- pTX: cannot be assessed
- pT0: no evidence of primary tumor
- pT1: tumor limited to the ipsilateral parietal surface with or without involvement of visceral pleura, mediastinal pleura or diaphragmatic pleura
- pT2: tumor involves each of the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic and visceral pleura) and has at least one of the following
- Diaphragmatic muscle involvement
- Extension from visceral pleura into the underlying pulmonary parenchyma
- pT3: tumor is locally advanced but potentially resectable; tumor involves all the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic and visceral pleura) and has at least one of the following
- Endothoracic fascia involvement
- Mediastinal fat involvement
- Solitary completely resectable focus of tumor extending into soft tissue of chest wall
- Nontransmural involvement of the pericardium
- pT4: tumor is locally advanced and technically unresectable; tumor involves all the ipsilateral pleural surfaces (parietal, mediastinal, diaphragmatic and visceral pleura) and has at least one of the following
- Diffuse extension or multifocal masses in chest wall
- Direct transdiaphragmatic extension to peritoneum
- Direct extension to the contralateral pleura
- Direct extension to mediastinal organs
- Direct extension into spine
- Extension to internal surface of the pericardium
- Myocardium involvement
Superpage
Superpage Topics
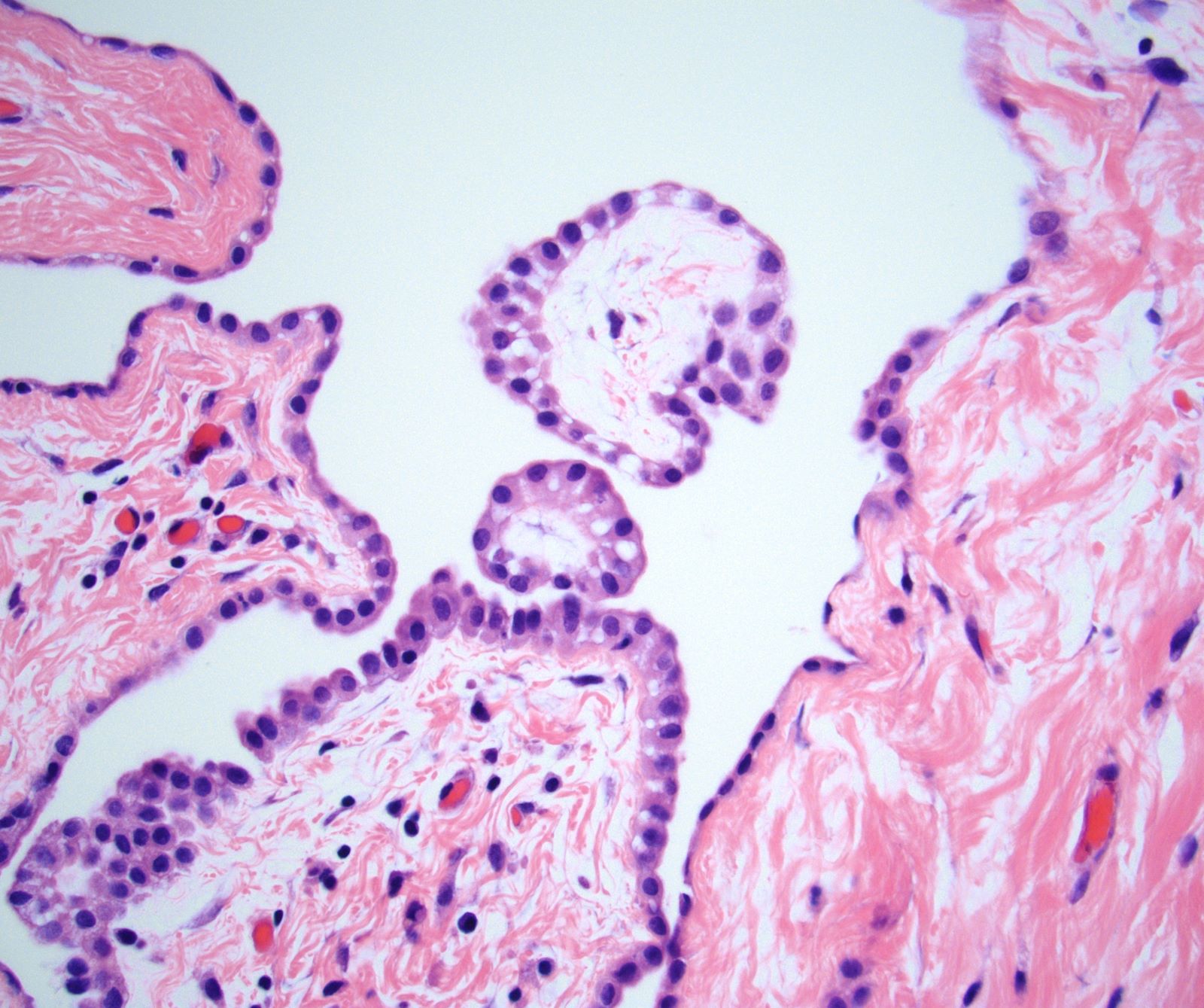
Adenomatoid tumor
Anatomy, history, grossing & features to report
Asbestos related disorders
Atypical mesothelial hyperplasia
Benign mesothelial proliferations
Diffuse mesothelioma
Endosalpingiosis
Localized mesothelioma
Mesothelial hyperplasia
Mesothelioma (peritoneum)-epithelioid
Mesothelioma (peritoneum)-overview
Mesothelioma (peritoneum)-sarcomatoid and biphasic (pending)
Mesothelioma (pleura)-epithelioid
Mesothelioma versus adenocarcinoma
Mesothelioma-biphasic
Mesothelioma-desmoplastic
Mesothelioma-sarcomatoid
Metastases
Nodular histiocytic hyperplasia
Peritoneal inclusion cyst
Pleural effusion
Pleural effusion
Pleural plaques
Pleuritis
Pneumothorax
Staging
Well differentiated papillary mesothelial tumor (peritoneum)
Well differentiated papillary mesothelioma tumor-pleura (pending)Adenomatoid tumor
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology / etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Differential diagnosis | Additional referencesDefinition / general
- Benign lesion, often incidental finding on oophorectomy specimen
- More frequently these lesion are found in males (epididymis, spermatic cord and testicular membrane); however, in females lesions are seen more commonly in fallopian tubes, broad ligament and uterus
- Thought to arise from mesothelial serosal cells
- First described by Golden and Ash in 1945 (Am J Pathol 1945;21:63)
Essential features
- Rarely found within ovary
- Typically small with 0.5 - 3 cm incidental lesions near hilum
Terminology
- Previously known as benign mesothelioma of the genital tract
Epidemiology
- Usually occurs in the third and fourth decades (Int J Gynecol Pathol 1991;10:364)
- Most commonly adults females (23 - 79 years old)
- Average age 54 reported in literature (Int J Gynecol Pathol 1991;10:364)
Sites
- Ovarian and juxtaovarian sites are rare
- Occur predominantly at the ovarian hilum and may extend into and replace the ovarian parenchyma
- Most frequently unilateral, found within fallopian tube, broad ligament or on uterine serosal surface
Pathophysiology / etiology
- Mesothelial origin supported by ultrastructural and immunohistochemical features (Pathol Res Pract 1983;176:258)
- Derivation from differentiated mesothelial cells by inclusion or embolization has been postulated (Arch Pathol Lab Med 2000;124:609)
- An origin from pluripotent Müllerian mesencyhmal stem cells has been suggested (Cancer 1958;11:337)
Clinical features
- Asymptomatic, discovered as an incidental finding
- Usually 0.5 - 3.0 cm, rarely larger and symptomatic
Diagnosis
- Histologic recognition, confirmed by immunophenotype
- Often incidental
Laboratory
- Nondiagnostic
- Rare reports have describe slightly elevated CEA with normal CA-125 (J Clin Ultrasound 2005;33:233)
- Other reports have described normal serum markers (Eur J Gynaecol Oncol 2014;35:91)
Radiology description
- Not routinely performed for primary diagnosis
- Case reports describe incidental lesions on transvaginal ultrasound displaying multilocular cystic mass often with vascularized central / solid portion
- Radiographic differential diagnosis, if provided, may include epithelial tumors, inclusion peritoneal cysts, and multiple large follicles
- CT imaging seldom describes lesion (J Clin Ultrasound 2005;33:233)
Prognostic factors
- Benign behavior, no reports of recurrence or malignant transformation
Case reports
- 26 year old woman with adenomatoid tumors involving uterus, ovary and appendix (J Obstet Gynaecol Res 2003;29:234)
- 52 year old woman with oxyphilic adenomatoid tumor of the ovary (Int J Gynecol Pathol 2007;26:16)
- 61 year old woman (Jpn J Clin Oncol 1988;18:159)
Treatment
- Excision results in complete cure
- Recurrence after excision is rare
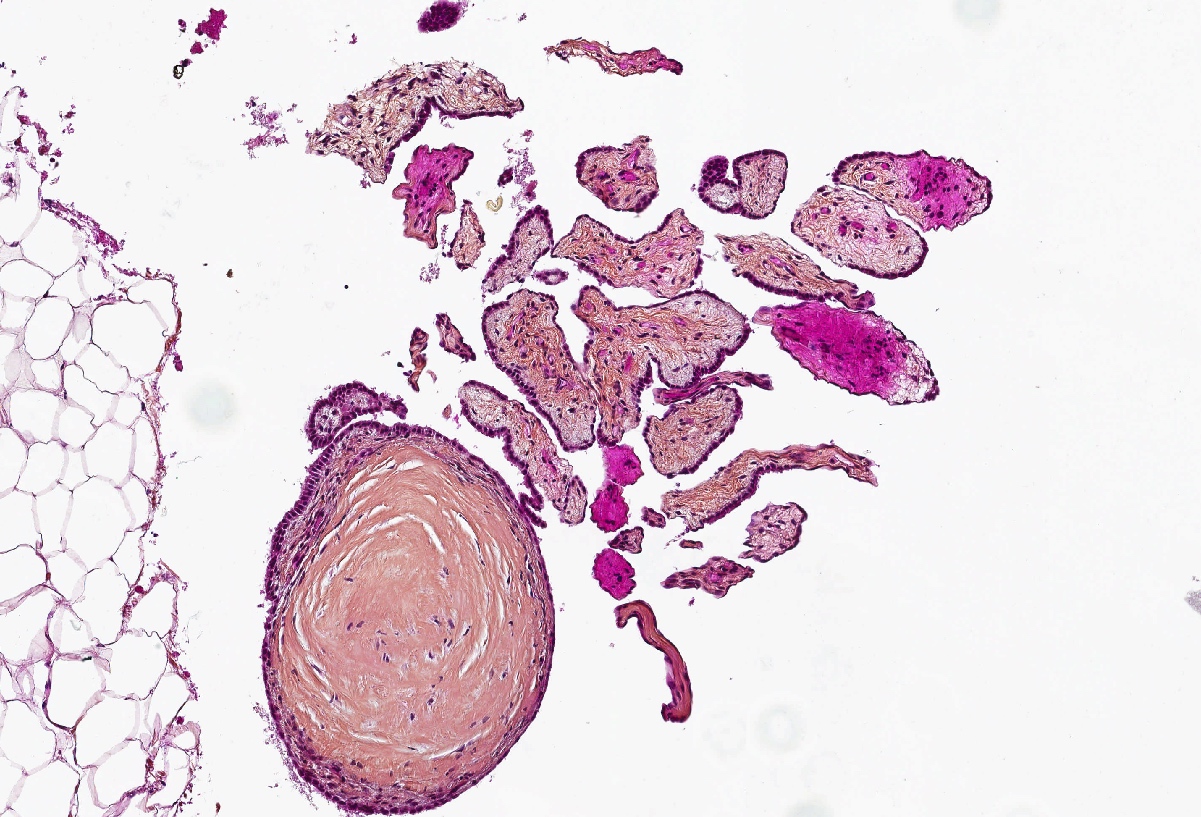
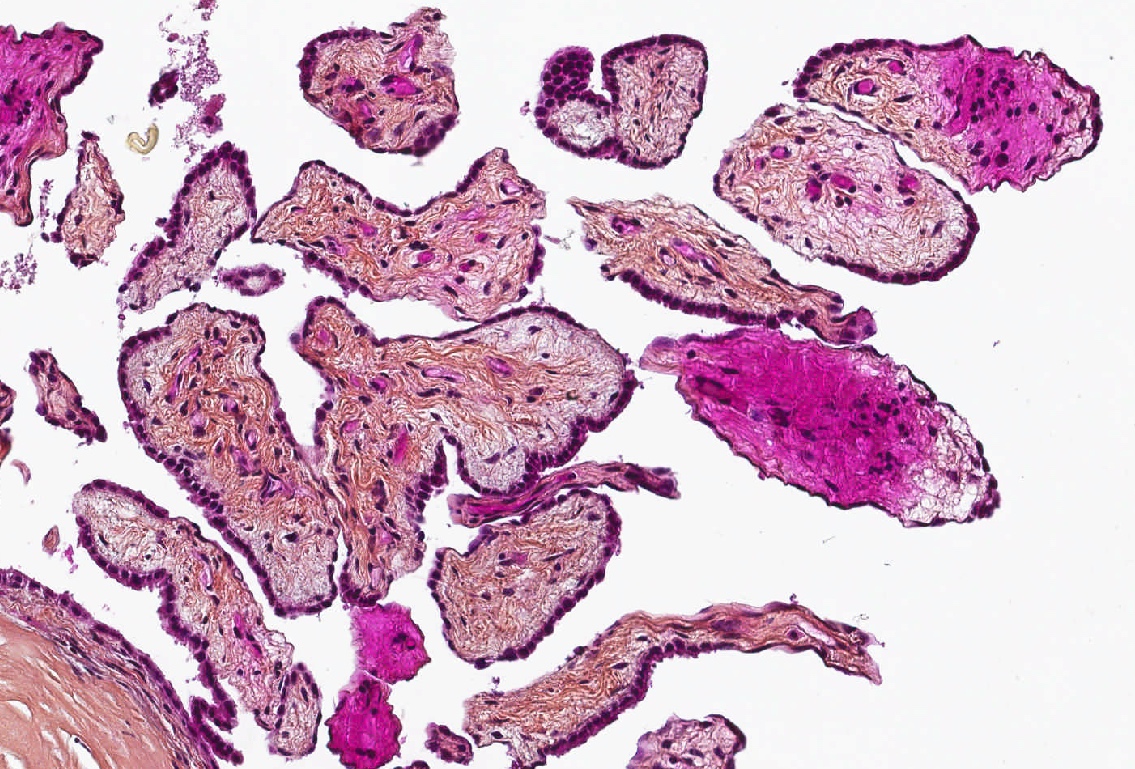
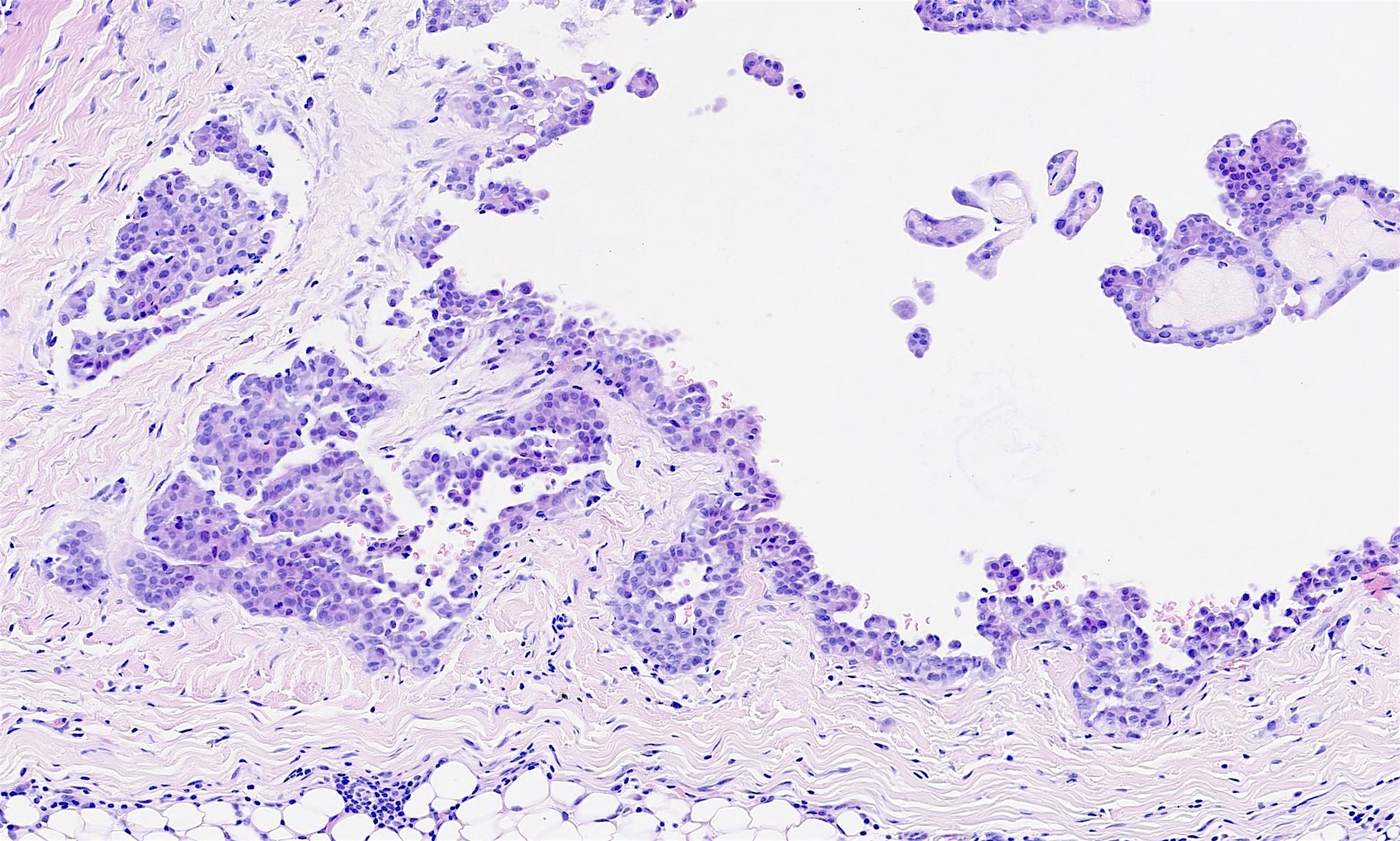
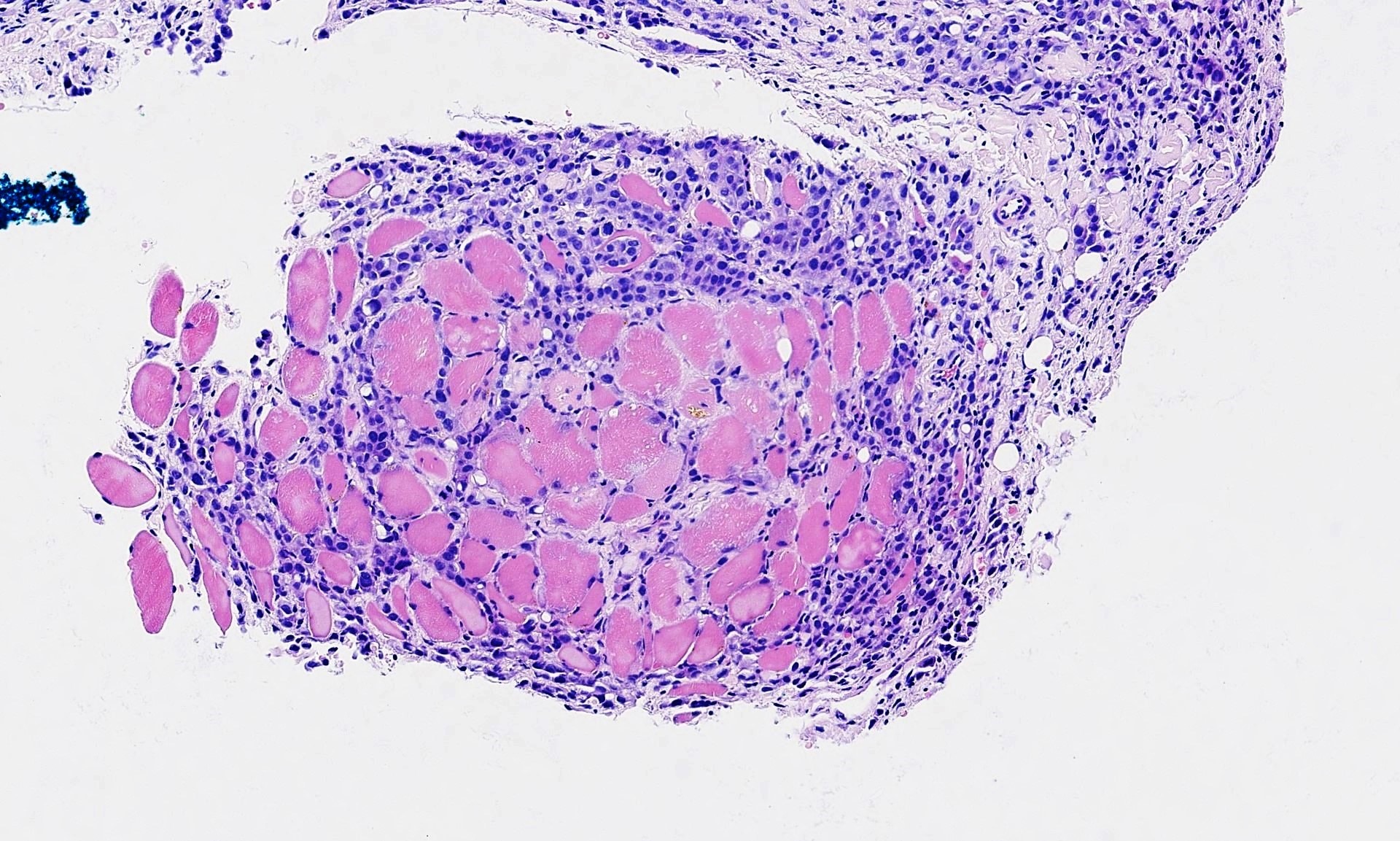
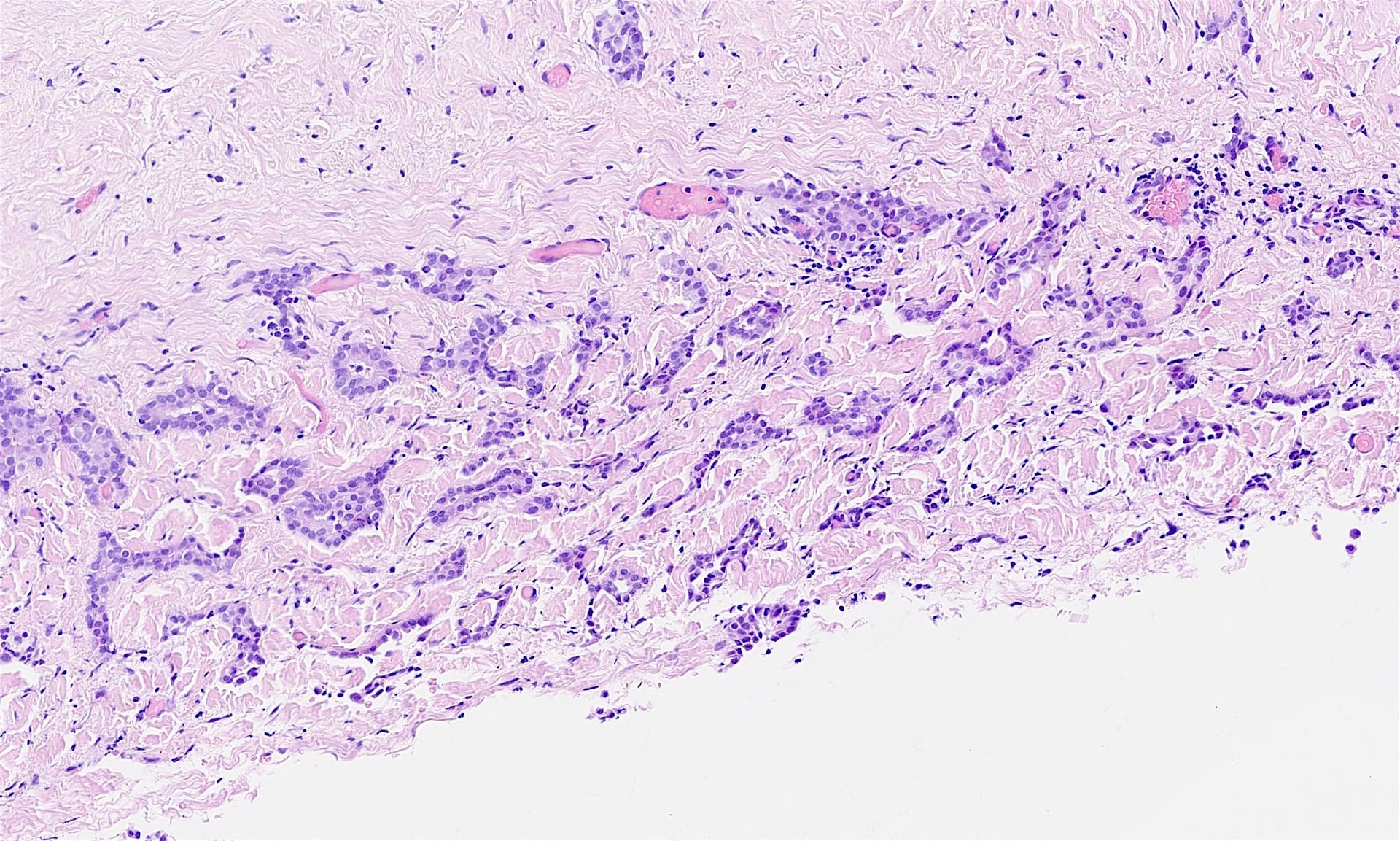
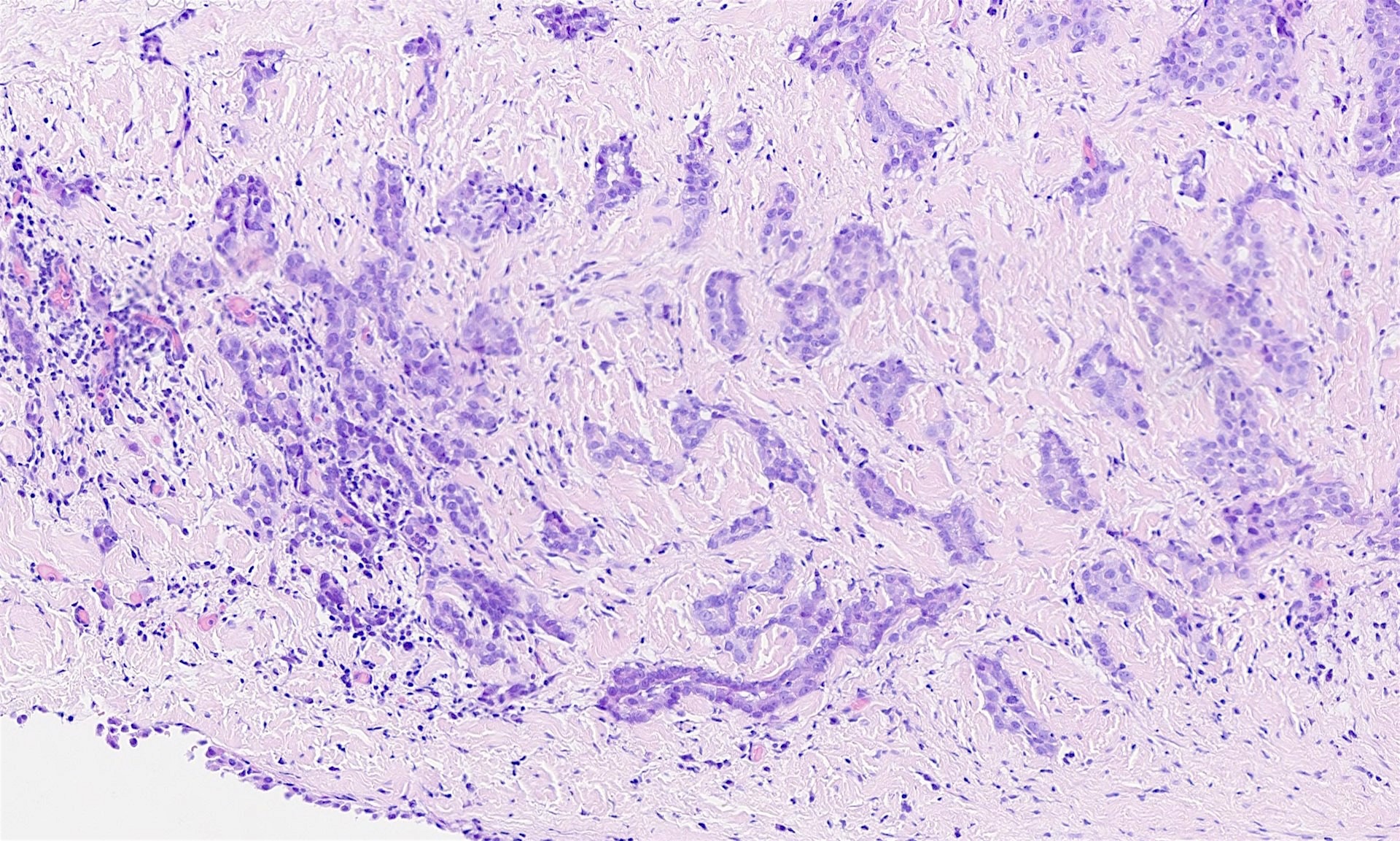
Gross description
- Small, round to oval, well circumscribed tumor
- Cut surface may have small cystic spaces
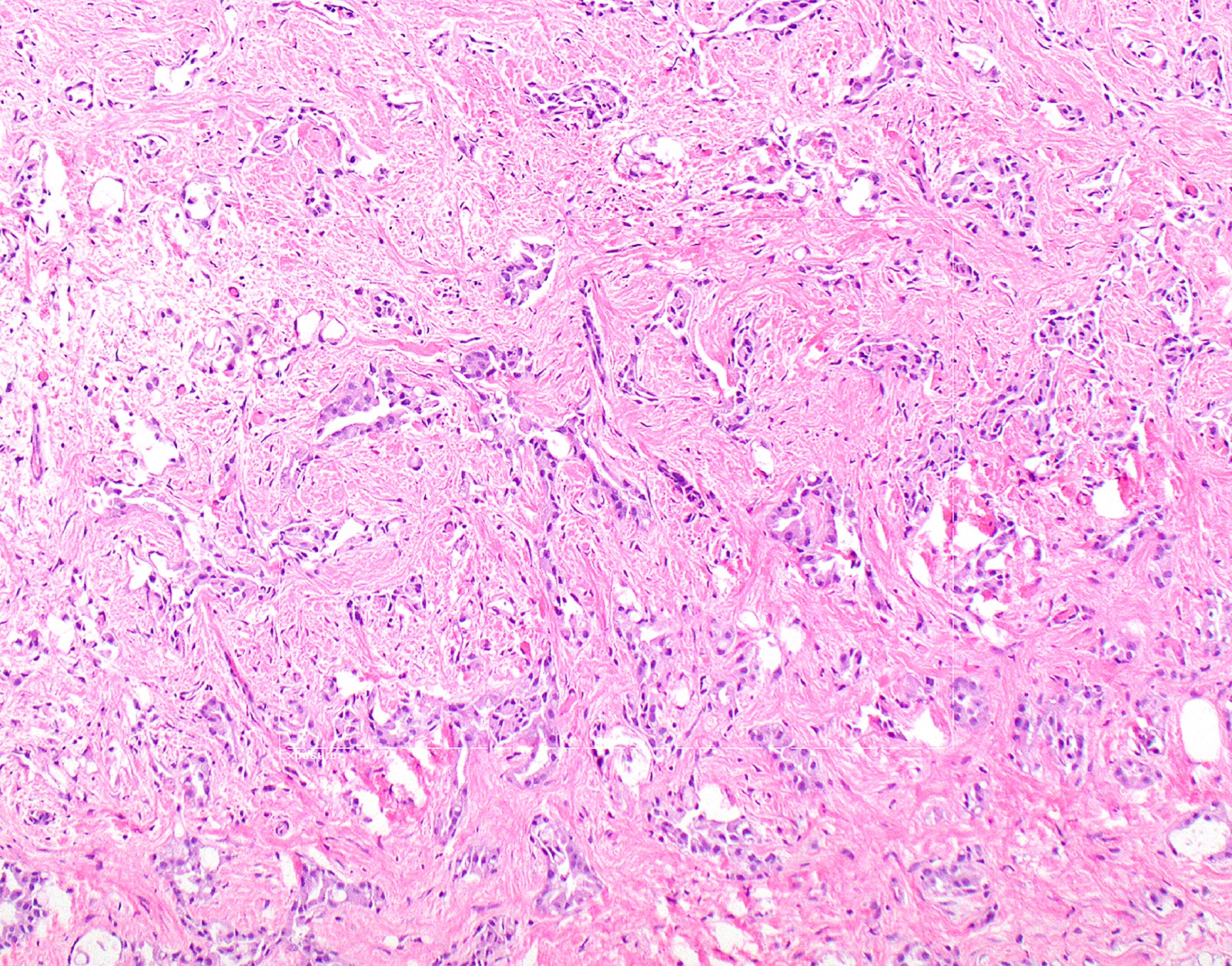
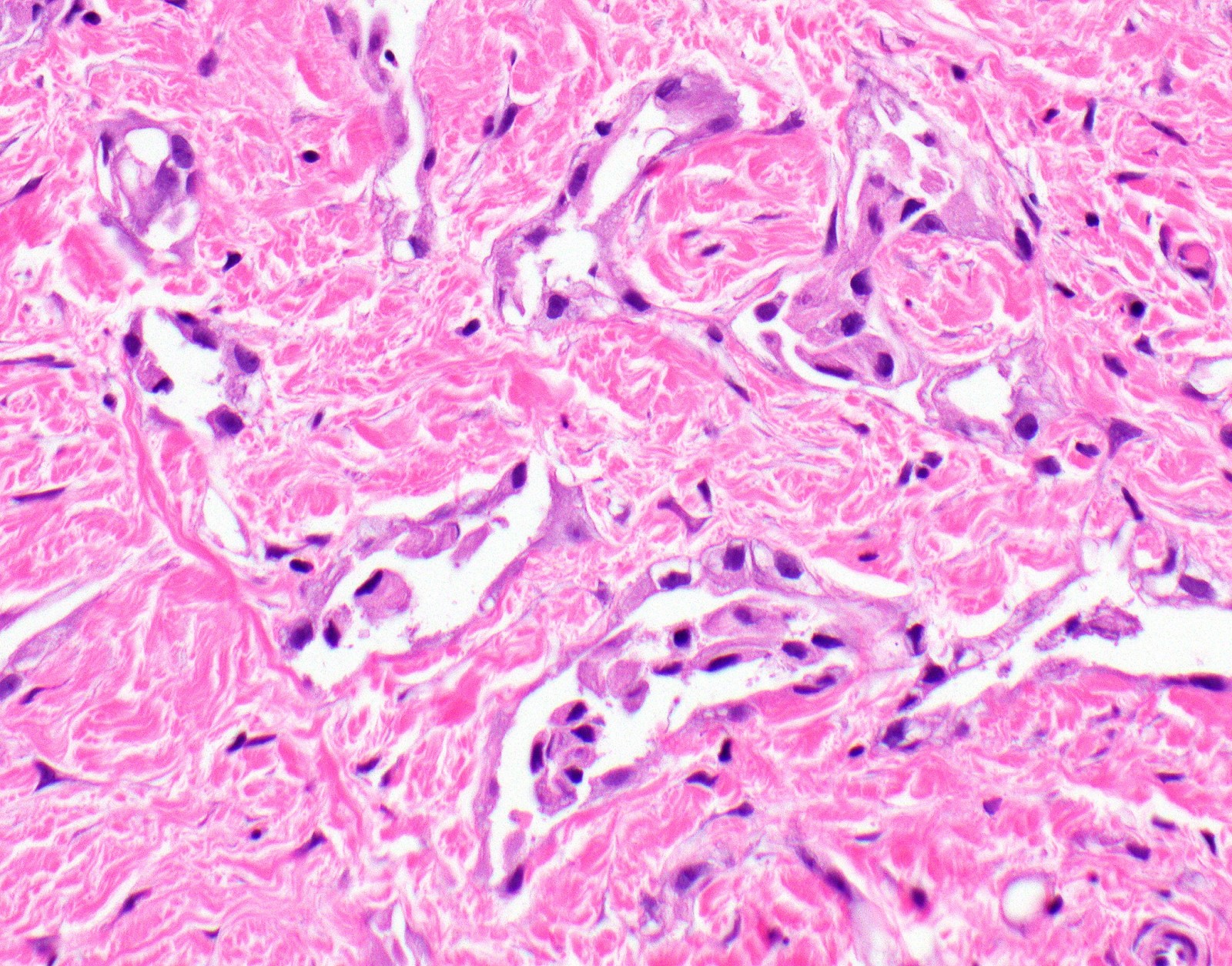
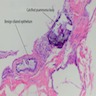
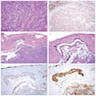
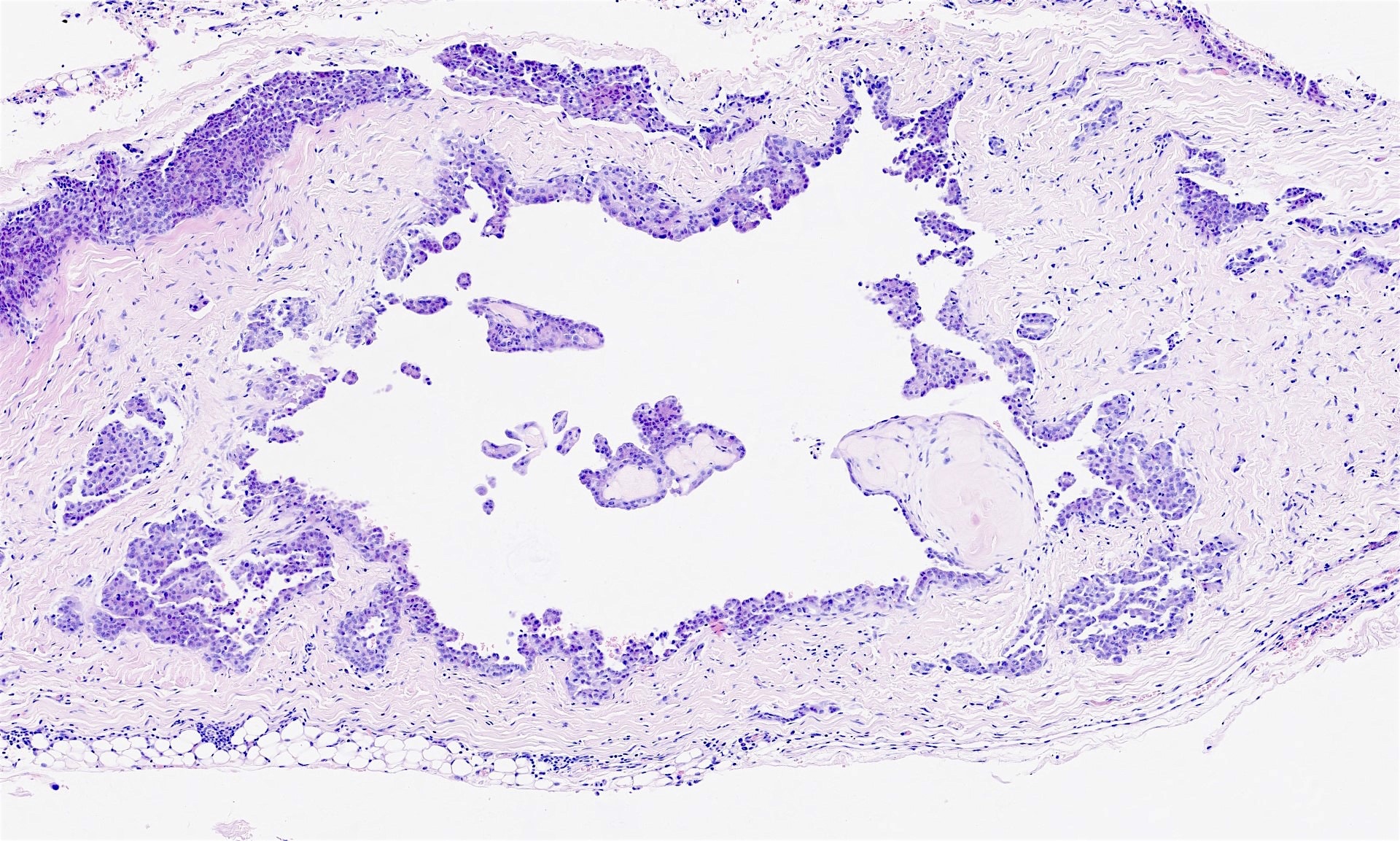
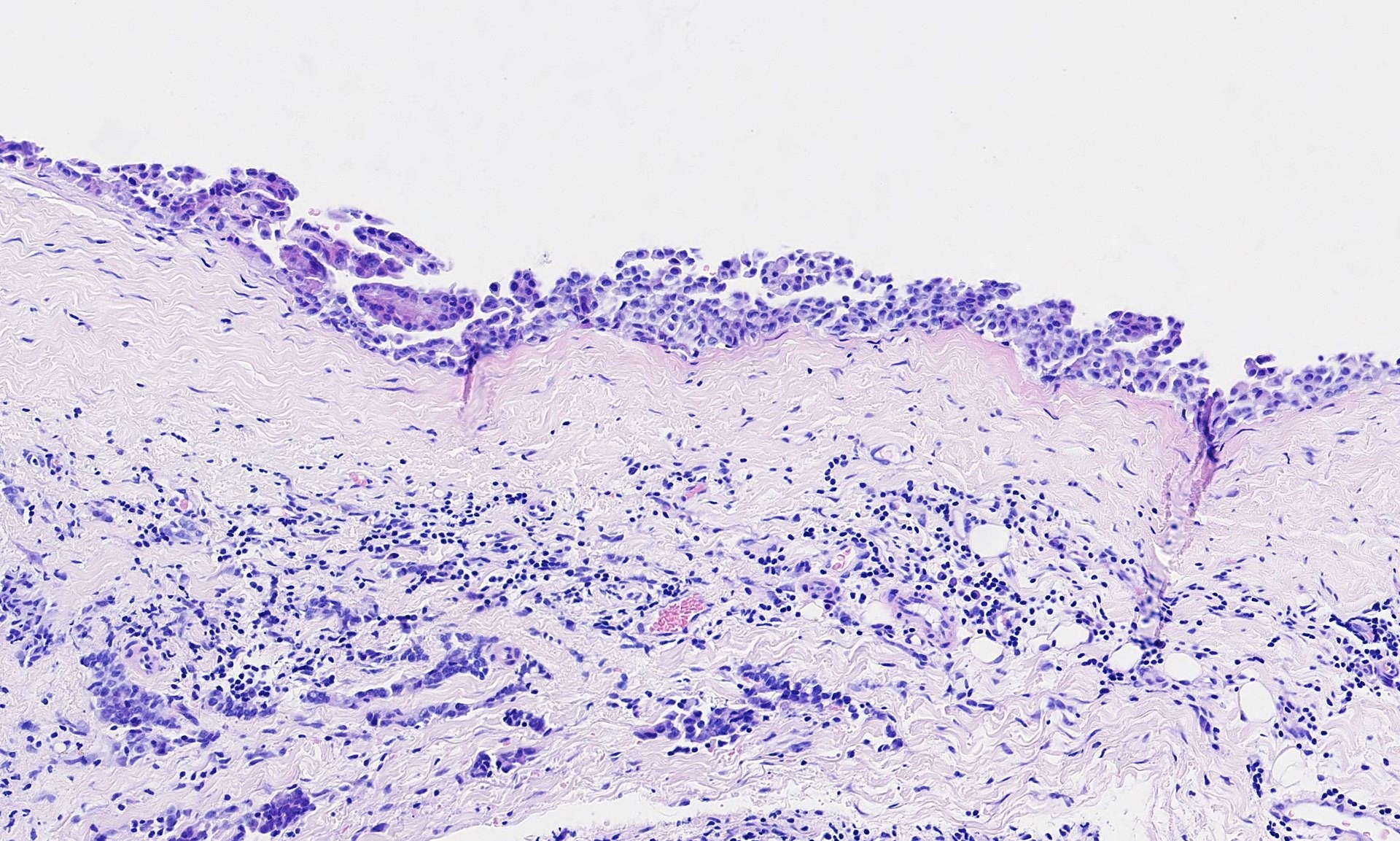
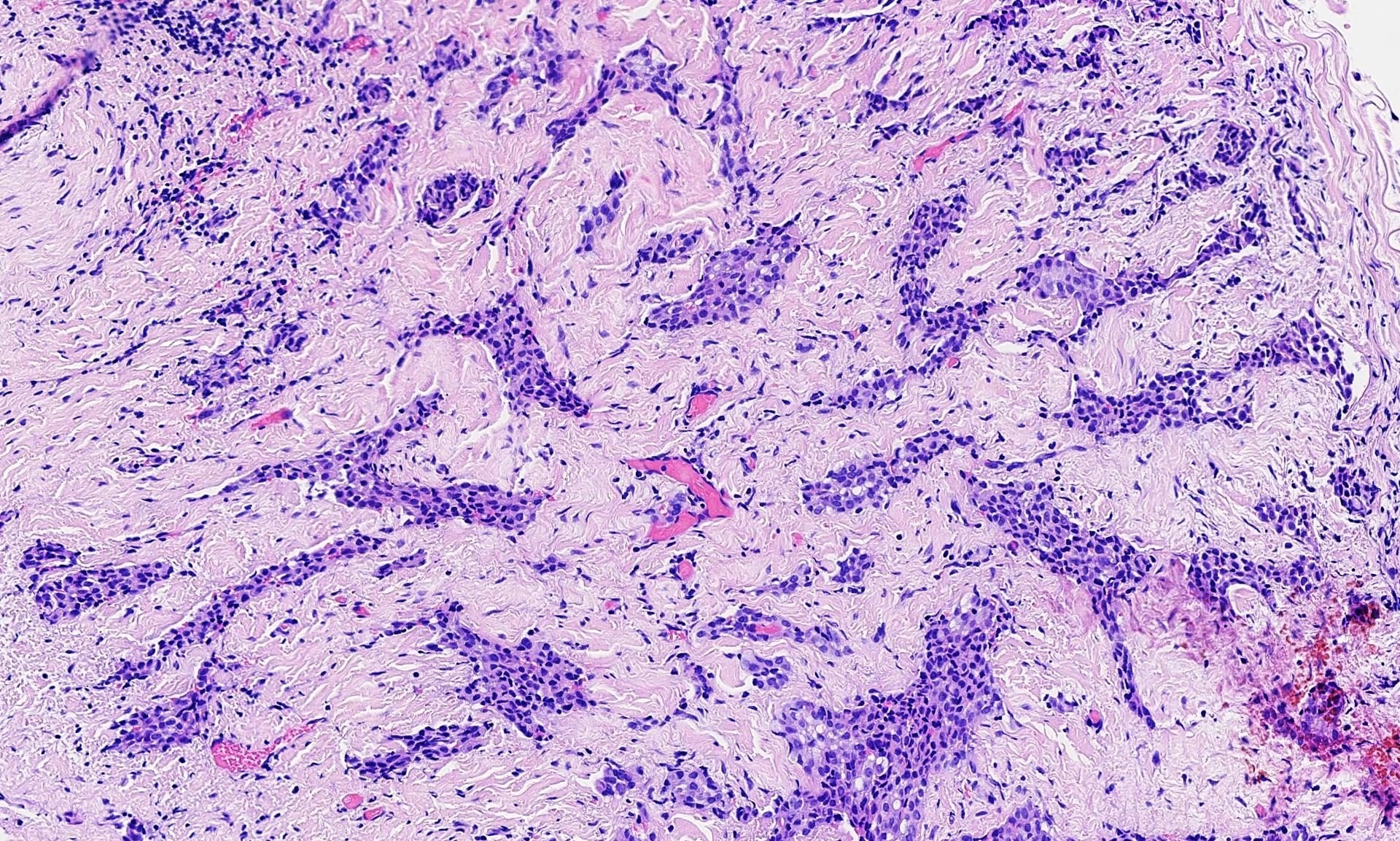
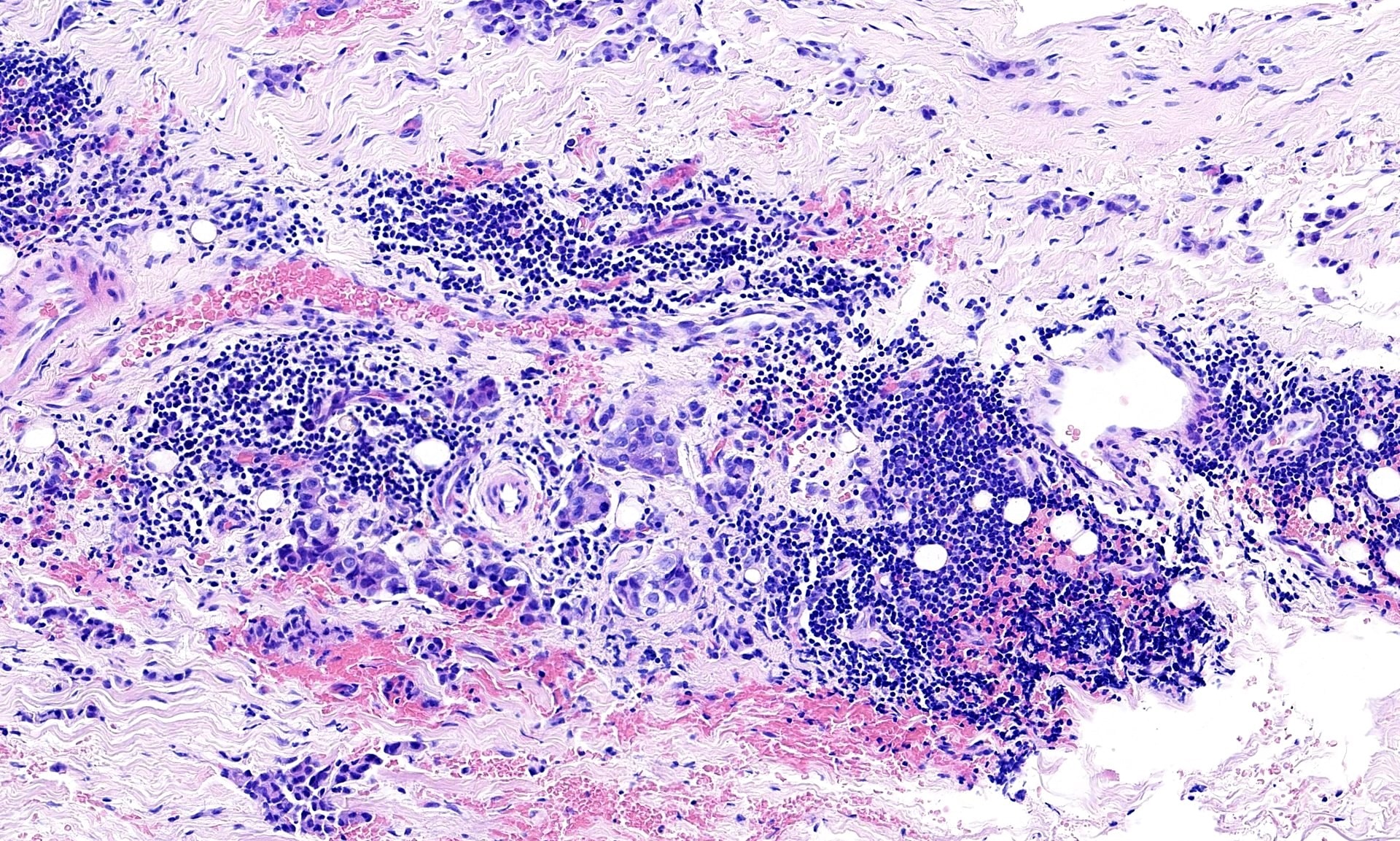
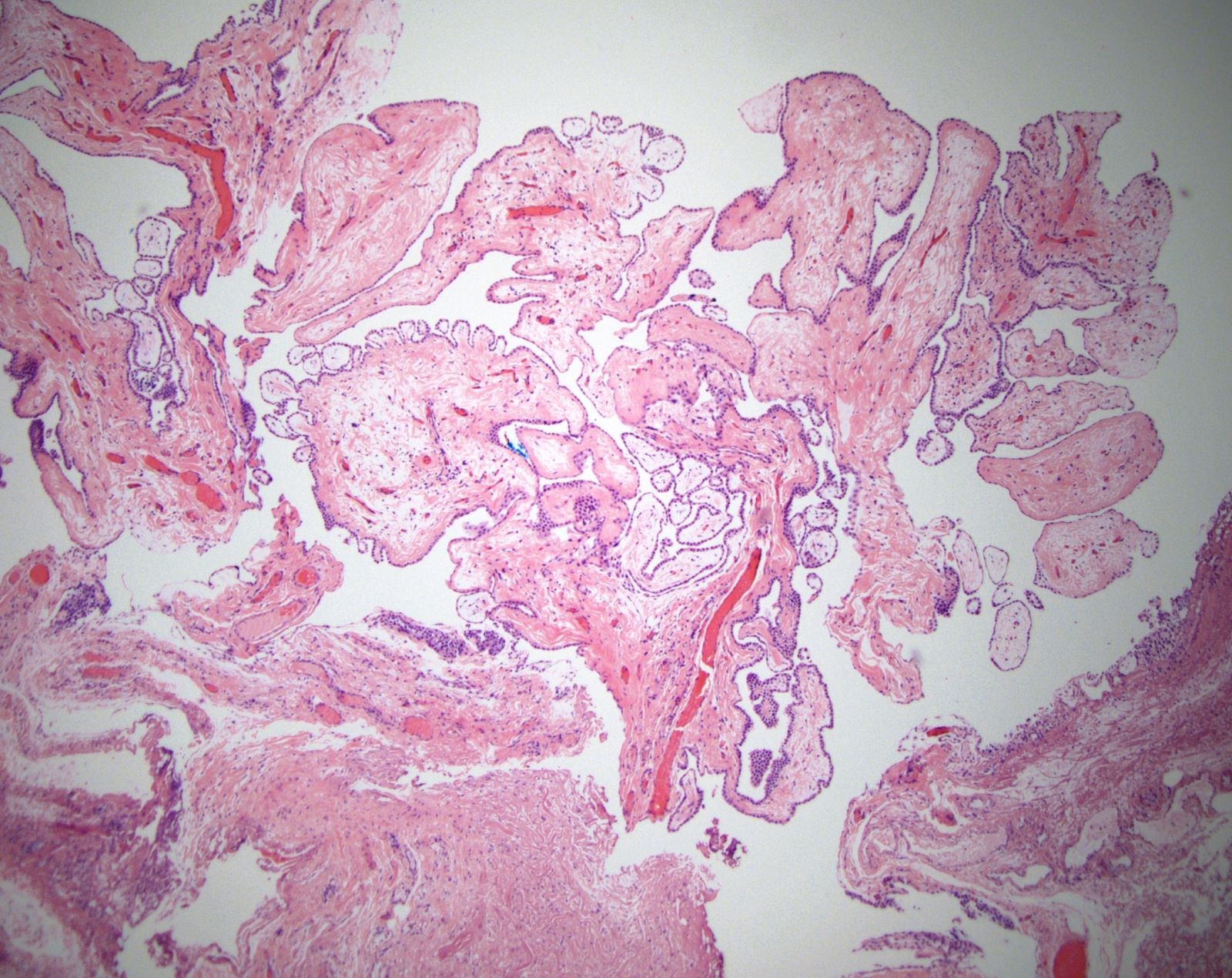
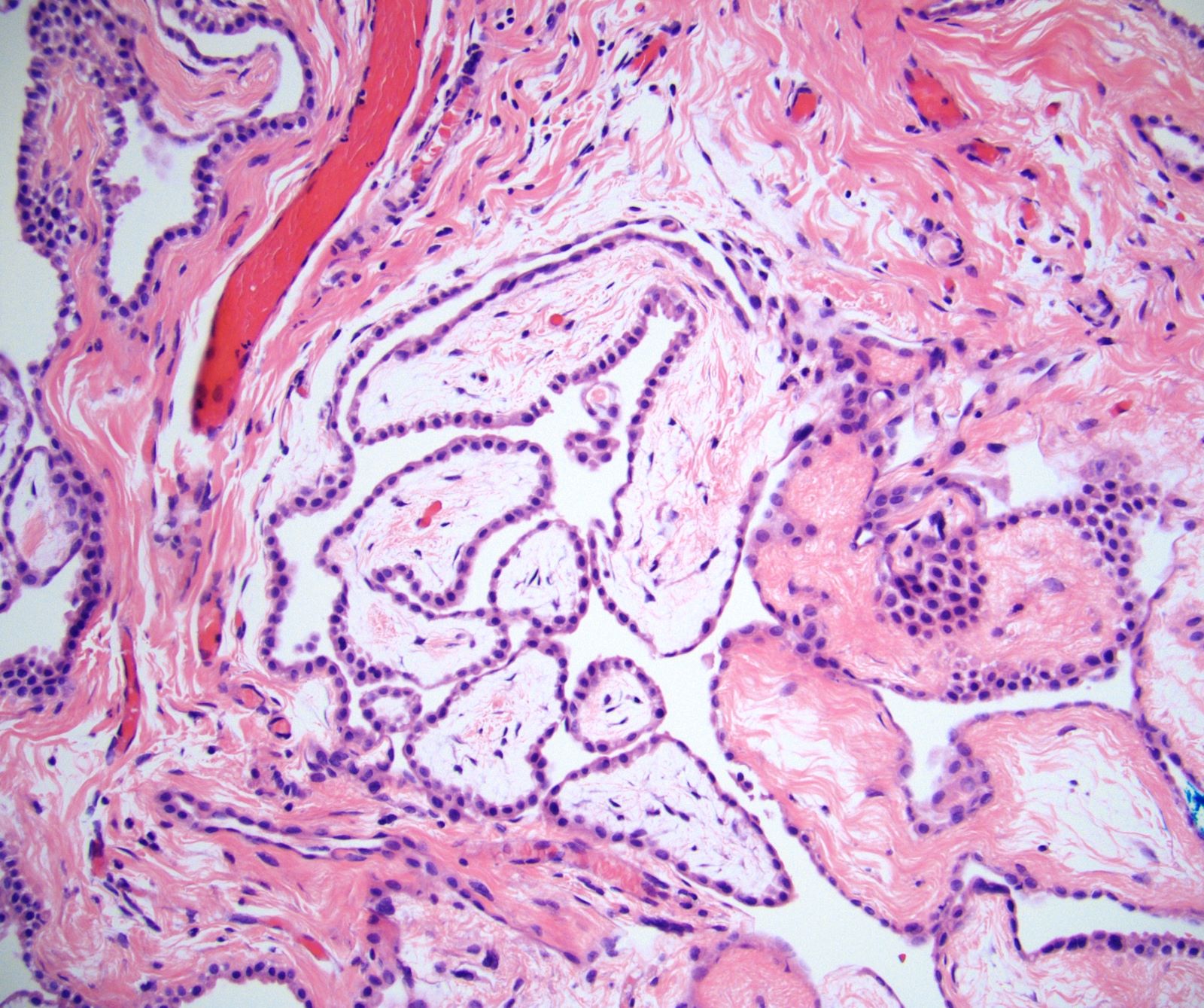
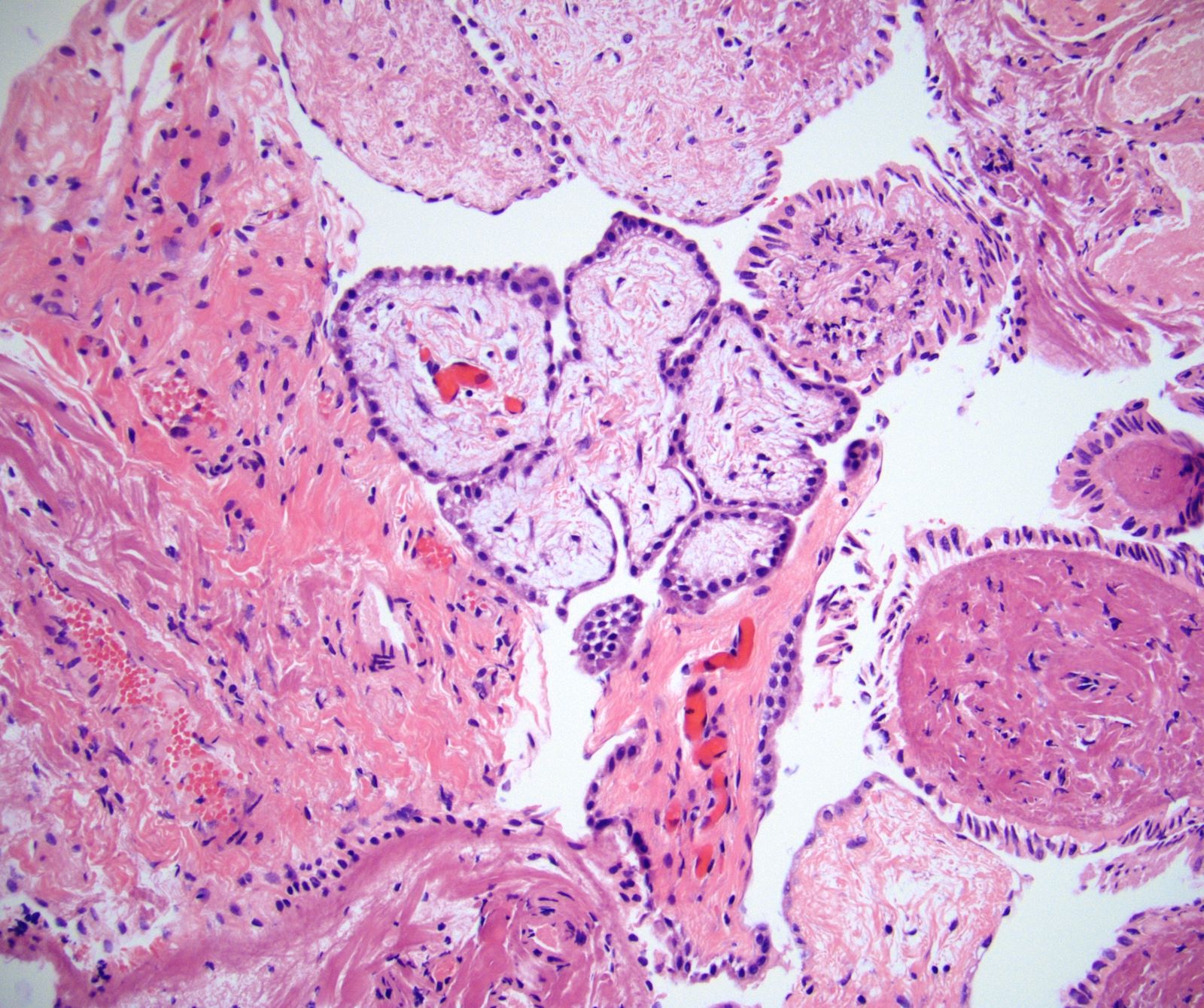
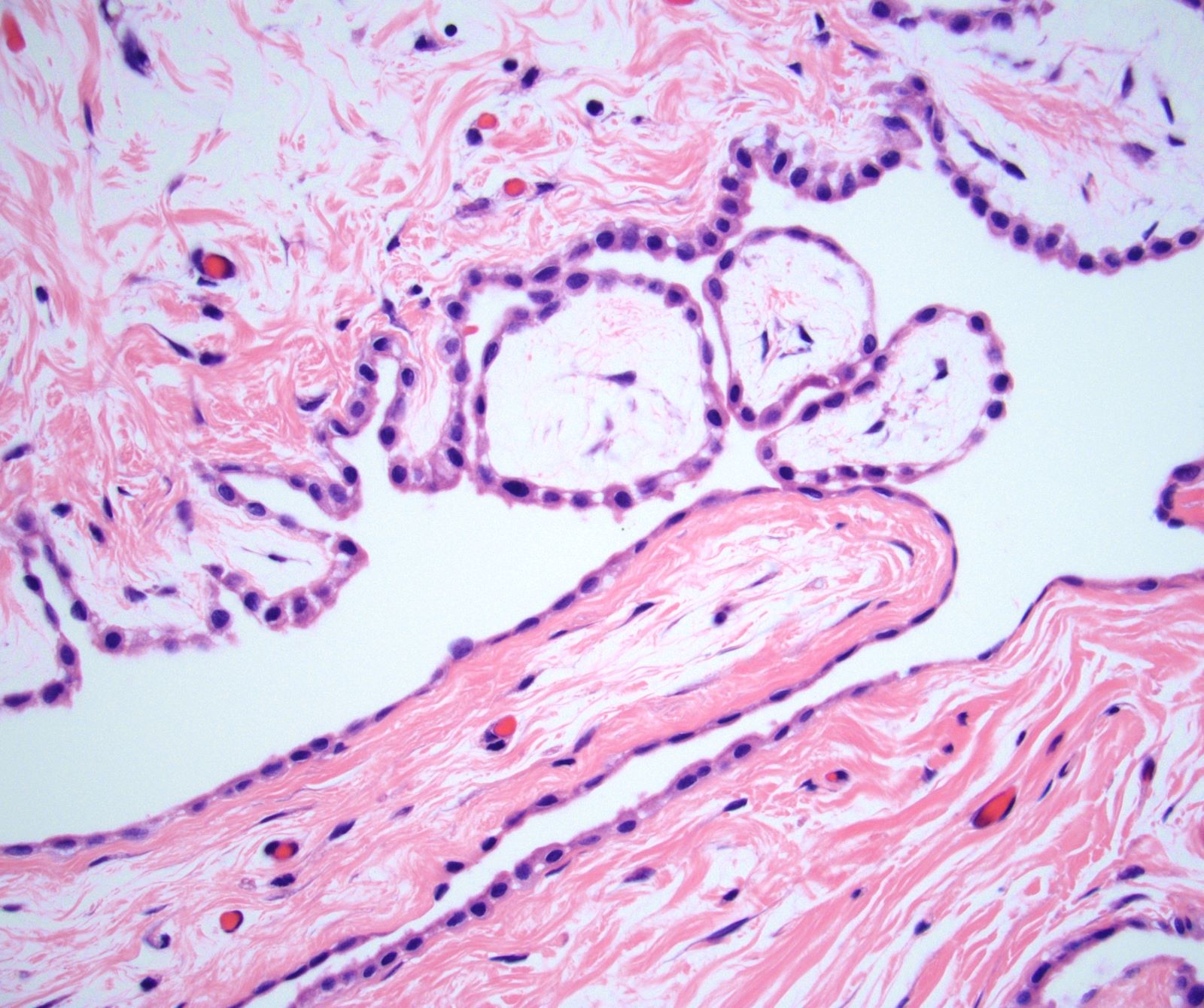
Microscopic (histologic) description
- Composed of clefts and spaces lined by cuboidal, low columnar or flattened epithelial-like cells
- Surrounded by connective tissue that varies from loose and edematous to dense and hyalinized
- The epithelial-like cells may exhibit marked vacoulation, which in some cases may contain weakly basophilic material
- A spotty lymphoid aggregate may be a low power clue to the diagnosis
- Distinctive thread-like bridging strands crossing the tubular spaces are useful diagnostic features
- Morphologic patterns:
- Adenoid
- Angiomatoid
- Cystic
- Glandular
- Solid
- Tubular
- Plexiform
- Canalicular
- Similar appearance to appearance found within other locations
- Relatively well demarcated, nonencapsulated solid aggregates of cells forming cleft-like spaces lined by low columnar to cuboidal flattened epithelial-like cells
- Cells often surrounded by stroma that ranges from dense / fibrotic to loose / edematous
- Epithelial-like cells may display marked vacuolization, signet ring cell-like appearance or oxyphilic cytoplasm
Microscopic (histologic) images
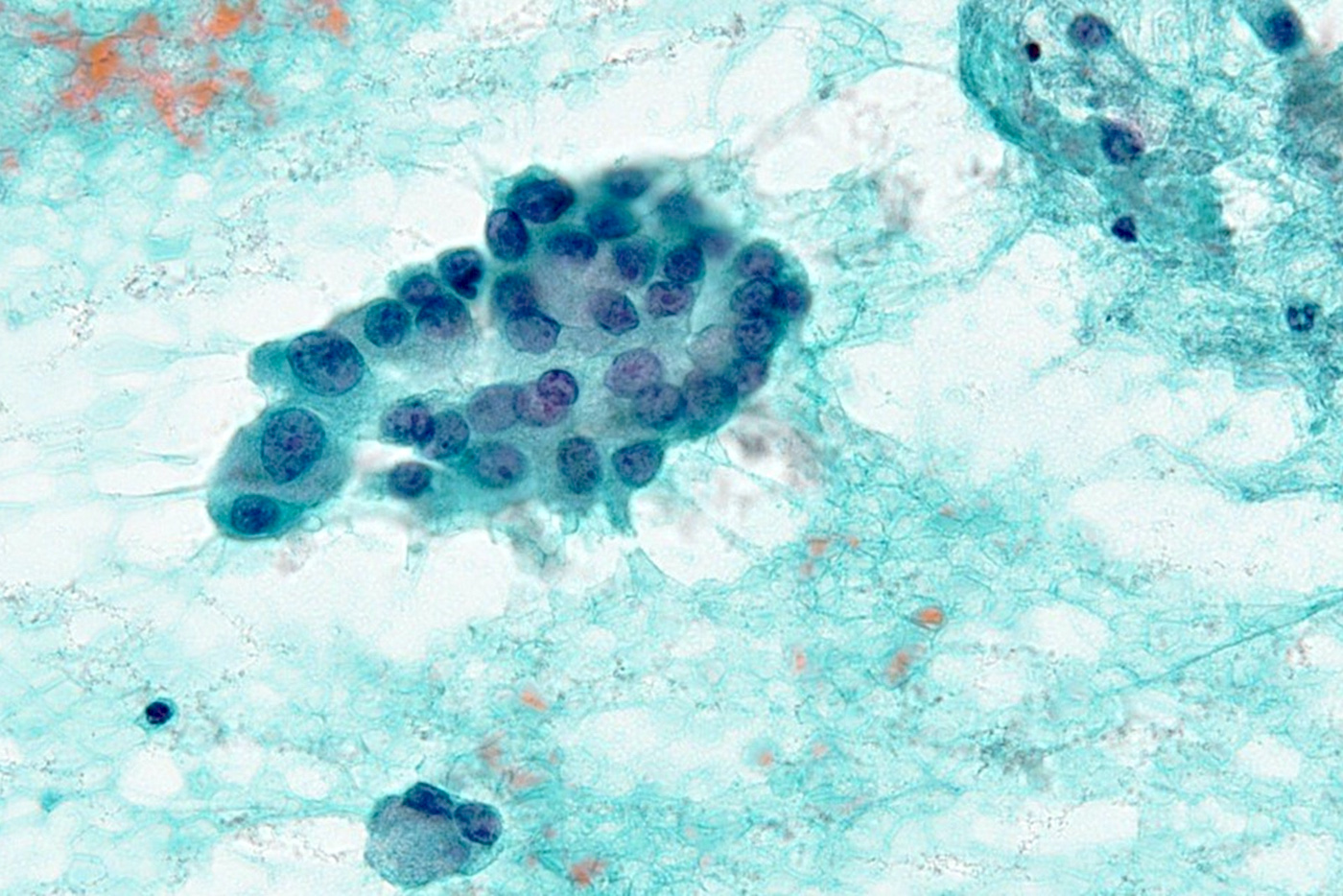
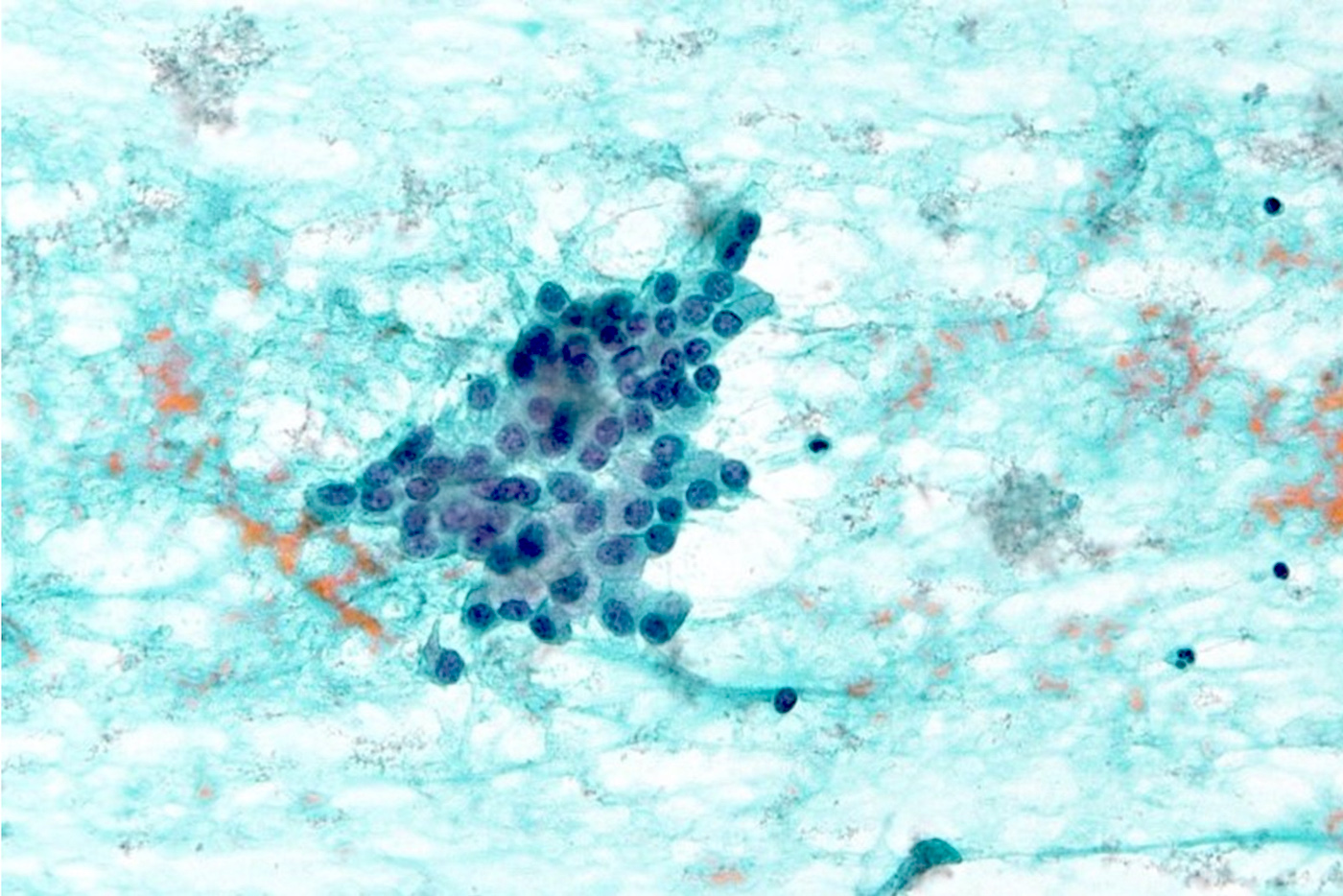
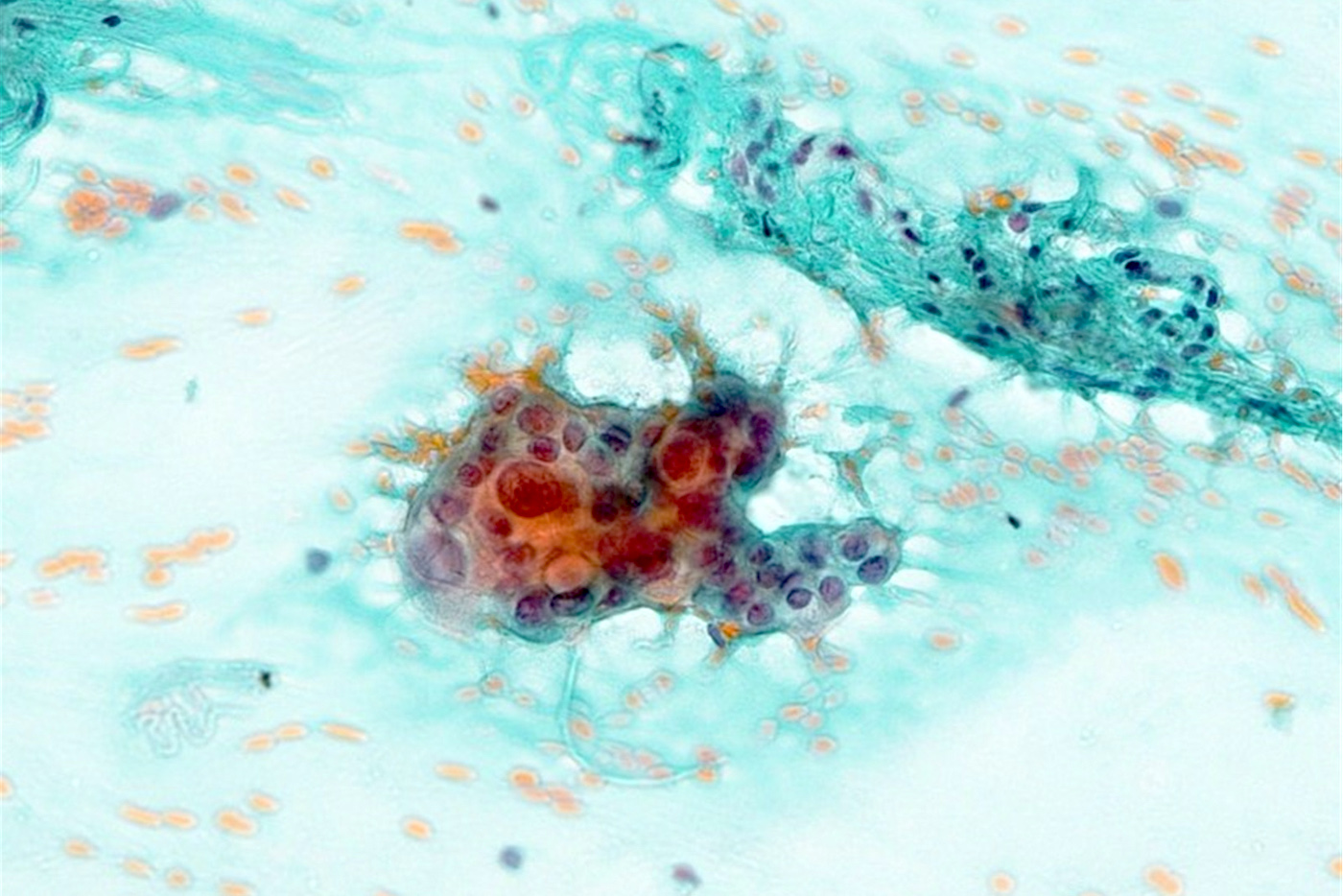
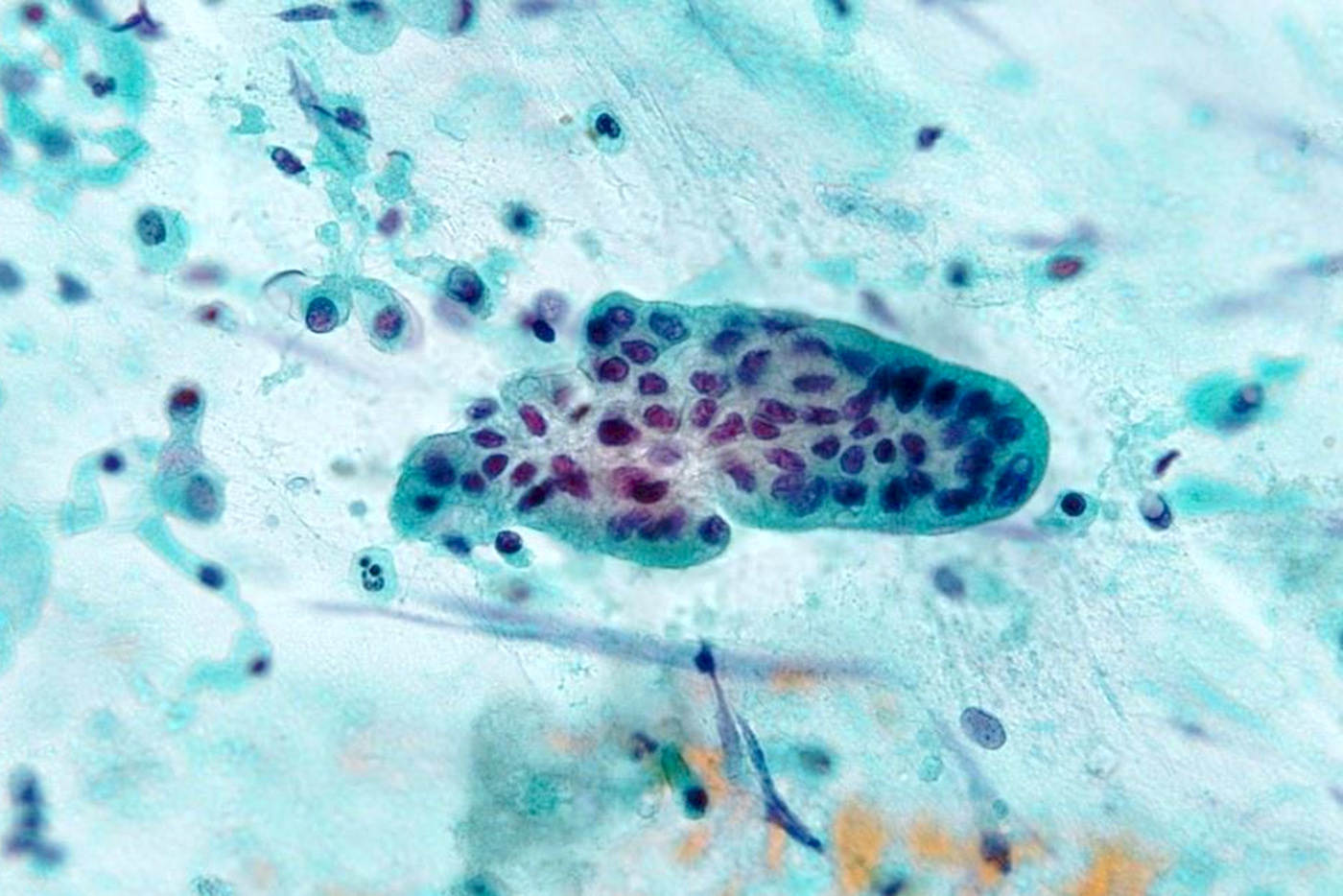
Cytology description
- Smears are moderately cellular with sheets of monotonous round to oval cells showing indistinct cell borders and moderate to abundant pale cytoplasm with vacuolations
- Nuclei are eccentric in location, but regular with inconspicuous nucleoli
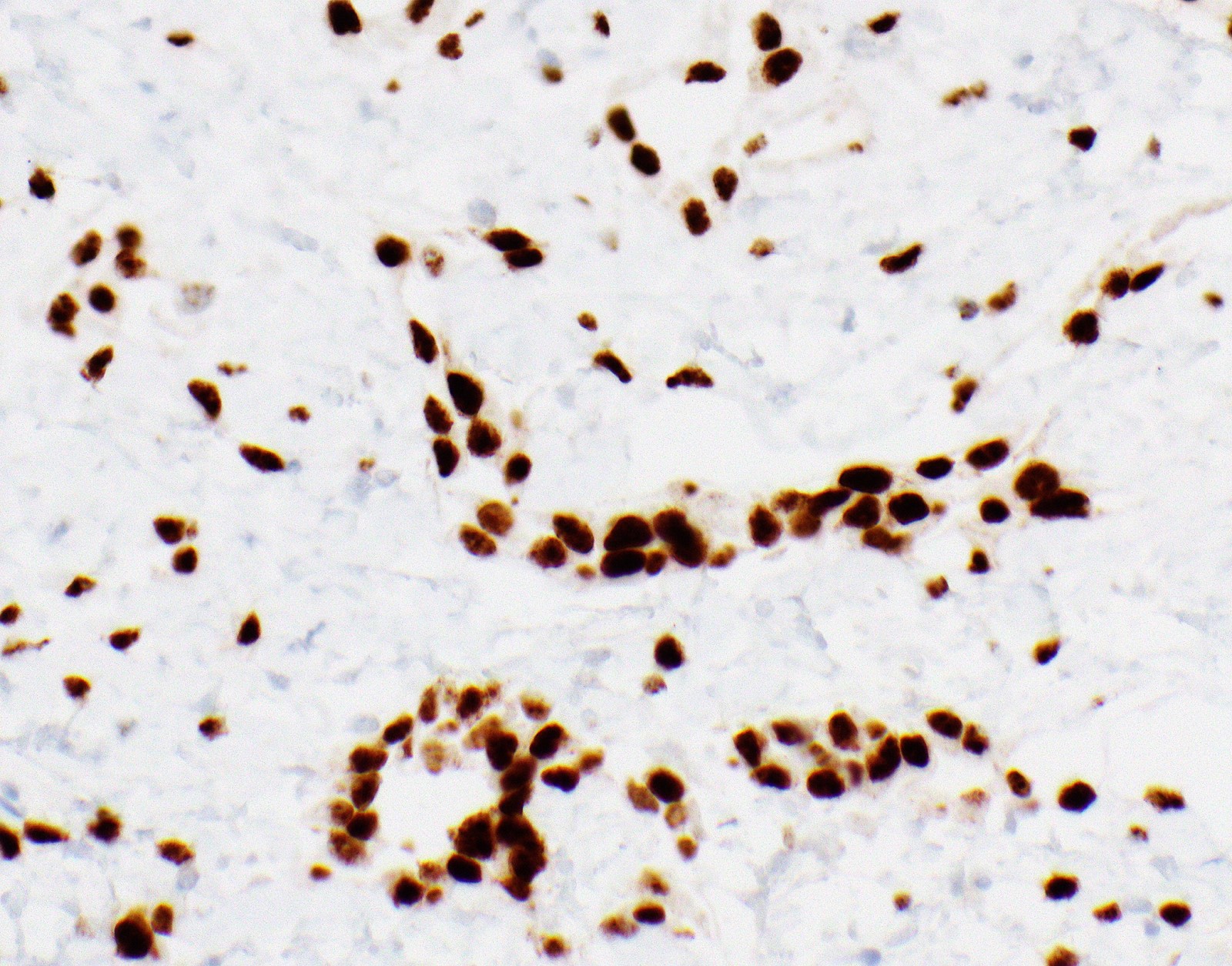
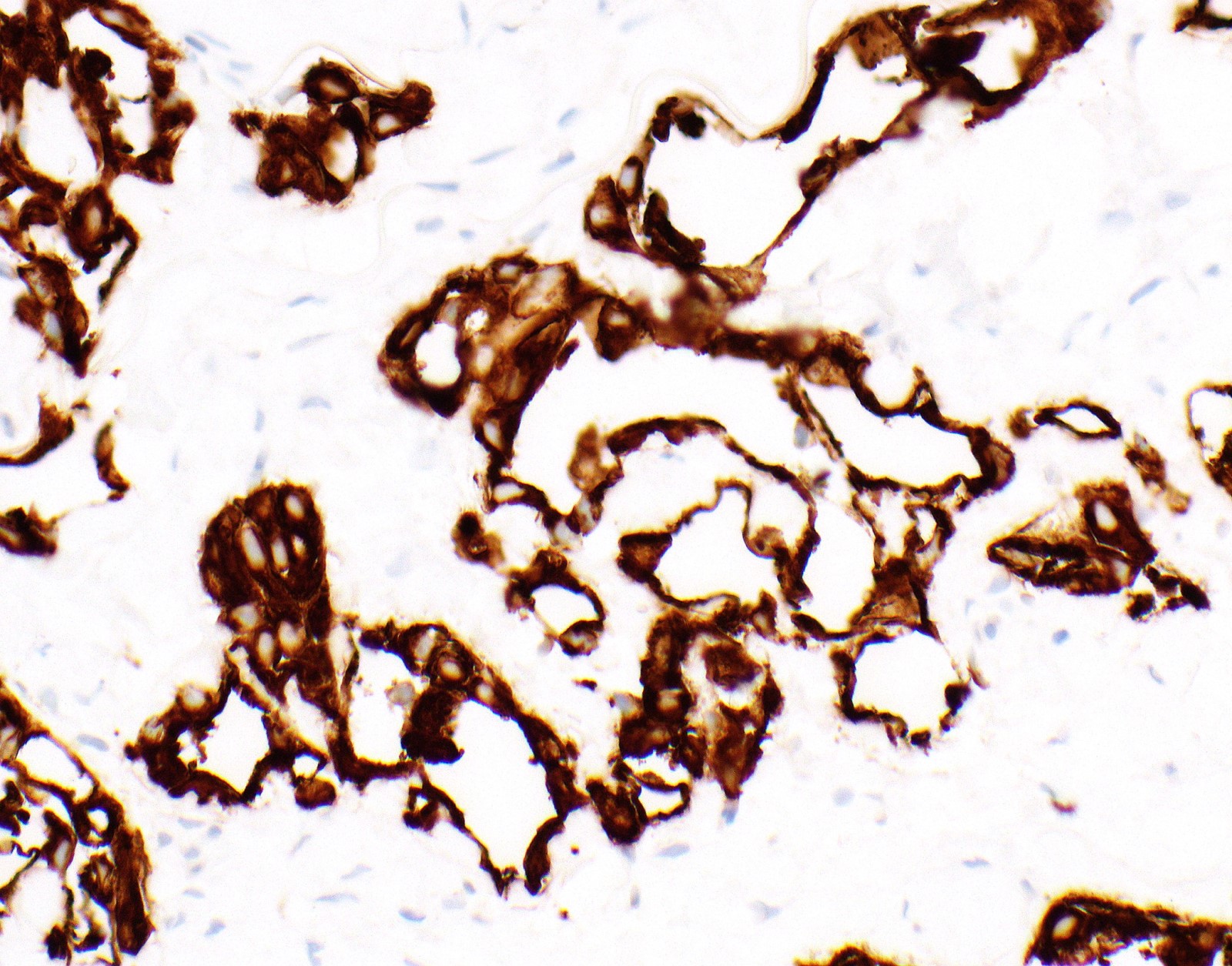
Positive stains
- Low molecular weight cytokeratin
- CK 5/6
- Calretinin
- WT1
- D240
- Alcian Blue (epithelial cells and within cleft-like spaces)
- Weak PAS
Electron microscopy description
- No microvilli, no bundles of cytoplasmic filaments, no tight junctional complexes, no intercellular spaces
