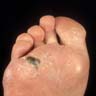
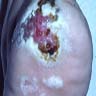
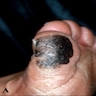
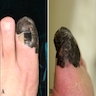
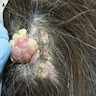
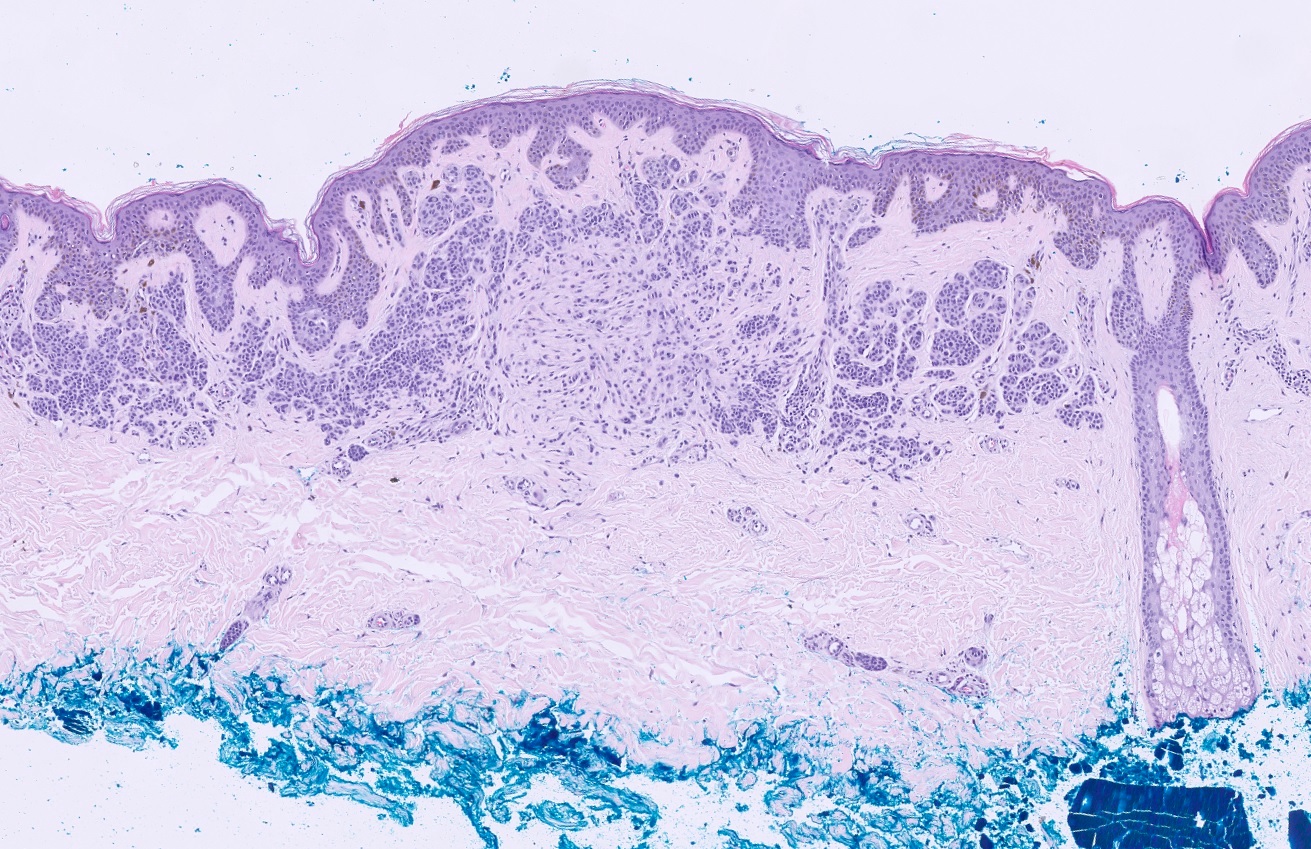
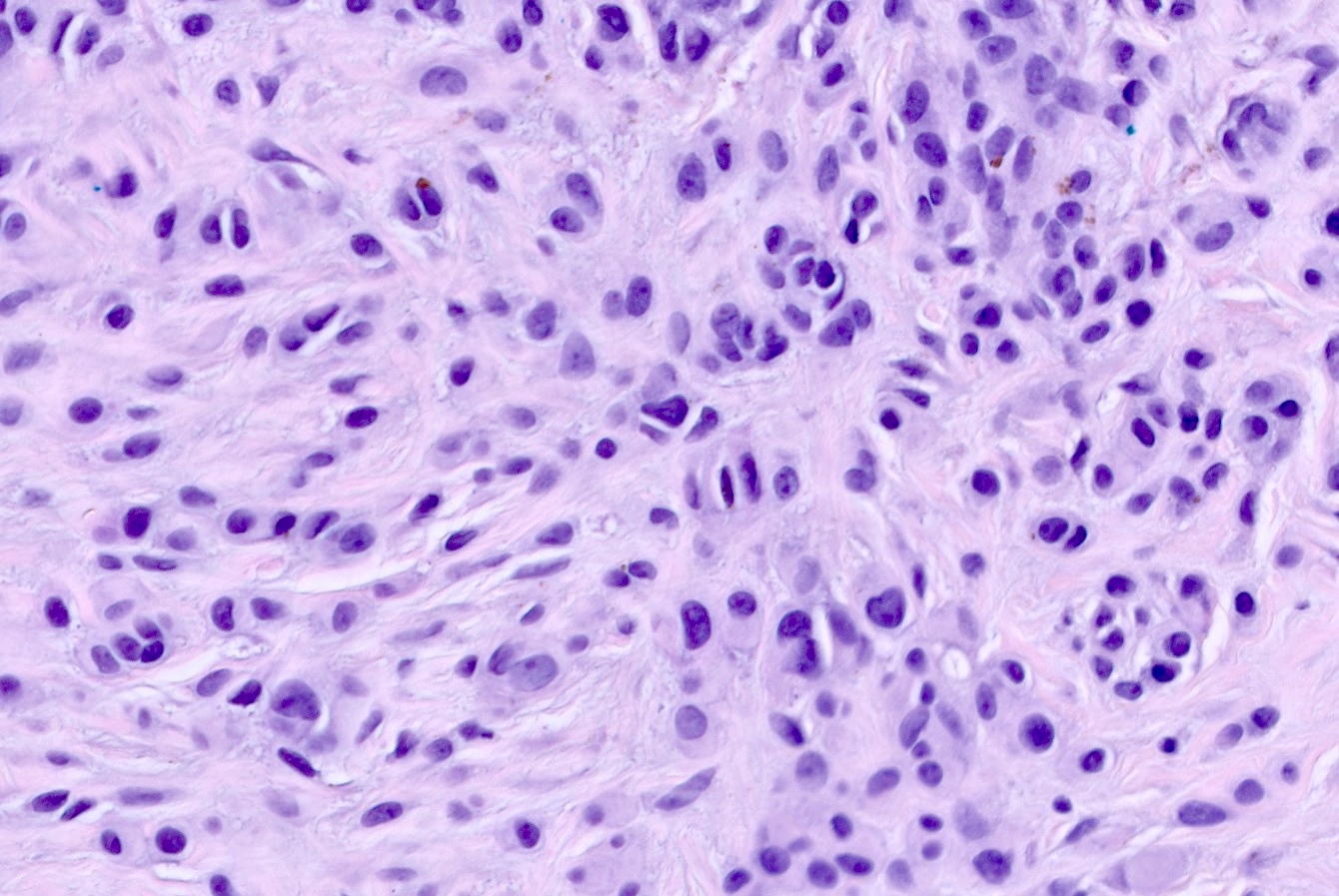
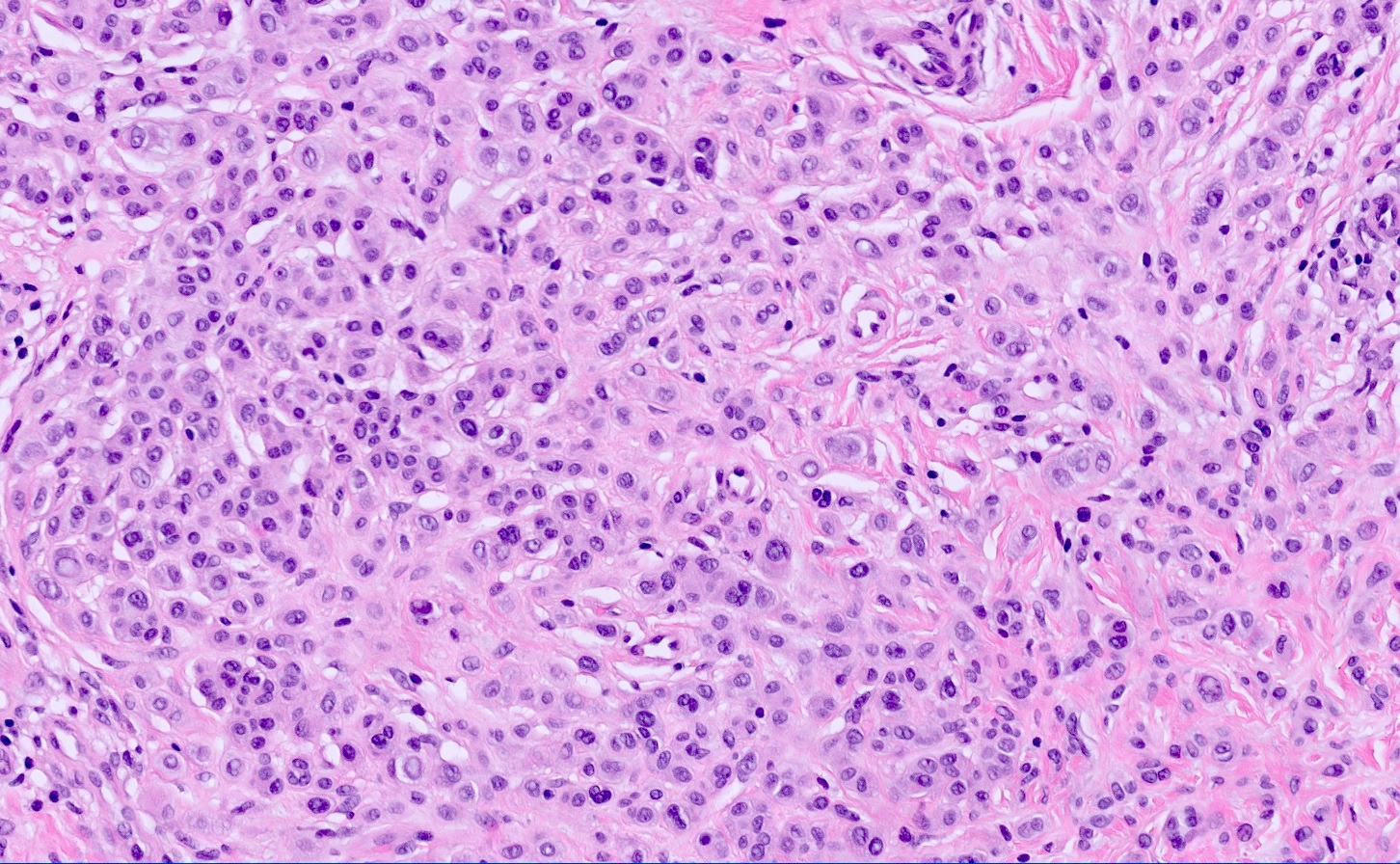
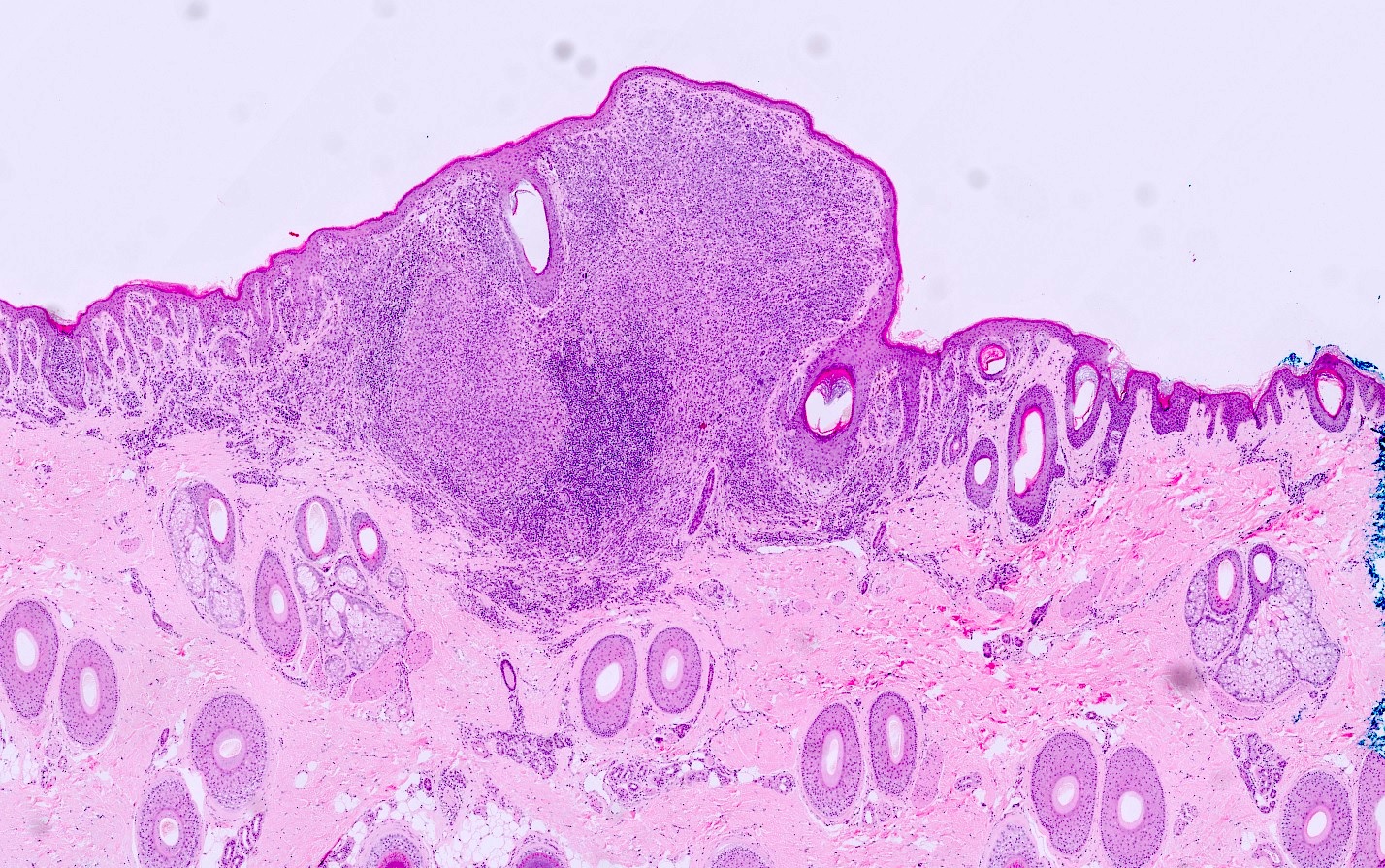
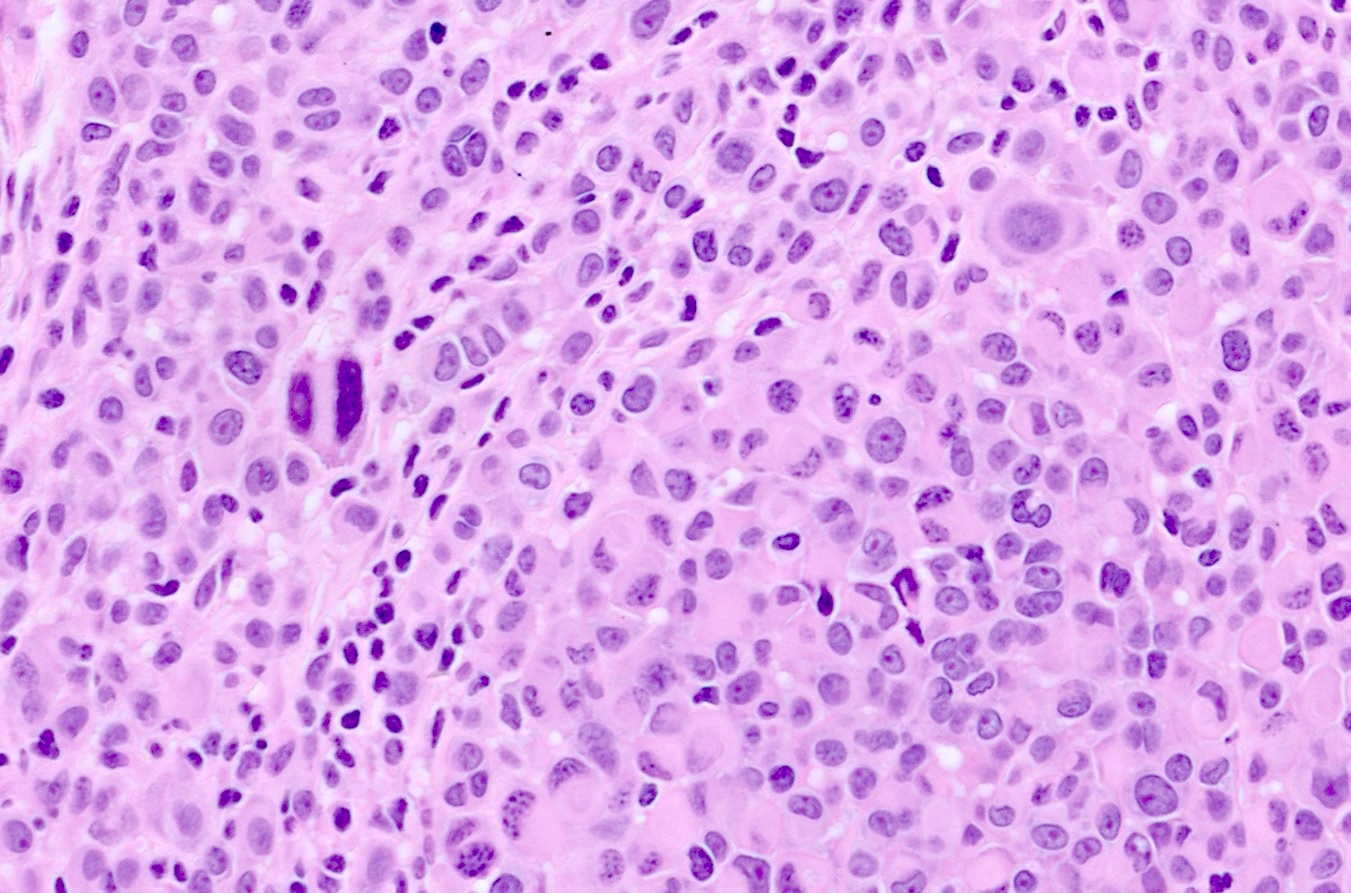
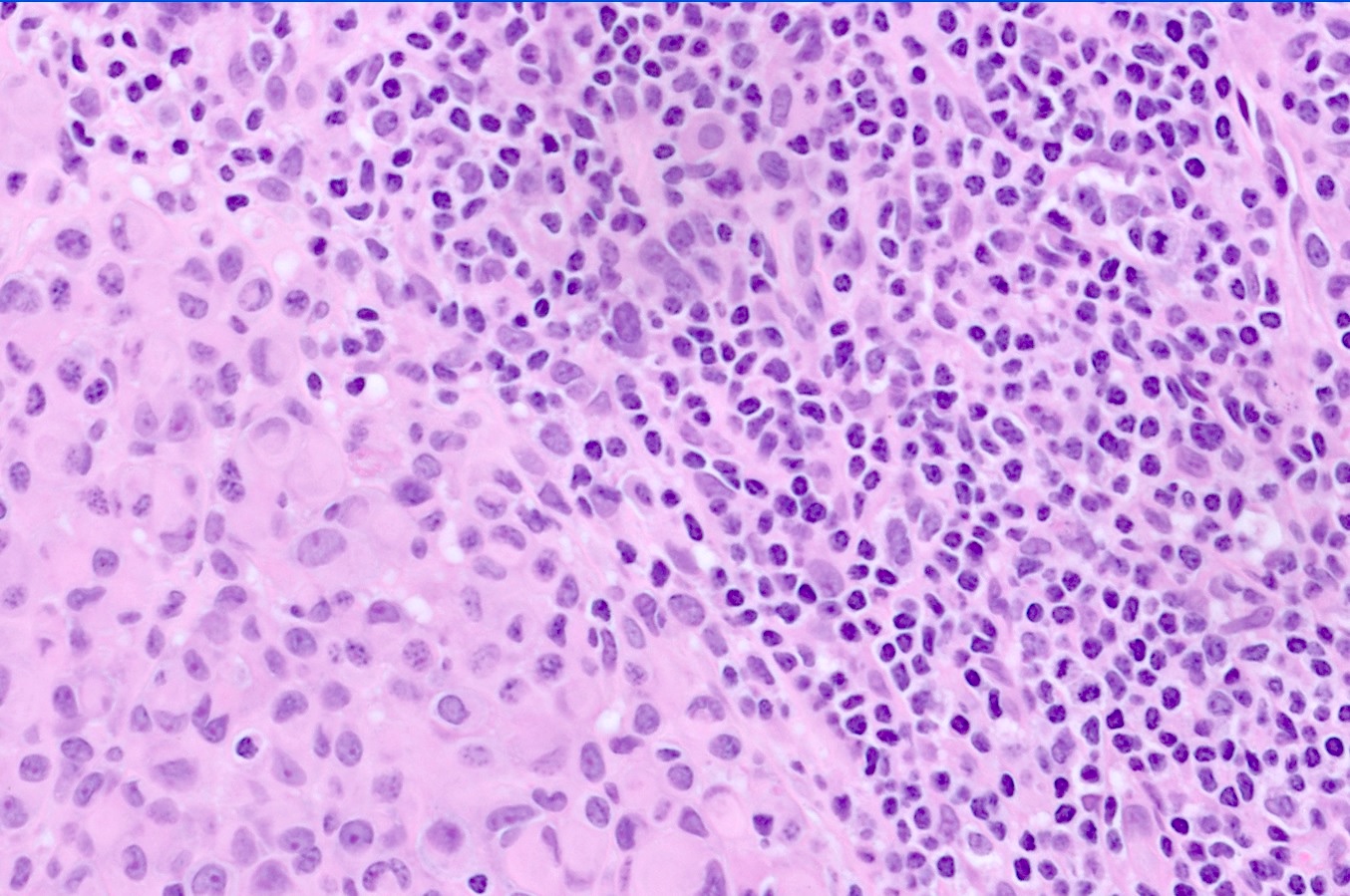
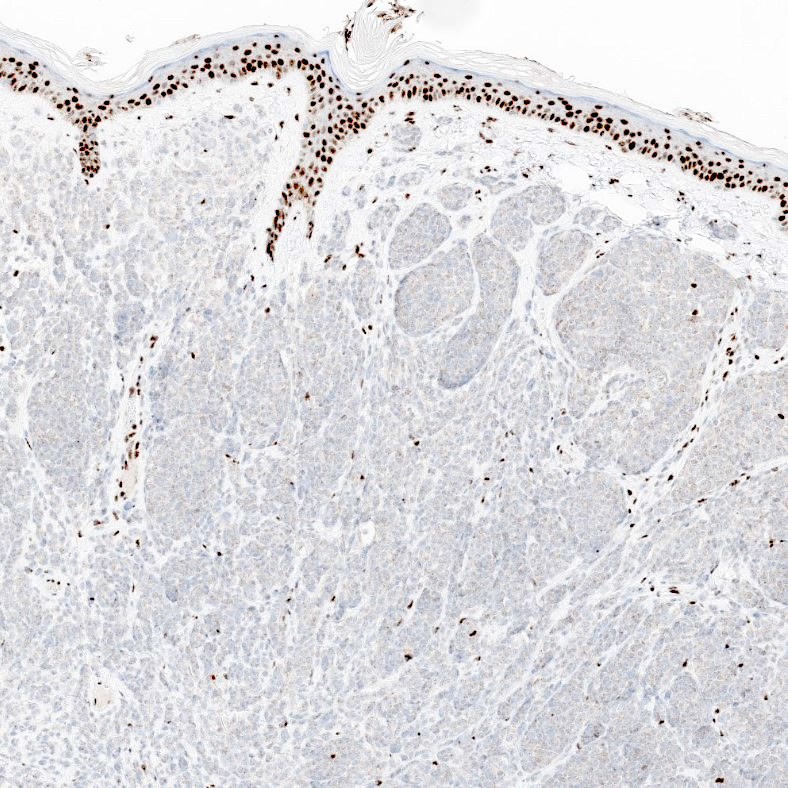
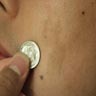
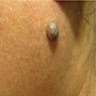
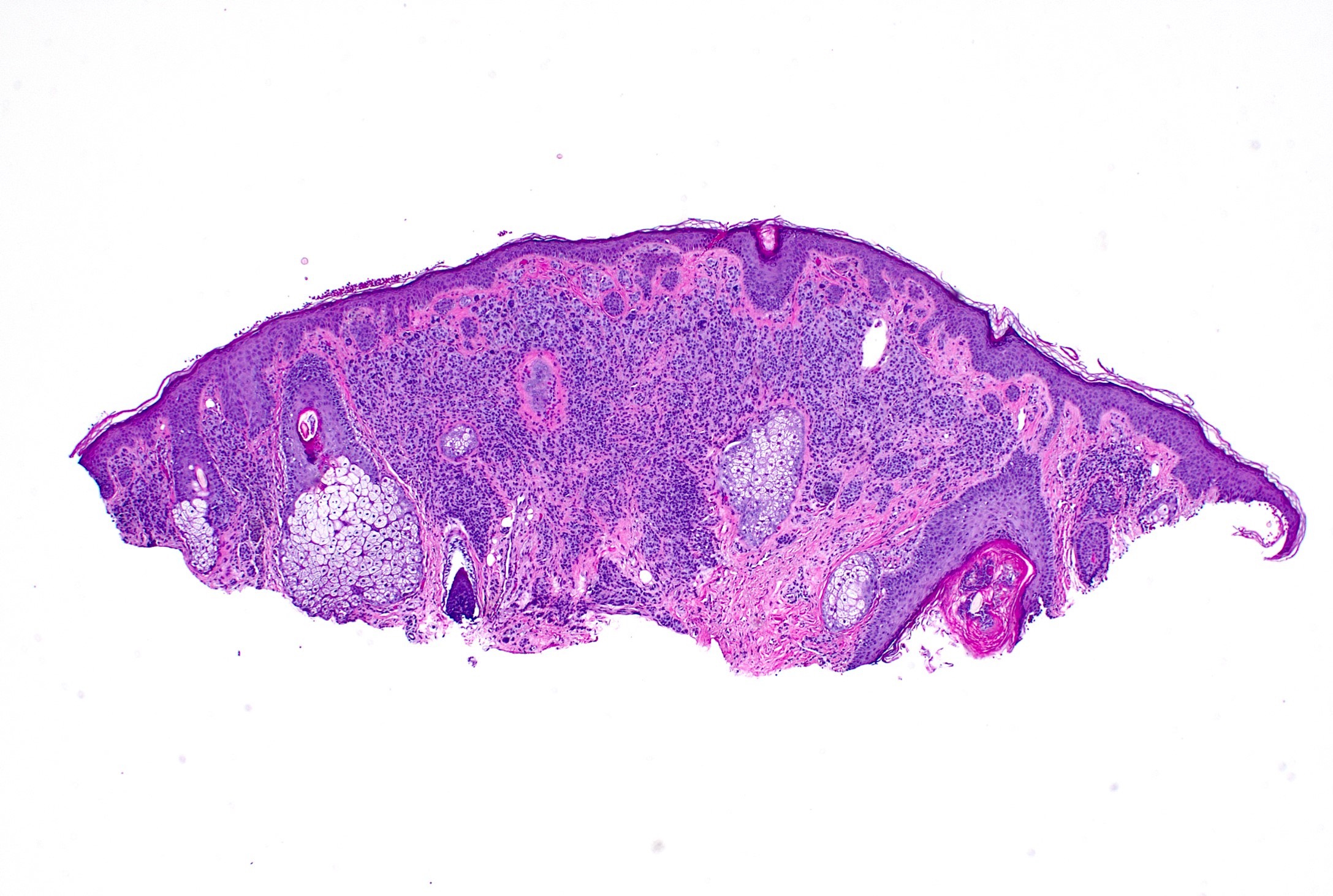
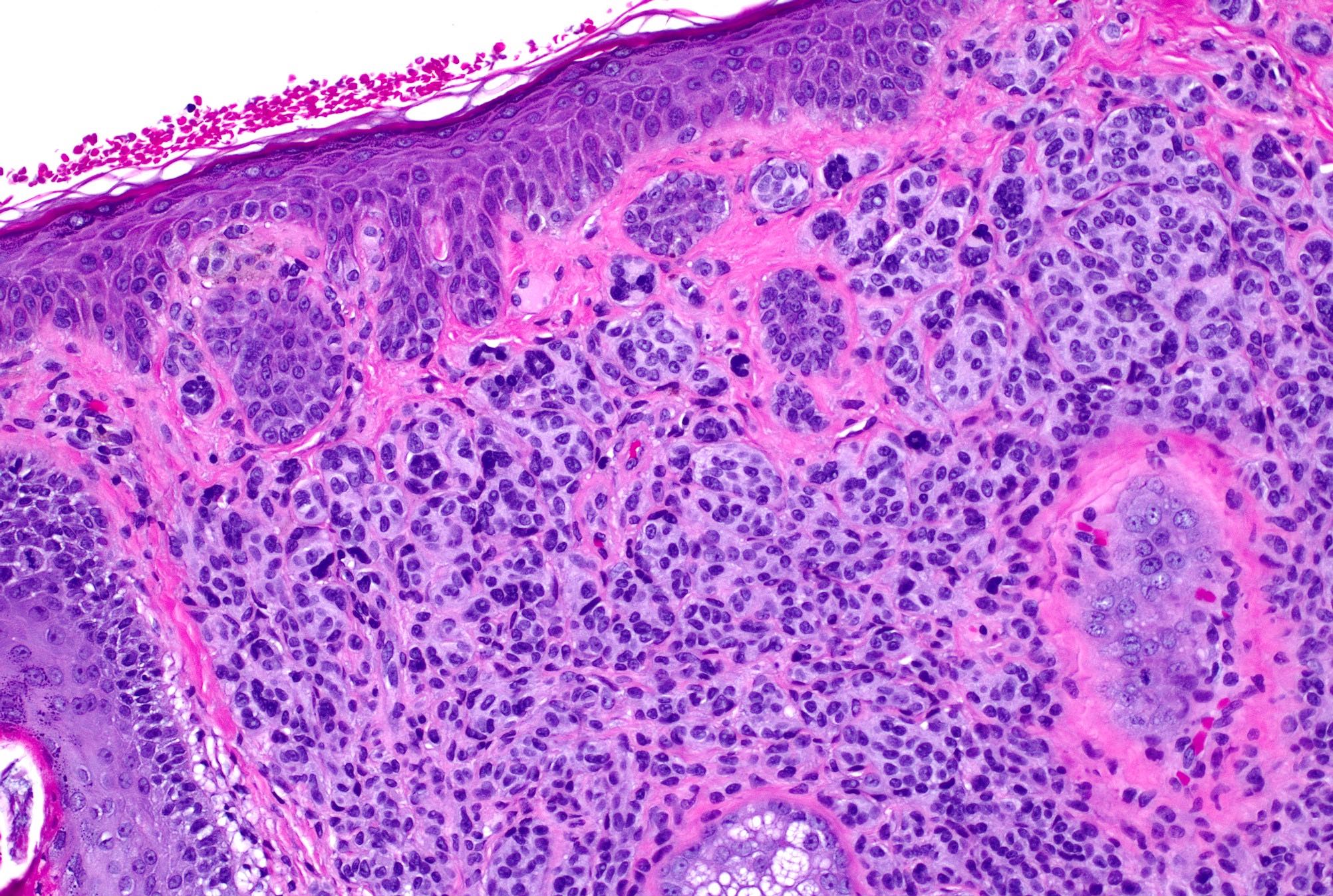
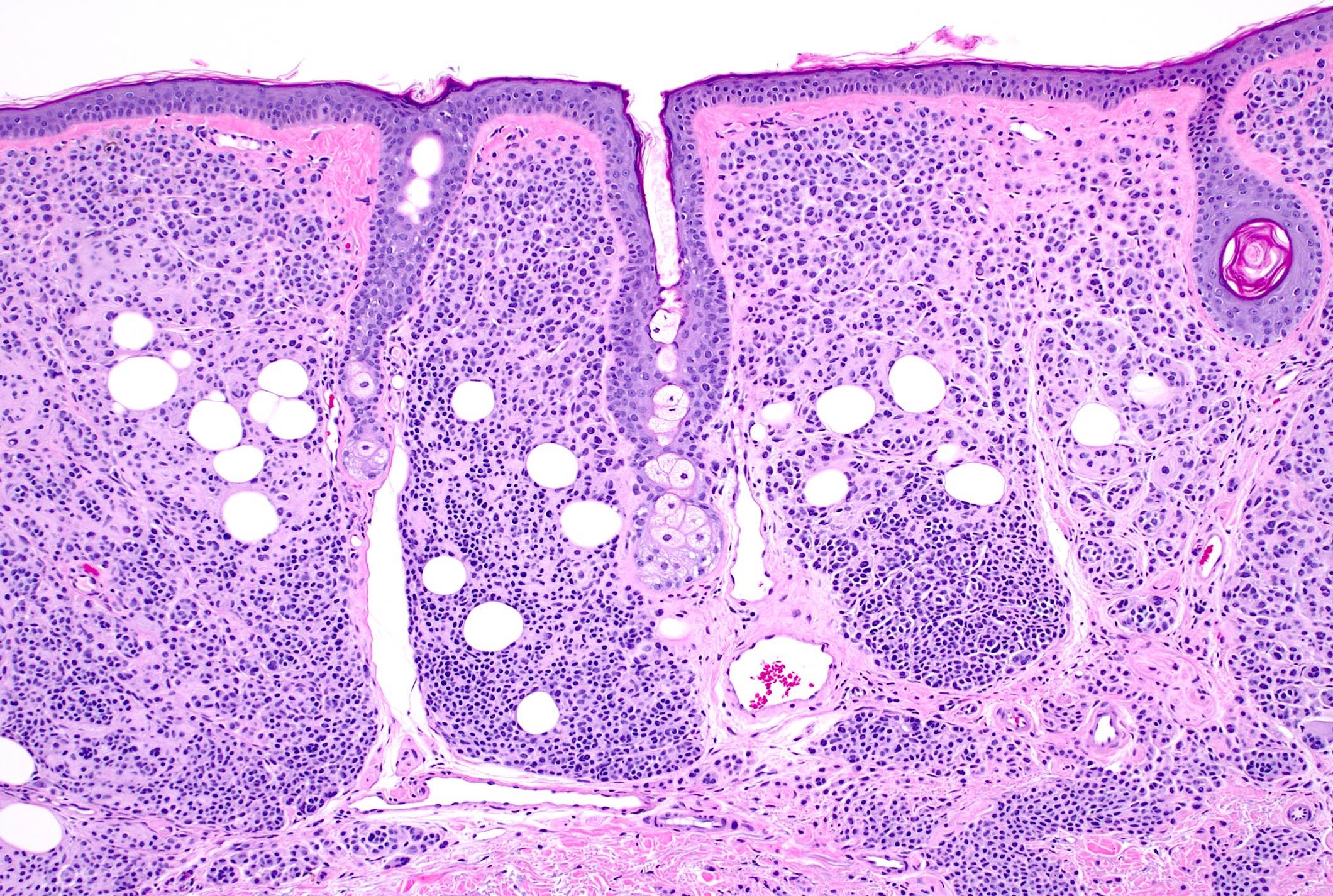
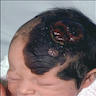
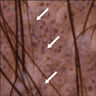
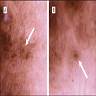
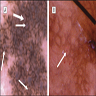
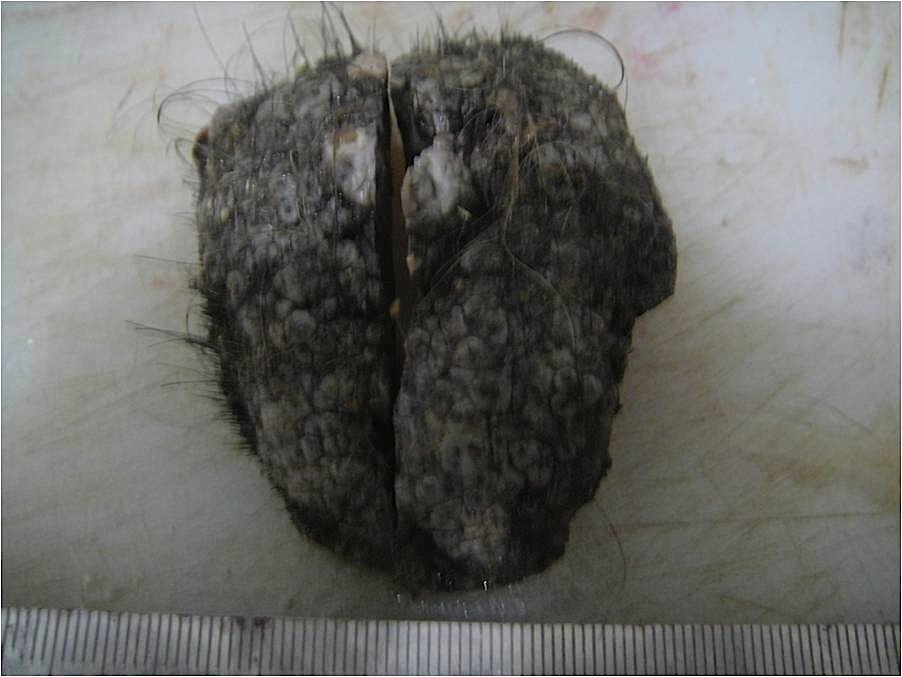
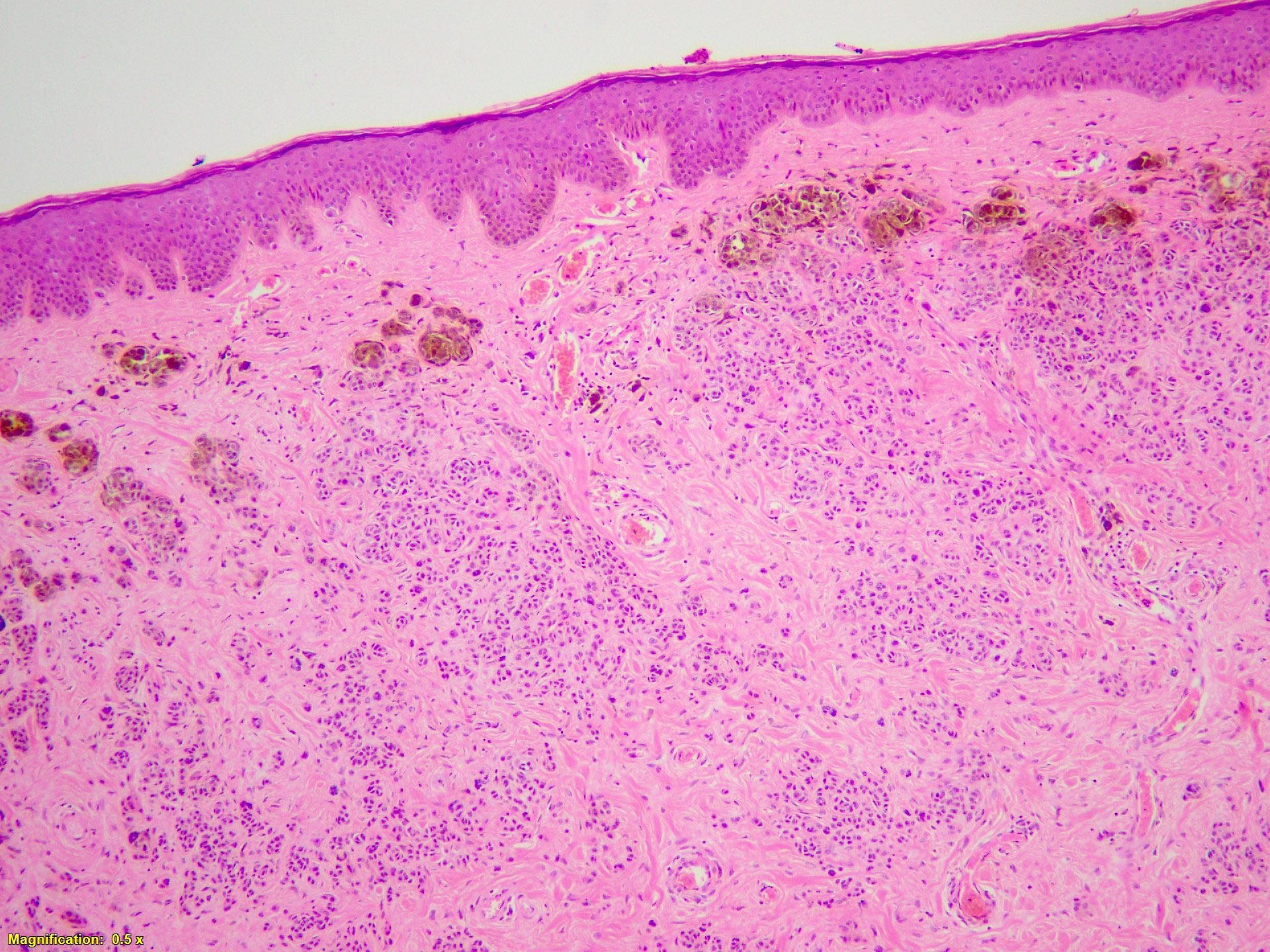
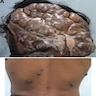
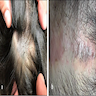
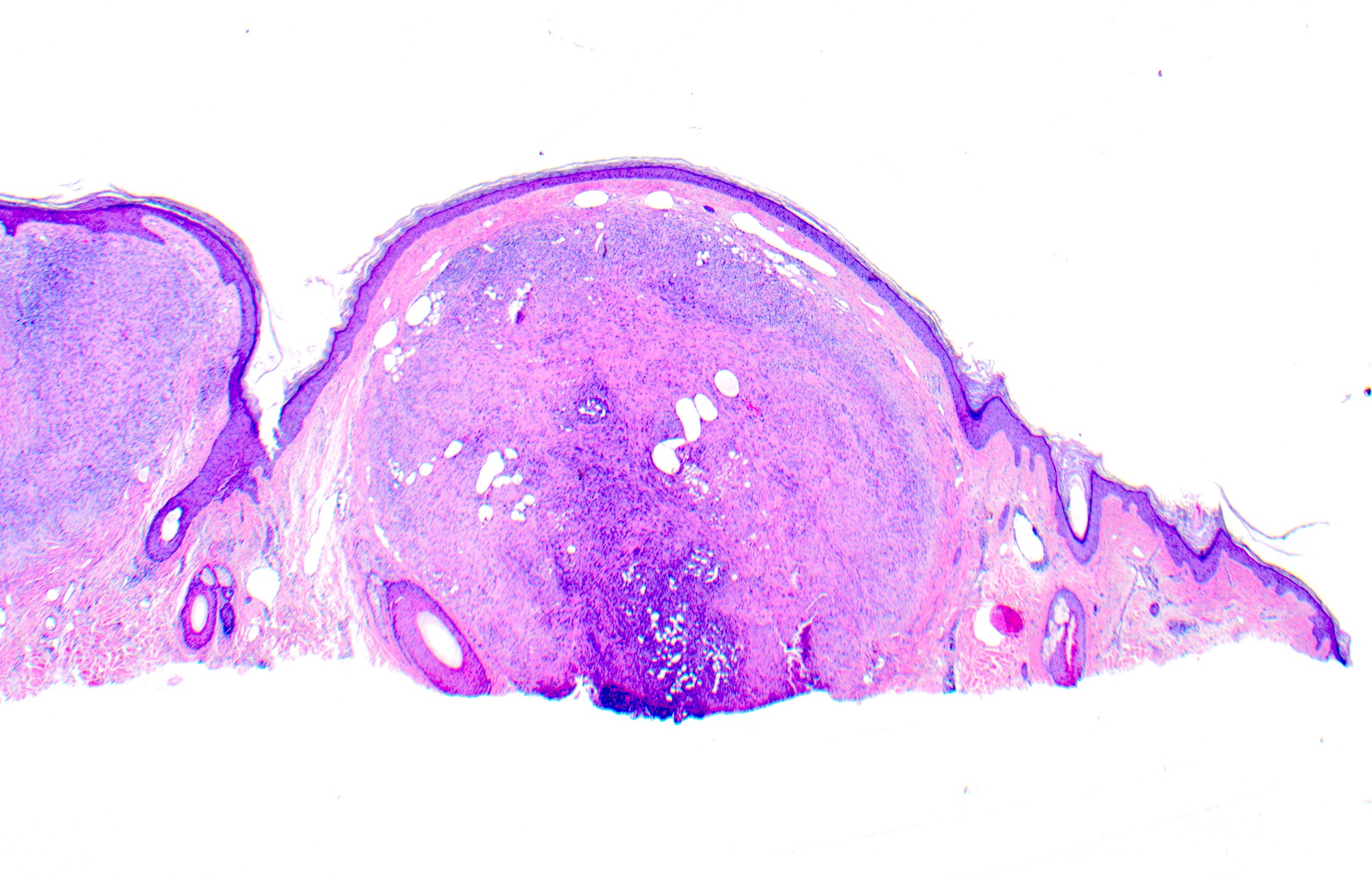
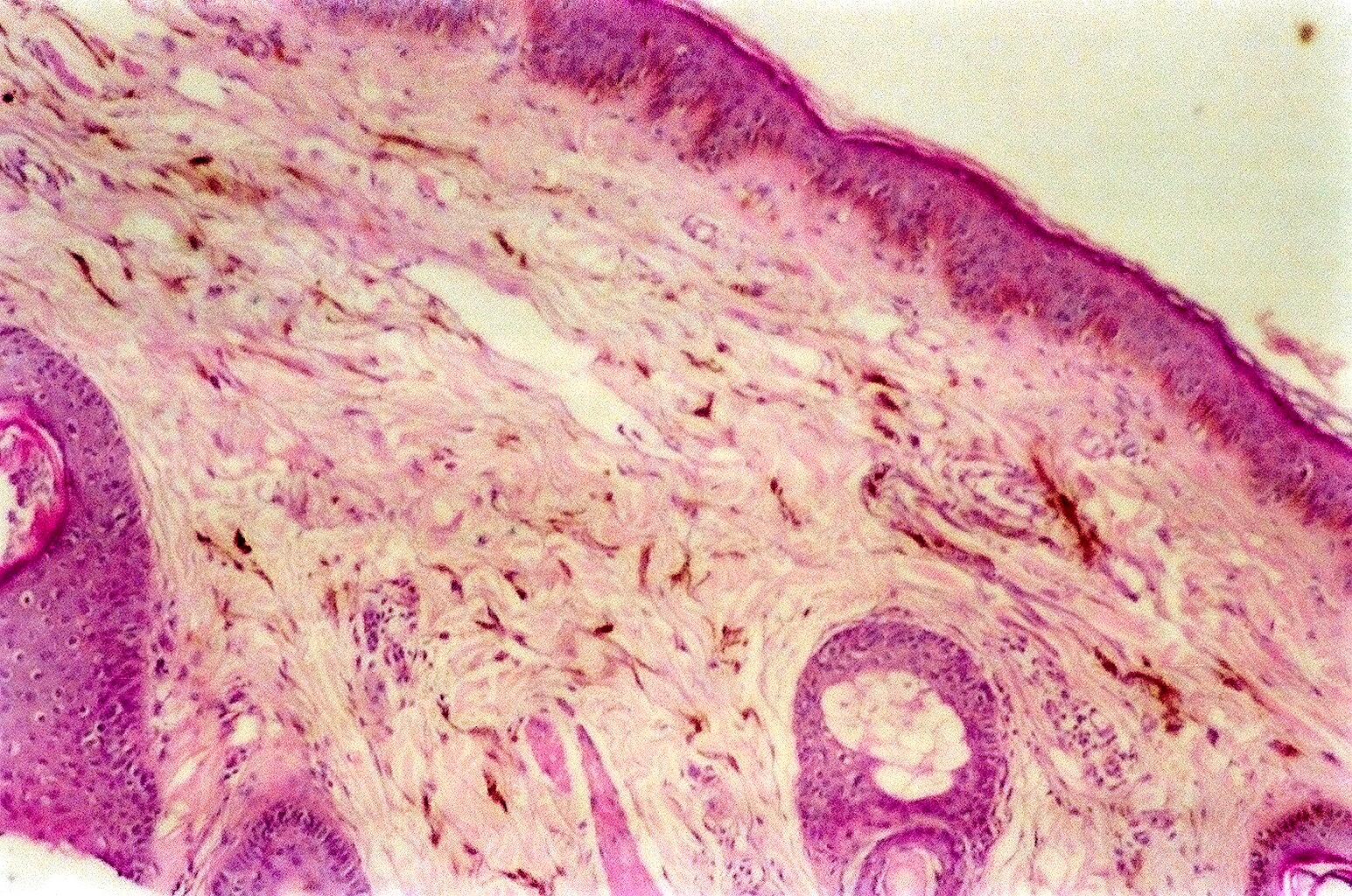
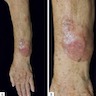
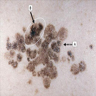
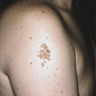
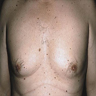
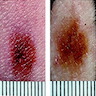
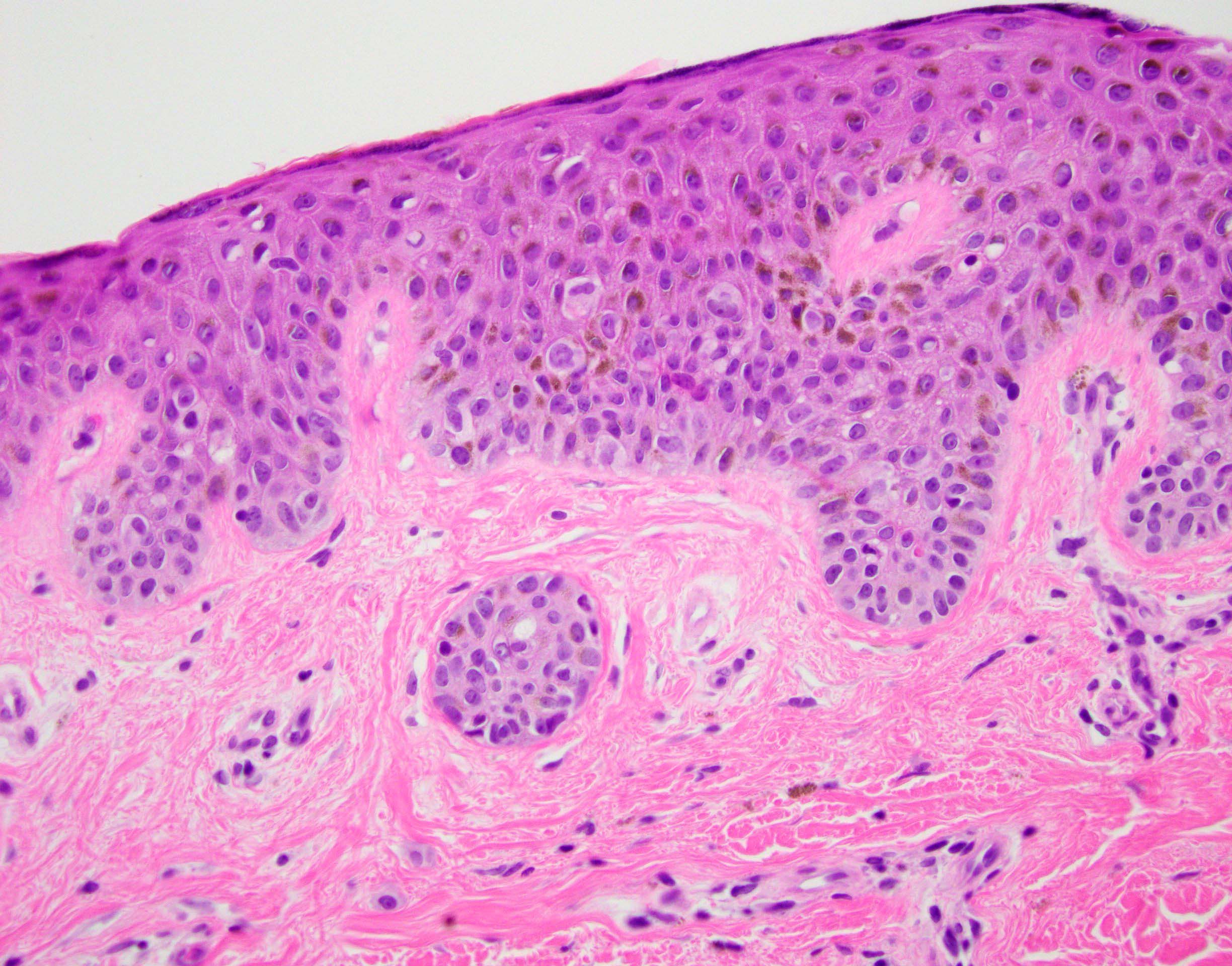
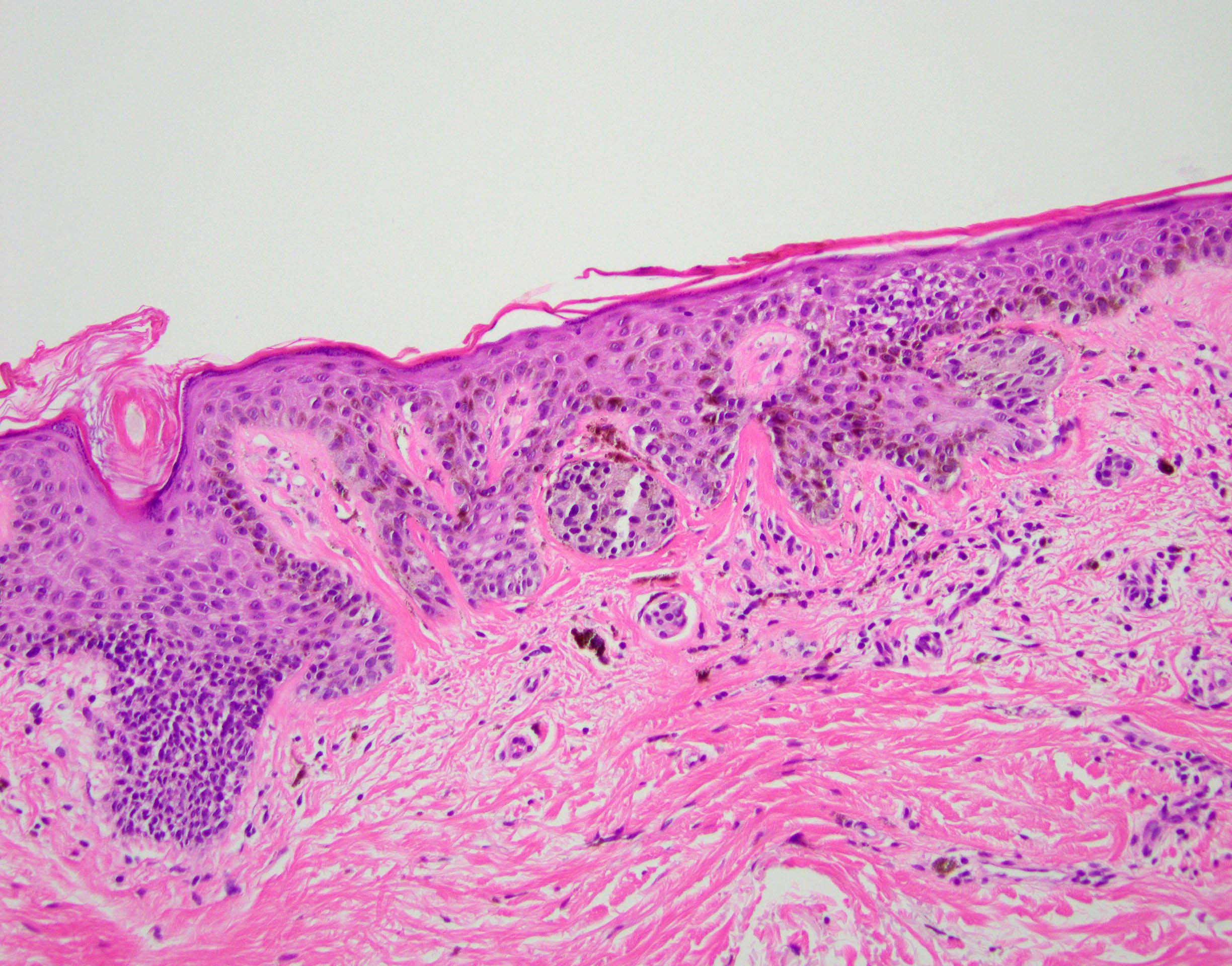
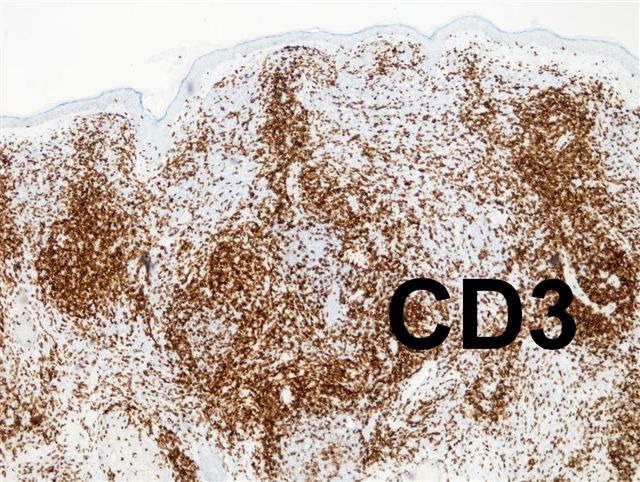
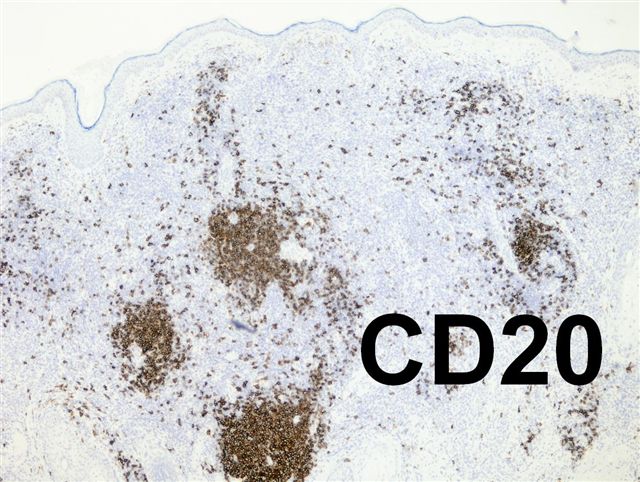
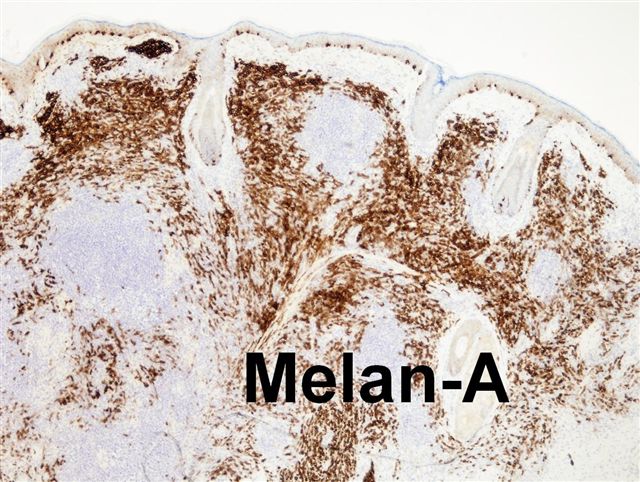
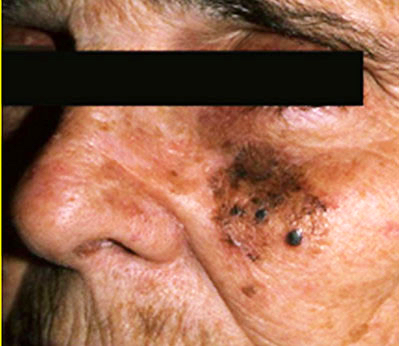
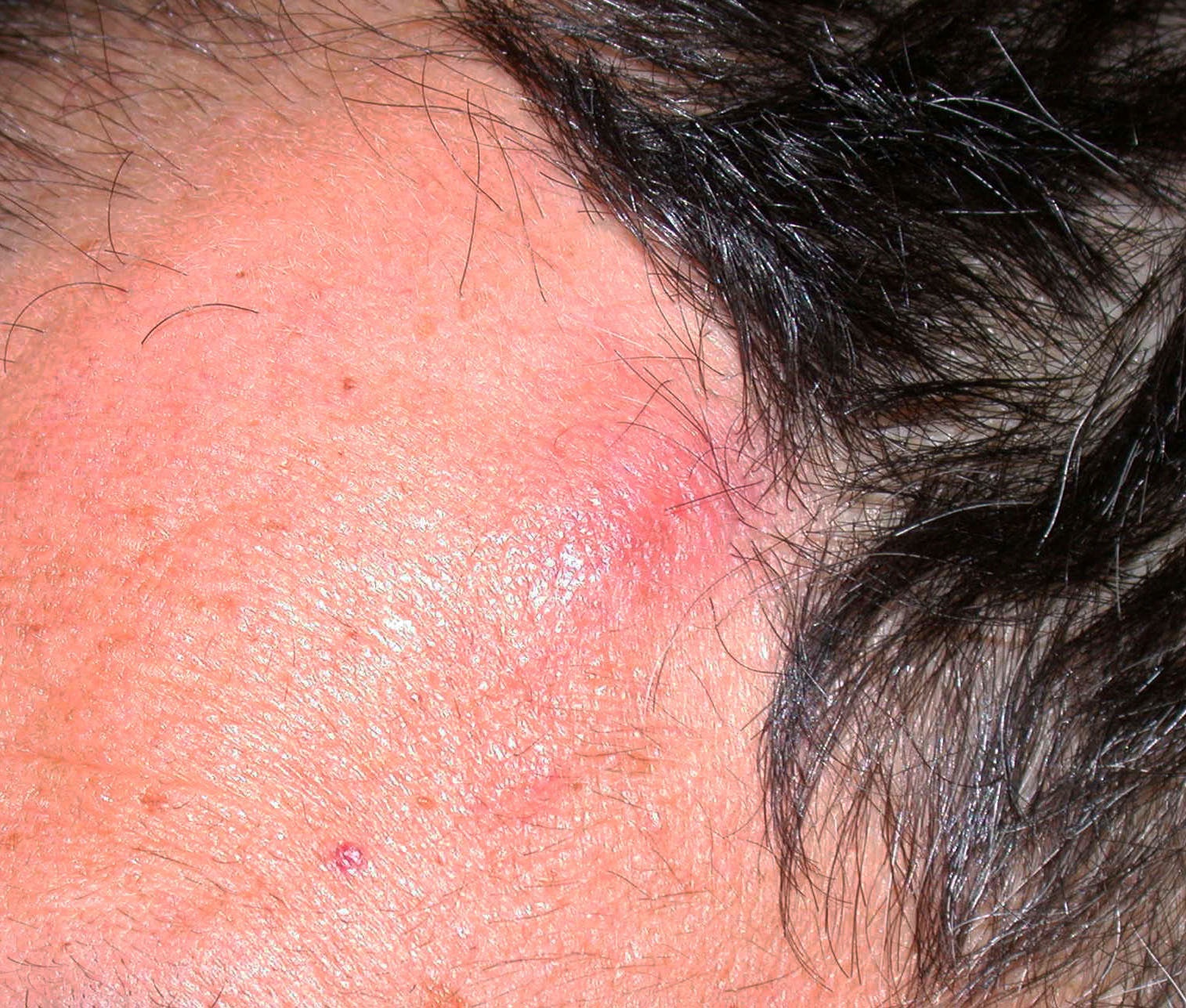
