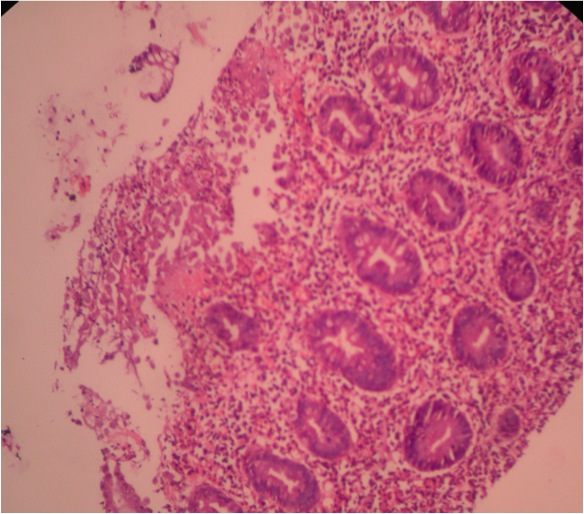
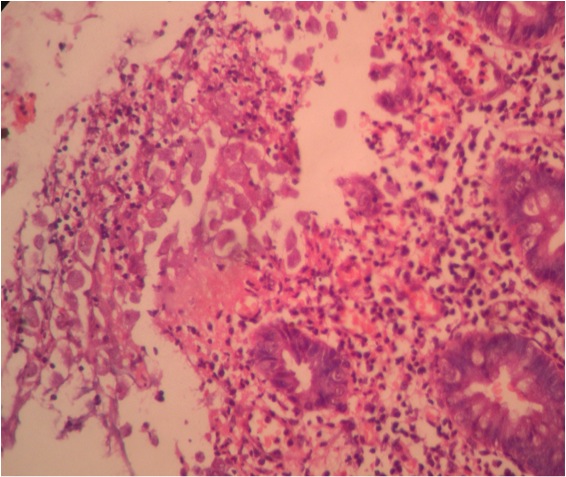
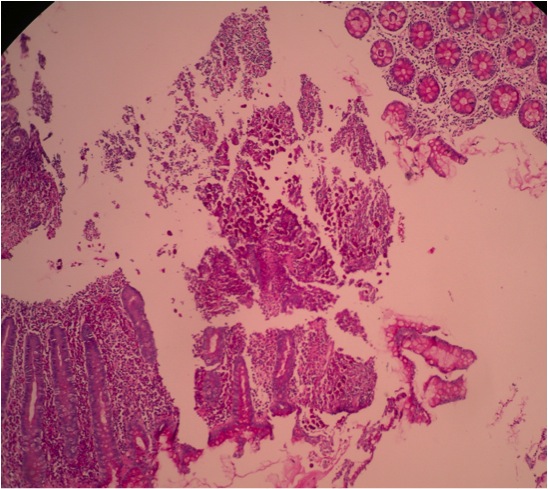
- TX: primary tumor cannot be assessed
- T0: no evidence of primary tumor
- Tis: carcinoma in situ
- T1: tumor limited to Ampulla of Vater of sphincter of Oddi or tumor invades beyond the sphincter of Oddi (perisphincteric invasion) or into the duodenal submucosa
- T1a: tumor limited to Ampulla of Vater of sphincter of Oddi
- T1b: tumor invades beyond the sphincter of Oddi (perisphincteric invasion) or into the duodenal submucosa
- T2: tumor invades into the muscularis propria of the duodenum
- T3: tumor directly invades into the pancreas (up to 0.5 cm) or tumor extends more than 0.5 cm into the pancreas or extends into peripancreatic or periduodenal tissue or duodenal serosa without involvement of the celiac axis or superior mesenteric artery
- T3a: tumor directly invades the pancreas (up to 0.5 cm)
- T3b: tumor extends more than 0.5 cm into the pancreas or extends into peripancreatic tissue or periduodenal tissue or duodenal serosa without involvement of the celiac axis or superior mesenteric artery
- T4: tumor involves the celiac axis, superior mesenteric artery or common hepatic artery, irrespective of size
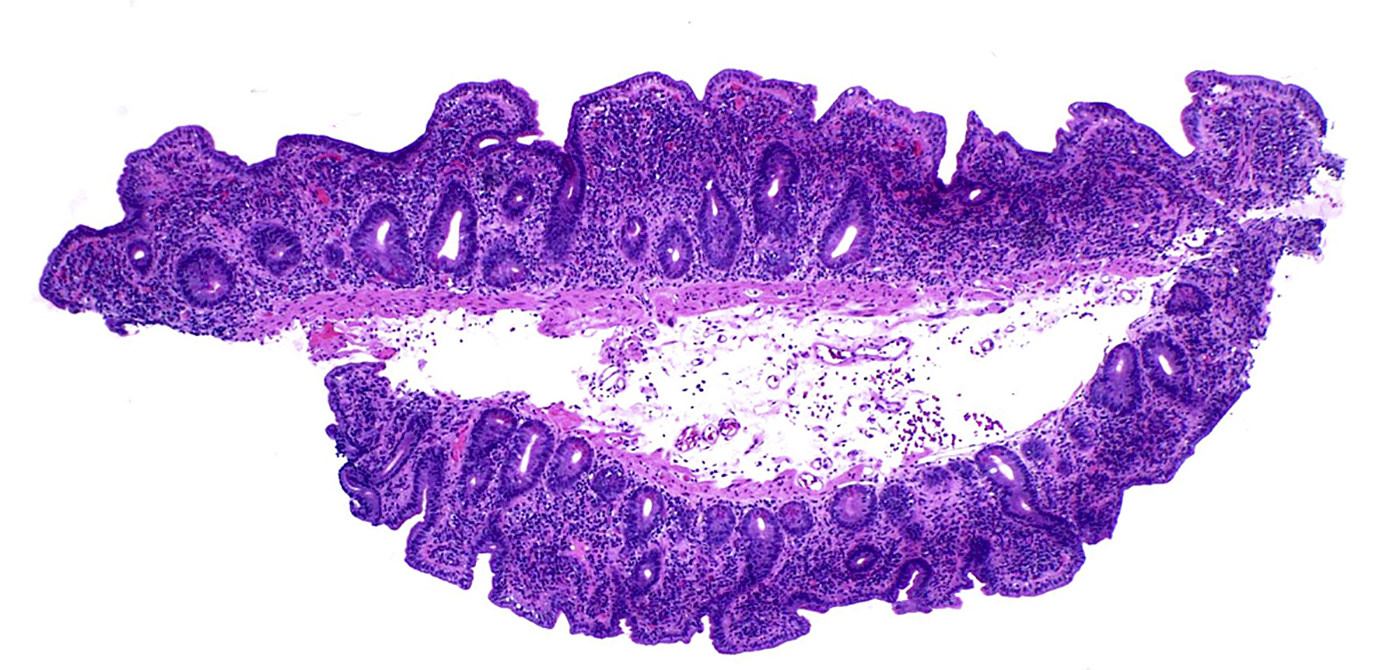
- Uncommon epithelial malignancy with glandular or mucinous differentiation that has an epicenter in the ampulla of Vater and displays an intestinal, pancreatobiliary or mixed phenotype
- While advanced duodenal, distal common bile duct or pancreatic carcinoma may extend to involve the ampulla, only those malignancies centered on or circumferentially surrounding the ampulla are regarded as ampullary carcinomas
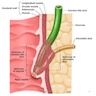
- Ampulla of Vater is a complex anatomical region that represents the junction of duodenal and pancreatobiliary type mucosa, resulting in a heterogenous group of malignancies that may arise from this site (Am J Surg Pathol 2012;36:1592)
- Distinguishing ampullary / periampullary primaries from duodenal, distal common bile duct and pancreatic ductal primaries is based on careful gross examination to assess the tumor epicenter
- Distinguishing intestinal from pancreatobiliary type tumors is an important prognostic factor; immunostains are helpful adjuncts to morphologic assessment (Int J Surg Pathol 2019;27:598)
- ICD-O
- ICD-11: 2B80.20 - adenocarcinoma of small intestine, site unspecified
- Rare, with an incidence of 4 - 10 cases/1 million population (Eur J Gastroenterol Hepatol 2000;12:75, J Surg Oncol 2009;100:598, Cancer Causes Control 2007;18:415, Cancer 2019;125:1489)
- M:F = 1.48:1 in one large series (J Surg Oncol 2009;100:598, Am J Surg Pathol 2012;36:1592)
- Commonly presents between the ages of 60 and 80 (median: 65 years) (J Surg Oncol 2009;100:598, Am J Surg Pathol 2012;36:1592)
- Associated with familial adenomatous polyposis (FAP), Lynch syndrome, Gardner syndrome, Peutz-Jeghers syndrome and neurofibromatosis (Gastroenterology 2001;121:1127, Mod Pathol 2001;14:1169, Gastroenterology 1992;102:1980, Am J Med Genet 1980;6:205)
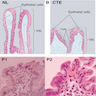
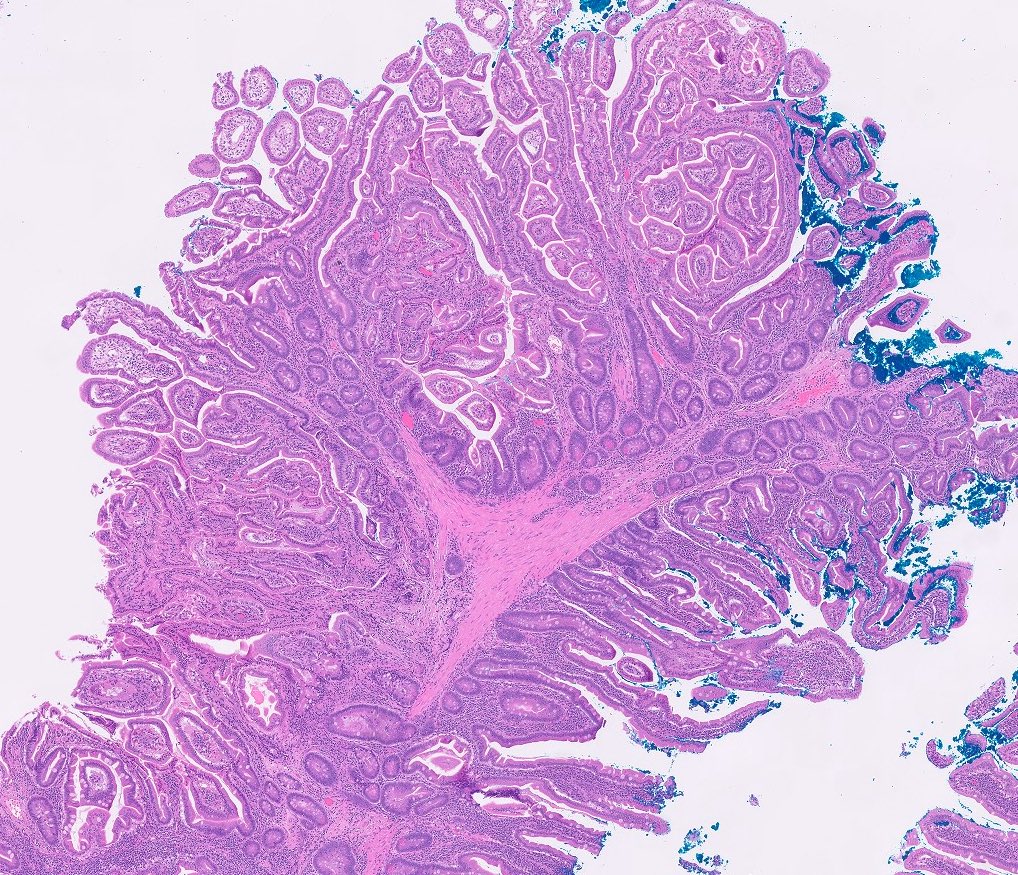
- Ampullary adenocarcinomas have 4 recognized subtypes (Am J Surg Pathol 2012;36:1592)
- Periampullary carcinomas are exophytic masses that arise from the duodenal surface of the ampulla and engulf the ampullary orifice (~5%)
- Intra-ampullary carcinomas arise from intra-ampullary papillary tubular neoplasms (IAPN, ~25%)
- Ampullary ductal carcinomas arise from the portions of the distal common bile or pancreatic ducts located within the papilla of Vater and circumferentially involve the duct within the papilla (~15%)
- Ampullary carcinomas, NOS are ulceronodular tumors located at the papilla of Vater that do not show the specific features of the 3 categories listed above (~55%)
- Experts theorize that the tendency for small bowel adenocarcinomas as well as familial adenomatous polyposis related adenocarcinomas to occur in the ampullary region may be related to exposure to bile and pancreatic secretions
- Known precursor lesions include (Am J Surg Pathol 2012;36:1592)
- Intra-ampullary adenocarcinomas: intra-ampullary papillary tubular neoplasms
- Periampullary adenocarcinoma: intestinal type adenoma
- Ampullary ductal adenocarcinomas: some tumors show intraepithelial neoplasia; however, this is difficult to distinguish from colonization of the surface epithelium by invasive carcinoma cells
- Ampullary carcinoma, NOS: precursor lesion unclear
- For the vast majority of tumors, a specific etiology cannot be determined (Am J Surg Pathol 2012;36:1592, Am J Surg Pathol 2010;34:1731, J Clin Oncol 2013;31:1348)
- Rare, accounting for 0.2% of gastrointestinal cancers and 6 - 9% of periampullary malignancies (Am Soc Clin Oncol Educ Book 2014:112)
- Patients often present with persistent jaundice, abdominal pain, pancreatitis or weight loss
