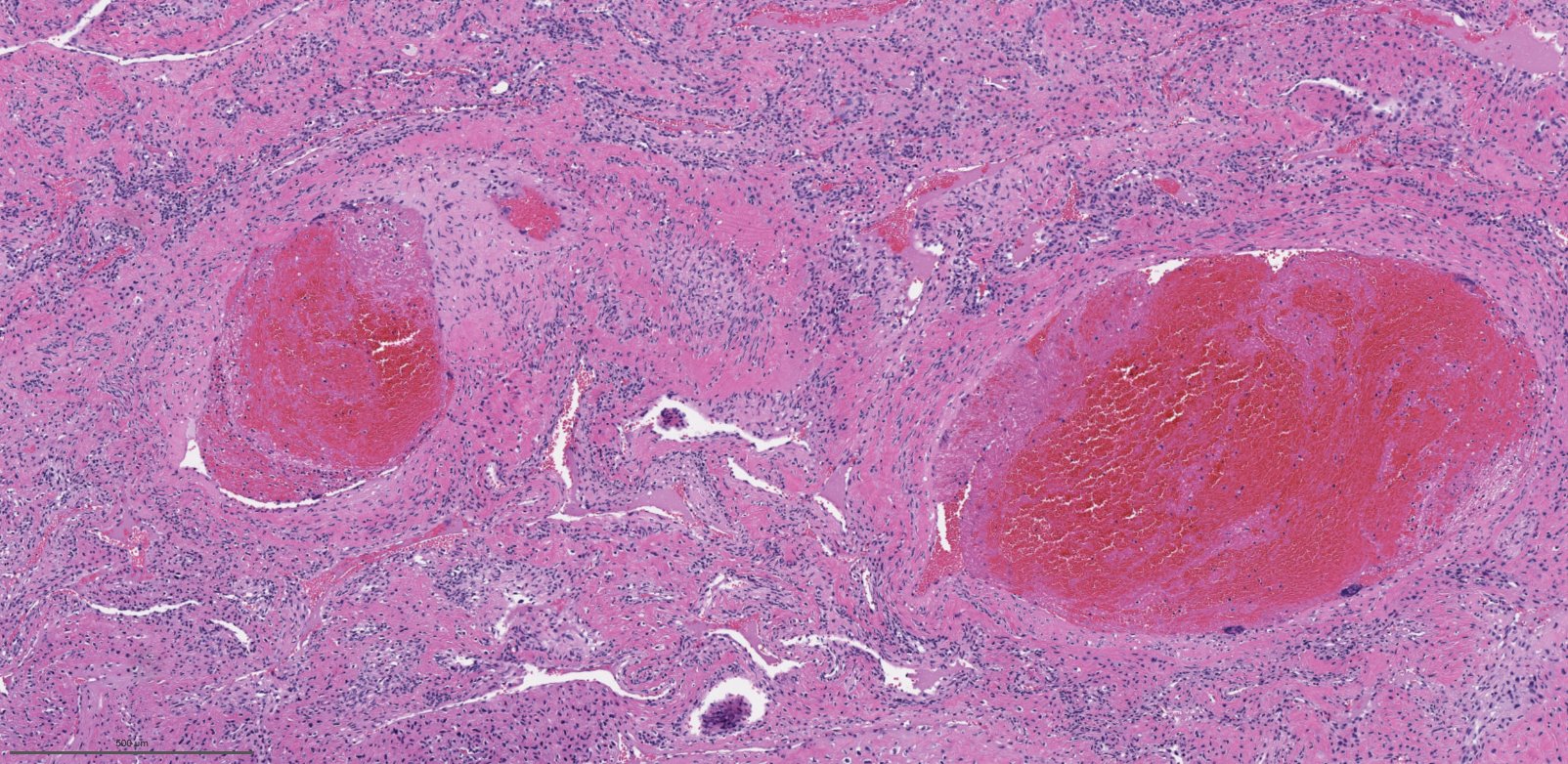
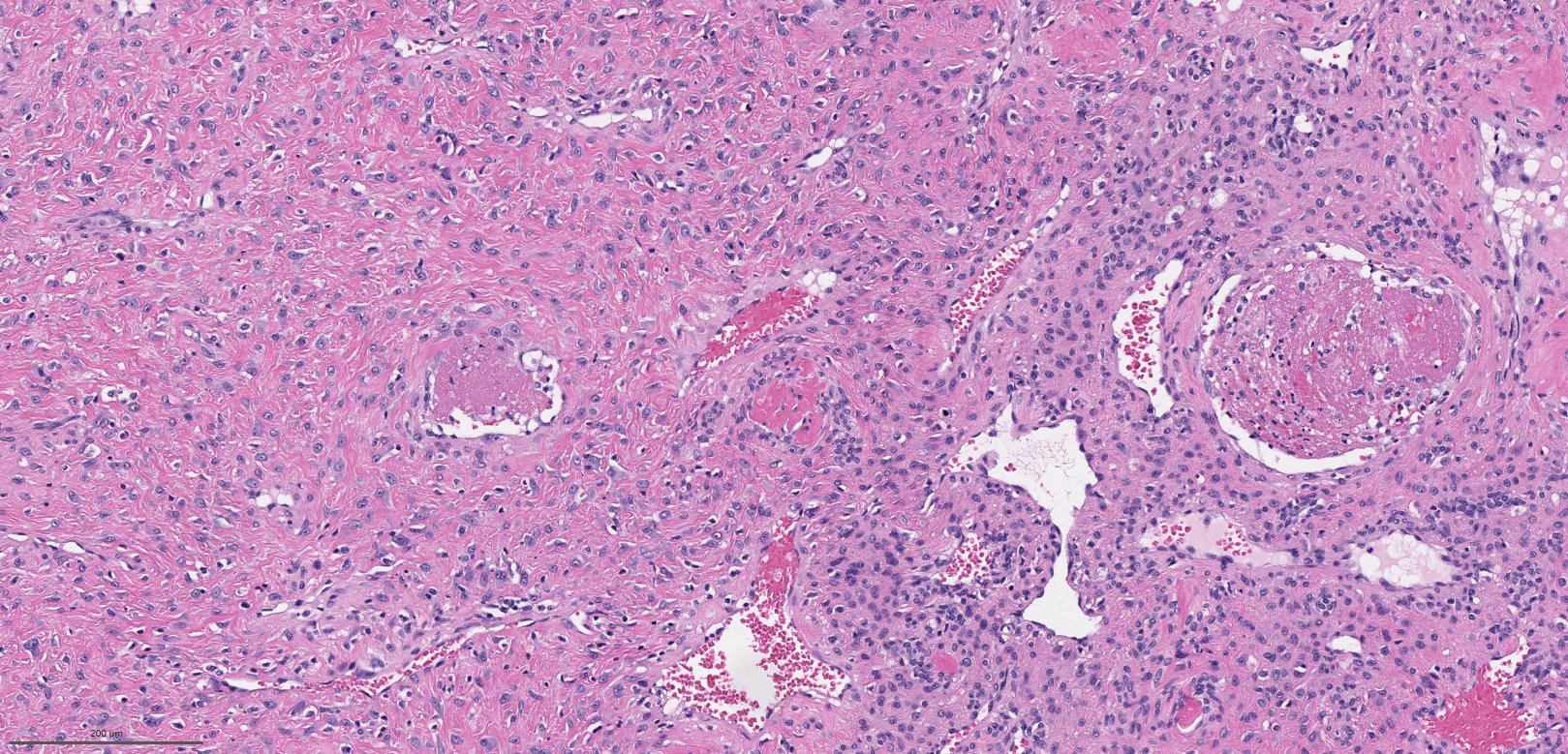
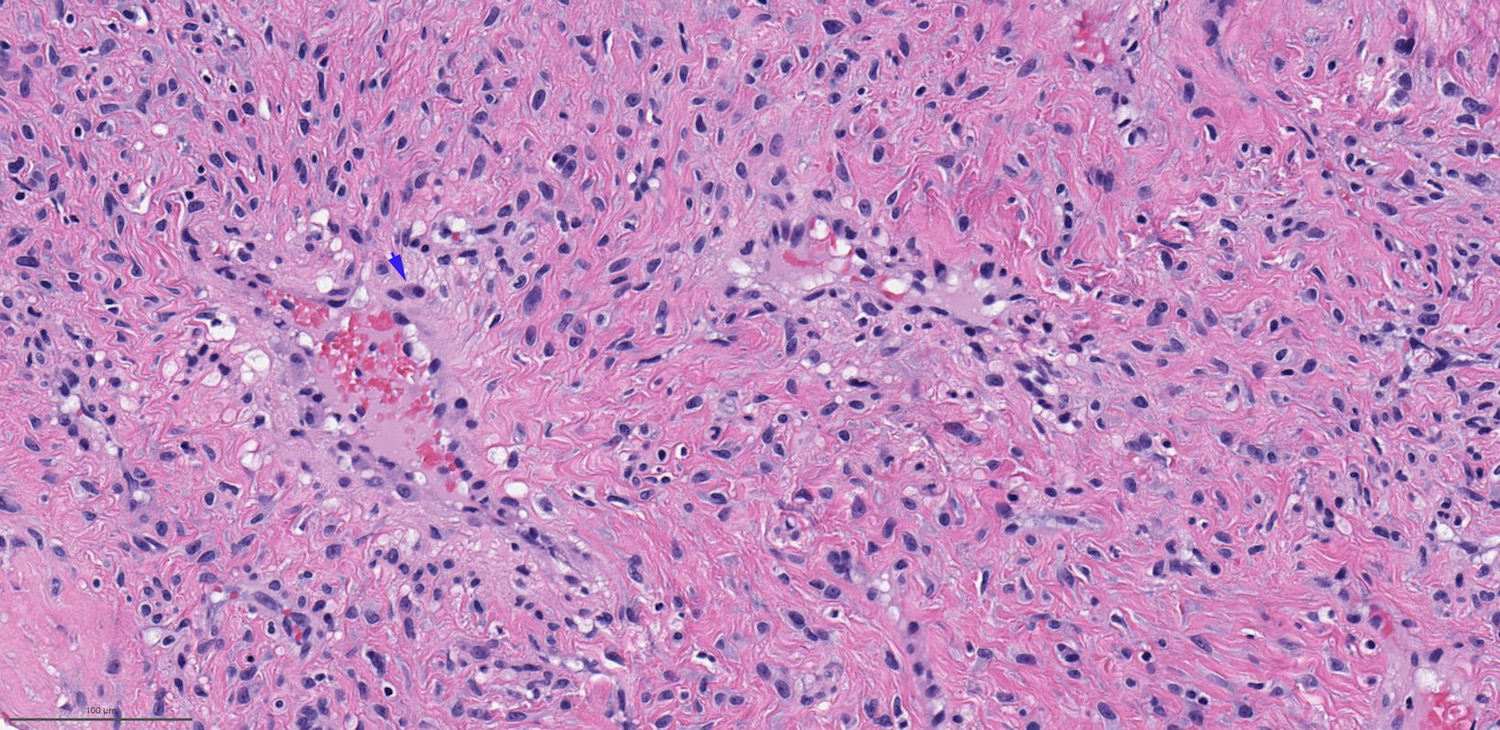
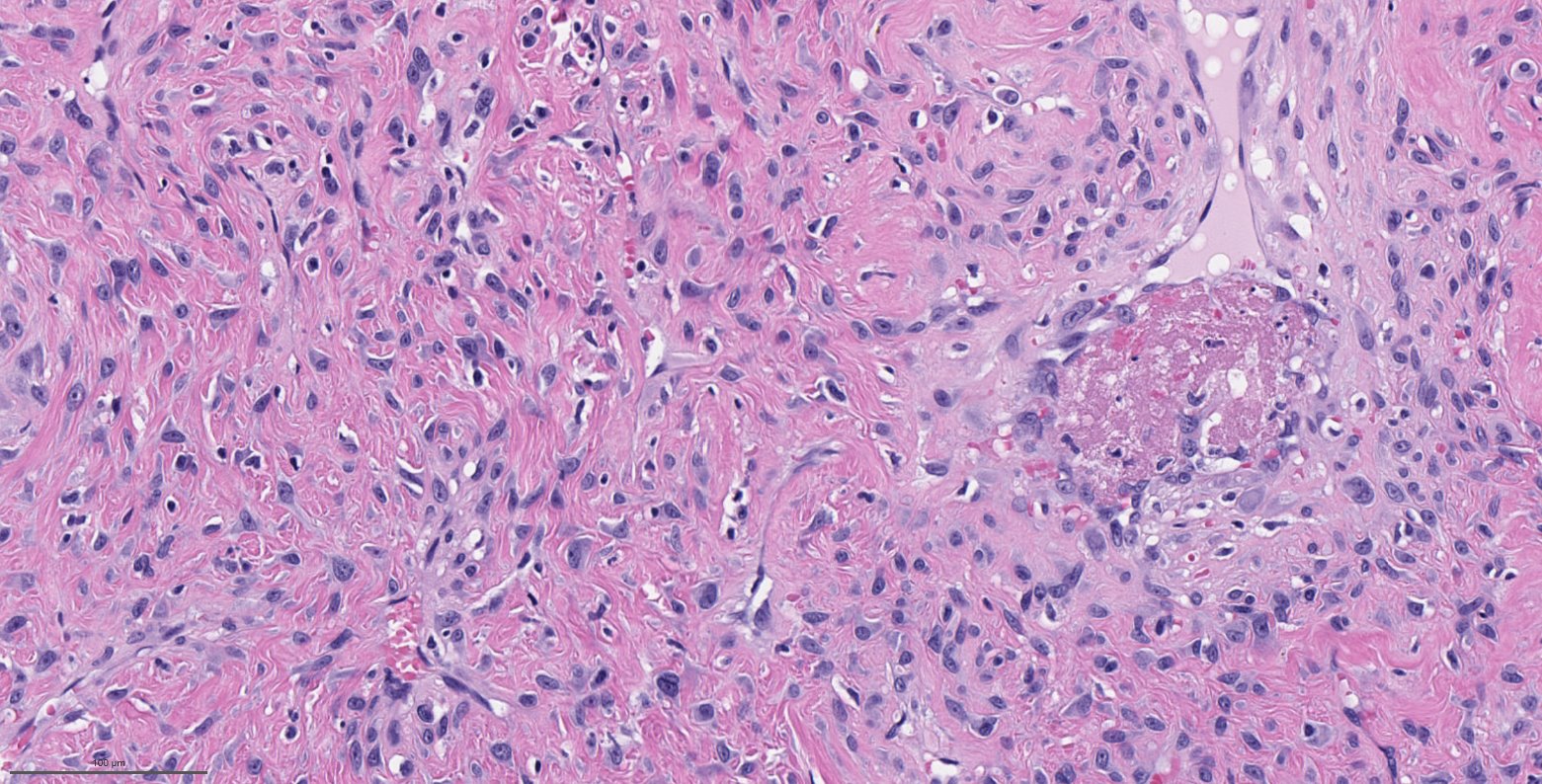
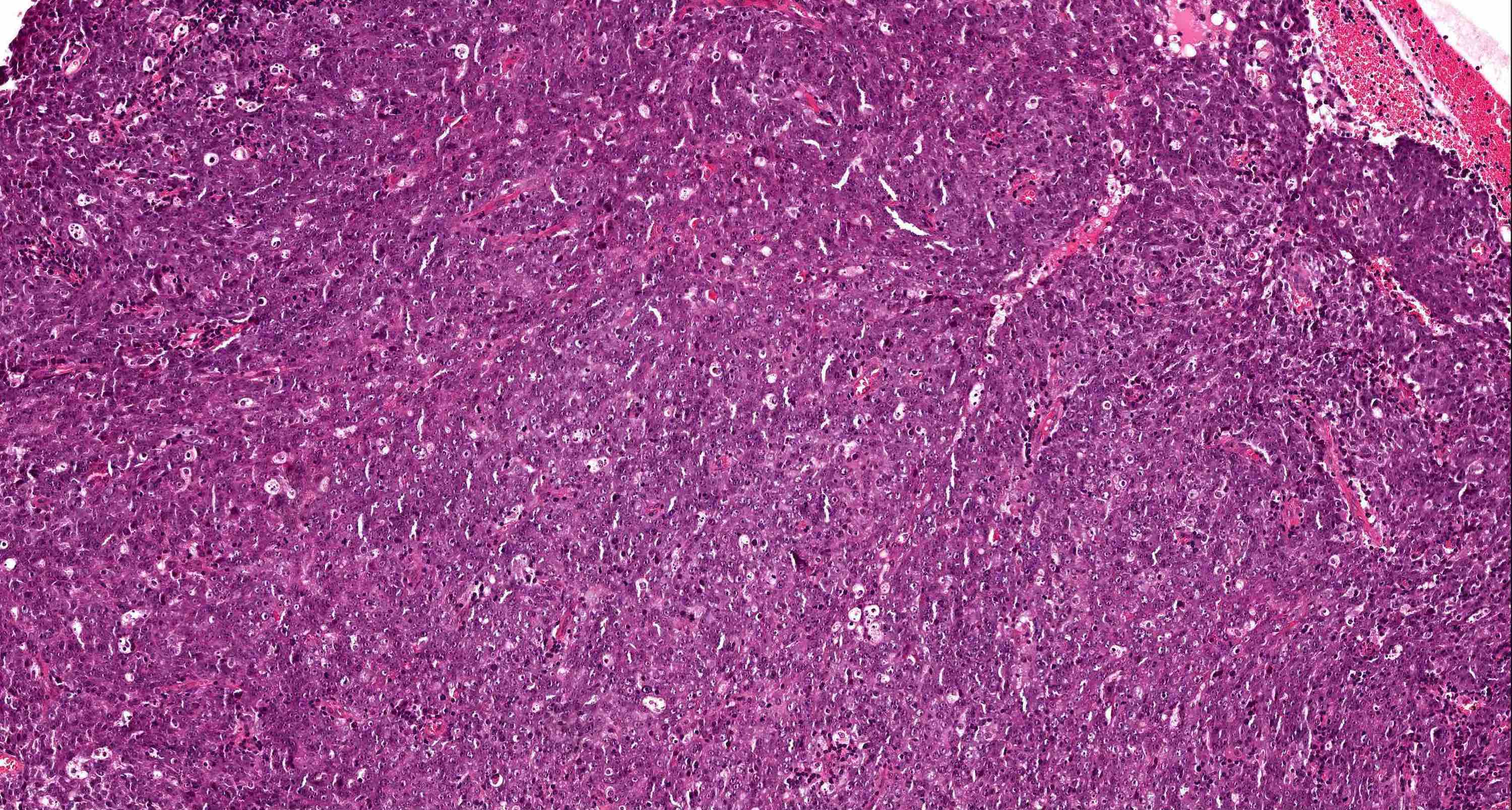
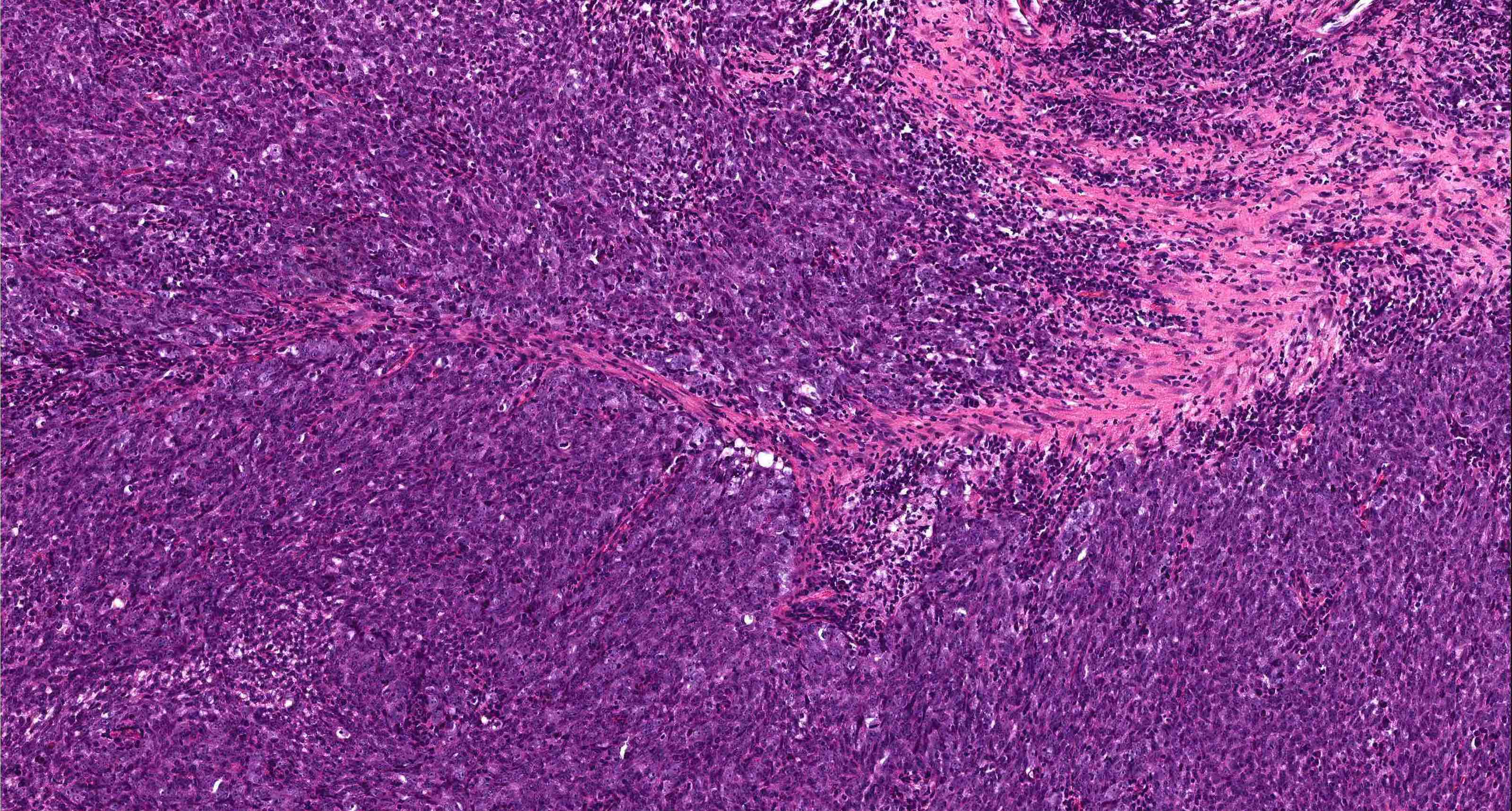
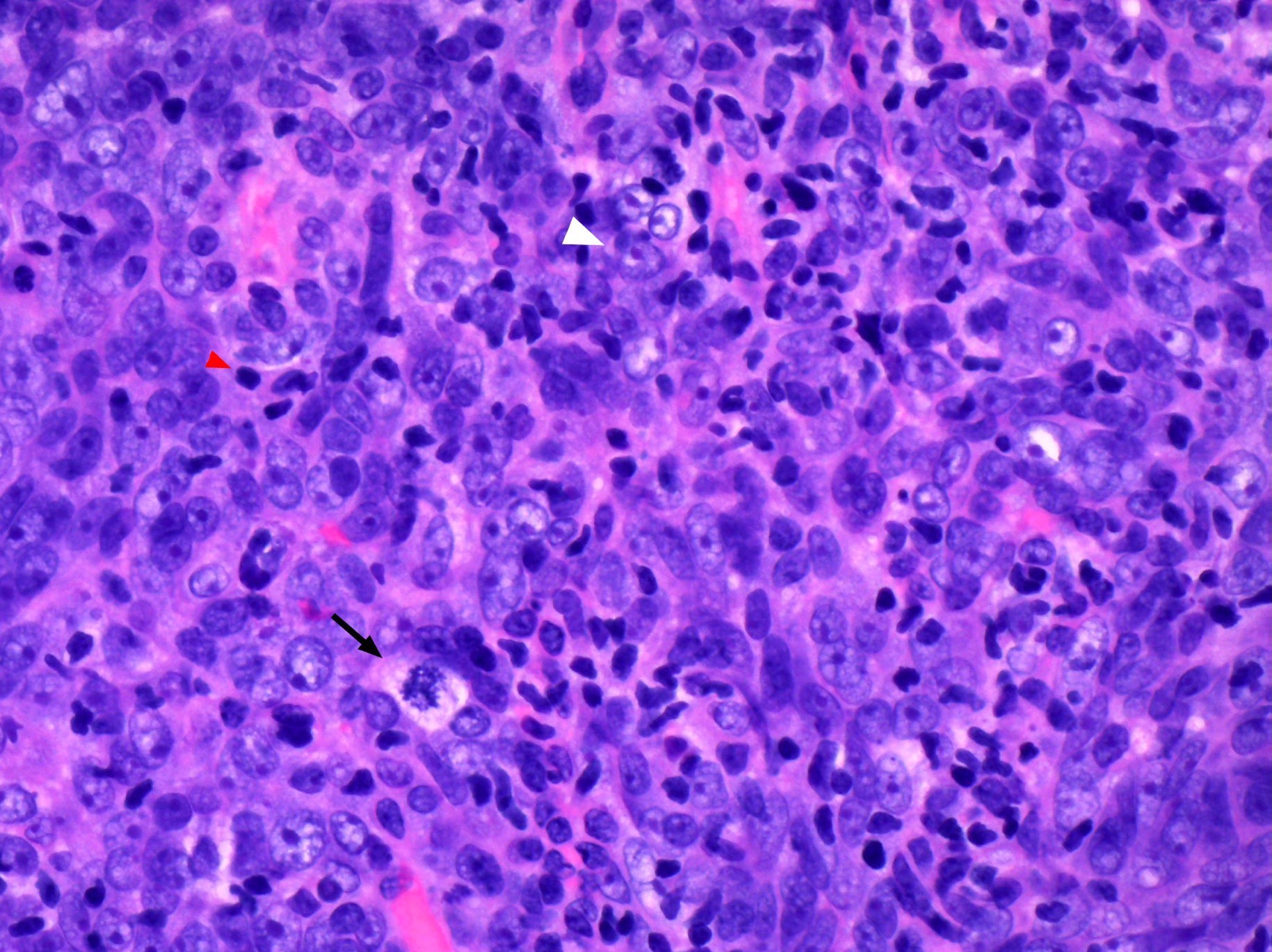
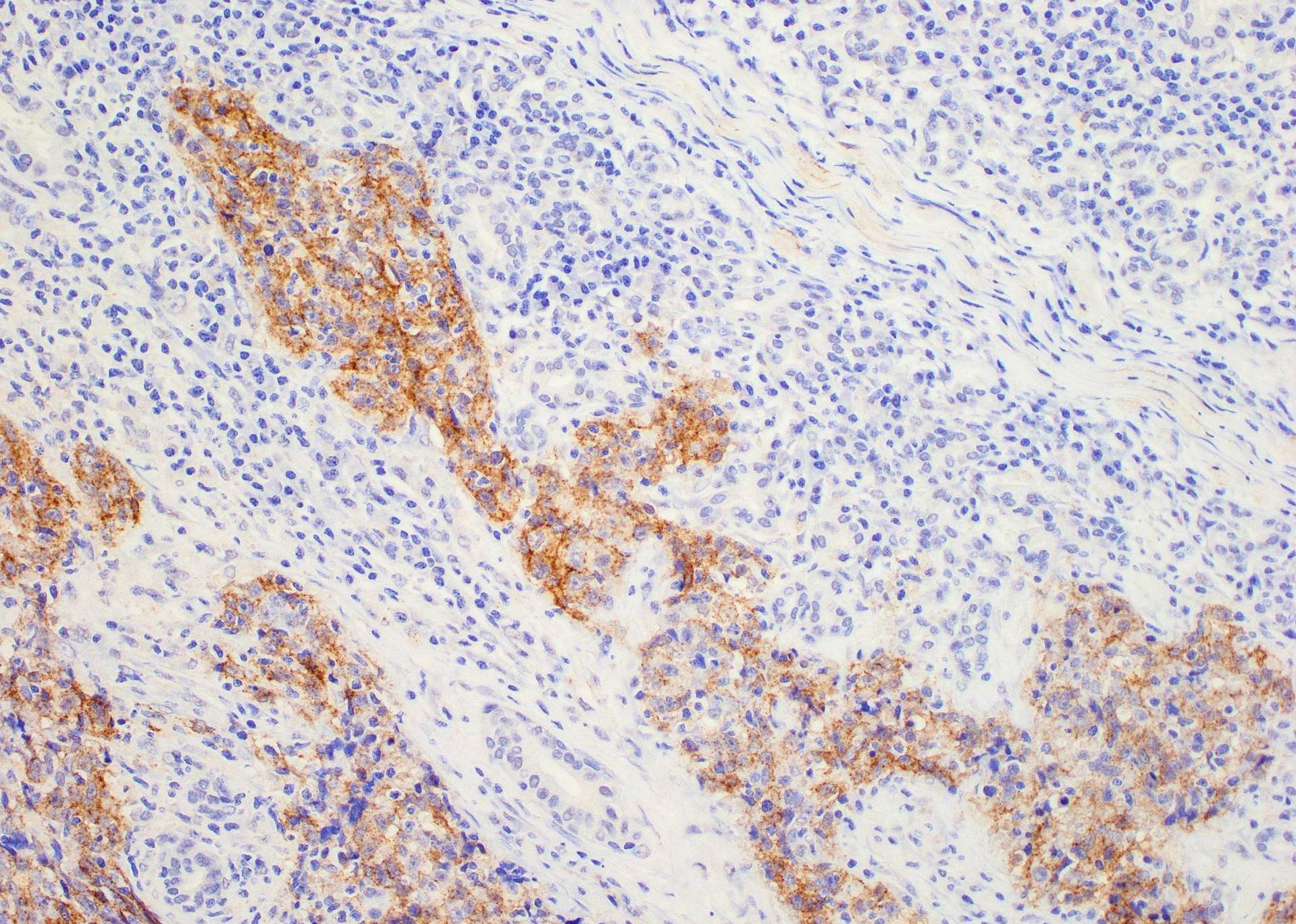
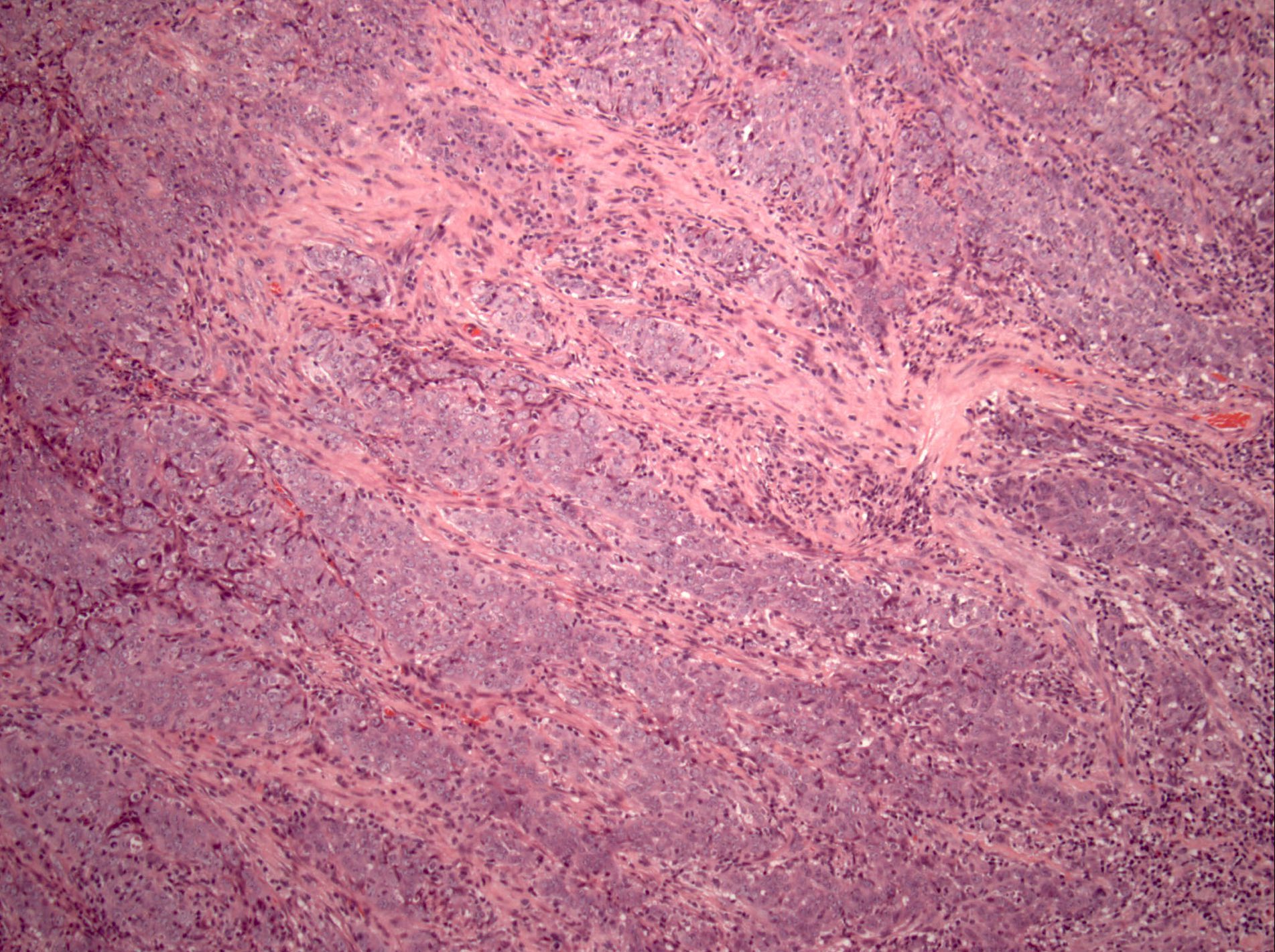
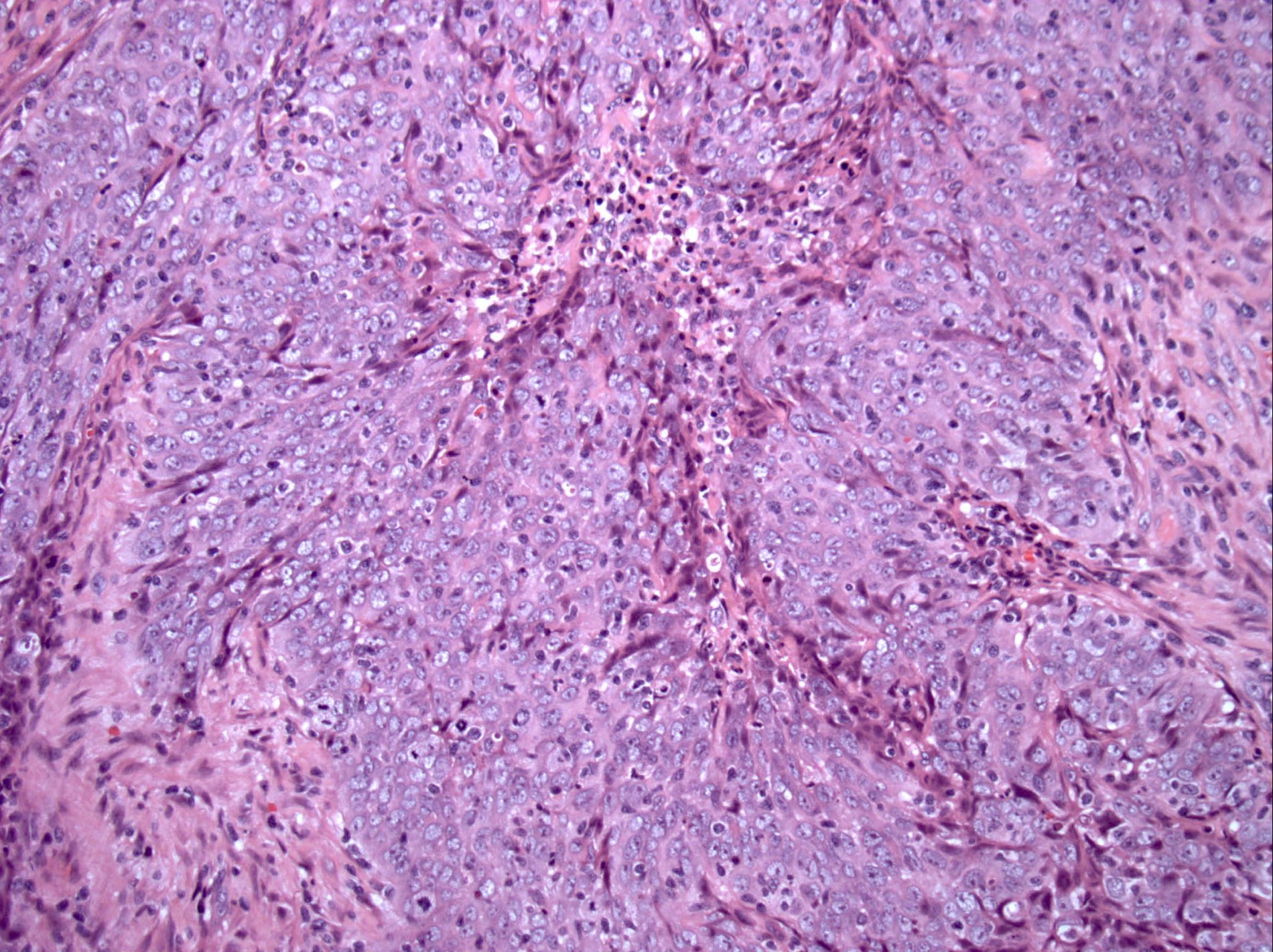
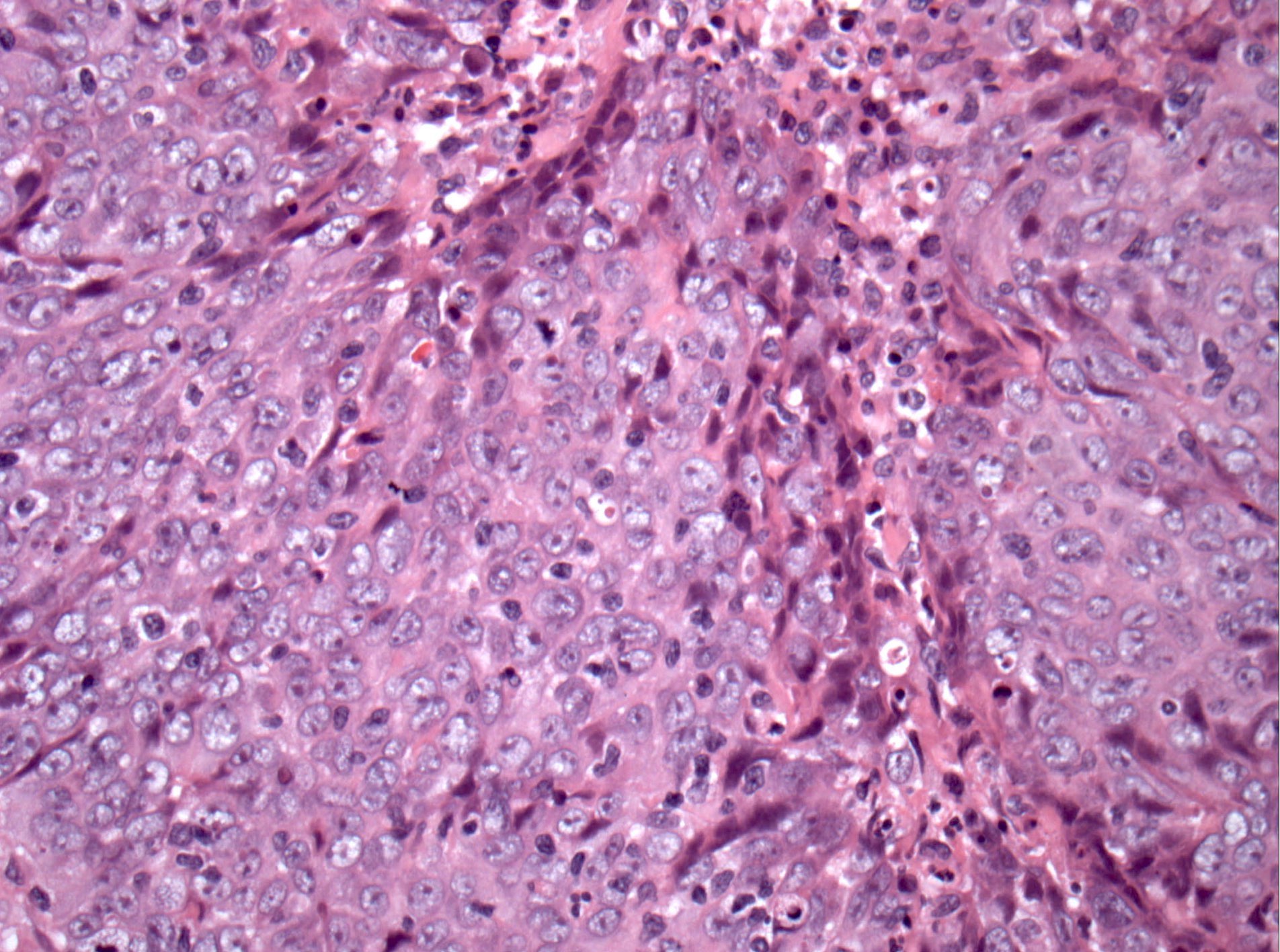
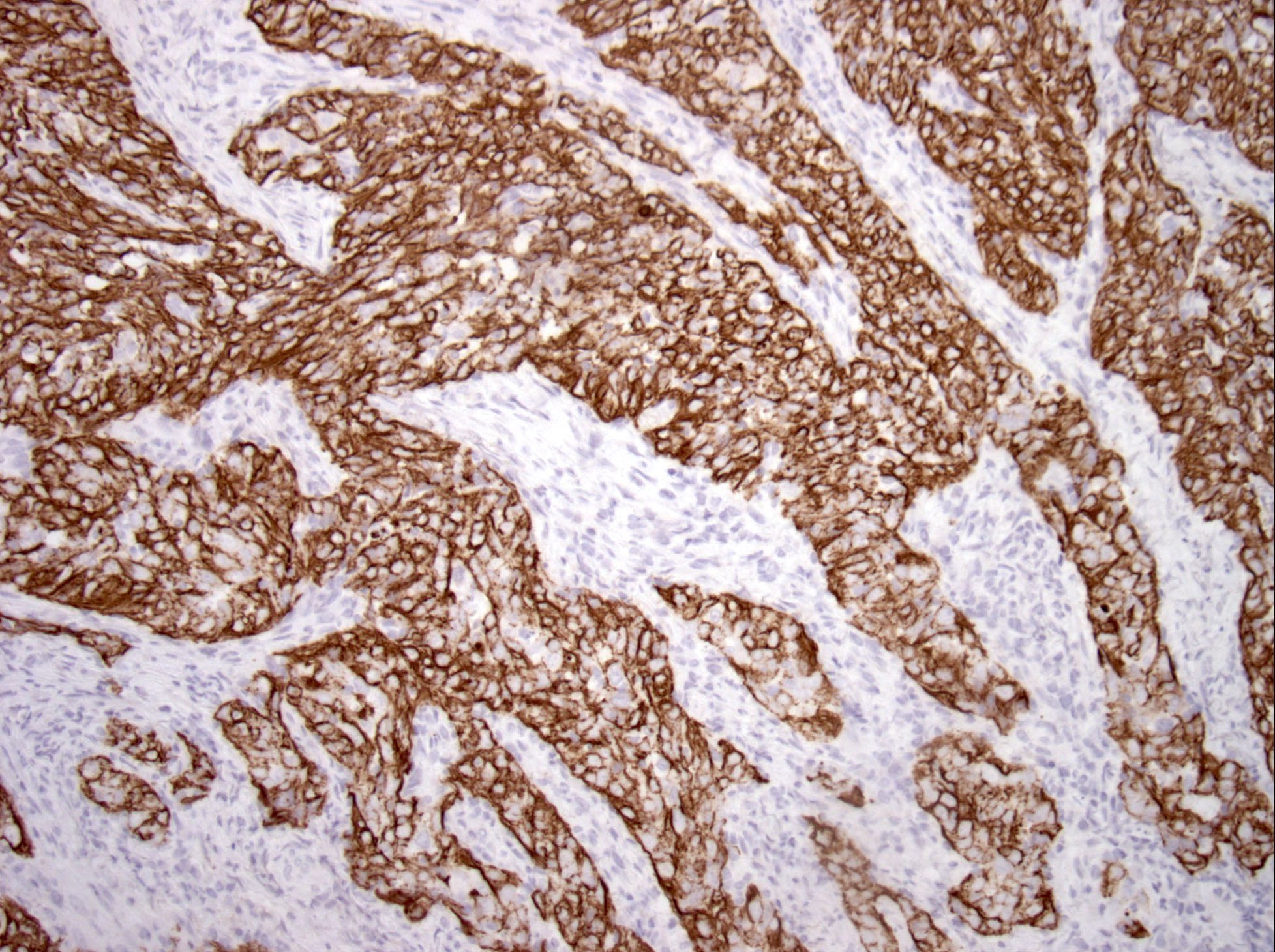
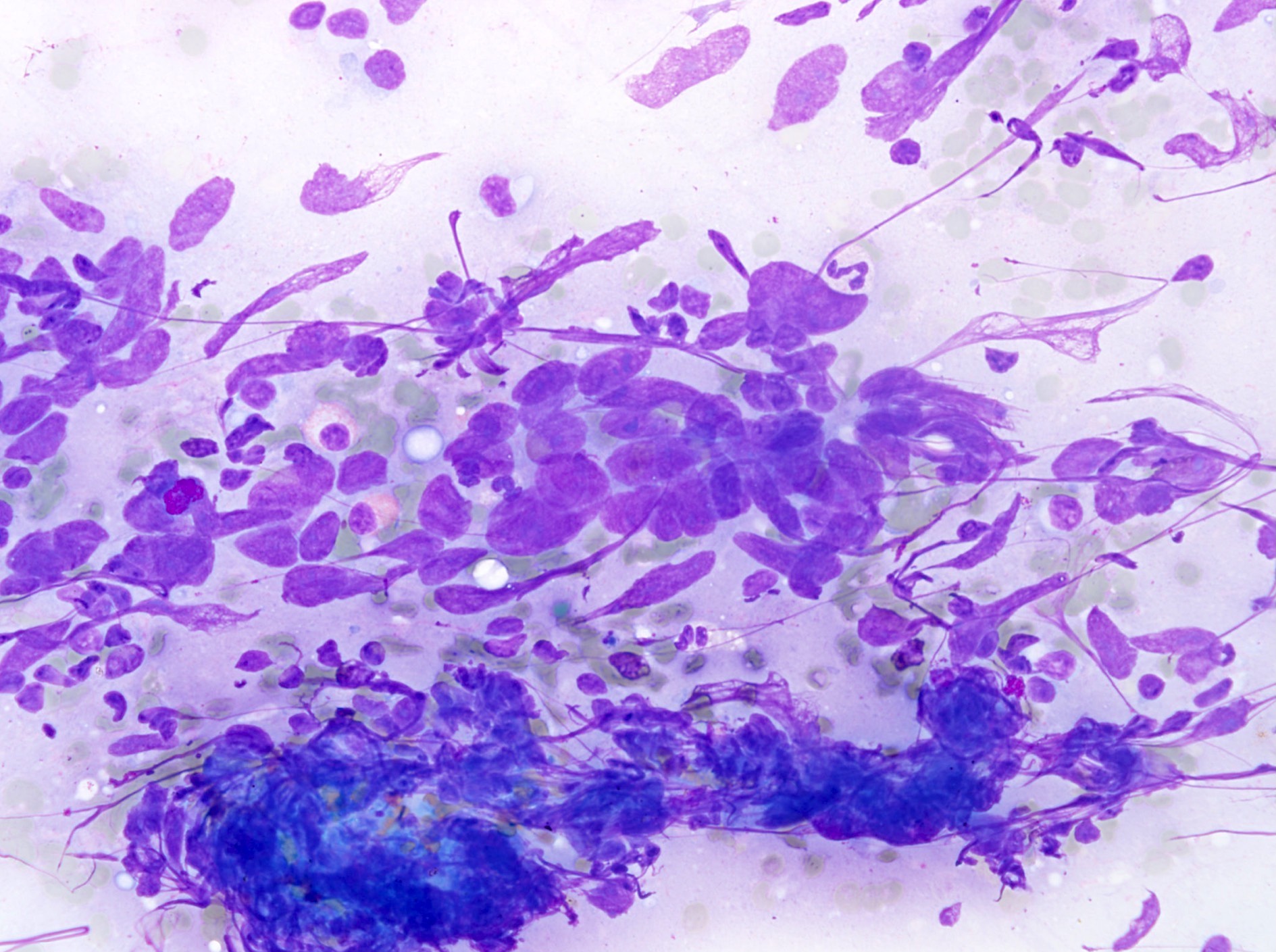
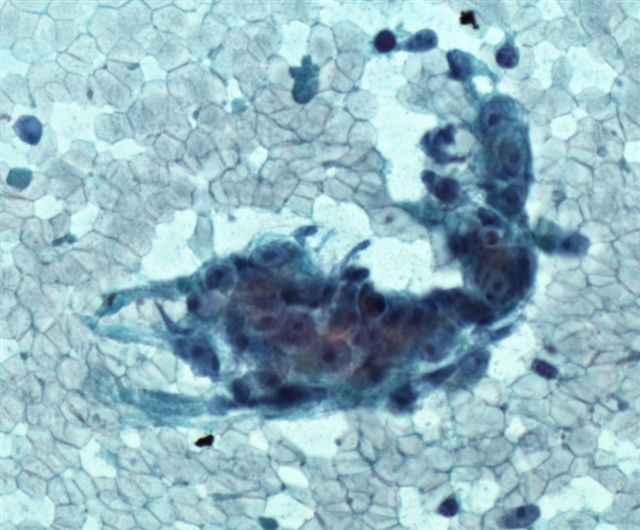
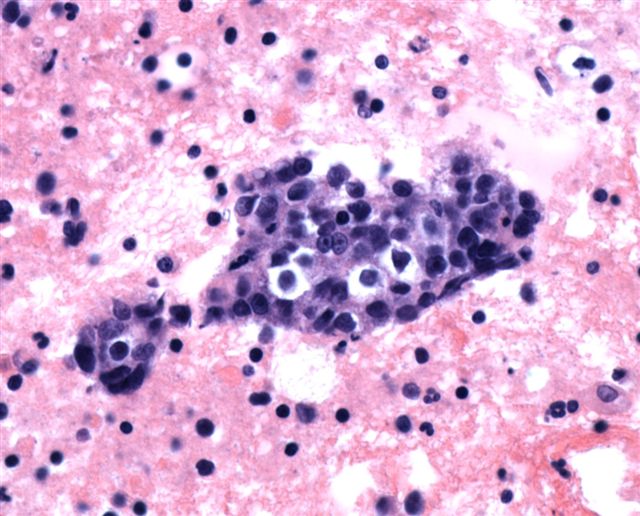
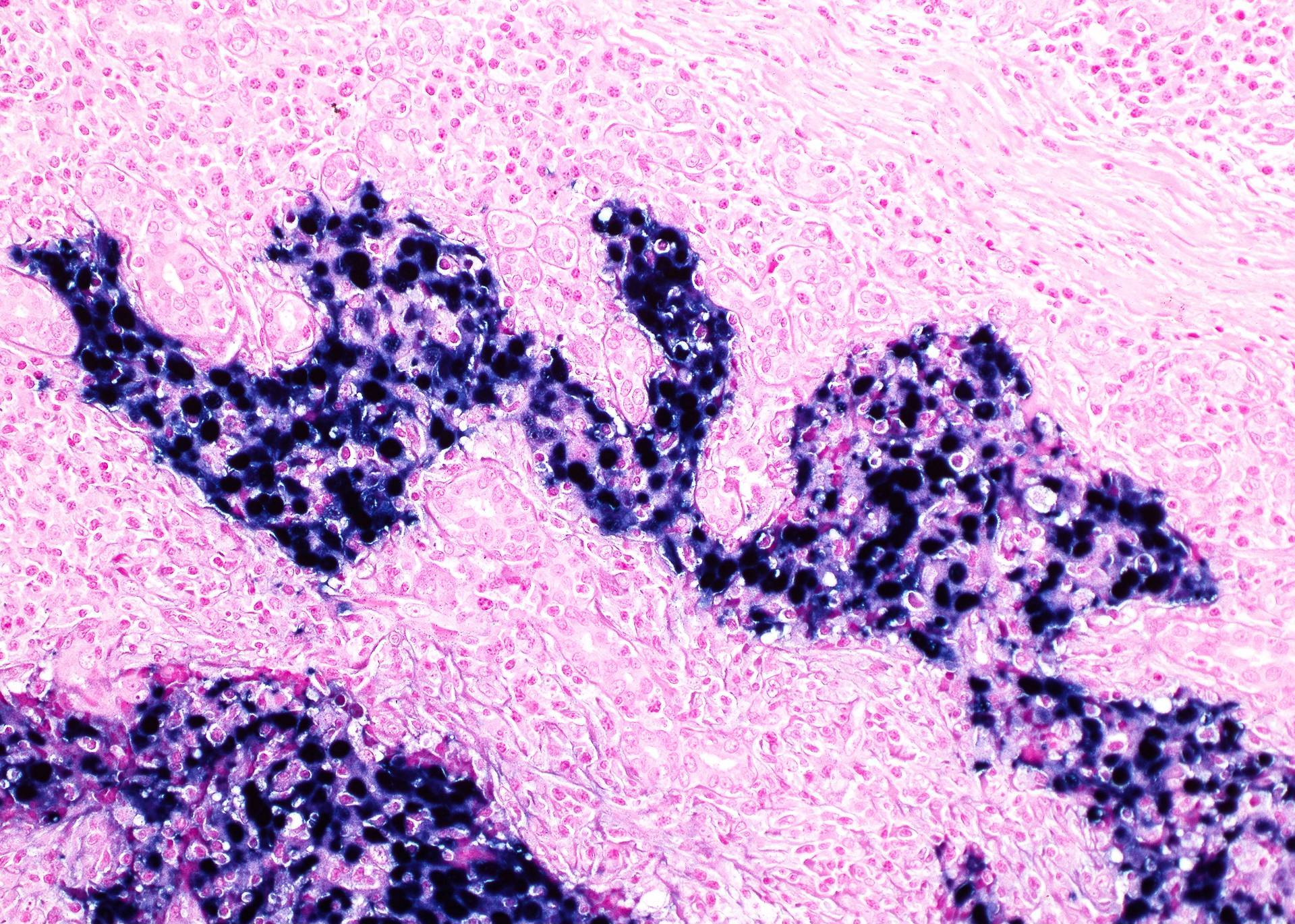
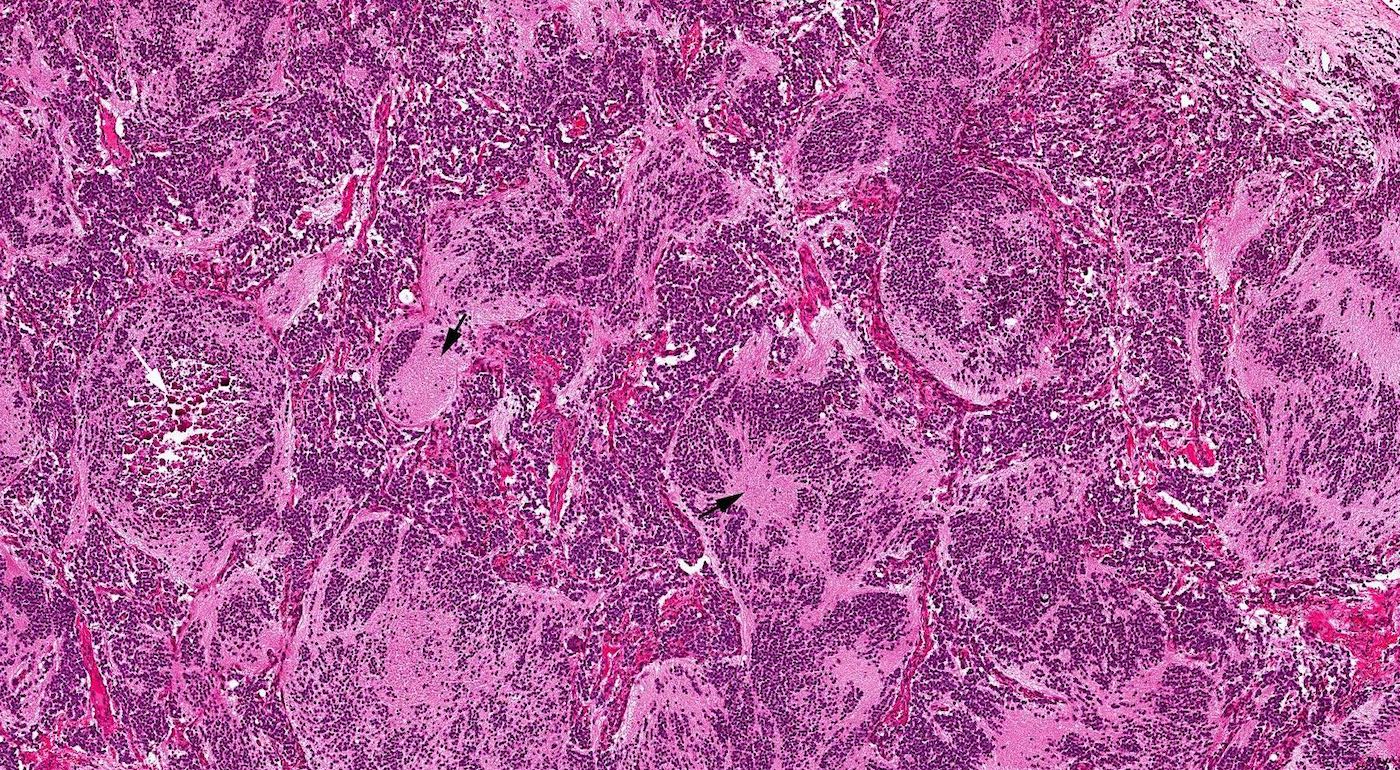
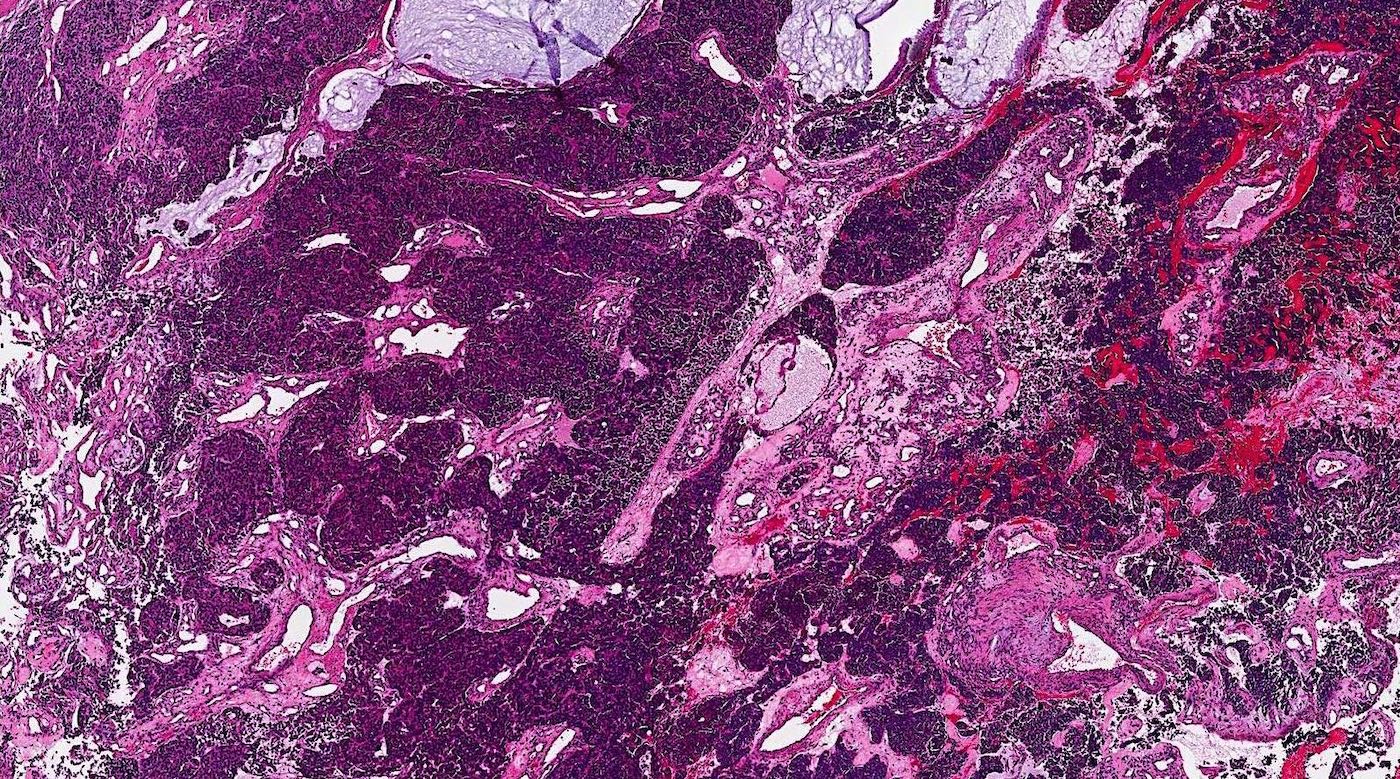
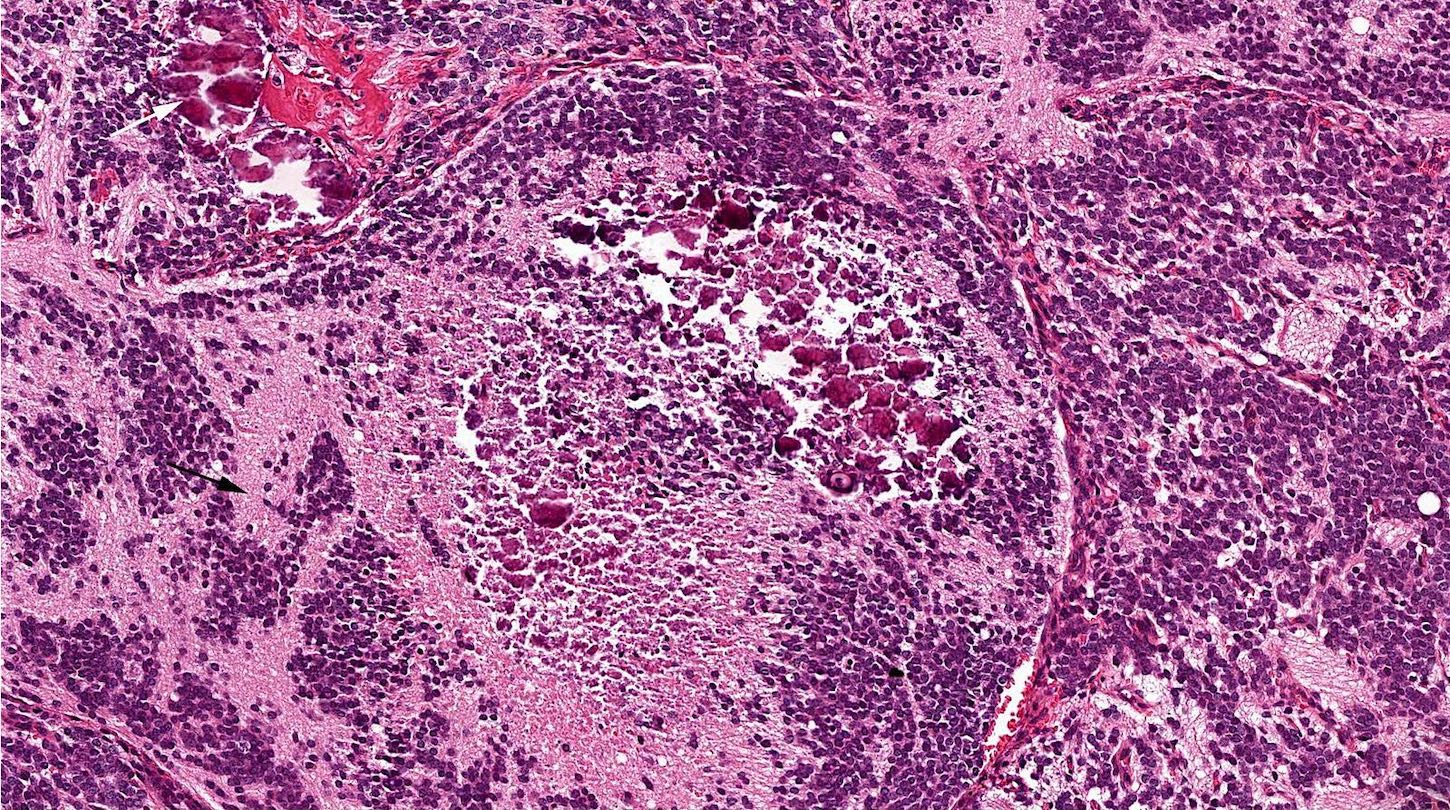
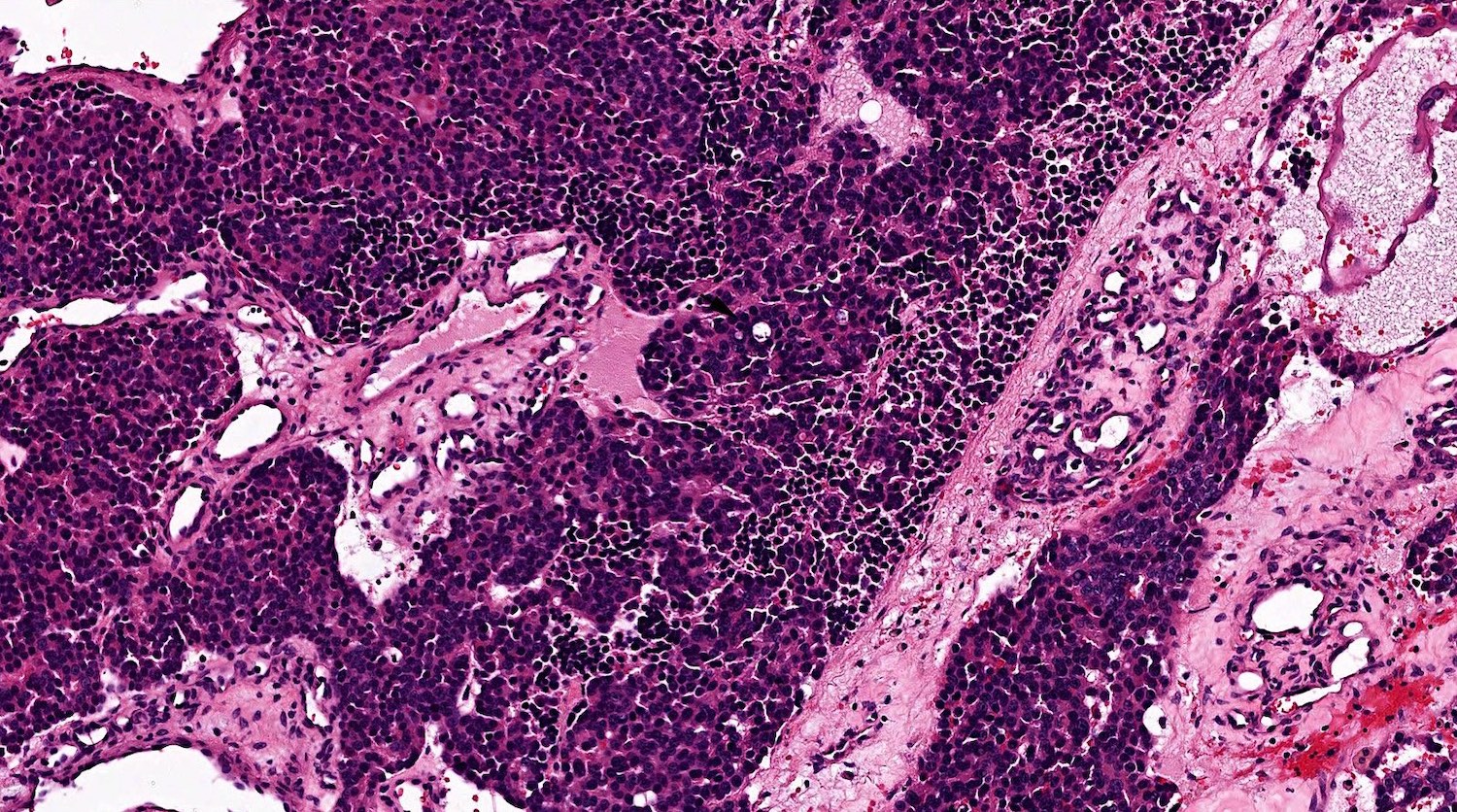
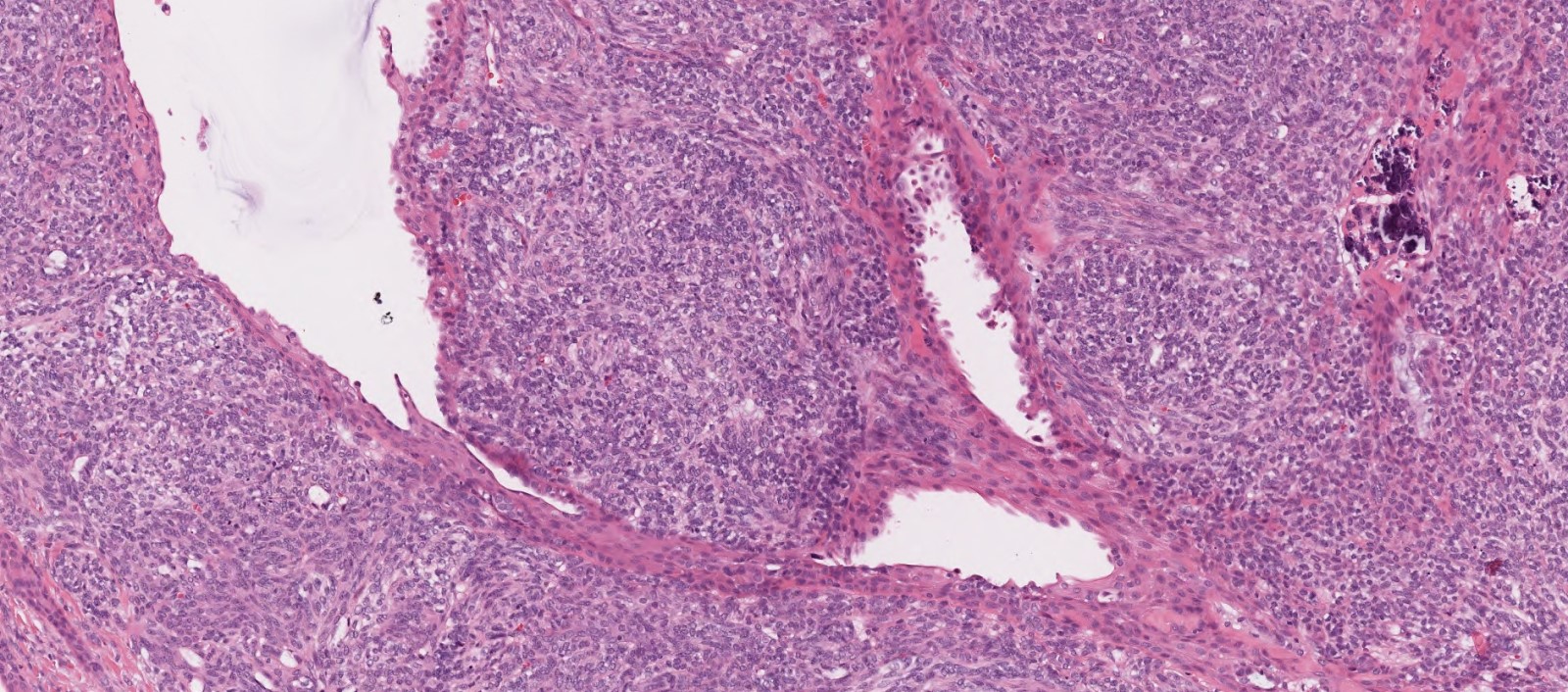
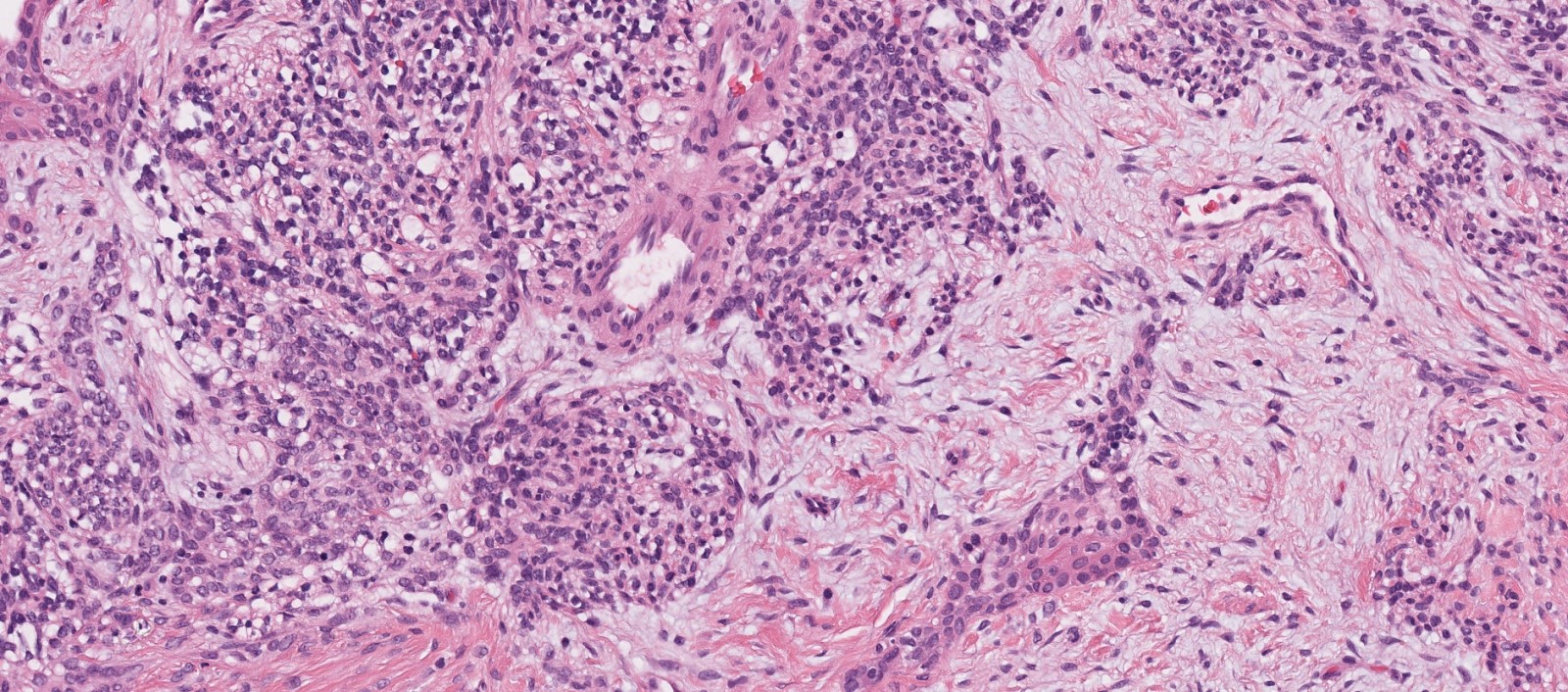
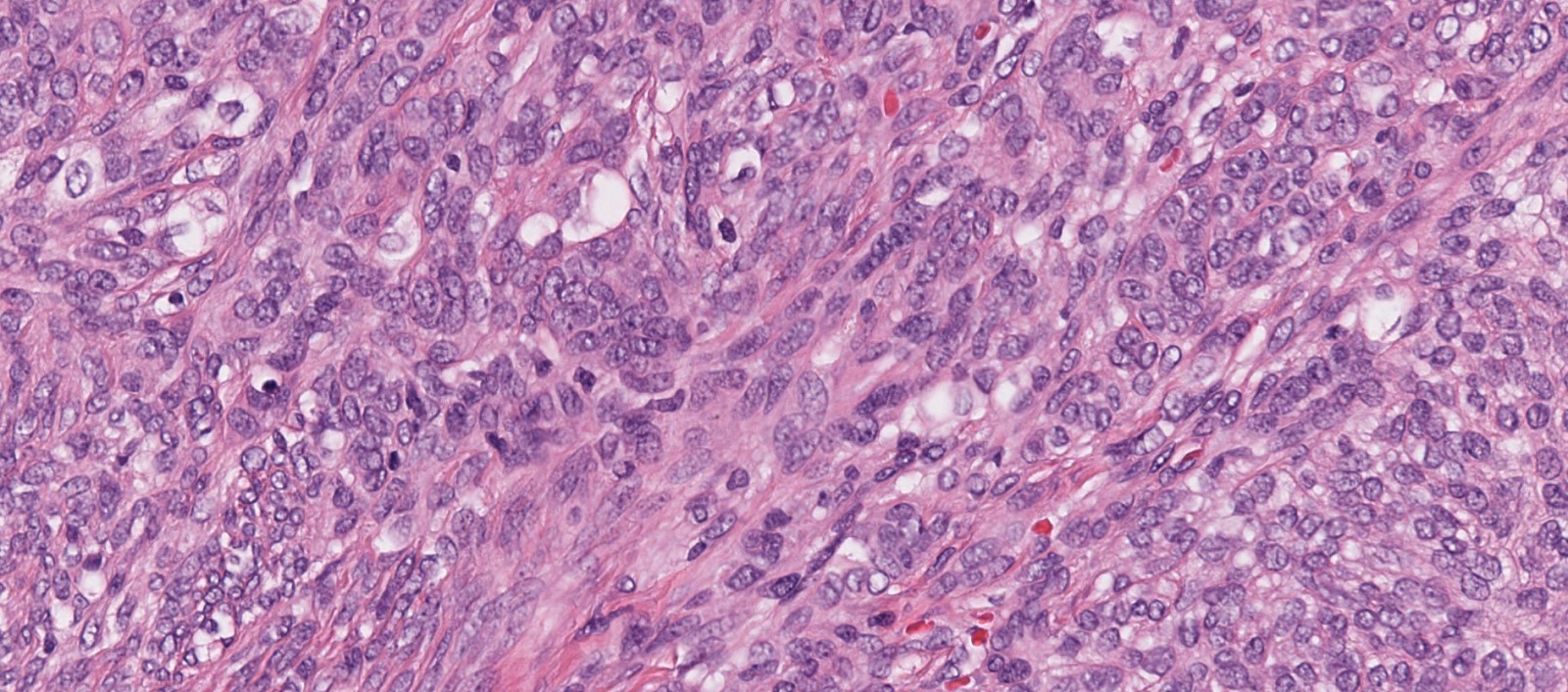
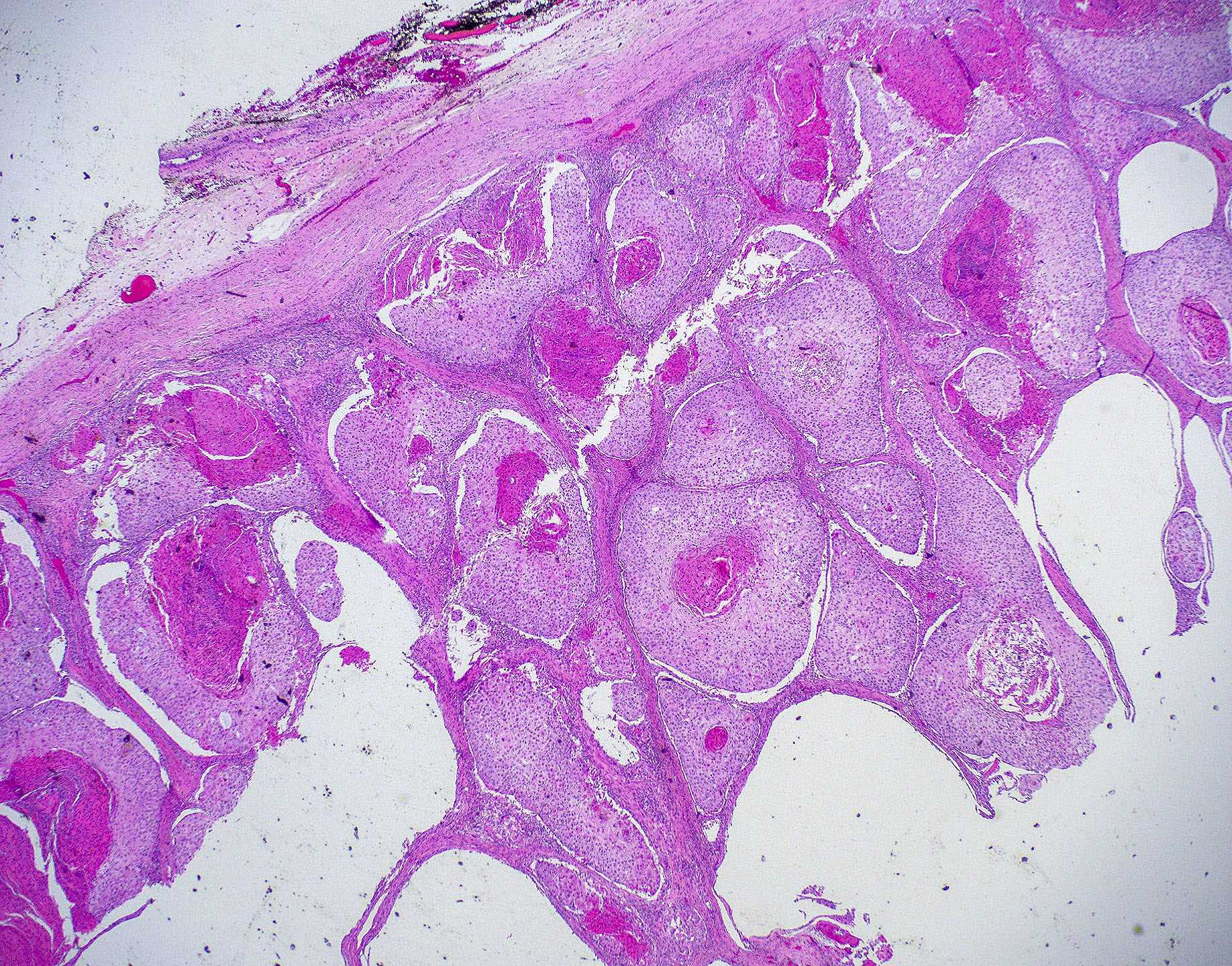
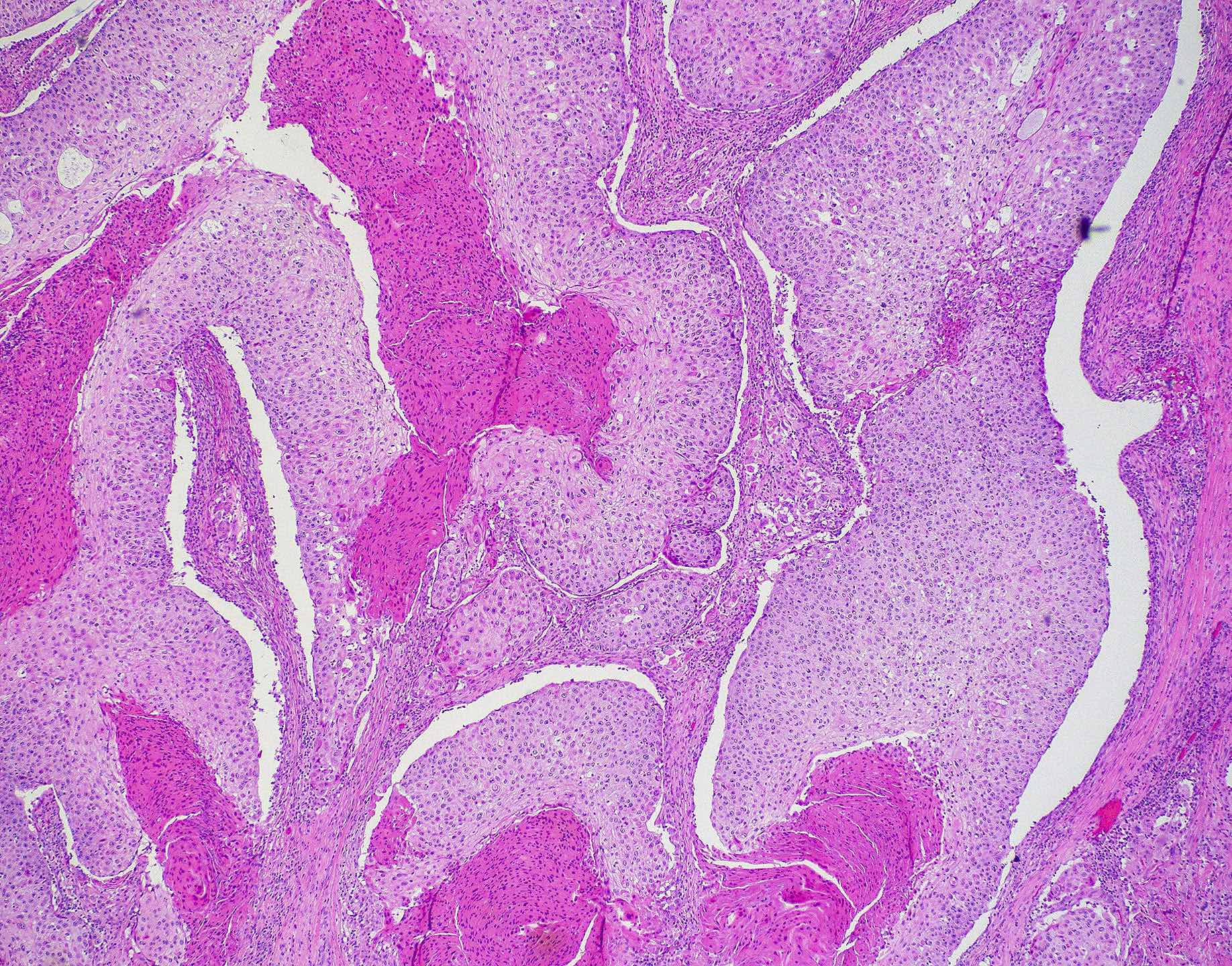
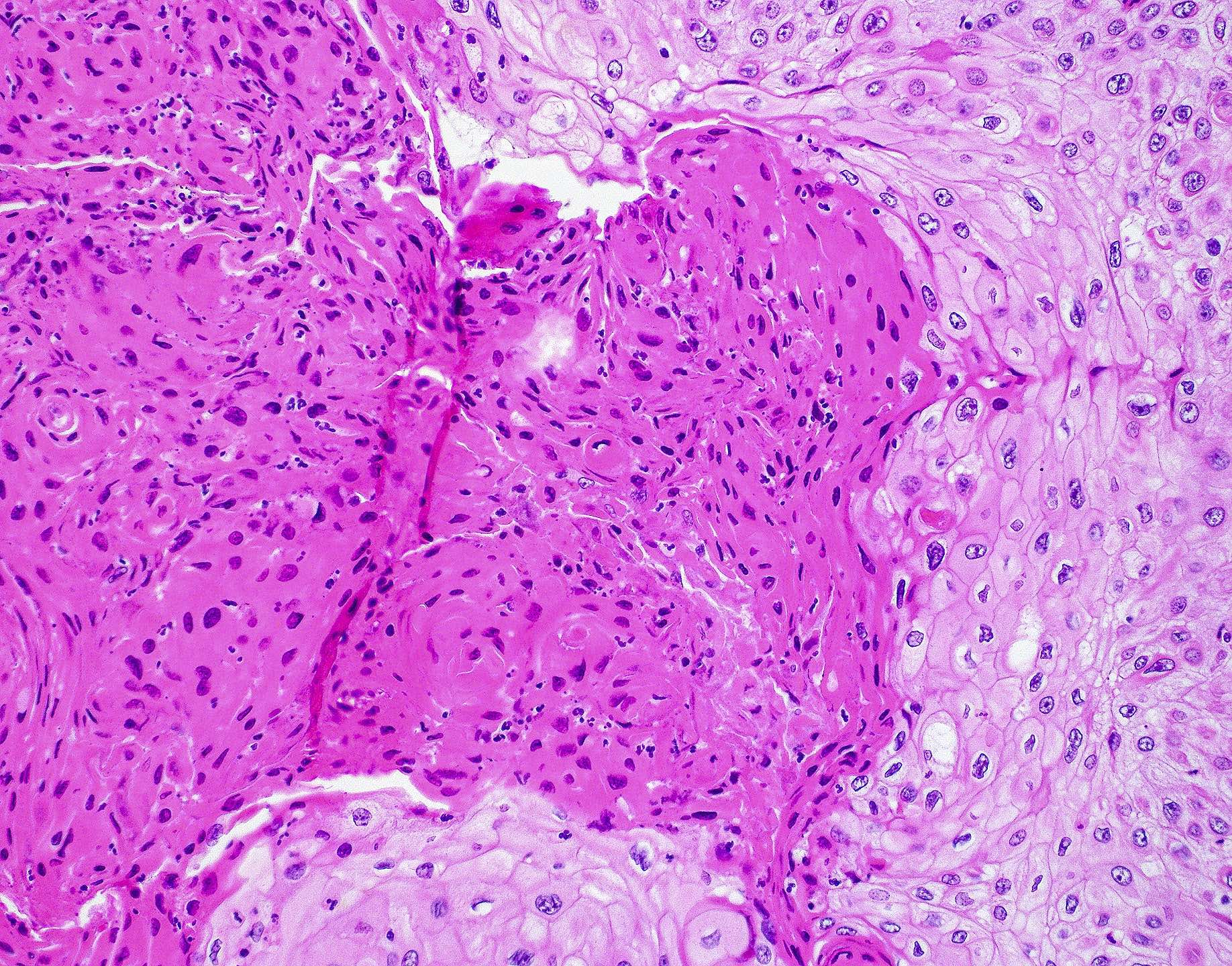
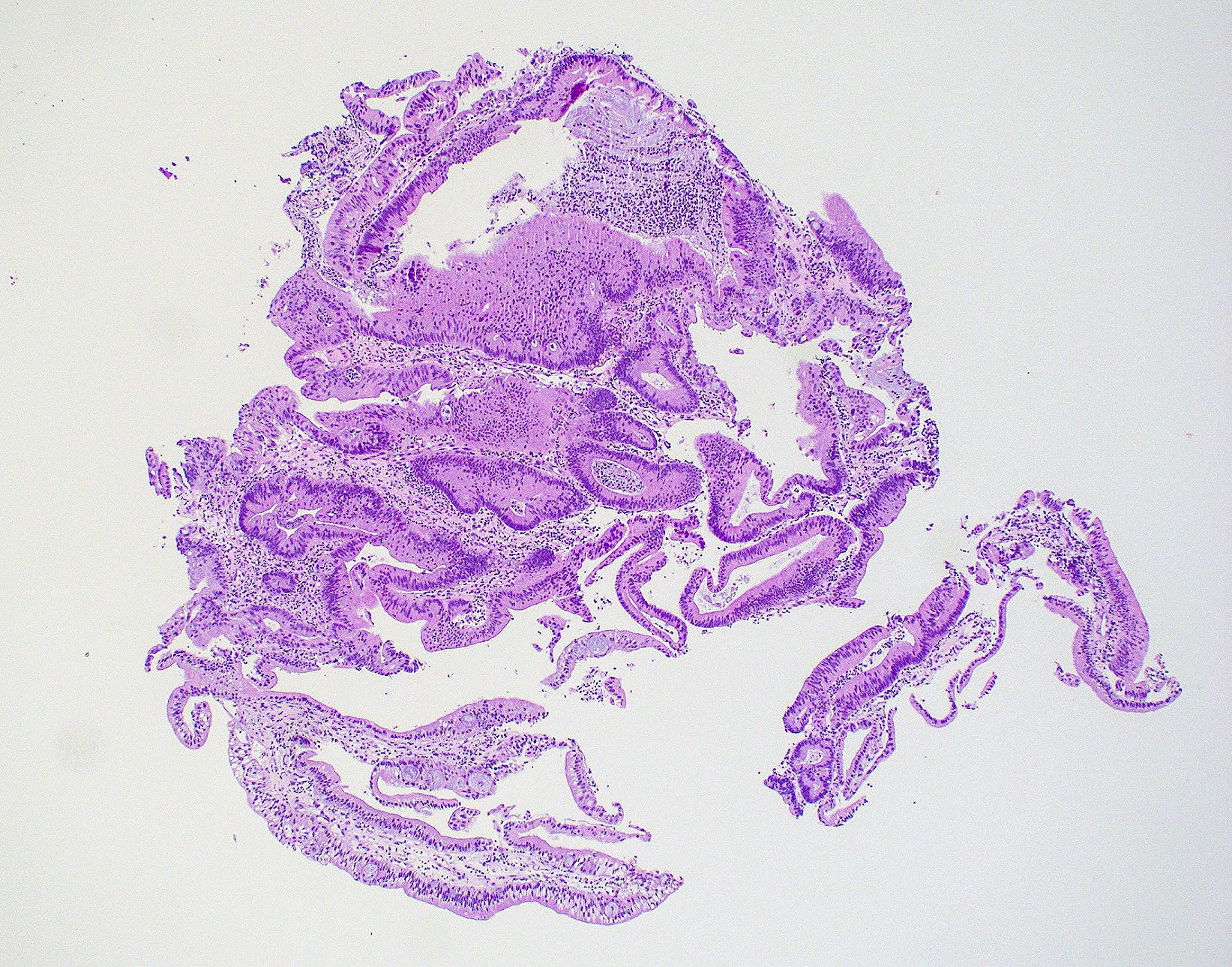
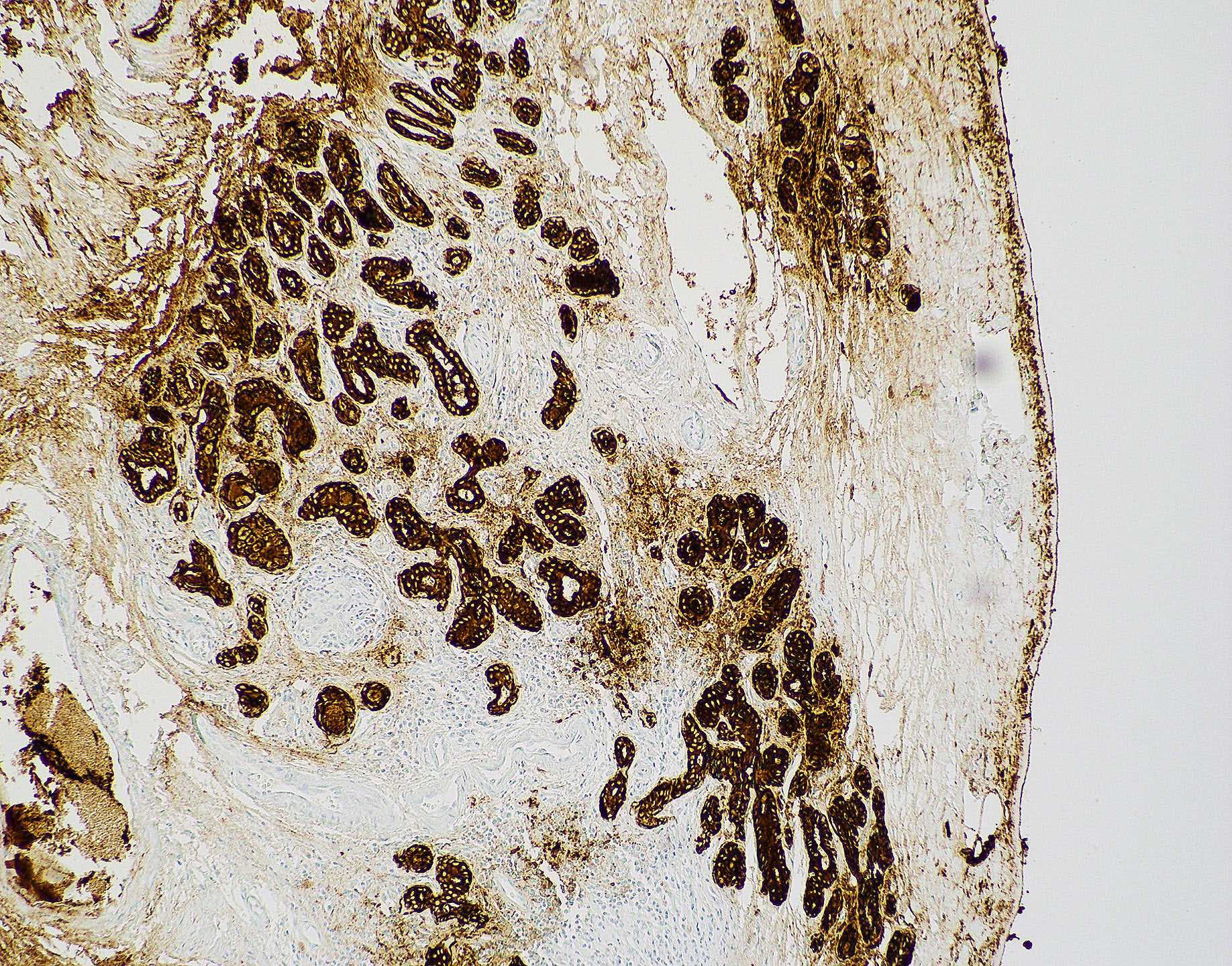
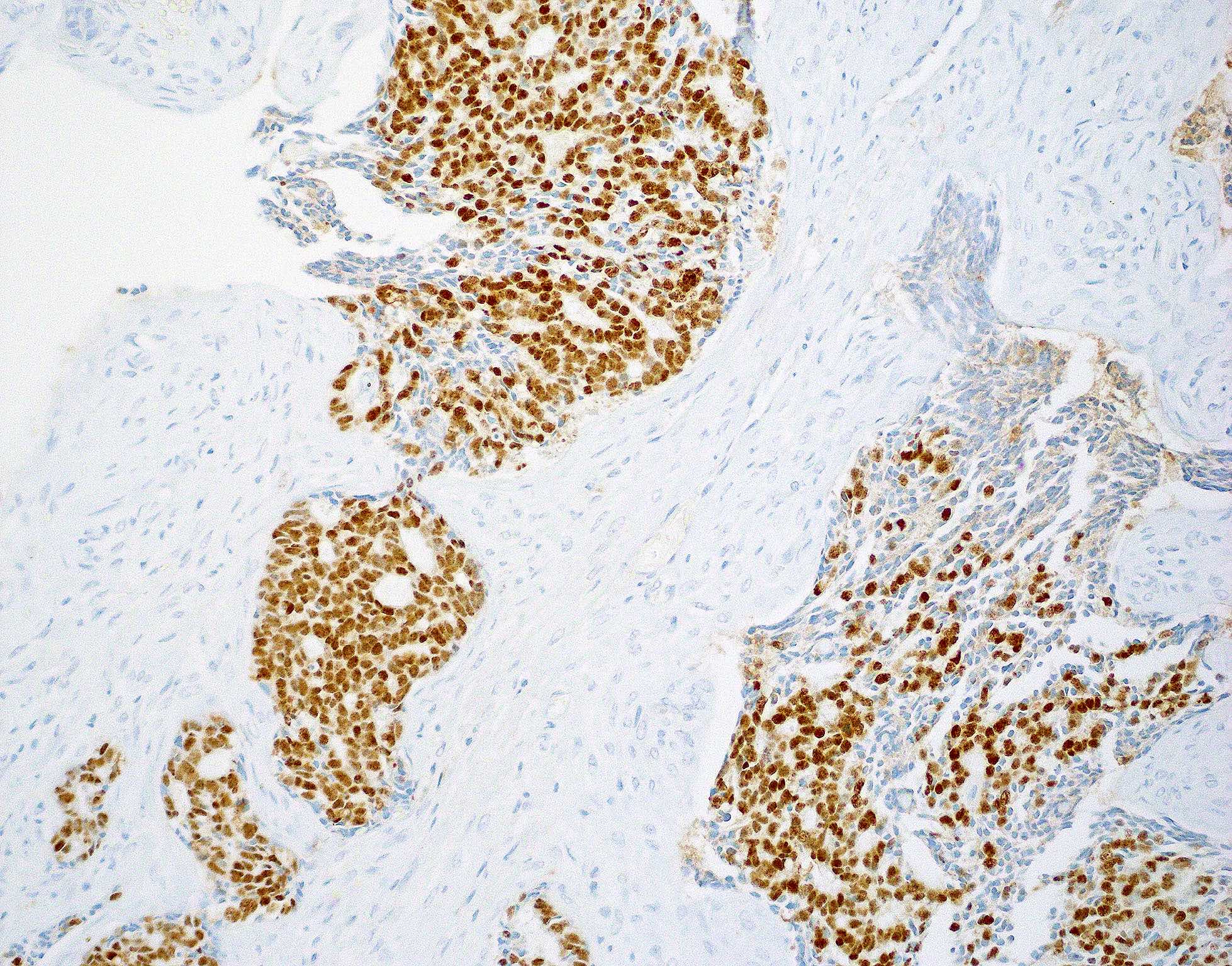
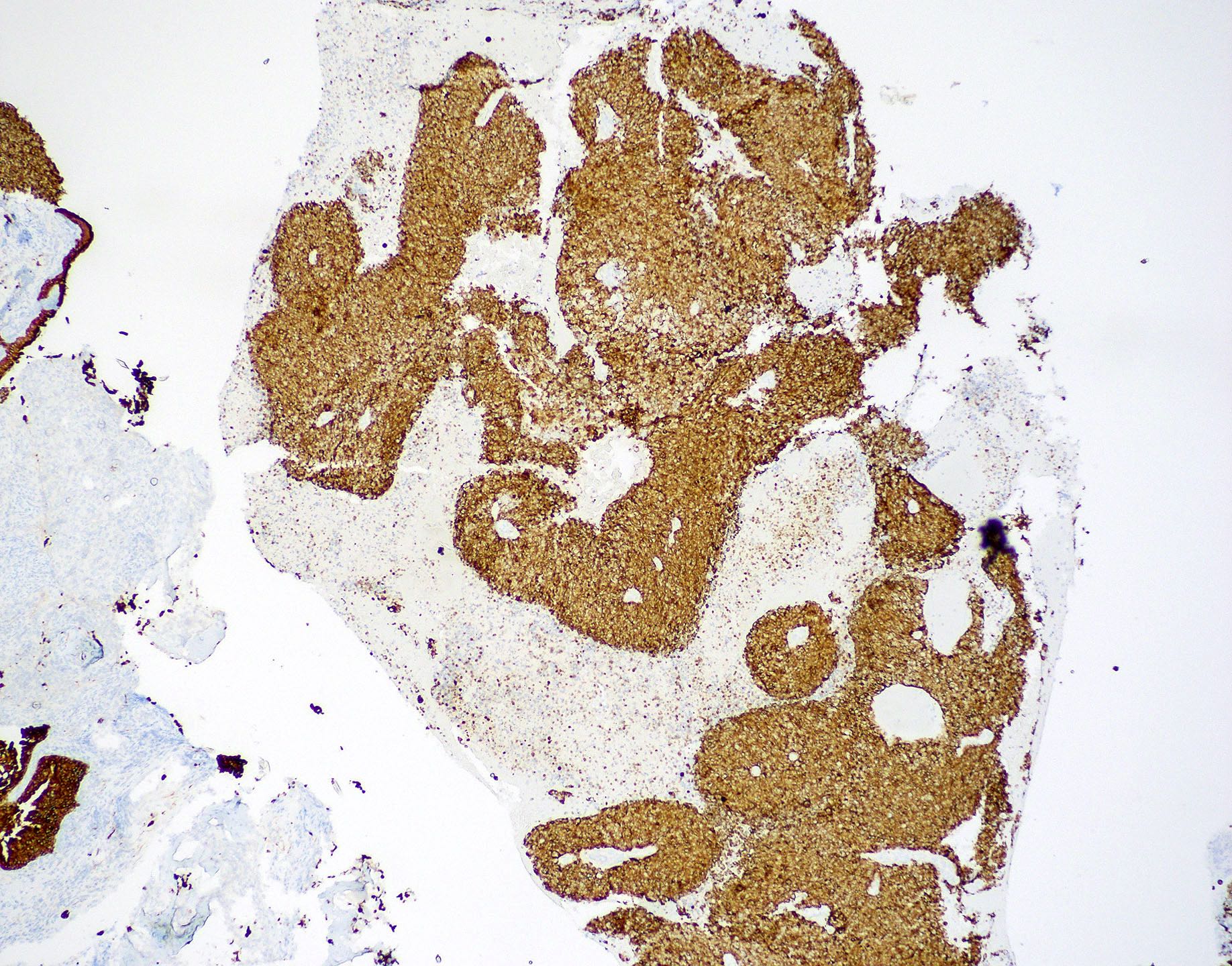
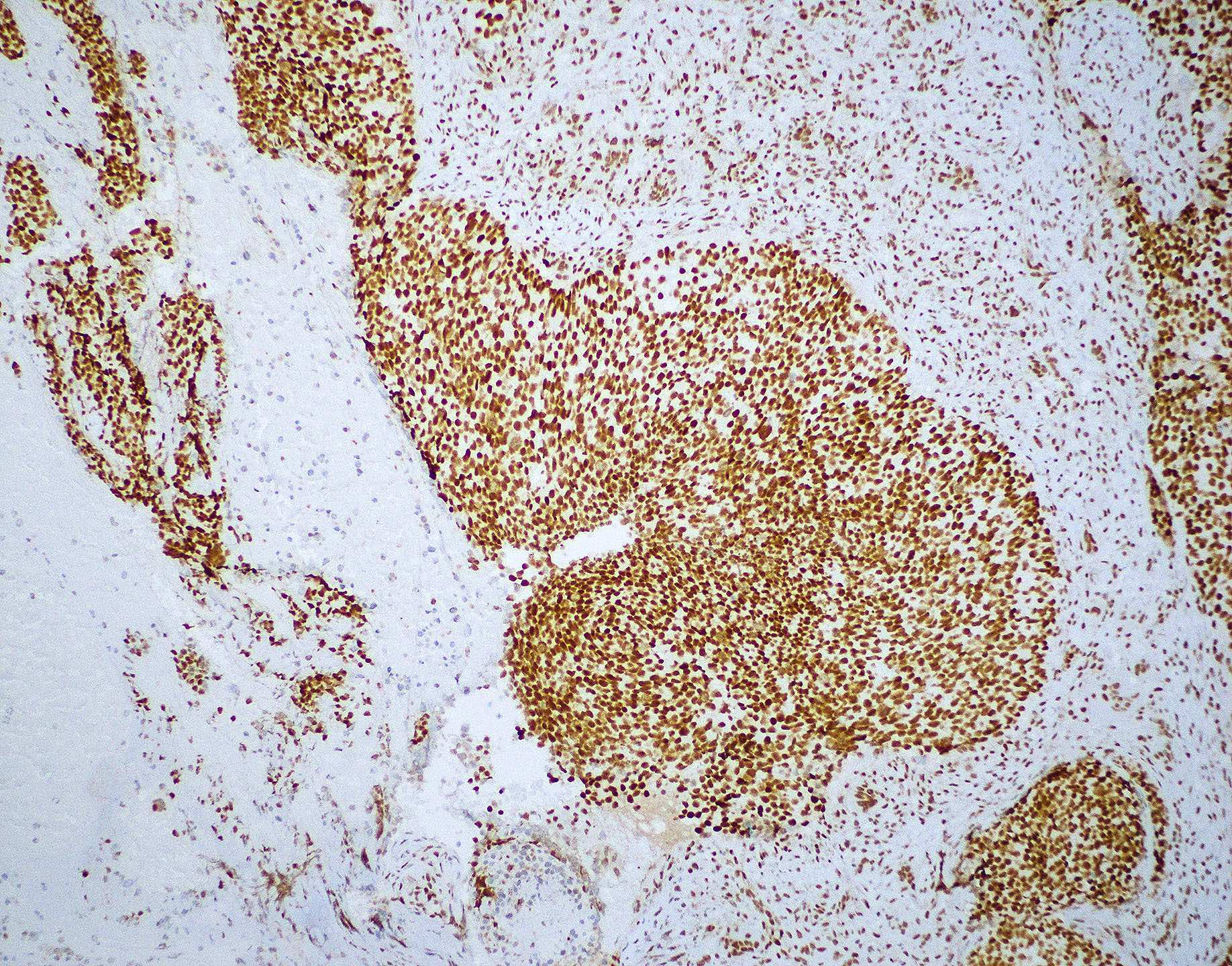
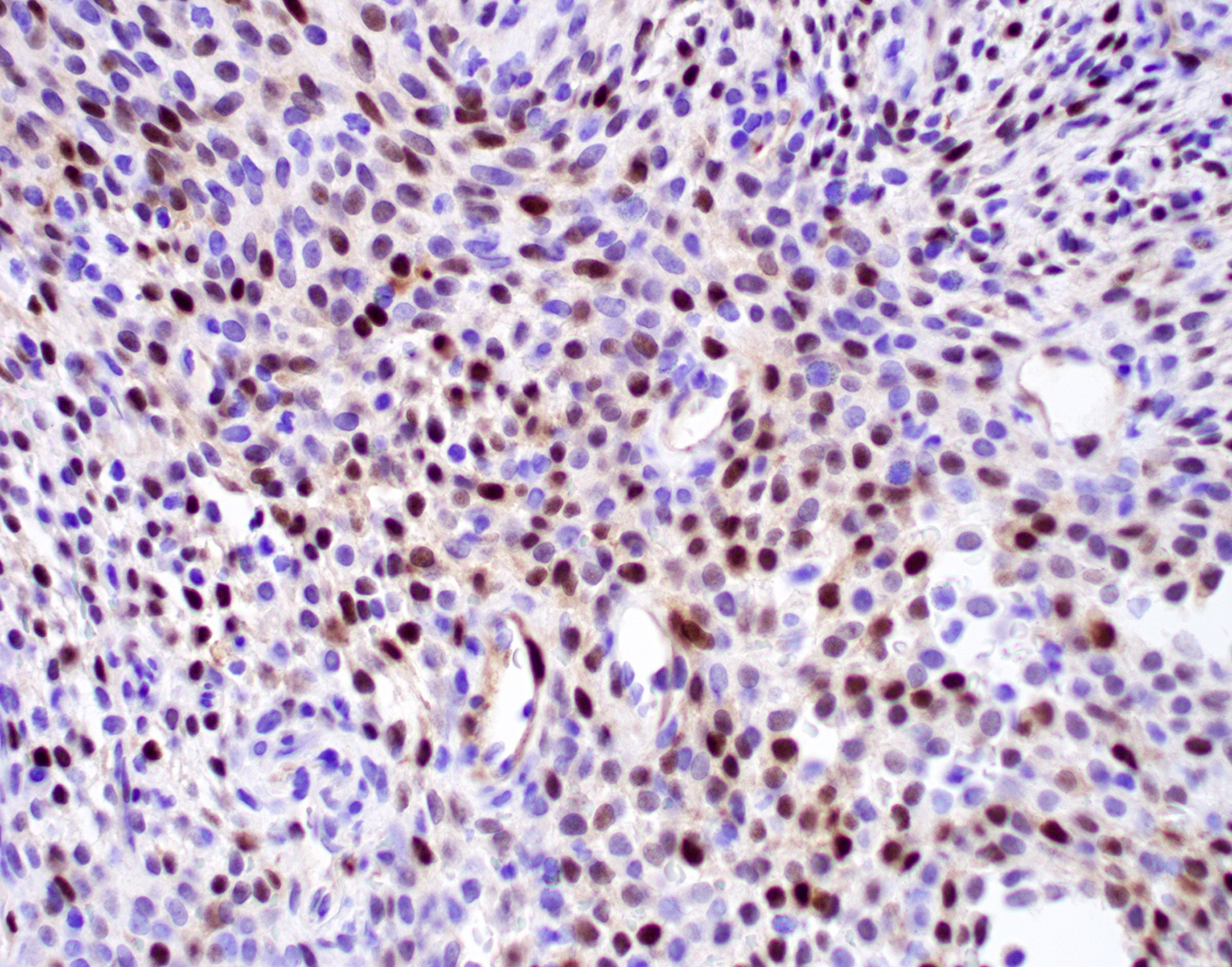
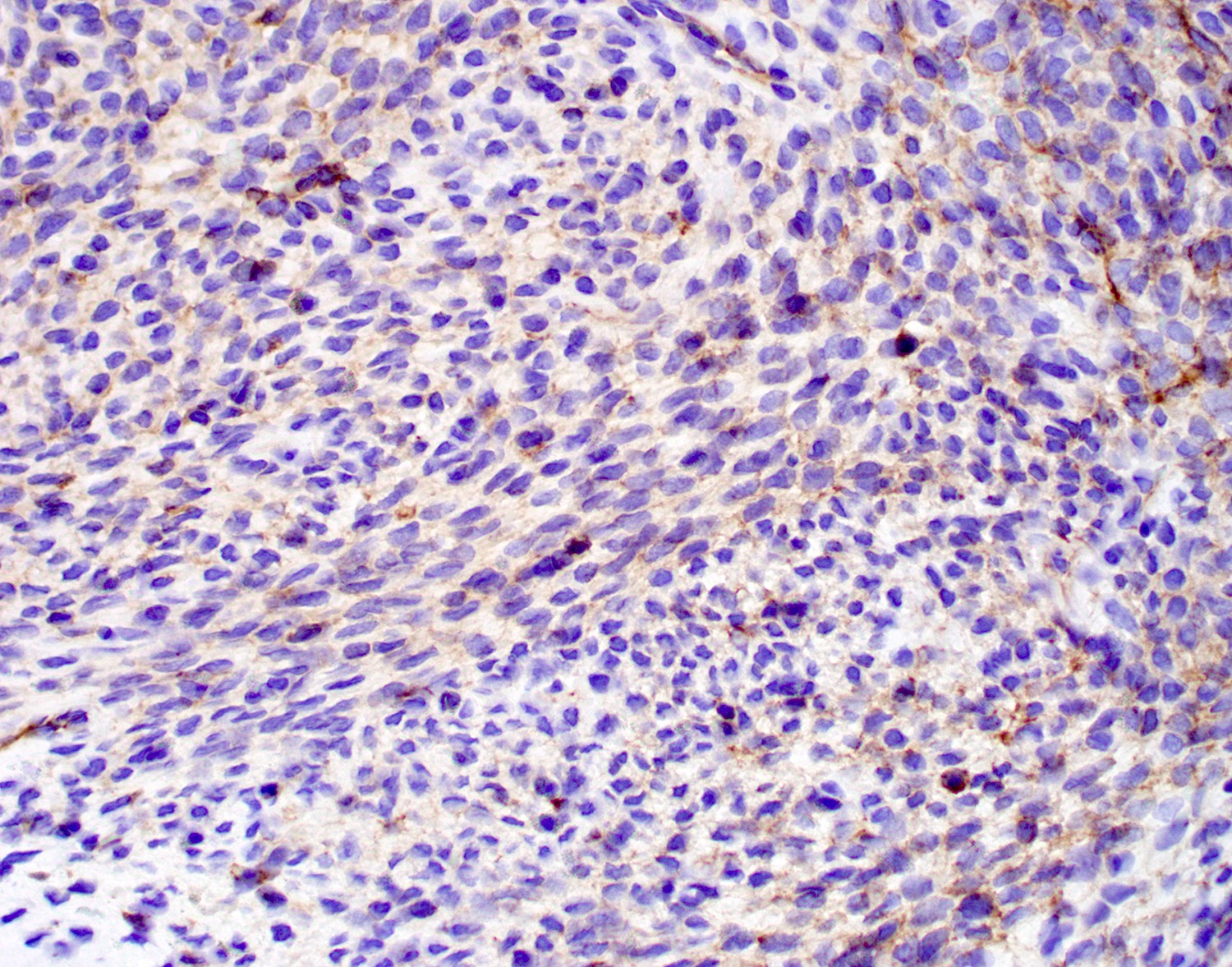
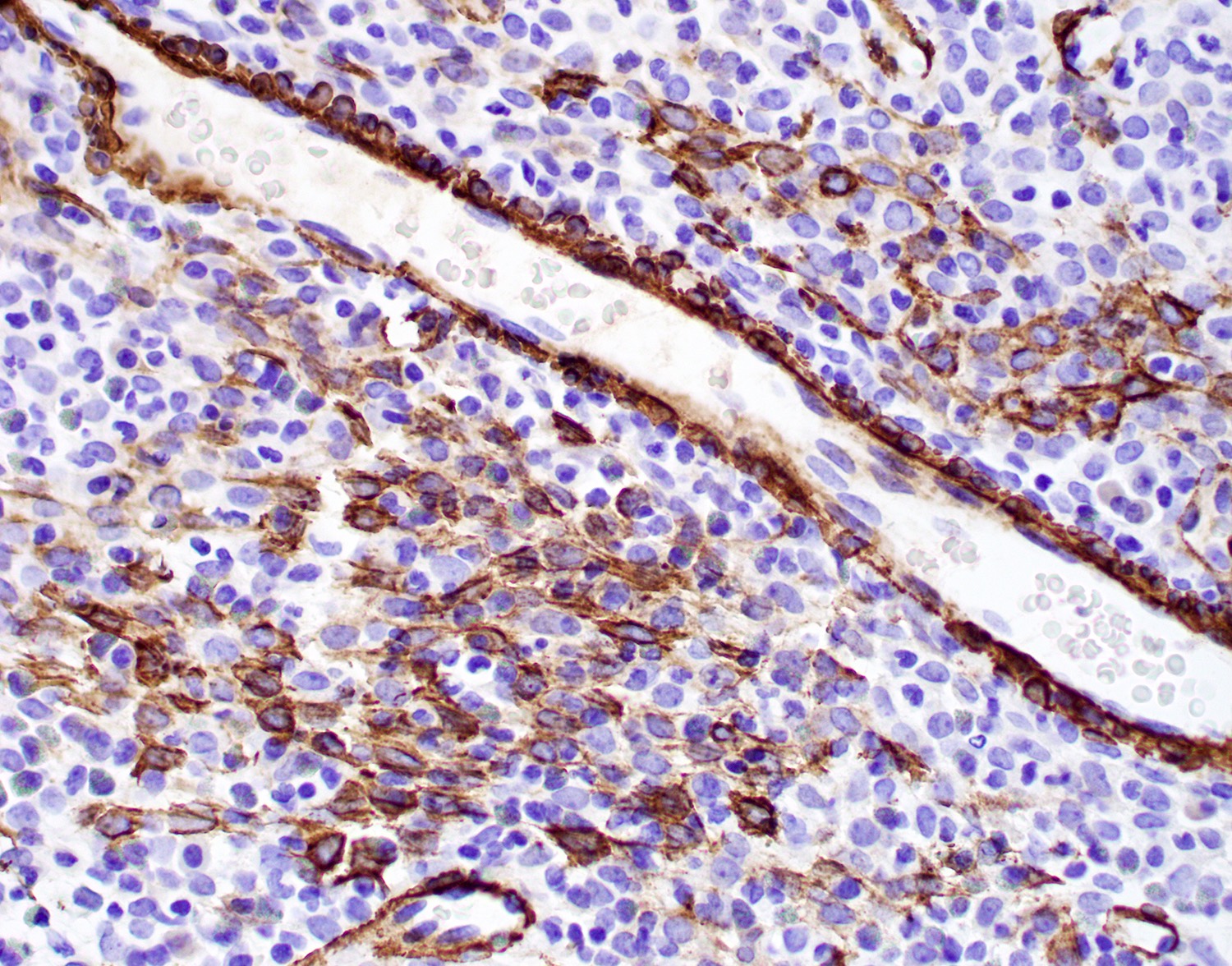
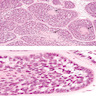
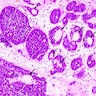
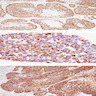
Microscopic (histologic) images
Contributed by Diana Bell, M.D.
Intestinal adenocarcinoma (ITAC)
Nonintestinal adenocarcinoma (non-ITAC)
Sinonasal renal cell-like adenocarcinoma
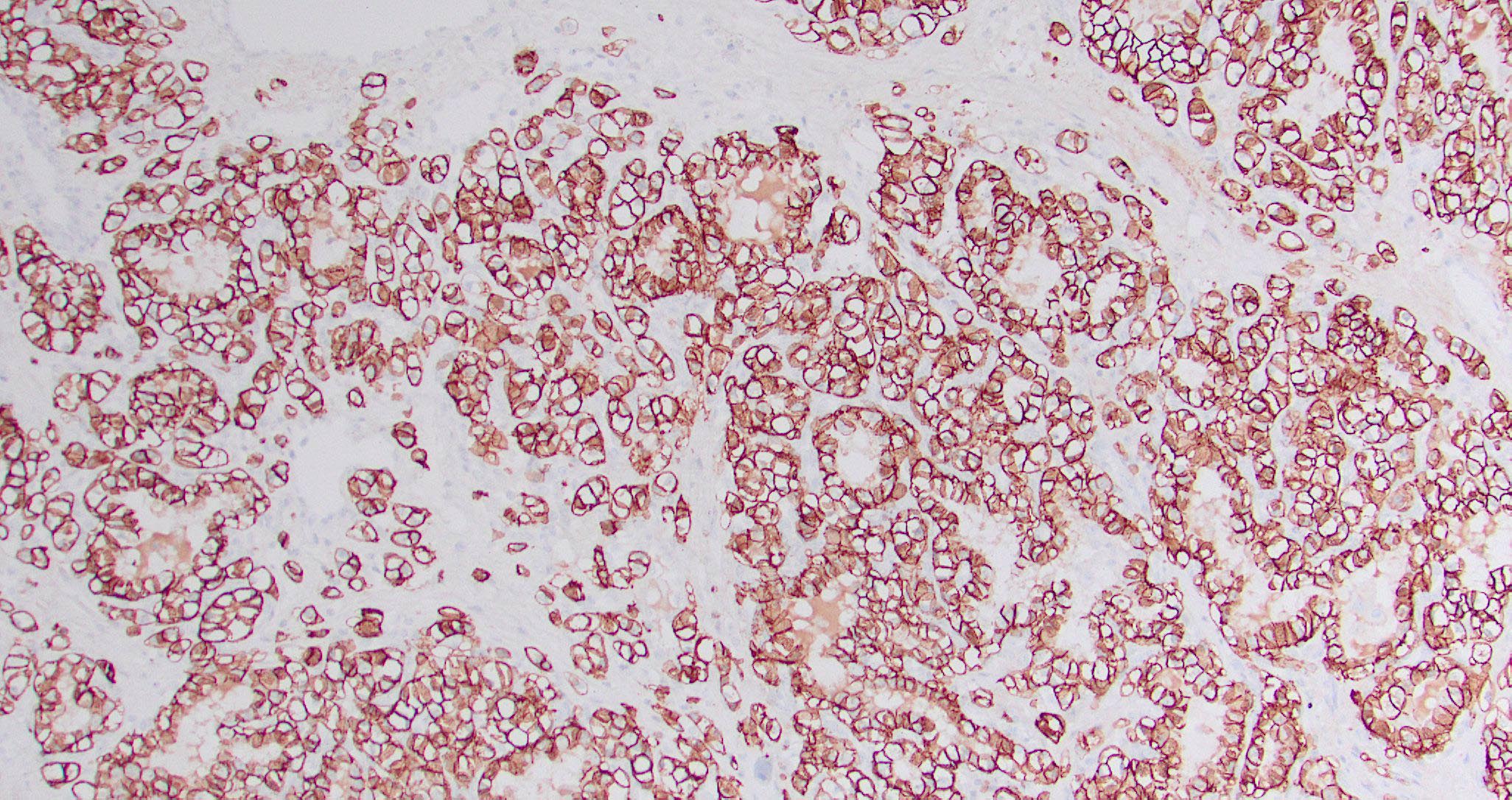
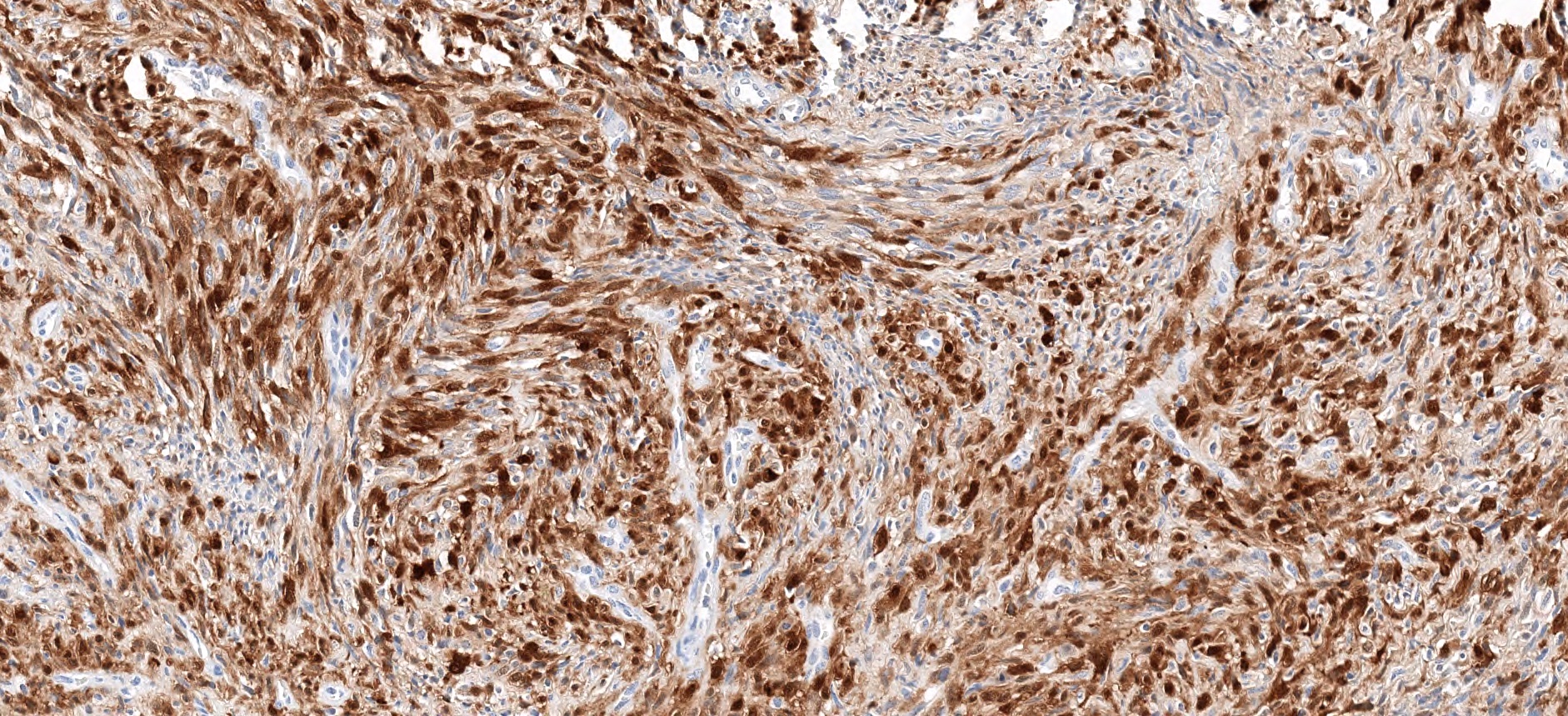
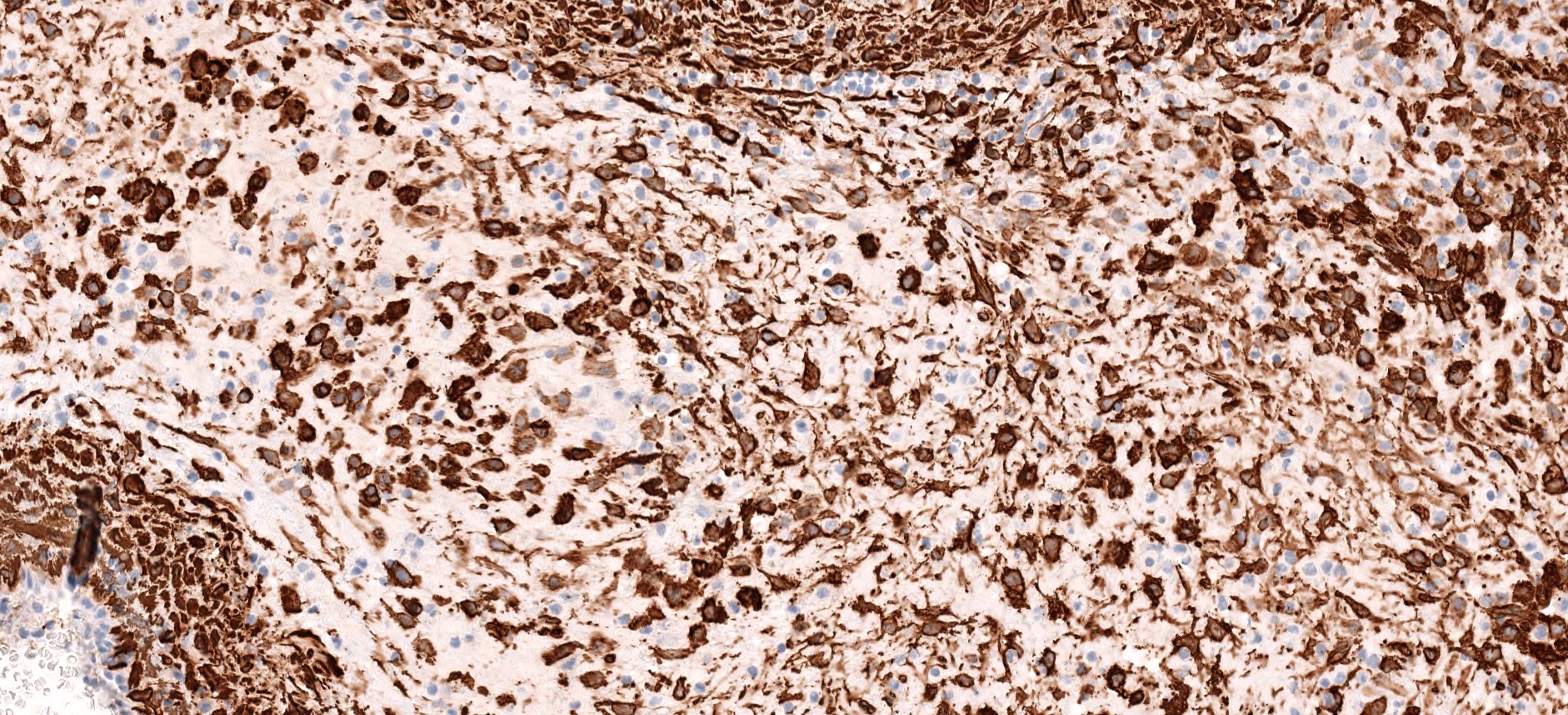
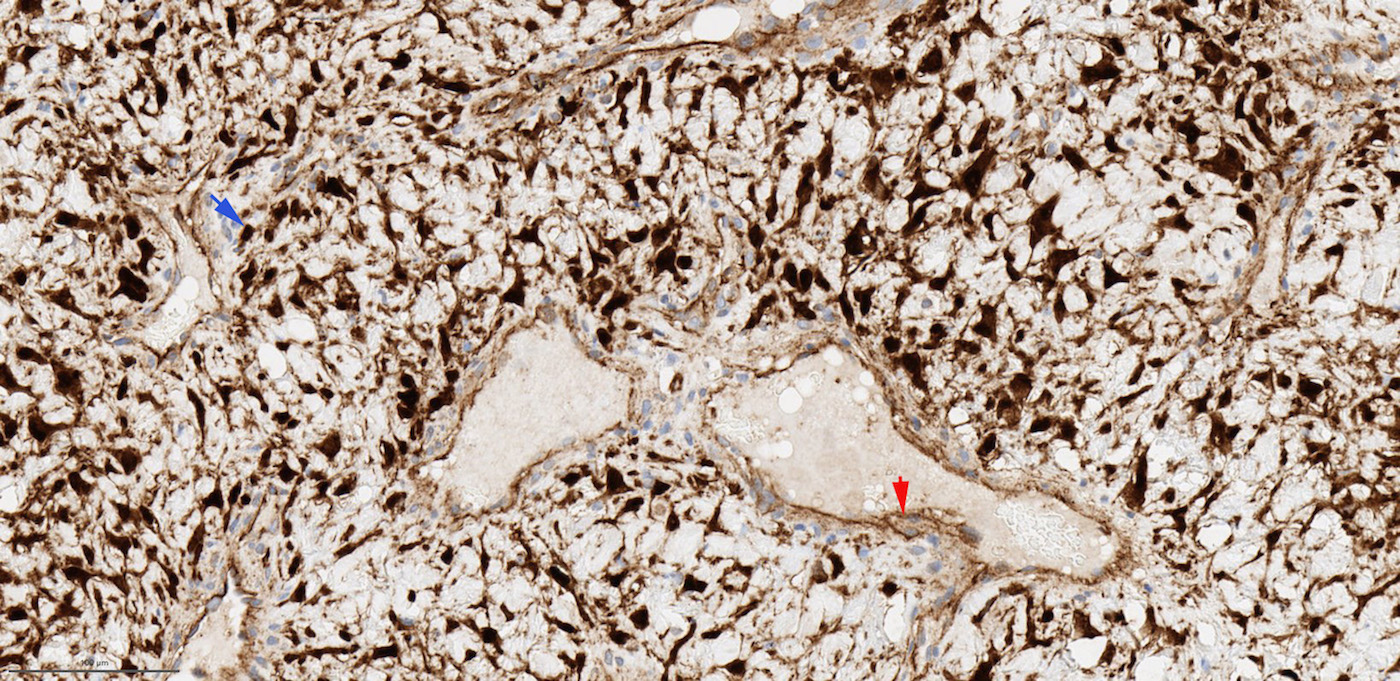
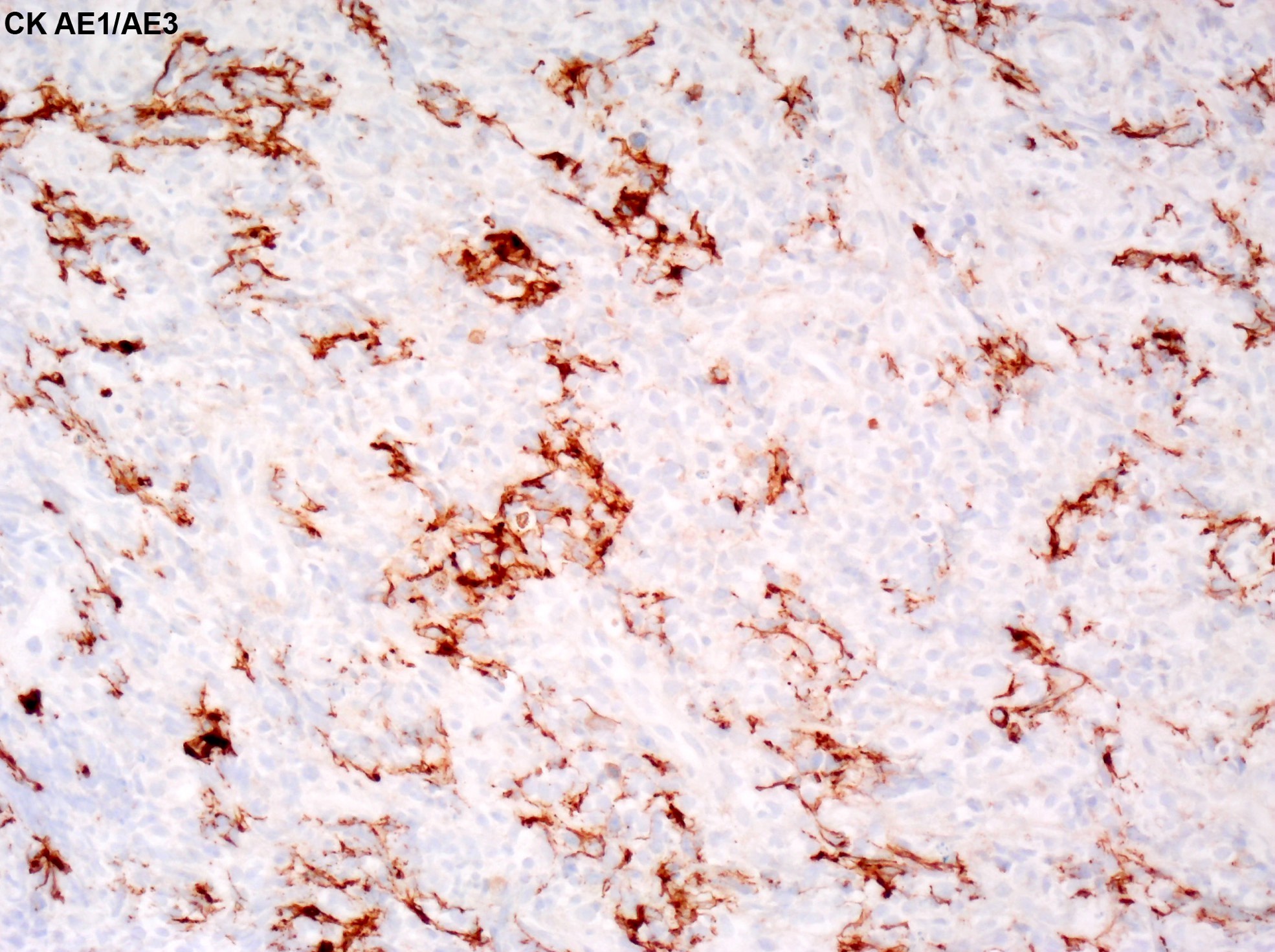
Positive stains
- Intestinal type adenocarcinoma
- Nonintestinal type adenocarcinoma
- Sinonasal renal cell-like adenocarcinoma
- Reference: Head Neck Pathol 2016;10:68
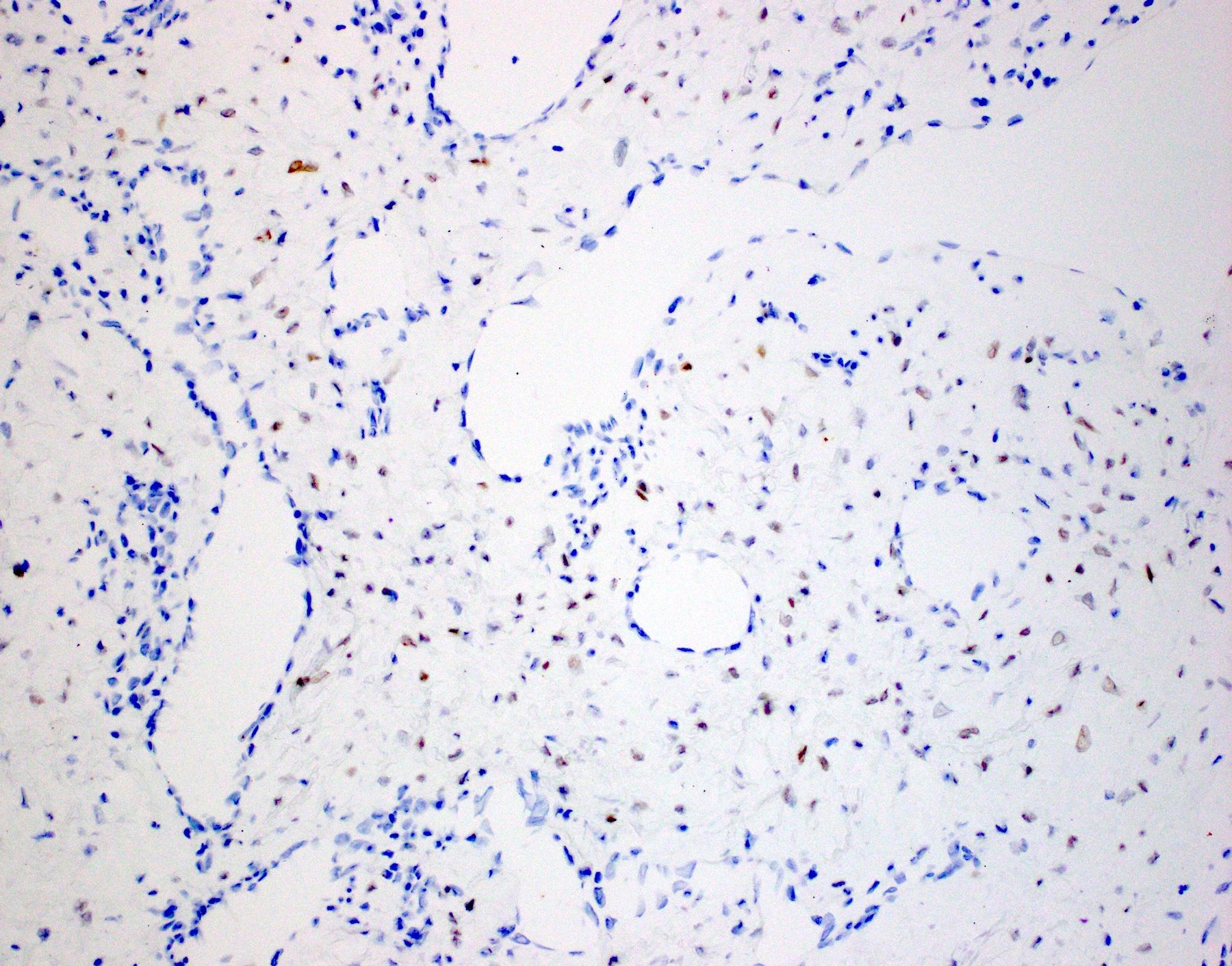
Negative stains
- Nonintestinal type adenocarcinoma
- Sinonasal renal cell-like adenocarcinoma
- Reference: Head Neck Pathol 2016;10:68
Molecular / cytogenetics description
- Intestinal type adenocarcinoma
- EGFR mutation frequently observed (Cell Oncol (Dordr) 2012;35:443)
- KRAS and BRAF mutations infrequent or absent (Oral Oncol 2012;48:692, Cell Oncol (Dordr) 2012;35:443)
- TP53 mutation in 41% of cases (J Cancer Res Clin Oncol 2021;147:1019)
- BRAF mutation in < 10% of cases
- Tumors are microsatellite stable (MSS)
- Variable beta catenin expression with aberrant nuclear expression of beta catenin in > 30% of cases
- Sinonasal renal cell-like adenocarcinoma rarely shows BRAF mutation (Curr Oncol Rep 2022;24:55)
Videos
Sinonasal carcinoma: updated phenotype and molecular characterization
Malignant tumors of the paranasal sinuses by Dr. Nadir Ahmad
Sample pathology report
- Ethmoidal sinus, biopsy:
- Adenocarcinoma, morphologically consistent with intestinal type adenocarcinoma
Differential diagnosis
- Metastatic gastrointestinal adenocarcinoma of intestinal origin:
- Clinical history is critical and mandatory
- Histology, histochemistry and IHC of ITAC and gastrointestinal (GIT) adenocarcinoma are identical
- Metastatic renal cell carcinoma:
- Must be excluded clinically and radiologically
- Adenosquamous carcinoma:
- High grade neoplasm showing squamous and glandular features
- Salivary gland neoplasm:
- Seromucinous hamartomas:
- Submucosal epithelial proliferation of small glands, serous acini and tubules growing in clusters and lobules
Additional references
Board review style question #1
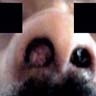
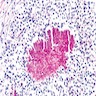
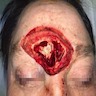
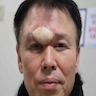
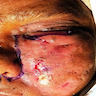
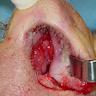
A 66 year old man presented with a rapidly growing soft mass on his glabellar region for 4 months. He was on medication for hypertension and recently had normal endoscopy findings for gastrointestinal malignancy. The findings of the transcutaneous open biopsy are given in the image shown above. What is the most likely diagnosis?
- Intestinal type adenocarcinoma
- Metastatic adenocarcinoma
- Mucocele
- Papillary rhinosinusitis
Board review style answer #1
A. Intestinal type adenocarcinoma. Microscopy is similar to colonic adenocarcinoma but the latest screening endoscopy of the colon was normal. Answer B is incorrect because despite having a morphology similar to primary intestinal adenocarcinoma, the patient's recent gastrointestinal endoscopy was normal. Answer C is incorrect because no epithelium lined cystic mass is seen. Answer D is incorrect because there is no inflammation with polyploidal structure.
Comment Here
Reference: Nasal cavity, paranasal sinuses, nasopharynx - Adenocarcinoma-general
Comment Here
Reference: Nasal cavity, paranasal sinuses, nasopharynx - Adenocarcinoma-general
Board review style question #2
Board review style answer #2
B. Kidney. As microscopy has clear cell architecture, it is crucial to rule out clear cell renal cell carcinoma. Answer A is incorrect because clear cell colorectal carcinoma is an extremely rare entity. Answer C is incorrect because clear cell carcinoma of the lung secretes mucus, which is absent here. Answer D is incorrect because the prostate does not have presentation as well as diagnosis of prostate cancer.
Comment Here
Reference: Nasal cavity, paranasal sinuses, nasopharynx - Adenocarcinoma-general
Comment Here
Reference: Nasal cavity, paranasal sinuses, nasopharynx - Adenocarcinoma-general
Allergic fungal sinusitis
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Laboratory | Radiology description | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Chronic allergic fungal sinusitis is an eosinophil mediated hypersensitivity reaction initiated by environmental fungi
Essential features
- Characterized by thick allergic mucin (with degranulated eosinophils and Charcot crystals) and hyphal fragments on GMS stain
Terminology
- Also called allergic fungal rhinosinusitis
Epidemiology
- 5 - 10% of all cases of chronic sinusitis (Am J Surg Pathol 1983;7:439)
Sites
- Multiple; nasal cavity or paranasal sinuses
Pathophysiology
- Environmental causes
- A. fumigatus, A. flavus or demateaceous fungi can trigger extreme eosinophil driven hypersensitivity to fungi in susceptible individuals
- Allergic fungal sinusitis is a TH 2-like lymphocyte mediated response
Clinical features
- Young adult with recurrent sinonasalpolyp, asthma, poor response to medical treatment
Laboratory
- Peripheral eosinophilia, elevated IgE
Radiology description
- CT: opacification of the nasal cavity and one or more paranasal sinuses
- Erosion of bone (skull base and orbit) is seen in 20 - 60% of cases
Case reports
- 24 year old woman with allergic bronchopulmonary aspergillosis with obstruction of the upper respiratory tracts (Chest 1976;70:788)
- Pathologic findings in allergic aspergillus sinusitis (Am J Surg Pathol 1983;7:439)
- Allergic aspergillosis of the maxillary sinuses (Thorax 1981;36:707 (abstract))
Treatment
- Complete endoscopic removal of the mucus and inflamed tissue followed by intranasal or systemic corticosteroids and possible maintenance therapy with fungal desensitization vaccines
Gross description
- Edematous polypoid respiratory mucosa with thick, tenacious mucus similar to peanut butter or wet clay
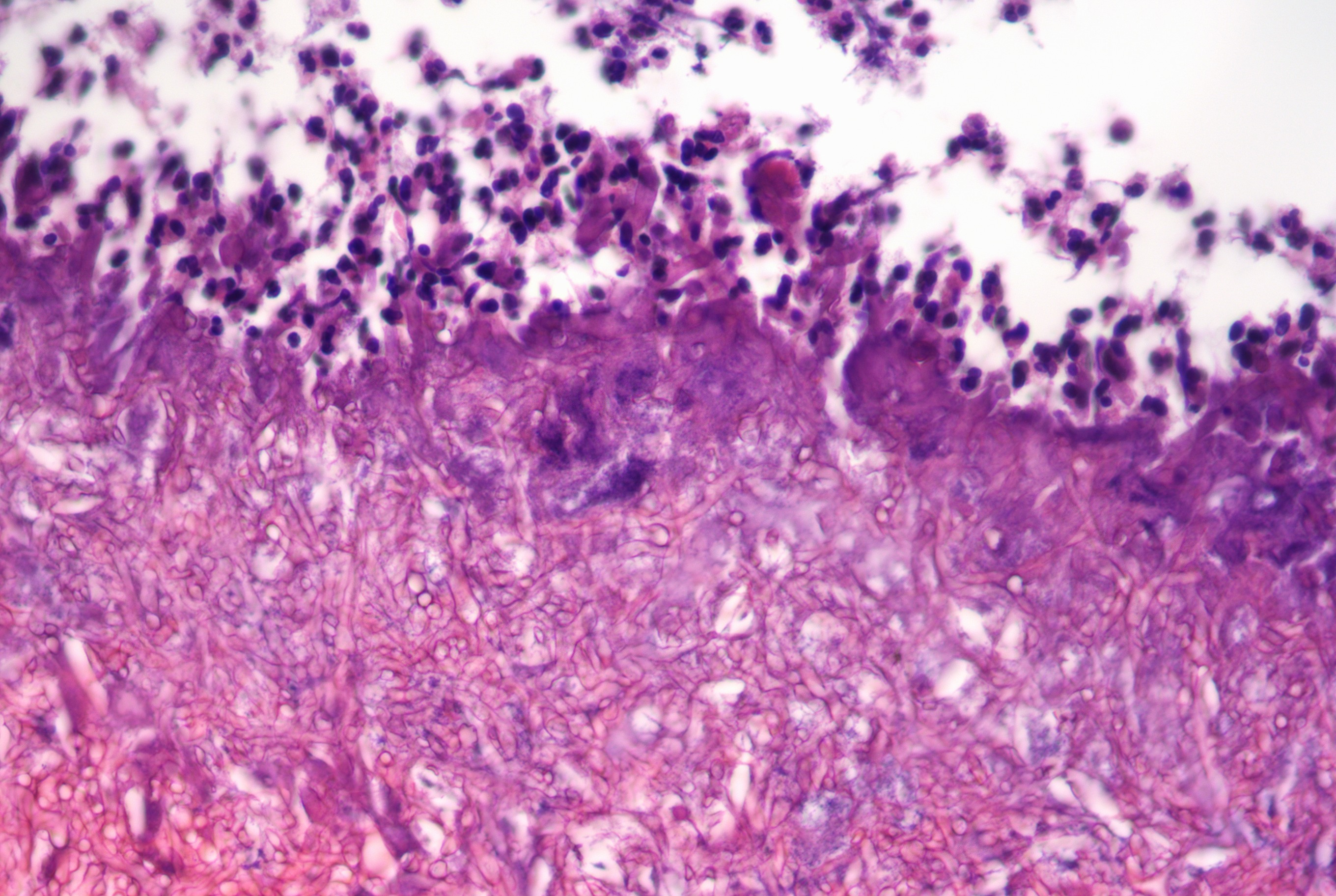
Microscopic (histologic) description
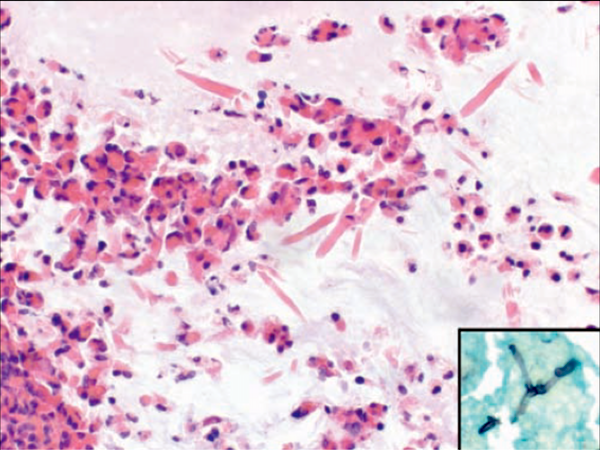
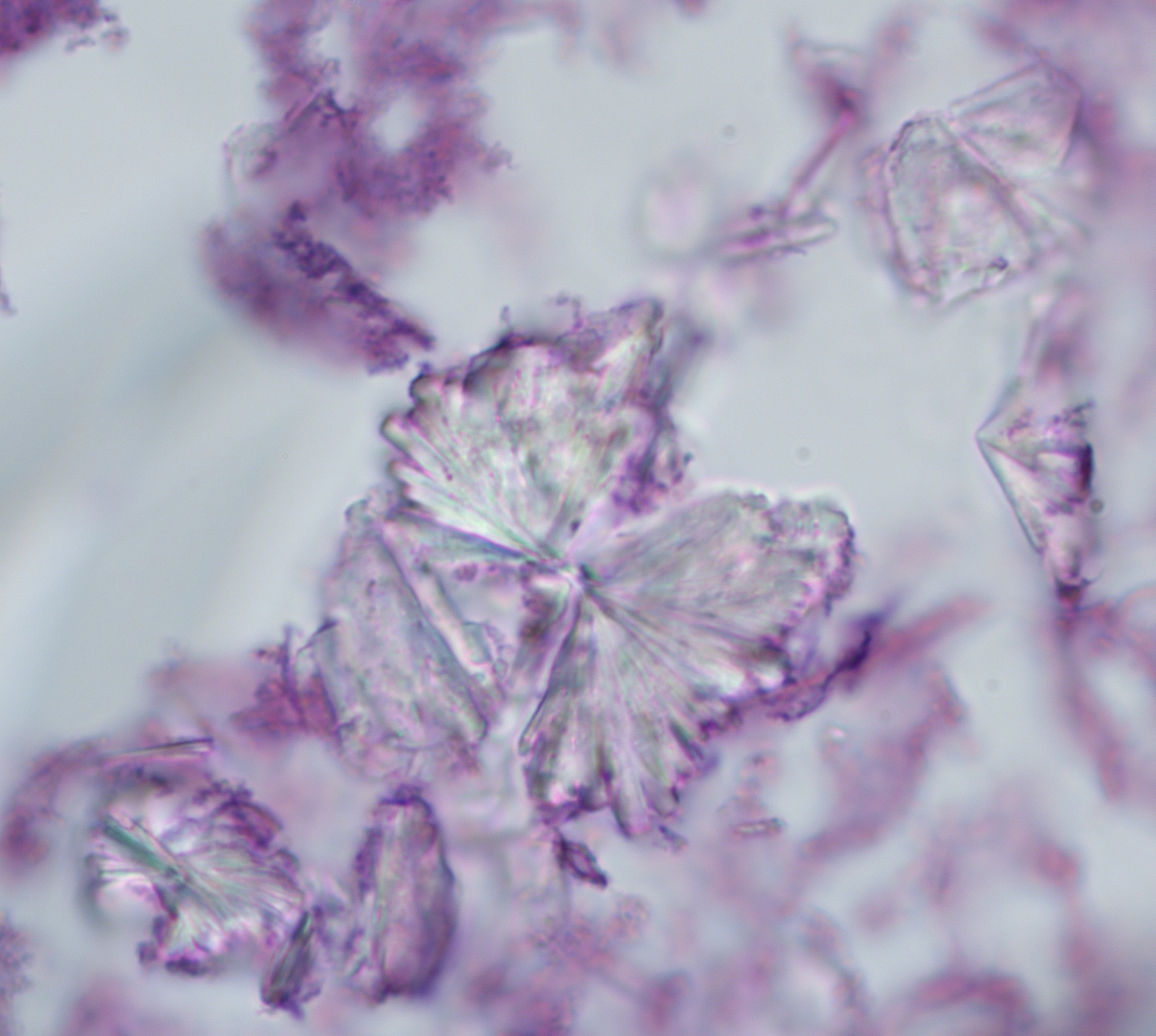
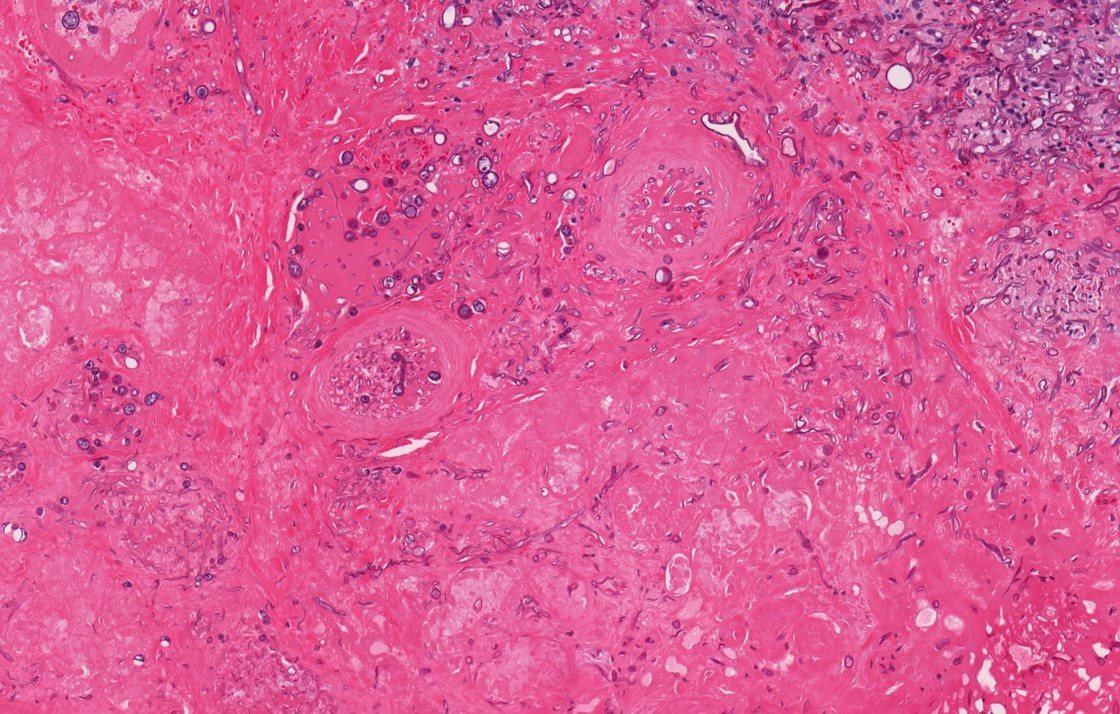
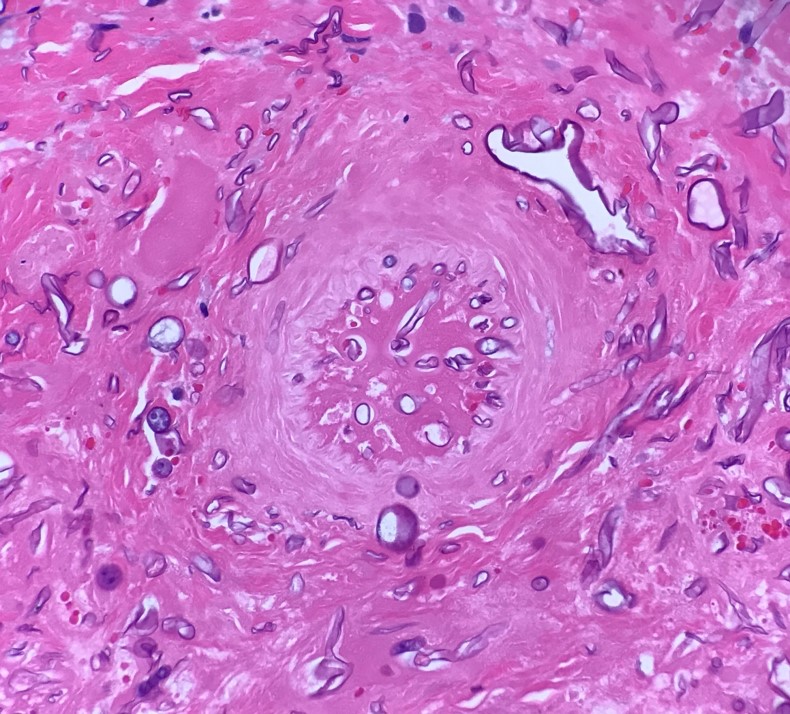
- Diagnostic features: eosinophilic mucin with red and blue ripples (laminations composed of cellular debris, epithelium, polymorphonuclear cells, degranulated eosinophils and Charcot Leyden crystals)
- Charcot Leyden crystals are pink/red refractive, and form long needle-like structures
- Rare noninvasive fungal hyphae (often found only with GMS stain)
- Schneiderian mucosa reveals thickened basement membrane with goblet cell hyperplasia, and numerous inflammatory cells with prominent eosinophils
- Eosinophils may have degenerative changes of smudged, elongated or basophilic nuclei
Microscopic (histologic) images
Differential diagnosis
- It is unclear if eosinophilic mucin rhinosinusitis (EMRS) is a distinct entity from allergic fungal sinusitis (AFS) because:
- Fungal hyphae are not always detected in allergic mucin, although the sensitivity for fungal detection by the gold standard Gomori methanamine silver (GMS) stain is dramatically improved by trypsin predigestion, which speaks against EMRS as a distinct entity
- On the other hand, aspirin sensitivity and bilateral sinus disease are more common types of eosinophilic mucin rhinosinusitis than allergic fungal sinusitis, consistent with the idea that ERMS represents a distinct clinical entity
- Thus, this issue remains unresolved
Additional references
Allergic rhinosinusitis
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Laboratory | Treatment | Microscopic (histologic) description | Cytology description | Differential diagnosis | Additional referencesDefinition / general
- Common IgE mediated sinonasal hypersensitivity reaction provoked by reexposure to a specific antigen, including plant pollen, fungi, dust mites, animal allergens
- May be acute or chronic
- Also called hay fever
Terminology
- Also called type I IgE immunological rhinitis
- Acute allergic rhinosinusitis: develops 2 to 5 minutes after antigen-antibody exposure, reaching its peak about 15 minutes later
- Chronic allergic rhinosinusitis: lasts more than 6 weeks; includes aspirin exacerbated respiratory disease
Epidemiology
- 10 - 20% of the U.S. adult population (Hosp Pract (Off Ed) 1991; 26:105)
Sites
- Bilateral nasal cavity and paranasal sinuses
Pathophysiology
- IgE antibody binding and mast cells release various mediators (histamine, prostaglandins and leukotrienes) causing vasodilation, edema, eosinophilia, nasal congenstion, rhinorrhea, sneezing and itching
Etiology
- Type I IgE hypersensitive reaction to allergens, including pollen, animal dander, dust mites, mold spores and food
- Aspirin exacerbated respiratory disease (AERD, also known as Samter triad) refers to the syndrome of allergic nasal polyps with eosinophils, aspirin intolerance and bronchial asthma, which affects 4 - 10% of asthmatics
- AERD is due to inhibition of the cyclooxygenase pathway in sensitive individuals; this shunts metabolism of arachidonic acid to the 5-lipooxygenase pathway, leading to increased proinflammatory leukotrienes and decreased PGE2, a protective prostaglandin
Clinical features
- Rhinorrhea, sneezing, itching
Laboratory
- Stained smears of nasal secretion slow > 25% eosinophils
- Elevated total serum IgE
Treatment
- Avoidance of allergens, use of environmental controls, sublingual immunotherapy
Microscopic (histologic) description
- Mucous secretions have neutrophils and prominent eosinophils
Cytology description
- Stained smears of nasal secretion: > 25% eosinphils
Differential diagnosis
Additional references
Anatomy
Definition / general
-
Nasal vestibule, nasal cavity, paranasal sinuses and nasopharynx
- Slight dilation inside anterior aperture of nostril, lined by skin containing hair and sebaceous glands
- Anterior boundary: nares
- Posterior boundary: line dropped perpendicular from the frontonasal suture through the anterior aspect of the inferior turbinate
- Lateral boundary: ala and lateral crus of greater alar cartilage
- Medial boundary: medial crus of greater alar cartilage
- Nares: anterior openings of nasal cavity
Nasal vestibule:
Nasal cavity:
- Nasal chambers are on either side of median plane formed by nasal septum
- Anterior boundary: continuous with the vestibule
- Posterior boundary: posterior choanae
- Superior boundary: cribriform plate
- Inferior boundary: hard palate
- Medial boundary: nasal septum
- Lateral boundary: lateral nasal wall with maxillary and ethmoid ostia and turbinates
- Divided into olfactory region (superior nasal turbinates and opposed septum) and respiratory region (rest of cavity)
- Bulla ethmoidalis: elevation on lateral wall of middle meatus, site of opening of middle ethmoid meatus
- Choanae: posterior opening of nasal cavity, communicates with nasopharynx
- Columella: anterior extreme nasal septum
- Crista galli: bony ridge which projects superiorly from cribriform plate
- Lateral wall: contains superior, middle and inferior nasal turbinates (conchae); below each is corresponding nasal passage or meatus
- Limen nasi: posterior lateral ridge separating the vestibule from the nasal cavity
- Middle meatus: below and lateral to middle turbinate
- Nasal septal swell body: thickened area of superior nasal septum containing nasal erectile vessels
- Olfactory cleft: narrow vertical aspect of superior nasal cavity
- Party wall: comprised of the lateral nasal wall and medial antral wall
- Sphenoethmoidal recess: above superior turbinate, site of opening of sphenoidal sinus
- Superior meatus: along upper border of middle turbinate, site of opening of posterior ethmoid meatus
- Turbinates (concha):
- Scroll-like projections of bone and vascular soft tissue
- The superior turbinate is smallest, the inferior turbinate is largest
- Attaches to the lateral nasal wall anteriorly, with a free edge posteriorly
Paranasal sinuses:
- Diverticula of nasal cavity that extend into neighboring bones
- Frontal Sinuses:
- Most anterior, above the orbits
- Small / rudimentary at birth
- Develop through puberty
- Paired sinuses between the interior and external cranial tables
- Ethmoid Complex:
- Between the orbits
- Well developed at birth
- Paired sinus complex composed of 3 to 18 cells that are grouped as anterior, middle or posterior, according to the location of their ostia
- Medial boundary: upper nasal fossa
- Lateral boundary: lamina papyracea of the orbit
- Superior boundary: fovea ethmoidalis, which is the medial extension of the orbital plate of the frontal bone
- Sphenoid sinuses:
- Most posterior at base of brain
- Small / rudimentary at birth
- Develop rapidly during childhood until permanent teeth develop
- Posterior to the ethmoid sinuses
- Superior boundary: floor of the anterior cranial fossa, anteriorly
- Posterior boundary: optic chiasm and the sella turcica, posteriorly
- Lateral boundary: orbital apex, the optic canal, the optic nerve and cavernous sinus
- Inferior boundary: nasopharynx
- Anterior boundary: nasal fossa
- Maxillary sinuses:
- Under the cheeks
- Small / rudimentary at birth
- Develop rapidly during childhood until permanent teeth develop