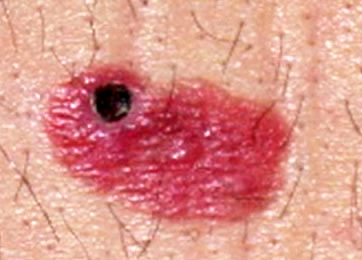
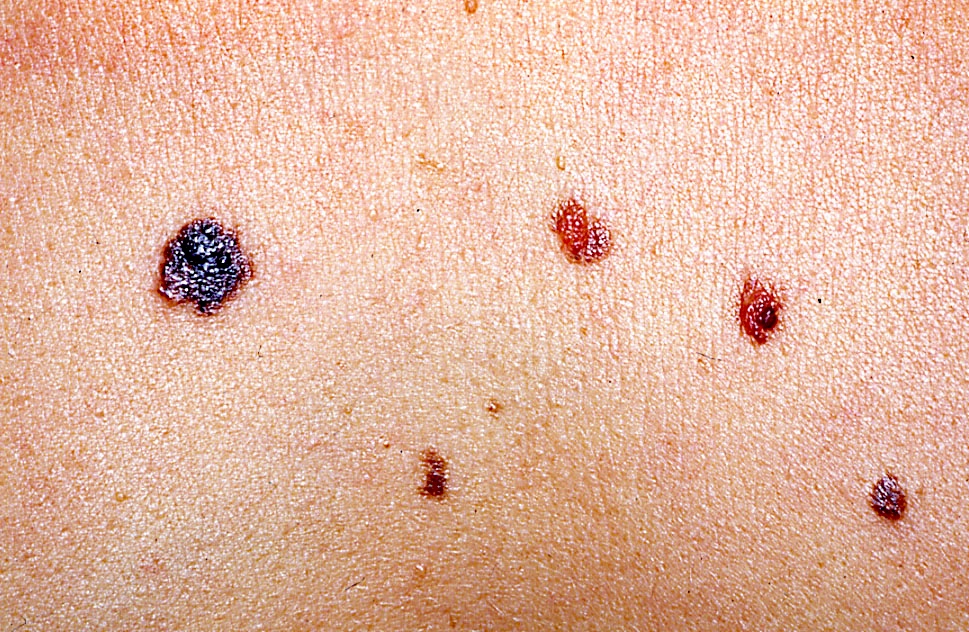
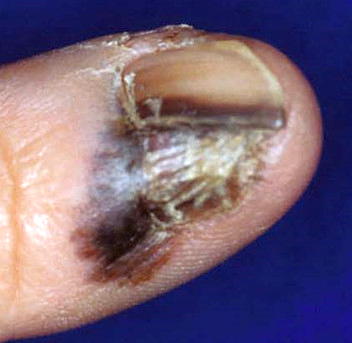
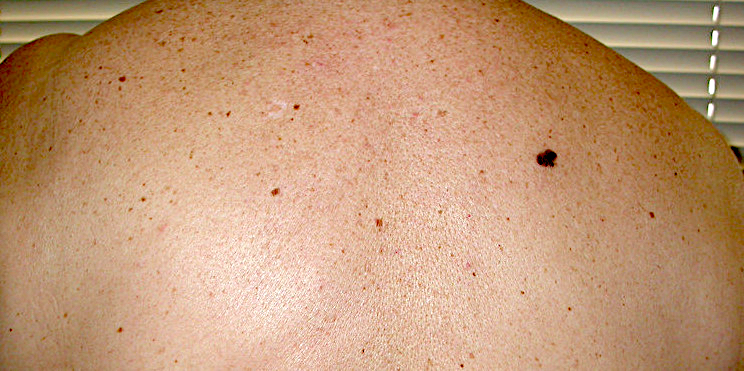
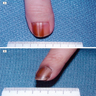
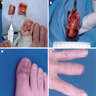
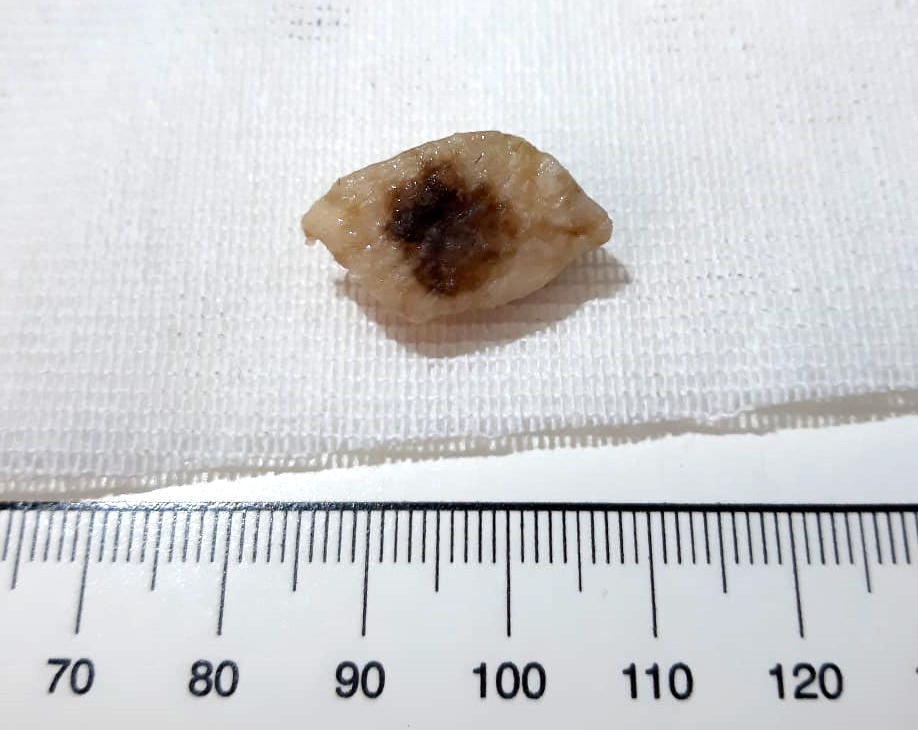
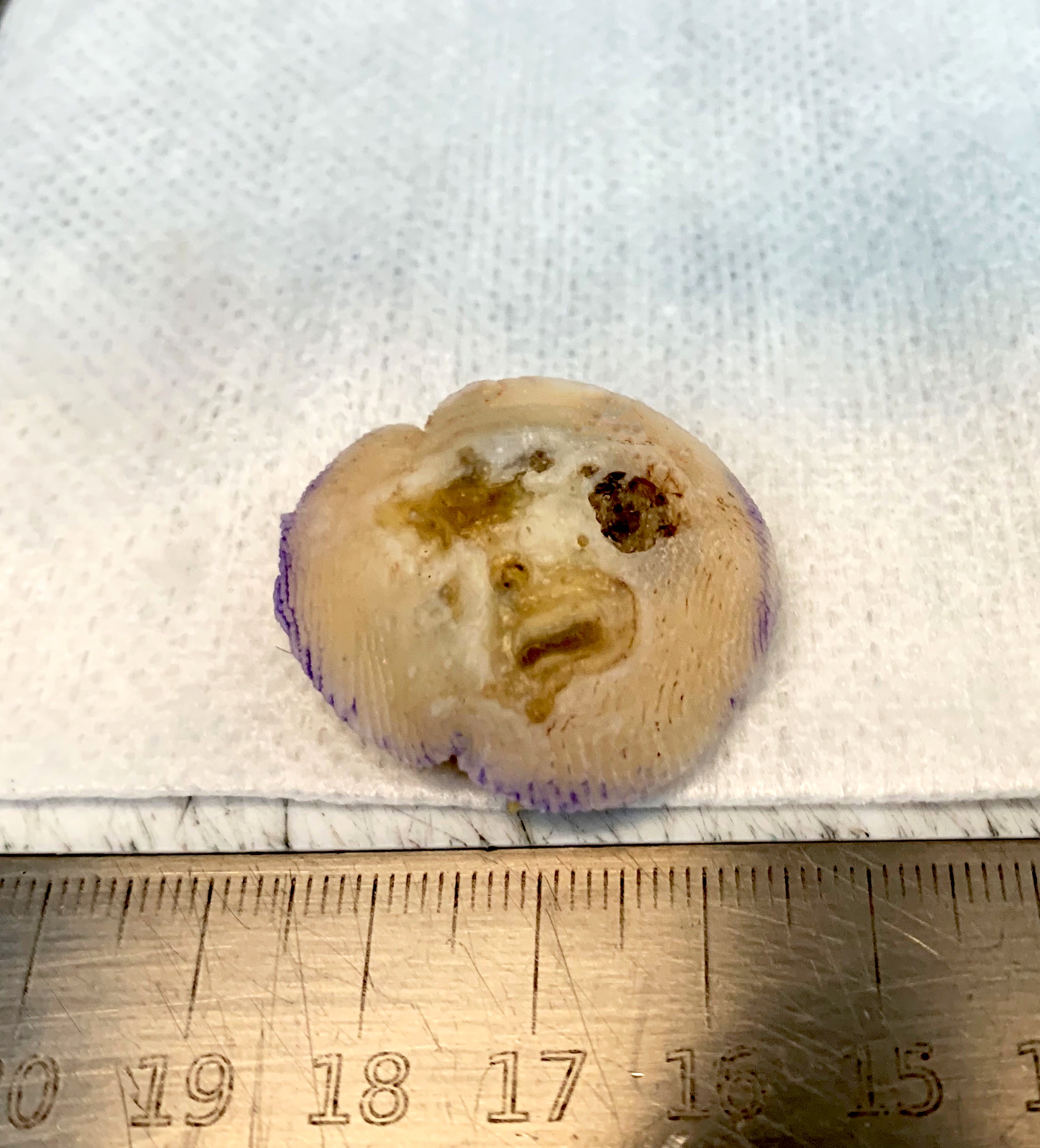
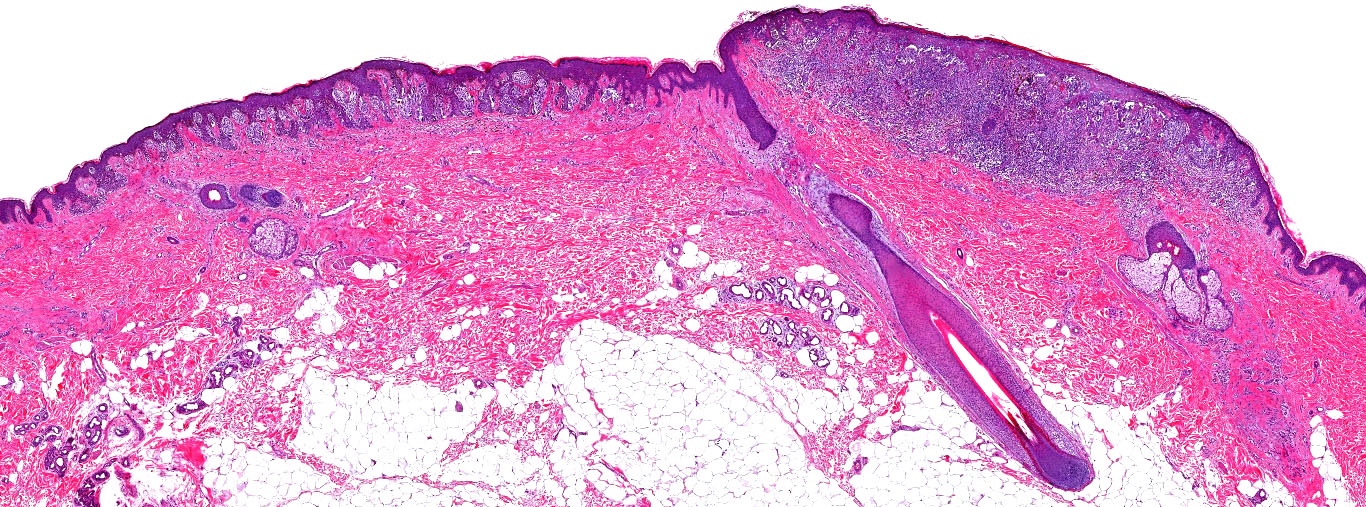
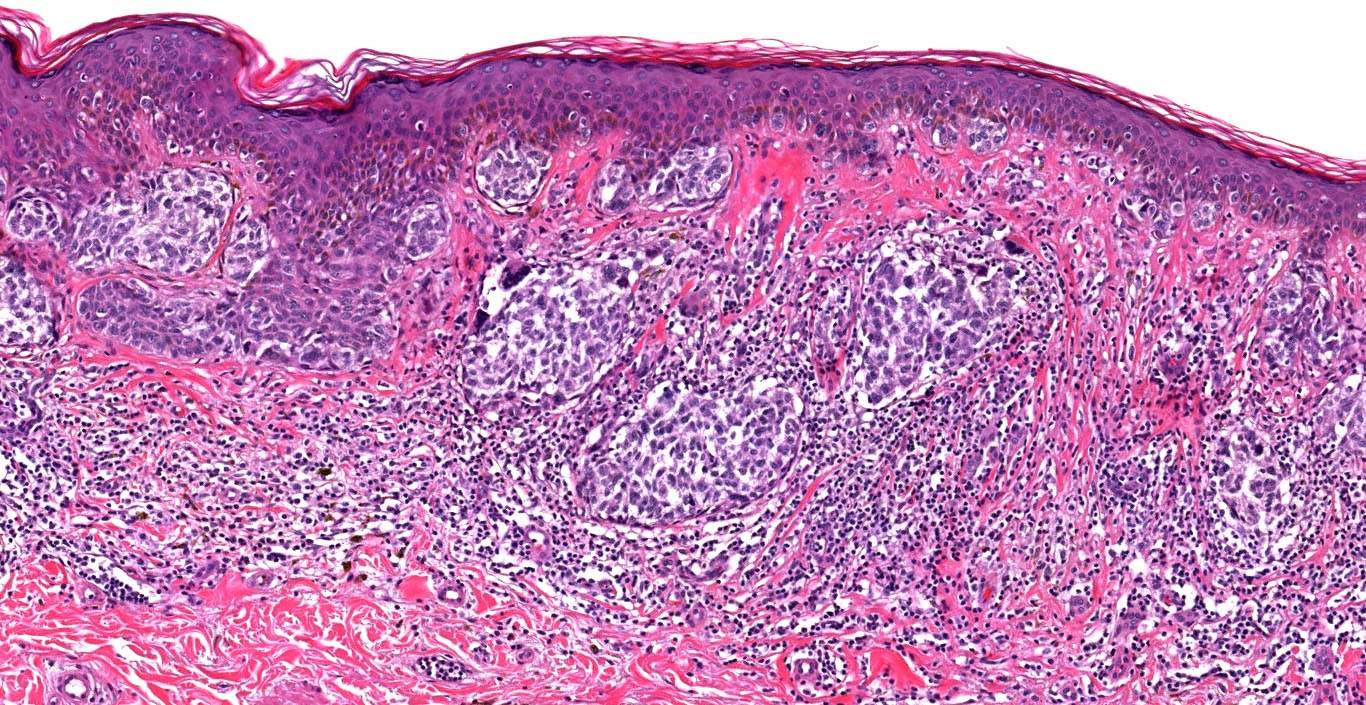
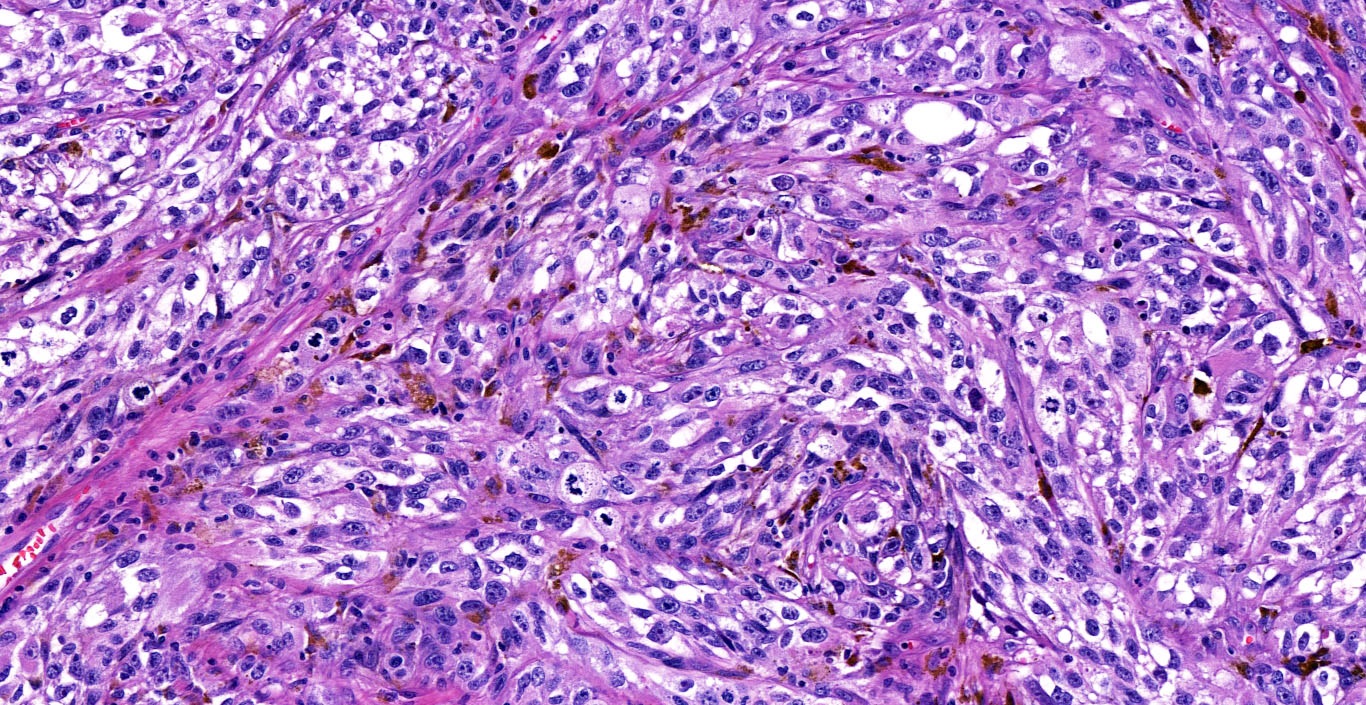
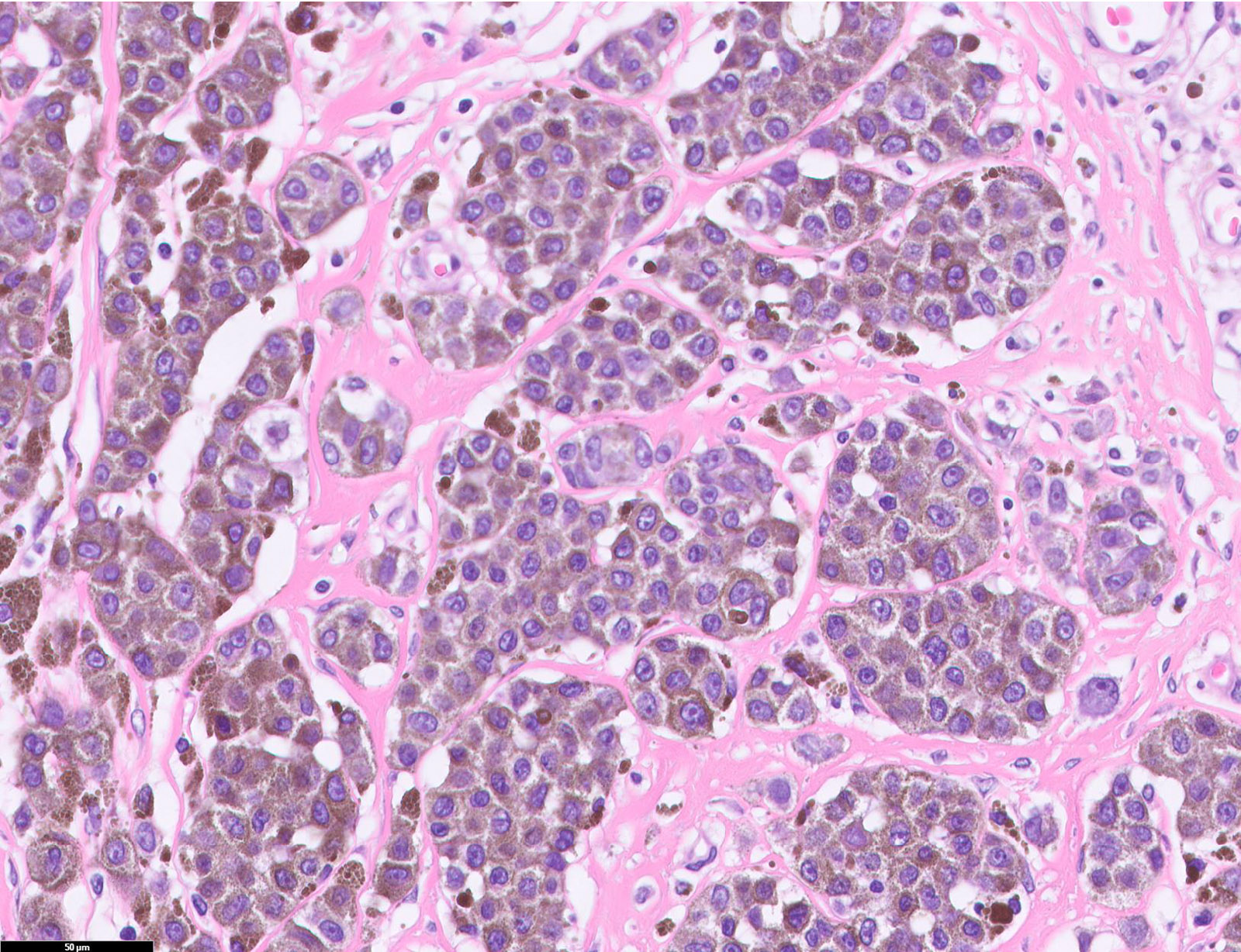
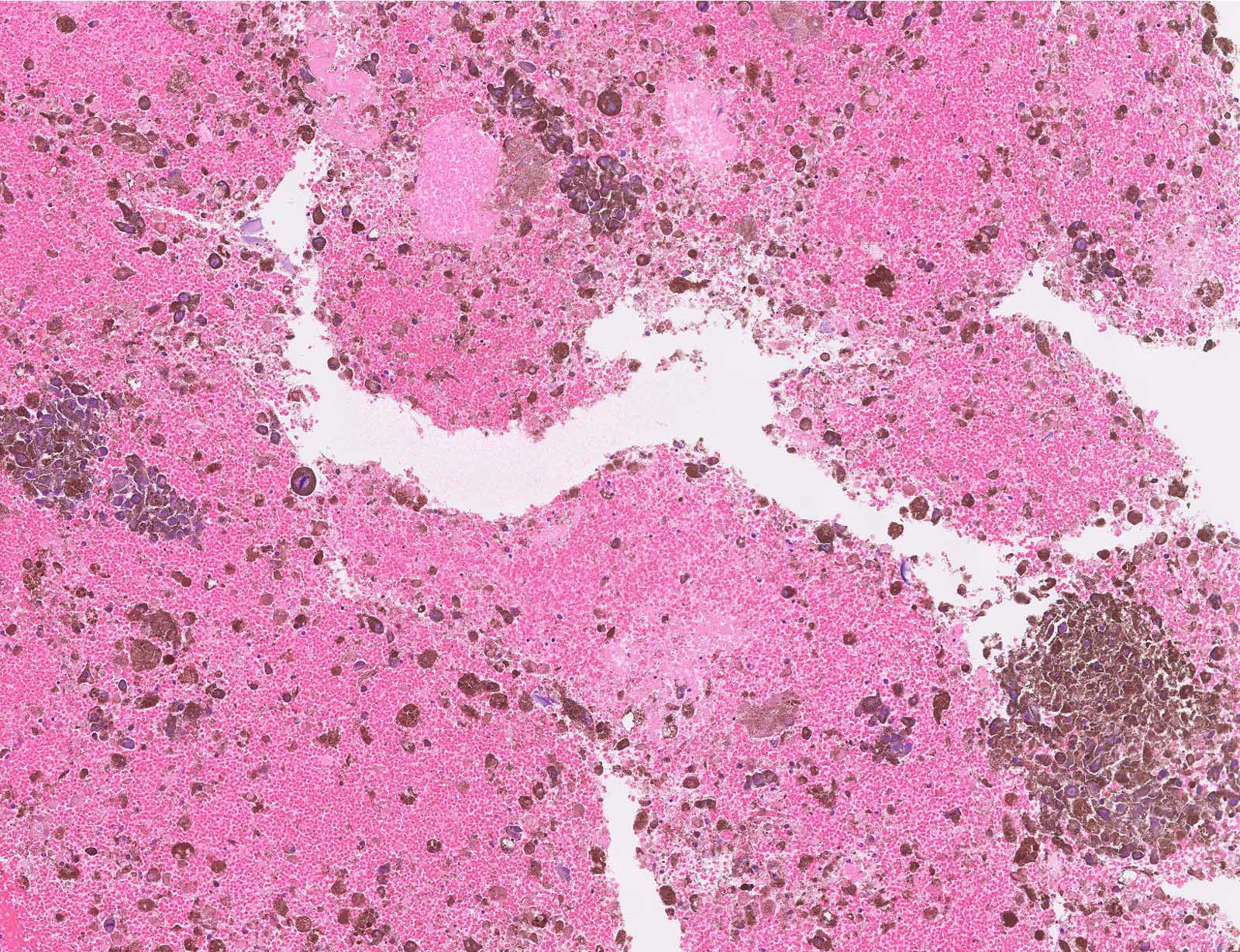
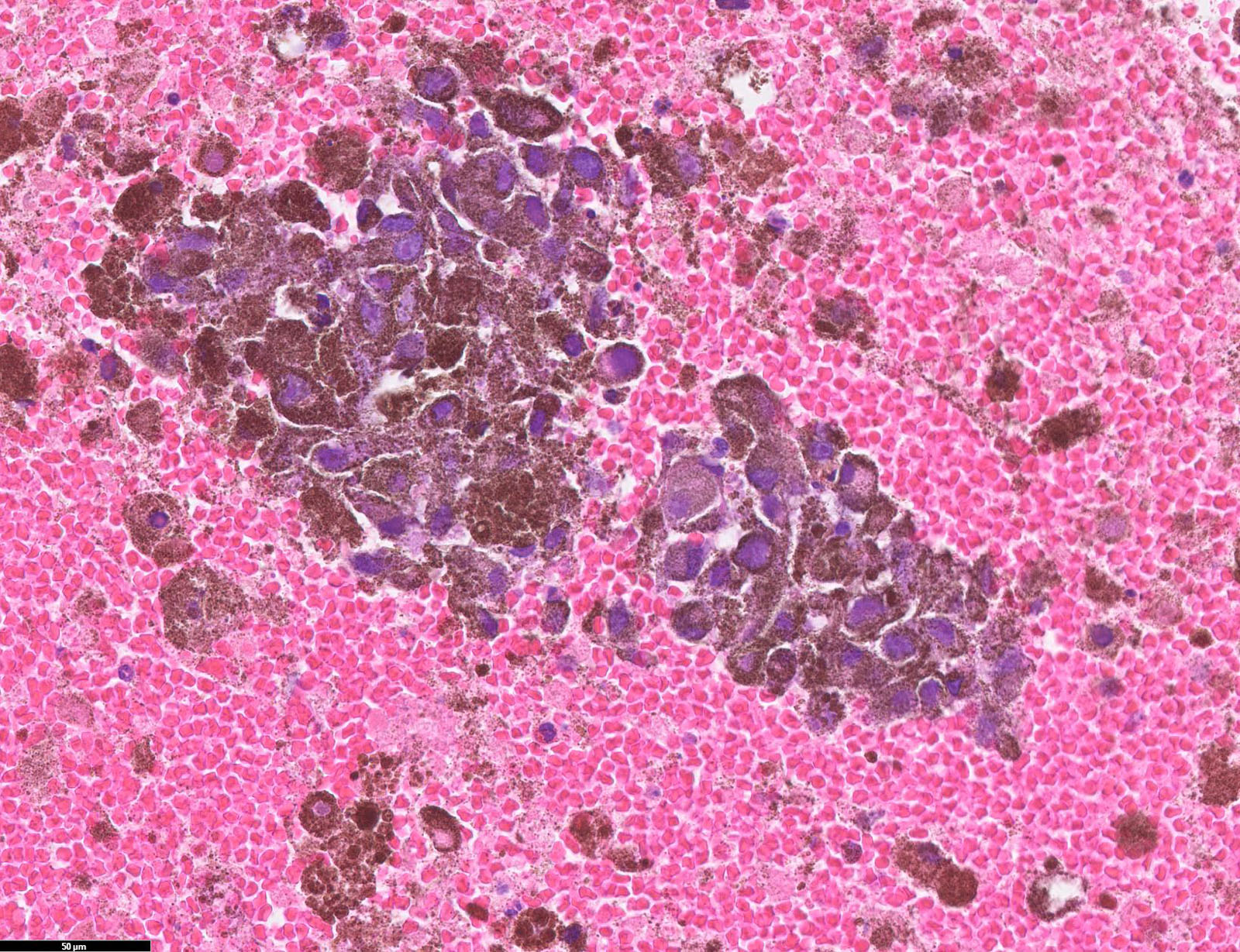
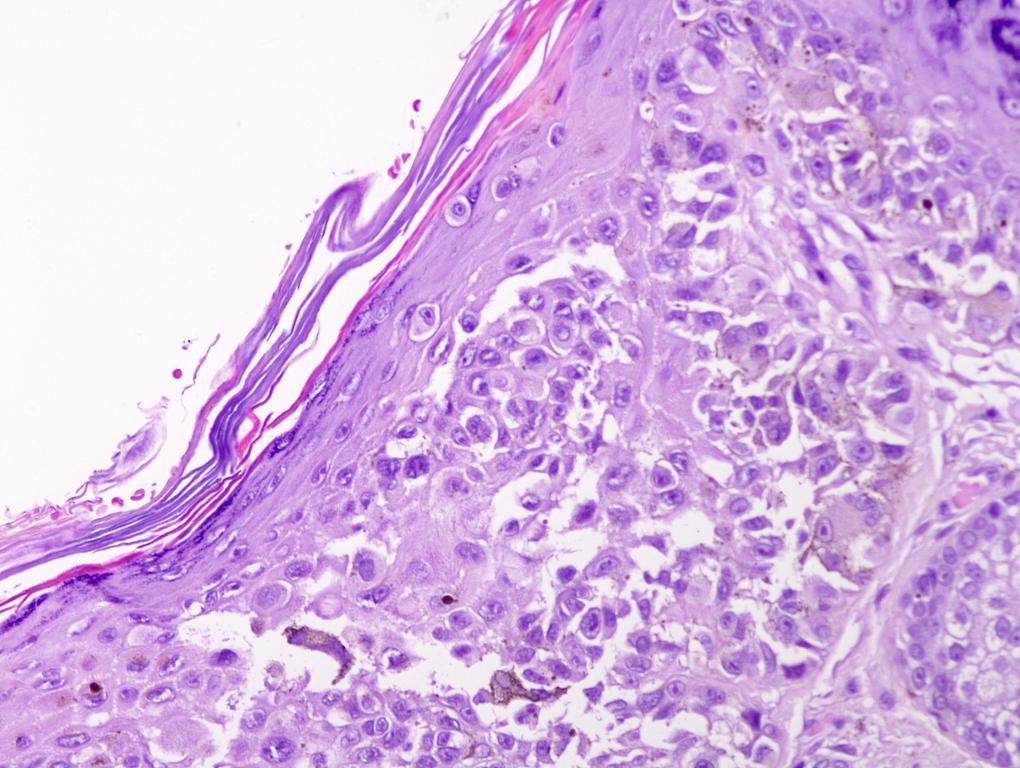
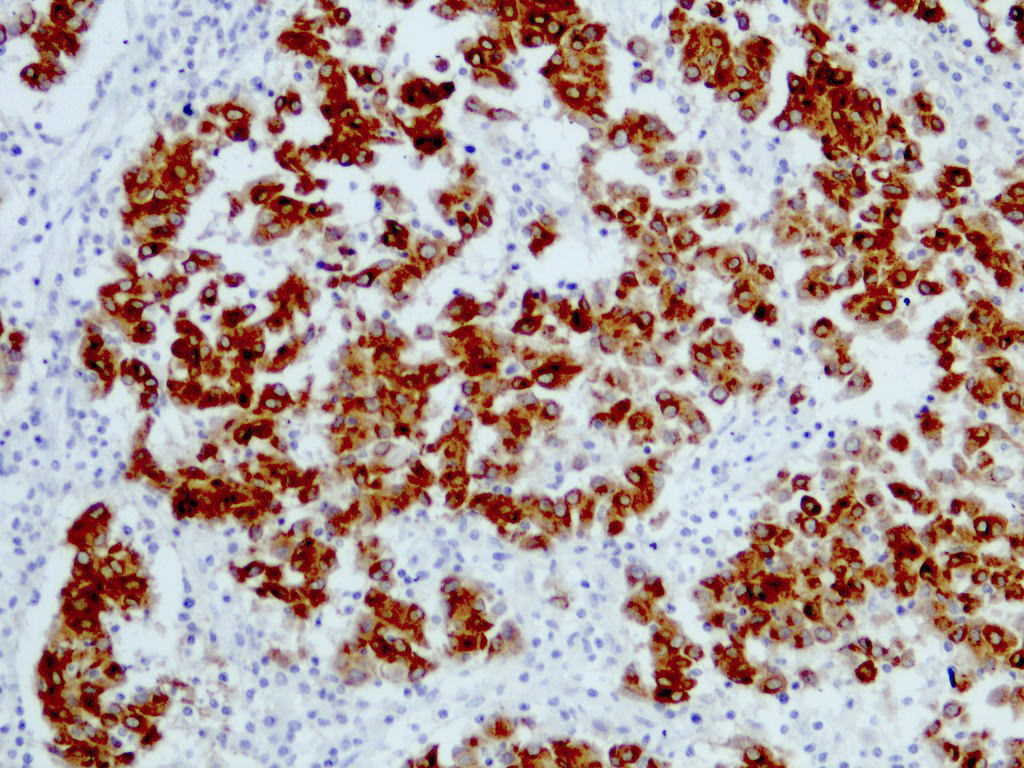
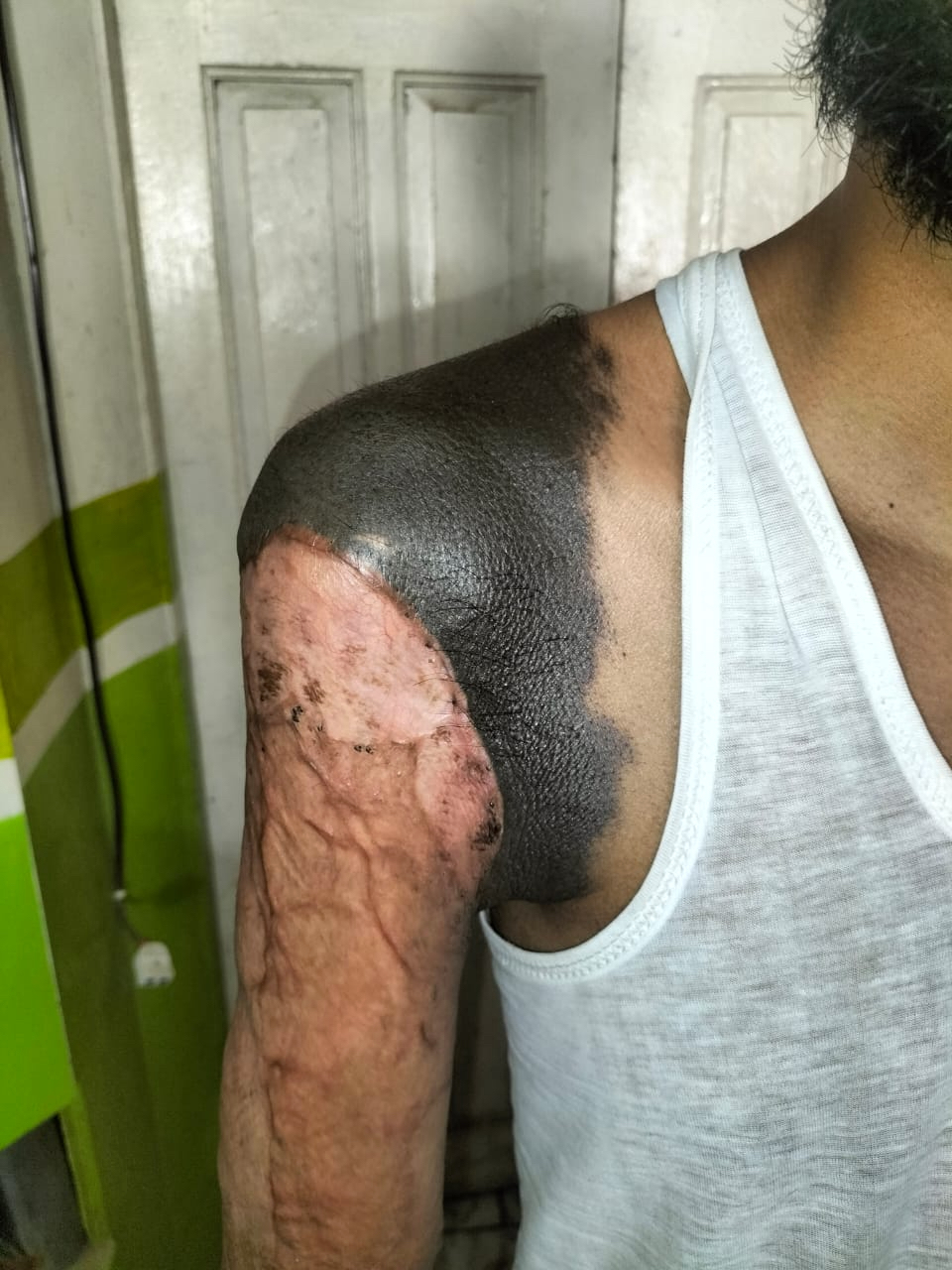
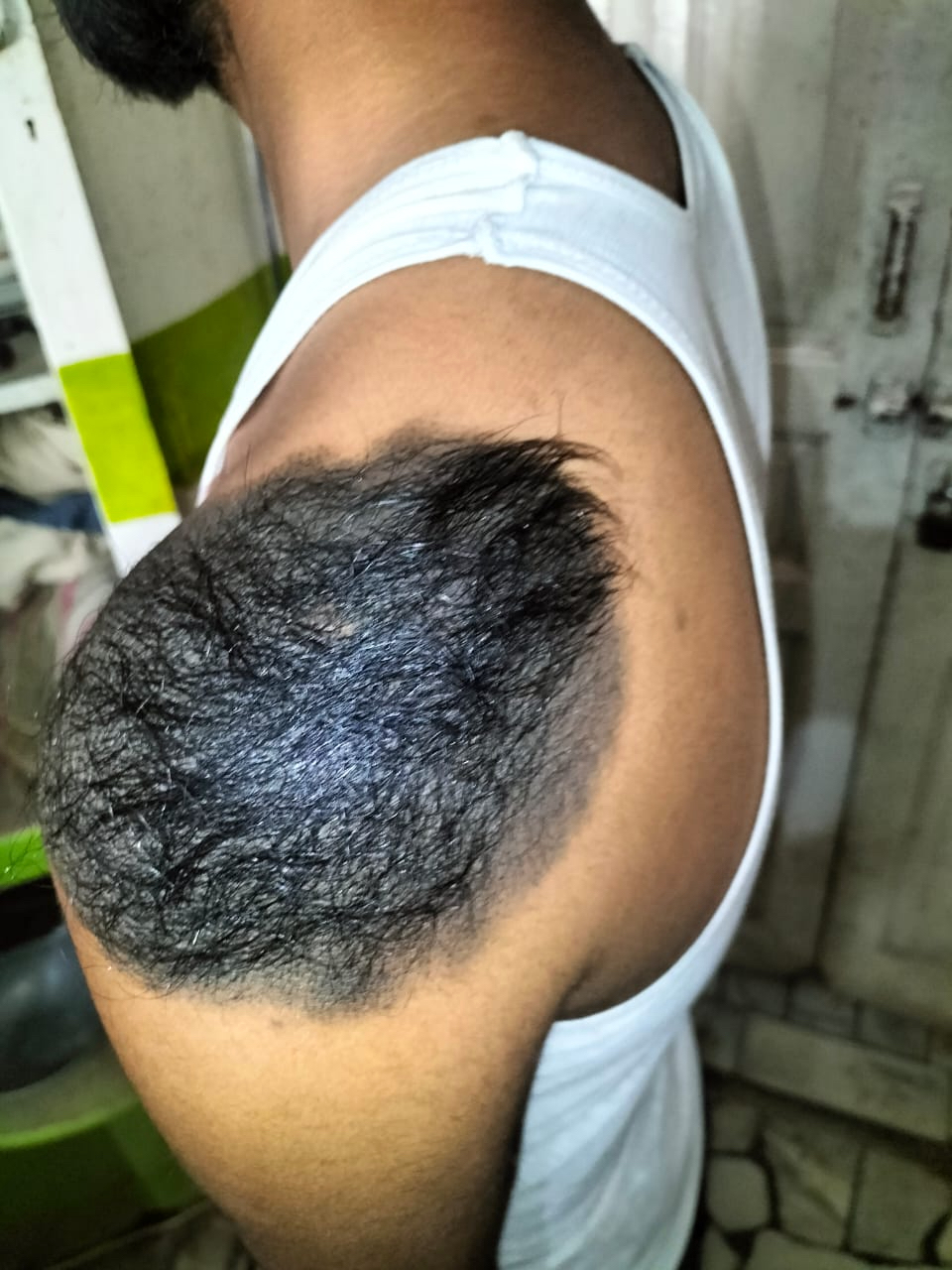
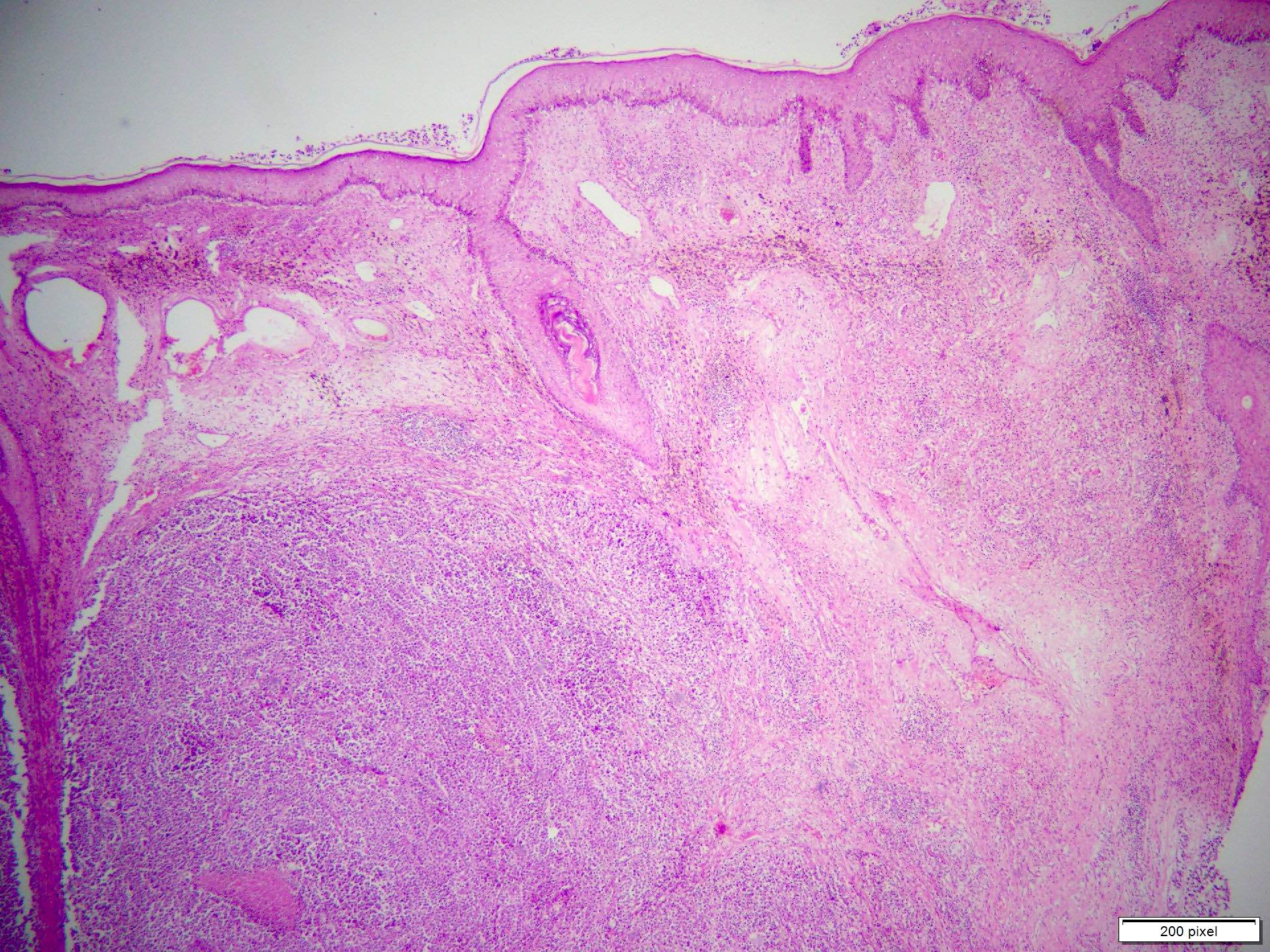
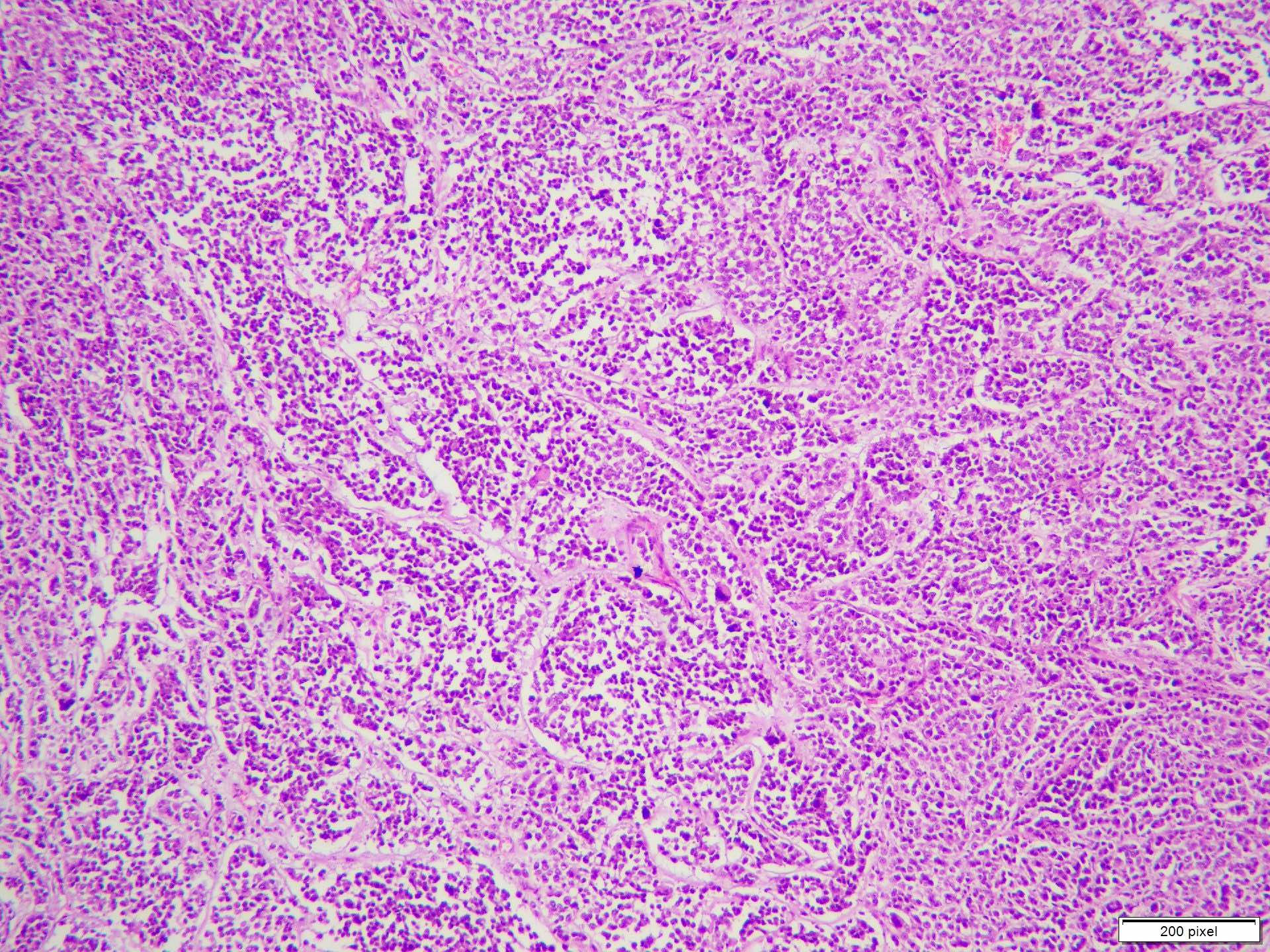
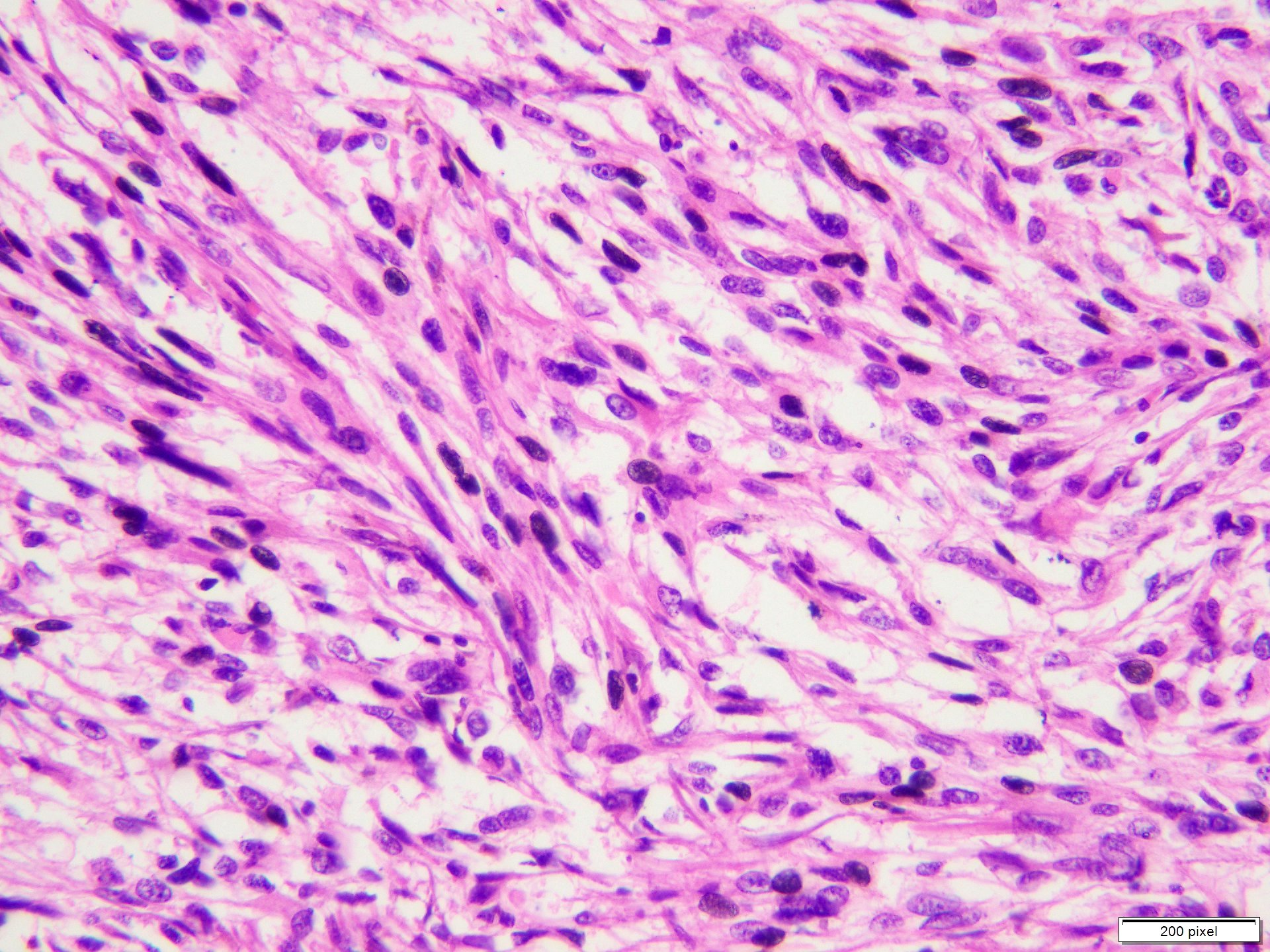
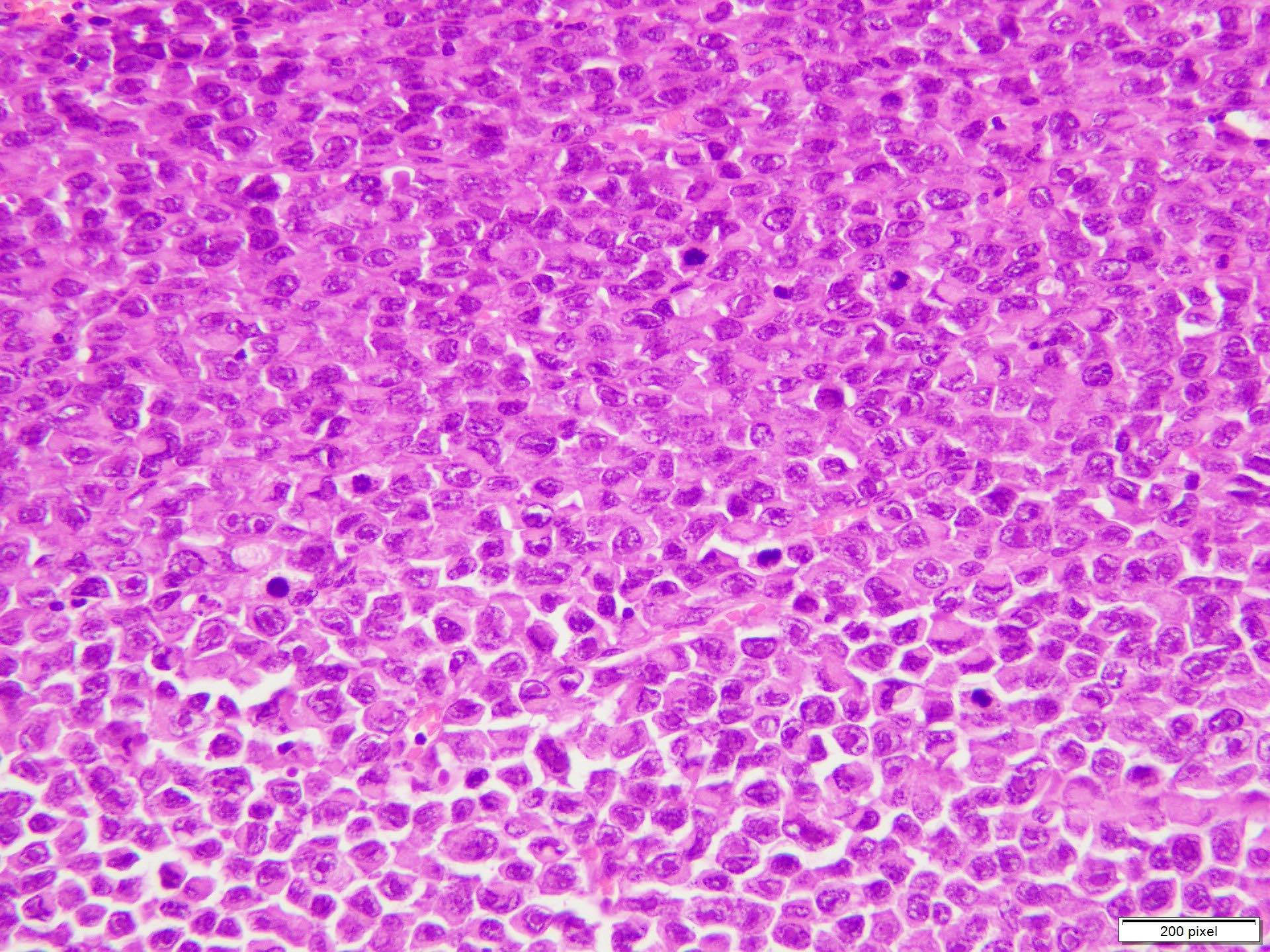
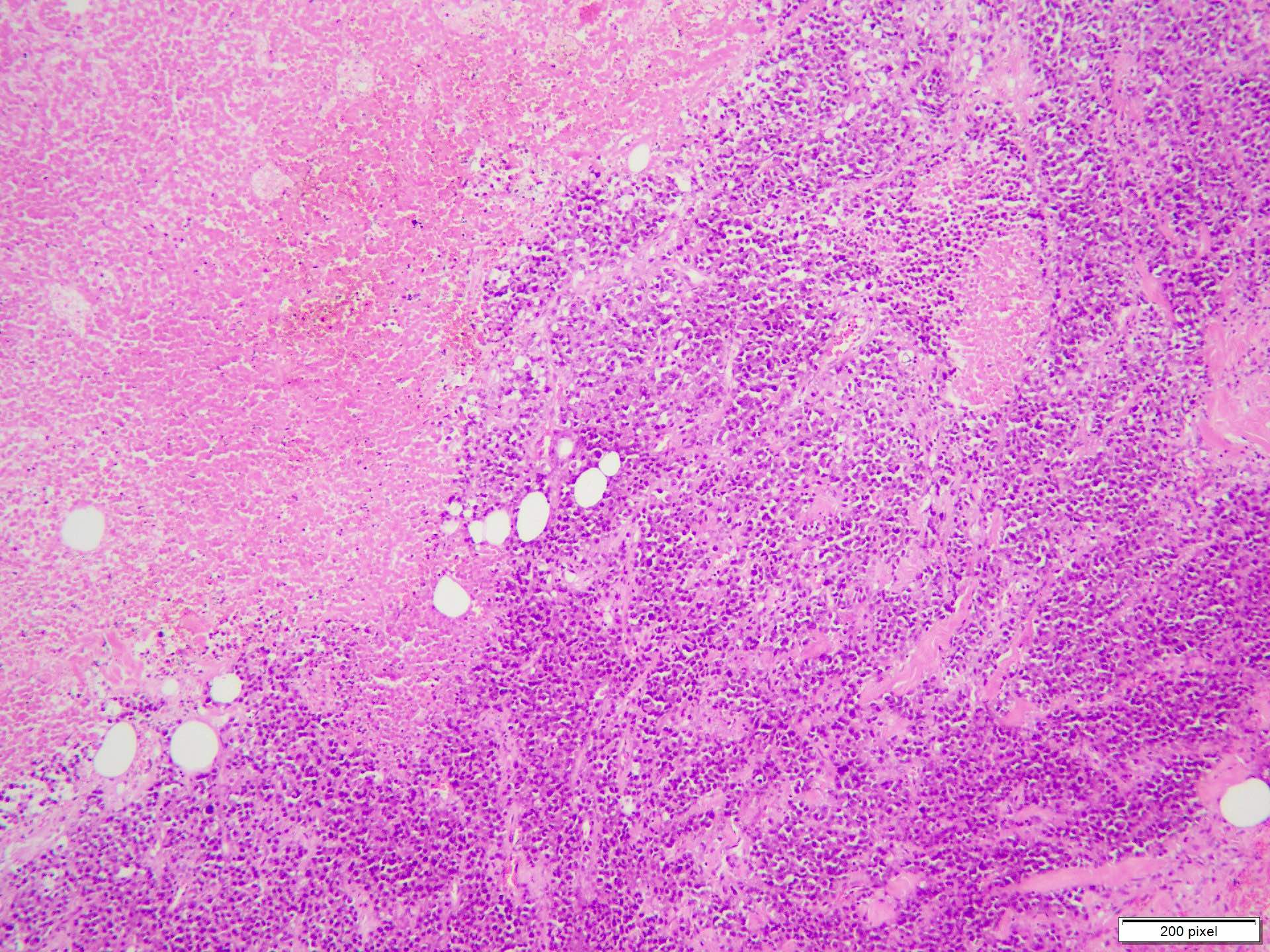
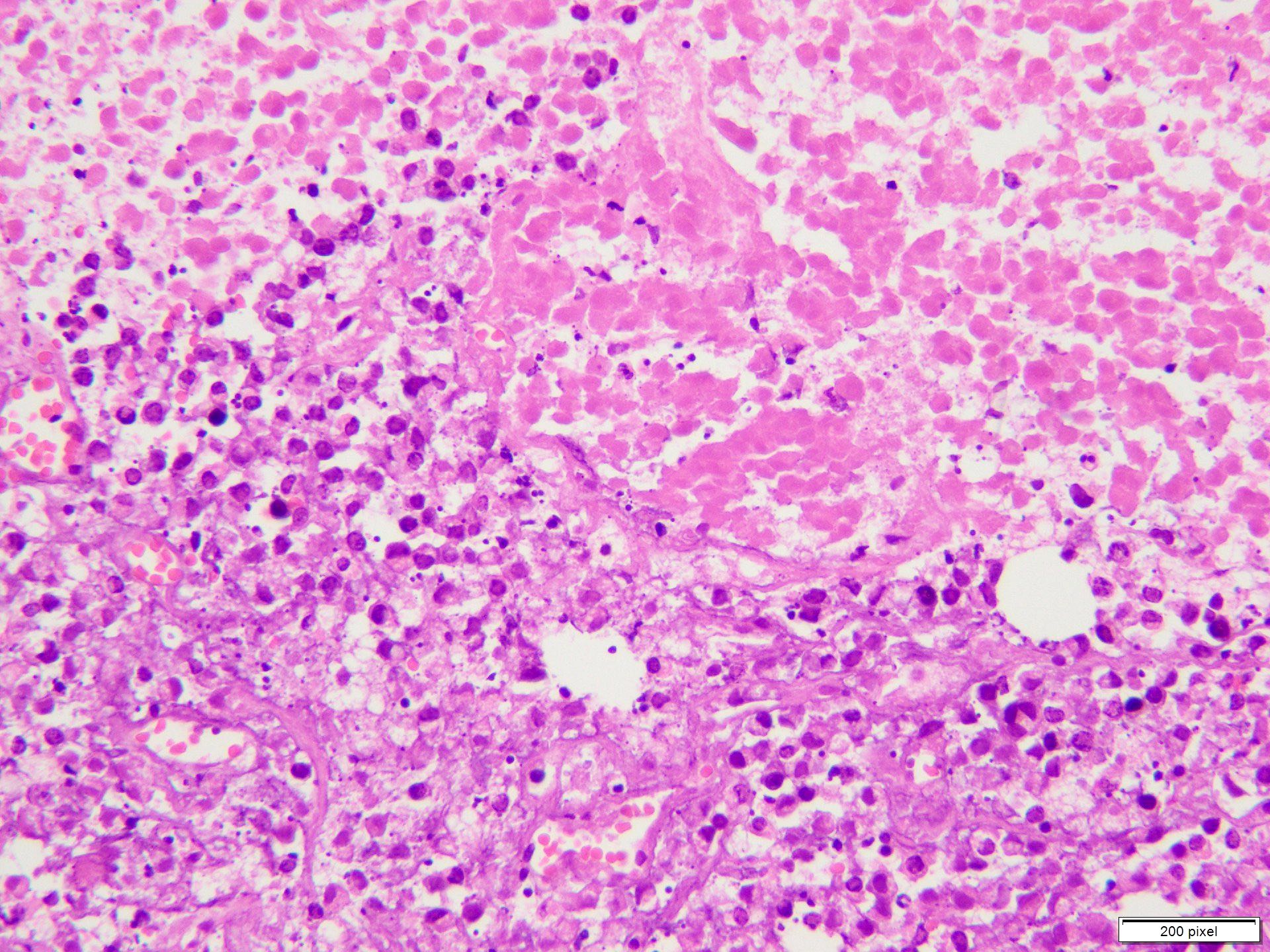
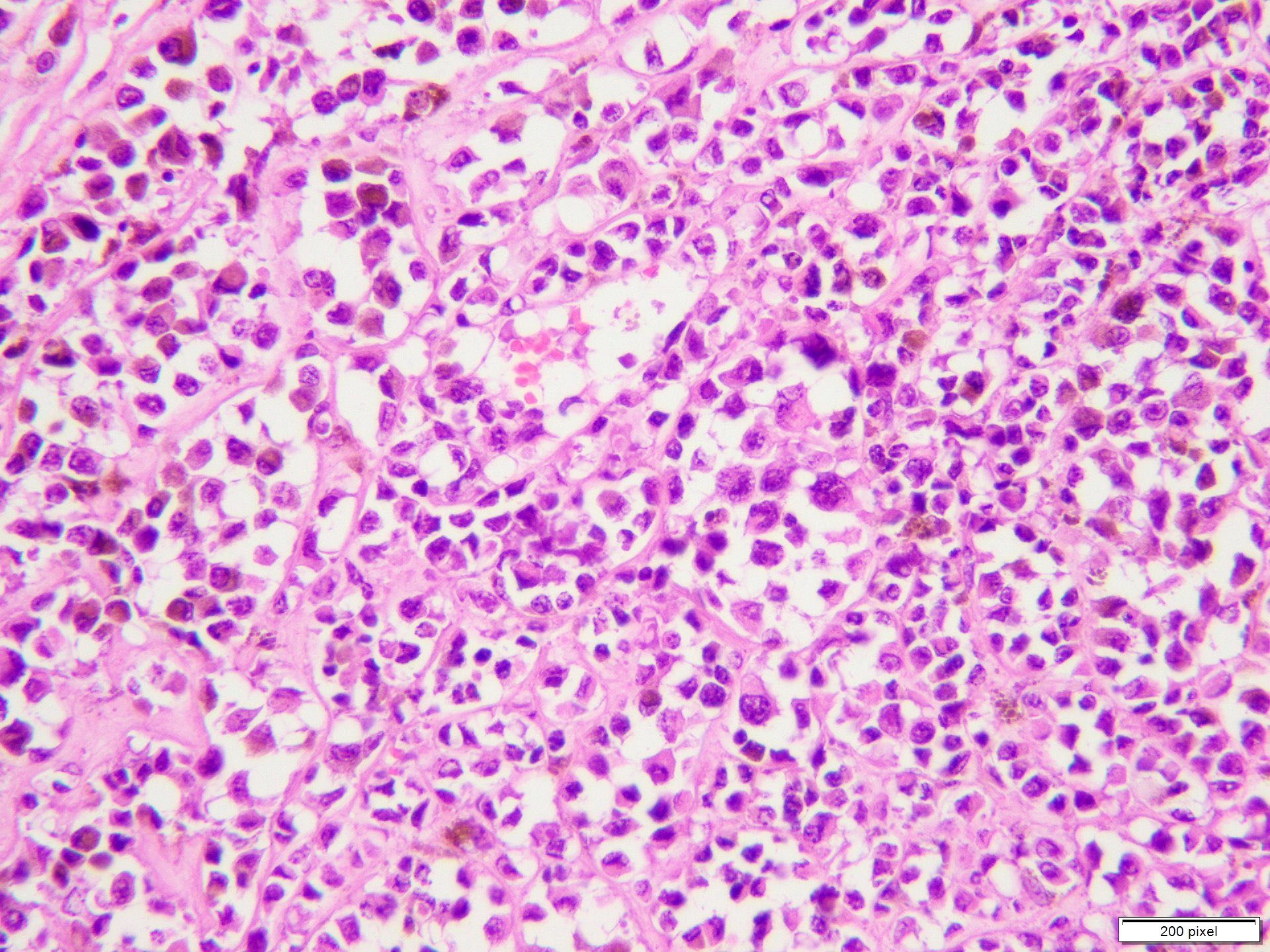
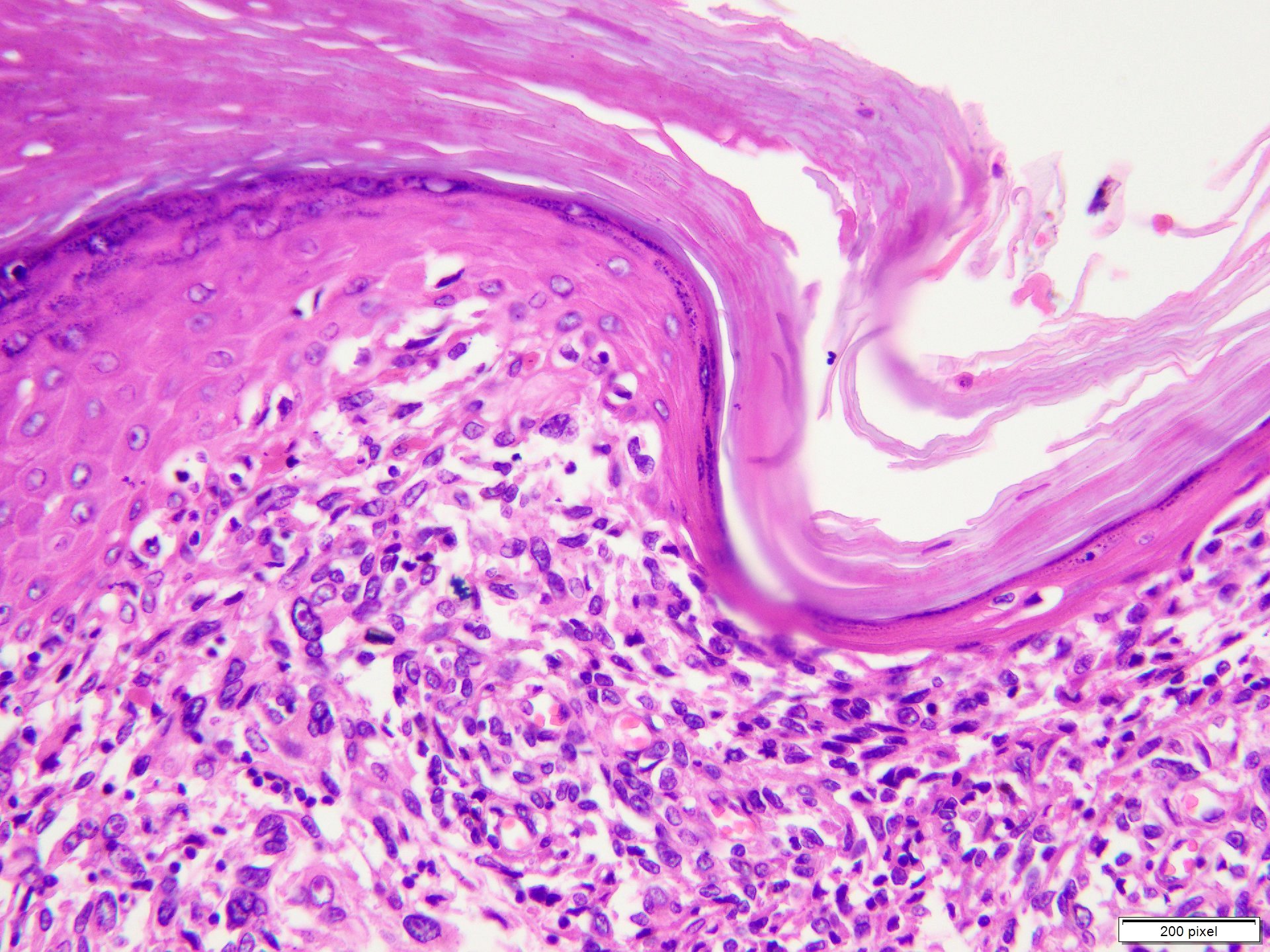
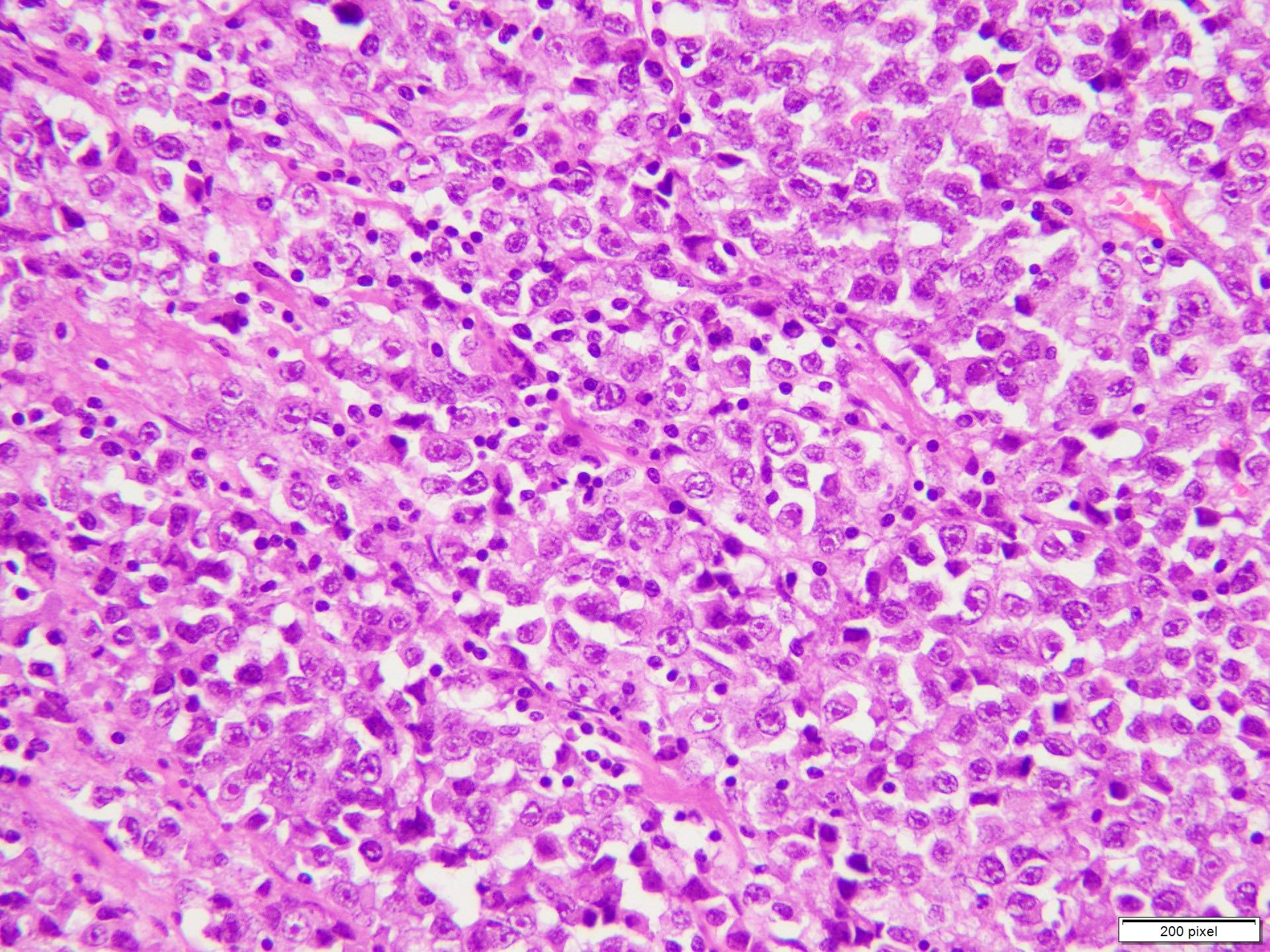
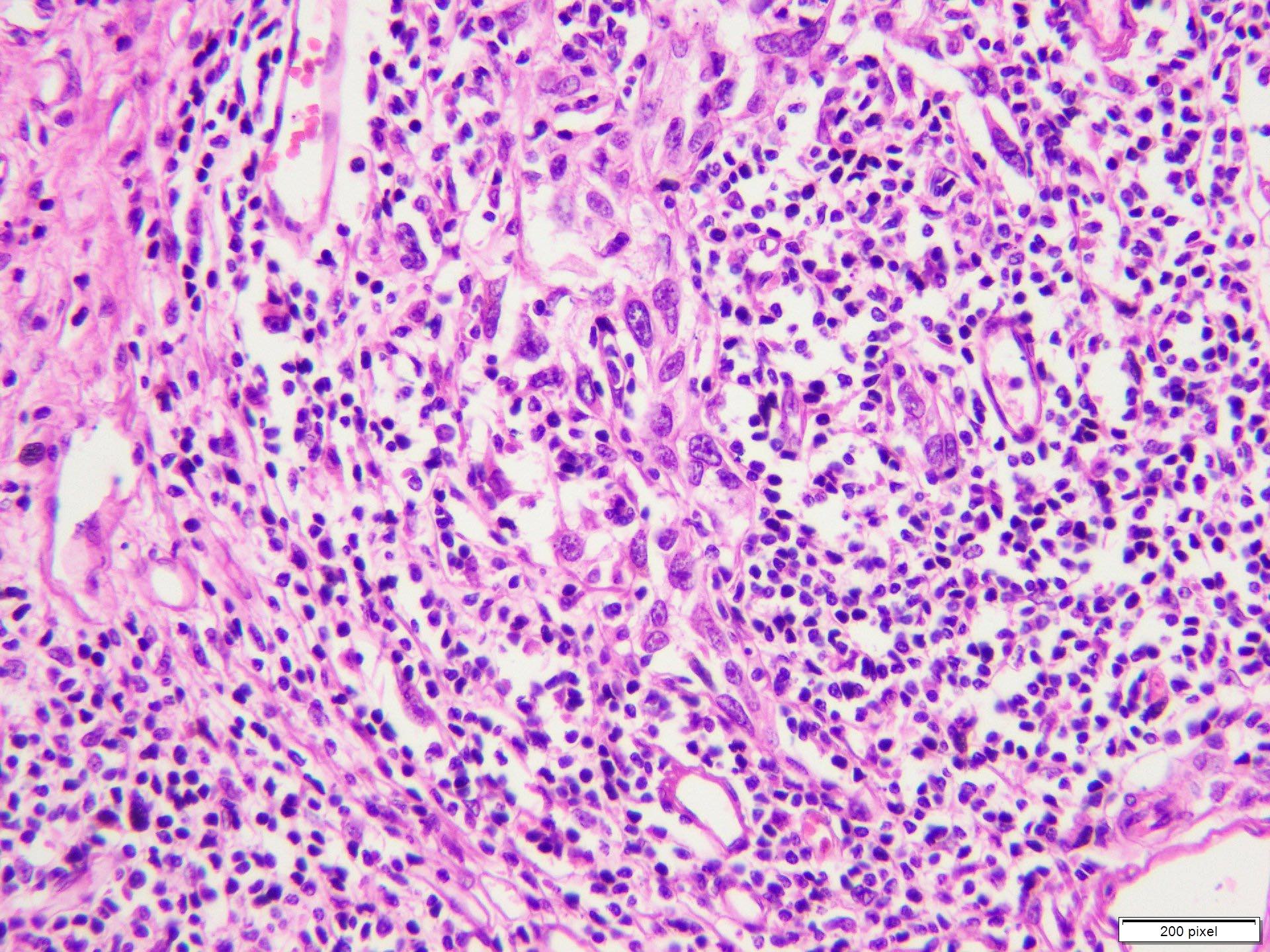
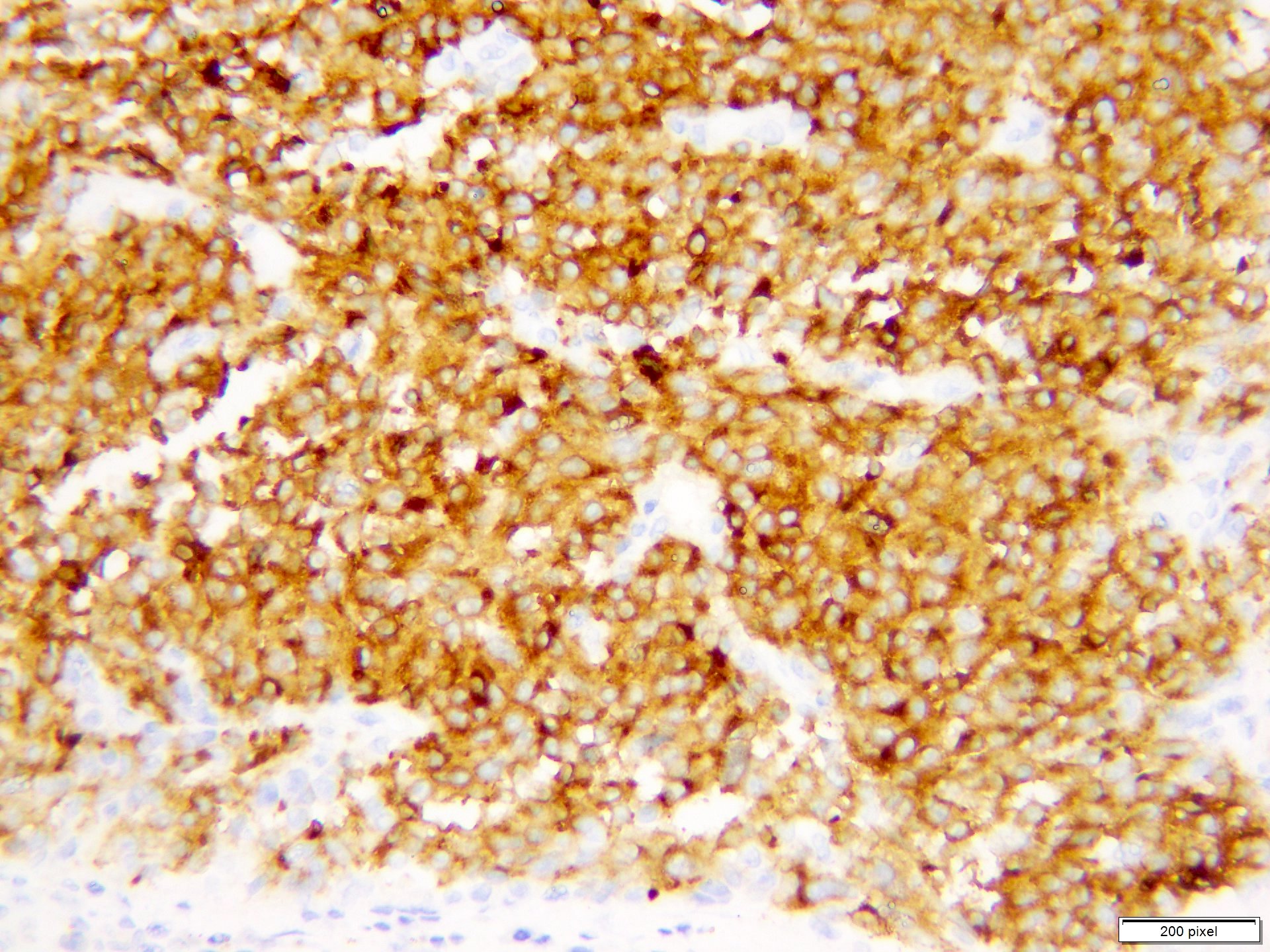
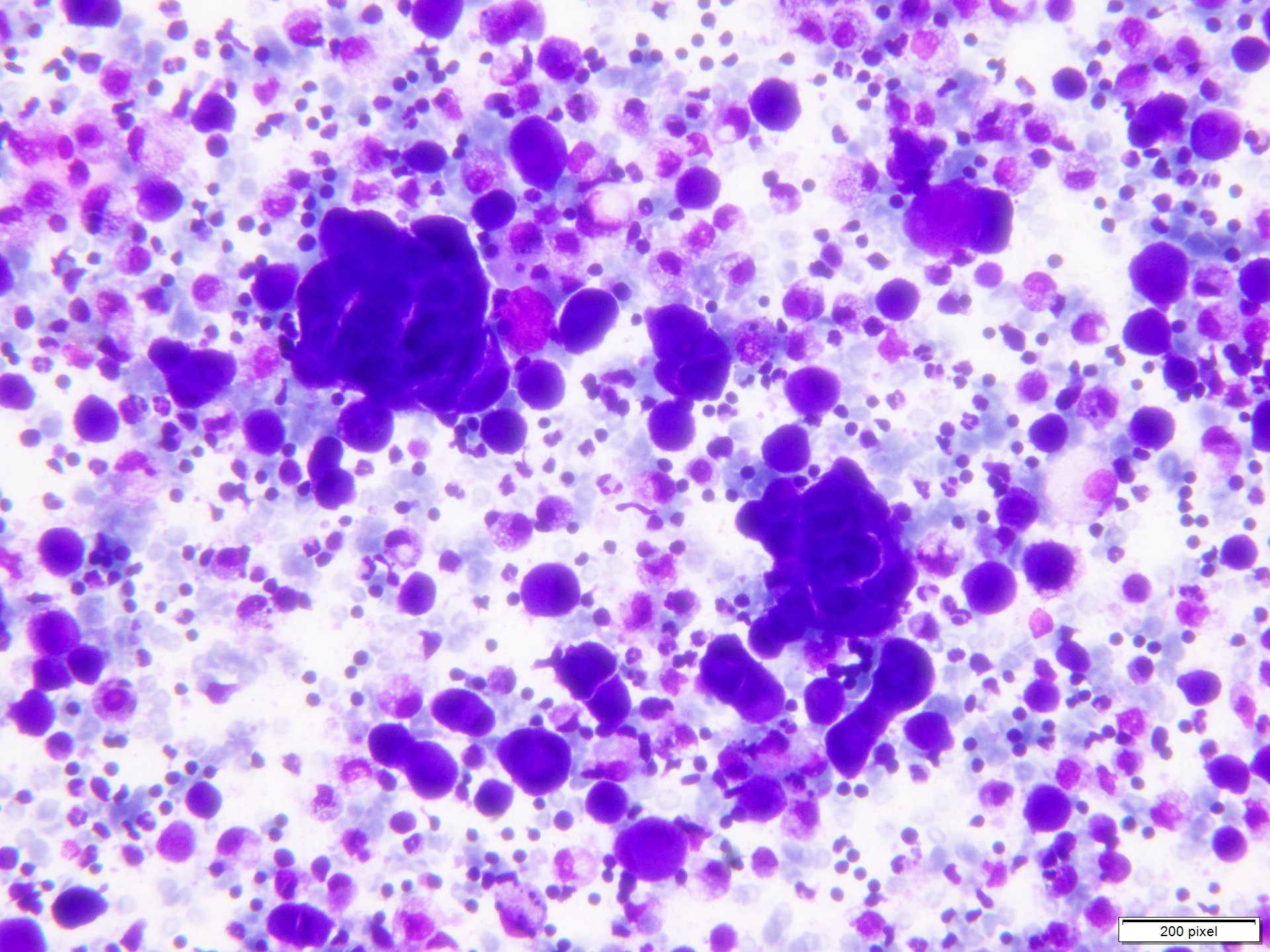
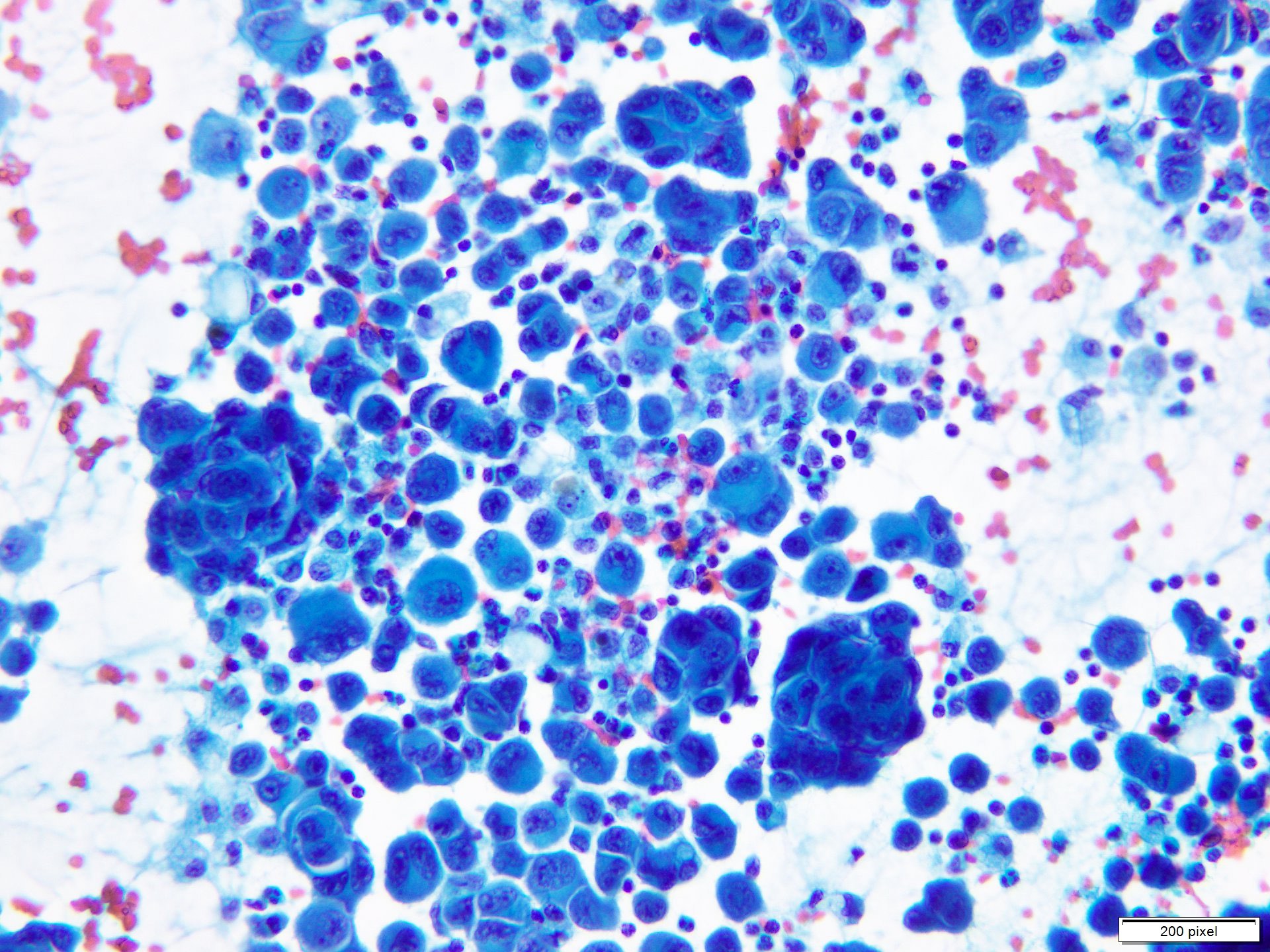
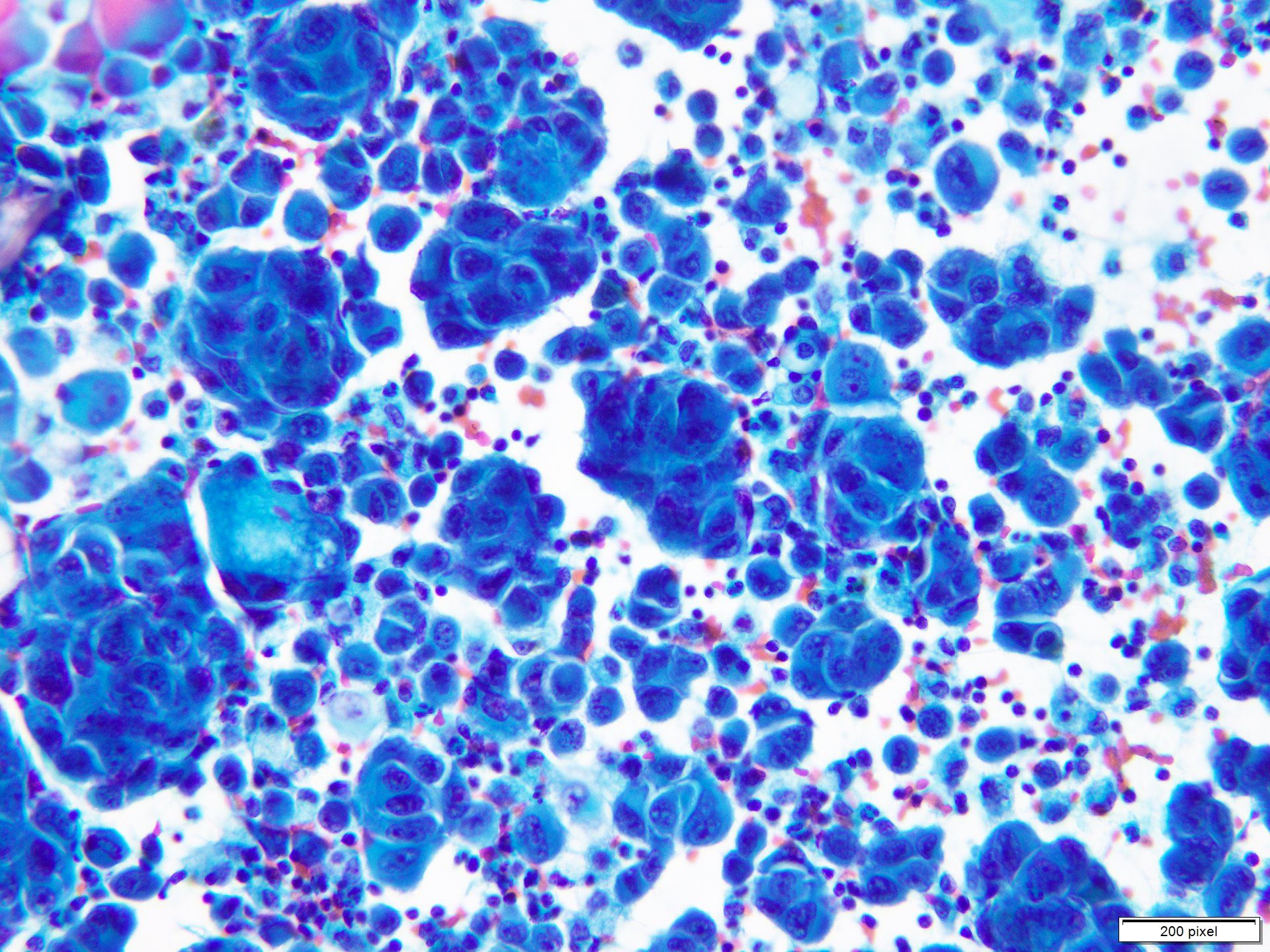
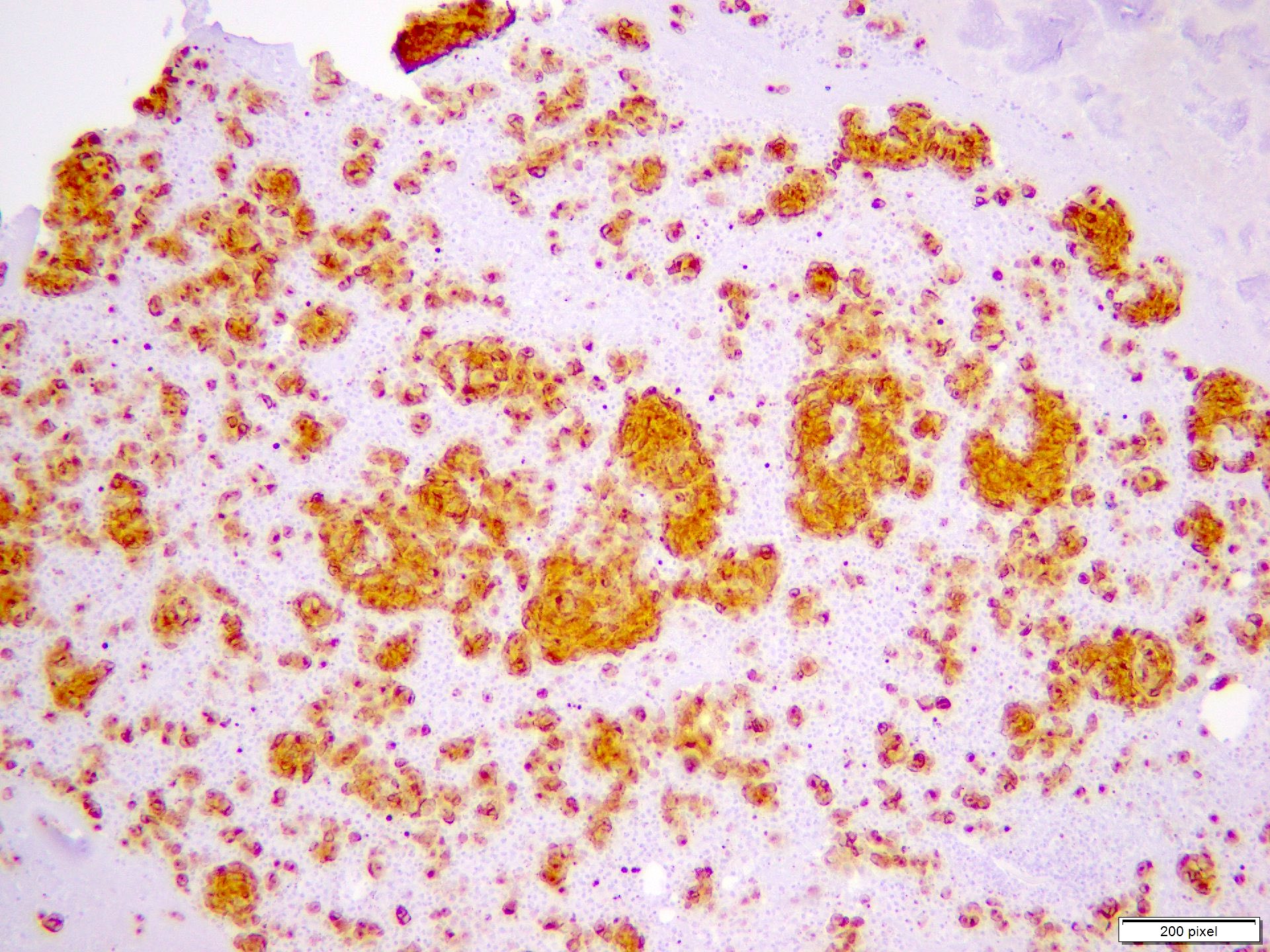
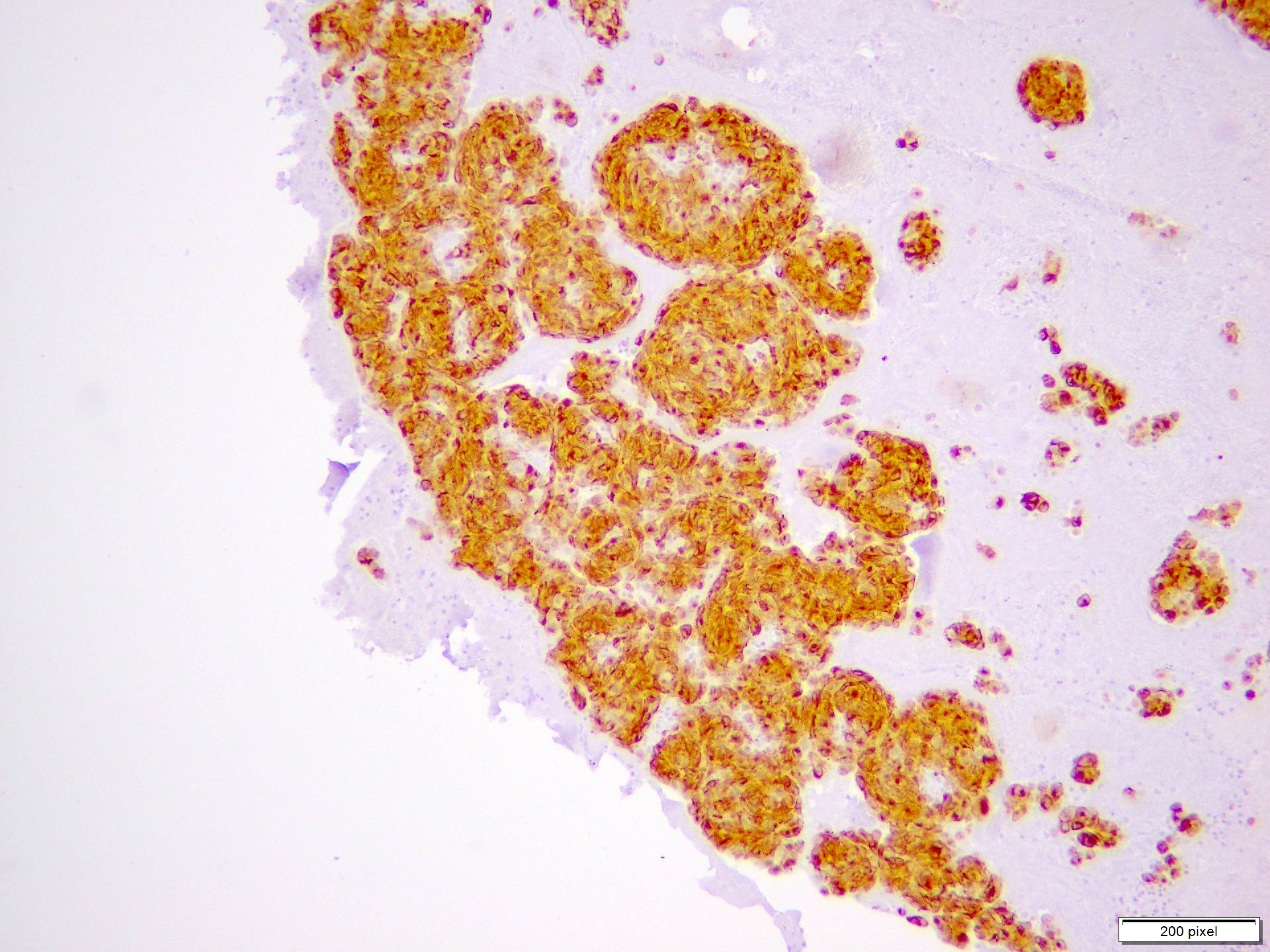
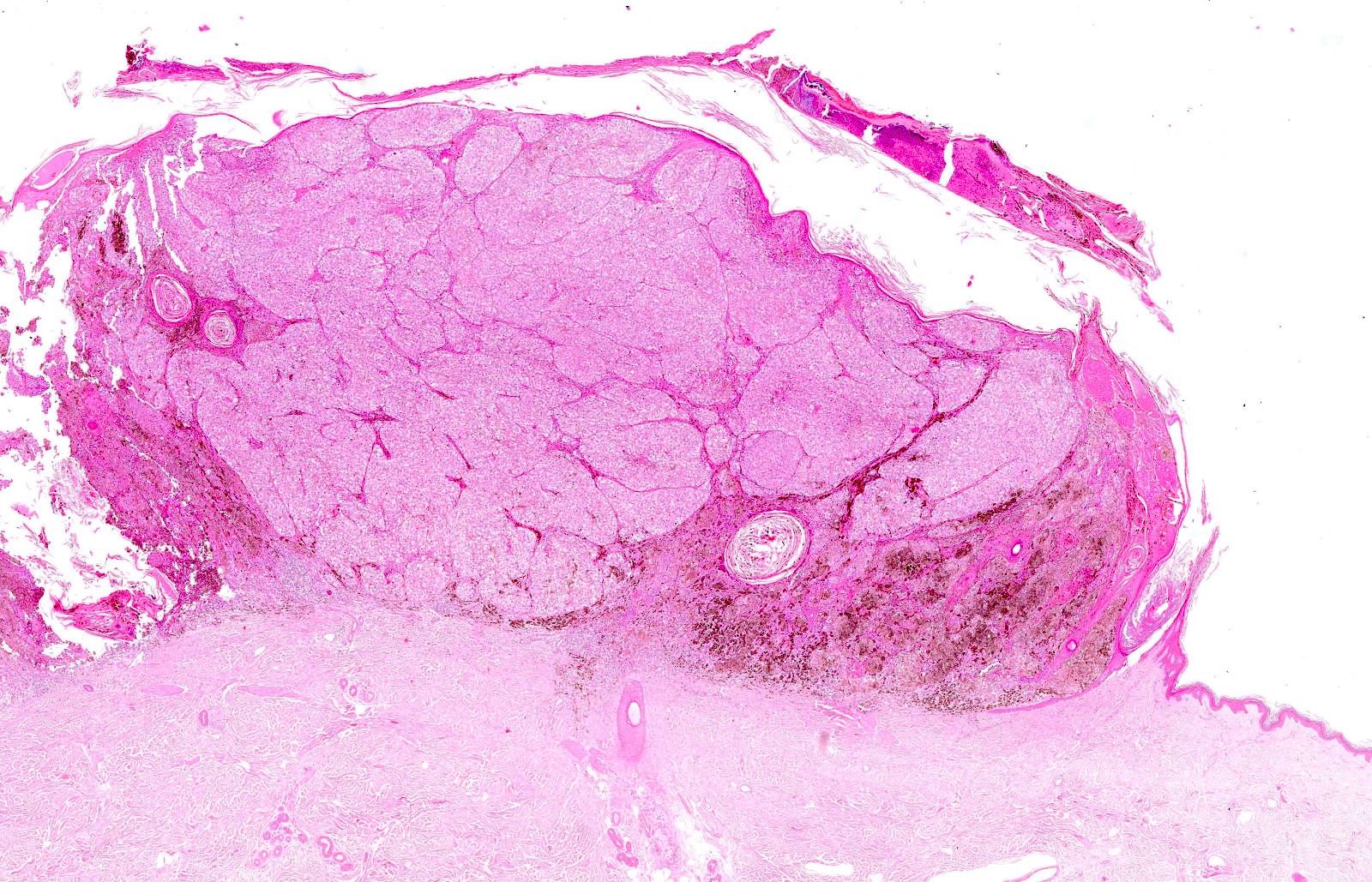
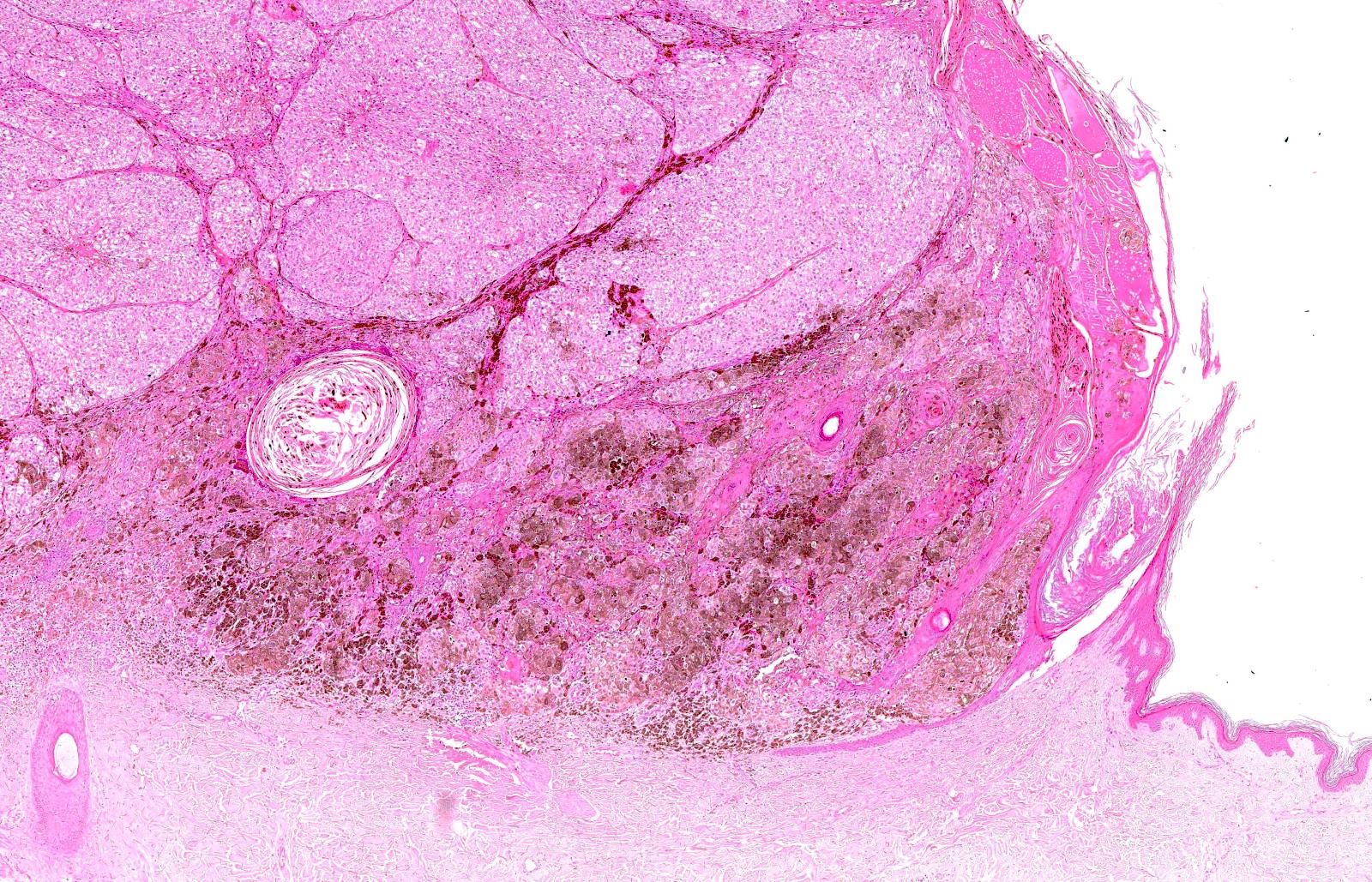
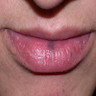
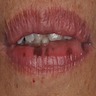
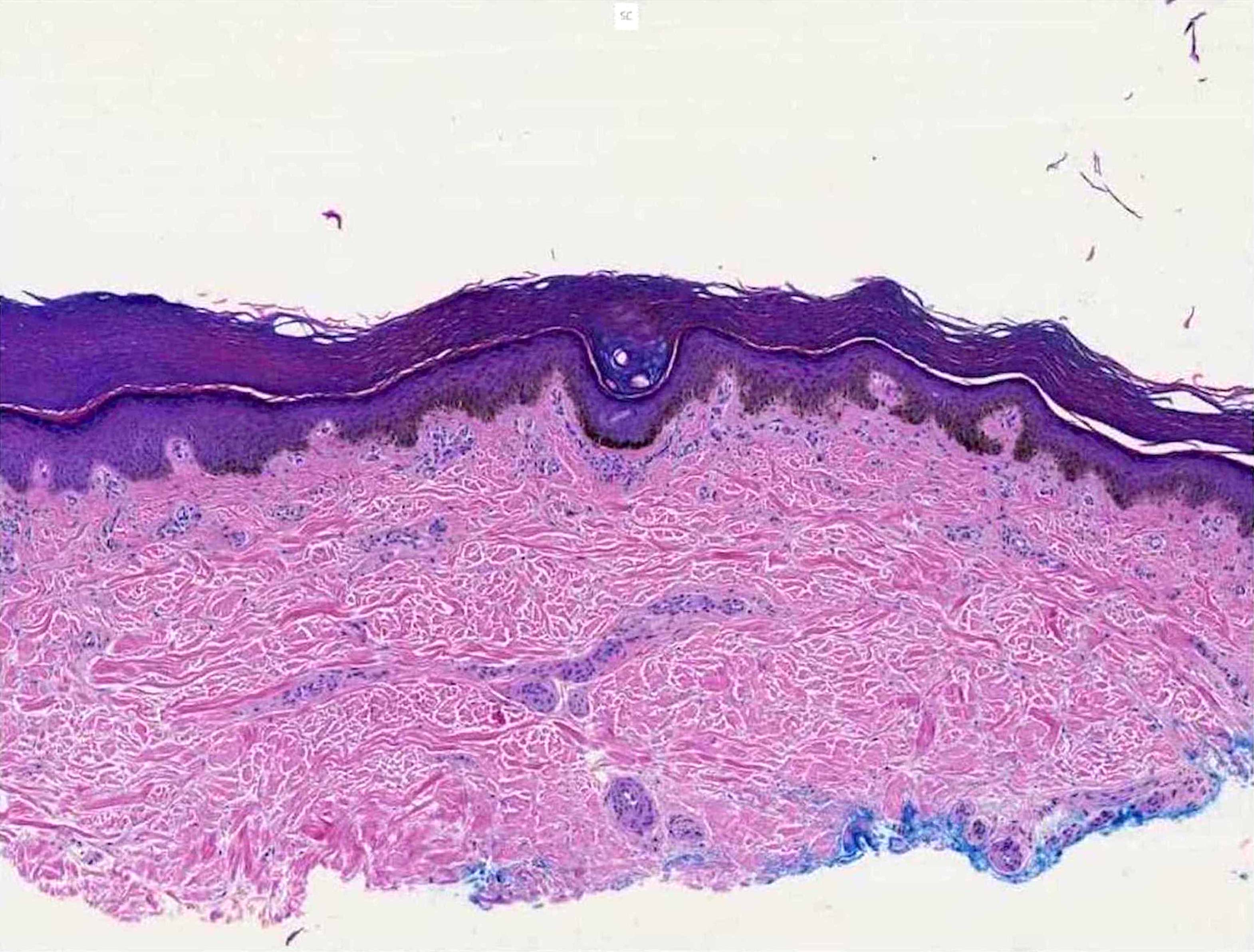