- Overall 5 year survival is 39 - 45% for patients who undergo resection (Hepatobiliary Pancreat Dis Int 2018;17:443, J Surg Oncol 2009;100:598, Am J Surg Pathol 2012;36:1592)
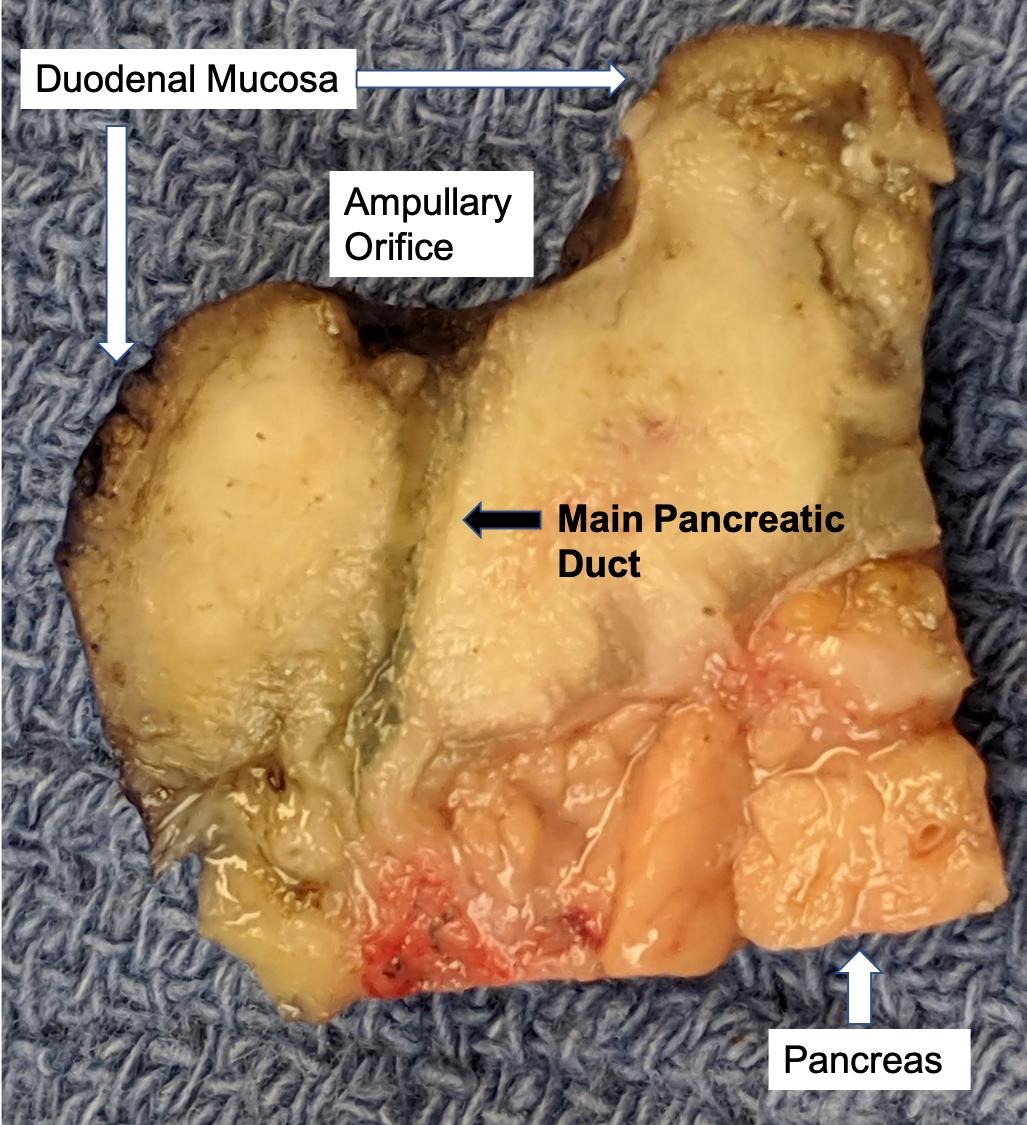
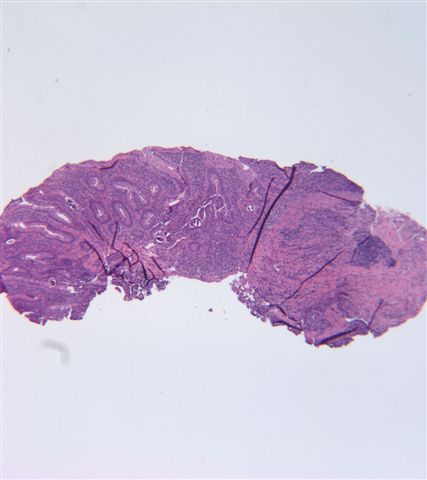
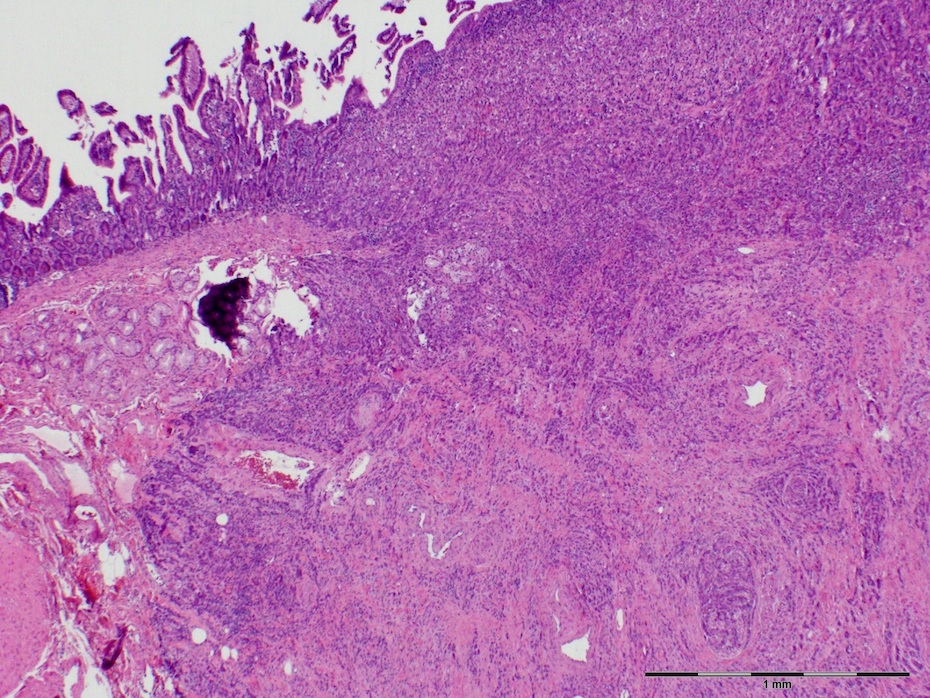
- Confirmation that a tumor is an ampullary carcinoma is best done on careful gross assessment of the resection specimen to determine the tumor epicenter (Am J Surg Pathol 2012;36:1592)
- It is also important to look for precursor lesions that can support the origin of a periampullary tumor: pancreatic intraepithelial neoplasms for pancreatic cancer, intestinal adenoma for duodenal cancer, biliary intraepithelial neoplasms or intraductal papillary neoplasms for biliary (distal) cancer (see Pathophysiology) (Am J Surg Pathol 2012;36:1592)
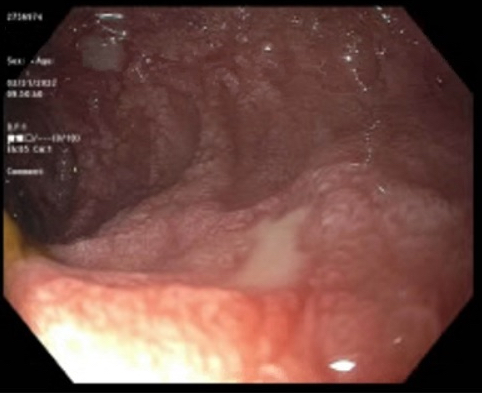
- Endoscopic retrograde cholangiopancreatography (ERCP) is the most useful endoscopic study for diagnosing ampullary carcinoma; it permits tumor identification, biopsy and biliary decompression with a single procedure (Am J Surg 1997;174:355)
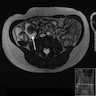
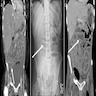
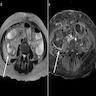
- MRI plus magnetic resonance cholangiopancreatography (MRCP) has shown good performance in differentiating between malignant and benign ampullary lesions (BMC Med Imaging 2019;19:77)
- MRI diagnostic accuracy for ampullary lesions has been reported to be as high as 91.17% (BMC Med Imaging 2019;19:77)
- Ampullary adenocarcinomas with pancreaticobiliary histology have a much worse outcome than those with intestinal histology (J Clin Oncol 2013;31:1348, J Am Coll Surg 2008;207:210, BMC Cancer 2013;13:428, World J Surg Oncol 2022;20:406)
- Decreased overall survival is associated with advanced age, tumor grade, tumor size, lymph node ratio, higher stage disease (pT3 - 4), lymph node metastasis, lymphovascular invasion, higher histologic grade tumors, perineural invasion and elevated serum CA 19-9 and CEA levels (Int J Radiat Oncol Biol Phys 2006;66:514, Ann Surg Oncol 2019;26:1079, J Am Coll Surg 2008;207:210, Am J Surg 2000;180:13, Br J Surg 2004;91:1600, Ann Surg Oncol 2003;10:1176, Pancreas 2019;48:70, J Gastrointest Surg 2008;12:1422)
- Positive prognostic indicators include serum bilirubin of 75 micromol/L or less and age < 70 years (Br J Surg 2004;91:1600)
- Prognosis (survival) by subtype: periampullary carcinoma > intra-ampullary > ampullary, NOS > ampullary ductal carcinoma (Am J Surg Pathol 2012;36:1592)
- 11 year old boy presented with obstructive jaundice due to ampullary adenocarcinoma (youngest patient reported) (J Pediatr Surg 1997;32:636)
- 45 year old man who presented with abdominal pain, nausea, vomiting and a fever was found to have ampullary adenocarcinoma (Cureus 2022;14:e29398)
- 58 year old man with familial adenomatous polyposis presented with ampullary adenocarcinoma (Bratisl Lek Listy 2019;120:908)
- 74 year old man who presented with decompensated cirrhosis and choledocholithiasis associated with an ampullary adenocarcinoma (Cureus 2023;15:e37566)
- 77 year old man who presented with 2 rare synchornous primaries: ampullary adenocarcinoma and ileal gastrointestinal stromal tumor (World J Gastrointest Oncol 2022;14:2253)
- Management of early stage disease is primarily surgical, typically pancreatoduodenectomy (Whipple procedure) followed by adjuvant chemoradiation (Am Soc Clin Oncol Educ Book 2014:112)
- Curative resection rates for early stage disease are as high as 80 - 90% with modern techniques at high volume centers (Am Surg 1999;65:1043, J Surg Oncol 2009;100:651)
- Local resection / ampullectomy is sometimes an option for patients who are poor surgical candidates / have significant comorbidities and is reported to offer lower surgical morbidity; however, recurrence rates are higher (Dig Surg 2003;20:511, Ann Surg Oncol 2005;12:971, Ann Surg 1996;224:621)
- Unresectable disease is treated systemically with gemcitabine combined with a platinum compound plus radiation therapy (Am Soc Clin Oncol Educ Book 2014:112, N Engl J Med 2010;362:1273)
- Small retrospective studies have suggested that patients with pancreatobiliary type carcinomas may benefit from gemcitabine therapy while those with intestinal type tumors may benefit from 5-fluorouracil (5-FU) based regimens (BMC Cancer 2008;8:170, Am J Surg Pathol 2014;38:1371)
- A large, multicenter European study group for pancreatic cancer (ESPAC)-3 periampullary randomized controlled trial demonstrated no significant overall survival benefit from adjuvant chemotherapy (fluorouracil or gemcitabine) (JAMA 2012;308:147)
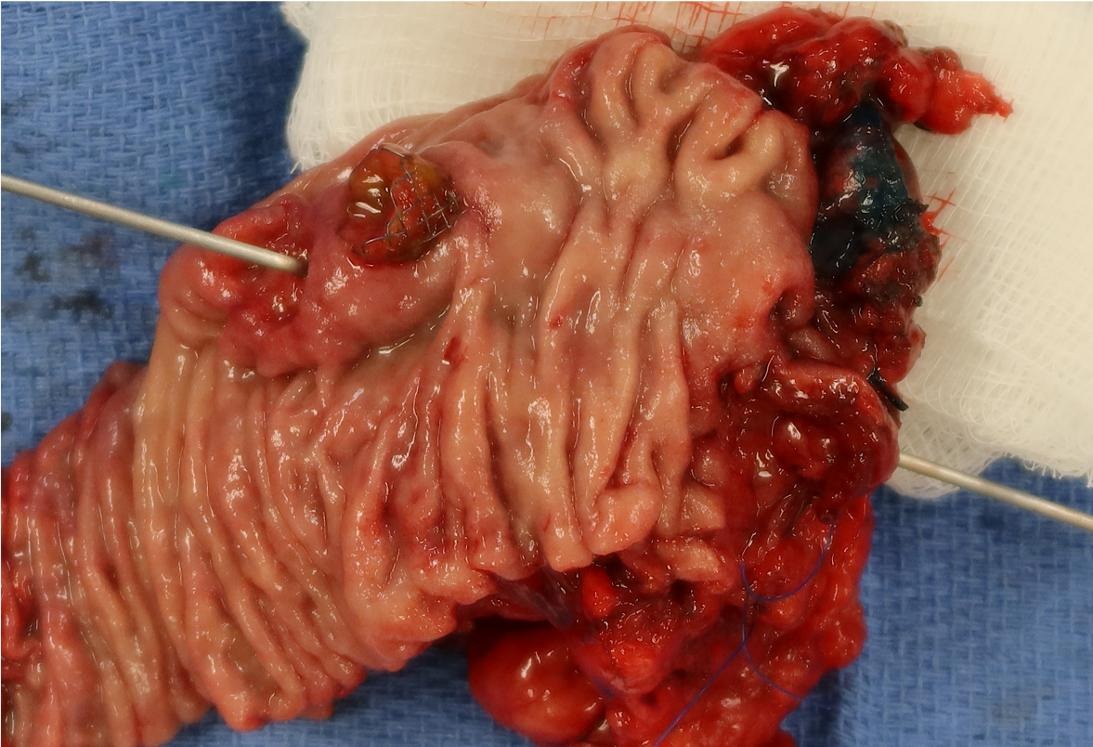
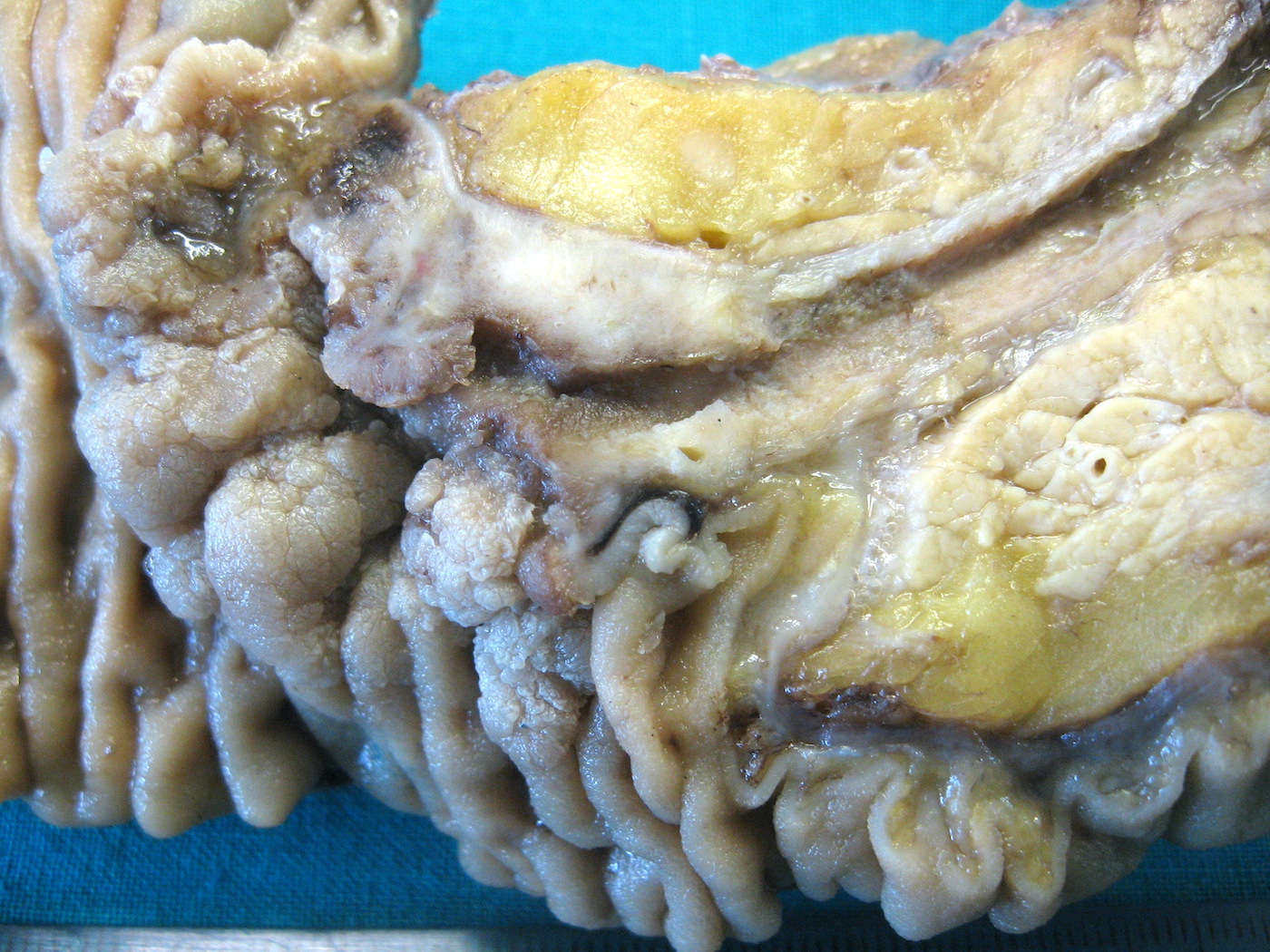
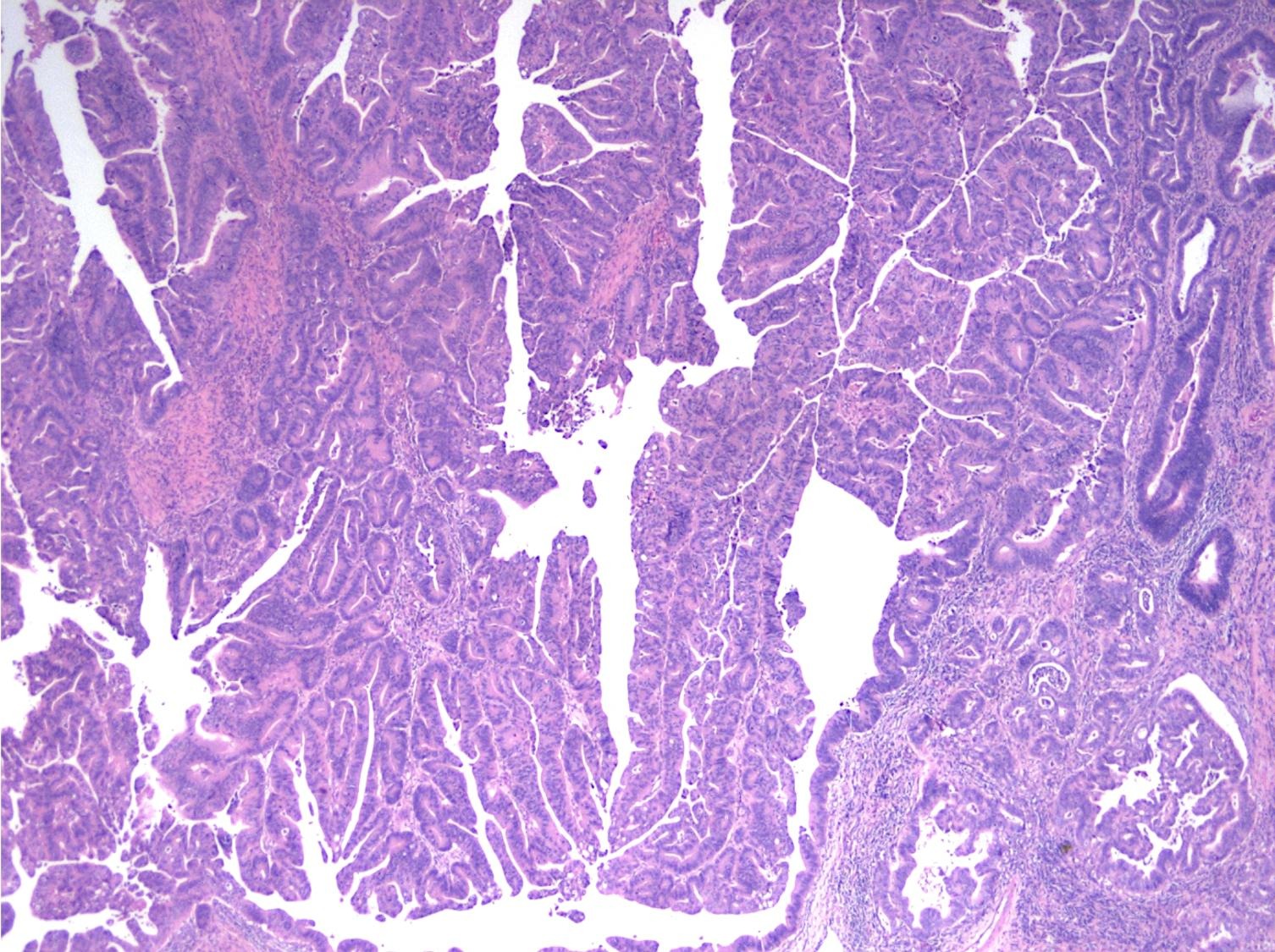
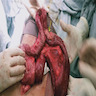
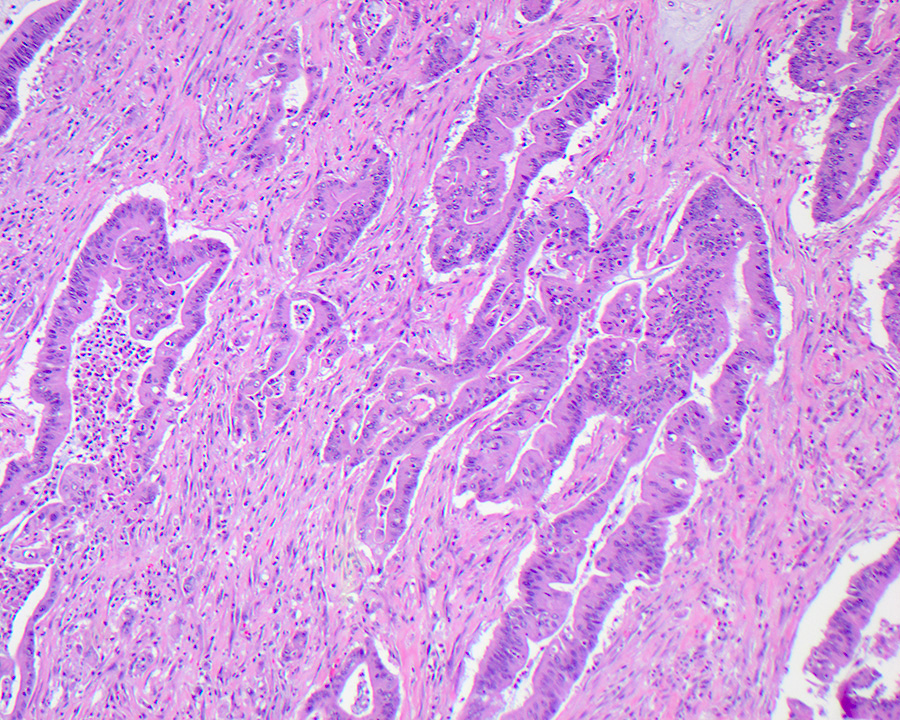
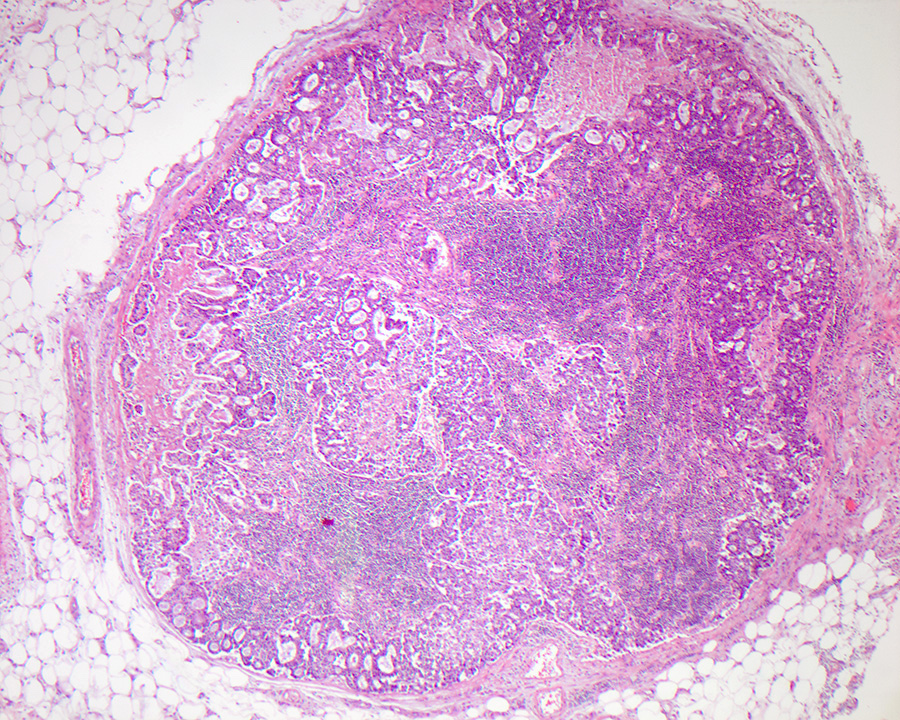
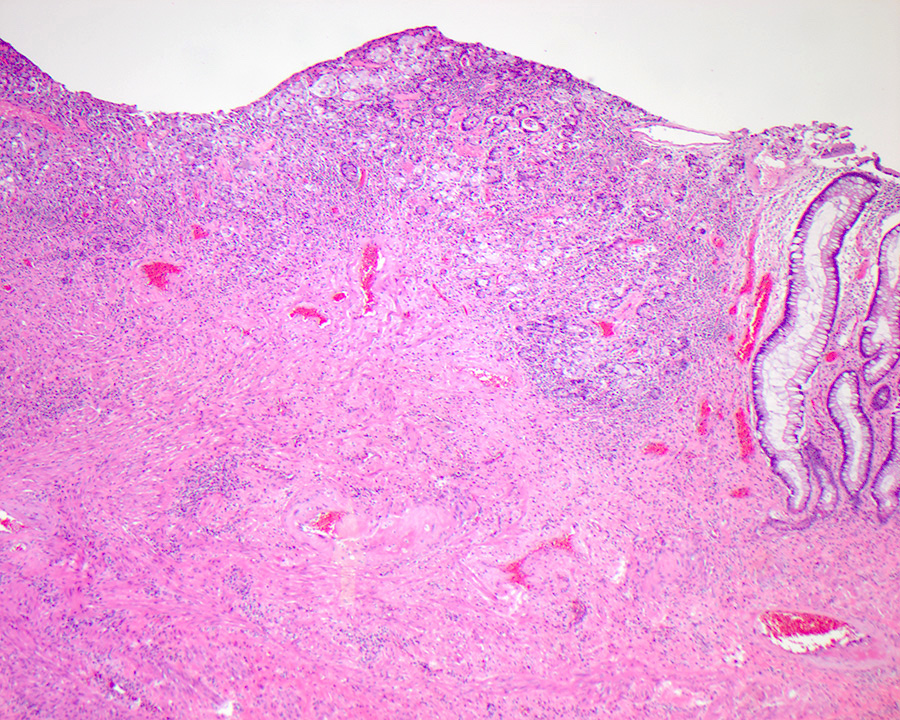
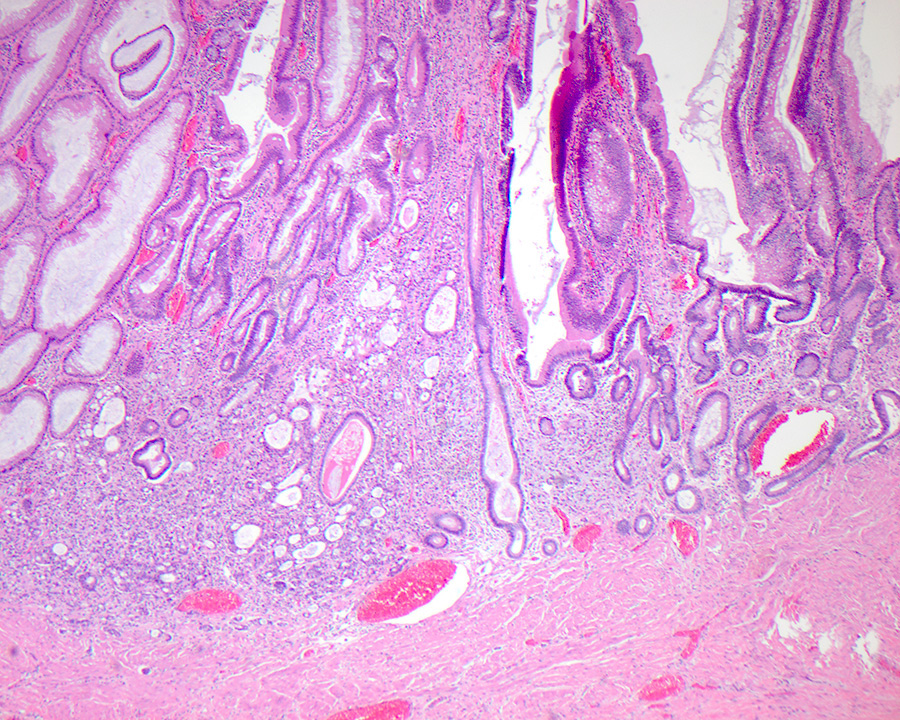
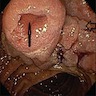