Molecular / cytogenetics description
- No specific genetic abnormality has been identified
Differential diagnosis
- Epithelioid hemagioendothelioma: positive for CD34 and factor VIII
- Leiomyoma
- Lymphangioma: positive for CD31, CD34 and factor VIII
- Malignant mesothelioma / adenomatoid-like mesothelioma: rare, marked cytologic atypia with features of invasion
- Metastatic adenocarcinoma: most likely from GYN origin
- Mucinous carcinoids: positive for neuroendocrine markers
- Signet ring cell carcinoma (Krukenberg tumor): positive for mucicarmine, EMA, BerEP4, cytological atypia, brisk mitosis
- Yolk sac tumor: AFP+, nuclei larger with prominent nucleoli and brisk mitotic activity
Additional references
Anatomy, history, grossing & features to report
Table of Contents
Anatomy | Drawings | Histology | Mesothelial cells | Connective tissue cells | Black spots | Types of specimen | Surgical procedures definition | Grossing biopsy | Grossing pleurectomy | Features to reportAnatomy
- Lungs are surrounded by visceral pleural, a delicate serous membrane arranged as a closed invaginated sac
- Inner chest cavity is lined by parietal pleural membrane
- Visceral and parietal pleura define the pleural space / cavity, which normally has minimal volume, unless lungs collapse or air / fluid collects between the 2 layers
- Only minimal contact between right and left pleural sacs
- Regional lymph nodes are internal mammary, intrathoracic, scalene and supraclavicular
Histology
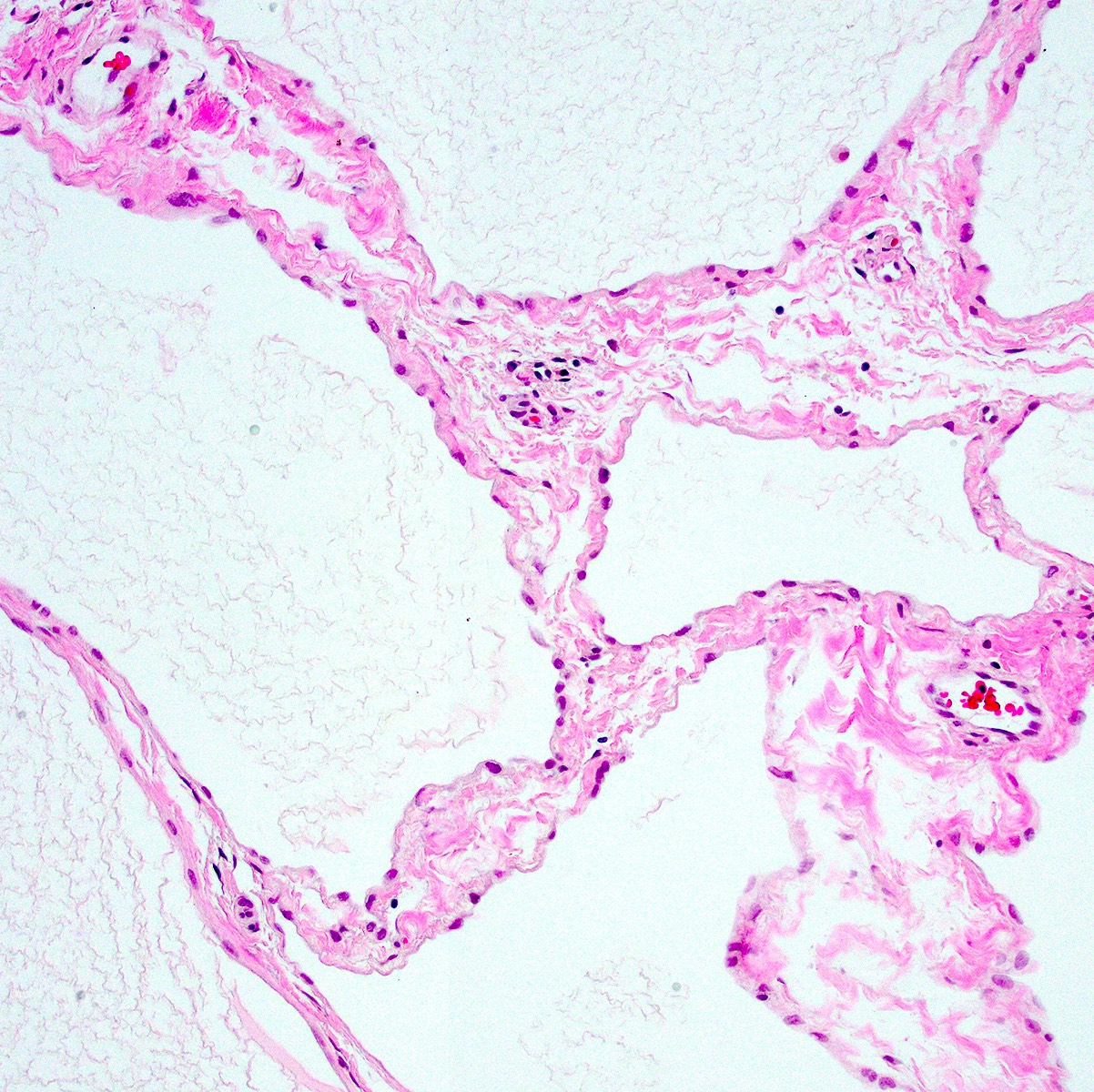
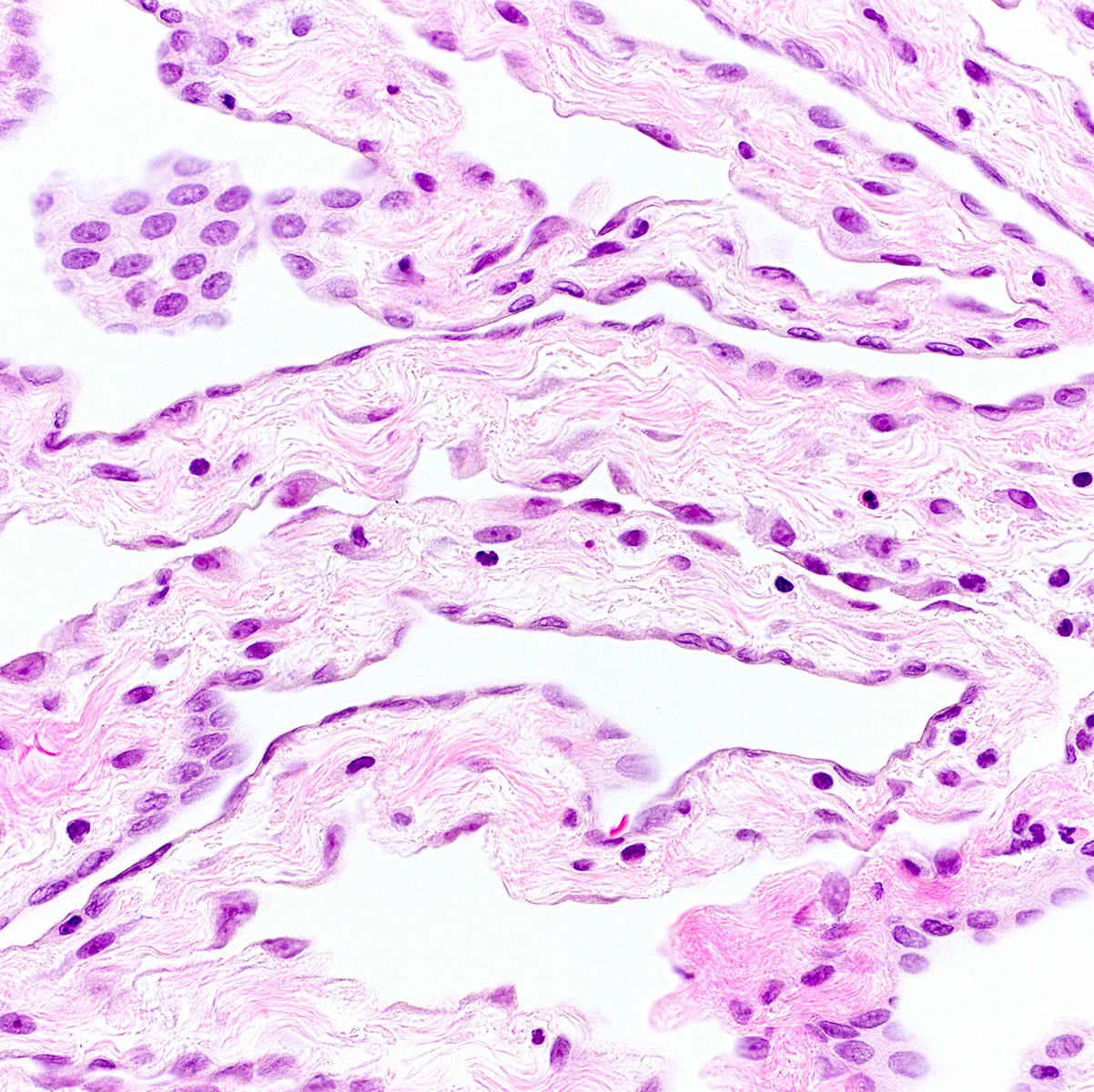
- Lined by mesothelial cells overlying vascularized connective tissue
- Mesothelium provides smooth, low friction surface to facilitate the gliding motion of lungs in pleural cavity, heart in pericardial cavity, viscera in abdominal cavity
- Gliding facilitated by numerous surface microvilli, thick glycocalyx, secretion of hyaluronic acid and other glycosaminoglycans
Mesothelial cells
- Microscopic (histologic) description
- Monolayer of flat or low cuboidal cells with bland and uniform nuclei, fine delicate chromatin, inconspicuous nucleoli
- In fine needle aspirates, have well defined cytoplasm and distinct cell borders
- Positive stains: keratin
- Electron microscopy description: apical tight junctions, desmosomes, surface microvilli, cytoplasmic tonofilaments in bundles
Connective tissue cells
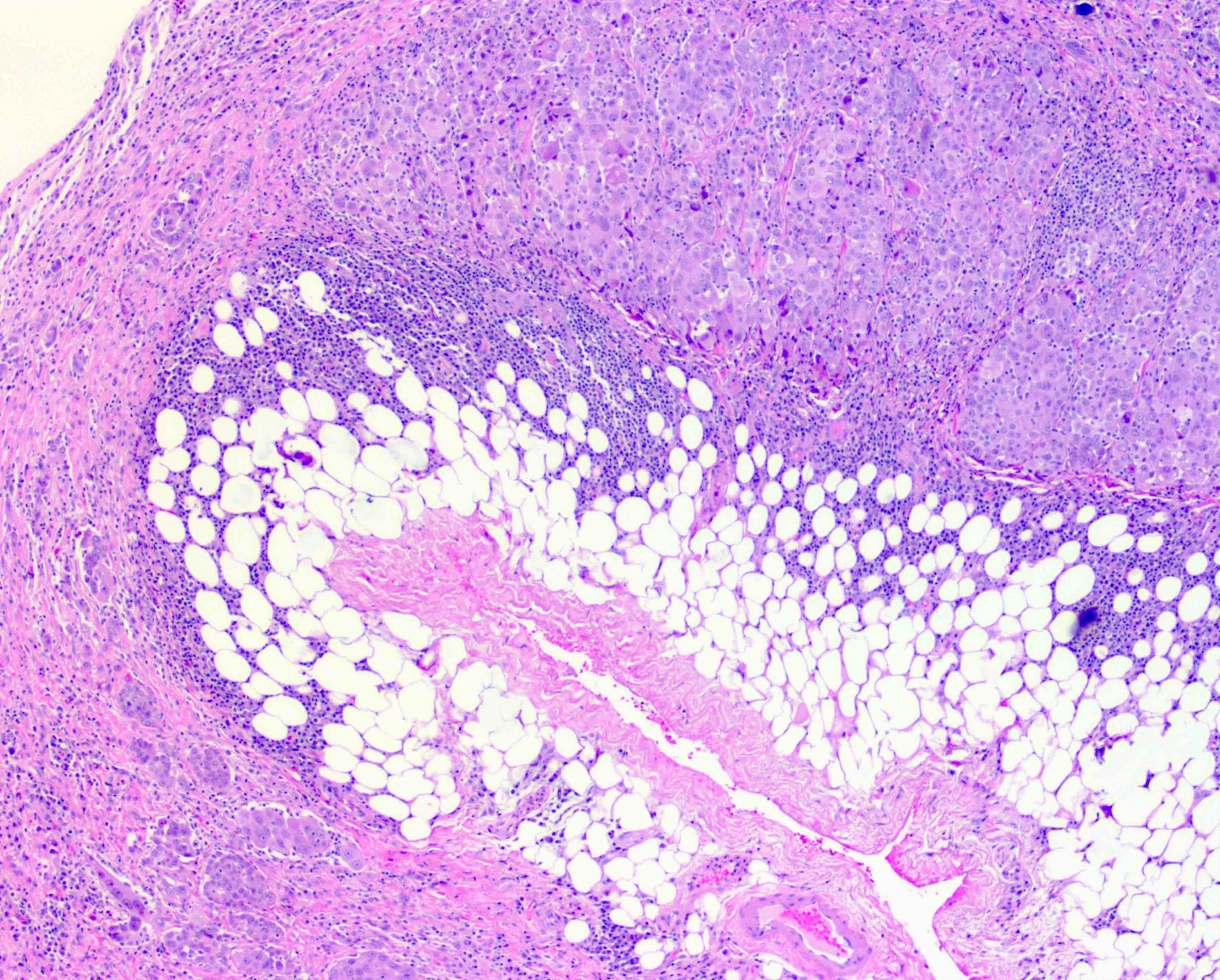
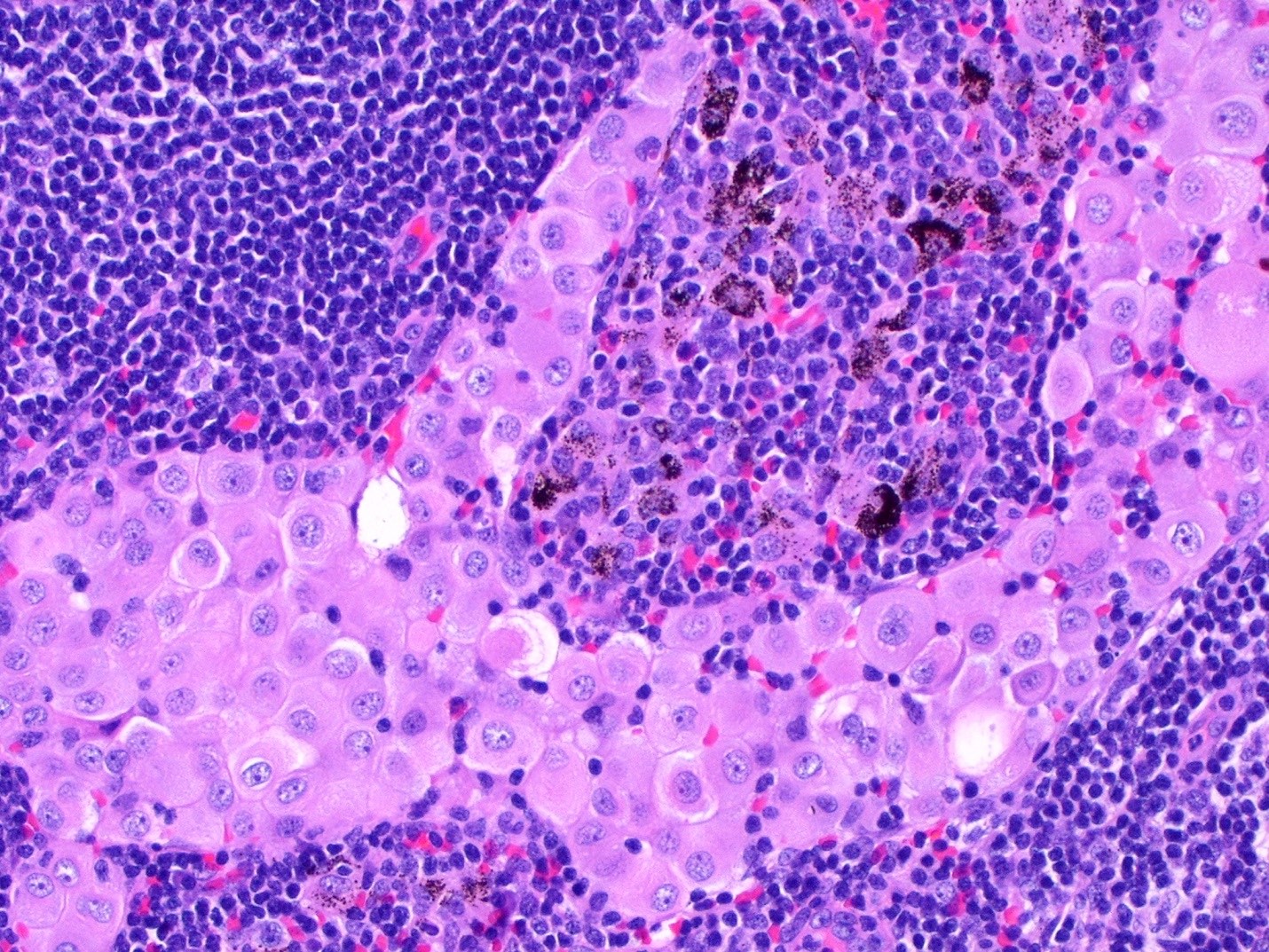
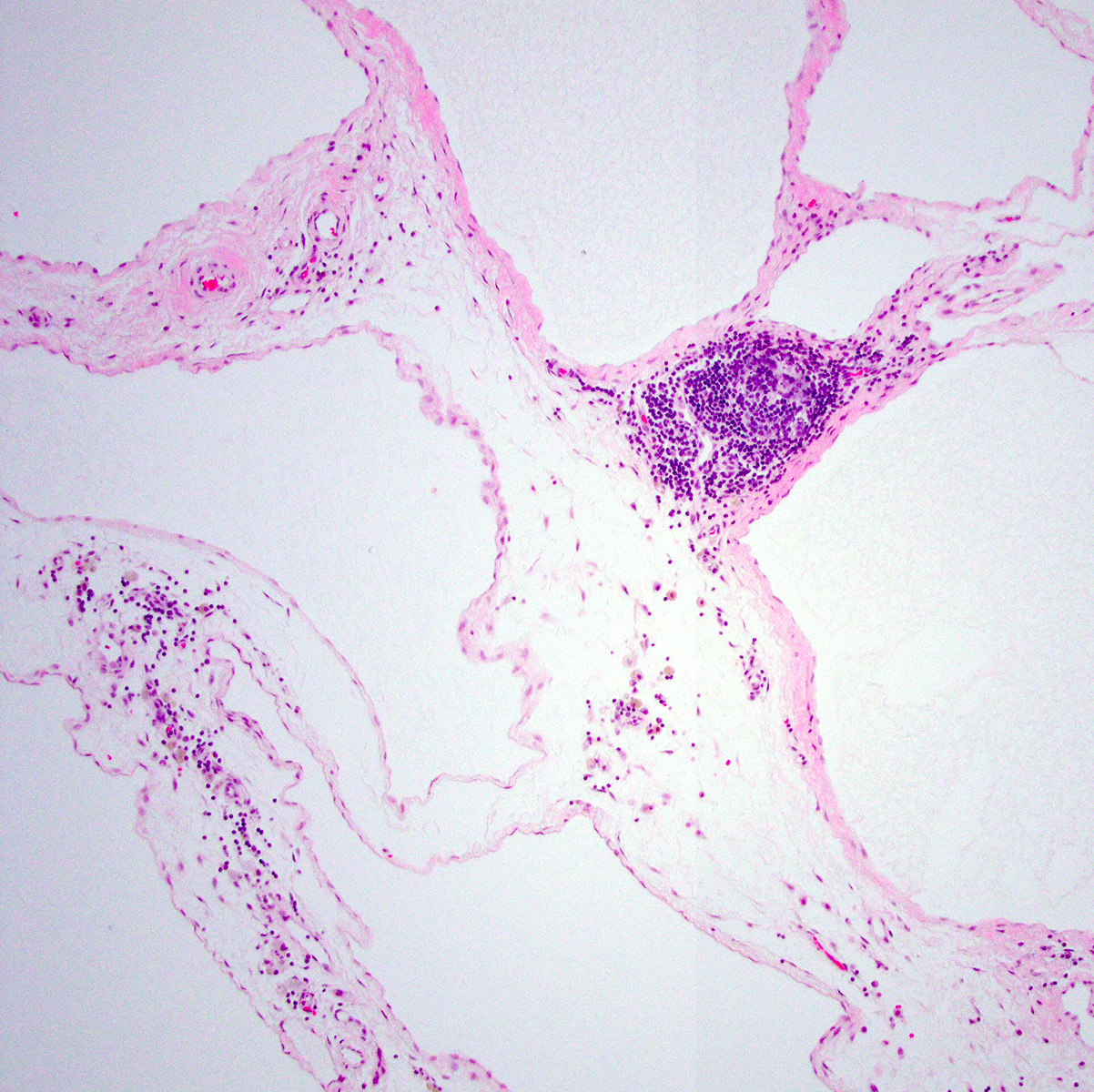
Black spots
- Carbonaceous / anthracotic pigments in parietal pleura
- Present in > 90% of urban dwellers in Belgium at autopsy
- Not related to hyaline pleural plaques (Am J Surg Pathol 2002;26:1198)
- Associated with lymphatic drainage
- Microscopic (histologic) description: deposits of opaque particles (intra or extracellular) of various sizes under an intact mesothelial layer, associated with chronic inflammatory cells
Types of specimen
- Biopsy
- Pleurectomy
Surgical procedures definition
- Pleurectomy / decortication with mediastinal lymph node sampling
- Complete removal of pleura and all gross tumor
- Extrapleural pneumonectomy
- En bloc resection of pleura, lung, ipsilateral diaphragm; may include pericardium
Grossing biopsy
- If received for frozen section, ensure enough lesional tissue is present
- Ask for additional tissue if tissue submitted needs to be entirely frozen
- Important because immunohistochemistry may be unreliable on previously frozen tissue
- May need to send for special studies including electron microscopy and cytogenetics
Grossing pleurectomy
- Describe dimension and number of fragments, any lesions present
- Note if pleural plaques are seen; describe
- Tumor involvement of adjacent structures - lung, diaphragm, pericardium, skeletal muscle
- Ink margins in sections closest to tumor
- Tumor
- One section per cm of tumor
- Extensive sampling if desmoplastic mesothelioma is suspected
- Additional sections of lung, if present (for asbestos fiber analysis)
- Recommended (up to 5)
- Sections for ancillary tests
- Electron microscopy, cytogenetics, etc., if necessary
- Lymph nodes
- References: NCCN: NCCN Guidelines [Accessed 23 March 2018], Lester: Manual of Surgical Pathology, 3rd Edition, 2010
Features to report
- Tumor size and location
- Histologic type
- Extent of invasion
- Surgical resection margins
- Involvement of pleura, pulmonary vessels, bronchus, mediastinal structures, diaphragm, chest wall, other
- Lymph nodes: total examined, number involved by tumor, extracapsular extension
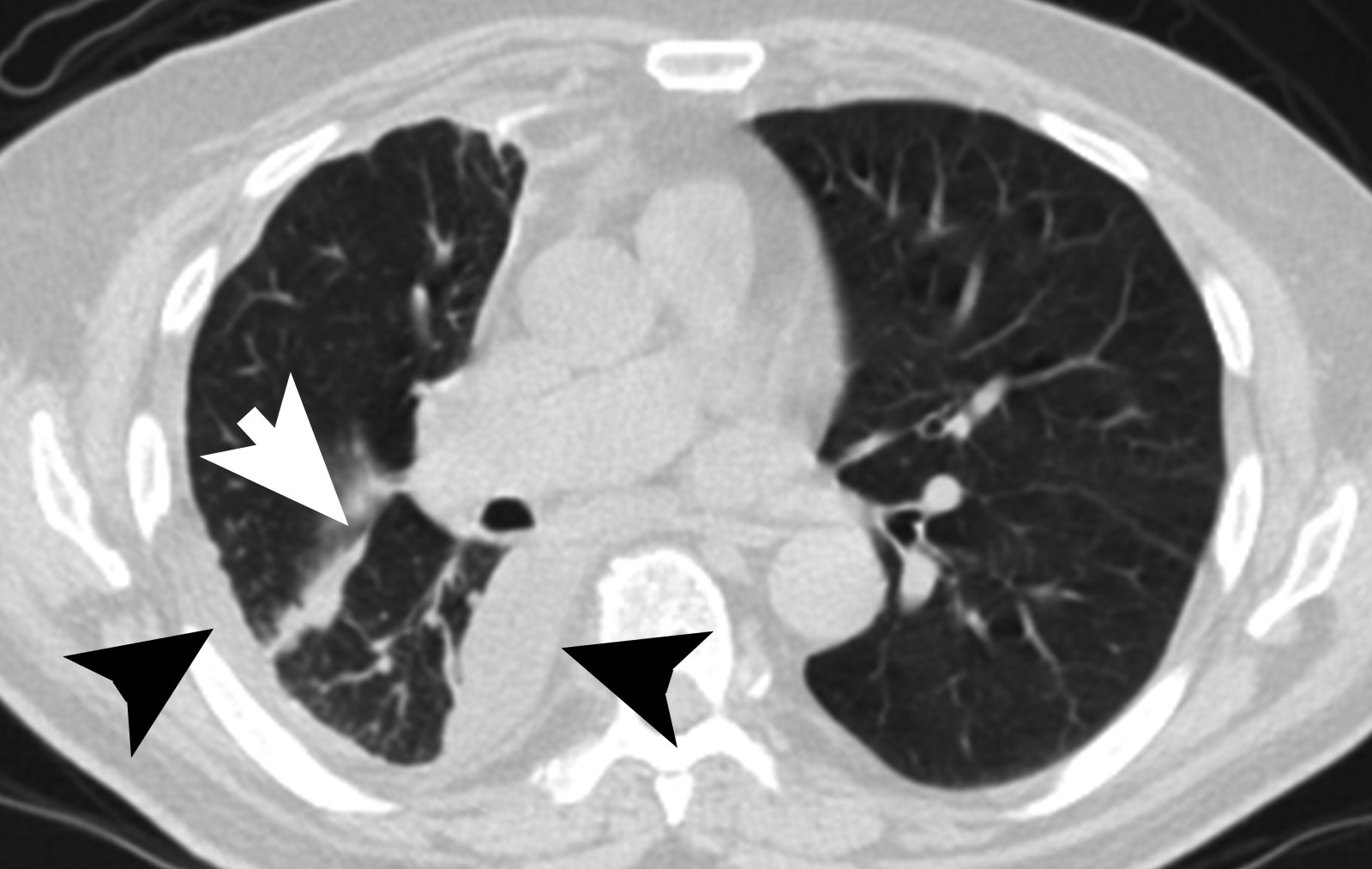
- Presence of pleural plaques, ferruginous bodies, pulmonary interstitial fibrosis, other significant findings
Asbestos related disorders
Table of Contents
Definition / generalDefinition / general
- Asbestos causes localized fibrous plaques, pleural effusions, parenchymal interstitial fibrosis (asbestosis), bronchogenic carcinoma, mesothelioma, laryngeal carcinoma, possibly colon carcinoma
- Exists in serpentine / chrysotile (curly, flexible) and amphibole (straight, stiff, brittle) forms; most asbestos in industry is serpentine but amphiboles are more pathogenic; link with mesothelioma is almost always with amphibole form
- Chrysotiles usually are caught in upper respiratory passages, removed by mucociliary elevator; they are soluble and leached from tissue if they reach alveoli
- Amphiboles go deeper into lungs; fibers > 8 mm and thinner than 0.5 mm are more injurious
- Both types are fibrogenic; act as tumor initiator and promoter; toxic chemicals such as tobacco smoke may be adsorbed to asbestos fibers; asbestos fibers may also generate reactive free radicals
- However, asbestos bodies are common in normals; present in 40% at autopsy in U.S. in lung smears
- Asbestos may act by countering antioxidant effect of vitamin C (ascorbic acid) (Hum Pathol 2003;34:737)
- In pleura, asbestos causes pleural plaques and mesothelioma
- Relative risk (RR) compared to normal population: for bronchogenic carcinoma, RR is 5x, increasing to 55x for asbestos exposure plus tobacco use; for mesothelioma (pleural, pericardial, peritoneal), RR is 1000x, with no change for asbestos plus tobacco use
Atypical mesothelial hyperplasia
Table of Contents
Definition / general | Terminology | Sites | Etiology | Clinical features | Radiology description | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Differential diagnosisDefinition / general
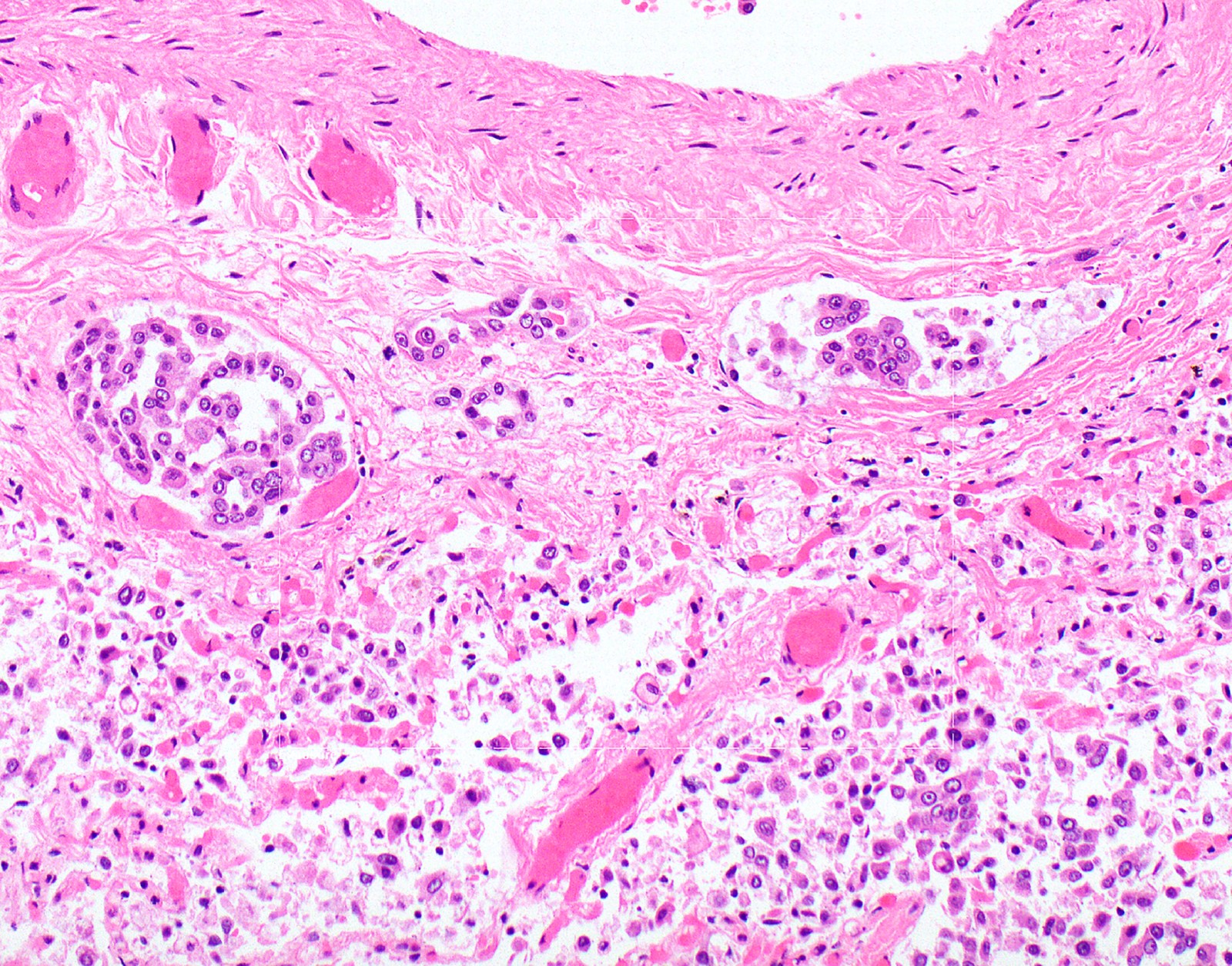
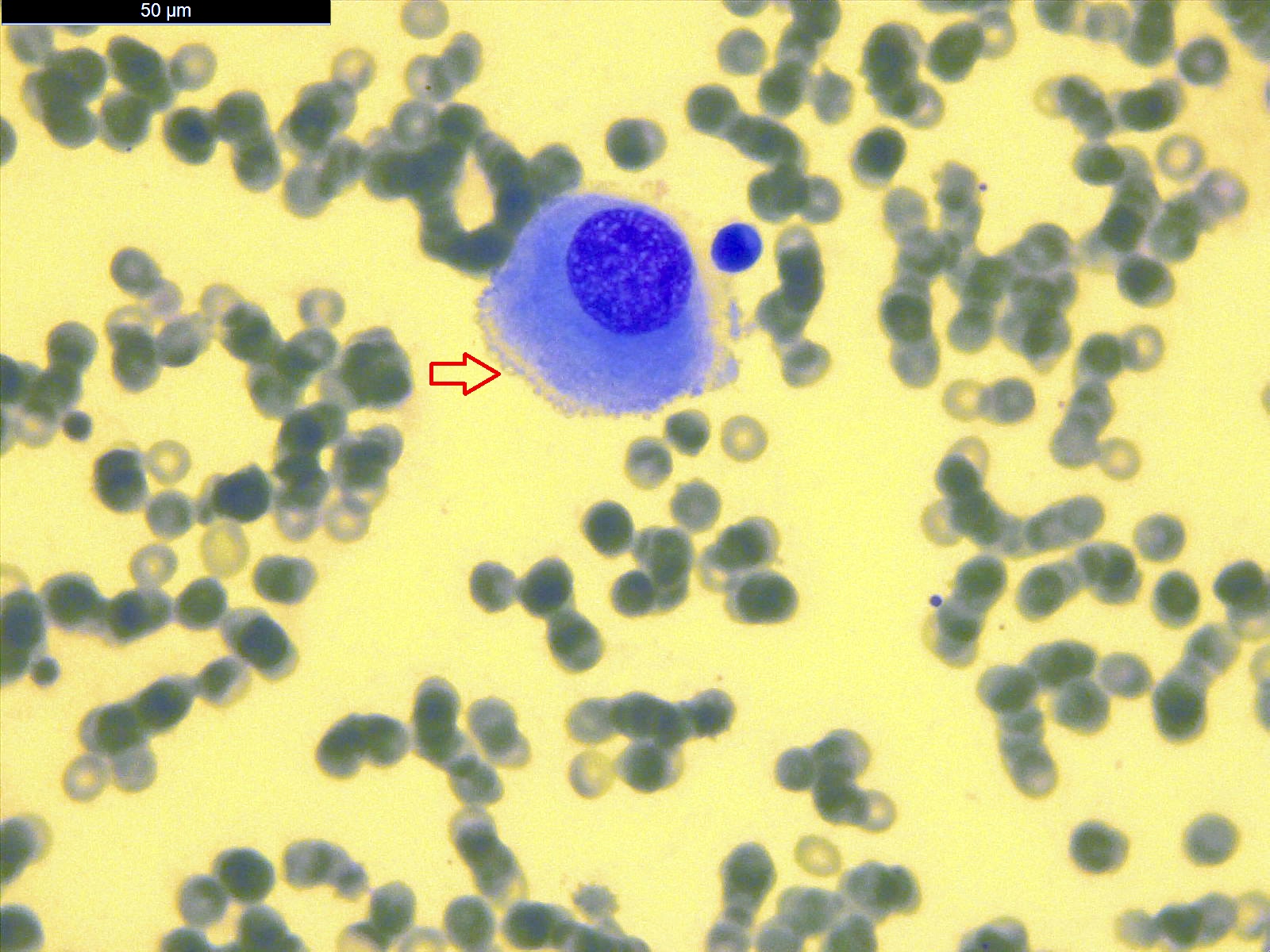
- Worrisome proliferations of mesothelial cells that are not unequivocally malignant are termed atypical mesothelial proliferation or atypical mesothelial hyperplasia
Terminology
- Pseudoneoplastic lesion of the pleural surface
- Are actually reactive mesothelial proliferations, associated with both benign and malignant conditions
Sites
- Any mesothelial surface, including pleura, peritoneum, tunica, etc.
Etiology
- Associated with anemia, bronchogenic carcinoma, cirrhosis, connective tissue diseases, pneumothorax (recurrent), viral infections
Clinical features
- History of pleural effusion or ascites, fluid may be hemorrhagic
Radiology description
- Description of pleura on imaging or pleuroscopy helps differentiate benign and malignant mesothelial proliferations
- Circumferential pleural thickening and nodular pleural thickening are highly suggestive of malignancy (Arch Pathol Lab Med 2012;136:1217)
Case reports
- 49 year old man with malignant mesothelioma eight years after a diagnosis of atypical mesothelial hyperplasia (J Clin Pathol 1999;52:535)
- Mesothelial hyperplasia with reactive atypia (Diagn Cytopathol 2000;22:113)
Microscopic (histologic) description
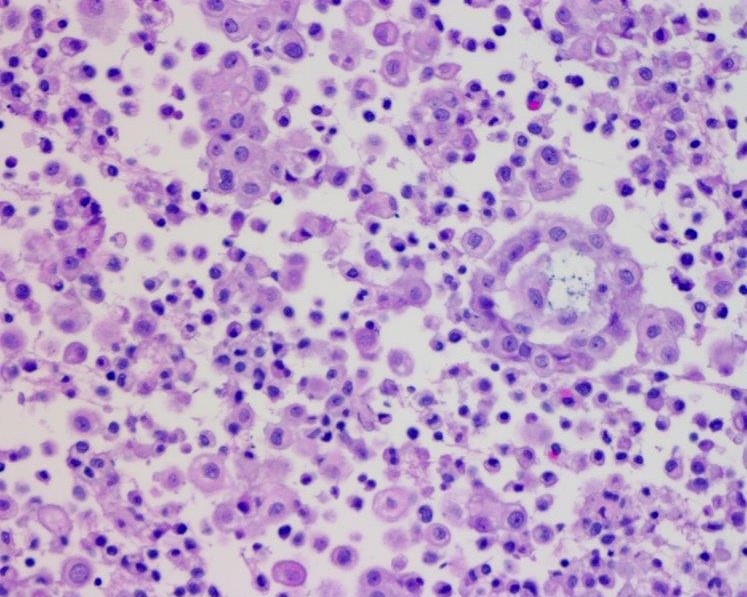
- Identification of neoplastic invasion is definitive criteria for diagnosis of malignant mesothelioma
- Finding of mesothelial cells in fat makes the proliferation malignant