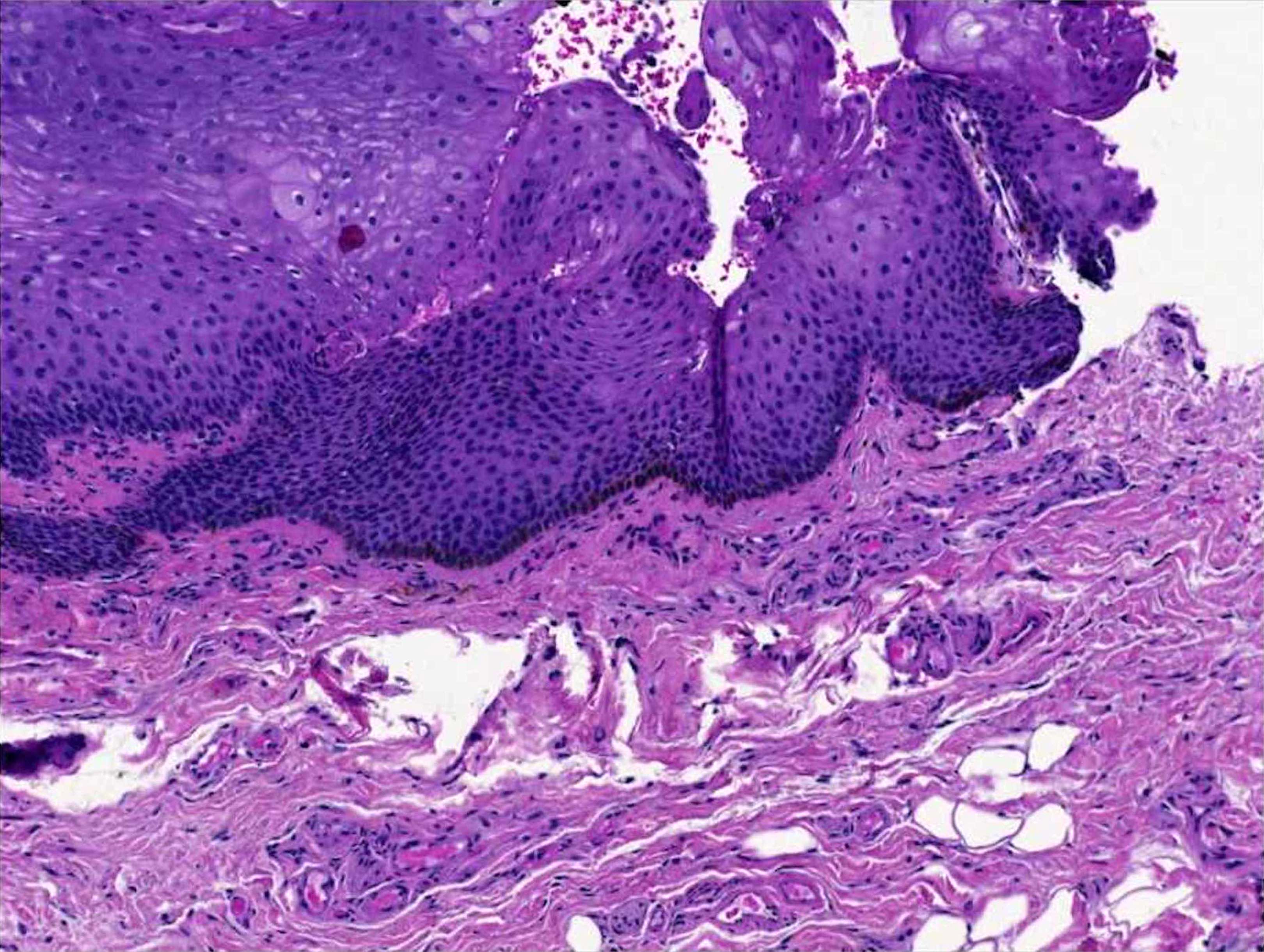
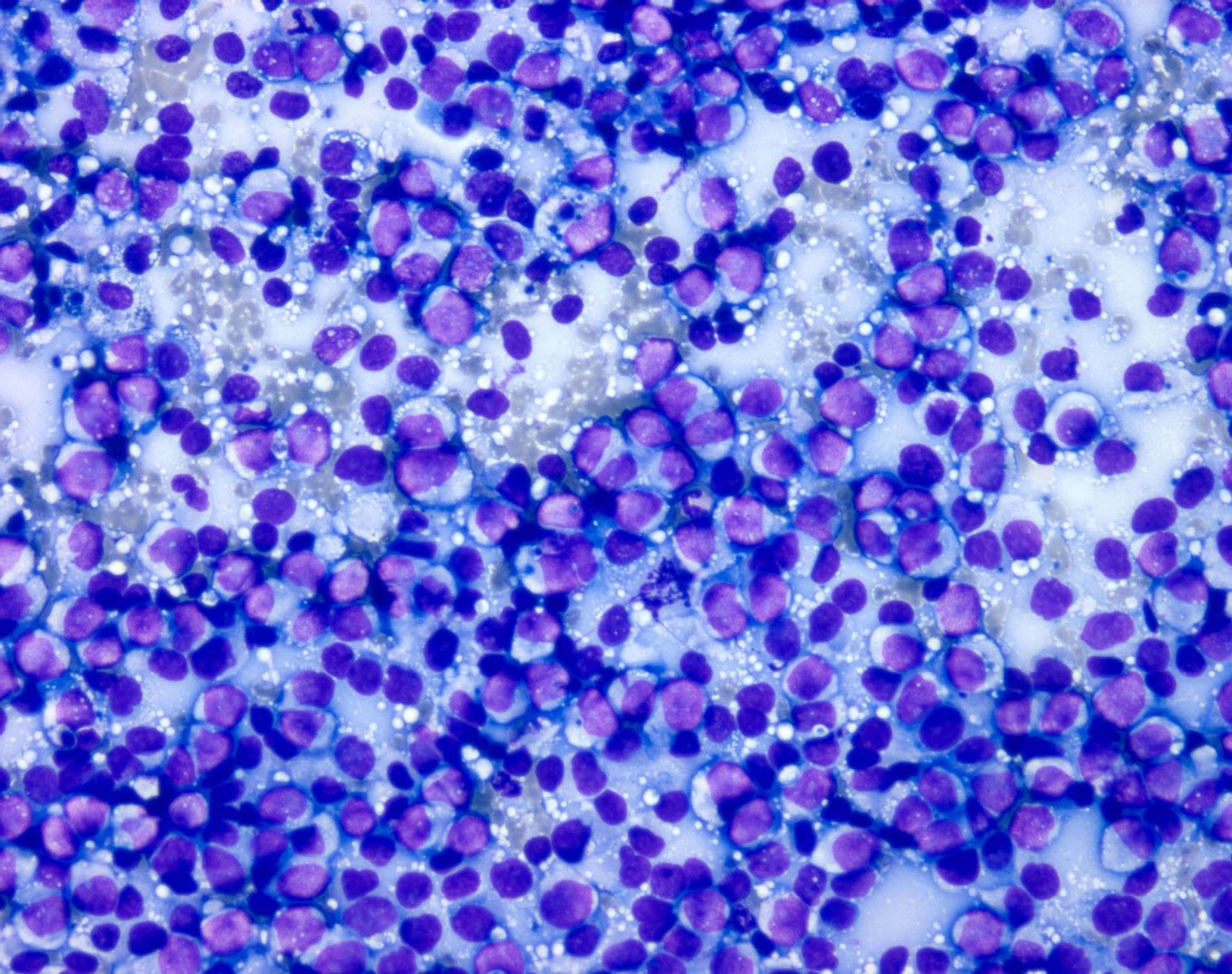
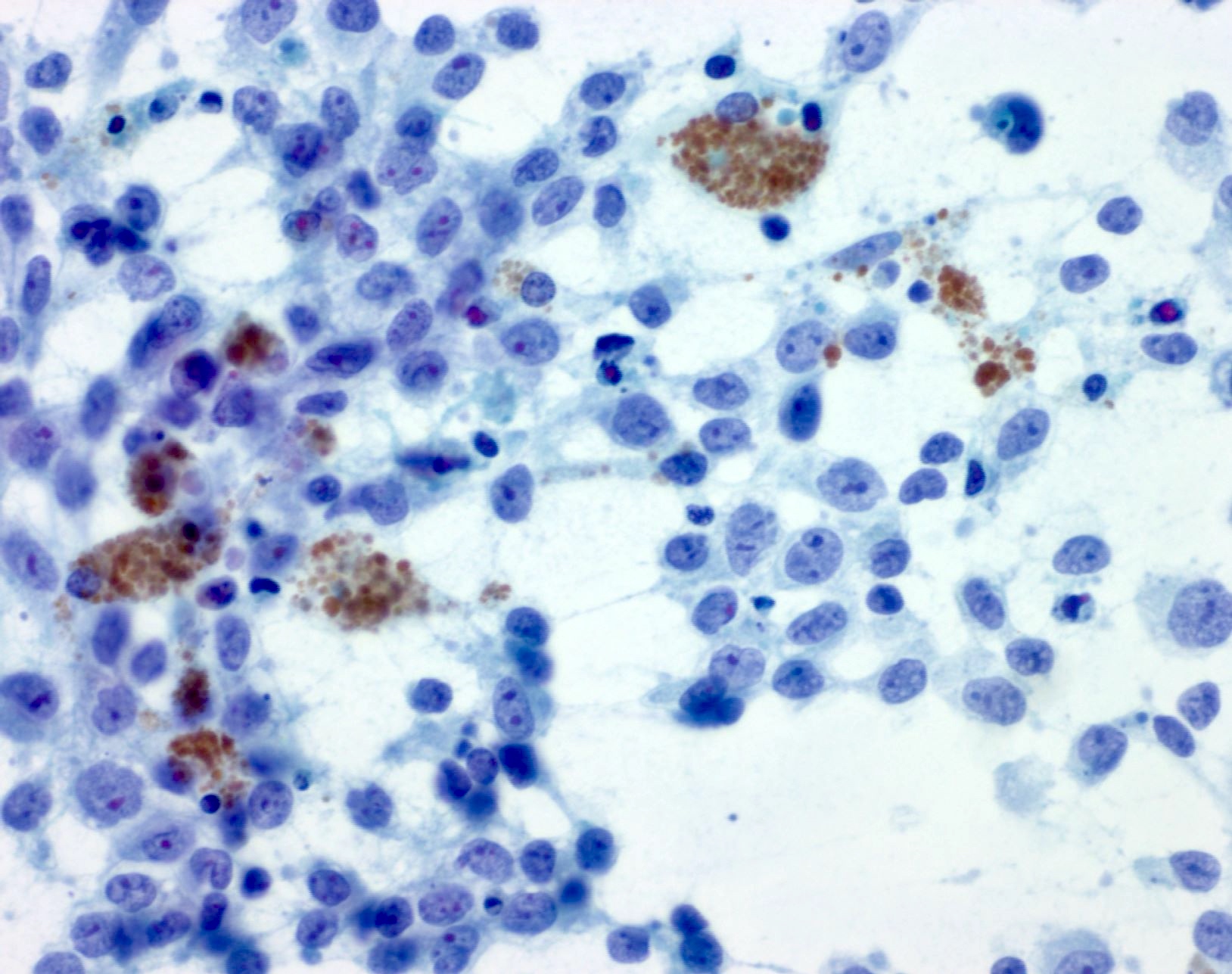
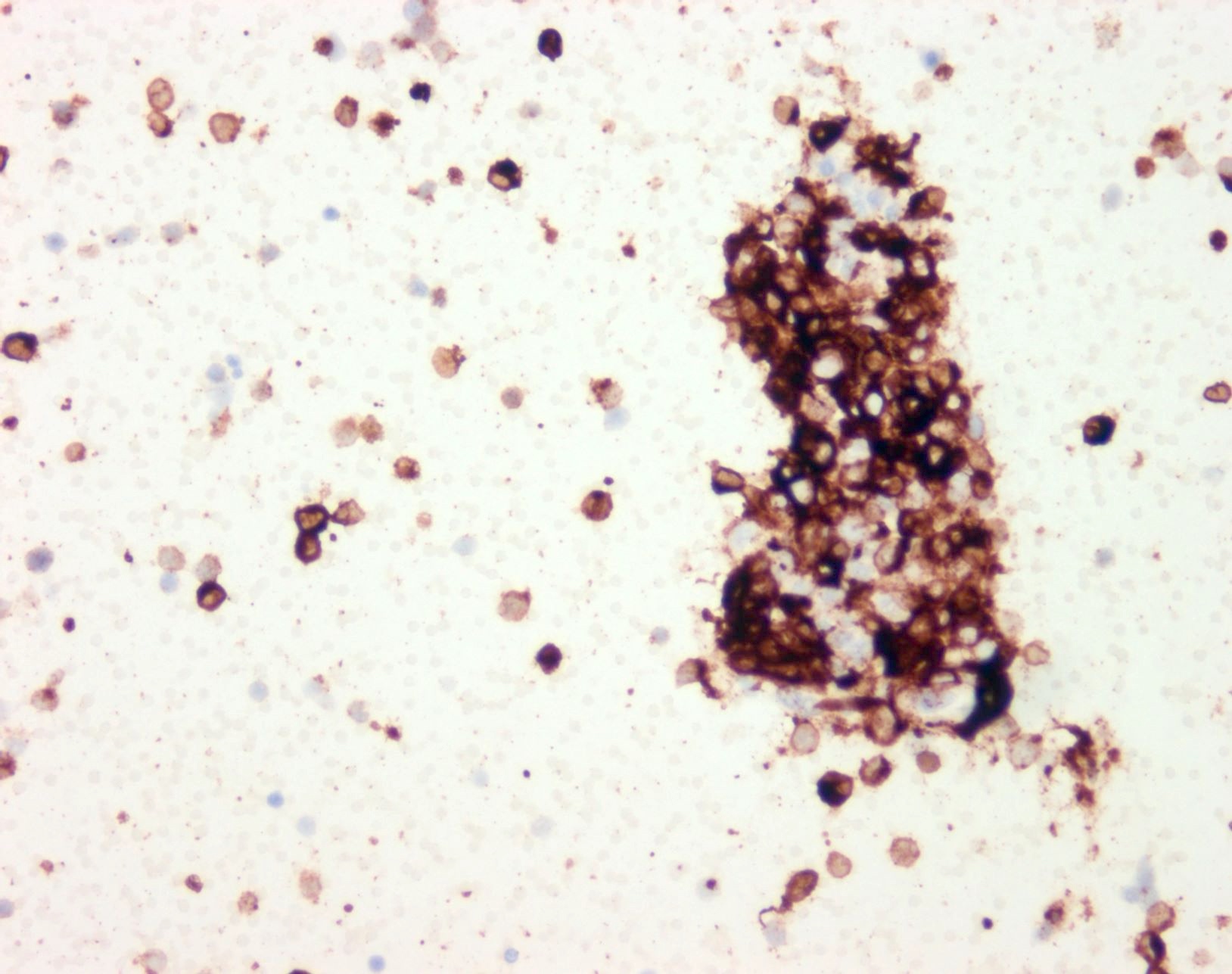
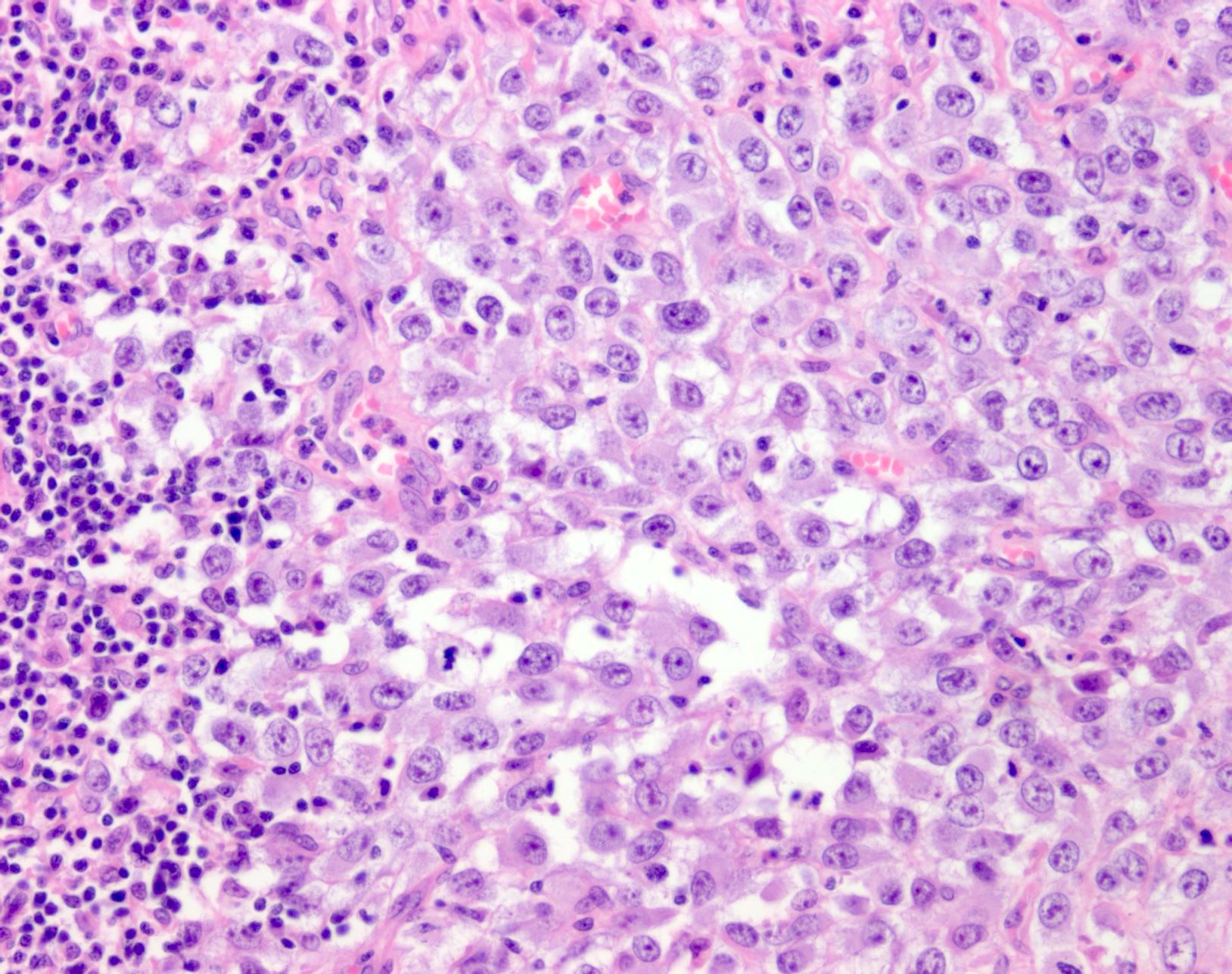
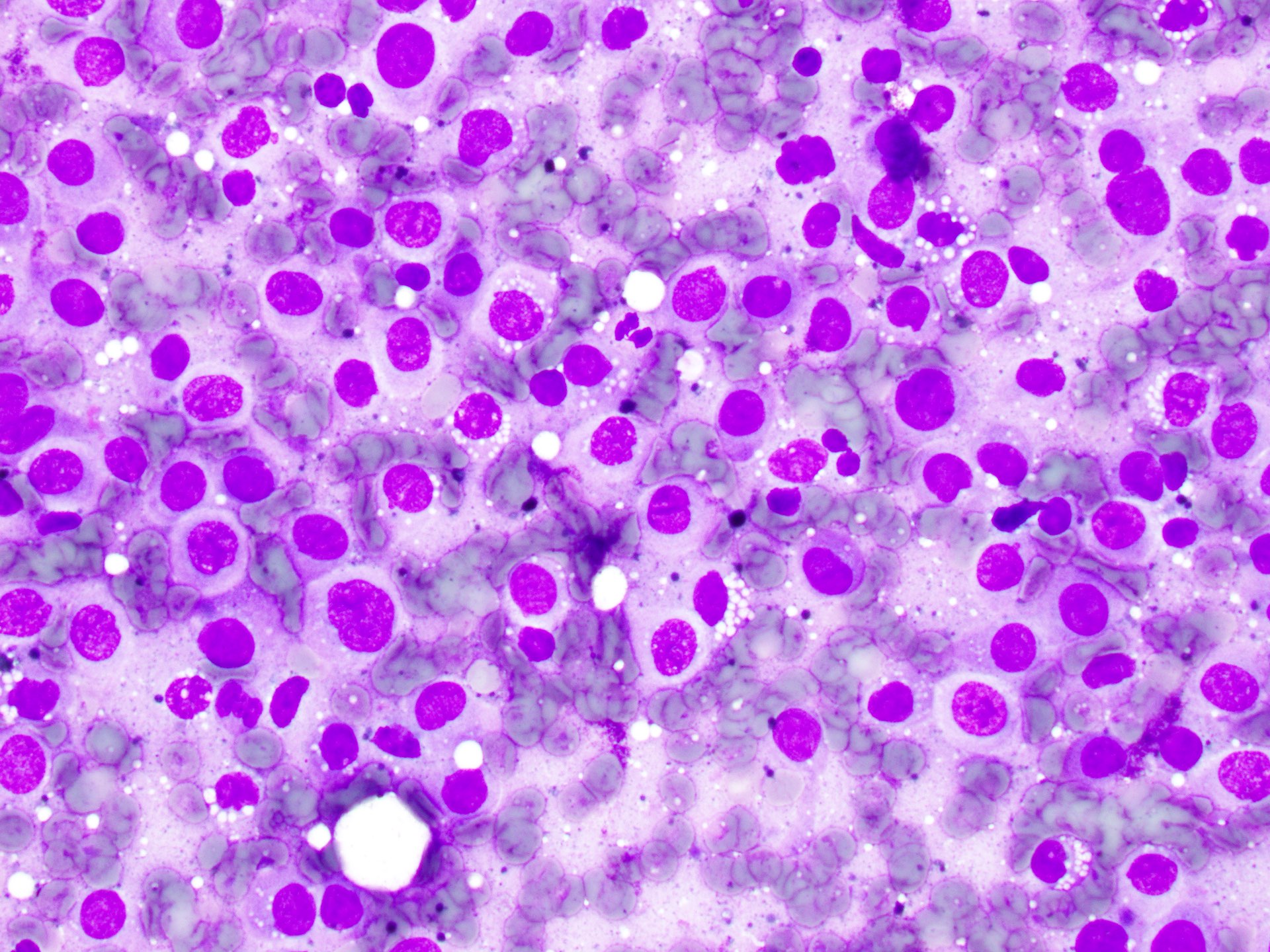
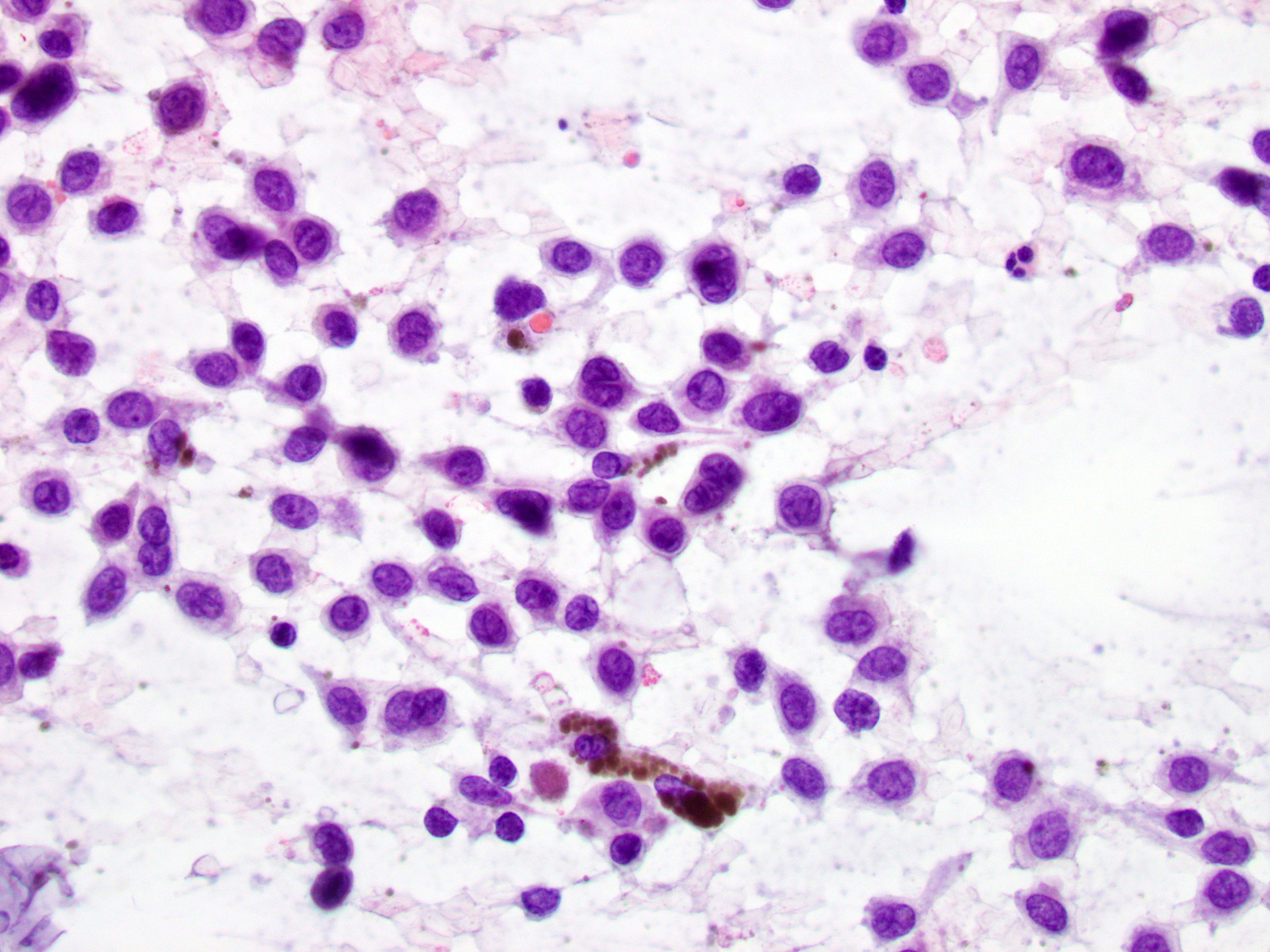
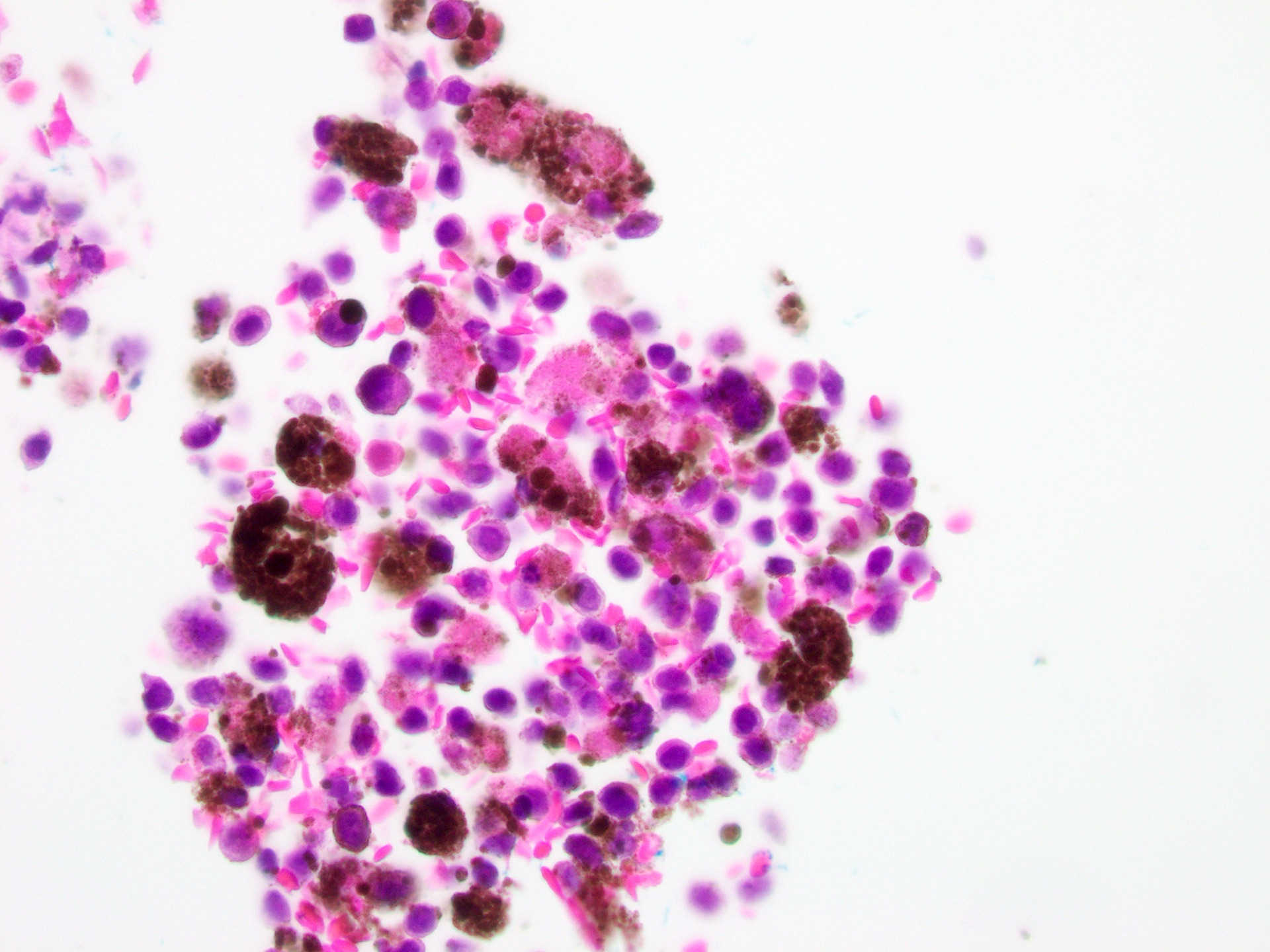
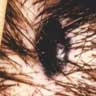
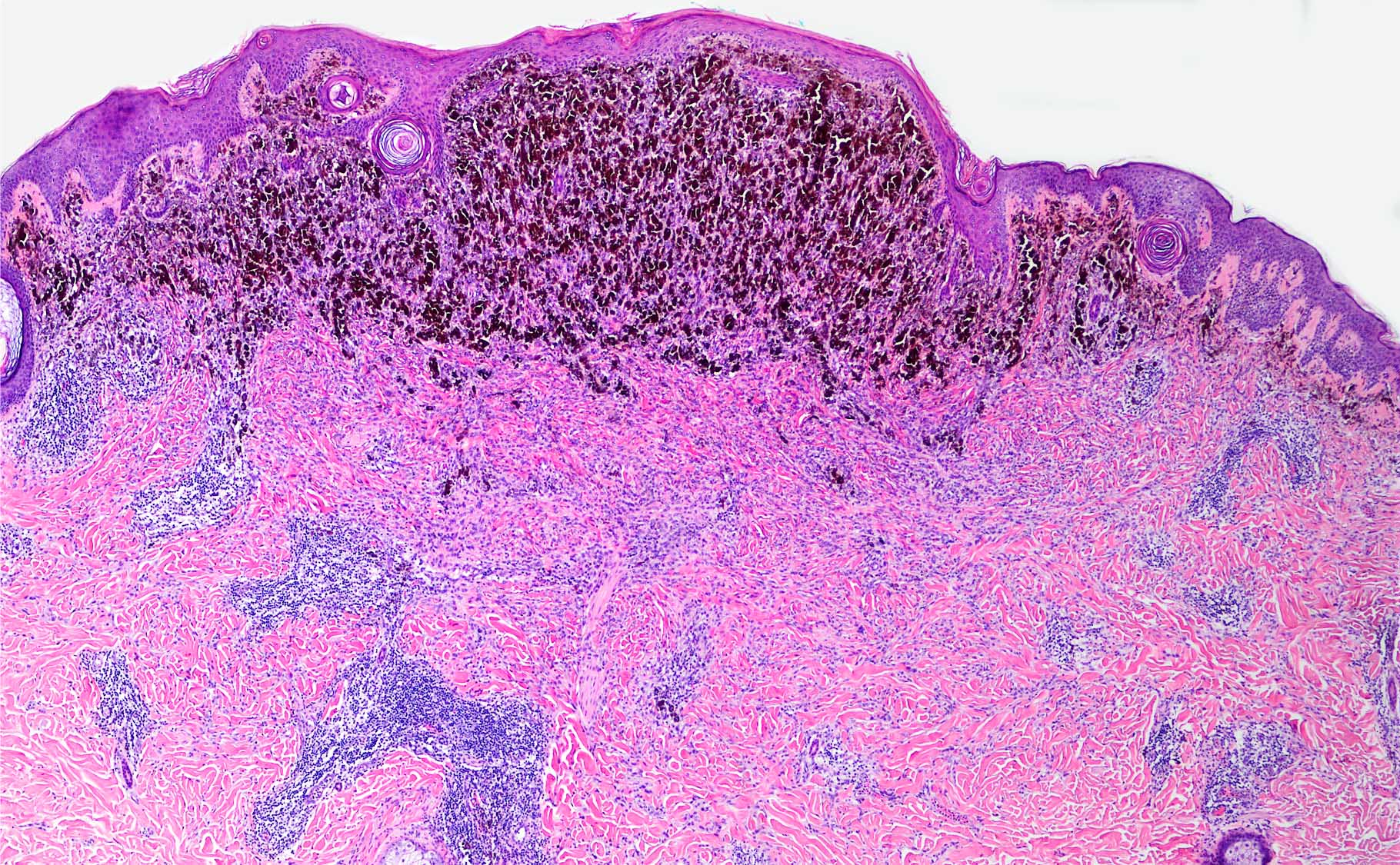
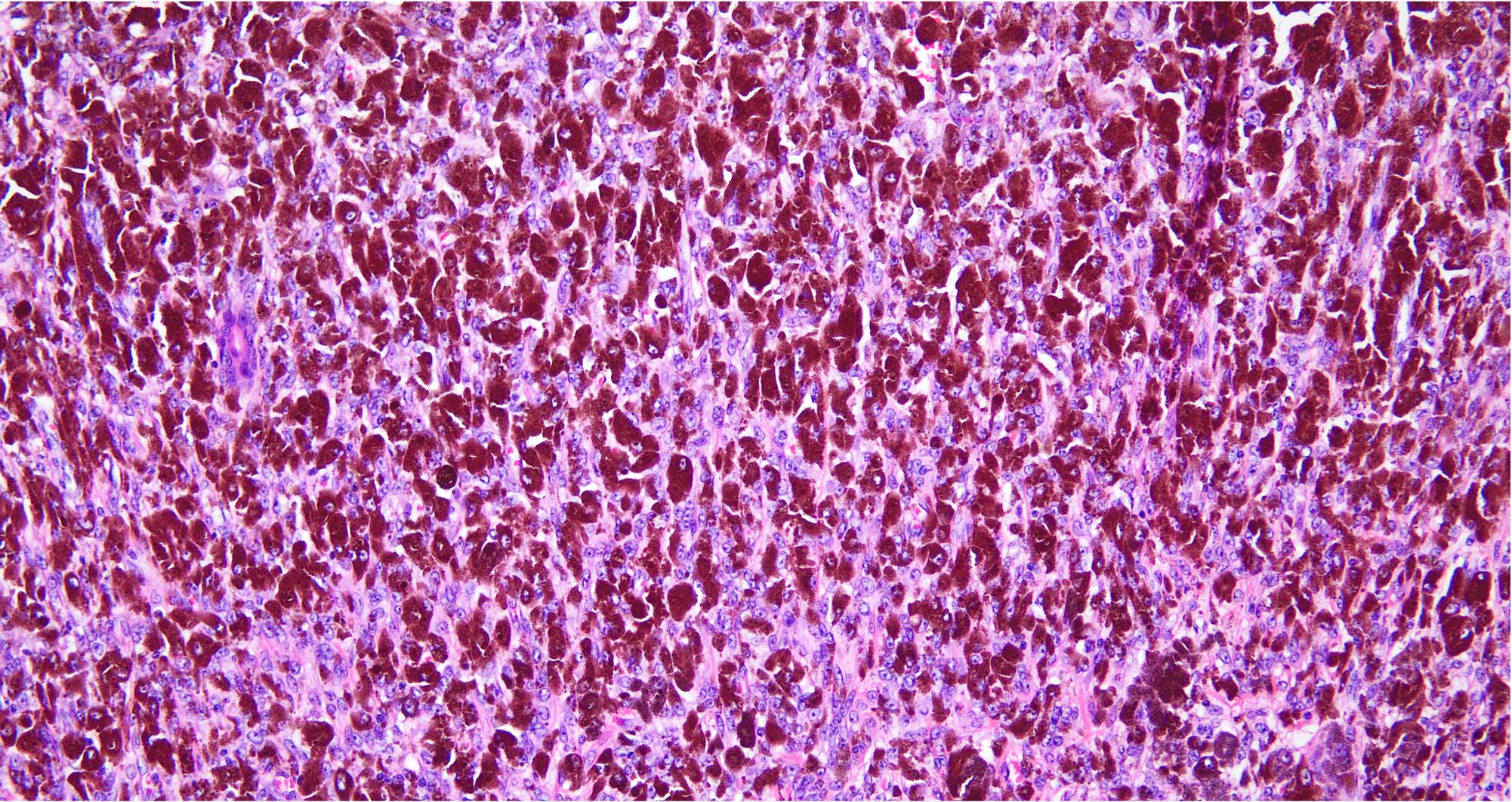
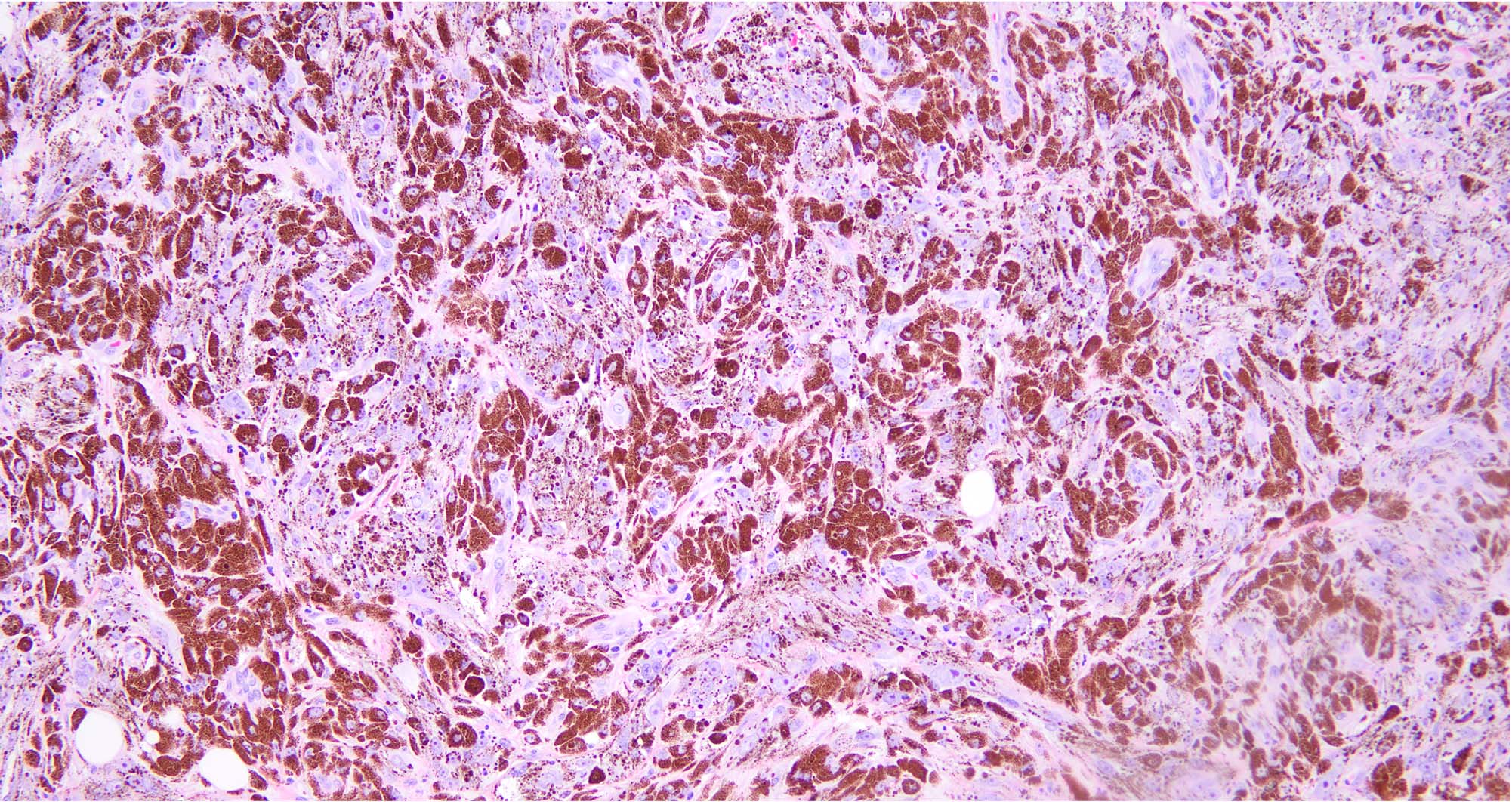
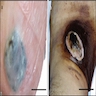
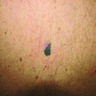
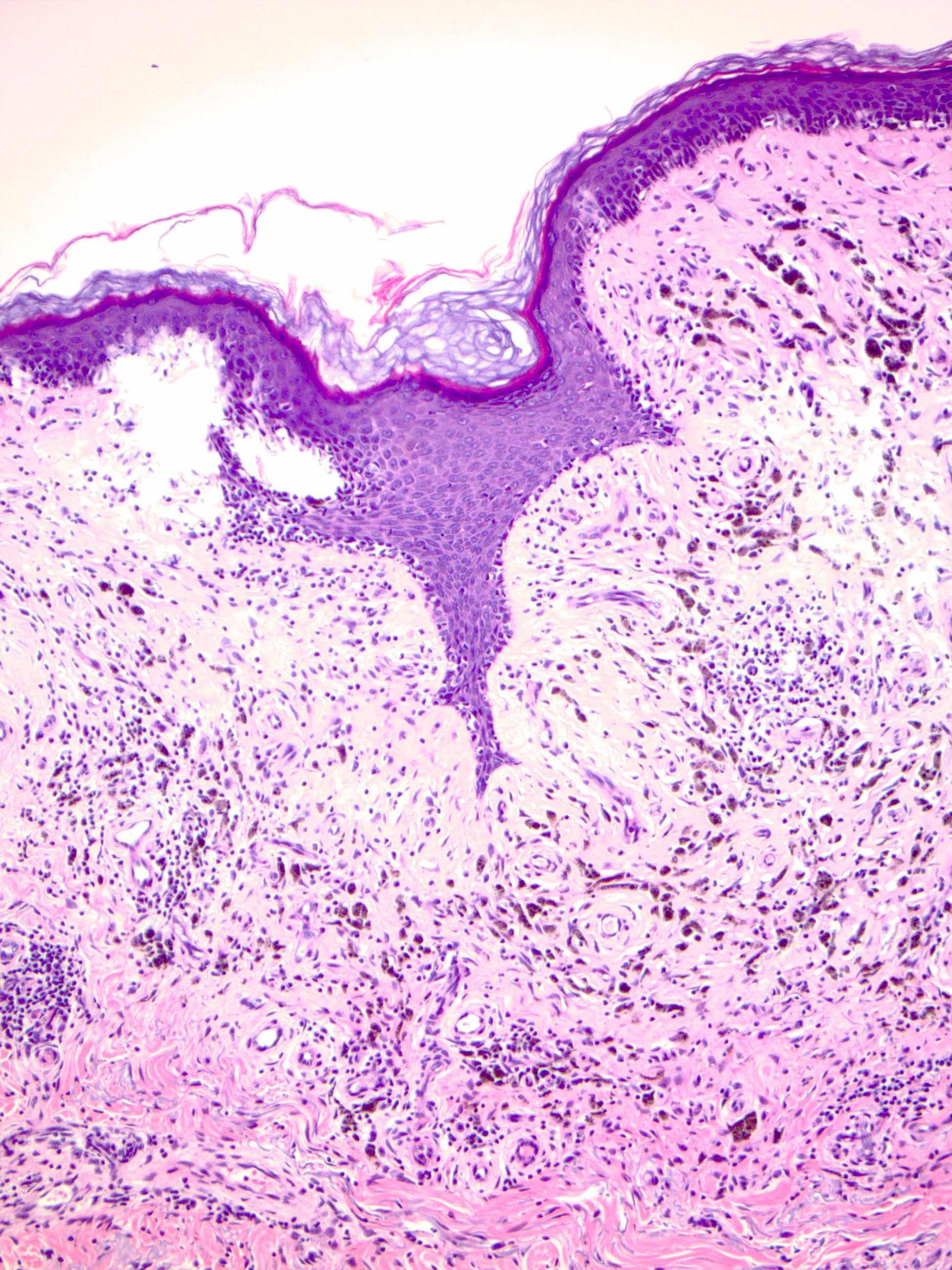
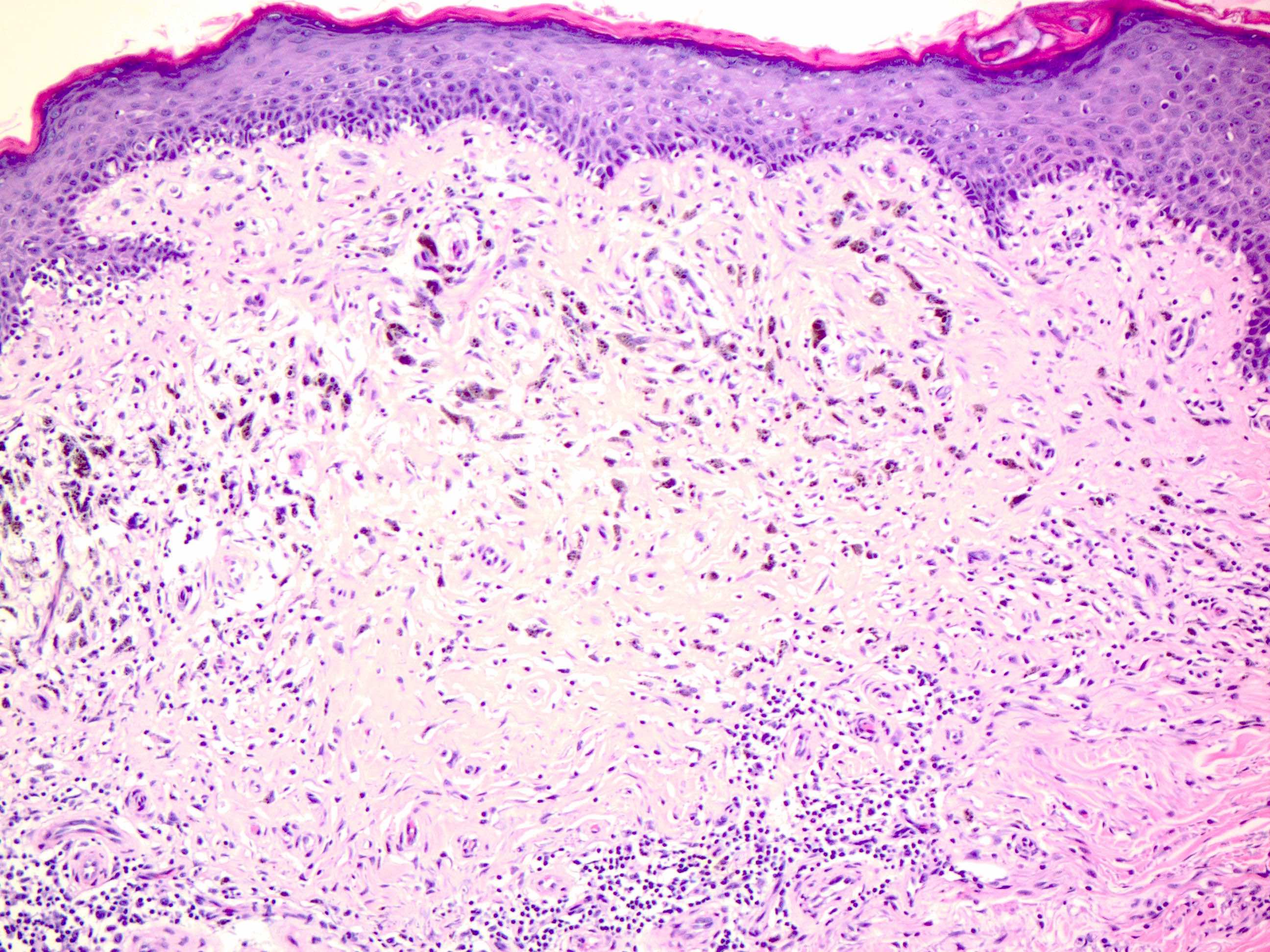
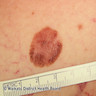
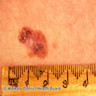
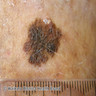
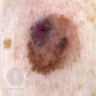
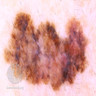
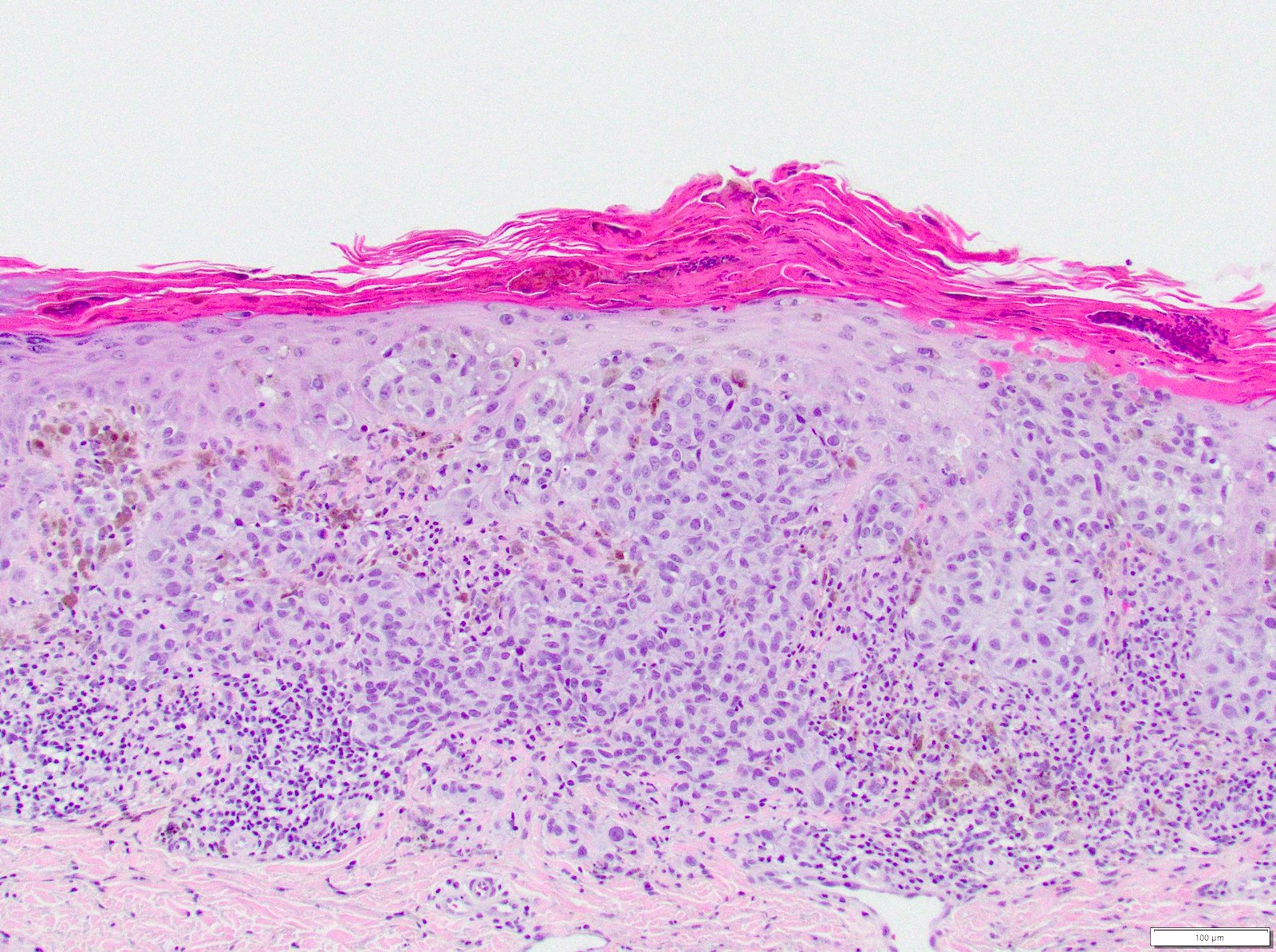
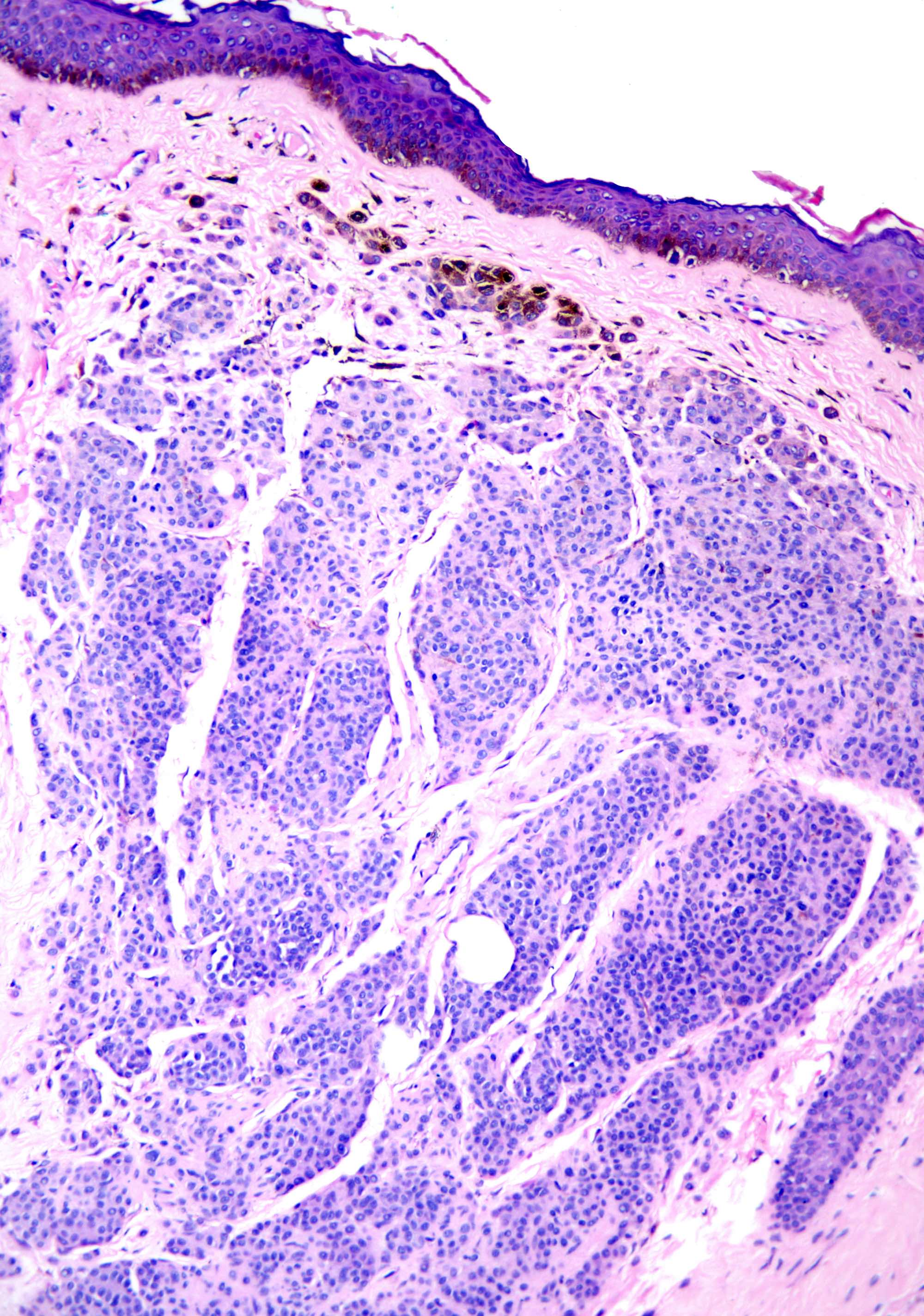
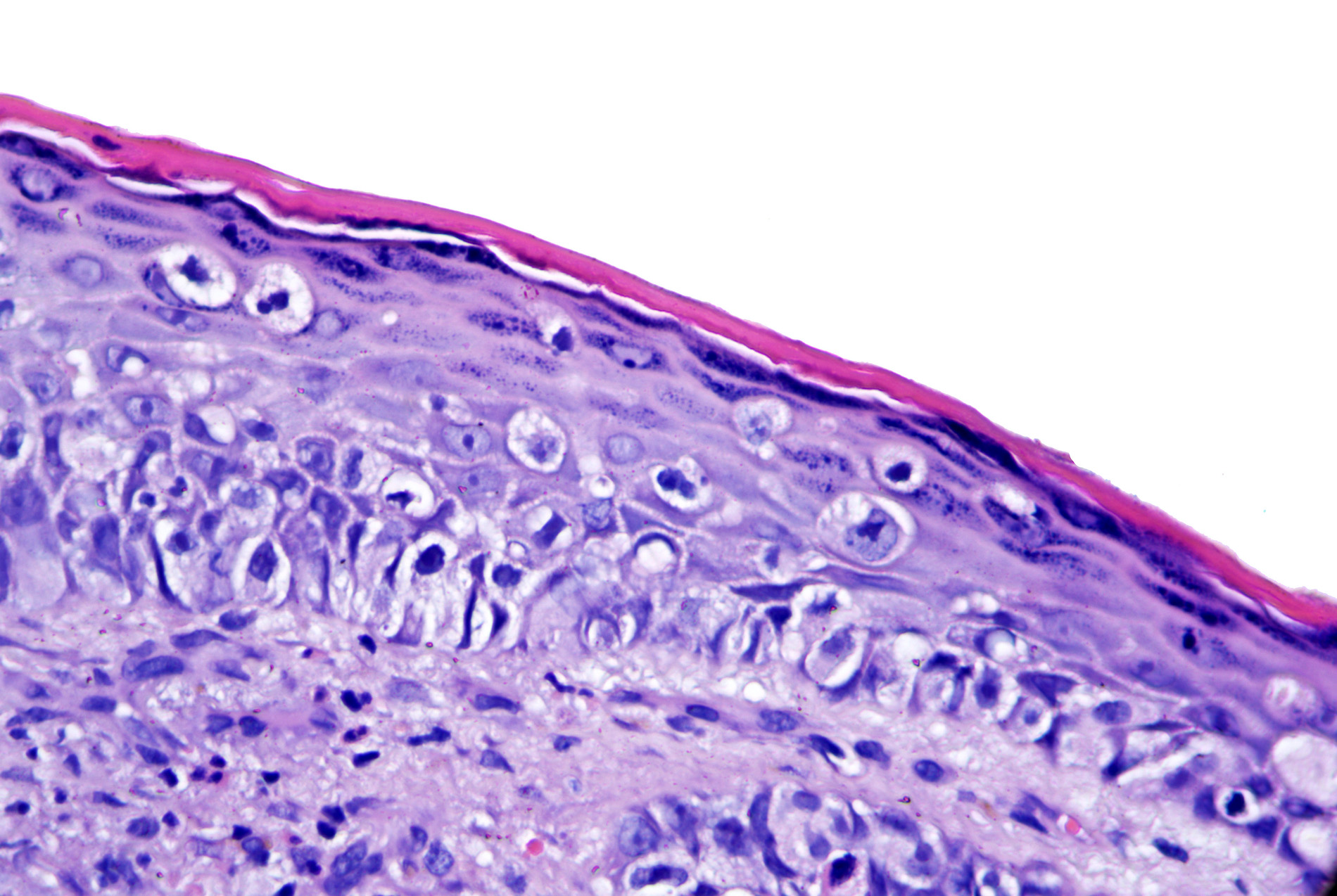
- pTX: thickness cannot be assessed (e.g. curettage specimen)
- pT0: no evidence of primary tumor (unidentified or completely regressed primary)
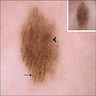
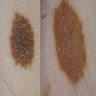
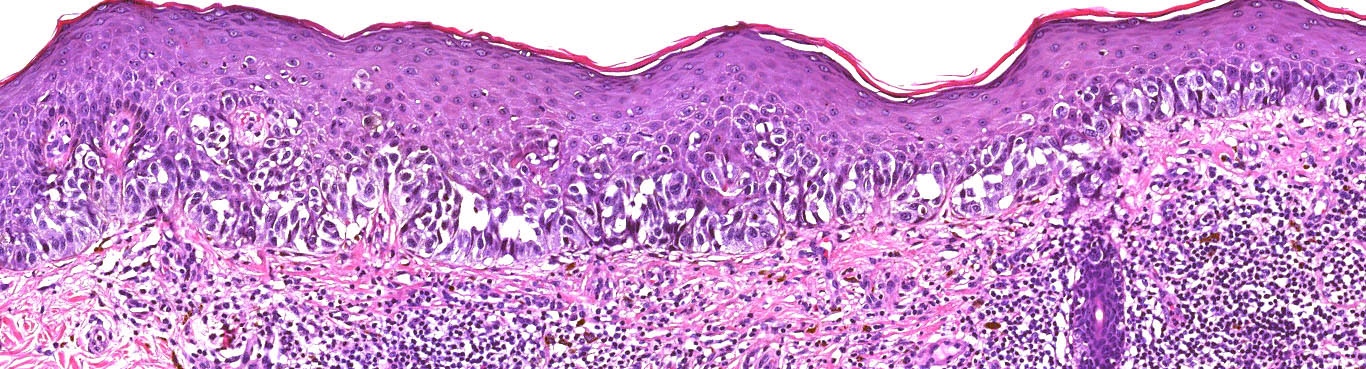
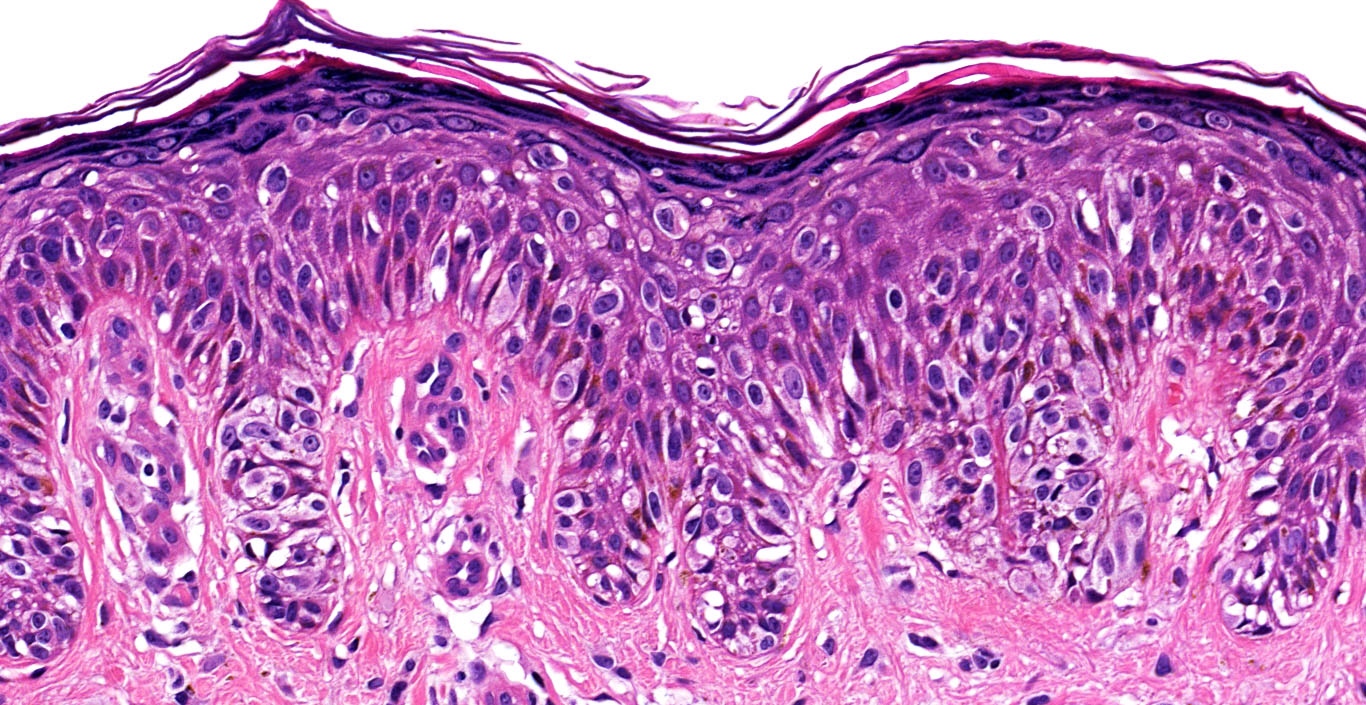
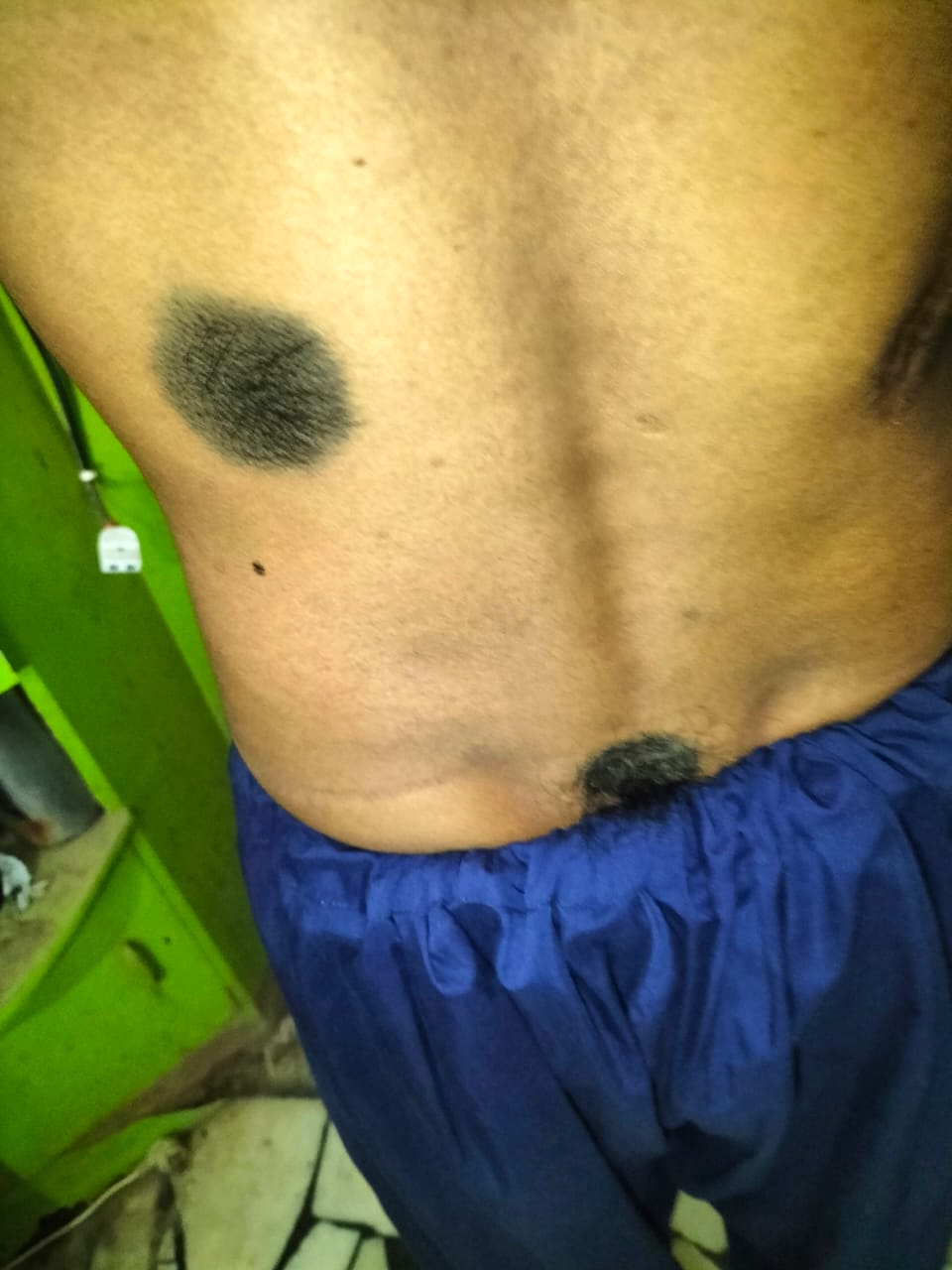
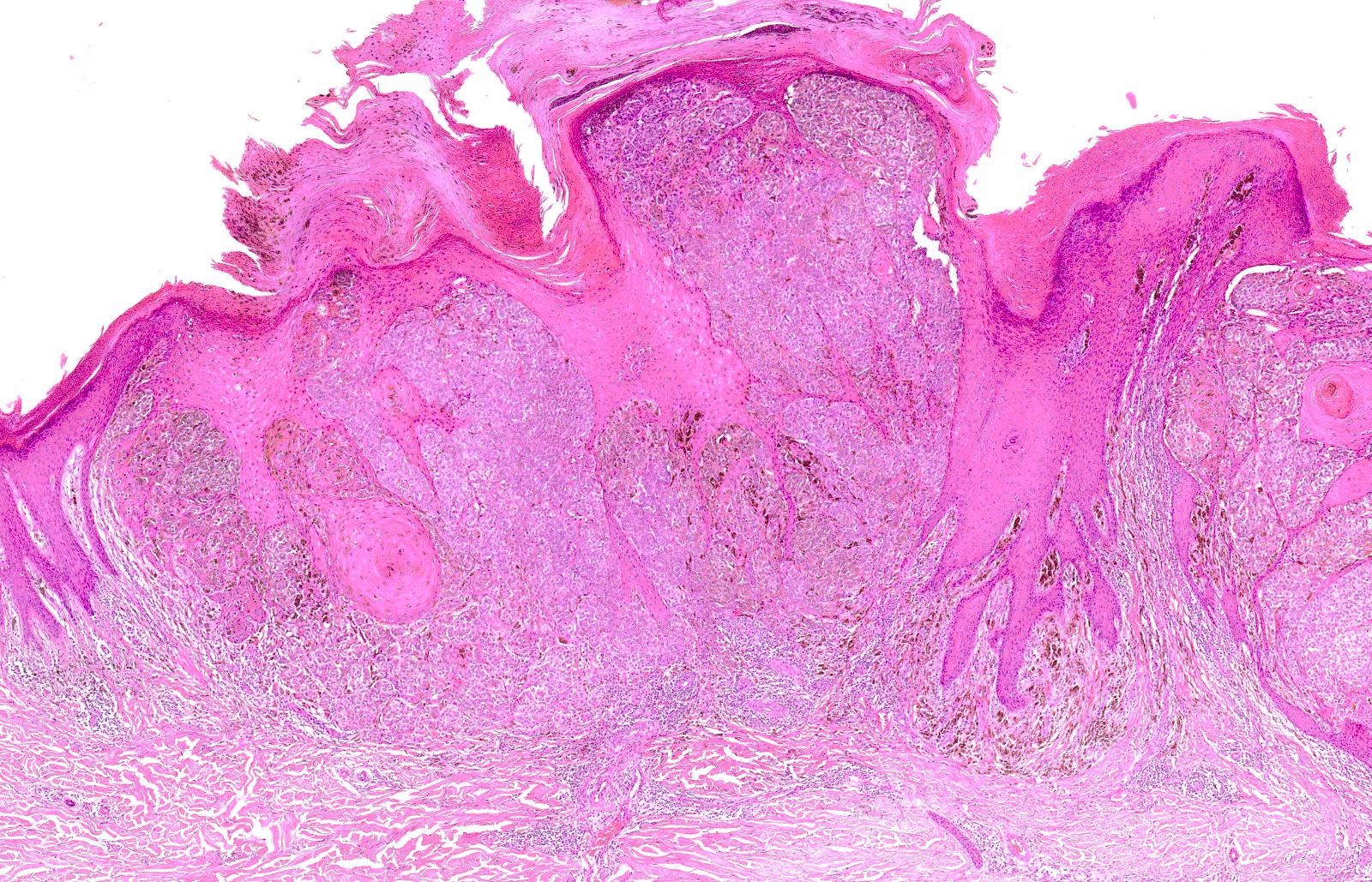
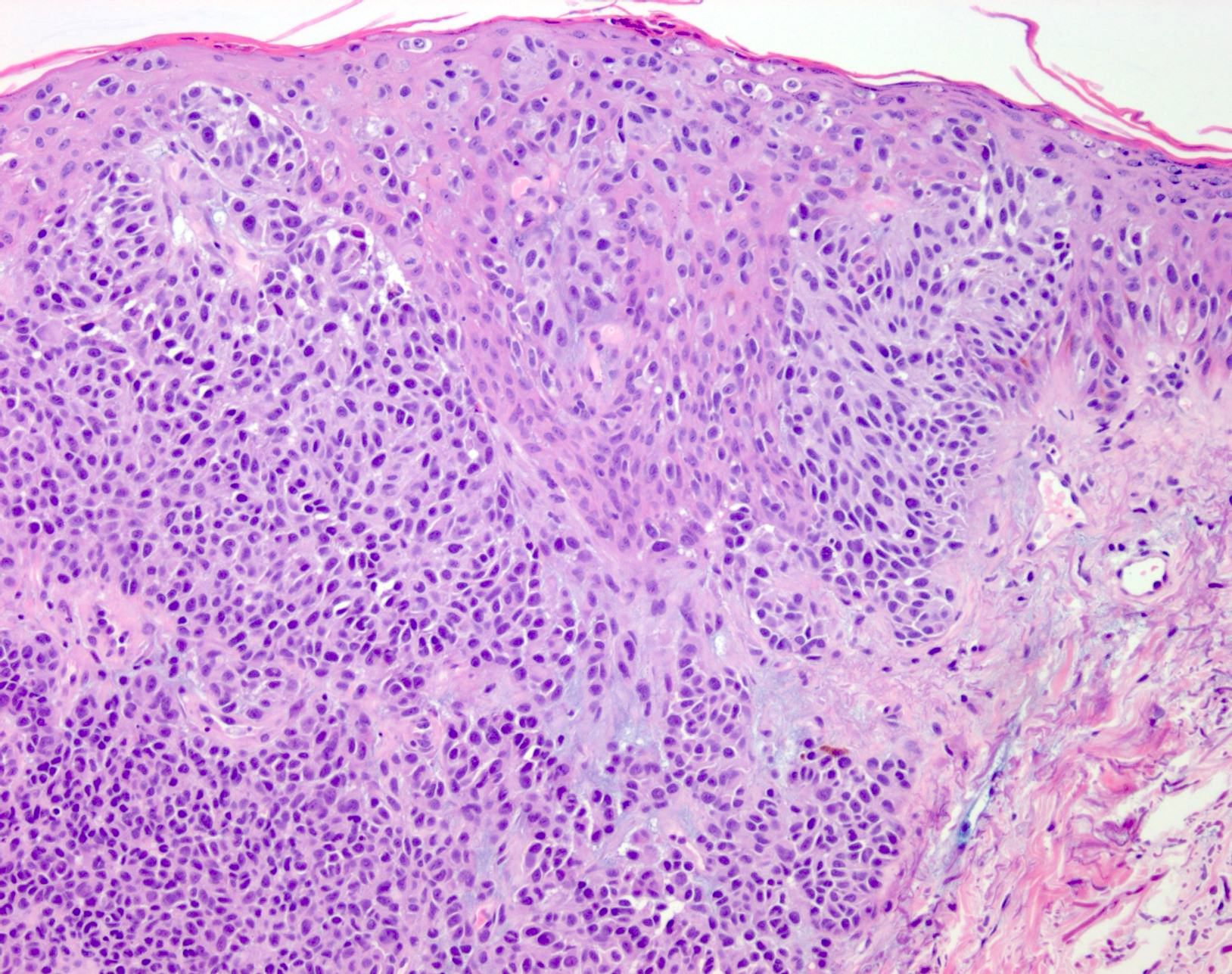
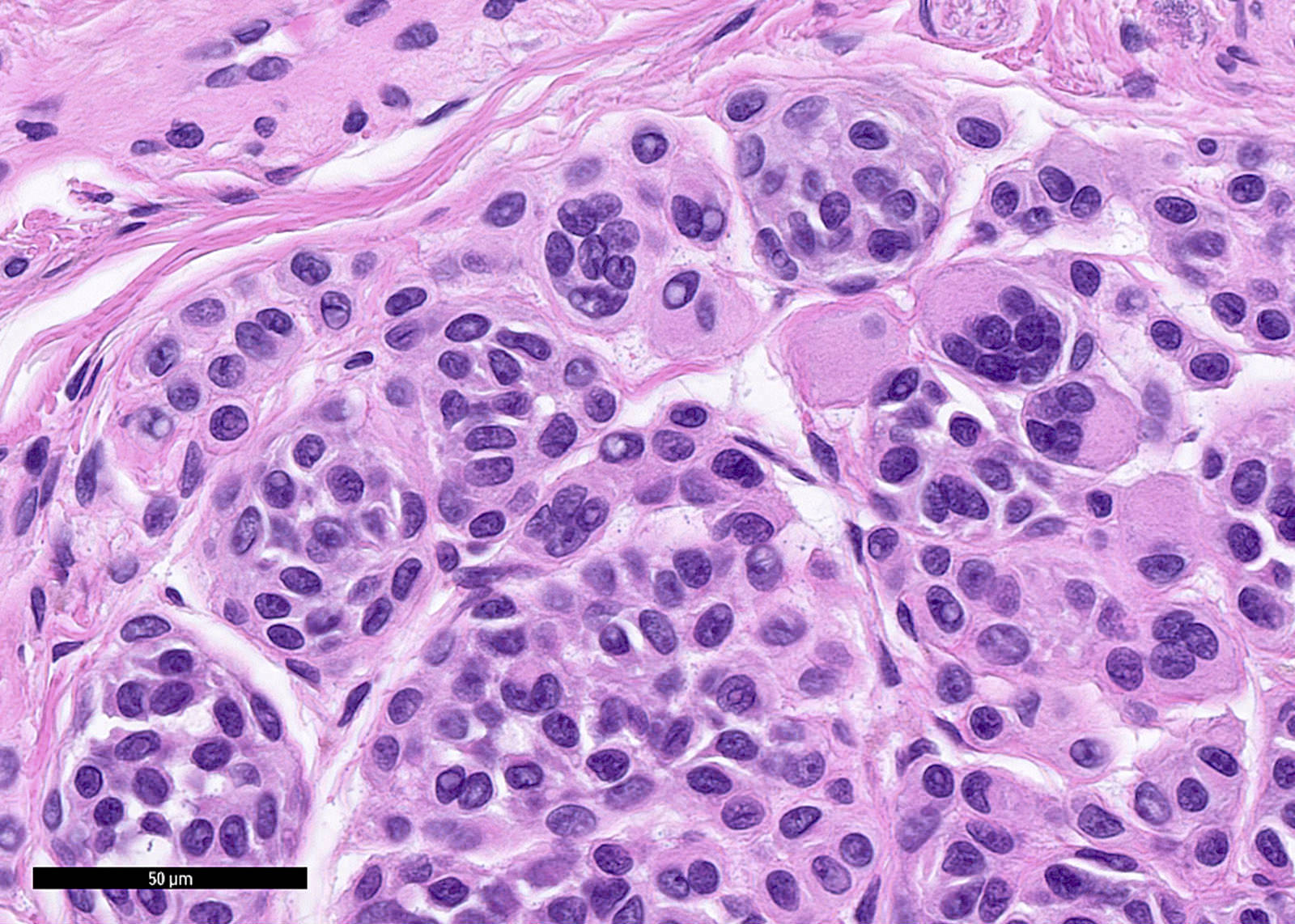
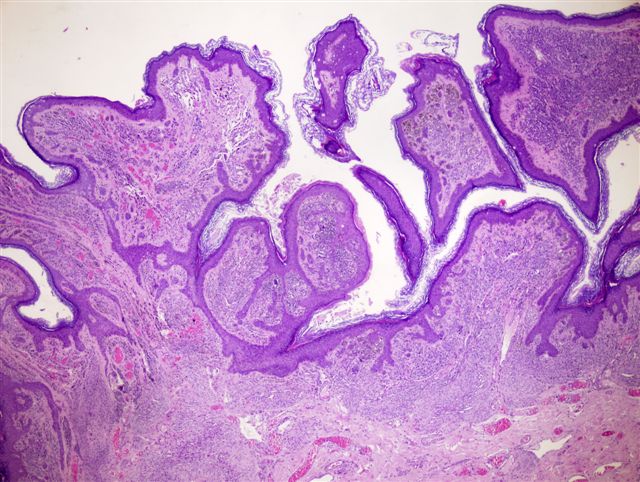
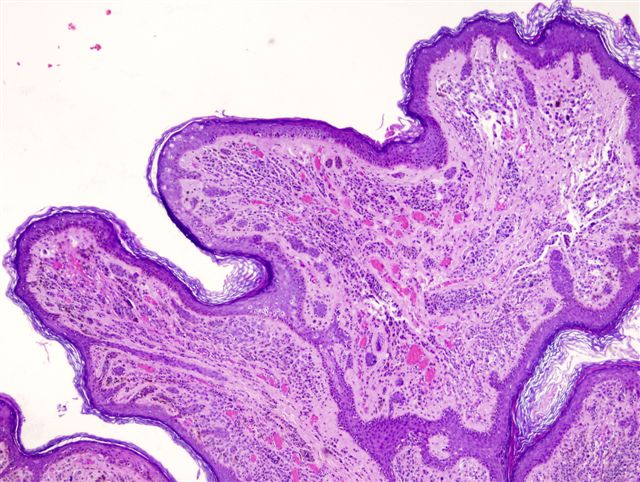
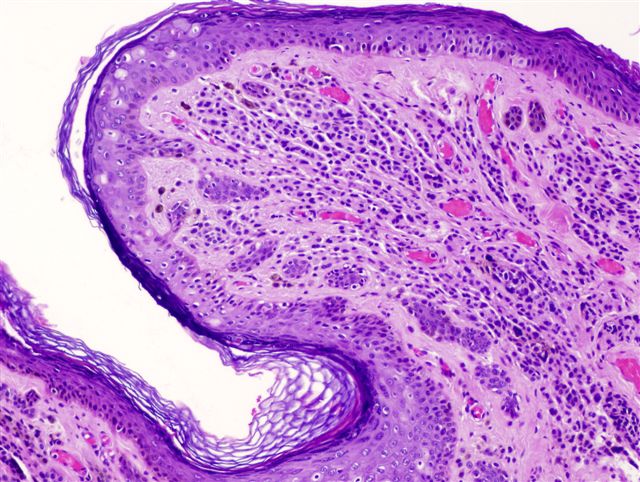