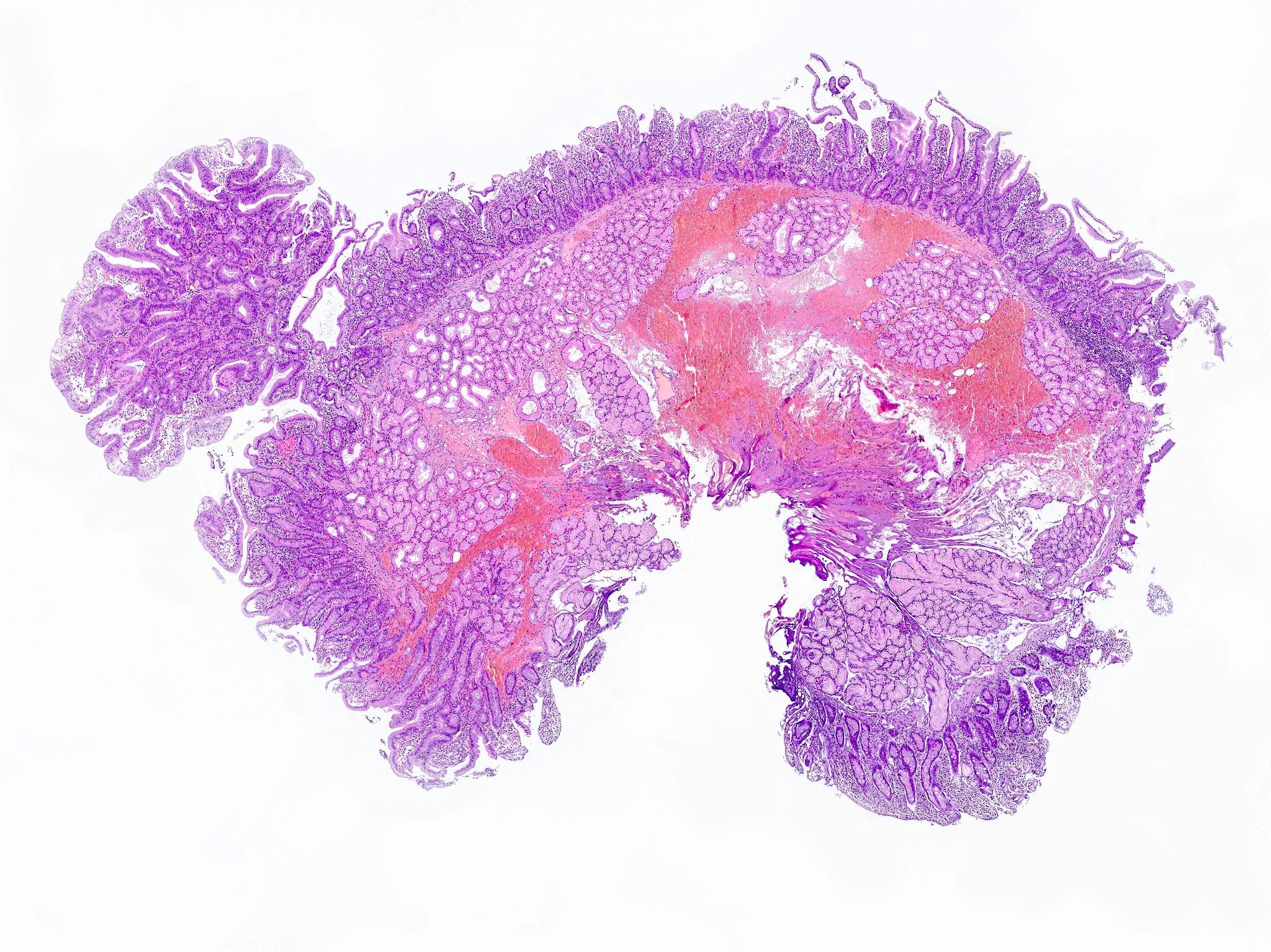
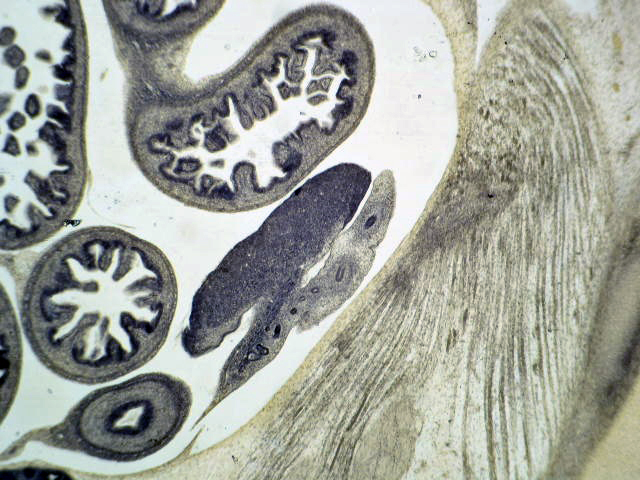
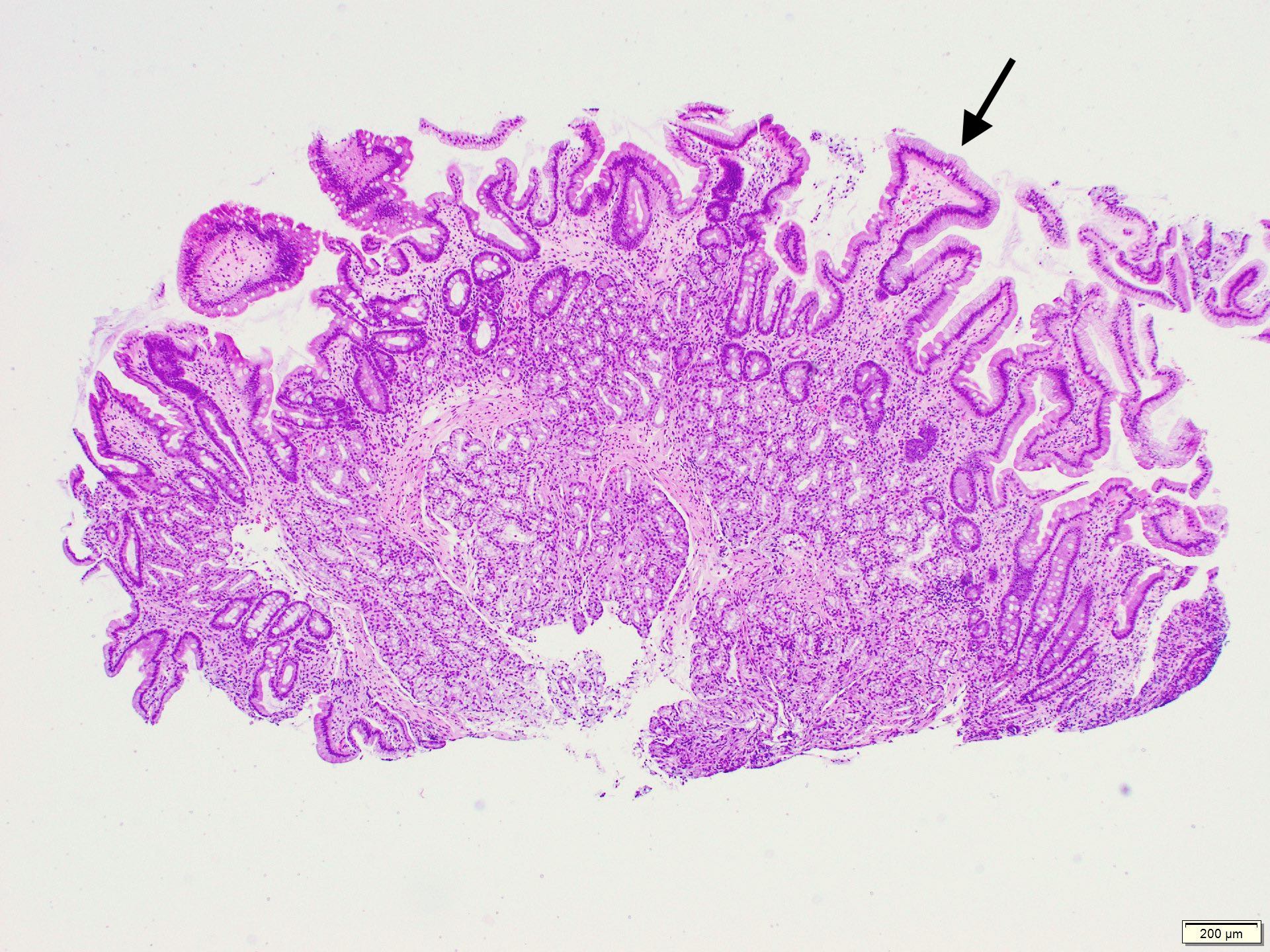
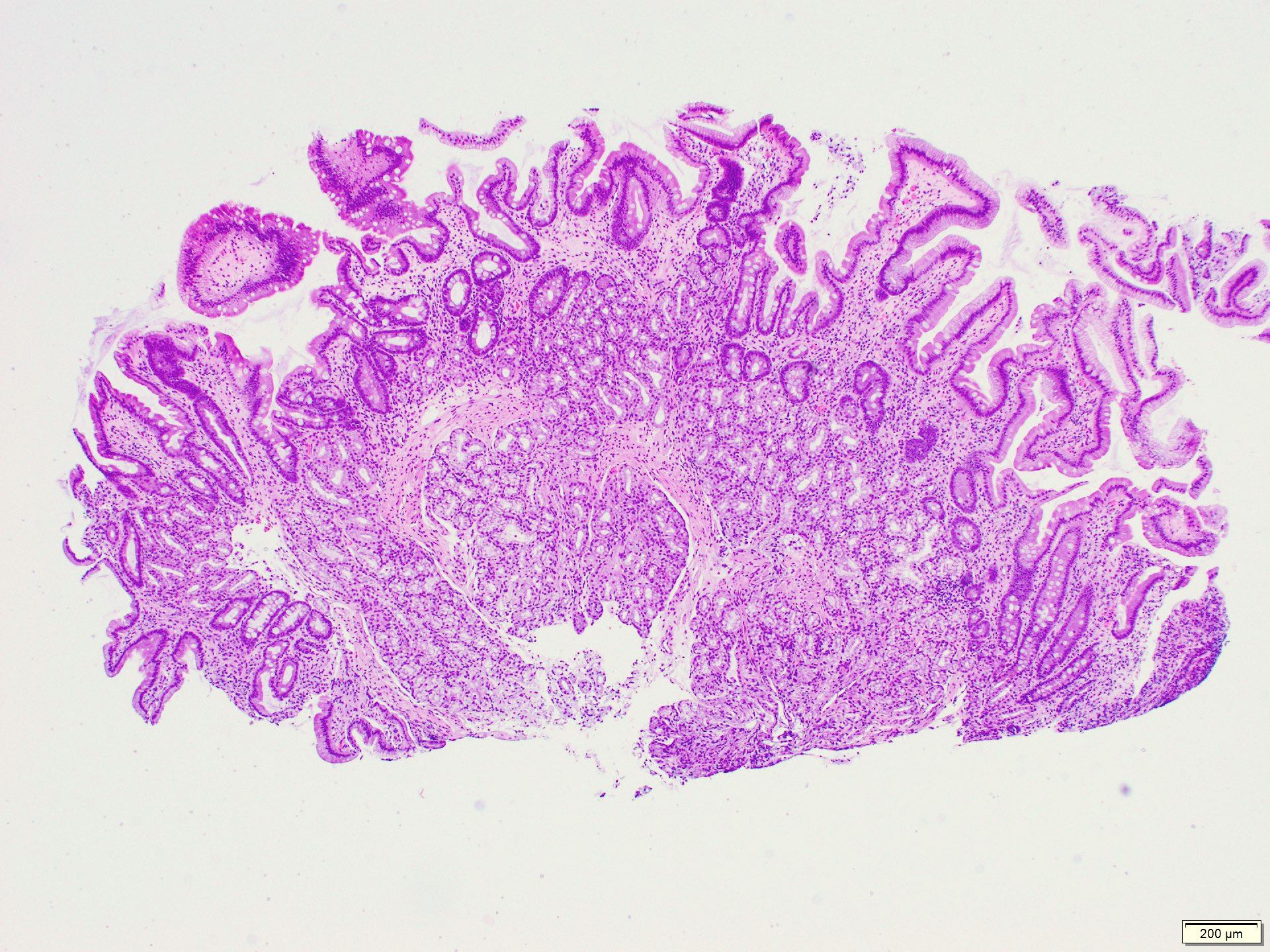
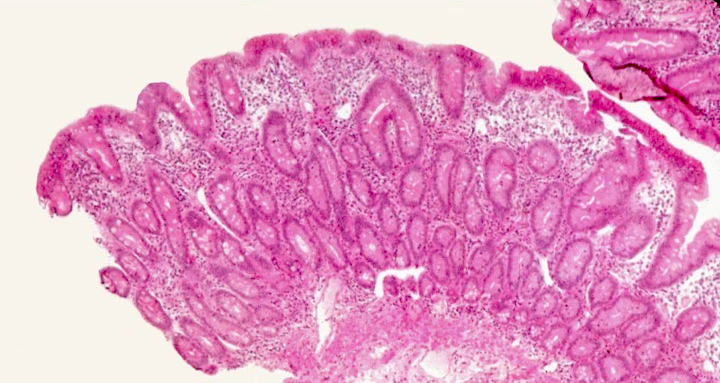
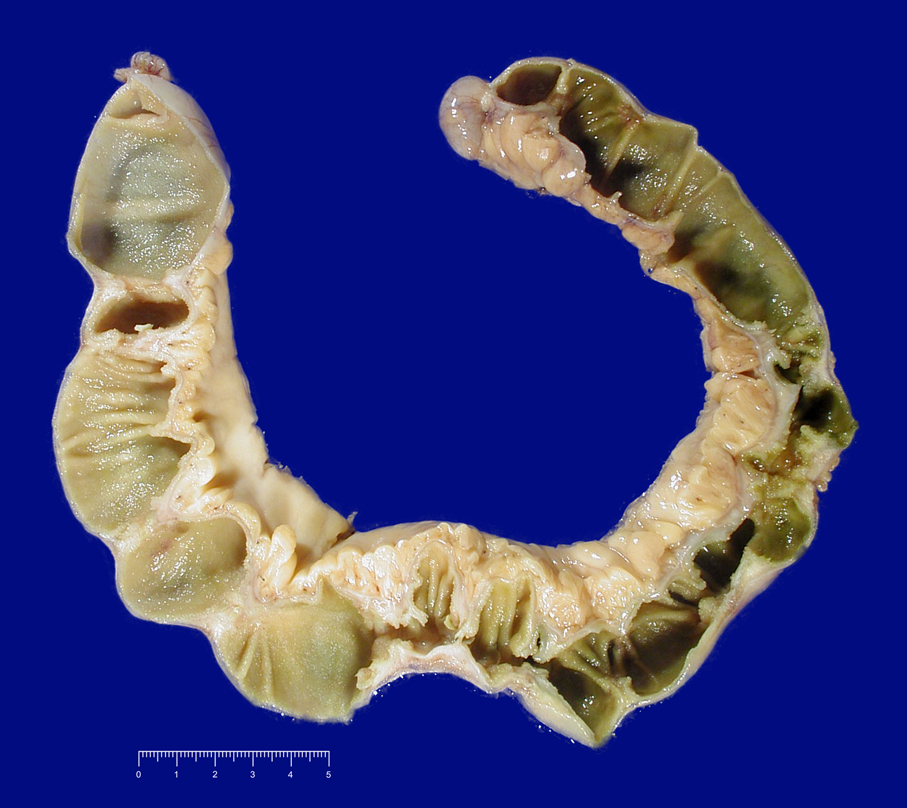
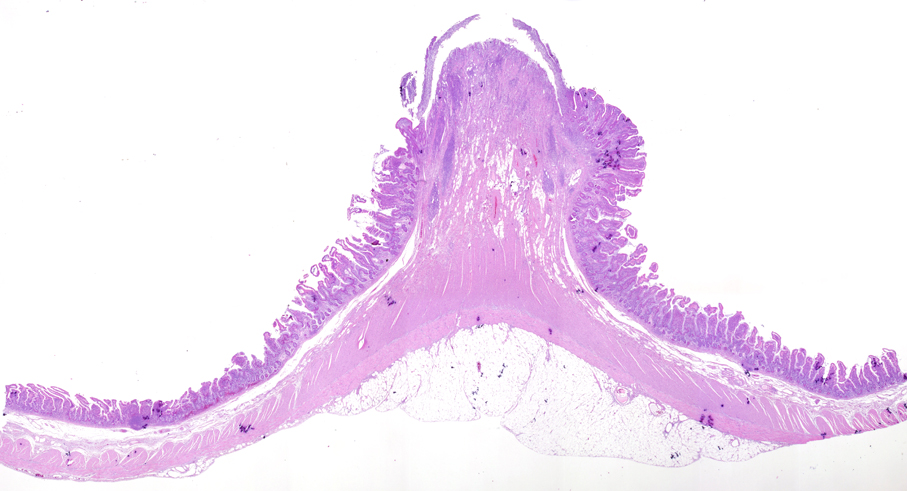
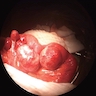
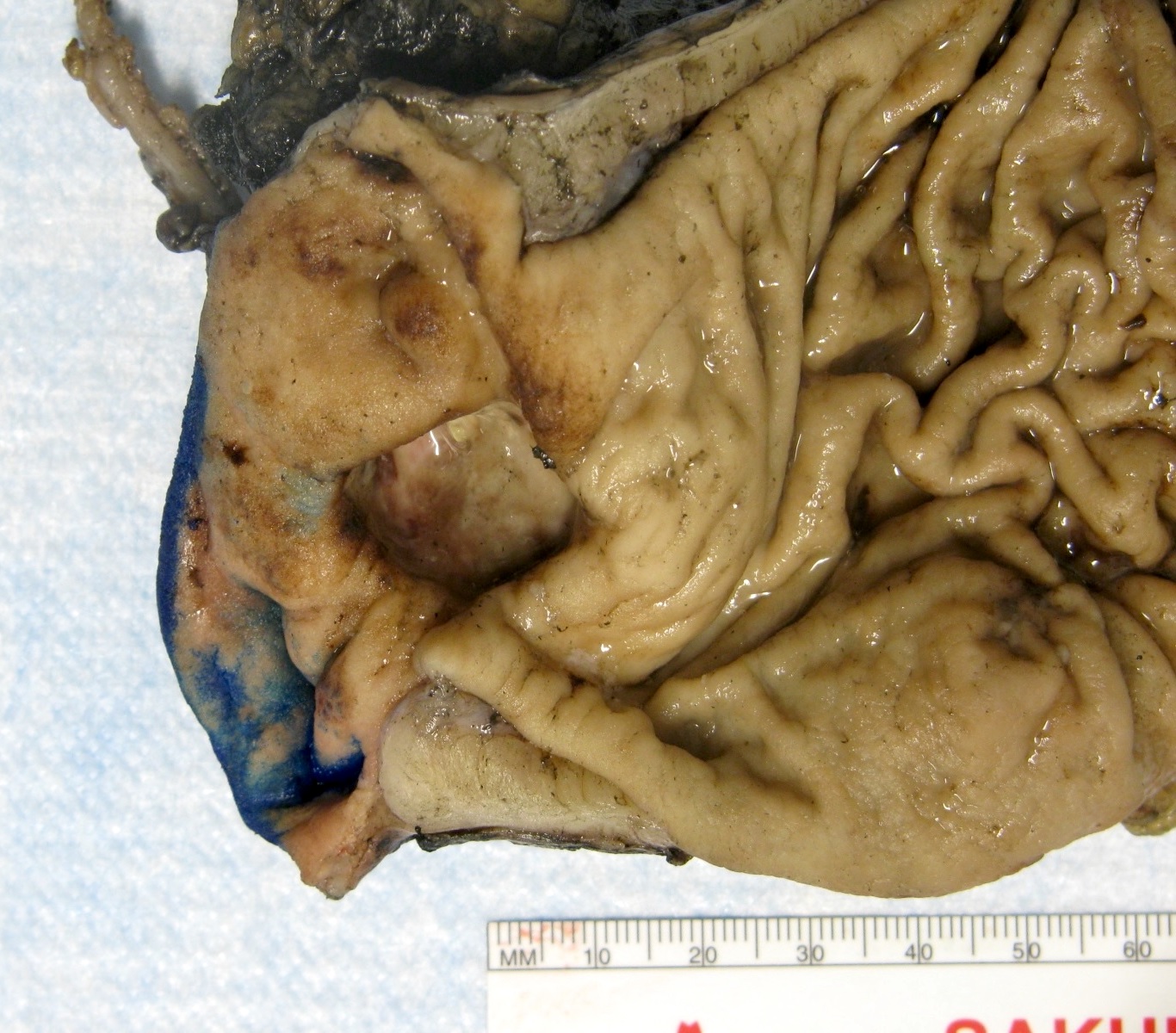
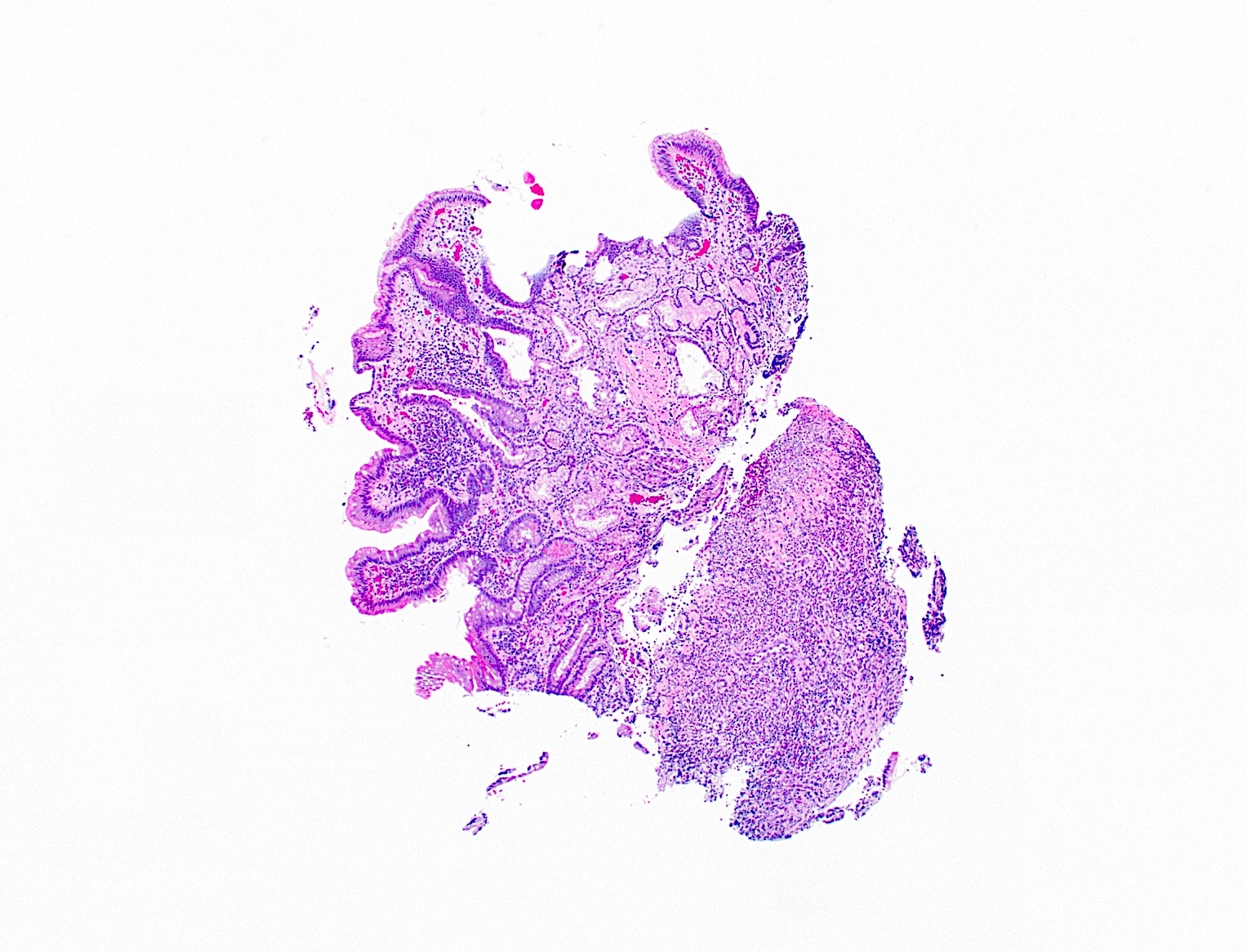
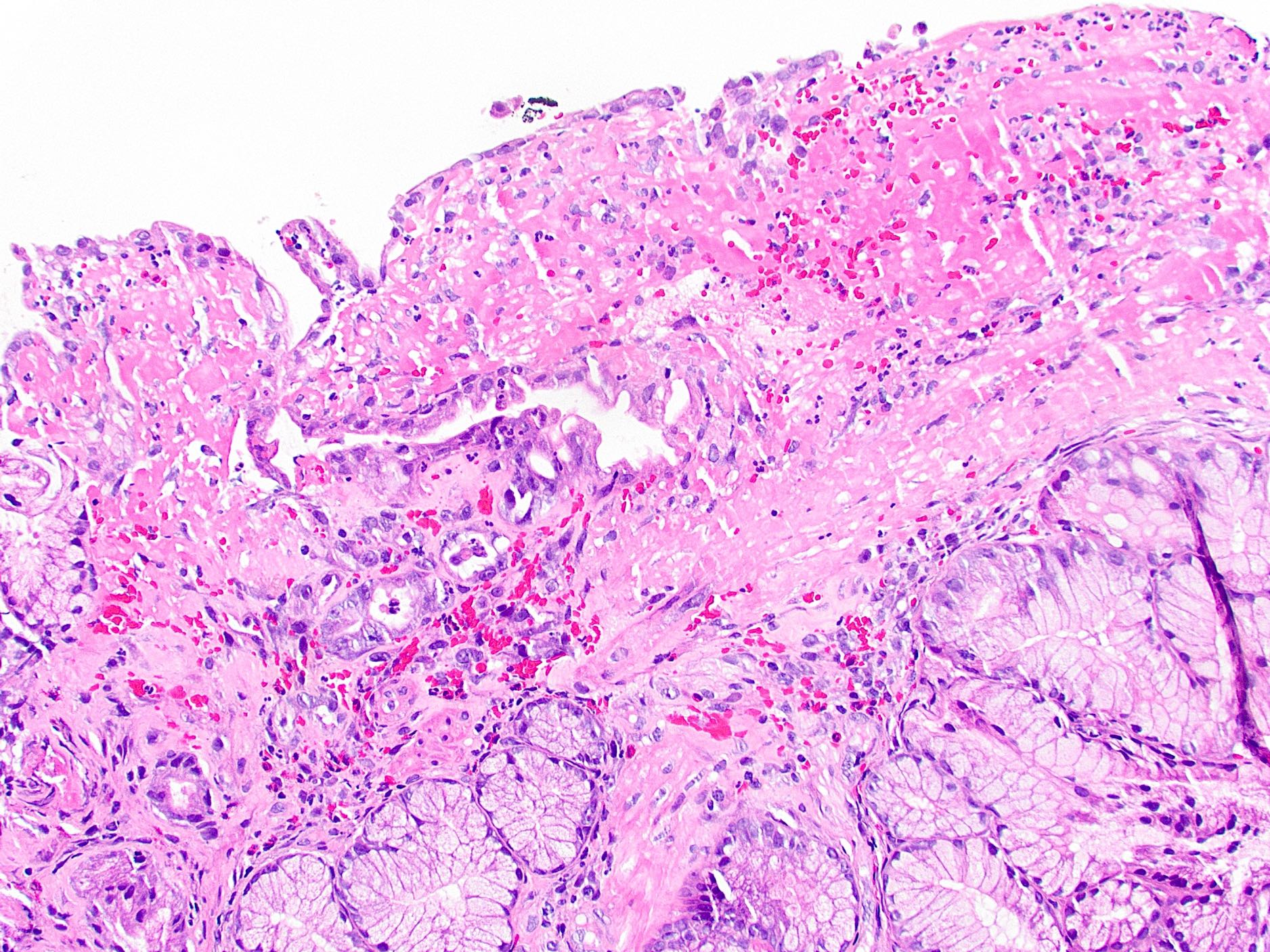
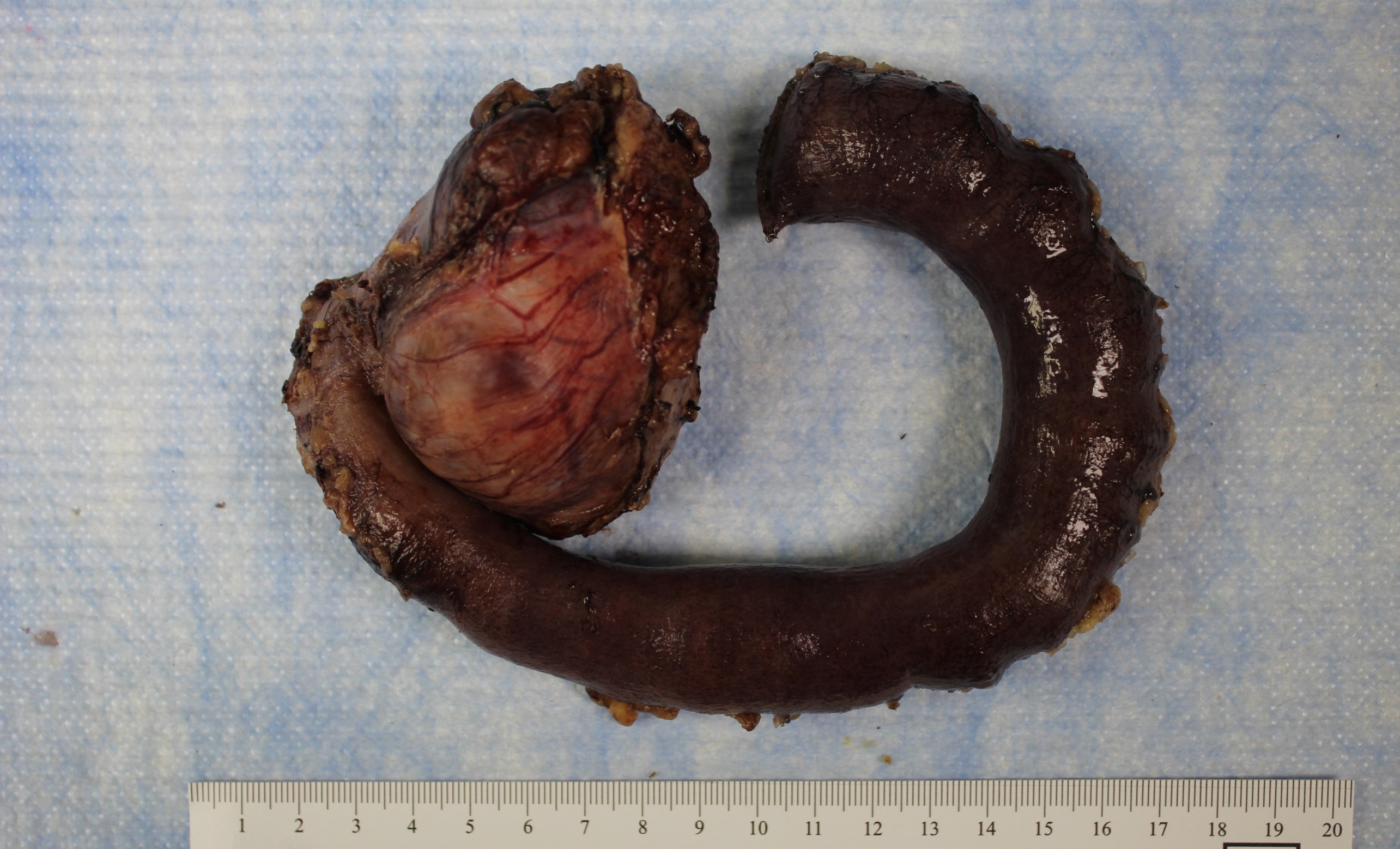
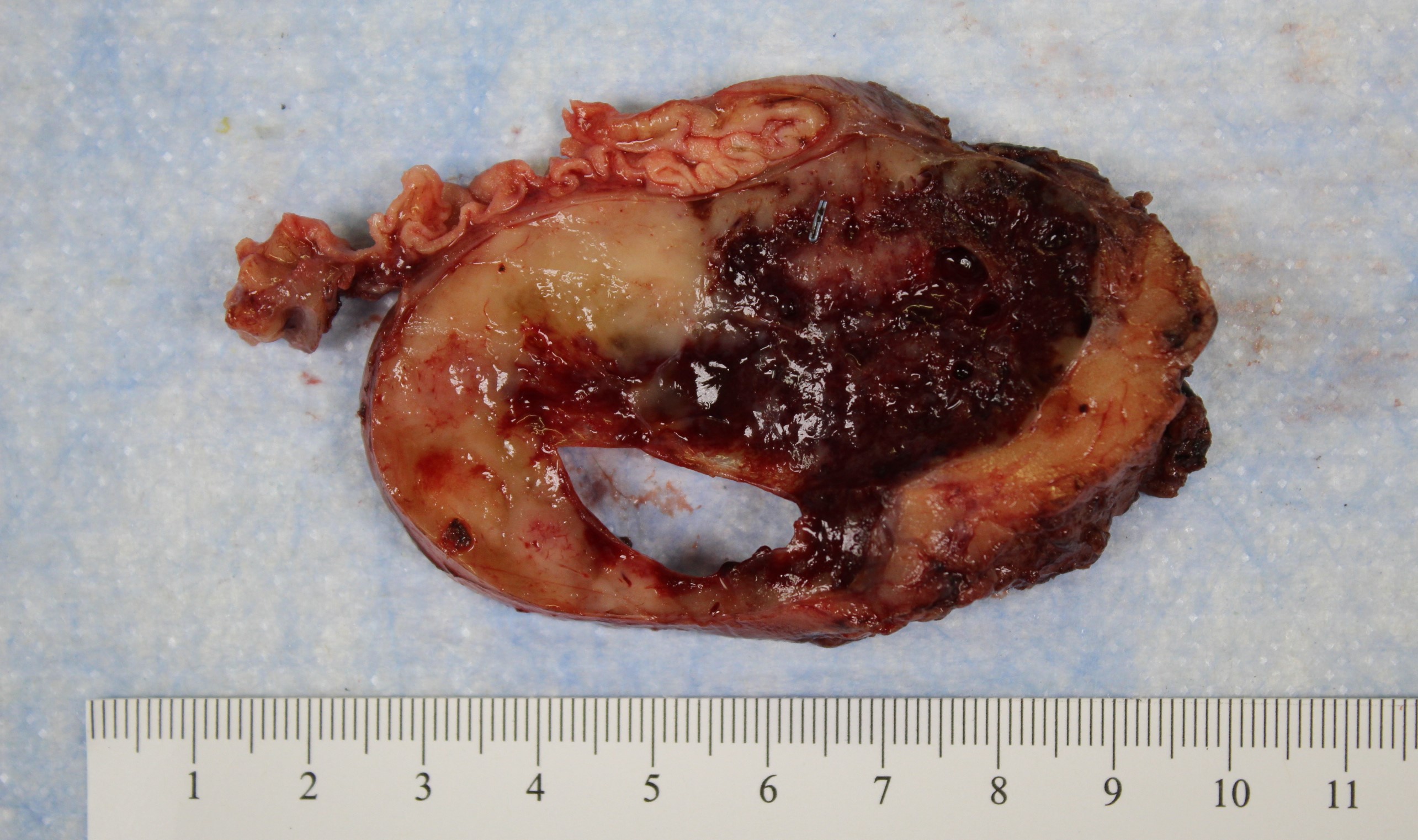
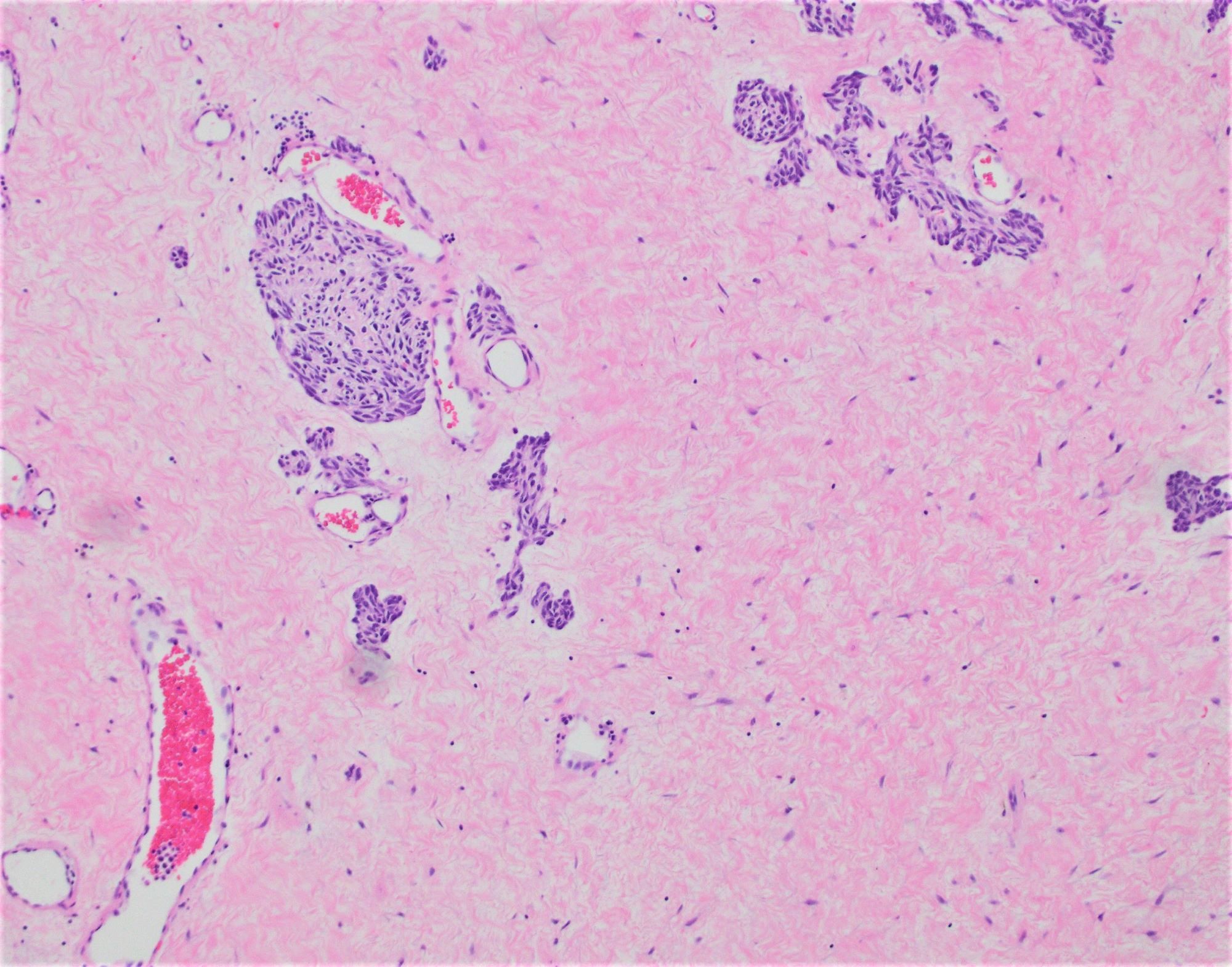
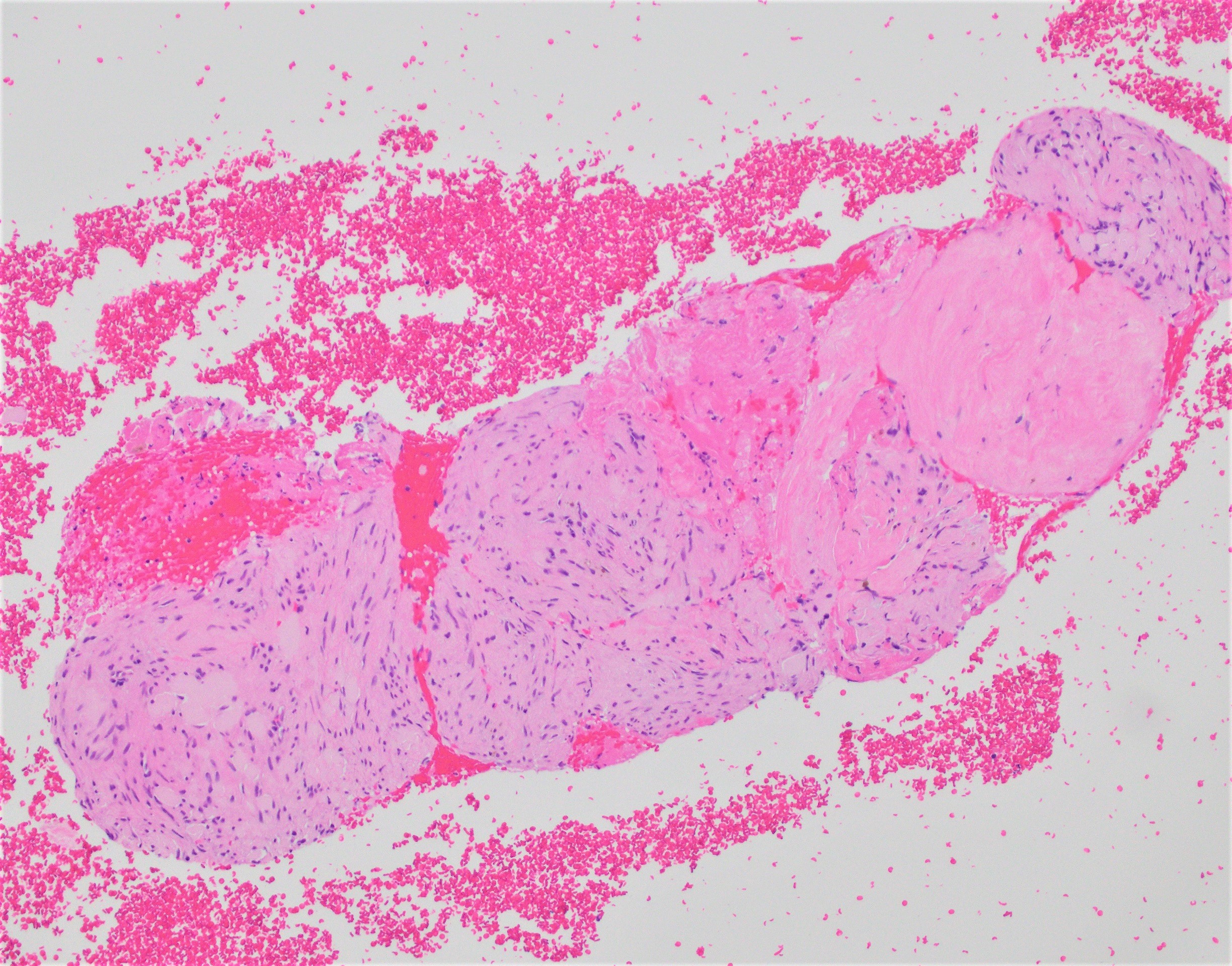
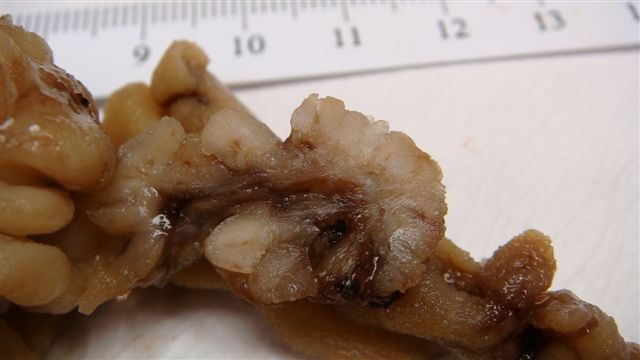
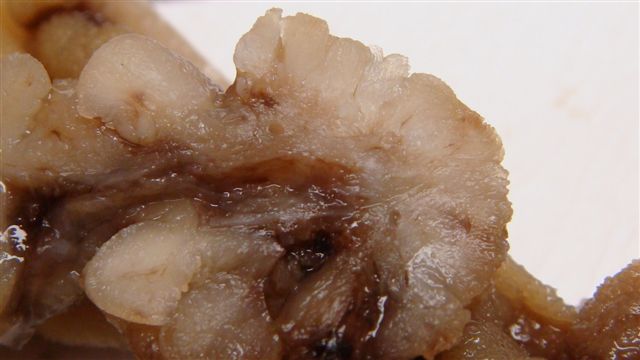
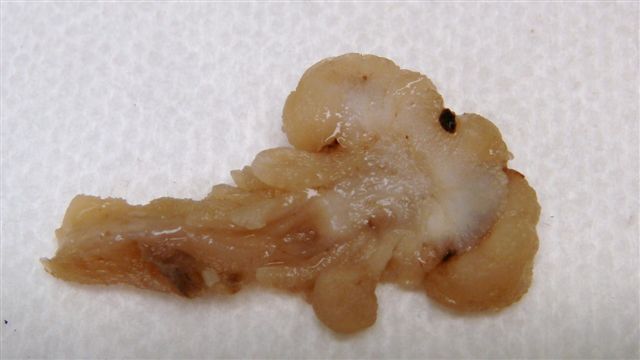
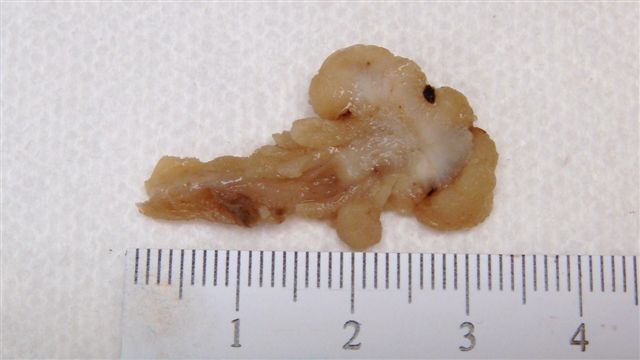
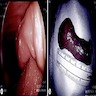
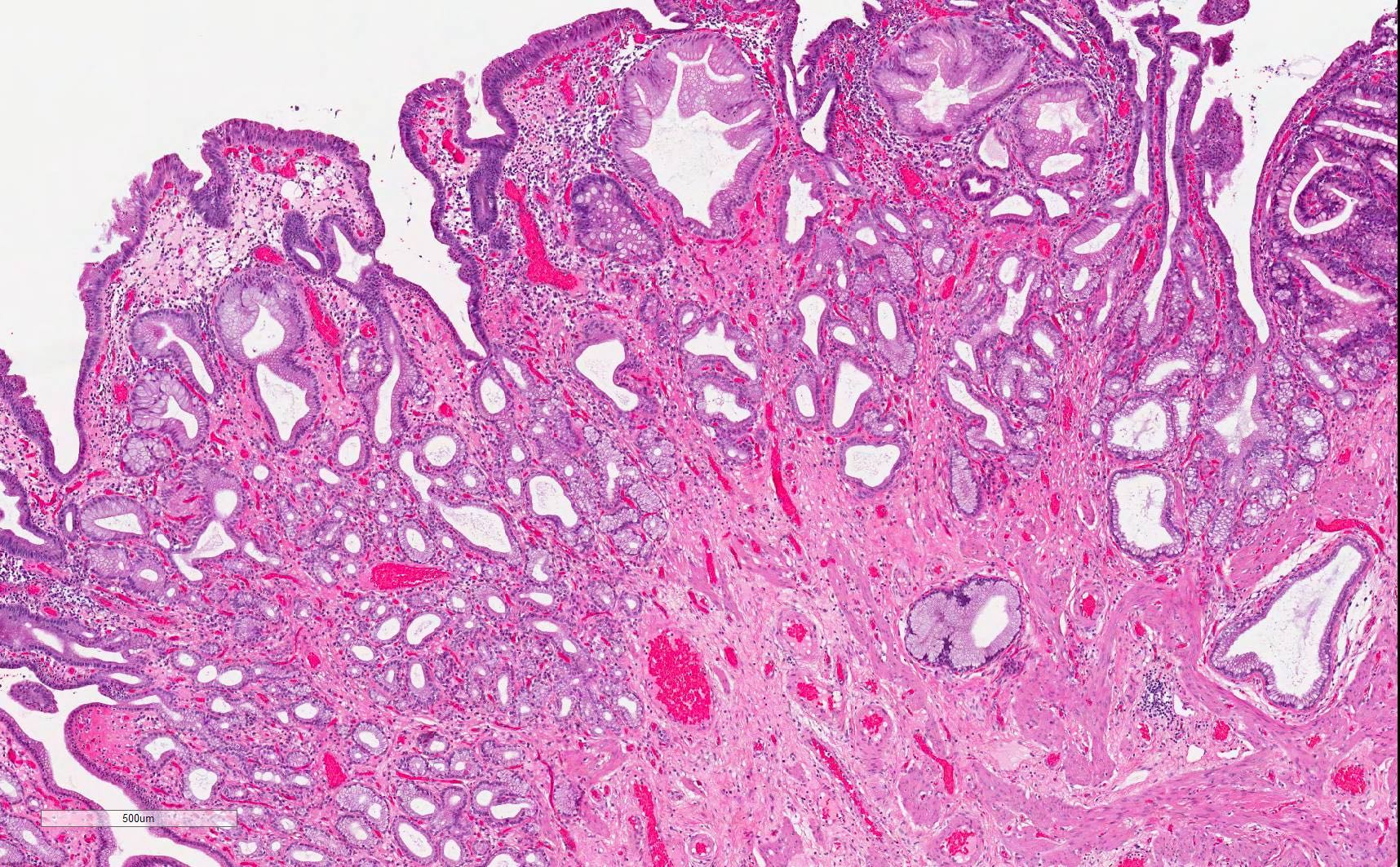
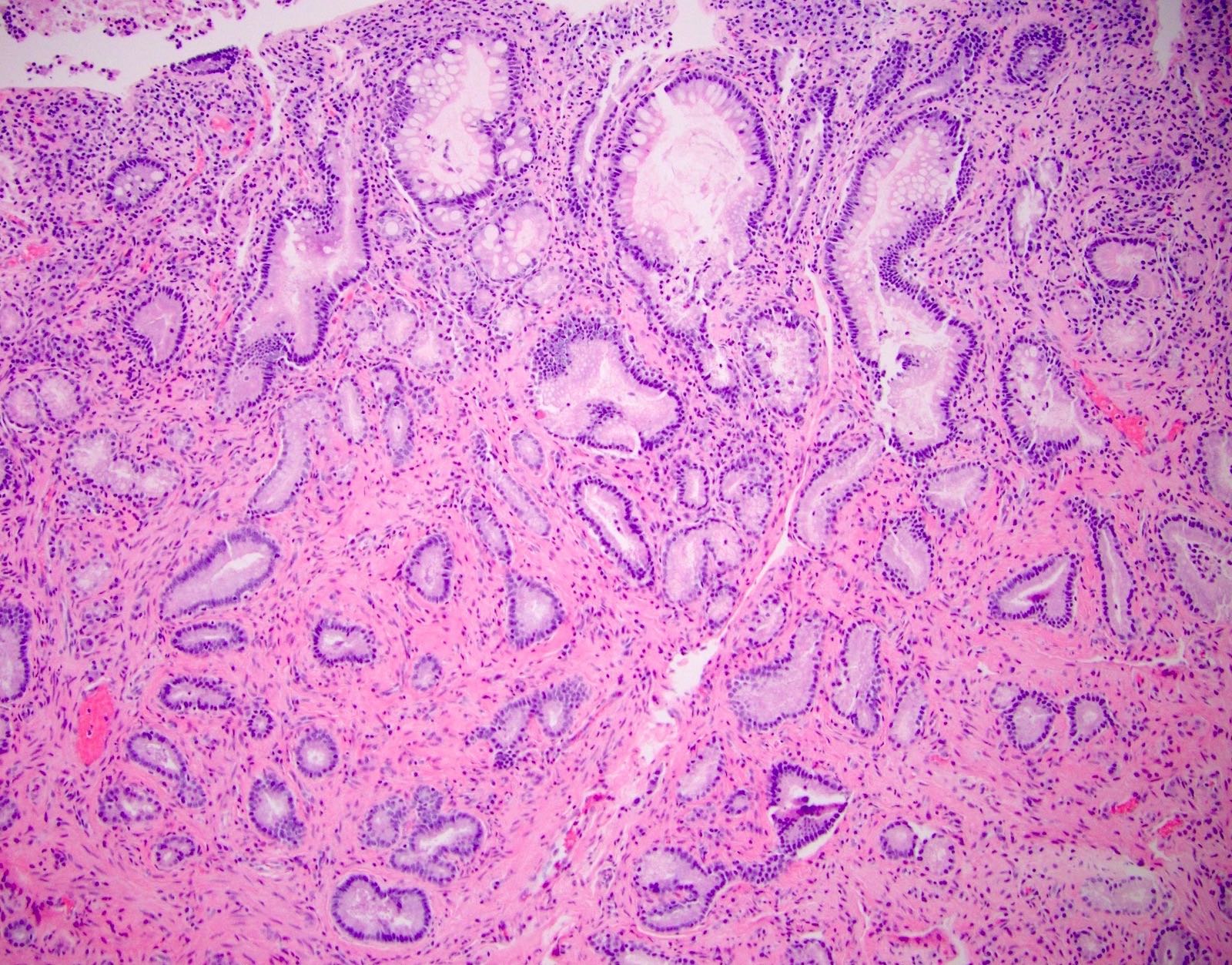
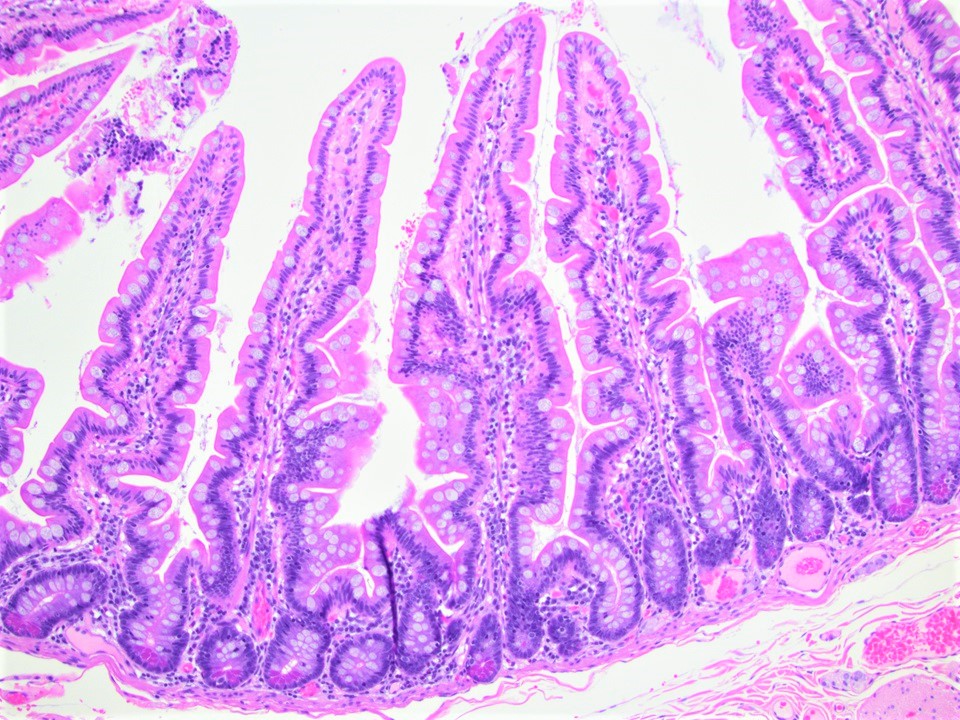
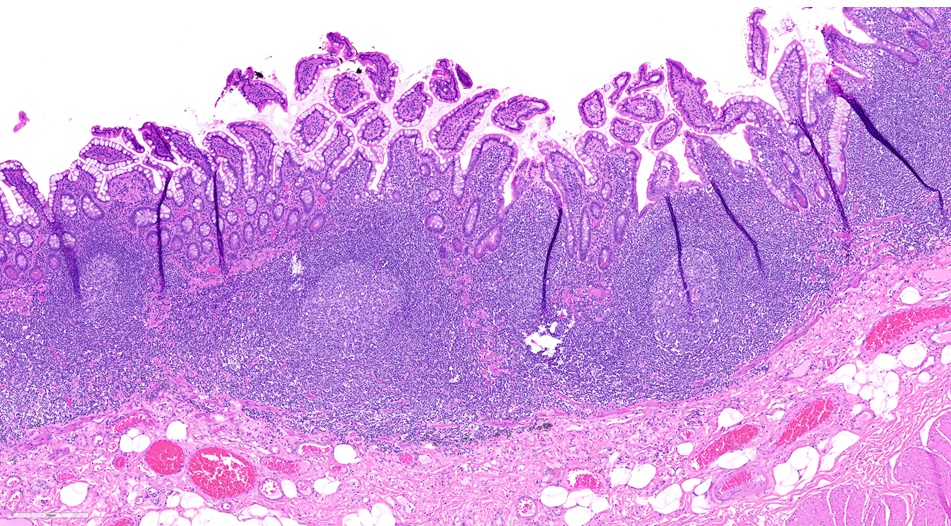
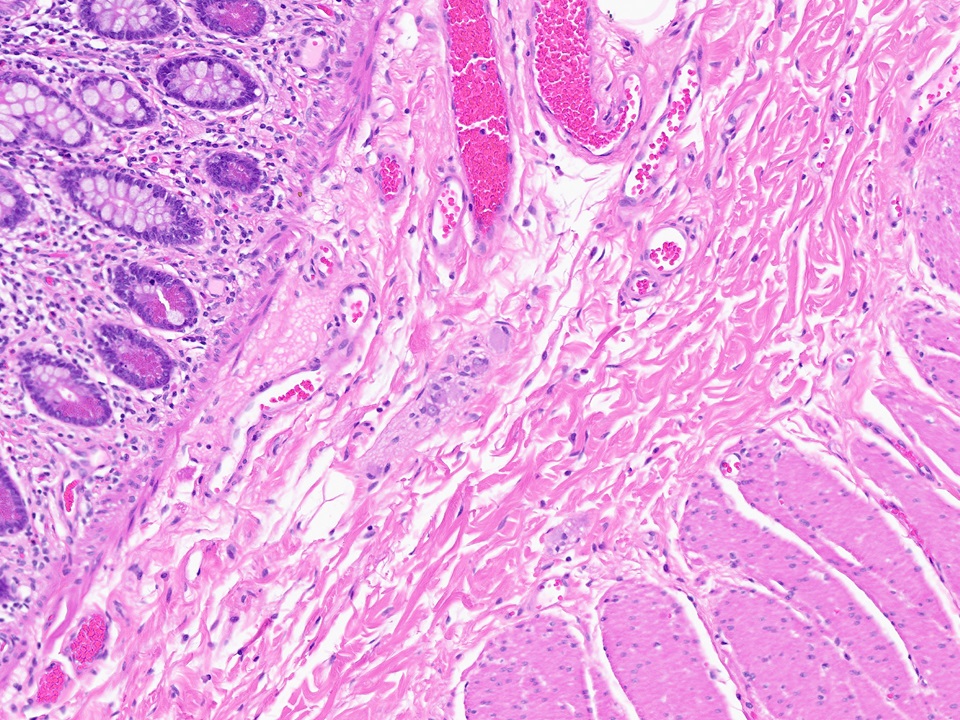
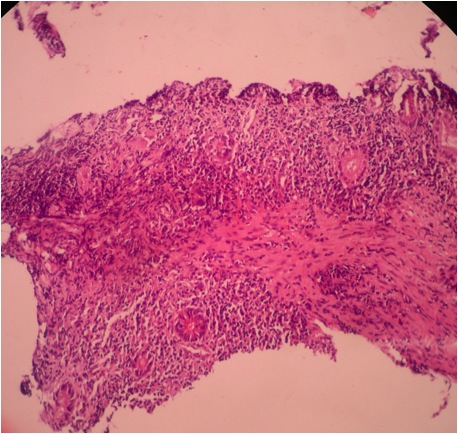
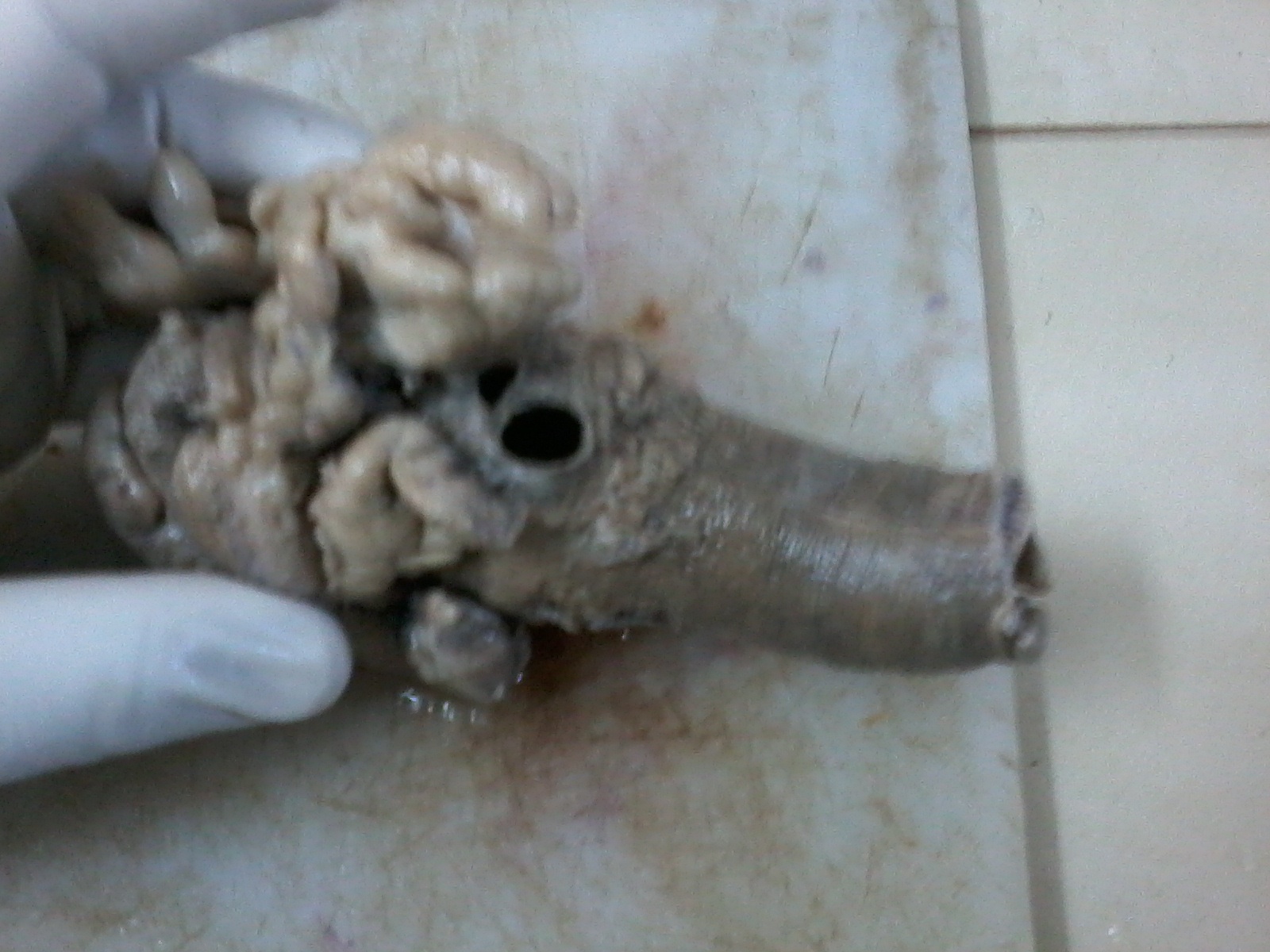
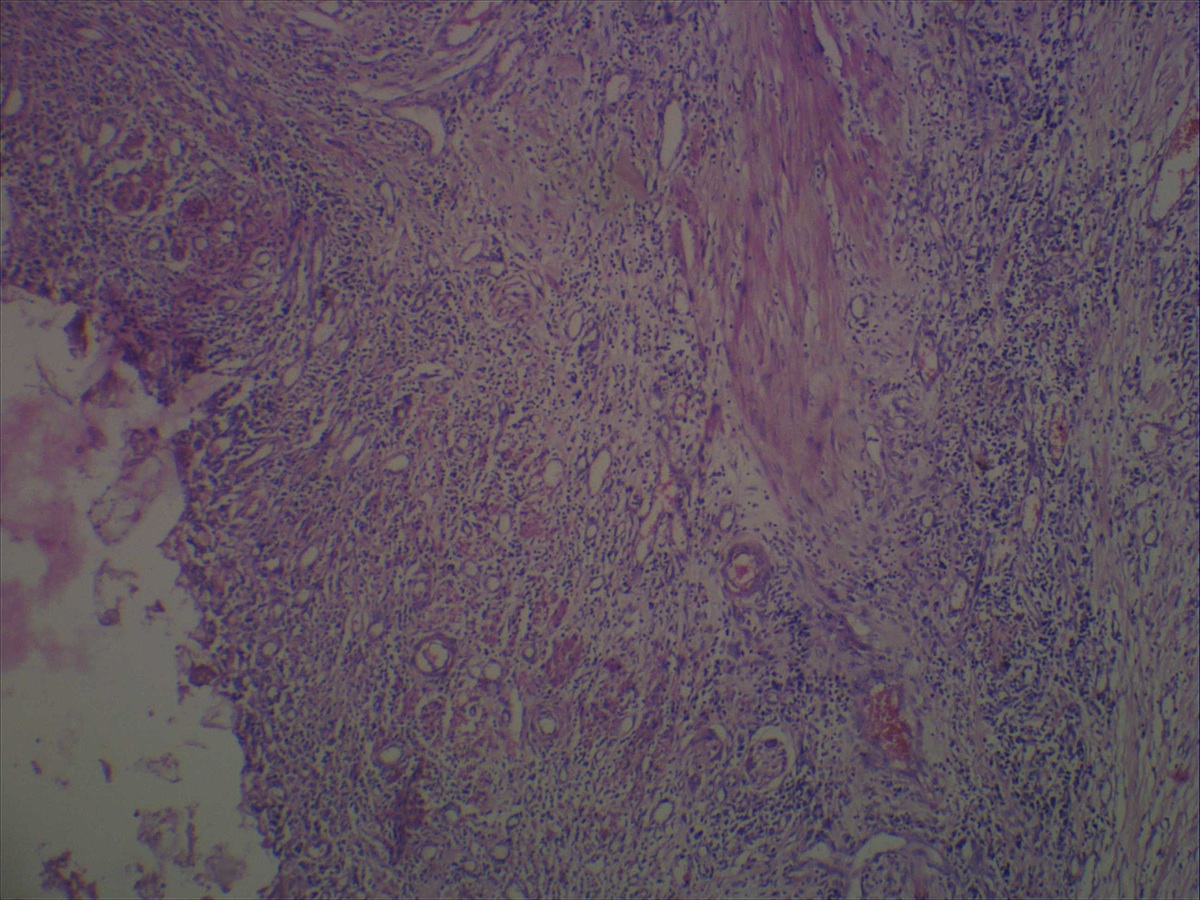
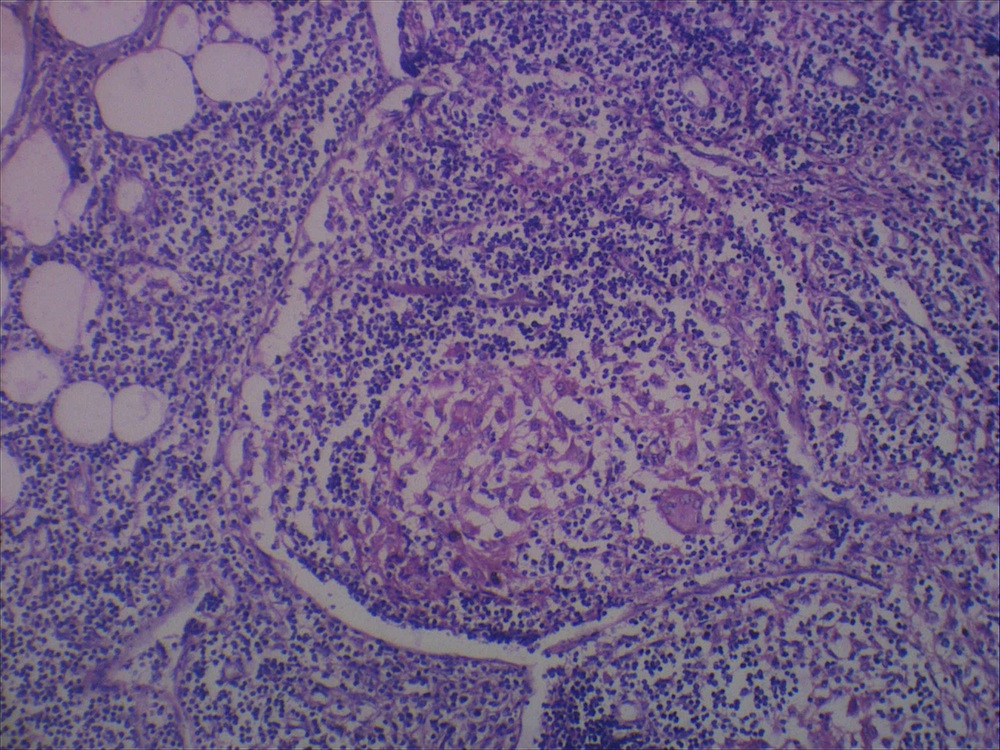
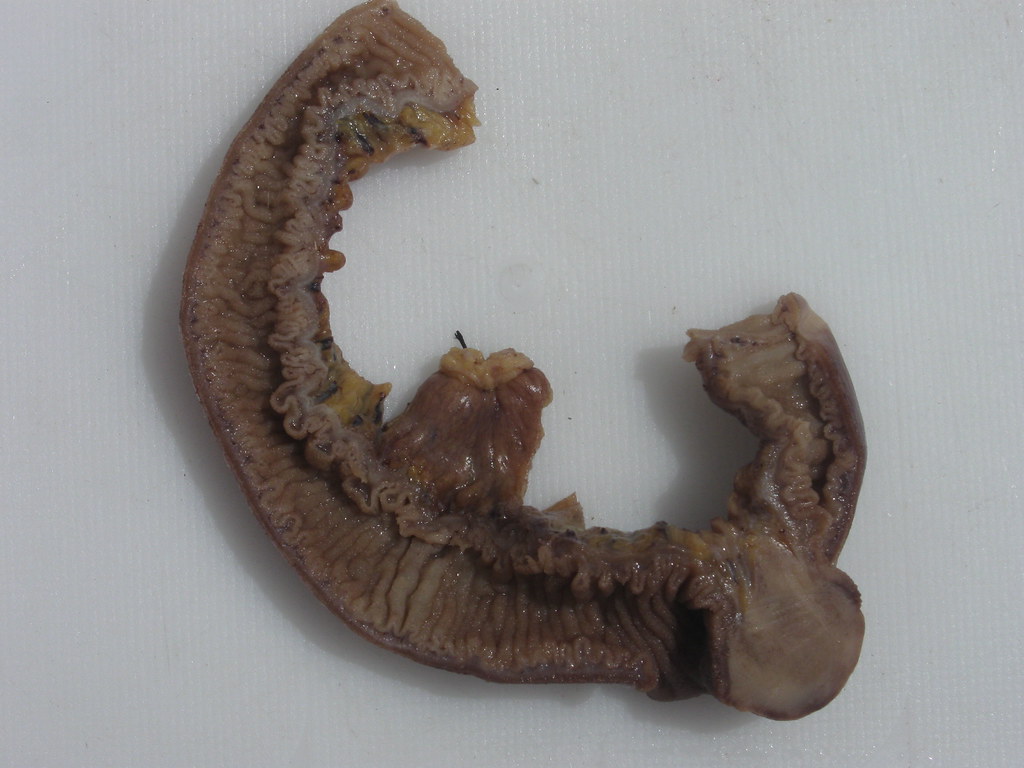
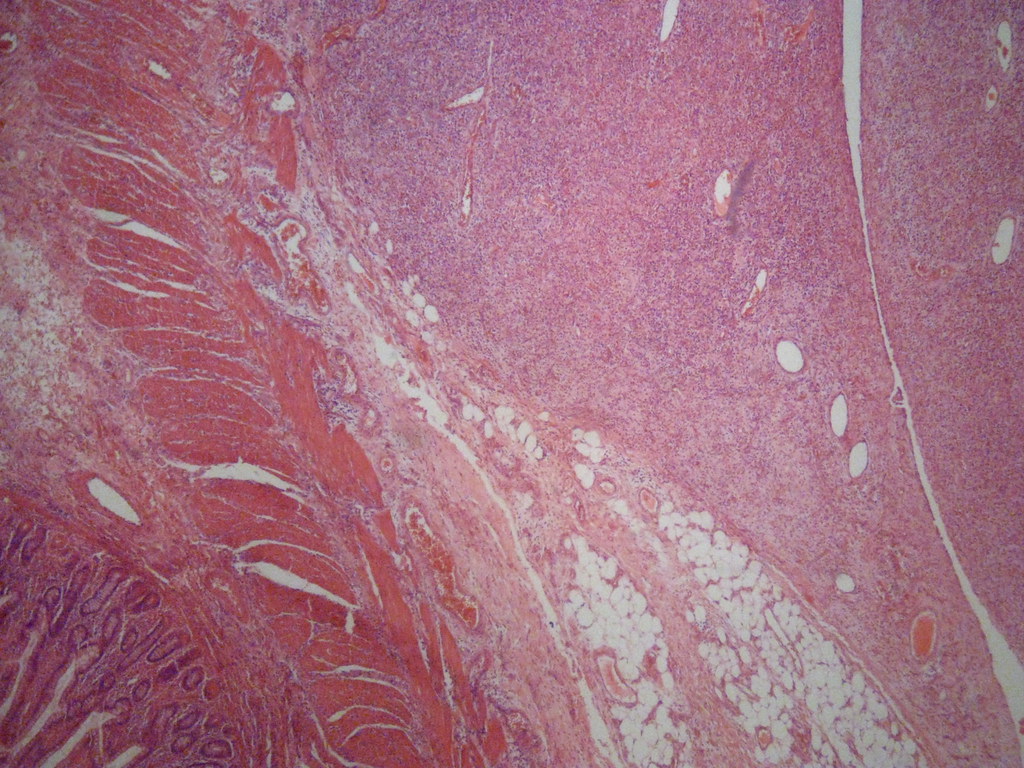
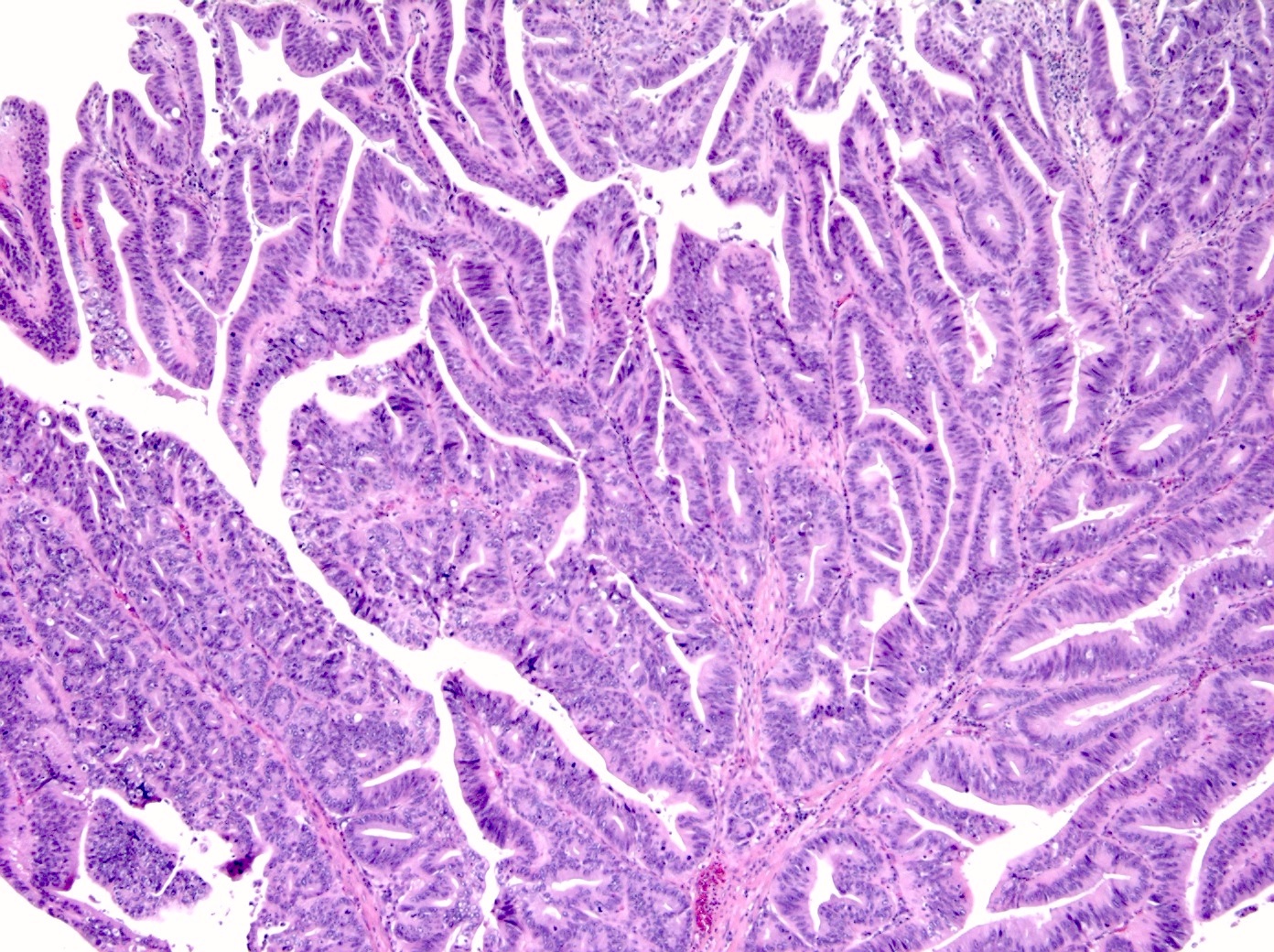
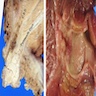
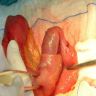
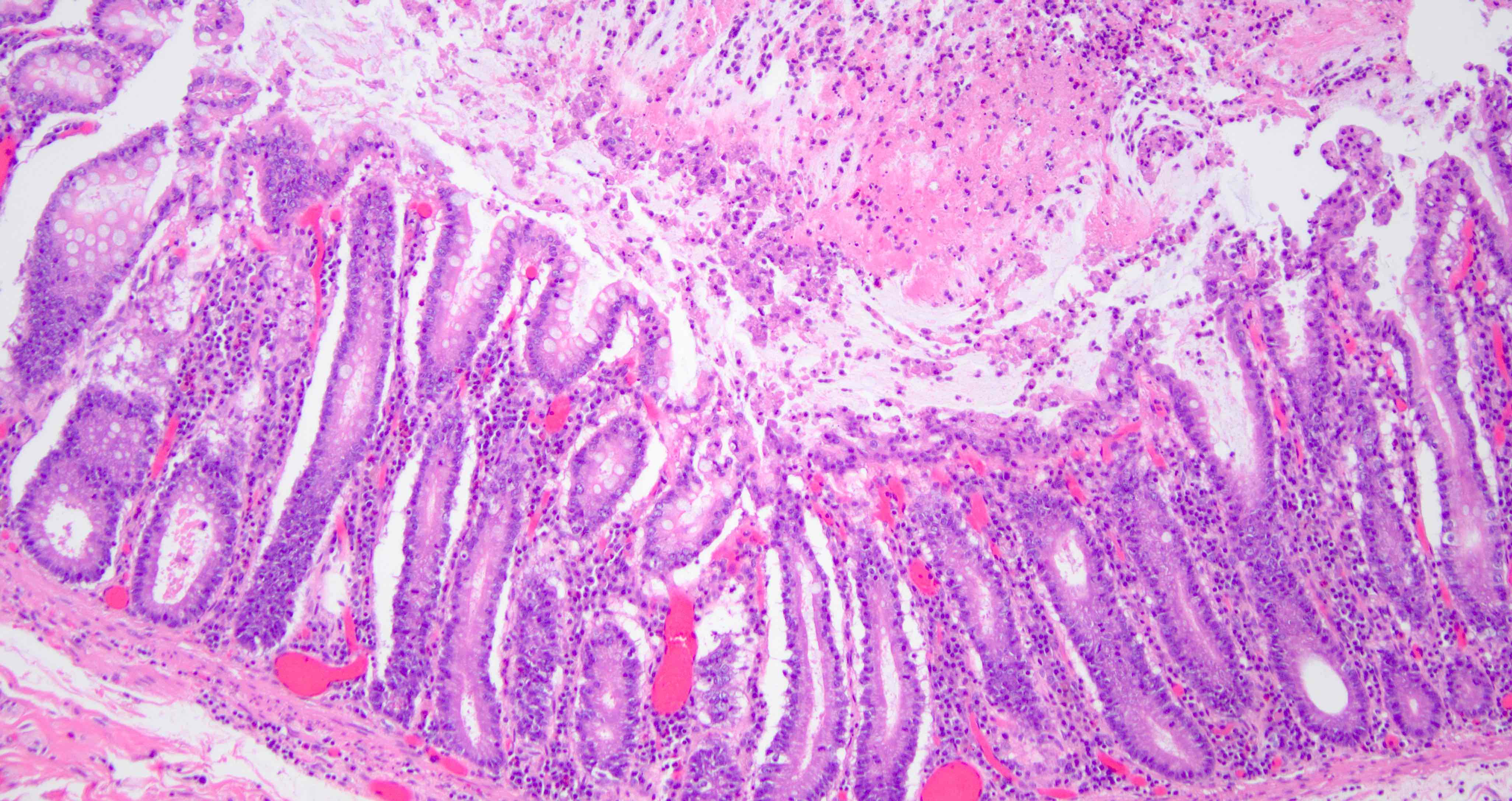
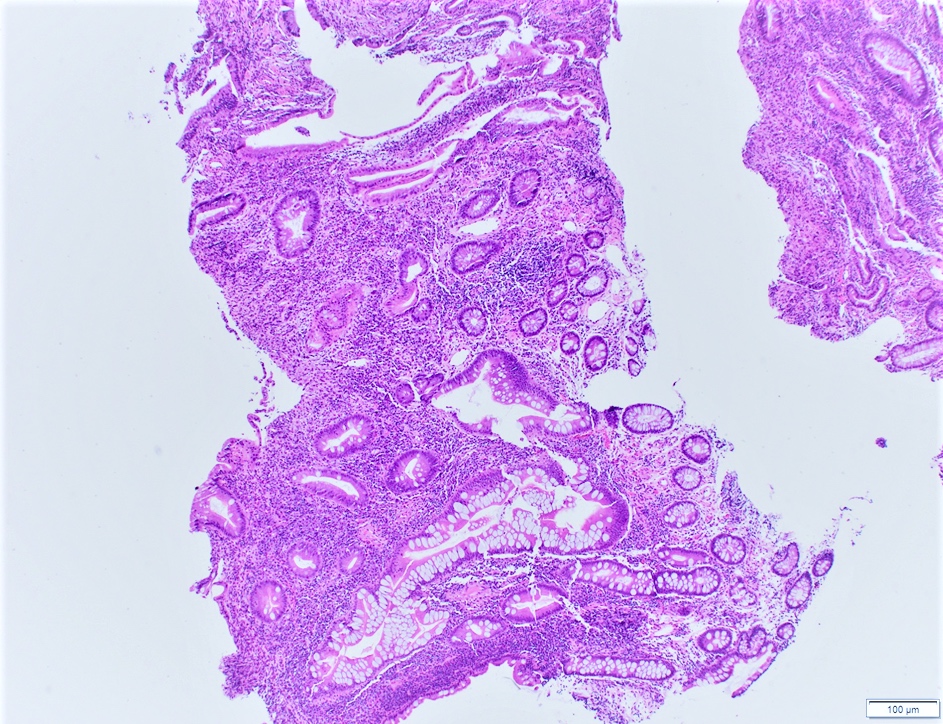
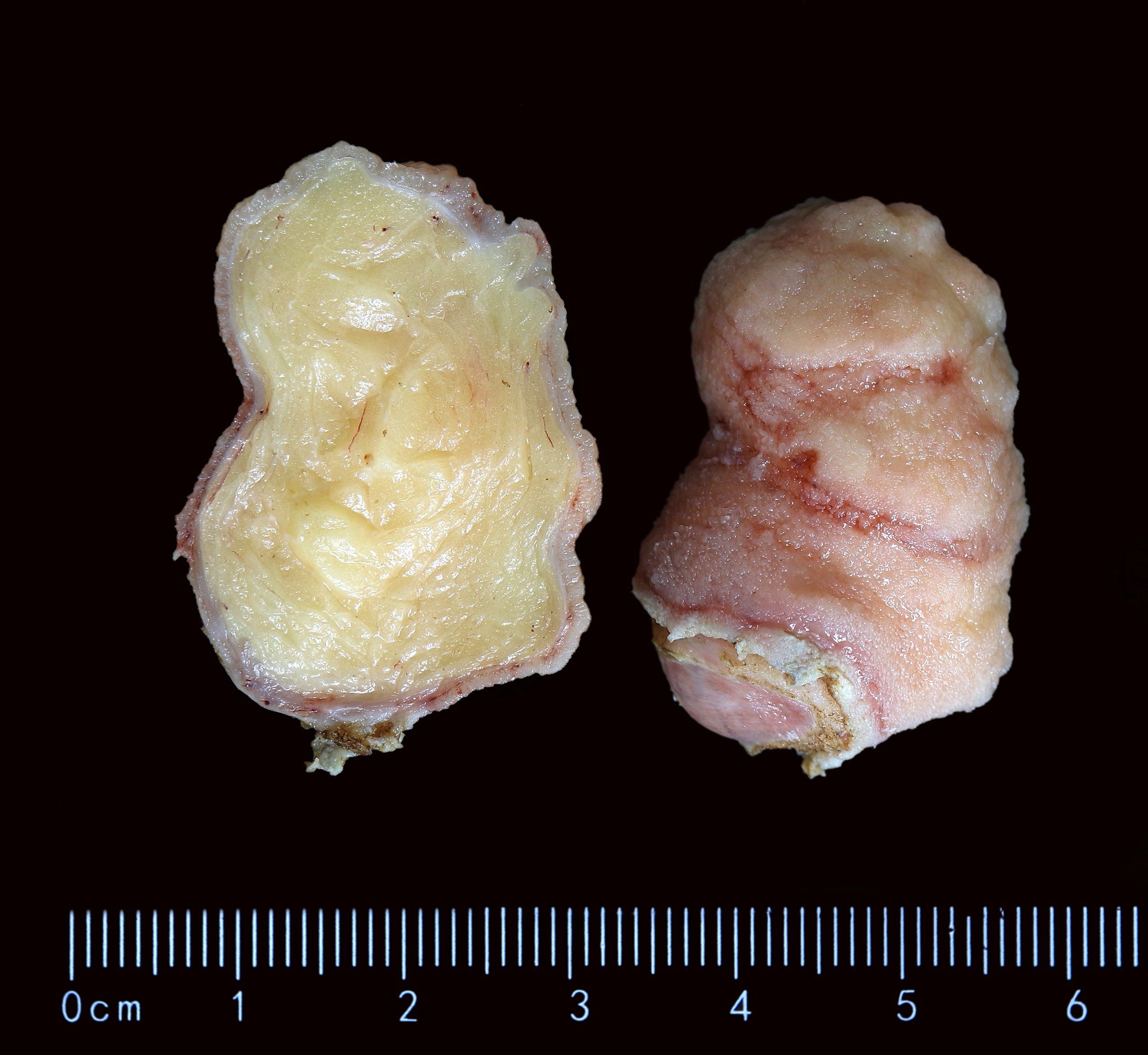
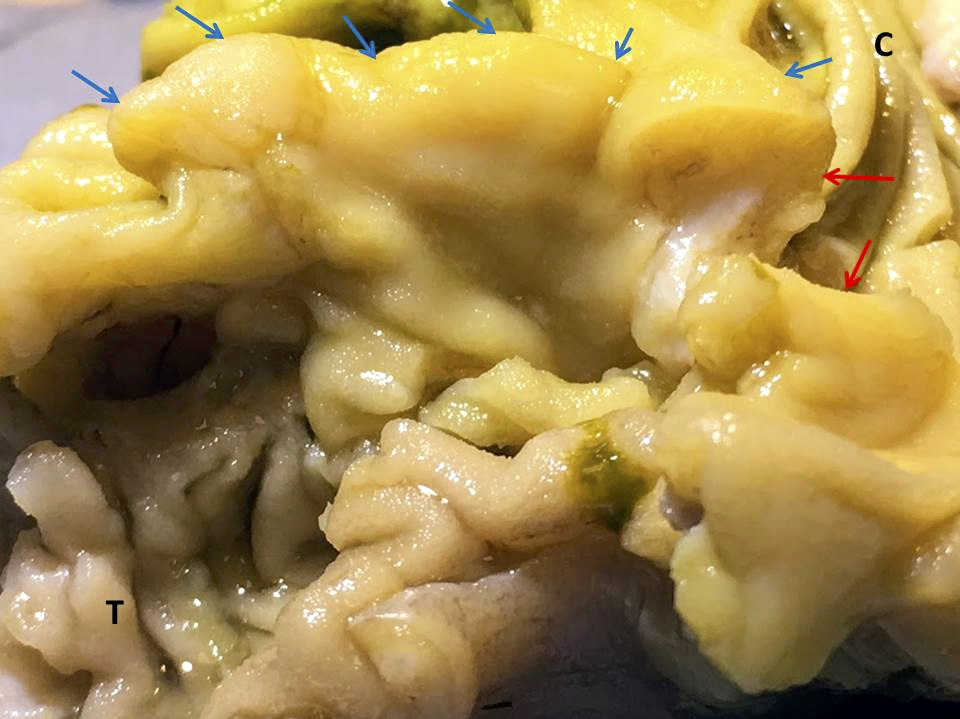
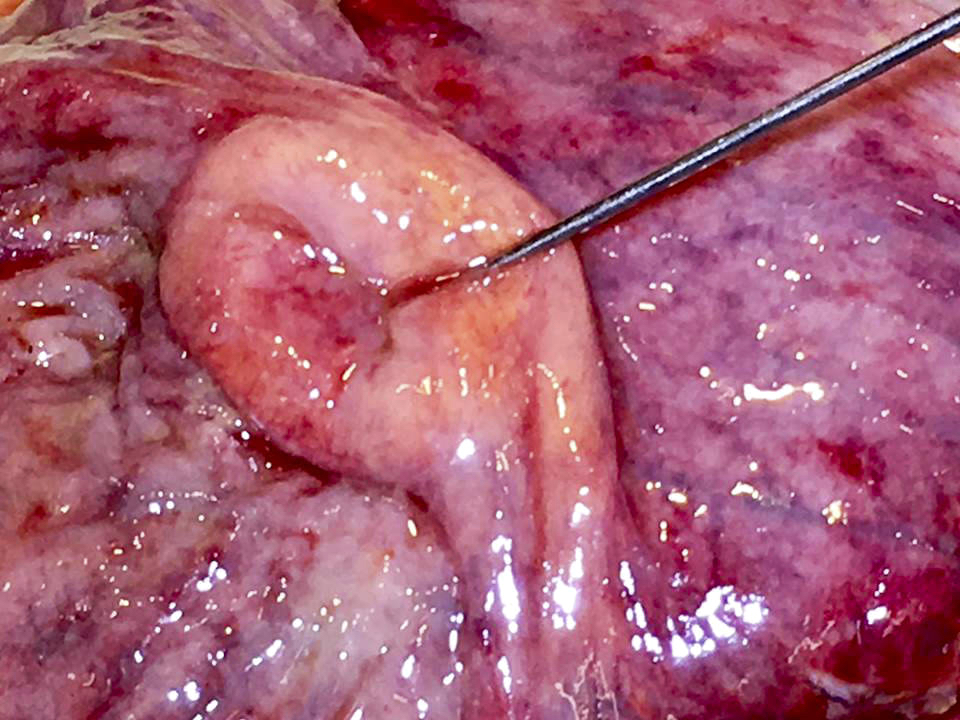
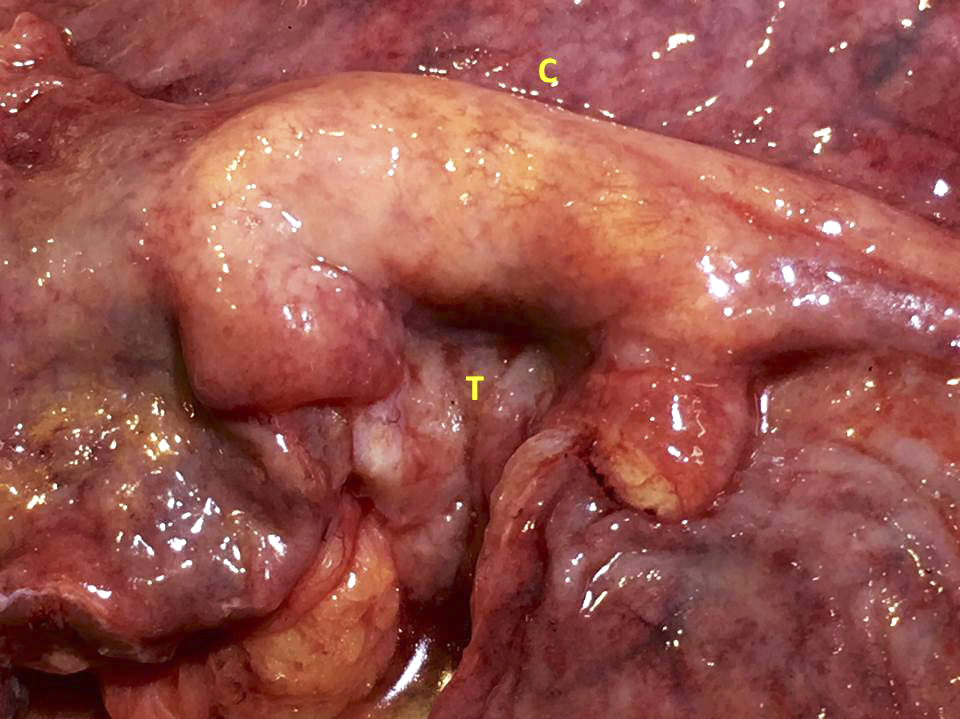
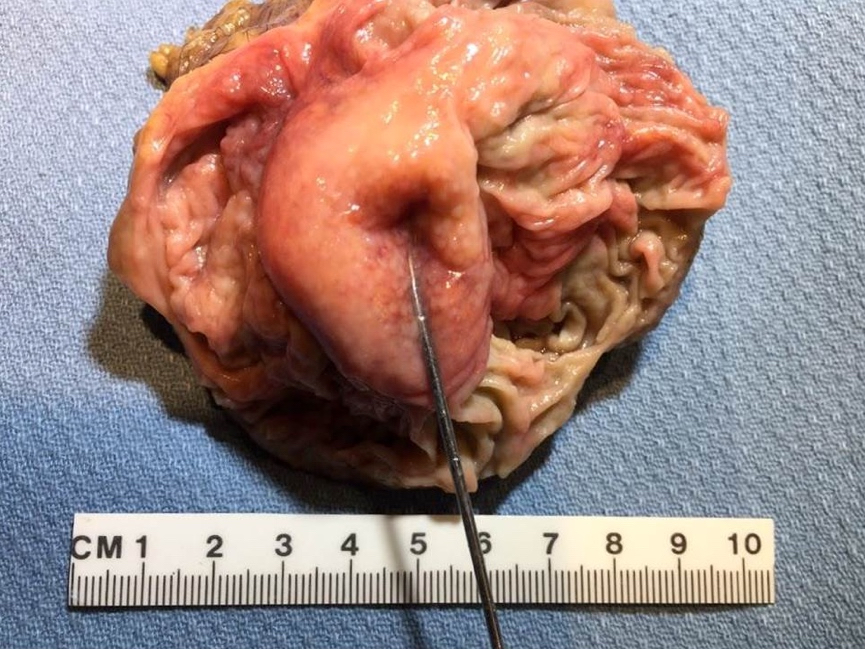
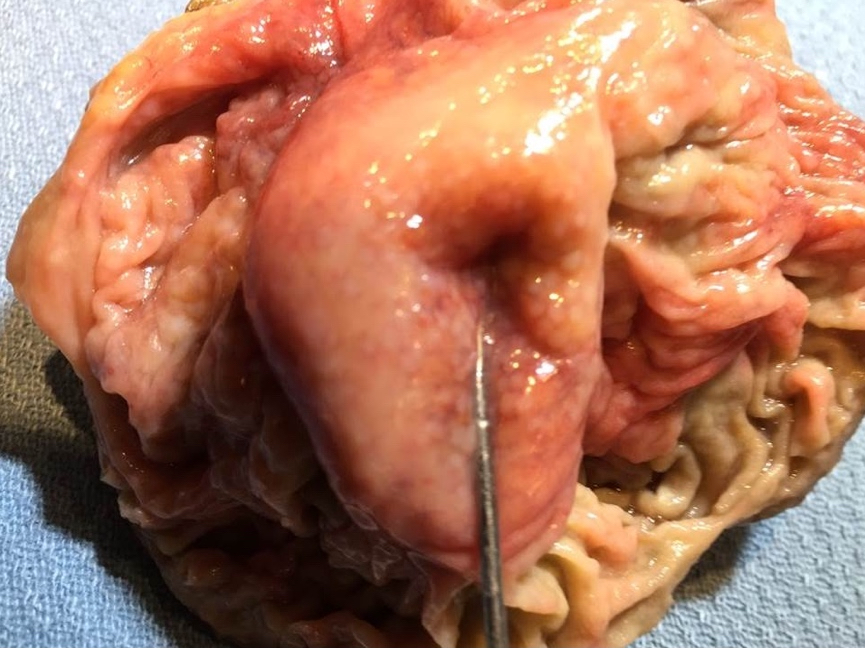
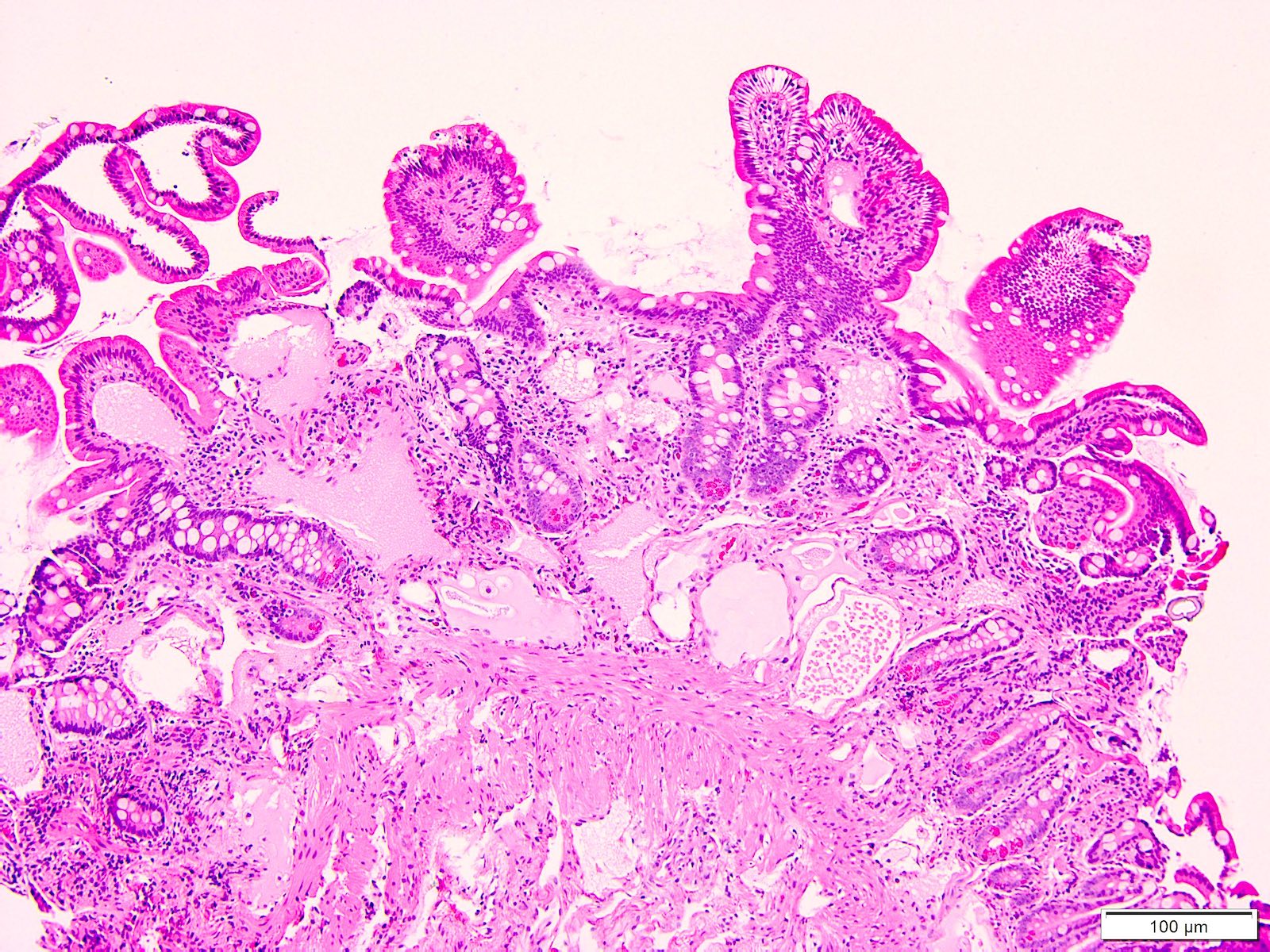
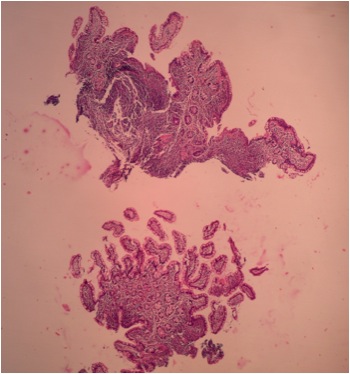
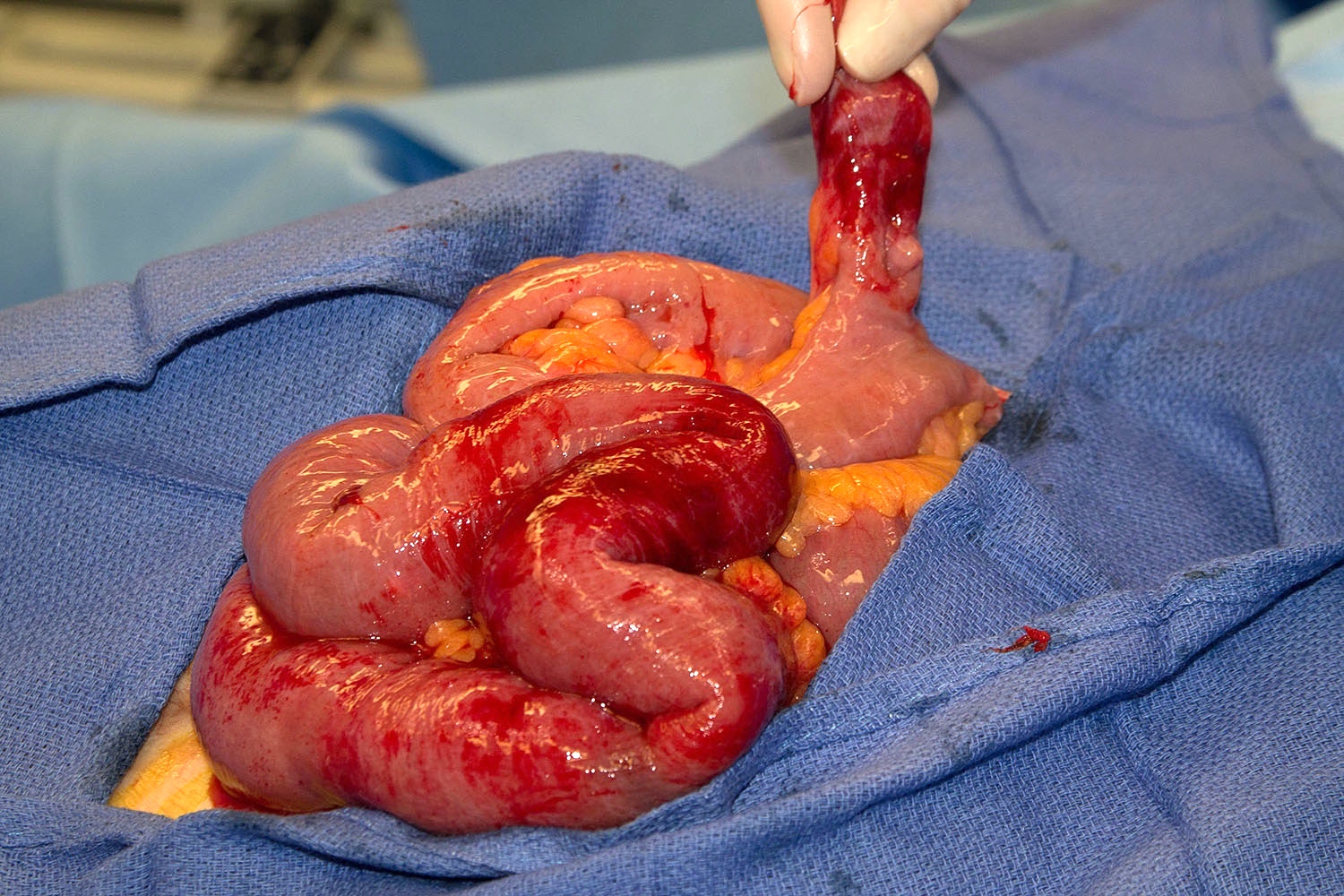
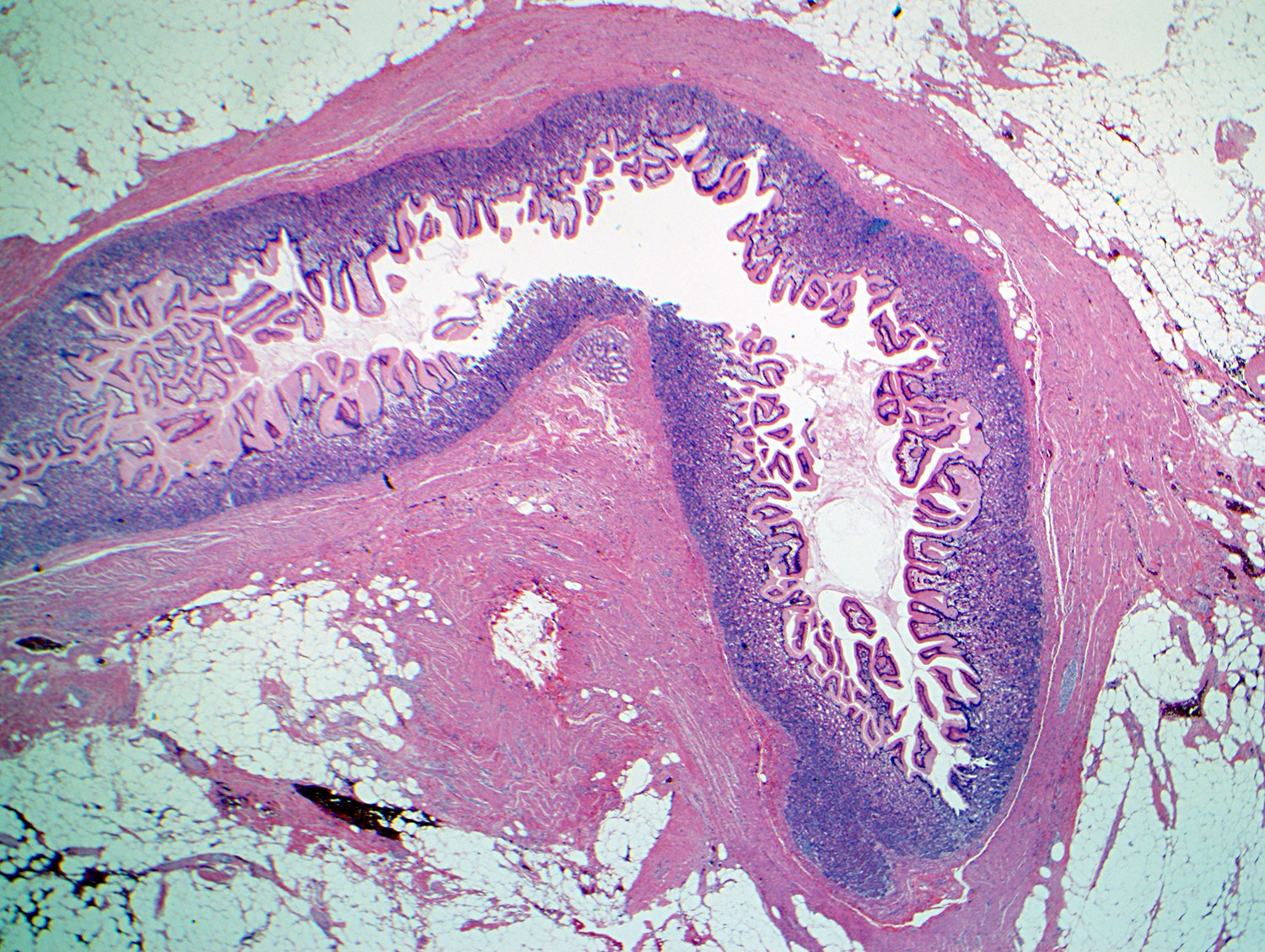
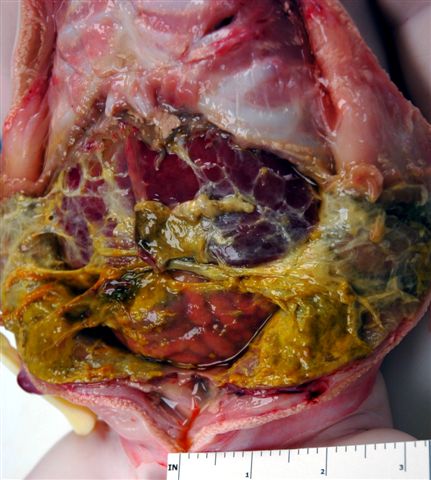
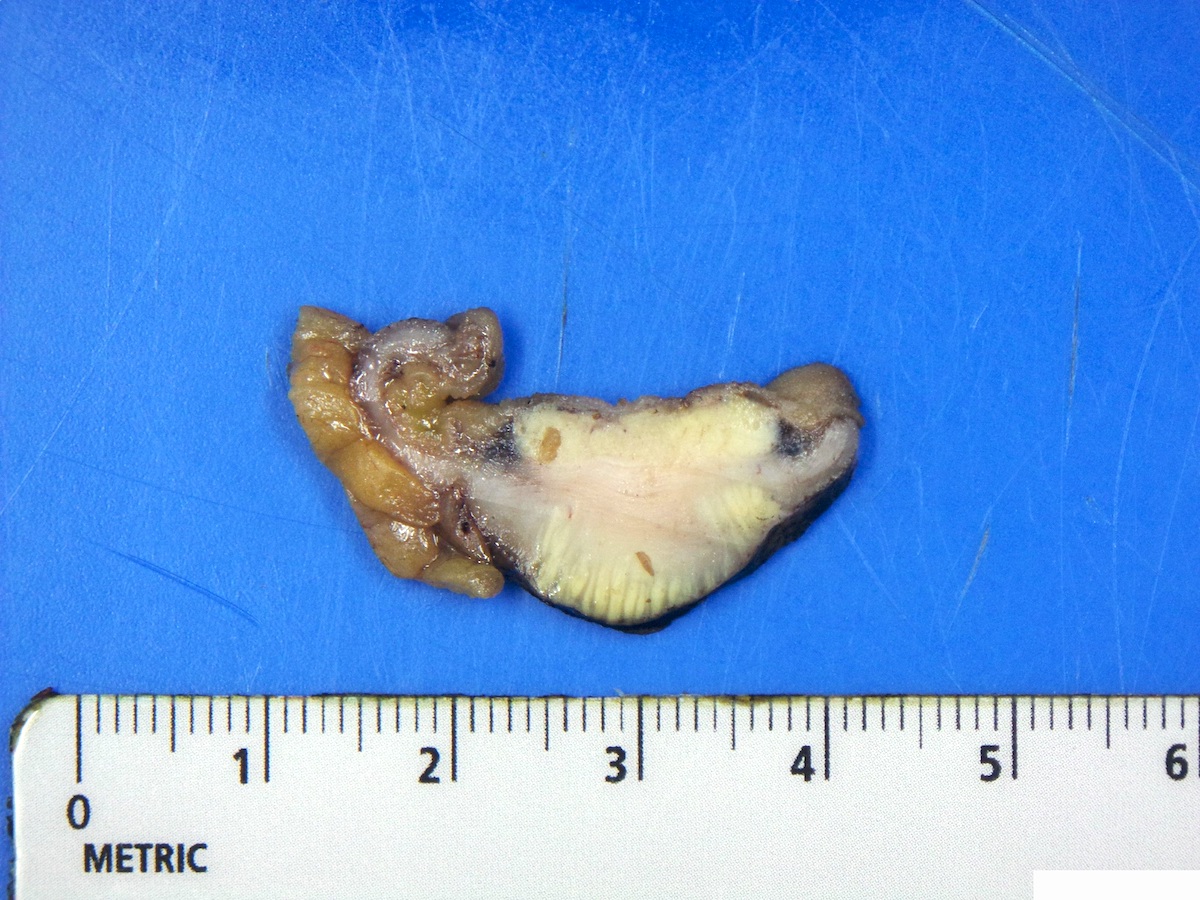
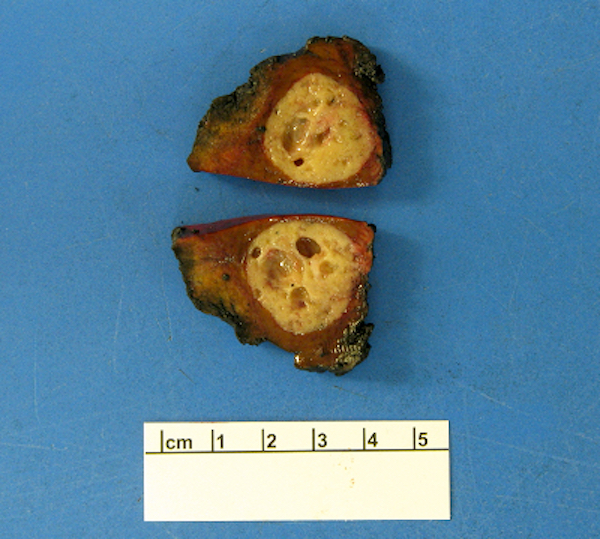
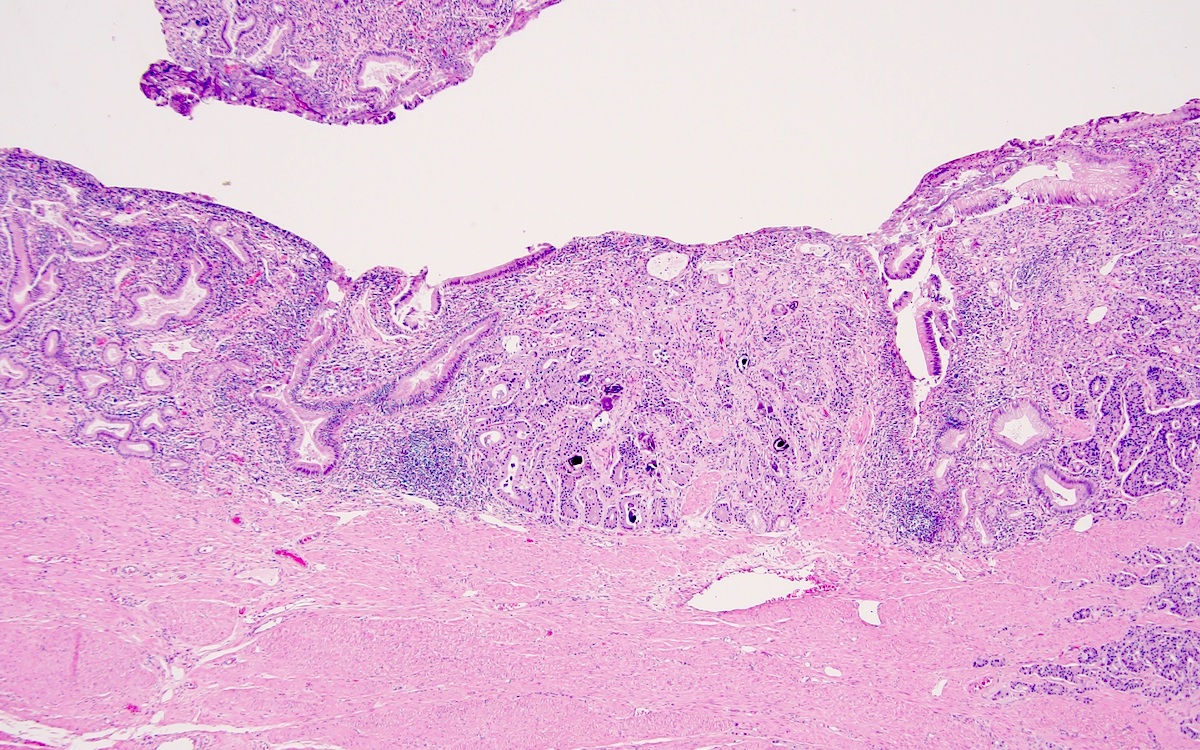
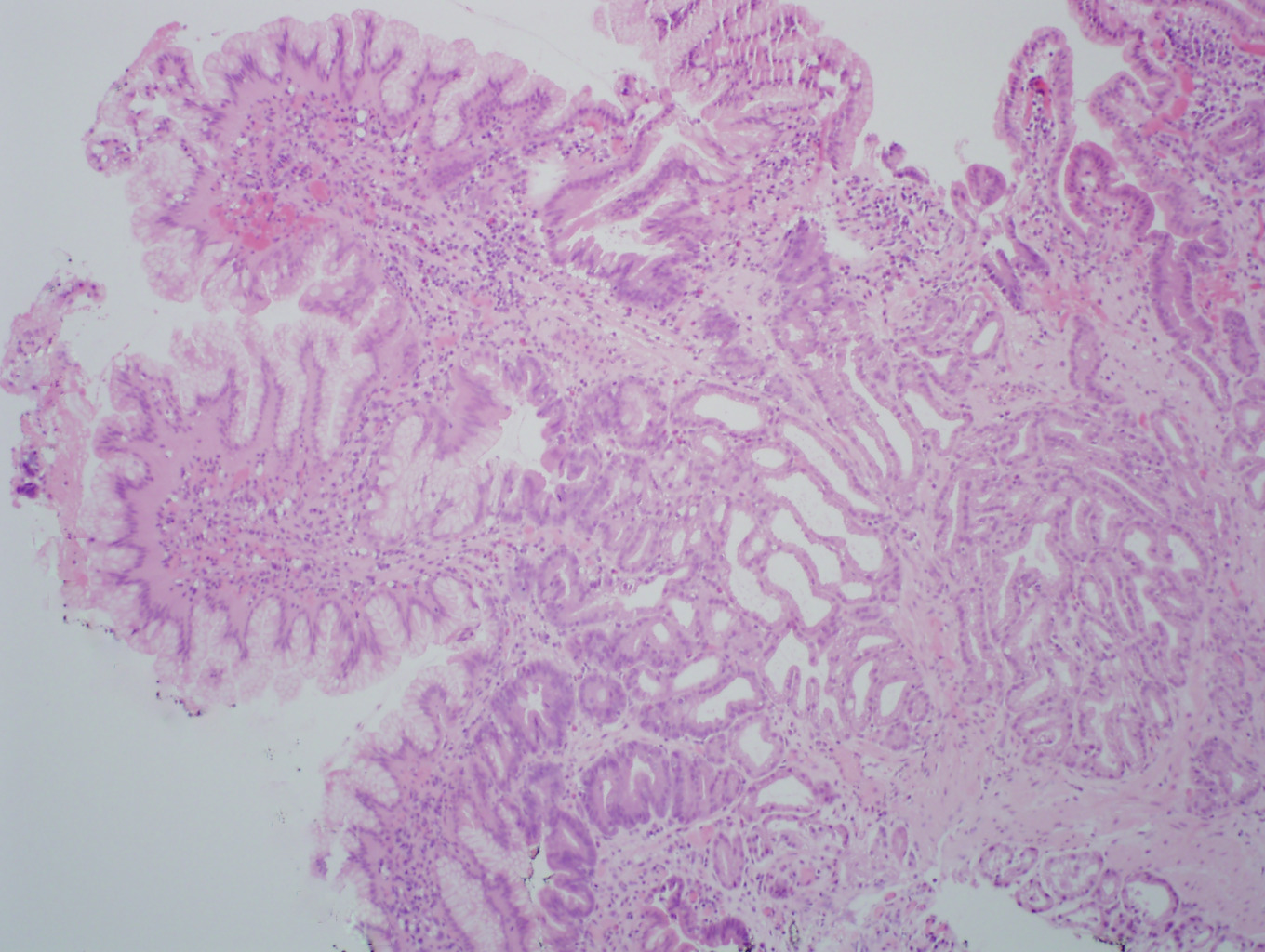
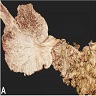
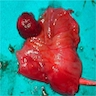
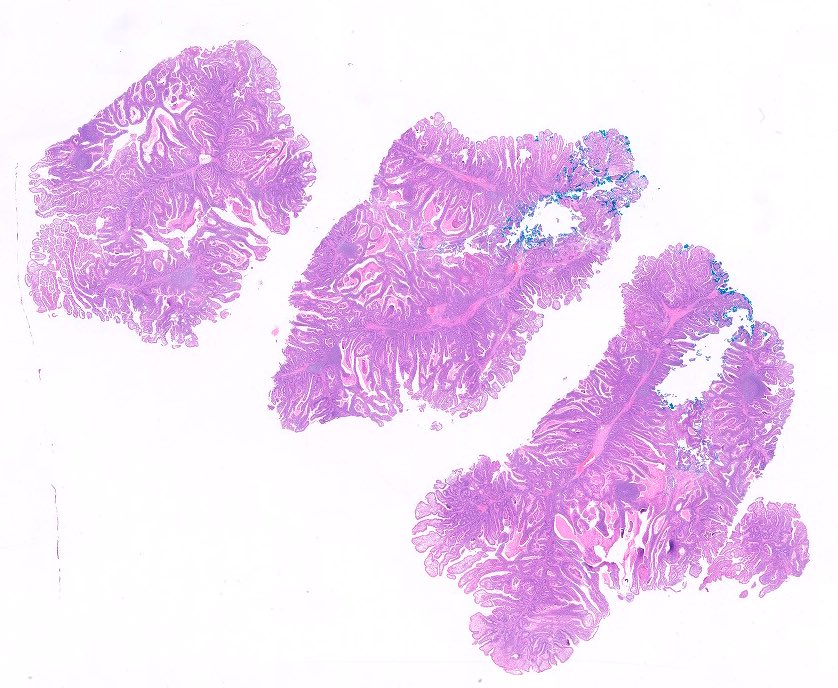
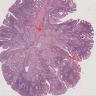
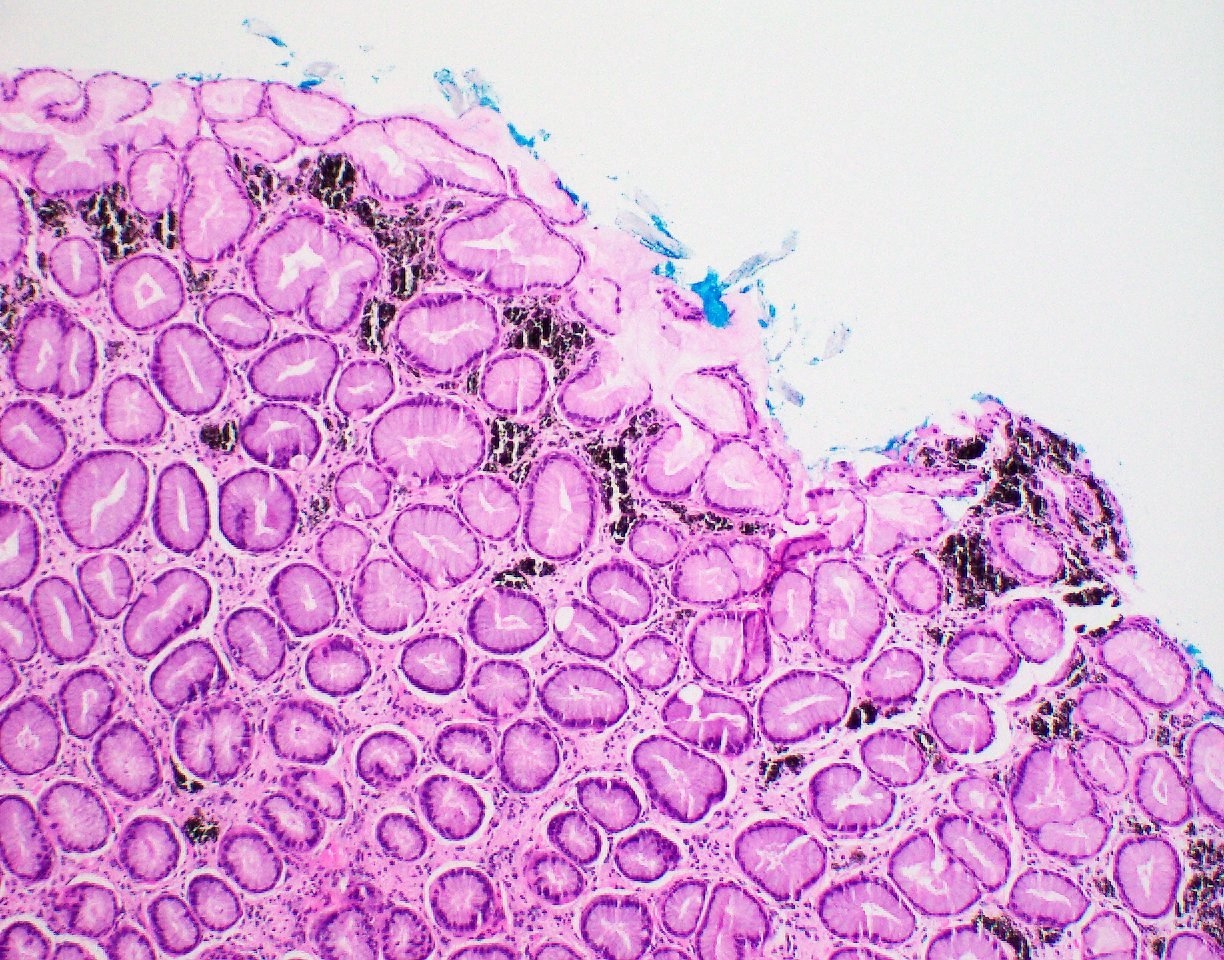
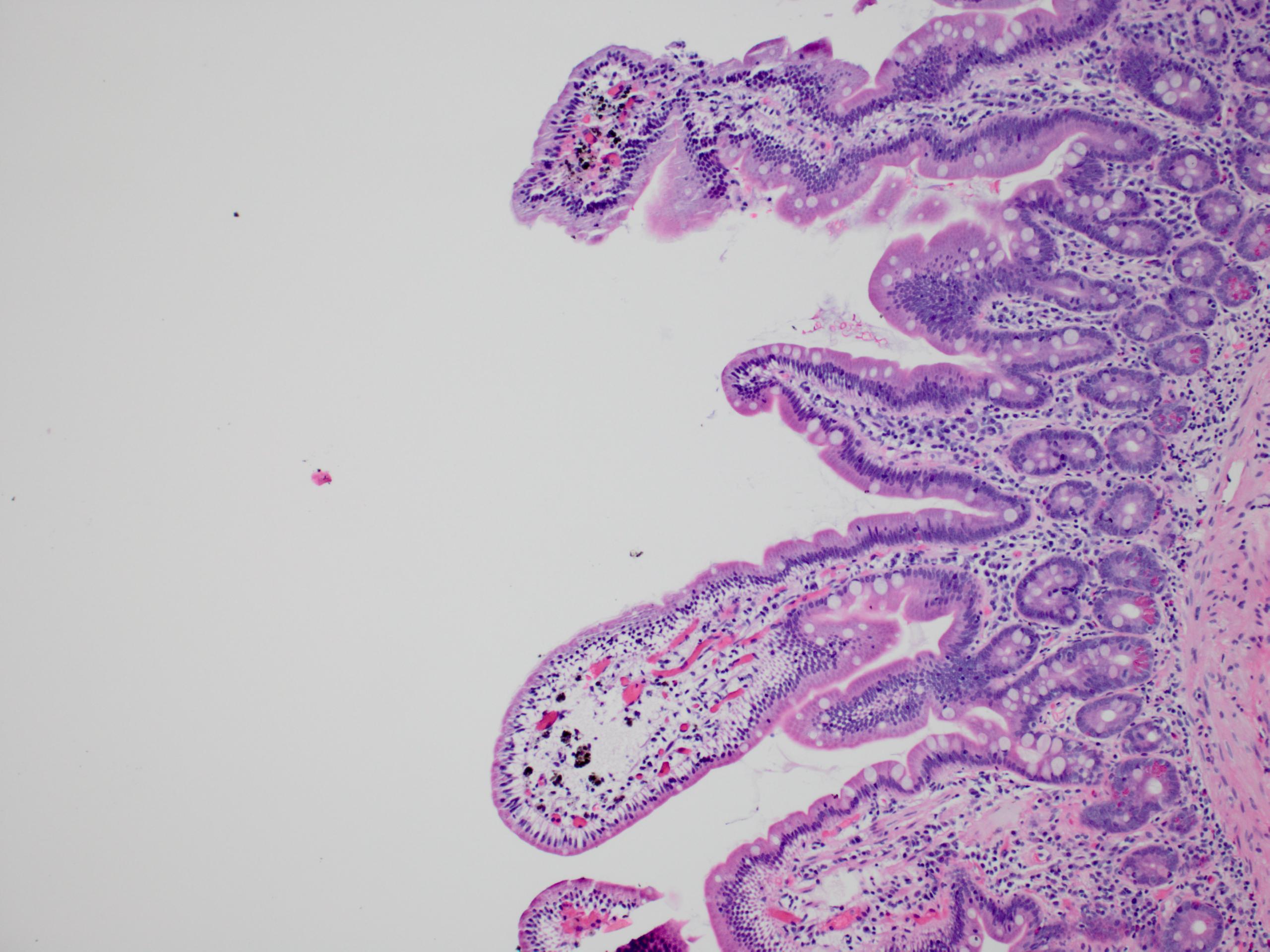
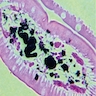
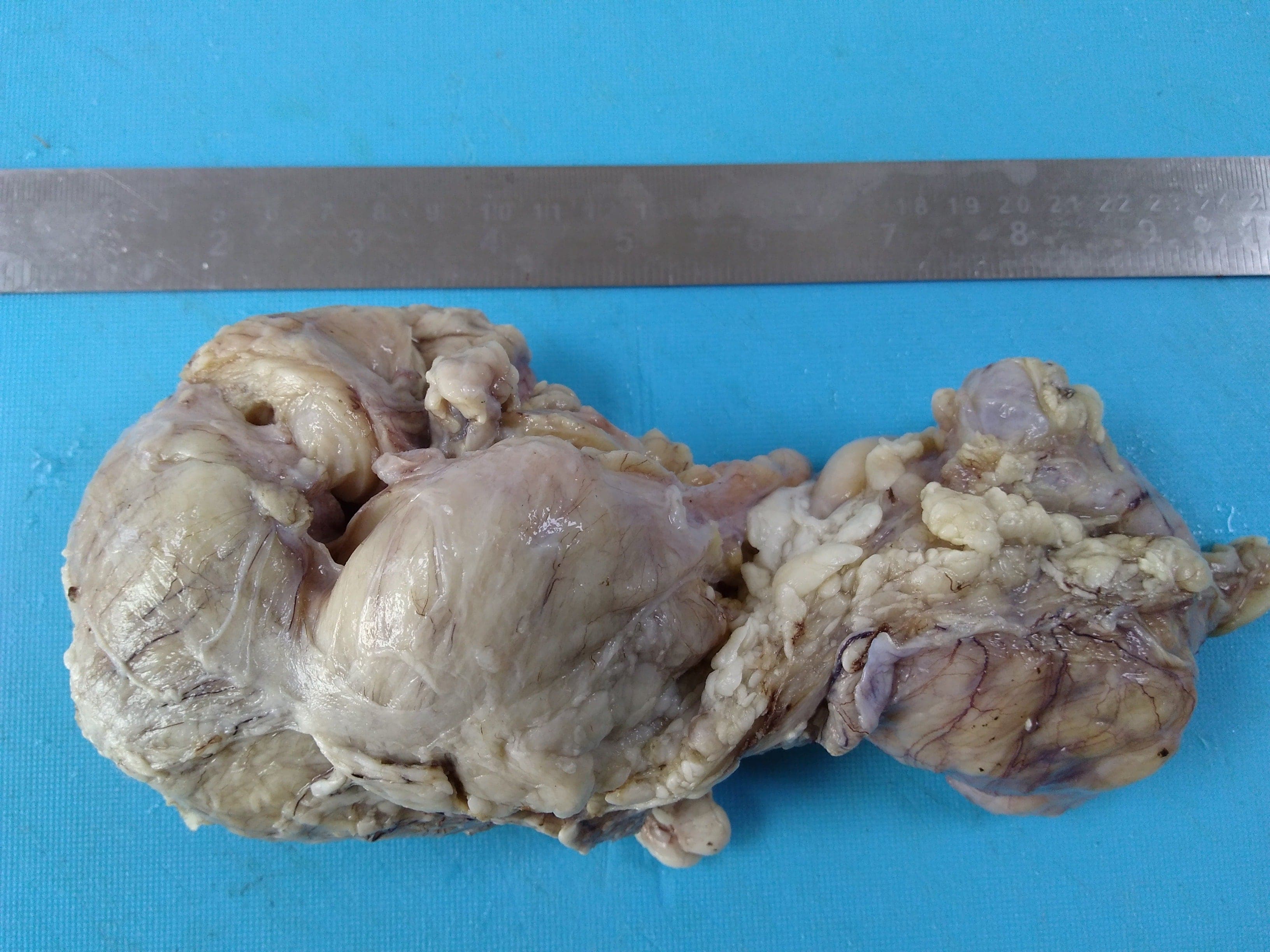
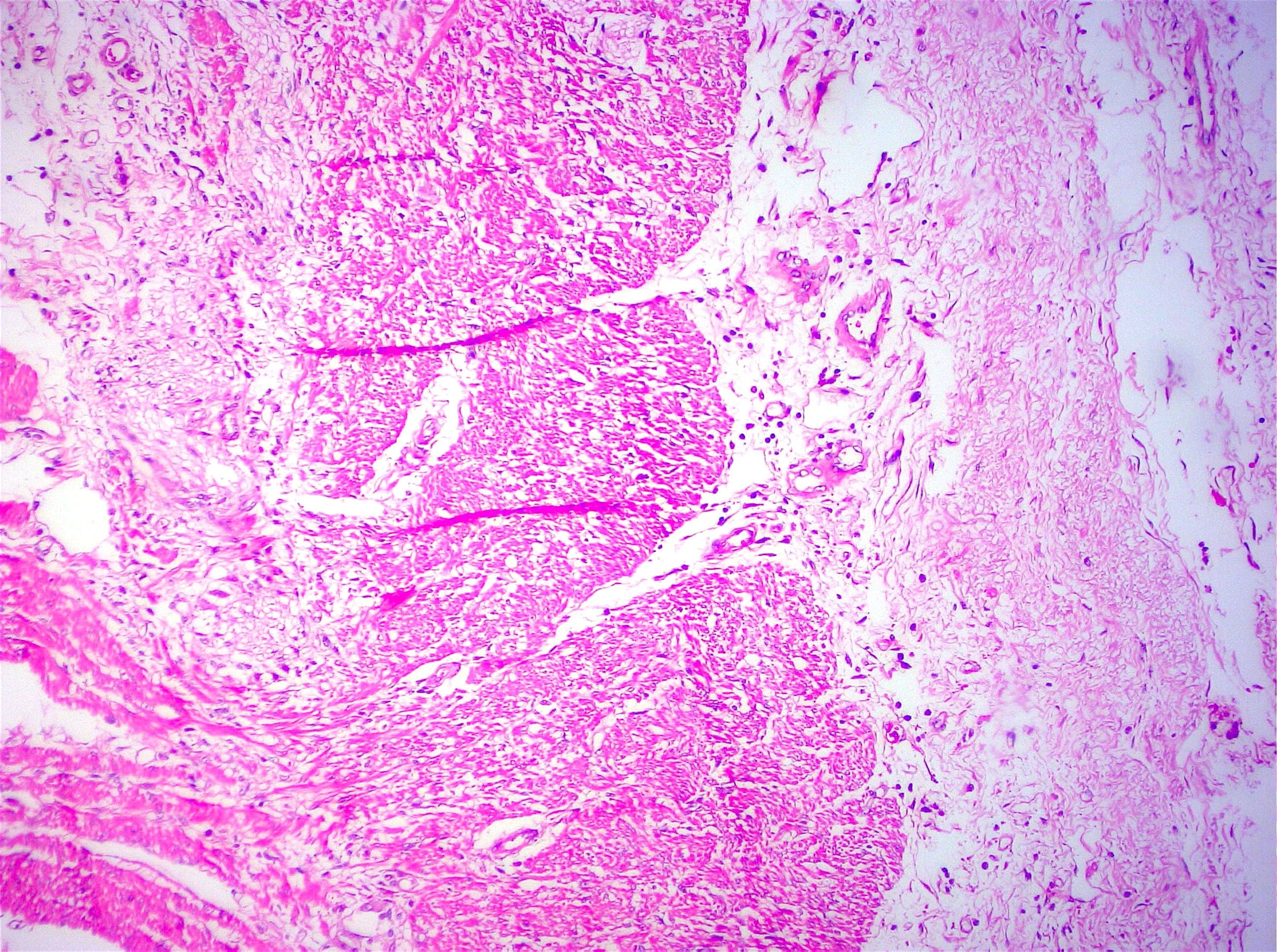
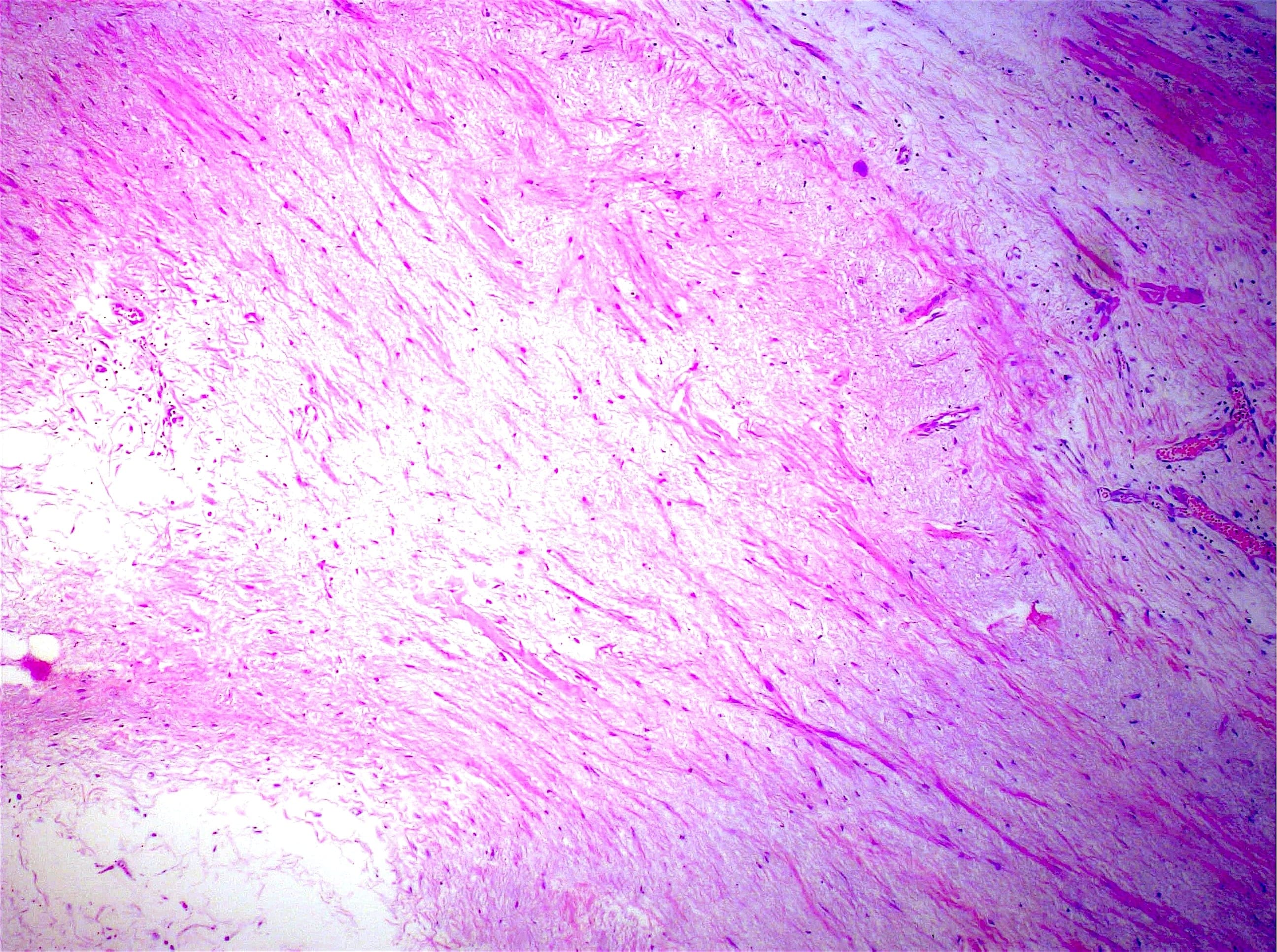
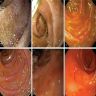
- Gross appearance depends on the region of the ampulla involved (Am J Surg Pathol 2012;36:1592)
- Periampullary adenocarcinomas
- Exophytic mass arising from the duodenal aspect of the ampulla
- Vegetating mass around the ampulla that may obscure the ampullary orifice
- Invasive component of the lesion may extend beyond the ampulla to involve the adjacent duodenal wall
- Large tumors: average size of 4.7 cm with a 2.4 cm invasive component
- 50% of cases have lymph node involvement at time of resection
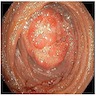
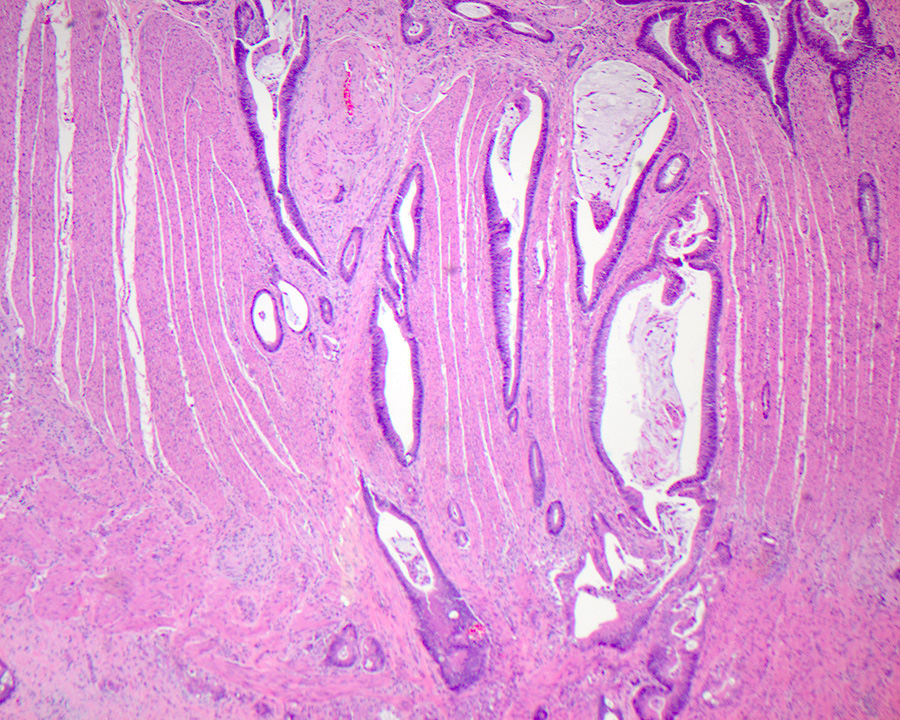
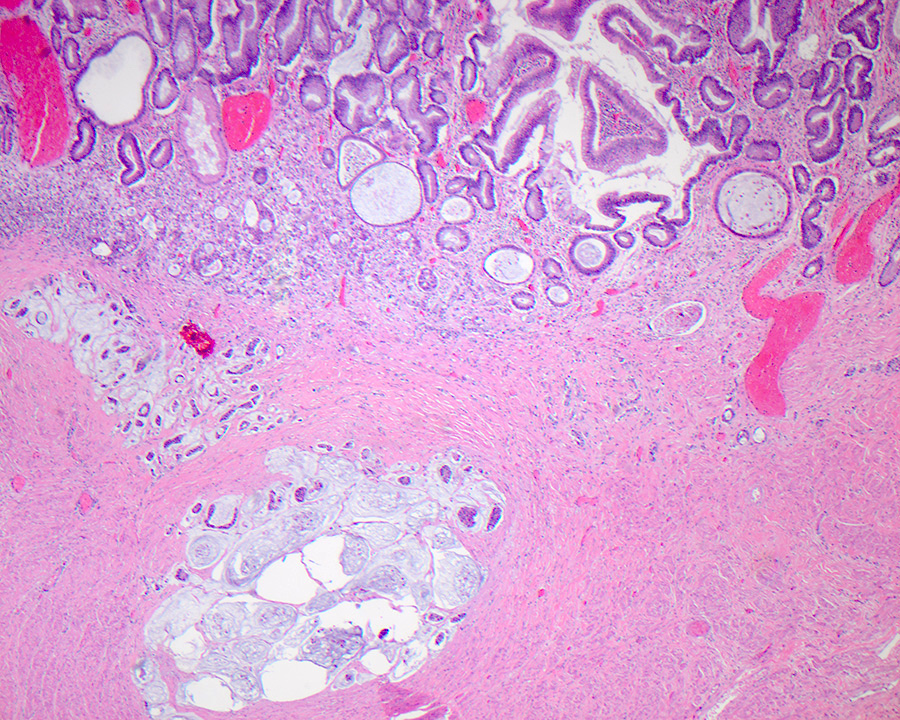
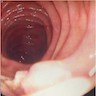
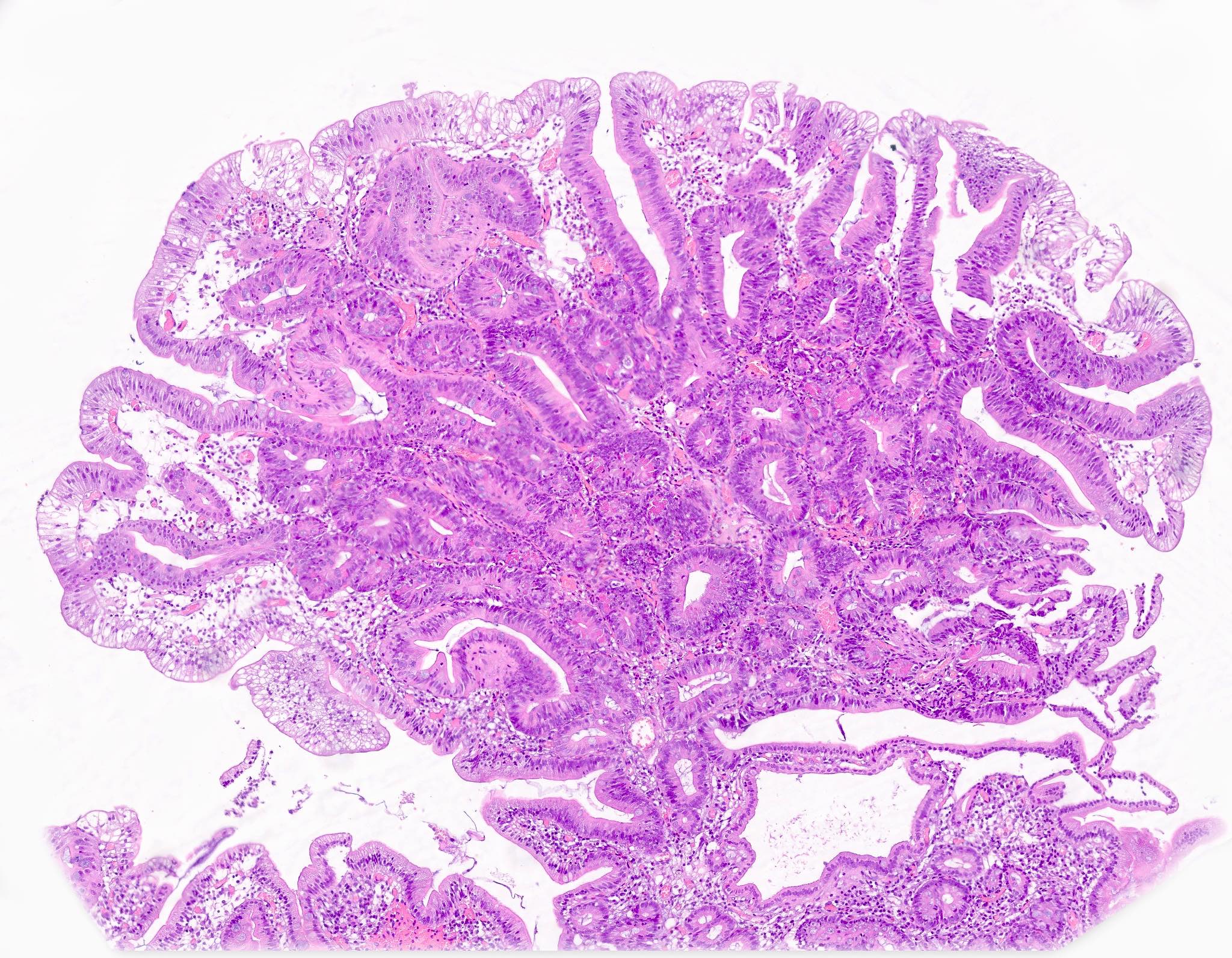
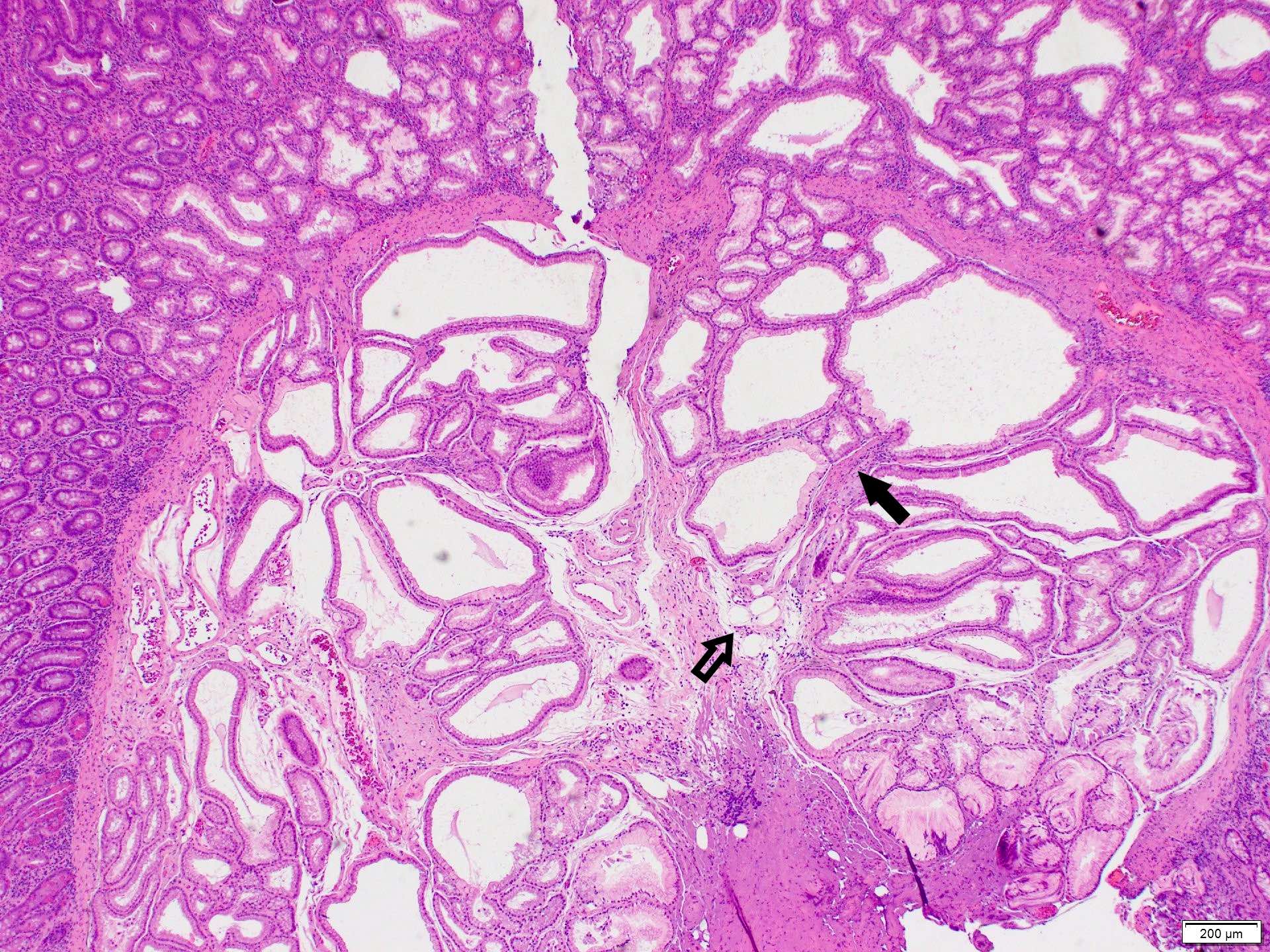
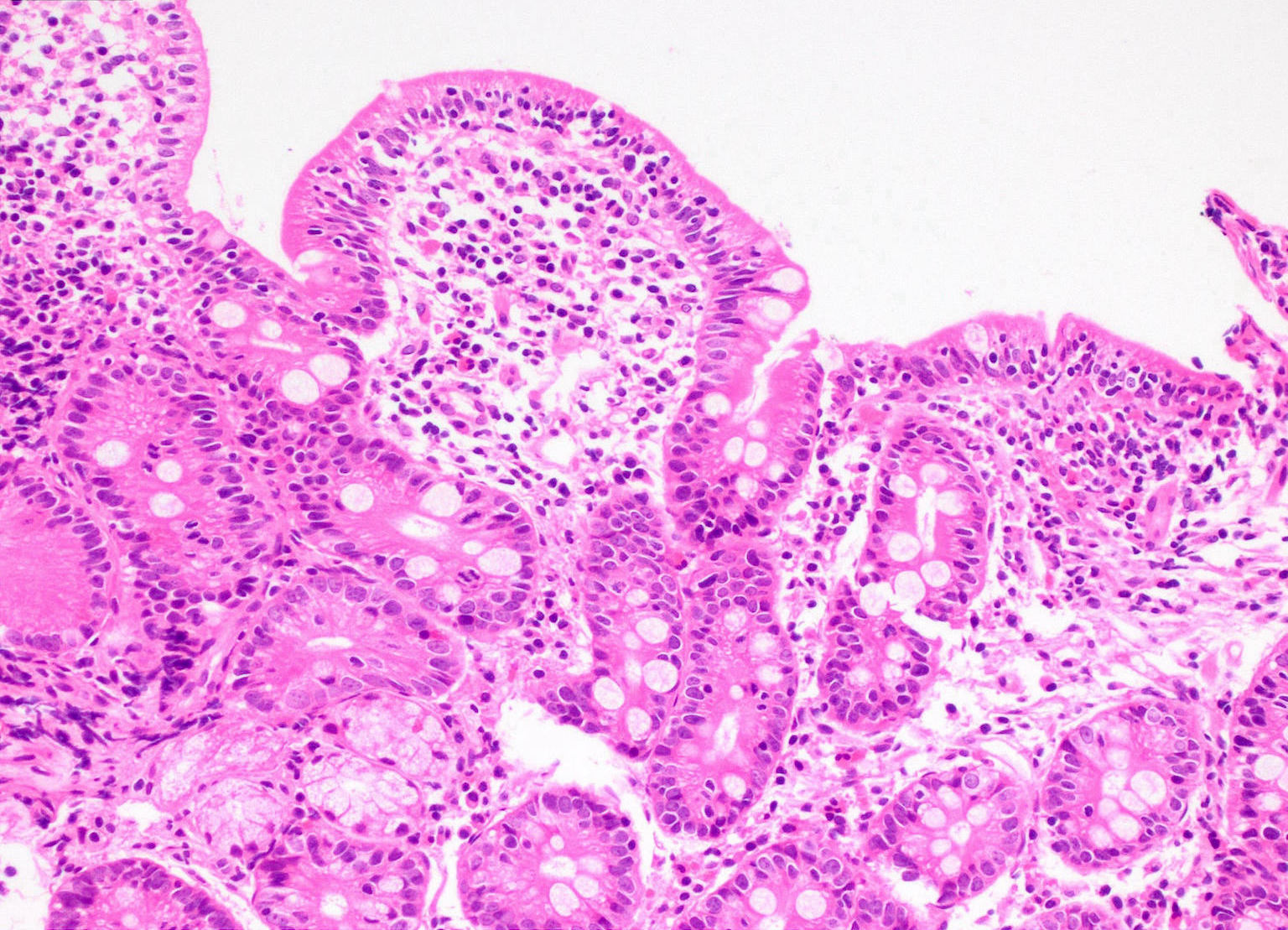
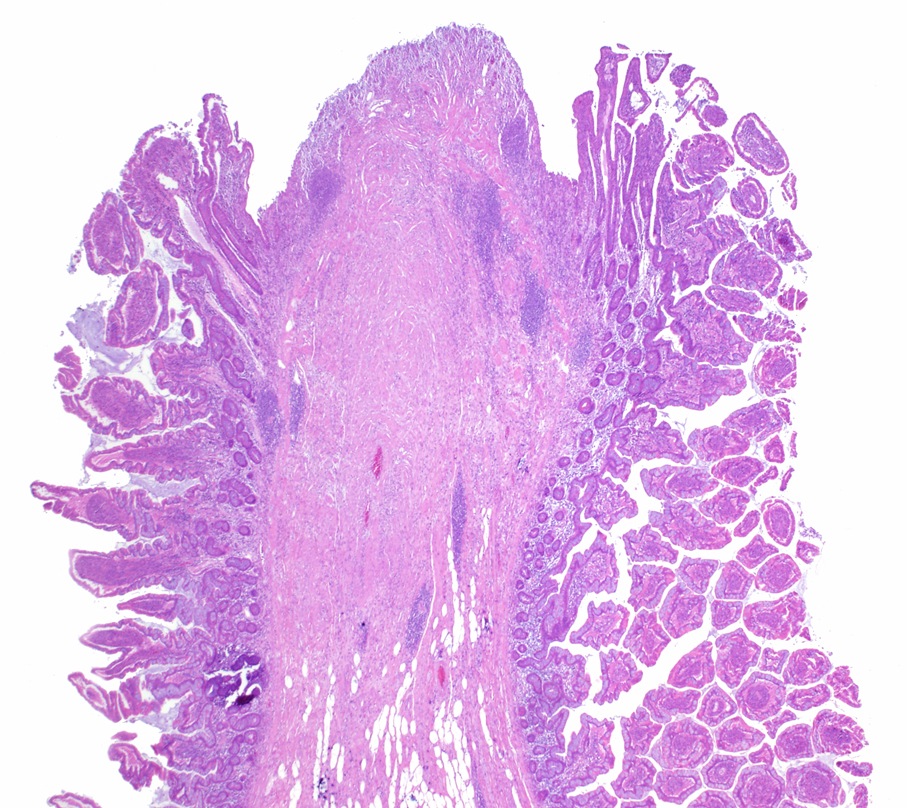
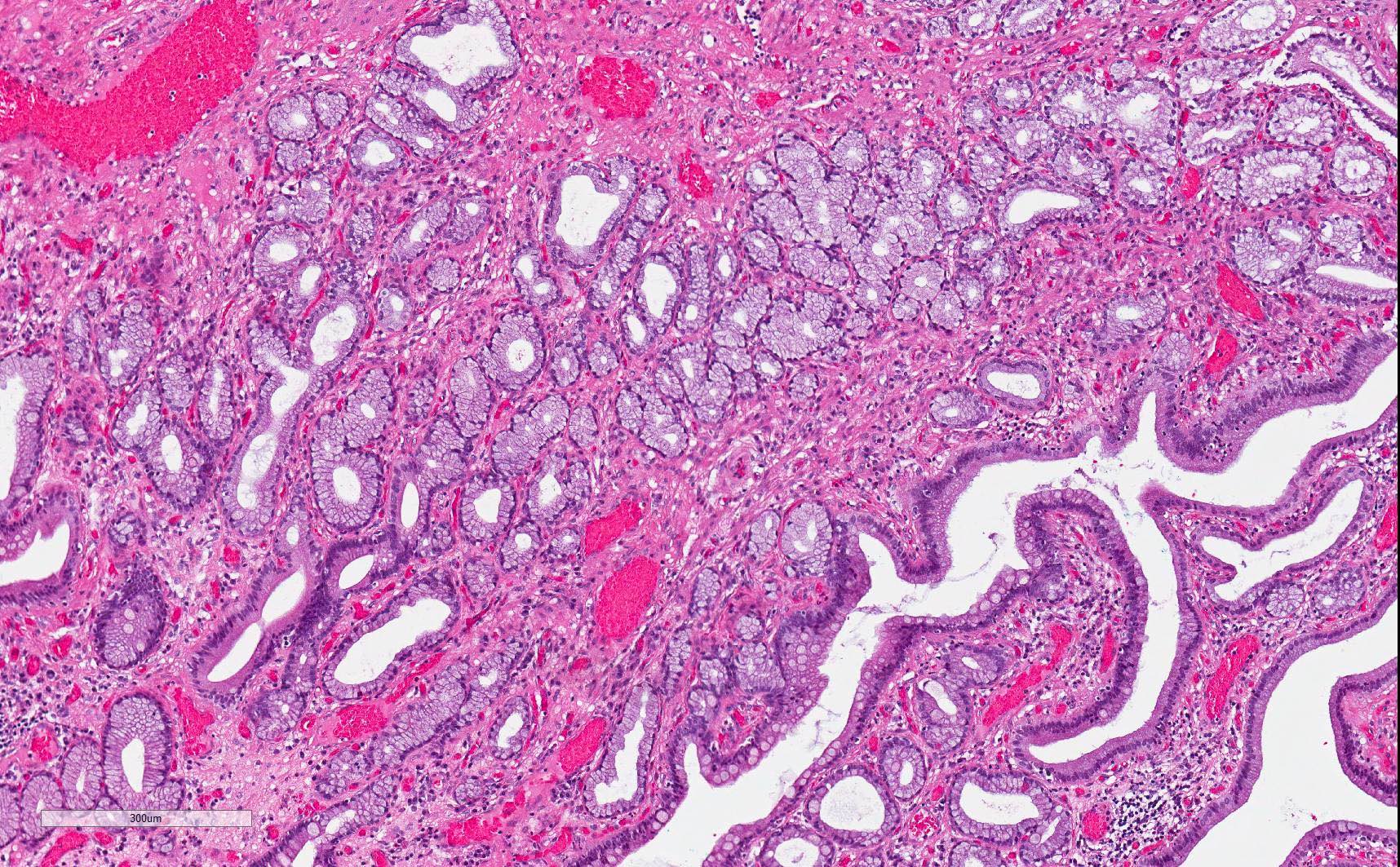
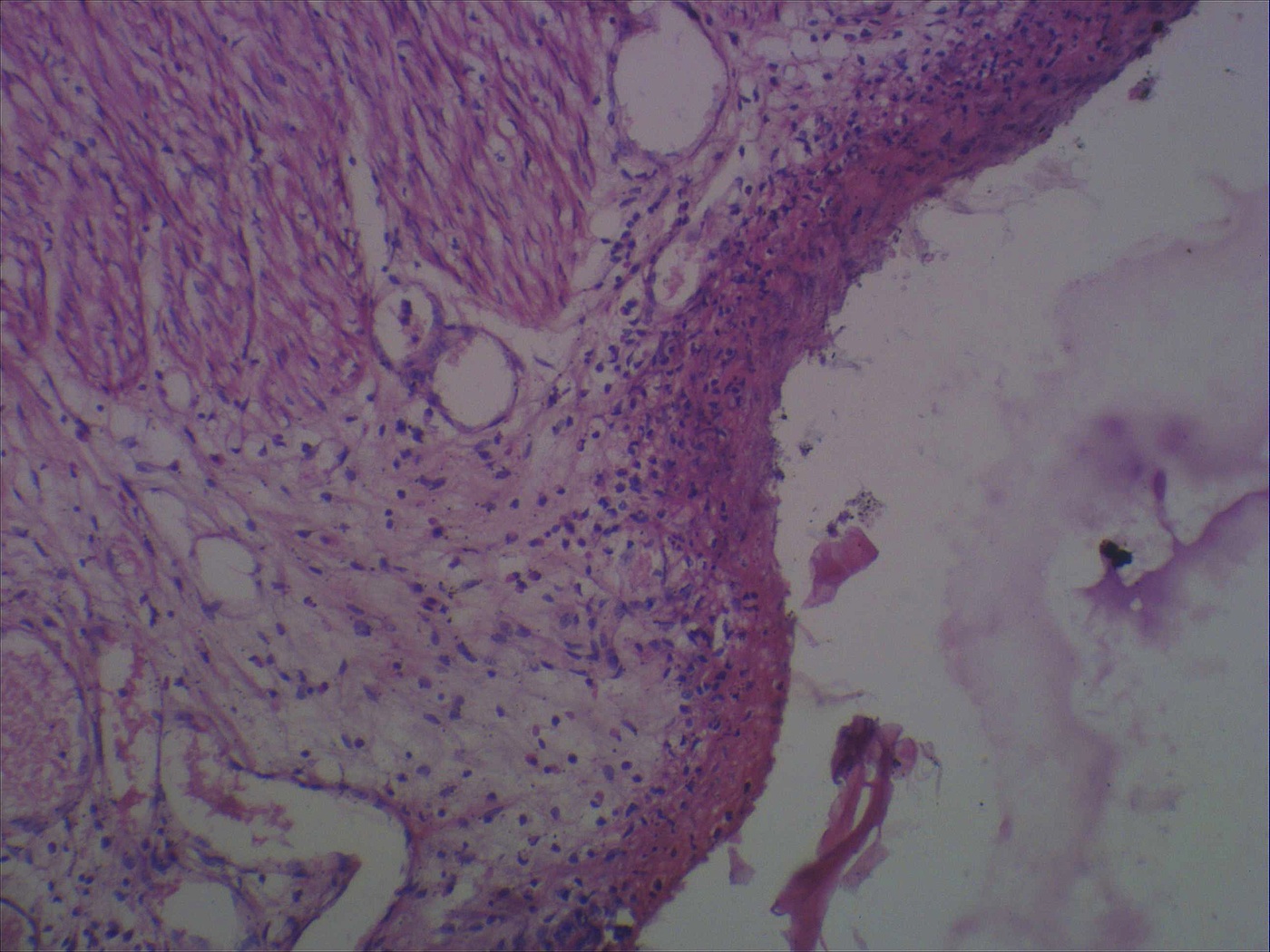
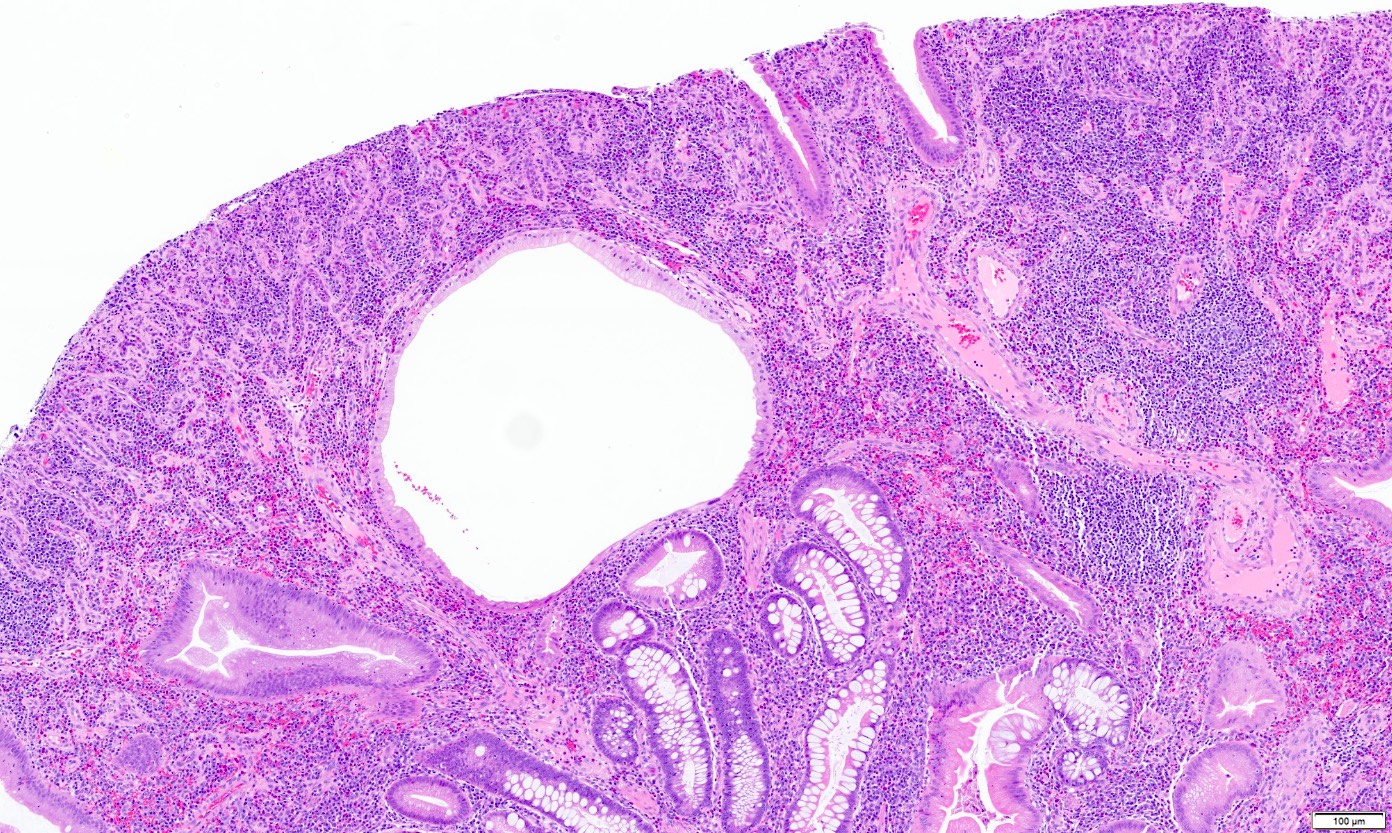
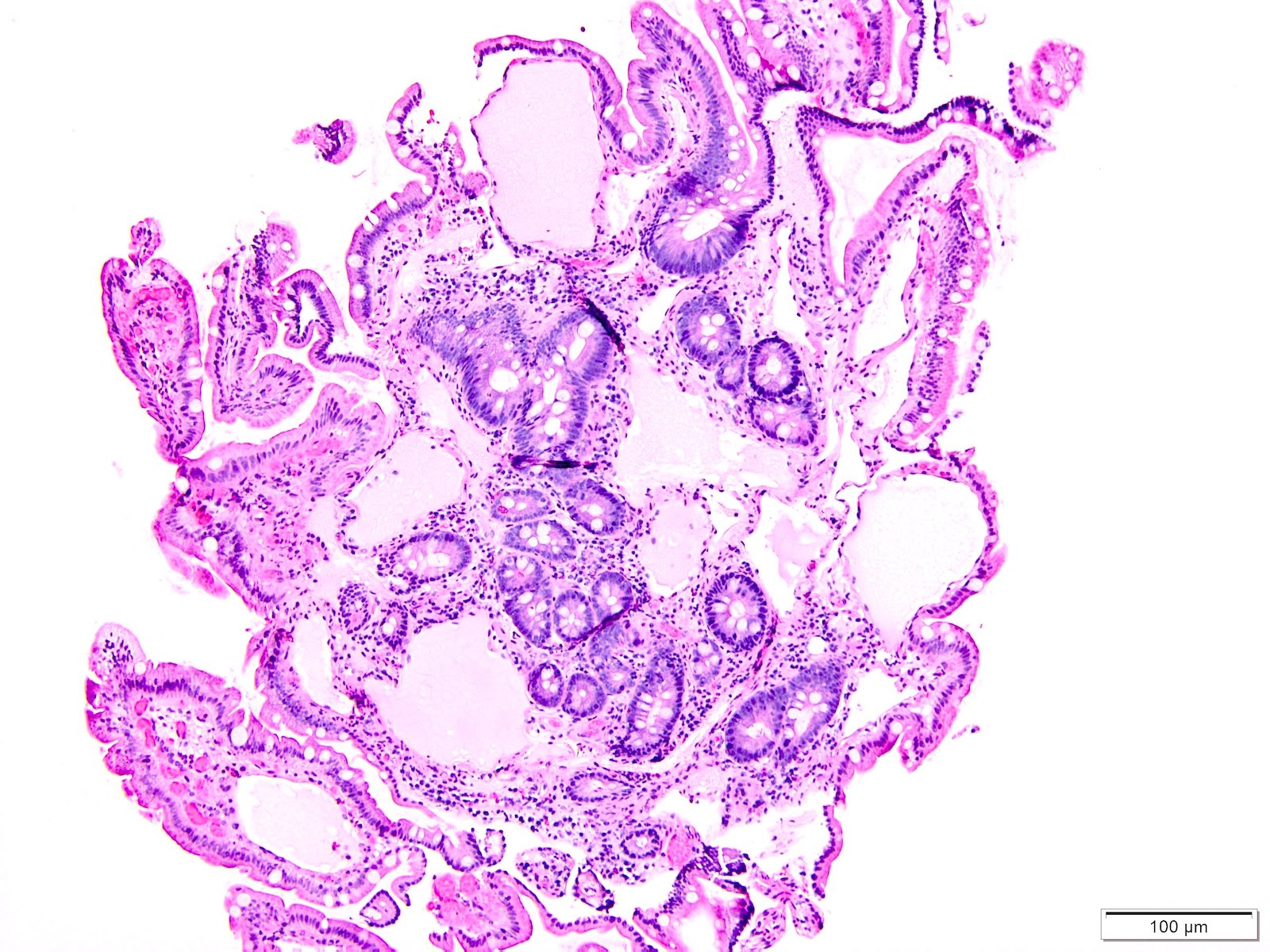
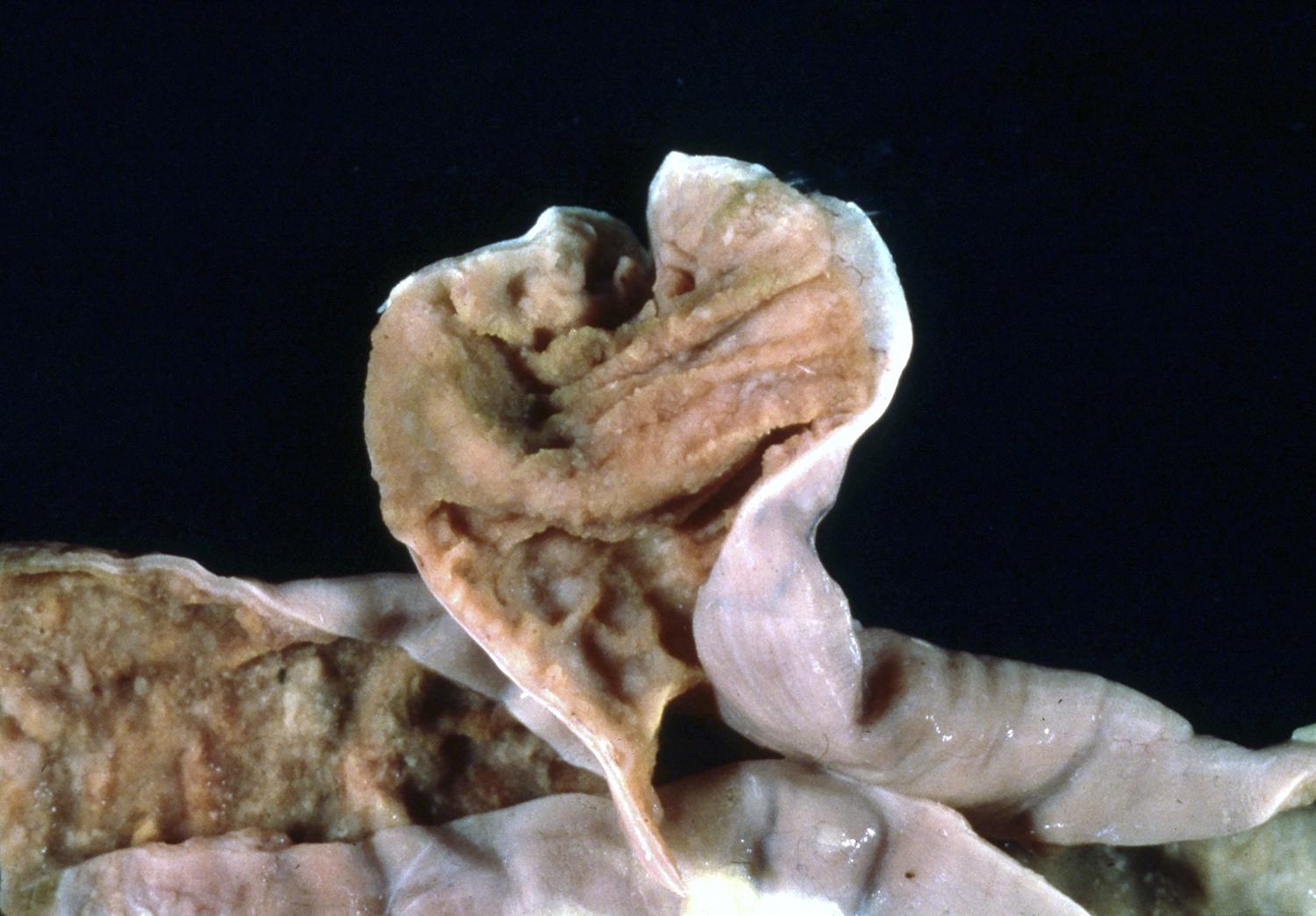
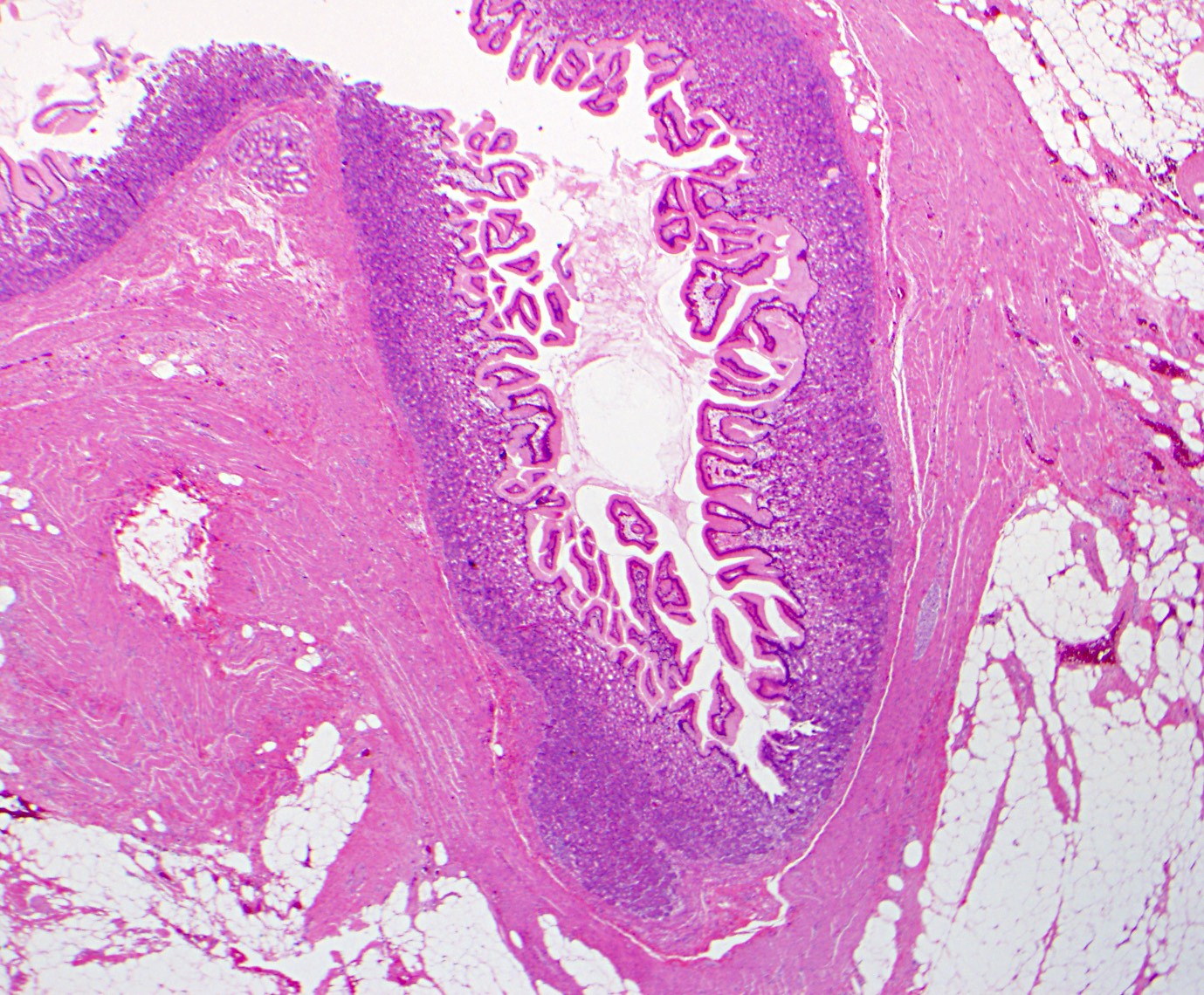
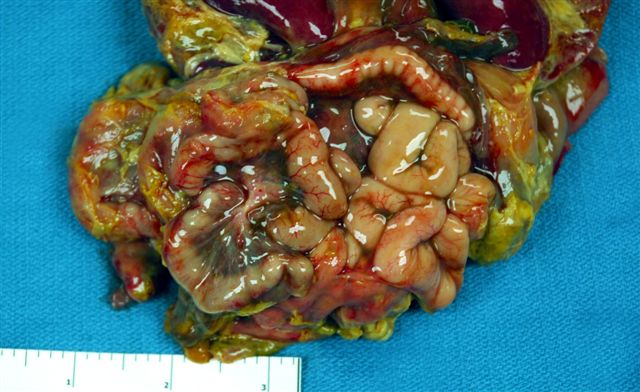
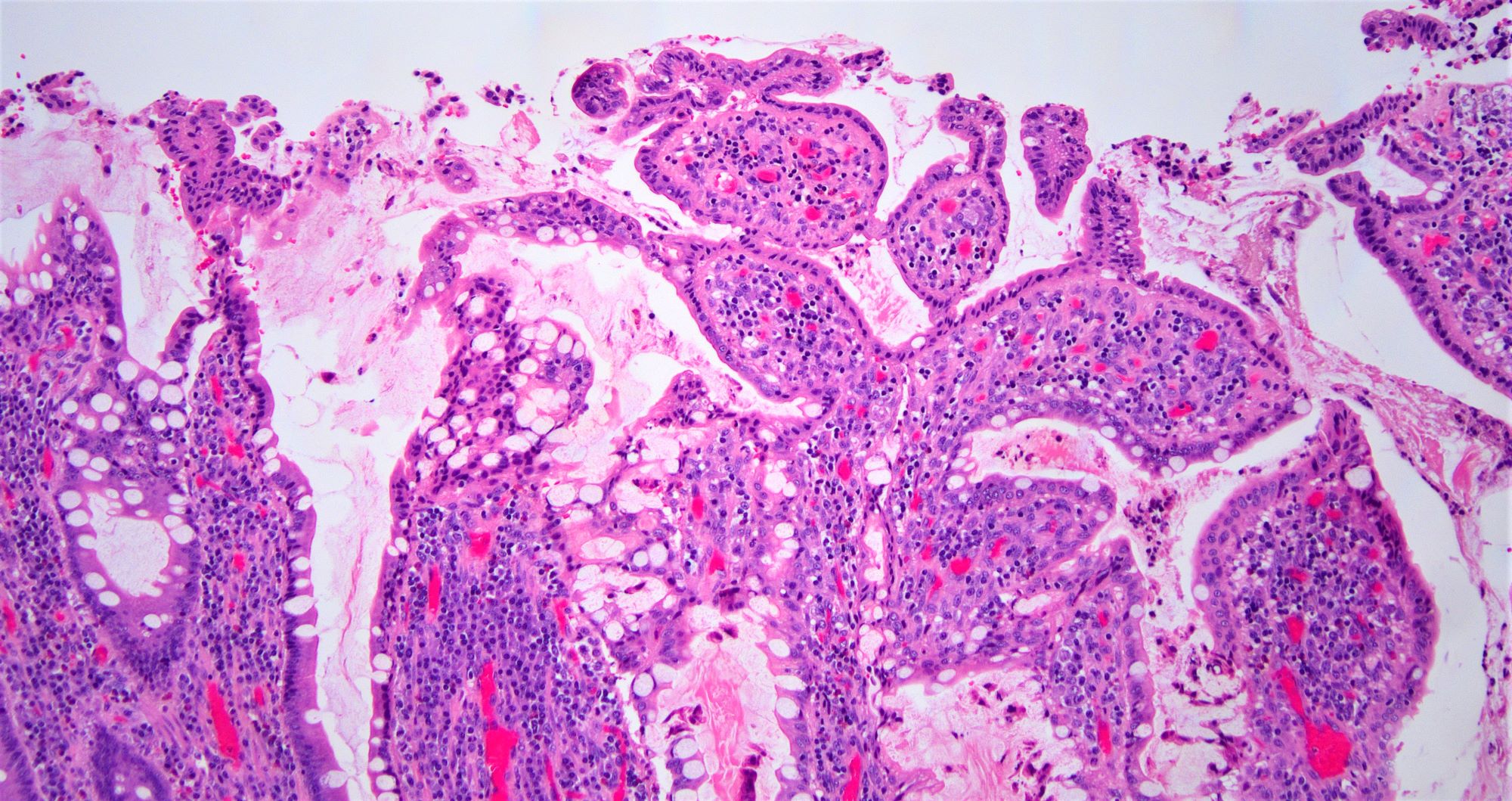
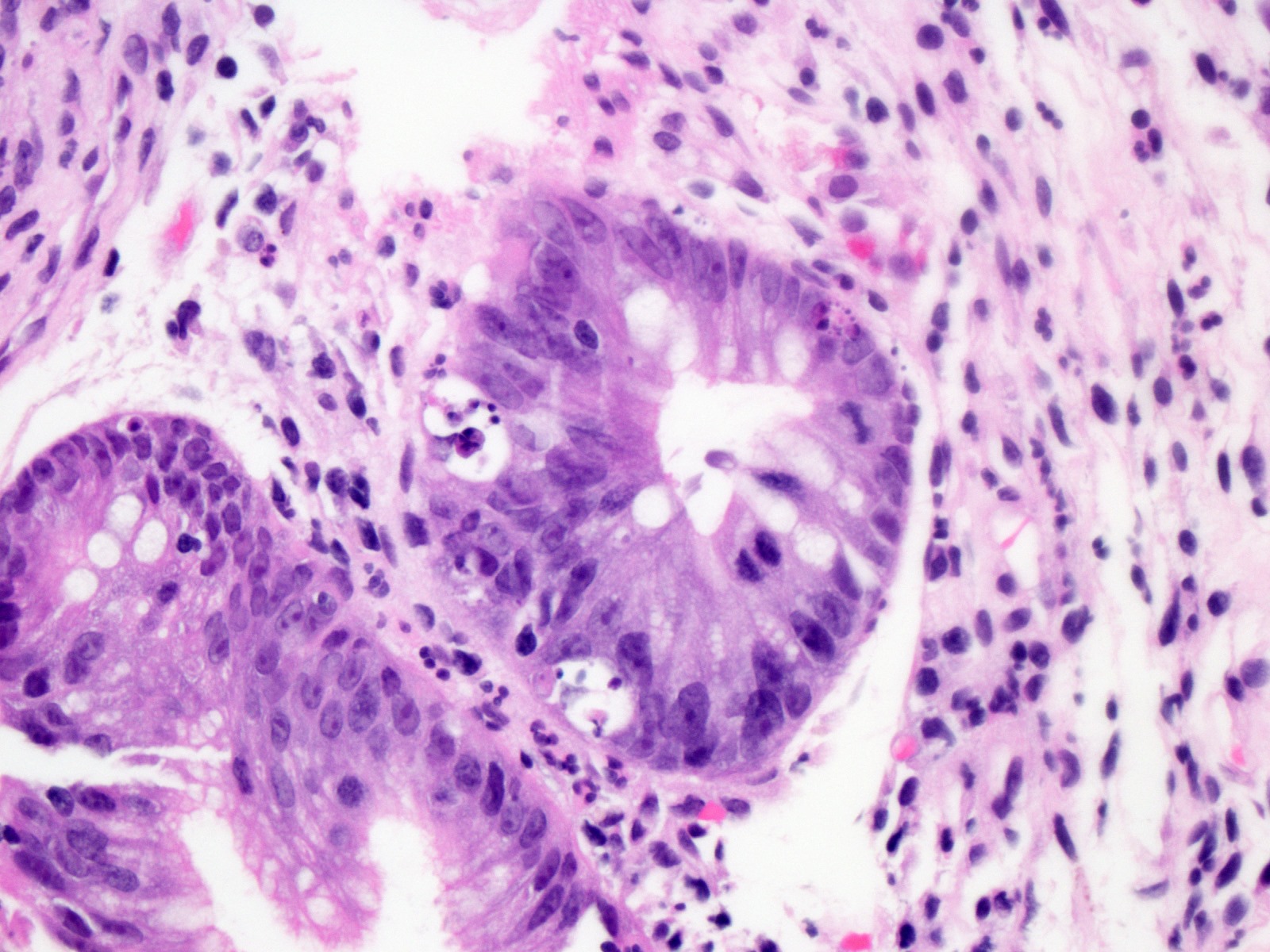
- Intra-ampullary adenocarcinomas
- Arising from an intra-ampullary papillary tubular neoplasm
- Appear as a mucosa covered bulge with a dilated ampullary orifice and bulky intraluminal growth within the ampulla
- Average tumor size of 2.9 cm with an invasive component of 1.5 cm
- 28% of cases have lymph node involvement at time of resection
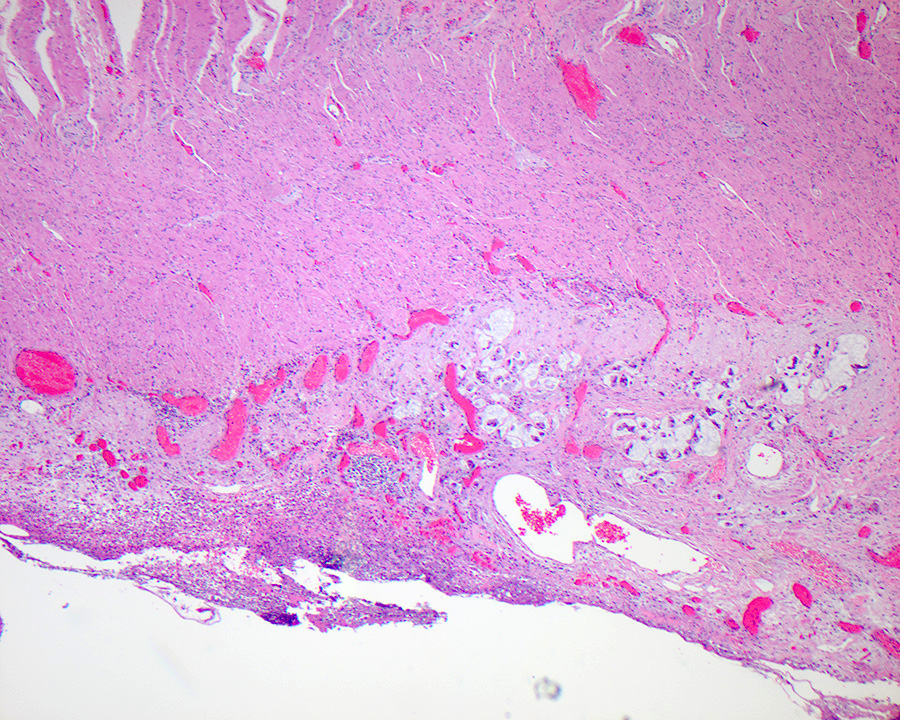
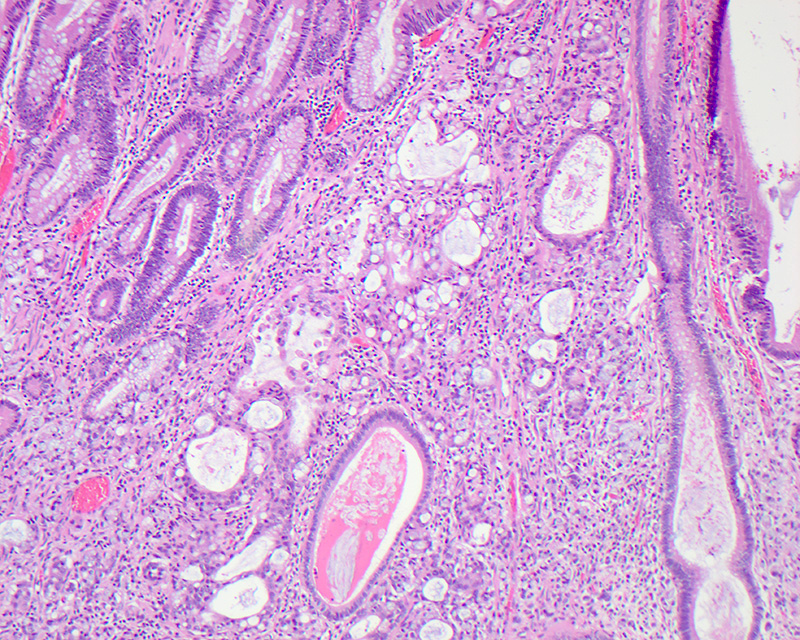
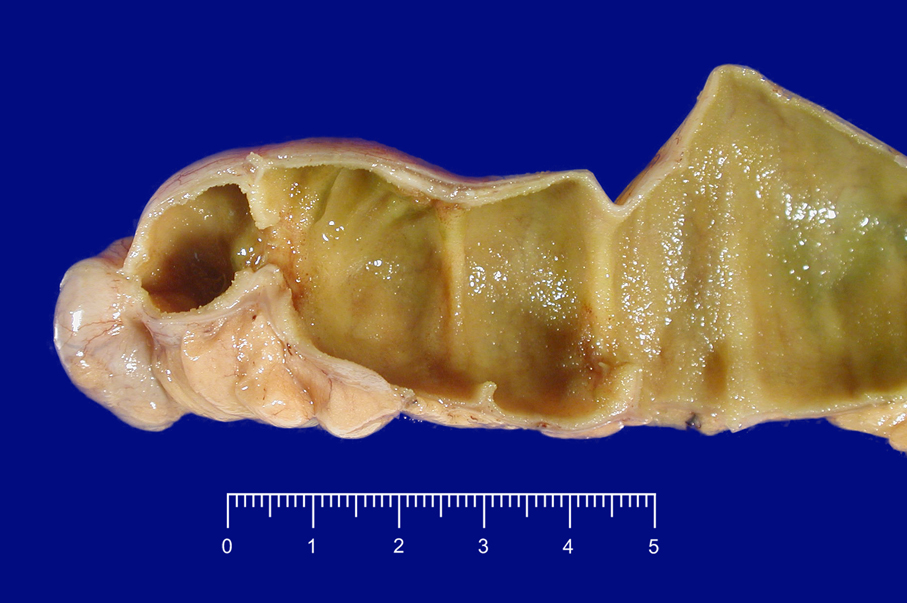
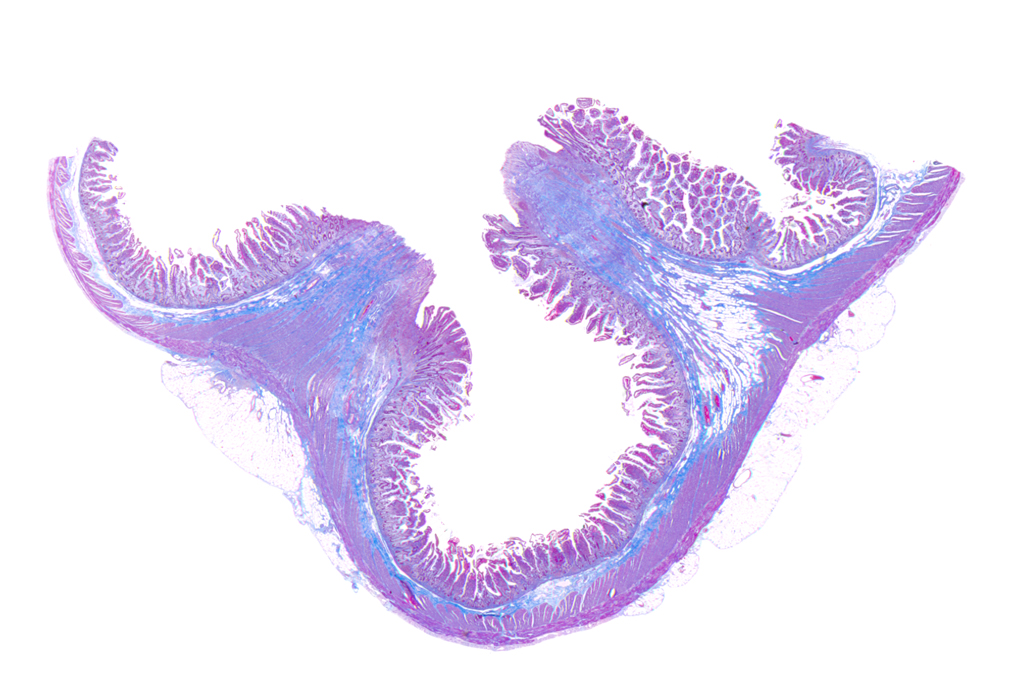
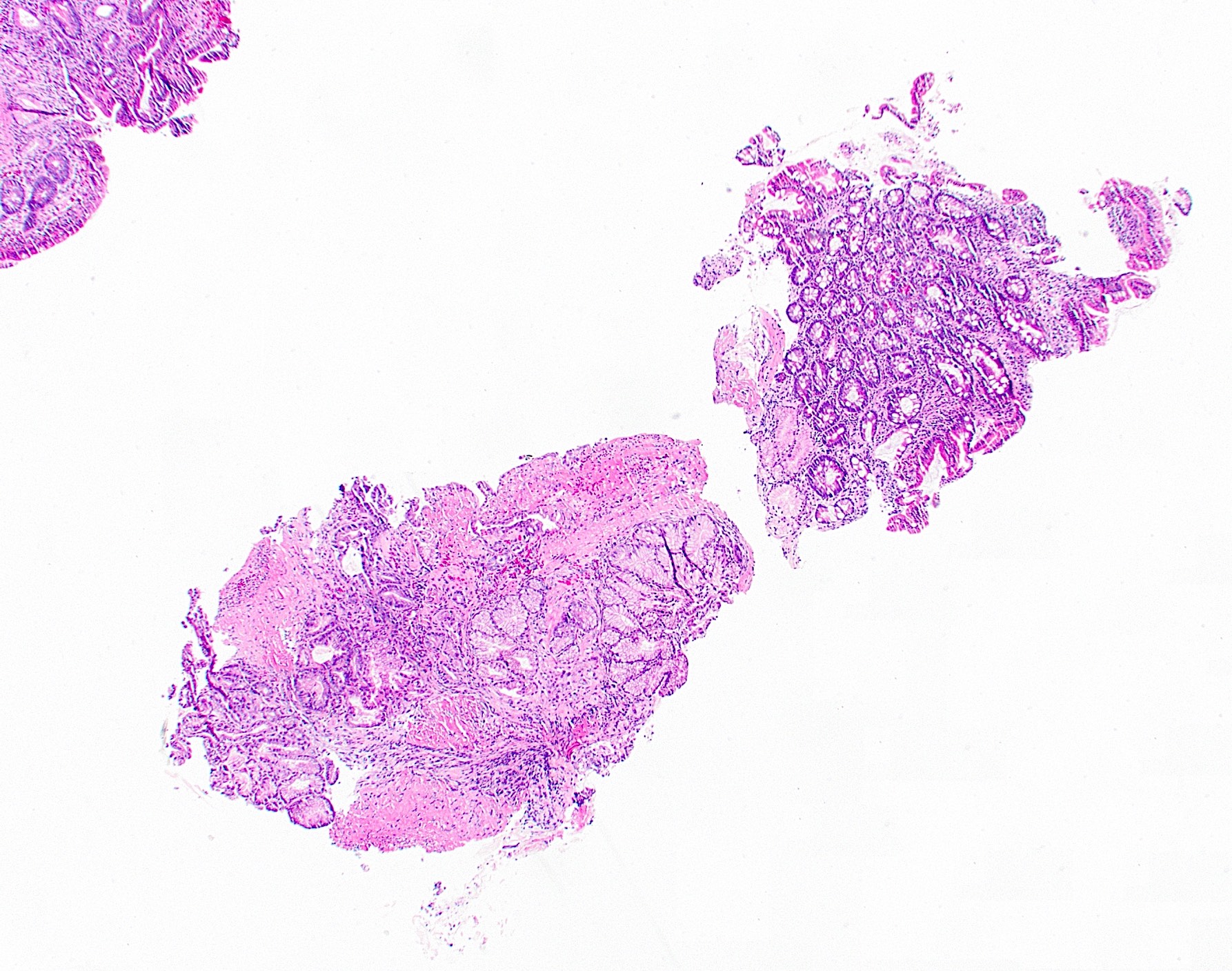
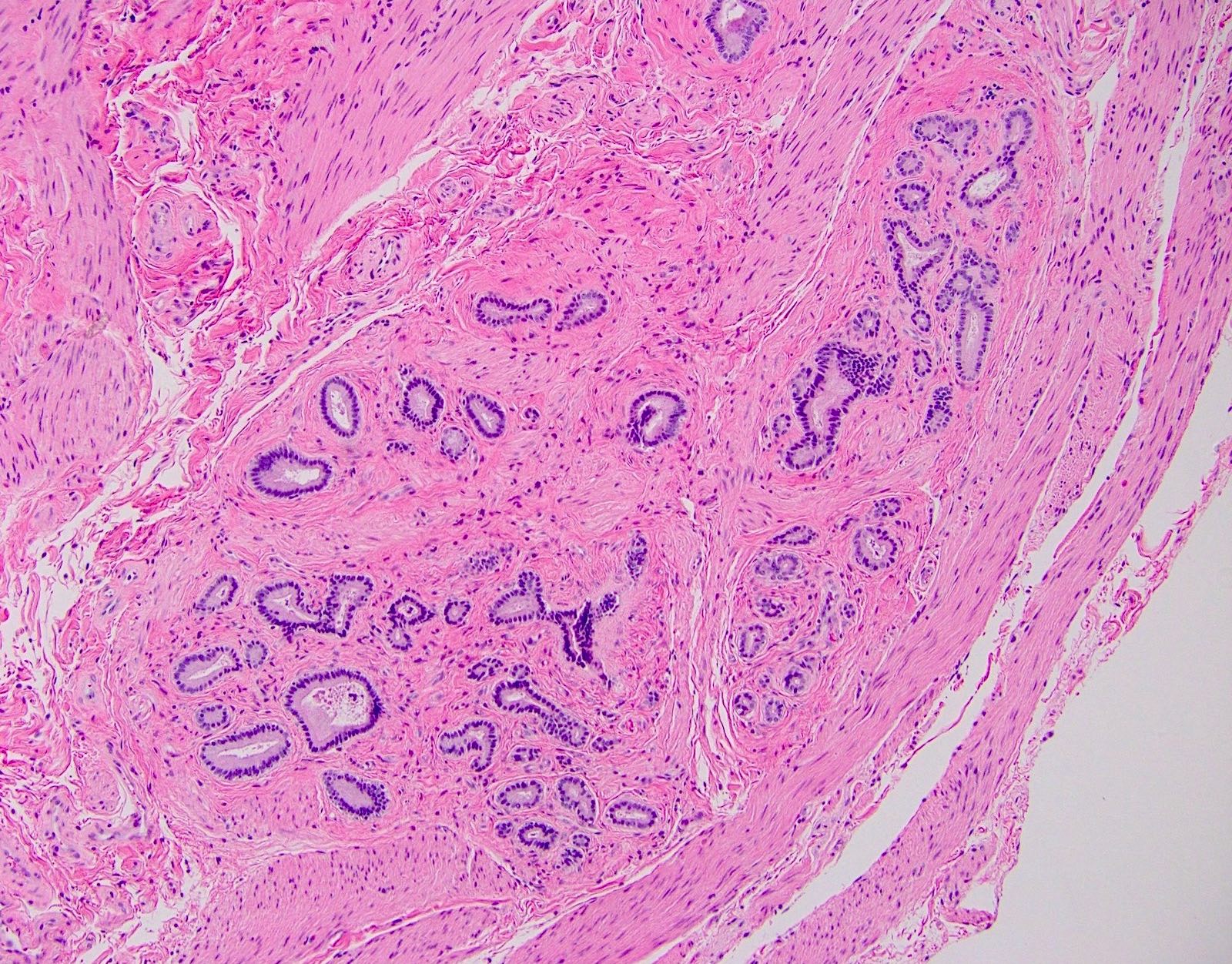
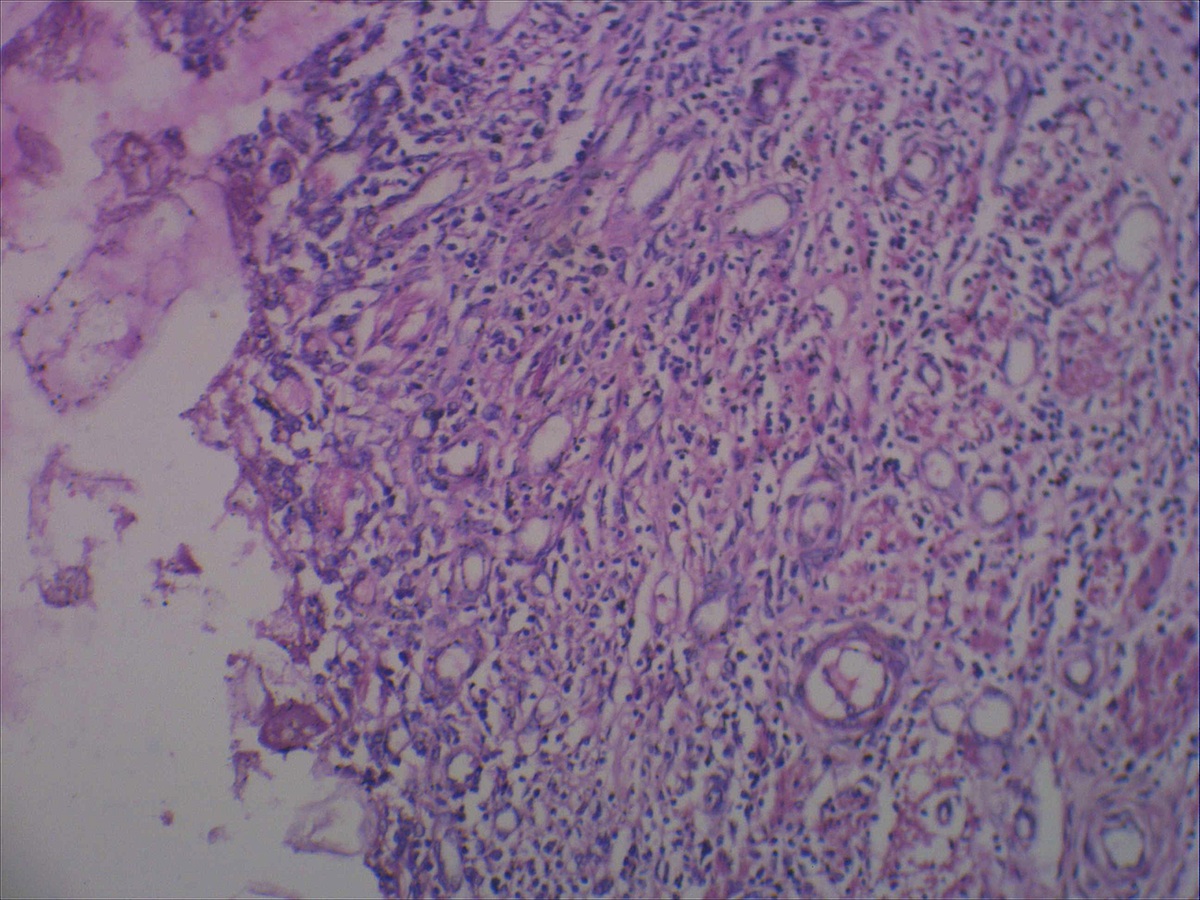
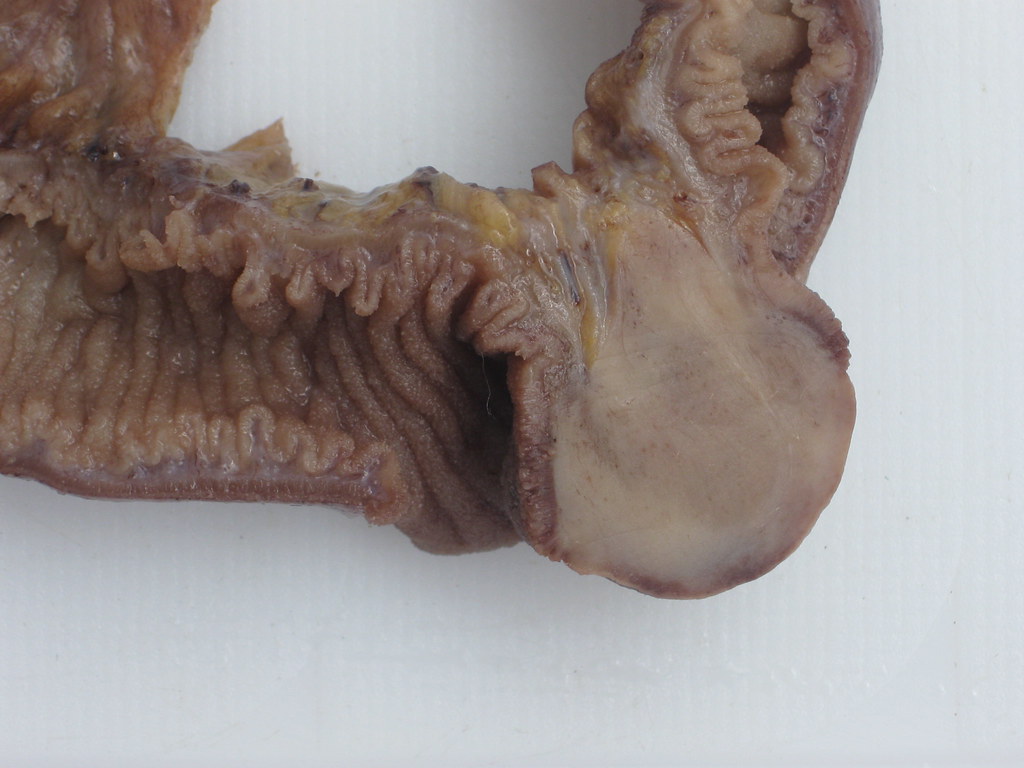
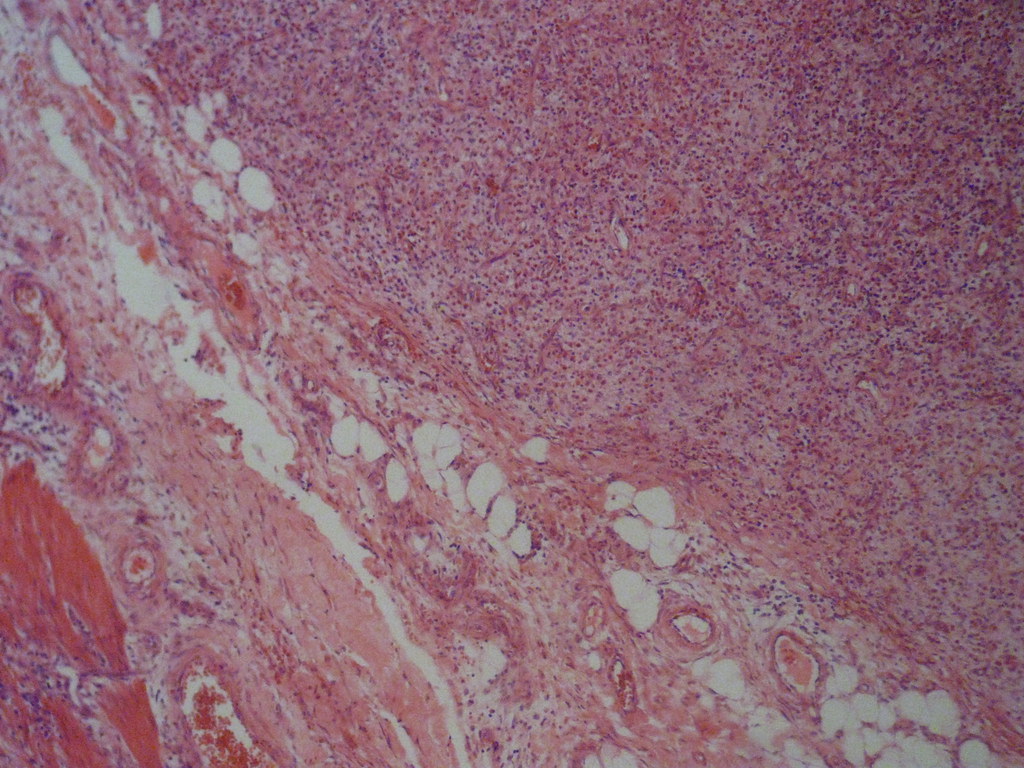
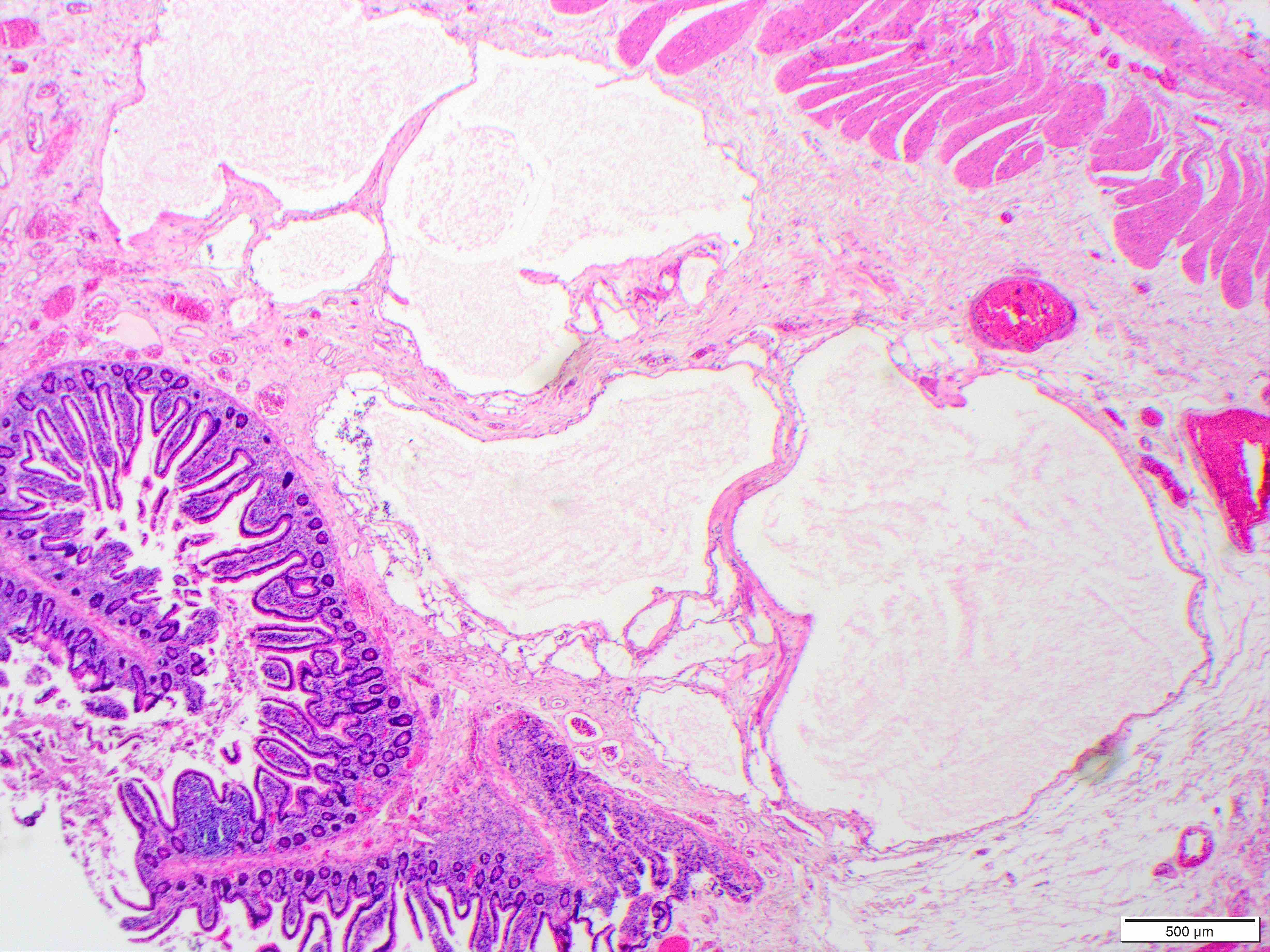
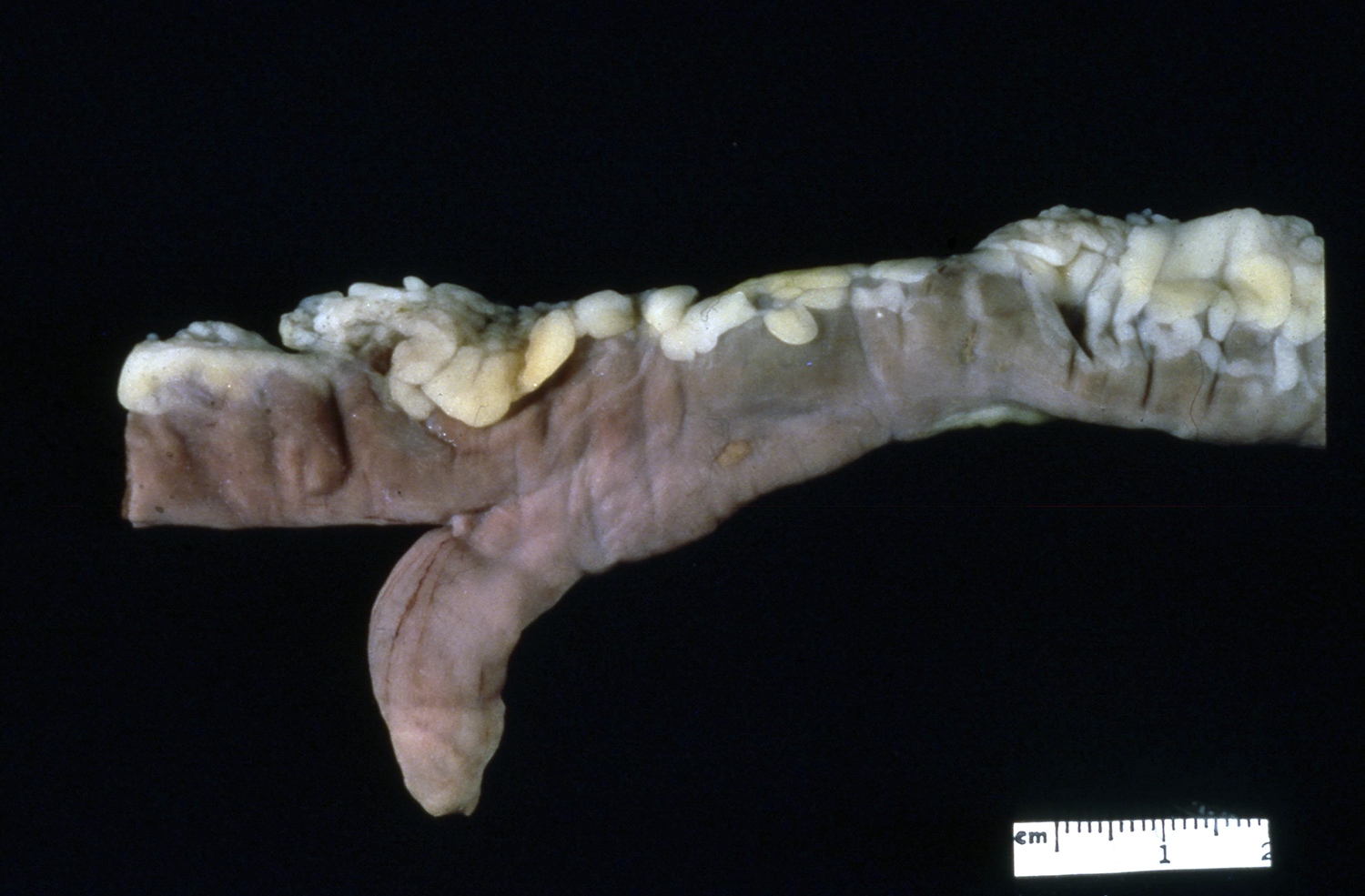
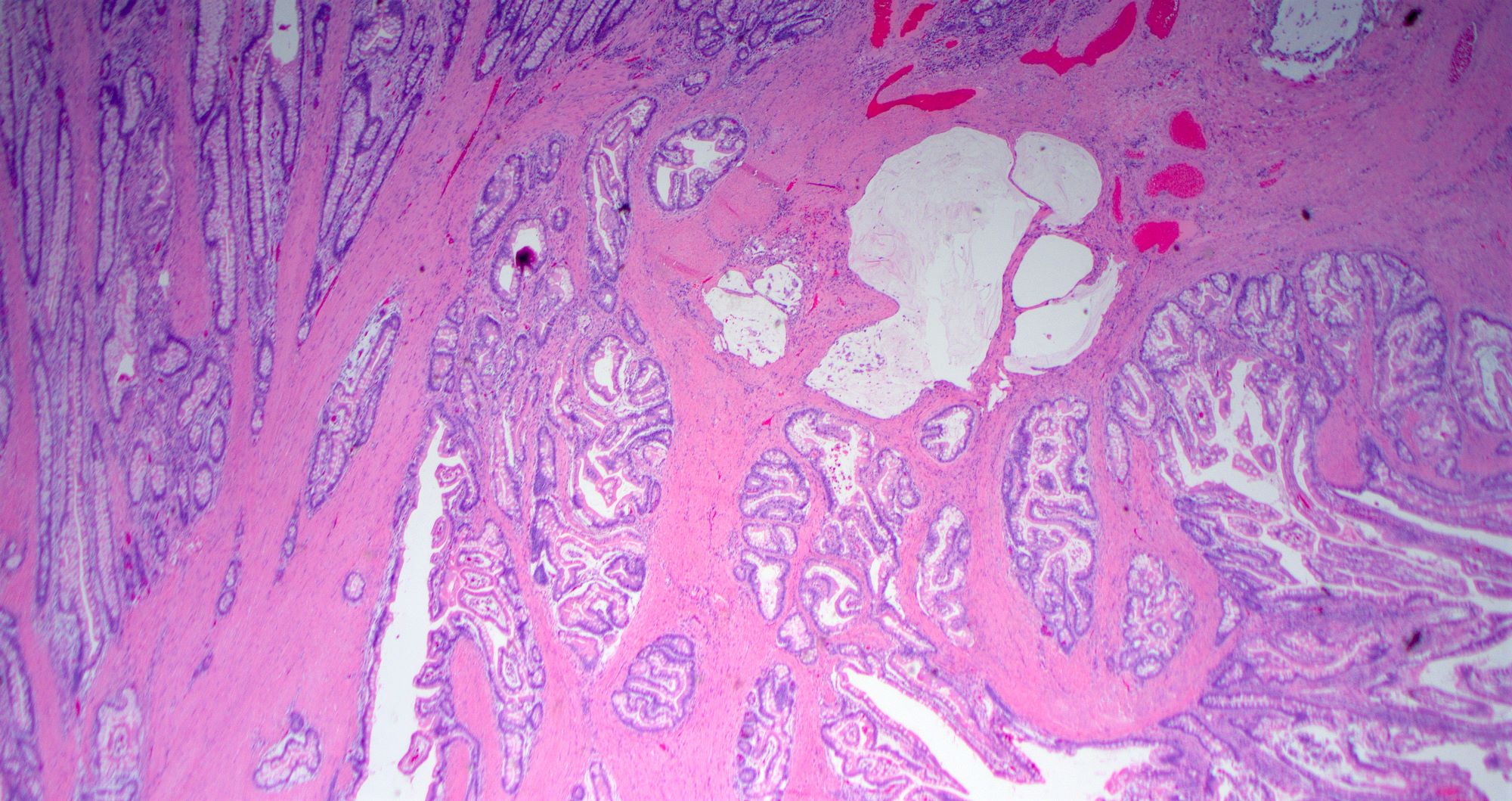
- Ampullary ductal adenocarcinomas
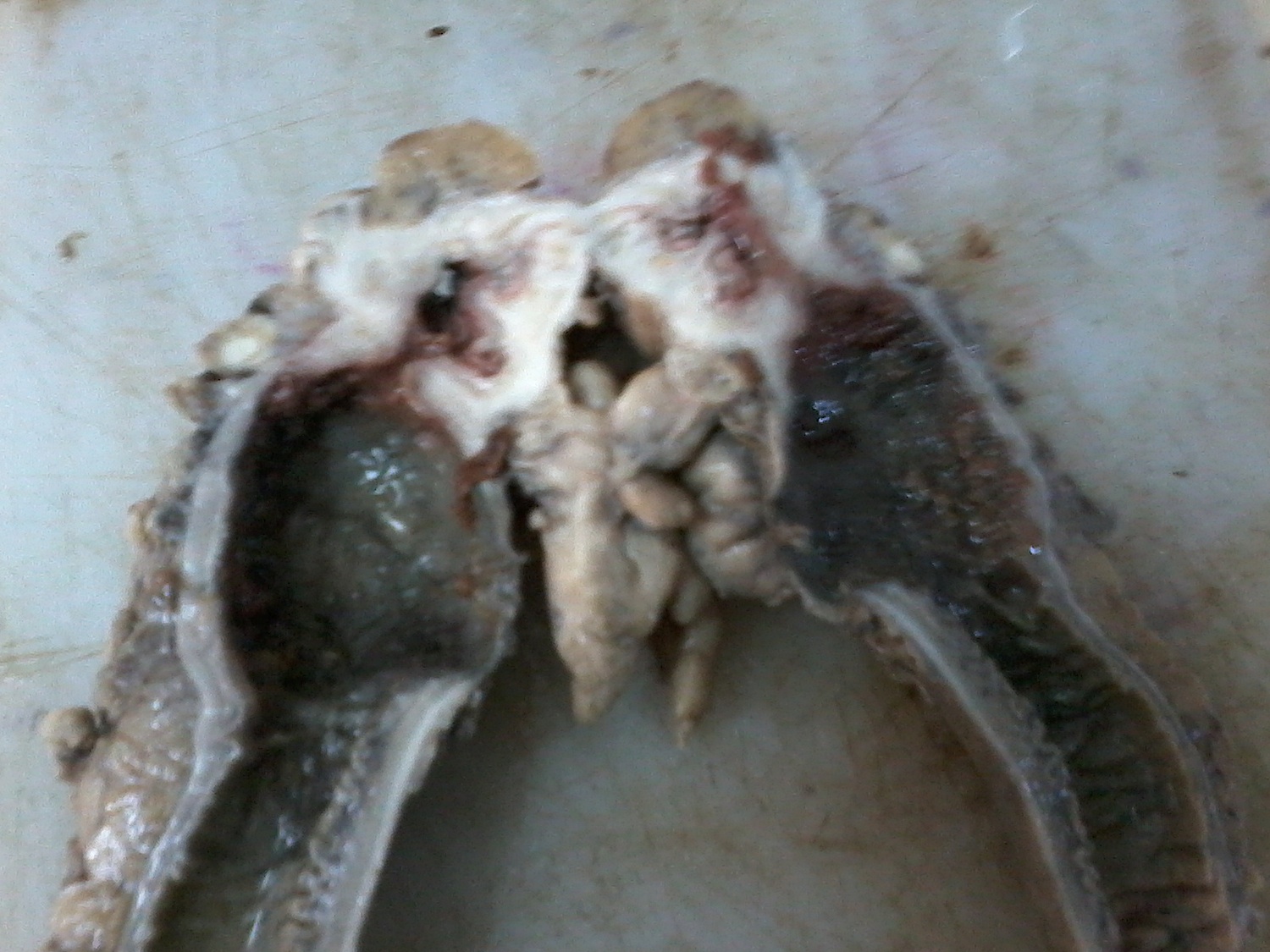
- Appear as small concentric elevations and ulcerating retractions around the ampullary orifice
- Upon bivalving the specimen along the duct, concentric thickening with or without stricturing of the intra-ampullary duct will be seen
- Small tumor size: average of 1.9 cm
- Low incidence of lymph node spread
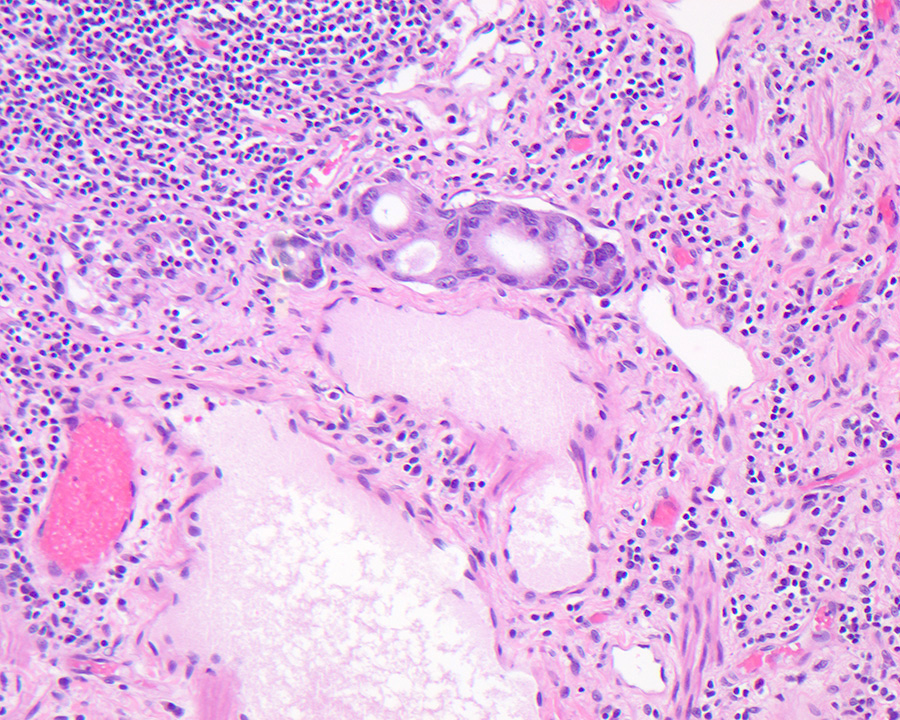