- Medial boundary: lateral wall of the nasal cavity ("party wall")
- The curved posterolateral wall separates the sinus from the infratemporal fossa
- Anterior boundary: the facial surface of the maxilla
- Inferior boundary: hard palate
- Superior boundary: orbital rim and orbital apex
- Ohngren line:
- Connects medial canthus of eye to angle of mandible
- Used to divide maxillary sinus into anteroinferior portion (infrastructure), associated with good prognosis for carcinoma and superoposterior portion (suprastructure), with a poor prognosis for carcinoma
Nasopharynx:
- Respiratory passage above and behind the soft palate
- Part of pharynx, which also includes oropharynx and hypopharynx
- Begins anteriorly at posterior turbinates and extends along plane of airway to the level of the free border of the soft palate
- Anterior wall is perforated by posterior nares (choanae)
- Posterior wall is also its roof, as well as the posterior base of skull
- Extends inferiorly to level of free border of soft palate where oropharynx begins
- Lateral wall contains ostium of eustachian tube, surrounded by mucosa covered cartilaginous prominence
- Ostium is anterior to pharyngeal recess (fossa of Rosenmuller)
Antrochoanal polyps
Table of Contents
Definition / general | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
- 4 - 6% of nasal polyps
- Frequently occur in childhood
- 90% solitary
- Arise from wall of maxillary antrum, extending through large primary or secondary maxillary ostium into nasal cavity
- May pass into choanae or nasopharynx
Case reports
- 27 year old woman with right nasal polyp (Case of the Week #390)
Gross description
- Long narrow stalk with firm, fibrous body
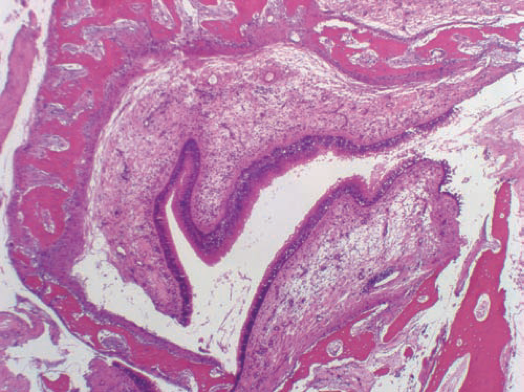
Microscopic (histologic) description
- Thin surface mucosa with no thickened basement membrane
- Stroma with stellate cells, less edema and fewer glands than inflammatory polyp
- May have prominent dilated vessels with thrombosis or infarct
- Prominent eosinophils in only 20%
Biphenotypic sinonasal sarcoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
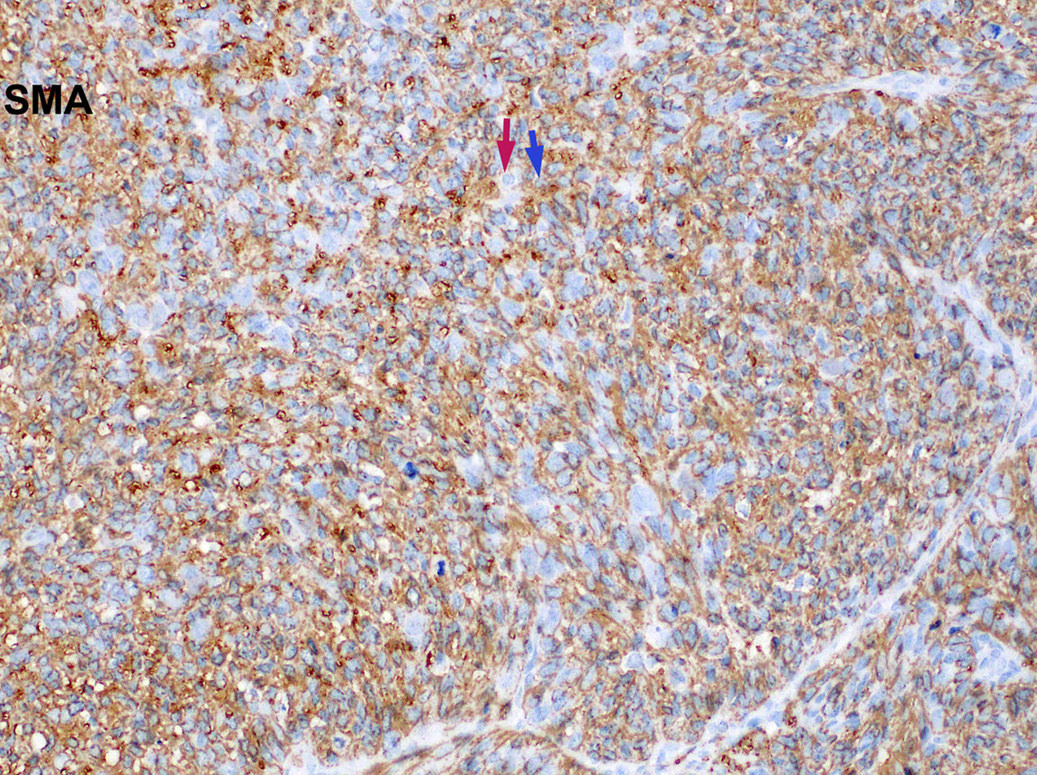
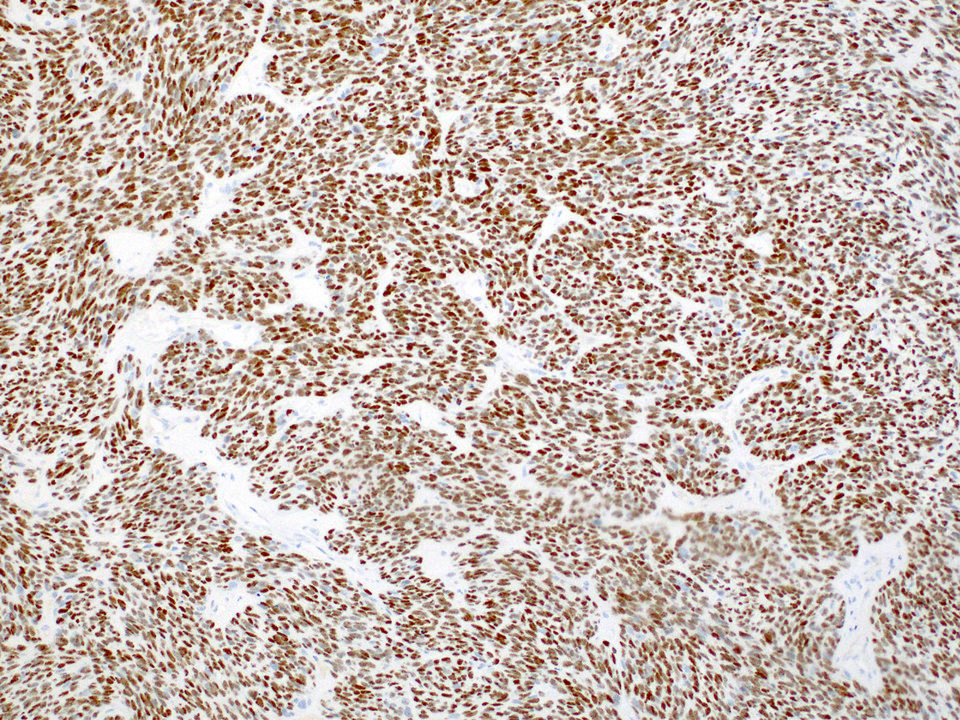
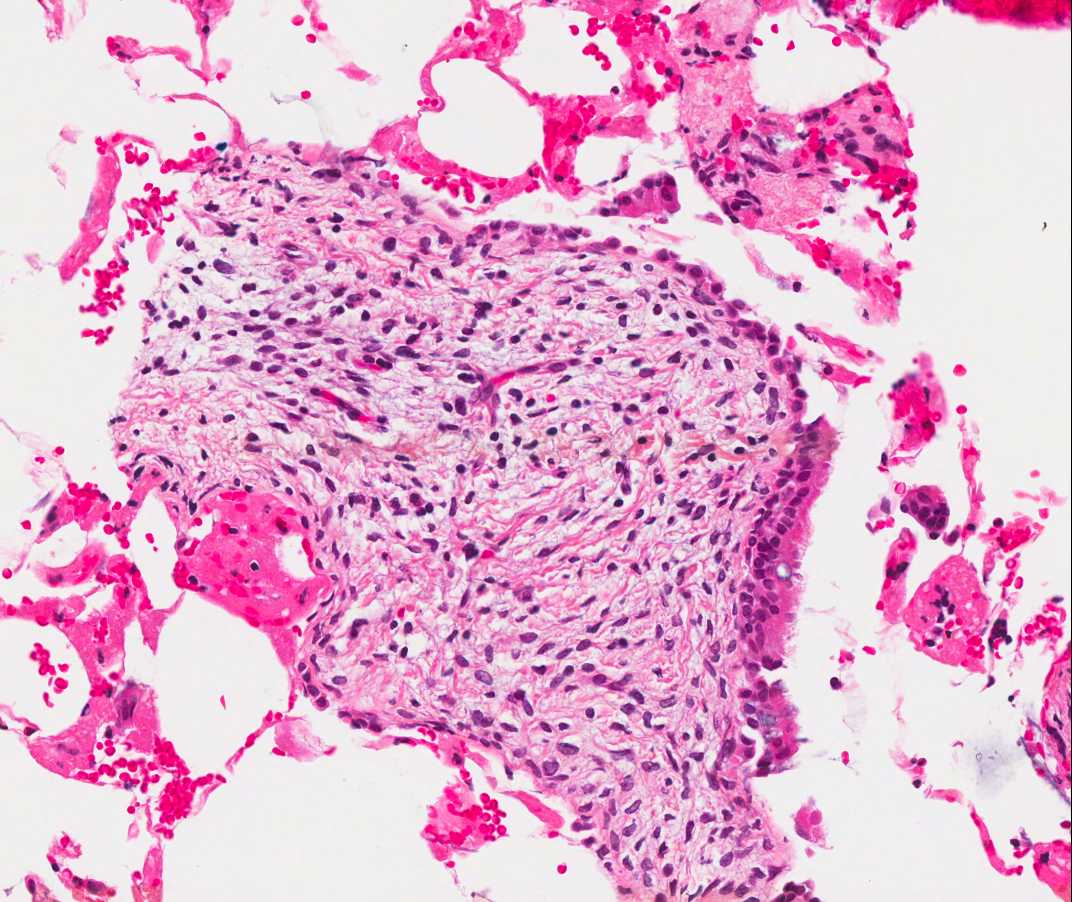
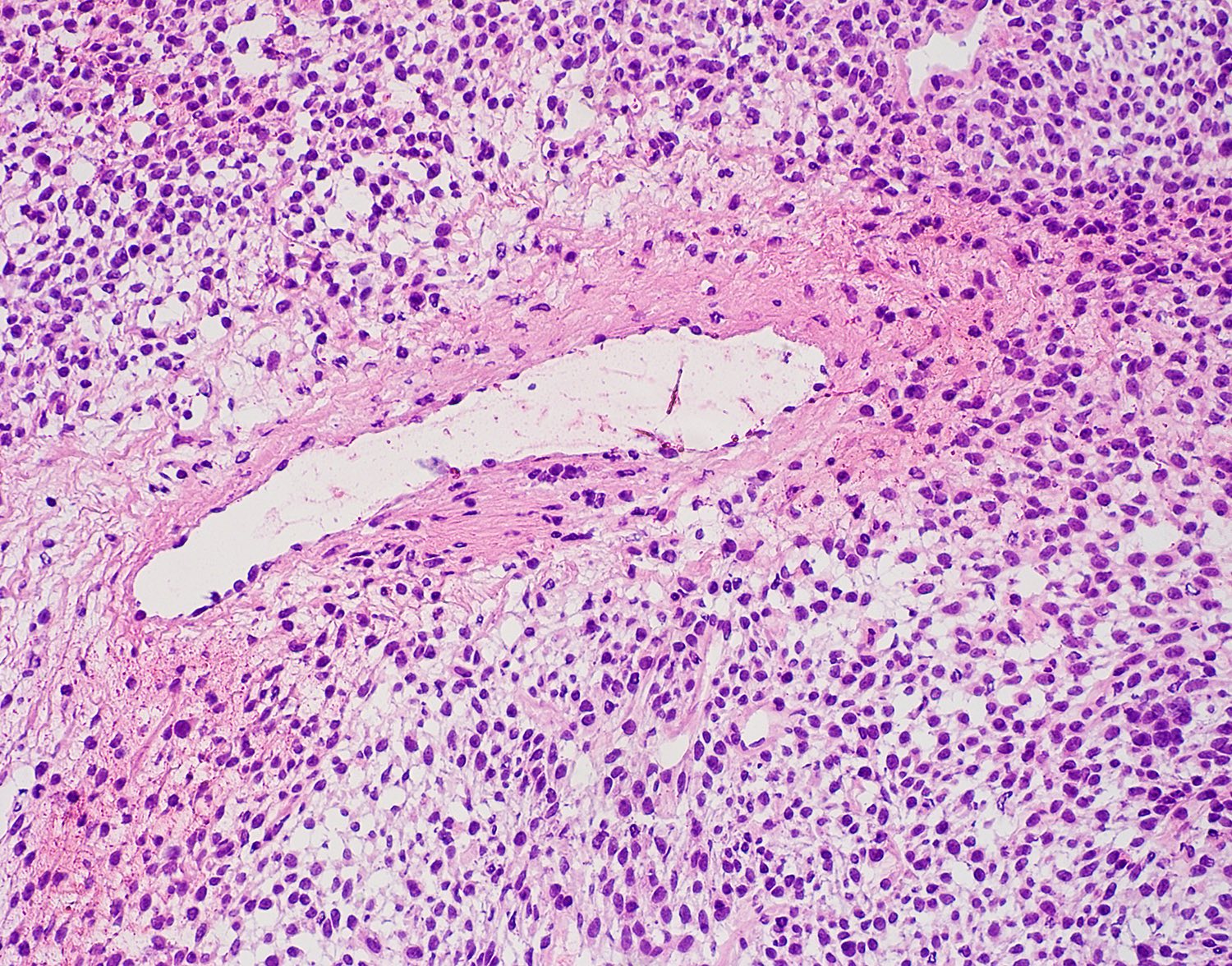
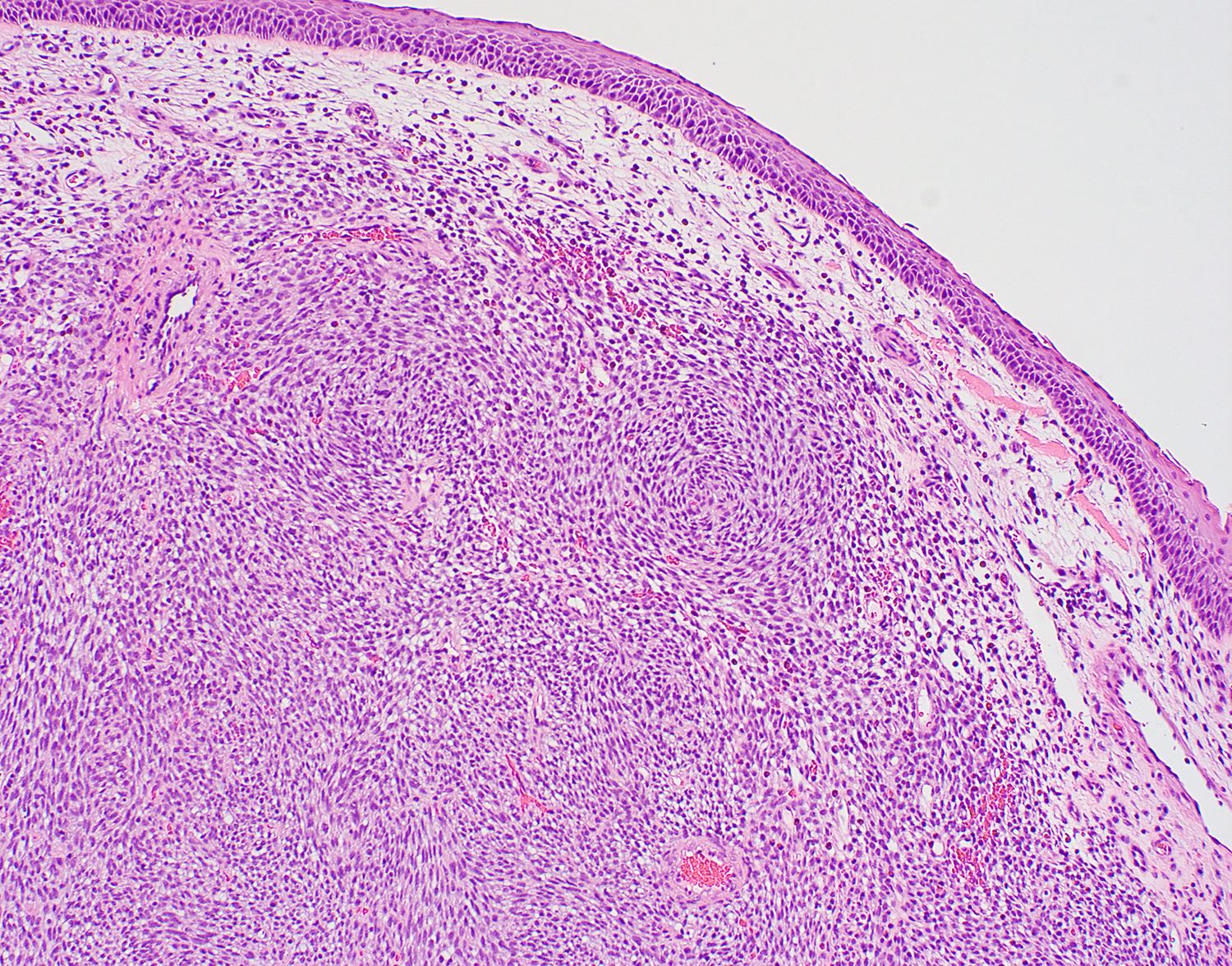
- Low grade sarcoma of the sinonasal tract which features both neural (S100) and myogenic (actin) differentiation and the majority have been associated with PAX3 translocations
Essential features
- Infiltrative, cellular, monomorphic spindled cell neoplasm with herringbone or fascicular architecture
- May have prominent stromal vasculature
- Positive for S100 (focal to diffuse), SMA/MSA, nuclear beta catenin immunohistochemical stains
- Often recurrent PAX3 translocations most commonly partnered with MAML3
Terminology
- Low grade sinonasal sarcoma with neural and myogenic features (Am J Surg Pathol 2012;36:517)
ICD coding
- ICD-10: C30.0 - Malignant neoplasm of nasal cavity and middle ear
Epidemiology
- M:F = 1:3
- Commonly presents in the fifth decade (range: 24 - 85 years) (Virchows Arch 2018;473:615)
- Rare (likely underreported)
Sites
- Sinonasal tract, with the upper nasal cavity or ethmoid sinus being the most commonly involved
- May extend to cribriform plate or orbit
Etiology
- Unknown
Clinical features
- Nonspecific, usually nasal obstructive symptoms manifesting as difficulty breathing, facial pressure, nasal congestion with or without pain
Diagnosis
- CT or MRI of the nasal sinuses
- Diagnosis is by endoscopic biopsy, debulking or surgical resection
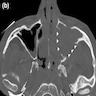
Radiology description
- Heterogenous, enhancing sinonasal tract polyp or mass
- Could present as destructive mass with extension into orbit or anterior skull base (J Neurol Surg Rep 2017;78:e15)
Radiology images
Prognostic factors
- Locally aggressive:
- Local recurrence rate 32 - 44%, majority within 5 years (Am J Surg Pathol 2019;43:747)
- Rare cases of distant metastasis death from disease (~ 2%) (Virchows Arch 2018;473:615)
Case reports
- 35 year old woman with history of right nasal obstruction (Ann Med Surg (Lond) 2018;37:4)
- 39 year old woman with frontal sinus mass who died 8 months after recurrence (Hum Pathol 2016;55:44)
- 47 year old woman with 4.8 cm ethmoid mass (Virchows Arch 2018;473:615)
- 65 year old man presents with epistaxis (Ann Diagn Pathol 2018;33:6)
- 67 year old woman with recurrent nasal polyps (Int J Clin Exp Pathol 2017;10:11743)
Treatment
- Complete surgical resection with or without radiation
Gross description
- Polypoid fragments of tan-yellow to soft gray tissue
Frozen section description
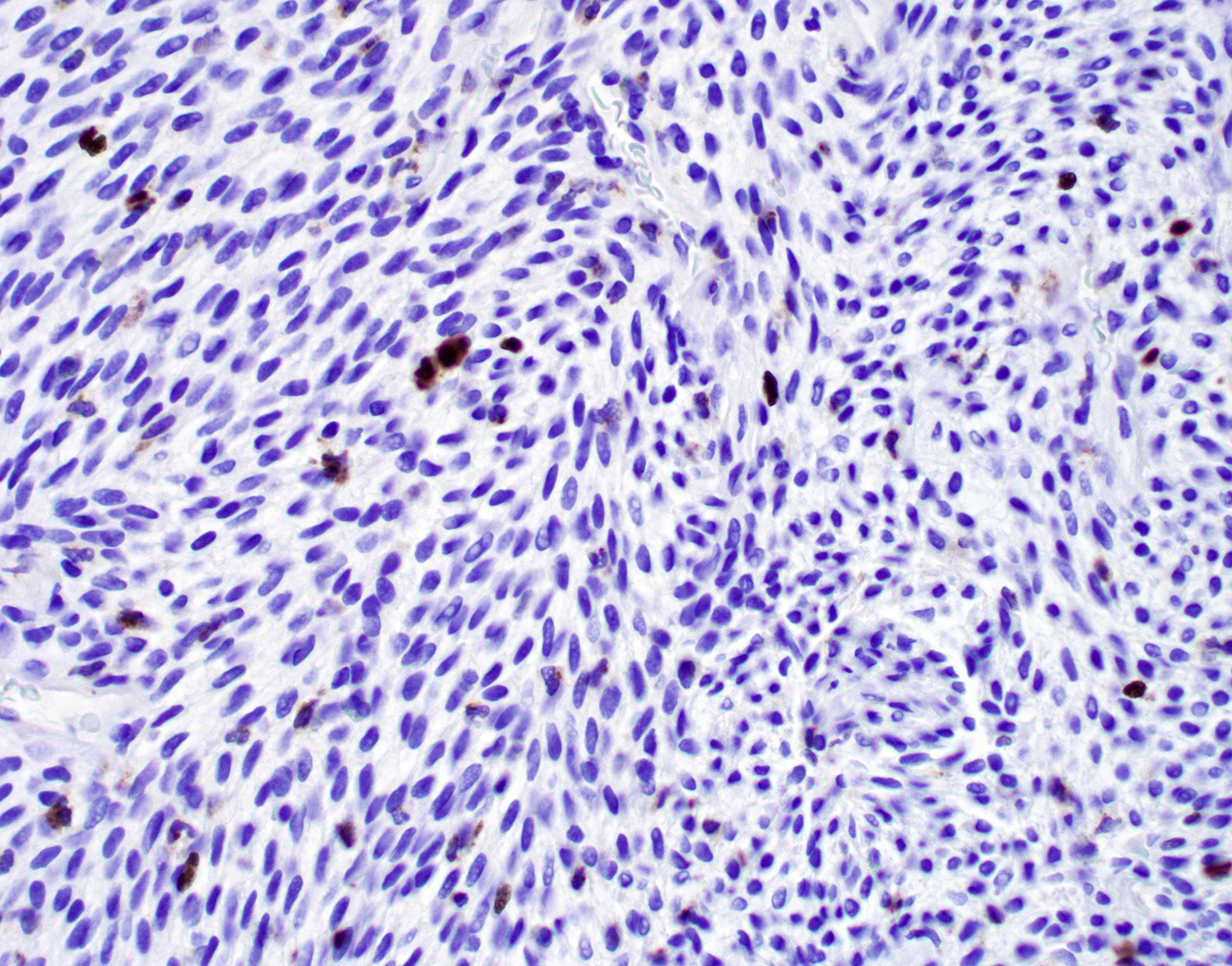
- Diagnostic stromal features could be subtle on frozen sections
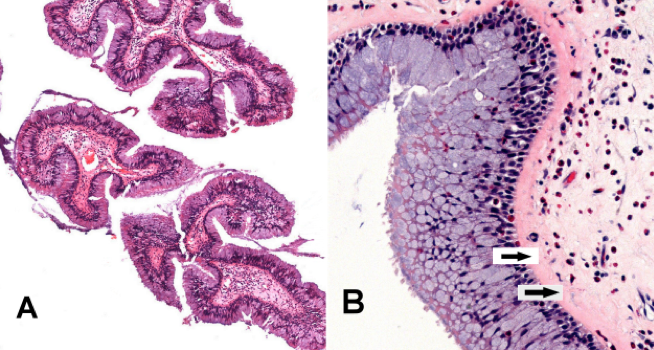
- In the presence of benign epithelial proliferation, could be mistaken for an epithelial tumor such as sinonasal papilloma or respiratory epithelial adenomatoid hamartoma on frozen section (Head Neck Pathol 2020;14:33)
Frozen section images
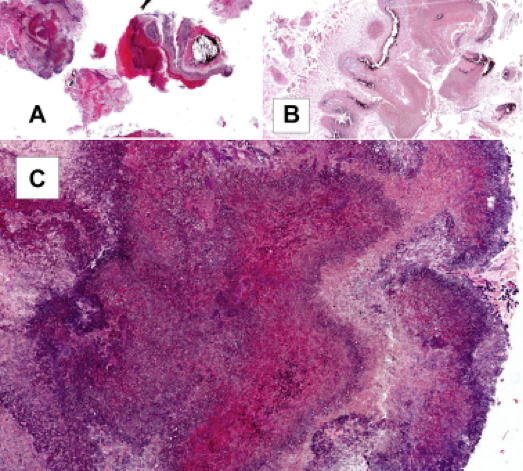
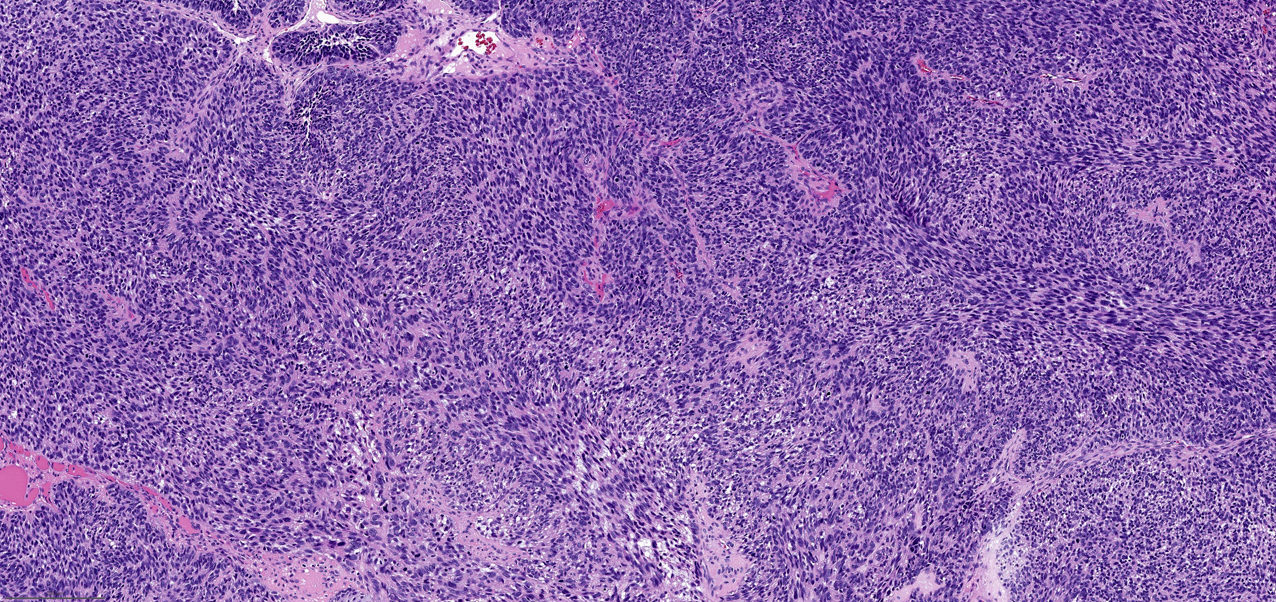
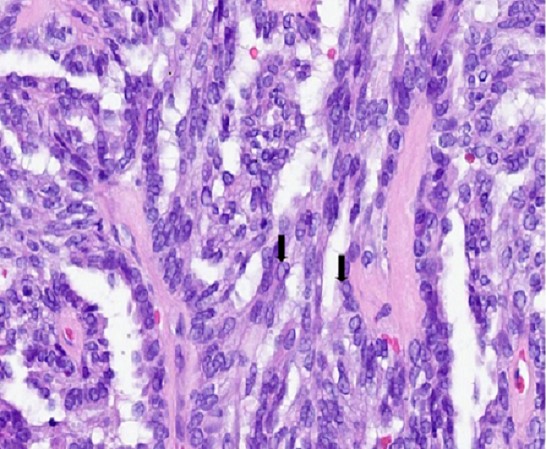
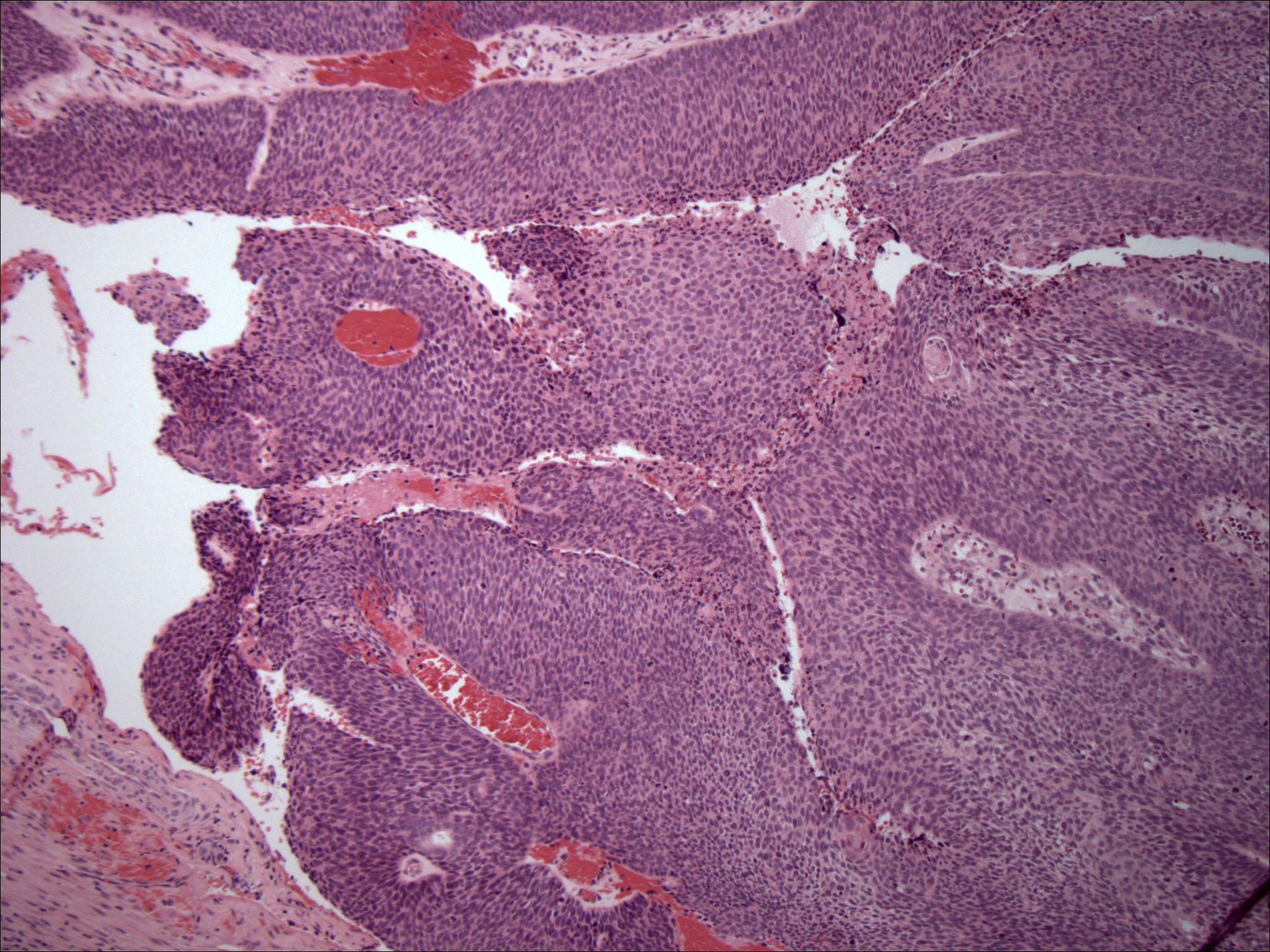
Microscopic (histologic) description
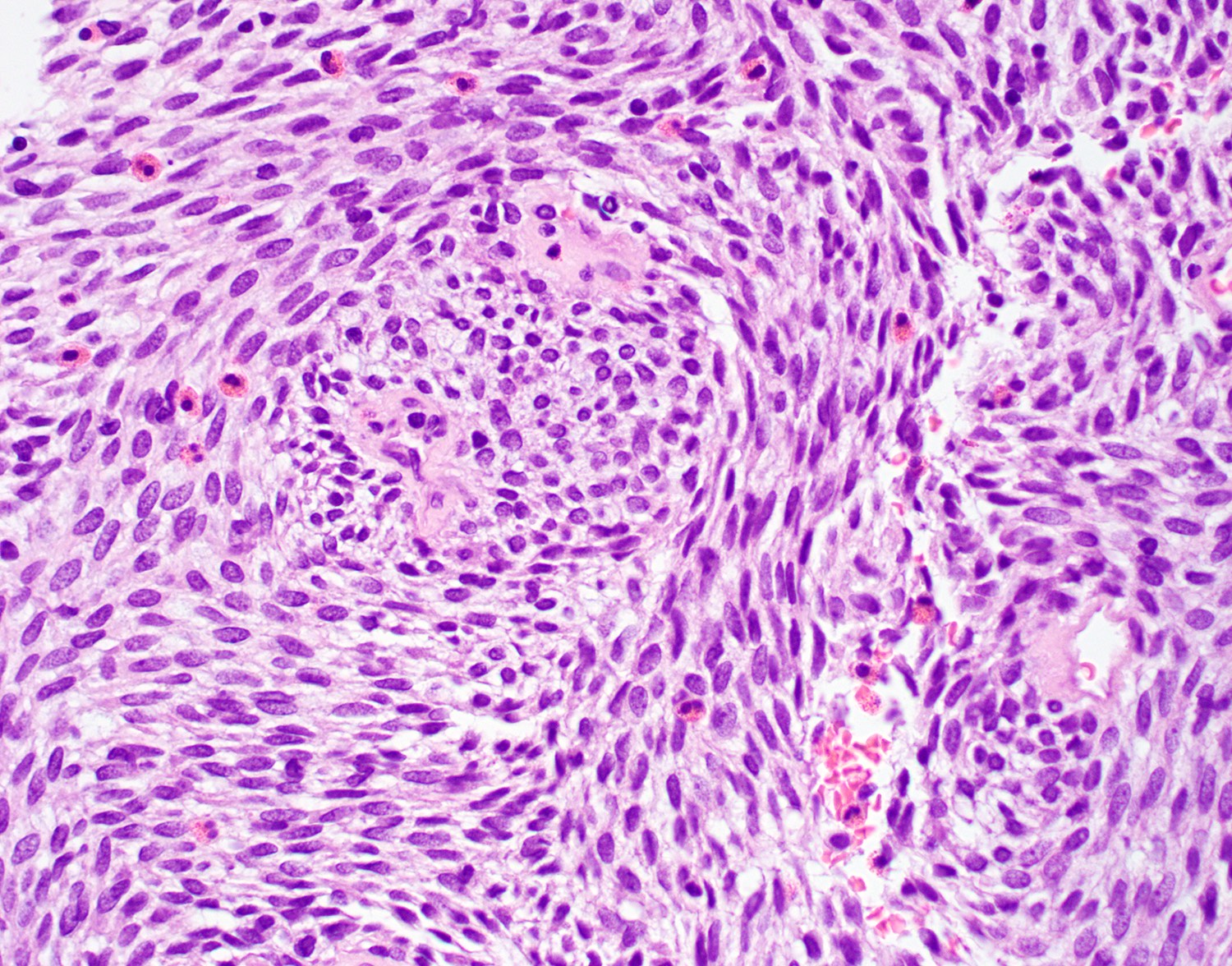
- Infiltrative, cellular spindled cell proliferation
- Medium to long fascicles with areas of herringbone pattern
- Low grade, monotonous spindled cells with ovoid to elongated nuclei and inconspicuous nucleoli
- Usually absence of high grade features such as nuclear pleomorphism, frequent mitoses, significant atypia, necrosis
- Invagination or entrapment of benign surface respiratory epithelial proliferation with or without squamous metaplasia
- Prominent stromal thick walled vasculature, could be hemangiopericytoma-like
- Delicate stromal collagen
- Subset of cases show focal rhabdomyoblastic elements (variable desmin, myogenin, myoD1) (Head Neck Pathol 2020;14:33)
Microscopic (histologic) images
Cytology description
- Bland, uniform population of spindle cells with mildly enlarged, oval to spindle shaped nuclei with fine nuclear chromatin and inconspicuous nucleoli
- Significant nuclear atypia or pleomorphism is typically absent (Diagn Cytopathol 2019;47:507)
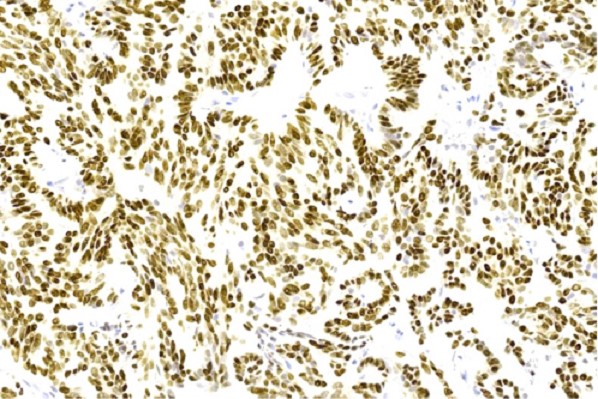
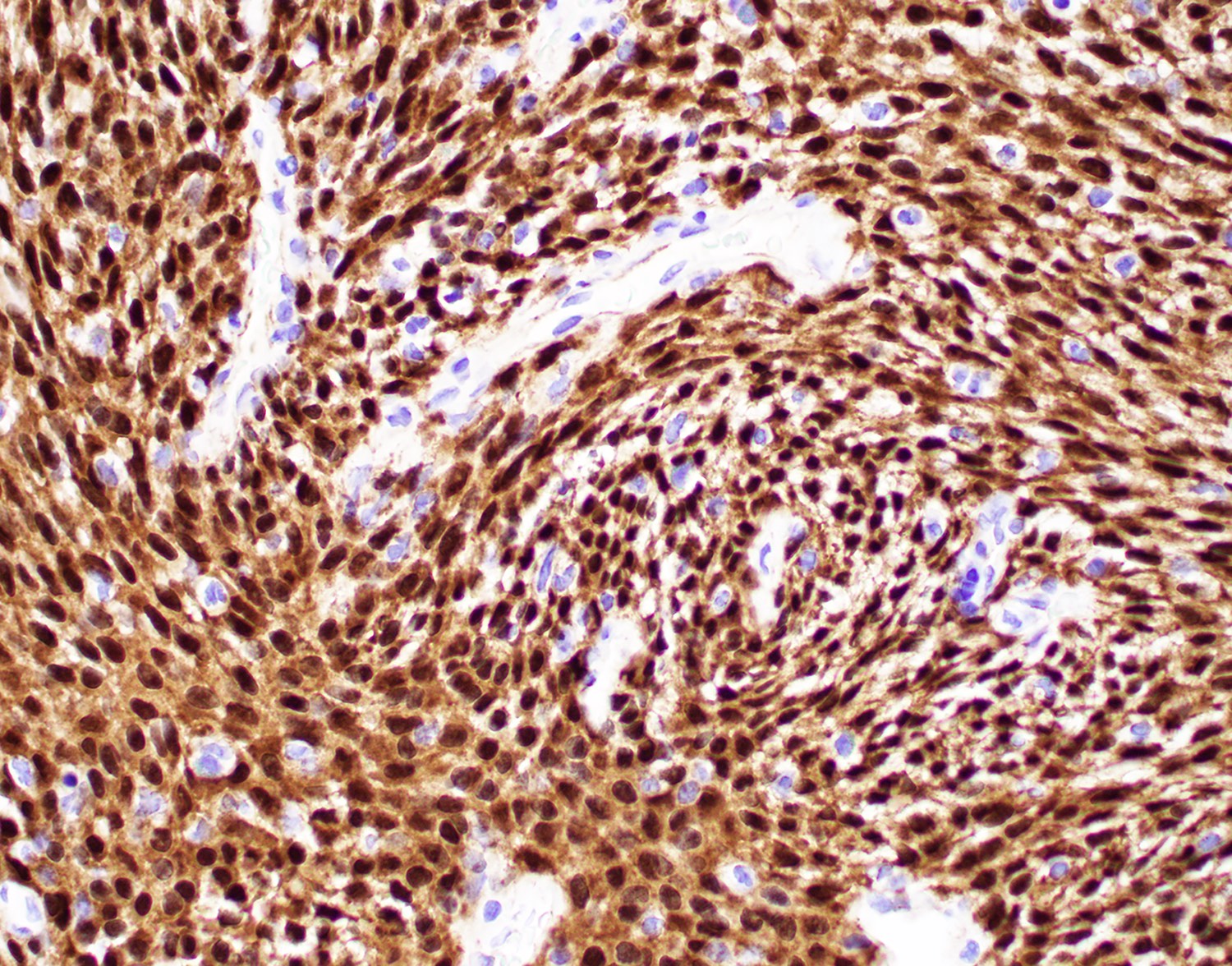
Positive stains
- S100 (may be focal), smooth muscle actin (may be diffuse or focal) or calponin, muscle specific actin (patchy to diffuse), nuclear beta catenin (may be focal), desmin (variable), myogenin (variable) (Ann Diagn Pathol 2018;33:6, Hum Pathol 2016;55:44)
- PAX3 (Am J Surg Pathol 2018;42:1275)
Negative stains
Molecular / cytogenetics description
- Recurrent PAX3 translocations (Am J Surg Pathol 2018;42:1275)
- PAX3 break apart signals on fluorescence in situ hybridization (Histopathology 2016;69:930)
- Most common rearrangement: t(2;4)(q35;q31) resulting in PAX3-MAML3 fusion detected by RNA-seq or RT-PCR (Nat Genet 2014;46:666)
- Other fusions reported: PAX3-NCOA1 (associated with rhabdomyoblastic differentiation), PAX3-FOXO1, PAX3-WWTR1, PAX3-NCOA2 (Am J Surg Pathol 2016;40:51, Genes Chromosomes Cancer 2016;55:25, Am J Surg Pathol 2019;43:747)
Sample pathology report
- Soft tissue mass, right nasal cavity, excision:
- Biphenotypic sinonasal sarcoma (see comment and synoptic report)
- Comment: The nasal cavity mass shows a cellular spindled proliferation with areas showing a herringbone pattern. The spindle cells are low grade with slender to ovoid nuclei and inconspicuous nucleoli. There are invaginations of benign epithelium with squamous metaplasia. The neoplastic spindle cells show patchy S100, SMA and nuclear beta catenin immunohistochemical staining. They are negative for CD34, STAT6, SOX10 and cytokeratin AE1/AE3. These findings support the diagnosis of biphenotypic sinonasal sarcoma, a low-grade spindle cell sarcoma commonly associated with a PAX3-MAML3 gene fusion. This tumor type usually shows slow progressive growth and local invasion. While local recurrence is frequent, distant metastasis is very rare.
Differential diagnosis
- Sinonasal glomangiopericytoma:
- Round to ovoid instead of elongated nuclei
- Diffuse smooth muscle actin and beta catenin nuclear staining positivity
- Absence of herringbone pattern
- Absence of S100 positivity
- Solitary fibrous tumor:
- Schwannoma:
- Malignant peripheral nerve sheath tumor:
- Leiomyoma or leiomyosarcomas:
- Variably cellular proliferation of cells with blunt ended, cigar shaped nuclei
- Positive for smooth muscle actin and desmin
- Absence of S100 positivity
- Spindle cell rhabdomyosarcoma:
- Synovial sarcoma:
- Variable positivity for cytokeratins and TLE1
- Negative for smooth muscle actin and muscle specific actin
- Characterized by t(X;18) SS18-SSX1/2 fusions
- Sinonasal papilloma, inverted type:
- Endophytic growth of markedly thickened squamous epithelial proliferation
- Absence of spindle cell proliferation
- Respiratory epithelial adenomatoid hamartoma (REAH)
- Noninvasive epithelial proliferation with surface invaginations with thickened basement membrane and ciliated respiratory epithelium
- Absence of spindle cell proliferation
Additional references
Board review style question #1
- A 45 year old woman presents with a 3 month history of nasal obstructive symptoms. An MRI of the nasal sinus shows a 3.8 cm polypoid mass in the ethmoid sinus with focal soft tissue extension without bony destruction of the cribriform plate. Histology reveals a low grade, monotonous spindle cell neoplasm. There is patchy immunoreactivity of S100 and SMA and negative staining for CD34, STAT6 and SOX10. Gene rearrangement involving which of the following genes is most commonly associated with this tumor?
- ETV6
- EWSR1
- NUTM1
- NTRK
- PAX3
Board review style answer #1
E. PAX3. Biphenotypic sinonasal sarcoma (shown here) is classically positive for S100 and actin, and is most frequently associated with PAX3 gene rearrangements, most commonly PAX3-MAML3. PAX3-NCOA1 and PAX3-FOXO1 translocations have also been reported.
Comment Here
Reference: Biphenotypic sinonasal sarcoma
Comment Here
Reference: Biphenotypic sinonasal sarcoma
Board review style question #2
- Which of the following features would favor a diagnosis of biphenotypic sinonasal sarcoma?
- Distant metastasis and death within several months of initial diagnosis
- Frequent mitoses and presence of necrosis
- Positive immunohistochemical reactivity for CD34
- Presentation in an adolescent or young male
- Uniform spindle cell proliferation with prominent stromal vasculature
Board review style answer #2
E. Uniform spindle cell proliferation with prominent stromal vasculature. Biphenotypic sinonasal sarcoma typically presents in middle aged women. Histologically, this entity is characterized by a cellular, monotonous spindle cell proliferation with low grade cytology that is positive for S100 and actin but negative for CD34 and STAT6. Prominent stromal vasculature could be present, sometimes reminiscent of hemangiopericytoma. Local recurrence is common but distant metastasis and death from disease remains rare.
Comment Here
Reference: Biphenotypic sinonasal sarcoma
Comment Here
Reference: Biphenotypic sinonasal sarcoma
Chordoma
Chronic rhinosinusitis
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
- Chronic inflammation of the nasal cavity (rhinitis) or the paranasal sinuses (sinusitis), symptoms lasting more than 6 weeks
- Sequel to acute rhinitis (symptoms lasting 6 weeks or less), with development of secondary bacterial infection
- Associated with deviated septum or nasal polyps; also ulceration and infection extending into sinuses
Essential features
- Thickened, hyalinized basement membrane (minimal criteria) directly beneath respiratory epithelium and around seromucinous glandular tubuli
Terminology
- Rhinosinusitis, sinusitis
Epidemiology
- Most common health problem in the United States
Sites
- Unilateral or bilateral, nasal cavity or paranasal sinus
Pathophysiology
- Associated with deviated septum or nasal polyps
- Ostial obstruction in osteomeatal compex causes anaerobic overgrowth
Etiology
- Allergy, vasomotor (constricted or dilated vessels), infection, diabetes mellitus, cystic fibrosis, Kartagener syndrome, aspirin intolerance, Churg-Strauss disease, nickel exposure
Clinical features
- Facial pain, pressure, congestion or fullness; nasal obstruction, blockage, discharge or purulence
Treatment
- Aeration or drainage, ensuring ostial patency
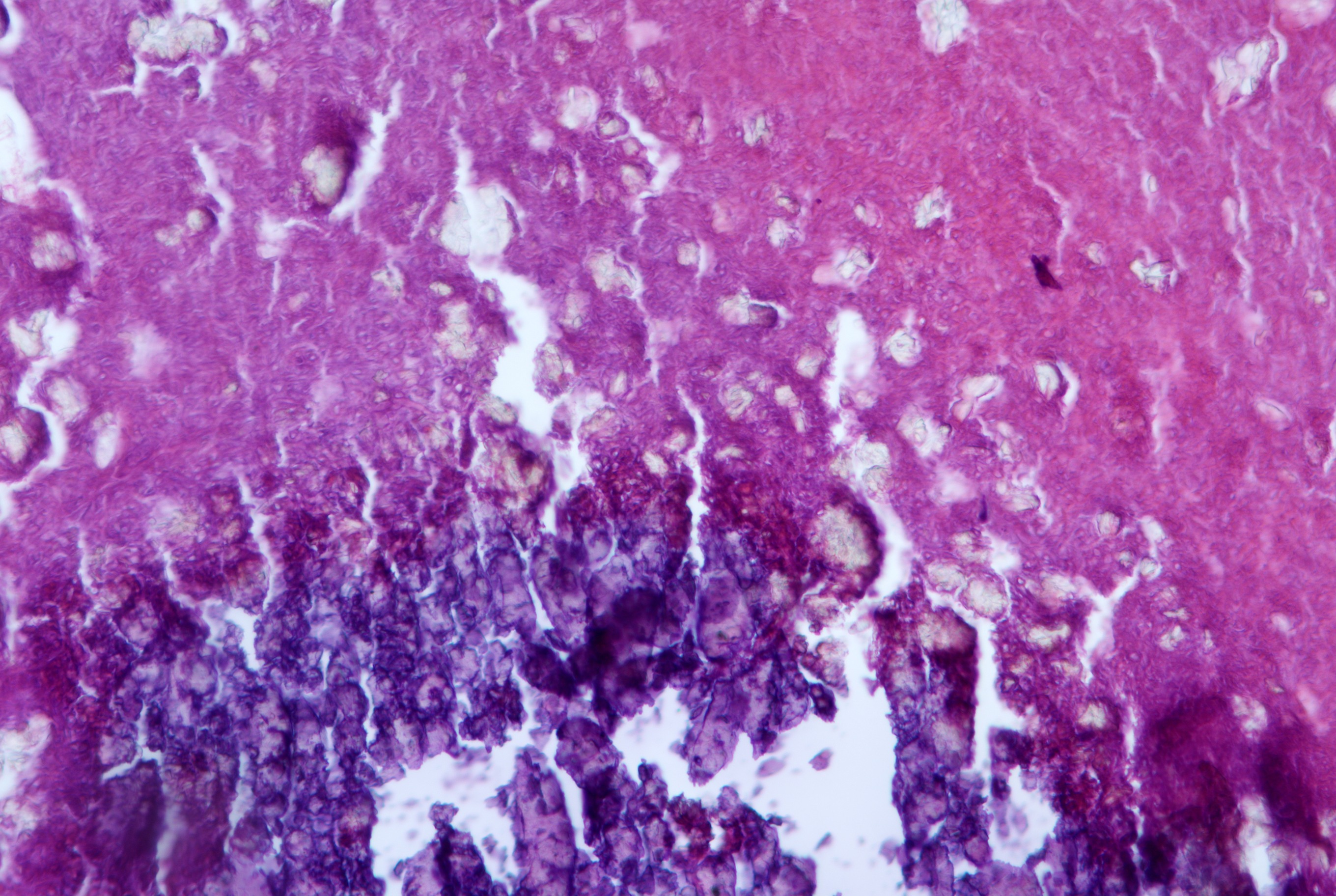
Microscopic (histologic) description
- Thickened basement membrane (minimal criteria) directly beneath respiratory mucosa and around seromucinous glandular tubuli
- Increased lymphoplasmacytic infiltrate
- Goblet cell hyperplasia and papillary hyperplasia
- Squamous metaplasia can be seen and is associated with cigarette exposure
Microscopic (histologic) images
Differential diagnosis
- Chronic allergic sinusitis
- Chronic infectious sinusitis
Additional references
Fungal ball
Table of Contents
Definition / general | Essential features | Terminology | Sites | Pathophysiology / etiology | Clinical features | Radiology description | Radiology images | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosis | Additional referencesDefinition / general
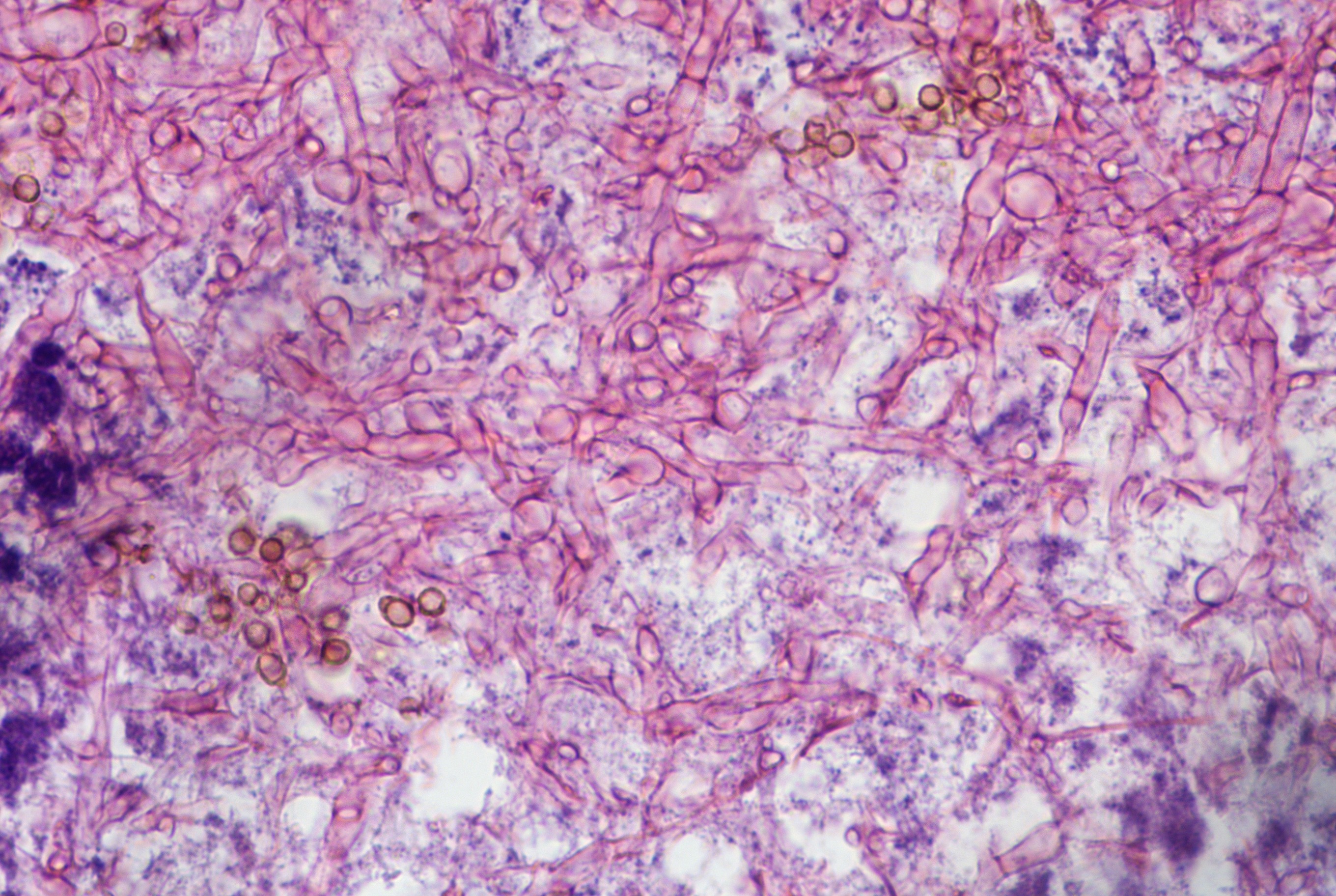
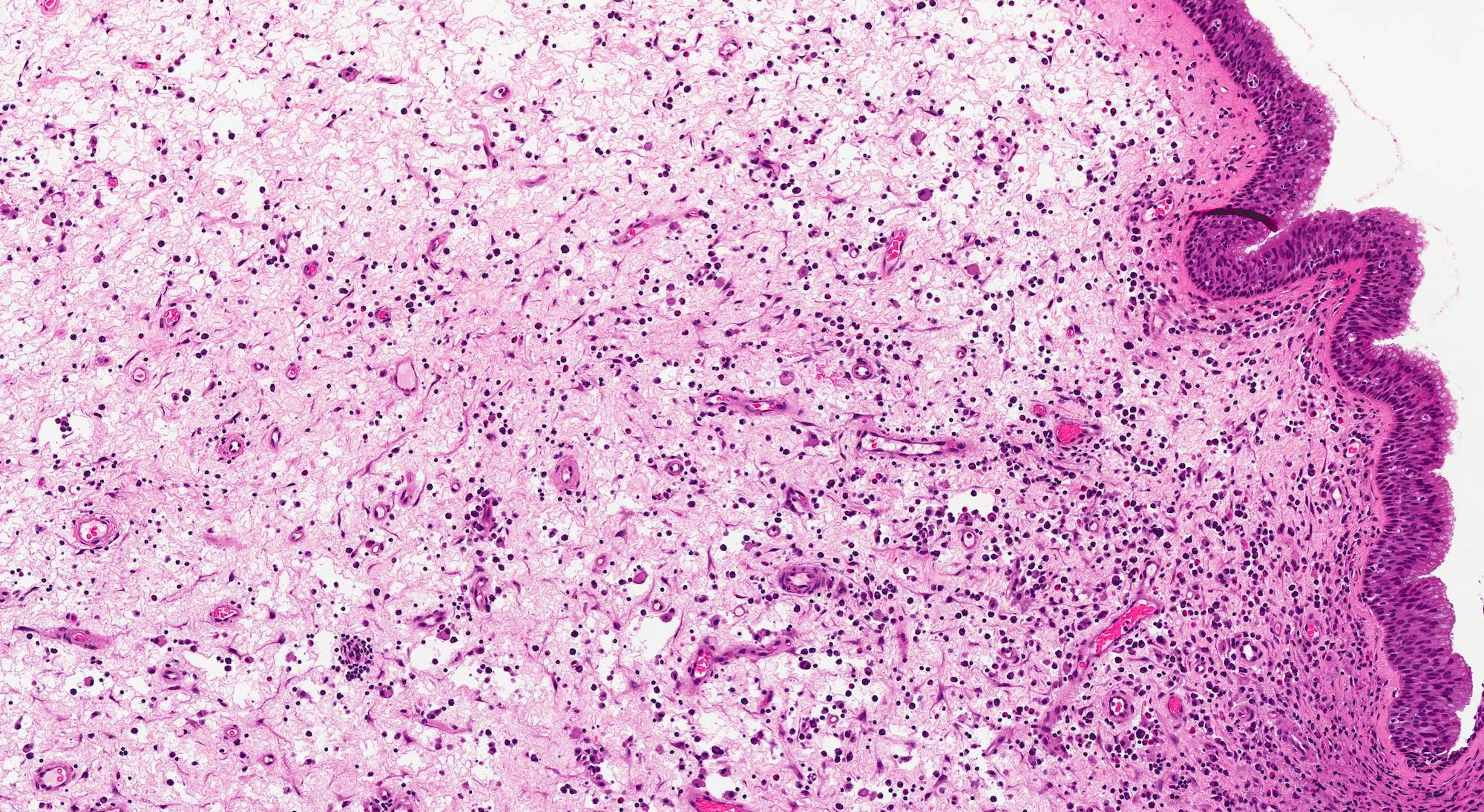
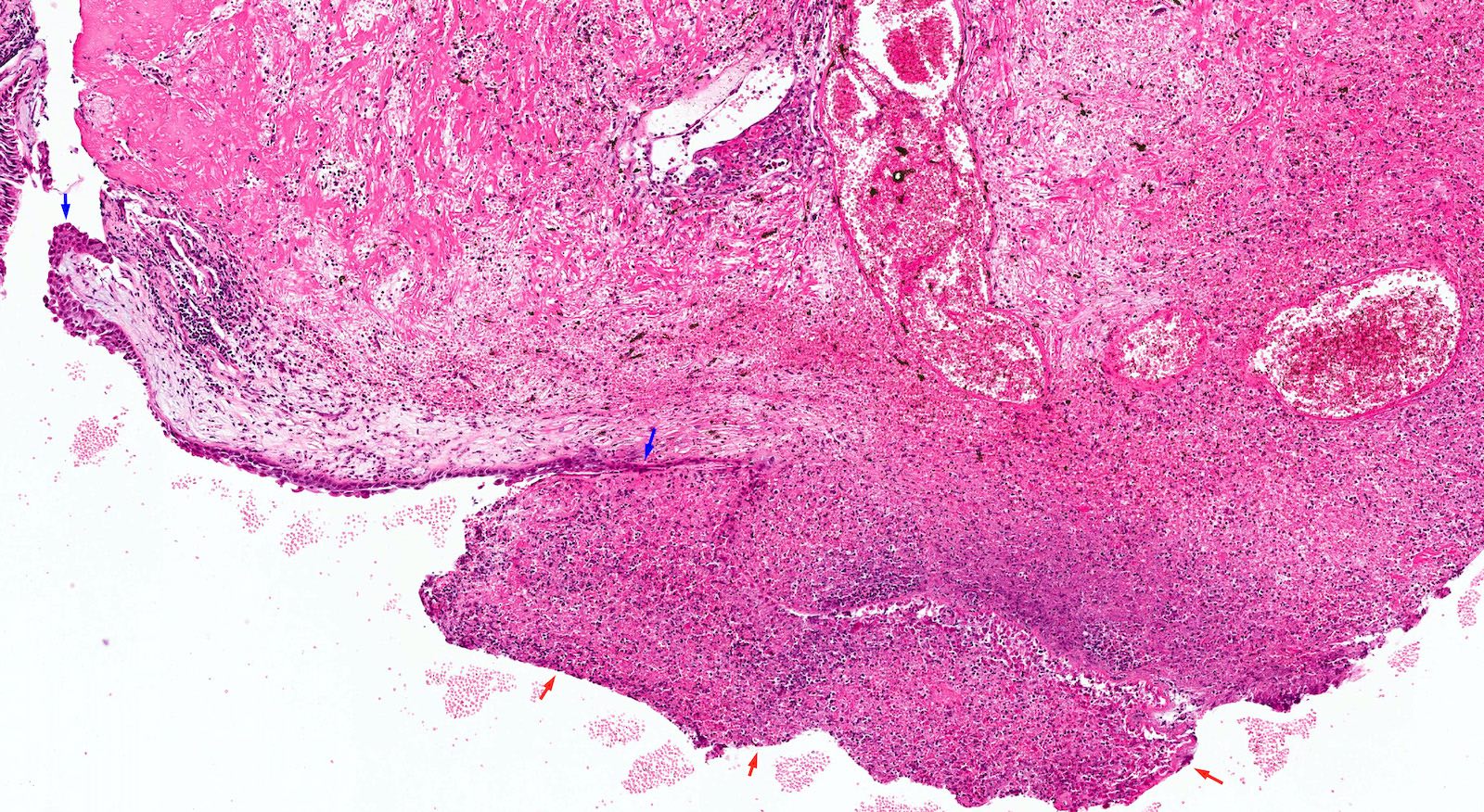
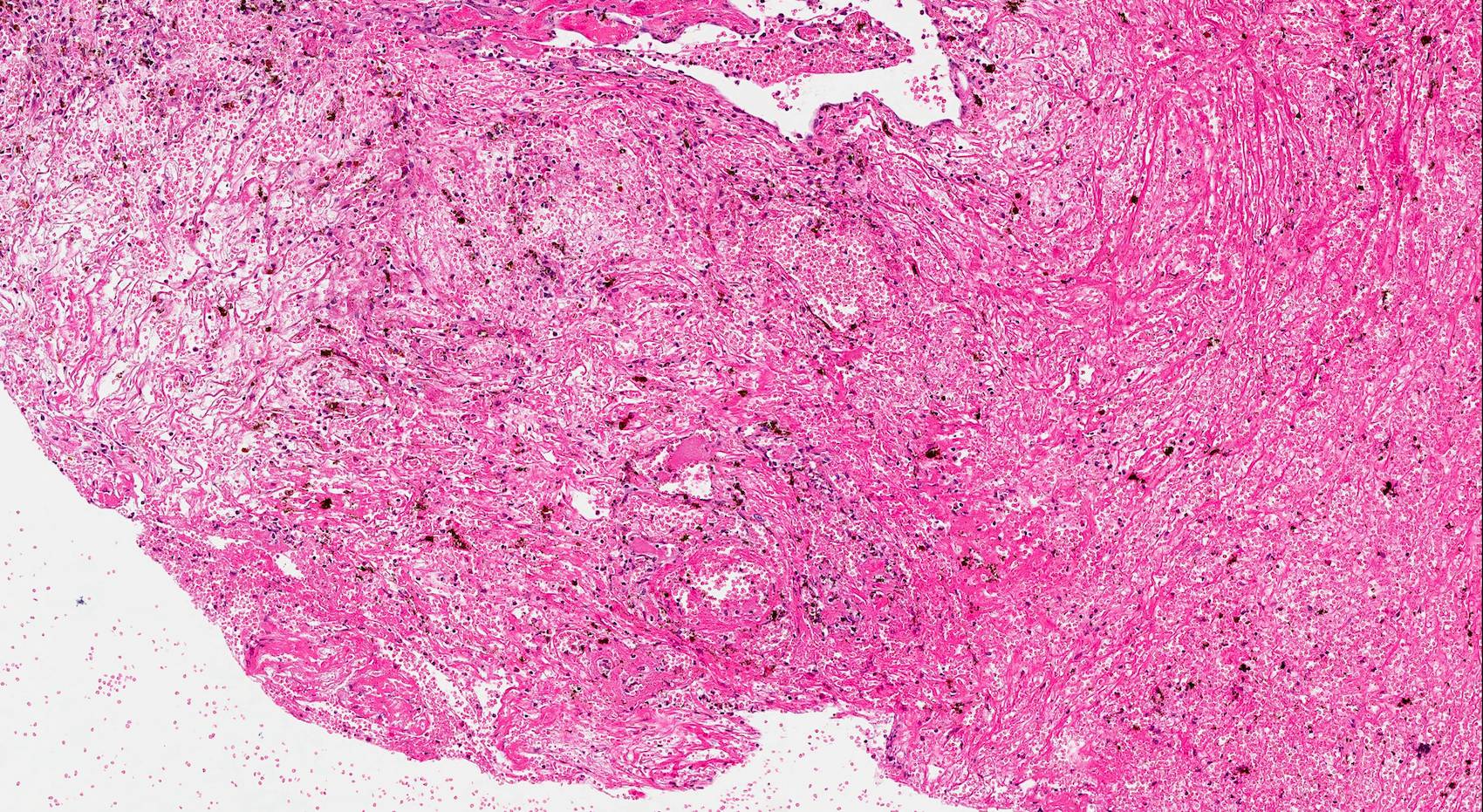
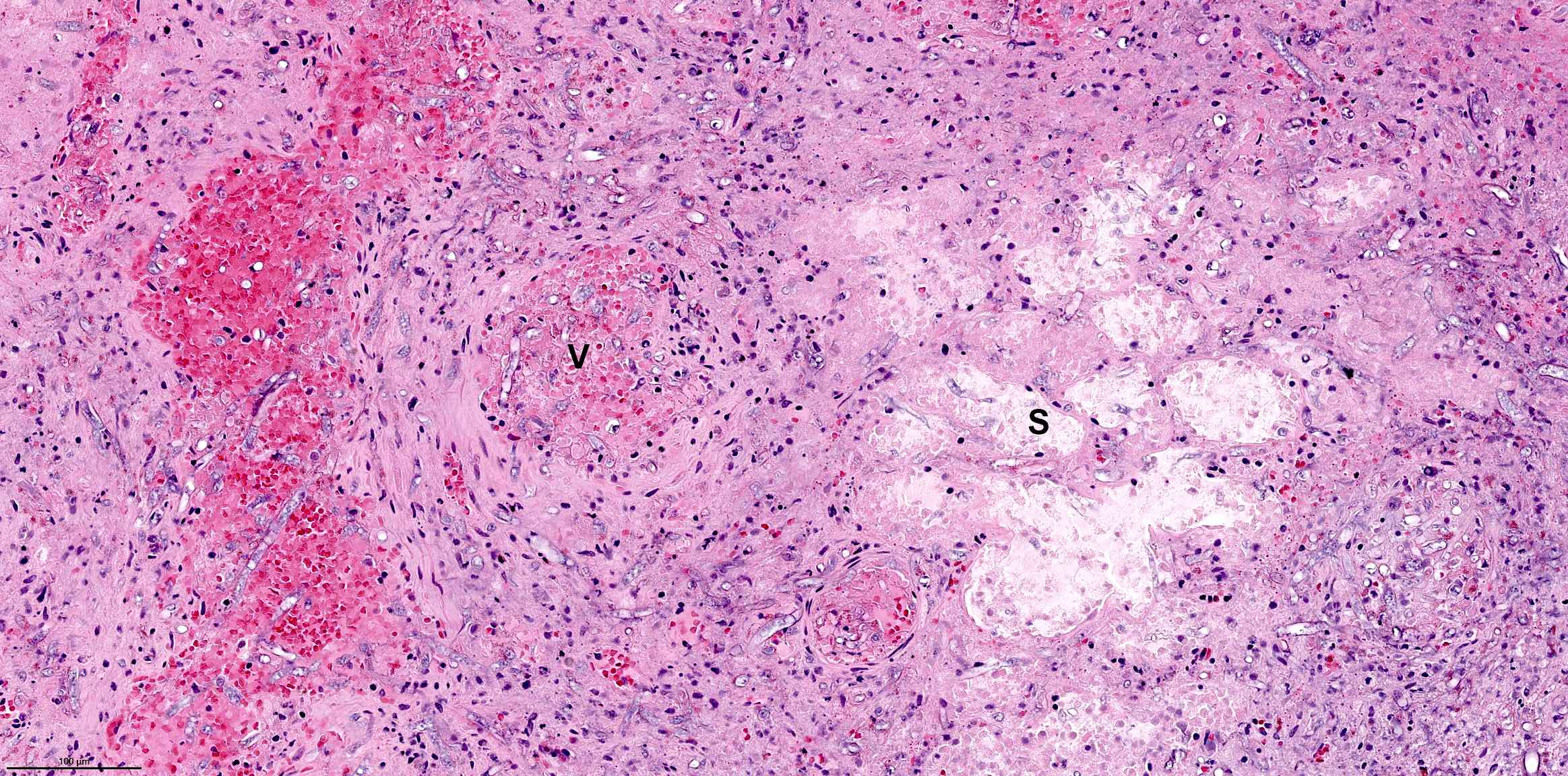
- Noninvasive accumulation of fungal hyphae that branch at 45 degrees
- Aspergillus causes fungus balls in nasal antrum of immunocompetent patients with minimal inflammatory response, microabscesses or multinucleated giant cells
- Also causes invasive aspergillosis, regardless of immune status, with extension into retroorbital region, cranium or parapharyngeal space; often fatal
- Also causes allergic fungal sinusitis
Essential features
- Dense fungal growth with no tissue invasion
- Fruiting heads (sexual reproduction) may be seen
Terminology
- Fungal ball, mycetoma, chronic noninvasive fungal sinusitis
Sites
- Maxilla is most commonly affected
Pathophysiology / etiology
- A. fumigatus and A. flavus are the most common isolates
- Usually immunocompetent patients, often prior history of sinus disease, trauma or foreign body
Clinical features
- Nasal congestion / obstruction
- Sinus pain
Radiology description
- Expansile massive process with bony remodeling
- MR / CT signaling reflects the iron, manganese and calcium content of fungal hyphae ("iron-like signalling")
Radiology images
Case reports
- 70 and 78 year old women with fungus ball of the paranasal sinuses (Int Arch Otorhinolaryngol 2012;16:286)
Treatment
- Conservative curettage, irrigation with saline or iodine solution, surgery
Gross description