- Challenges and controversies in diagnosis of mesothelioma discussed at J Clin Pathol 2013;66:847
Microscopic (histologic) images
Cytology description
- Criteria are defined for malignant mesothelioma (high specificity - 99% when all criteria are fulfilled); refer to malignant mesothelioma
- Cytology of atypical mesothelial cells:
- Mesothelial cells in large groups
- Cell groups with scalloped borders
- Nuclear hyperchromasia
- High N:C ratio
- Coarse chromatin
- Prominent nucleoli
- Diagnostic problems in serous effusions discussed at Diagn Cytopathol 1998;19:131
Positive stains
- All active mesothelial proliferations, benign or malignant, are pankeratin+
- EMA, GLUT1 and IMP3 can be positive in both benign and malignant mesothelial proliferation so cannot be used to differentiate reliably
- Homozygous deletion of p16 / CDKN2A demonstrated by FISH may be specific for malignant proliferations
- p16 FISH staining usually negative in benign proliferations, 59% sensitive for malignant mesothelioma (Am J Clin Pathol 2011;135:619)
Differential diagnosis
- Adenocarcinoma: positive for CEA, CD15, B72.3, BerEp4
- Malignant mesothelioma: epithelioid, sarcomatoid, well differentiated papillary mesothelioma
- Site specific tumors: related to the peritoneum, tunica, etc.
Benign mesothelial proliferations
Table of Contents
Terminology | Pathophysiology | Etiology | Uses by pathologists | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Differential diagnosis | Additional referencesTerminology
- Also called simple mesothelial hyperplasia
Pathophysiology
- Normal mesothelial surface has single layer of flat cuboidal epithelium
- Irritation of pleural surface causes simple hyperplasia of mesothelium
Etiology
- Due to asbestos, benign pleural effusion, benign pleural plaque, collagen infections, drug reactions, pneumothorax, pulmonary infarct, trauma, vascular disease
Uses by pathologists
- If malignancy cannot be excluded, use diagnosis of "atypical mesothelial hyperplasia" and recommend rebiopsy if clinically suspicious (Arch Pathol Lab Med 2012;136:1217)
Case reports
- 43 year old woman with pleural multicystic mesothelial proliferation (Tuberk Toraks 2013;61:47)
Microscopic (histologic) description
- Mesothelial cells form conspicuous layer of regularly spaced, bland cuboidal cells along pleural surface; normally, mesotheial cells present only along surface and not in underlying tissue
- Distinct nucleoli may be present
- Likely benign if papillary excrescences with tufts of cells with bland tubule-like nonbranching structures, no fibrovascular cores
- Capillaries are parallel to each other and perpendicular to pleural surface (in malignancy, the capillaries are haphazard)
- Necrosis may be seen but usually accompanied with inflammatory cells and debris (Arch Pathol Lab Med 2005;129:1421)
Microscopic (histologic) images
Cytology description
- Usually mesothelial cells will be numerous, dispersed or present in small clusters
- Clusters of > 12 cells is unusual in simple hyperplasia
- Binucleation, multinucleation, mitosis, prominent nucleolus can be seen in benign proliferations
- Two or more mesothelial cells are often separated by "window" or a narrow space
- Benign mesothelial cells usually have characteristic "skirt" or "halo" at pale outer rim of cell
Cytology images
Positive stains
- Immunostains do not differentiate benign and malignant mesothelial cells as both are positive for keratin
- However, immunostains can demonstrate invasion into underlying tissues
Differential diagnosis
Additional references
Diffuse mesothelioma
Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
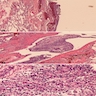
- Malignant neoplasm of mesothelial differentiation that arises from mesothelial lining cells of the pleura
- Can be of epithelioid or sarcomatoid cytology or a combination thereof
Essential features
- Aggressive neoplasm of mesothelial differentiation
- Epithelioid, biphasic or sarcomatoid subtype
- Overall survival, 4 - 27 months, depending on subtype
- Most commonly associated with remote (up to 40 years prior) asbestos exposure
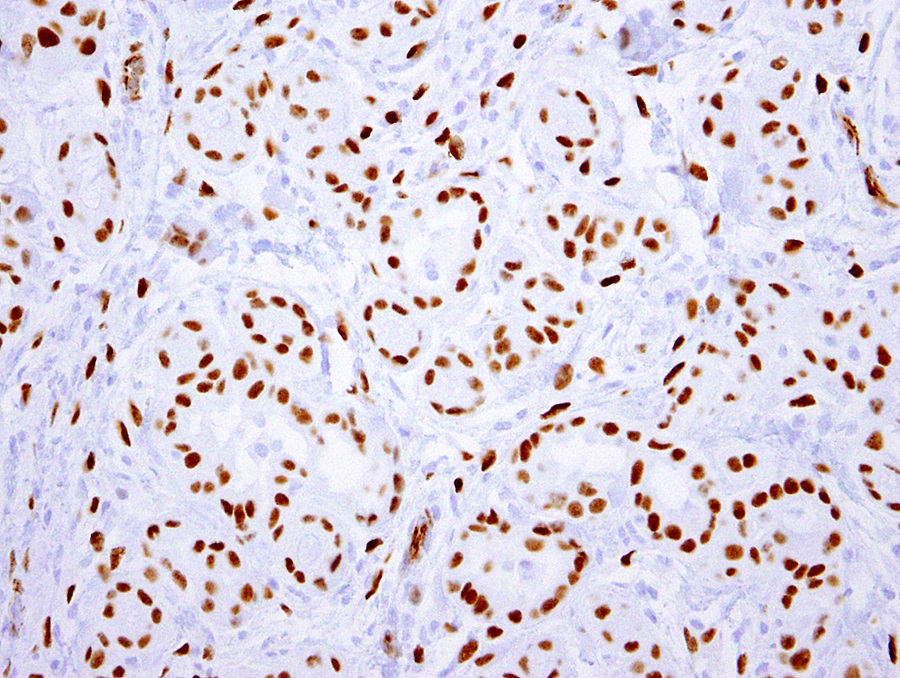
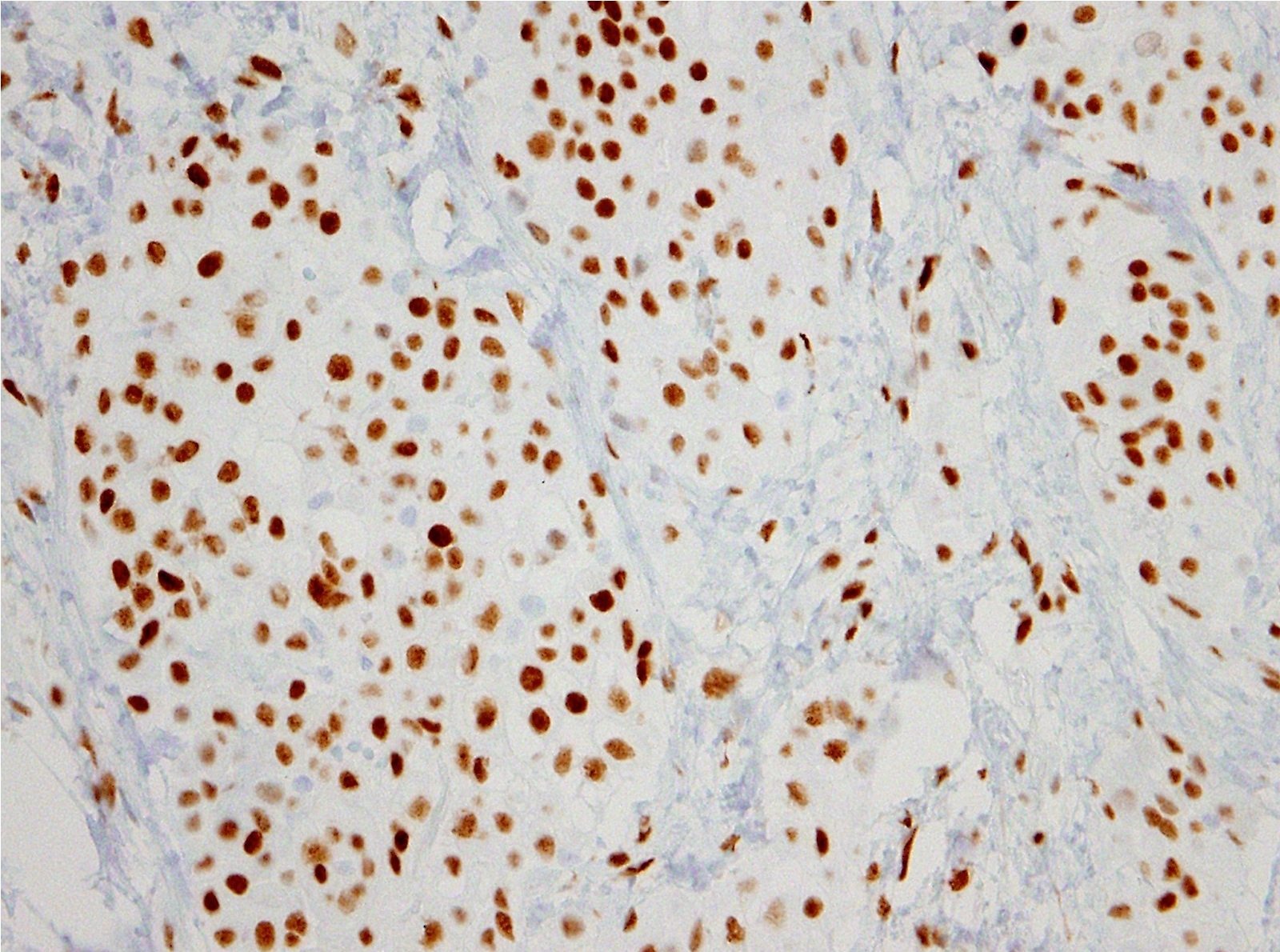
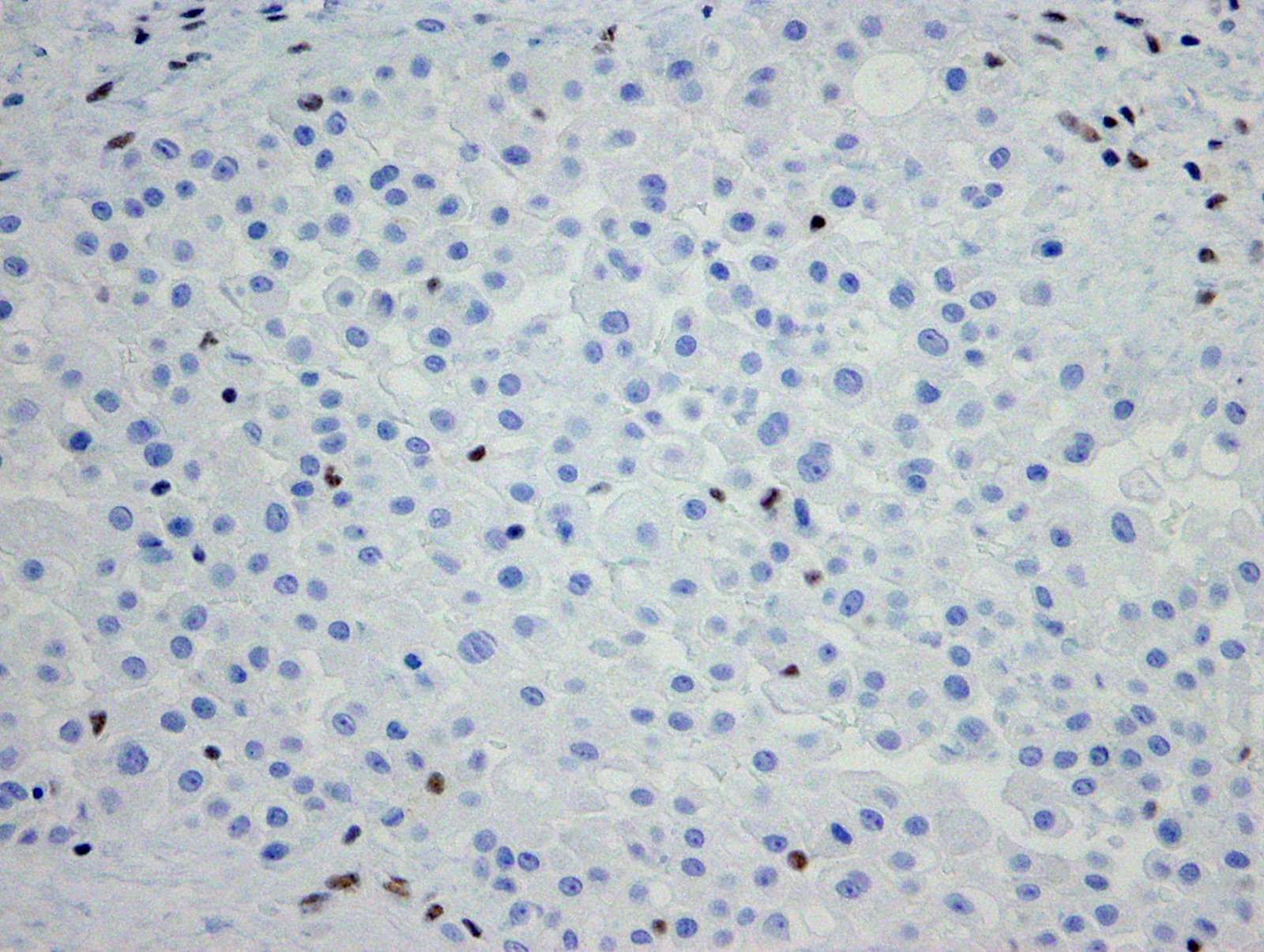
- Loss of expression of BRCA1 associated protein (BAP1) or methylthioadenosine phosphorylase (MTAP) or homozygous deletion of cyclin dependent kinase inhibitor 2A (CDKN2A) (p16) by FISH helps to distinguish reactive mesothelial proliferation from malignant pleural mesothelioma
Epidemiology
- Incidence varies by geographic location; up to 30 cases/million people/year in Australia, Great Britain; 2,000 - 3,000 new cases per year in the United States (Surg Pathol Clin 2020;13:73, Semin Respir Crit Care Med 2019;40:347)
- An estimated 28,000 - 43,000 people die from malignant pleural mesothelioma worldwide every year (Semin Respir Crit Care Med 2019;40:347)
- Elderly patients; median age, 63 - 70 years (range, 26 - 95 years) (Thorac Cancer 2019;10:1193, Ann Thorac Surg 2018;105:432, Surg Pathol Clin 2020;13:73)
- Male predominance; 75 - 87% male (Thorac Cancer 2019;10:1193, Ann Thorac Surg 2018;105:432, Surg Pathol Clin 2020;13:73)
- Latency period between asbestos exposure and development of disease of 20 to > 40 years, depending on severity and duration of exposure (Surg Pathol Clin 2020;13:73)
- Strong association with asbestos exposure either occupational (e.g., shipbuilding, construction, automotive industry) or residential (industrial contamination, environmental / domestic) (Br J Ind Med 1960;17:260, Lung Cancer 2004;45:S3)
- Rare in young patients (≤ 35 years old); in that population equal sex distribution, more commonly history of prior mantle radiation and family history of breast cancer, history of asbestos exposure less common than in older patients (Mod Pathol 2018;31:122)
Sites
- Parietal, visceral, mediastinal or diaphragmatic pleura
Pathophysiology
- Hypotheses for how asbestos causes malignant pleural mesothelioma include (Respirology 2005;10:2):
- Toxic oxygen radical generation
- Chronic pleural irritation
- Persistent kinase mediated signaling
- Asbestos fibers exert cytotoxic and genotoxic effects
- Long and thin fibers associated with higher mutagenesis (Transl Lung Cancer Res 2020;9:S39)
- Erionite and zeolite fibers are specifically linked to malignant pleural mesothelioma and likely more potent carcinogens than other asbestos fibers (Semin Oncol 2002;29:2)
- Common inactivated tumor suppressors in malignant pleural mesothelioma: BAP1, neurofibromin 2 (NF2), CDKN2A
- Malignant mesothelioma in situ has high risk of developing invasive mesothelioma (Mod Pathol 2020;33:297)
Etiology
- Asbestos exposure
- Erionite exposure in genetically predisposed people thought to be associated with unusual high prevalence of malignant mesothelioma in region of Cappadocia, Turkey (Cancer Res 2006;66:5063)
- Ionizing radiation for treatment of malignancy (Hodgkin lymphoma, non-Hodgkin lymphoma, testicular cancer) might be a risk factor (Cancer 2006;107:108, Cancer 2007;109:1432, J Natl Cancer Inst 2005;97:1354)
- Close family members of patients with malignant pleural mesothelioma might have increased risk (Eur Respir J 2016;48:873)
- Molecular aberrations / familial - germline pathogenic variants identified in 12% of patients including mutations in BAP1 (1 - 4%), MutS homolog 3 (MSH3), breast cancer gene 1 associated ring domain 1 (BARD1), RecQ-like helicase 4 (RECQL4), breast cancer gene 2 (BRCA2), MRE11 homolog, double strand break repair nuclease (MRE11A), SHQ1, H/ACA ribonucleoprotein assembly factor (SHQ1) (1% each) (J Thorac Oncol 2020;15:655)
- Association with SV-40 is controversial (Respirology 2005;10:2)
Diagrams / tables
Images hosted on other servers:
Table 1. Commonly used immunohistochemical stains for the diagnosis of malignant pleural mesothelioma