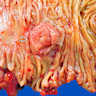
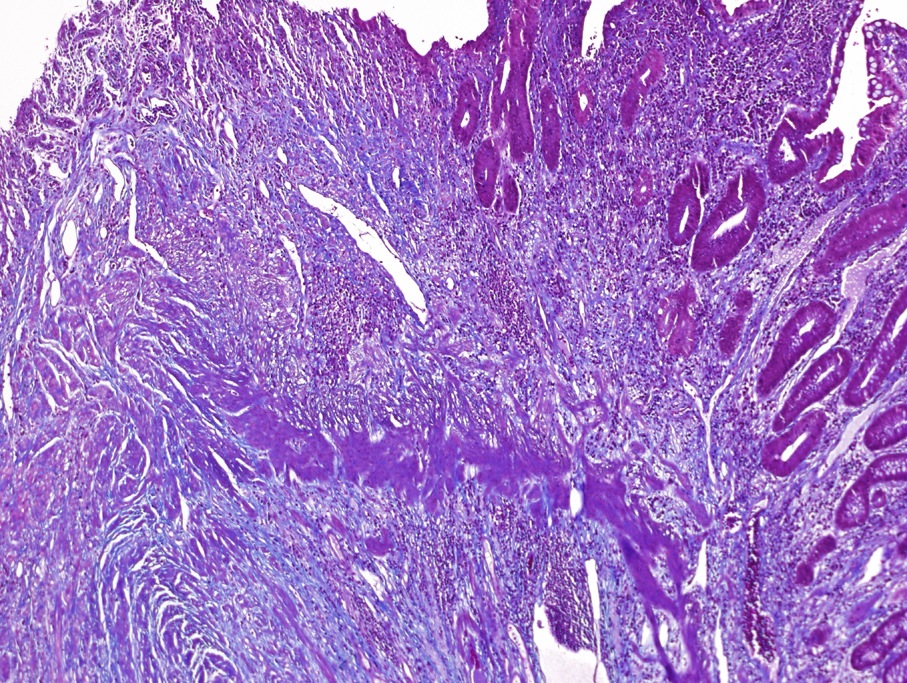
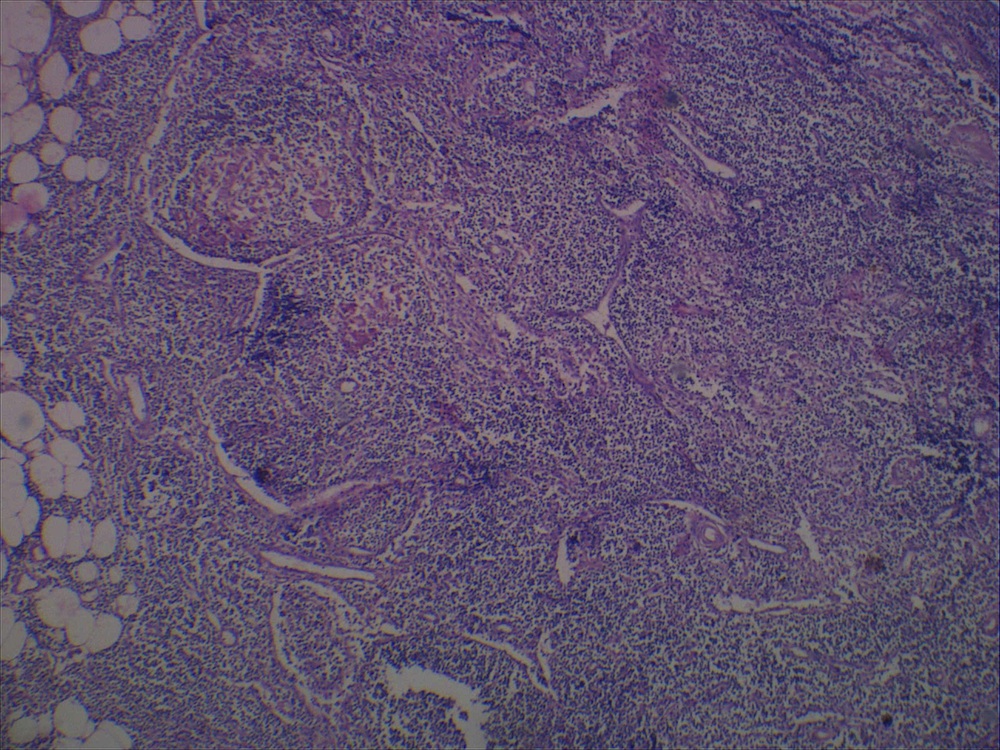
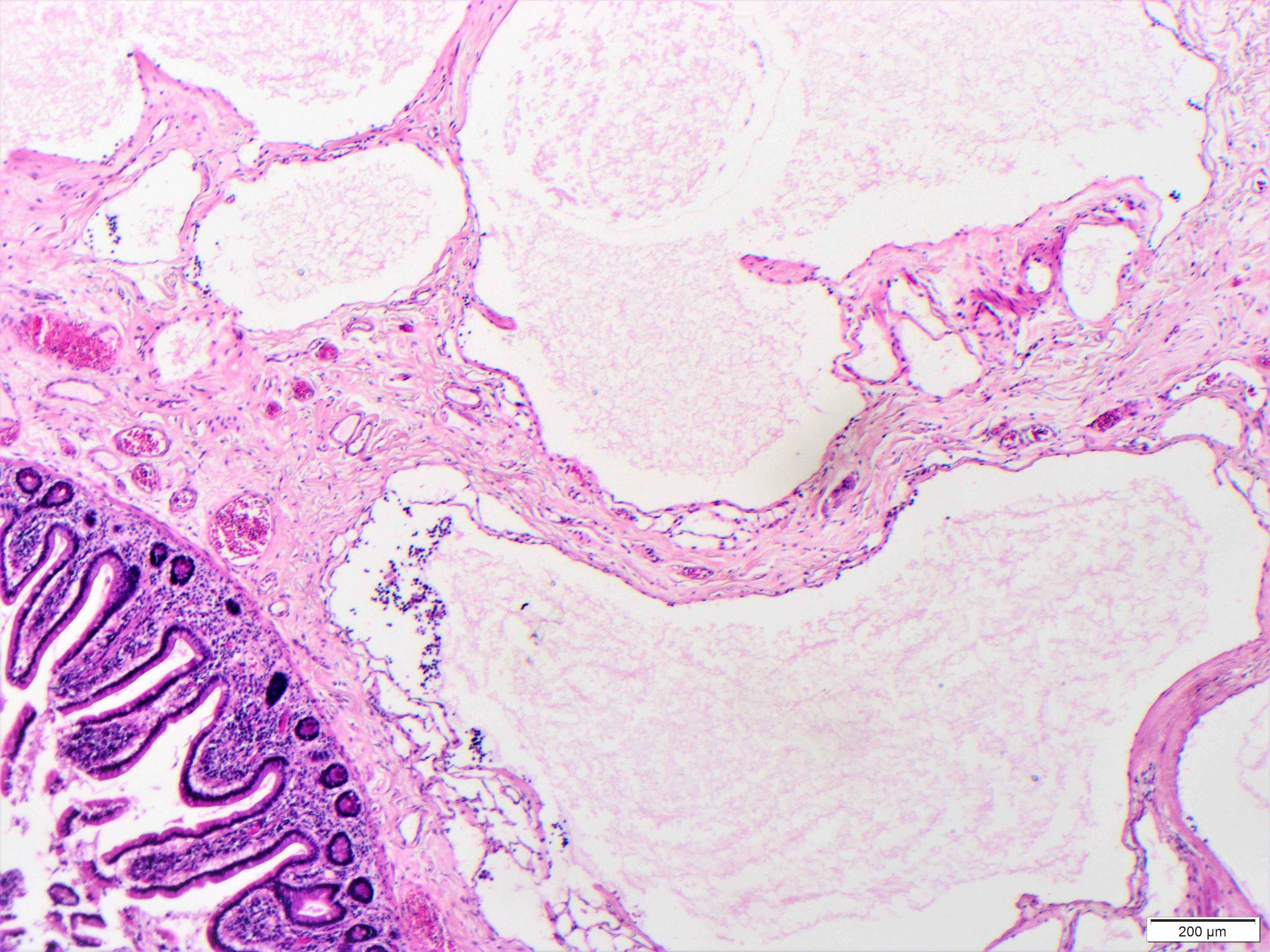
- Ampullary carcinoma, NOS
- Tumor is grossly centered in the ampulla
- Lacks the specific characteristics of the subtypes listed above
- Often presents as an ulceration of the papilla with dilation of both the common bile duct and main pancreatic duct
- Periampullary adenocarcinomas
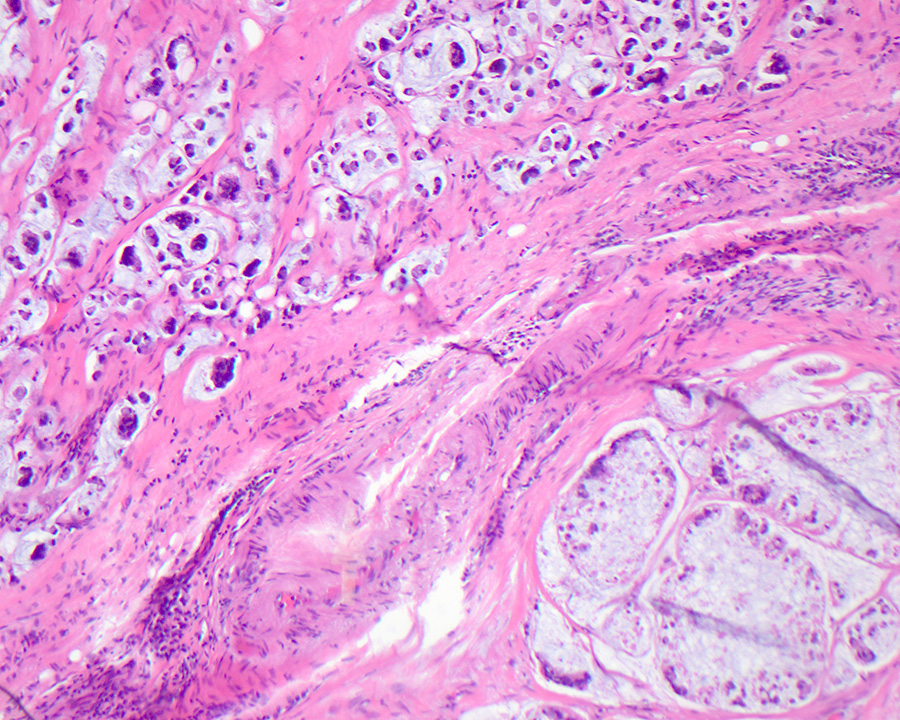
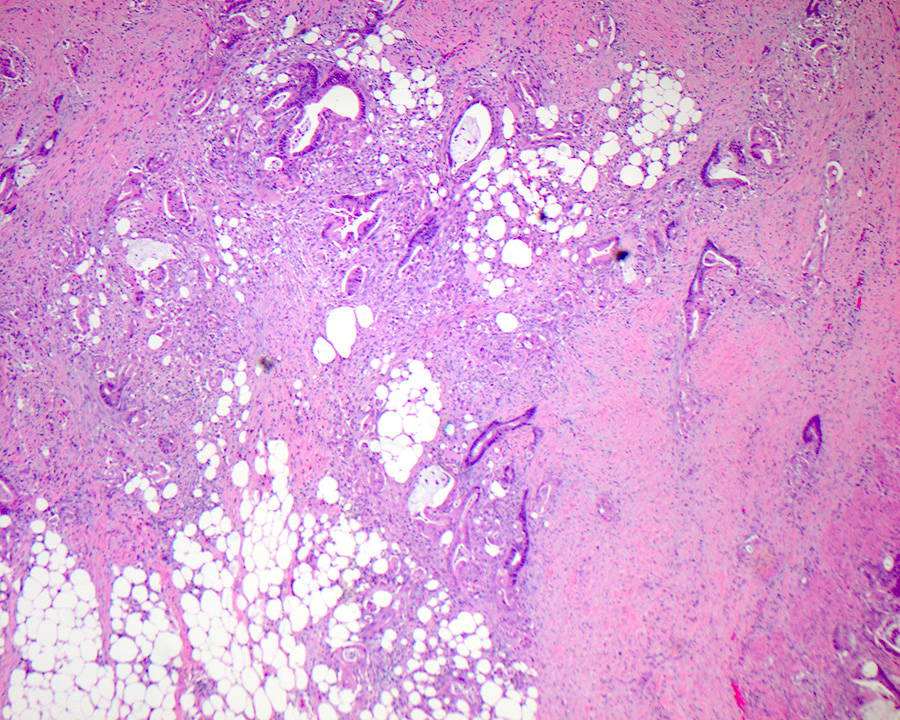
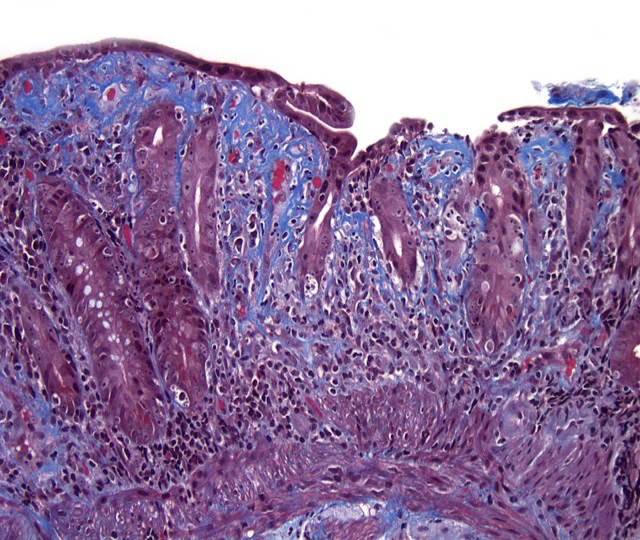
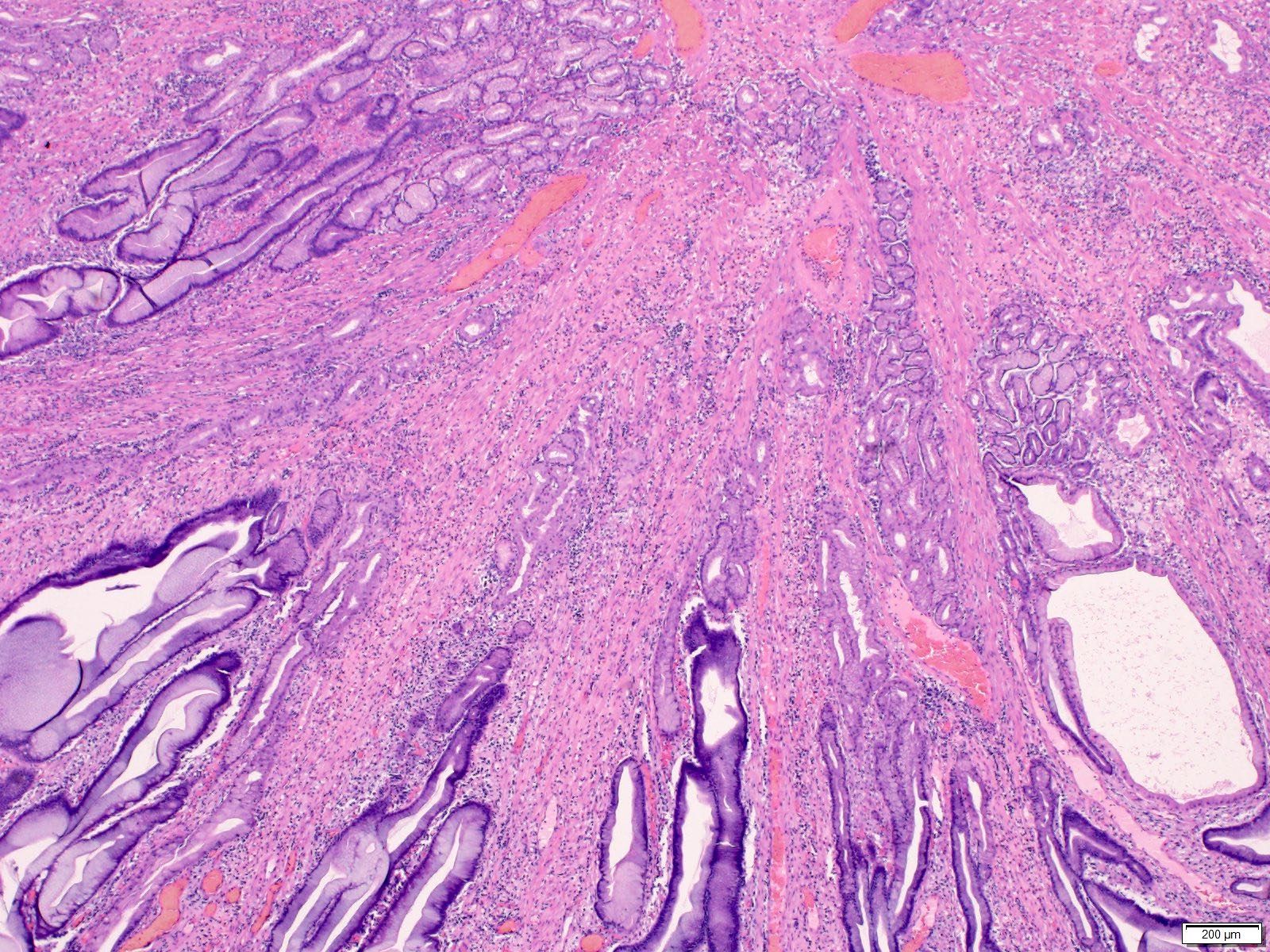
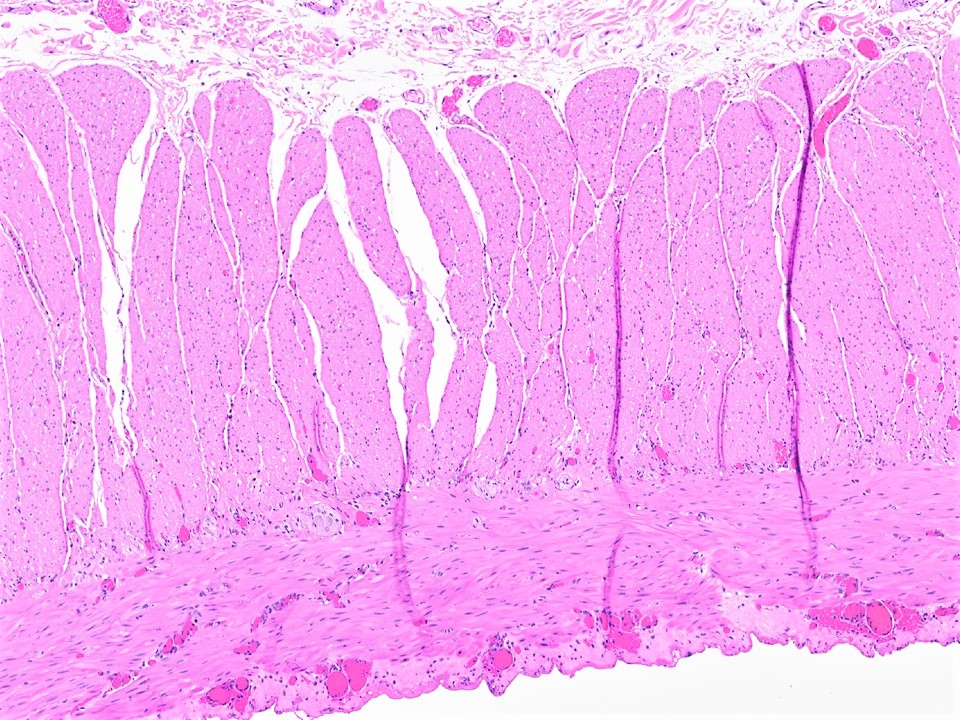
- Careful gross assessment of tumor extension into the duodenal wall and the pancreas or peripancreatic soft tissue as well as any major vessels is important for correct staging
- Average gross size of tumors: 2.6 cm with invasive component measuring 1.8 cm (Am J Surg Pathol 2012;36:1592)
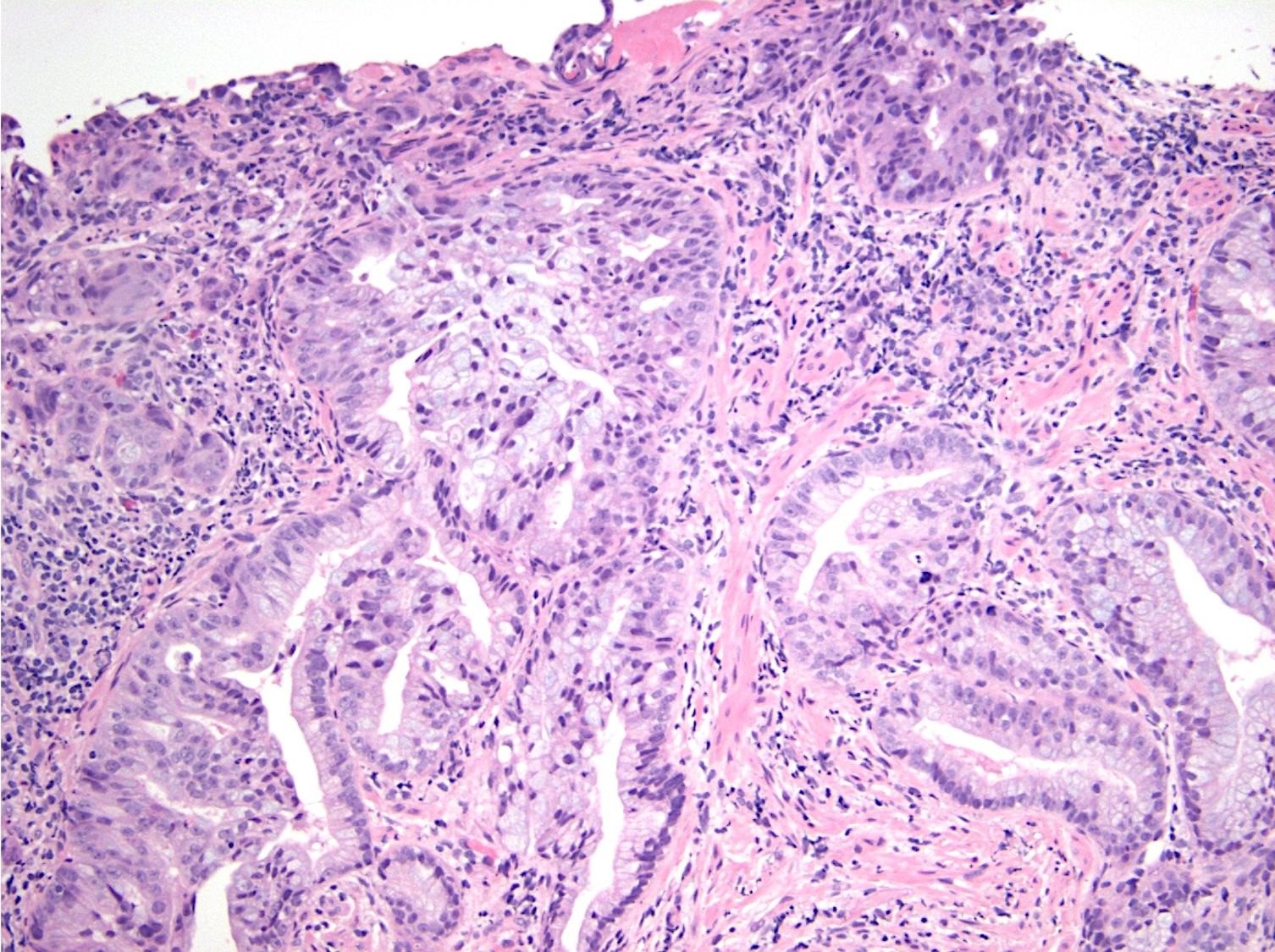
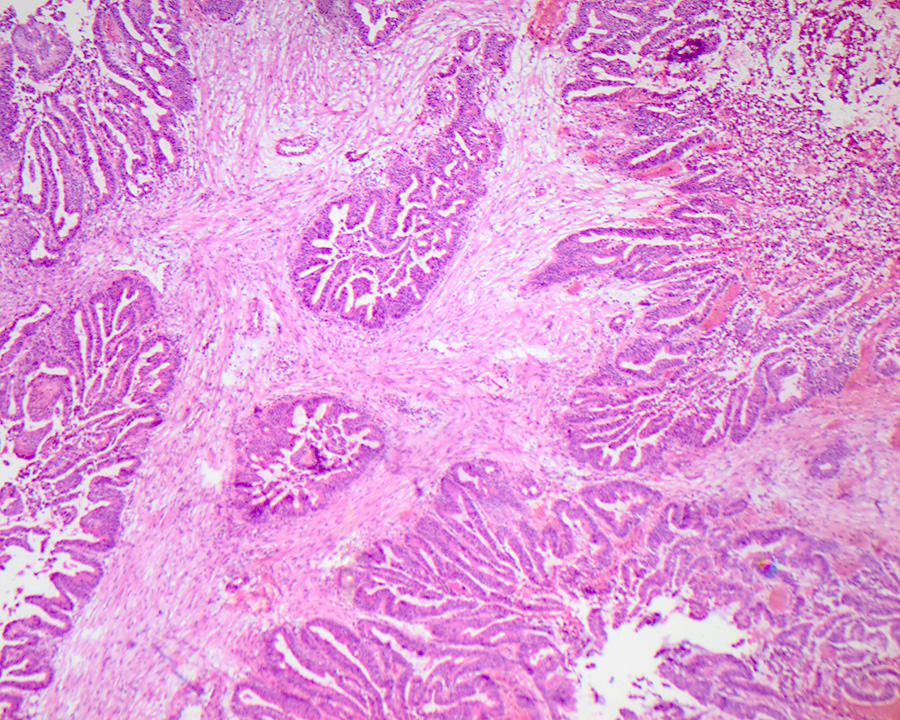
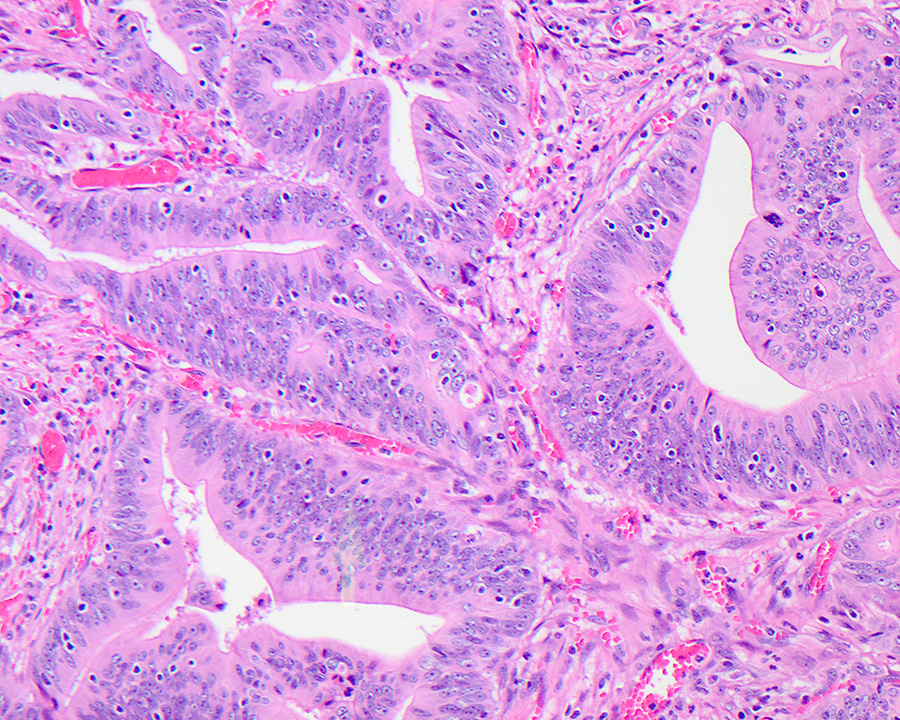
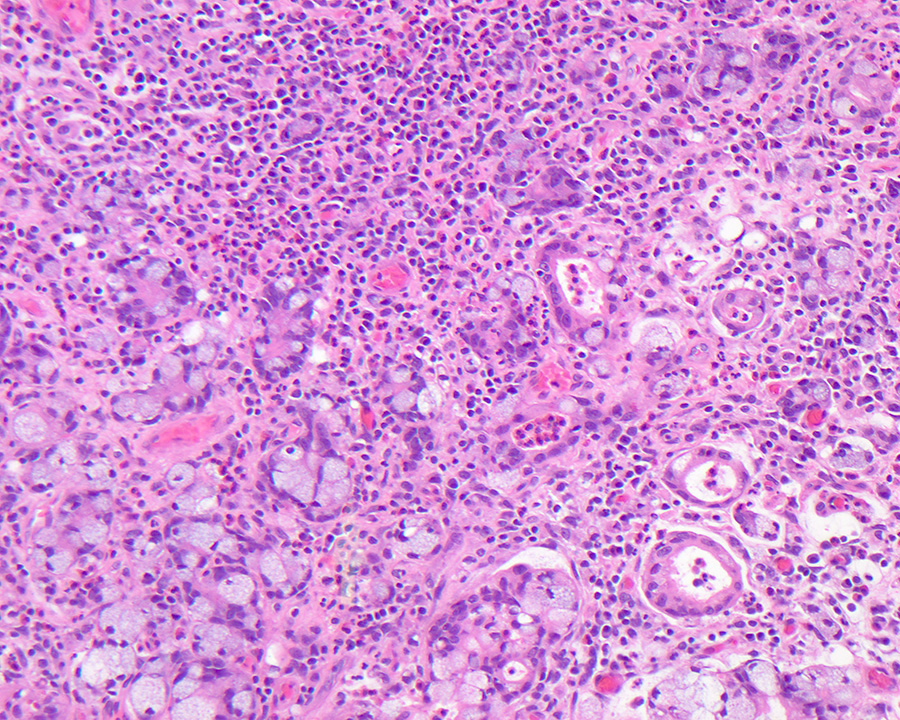
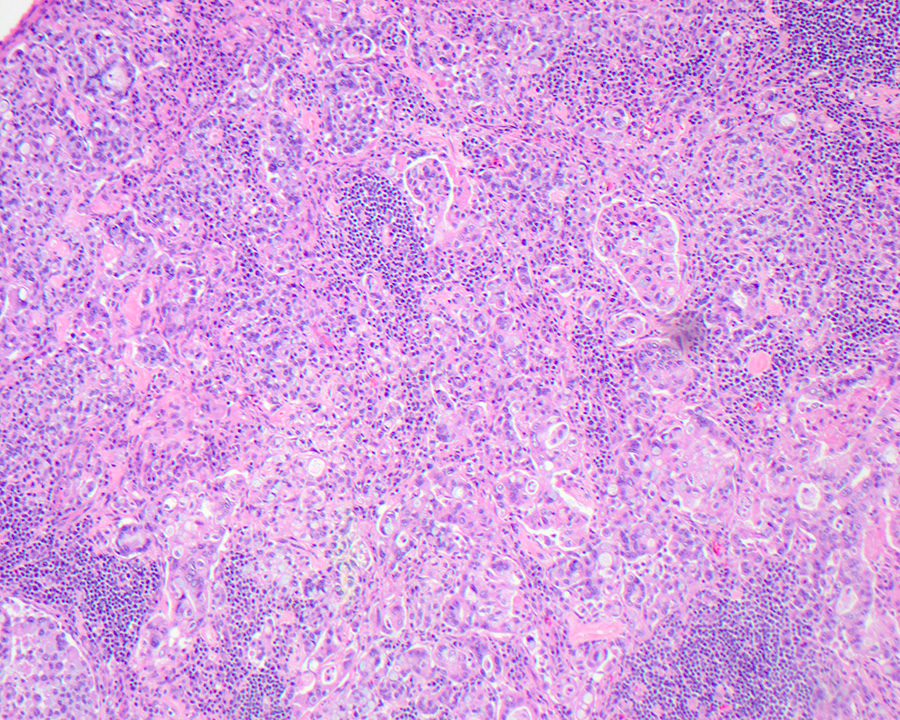
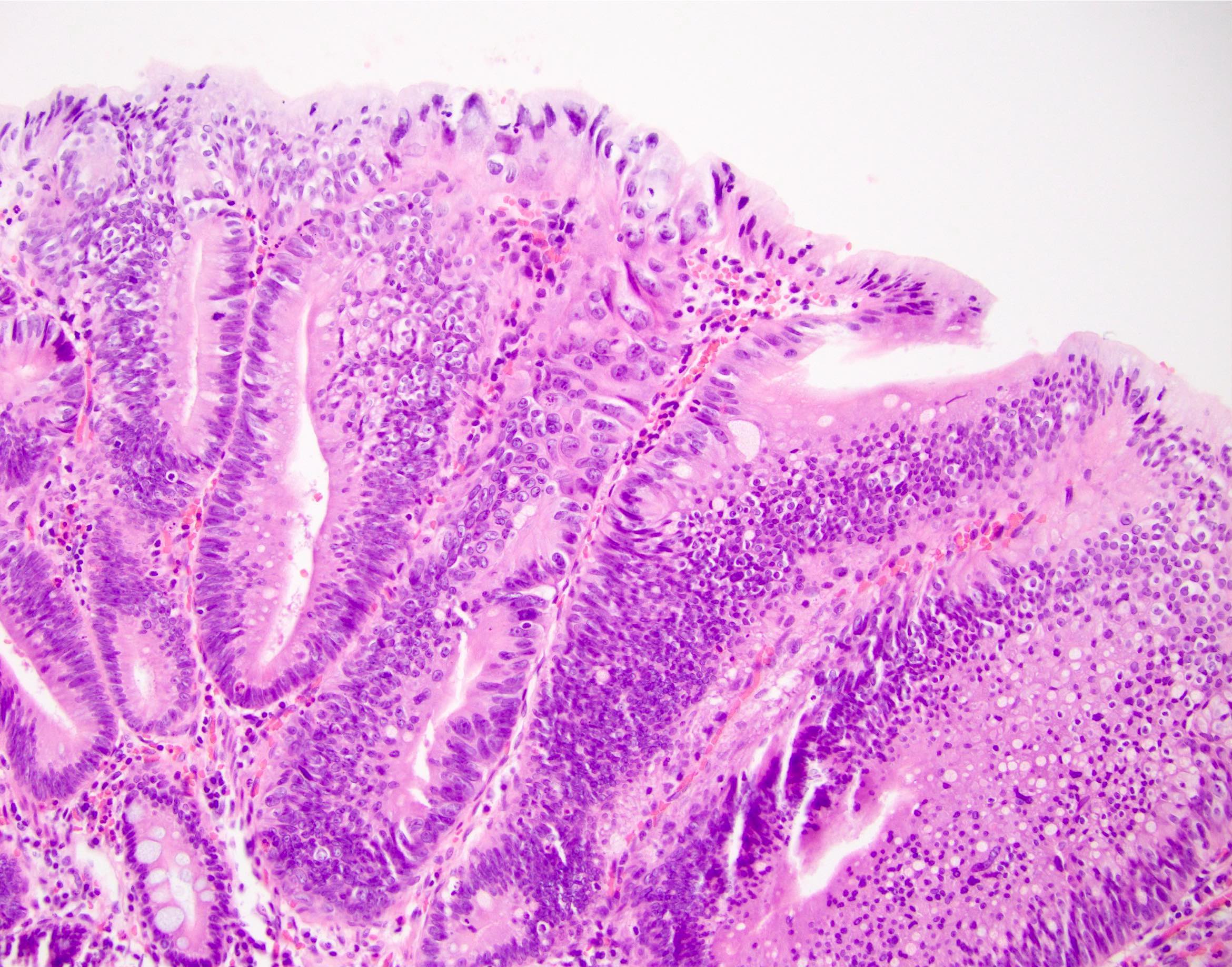
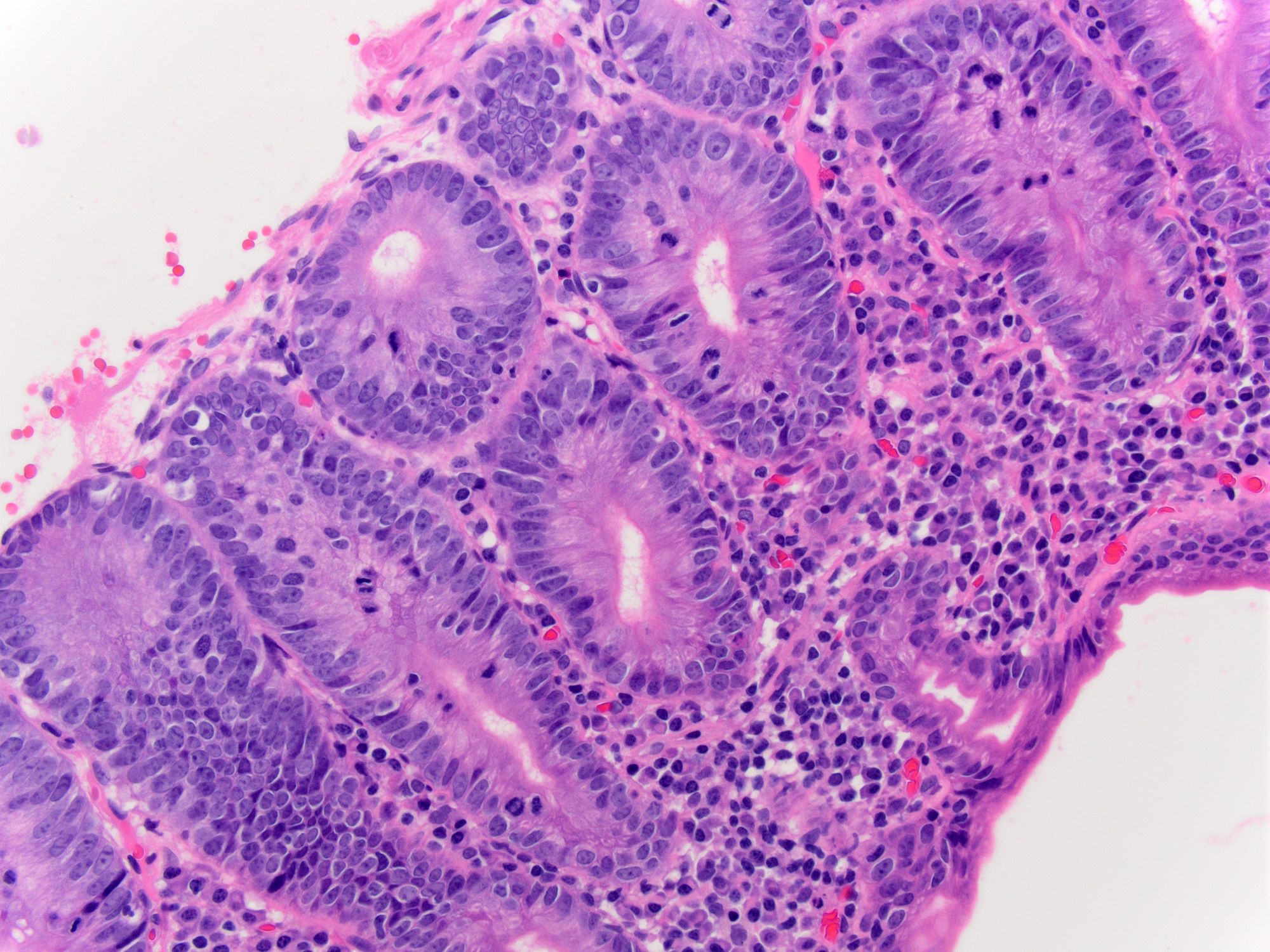
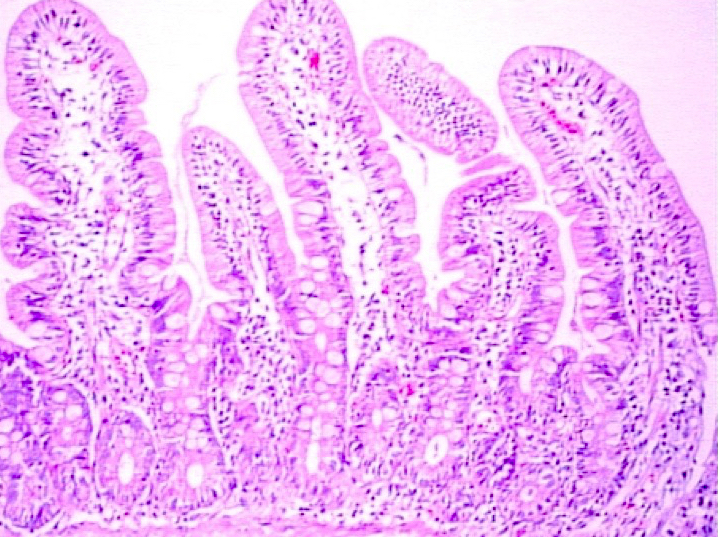
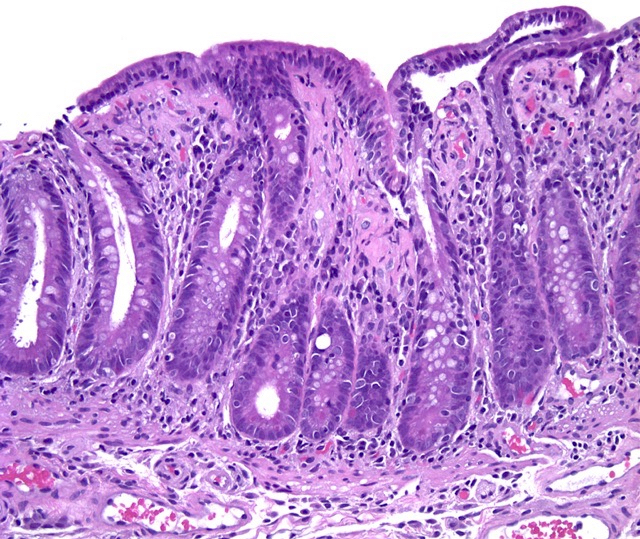
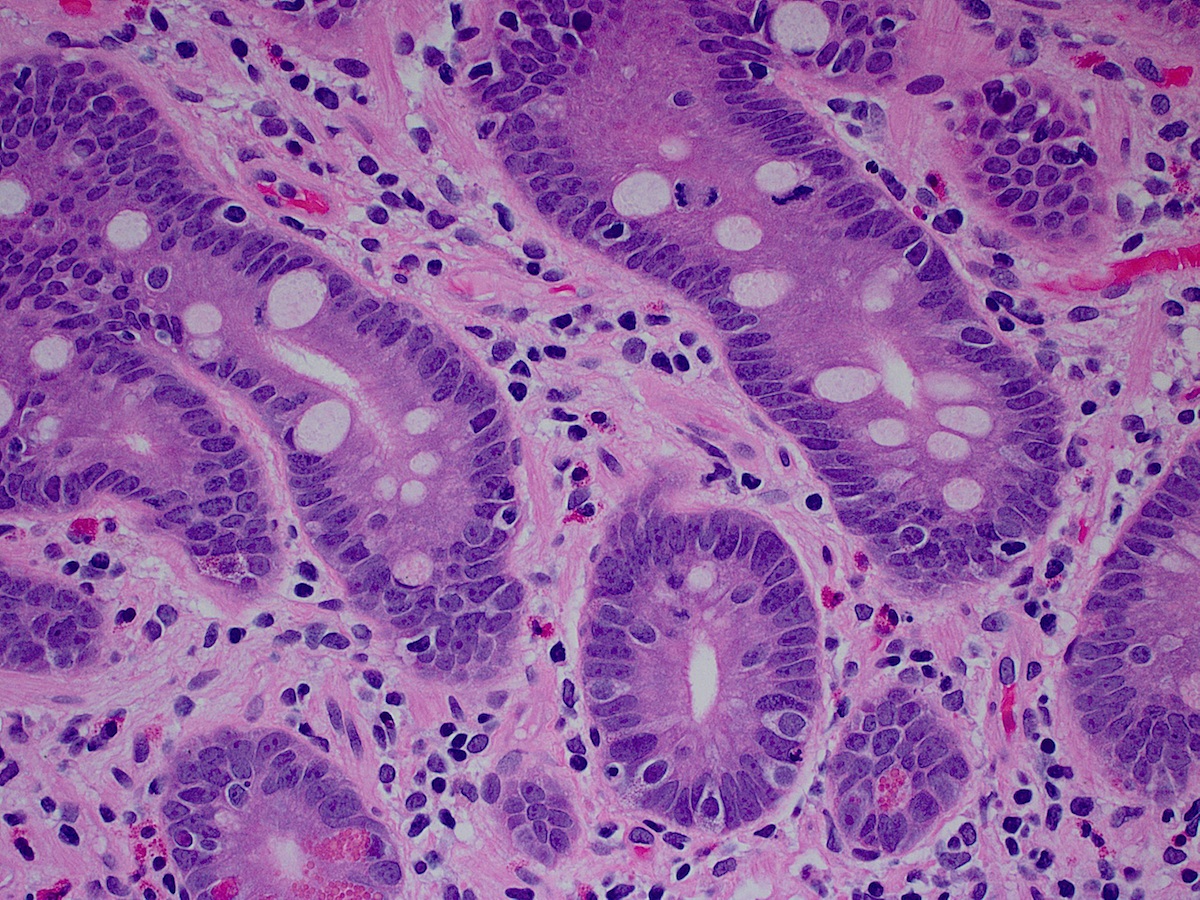
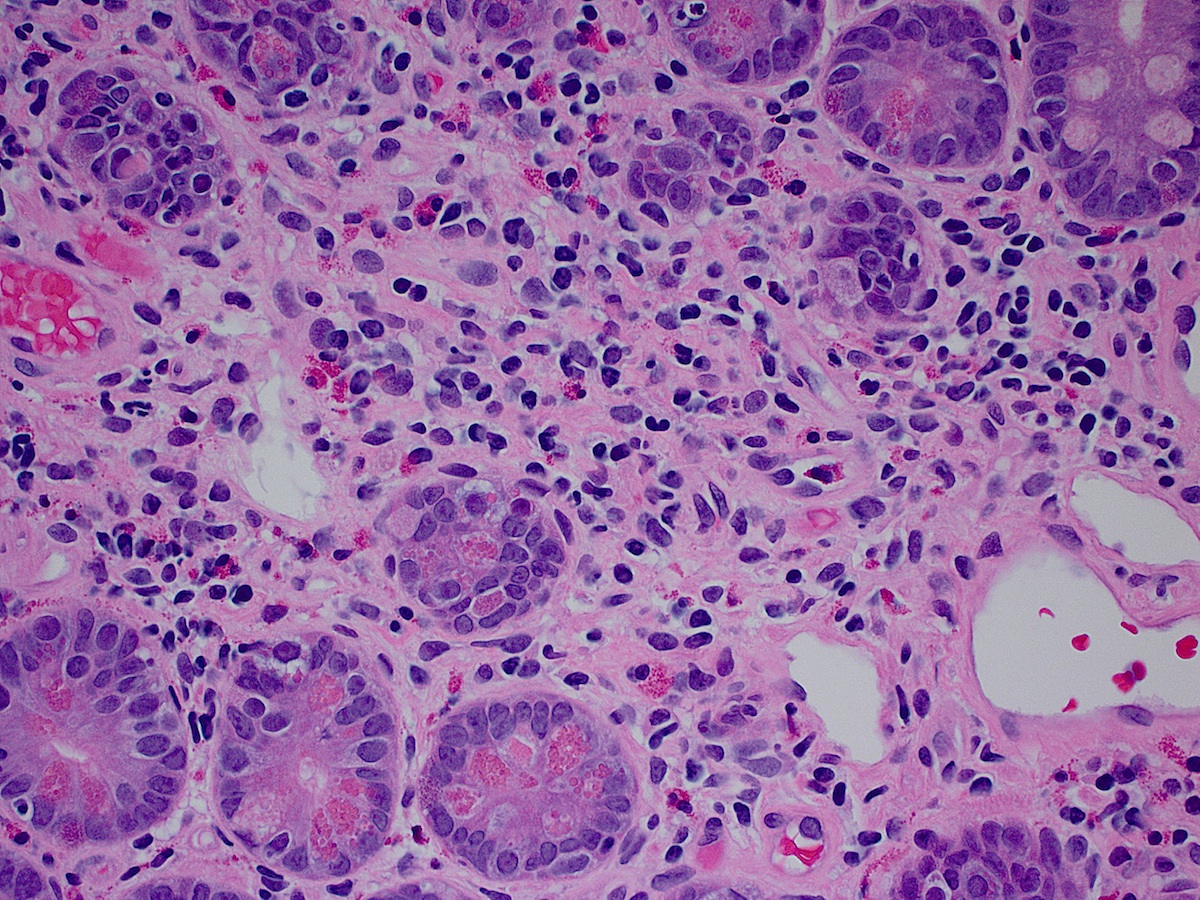
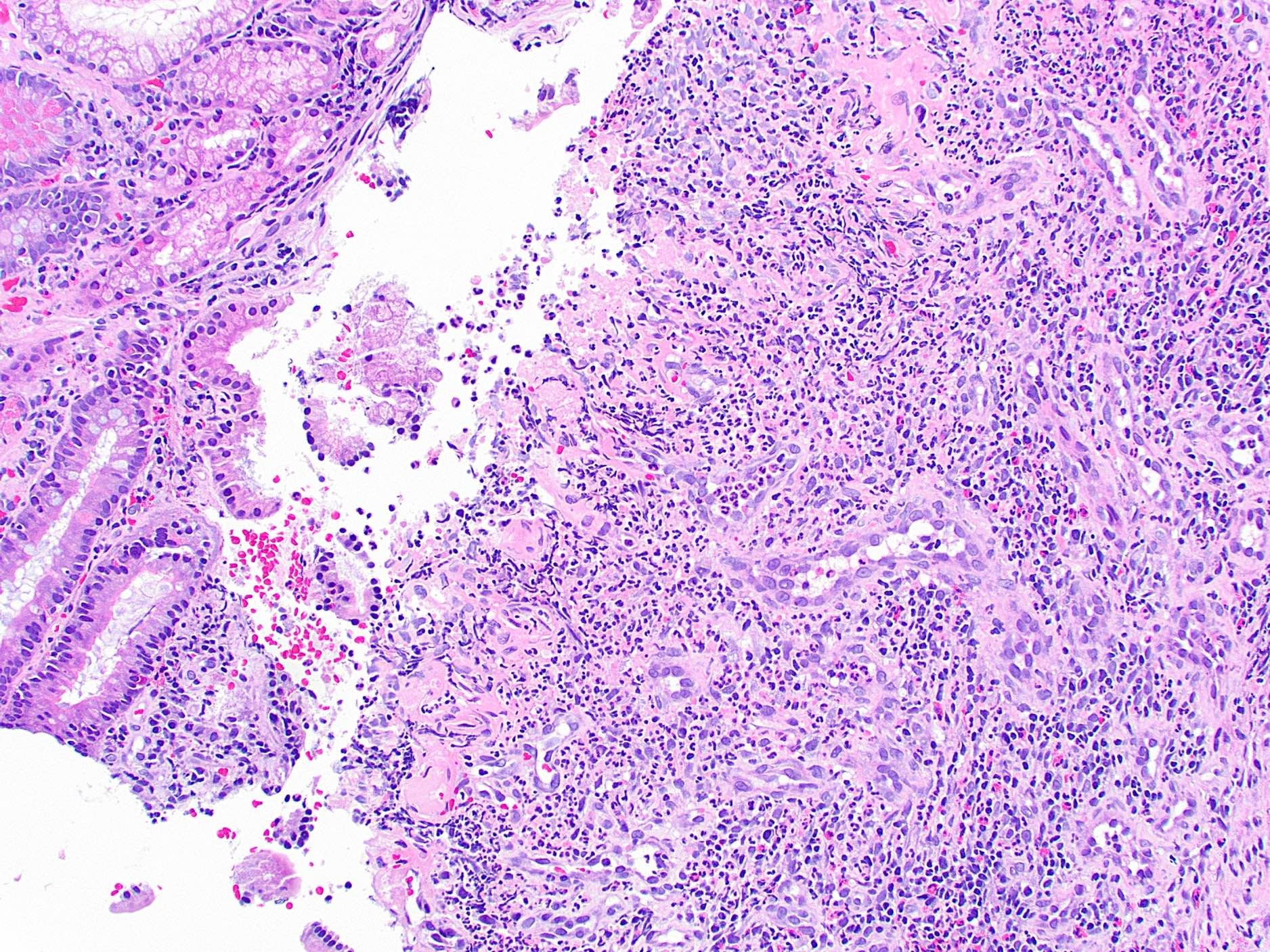
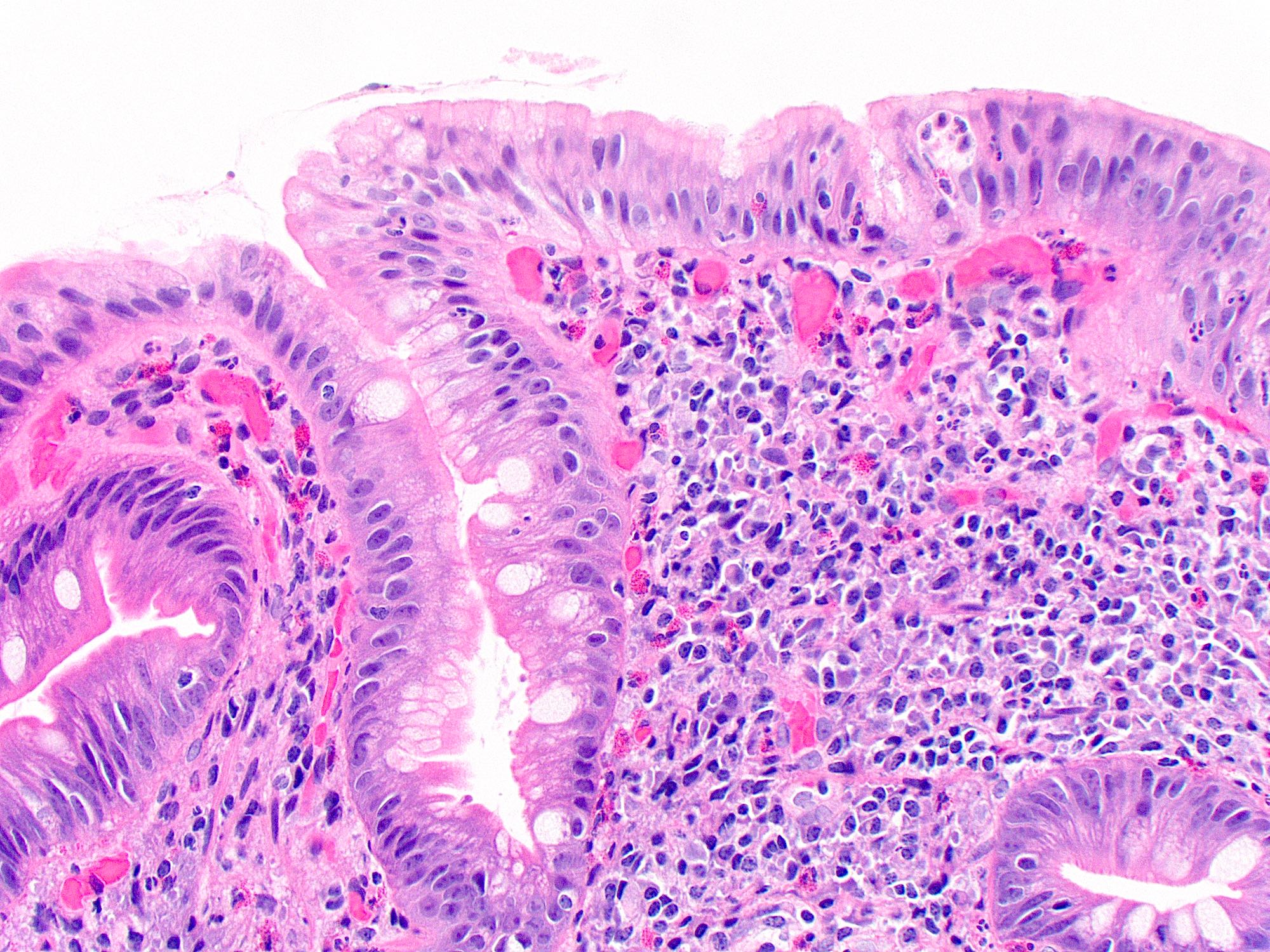
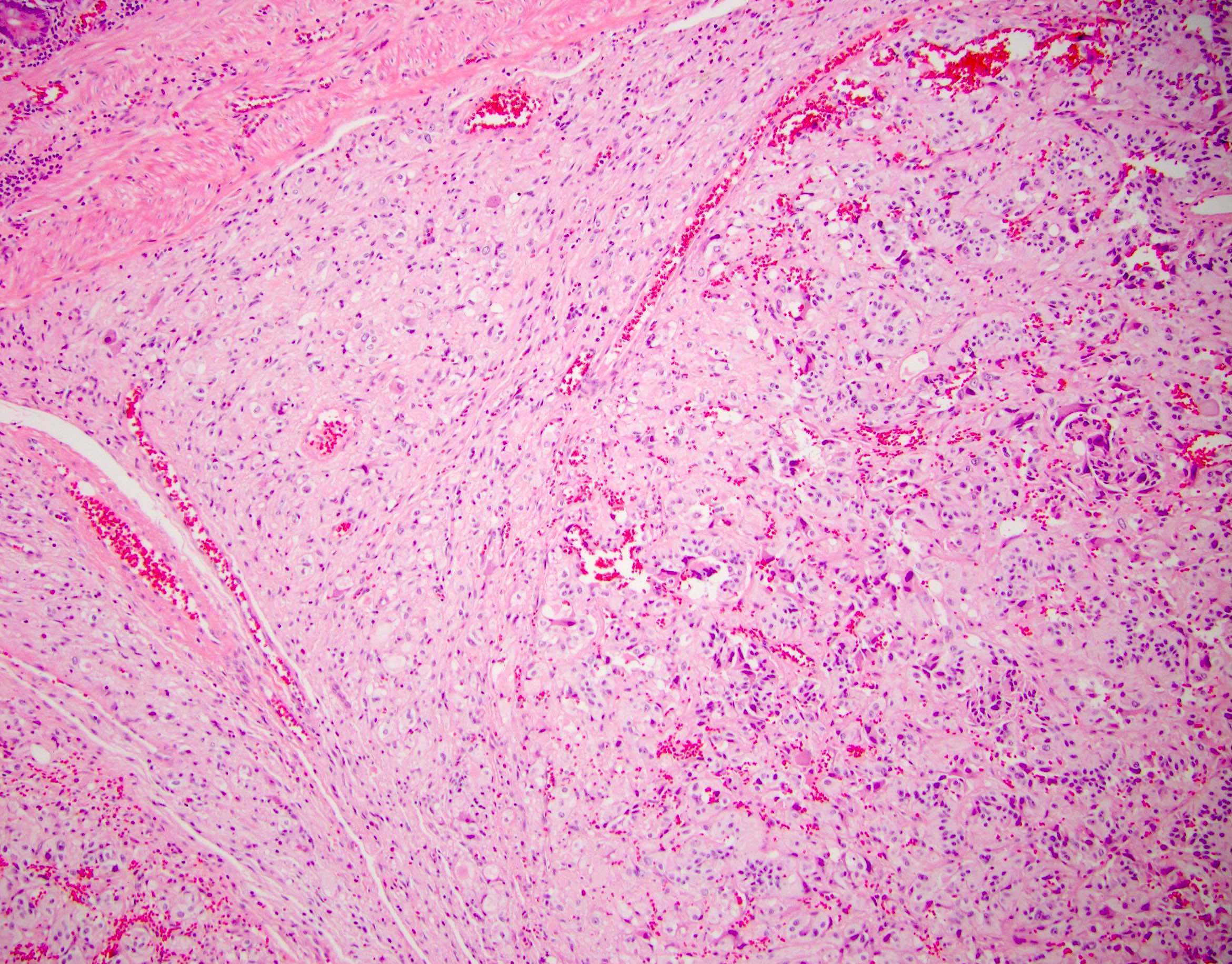
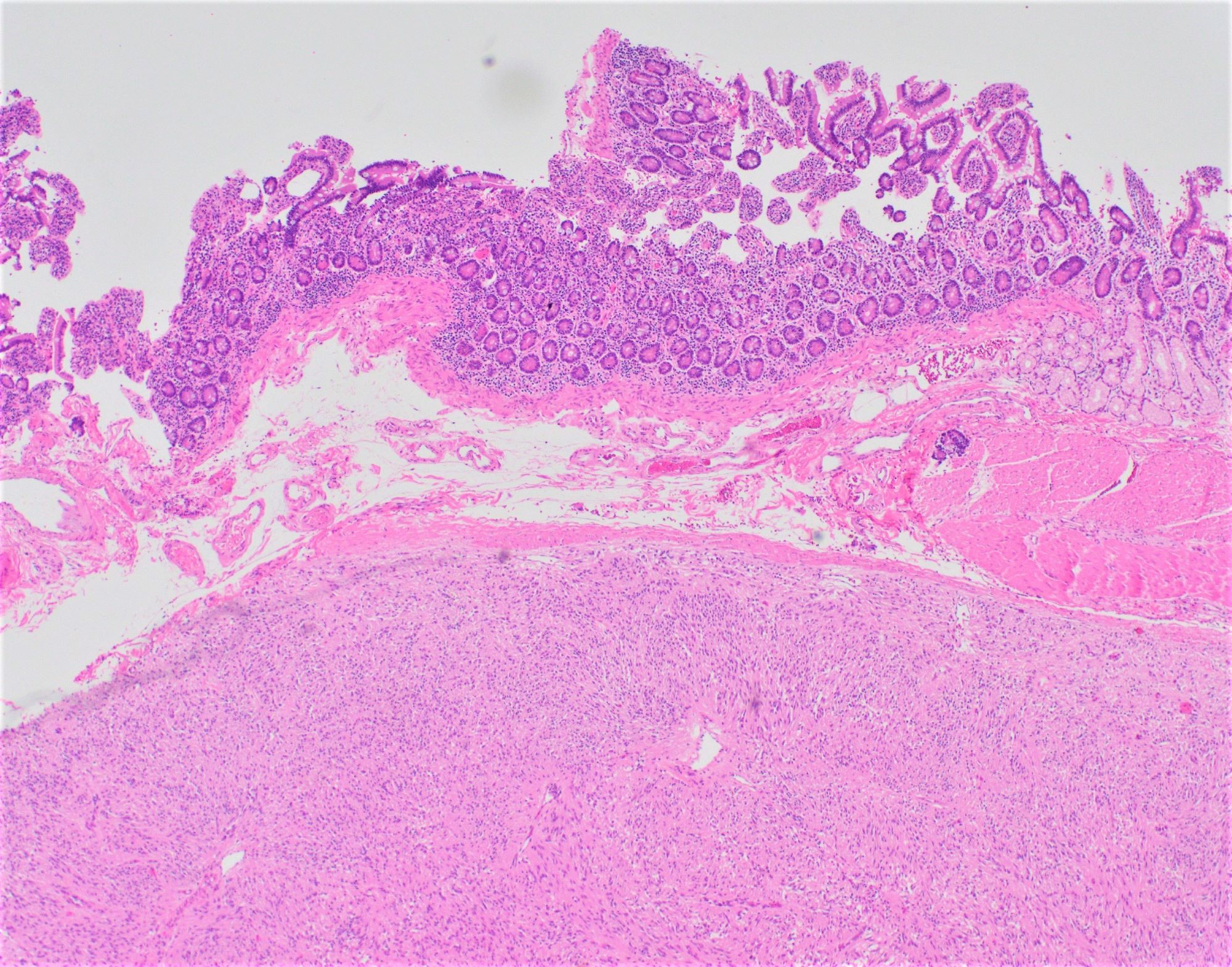
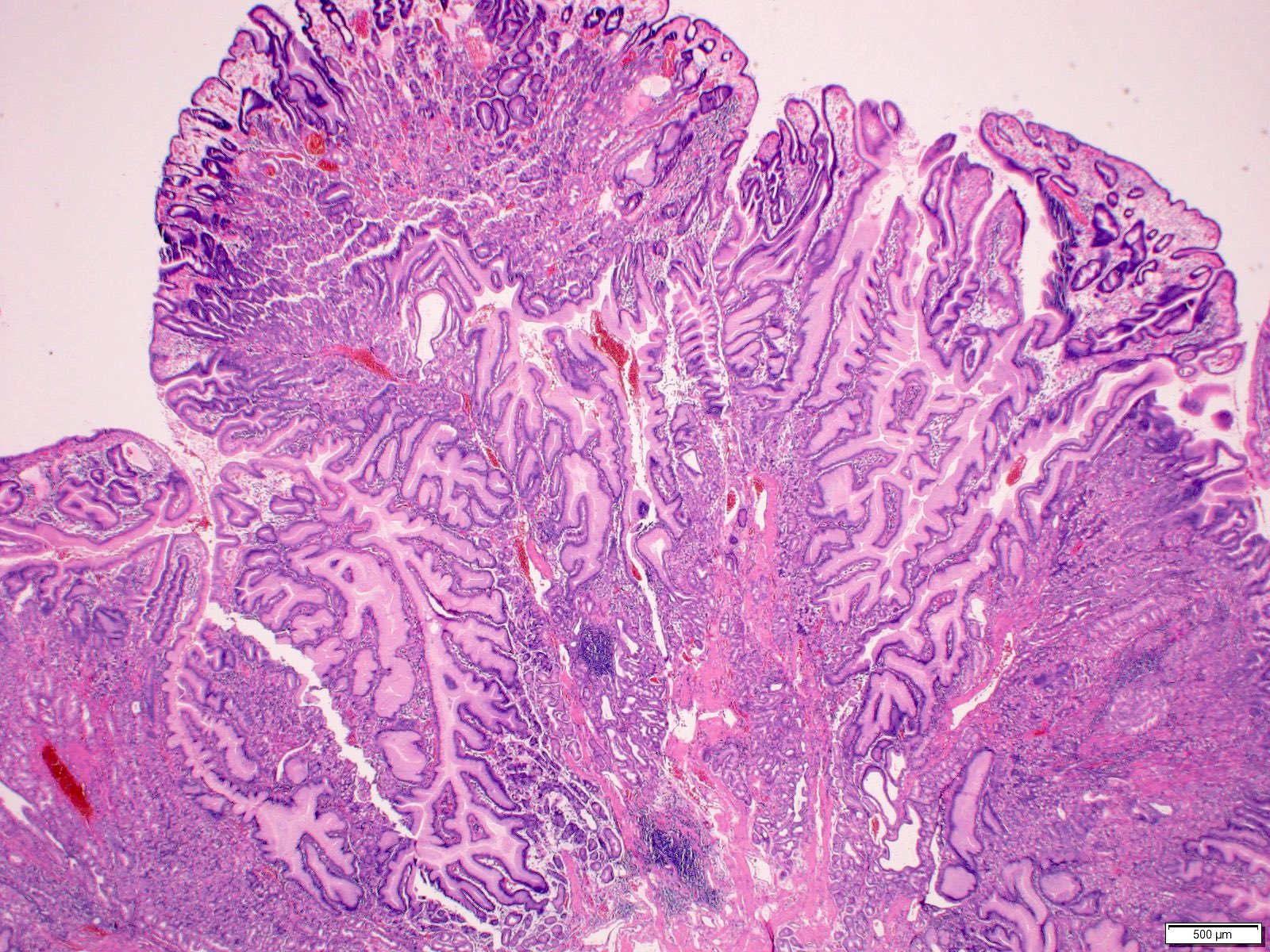
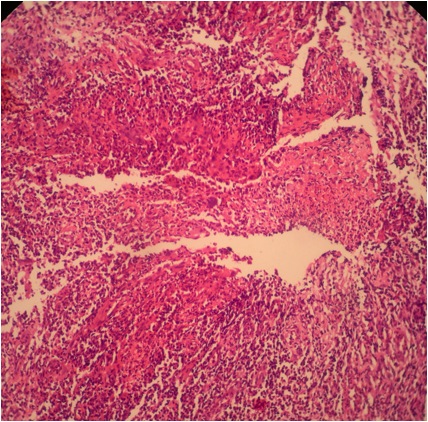
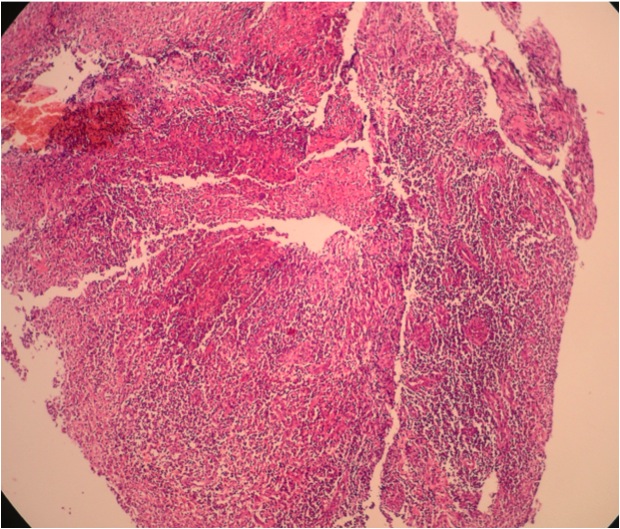
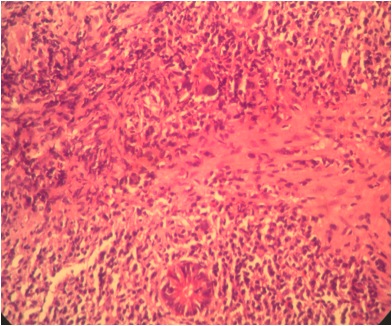
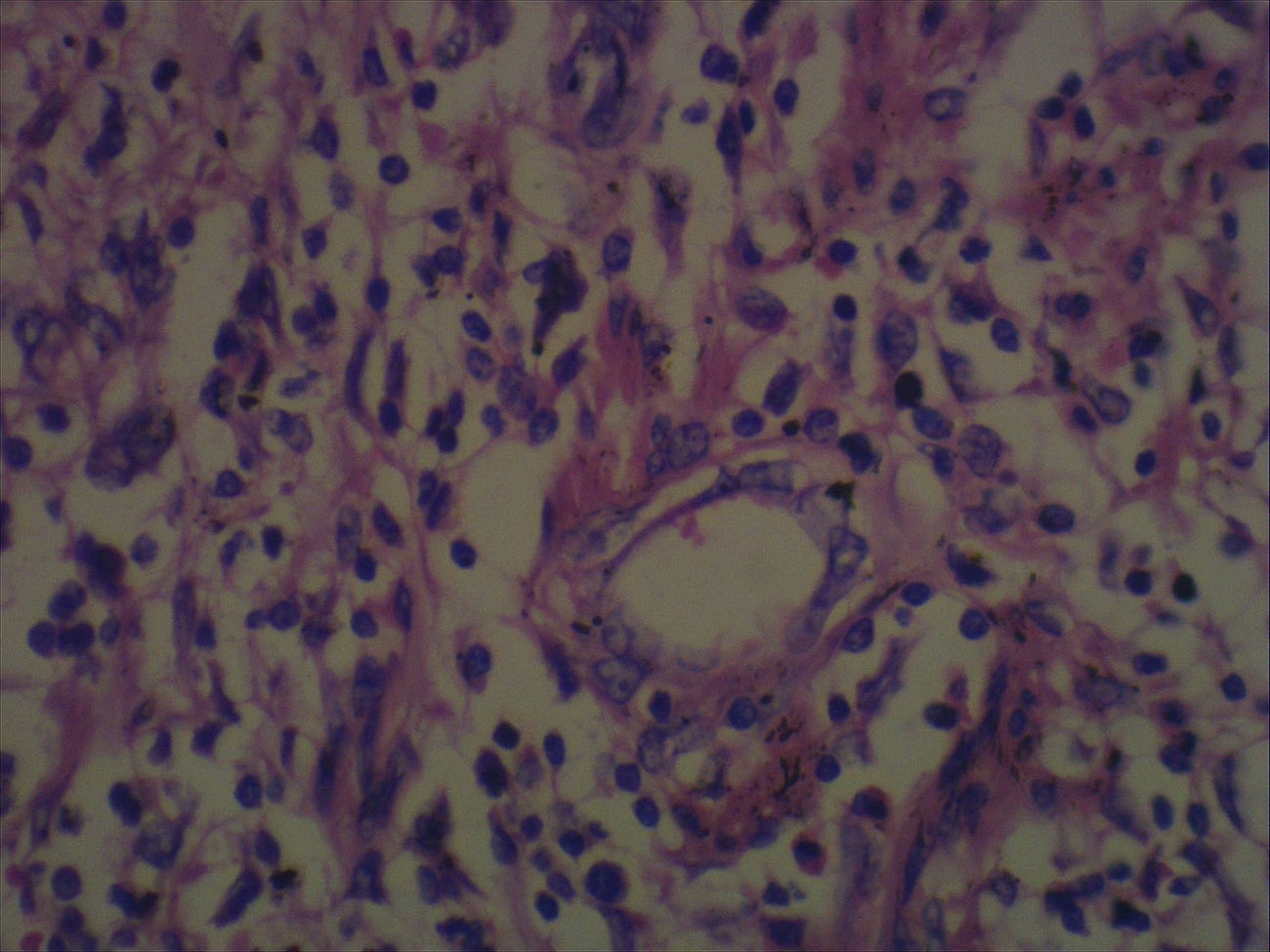
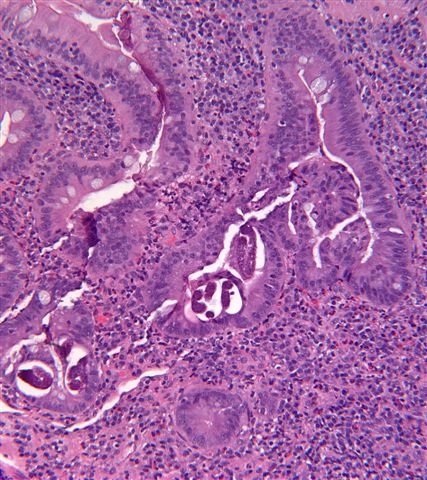
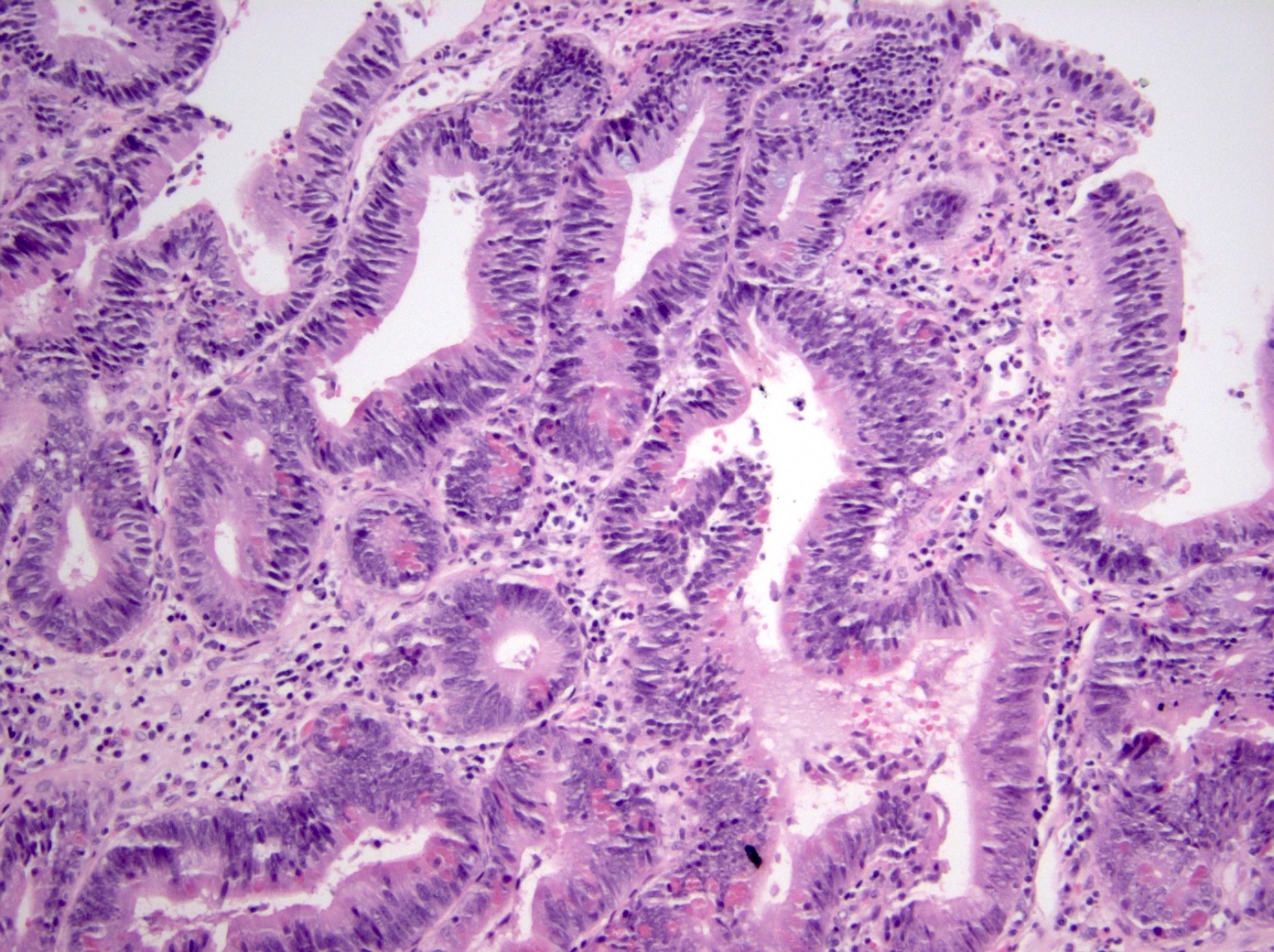
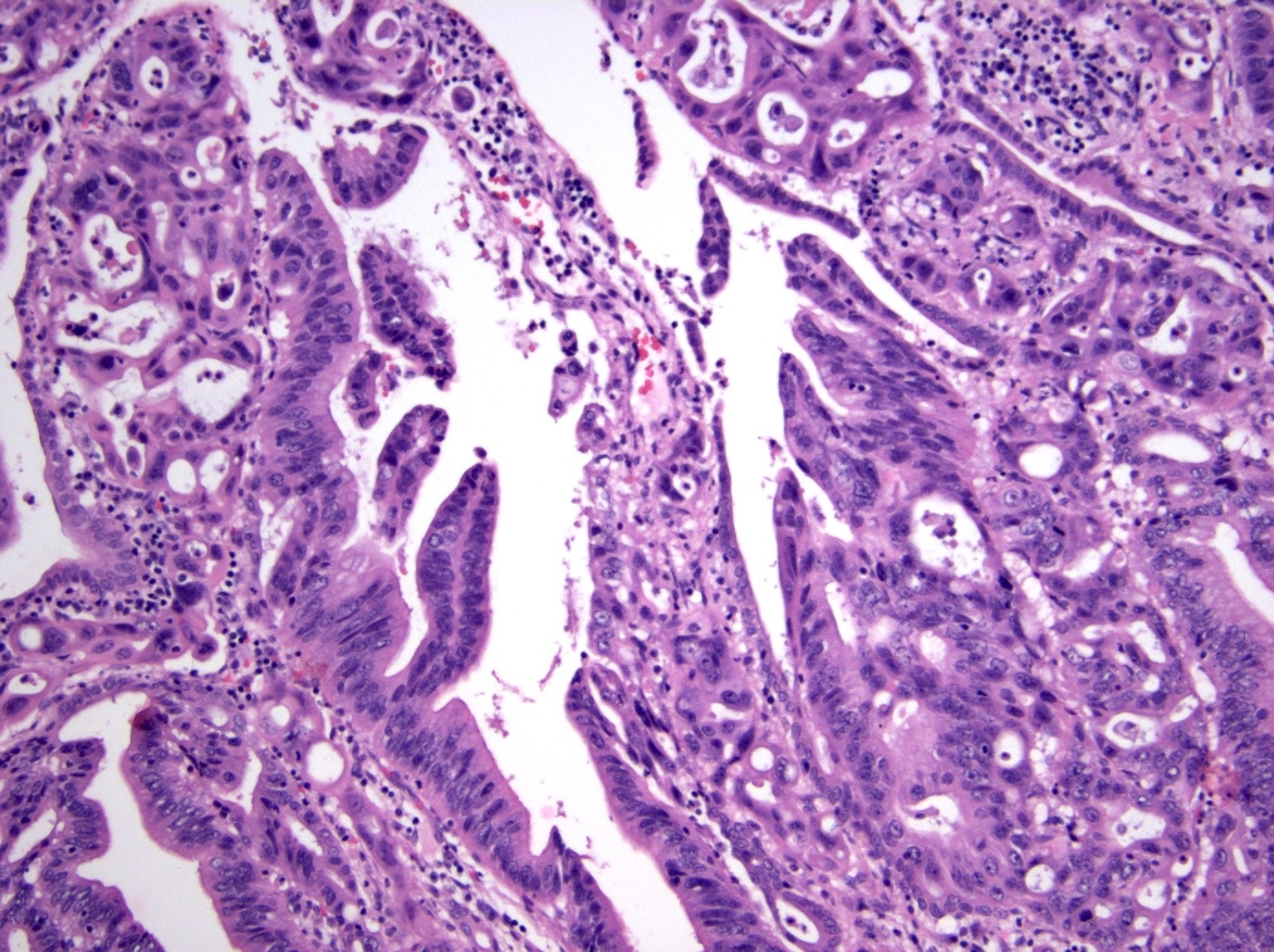
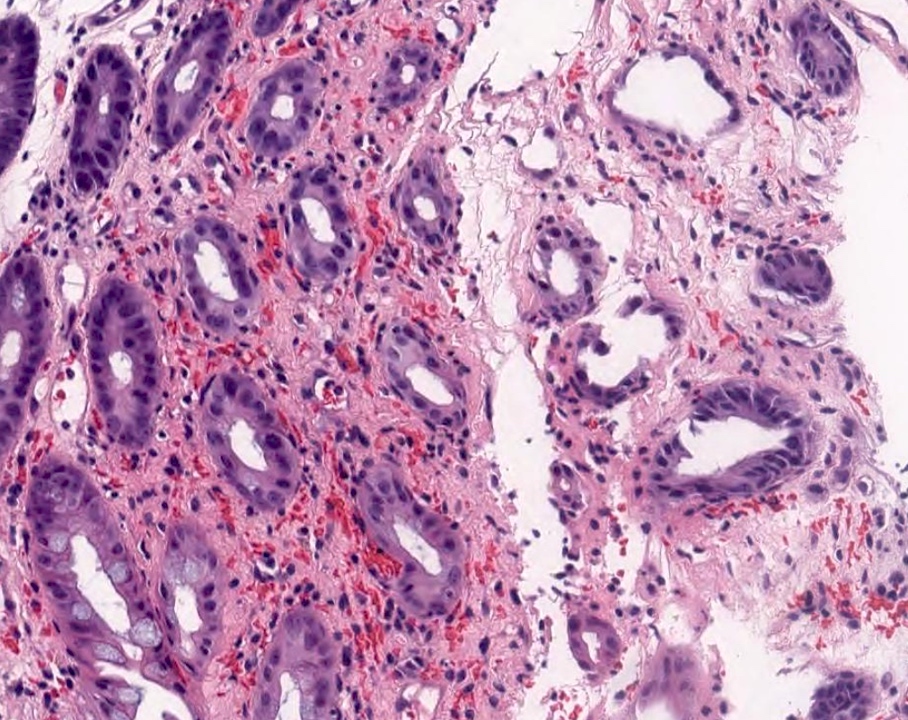
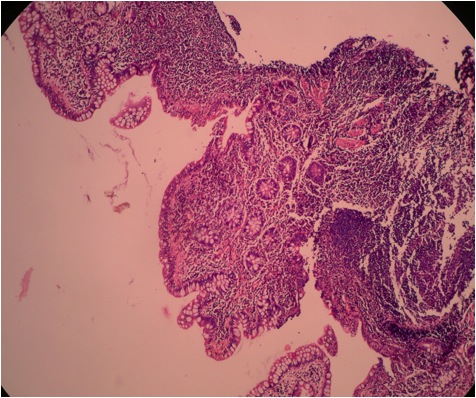
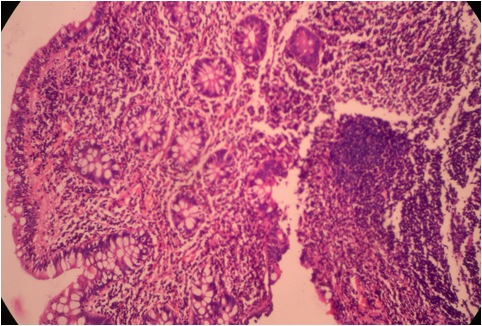
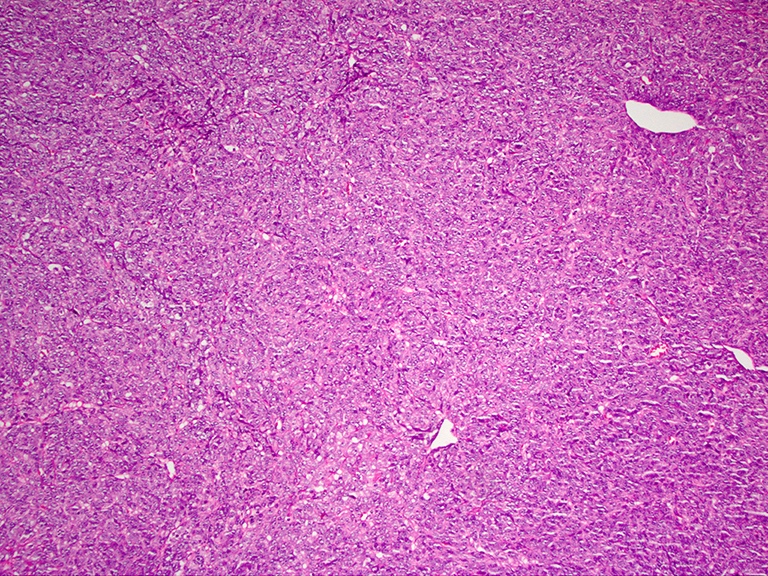
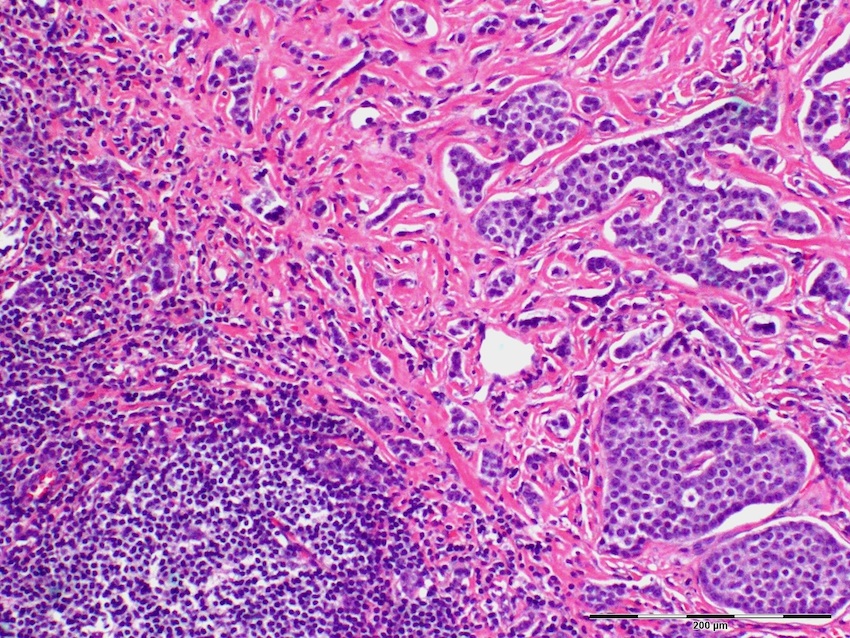
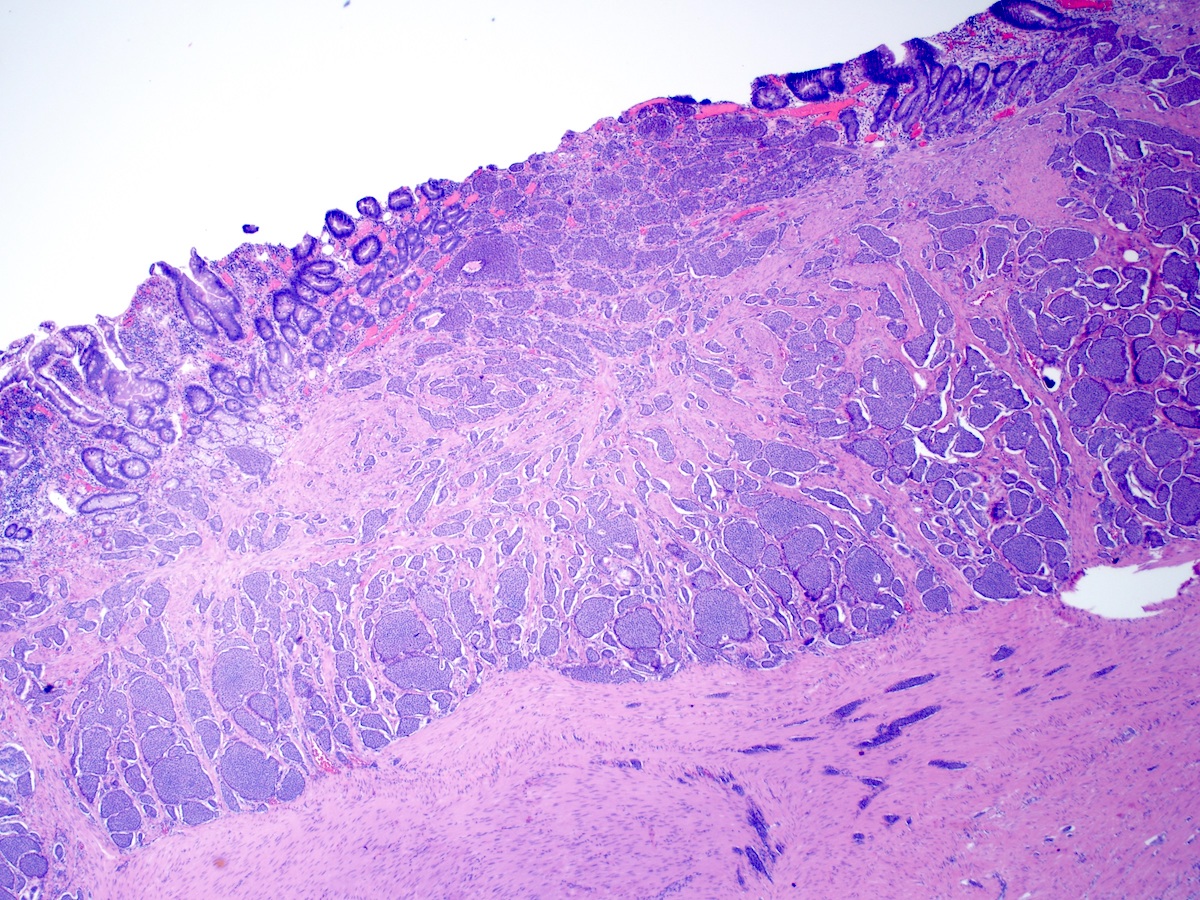
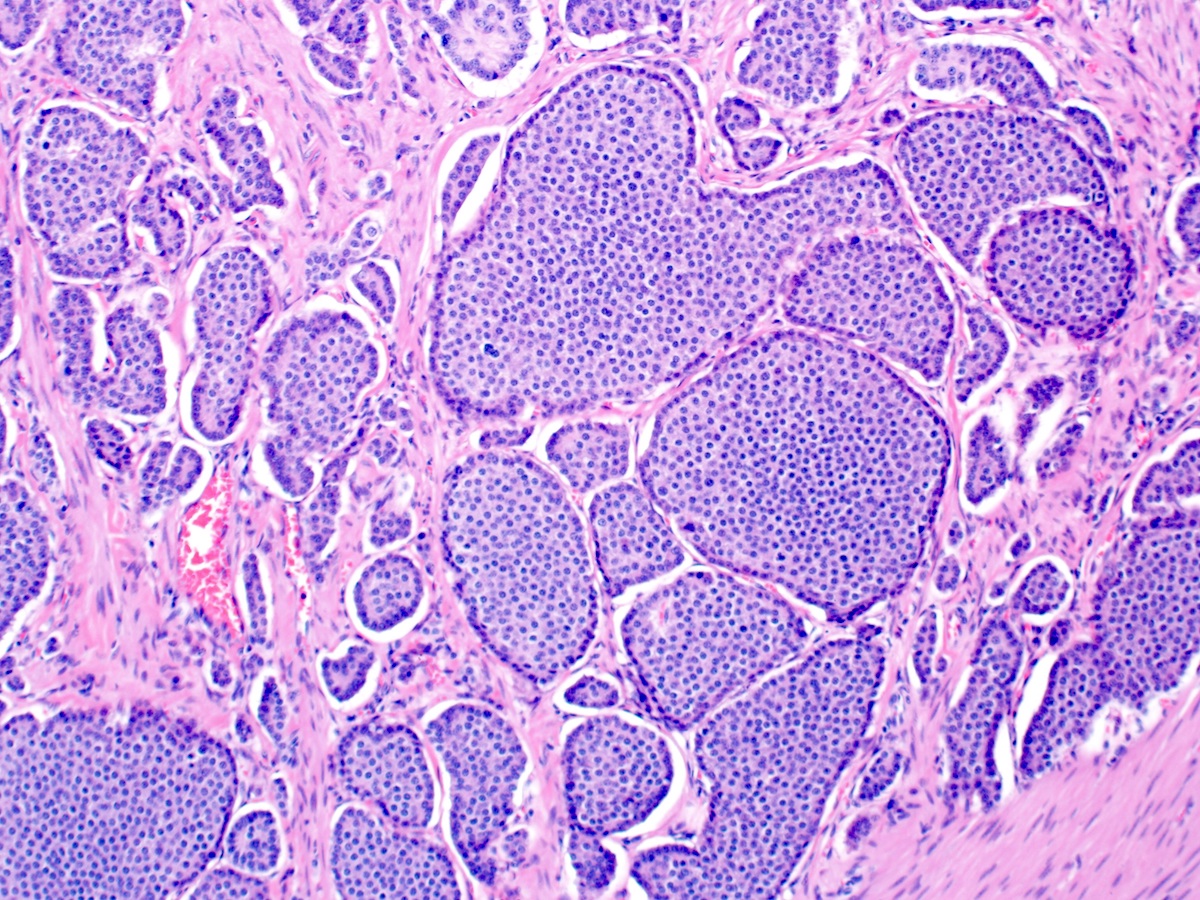
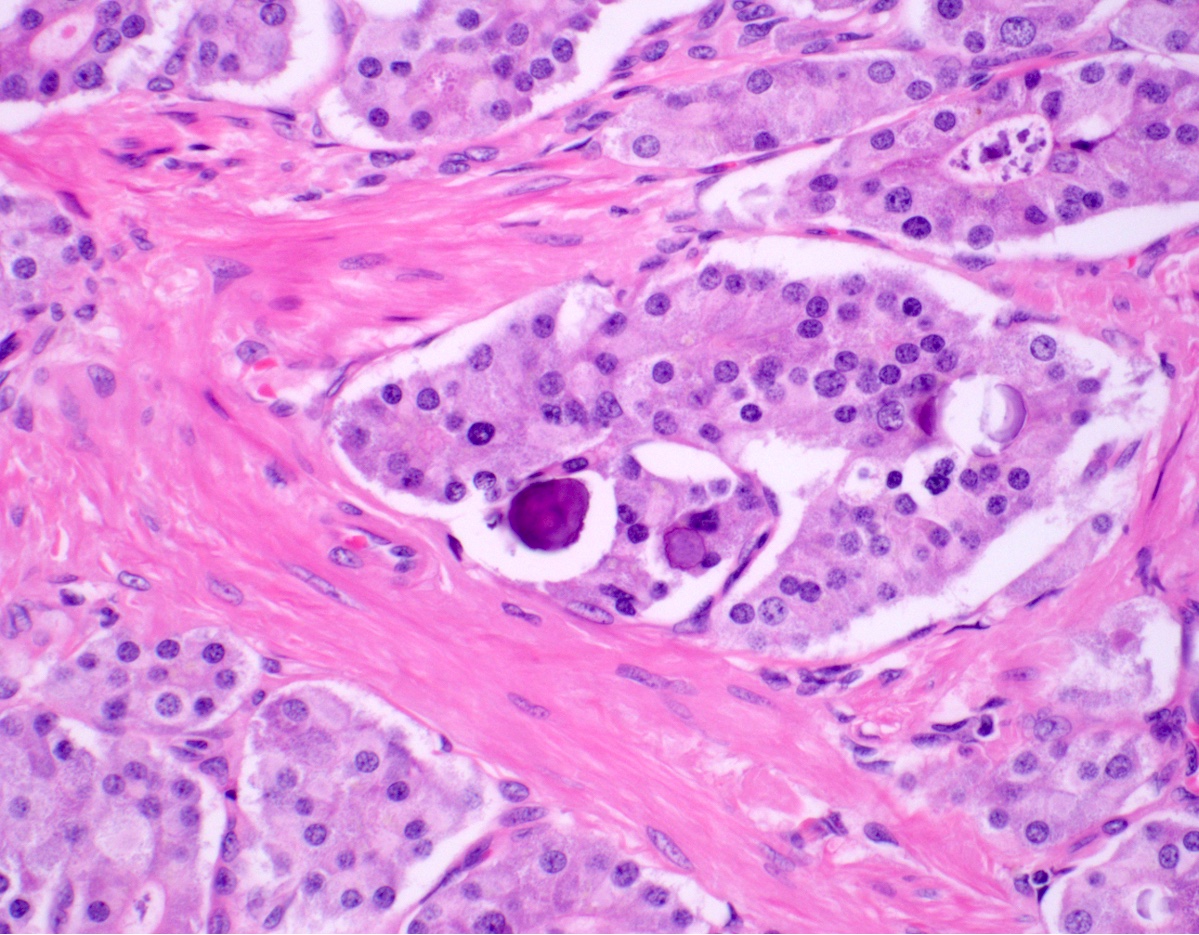
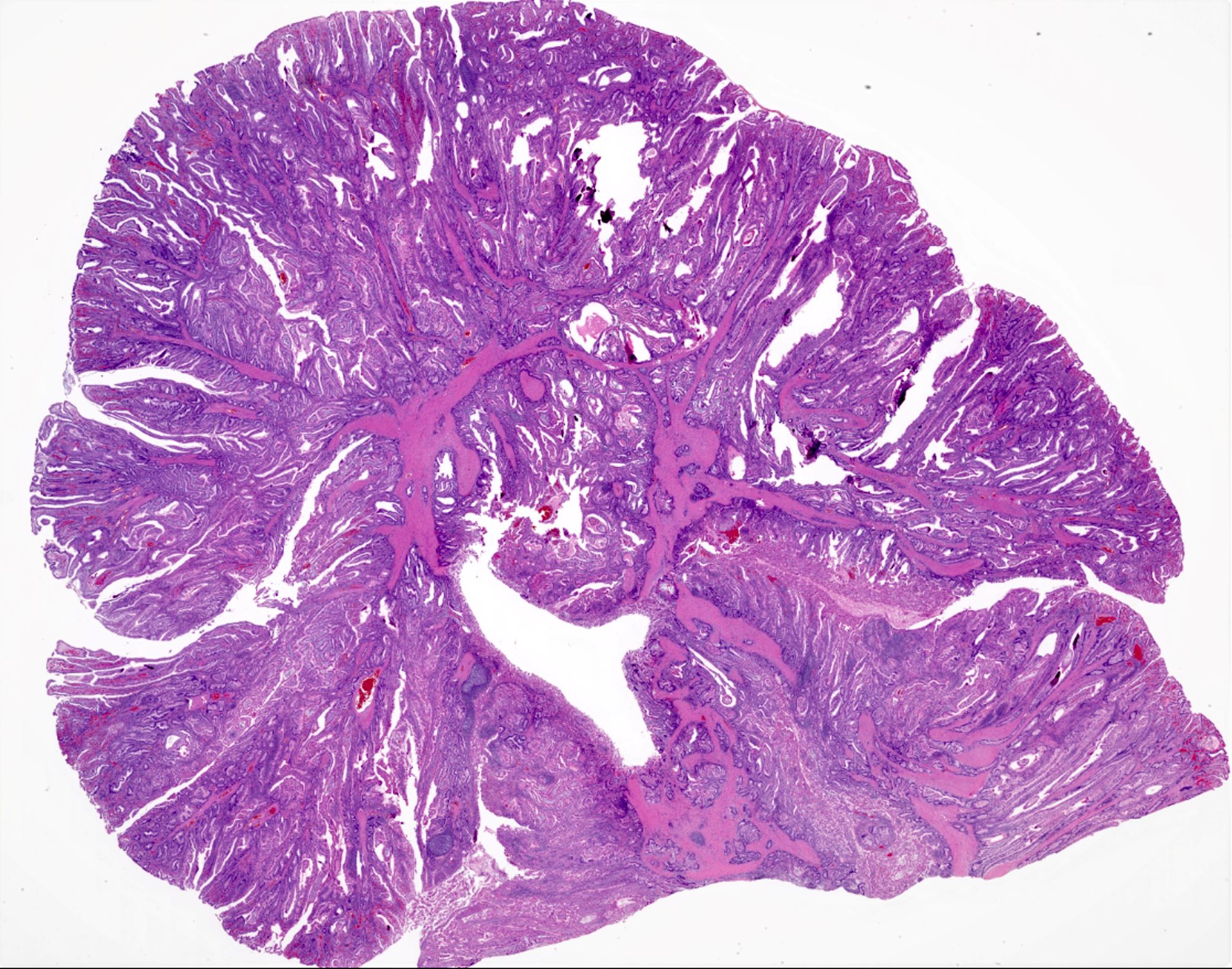
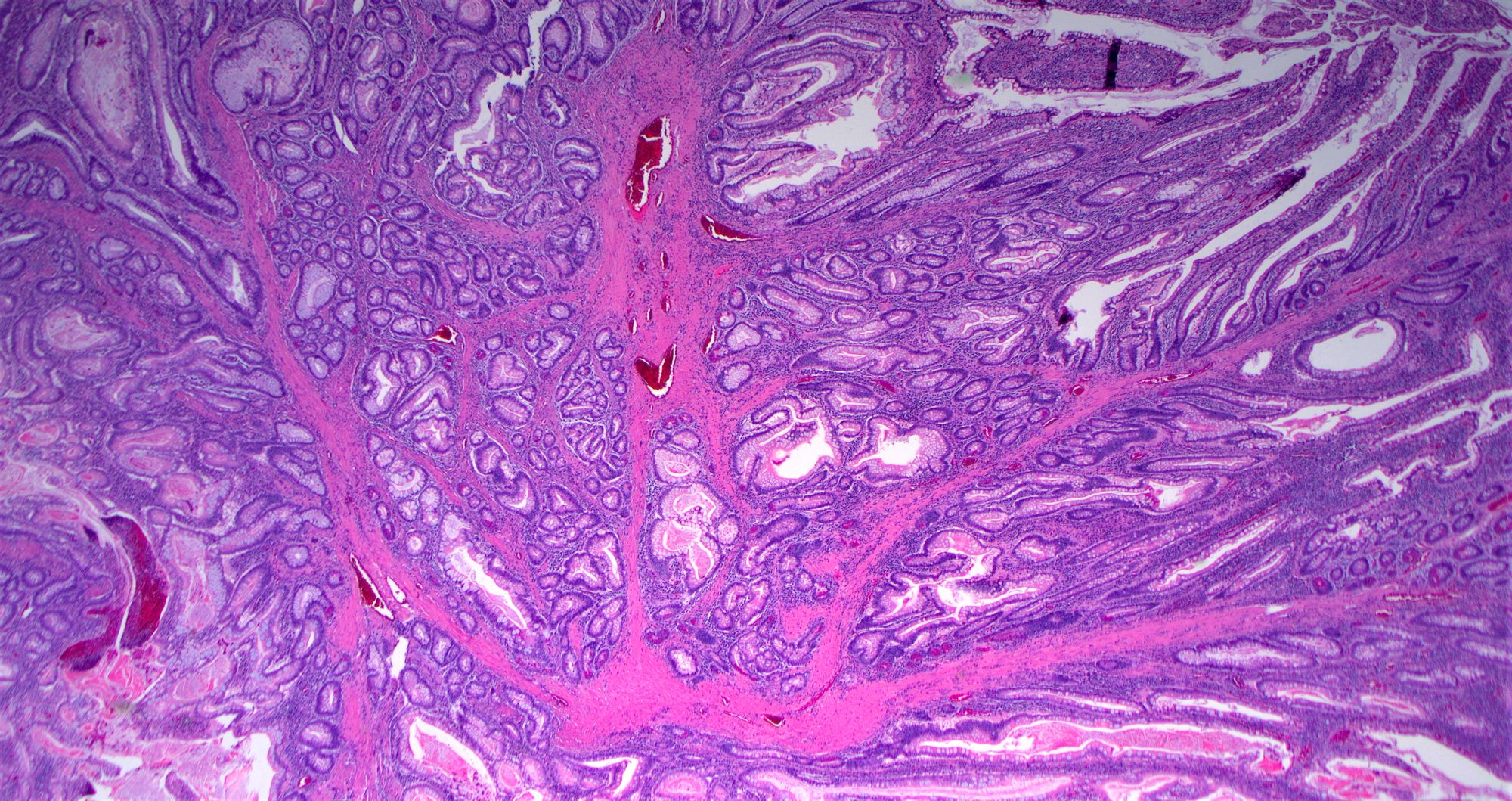
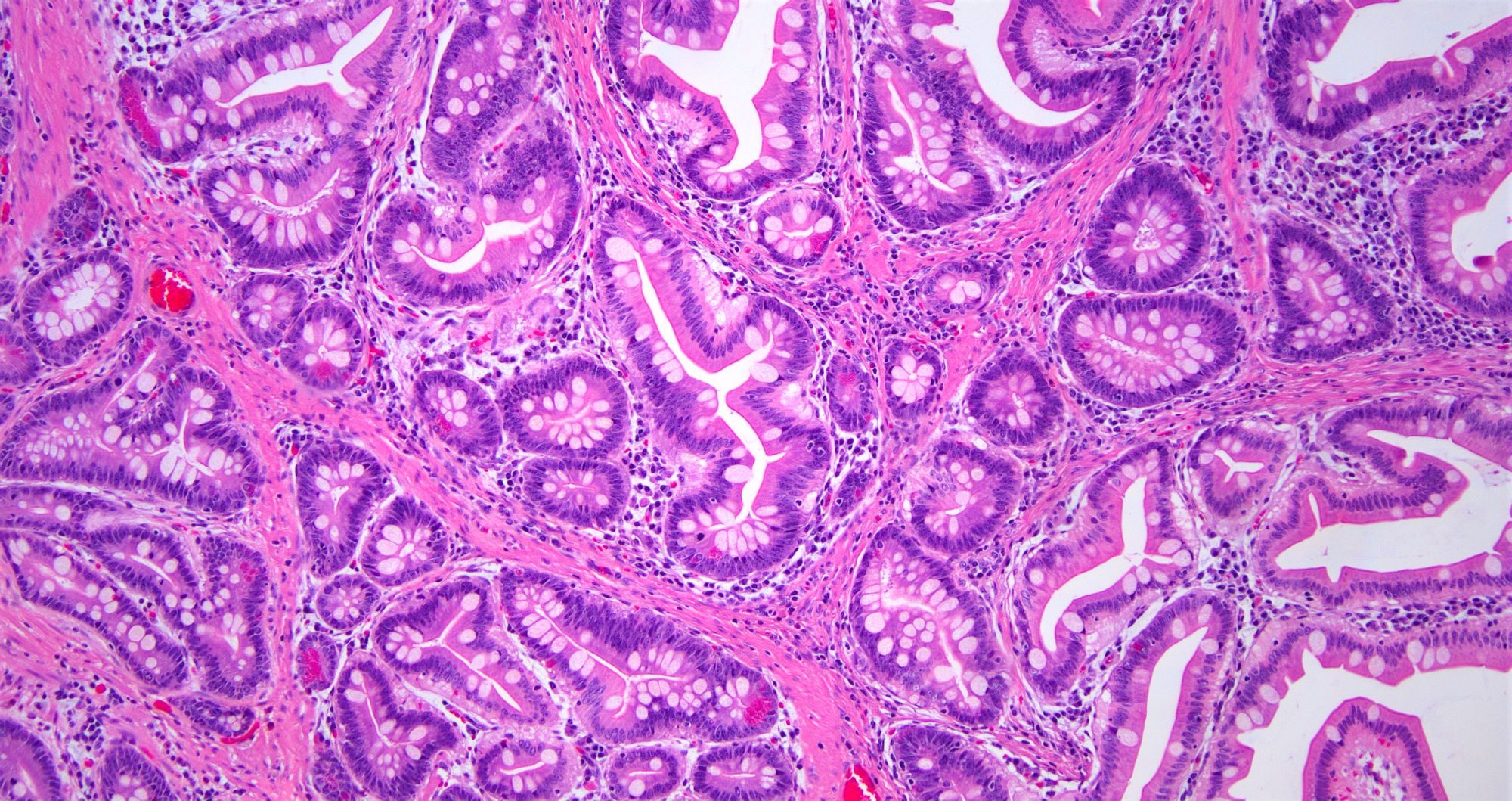
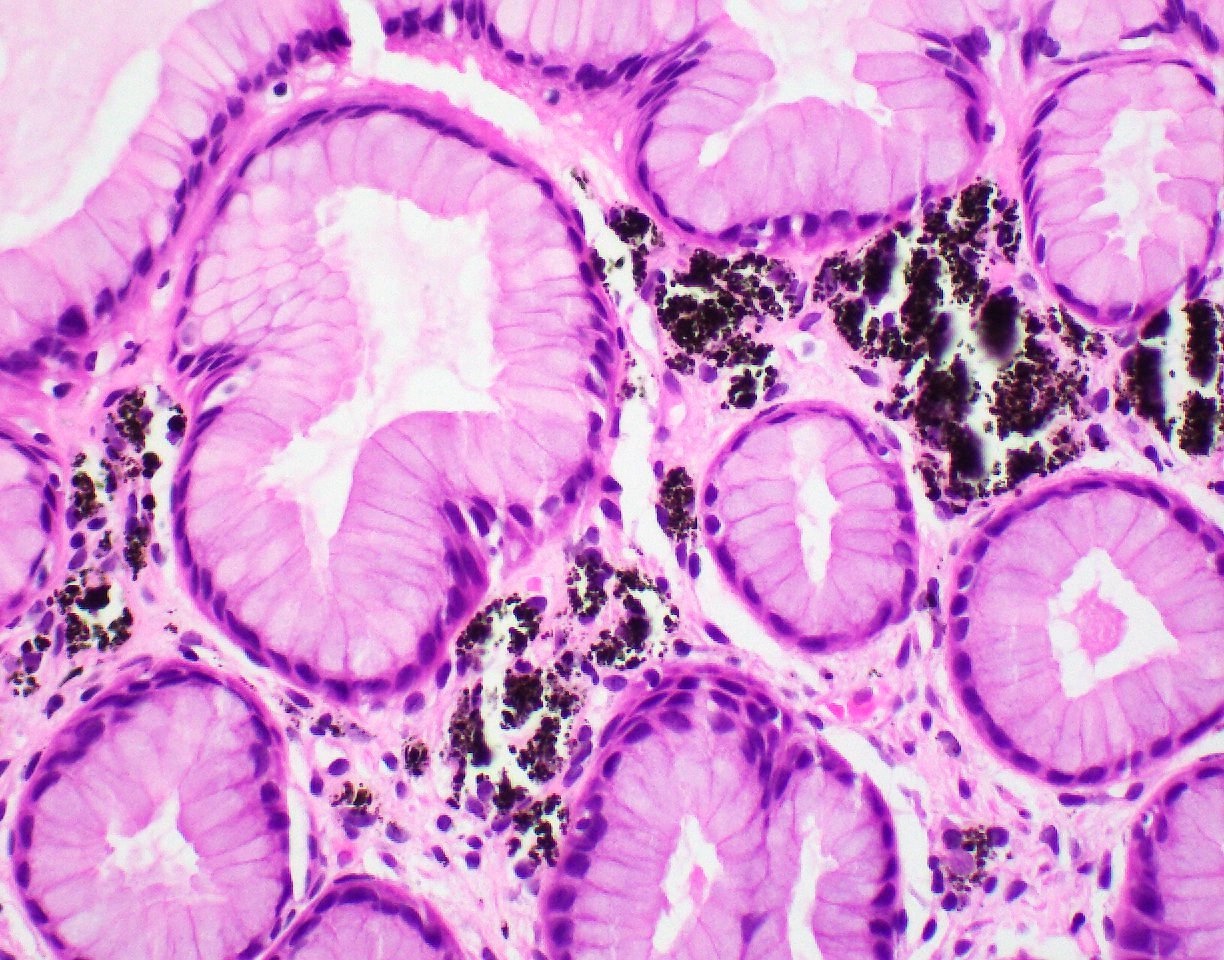
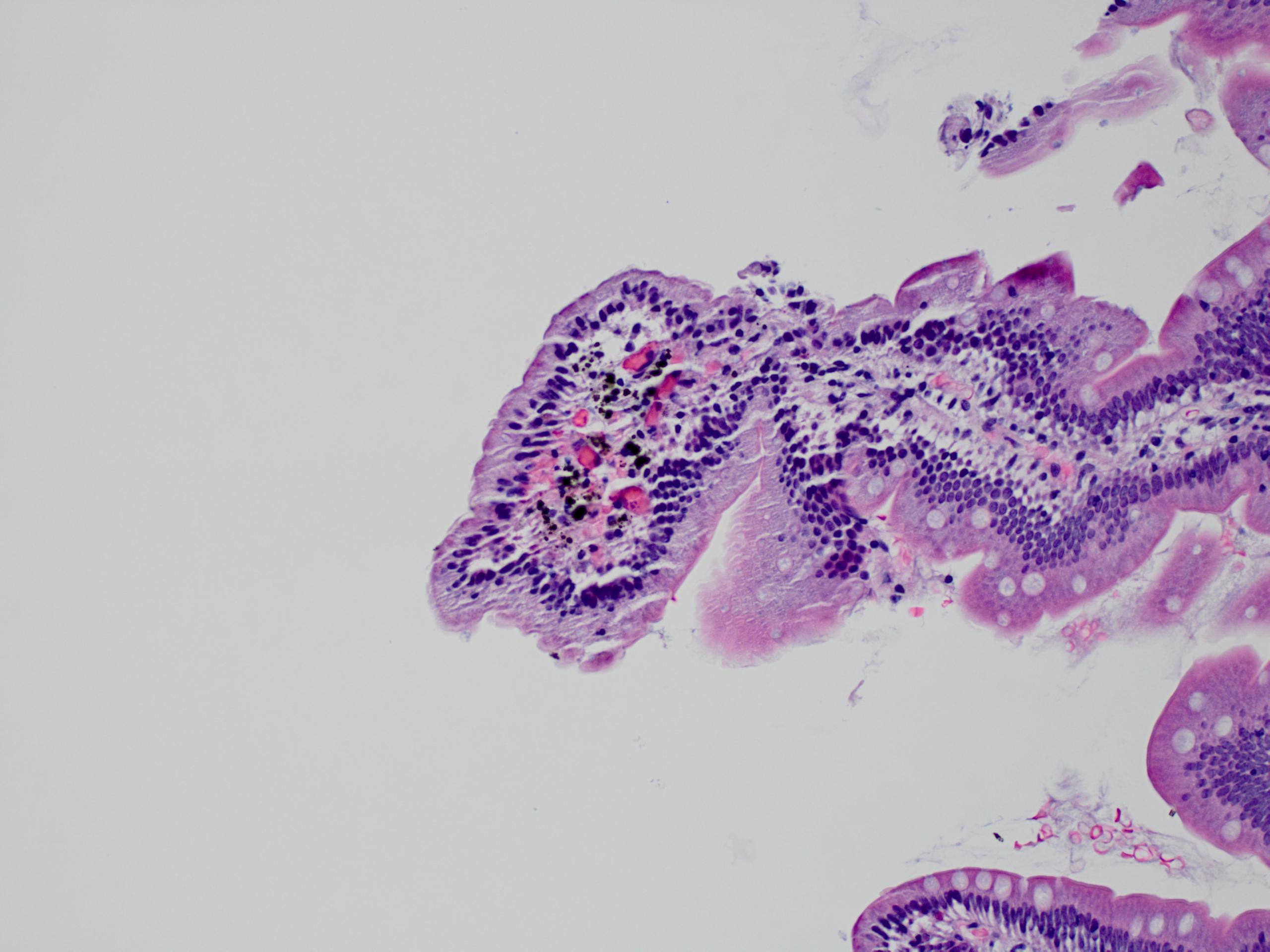
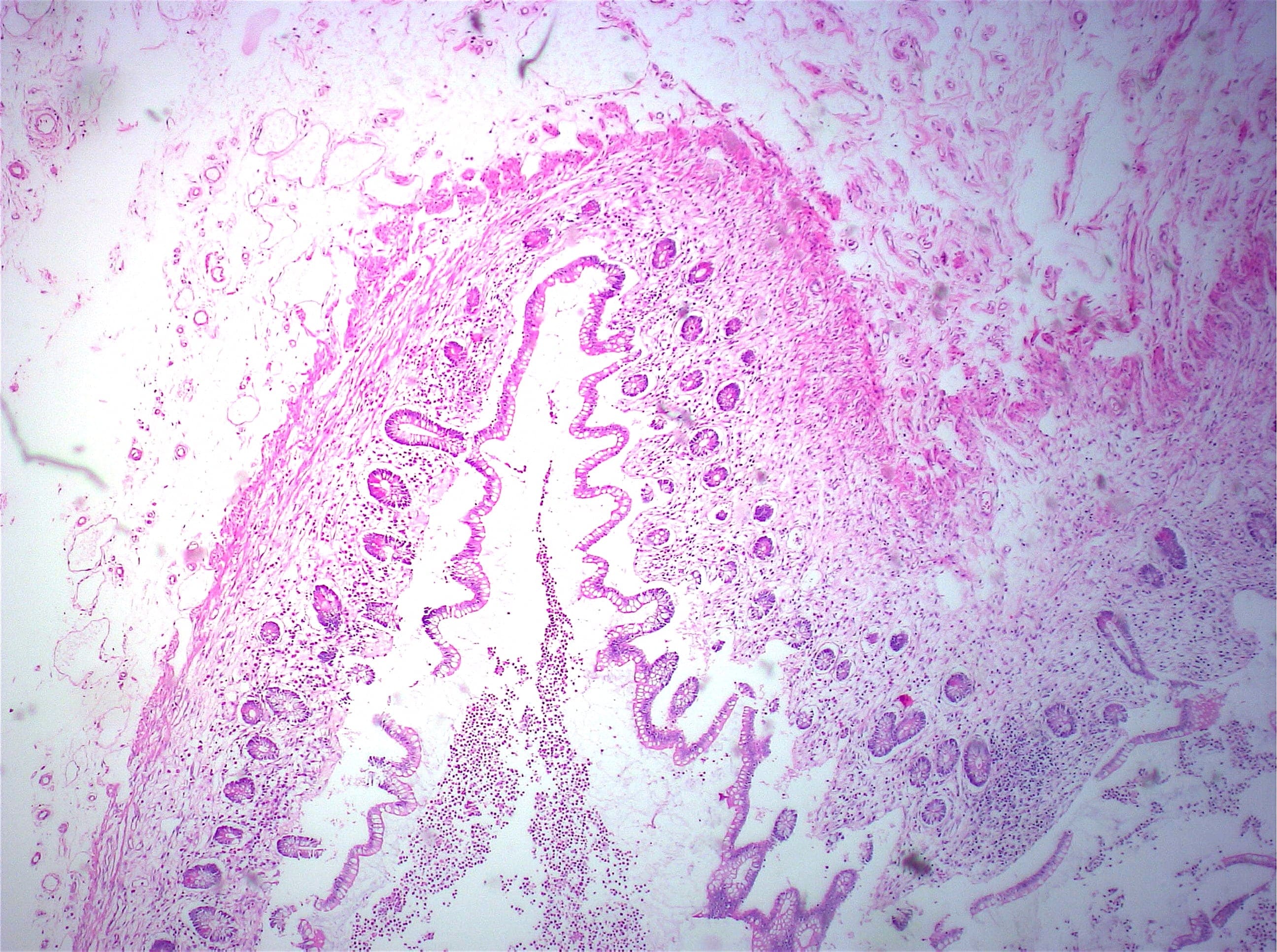
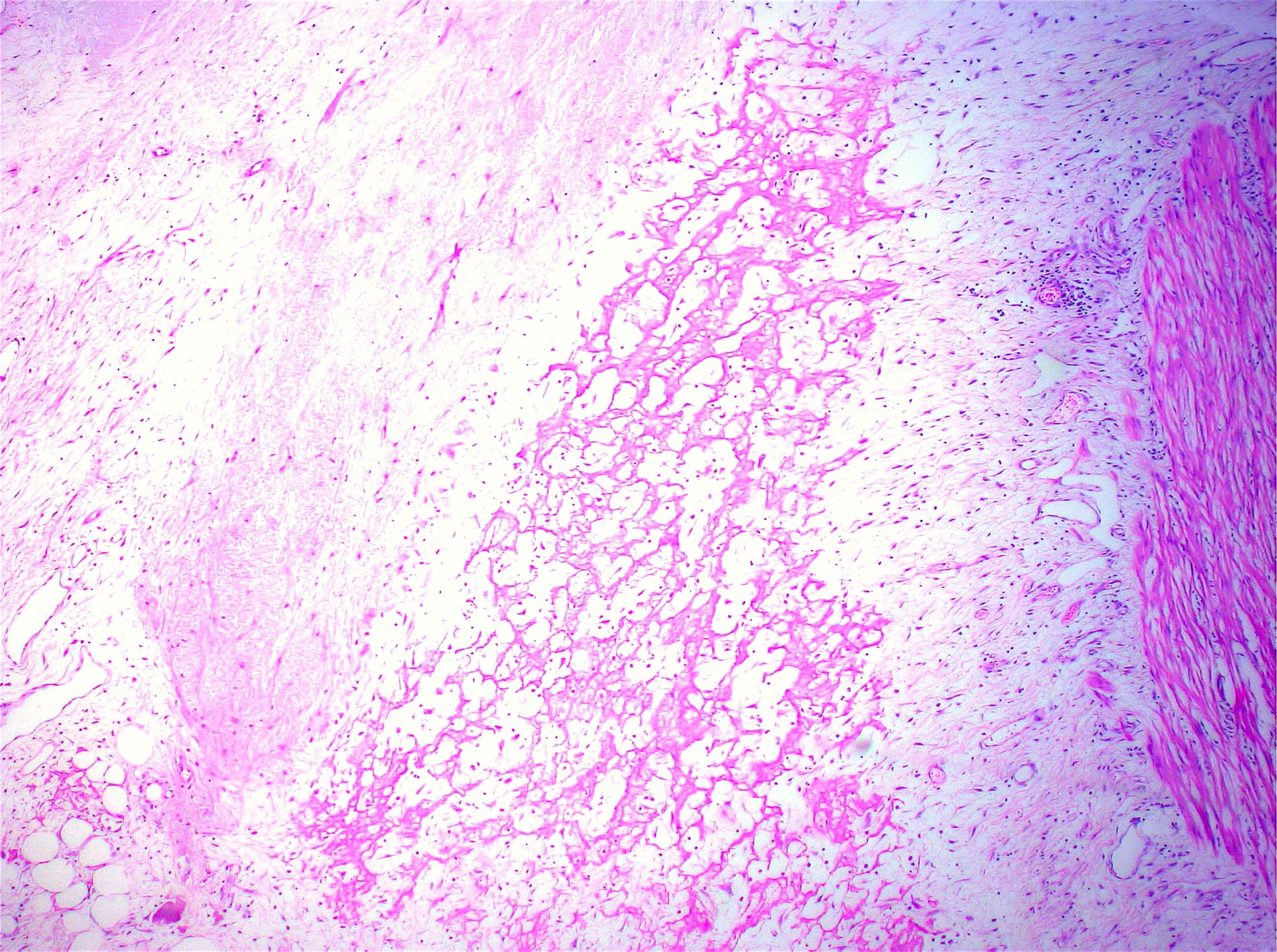
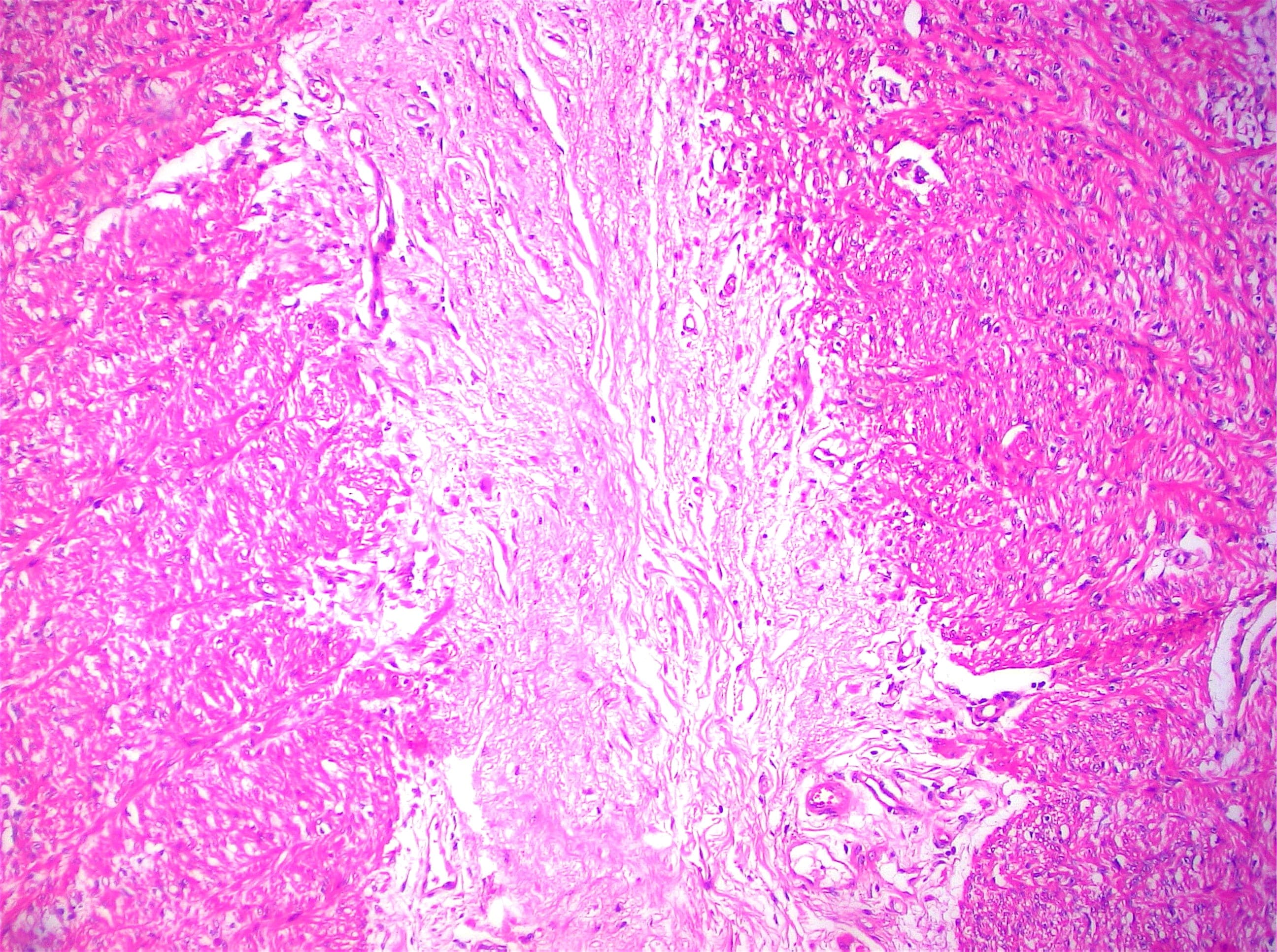
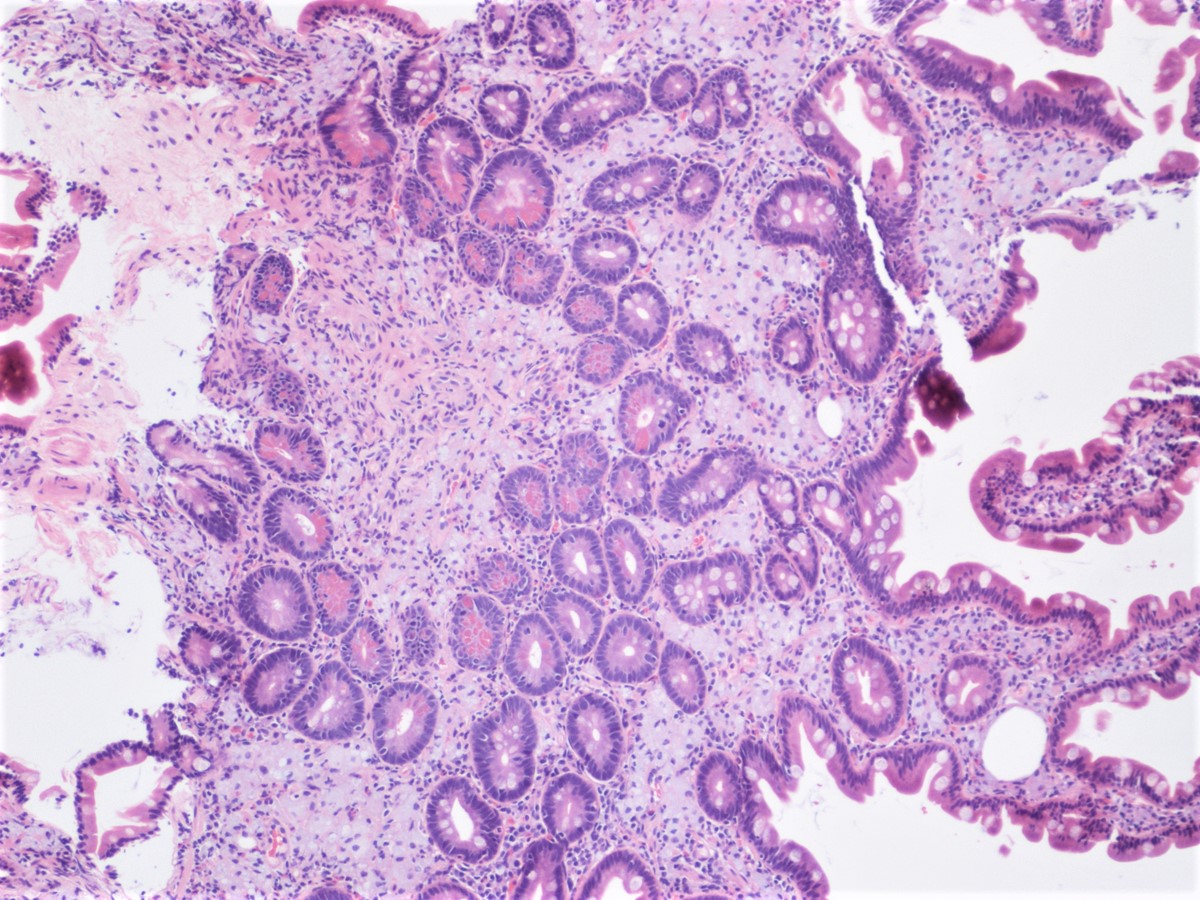
- Majority are gland forming (tubular adenocarcinoma)
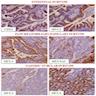
- 60% show either intestinal or biliary phenotypes while 40% have a mixed phenotype (Mod Pathol 2016;29:1575)
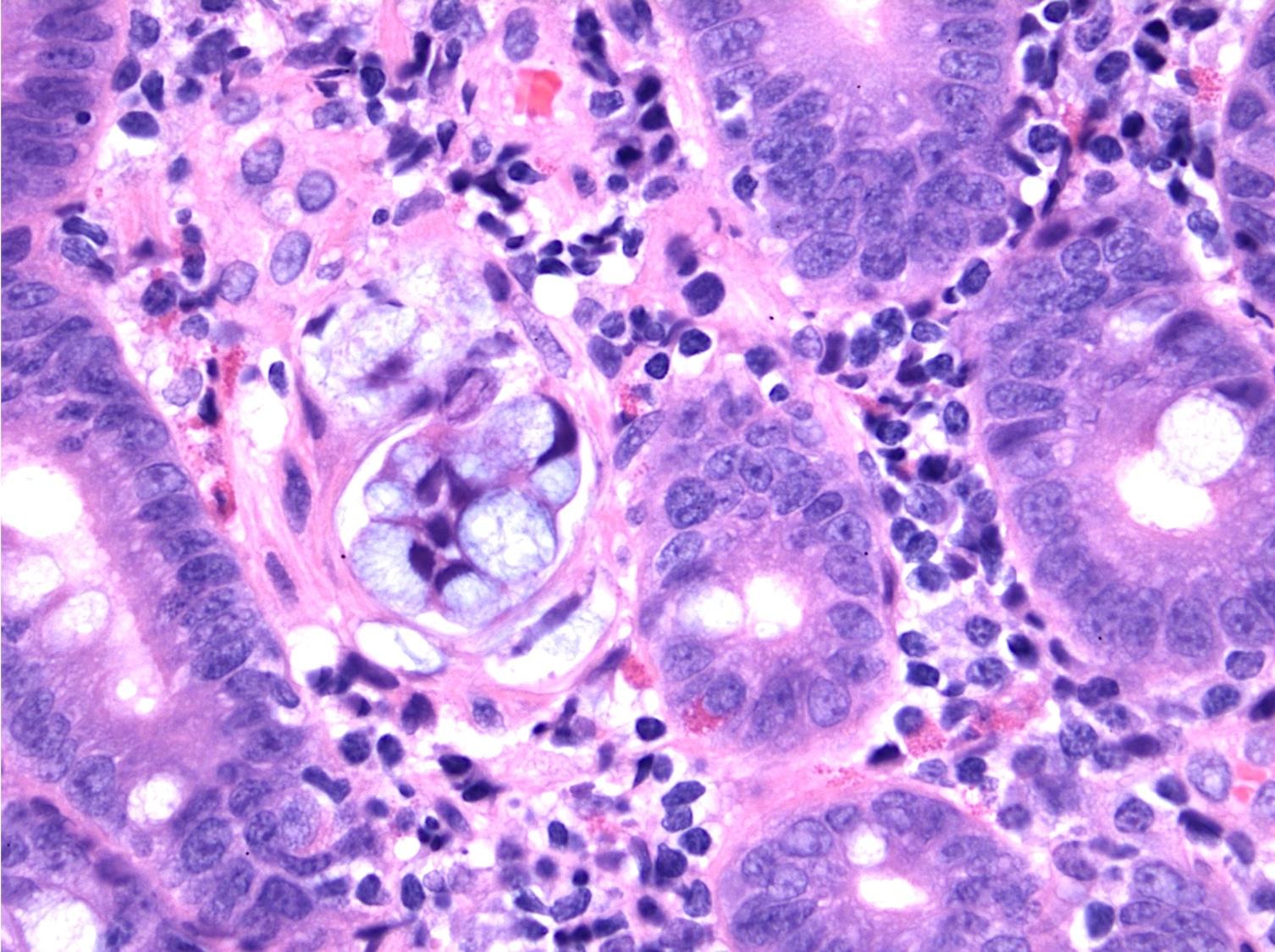
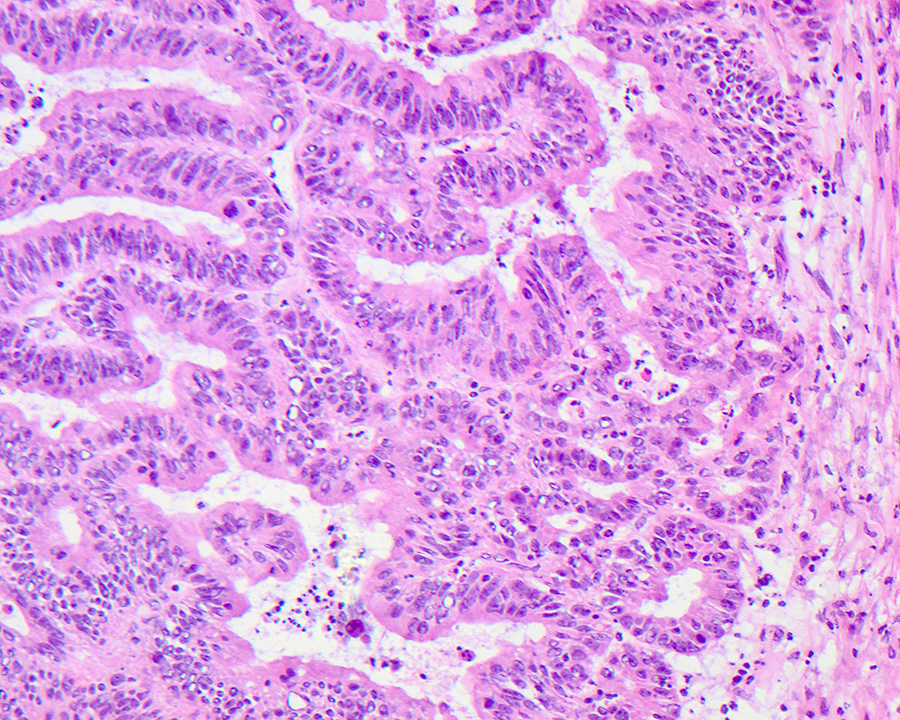
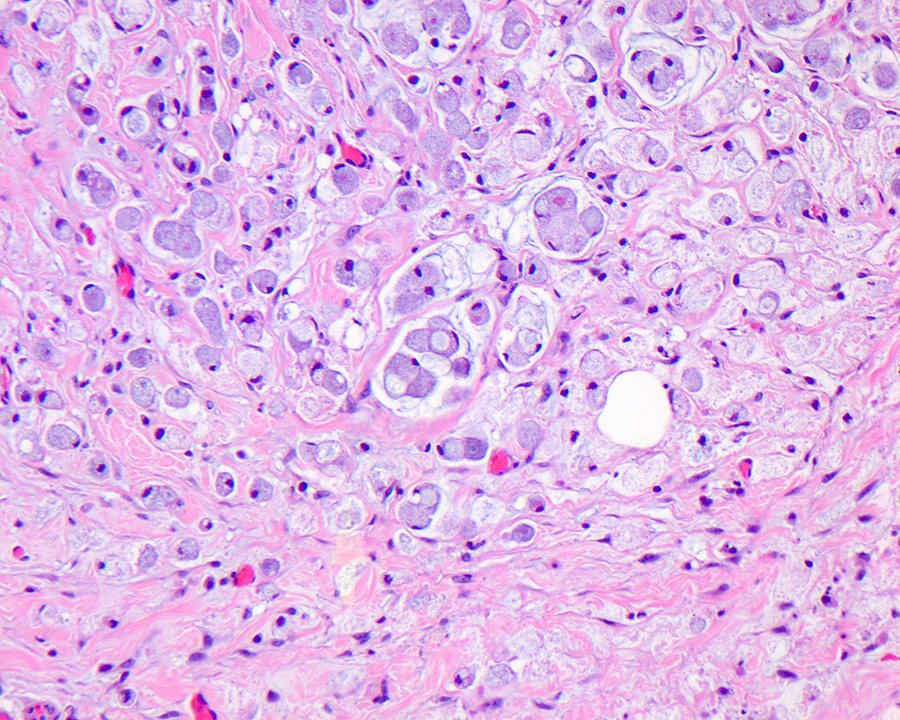
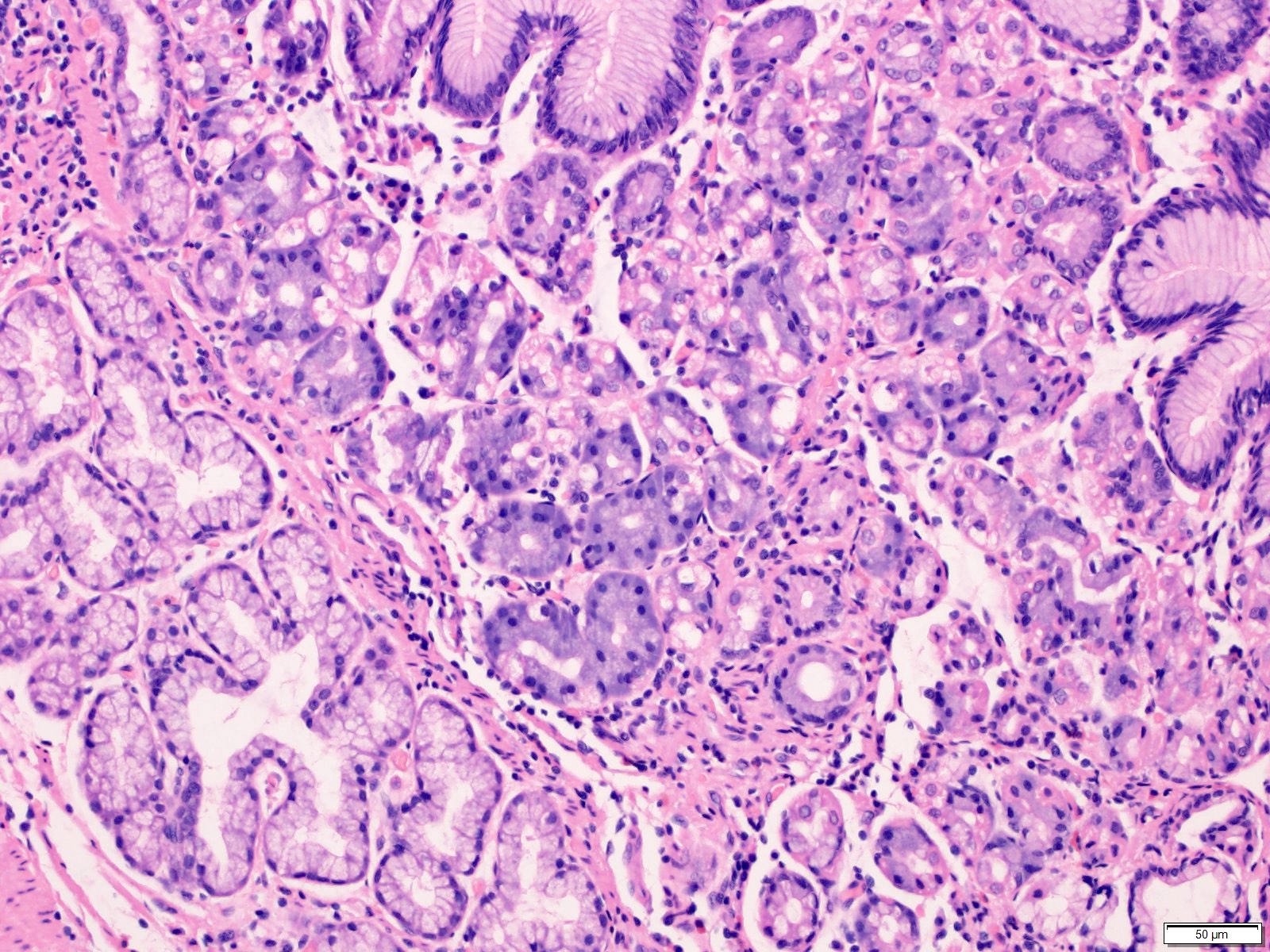
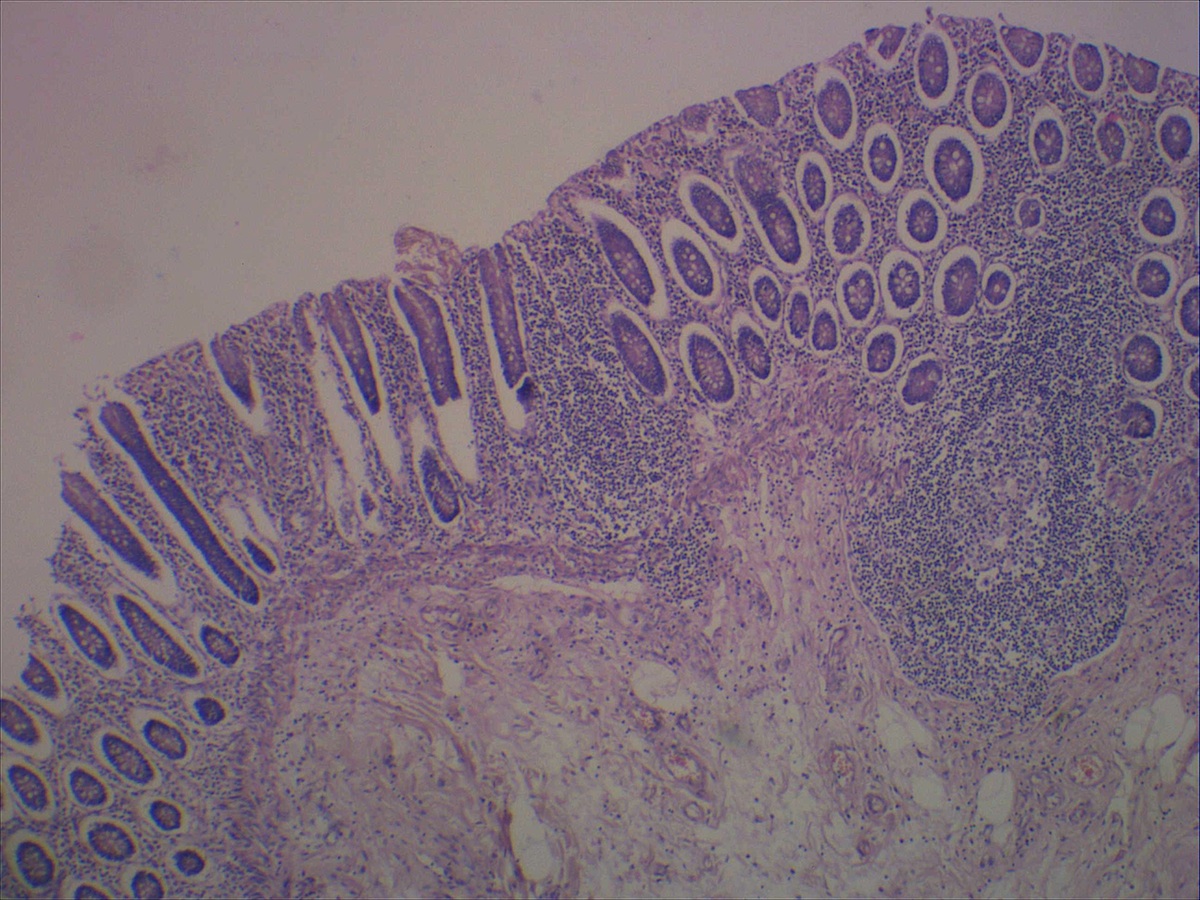
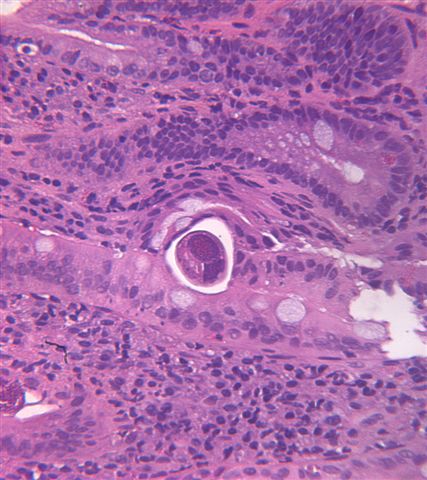
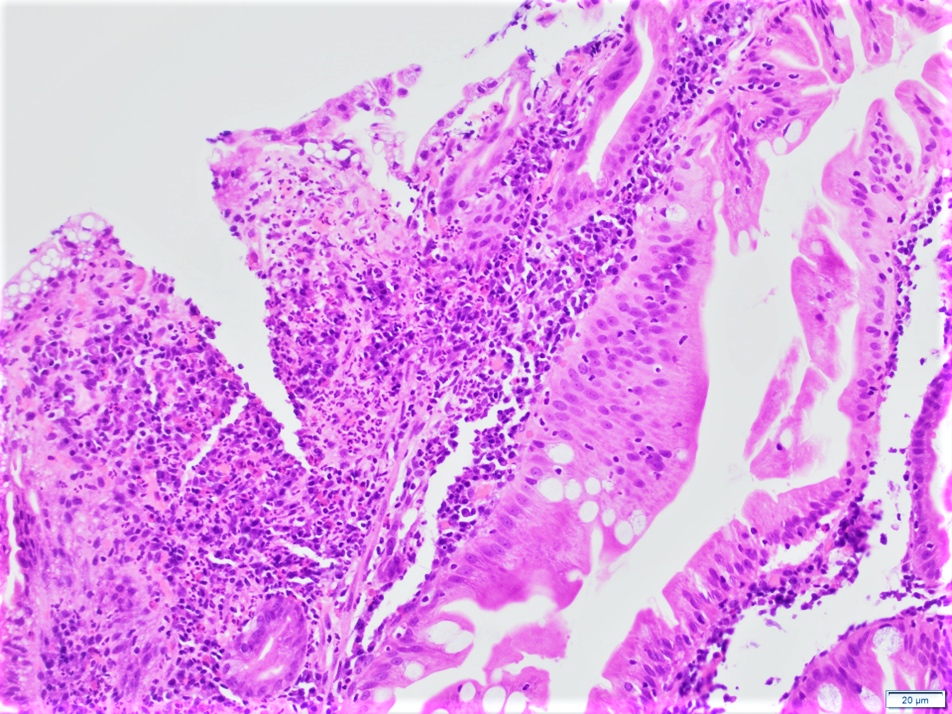
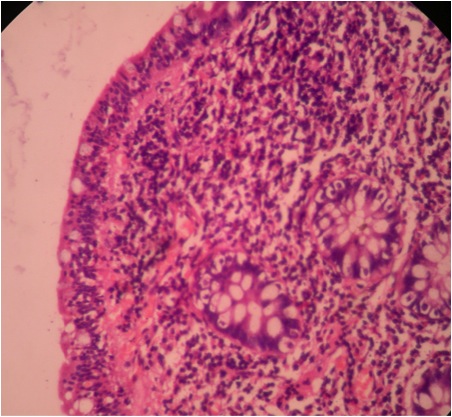
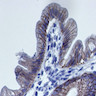
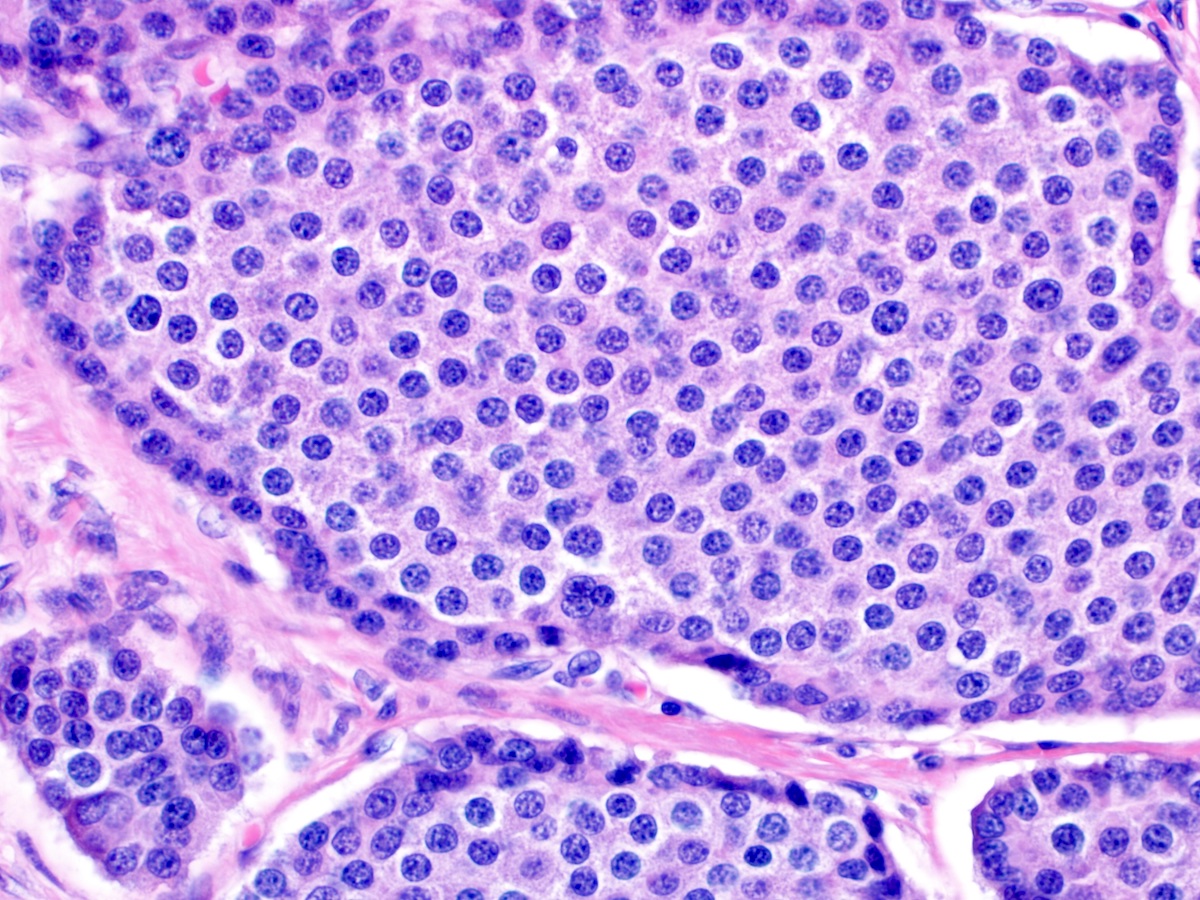
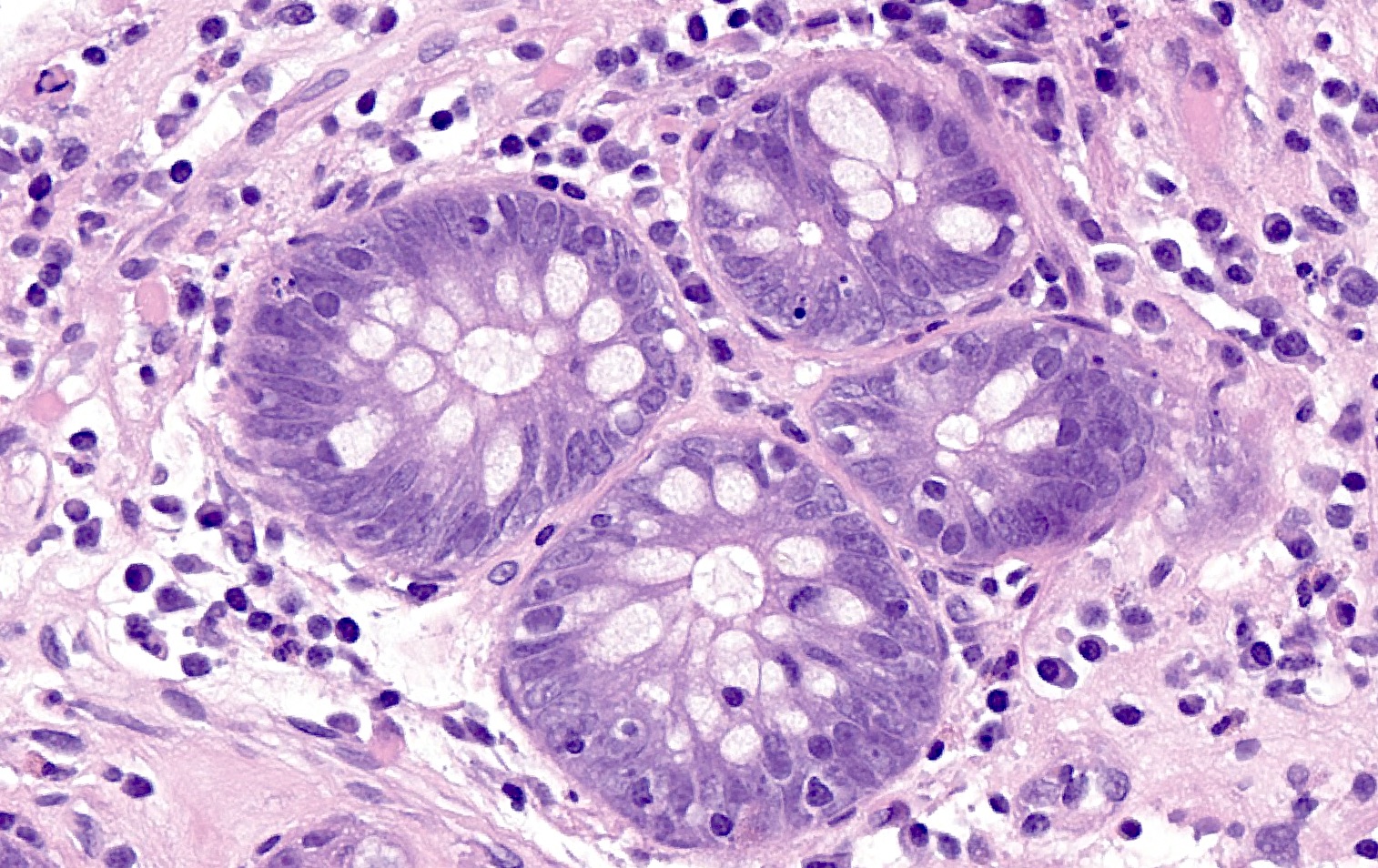
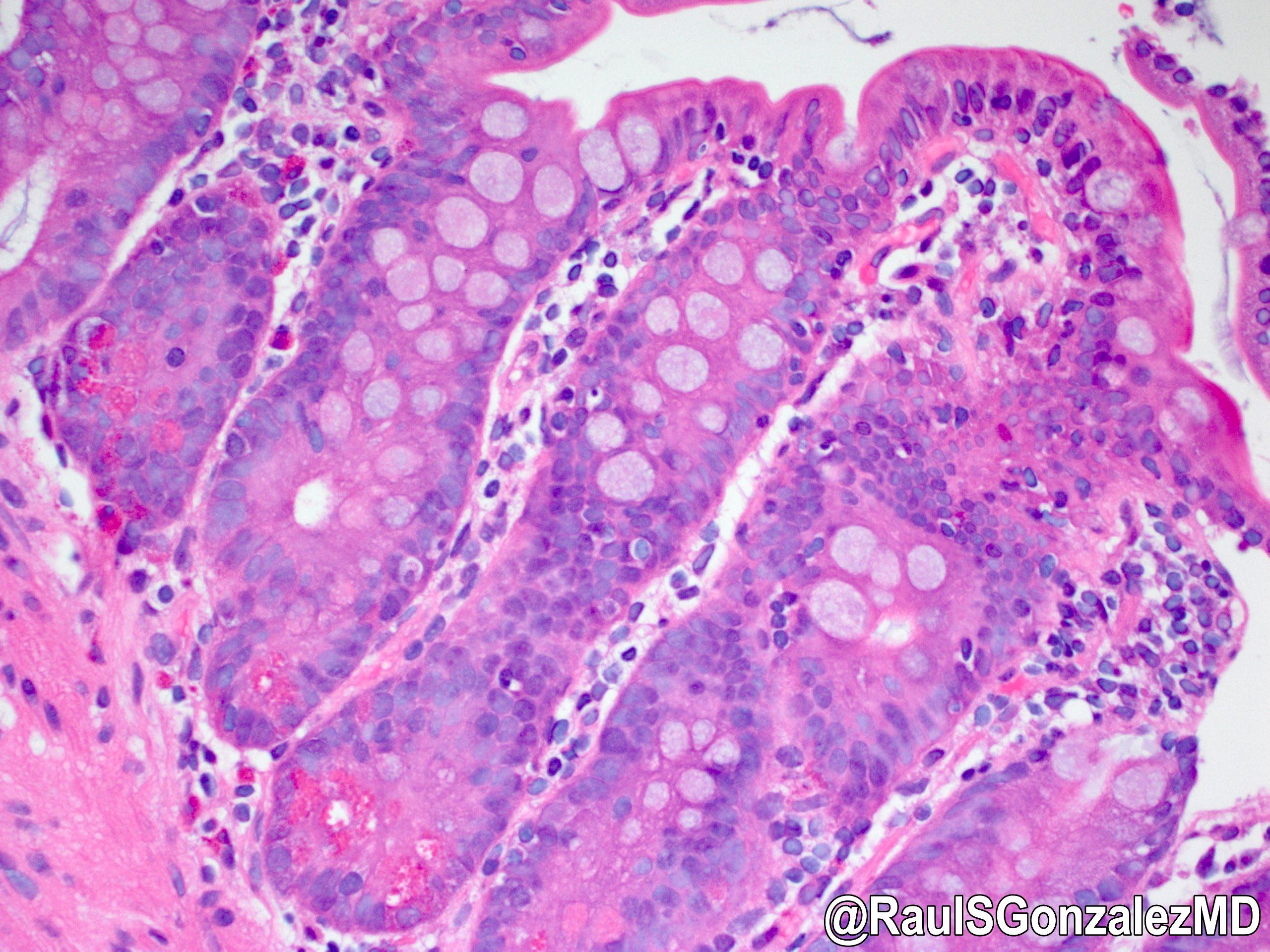
- Intestinal type: columnar cells with elongated, pseudostratified nuclei with scattered goblet cells and Paneth cells
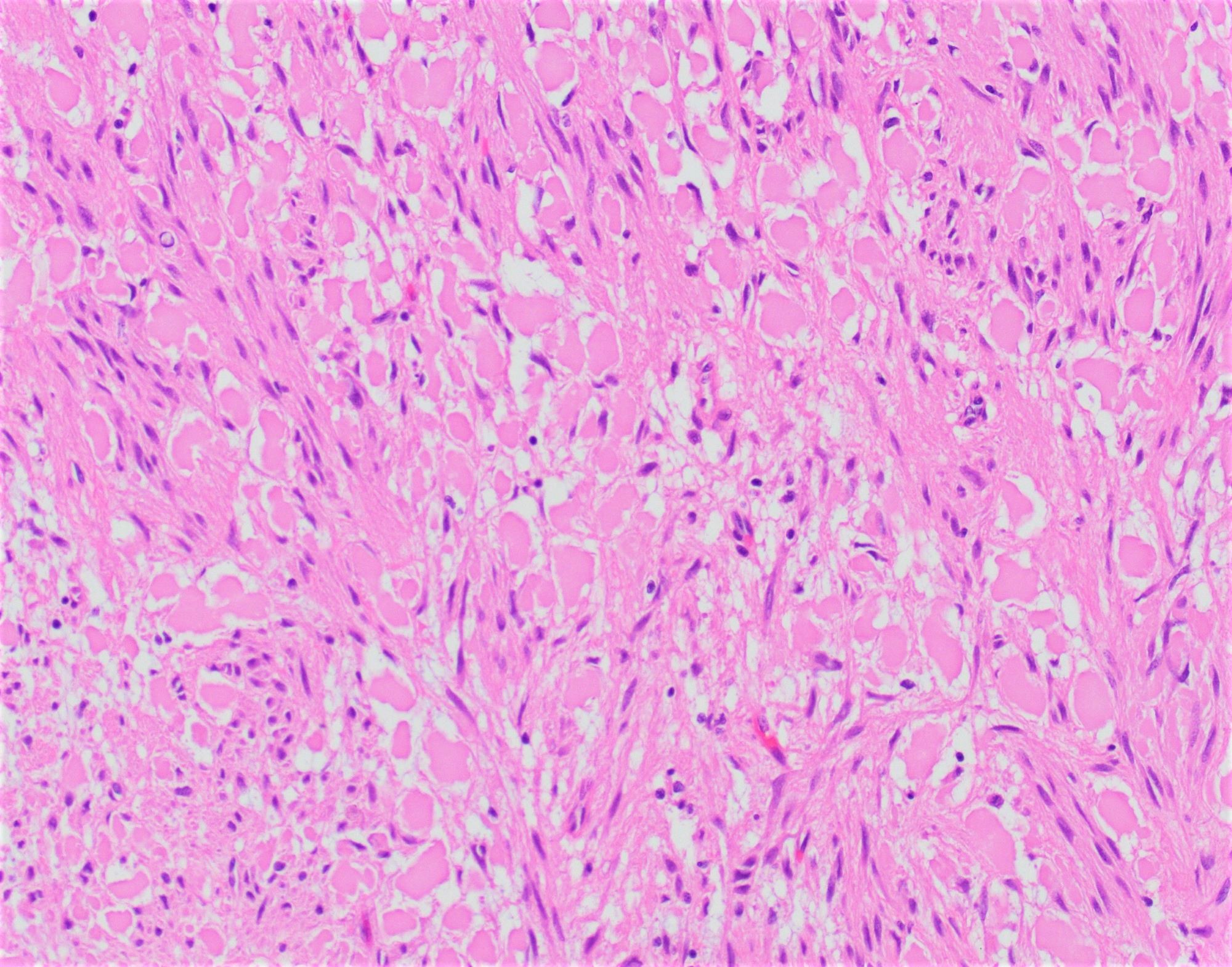
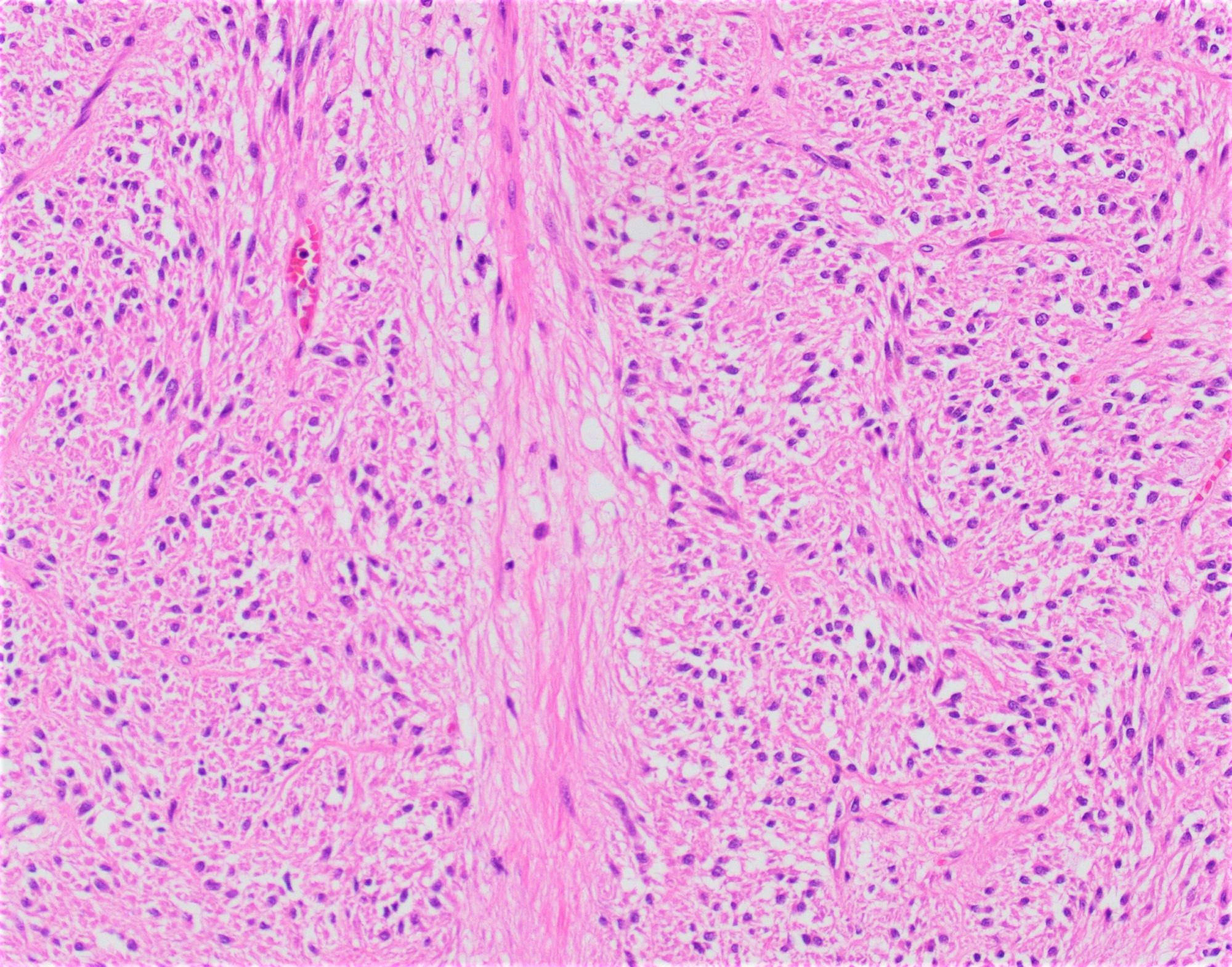
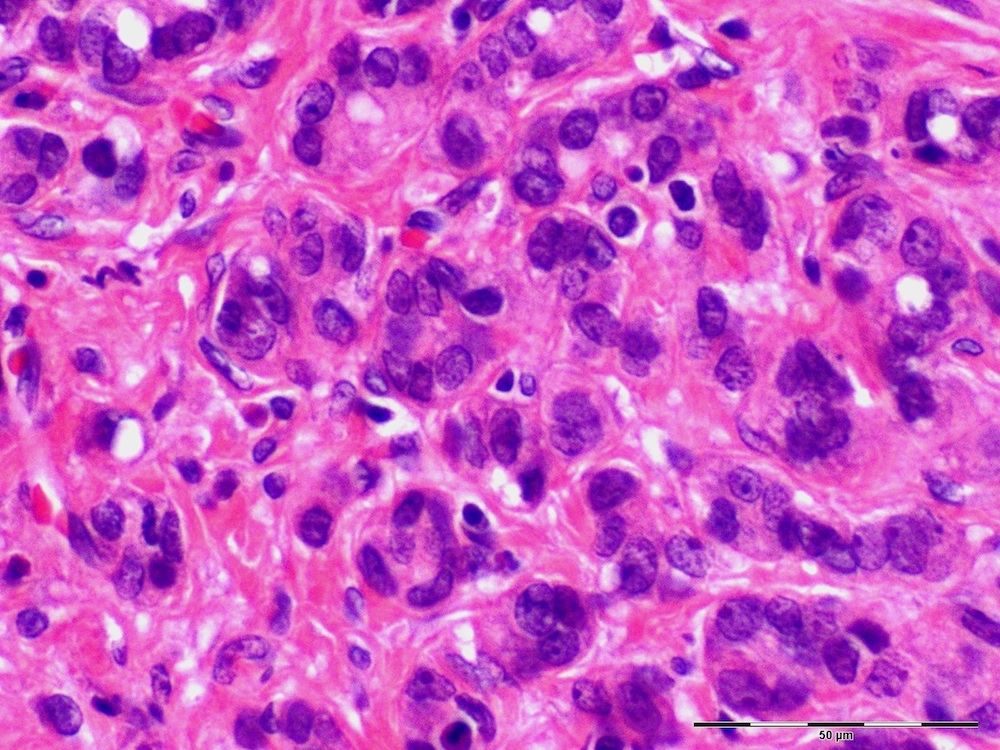
- Pancreatobiliary type: cuboidal cells with pleomorphism forming small glands in desmoplastic stroma
- Mixed type: shows mix of intestinal and pancreatobiliary types
- Nonglandular patterns include:
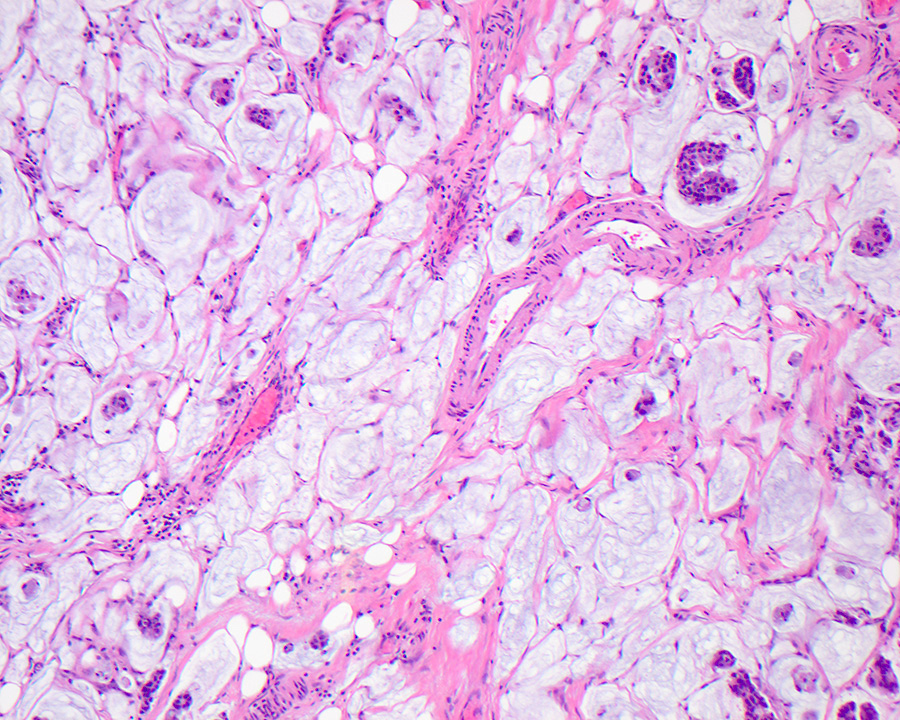
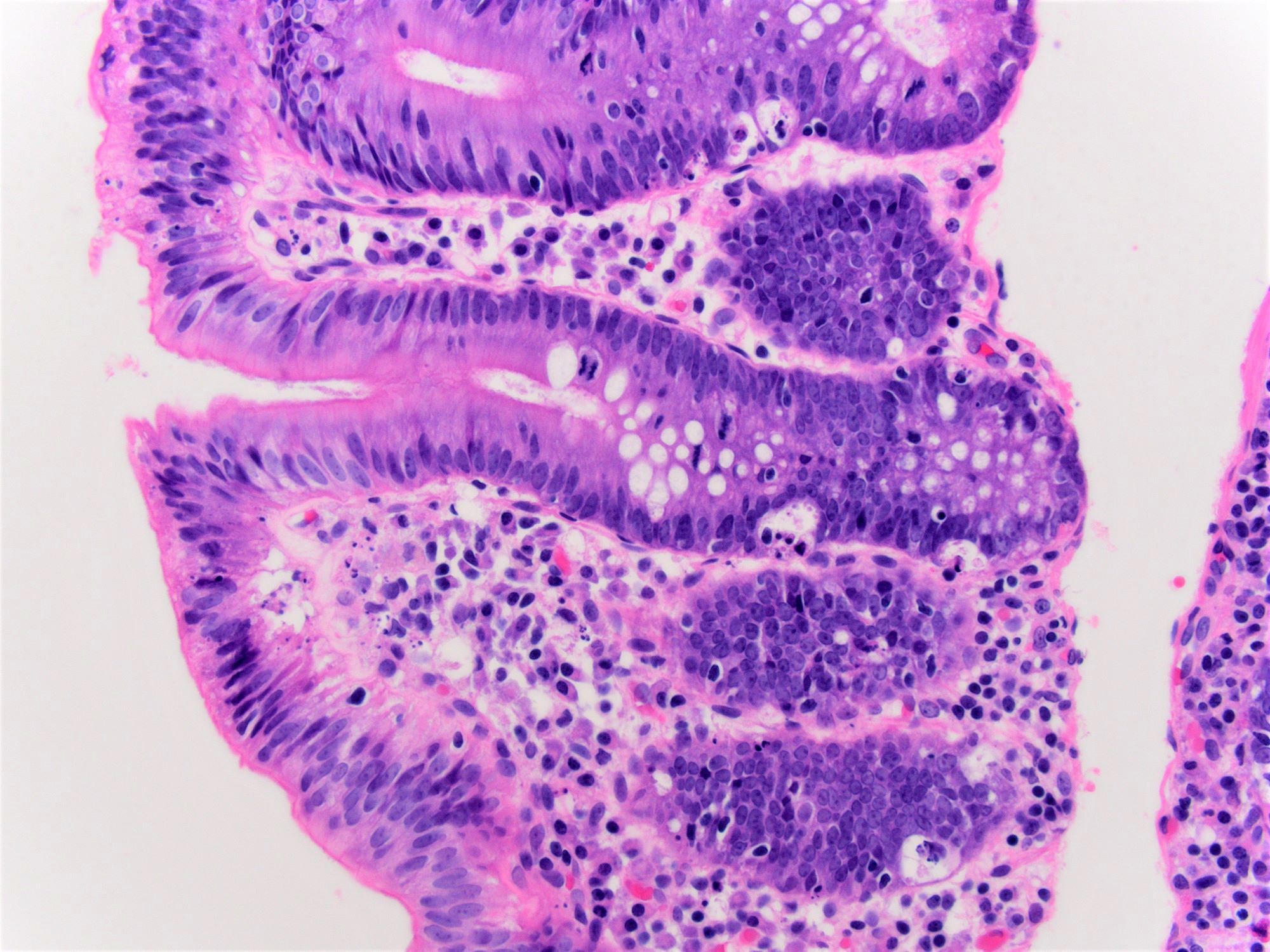
- Mucinous adenocarcinoma: > 50% stromal mucin pools containing floating tumor cells / glands with an intestinal phenotype
- Poorly cohesive cell carcinoma
- Medullary carcinoma
- Adenosquamous carcinoma: this extremely rare mixed tumor shows both morphologic and immunophenotypic evidence of both glandular and squamous differentiation (World J Surg Oncol 2015;13:287, World J Surg Oncol 2013;11:124)
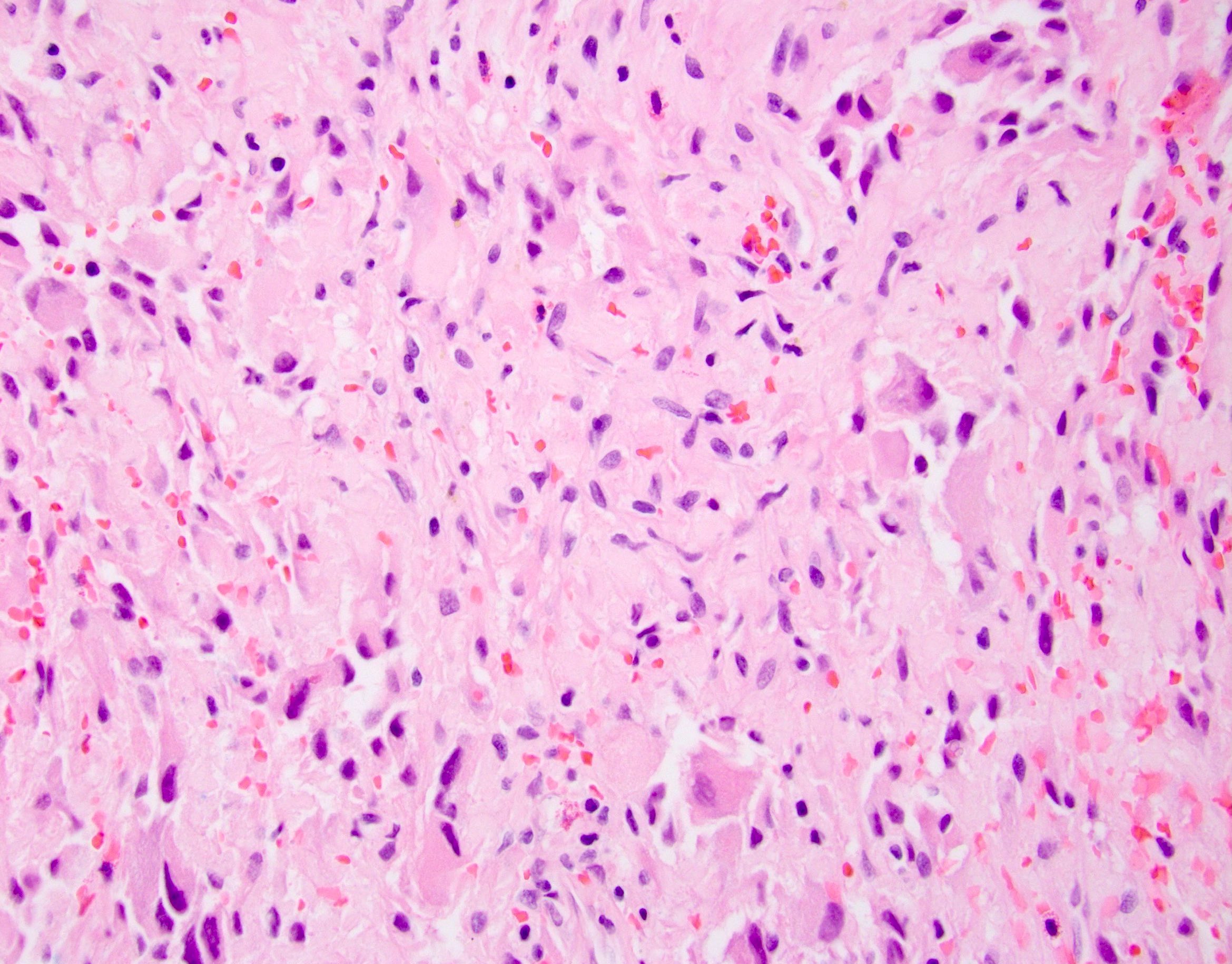
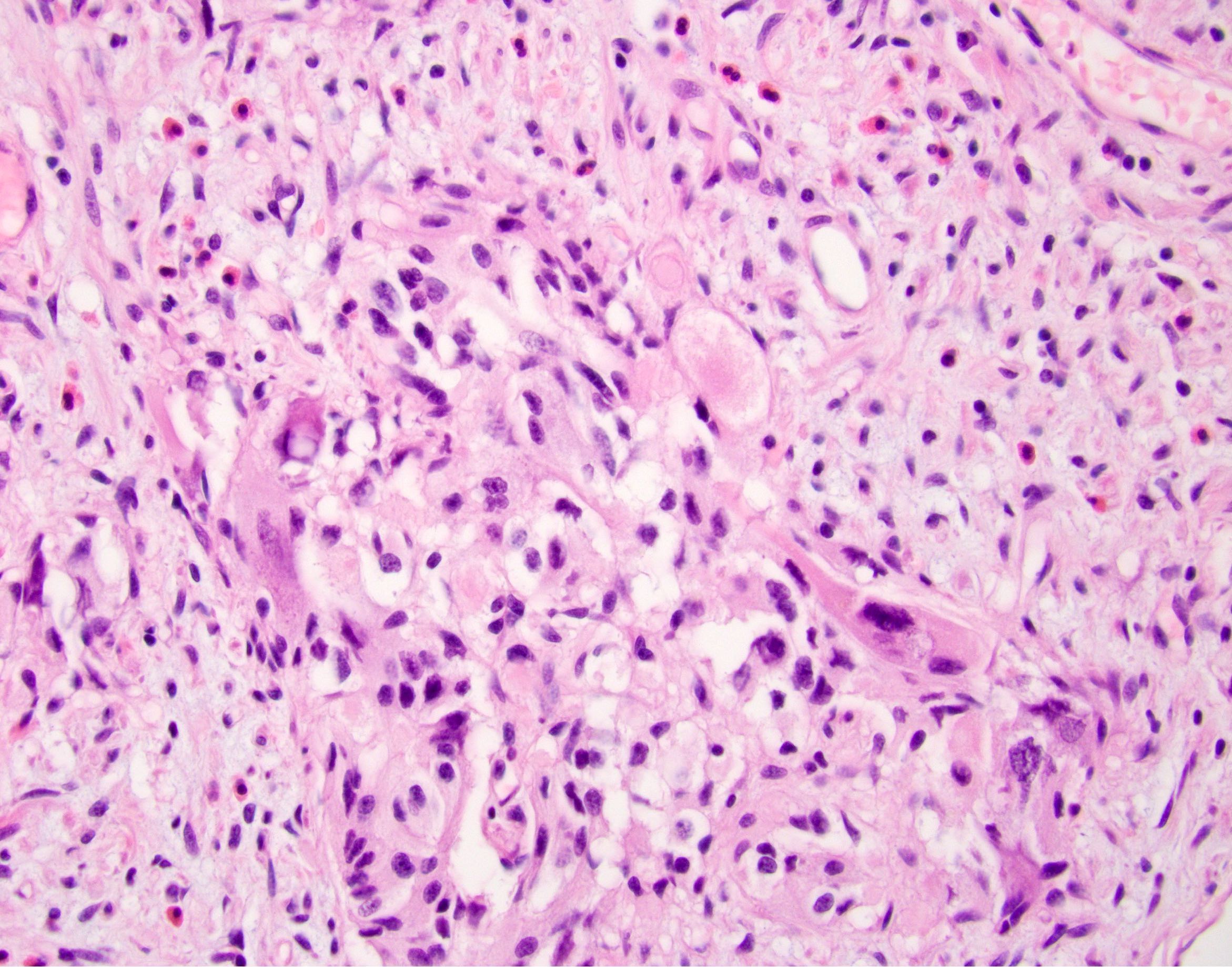
- Undifferentiated carcinoma
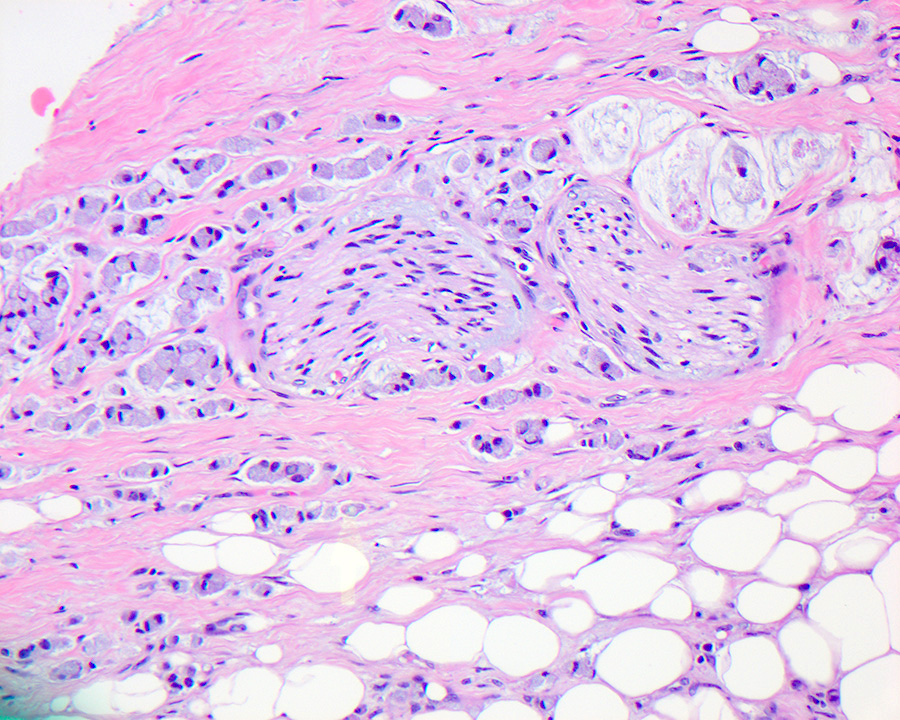
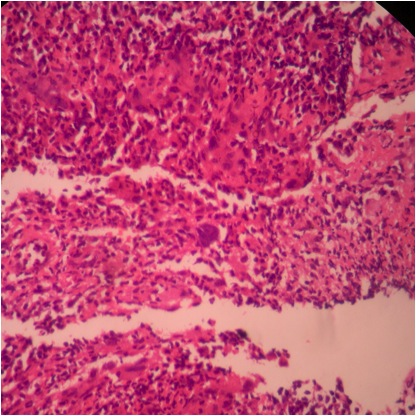
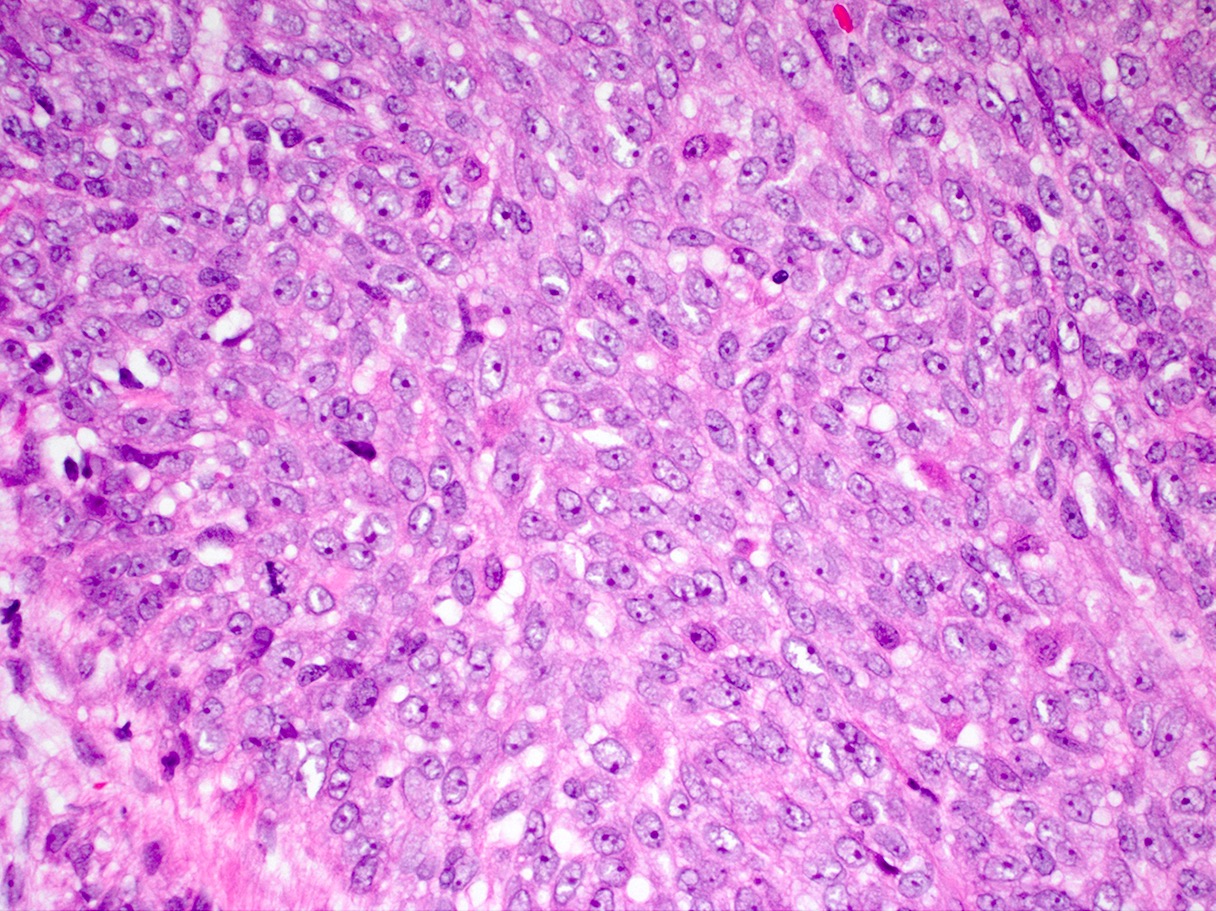
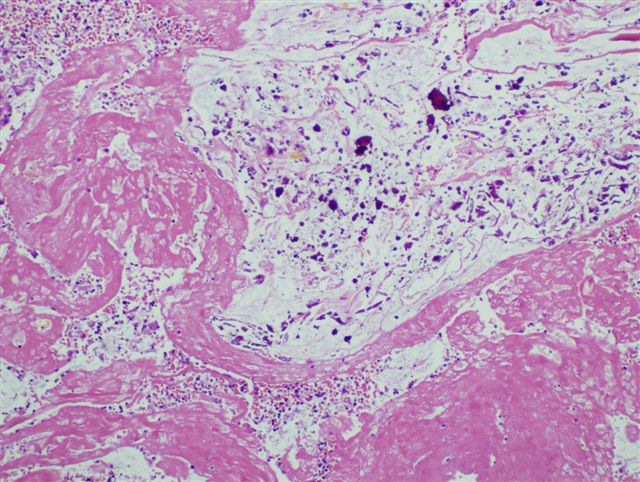
- Undifferentiated carcinoma with osteoclast-like giant cells: tumor that is comprised of sarcomatoid appearing mononuclear cells and contains osteoclast-like giant cells
- Undifferentiated carcinoma with rhabdoid phenotype: discohesive tumor cells show abundant eosinophilic intracytoplasmic rhabdoid bodies and are present in a myxoid matrix (Am J Surg Pathol 2016;40:544)
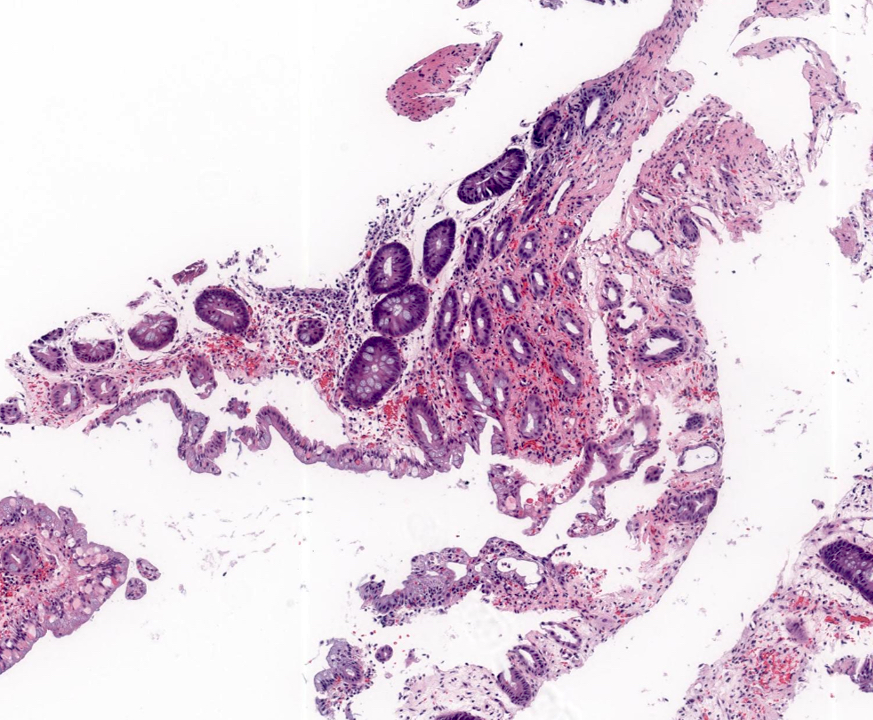
- Histologic features by subtype (Am J Surg Pathol 2012;36:1592)
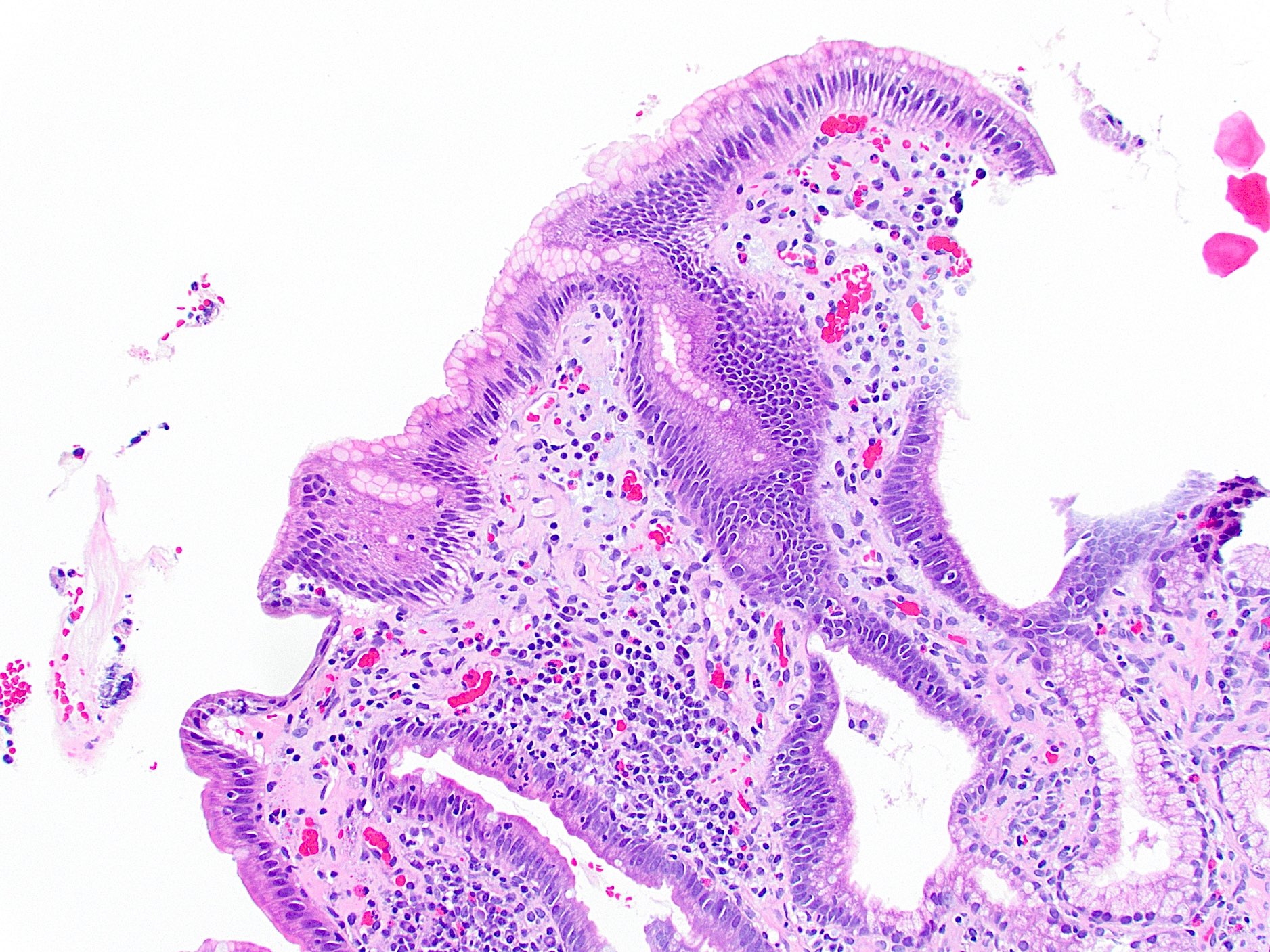