- May present as a large, expansile mass, without involvement of the underlying mucous membrane
- Grumous, friable, gray-brown-black mass, often with clotted blood
Gross images
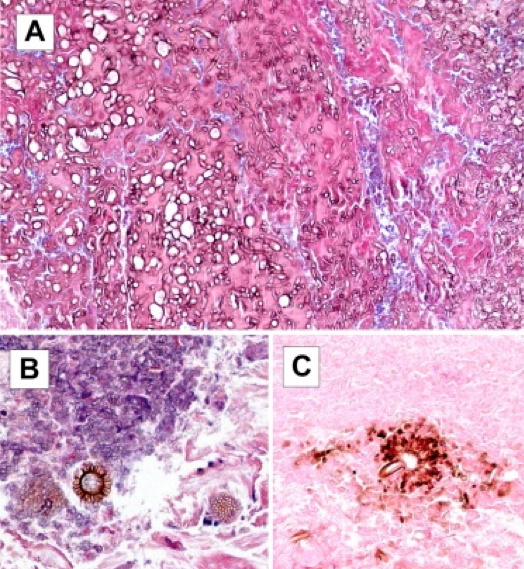
Microscopic (histologic) description
- Tightly packed laminated hyphae with inflammatory exudates and cell debris
- Pigmented hyphae may be dematiaceous group of fungi
- Presence of characteristic fruiting heads is diagnostic for Aspergillus sp
- Black conidia specifically indicate Aspergillus niger
- Fungal invasion of tissue is usually not seen, although it has been reported
- No / minimal host response in mucosa
Microscopic (histologic) images
Contributed by Margie Brandwein-Gensler, M.D. and @Andrew_Fltv on Twitter
Images hosted on other servers:
Differential diagnosis
- Chronic sinusitis
- Sinonasal neoplasm
- Of fungal infections:
- Alternaria
- Cladosporium trichoides
- Fusarium
- Paecilomyces
- Pseudallescheria boydii
- Zygomycetes
Additional references
Glial heterotopia
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Glial heterotopia is a benign, congenital, nonneoplastic displacement of mature neuroglial tissue at extracranial sites without intracranial connection (Acta Otorhinolaryngol Ital 2022;42:317, Int J Pediatr Otorhinolaryngol 2020;129:109728, J Cutan Aesthet Surg 2020;13:233)
- Very rare malformation that causes tumor-like conditions in newborns and older infants
Essential features
- Most frequent in the nasal area
- Mature brain tissue in the nasal cavity without connection to the dura / meninges and absence of a skull defect
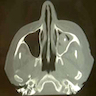
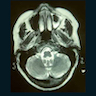
- Radiography is essential to rule out communication of mass with intracranial space and assess bony defects (J Cutan Aesthet Surg 2020;13:233, Medicine (Baltimore) 2018;97:e12000)
Terminology
- Acceptable: ectopic glial - meningeal tissue, neuroglial heterotopia, nasal glial heterotopia, nasal glioma
- Not recommended: heterotopic brain choristoma, ectopic brain tissue
ICD coding
Epidemiology
- Incidence of congenital nasal masses is 1 in 20,000 - 40,000 live births; nasal glial heterotopia accounts for ~5% of them (Head Neck Surg 1980;2:222, Clin Otolaryngol Allied Sci 1982;7:87)
- Rare association with brain or systemic anomalies
- Most patients are diagnosed at birth or before the age of 3 (Int J Pediatr Otorhinolaryngol 2020;129:109728)
- 8% of cases are diagnosed in adults (Acta Otorhinolaryngol Ital 2022;42:317)
- M:F = 3:2
Sites
- Based on location related to nose, glial heterotopia can be
- Intranasal: located in the nasal cavity or sinuses
- Extranasal: protruding from the nasal root within the subcutaneous tissue of the nose
- Mixed type: present in both locations (Int J Pediatr Otorhinolaryngol 2020;129:109728)
- Bridge of nose is the most commonly involved site (Int J Pediatr Otorhinolaryngol 2020;129:109728)
- Rare occurrence at other sites, including orbit, lung, uterine cervix, endometrium, lip, tongue, pharynx, tonsil, palate and maxilla (Ophthalmic Plast Reconstr Surg 2020;36:2, J Cutan Aesthet Surg 2020;13:233, Ophthalmic Plast Reconstr Surg 2015;31:e26, Arch Ophthalmol 2003;121:119)
Pathophysiology
- Congenital, nonhereditary malformation
- Development of nasal cerebral heterotopia is embryologically similar to that of nasal encephalocele or dermoid
- During retraction of the embryonic dural diverticulum, remnants of neural glial tissue become sequestered when their connections to the subarachnoid space are pinched off and obliterated
- Lack of subarachnoid communication distinguishes heterotopia from anterior encephalocele
- Distinction between glioma and encephalocele is not possible based on histopathologic findings because glial tissue may be the predominant or exclusive component in both types of lesions (Acta Otorhinolaryngol Ital 2022;42:317)
Etiology
- See Pathophysiology
Clinical features
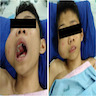
- Intranasal: nasal congestion, mass, obstruction or deformity, may be asymptomatic (Ann Diagn Pathol 2003;7:354)
- Extranasal: firm, smooth masses that do not pulsate or expand during crying, coughing or straining (Acta Otorhinolaryngol Ital 2009;29:218)
Diagnosis
- Diagnosis requires strong clinical and radiologic correlation in addition to histology of lesion
- Magnetic resonance imaging (MRI) to exclude communication with the central nervous system and plan a surgical approach
- Additional computed tomography (CT) performed if bony involvement is suspected or necessary for endoscopic surgery (Acta Otorhinolaryngol Ital 2022;42:317)
Radiology description
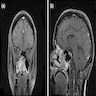
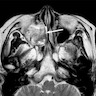
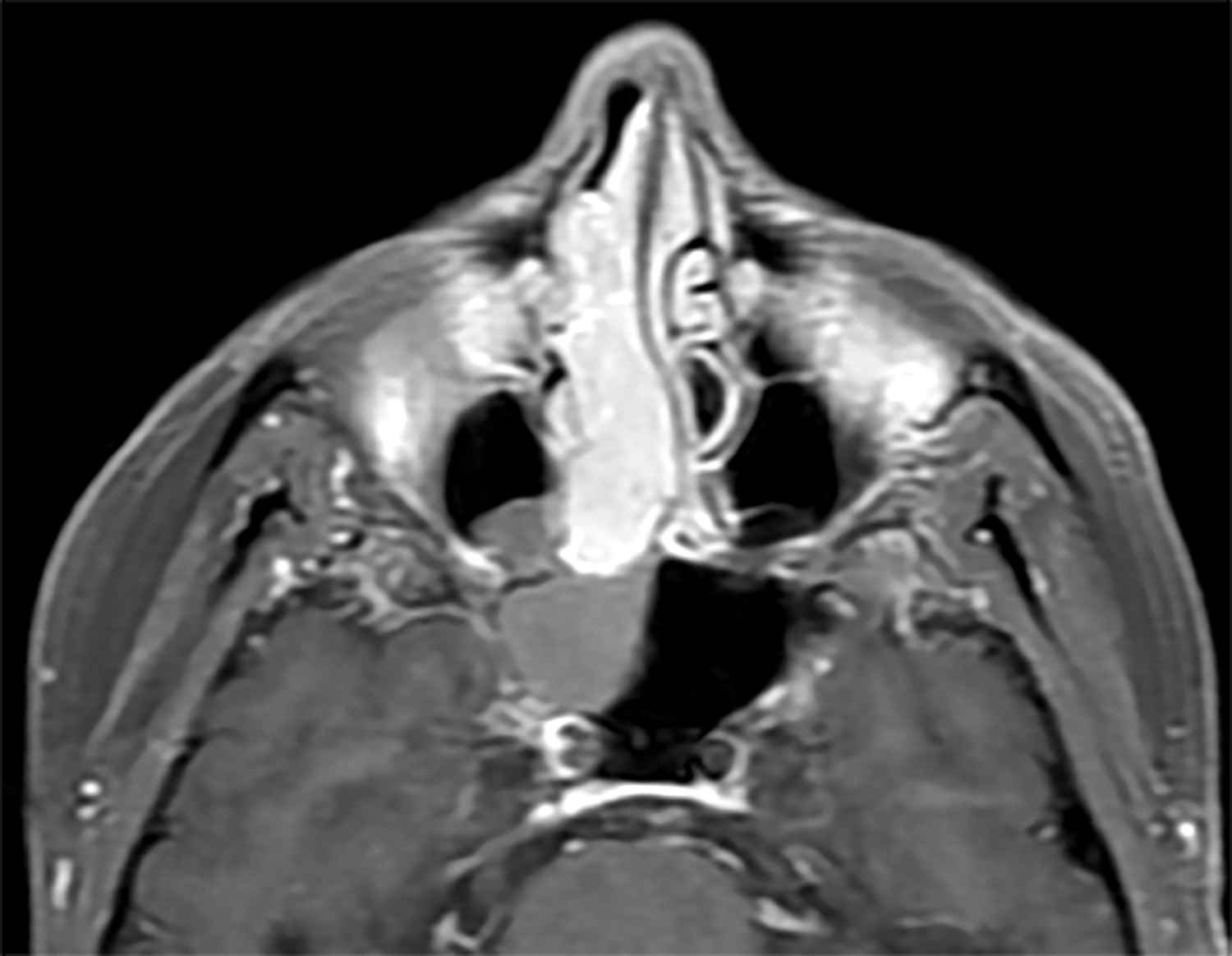
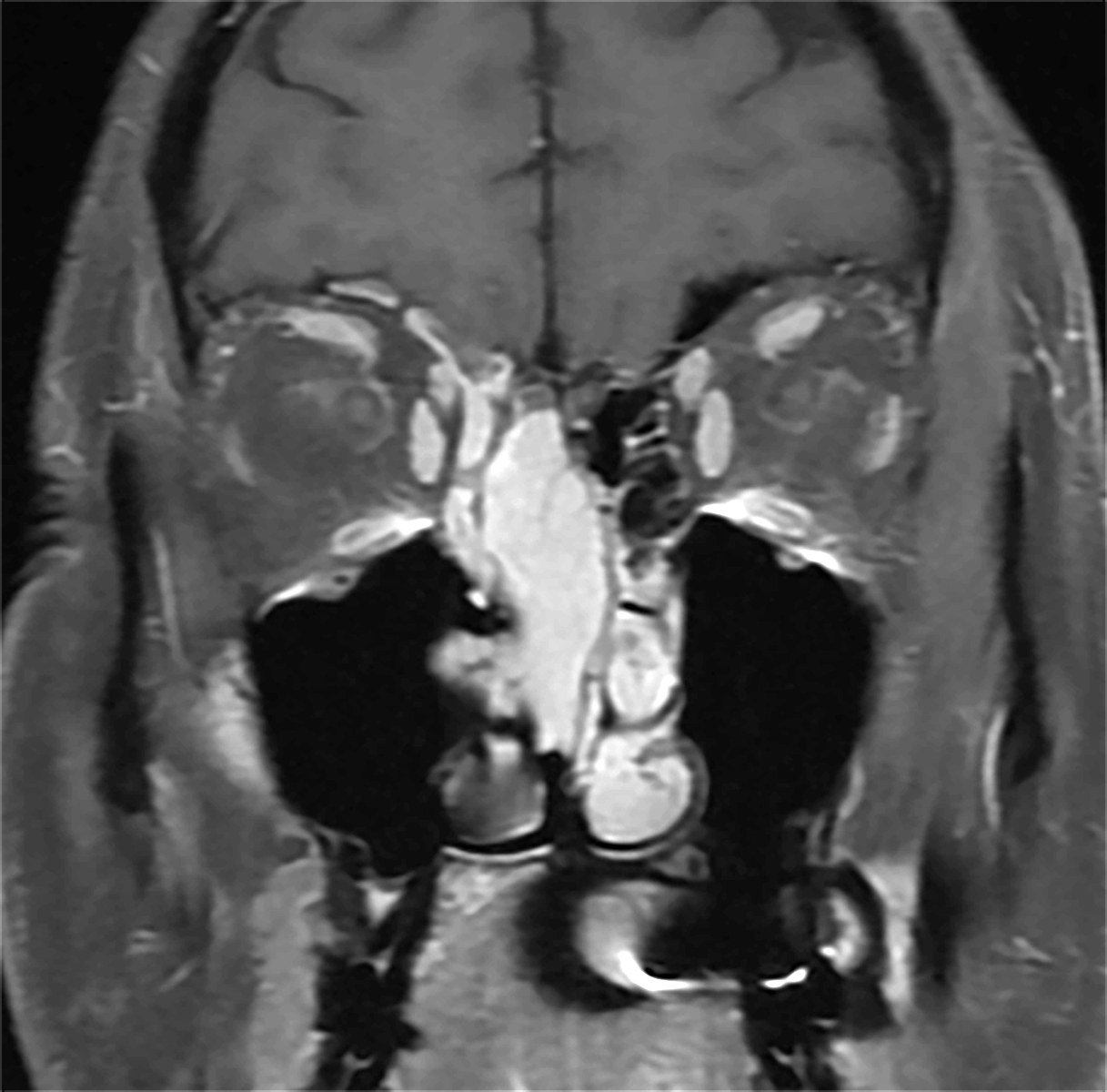
- Radiology is necessary to determine the location of the mass and its relationship to the skull base
- MRI characteristics of glial heterotopia resemble normal brain tissue in all pulse sequences
- Cystic elements might be present and represent cerebrospinal fluid (CSF)-like fluid filled spaces
- MRI findings (J Cutan Aesthet Surg 2020;13:233, Acta Otorhinolaryngol Ital 2022;42:317)
- Circumscribed, ovoid soft tissue intensity lesion
- Isointense signal in T1 weighted imaging
- Heterogeneously hyperintense signal in T2 weighted imaging
- Moderate enhancement postintravenous gadolinium contrast
- No connection between the mass and the dura or brain
- Noncontrast computed tomography (NCCT): soft tissue density, space occupying lesion with no communication with nasal cavity or brain (J Cutan Aesthet Surg 2020;13:233)
Radiology images
Prognostic factors
- Benign lesion with good prognosis (Int J Pediatr Otorhinolaryngol 2020;129:109728)
- Local recurrence rate: up to 30% with incomplete excision
- Histological evaluation of surgical margins can reduce the risk of recurrence
- Most recurrences occur within the first 12 months
Case reports
- 2 month old boy presented with nasal obstruction and shortness of breath since birth (Medicine (Baltimore) 2020;99:e21200)
- 6 month old infant with a congenital mass located at the root of the nose (J Cutan Aesthet Surg 2020;13:233)
- 16 year old girl presented with sore throat and feeling of lump in her throat (BJR Case Rep 2020;6:20190116)
- 37 year old man presented with right nasal obstruction, epistaxis and headache (Head Neck 2008;30:549)
Treatment
- Complete surgical resection is the curative treatment (Int J Pediatr Otorhinolaryngol 2020;129:109728, J Cutan Aesthet Surg 2020;13:233)
- Surgical approach depends on the location and extent of lesion: external rhinotomy, endoscopic resection or combined approach are the most common techniques
Clinical images
Gross description
- Size range: 1 - 3 cm; can grow up to 7 cm
- Firm, globular to polypoidal smooth mass with homogenous gray to pearly white cut surface (J Cutan Aesthet Surg 2020;13:233)
- Grows slowly in proportion to adjacent tissue
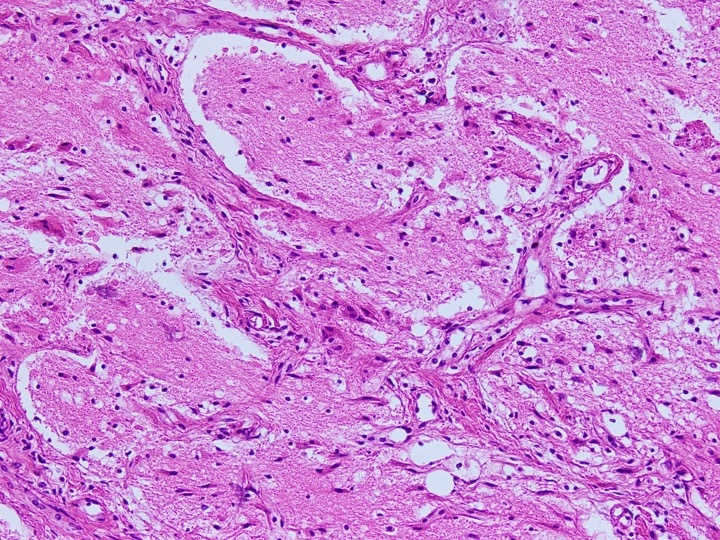
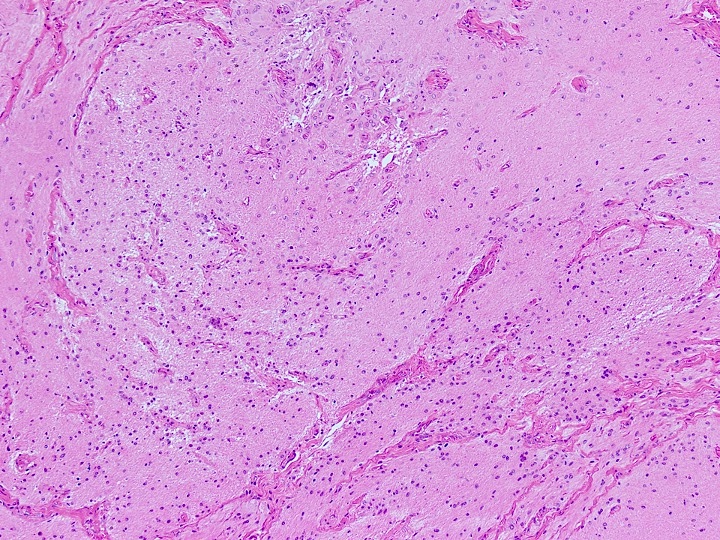
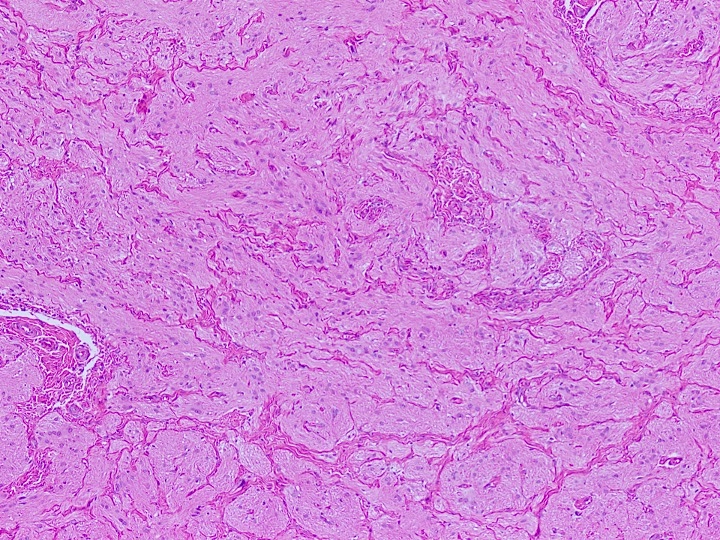
Microscopic (histologic) description
- Nonencapsulated lesion with ill defined edges
- Composed of variable sized mats and nests of benign and mature brain tissue in a background of vascularized connective tissue
- Histologically, brain tissue shows a predominant glial component and minimal neuronal component if present
- Glial component shows evenly dispersed astrocytes in a fine, neurofibrillary matrix
- Interspersed large gemistocytes, ependyma, choroid plexus, retinal epithelium, cerebellar tissue and leptomeninges may be present (J Cutan Aesthet Surg 2020;13:233)
- Rare proliferation of eccrine ducts
- Longstanding lesions show fibrosis and collagen
- Large amount of fibrotic tissue can undermine the glial tissue in H&E sections
- Inflammation, focal calcification may be seen
- Mitoses are absent
Microscopic (histologic) images
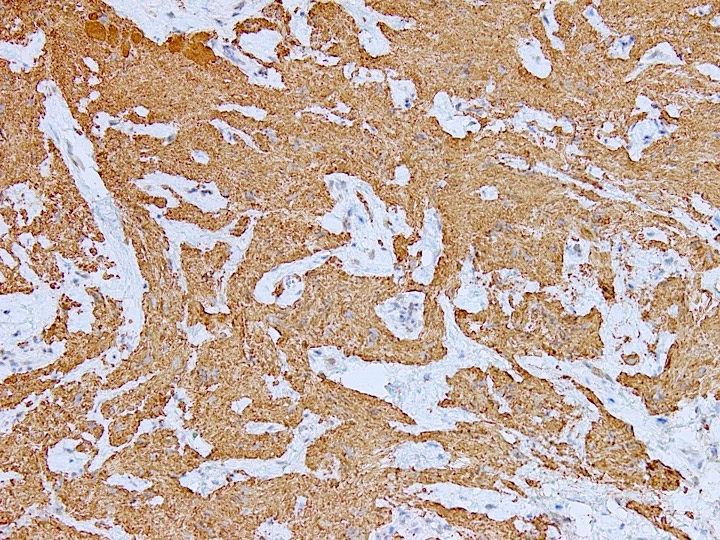
Positive stains
- GFAP (cytoplasmic) (Br J Ophthalmol 1993;77:817)
- S100
- Neurofilament: stains axons
- NeuN: stains neurons
- SSTR2A: highlights leptomeninges and arachnoid cells (Childs Nerv Syst 2022;38:63)
- Special stain Masson trichrome shows red staining of the glial tissue, whereas the background fibrosis appears blue (J Cutan Aesthet Surg 2020;13:233)
Negative stains
Molecular / cytogenetics description
- Not required for routine diagnosis
- Copy number profiling from global DNA methylation data showed a balanced genome
- One case showed significant copy number variation involving loss of chromosomes 16, 17 and 19 and gain of chromosomes 4 and 5 (Childs Nerv Syst 2022;38:63)
Sample pathology report
- Nasal swelling, excision:
- Glial heterotopia, intranasal type (see comment)
- Resection margin is free
- Comment: The tissue shows respiratory epithelium covered unencapsulated polypoid lesion with poorly defined borders. The lesion is composed of nests and patches of mature glial tissue showing evenly dispersed astrocytes in a fine, neurofibrillary matrix. Mitosis is absent. Based on combined radiologic features and histologic findings, the lesion is best characterized as nasal glial heterotopia. This lesion is typically benign with recurrence associated with incomplete excision.
Differential diagnosis
- True encephalocele:
- Extracranial hernias of the meninges or brain caused by congenital defects in the skull
- Intracranial connection present (Medicine (Baltimore) 2018;97:e12000)
- Histologically similar to heterotopia; imaging required for correlation
- Due to shared molecular alterations in a subset of nasal glial heterotopia and encephalocele, there is a strong suggestion that these 2 entities represent the same histopathologic entity (Childs Nerv Syst 2022;38:63)
- Teratoma:
- Congenital or developmental tumor derived from multipotent cells
- Extremely rare (2%) in head and neck region (Int J Pediatr Otorhinolaryngol 2015;79:1991)
- May present as a congenital nasal midline mass
- Differentiate into diverse types of tissue derived from the 3 germ layers (Chest 2005;128:2893)
- Histology shows derivatives of germ layers in variable combination
- Mature type contains neural tissue, teeth, skin, hair, fat, bone, cartilage, respiratory or intestinal epithelium
- Immature type has additional primitive neuroepithelium with rosettes, pseudorosettes or neurofibrillary matrix in some tumors (Iran J Otorhinolaryngol 2018;30:355)
- Neurofibroma:
- Neural tumor occurs in second or third decades of life
- Wide anatomic distribution but diffuse type neurofibromas usually arise in the head and neck region
- Histology shows spindle cells with wavy, tapering nuclei arranged haphazardly or in fascicles
- Stroma shows collagenous (shredded carrot collagen or homogeneous pink) rather than fibrillary appearance
- CD34 positive
- Ectopic meningioma / meningothelial hamartoma:
- Meningothelial neoplasm arises in children and young adults
- Usually occurs in the head and neck
- Histology shows syncytial lobules and whorls of spindle cells with regular nuclei and pale intranuclear cytoplasmic inclusions
- May show psammomatous calcifications
- Meningothelial hamartoma arises in infants; most common site is posterior scalp
- Shows slit-like pseudovascular spaces lined by spindle cells along with small clusters in a fibrocollagenous stroma
- EMA positive
- Congenital hemangioma:
- Benign vascular tumor, fully developed at birth (BMJ Paediatr Open 2020;4:e000816)
- Superficial, occurs commonly in head and neck region
- Have similar clinical presentations as heterotopia
- Usually presents as a reddish purple limited lesion covered with fine or coarse telangiectasia
- Frequently shows a pale peripheral halo (BMJ Paediatr Open 2020;4:e000816)
- Prenatal ultrasound diagnosis between nasal glial heterotopia and congenital hemangioma is difficult
- Fetal MRI is not very specific for distinguishing between the 2 lesions but excludes the presence of an intracerebral connection in case of heterotopia
- Postnatal exams are more specific (J Stomatol Oral Maxillofac Surg 2017;118:298)
- Histology shows poorly canalized vessels and mitotically active endothelium in rapid growth phase
- Lumina become prominent with maturation and a combination of solid and vascular areas in varying proportions may be seen
- Ganglioglioma:
- Glioneuronal tumor that makes up ~2% of all primary intracranial tumors (Childs Nerv Syst 2016;32:1839)
- Predilection for children and young adults (median age: 12 years)
- Mostly occurs in temporal lobe
- Histology shows biphasic tumor with variable mixture of mature appearing ganglion-like cells and atypical glial cells
- Large ganglion-like cells may show bi or multinucleation
- Atypical glial cells show moderate enlargement with hyperchromasia and mimic those of fibrillary astrocytomas, pilocytic astrocytoma or oligodendroglioma
- May show perivascular lymphocytic cuffing, eosinophilic granular bodies and dystrophic calcifications (Childs Nerv Syst 2022;38:63)
Additional references
Board review style question #1
A 6 month old infant presented with swelling in the nose since birth. Radiology showed a well circumscribed lesion with no connection to brain or dura. Histological examination revealed nests of glial tissue embedded in a fibrillary stroma. Which immunostain will be most likely expressed by the lesional cells?
- CD34
- Desmin
- EMA
- GFAP
- SMA
Board review style answer #1
D. GFAP. The glial tissue will be immunopositive for GFAP (J Cutan Aesthet Surg 2020;13:233, Br J Ophthalmol 1993;77:817). Answer A is incorrect because CD34 is a vascular marker and also shows positive expression in some fibrohistiocytic lesions. Answer B is incorrect because desmin is a myogenic marker expressed in skeletal muscle and smooth muscle lesions. Answer C is incorrect because EMA is positive in meningeal and epithelial cells (Oncol Lett 2013;5:768, J Oral Maxillofac Pathol 2023;27:604). Answer E is incorrect because SMA is expressed in smooth muscle cells and myofibroblasts in normal, reactive or neoplastic tissue.
Comment Here
Reference: Glial heterotopia
Comment Here
Reference: Glial heterotopia
Board review style question #2
Board review style answer #2
A. Benign. Nasal glial heterotopia is a benign lesion with a very good prognosis. Recurrence rate is low and related to incomplete excision (Int J Pediatr Otorhinolaryngol 2020;129:109728). Answers B - E are incorrect because the lesion does not show intermediate or malignant features.
Comment Here
Reference: Glial heterotopia
Comment Here
Reference: Glial heterotopia
Grossing & features to report
Grossing
Grossing of the sinonasal tract is extremely complicated, as the extent of each surgery (composite resection) varies. Therefore, the sampling of each specimen is unique, and consultation with the surgeon and an experienced head and neck pathologist is recommended.