| Immunohistochemical stain | in malignant mesothelioma, % | Relevant other neoplasms that might express that marker at least in a subset of cases |
| Keratin AE1 / AE3a | Carcinomas | |
| CAM 5.2 | Carcinomas | |
| WT1 (nuclear)a | Carcinomas of the gynecologic tract (e.g., serous carcinomas), Wilms tumor | |
| Calretinin (nuclear and cytoplasmic)a | Granulosa cell tumor, squamous cell carcinoma | |
| CK5, CK5/6a | Squamous cell carcinoma, NUT carcinoma, breast carcinoma, urothelial carcinoma | |
| Podoplanin (D2-40)a | Squamous cell carcinoma, follicular dendritic cell tumor, angiosarcoma, seminoma, serous carcinoma | |
| GATA3 (nuclear)b | Breast carcinoma, urothelial carcinoma | |
| pCEA | Adenocarcinoma, squamous cell carcinoma, neuroendocrine tumors | |
| MOC31 | Adenocarcinoma, squamous cell carcinoma | |
| BerEP4 | Adenocarcinoma, squamous cell carcinoma | |
| B72.3 | Adenocarcinoma, squamous cell carcinoma | |
| Claudin 4 | Adenocarcinoma, squamous cell carcinoma, small cell carcinoma, sarcomatoid carcinoma | |
| MUC4 | Adenocarcinoma, squamous cell carcinoma | |
| TTF1 (8G7G3/1) | Lung adenocarcinoma, thyroid carcinoma | |
| TTF1 (SP141) | Lung adenocarcinoma, thyroid carcinoma | |
| Napsin A | Lung adenocarcinoma, renal cell carcinoma | |
| p40 | Squamous cell carcinoma, thymic carcinoma, NUT carcinoma, urothelial carcinoma | |
| CDX2 | Pancreatobiliary adenocarcinoma, intestinal type adenocarcinoma colorectal adenocarcinoma | |
| PAX8 | Renal cell carcinoma, thyroid carcinoma, thymic carcinoma (polyclonal antibody), carcinoma of gynecologic tract | |
| Estrogen receptor | Breast carcinoma, carcinomas of gynecologic tract |
Table 2. Markers that aid in the distinction between reactive and malignant mesothelial proliferation
Mod Pathol 2015;28:1043,
Am J Surg Pathol 2015;39:977,
Am J Surg Pathol 2016;40:714,
Arch Pathol Lab Med 2018;142:1549,
Hum Pathol 2017;60:86,
Lung Cancer 2017;104:98,
J Thorac Oncol 2015;10:565,
Lung Cancer 2018;125:198,
Mod Pathol 2020;33:245,
Ann Diagn Pathol 2017;26:31
| Markers | Sensitivity | Specificity |
| Loss of expression of nuclear BAP1 All mesothelioma Epithelioid mesothelioma Sarcomatoid mesothelioma | 56 - 81 0 - 63 | |
| Loss of expression of cytoplasmic MTAP Epithelioid mesothelioma Sarcomatoid mesothelioma | 80 | |
| Homozygous deletion of CDKN2A (p16) by FISH All mesothelioma Epithelioid mesothelioma Sarcomatoid mesothelioma | 48 - 78 67 | |
| Loss of BAP expression or homozygous deletion of CDKN2A All mesothelioma Epithelioid mesothelioma Sarcomatoid mesothelioma | 92 67 - 100 | |
| Loss of expression of BAP1 or MTAP All mesothelioma Sarcomatoid mesothelioma | 90 | |
Clinical features
- Shortness of breath
- Chest wall pain, pleurisy
- Cough
- Weight loss
- Recurrent unilateral pleural effusion; might be hemorrhagic
- Occasionally asymptomatic when discovered at early stage
Diagnosis
- Pleural thickening or recurrent pleural effusion on chest Xray followed up with contrast enhanced chest CT scan
- Thoracocentesis acquiring pleural fluid for cytology
- In the past, was often considered not sufficient for definite diagnosis
- With BAP1 and MTAP immunostaining and FISH for homozygous deletion of CDKN2A, diagnosis of malignant pleural mesothelioma possible on at least a subset of fluids) (Cancer Cytopathol 2018;126:54)
- Pleural biopsy (e.g., video assisted thoracoscopic surgery [preferred], CT guided core biopsy, open biopsy
- Mediastinoscopy with lymph node sampling
Laboratory
- Serology for mesothelin and fibulin-3 might be useful as screening markers for malignant pleural mesothelioma, osteopontin lacks specificity (Curr Opin Pulm Med 2015;21:352, Carcinogenesis 2019;40:1320)
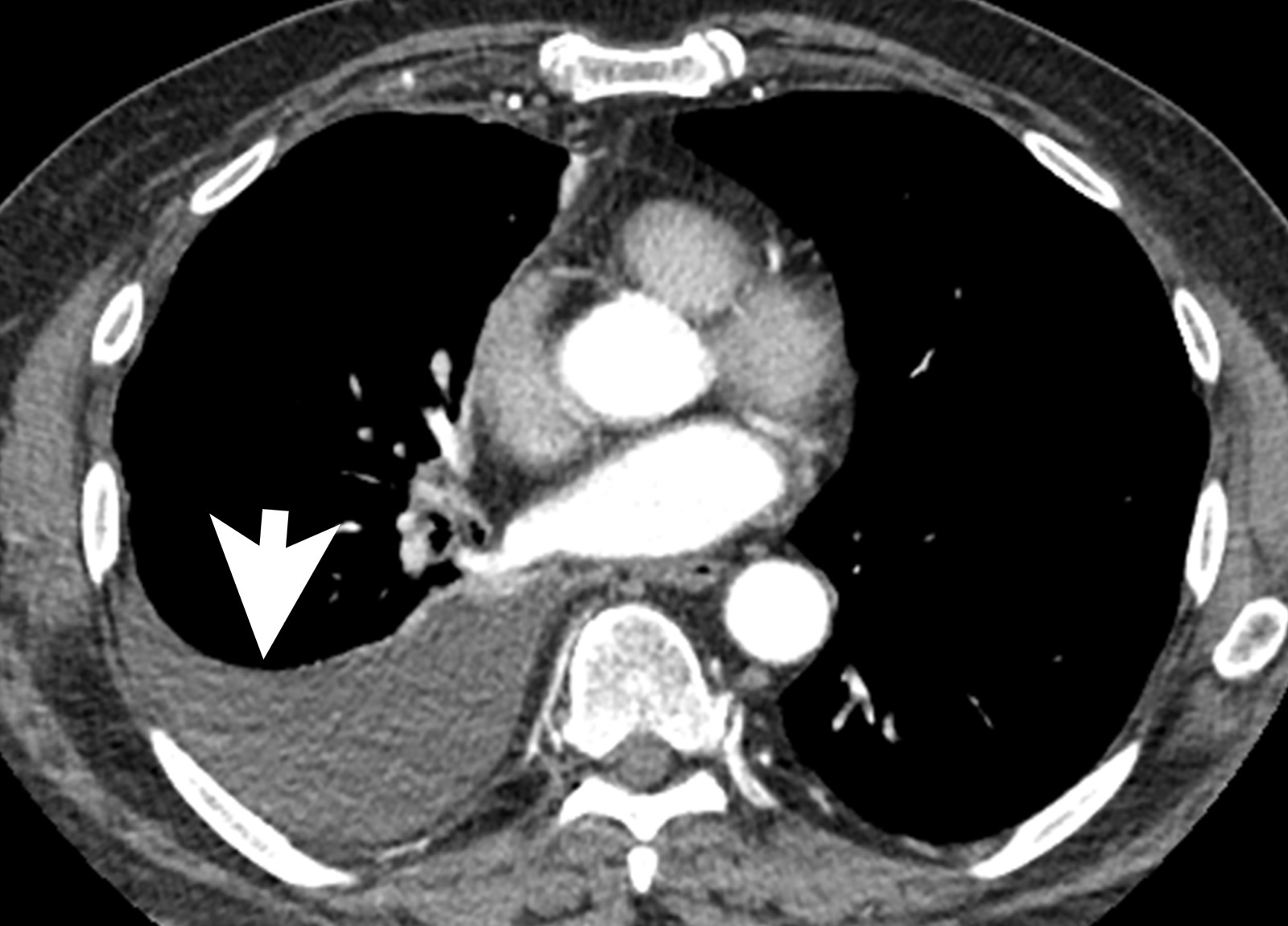
Radiology description
- Chest Xray:
- Unilateral effusion
- CT scan ideally with contrast enhancement (Surg Pathol Clin 2020;13:73):
- Unilateral pleural effusion
- Loculated, nodular or diffuse pleural thickening
- Multifocal nodules studding pleural surfaces including visceral, parietal and diaphragmatic pleura and possibly extending into fissures
- Thick rind of pleura
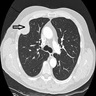
- Localized pleural or subpleural mass, rare, might measure up to 15 cm (Surg Pathol Clin 2020;13:73)
- Anterior mediastinal mass, rare, might invade into abdomen including liver
- Benign pleural plaques of parietal pleura, usually bilateral, might be seen in addition to malignant pleural mesothelioma; indicate exposure to asbestos; they are not a sign of malignant pleural mesothelioma
- MRI and PET / CT: complementary to contrast enhanced CT
- MRI might help to better delineate relationship of malignant pleural mesothelioma to adjacent structures and organs
- PET / CT might help to identify metastatic disease
Prognostic factors
- Morphologic subtype: epithelioid subtype (best prognosis) > biphasic > sarcomatoid / desmoplastic subtype (worst prognosis) (Ann Thorac Surg 2018;105:432)
- Epithelioid: overall survival, 12 - 27 months, lowest T stage (23% T1), 12% M1 (Ann Thorac Surg 2018;105:432, J Surg Res 2015;196:23, Thorac Cancer 2019;10:1193, Semin Respir Crit Care Med 2019;40:347)
- Biphasic: overall survival, 8 - 21 months; % epithelioid component might be associated with outcome (Semin Respir Crit Care Med 2019;40:347)
- Sarcomatoid: overall survival, 4 - 18 months, highest T stage (29% T4), 17% M1
- Overall survival is independently associated with (Thorac Cancer 2019;10:1193, Mod Pathol 2012;25:260):
- Male gender
- Histology (biphasic and sarcomatoid)
- Lymph node metastasis
- Treatment without chemotherapy
- Lymph node dissection: independently associated with worse overall survival
- Proposed nuclear grading of epithelioid malignant pleural mesothelioma
- Advanced age: associated with worse overall survival (Thorac Cancer 2019;10:1193)
- Staging: prognostic significance is controversial (Thorac Cancer 2019;10:1193)
Case reports
- 45 year old woman with epithelioid malignant pleural mesothelioma with brain metastasis and peritoneal carcinomatosis with response to pembrolizumab (Lung Cancer 2020;142:47)
- 65 year old man with metastatic epithelioid malignant pleural mesothelioma harboring a BRAF V600E mutation (Lung Cancer 2018;116:96)
- 68 year old man with epithelioid malignant pleural mesothelioma diffusely expressing CK20 (Appl Immunohistochem Mol Morphol 2019;27:e93)
- 73 year old man with malignant pleural mesothelioma in situ (Virchows Arch 2020;476:469)
- 77 year old man with epithelioid malignant pleural mesothelioma colliding with lung adenocarcinoma (Diagn Pathol 2016;11:38)
Treatment
- Management by multidisciplinary team
- Most patients (40%): no specific modality of treatment (Ann Thorac Surg 2018;105:432)
- Chemotherapy alone: most common treatment (Ann Thorac Surg 2018;105:432)
- Trimodality treatment (chemotherapy, surgical resection, radiation therapy): best survival > combination chemotherapy and resection (Ann Thorac Surg 2018;105:432)
- Surgery within multimodal therapy suggested only to suitable patients (i.e., young patients with early stage epithelioid histology; good performance status, epithelioid or possibly biphasic morphology) (Thorac Cancer 2019;10:1193)
- NCCN guidelines (Version 1.2020):
- Clinical stage I - IIIA and epithelioid or biphasic morphology (surgery should be considered for biphasic if early stage disease)
- Induction chemotherapy followed by surgical exploration (pleurectomy / decortication or extrapleural pneumonectomy; mediastinal lymph node sampling)
- If pleurectomy / decortication: followed by observation or radiation
- If extrapleural pneumonectomy: followed by hemithoracic radiation; if found unresectable, chemotherapy
- Surgical exploration (pleurectomy / decortication or extrapleural pneumonectomy; mediastinal lymph node sampling)
- If pleurectomy / decortication: followed by chemotherapy followed by observation or radiation