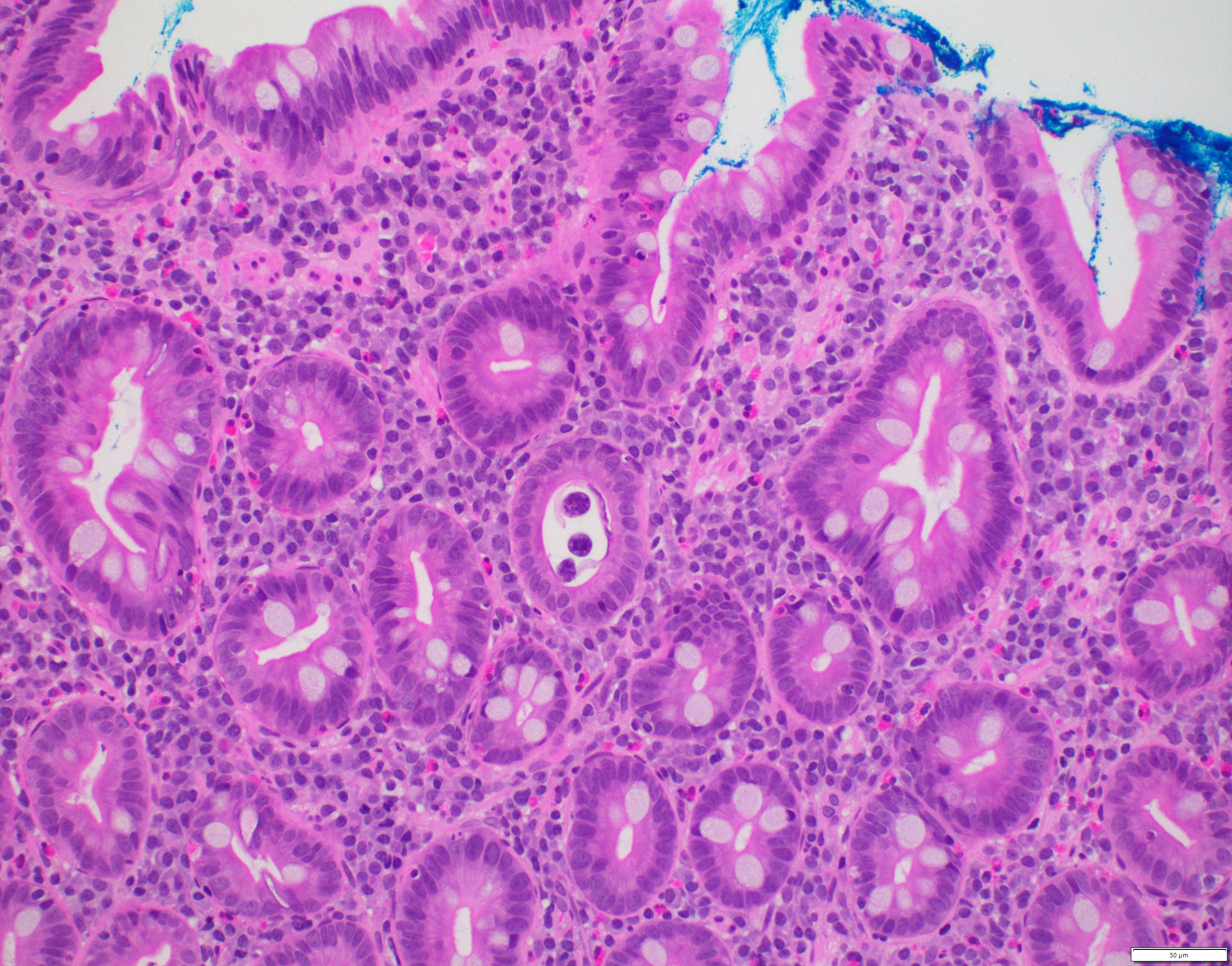
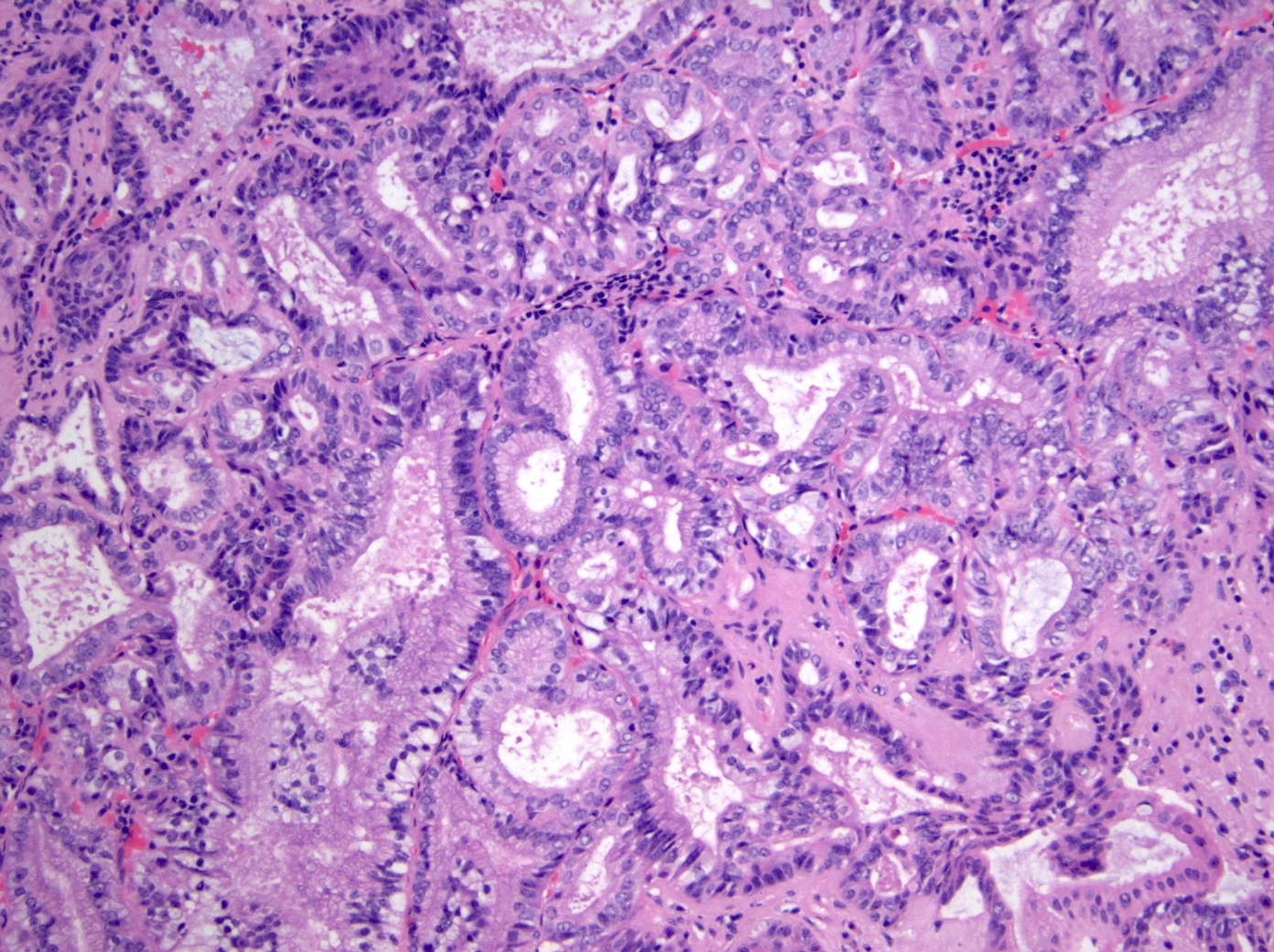
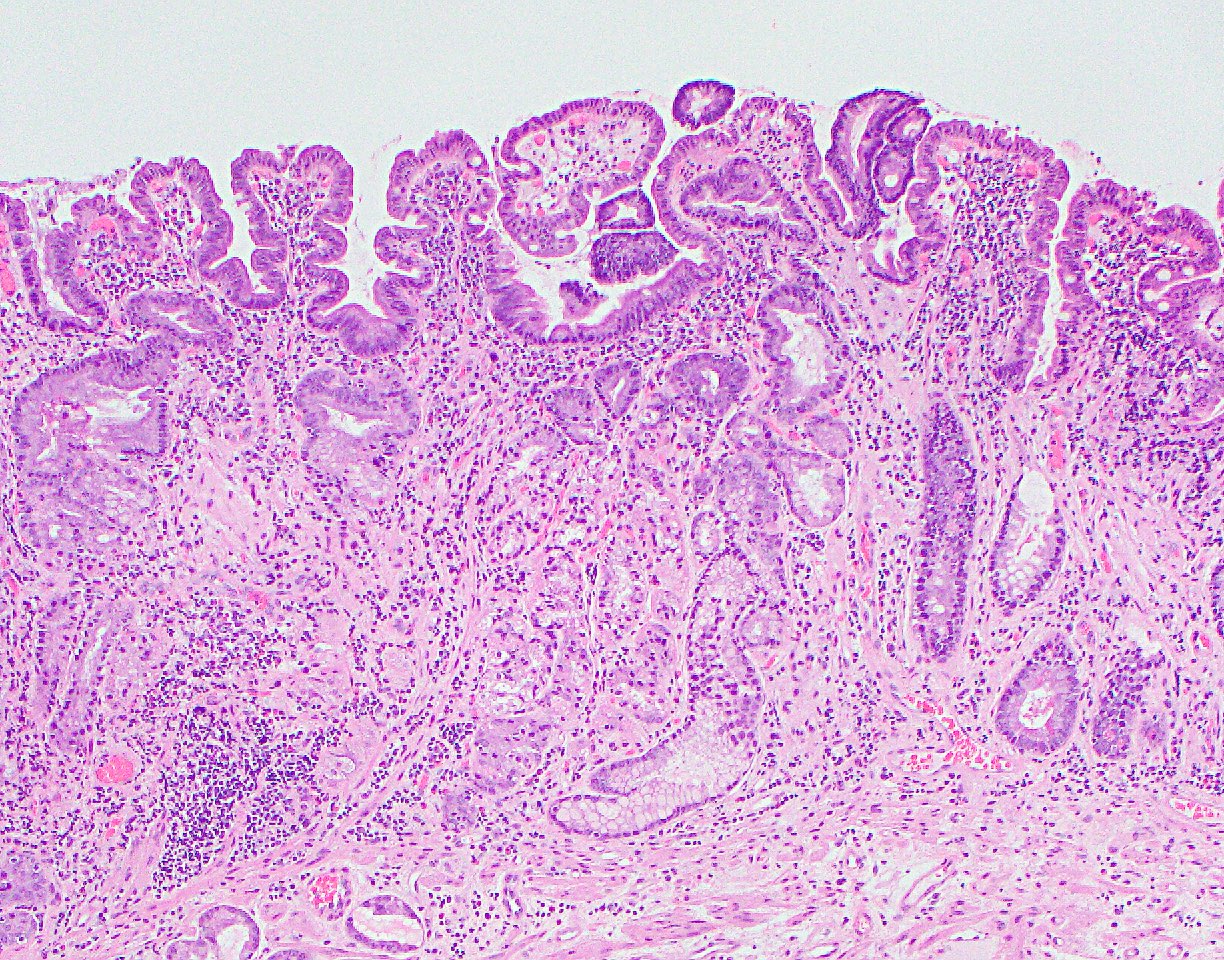
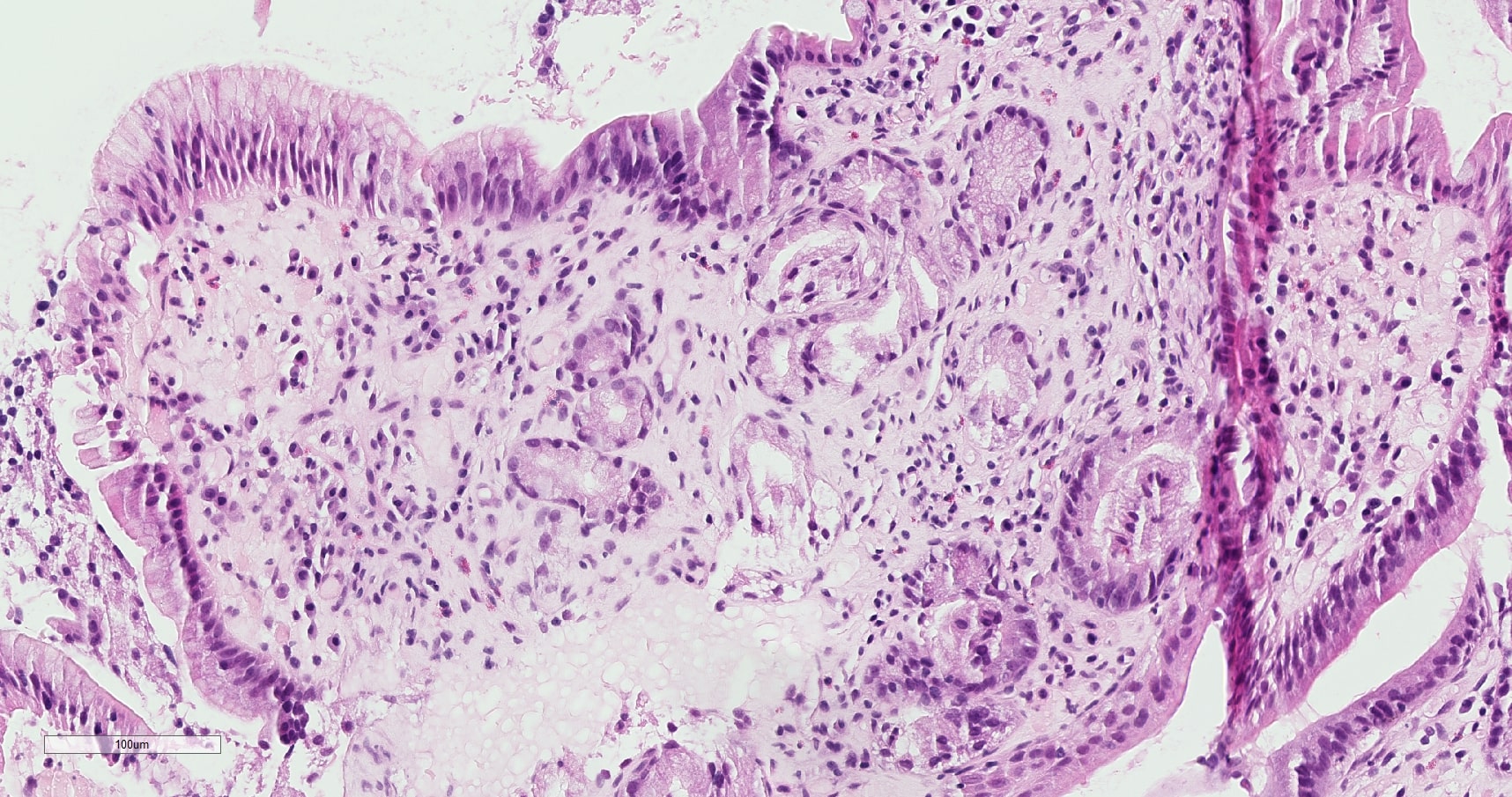
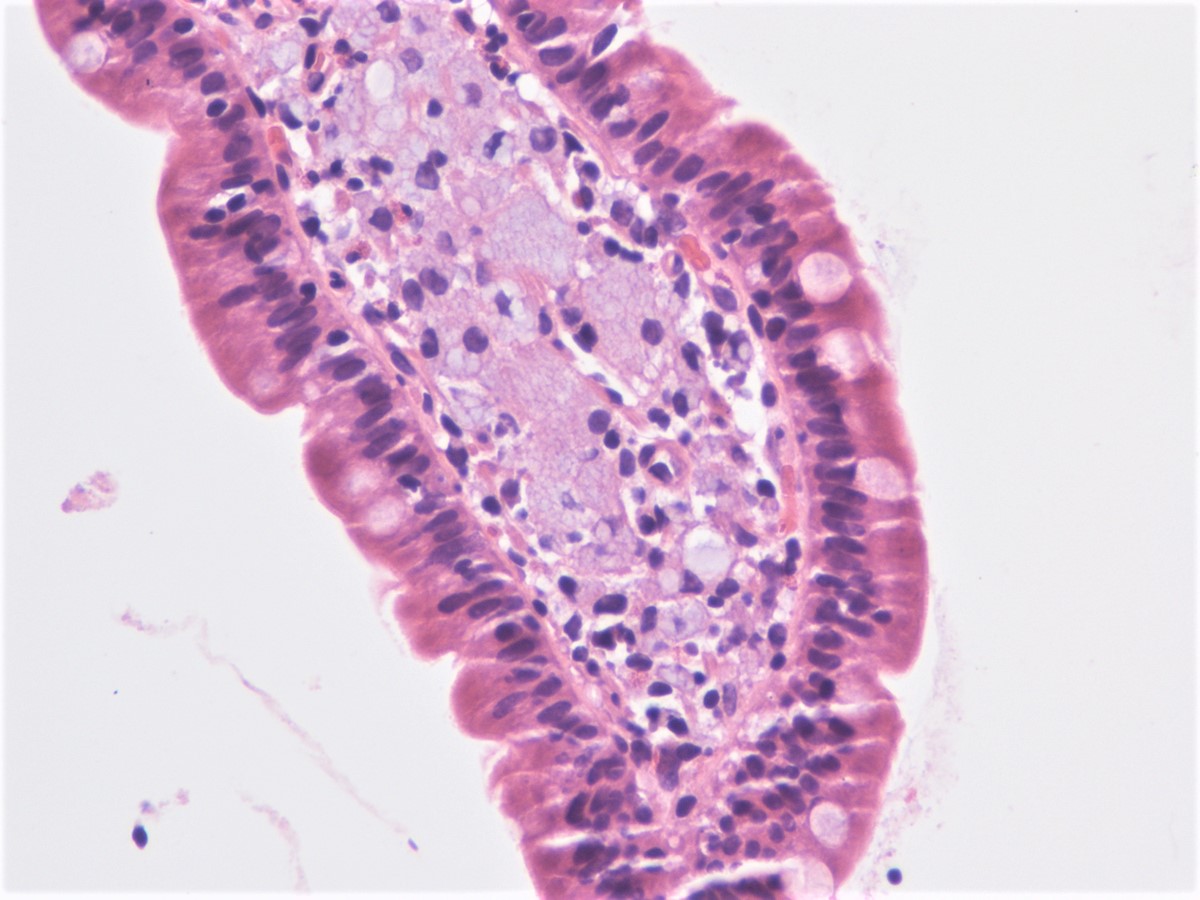
- Intra-ampullary adenocarcinomas
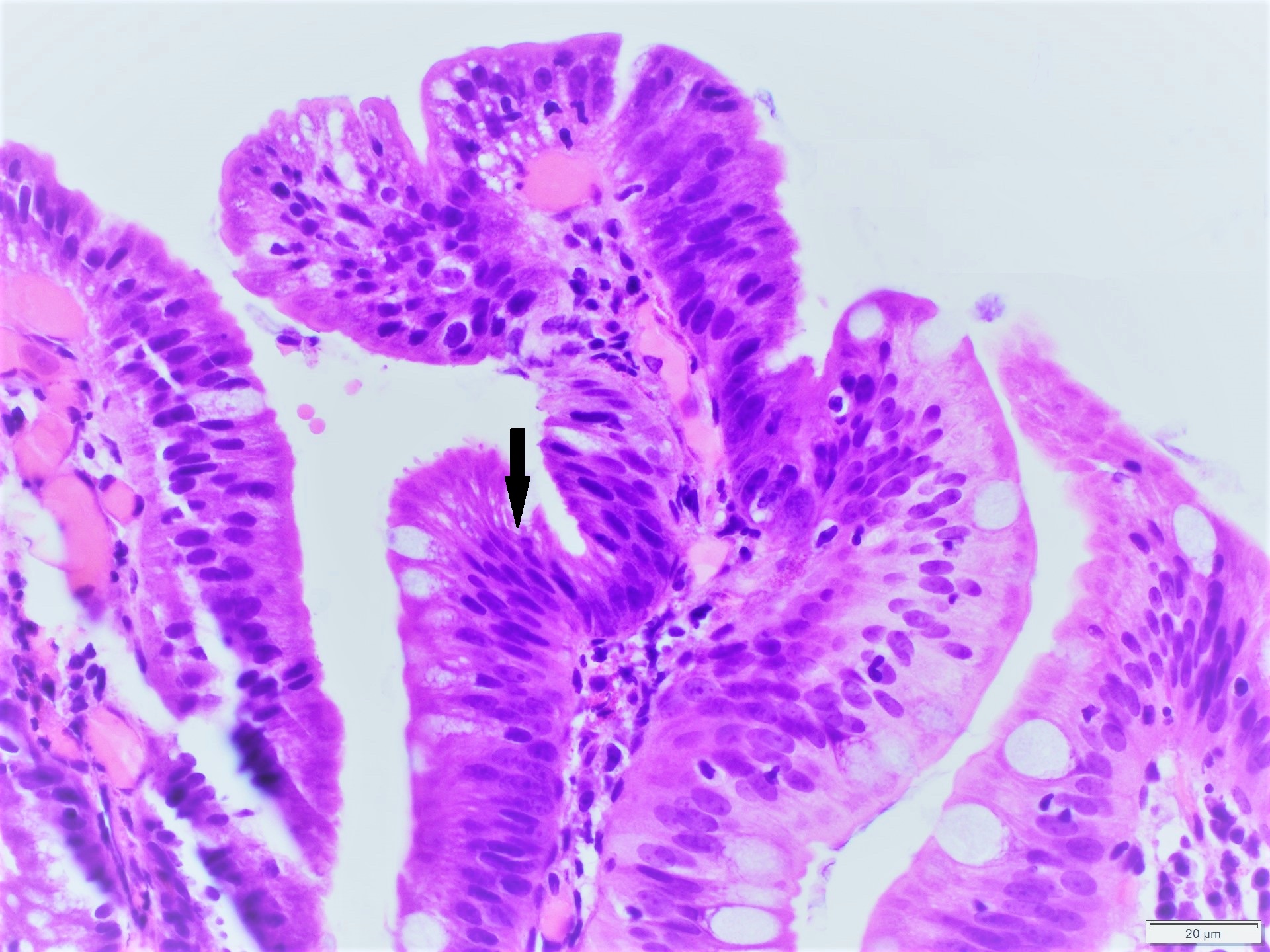
- Majority of the lesion often consists of the precursor intra-ampullary papillary tubular neoplasm
- Majority show an intestinal phenotype
- Growth patterns include papillary, tubular and tubulopapillary
- Noninvasive precursor component may display a different epithelial phenotype than the invasive component
- Periampullary adenocarcinomas
- Majority are intestinal type
- May show mucinous or signet ring cell patterns
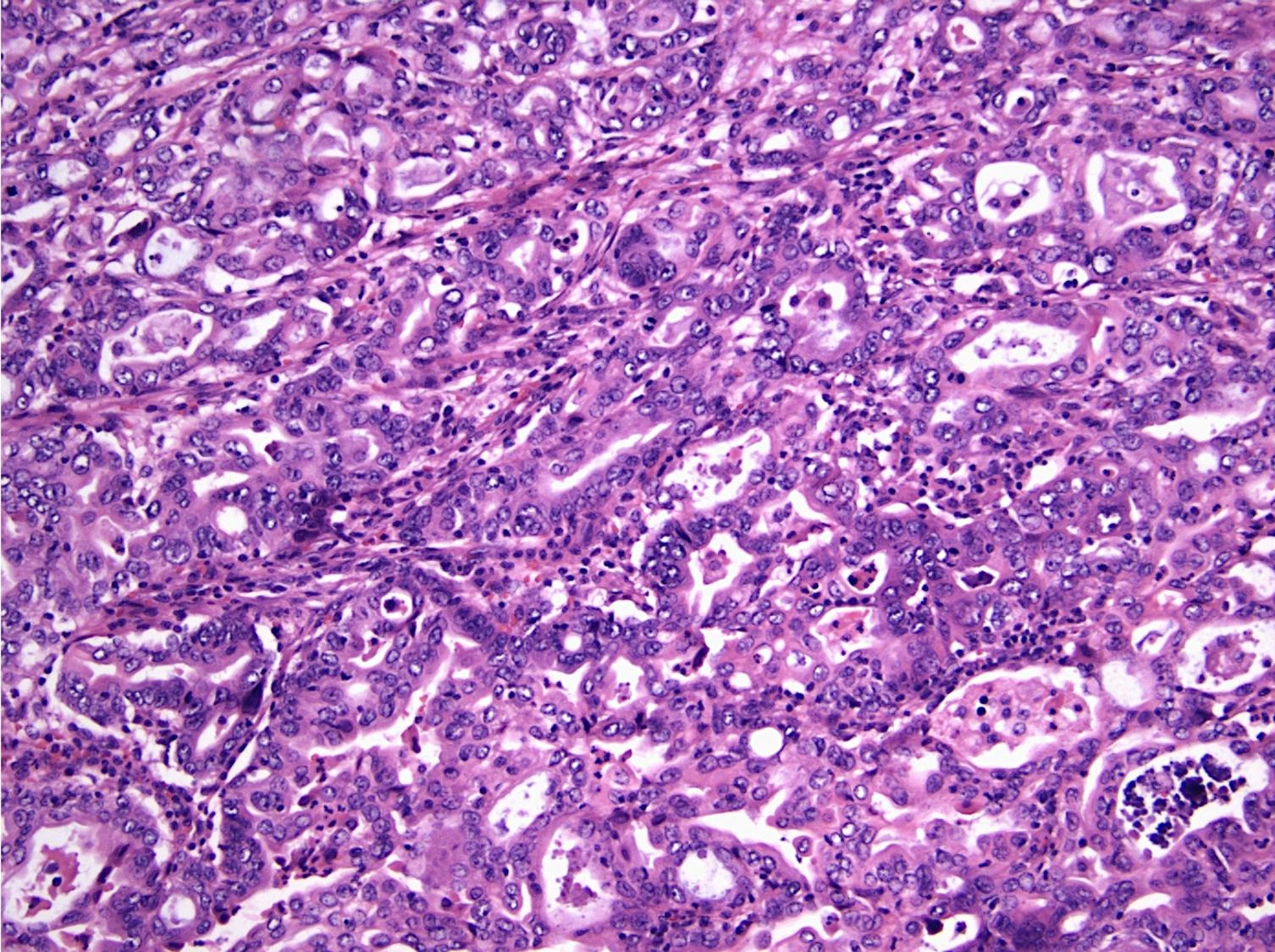
- Ampullary ductal carcinomas
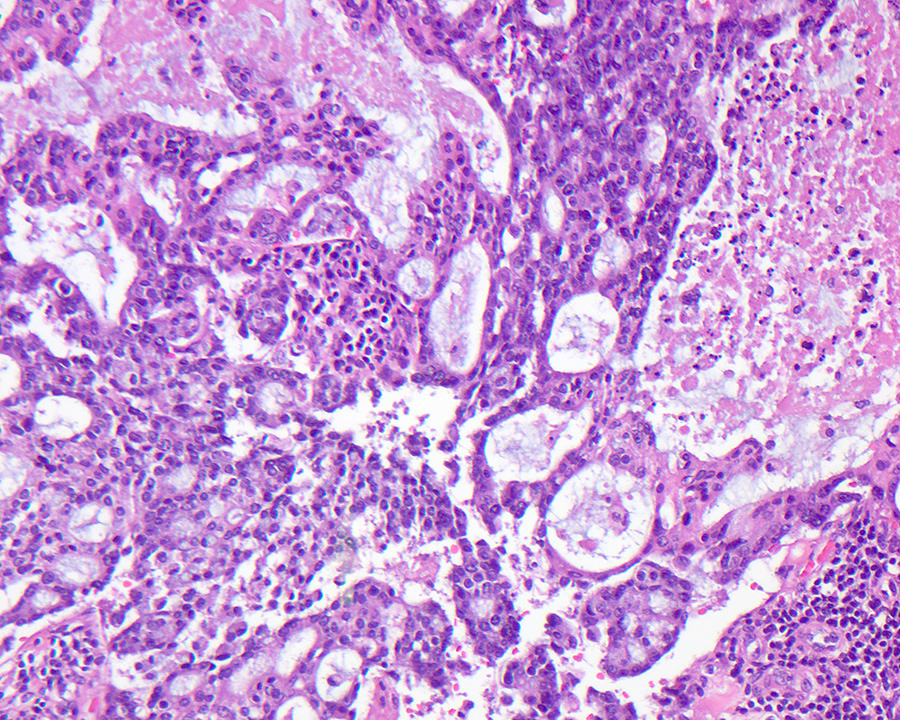
- Pancreatobiliary type
- May show focal micropapillary or sarcomatoid areas
- Ampullary carcinoma, NOS
- Lacks the specific characteristics of the above subtypes
- Heterogenous histologic types: 45% pancreatobiliary type, 27% intestinal type, 28% mixed or other type
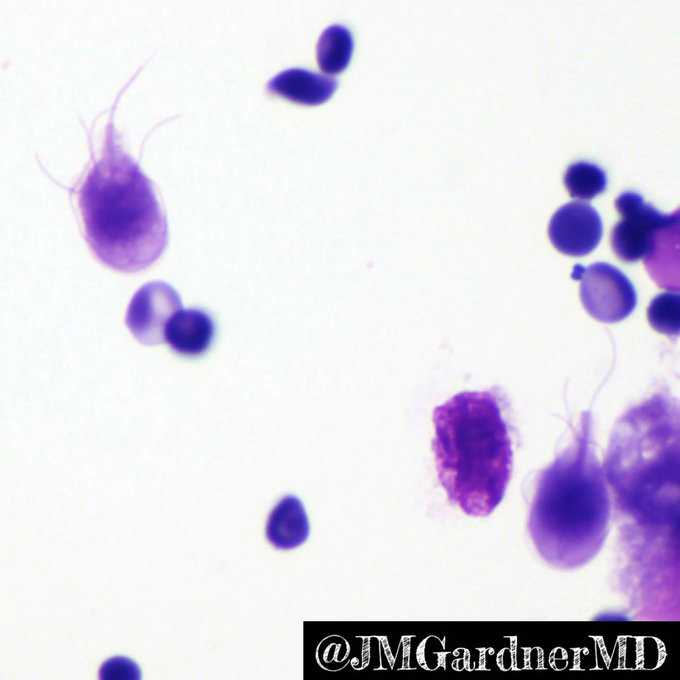
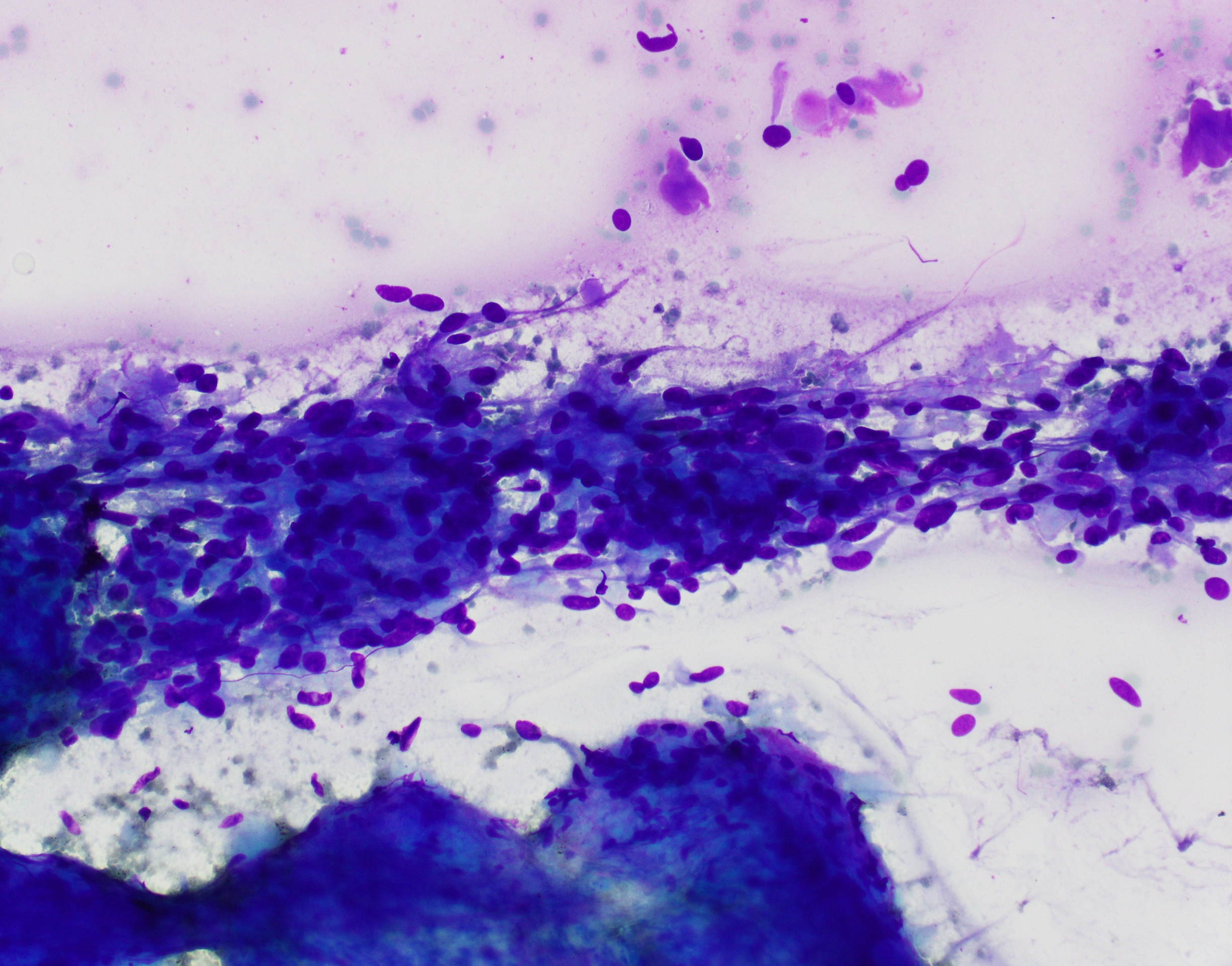
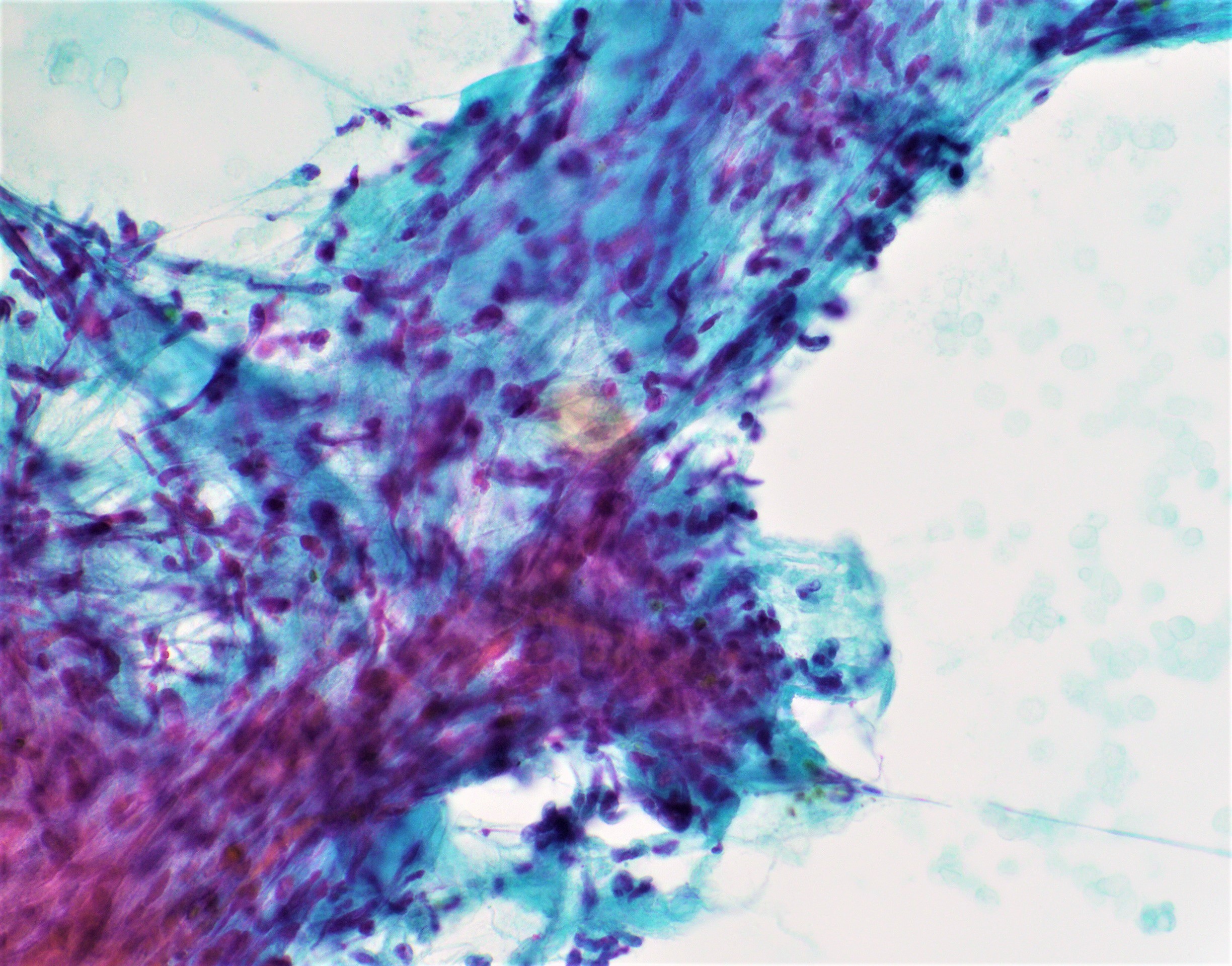
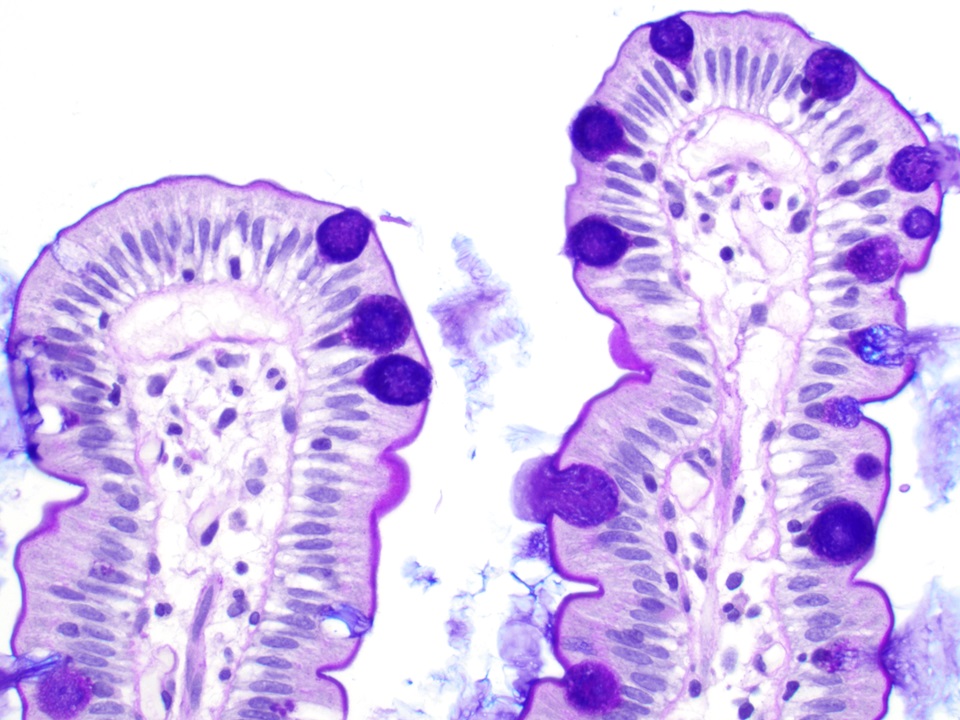
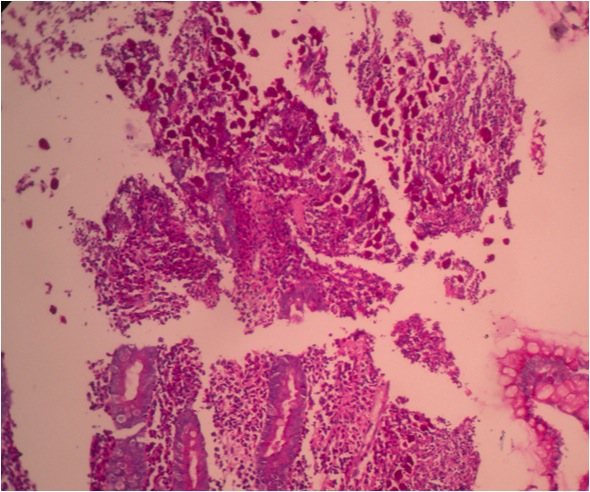
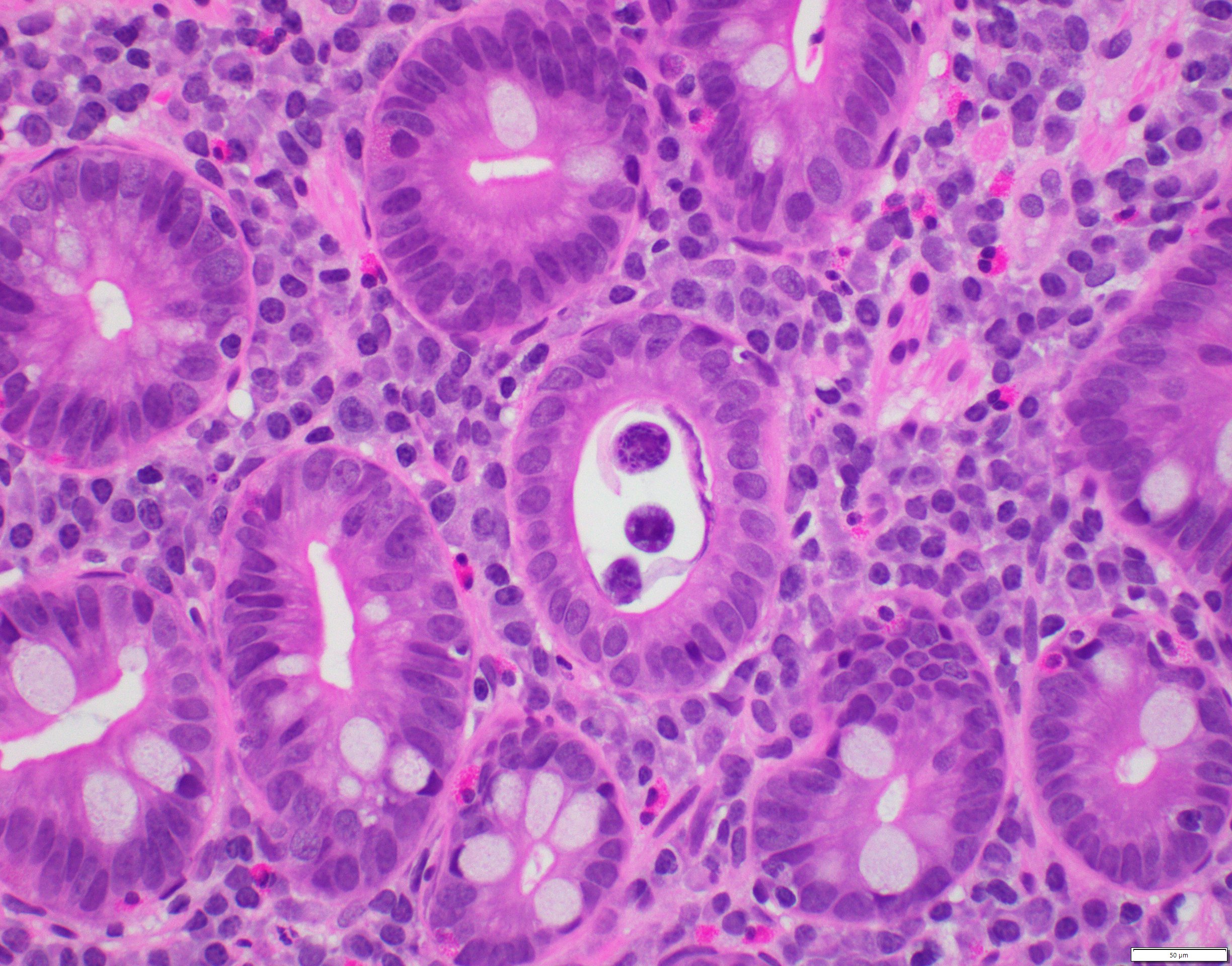
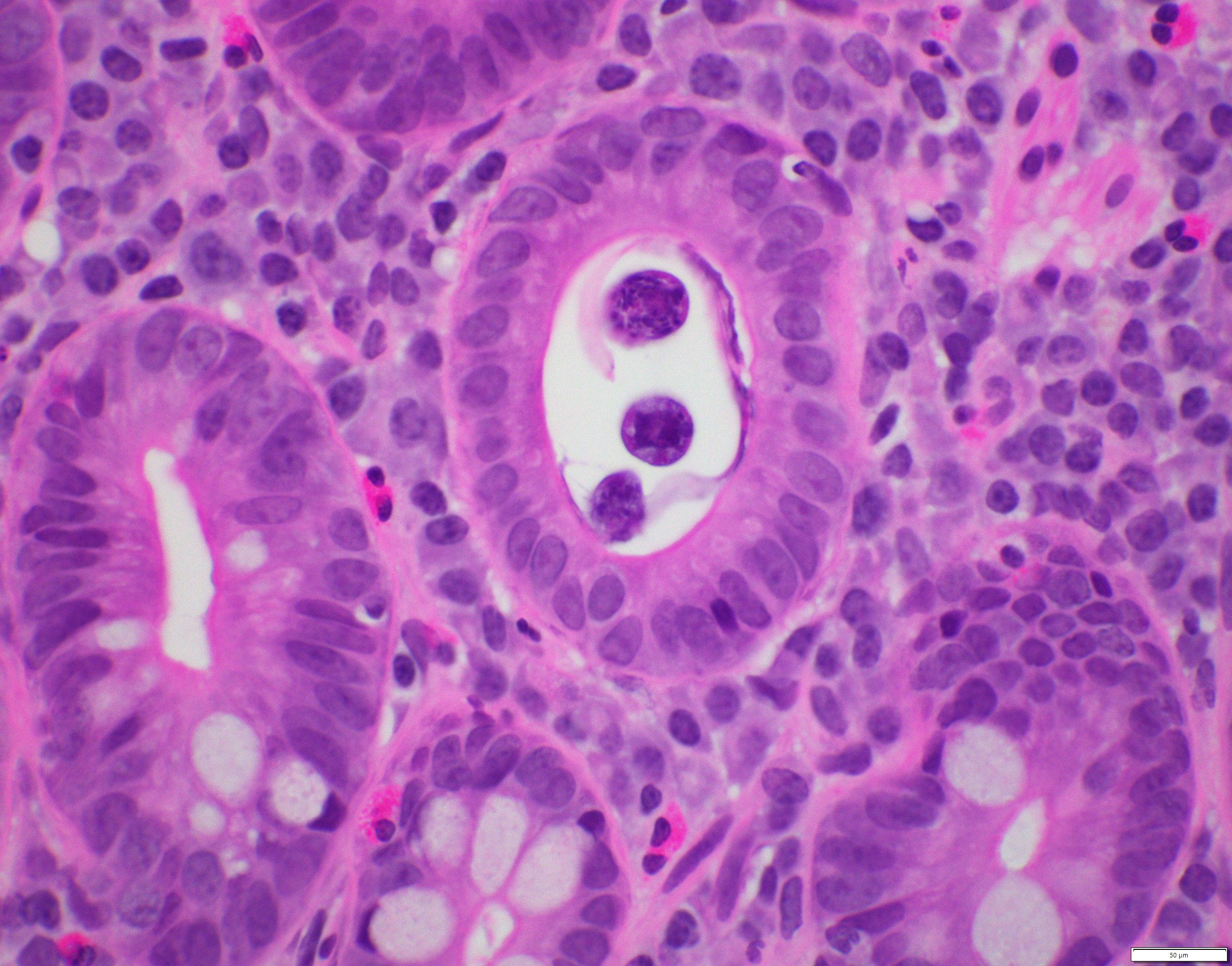
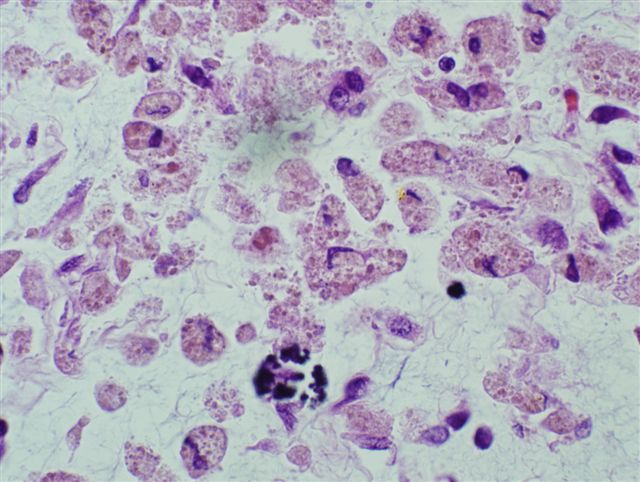
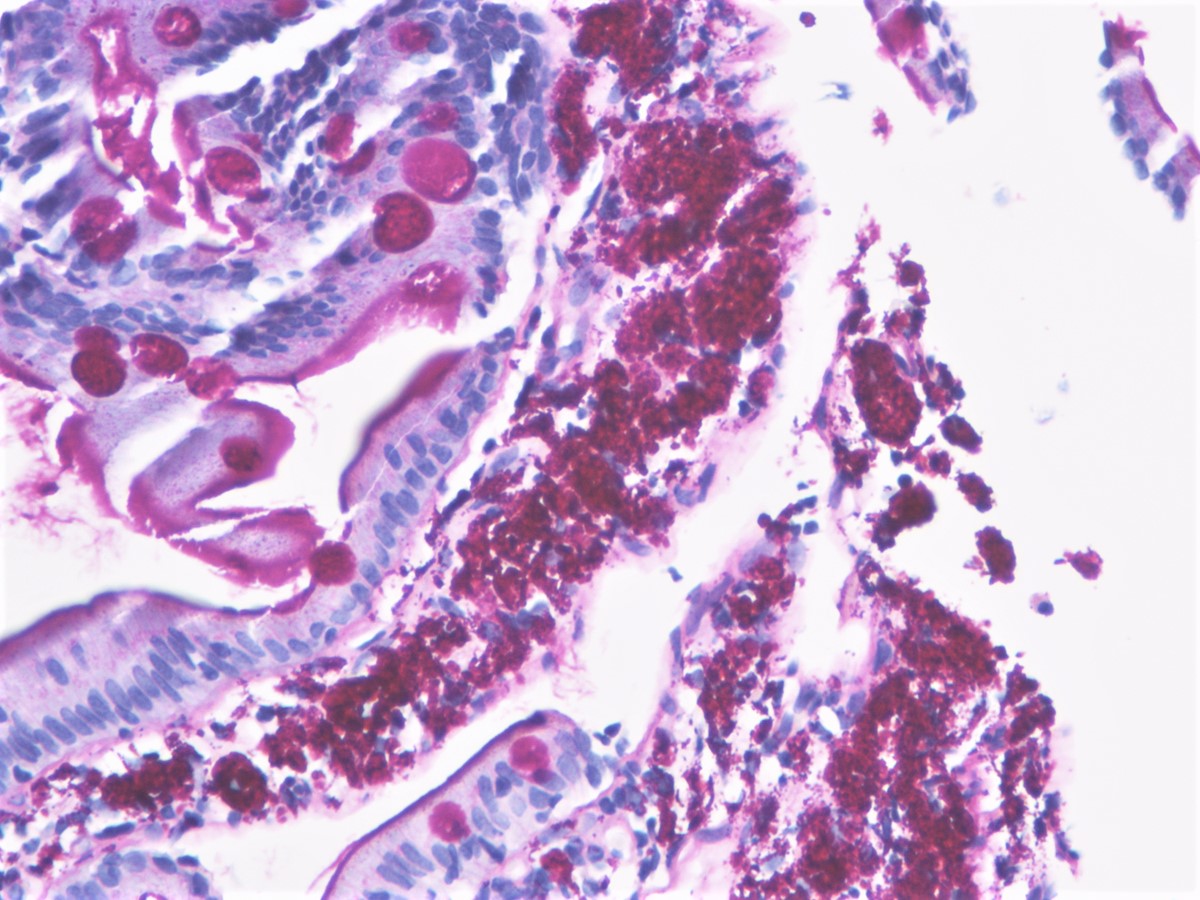
- Intra-ampullary adenocarcinomas
- Cellular to moderately cellular preparations
- Malignant cells when grouped are typically crowded and present in 3 dimensional clusters
- Single pleomorphic cells are often present
- Nuclei are typically enlarged and irregular with an increased N:C ratio, coarse chromatin and prominent nucleoli are often present
- Necrosis can occasionally be seen (J Clin Pathol 2001;54:449, Cancer 2005;105:289)
- In one study, 13/35 ampullary adenocarcinomas were identified via EUS FNA sampling (Cancer 2005;105:289)
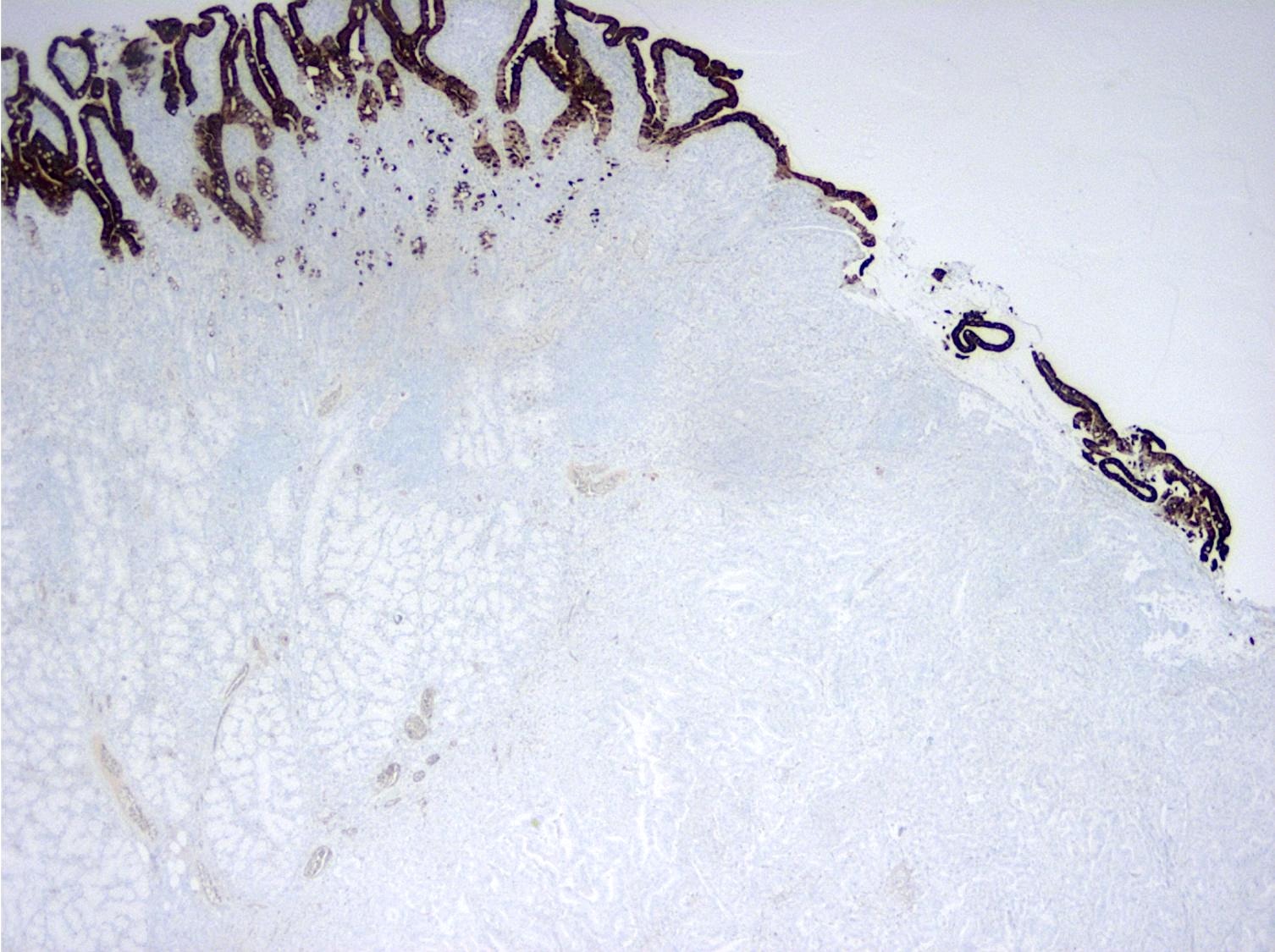
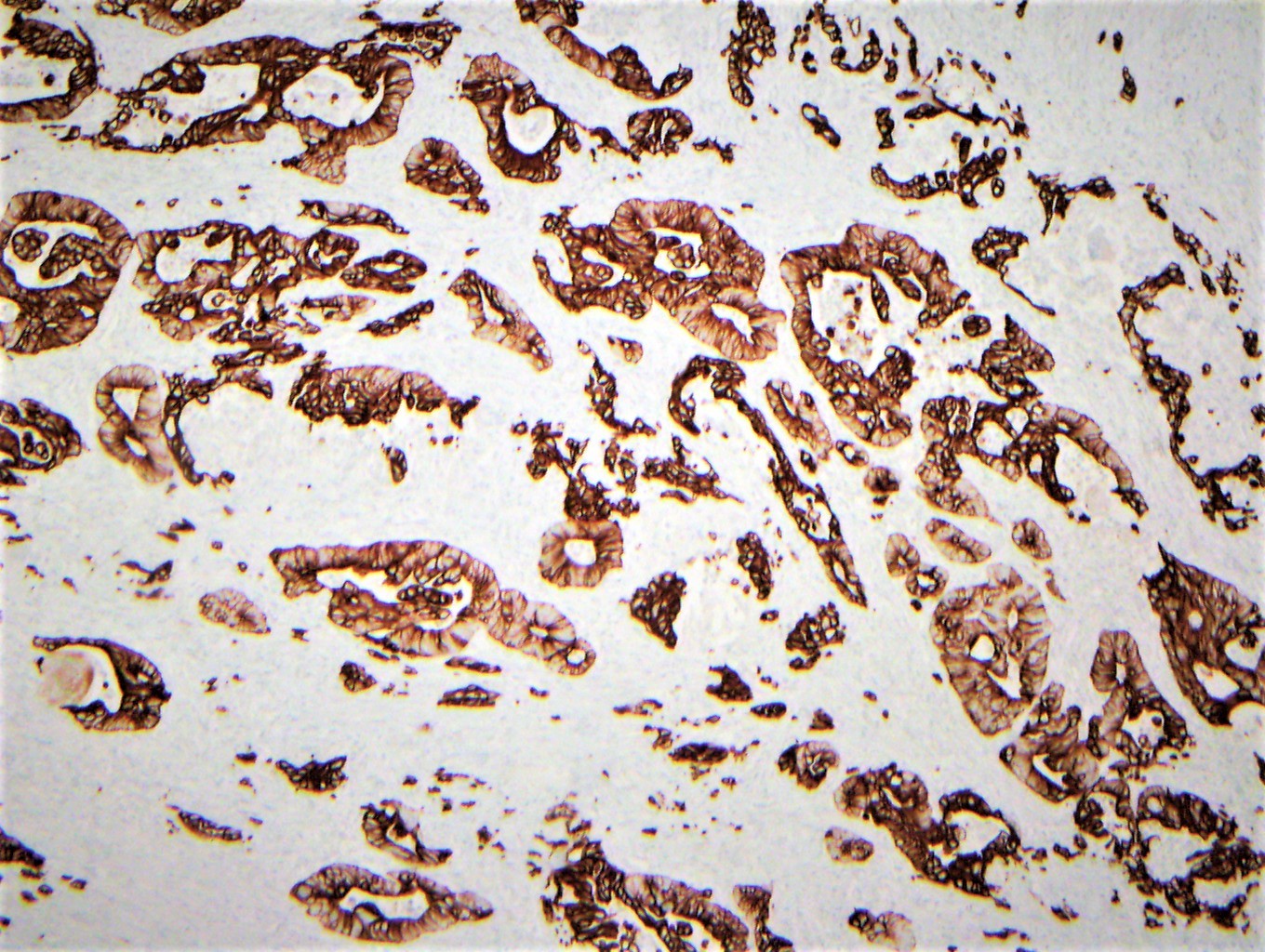
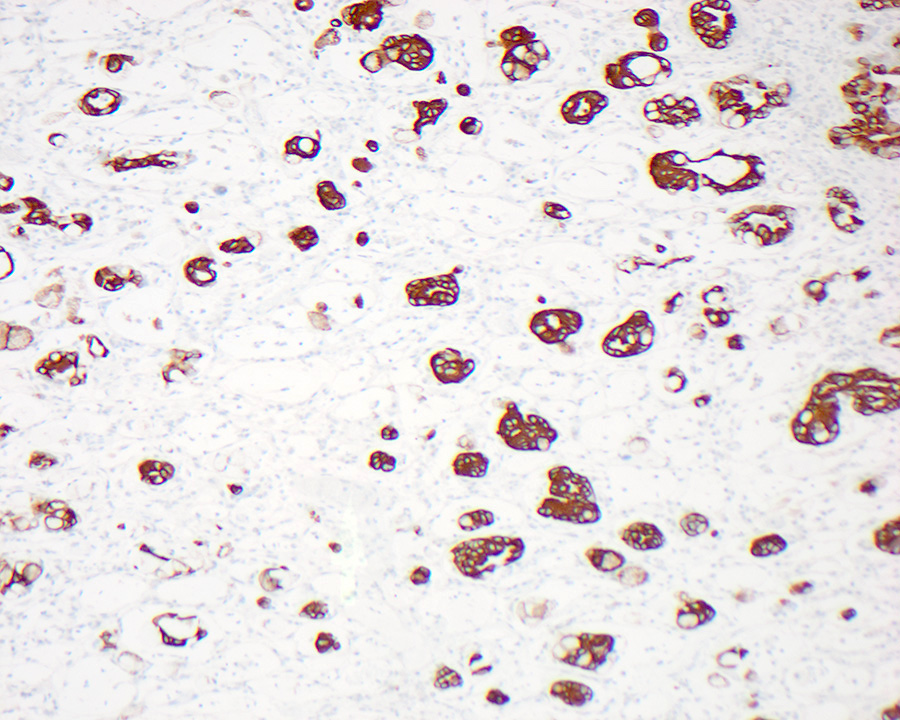
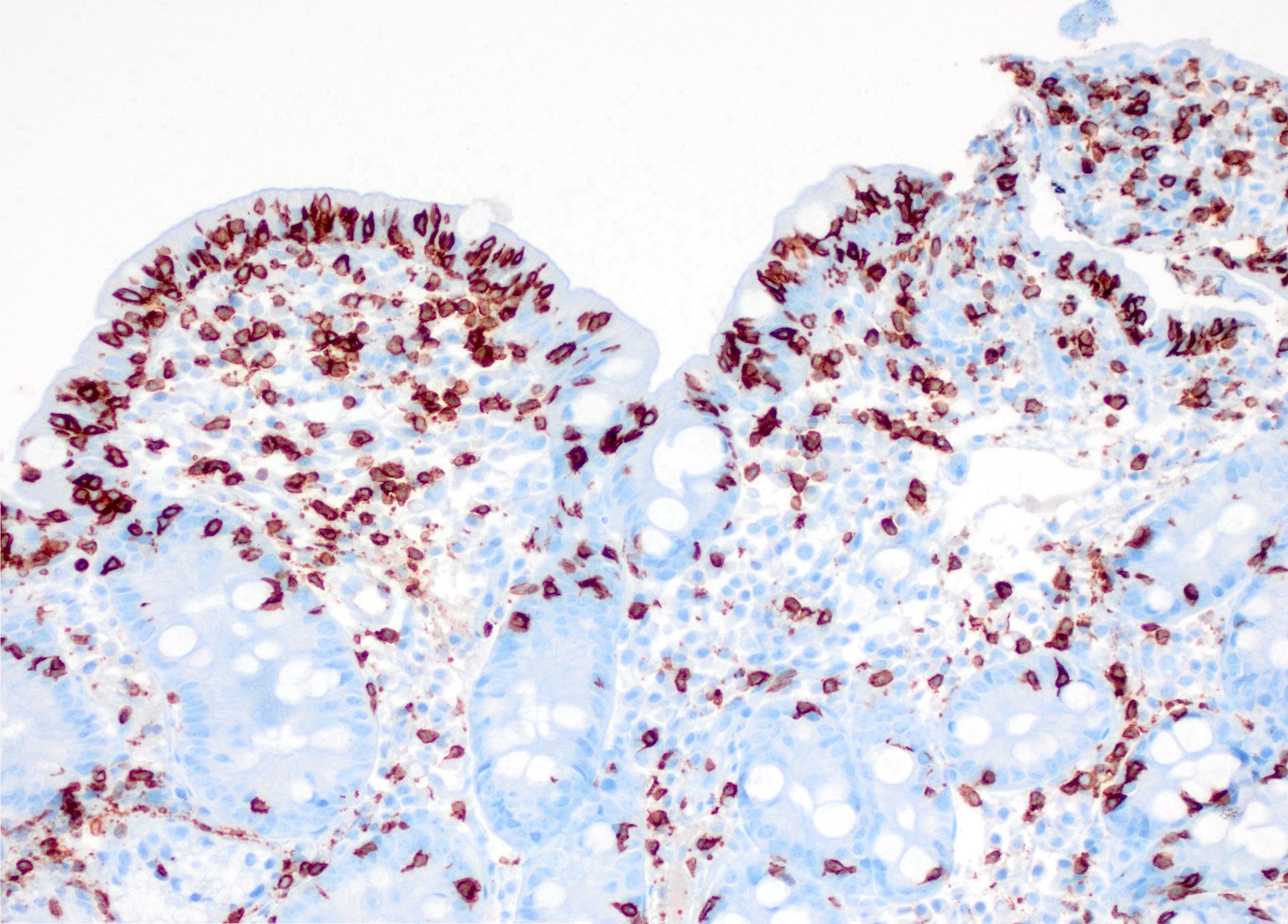
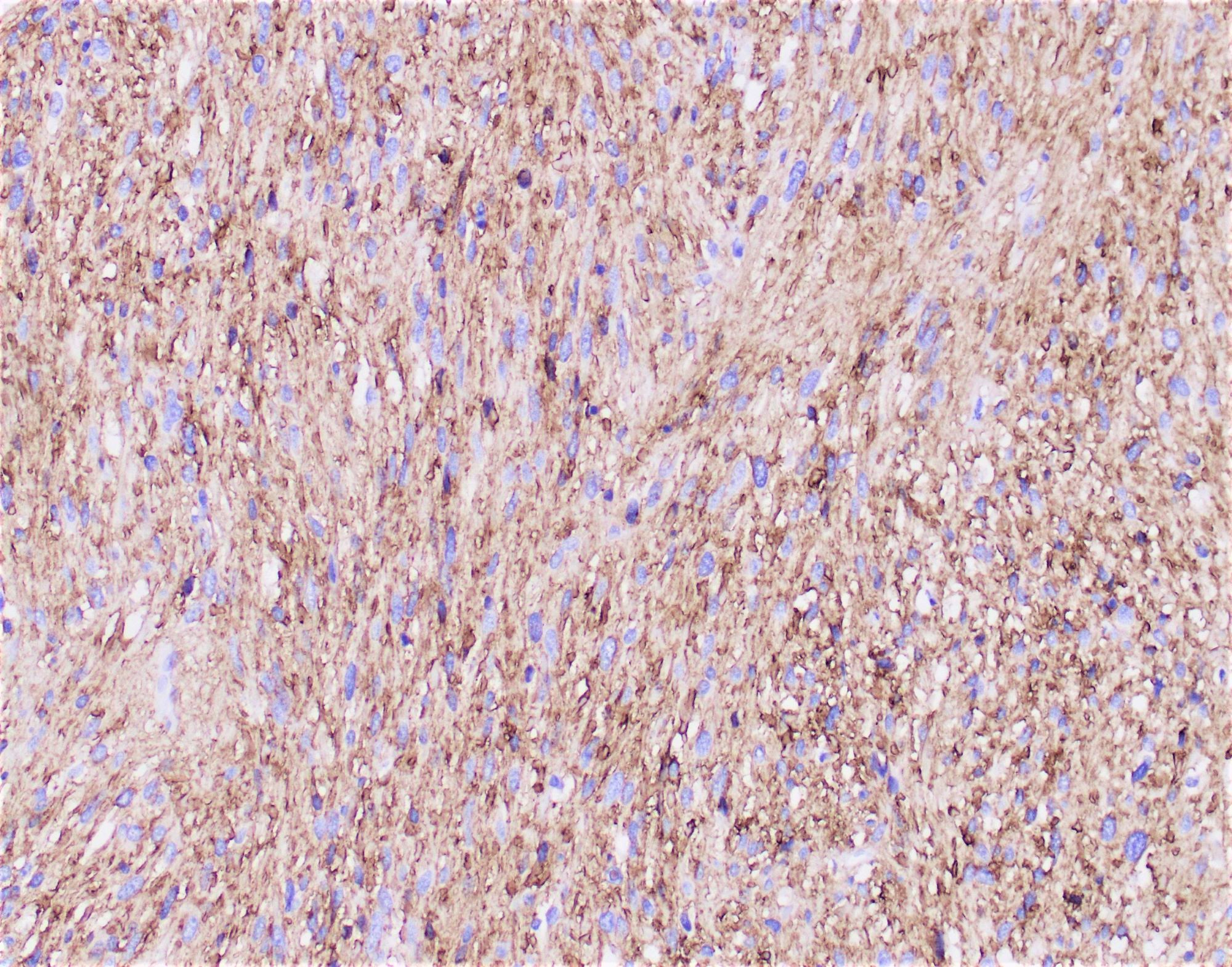
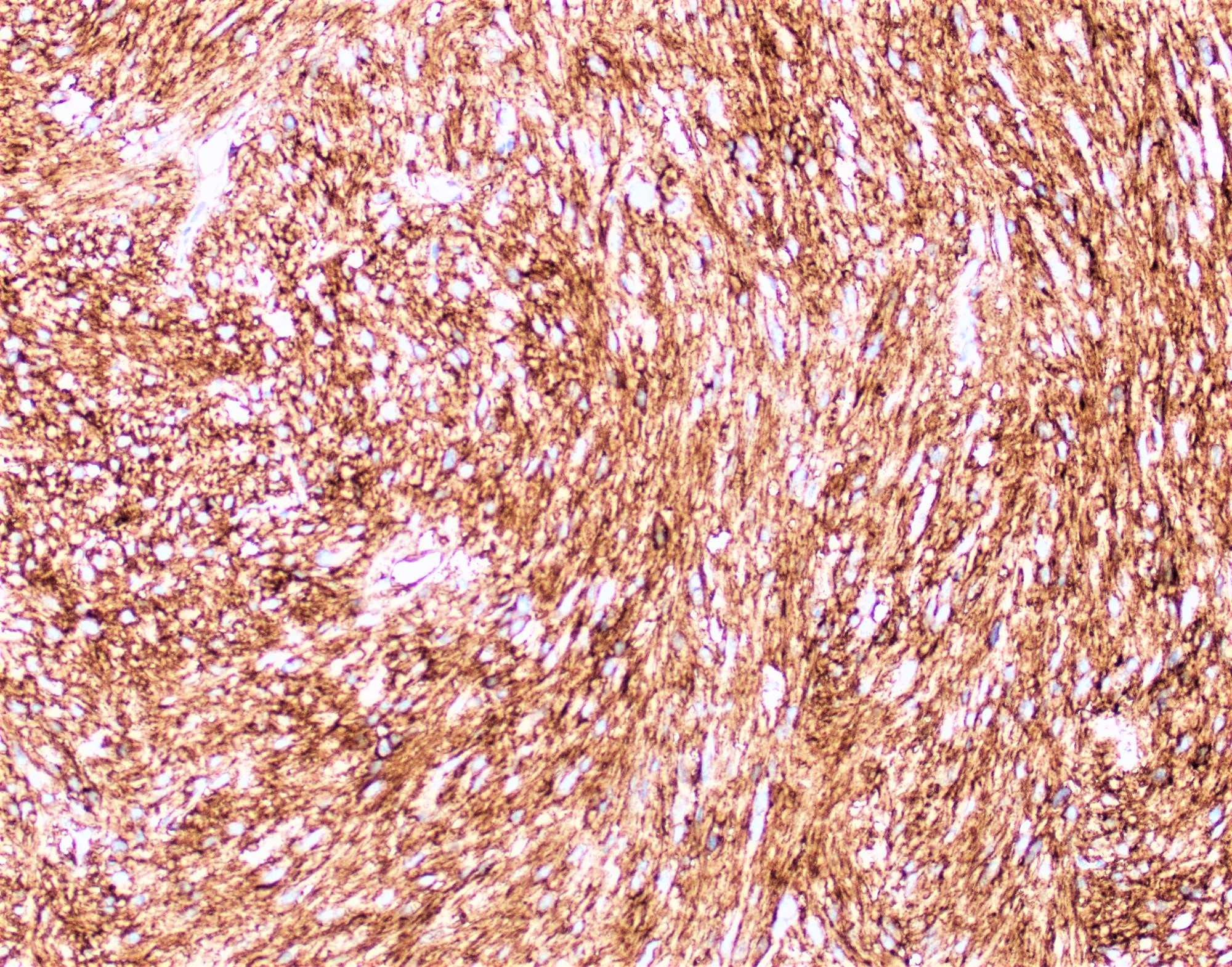
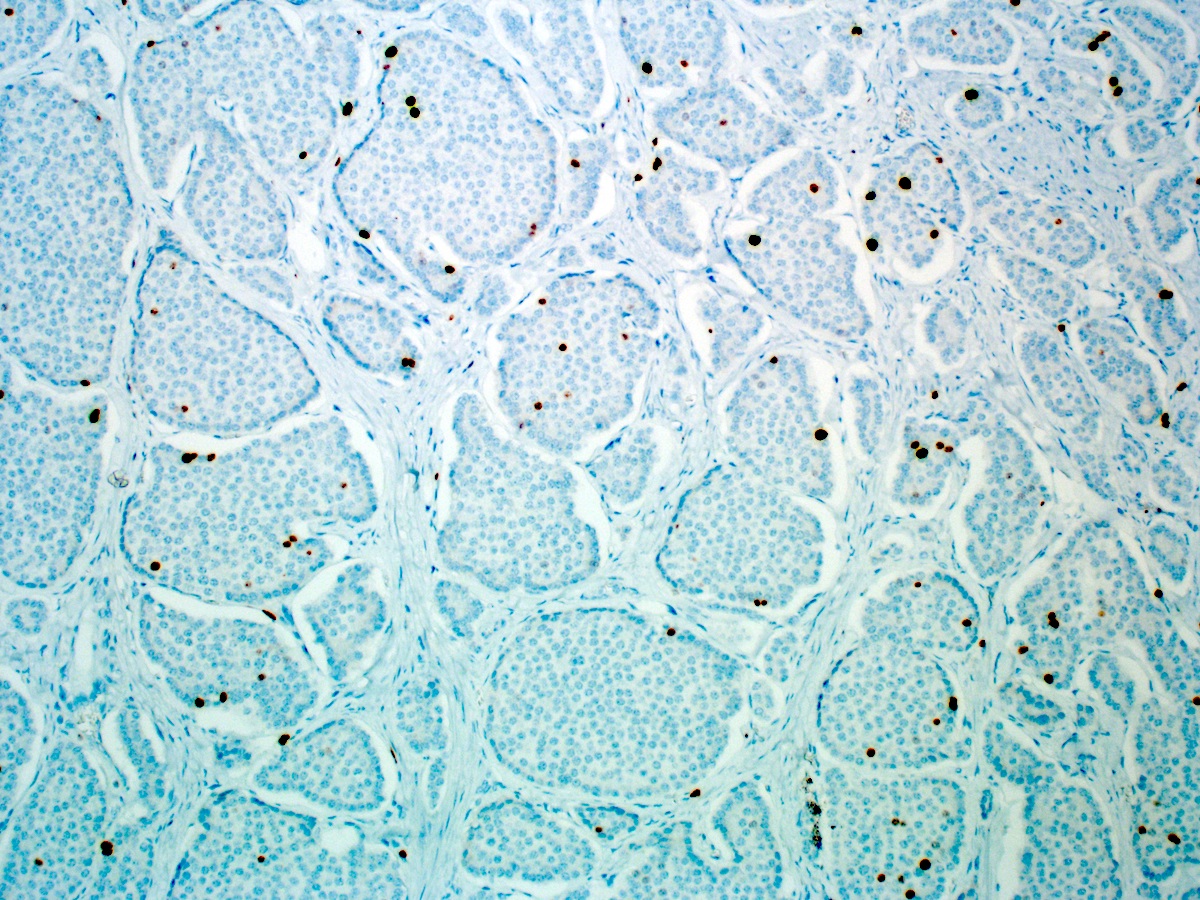
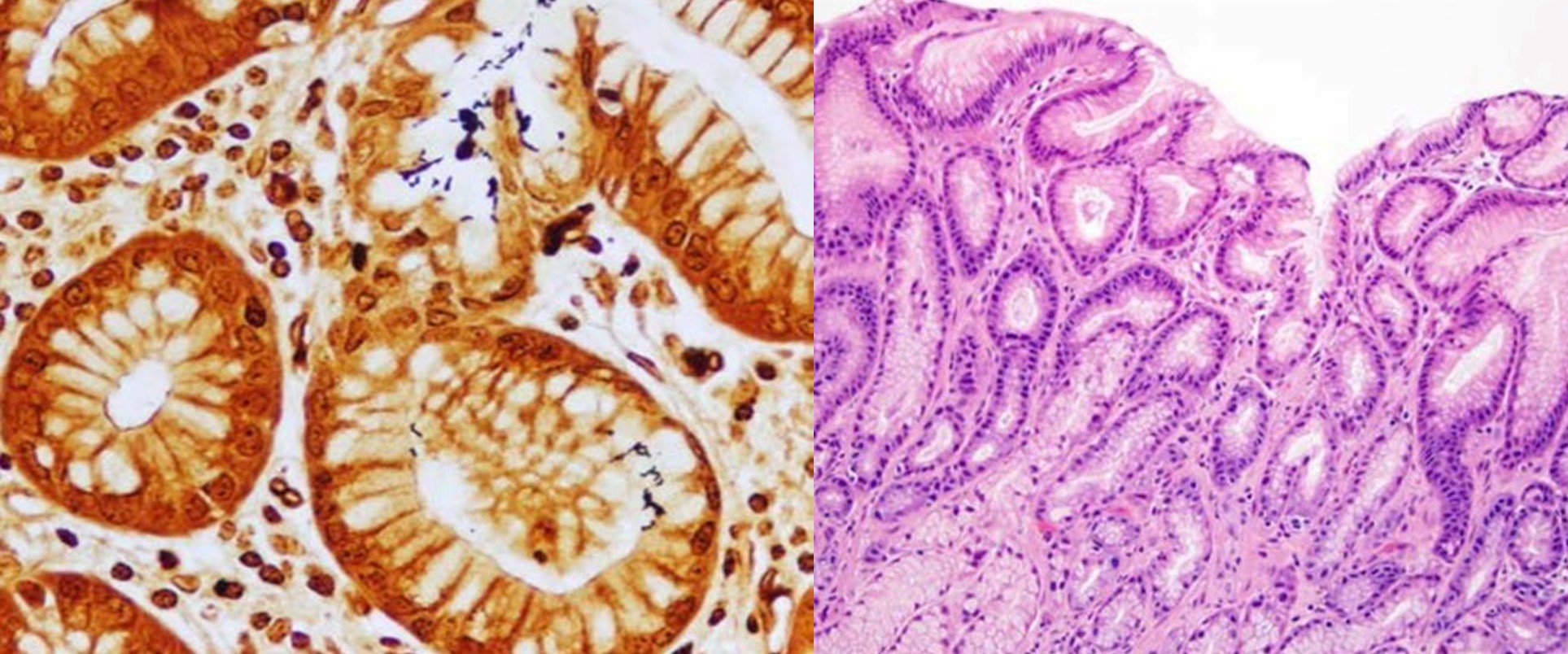
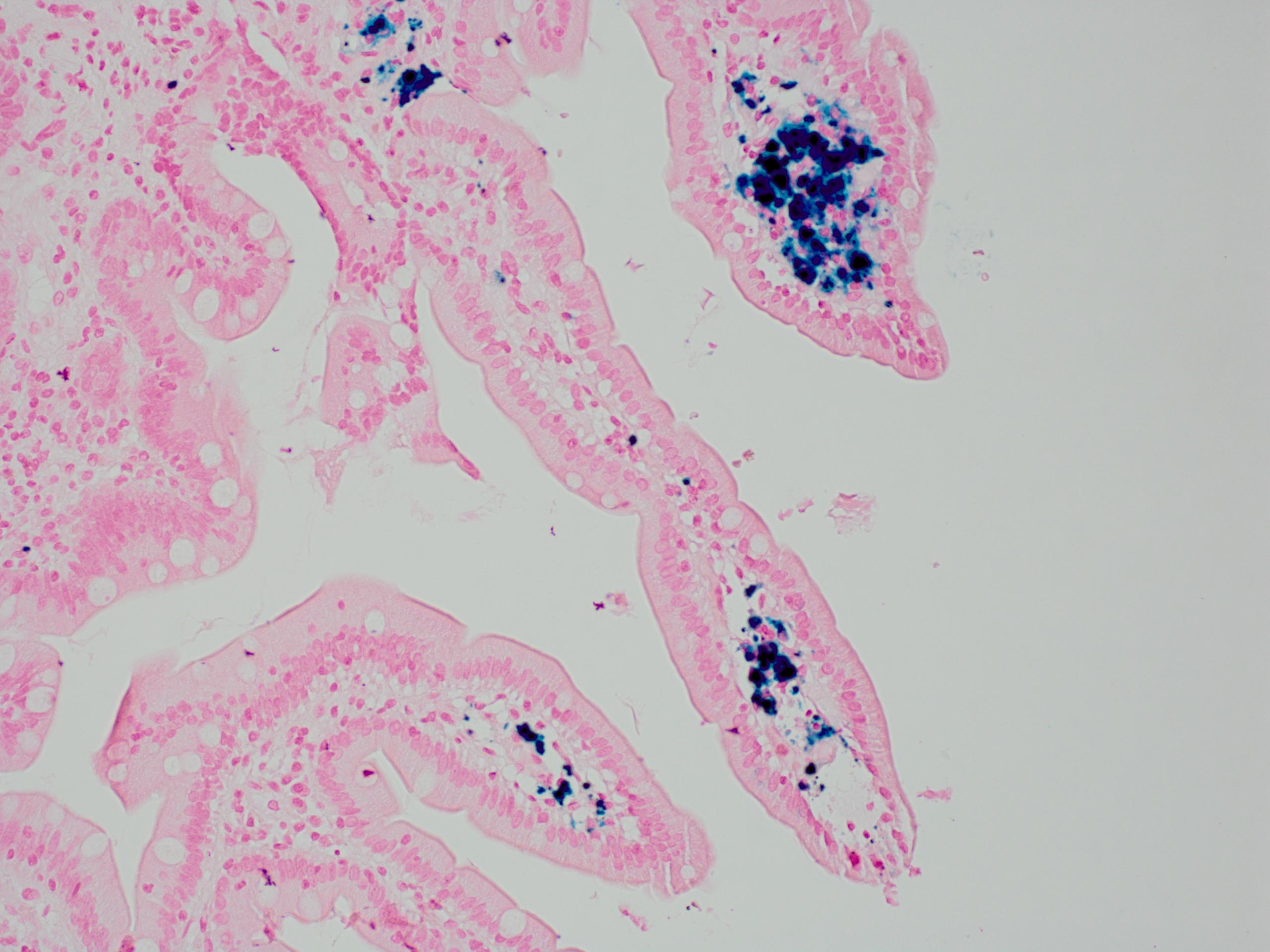
- Intestinal type tumors are typically positive for CK20 (50%), CDX2 (> 25% of tumor staining, 89.5%) and S100P (63%) with variable CK7 (70 - 80%) (Am J Surg Pathol 2014;38:1371, Hum Pathol 2013;44:2213, J Clin Pathol 2019;72:762)
- Pancreatobiliary type tumors are positive for MUC1 (100%), CK7 (91%) (Am J Surg Pathol 2014;38:1371)
- 18 - 20% can be ambiguous (mixed type or a nonglandular tumor subtype) by IHC (Am J Surg Pathol 2014;38:1371, Br J Cancer 2019;120:697)
- Undifferentiated carcinoma with osteoclast-like giant cells: often show mutational p53 staining pattern with giant cells showing immunoreactivity for CD68 (Am J Surg Pathol 1998;22:1247)
- Undifferentiated carcinoma with rhabdoid phenotype: loss of nuclear SMARCB1 / INI1 expression (Am J Surg Pathol 2016;40:544)
- Intestinal type tumors are typically positive for MUC2 (39.5%), MUC5AC (41%) (Am J Surg Pathol 2014;38:1371, Hum Pathol 2013;44:2213, J Clin Pathol 2019;72:762)
- Intestinal type tumors are typically negative for MUC1 (EMA) (Am J Surg Pathol 2014;38:1371)
- Pancreatobiliary type tumors are typically negative for CDX2 (< 25% of tumor staining), MUC2 and CK20 (Am J Surg Pathol 2014;38:1371, Hum Pathol 2013;44:2213)
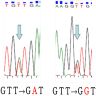
- KRAS mutations are present in 30 - 40%, may be associated with worse disease free survival and are more frequently found in pancreatobiliary type tumors (Oncotarget 2016;7:58001, Br J Cancer 2019;120:697)
- TP53 is also a negative predictor of survival, regardless of phenotype (Ann Surg 2018;267:149)
- Microsatellite instability is present in 10 - 15%, usually in intestinal type tumors (Am J Surg Pathol 2009;33:691)
- Other mutations detected include: APC, PIK3CA, SMAD4, BRAF, CDKN2A, FBXW7, TP53, APC, ELF3 and RAS mutations (Br J Cancer 2019;120:697, J Pathol Transl Med 2021;55:192, Cancer Cell 2016;29:229)
- Targetable mutations have been identified in both intestinal and pancreatobiliary phenotype tumors (J Pathol Transl Med 2021;55:192, Ann Surg 2018;267:149)
- General: ERBB, WNT and PI3K
- Intestinal phenotype: PI3 / AKT
- Pancreatobiliary phenotype: RAS / RAF and PI3 / AKT
- BRAF and KRAS are mutually exclusive (Br J Cancer 2019;120:697)
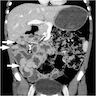
- Pancreas, small bowel and distal common bile duct, pancreatoduodenectomy:
- Ampullary adenocarcinoma, pancreatobiliary type, poorly differentiated, invasive into the pancreatic head (pT3a) (see synoptic report)
- 3 of 15 lymph nodes positive for carcinoma and 1 tumor deposit (3/15, pN1)
- Perineural as well as extensive lymphovascular and large vessel invasion is present