- At least one section per 1 cm of tumor for large tumors, including tumor center and periphery
- Submit entire tumor, if possible in < 5 sections
- Submit resection margins
- Submit samples of tissue from other sites
Features to report
- Tumor histologic type and pattern
- Tumor size and location
- Tumor histologic grade
- Tumor extension to adjacent structures
- Status of resection margins
- Vascular invasion
- Perineural invasion
- Lymph nodes: for each level, number obtained, number involved by tumor, size of nodal metastases, presence of extracapsular spread
Hairy polyp
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
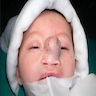
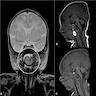
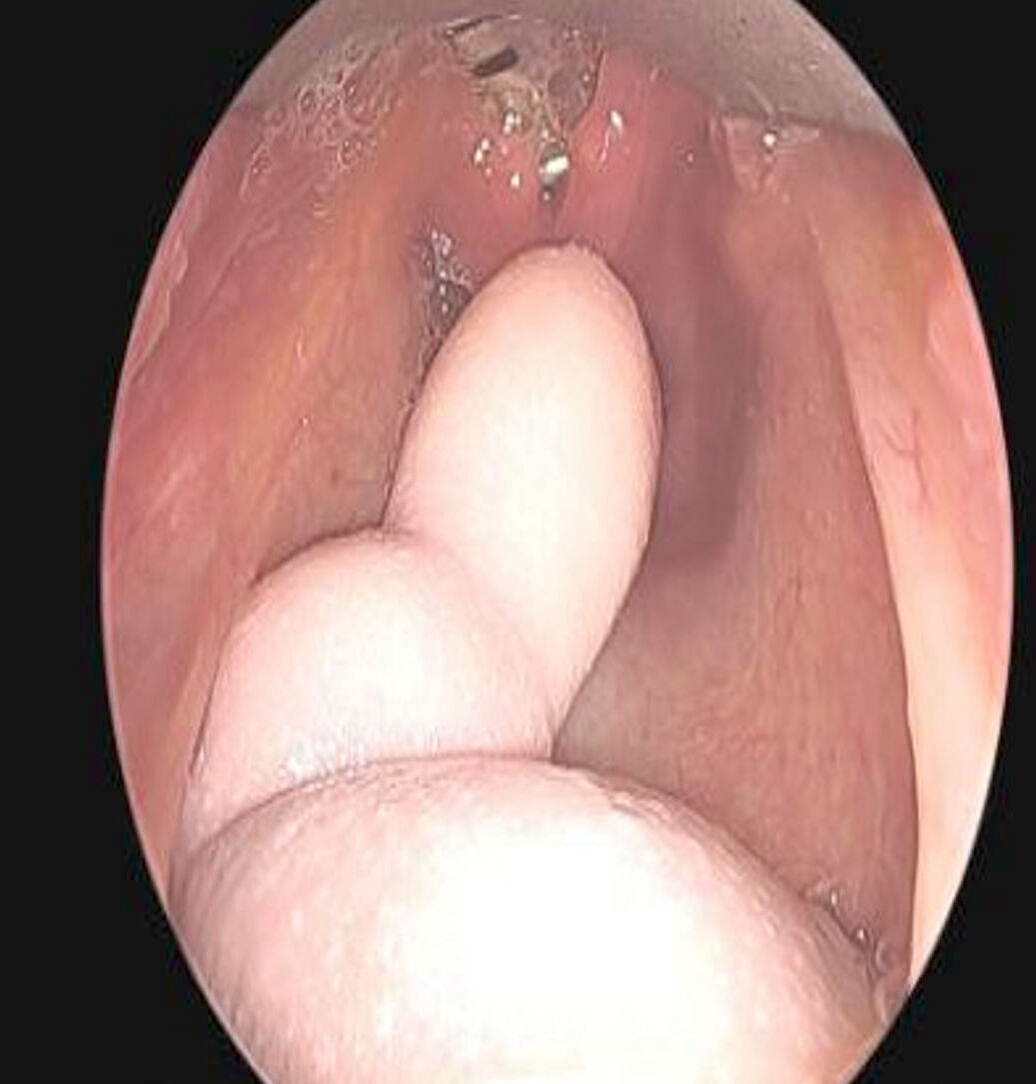
- Benign nasopharyngeal lesion
- Polypoid lesion containing both ectodermal and mesodermal components
Essential features
- Polypoidal lesions are often pedunculated with bigerminal origin
- Congenital, usually presents in newborns
- Comprised of only ectoderm and mesodermal cell lines; no endoderm
- Skin covered polypoid mass with mesoderm core
Terminology
- Not recommended: dermoid polyp, teratoid polyp, naso-oropharyngeal choristoma
ICD coding
- ICD-11: 2E90.6 - benign neoplasm of nasopharynx
Epidemiology
- Rare (1/40,000 live births) (Am J Case Rep 2021;22:e930200)
- Usually presents in neonates and young children
- F > M
Sites
- Lateral wall of nasopharynx (most common) (Medicine (Baltimore) 2019;98:e14305)
- Oropharynx, lip, tongue, palate, tonsillar areas and middle ear (Head Neck Pathol 2013;7:232)
Pathophysiology
- Pathogenesis is unclear
- May be branchial arch anomaly or misdirected totipotent cells (Medicine (Baltimore) 2019;98:e14305)
- Closure failure of naso-oropharyngeal plates during organogenesis (Head Neck Pathol 2013;7:232)
Etiology
- Unknown
Clinical features
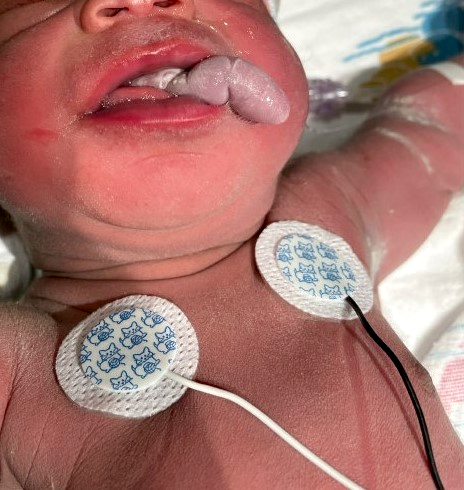
- Varies based on extent, site and mobility of the lesion
- Spectrum ranges from asymptomatic to lethal presentation
- Presents as polyhydramnios prenatally in 20 - 30% of cases (Ear Nose Throat J 2022;101:NP68)
- Most common symptom is respiratory obstruction (Am J Case Rep 2021;22:e930200)
- Others include snoring, feeding difficulties, cyanosis, cough, stridor, dyspnea, vomiting and recurrent middle ear infections (BMJ Case Rep 2015;2015:bcr2015209825)
- Smaller polyps and polyps in nasopharynx and middle ear remain silent until adulthood
- Rare associations: branchial arch anomalies, cleft palate, choanal atresia or Dandy-Walker syndrome (Int J Clin Pediatr Dent 2009;2:46, J Med Genet 1990;27:788)
- Autoamputation occurs infrequently (Radiol Case Rep 2021;16:1570)
- Can be occasionally bilateral (Braz J Otorhinolaryngol 2017;83:117)
Diagnosis
- Ear, nose and throat (ENT) examination (Ear Nose Throat J 2022;101:NP68)
- Flexible endoscopy (Am J Respir Crit Care Med 2019;200:924)
- CT or MRI to assess the extent of lesion and to exclude intracranial extension (Ear Nose Throat J 2022;101:NP105, BMJ Case Rep 2015;2015:bcr2015209825)
- Histopathological examination is confirmatory (Am J Case Rep 2021;22:e930200)
Laboratory
- No specific laboratory findings
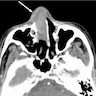
Radiology description
- CT findings: sausage shaped mass with fat attenuation (Diagnostics (Basel) 2023;13:1328)
- MRI: multilayered mass with hyperintense signal on both T1 and T2 with a linear internal low signal (Eurorad: Nasopharyngeal Hairy Polyp in an Infant with Apnoiec Spells [Accessed 6 September 2023])
- Contrast: variable stalk enhancement with no lesion enhancement on both CT and MRI
Radiology images
Prognostic factors
- Excellent prognosis
- No recurrence or malignancy reported (Case Rep Otolaryngol 2013;2013:374681)
- Rarely may be life threatening due to bleeding, choking or autoamputation (Int J Otolaryngol 2022;11:75)
Case reports
- Newborn girl with severe respiratory distress due to hairy polyp of eustachian tube (Congenit Anom (Kyoto) 2015;55:158)
- Newborn boy with nasopharyngeal hairy polyp arising from eustachian tube (Head Neck Pathol 2016;10:213)
- Full term newborn boy with intermittent dyspnea and cyanosis due to hairy polyp (Pediatr Neonatol 2014;55:231)
- 2 day old preterm infant girl with oral mass (Case Rep Otolaryngol 2013;2013:374681)
- 15 month old boy with rare occurrence of 2 separate hairy polyps with meningothelial components (Case Rep Otolaryngol 2021;2021:1844244)
- 3 year old boy with respiratory distress due to nasopharyngeal hairy polyp from supratonsillar region (Int Arch Otorhinolaryngol 2015;19:90)
Treatment
- Surgical excision is curative (Head Neck Pathol 2016;10:213)
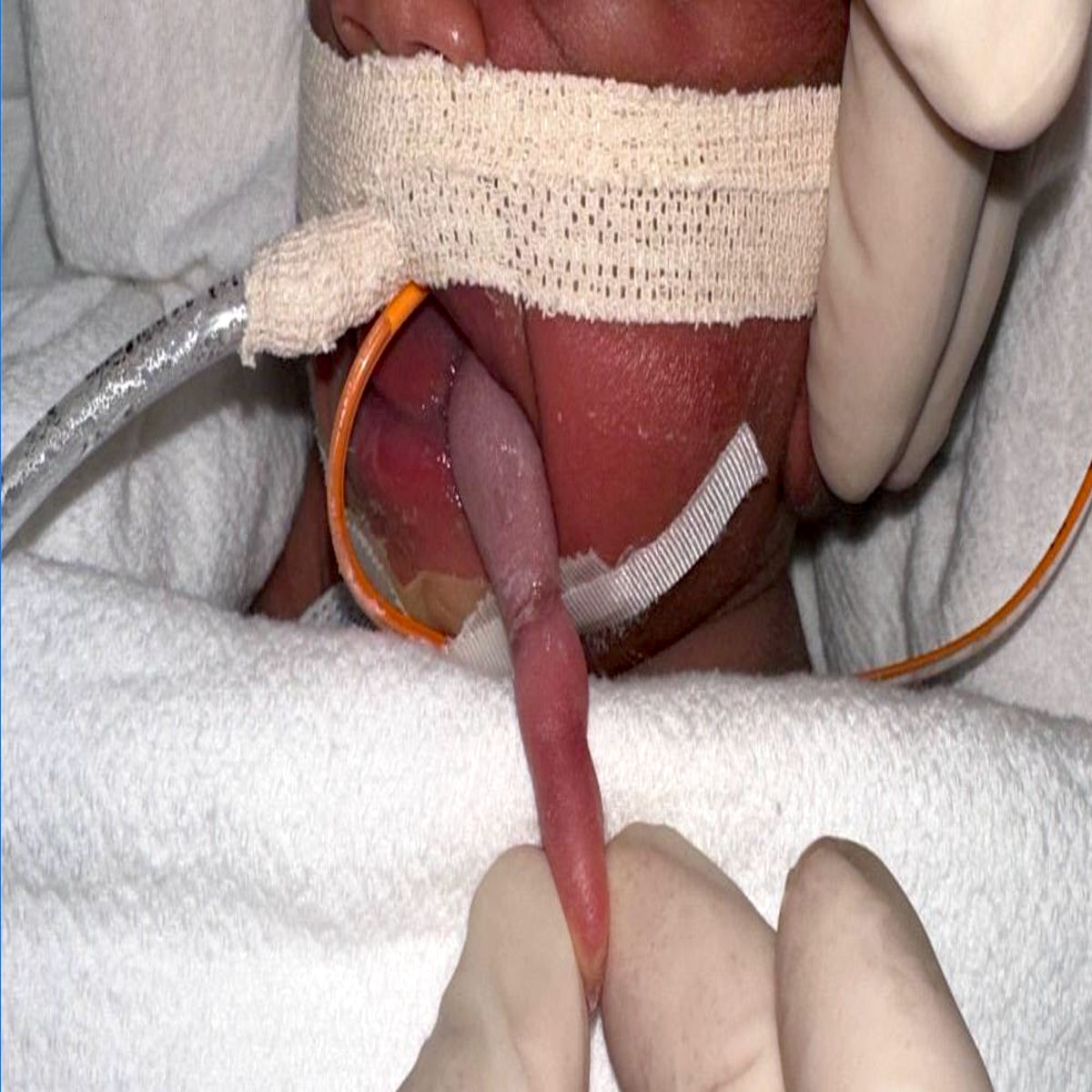
Clinical images
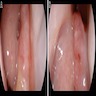
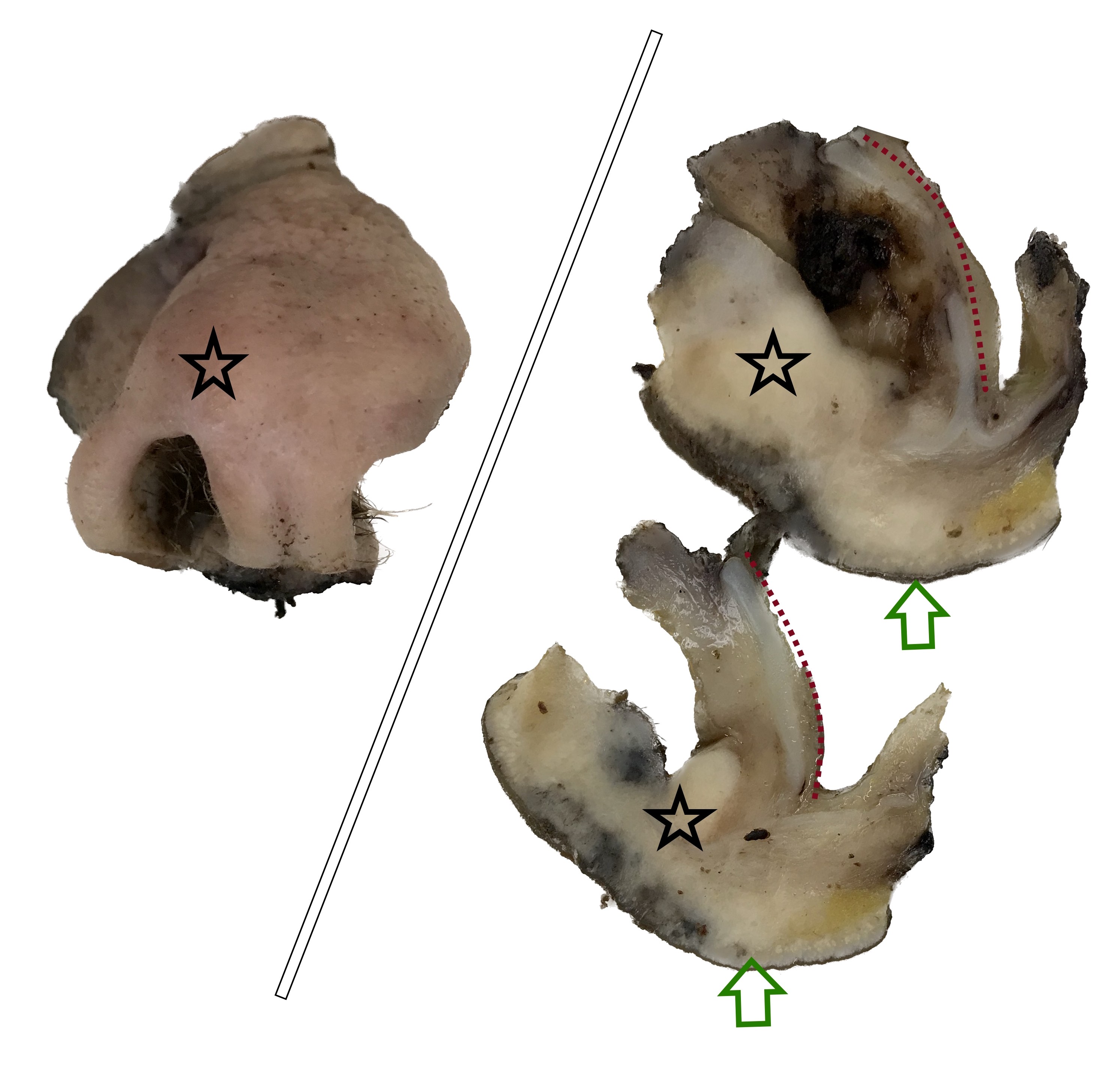
Gross description
- Grossly, skin covered polypoid and pedunculated / sessile lesion (Medicine (Baltimore) 2019;98:e14305)
- Hair growth on the external surface (Medicine (Baltimore) 2019;98:e14305)
- Cut surface: tan-yellow and mostly solid
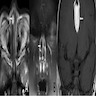
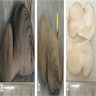
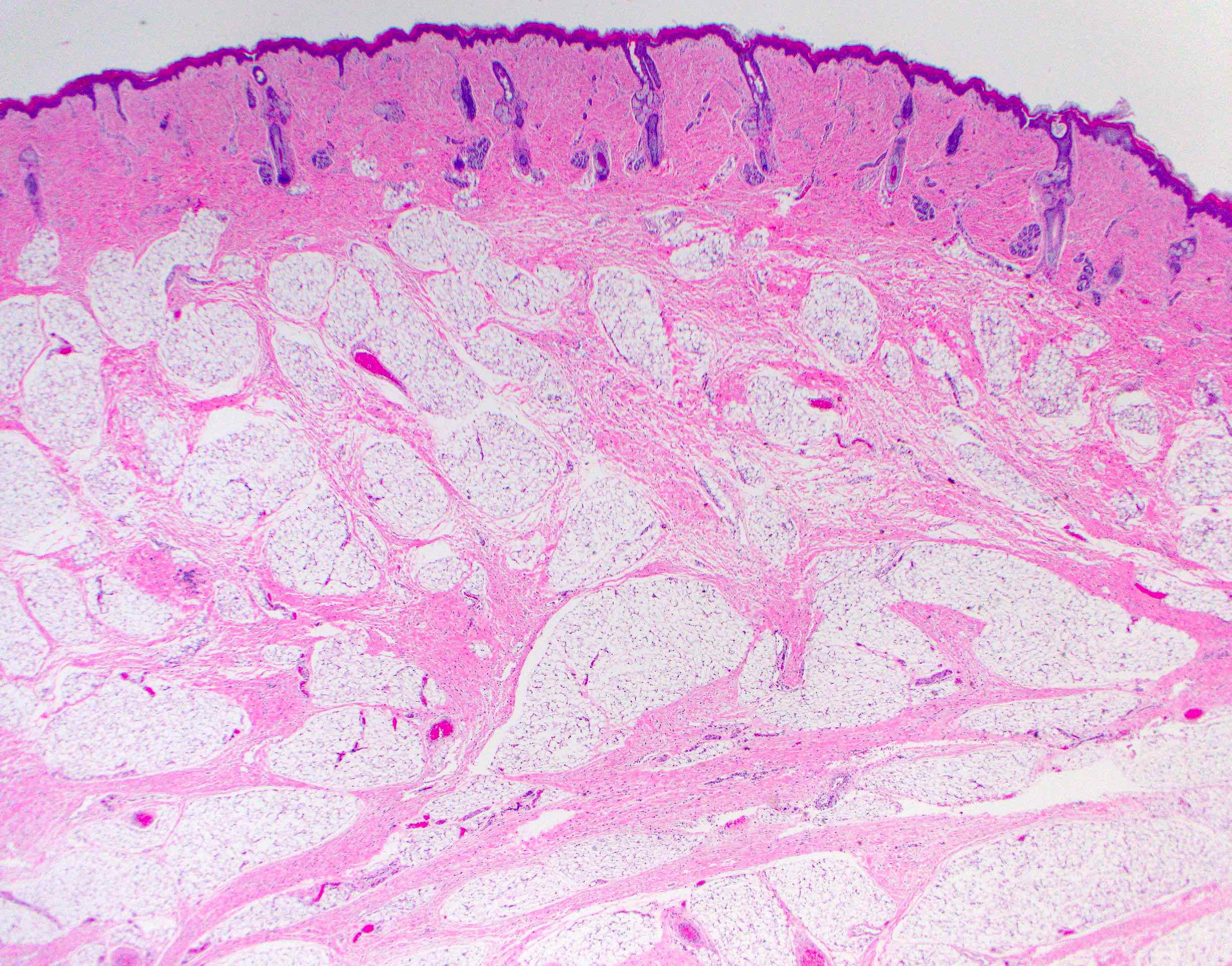
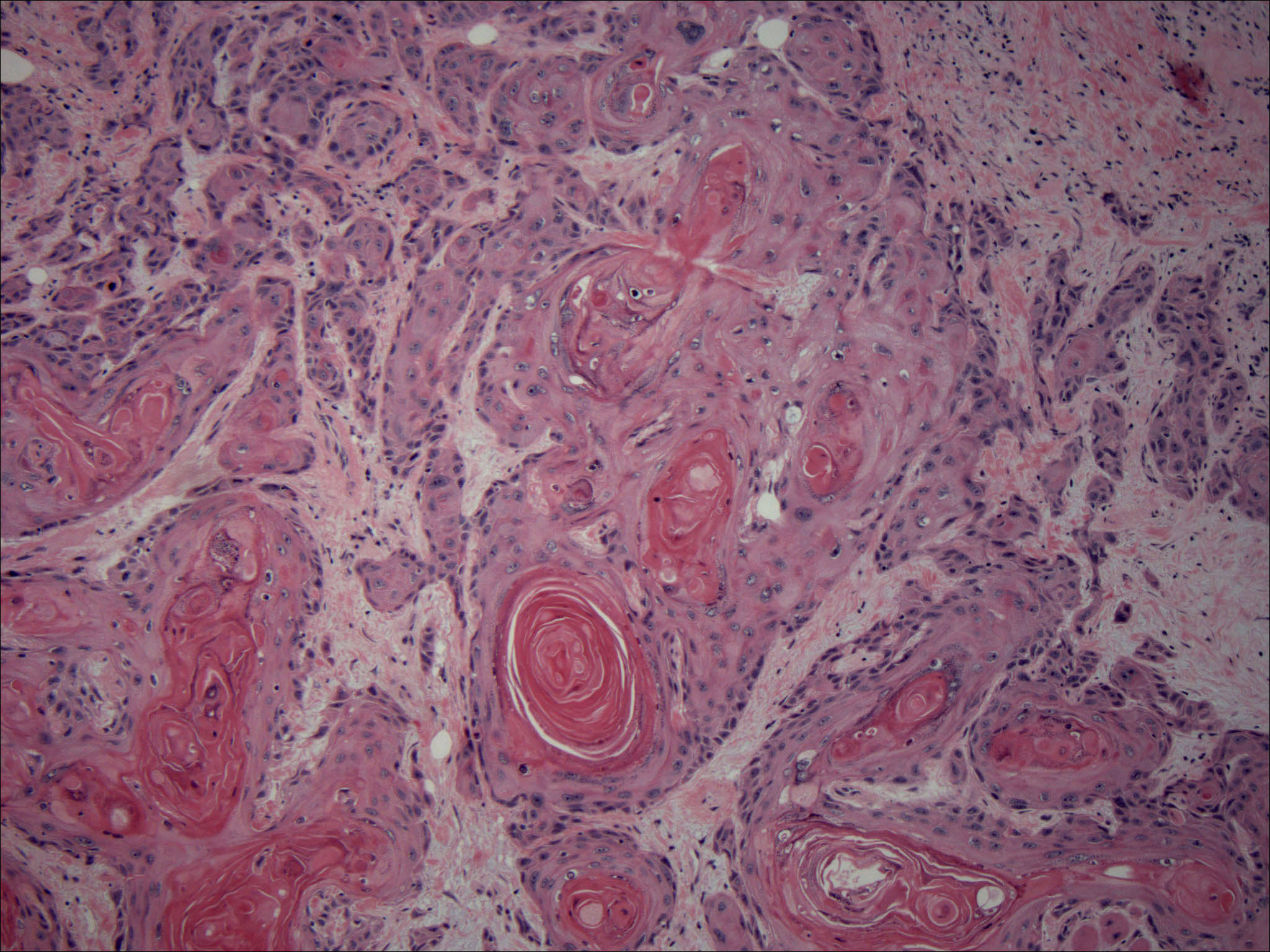
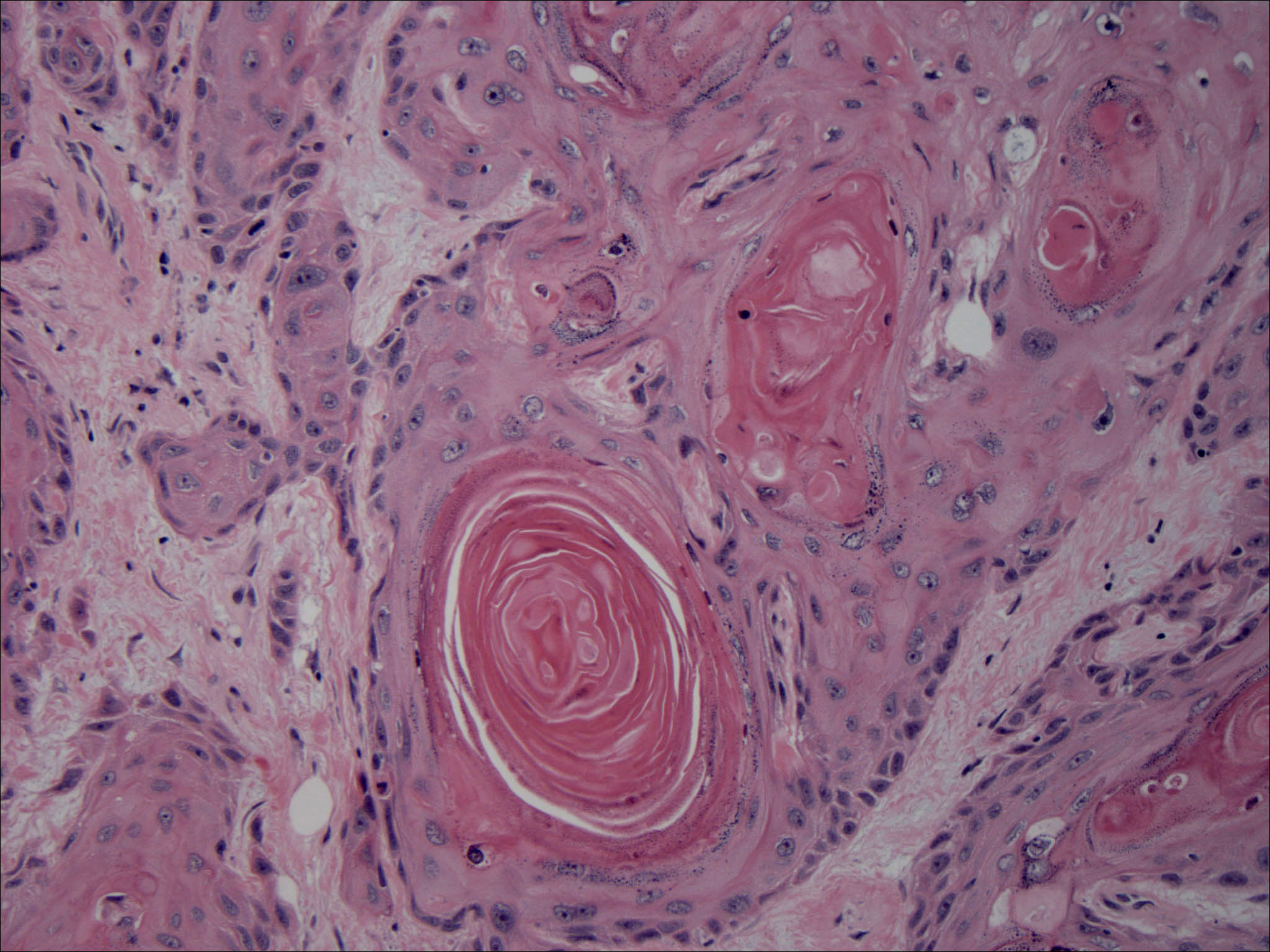
Microscopic (histologic) description
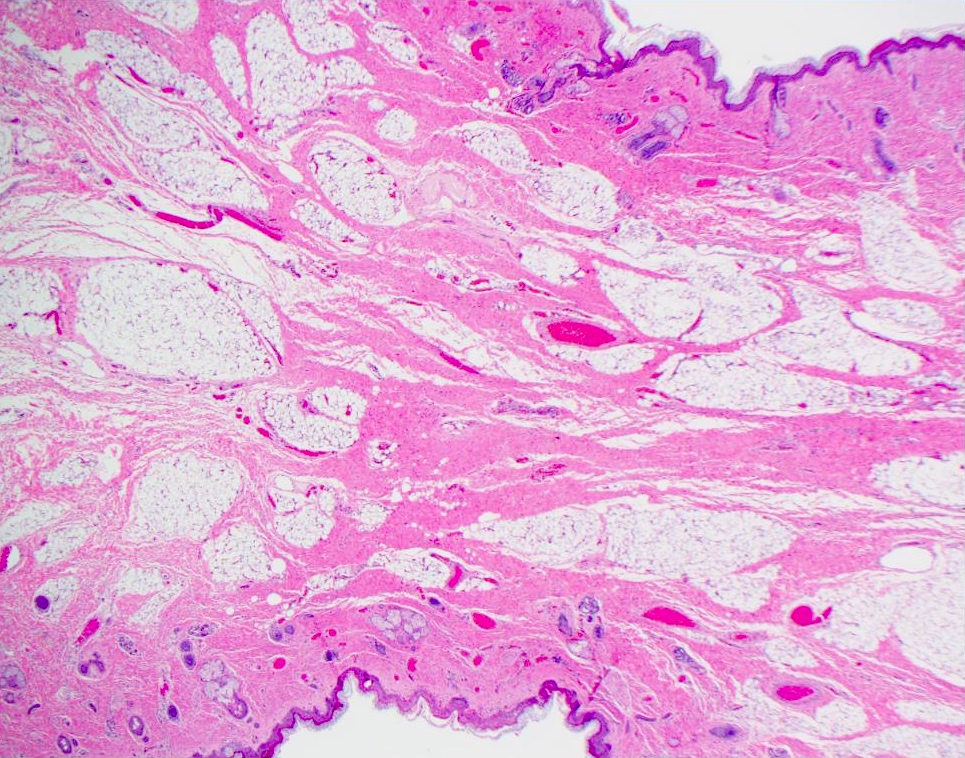
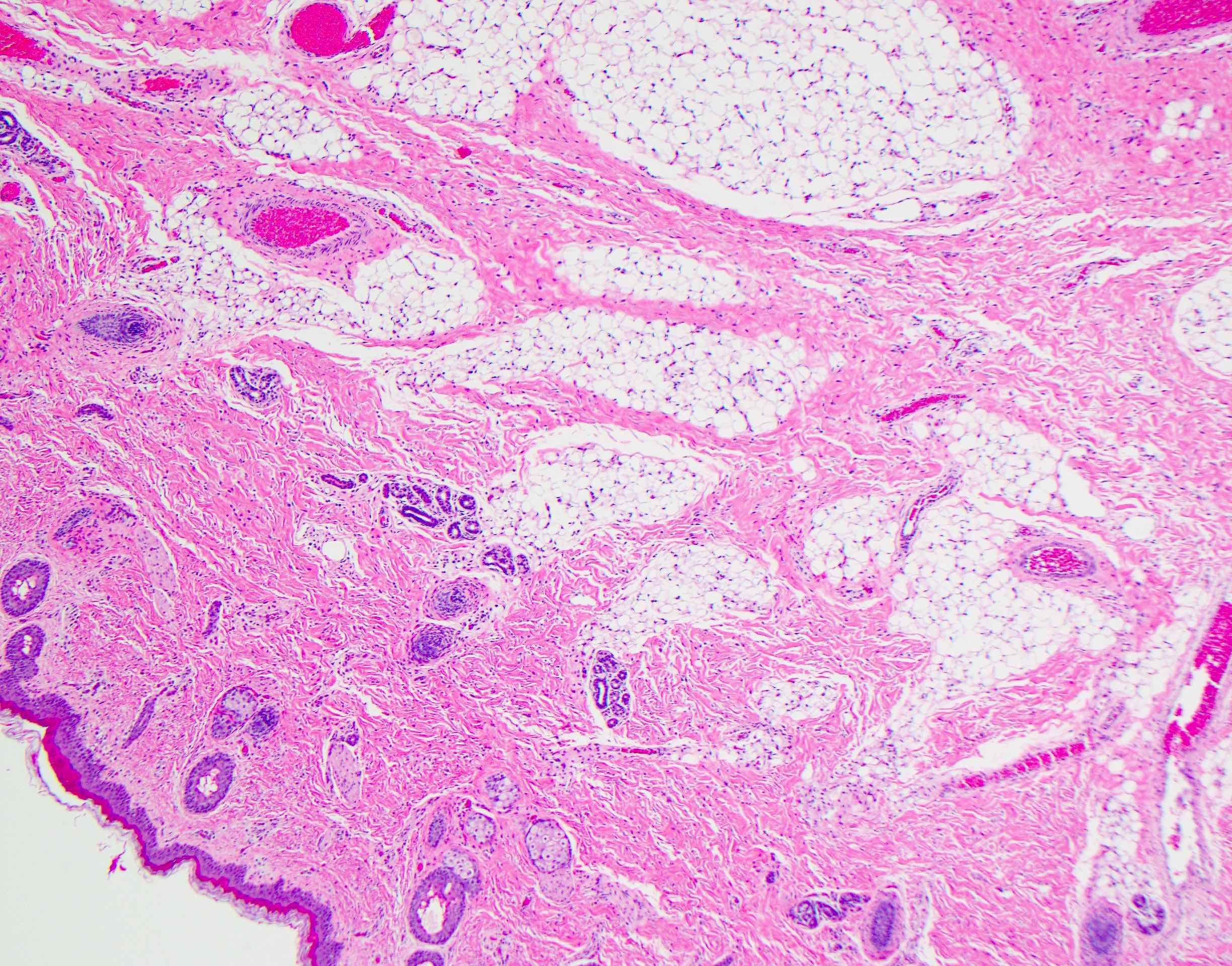
- Comprised of tissue derived from ectoderm and mesoderm
- Consists of keratinizing squamous epithelium with underlying adnexa which includes pilosebaceous units
- Mesoderm derivatives include fibroadipose tissue, skeletal muscle, smooth muscle, seromucous glands and cartilage
- Meningothelial cells have been reported in literature occasionally (Case Rep Otolaryngol 2021;2021:1844244, Head Neck Pathol 2021;15:25)
- No endodermal elements are present
Microscopic (histologic) images
Sample pathology report
- Right oral cavity lesion, excision:
- Hairy polyp (see comment)
- Comment: Squamous epithelium with underlying adnexal structures, cartilage and fibroadipose tissue are present.
Differential diagnosis
- Teratoma (Turk Arch Otorhinolaryngol 2015;53:188):
- Comprised of 3 germ layers
- Solid or cystic with mature and immature components
- Hamartoma:
- Disordered growth of cells or tissue in normal anatomical location
- Dermoid cyst (Ear Nose Throat J 2022;101:NP105):
- Consists of squamous epithelium with adnexal structures
- No other derivatives present
- Choristoma (Turk J Pediatr 2018;60:460, Eurorad: Nasopharyngeal Hairy Polyp in an Infant with Apnoiec Spells [Accessed 6 September 2023]):
- Histologically normal tissue in abnormal anatomical location
Additional references
Board review style question #1
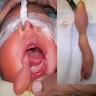
A 2 day old neonate presents with airway obstruction and oropharyngeal mass since birth. There are no underlying congenital deformities. Flexible endoscopy demonstrates a mass extending from the left torus tubarius into the oral cavity which is excised for histopathological examination. Microscopic examination displays epidermal squamous epithelium with underlying pilosebaceous units and mesenchymal core (depicted in the image above). What is the diagnosis?
- Dermoid cyst
- Hairy polyp
- Squamous papilloma
- Teratoma
Board review style answer #1