- If extrapleural pneumonectomy: followed by sequential chemotherapy and hemithoracic radiation; if found unresectable chemotherapy
- Surgery associated with morbidity and mortality; extrapleural pneumonectomy and extended pleurectomy / decortication: perioperative mortality of 6.8% and 2.9%; morbidity of 62% and 27.9%, respectively (Lung Cancer 2014;83:240)
- Sarcomatoid and desmoplastic mesothelioma or patients with poor performance status: chemotherapy or supportive care only
- Supportive care: pleurodesis or pleural catheter if recurrent pleural effusion
- Anti-PD1 or anti-PDL1 treatment on occasion, not standardized
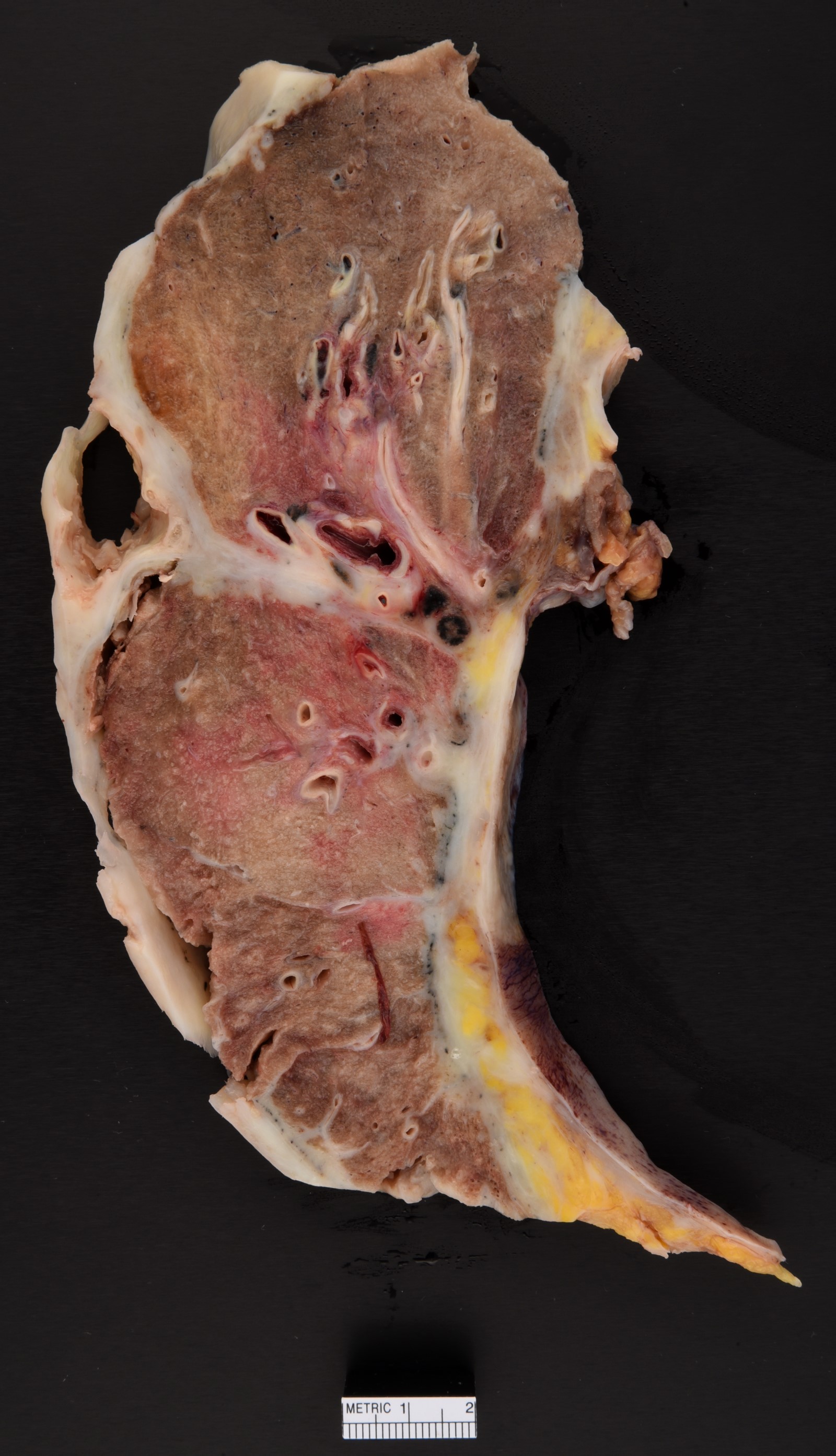
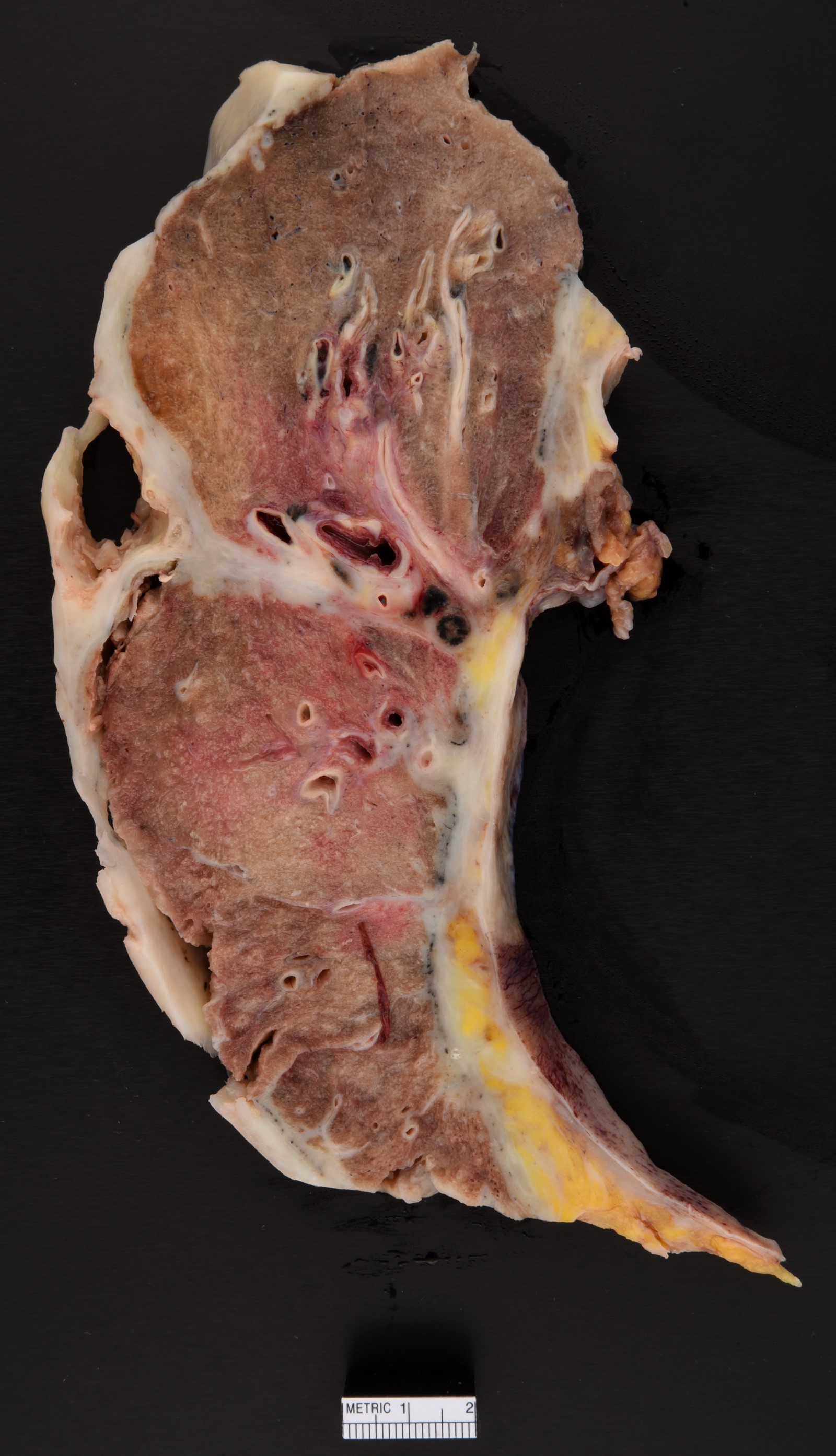
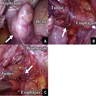
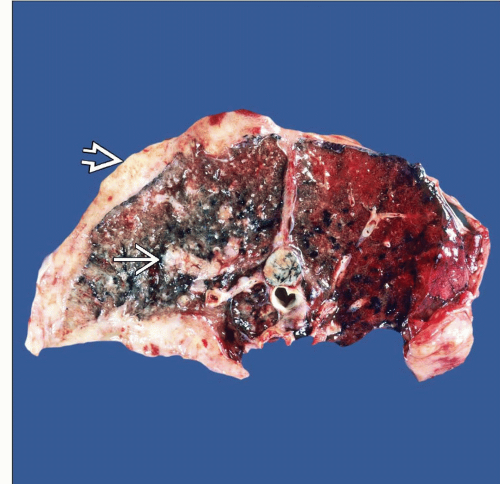
Gross description
- Thick rind of pleura possibly extending into fissures
- Studding of pleura
- Multiple pleural nodules
- Single pleural based mass rare (localized malignant pleural mesothelioma) (Mod Pathol 2020;33:281)
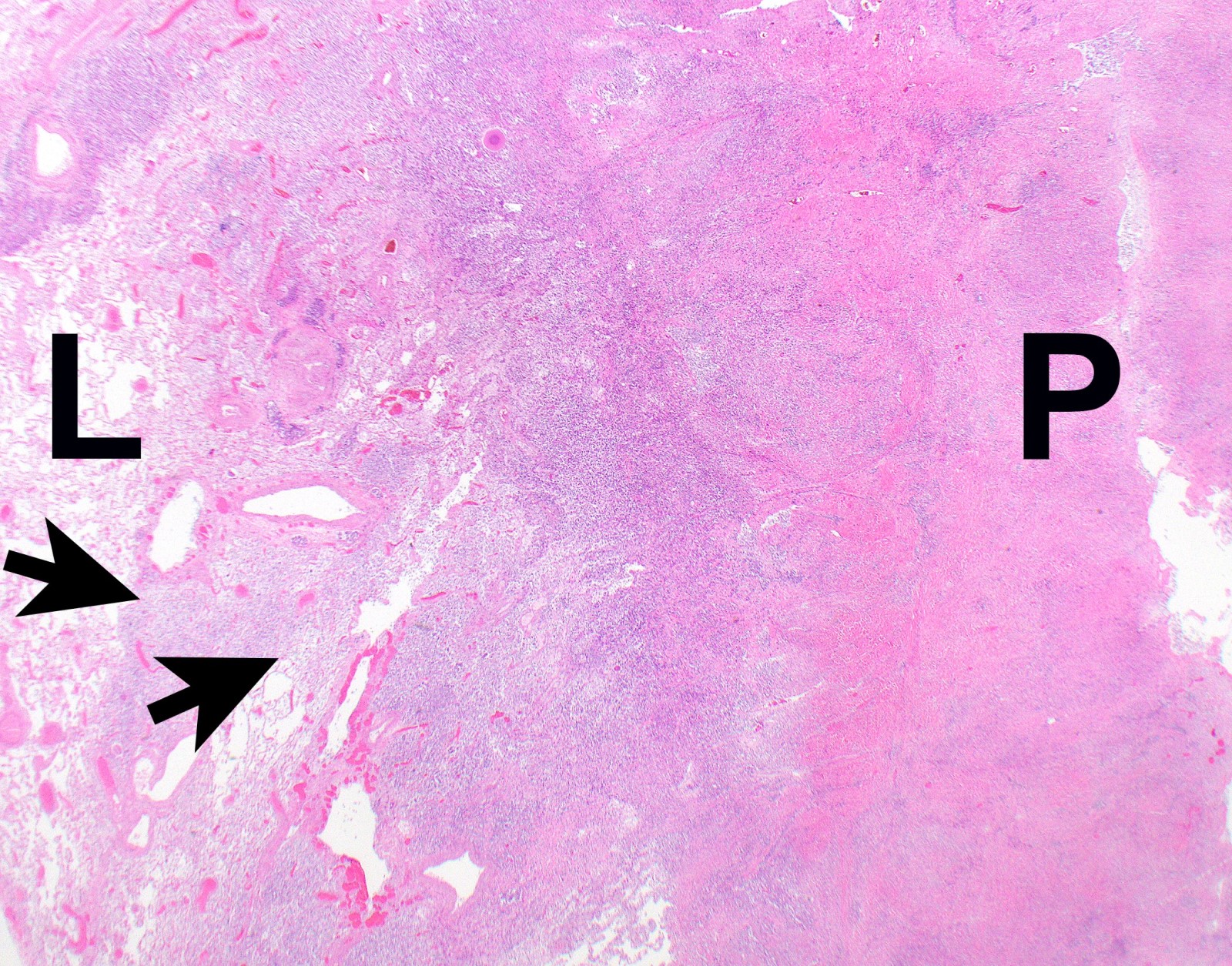
- Optimal orientation of specimen important for assessment of invasion: pleural sections submitted perpendicular to surface; should contain entire pleural thickness together with adjacent structures such as lung, adipose tissue or skeletal muscle
Frozen section description
- Assess adequacy of sampled tissue that includes sufficient tissue to assess growth pattern and invasion
- Distinction between metastatic carcinoma, sarcoma, melanoma and mesothelioma is in general not possible; neither is it important at time of frozen section evaluation
Frozen section images
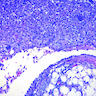
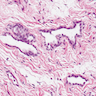
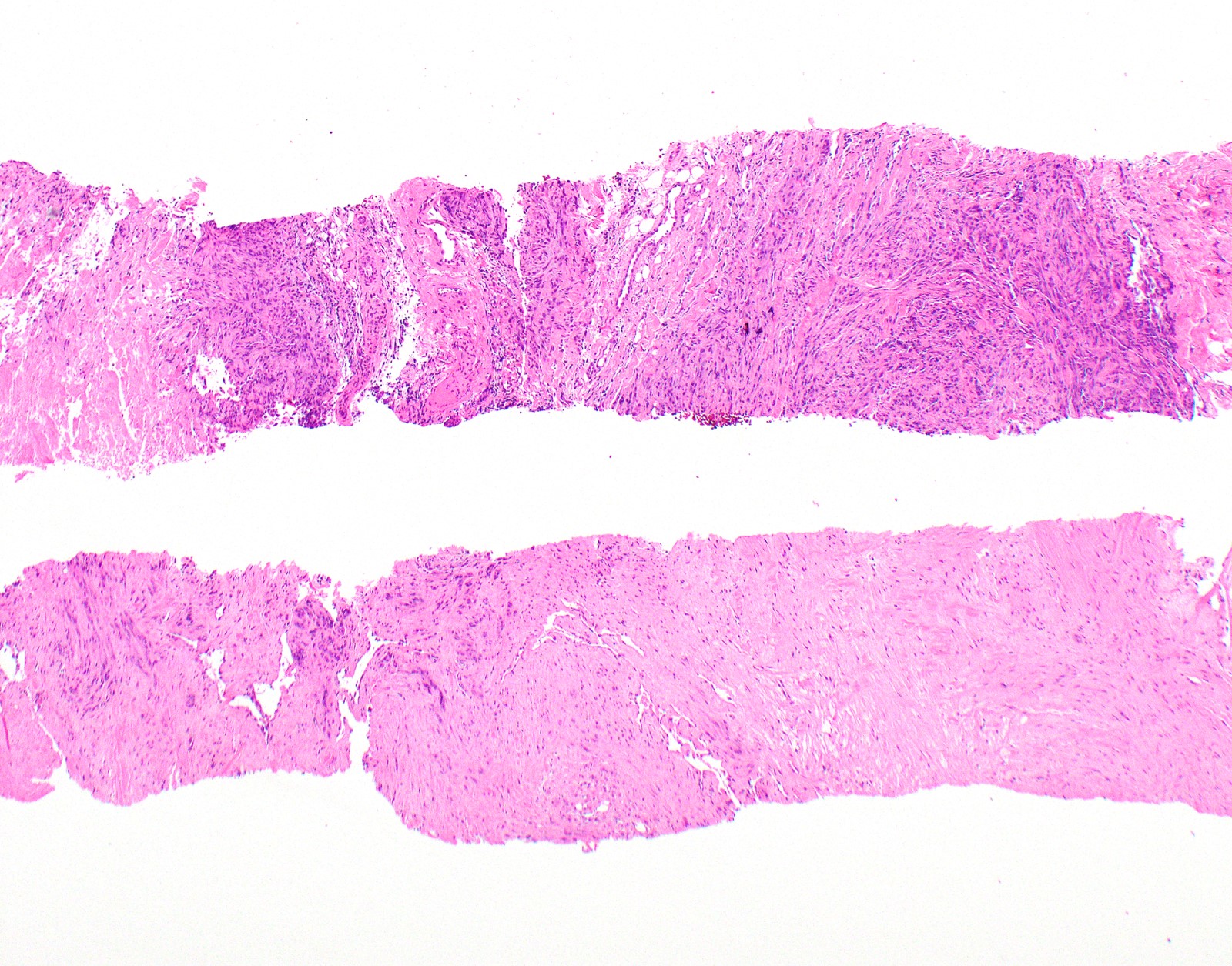
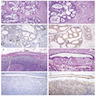
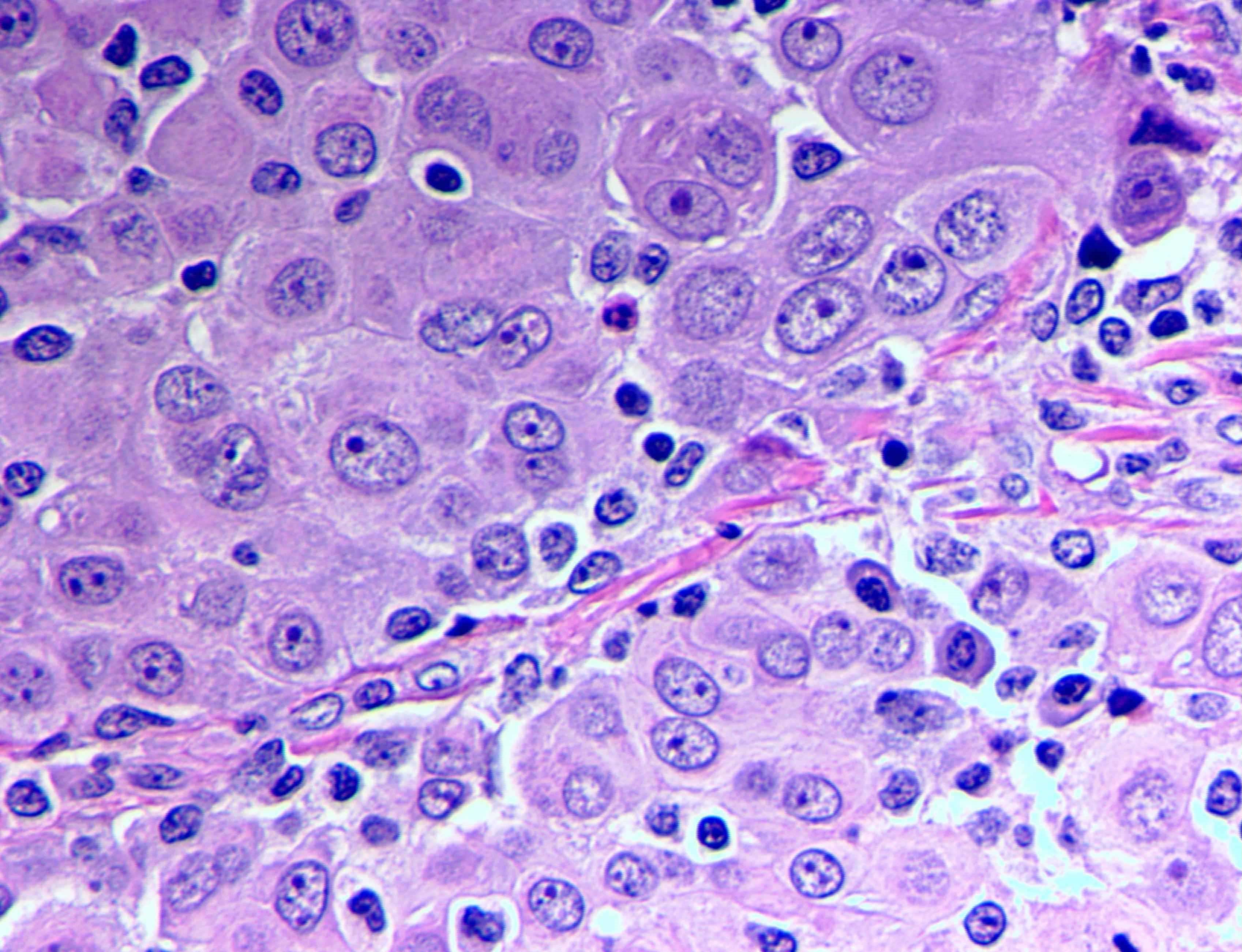
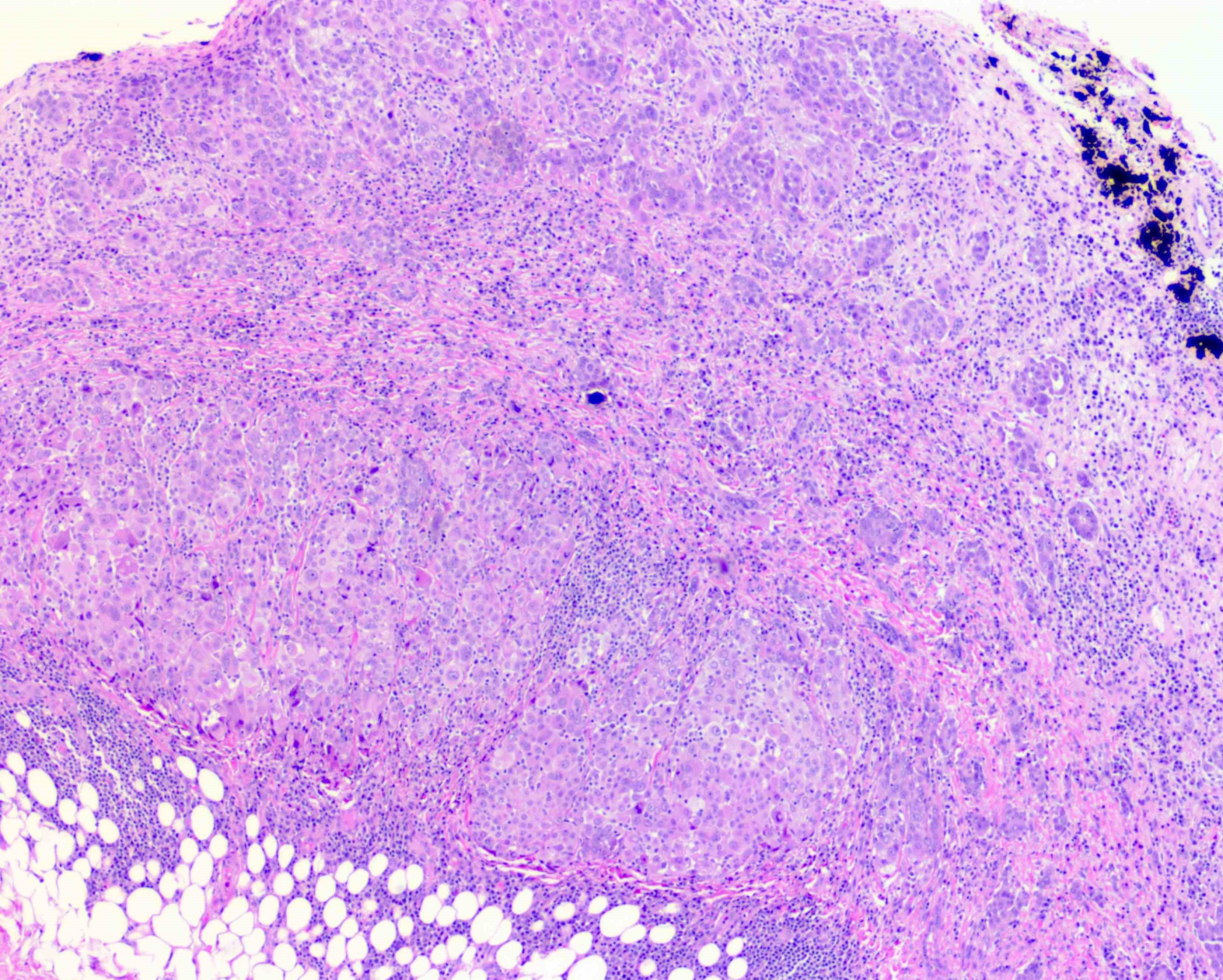
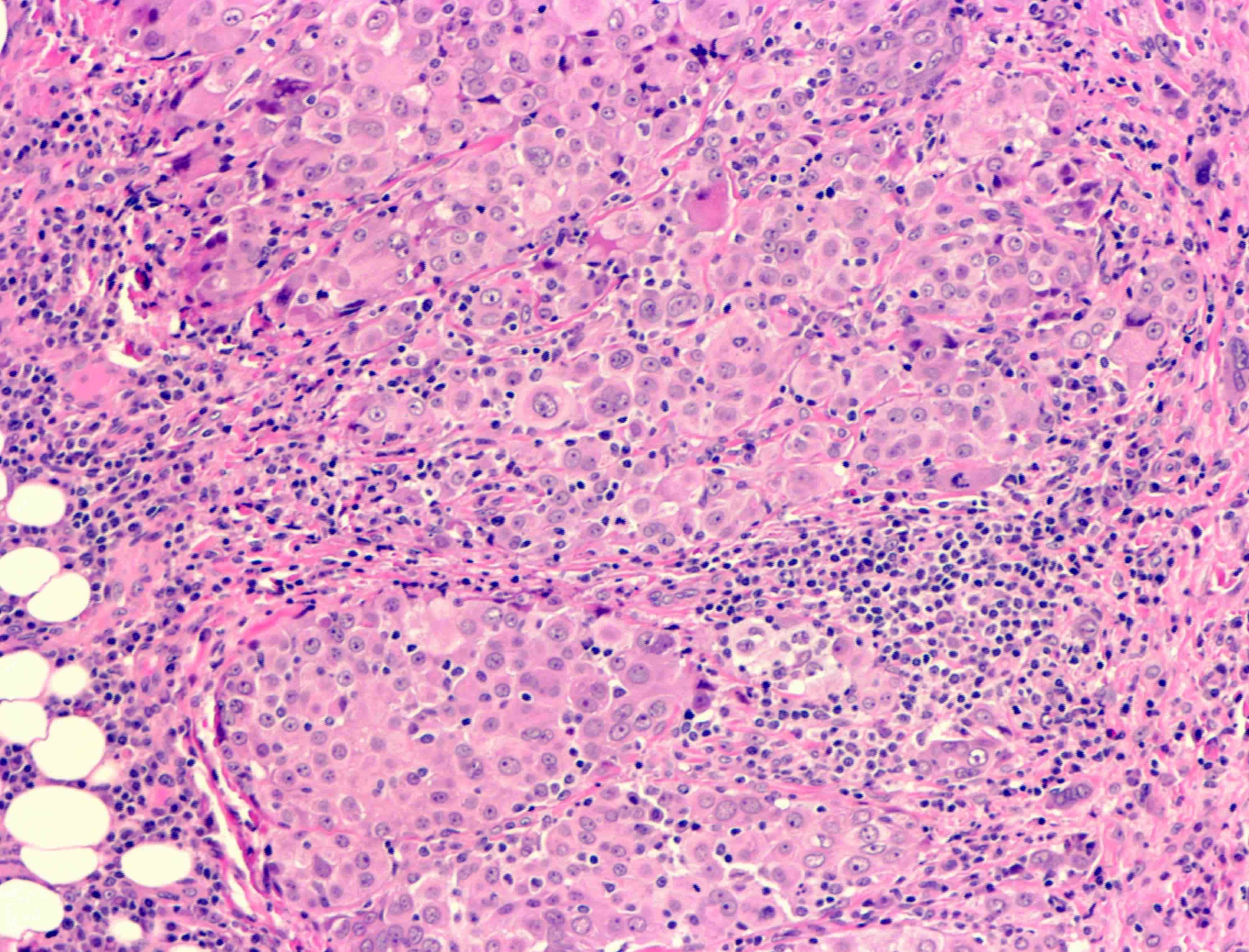
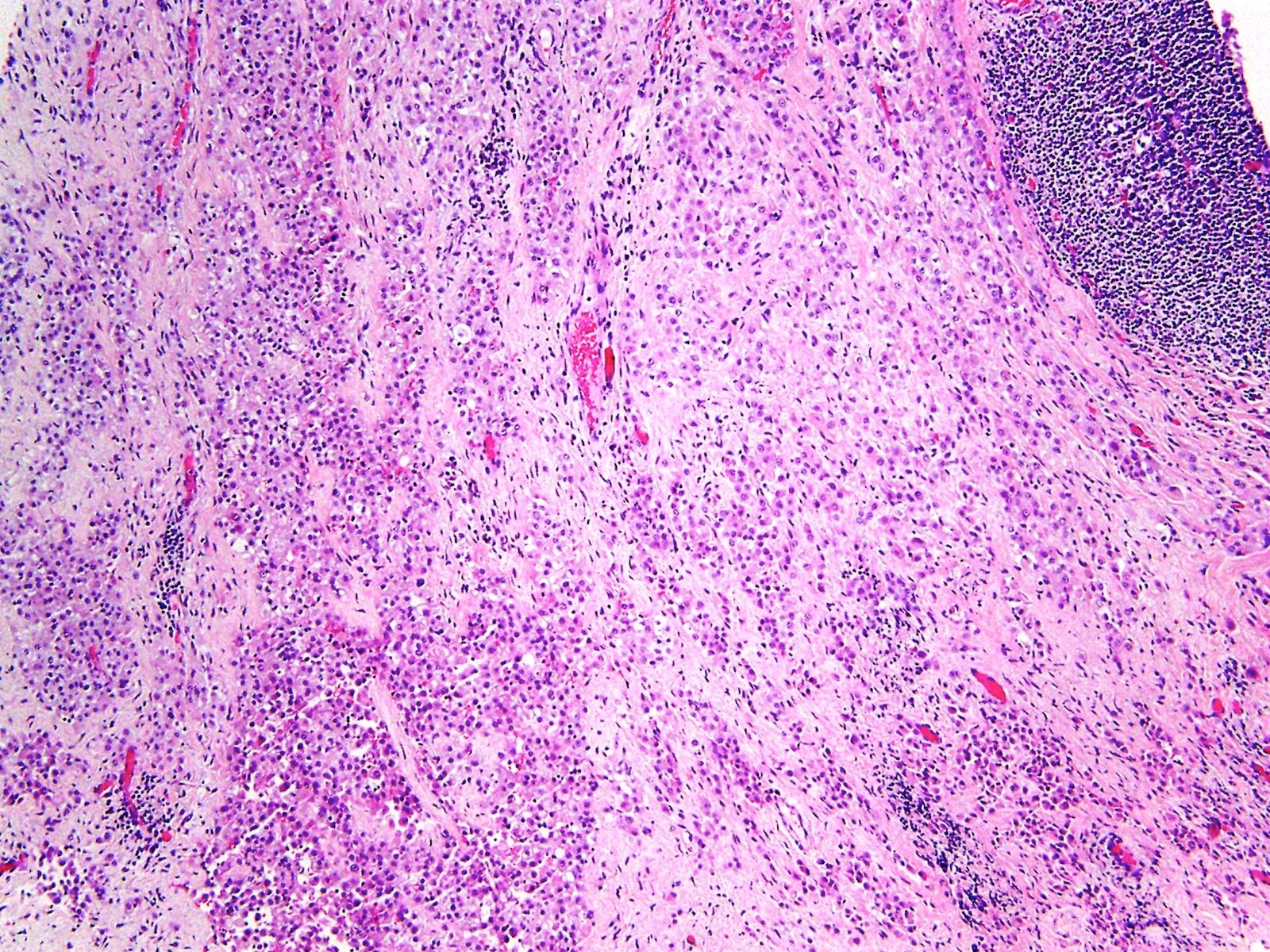
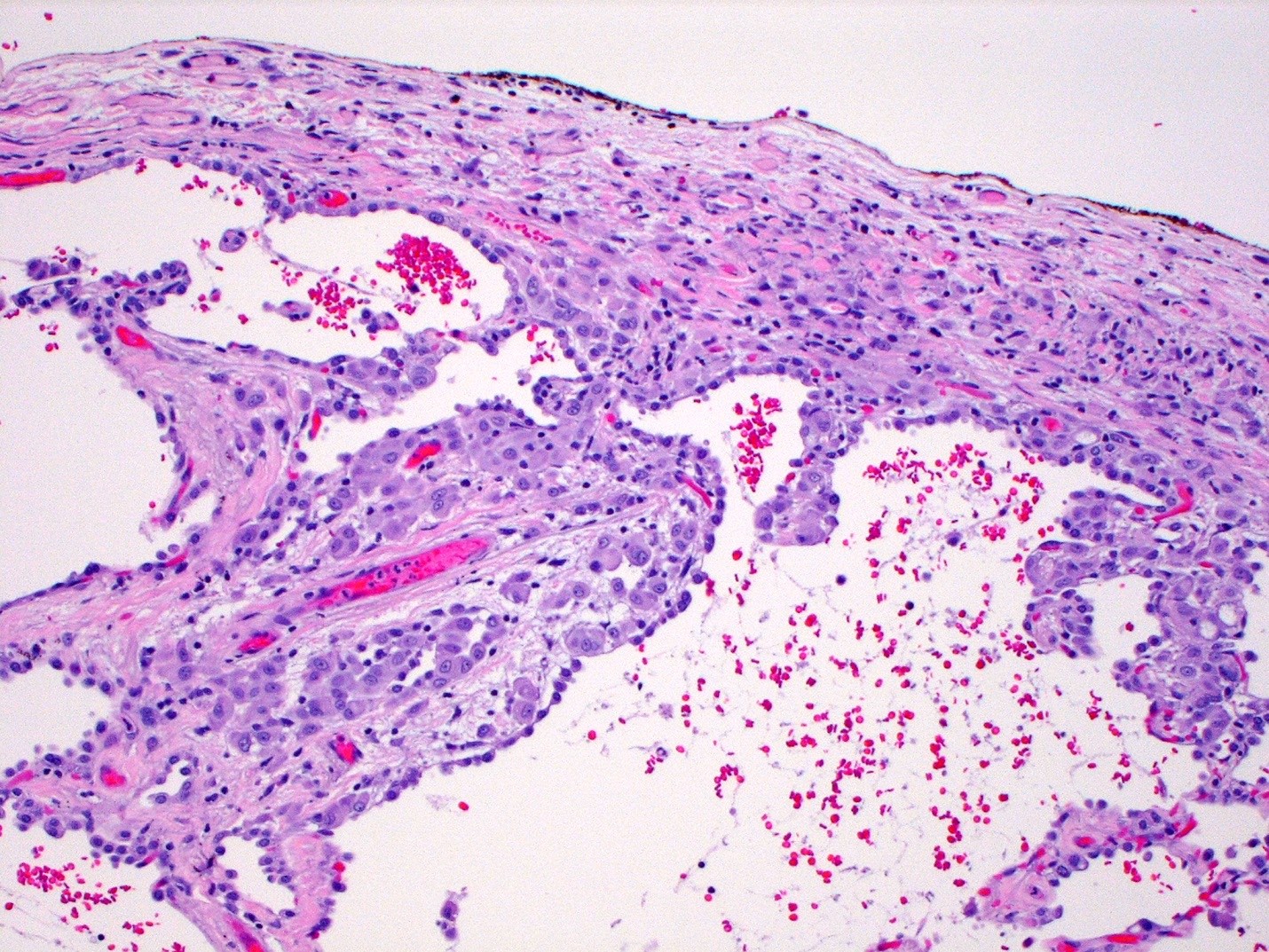
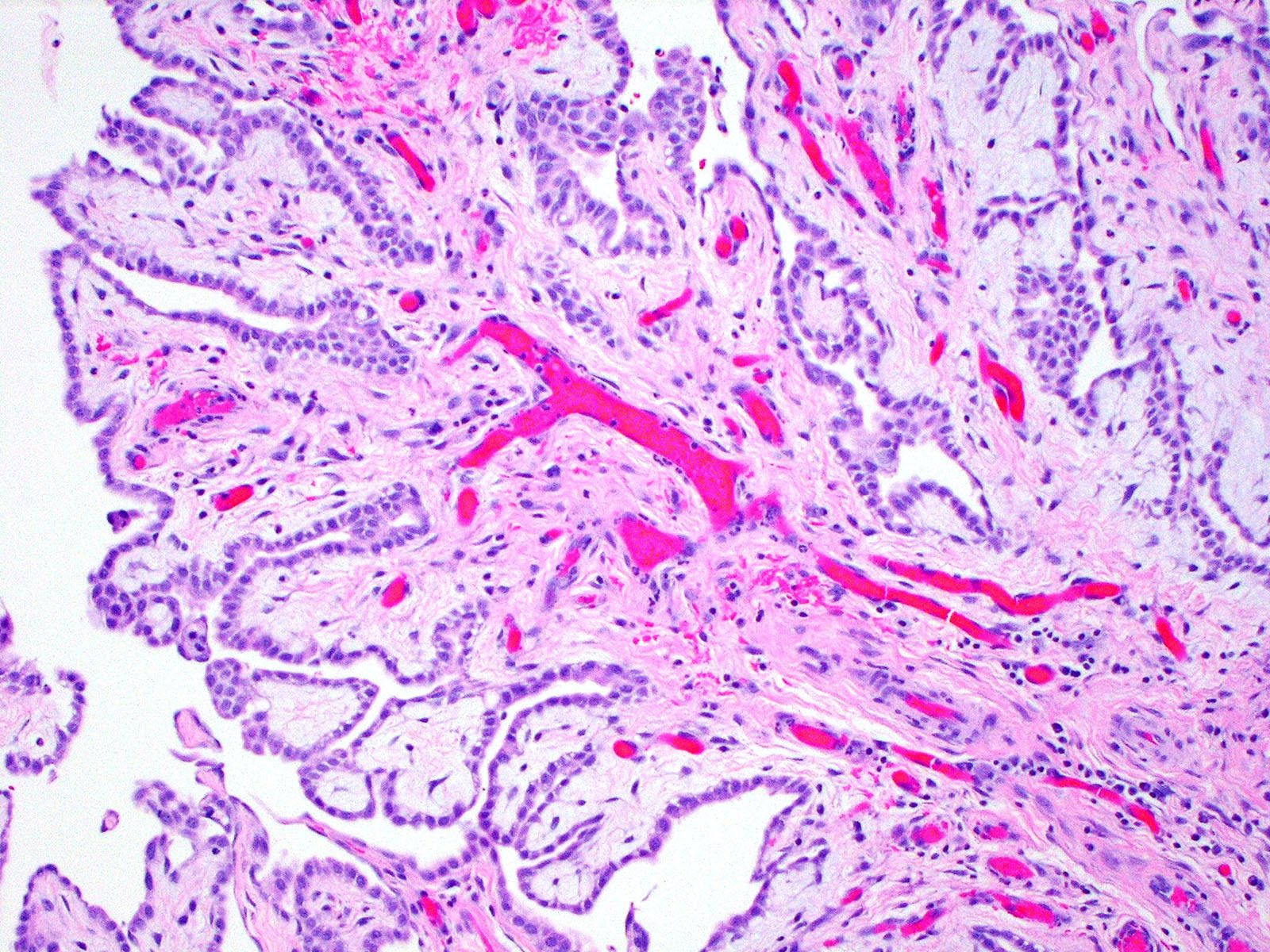
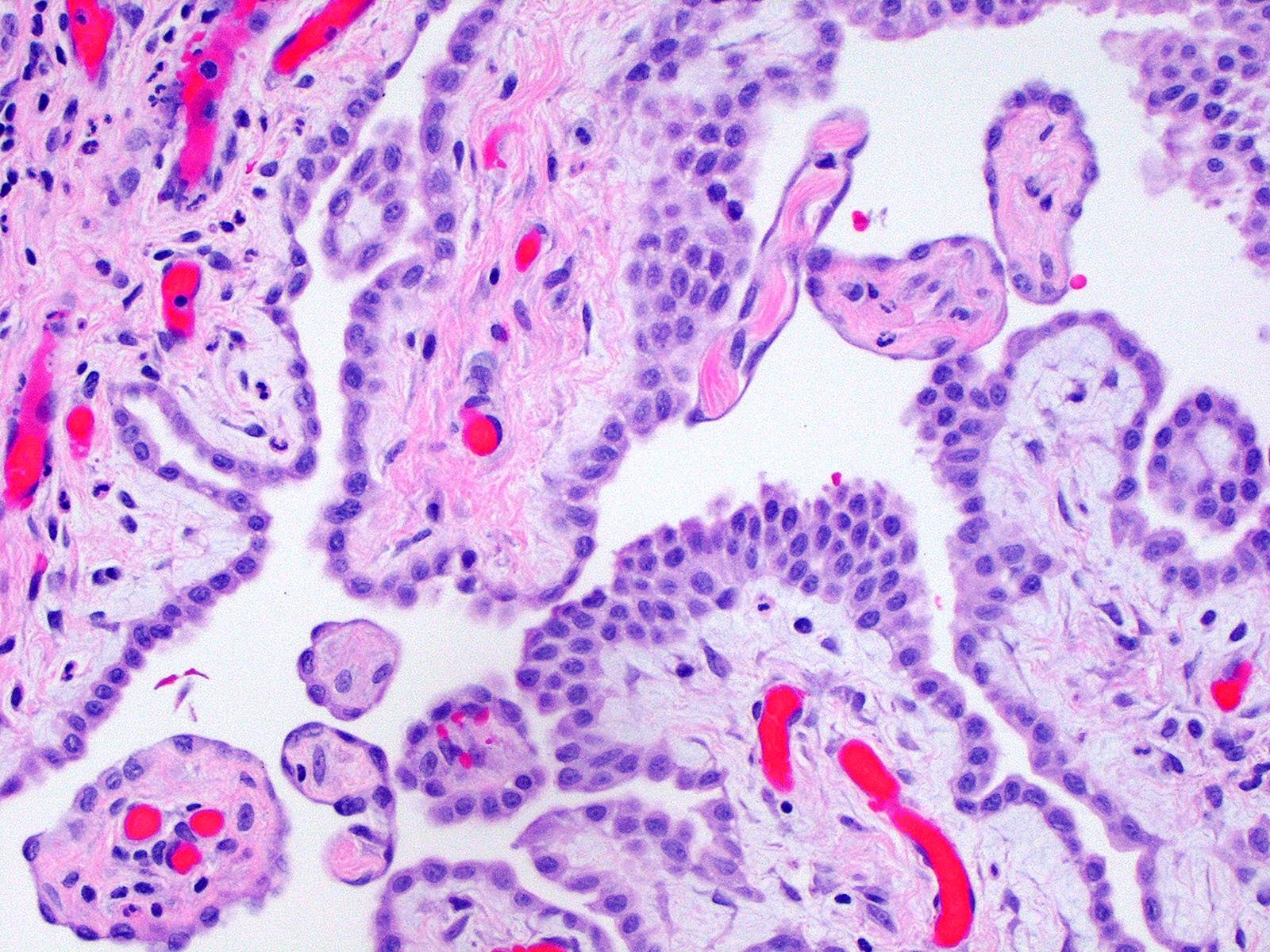
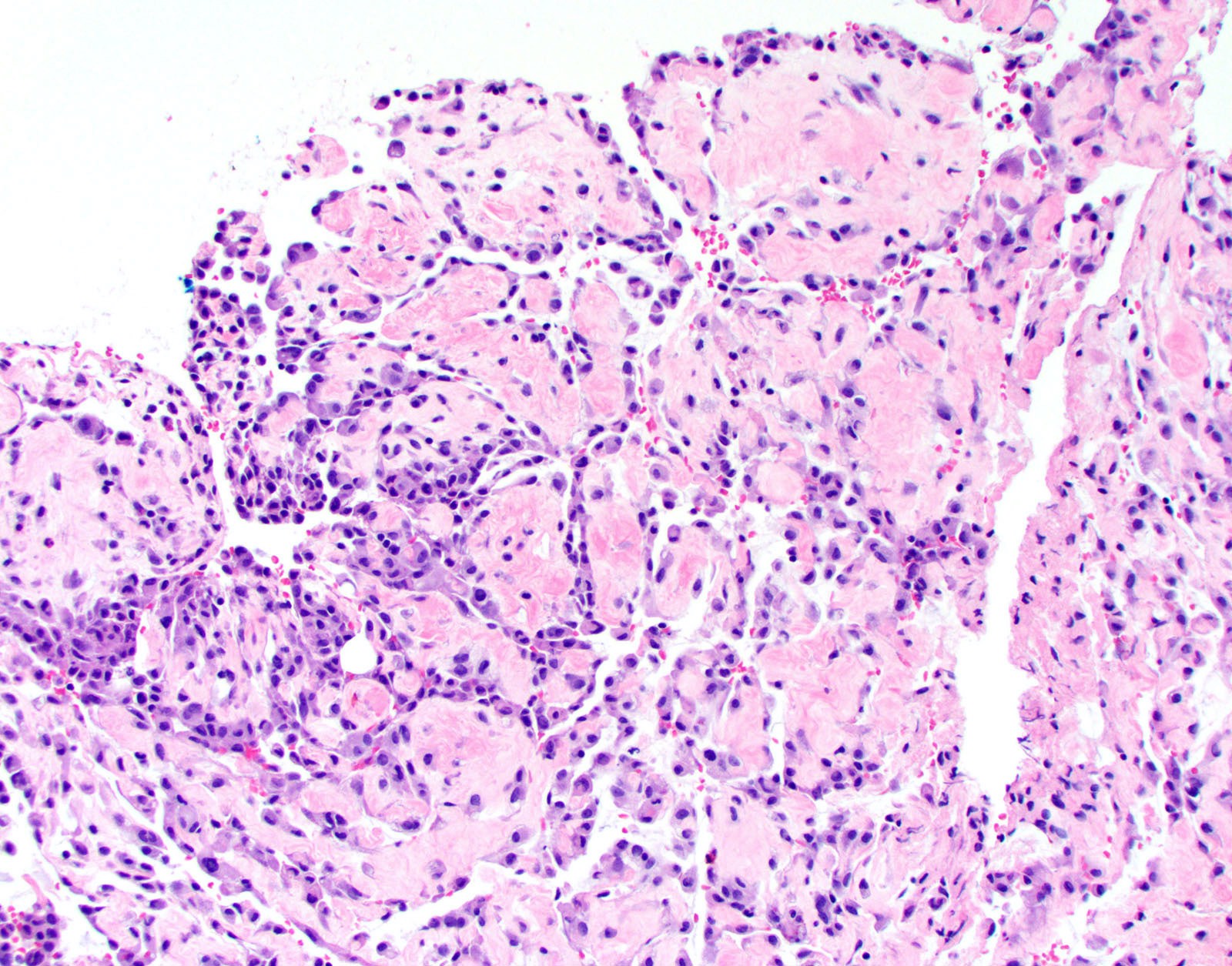
Microscopic (histologic) description
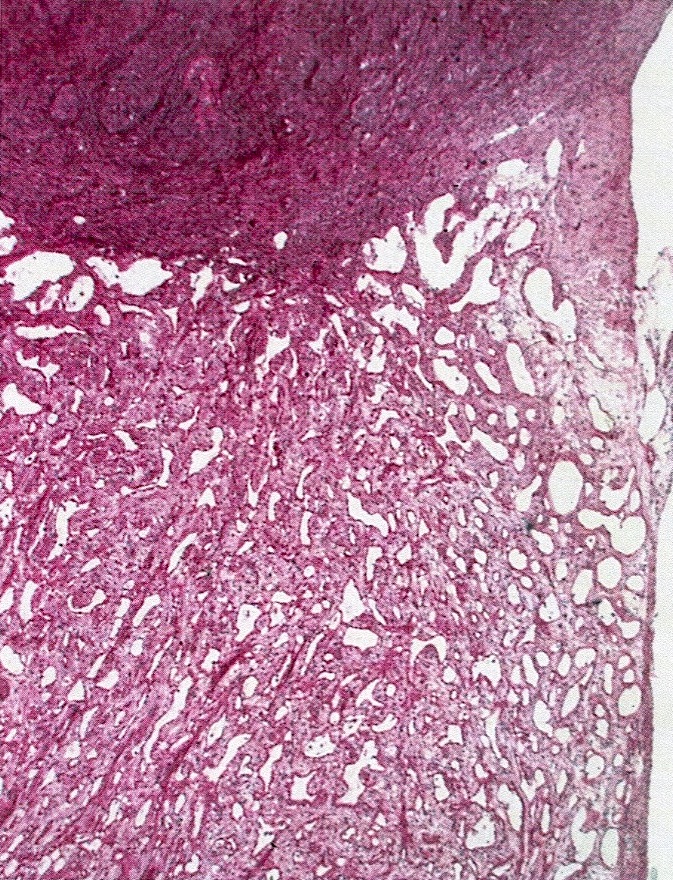
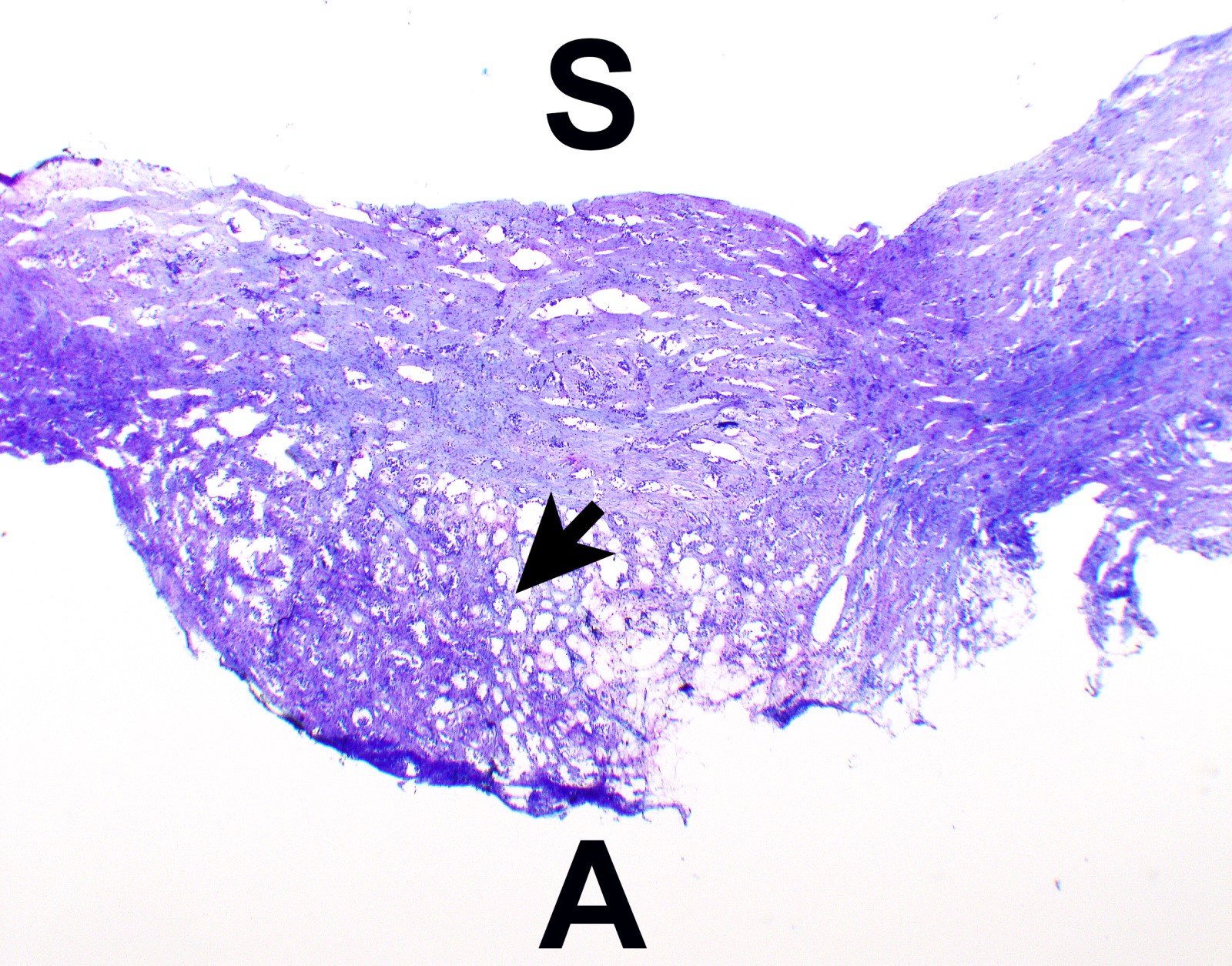
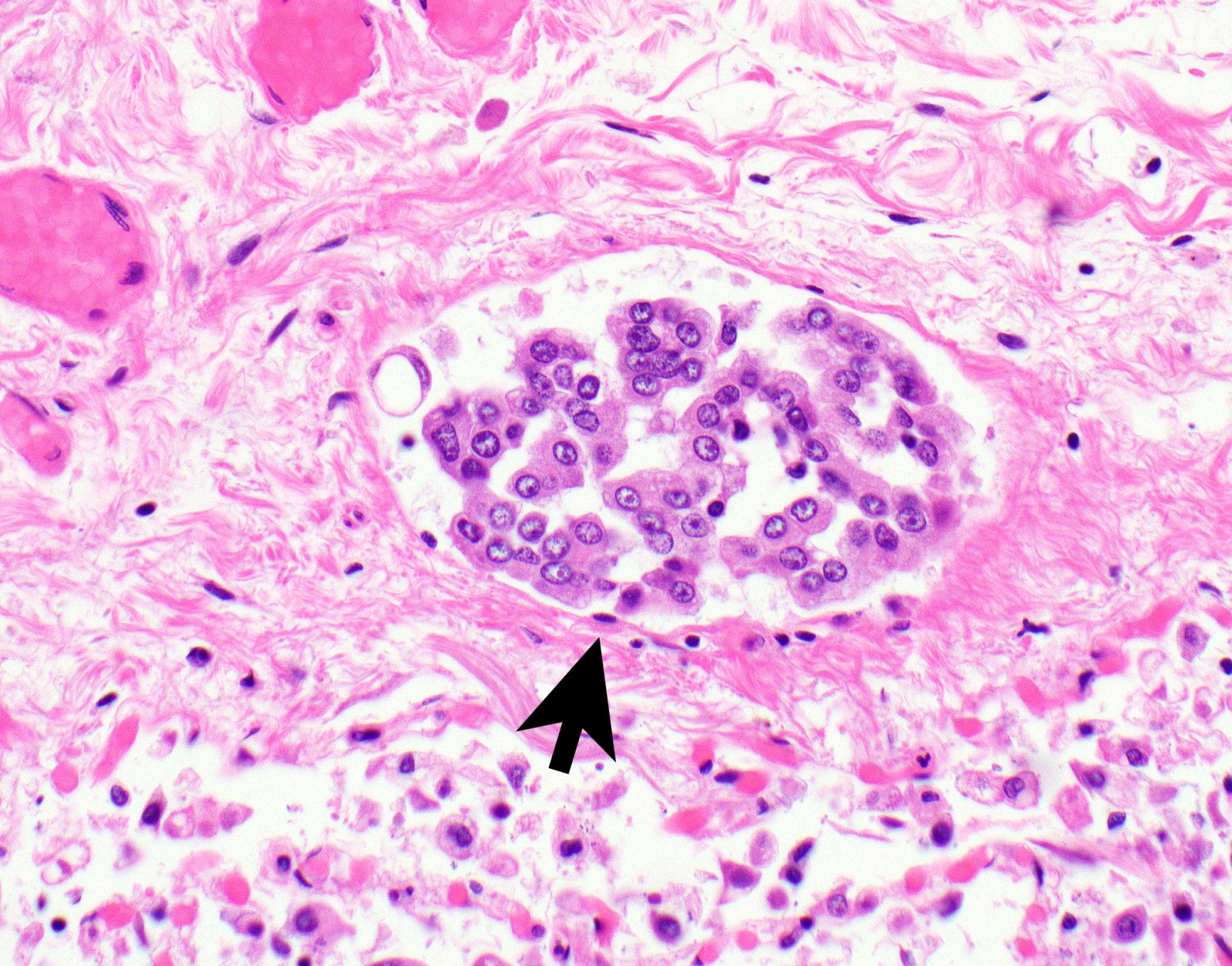
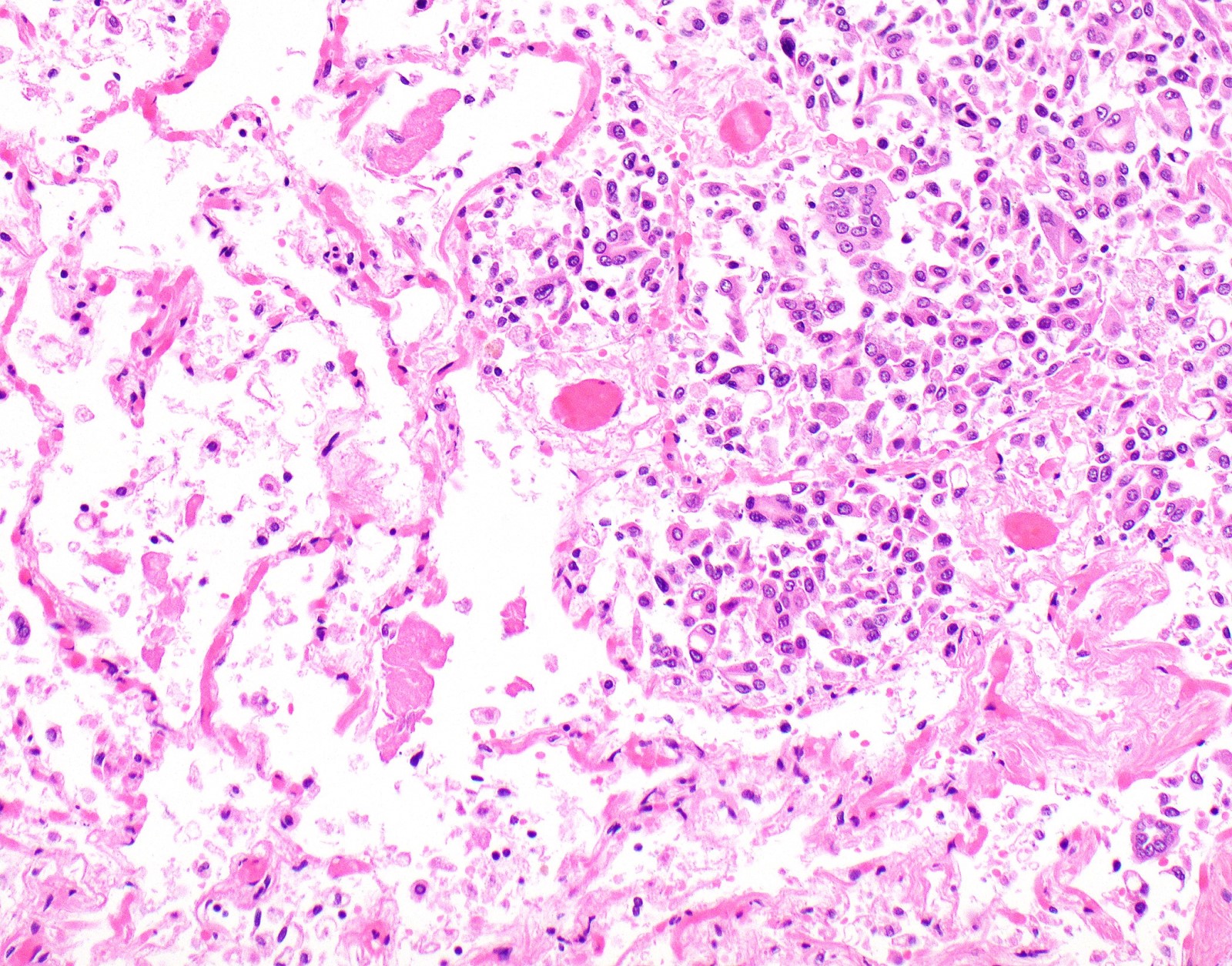
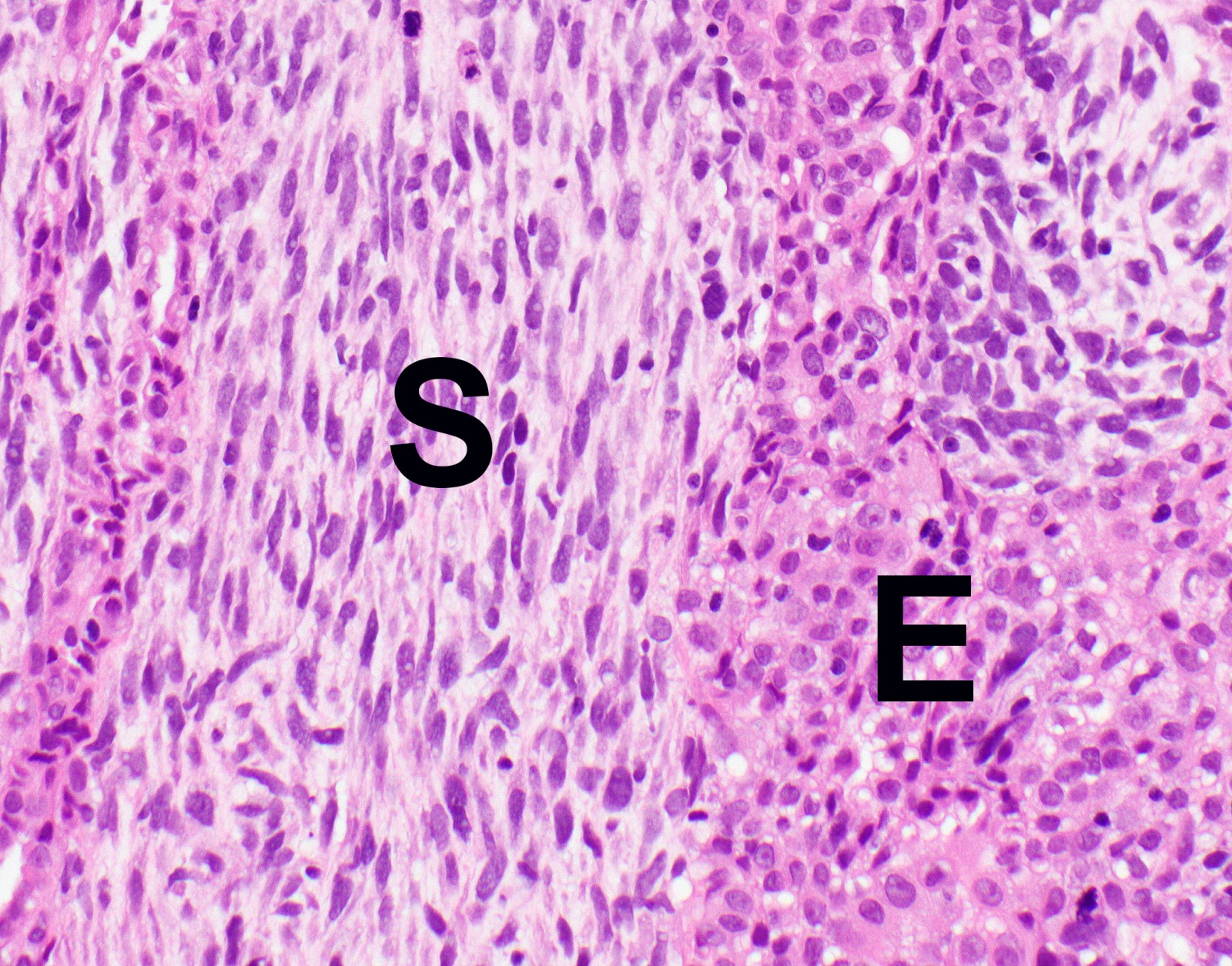
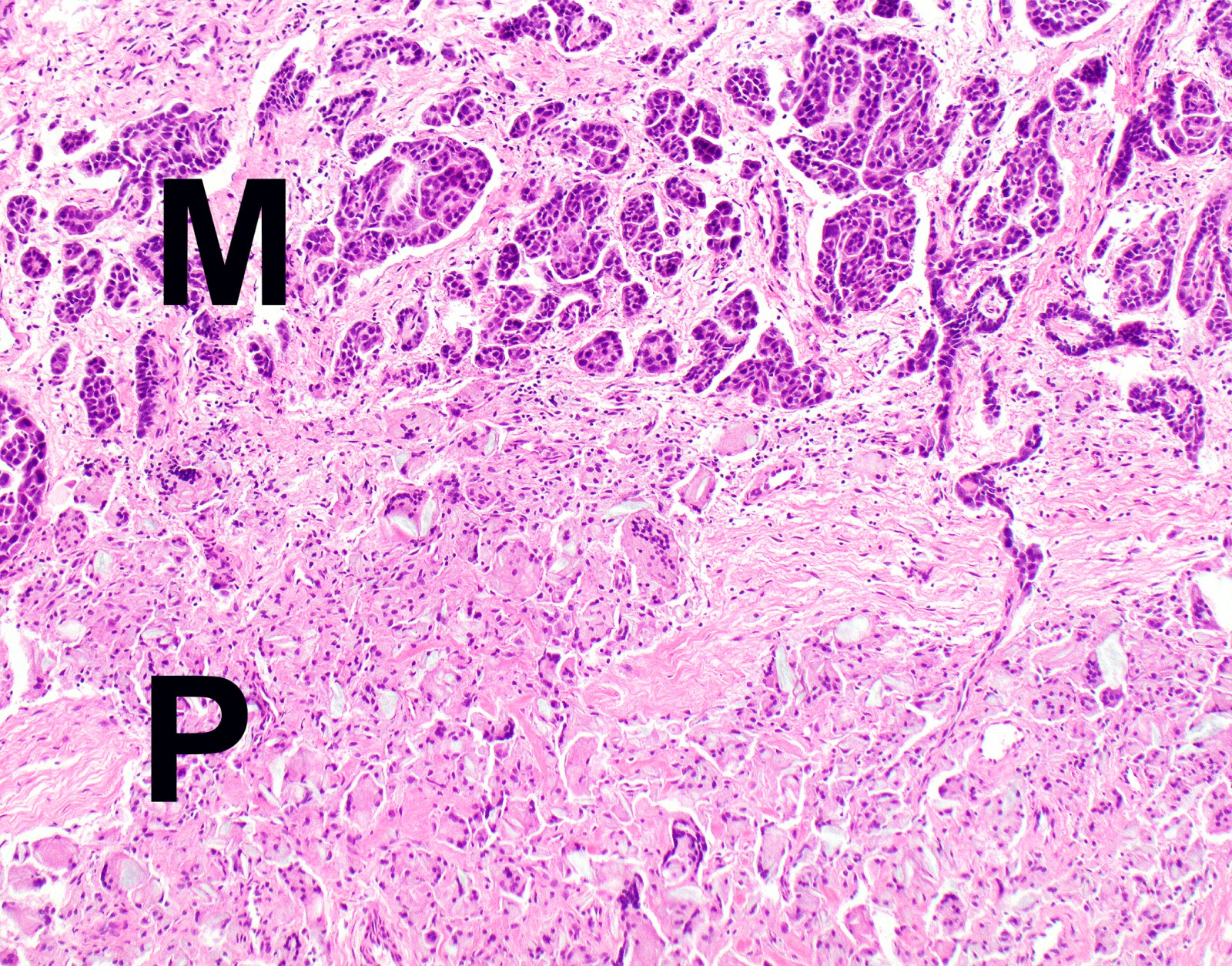
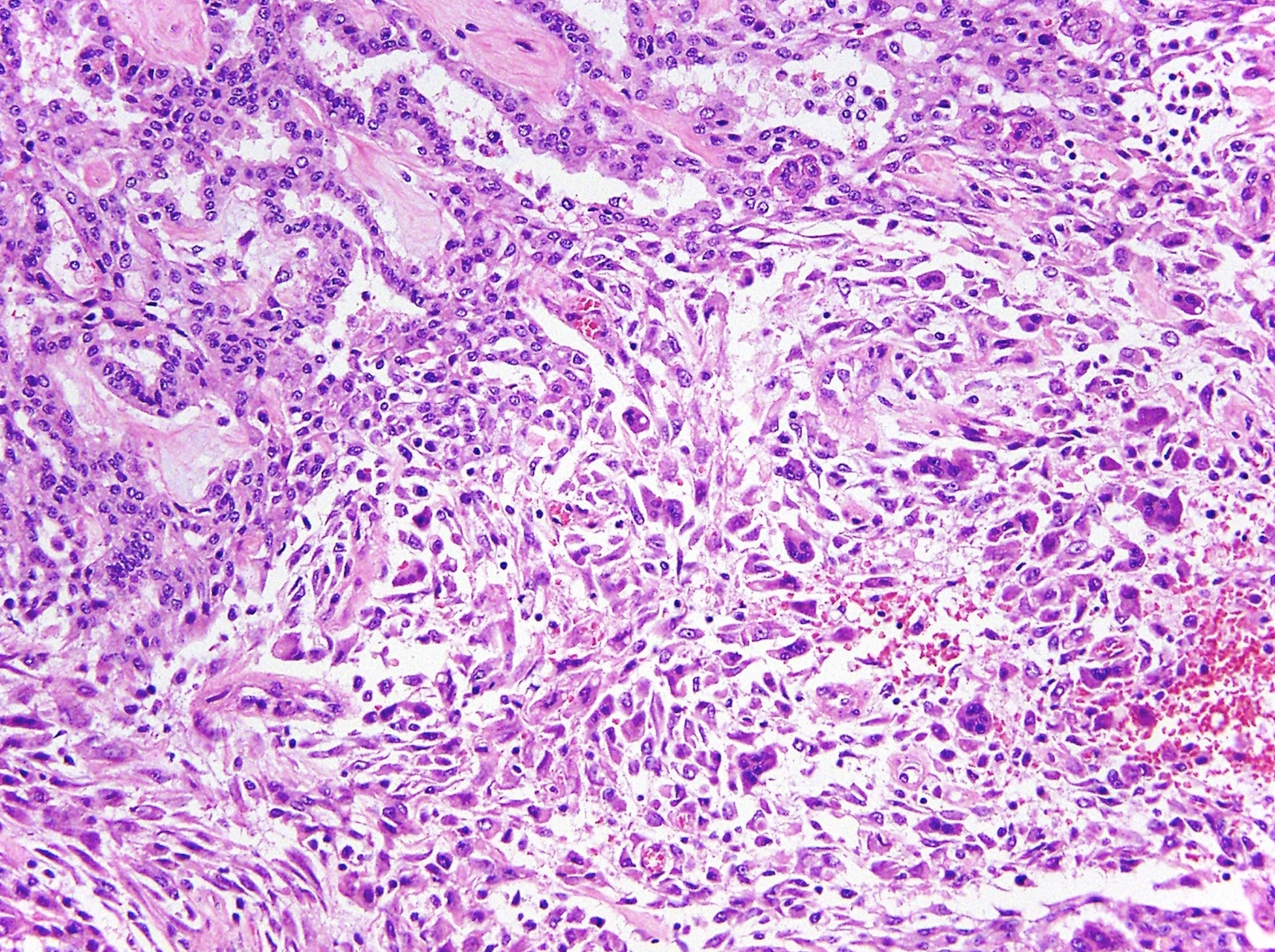
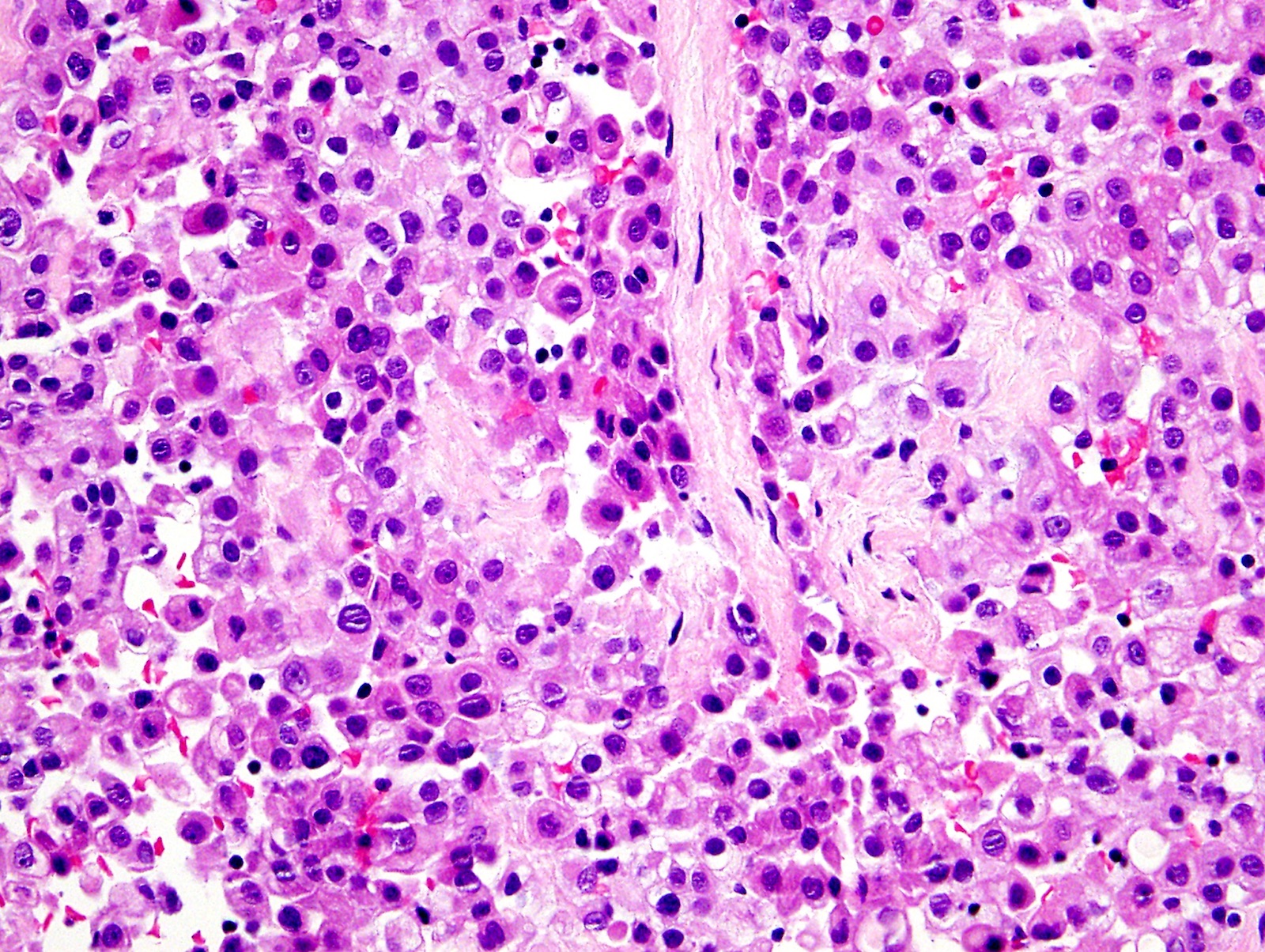
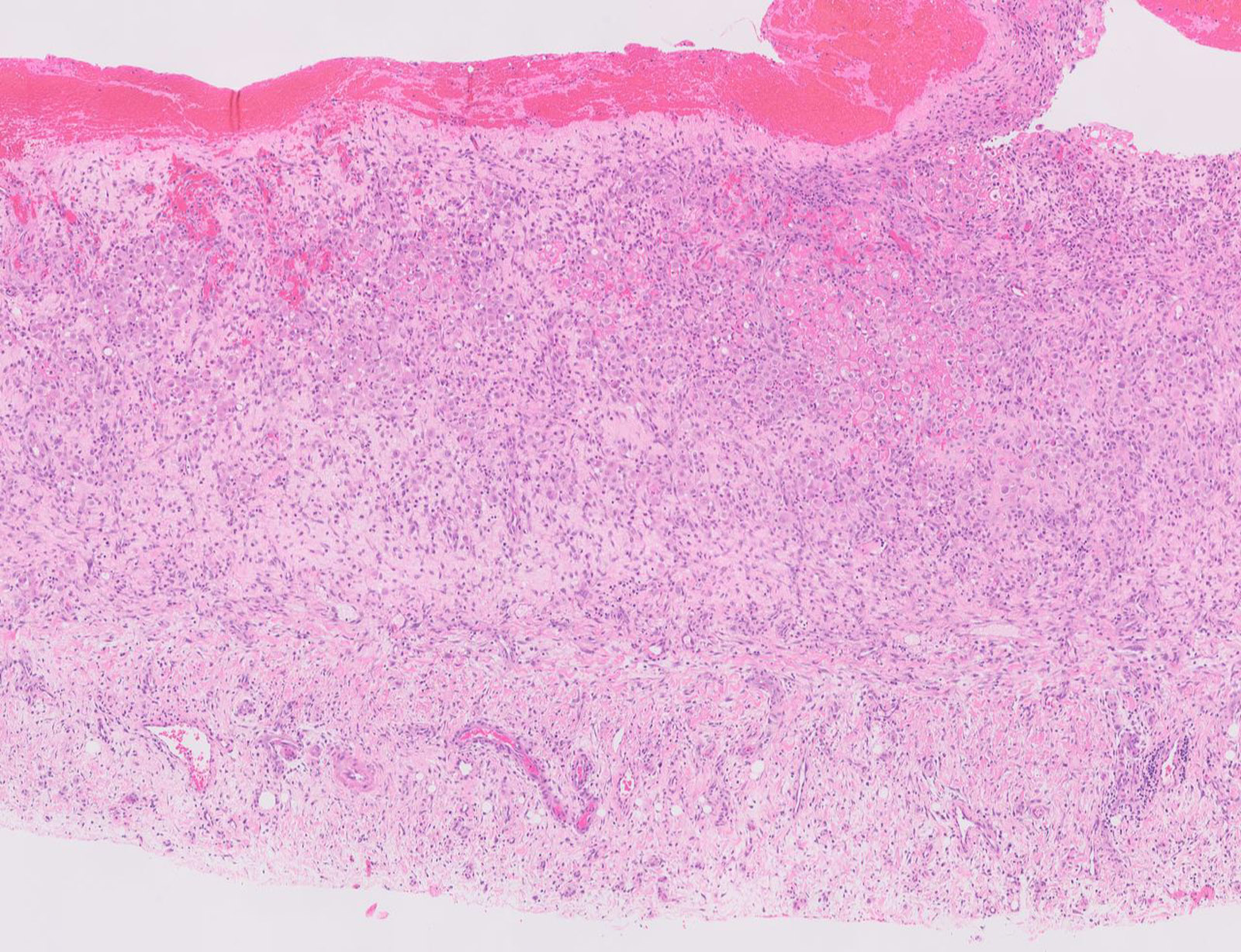
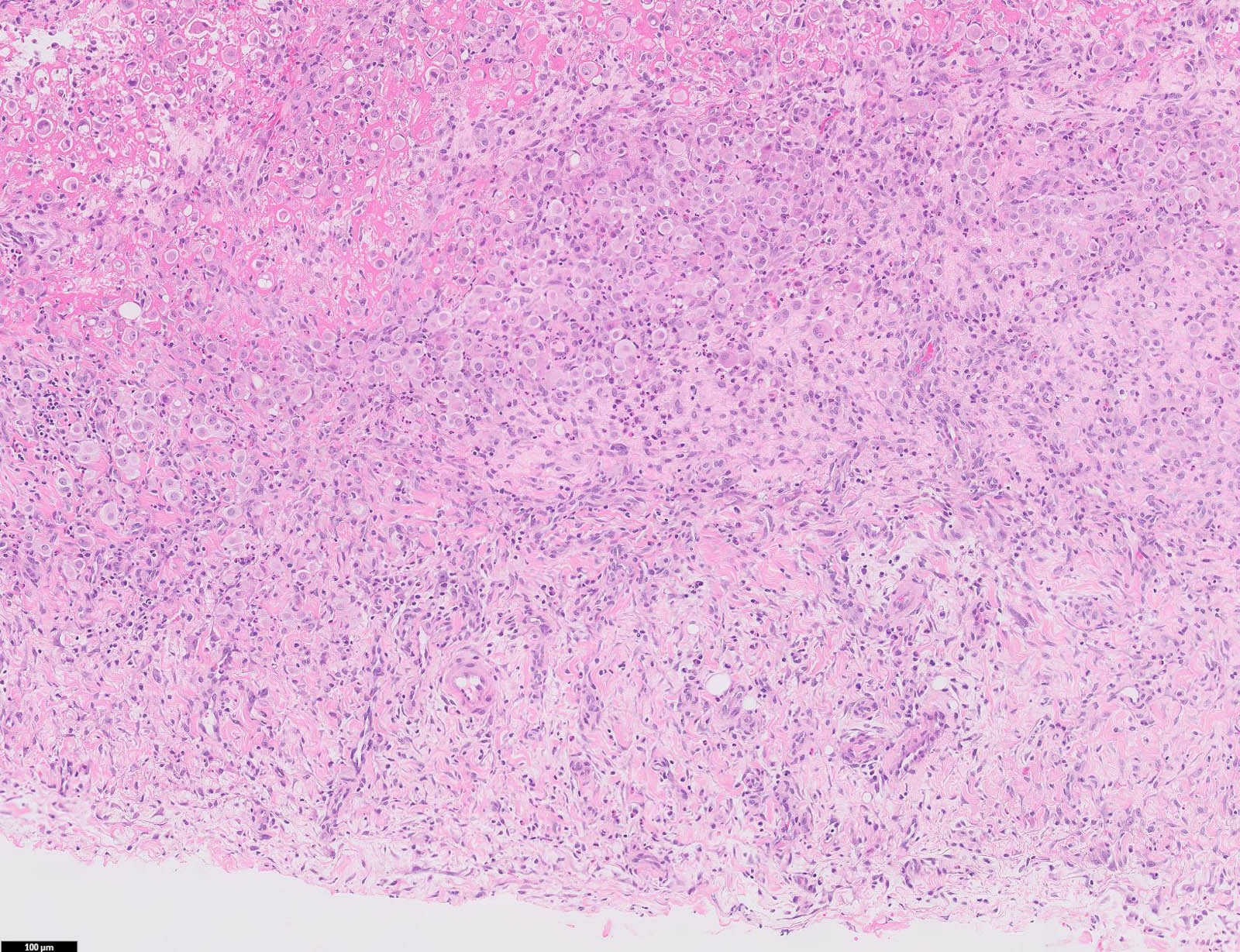
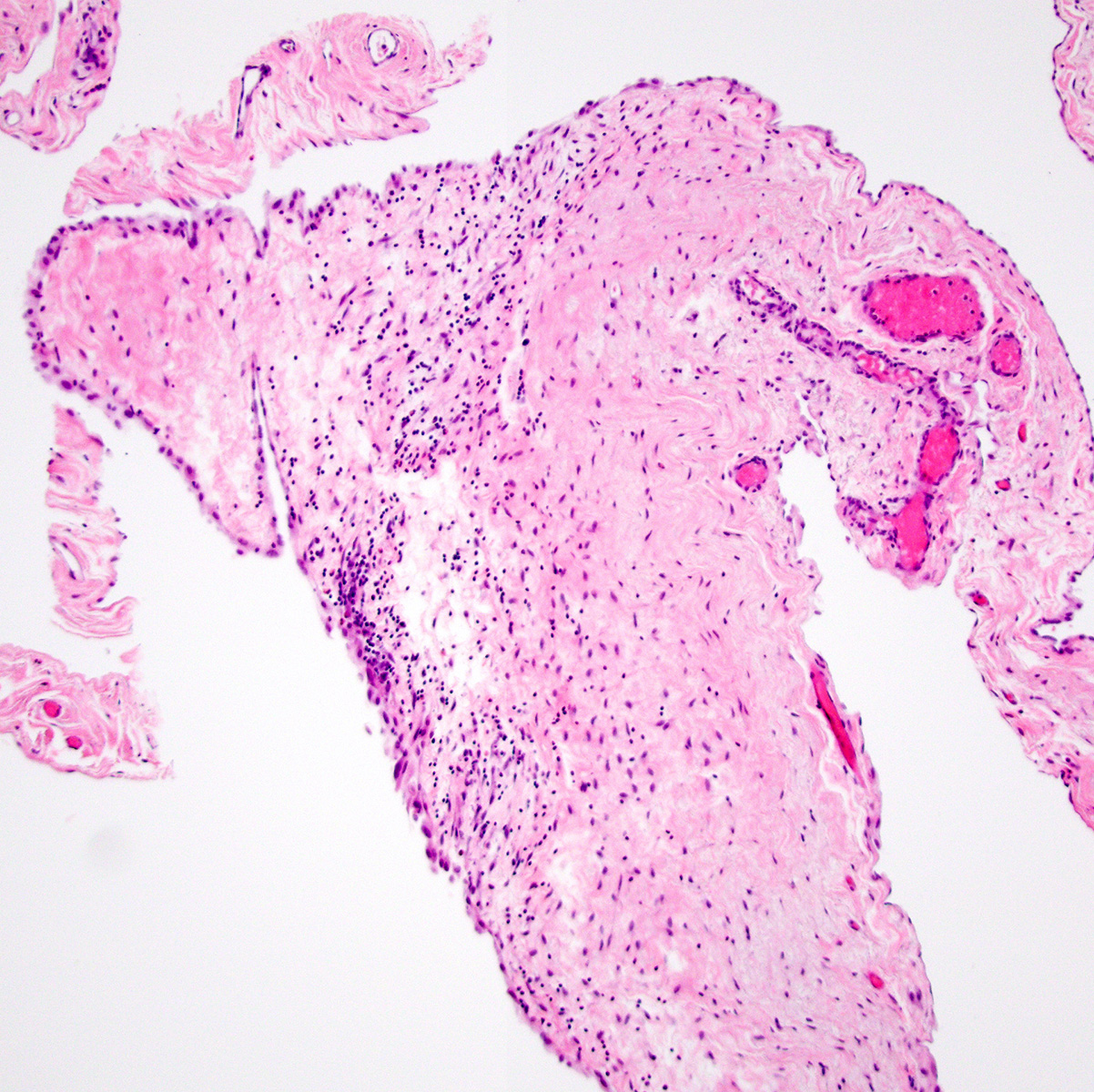
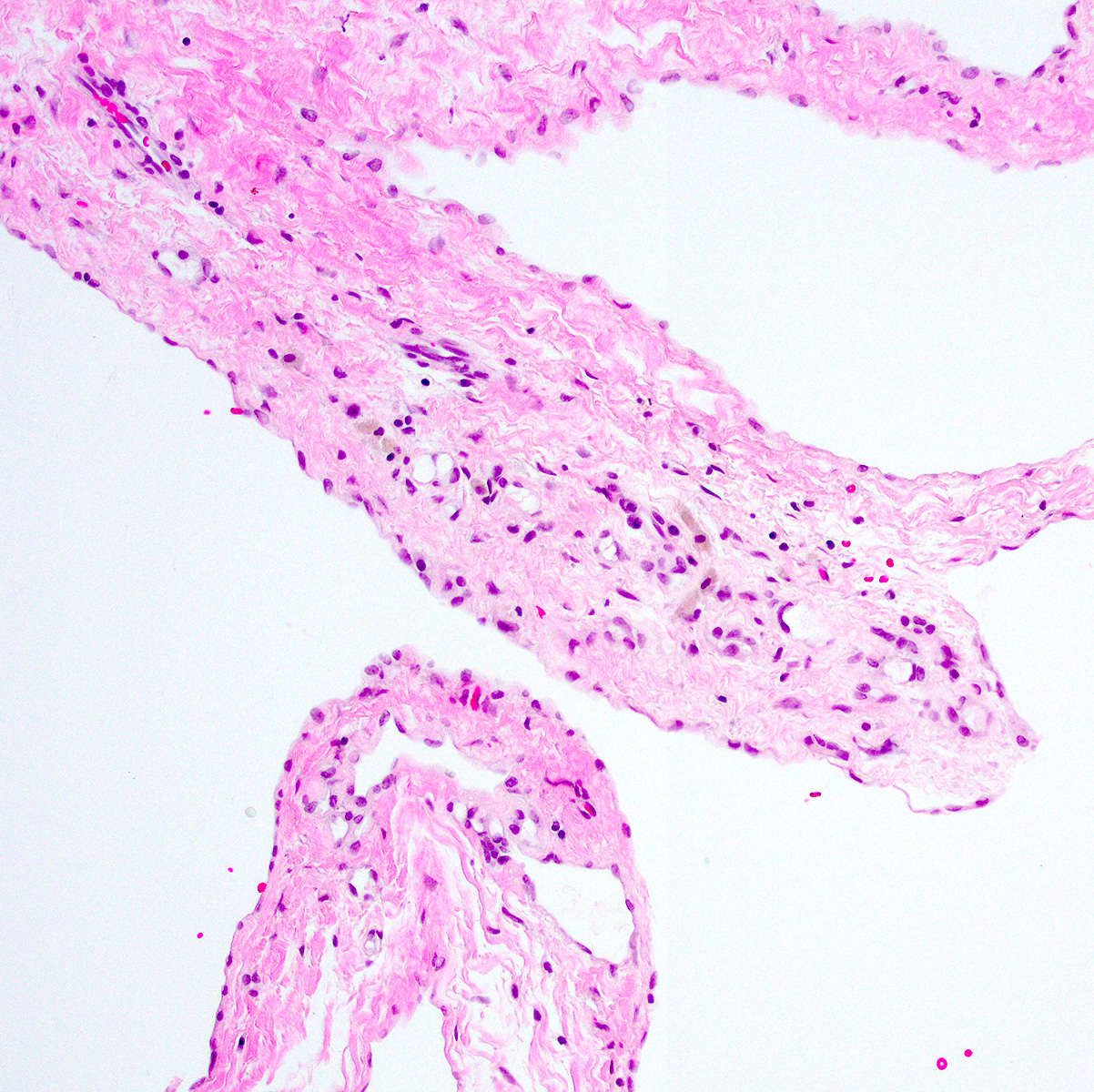
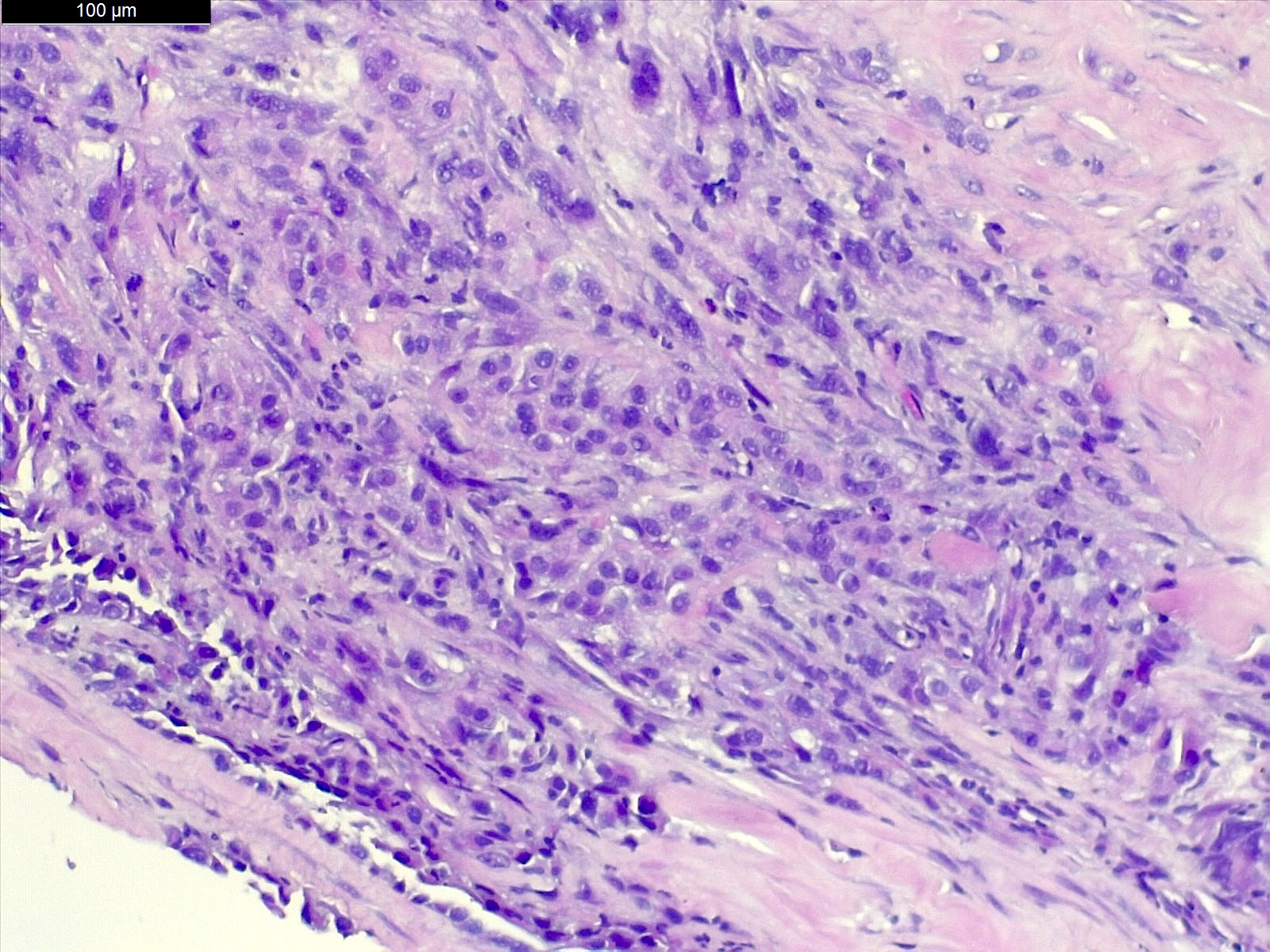
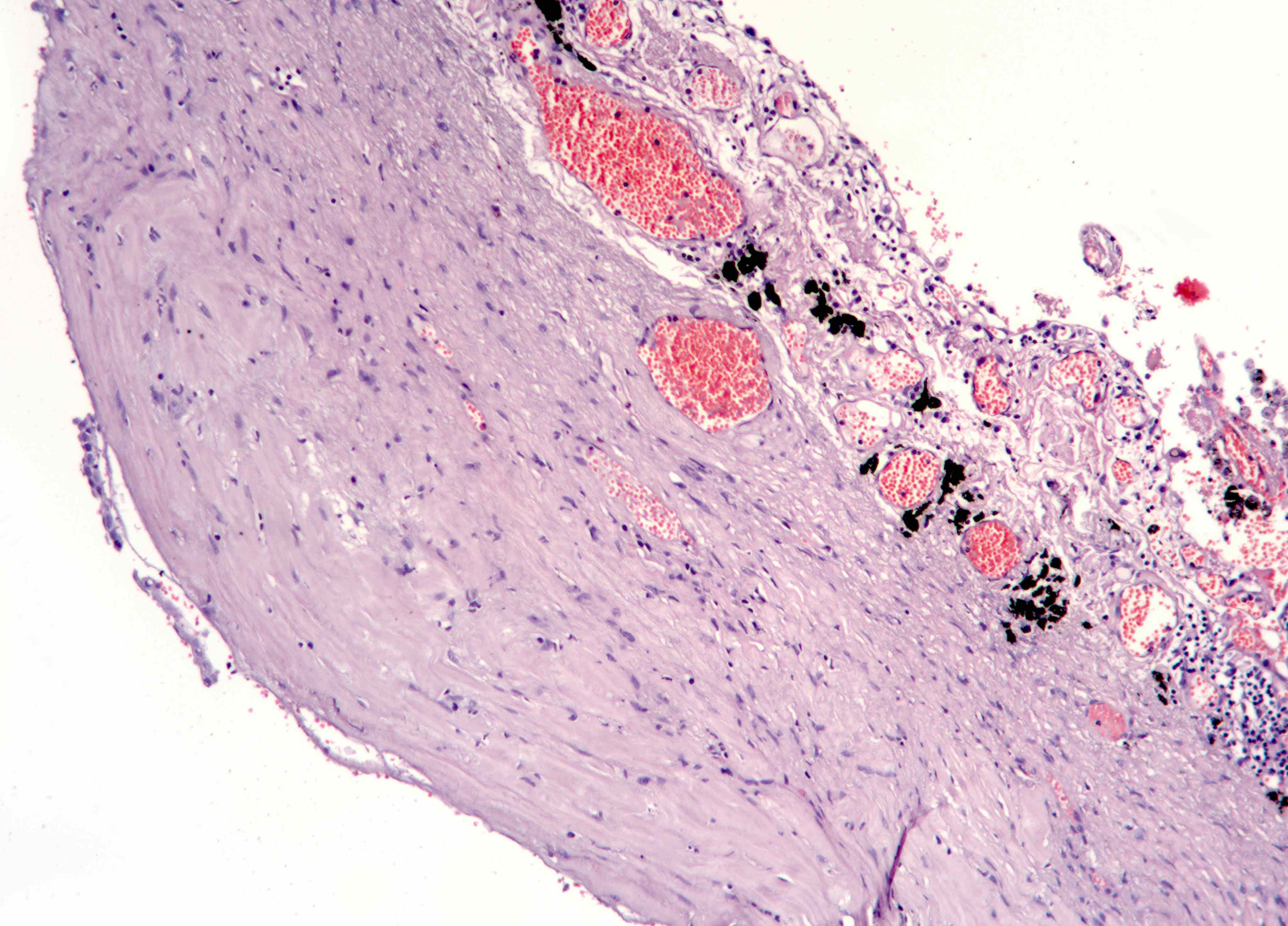
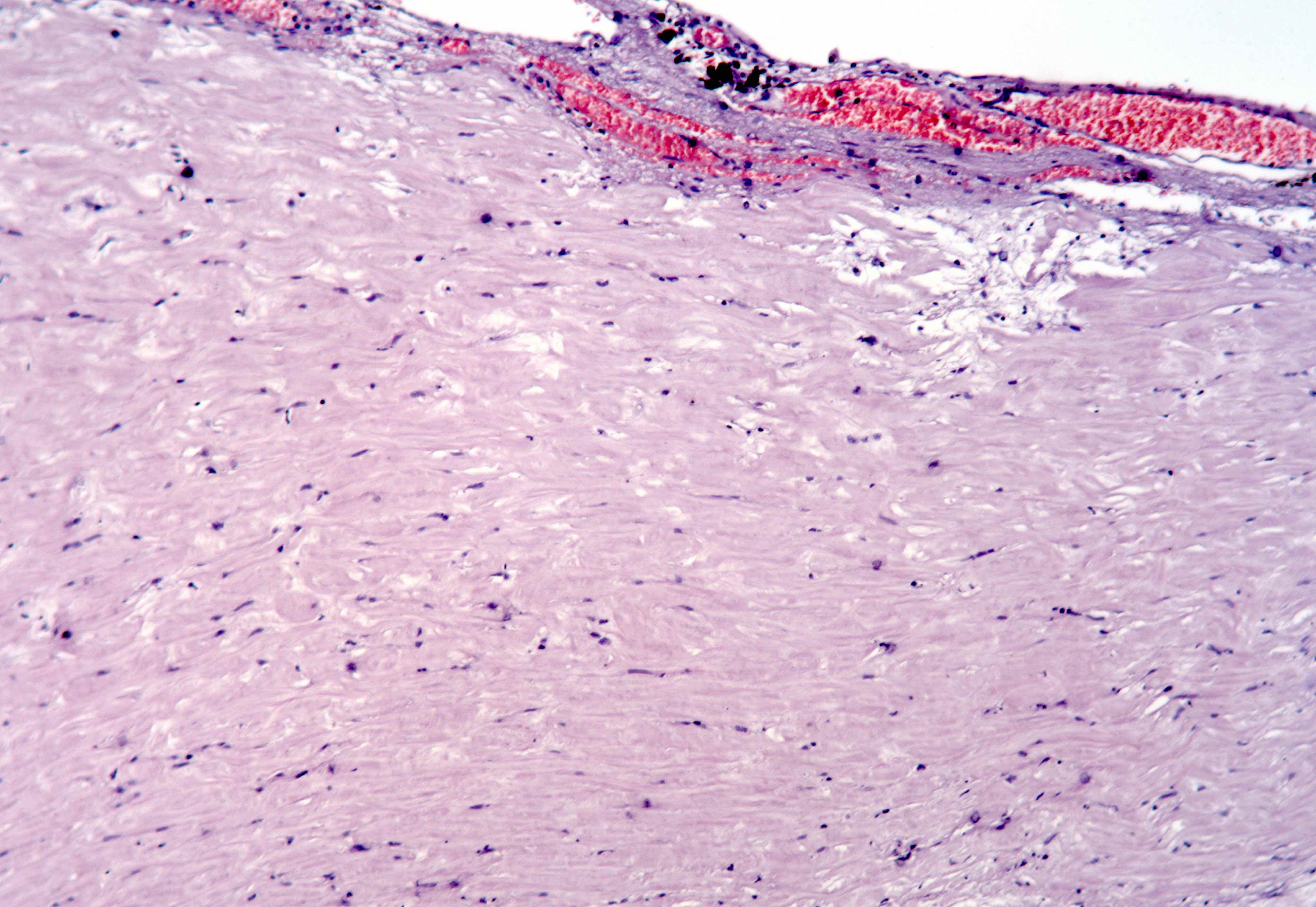
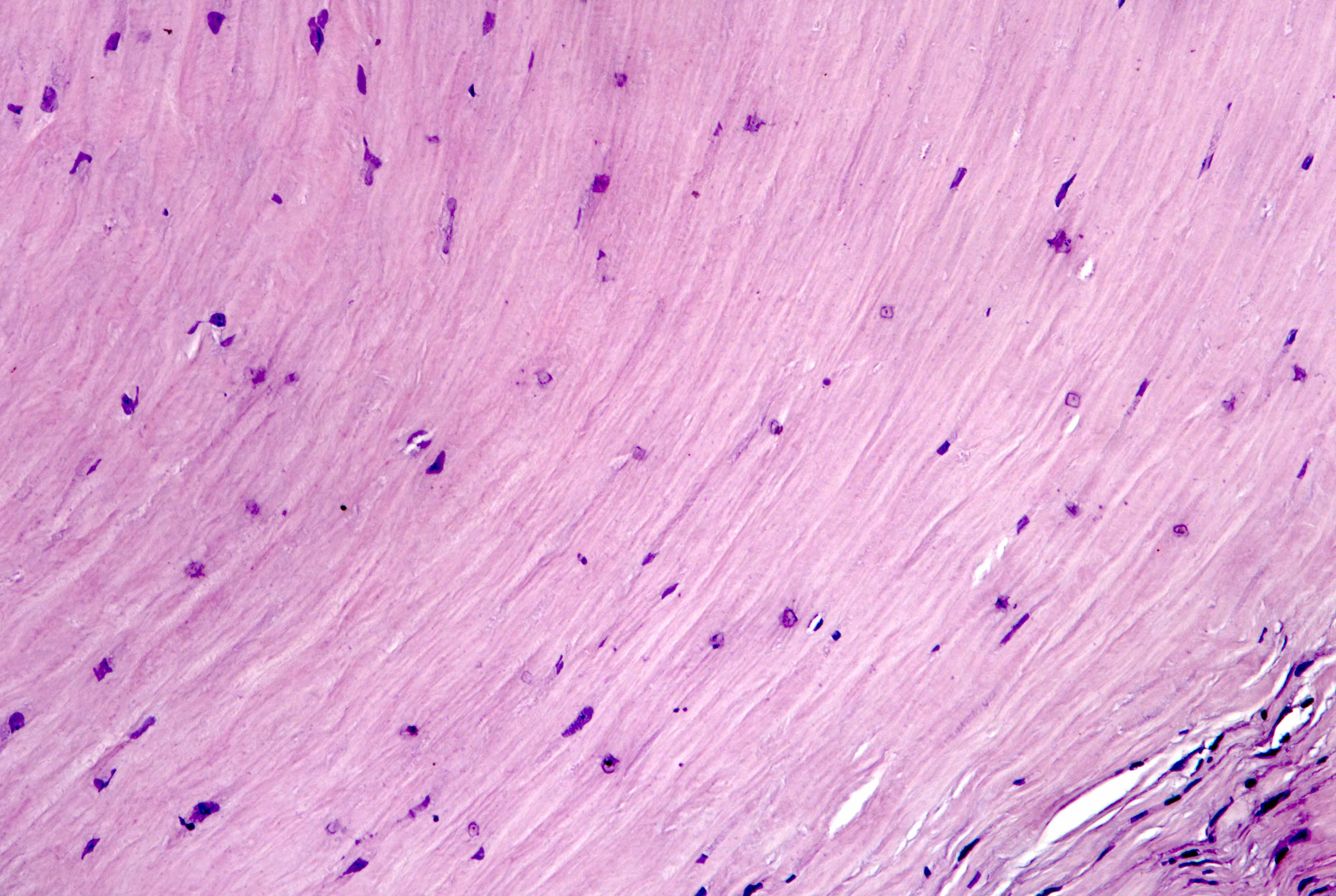
- Thickened pleura due to neoplastic cells and desmoplastic stromal reaction with or without necrosis
- In contrast, benign pleura: mesothelial cells are horizontally oriented, venules are perpendicular oriented, zonation of mesothelial cells [highest cellularity near serosal surface, trailing off toward chest wall], no invasion, no tumefactive growth (J Clin Pathol 2006;59:564)
- Invasion of neoplastic cells into adipose tissue, skeletal muscle or lung
- Tumefactive growth of malignant cells
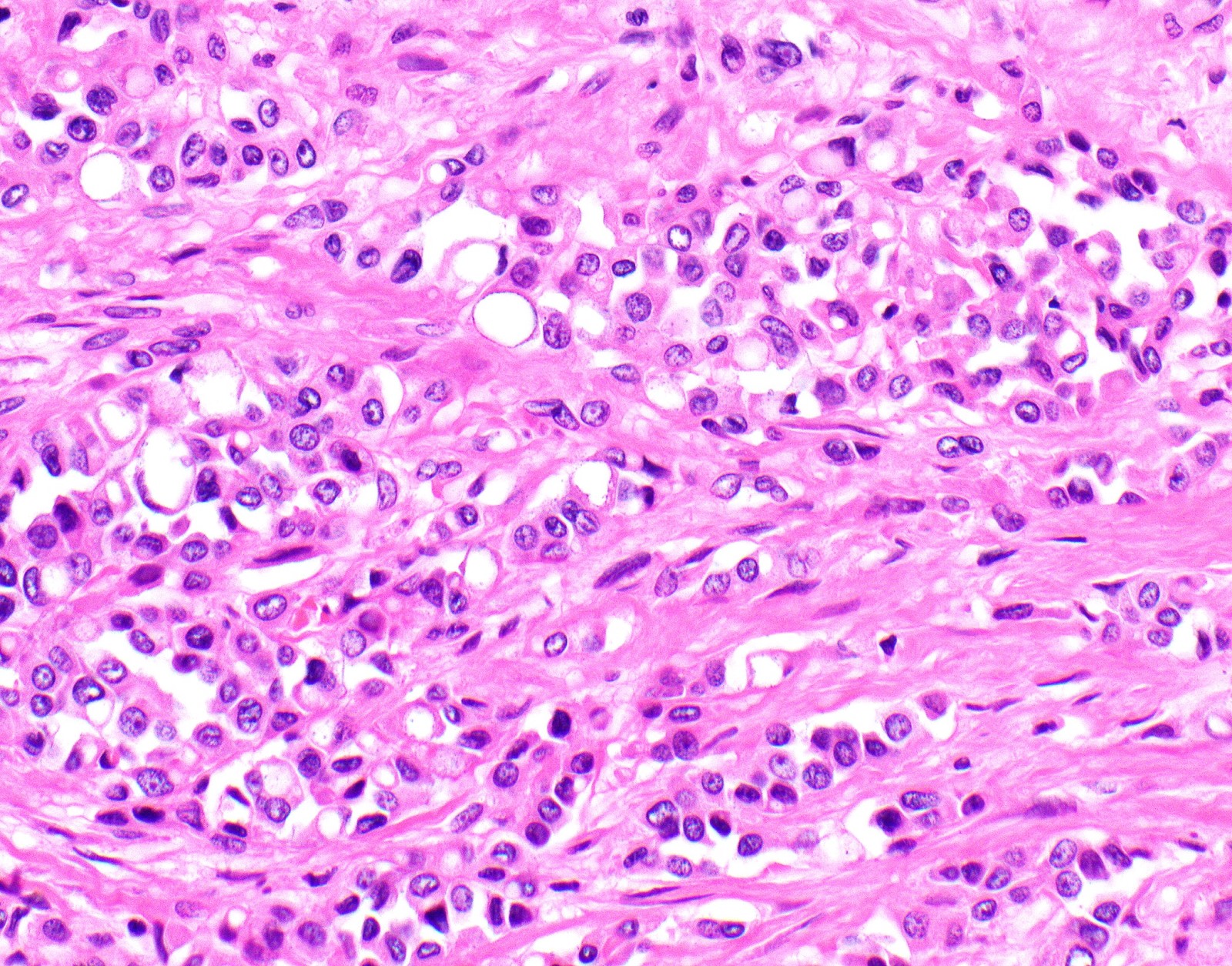
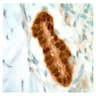
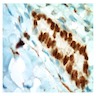
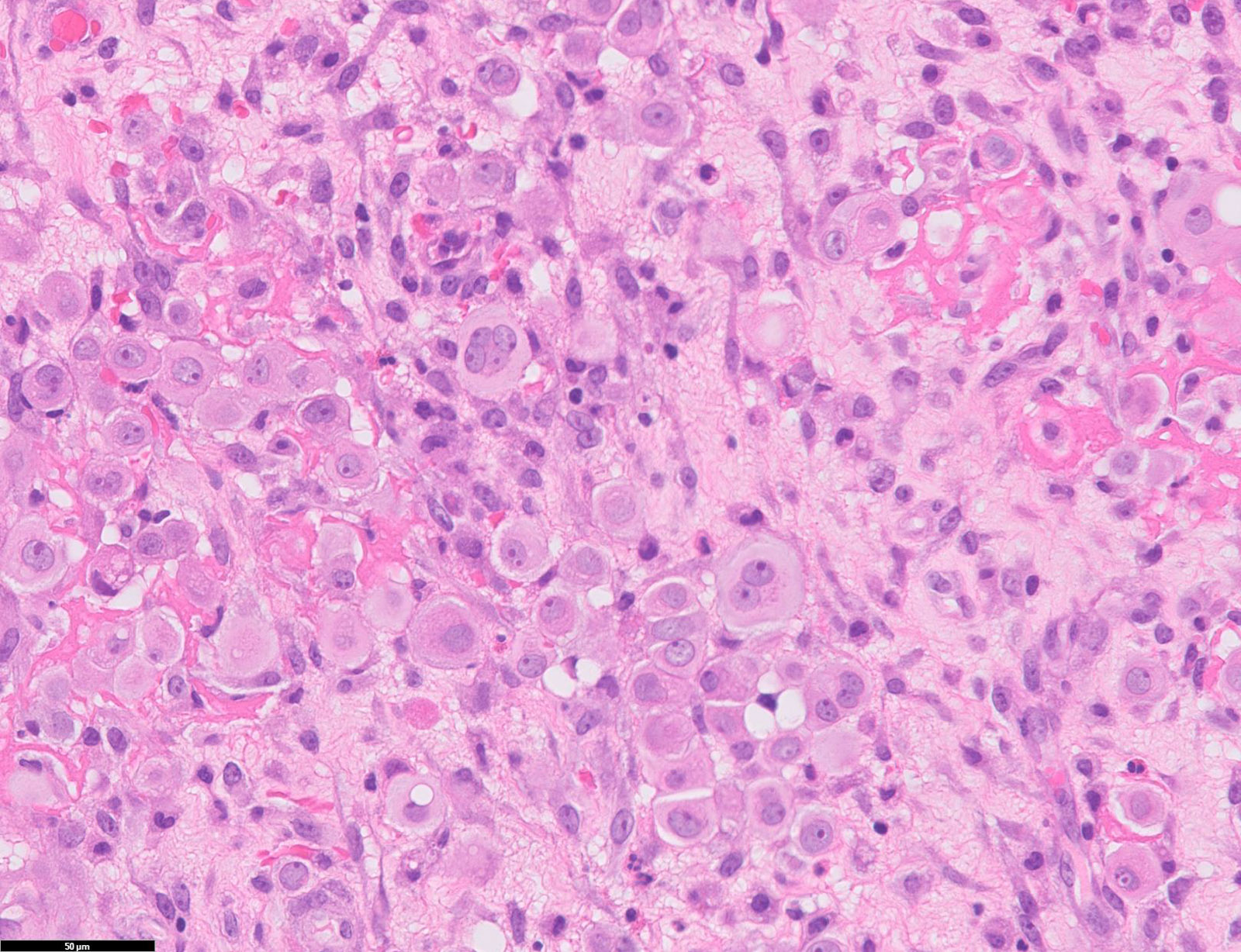
- Malignant mesothelial cells are either of epithelioid (epithelioid subtype) or spindled (sarcomatoid / desmoplastic subtype) cytology or a combination thereof (biphasic)
- Epithelioid subtype most common, 36 - 53%; sarcomatoid subtype, 12 - 27%, biphasic 11 - 19% (Thorac Cancer 2019;10:1193, Ann Thorac Surg 2018;105:432)
- If biphasic: provide percentage of sarcomatoid component
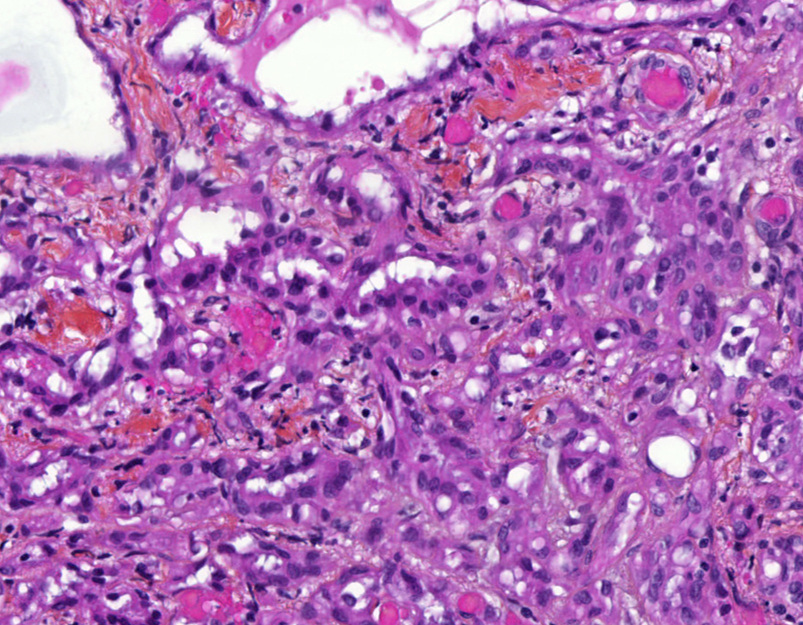
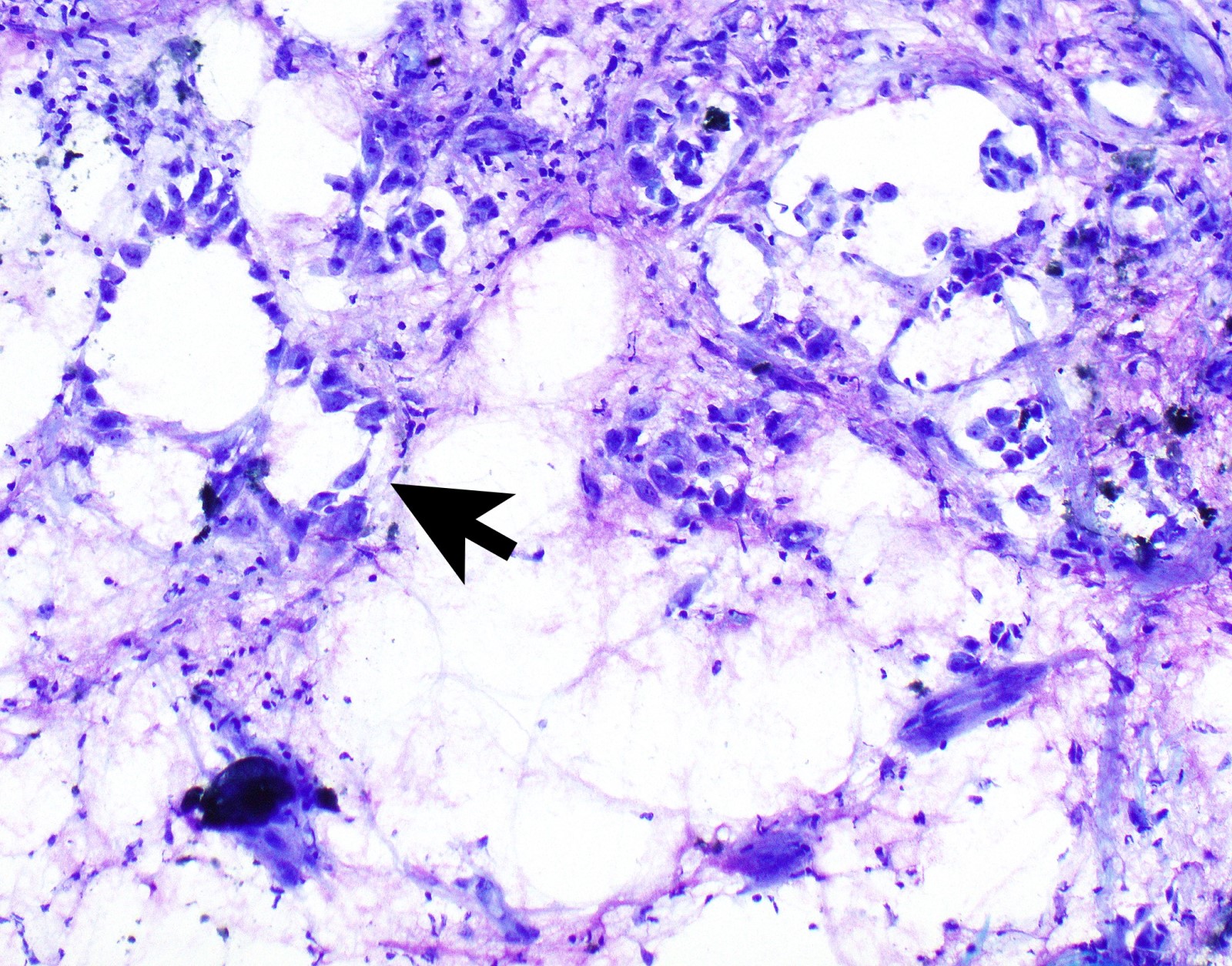
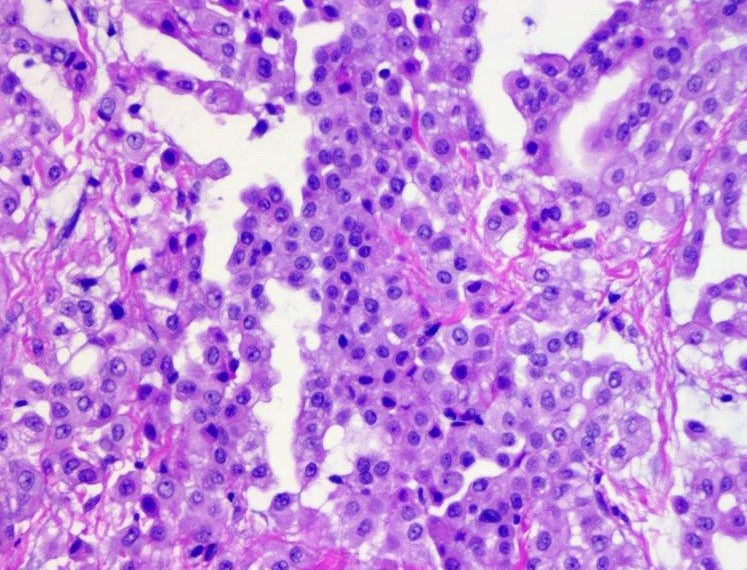
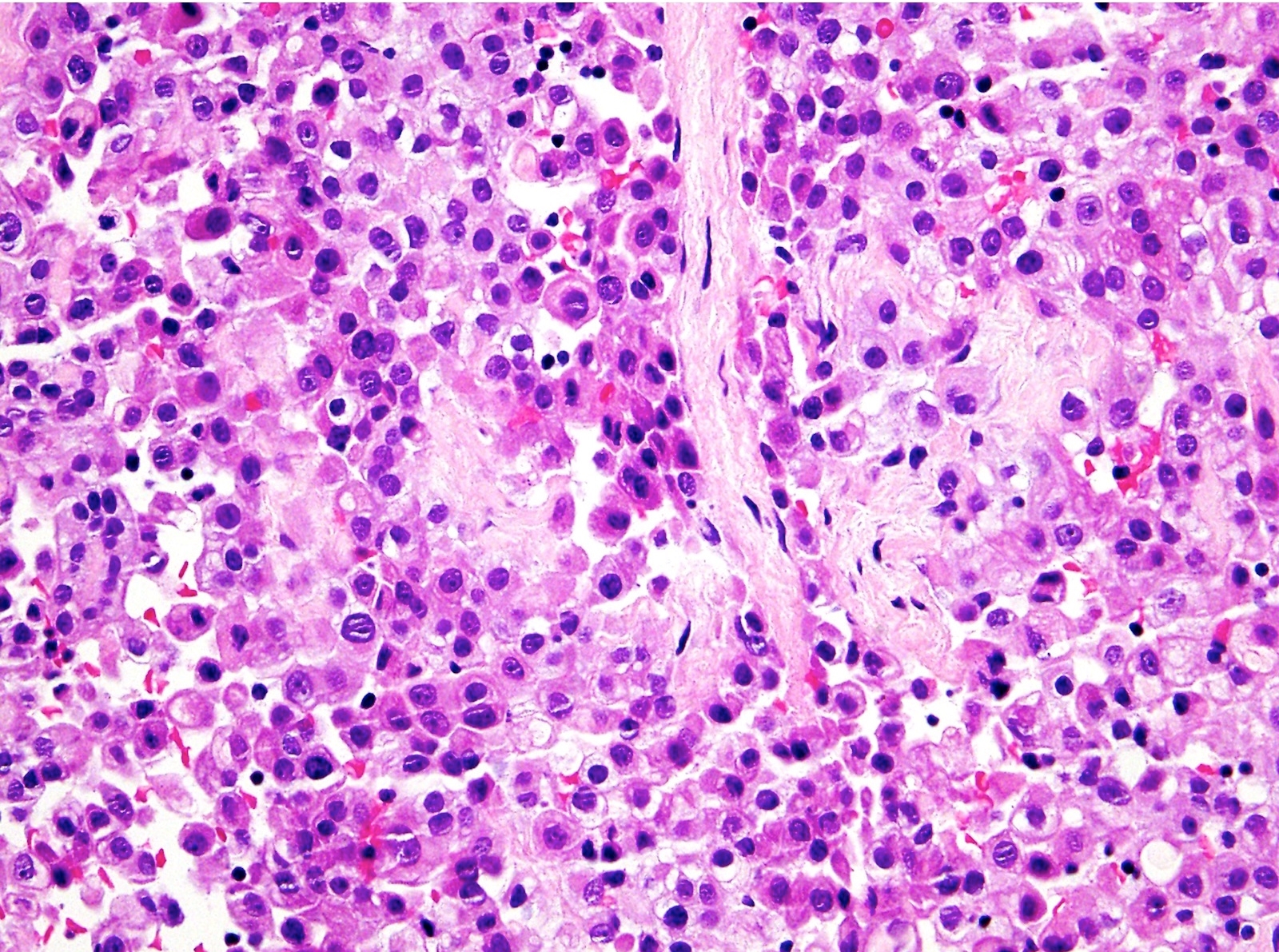
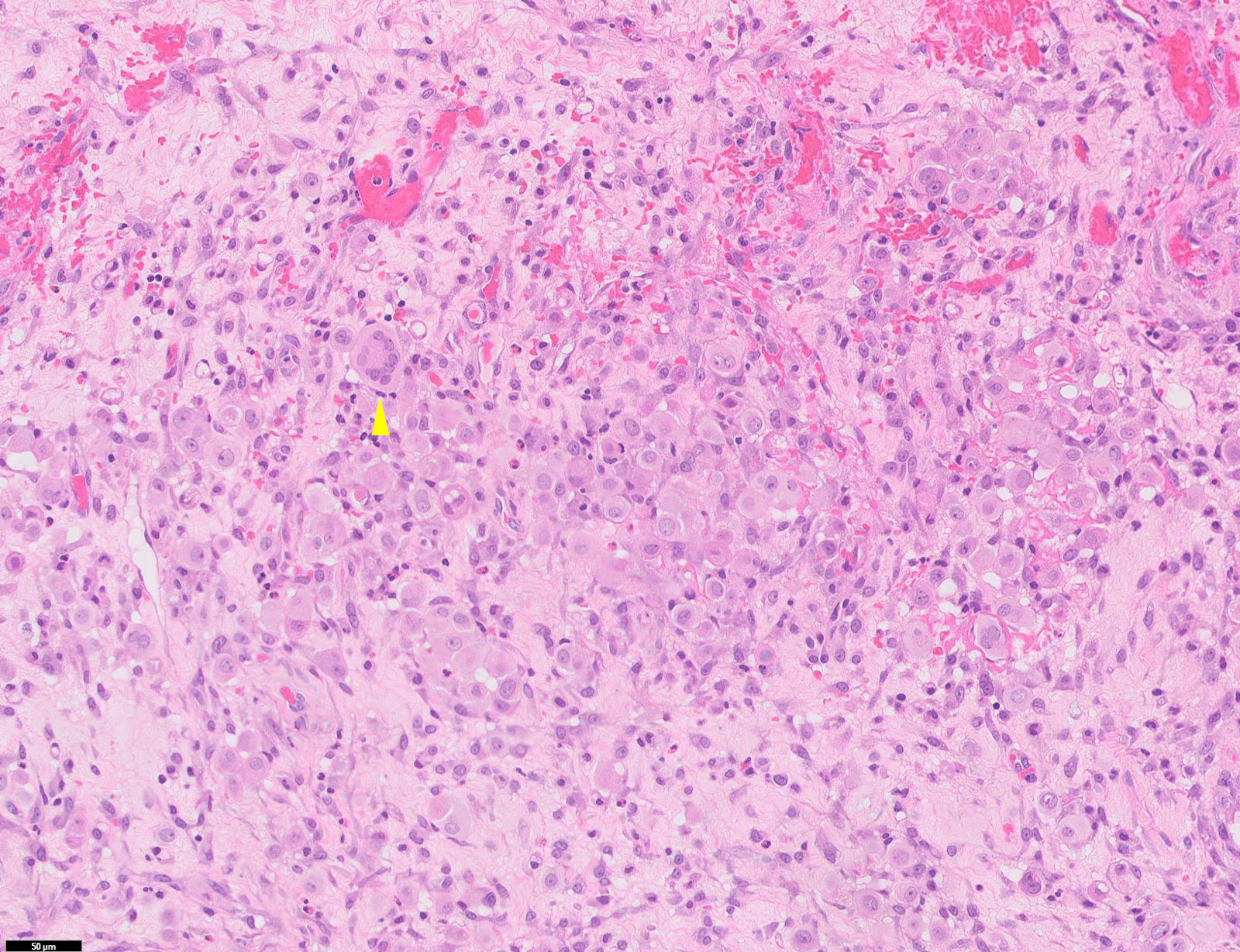
- Epithelioid:
- Cytology usually bland
- Patterns: solid, acinar (glandular), tubulopapillary, trabecular, micropapillary, microcystic (adenomatoid), clear cell, deciduoid, small cell
- Psammoma bodies might be present in any pattern
- Be aware of reactive spindle cells that might mimic sarcomatoid component
- 2 - 5% show cytoplasmic mucin (Ultrastruct Pathol 2006;30:3)
- Proposed nuclear grading (Mod Pathol 2012;25:260):
- Grade I - III
- Scoring:
- Mitotic count: score 1 (0 - 1 mitoses/10 HPF), score 2 (2 - 4/10 HPF), score 3 (≥ 5/10 HPF)
- Nuclear atypia: score 1, mild atypia, score 2, moderate atypia, score 3, severe atypia
- Composite score: 2 - 3 (grade I), 4 - 5 (grade II), 6 (grade III)
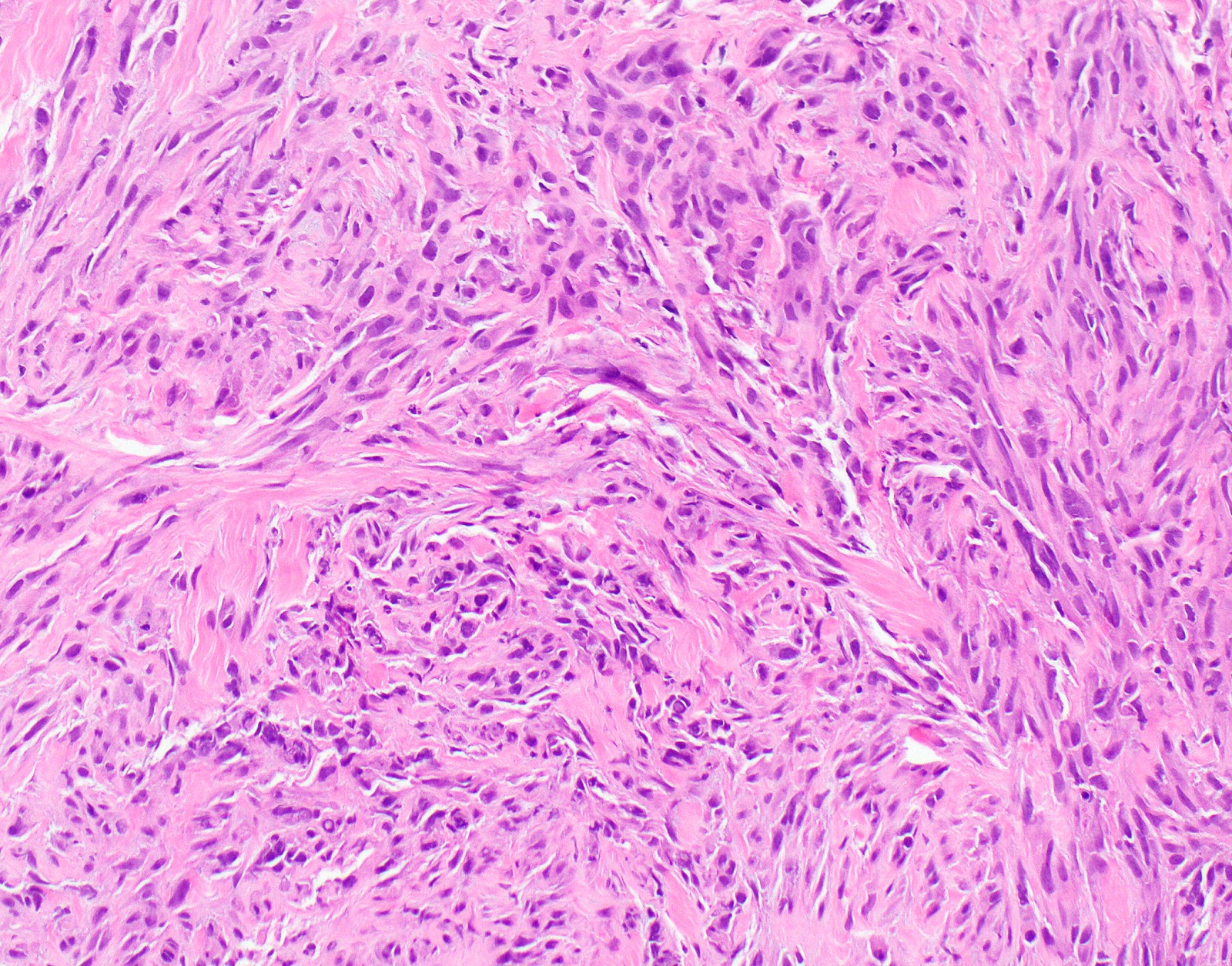
- Sarcomatoid:
- Haphazard growth of malignant cells
- Desmoplastic variant: paucicellular, usually bland appearing spindle cells growing in a storiform pattern in a collagenized background with stromal invasion and possible bland necrosis (Arch Pathol Lab Med 2018;142:89)
- Transitional pattern appears to group closer together with sarcomatoid than epithelioid subtype (J Thorac Oncol 2020;15:1037)
- Malignant mesothelioma in situ (Histopathology 2018;72:1033):
- Defined by
- Single layer of surface mesothelial cells that lost BAP1 expression
- Usually presenting as unilateral pleural effusion
- No evidence of tumor by imaging or by direct examination of pleura
- No invasive mesothelioma developing for at least 1 year
- CDKN2A homozygous deletion is rare in these tumors
- Defined by
- Diffuse intrapulmonary malignant mesothelioma; rare, predominantly intrapulmonary growth and minimal pleural involvement clinically simulating interstitial lung disease (Am J Surg Pathol 2019;43:147)
- Recommendations by European Network for Rare Adult Solid Cancers (EURACAN) and International Association for the Study of Lung Cancer (IASLC) to update histologic classification of malignant pleural mesothelioma using a multidisciplinary approach include (J Thorac Oncol 2020;15:29):
- To update classification to include architectural patterns and stromal and cytologic features that refine prognostication
- Malignant mesothelioma in situ could be an additional category
- Routinely grade epithelioid malignant pleural mesothelioma
- Routinely stage resection specimens; amongst others
- Many of these recommendations will likely be included in the upcoming WHO classification
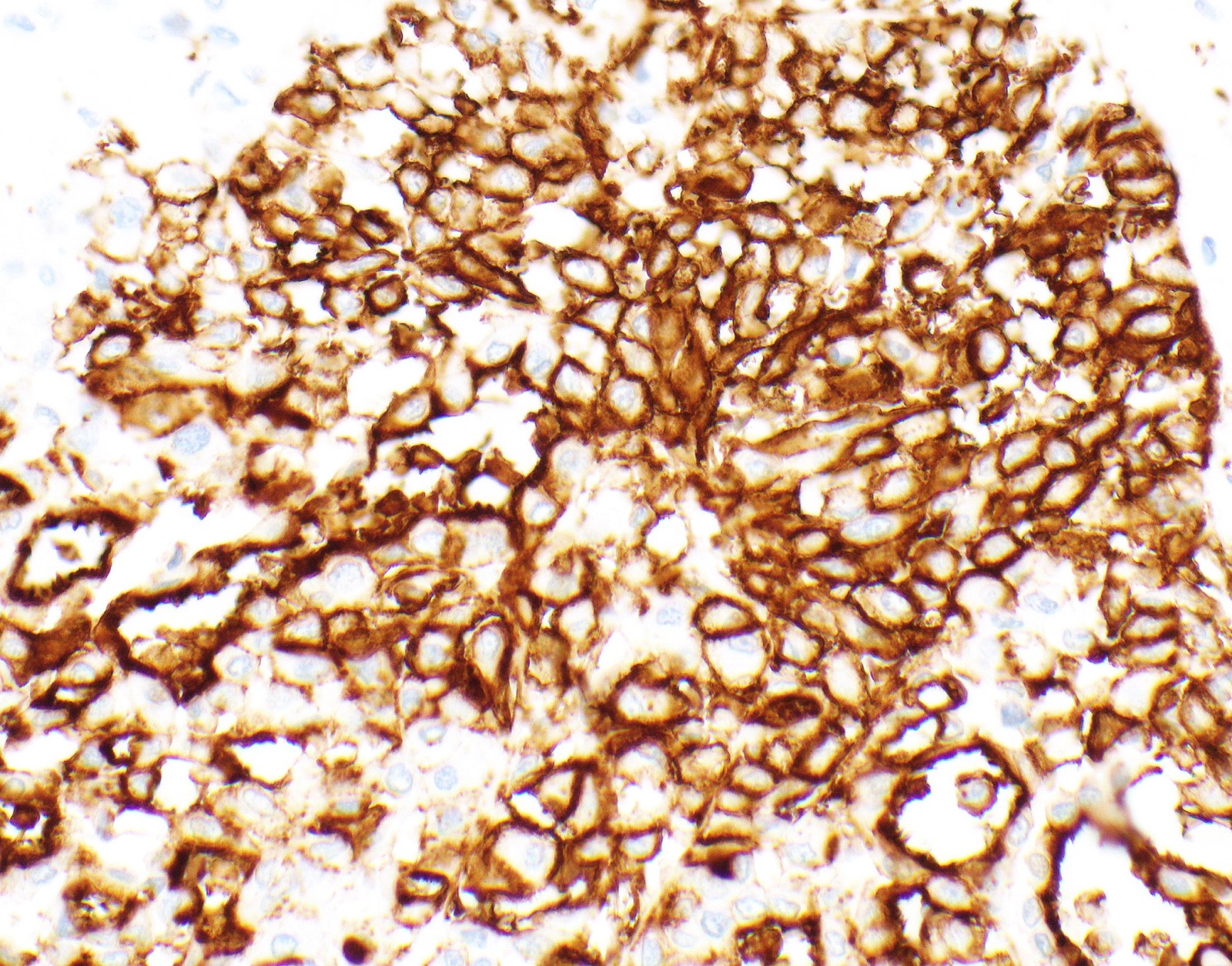
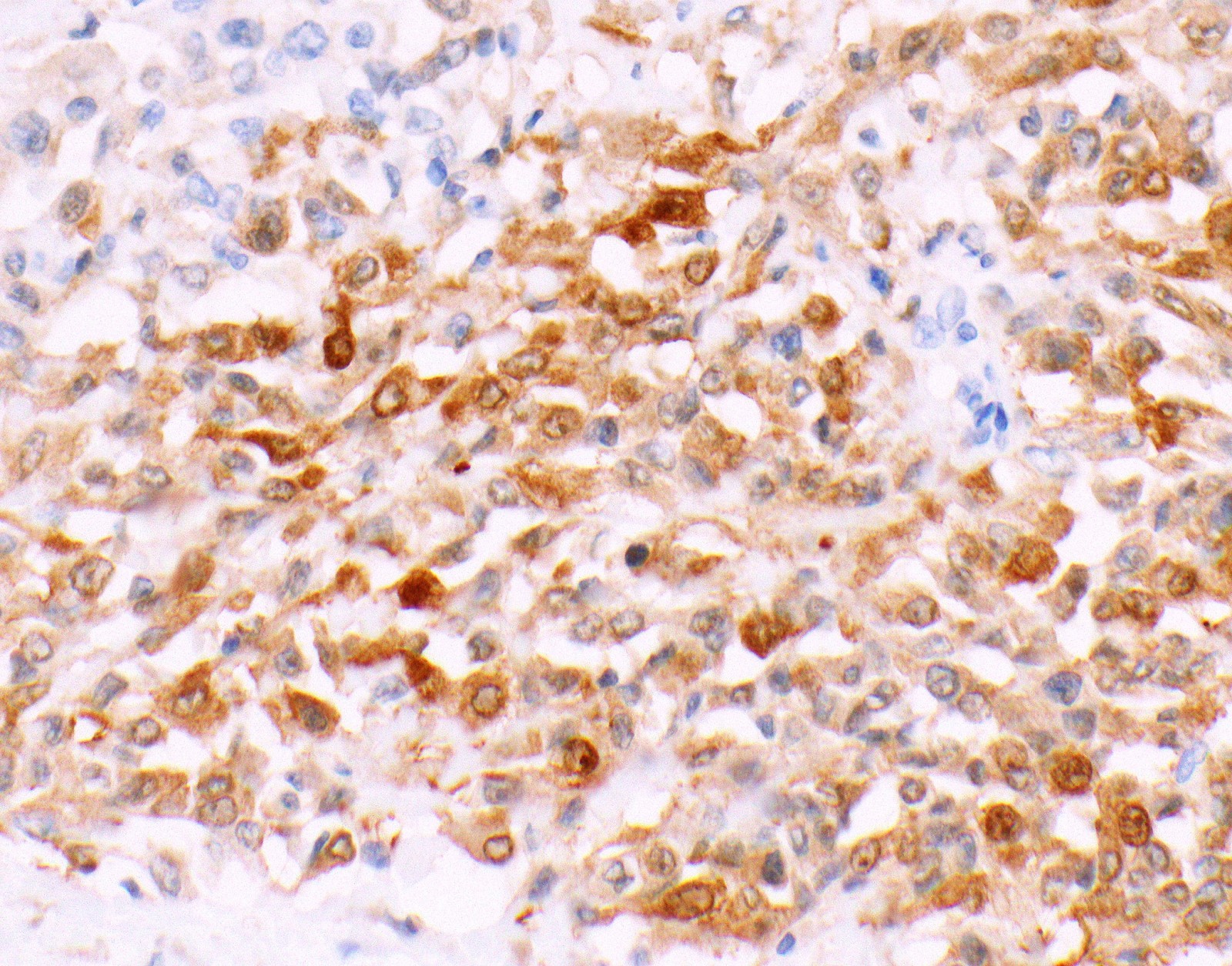
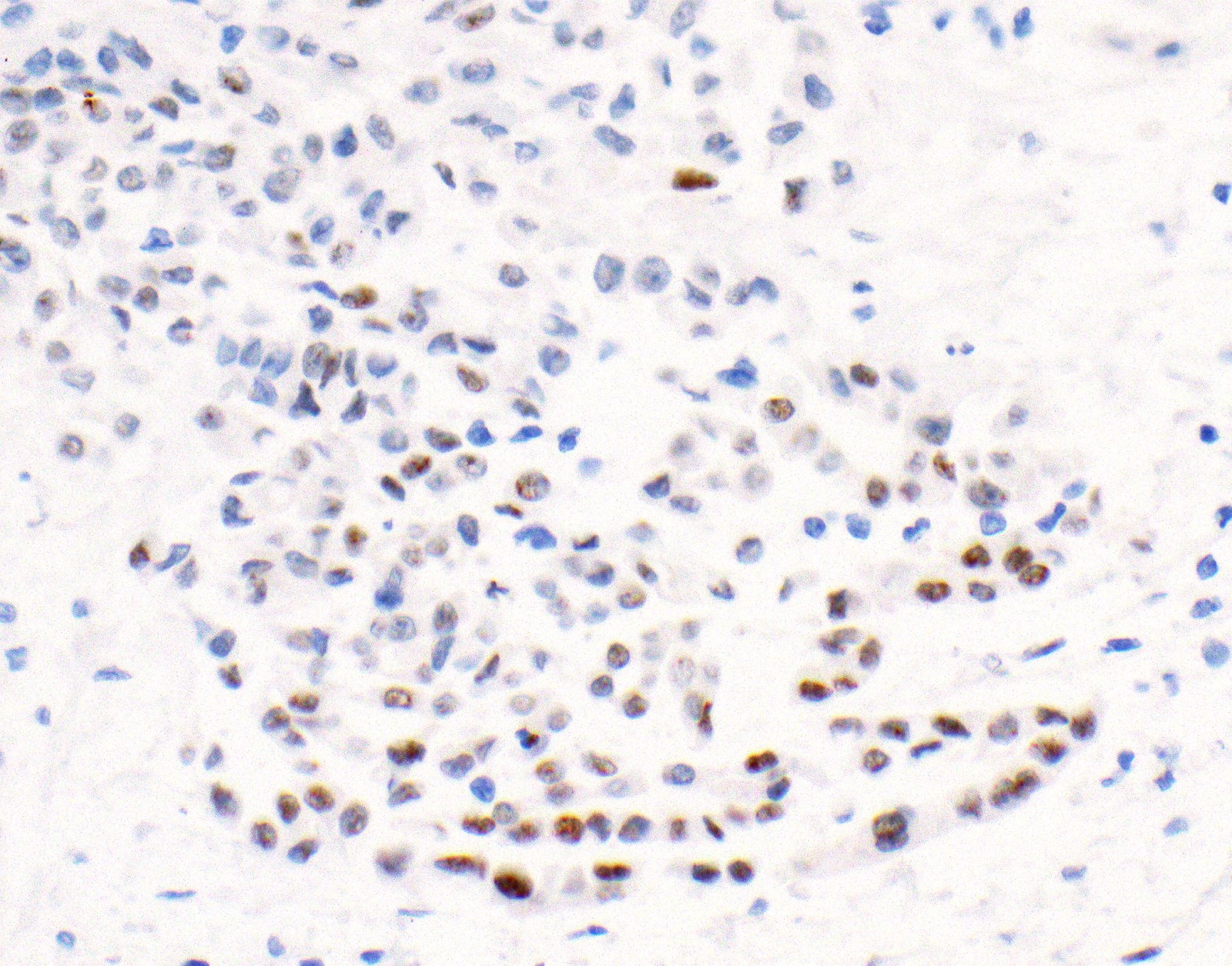
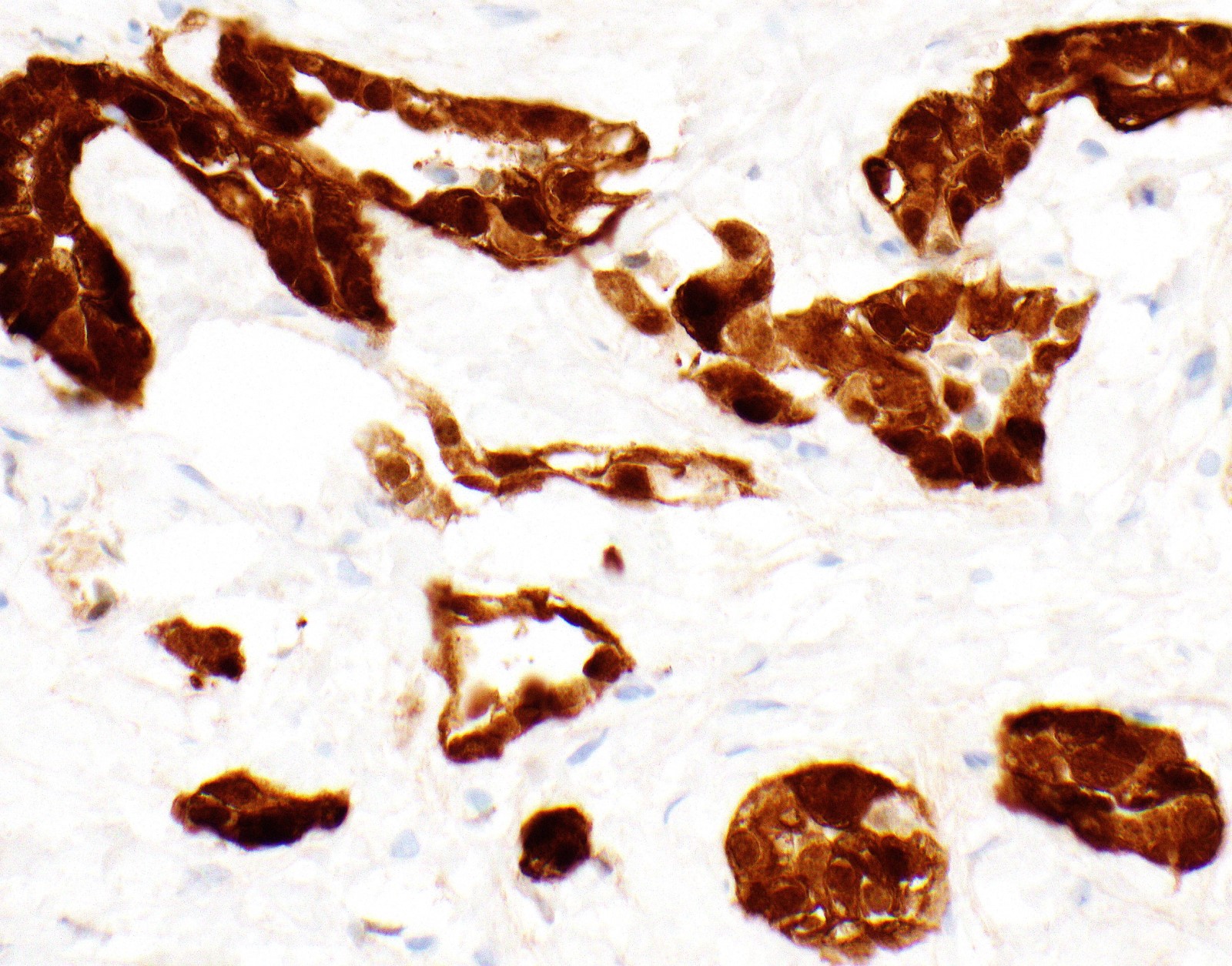
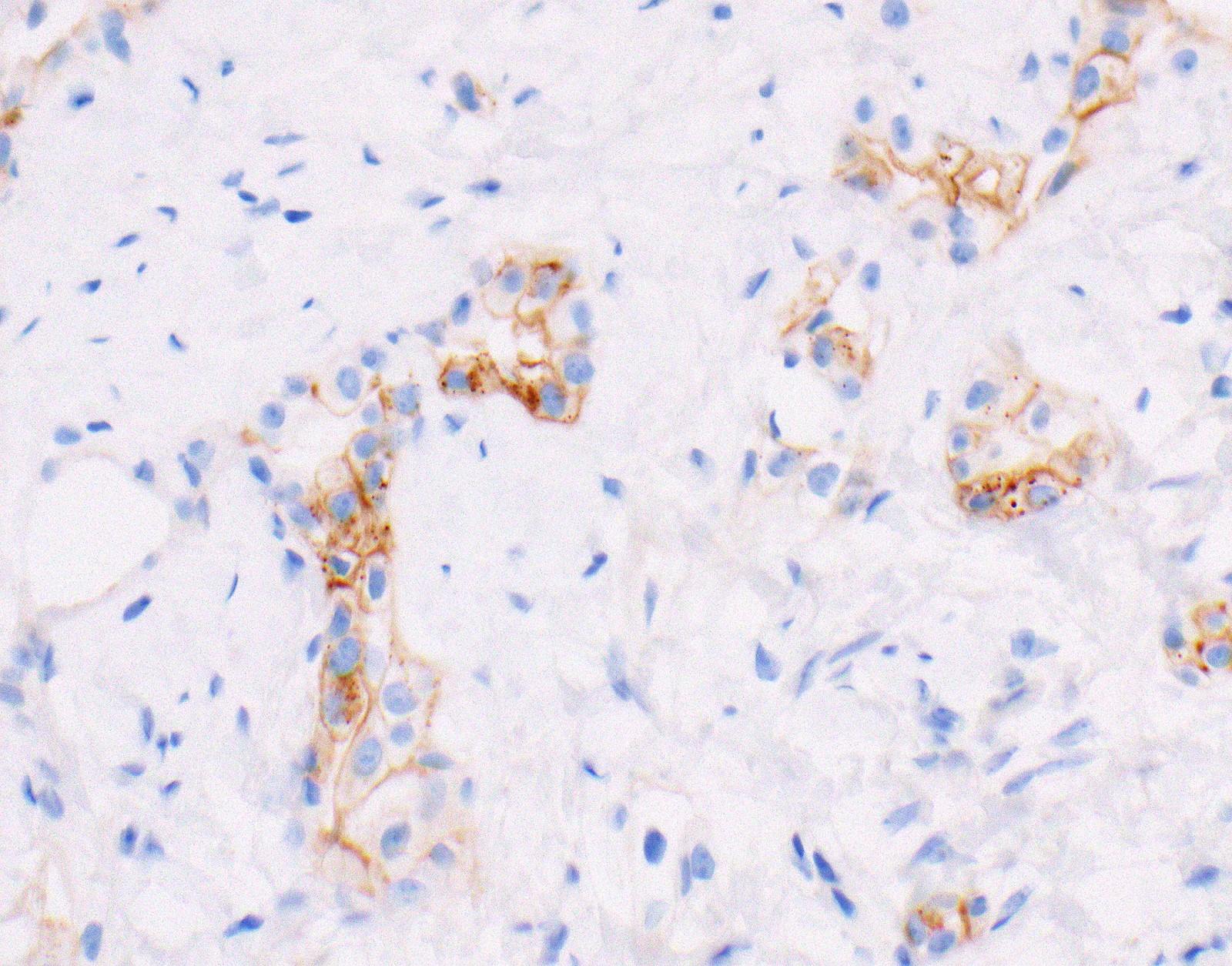
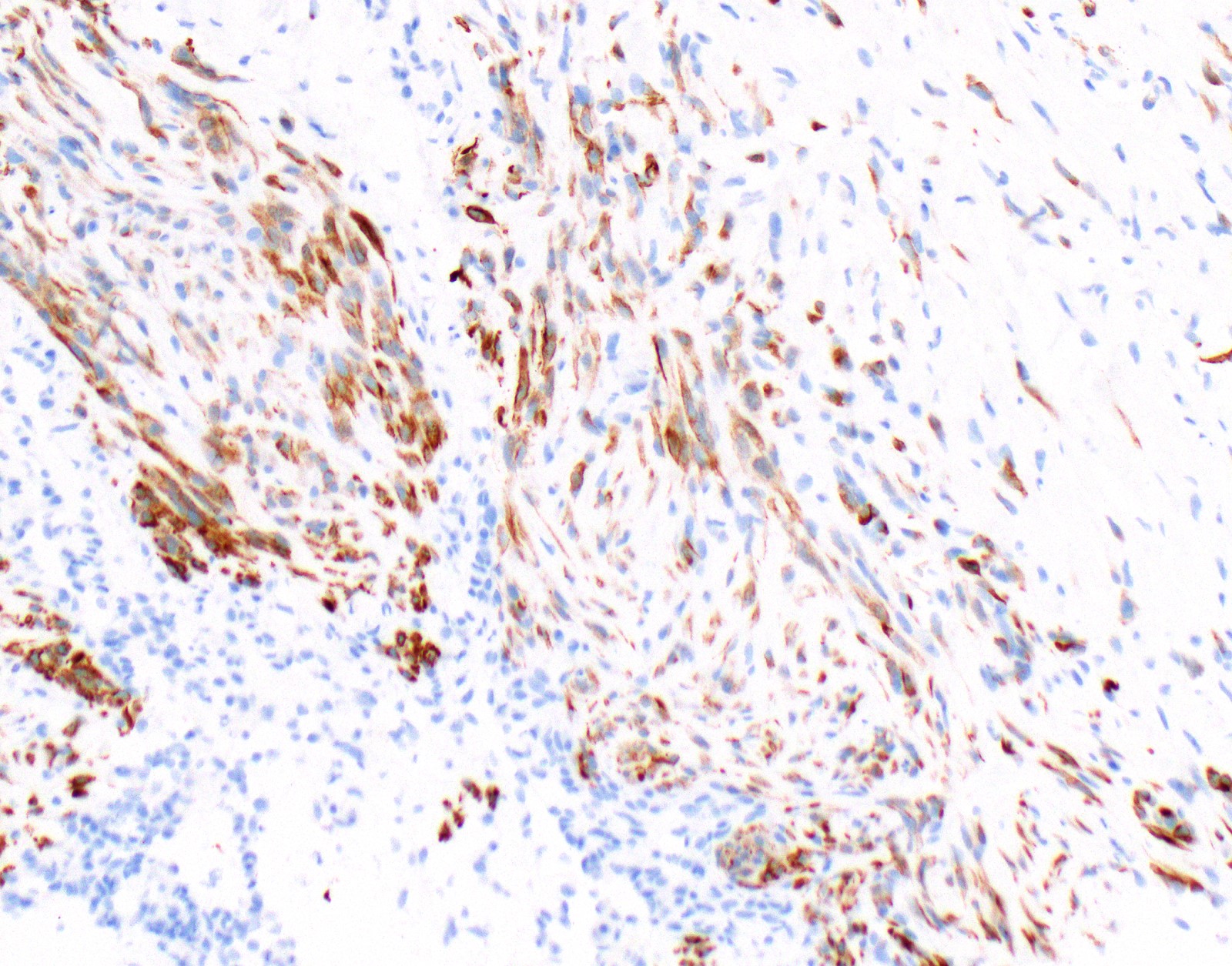
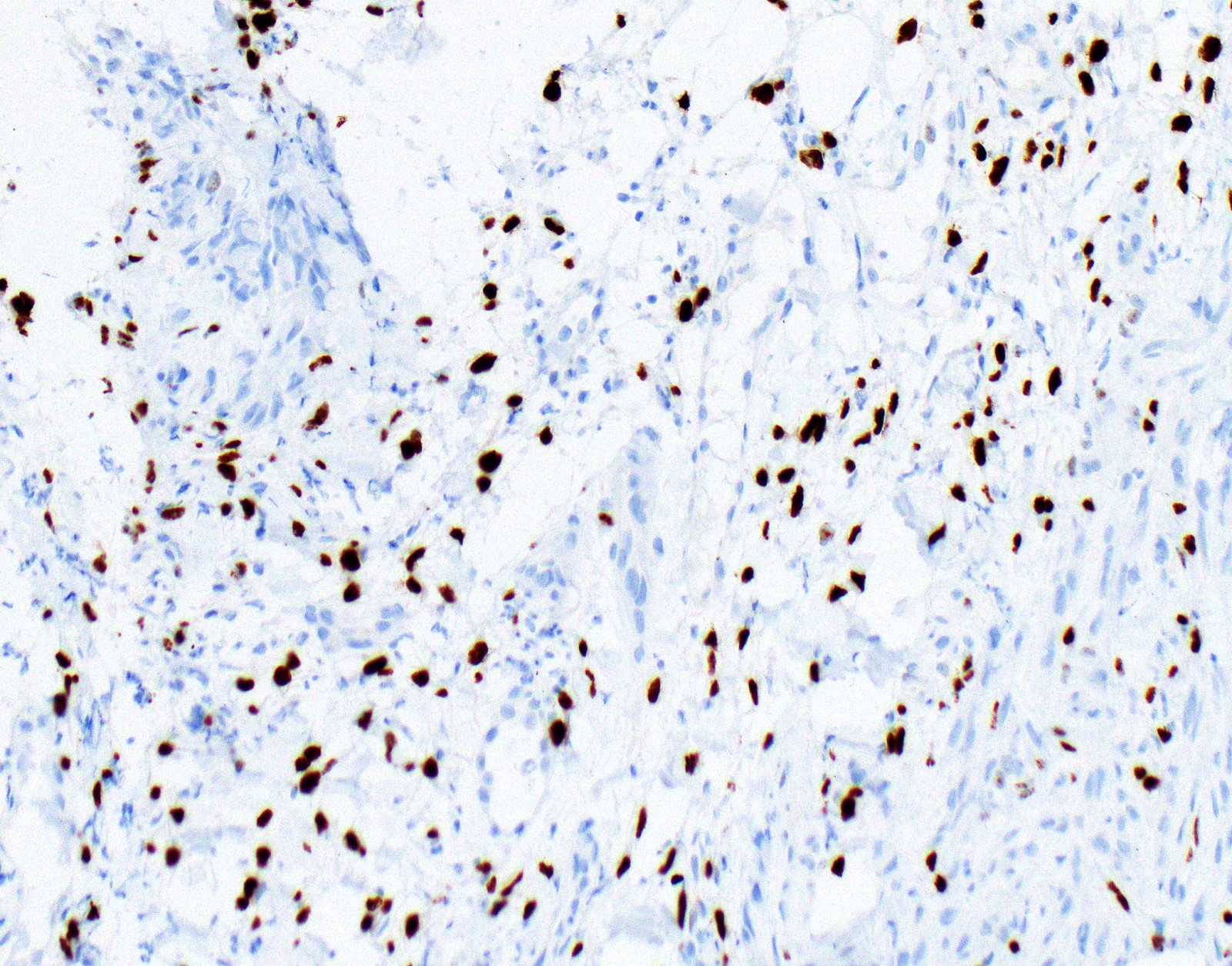
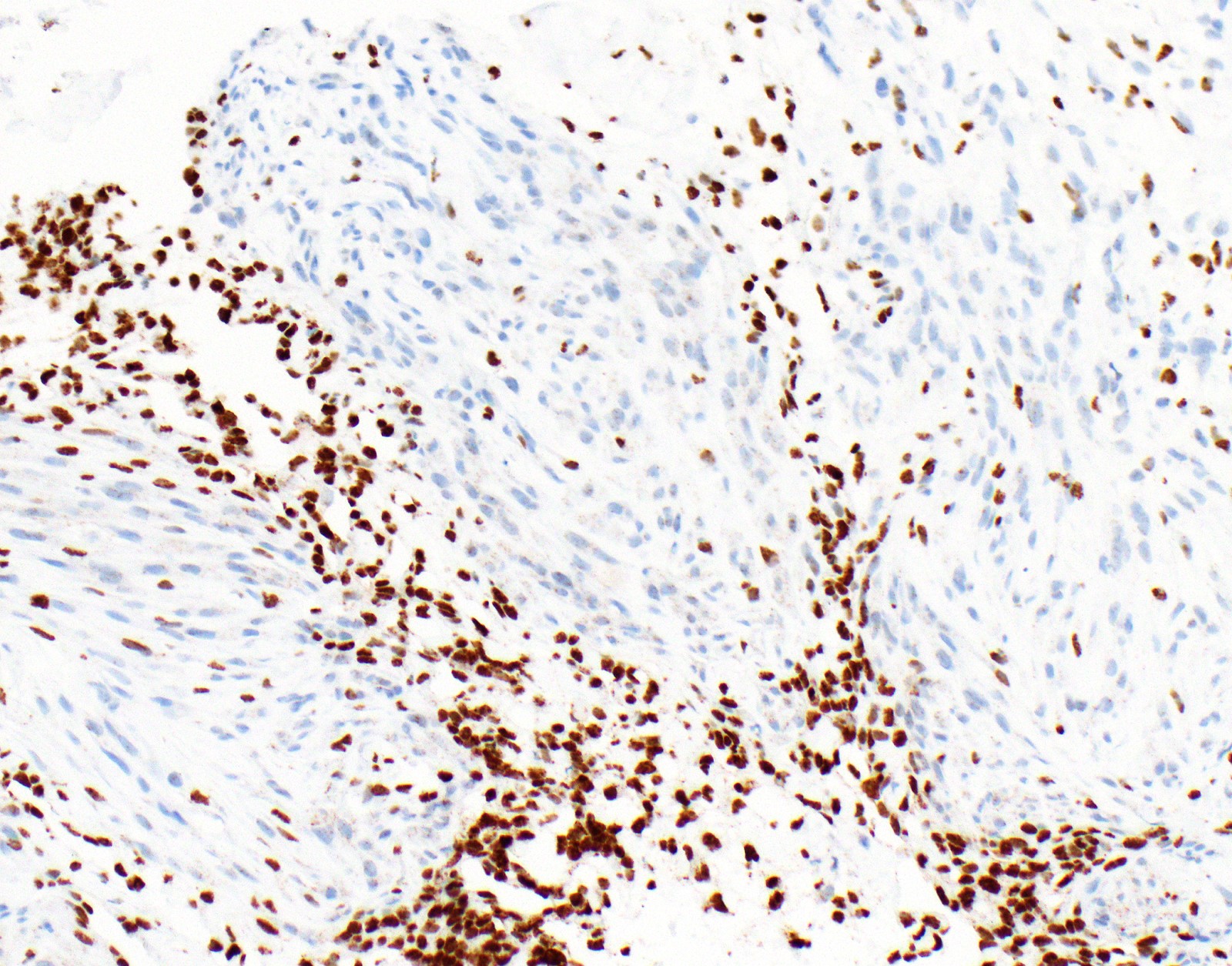
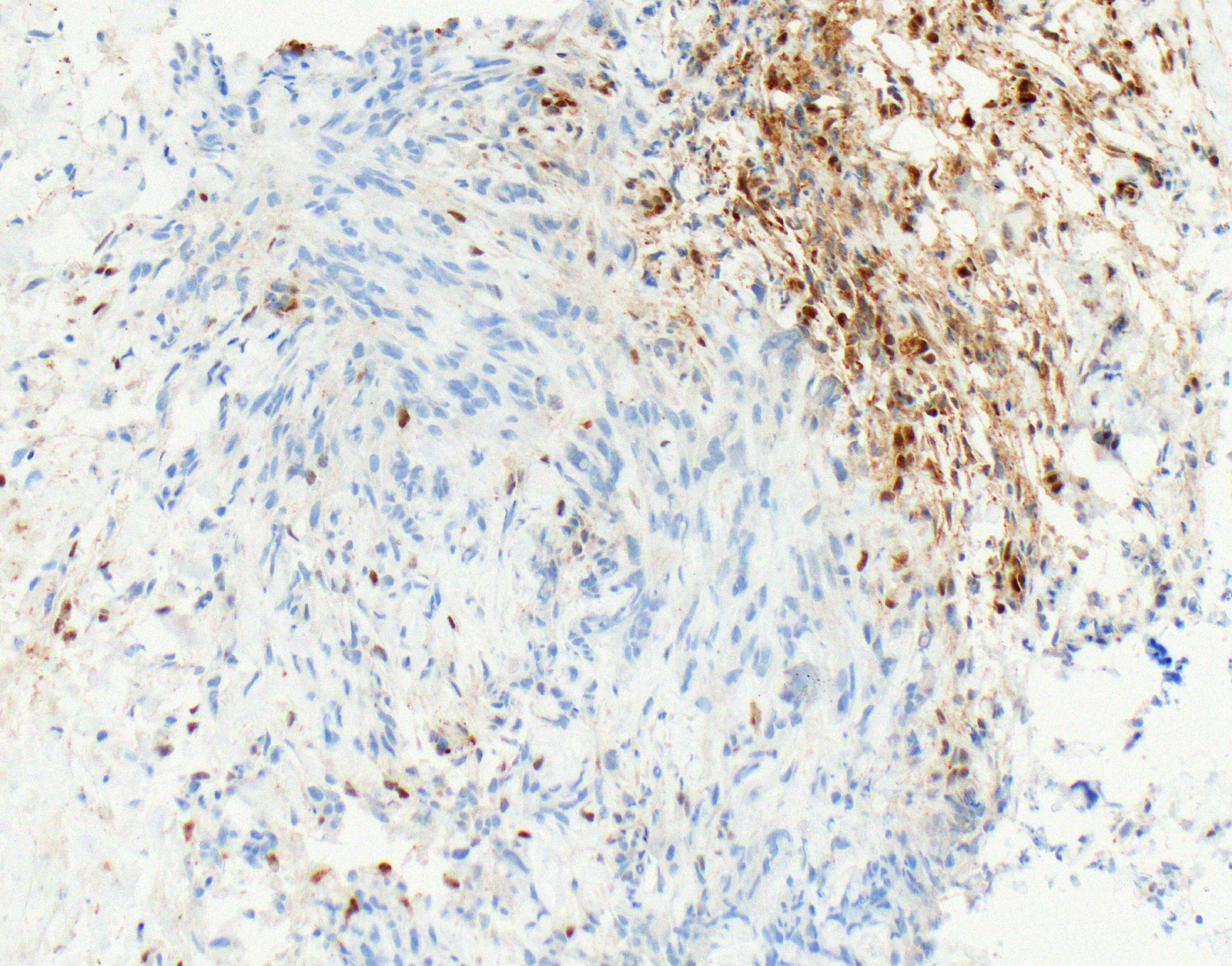
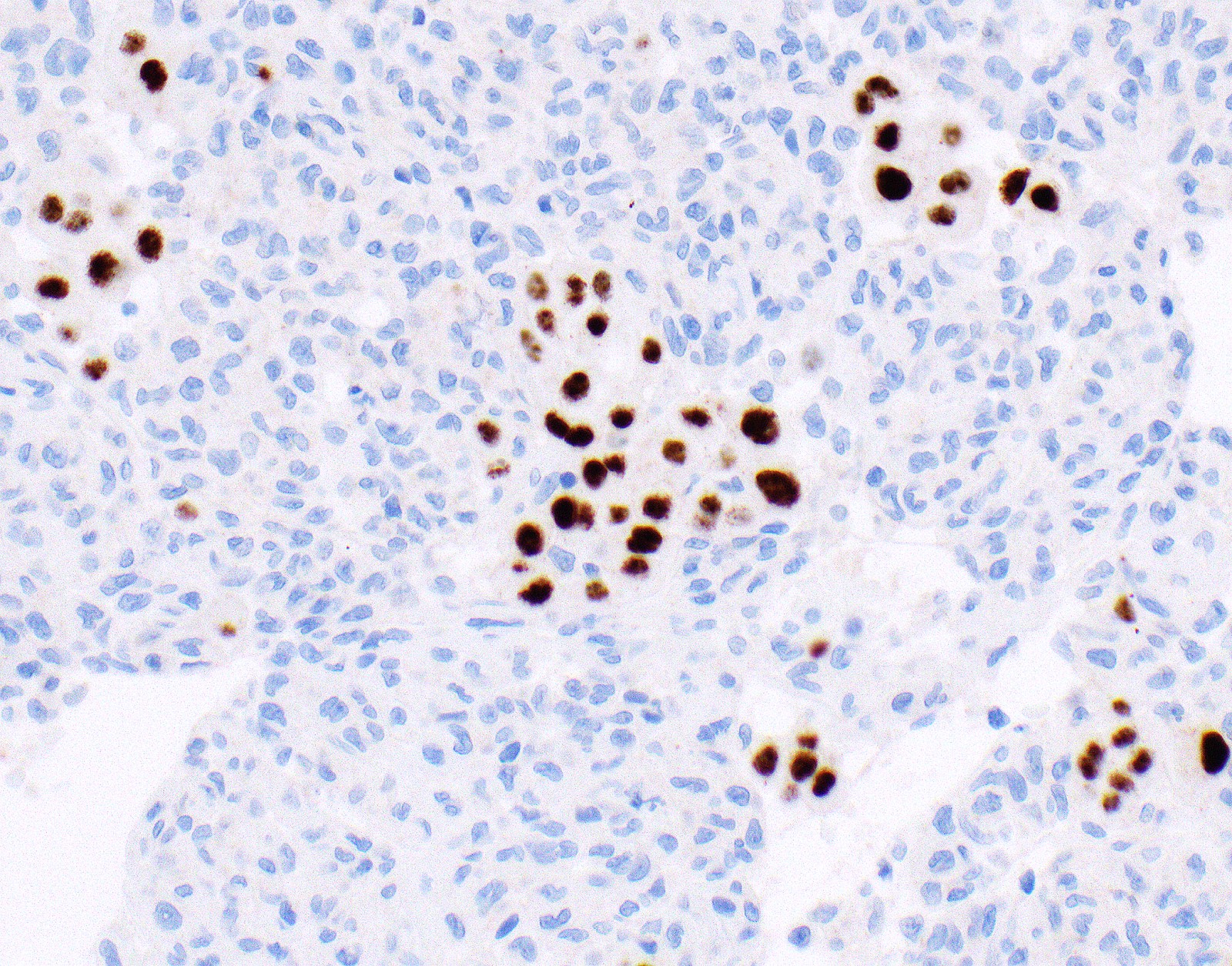
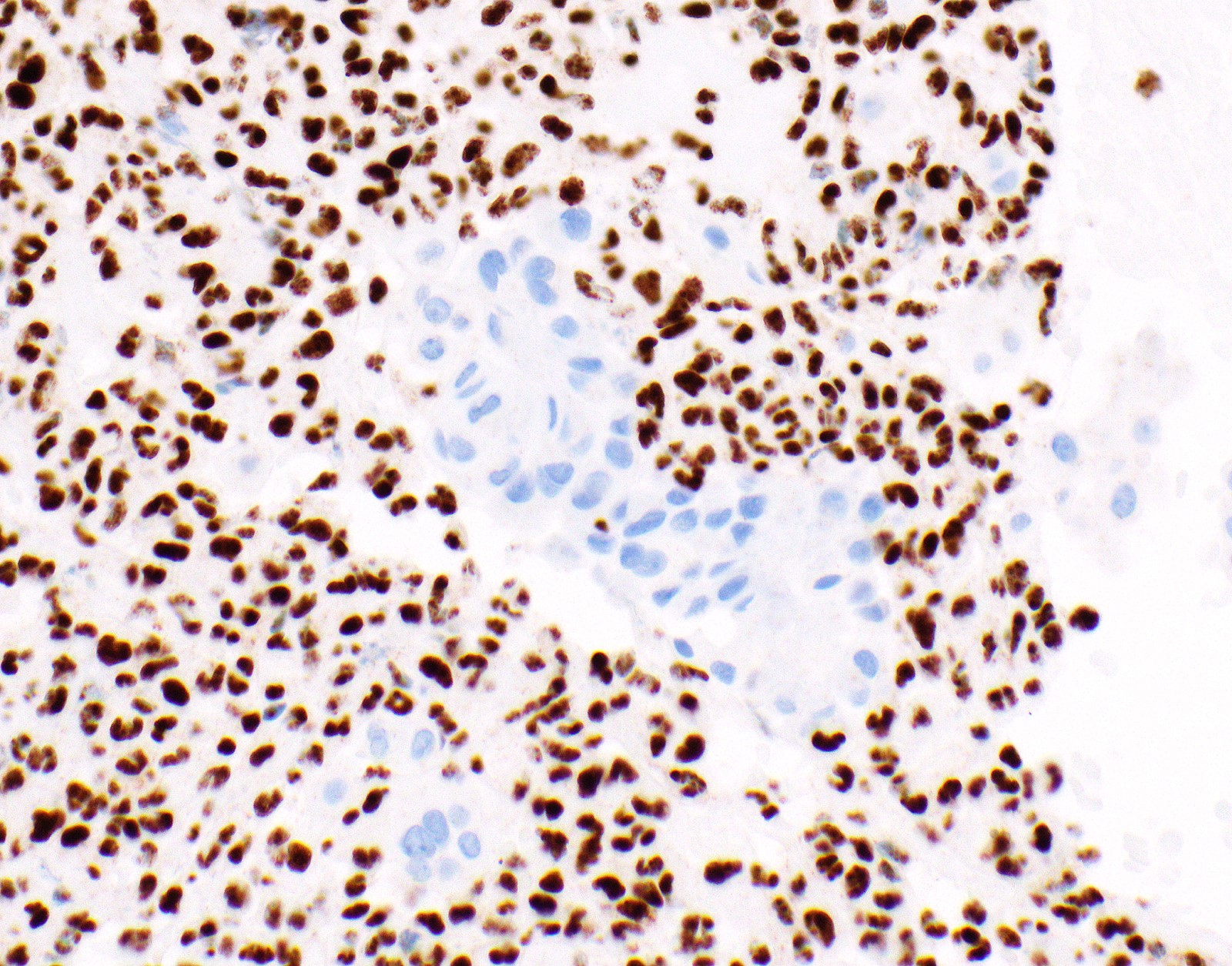
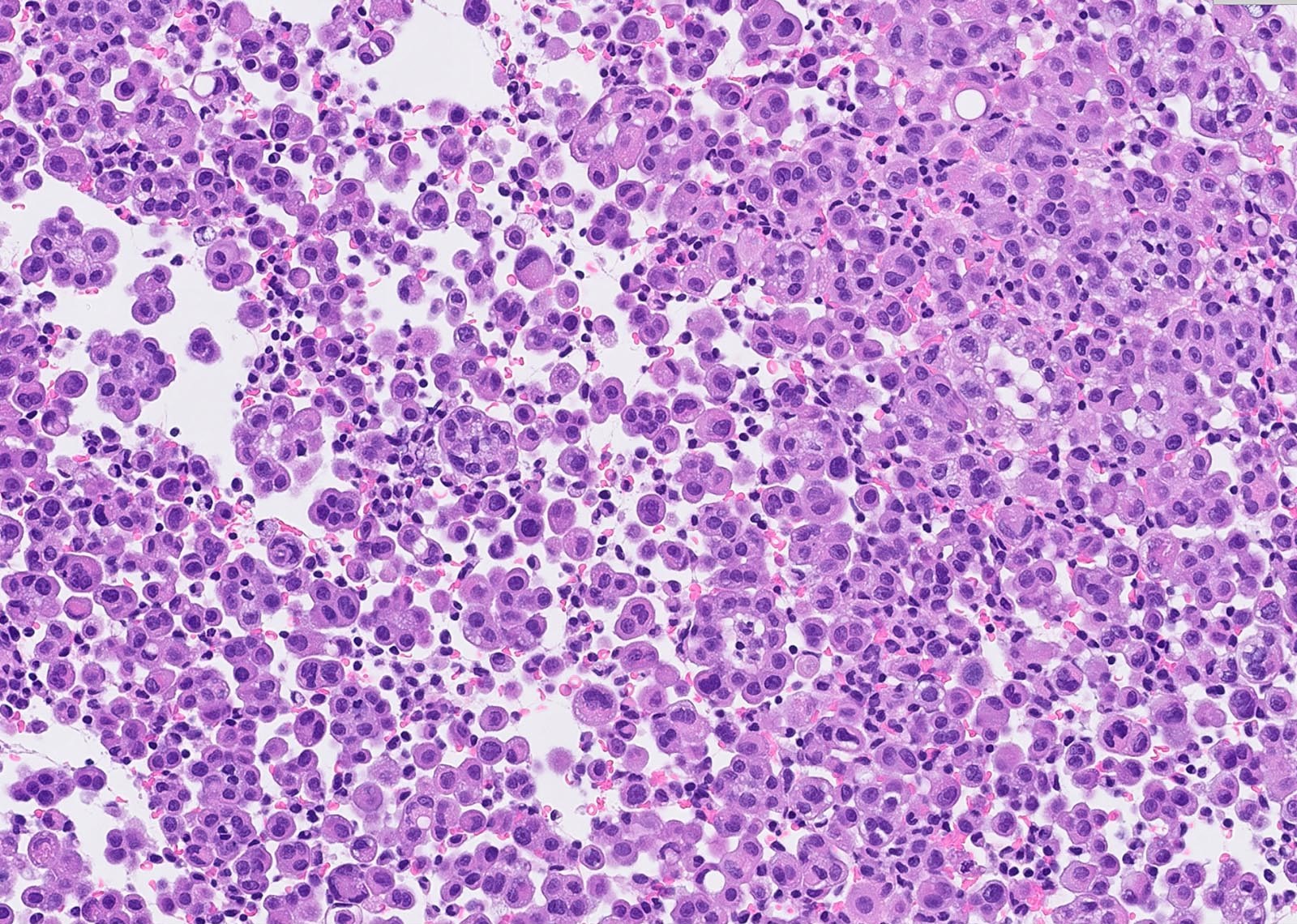
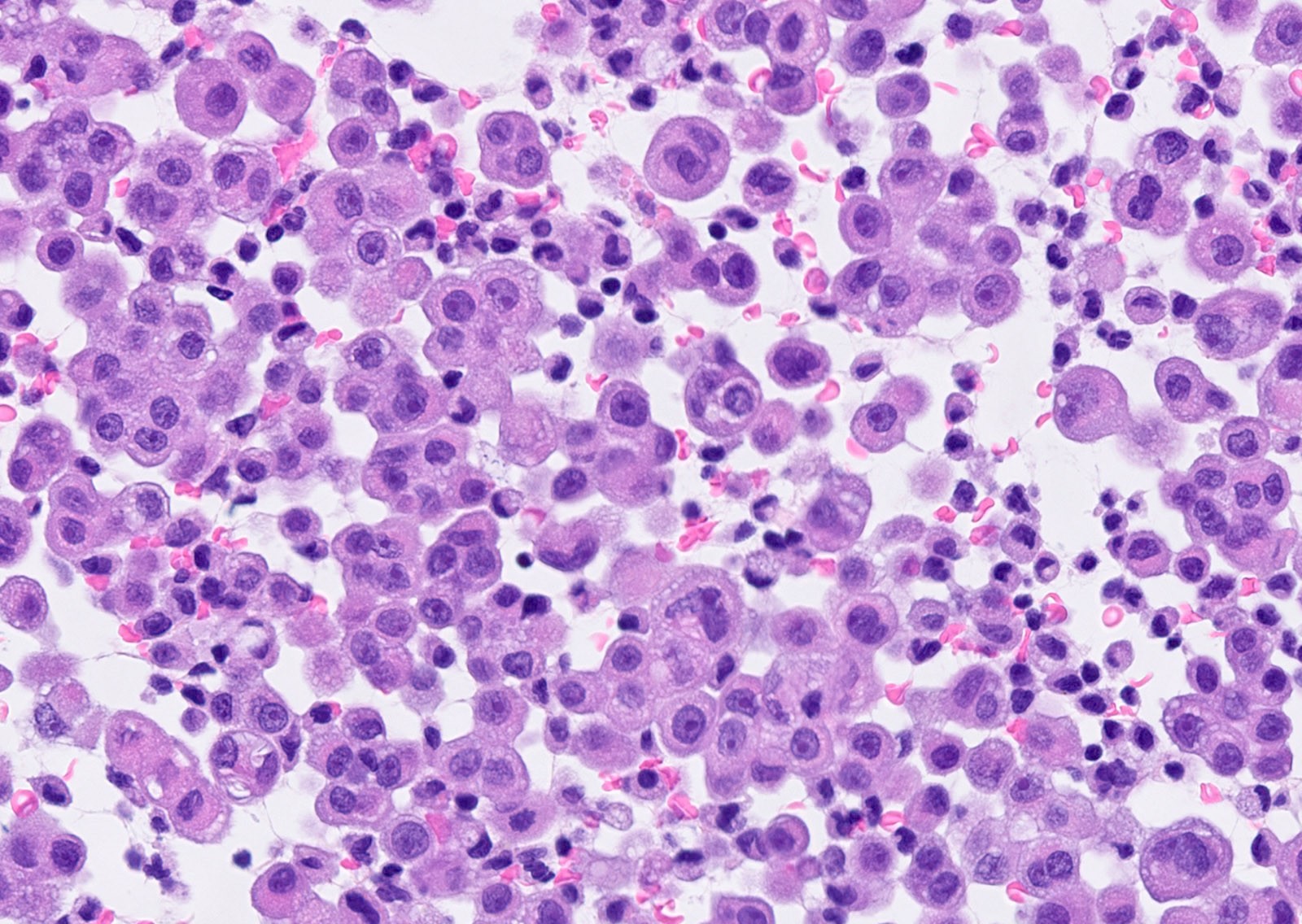
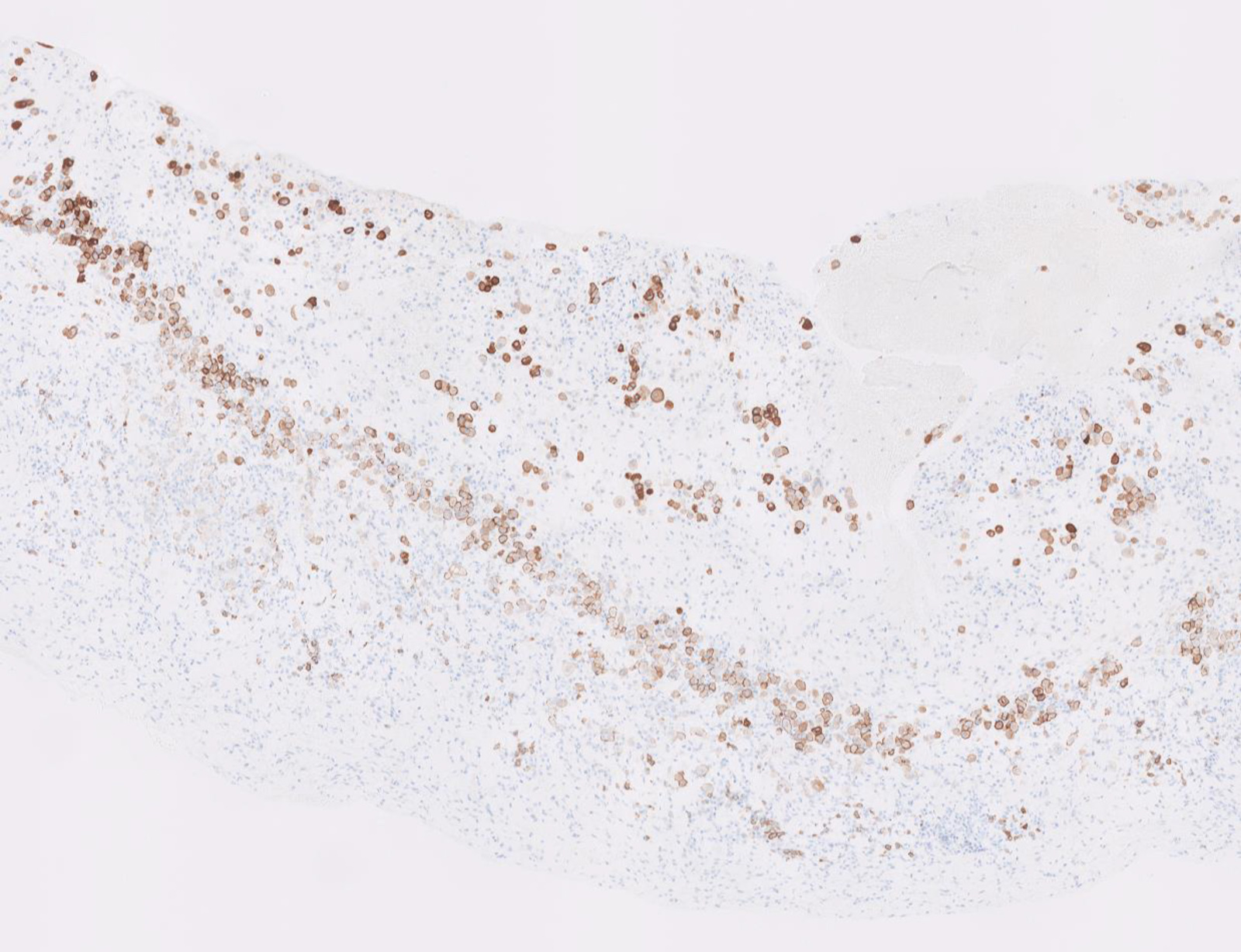
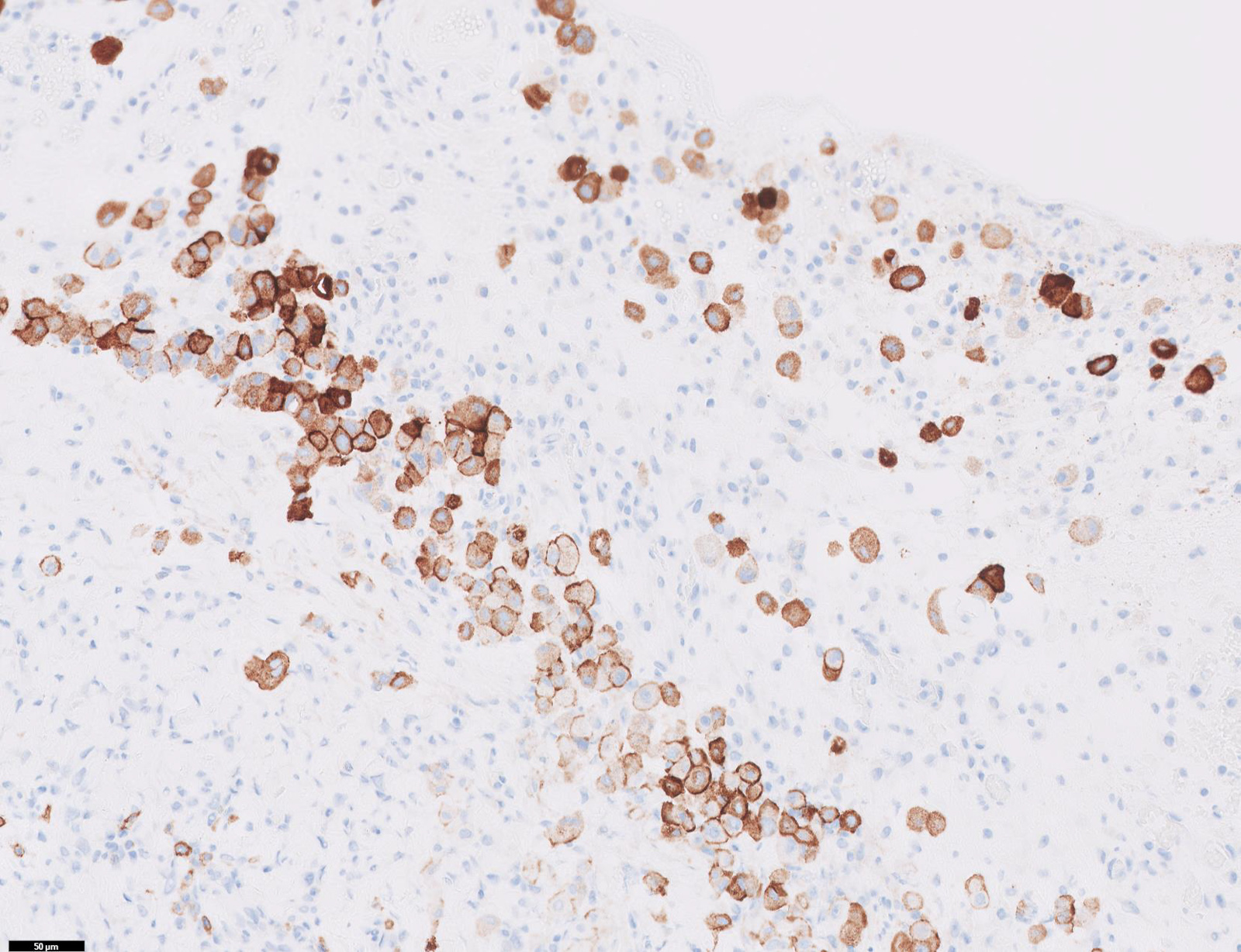
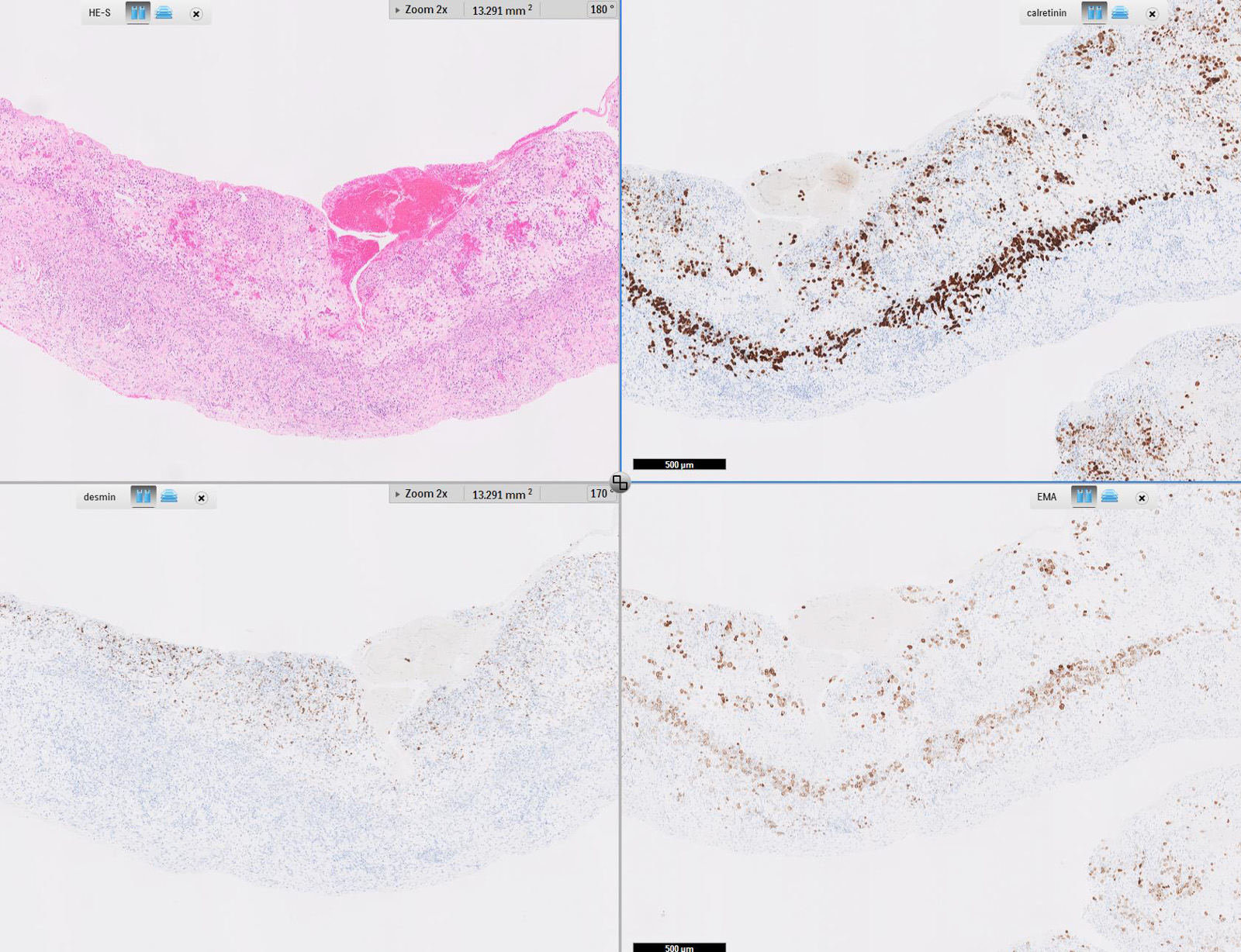
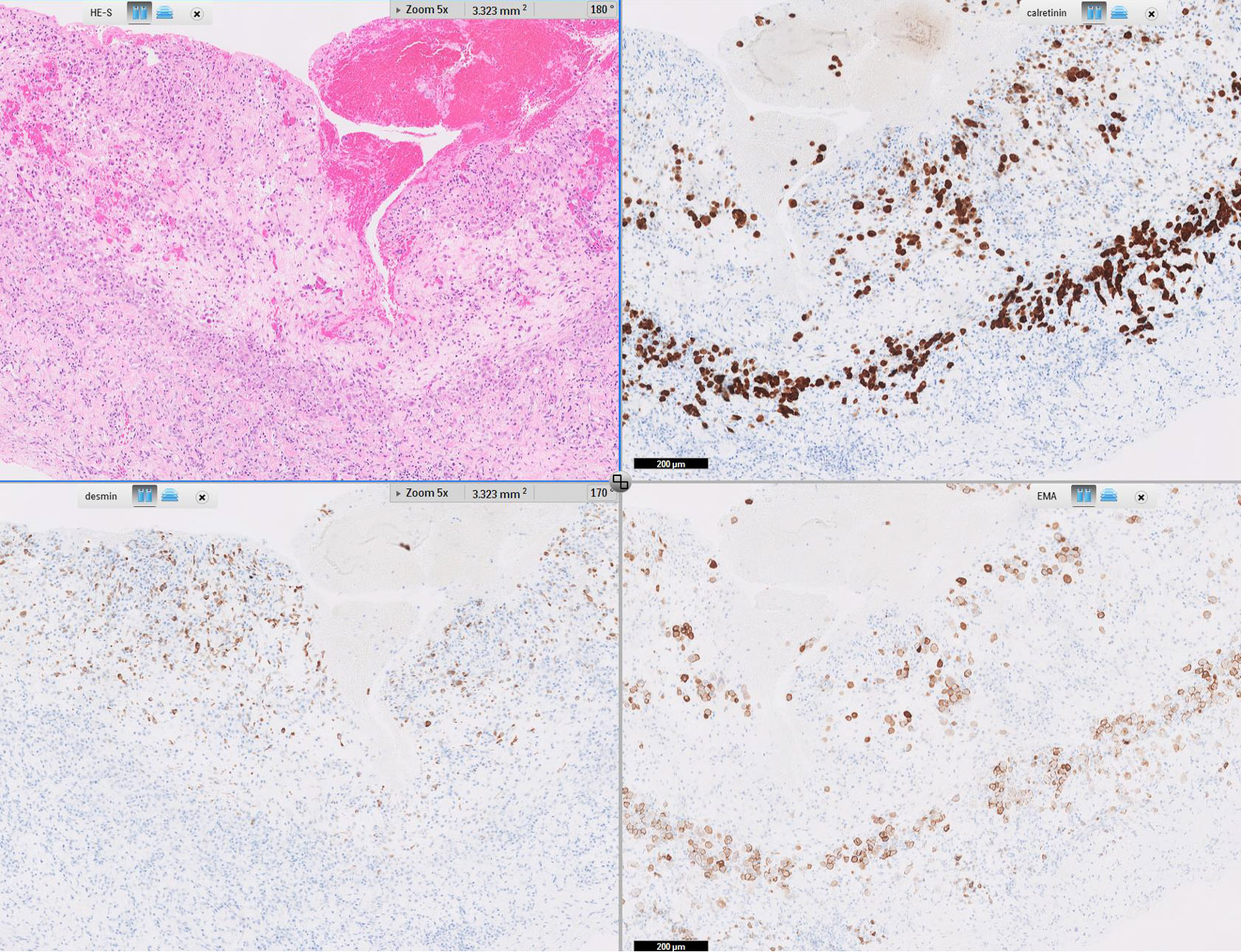
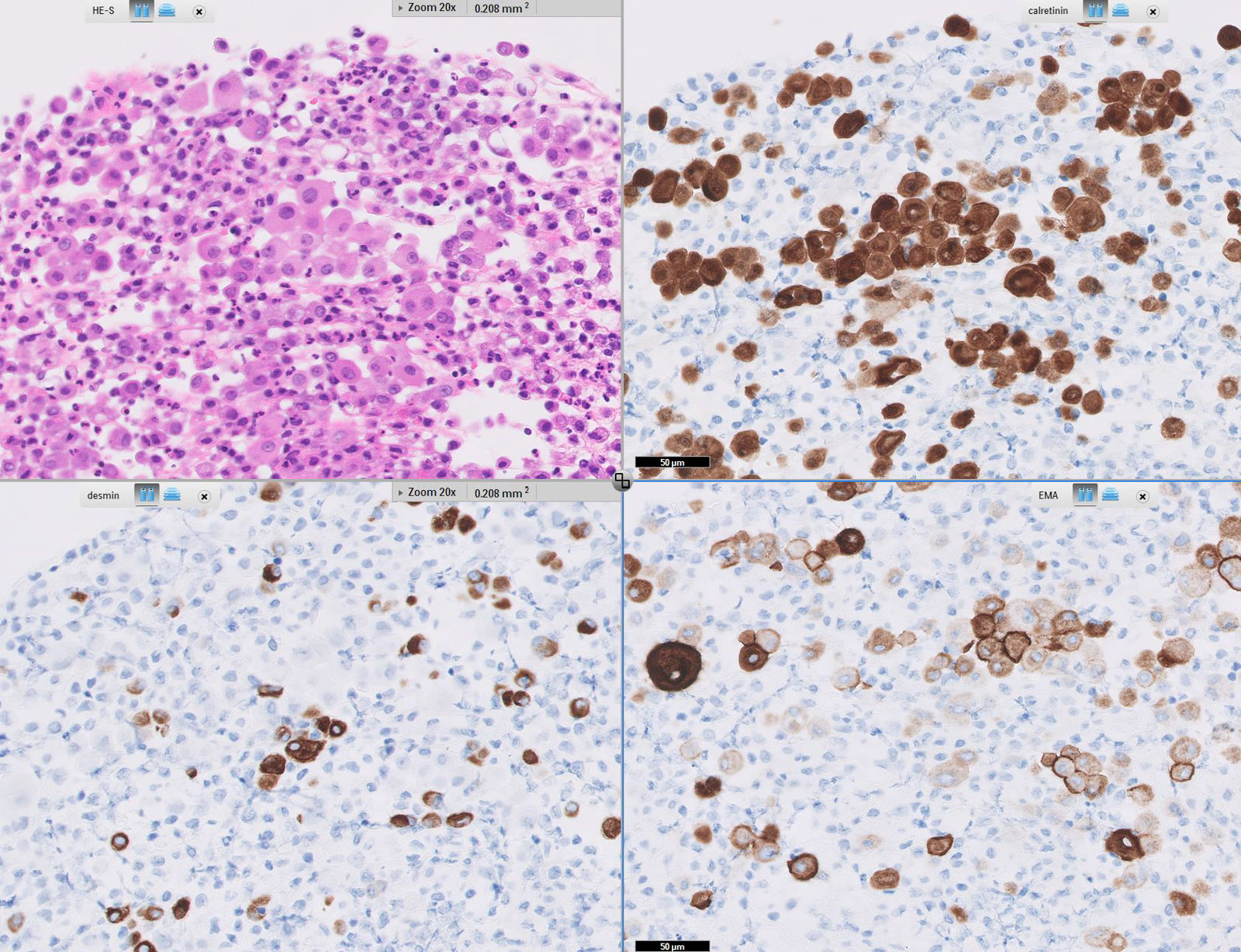
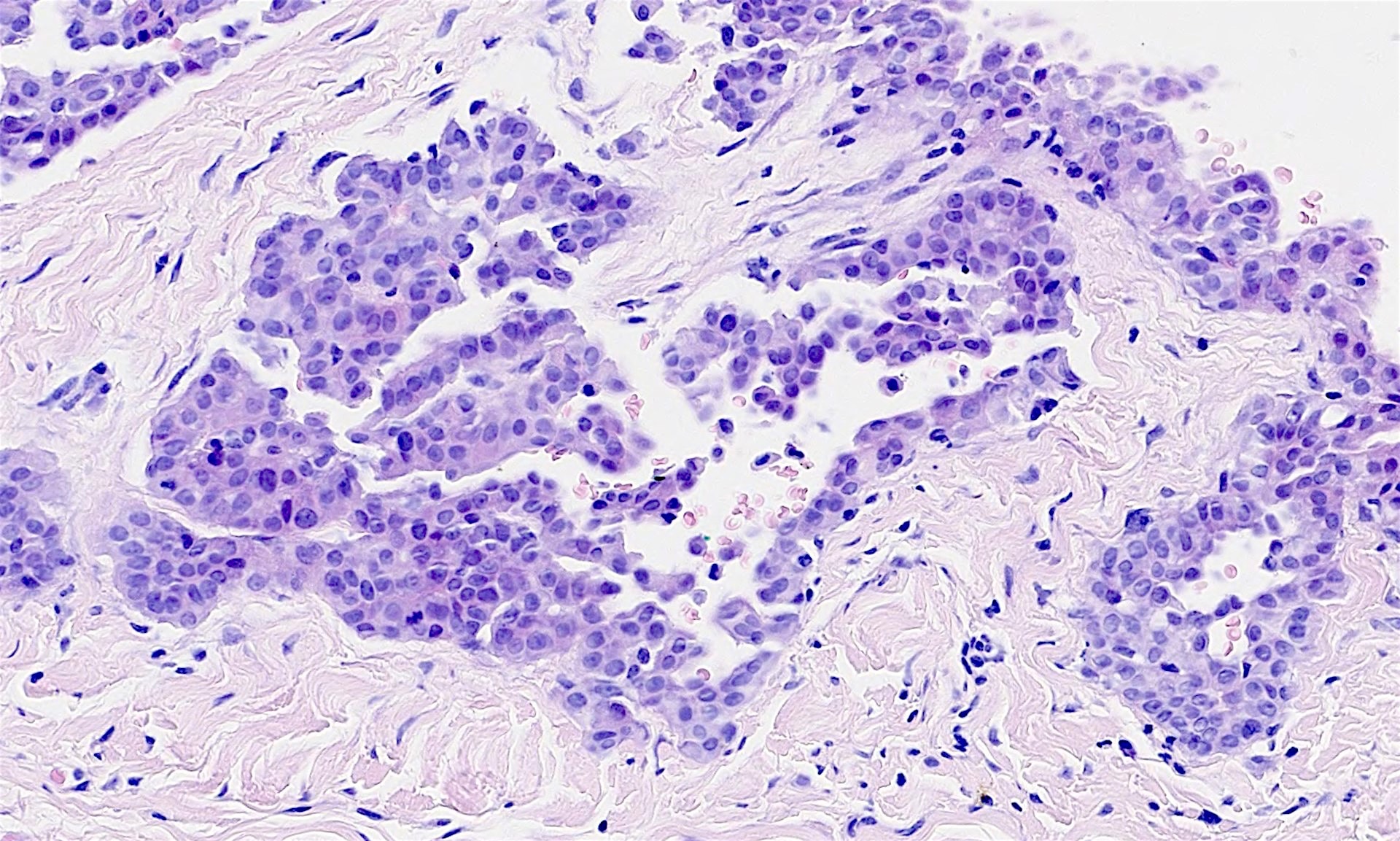
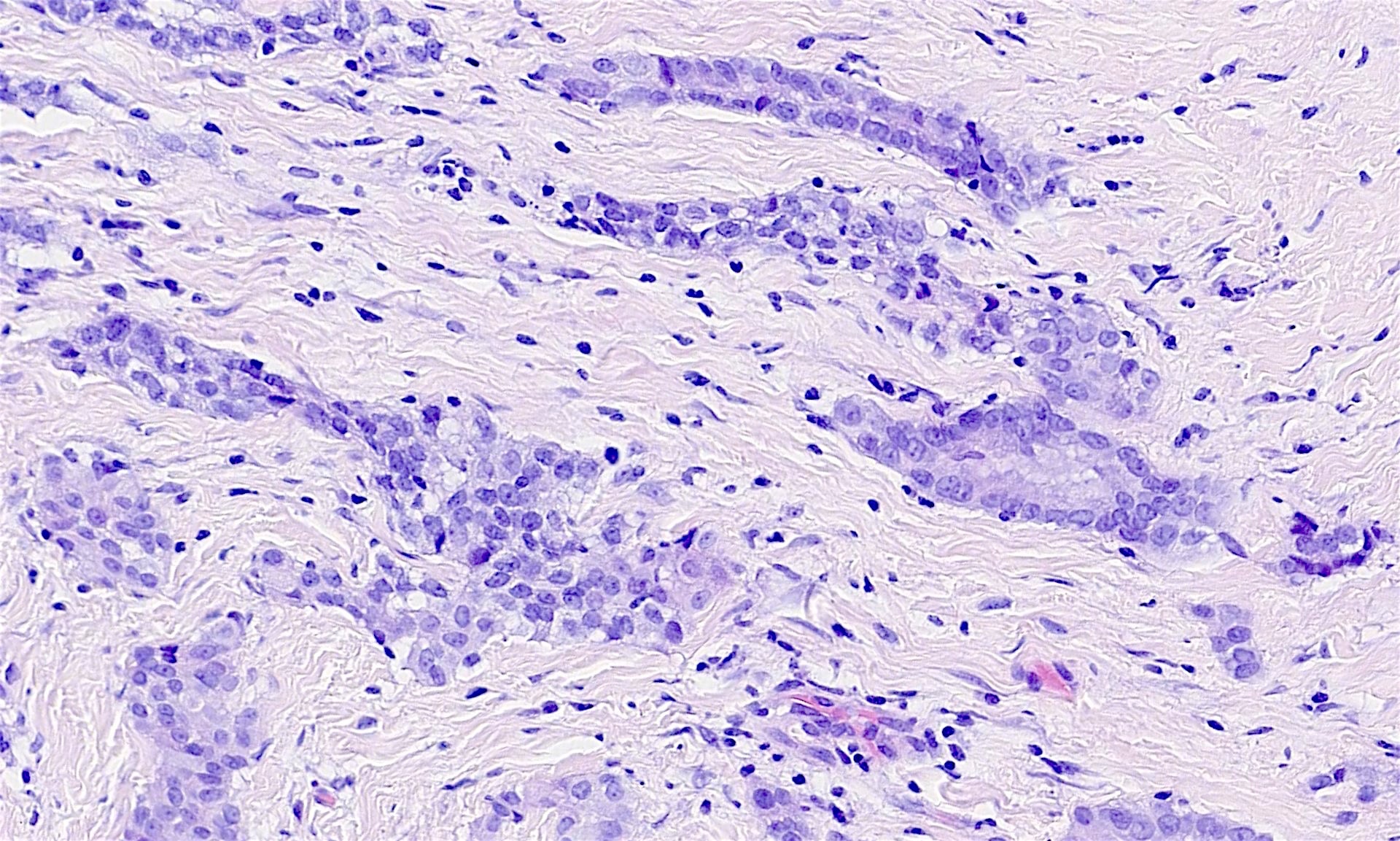
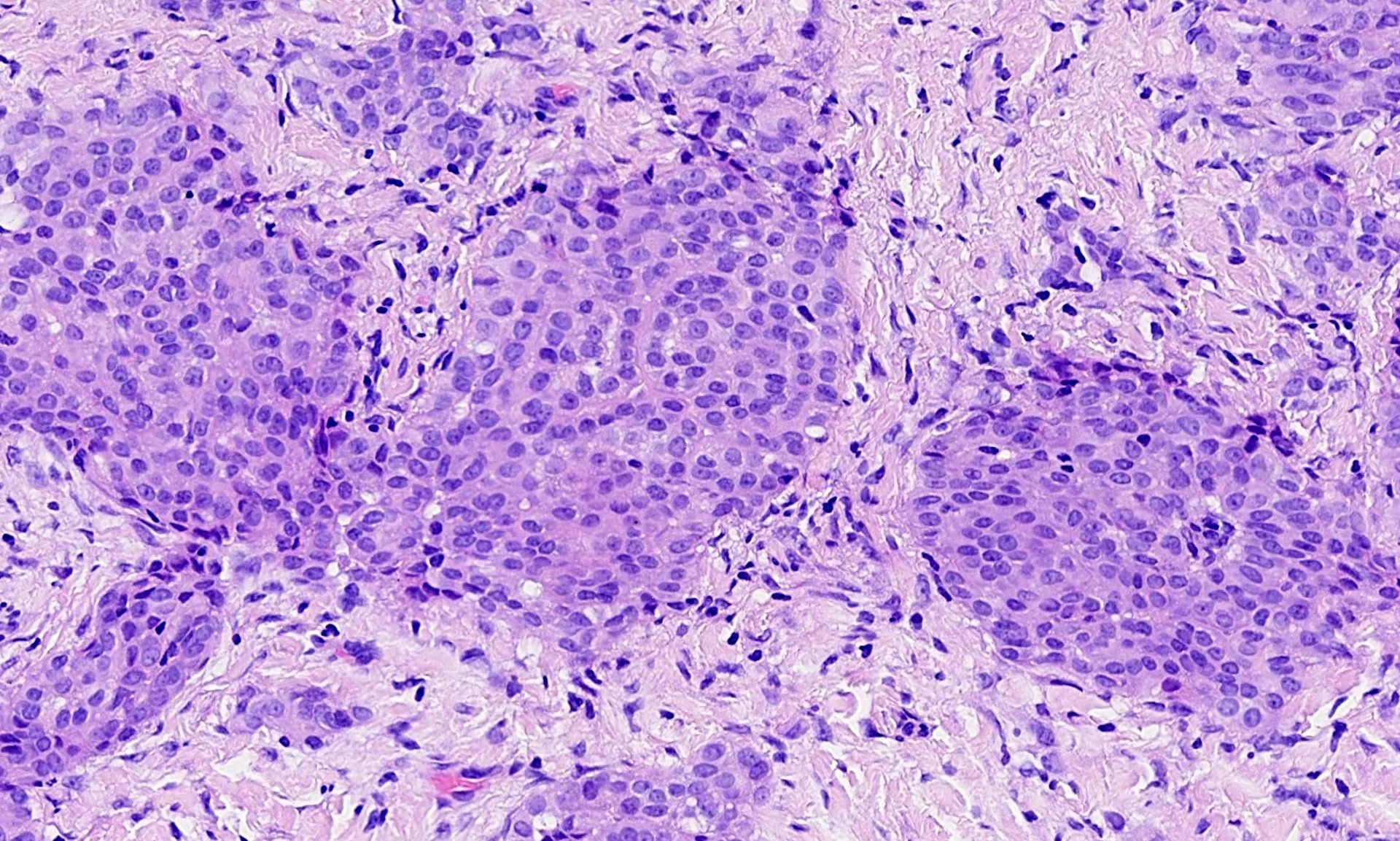
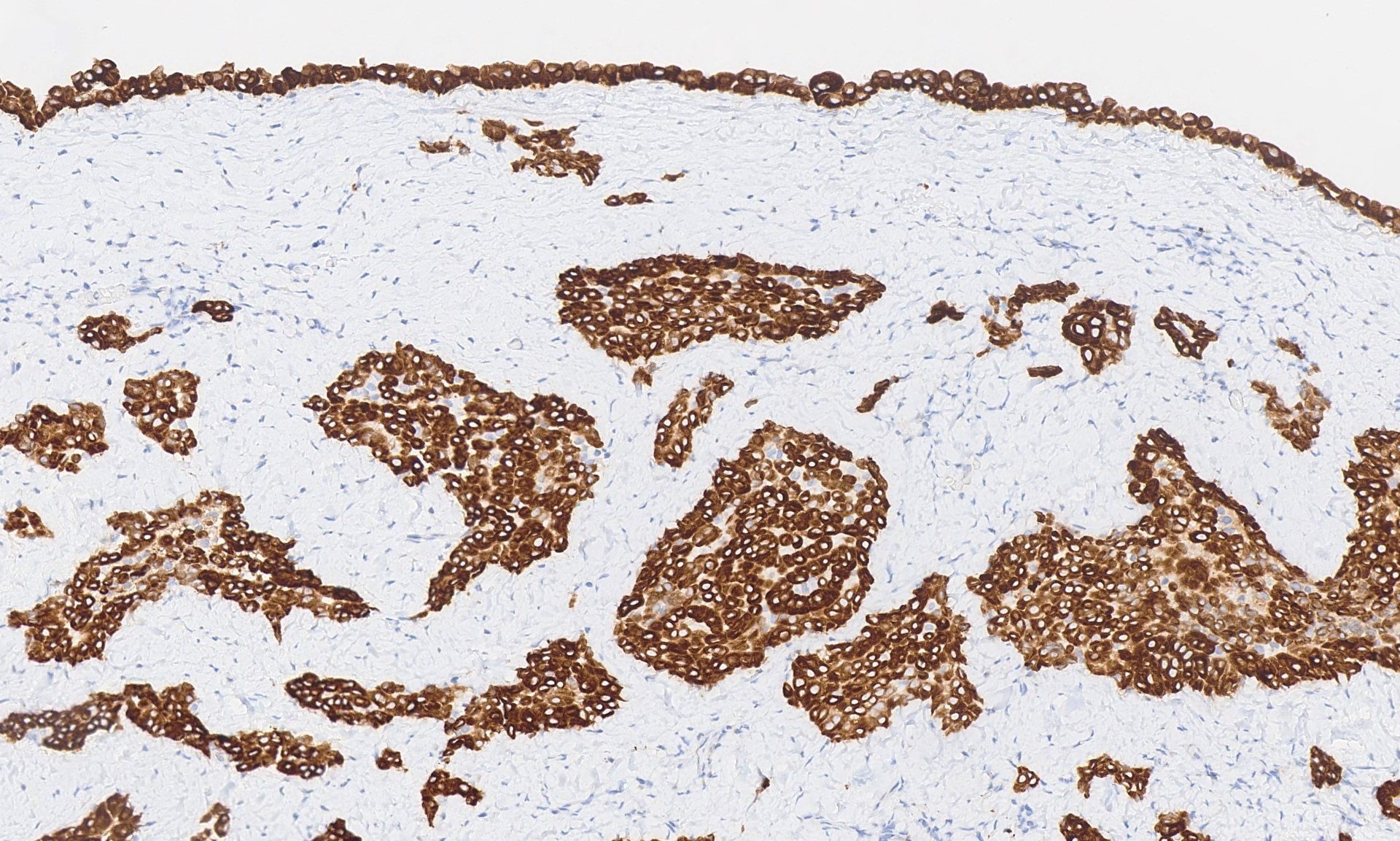
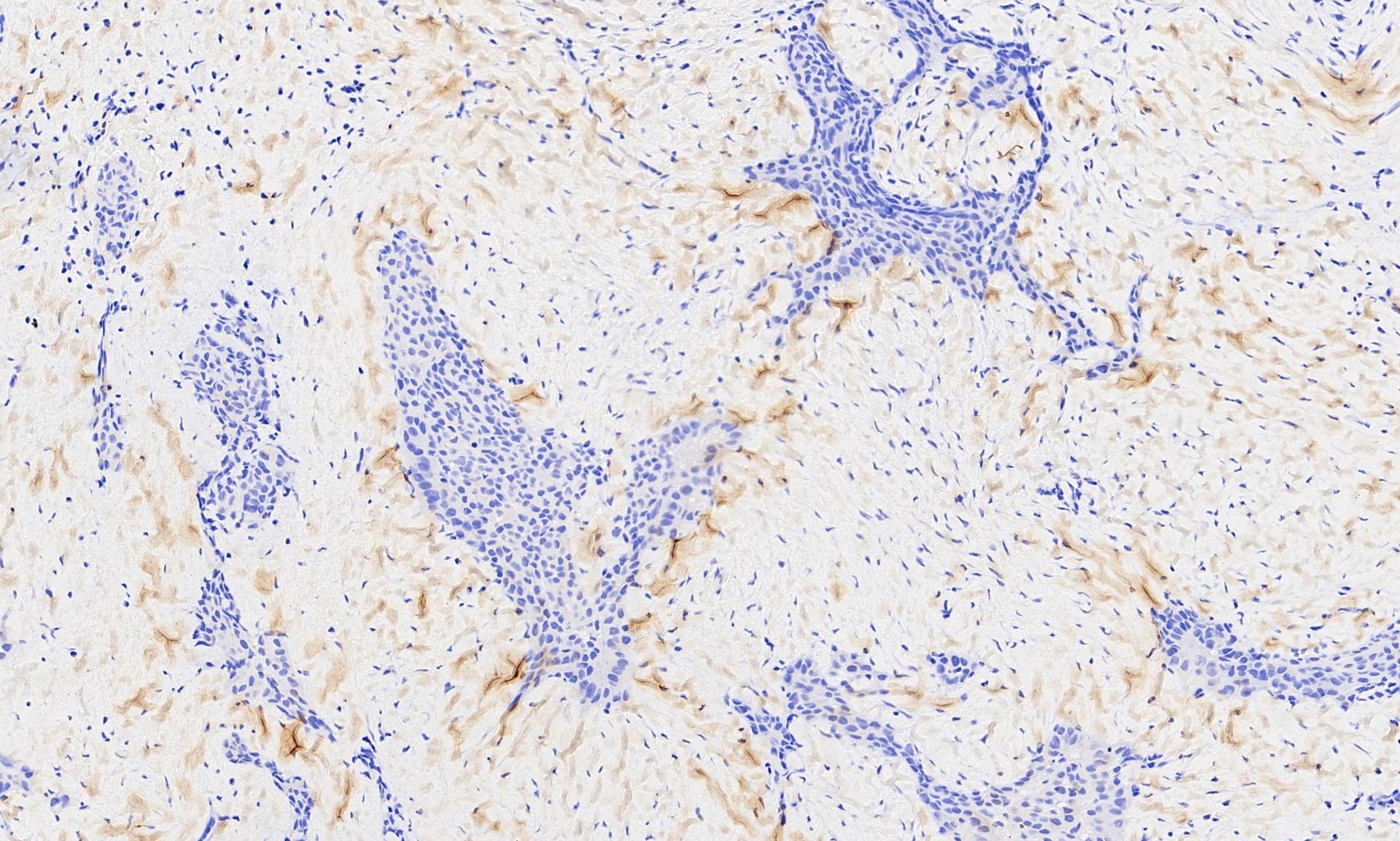
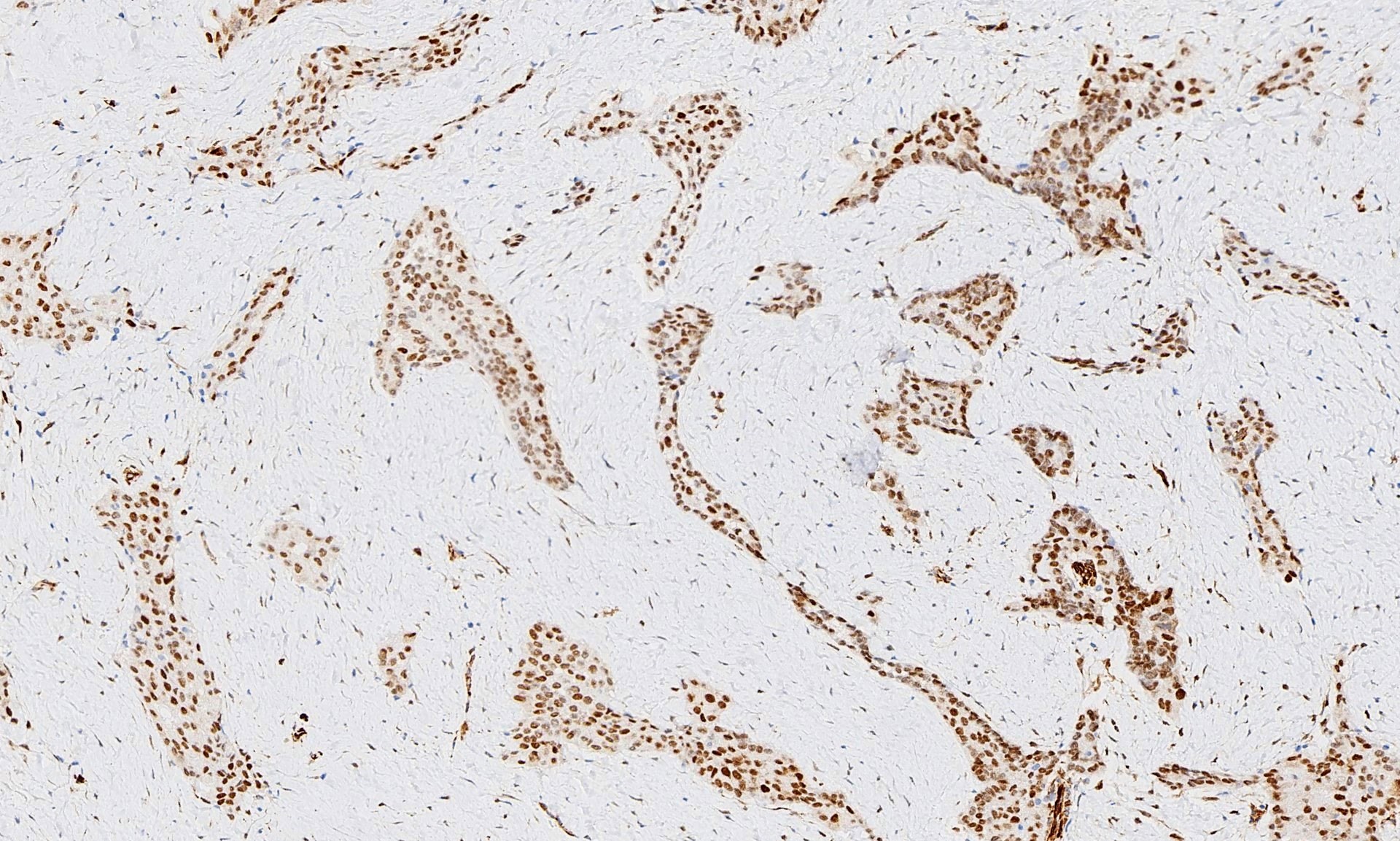
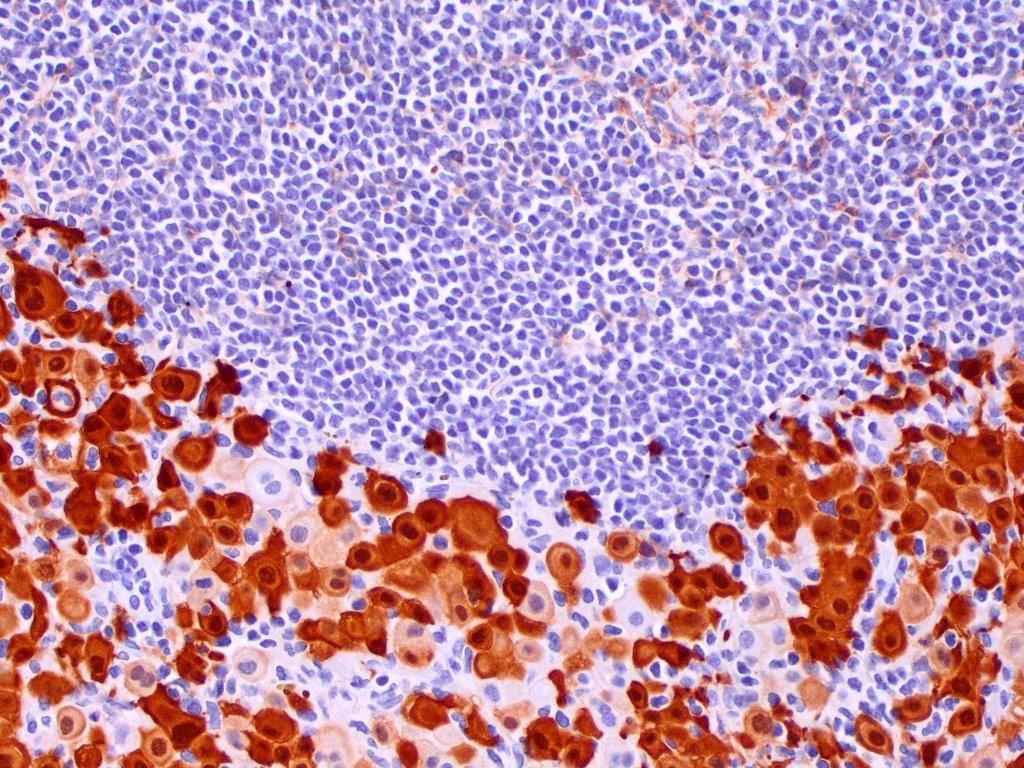
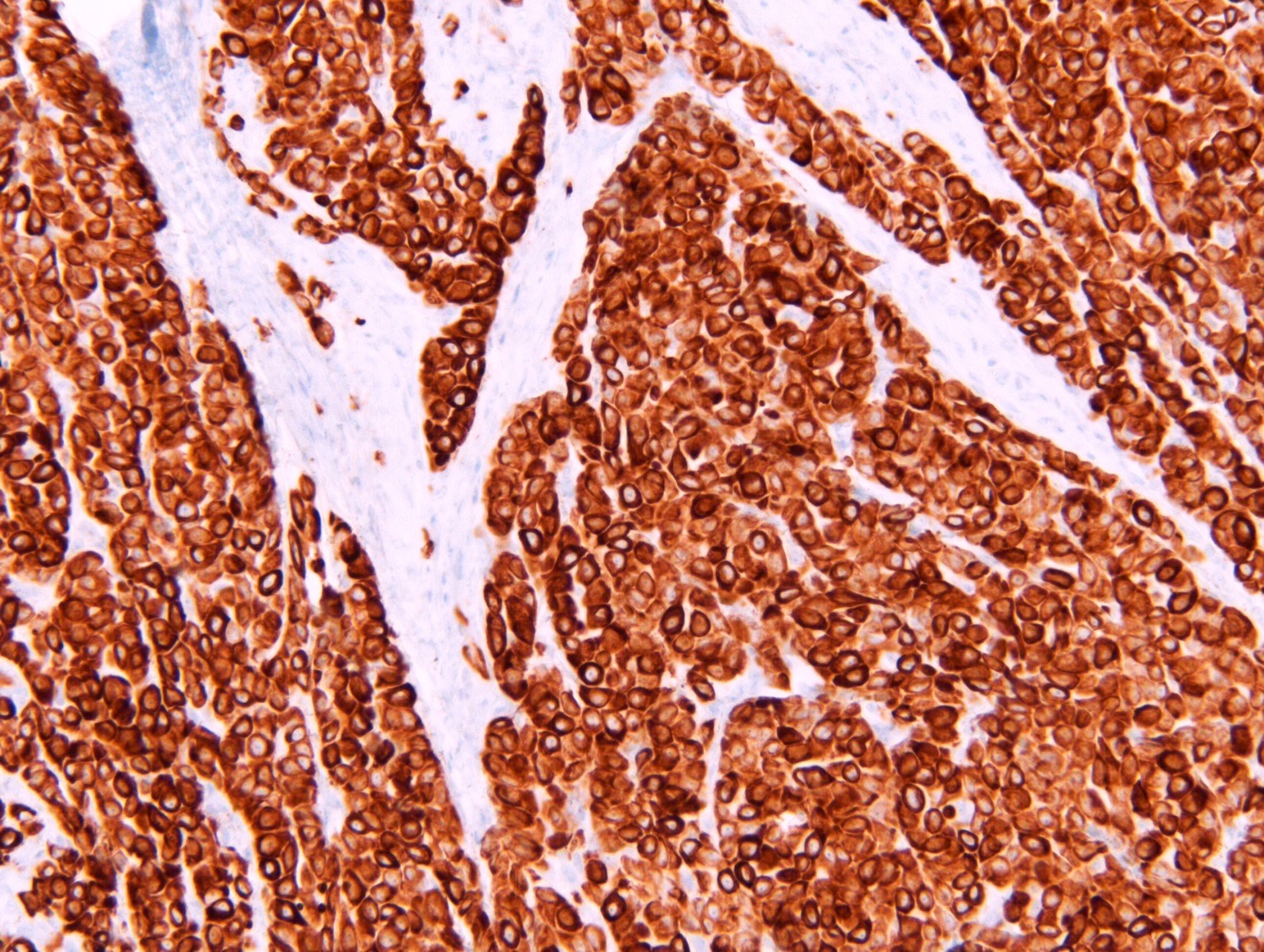
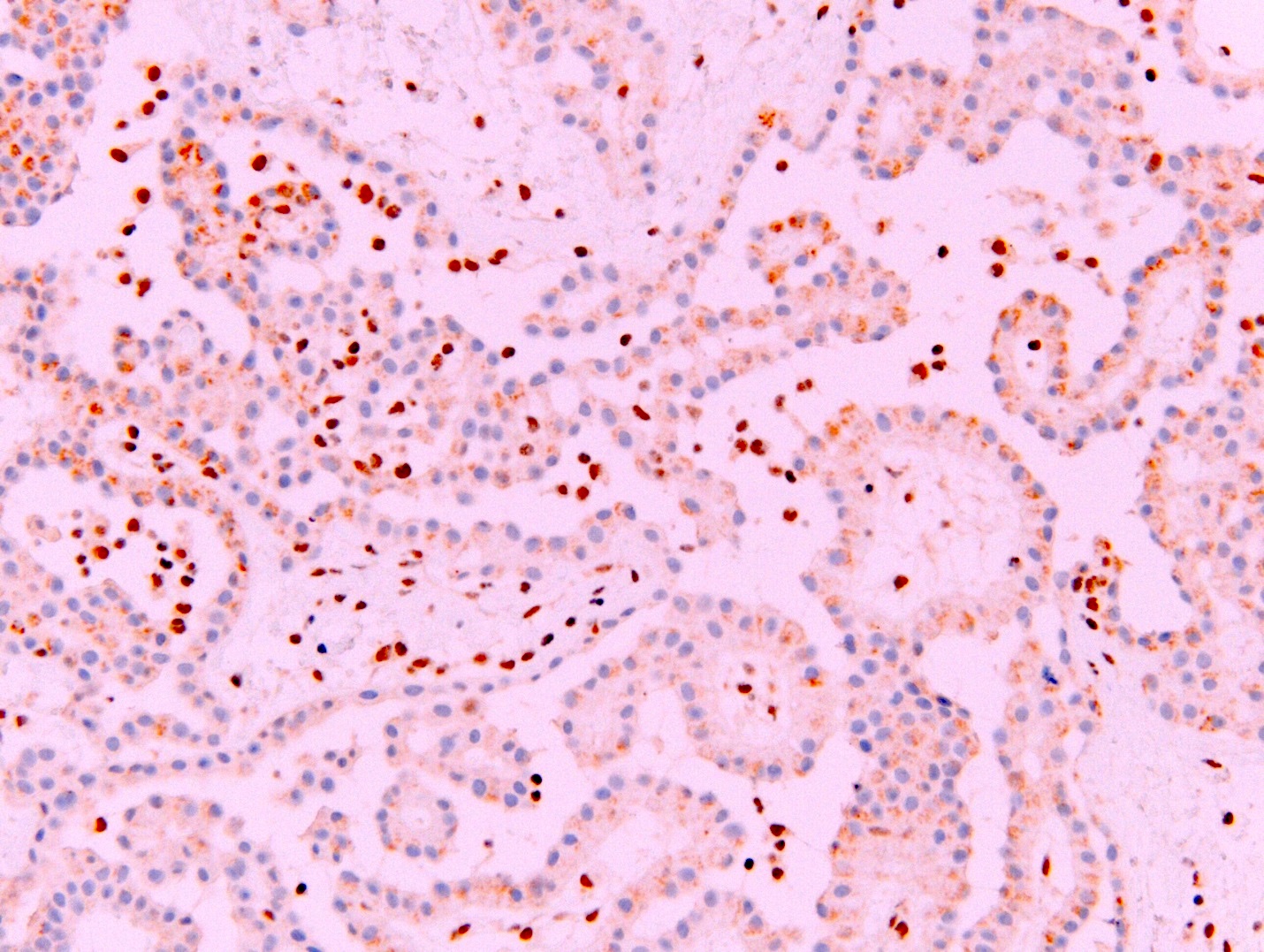
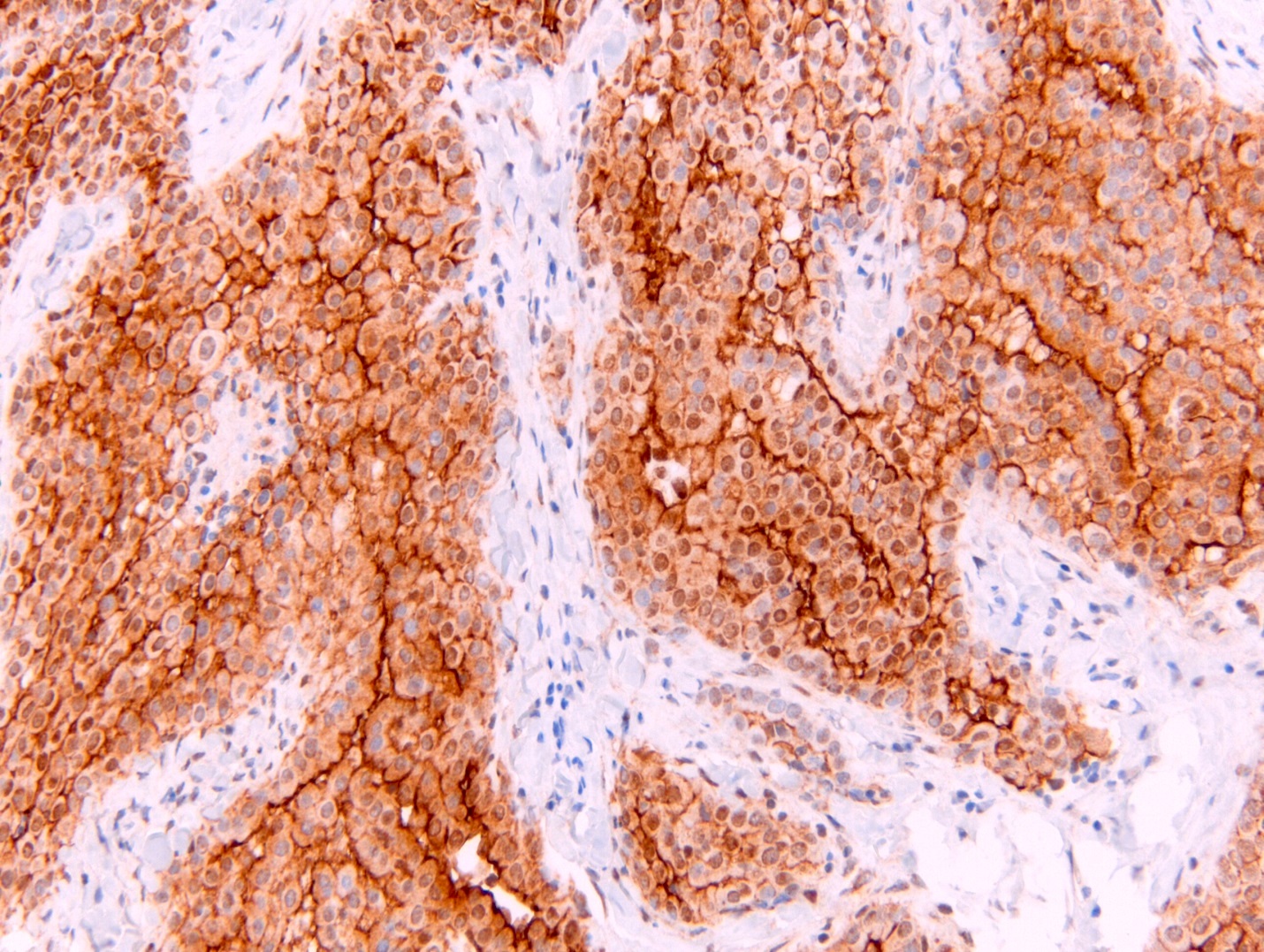
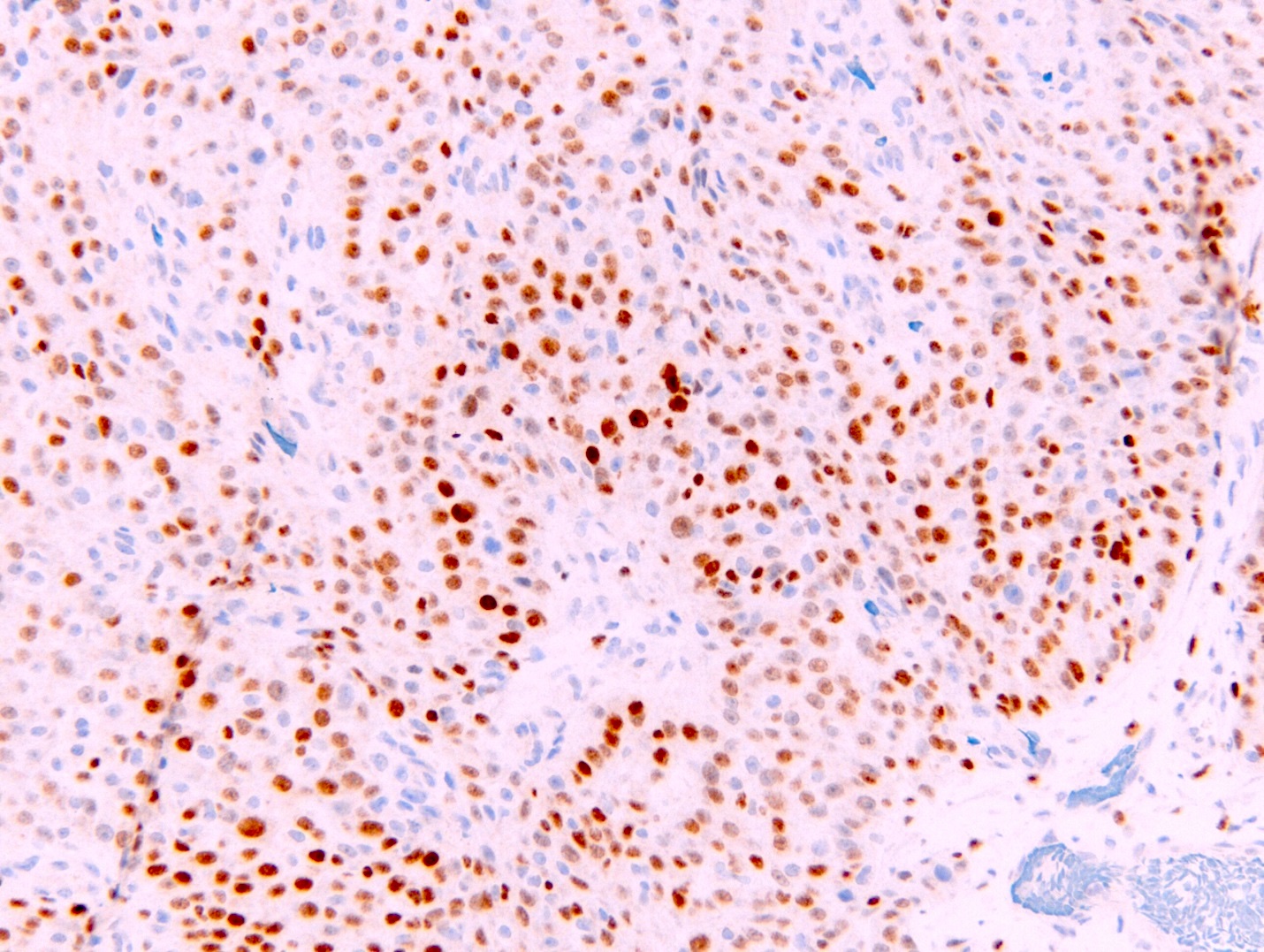
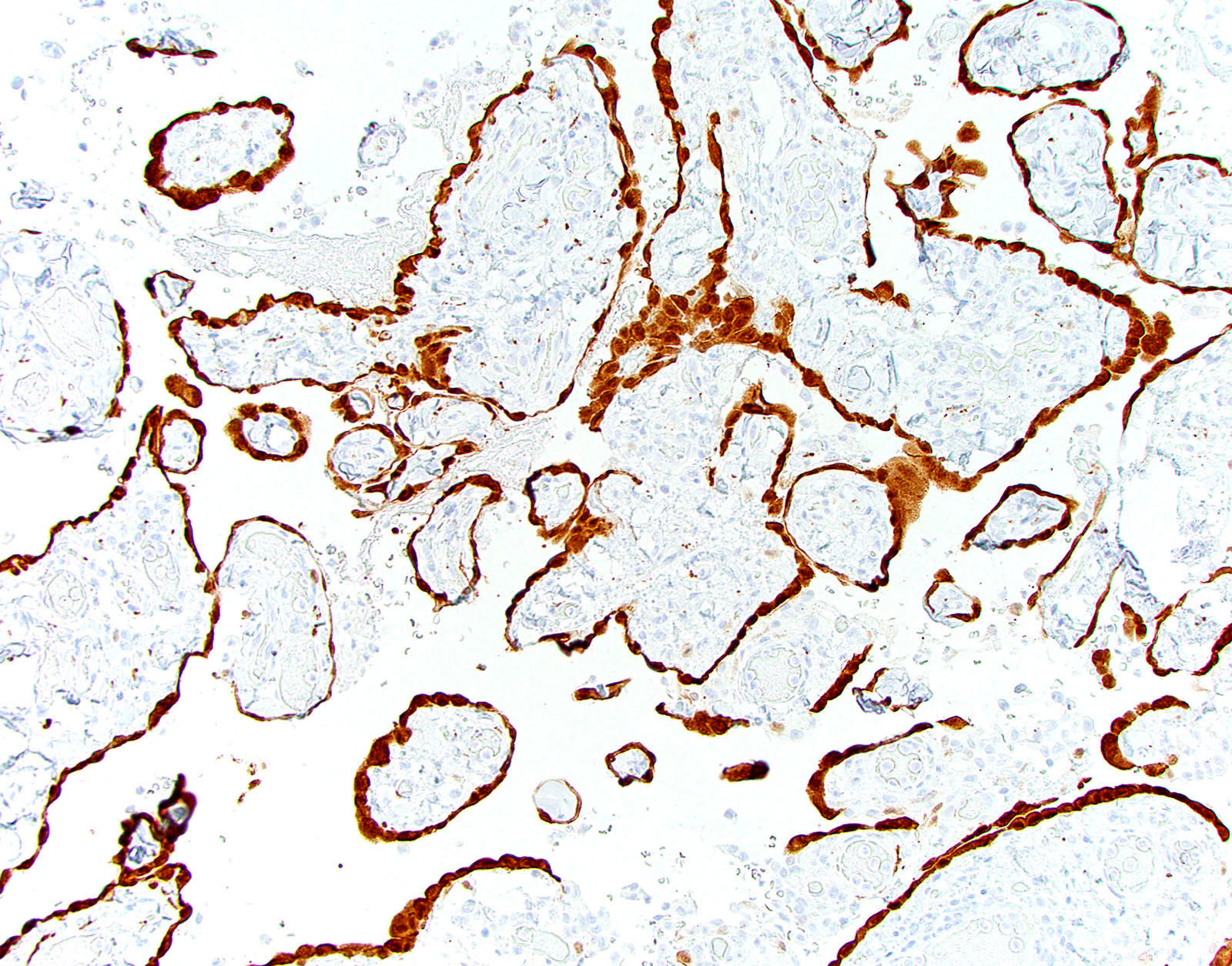
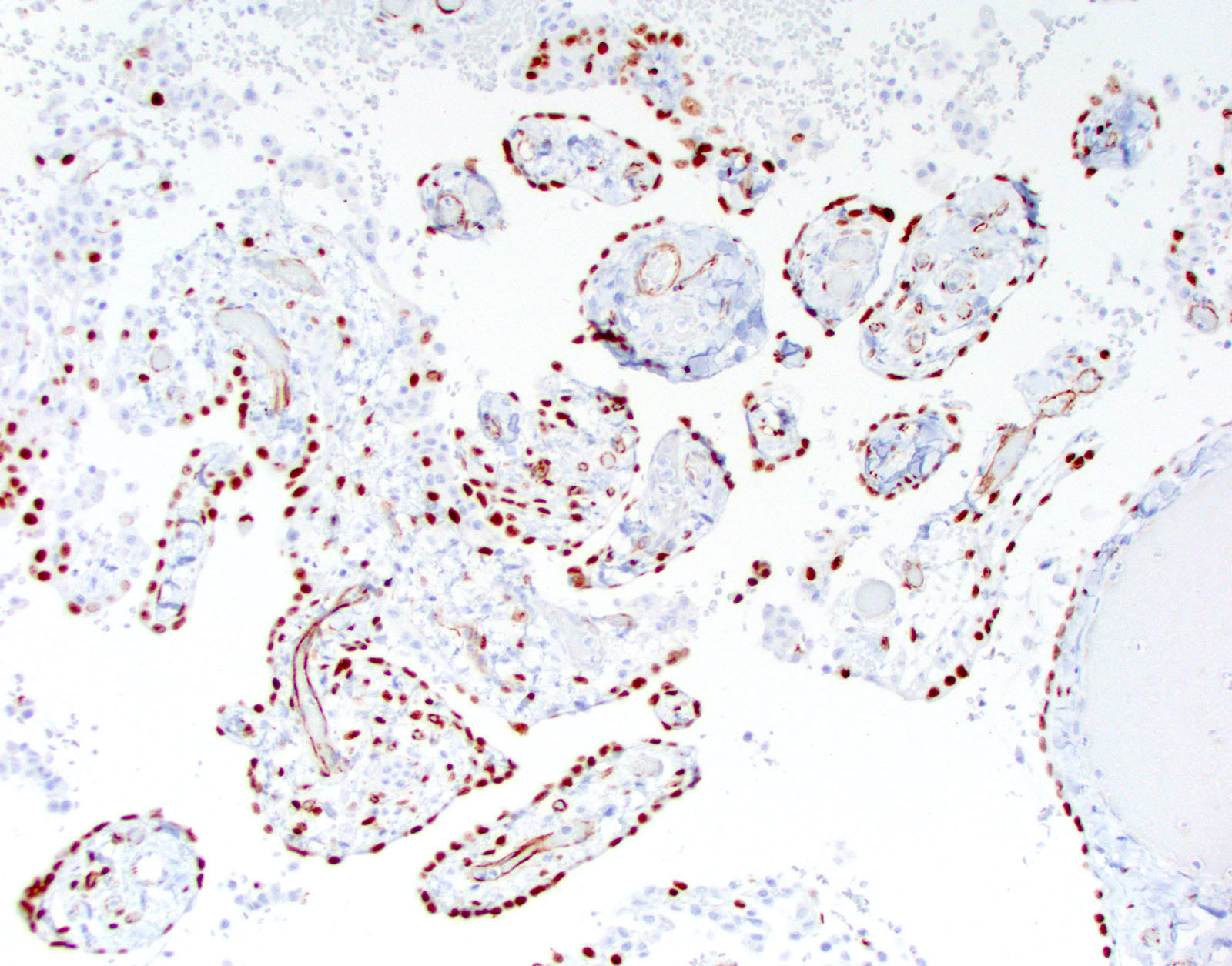
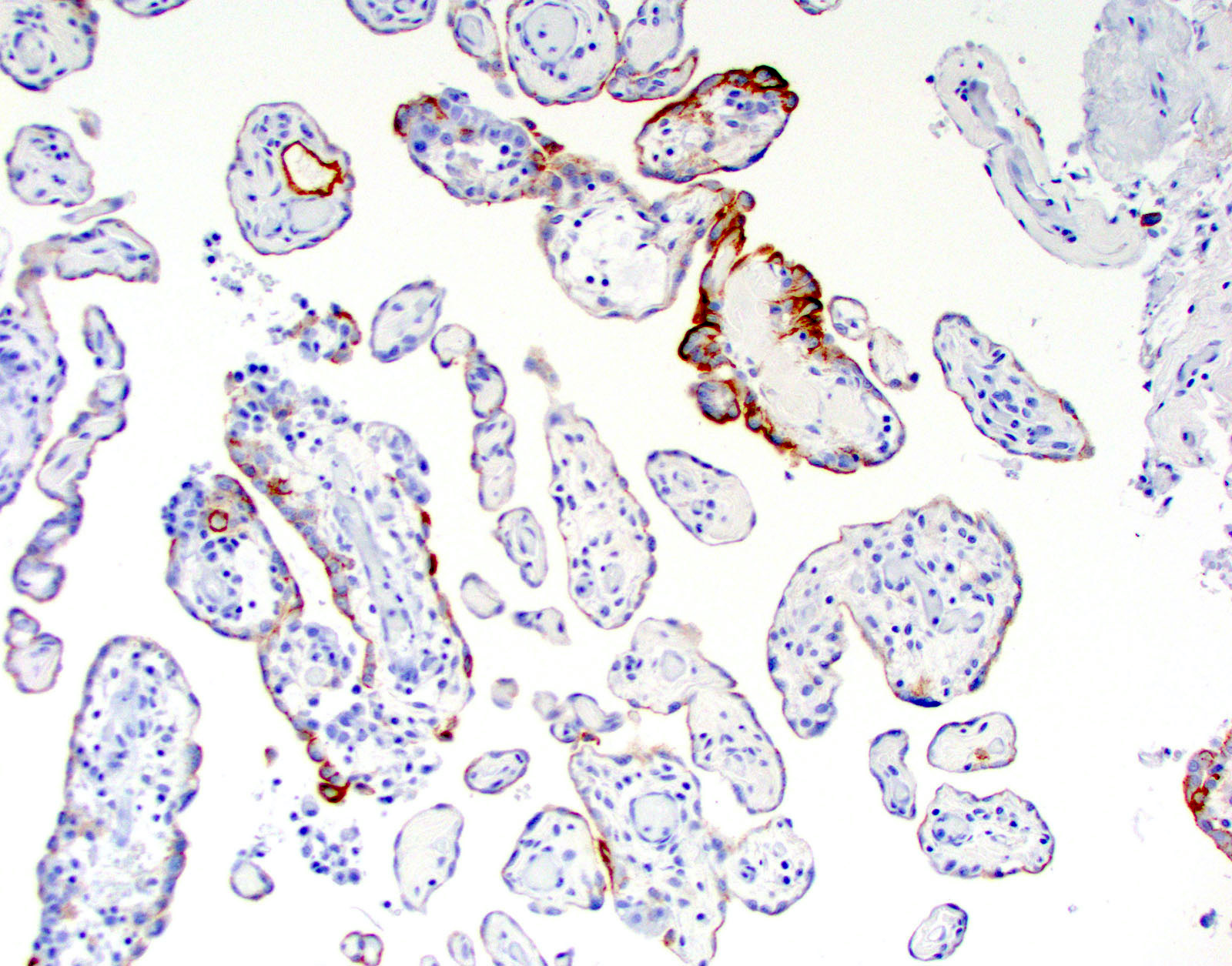
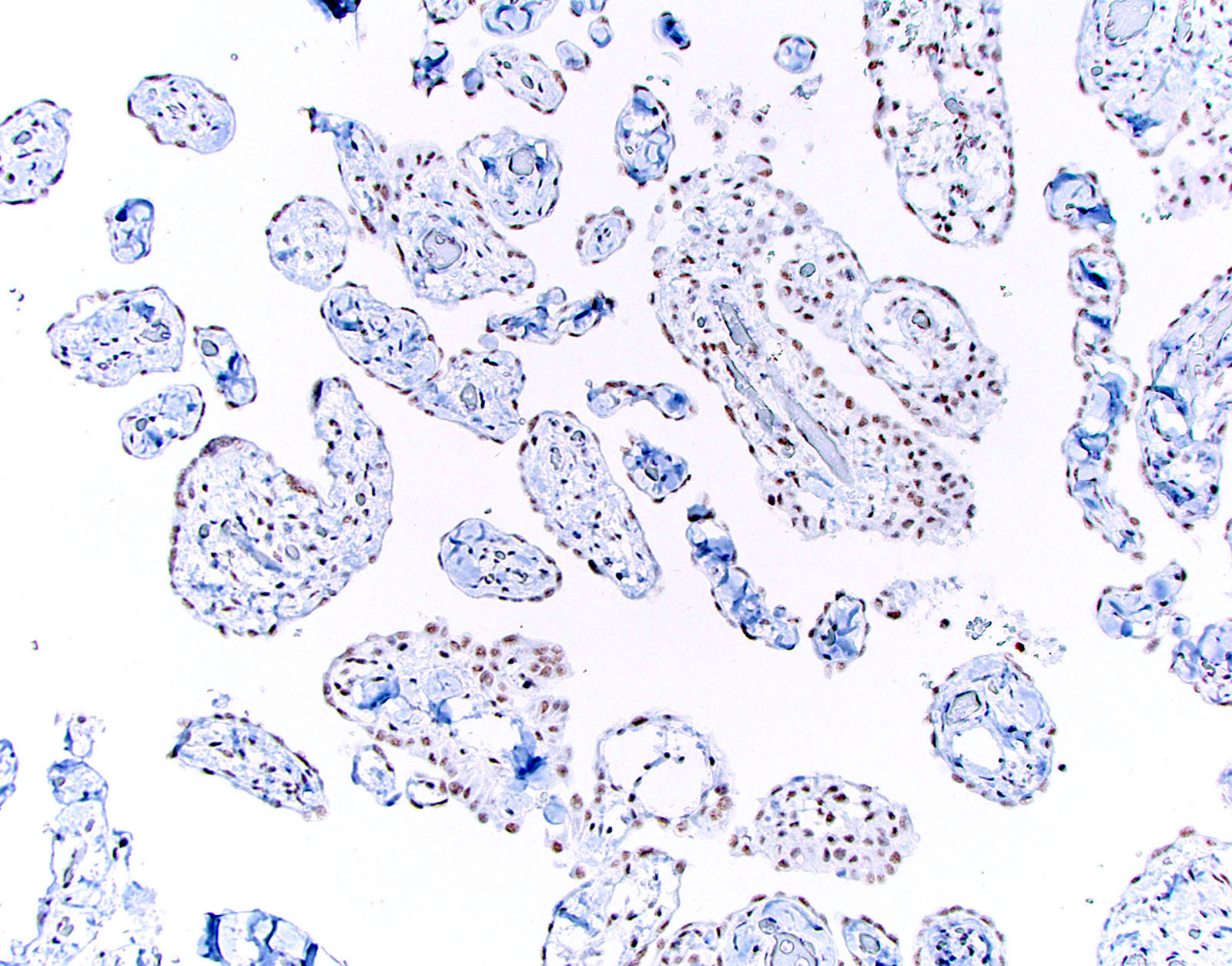
Microscopic (histologic) images
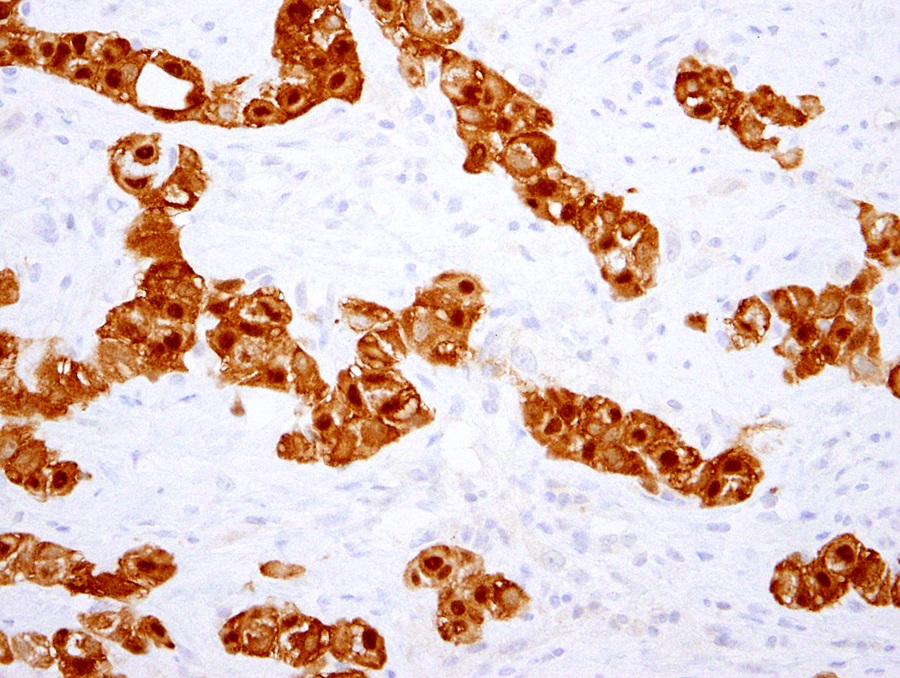
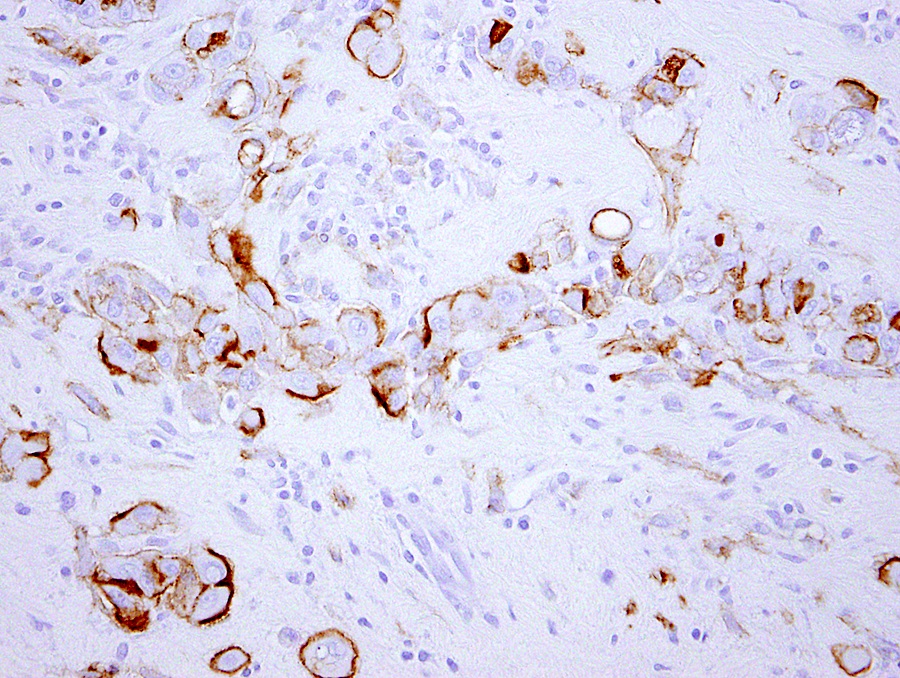
Contributed by Anja C. Roden, M.D.