- Resection margins are free of high grade dysplasia and carcinoma (closest approximation: 0.3 cm to retroperitoneal margin)
- Use ampulla pTMN for staging
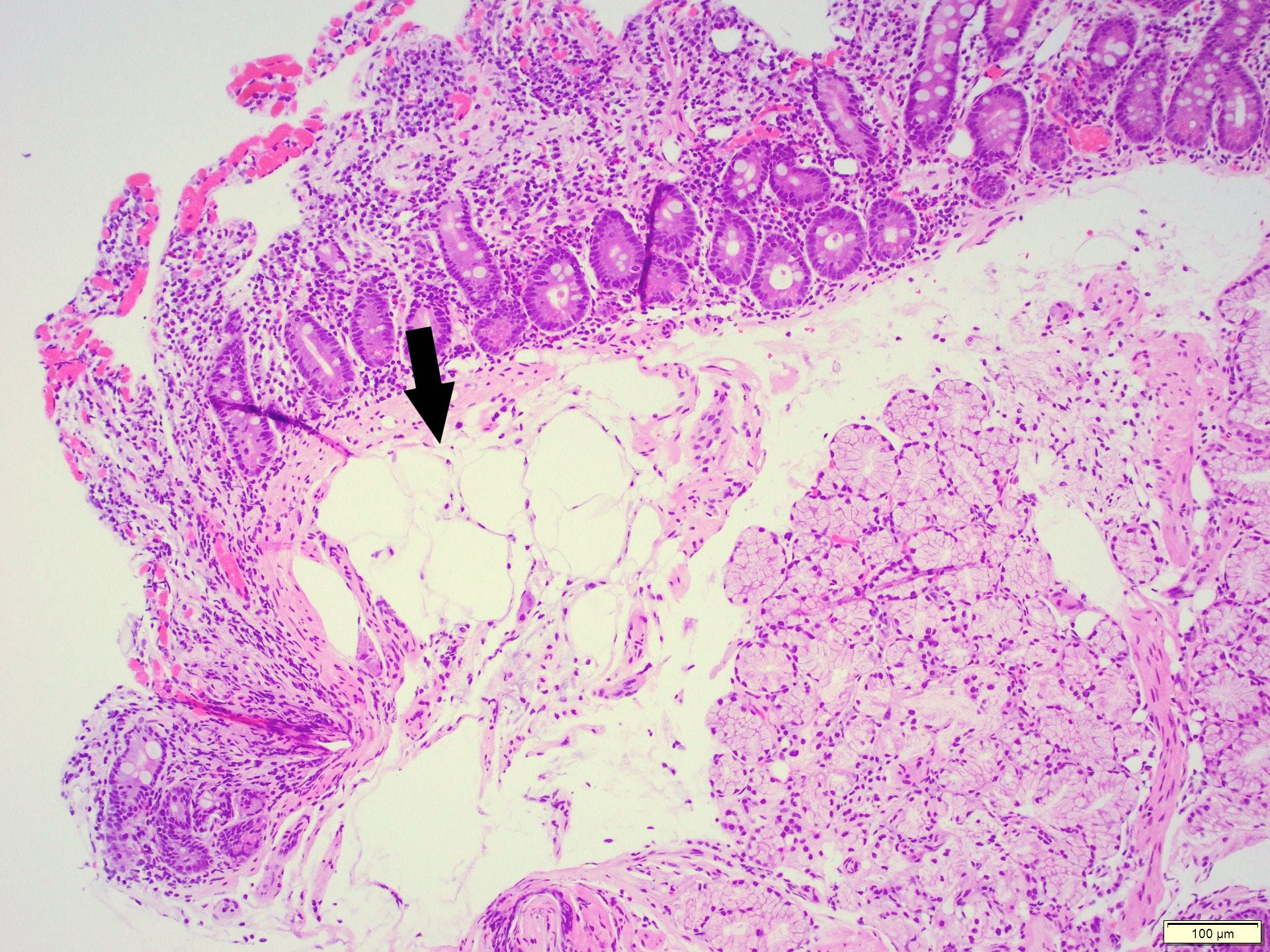
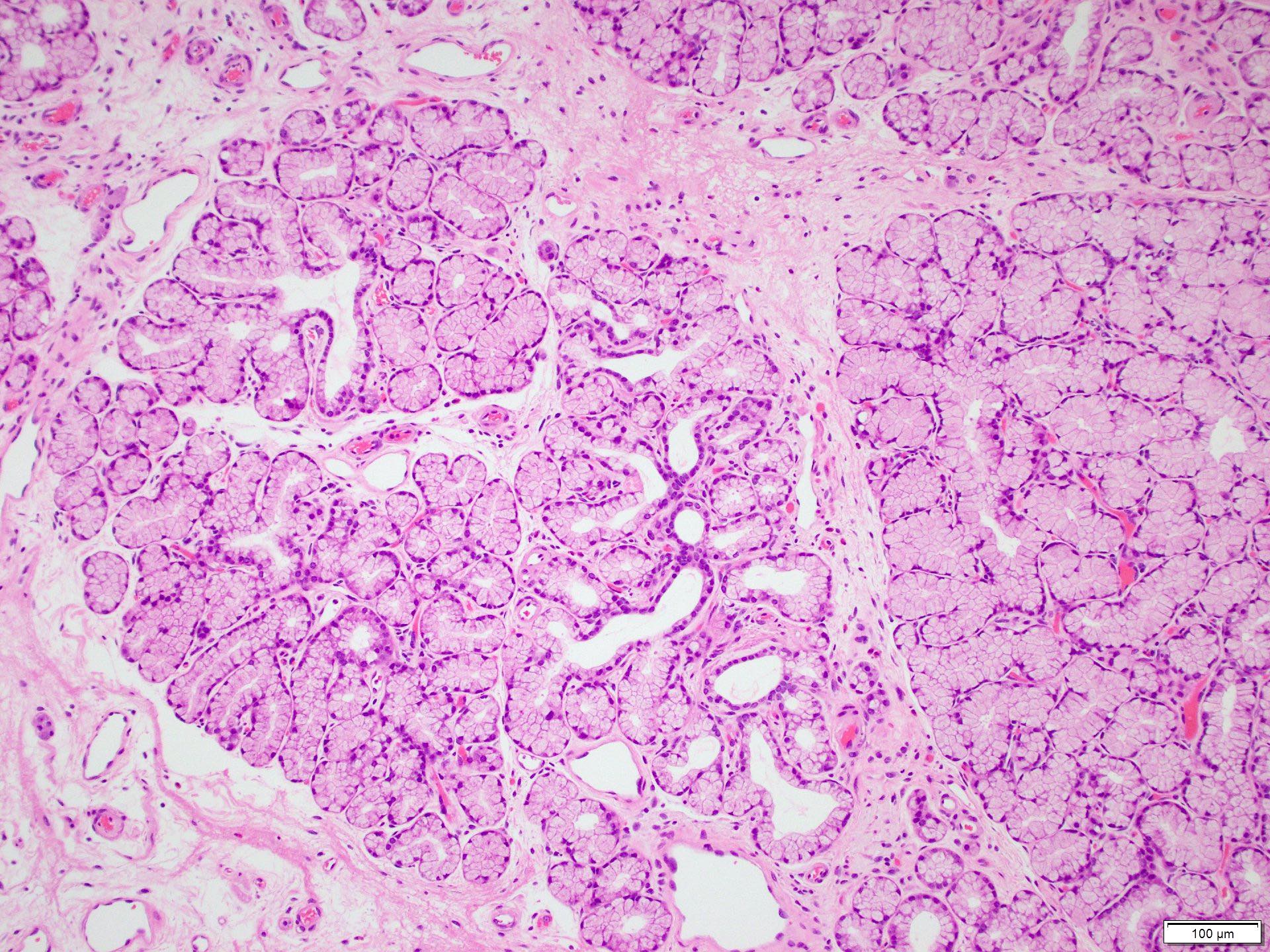
- Intra-ampullary papillary tubular neoplasm:
- No invasion present
- Adenomatous changes in submucosal glands / ductules simulating invasion:
- No desmoplastic stroma or single cell invasion present to indicate a truly invasive process
- Dysplastic ducts will have round, regular contours and be present in a lobular configuration in most cases
- Extrahepatic cholangiocarcinoma:
- Tumor may show concentric thickening of the distal common bile duct but tumor will not be centered in the ampulla
- Duodenal adenocarcinoma:
- Tumor may also arise in intestinal type adenomatous mucosa near the ampullary orifice and extend to involve the ampulla but the tumor will not be centered around the orifice
Comment Here
Reference: Adenocarcinoma - ampulla
- Nonampullary primary malignant epithelial neoplasm of the small intestine showing glandular differentiation
- Small bowel adenocarcinomas are histologically very similar to colorectal adenocarcinomas, with complex glandular formations
- Gross identification of the tumor epicenter is essential in duodenal tumors that involve the ampulla to exclude a primary ampullary adenocarcinoma or extension from pancreatic or bile duct malignancies
- Adenocarcinomas can arise in a variety of inflammatory, autoimmune and familial conditions
- 30 - 40% of small intestinal cancers are adenocarcinoma and 3.3% of gastrointestinal cancers were from the small intestines in 2020 (CA Cancer J Clin 2020;70:7)
- More frequently seen in men, African Americans and patients in their early to mid 60s (Cancer Causes Control 2005;16:781)
- Most common locations include the periampullary region of the duodenum (50 - 64%) followed by the jejunum (18 - 20%) and finally the ileum (15%) (Ann Surg 2009;249:63, Cancer 1999;86:2693)
- Patients with Crohn's disease have adenocarcinomas in a predominantly ileal location
- Inflammatory, autoimmune, genetic and familial diseases have been recognized as common risk factors:
- Crohn's disease: the cumulative risk increases after 10 years of disease and there is an absolute risk of 2.2% at 25 years
- Risk increases with longstanding ileal inflammation, younger than average age of onset, previous immunosuppressant use, male sex and stricture and fistula formation (Dis Colon Rectum 2007;50:839, Am J Gastroenterol 2005;100:2724, Am Surg 2007;73:1181, J Crohns Colitis 2014;8:19)
- Celiac disease: there is an increased relative risk of 10 to 80 times compared with the general population in longstanding, untreated disease (QJM 2003;96:345)
- Most adenocarcinomas occur in the jejunum
- Familial adenomatous polyposis (FAP): there is a 3 - 5% lifetime risk
- Most tumors occur in the duodenum and periampullary regions
- Risk increases with a higher number of polyps, larger polyps and those with poorer histologic features (Lancet 1989;2:783)
- Peutz-Jeghers syndrome: there is a 1.7 - 13% lifetime risk (Gastroenterology 2000;119:1447, Clin Cancer Res 2006;12:3209)
- Adenocarcinomas most commonly occur in the jejunum and ileum and can arise from hamartomatous or adenomatous polyps
- Lynch syndrome: there is a 4% lifetime risk (Cancer 1998;83:240)
- Adenocarcinomas most commonly occur in the duodenum and jejunum
- Crohn's disease: the cumulative risk increases after 10 years of disease and there is an absolute risk of 2.2% at 25 years
- Other associations and risk factors include:
- Ileal conduits / reservoirs which expose the small intestine to its nonnative milieu (Oncol Rep 2012;27:371)
- Exposure to acid and bile, especially in the vicinity of ampulla and pylorus (Int J Cancer 1997;70:512)
- Smoking, alcohol and dietary factors (low fiber, high red meat consumption, sugary drinks) (Int J Cancer 1997;70:512, Cancer Epidemiol 2015;39:265)
- Congenital bowel duplication, ileostomy, duodenal or jejunal bypass surgery (Radiographics 1998;18:379)
- Patients are typically asymptomatic or exhibit nonspecific symptoms in the early stages of disease
- Some symptoms include abdominal pain, anemia, GI bleeding, weight loss, nausea and vomiting
- Intestinal obstruction may develop in ileal and jejunal cancers as the disease progresses, necessitating surgical intervention (Cancer 2004;101:518)
- Initial workup for primarily duodenal adenocarcinomas includes esophagogastroduodenoscopy (EGD) with endoscopic ultrasound (EUS) and biopsy for diagnosis and staging (J Natl Compr Canc Netw 2019;17:1109)
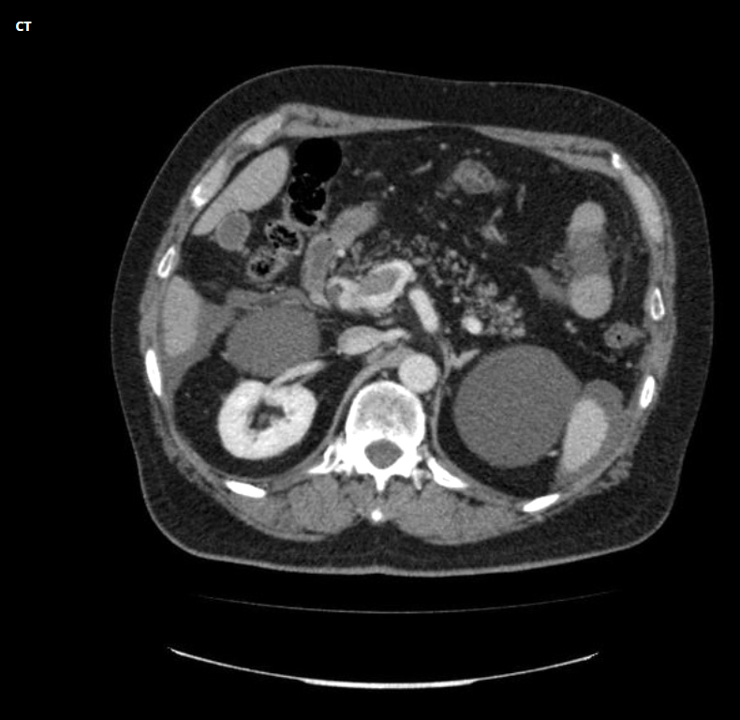
- CT or MRI can be used to evaluate local tumor invasion and assess for metastatic disease
- Balloon assisted and video capsule endoscopy allow for a detailed examination of entire small bowel if nonobstructed (J Natl Compr Canc Netw 2019;17:1109)
- Complete blood count (CBC), chemistry profile, carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA) (J Natl Compr Canc Netw 2019;17:1109)