B. Hairy polyp. Hairy polyp is comprised of 2 germ layers, which are ectoderm and mesoderm derivatives. Answer D is incorrect because teratoma contains derivative from all 3 layers. Answer C is incorrect because squamous papillomas show finger-like projections with squamous epithelium and fibrovascular cores. Answer A is incorrect because it is a midline cyst with squamous epithelium and adnexal structures only.
Comment Here
Reference: Hairy polyp
Comment Here
Reference: Hairy polyp
Board review style question #2
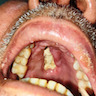
A neonate presents with a finger-like polypoidal extension of soft tissue mass from oral cavity (depicted in the clinical picture above) with feeding difficulties and airway obstruction requiring intubation. The baby has had this lesion since birth. The microscopic examination shows keratinizing squamous epithelium with adnexal structures, adipose tissue, skeletal muscle and cartilaginous core. What is the most likely diagnosis in this case?
- Hairy polyp
- Hamartoma
- Lipoma
- Teratoma
Board review style answer #2
A. Hairy polyp. Hairy polyps occur congenitally and are comprised of mesodermal core with ectodermal lining. Answer C is incorrect because lipomas contain mature adipose tissue. Answer D is incorrect because teratomas contain derivatives from all 3 germ layers. Answer B is incorrect because hamartomas have a haphazard growth of cells in a normal anatomical location.
Comment Here
Reference: Hairy polyp
Comment Here
Reference: Hairy polyp
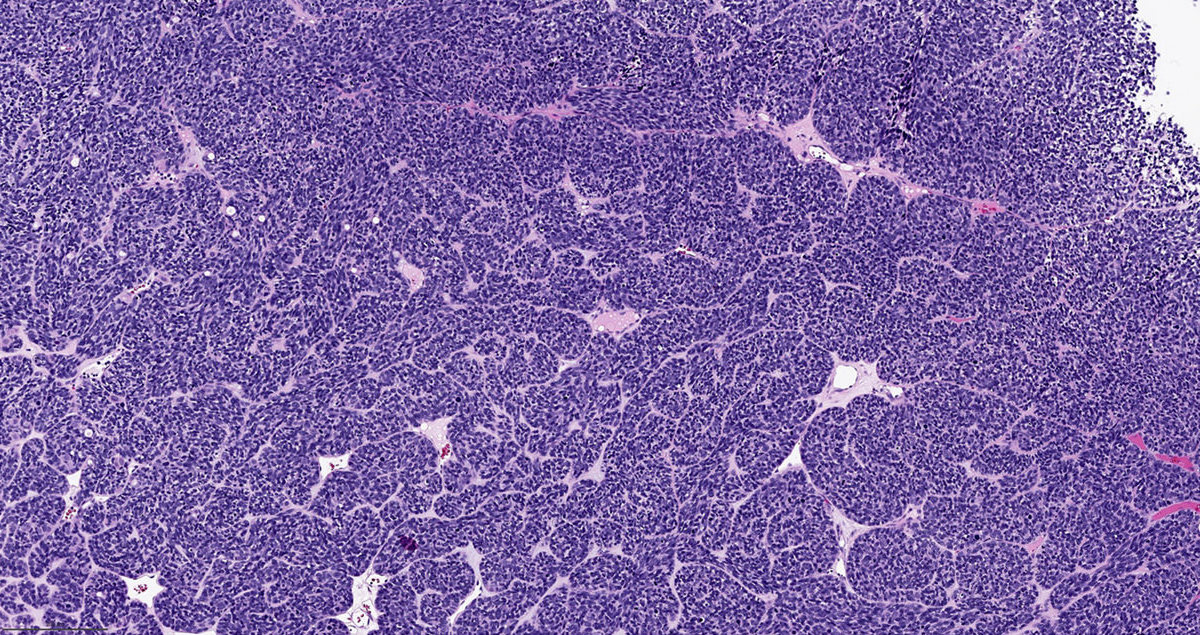
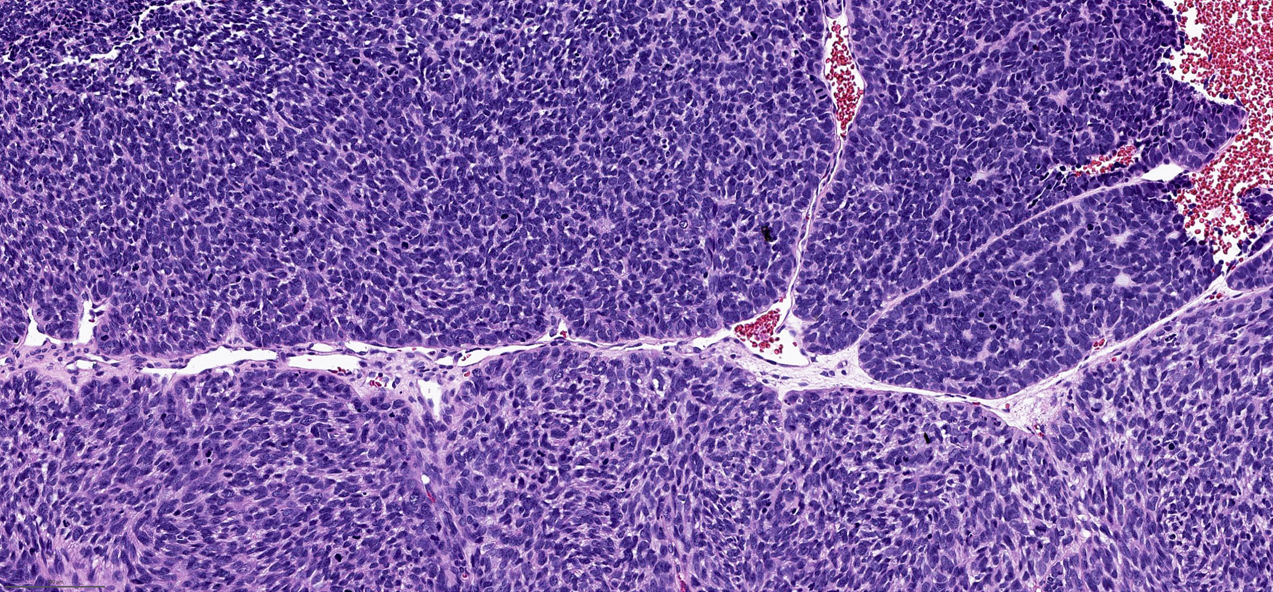
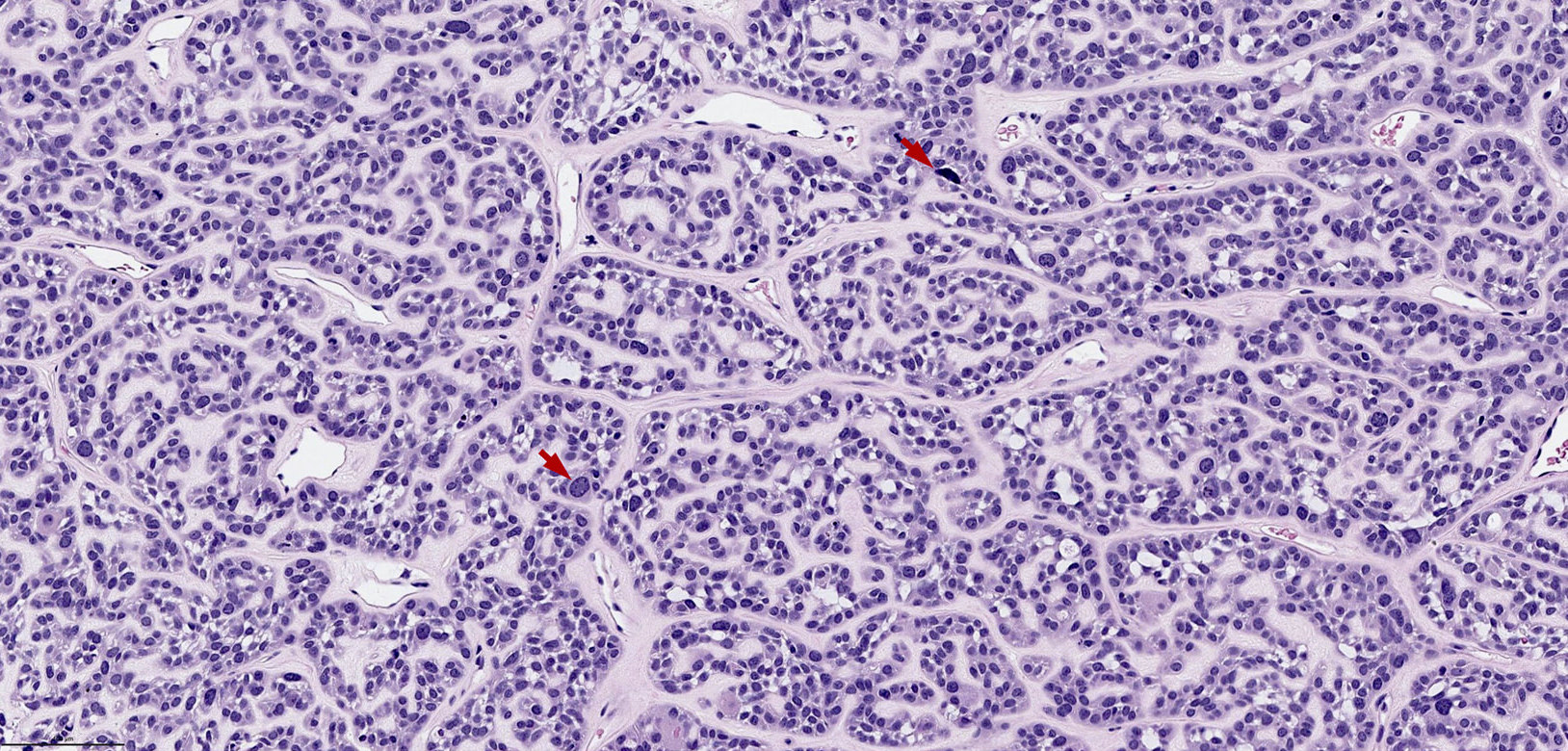
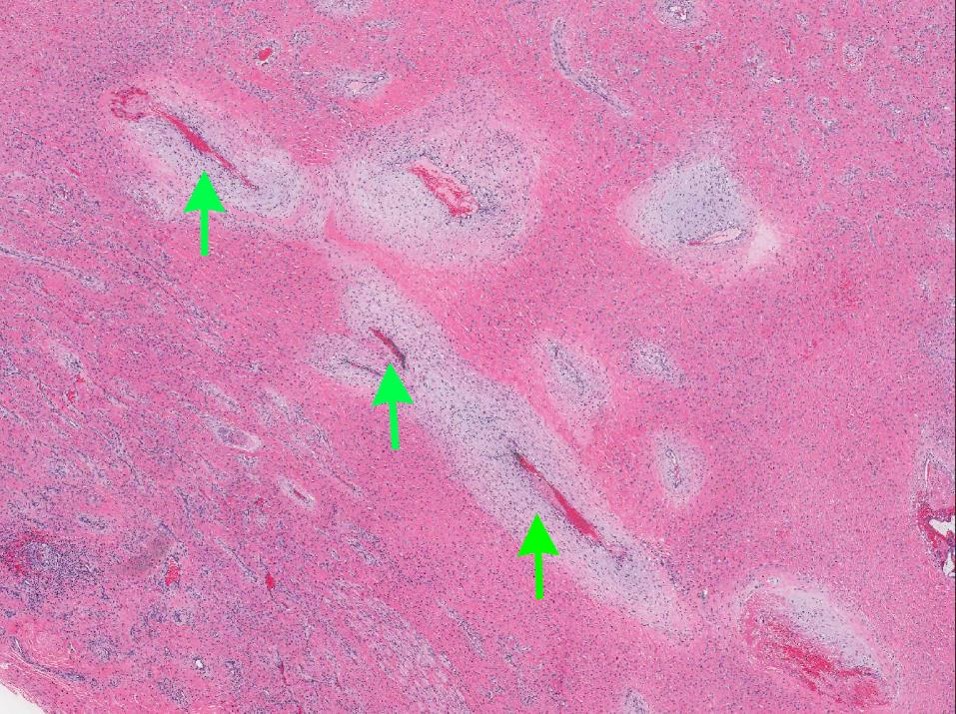
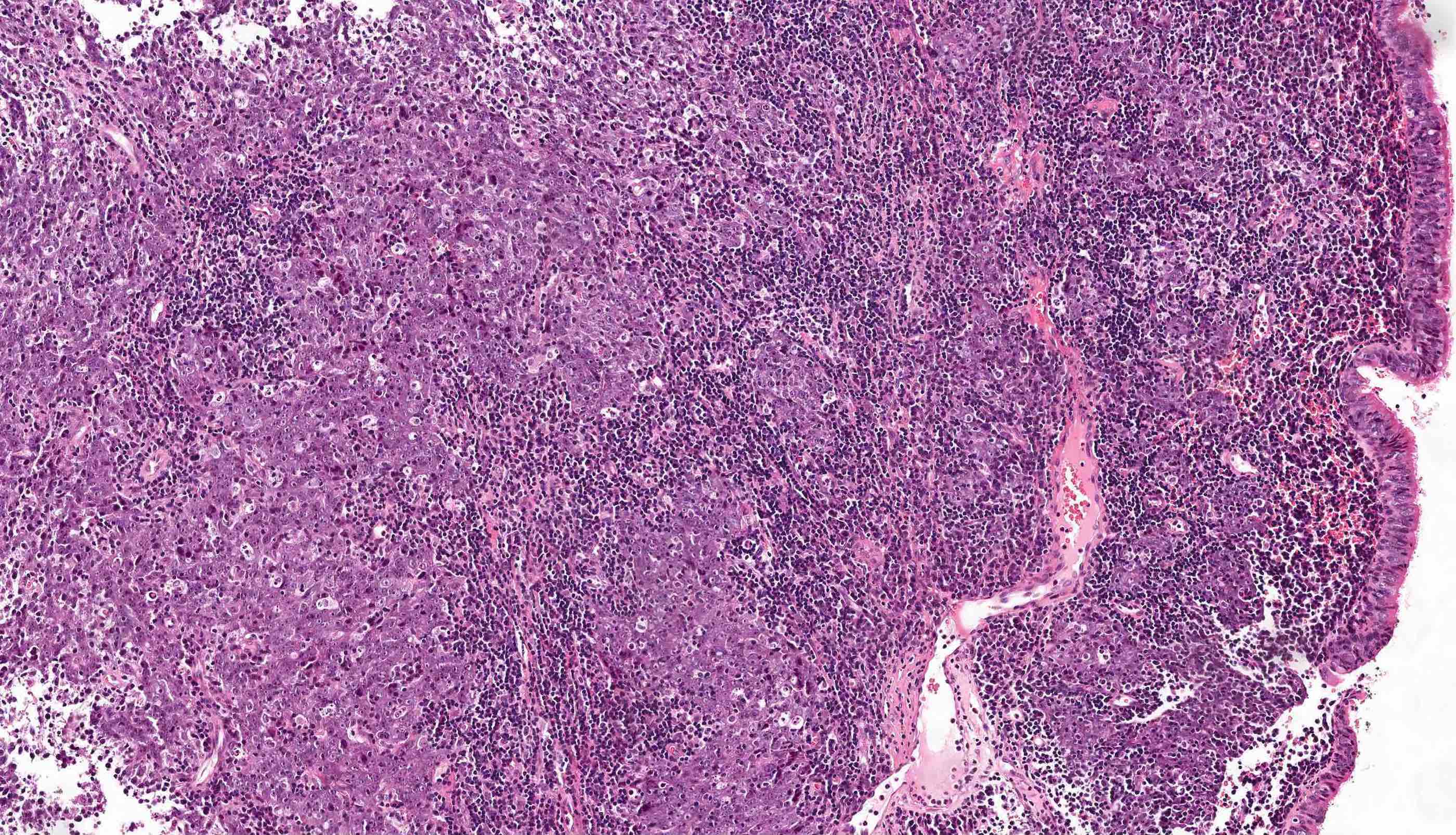
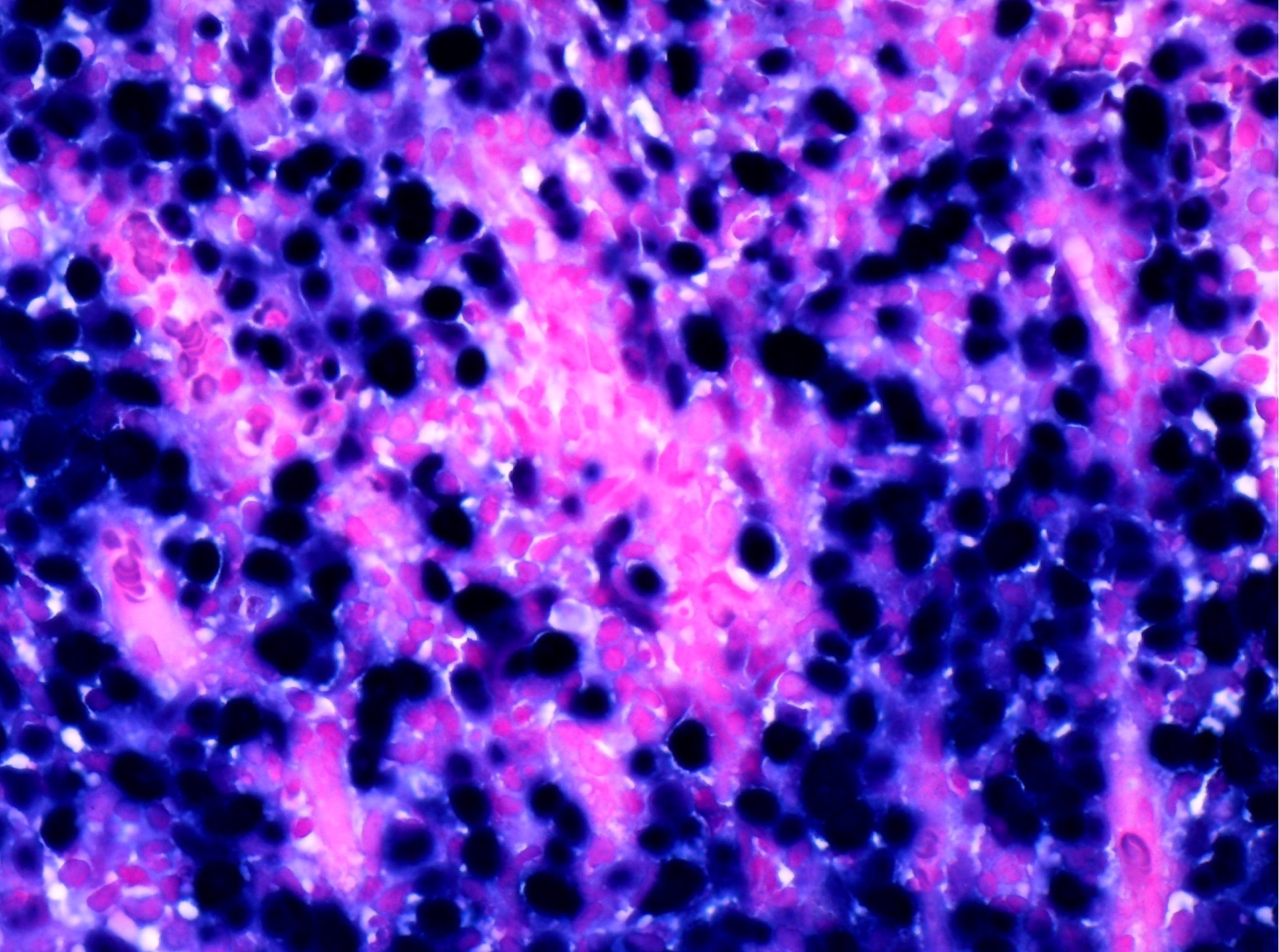
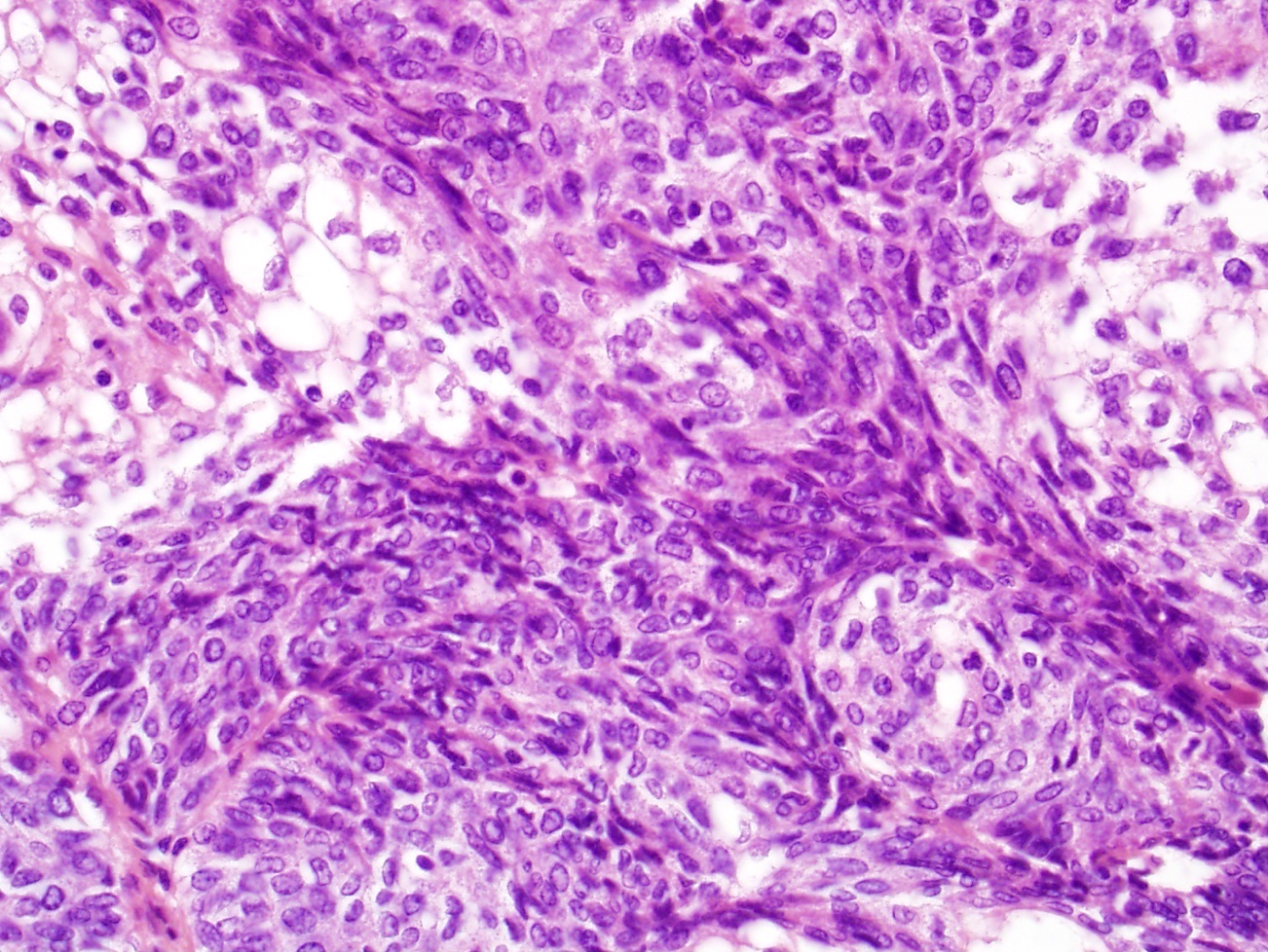
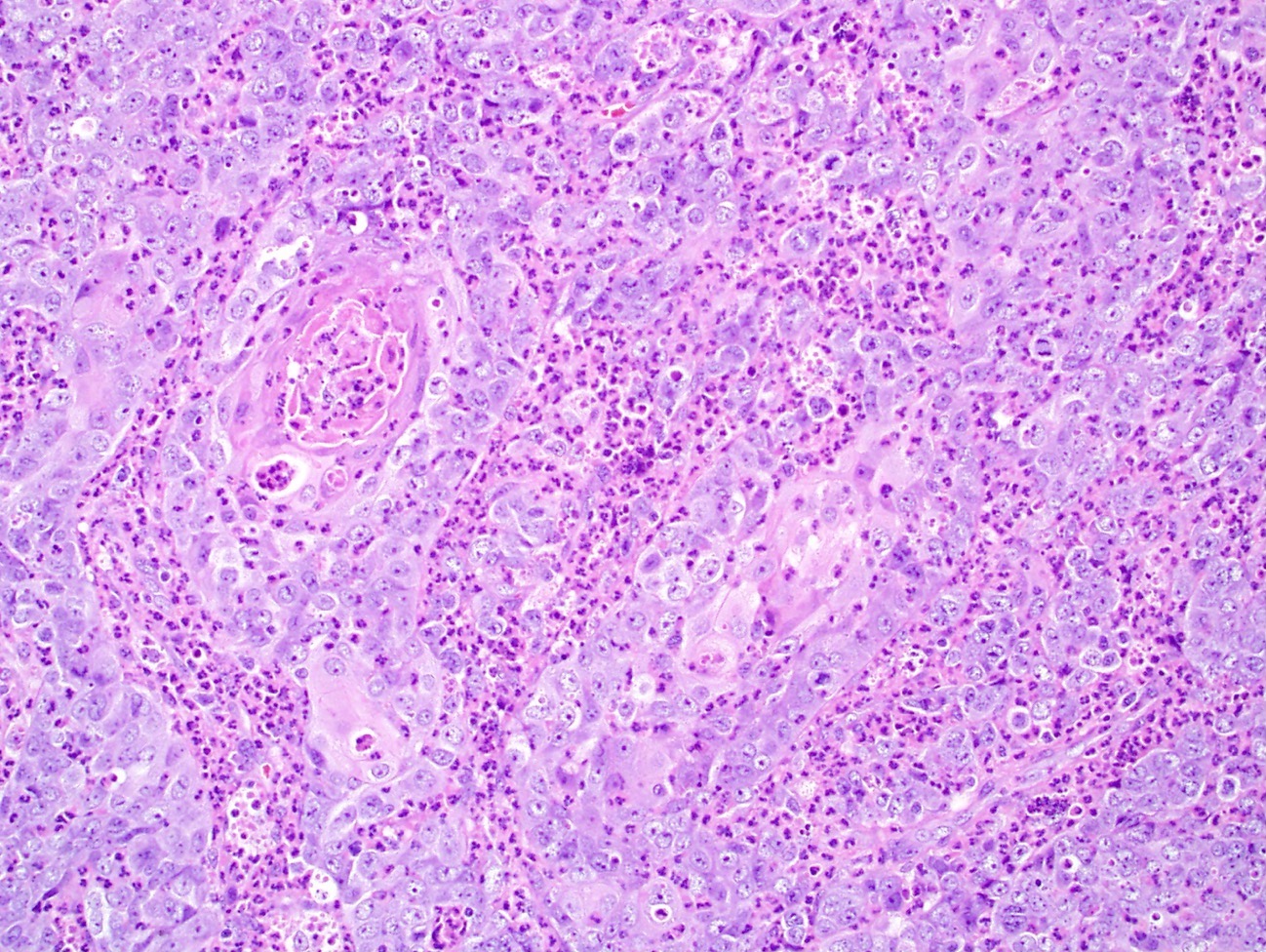
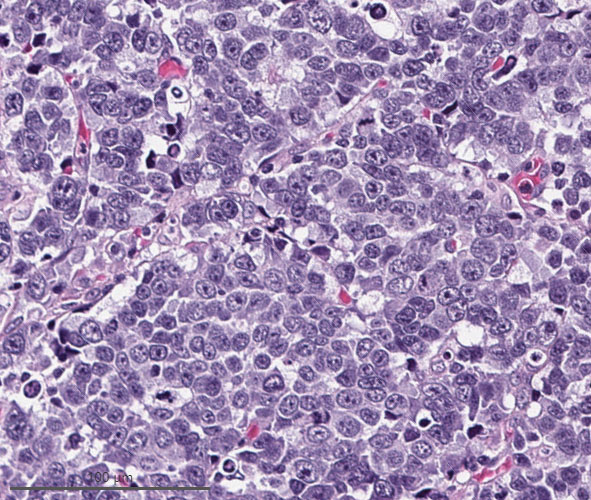
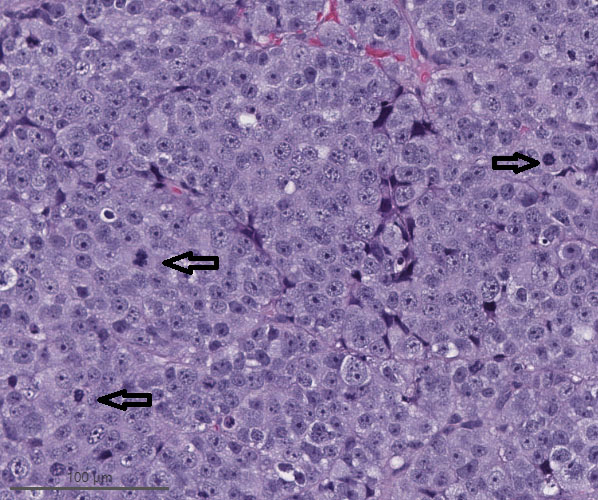
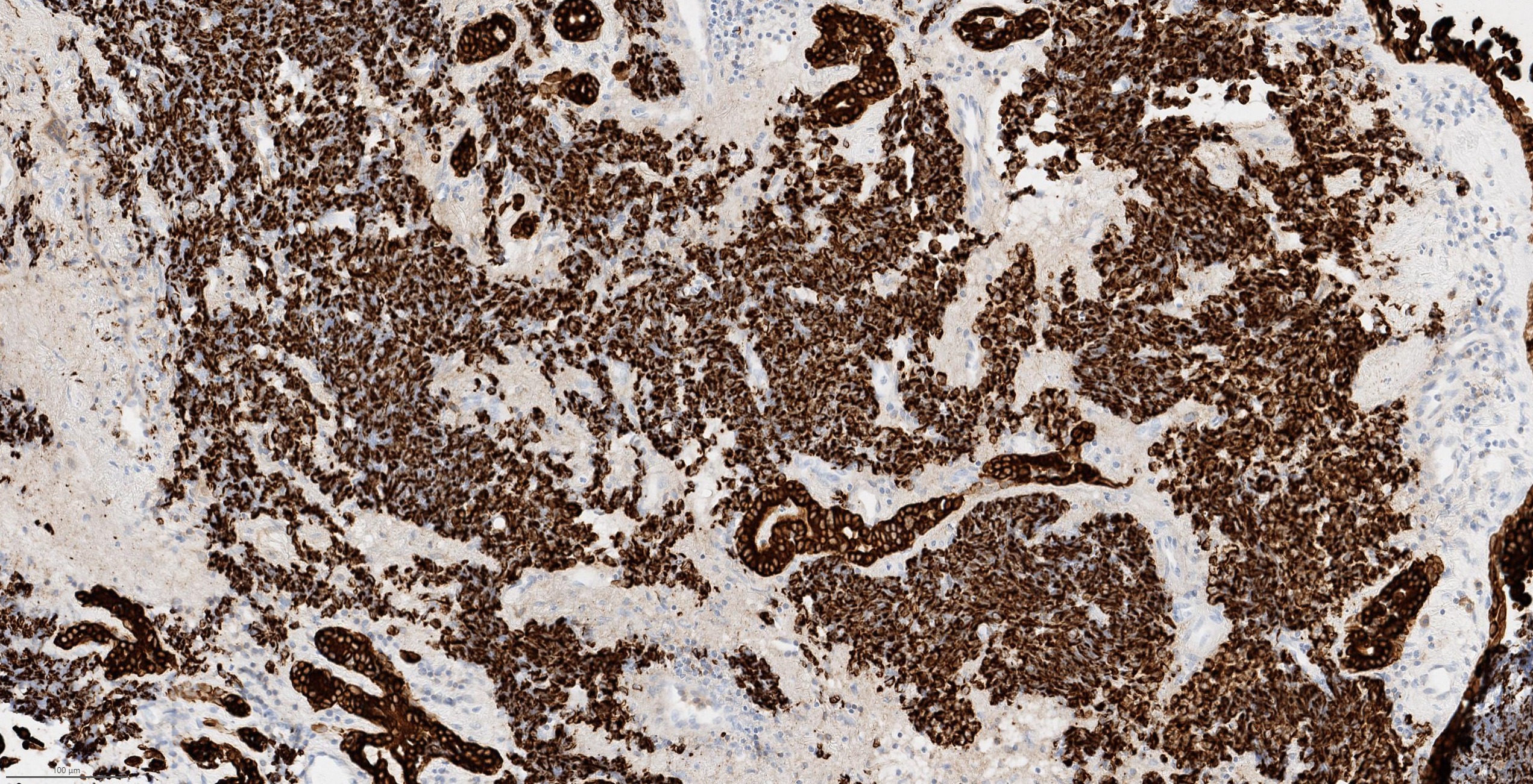
High grade neuroendocrine carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
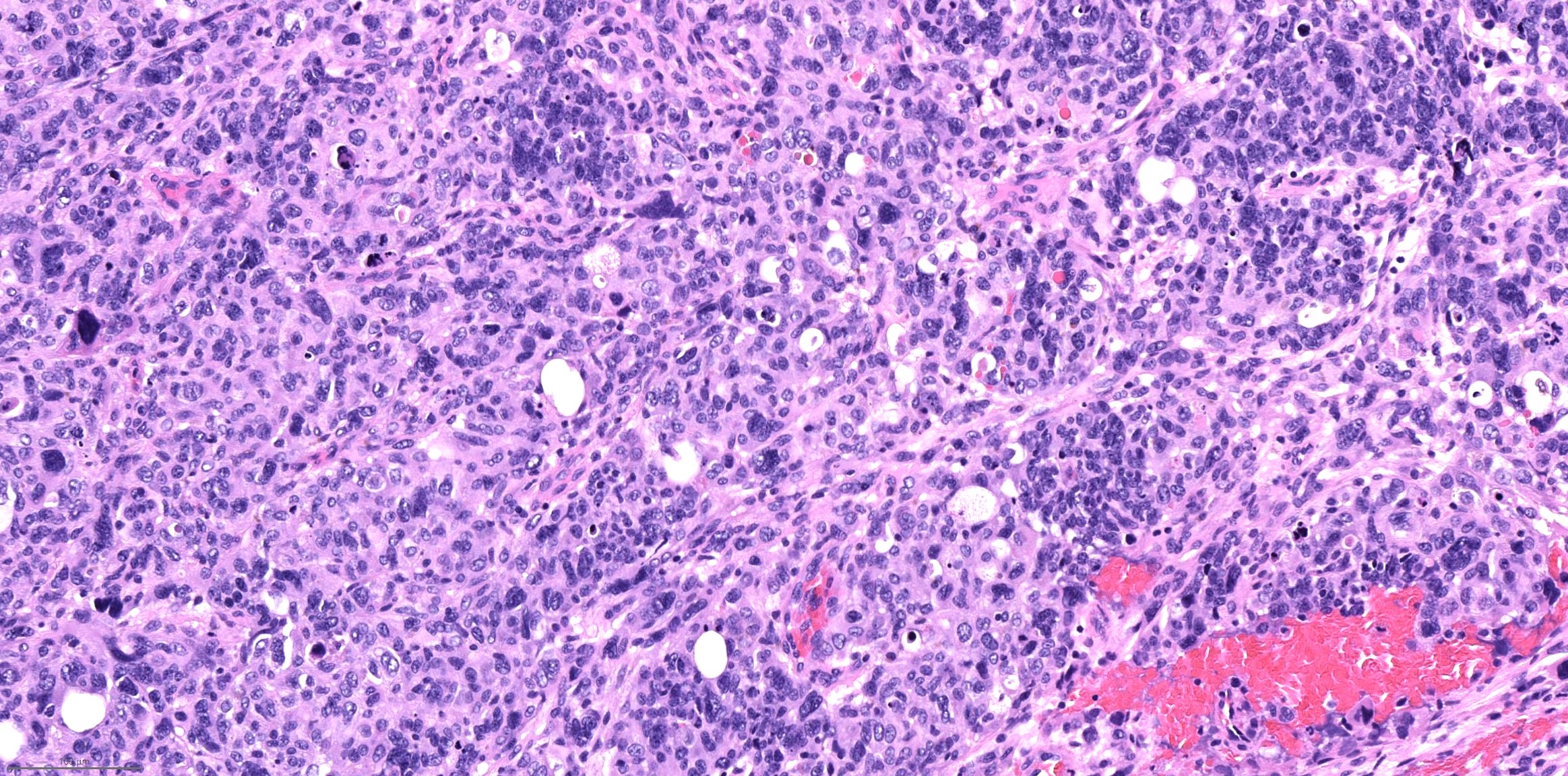
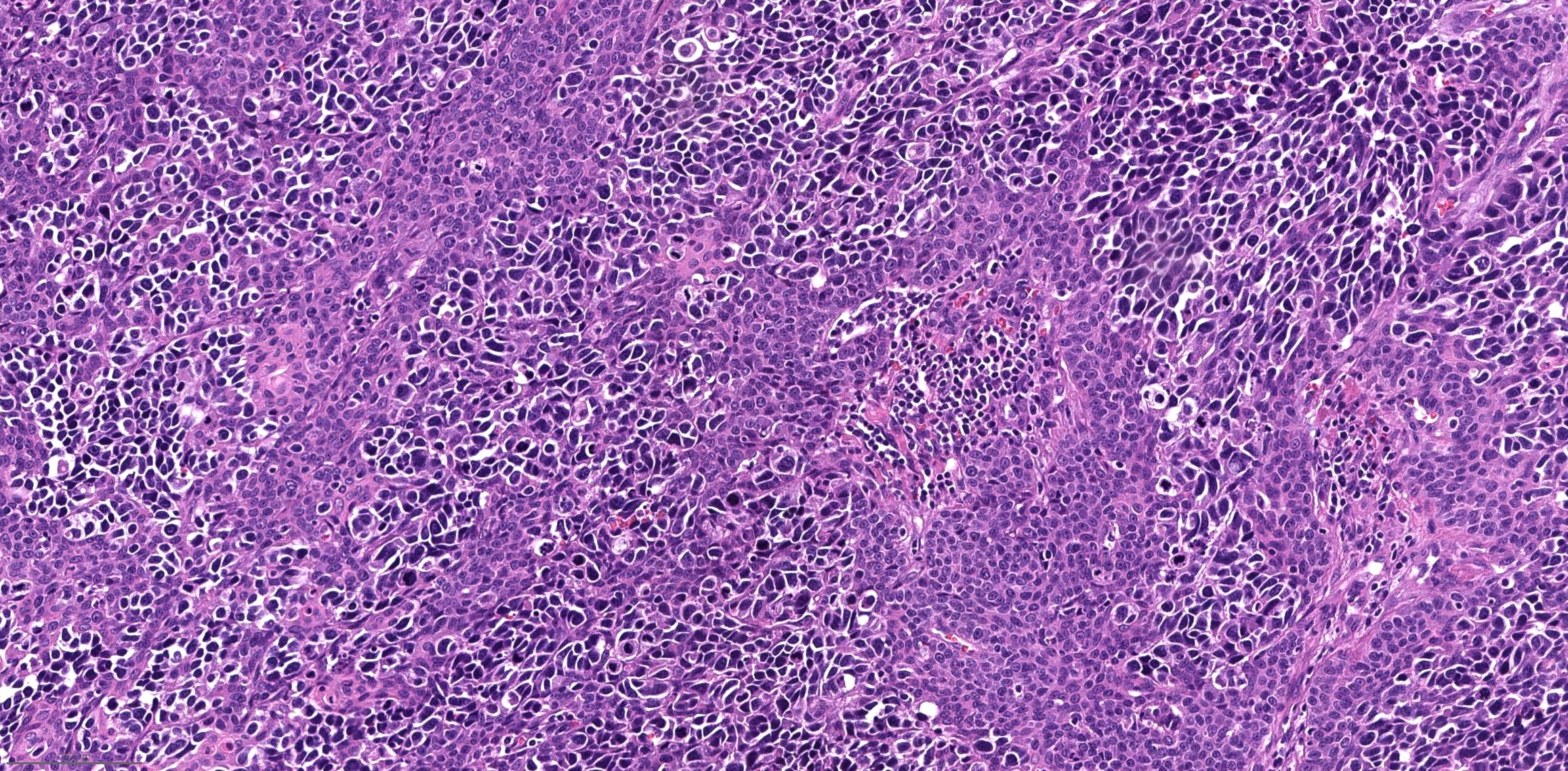
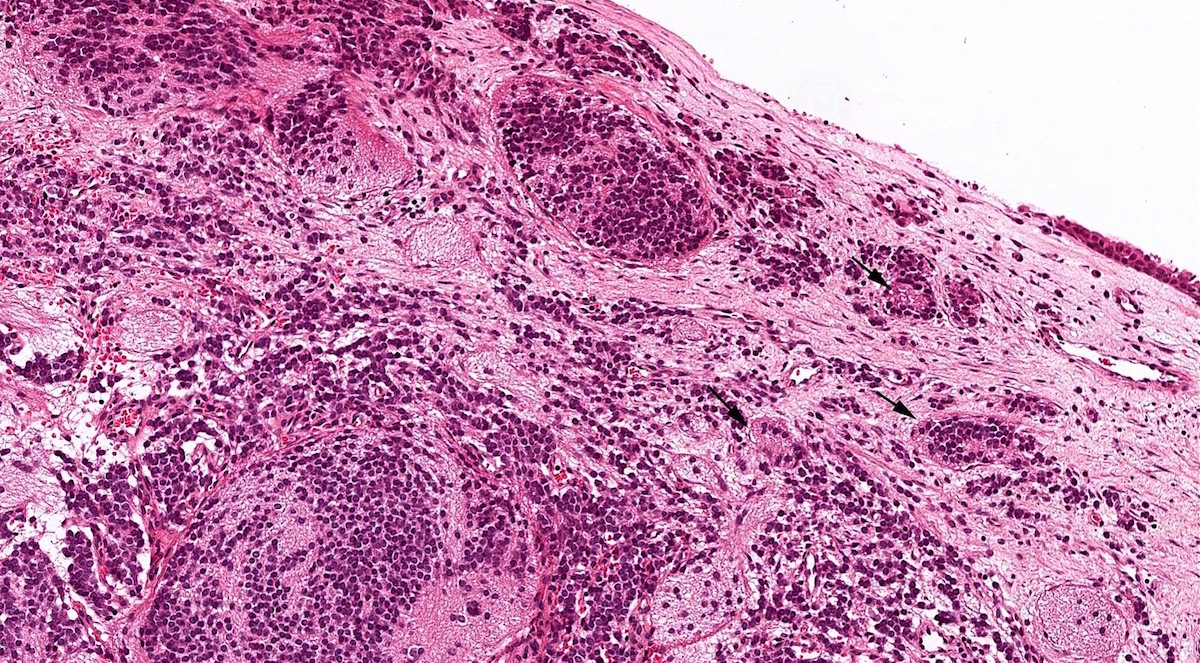
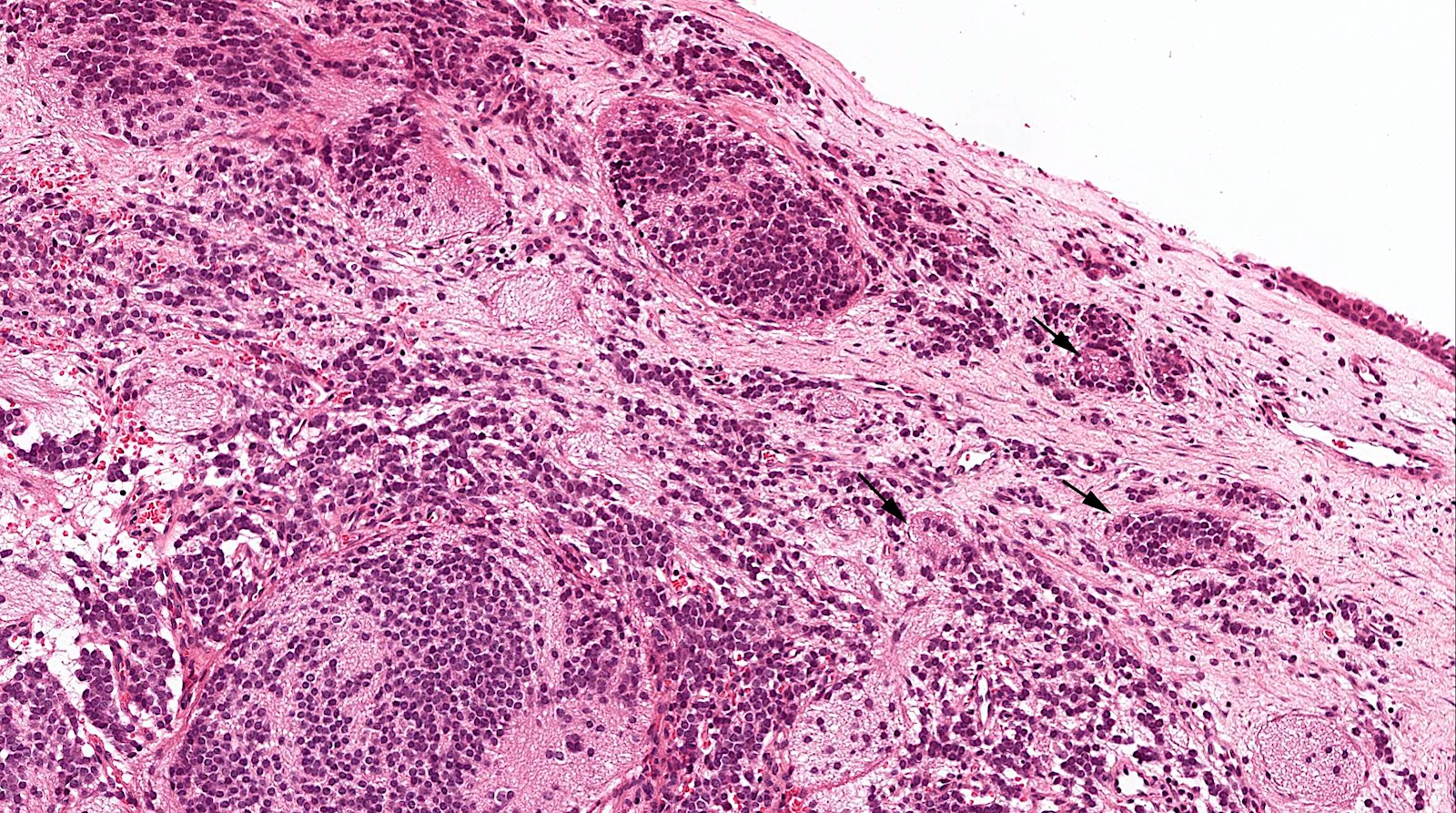
- High grade / poorly differentiated neuroendocrine carcinoma, characterized by high mitotic count and (comedo type) tumor necrosis, morphologically similar to high grade neuroendocrine carcinoma occurring in other body sites
Essential features
- Diagnosis dependent on histologic and immunohistochemical evidence of neuroendocrine differentiation
- Can be further classified as small cell (neuroendocrine) carcinoma and large cell neuroendocrine carcinoma
- May be combined with another type of nonneuroendocrine carcinoma, most frequently squamous cell carcinoma, termed as combined carcinoma
Terminology
- Poorly differentiated neuroendocrine carcinoma
- Small cell carcinoma
- Small cell neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Neuroendocrine carcinoma, large cell type
ICD coding
Epidemiology
- Sinonasal small cell carcinoma accounts for ~35% of all head and neck small cell carcinomas (Laryngoscope 2017;127:1785)
- Mean age of presentation is in the 50s (Oral Oncol 2016;63:1, Int Forum Allergy Rhinol 2016;6:744, Laryngoscope 2017;127:1785)
Sites
- Nasal cavity, including nasal septum, is the most common site, being involved in 32 - 45% of cases (Int Forum Allergy Rhinol 2016;6:744, Laryngoscope 2017;127:1785)
- Other sites in descending order include maxillary sinus, ethmoid sinus, frontal sinus and sphenoid sinus
Etiology
- Subset is associated with high risk human papillomavirus (HPV) (Am J Surg Pathol 2013;37:185, Virchows Arch 2015;467:405)
Clinical features
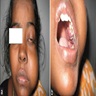
- Nasal congestion and obstruction and epistaxis are the most common presenting symptoms, followed by facial pain, palpable facial mass and exophthalmos (Int Forum Allergy Rhinol 2016;6:744)
Diagnosis
- Diagnosis relies on demonstration of neuroendocrine differentiation, histologic features of small cell or large cell neuroendocrine carcinoma, high mitotic index (> 10 per 2 mm2) and tumor necrosis
Radiology description
- Destructive soft tissue mass of the sinonasal tract
Prognostic factors
- Diagnosis designates a poor prognosis: 5 year disease specific survival of sinonasal small cell carcinoma is 46% (Oral Oncol 2016;63:1)
- Frequent local recurrence and distant metastasis despite multimodal therapy
- Stage does not appear to affect the prognosis (Oral Oncol 2016;63:1)
Case reports
- 22 year old woman with small cell carcinoma of the maxillary sinus (Natl J Maxillofac Surg 2013;4:111)
- 50 year old woman with HPV associated combined neuroendocrine carcinoma and squamous cell carcinoma in the nasal cavity (Head Neck Pathol 2022;16:1227)
- 56 year old woman with large cell neuroendocrine carcinoma of the nasal cavity (NMC Case Rep J 2021;8:485)
- 68 year old man with small cell carcinoma of the paranasal sinus with intraoral extension (J Oral Maxillofac Pathol 2017;21:286)
Treatment
- There are no specific management guidelines due to rarity of the tumor
- Multimodality treatment has been used with variable results
- Most common treatment modality is combined chemotherapy and radiation therapy (Int Forum Allergy Rhinol 2016;6:744)
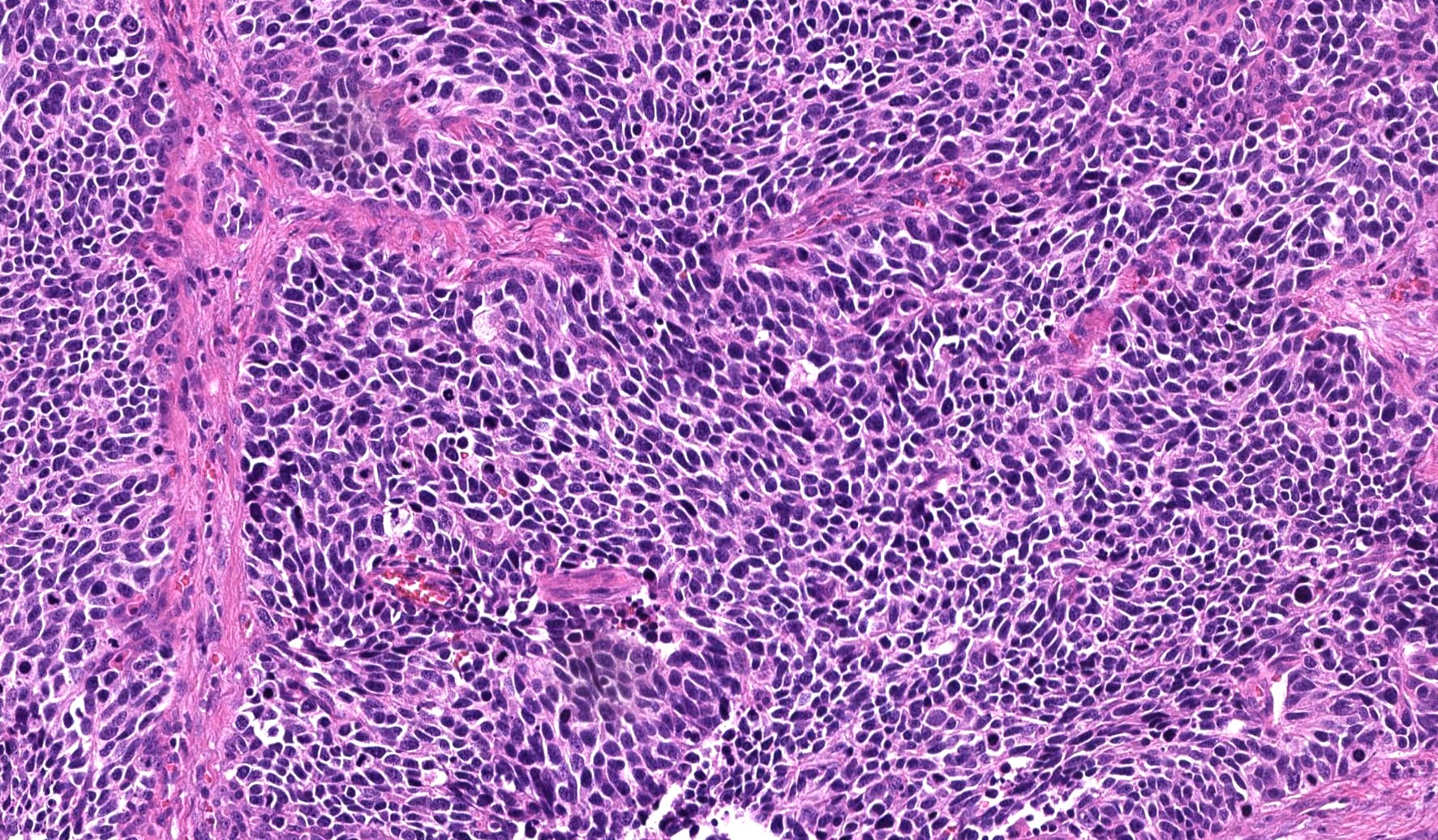
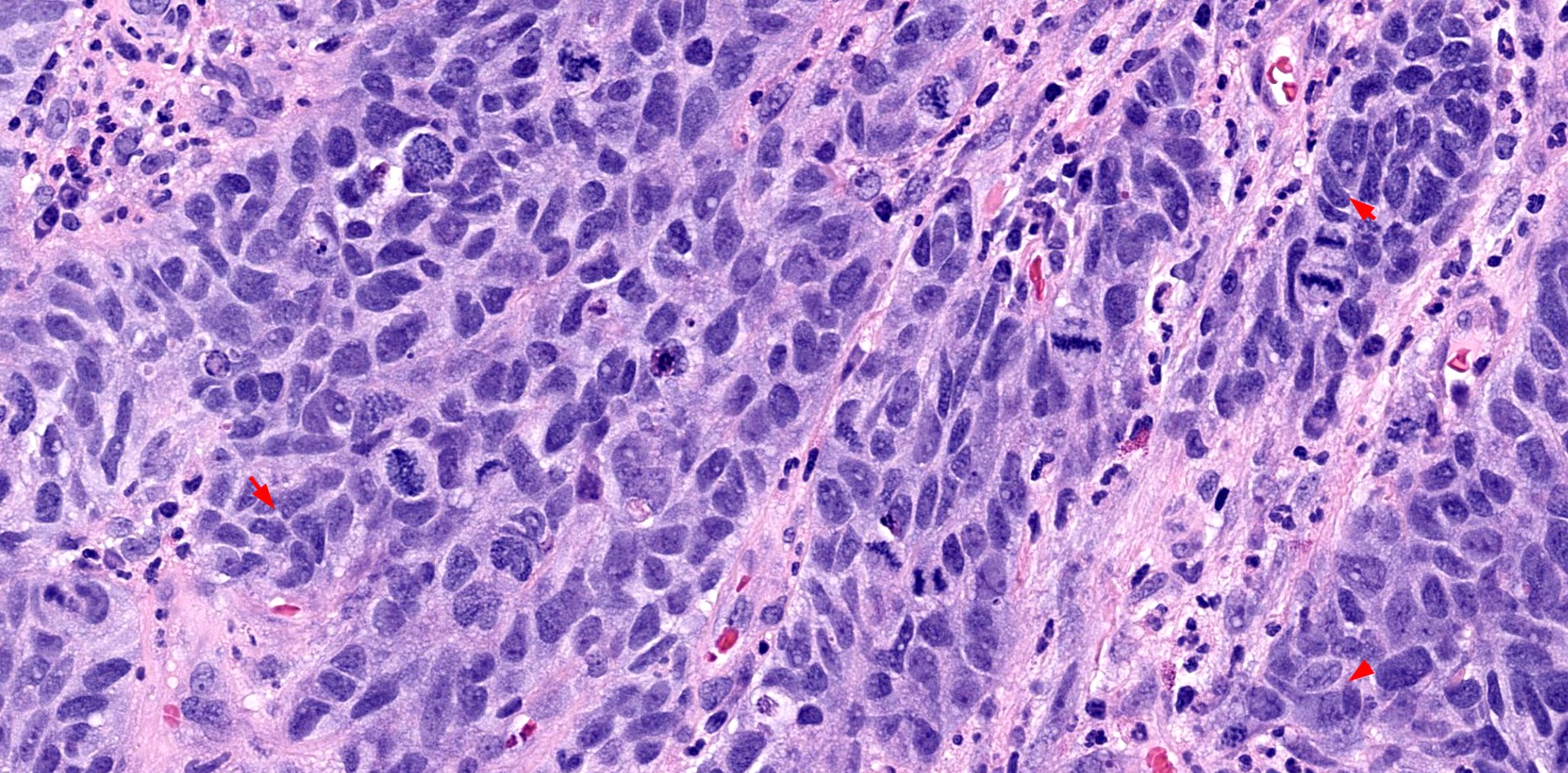
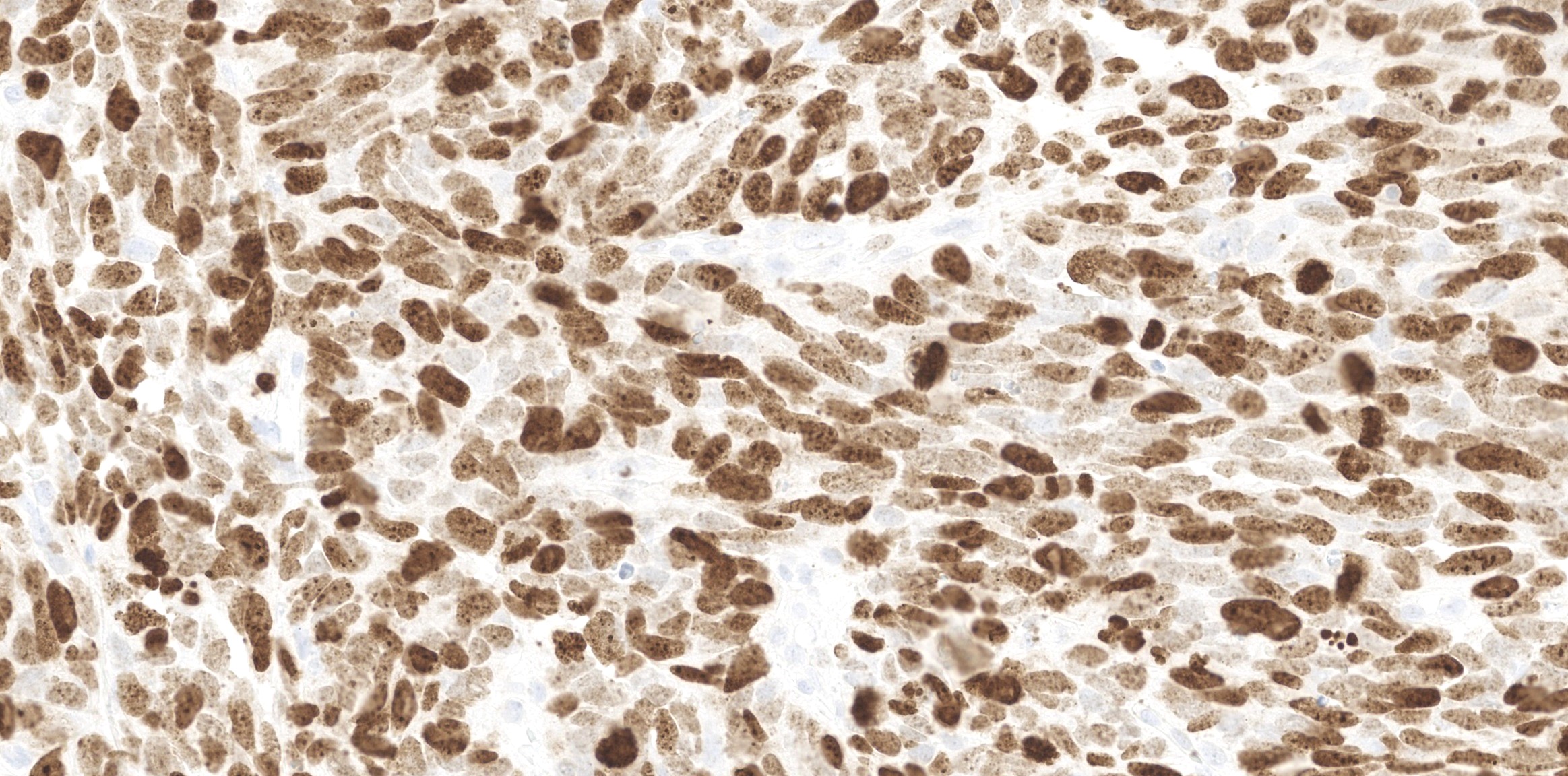
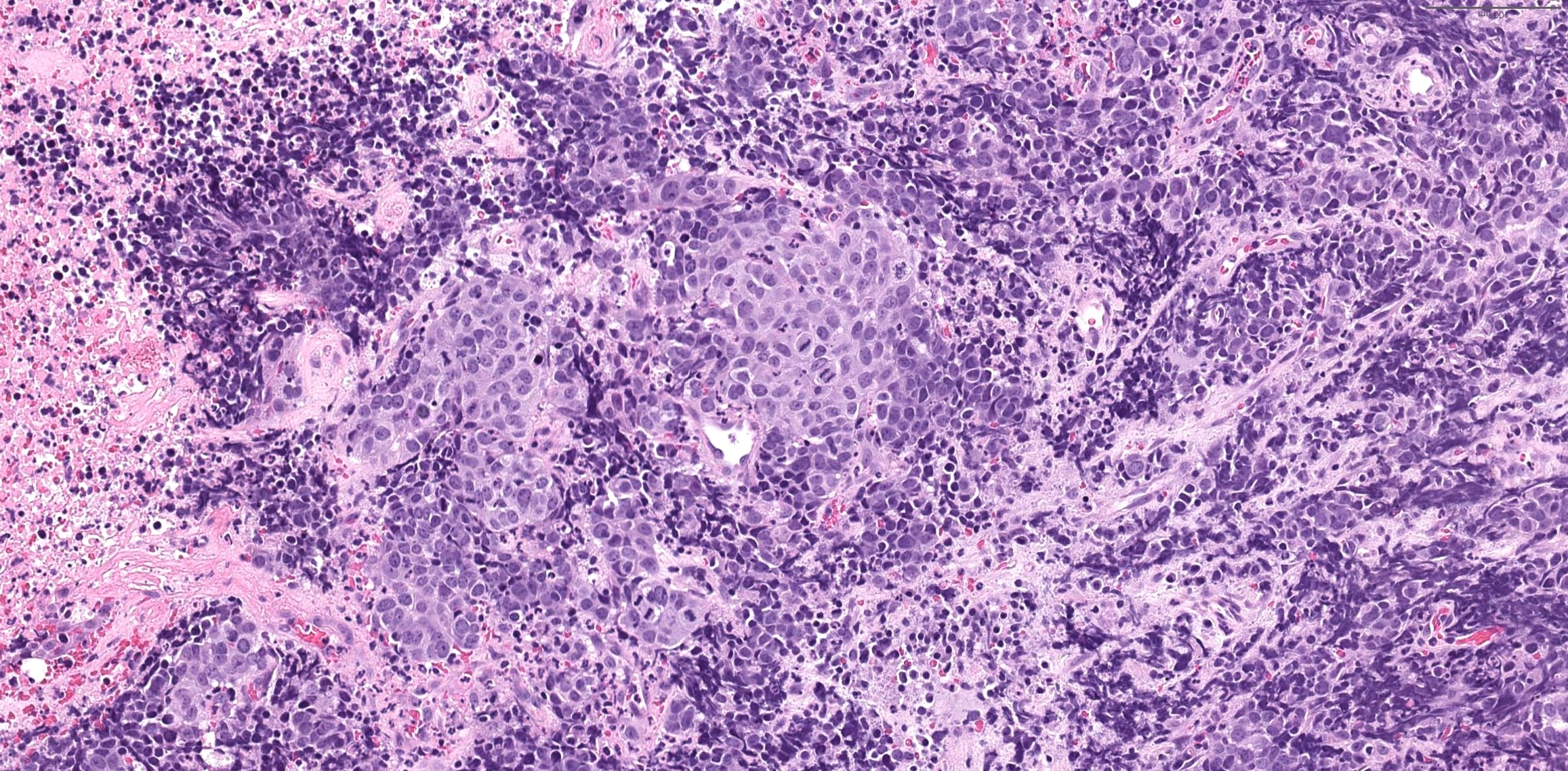
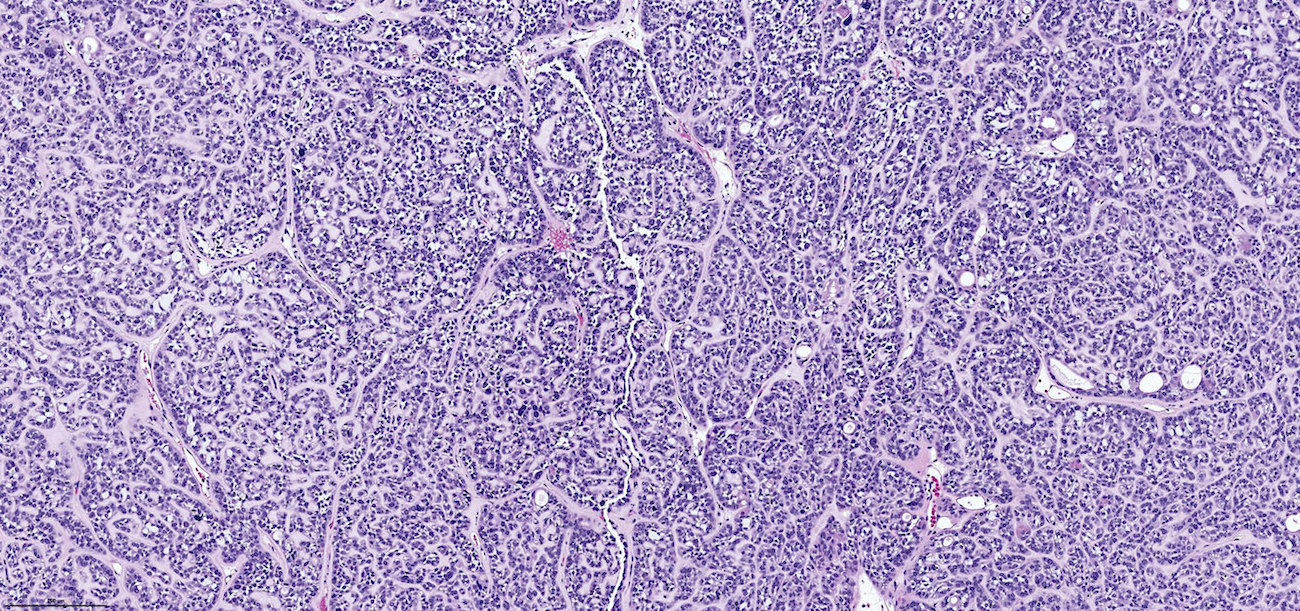
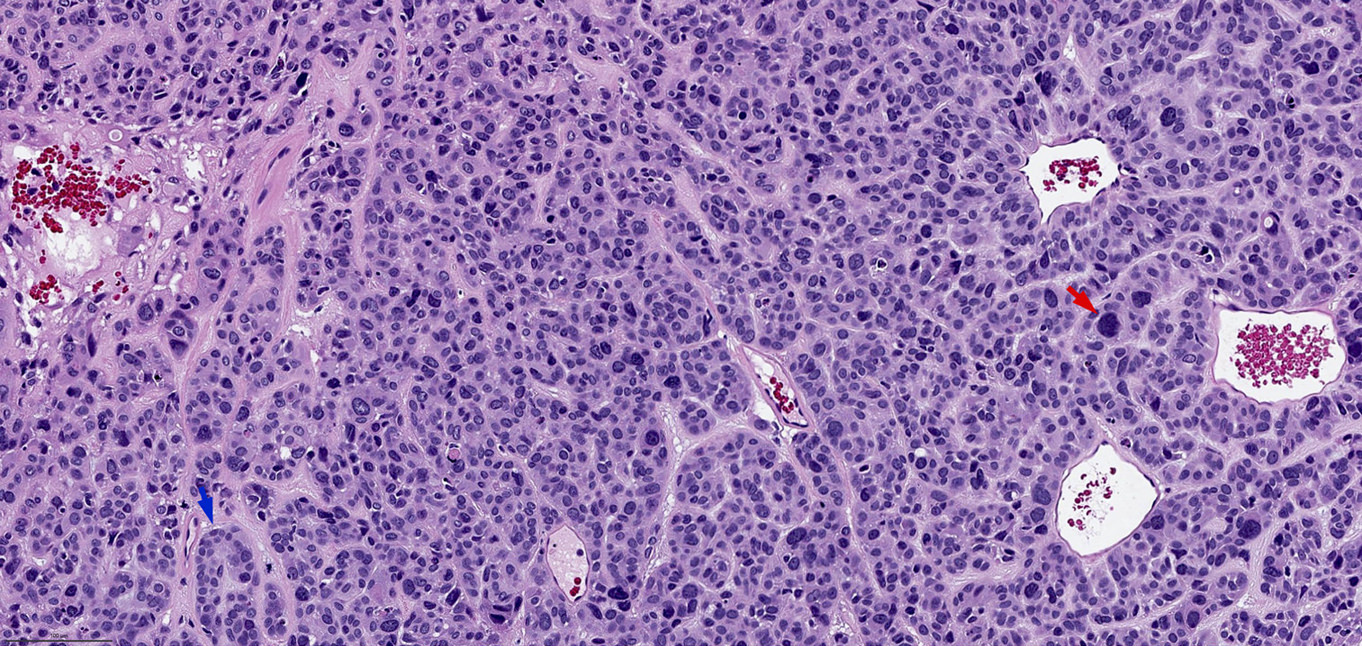
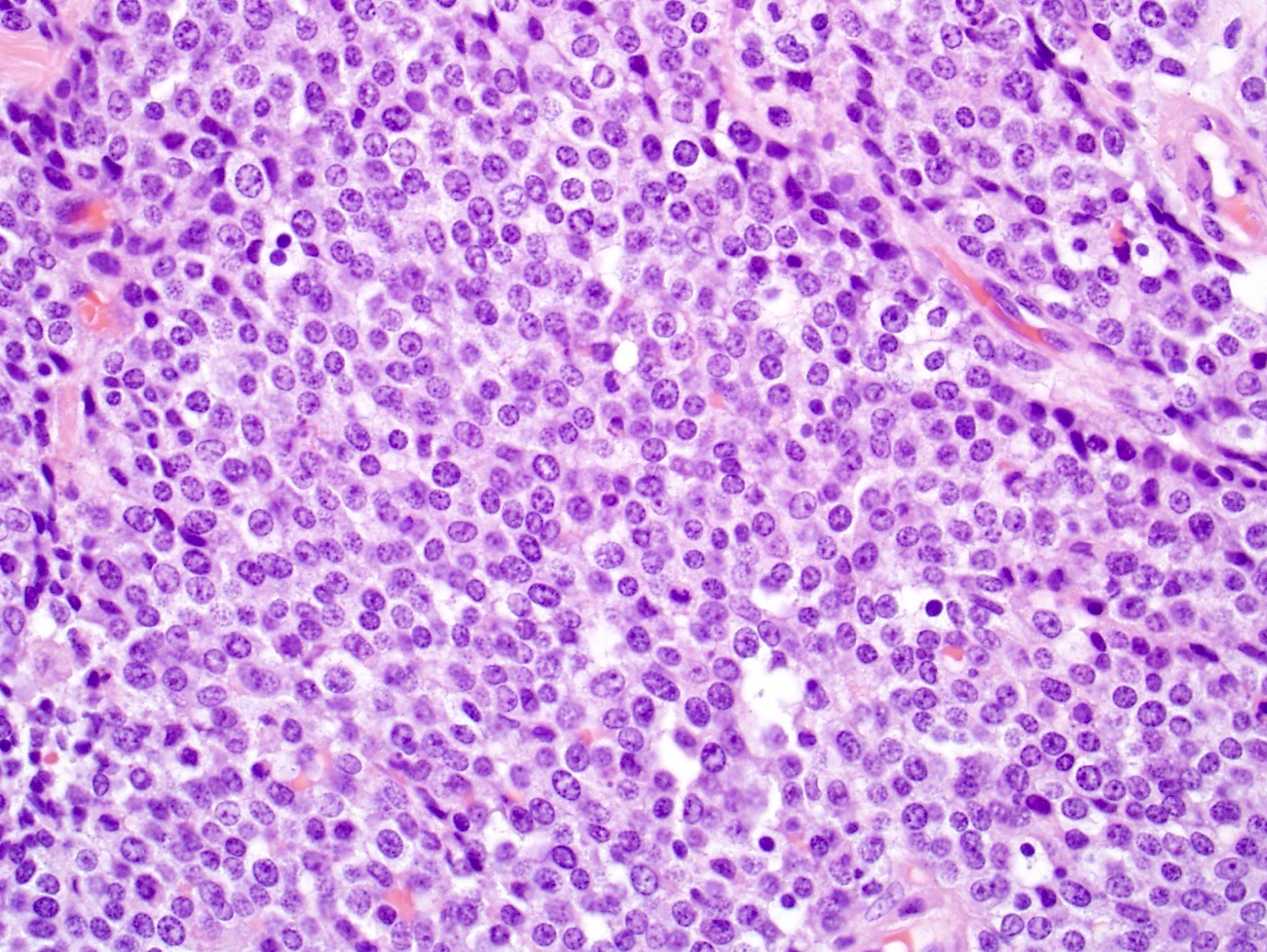
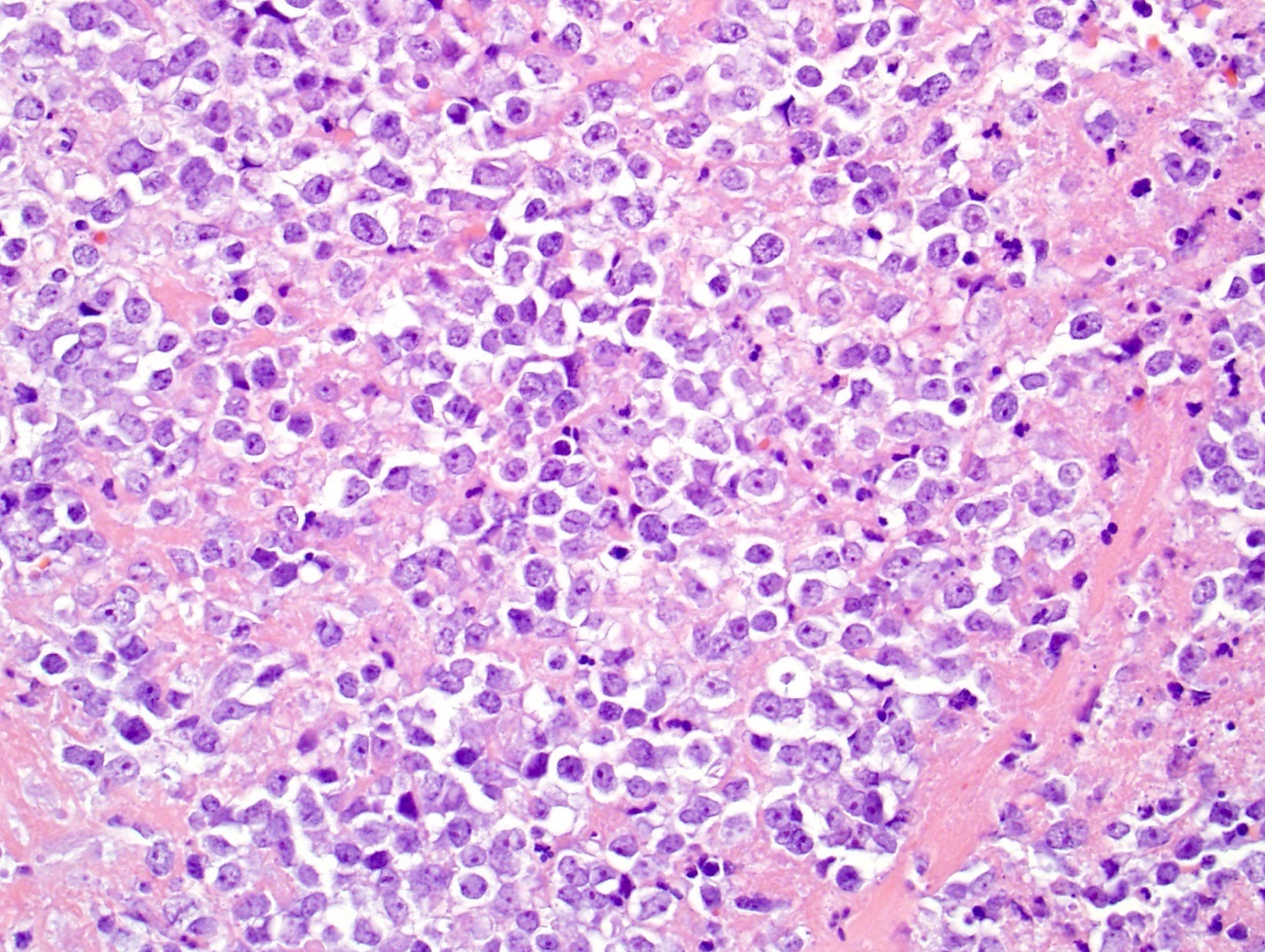
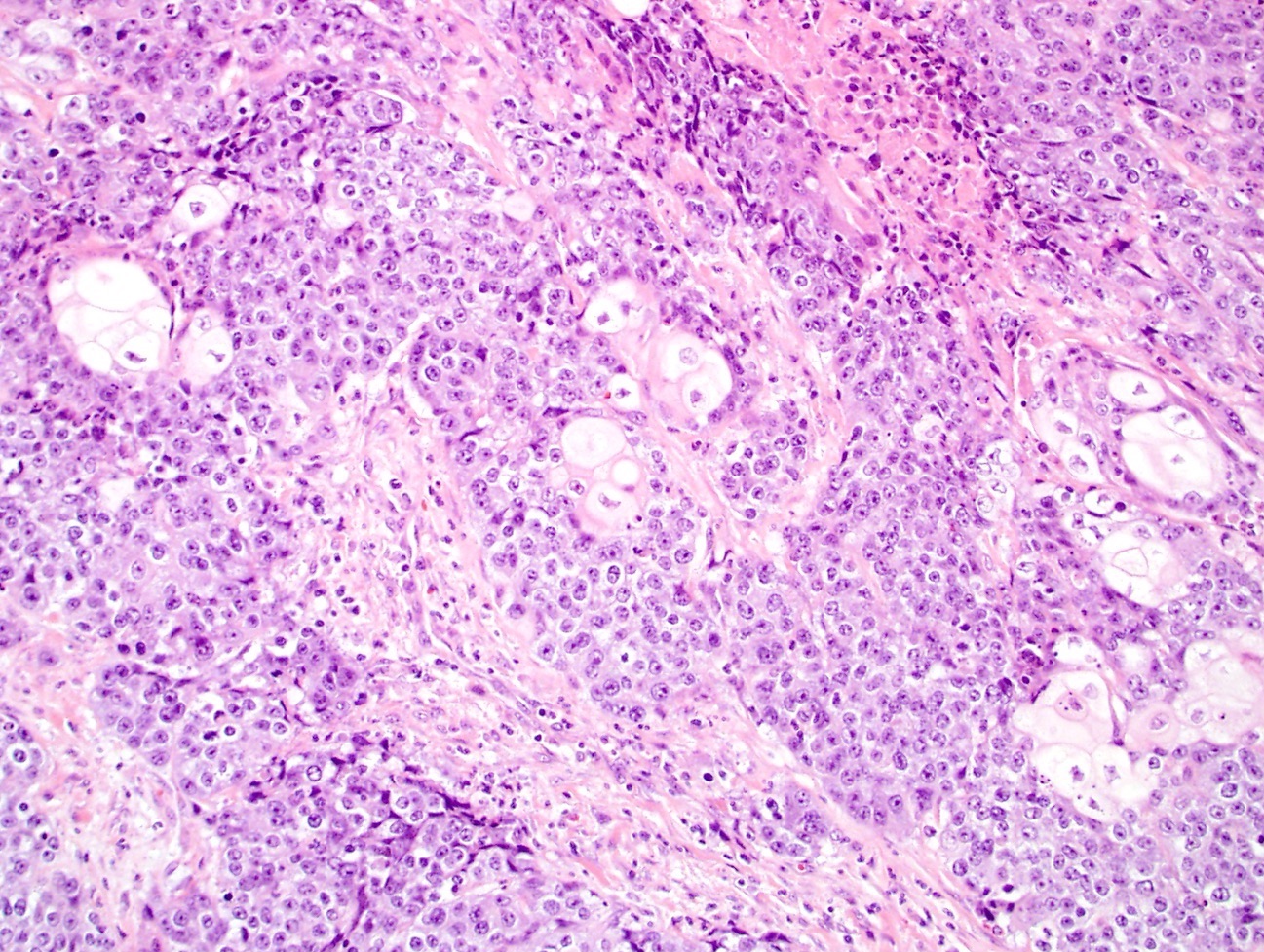
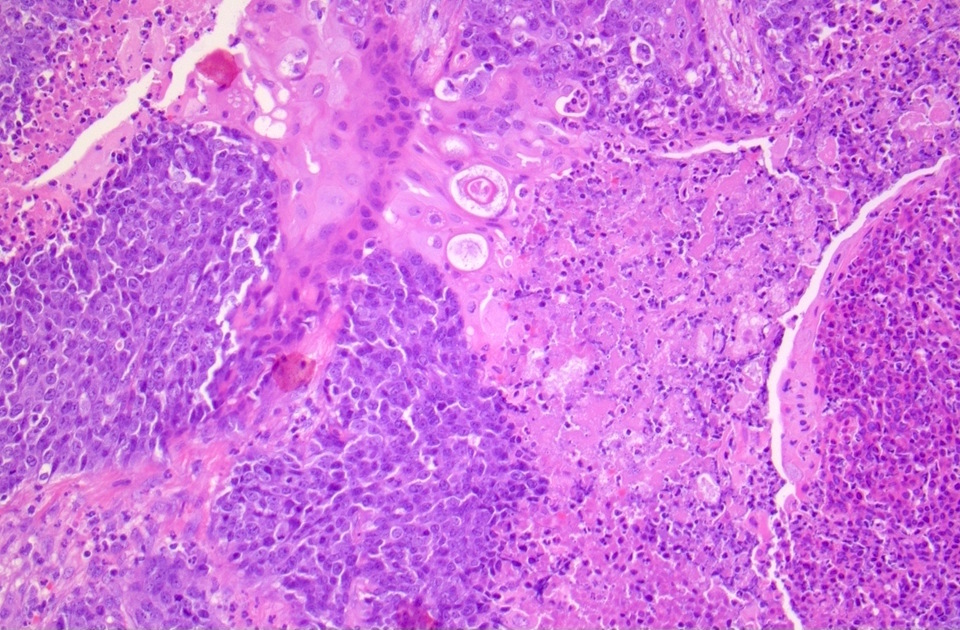
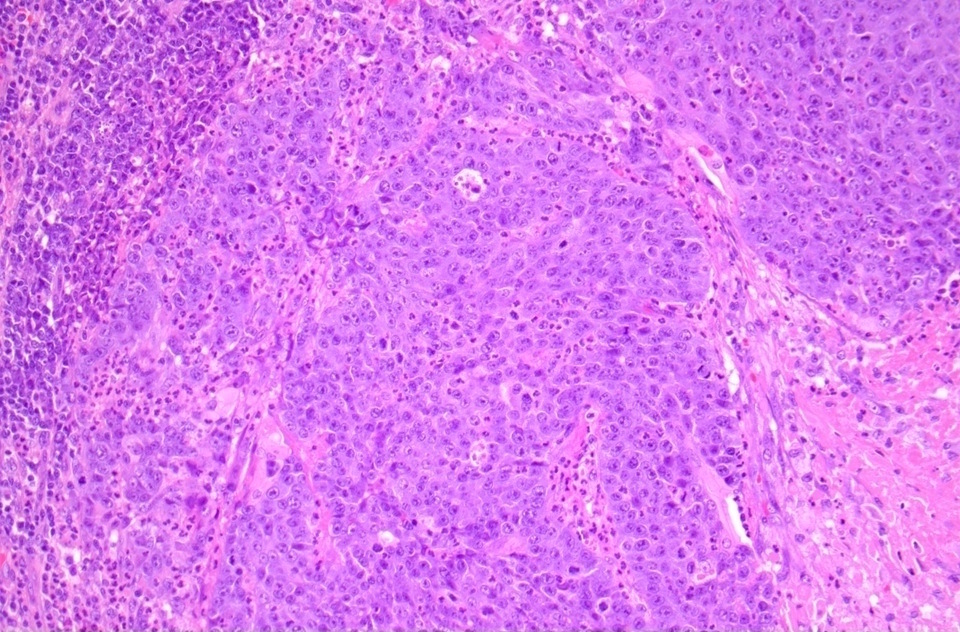
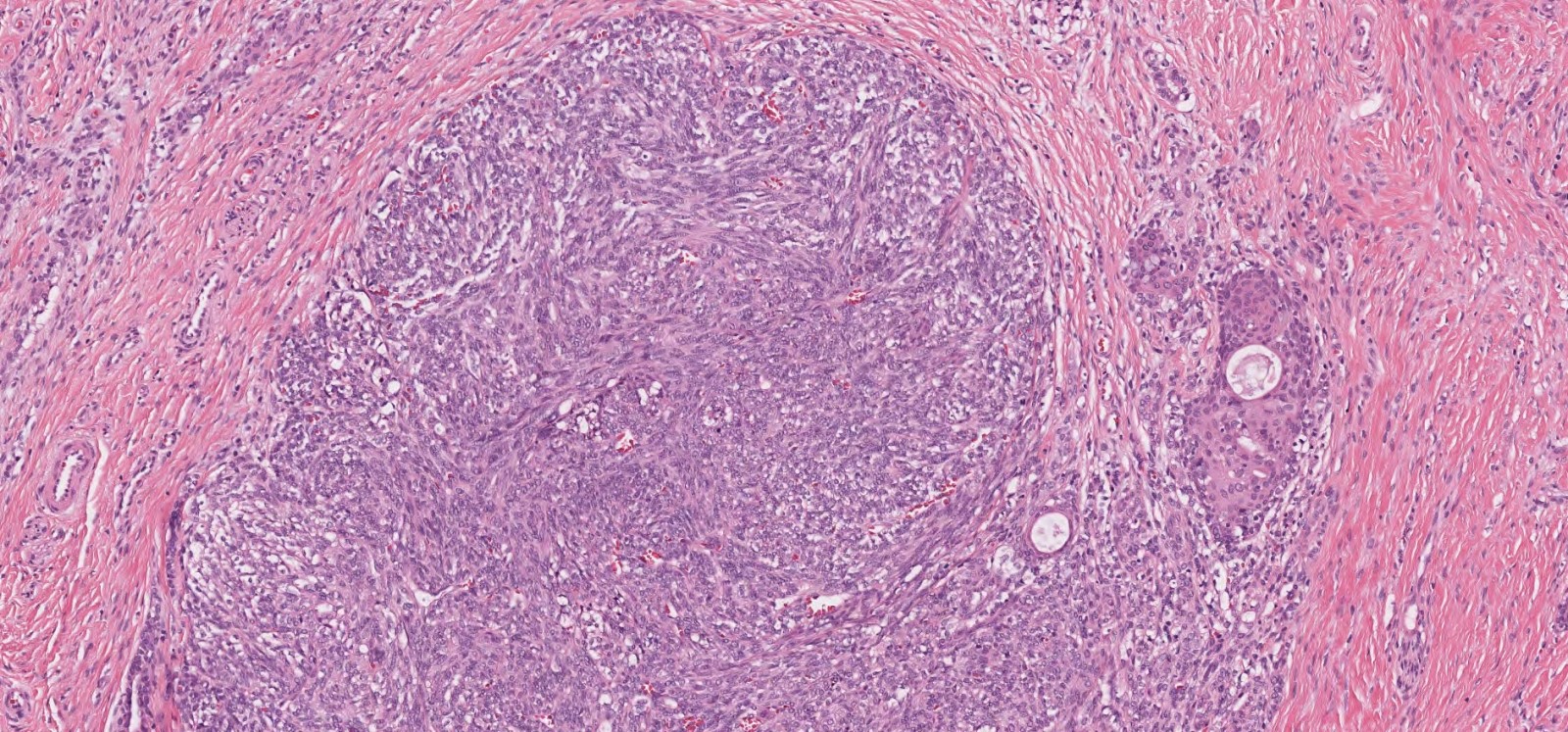
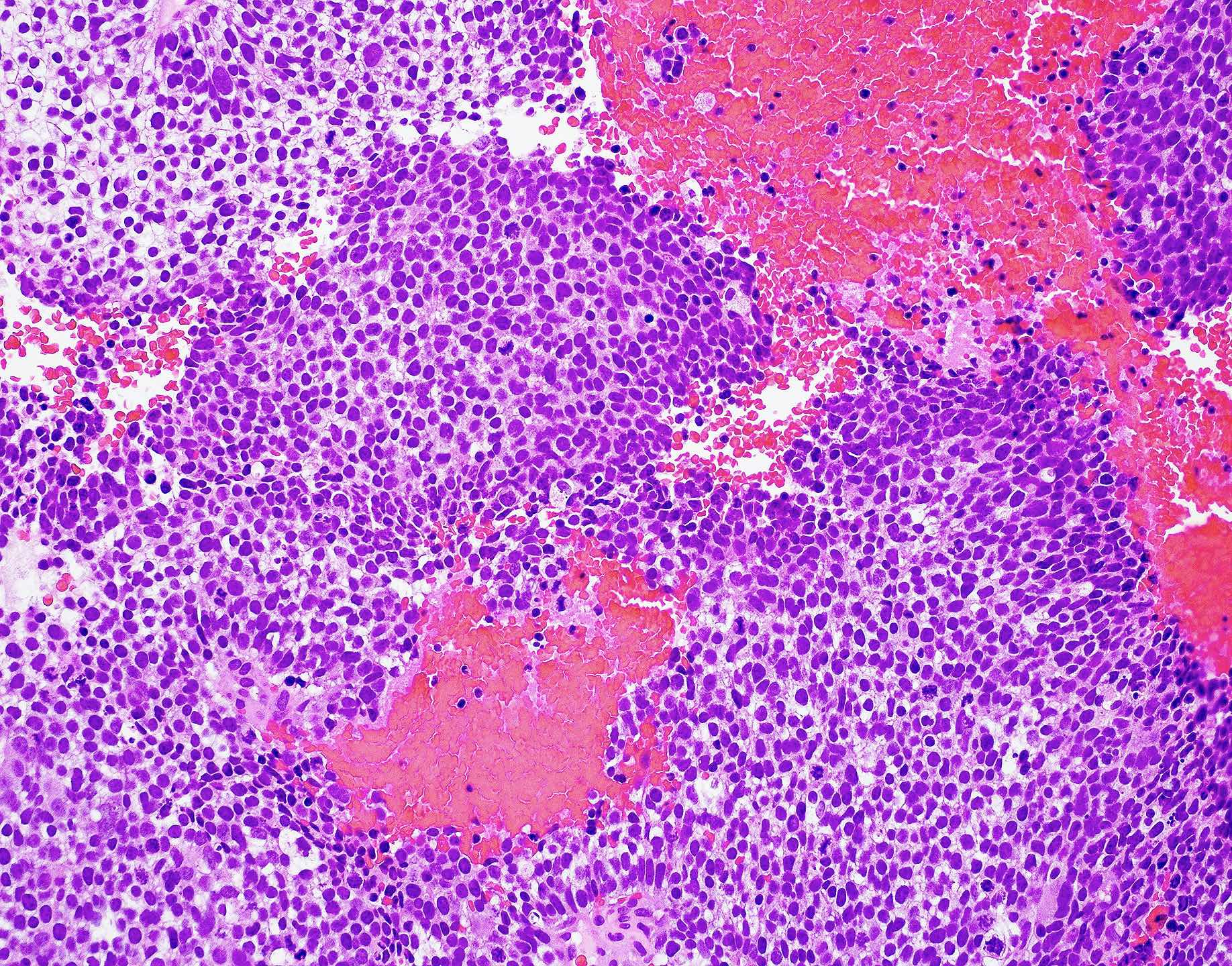
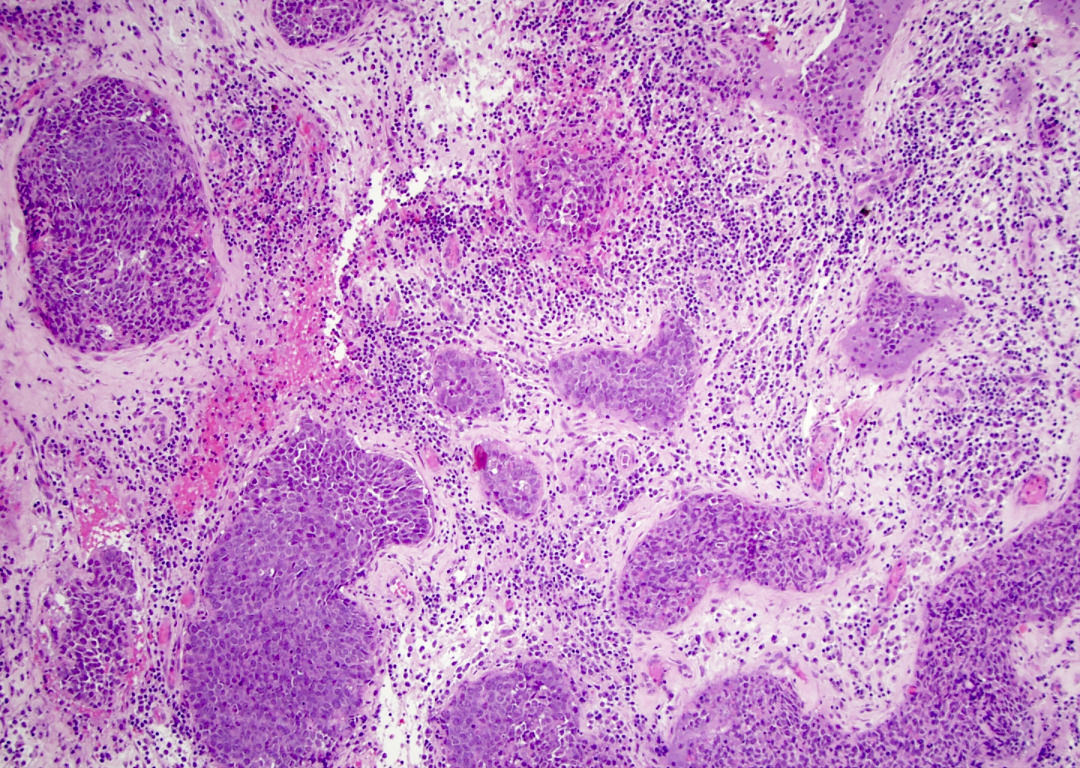
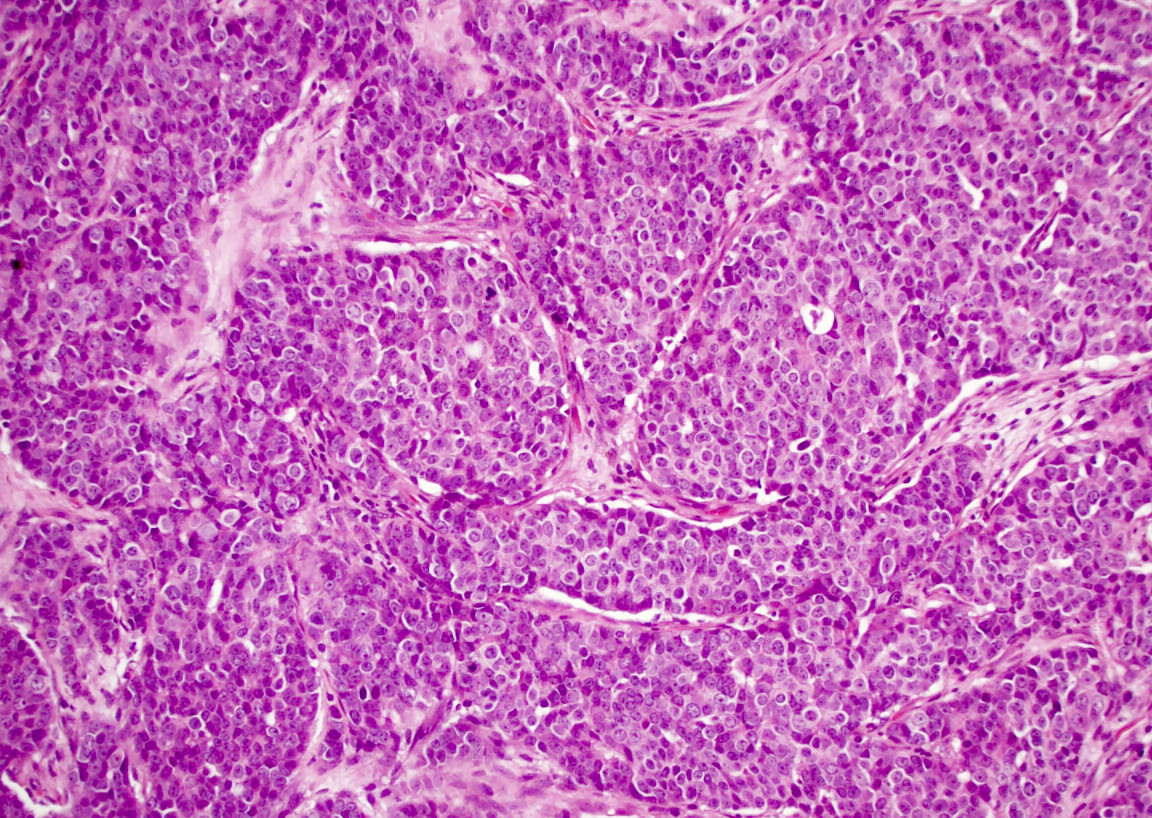
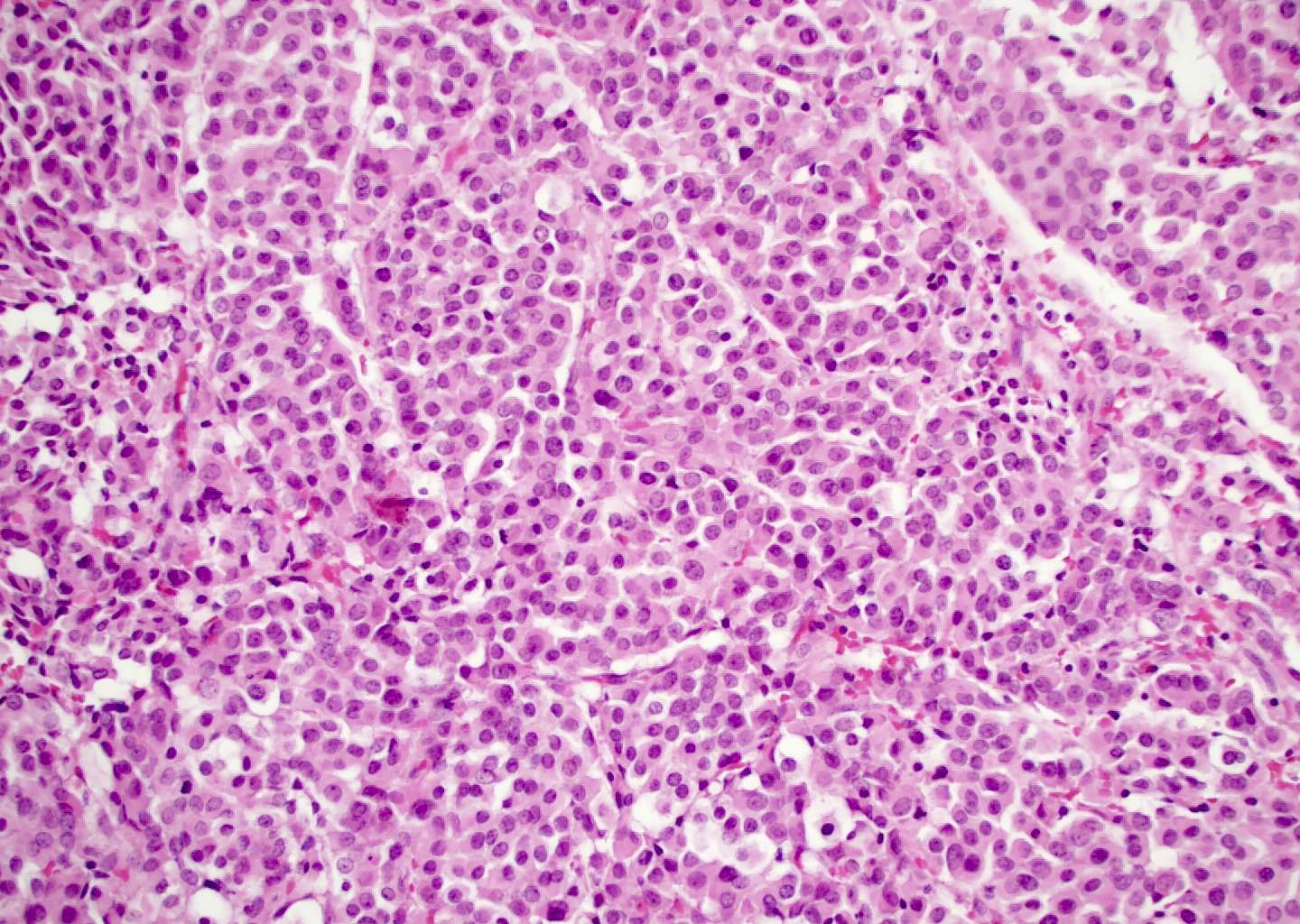
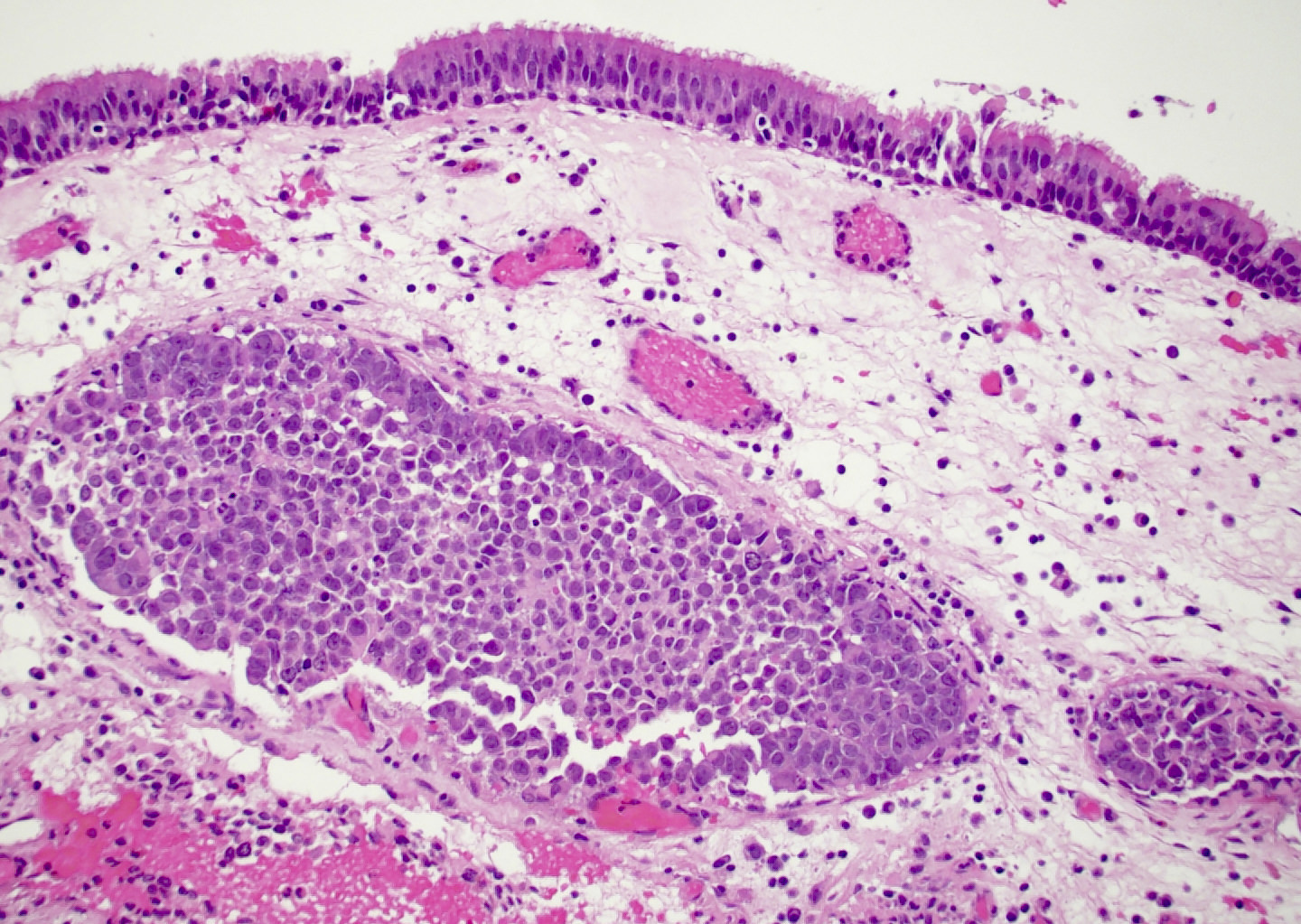
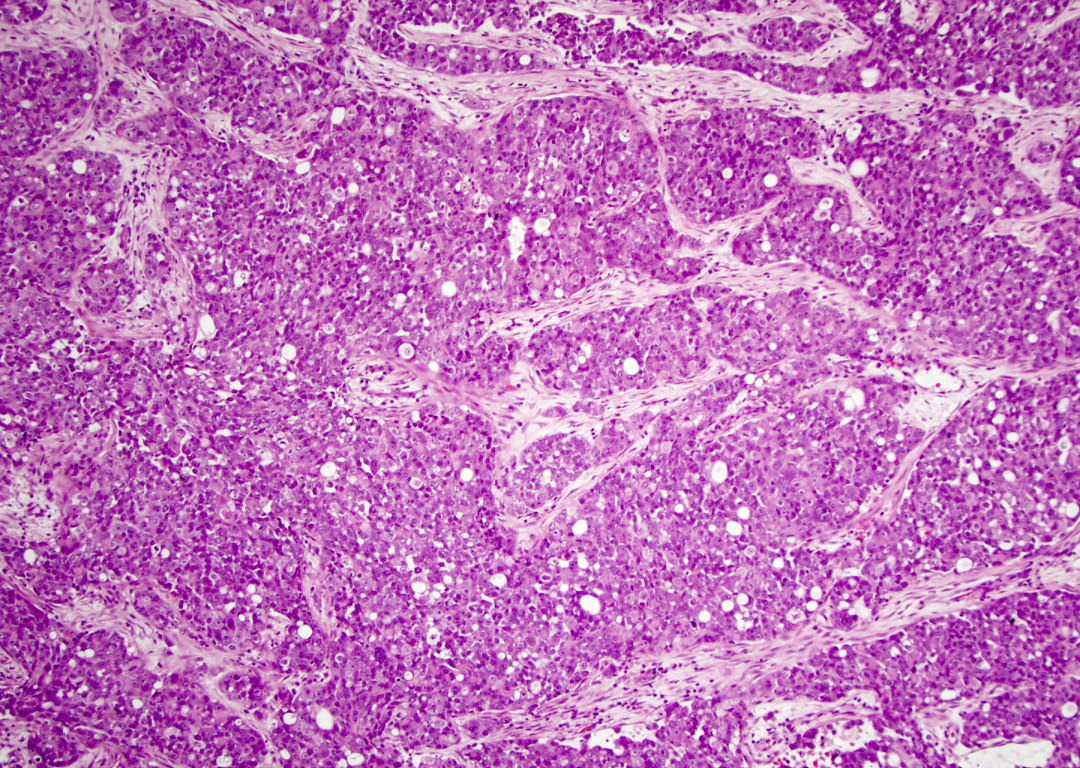
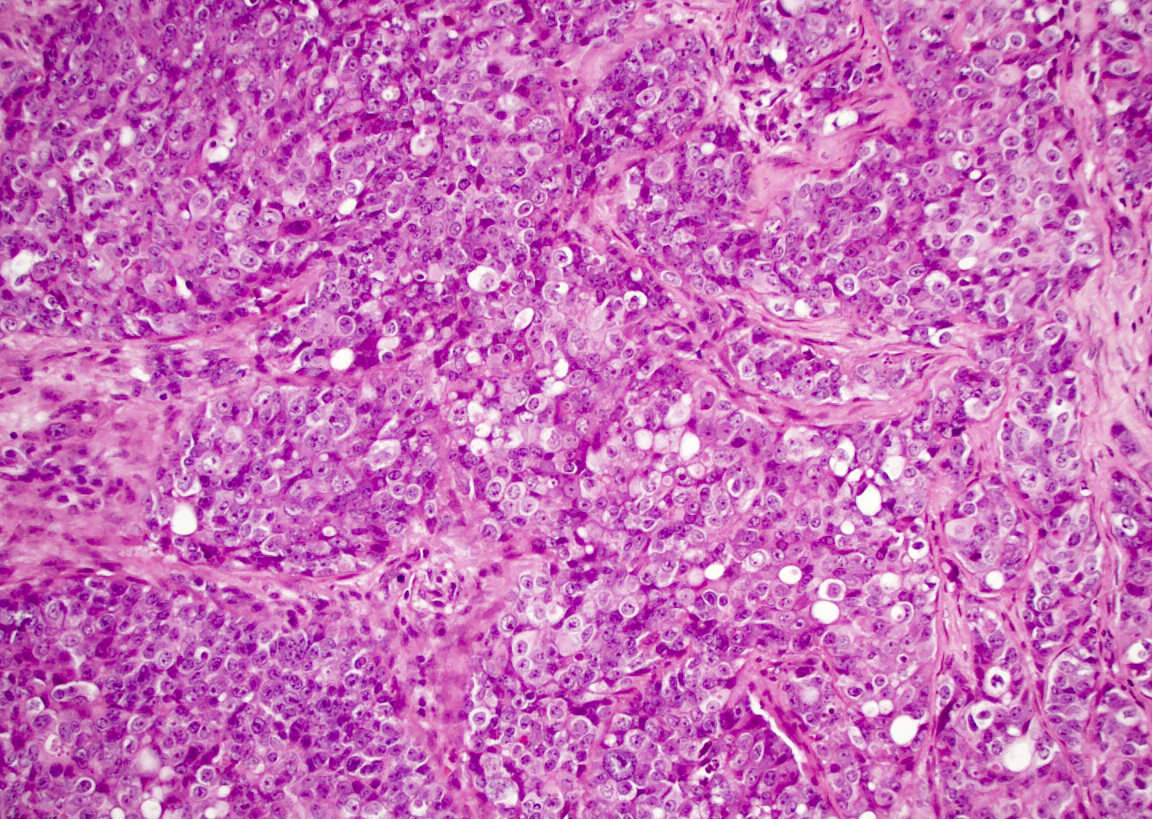
Microscopic (histologic) description
- High grade neuroendocrine carcinoma (general features)
- Mitotic index > 10 mitoses per 2 mm2, frequent apoptotic bodies and tumor necrosis
- Solid sheets or trabeculae of tumor cells, sometimes with peripheral palisading or rosette formation
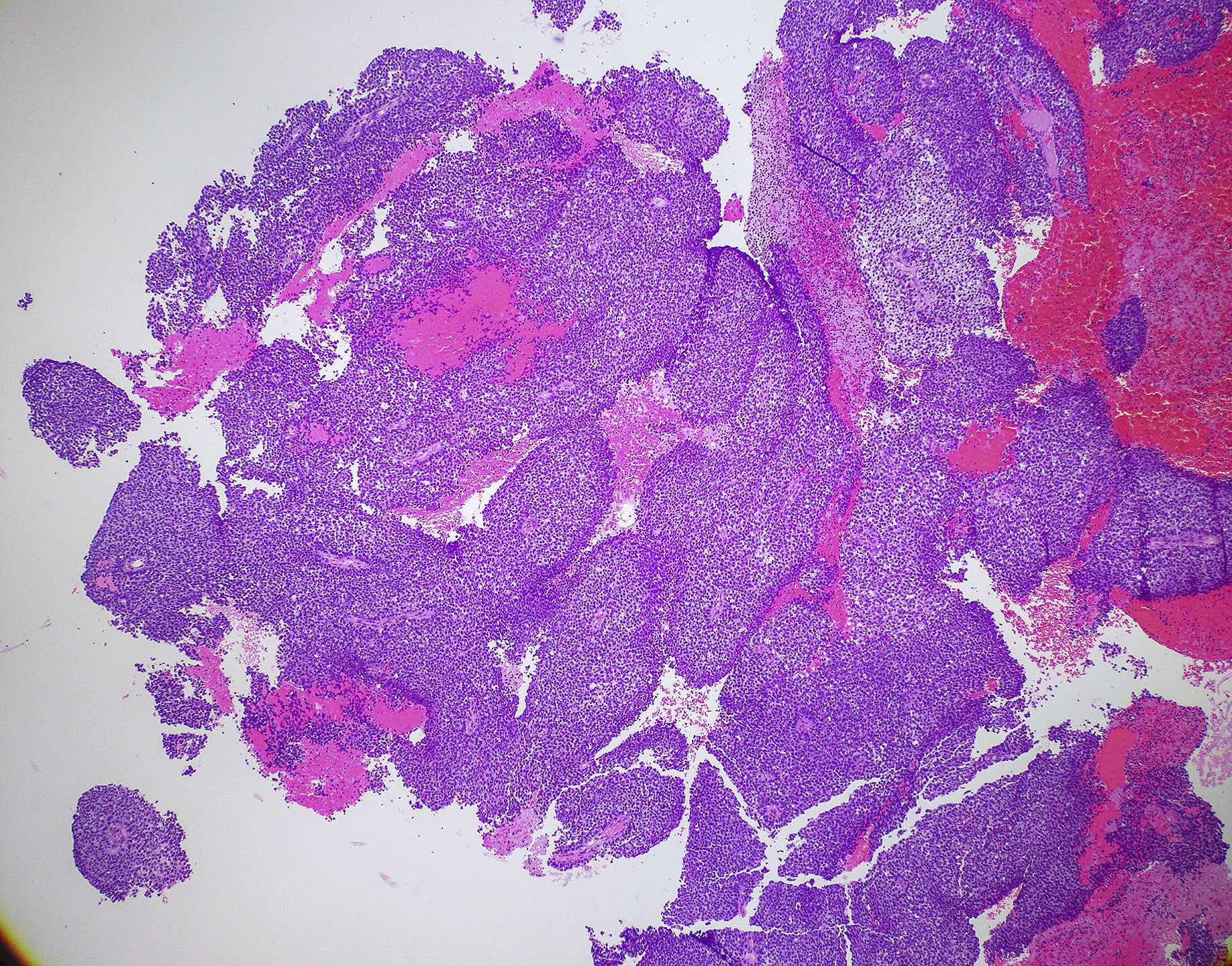
- Small cell carcinoma
- Small to medium sized cells: the diameter of tumor cells is < 3 times the diameter of a lymphocyte
- Scanty cytoplasm and indistinct cell boundary
- Finely granular salt and pepper chromatin
- No or inconspicuous nuclei
- Nuclear molding (conformity of adjacent nuclei to one another), nuclear spindling and crush artifacts of the nuclei may be seen
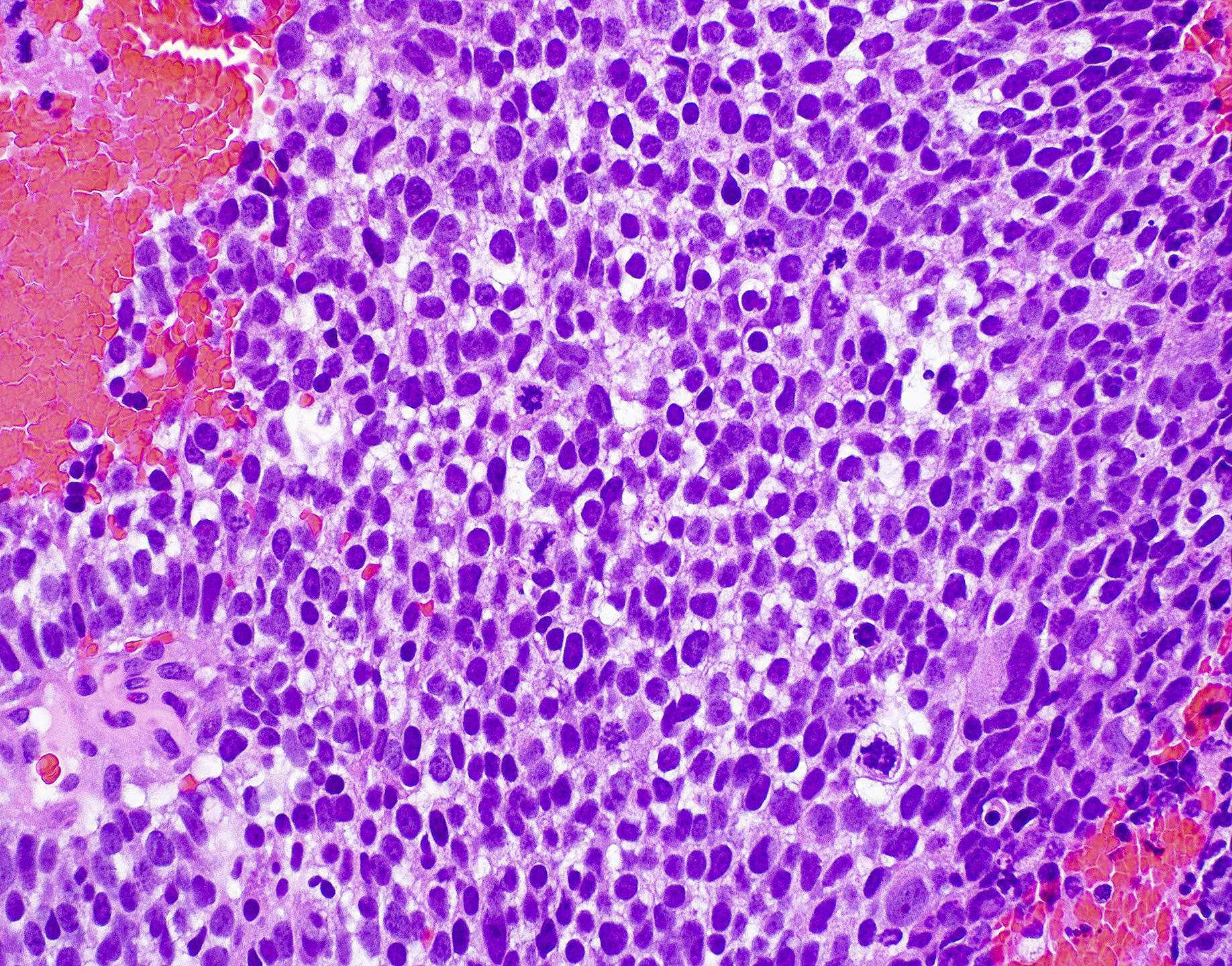
- Large cell neuroendocrine carcinoma
- Large sized cells: the diameter of tumor cells is > 3 times the diameter of a lymphocyte
- Abundant amphophilic to eosinophilic cytoplasm and distinct cell membrane
- Large nuclei with prominent nucleoli; chromatin pattern is variable, ranging from granular, vesicular, to coarse
- Combined neuroendocrine carcinoma
- Tumor that is composed of a high grade neuroendocrine carcinoma and a nonneuroendocrine carcinoma, commonly a squamous cell carcinoma
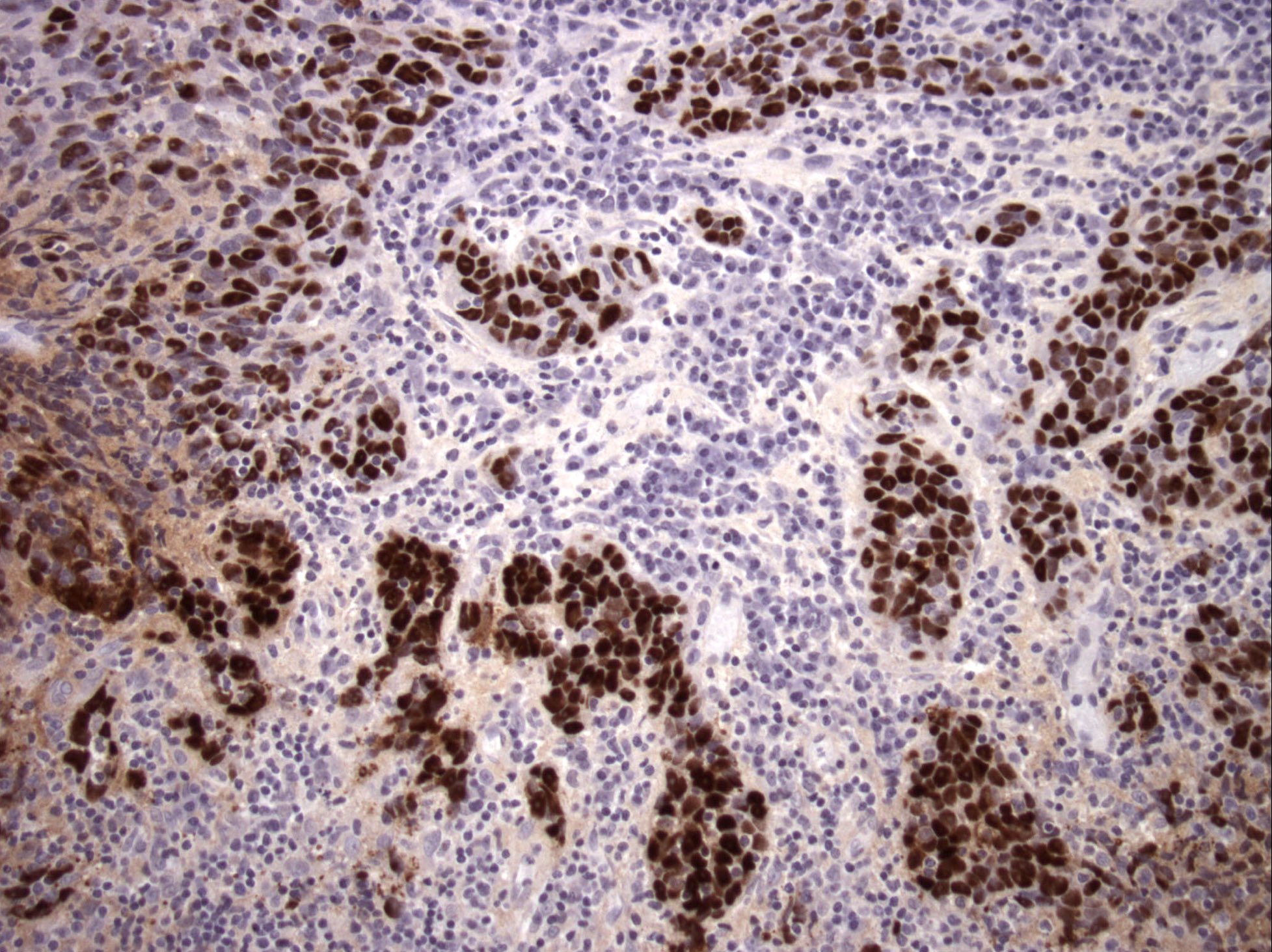
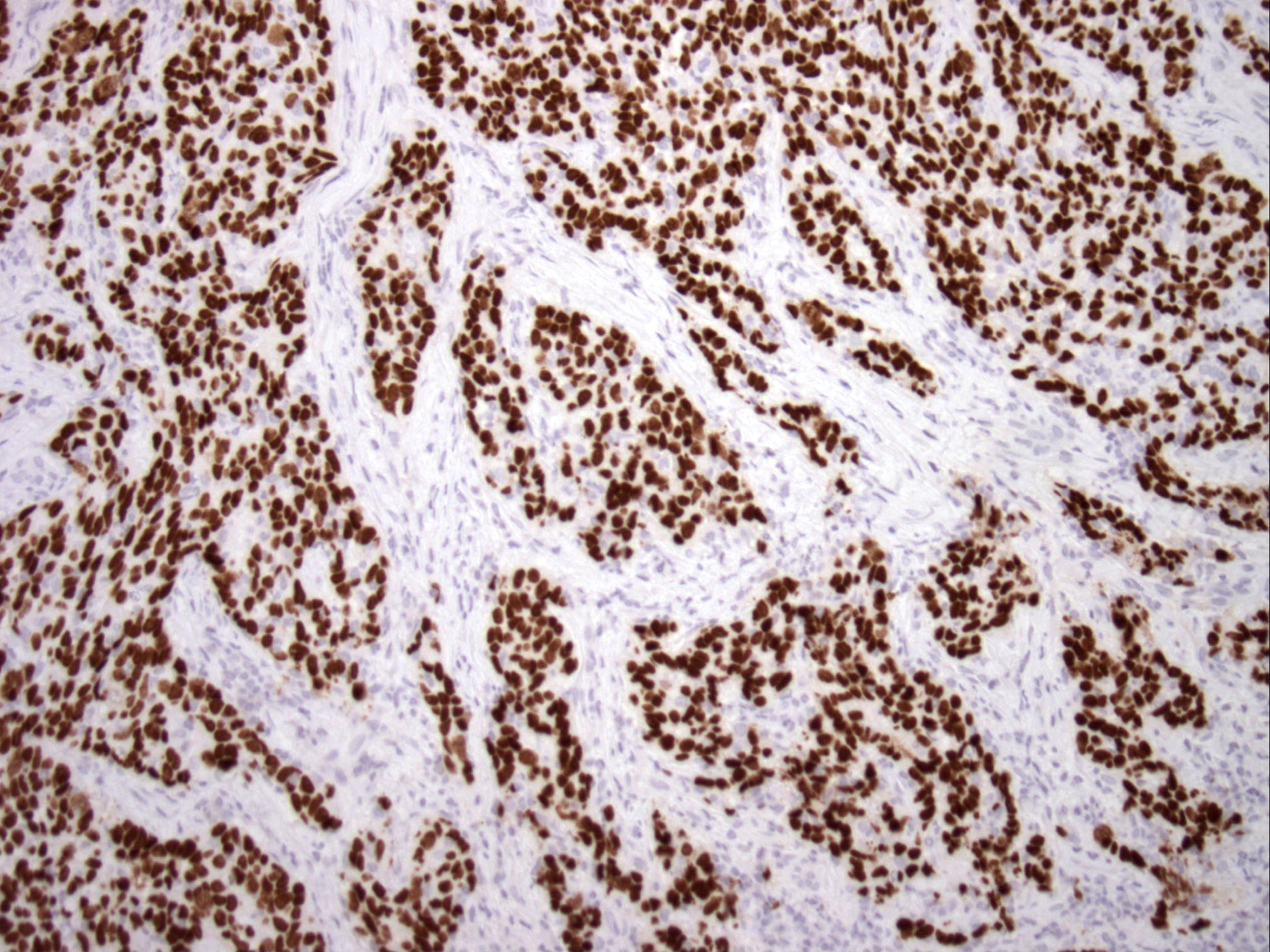
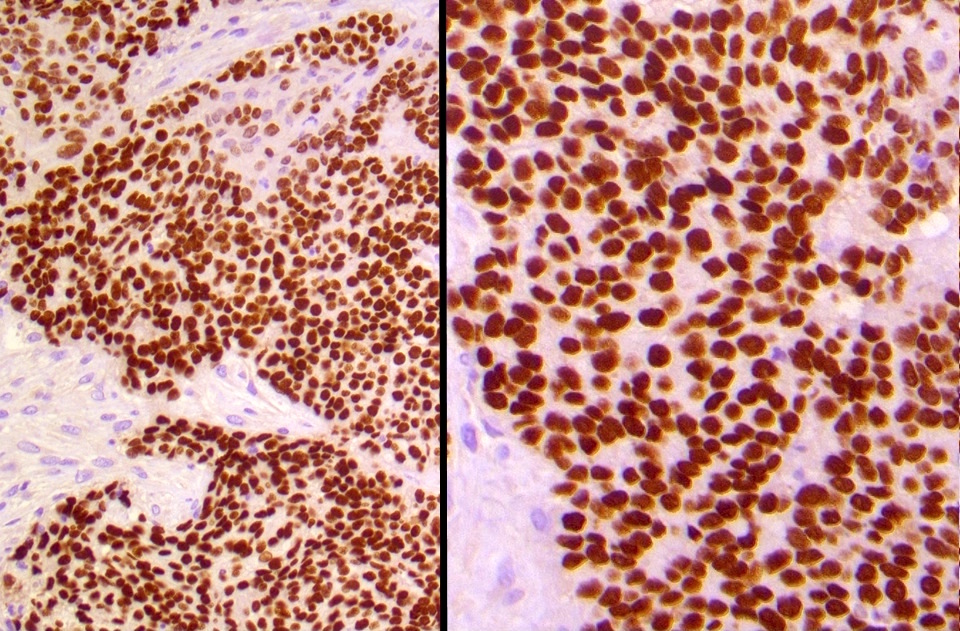
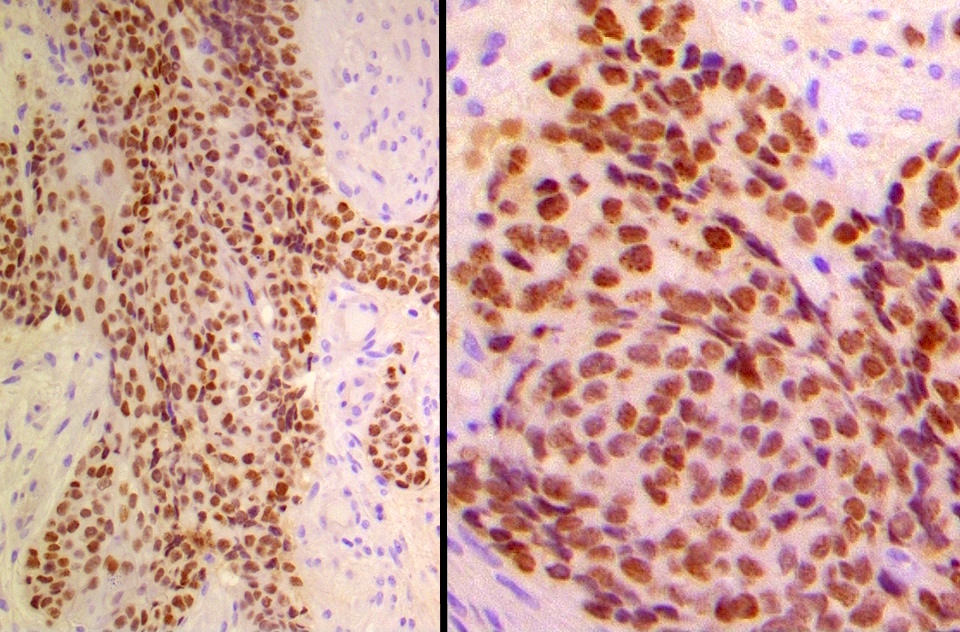
Microscopic (histologic) images
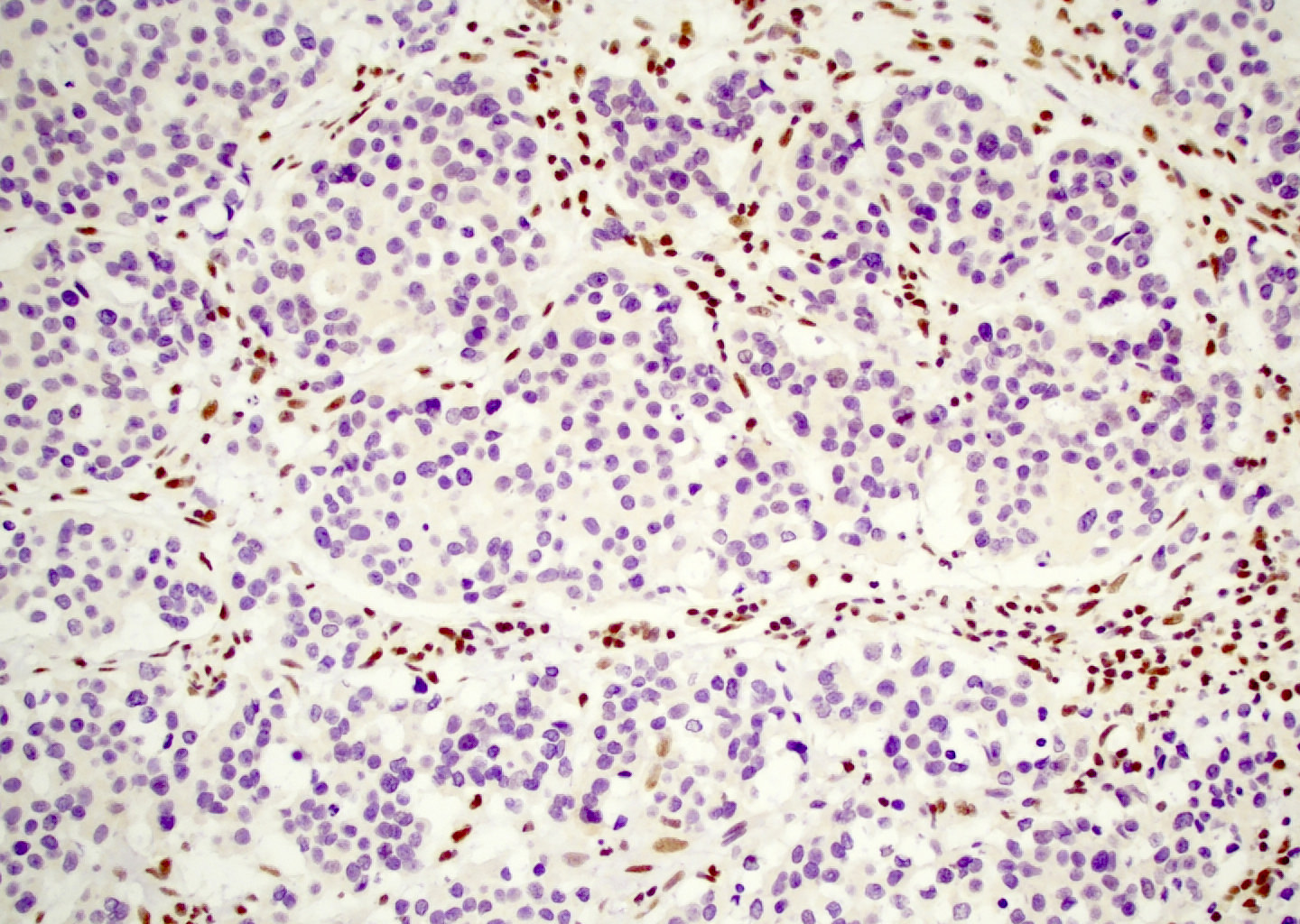
Contributed by Bin Xu, M.D., Ph.D.