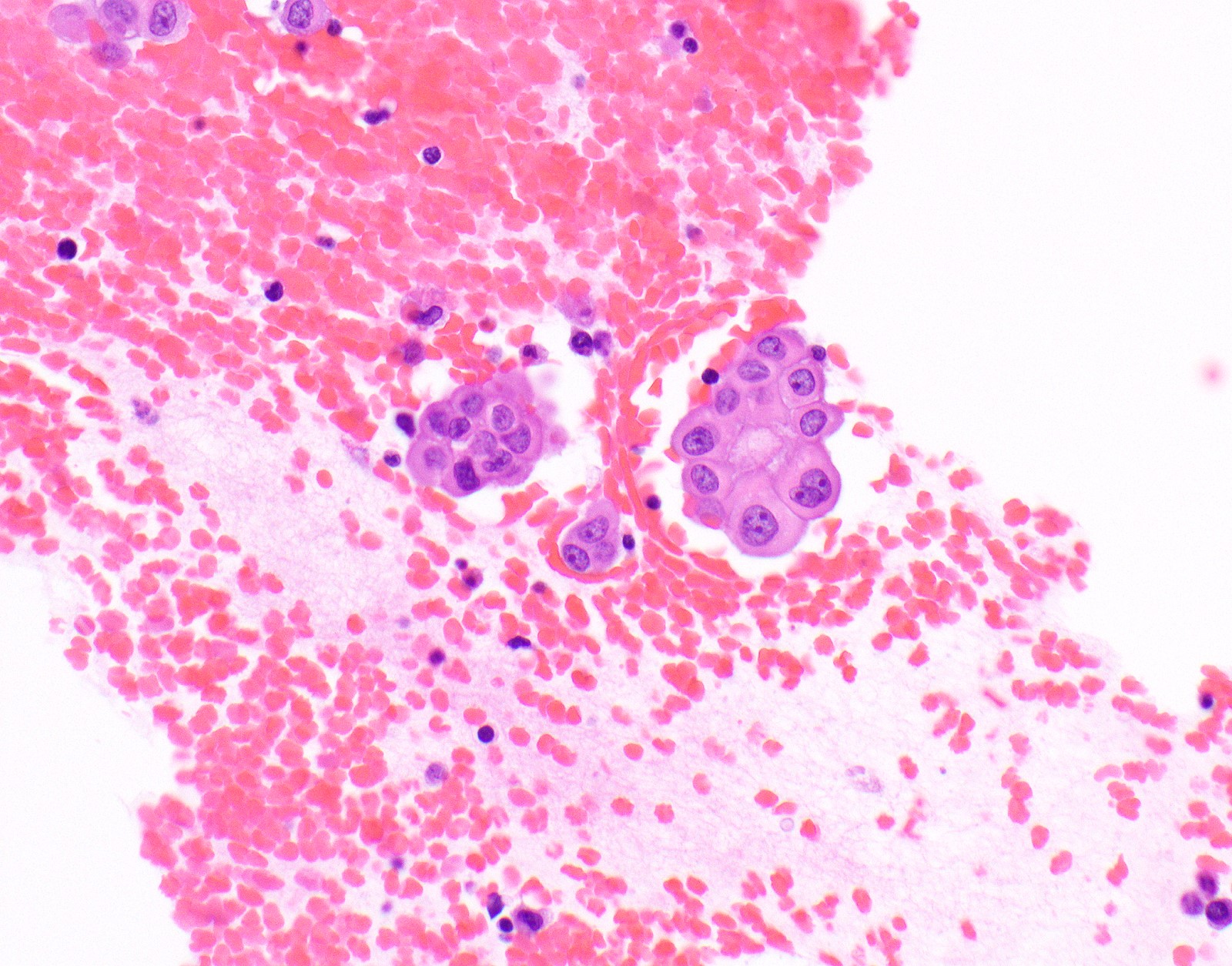
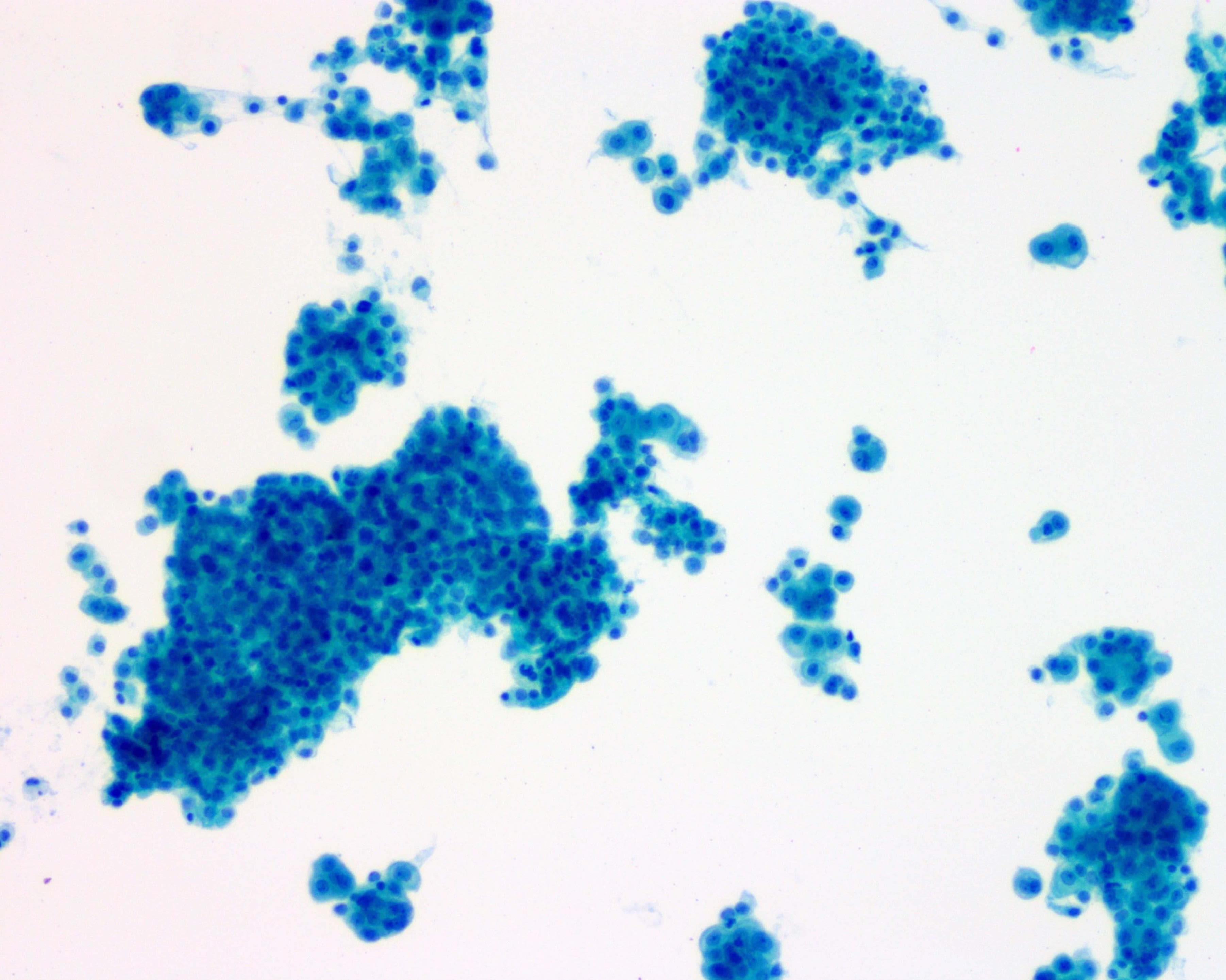
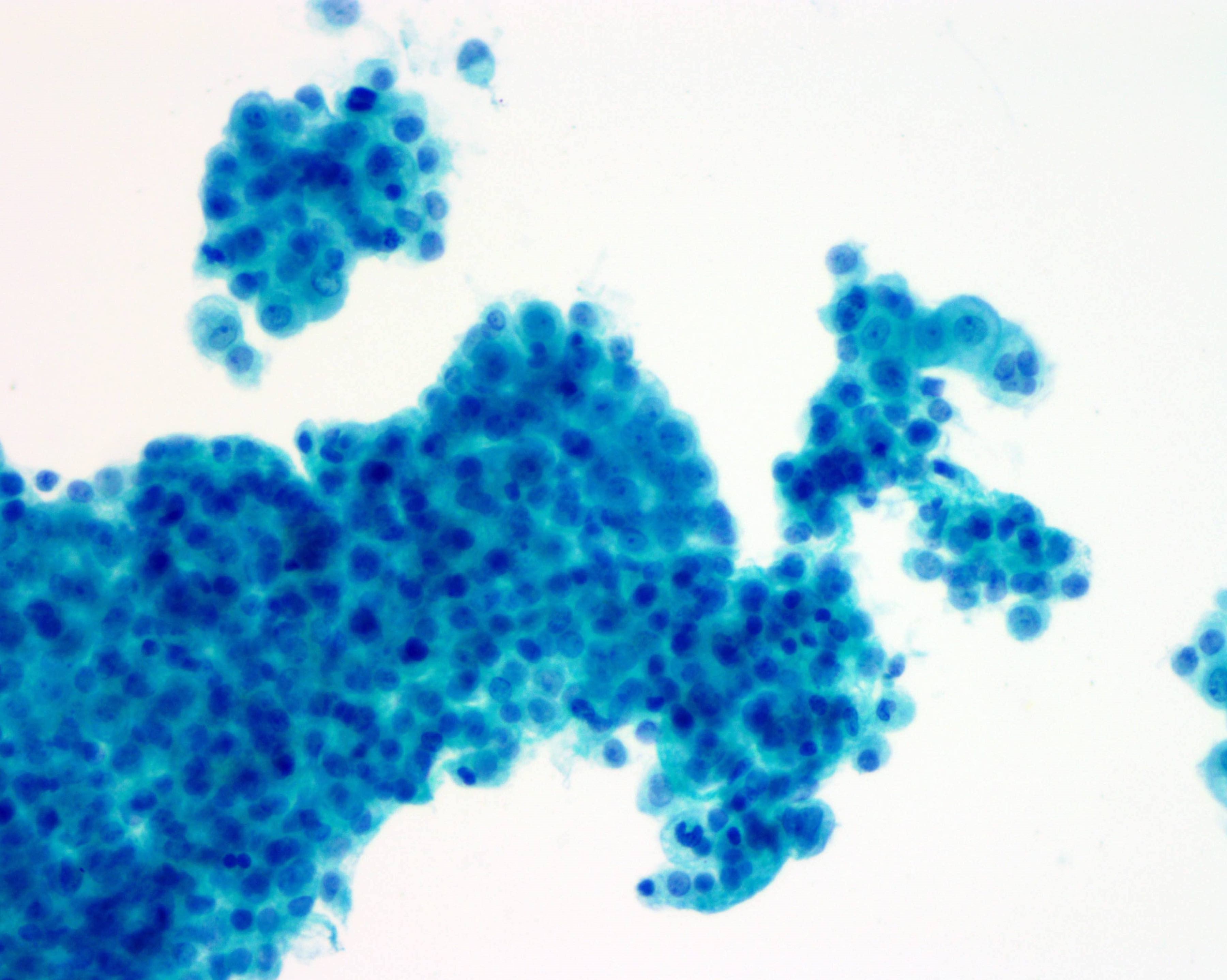
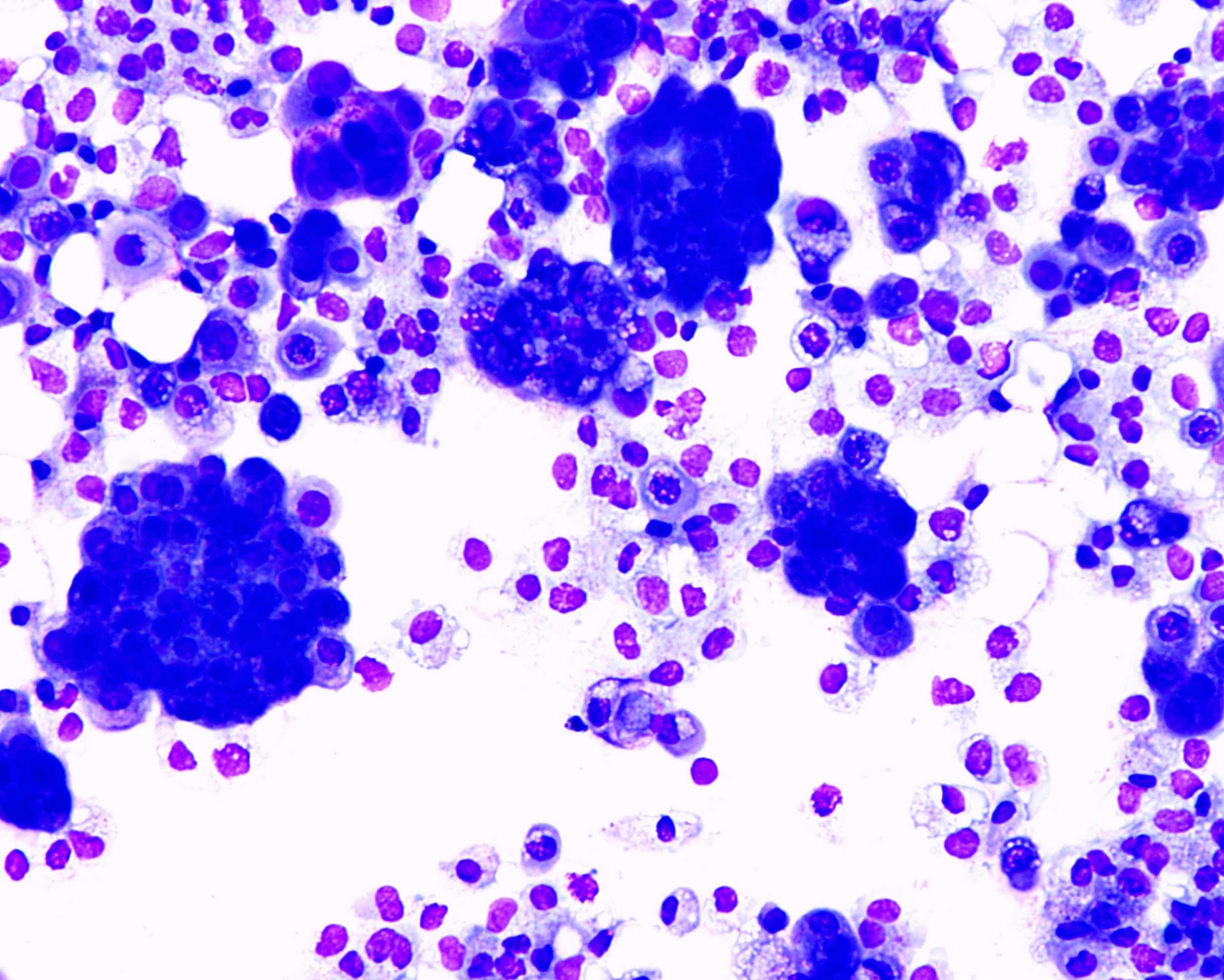
Cytology description
- In general, only epithelioid malignant pleural mesothelioma shed into pleural effusion
- Epithelioid cells in sheets, clusters, morules, papillae
- Usually bland cytology, can be pleomorphic
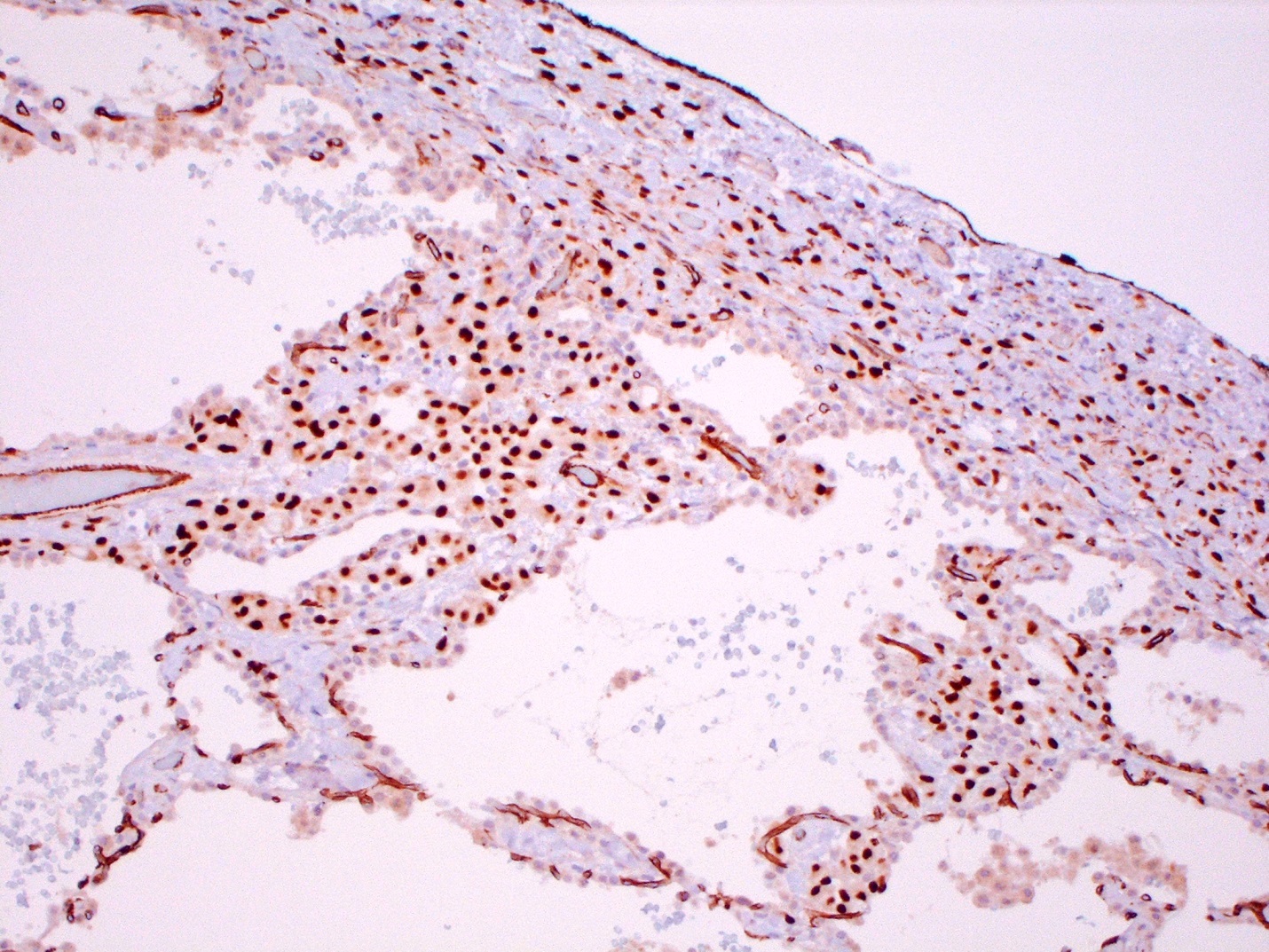
- Psammoma bodies possible
- Loss of BAP1 or MTAP expression or homozygous deletion of CDKN2A helpful in establishing the diagnosis of malignant pleural mesothelioma (see below) (Cancer 2003;99:51)
- On cytology specimens (Klin Khir 1995;2:53):
- Lack of MTAP and BAP1 expression by immunohistochemistry: 100% specific for malignant pleural mesothelioma (versus reactive mesothelial proliferation); 42 and 60% sensitive, respectively
- Loss of MTAP or BAP1 expression: 78% sensitive for malignant pleural mesothelioma
- Homozygous deletion of CDKN2A by FISH: 62% sensitive for malignant pleural mesothelioma, 100% specific
- Combination of loss of BAP1 expression or homozygous deletion of CDKN2A by FISH 84% sensitive for malignant pleural mesothelioma
- Loss of MTAP expression: sensitivity and specificity for homozygous deletion of CDKN2A by FISH; 68 and 100%, respectively
- Exclude metastatic carcinoma using immunostains (see below)
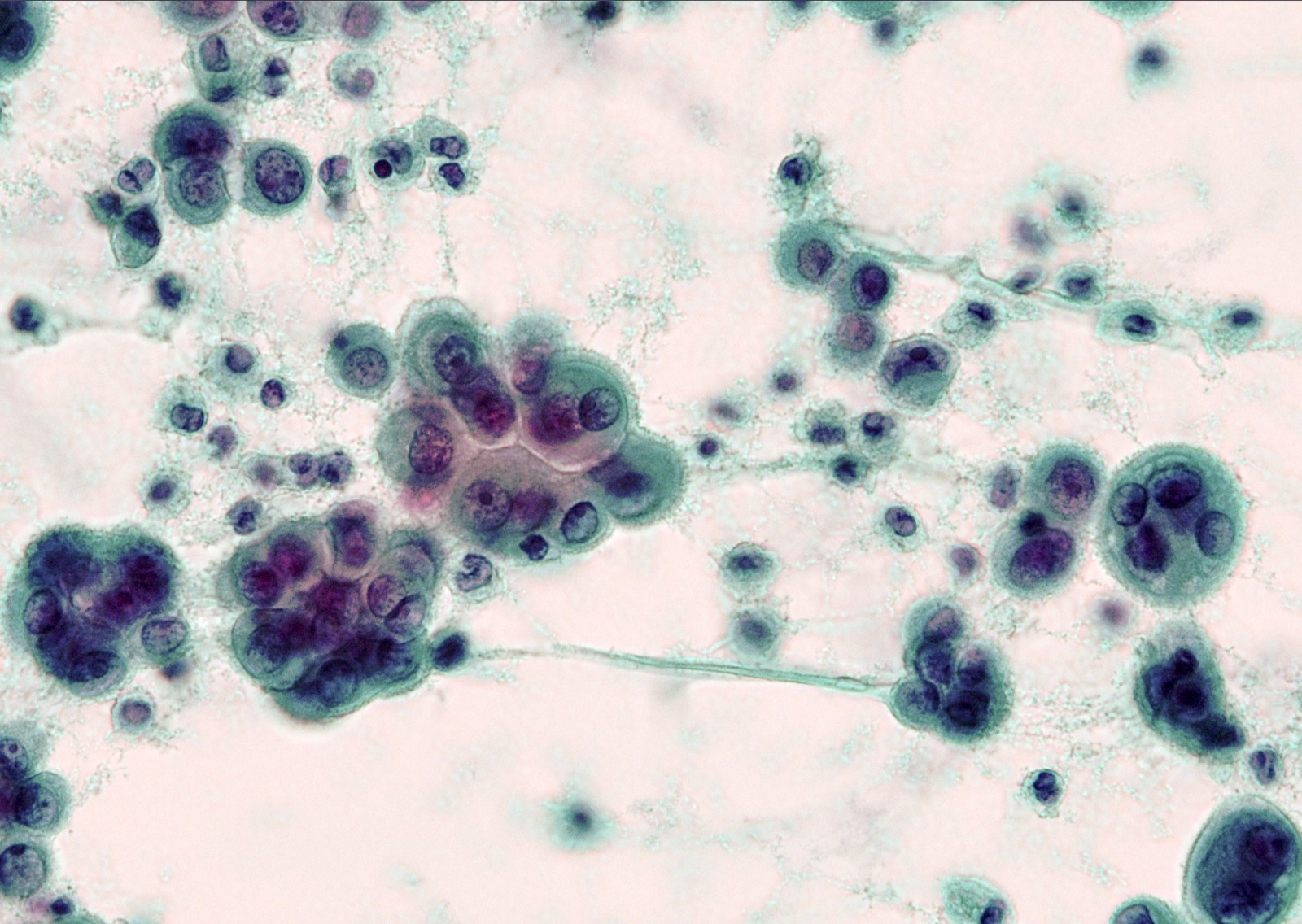
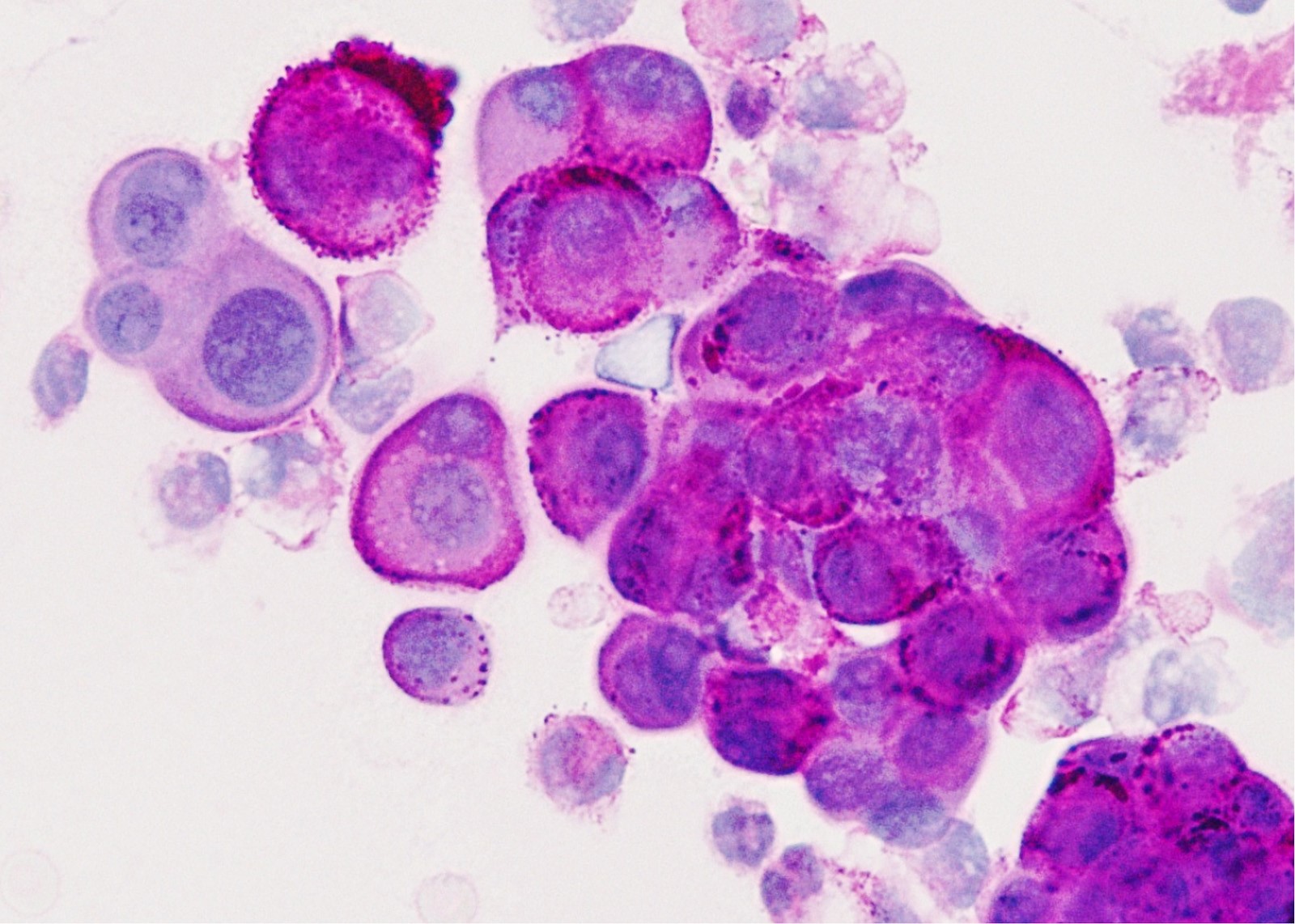
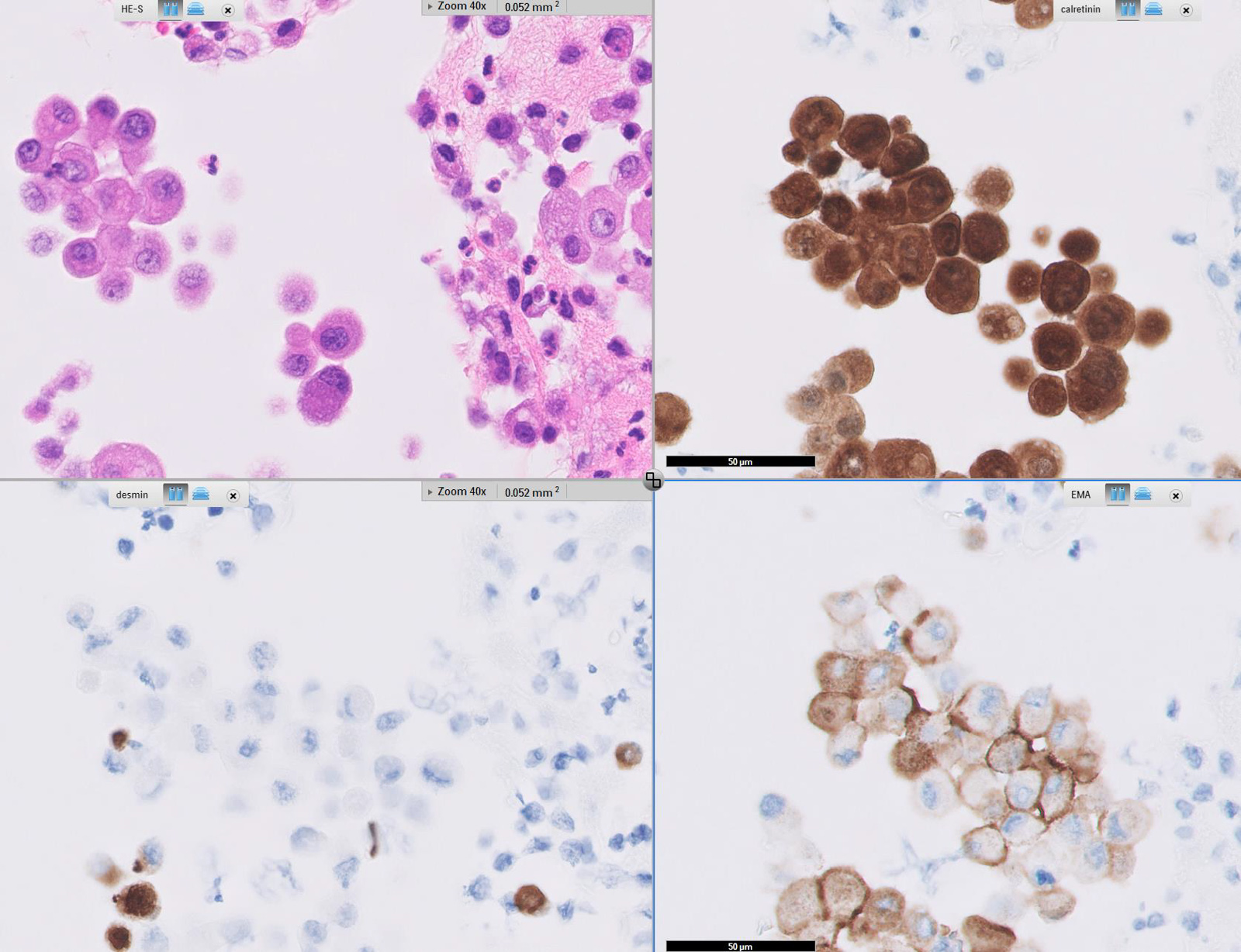
Cytology images
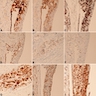
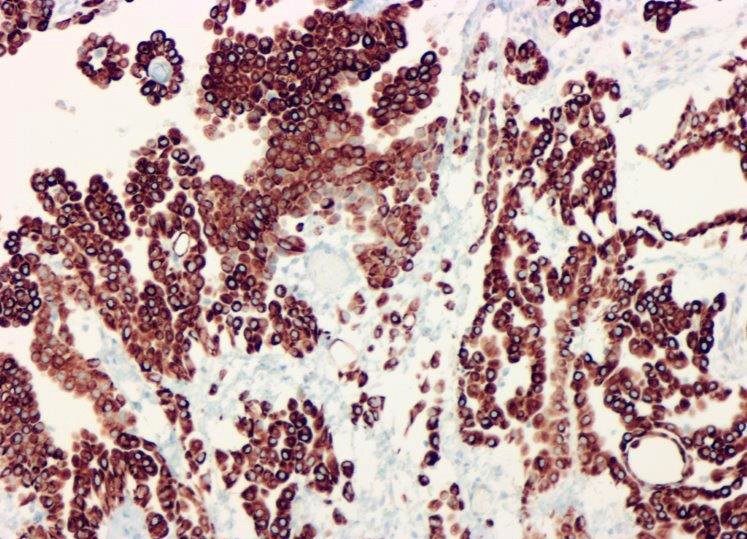
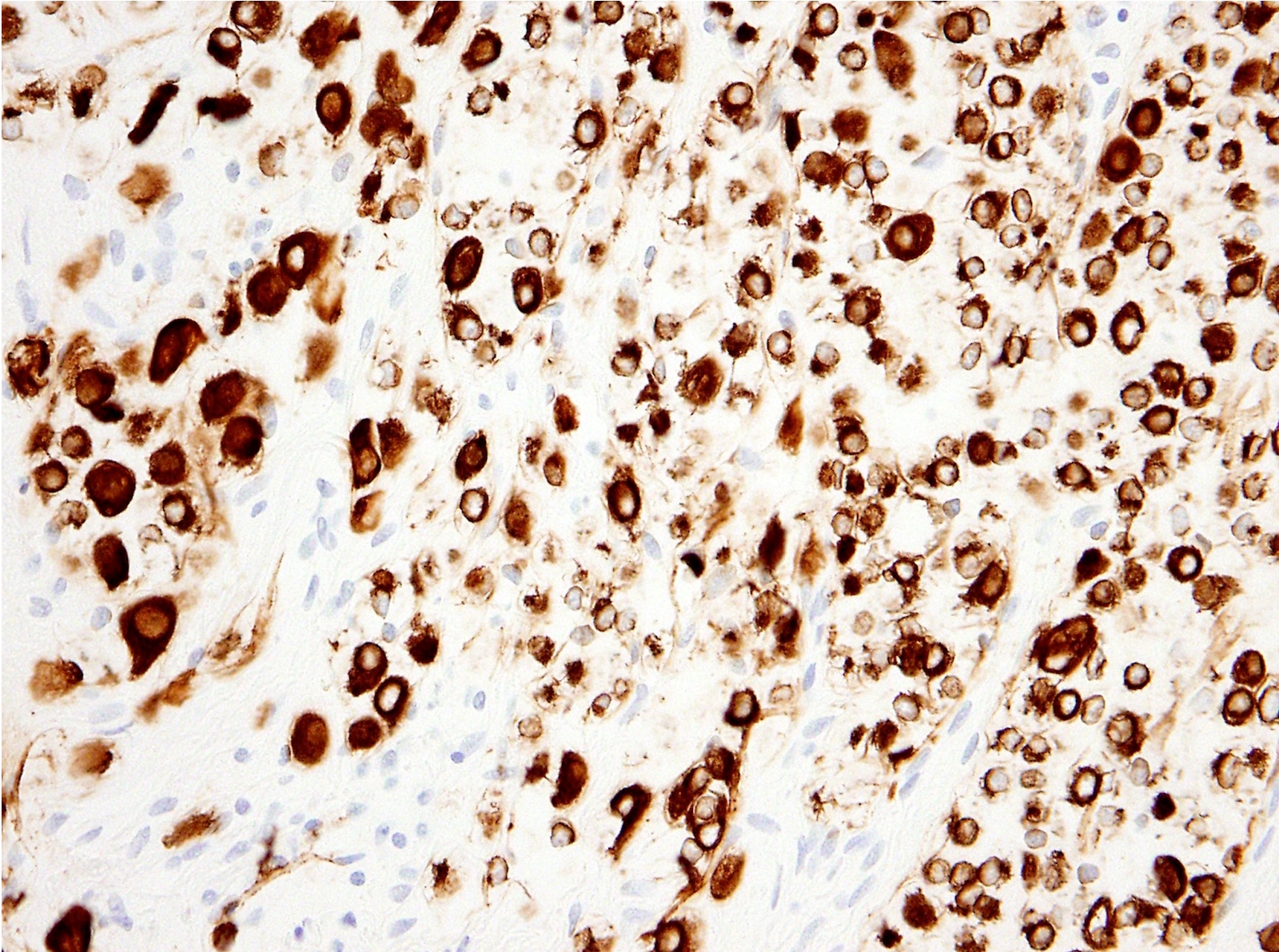
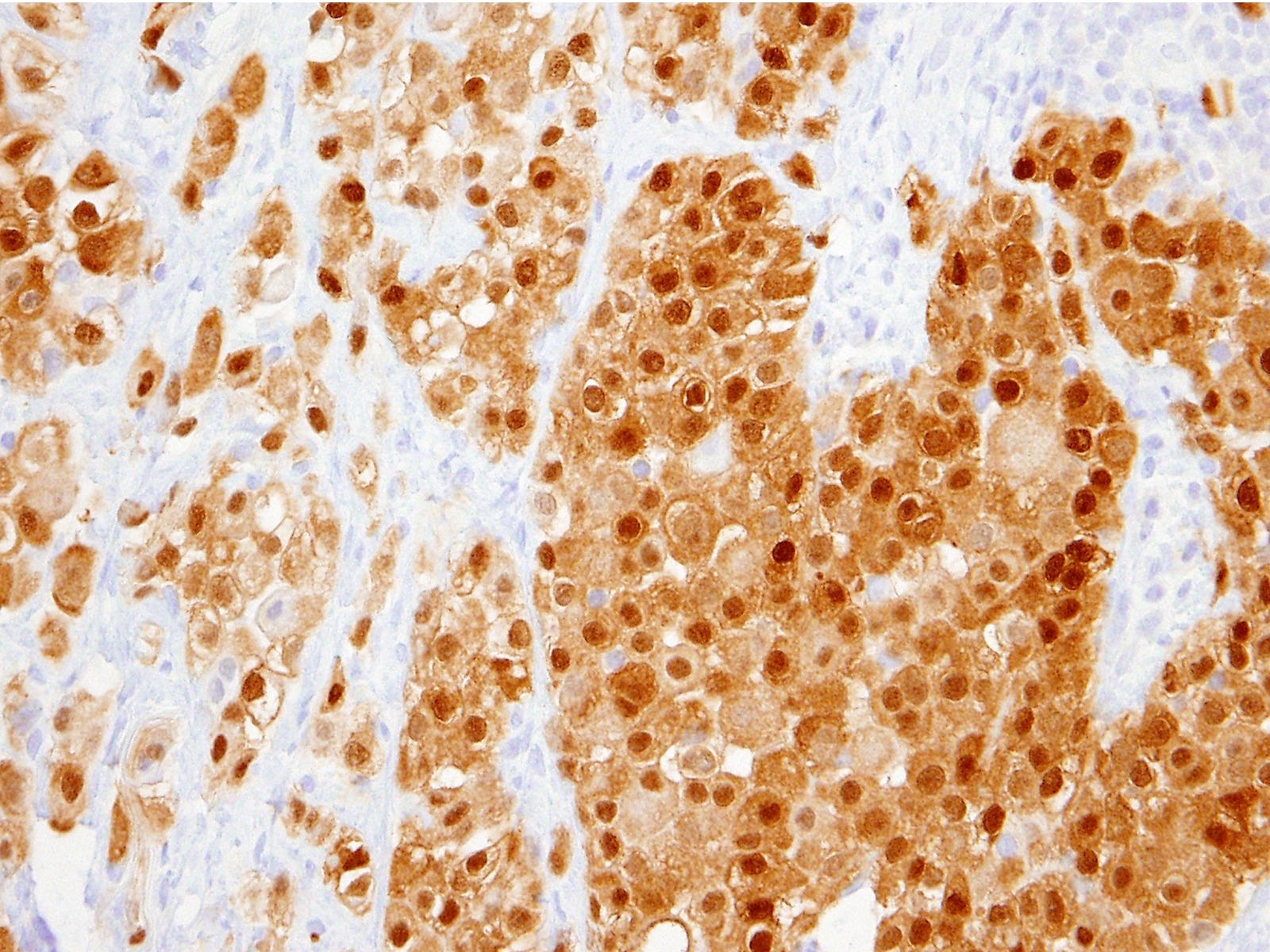
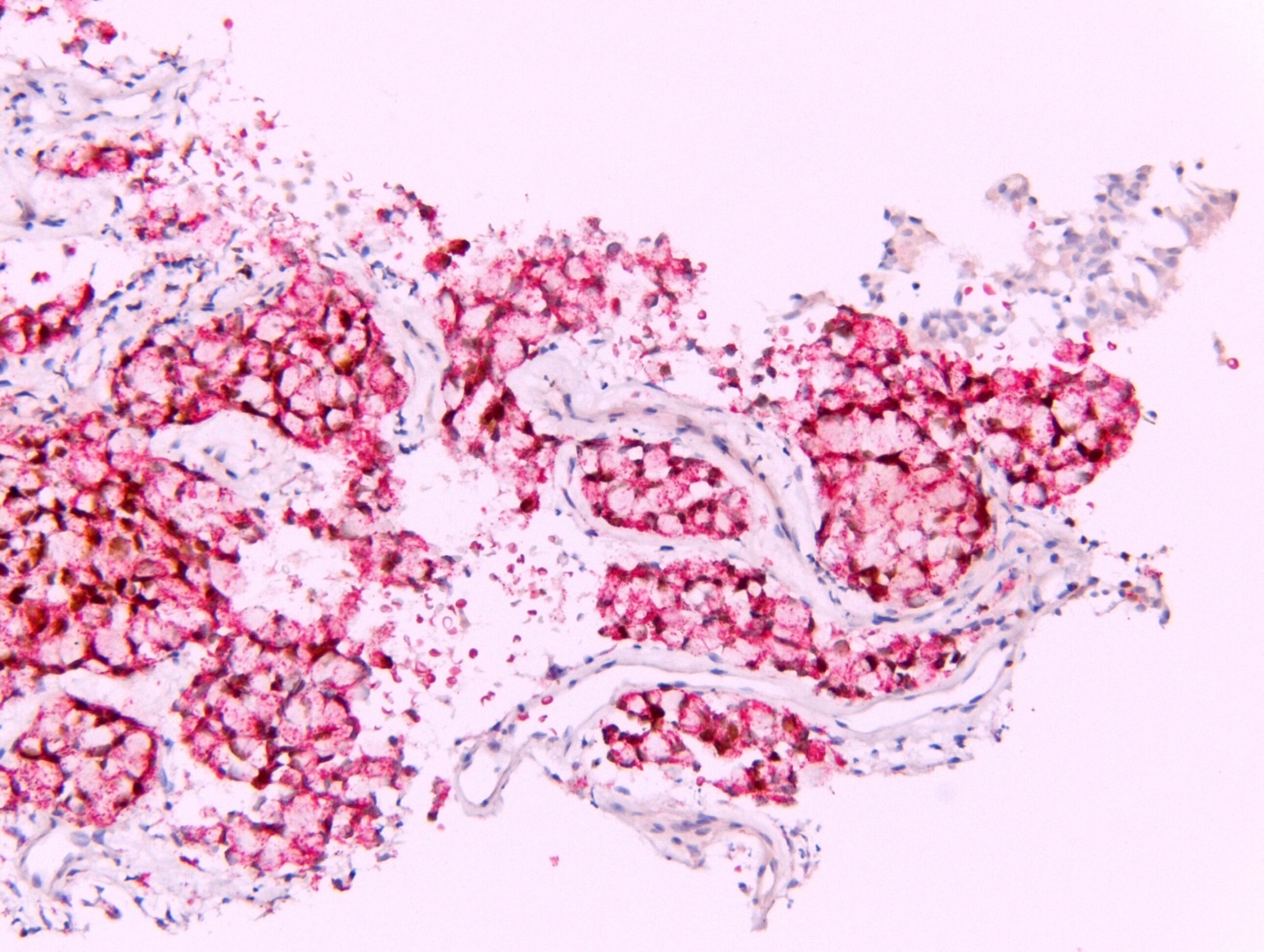
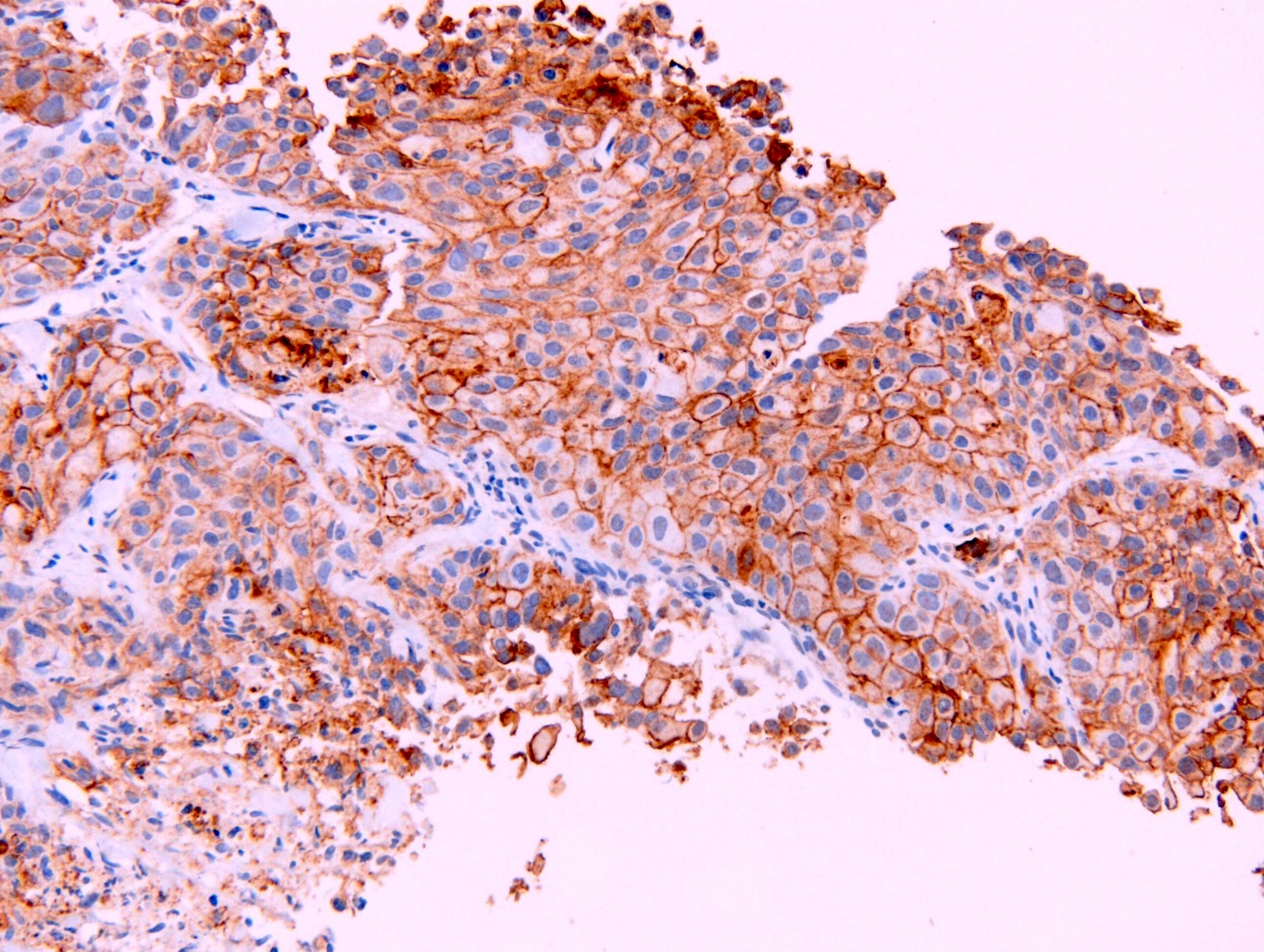
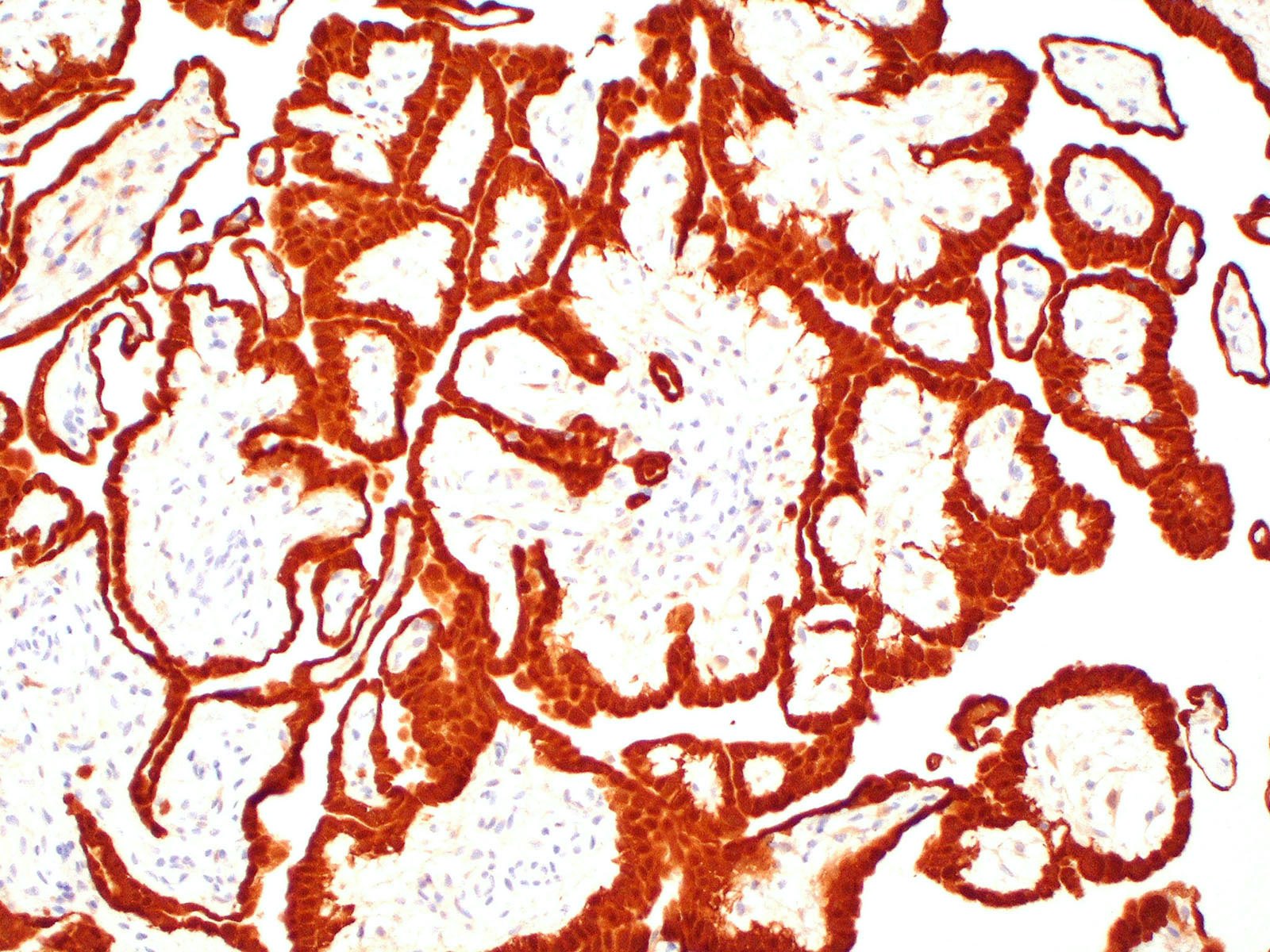
Positive stains
- See Table 1 and Table 2
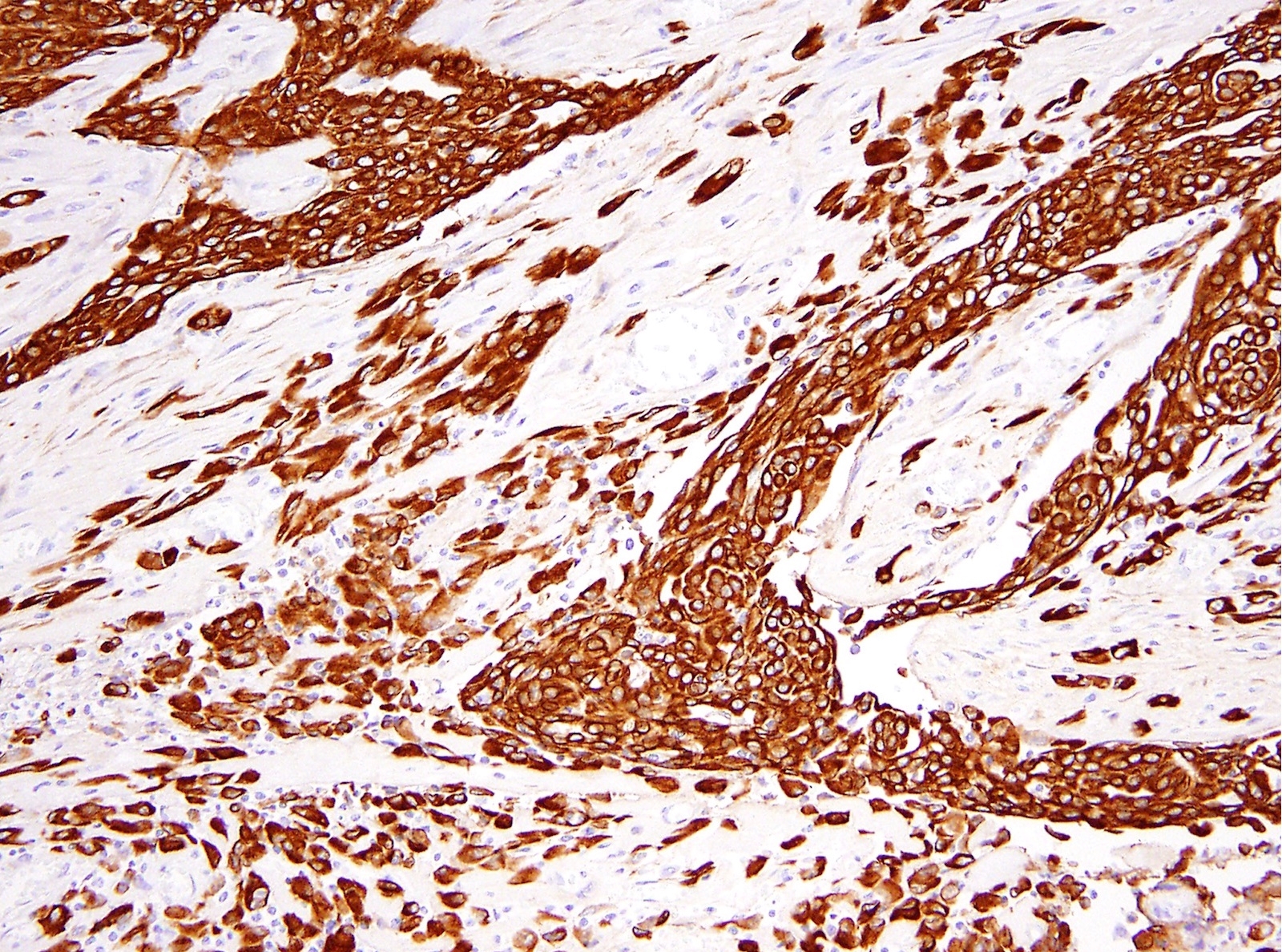
- Keratin (e.g., AE1 / AE3):
- Expressed in virtually all epithelioid malignant pleural mesothelioma and almost all sarcomatoid malignant pleural mesothelioma (if multiple keratins are negative consider other diagnosis)
- Useful to:
- Distinguish from sarcoma, malignant melanoma and other differential diagnoses
- Identify or confirm invasion
- Markers for mesothelial differentiation:
- No single marker sufficiently sensitive or specific for mesothelial differentiation → panel of at least two carcinoma markers (e.g., pCEA, TTF1, among others) and two mesothelial markers (i.e., WT1, calretinin, CK5, CK5/6, D2-40) recommended (Table 1)
- MOC31 and BerEP4, although considered carcinoma markers, often at least focally expressed in malignant pleural mesothelioma
Negative stains
- See Positive stains, Table 1 and Table 2
- Loss of nuclear BAP1 or cytoplasmic (or substantially weaker than positive internal control) MTAP expression confirm malignancy but are not specific for malignant pleural mesothelioma (see Table 2 above)
- Loss of cytoplasmic MTAP expression is a reasonable alternative for homozygous deletion of CDKN2A, k = 0.63 - 0.69; sensitivity and specificity of loss of cytoplasmic MTAP expression for homozygous deletion of CDKN2A, 78 - 93% and 96 - 100%, respectively; if MTAP expression is preserved but morphology and immunophenotype are highly suspicious for malignant pleural mesothelioma: CDKN2A by FISH might be performed (Lung Cancer 2018;125:198, Mod Pathol 2020;33:245, Lung Cancer 2018;125:198)
- Aberrant expression of CK20 rare (Appl Immunohistochem Mol Morphol 2019;27:e93)
Electron microscopy description
- In general not used anymore in the distinction from adenocarcinoma (Arch Pathol Lab Med 2018;142:89)
- Numerous long, thin, sinuous microvilli that are not covered by a glycocalyx (in contrast to adenocarcinoma in which microvilli are usually also shorter) (Ultrastruct Pathol 2006;30:3)
- Large desmosomes and prominent junctional complexes
- Tonofilaments frequently in a perinuclear distribution
Molecular / cytogenetics description
- See also Etiology and Table 2
- In a subset of malignant pleural mesothelioma
- Somatic inactivating mutations in BAP1 gene
- Germline mutations in BAP1 gene
- Homozygous deletion of CDKN2A
- Deletion of NF2 (53 - 76%) (Mod Pathol 2020;33:235, Mod Pathol 2018;31:122)
Sample pathology report
- Pleura, right, pleurectomy:
- Malignant mesothelioma, epithelioid type (see synoptic report)
- 1 of 2 lymph nodes involved by malignant mesothelioma
Differential diagnosis
- Metastatic disease:
- Immunohistochemistry (Table 1) and history required
- Metastatic adenocarcinoma
- Metastatic squamous cell carcinoma
- Keratinization or intercellular bridges in a subset
- Expression of p40
- Metastatic sarcomatoid carcinoma
- Expression of claudin 4 might be helpful
- Sarcoma (primary or metastatic)
- Lack of expression of keratin
- Expression of lineage specific markers
- Presence of disease defining molecular alterations such as t(X;18) and rearrangements of SS18 gene
- Metastatic carcinoma
- Expression of carcinoma markers in carcinoma component
- Possible expression of lineage specific markers in sarcoma component
- Malignant melanoma
- Epithelioid hemangioendothelioma (EHE)
- Reactive mesothelial proliferation:
- Lack of invasion, tumefactive or haphazard growth (see Microscopic (histologic) description)
- Expression of BAP1 and MTAP preserved (Table 2)
- Wild type of CDKN2A (Table 2)
- Lung adenocarcinoma directly extending into pleura:
Board review style question #1
- Which clinical history would be most likely for this patient?
- 30 year old man who underwent orchiectomy for immature teratoma as a child
- 55 year old man who is on dialysis for diabetic nephropathy
- 60 year old man who worked in a shipyard 35 years ago
- 65 year old man who worked in a coal mine for 30 years
- 65 year old woman with history of breast carcinoma
Board review style answer #1
C. This patient was likely exposed to asbestos 35 years ago. Unilateral pleural thickening is highly suspicious for malignant pleural mesothelioma.
Answer A is false; metastases from germ cell tumors would be usually bilateral. B is false; this patient would likely present with bilateral pleural effusion and possible bilateral pleural thickening. D is false; exposure to coal dust is not a risk factor for malignant pleural mesothelioma. E is false; metastases from breast carcinoma would be usually bilateral.
Comment Here
Reference: Diffuse malignant mesothelioma
Answer A is false; metastases from germ cell tumors would be usually bilateral. B is false; this patient would likely present with bilateral pleural effusion and possible bilateral pleural thickening. D is false; exposure to coal dust is not a risk factor for malignant pleural mesothelioma. E is false; metastases from breast carcinoma would be usually bilateral.
Comment Here
Reference: Diffuse malignant mesothelioma
Board review style question #2
- This patient has a history of ovarian carcinoma. Which would be the most useful panel of immunostains to rule out malignant mesothelioma in this patient?
- Calretinin, CK5, PAX8, pCEA
- CAM 5.2, WT1, MOC31, D2-40
- D2-40, WT1, pCEA, MOC31
- Keratin, AE1 / AE3, WT1, estrogen receptor, MOC31
- WT1, pCEA, MOC31, BerEP4
Board review style answer #2
A. Expression of calretinin and CK5 would indicate malignant pleural mesothelioma while PAX8 and pCEA indicate metastatic ovarian carcinoma. Be aware that PAX8 can be positive in a small subset of malignant pleural mesothelioma.
Answer B is false; CAM5.2 and WT1 will be positive in both, malignant pleural mesothelioma and metastatic ovarian carcinoma; MOC31 will be expressed in metastatic carcinoma but can be at least focally expressed in malignant pleural mesothelioma. D2-40 can be expressed in serous carcinomas. C is false; although two malignant pleural mesothelioma markers (D2-40, WT1) and two carcinoma markers (pCEA and MOC31), WT1 can be positive in ovarian carcinomas and MOC31 is sometimes at least focally expressed in malignant pleural mesothelioma. D is false; keratin AE1/AE3 and WT1 will be positive in both, malignant pleural mesothelioma and metastatic ovarian carcinoma; MOC31 will be expressed in metastatic carcinoma but can be at least focally expressed in malignant pleural mesothelioma. E is false; these are three carcinoma markers (pCEA, MOC31, BerEP4) and only one malignant pleural mesothelioma marker which also is frequently expressed in ovarian carcinomas. For workup of malignant pleural mesothelioma, at least two carcinoma markers and two mesothelial markers should be used.
Comment Here
Reference: Diffuse malignant mesothelioma
Answer B is false; CAM5.2 and WT1 will be positive in both, malignant pleural mesothelioma and metastatic ovarian carcinoma; MOC31 will be expressed in metastatic carcinoma but can be at least focally expressed in malignant pleural mesothelioma. D2-40 can be expressed in serous carcinomas. C is false; although two malignant pleural mesothelioma markers (D2-40, WT1) and two carcinoma markers (pCEA and MOC31), WT1 can be positive in ovarian carcinomas and MOC31 is sometimes at least focally expressed in malignant pleural mesothelioma. D is false; keratin AE1/AE3 and WT1 will be positive in both, malignant pleural mesothelioma and metastatic ovarian carcinoma; MOC31 will be expressed in metastatic carcinoma but can be at least focally expressed in malignant pleural mesothelioma. E is false; these are three carcinoma markers (pCEA, MOC31, BerEP4) and only one malignant pleural mesothelioma marker which also is frequently expressed in ovarian carcinomas. For workup of malignant pleural mesothelioma, at least two carcinoma markers and two mesothelial markers should be used.
Comment Here
Reference: Diffuse malignant mesothelioma
Endosalpingiosis
Table of Contents
Definition / general | Essential features | Case reports | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Glands lined by epithelium with morphology and immunophenotype resembling fallopian tube epithelium, identified outside of the fallopian tube proper (Obstet Gynecol 1980;55:57S)
- Ciliated and unciliated columnar cells, intercalated cells and peg cells are identified
- Typically affects pelvic and abdominal peritoneum, usually as an incidental microscopic finding
- Also known to involve ovary, uterus, cervix, bowel, omentum and skin
- Rarely occurs in inguinal, mediastinal or axillary lymph nodes
- Rarely forms a cystic mass, entitled florid cystic endosalpingiosis (Hum Pathol 2002;33:944, Am J Surg Pathol 1999;23:166)
Essential features
- Multiple theories of origin including direct implantation of normal fallopian tube epithelium, involution of ovarian surface epithelium and metaplastic changes in multipotential peritoneal cells
- Typically an incidental finding, except in cystic lesions which may be grossly seen
- Seen in association with ovarian serous tumors (usually of borderline type), in conjunction with implants or alone
- Theorized to be the precursor lesion in borderline and low grade serous neoplasms found on the peritoneum or in lymph nodes (Am J Surg Pathol 2010;34:1442)
- May be seen in isolation but more commonly seen in conjunction with other nonneoplastic lesions of the Müllerian system, such as endometriosis and endocervicosis
Case reports
- Adolescent girl with endosalpingiosis of the appendix (Gynecol Obstet Fertil 2016;44:669)
- 40 year old and 54 year old women with endosalpingiosis of the urinary bladder (Aktuelle Urol 2017 Jun 20 [Epub ahead of print], Int J Surg Pathol 2010;18:381)
- 70 year old woman with endosalpingiosis of axillary lymph nodes (Case Rep Pathol 2016;2016:2856358)
- Endosalpingiosis of lumbar nerve root (Clin Neuropathol 2017;36:108)
Gross description
- Usually not grossly discernible
- Cystic appearance when seen
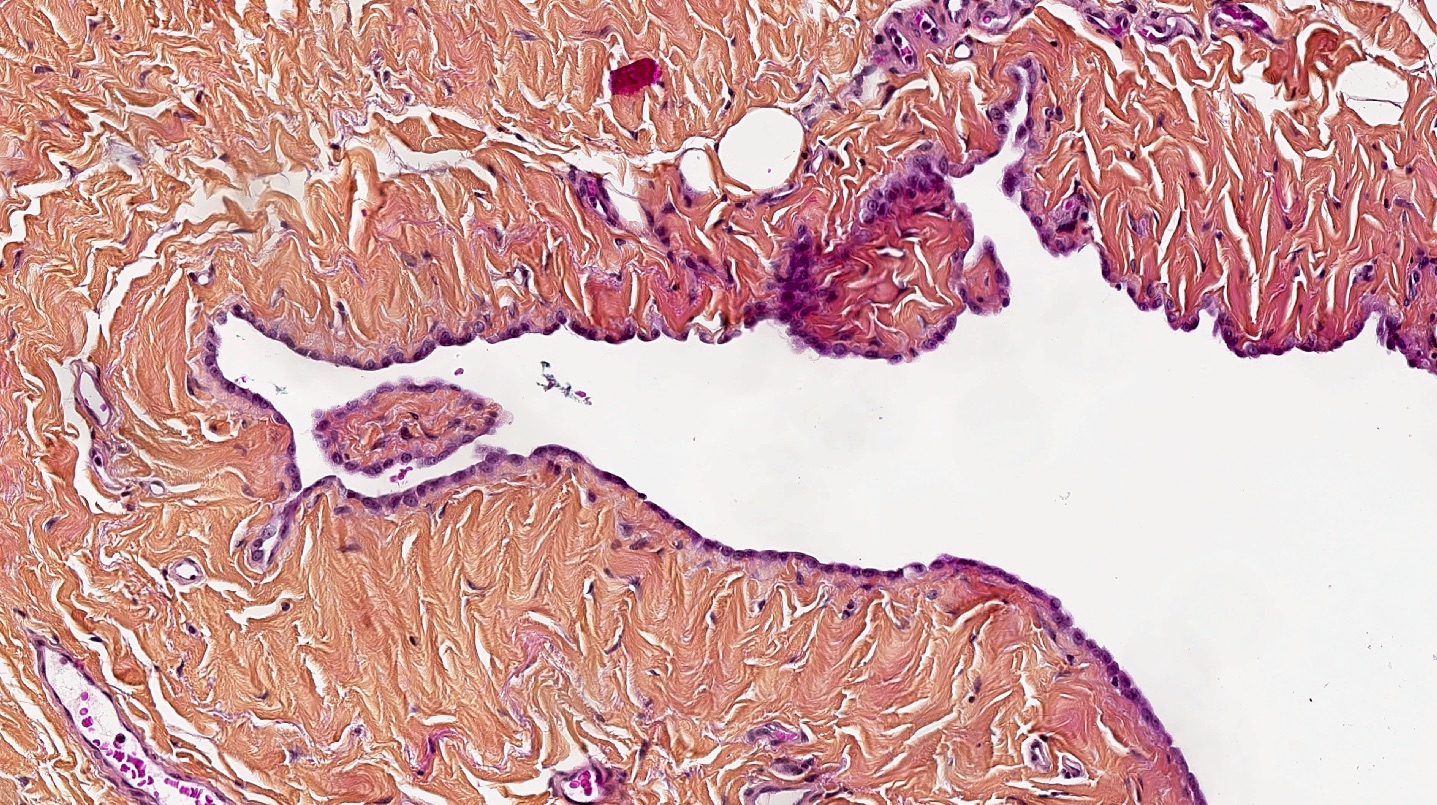
Microscopic (histologic) description
- Glands and tubules lined by low columnar or cuboidal cells, typically with cilia
- Psammoma bodies (J Reprod Med 2000;45:526) and papillae may be present
Microscopic (histologic) images
Cytology description
- Ciliated epithelium similar to fallopian tube, surrounded by fibrous stroma
- May have psammoma bodies (J Reprod Med 1991;36:675, J Reprod Med 2000;45:526)
- May have crowding and hyperchromasia
Negative stains
- Calretinin, CK20, p53 (wild type), D2-40
Differential diagnosis
- Adenocarcinoma: has atypia, mitoses, necrosis, desmoplasia
- Benign lesions known to occur in lymph nodes include salivary gland inclusions, thyroid follicles, capsular nevi: use morphology and immunohistochemistry to aid in the distinction
- Endometriosis: no endometrial stroma or hemorrhage is seen with endosalpingiosis
- Extraovarian serous cystadenoma
- Implant: from serous borderline tumor
- Distinction between these two abnormalities is not always sharp
- Multicystic mesothelioma: for cystic endosalpingiosis
- Peritoneal inclusion cyst
Board review style question #1
What is the expected immunohistochemical staining profile for endosalpingiosis?
- WT1+, PAX8+, calretinin+, BCL2-
- WT1+, PAX8-, calretinin+, BCL2+
- WT1+, PAX8+, calretinin-, BCL2+
- WT1-, PAX8+, calretinin-, BCL2+
- WT1-, PAX8-, calretinin-, BCL2-
Board review style answer #1
C. WT1+, PAX8+, calretinin-, BCL2+. As endosalpingiosis is composed of tubal type epithelium and thought to be derived from the Müllerian system, it is not surprising that this lesion is positive for PAX8 and WT1. Calretinin would be expected to stain lesions of mesothelial origin and ovarian theca origin. Studies have shown that BCL2, in addition to new contemporary markers such as FOXJ1 and phospho-SMAD2, are contemporary and promising markers for tubal epithelium and endosalpingiosis (Gynecol Oncol 2014;132:316).
Comment Here
Reference: Endosalpingiosis
Comment Here
Reference: Endosalpingiosis
Localized mesothelioma
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
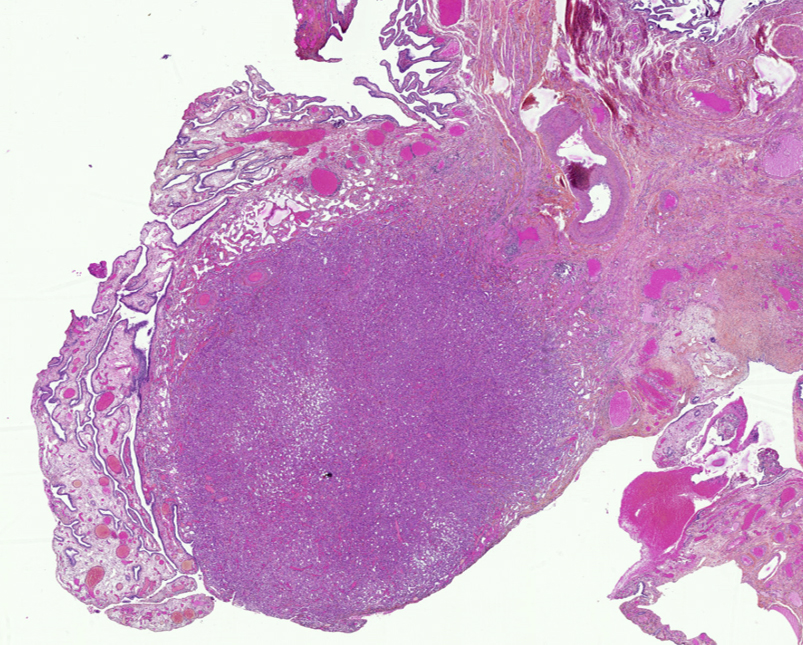
- Localized mesothelioma is a rare malignant mesothelial tumor that is microscopically identical to diffuse mesothelioma
- Unlike diffuse mesothelioma, localized mesothelioma is solitary and circumscribed, with no radiologic, intraoperative, gross or microscopic evidence of diffuse serosal involvement
Essential features
- Solitary and circumscribed, with no radiologic, intraoperative, gross or microscopic evidence of diffuse serosal involvement
- Microscopically identical to diffuse pleural mesothelioma
- Can be subclassified into epithelioid, biphasic and sarcomatoid histotypes
- Mesothelial immunophenotype
Terminology
- Not recommended: localized malignant mesothelioma
- Not recommended: solitary malignant mesothelioma
ICD coding
Epidemiology
- < 1% of all mesothelial tumors (Mod Pathol 2020;33:271)
- < 200 published cases of localized pleural mesothelioma (Mod Pathol 2020;33:281)
Sites
- Most cases reported involve the pleura; the remainder of the cases involve the peritoneum, including mesenteric, perigastric, perisplenic or other intra-abdominal sites (Mod Pathol 2020;33:281, Am J Surg Pathol 2022;46:1352)
Pathophysiology
- Unknown at this time
Etiology
- Associations with exposure to asbestos reported in some cases (Mod Pathol 2020;33:281)
Clinical features
- Wide age range and male predilection (Am J Surg Pathol 1994;18:357, Am J Surg Pathol 2005;29:866, Mod Pathol 2020;33:281)
Diagnosis
- Diagnosis of localized mesothelioma cannot be rendered based on the histologic findings alone and requires correlation with clinical and radiologic findings, in order to ascertain that the tumor is solitary and circumscribed, with no evidence of diffuse serosal involvement
Radiology description
- Solitary localized serosal or subserosal mass (Mod Pathol 2020;33:281)
Prognostic factors
- More indolent than diffuse mesothelioma (Interact Cardiovasc Thorac Surg 2013;16:533)
- Potential favorable prognostic factors: epithelioid histotype, small tumor size and low grade cytology / mitotic index (Mod Pathol 2020;33:281, Am J Surg Pathol 2022;46:1352)
Case reports
- 48 year old man with a localized splenic mass (Int J Surg Pathol 2014;22:451)
- 69 year old woman with a solitary intra-abdominal mass (Case Rep Pathol 2019;2019:2732674)
- 72 year old woman with a solitary mass adjacent to the diaphragm and esophagus (Ann Thorac Surg 2021;112:e57)
Treatment
- Surgical resection
- Adjuvant chemotherapy or radiotherapy in some patients (Mod Pathol 2020;33:281, Am J Surg Pathol 2022;46:1352)
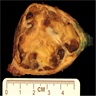
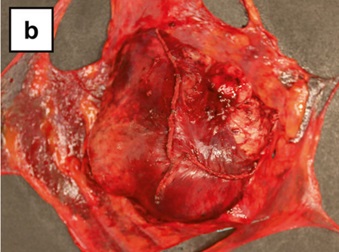
Gross description
- Circumscribed; can be encapsulated or nonencapsulated
- Tumor size ranging from < 2 cm to ~ 20 cm (Mod Pathol 2020;33:281)
Microscopic (histologic) description
- Histologically identical to diffuse mesothelioma
- Comprises epithelioid, biphasic and sarcomatoid histologic types
Microscopic (histologic) images
Positive stains
- WT1
- Calretinin
- D2-40
- HEG1 (membranous)
- BAP1 (loss of nuclear staining) seen in a subset of cases
- References: Mod Pathol 2020;33:271, Am J Surg Pathol 2020;44:1143
Negative stains
- Claudin4
- MOC31
- BerEP4
- TTF1
- PAX8 (expression in 10 - 20% of mesotheliomas)
- References: Virchows Arch 2007;451:669, Am J Surg Pathol 2017;41:1675
Molecular / cytogenetics description
- Genomically heterogeneous with multiple subgroups (Mod Pathol 2020;33:271)
- Alterations involving BAP1, CDKN2A or NF2
- Mutations involving TRAF7
- Genomic near haploidization, with extensive loss of heterozygosity involving most chromosomes except chromosomes 5 and 7
Sample pathology report
- Pleura, pleurectomy:
- Localized pleural biphasic mesothelioma (see comment)
- Comment: Radiologic findings and intraoperative findings were reviewed. Radiologically, intraoperatively and grossly, this tumor is solitary and circumscribed. Microscopically, it is biphasic, with ~70% epithelioid component and ~30% sarcomatoid component. By immunohistochemistry, the tumor cells in both components are positive for AE1 / AE3 keratins, WT1, calretinin and D2-40 (patchy) and are negative for TTF1, MOC31 and claudin4, with complete loss of BAP1 nuclear staining, supporting the above diagnosis. Resection margin is negative for tumor. Tumor is 0.2 cm from the resection margin.
Differential diagnosis
- Metastatic carcinoma:
- Diffuse mesothelioma:
- Microscopically identical to localized mesothelioma
- Diffuse involvement of the serosa, as noted radiologically or intraoperatively
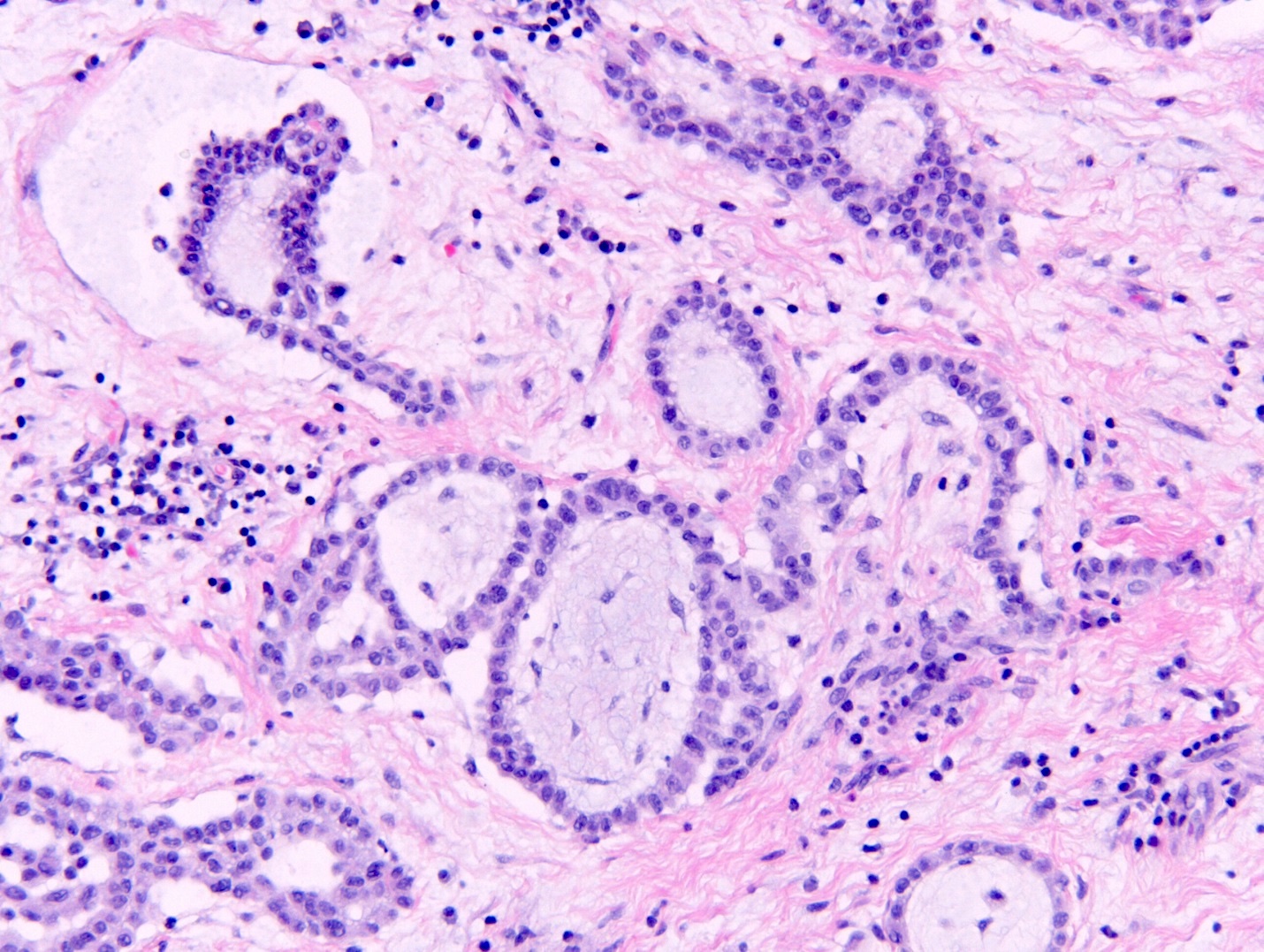
- Adenomatoid tumor:
- Benign mesothelial tumor that arises most commonly near the genital tract
- Microcystic appearance at low magnification
- Recurrent mutations in TRAF7 (Mod Pathol 2018;31:660, Hum Pathol 2021;111:59)
Board review style question #1
Which of the following statements is true about localized mesothelioma?