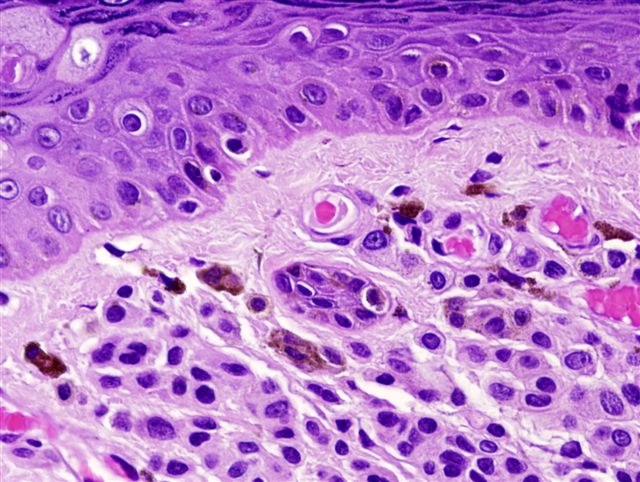
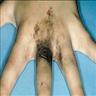
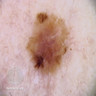
- pTis: melanoma in situ
- pT1a: < 0.8 mm thickness without ulceration
- pT1b: < 0.8 mm thickness with ulceration or 0.8 - 1.0 mm thickness with or without ulceration
- pT2a: > 1.0 - 2.0 mm thickness without ulceration
- pT2b: > 1.0 - 2.0 mm thickness with ulceration
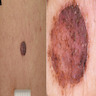
- pT3a: > 2.0 - 4.0 mm thickness without ulceration
- pT3b: > 2.0 - 4.0 mm thickness with ulceration
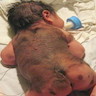
- pT4a: > 4.0 mm thickness without ulceration
- pT4b: > 4.0 mm thickness with ulceration
Notes:
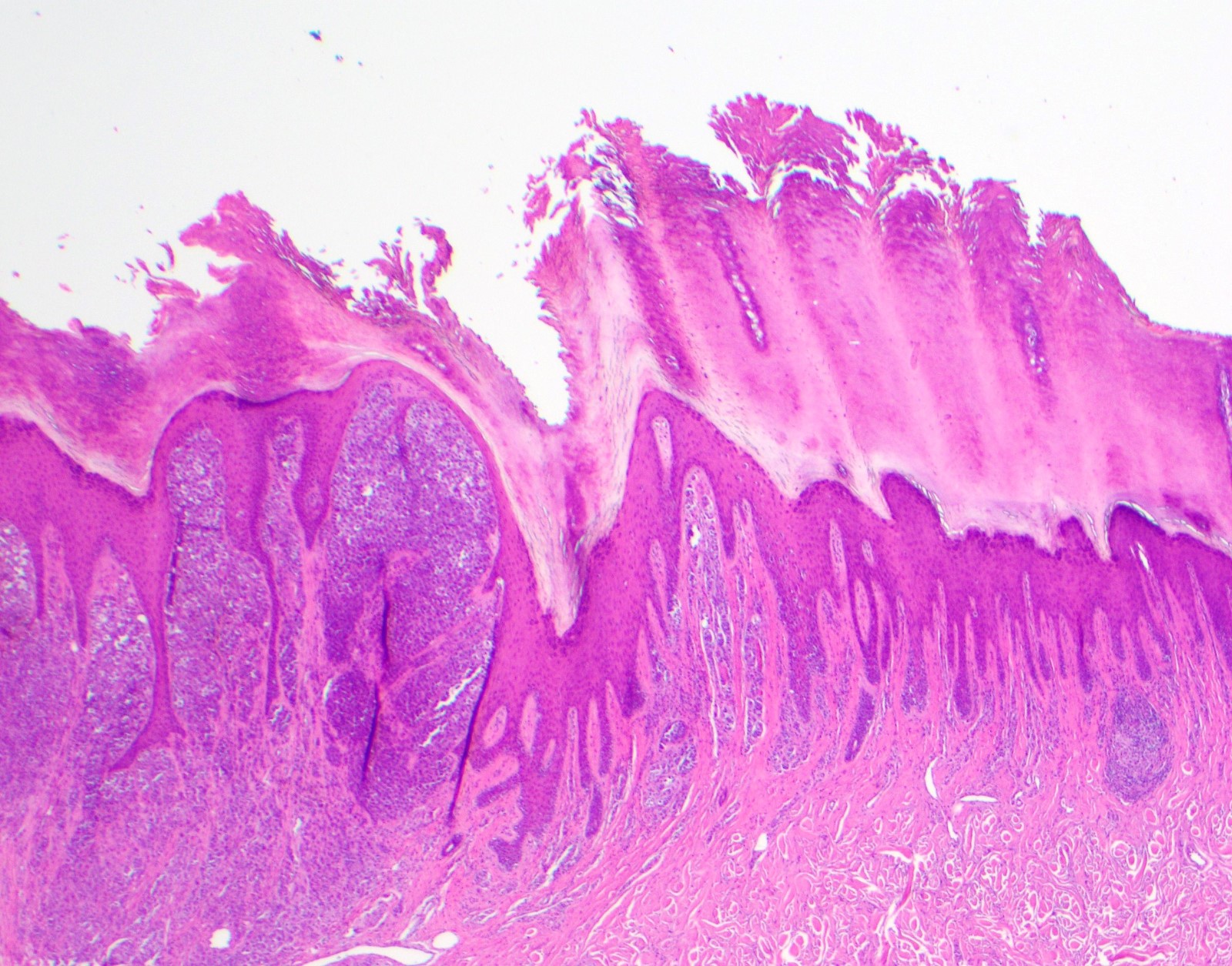
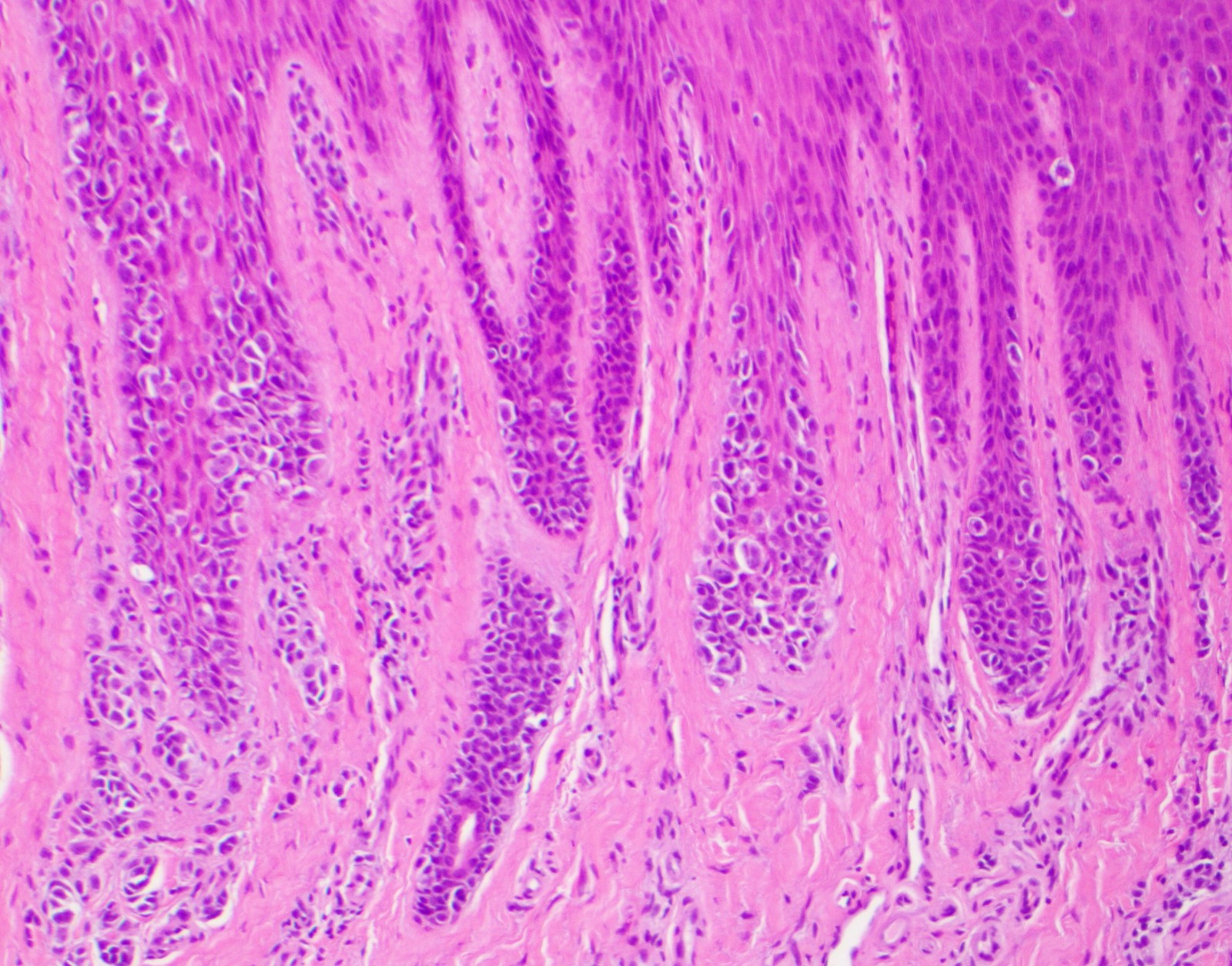
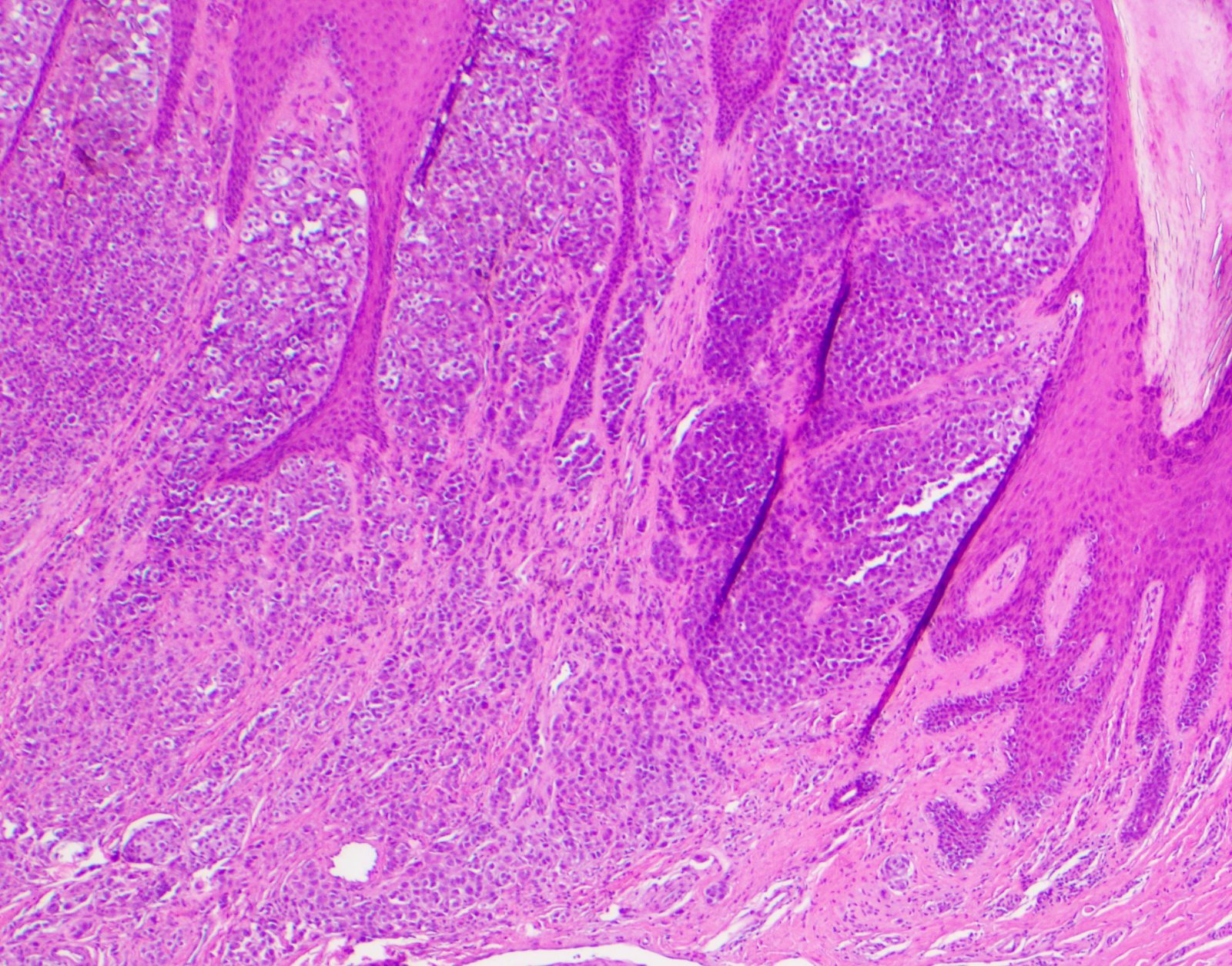
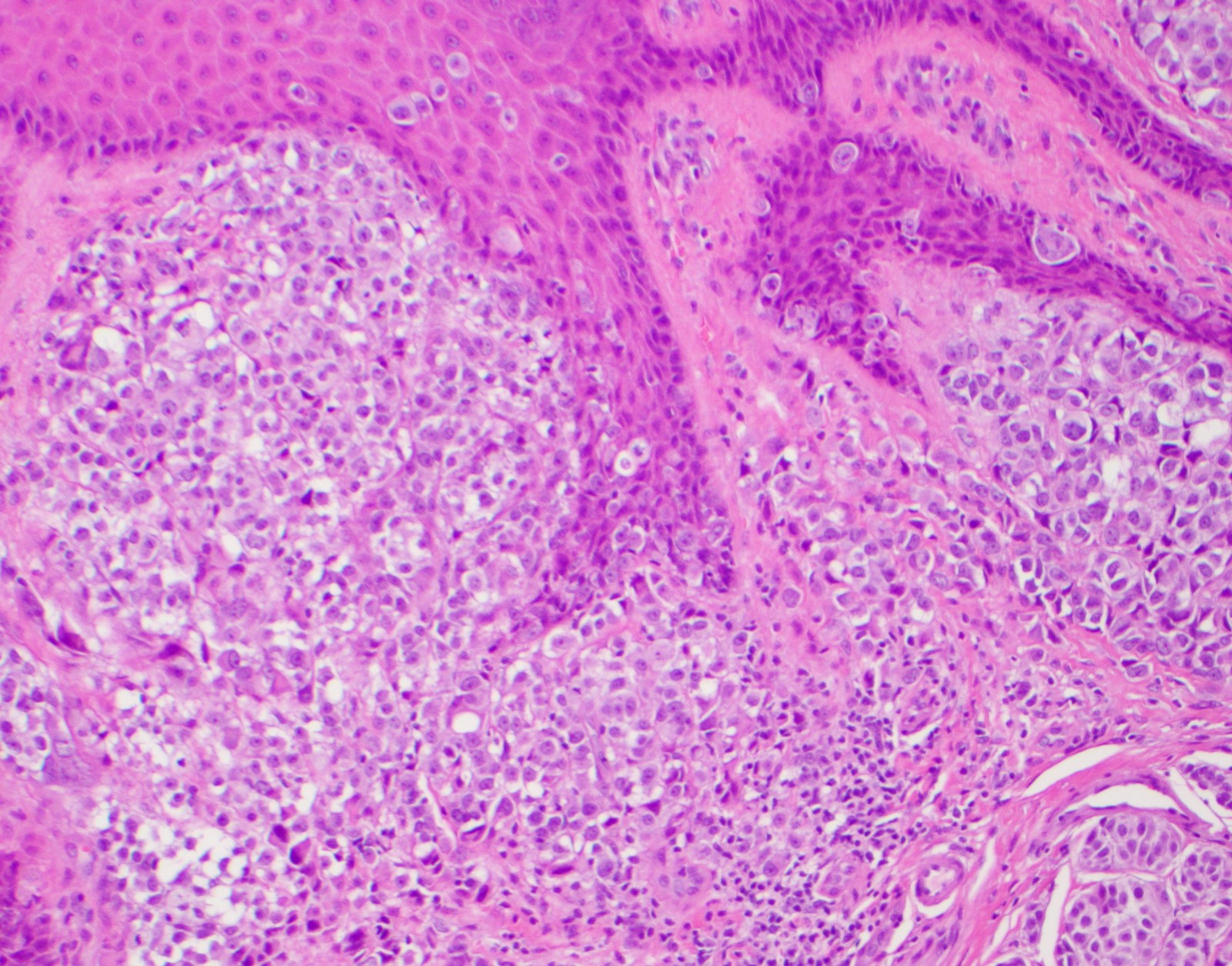
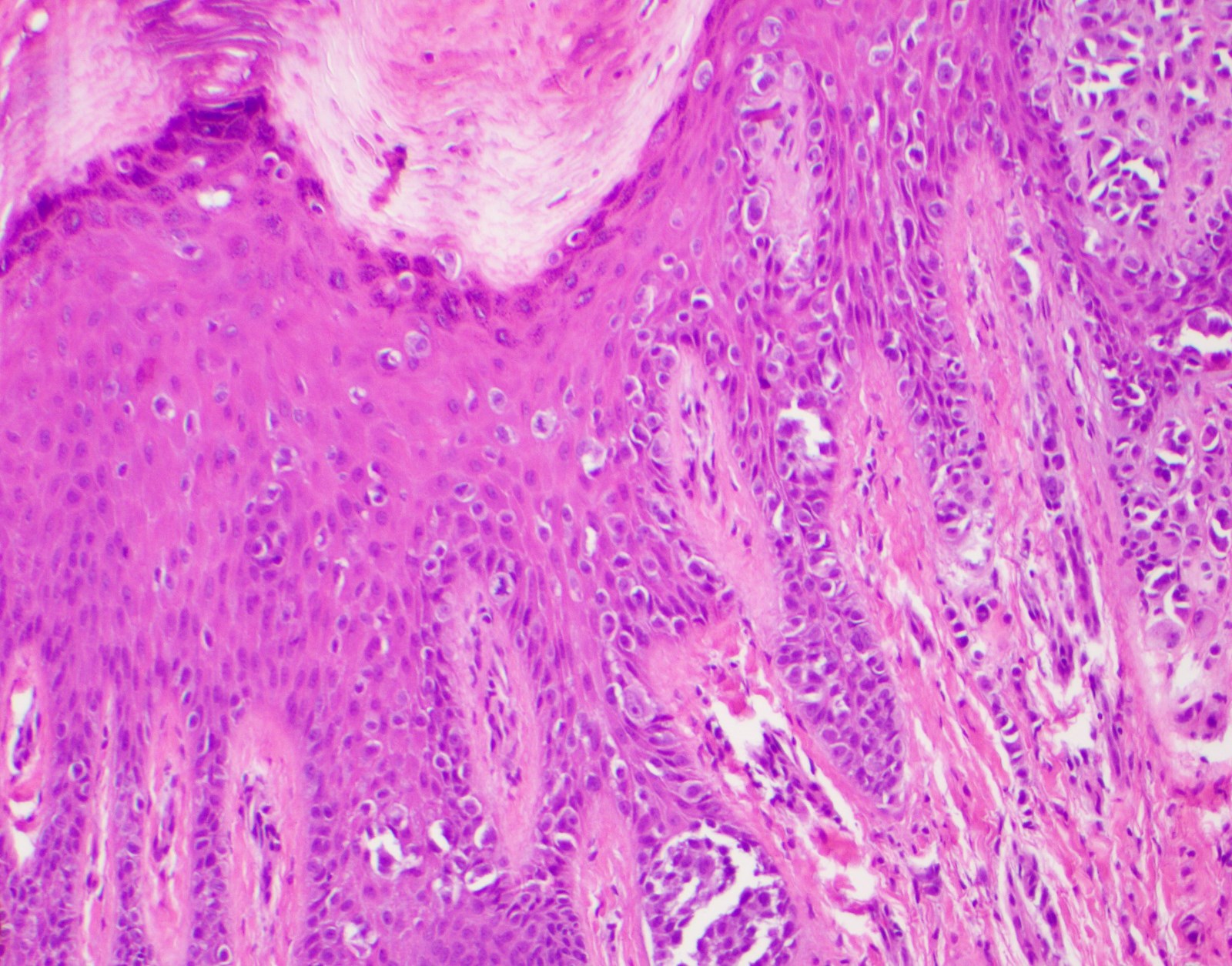
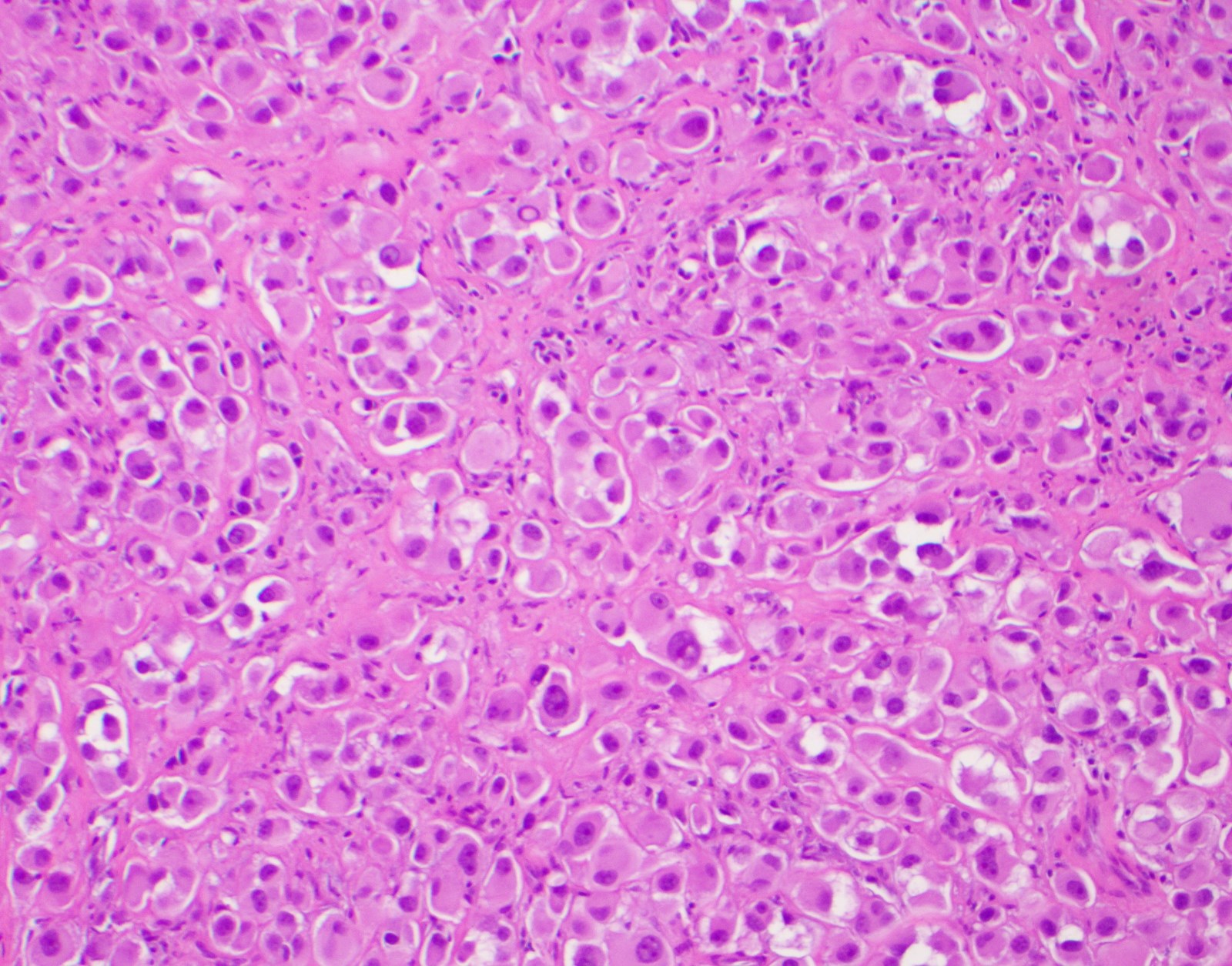
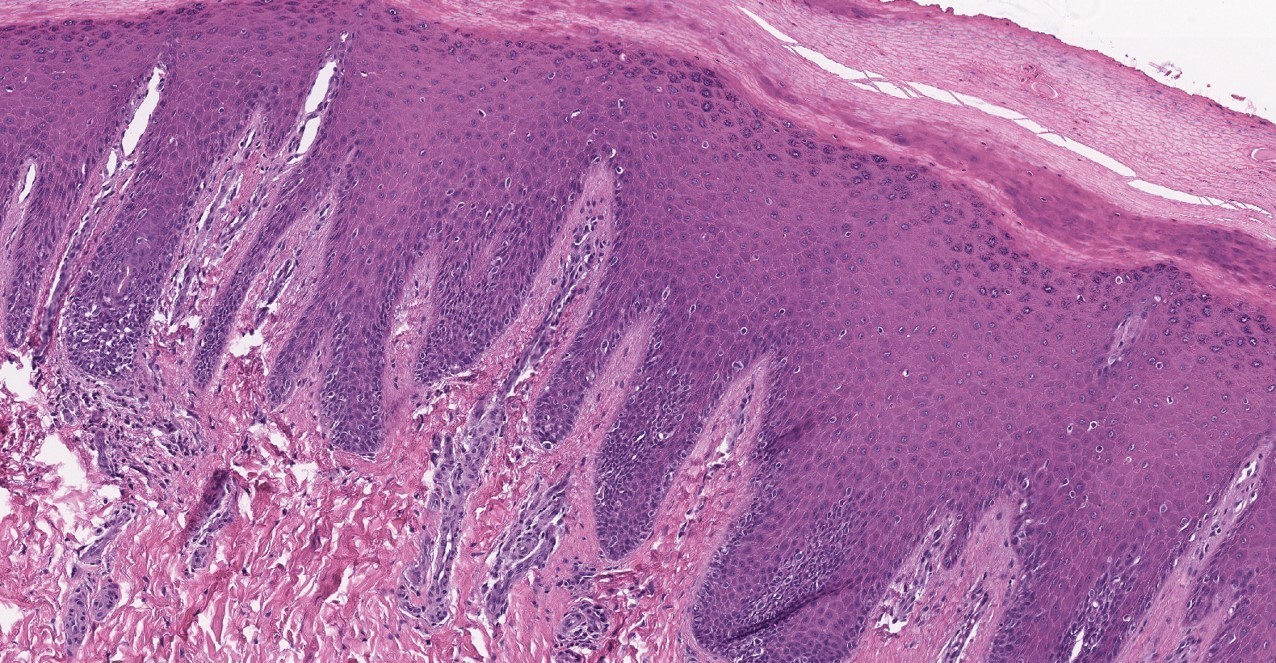
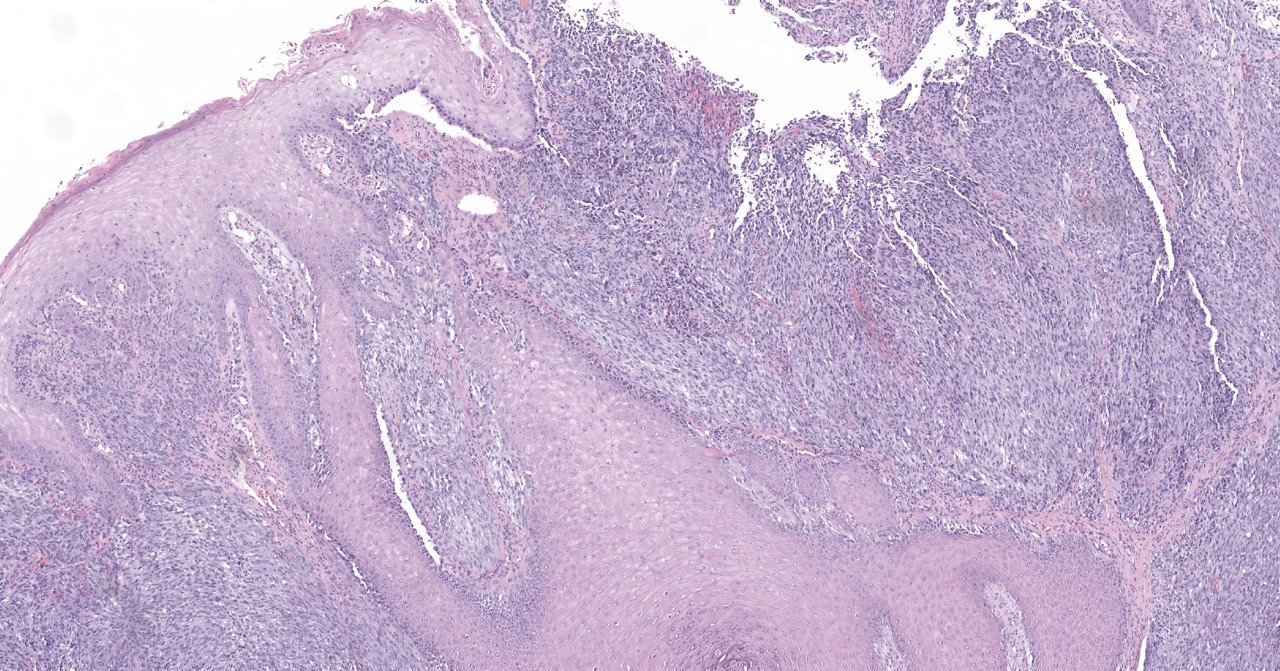
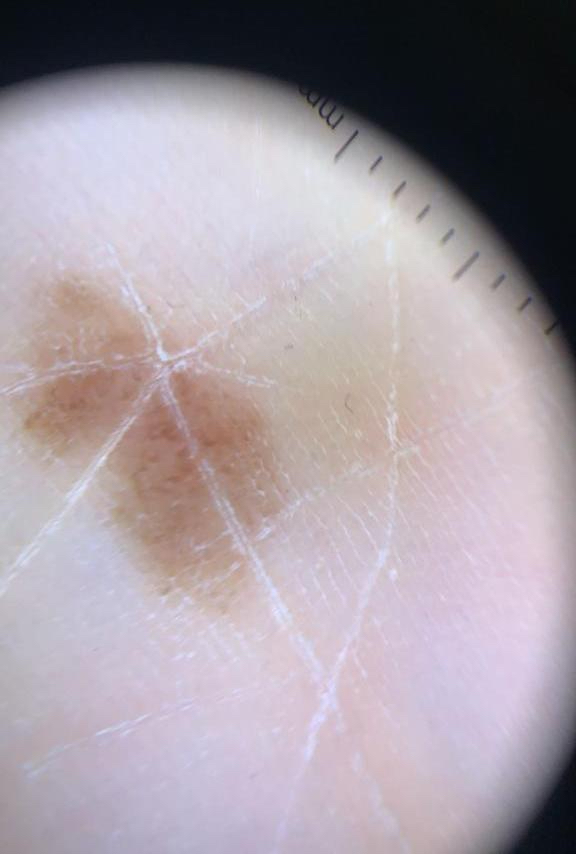
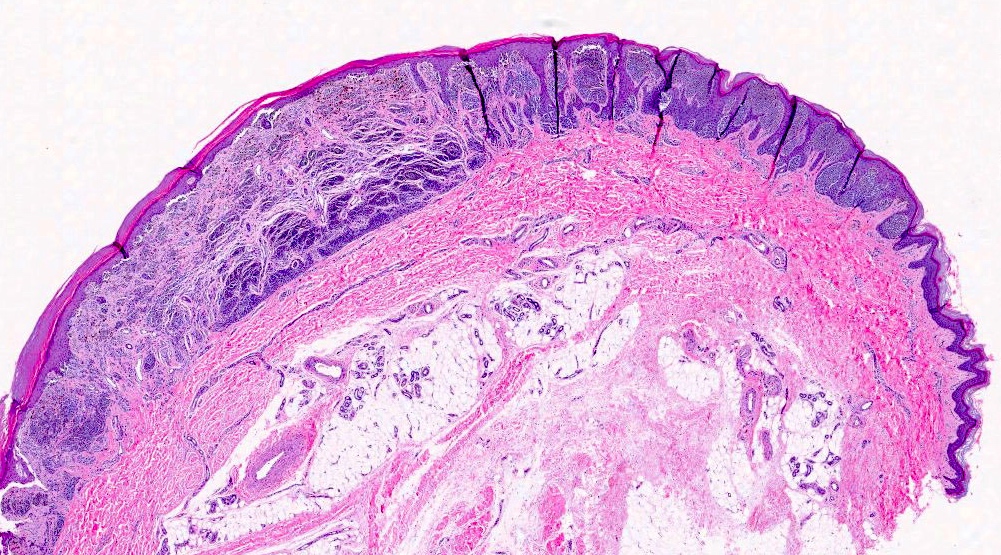
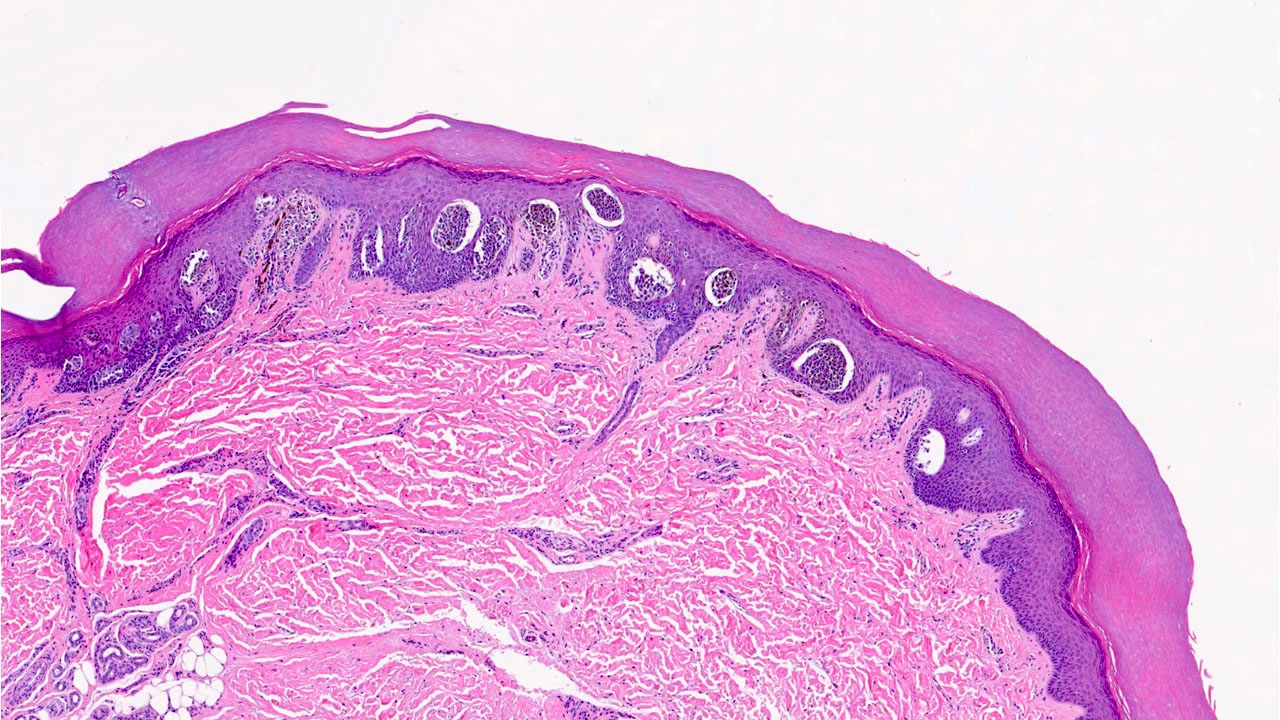
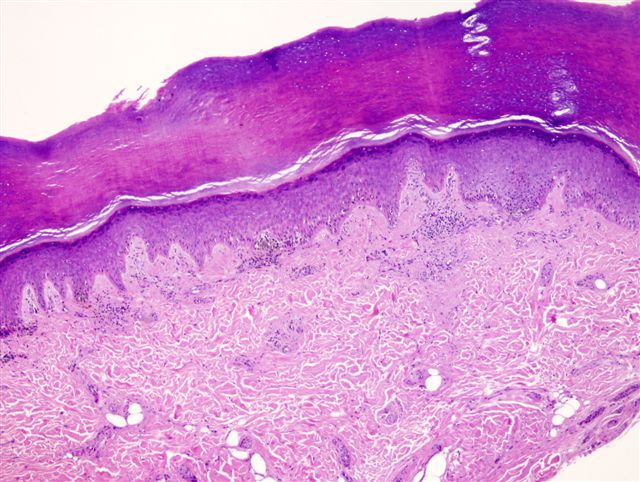
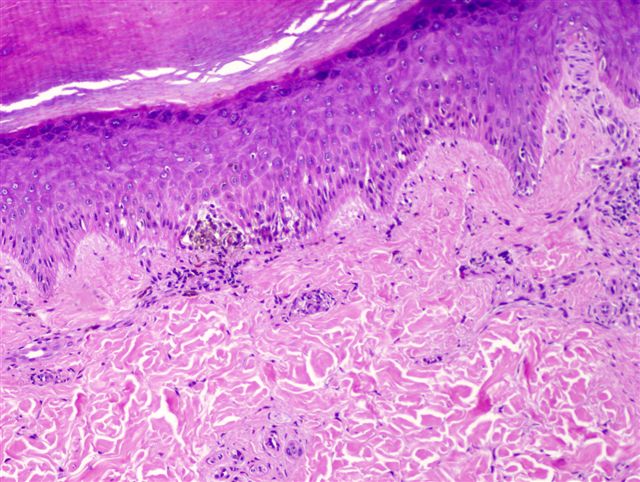
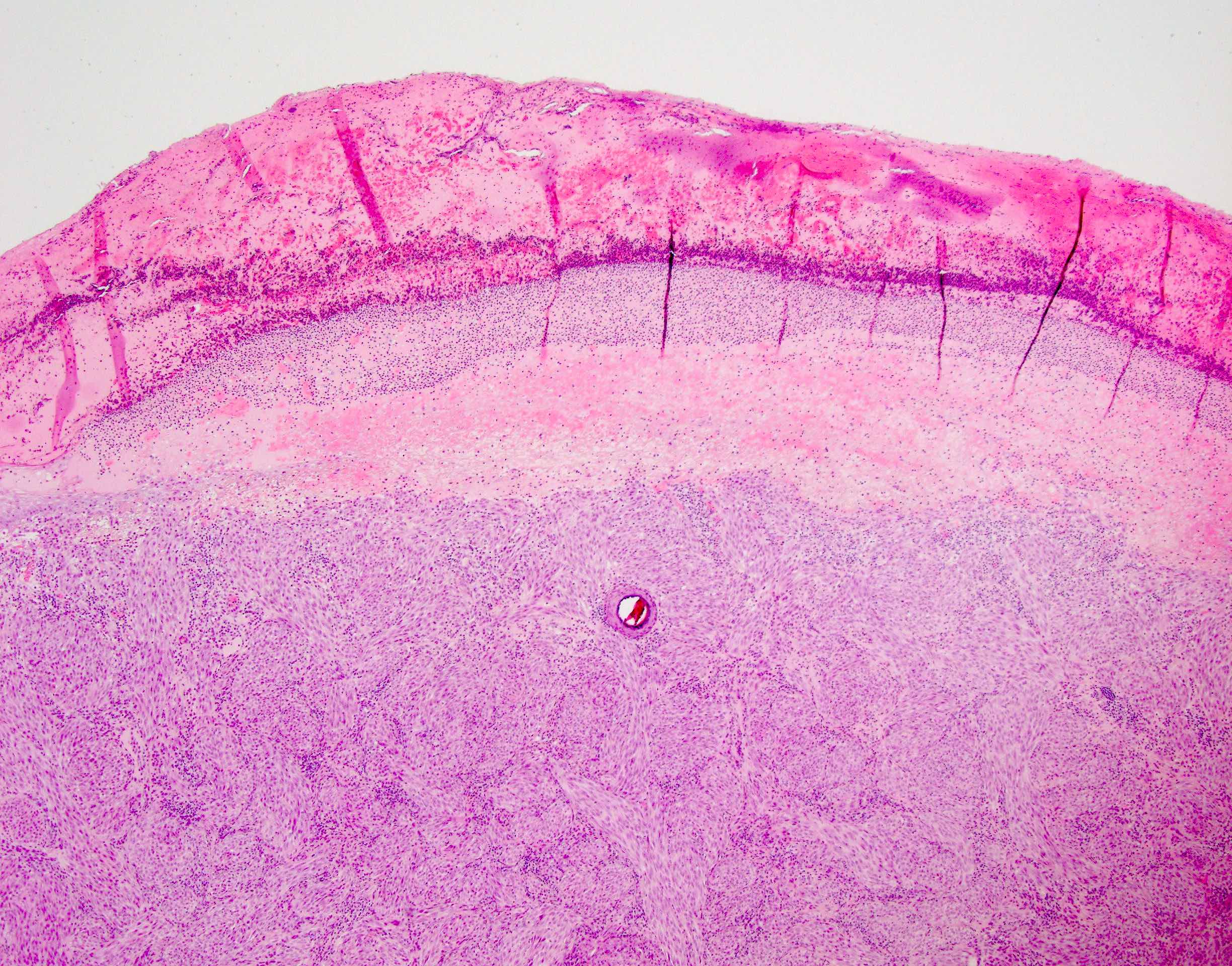
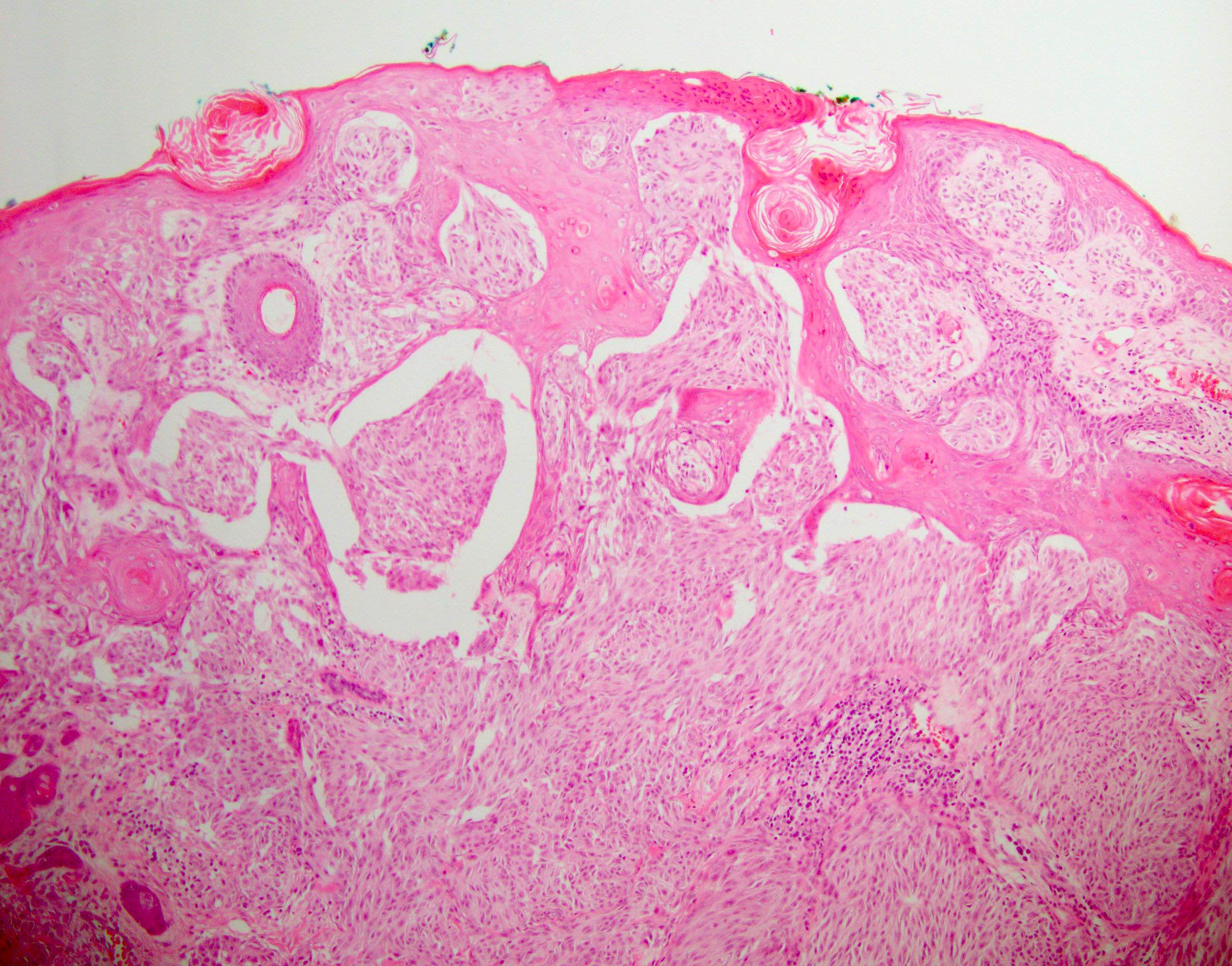
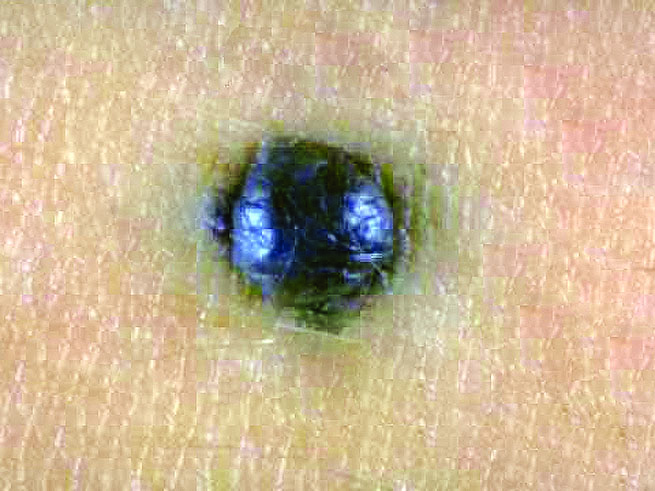
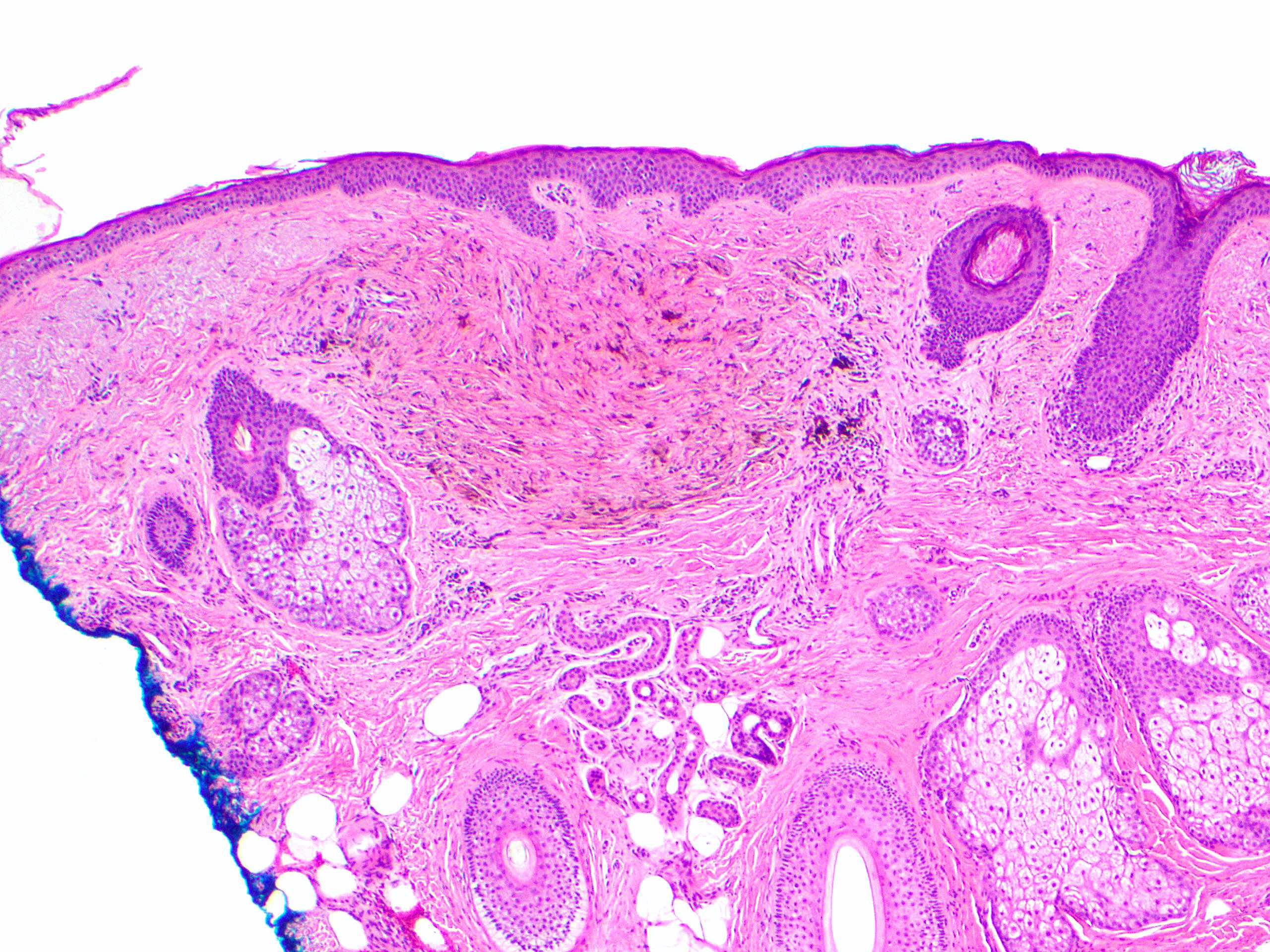
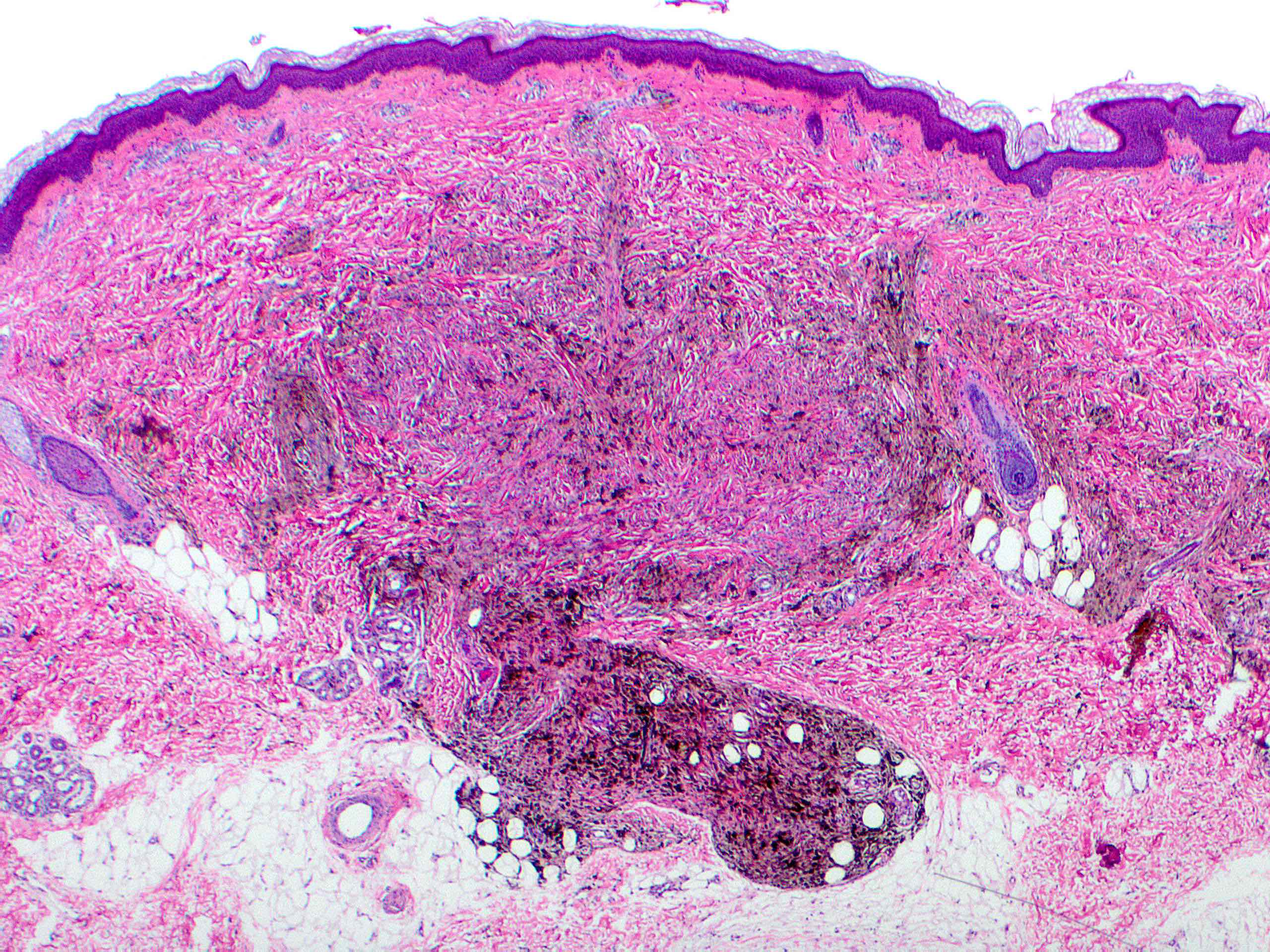
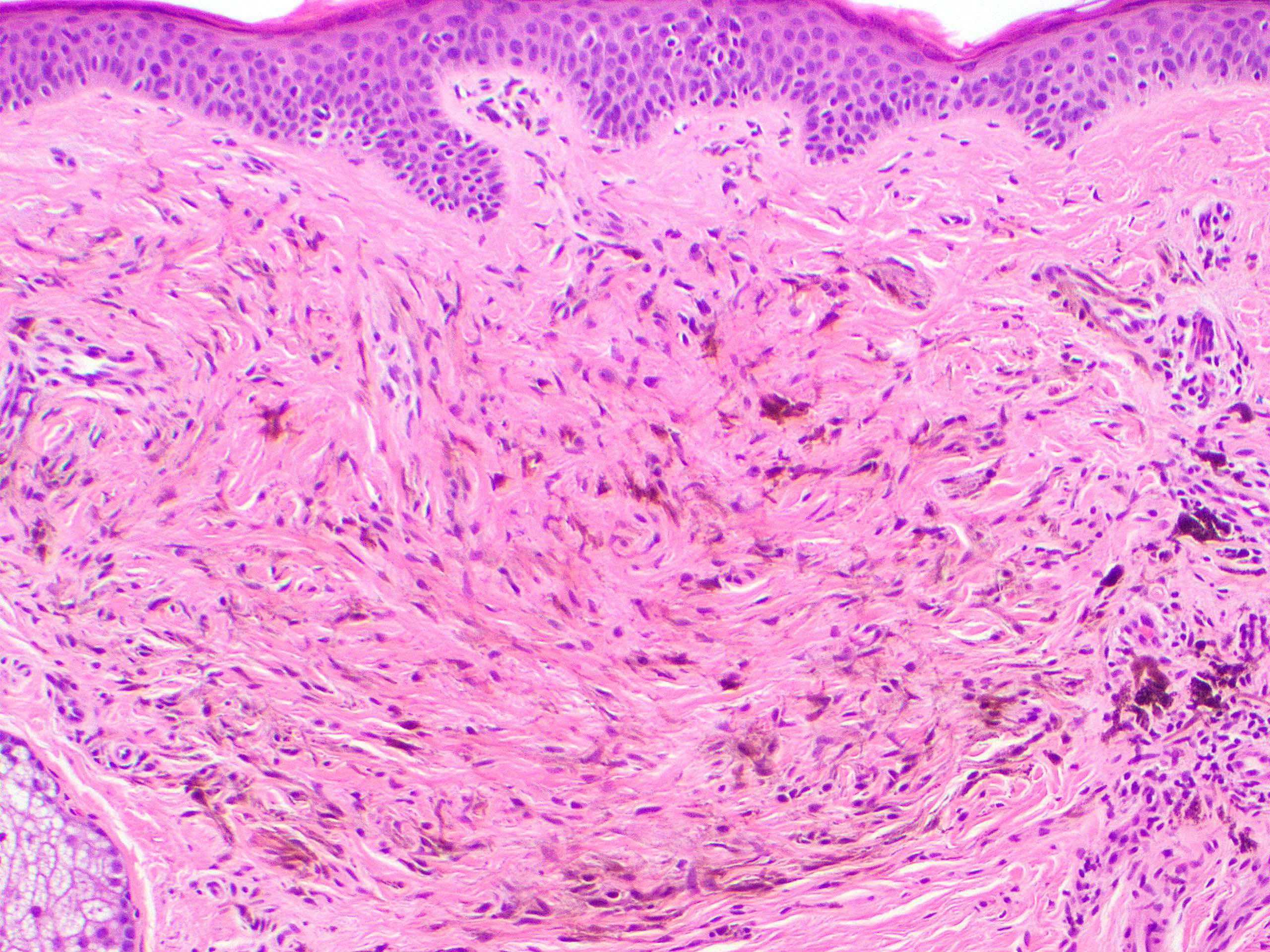
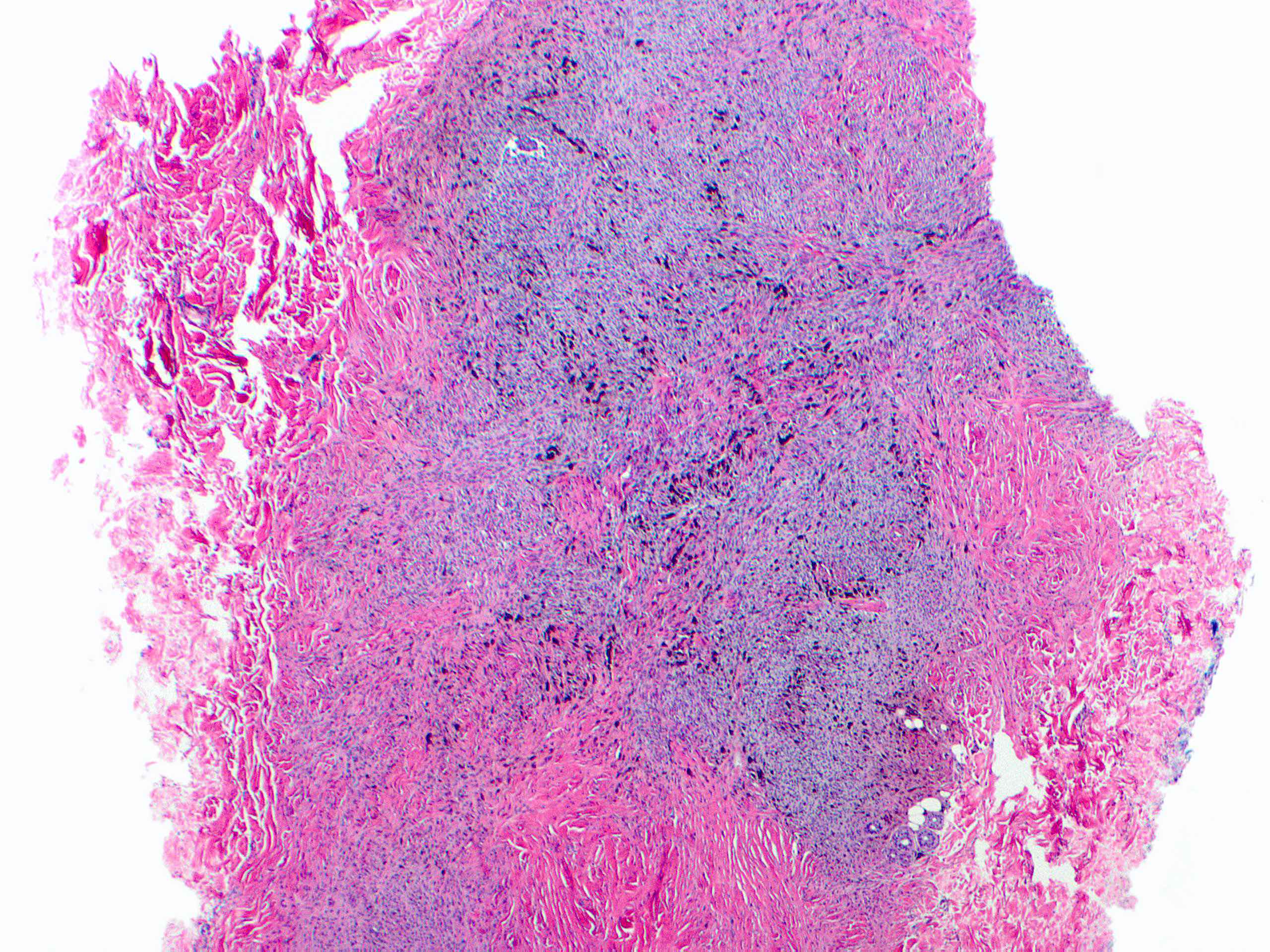
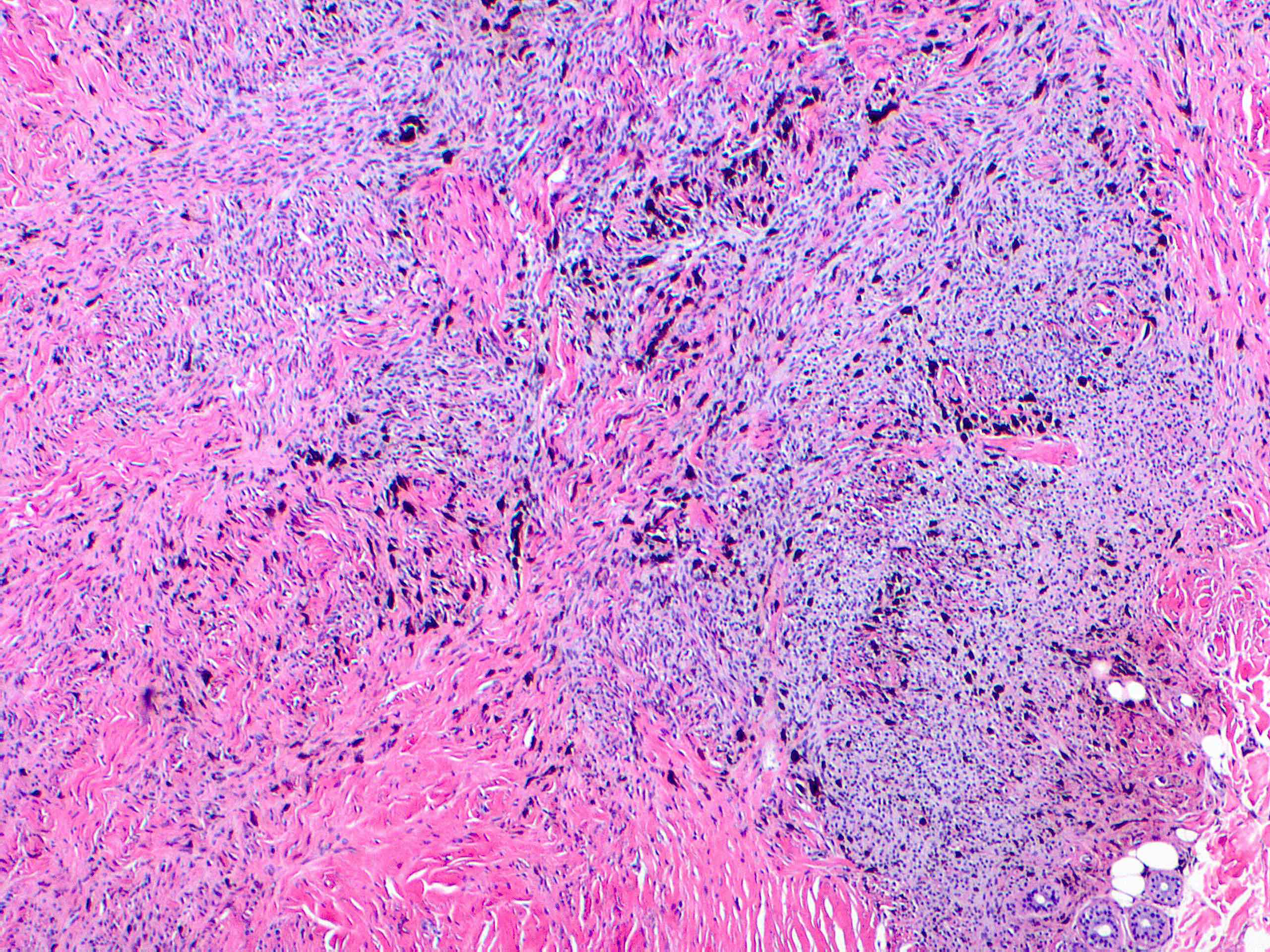
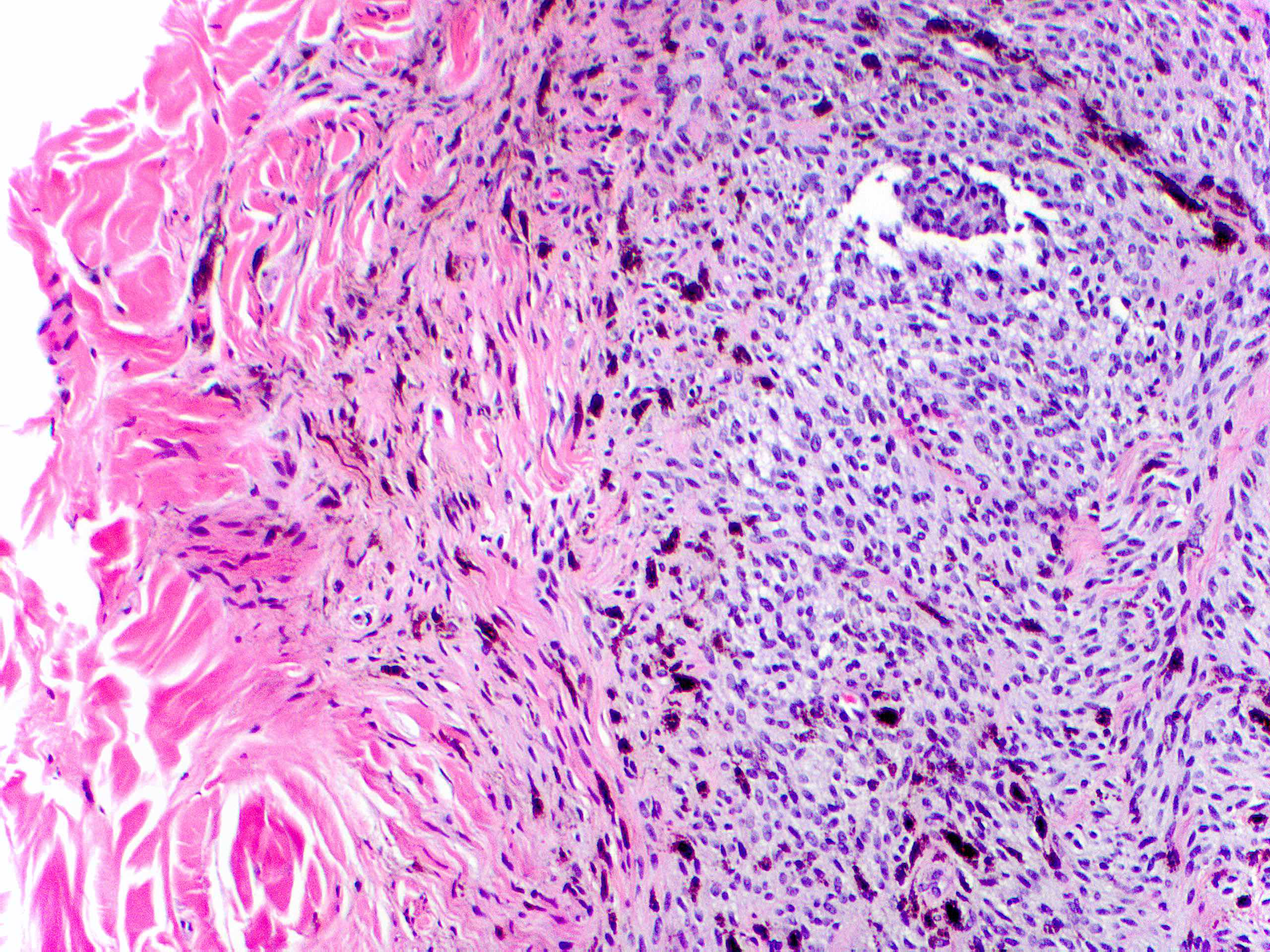