Small cell carcinoma
Large cell neuroendocrine carcinoma
Combined small cell carcinoma and squamous cell carcinoma
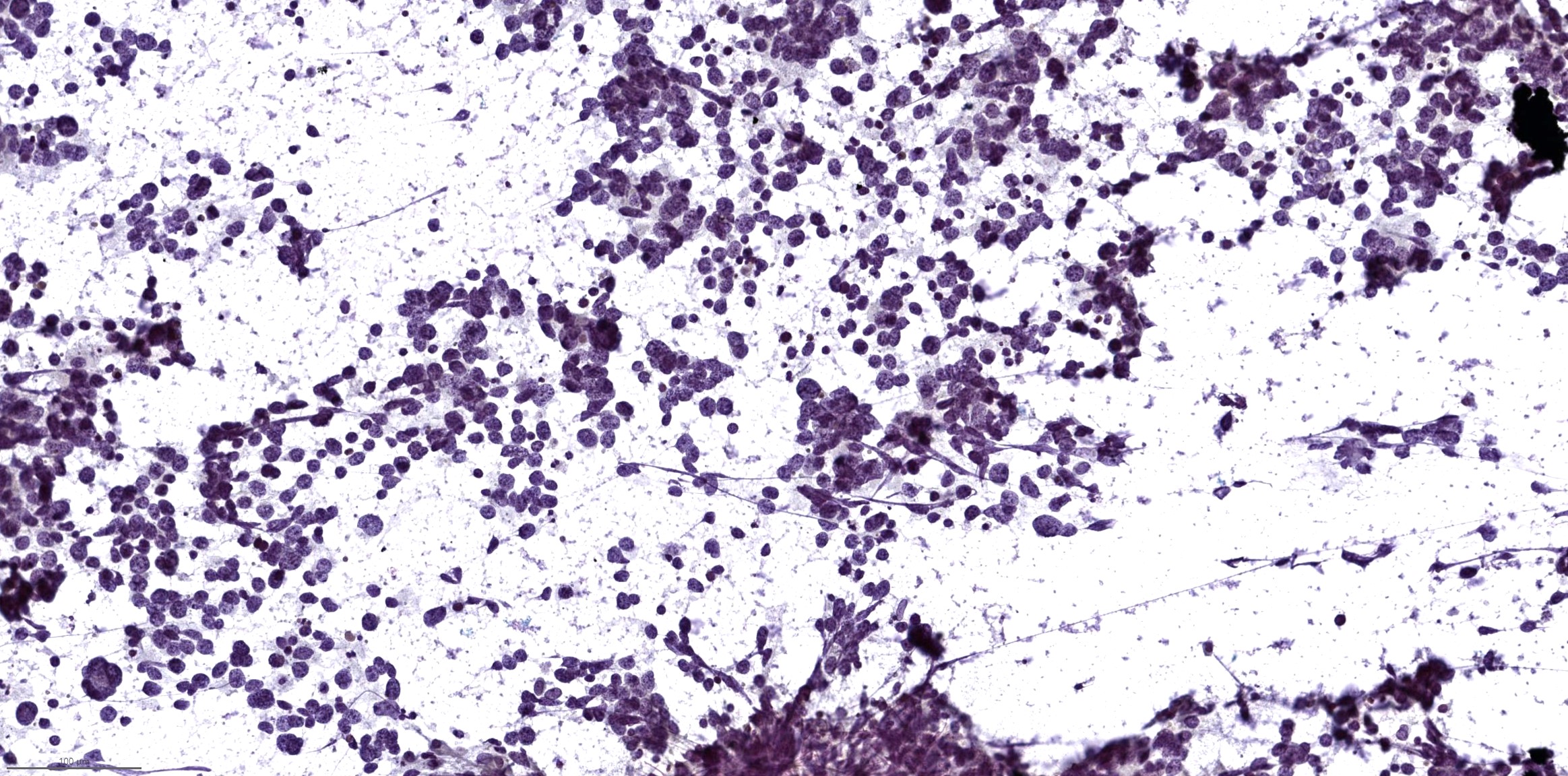
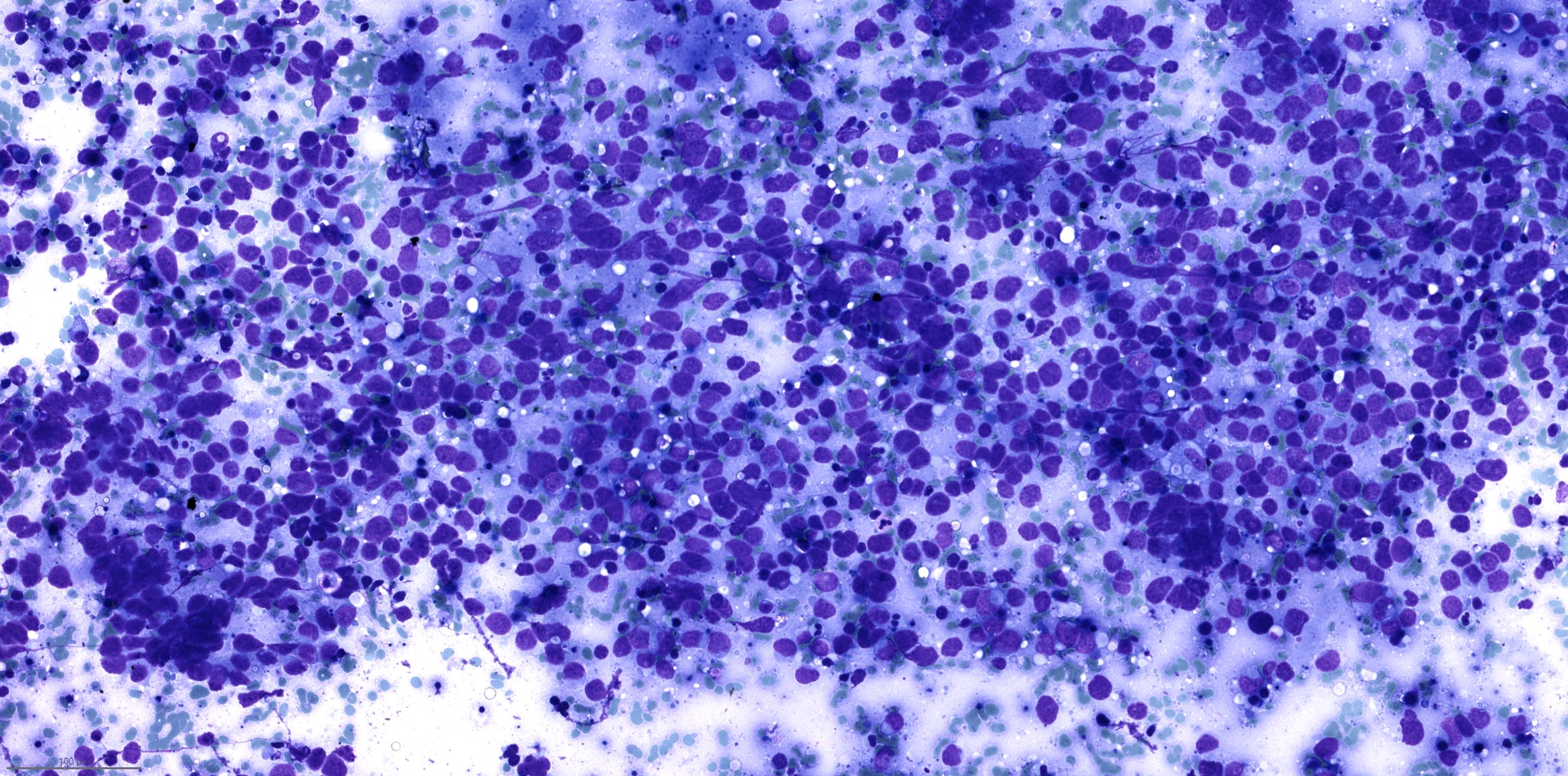
Cytology description
- Small cell carcinoma: hypercellular sample, individual cells or small loose clusters, hyperchromatic nuclei, no nucleoli, nuclear molding, crush artifact and necrotic background
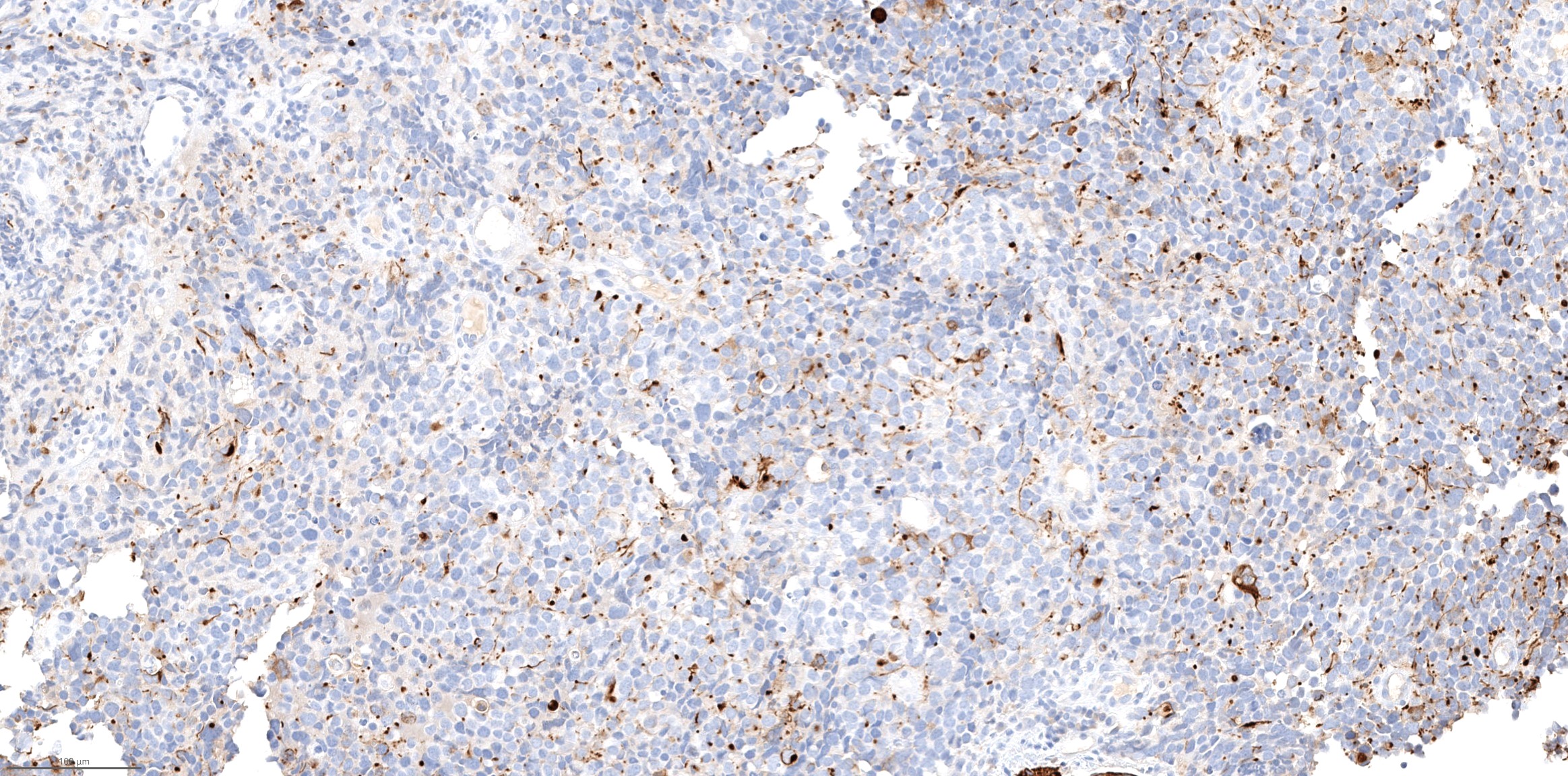
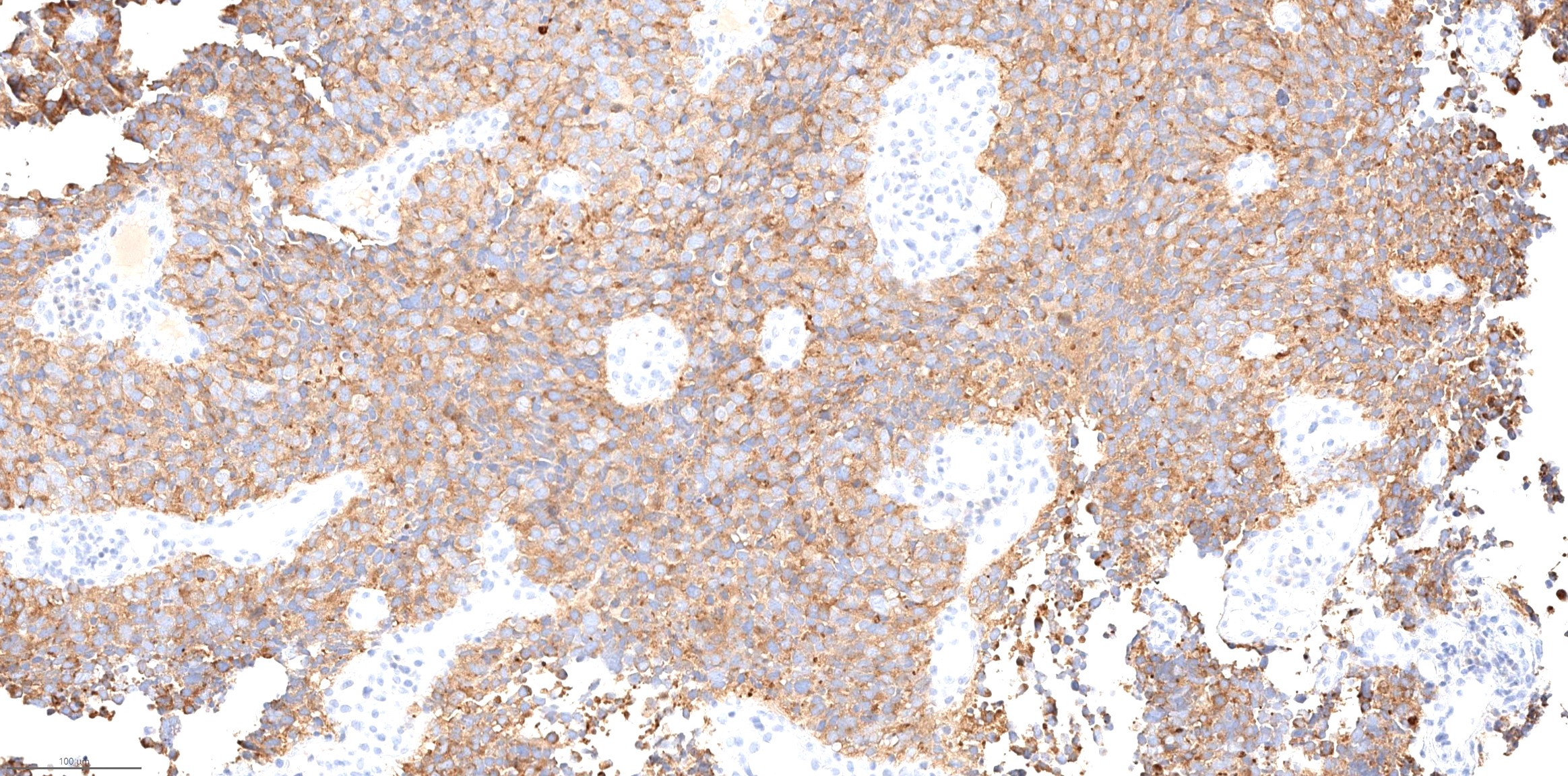
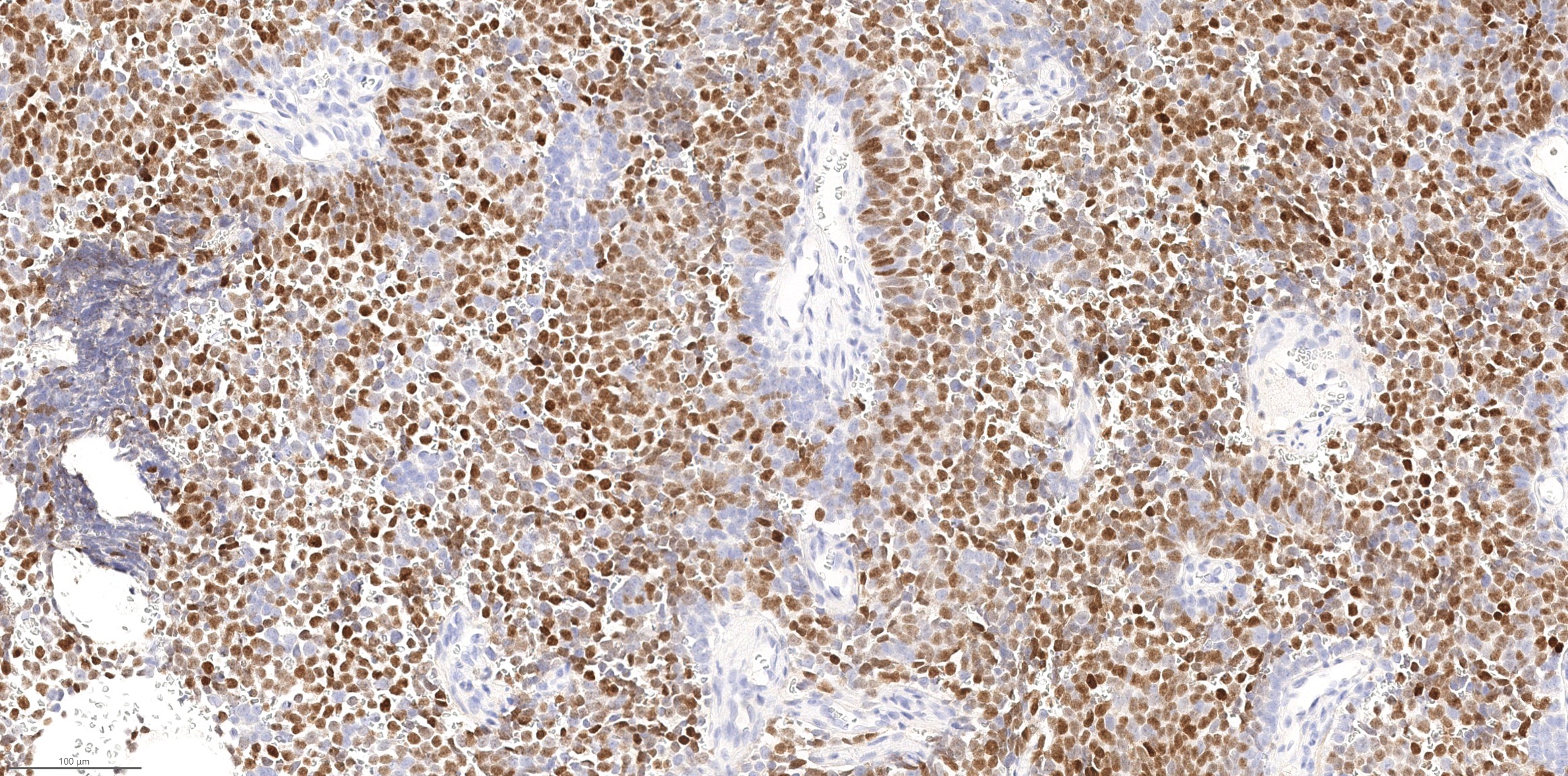
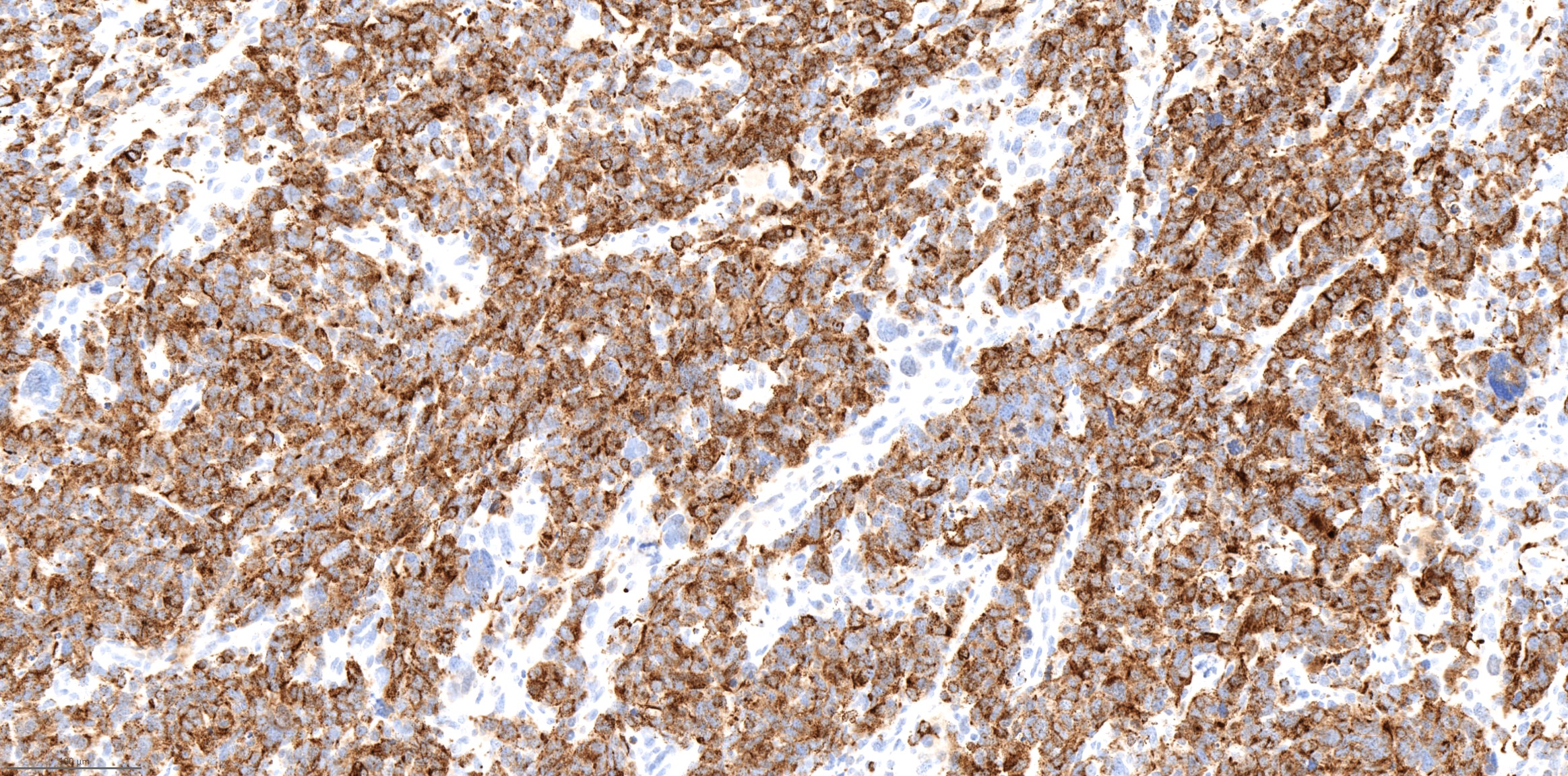
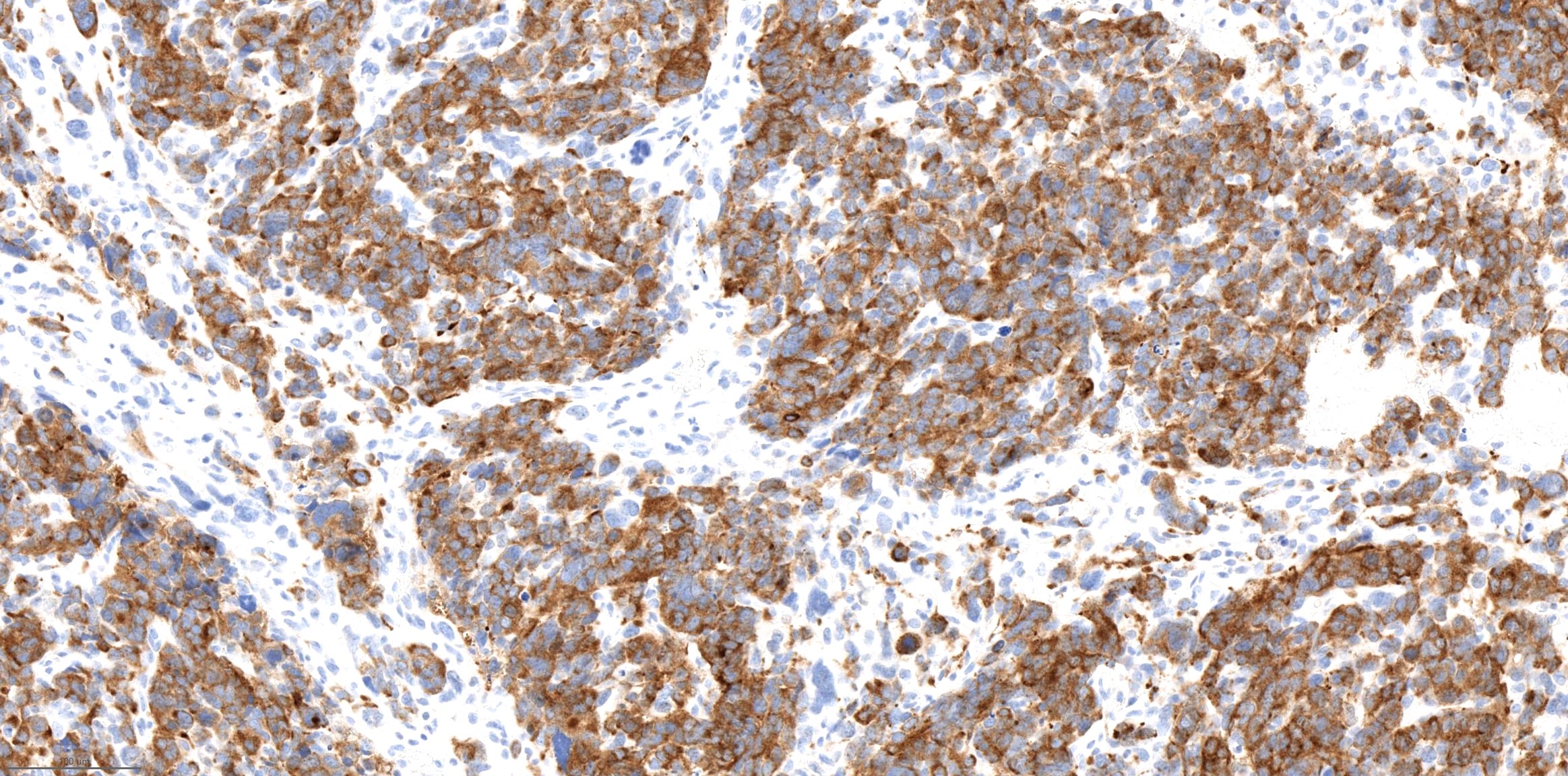
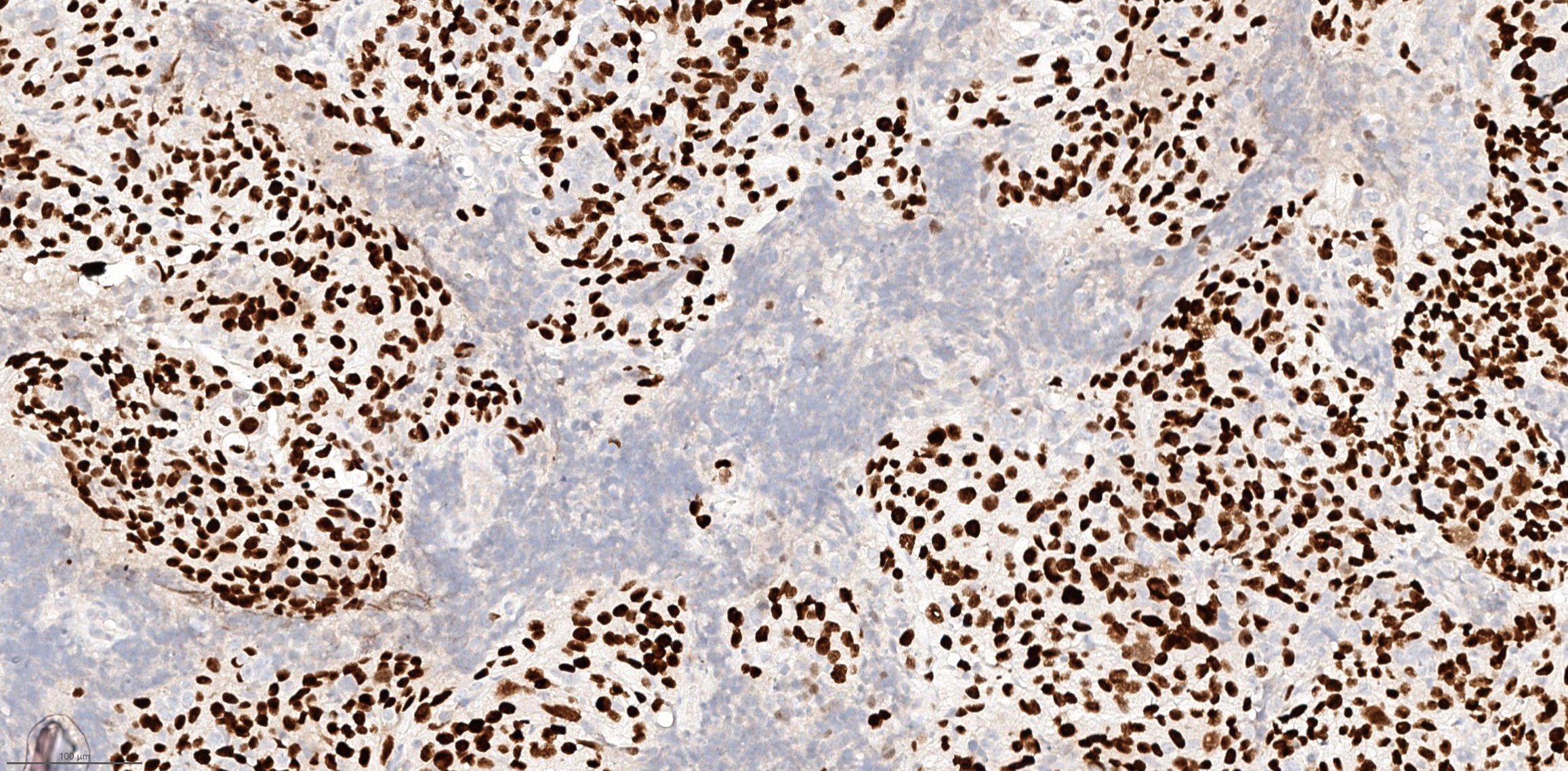
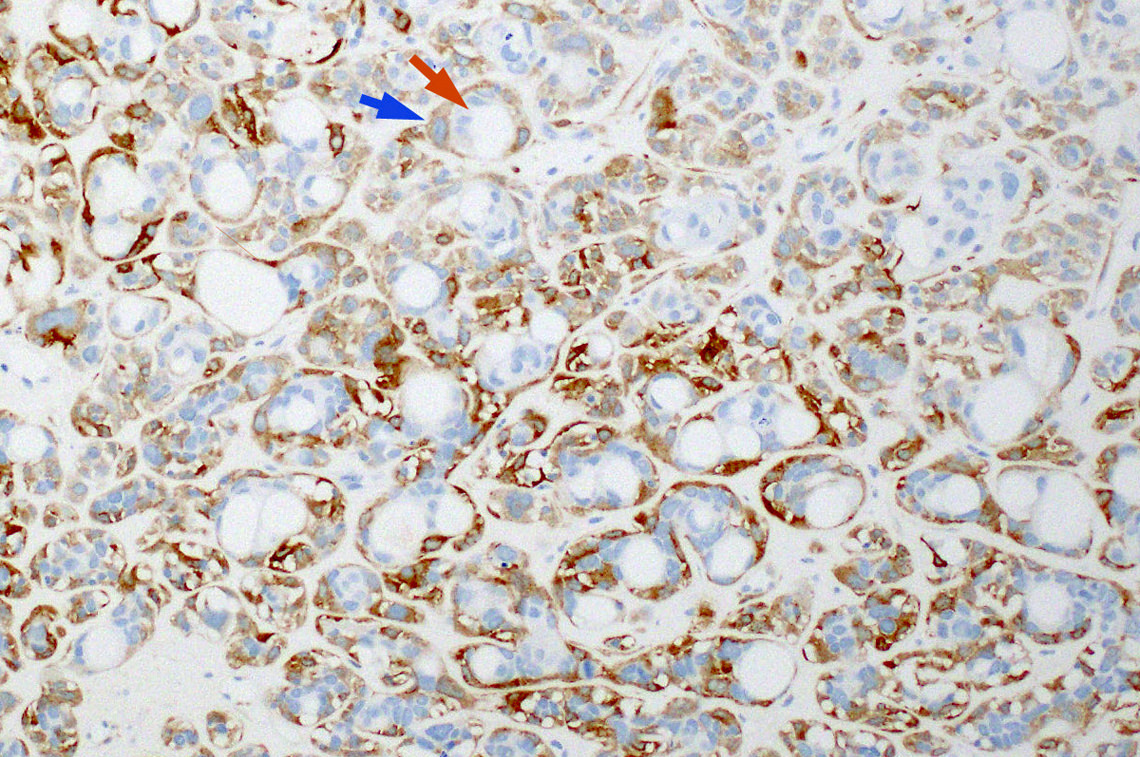
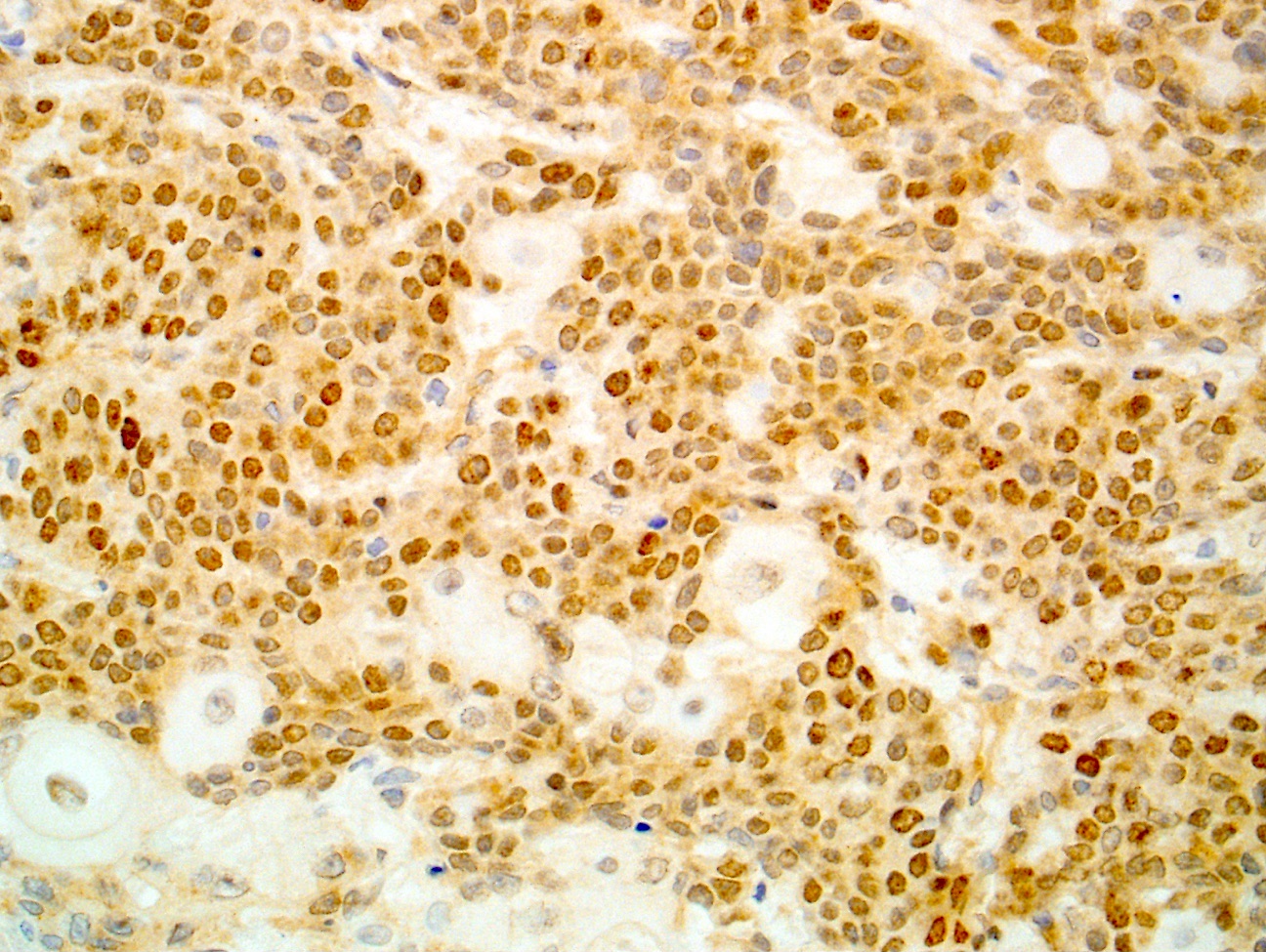
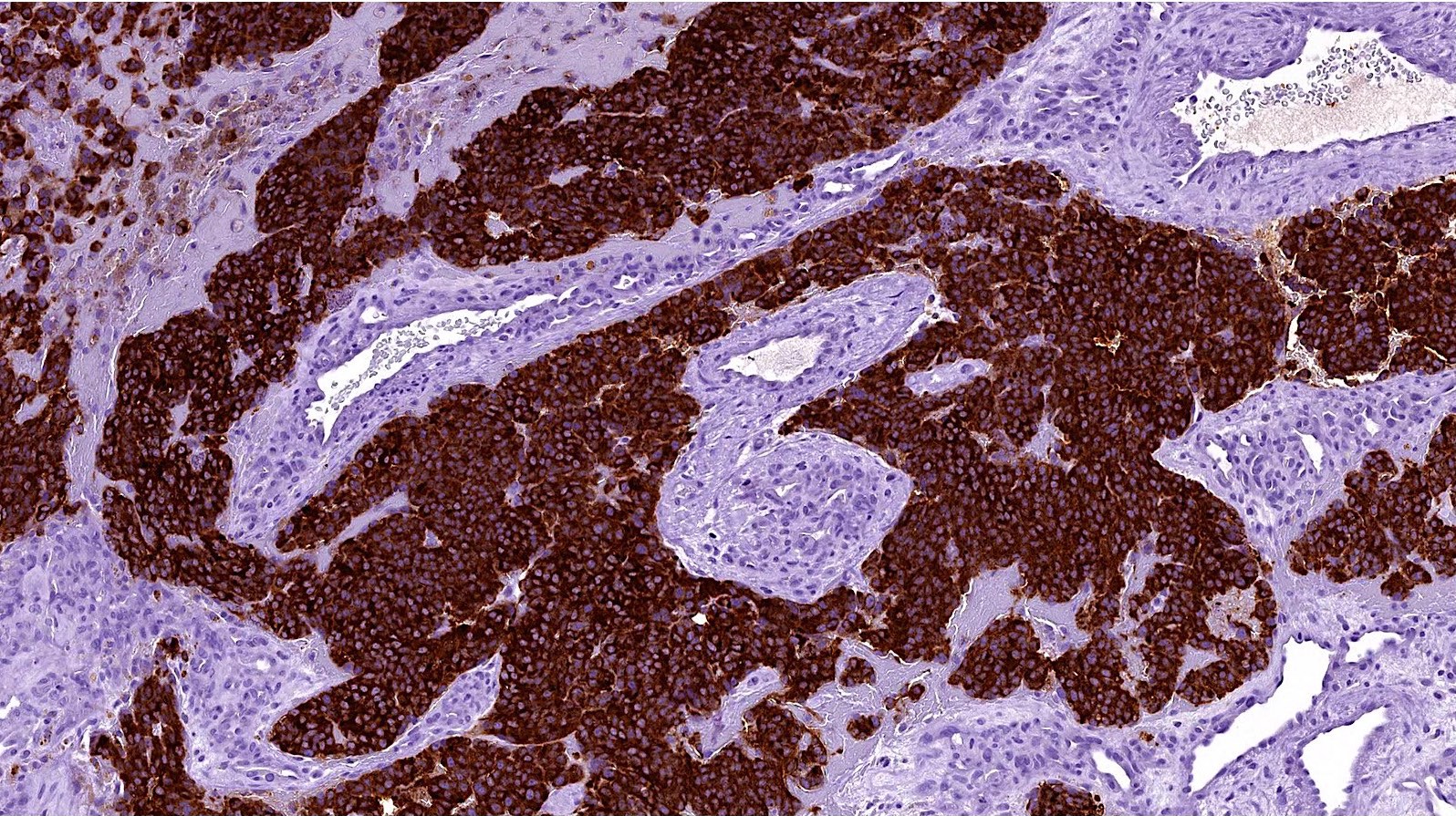
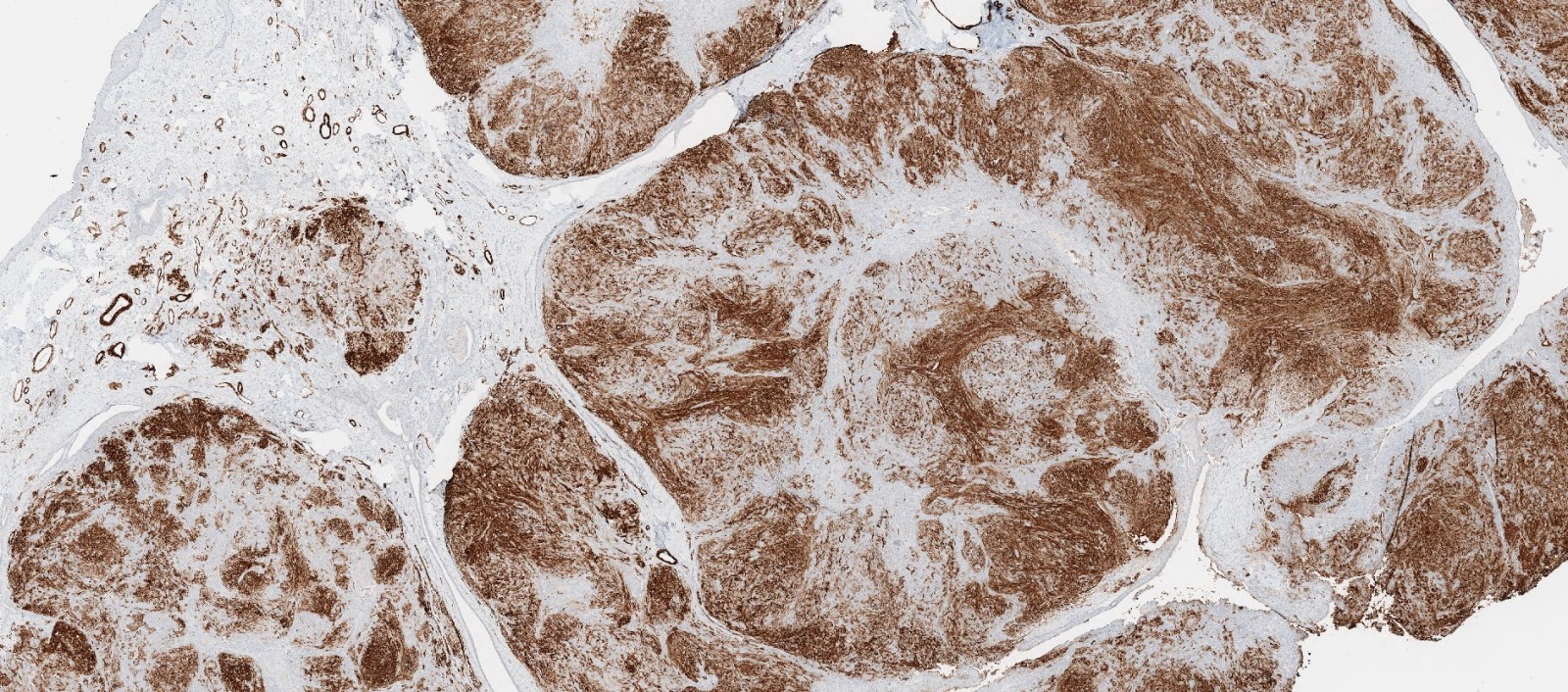
Positive stains
- Neuroendocrine markers such as chromogranin, synaptophysin and INSM1
- Cytokeratin AE1 / AE3 and CAM5.2: often show perinuclear dot-like staining pattern
- EMA
- TTF1 can be positive in high grade neuroendocrine carcinoma of sinonasal tract (Hum Pathol 2002;33:642)
- Ki67 proliferation index: by definition > 20% but is often much higher (> 50%)
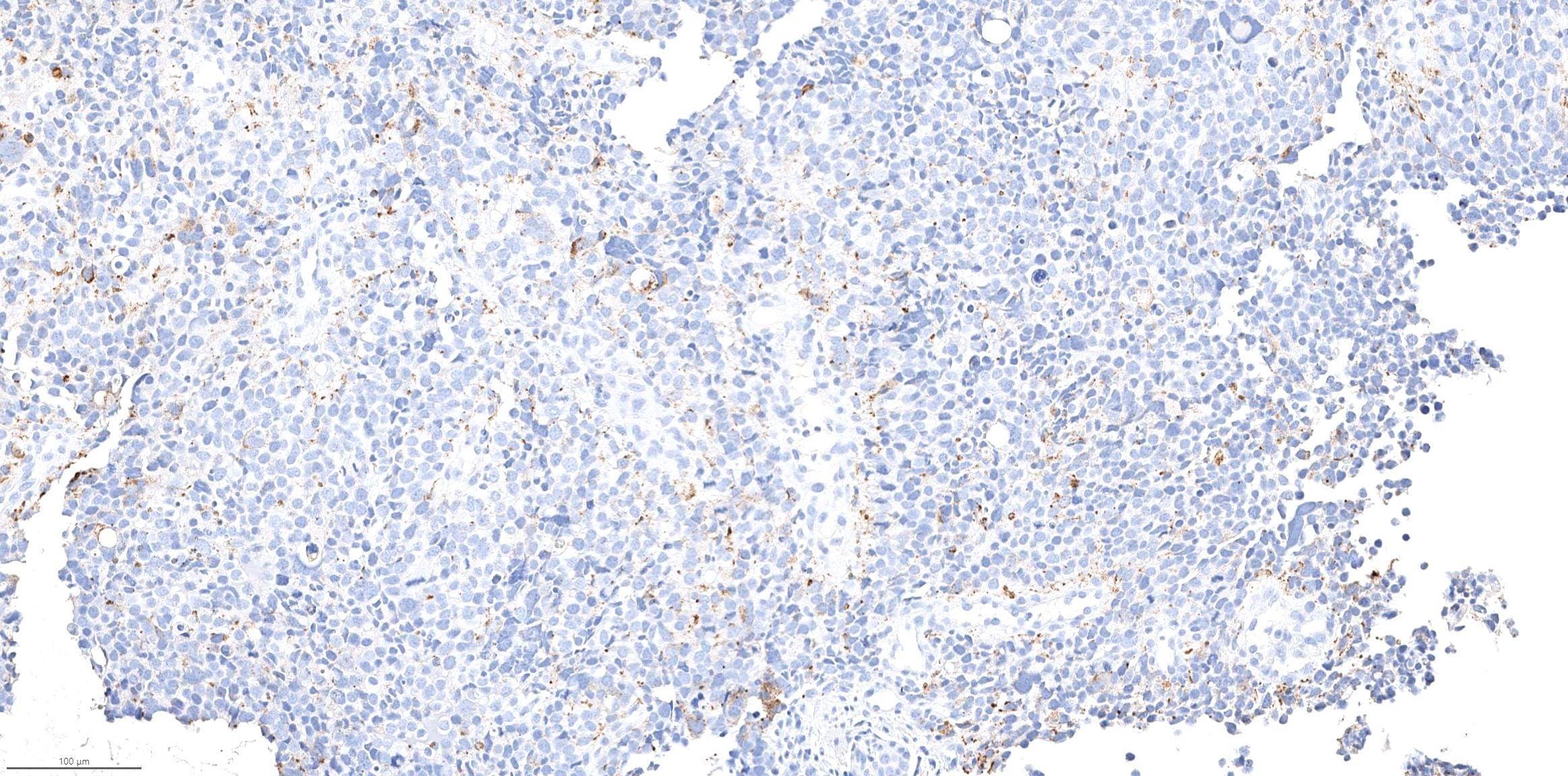
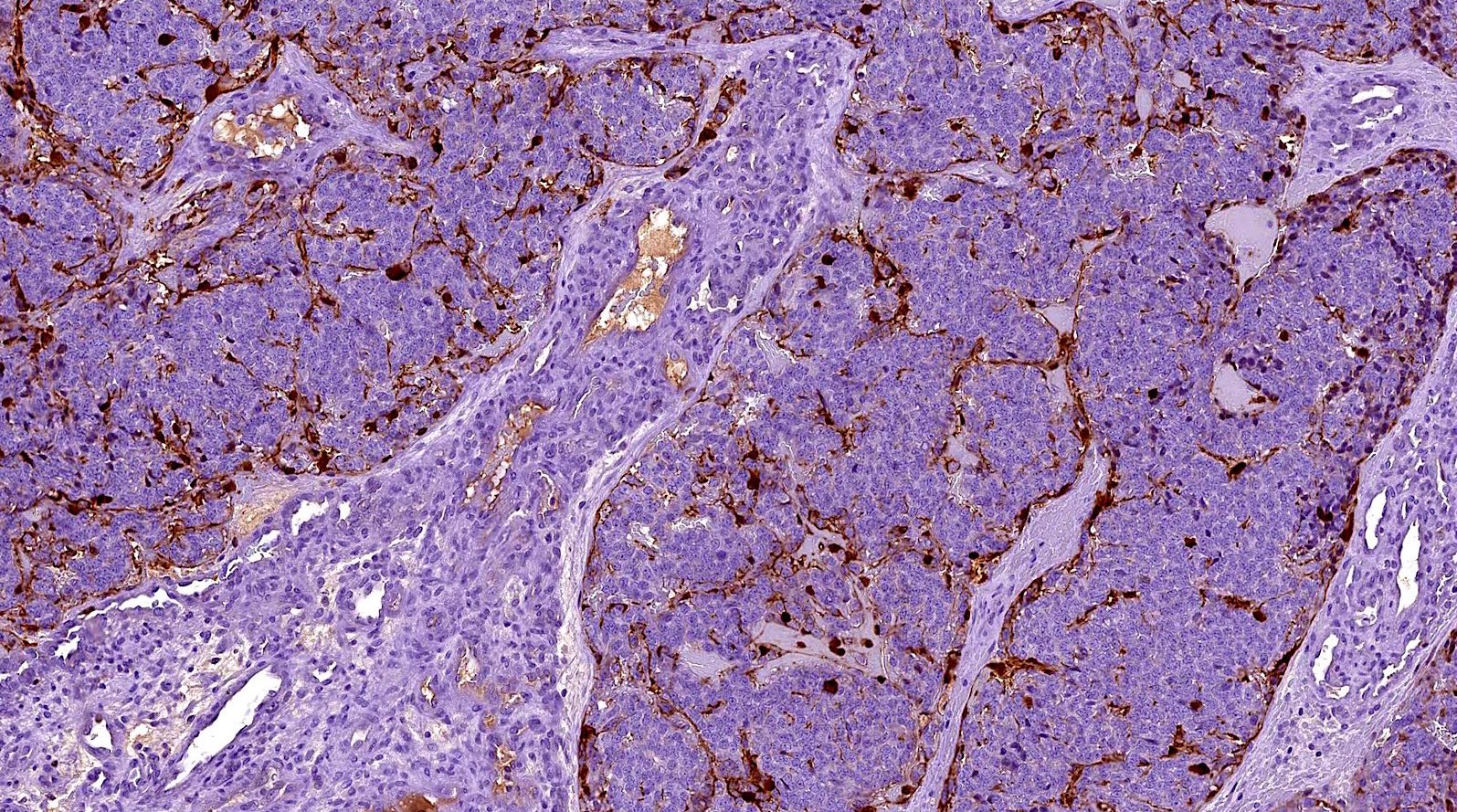
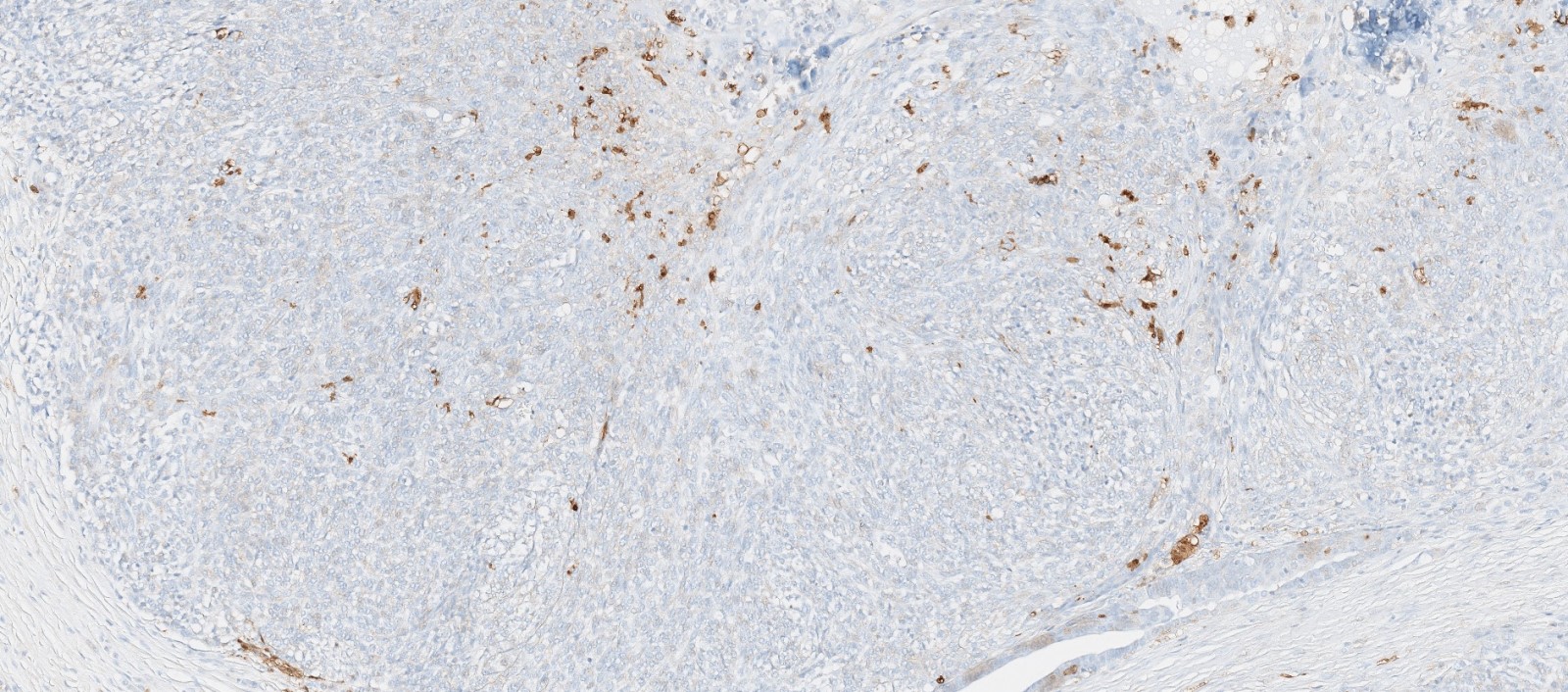
Negative stains
- NUT
- No loss of SWF / SNF pathway proteins: immunoexpression of INI1 / SMARCB1 or SMARCA4 / BRG1 is retained
- S100
Molecular / cytogenetics description
- Subset of large cell neuroendocrine carcinoma has IDH2 R172 mutation (Mod Pathol 2019;32:1447)
- Small cell carcinoma does not have IDH2 mutation but may harbor ARID1A mutation (Mod Pathol 2019;32:1447)
Sample pathology report
- Nasal cavity, biopsy:
- Small cell carcinoma (high grade neuroendocrine carcinoma, small cell carcinoma type) (see comment)
- Comment: Immunohistochemistry studies show that the tumor is diffusely positive for synaptophysin, chromogranin, INSM1, cytokeratin AE1 / AE3 and CAM5.2, whereas it is negative for S100 and NUT. Immunoexpression of SMARCA2, SMARCA4 and SMARCB1 is retained in this tumor. Ki67 proliferation index is > 90%. The overall histologic features and immunoprofile support the diagnosis.
Differential diagnosis
- Olfactory neuroblastoma:
- Both entities are positive for synaptophysin and chromogranin
- Olfactory neuroblastoma usually has S100 positive sustentacular network and is negative for keratins (such as cytokeratin AE1 / AE3 and CAM5.2) and EMA
- Low grade olfactory neuroblastoma lacks necrosis and has a low mitotic index
- NUT carcinoma:
- Synaptophysin and chromogranin positivity can be seen in NUT carcinoma (Hum Pathol 2022;126:87)
- NUT immunopositivity or detection of NUTM1 translocation is essential for the diagnosis
- SWI / SNF complex deficient sinonasal carcinoma:
- 52% of SMARCB1 deficient sinonasal carcinomas express synaptophysin or chromogranin (Hum Pathol 2020;104:105)
- Loss of immunoexpression of SMARCB1, SMARCA2 or SMARCA4 is required for the diagnosis
- Sinonasal undifferentiated carcinoma (SNUC):
- SNUC can show very focal synaptophysin and chromogranin positivity (Am J Surg Pathol 2001;25:156)
- More than focal or diffuse synaptophysin and chromogranin immunopositivity excludes a diagnosis of SNUC
Additional references
Board review style question #1
Board review style answer #1
D. Small cell carcinoma. The tumor shows diffuse synaptophysin expression and perinuclear dot-like expression of CAM5.2. The tumor cells have scanty cytoplasm, salt and pepper chromatin, inconspicuous nuclei, frequent mitoses and apoptotic bodies. Answer C is incorrect because diffuse synaptophysin expression excludes a diagnosis of sinonasal undifferentiated carcinoma. Answer B is incorrect because olfactory neuroblastoma is cytokeratin negative, while this tumor expresses CAM5.2. Answer A is incorrect because the tumor cells lack abundant cytoplasm and prominent nucleoli.
Comment Here
Reference: High grade neuroendocrine carcinoma
Comment Here
Reference: High grade neuroendocrine carcinoma
Board review style question #2
Which of the following statements is true for high grade neuroendocrine carcinoma of the sinonasal tract?
- It is generally positive for INSM1 and S100
- It is universally negative for high risk human papillomavirus (HPV), distinguishing it from HPV related squamous cell carcinoma and HPV related multiphenotypic sinonasal carcinoma
- It may coexist with a squamous cell carcinoma
- Ki67 proliferation index is between 2% and 20%
Board review style answer #2
C. It may coexist with a squamous cell carcinoma. High grade neuroendocrine carcinoma may coexist with a nonneuroendocrine carcinoma component (i.e., combined carcinoma). Answer B is incorrect because a subset can be positive for high risk human papillomavirus. Answer D is incorrect because Ki67 by definition should be > 20%. Answer A is incorrect because it is generally negative for S100.
Comment Here
Reference: High grade neuroendocrine carcinoma
Comment Here
Reference: High grade neuroendocrine carcinoma
Histology
Table of Contents
Definition / generalDefinition / general
Nasal cavity
Nasopharynx
Paranasal sinuses
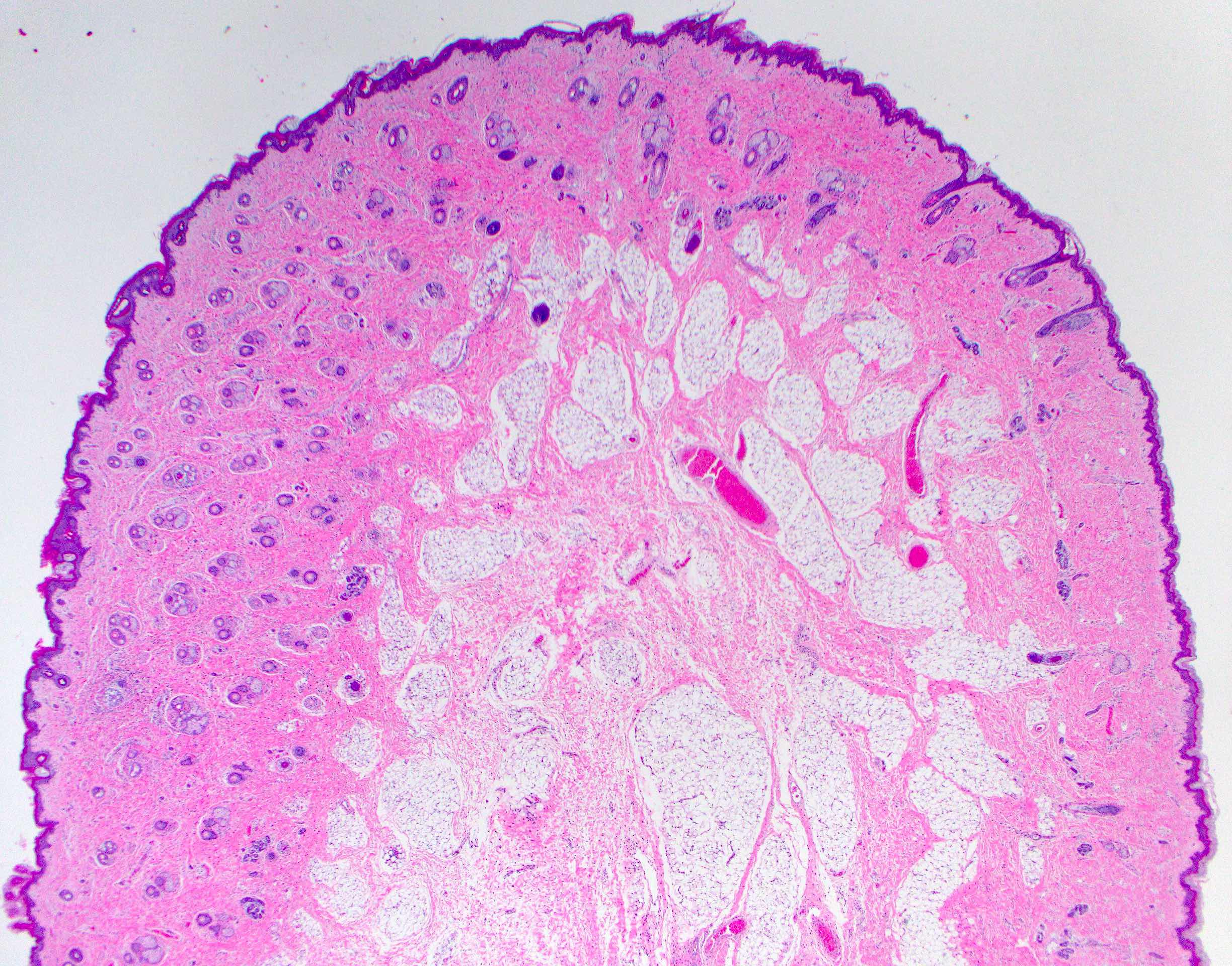
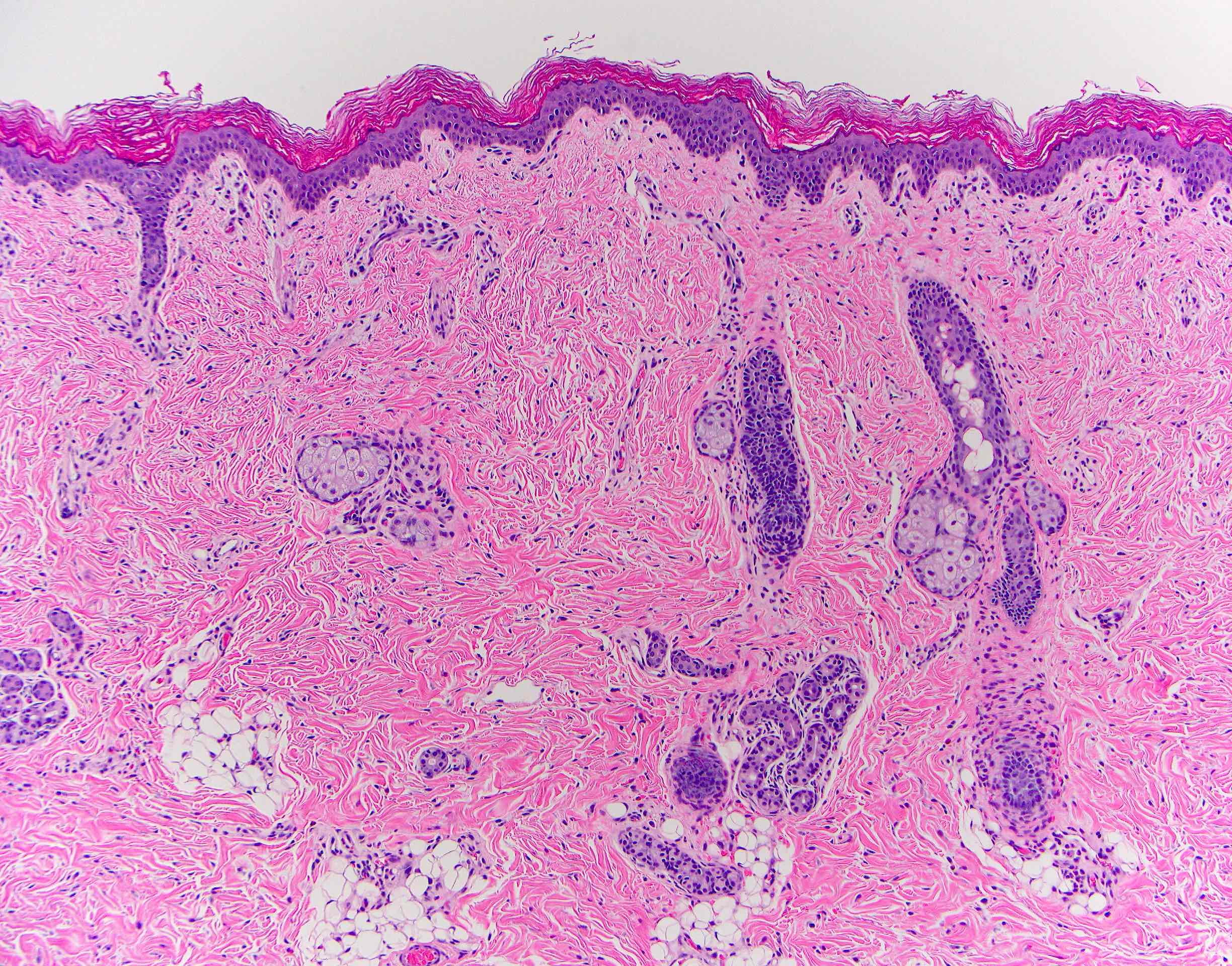
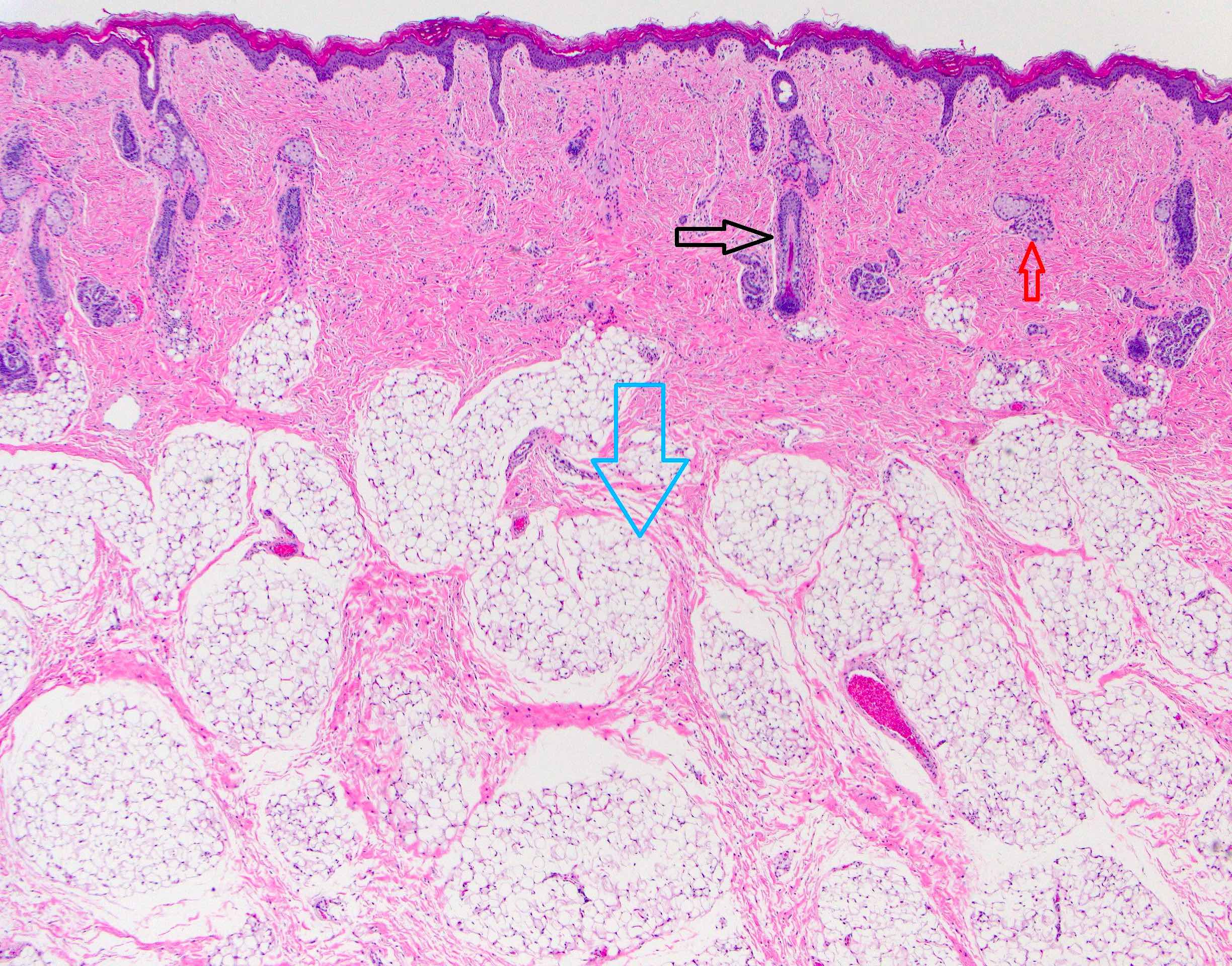
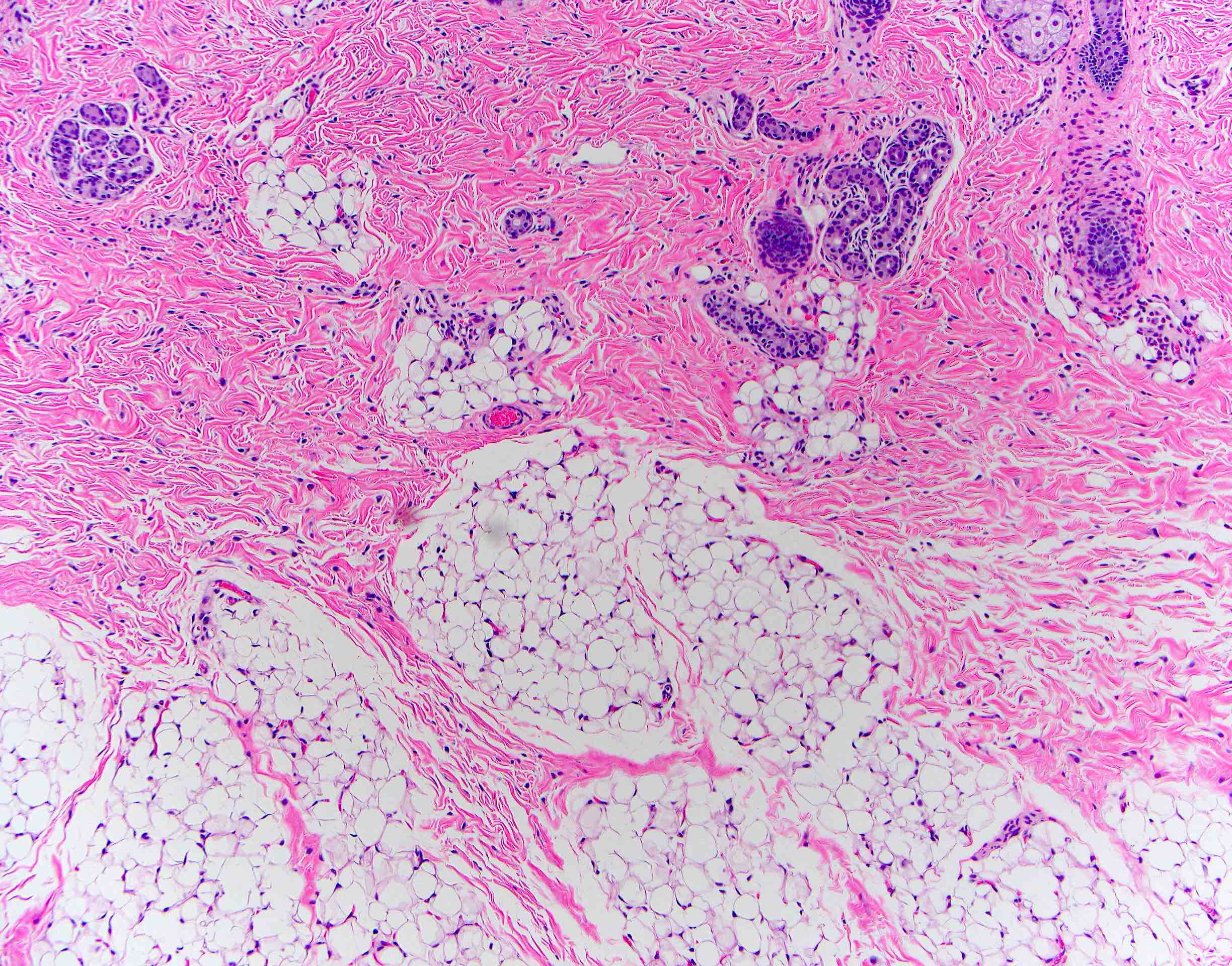
- Lined by stratified squamous and respiratory type pseudostratified columnar epithelium, separated by transitional epithelium in some places
- Respiratory mucosa (also called Schneiderian membrane) may contain goblet cells; may undergo squamous metaplasia
- Superior third of nasal septum, superior turbinate and cribriform plate are covered with thinner olfactory mucosa, usually patchy in adults, which has neuroendocrine features
- Seromucinous glands (resembling salivary glands) are present in submucosa, numerous near eustachian tube opening of nasopharynx, may undergo oncocytic metaplasia with increasing age
- Normally no lymphoid tissue
Nasopharynx
- Lined by stratified squamous epithelium (inferior anterior and posterior walls and anterior lateral walls) and respiratory type epithelium (around nasal choanae and roof of posterior wall); remaining areas have mixtures of squamous and respiratory or intermediate epithelium (also called transitional although it does not resemble urothelium ultrastructurally)
- Intermediate epithelium is usually concentrated as a wavy ring at junction of nasopharynx and oropharynx
- Seromucinous glands may undergo oncocytic metaplasia and rarely form a mass or obstruct eustachian tube
- Abundant lymphoid tissue present, particularly at rim of eustachian tube opening (Gerlach tonsil); functionally equivalent to that of GI tract or mucosal associated lymphoid tissue (MALT)
Paranasal sinuses
- Mucosa is continuous with nasal cavity and identical (respiratory type epithelium) but thinner and with fewer goblet cells and seromucinous glands
- Normally no lymphoid tissue
HPV related multiphenotypic sinonasal carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Unique type of carcinoma characterized by its association with high risk human papillomavirus (HPV, in particular type 33), exclusive sinonasal location and mixed histologic phenotype (resembling both adenoid cystic carcinoma and squamous cell carcinoma)
Essential features
- Located exclusively within the sinonasal tract
- Positive for HPV, in particular type 33 and negative for MYB / MYBL1 / NFIB translocation
- Exhibits various histologic features, including areas that resemble adenoid cystic carcinoma, squamous cell carcinoma, carcinoma with basaloid appearance
Terminology
- HPV related sinonasal carcinoma with adenoid cystic carcinoma-like features
ICD coding
- Newly described in 2013 and does not have its own ICD code (Am J Surg Pathol 2013;37:185)
- Included in the current WHO classification as a provisional entity under sinonasal nonkeratinizing squamous cell carcinoma
- ICD-0: 8072/3 - squamous cell carcinoma, large cell, nonkeratinizing, NOS
Epidemiology
- Age of initial presentation: from 28 - 90 (mean age: 54)
- M:F = 1:1.6 (Am J Surg Pathol 2017;41:1690)
Sites
- Occurs exclusively in the sinonasal tract (Am J Surg Pathol 2017;41:1690)
- Approximately 57% affects nasal cavity, 10% paranasal sinus and the rest affects both sites
- Involvement of adjacent sites, e.g. lacrimal duct, cranial fossa and orbit, is rare but has been reported (Arch Pathol Lab Med 2019 Mar 6 [Epub ahead of print])
Etiology
- High risk HPV is detected in all reported cases (Am J Surg Pathol 2017;41:1690)
- HPV type 33 detected in 68% of cases; other uncommon HPV types are 35 (6% of cases), 56, 16, 52 and 26 (Head Neck Pathol 2018 Sep 26 [Epub ahead of print])
Clinical features
- Usually present with nonspecific symptoms, e.g. nasal congestion, discharge, nasal obstruction, epistaxis and pain
Radiology description
- Locally destructive mass involving nasal cavity or paranasal sinus (AJNR Am J Neuroradiol 2019;40:584)
Prognostic factors
- No known prognostic factor
- Overall appears to be more indolent than sinonasal squamous cell carcinoma
- Based on the reported cases
- Associated with high frequency (36%) of local recurrence, including late local recurrence decades after the initial surgery (J Pathol Transl Med 2019 Apr 25 [Epub ahead of print], Head Neck Pathol 2018;12:623)
- Distant metastases are rare, occurring in 5% of patients 8 - 17 years after the initial resection
- No reported case with regional lymph node metastasis (Am J Surg Pathol 2017;41:1690)
Case reports
- 36 year old woman with nasal cavity mass that recurred 33 years after the initial resection (Head Neck Pathol 2018;12:623)
- 48 year old woman with epistasis and a nasal cavity mass (Head Neck Pathol 2018 Sep 26 [Epub ahead of print])
- 66 year old man with a polypoid lesion in the right nasal cavity (J Pathol Transl Med 2019 Apr 25 [Epub ahead of print])
Treatment
- Knowledge on the appropriate treatment for this rare tumor is limited; based on the reported cases (Am J Surg Pathol 2017;41:1690):
- Surgical resection with appropriate margin is the main treatment approach
- Approximately 40% of patients also receive adjuvant radiation therapy
- Given the reported negligible risk of regional lymph node metastasis and low risk of distant metastasis, routine regional lymph node dissection or chemotherapy may not be indicated
- Late local recurrence and distant metastasis may occur, therefore long term clinical follow up may be warranted
Microscopic (histologic) description
- Typical microscopic features include:
- Areas resemble adenoid cystic carcinoma of salivary gland origin, e.g. tubular, cribriform and solid architecture
- Biphasic ductal and myoepithelial differentiation may be evident on H&E and on immunostain for myoepithelial markers (e.g. calponin, S100, SOX10 and smooth muscle actin)
- Areas showing definite squamous differentiation may be seen in the forms of true keratinization, keratin pearls and intracellular bridges
- Focally, the tumor may show basaloid features resembling cylindroma with peripheral palisading and high nuclear to cytoplasmic ratio, as well as tumor forming solid nests and lobules separated by hyalinized fibrous bands
- Surface involvement with marked nuclear pleomorphism may be seen in about 70% of cases
- At high power, typically shows randomly distributed tumor cells with marked nuclear pleomorphism and hyperchromasia
- Other rare features that may be seen
- Areas with pure sarcomatoid / spindle cell proliferation with or without dilated staghorn shaped vasculature
- Heterologous mesenchymal component e.g. chondroid or osseous differentiation
Microscopic (histologic) images
Positive stains
- p16 consistently shows diffuse and strong nuclear and cytoplasmic staining
- Myoepithelial differentiation by immunohistochemistry, i.e. it is typically positive for myoepithelial markers, e.g. calponin, S100 and smooth muscle actin
- Also shows squamous differentiation by immunohistochemistry, i.e. it is typically positive for p40, p63 and high molecular weight cytokeratin (e.g. CK5 / 6 and 34βE12)
- MYB immunohistochemistry can be positive (although negative for MYB fusion by FISH) (Head Neck Pathol 2019;13:220)
- INI1 (retained)
Negative stains
- Melanocytic markers, e.g. melanA and HMB45
- Neuroendocrine markers, e.g. synaptophysin, chromogranin and INSM1
Molecular / cytogenetics description
- All reported cases are positive for high risk HPV using various detection methods, e.g. DNA in situ hybridization (ISH), RNA ISH and PCR based essays
- EBV ISH (EBER)
- Does not have any characteristic molecular or cytogenetic findings
- Consistently negative for fusions characteristics for adenoid cystic carcinoma, e.g. MYB, MYBL1 and NFIB (Am J Surg Pathol 2017;41:1690)
Sample pathology report
- Nasal cavity, left, resection:
- HPV related multiphenotypic sinonasal carcinoma (see comment)
- Comment: The tumor shows biphasic differentiation with ductal and myoepithelial cells arranged as tubular, cribriform and solid architecture. There are scattered tumor cells with marked nuclear pleomorphism. There is surface involvement. Immunohistochemistry studies show that the tumor is positive for S100, SMA, calponin and p16 (diffuse and strong nuclear and cytoplasmic). RNA in situ hybridization for high risk HPV is positive in this tumor. The overall histologic features and immunophenotype are consistent with the above diagnosis. In the current WHO classification (4th edition), HPV related multiphenotypic sinonasal carcinoma is considered as a provisional diagnosis in the category of sinonasal squamous cell carcinoma (Am J Surg Pathol 2017;41:1690).
Differential diagnosis
- Adenoid cystic carcinoma:
- No confirmed association with HPV infection (Am J Surg Pathol 2017;41:1422)
- 60 - 80% positive for MYB / MYBL1 / NFIB translocation
- Both HPV related multiphenotypic sinonasal carcinoma and adenoid cystic carcinoma can show
- Biphasic differentiation with ductal and myoepithelial elements
- Immunopositivity for myoepithelial markers (e.g. calponin, smooth muscle actin and S100)
- Tubular, cribriform and solid architecture
- Squamous differentiation (in adenoid cystic carcinoma with high grade transformation) (Am J Surg Pathol 2007;31:1683)
- Sinonasal squamous cell carcinoma:
- 15 - 30% can be positive for high risk HPV (Am J Surg Pathol 2013;37:185)
- Lacks biphasic or myoepithelial differentiation by histology and immunohistochemistry
- Both tumors can have squamous differentiation
- SMARCB1 deficient sinonasal carcinoma:
- Sinonasal undifferentiated carcinoma (SNUC):
- HPV negative
- Diagnosis of exclusion, i.e. the diagnosis can only be made when any specific diagnostic entity (e.g. HPV related multiphenotypic sinonasal carcinoma) has been excluded
Board review style question #1
A 45 year old man is present with a nasal cavity mass. A representative H&E of the mass is shown in the picture. Which of the following statements is true?
- Differential diagnoses include adenoid cystic carcinoma of salivary gland origin and HPV related multiphenotypic sinonasal carcinoma
- Tumor is consistently negative for myoepithelial markers, e.g. SMA, calponin and S100
- Tumor may be positive for EBV
- 80% are positive for EWSR1 fusion
Board review style answer #1
A. Differential diagnoses include adenoid cystic carcinoma of salivary gland origin and HPV related multiphenotypic sinonasal carcinoma
Comment here
Reference: HPV related multiphenotypic sinonasal carcinoma
Comment here
Reference: HPV related multiphenotypic sinonasal carcinoma
Board review style question #2
Which of the following statements is true for HPV related multiphenotypic sinonasal carcinoma?
- May contain areas resembling adenoid cystic carcinoma
- May show neuroendocrine differentiation
- Related with low risk HPV, e.g. type 6 and 11
- Typically negative for p40, p63 and high molecular weight cytokeratin
Board review style answer #2
A. May contain areas resembling adenoid cystic carcinoma
Comment here
Reference: HPV related multiphenotypic sinonasal carcinoma
Comment here
Reference: HPV related multiphenotypic sinonasal carcinoma
Inflammatory sinonasal polyp
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Benign, nonneoplastic inflammatory outgrowth of sinonasal mucosa
- Most common type of sinonasal polyp
- Most common space occupying lesion of the sinonasal tract (J Clin Diagn Res 2014;8:FC04)
Essential features
- Benign, nonneoplastic polyp characterized by edematous stroma infiltrated by mixed inflammatory cells
Terminology
- Chronic rhinosinusitis with nasal polyps
- Nasal polyps
ICD coding
- ICD-10: J33.9 - nasal polyp, unspecified
Epidemiology
- Occurs in 2 - 10% of population (J Allergy Clin Immunol Pract 2021;9:4117, StatPearls: Nasal Polyps [Accessed 12 January 2022])
Sites
- Commonly bilateral and multifocal (Pak J Med Sci 2020;36:146)
- Most frequently involves nasal cavity and ethmoid sinus (J Allergy Clin Immunol Pract 2016;4:565)
Pathophysiology
- Defected epithelial cell barrier, increased exposure to bacteria and dysfunctioning host immune system may play a role in the pathogenesis (J Allergy Clin Immunol Pract 2016;4:565)
Etiology
- Most frequently occurs in the setting of chronic rhinosinusitis
- Other risk factors include allergy, systemic vasculitis, cystic fibrosis, sensitivity to aspirin and nonsteroid anti-inflammatory drugs (StatPearls: Nasal Polyps [Accessed 12 January 2022])
Clinical features
- Most common symptoms: obstruction, nasal congestion, rhinorrhea, facial pressure and pain (J Allergy Clin Immunol Pract 2016;4:565)
Diagnosis
- Diagnosis can be rendered based on the typical endoscopic or histologic findings
Radiology description
- Smooth soft tissue masses commonly originate in the ethmoid sinuses and protrude into the nasal cavity (Eur Radiol 2006;16:872)
- Hypodense but may be hyperdense due to concurrent fungal infection or increased protein content
- May demonstrate adjacent bony remodeling or erosion
Prognostic factors
- Recurrence rate is approximately 60% in patients followed for at least 10 years
- Presence of asthma is associated with increased risk of recurrence
- Reference: Am J Rhinol Allergy 2021;35:449
Case reports
- 21 and 32 year old men with infarcted angiomatous nasal polyps (Eur Arch Otorhinolaryngol 2005;262:225)
- 4 cases of angiomatous nasal polyps (Cureus 2020;12:e7642)
Treatment
- Medical treatments include nasal corticosteroid and high volume saline irrigation (JAMA 2015;314:926)
- Sinus surgery for patients failed medical management (J Allergy Clin Immunol Pract 2016;4:565)
Gross description
- Usually multiple and bilateral and involve nasal cavity and paranasal sinuses
- Have translucent, moist or edematous cut surface
- Broad base of attachment is present
- Usually not destructive
Microscopic (histologic) description
- Edematous, fibrotic or loosely myxoid stroma covered by respiratory epithelium
- Infiltrated by mixed inflammatory cells, including lymphocytes, plasma cells, eosinophils, neutrophils and mast cells
- Surface epithelium can show ulceration or squamous metaplasia
- May have bizarre stromal cells (large and pleomorphic)
- Submucosal glands are decreased or absent
- Concurrent fungal infection may be seen
- Rarely, osseous metaplasia may be present
Microscopic (histologic) images
Sample pathology report
- Nasal cavity, left, excision:
- Inflammatory sinonasal polyp
Differential diagnosis
- Sinonasal papilloma:
- Inflammatory polyp lacks the epithelial proliferation, the thickened epithelial lining or the inverted growth pattern seen in sinonasal papilloma
- Respiratory epithelial adenomatoid hamartoma:
- Inflammatory polyp lacks the proliferation of respiratory epithelium and thickened basement membrane typical of respiratory epithelial adenomatoid hamartoma
- Biphenotypic sinonasal sarcoma:
- Can be polypoid but characterized by cellular spindle cells in the stroma showing biphenotypic neural and myoid differentiation
- Rhabdomyosarcoma, embryonal (botryoid) type:
Board review style question #1
What is the most common polypoid lesion in the sinonasal cavity?
- Angiofibroma
- Inflammatory sinonasal polyp
- Respiratory epithelial adenomatoid hamartoma
- Sinonasal papilloma
Board review style answer #1
B. Inflammatory sinonasal polyp is the most common polypoid lesion and space occupying lesion in the sinonasal tract.
Comment Here
Reference: Inflammatory sinonasal polyp
Comment Here
Reference: Inflammatory sinonasal polyp
Board review style question #2
Board review style answer #2
B. This is an inflammatory sinonasal polyp, a nonneoplastic benign lesion characterized by edematous stroma and inflammatory infiltrates.
Comment Here
Reference: Inflammatory sinonasal polyp
Comment Here
Reference: Inflammatory sinonasal polyp
Intestinal type
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Sinonasal intestinal type adenocarcinoma (ITAC) is an epithelial tumor of the nasal cavity and the paranasal sinuses, often related to professional exposure to organic dust (such as wood or leather) and histologically resembling intestinal adenocarcinoma (Head Neck 2016;38:1564)
- ITACs can be present in 5 morphologic patterns: colonic, papillary, solid, mucinous and mixed (Head Neck Pathol 2017;11:295)
Essential features
- Incidence is correlated with organic dust exposure (Cancers (Basel) 2021;13:5245)
- ITAC is usually located in the olfactory mucosa of the ethmoid sinus (Cancers (Basel) 2021;13:5022)
- A characteristic feature of this tumor is an increased production of intracellular or extracellular mucin, similar to mucinous adenocarcinoma of the intestinal tract (Cancers (Basel) 2021;13:5022)
Terminology
- Colloid type adenocarcinoma, colonic type adenocarcinoma, enteric type adenocarcinoma (these terms are not recommended for use by WHO)
ICD coding
- ICD-O: 8144/3 - intestinal type adenocarcinoma
- ICD-11:
- 2C20.0 & XH0349 - adenocarcinoma of nasal cavity & adenocarcinoma, intestinal type
- 2C22.0 & XH0349 - adenocarcinoma of accessory sinuses & adenocarcinoma, intestinal type
Epidemiology
- Male predominance (M:F ratio can vary from 4:1 to 21:1 in different geographical regions) with a median age of 60 - 65 years (Cancers (Basel) 2021;13:5245, Head Neck 2016;38:1564)
- Strong correlation to professional exposure to organic dust, such as wood or leather (Head Neck 2016;38:1564)
- Incidence of ITAC varies between geographical regions, presumably due to differences in dust exposure (Cancers (Basel) 2021;13:5245)
- Reported average exposure period is ~40 years
- In contrast to squamous cell carcinoma of a similar region, alcohol, smoking and human papillomavirus do not seem to be risk factors for the development of ITAC (Head Neck 2016;38:1564)
Sites
- Most common location is the olfactory mucosa of the ethmoid sinus but can occur in the nasal cavity and maxillary antrum (Cancers (Basel) 2021;13:5022)
- Cases related to industrial wood dust exposure show predilection for ethmoid sinus
- Sporadic tumors are more common in maxillary antrum
Pathophysiology
- ITAC is considered to develop through intestinal metaplasia of the sinonasal epithelium (Patholog Res Int 2011;2011:230147)
- Wood dust does not seem to have direct mutagenic properties of its own but prolonged exposure to and irritation by wood dust particles can stimulate cellular turnover by inflammatory pathways
- Chronic inflammation is recognized as an important mechanism in tumor initiation and progression (Head Neck 2016;38:1564)
Etiology
- Exposure to organic dust (such as wood or leather) for several decades is the major risk factor (Head Neck 2016;38:1564)
Clinical features
- Unilateral nasal obstruction, rhinorrhea, epistaxis are common presentations (Arch Craniofac Surg 2018;19:210)
- Tumor tends to be very locally aggressive but rarely metastasizes (Arch Craniofac Surg 2018;19:210, Pathol Res Pract 2019;215:152432)
- Advanced tumors may cause pain, neurologic disturbances, exophthalmos, visual impairment
Diagnosis
- CT and MRI are used for diagnosing early lesions, defining disease extent and detecting early recurrences
- Ultrasound guided fine needle aspiration plays an important role in confirming the diagnosis before treatment because it is frequently misdiagnosed as a salivary gland tumor (Arch Craniofac Surg 2018;19:210)
Radiology description
- CT or MRI may reveal soft tissue mass in the ethmoid sinus, frontal sinus or other locations (Arch Craniofac Surg 2018;19:210)
Prognostic factors
- Locally aggressive with ~50% local recurrence
- Locoregional / distant metastasis occurs in 10 - 20% cases
- Mean disease free survival is 32 months (Cancers (Basel) 2021;13:5022)
- Papillary subtype is associated with better prognosis, while mucinous and solid subtypes carry poor prognosis (Cancers (Basel) 2021;13:5022)
- Prognosis and response to the chemotherapy can be associated with genotoxic agents, such as TP53 mutations
- Significantly higher complete response rate and disease free survival rate were reported in patients carrying tumors with wild type TP53 compared to patients bearing mutated TP53 (Head Neck 2016;38:1564)
- There is no significant prognostic difference between occupational and sporadic tumors
Case reports
- 36 year old man with ITAC that developed from inverted papilloma (J Clin Diagn Res 2016;10:ED12)
- 50 year old man with right nasal block, mass in right nose and frontal headache (J Clin Diagn Res 2014;8:149)
- 63 year old man with a rapidly growing soft mass on his glabellar region for 4 months (Arch Craniofac Surg 2018;19:210)
Treatment
- Standard treatment is surgical resection, with a minimally invasive endoscopic approach when possible (Cancers (Basel) 2021;13:5022)
- Due to the high recurrence rate of ITAC, postoperative radiation is commonly administered
- Neoadjuvant chemotherapy is used in advanced stage tumors (Cancers (Basel) 2021;13:5022)
- Because local recurrences and metastases of these tumors occur frequently, palliative treatment is most often the only option (Head Neck 2016;38:1564)
Clinical images
Gross description
- Irregular exophytic tan-pink mass bulging into nasal cavity or paranasal sinuses, often with necrotic and friable appearance
- Some lesions are gelatinous
Gross images
Microscopic (histologic) description
- ITACs can be present in 5 morphologic patterns: colonic, papillary, solid, mucinous and mixed (Head Neck Pathol 2017;11:295)
- Papillary pattern: predominantly papillary growth with tubular elements
- Colonic pattern: tubuloglandular architecture with minor papillary elements; neoplastic cells have palisaded hyperchromatic nuclei and a few goblet cells
- Solid pattern: poorly differentiated; trabecular and solid proliferation of neoplastic cells
- Mucinous pattern: mucin laden neoplastic glands or tumor cell clusters within pools of extracellular mucin
- Mixed pattern: variable admixture of 2 or more subtypes
- Goblet cells, Paneth cells and argentaffin cells may be found in all patterns
- Exceptionally well differentiated ITAC may resemble normal small intestinal mucosa with well formed villi and muscularis mucosae
Microscopic (histologic) images
Positive stains
- Consistently positive for CK20, CDX2, villin, MUC2, SATB2 (Head Neck Pathol 2022;16:670)
- Variably positive for CK7 and CEA
- Chromogranin+ cells may be present, scattered or in clusters
Molecular / cytogenetics description
- Frequent KRAS and p53 mutations (Head Neck Pathol 2022;16:670, Head Neck 2016;38:1564)
- Tumors with occupational exposure to wood dust show p53, p14 ARF, p16 INK4a gene deregulation
- EGFR alterations also reported (Cancers (Basel) 2021;13:5022)
- Mutations in the genes in Wnt, MAPK and PI3K pathways were reported in 20%, 20% and 24% of cases, respectively (Cancers (Basel) 2021;13:5022)
Videos
Update on sinonasal carcinoma
Malignant tumors of the paranasal sinuses
Sample pathology report
- Ethmoid sinus, biopsy:
- Invasive adenocarcinoma, intestinal type (see comment)
- Comment: The patient's clinical history of a mass in the right ethmoid sinus is noted. Morphology and immunophenotypic features (positive for CK7, CK20, villin and MUC2) are most consistent with intestinal type sinonasal adenocarcinoma. However, a metastasis from elsewhere, particularly the digestive tract, cannot be absolutely ruled out. Clinical and imaging correlation is suggested.
Differential diagnosis
- Nonintestinal sinonasal adenocarcinoma:
- Metastasis from colonic adenocarcinoma:
- Rare but most important differential
- Usually CEA+, CK7-, chromogranin-
- Clinical features and colonoscopy are helpful
- Papillary sinusitis:
- May have abundant mucinous material but has short and blunt papillae with clean background
- Thick and hyalinized basement membrane
- Ciliated surface cells
- Prominent eosinophils and no significant cytological atypia
Board review style question #1
A 70 year old man presented with a mass in the right nose and a frontal headache. A representative image of the mass is shown in the image above. Which of the following statements is true?
- Chemotherapy is the standard treatment
- Cigarette smoking is the number one risk factor for this tumor
- The tumor can be locally aggressive but rarely metastasizes
- The tumor is most commonly located in the nasal cavity
Board review style answer #1
C. The tumor can be locally aggressive but rarely metastasizes.
The image depicts intestinal type adenocarcinoma, with colonic type morphology. These tumors are known to be locally aggressive but metastasis is very uncommon (answer C). Ethmoid sinus is the most common location, not nasal cavity (answer D). Exposure to organic wood dust is considered as a strong risk factor. Unlike squamous cell carcinoma, cigarette smoking or human papillomavirus is not etiologically linked to ITAC (answer B). Surgical resection is the standard treatment, not chemotherapy (answer A).
Comment Here
Reference: Intestinal type adenocarcinoma
Comment Here
Reference: Intestinal type adenocarcinoma
Board review style question #2
Which of the following statements is true for intestinal type sinonasal adenocarcinoma?
- Signet ring cell morphology is the most common histologic pattern
- Tumor cells have uniform nuclei with salt and pepper chromatin
- Tumor may be positive for EBV
- Tumor usually demonstrates positive staining for CK20, villin or SATB2
Board review style answer #2
D. Tumor usually demonstrates positive staining for CK20, villin or SATB2. ITAC shows intestinal phenotype and shows immunoreactivity for CK20, villin and SATB2 (answer D). There is no association with EBV (answer C). Common histologic subtypes include colonic, papillary, solid, mucinous and mixed patterns. Signet ring cell morphology is uncommon (answer A). Salt and pepper nuclear chromatin is characteristically observed in neuroendocrine tumors and not in ITAC (answer B).
Comment Here
Reference: Intestinal type adenocarcinoma
Comment Here
Reference: Intestinal type adenocarcinoma
Invasive fungal rhinosinusitis
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Acute aggressive life threatening invasive fungal infection involving sinonasal tract and adjacent organs (such as orbit or brain); the mortality rate is ~50% (Laryngoscope 2013;123:1112)
Essential features
- Definite diagnosis is made on biopsy by identifying the essential histologic feature, which is invasion of tissue and vessels by fungal organisms, often associated with tissue necrosis and infarction
- Mostly occurs in immunocompromised hosts, although immunocompetent patients may also be affected (Laryngoscope 2013;123:1112, Radiographics 2022;42:2075)
- Caused by a diverse group of fungal organisms, most commonly Zygomycetes (such as Mucor and Rhizopus) and Aspergillus (Laryngoscope 2013;123:1112, Radiographics 2022;42:2075)
- 1 of the 3 pathologic manifestations of fungi in the sinonasal tract; the other 2 are allergic fungal sinusitis and mycetoma (fungus ball)
Terminology
- Acute invasive fungal (rhino)sinusitis
- Angioinvasive fungal (rhino)sinusitis
ICD coding
Epidemiology
- Mostly occurs in immunocompromised patients
- Underlying risk factors in a descending order of frequency include (Laryngoscope 2013;123:1112)
- Diabetes (in ~50%), including those with ketoacidosis
- Hematologic malignancy (in ~40%), such as acute lymphoblastic leukemia and acute myeloid leukemia
- Corticosteroid medication
- Renal / liver failure
- Organ transplant (solid organ or allogeneic stem cell transplant)
- HIV / AIDS
- Anaplastic anemia
- Autoimmune disease
- May occur in pediatric patients (J Pediatric Infect Dis Soc 2017;6:S22, Int J Pediatr Otorhinolaryngol 2016;90:231)
Sites
- Sinonasal tract
- Adjacent vital organs, including orbit (in ~50%), intracranial and hard palate (in ~20%) and cavernous sinus (in 9%) (Laryngoscope 2013;123:1112)
Etiology
- Immunocompromised host
- Caused by hyphae form fungi, commonly Zygomycetes (such as Rhizopus and Mucor) and Aspergillus
- Coinfection with COVID-19 and post-COVID-19 infection were reported during COVID-19 pandemic (Laryngoscope Investig Otolaryngol 2022;7:913)
Clinical features
- Most common symptoms presenting in > 50% of patients include facial swelling, fever, nasal congestion and ophthalomoplegia (Laryngoscope 2013;123:1112)
- Other symptoms include proptosis, vision loss, nasal discharge, facial pain, headache, cranial nerve palsy, altered mental status and palatal necrosis / ulcer
Diagnosis
- Diagnosis of invasive fungal rhinosinusitis is rendered on tissue biopsy by identification of fungal hyphae in tissue (such as mucosa, submucosa, bone or vessels)
- Tissue culture is recommended for genus of the fungi and antifungal susceptibility testing (Lancet Infect Dis 2019;19:e405, Clin Infect Dis 2016;63:e1)
- In situ hybridization and RT-PCR for various fungi can be used in formalin fixed tissue for genus of the fungi
Radiology description
- Mucosal thickening
- Soft tissue invasion into adjacent tissue / organ(s), such as orbit and intracranial extension
Radiology images
Prognostic factors
- Unfavorable prognostic factors include older age, altered mental status, underlying conditions of anaplastic anemia or renal / liver failure, intracranial or cavernous sinus involvement, neutropenia and treatment delay (Laryngoscope 2013;123:1112, Otolaryngol Head Neck Surg 2018;159:386)
- Favorable prognostic factors are surgery and liposomal amphotericin B antifungal therapy
Case reports
- 27 year old woman with acute lymphoblastic leukemia developed rhinosinusitis after induction chemotherapy (Clin Hematol Int 2022;4:60)
- 29 year old man post-bone marrow transplant with psychosis and a gingival lesion (Arch Pathol Lab Med 2000;124:883)
- 33 year old male intranasal cocaine user presented with facial and nasal pain and was found to have invasive Aspergillus flavus sinusitis (IDCases 2021;26:e01327)
- 36 year old woman, 45 year old woman and 62 year old man with acute invasive fungal sinusitis associated with COVID-19 infection (Indian J Otolaryngol Head Neck Surg 2022;74:3359)
Treatment
- Combination of surgery and antifungal treatment (Laryngoscope 2013;123:1112)
- Surgery can be performed via endoscopic or open approach
- The most common antifungal treatment is amphotericin B
- Correcting the source of immunosuppression may also be useful
Gross description
- Necrotic and hemorrhagic tissue fragments
Frozen section description
- Fungal hyphae in tissue are diagnostic for invasive fungal sinusitis on frozen sections
- Free floating fungal elements with or without mucus, blood, debris or fibrin are insufficient to confirm the invasive nature of the fungi and are therefore insufficient for the diagnosis
Microscopic (histologic) description
- Fungal hyphae in mucosa, submucosa, vessels and bone
- Zygomycetes: broad nonseptate hyphae branching at 90 degrees
- Aspergillus: slender septate hyphae branching at 45 degrees
- Preoperative antifungal treatment may significantly alter the morphology of the hyphae
- Classification of the fungal genus is not reliable on histology: tissue fungal culture or RT-PCR test using paraffin blocks are more reliable tools
- Often associated with tissue necrosis, acute inflammation, ulceration or granulation tissue
- Grocott-Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains are useful special stains to highlight fungal organisms
Microscopic (histologic) images
Cytology description
- Cytology (such as nasal swab) can only detect the presence of fungal hyphae
- Invasive nature of the fungal elements cannot be established in cytologic samples
Sample pathology report
- Maxillary sinus, biopsy:
- Invasive and angioinvasive fungal rhinosinusitis associated with tissue necrosis, acute inflammation and ulceration (see comment)
- Comment: GMS and PAS stains highlight fungal hyphae.