- It involves only the pleura
- Its histopathologic features are identical to those of diffuse mesothelioma
- Loss of BAP1 nuclear staining is present in all cases
- Radiologic and intraoperative correlation is not needed to render this diagnosis
Board review style answer #1
B. Its histopathologic features are identical to those of diffuse mesothelioma. As such, its diagnosis requires correlation with radiologic, intraoperative and gross findings to ascertain that the tumor is indeed solitary with no diffuse serosal involvement. Answer D is incorrect because radiologic and intraoperative correlation is needed to render the diagnosis of localized mesothelioma. Answer A is incorrect because localized mesothelioma can involve the pleura and other serosal membranes including the peritoneum. Answer C is incorrect because the loss of BAP1 nuclear staining is seen in only a subset of localized mesotheliomas.
Comment Here
Reference: Localized mesothelioma
Comment Here
Reference: Localized mesothelioma
Mesothelial hyperplasia
Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Diagnosis | Radiology description | Case reports | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Reactive proliferation of mesothelial cells with no or minimal cytologic atypia and without invasion
Essential features
- Common response to inflammation occurring in any process that leads to irritation of peritoneal surface
- Reactive proliferation of mesothelial cells with minor degrees of nuclear atypia
- Without invasion, positive for mesothelial markers (CK5/6, calretinin and WT1)
- BAP1 and MTAP retained
Terminology
- Simple mesothelial hyperplasia
- Florid mesothelial peritoneal hyperplasia
Epidemiology
- Exact incidence is unknown but mesothelial cell hyperplasia is not an uncommon phenomenon
- May occur at any age, whatever the sex (J Clin Pathol 2011;64:313)
Sites
- May occur at any site of the peritoneum
Pathophysiology
- Common response to inflammation occurring in any process that leads to irritation of peritoneal surface
Etiology
- Chronic effusions (ascites)
- Inflammatory processes
- Endometriosis
- Hernias
- Neoplasms (e.g. ovarian tumor, colorectal tumor)
- Reference: Int J Gynecol Pathol 2014;33:393
Diagnosis
- Exploratory laparoscopy with tissue sampling
- Often incidentally identified in peritoneal tissue obtained for other purposes
Radiology description
- No gross evidence of disease on imaging
Case reports
- 32 year old woman with a left ovarian microinvasive serous borderline tumor (Eur J Gynaecol Oncol 2004;25:236)
- 35, 53 and 59 year old women with tubal ectopic pregnancy, ovarian serous cystadenoma and ovarian adenosquamous carcinoma, respectively (Histopathology 2013;63:598)
- 36 and 37 year old women with dysmenorrhea and secondary infertility and endometriosis, respectively (Ann Diagn Pathol 2004;8:115)
Gross description
- Not commonly visible upon gross examination (incidental findings on microscopic examination)
- Occasionally observed as small nodules or flat plaques from the peritoneum (Int J Gynecol Pathol 2014;33:393)
Microscopic (histologic) description
- Variety of architectural patterns (solid sheets, nests, discrete small papillary or tubulopapillary growths, gland-like structures, cord-like linear arrays or single cells) (Int J Gynecol Pathol 2014;33:393)
- Minor degrees of nuclear atypia (small, regular, round or oval and exhibit central nucleoli) without invasion; more common to be uniform in appearance
- Reactive changes may be present that are worrisome for malignancy (enlarged vesicular nuclei, multinucleation, conspicuous nucleoli and rare mitotic figures)
- Usually accompanied with inflammatory cells
- Sometimes florid mesothelial peritoneal hyperplasia or psammoma bodies may be seen (Int J Gynecol Pathol 1993;12:51)
- Rare findings:
- Eosinophilic strap-like cells resembling rhabdomyoblasts (Cancer 1975;35:165)
- Deciduoid morphology with glassy eosinophilic cytoplasm (Histopathology 2013;63:598)
Microscopic (histologic) images
Virtual slides
Cytology description
- Usually mesothelial cells may be numerous or not, dispersed or present in small clusters
- Binucleation, multinucleation, mitosis, prominent nucleolus can be present in benign proliferations (Cytojournal 2013;10:7)
- 2 or more mesothelial cells are often separated by window or a narrow space (Cytopathology 2004;15:131)
- Benign mesothelial cells usually have recognizable halo at outer rim of cell
- Complex papillary or branching clusters should not be present (Cytojournal 2013;10:7)
Positive stains
- Positive immunostains in mesothelial cells (CK AE1 / AE3, CK7, CK5/6, calretinin, WT1, D2-40)
- Reported positivity for desmin but nonspecific (more common in reactive mesothelial cells [84%] than mesothelioma [8%] or carcinoma [2%]) (Am J Surg Pathol 2001;25:1405)
- BAP1 and MTAP are retained (positive)
Negative stains
Molecular / cytogenetics description
- FISH for CDKN2A: intact
Videos
Mesothelial proliferations
Sample pathology report
- Peritoneal biopsy:
- Mesothelial hyperplasia of the peritoneum (see comment)
- Comment: There are small papillary structures containing myxoid stroma, which are lined by a single layer of uniform cuboidal mesothelial cells without any infiltrative cells or necrosis. Immunohistochemically, the mesothelial cells are positive for calretinin and WT1. BAP1 and MTAP are retained.
Differential diagnosis
- Mesothelioma:
- Generally, diffuse involvement of the peritoneum
- Infiltration of underlying pre-existing tissue is classical
- At least moderate cytologic atypia in most cases
- Solid architecture may be present
- BAP1 loss by immunostaining or CDKN2A homozygous loss is required for diagnosis (Virchows Arch 2021;478:59)
- Mesothelioma in situ:
- Similar morphology
- BAP1 loss by immunostaining or CDKN2A homozygous loss is required for diagnosis (Virchows Arch 2021;478:59)
- Well differentiated papillary mesothelioma:
- Papillae larger with myxoid cores, each lined by a single mesothelial cell layer
- Invasion is rarely observed (Am J Surg Pathol 2014;38:990)
- Recurrent mutations in TRAF7 or CDC42 (Mod Pathol 2019;32:88)
- Found by positive L1CAM immunostain
- Adenomatoid tumor:
- Organized in vascular-like, gland-like, complex slit-like and cystic branching spaces; also tubules or combination of above
- Positive immunostain for L1CAM
- Sertoli cell tumor:
- Usually well circumscribed
- Different architecture to reactive mesothelial proliferations: often mixed patterns of tubular, cords, macro or microcystic, nested, trabecular, whorled, solid, retiform, pseudopapillary
- Often presence of cells with cytoplasmic vacuoles
- Metastatic carcinoma:
- Negative for calretinin
- Positive for claudin4, MOC31, BerEP4 and other epithelial lineage markers
Additional references
Board review style question #1
Which of the following statements regarding mesothelial hyperplasia of the peritoneum is true?
- Approximately 10% of patients progress to malignant mesothelioma over 10 years
- BAP1 immunostain can be lost
- Common response to inflammation occurring in any process that leads to irritation of peritoneal surface
- Lesions harbor consistently mutation of TRAF7
- Psammoma bodies are never seen
Board review style answer #1
C. Common response to inflammation occurring in any process that leads to irritation of peritoneal surface
Comment Here
Reference: Mesothelial hyperplasia
Comment Here
Reference: Mesothelial hyperplasia
Board review style question #2
A small focal nodule depicted in the above photomicrograph was found incidentally during a resection of an ovarian serous cystadenoma in a 26 year old woman. Lesional cells were positive for calretinin. Which of the following is true about the depicted entity?
- CDKN2A homozygous deletion is never seen
- Deciduoid morphology can be occasionally seen
- Immunohistochemical loss of BAP1 is seen in 10% of cases
- Lesions harbor consistently mutation of TRAF7
- Most cases are causally linked to asbestos exposure
Board review style answer #2
A. CDKN2A homozygous deletion is never seen. This is a peritoneal mesothelial hyperplasia.
Comment Here
Reference: Mesothelial hyperplasia
Comment Here
Reference: Mesothelial hyperplasia
Mesothelioma (peritoneum)-epithelioid
Table of Contents
Definition / general | Essential features | Epidemiology | Pathophysiology | Clinical features | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Definition / general
- Mesothelioma is a neoplasm arising from mesothelial cells that line serous cavities, such as the pleura and peritoneum
- Pleural mesothelioma is much more common than peritoneal mesothelioma
- Epithelioid mesothelioma is the most frequent histologic type of malignant mesothelioma; sarcomatoid and biphasic subtypes are less common
- 20 - 33% of malignant mesothelioma arises in the peritoneum (Semin Oncol 2002;29:51)
Essential features
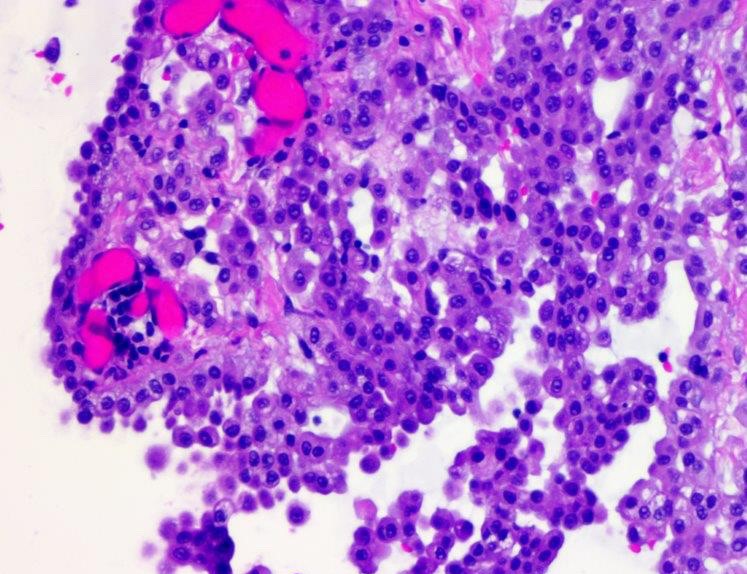
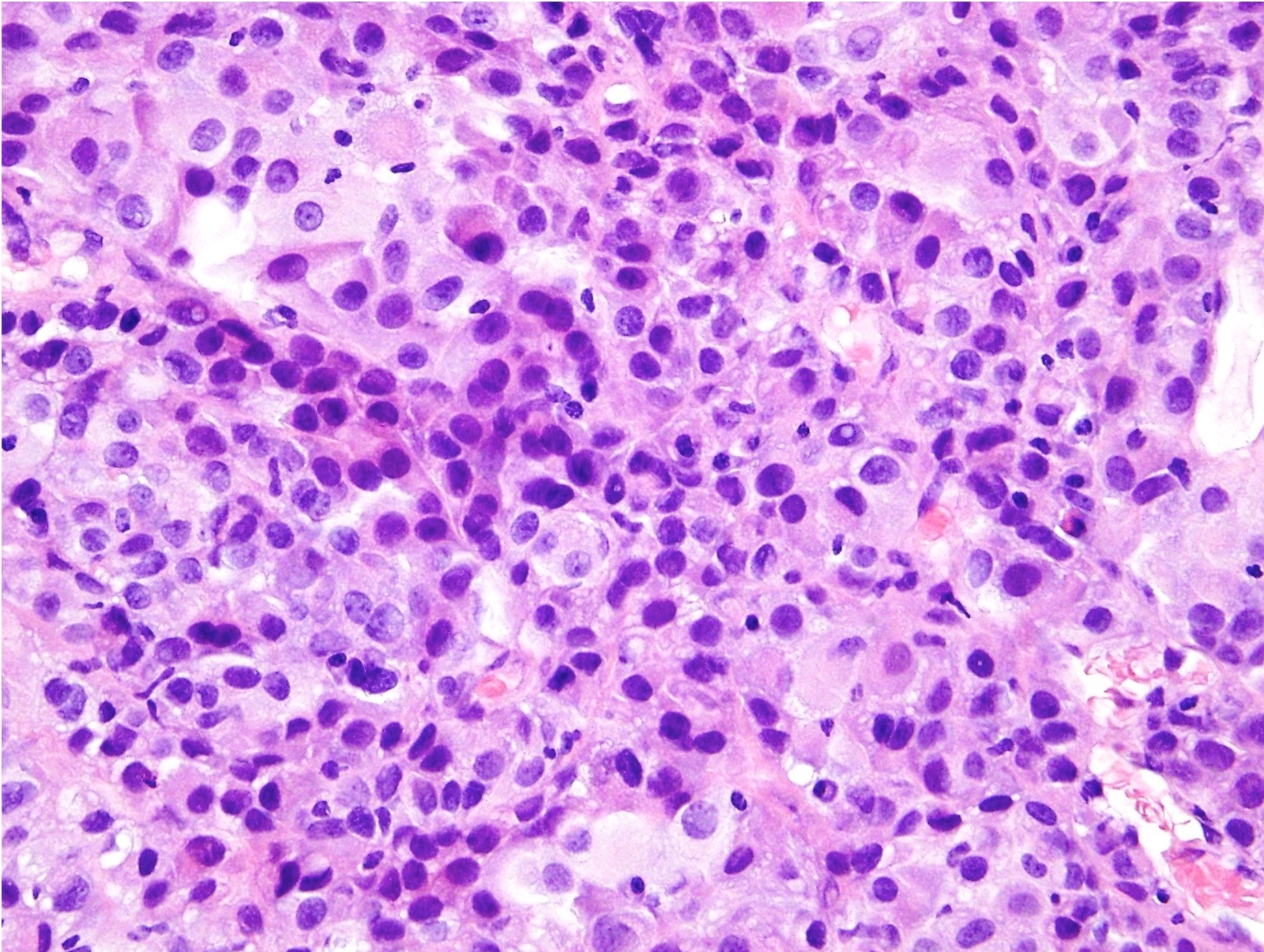
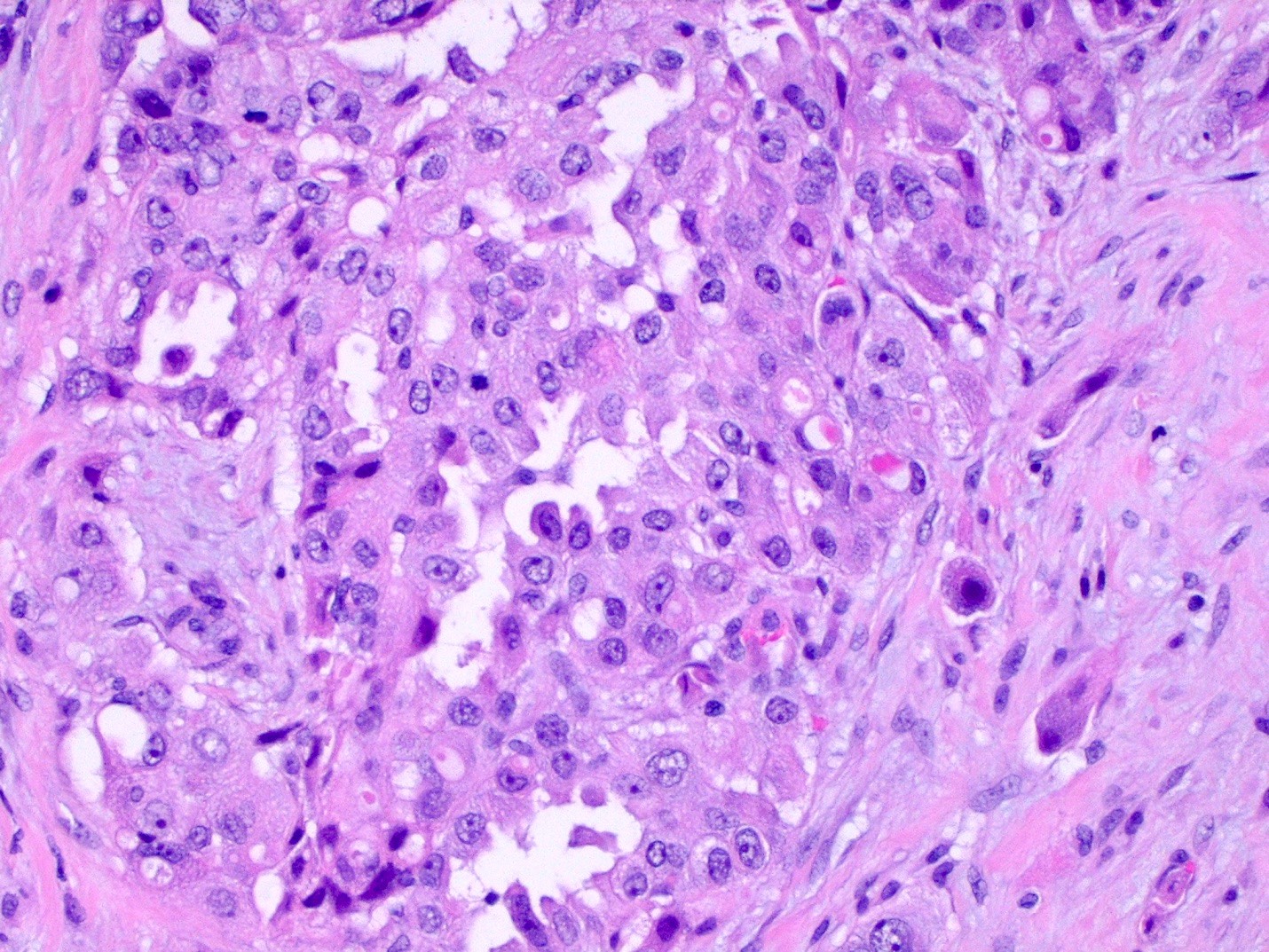
- Hallmark of epithelioid mesothelioma is the epithelioid cells which are polygonal cells with moderate to abundant eosinophilic cytoplasm, vesicular round nuclei and prominent nucleolus; often mimic nonneoplastic, reactive mesothelial cells (Arch Pathol Lab Med 2018;142:89)
- The most common histologic patterns of epithelioid mesothelioma are tubulopapillary, adenomatoid, solid well differentiated, solid poorly differentiated and acinar (Arch Pathol Lab Med 2012;136:241)
- Myxoid variant of peritoneal epithelioid mesothelioma is extremely rare with only 5 reported cases
Epidemiology
- Often due to asbestos exposure, whether in the pleura, peritoneum and pericardium; cumulative asbestos exposure is directly proportional to risk of cancer (J Med Case Rep 2008;2:121)
Pathophysiology
- Asbestos fibers lead to chronic inflammation, which causes the release of free radicals
- Latent period between asbestos exposure and disease averages 20 - 30 years (Cancer Treat Rev 2012;38:605)
Clinical features
- No distinctive symptoms, causing difficulties in diagnosis and treatment
- When symptomatic, usually present with abdominal pain, ascites and abdominal distention
Prognostic factors
- Prognosis is poor
- Survival rate of myxoid variant appears to be better than epithelioid mesothelioma in general (Virchows Arch 2005;447:828)
- Suggested favorable prognostic factors are small nuclear size and low Ki67 labeling index (World J Gastroenterol 2009;15:4856, Med Mol Morphol 2010;43:53)
Case reports
- 34 year old woman with epithelioid mesothelioma after radiation for cervical cancer (Mol Clin Oncol 2018;8:302)
- 44 year old woman with extensive myxoid change in well differentiated papillary mesothelioma (Ann Diagn Pathol 2002;6:164)
- 59 year old man with abdominal bloating and vague abdominal pain (Case #441)
- 60 year old woman with myxoid variant of epithelioid malignant mesothelioma (Cesk Patol 2014;50:149)
- 76 year old woman with small bowel obstruction secondary to carcinomatosis caused by primary peritoneal mesothelioma (Am J Case Rep 2015;16:496)
Treatment
- Systemic chemotherapy
- Cytoreductive and palliative surgery
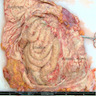
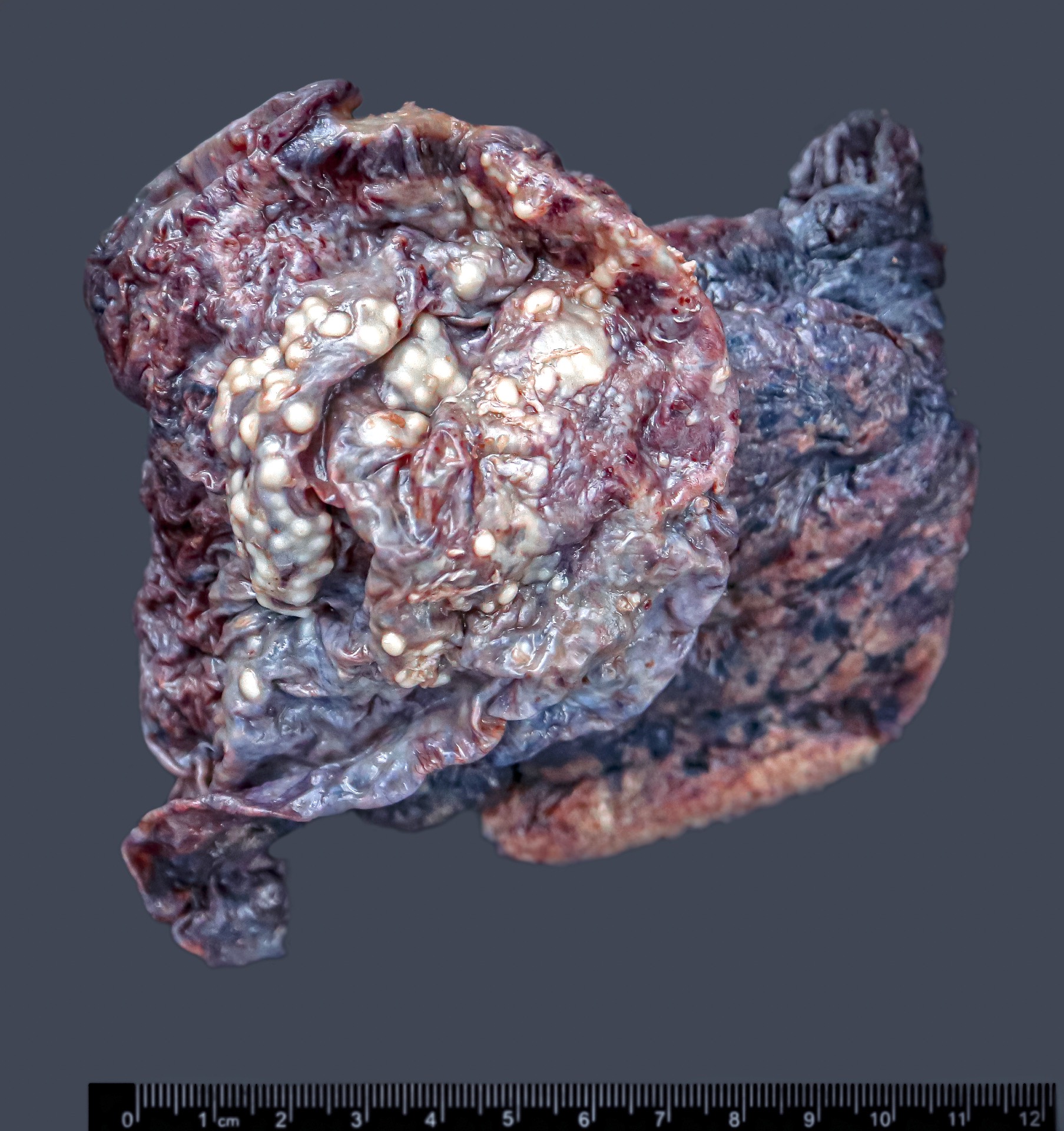
Gross description
- Diffuse thickening or multiple nodules on the peritoneum
- Myxoid variant is gelatinous
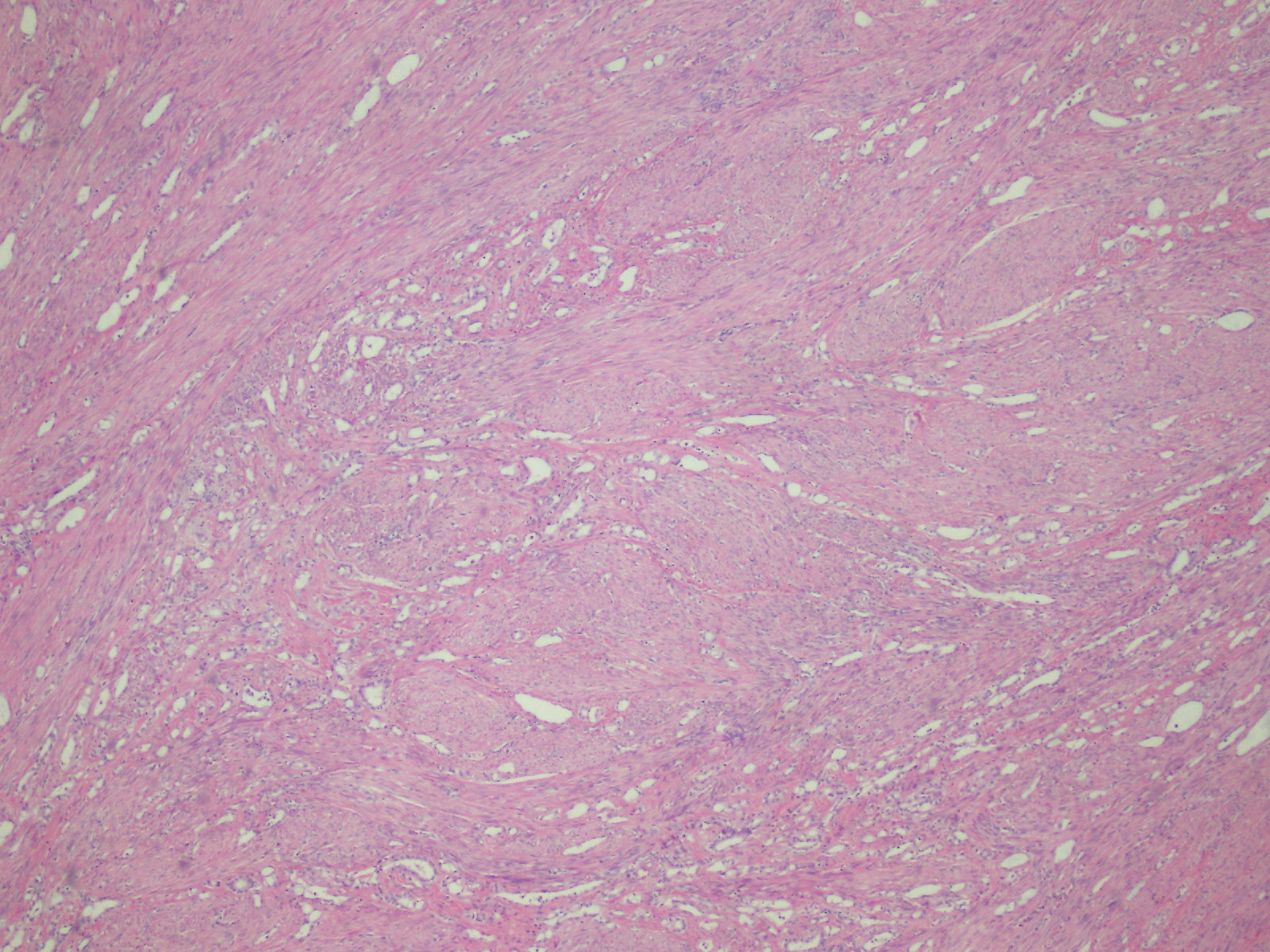
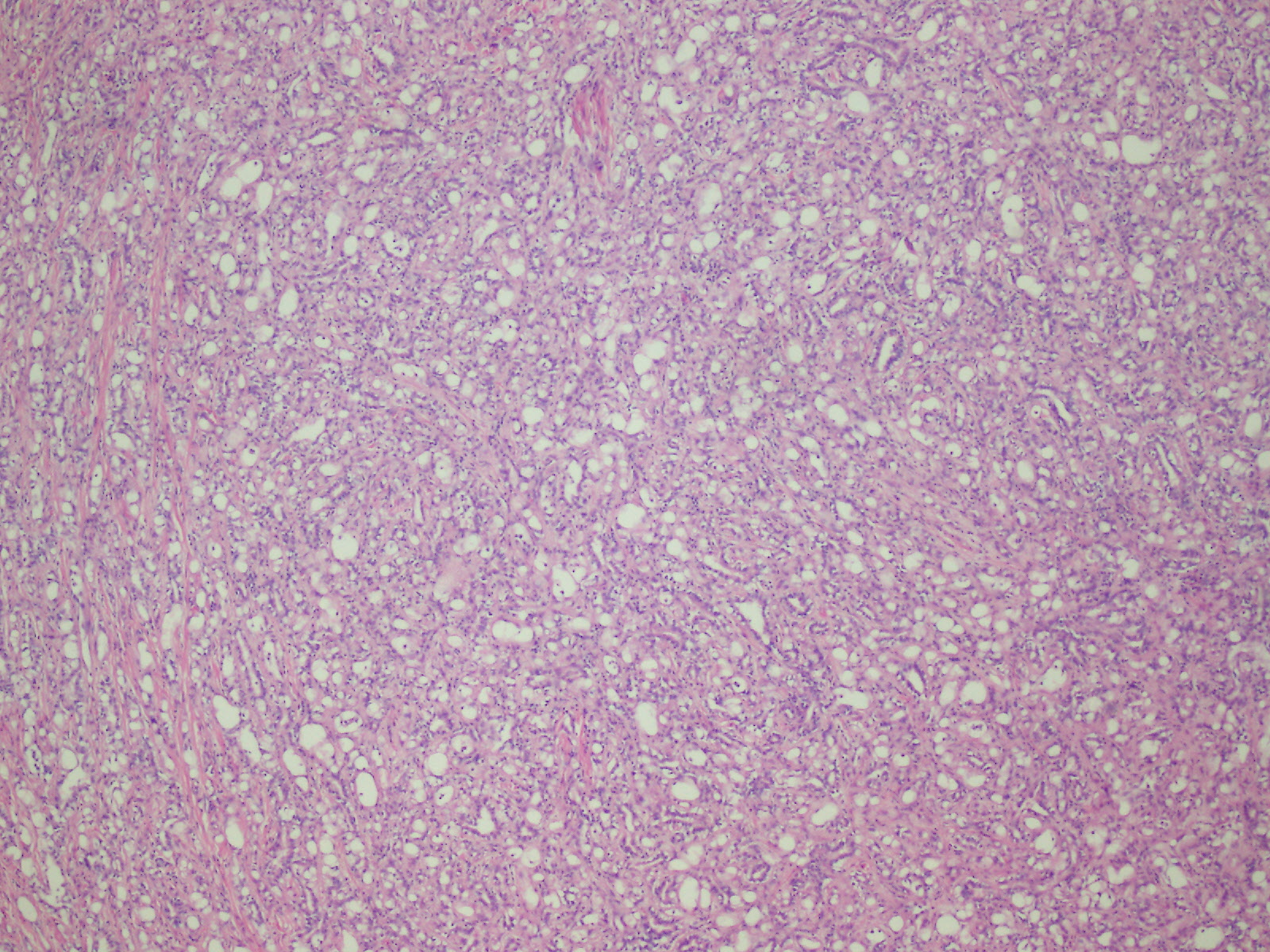
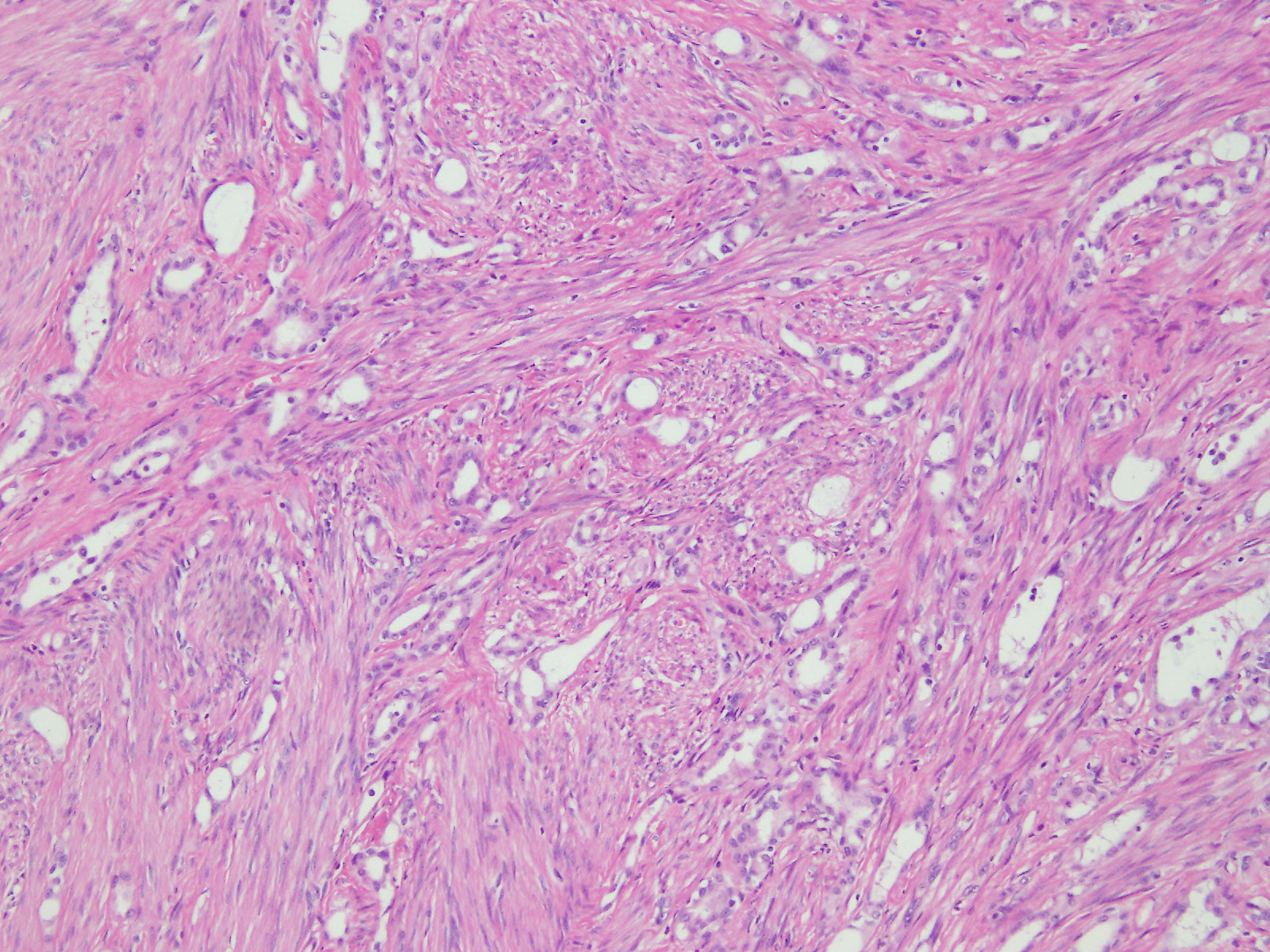
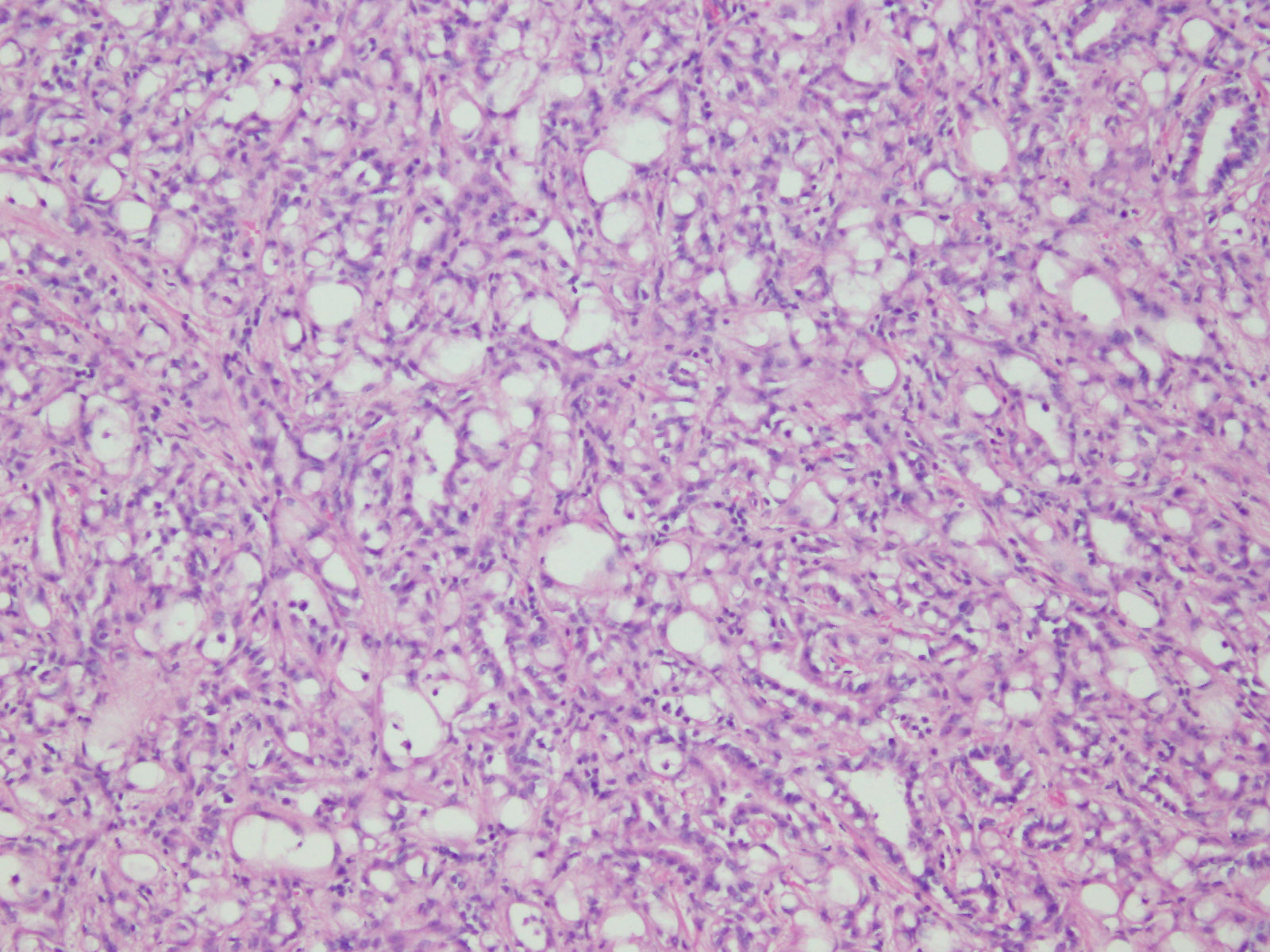
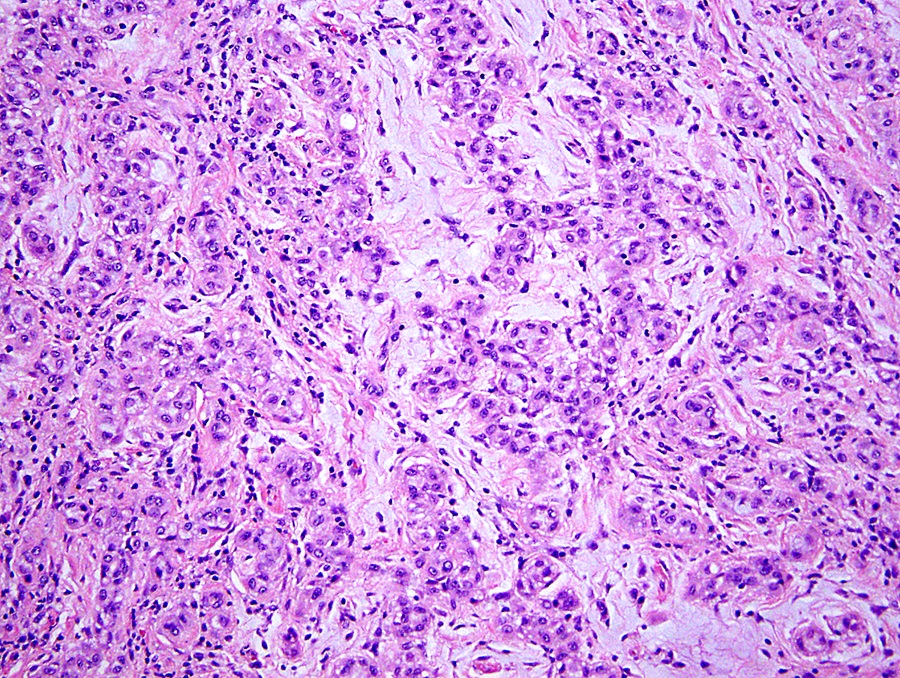
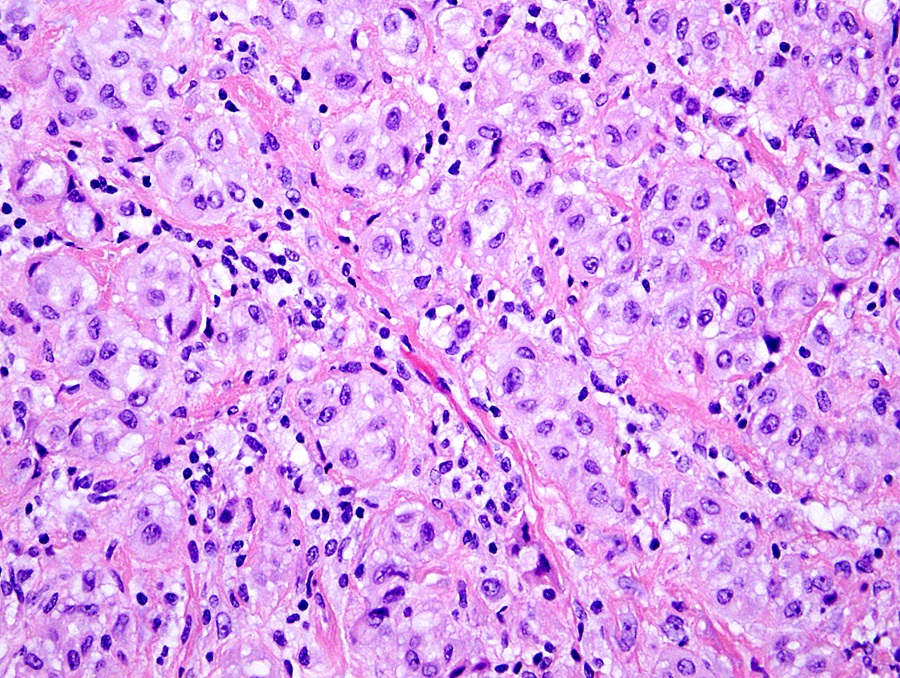
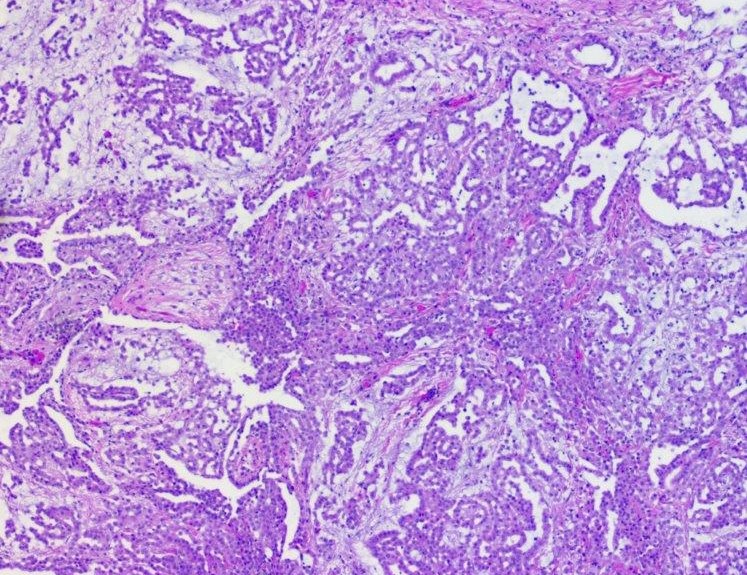
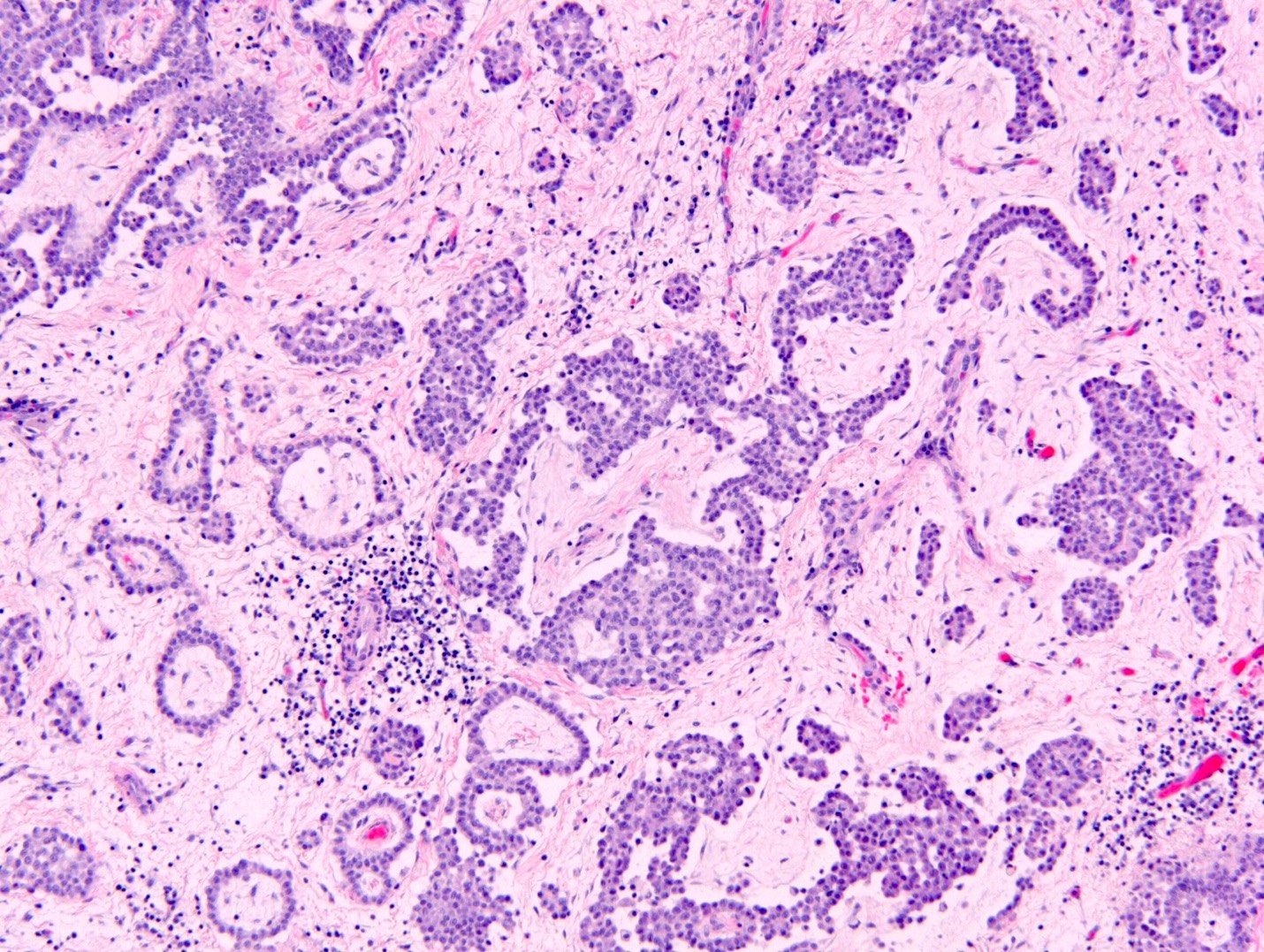
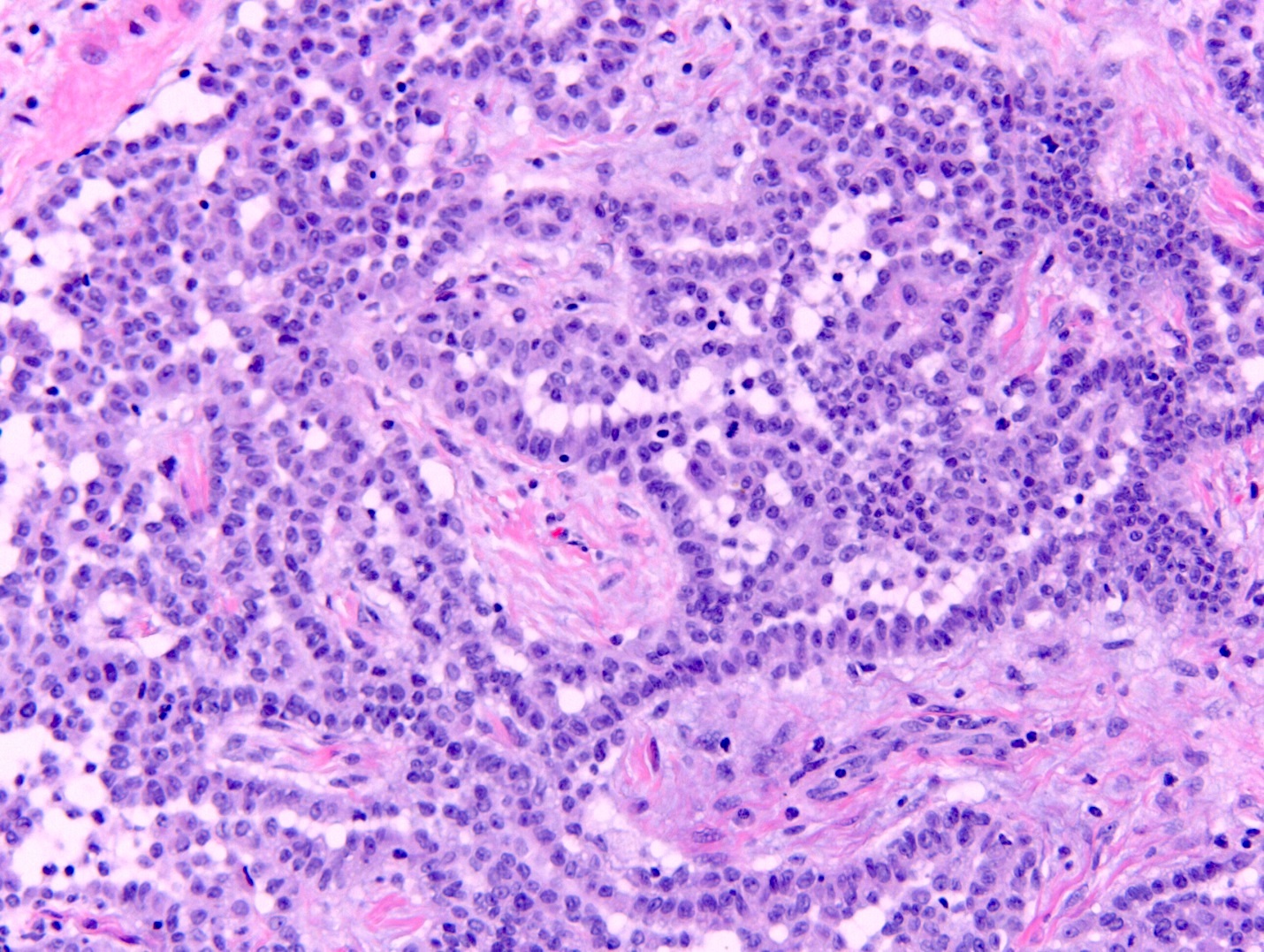
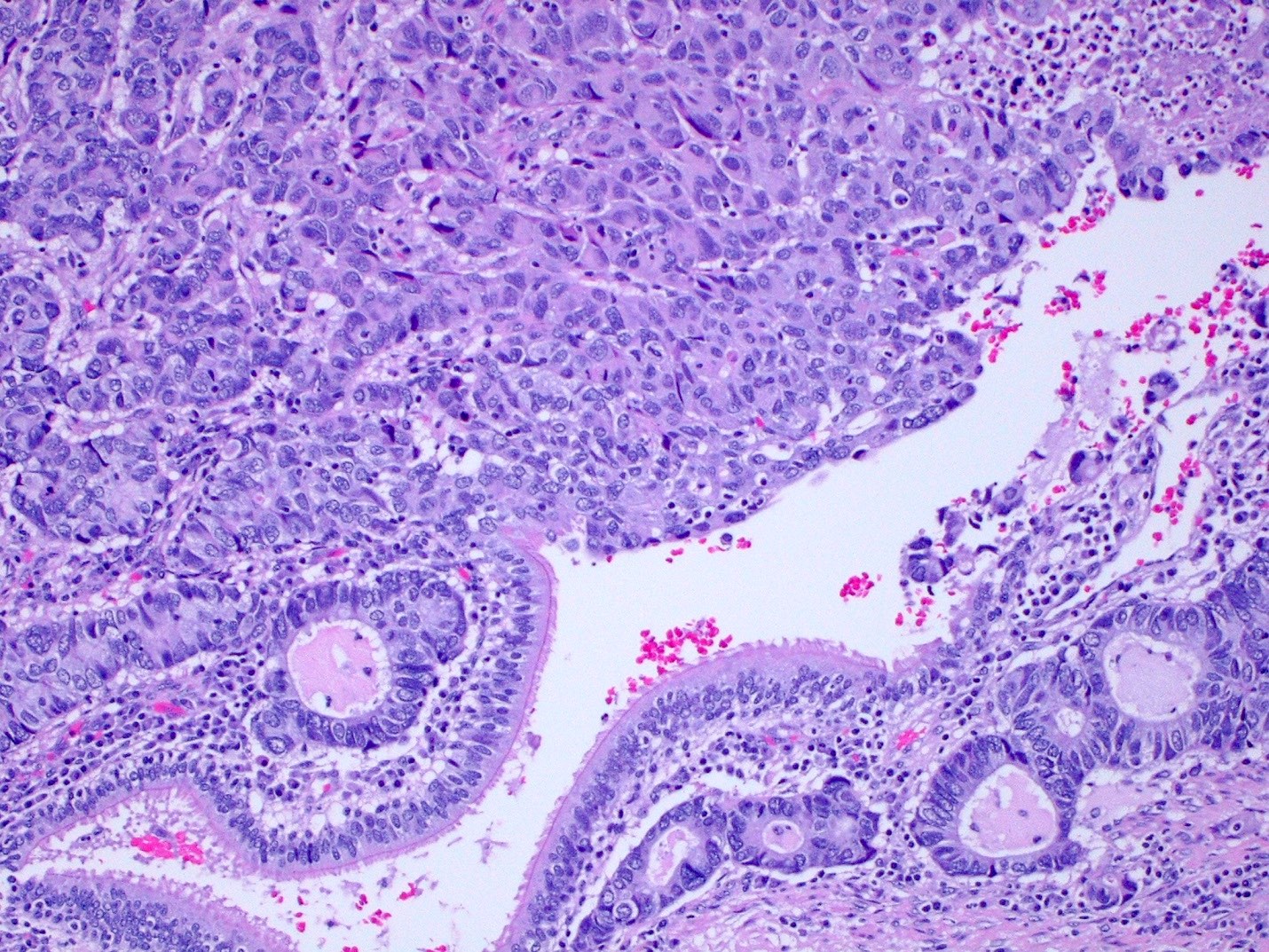
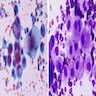
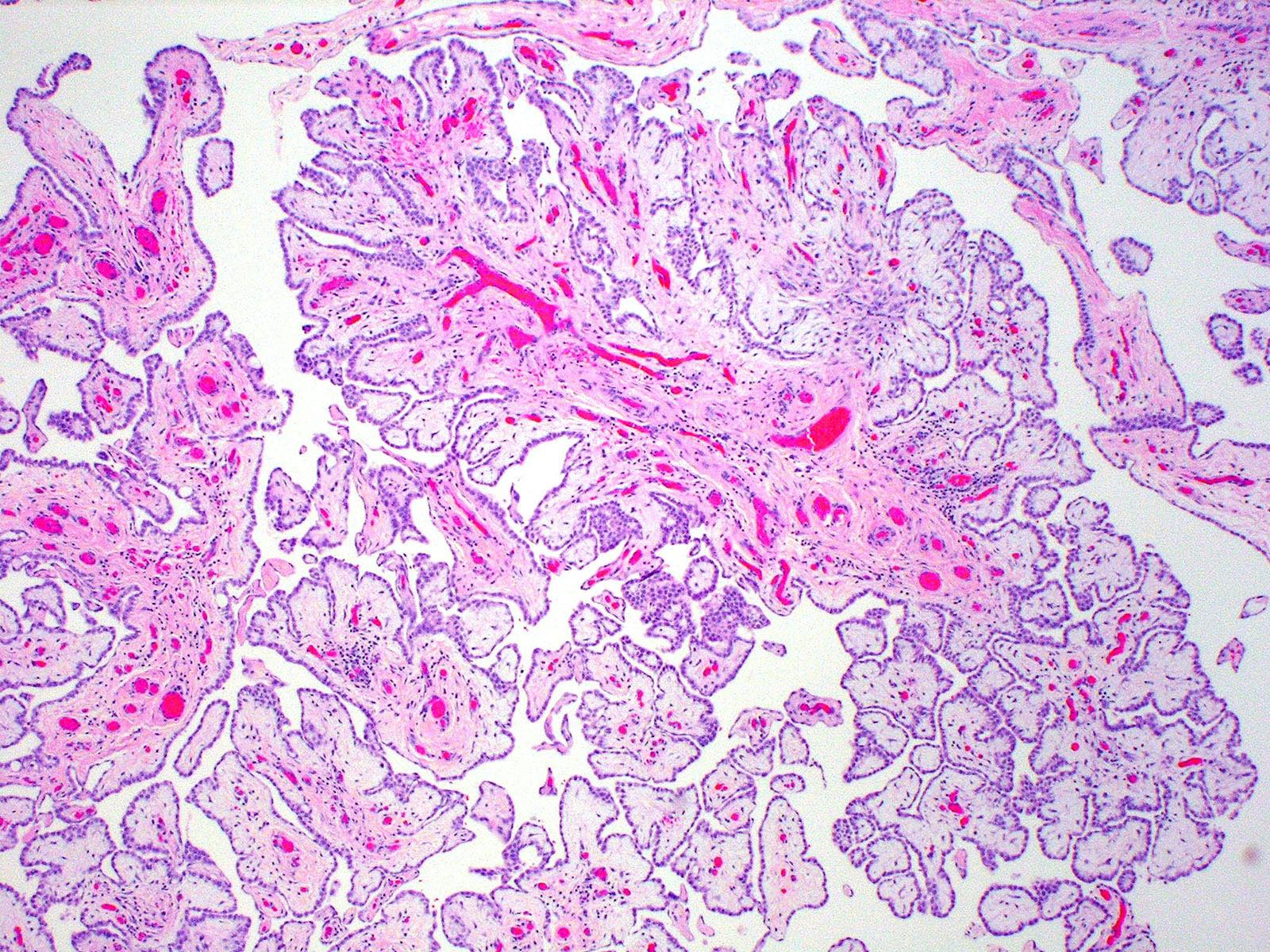
Microscopic (histologic) description
- Invasive epithelioid cells are arranged in different patterns (which form the basis of the different subtypes of epithelioid mesothelioma)
- Form tubules and papillae with / without psammoma bodies (tubulopapillary variant), gland-like structures (acinar variant) and are in solid sheets, nests or cords (solid variant)
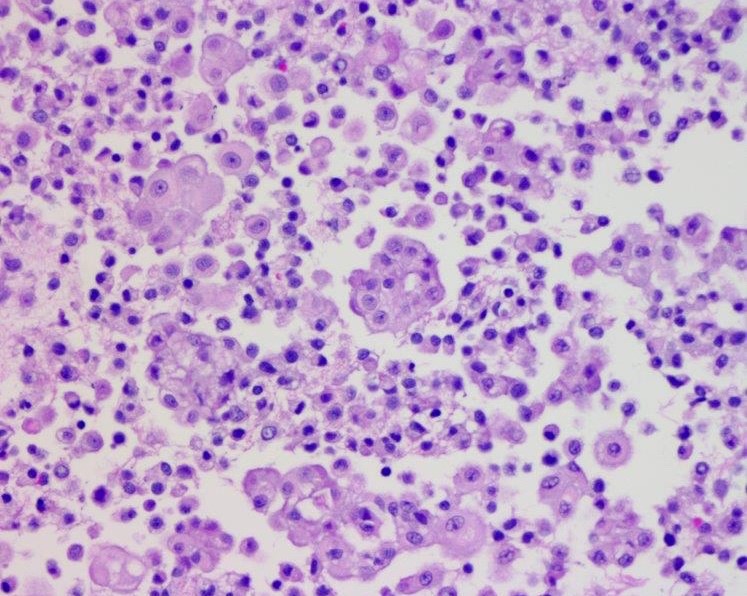
- Myxoid variant:
- Dyscohesive medium to large epithelioid cells with a moderate to abundant amount of eosinophilic cytoplasm dispersed in a myxoid background
- Some cells can have intracytoplasmic clear vacuoles
- Nuclei with coarse chromatin and prominent nucleoli
- Mitotic figures are usually inconspicuous
- Difficulty to differentiate from other myxoid lesions of the peritoneum (e.g. adenocarcinoma) thus panel of immunohistochemical markers is generally required
Microscopic (histologic) images
Virtual slides
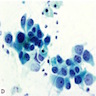
Cytology description
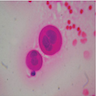
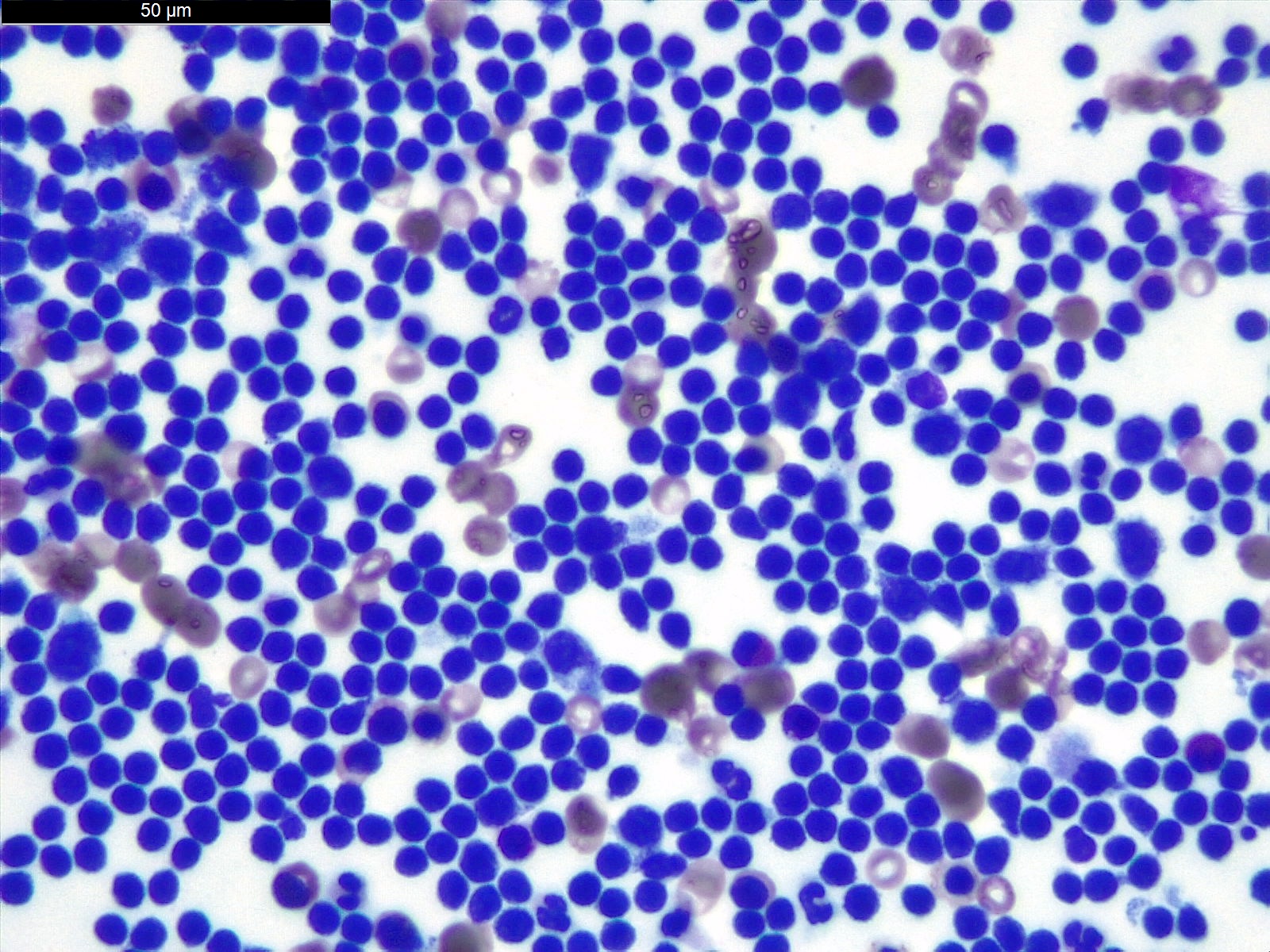
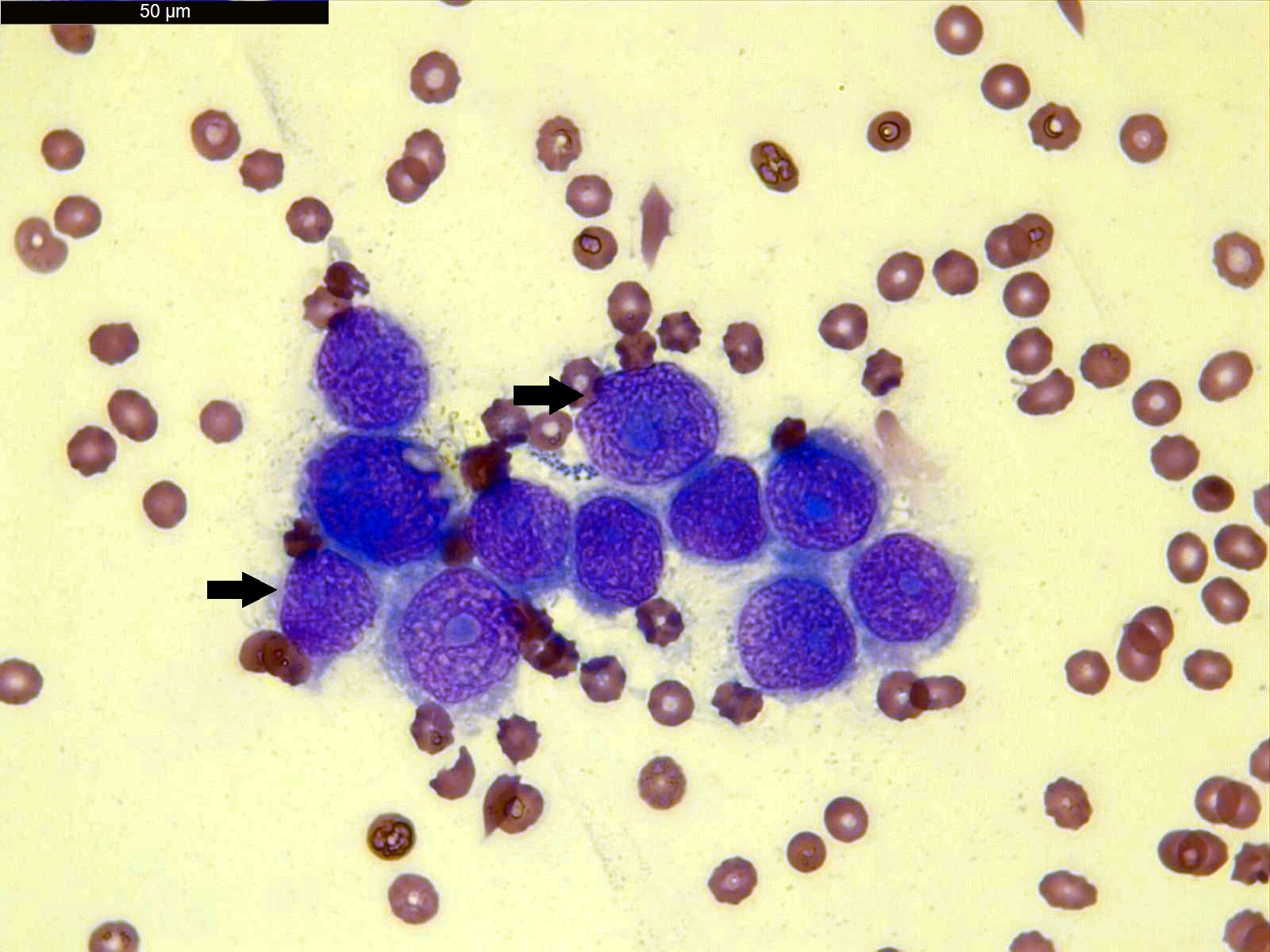
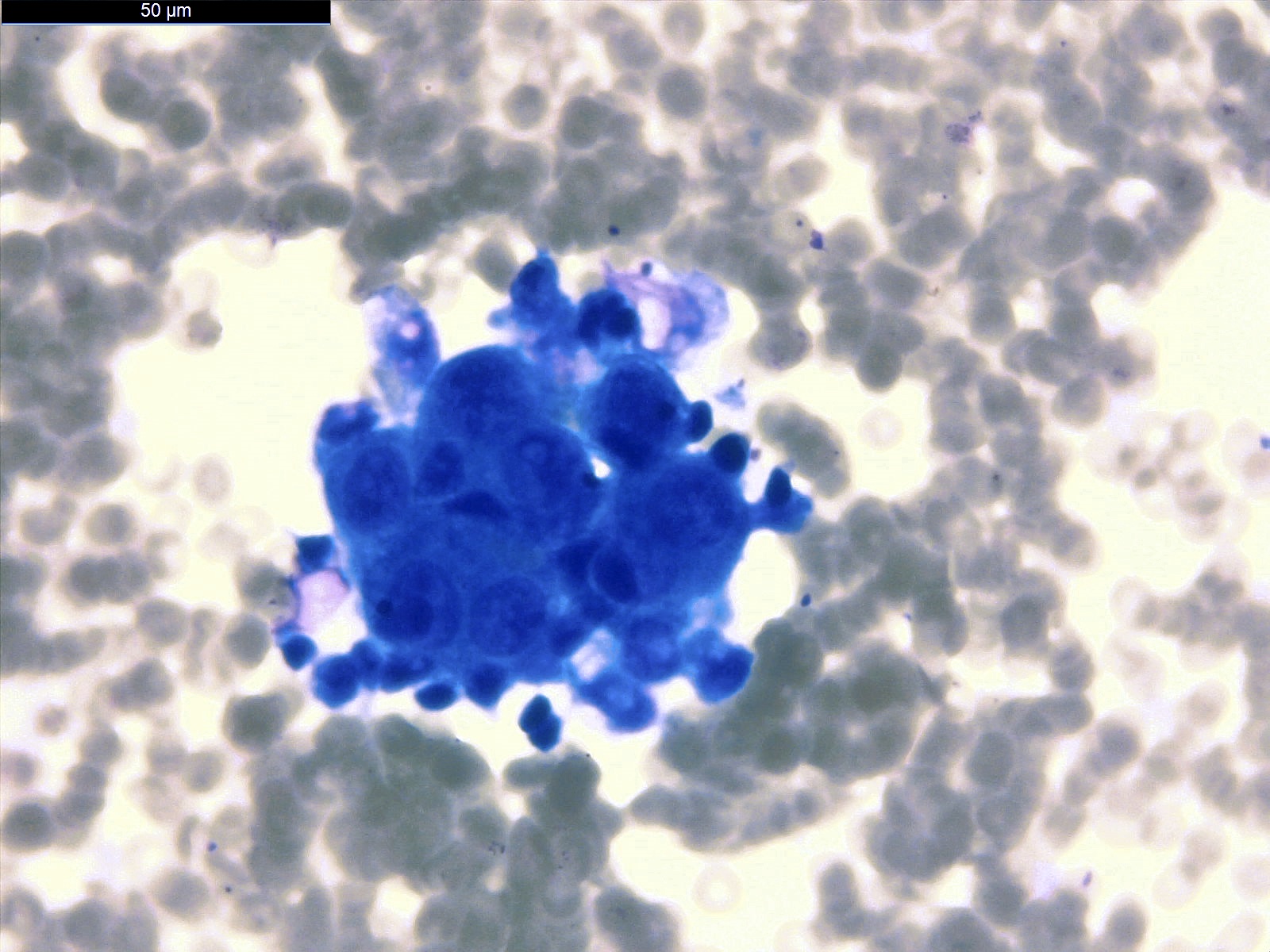
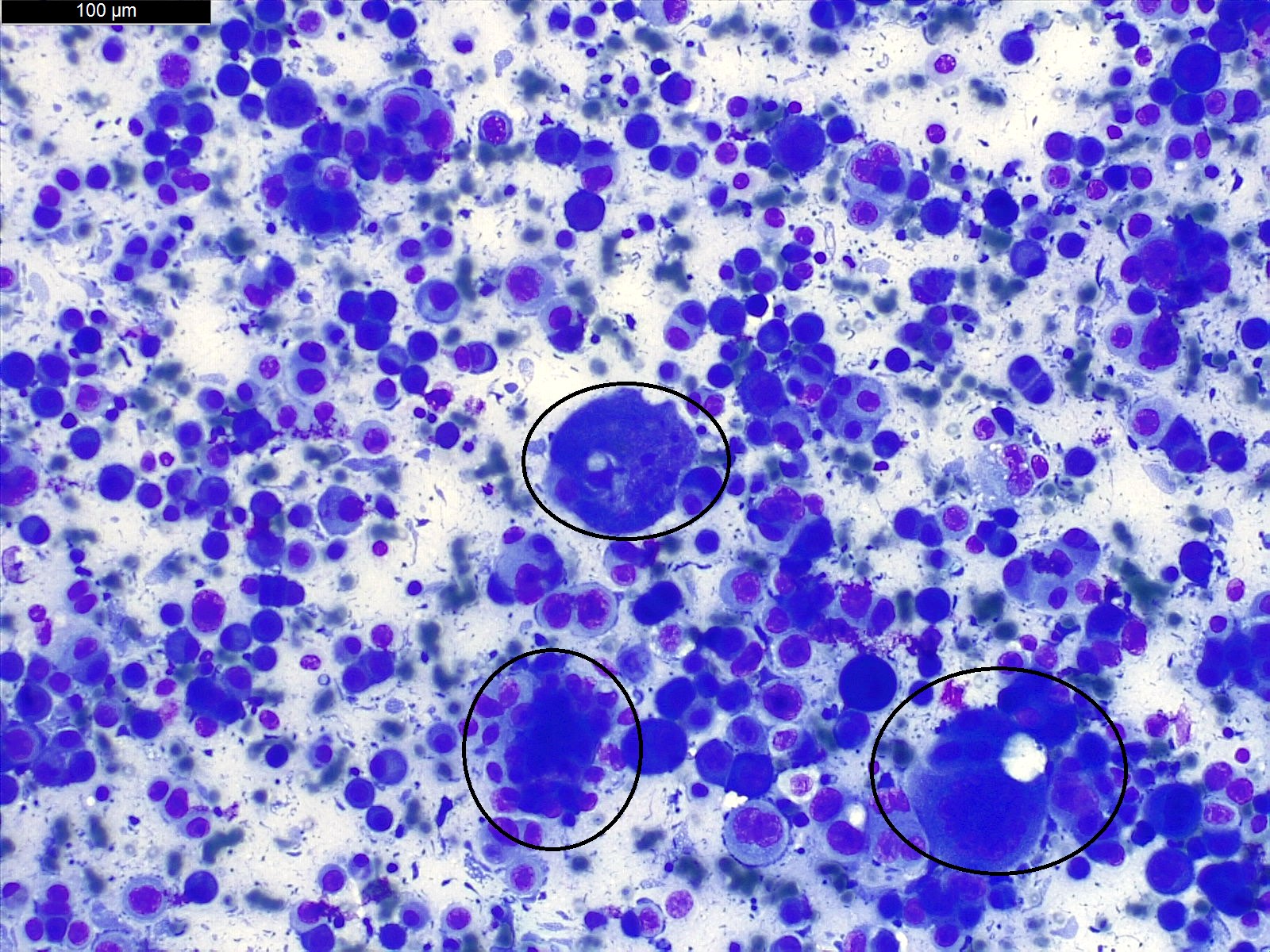
- Clusters of epithelioid cells (morulae) with knobby contour
- Abundant cytoplasm, round nuclei and prominent nucleoli
- Mild atypia
Cytology images
Electron microscopy description
- Very long, thin apical microvilli and the absence of glycocalyx (compared to adenocarcinoma, which has shorter villi)
Sample pathology report
- Peritoneum, resection:
- Multifocal epithelioid mesothelioma (largest focus 4.5 cm) (see comment)
- Margins of resection unremarkable.
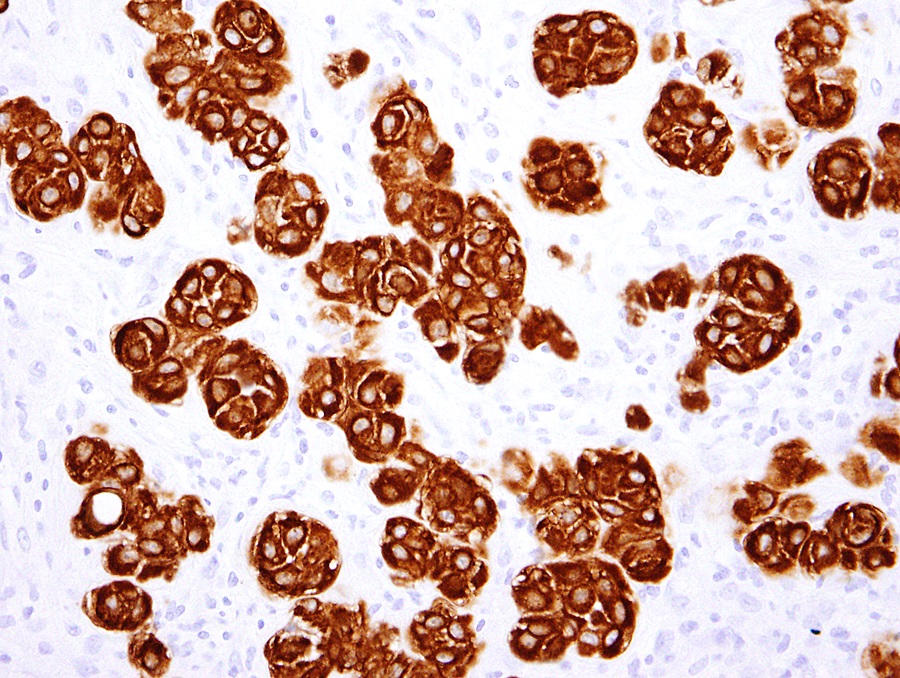
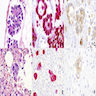
- Comment: There is not currently an AJCC TNM cancer staging system for peritoneal mesothelioma. Immunohistochemical stains for calretinin and D2-40 are positive in the tumor.
Differential diagnosis
- Mucinous adenocarcinoma
- Papillary serous carcinoma involving the peritoneum (Am J Surg Pathol 2007;31:1139)
- Pleura - mesothelioma versus adenocarcinoma
- Pseudomyxoma peritonei
Additional references
Board review style question #1
Which of the following stains is positive in the myxoid variant of epithelioid mesothelioma and helps to differentiate it from mucinous adenocarcinoma?