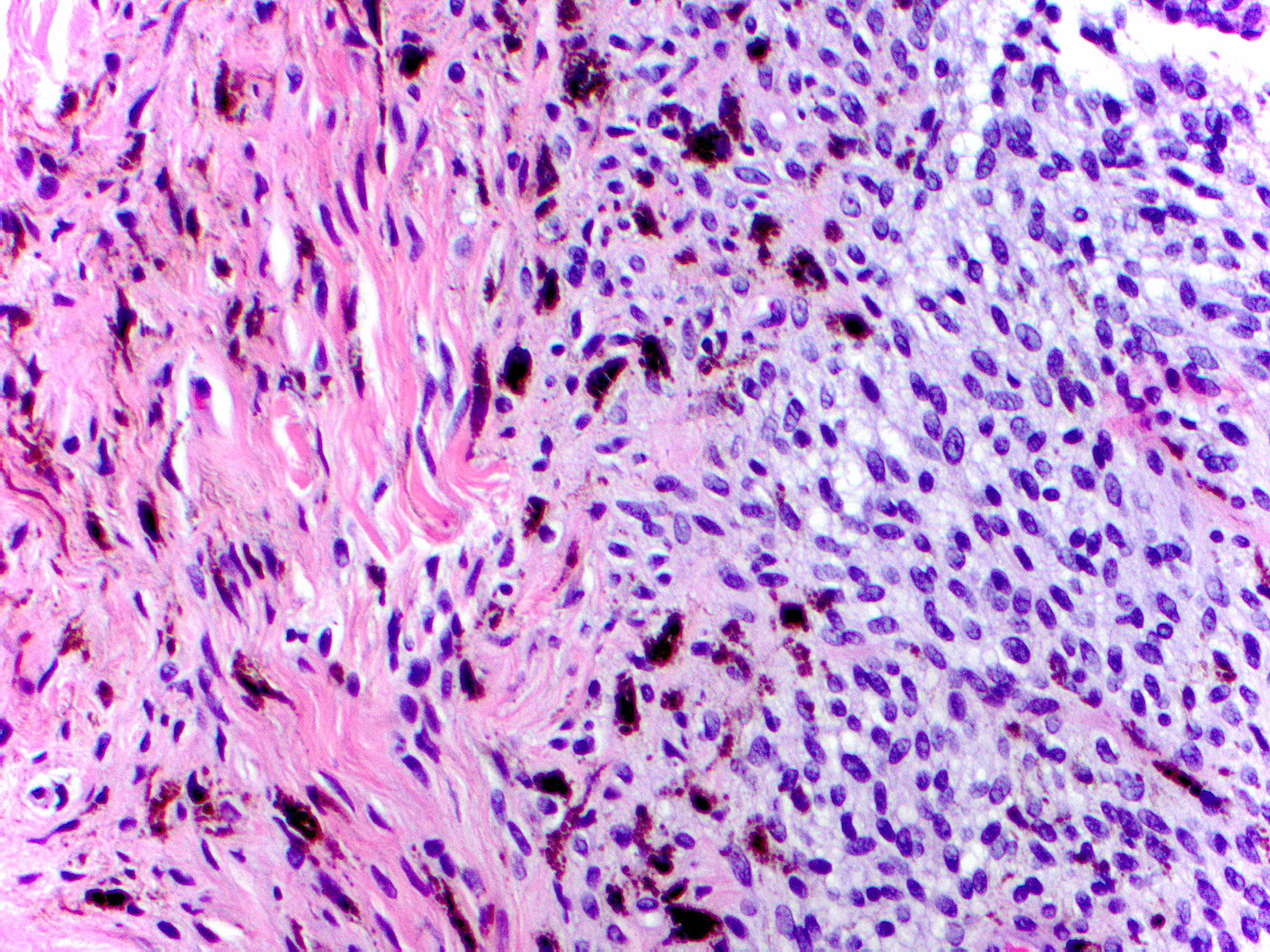
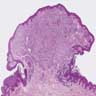
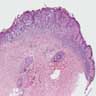
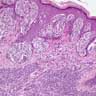
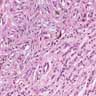
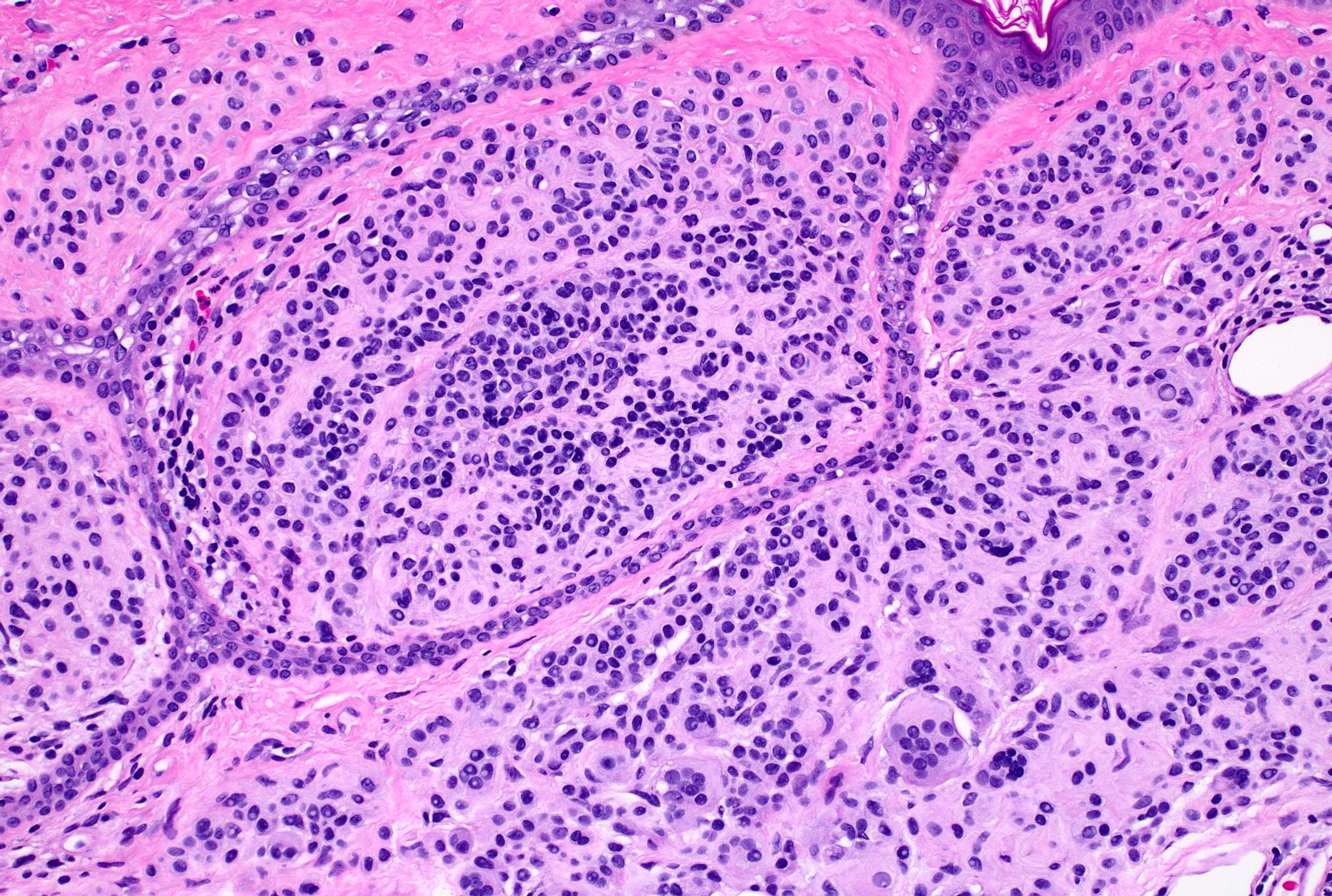
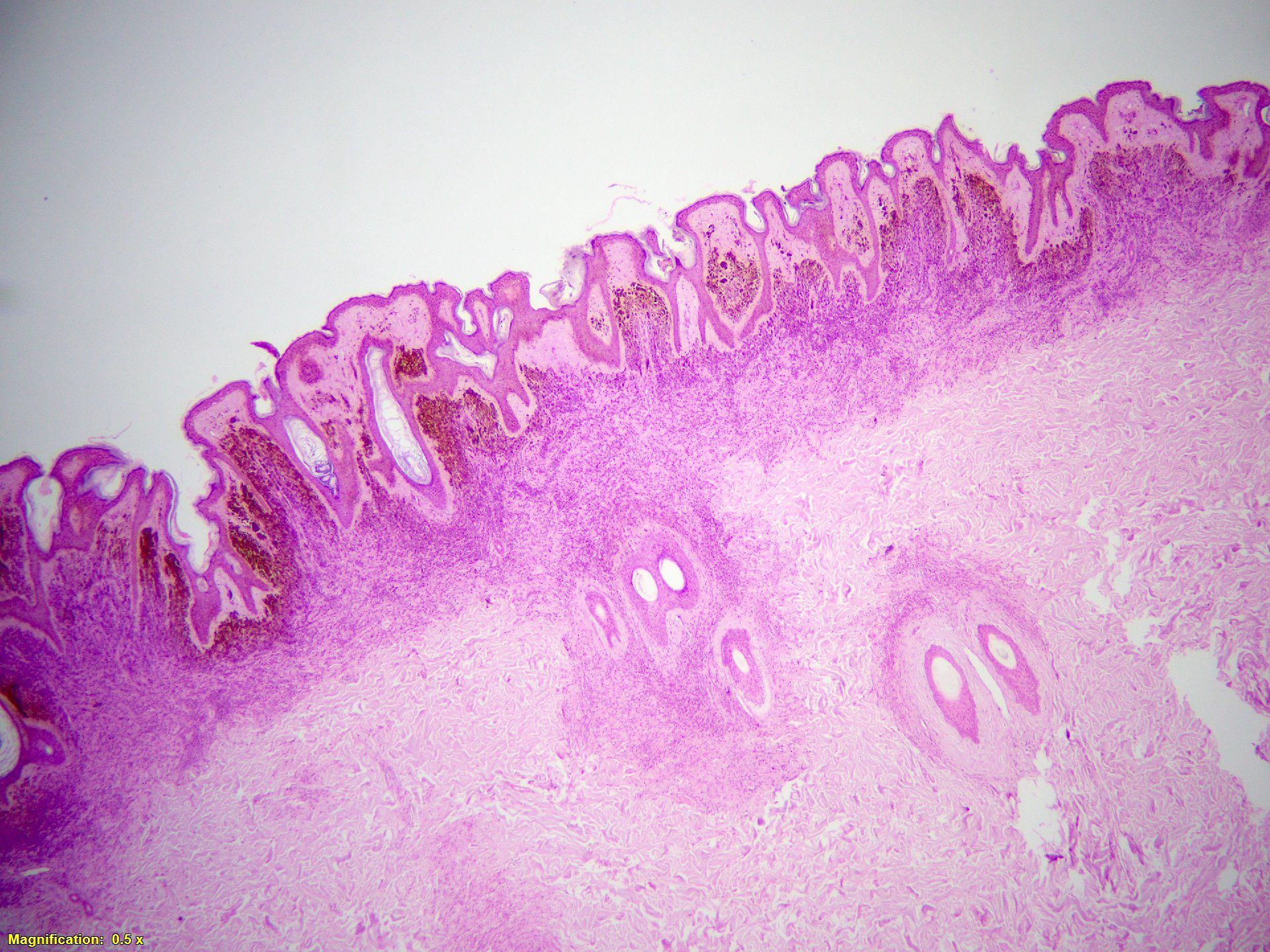
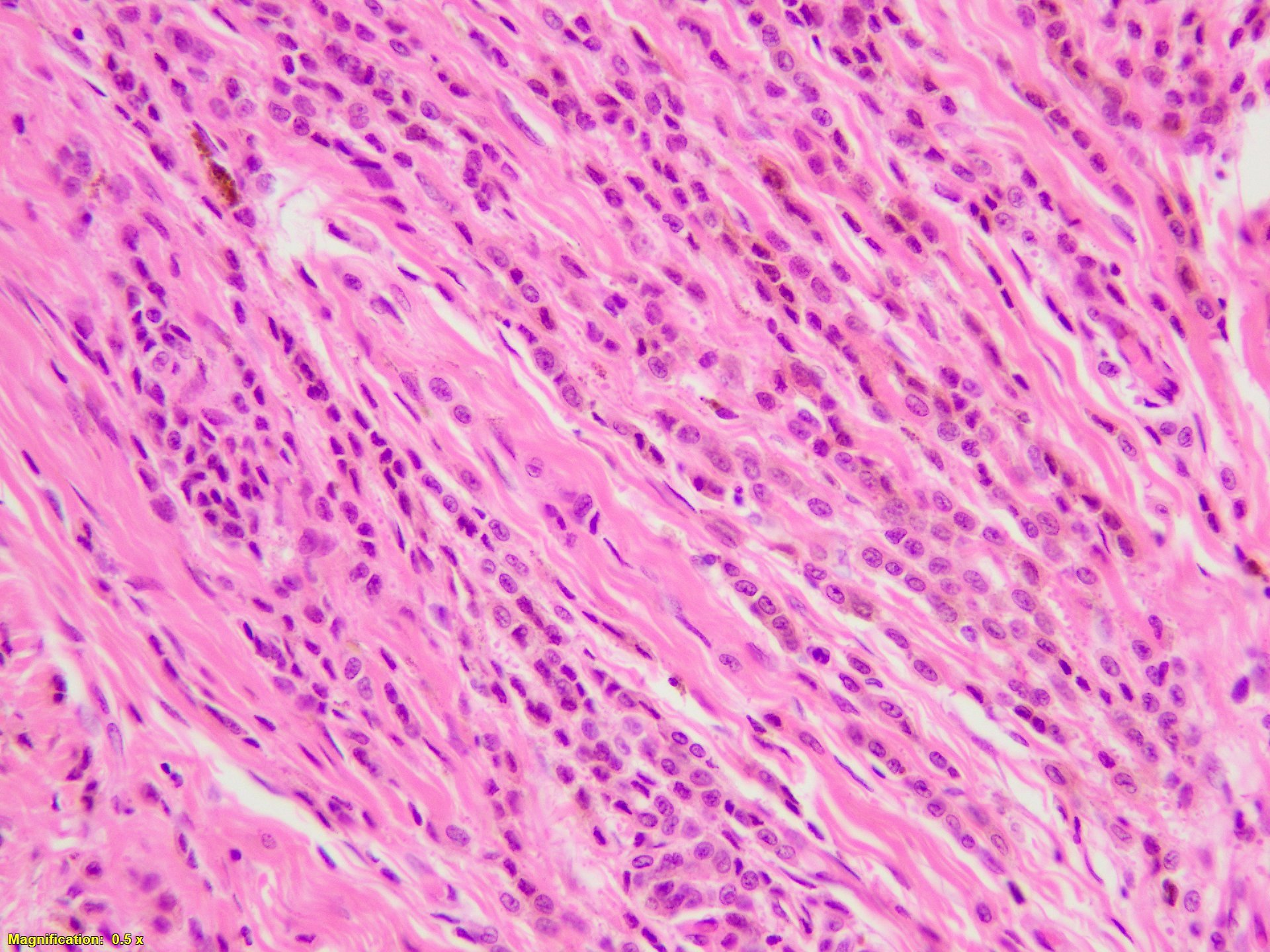
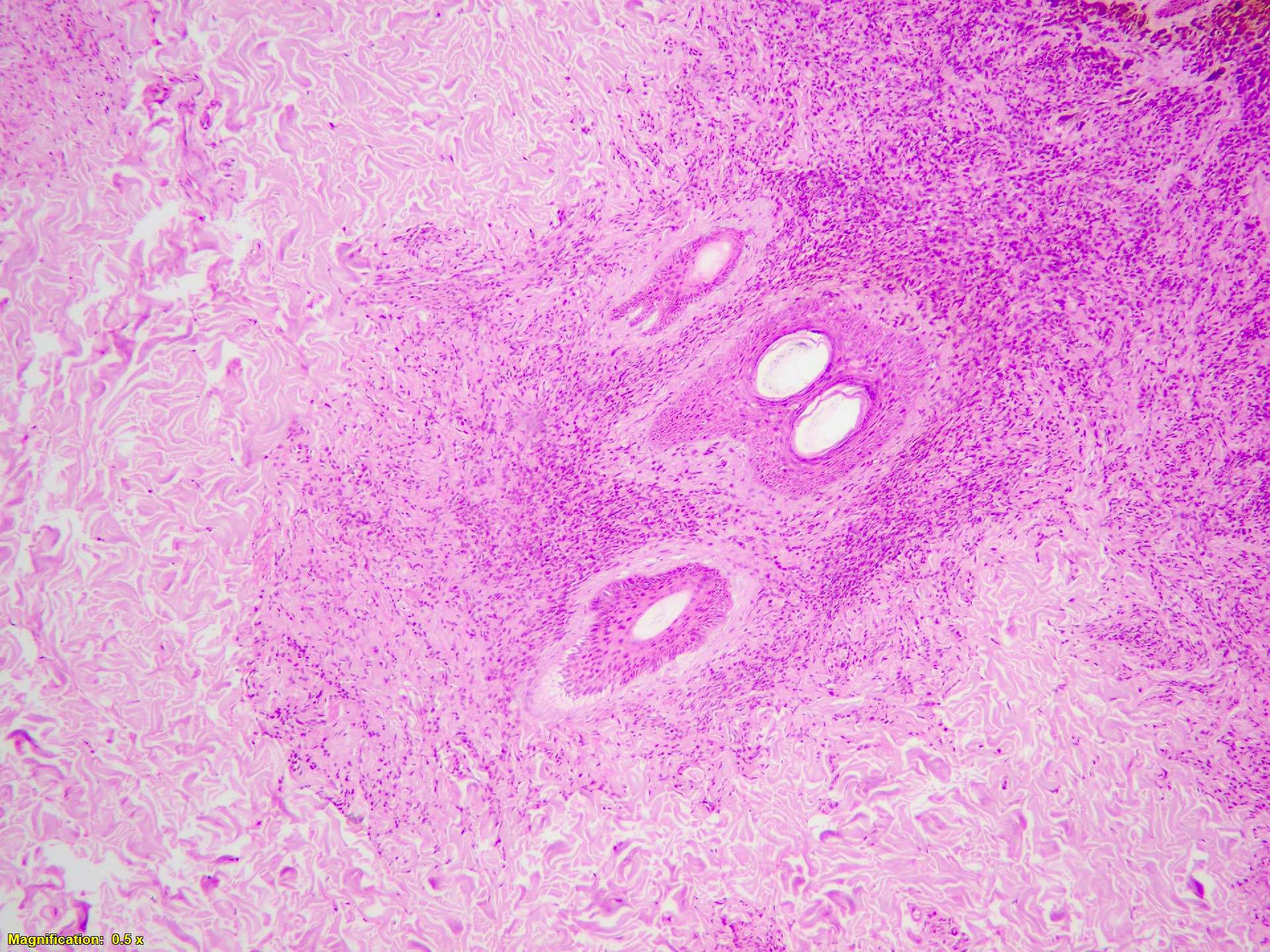
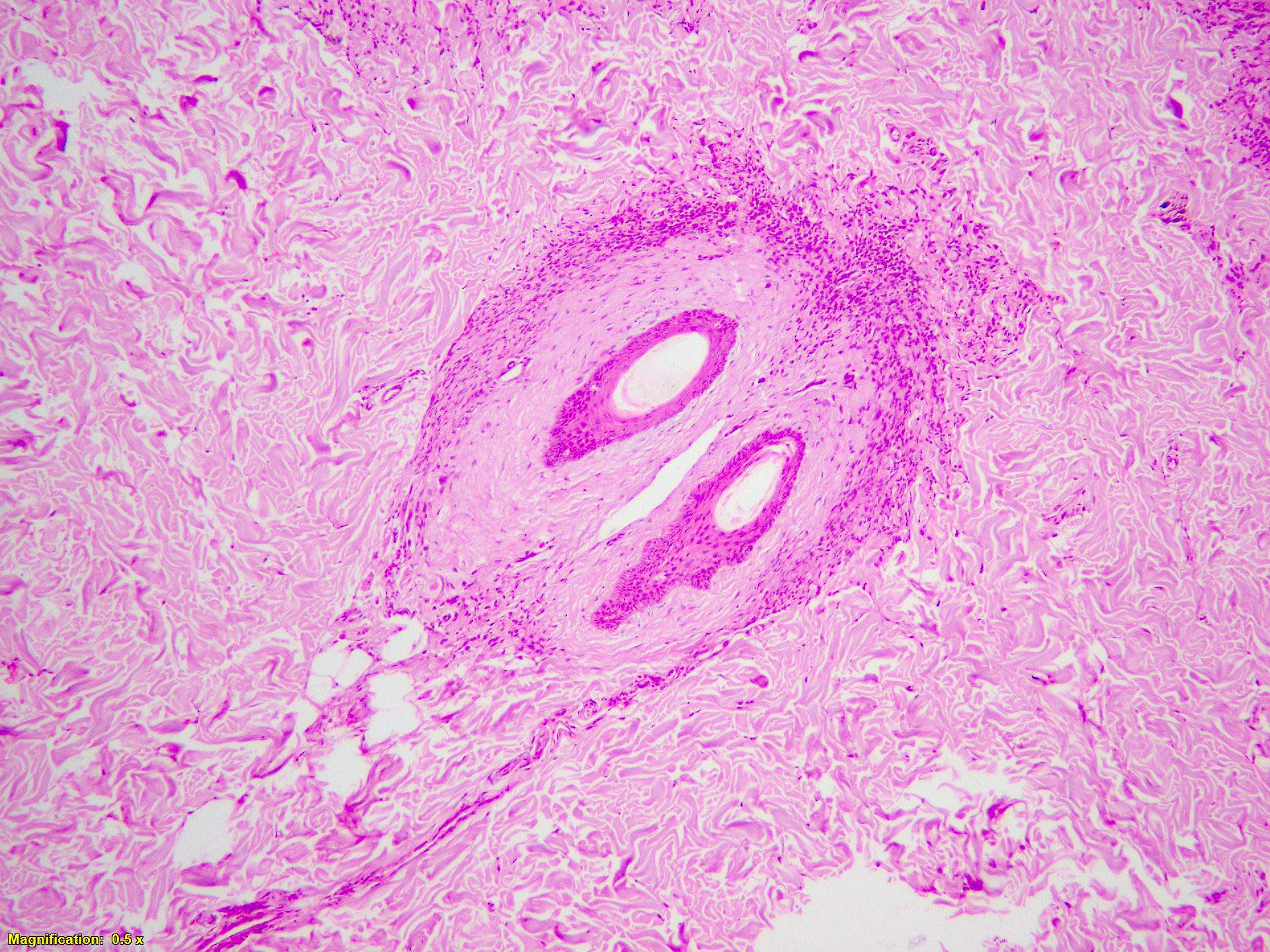
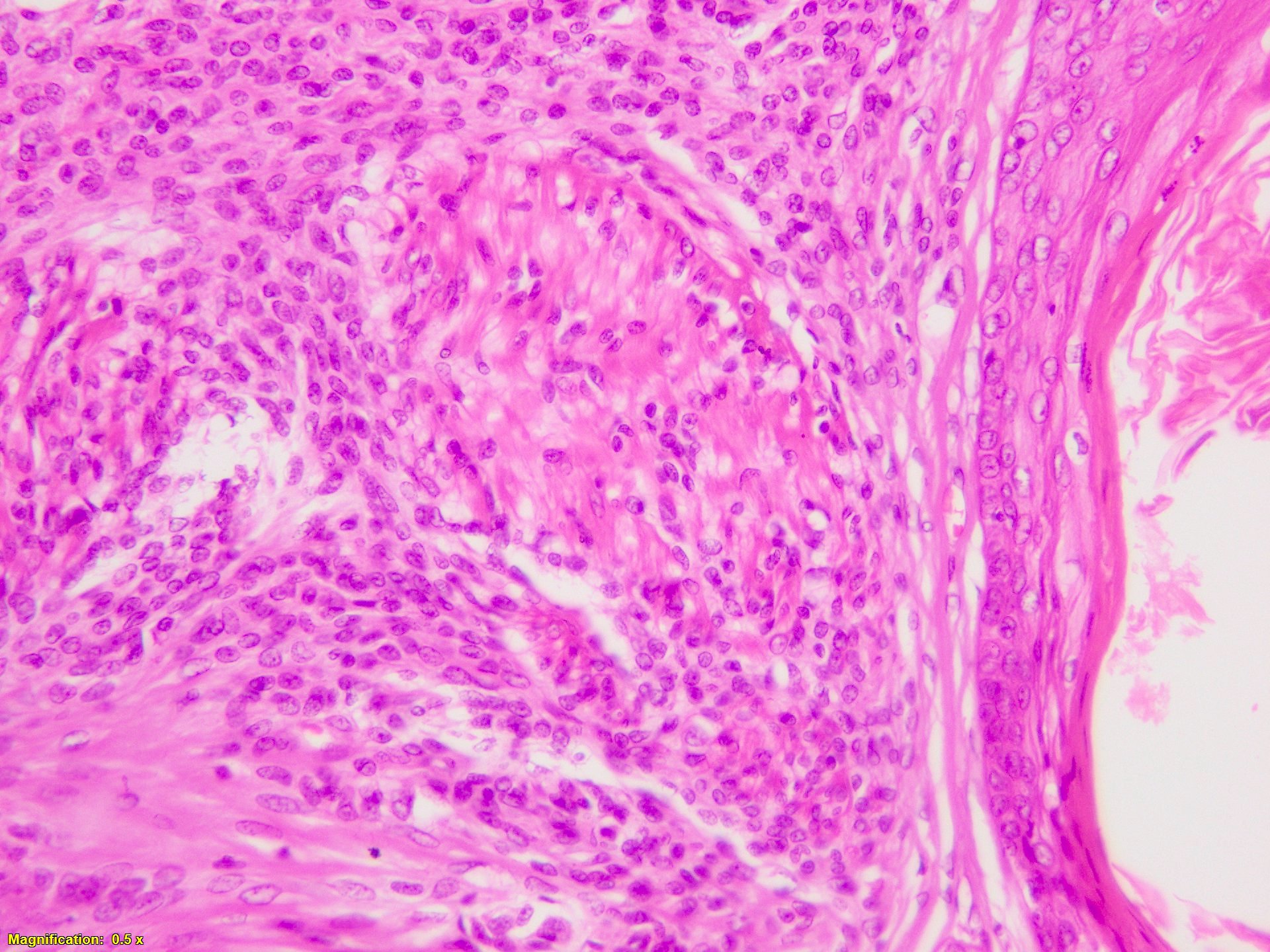
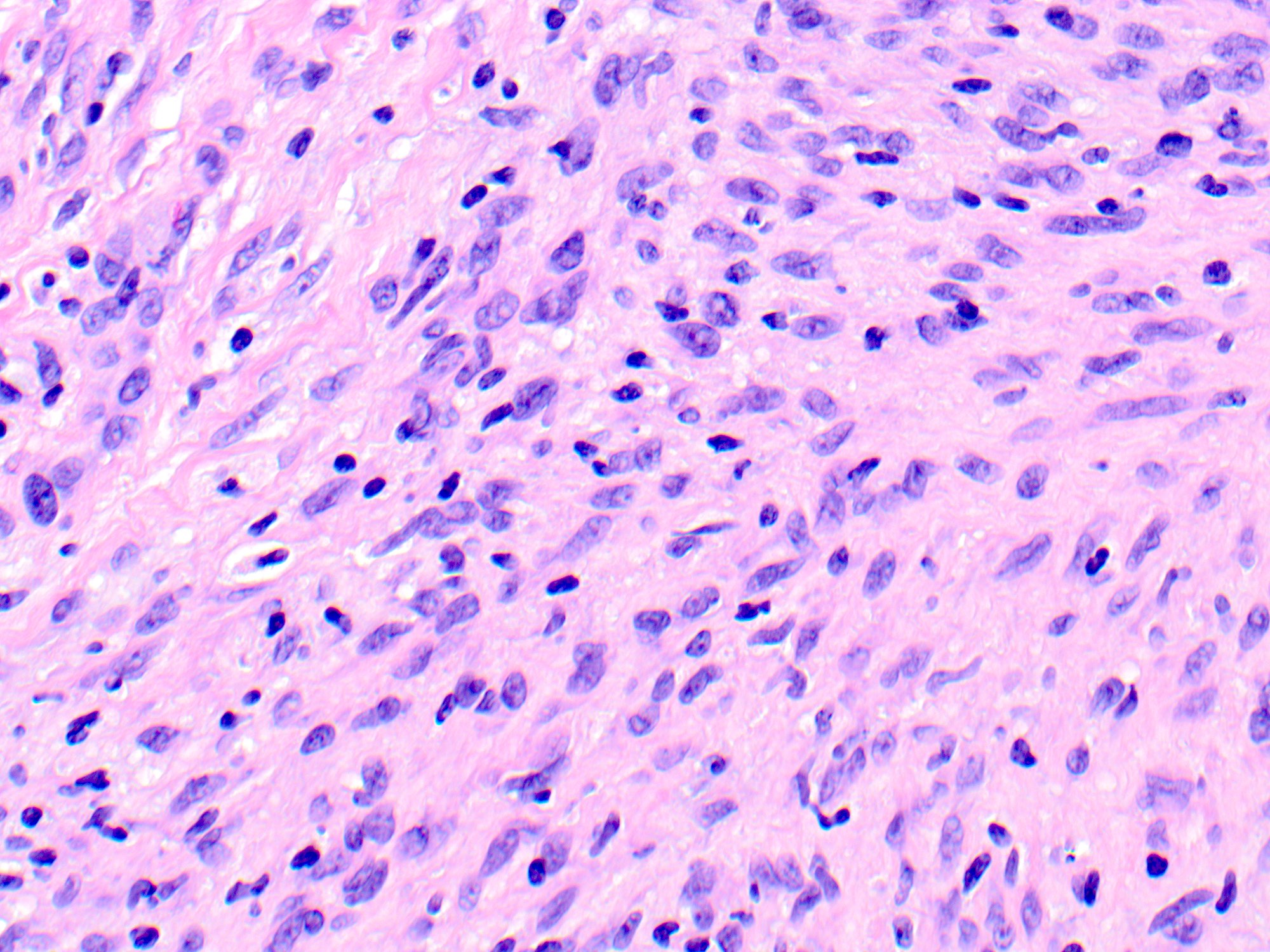
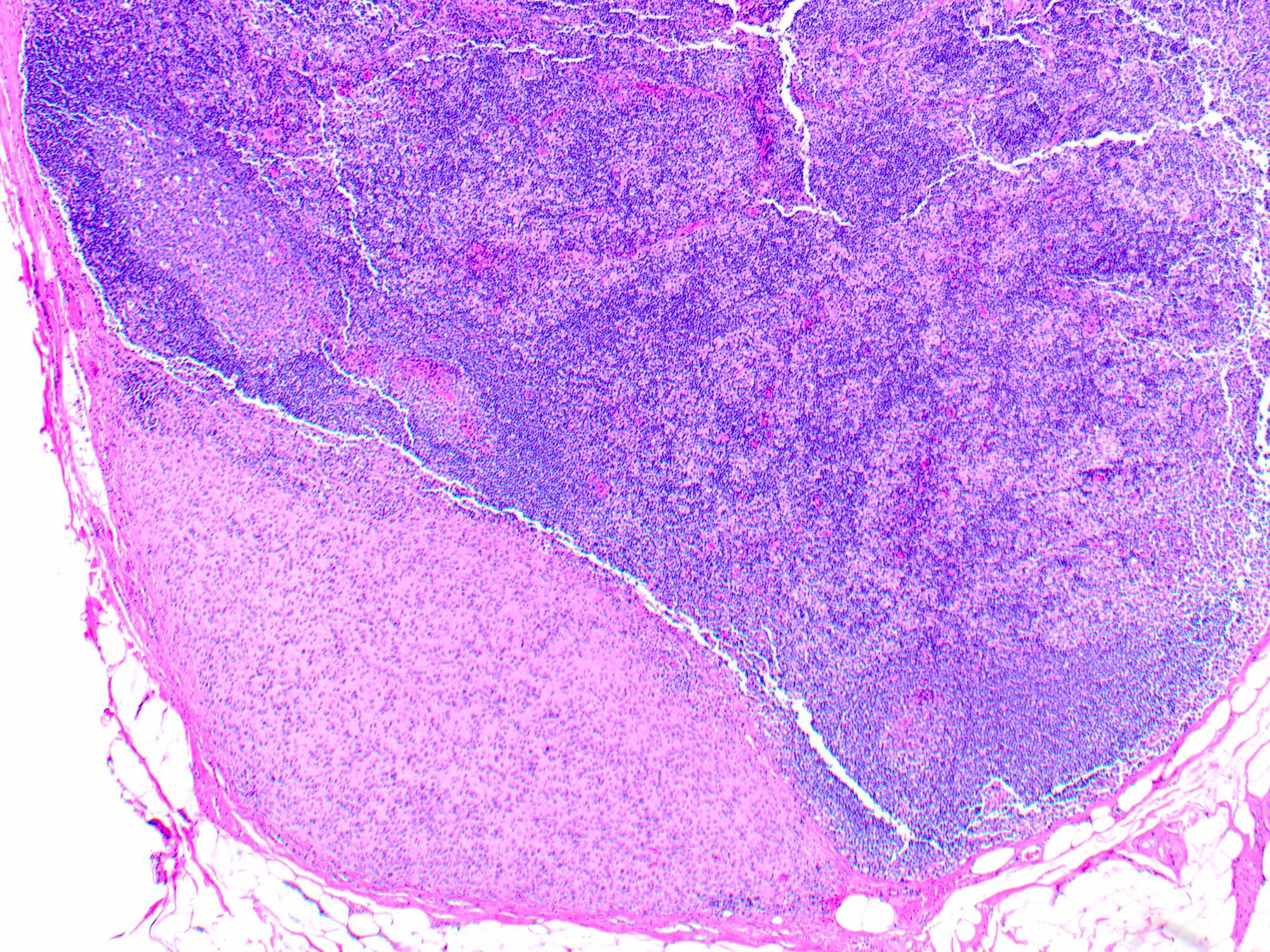
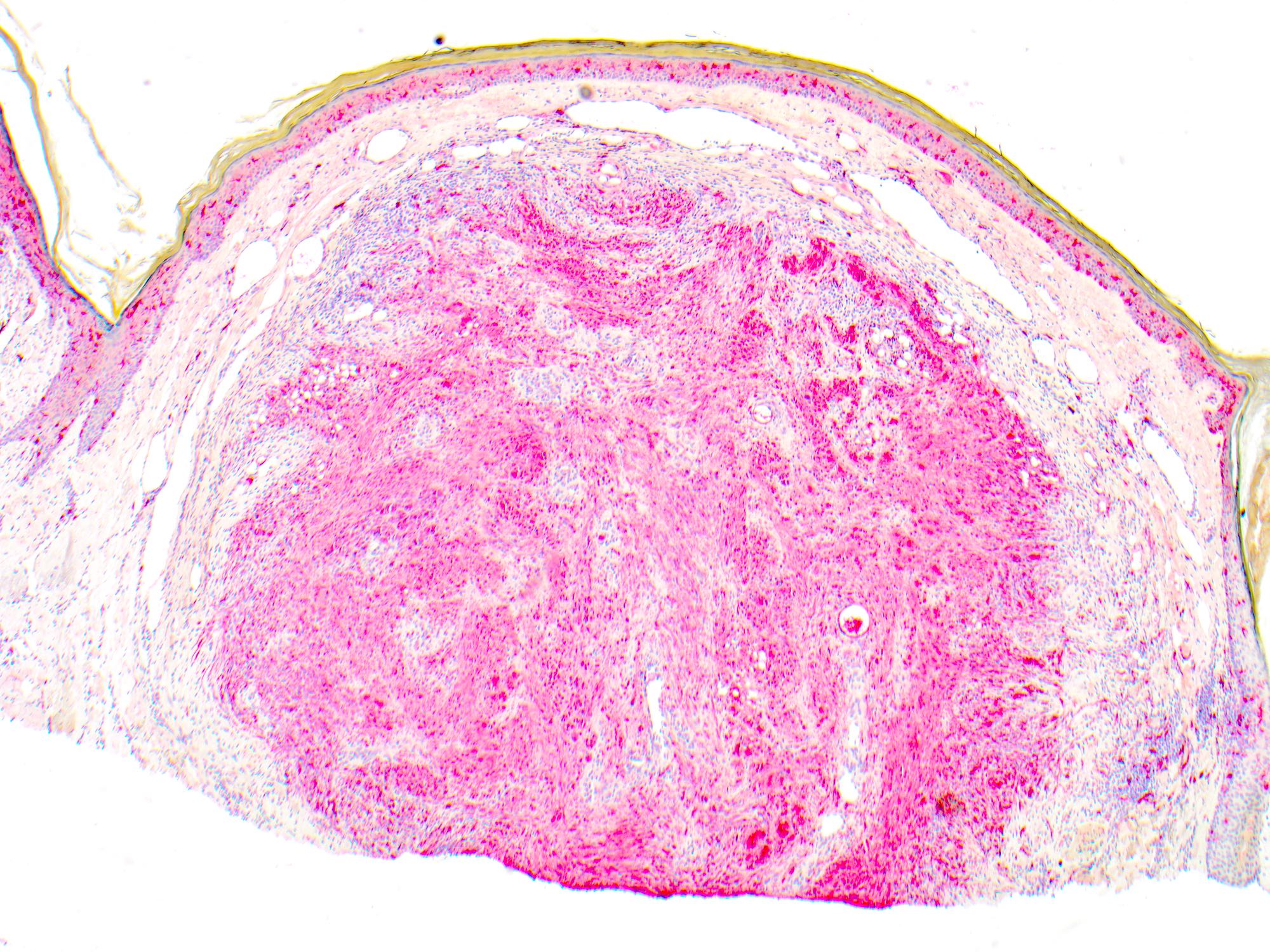
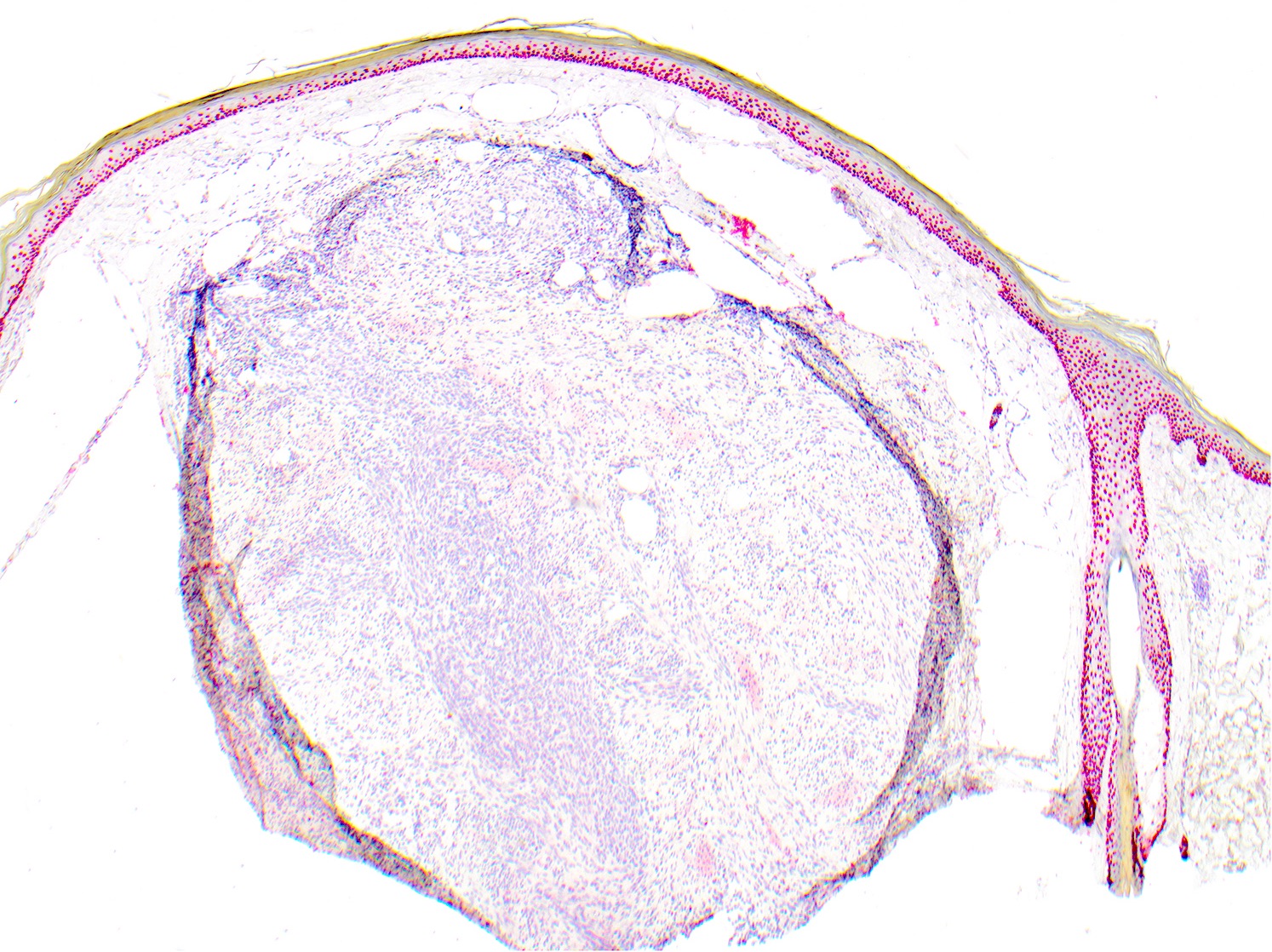
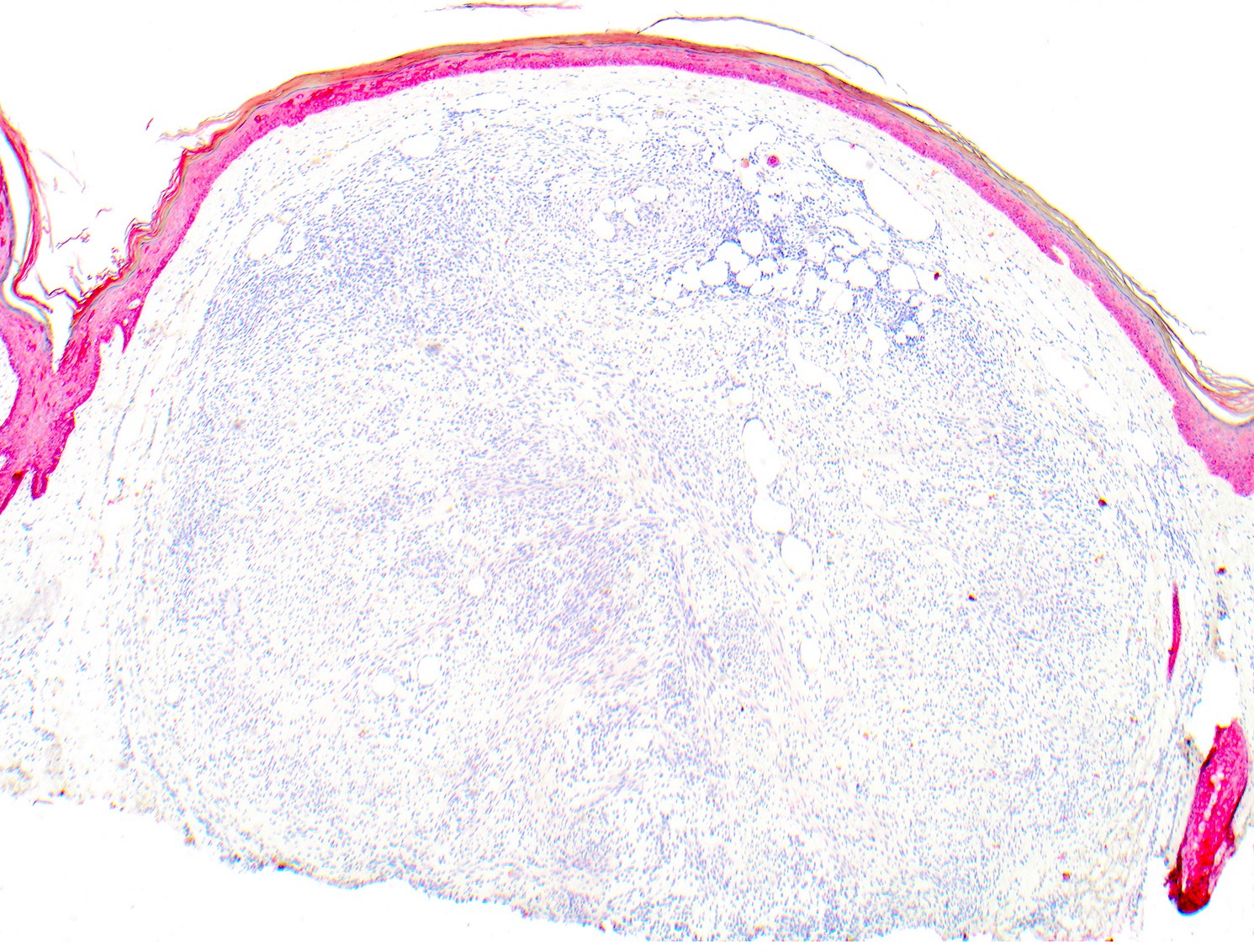
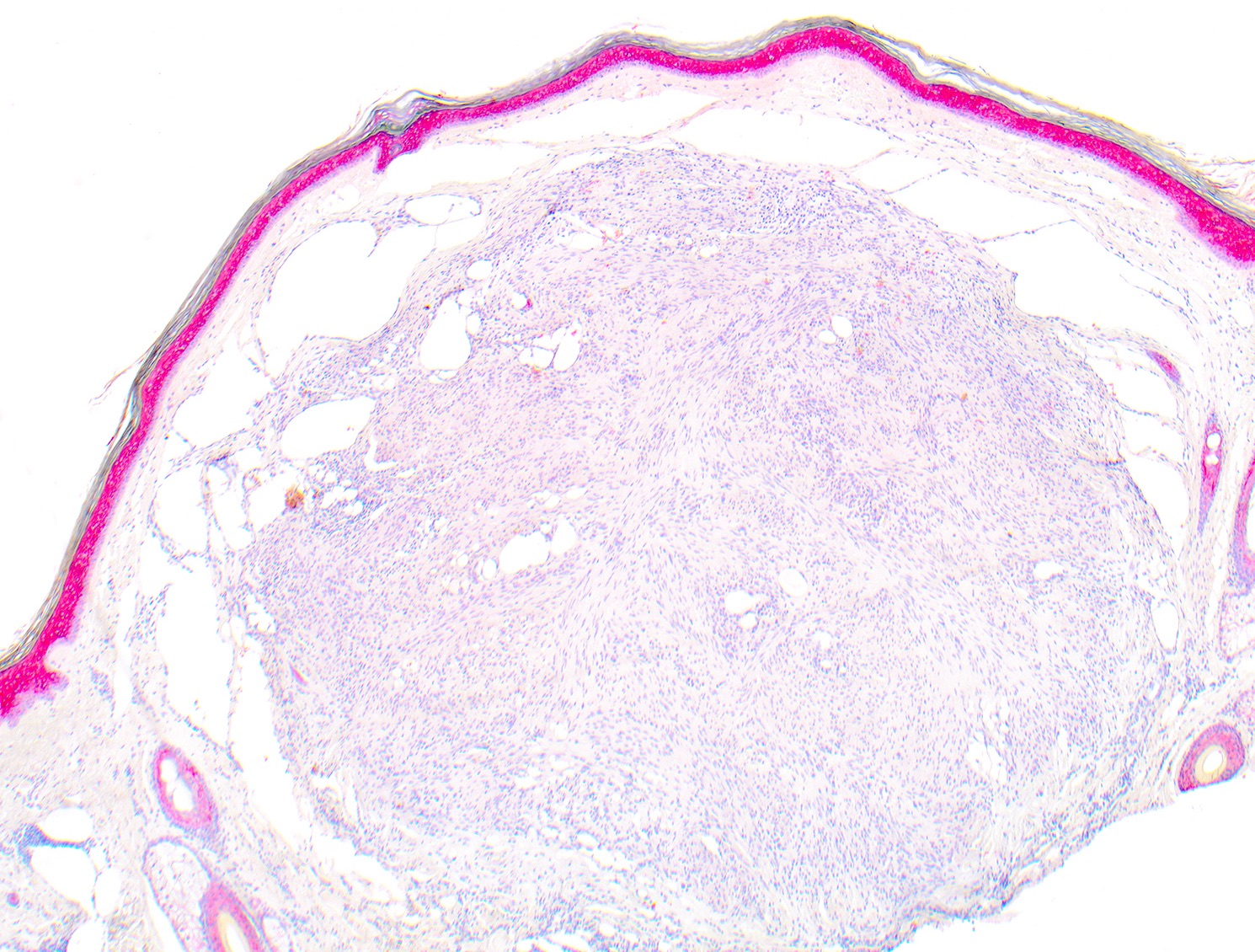
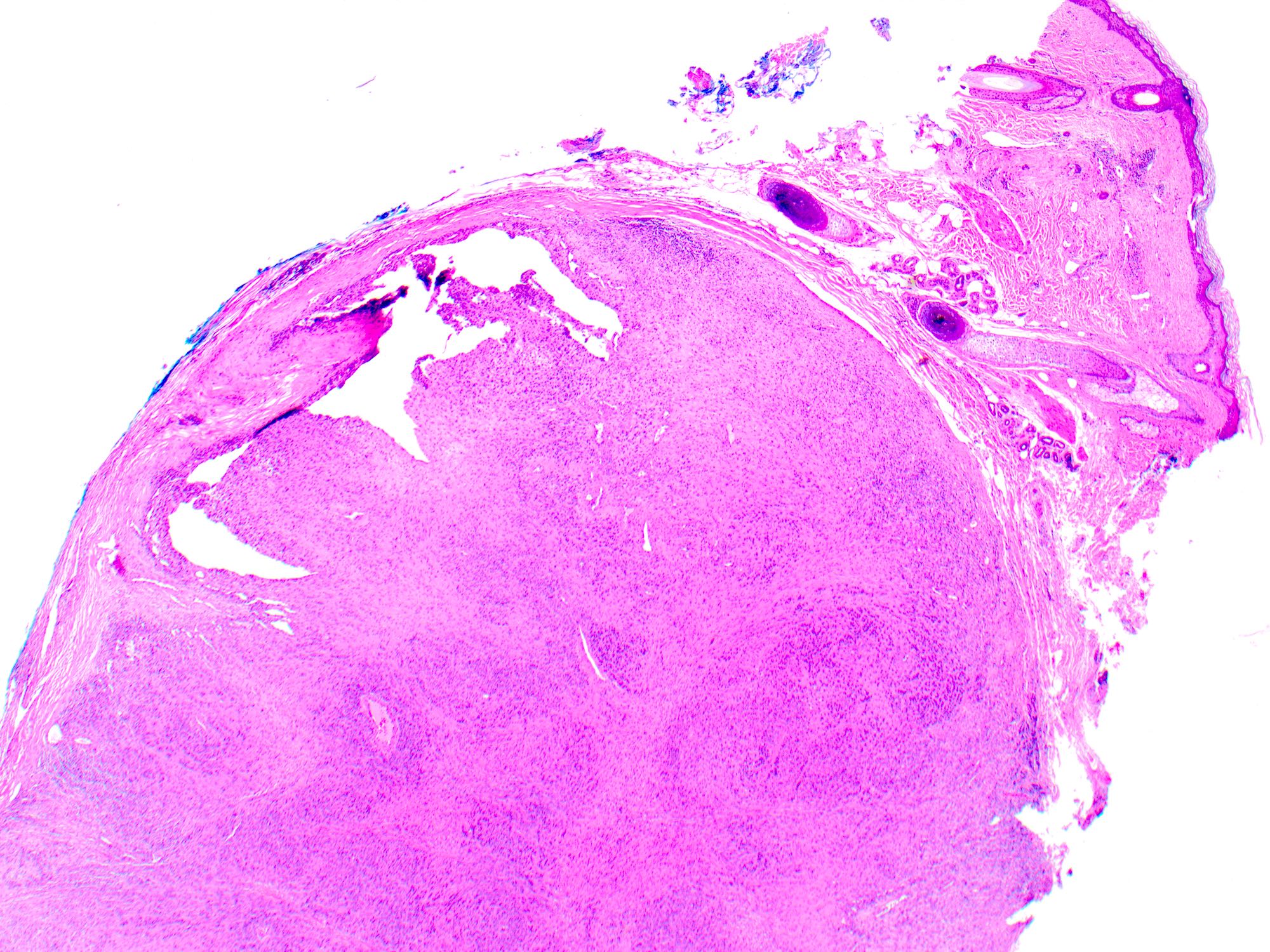
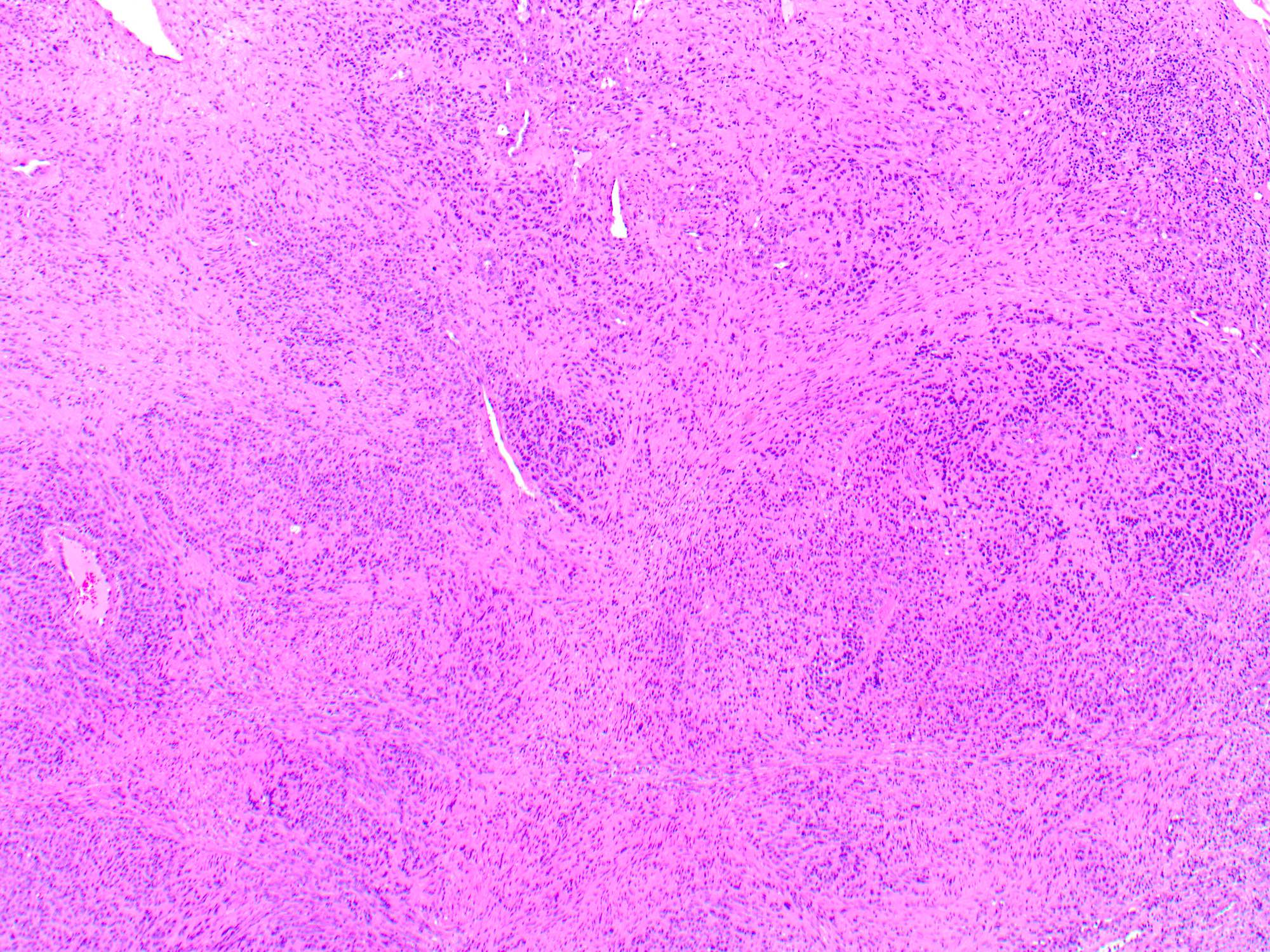
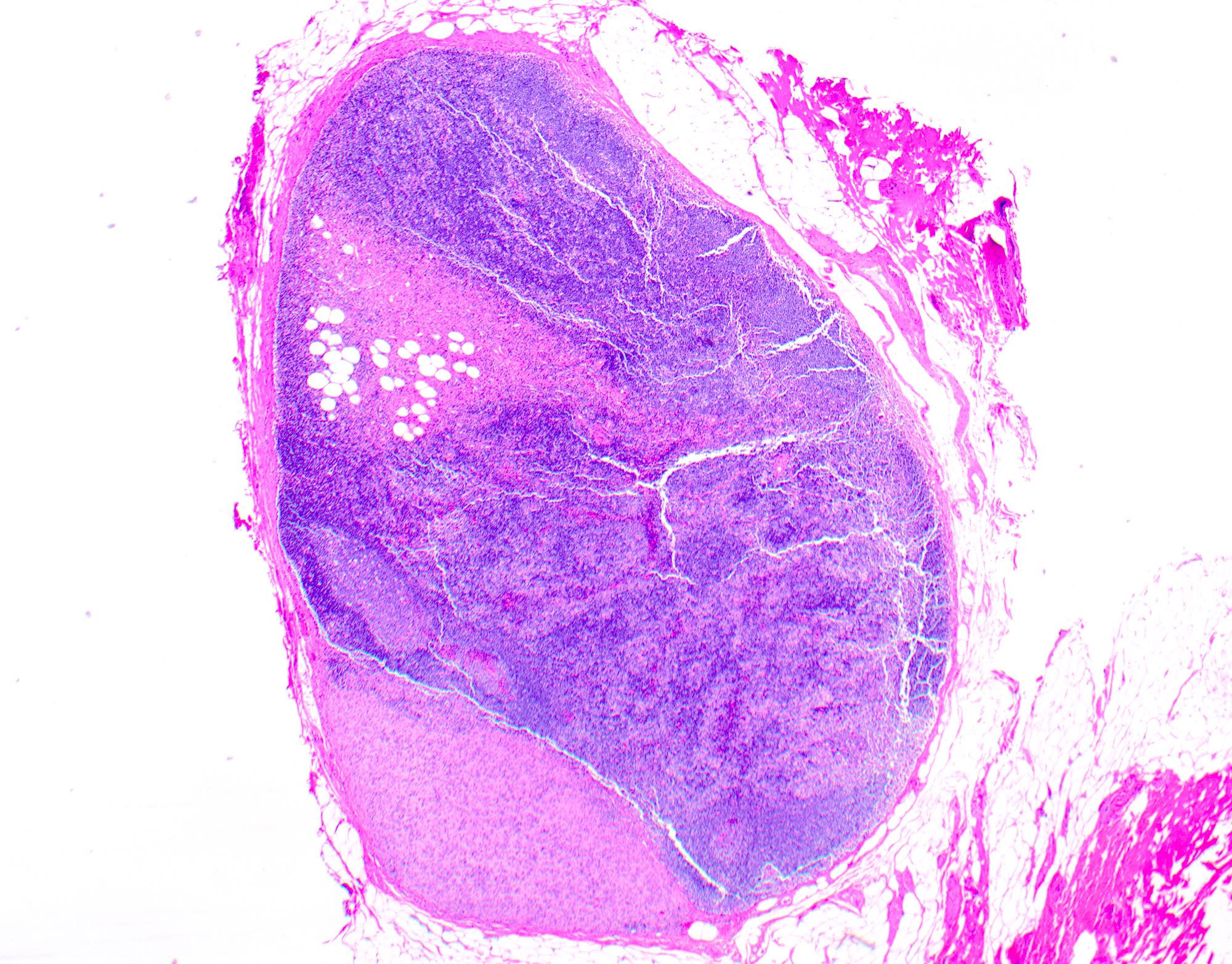
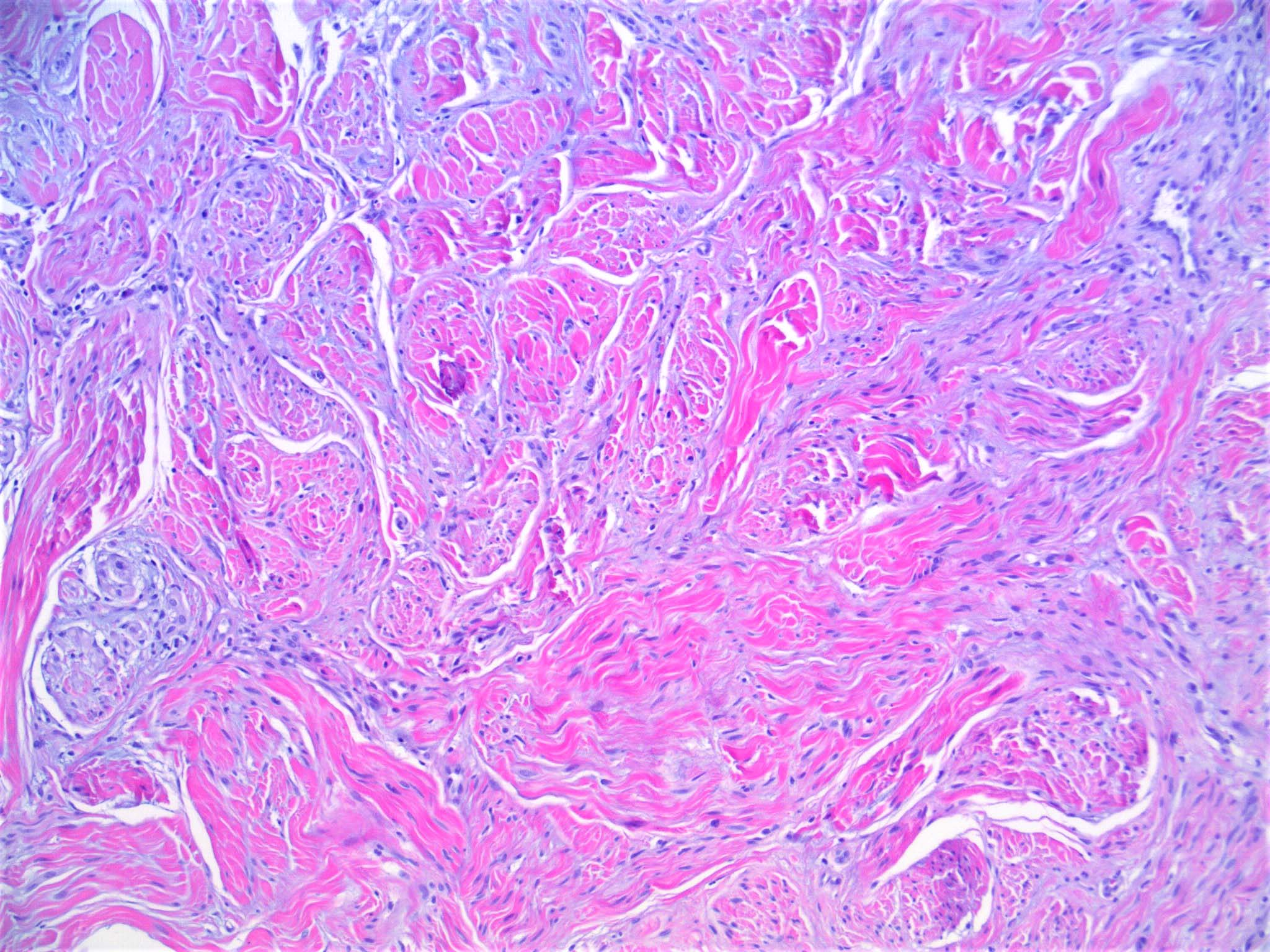
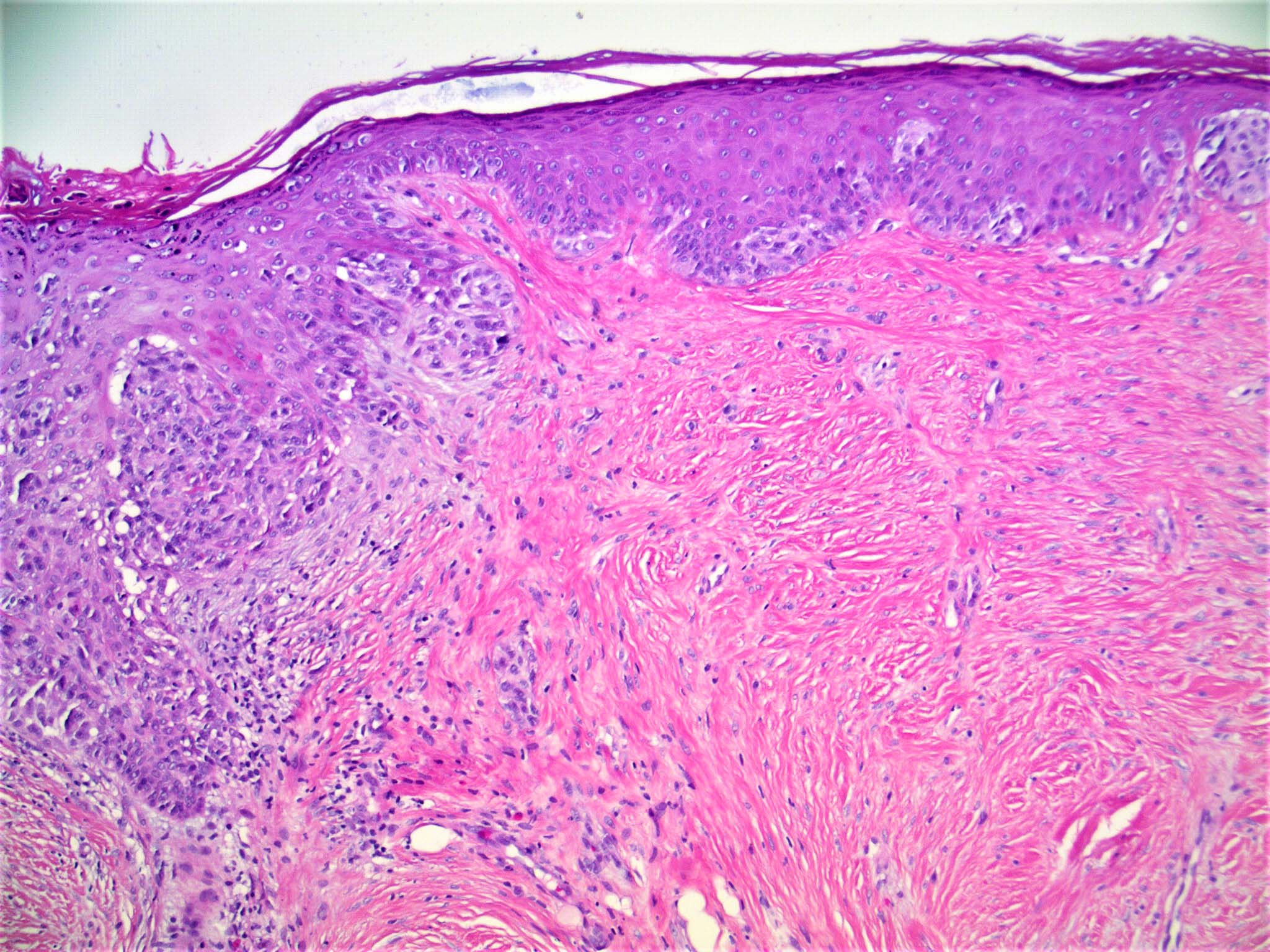
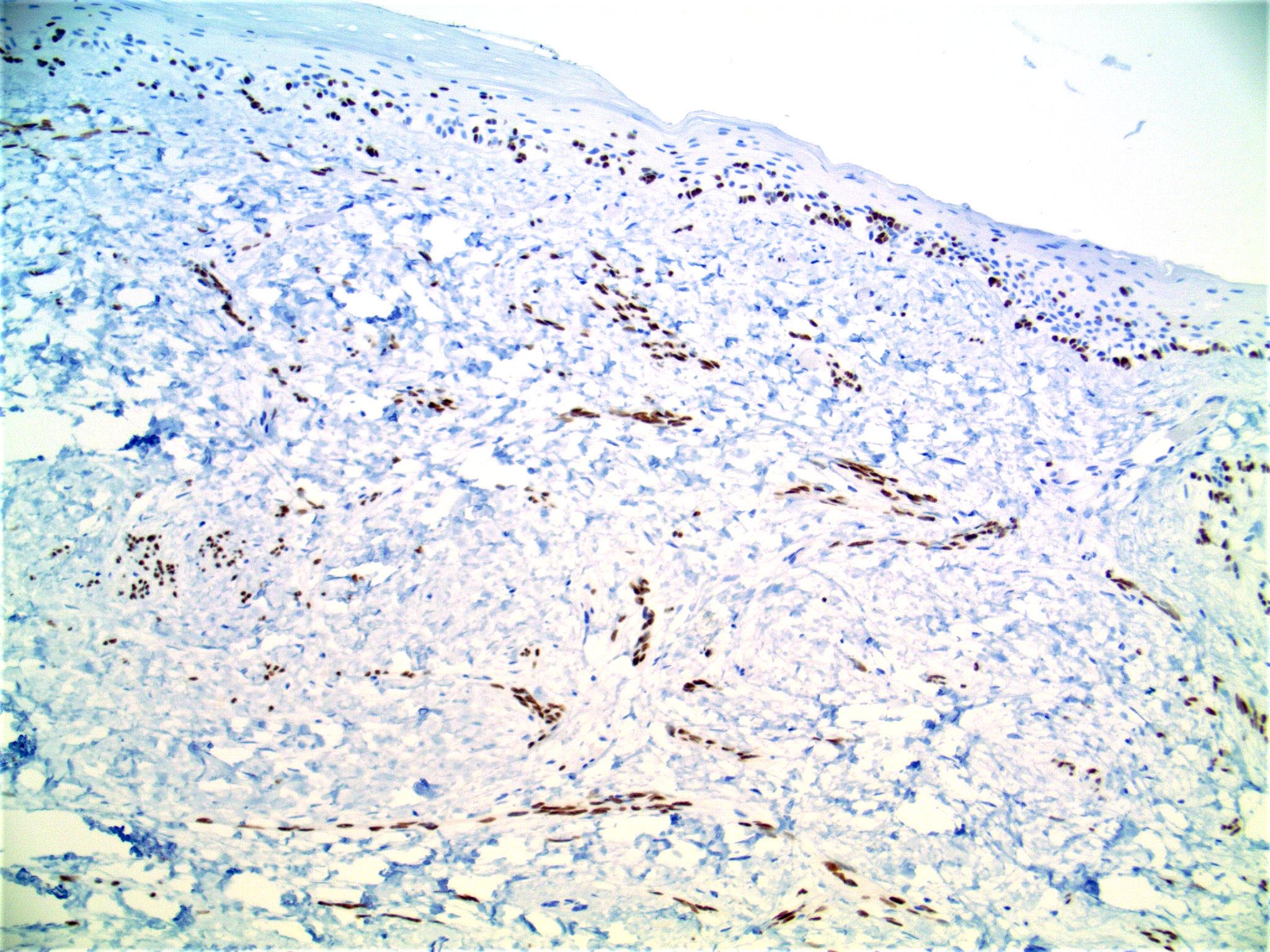
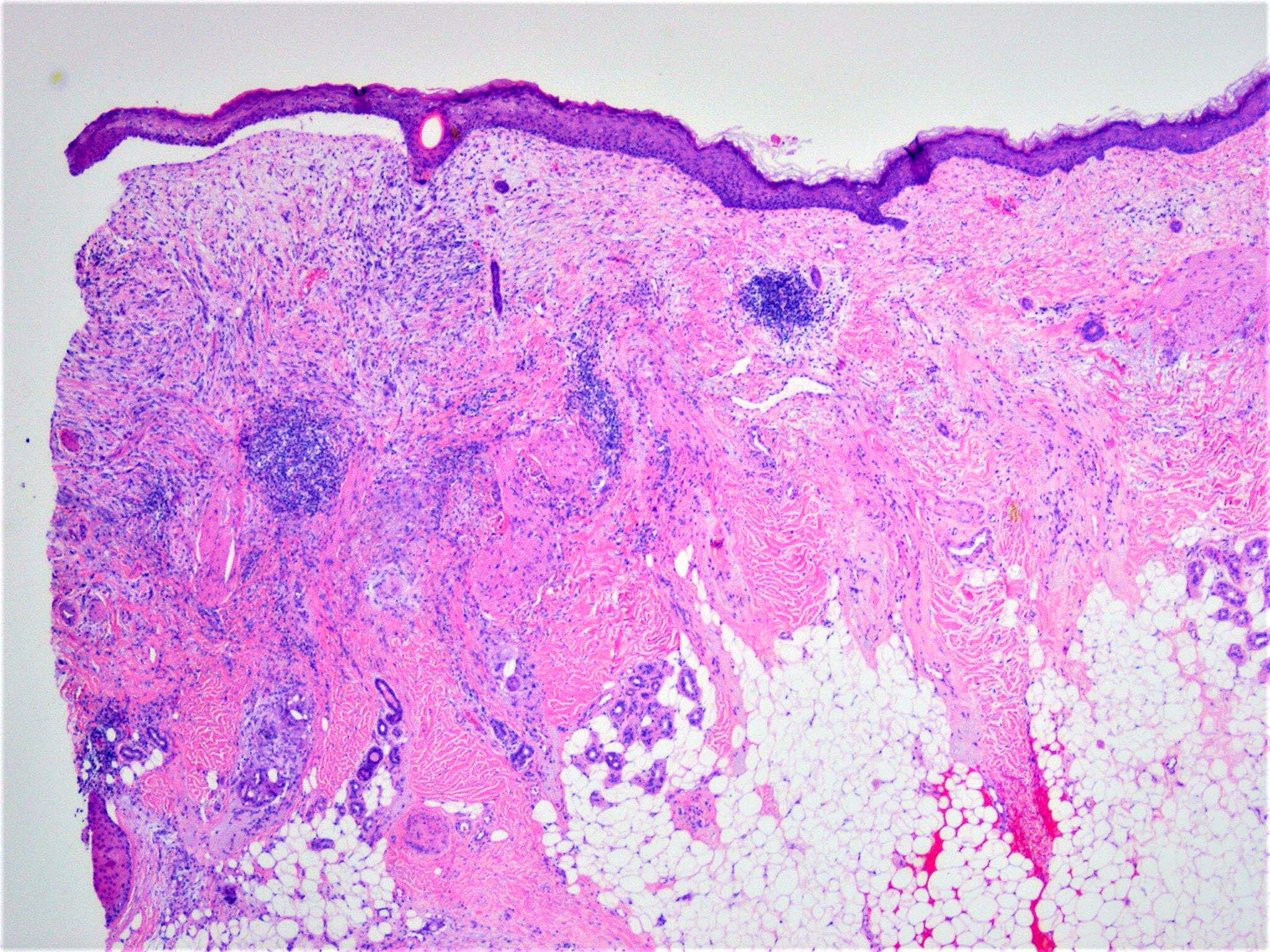
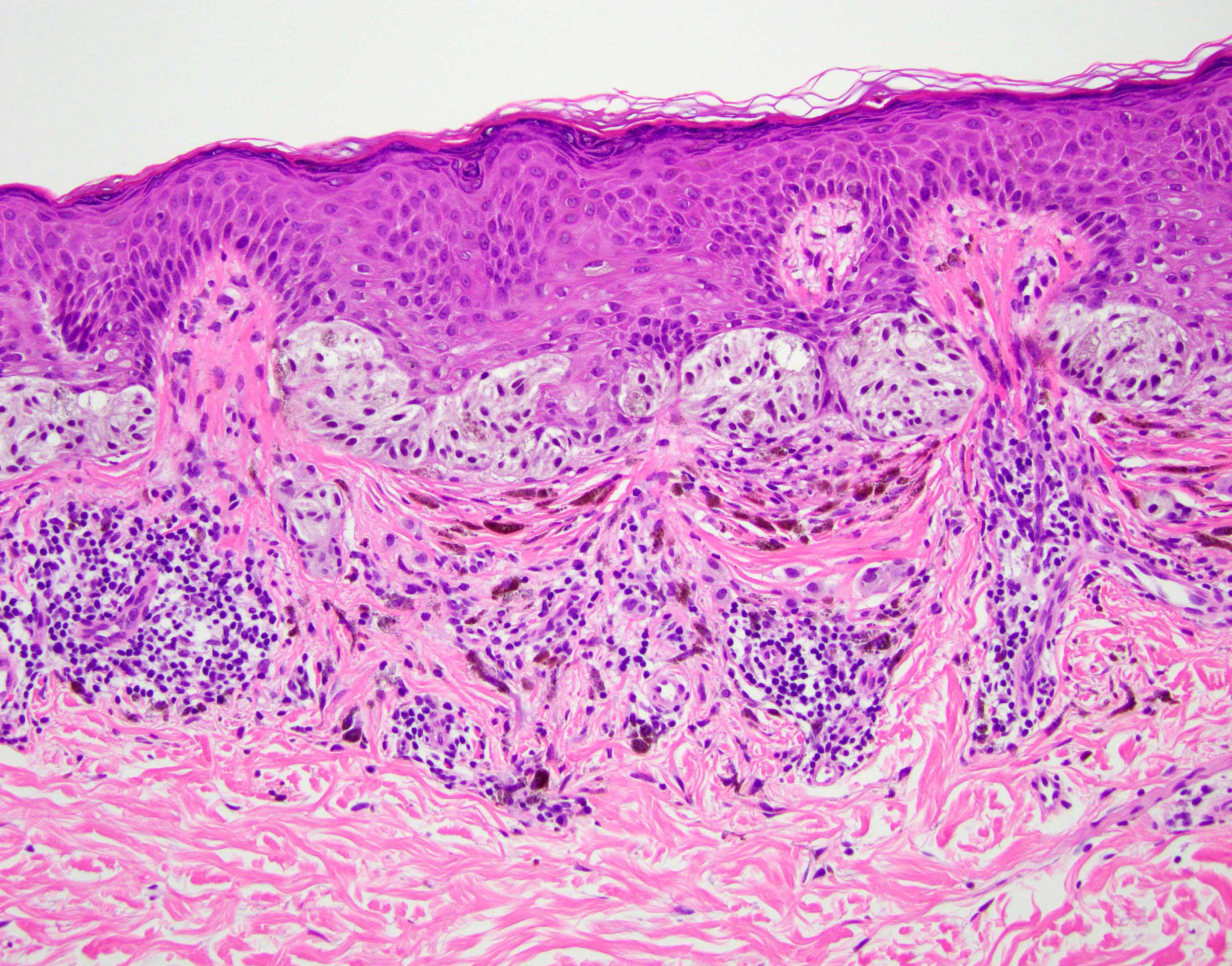
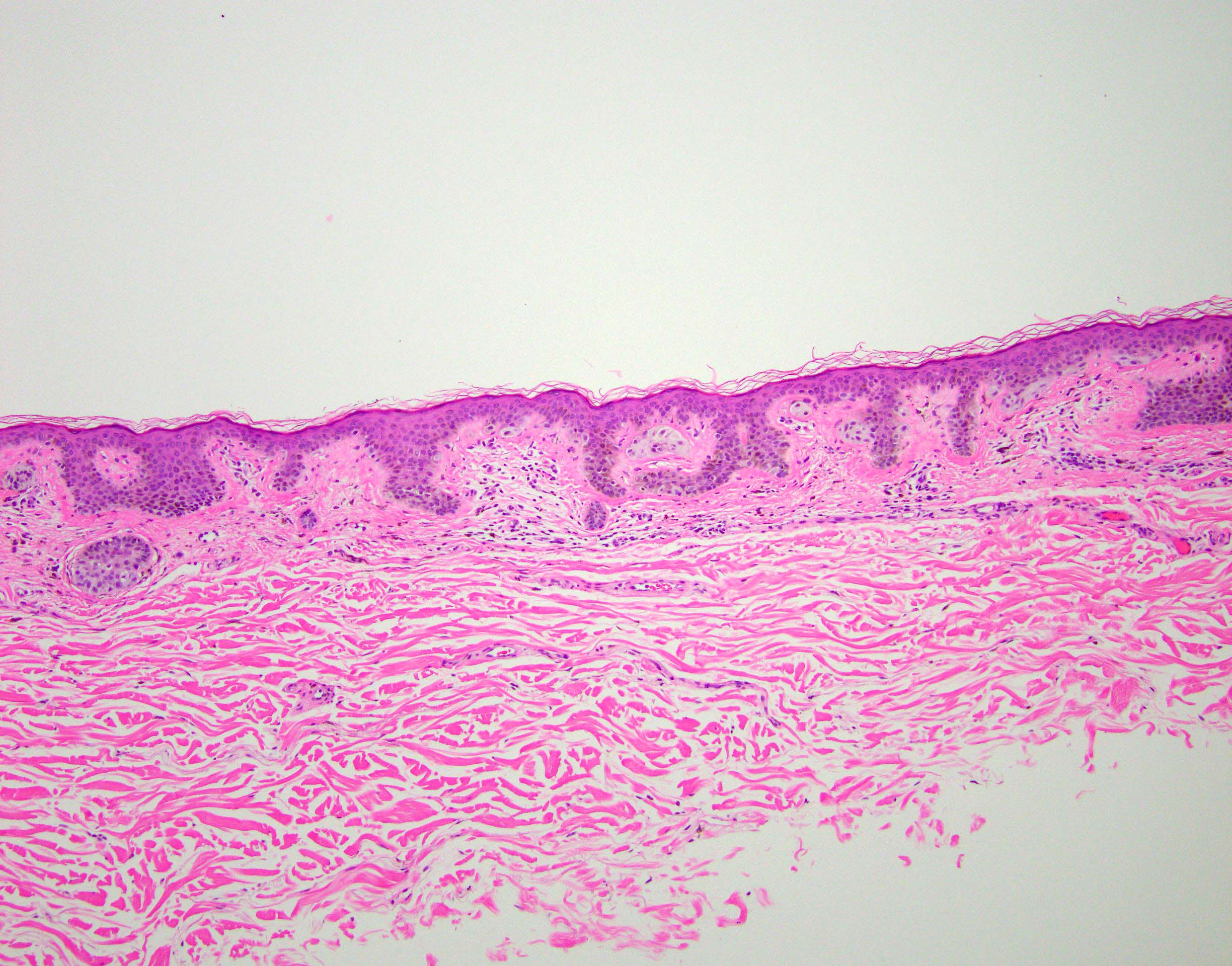
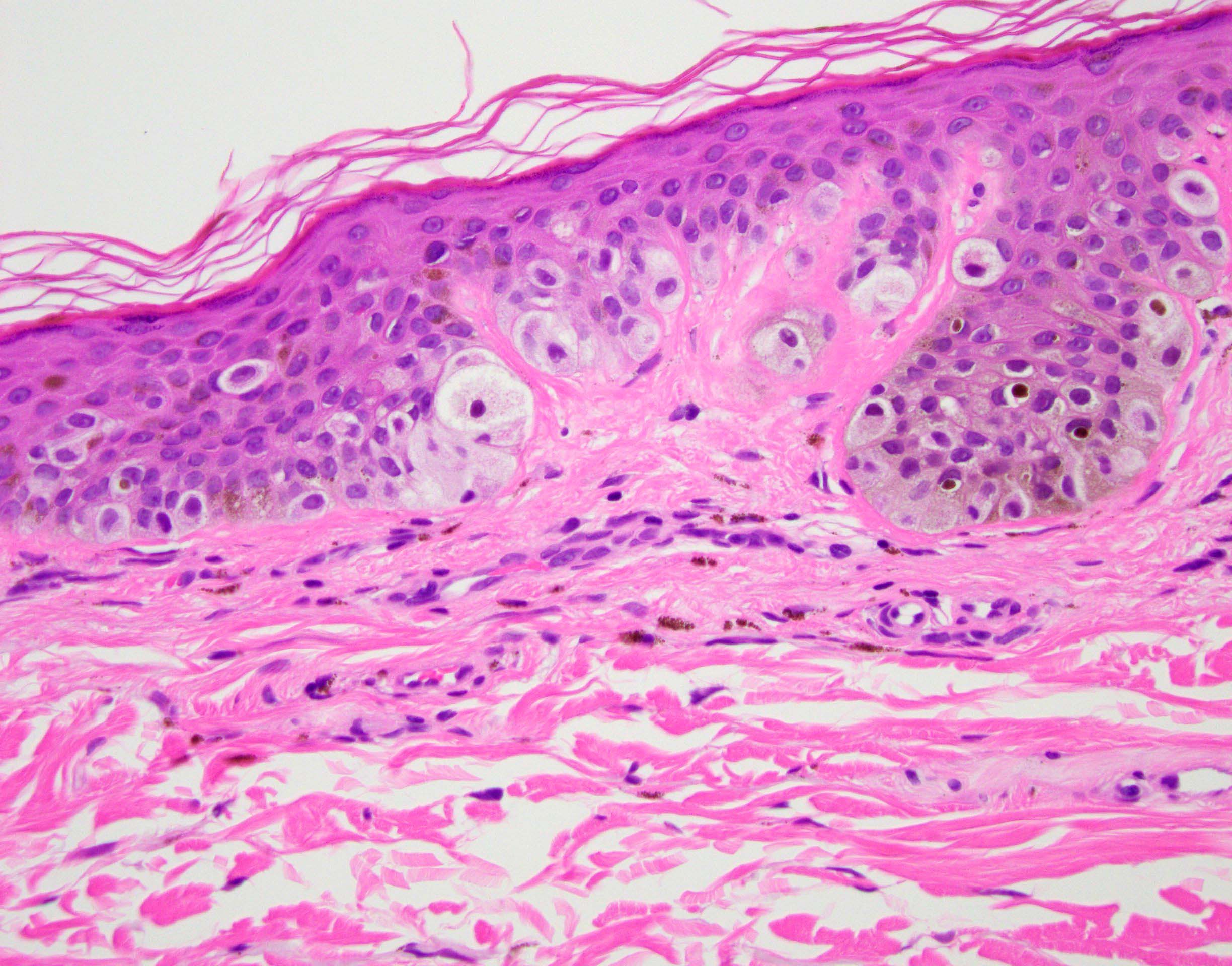
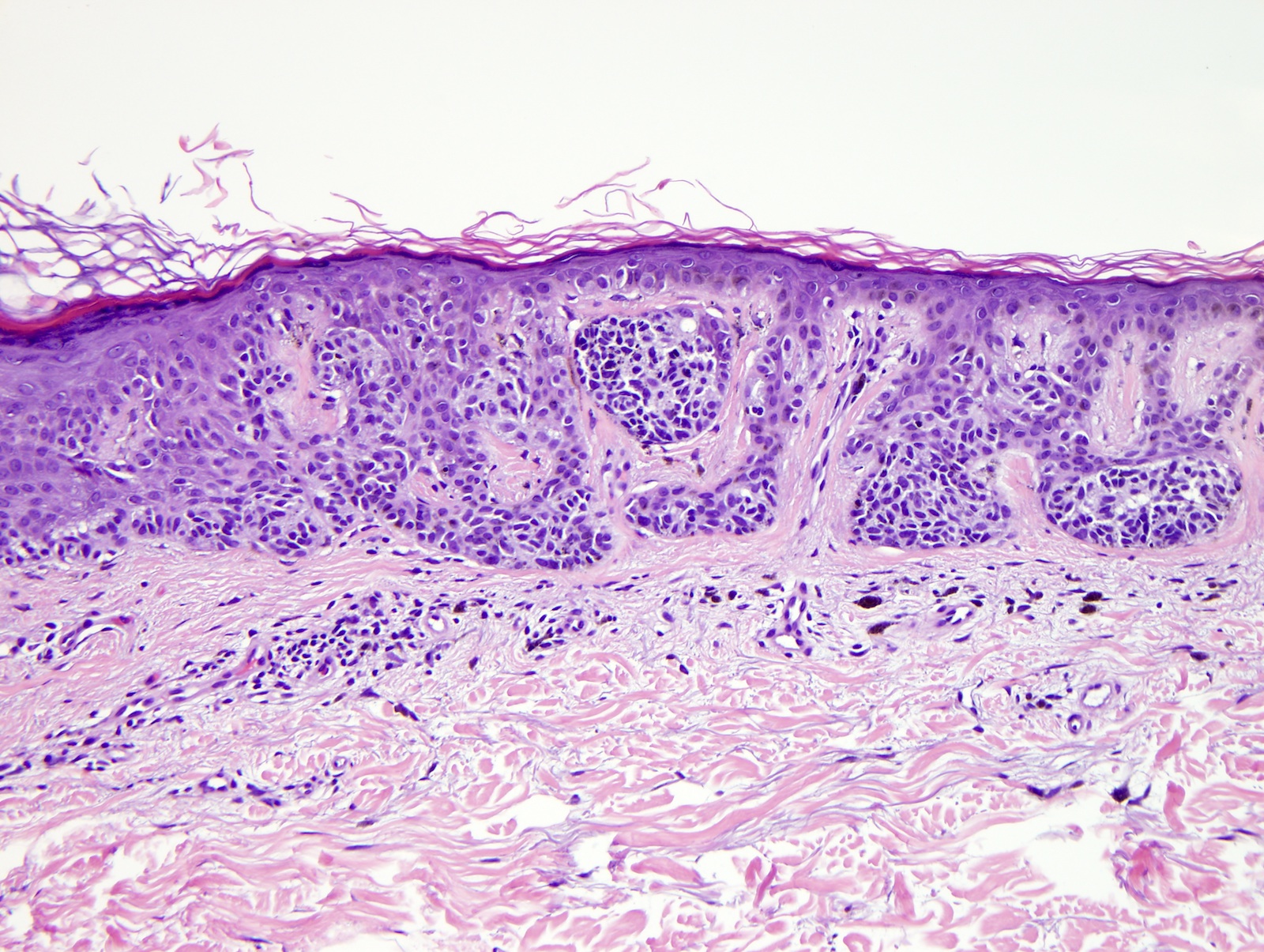
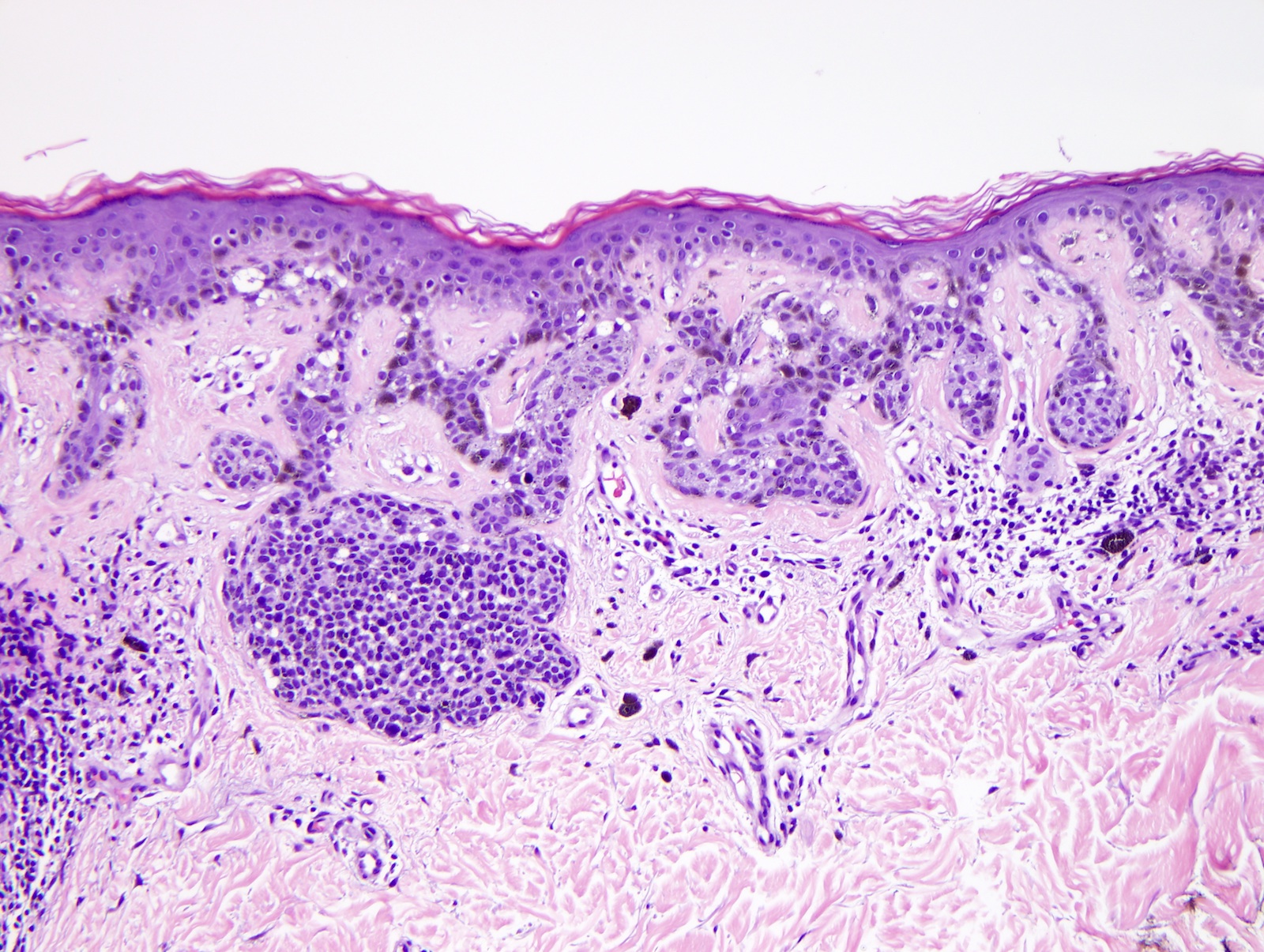
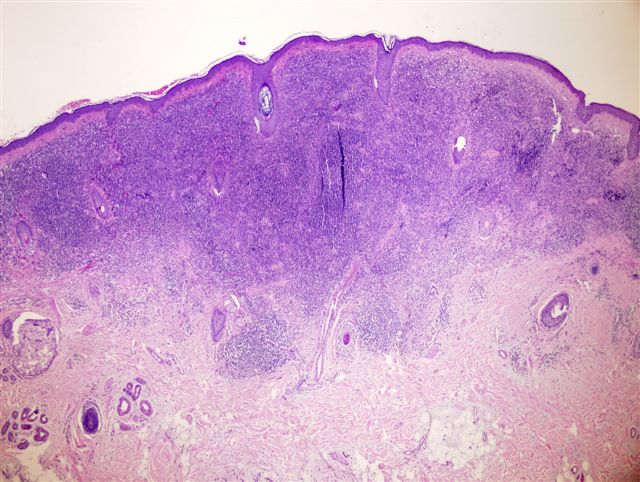
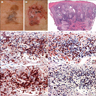
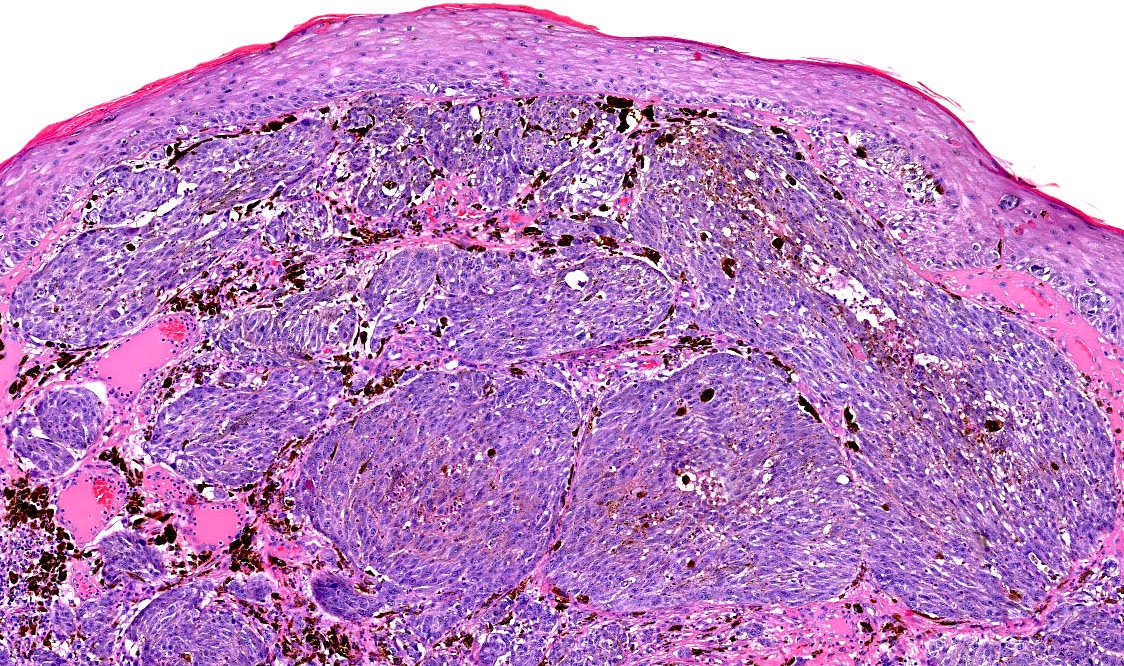
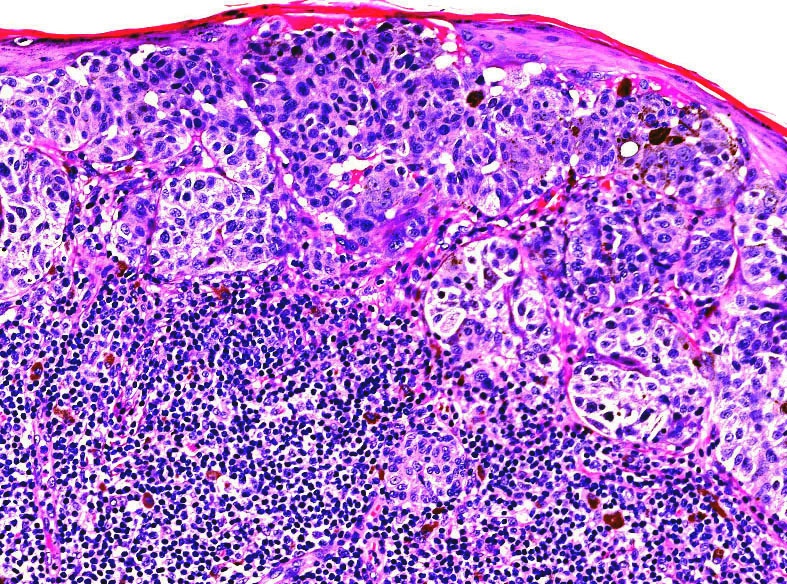
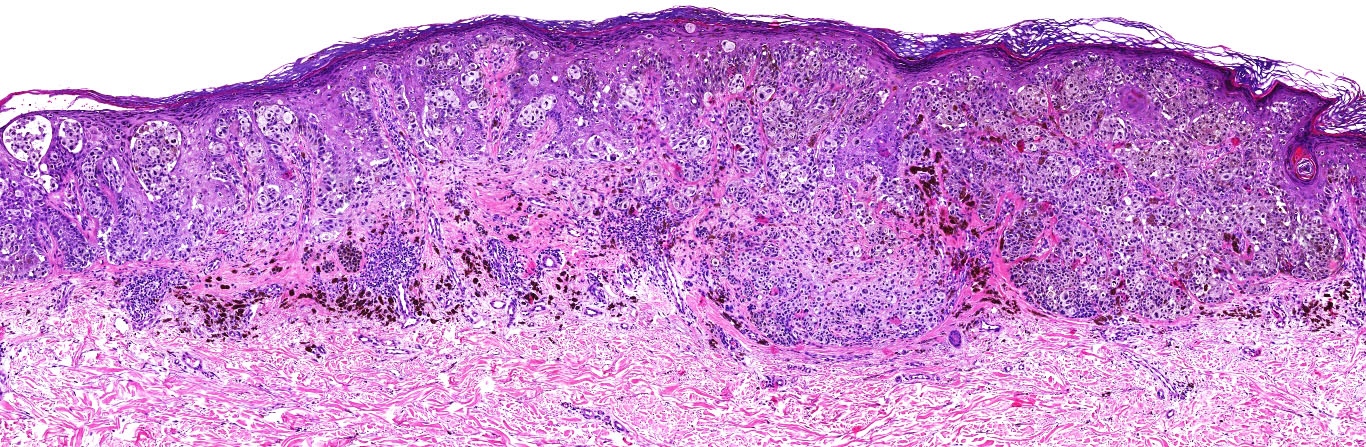
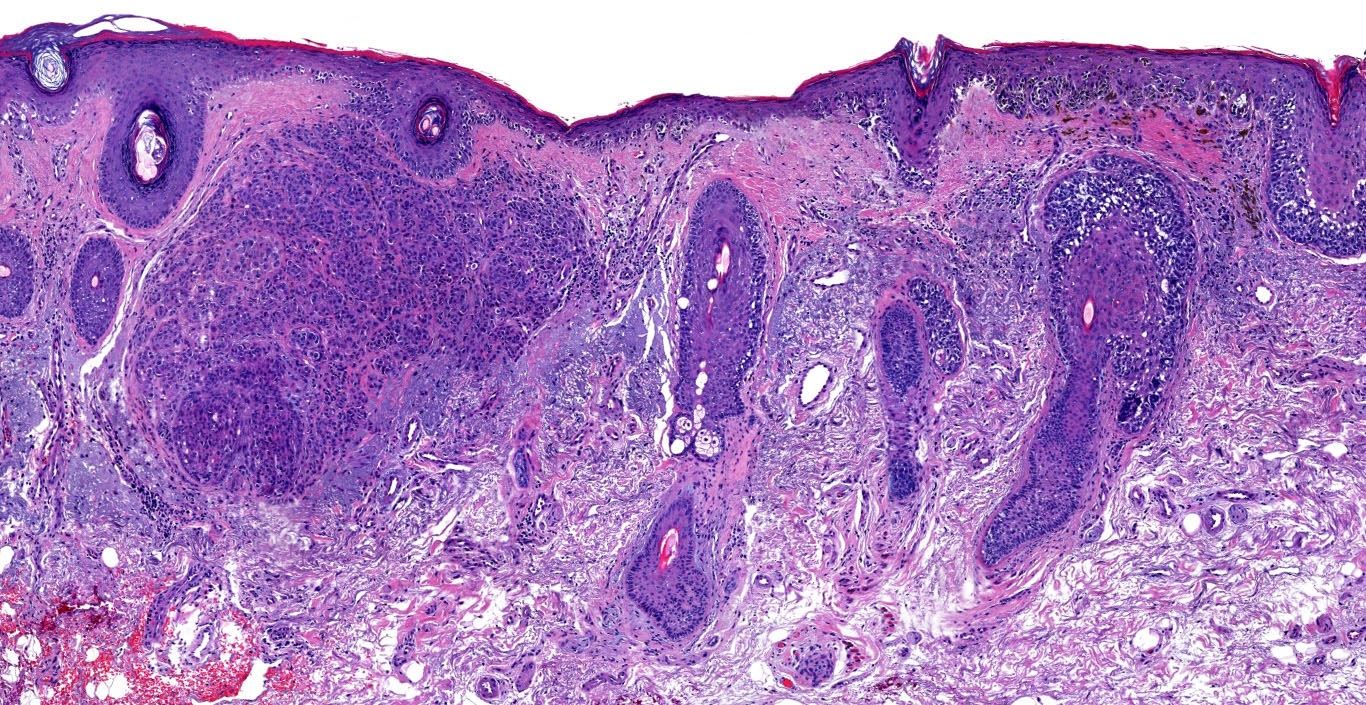
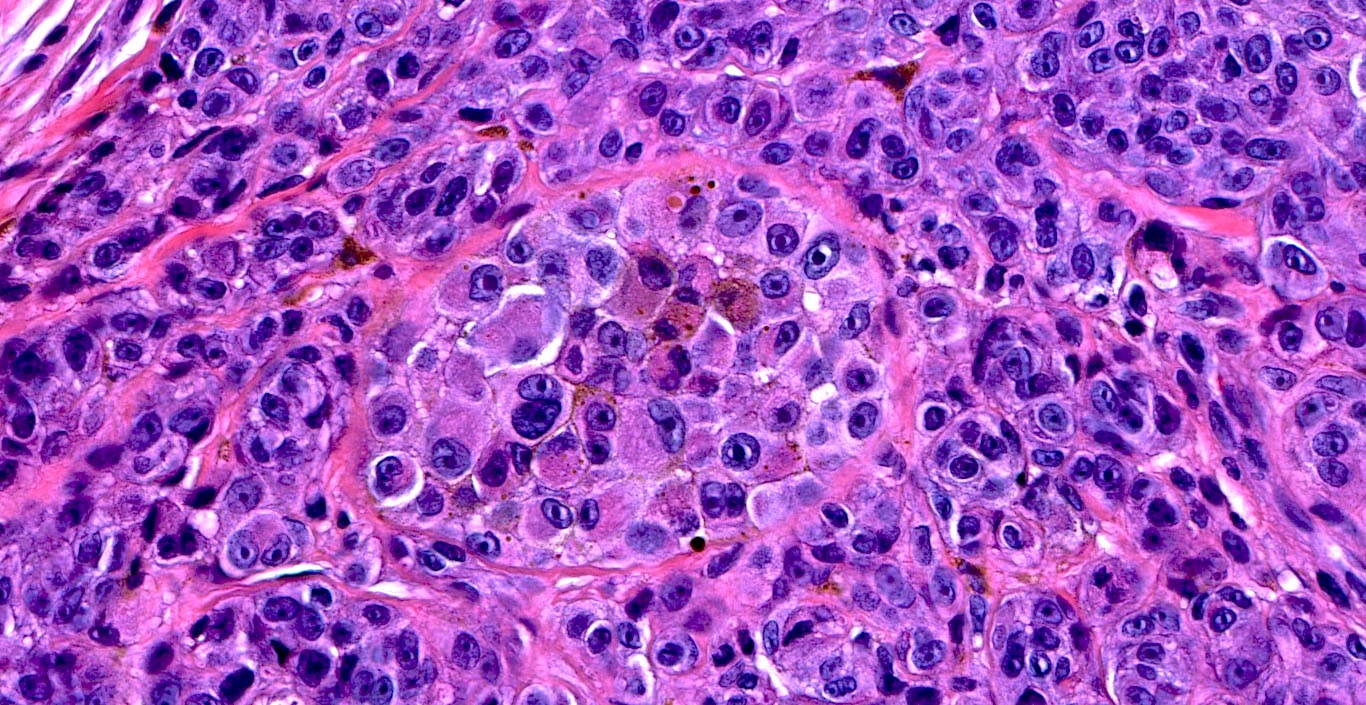
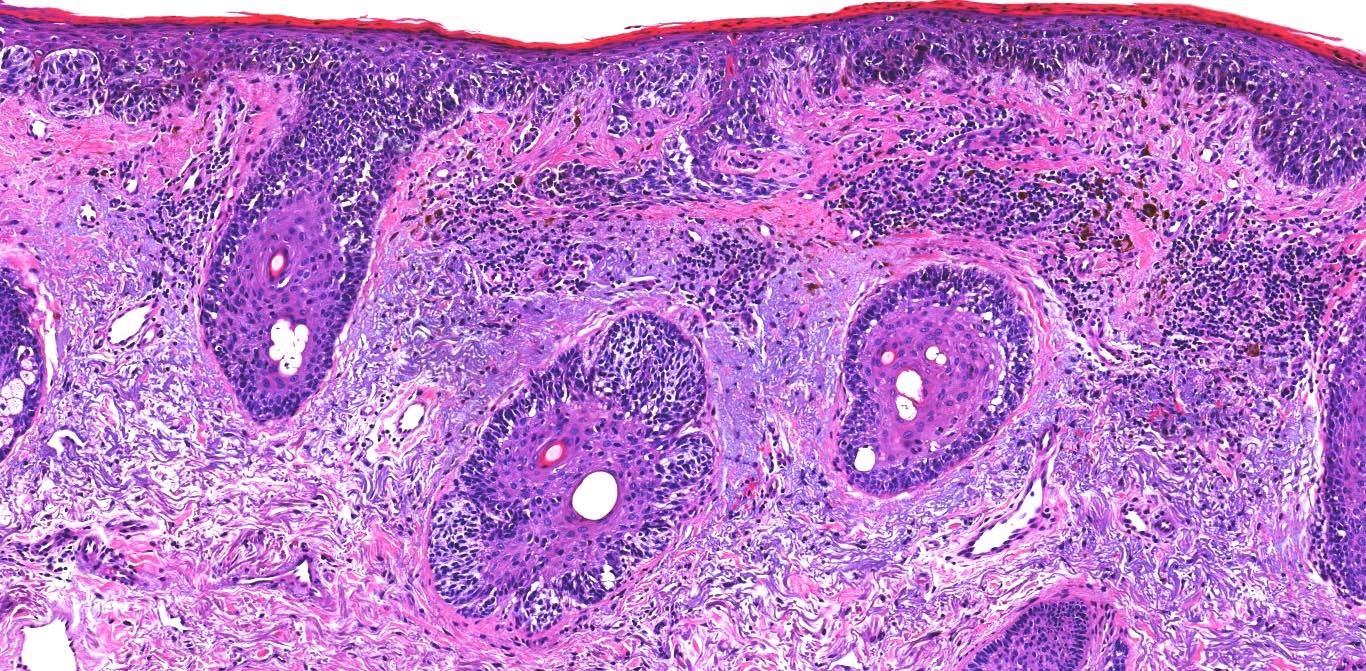
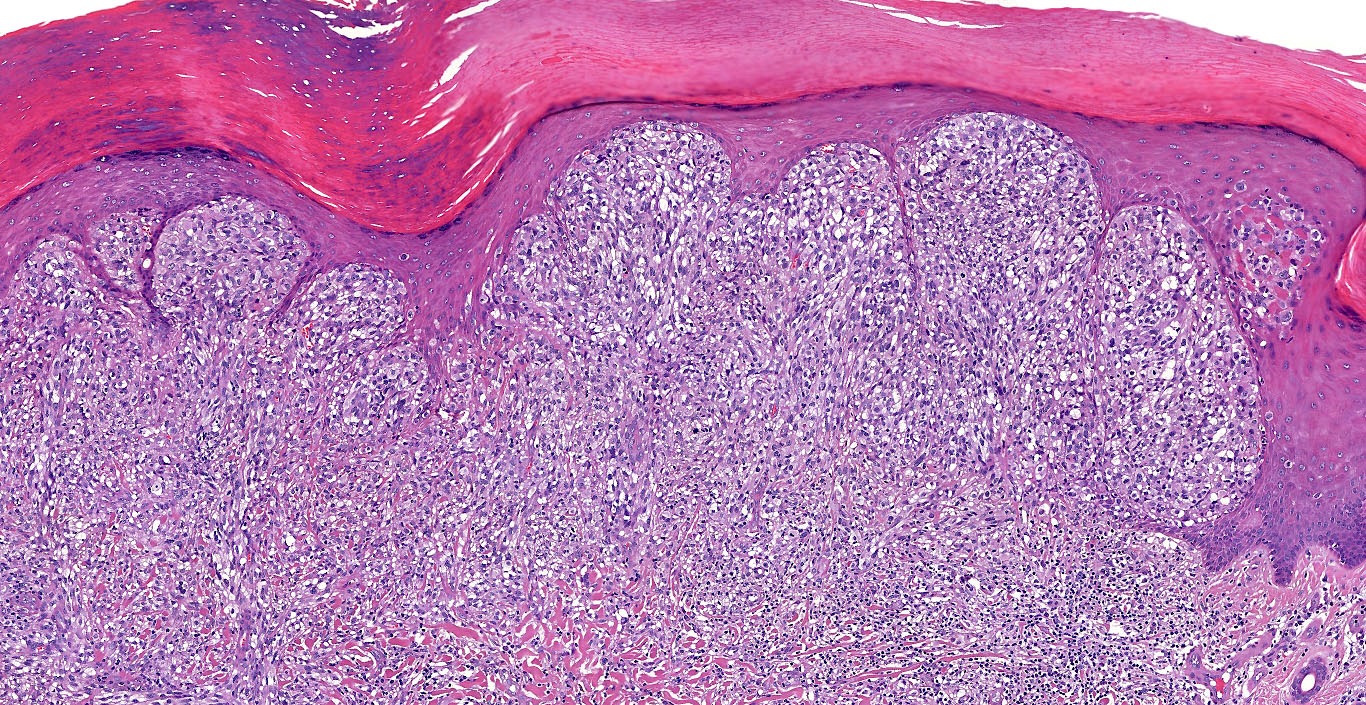
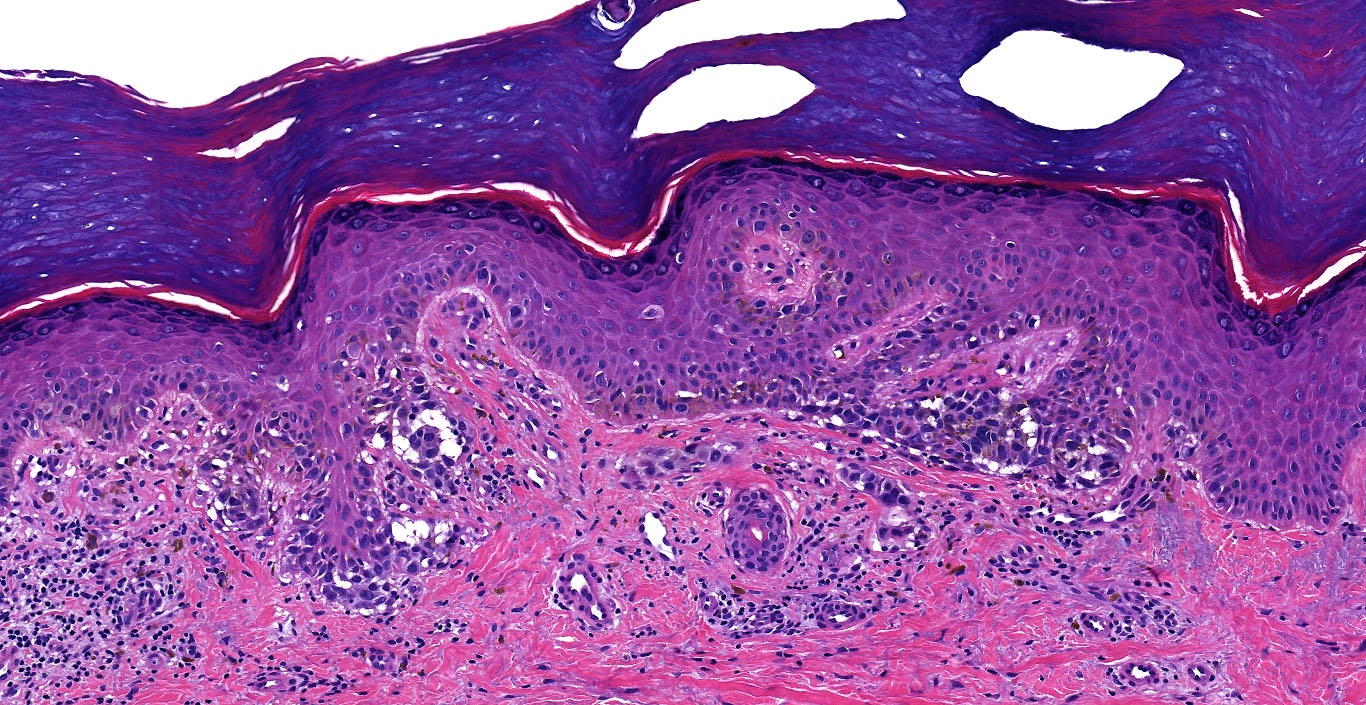