- Distal adenocarcinomas may manifest as annular narrowing with abrupt concentric or irregular overhanging edges, a discrete tumor mass or an ulcerative lesion, while duodenal adenocarcinomas tend to be papillary or polypoid on CT scan (Radiographics 1998;18:379)
- Prognosis is related to the stage of disease at diagnosis, resectability, margin status, differentiation and lymph node status (Langenbecks Arch Surg 2019;404:439, Dis Colon Rectum 2002;45:1496, Am J Surg Pathol 2014;38:1484)
- Special morphologic variants (e.g. mucinous, adenosquamous) and tumor size are not strong predictors of outcome (Dis Colon Rectum 2002;45:1496)
- Duodenal tumors have a worse prognosis compared with tumors occurring in the remainder of small bowel (Cancer 2004;101:518)
- Mismatch repair deficient adenocarcinomas of the small bowel may present at an earlier stage of disease with lower recurrence rates (Clin Cancer Res 2021;27:1429)
- 7 year old boy with Peutz-Jeghers syndrome (BMJ Case Rep 2018;11:e225076)
- 37 year old woman with bilateral ovarian metastasis (Gastroenterology Res 2017;10:366)
- 38 year old woman with coexisting ileal diverticulosis, Crohn’s disease and small bowel adenocarcinoma (In Vivo 2018;32:191)
- 47 year old man with adenocarcinoma arising in jejunal adenomyoma (Pathol Int 2019;69:556)
- 49 year old morbidly obese man with recurrent adenocarcinoma (BMJ Case Rep 2018;2018:bcr2018225273)
- Surgery is the mainstay of treatment with regional lymph node removal for stage I - III disease (J Natl Compr Canc Netw 2019;17:1109)
- Segmental resection is usually performed for jejunal, ileal and some duodenal primaries; pancreaticoduodenectomy may be indicted for some duodenal tumors that invade the pancreas or ampulla
- Adjuvant chemotherapy and radiation have mixed, limited results and are still being evaluated
- A substantial number of duodenal adenocarcinomas show plaque-like growth but they can also present with polypoid growth in approximately 33% of cases (Mod Pathol 2017;30:255)
- Jejunal and ileal adenocarcinomas present as large, annular, constricting apple core lesions with circumferential bowel wall involvement (Cancer 1975;36:1876)
- Gross appearance can also be influenced by background mucosa in adenocarcinomas that arise in inflammatory conditions (Crohn's disease), polyposis syndromes or intestinal duplications (Virchows Arch 2018;473:265)
- Frequent serosal involvement or extension into other organs is not uncommon
- Intraoperative consultation (gross or microscopic) may be requested to evaluate margin status for pancreaticoduodenectomy or segmental resection specimens
- In patients undergoing surgery for inflammatory bowel disease, intraoperative findings concerning for malignancy (i.e. abscess formation, strictures, fistula tracts, perforation) may prompt a frozen section analysis
- Microscopic features compatible with malignancy include complex glandular architecture, invasion, desmoplastic reaction, cellular and nuclear pleomorphism, loss of epithelial polarity and luminal necrosis
- Adenocarcinomas, not otherwise specified, are characterized by columnar epithelial cells with elongated, pseudostratified nuclei forming complex glandular architecture with nuclear pleomorphism, loss of epithelial polarity and luminal dirty necrosis
- Histologic grading system, based on the extent of glandular formation in the tumor, is recommended; grading is done as well differentiated (with more than 95% of tumor composed of glands), moderately differentiated (with 50% to 95% of tumor composed of glands) and poorly differentiated (with less than 50% of tumor composed of glands)
- Additional histologic characterizations include mucinous adenocarcinoma (> 50% mucin), poorly cohesive cell carcinoma (with or without signet ring cells), medullary carcinoma, adenosquamous carcinoma (squamous and adenocarcinoma components), undifferentiated carcinoma or mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN)
- Signet ring cell / poorly differentiated carcinomas, presenting as late stage disease, are more common in Crohn's disease than as de novo small intestinal carcinomas (Inflamm Bowel Dis 2005;11:828)
- Lynch syndrome associated small intestinal adenocarcinomas show similar features to their colorectal counterparts; tumors often show a high number of intratumoral lymphocytes and Crohn's-like lymphoid reaction (Gastroenterology 2005;128:590)
- Preexisting adenoma is present in the majority of proximal tumors but sometimes cannot be identified in large distal small intestinal adenocarcinomas due to tumor overgrowth
- Determining a background of Crohn's disease can sometimes be difficult because the histologic characteristics of Crohn's disease such as transmural inflammation can be caused by the tumor; oftentimes patients present without an established diagnosis of inflammatory bowel disease (J Crohns Colitis 2014;8:19)
Contributed by Krutika S. Patel, M.B.B.S., M.D. and Annika L. Windon, M.D.