Differential diagnosis
- Key differential diagnosis is noninvasive fungal infection of the sinonasal tract, including
- Allergic fungal sinusitis:
- Mycetoma (fungus ball):
- Mass forming growth of densely packed fungal hyphae in sinus cavity
- Tissue invasion by fungi is absent
Board review style question #1
Board review style answer #1
C. Invasive fungal sinusitis. The H&E shows invasive fungal infection with fungal hyphae in a blood vessel and fibrous tissue (i.e., invasive fungal sinusitis). Answers A and D are incorrect as both are noninvasive fungal diseases. Answer B is incorrect because the presence of fungal organisms excludes the diagnosis of granulomatosis with polyangiitis.
Comment Here
Reference: Invasive fungal rhinosinusitis
Comment Here
Reference: Invasive fungal rhinosinusitis
Board review style question #2
What is the most common causal fungal organism for invasive fungal sinusitis?
- Candida albicans
- Cryptococcus
- Histoplasma capsulatum
- Mucor
Board review style answer #2
D. Mucor. The most common causal fungal organisms for invasive fungal sinusitis are Zygomycetes (such as Mucor and Rhizopus) and Aspergillus. Answers A - C are incorrect because Cryptococcus, Histoplasma and Candida are all fungal organisms but are not the common cause for invasive fungal sinusitis.
Comment Here
Reference: Invasive fungal rhinosinusitis
Comment Here
Reference: Invasive fungal rhinosinusitis
Low grade nasopharyngeal papillary adenocarcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Low grade primary adenocarcinoma of nasopharynx
Essential features
- Relatively rare nasopharyngeal tumor
- No known etiologic agents; lacks Epstein-Barr virus (EBV) association
- Morphologically, an adenocarcinoma with predominant papillary architecture and hyalinized cores
- Mimics papillary thyroid carcinoma; may be TTF1+, however, PAX8- and thyroglobulin-
- Excellent prognosis with no known metastases
Terminology
- Thyroid-like low grade nasopharyngeal papillary adenocarcinoma
- Low grade nasopharyngeal papillary adenocarcinoma
ICD coding
- ICD-10: C11.9 - malignant neoplasm of nasopharynx, unspecified
Epidemiology
- Uncommon tumor of nasopharynx (Ear Nose Throat J 2017;96:456)
- < 1% of nasopharyngeal malignancies
- Median age of 37 years but occurs over a wide age range (reported range: second - seventh decade)
- No gender predilection
Sites
- May occur anywhere in nasopharynx but common locations are roof, posterior wall and lateral wall of nasopharynx
Pathophysiology
- Exact pathogenesis is unclear
Etiology
- No known etiologic agents
- Not associated with wood dust exposure or other known factors
- Lacks any EBV or human papillomavirus (HPV) association
- Association with Turner syndrome in a rare case (Med J Malaysia 1998;53:104)
Clinical features
- Nasal obstruction is the most common presenting symptom (Am J Clin Pathol 2019;152:582)
- Tumors usually remain confined within the nasopharynx
- Subset of patients with tumor involving the posterior and lateral nasopharyngeal walls present with otitis media or postnasal drip
- Slow growing and indolent
Diagnosis
- Imaging followed by biopsy
Radiology description
- Nasopharyngeal endoscopy typically reveals polypoid or pedunculated masses
- Sinus computed tomography (CT) images show circular or nodular mass at the nasopharynx or the edge of the nasal septum, moderately to strongly enhancing on contrast enhanced CT (CECT) with no bone destruction (Cancer Manag Res 2021;13:1271)
- Sinus magnetic resonance imaging (MRI) reveals masses with moderate signals in plain T1 weighted images and strong signals in T2 weighted images
Prognostic factors
- Excellent prognosis with complete surgical excision
- Rarely recurs (if incomplete excision)
- No metastases reported to date (Ear Nose Throat J 2017;96:456)
Case reports
- 22 year old woman with nasopharyngeal papillary adenocarcinoma harboring a ROS1::GOPC fusion (Medicine (Baltimore) 2021;100:e24377)
- 42 year old nonsmoking woman presented with foreign body sensation in pharynx and dry throat for 1 month (Transl Cancer Res 2020;9:4457)
- 50 year old woman harboring biphasic thyroid-like low grade nasopharyngeal papillary adenocarcinoma with a prominent spindle cell component (Diagnostics (Basel) 2020;10:323)
Treatment
- Complete surgical excision is usually curative
- Some patients may receive radiation, though role of radiotherapy is unclear
Gross description
- Soft, exophytic mass with papillary, polypoidal or nodular appearance (Ear Nose Throat J 2017;96:456)
- Size can vary from a few millimeters to 3 cm
- Consistency may be soft or gritty
Microscopic (histologic) description
- Invasive tumor involving surface epithelium, at times merging with adjacent nonneoplastic epithelium
- Papillary and glandular growth patterns (Cancer Manag Res 2021;13:1271)
- Papillary structures are complex with arborization and hyalinized fibrovascular cores
- Glandular growth pattern has cribriform or back to back glands
- Lined by single layer of cuboidal to columnar cells with moderate amount of eosinophilic cytoplasm and round to oval nuclei
- Nuclei are vesicular or optically clear showing grooves or membrane irregularities mimicking papillary thyroid carcinoma
- Mild to moderate nuclear pleomorphism, no / rare nucleoli
- Mitotic figures are inconspicuous
- Psammoma bodies may be present in 33% of cases
- Necrosis is rare and usually absent
- No angiolymphatic invasion or perineural invasion
Microscopic (histologic) images
Contributed by Diana Bell, M.D. and Lester Thompson, M.D.
Positive stains
- PAS (intracytoplasmic, diastase resistant granules), mucin stains like mucicarmine (intracellular or luminal)
- Pancytokeratin (diffuse), CK7 (diffuse), EMA (diffuse), CK5/6, CEA (focal), CK19 (subset of cases), S100 (focally in few cases) (Cancer Manag Res 2021;13:1271)
- TTF1 (J Formos Med Assoc 2015;114:473)
- Low proliferation indices (Ki67 < 5%)
Negative stains
- PAX8, thyroglobulin, CK20, CDX2, villin, GFAP, BCL2 (Hum Pathol 2023;134:66)
- EBV encoded small nuclear RNA in situ hybridization
Molecular / cytogenetics description
- No defined genetic alterations to date
- No BRAF or NRAS mutations
- One case was reported in a patient with Turner syndrome (Med J Malaysia 1998;53:104)
- ROS1 gene rearrangements with presence of ROS1::GOPC fusion has been described in a patient (Medicine (Baltimore) 2021;100:e24377)
Sample pathology report
- Roof of nasopharynx, endoscopic resection:
- Nasopharyngeal papillary adenocarcinoma (1 cm) (see comment)
- Margins negative
- Comment: Microscopic examination reveals complex, interwoven papillary architecture along with crowded back to back glands. The tumor nuclei are oval and exhibit mild degree of pleomorphism with presence of optically clear chromatin. Lymphovascular or perineural invasion are not identified. On immunohistochemistry, the tumor cells are immunopositive for pan-CK, CK7 and TTF1, while they are negative for PAX8 and thyroglobulin. The findings are consistent with nasopharyngeal papillary adenocarcinoma.
Differential diagnosis
- Metastatic papillary thyroid carcinoma:
- No surface epithelial dysplasia
- PAX8+ and thyroglobulin+
- TTF1+ in both tumor types (Head Neck Pathol 2019;13:661)
- Sinonasal intestinal type adenocarcinoma, papillary variant:
- Nasal cavity and paranasal sinuses
- Usually wood dust exposure
- More papillary and less glandular
- Tall columnar and goblet cells
- Lack of nuclear features of papillary thyroid carcinoma
- Dirty background with hemorrhage and inflammation
- Mitotic activity
- Usually positive for CK20, CDX2, villin and MUC2 (Ann Diagn Pathol 2006;10:215)
- Polymorphous adenocarcinoma of salivary gland origin:
- Papilloma of surface epithelial or minor salivary gland origin:
- Lacks infiltrative growth pattern
Board review style question #1
A 45 year old woman presented with a nasopharyngeal mass. Based on the H&E and immunohistochemistry (TTF1) images above, what is the diagnosis?
- Low grade nasopharyngeal papillary adenocarcinoma
- Metastasis from papillary thyroid carcinoma
- Polymorphous adenocarcinoma
- Sinonasal intestinal type adenocarcinoma, papillary variant
Board review style answer #1
A. Low grade nasopharyngeal papillary adenocarcinoma. The pictures show a tumor arranged as glands and interwoven papillae with hyalinized cores. The tumor nuclei show pseudoinclusions, nuclear clearing, longitudinal grooves and overlapping. No mitotic figures are identified. Diffuse nuclear immunopositivity for TTF1 is seen. These features are consistent with a low grade nasopharyngeal papillary adenocarcinoma. Though metastasis from papillary thyroid carcinoma is also TTF1 positive and shows similar nuclear features, answer B is incorrect because it does not fit the given clinical history of presence of only a nasopharyngeal mass. However, in case of diagnostic dilemma, other immunohistochemical markers like PAX8 and thyroglobulin can be used, which are immunopositive in papillary thyroid carcinoma but not in low grade nasopharyngeal papillary adenocarcinoma. Answer C is incorrect because polymorphous adenocarcinoma usually shows highly variable architectural patterns in different proportions including solid, trabecular, tubular, reticular, cribiform and papillary arrangements. The tumor cells usually have monotonous pale nuclei and are immunonegative for TTF1. Answer D is incorrect because sinonasal intestinal type adenocarcinoma, papillary variant shows tumor cells arranged in papillary architecture with presence of goblet cells. The tumor cells are immunopositive for CD20 and CDX2, while TTF1 is negative in this tumor.
Comment Here
Reference: Low grade nasopharyngeal papillary adenocarcinoma
Comment Here
Reference: Low grade nasopharyngeal papillary adenocarcinoma
Board review style question #2
Regarding low grade nasopharyngeal papillary adenocarcinoma, which of the following statements is true?
- Complete surgical excision is usually curative
- PAX8 is immunopositive in this tumor
- This tumor has EBV association
- This tumor has a high rate of metastasis
Board review style answer #2
A. Complete surgical excision is usually curative. This tumor exhibits indolent biological behavior and complete surgical excision is usually curative.
Answer C is incorrect because this tumor does not have any known etiological agent or any viral association.
Answer B is incorrect as PAX8 (which is a transcription factor involved in development of the central nervous system, eye, kidney, thyroid gland, organs derived from the mesonephric [Wolffian] duct and organs derived from the Müllerian duct) is usually immune negative in nasopharyngeal tumors.
Answer D is incorrect because this tumor usually has anexcellent prognosis with no known metastases.
Comment Here
Reference: Low grade nasopharyngeal papillary adenocarcinoma
Comment Here
Reference: Low grade nasopharyngeal papillary adenocarcinoma
Nasal chondromesenchymal hamartoma
Table of Contents
Definition / general | Radiology images | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Additional referencesDefinition / general
- Rare; intranasal and paranasal polypoid mass in infants and occasionally older children
- 2/3 male, mean age 14 months, range 1 day to 7 years
- Benign but fills nasal cavity and usually extends into ethmoid sinuses
- May erode bone, including cribriform plate and have an intracranial component
Case reports
- 47 year old woman with endoscopic surgery for nasal polyp (Case of the Week #291)
Treatment
- Excision
Microscopic (histologic) description
- Well demarcated mature cartilage, myxoid stroma, focal osteoclast-like giant cells, aneurysmal bone cyst-like formations, spindle cells, collagen fibers
Microscopic (histologic) images
Electron microscopy description
- Fibroblastic and myofibroblastic differentiation
Additional references
Nasopharyngeal angiofibroma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Locally aggressive fibrovascular neoplasm of the nasopharynx developed almost exclusively in adolescent and young male patients
Essential features
- Nasopharyngeal location
- Affecting adolescent or young male patients
- Histologically composed of vasculature of various sizes and cellular fibrotic stroma with fibroblasts
Terminology
- Juvenile nasopharyngeal angiofibroma, juvenile fibroma, angiofibroma
Epidemiology
- 0.05 - 0.5% of all head and neck tumors (StatPearls: Nasopharyngeal Angiofibroma [Accessed 10 June 2020])
- Occurs almost exclusively in adolescent and young males, with a median age of diagnosis of 15 years (range: 8 - 27 years) (Acta Otorrinolaringol Esp 2019;70:279, Head Neck Pathol 2018;12:52)
- Although a handful of case reports describe nasopharyngeal angiofibroma in female patients, the documentation of these cases is not convincing to represent this particular entity (Mol Clin Oncol 2018;9:702, J Laryngol Otol 2018;132:184, Eur Arch Otorhinolaryngol 2006;263:657, Indian J Dent Res 2004;15:145, AMA Arch Otolaryngol 1951;54:620)
- May occur in patients with familial adenomatous polyposis (Gastroenterology 1993;105:1550, Otolaryngol Head Neck Surg 1995;113:435, ANZ J Surg 2013;83:387)
Sites
- Arises in the nasopharynx or posterolateral wall of nasal cavity (Otolaryngol Head Neck Surg 2019;161:352)
- May be locally aggressive, show extension into paranasal sinus, pterygopalatine fossa, infratemporal fossa and orbit (Otolaryngol Head Neck Surg 2019;161:352)
- 10 - 37% have intracranial extension, usually into the middle cranial fossa (Otolaryngol Head Neck Surg 2019;161:352, StatPearls: Nasopharyngeal Angiofibroma [Accessed 10 June 2020])
Etiology
- Etiology remains unclear
- May be hormonally affected given the predilection for adolescent male
Clinical features
- Highly vascular lesion with one or more feeding arterials
- The most common feeding vessel is the internal maxillary artery, a branch of the external carotid artery (StatPearls: Nasopharyngeal Angiofibroma [Accessed 10 June 2020])
- Most frequently presents with epistaxis followed by nasal obstruction and rhinorrhea (Head Neck Pathol 2018;12:52, J Oral Maxillofac Pathol 2016;20:330)
Diagnosis
- Diagnosis is typically rendered using angiography, which allows visualization of the feeding vessels and preoperative embolization
- Given the risk of massive hemorrhage, preoperative biopsy and fine needle aspiration is strongly discouraged
Radiology description
- Angiography allows identification of a highly vascularized mass with a central feeding vessel
Prognostic factors
- Although considered as a benign mesenchymal neoplasm, nasopharyngeal angiofibroma is locally aggressive with approximately 20% local recurrence (Otolaryngol Head Neck Surg 2019;161:352, J Laryngol Otol 2018;132:978)
- Cranial or orbital involvement and postoperative residual vascularity on imaging study are associated with increased risk of local recurrence (Otolaryngol Head Neck Surg 2019;161:352)
Case reports
- 17 year old boy with nasopharyngeal angiofibroma extending intraorally (J Oral Maxillofac Pathol 2016;20:330)
- 18 year old man with nasal mass, recurrent epistaxis and nasal obstruction (J Clin Diagn Res 2017;11:MD03)
- 21 year old man with bilateral nasopharyngeal angiofibroma (Eur Arch Otorhinolaryngol 2016;273:3435)
- 31 year old man with Proteus syndrome and nasopharyngeal angiofibroma (Hippokratia 2017;21:147)
- 32 year old man with severe epistaxis and nasopharyngeal mass (BMJ Case Rep 2018)
Treatment
- Complete surgical resection is the treatment of choice
- Preoperative embolization through angiography may be performed
Gross description
- Polypoid firm mass
- Color varies from yellow to dark red or black, depending on the extent of intraoperative hemorrhage
Gross images
Microscopic (histologic) description
- Benign fibrovascular lesion composed of 2 components
- Vascular space of various sizes, ranging from dilated branching vessel of various thickness to slit-like capillaries
- Fibrous or collagenous stroma with fibroblasts
- Central area of the tumor is typically cellular, composed of fibroblasts or myofibroblasts with spindle, round or stellate morphology
- Stroma can be fibrous, edematous or collagenized
- Fibrinous thrombi may be seen in dilated vessels
- Frequently contain (abundant) mast cells
- Mitotic figures are usually absent
- Reference: Chan: WHO Classification of Head and Neck Tumours, 4th Edition, 2017
Microscopic (histologic) images
Contributed by Bin Xu, M.D., Ph.D.
Contributed by Kelly Magliocca, D.D.S., M.P.H.
Cytology description
- Given the nasopharyngeal location and the highly vascular nature of the lesion, fine needle aspiration should be avoided
Positive stains
- SMA, HHF35 / h-caldesmon in smooth muscle wall of large vasculature (Pathology 2008;40:396, Head Neck Pathol 2018;12:52)
- CD31, CD34 and ERG highlight endothelial cells of vasculature
- AR is positive in stroma cells in 40 - 75% cases; the expression may be focal and weak (Acta Otolaryngol 2015;135:51, Am J Clin Pathol 2006;125:832, Mod Pathol 1998;11:1122)
- Reported positive rate of ER is highly variable, ranging from 0% to over 90% partially depending on the subunits stained (Acta Otolaryngol 2015;135:51, Am J Clin Pathol 2006;125:832)
- Beta catenin: nuclear staining in stromal cells (fibroblasts) in 70 - 90% of cases, membranous / cytoplasmic staining in endothelial cells (J Laryngol Otol 2016;130:907)
Negative stains
Molecular / cytogenetics description
- Somatic mutation of CTNNB1 (beta catenin) gene in 75% of cases (Ann Otol Rhinol Laryngol 2019;128:1061)
Sample pathology report
- Nasopharynx, resection:
- Nasopharyngeal angiofibroma, 1.8 cm, margin negative for tumor (see comment)
- Comment: Immunostain shows that the tumor is focally positive for AR. There is abnormal nuclear accumulation of beta catenin. The immunoprofile supports the diagnosis.
Differential diagnosis
- Hemangioma:
- May affect both genders, any age and is not limited to nasopharynx
- Lacks cellular stroma enriched with fibroblasts
- Vessels are typically uniform in size, whereas angiofibroma usually contains central large caliber vessels and peripheral slit-like vascular space
- Does not show AR immunopositivity or nuclear beta catenin
- Inflammatory sinonasal polyp:
- May contain fibrous to edematous stroma but usually hypocellular
- Lack rich vasculature seen in angiofibroma
- Does not show AR immunopositivity or nuclear beta catenin
- Occurs in nasal cavity or paranasal sinuses, rather than nasopharynx
- Nasal turbinate:
- Normal nasal turbinate is vascular rich, containing large caliber blood vessels with muscle wall
- Lacks hypercellular stroma and slit-like vasculatures of angiofibroma
Board review style question #1
Board review style answer #1
B. It is locally aggressive with 20% risk of local recurrence
Comment Here
Reference: Nasopharyngeal angiofibroma
Comment Here
Reference: Nasopharyngeal angiofibroma
Board review style question #2
- Which of the following statements of nasopharyngeal angiofibroma is true?
- It generally affects adolescent women
- It has a hormonal profile of ER+, PR+, AR+
- It is a benign lesion that can be safely managed by long term observation even when large
- The most frequent molecular alteration in this lesion is CTNNB1 somatic mutation
Board review style answer #2
D. The most frequent molecular alteration in this lesion is CTNNB1 somatic mutation
Comment Here
Reference: Nasopharyngeal angiofibroma
Comment Here
Reference: Nasopharyngeal angiofibroma
Nasopharyngeal carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Nasopharyngeal carcinoma originates from the nasopharyngeal mucosa showing histologic or immunophenotypic evidence of squamous differentiation
- WHO classification of nasopharyngeal carcinoma:
- Nonkeratinizing squamous cell carcinoma
- Differentiated subtype
- Undifferentiated subtype
- Keratinizing squamous cell carcinoma
- Basaloid squamous cell carcinoma
- Nonkeratinizing squamous cell carcinoma
Essential features
- Originates from nasopharynx
- Showing squamous differentiation on H&E or immunohistochemistry
- Most are positive for Epstein-Barr virus (EBV) / Epstein-Barr encoded RNA (EBER) in endemic areas (China, Southeast Asia and North Africa)
- Nonkeratinizing, undifferentiated subtype is the most common, showing syncytial growth pattern, vesicular nuclei with prominent central nucleoli and reactive lymphoid stroma
Terminology
- Nonkeratinizing, undifferentiated: lymphoepithelioma, lymphoepithelial carcinoma, undifferentiated carcinoma
- Nonkeratinizing, differentiated: transitional carcinoma
- Keratinizing: squamous cell carcinoma
ICD coding
Epidemiology
- High Incidence in China, Southeast Asia, North Africa, natives of Arctic region and part of the Mediterranean Basin (Semin Diagn Pathol 2015;32:54, Surg Oncol Clin N Am 2015;24:547)
- Rare in US: incidence 0.2 to 0.5 per 100,000 (Semin Diagn Pathol 2015;32:54, Surg Oncol Clin N Am 2015;24:547)
- Undifferentiated is the most common subtype, accounting for > 60% of nasopharyngeal carcinoma
- It is also the most frequent pediatric subtype
- Other subtypes in a descending order of frequency are keratinizing, nonkeratinizing differentiated and basaloid
- Peak incidence in fourth to sixth decades; less than 20% occur in pediatric age group
Sites
- As the name implies, nasopharyngeal carcinoma affects nasopharynx, with nasopharyngeal recess (i.e. fossa of Rosenmüller) as the most common affected site, followed by superior posterior wall
- Extension to adjacent organs (e.g., nasal cavity, paranasal sinus, oropharynx, bone, orbit and infratemporal fossa) is common (Int J Radiat Oncol Biol Phys 2004;59:21)
Etiology
- Strongly associated with Epstein-Barr virus (EBV) in endemic regions (Semin Diagn Pathol 2015;32:54, Mod Pathol 2017;30:S68)
- Subset may be attributed to high risk human papillomavirus (HPV) in nonendemic and endemic regions (Head Neck 2014;36:511, Neoplasma 2016;63:107, Acta Virol 1988;32:261)
- Other risk factors (Semin Diagn Pathol 2015;32:54):
- Genetic susceptibility; e.g., certain human leukocyte antigen (HLA) class I genotypes (Semin Diagn Pathol 2015;32:54)
- High levels of nitrosamines in preserved food (e.g., salted fish)
- Cigarette smoking
- Occupational exposure to chemical fumes, smoke, formaldehyde and wood dust
- Keratinizing subtype can arise as secondary malignancy years after radiation therapy for nonkeratinizing nasopharyngeal carcinoma (Hum Pathol 2000;31:227)
Clinical features
- Commonly presents with painless upper cervical lymphadenopathy secondary to lymph node metastasis or obstructive symptoms related to nasopharyngeal mass (e.g., postnasal drip, nasal discharge, epistaxis, serous otitis media and tinnitus) (Hong Kong Med J 1997;3:355)
- Headache and symptoms of cranial nerve involvement in advanced disease with skull base involvement
Diagnosis
- Biopsies are recommended if diagnosis is suspected (70% sensitive)
Laboratory
- Positive Epstein-Barr virus (EBV) serology (Int J Cancer 2020;146:2923)
- Elevated circulating EBV DNA or RNA; e.g., plasma or serum EBV encoded small RNA (EBERs)
Radiology description
- MRI covering the nasopharynx and cervical regions is the preferred imaging modality to assess extent of disease and presence of intracranial extension (Radiopaedia: Nasopharyngeal Carcinoma [Accessed 5 April 2018])
Radiology images
Prognostic factors
- 5 year survival is ~ 65% for nonkeratinizing differentiated and undifferentiated subtypes
- Keratinizing type has a poorer outcome, with frequent nodal metastasis and high mortality (Semin Diagn Pathol 2015;32:54)
- Clinical stage is the most important prognostic factor: 5 year disease specific survival is 98% for stage I, 95% for stage II, 86% for stage III and 73% for stage IV
- Other adverse prognostic factors include older age, male gender, large tumor volume and cranial nerve palsy
Case reports
- 49 year old woman with breast metastasis from nasopharyngeal carcinoma (Oncol Lett 2013;5:1859)
- 50 year old man with bilateral enlarging neck masses for 10 months (Case #100)
- 56 and 58 year old men with glandular differentiation in EBV positive nasopharyngeal carcinoma (Arch Pathol Lab Med 2000;124:1369)
Treatment
- Radiation therapy is the mainstay of treatment for all histologic types of nasopharyngeal carcinoma (NCCN: NCCN Guidelines - Head and Neck Cancers [Accessed 14 October 2020])
- Surgery is reserved for patients who fail to respond to radiation therapy
Gross description
- Most specimens are endoscopic biopsy without specific gross findings
- In resection specimens, the gross appearance is variable: smooth mucosal bulge, raised nodule with or without surface ulceration, infiltrative mass lesion or occult lesion identified only on microscopy
Microscopic (histologic) description
- Nonkeratinizing, undifferentiated subtype
- Either syncytial arrangement of cohesive cells with indistinct cell margins (Regaud pattern) or diffuse cellular infiltrate of noncohesive cells (Schmincke pattern) resembling non-Hodgkin lymphoma (growth patterns have no clinical significance)
- Tumor cells have moderate eosinophilic to amphophilic cytoplasm, round nuclei, prominent eosinophilic nucleoli, vesicular chromatin
- No significant keratinization
- Apoptosis and brisk mitotic activity are invariably present
- Usually (but not always) prominent nonneoplastic lymphoplasmacytic infiltrate accompanying the tumor
- Necrosis is uncommon
- Nonkeratinizing, differentiated subtype
- Usually interconnecting cords or trabeculae, with little or no keratinization; growth pattern is similar to urothelial carcinoma
- Tumor cells show well defined cell borders with variable intercellular bridges
- Background stroma demonstrates variable lymphoplasmacytic infiltrate
- Usually no desmoplastic response to invading tumor
- Subclassification of nonkeratinizing nasopharyngeal carcinoma into differentiated and undifferentiated subtypes is optional as it is not clinically or prognostically significant; if there is overlapping histology within the same tumor, classify according to the dominant component
- Keratinizing subtype
- Indistinguishable from keratinizing squamous cell carcinoma of other body sites
- Obvious squamous differentiation as intercellular bridges and keratinization
- Can grade as well differentiated, moderately differentiated or poorly differentiated
- Desmoplastic response common
- Basaloid subtype
- Indistinguishable from basaloid squamous cell carcinoma of other body sites
- Tumor cells have high nuclear / cytoplasmic ratio with scanty cytoplasm, giving the basaloid appearance
- Basaloid tumor nests and sheets, often with peripheral palisading and central comedo type necrosis
Microscopic (histologic) images
Contributed by Bin Xu, M.D., Ph.D. and Andrey Bychkov, M.D., Ph.D. and Songyang Yuan, M.D., Ph.D.
Nonkeratinizing, undifferentiated subtype
Cytology description
- Nonkeratinizing undifferentiated type shows clusters of tumor cells with indistinct cell border, vesicular nuclei and prominent central nucleoli
- Background lymphocytes and plasma cells are commonly seen
Cytology images
Positive stains
- EBV LMP is less specific and less widely used in North America but is used elsewhere
- Pancytokeratin, high molecular weight CK, p63 and p40 are strongly positive
- Rarely negative for high molecular weight CK and p40
Negative stains
Molecular / cytogenetics description
- Detection of Epstein-Barr virus (EBV) by PCR or ISH (EBER) found in 75 - 100% of cases
Molecular / cytogenetics images
Sample pathology report
- Nasopharynx, biopsy:
- Nasopharyngeal carcinoma, nonkeratinizing, undifferentiated type (see comment)
- Comment: In situ hybridization for Epstein-Barr virus (EBER) is positive in this tumor.
Differential diagnosis
- Diffuse large B cell lymphoma (DLBCL):
- Mucosal malignant melanoma:
- Primary mucosal melanoma of nasopharynx is exceedingly rare, with only a few case reports (J Cancer Res Ther 2014;10:416, J Craniofac Surg 2014;25:e567, J Contemp Brachytherapy 2013;5:157)
- Epithelioid and amelanotic melanoma may share some cytologic similarity; e.g. prominent nucleoli and syncytial sheet-like growth pattern
- Positive for S100, HMB45, MelanA, negative for CK and EBV
- Oropharyngeal nonkeratinizing squamous cell carcinoma:
- Usually nonkeratinizing and shares certain histologic similarities
- Over 85% are p16+ and HPV+ but negative for EBV, compared with nasopharyngeal carcinomas that are commonly related to EBV
- In the rare incidence of HPV related nasopharyngeal carcinoma, radiologic correlation to determine the location of the primary tumor is needed
- Rhabdomyosarcoma:
- Several subtypes of rhabdomyosarcoma, e.g., embryonal rhabdomyosarcoma (including the botryoid variant), alveolar rhabdomyosarcoma, spindle cell rhabdomyosarcoma and pleomorphic rhabdomyosarcoma, have been reported in sinonasal tract / nasopharynx
- Embryonal rhabdomyosarcoma and alveolar rhabdomyosarcoma can demonstrate a primitive small blue round cell morphology that could mimic undifferentiated nasopharyngeal carcinoma
- Positive for myogenic markers (desmin, myoglobin and myogenin), negative for CK and EBV
- Sinonasal undifferentiated carcinoma:
- Arises in nasal cavity and paranasal sinuses only, hyperchromatic tumor cells with coarse chromatin, individual cell necrosis and necrosis of cell nests
- EBER- (Am J Surg Pathol 2002;26:371)
Additional references
Board review style question #1
A recent immigrant from Hong Kong presented with nasal discharge and a nasopharyngeal mass was biopsied. Which of the following statements is true?
- In the United States, the lesion most commonly affects African Americans
- Lesion commonly shows a squamous immunophenotype (i.e. positive for high molecular weight cytokeratin and p40)
- Lesion is most commonly associated with high risk human papillomavirus (HPV)
- Surgery is the standard treatment modality for this lesion
Board review style answer #1
B. Lesion commonly shows a squamous immunophenotype (i.e. positive for high molecular weight cytokeratin and p40)
Comment Here
Reference: Nasopharyngeal carcinoma
Comment Here
Reference: Nasopharyngeal carcinoma
Board review style question #2
Which of the following statements about nasopharyngeal carcinoma is true?
- B cell clonality may be demonstrated in the undifferentiated type of nonkeratinizing nasopharyngeal carcinoma
- Most common subtype of nasopharyngeal carcinoma is the keratinizing type
- Nasopharyngeal carcinoma may be related to EBV or HPV
- Nasopharyngeal carcinoma predominantly affects patients from South America
Board review style answer #2
C. Nasopharyngeal carcinoma may be related to EBV or HPV
Comment Here
Reference: Nasopharyngeal carcinoma
Comment Here
Reference: Nasopharyngeal carcinoma
Nonintestinal type
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Malignant tumor showing glandular differentiation, with presence of intraluminal mucin (in most cases)
Essential features
- Gland forming malignancy of the sinonasal tract
- Morphologically, lacks intestinal type features or characteristics of a salivary gland specific neoplasm
- Histologically divided into 2 grades: low grade (LG) and high grade (HG)
- Sinonasal renal cell-like adenocarcinoma (SRCLA) constitutes a definite subtype
- ~25% of LG sinonasal adenocarcinoma cases recur (Cancer 1982;50:312)
- HG sinonasal adenocarcinoma fares worse with reports of occasional metastases
Terminology
- Terminal tubulus adenocarcinoma
- Tubulopapillary low grade adenocarcinoma
- Low grade adenocarcinoma
- Seromucinous adenocarcinoma
ICD coding
- ICD-11
- 2C20.0 & XH74S1 - adenocarcinoma of nasal cavity & adenocarcinoma, NOS
- 2C22.0 & XH74S1 - adenocarcinoma of paranasal sinuses & adenocarcinoma, NOS
Epidemiology
- LG sinonasal adenocarcinoma is uncommon and does not exhibit any sex predilection
- HG sinonasal adenocarcinoma is rarer and affects men more frequently
- Both LG and HG sinonasal adenocarcinoma occur over a wide age range, with a peak in the sixth decade
Sites
- LG sinonasal adenocarcinoma arises most frequently in the middle turbinate (64%), followed by ethmoid sinus (20%) and other individual sinuses (Am J Surg Pathol 2009;33:401)
- ~50% of all HG sinonasal adenocarcinoma cases are locally advanced at presentation with involvement of multiple sites (Am J Surg Pathol 2011;35:971)
Pathophysiology
- Exact pathogenesis of HG sinonasal adenocarcinoma is still unknown
- Some LG sinonasal adenocarcinoma cases have been found to be enriched in ETV6::NTRK3 / ETV6::RET fusions and CTNNB1 mutations (Am J Surg Pathol 2017;41:1552, Case Rep Pathol 2018;2018:8741017)
Etiology
- Exact etiology of LG sinonasal adenocarcinoma is yet to be unveiled
- HG sinonasal adenocarcinoma may be related to exposure to carcinogens (such as wood dusts and other solvents) and occasionally also harbors association with high risk human papillomavirus (HPV) (Am J Surg Pathol 2011;35:971)
Clinical features
- Nasal obstruction is most common presenting symptom
- Patients harboring HG sinonasal adenocarcinoma additionally also present with epistaxis, pain, deformity and proptosis
Diagnosis
- Imaging followed by biopsy
Radiology description
- Nasopharyngeal endoscopy typically shows soft tissue mass
- Bone destruction can be seen in HG sinonasal adenocarcinoma
- Mucin producing adenocarcinoma shows hyperintensity on T2 weighted images and exhibits gradual enhancement on contrast enhanced T1 weighted images
- Adenocarcinoma without mucin production shows isointensity to hypointensity on T2 weighted images (J Clin Med 2017;6:116)
Radiology images
Prognostic factors
- ~25% of LG sinonasal adenocarcinoma cases recur; 6% of patients die due to tumor related morbidity (Cancer 1982;50:312)
- Most patients of HG sinonasal adenocarcinoma die within 5 years, with evidence of local and distal metastasis
- Few reported cases of SRCLA have recurred or have evidence of metastasis (Head Neck Pathol 2008;2:75)
Case reports
- 29 year old woman presented with a round polypoid mass at the posterior end of nasal septum (Ear Nose Throat J 2022 Aug 10 [Epub ahead of print])
- 35 year old African American man with von Hippel-Lindau (VHL) syndrome who developed SRCLA (Oral Oncol 2022;125:105705)
- 39 year old woman and 39 year old man with HG sinonasal adenocarcinoma exhibiting a distinct morphological and immunohistochemical phenotype and harboring ETV6::NTRK3 fusion (Virchows Arch 2023;483:187)
- 67 year old woman presented with a nasopharyngeal mass with an accompanying parapharyngeal mass (Curr Oncol 2017;24:e55)
Treatment
- No standardized treatment algorithm; however, surgery followed by radiation is a mainstay in management
- Tumor free resection margin is of utmost importance (World Neurosurg 2018;120:e962)
Gross description
- Variable; however, usually appears as a red, polypoidal mass or firm, raspberry-like growth
Microscopic (histologic) description
- LG sinonasal adenocarcinoma
- Papillary and glandular growth patterns (Cancer Manag Res 2021;13:1271)
- Complex papillary structures with fibrovascular cores
- Glandular (tubular) growth pattern has cribriform or back to back glands with little intervening stroma (Cancer 1982;50:312)
- Lining mucinous cuboidal to columnar epithelial cells have eosinophilic cytoplasm with uniform basally located rounded nuclei with finely granular chromatin
- Intraluminal mucin or material present
- Mitotic figures are rare
- Necrosis is absent
- Invasion into deeper soft tissue as well as into bone may be present
- Calcospherites and squamous morules may sometimes be seen (Case Rep Pathol 2018;2018:8741017)
- Papillary and glandular growth patterns (Cancer Manag Res 2021;13:1271)
- HG sinonasal adenocarcinoma
- Exhibits more diversity in histomorphological patterns (Am J Surg Pathol 2011;35:971)
- Predominately solid or nested growth pattern
- Dispersed glandular structures or small cysts with papilla projecting into them
- Cells have intracytoplasmic mucin
- Destructive infiltrative growth pattern with invasion into bone
- Brisk mitotic activity with foci of necrosis
- SRCLA
- Histological subtype reminiscent of renal cell carcinoma
- Tubulopapillary proliferation of tumor cells
- Predominately composed of polygonal cells, with clear or slightly eosinophilic cytoplasm (Head Neck Pathol 2008;2:75, Hum Pathol 2015;46:1598, Head Neck Pathol 2017;11:333)
- Tumor cells are glycogen rich, lacking mucin production
- Small, uniform, round nuclei with presence of intranuclear inclusions commonly
- Perineural or lymphovascular invasion, necrosis or severe pleomorphism are not seen
Microscopic (histologic) images
Contributed by Diana Bell, M.D. and Wai Szeto, M.D., M.S.
Positive stains
- PAS (intracytoplasmic, diastase resistant granules), mucin stains, such as mucicarmine (intracellular or luminal)
- Pancytokeratin (diffuse), CK7 (diffuse), p40 (focally, in few cases) (Virchows Arch 2003;443:152)
- Subset of cases can also express DOG1, SOX10 and S100 protein (Head Neck Pathol 2015;9:436)
- LG sinonasal adenocarcinoma: beta catenin (nuclear localization) (Case Rep Pathol 2018;2018:8741017)
- HG sinonasal adenocarcinoma: focal expression of neuroendocrine antigens, such as synaptophysin, chromogranin, CD56 (Am J Surg Pathol 2011;35:971)
- SRCLA: carbonic anhydrase IX (CAIX) and CD10 (diffuse) (Hum Pathol 2015;46:1598)
Negative stains
- CDX2, SATB2, villin, MUC2, CK20 (infrequently positive)
- HG sinonasal adenocarcinoma: beta catenin and mismatch repair (MMR) protein (wild type expression) (Mod Pathol 2005;18:315)
- SRCLA: PAX8 or renal cell carcinoma (RCC) antibody
Molecular / cytogenetics description
- No defined diagnostic molecular alterations to date
- Some LG sinonasal adenocarcinoma cases have been found to be enriched in ETV6::NTRK3 / ETV6::RET fusions or CTNNB1 mutations (Am J Surg Pathol 2017;41:1552, Case Rep Pathol 2018;2018:8741017)
- Occasional BRAF mutations (Pathol Oncol Res 2014;20:571)
- No KRAS mutation
- SRCLA: a couple of cases reported in patients with von Hippel-Lindau (VHL) syndrome (Oral Oncol 2022;125:105705)
Sample pathology report
- Middle turbinate mass, biopsy:
- Invasive adenocarcinoma (low grade), nonintestinal type (see comment)
- Comment: The patient's clinical history of a mass in the middle turbinate is noted. Microscopic examination reveals complex, interwoven papillary architecture along with crowded back to back glands. The lining mucinous cuboidal to columnar epithelial cells have eosinophilic cytoplasm with uniform, basally located, rounded nuclei with finely granular chromatin. Occasional mitotic figures are present. Necrosis is absent. Lymphovascular or perineural invasion is not identified. On immunohistochemistry, the tumor cells are immunopositive for pancytokeratin and CK7 while negative for CK20, CDX2 and SATB2. The findings are consistent with low grade nonintestinal type sinonasal adenocarcinoma.
Differential diagnosis
- Sinonasal intestinal type adenocarcinoma:
- Metastatic papillary thyroid carcinoma:
- No surface epithelial dysplasia
- PAX8, TTF1 and thyroglobulin positive (Head Neck Pathol 2019;13:661)
- Sinonasal papilloma, oncocytic type:
- Noninvasive papillary epithelial neoplasm
- Mixed exophytic and endophytic growth patterns
- Multiple layers of cuboidal to columnar cells
- Abundant oncocytic cytoplasm, scattered admixed ciliated or mucous columnar cells
- Intraepithelial neutrophilic inflammation, microcysts with mucin or neutrophilic microabscesses are characteristic
- No epithelial dysplasia
- Respiratory epithelial adenomatoid hamartoma (REAH):
- Associated with allergy and inflammatory disorders
- Predilection for posterior nasal septum
- Lobular / polypoid growth pattern
- Proliferation of medium sized, elongated to rounded glands that expand from surface epithelium downward into the stroma
- Absence of complex glandular architecture, true invasion or dysplasia (Ear Nose Throat J 2021;100:495S)
Board review style question #1
The H&E images shown above depict a mass in the middle turbinate of a 45 year old man. Based on these images, what is the diagnosis?
- Respiratory epithelial adenomatoid hamartoma (REAH)
- Sinonasal nonintestinal type adenocarcinoma
- Sinonasal papilloma, oncocytic type
- Sinonasal renal cell-like adenocarcinoma
Board review style answer #1
B. Sinonasal nonintestinal type adenocarcinoma. The pictures show a tumor arranged as glands and interwoven papillae and infiltrating the underlying stroma. These features are consistent with a sinonasal nonintestinal type adenocarcinoma. Answers A and C are incorrect because these neoplasms lack true invasion into the underlying stroma. Answer D is incorrect because sinonasal renal cell-like adenocarcinoma is predominately composed of polygonal cells with clear cytoplasm, resembling a renal cell carcinoma.
Comment Here
Reference: Nonintestinal type sinonasal adenocarcinoma
Comment Here
Reference: Nonintestinal type sinonasal adenocarcinoma
Board review style question #2
Regarding sinonasal renal cell-like adenocarcinoma (SRCLA), which of the following statements is true?
- Carbonic anhydrase IX (CAIX) and CD10 are diffusely immunopositive
- PAX8 is immunopositive in this tumor
- Renal cell carcinoma (RCC) marker is immunopositive
- This tumor has high rate of metastasis
Board review style answer #2
A. Carbonic anhydrase IX (CAIX) and CD10 are diffusely immunopositive. Answers B and C are incorrect because unlike renal cell carcinomas, PAX8 (which is a transcription factor involved in development of the kidney) and RCC immunomarkers are immunonegative in SRCLA. Answer D is incorrect because no reported cases have metastases to date.
Comment Here
Reference: Nonintestinal type sinonasal adenocarcinoma
Comment Here
Reference: Nonintestinal type sinonasal adenocarcinoma
NUT carcinoma
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Clinical features | Radiology images | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Aggressive carcinoma defined by translocations involving the NUT gene and frequent squamous differentiation
Essential features
- Highly aggressive malignancy with median survival of < 1 year
- Histologically defined by primitive round cells with areas of abrupt keratinization
- Caused by translocations involving the NUT gene (15q14), most commonly with BRD4 (19p13)
- Speckled nuclear positivity for NUT1 immunohistochemistry is sensitive and specific for diagnosis
Terminology
- NUT stands for nuclear protein in testis
- Formerly called NUT midline carcinoma due to proclivity for midline sites
Epidemiology
- Although originally described in children, affects people of all ages (Head Neck Pathol 2013;7:11)
- No sex or geographic predilection (Head Neck Pathol 2013;7:11)
- No clear risk factors established to date (Head Neck Pathol 2013;7:11)
Clinical features
- Aggressive malignancy with median survival of < 1 year (Head Neck Pathol 2013;7:11)
- ~35% involve the head and neck, most commonly in the sinonasal tract (Head Neck Pathol 2013;7:11)
- Although also common in other midline sites such as the mediastinum, can occur anywhere in the body (Head Neck Pathol 2013;7:11)
Case reports
- 14 year old girl with ethmoid sinus mass (Oncogenesis 2015;4:e174)
- 18 year old woman with undifferentiated sinonasal mass (Pathol Res Pract 2014;210:383)
- 48 year old man with vision changes (Saudi J Ophthalmol 2018;32:62)
- 53 year old man with nasal cavity mass (Head Neck Pathol 2017;11:389)
- 54 year old woman with nasal mass (Arch Pathol Lab Med 2011;135:1494)
Treatment
- Tumors generally show minimal response to radiation and conventional chemotherapy (Head Neck Pathol 2013;7:11)
- In clinical trials, some patients show rapid response to extraterminal bromodomain inhibitors although prognosis remains poor (Cancer Discov 2016;6:492)
Gross description
- Highly infiltrative tumor frequently involving multiple anatomic subsites
- Nonspecific white-tan cut surface commonly demonstrating prominent necrosis
Microscopic (histologic) description
- Primitive small to medium sized cells with minimal indistinct to clear cytoplasm
- Monotonous round to oval nuclei with variably prominent nucleoli
- High mitotic rate with prominent tumor necrosis
- Foci of abrupt squamous differentiation with clear to eosinophilic cytoplasm and keratin pearl formation
- Areas of spindled cells frequently present
- Dense neutrophil infiltrate seen in subset of cases
Microscopic (histologic) images
Contributed by Lisa Rooper, M.D.
Contributed by Brendan Dickson, M.D.
Positive stains
- NUT1 diffuse speckled nuclear positivity is 87% sensitive and 100% specific (Am J Surg Pathol 2009;33:984)
- Pancytokeratin, EMA consistently positive (Am J Surg Pathol 2012;36:1216)
- p63 / p40 ~67% (Am J Surg Pathol 2012;36:1216)
- CD34 ~30 - 50% (Am J Surg Pathol 2012;36:1216)
- INI1 retained (Surg Pathol Clin 2017;10:103)
Negative stains
- CD99, NKX2.2
- S100, synaptophysin, chromogranin focal expression in rare cases (Am J Surg Pathol 2012;36:1216)
Electron microscopy description
- Large, irregular nuclei with prominent compact nucleoli (Ultrastruct Pathol 2012;36:280)
- Abundant cytoplasm with prominent tonofilament bundles, scattered pleomorphic granules and numerous desmosomal junctions (Ultrastruct Pathol 2012;36:280)
Molecular / cytogenetics description
- Defined by translocations involving the NUT gene on 15q14 (Cancer Discov 2014;4:928)
- BRD4-NUT (19p13) fusion is present in 67% of cases (Cancer Res 2003;63:304)
- Other cases show BRD3-NUT or NSD3-NUT fusions (Oncogene 2008;27:2237, Cancer Discov 2014;4:928)
- Fusions block epithelial differentiation and maintain proliferation in tumor cells (Oncogene 2008;27:2237)
- In situ hybridization for high risk HPV and EBV is uniformly negative (Int J Surg Pathol 2013;21:102)
Sample pathology report
- NUT carcinoma; see note:
- Note: Immunostains show that the tumor cells are positive for AE1 / AE3, p40 and NUT1 and negative for CD99 and synaptophysin, supporting the above diagnosis. NUT carcinoma is a rare, highly aggressive malignancy that is defined by translocations involving the NUT gene. Please see www.nmcregistry.org for information about enrolling the patient into the International NUT Carcinoma Registry and opportunities for clinical trials.
Differential diagnosis
- Basaloid squamous cell carcinoma: can also show abrupt keratinization and p63 / p40 positivity; negative for NUT1
- Sinonasal undifferentiated carcinoma: also high grade tumor; has more cytologic atypia, lacks keratinization, negative for NUT1 and p63 / p40, frequent IDH2 mutations
- High grade neuroendocrine carcinoma: also high grade tumor; lacks keratinization, negative for NUT1 and p63 / p40 and positive for synaptophysin, chromogranin and INSM1
- Adamantinoma-like Ewing sarcoma: can also show abrupt keratinization and p63 / p40 positivity; negative for NUT1 and positive for CD99 and NKX2.1
- SMARCB1 deficient sinonasal carcinoma: also high grade tumor that can be positive for p63 / p40; lacks keratinization, negative for NUT1 and shows loss of INI1
Board review style question #1
An 18 year old woman presented with a destructive sinonasal tumor; biopsy findings are shown below. What immunohistochemical finding would you expect?


- Cytoplasmic positivity for cytokeratin without significant reactivity for any other markers
- Diffuse strong positivity for synaptophysin and INSM1
- Loss of nuclear INI1 expression
- Speckled nuclear positivity for NUT1
Board review style answer #1
D. Speckled nuclear positivity for NUT1. The picture depicts a NUT carcinoma, diagnosis of which is suggested by the presence of monotonous primitive cells interspersed with areas of abrupt keratinization. The correct immunohistochemical finding for this tumor is speckled nuclear positivity for NUT1, a sensitive and specific marker of this tumor type. The other immunoprofiles listed would be more characteristic of tumors that lack the characteristic keratinization pattern depicted in this photomicrograph, including sinonasal undifferentiated carcinoma (A. Cytoplasmic positivity for cytokeratin without significant reactivity for any other markers), small cell carcinoma (B. Diffuse strong positivity for synaptophysin and INSM1) and SMARCB1 deficient sinonasal carcinoma (C. Loss of nuclear INI1 expression).
Comment Here
Reference: NUT carcinoma
Comment Here
Reference: NUT carcinoma
Board review style question #2
What is the most common genetic abnormality identified in NUT carcinoma?
- BRD3-NUT
- BRD4-NUT
- Deletion of NUT
- NSD3-NUT
Board review style answer #2
B. BRD4-NUT. The most common genetic abnormality identified in NUT carcinoma is BRD4-NUT fusion, which is present in ~67% of cases. The other translocations listed, BRD3-NUT and NSD3-NUT, are less common variant fusions seen in NUT carcinoma; deletion of NUT has not been reported as a cause of this tumor type.
Comment Here
Reference: NUT carcinoma
Comment Here
Reference: NUT carcinoma
Olfactory neuroblastoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Radiology description | Radiology images | Prognostic factors | Diagrams / tables | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Malignant neuroectodermal tumor commonly located at superior aspect of nasal cavity showing neuroblastic differentiation (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Thought to arise from olfactory membrane or olfactory placode (plate-like thickening of embryonic ectoderm from which a nerve ganglion or sensory organ will develop) which extends from roof of nasal cavity in fetus to midnasal septum and superior turbinate
- Not related to neuroblastoma elsewhere in body
Essential features
- Originated and centered around cribriform plate of nasal cavity
- Small blue round cell tumor with lobulated growth pattern and abundant neuropil
- Homer Wright pseudorosettes and Flexner-Wintersteiner rosettes may be seen
- The most important prognostic factors are Hyams grade and Kadish stage
Terminology
- Also called esthesioneuroblastoma, esthesioneuroepithelioma or olfactory placode tumor
ICD coding
Epidemiology
- Patients of all ages, 2 to 90 years
- No obvious gender, ethnic or familial predilection
- Annual incidence of 0.4 per million (Mod Pathol 2017;30:S1, Head Neck Pathol 2009;3:252)
- 2% of all sinonasal neoplasms
Sites
- Usually confined to the cribriform plate and upper nasal vault; rarely in nasopharynx, maxillary or ethmoid sinus (Mod Pathol 2017;30:S1, Head Neck Pathol 2009;3:252)
- May become locally invasive into paranasal sinuses, nasopharynx, palate, orbit, skull base, brain
Pathophysiology
- Theorized to originate from specialized sensory neuroepithelium located in superior nasal cavity, including cribriform plate of ethmoid, nasal roof, superior nasal concha and superior nasal septum (Mod Pathol 2017;30:S1, Head Neck Pathol 2009;3:252)
Etiology
- None identified
Clinical features
- Usually presents with nonspecific syndromes of nasal obstruction, epistaxis, headache and rhinorrhea (Mod Pathol 2017;30:S1, Head Neck Pathol 2009;3:252)
- Rare symptoms include anosmia, visual disturbance, paraneoplastic syndromes (e.g. syndrome of inappropriate antidiuretic hormone secretion (SIADH))
Radiology description
- Typical finding is a dumbbell shaped mass centered at cribriform plate
Radiology images
Prognostic factors
- 20% develop regional or distant metastases, usually to cervical lymph nodes and lung; late recurrence (after 10 years) is common
- Grade and stage are the most important prognostic factors
- Hyams histologic grade (Table 1) is the most widely used grading system which encompasses six histologic features and is an independent prognostic factor (Head Neck Pathol 2015;9:51, Hyams VJ. Olfactory neuroblastoma (Case 6) In: Batsakis JG, Hyams VJ, Morales AR, editors, Special tumors of the head and neck, Chicago: ASCP Press; 1982. pp. 24-29)
- Kadish staging system (Cancer 1976;37:1571, Head Neck 2017;39:1962, Head Neck Pathol 2015;9:51) and Morita modification (Table 2) (Head Neck Pathol 2015;9:51, Neurosurgery 1993;32:706) are the staging systems that are most commonly used: the 5 year survival for stages A, B and C are 75%, 68% and 41%, respectively (Mod Pathol 2017;30:S1, Head Neck Pathol 2009;3:252)
Diagrams / tables
Table 1: Hyams grade for olfactory neuroblastoma
Table 2: Staging systems of olfactory neuroblastoma
| Grade I | Grade II | Grade III | Grade IV | |
| Architecture | Lobular | Lobular | Variable | Variable |
| Fibrillary matrix | Prominent | Present | Minimal | Absent |
| Mitosis | Absent | Present | Prominent | Marked |
| Necrosis | Absent | Absent | May present | Common |
| Nuclear pleomorphism | Absent | Moderate | Prominent | Marked |
| Rosettes | Homer Wright | Homer Wright | Flexner- Wintersteiner |
Flexner- Wintersteiner |
Table 2: Staging systems of olfactory neuroblastoma
Kadish staging:
Morita modification:
|
Case reports
- 30 year old woman and 67 year old woman with varied atypical clinical presentation (J Clin Diagn Res 2016;10:PD01)
- 35 year old man with divergent differentiation (Head Neck Pathol 2017;11:531)
- 41 year old man with multifocal ectopic olfactory neuroblastoma (Am J Case Rep 2016;17:268)
- 43 year old woman with a mass in the right nasal cavity (Head Neck Pathol 2016;10:256)
- 44 year old man with aberrant pattern of cytokeratin expression (Head Neck Pathol 2017;11:262 )
Treatment
- Complete surgical excision (may require craniofacial resection), with radiation therapy or chemotherapy
Gross description
- Red-gray, highly vascular, polypoid mass
Microscopic (histologic) description
- Low grade olfactory neuroblastoma usually contains nests and lobules of monotonous tumor cells with round nuclei, indistinct nucleoli and scanty cytoplasm in association with a vascular-rich to hyalinized stroma; fibrillary neural matrix may be present
- High grade tumors may show solid growth, marked mitotic activity, nuclear pleomorphism, necrosis and no neuropil
- Homer Wright pseudorosettes: neoplastic cells palisading around a central zone of fibrillar neural matrix; their presence in a nasal tumor is characteristic for olfactory neuroblastoma
- Flexner-Wintersteiner rosettes: palisading tumor cells surrounding a true central lumen
- Perivascular pseudorosettes may be present but are non-specific
- Other findings: melanin pigment, neurons, divergent differentiation (e.g. rhabdomyoblastic, glandular and squamous differentiation)
Microscopic (histologic) images
Contributed by Bin Xu, M.D., Ph.D.
Positive stains
- Synaptophysin, chromogranin, CD56, neurofilament, S100 in sustentacular cells at periphery of cell nests
- Keratins, e.g. cytokeratin AE1/AE3, CAM5.2 and CK18, are commonly negative, but up to a third may show focal positivity
- INI1
- Area of rhabdomyoblastic differentiation if present is positive for desmin and myogenin
- Calretinin (Am J Surg Pathol 2011;35:1786)
Negative stains
Electron microscopy description
- Dense core neurosecretory granules, microtubules, neuritic processes, neurofilaments (Mod Pathol 2017;30:S1, Head Neck Pathol 2009;3:252)
Molecular / cytogenetics description
- Recent next generation sequencing studies have shown that olfactory neuroblastoma has high level but heterogenous chromosomal instability and copy number alterations (Oncotarget 2016;7:52584)
- Absence of characteristic fusions of other small blue round cell tumors that may occur in the sinonasal tract, e.g. Ewing sarcoma EWSR1 fusion and alveolar rhabdomyosarcoma PAX3 or PAX7 fusion
Sample pathology report
- Nasal cavity, left; biopsy:
- Olfactory neuroblastoma, Hyams grade II (see comment)
- Comment: Immunohistochemistry studies show that the tumor is positive for synaptophysin and chromogranin, whereas negative for cytokeratin. S100 highlighted the sustentacular network. The overall immunoprofile supports the diagnosis.
Differential diagnosis
-
Quite broad; includes all sinonasal small blue round cell tumors (Surg Pathol Clin 2017;10:103)
- Lymphoma: positive for CD45 and other lymphoid markers
- Melanoma: positive for S100, SOX10, MelanA, HMB45
- Small round cell sarcoma:
- Ewing sarcoma: positive for CD99, FLI1, t(11,22) / FLI1::EWSR1 fusion
- Embryonal rhabdomyosarcoma: diffusely positive for desmin and myogenin
- Alveolar rhabdomyosarcoma: diffusely positive for desmin and myogenin, PAX3 or PAX7 fusion
- Mesenchymal chondrosarcoma: NCOA2 fusion
- Carcinoma:
- Small cell carcinoma / poorly differentiated neuroendocrine carcinoma: diffusely positive for cytokeratin, negative for neurofilament and S100
- Sinonasal undifferentiated carcinoma: negative for synaptophysin and chromogranin
- NUT midline carcinoma: positive for NUT
- Adenoid cystic carcinoma: negative for synaptophysin and chromogranin; positive for myoepithelial markers calponin, SMA and S100 and MYB::NFIB fusion
- SMARCB1-deficient sinonasal carcinoma: loss of INI1
Board review style question #1
-
A biopsy of a sinonasal mass from a 50 year old man is shown here:
- The tumor most commonly locates to the lower portion of nasal cavity
- Tumor necrosis, nuclear pleomorphism and high mitotic index are part of the staging system
- The tumor is not associated with similar appearing tumors elsewhere in the body
- SMARCB1 (INI1) immunohistochemistry is lost in this tumor
Which of the following statements is true?
Board review style answer #1
C. Olfactory neuroblastoma is not associated with neuroblastoma elsewhere in the body
Comment Here
Reference: Olfactory neuroblastoma
Comment Here
Reference: Olfactory neuroblastoma
Board review style question #2
-
Which statement about olfactory neuroblastoma is true?
- The most important prognostic factor is N-MYC amplification
- It is most commonly originated from the medial wall of maxillary sinus
- Most tumors are diffusely positive for CAM5.2
- It may contain areas with rhabdomyoblastic differentiation
Board review style answer #2
D. It may contain areas with rhabdomyoblastic differentiation
Comment Here
Reference: Olfactory neuroblastoma
Comment Here
Reference: Olfactory neuroblastoma
Respiratory epithelial adenomatoid hamartoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Acquired overgrowth of surface glands characterized by pseudostratified epithelium with cilia surrounded by a thickened basement membrane
- Currently classified as nonneoplastic hamartoma in the WHO classification 5th edition (Head Neck Pathol 2022;16:1)
- Chondro-osseous respiratory epithelial (CORE) hamartoma is considered a subtype of respiratory epithelial adenomatoid hamartoma (REAH) (Head Neck Pathol 2022;16:1)
Essential features
- Overgrowth of medium sized glands displacing normal elements (such as seromucinous glands)
- Lining of glands is pseudostratified respiratory epithelium with cilia
- Glands are surrounded by thickened basement membrane
Terminology
- Glandular hamartoma (not recommended)
- Subtype: chondro-osseous and respiratory epithelial hamartoma
ICD coding
- ICD-10: Q85.9 - phakomatosis, unspecified
Epidemiology
- M:F = 3:1 to 7:1 (Ann Otol Rhinol Laryngol 1995;104:639, Am J Rhinol Allergy 2013;27:322)
- Median age: 58 years (range: 27 - 81 years) (Ann Otol Rhinol Laryngol 1995;104:639, Am J Rhinol Allergy 2013;27:322)
Sites
- Most commonly occurs in the posterior septum of nasal cavity, particularly the olfactory cleft (Ann Otol Rhinol Laryngol 1995;104:639, Am J Rhinol Allergy 2013;27:322)
- Other sites of involvement include lateral wall of nasal cavity, middle meatus, nasal turbinate, nasopharynx, paranasal sinuses (ethmoid, maxillary or frontal) (Laryngoscope Investig Otolaryngol 2022;7:1292, Ann Otol Rhinol Laryngol 1995;104:639, Am J Rhinol Allergy 2013;27:322)
Etiology
- > 66% are associated with atopic disease or chronic rhinosinusitis (Am J Rhinol Allergy 2020;34:610, Am J Rhinol Allergy 2013;27:322)
Clinical features
- Patients commonly present with nonspecific symptoms (e.g., nasal congestion / obstruction, headache, rhinorrhea and epistaxis)
- Often has a longstanding history of chronic rhinosinusitis (Ann Otol Rhinol Laryngol 1995;104:639)
- May be unilateral or bilateral (Ann Otol Rhinol Laryngol 1995;104:639, AJNR Am J Neuroradiol 2013;34:1086)
Diagnosis
- Identifying diagnostic histologic features in an endoscopic resection specimen is necessary for the diagnosis
Radiology description
- Expansion of the maximum olfactory cleft width (especially when > 10 mm) is a common finding on computed tomography (CT) (Am J Rhinol Allergy 2013;27:322, AJNR Am J Neuroradiol 2013;34:1086)
- No evidence of destructive growth or bone invasion
Prognostic factors
- Benign lesion: negligible risk of recurrence if resected completely (Am J Rhinol Allergy 2013;27:322)
- Local recurrence is rare
Case reports
- 17 year old boy with respiratory epithelial adenomatoid hamartoma (Ear Nose Throat J 2022 May 9 [Epub ahead of print])
- 62 year old man with respiratory epithelial adenomatoid hamartoma of the maxillary sinus (Acta Otorhinolaryngol Ital 2006;26:225)
- 75 year old man with bilateral nasal mass (Proc (Bayl Univ Med Cent) 2017;30:221)
- 83 year old woman with chondro-osseous respiratory epithelial adenomatoid hamartoma (Case Rep Otolaryngol 2019;2019:5247091)
Treatment
- Complete excision, often through the endoscopic approach, is considered curative
Gross description
- Polypoid or exophytic, rubbery, tan-brown, with a firm to gritty consistency
Microscopic (histologic) description
- Respiratory epithelial adenomatoid hamartoma (REAH)
- Proliferation (often polypoid) of medium sized glands lined by ciliated epithelium
- Glands are surrounded by thick eosinophilic basement membranes
- Lesion replaces background normal elements, often resulting in a decreased number of seromucinous glands
- Features of chronic rhinosinusitis (chronic inflammation) and inflammatory sinonasal polyp (edematous stroma admixed with chronic inflammation) may be seen in the background
- Chondro-osseous respiratory epithelial (CORE) hamartoma subtype (Int Arch Otorhinolaryngol 2013;17:218, Int J Surg Pathol 2022;30:448)
- CORE hamartoma is composed of 2 components, often intimately intermixed with each other
- Glandular component composed of glands lined by respiratory type epithelium with cilia; the extent of glandular component is often less prominent compared with REAH
- Mesenchymal component that ranges from immature mesenchyme, cartilage, bone trabeculae or chondromyxoid matrix
- CORE hamartoma is composed of 2 components, often intimately intermixed with each other
Microscopic (histologic) images
Contributed by Bin Xu, M.D., Ph.D.
Respiratory epithelial adenomatoid hamartoma (REAH)
Chondro-osseous respiratory epithelial (CORE) hamartoma
Positive stains
- Diagnosis is typically rendered based on H&E alone; immunohistochemistry is not required for this entity
- CK7 (Head Neck Pathol 2008;2:203)
Negative stains
Molecular / cytogenetics description
- Currently considered as a nonneoplastic hamartoma in the WHO classification 5th edition; however, increased fractional allelic loss is detected, suggesting a possible neoplastic nature (Am J Surg Pathol 2006;30:1576)
Sample pathology report
- Left nasal cavity, excision:
- Respiratory epithelial adenomatoid hamartoma
Differential diagnosis
- Inflammatory sinonasal polyp:
- May have occasional invagination of surface epithelium but lacks proliferation of glandular component and the thickened surrounding basement membrane
- Sinonasal papilloma, inverted type:
Respiratory epithelial adenomatoid hamartoma Sinonasal papilloma, inverted type Location Commonly on the posterior septum close to the olfactory cleft Typically on the lateral nasal wall High risk HPV Negative May be positive Thickened basement membrane Present Absent Epithelial lining Respiratory type epithelium of normal thickness with cilia Hyperplastic (thickened) squamous, transitional or respiratory type epithelium Transmigrating neutrophils / neutrophilic microabscesses Absent Present
- Sinonasal adenocarcinoma, nonintestinal type:
- Complex architecture manifested as densely packed back to back tubular glands or fused / cribriform glandular architecture
- Lacks cilia and thickened basement membrane
- May show other malignant features (e.g., nuclear pleomorphism, prominent mitotic activity, necrosis, perineural invasion and lymphovascular invasion)
- May harbor diagnostic mutations or fusions involving NTRK3, MET, NRG1, PRKACA, AKT1, BRAF and CTNNB1 genes (Mod Pathol 2022;35:1160)
- Biphenotypic sinonasal sarcoma:
Board review style question #1
Board review style answer #1
B. Respiratory epithelial adenomatoid hamartoma (REAH). The H&E shows typical histologic findings of respiratory epithelial adenomatoid hamartoma, characterized by proliferation of medium sized glands with cilia surrounded by thickened basement membrane. See Differential diagnosis section for features to distinguish REAH from its diagnostic mimickers, such as inverted papilloma, inflammatory polyp and nonintestinal sinonasal adenocarcinoma.
Comment Here
Reference: Respiratory epithelial adenomatoid hamartoma
Comment Here
Reference: Respiratory epithelial adenomatoid hamartoma
Board review style question #2
Which of the following statements about respiratory epithelial adenomatoid hamartoma (REAH) is true?
- 30% of REAH is associated with high risk human papillomavirus
- Classified as a benign neoplasm in the WHO classification
- Commonly located on the nasal septum rather than lateral nasal wall
- Has ~15% risk of recurrence and a small risk of distant metastasis
Board review style answer #2
C. Commonly located on the nasal septum rather than lateral nasal wall. Answer A is incorrect because REAH is not associated with high risk human papillomavirus. Answer B is incorrect because REAH is classified as a nonneoplastic lesion in WHO classification. Answer D is incorrect because REAH has a very low (if any) risk of recurrence if completely excised and no distant metastasis has been reported.
Comment Here
Reference: Respiratory epithelial adenomatoid hamartoma
Comment Here
Reference: Respiratory epithelial adenomatoid hamartoma
Rhinosclerosis
Table of Contents
Definition / general | Treatment | Case reports | Microscopic (histologic) description | Positive stains | Electron microscopy description | Differential diagnosisDefinition / general
- Rare; chronic granulomatous disease of nasal cavity (95 - 100%), nasopharynx (18 - 43%), larynx (15 - 40%), trachea (12%) or bronchi (2 - 7%) caused by Klebsiella rhinoscleromatis
- Usually low socioeconomic environments of central / South America, Africa, Middle East, Philippines and India; rare in US (usually immigrants)
- Most common in young adults
- Slowly progressive with remission and relapse; not fatal unless it obstructs the airway
- Microbiology: MacConkey agar cultures are 50 - 60% sensitive; bacteria is gram negative, encapsulated, nonmotile, diplobacillus, member of Enterobacteriaceae, not normal flora, infective via drops or contamination of material that is inhaled
Treatment
- Antibiotics for months to years
- Possibly steroids, surgery to treat airway compromise and tissue deformity
Case reports
- 32 year old woman with supraglottic granulomas (Arch Pathol Lab Med 2001;125:159)
Microscopic (histologic) description
- Catarrhal / atrophic, granulomatous and sclerotic stages
- Initially: squamous metaplasia and inflamed granulation tissue
- Later: pseudoepitheliomatous squamous hyperplasia with foamy macrophages (Mikulicz cells containing bacteria), plasma cells with Russell bodies and granulomatous inflammation
- Late: fibrosis, lymphocytes and plasma cells but no Mikulicz cells
Electron microscopy description
- Large phagosomes containing bacilli and finely granular material (antibodies on bacterial surface and aggregates of bacterial mucopolysaccharides)
Differential diagnosis
- Leprosy
- Other granulomatous processes
- Rosai-Dorfman disease
Rhinosporidiosis
Table of Contents
Definition / general | Risk factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosisDefinition / general
- Caused by Rhinosporidium seeberi, traditionally thought to be a fungus but actually an aquatic protistan parasite (J Clin Microbiol 1999;37:2750)
- Endemic in southern India, Sri Lanka and emigrants from this region (Diagn Pathol 2006;1:25, Singapore Med J 2004;45:224); rare indigenous cases in U.S. (South Med J 1996;89:65)
- Natural aquatic habitat, transmitted through traumatized epithelium, most commonly in nose and eye, also skin, ear, genitals and rectum
Risk factors
- Bathing or working in stagnant water
- Rarely presents with disseminated skin disease (Indian J Dermatol Venereol Leprol 2007;73:185, Indian J Dermatol Venereol Leprol 2001;67:332)
- Not transmitted person to person
Case reports
- 29 year old man from India with progressively increasing nasal obstruction (Case of the Week #97)
Treatment
- Surgical excision, may recur
- No effective medical treatment
Gross description
- Hyperplastic, polypoid, red, granular masses of nasal cavity
- Yellow pinhead spots represent mature sporangia
- Superficial mucus is common
Microscopic (histologic) description
- Large (100 - 450 microns), thick walled sporangia with 1000+ endospores, each 6 - 10 microns, accompanied by a mixed inflammatory infiltrate
- May not be present in all sections and may need additional sampling for diagnosis
Microscopic (histologic) images
Contributed by Hanni Gulwani, M.B.B.S. (Case #97), Veena Maheshwari, M.D., Kiran Alam, M.D., Anshu Jain, M.D., Alia Albawardi, M.B.B.S. and @DrTravisBrown on Twitter
Differential diagnosis
- Coccidiodes immitis: smaller spherules (30 - 60 microns), smaller endospores (2 - 5 microns)
- Myospherulosis: large tissue spaces with saclike structures containing brown spherules that resemble Prototheca but are actually clumped red blood cells, GMS-
- Often arthroconidia and hyphae
Salivary gland anlage tumor
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Extremely rare, benign, congenital, biphasic tumor with mixed epithelial and myoepithelial elements of midline nasopharynx
Essential features
- Presents within first 6 weeks of life with a midline nasopharyngeal mass and symptoms related to airway / nasal obstruction (Pediatrics 2003;112:e66)
- Covered by nonkeratinizing squamous epithelium
- Peripheral squamous nests and duct-like structures
- Central stromal nodules consisting of uniform ovoid to spindled cells (Am J Surg Pathol 1994;18:25)
- Good prognosis; excision is curative (Int J Pediatr Otorhinolaryngol 2005;69:149)
Terminology
- Salivary gland anlage tumor (SGAT)
- Congenital pleomorphic adenoma
ICD coding
- ICD-10: D49.0 - neoplasm of unspecified behavior of digestive system
Epidemiology
- Extremely rare
- Presents in immediate neonatal period or in early infancy (by age of 6 weeks)
- Male predilection (Int J Pediatr Otorhinolaryngol 2005;69:149)
Sites
- Located or near midline in the nasopharynx or posterior nasal septum
Pathophysiology
- Congenital / developmental anomaly, likely represents hamartoma of minor salivary gland origin
Clinical features
- Obstructive symptoms manifesting as respiratory distress, feeding difficulties or nasal airway obstruction
Diagnosis
- CT or MRI of the nasal sinuses
- Diagnosis is by endoscopic biopsy / debulking or surgical resection
Radiology description
- Lobular, midline soft tissue mass in the naso-oropharynx without infiltration into surrounding structures
- T1 isointense, T2 hyperintense, homogeneously enhancing mass measuring 1 to 2 cm on MRI (Pediatr Radiol 2015;45:453, AJNR Am J Neuroradiol 2009;30:1022)
Radiology images
Prognostic factors
- Excellent prognosis
- Curable by surgical resection
Case reports
- Newborn boy and 6 week old girl with nasopharyngeal mass (Fetal Pediatr Pathol 2011;30:116)
- Newborn boy with life threatening nasal obstruction (Pediatrics 2003;112:e66)
- Newborn boy with acute airway obstruction secondary to nasopharyngeal mass (Int J Pediatr Otorhinolaryngol 2005;69:149)
- 2 week old infant with difficulty breathing since birth (AJNR Am J Neuroradiol 2009;30:1022)
- Congenital salivary gland anlage tumor causing severe neonatal respiratory distress (Fetal Pediatr Pathol 2010;29:323)
- Salivary gland anlage tumor with widespread necrosis and large cyst formation (Pathology 1996;28:128)
Treatment
- Simple surgical excision
Gross description
- Polypoid, gray, rubbery, grossly encapsulated tissue fragment with homogenous cut surface without necrosis or hemorrhage
Microscopic (histologic) description
- Surface covered by nonkeratinizing squamous mucosa
- Multiple submucosal, cellular, solid stromal nodules separated by hypocellular stroma in the center
- Duct-like epithelial structures and squamous nests with variable keratinization more prominent toward periphery of stromal nodules (Int J Pediatr Otorhinolaryngol 2005;69:149)
- Internodular epithelial components blend into cellular nodules
- Cellular stromal nodules composed of mesenchymal appearing, fusiform to spindled to ovoid cells forming short fascicles or trabeculae (Pediatr Pathol Lab Med 1996;16:973)
- Stromal cells are uniform and show bland cytology with dispersed chromatin, scant eosinophilic cytoplasm and indistinct cell borders (Fetal Pediatr Pathol 2011;30:116)
- Background hypocellular collagenous stroma with focal chondromyxoid areas
- Mitotic figures and hemorrhagic necrosis may be present
Microscopic (histologic) images
Contributed by Josephine K. Dermawan, M.D., Ph.D. and Laura O. Rabinowitz, M.D.
Positive stains
- Epithelial components:
- Cytokeratins (pancytokeratins, CK7)
- p63
- Mesenchymal components:
- Variable for cytokeratins (pancytokeratins, CK7)
- Alpha smooth muscle actin, muscle specific actin, smooth muscle myosin
- May have focal S100 (Pediatr Pathol Lab Med 1996;16:973, Am J Surg Pathol 1994;18:25)
Electron microscopy description
- Stromal-like cells have ultrastructural features of myoepithelial cells (Am J Surg Pathol 1994;18:25)
Sample pathology report
- Soft tissue mass, nasopharynx, excision:
- Salivary gland anlage tumor (see comment)
- Comment: Histologic sections demonstrate a lobulated nasopharyngeal proliferation which shows mixed epithelial and myoepithelial components. The tumor shows tubules and ducts with variable keratinization with attachment to surface squamous epithelium and multiple cellular spindle cell nodules. Focal chondromyxoid matrix is noted. The epithelial component shows diffuse, strong immunoreactivity for cytokeratin and CK7. The spindle cells show variable to strong immunoreactivity with CK7, p63, SMMS and S100 (rare cells).
Differential diagnosis
- Dermoid cyst:
- Cystic lesion lined by keratinizing squamous epithelium
- Epithelium associated with skin adnexal structures, such as sebaceous glands
- Craniopharyngioma:
- Nests and cords of squamoid cells
- Peripheral palisaded nuclei
- Calcification and keratin material
- Leiomyoma or leiomyosarcomas:
- Spindle cell rhabdomyosarcoma:
- Synovial sarcoma:
- Variable positivity for cytokeratins and TLE1
- Negative for SMA and MSA
- Characterized by t(X;18) SS18-SSX1/2 fusions
- Respiratory epithelioid adenomatoid hamartoma (REAH):
- Noninvasive epithelial proliferation with surface invaginations with thickened basement membrane and ciliated respiratory epithelium
- Lack of spindle cell proliferation
Board review style question #1
- A neonate presents with respiratory distress shortly after delivery. Direct laryngoscopy shows an obstructing nasopharyngeal mass. An MRI of the nasal sinus shows a 1.8 cm, polypoid mass in the in the naso-oropharynx. Histology reveals a biphasic, epithelial and mesenchymal neoplasm with ducts and squamous nests and cellular stromal nodules consisting of spindle cells with a myoepithelial immunophenotype. What is the best management for this tumor?
- Close observation
- Cryotherapy
- Neoadjuvant chemotherapy followed by surgical resection
- Radiation therapy
- Simple endoscopic or surgical resection
Board review style answer #1
E. Simple endoscopic or surgical resection
Salivary gland anlage tumor is a benign tumor with excellent prognosis following simple surgical resection. Of all the cases that have been reported, none has recurred following surgical excision.
Comment Here
Reference: Salivary gland anlage tumor
Salivary gland anlage tumor is a benign tumor with excellent prognosis following simple surgical resection. Of all the cases that have been reported, none has recurred following surgical excision.
Comment Here
Reference: Salivary gland anlage tumor
Board review style question #2
- Which of the following features would favor a diagnosis of salivary gland anlage tumor?
- Diffuse S100 immunoreactivity in the spindled component
- Distant metastasis and death within several months of initial diagnosis
- Mesenchymal component with pleomorphic spindle cells showing significant cytologic atypia
- Presence of ducts and squamous nests that blend into nodules of spindled cells
- Presentation in an adolescent or young male
Board review style answer #2
D. Presence of ducts and squamous nests that blend into nodules of spindled cells
Salivary gland anlage tumor is a benign congenital tumor of infancy characterized histologically by the presence of both epithelial and mesenchymal components. Lined by nonkeratinizing squamous epithelium, in the submucosa are cellular stromal nodules composed of uniform, spindled to ovoid cells forming short fascicles, with duct-like epithelial structures and squamous nests in the periphery that blend into the cellular nodules. The spindle cells show variable to strong immunoreactivity with CK7, p63, SMMS and S100 (rare cells).
Comment Here
Reference: Salivary gland anlage tumor
Salivary gland anlage tumor is a benign congenital tumor of infancy characterized histologically by the presence of both epithelial and mesenchymal components. Lined by nonkeratinizing squamous epithelium, in the submucosa are cellular stromal nodules composed of uniform, spindled to ovoid cells forming short fascicles, with duct-like epithelial structures and squamous nests in the periphery that blend into the cellular nodules. The spindle cells show variable to strong immunoreactivity with CK7, p63, SMMS and S100 (rare cells).
Comment Here
Reference: Salivary gland anlage tumor
Seromucinous hamartoma
Table of Contents
Case reportsCase reports
- 54 year old man with large posterior nasal septal mass (Case of the Week #191)
Sinonasal carcinoma-general
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Rare and heterogenous group of malignancies that originate in the nasal cavity and paranasal sinuses and present with different histologic features and clinical behavior
Essential features
- Sinonasal carcinomas are a rare and heterogeneous group of malignancies that develop in the nasal cavity and the paranasal sinuses with diverse histology
- Most common are sinonasal squamous cell carcinoma and intestinal type adenocarcinomas
- Clinical presentation of sinonasal malignancies is usually nonspecific and diagnosed at advanced stages with poor prognosis
- Persistent exposure to wood and leather dust, textiles and organic solvents over a period of time is a strong risk factor for developing sinonasal carcinomas and is thought to be the result of chronic inflammation
ICD coding
- ICD-10: C30.0 - malignant neoplasm of the nasal cavity
Epidemiology
- Rare, < 1% of all human malignancies (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420, J Clin Med 2017;6:116)
- Sinonasal cancers comprise 3% of all head and neck cancers (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420, J Clin Med 2017;6:116)
- Affects 1 in 100,000 people per year worldwide (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Average age of presentation is between 50 - 70 years (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Epithelial tumors are the most common (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Originate from epithelial lining, accessory salivary glands, neuroendocrine tissue and olfactory epithelium
- Represent > 80% of all sinonasal tumors
- The 2022 5th edition of the WHO Classification of Tumours of the nasal cavity, paranasal sinuses and skull base include the following epithelial malignancies (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420, Cancers (Basel) 2021;13:2835, Head Neck Pathol 2022;16:1)
- Squamous cell carcinoma
- 50 - 80% of all sinonasal malignancies
- ~60% of these are keratinizing, ~40% are nonkeratinizing, smaller number of other variants
- NUT carcinoma
- Up to ~18% of upper aerodigestive tract poorly differentiated carcinomas
- SWI / SNF complex deficient sinonasal carcinoma
- Rare, with most tumors showing loss of SMARCB1 (INI1)
- Sinonasal lymphoepithelial carcinoma
- Strongly associated with Epstein-Barr virus infection, especially in endemic areas
- Sinonasal undifferentiated carcinoma
- ~3 - 5% of sinonasal carcinomas
- Teratocarcinosarcoma (Turk Neurosurg 2017;27:468, Brain Tumor Res Treat 2020;8:57)
- Extremely rare
- Mostly middle aged adults with a mean age of 54.5 years
- M:F = 7 - 8:1
- HPV related multiphenotypic sinonasal carcinoma (Ear Nose Throat J 2020;99:94)
- Strong association with HPV, particularly HPV 33
- Women affected more than men
- Mean age at presentation in sixth decade
- Adenocarcinomas
- Intestinal type adenocarcinoma represents ~20% of sinonasal malignancies
- Squamous cell carcinoma
- Sinonasal carcinomas occur more commonly in men (Nat Rev Clin Oncol 2014;11:460)
- M:F = 2:1 in squamous cell carcinoma
- M:F = 6:1 in intestinal type adenocarcinoma
- Male predominance is thought to be a result of occupational exposure (Nat Rev Clin Oncol 2014;11:460)
- Woodworkers have 500 - 900 times and 20 times the risk of developing intestinal type adenocarcinoma and squamous cell carcinoma, respectively (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Persistent exposure lasting longer than 20 years
- Association with woodworking not as strong outside of Europe, may reflect other susceptibility factors or exposure to different types of wood
- Leather dust, glues, formaldehyde, chrome, nickel, flour, textiles and organic solvents have also been associated with sinonasal carcinomas, mostly squamous cell carcinoma (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Smoking is also a risk factor, although not as strongly associated as other head and neck cancers (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Smoking can increase the risk of squamous cell carcinoma 2 to 3 fold
- HPV 16 and 18 are associated with squamous cell carcinoma (~30% of cases) (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Favorable prognosis
- Other lower risk factors include nasal polyposis, inverted sinonasal papilloma, chronic sinusitis and radiotherapy used in the treatment of retinoblastoma (Curr Oncol 2021;28:2420)
- Squamous cell carcinoma can arise from the 3 types of Schneiderian papillomas (exophytic, inverted and oncocytic) (Head Neck Pathol 2016;10:60)
- Very rare in exophytic type
- Most commonly arise in inverted papillomas (~10%, although reportedly as high as 27%)
- 4 - 17% in oncocytic type
- Squamous cell carcinoma can arise from the 3 types of Schneiderian papillomas (exophytic, inverted and oncocytic) (Head Neck Pathol 2016;10:60)
Sites
- Sinonasal tract comprises the nasal cavity, maxillary sinus, ethmoid sinus and sphenoid sinus (J Clin Med 2017;6:116)
- Includes the maxillary, ethmoid, nasal, frontal, palatine, sphenoid and lacrimal bones
- Lined with ciliated respiratory epithelium
- Sinonasal squamous cell carcinoma occurs predominantly in the nasal cavity and maxillary sinuses (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Virtually all intestinal type adenocarcinomas are located in the olfactory groove of the ethmoid sinuses (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Other histologic subtypes can occur anywhere within the sinonasal cavity (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
Pathophysiology
- Organic dust and occupational exposures associated with tumor development are not considered directly mutagenic (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- These substances may cause chronic inflammation due to continuous mucosal irritation
- Chronic inflammation is a recognized mechanism of malignancy in several cancer types
- Phagocytosis of inhaled organic dust, mineral fibers or fungal spores induce alveolar macrophages to secrete cytokines and chemokines involved in the inflammatory response, including (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Tumor necrosis factor (TNF) and IL1β, resulting in activation of transcription factor κβ (NFκβ)
- Expression of both NFκβ and cyclo-oxygenase 2 (COX2) are elevated in sinonasal carcinomas
- Stimulation by wood dust results in the release of reactive oxygen species and reactive nitrogen species by alveolar macrophages (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Peroxynitrites and nitrogen oxides can generate mutagenic DNA adducts
- Strong relationship between nitric oxide production through inducible nitric oxide synthase activity and G > A nucleotide transitions in TP53 gene
- TP53 G > T transitions have been observed virtually exclusively in nonsmoking woodworkers
- G > A transitions in KRAS was the most frequent mutation found in patients with intestinal type adenocarcinoma with a history of exposure to wood and leather dust
- Wnt / beta catenin pathway is also frequently affected (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Normally, beta catenin interacts with antigen presenting cell (APC) receptors, leading to ubiquitination of beta catenin
- When Wnt is overexpressed, beta catenin interacts with it and triggers translocation to the nucleus
- Acts as a transcription factor for genes encoding cyclin D1 and c-MYC, leading to cell cycle dysregulation
- Activating mutations of Wnt are detected in 30 - 50% of paranasal sinus tumors
- EGFR overexpression is found in 20 - 30% of sinonasal tumors (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Overexpression correlates with either amplification of EGFR gene or with hyperactivating point mutations
- EGFR overexpression is mutually exclusive with overexpression of p16, a surrogate marker of HPV infection
- Metaplasia of the normal respiratory or olfactory epithelium is thought to occur in early stages of development (Nat Rev Clin Oncol 2014;11:460)
- Histopathologic change that represents cellular and tissue response to chronic inflammation
- Squamous metaplasia followed by dysplasia are histologic changes that precede sinonasal squamous cell carcinoma
- Inverted papillomas are associated with a small proportion of sinonasal squamous cell carcinoma
- HPV 16 infection has been described in ~30% of malignant tumors, suggestive of inverted papilloma as a precursor lesion to this subset of squamous cell carcinoma
- Cuboidal and intestinal metaplasia are proposed to be a precursor to intestinal type adenocarcinoma
- These metaplastic tissues have been observed adjacent to these tumors and in people with exposure to environmental or occupational risk factors
- Metaplastic tissues show a switch from normal sinonasal epithelial immunophenotype (CK7+ / CK20- / CDX2- / villin-) to an abnormal intestinal epithelium immunophenotype (CK7- / CK20+ / CDX2+ / villin+)
Etiology
- Persistent environmental or occupational exposure to dust produced in the processing of wood, leather, flour, textiles, nickel and chrome (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Persistent exposure to glue, formaldehyde and organic solvents (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Smoking (Nat Rev Clin Oncol 2014;11:460)
- Human papillomavirus infection (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Radiotherapy for retinoblastoma (Curr Oncol 2021;28:2420)
Clinical features
- Tumors grow locally and may extend into adjacent structures (Curr Oncol 2021;28:2420)
- Clinical symptoms are often nonspecific (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Nasal obstruction, facial pain, persistent rhinorrhea or epistaxis
- Proptosis, diplopia or other neurologic symptoms may be seen in patients with advanced disease
- Due to nonspecific nature of symptoms in early stages of disease, sinonasal malignancies may have a prolonged period of time before diagnosis (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
- Lymph node metastasis at diagnosis in 10 - 20% of patients with sinonasal squamous cell carcinoma
- Lymphatic metastasis is rare in intestinal type adenocarcinoma, likely due to location and pattern of lymphatic drainage
- On follow up, 10% of patients develop distant metastasis; rarely occurs without local recurrence (Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420)
Diagnosis
- Rigid nasal endoscopy
- Noninvasive imaging is essential in establishing the extent of the tumor (Nat Rev Clin Oncol 2014;11:460)
- Both CT and MRI should be performed to determine anatomic details on tumor localization and extension
- Critical in determining operability or planning radiotherapy
- MRI is standard imaging modality for postoperative surveillance (Nat Rev Clin Oncol 2014;11:460)
- Role of PET CT is important in staging of squamous cell carcinoma, allowing the identification of regional and distant metastases (Cancers (Basel) 2021;13:2835, Nat Rev Clin Oncol 2014;11:460)
- Biopsy
Radiology description
- Radiologic differentiation of sinonasal carcinomas is challenging due to overlap in imaging findings
- Sinonasal squamous cell carcinomas show a soft tissue mass characterized by prominent bony destruction on CT (J Clin Med 2017;6:116, Head Neck Pathol 2016;10:1)
- MRI shows intermediate T1 signal intensity and hypointense T2 signal
- Variable enhancement on contrast enhanced T1 images that are less than half that of sinus mucosa
- Small lesions are homogenous in signal intensity
- Large tumors are more heterogenous with areas of necrosis and hemorrhage
- Many imaging manifestations of adenocarcinoma are often indistinguishable from squamous cell carcinoma (J Clin Med 2017;6:116, Head Neck Pathol 2016;10:1)
- May show areas of calcification corresponding with mucin content
- Mucin producing adenocarcinomas may show hyperintensity on T2, while those without mucin may show isointensity to hypointensity on T2
- Sinonasal undifferentiated carcinoma (J Clin Med 2017;6:116, Head Neck Pathol 2016;10:1)
- Noncalcified mass with variable contrast enhancement and areas of central necrosis on CT
- MRI shows isointensity on T1, isointensity to hyperintensity on T2 and heterogeneous enhancement on contrast enhanced T1
- Bony destruction and invasion into adjacent structures
- Difficult to distinguish from squamous cell carcinoma due to nonspecific findings
Radiology images
Prognostic factors
- Prognostic factors include age, performance status, tumor location and local extension, the histologic subtype and whether perineural invasion is present
- Carcinomas originating from the nasal cavity have a better prognosis than those from the paranasal sinuses
- This is attributed to nasal carcinomas producing symptoms that require earlier clinical attention
- Among carcinomas arising from the maxillary sinuses, those originating from the anterior inferior portion have a better prognosis than those from the superior posterior portion
- This is attributed to superior posterior tumors having accessible structures such as the orbit or skull base
- In T1 disease, 5 year survival is 80%; 30% in T4 disease
- Extensive local disease increases both surgical morbidity and local recurrence within 2 years; for example, maxillary sinus tumors have a 30 - 70% 5 year survival with resection but this drops to 10 - 20% in unresectable cases
- Intestinal type adenocarcinoma have a 50% 5 year survival after surgery and chemoradiation
- They usually grow locally, rarely involve regional lymph nodes and even more rarely give rise to distant metastases
- Papillary type and colonic type tumors have a better prognosis than solid or mucinous subtypes
- p53 and nuclear expression of beta catenin is associated with worse prognosis
- Histologic grade does not have prognostic value in sinonasal squamous cell carcinoma but molecular markers do
- EGFR overexpression and HPV negativity in squamous cell carcinoma is associated with worse prognosis
- p16 overexpression with EGFR negativity has a better prognosis
- References: Nat Rev Clin Oncol 2014;11:460, Curr Oncol 2021;28:2420
Case reports
- 36 year old man with a history of nasal blockage and left sided polypoid mass (J Clin Diagn Res 2016;10:ED12)
- 43 year old woman with disfiguring facial swelling with exophytic lesions protruding from nostrils (Cureus 2017;9:e1386)
- 61 year old man with squamous cell carcinoma arising from sinonasal inverted papilloma (AJNR Am J Neuroradiol 2020;41:1156)
- 68 year old man with rapidly progressive visual loss (Case Rep Oncol 2019;12:277)
- 69 year old woman with progressively worsening right eye visual acuity (Cureus 2019;11:e5281)
Treatment
- Surgical resection has been the mainstay of sinonasal cancer management (Curr Oncol 2021;28:2420, Cancers (Basel) 2021;13:2835)
- Resection results in excellent control rates for T1 and T2 tumors
- For small, localized tumors, complete removal is possible with less invasive techniques (such as endoscopically)
- Radiotherapy is given postoperatively in most cases, even if margins are negative (Curr Oncol 2021;28:2420)
- Adjuvant chemotherapy is considered in cases of inadequate resection margin (Curr Oncol 2021;28:2420)
- Radical neck dissection or elective radiation therapy of the whole neck for patients with positive neck lymph nodes (Curr Oncol 2021;28:2420)
- Cisplatin or carboplatin with external beam radiation can be used for locally advanced and unresectable squamous cell carcinomas (Curr Oncol 2021;28:2420)
- Concurrent chemoradiation can be used for those with comorbidities contraindicating surgery (Curr Oncol 2021;28:2420)
- Intestinal type adenocarcinoma is typically less responsive to chemotherapy; use of radiotherapy is preferred (Curr Oncol 2021;28:2420)
- Radiotherapy in conjunction with chemotherapy, when possible, is beneficial in locally advanced or unresectable disease (Curr Oncol 2021;28:2420)
- Significant improvement in survival in patients treated with a combination of 2 or more multidisciplinary approaches (e.g., surgery, chemotherapy, radiotherapy) (Curr Oncol 2021;28:2420)
Clinical images
Gross description
- Squamous cell carcinoma
- Soft, friable tan-white solid tumor
- Exophytic, papillary, polypoid, nodular or ulcerated mass
- May show hemorrhage and necrosis
- Adenocarcinoma
- Tan-white cut surface
- May be flat, polypoid, exophytic, papillary, ulcerated, mucinous
Gross images
Contributed by Kelly Magliocca, D.D.S., M.P.H.