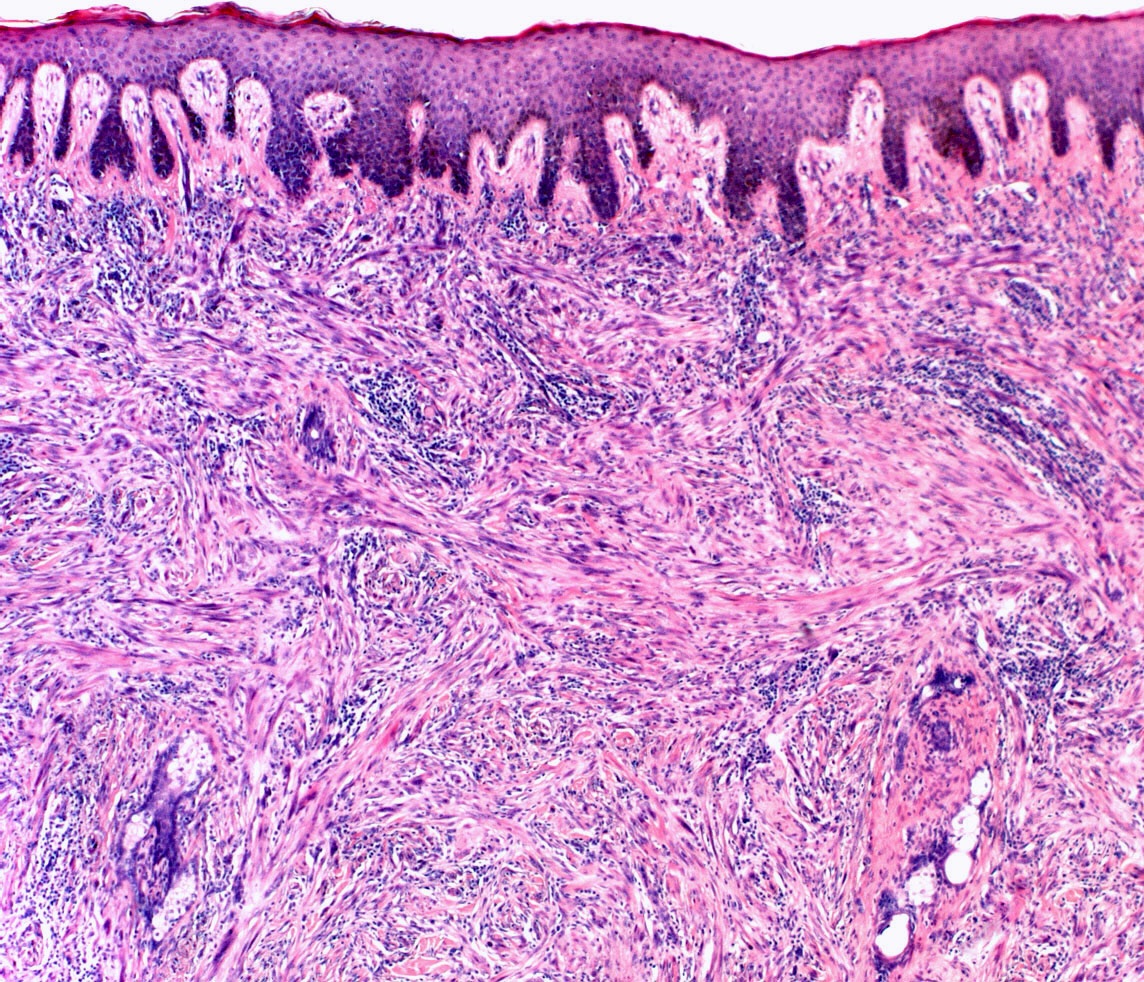
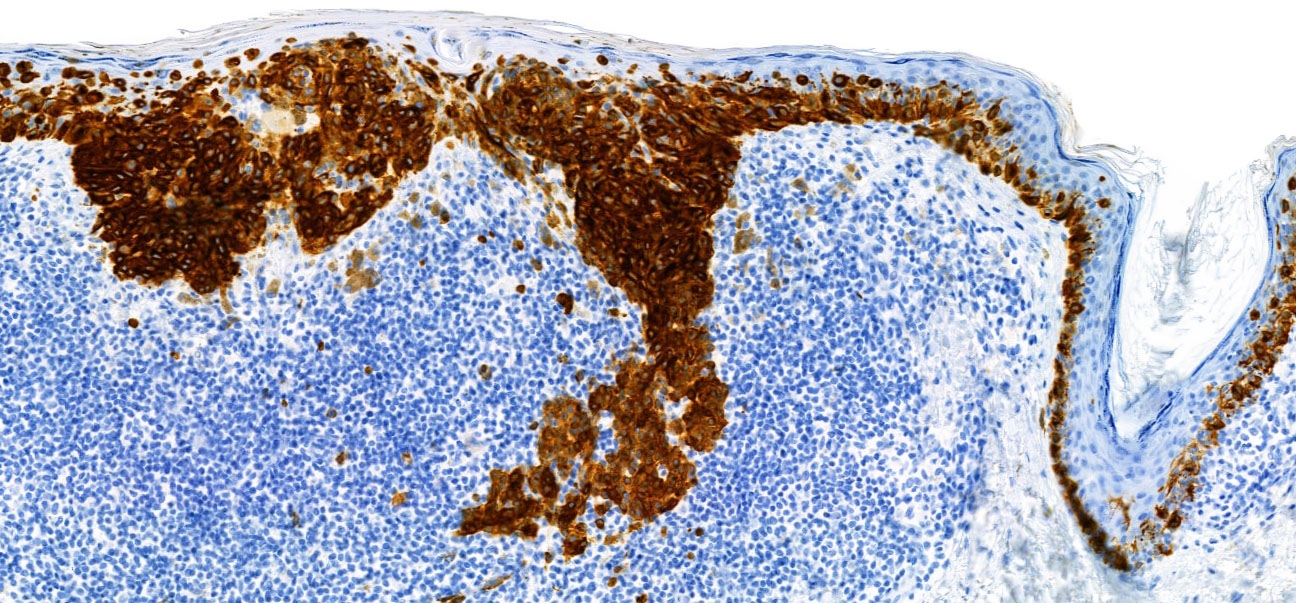
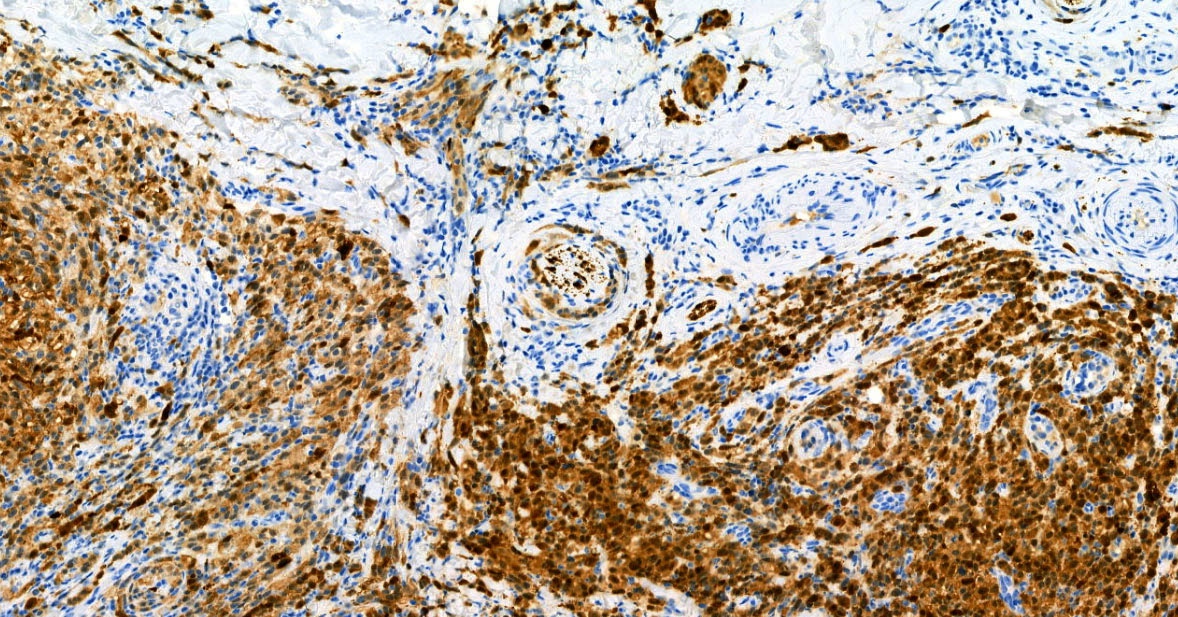
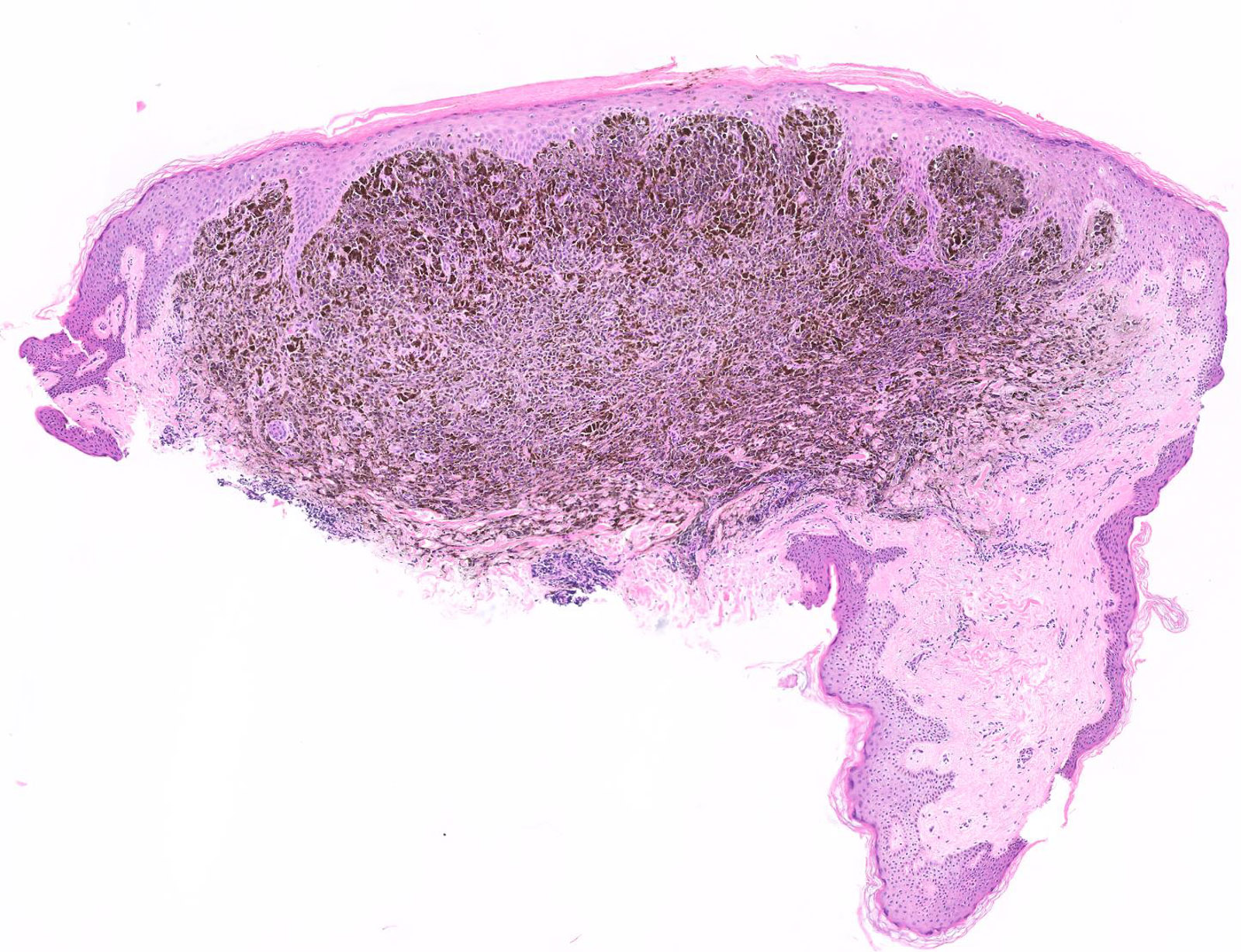
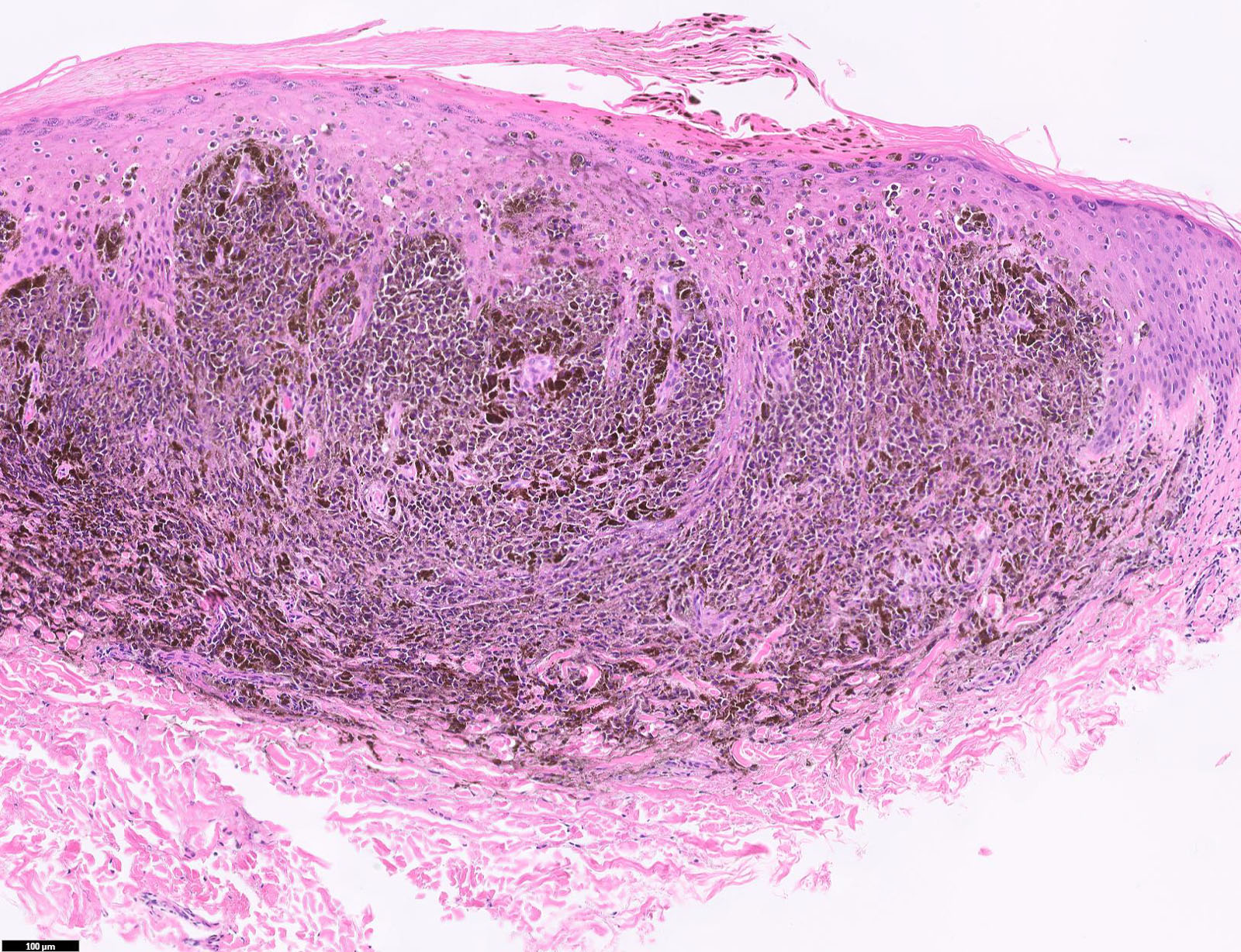
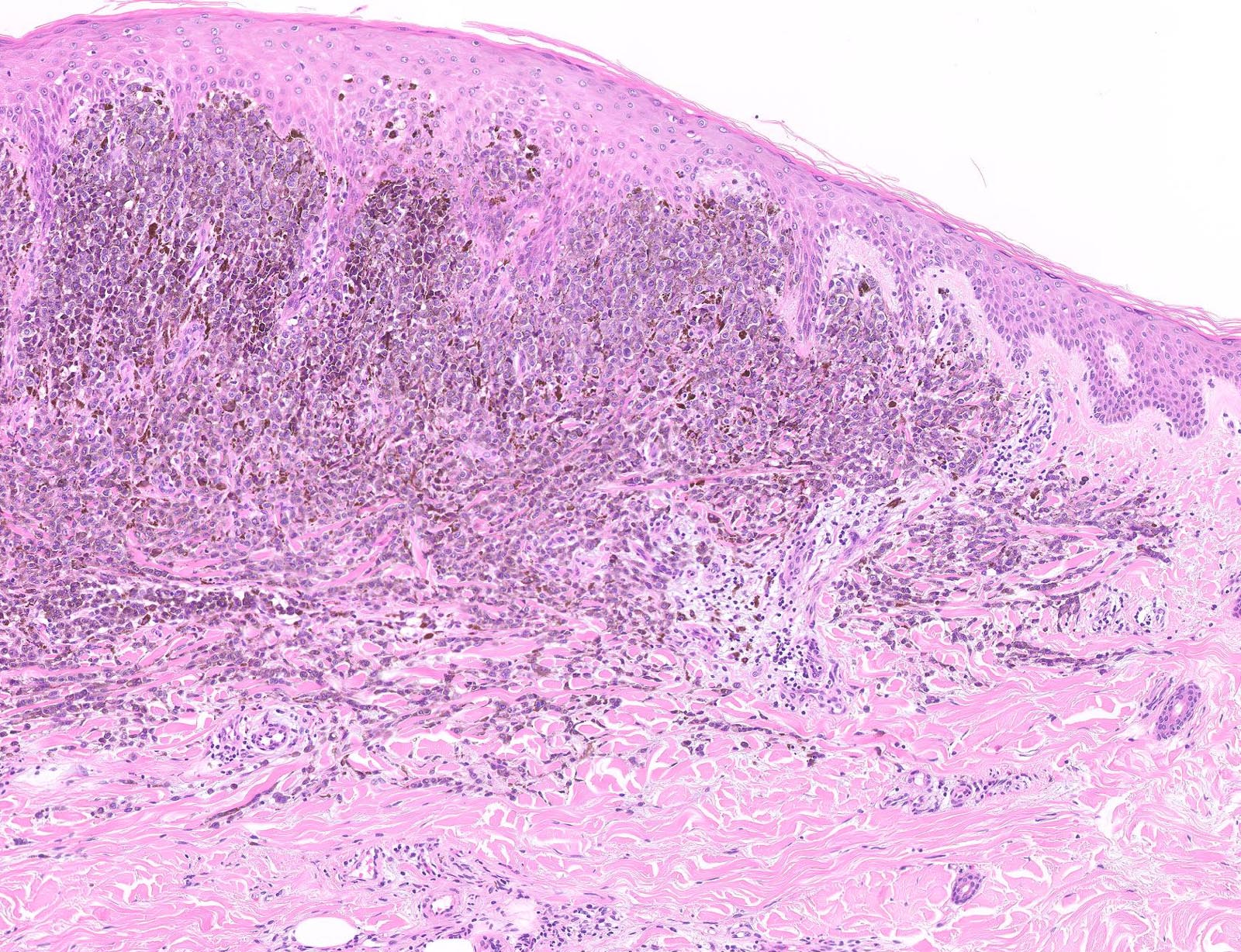
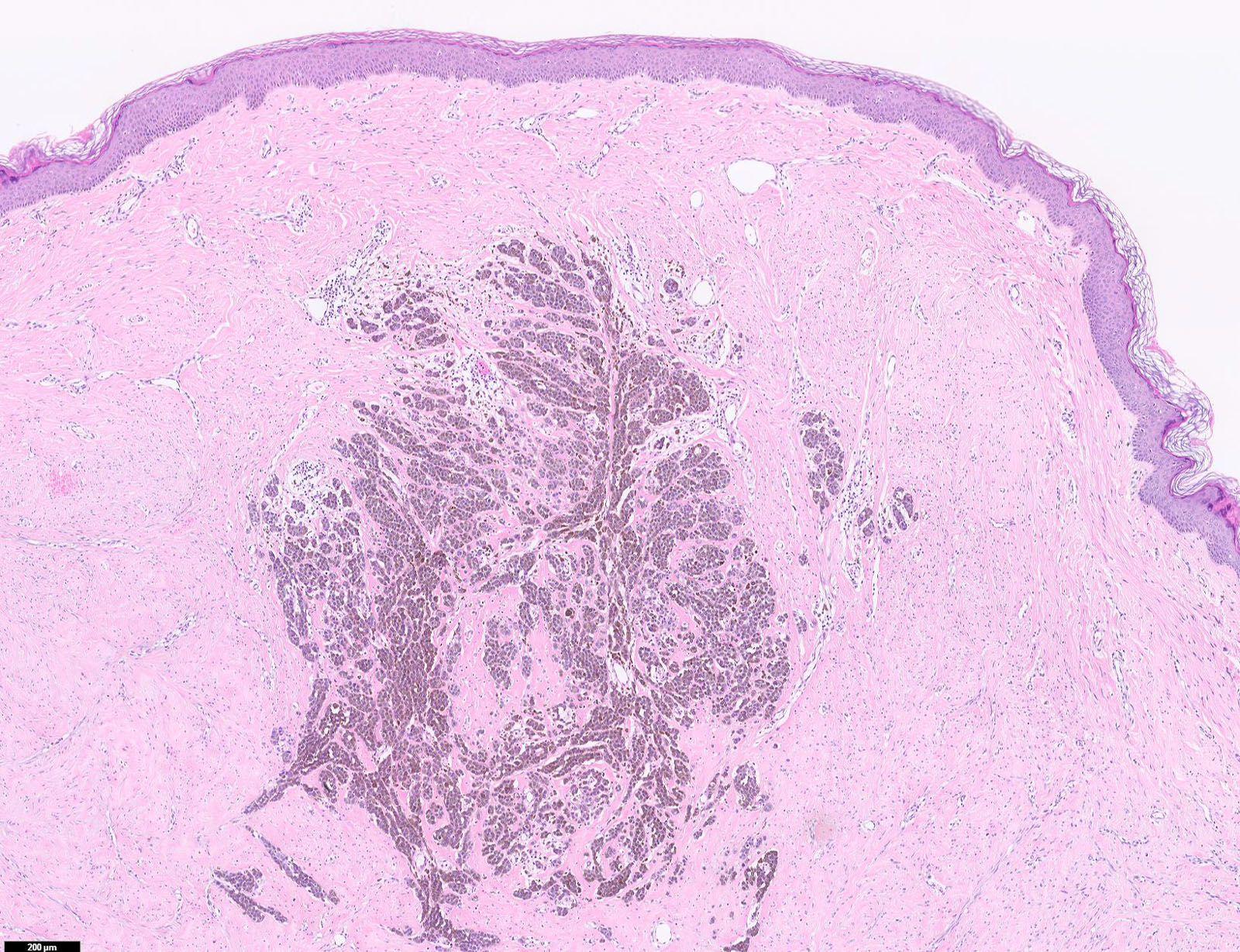
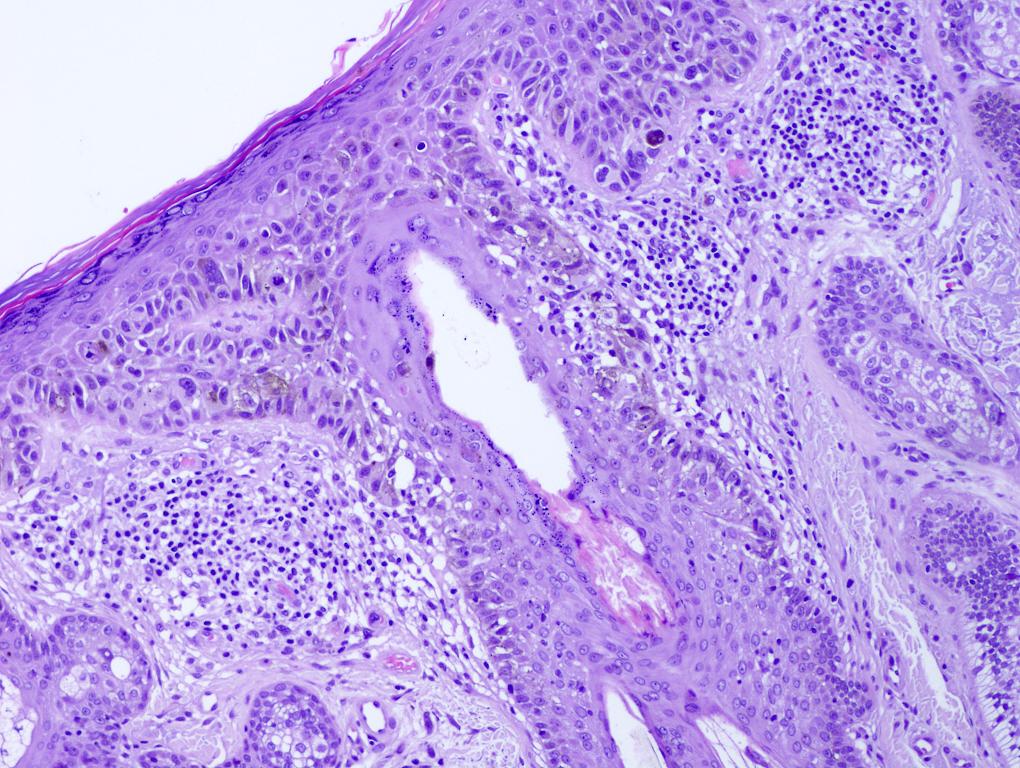
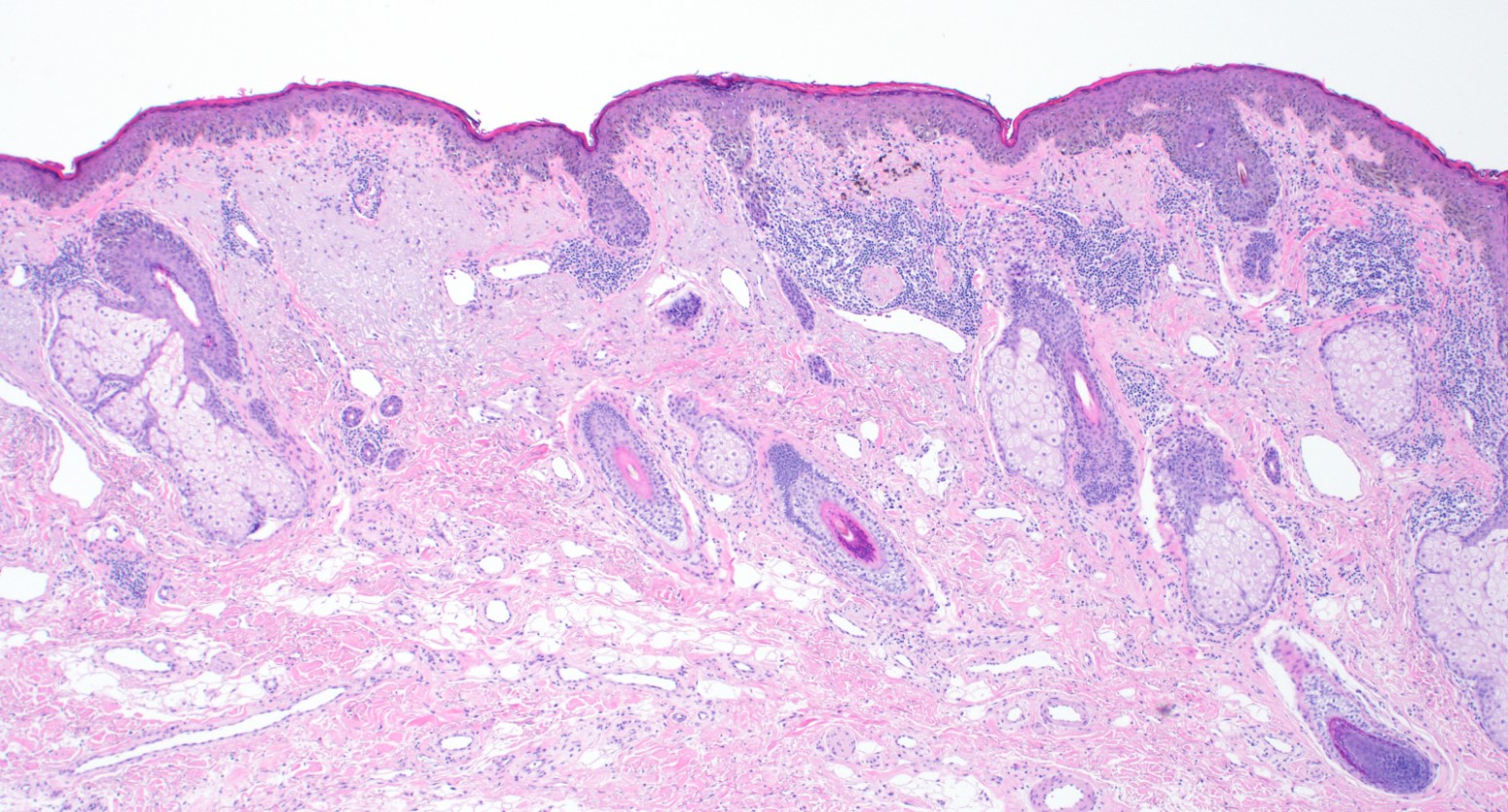
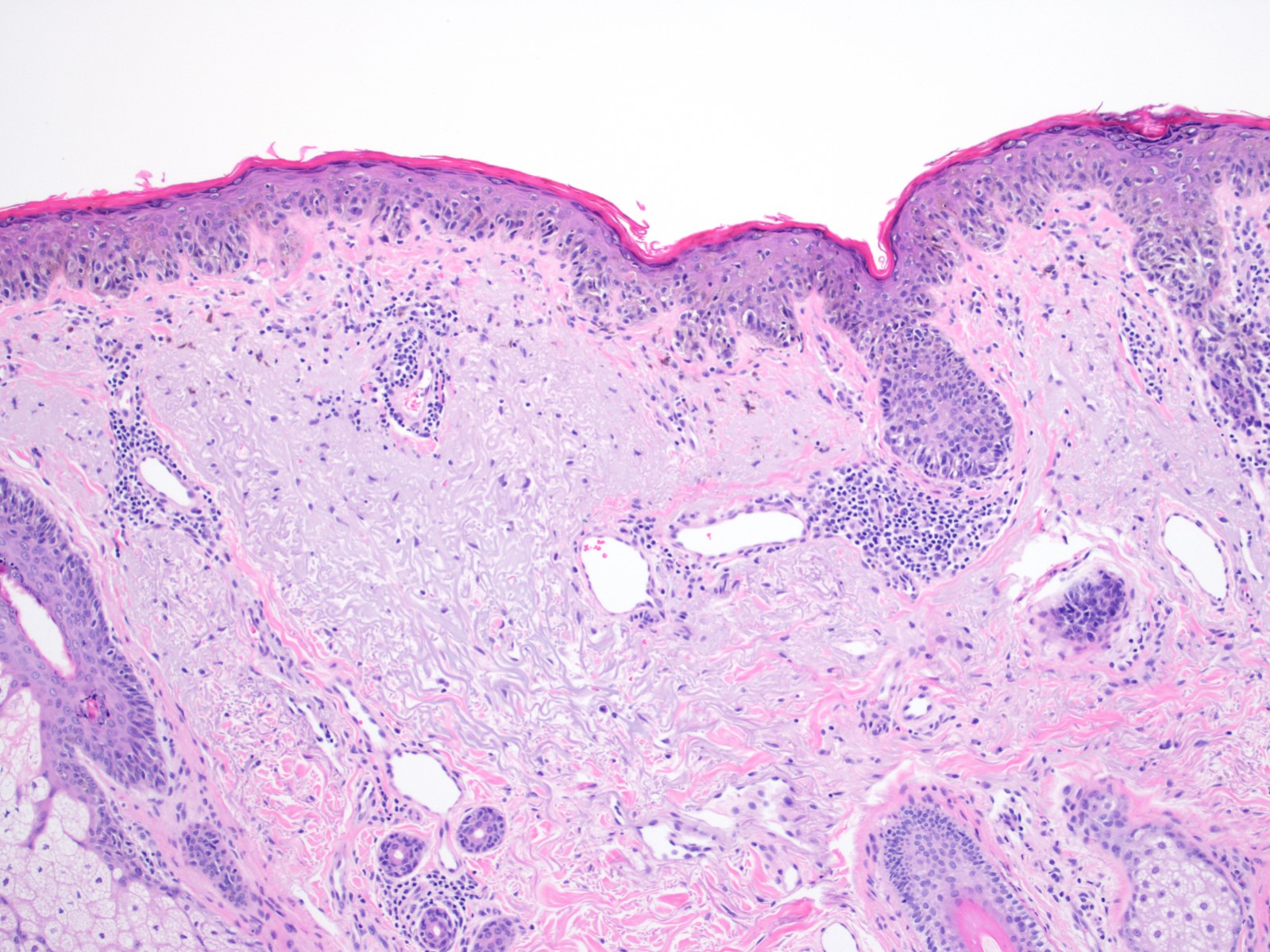
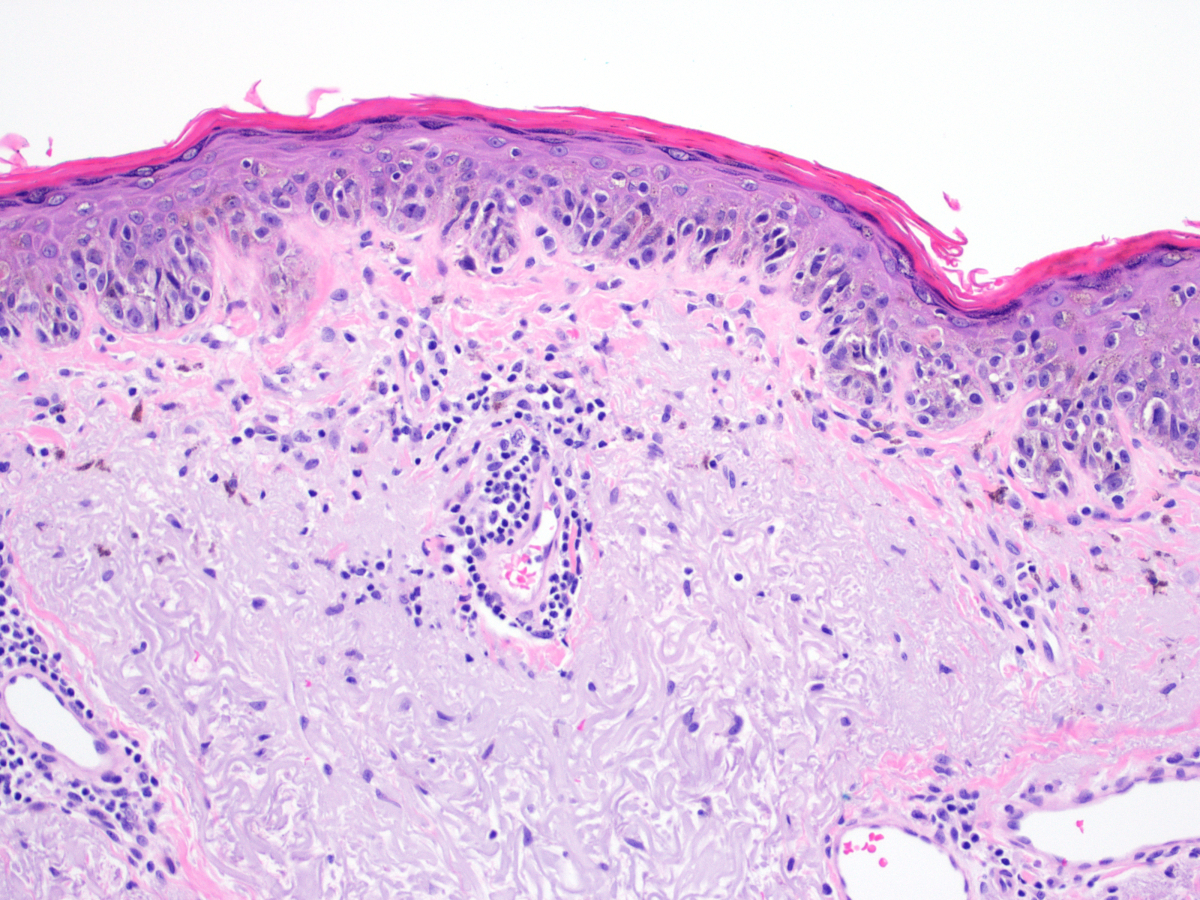
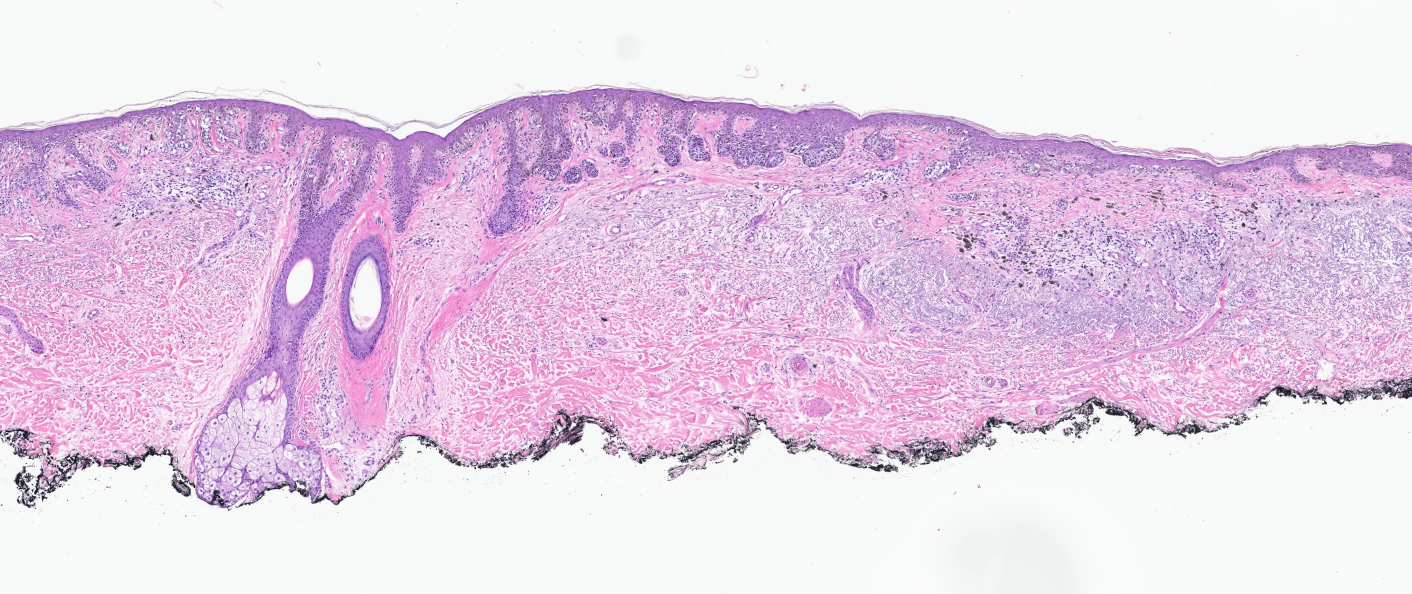
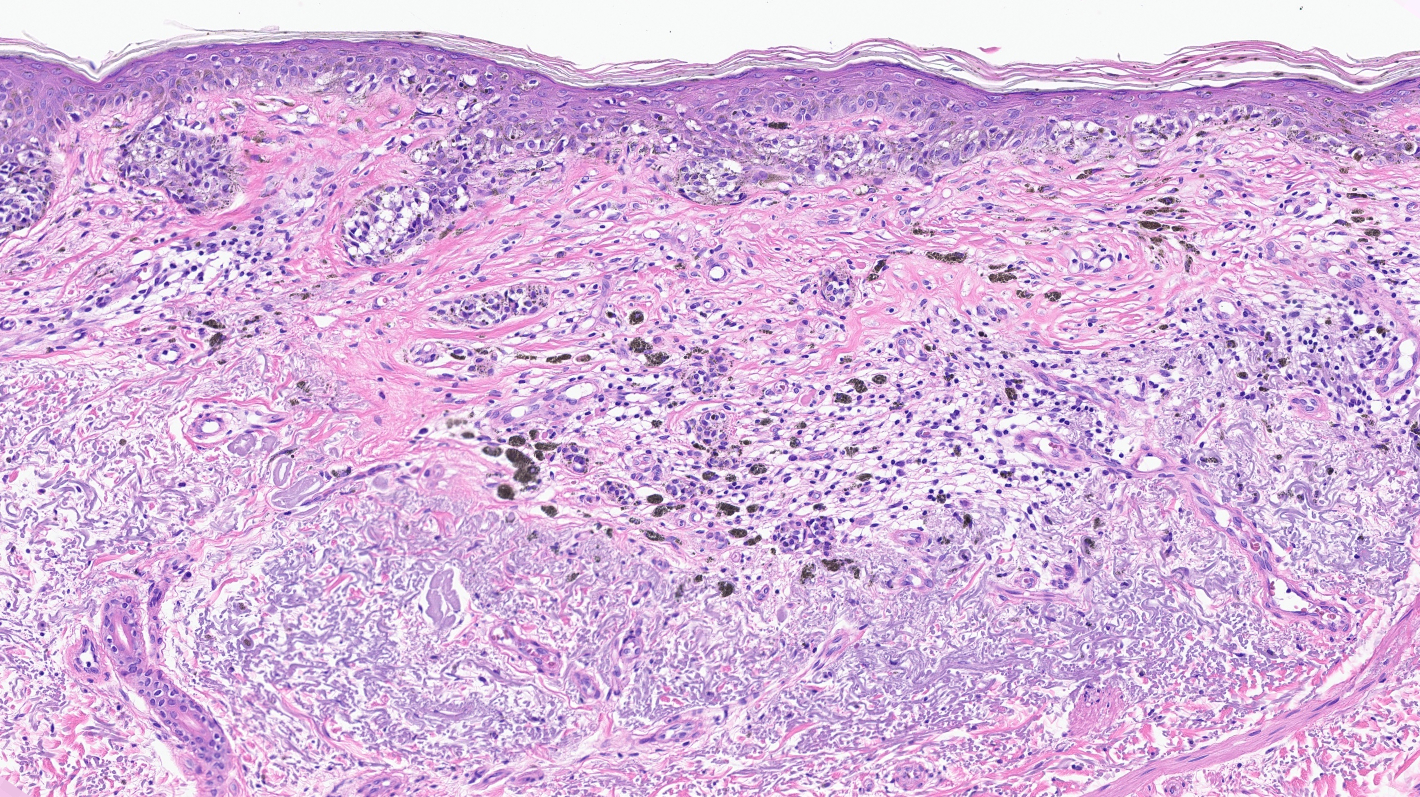
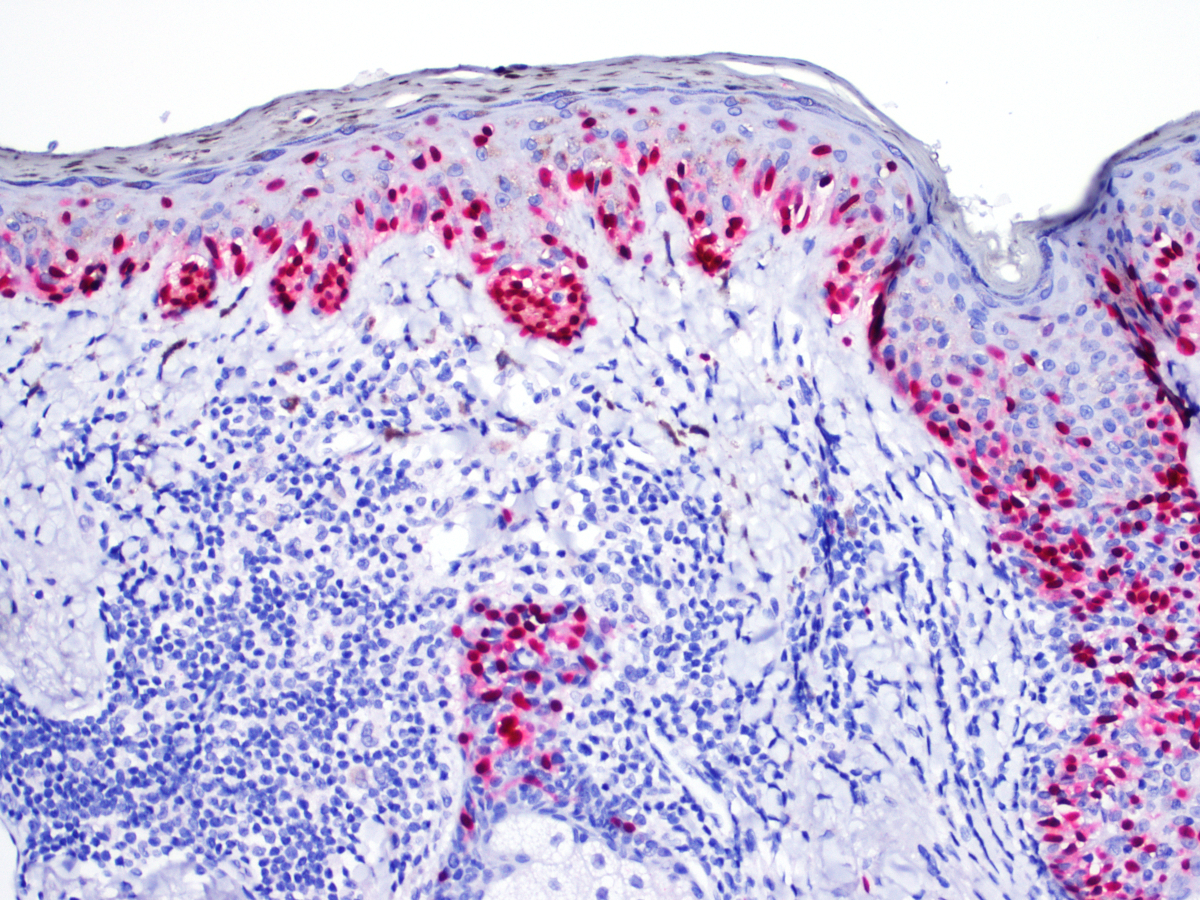
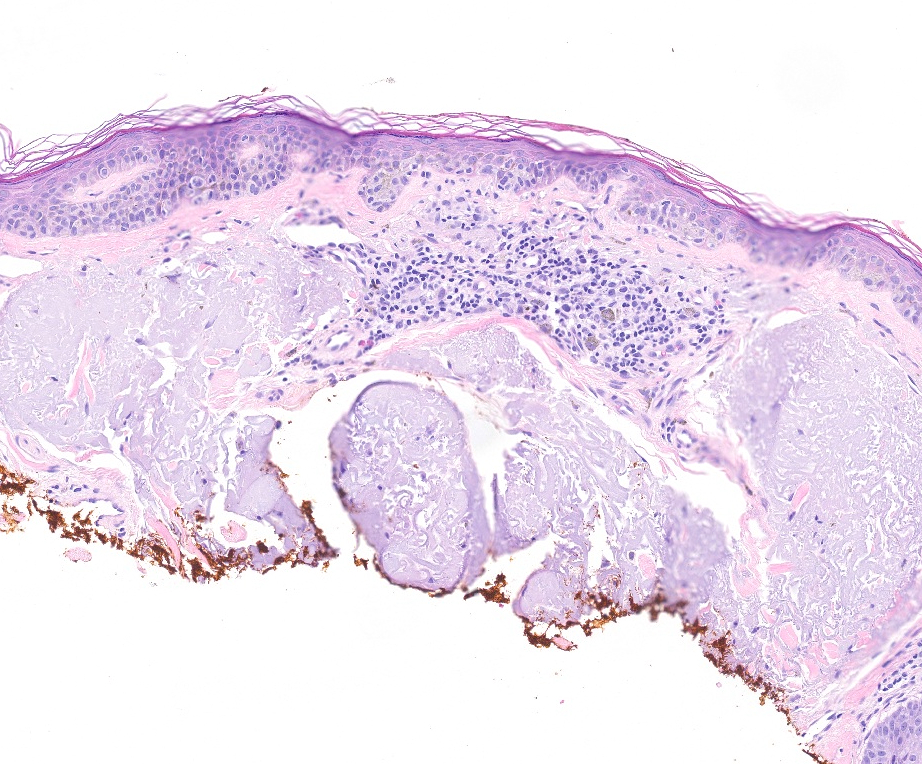
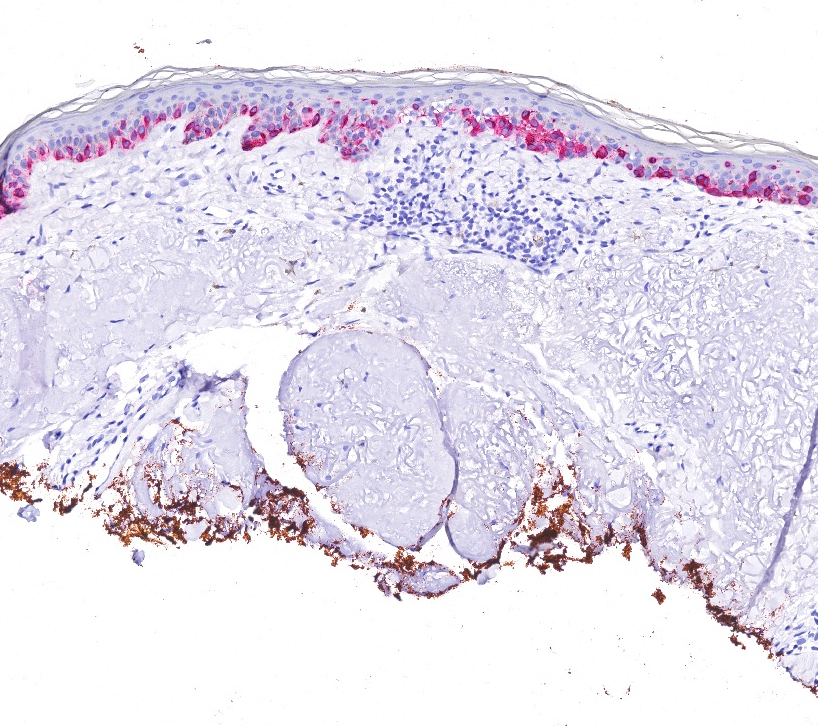
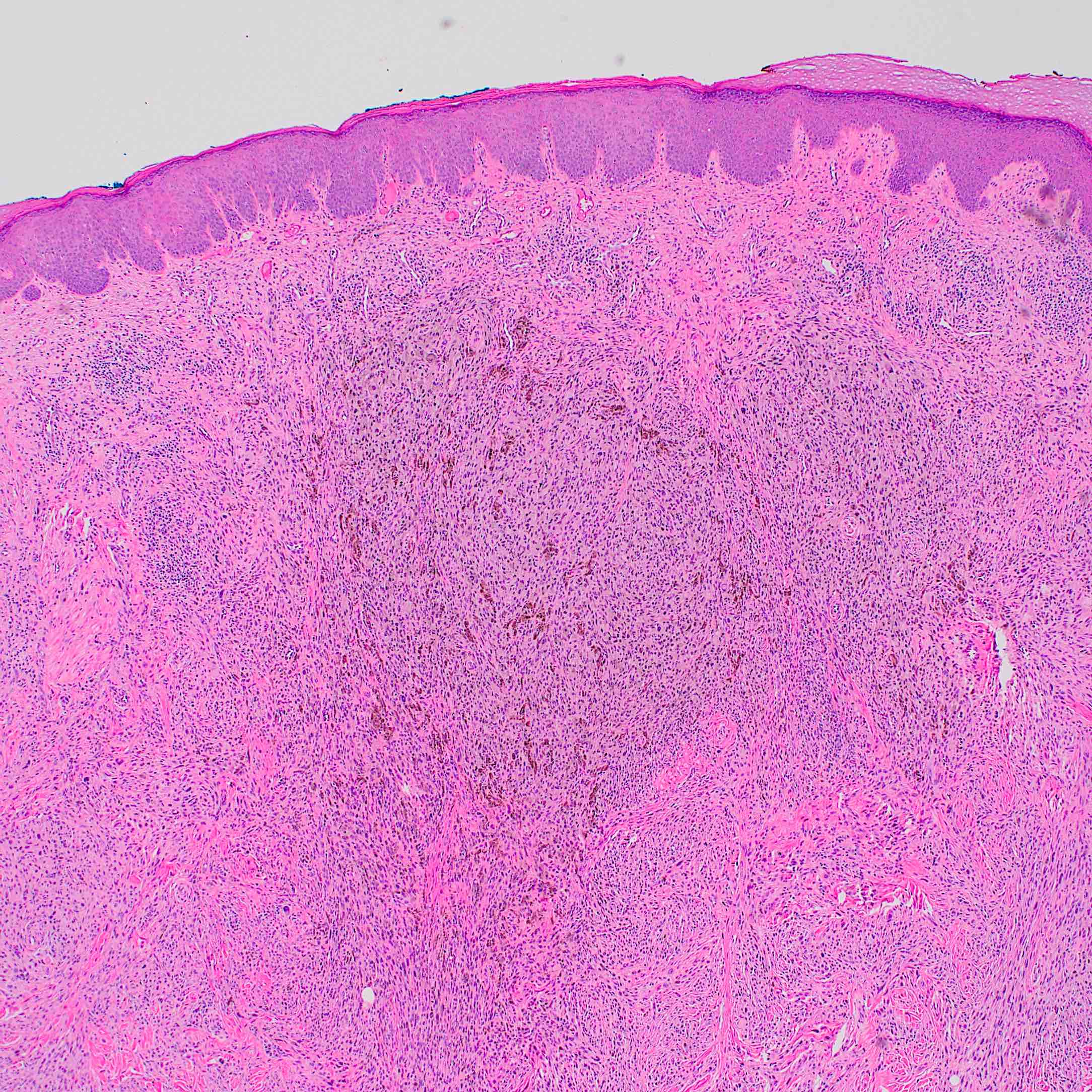
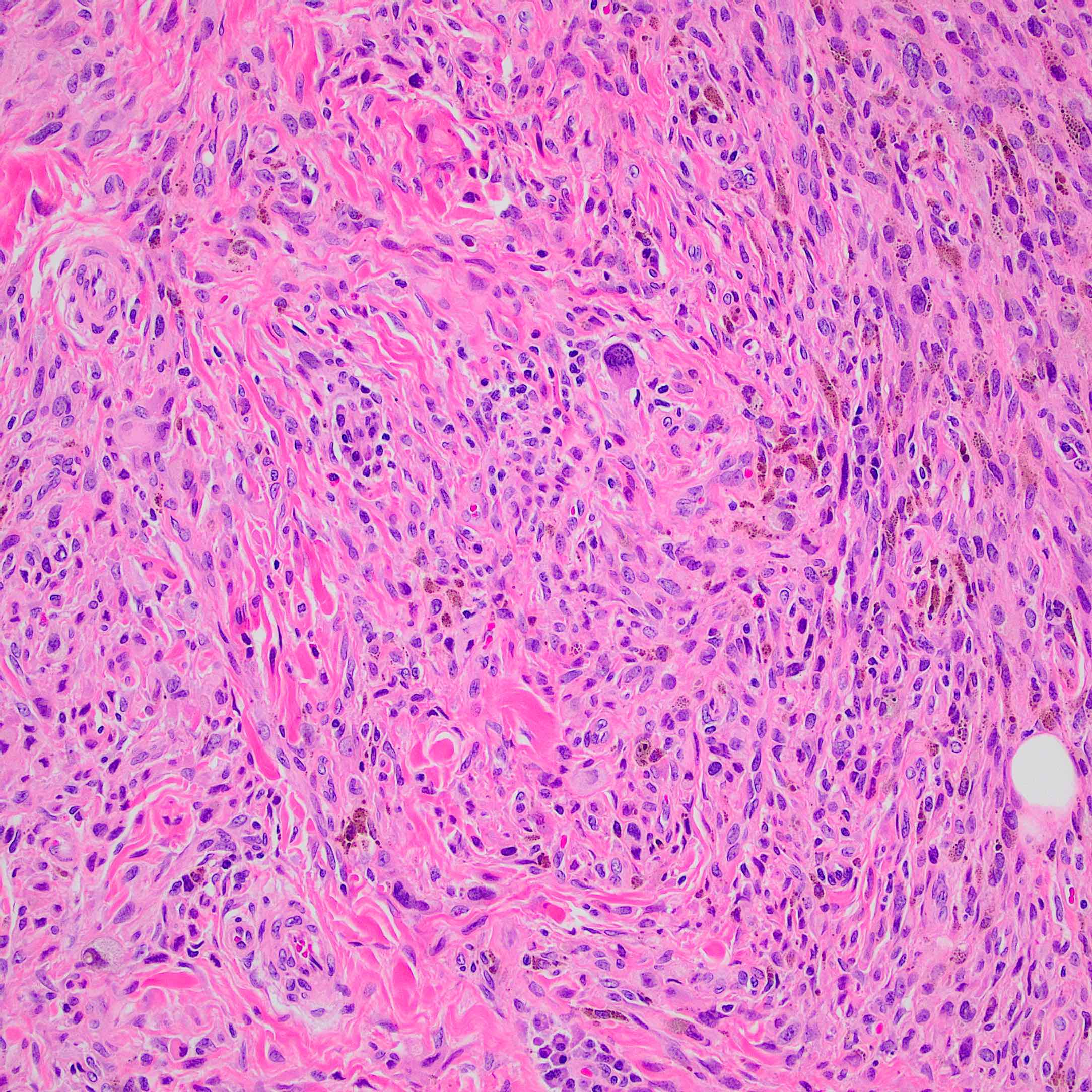
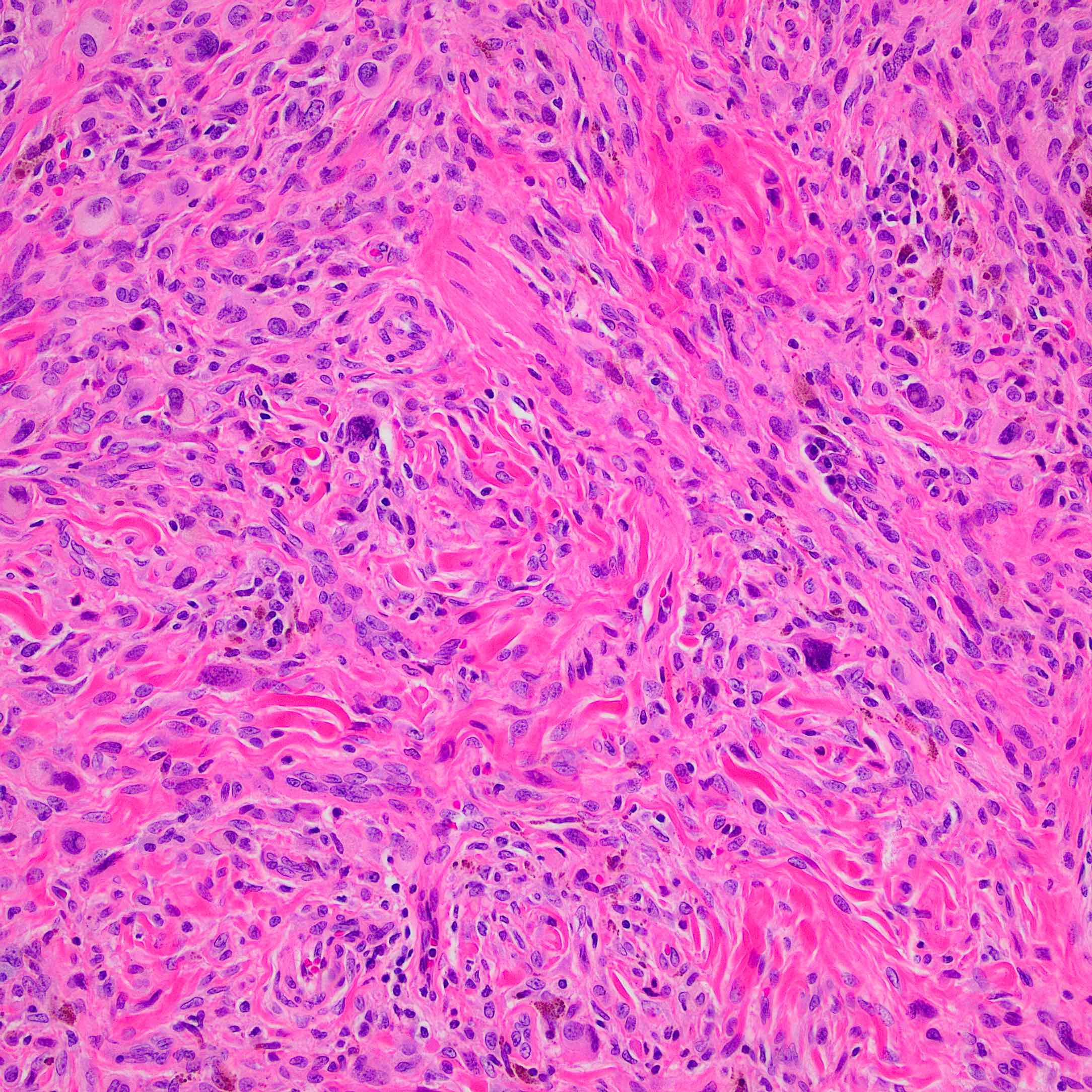
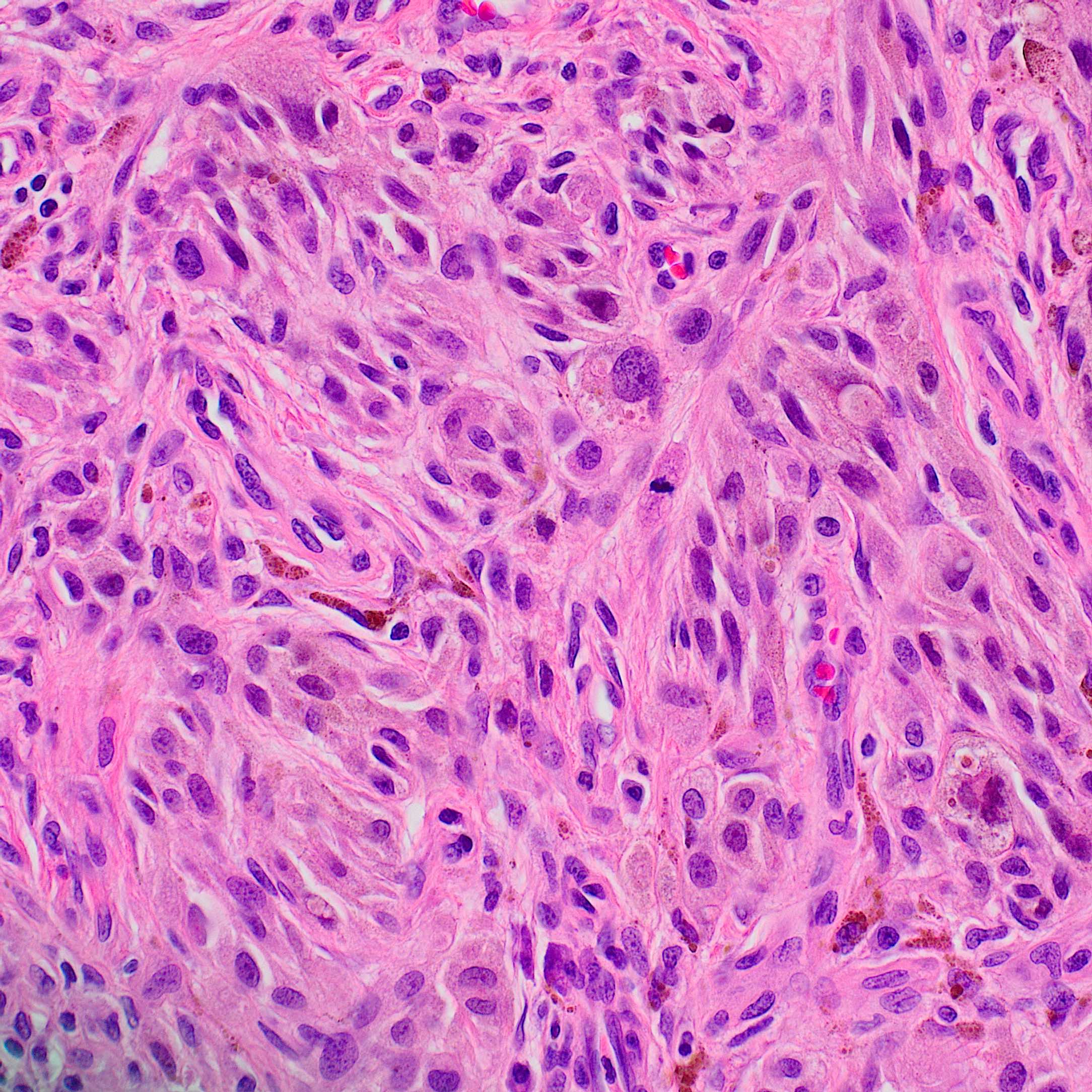
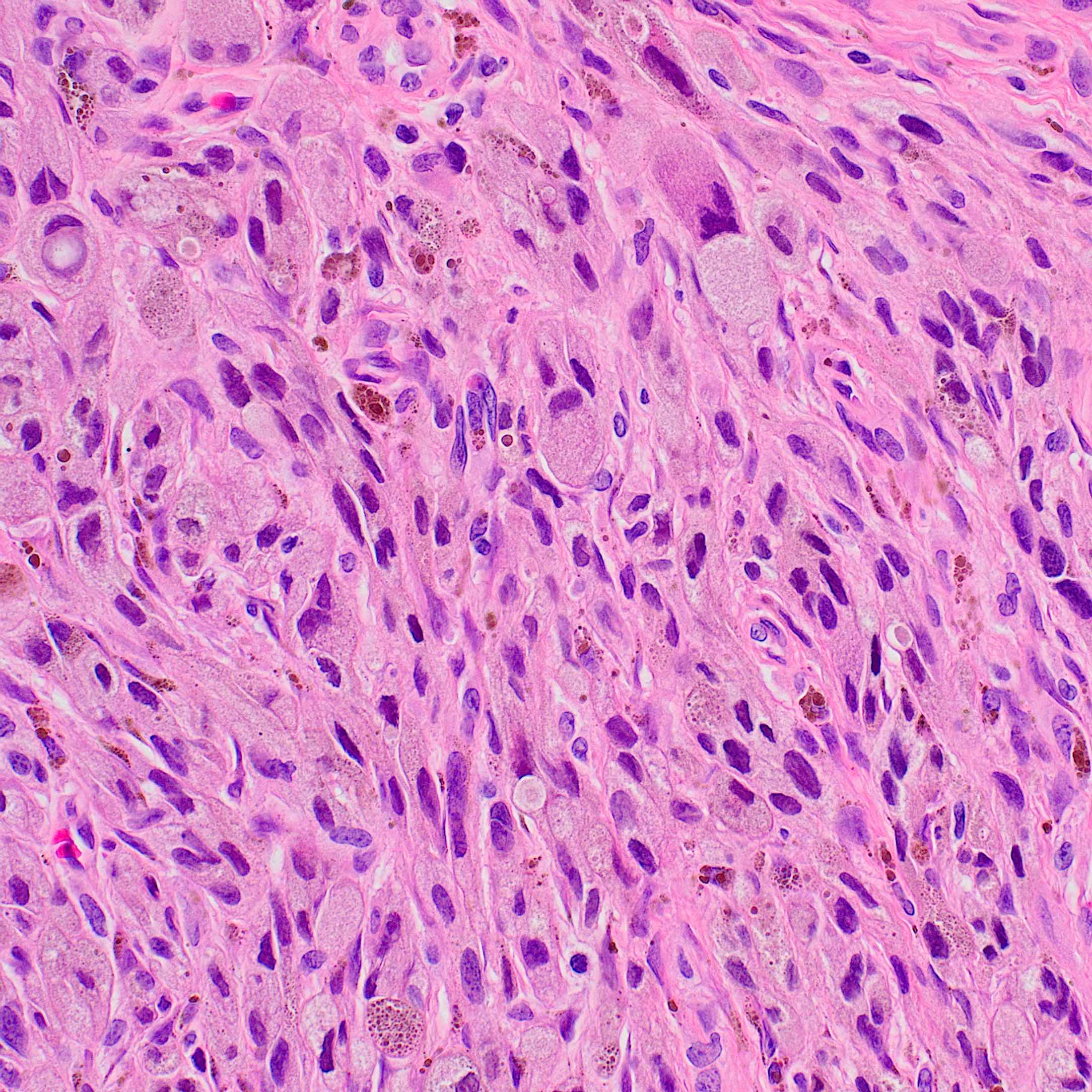
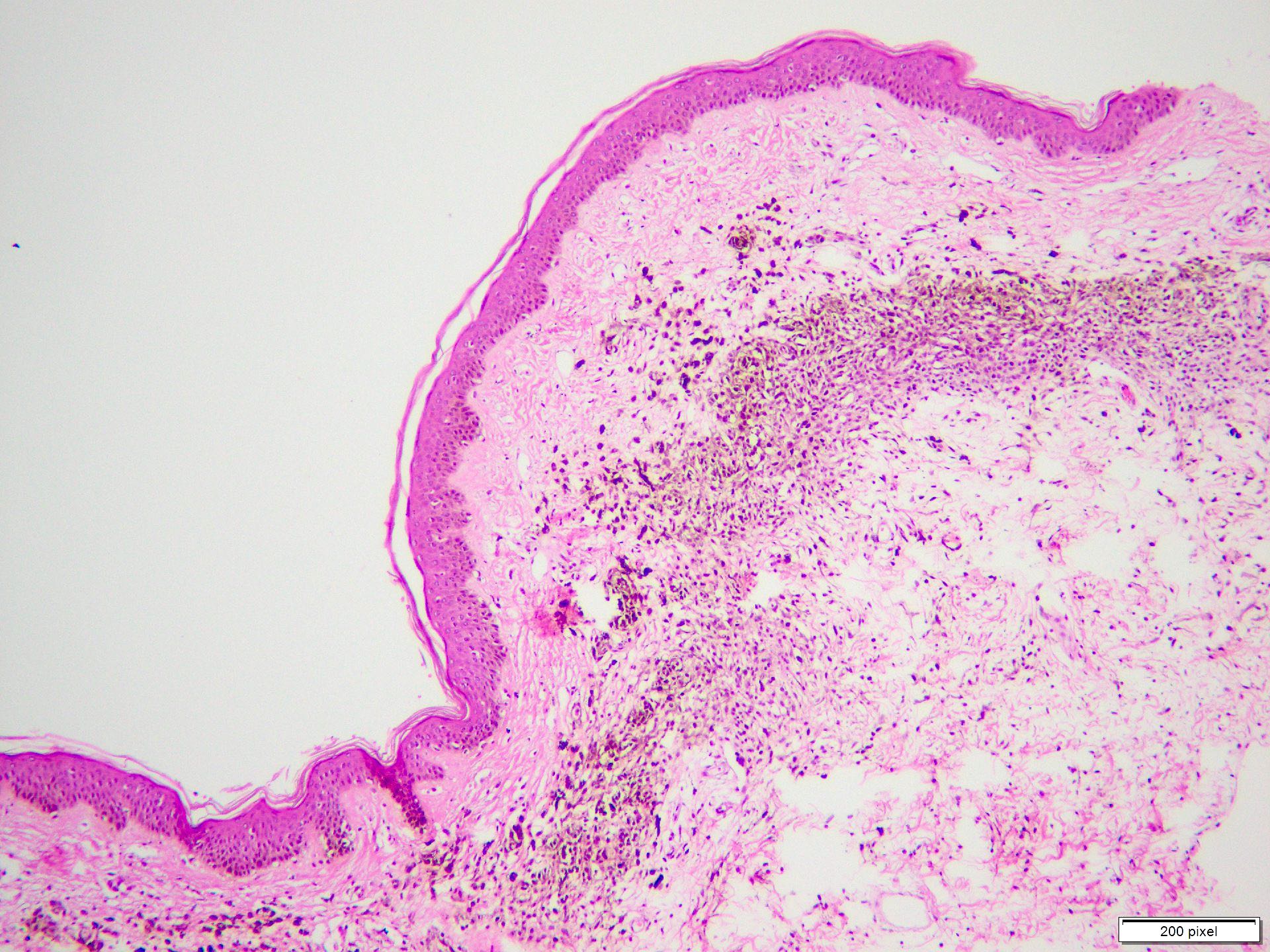
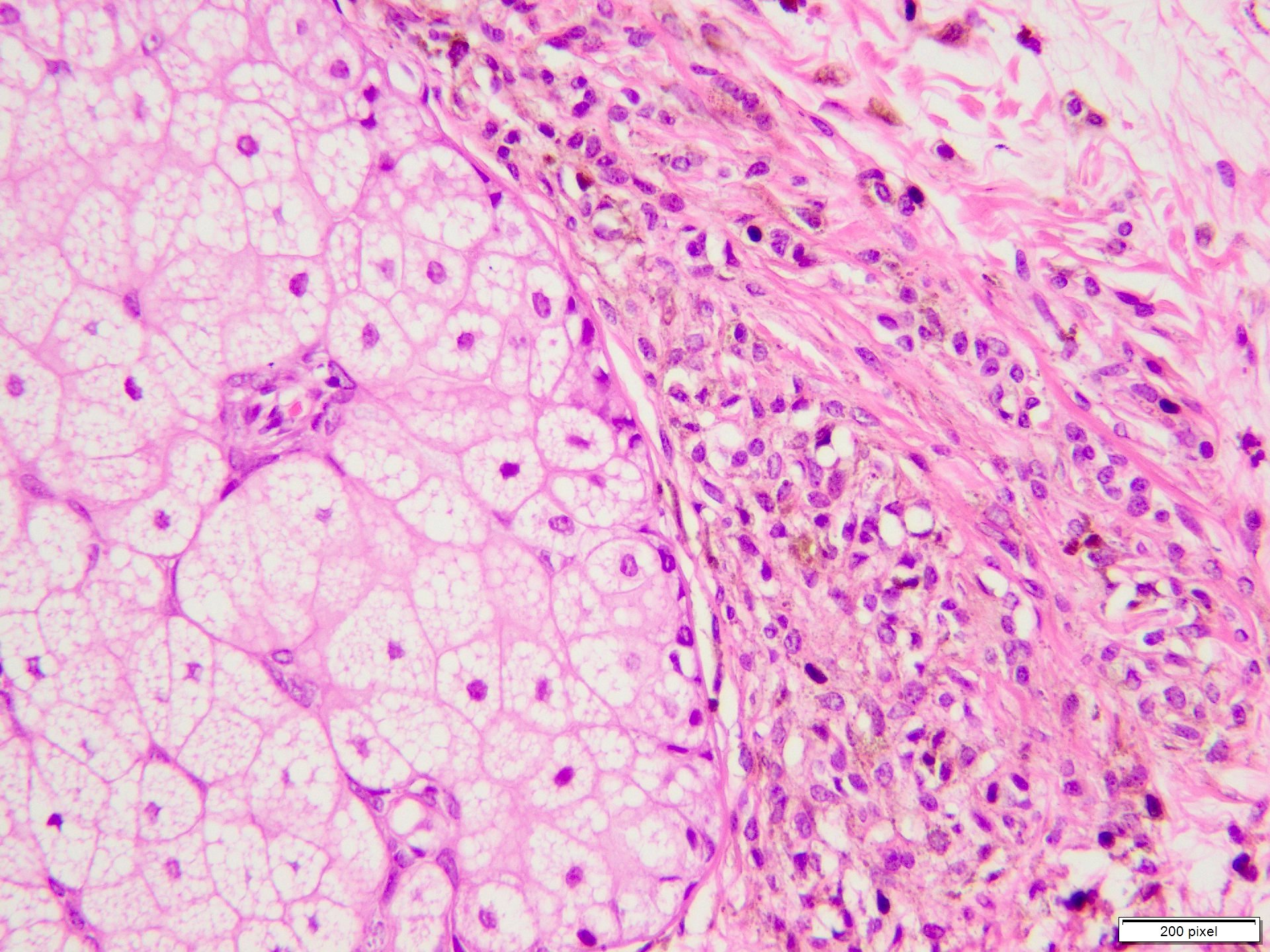
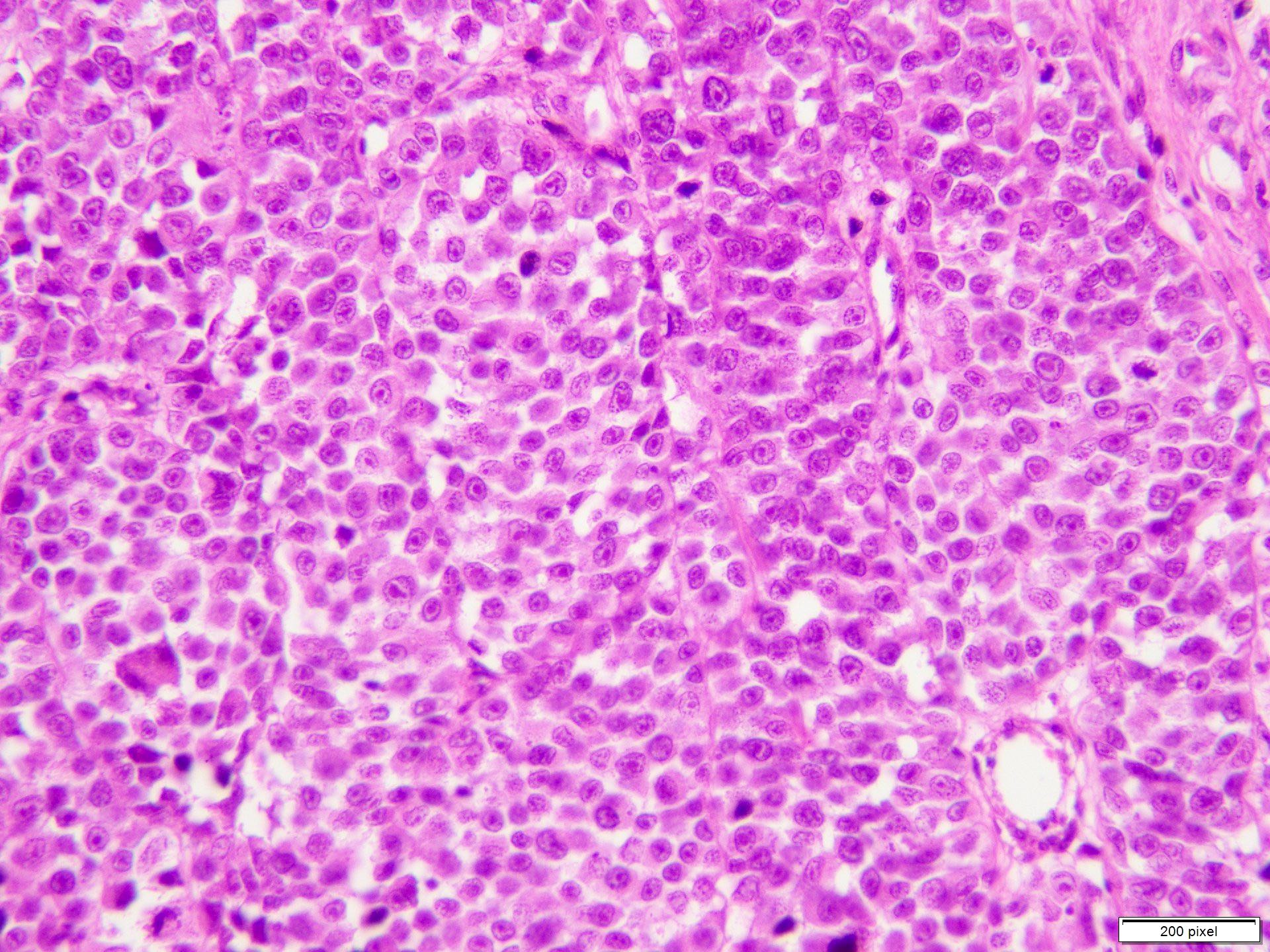
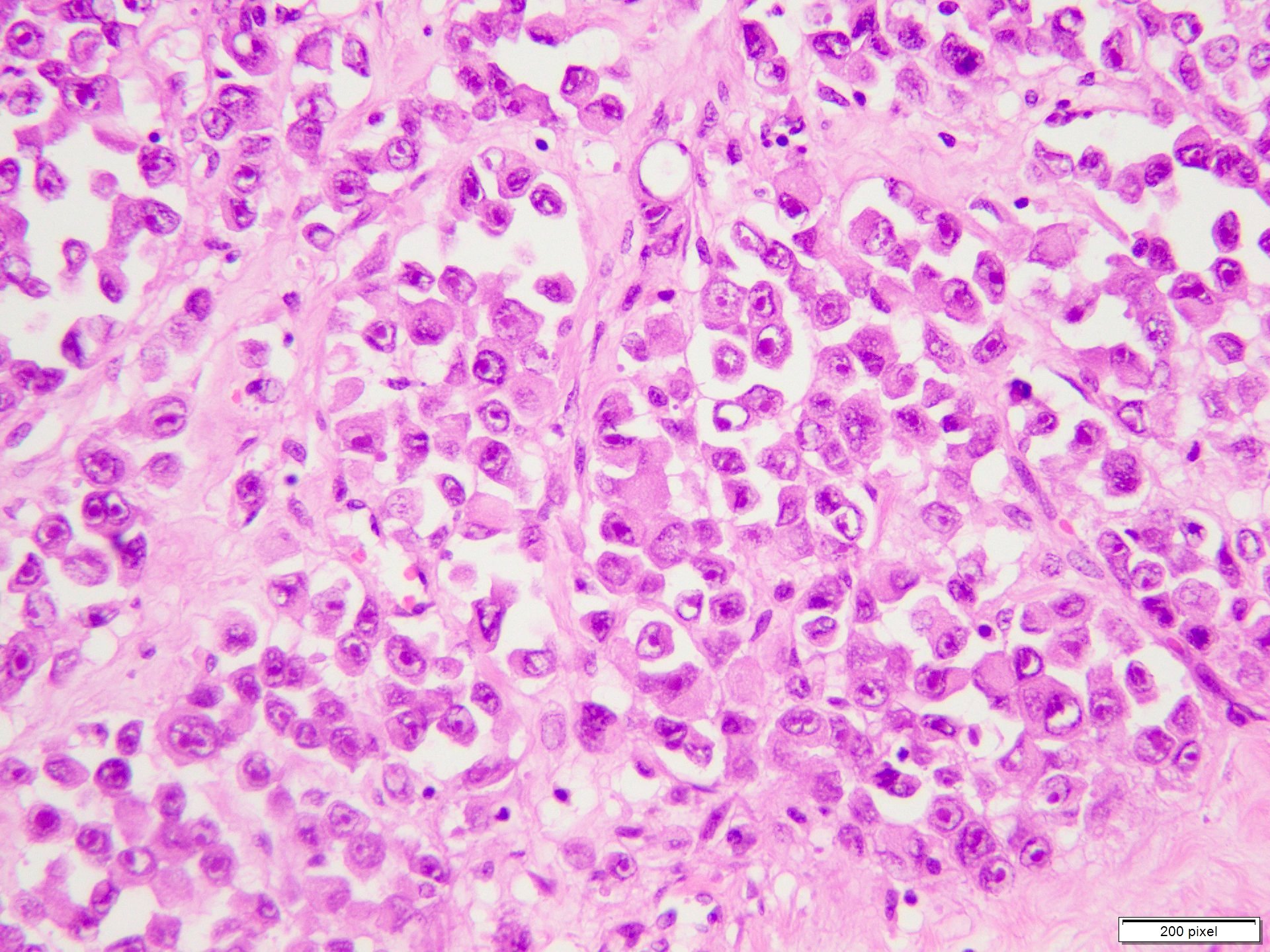
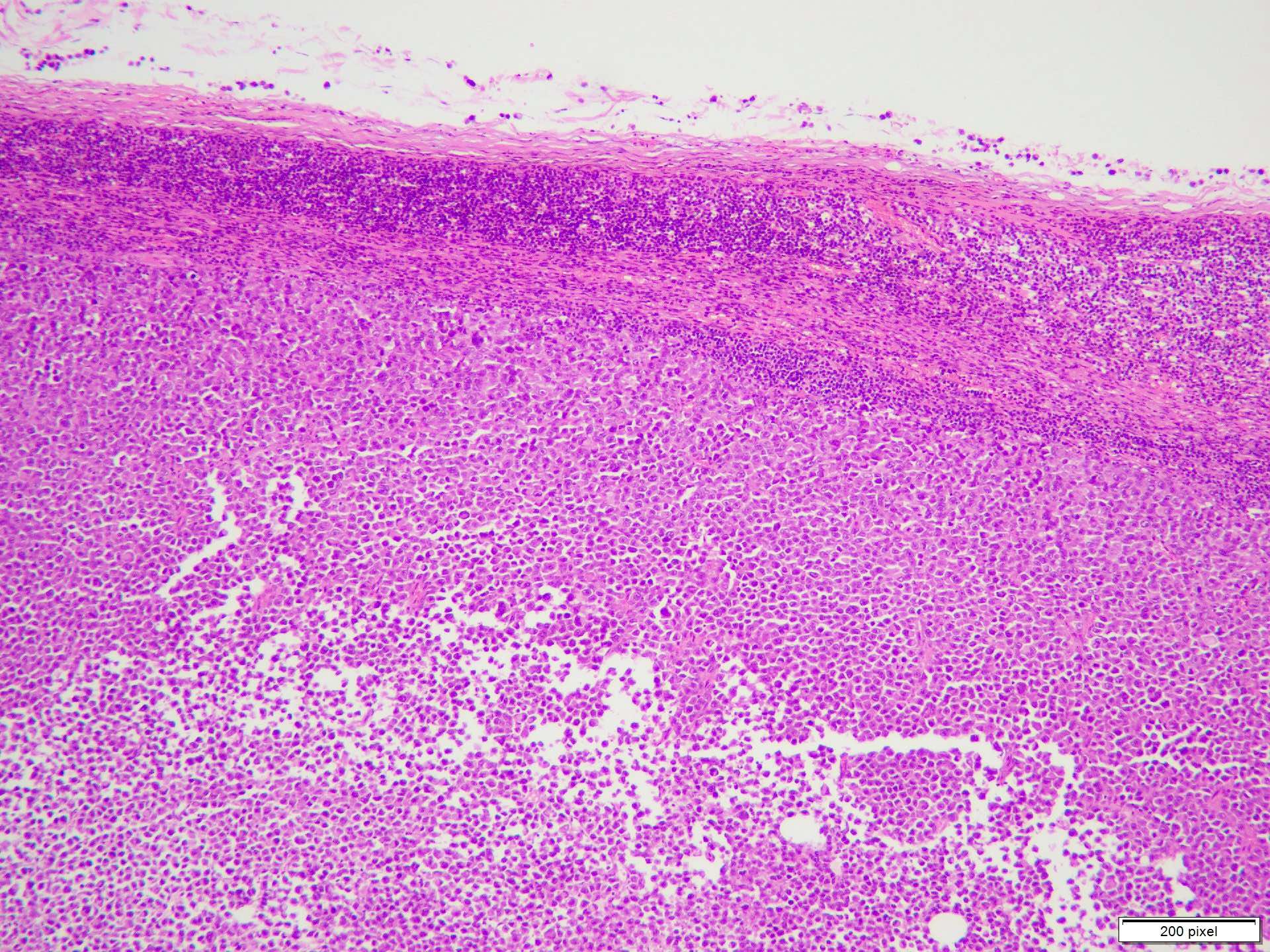
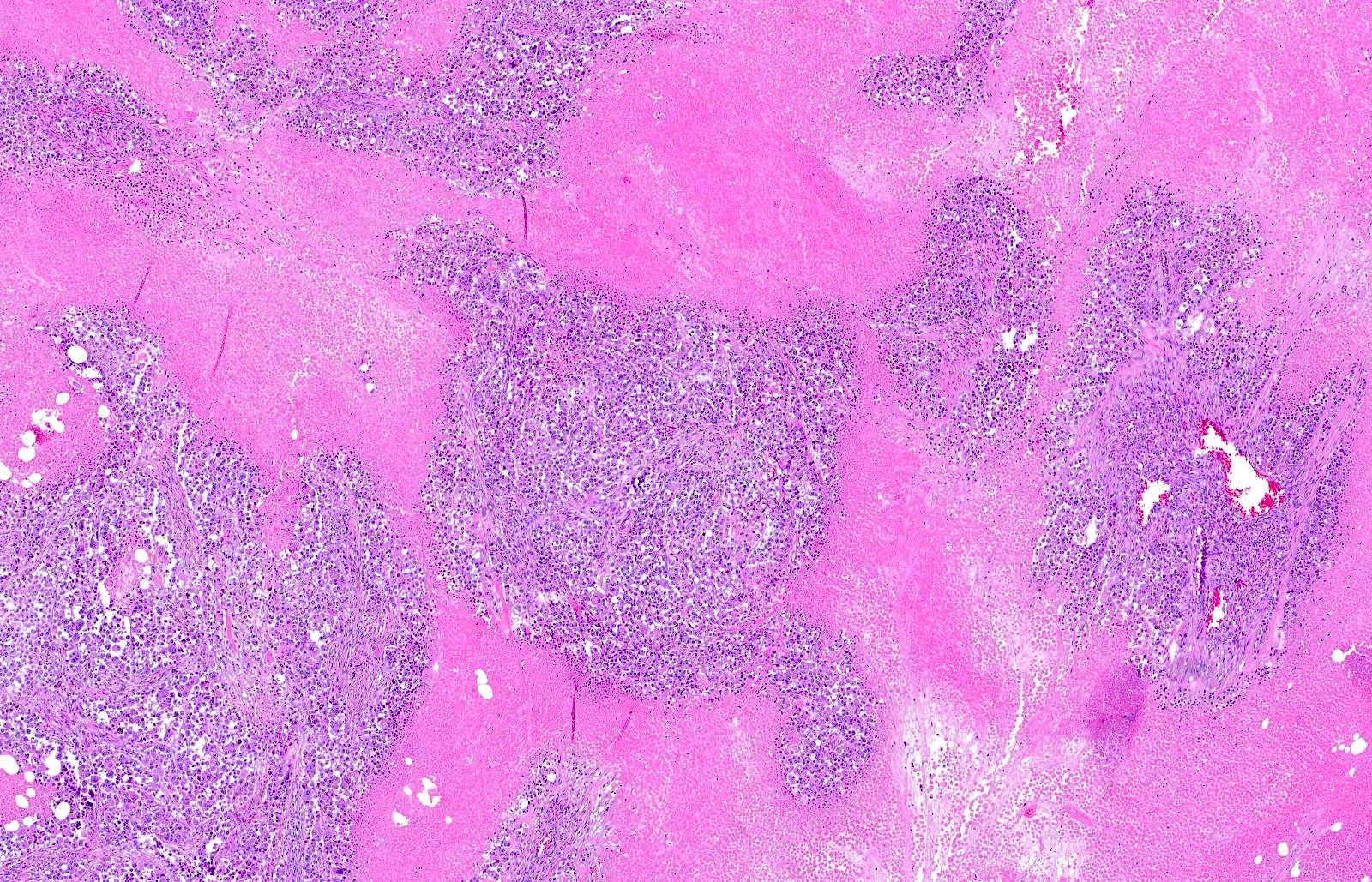
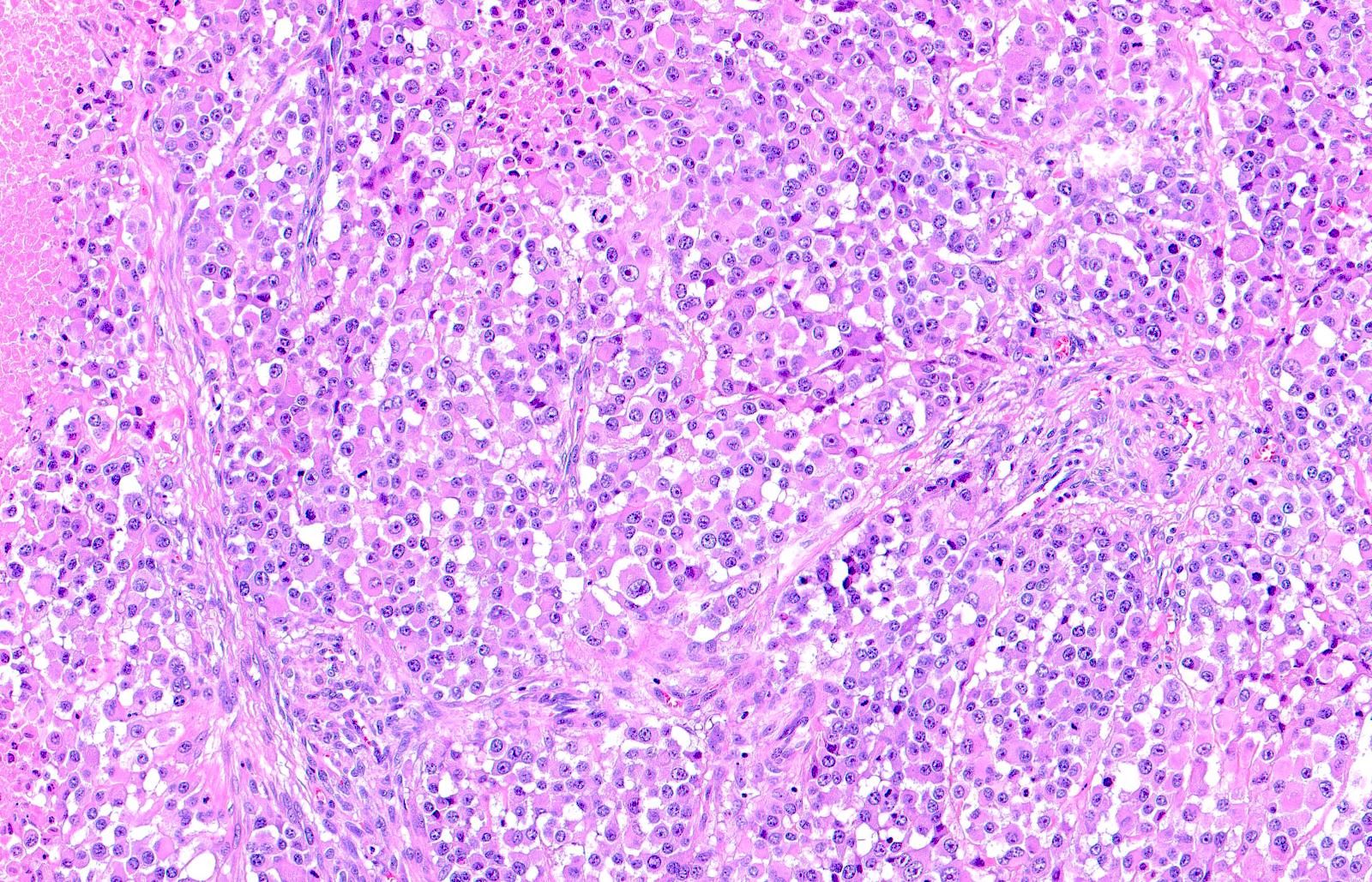
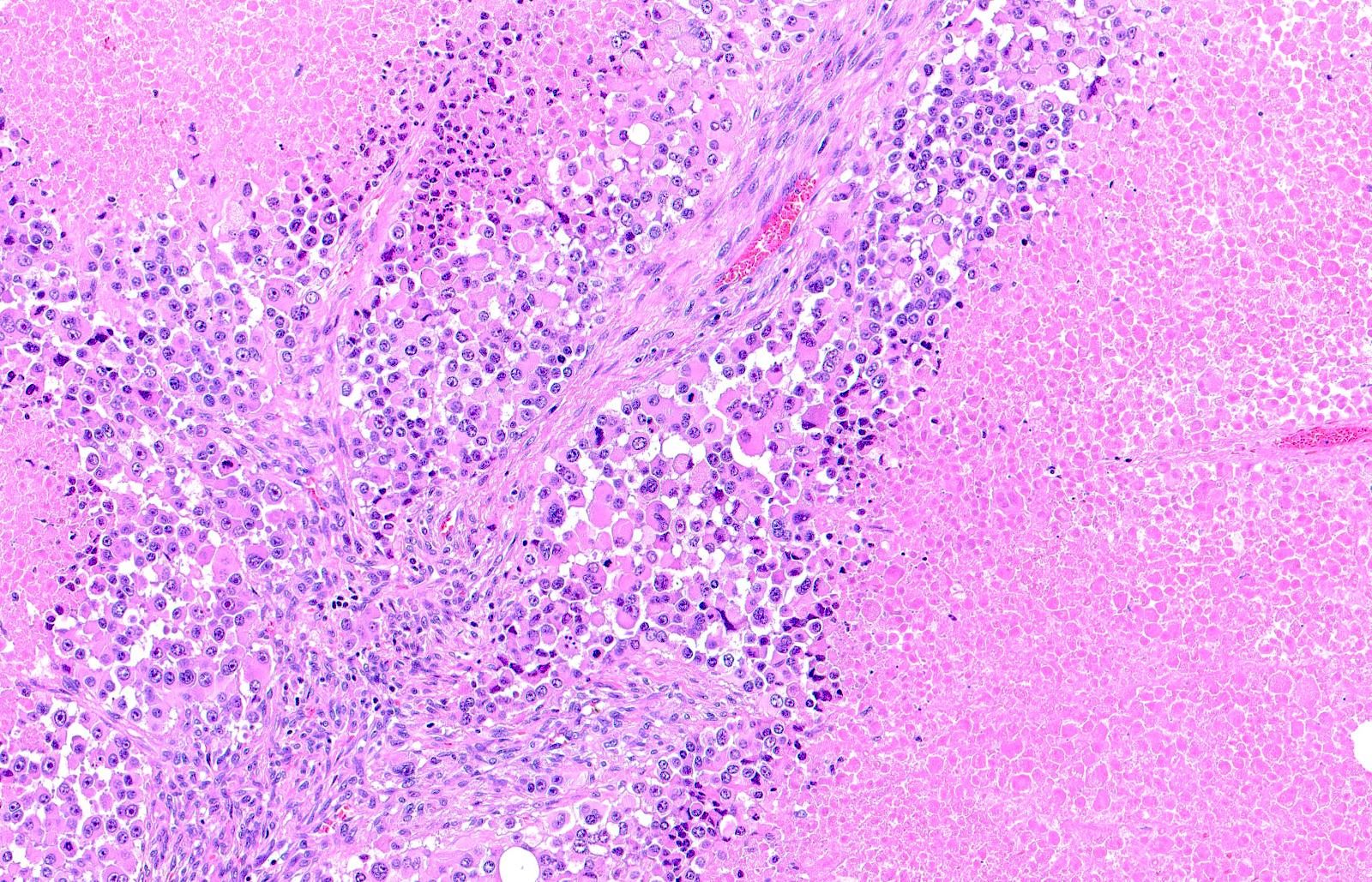
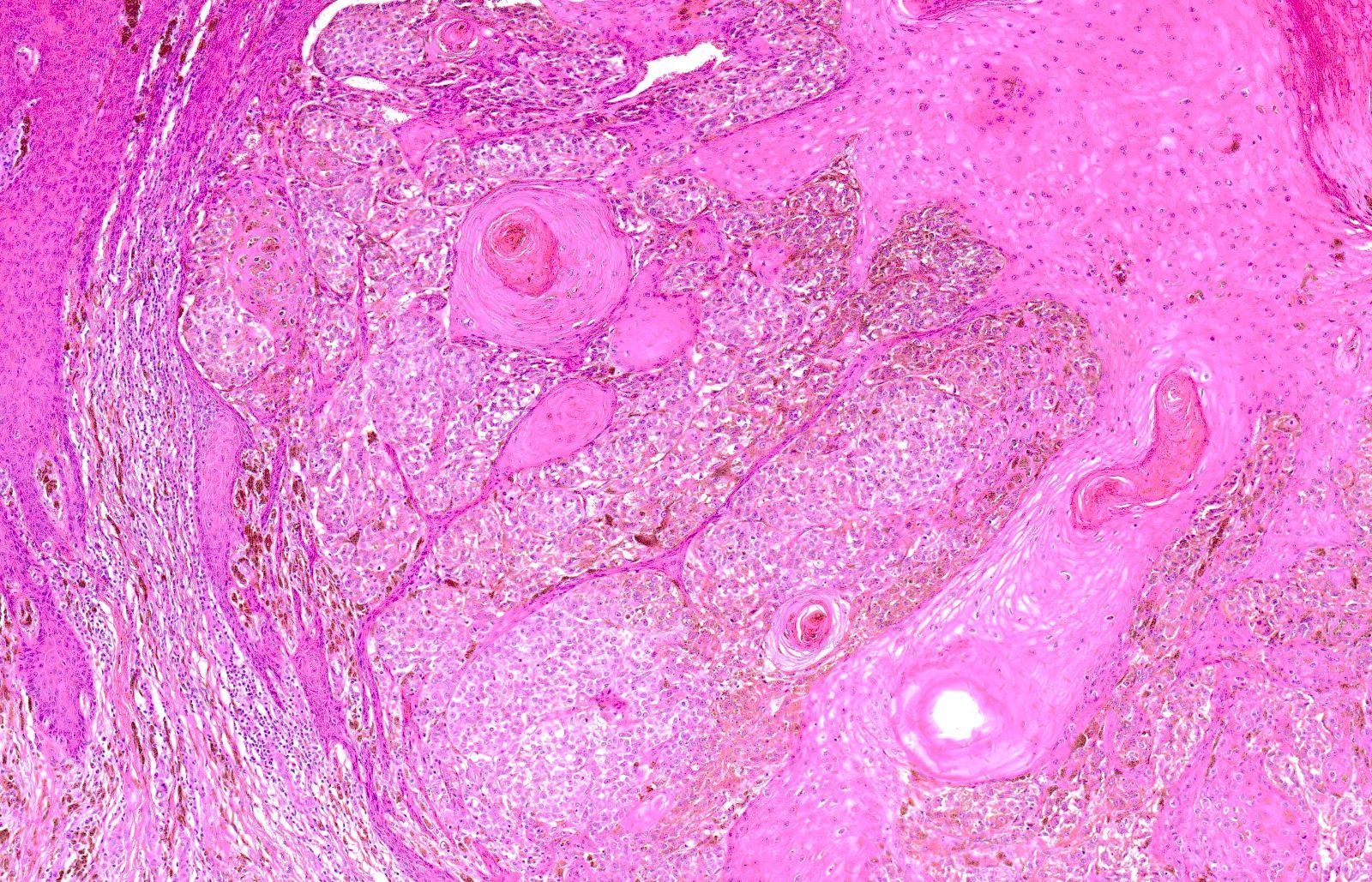
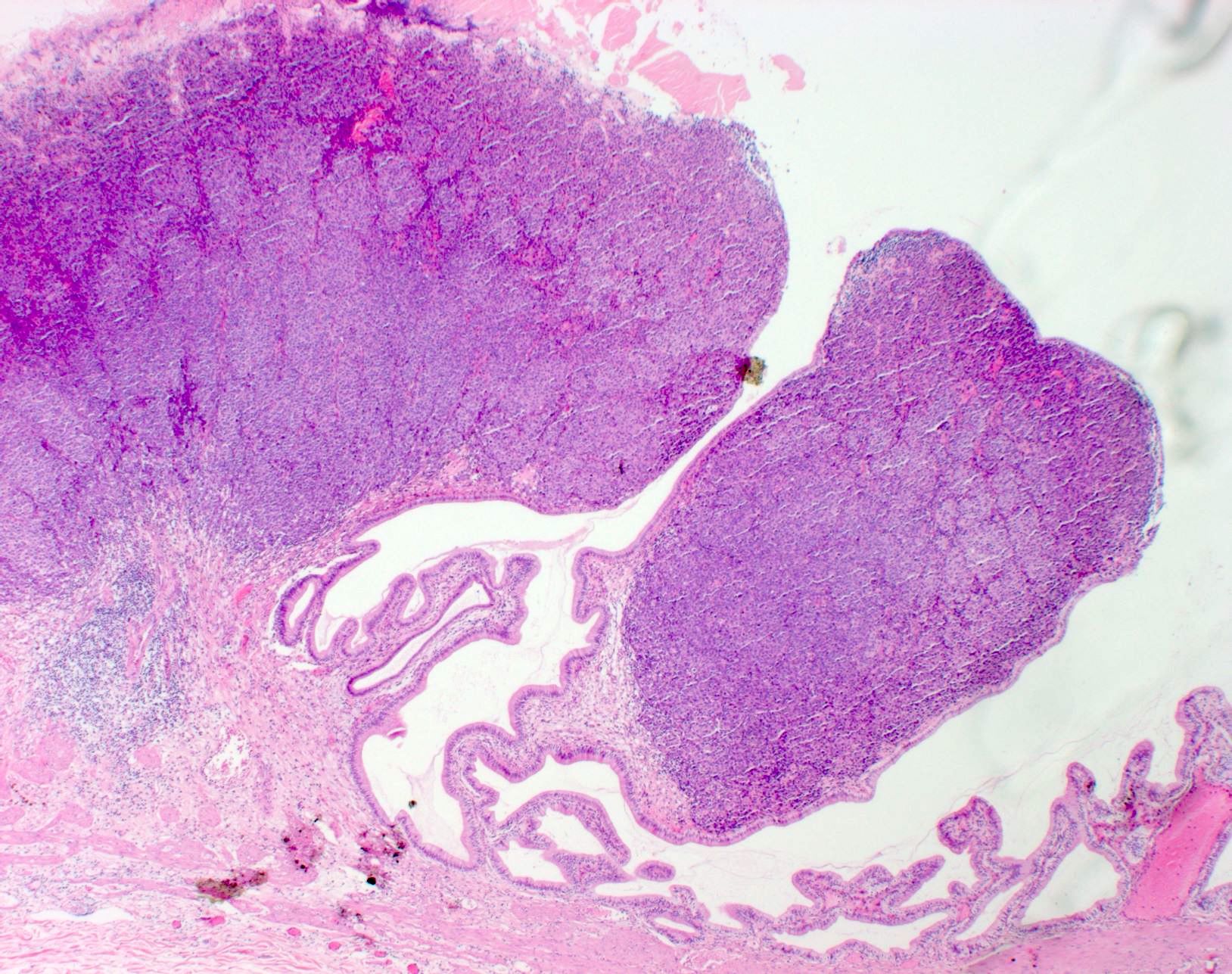
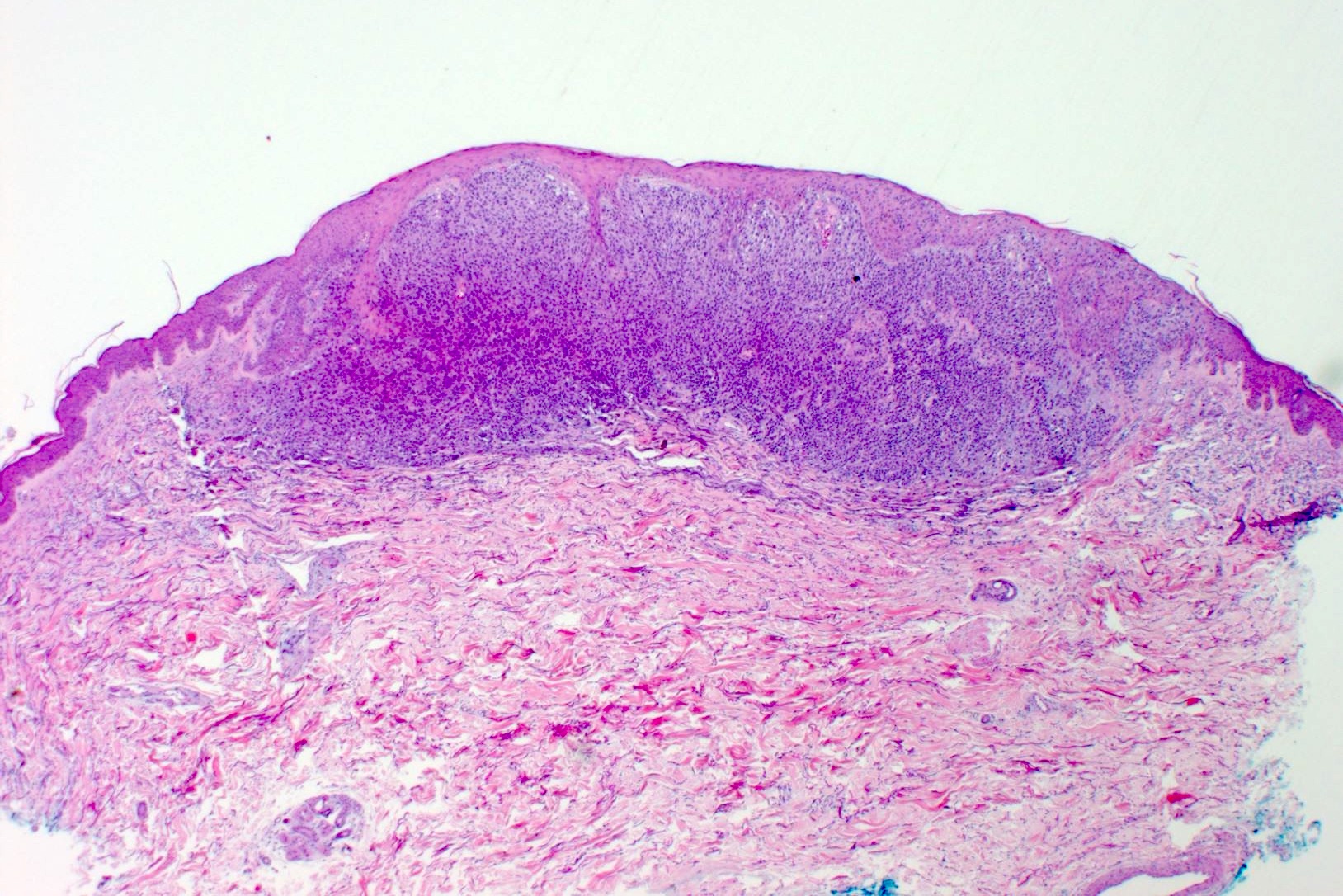
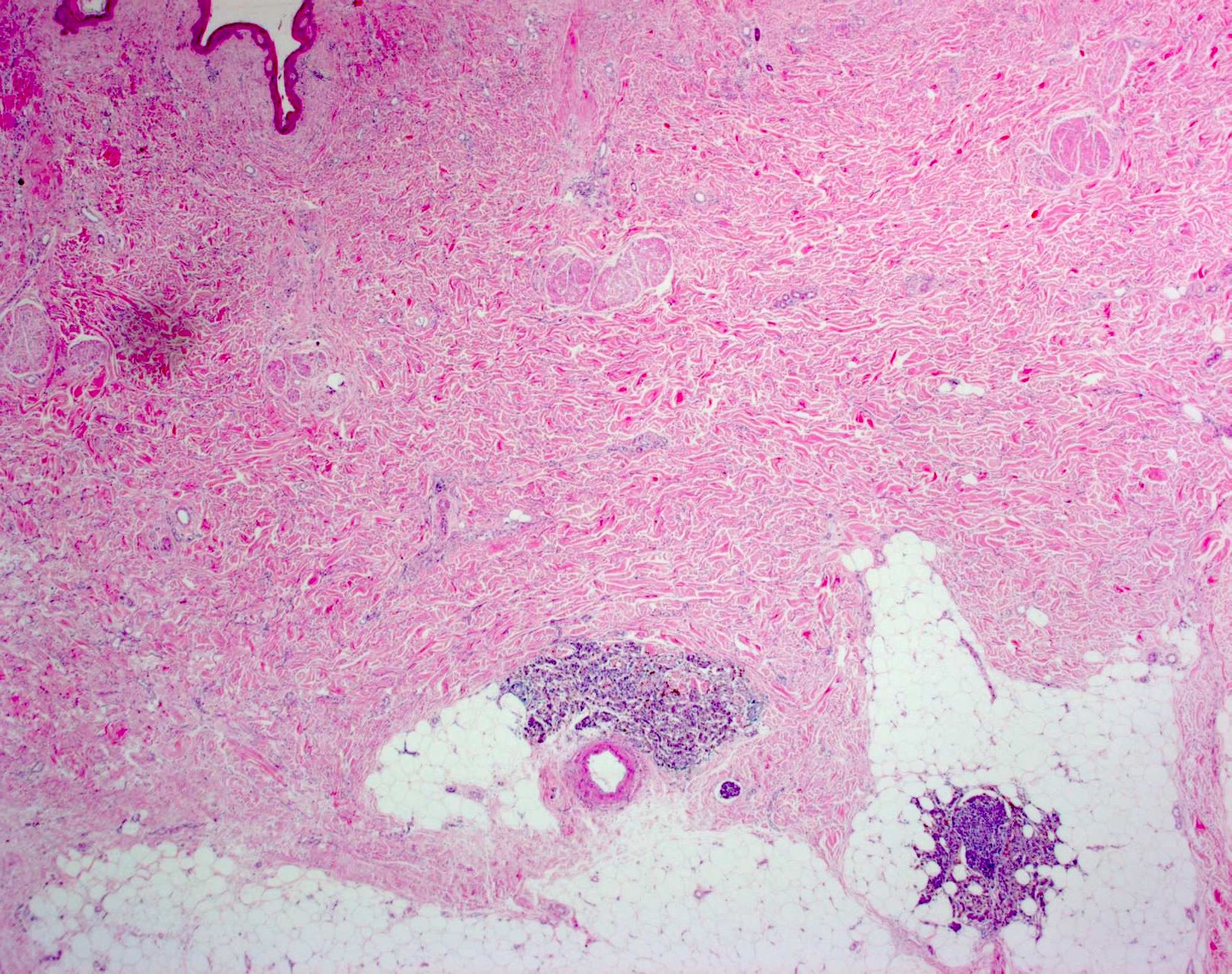
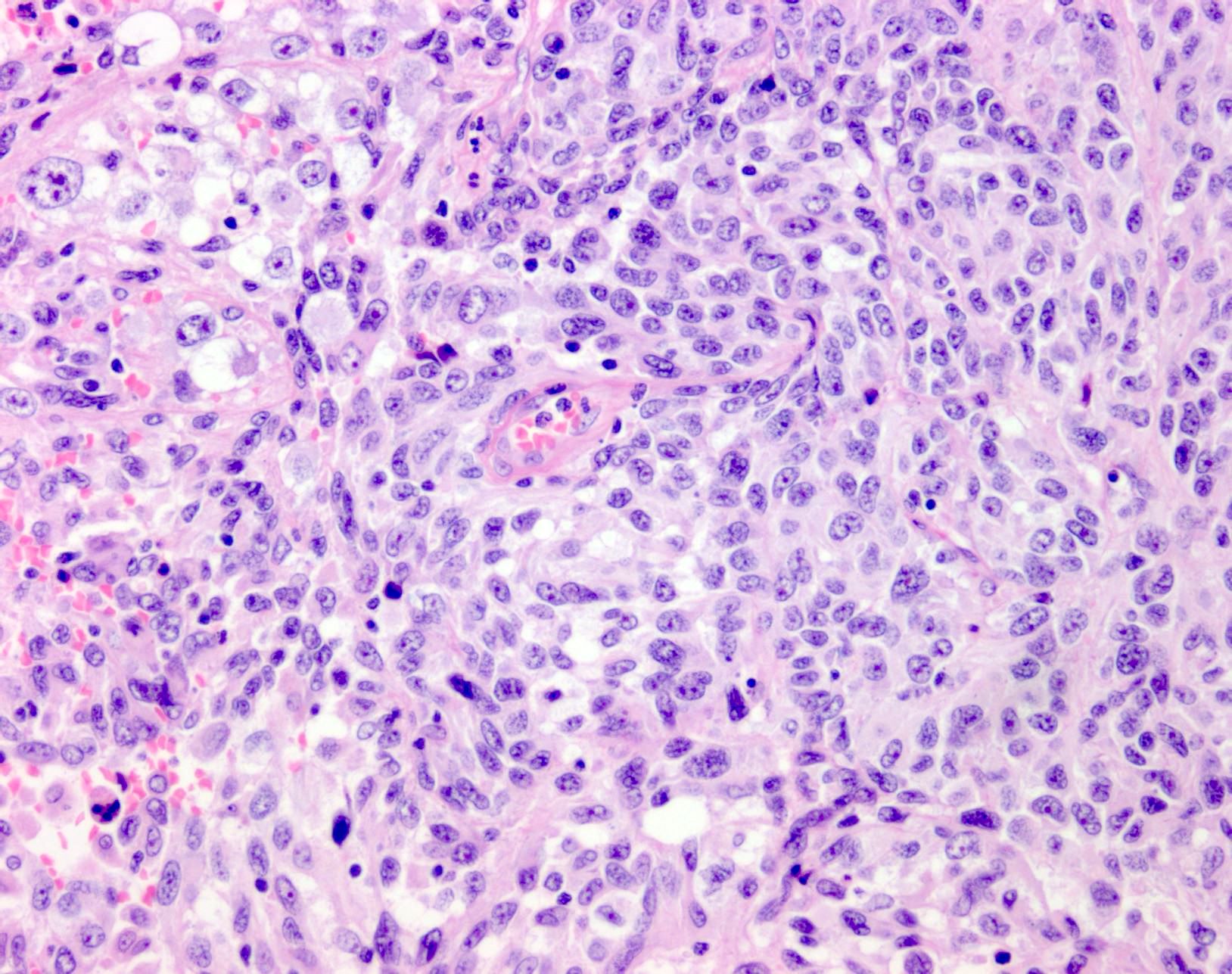
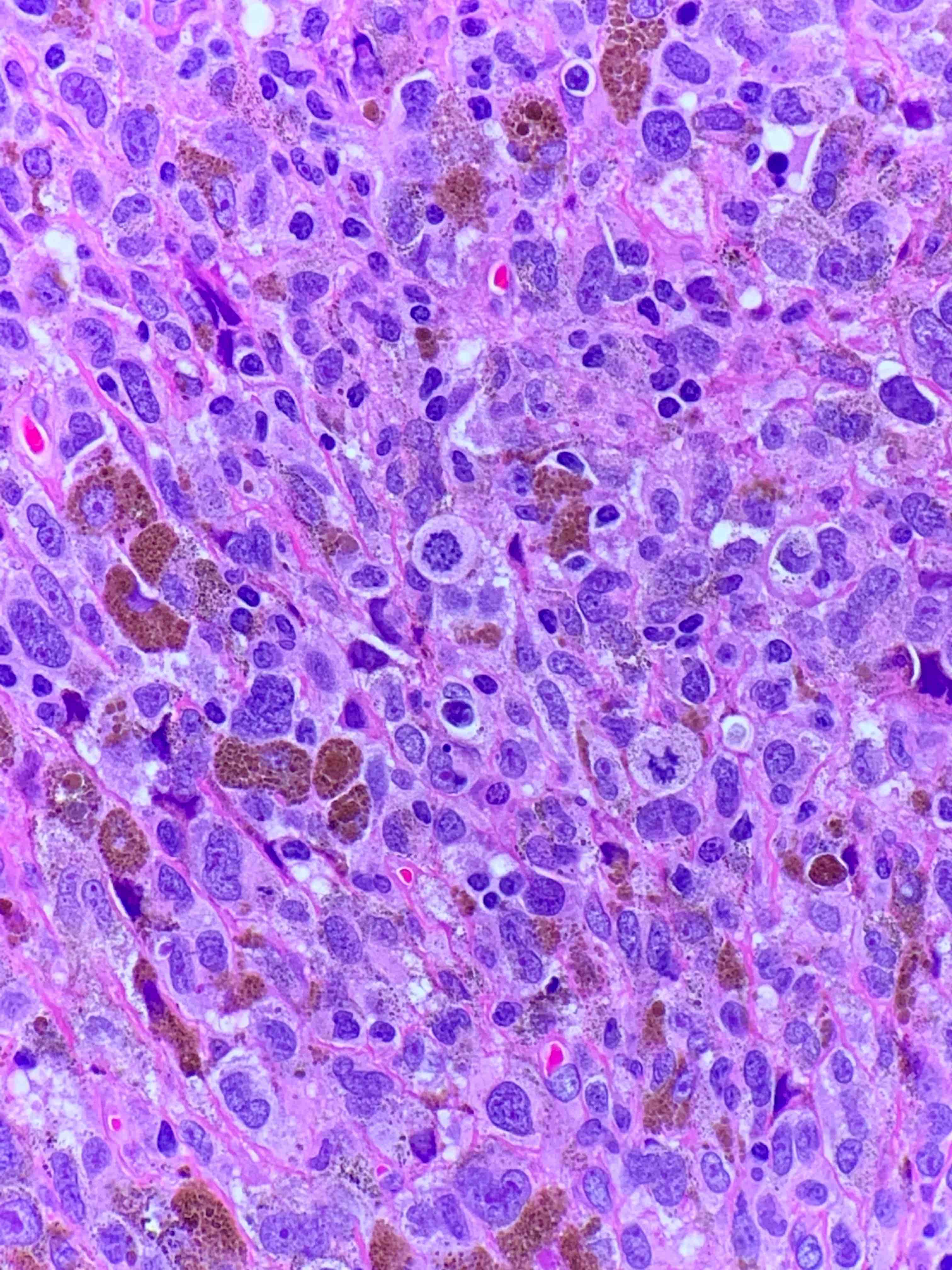
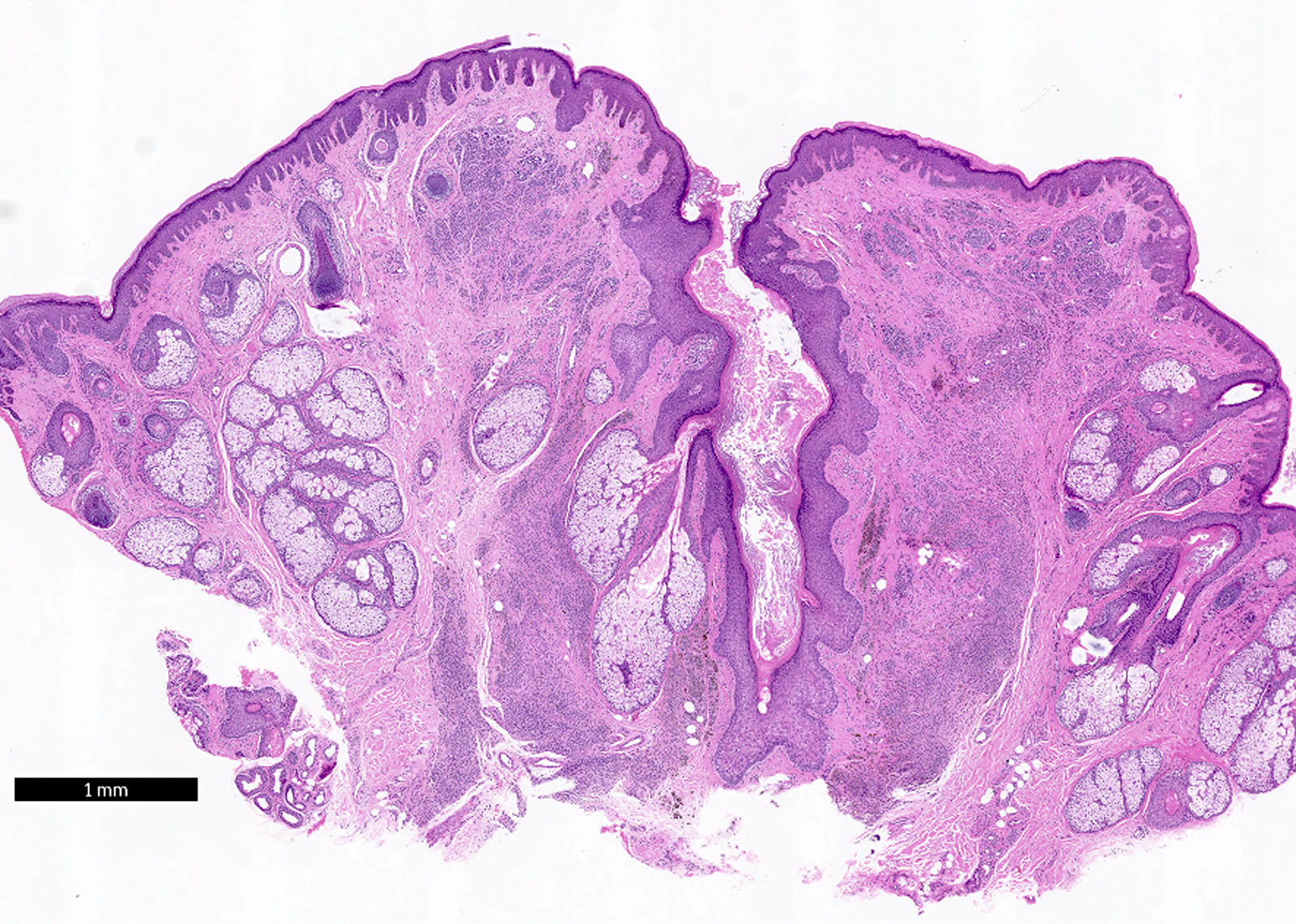
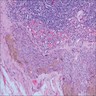
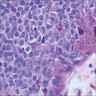
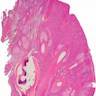