- B72.3
- Claudin4
- D2-40
- MOC31
Board review style answer #1
Mesothelioma (peritoneum)-overview
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Tumor that originates from the serosal lining of the peritoneal cavity
Essential features
- Peritoneal mesothelioma shows weaker association with asbestos exposure and more likely involves women / young patients than pleural mesothelioma
- Histologic variants (epithelioid, biphasic and sarcomatoid) impart prognostic information with treatment implications
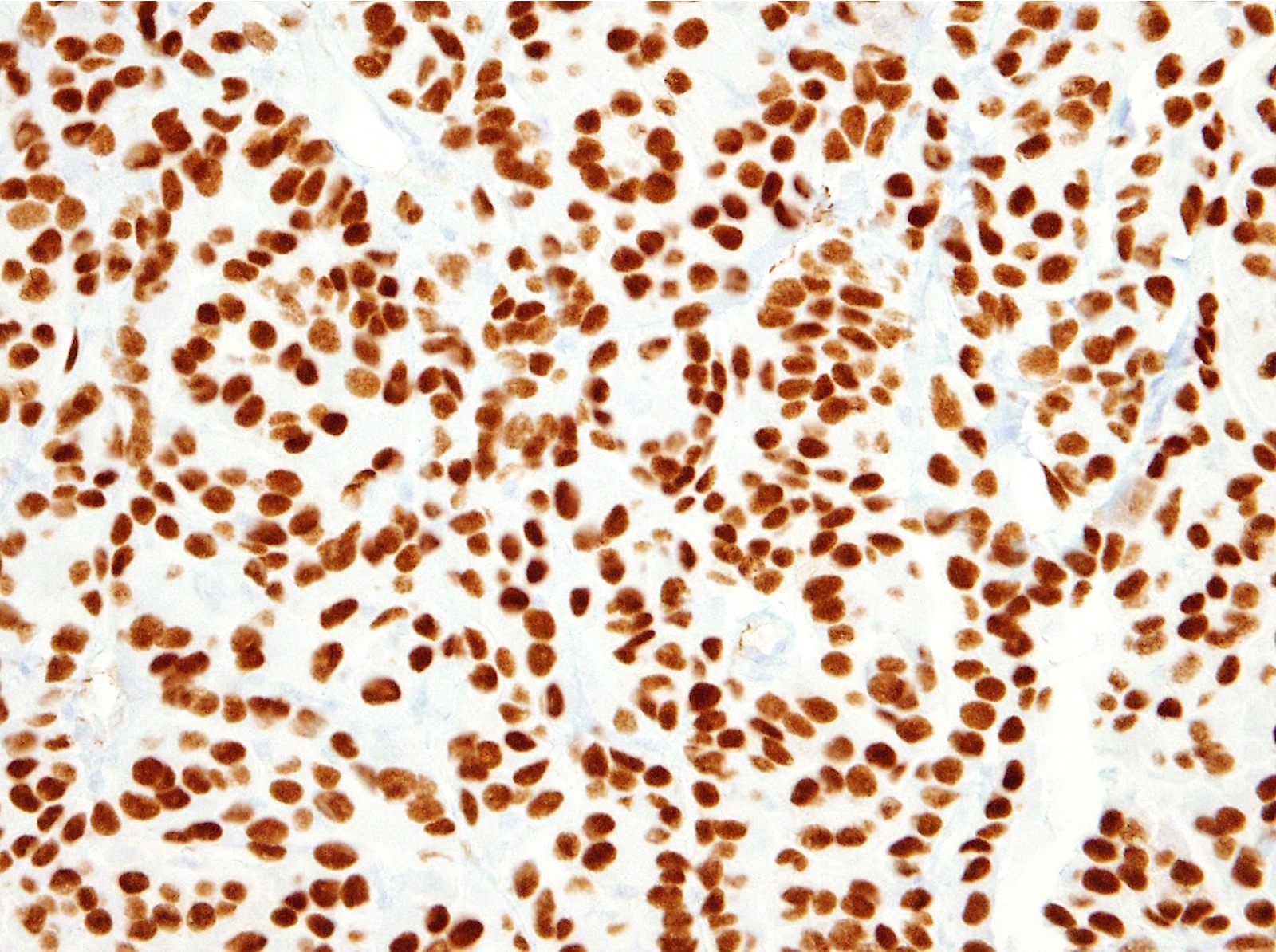
- Loss of BAP1 expression is seen in 40 - 60% of peritoneal mesothelioma, not sensitive but fairly specific for the diagnosis of mesothelioma in the appropriate histologic context
Terminology
- Peritoneal mesothelioma
- Malignant peritoneal mesothelioma
- Diffuse malignant peritoneal mesothelioma
- Abdominal mesothelioma
ICD coding
Epidemiology
- Accounts for ~10% of all mesotheliomas
- Incidence: ~300 new cases annually in the United States
- Presents with a wide age range and no apparent sex predilection (Cancer Causes Control 2009;20:935, Bull World Health Organ 2011;89:716, Clin Epidemiol 2016;8:743)
Sites
- Involves the serosal lining in the peritoneal cavity, often of multiple intra-abdominal organs
- Generally diffuse, rarely solitary / localized (Am J Surg Pathol 2005;29:866)
Etiology
- Associated with exposure to asbestos fibers in a subset of patients, typically with a long latency (median ~32 years); the association is weaker than in pleural mesothelioma (J Clin Oncol 1983;1:386, J Occup Med 1992;34:718, Arch Pathol Lab Med 2018;142:753)
- Rarely associated with exposure to non asbestos mineral fibers: erionite, fluoro-edenite and others (Arch Pathol Lab Med 2018;142:753)
- Rarely associated with prior exposure to therapeutic radiation for other malignancy with a latency of years to decades (J Clin Oncol 1983;1:695, Cancer 1988;61:2019, Arch Pathol Lab Med 2018;142:753)
- Rarely associated with recurrent peritonitis / chronic serosal inflammation secondary to Crohn disease, endometriosis or Familial Mediterranean Fever (J Clin Pathol 2017;70:228, J Clin Pathol 2018;71:971, Arch Pathol Lab Med 2018;142:753)
- Rarely associated with germline mutations and characteristic gene rearrangements (see molecular/cytogenetics section below)
Clinical features
- More likely to affect women and young patients as compared to pleural mesothelioma (Cancer Causes Control 2009;20:935)
- Symptoms can be nonspecific and depend on the extent of involvement
- Presentation includes most commonly abdominal pain, distension or ascites; rarely incidental or with new hernia, bowel obstruction or perforation (Tumori 2003;89:269, Ann Gastroenterol 2018;31:659)
- Morbidity / mortality primarily due to locoregional spread with extra-abdominal metastasis rare
- Median overall survival of 3 - 7 years with a 5 year survival rate of 40 - 60% with treatment (Ann Gastroenterol 2018;31:659)
Diagnosis
- Radiologic assessment of disease extent by computed tomography (CT) or magnetic resonance imaging (MRI)
- Cytologic analysis of peritoneal fluid, though this is not entirely sensitive
- Definitive diagnosis is most commonly based on histologic analysis of surgical specimen from laparoscopic / open or core needle biopsy
- Peritoneal mesothelioma, particularly the sarcomatoid variant, is difficult to diagnose and requires multiple immunohistochemical markers to exclude mimics
- Since no single immunohistochemical marker is entirely sensitive and specific for the diagnosis, a panel of at least 2 positive markers and 2 negative markers is recommended (Hum Pathol 2017;67:160)
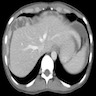
Radiology description
- Multiple nodular lesions involving omentum and the mesentery, peritoneal thickening and accumulation of ascites (Anticancer Res 2016;36:1067)
Prognostic factors
- Improved survival has been associated with the following:
- Age < 60 years (J Clin Oncol 2003;21:4560, Ann Surg Oncol 2018;25:2159, Ann Surg Oncol 2018;25:2018)
- Female gender (Ann Surg Oncol 2018;25:2159, Ann Surg Oncol 2018;25:2018)
- Epithelioid variant (J Clin Oncol 2009;27:6237, Pathology 2014;46:604, Ann Surg Oncol 2018;25:2018)
- Complete cytoreduction (J Clin Oncol 2003;21:4560, J Clin Oncol 2009;27:6237, Ann Surg Oncol 2015;22:1686, J Gastrointest Oncol 2017;8:915)
- Lack of lymph node metastasis (J Clin Oncol 2009;27:6237, J Gastrointest Oncol 2017;8:915)
- Low peritoneal cancer index (PCI) (Cancer 2011;117:1855, J Gastrointest Oncol 2017;8:915)
- Absence of loss of chromosomal region 9p21 / CDKN2A (Mod Pathol 2010;23:531, JAMA Oncol 2018;4:235)
- Worse survival has been noted to be associated with the following, although more data is needed for definitive conclusion:
- BAP1 molecular or expression status (J Thorac Oncol 2017;12:724)
- Elevated mitotic count (Clin Cancer Res 2005;11:3303, Histopathology 2016;68:729)
- High nuclear grade (Am J Surg Pathol 2016;40:1243)
- Solid pattern in epithelioid variant (Histopathology 2016;68:729)
Case reports
- 19 year old woman with abdominal pain and cachexia (epithelioid) (Cold Spring Harb Mol Case Stud 2019;5:a003566)
- 35 year old woman with omentum caking (epithelioid, deciduoid) (BMC Clin Pathol 2017;17:13)
- 40 year old woman with abdominal pain and distension (epithelioid) (Gastroenterology Res 2019;12:48)
- 61 year old woman with abdominal distension and ascites (epithelioid, clear cell pattern) (Pathol Res Pract 2017;213:580)
- 63 year old woman with abdominal pain (epithelioid, clear cell pattern with VHL mutation) (Hum Pathol 2019;83:199)
- 81 year old man with refractory ascites (biphasic) (Intern Med 2017;56:861)
Treatment
- Cytoreductive surgery, often combined with hyperthermic intraoperative chemotherapy, generally recommended for treating epithelioid variant (and some biphasic) but not sarcomatoid variant (Ann Surg Oncol 2015;22:1686, Ann Surg Oncol 2018;25:667)
- Chemotherapy types: hyperthermic intraoperative chemotherapy (HIPEC), early postoperative intraperitoneal chemotherapy (EPIC), long term intraperitoneal (IP) chemotherapy and systemic chemotherapy (Eur J Cancer 2016;65:69, Eur J Surg Oncol 2017;43:1228)
- Radiation
- Immunotherapy: clinical trial on using tremelimumab (anti-CTLA4 monoclonal antibody) and durvalumab (PD-L1 blockade) ongoing (Lancet Respir Med 2018;6:451)
Gross description
- Multiple omental / serosal nodules and thickened peritoneum
Microscopic (histologic) description
- Unequivocal indicator of malignancy: invasion into adipose tissue or stromal invasion (Am J Surg Pathol 2000;24:1183, Arch Pathol Lab Med 2018;142:89)
- Histologically classified into epithelioid, biphasic and sarcomatoid variants with implications on prognosis and treatment planning
- Epithelioid mesothelioma is characterized by epithelioid to round tumor cells, which are often more monotonous than what are seen in most carcinomas
- Sarcomatoid mesothelioma is characterized by spindled tumor cells
- Biphasic mesothelioma is characterized by the presence of both epithelioid and sarcomatoid components, each comprising at least 10% of the tumor
- In epithelioid mesothelioma, architectural / cytologic features that can be seen are diverse; histologic patterns most commonly seen are tubular, papillary and solid and more rarely micropapillary, trabecular, acinar, adenomatoid-like, clear cell, deciduoid, adenoid cystic-like, signet ring cell, small cell and rhabdoid (Arch Pathol Lab Med 2013;137:647)
- In sarcomatoid mesothelioma, histologic patterns include conventional, desmoplastic, lymphohistiocytoid and those with heterologous differentiation (Arch Pathol Lab Med 2013;137:647, Am J Surg Pathol 2015;39:1568)
Microscopic (histologic) images
Cytology description
- Large clusters to sheets of fairly monotonous mesothelial tumor cells
- Limitation of cytologic diagnosis: rarely definitive, since tissue invasion is difficult to assess
Positive stains
- Mesothelial markers: calretinin, WT1 and D2-40 (podoplanin), CAIX (J Clin Pathol 2016;69:706, Arch Pathol Lab Med 2018;142:236, Arch Pathol Lab Med 2018;142:89)
- Keratins such as AE1 / AE3, CAM 5.2 and broad spectrum pankeratin
- Keratin 5 / 6: expressed in most epithelioid mesotheliomas (rarely adenocarcinomas)
- Mesothelin, thrombomodulin, HBME1
- GATA3: expressed in a large subset of sarcomatoid mesothelioma (also in breast and urothelial carcinoma)
Negative stains
- Epithelial markers: claudin4, BerEP4, MOC31, B72.3, CEA
- TTF1
- BAP1: complete loss of BAP1 expression in 40 - 60% (lost in < 1% of carcinomas and not in reactive mesothelial proliferation) (Am J Surg Pathol 2015;39:977, Hum Pathol 2016;51:9, J Thorac Oncol 2017;12:724)
- PAX8: expression in 10 - 20% (Hum Pathol 2018;72:160, Am J Surg Pathol 2017;41:1675, Arch Pathol Lab Med 2018;142:89)
Electron microscopy description
- Microvilli and desmosomes
Molecular / cytogenetics description
- BAP1 somatic mutations in 40 - 70% (J Transl Med 2015;13:122, Mod Pathol 2017;30:246, J Thorac Oncol 2017;12:724)
- Copy number loss of BAP1 in 3p21, CDKN2A in 9p21 and NF2 in 22q12 in 20 - 40% (Mod Pathol 2010;23:531, Hum Pathol 2016;55:72)
- Somatic mutations in SETD2, DDX3X and others in a subset (Mod Pathol 2017;30:246)
- Germline mutations in BAP1, ATM or other regulators in DNA repair in a subset (J Clin Oncol 2018;36:2863, J Pediatr Hematol Oncol 2018;40:e511)
- EWSR1-ATF1 or FUS-ATF1 gene fusion in rare cases (Am J Surg Pathol 2017;41:980)
- ALK gene rearrangement in rare cases but enriched in young patients (JAMA Oncol 2018;4:235)
Sample pathology report
- Peritoneum and omentum, resection:
- Mesothelioma, epithelioid variant, 5.0 cm in greatest dimension
- Surgical margins, negative for tumor
- Comment: The tumor cells are positive for WT1, calretinin and D2-40, and are negative for claudin4, PAX8, MOC31 and CEA.
Differential diagnosis
- Metastatic adenocarcinoma
- Variable histologic overlap
- Typically expresses other epithelial markers: claudin4, BerEP4, polyclonal CEA, MOC31 and B72.3
- Rarely express keratin 5 / 6 and mesothelial markers (calretinin and D2-40), though WT1 is commonly expressed in carcinomas of Müllerian primary and CAIX is expressed in renal cell carcinoma, clear cell type
- Expression of PAX8 favors renal or gynecologic origin and TTF1 favors lung origin
- Sclerosing peritonitis
- Reactive proliferation of stromal myofibroblasts, often associated with adjacent linear arrangement of mesothelial cells, chronic inflammation, surface fibrin deposition and occasionally entrapped fat
- Associated with various conditions including luteinized thecomas (Am J Surg Pathol 1994;18:1)
- Distinction can be challenging, particularly in small biopsies, cases with tangential sectioning or fat entrapment (Am J Surg Pathol 2000;24:1183)
- Well differentiated papillary mesothelial tumor
- Most commonly in the peritoneum, rarely pleura and other sites
- Histology: papillae with myxoid cores, each lined by a single mesothelial cell layer
- Invasion is typically not present (Am J Surg Pathol 2014;38:990)
- Overall more indolent than peritoneal mesothelioma (Ann Surg Oncol 2019;26:852)
- Recurrent mutations in TRAF7 or CDC42 (Mod Pathol 2019;32:88)
- Adenomatoid tumor of genital type
- Benign mesothelial tumor that arises mostly commonly near the genital tract
- Histology: a microcystic low power appearance with poor margination and tubules / cords of epithelioid cells with characteristic cytoplasmic vacuolation
- Associated with immunosuppressive state in some cases (Histopathology 2018;73:1013)
- Recurrent mutations in TRAF7 (Mod Pathol 2018;31:660)
- Peritoneal inclusion cyst
- Also known as multilocular inclusion cyst
- Histology: multiple cysts of various sizes, each with thin fibrous walls lined by flattened mesothelial cells (Cancer 1989;64:1336)
- TNS3-MAP3K3 or ZFPM2-ELF5 gene fusion in rare cases (Cancer Lett 2015;357:502)
- Epithelioid hemangioendothelioma
- Rare distinctive malignant vascular tumor
- Most commonly involves liver, lung / pleura, bone or soft tissue
- Histology: cord-like pattern, myxohyaline matrix, epithelioid cells with intracytoplasmic vacuoles
- Immunohistochemistry: typically positive for CAMTA1 and endothelial markers (ERG, CD31, CD34 and D2-40) but negative for calretinin and WT1 (Am J Surg Pathol 2016;40:94)
- WWTR1-CAMTA1 gene fusion in most cases, YAP1-TFE3 fusion in rare cases (Sci Transl Med 2011;3:98ra82, Genes Chromosomes Cancer 2011;50:644, Genes Chromosomes Cancer 2013;52:775)
Board review style question #1
- Which of the following is true regarding peritoneal mesothelioma?
- All cases are associated with asbestos exposure
- Compared to pleural mesothelioma, peritoneal mesothelioma more likely involves women and children
- Copy number loss of CDKN2A and NF2 is detected in all cases
- Histologic subtyping has no prognostic value
- The presence of intact BAP1 protein expression rules out malignancy entirely
Board review style answer #1
B. Compared to pleural mesothelioma, peritoneal mesothelioma more likely involves women and children
Comment Here
Reference: Peritoneal mesothelioma
Comment Here
Reference: Peritoneal mesothelioma
Board review style question #2
- Which of the following is true regarding the two images (the tumor and the corresponding BAP1 immunostain) illustrated?
- This indicates germline BAP1 mutation(s) in all cases
- This is a metastatic adenocarcinoma
- This is a reactive mesothelial proliferation
- This is a sarcomatoid mesothelioma
- This is an epithelioid mesothelioma
Board review style answer #2
Mesothelioma (peritoneum)-sarcomatoid and biphasic (pending)
[Pending]
Mesothelioma (pleura)-epithelioid
Table of Contents
Definition / general | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Differential diagnosis | Board review style question #1 | Board review style answer #1Definition / general
- Most frequent histologic type of malignant mesothelioma
Case reports
- 73 year old man with bilateral pleural effusions, lung nodules and pleural thickening (Case of the Week #476)
Microscopic (histologic) description
- Composed of oval, polygonal or cuboidal cells, with multiple secondary patterns:
- Tubulopapillary:
- Characteristic papillary growth pattern
- Tumor cells with round nuclei, moderate amounts of eosinophilic cytoplasm and conspicuous nucleoli
- Elongated tubular structures may also be seen
- Must distinguish from micropapillary pattern, which has a poorer prognosis
- Tubulopapillary:
- Other patterns:
- Acinar: elongated or branching gland-like lumina lined by relatively bland cuboidal cells
- Adenomatoid
- Adenoid cystic: cribriform and tubular patterns separated by fibrous stroma
- Clear cell: mesothelial cells with clear cytoplasm
- Deciduoid: sheets of large polygonal cells with abundant glassy cytoplasm, round vesicular nuclei and prominent nucleoli
- Micropapillary: has higher incidence of lymphatic invasion
- Pleomorphic: marked nuclear pleomorphism; if this component is > 10%, has an adverse prognosis
- Rhabdoid: dyscohesive cells with abundant eosinophilic cytoplasm, eccentric nuclei, prominent nucleoli; variable eosinophilic cytoplasmic inclusion
- Signet ring cell: clusters or sheets of cells with cytoplasmic vacuoles
- Small cell: very rare; uniform small round cells with high N:C ratio
- Solid
- Trabecular
Microscopic (histologic) images
Cytology description
- Papillary epithelial type:
- Papillary fragments and cohesive cell clusters
- Moderate amount of cytoplasm
- Round to ovoid nuclei, prominent nuclei
- Psammoma bodies may be seen (Cancer Cytopathol 2013;121:703)
- Cohesive epithelioid type:
- Cohesive groups of cells
- Nuclei round or oval with some pleomorphism
- Eccentrically located nuclei occasionally with coarse chromatin
- Multinucleation is common
- Variable mitotic figures
- Asbestos bodies maybe seen
Positive stains
- Calretinin: both nuclear and cytoplasmic staining; useful to distinguish mesothelioma and lung adenocarcinoma
- Keratin 5 / 6: expressed in epithelioid mesothelioma but negative in sarcomatoid mesothelioma
- Podoplanin (D2-40): membranous and apical staining; may be expressed in squamous cell carcinoma of lung and serous carcinoma, synovial sarcoma and angiosarcoma
- WT1: useful to distinguish mesothelioma from renal cell carcinoma and squamous cell carcinoma (Hum Pathol 2013;44:1)
Negative stains
- TTF1 and Napsin A: positive in lung adenocarcinoma
- PAX8: positive in tumors of Müllerian origin
- GATA3 / GCDFP-15 / mammaglobin: positive in breast cancers
- MOC31, BerEp4, CEA: positive in adenocarcinoma
- Claudin4 may distinguish epithelioid mesothelioma (negative) from metastatic carcinoma to serosal membranes (Am J Clin Pathol 2013;139:611)
Electron microscopy description
- Very long, thin apical microvilli that do not have a glycocalyx
- Adenocarcinomas have shorter microvilli, have a glycocalyx and perinuclear tonofilament bundles
Differential diagnosis
Board review style question #1
Which set of antibodies is useful for distinguishing pleural malignant mesothelioma from lung adenocarcinoma?
A. WT1, CA125, BerEP4 (EpCAM)
B. CA125, BerEP4, Calretinin
C. BerEP4, Calretinin, AE1/AE3
D. WT1, BerEP4, Calretinin
E. WT1, BerEP4, AE1/AE3
A. WT1, CA125, BerEP4 (EpCAM)
B. CA125, BerEP4, Calretinin
C. BerEP4, Calretinin, AE1/AE3
D. WT1, BerEP4, Calretinin
E. WT1, BerEP4, AE1/AE3
Board review style answer #1
D. WT1 and calretinin are specific for mesothelial cells and BerEP4 is specific for lung adenocarcinoma. CA125 and AE1/AE3 are immunoreactive in both mesothelioma and adenocarcinoma.
Comment Here
Reference: Mesothelioma (pleura) - epithelioid
Comment Here
Reference: Mesothelioma (pleura) - epithelioid
Mesothelioma versus adenocarcinoma
Table of Contents
Definition / general | ICD coding | Epidemiology | Etiology | Clinical features | Diagnosis | Laboratory findings / pleural fluid measurements | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Immunohistochemical stains (positive and negative stains) | Special stains | Electron microscopy description | Molecular / cytogenetics description | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Mesothelial proliferations are a spectrum of benign to malignant lesions of the lining of the serosal cavities
- Adenocarcinoma is a malignant epithelial neoplasm with glandular differentiation
ICD coding
Epidemiology
- Age and gender are unreliable distinguishing factors, as both tumors have a slight male predominance and peak incidence in patients over 65
- History of asbestos exposure should not be taken into consideration by the pathologist when confirming or excluding mesothelioma; diagnosis should be made using morphology and immunohistochemical studies (Arch Pathol Lab Med 2009;133:1317)
- Unlike adenocarcinoma, tobacco is not implicated in the causation of mesothelioma; however, tobacco (especially cigarette) smoke and asbestos can synergistically interact in the causation of lung cancer (Int J Environ Res Public Health 2019;17:258)
- Etiologies and epidemiology for metastases are dependent on site of origin
Etiology
- See epidemiology
Clinical features
- Wide variety of symptoms can lead to a diagnosis of lung cancer and are not specific to a pathologic entity
- Presenting symptoms include chest wall pain (unilateral or bilateral), pleurisy, cough and progressive dyspnea secondary to pleural effusion (Surg Pathol Clin 2020;13:73)
- Both commonly present with insidious onset of chest pain or dyspnea
- Other site specific symptoms may or may not be present in patients with metastatic carcinoma
Diagnosis
- In the initial evaluation of patients with suspected malignant pleural mesothelioma, radiological imaging is essential
- Computed tomography (CT) of the chest is the primary imaging modality but magnetic resonance imaging (MRI) and positron emission tomography (PET) can provide additional information for surgical planning (Radiographics 2004;24:105)
- Further investigation of suspected malignant pleural mesothelioma includes thoracentesis of any existing pleural effusion with cytological examination and closed pleural biopsy
- If there is not enough tissue to reliably distinguish mesothelioma and adenocarcinoma on closed pleural biopsy, surgical intervention via video assisted thoracoscopic, biopsy or open thoracotomy can be pursued (Eur Respir Rev 2016;25:472)
- Concurrent bronchoscopy at the time of surgery can also be considered, as endobronchial lesions are typically not seen in mesothelioma and their presence argues against this diagnosis (Tuberc Respir Dis (Seoul) 2015;78:297)
Laboratory findings / pleural fluid measurements
- High levels of hyaluronic acid (concentrations exceeding 100,000 ng/ml) are suggestive of mesothelioma (Respir Investig 2013;51:92)
- Carcinoembryonic antigen elevation (CEA) in both serum and malignant pleural effusions has been associated with pulmonary adenocarcinoma (J Thorac Dis 2018;10:E340)
- When malignant pleural effusions occur in either mesothelioma or adenocarcinoma, they are typically exudative effusions (Dtsch Arztebl Int 2019;116:377)
Radiology description
- Mesothelioma
- Most often presents with unilateral disease
- Diffuse thick rind-like or nodular pleural thickening which may extend into the lung (Indian J Radiol Imaging 2013;23:313)
- May be associated with unilateral pleural effusion and ipsilateral lung volume loss
- Adenocarcinoma of lung / metastatic carcinoma
- Lung primary: main finding is a lung mass / nodule which may extend to the pleura
- Metastatic adenocarcinoma: often multiple nodules in the lung or pleura
- Pitfalls
- Both may have bilateral disease or mediastinal lymph node involvement
- Mesothelial diaphragmatic invasion with liver involvement can mimic a primary liver tumor
Radiology images
Prognostic factors
- 75% of mesotheliomas are detected at stage III - IV at diagnosis
- CT screening detected adenocarcinoma is usually at an earlier stage and has a better prognosis (J Natl Compr Canc Netw 2018;16:412)
- Metastatic adenocarcinoma, is, by definition, high stage and has poor prognosis
Case reports
- 45 year old woman with pleural epithelioid mesothelioma and ALK translocation with response to targeted immunotherapy (Lung Cancer 2020;142:47)
- 61 year old man with pleural fluid asbestos bodies and lung adenocarcinoma (Rom J Morphol Embryol 2016;57:1171)
- 70 year old man with concurrent mesothelioma and lung adenocarcinoma (Respir Med Case Rep 2018;26:45)
Treatment
- Surgical management depends on stage and resectability of the tumor
Gross description
- Diffuse pleurotropic growth pattern is most common in mesothelioma (J Carcinog 2008;7:3)
- Peripheral adenocarcinoma encasing the lung, so called pseudomesotheliomatous growth, will usually have a component involving the lung parenchyma
Gross images
Frozen section description
- There are no distinctive features that distinguish mesothelioma and adenocarcinoma on frozen section
Microscopic (histologic) description
- Epithelioid variant is the most common histological subtype of mesothelioma and its principal differential consideration is adenocarcinoma; they are difficult to differentiate histologically if adenocarcinoma does not produce obvious mucin
- Morphologic features of mesothelioma (J Clin Pathol 2006;59:564):
- Round or cuboidal tumor cells, paracentric nuclei, small nucleoli, moderate to abundant amount of cytoplasm with low nuclear to cytoplasmic ratio
- Often deceptively bland cytologic features
- Morphologic features of adenocarcinoma (J Clin Pathol 2006;59:564):
- Tend to have columnar morphology with more pleomorphism, eccentric nuclei, prominent nucleoli, nuclear molding, cellular crowding and variable amount of cytoplasm
- More likely to have high nuclear to cytopasmic ratio
- Background myxoid stromal change favors a diagnosis of mesothelioma but is not a definitive feature
- Pitfalls:
- Papillary formations or psammoma bodies can be seen in reactive and malignant mesothelial proliferations as well as adenocarcinoma
- Intracytoplasmic vacuoles can be seen in both entities
Microscopic (histologic) images
Contributed by Aliya N. Husain, M.D.
Cytology description
- Adenocarcinoma appears as a distinct population from background mesothelial cells, while mesothelioma appears as a uniform population
- Adenocarcinoma is the likeliest lung cancer cell type to generate a malignant pleural effusion and it is also associated with the highest cytological yield (Ann Transl Med 2019;7:352)
- In adenocarcinoma, the cells may line up to form a picket fence arrangement
- Cytomorphology of mesothelial cells consists of sheets or individual cells with windows between the cells
- In 1 study, the 3 features that were statistically significant to distinguish mesothelioma from adenocarcinoma were: a giant atypical mesothelial cell being indicative of mesothelioma, in contrast to increased nuclear pleomorphism and acinar structures being indicative of adenocarcinoma (Diagn Cytopathol 2009;37:4)
- Cytoplasmic vacuoles in reactive mesothelial cells tend to be paranuclear without indentation of the nuclear membrane, unlike in adenocarcinoma
- Cytoplasmic vacuoles of mesothelial cells contain hyaluronic acid, while those of adenocarcinoma contain epithelial mucin
- Pitfalls: both entities can occur as cell balls with smooth or knobby contours
Cytology images
Contributed by Takashi Hori, C.T., Akira Yoshikawa, M.D., Anja C. Roden, M.D. and Andrey Bychkov, M.D., Ph.D.
Images hosted on other servers:
Immunohistochemical stains (positive and negative stains)
- International Mesothelioma Interest Group recommends the initial diagnostic immunohistochemical panel should include at least 2 mesothelial markers and 2 general epithelial markers
- For other tumors in the differential diagnosis, on the basis of morphology, specific markers can be added (e.g. TTF1, PAX8, etc.)
- Use more markers if results inconclusive
- Best positive mesothelioma markers: calretinin, CK5 or CK5/6, WT1, D2-40
- Best positive carcinoma markers: claudin 4, MOC31, BerEP4, CEA and BG8 (Lewis antigen blood group)
- Best positive lung adenocarcinoma markers: TTF1 and napsin (Arch Pathol Lab Med 2013;137:647)
- Metastatic adenocarcinoma (Arch Pathol Lab Med 2009;133:1317)
- PAX8 is very useful for renal cell carcinoma; about 85 - 100% of renal cell carcinomas are positive; mesotheliomas are mostly negative
- Claudin 4 is positive in almost all lung adenocarcinomas, 90% of renal cell carcinomas, 98% of papillary serous carcinomas, 100% of gastric, pancreatic, colonic and biliary adenocarcinomas; mesotheliomas are always negative
- CDX2 is positive in 90 - 100% of colon, 80% of small intestine and 70% of gastric carcinomas and negative in mesothelioma
- PSA is often positive in metastatic prostatic adenocarcinoma and is always negative in mesothelioma
- Estrogen receptor is positive in 60 - 93% in serous and breast carcinomas and negative or has a very low positive rate (0 - 8%) in mesothelioma
- GATA3 is frequently positive in breast and urothelial carcinomas; however, 33 - 50% of epithelioid mesotheliomas also express GATA3
- Strong diffuse staining for GATA3 favors a diagnosis of sarcomatoid / desmoplastic mesothelioma over metastatic sarcomatoid carcinoma of the lung (Am J Surg Pathol 2017;41:1221)
| IHC stain | Mesothelioma | Adenocarcinoma | Staining pattern in positive cells |
| Calretinin | Positive | Negative | Nuclear and cytoplasmic |
| D2-40 (podoplanin) | Positive | Negative | Membranous |
| WT1 | Positive | Negative | Nuclear |
| Cytokeratin 5 / 6 | Positive | Negative | Cytoplasmic |
| Claudin 4 | Negative | Positive | Membranous |
| MOC31 | Negative | Positive | Membranous |
| TTF1 | Negative | Positive | Nuclear |
| Napsin A | Negative | Positive | Granular cytoplasmic |
| B72.3 | Negative | Positive | Membranous, cytoplasmic or both |
| BG8 | Negative | Positive | Cytoplasmic |
| CEA (monoclonal) | Negative | Positive | Cytoplasmic with membrane enhancement |
| BerEP4 | Negative | Positive | Membranous |
| BAP1 | Loss in 60% of epithelioid MM | Retained | Nuclear |
Special stains
- With the advent of specific histochemical markers, these are now mostly historical and not useful in everyday practice
- PAS after pretreatment with diastase or Alcian blue after hyaluronidase, positivity indicates neutral mucin which favors adenocarcinoma (Alcian blue without hyalurinadase is not helpful) (J Surg Oncol 1987;35:30)
- Mucicarmine should not be used as it sometimes cross reacts with hyaluronic acid
- Histochemical methods are most useful in epithelioid mesotheliomas and not useful in nonepithelioid mesotheliomas
Electron microscopy description
| EM features | Mesothelioma | Adenocarcinoma |
| Apical microvilli | Long and thin, no glycocalyx | Shorter and have microvilli |
| Perinuclear tonofilament bundles | Present | Absent |
| Basal lamina | Present | Absent |
| Long desmosomes | Present | Absent |
- Helpful in distinguishing mesothelioma from adenocarcinoma in well differentiated tumors
- Not helpful in distinguishing benign / reactive from malignant mesothelial proliferations
- Reference: Ultrastruct Pathol 2006;30:3
Molecular / cytogenetics description
- Most mesotheliomas (> 80%) harbor somatic alterations of the CDKN2A locus, 60% harbor somatic mutations and exonic deletions of the BAP1 gene and 30 - 50% show inactivation of NF2 (Transl Lung Cancer Res 2017;6:270)
- Several driver gene alterations are known in lung adenocarcinomas including EGFR, KRAS and ALK
Additional references
Board review style question #1
Board review style answer #1
C. WT1, calretinin, claudin 4, MOC31 is the only answer choice that has 2 mesothelioma markers (WT1, calretinin) and 2 adenocarcinoma markers (claudin 4, MOC31). BAP1 is lost in the majority of epithelioid mesotheliomas and is indicative of malignancy but is not a sensitive initial diagnostic marker. GATA3 and p16 are not specific to mesothelioma. ER and CDX2 are specific to certain metastatic adenocarcinomas but not other adenocarcinomas.
Comment Here
Reference: Mesothelioma versus adenocarcinoma
Comment Here
Reference: Mesothelioma versus adenocarcinoma
Board review style question #2
Molecular alterations of which of the following genes is least likely to be occur in pleural mesothelioma?
- ALK
- BAP1
- CDKN2A
- NF2
Board review style answer #2
A. ALK. Genetic alterations in NF2, CDKN2A and BAP1 are commonly associated with epithelioid mesothelioma of the pleura. ALK-EML4 translocations are seen in 3 - 7% of lung adenocarcinomas. ALK alterations rarely occur in a subset of peritoneal mesotheliomas. Rare case reports of pleural mesothelioma with ALK translocations have now recently been described and although very uncommon are important to recognize because they may impact treatment options. (JCO Precis Oncol 2019;3:PO.19.00048, Transl Lung Cancer Res 2018;7:537)
Comment Here
Reference: Mesothelioma versus adenocarcinoma
Comment Here
Reference: Mesothelioma versus adenocarcinoma
Mesothelioma-biphasic
Table of Contents
Definition / general | Epidemiology | Radiology images | Case reports | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Differential diagnosisDefinition / general
- Biphasic / mixed: both epithelioid and sarcomatoid components, each at least 10%
- May represent epithelial mesenchymal transition process (Mod Pathol 2012;25:86)
Epidemiology
- Biphasic pattern occurs in 30%, less common than epithelioid variant but more common than the pure sarcomatoid variant
Case reports
- 66 year old man with hepatic nodule (World J Gastroenterol 2009;15:615)
Microscopic (histologic) description
- Epithelioid and sarcomatoid areas, juxtaposed to each other or intermingled
Microscopic (histologic) images
Differential diagnosis
- Metastatic pleomorphic carcinoma, most commonly from lung
- Reactive proliferation in response to serosal metastases
- Synovial sarcoma
Mesothelioma-desmoplastic
Table of Contents
Definition / general | Case reports | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Differential diagnosisDefinition / general
- > 50% of tumor shows desmoplastic pattern
- 5 - 10% of malignant mesotheliomas
- Note: DMM may refer to desmoplastic or diffuse malignant mesothelioma
Case reports
- 2 cases with rapid decline (Med Electron Microsc 2003;36:173)
Microscopic (histologic) description
- Characteristic dense collagenized tissue separated by atypical cells arranged in a storiform or haphazard pattern (J Clin Pathol 1992;45:295), comprising > 50% of tumor
- Presence of sarcomatoid areas, bland collagen, necrosis, stromal / fat invasion help differentiate from organizing pleuritis (Am J Surg Pathol 2011;35:1823)
- Paucicellularity of lesion may lead to misdiagnosis
Positive stains
- Keratin stains:
- Pancytokeratin is positive in > 70% of sarcomatoid mesotheliomas (including desmoplastic)
- Use multiple cytokeratin antibodies (AE1 / AE3, CAM 5.2 and CK7)
- If only focal cytokeratin staining, additional blocks should be stained along with vimentin to check for tissue antigen integrity
- D2-40, calretinin
- GLUT1: 53% sensitivity, 98% specific
Negative stains
- CD31 (positive in angiosarcoma), CD34 (positive in solitary fibrous tumor, Hum Pathol 1995;26:428)
- S100 (positive in neurogenic tumors, mesotheliomas may show focal staining)
- CD45
- Some muscle markers are focally positive
Electron microscopy description
- Sarcomatous mesotheliomas including desmoplastic type have no specific ultrastructural features
Differential diagnosis
- Calcifying fibrous pseudotumor of pleura / localized fibrous tumor:
- Whorls and haphazard arrays of keloidal collagen
- Also psammomatous and dystrophic calcification
- Fibrous pleurisy / pleuritis:
- Cytokeratin highlights malignant cells of mesothelioma; if present in fat, skeletal muscle or connective tissue, is an important clue to desmoplastic mesothelioma
- "Fake fat" may be seen in organizing pleuritis due to artefactual changes in dense fibrous connective tissue
- S100, laminin and collagen are negative in fake fat (Arch Pathol Lab Med 2013;137:647)
- Solitary fibrous tumor: CD34+
Mesothelioma-sarcomatoid
Table of Contents
Definition / general | Epidemiology | Radiology images | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Differential diagnosis | Additional referencesDefinition / general
- Defined as > 90% spindle shaped tumor cells
- Termed desmoplastic if > 50% of the tumor shows desmoplastic pattern
Epidemiology
- 10 - 20% of all mesotheliomas
Radiology images
Case reports
- 61 year old woman with shortness of breath (Intern Med 2013;52:249)
- Osteoblastic variant (Br J Radiol 2011;84:e106)
Treatment
- Sarcomatoid histology (pure or mixed) is poor prognostic factors after extrapleural pneumonectomy
- Chemotherapy is recommended according to NCCN guidelines (National Comprehensive Cancer Network: NCNN Guidelines for Treatment of Cancer by Site [Accessed 23 March 2018])
Microscopic (histologic) description
- Conventional, spindle cell type: spindle cells arranged in fascicles or having a haphazard distribution
- Desmoplastic variant
- Heterologous differentiation: osteosarcoma, chondrosarcoma or other sarcoma-like areas
- Lymphohistiocytoid: diffuse large histiocyte-like cells with dense infiltrate of lymphocytes and plasma cells; behaves similar to epithelioid type, some prefer classifying as such (Am J Surg Pathol 2007;31:711)
Microscopic (histologic) images
Cytology description
- Tumor cells not shed in pleural fluid, which typically contains only reactive mesothelial cells
Positive stains
- Keratin: pancytokeratin positive in > 70%; use multiple antibodies (AE1 / AE3, CAM 5.2 and CK7); if only focal cytokeratin staining is present, stain additional blocks with vimentin to check tissue antigen integrity
- D2-40, calretinin
- GLUT1: overall sensitivity and specificity in mesothelioma is 53% and 98% respectively (Arch Pathol Lab Med 2012;136:804)
Negative stains
Electron microscopy description
- No specific ultrastructural features
Differential diagnosis
- Other biphasic tumors:
- Desmoid tumor
- Fibrous pleuritis
- Sarcoma
- Sarcomatoid carcinoma:
- Kidney may be difficult to differentiate
- Lung
- Solitary fibrous tumor: CD34+, BCL2+, CD99+, keratin-
Additional references
Metastases
Table of Contents
Definition / general | Sites | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology images | Case reports | Microscopic (histologic) images | Cytology description | Cytology images | Positive stainsDefinition / general
- Metastases to pleura are more common than primary tumors of pleura
Sites
- Most common primary pleural neoplasms originate from chest wall, mediastinum, lungs
- Lung cancer: most common type in pleural fluid is adenocarcinoma, followed by small cell neuroendocrine carcinoma; squamous cell carcinoma is rare
- Extrathoracic malignancy: most common primary carcinomas are breast, gastrointestinal tract and ovary
- Metastases: lung is most common primary site in men, breast in women (see table below)
- For lung, breast and ovarian metastases, 92% of pleural effusions are ipsilateral to primary lesion
Pathophysiology
- Adenocarcinoma of lung spreads to parietal pleura from visceral pleura along adhesions
- Pleural metastases from extrathoracic sites occur via hematogenous or lymphatic spread
- Malignant pleural effusions from breast occur via chest wall lymphatics or hepatic metastases, resulting in contralateral or bilateral effusions
Diagrams / tables
Clinical features
- Most malignant effusions are symptomatic; dyspnea is most common symptom and may be associated with chest pain and cough
Diagnosis
- Cytologic examination of pleural fluid leads to diagnosis in 2/3 of malignant pleural effusions
- Three specimen are recommended if clinical suspicion of metastases is high
- Note: British Thoracic Society guidelines do not recommend more than 2 specimens (British Thoracic Society: BTS Pleural Disease Guideline 2010 [Accessed 11 January 2021])
Laboratory
- Recommended tests for all sampled pleural effusions:
- Biochemistry: LDH and protein (send pleural fluid and blood simultaneously so that Light's criteria can be applied, Thorax 2010;65:ii4)
- Microscopy and culture
- Cytological examination
- Differential count
Case reports
- 64 year old man with late multiple pleural metastases of renal cell carcinoma (Intern Med 2013;52:2475)
- 76 year old woman with early gastric cancer with solitary metastasis to pleura (Clin Endosc 2013;46:666)
- 78 year old man with pleural metastases from papillary thyroid carcinoma mimicking mesothelioma (Intern Med 2014;53:163)
Microscopic (histologic) images
Cytology description
- Cytology helpful since associated pleural effusion contains tumor cells
Positive stains
- Commonly used IHC markers: CK AE1 / AE3, CK7, CK20, mCEA, BerEP4, calretinin, PAX2, PAX8 (depending on history and differential diagnosis)
- Cytochemical stains can also be used: mucicarmine, PAS, D-PAS, Alcian blue
Nodular histiocytic hyperplasia
Table of Contents
Definition / general | Terminology | Sites | Pathophysiology | Clinical features | Case reports | Treatment | Clinical images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Differential diagnosisDefinition / general
- Small nodular aggregates of histiocytes with mesothelial cells scattered in the aggregates or in clusters
Terminology
- Nodular mesothelial hyperplasia, mesothelial / monoytc incidental cardiac excrescences (MICE), nodular histiocytic / mesothelial hyperplasia
Sites
- Lesions have been described in lung, pleura, hernia sac, pericardium, peritoneum
Pathophysiology
- Hypothesis: trauma, tumor or inflammation lead to histiocyte / mesothelial expression of CD34 and adhesion molecules, which lead to aggregates of histiocytes and mesothelial cells through cell-cell interactions
Clinical features
- Almost always an incidental finding
Case reports
- 2 year old boy, 23 and 78 year old women, 74 year old man (Am J Surg Pathol 1998;22:285)
- 5 cases: peritoneal, pulmonary, pericardial (Ann Diagn Pathol 2004;8:115)
Treatment
- None required; an incidental finding
Microscopic (histologic) description
- Compact nodular collections of polygonal to oval cells with indistinct cell borders and moderate cytoplasm
- Nuclei are oval to angulated with grooves (usually) and inconspicuous nucleoli
- Mitoses may be present but no atypical mitoses
- Hemosiderin laden macrophages may be present
- Also present are scattered indistinguishable bland cuboidal to polygonal cells with moderate cytoplasm, which show cytokeratin and calretinin staining, indicating their mesothelial origin
Microscopic (histologic) images
Cytology description
- Similar nodular aggregates have also been described in cell block material from serous effusions - this is the most important pitfall in cytodiagnosis of serous effusions (Diagn Cytopathol 2002;26:68)
Positive stains
- Two populations: histiocytes (CD68+), mesothelial cells (cytokeratin+, calretinin+)
Differential diagnosis
- Endometriosis
- Leukemia / lymphoma
- Metastatic adenocarcinoma / carcinoma
- Mesothelioma
- Reactive / florid mesothelial hyperplasia: generalized, in contrast to the localized nodular histiocytic proliferation in this lesion
Peritoneal inclusion cyst
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Peritoneal inclusion cyst(s) are comprised of one or more cystic spaces lined by bland mesothelial cells
- This is a broad diagnostic category of uncertain pathogenesis, ranging from small (0.1 cm) incidental unilocular cysts to florid (> 30 cm) symptomatic multilocular tumors
Essential features
- F > M
- Uniloculated or multiloculated cysts lined by bland mesothelial cells
- Complete resection is mainstay of treatment
- Recurrence rates range from 3% (in series enriched for smaller, unilocular lesions) to 50% (in series emphasizing florid, multilocular lesions) but death from disease is exceptionally rare in either scenario
- Pathogenesis (reactive versus neoplastic) is uncertain
- Knowledge is limited by shifting definitions and variable inclusion criteria in studies across time
Terminology
- WHO advocates for (multiloculated) peritoneal inclusion cyst(s) and discourages (benign) multicystic mesothelioma and (benign) cystic mesothelioma
ICD coding
- ICD-10: D19.1 - benign neoplasm of mesothelial tissue of peritoneum
Epidemiology
- Rare; accounts for 3 - 5% of peritoneal mesothelial lesions (Pleura Peritoneum 2019;4:20190024)
- 80 - 90% in women (Cancer 1982;50:1615, Am J Surg Pathol 1988;12:737, Hum Pathol 2003;34:369, Eur J Surg Oncol 2018;44:1100)
- Age at diagnosis is 11 - 77 years
- In women, most common in the third and fourth decades (Cancer 1982;50:1615, Am J Surg Pathol 1988;12:737, Hum Pathol 2003;34:369, J Pediatr Adolesc Gynecol 2009;22:41, Eur J Surg Oncol 2018;44:1100)
- In men, most common in the seventh decade (Hum Pathol 2003;34:369, Case Rep Pathol 2017;2017:9752908)
Sites
- Pelvis is the most common site (Am J Surg Pathol 1988;12:737, Cancer 1989;64:1336, Am J Clin Pathol 2021;155:853)
- Ovarian surface, peritoneal cul de sac and rectal serosa are frequently involved
- ~50% also involve the abdominal peritoneum (Am J Surg Pathol 1988;12:737, Cancer 1989;64:1336, Am J Clin Pathol 2021;155:853)
- Minority (~20%) also involve the retroperitoneum (Cancer 1989;64:1336)
- Rare cases involve splenic capsule or perinephric region (Am J Surg Pathol 1997;21:334, Arch Pathol Lab Med 2000;124:766)
Pathophysiology
- Unclear if neoplastic or reactive
- Factors favoring neoplastic pathogenesis (Am J Surg Pathol 1988;12:737, Eur J Surg Oncol 2018;44:1100)
- Progressive nature of untreated cases
- Rare transformation to malignant mesothelioma
- Inconstant association with prior surgery or inflammatory process
- Occasional co-occurrence with adenomatoid tumor or well differentiated papillary mesothelioma (Histopathology 1996;29:375, Curr Probl Cancer 2017;41:340, Ann Diagn Pathol 2019;38:43, Medicine (Baltimore) 2019;98:e15746)
- Factors favoring reactive pathogenesis (Cancer 1989;64:1336, Am J Surg Pathol 1986;10:844, Hum Pathol 2003;34:369)
- Frequent association with prior surgery, pelvic inflammatory disease, endometriosis or peritoneal dialysis
Clinical features
- May present with abdominal fullness, pain, urinary / bowel symptoms or palpable mass (Cancer 1982;50:1615)
- Up to half present as incidental finding (Am J Surg Pathol 1988;12:737, Cancer 1989;64:1336, Am J Clin Pathol 2021;155:853)
Diagnosis
- Laparoscopic exploration with tissue sampling (Cancer 1982;50:1615)
Laboratory
- CA125 may be mildly elevated (Case Rep Obstet Gynecol 2014;2014:852583, Obstet Gynecol Surv 2009;64:321)
Radiology description
- Ultrasound: multiloculated anechoic cysts (Curr Probl Cancer 2017;41:340, Obstet Gynecol Surv 2009;64:321)
- CT: multilocular low density, thin walled multicystic mass (Tumori 2008;94:14, Obstet Gynecol Surv 2009;64:321)
- MRI: multiple cysts that are hypointense on T1, hyperintense on T2 (Clin Imaging 2017;42:133, Obstet Gynecol Surv 2009;64:321)
Prognostic factors
- Peritoneal recurrence rates range from 3% in a population based series with more frequent small unilocular cysts to 50% in a consultation heavy series enriched for florid multicystic lesions (Am J Clin Pathol 2021;155:853, Cancer 1982;50:1615, Cancer 1989;64:1336)
- Based on inclusion criteria for these studies, the true recurrence rate is likely closer to 3% than 50%, though data remains limited and the WHO (5th edition) retains the 50% figure
- Recurrence free interval may range from 6 months to 21 years (Cancer 1989;64:1336, Am J Clin Pathol 2021;155:853)
- Recurrences are frequently multiple (Cancer 1989;64:1336, Hum Pathol 2003;34:369)
- High peritoneal carcinomatosis index and incomplete resection are associated with increased recurrence risk (Cancer 1989;64:1336, Eur J Surg Oncol 2018;44:1100)
- In surgical series, complete cytoreduction with hyperthermic intraperitoneal chemotherapy has been associated with reduced recurrence risk (21% at 10 years) (Eur J Surg Oncol 2018;44:1100)
- Death from disease is extremely rare (Am J Surg Pathol 1988;12:737, Cancer 1989;64:1336, Hum Pathol 2003;34:369)
- Very rare cases present with or progress to epithelioid malignant mesothelioma (Am J Surg Pathol 1988;12:737, BMJ Case Rep 2014;2014:bcr2013200212, J Surg Oncol 2002;79:243)
Case reports
- 28 year old woman with peritoneal inclusion cyst of the pelvis (Obstet Gynecol Sci 2013;56:126)
- 47 year old woman with peritoneal inclusion cyst presenting as a pelvic mass (Obstet Gynecol Sci 2018;61:170)
- 47 year old woman peritoneal inclusion cyst of the pelvis (Int J Surg Case Rep 2020;74:152)
- 68 year old man with peritoneal inclusion cyst of the abdomen and pelvis (Case Rep Pathol 2017;2017:9752908)
- 84 year old man with peritoneal inclusion cyst (Diagn Interv Imaging 2016;97:361)
Treatment
- Early complete surgical resection is first line therapy (Am J Surg Pathol 1988;12:737, Eur J Surg Oncol 2018;44:1100)
- Recurrence(s) is managed by repeat resection
- Hyperthermic intraperitoneal chemotherapy is advocated by some for select clinical scenarios (Eur J Surg Oncol 2018;44:1100, Br J Surg 2011;98:60)
- Other options include hormonal therapy, sclerotherapy or laser ablation for select clinical scenarios (Korean J Radiol 2001;2:164, Curr Probl Cancer 2017;41:340)
- No role for radiotherapy or systemic chemotherapy
Gross description
- Uniloculated or multiloculated cystic lesion ranging from 0.1 cm to > 30 cm (Cancer 1982;50:1615, Cancer 1989;64:1336, Am J Clin Pathol 2021;155:853)
- In one population based study, ~66% were unifocal and unilocular while 33% were multifocal or multilocular (Am J Clin Pathol 2021;155:853)
- Multilocular lesions are larger than unilocular (average, 4.5 cm versus 2.0 cm, respectively)
- Occasional cases present with numerous discrete cysts studding the peritoneum (Am J Surg Pathol 1988;12:737)
- Rare cases present with free floating intraperitoneal cysts (Cancer 1989;64:1336)
- Individual locules range from 0.1 - 15 cm (Cancer 1982;50:1615, Cancer 1989;64:1336, Am J Surg Pathol 1988;12:737, Hum Pathol 2003;34:369)
- Pink-tan to green-gray to purple-red
- Cysts filled with serous fluid; intracystic hemorrhage is common
Microscopic (histologic) description
- 1 or multiple small cysts lined by a single layer of bland, flat to cuboidal cells
- Delicate mesothelial papillae or buds may project into cyst lumina (Am J Surg Pathol 1988;12:737, Cancer 1989;64:1336)
- Cysts separated by scant loose to collagenous stromal septa
- No infiltrative invasion of underlying tissues (e.g., fat, bowel)
- Chronic inflammation and hemorrhage are common
- Uncommon features (Am J Surg Pathol 1988;12:737)
- Adenomatoid tumor-like foci
- Squamous metaplasia of the mesothelial lining
- Hobnail change of mesothelial lining
- Brisk acute inflammation with mesothelial denudation and granulation type change
- Small mesothelial nests, cords and single cells in lesional stroma; often associated with brisk inflammation
- Mesothelial nests, cords and single cells may be seen in stromal septa; often associated with brisk inflammation (termed mural mesothelial proliferation) (Cancer 1989;64:1336, Am J Surg Pathol 1986;10:844, Hum Pathol 2003;34:369)
- Considered to be a superimposed reactive change
- No diffuse sheet-like growth or infiltration of underlying tissues
- Recurrences histologically similar to primary lesions (Cancer 1982;50:1615, Cancer 1989;64:1336)
Microscopic (histologic) images
Cytology description
- Nonspecific sheets or strips of flattened or squamoid cells with distinct cell borders and small bland nuclei (Diagn Cytopathol 2010;38:192, Cytopathology 2008;19:224, Obstet Gynecol Surv 2009;64:321)
Positive stains
- Cytokeratin
- Calretinin (Hum Pathol 2003;34:369, J Pediatr Adolesc Gynecol 2009;22:41)
- CK5/6
- WT1
- D2-40
- BAP1 (normal / retained)
- MTAP (normal / retained)
Negative stains
- ER, PR (focal weak staining in ~10%) (Hum Pathol 2003;34:369)
- Claudin4
- MOC31 / BerEP4
Electron microscopy description
- Well developed desmosomal attachments
- Numerous luminal microvilli
- Abundant cytoplasmic keratin filaments
- Ovoid nuclei with even chromatin
- Thin, intact basal lamina (Cancer 1982;50:1615, Cancer 1989;64:1336)
Sample pathology report
- Pelvic mass, excision:
- Multiloculated peritoneal inclusion cysts (6.5 cm) (see comment)
- Comment: On microscopic examination of this multiloculated cystic lesion, individual cysts are lined by bland, flattened mesothelial cells. Mitoses are not identified. The cysts are separated by fibrous septa with patchy chronic inflammation. There is no infiltration of underlying tissues. The gross and microscopic findings are most consistent with a diagnosis of multiloculated peritoneal inclusion cyst (previously termed multicystic mesothelioma). Although peritoneal inclusion cysts are generally regarded as benign, local recurrence has been documented in up to 50% of cases. Recurrence is more common after incomplete excision. Progression to malignant mesothelioma has been reported in exceptionally rare cases. Clinical and radiographic correlation is advised with appropriate clinical follow up.
Differential diagnosis
- Malignant mesothelioma:
- Lymphangioma (Cancer 1982;50:1615, Am J Surg Pathol 1988;12:737, Hum Pathol 2003;34:369):
- Unifocal lesion
- Primarily seen in in retroperitoneum, mesentery or omentum
- Uniform cyst size without intraluminal mesothelial budding
- Endothelial lining positive for CD31, CD34, ERG
- Negative for cytokeratin, calretinin, WT1
Additional references
Board review style question #1
A 36 year old woman presents with pelvic discomfort, worsening over 6 months. Transvaginal ultrasound shows a multicystic lesion involving the cul de sac, left uterine adnexa and rectal serosa. The lesion is resected by laparoscopy. A representative H&E stained slide is shown. The cyst lining cells are positive for cytokeratin AE1 / AE3, calretinin and WT1 and negative for MOC31. The lesion is extensively sampled and no infiltrative growth is identified. Which of following is true?
- ~70% of such lesions harbor BAP1 mutations
- Complex copy number alterations are characteristic of such lesions
- Excision is curative; no clinical follow up is required
- Local recurrence affects ~50% of patients
- Men are affected ~4 times as often as women
Board review style answer #1
D. Local recurrence affects ~50% of patients. This is a multiloculated peritoneal inclusion cyst. The published literature and WHO blue book indicate a local recurrence rate as high as 50% (answer D). Answer A is incorrect as peritoneal inclusion cysts lack BAP1 mutations; rather, ~70% of peritoneal mesotheliomas harbor BAP1 mutations. Answer B is incorrect because peritoneal inclusion cysts lack recurrent copy number alterations. The molecular underpinnings of these lesions remain uncertain. Answer C is incorrect as the potential for local recurrence (particularly in larger multiloculated lesions) warrants clinical follow up. Answer E is incorrect as peritoneal inclusion cysts predominantly affect women, not men.
Comment Here
Reference: Peritoneal inclusion cyst
Comment Here
Reference: Peritoneal inclusion cyst
Board review style question #2
Which of the following statements regarding peritoneal inclusion cysts is true?
- ~20% of patients progress to malignant mesothelioma over 10 years
- 40 - 50% of lesions harbor homozygous deletion of CDKN2A
- > 90% of lesions express estrogen receptor and progesterone receptor
- Destructive infiltration of underlying structures (e.g., omental fat) is seen in ~33% of cases and does not affect prognosis
- Surgical excision is considered the mainstay of treatment
Board review style answer #2
E. Surgical excision is considered the mainstay of treatment. Answer A is incorrect. Although the literature contains rare reports of apparent progression from peritoneal inclusion cyst to malignant mesothelioma, the rate of progression is much lower than 20%. Answer B is incorrect as peritoneal inclusion cysts lack CDKN2A deletions. Instead, ~40 - 50% of pleural mesotheliomas harbor CDKN2A deletion. Answer C is incorrect as ER and PR expression are rarely seen in peritoneal inclusion cysts. Answer D is incorrect as peritoneal inclusion cysts lack destructive invasion of underlying tissues. The presence of destructive invasion is consistent with a diagnosis of mesothelioma.
Comment Here
Reference: Peritoneal inclusion cyst
Comment Here
Reference: Peritoneal inclusion cyst
Pleural effusion
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Pleural effusion is an excessive buildup of fluid between the layers of the pleura outside the lungs
Essential features
- The most common causes of pleural effusion are congestive heart failure, cancer, pneumonia and pulmonary embolism
- Pleural fluid is classified as a transudate or exudate based on modified Light’s criteria
- The International System for Serous Fluid Cytopathology standardized the way to report cytopathology diagnoses of serous fluids, which includes 5 different categories: nondiagnostic (ND), negative for malignancy (NFM), atypia of undetermined significance (AUS), suspicious for malignancy (SFM) and malignant (MAL)
- Treatment is based on the underlying condition and whether the effusion is causing severe respiratory symptoms
Terminology
Not relevant
ICD coding
Epidemiology
- An estimated 1.5 million patients in the U.S. experience pleural effusion each year (JAMA Netw Open 2021;4:e2120306)
- Age of pleural effusion varies depending on the underlying cause
Sites
- Pleura
Pathophysiology
- A pleural effusion represents a disturbance of equilibrium between production and resorption of pleural fluid (Dtsch Arztebl Int 2019;116:377)
Etiology
- There are > 50 causes of pleural effusion; the most common are congestive heart failure, cancer, pneumonia and pulmonary embolism (Arch Bronconeumol 2014;50:161)
Diagrams / tables
Clinical features
- Patients can be asymptomatic or can present with cough, dyspnea and pleuritic chest pain (Am Fam Physician 2014;90:99)
- Depending on the cause, the fluid may be either transudative or exudative
- Pleural fluid is classified as a transudate or exudate based on modified Light’s criteria; pleural fluid is considered an exudative effusion if at least 1 of the criteria is met
- Pleural fluid protein / serum protein ratio of > 0.5
- Pleural fluid lactate dehydrogenase (LDH) / serum LDH ratio of > 0.6
- Pleural fluid LDH is > 66% of the upper limits of normal laboratory value for serum LDH
Diagnosis
- A detailed medical history should be obtained from all patients presenting with a pleural effusion, as this may help to establish the etiology
- Physical examination includes inspection, palpation, percussion and auscultation (Cleve Clin J Med 2008;75:297)
- Thoracentesis is a procedure for aspirating fluid from the pleural space by percutaneous insertion of a needle through the chest wall with or without the insertion of a catheter; it can be ultrasound guided (J Hosp Med 2018;13:126)
Laboratory
- For distinguishing transudates from exudates, serum and pleural fluid levels of protein and LDH are taken
- Pleural fluid drainage is recommended if the pH is < 7.20 (2,3); the equivalent glucose level considered low is < 3.30 mmol/L (60 mg/dL) (J Thorac Dis 2023;15:2885)
- Amylase rich pleural effusion (ARPE) is defined as either a pleural amylase greater than the upper limit of serum amylase or a pleural fluid to serum amylase ratio of > 1 (J R Coll Physicians Edinb 2010;40:119)
- B type natriuretic peptide (BNP), N terminal proBNP (NTproBNP) and Mid regional pro atrial NP (MRproANP), either in blood or pleural effusion, are effective tools for diagnosis of heart failure (PLoS One 2015;10:e0134376)
Radiology description
- Chest radiographs: a posteroanterior view reveals effusions of volume 200 mL or larger, a lateral view shows effusions of volume 50 mL or larger
- Radiographic signs
- Meniscus sign on a frontal radiograph (> 200 mL)
- Blunting of costophrenic sulcus on lateral view (> 50 mL)
- Subpulmonic effusion: lateral displacement of the apex of the pseudodiaphragm
- Ultrasound allows the detection of small amounts of pleural locular fluid, with positive identification of amounts as small as 3 - 5 mL
- According to the characteristics of the pleural effusion on ultrasound, it can appear as anechoic (black), complex nonseptated (black with white strands), complex septated (black with white septa) or homogeneously echogenic (white) (J Bras Pneumol 2014;40:1)
- On chest computed tomography (CT), free flowing fluid will collect initially in deep lateral and posterior pleural recesses (Respir Med 2017:124:88)
Prognostic factors
Not relevant
Case reports
- 7 year old Turkish girl presented to a local hospital with fever, chest pain and vomiting (J Med Case Rep 2019;13:344)
- 30 year old man presented with complaints of low grade, intermittent fever for 2 years, right sided chest pain for 2 months and weight loss (Trop Doct 2021;51:111)
- 33 year old man with a history of sudden onset, pleuritic, right sided chest pain of 2 days duration (Breathe (Sheff) 2017;13:e46)
Treatment
- Treatment is based on the underlying condition and whether the effusion is causing severe respiratory symptoms
Clinical images
Not relevant
Gross description
Not relevant
Gross images
Not relevant
Frozen section description
Not relevant
Frozen section images
Not relevant
Microscopic (histologic) description
Not relevant
Microscopic (histologic) images
Not relevant
Cytology description
- For cytology, different cytological preparation methods can be used, including conventional direct smear, cytospin, liquid based preparations and cell blocks, each with its own strengths and limitations (Am J Cancer Res 2021;11:43)
- Differential count of pleural fluid white blood cells (WBCs) aids differentiation of the causal diseases
- It is determined by counting 100 cells under a light microscope (400x)
- Lymphocytes, neutrophils, histiocytes, eosinophils, mesothelial cells, malignant cells and atypical cells are differentiated (World J Surg Oncol 2021;19:180)
- The International System for Reporting Serous Fluid Cytopathology standardized the way to report cytopathology diagnoses of serous fluids, which includes 5 different categories: nondiagnostic (ND), negative for malignancy (NFM), atypia of undetermined significance (AUS), suspicious for malignancy (SFM) and malignant (MAL) (see International system for reporting serous fluid cytopathology)
Cytology images
Contributed by Gheorghe-Emilian Olteanu, M.D., Ph.D.
Immunofluorescence description
Not relevant
Immunofluorescence images
Not relevant
Positive stains
- The selection of an appropriate panel of immunostains is dependent upon the clinical profile of the patient, the site of effusion, imaging findings and serum tumor markers (Cytopathology 2022;33:678, Surg Pathol Clin 2018;11:523)
Negative stains
Not relevant
Electron microscopy description
Not relevant
Electron microscopy images
Not relevant
Molecular / cytogenetics description
Not relevant
Molecular / cytogenetics images
Not relevant
Videos
Pleural effusion causes, signs and symptoms, diagnosis and treatment
Pleural effusion: explanation of Xray findings
The international system of serous effusion cytopathology
Sample pathology report
- International system for reporting serous fluid cytopathology was an effort to standardize terminology with standardized cytomorphologic criteria for reporting pleural, peritoneal and pericardial effusion (see International system for reporting serous fluid cytopathology)
Differential diagnosis
Not relevant
Additional references
Not relevant
Board review style question #1
The distinction between a transudate and an exudate is based on Light's criteria and is determinative for the evaluation and treatment of a pleural effusion. What parameter(s) among the listed is / are the Light's criteria for drawing this distinction?
- Amylase in the effusion fluid
- NTproBNP in the effusion fluid
- pH level in the effusion fluid
- Protein and LDH in the effusion fluid and in the serum
Board review style answer #1
D. Protein and LDH in the effusion fluid and in the serum. The difference between transudate and exudate are based on modified Light's criteria, which includes: pleural fluid protein / serum protein ratio of > 0.5; pleural fluid lactate dehydrogenase (LDH) / serum LDH ratio of > 0.6; pleural fluid LDH is > 66% of the upper limits of normal laboratory value for serum LDH. Answers A, B and C are incorrect because analyzing amylase, NTproBNP and pH level in effusion fluid can help in detecting the cause of pleural effusion but these do not help in differentiating between transudate and exudate.
Comment Here
Reference: Pleural effusion
Comment Here
Reference: Pleural effusion
Board review style question #2
Regarding pleural effusion, which of the following is true?
- Pleural fluid is classified as a transudate or exudate based on Bethesda criteria
- The International System for Reporting Serous Fluid Cytopathology standardized the way to report cytopathology diagnosis of serous fluids, which includes 5 different categories
- Treatment is the same, regardless of the underlying condition and whether the effusion is causing respiratory symptoms
- Uncommon causes of pleural effusion are congestive heart failure and cancer
Board review style answer #2
B. The International System for Reporting Serous Fluid Cytopathology standardized the way to report cytopathology diagnosis of serous fluids, which includes 5 different categories: nondiagnostic (ND), negative for malignancy (NFM), atypia of undetermined significance (AUS), suspicious for malignancy (SFM) and malignant (MAL). Answer D is incorrect because the most common causes of pleural effusion are congestive heart failure, cancer, pneumonia and pulmonary embolism. Answer A is incorrect because pleural fluid is classified as a transudate or exudate based on modified Light’s criteria. Answer C is incorrect because treatment is based on the underlying condition and whether the effusion is causing severe respiratory symptoms.
Comment Here
Reference: Pleural effusion
Comment Here
Reference: Pleural effusion
Pleural effusion
Table of Contents
Definition / generalDefinition / general
- 15 cc or more of fluid within pleural cavities
- Most common causes are congestive heart failure and metastatic disease
- Due to increased hydrostatic pressure (congestive heart failure), increased vascular permeability (pneumonia), decreased oncotic pressure (nephrotic syndrome), increased intrapleural negative pressure (atalectasis), decreased lymphatic drainage (carcinomatosis)
- May or may not be inflammatory
- Malignant effusions are usually greater than 500 ml; are often first evidence of malignancy
- For lung, breast and ovarian metastases, 92% of pleural effusions are ipsilateral to primary lesion
Inflammatory pleural effusion:
- Either serous, serofibrinous or fibrinous
- Due to inflammation in lung (tuberculosis, pneumonia, infarct, abscess, bronchiectasis), collagen vascular disease (rheumatoid arthritis, systemic lupus erythematosis, uremia) or radiation therapy
- Empyema: pus in pleural cavity; due to bacteria or fungal seeding of pleural space; often from lung infection; usually organizes into dense adhesions but does not disappear
Noninflammatory pleural effusion:
- Hydrothorax: clear / straw colored fluid; usually due to congestive heart failure
- Isolated right sided hydrothorax seen in Meigs syndrome (hydrothorax, ascites, ovarian fibroma)
- Usually is NOT loculated, collects basally
- Hemothorax: usually due to rupture of aneurysm or vascular trauma; associated with large clots
- Chylothorax: accumulation of milky white fluid, usually lymph, contains fat (DD: turbid serous fluid); usually left sided, caused by thoracic duct trauma / obstruction due to malignancy
Pleural plaques
Table of Contents
Definition / general | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) imagesDefinition / general
- Due to chronic injury of pleural surface
- Do not contain asbestos bodies but rare if no asbestos history (may be from other pneumoconiosis)
- May induce pleural effusions but usually no symptoms
Gross description
- On parietal pleura near vertebral column, lower lung, dome of diaphragm
Gross images
Microscopic (histologic) description
- Well circumscribed plaques of dense, hyalinized, acellular collagen, may have with basketweave morphology, often calcified
Microscopic (histologic) images
Pleuritis
Definition / general
- May be secondary to lung disease, hematoma
- Fibrosis may cause pleural symphysis (fusion of visceral and parietal layers)
- Thickened pleura may prevent lung expansion; treat with decortication
- Hemorrhagic pleuritis: rare, associated with hemorrhage, Rickettsiae infection, tumor
Microscopic (histologic) description
- Thickened pleura is composed of fibrous tissue without elastic fibers
Negative stains
- Telomerase reverse transcriptase (Am J Surg Pathol 2002;26:365)
Pneumothorax
Table of Contents
Definition / generalDefinition / general
- All types may cause compression, collapse, atelectasis of lung and associated respiratory distress
- Either spontaneous, traumatic or therapeutic
- Spontaneous: due to abscess, tuberculosis, emphysema, asthma
- Traumatic: due to puncture of chest wall or lung
- Therapeutic: formerly used to treat tuberculosis
- Spontaneous idiopathic: young people, rupture of small, peripheral, apical subpleural blebs, usually subsides spontaneously but may recur and be disabling
- Tension pneumothorax: acts as one way valve to trap air during inhalation; may compress contralateral lung, mediastinal structures
Staging
Table of Contents
Definition / general | Essential features | ICD coding | Diagrams / tables | Clinical features | Primary tumor (pT) | Regional lymph nodes (pN) | Distant metastasis (pM) | Prefixes | AJCC prognostic stage groups | Registry data collection variables | Histologic grade (G) | Histopathologic type | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- All diffuse malignant pleural mesothelioma are covered by this staging system
- This staging system does not apply to localized malignant pleural mesothelioma, solitary fibrous tumor, peritoneal mesothelioma, lymphoma or sarcoma
- Clinical staging relies primarily on imaging, most frequently CT, sometimes FDG PET / CT and MRI
- Pathological staging is based on surgical resection
- T category staging is descriptive without a size criterion
- Other staging systems also exist (Brigham staging, Butchart staging) (Semin Thorac Cardiovasc Surg 1997;9:356)
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- T, N and M categories predict outcome of patients with malignant pleural mesothelioma
- Tumor histology (epithelioid versus sarcomatoid) is an important prognostic factor beyond TNM
Clinical features
- The most frequent sites of metastatic disease are the contralateral pleura and lung, peritoneum, extrathoracic lymph nodes, bones and liver
- Tumor histology is an important prognostic factor: epithelioid tumors have a better prognosis than biphasic or sarcomatoid tumors, while desmoplastic malignant mesothelioma has the worst prognosis (J Thorac Oncol 2012;7:1631)
- Major variables from the largest international database (J Thorac Oncol 2012;7:1631):
- Median age: 63 years; 79% men, 62% epithelioid tumor
- Stage I (11%), stage II, (21%), stage III (48%) and stage IV (20%)
- Median survival by TNM stage: 21 months (stage I), 19 months (stage II), 16 months (stage III) and 12 months (stage IV)
- Median survival by histology: epithelioid 19 months, biphasic 13 months and sarcomatoid 8 months
Primary tumor (pT)
Regional lymph nodes (pN)
- pNX: cannot be assessed
- pN0: no regional lymph node metastasis
- pN1: metastases in the ipsilateral bronchopulmonary hilar or mediastinal (including the internal mammary, peridiaphragmatic, pericardial fat pad or intercostal) lymph nodes
- pN2: metastases in the contralateral mediastinal, ipsilateral or contralateral supraclavicular lymph nodes
Distant metastasis (pM)
- pM0: no distant metastasis
- pM1: distant metastasis
Prefixes
- y: preoperative radiotherapy or chemotherapy
- r: recurrent tumor stage
AJCC prognostic stage groups
| Stage group IA: | T1 | N0 | M0 |
| Stage group IB: | T2 - 3 | N0 | M0 |
| Stage group II: | T1 - 2 | N1 | M0 |
| Stage group IIIA: | T3 | N1 | M0 |
| Stage group IIIB: | T1 - 3 | N2 | M0 |
| T4 | any N | M0 | |
| Stage group IV: | any T | any N | M1 |
Registry data collection variables
- Histologic type
- Age, sex
- Performance status
- Laboratory parameters (white blood cells, platelet count and hemoglobin)
- Surgical resection with curative intent (pleurectomy / decortications, extended pleurectomy / decortications or extrapleural pneumonectomy)
- For patients undergoing multimodality therapy, use of chemotherapy or radiotherapy
- Comment: these are supplementary factors recommended for clinical care owing to their prognostic significance (J Thorac Oncol 2014;9:856)
Histologic grade (G)
- GX: cannot be assessed
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
- G4: undifferentiated
Histopathologic type
- Epithelioid
- Biphasic (at least 10% of epithelioid and sarcomatoid)
- Sarcomatoid
- Desmoplastic
Board review style question #1
A 59 year old man presented with a 2 cm malignant pleural tumor with focal extension to the underlying lung parenchyma. Biopsy revealed well differentiated epithelioid malignant pleural mesothelioma confirmed by BAP1 immunostaining. What is the pT category using the AJCC / TNM 8th edition?
- pTX
- pT1
- pT2
- pT3
- pT4
Board review style answer #1
C. pT2. Extension of malignant mesothelioma from visceral pleura into the underlying pulmonary parenchyma qualifies as the pT2 category.
Comment Here
Reference: Pleura - Staging
Comment Here
Reference: Pleura - Staging
Board review style question #2
Which of the following is a major prognostic factor of malignant mesothelioma (other than TNM variables)?
- Duration of asbestos exposure
- Family history
- Genetic / mutation signature
- PDL1 expression by microenvironment
- Tumor histology
Board review style answer #2
E.
Tumor histology is the major prognostic factor beyond TNM (epithelioid tumors have better prognosis than sarcomatoid).
Comment Here
Reference: Pleura - Staging
Comment Here
Reference: Pleura - Staging
Well differentiated papillary mesothelial tumor (peritoneum)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Well differentiated papillary mesothelioma (WDPM) is a neoplastic mesothelial proliferation of low malignant potential, which predominantly involves the peritoneum of young women
Essential features
- Most cases diagnosed incidentally during surgery for an unrelated indication
- Composed of branching papillae lined by a single layer of bland cuboidal mesothelial cells
- Invasion of underlying pre-existing tissues (e.g. fat, muscle, ovarian stroma) precludes the diagnosis (→ diagnosis of malignant mesothelioma)
- BAP1 loss and CDKN2A (p16) homozygous deletion preclude the diagnosis (→ diagnosis of malignant mesothelioma)
Terminology
- Localized mesothelioma (confusing terminology; use not recommended) (Am J Surg Pathol 1995;19:1124)
ICD coding
- ICD-10: D19.9 - Benign neoplasm of mesothelial tissue, unspecified
Epidemiology
- F:M = ~5:1
- Median age at diagnosis: ~40 years (range 7 - 75)
Sites
- Peritoneum is most commonly affected site
- Rare cases involve the pleura or tunica vaginalis (Hum Pathol 2019;92:48, Am J Surg Pathol 2004;28:534)
- Cases in the pleura or tunica vaginalis more evenly distributed between men and women
- Pleural cases diagnosed at older age (median ~60 years)
- Pleural cases have higher rate of asbestos exposure (~50%)
Pathophysiology
- Molecularly distinct from epithelioid malignant peritoneal mesothelioma (Cancers (Basel) 2020;12:E1568)
Etiology
- Asbestos exposure reported in only ~1% (causality not established)
Clinical features
- Most cases (> 90%) incidentally identified during abdominal surgery for an unrelated cause
- Rare florid cases (< 10%) present with abdominal pain, weight loss or ascites
Diagnosis
- Tissue sampling followed by microscopic evaluation
Radiology description
- In patients with florid or symptomatic disease, computed tomography (CT) may show ascites or thickening of the bowel wall or mesentery
Prognostic factors
- Most cases follow a benign clinical course
- Recurrence rate in morphologically classical cases is low (< 5%)
- Recurrence rate reportedly higher in cases with invasive foci in papillary cores and with higher disease burden (Am J Surg Pathol 2014;38:990, Eur J Surg Oncol 2019;45:371)
- Fatal outcomes rare
- Progression to malignant peritoneal mesothelioma is exceptionally rare (< 5%) and causality is unclear (may occur years to decades later)
Case reports
- 28 year old woman with a large peritoneal tumor, presenting with ascites (J Lab Physicians 2018;10:248)
- 48 year old man with a tumor in an inguinal hernia (Indian J Pathol Microbiol 2012;55:546)
Treatment
- Observation
- Surgical cytoreduction and hyperthermic intraperitoneal chemotherapy performed in some cases with diffuse, symptomatic or recurrent disease (Ann Surg Oncol 2019;26:852)
- Randomized clinical trials not available
Gross description
- Discrete nodular implants
- Papillary fronds macroscopically evident in some (but not all) cases
- Individual implants usually 0.2 - 2.0 cm but may be up to several centimeters (Am J Surg Pathol 2012;36:117)
- Approximately 50% of cases are unifocal and 50% are multifocal (Ann Diagn Pathol 2019;38:43)
- Ovarian or fallopian tube surface involved in a minority of cases
Gross images
Frozen section description
- Papillary architecture (Am J Surg Pathol 2012;36:117)
- Single nonstratified layer of low cuboidal cells with bland cytomorphology
- No solid growth
- No invasion of underlying tissues
- Correlate with surgical findings, re: extent of peritoneal involvement
Frozen section images
- See Virtual slides
Microscopic (histologic) description
- Branching plump papillae lined by a single layer of bland cuboidal cells (Am J Surg Pathol 2012;36:117, Ann Diagn Pathol 2019;38:43, Histopathology 2013;62:805)
- Minimal cytologic atypia; single small nucleoli may be present
- Mitoses usually absent (or at most 1 mitosis per 10 high power fields)
- Papillary cores may show myxoid change, edema, psammomatous calcifications, fibrosis, foamy macrophages or chronic inflammation
- Invasion of underlying pre-existing tissues is absent
- Unusual patterns
- Some cases associated with a discrete adenomatoid tumor or multicystic mesothelioma (no apparent effect on prognosis)
- Invasive foci composed of tubules, single cells, small solid nests or cords within papillary cores (see Prognostic factors)
Microscopic (histologic) images
Virtual slides
Positive stains
- Broad spectrum cytokeratin (100%)
- Calretinin (virtually 100%) (cytoplasmic + nuclear staining is most specific)
- WT1 (virtually 100%)
- D2-40 (virtually 100%)
- Cytokeratin 5/6 (~90%)
- PAX8 (60 - 95% show at least partial staining) (Hum Pathol 2018;72:160, Ann Diagn Pathol 2019;38:43)
- Ki67 < 5%
- BAP1 retained in virtually all cases (loss strongly suggests malignant mesothelioma with WDPM-like features) (Hum Pathol 2018;79:168)
Negative stains
Electron microscopy description
- Mesothelial cells lining papillary cores show (Am J Surg Pathol 2001;25:1304):
- Long slender microvilli (length to diameter ratio > 10)
- Abundant mitochondria and rough endoplasmic reticulum
- Well formed desmosomes
Molecular / cytogenetics description
- All cases appear to harbor mutation in TRAF7 or CDC42 (Mod Pathol 2019;32:88)
- TRAF7 mutations also found in adenomatoid tumors, suggesting biologic connection
- BAP1 mutation or deletion exceptionally rare
- Lack homozygous CDKN2A (p16) deletion
- Abnormal karyotypes seen in a subset (Am J Surg Pathol 2014;38:990)
Sample pathology report
- Peritoneal nodule, biopsy:
- Well differentiated papillary mesothelioma (0.6 cm) (see comment)
- Comment: Microscopic examination shows branching papillae lined by bland cuboidal mesothelial cells. No mitoses are seen. These morphologic features are consistent with well differentiated papillary mesothelioma. Small unifocal lesions are generally regarded as benign, although recurrences are reported in rare cases. Clinical correlation is advised with appropriate follow up to exclude regrowth or recurrence.
- Peritoneal lesion, excision:
- Well differentiated papillary mesothelioma (2.0 cm) with invasive foci (see comment)
- Comment: Microscopic examination reveals a bland mesothelial lesion with features of well differentiated papillary mesothelioma, including branching papillae lined by a single layer of bland cuboidal mesothelial cells. However, the papillary cores are infiltrated in few foci by nests and cords of bland mesothelial cells. There is no invasion of the underlying peritoneal fibroadipose tissue. Such lesions have been termed "well differentiated papillary mesothelioma with invasive foci" and there is evidence suggesting that such lesions have a higher rate of recurrence compared with banal well differentiated papillary mesothelioma. Clinical and radiographic correlation is advised with appropriate follow up to exclude recurrence.
Differential diagnosis
- Diffuse malignant peritoneal mesothelioma:
- Diffuse involvement of the peritoneum
- Infiltration of underlying pre-existing tissue is typical
- At least moderate cytologic atypia in most cases
- Increased mitotic activity (> 1 mitosis per 10 high power fields is typical)
- Solid architecture may be present
- BAP1 loss frequent (~70%)
- May contain WDPM-like foci so thorough sampling is necessary
- Localized malignant peritoneal mesothelioma:
- Exceptionally rare diagnosis
- Localized disease (resembling WDPM)
- Complex papillary or solid architecture, identical to that seen in diffuse malignant peritoneal mesothelioma
- At least moderate cytologic atypia in most cases
- Increased mitotic activity (> 1 mitosis per 10 high power fields is typical)
- May contain WDPM-like foci so thorough sampling is necessary
- Reactive mesothelial hyperplasia:
- History of surgery, inflammatory disease or cardiovascular disease
- Reactive or inflammatory changes also seen in adjacent flat serosa
- Serous borderline tumor:
Additional references
Board review style question #1
The unifocal lesion depicted in the photomicrograph was discovered incidentally during an exploratory laparoscopy in a 38 year old woman with endometriosis. It measured 0.6 cm on gross examination. On immunohistochemical stains, lesional cells were positive for calretinin (shown above) but negative for MOC31. Which of the following is true about the depicted entity?
- Immunohistochemical loss of BAP1 is seen in ~70% of cases
- Men are more frequently affected than women
- Most cases are causally linked to asbestos exposure
- Most cases of this entity result in patient death within 10 years
- WT1 expression is seen in virtually 100% of cases
Board review style answer #1
E. WT1 expression is seen in virtually 100% of cases. This is a well differentiated papillary mesothelioma.
Comment Here
Reference: Well differentiated papillary mesothelioma
Comment Here
Reference: Well differentiated papillary mesothelioma
Board review style question #2
Which of the following molecular alterations is most frequently seen in well differentiated papillary mesothelioma of the peritoneum?
- BAP1 missense mutation
- CDKN2A homozygous deletion
- NF2 homozygous deletion
- TP53 missense mutation
- TRAF7 mutation
Board review style answer #2
Well differentiated papillary mesothelioma tumor-pleura (pending)
Table of Contents
Definition / generalDefinition / general
[Pending]
Recent Pleura & peritoneum Pathology books
Find related Pathology books: cardiovascular, gynecologic, lung, mediastinum/serosa