Images hosted on other servers:
Microscopic (histologic) description
Squamous cell carcinoma (Head Neck Pathol 2016;10:60, Head Neck Pathol 2022;16:1)
NUT carcinoma (Head Neck Pathol 2022;16:1)
SWI / SNF complex deficient sinonasal carcinoma (Head Neck Pathol 2022;16:1, Am J Case Rep 2023;24:e939244)
Lymphoepithelial carcinoma (Curr Oncol 2021;28:2420, Ear Nose Throat J 2022;101:386)
Sinonasal undifferentiated carcinoma (Curr Oncol 2021;28:2420, Adv Anat Pathol 2020;27:51)
Teratocarcinosarcoma (J Oral Maxillofac Pathol 2016;20:147, Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1)
HPV associated multiphenotypic sinonasal carcinoma (Ear Nose Throat J 2020;99:94, Head Neck Pathol 2022;16:1)
Adenocarcinoma (Curr Oncol 2021;28:2420, Head Neck Pathol 2016;10:68)
- Keratinizing squamous cell carcinoma
- Most common type, ~60%
- Morphologically identical to squamous cell carcinomas arising elsewhere
- Abundant, eosinophilic cytoplasm filled with keratin filaments
- Intercellular bridges prominent
- Stellate, irregular nests, cords or single tumor cells in desmoplastic stroma
- Keratinization and keratin pearl formation readily identifiable
- Graded as well, moderately or poorly differentiated
- Well differentiated shows squamous epithelium with abundant keratinization, prominent intercellular bridges, with minimal pleomorphism and low mitotic activity
- Moderately differentiated shows features between well and poorly differentiated, with focal keratinization
- Poorly differentiated carcinomas have more nuclear atypia, pleomorphism, increased mitotic activity and minimal keratinization
- Nonkeratinizing squamous cell carcinoma
- Squamous differentiation with minimal keratinization
- ~40% of all sinonasal squamous cell carcinomas
- Similar to the same tumor type arising in the oropharynx
- Blue cell tumor appearance with high N:C ratio, arranged in large, rounded nests or ribbons with smooth, usually well demarcated borders with little stromal desmoplasia
- Exophytic or inverted papillary architecture is common
- Minimal maturing squamous differentiation
- Prominent mitotic activity and apoptosis
- Central necrosis is common in nests
- Tumors tend to coat the mucosal surface with undulating, irregular contour
- In areas where tumor invades downward, has inverted appearance with rounded nests, mimicking Schneiderian papilloma with carcinoma in situ
- Combined with lack of stromal desmoplasia, gives a noninvasive appearance
- DEK::AFF2 subtype shows complex exophytic and endophytic growth
- Broad papillary fronds, anastomosing lobules, ribbons and nests
- Nuclear monotony and inflammatory infiltrate, especially neutrophils, typically present (Head Neck Pathol 2022;16:1)
- Requires confirmation with DEK::AFF2 FISH break apart probe
NUT carcinoma (Head Neck Pathol 2022;16:1)
- Composed of monotonous evenly spaced sheets of evenly sized nuclei that have vesicular chromatin and prominent nucleoli
- Inflammatory infiltrate commonly seen
- Abrupt keratinization may be seen in up to a third of cases
SWI / SNF complex deficient sinonasal carcinoma (Head Neck Pathol 2022;16:1, Am J Case Rep 2023;24:e939244)
- Most common subtype is SMARCB1 deficient
- Small to medium sized monomorphic basaloid cells, undifferentiated appearance
- Indistinct cytoplasmic borders and round nuclei with variably prominent nucleoli
- Up to a third of cases are more eosinophilic with plasmacytoid morphology, cytoplasmic vacuoles commonly seen
- SMARCB1 deficient sinonasal carcinoma may occasionally be gland forming or yolk sac-like
Lymphoepithelial carcinoma (Curr Oncol 2021;28:2420, Ear Nose Throat J 2022;101:386)
- Undifferentiated carcinoma with epithelial neoplastic cells accompanied by strong lymphocytic infiltrate
- Characterized by medium to large sized polygonal epithelioid cells with vesicular nuclei and prominent nucleoli
- Epithelioid cells form well circumscribed lobules or nests with dense mixed lymphoplasmacytic infiltrate
- Increased mitoses with atypical forms
- Dense lymphoid infiltrate may obscure epithelioid cells and mimic a lymphoproliferative disorder
- Strong association with EBV, positive by in situ hybridization
Sinonasal undifferentiated carcinoma (Curr Oncol 2021;28:2420, Adv Anat Pathol 2020;27:51)
- Undifferentiated polygonal medium to large cells arranged in nests, sheets, trabecular or solid patterns
- Round blue cell tumor
- Cells have round to oval hyperchromatic nuclei with variably prominent eosinophilic nucleoli and indistinct cell membranes
- High N:C ratio
- Strong mitotic activity with atypical forms
- Frequently with necrosis, lymphovascular invasion and perineural invasion
- Squamous, glandular or neuroectodermal differentiation should not be present
Teratocarcinosarcoma (J Oral Maxillofac Pathol 2016;20:147, Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1)
- Has features of carcinosarcoma and teratoma with varying degrees of maturity
- Epithelial component typically consists of squamous epithelium and glandular structures
- May be benign or malignant
- Fetal appearing clear cell squamous epithelium is a characteristic finding
- Mesenchymal component may show myofibroblastic cell proliferation with a loose myxoid stroma
- Mature or immature mesenchymal elements seen (e.g., osteoid, cartilage, smooth or striated muscle)
- Primitive neuroepithelial cells in sheets and nests
- Cytologic atypia ranges from minimal to sarcomatous differentiation
HPV associated multiphenotypic sinonasal carcinoma (Ear Nose Throat J 2020;99:94, Head Neck Pathol 2022;16:1)
- Histologic and immunophenotypic features of both salivary gland type carcinoma and squamous cell carcinoma
- Biphasic ductal and myoepithelial differentiation, frequently in a cribriform arrangement reminiscent of adenoid cystic
- Frequent squamous cell carcinoma in situ, occasional squamous differentiation within invasive tumor
- Myoepithelial features show cytoplasmic clearing, plasmacytoid appearance and spindling
- May show sarcomatous transformation and cartilaginous differentiation
- May see surface squamous dysplasia
- High mitotic rate and areas of necrosis are frequently seen
Adenocarcinoma (Curr Oncol 2021;28:2420, Head Neck Pathol 2016;10:68)
- 20% of malignant neoplasms in the sinonasal tract
- Arises from either lining surface epithelium or seromucinous glands
- 3 main types: intestinal type, nonintestinal type and salivary type
- Intestinal type adenocarcinoma
- Originates from Schneiderian mucosa and is the most frequent type of adenocarcinoma (~6 - 13%)
- May recapitulate normal intestinal mucosa with villi and specialized cell types (e.g., goblet, Paneth)
- Similar features to adenomas and colorectal adenocarcinoma
- Cribriform glands with necrotic debris in lumen (dirty necrosis)
- Histologic types (modified Barnes classification): colonic, papillary, solid, mucinous, mixed
- Colonic type is most common (~40 - 50%)
- Glandular, tubular and trabecular architecture
- Rare papillae
- Glands lined by crowded columnar cells with anisonucleosis
- Intracellular and extracellular mucin and goblet cells may be present
- Papillary type
- Papillary architecture predominant with occasional tubular elements with minimal cytologic atypia
- May have columnar goblet cells mimicking intestinal adenomas
- Solid type
- Less differentiated with solid and trabecular architecture
- Minimal cytologic atypia with rare mitosis
- Mucinous
- Mimics mucinous variants of colorectal carcinoma
- Mucin filled glands or cell clusters that float in pools of extracellular mucin
- Colonic type is most common (~40 - 50%)
- Nonintestinal type adenocarcinoma
- Does not display features of either intestinal type or salivary type
- Morphologically heterogeneous
- Back to back small glands or acini
- Divided into high grade and low grade
- High grade
- More poorly differentiated with marked nuclear pleomorphism
- Glandular and papillary growth patterns
- Mitoses readily identifiable
- Tumor necrosis and apoptosis are frequently seen
- Low grade
- Varied with exophytic papillae and tubular or glandular patterns
- Papillae and glands lined by single layer of uniform columnar or cuboidal cells with minimal cytologic atypia and inconspicuous nucleoli
- Mild to moderate cellular pleomorphism and rare mitoses
- Tumor necrosis is not typically seen
- Sinonasal renal cell-like adenocarcinoma (Cureus 2021;13:e14285, Head Neck Pathol 2008;2:75, Head Neck Pathol 2022;16:1)
- Rare
- Morphologically similar to clear cell renal cell carcinoma with monotonous cuboidal to polyhedral cells with abundant clear cytoplasm
- Intranuclear holes may be seen
- Predominantly solid to nested, glandular growth pattern with fibrous septa and prominent vascularity
- Immunohistochemistry can distinguish from clear cell renal cell carcinoma
- Positive: CK7, CAIX, CD10; negative: RCC, PAX8 (Medicine (Baltimore) 2017;96:e7711)
Microscopic (histologic) images
Contributed by Wai Szeto, M.D., M.S.
Positive stains
- Squamous cell carcinoma (Head Neck Pathol 2014;8:269, Am J Surg Pathol 2017;41:458)
- AE1 / AE3, CK5/6, CK7, CK903
- p40, p63
- EMA
- Nonkeratinizing squamous cell carcinoma
- p16 should be diffuse, block-like, with nuclear and cytoplasmic staining in > 70% of neoplastic cells
- SMARCB1 (INI1) and SMARCA4 (BRG1) retained
- NUT carcinoma (Am J Surg Pathol 2012;36:1216, Head Neck Pathol 2022;16:1)
- NUT1 in diffuse speckled pattern
- Pancytokeratin, p40 and p63 in majority of cases
- INI1 retained
- SWI / SNF complex deficient sinonasal carcinoma (Am J Surg Pathol 2017;41:458, Am J Case Rep 2023;24:e939244)
- Pancytokeratin
- p63, CK5 and CK7 in ~50% of cases
- Synaptophysin, chromogranin, CD56 and CD117 may occasionally show positivity
- p16 may show diffuse positivity but negativity for HPV by in situ hybridization
- Lymphoepithelial carcinoma (Semin Diagn Pathol 2015;32:74)
- Pancytokeratin
- EBER by in situ hybridization
- Sinonasal undifferentiated carcinoma (Mod Pathol 2017;30:S1, Mod Pathol 2019;32:205, Arch Pathol Lab Med 2015;139:55, Curr Oncol 2021;28:2420)
- Teratocarcinosarcoma (J Oral Maxillofac Pathol 2016;20:147, Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1, Head Neck Pathol 2022;16:229)
- Complex immunoprofile due to many immunohistochemical stains highlighting different components
- Cytokeratins, AE1 / AE3, CAM5.2, CK5/6, EMA in epithelial components
- Desmin, vimentin, SMA in mesenchymal components
- S100, synaptophysin, chromogranin, CD56, CD99, INSM1, GFAP, NSE in immature neuroectodermal components
- HPV associated multiphenotypic sinonasal carcinoma (Head Neck Pathol 2019;13:331, Am J Surg Pathol 2017;41:1690, Head Neck Pathol 2019;13:220, Ear Nose Throat J 2020;99:94)
- Intestinal type adenocarcinoma (Head Neck Pathol 2017;11:295, Head Neck Pathol 2016;10:68)
- Pancytokeratin
- CK7, CEA and EMA are variably positive
- Intestinal markers: CK20, CDX2, villin, SATB2, MUC2
- Neuroendocrine markers may be focally positive (chromogranin, synaptophysin)
- Aberrant p53 expression
- Nonintestinal type adenocarcinoma (Head Neck Pathol 2016;10:68)
Negative stains
- Squamous cell carcinoma (Head Neck Pathol 2014;8:269, Am J Surg Pathol 2017;41:458)
- NUT carcinoma (Am J Surg Pathol 2012;36:1216, Head Neck Pathol 2022;16:1)
- Synaptophysin, chromogranin, S100 (may be focal)
- SWI / SNF complex deficient sinonasal carcinoma (Am J Surg Pathol 2017;41:458, Am J Case Rep 2023;24:e939244)
- Lymphoepithelial carcinoma (Semin Diagn Pathol 2015;32:74)
- p16 typically negative
- Sinonasal undifferentiated carcinoma (Mod Pathol 2017;30:S1, Mod Pathol 2019;32:205, Arch Pathol Lab Med 2015;139:55, Curr Oncol 2021;28:2420)
- Absent to minimal expression of lineage specific markers
- p40, p63, CK5/6, CEA, CD34, desmin, SMA, SMMHC, S100, calretinin, CD45
- May show weak or patchy expression of neuroendocrine markers (synaptophysin, chromogranin, CD56)
- Absent to minimal expression of lineage specific markers
- Teratocarcinosarcoma (J Oral Maxillofac Pathol 2016;20:147, Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1, Head Neck Pathol 2022;16:229)
- SMARCA4 (BRG1) loss
- Β hCG, CD45
- HPV associated multiphenotypic sinonasal carcinoma (Head Neck Pathol 2019;13:331, Am J Surg Pathol 2017;41:1690, Head Neck Pathol 2019;13:220, Ear Nose Throat J 2020;99:94)
- Intestinal type adenocarcinoma (Head Neck Pathol 2017;11:295, Head Neck Pathol 2016;10:68)
- Annexin A1 loss
- Annexin A2 decreased
- Nonintestinal type adenocarcinoma (Head Neck Pathol 2016;10:68)
Molecular / cytogenetics description
- Nonkeratinizing squamous cell carcinoma
- p16 positive cases should be followed by high risk HPV ISH or PCR for confirmation (Cancers (Basel) 2022;14:1874)
- DEK::AFF2 fusion with RNA sequencing or DEK break apart FISH in DEK::AFF2 carcinoma (Am J Surg Pathol 2021;45:1682)
- Lymphoepithelial carcinoma (Head Neck Pathol 2022;16:1)
- EBER ISH positive
- Sinonasal undifferentiated carcinoma
- IDH2 R172 mutation is common (> 80%) (Mod Pathol 2019;32:205)
- NUT carcinoma (Head Neck Pathol 2013;7:11, Head Neck Pathol 2022;16:1)
- Rearrangement of NUT gene, most commonly BRD4::NUT [t(15;19)(q14, p13.1)]
- Remaining cases are BRD3::NUT [t(9;15)(q34.2;q14)] or other NUT variant fusions
- HPV associated multiphenotypic sinonasal carcinoma (Head Neck Pathol 2019;13:331, Am J Surg Pathol 2017;41:1690, Head Neck Pathol 2019;13:220, Ear Nose Throat J 2020;99:94)
- High risk HPV positive by DNA or RNA ISH and PCR
- HPV 33 is most commonly isolated
- Negative for MYB, MYBL1 or NFIB gene fusions that are seen in adenoid cystic carcinoma
Sample pathology report
- Nasal contents, biopsy:
- Undifferentiated carcinoma, most consistent with sinonasal undifferentiated carcinoma (see comment)
- Comment: Sections show a poorly differentiated epithelioid neoplasm growing in nests, sheets and trabeculae. Numerous mitotic figures are identified. Focal necrosis is present. The tumor cells have mild nuclear pleomorphism and prominent nucleoli. No overt squamous or glandular differentiation is identified. By immunohistochemical staining, the tumor is positive for pankeratin and CK7. The tumor is negative for synaptophysin, chromogranin, CK5/6, p40, p63, SMMS, S100, SOX10, myogenin and desmin. INI1 immunostain is retained. EBV EBER in situ hybridization is negative. Overall morphology and immunophenotypic profile are most consistent with sinonasal undifferentiated carcinoma.
Differential diagnosis
- Squamous cell carcinoma (Head Neck Pathol 2016;10:68):
- Schneiderian papillomas (inverted, exophytic, oncocytic):
- Lacks invasive growth
- Mild atypia
- Basaloid squamous cell carcinoma:
- Basaloid variant that grows in nests / nodules with peripheral palisading
- Schneiderian papillomas (inverted, exophytic, oncocytic):
- NUT carcinoma (Mod Pathol 2019;32:205, Head Neck Pathol 2022;16:1, Int J Surg Pathol 2022;30:273, Head Neck Pathol 2016;10:85, Curr Oncol 2021;28:2420):
- Basaloid squamous cell carcinoma:
- Sinonasal undifferentiated carcinoma:
- NUT1 negative
- IDH2 mutations frequently present
- Neuroendocrine carcinoma:
- Lacks keratinization
- Positivity for synaptophysin, chromogranin, CD56, NSE
- SWI / SNF complex deficient sinonasal carcinoma:
- SWI / SNF complex deficient sinonasal carcinoma (Am J Surg Pathol 2017;41:458, Am J Case Rep 2023;24:e939244, Head Neck Pathol 2022;16:1, Head Neck Pathol 2016;10:85, Curr Oncol 2021;28:2420):
- Sinonasal undifferentiated carcinoma:
- INI1 retained
- IDH2 mutations frequently present
- NUT carcinoma:
- NUT1 positive
- Neuroendocrine carcinoma:
- Positivity for synaptophysin, chromogranin, CD56, NSE
- HPV associated multiphenotypic sinonasal carcinoma:
- Sinonasal undifferentiated carcinoma:
- Lymphoepithelial carcinoma (Cureus 2022;14:e31103, Mod Pathol 2017;30:S1):
- Sinonasal undifferentiated carcinoma:
- Typically, does not have a prominent lymphoid infiltrate
- EBV negative
- Nonkeratinizing squamous cell carcinoma:
- Difficult to distinguish as the immunohistochemical profile will be virtually identical
- Melanoma:
- Lymphoma:
- Hematolymphoid markers showing B or T cell lineage
- Sinonasal undifferentiated carcinoma:
- Sinonasal undifferentiated carcinoma (CA Cancer J Clin 2023;73:72, Head Neck Pathol 2016;10:68):
- Neuroendocrine carcinoma:
- Shows nuclear molding, salt and pepper chromatin
- Strong positivity with neuroendocrine marker (synaptophysin, chromogranin, NSE)
- Nonkeratinizing squamous cell carcinoma:
- Keratinizing squamous cell carcinoma:
- p40, p63 and CK5/6 positive in keratinizing squamous cell carcinoma; negative in sinonasal undifferentiated carcinoma
- NUT carcinoma:
- Diffuse nuclear staining with NUT
- Lymphoma:
- Hematolymphoid markers showing B or T cell lineage
- Neuroendocrine carcinoma:
- Teratocarcinosarcoma (Brain Tumor Res Treat 2020;8:57, Head Neck Pathol 2022;16:1):
- Olfactory neuroblastoma:
- Mesenchymal differentiation not present
- Carcinosarcoma:
- Neuroectodermal differentiation not present
- Olfactory neuroblastoma:
- HPV associated multiphenotypic sinonasal carcinoma (Ear Nose Throat J 2020;99:94, Head Neck Pathol 2022;16:1)
- Adenoid cystic carcinoma:
- MYB, MYBL1 or NFIB translocations present
- Squamous cell carcinoma:
- Lacks biphasic ductal and myoepithelial cell differentiation
- SWI / SNF complex deficient sinonasal carcinoma:
- Sinonasal undifferentiated carcinoma:
- IDH2 mutations frequently present
- HPV negative
- Diagnosis of exclusion
- Adenoid cystic carcinoma:
- Intestinal type adenocarcinoma (Head Neck Pathol 2016;10:68):
- Metastatic adenocarcinoma of colonic origin:
- Rare but must be considered
- Morphology and immunohistochemistry are virtually indistinguishable from a GI primary
- Exclusion primarily based on clinical and radiographic correlation
- Sinonasal nonintestinal type adenocarcinoma:
- Salivary gland type adenocarcinomas:
- Metastatic adenocarcinoma of colonic origin:
- Nonintestinal type adenocarcinoma (Head Neck Pathol 2016;10:68):
- Sinonasal intestinal type adenocarcinoma:
- Salivary gland type adenocarcinomas:
- Morphologically diverse
- Most common is adenoid cystic
- Follows immunophenotype of respective salivary gland type adenocarcinoma
Board review style question #1
Board review style answer #1
D. Sinonasal undifferentiated carcinoma. IDH1 or IDH2 mutations are common in sinonasal undifferentiated carcinoma and can be seen in more than 80% of cases. The most common is the IDH2 R172 mutation, which can be detected with immunohistochemistry. Answers A - C are incorrect because these mutations are not typically associated with those entities.
Comment Here
Reference: Sinonasal carcinoma-general
Comment Here
Reference: Sinonasal carcinoma-general
Board review style question #2
A biopsy from a mass in the maxillary sinus showed histologic features of squamous cell carcinoma with a complex exophytic and endophytic growth pattern with broad papillary fronds. There is minimal keratinization. Which of the following gene rearrangements is frequently observed in this type of tumor?
- CASG::PRKD1 fusion
- CRTC1::MAML2 fusion
- DEK::AFF2 fusion
- MYB::NFIB fusion
Board review style answer #2
C. DEK::AFF2 fusion. DEK::AFF2 is a novel fusion identified in a type of nonkeratinizing squamous cell carcinoma of the sinonasal tract called DEK::AFF2 fusion associated papillary squamous cell carcinoma that exhibits complex exophytic and endophytic growth with broad papillary fronds, anastomosing lobules, ribbons and nests. Detectable with DEK::AFF2 FISH break apart probe or RNA sequencing. Answer A is incorrect because CASG::PRKD1 is associated with polymorphous adenocarcinoma. Answer B is incorrect because CRTC::MAML2 is associated with mucoepidermoid carcinoma. Answer D is incorrect because MYB::NFIB is associated with adenocystic carcinoma.
Comment Here
Reference: Sinonasal carcinoma-general
Comment Here
Reference: Sinonasal carcinoma-general
Sinonasal glomangiopericytoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Uncommon soft tissue tumor of perivascular myoid differentiation with hemangiopericytoma-like vasculature
Essential features
- Also called glomangiopericytoma, sinonasal type
- Nasal cavity is usually affected, present with nasal obstruction, mass, epistaxis and sinusitis
- CTNNB1 mutation with beta catenin oncogenic activation (beta catenin nuclear staining on IHC) (Head Neck Pathol 2019;13:298, Yale J Biol Med 2021;94:593)
- Excellent long term outcome with surgery alone, recurrence rate ~27%
Terminology
- Previous name: sinonasal type hemangiopericytoma
- Glomus tumor
- Intranasal myopericytoma
ICD coding
- ICD-10: D38.5 - neoplasm of uncertain behavior of other respiratory organs
Epidemiology
- Rare, comprising < 0.5% of sinonasal primary neoplasms (Thompson: Diagnostic Pathology - Head and Neck, 2nd Edition, 2016)
- Broad age range (5 - 90 years); mean in seventh decade
- F > M (1.2:1)
- No difference in outcome based on gender after surgery
Sites
- Nasal cavity is usually affected, turbinate and septum occasionally affected in isolation (Thompson: Diagnostic Pathology - Head and Neck, 2nd Edition, 2016)
- Maxillary and ethmoid sinuses may also be affected in conjunction with nasal cavity
- Bilateral tumor uncommon (~5%)
Pathophysiology
- May arise from pericytes associated with vessels of nasal cavity
- Mutational activation of beta catenin (Head Neck Pathol 2019;13:298, Yale J Biol Med 2021;94:593)
- Associated with cyclin D1 overexpression (Pathol Res Pract 2019;215:983)
Etiology
- CTNNB1 mutations
Clinical features
- Nasal obstruction, mass, polyps, difficulty breathing
- Sinusitis, discharge, change in smell
- Nasal bleeding (J Pak Med Assoc 2020;70:2469)
- Headache
- Rare association with oncogenic osteomalacia (BMC Endocr Disord 2022;22:31)
Diagnosis
- Clinical symptoms and polypoid mass identified in the nasal cavity
- CT and MRI showing a polypoid mass of nasal cavity (paranasal sinus) accompanied with bone erosion or sclerosis
- Biopsy showing soft tissue with perivascular myoid differentiation, positive for beta catenin nuclear staining, cyclin D1 and SMA, negative for vascular markers in the tumor cells (Pathol Res Pract 2019;215:983)
Laboratory
- In the majority of cases, lab tests do not help
- In rare cases, associated with osteomalacia: serum hypophosphatemia and elevated alkaline phosphatase (BMC Endocr Disord 2022;22:31)
Radiology description
- CT and MRI showing a mass of nasal cavity (paranasal sinus), could be lobulated
Radiology images
Prognostic factors
- Excellent prognosis after surgery (5 year survival ~90%)
- Recurrence rate ~27%, long term clinical follow up advocated as recurrences may develop late (Thompson: Diagnostic Pathology - Head and Neck, 2nd Edition, 2016)
- Recurrences are associated with long duration of the symptoms, bone invasion and profound nuclear pleomorphism
Case reports
- 42 year old woman with osteomalacia caused by sinonasal glomangiopericytoma and coexisting parathyroid adenoma (BMC Endocr Disord 2022;22:31)
- 57 year old man with sinonasal FUS::ERG rearranged Ewing sarcoma mimicking glomangiopericytoma (Case Rep Oncol 2020;13:1393)
- 60 year old man with new onset, right arm and leg weakness / numbness, was found to have a left ethmoid sinus glomangiopericytoma with extension in the olfactory fossa (Yale J Biol Med 2021;94:593)
- 70 year old man with nasal blockage and epistaxis underwent endoscopic sinus surgery (J Pak Med Assoc 2020;70:2469)
- 74 year old man with a reddish nasal mass with CTNNB1 p.S37C mutation (Head Neck Pathol 2019;13:298)
Treatment
- Surgery (polypectomy or wide surgical resection) is the treatment of choice (J Pak Med Assoc 2020;70:2469)
- Radiation could be used for nonsurgical candidates
Gross description
- Polypoid mass, mean 3 cm, beefy red to grayish pink with hemorrhage
- Soft, edematous and fleshy cut surface
Frozen section description
- Frozen sections of the tumor show subepithelial cytologically bland spindle and round cell proliferation separated from the surface respiratory epithelium, hemorrhagic stromal, perivascular hyalinization and staghorn vessels
Frozen section images
Microscopic (histologic) description
- Surface respiratory epithelium or metaplastic squamous epithelium is intact
- Subepithelial proliferation separated from surface (grenz zone)
- Diffuse growth with fascicular, solid or focally whorled pattern of spindled or round / oval tumor cells that arrange themselves around prominent, small, thin walled submucosal blood vessels
- Cells have variable cytoplasm and nuclei, indistinct cell borders and occasionally are multinucleated
- Minimal atypia, no necrosis, no / rare mitotic activity
- Vessels are prominent with staghorn appearance and perivascular hyalinization
- Inflammatory cells including mast cells and eosinophils are often present
Microscopic (histologic) images
Cytology description
- Smears show spindled, epithelioid or round cells with indistinct cell borders
Positive stains
- Beta catenin: nearly all tumor cells with nuclear and cytoplasmic staining (Pathol Res Pract 2019;215:983, Yale J Biol Med 2021;94:593)
- Cyclin D1, CD99 (Pathol Res Pract 2019;215:983)
- Vimentin (98%), smooth muscle actin (92%), muscle specific actin (77%), factor XIIIa (78%), laminin (52%)
- D2-40 (Virchows Arch 2006;448:459)
Negative stains
- ERG, CD31, CD34 (usually), factor VIII, keratin
Electron microscopy description
- Basal lamina surrounds individual cells, tapered cytoplasmic extensions, orderly bundles of filaments (Thompson: Diagnostic Pathology - Head and Neck, 2nd Edition, 2016)
Molecular / cytogenetics description
- CTNNB1 mutation: PCR and gene sequencing show single nucleotide substitutions with exon 3 codons of the CTNNB1 glycogen serine kinase 3 beta phosphorylation region
Sample pathology report
- Right nasal cavity mass, biopsy:
- Nasal glomangiopericytoma (see comment)
- Comment: Sections of the nasal mass biopsy show cytologically bland spindle cell proliferation with scattered staghorn vessels and perivascular hyalinization. The tumor cell proliferation is separated from the overlying respiratory epithelium. No mitotic figures or tumor necrosis is identified. The tumor cells are positive for beta catenin (nuclear staining), cyclin D1, CD99 and SMA, negative for desmin, CD31, ERG, CD34 and AE1 / AE3. The histologic features and immunoprofile support the diagnosis.
Differential diagnosis
- Glomus tumor:
- Also a pericytic tumor with similar staining but extremely rare in the sinonasal region
- Composed of compact epithelioid cells
- Positive for SMA but negative for beta catenin nuclear staining
- Lobular capillary hemangioma:
- Lobular pattern at low power
- Has spindled fibroblasts and prominent vessels but overall granulation tissue-like appearance with only small capillaries
- Positive for CD34 and CD31, negative for beta catenin nuclear staining
- Solitary fibrous tumor:
- Similar vascular pattern but tumor has patternless pattern, with ropy keloidal collagen and thin walled vascular spaces
- Positive for CD34 and STAT6, negative for beta catenin nuclear staining
- Ewing or Ewing-like sarcoma:
- Tumor cells have clear cytoplasm and similar histology with positive CD99 immunoreactivity
- Negative beta catenin nuclear staining and positive for EWSR1 rearrangement (Case Rep Oncol 2020;13:1393)
Additional references
Board review style question #1
Regarding sinonasal glomangiopericytoma, which of the following statements is true?
- Bilateral tumor is seen in 10% of patients
- It is much more common in male patients
- Majority of the tumors have a poor prognosis
- Maxillary sinus is the most affected
- Nasal cavity is usually affected
Board review style answer #1
E. Nasal cavity is usually affected; maxillary and ethmoid sinuses may also be affected in conjunction with nasal cavity. The female to male ratio is 1.2 to 1. Patients with bilateral tumor are uncommon (~5%). The majority of the tumors have a good prognosis.
Comment Here
Reference: Sinonasal glomangiopericytoma
Comment Here
Reference: Sinonasal glomangiopericytoma
Board review style question #2
A 50 year old woman presented with right nasal obstruction and occasional nose bleeding. Physical examination showed a red to grayish polypoid soft mass in her right nasal cavity and a biopsy was performed. A histology photo is shown above. Which of the following immunostains should be positive for the diagnosis of nasal glomangiopericytoma?
- Beta catenin nuclear staining
- CD31
- CD34
- S100 nuclear staining
- STAT6 nuclear staining
Board review style answer #2
A. Beta catenin nuclear staining should be positive in the tumor cells, as sinonasal glomangiopericytoma is related to mutational beta catenin activation. Positive CD34 and STAT6 are helpful for diagnosis of solid fibrous tumor. Positive S100 and muscular markers are helpful for diagnosis of biphenotypic sinonasal sarcoma.
Comment Here
Reference: Sinonasal glomangiopericytoma
Comment Here
Reference: Sinonasal glomangiopericytoma
Sinonasal lymphoepithelial carcinoma (pending)
[Pending]
Sinonasal papilloma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Sinonasal papilloma is a benign epithelial neoplasm of sinonasal tract
- WHO has divided sinonasal papilloma into 3 distinct types (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017):
- Inverted papilloma
- Exophytic papilloma
- Oncocytic papilloma
Essential features
| Comparison of essential features of the 3 types of sinonasal papilloma | |||
| Inverted papilloma | Exophytic papilloma | Oncocytic papilloma | |
| Frequency | Most common | Second most common | Least common |
| Location | Lateral nasal wall / paranasal sinus | Nasal septum | Lateral nasal wall / paranasal sinus |
| Male to female ratio | 2 - 3:1 | 10:1 | 1:1 |
| Most common age of presentation | 5th to 6th decades | 3rd to 5th decades | 5th to 6th decades |
| Association with human papillomavirus (HPV) | High risk HPV Low risk HPV | Low risk HPV | No association |
| Architectural pattern | Endophytic (inverted) | Exophytic (filiform) | Exophytic or endophytic |
| Epithelial lining | Squamous, transitional or respiratory | Squamous, transitional or respiratory | Oncocytic |
| Molecular alterations | EGFR activating mutation | None reported | KRAS mutation |
| Risk of malignant transformation | 5 - 15% | ~0% | 4 - 17% |
Terminology
- Other terminologies that have been used in the literature and previous versions of WHO classification include:
- All types: Schneiderian papilloma and epithelial papilloma
- Exophytic type: transitional cell papilloma, fungiform papilloma, Ringertz papilloma and septal papilloma
- Oncocytic type: cylindrical cell papilloma and columnar cell papilloma
Epidemiology
- Annual incidence is 0.74 - 2.3 per 100,000 population (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Inverted papilloma is the most common subtype, followed by exophytic papilloma; oncocytic papilloma is the least common
- Inverted and oncocytic papilloma most commonly affect patients in their 5th to 6th decades, while exophytic papilloma occurs in 3rd to 5th decades
- Inverted and exophytic papilloma occur more frequently in males, with a male to female ratio of 2 - 3:1 and 10:1, respectively; oncocytic papilloma affects both genders equally (Head Neck Pathol 2017;11:269)
Sites
- Sinonasal papilloma commonly affects nasal cavity or paranasal sinuses
- Exophytic papilloma is typically located in the nasal septum, while the inverted and oncocytic types predominantly affect lateral nasal wall or paranasal sinuses
- Inverted papilloma may secondarily extend to nonsinonasal sites, e.g. pharynx, ear, cranial cavity
- Sinonasal papilloma is usually unilateral; bilateral involvement is rare
Etiology
- Role of high risk human papillomavirus (HPV) in inverted papilloma remains controversial
- Reported rate of high risk HPV in inverted papilloma and carcinoma ex inverted papilloma ranges from 0 - 100% (World J Otorhinolaryngol Head Neck Surg 2016;3:54, Head Neck Pathol 2017;11:269)
- WHO gives a frequency of 38.5% in inverted papilloma (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Low risk HPV, especially types 6 and 11, has also been detected in inverted papilloma and is more common than high risk HPV
- Other etiologic factors implicated in inverted papilloma include exposure to welding, organic solvents and smoking (World J Otorhinolaryngol Head Neck Surg 2016;3:54)
- Exophytic papilloma may be related to low risk HPV, especially types 6 and 11
- No significant association between oncocytic papilloma and HPV (Head Neck Pathol 2017;11:269)
Clinical features
- Symptoms are usually nonspecific, including nasal congestion, nasal obstruction, nasal discharge or epistaxis
Diagnosis
- Relies on histology in biopsy or surgical resection
Radiology images
Prognostic factors
- Increased risk of recurrence if involvement of sphenoid sinus, frontal sinus or maxillary sinus walls other than medial or extrasinus extension (Biomed Res Int 2017;2017:9195163)
- Major cause of recurrence is incomplete resection
- Risk of malignant transformation is ~9% in inverted papilloma (range: 5 - 15%) (Eur Arch Otorhinolaryngol 2017;274:2991)
- 4 - 17% of oncocytic papilloma undergo malignant transformation
- Exophytic papilloma is not associated with an increased incidence of carcinoma
Case reports
- 46 year old man with inverted papilloma of the middle ear (Braz J Otorhinolaryngol 2012;78:122)
- 48 year old man and 56 year old man with inverted papilloma of the maxillary sinus (Rom J Morphol Embryol 2016;57:289)
- 60 year old man with inverted papilloma of the lacrimal sac (J Cancer Res Ther 2015;11:238)
- 74 year old woman with inverted papilloma of the temporal bone (BMJ Case Rep 2013;2013:bcr2013201219)
- 82 year old man with oncocytic papilloma of the maxillary sinus mimicking lymphoma (Medicine (Baltimore) 2016;95:e4646)
Treatment
- Complete surgical excision through endoscopic surgery or open radical procedure is the treatment of choice (Clin Otolaryngol 2006;31:499)
- Aim is to completely remove all diseased mucosa; lateral rhinotomy and medial maxillectomy may be required for inverted or oncocytic papilloma
- If treated with local excision only, 50 - 70% may recur, particularly for inverted subtype
Clinical images
Gross description
- Inverted papilloma: pink-tan-gray, soft to moderately firm polypoid growth with convoluted or wrinkled surface
- Exophytic papilloma: usually mushroom shaped and exophytic with papillary appearance
- Oncocytic papilloma: fleshy polypoid growth of variable color
- Generous or complete sampling is advised to search for focus of malignant transformation
Microscopic (histologic) description
Inverted papilloma:
Exophytic papilloma:
Oncocytic papilloma:
Malignant transformation (carcinoma ex sinonasal papilloma):
- Architecture: prominent downward endophytic growth of round to elongated interconnected epithelial nests with smooth outer contour
- Epithelium is hyperplastic (5 - 30 cell layers in thickness) and may be of squamous, transitional or respiratory type
- Transmigrating neutrophils and neutrophilic microabscesses may be seen
- Stroma may have edema or chronic inflammation
- Seromucinous gland in the lamina propria is commonly decreased or absent
Exophytic papilloma:
- Architecture: filiform or papillary arrangement with delicate fibrovascular core
- Epithelial lining can be either squamous, respiratory or transitional; it may contain mucus secreting cells and goblet cells
- Variable koilocytosis may be present
- Usually no / scant surface keratinization
- Mitotic figures are absent or limited to the basal layer
- Minimal inflammatory cells
Oncocytic papilloma:
- Architecture: may have endophytic (inverted) or exophytic growth patterns
- Epithelial lining is pseudostratified and columnar with abundant eosinophilic granular cytoplasm and hyperchromatic uniform nuclei
- Intraepithelial mucin filled cysts with neutrophilic microabscesses may be seen
Malignant transformation (carcinoma ex sinonasal papilloma):
- Most common type of carcinoma arising in sinonasal papilloma is squamous cell carcinoma
- Other types of carcinoma that have been reported include verrucous carcinoma, mucoepidermoid carcinoma, small cell carcinoma and sinonasal undifferentiated carcinoma (Head Neck Pathol 2017;11:269)
- Carcinoma is characterized by marked nuclear pleomorphism and hyperchromasia, frequent mitotic figures beyond basal layer including the atypical forms, tumor necrosis and frank invasion with infiltrative irregular nests and desmoplastic stromal reaction
Microscopic (histologic) images
Positive stains
- Cytokeratin AE1 / AE3, CK7, CK5 / 6 (in squamous lining), p40 (in squamous lining)
Molecular / cytogenetics description
- Inverted papilloma may be positive for low risk or high risk HPV by in situ hybridization
- Exophytic papilloma may be positive for low risk HPV by in situ hybridization
- 88% of inverted papilloma and 77% of carcinoma ex inverted papilloma harbor EGFR mutations (Cancer Res 2015;75:2600)
- Most oncocytic papillomas, including those with malignant transformation, have KRAS mutations (J Pathol 2016;239:394)
Sample pathology report
- Maxillary sinus, left; biopsy:
- Sinonasal papilloma, inverted type
Differential diagnosis
- Cutaneous squamous papilloma:
- May involve nasal vestibule and become a differential diagnosis for exophytic papilloma
- May show surface keratinization
- Lacks mucocytes and ciliated respiratory epithelium
- May contain skin adnexa but lacks seromucinous glands
- Nonintestinal type sinonasal adenocarcinoma:
- Has oncocytic cytomorphologic features and is a differential diagnosis for oncocytic papilloma
- Characterized by complex, confluent, back to back glandular proliferation, while oncocytic papilloma has a simple exophytic or endophytic growth pattern without architectural complexity
- High grade tumors may show marked nuclear atypia
- Nonkeratinizing squamous cell carcinoma:
- Infiltrative growth, irregular border, marked nuclear atypia, frequent mitoses, desmoplastic reaction, unequivocal invasion and tumor necrosis
- Respiratory epithelial adenomatoid hamartoma:
- Downward proliferation of respiratory mucosa can mimic inverted papilloma
- Surrounded by a thickened basement membrane
- Absence of hyperplastic changes in the epithelial lining
- Associated with an increase of seromucinous glands
- Rhinosporidiosis:
- Endospores involving the submucosa that may mimic microcysts of oncocytic papilloma
- Endospores are located within stroma rather than epithelium
- Sinonasal inflammatory polyp:
- Has epithelial lining commonly of respiratory type and lacks hyperplastic changes seen in sinonasal papilloma
- Lacks microcysts, transmigrating neutrophils and microabscesses seen in sinonasal papilloma
Additional references
Board review style question #1
Which of the following statements about this sinonasal lesion is false?
- Approximately 30% of cases are associated with high risk human papillomavirus (HPV)
- It affects lateral nasal wall rather than nasal septum
- It affects males and females equally
- It carries approximately 10% risk of malignant transformation
Board review style answer #1
A. This is an oncocytic papilloma. It is not associated with HPV, so "Approximately 30% of cases are associated with high risk human papillomavirus (HPV)" is false.
Comment Here
Reference: Sinonasal papilloma
Comment Here
Reference: Sinonasal papilloma
Board review style question #2
Which of the following statements about sinonasal papilloma is true?
- Both inverted and oncocytic papilloma may show endophytic growth pattern
- Oncocytic sinonasal papilloma has a negligible risk of malignant transformation
- Standard of care for sinonasal papilloma is observation or excision for symptom control
- Subtypes of papilloma that may be related to human papillomavirus (HPV) are oncocytic and exophytic subtypes; inverted papilloma has no significant association with HPV
Board review style answer #2
A. Both inverted and oncocytic papilloma may show endophytic growth pattern
Comment Here
Reference: Sinonasal papilloma
Comment Here
Reference: Sinonasal papilloma
Sinonasal undifferentiated carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- WHO definition: undifferentiated carcinoma lacking evidence of differentiation (such as squamous, glandular or neuroendocrine differentiation) by histology and immunohistochemistry (El-Naggar: WHO Classification of Head and Neck Tumours, 4th Edition, 2017)
- Diagnosis of exclusion
Essential features
- Undifferentiated carcinoma demonstrating epithelial differentiation but lacking specific squamous, glandular, neuroectodermal, mesenchymal, melanocytic or other lines of differentiation
- Lacks specific viral etiologies, such as HPV and EBV
- Lacks other specific genotypes, such as NUT (NUTM1) fusions, SWI/SNF deficiency and others (JNCI Cancer Spectr 2019;4:pkz094, Semin Diagn Pathol 2021;38:175, Hum Pathol 2020;104:105)
- Lacks neuroendocrine differentiation that would justify for high grade neuroendocrine carcinoma, including large cell and small cell neuroendocrine carcinoma
- Displays oncogenic IDH2 or IDH1 mutations in 80% of cases: IDH2 R172T is the most common mutation (Mod Pathol 2019;32:205, Am J Surg Pathol 2018;42:1067)
Terminology
- Sinonasal undifferentiated carcinoma (SNUC)
ICD coding
Epidemiology
- Rare; 3 - 5% of all sinonasal carcinomas (Nat Rev Clin Oncol 2014;11:460)
- Median age at presentation: 50 - 60 years (Curr Oncol Rep 2019;21:26)
- Male predominance with M:F = 2 - 3:1 (Curr Oncol Rep 2019;21:26)
- No clear clinical risk factors documented to date
Sites
- Nasal cavity most common; may also affect paranasal sinus, such as ethmoid sinus and maxillary sinus (Am J Surg Pathol 2002;26:371)
Etiology
- No consistent etiology has been reported
Clinical features
- Presenting symptoms are variable, including nasal obstruction, epistaxis, facial pain and headache to severe visual symptoms (proptosis, diplopia and impaired visual acuity) and cranial nerve palsies (J Otolaryngol Head Neck Surg 2013;42:2)
- Usually presents with stage 4 disease and involves the skull base and orbit (Curr Oncol Rep 2019;21:26)
Prognostic factors
- Dismal prognosis
- Overall, 5 and 10 year relative survival rates for SNUC patients were 34.9% and 31.3%, respectively (J Neurol Surg B Skull Base 2015;76:94)
Case reports
- 50 year old woman with SNUC of maxillary sinus metastasizing to the liver (Oncol Lett 2019;17:5811)
- Man in his early to mid 50s with SNUC presenting with bilateral compressive optic neuropathy (JAMA Ophthalmol 2016;134:e155494)
- 60 year old man with a SNUC of maxillary sinus metastasizing to brain, lung and periaortic area (Autops Case Rep 2020;10:e2020222)
- 63 year old woman with SNUC arising in sinonasal inverted papilloma in the left maxillary sinus (Medicine (Baltimore) 2017;96:e8584)
- 80 year old man with SNUC arising in the left anterior nasal cavity involving the ethmoid, sphenoid and maxillary sinuses with brain metastasis (Cureus 2018;10:e2320)
Treatment
- Multimodal therapy regimens incorporating intensified radiotherapy or chemotherapy after surgery (Head Neck 2017;39:1819)
Gross description
- Usually > 4 cm
- Tend to be fungating, with ill defined borders and frequent invasion into adjacent structures (Arch Pathol Lab Med 2009;133:699, Radiology 1997;202:477)
Microscopic (histologic) description
- Nests, lobules, trabeculae and sheets of medium sized cells
- Cells contain small amounts of eosinophilic cytoplasm, medium to large sized hyperchromatic to vesicular nuclei, inconspicuous to prominent nucleoli and poorly defined cell membrane
- Mitotic activity is very high
- Tumor necrosis and apoptoses are frequent
- Lymphovascular invasion and perineural invasion are common
- Lacks any definite lineage of differentiation
- Squamous differentiation (e.g. keratin pearls and intracellular bridges)
- Glandular differentiation (e.g. glandular lumen or acinar architecture)
- Neuroectodermal differentiation (e.g. neurofibrillary material and true neural rosettes)
- Reference: Adv Anat Pathol 2020;27:51
Microscopic (histologic) images
Positive stains
- Pancytokeratin AE1 / AE3, low molecular weight cytokeratin (CAM 5.2)
- CK7: expressed in half of SNUC (Am J Surg Pathol 2002;26:1597)
- Retain (i.e. no aberrant loss) of SWI/SNF complex, including SMARCB1 / INI1, SMARCA4 / BRG1 and SMARCA2 / BRM
- IDH mutation protein such as IDH2 R172T can be detected by immunohistochemistry in 70% (Mod Pathol 2019;32:205, Am J Surg Pathol 2018;42:1067)
Negative stains
- Minimal to absent expression of lineage markers, such as:
- Neuroendocrine markers: synaptophysin, chromogranin and INSM1 (Am J Surg Pathol 2001;25:156)
- Squamous markers: e.g. CK5/6, p63 and p40 (Ann Diagn Pathol 2014;18:261)
- NUT
- Negative for immunomarkers specific for sarcoma (e.g. CD99, desmin and myogenin), lymphoma (e.g. CD45), melanoma (e.g. MelanA and HMB45)
Molecular / cytogenetics description
- IDH2 or IDH1 mutations in > 80% cases, the most common being IDH2 R172 mutation (Mod Pathol 2019;32:1447)
- IDH2 mutant large cell neuroendocrine carcinoma is genetically similar to the IDH2 mutant SNUC
- In situ hybridization for high risk HPV and EBV is consistently negative
Sample pathology report
- Nasal cavity, biopsy:
- Poorly differentiated carcinoma, most consistent with sinonasal undifferentiated carcinoma (SNUC), at least 1.15 cm in greatest dimension, invading bone (see comment)
- Comment: Immunohistochemical stains show that the tumor cells are focal staining of AE1 / AE3, Cam 5.2 and IDH2 R172S, while they are negative for CK5/6, synaptophysin, chromogranin, IDH1-R132H, calretinin, NUT, P40, S100 and calponin. The immunostaining for BAF47, BRM and BRG1 is retained in the tumor. In situ hybridization for high risk HPV and EBV (EBER) are negative in the tumor.
Differential diagnosis
- Differential diagnoses of SNUC are broad and encompass a wide range of poorly differentiated carcinomas, sarcoma, melanoma and lymphoma
- Squamous cell carcinoma:
- Adenocarcinoma:
- Shows glandular differentiation by histology
- High grade salivary gland carcinoma:
- Shows histologic, immunohistochemical and molecular characteristics of salivary gland carcinoma
- High grade neuroendocrine carcinoma:
- Large cell neuroendocrine carcinoma has coarse or specked chromatin, prominent nucleoli
- Small cell undifferentiated neuroendocrine carcinoma has salt and pepper chromatin and nuclear molding
- Nonfocal immunoexpression of neuroendocrine markers (e.g. synaptophysin, chromogranin and INSM1)
- Like SNUC, might harbor IDH2 mutation
- Olfactory neuroblastoma:
- SWI/SNF deficient sinonasal carcinoma:
- NUT carcinoma:
- Detection of NUT (NUTM1) fusion by molecular testing or abnormal diffuse speckled NUT nuclear immunopositivity by immunohistochemistry
- HPV related multiphenotypic sinonasal carcinoma:
- Shows salivary / myoepithelial differentiation by histology
- Positive for high risk HPV
- Sarcoma
- Melanoma:
- Lymphoma:
- CD45 positive
Board review style question #1
Which of the following statement about sinonasal undifferentiated carcinoma (SNUC) is true?
- 20% of SNUC are positive for high risk HPV
- It is a diagnosis of exclusion
- It is commonly diffusely positive for synaptophysin
- SMARCB1 (INI1) immunostain is lost in this tumor
Board review style answer #1
B. It is a diagnosis of exclusion. SNUC can be focally positive for neuroendocrine markers (e.g. synaptophysin). However, diffuse synaptophysin positivity hints towards olfactory neuroblastoma, paraganglioma or neuroendocrine neoplasm. SMARCB1 loss is characteristic for SMARCB1 deficient sinonasal carcinoma. SNUC has no association with high risk HPV. The correct answer is B (SNUC is a diagnosis of exclusion).
Comment Here
Reference: Sinonasal undifferentiated carcinoma
Comment Here
Reference: Sinonasal undifferentiated carcinoma
Board review style question #2
Board review style answer #2
C. Sinonasal undifferentiated carcinoma (SNUC)
Comment Here
Reference: Sinonasal undifferentiated carcinoma
Comment Here
Reference: Sinonasal undifferentiated carcinoma
Squamous cell carcinoma
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Differential diagnosis | Additional referencesDefinition / general
- Epithelial malignancy originating from sinonasal surface epithelium
Terminology
- Two broad histologic subtypes: keratinizing and nonkeratinizing
Epidemiology
- ~3% of malignancies of head and neck; < 1% of malignant neoplasms at all sites
- Most common malignant epithelial neoplasm of sinonasal tract
- More common in Japan than in the West
- More common in adult men
- Average age at presentation is 55 - 65 years
- Rare in patients < 40 years old
Sites
- Maxillary sinus (60 - 70%), nasal cavity (12 - 25%), ethmoid sinus (10 - 15%), ethmoid and frontal sinuses (1%)
Etiology
- Reported risk factors include exposure to nickel, chlorophenols, textile dust, thorotrast and smoking (Int J Otolaryngol 2013;2013:672621)
- May develop from Schneiderian papilloma and be associated with HPV (Laryngoscope 2001;111:1104)
Clinical features
- Nasal fullness, epistaxis, rhinorrhea, pain, nasal / facial swelling are common presentations
- Advanced cases may present with proptosis, diplopia or lacrimation
Prognostic factors
- Patients with nasal tumors usually present earlier and have better prognosis than those with maxillary sinus tumors
- Overall 5 year survival of nasal squamous cell carcinoma is ~60% versus 42% for maxillary sinus tumor
- Prognosis usually correlates with tumor stage (Arch Otolaryngol Head Neck Surg 2007;133:131)
- Nonkeratinizing squamous cell carcinoma usually has better prognosis than keratinizing type
Case reports
- 60 and 85 year old patients with basaloid squamous cell carcinoma of maxillary sinus (Oncol Lett 2013;5:1755)
Treatment
- Complete surgical resection and adjuvant radiotherapy
Gross description
- Variegated appearance: exophytic, fungating or papillary; friable, hemorrhagic or necrotic mass
Microscopic (histologic) description
- Two broad microscopic subtypes:
- Keratinizing squamous cell carcinoma:
- 80 - 85% cases
- Histologically identical to squamous cell carcinoma at other mucosal sites in head and neck; either well, moderately or poorly differentiated
- Nonkeratinizing (cylindrical cell / transitional) carcinoma
- Keratinizing squamous cell carcinoma:
- Morphologic variants:
- Adenosquamous carcinoma
- Basaloid squamous cell carcinoma
- Papillary squamous cell carcinoma
- Spindle cell carcinoma
- Verrucous carcinoma
Microscopic (histologic) images
Differential diagnosis
- Postchemotherapy changes
- Scheinederian papillomas
Additional references
Staging terminology
Anatomy
- Anatomic sites and subsites for the nasal cavity and paranasal sinuses
- Nasal cavity is divided in the midline to right and left halves by the septum; each half opens on the face via the nares or nostrils and communicates behind with the nasopharynx through the posterior nasal apertures or the choanae
- Nasal cavity is divided into 4 subsites including the septum, floor, lateral wall and vestibule
- Paranasal sinuses represent a grouping of 4 paired sinuses including the maxillary sinuses, ethmoid sinuses, frontal sinuses and sphenoid sinuses
- Nasoethmoidal complex is divided into 2 sites including the nasal cavity and the ethmoid sinuses
- Nasal cavity is divided in the midline to right and left halves by the septum; each half opens on the face via the nares or nostrils and communicates behind with the nasopharynx through the posterior nasal apertures or the choanae
Surgical margins
- Surgical margins (AJCC: Cancer Staging [Accessed 3 December 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Nasal Cavity and Paranasal Sinuses [Accessed 3 December 2018]):
- Definition of a positive margin is somewhat controversial given the varied results from prior studies
- This is made even more challenging and nebulous for sinonasal tumors, which are often received piecemeal with margins submitted separately
- But for squamous cell carcinoma, data is essentially extrapolated from other sites
- Here, overall, several studies support the definition of a positive margin to be invasive carcinoma or carcinoma in situ / high grade dysplasia present at margins (microscopic cut through of tumor)
- Furthermore, reporting of surgical margins should also include information regarding the distance of invasive carcinoma, carcinoma in situ or high grade dysplasia (moderate to severe) from the surgical margin
- Tumors with "close" margins also carry an increased risk for local recurrence
- Definition of a "close" margin is not standardized as the effective cutoff varies between studies and between anatomic subsites
- Commonly used cutpoints to define close margins are 5 mm in general and 2 mm with respect to glottic larynx
- However, values ranging from 3 to 7 mm have been used with success and for glottic tumors as low as 1 mm
- Thus, distance of tumor from the nearest margin should be recorded
- Reporting of surgical margins for carcinomas of the minor salivary glands should follow those used for squamous cell carcinoma of oral cavity
- While there is no standard recommendation for the other histologic types of carcinoma encountered, adherence to the recommendations for squamous cell carcinoma is acceptable
- Definition of a positive margin is somewhat controversial given the varied results from prior studies
Extranodal extension (ENE)
- Extranodal extension (ENE) (AJCC: Cancer Staging [Accessed 3 December 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Nasal Cavity and Paranasal Sinuses [Accessed 3 December 2018]):
- 8th edition introduces the use of extranodal extension in categorizing the "N" category for metastatic cancer to neck nodes
- Effect of ENE on prognosis in head and neck cancers is profound, except for those tumors associated with HR HPV
- Pathological ENE also must be clearly defined as extension of metastatic tumor (tumor present within the confines of the lymph node and extending through the lymph node capsule into the surrounding connective tissue, with or without associated stromal reaction)
- Histopathologic designations for ENE are as follows (Amin: AJCC Cancer Staging Manual, 8th Edition, 2018):
- ENEn (none)
- ENEmi (microscopic ENE ≤ 2 mm)
- ENEma (ENE > 2 mm or gross ENE)
- ENEmi and ENEma are used to define pathological ENE nodal status
- ENEmi versus ENEn will affect current nodal staging but data collection is recommended to allow standardization of data collection and future analysis (AJCC: Cancer Staging [Accessed 3 December 2018])
TNM descriptors
- TNM descriptors:
- By AJCC / UICC convention, the designation "T" refers to a primary tumor that has not been previously treated
- "p" symbol refers to the pathologic classification of the TNM, as opposed to the clinical classification and based on clinical stage information supplemented / modified by operative findings and gross and microscopic evaluation of the resected specimens
- pT entails a resection of the primary tumor or biopsy adequate to evaluate the highest pT category, pN entails removal of nodes adequate to validate lymph node metastasis and pM entails microscopic examination of distant lesions
- Clinical classification (cTNM) is usually carried out by the referring physician before treatment during initial evaluation of the patient or when pathologic classification is not possible
- Pathologic staging is usually performed after surgical resection of the primary tumor
- Pathologic staging depends on pathologic documentation of the anatomic extent of disease, whether or not the primary tumor has been completely removed
- If a biopsied tumor is not resected for any reason (e.g. when technically unfeasible) and if the highest T and N categories or the M1 category of the tumor can be confirmed microscopically, the criteria for pathologic classification and staging have been satisfied without total removal of the primary cancer
- Use of pT0:
- Additional change in AJCC 8th edition is the elimination of the T0 category for all oral cavity, skin, larynx, HPV- oropharynx, hypopharynx and sinus
- This change affects cases where a cervical lymph node has metastatic squamous cell carcinoma but no primary tumor is identified despite thorough history, examination and available imaging studies
- Assigning these cases to a specific head and neck site is not possible
- Previous editions of TNM staging included a T0 category in each of these disease sites
- However, it is seldom used and if it is, the cancer could not be assigned to a stage group
- Therefore, for the 8th edition, the expert panel eliminated the T0 category from the head and neck staging systems
- Additional TNM descriptors:
- For identification of special cases of TNM or pTNM classifications, the "m" suffix and "y" and "r" prefixes are used; although they do not affect the stage grouping, they indicate cases needing separate analysis
- "m" suffix indicates the presence of multiple primary tumors in a single site and is recorded in parentheses: pT(m)NM
- "y" prefix indicates those cases in which classification is performed during or following initial multimodality therapy (i.e. neoadjuvant chemotherapy, radiation therapy or both chemotherapy and radiation therapy)
- cTNM or pTNM category is identified by a "y" prefix
- ycTNM or ypTNM categorize the extent of tumor actually present at the time of that examination
- "y" categorization is not an estimate of tumor prior to multimodality therapy (i.e. before initiation of neoadjuvant therapy)
- "r" prefix indicates a recurrent tumor when staged after a documented disease free interval and is identified by the "r" prefix: rTNM
Staging-nasal cavity & sinuses
Table of Contents
Definition / general | Essential features | ICD coding | Clinical features | Primary tumor (pT) - maxillary sinus | Primary tumor (pT) - nasal cavity and ethmoid sinus | Pathological regional lymph nodes (pN) | Distant metastasis (pM) | AJCC prognostic stage grouping | Registry data collection variables | Histologic grade | Histologic type | Board review style question #1 | Board review style answer #1Definition / general
- Staging of definitive resections for carcinoma (squamous cell carcinoma, neuroendocrine carcinoma and minor salivary gland carcinoma) of the nasal cavity and paranasal sinuses should use this system (AJCC: Cancer Staging [Accessed 24 September 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Nasal Cavity and Paranasal Sinuses [Accessed 24 September 2018])
- Cancer of the maxillary sinus is the most common of the sinonasal malignancies
- Ethmoid sinus and nasal cavity cancers are equal in frequency but considerably less common than maxillary sinus cancers
- Tumors of the sphenoid and frontal sinuses are rare
- Staging protocols such as those of CAP are to document procedure, tumor site, tumor laterality, tumor focality, tumor size, histologic type, histologic grade where applicable, margin status, lymphovascular invasion, perineural invasion, regional lymph node findings and summarize these pathologic findings using TNM system
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- Use of the CAP protocol is not required for recurrent tumors or for metastatic tumors that are resected at a different time than the primary tumor
- Use of this protocol is also not required for pathology reviews performed at a second institution (i.e. secondary consultation, second opinion or review of outside case at second institution)
- Reporting of surgical margins for carcinomas of the minor salivary glands should follow those used for squamous cell carcinoma
- CAP staging protocol: CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Nasal Cavity and Paranasal Sinuses [Accessed 24 September 2018]
- Alternatively for providers practicing in different geographic regions, there may be interest in the International Collaboration on Cancer Reporting (ICCR) guidelines: ICCR: Carcinomas of the Nasal Cavity and Paranasal Sinuses (TNM8) [Accessed 24 September 2018]
Clinical features
- Cancers of the maxillary sinuses are the most common sinonasal malignancies, followed by cancers of the ethmoid sinuses, which are much less common
- Cancers of the frontal and sphenoid sinuses are rare
- When considering the nasal cavity and paranasal sinuses, 60% of malignant neoplasms originate from the maxillary sinus, 20 - 30% from the nasal cavity, 10 - 15% from the ethmoid sinus and 1% from the sphenoid and frontal sinuses
- When only considering the paranasal sinuses, 77% of malignant neoplasms originate from the maxillary sinus, 22% from the ethmoid sinus and 1% from the sphenoid and frontal sinuses
- Location as well as the extent of the mucosal lesion in the maxillary sinus has prognostic importance
- Ohngren's line, connecting the medial canthus of the eye to the angle of the mandible, divides the maxillary sinus into an anterioinferior portion (infrastructure) and superioposterior portion (suprastructure) structures
- Carcinomas of the infrastructure are associated with a good prognosis; carcinomas of the suprastructure are associated with a poor prognosis
- Poorer prognosis with carcinomas of the suprastructure reflects early access of these tumors to critical structures, including the eye, skull base, pterygoids and infratemporal fossa (AJCC: Cancer Staging [Accessed 24 September 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Nasal Cavity and Paranasal Sinuses [Accessed 24 September 2018])
Primary tumor (pT) - maxillary sinus
Primary tumor (pT) - nasal cavity and ethmoid sinus
- pTX: Primary tumor cannot be assessed
- pTis: Carcinoma in situ
- pT1: Tumor restricted to any 1 subsite, with or without bone invasion
- pT2: Tumor invading 2 subsites in a single region or extending to involve an adjacent region within the nasoethmoidal complex, with or without bone invasion
- pT3: Tumor extends to invade the medial wall or floor of the orbit, maxillary sinus, palate or cribriform plate
- pT4: Moderately advanced or very advanced local disease
- pT4a: Moderately advanced local disease; tumor invades any of the following:
- Anterior orbital contents, skin of nose or cheek, minimal extension to anterior cranial fossa, pterygoid plates, sphenoid or frontal sinuses
- pT4b: Very advanced local disease; tumor invades any of the following:
- Orbital apex, dura, brain, middle cranial
- pT4a: Moderately advanced local disease; tumor invades any of the following:
Pathological regional lymph nodes (pN)
- pNX: Regional lymph nodes cannot be assessed
- pN0: No regional lymph node metastasis
- pN1: Metastasis in a single ipsilateral lymph node, ≤ 3 cm in greatest dimension and extranodal extension negative (ENE-)
- pN2a: Metastasis in:
- Single ipsilateral lymph node that is ≤ 3 cm and ENE+ or
- Single ipsilateral lymph node that is > 3 cm but ≤ 6 cm in greatest dimension and ENE-
- pN2b: Metastasis in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension and ENE-
- pN2c: Metastasis in bilateral or contralateral lymph node(s), none > 6 cm in greatest dimension and ENE-
- pN3a: Metastasis in a lymph node that is > 6 cm in greatest dimension and ENE-
- pN3b: Metastasis in:
- Single ipsilateral lymph node, > 3 cm and ENE+ or
- Multiple ipsilateral, contralateral or bilateral lymph nodes, any with ENE+ or
- Single contralateral lymph node of any size and ENE+
Notes:
- Designation of "U" or "L" may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L)
- A selective neck dissection will ordinarily include 10+ lymph nodes and a comprehensive neck dissection (radical or modified radical neck dissection) will ordinarily include 15+ lymph nodes
- Negative pathologic examination of a smaller number of nodes still mandates a pN0 designation
- Midline nodes are considered ipsilateral nodes
Distant metastasis (pM)
- pM0: No distant metastasis
- pM1: Distant metastasis
Notes:
- Measurement of tumor metastasis (AJCC: Cancer Staging [Accessed 24 September 2018], CAP: Protocol for the Examination of Specimens from Patients with Cancers of the Nasal Cavity and Paranasal Sinuses [Accessed 24 September 2018]):
- Cross sectional diameter of the largest lymph node metastasis (not the lymph node itself) is measured in the gross specimen at the time of macroscopic examination or if necessary, on the histologic slide at the time of microscopic examination
- Measurement of the metastatic focus in the lymph nodes is based on the largest metastatic deposit size, which may include matted or fused lymph nodes
AJCC prognostic stage grouping
| Stage 0: | Tis | N0 | M0 |
| Stage I: | T1 | N0 | M0 |
| Stage II: | T2 | N0 | M0 |
| Stage III: | T3 | N0 | M0 |
| T1 - 3 | N1 | M0 | |
| Stage IVA: | T4a | N0 - 1 | M0 |
| T1 - 4a | N2 | M0 | |
| Stage IVB: | Any T | N3 | M0 |
| T4b | Any N | M0 | |
| Stage IVC: | Any T | Any N | M1 |
Registry data collection variables
- Extranodal extension (ENE) clinical (present versus absent)
- ENE pathological (present versus absent)
- Extent of microscopic ENE (distance of extension from the native lymph node capsule to the farthest point of invasion in extranodal tissue)
- Perineural invasion
- Lymphovascular invasion
- Performance status
- Tobacco use and pack years
- Alcohol use
- Depression diagnosis
Histologic grade
- GX: Cannot be assessed
- G1: Well differentiated
- G2: Moderately differentiated
- G3: Poorly differentiated
Notes:
- For histologic types of carcinomas that are amenable to grading, 3 histologic grades are suggested, as shown above
- For conventional squamous cell carcinoma, histologic grading as a whole does not perform well as a prognosticator
- Nonetheless, it should be recorded when applicable, as it is a basic tumor characteristic
- Selecting either the most prevalent grade or the highest grade for this synoptic protocol is acceptable
- Variants of squamous cell carcinoma (i.e. verrucous, basaloid, etc.) have an intrinsic biologic potential and currently do not appear to require grading
- Histologic (microscopic) grading of salivary gland carcinomas has been shown to be an independent predictor of behavior and plays a role in optimizing therapy
- However, most salivary gland carcinoma types have an intrinsic biologic behavior and attempted application of a universal grading scheme is merely a crude surrogate
- Thus a generic grading scheme is no longer recommended for salivary gland carcinomas
- Carcinoma types for which grading systems exist and are relevant are incorporated into histologic type
- 3 major categories that are amenable to grading include adenoid cystic carcinoma, mucoepidermoid carcinoma and adenocarcinoma, not otherwise specified
- In some carcinomas, histologic grading may be based on growth pattern, such as in adenoid cystic carcinoma, for which a histologic high grade variant has been recognized based on the percentage of solid growth
- Those adenoid cystic carcinomas showing ≥ 30% of solid growth pattern are considered to be histologically high grade carcinomas
- Histologic grading of mucoepidermoid carcinoma includes a combination of growth pattern characteristics (e.g. cystic, solid, neurotropism) and cytomorphologic findings (e.g. anaplasia, mitoses, necrosis)
- Adenocarcinomas, not otherwise specified do not have a formalized grading scheme and are graded intuitively based on cytomorphologic features
- For nonsalivary sinonasal adenocarcinoma, intestinal type adenocarcinoma grading is based on growth pattern
- Generally, papillary patterns correspond to low grade tumors, colonic to intermediate grade and solid to high grade
- Mucinous and mixed types have variable behavior
- Nonintestinal type adenocarcinomas are graded intuitively into low, intermediate and high grade tumors
Histologic type
- CAP protocol applies only to carcinomas and melanomas and does not apply to lymphomas, sarcomas or neuroectodermal tumors (e.g. olfactory neuroblastoma, primitive neuroectodermal tumor [PNET], others)
- Nasal cavity and paranasal sinuses
- Squamous cell carcinoma, keratinizing
- Squamous cell carcinoma, nonkeratinizing
- Adenosquamous carcinoma
- Basaloid squamous cell carcinoma
- Papillary squamous cell carcinoma
- Spindle cell squamous cell carcinoma
- Verrucous squamous cell carcinoma
- Lymphoepithelial carcinoma (nonnasopharyngeal)
- Sinonasal undifferentiated carcinoma (SNUC)
- NUT carcinoma
- Adenocarcinoma, non salivary gland type
- Intestinal type
- Nonintestinal type
- Carcinomas of minor salivary glands
- Mucoepidermoid carcinoma, low grade
- Adenoid cystic carcinoma
- Acinic cell carcinoma
- Polymorphous adenocarcinoma
- (Mammary analogue) secretory carcinoma
- Salivary duct carcinoma
- Carcinoma ex pleomorphic adenoma
- Epithelial myoepithelial carcinoma
- (Hyalinizing) clear cell carcinoma
- Adenocarcinoma, not otherwise specified
- Neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Small cell neuroendocrine carcinoma
Board review style question #1
Based on the gross and microscopic examination of the 2.2 cm nasal cavity adenosquamous carcinoma originating along the right nasal septum in the following images, which of the pT category criteria in the 8th edition AJCC staging guide is satisfied?
- pT1 due to tumor size
- pT2 due to tumor size
- pT3 due to extension to overlying nasal skin
- pT4 due to extension to overlying nasal skin
Board review style answer #1
D. pT4 due to extension to overlying nasal skin
Comment Here
Reference: Pathologic TNM staging of nasal cavity and paranasal sinuses (AJCC 8th edition)
Comment Here
Reference: Pathologic TNM staging of nasal cavity and paranasal sinuses (AJCC 8th edition)
Staging-nasopharynx
Table of Contents
Definition / general | Essential features | ICD coding | Primary tumor (pT) | Regional lymph nodes (pN) | Distant metastasis (pM) | Prefixes | AJCC prognostic stage groups | Registry data collection variables | Histologic grade (G) | Histopathologic type | Board review style question #1 | Board review style answer #1Definition / general
- All primary carcinomas of the nasopharynx are covered by this staging system
- These topics are not covered: metastasis, mucosal melanoma, lymphoma and sarcoma
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
ICD coding
Primary tumor (pT)
- pTX: cannot be assessed
- pT0: no evidence of primary tumor, with proven EBV positive lymph node metastasis
- pTis: carcinoma in situ
- pT1: confined to nasopharynx or involvement to oropharynx or nasal cavity without parapharyngeal extension
- pT2: parapharyngeal extension or involvement of adjacent soft tissue including medial pterygoid / lateral pterygoid / prevertebral muscles
- pT3: bone invasion into skull base, cervical vertebra, pterygoid structures or paranasal sinuses
- pT4: involvement of intracranial structures, cranial nerve, hypopharynx, orbit or parotid gland or invasion beyond the lateral surface of the lateral pterygoid muscle
Regional lymph nodes (pN)
- pNX: cannot be assessed
- pN0: no regional lymph node metastasis
- pN1: metastasis to unilateral cervical lymph node(s) or unilateral / bilateral retropharyngeal lymph node(s), above the inferior border of cricoid cartilage, ≤ 6 cm in size
- pN2: metastasis to bilateral cervical lymph nodes, above the inferior border of cricoid cartilage, ≤ 6 cm in size
- pN3: metastasis > 6 cm in size or below the inferior border of cricoid cartilage
Distant metastasis (pM)
- pM1: distant metastasis
Prefixes
- y: preoperative radiotherapy or chemotherapy
- r: recurrent tumor stage
AJCC prognostic stage groups
| Stage group 0: | Tis | N0 | M0 |
| Stage group I: | T1 | N0 | M0 |
| Stage group II: | T0 or T1 | N1 | M0 |
| T2 | N0 or N1 | M0 | |
| Stage group III: | T0, T1 or T2 | N2 | M0 |
| T3 | N0, N1 or N2 | M0 | |
| Stage group IV: | T4 | any N | any M |
| any T | N3 | any M | |
| any T | any N | M1 | |
| Stage group IVA: | T4 | any N | M0 |
| any T | N3 | M0 | |
| Stage group IVB: | any T | any N | M1 |
Registry data collection variables
- Not applicable
Histologic grade (G)
- Not applicable for nasopharyngeal carcinoma
Histopathologic type
- Nasopharyngeal carcinoma
- Keratinizing squamous cell carcinoma
- Nonkeratinizing squamous cell carcinoma
- Basaloid squamous cell carcinoma
- Nasopharyngeal papillary adenocarcinoma
- Salivary gland carcinoma
Board review style question #1
Which of the following statements about the AJCC staging (8th edition) for nasopharyngeal carcinoma is true?
- pN is determined using a combination of laterality and location of involved lymph node and size of the metastasis
- The presence of extranodal extension increases the pN category
- pT is determined by tumor size and site of involvement
- A cervical lymph node with EBV positive squamous cell carcinoma and no primary tumor detected after extensive sampling is pTX
Board review style answer #1
A. pN is determined using a combination of laterality and location of involved lymph node and size of the metastasis
Comment Here
Reference: Pathologic TNM staging of nasopharynx (AJCC 8th edition)
Comment Here
Reference: Pathologic TNM staging of nasopharynx (AJCC 8th edition)
SWI / SNF complex deficient sinonasal carcinoma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Radiology description | Radiology images | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Aggressive carcinoma defined by SMARCB1 gene mutations and associated loss of immunohistochemical expression
- Provisional entity in El-Naggar: WHO Classification of Head and Neck Tumors, 4th Edition, 2017
Essential features
- Sinonasal carcinoma defined by inactivation of SMARCB1 gene
- Loss of SMARCB1 (INI1) immunohistochemical expression is highly sensitive and specific for diagnosis in this site
- Tumors are high grade but demonstrate cytological uniformity
- Tumors have variable proportions of basaloid and rhabdoid / plasmacytoid cells
- Highly aggressive clinical behavior
Terminology
- SMARCB1 stands for SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1
- The protein product of the SMARCB1 gene is also sometimes known as INI1, which stands for integrase interactor 1
ICD coding
Epidemiology
- Described in patients from 16 to 89 years old (Virchows Arch 2015;467:649, Pathol Res Pract 2017;213:133)
- M:F = 3:2 (Am J Surg Pathol 2017;41:458)
- No clear clinical risk factors documented to date
Sites
- Exclusively occurs in sinonasal tract
- Most commonly arises in nasal cavity or ethmoid sinus but frequently involves multiple sites (Am J Surg Pathol 2017;41:458)
Clinical features
- Most present with symptoms of mass effect such as nasal obstruction, headache, epistaxis, proptosis or visual deficits (Am J Surg Pathol 2017;41:458)
- More than 80% present with stage T4 disease with bone, skull base or periorbital invasion
Radiology description
- Avid contrast enhancement with intermediate to low T2 signal and fluorodeoxyglucose (FDG) avidity
- Frequent calcification with occasional hair on end periosteal reaction (Am J Surg Pathol 2014;38:1274)
Case reports
- 44 year old woman with tumor showing yolk sac differentiation (Int J Surg Pathol 2018;26:245)
- 53 year old man with highly necrotic tumor (Ann Otol Rhinol Laryngol 2019;128:676)
- 53 year old man with endobronchial metastases (Acta Cytol 2019 May 27 [Epub ahead of print])
- 61 year old woman with orbital compartment syndrome (Surv Ophthalmol 2019 Apr 10 [Epub ahead of print])
- 77 year old man with retropharyngeal lymph node metastasis (Diagn Cytopathol 2016;44:700)
Treatment
- Best outcomes with trimodality therapy including surgical resection, external beam radiation and systemic chemotherapy (Head Neck Pathol 2017;11:256, Am J Surg Pathol 2017;41:458)
Gross description
- Highly infiltrative tumor that frequently invades multiple sinonasal sites (Head Neck Pathol 2017;11:256)
Microscopic (histologic) description
- Although tumors show high grade histologic features including prominent necrosis and mitoses, cytology is relatively uniform
- Nuclei are round to oval with smooth nuclear contours
- Two common morphologic variants: plasmacytoid / rhabdoid (40% of cases) and basaloid (60% of cases) (Am J Surg Pathol 2017;41:458)
- Plasmacytoid / rhabdoid variant composed entirely of sheets of plasmacytoid / rhabdoid cells with abundant eosinophilic cytoplasm and eccentric nuclei
- Basaloid variant composed of nests and lobules of basaloid cells with high nuclear cytoplasmic ratio and peripheral palisading and scattered plasmacytoid / rhabdoid cells
- No overt squamous differentiation should be identified (Am J Surg Pathol 2014;38:1282)
- Frequent formation of nonspecific vacuoles and occasional overt glandular differentiation (Hum Pathol 2019;83:59)
- Subset shows papillary / exophytic growth (Virchows Arch 2015;467:649)
- No associated surface dysplasia but pagetoid spread in overlying epithelium can be seen (Am J Surg Pathol 2014;38:1274)
Microscopic (histologic) images
Cytology description
- Hypercellular smears with clusters of cohesive polygonal cells
- Variable proportion of individual rhabdoid / plasmacytoid cells with abundant cytoplasm
- Uniform nuclei with fine chromatin and small nucleoli
- No overt keratinization or other evidence of squamous differentiation (Diagn Cytopathol 2016;44:700)
- Apoptotic bodies common with abundant background necrotic debris (Cancer Cytopathol 2018;126:567)
Positive stains
- Virtually always diffusely positive for pancytokeratin
- Squamous markers p63 / p40 positive in > 50% of cases
- Neuroendocrine markers synaptophysin / chromogranin positive in 30% of cases
- Small subset of cases express diffuse p16 (Am J Surg Pathol 2017;41:458)
Negative stains
Molecular / cytogenetics description
- Defined by inactivating mutations of SMARCB1 gene, a tumor suppressor gene located on chromosome 22q11.2 (Am J Surg Pathol 2014;38:1274, Am J Surg Pathol 2014;38:1282)
- FISH less sensitive for diagnosis than immunohistochemistry, likely due to heterozygous deletions
- Next generation sequencing has not identified other driver mutations within the limited number of cases evaluated (Pathol Res Pract 2017;213:133)
- In situ hybridization for high risk HPV and EBV is consistently negative (Am J Surg Pathol 2017;41:458)
Sample pathology report
- Nasal mass, biopsy:
- SMARCB1 deficient sinonasal carcinoma (see comment)
- Comment: Immunostains show that the tumor cells are positive for AE1/AE3 and p40, focally positive for synaptophysin and INSM1 and negative for NUT1, CD99, p16, S100 and calponin with loss of SMARCB1 (INI1) expression. SMARCB1 deficient sinonasal carcinoma is an aggressive tumor unique to the sinonasal tract that is defined by loss of SMARCB1 (INI1) expression (Am J Surg Pathol 2017;41:458).
Differential diagnosis
- Sinonasal undifferentiated carcinoma:
- Also high grade but shows more prominent cytologic atypia and lacks rhabdoid / plasmacytoid cells
- Negative for p63 / p40 and synaptophysin / chromogranin with frequent IDH2 mutations
- NUT carcinoma:
- Also high grade and frequently positive for p63 / p40
- Shows abrupt keratinization and positivity for NUT1 with retained SMARCB1 (INI1)
- Myoepithelial carcinoma:
- Also demonstrates rhabdoid / plasmacytoid morphology and a subset show loss of SMARCB1 (INI1)
- Positive for myoepithelial markers S100, SMA and calponin
- Adamantinoma-like Ewing sarcoma:
- Also high grade and frequently positive for p63 / p40 and synaptophysin / chromogranin
- Positive for CD99 / NKX2.1 with retained SMARCB1 (INI1)
- High grade neuroendocrine carcinoma:
- Also high grade and positive for synaptophysin / chromogranin
- Speckled chromatin and retained SMARCB1 (INI1)
- HPV related multiphenotypic sinonasal carcinoma:
- Also high grade with basaloid features and positivity for p63 / p40
- Distinct biphasic basal and ductal populations, positive for HPV and retained SMARCB1 (INI1)
Board review style question #1
Board review style answer #1
D. Inactivation of SMARCB1. This photomicrograph depicts a SMARCB1 deficient sinonasal carcinoma, an aggressive sinonasal tumor that is defined by genetic inactivation of the SMARCB1 gene with corresponding immunohistochemical loss of SMARCB1 (INI1) protein expression. The other genetic abnormalities listed here are characteristic of other high grade sinonasal tumors that fall into the differential diagnosis of SMARCB1 deficient sinonasal carcinoma, including IDH2 mutations in sinonasal undifferentiated carcinoma, EWSR1 translocation in adamantinoma-like Ewing sarcoma and NUT1 translocation in NUT carcinoma; these other tumor types all demonstrate retained immunohistochemical expression of SMARCB1.
Comment here
Reference: SMARCB1 deficient sinonasal carcinoma
Comment here
Reference: SMARCB1 deficient sinonasal carcinoma
Board review style question #2
What feature would essentially exclude the diagnosis of SMARCB1 deficient sinonasal carcinoma?
- Basaloid cell nests with peripheral palisading and strong p40 positivity
- Diffuse positivity for p16 immunohistochemistry and high risk HPV RNA in situ hybridization
- Formation of glandular lumina with mucin production
- Retained SMARCB1 signals by FISH
Board review style answer #2
B. Diffuse positivity for p16 immunohistochemistry and high risk HPV RNA in situ hybridization. SMARCB1 deficient sinonasal carcinoma can show a variety of morphologic features and a highly variable immunohistochemical profile. Histologically, SMARCB1 deficient sinonasal carcinoma is composed of various proportions of basaloid and plasmacytoid / rhabdoid cells; a subset of cases can show glandular differentiation. The most sensitive and specific feature for diagnosis is consistent loss of SMARCB1 (INI1) expression by immunohistochemistry; while FISH generally shows loss as well, signals can be retained in rare cases due to heterozygous mutations. Otherwise SMARCB1 deficient sinonasal carcinoma is immunohistochemically heterogeneous with variable positivity for p63 / p40 and synaptophysin / chromogranin; a subset of cases can even demonstrate diffuse p16 positivity. However, in situ hybridization for high risk HPV should be consistently negative.
Comment here
Reference: SMARCB1 deficient sinonasal carcinoma
Comment here
Reference: SMARCB1 deficient sinonasal carcinoma
Teratocarcinosarcoma
Table of Contents
Definition / general | Terminology | Microscopic (histologic) description | Positive stains | Negative stains | Additional referencesDefinition / general
- Features of carcinosarcoma and immature teratoma
- Adults; poor prognosis with 3 year survival of 40% due to uncontrollable local growth
Terminology
- Also known as teratoid carcinosarcoma
Microscopic (histologic) description
- Primitive neuroepithelial elements with well formed rosettes
- Well formed glands with atypical epithelium
- Myxoid tissue
- Rhabdomyosarcomatous differentiation
- Benign and malignant cartilage
- Cellular areas
Positive stains
- CD99, NSE, vimentin, keratin, EMA, GFAP, chromogranin, synaptophysin
Negative stains
Additional references
WHO classification
Table of Contents
Definition / general | Major updates | WHO (2017) nasal cavity, paranasal sinuses and skull base | WHO (2017) nasopharynx | Videos | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Classification is based on WHO classification of tumors of the nasal cavity, paranasal sinuses and skull base (4th edition, 2017)
Major updates
- Several new entities and provisional entities were added to the chapter of nasal cavity, paranasal sinuses and skull base, including:
- HPV related multiphenotypic sinonasal carcinoma
- SMARCB1 deficient sinonasal carcinoma
- Renal cell-like adenocarcinoma
- Seromucinous hamartoma
- NUT carcinoma
- Biphenotypic sinonasal sarcoma
WHO (2017) nasal cavity, paranasal sinuses and skull base
-
Carcinomas ICD-O codes
- Keratinizing squamous cell carcinoma 8071/3
- Nonkeratinizing squamous cell carcinoma 8072/3
- Provisional entity: HPV related multiphenotypic sinonasal carcinoma
- Spindle cell squamous cell carcinoma 8074/3
- Lymphoepithelial carcinoma 8082/3
- Sinonasal undifferentiated carcinoma 8020/3
- Provisional entity: SMARCB1 deficient sinonasal carcinoma
- NUT carcinoma 8023/3
- Neuroendocrine carcinoma
- Small cell neuroendocrine carcinoma 8041/3
- Large cell neuroendocrine carcinoma 8013/3
- Adenocarcinoma
- Intestinal type adenocarcinoma 8144/3
- Nonintestinal type adenocarcinoma 8140/3
- Provisional entity: renal cell-like adenocarcinoma
-
Teratocarcinosarcoma 9081/3
Sinonasal papillomas ICD-O codes
-
Respiratory epithelial lesions
-
Salivary gland tumors ICD-O codes
- Pleomorphic adenoma 8940/0
-
Malignant soft tissue tumors ICD-O codes
- Fibrosarcoma 8810/3
- Undifferentiated pleomorphic sarcoma 8802/3
- Leiomyosarcoma 8890/3
- Rhabdomyosarcoma, NOS 8900/3
- Embryonal rhabdomyosarcoma 8910/3
- Alveolar rhabdomyosarcoma 8920/3
- Pleomorphic rhabdomyosarcoma, adult type 8901/3
- Spindle cell rhabdomyosarcoma 8912/3
- Angiosarcoma 9120/3
- Malignant peripheral nerve sheath tumor 9540/3
- Biphenotypic sinonasal sarcoma
- Synovial sarcoma 9040/3
-
Borderline / low grade malignant soft tissue tumors ICD-O codes
- Desmoid type fibromatosis 8821/1
- Sinonasal glomangiopericytoma 9150/1
- Solitary fibrous tumor 8815/1
- Epithelioid hemangioendothelioma 9133/3
-
Benign soft tissue tumors ICD-O codes
- Leiomyoma 8890/0
- Hemangioma 9120/0
- Schwannoma 9560/0
- Neurofibroma 9540/0
- Meningioma 9530/0
- Sinonasal ameloblastoma 9310/0
- Chondromesenchymal hamartoma
- Extranodal NK / T cell lymphoma 9719/3
- Extraosseous plasmacytoma 9734/3
-
Other tumors ICD-O codes
-
Hematolymphoid tumors ICD-O codes
-
Neuroectodermal / melanocytic tumors ICD-O codes
WHO (2017) nasopharynx
-
Nasopharyngeal carcinoma ICD-O codes
-
Salivary gland tumors ICD-O codes
- Adenoid cystic carcinoma 8200/3
- Salivary gland anlage tumor
-
Benign and borderline lesions ICD-O codes
- Hairy polyp
- Ectopic pituitary adenoma 8272/0
- Craniopharyngioma 9350/1
-
Soft tissue tumors ICD-O codes
- Nasopharyngeal angiofibroma 9160/0
-
Hematolymphoid tumors ICD-O codes
- Diffuse large B cell lymphoma 9680/3
- Extraosseous plasmacytoma 9734/3
- Extramedullary myeloid sarcoma 9930/3
-
Notochord tumors ICD-O codes
- Chordoma 9370/3
Videos
WHO's new in sinonasal tract pathology
Dr. Thompson
Additional references
Board review style question #1
What are the three subtypes of nasopharyngeal carcinoma in the WHO classification?
- Keratinizing, nonkeratinizing, basaloid
- Keratinizing, nonkeratinizing, neuroendocrine carcinoma
- Keratinizing, nonkeratinizing, nasopharyngeal papillary adenocarcinoma
- Keratinizing, nonkeratinizing, HPV-positive
Board review style answer #1
Recent Nasal cavity, paranasal sinuses, nasopharynx Pathology books
Find related Pathology books: head & neck/endocrine