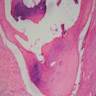
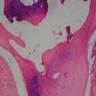
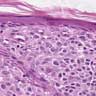
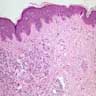
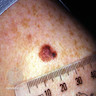
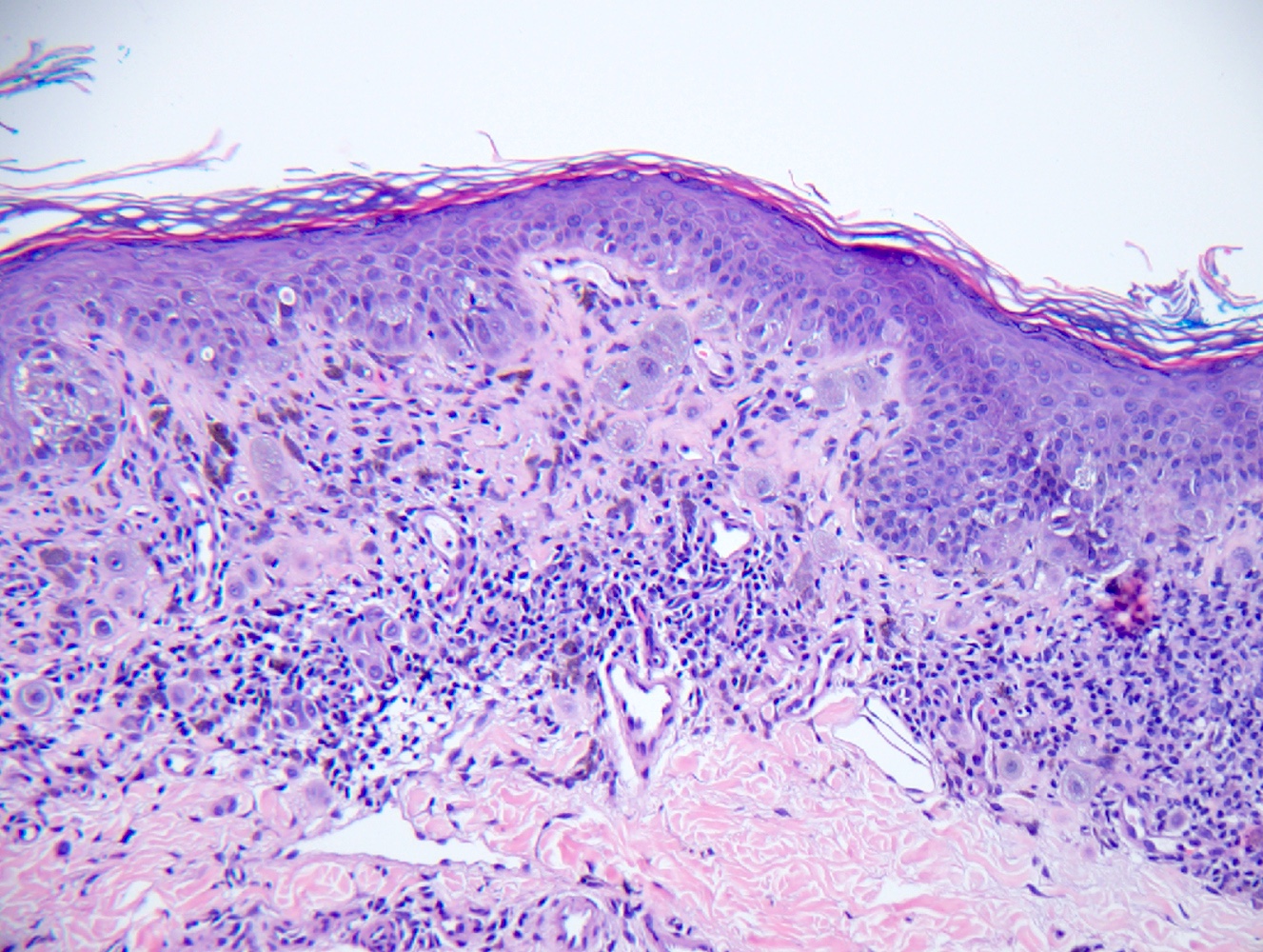
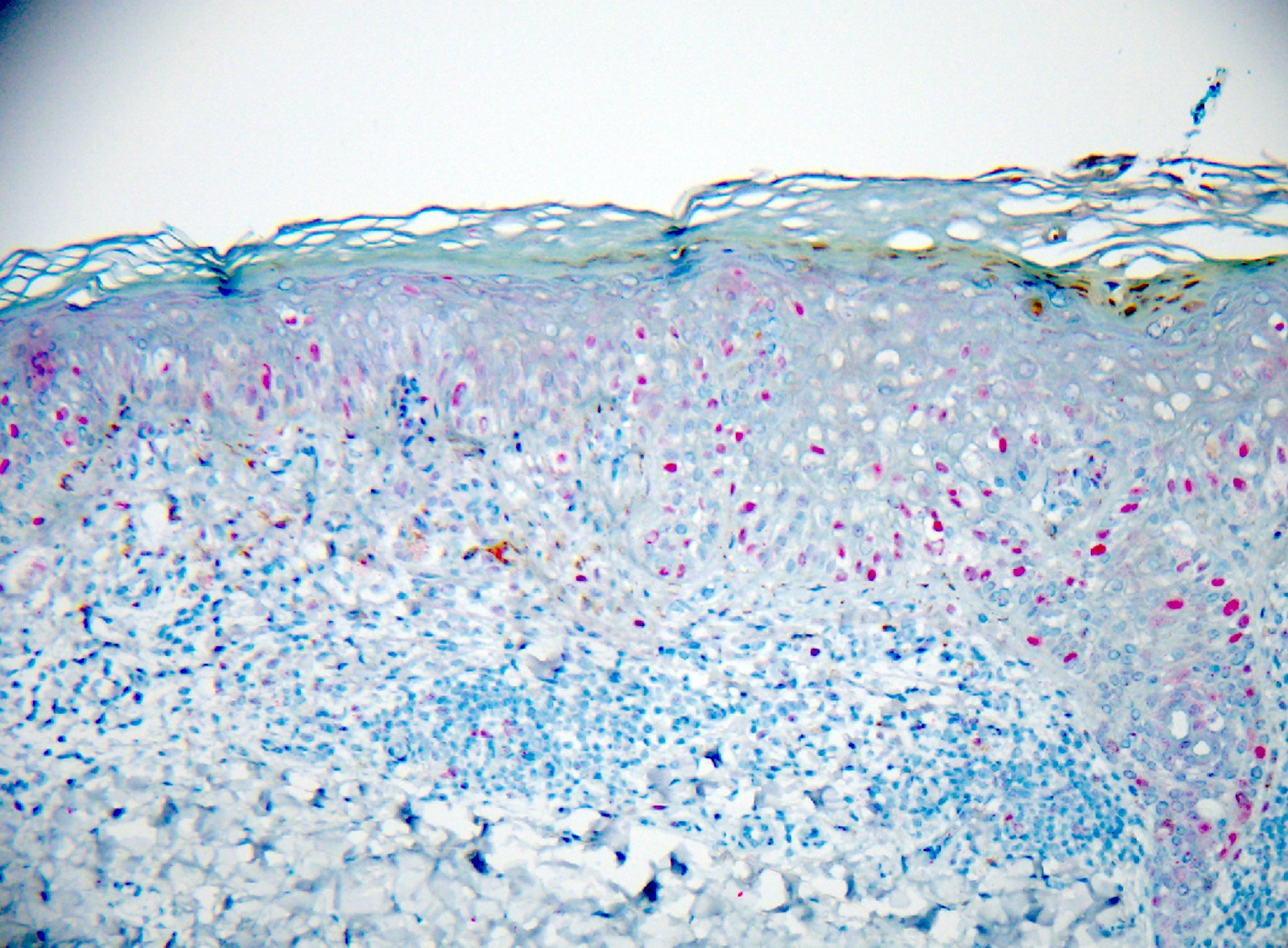
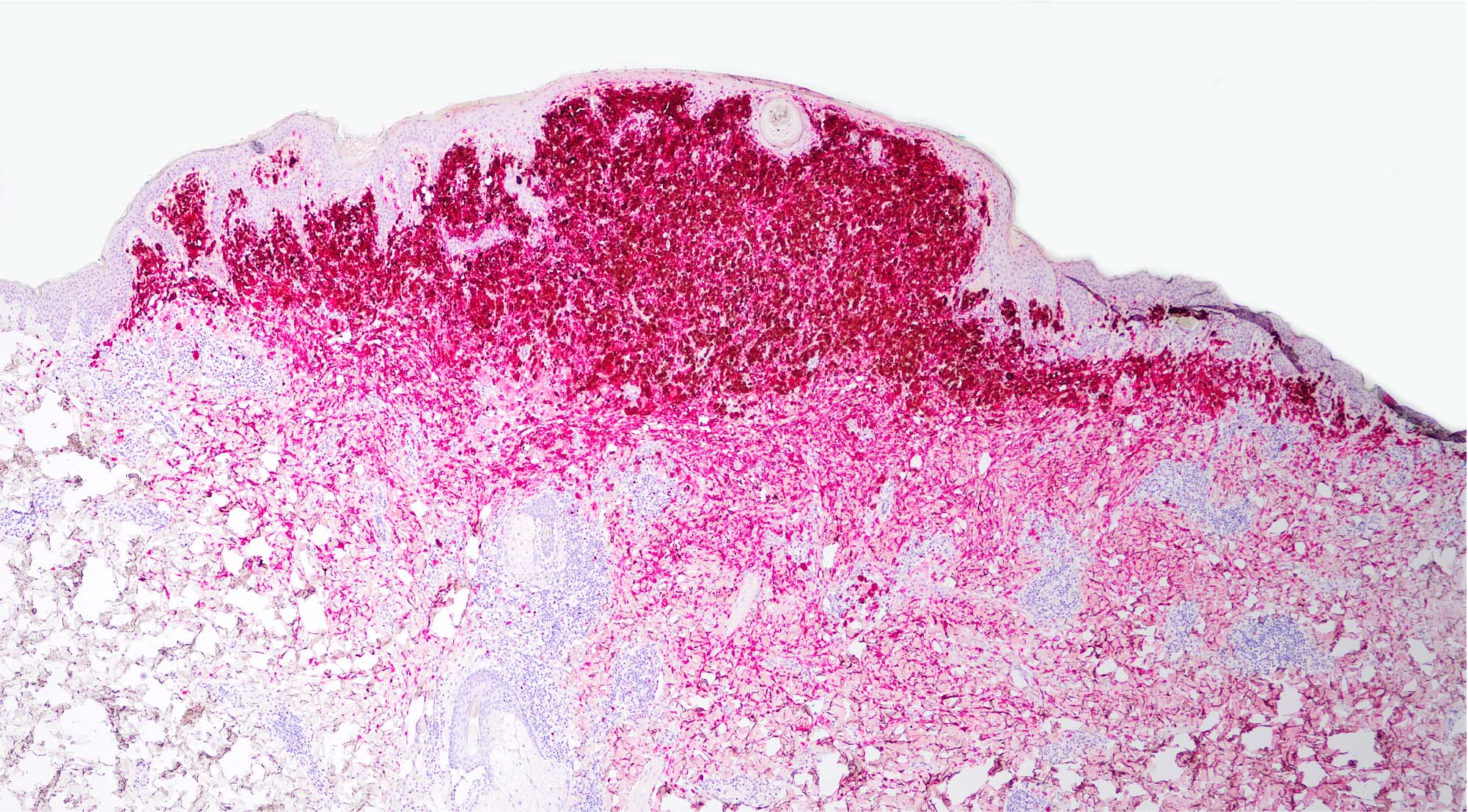
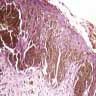
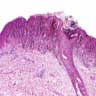
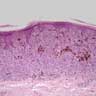
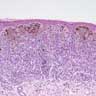
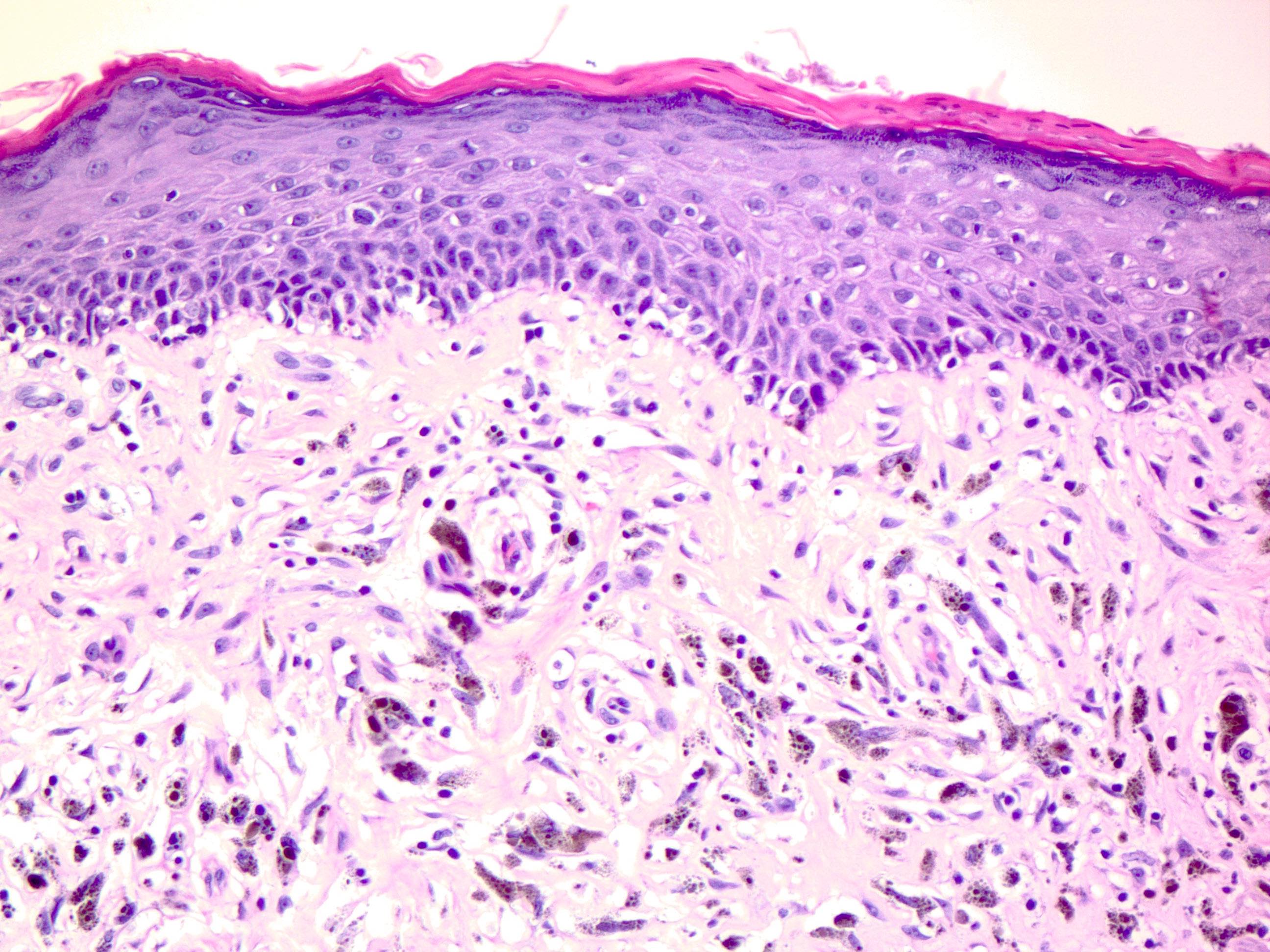
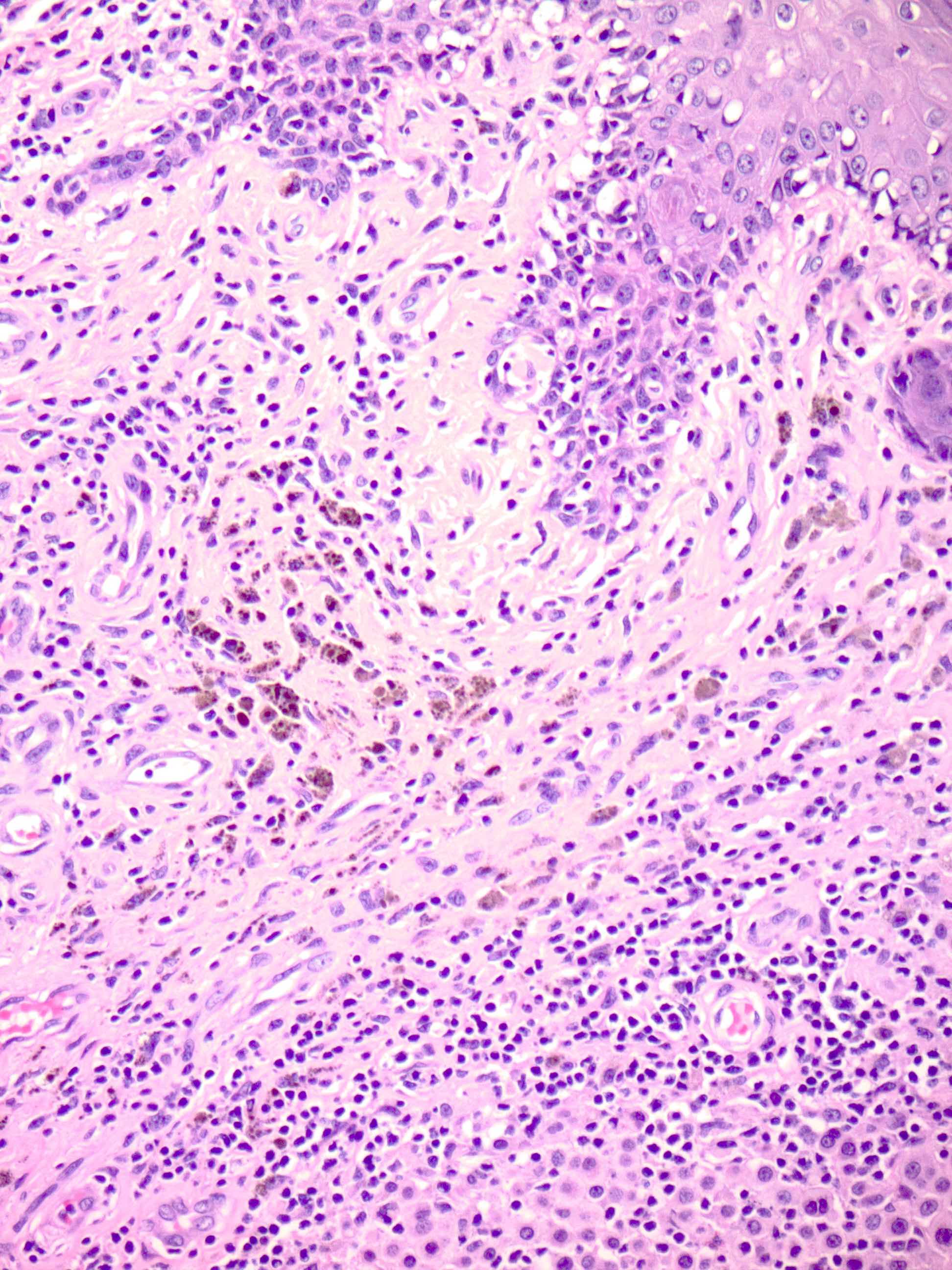
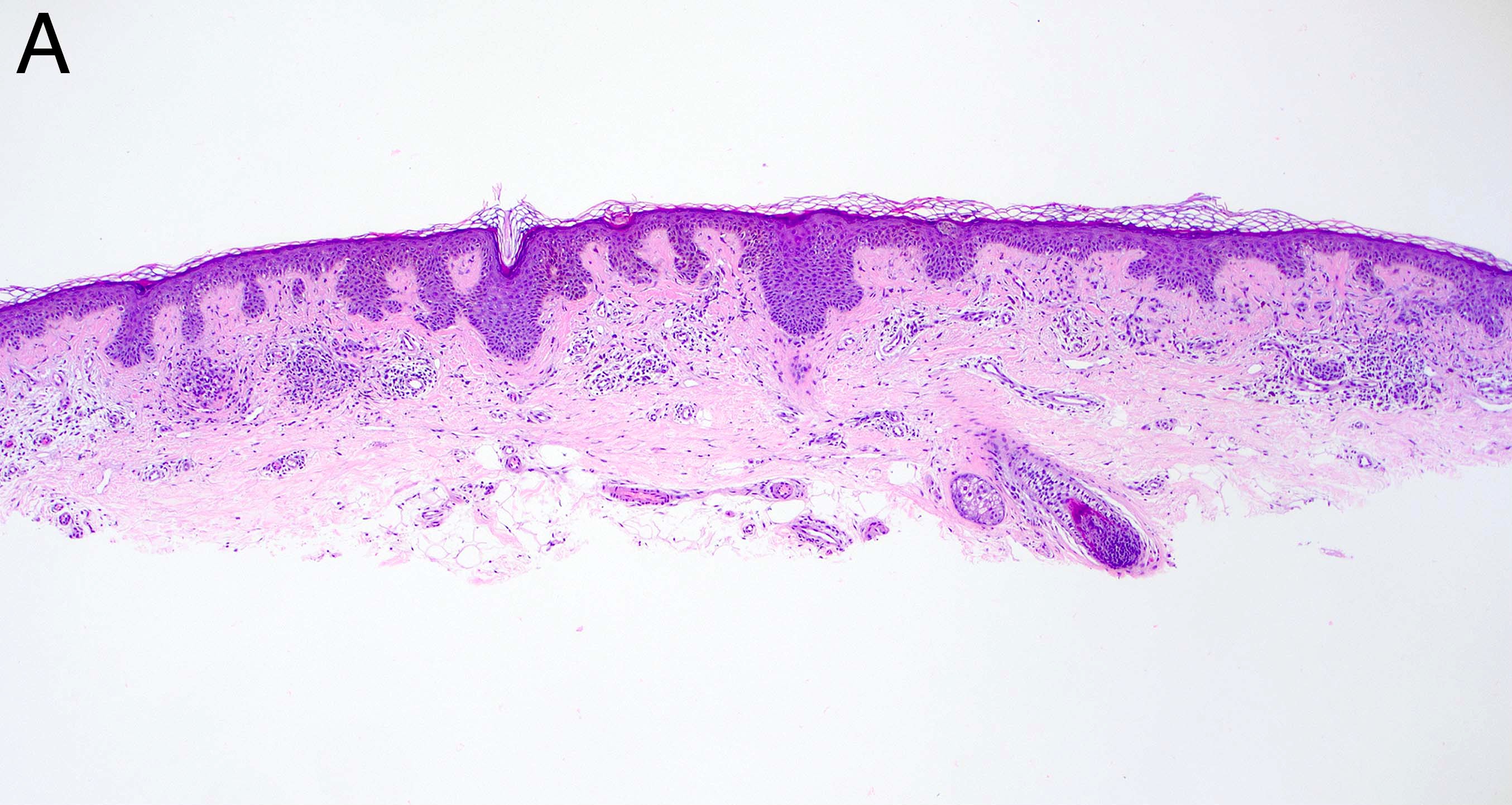
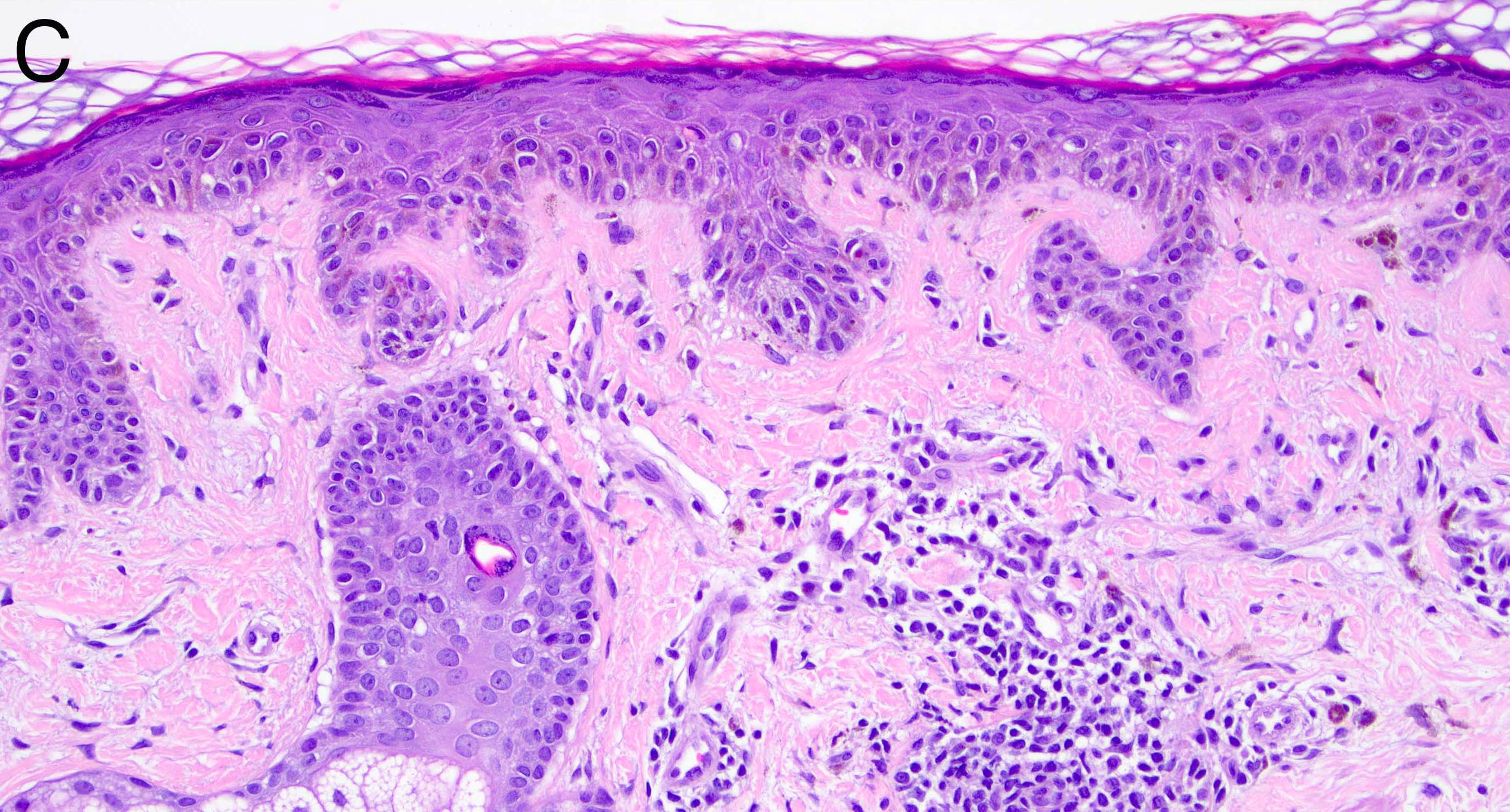
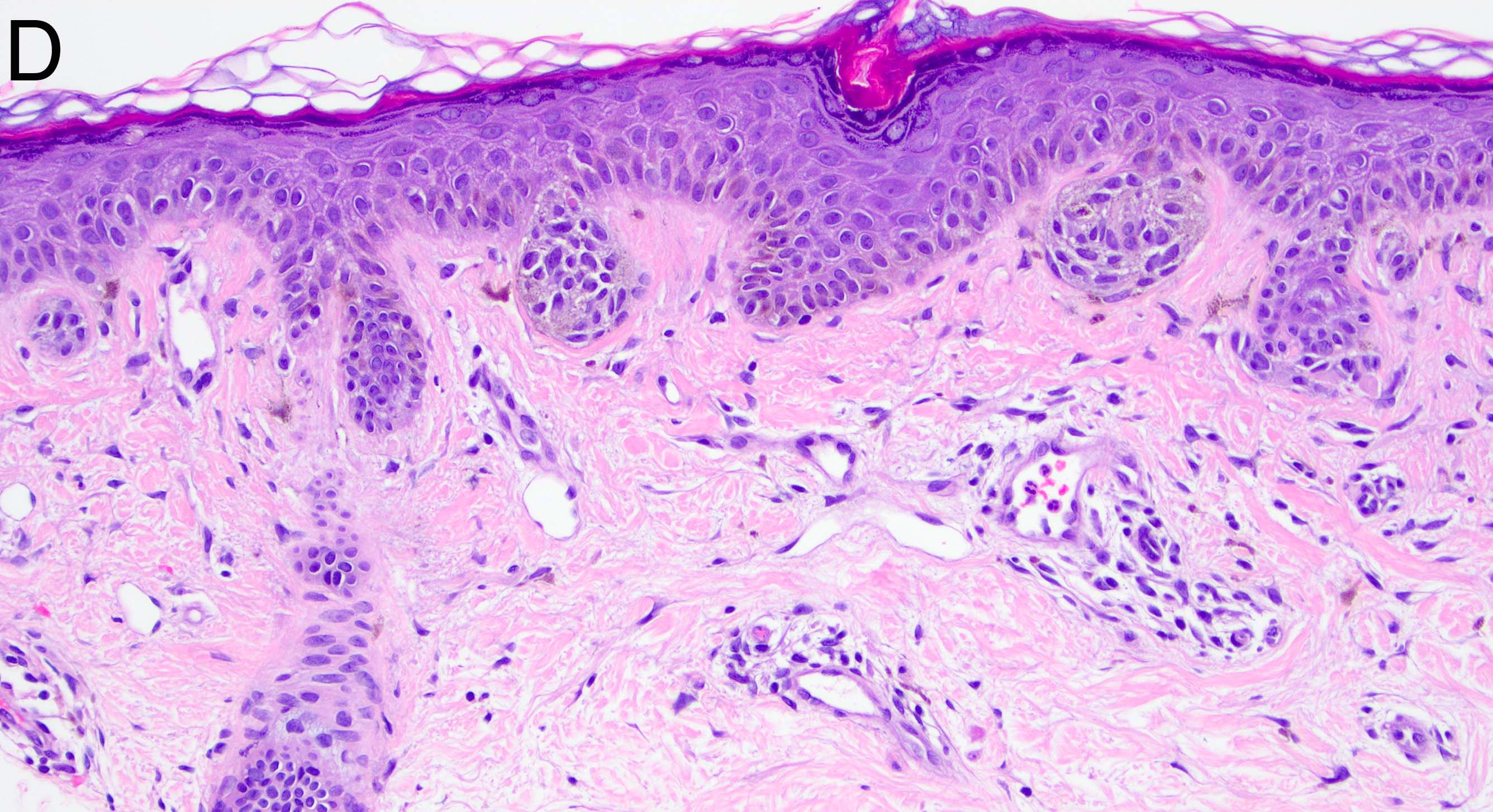
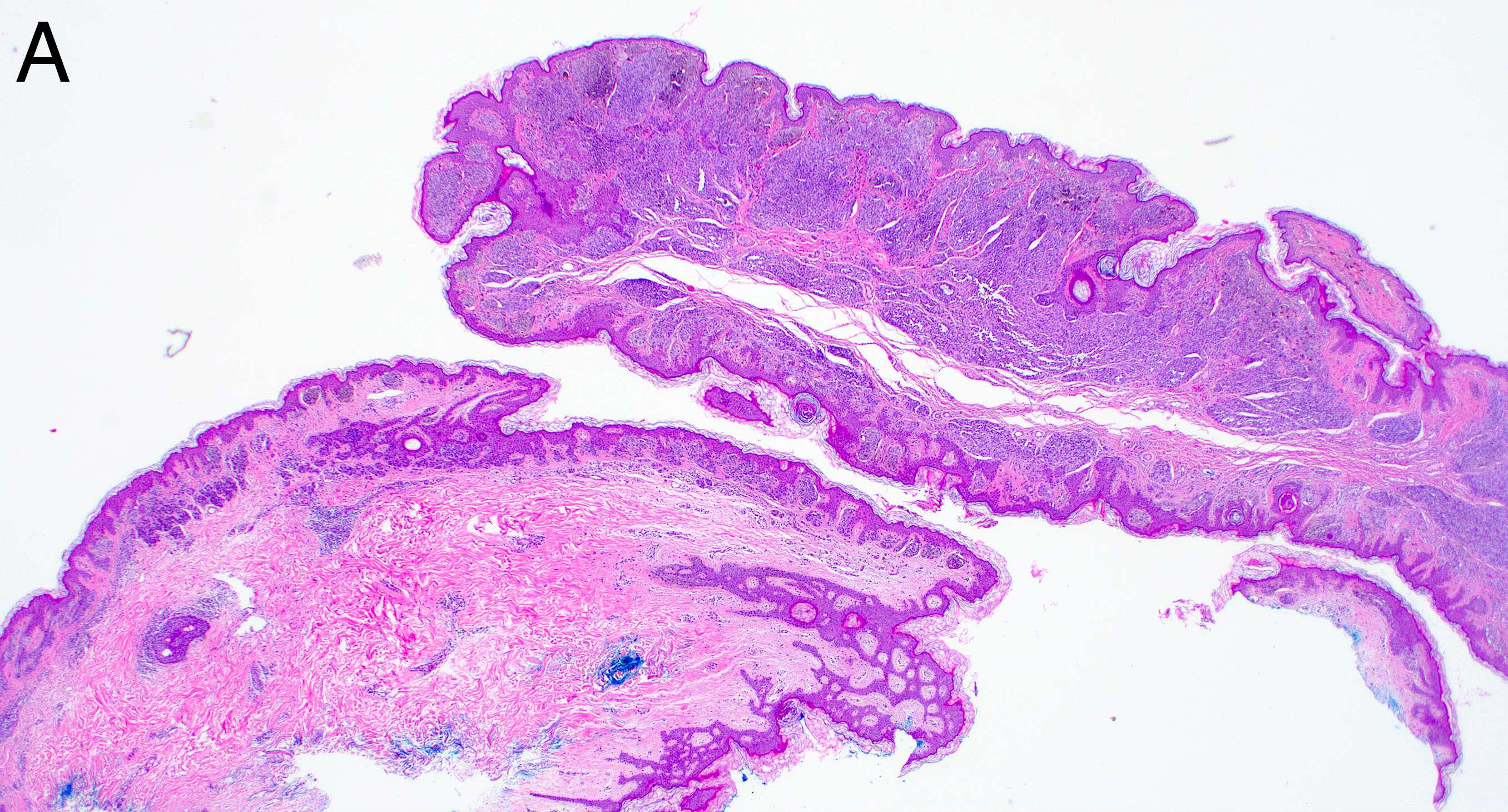
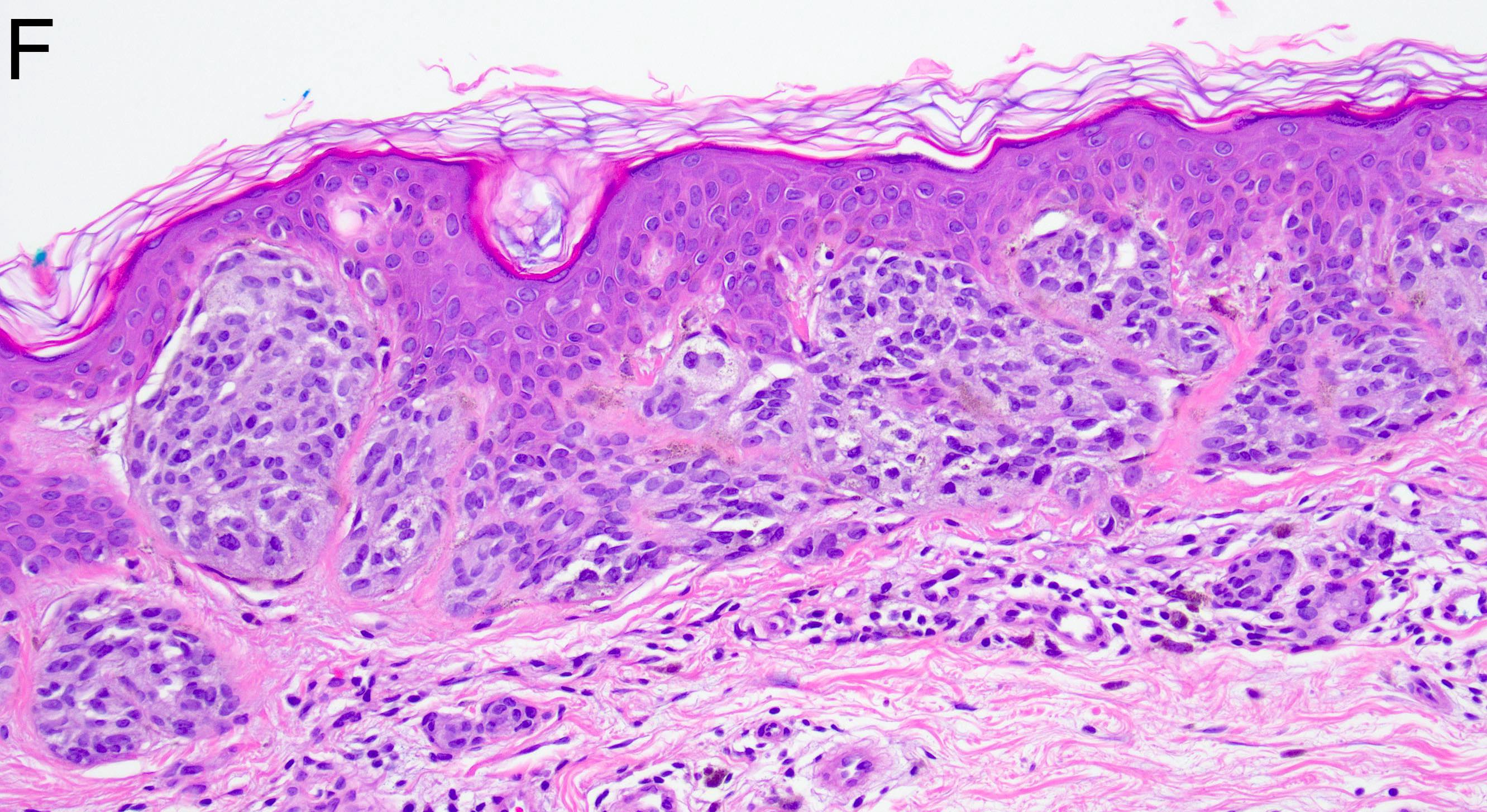
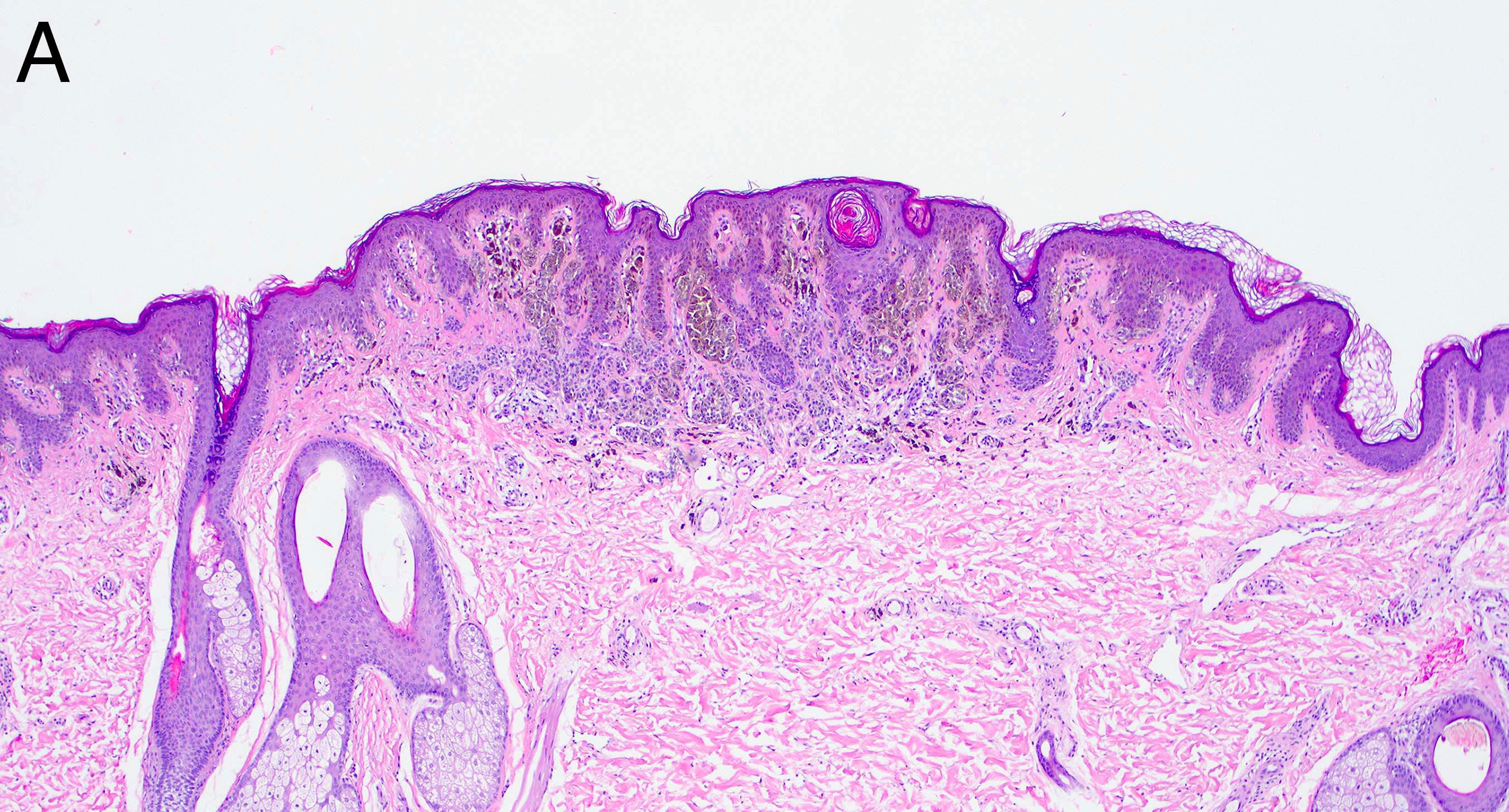
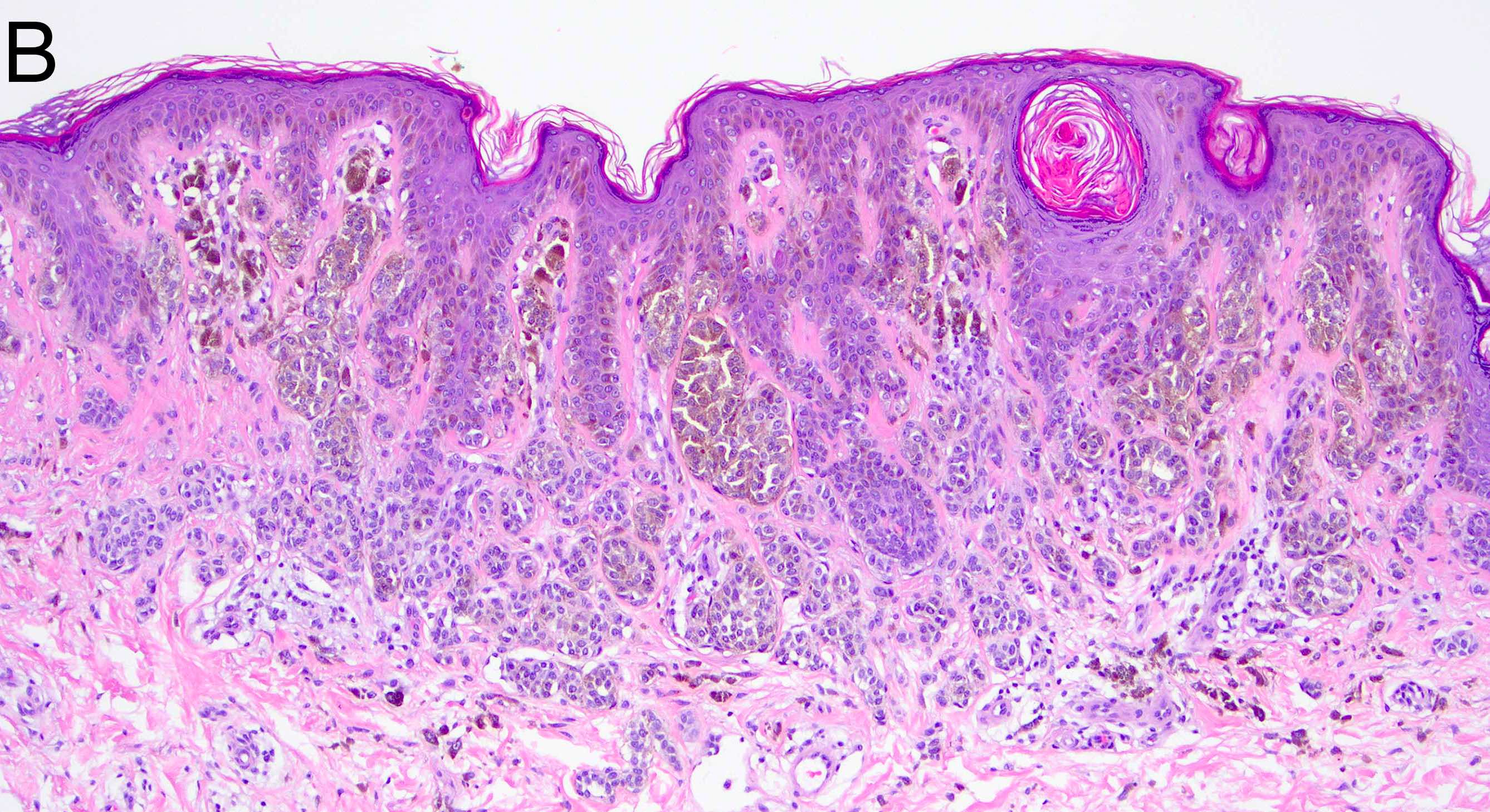
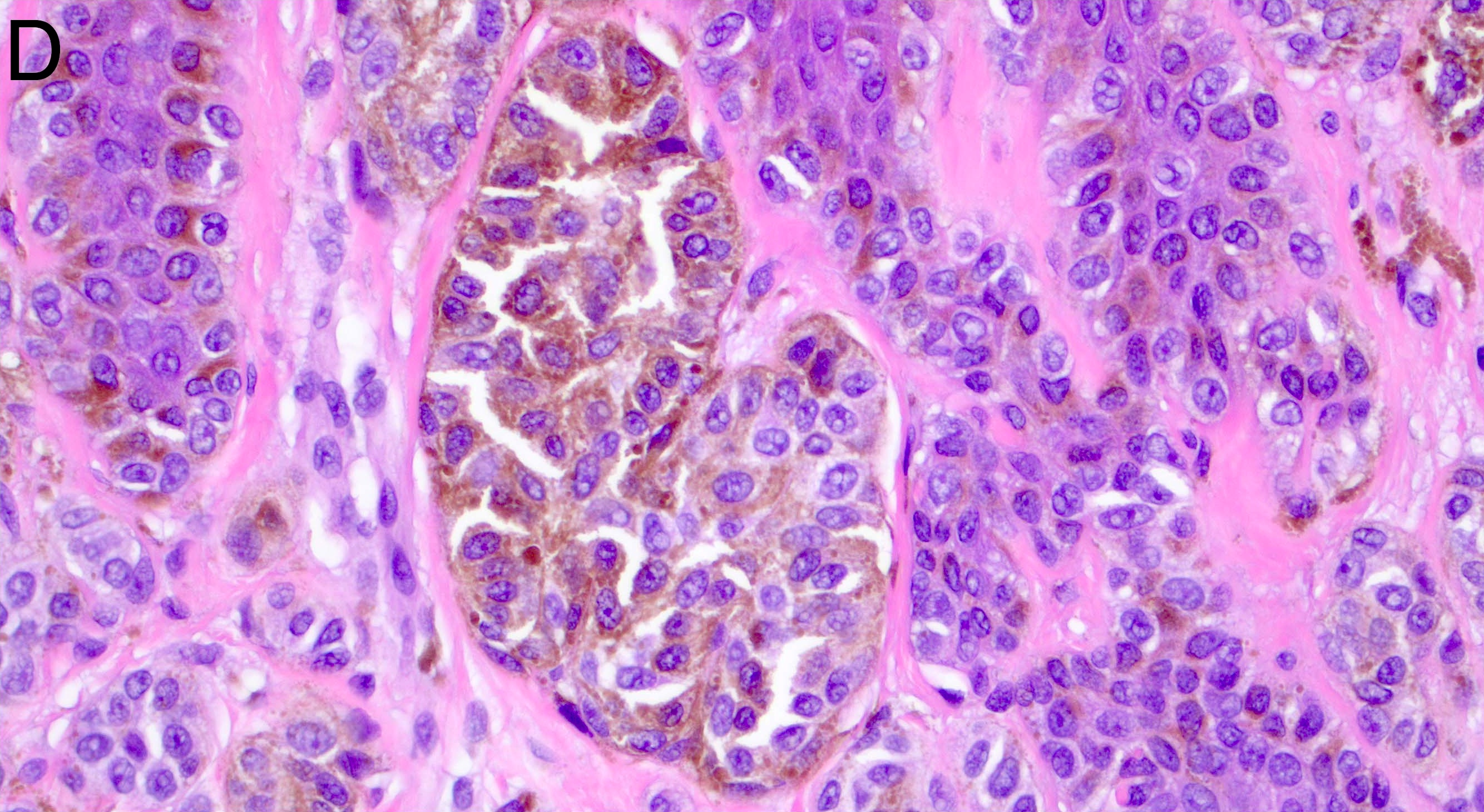
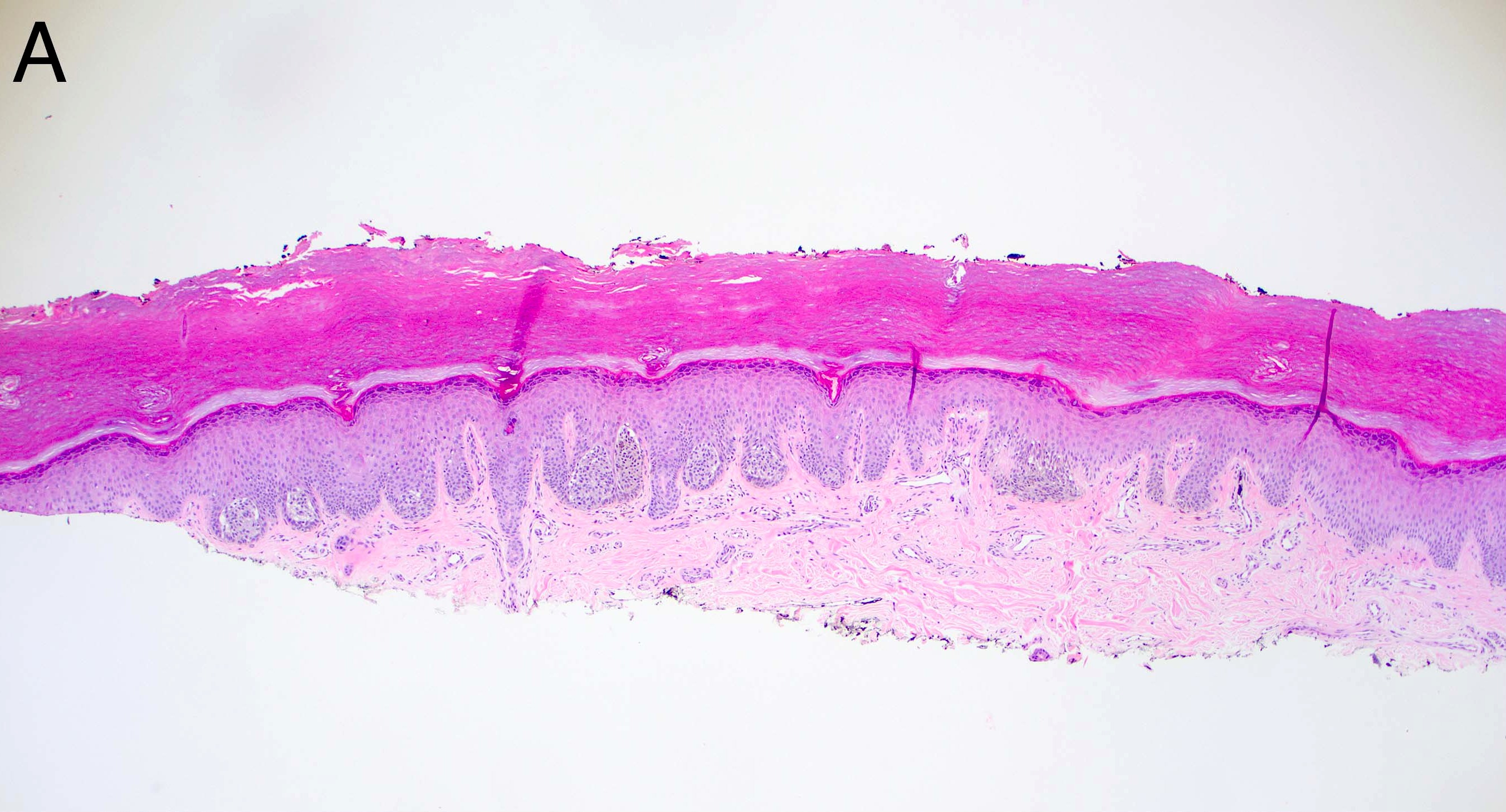
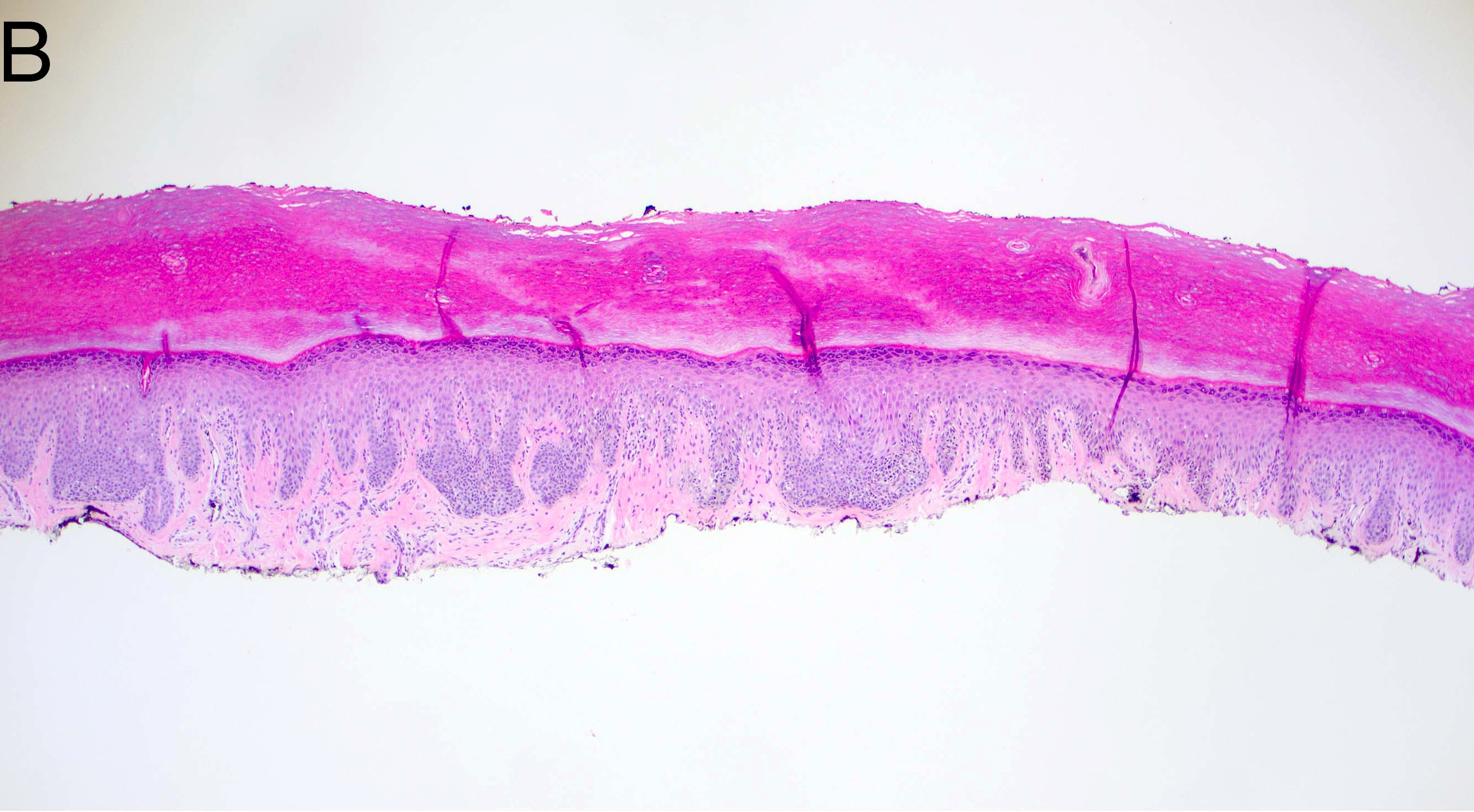
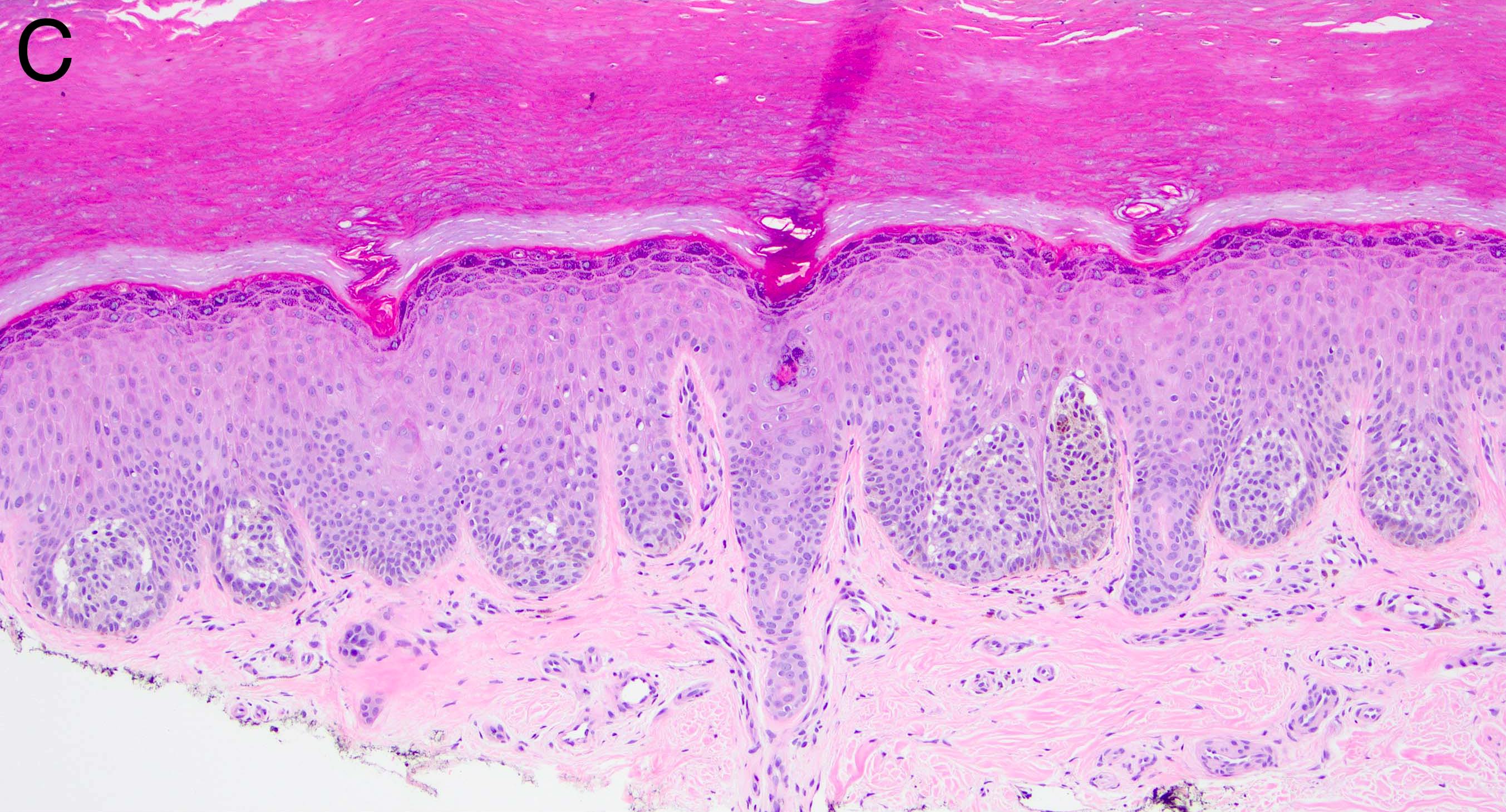
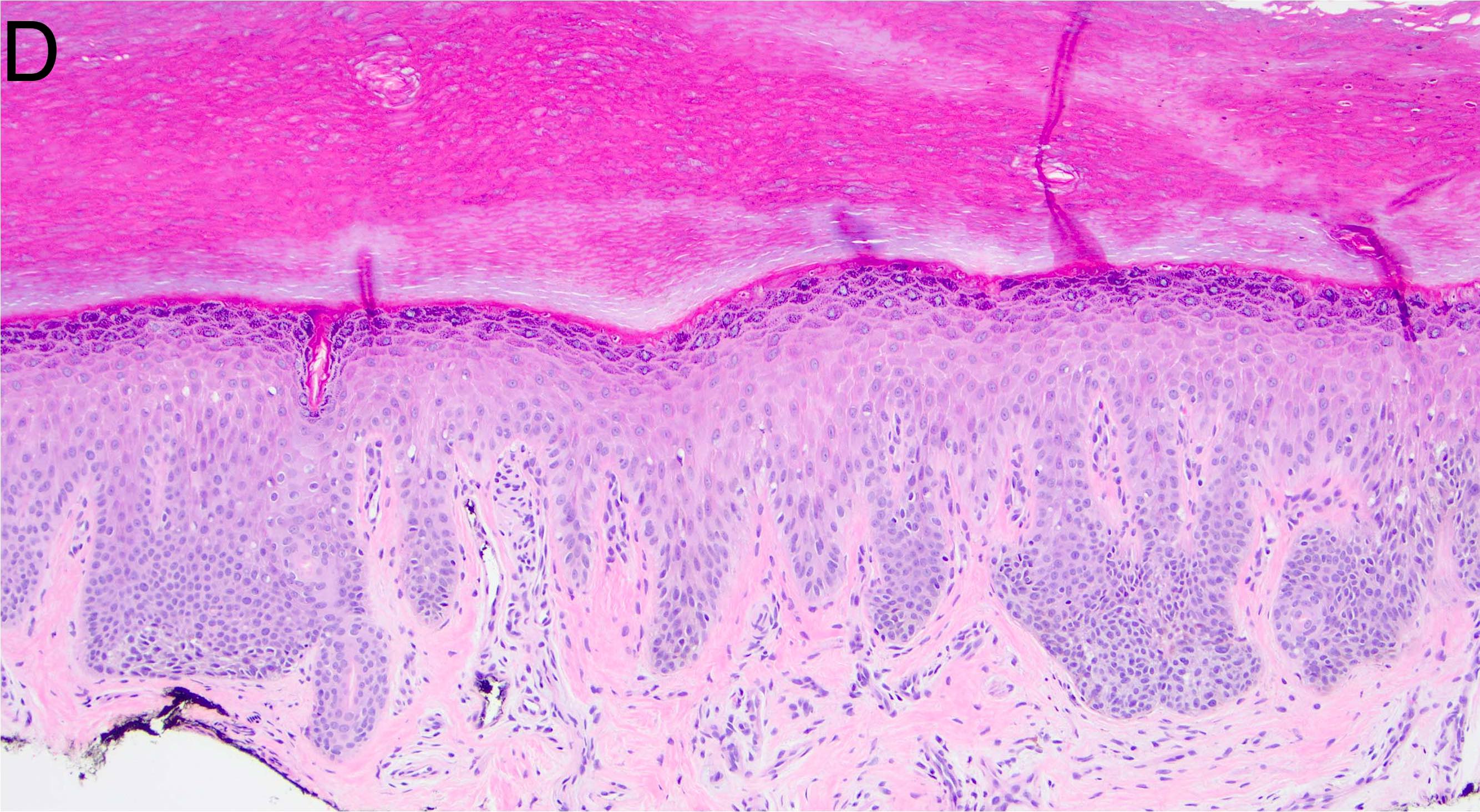
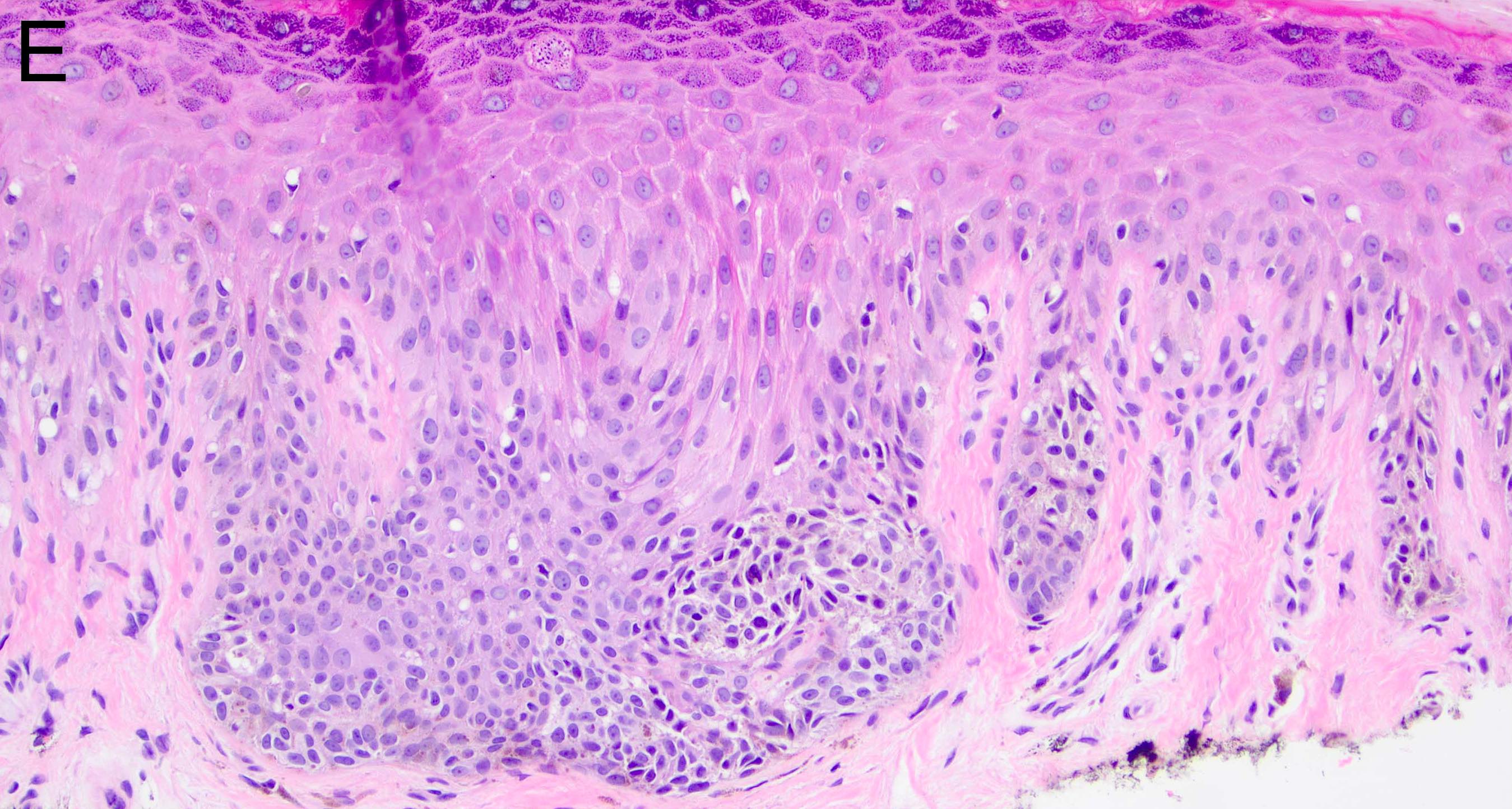
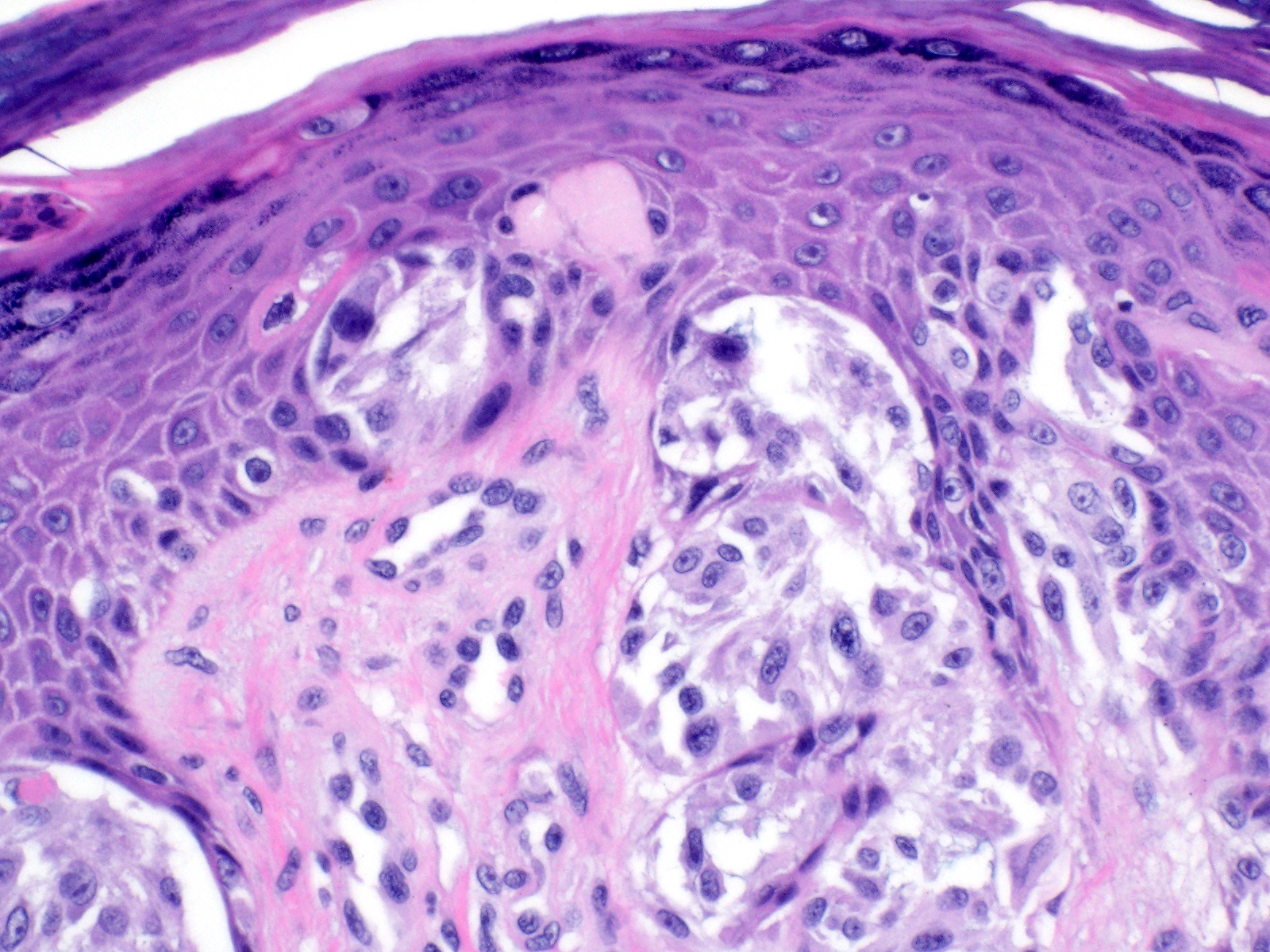
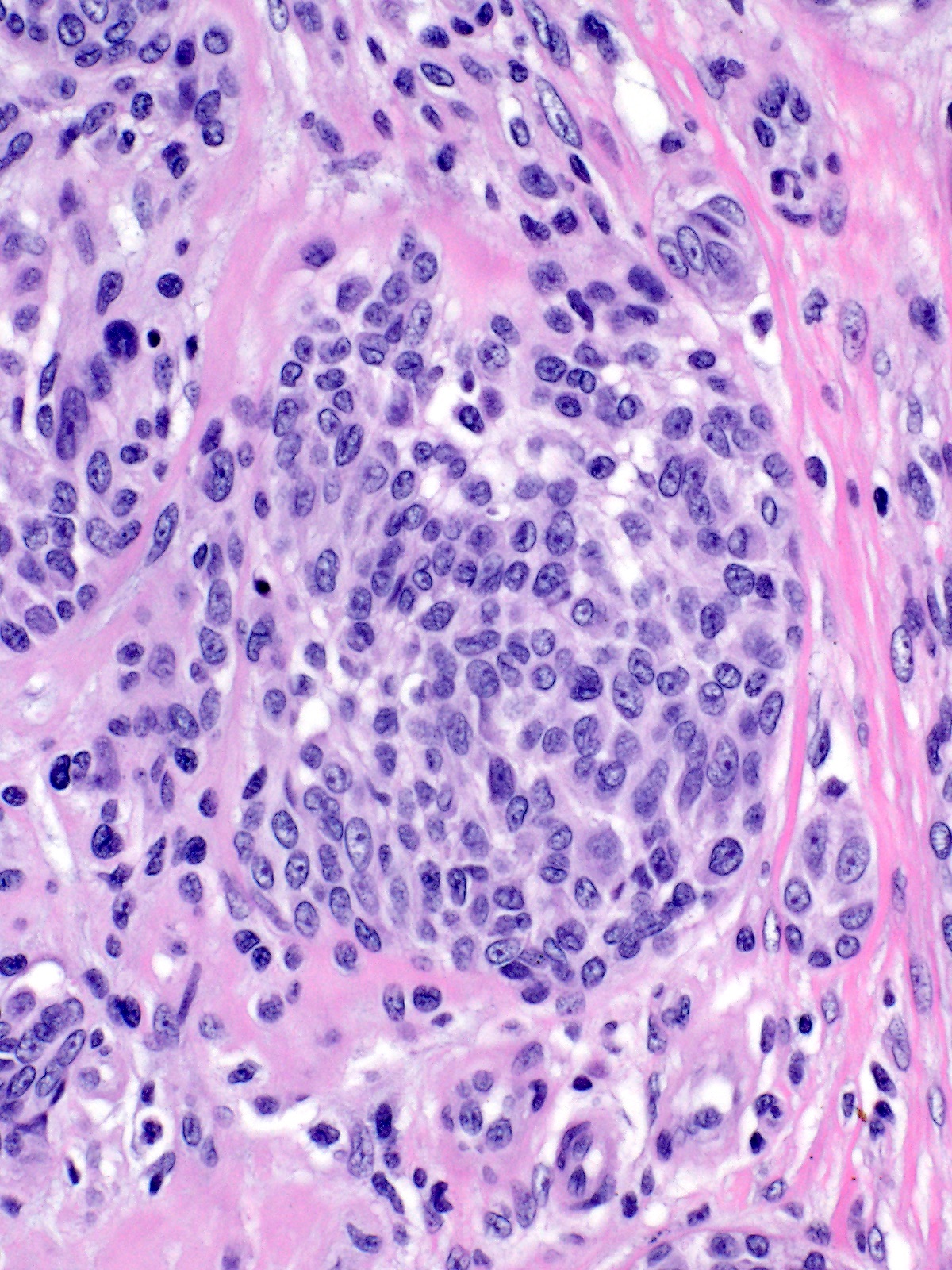
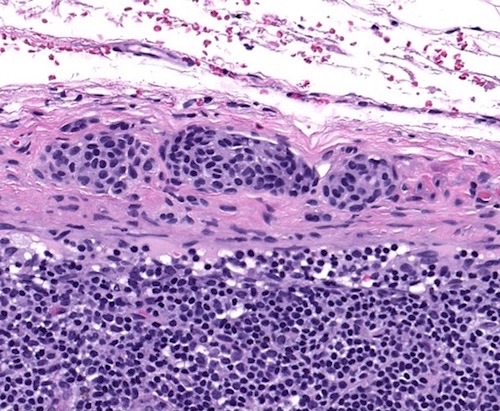
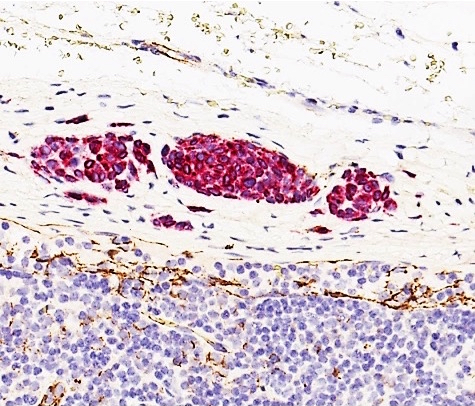
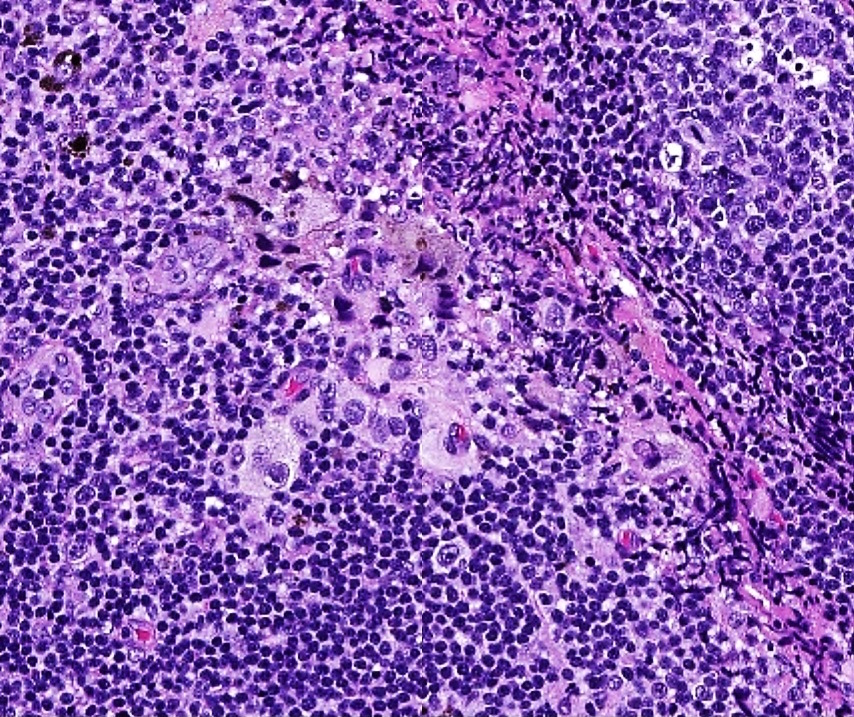
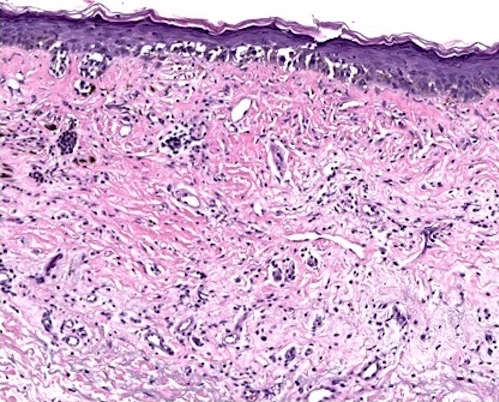
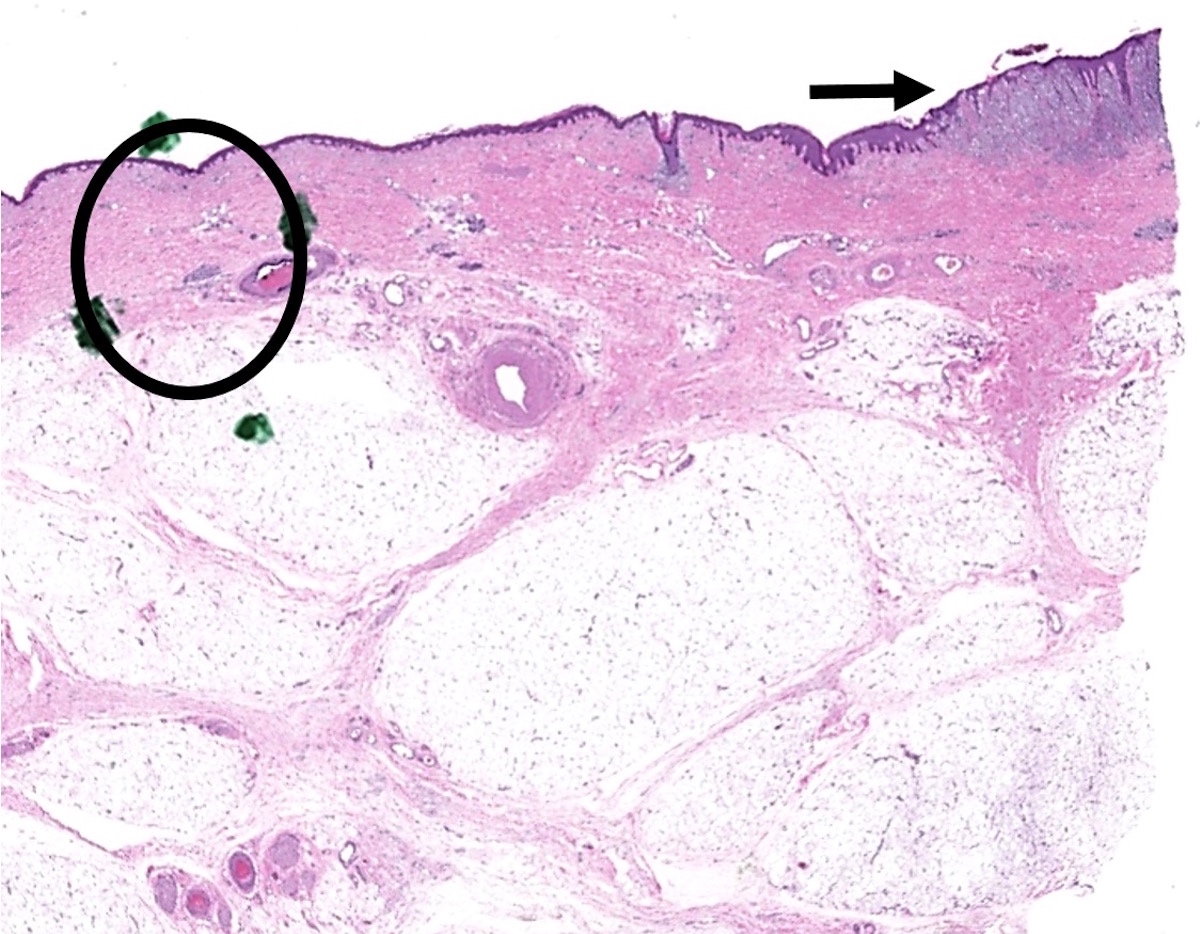
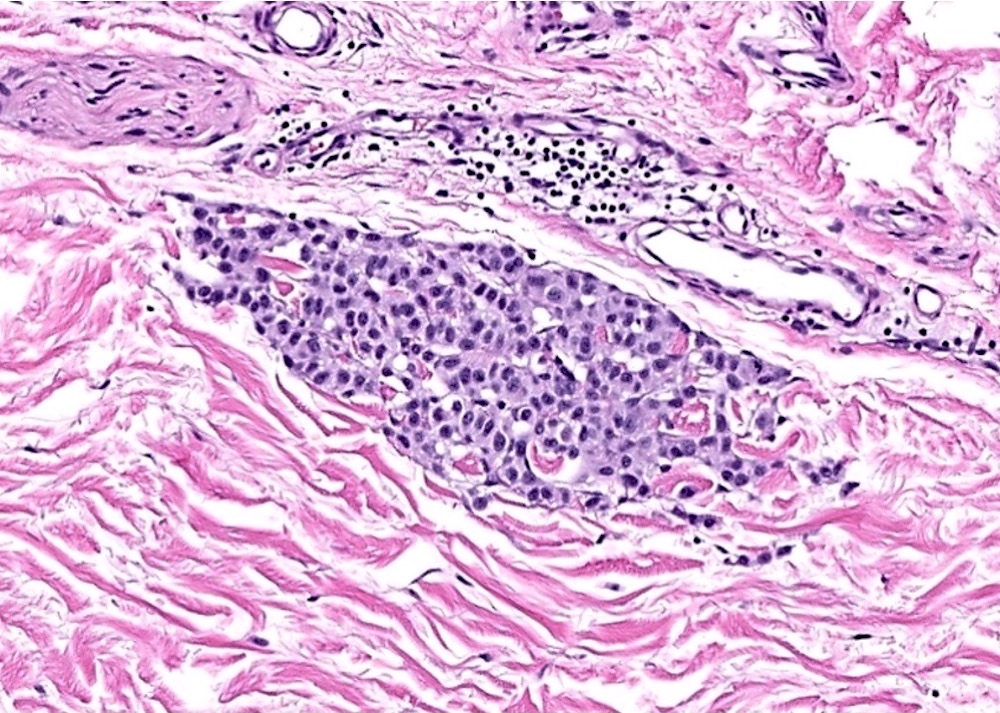
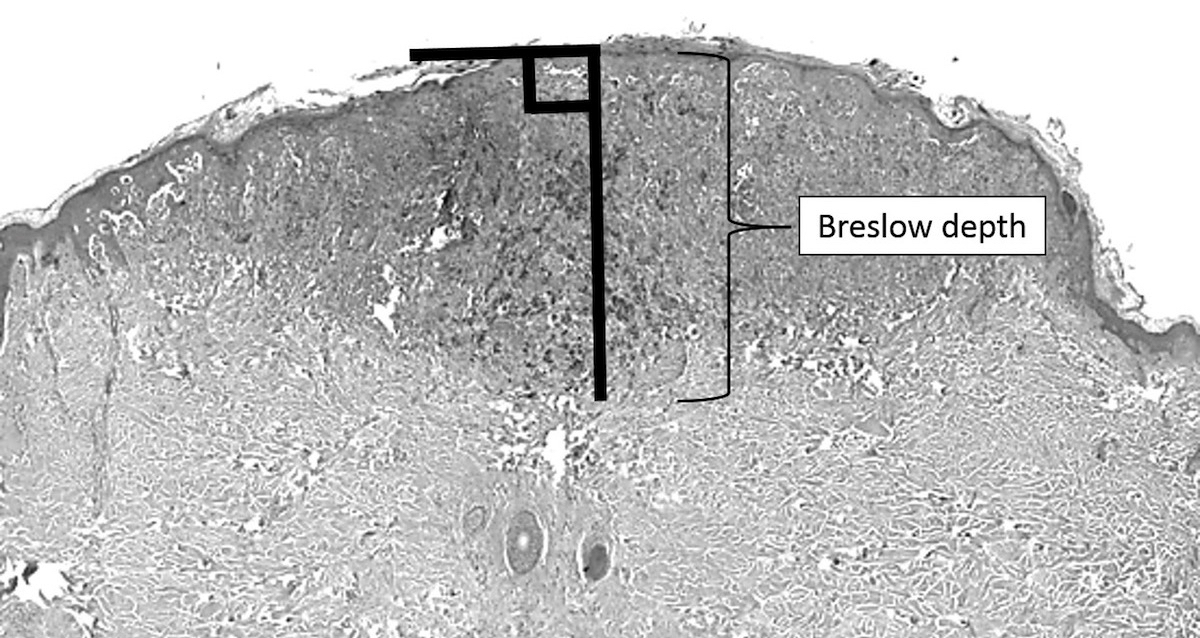
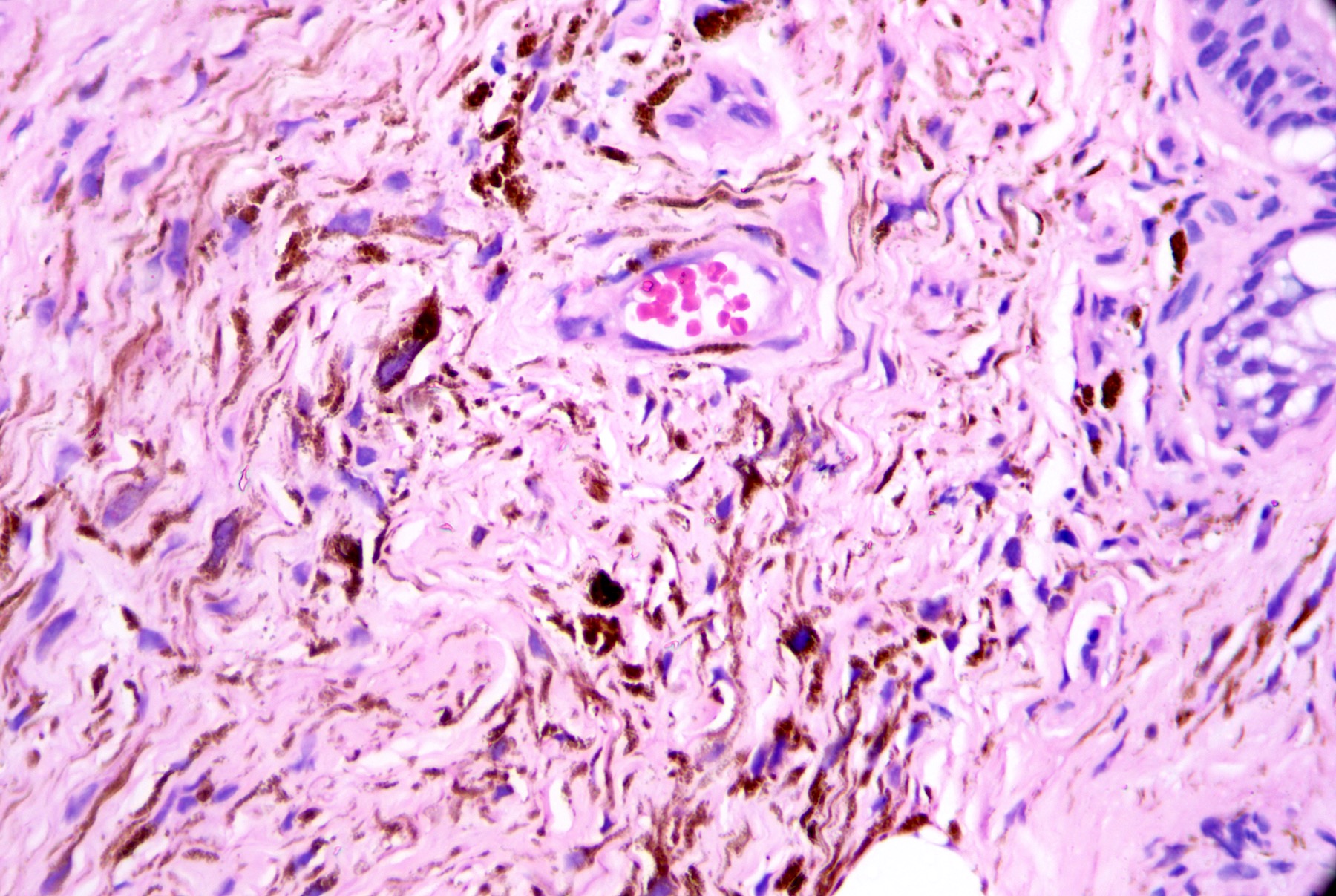
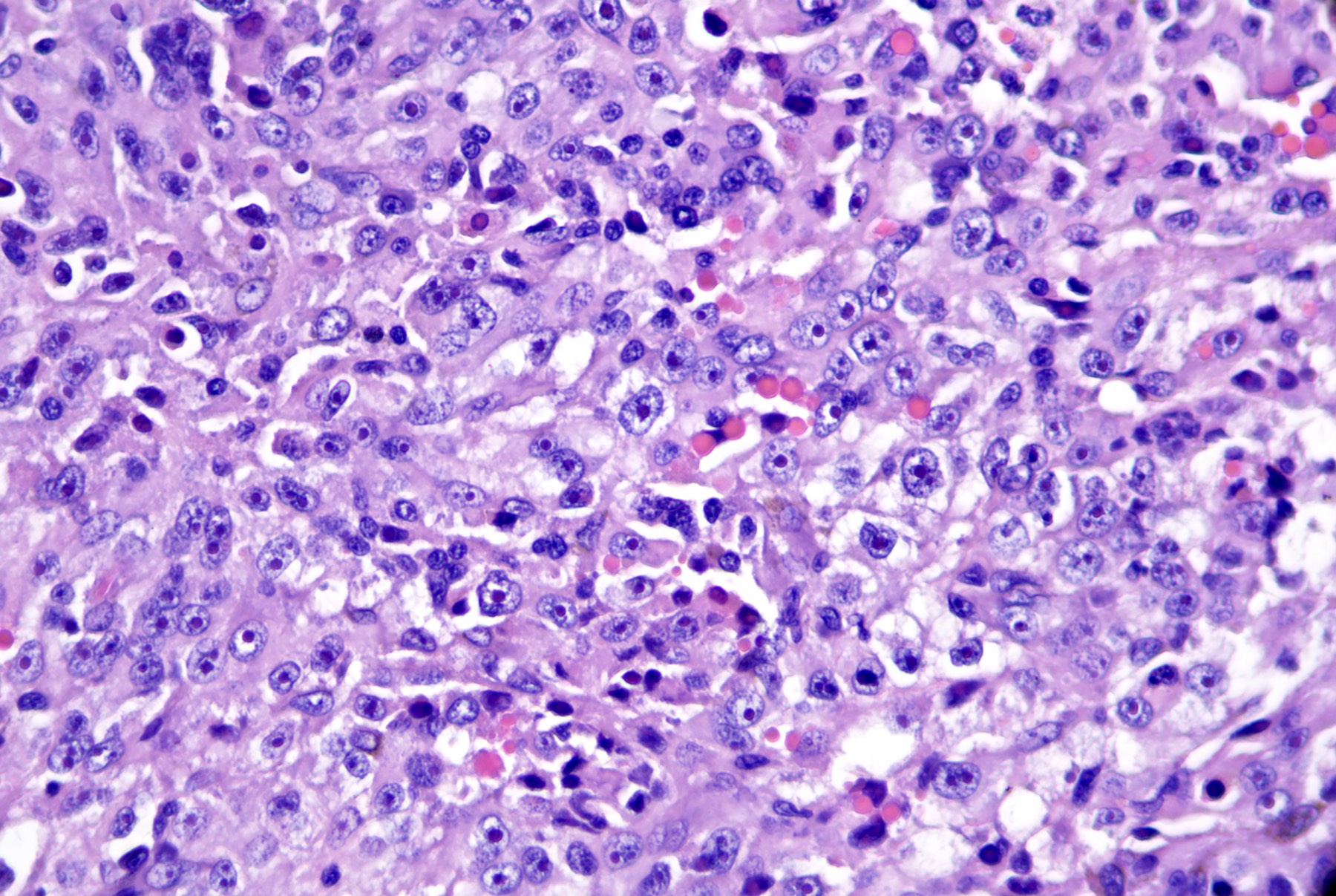
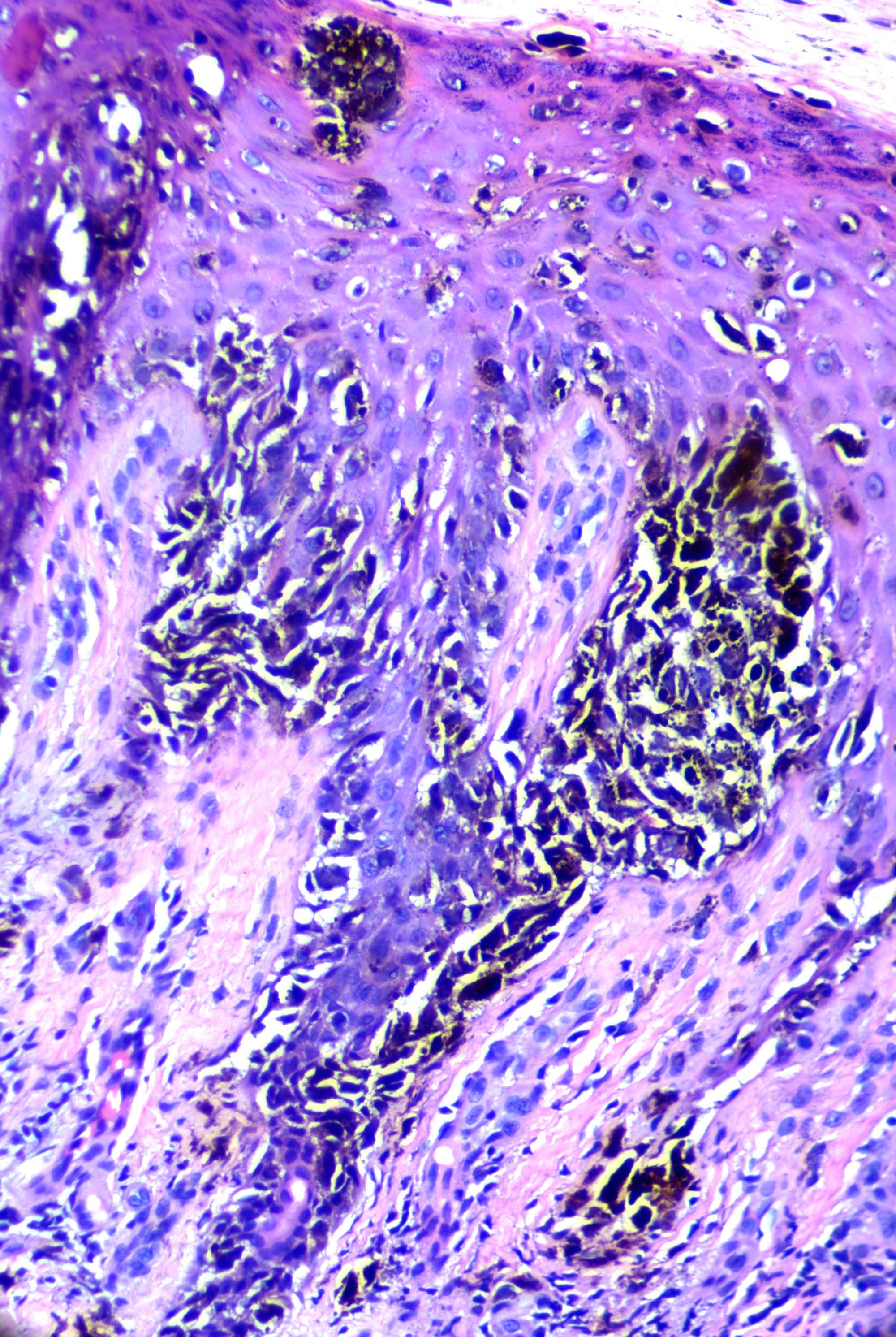
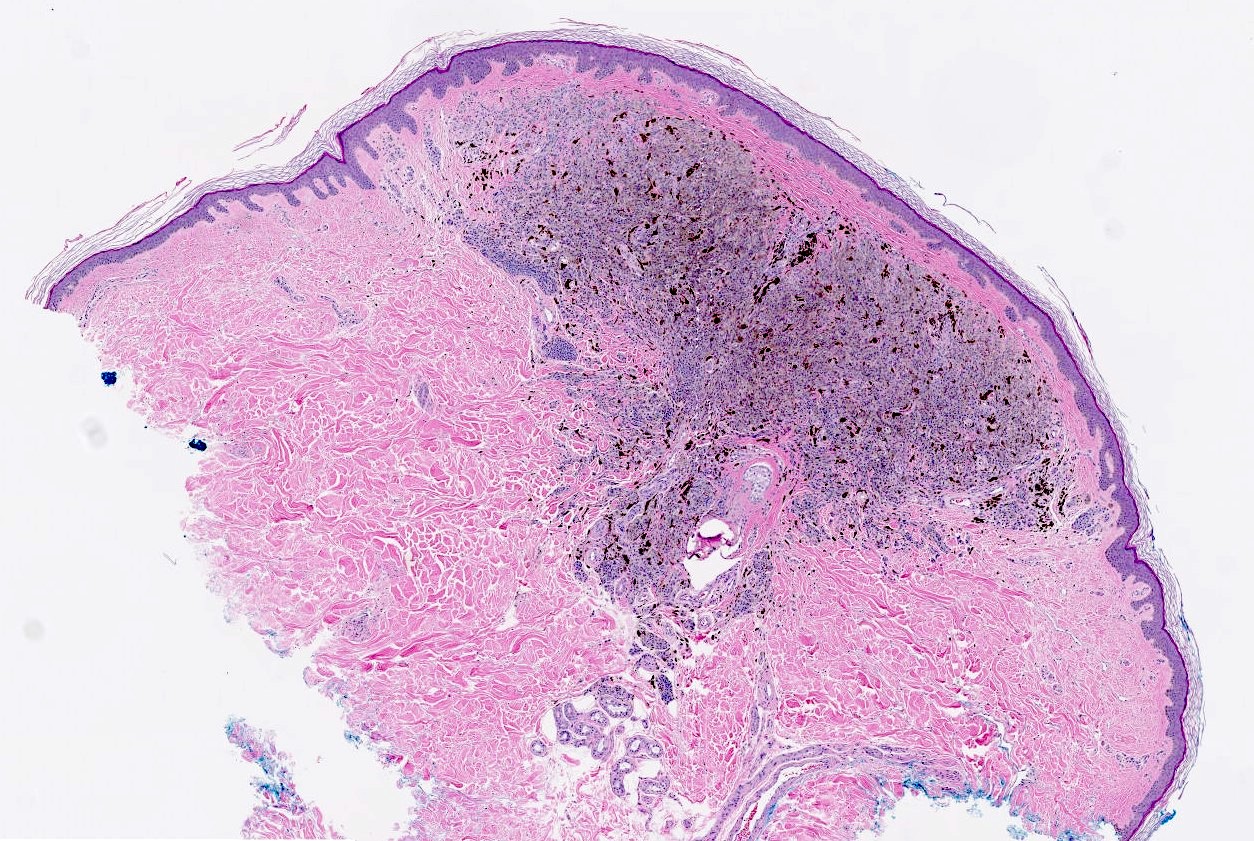
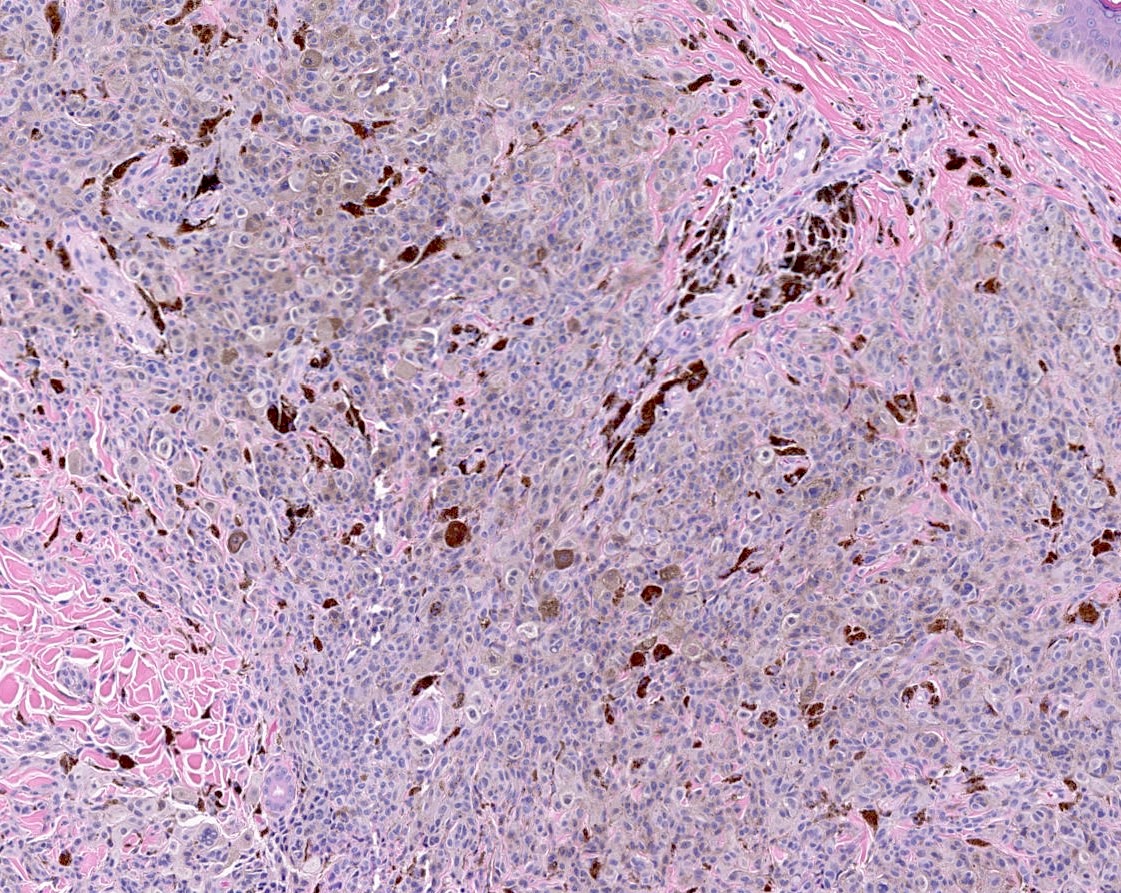
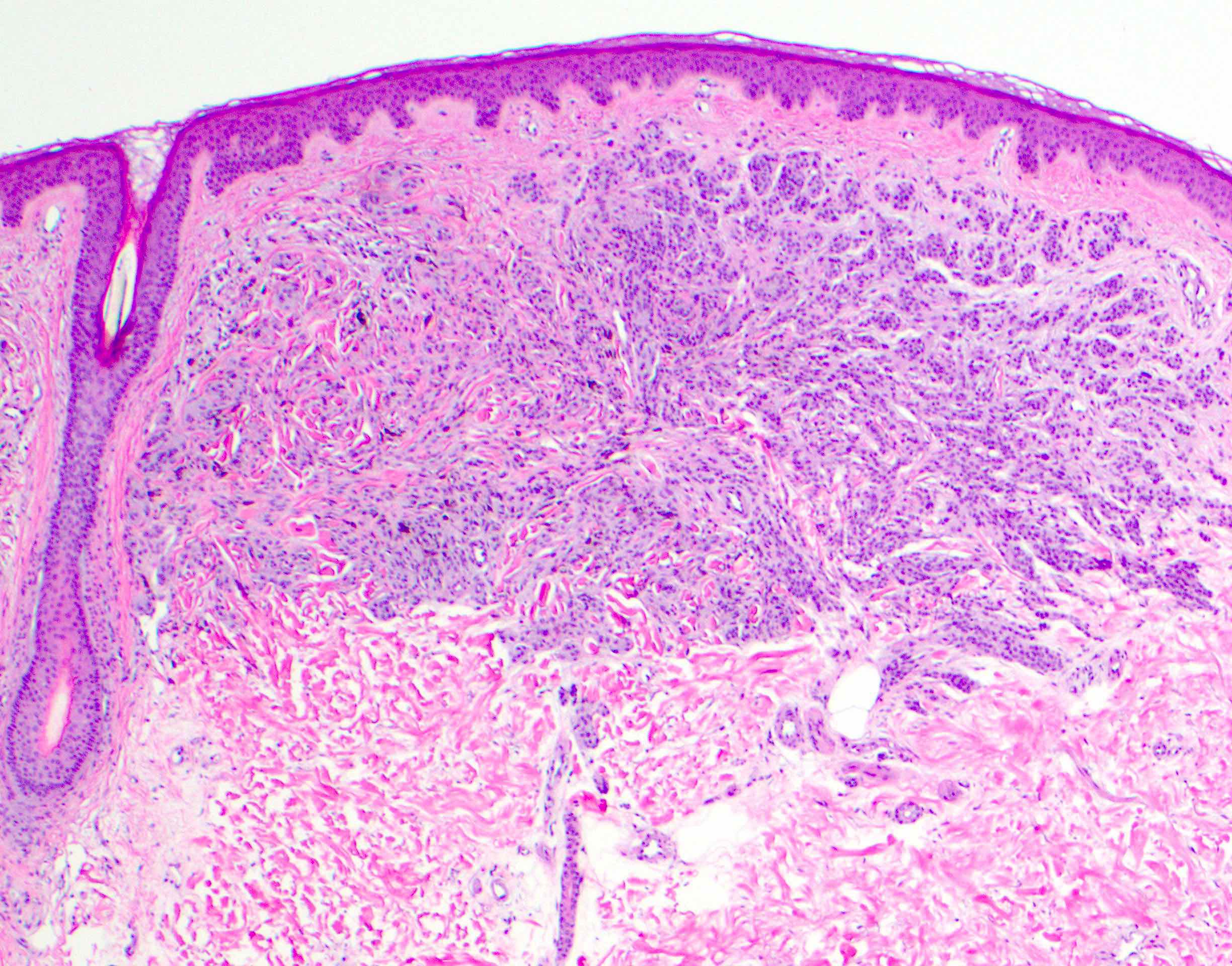
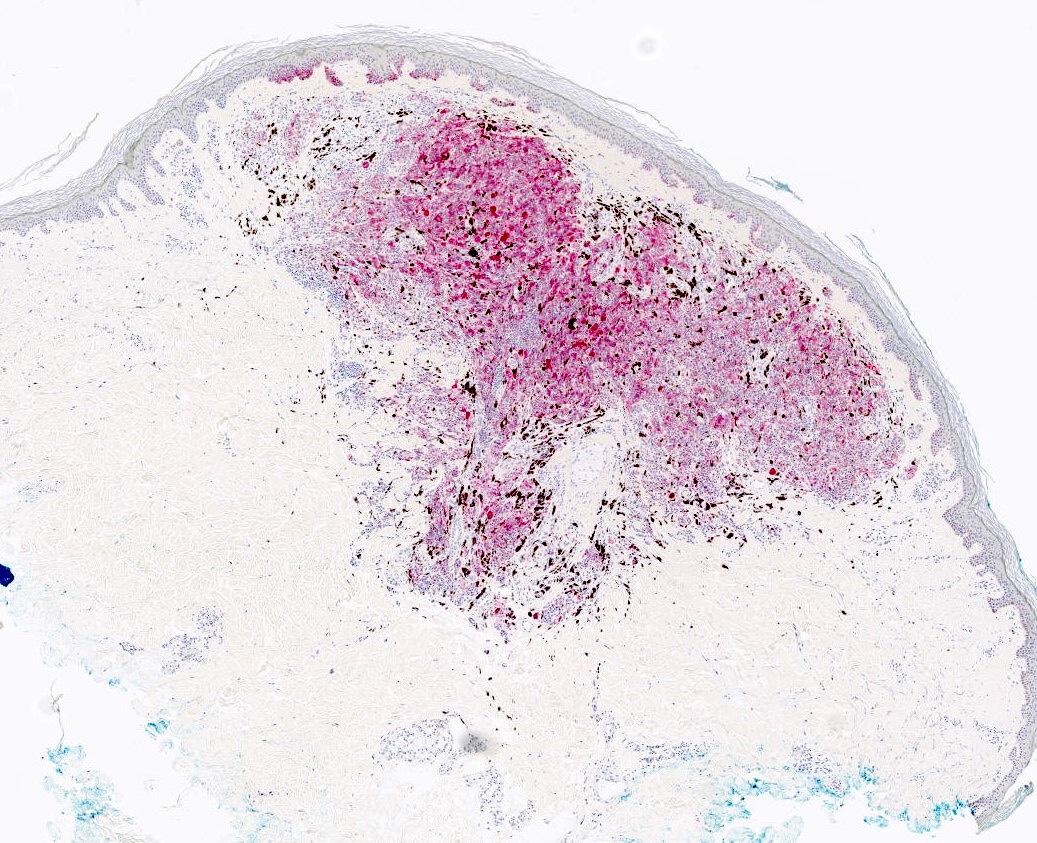
- Breslow depth: measure from the surface of the epidermal granular layer to the point of maximum tumor thickness at a right angle to adjacent epidermis
- Measured with a calibrated ocular micrometer
- Measurement is rounded to the nearest 0.1 mm
- Report as "at least _ mm" if the maximum tumor thickness cannot be determined, most commonly when invasive melanoma is present at the edge of a shave biopsy
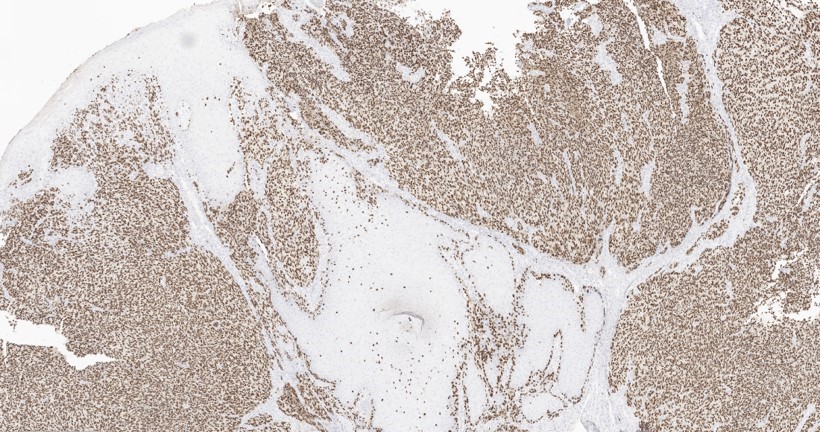
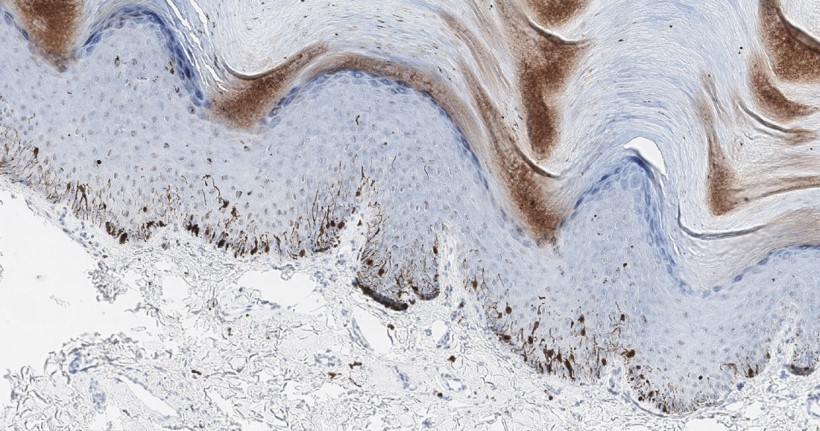
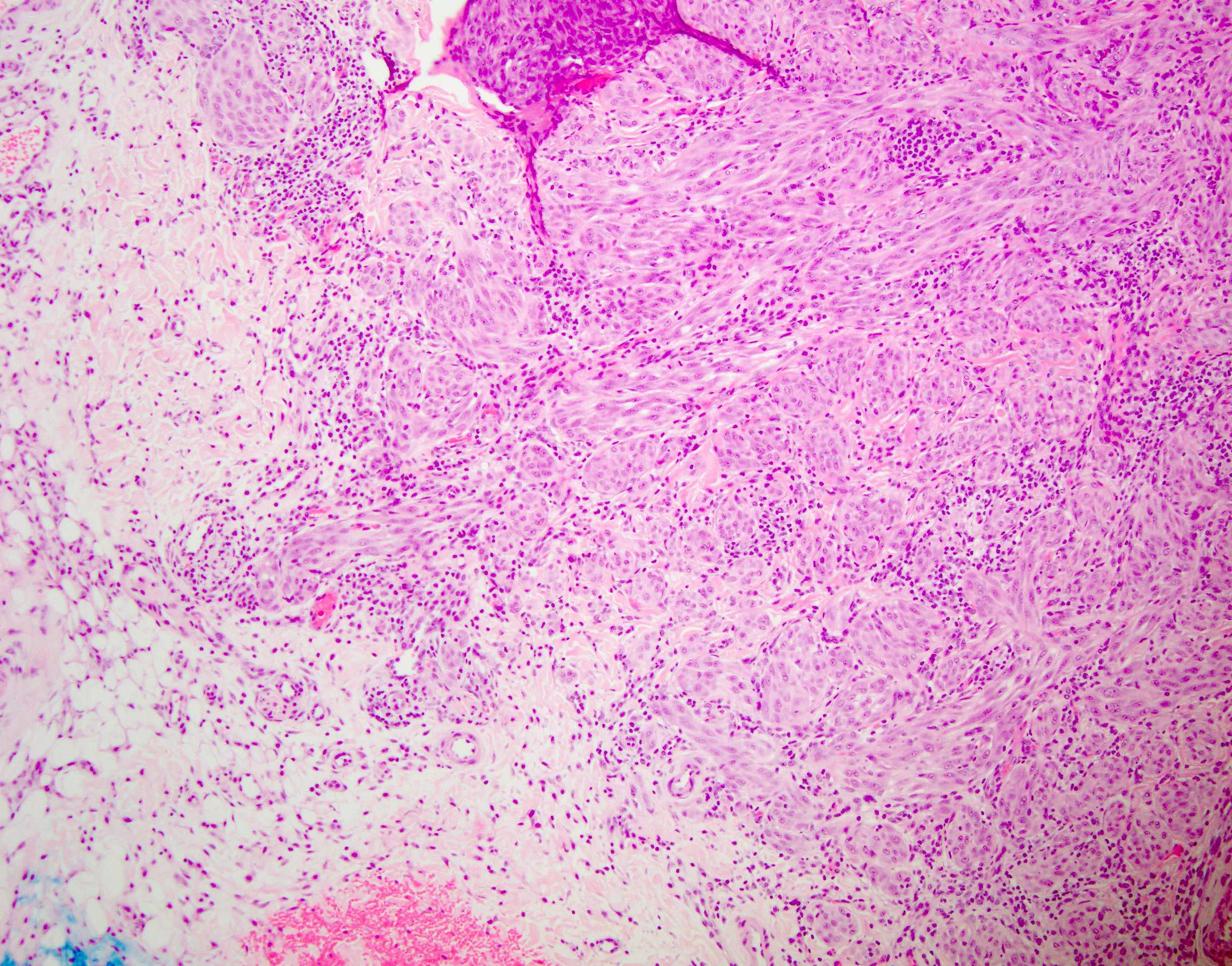
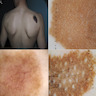
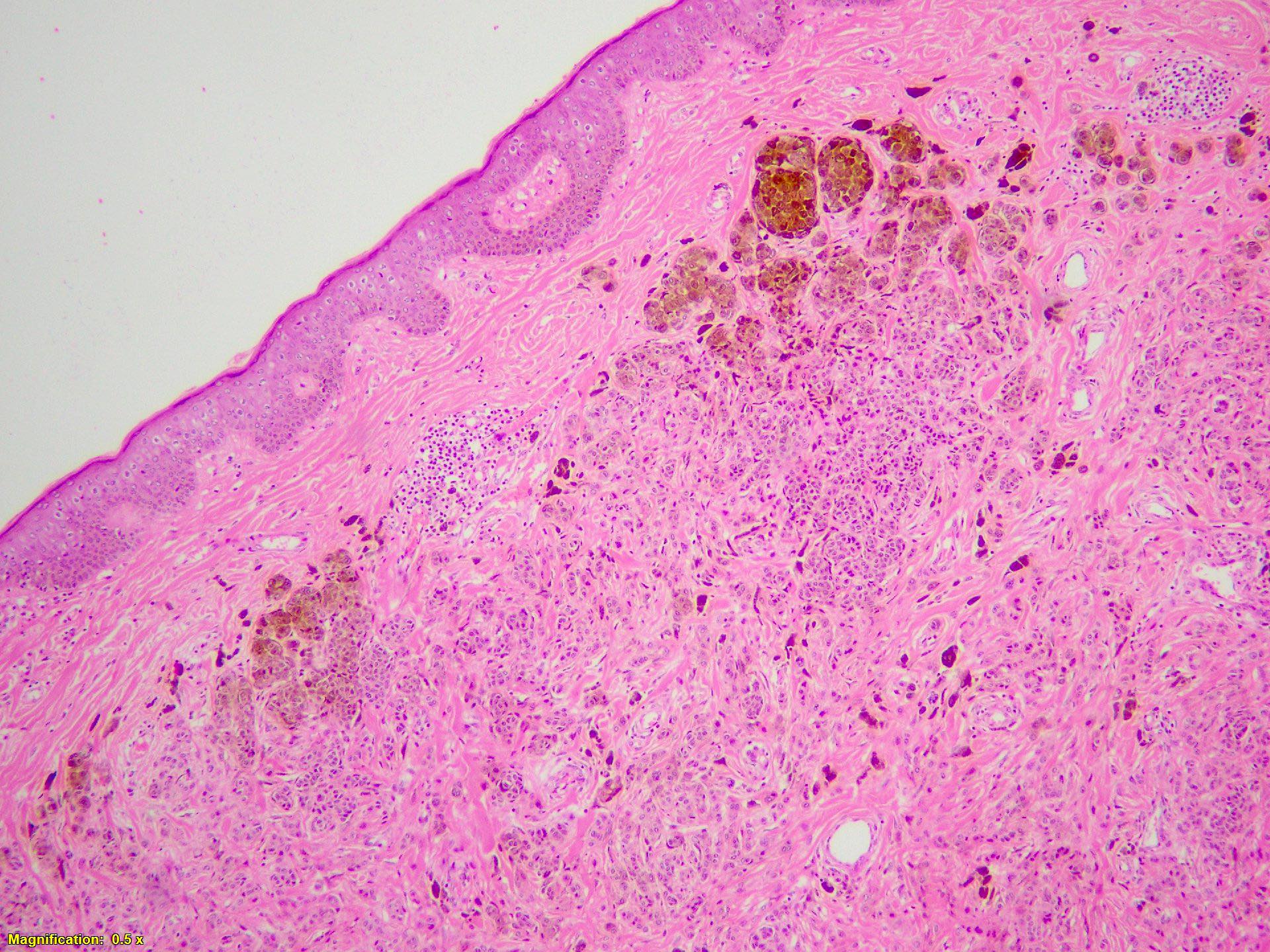
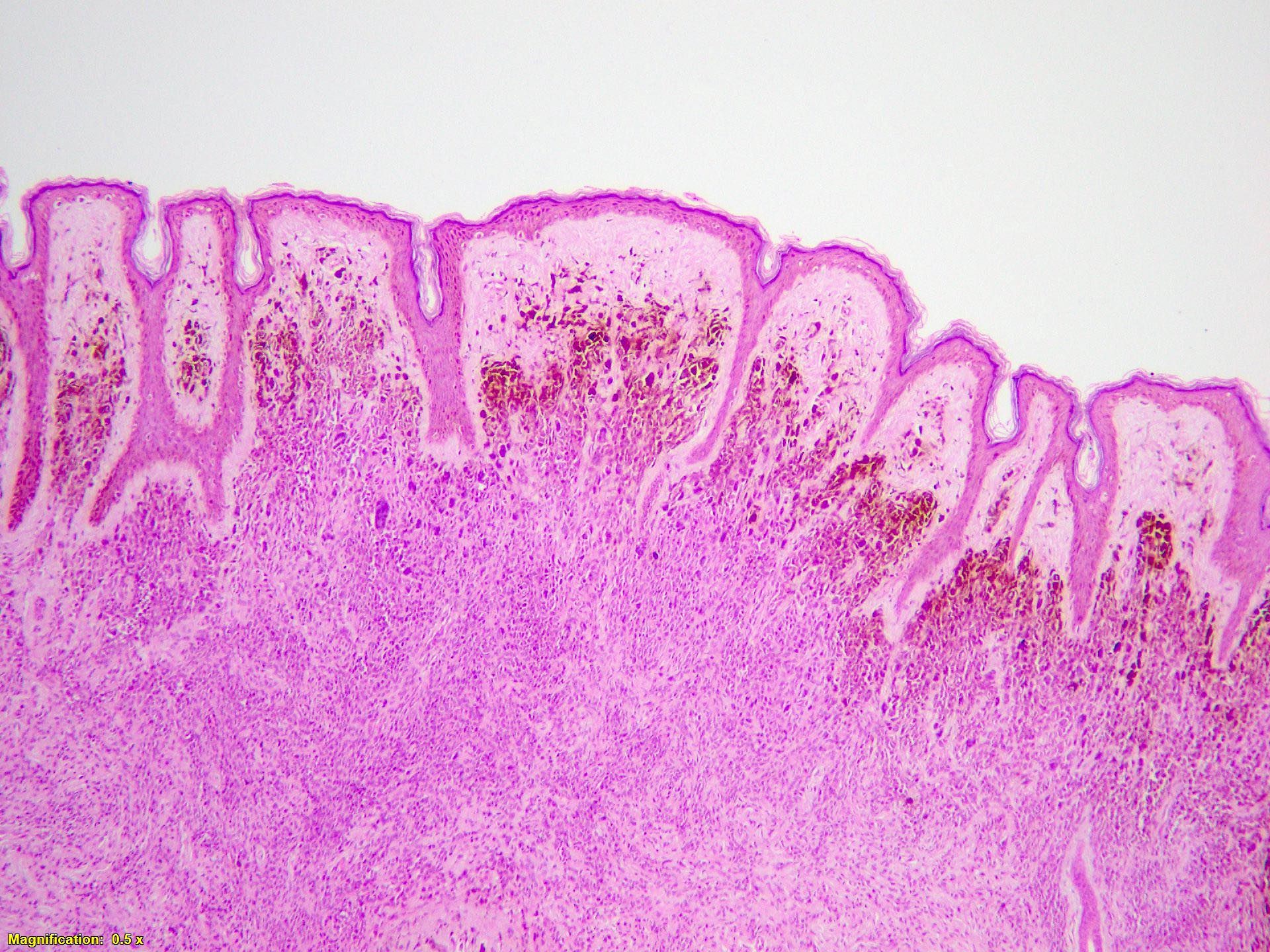
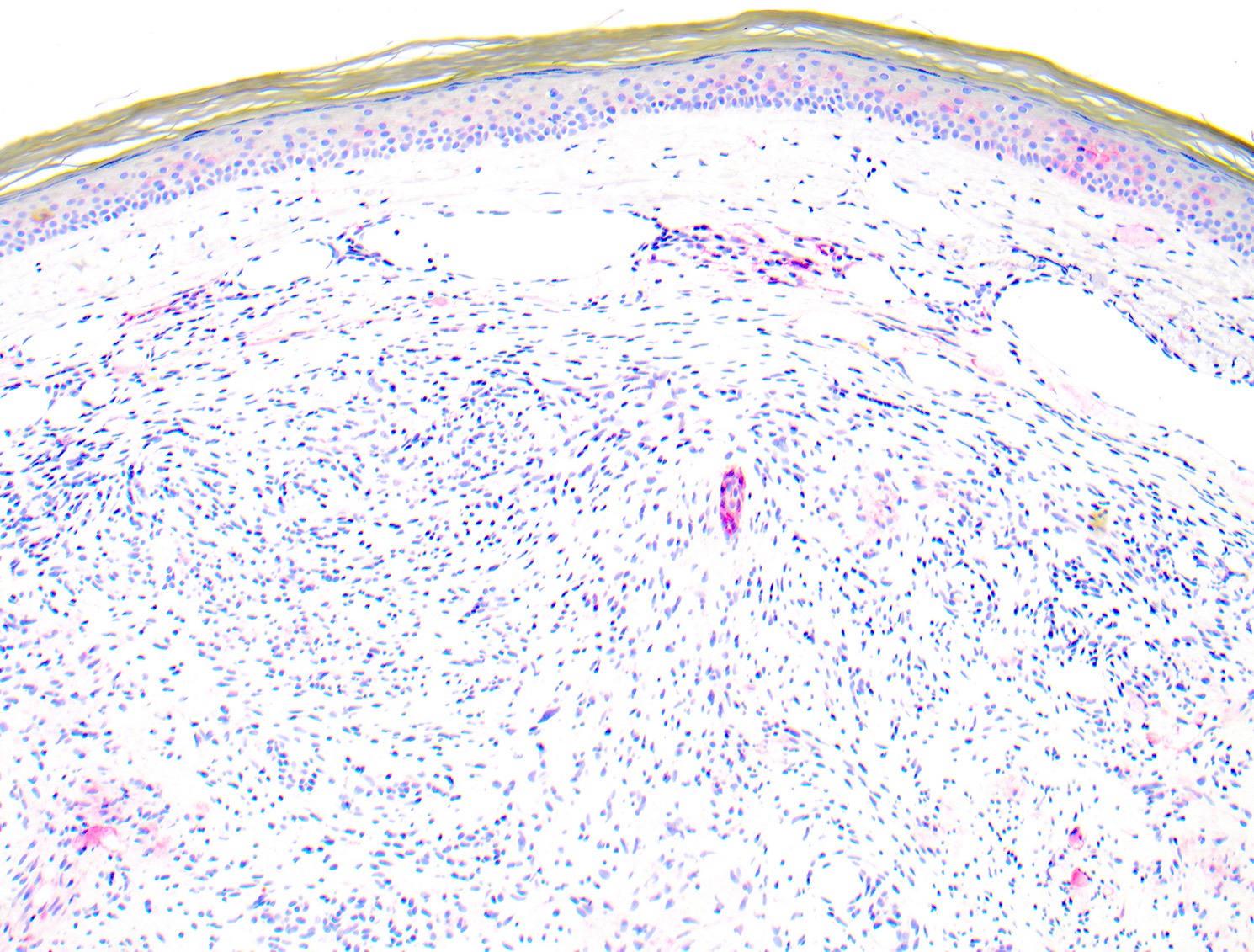
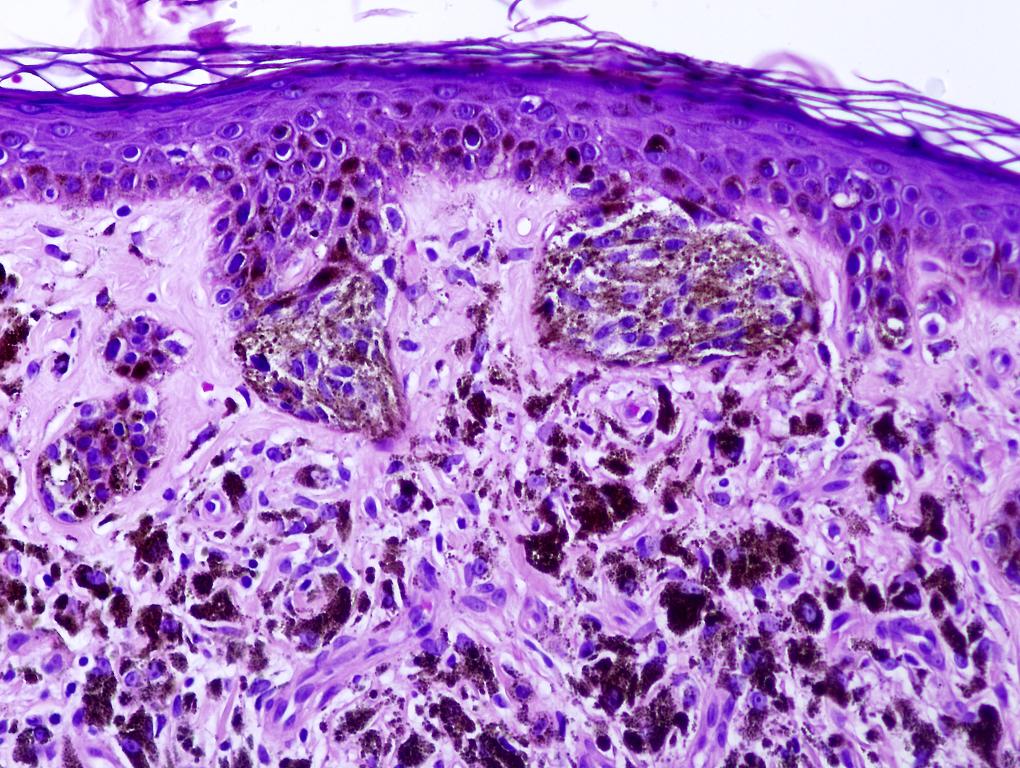
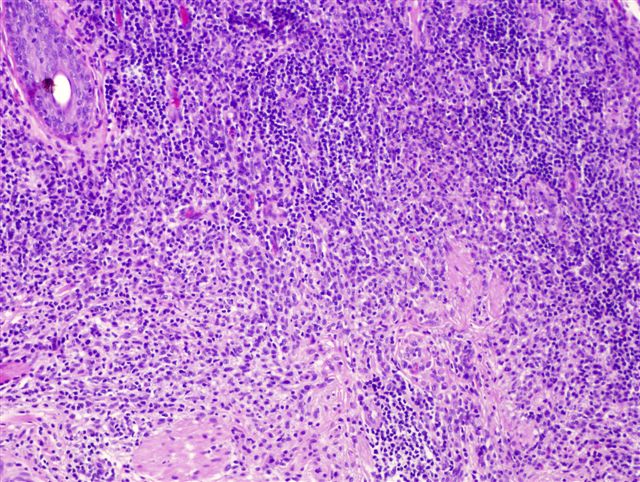
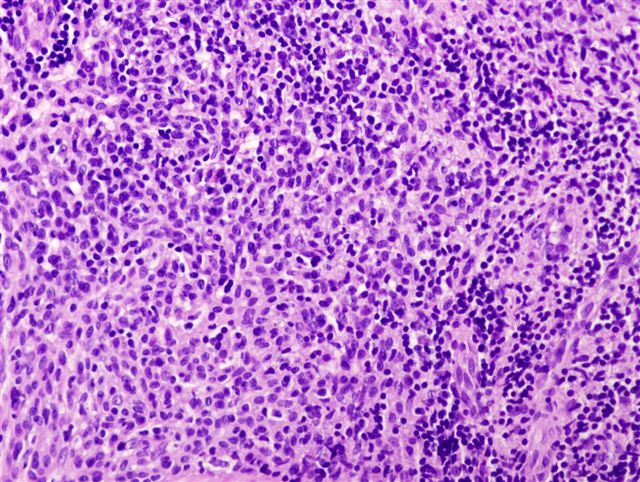
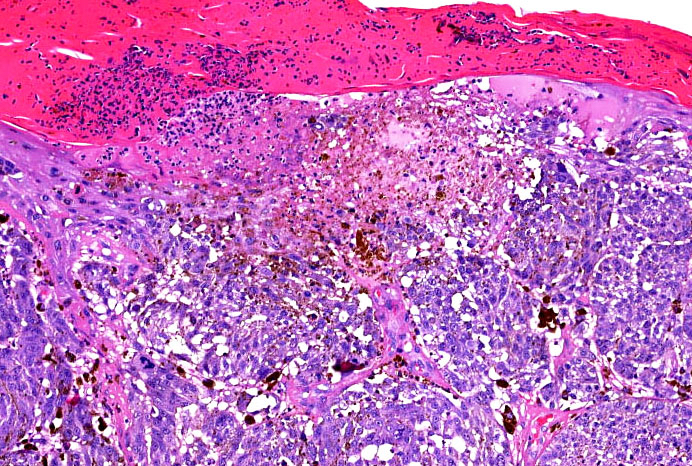
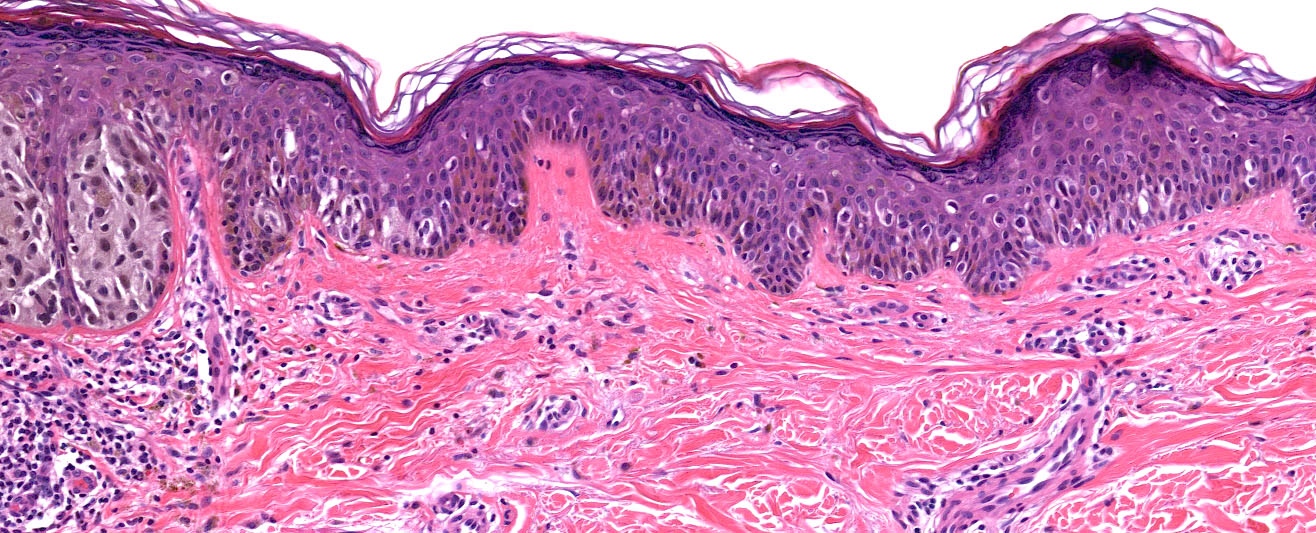
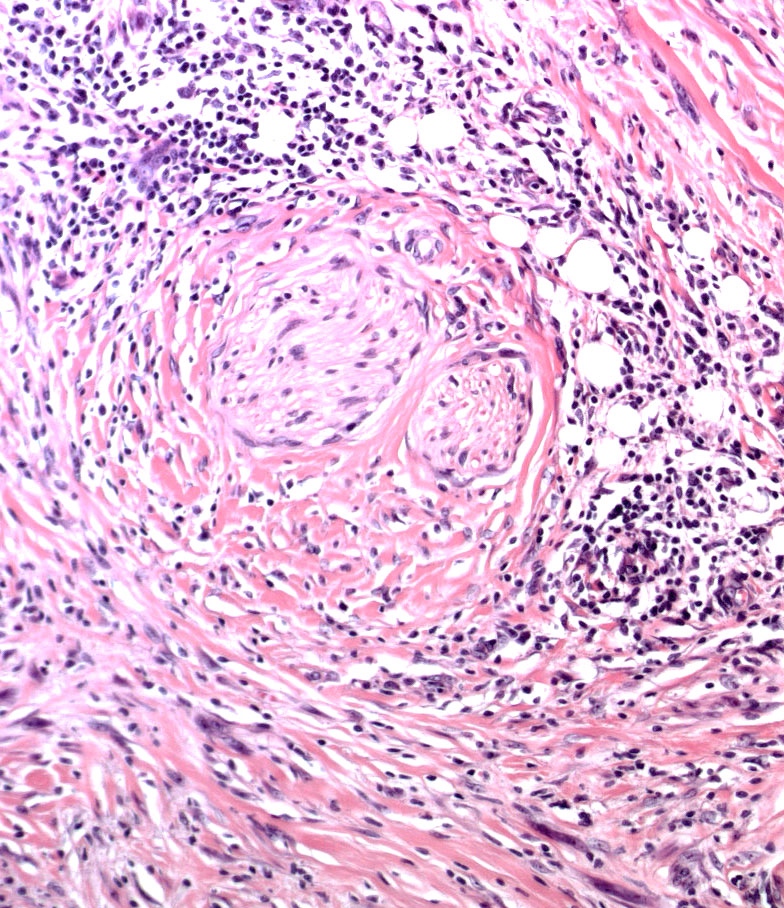
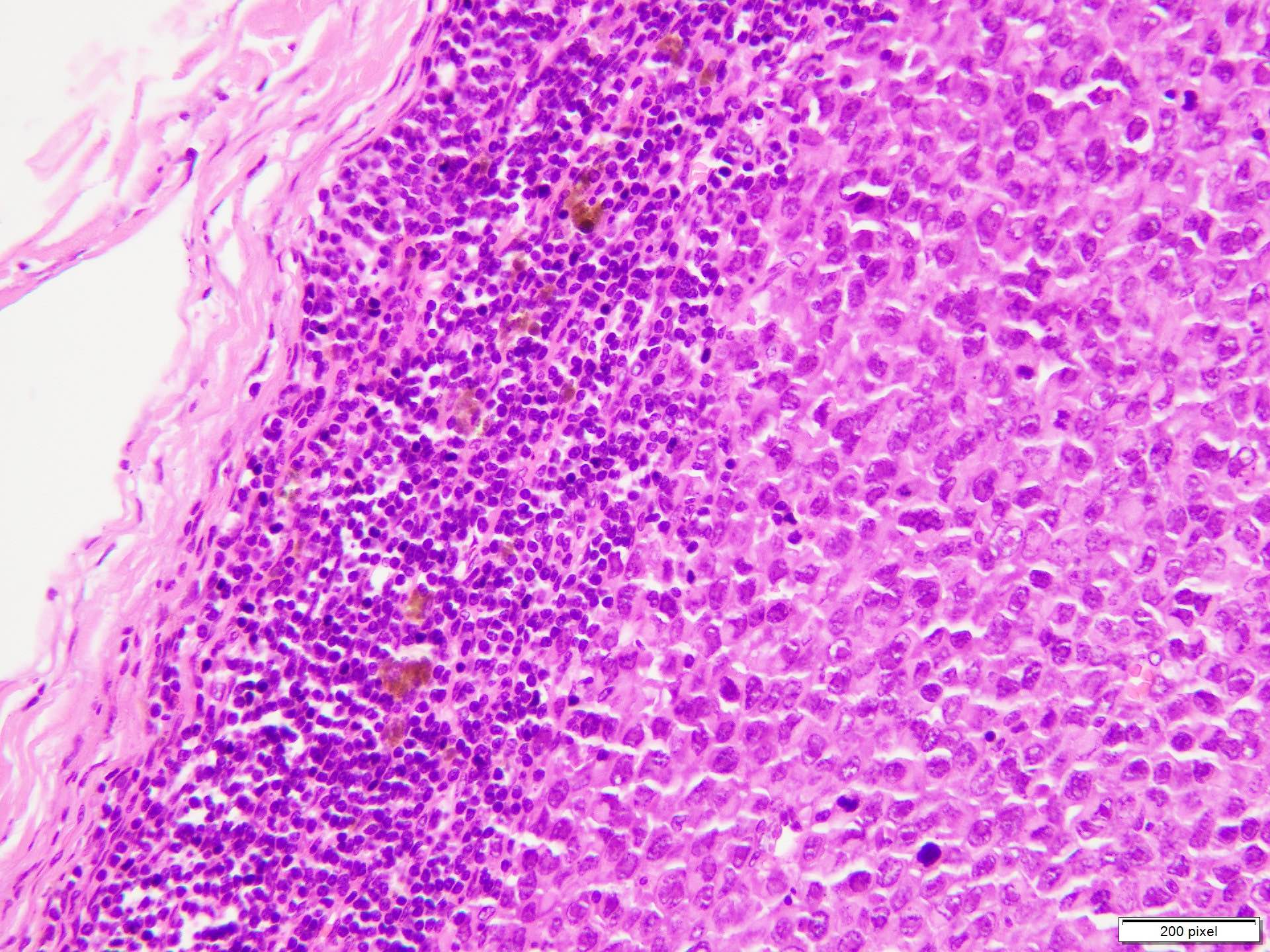
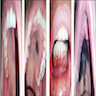
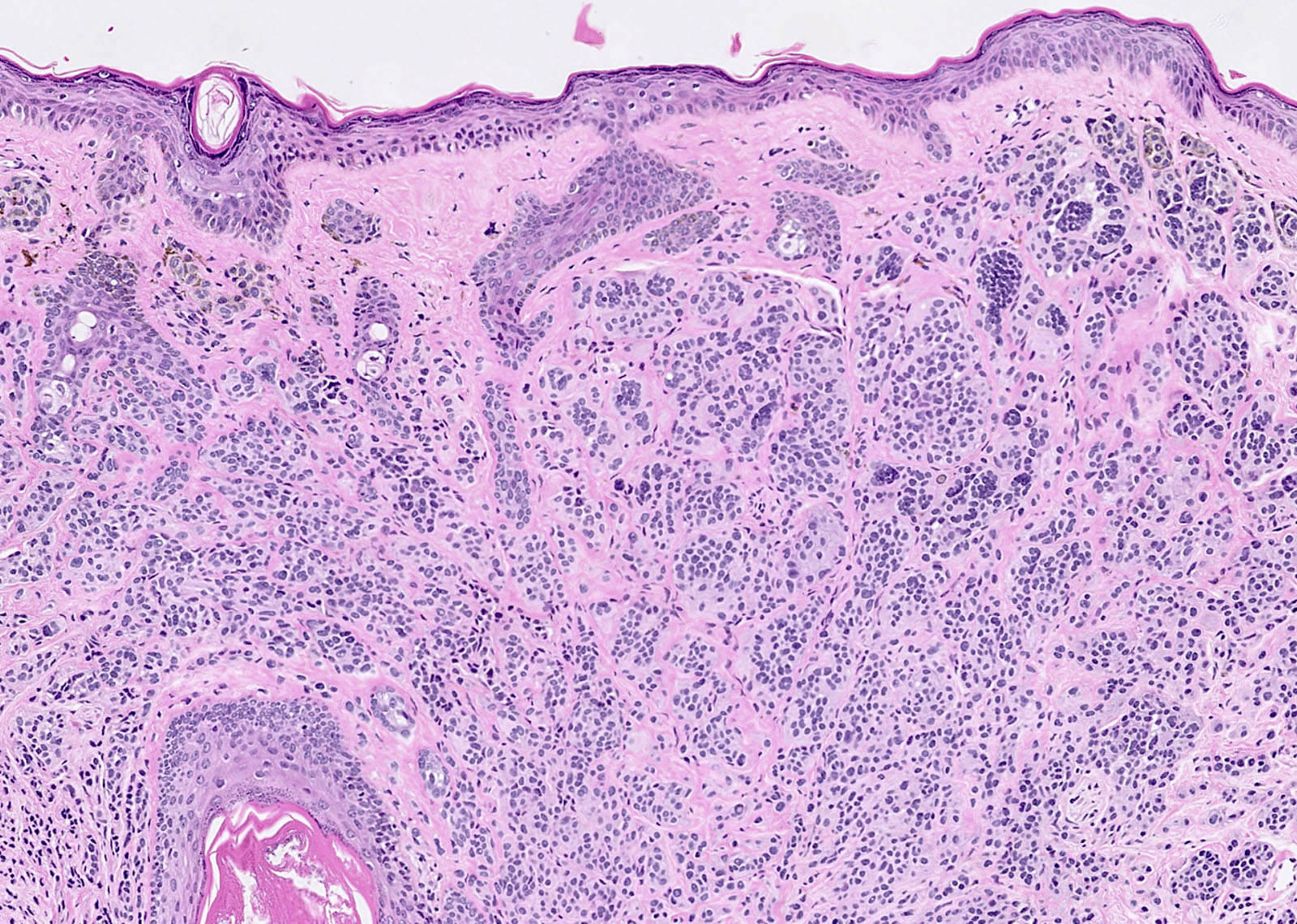
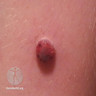
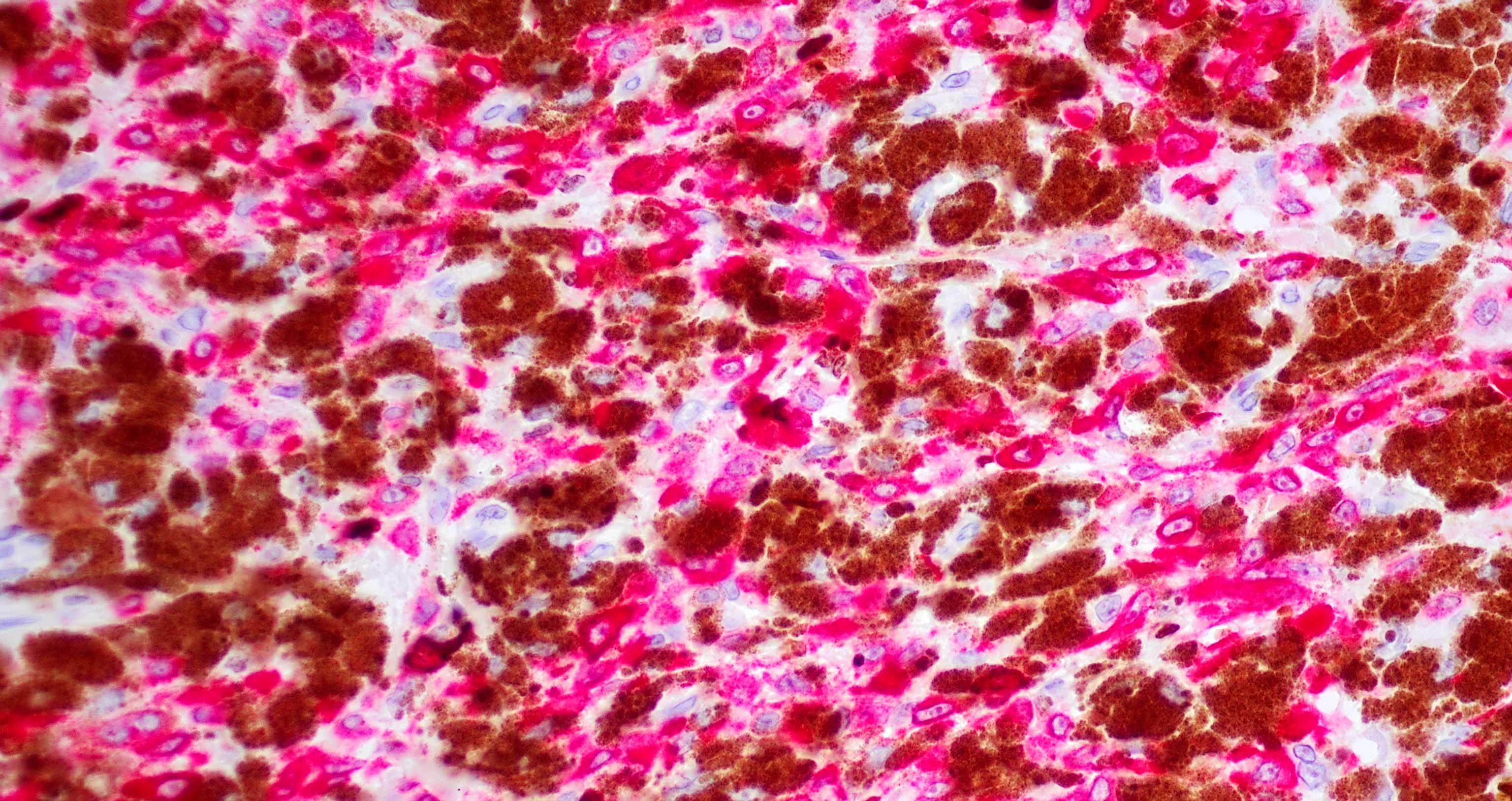
- Ulceration: full thickness epidermal defect including absence of stratum corneum and basement membrane, fibrin deposition and neutrophils and thinning, effacement or reactive hyperplasia of the adjacent epidermis in the absence of a trauma or recent surgical procedure