- Cytopathologic analysis (FNA or brushing) is rarely used in the small intestine, except for occasionally diagnosing duodenal tumors in the ampullary or pyloric region
- Malignant cells are arranged in loose 3 dimensional clusters of crowded epithelial cells without goblet cells
- Alternatively, many single atypical cells with mitoses, increased nuclear to cytoplasmic ratio and marked nuclear pleomorphism are present
- Reference: J Gastrointest Oncol 2012;3:285
- CK7, CK20 (variable) (Am J Surg Pathol 2004;28:1352)
- CDX2 in up to 70% of cases (Arch Pathol Lab Med 2017;141:1155)
- SATB2 in up to 46% of cases, with patchy and weak staining in most cases, strong and diffuse staining in < 10% of cases (Arch Pathol Lab Med 2017;141:1155)
- MUC1 (EMA): positive in up to 53% of cases (Am J Clin Pathol 2007;128:808)
- MUC2: positive in up to 57% of cases
- Villin: positive in up to 67% of cases (can be focal)
- Small intestinal mucin antigen (SIMA): variably positive in up to 50% of cases
- p53: strong, diffuse overexpression or completely negative (mutated phenotype) in a subset of mutated tumors
- MUC5AC: focally in up to 40% of cases
- MUC6: focally in up to 30% of cases
- AMACR: usually negative (Am J Surg Pathol 2005;29:890)
- CDH17 (Arch Pathol Lab Med 2017;141:1155)
- 2 well defined molecular pathways are reported in small bowel adenocarcinomas, similar to colorectal adenocarcinoma tumorigenesis
- First pathway includes APC, KRAS, TP53 (Int J Cancer 1997;70:390)
- Prevalence of mutations in KRAS is similar between colorectal and small bowel adenocarcinomas, typically 30 - 60%
- Prevalence of TP53 mutations is also comparable, 20 - 50%
- Fewer than 20% of small bowel adenocarcinomas have mutations in APC, in contrast to 80% of colorectal carcinomas
- Second pathway includes inactivation of mismatch repair (MMR) genes, either by germline mutations (Lynch syndrome) or promoter hypermethylation (sporadic), which occurs in up to 38.5% of small bowel adenocarcinomas (Clin Cancer Res 2021;27:1429)
- A number of other genetic alterations have been reported in small bowel adenocarcinomas, including mutations in CTNNB1, SMAD4, IDH1, CDH1, KIT, FGFR2, FLT3, NPM1, PTEN, MET, AKT, RET, ERBB2 (HER2), NOTCH1 and ERBB4 (Gut 2002;50:218, Scand J Gastroenterol 2004;39:748, Int J Cancer 1997;70:390, Am J Gastroenterol 2000;95:1576, Oncotarget 2015;6:20863)
- Small bowel, duodenum, biopsy:
- Invasive moderately differentiated adenocarcinoma arising in a tubular adenoma; lymphovascular invasion is present (see comment)
- Comment: Mucosal colonization from an ampullary, biliary or pancreatic origin must be excluded. Clinical and radiographic correlation is recommended. Immunohistochemical testing for mismatch repair (MMR) proteins shows intact nuclear expression of MLH1, PMS2, MSH2, MSH6 in the tumor cells. Background nonneoplastic tissue / internal control shows intact nuclear expression. Based on these results, there is low probability of MSI-H (MSI = microsatellite instability; H = high).
- Metastatic adenocarcinoma (e.g. colon, breast, ovary, lung):
- Adenoma with high grade dysplasia:
- Differentiating prolapse from invasion around ampulla can be challenging
- Presence of desmoplasia and single cells supports invasion
- Ectopic pancreas:
- Presence of only ducts in a small biopsy specimen may be misinterpreted as neoplasm
- Endometriosis:
- Presence of endometrial stroma and ciliated epithelium helps to make this diagnosis
- Ampullary adenocarcinoma:
- Advanced duodenal carcinoma may extend to involve the ampulla but only those centered on or circumferentially surrounding the ampulla are regarded as ampullary carcinomas
- Careful gross examination to assess the tumor epicenter helps in distinguishing ampullary / periampullary primary from duodenal primary
Comment Here
Reference: Adenocarcinoma-small intestine
- Carcinoma that develops in Crohn's disease is usually seen in older individuals in their 80s
- Carcinoma that presents in Crohn's disease tends to present as early stage disease
- Carcinomas in Crohn's disease usually are well differentiated
- Risk of developing carcinoma is related to duration of disease
- There is no increased risk when compared to the general population
Comment Here
Reference: Adenocarcinoma-small intestine
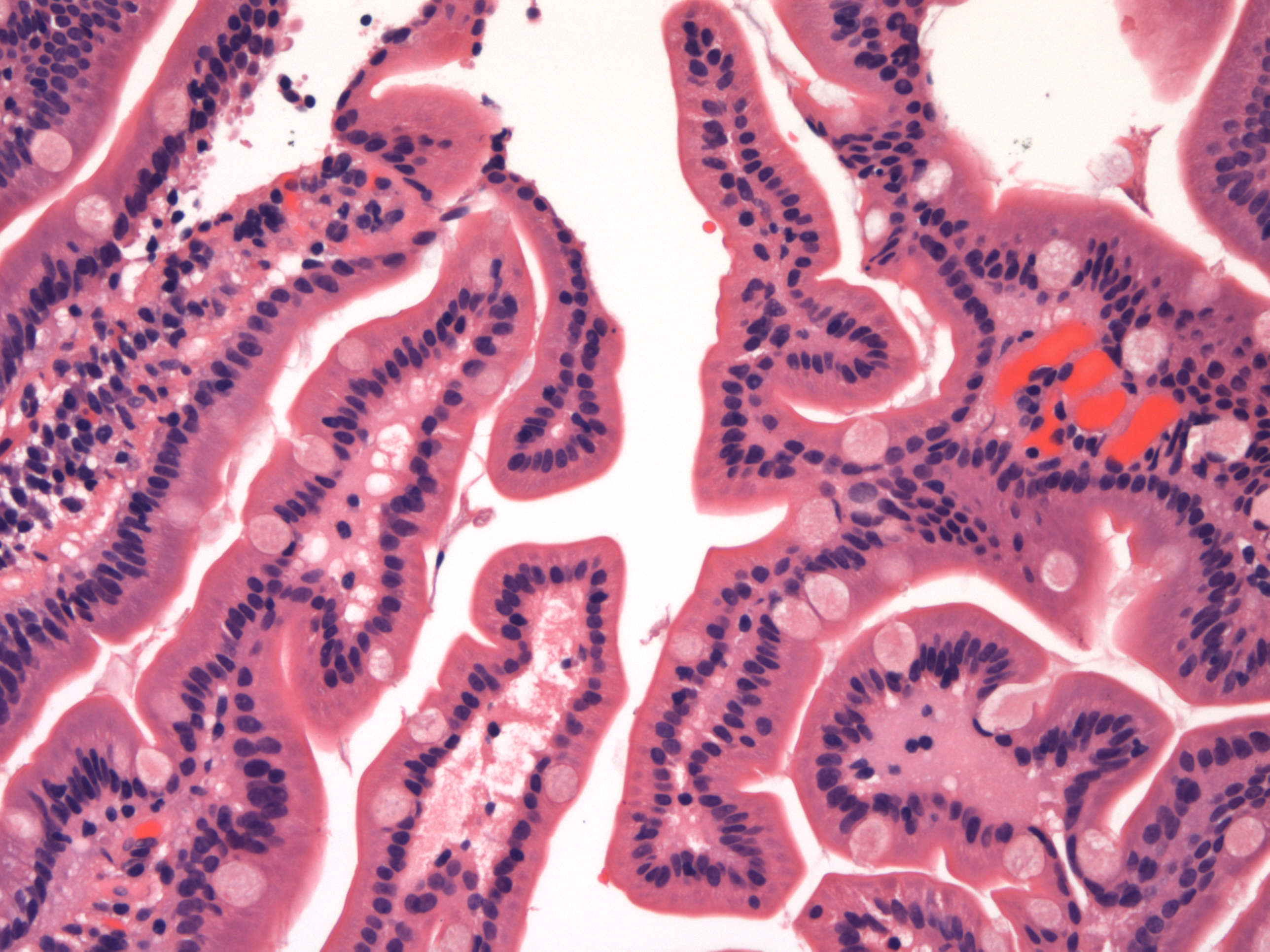
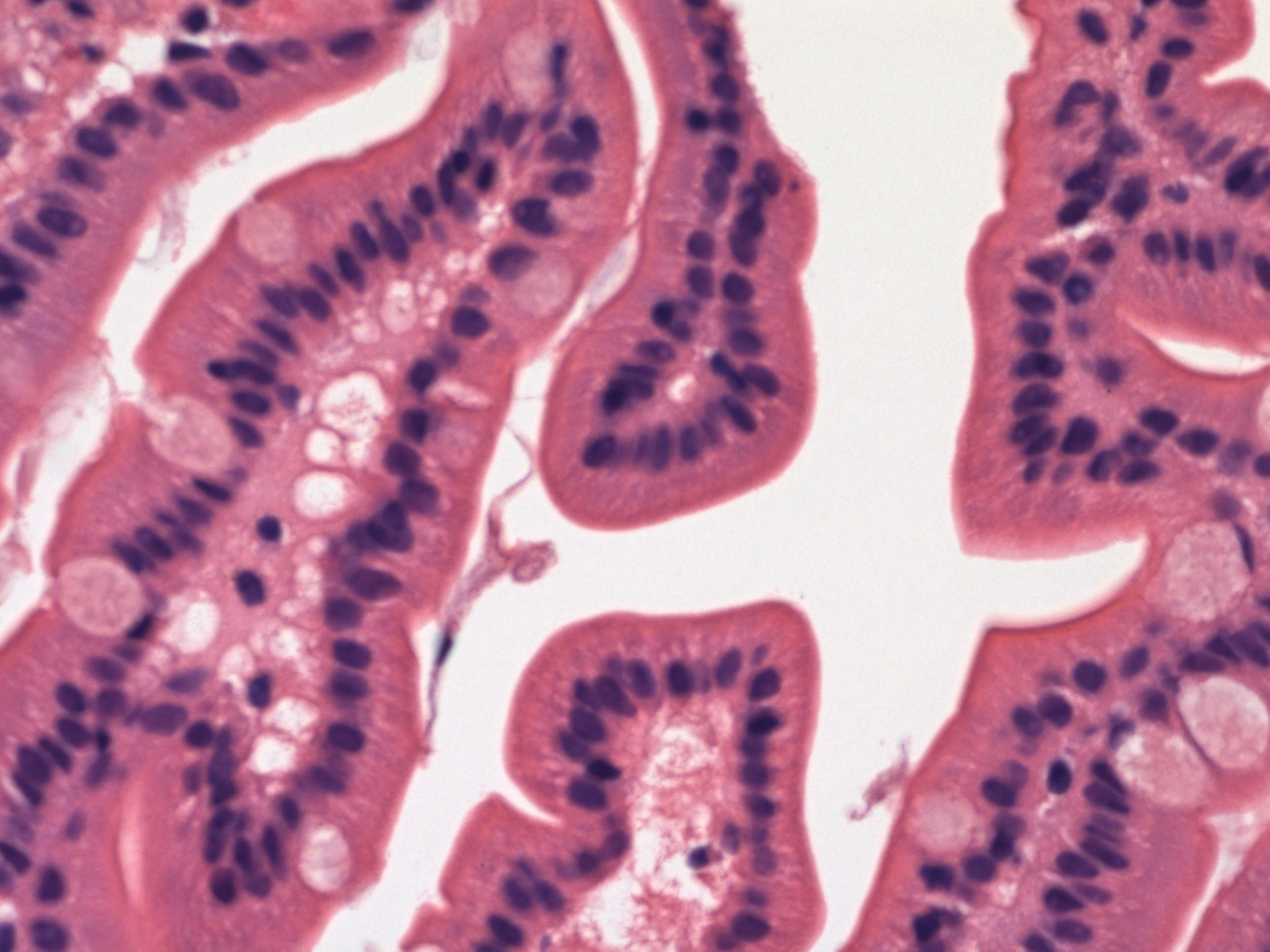
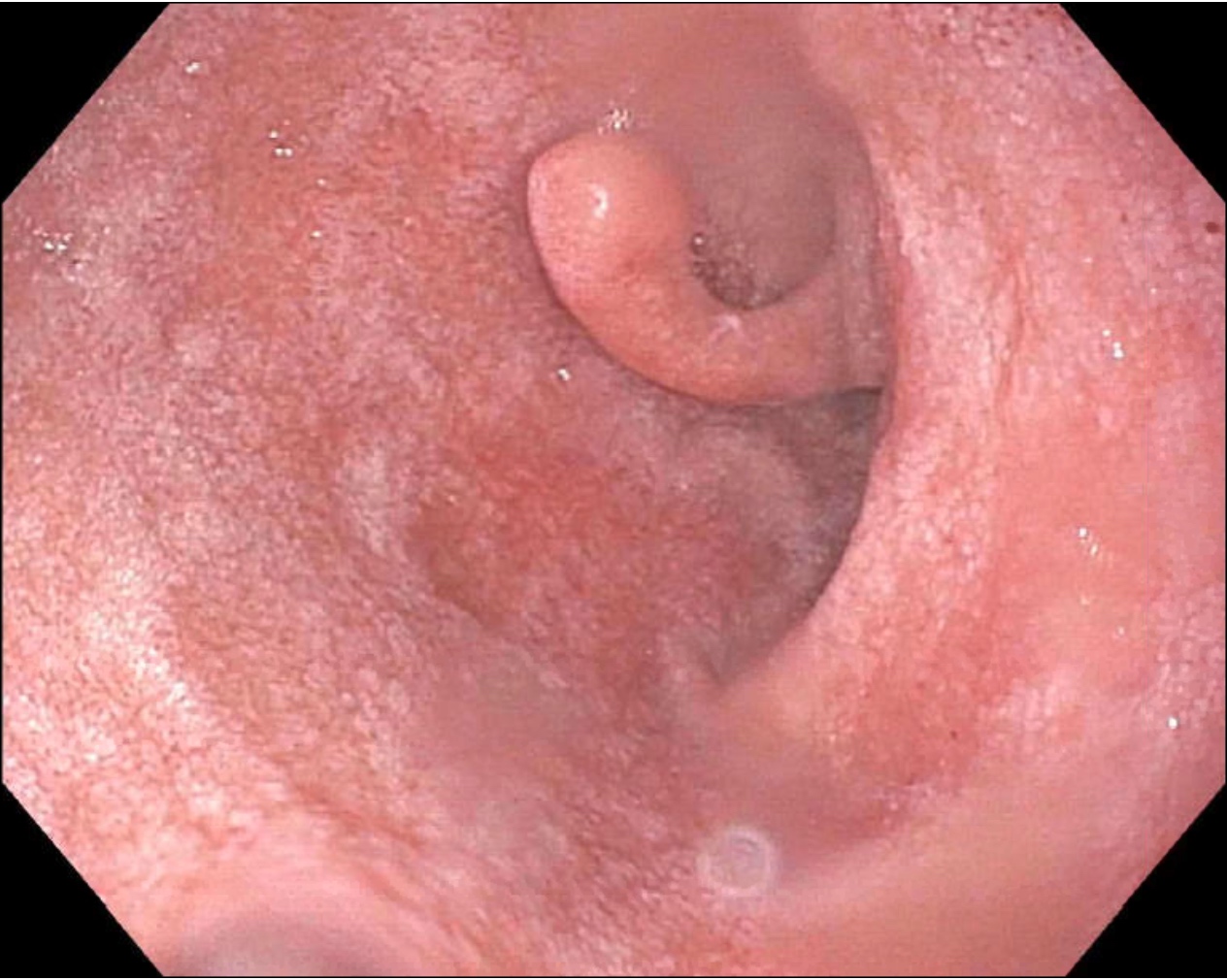
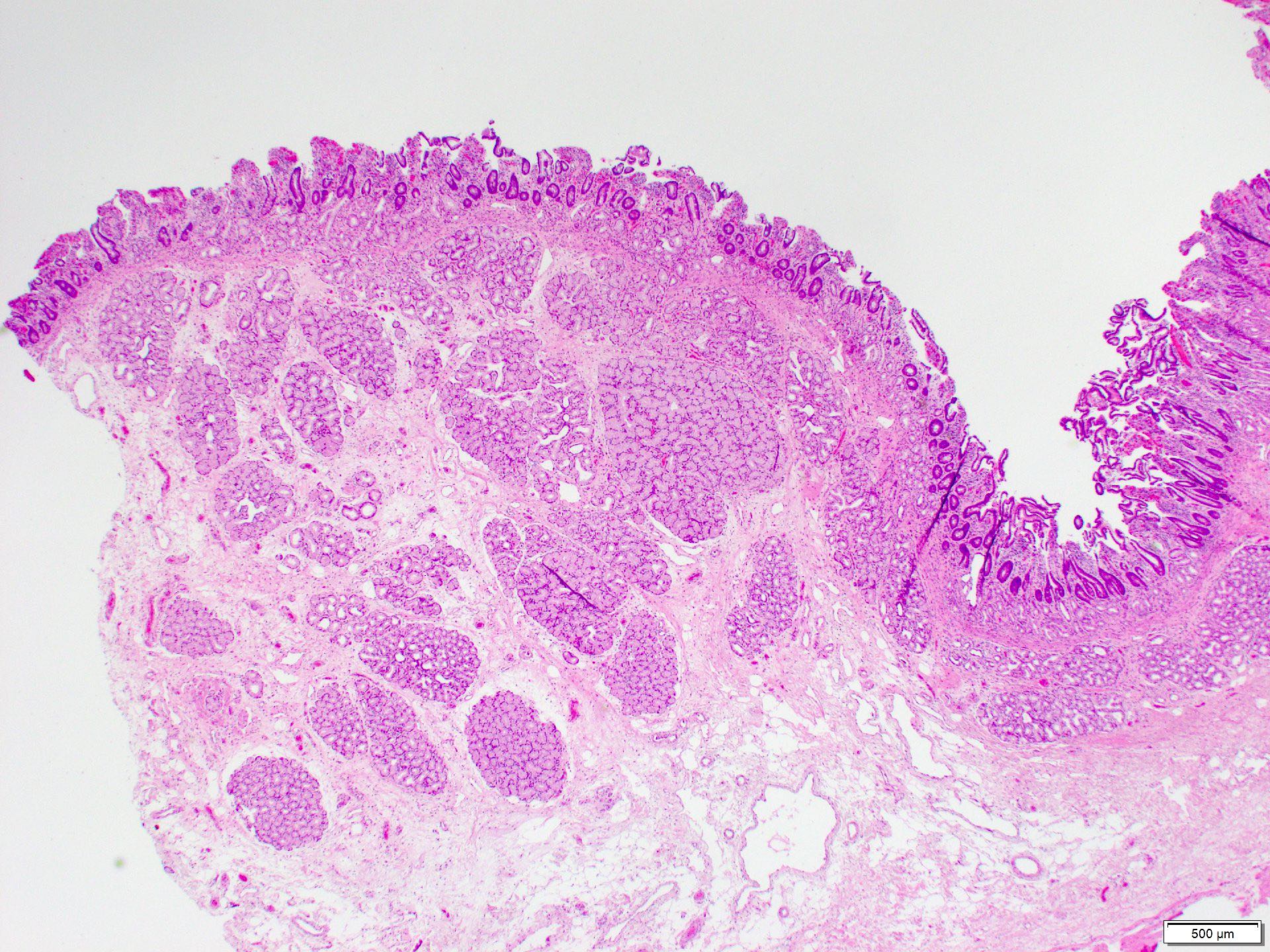
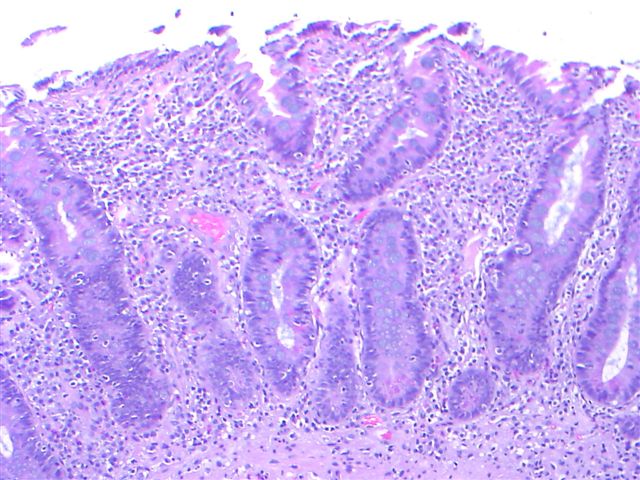
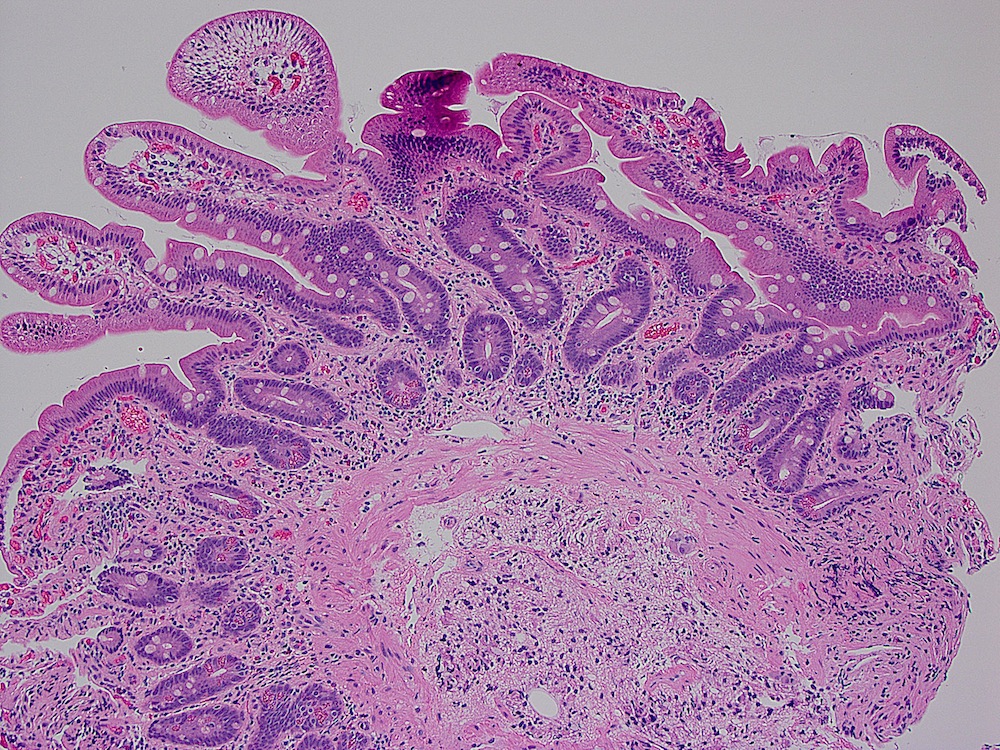
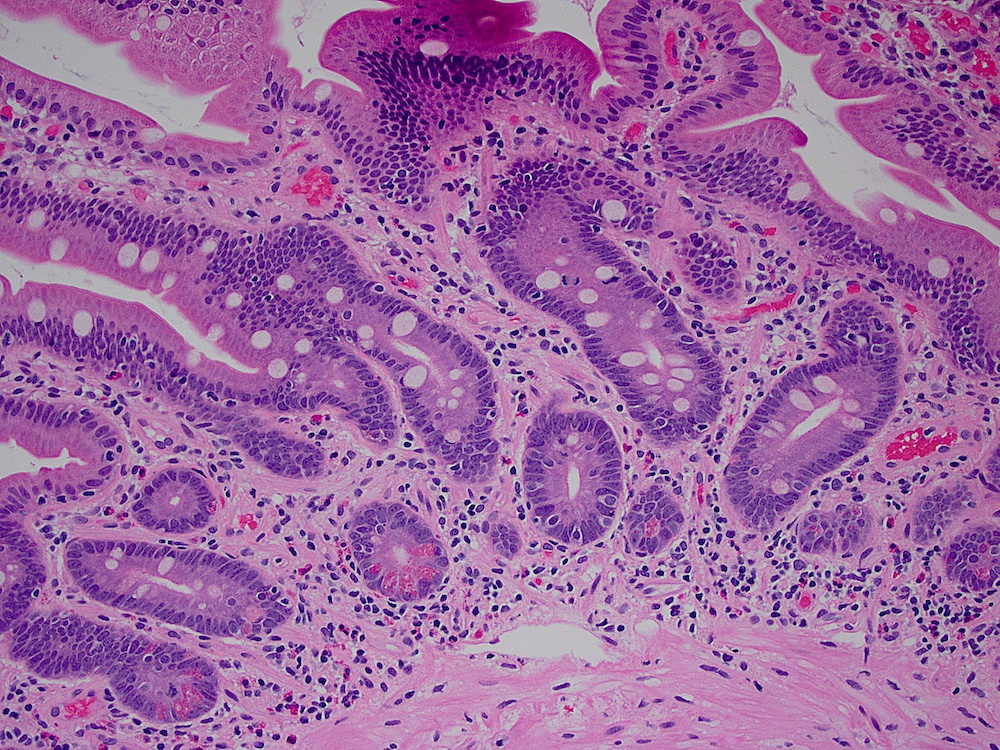
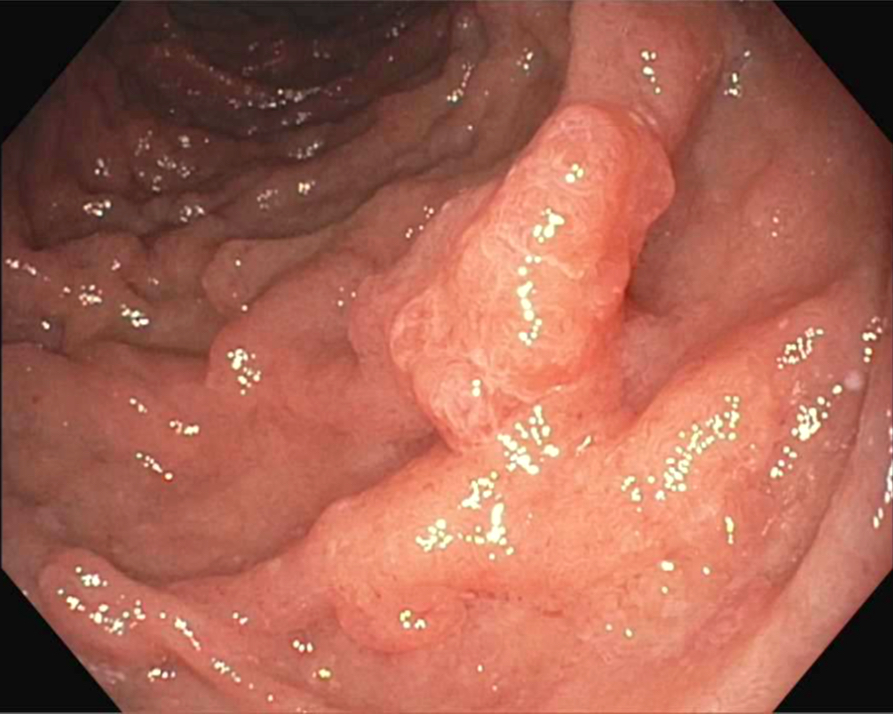
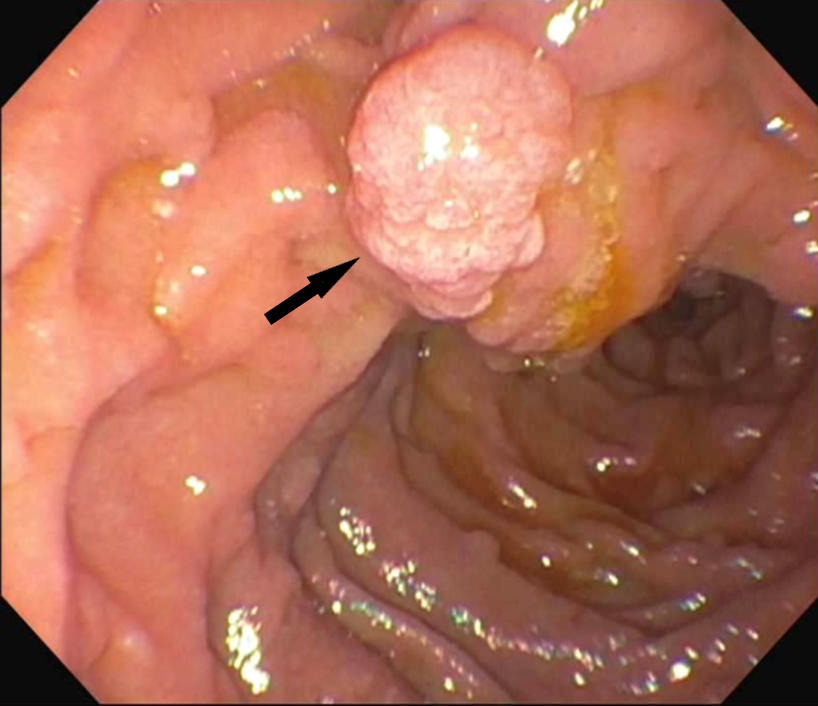
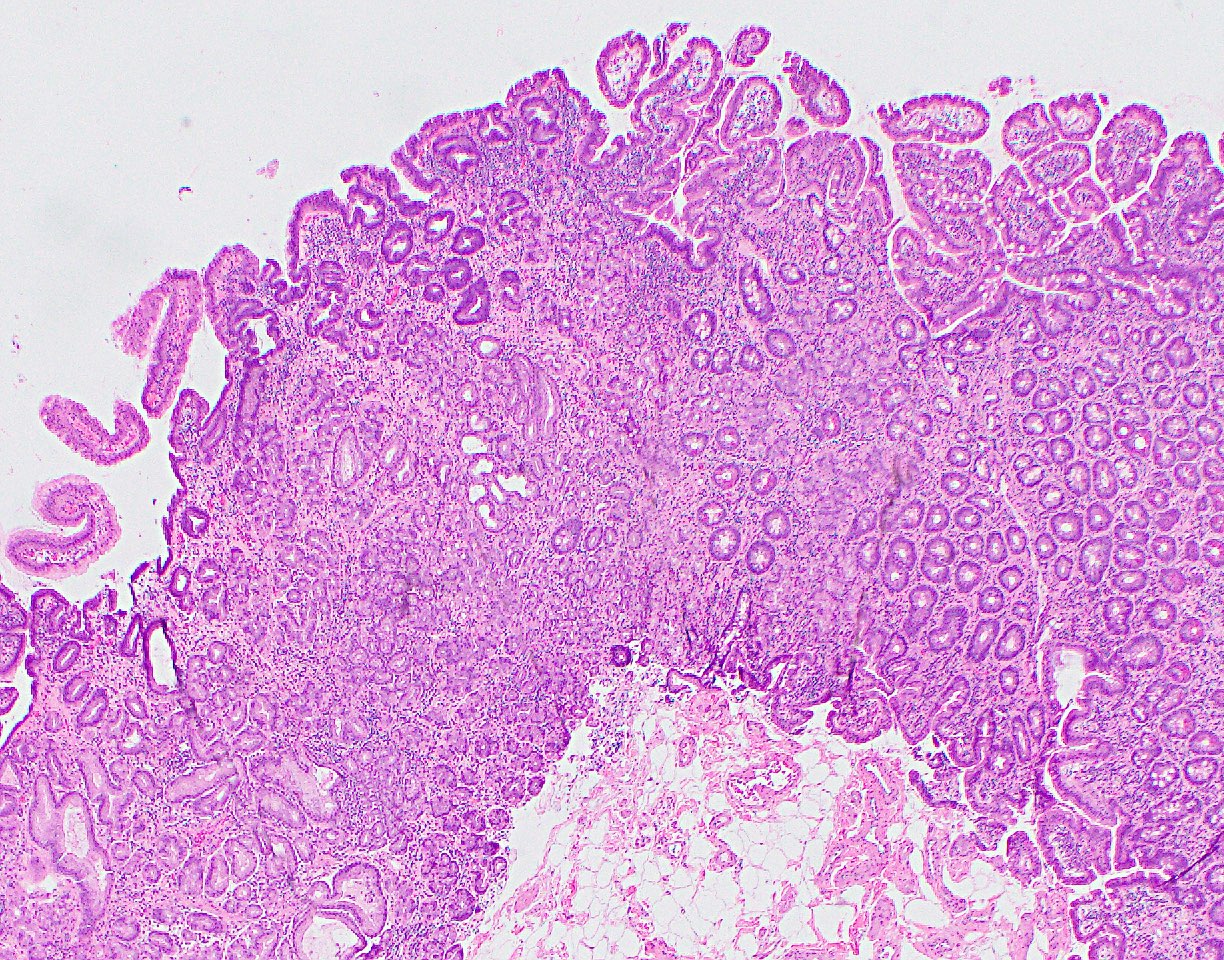
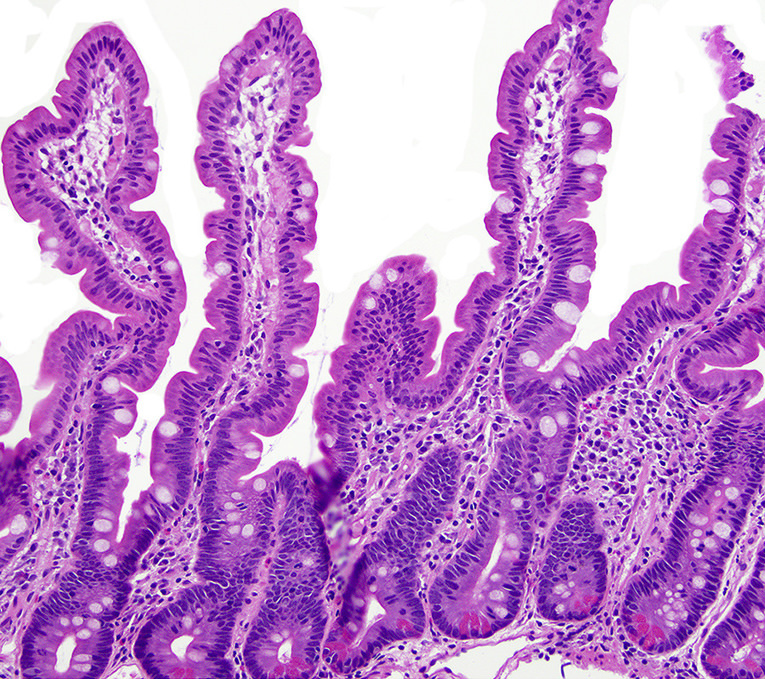
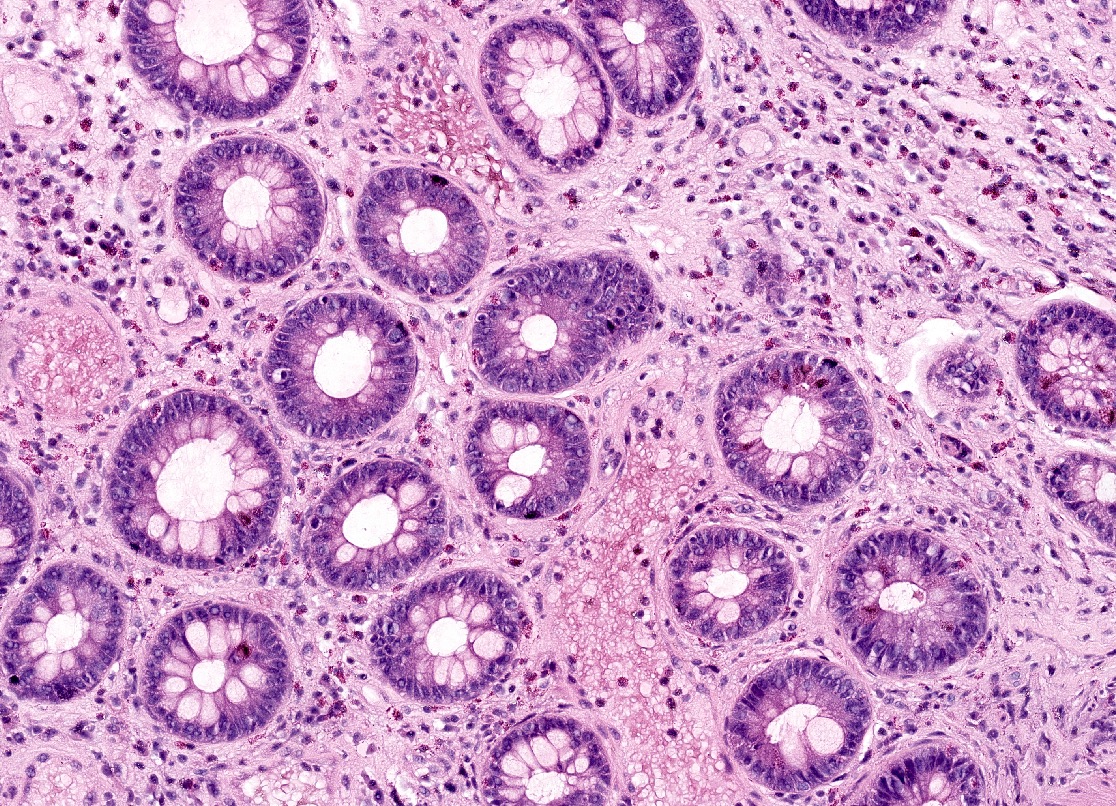
- Dysplastic, premalignant lesion of ampulla of Vater
- Essentially the ampullary counterpart to colonic tubular / tubulovillous adenoma
- ICD-10: D37.6 - Neoplasm of uncertain behavior of ampulla of Vater
- < 10% of periampullary neoplasms
- Age range 32 - 86 years (Am J Clin Pathol 2009;132:506)
- Incidence 1:1,000 - 2,000 based on autopsy studies
- Generally occurs in older patients
- Increased incidence in familial adenomatous polyposis syndrome
- Usually asymptomatic but can cause gastric outlet obstruction or bleeding and rarely acute pancreatitis, biliary obstruction or intussusception
- Excellent prognosis if completely excised
- Often associated with concurrent pancreatic intraepithelial neoplasia (Mod Pathol 2001;14:139)
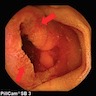
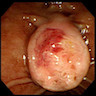
- Endoscopic impression, confirmed on biopsy
- 49 year old woman with intussusception (Case Rep Gastroenterol 2016;10:545)
- 53 year old man with common bile duct stricture (Cytojournal 2017;14:19)
- 74 year old man with concurrent ampullary small cell neuroendocrine carcinoma (World J Gastroenterol 2008;14:4709)
- 78 year old man with ampullary adenoma (J Med Case Rep 2014;8:228)
- 81 year old man with progression to carcinoma (Am J Case Rep 2015;16:586)
- Early or relatively confined lesions may be excised by endoscopic polypectomy or ampullectomy (Am J Clin Pathol 2009;132:506)
- Otherwise, requires pancreatoduodenectomy
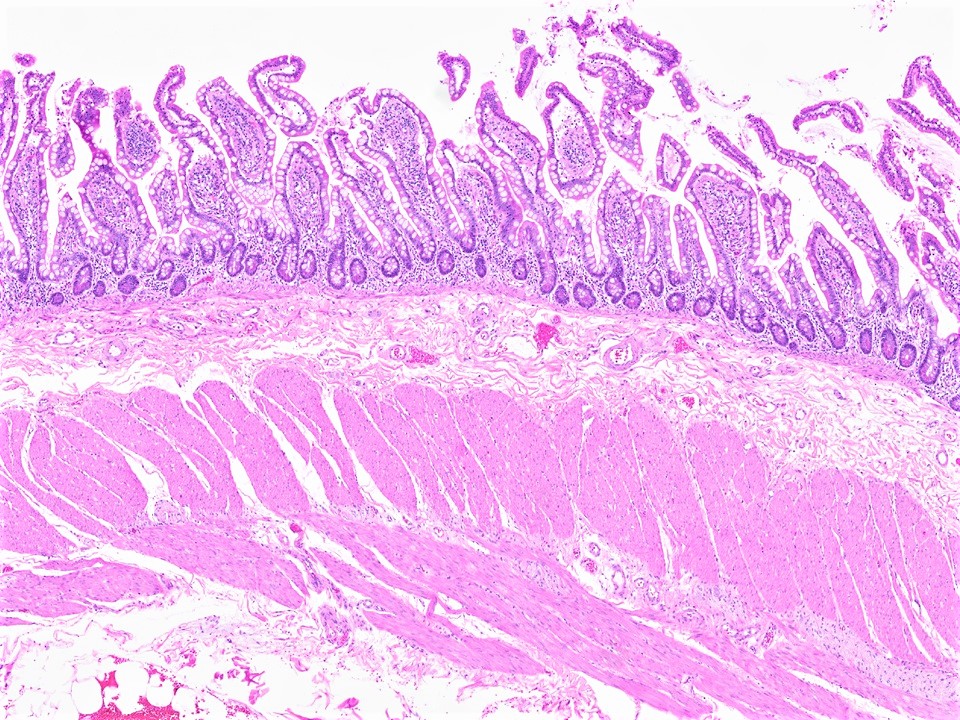
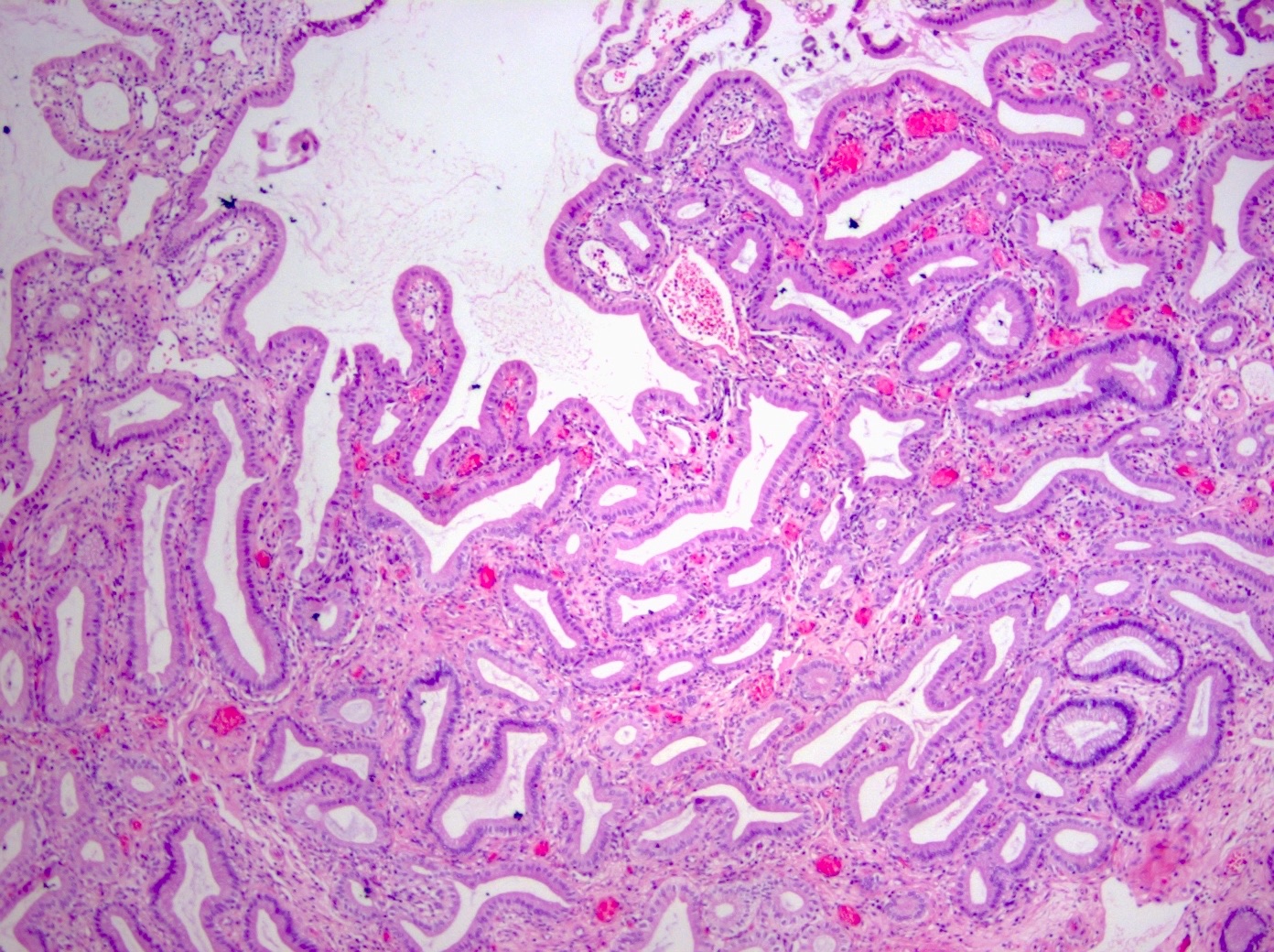
- Polypoid / exophytic mass
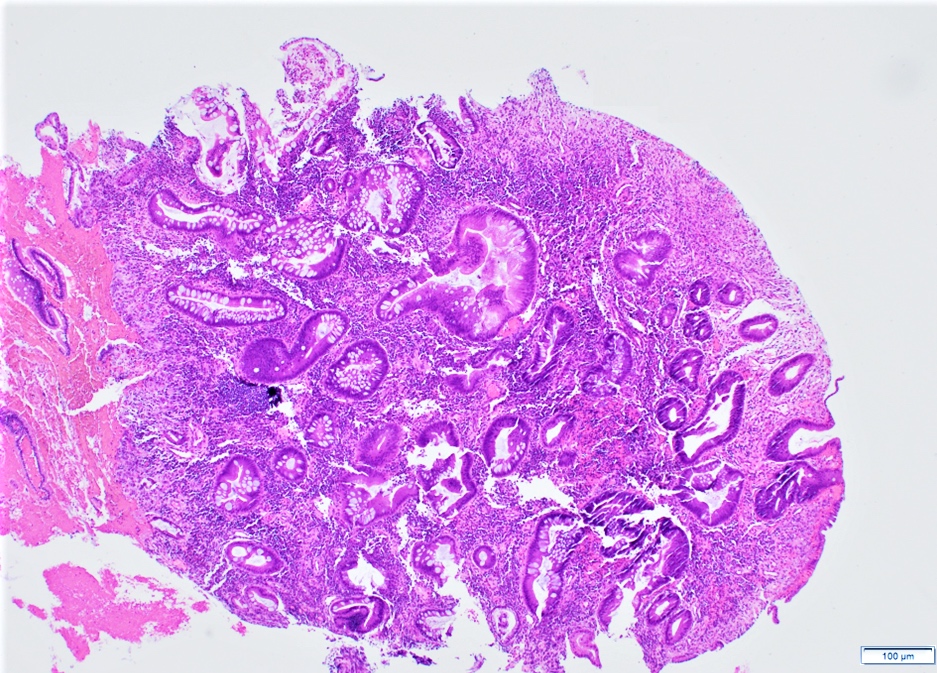
- Tubular, villous or mixed, similar to adenomas in colon, with approximately half tubular and half villous (Am J Clin Pathol 2009;132:506)
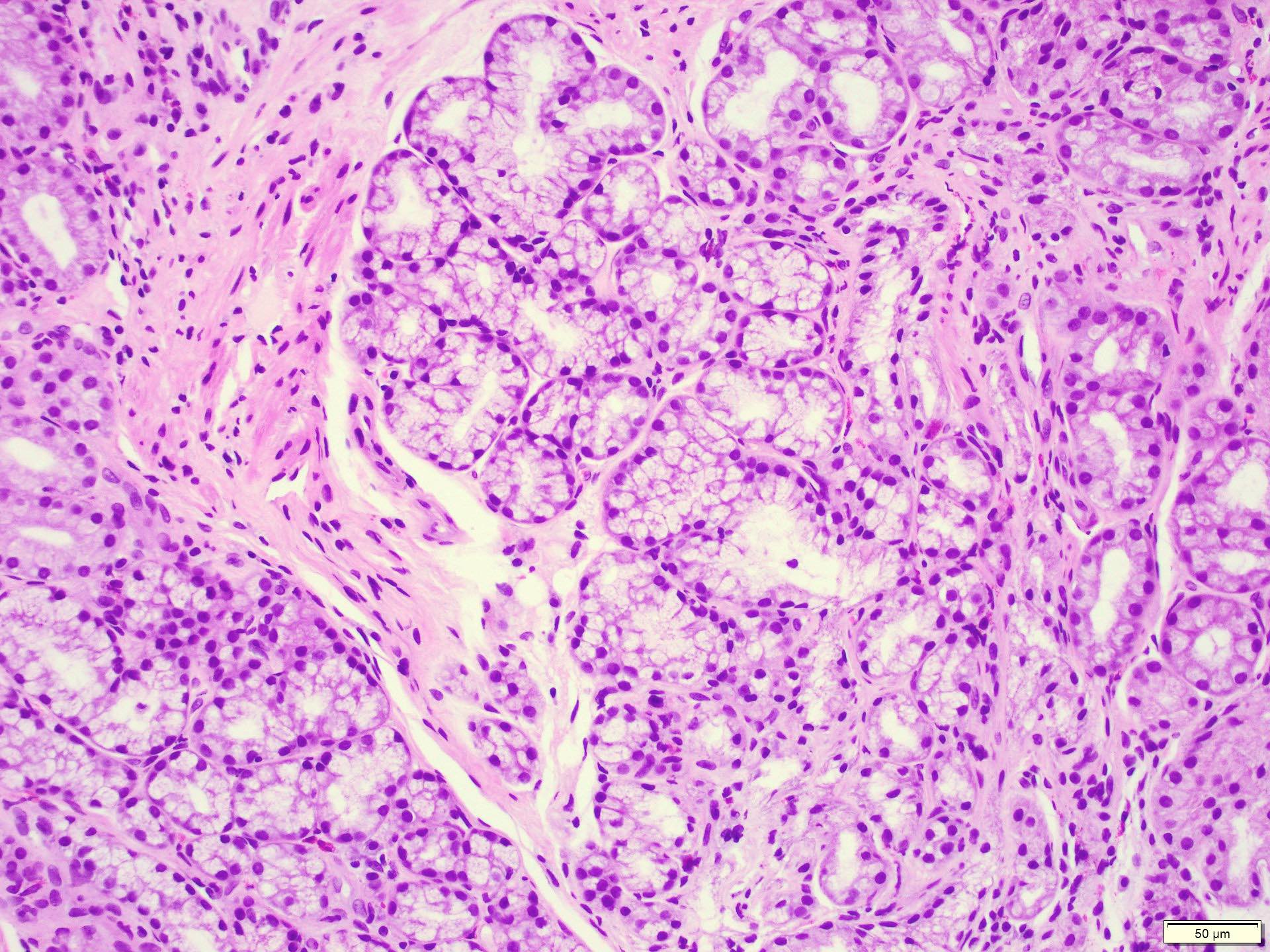
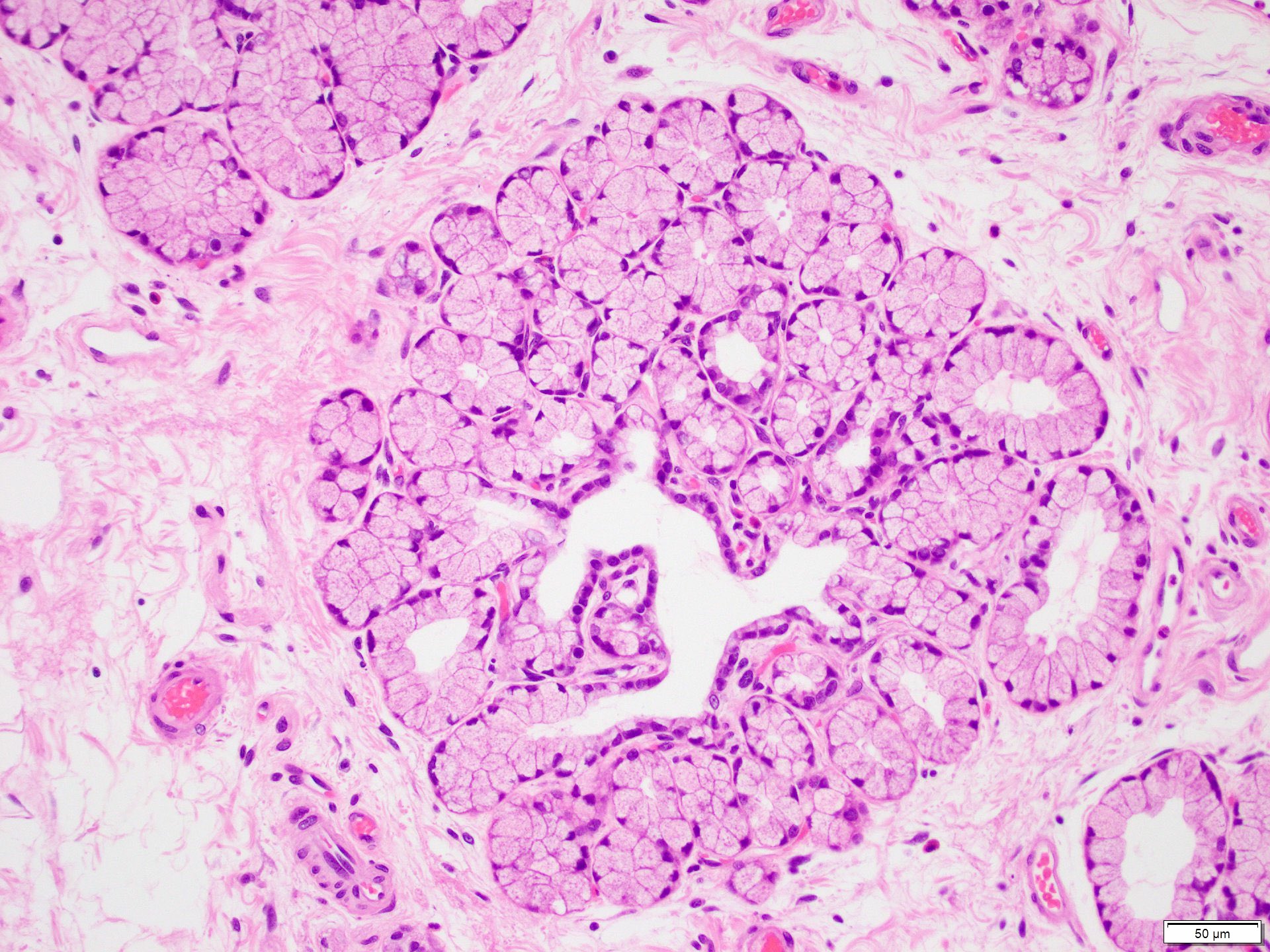
- Dysplastic epithelium may have only subtle changes of mild cellular stratification and fine chromatin pattern
- Often contain prominent Paneth cells (with coarse, large, red-pink, refractile granules in supranuclear cytoplasm), endocrine cells (dark, red-purple, fine small granules in basal cytoplasm) and goblet cells
- May show high grade dysplasia or give rise to adenocarcinoma
- Endoscopic brush cytology is sensitive and specific for adenoma / carcinoma, although diagnosis of adenoma does not exclude coexisting carcinoma (Am J Clin Pathol 1998;109:540)
- Sporadic and familial adenomatous polyposis related adenomas show similar molecular features to colorectal adenoma, with presence of APC and KRAS mutations
- BRAF mutations, p53 alterations and DNA mismatch repair abnormalities are rare (Am J Surg Pathol 2008;32:1388)
- Ampulla, polypectomy:
- Ampullary adenoma (low grade dysplasia)
- Duodenal (nonampullary) adenoma:
- Anatomic site is only distinction
- Intra-ampullary papillary tubular neoplasm:
- Occurs deeper within the ampulla
- May have pancreatobiliary type or intestinal type epithelium (Am J Surg Pathol 2010;34:1731)
- BRAF mutations are rarely seen
- Cases must be managed with pancreatoduodenectomy
- Patients with Lynch syndrome are at significantly increased risk
- Progression to adenocarcinoma is unusual
Comment Here
Reference: Adenoma
Comment Here
Reference: Adenoma
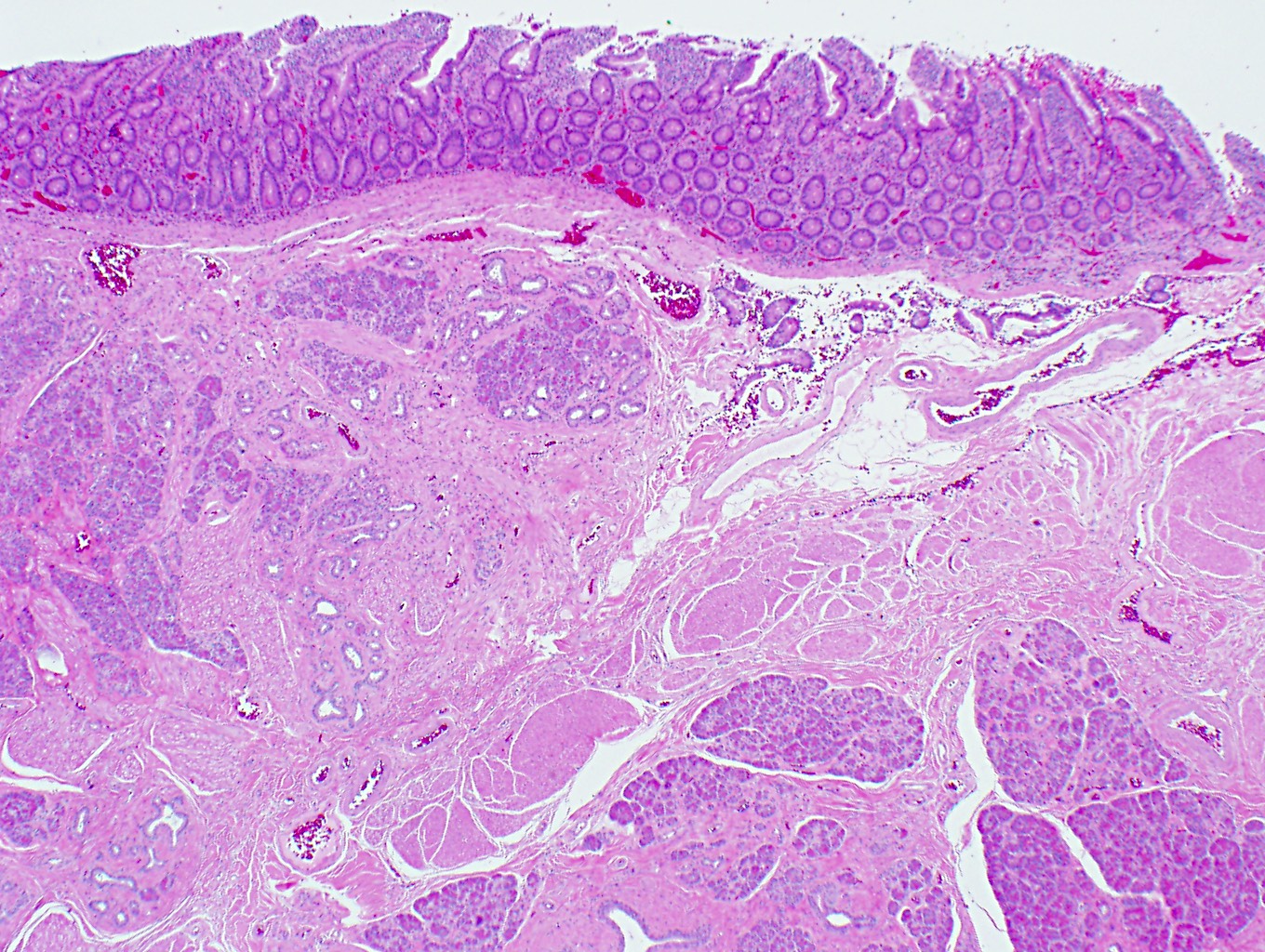
- Also called adenomyomatous hyperplasia
- Rare, benign
- Usually stomach, duodenum, jejunum
- Often causes pain in right upper quadrant, radiating to back
- May cause biliary obstruction and common bile duct dilation (Arch Pathol Lab Med 1987;111:388)
- 55 year old woman with history of duodenal ulcers (Arch Pathol Lab Med 2001;125:701)
- 74 year old woman presenting as acute recurrent pancreatitis (World J Gastroenterol 2007;13:2892)
- Excision
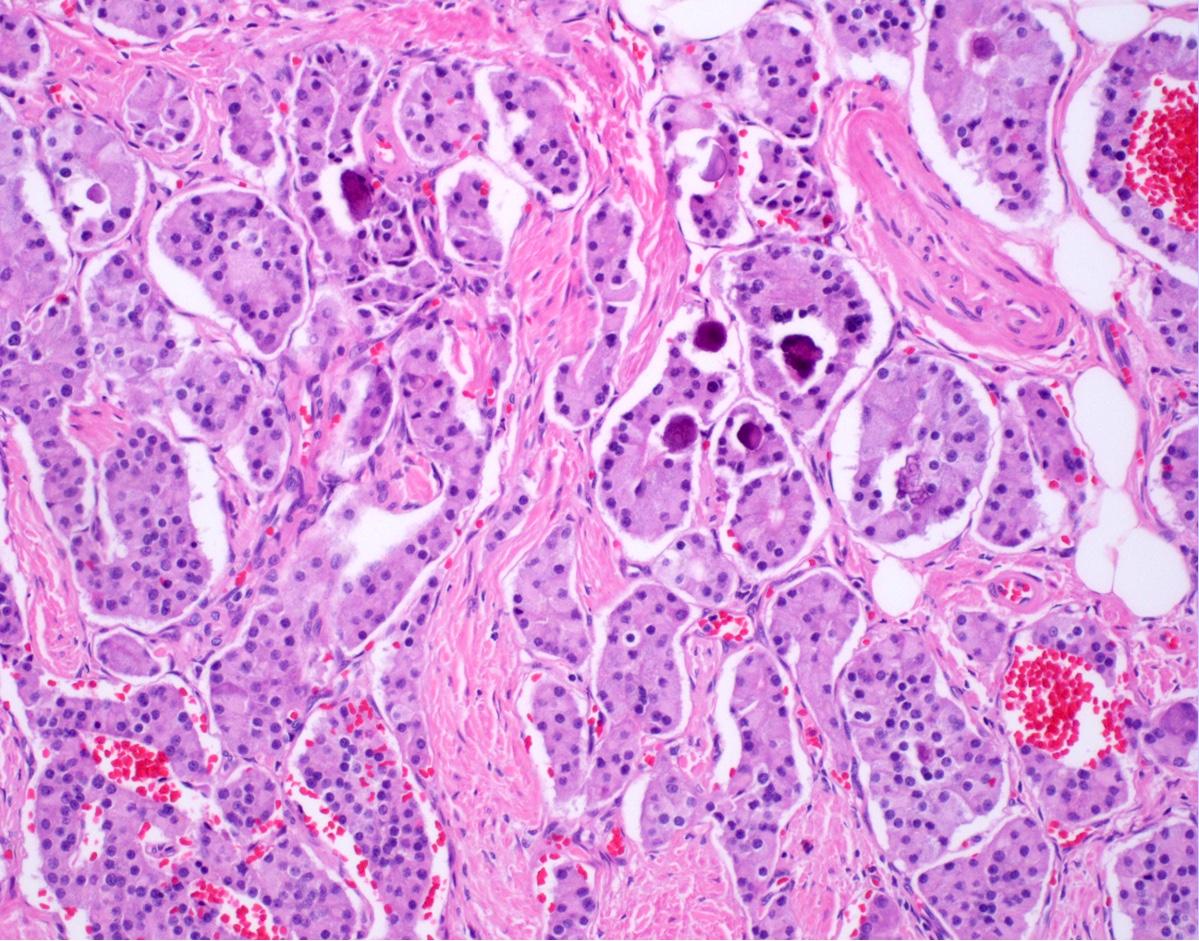
- Mass at head of pancreas, usually 0.5 cm or more
- Well circumscribed, nodular proliferation of smooth muscle cells, ducts and glands, clearly disorganized compared to normal
- Ducts and glands are lined by columnar/cuboidal cells
- No atypia, no mitoses, usually no pancreatic tissue
- MUC6, focal surface positivity for MUC5AC (J Hepatobiliary Pancreat Sci 2010;17:275)
- MUC1, MUC4
- Normal intraampullary common bile duct (normally has dense muscular layer)
- Ectopic pancreatic tissue
- Fibroadenoma
- Brunner gland hyperplasia
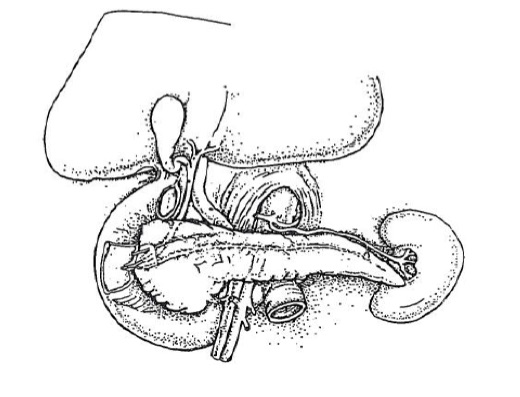
- Small intestine extends from gastric pylorus to ileocaecal valve
- 6 meters long, divided into duodenum, jejunum, ileum
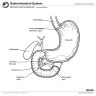
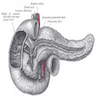
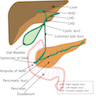
- Ampulla means flask like dilatation (spreading or stretching) of a tubular structure
- May refer to Ampulla of Vater or portion of fallopian tube, vas deferens, semicircular canal or colon
- Vater ("fah-ter") is German anatomist Abraham Vater (1684-1751) who first described this structure
- Usually refers to confluence of distal common bile duct and main pancreatic duct in second portion of duodenum near pancreatic head, although in 42% of patients, ampulla is termination of common bile duct only as the pancreatic duct enters the duodenum separately next to ampulla; in these cases, ampulla may be difficult to locate or nonexistent
- Ampulla is 1.5 cm long or less, traverses duodenal wall, opens into the duodenal lumen through (major) duodenal papilla (papilla of Vater), a 0.5 cm in diameter mucosal elevation with mucosal reduplications (valves of Santorini) that probably prevent regurgitation
- Minor papilla, also called accessory pancreatic duct (APD) of Santorini, is 2 cm proximal and slightly anterior to major papilla
- APD is Patent in 50% cases, pancreatic tissue is noted in 80% of cases in minor papilla (Dig Surg 2010;27:137)
- Ampulla is surrounded by muscular fibers of sphincter of Oddi
- Reference: Dig Surg 2010;27:90
- 25 cm long, from pyloric sphincter to ligament of Treitz, mostly retroperitoneal, fixed in position
- Common bile duct (CBD) and pancreatic duct enter second part of duodenum posteromedially at ampulla of Vater (eMedicine: Duodenal Anatomy [Accessed 9 February 2018)
- 240 cm long, 40% of remainder of bowel, begins at ligament of Treitz
- Has prominent circular mucosal folds (folds of kerckring) that increase absorptive surface
- 360 cm long, distal 60% of postduodenal bowel
- Mucosa has transverse folds, prominent in proximal ileum, flat / absent at terminal ileum
- At end of small bowel
- 2 lip structure containing adipose tissue and lymphoid tissue
- Duodenum drains to portal and pyloric nodes
- Jejunum and proximal ileum drain to mesenteric nodes and nodes around superior mesenteric artery, terminal ileum drains to ileocolic nodes
- Lacteals are lymphatic channels in villi for chylomicrons
- Peyer patches in ileum (ovoid lymphoid follicles, partly mucosal and partly submucosal, in antimesenteric side of terminal ileum)
- Small intestinal goblet cells, which deliver low molecular weight soluble antigens from intestinal lumen to CD103+ lamina propria dendritic cells, which regulates development of T cells (Nature 2012;483:345)
- M (membranous) cells, part of follicle associated epithelia (MALT) in small bowel and colon, which transfer antigen macromolecules from lumen to lymphocytes
- T cells, usually CD8+ and scattered in surface epithelium
- Lamina propria contains CD4+ T cells and B cells
- Mucosa associated lymphoid tissue: lymphoid nodules, mucosal lymphocytes, appendiceal lymphoid follicles and mesenteric nodes (Annu Rev Cell Dev Biol 2000;16:301)
- Anterograde and retrograde peristalsis mixes food and promotes maximal contact of nutrients with mucosa
- Colonic peristalsis prolongs contact with mucosa
- Peristalsis is mediated via myenteric plexus and autonomic innervation (sympathetic thoracolumbar, parasympathetic vagal)
- Also through interstitial cells of Cajal (pacemaker cells) and smooth muscle cells
- Vagal receptors are abundant in duodenum and scattered throughout wall
- Atresia: imperforate mucosal diaphragm or string-like segment of bowel
- Stenosis: narrowing of lumen; less common
- Complications: perforation, meconium peritonitis, brown bowel syndrome
- Developmental failure, intrauterine vascular accidents, intussusceptions
- 46 day old boy with multiple areas of jejunal atresia with apple peel deformity (twisted around an artery) associated with 22q11 abnormality (Arch Pathol Lab Med 2000;124:880)
- Immune mediated disorder causing small intestinal atrophy and resulting in severe diarrhea
- Intractable diarrhea leading to malabsorption and electrolyte imbalances
- Histologic findings include intestinal atrophy with variable inflammation (acute and chronic), crypt apoptosis and absence of goblet / Paneth cells
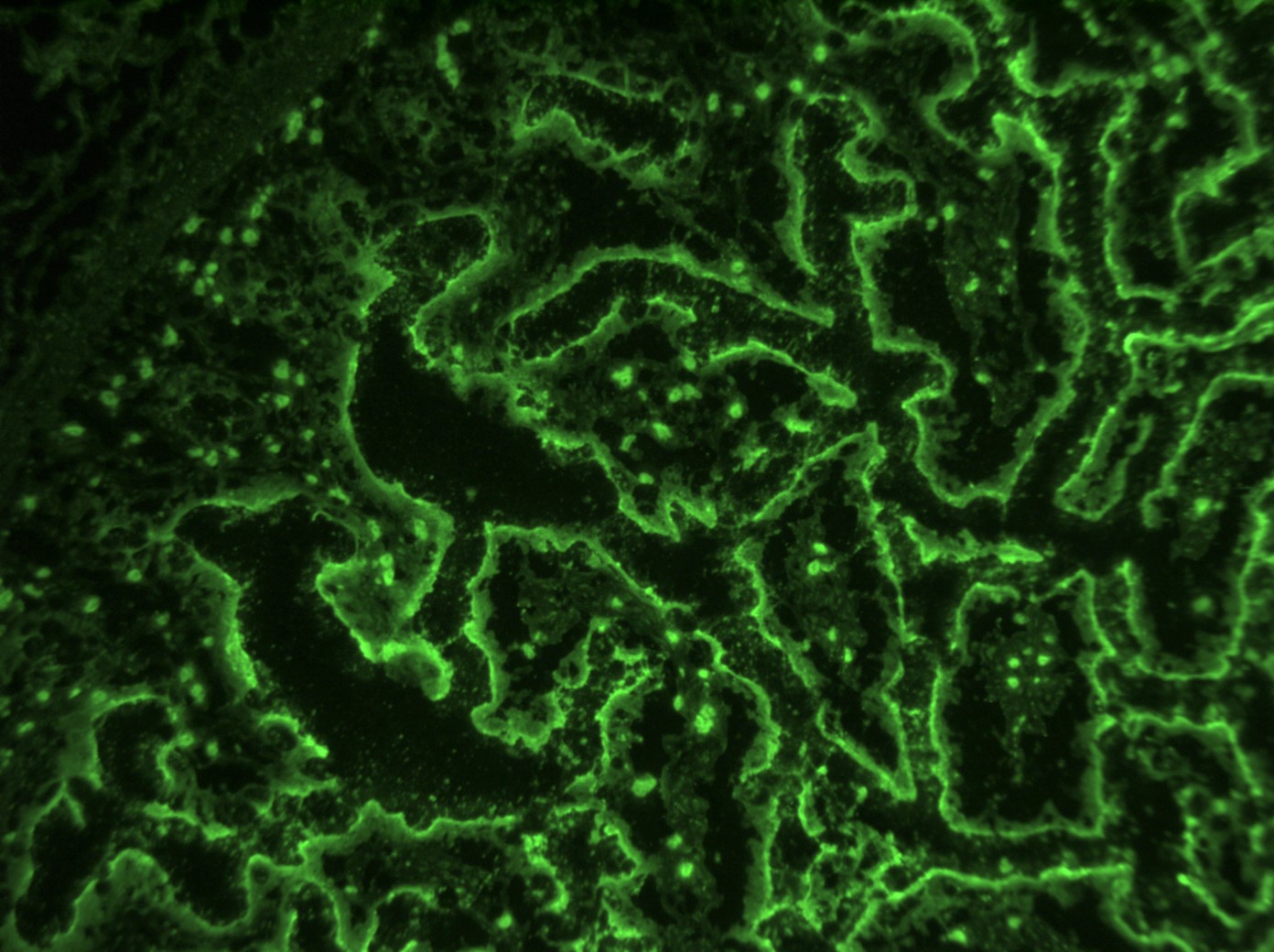
- Indirect immunofluorescence on normal intestinal tissue using patient’s serum is useful in detecting circulating anti-enterocyte and antigoblet cell antibodies
- Extraintestinal autoimmune manifestations are possible
- Prognosis is much improved with immunomodulatory therapy
- Autoimmune enteropathy
- Syndromic forms: immune dysregulation, polyendocrinopathy, enteropathy and X linked (IPEX) syndrome; autoimmune polyglandular syndrome (APS 1) also known as autoimmune phenomena, polyendocrinopathy, candidiasis and ectodermal dystrophy (APECED) syndrome
- Age at diagnosis: variable, predominantly infants and young children, median age at presentation ~6 months; adult presentation documented (Mod Pathol 2014;27:543, Dig Dis Sci 2019;64:643)
- < 1/100,000 children (Scand J Gastroenterol 2008;43:1102)
- M > F
- Exact pathogenesis is unclear
- Intestinal epithelial damage from anti-enterocyte antibodies and antigoblet cell antibodies
- Complex, multifactorial
- IPEX syndrome: FOXP3 mutation leading to impaired function / number of regulatory T cells, X linked
- APECED syndrome: AIRE mutation resulting in lack of suppression of autoreactive T cells, autosomal recessive
- IPEX-like syndrome: mutations in genes related to regulatory T cell functions such as CD25, STAT5b, STAT1, LRBA and CTLA4 (Front Immunol 2018;9:2411)
- Severe intractable diarrhea, unresponsive to dietary modifications (Dig Dis Sci 2020 Aug 24 [Epub ahead of print])
- Failure to thrive, weight loss, growth restriction
- IPEX and APECED: endocrinopathies and cutaneous manifestations (see Sites)
- Combination of clinical presentation, histologic findings and laboratory studies
- Anti-enterocyte and antigoblet cell antibodies demonstrable by indirect immunofluorescence; predominantly IgG, less commonly IgM and IgA
- Sequencing for known mutations
- Can be fatal if untreated; advances in treatment and aggressive nutritional care have improved outcomes
- 13 year old girl with autoimmune enteropathy successfully treated with infliximab (J Clin Gastroenterol 2014;48:264)
- 18 year old Caucasian man with APECED (BMJ Case Rep 2013;2013:bcr2012008116)
- 55 year old man with autoimmune enteropathy and bowel transplantation (Transplant Proc 2020;S0041:32669)
- Supportive nutrition, enteral and parenteral
- Immunosuppression (steroids, immunomodulatory chemotherapeutic agents, biologic medications)
- Stem cell transplant (IPEX syndrome) (Dig Dis Sci 2019;64:643)
- Villous architectural abnormalities
- Villous blunting, severe villous atrophy and crypt hyperplasia
- Absence of an epithelial cell subtype
- Diminished / absent goblet cells and Paneth cells
- Observed in a subset of cases; not a requisite for diagnosis (Am J Surg Pathol 2014;38:1319, Mod Pathol 2014;27:543)
- Inflammation:
- Increased intraepithelial lymphocytes
- Active (acute) inflammation and cryptitis
- Increased crypt apoptosis resembling graft versus host disease
- Findings pronounced in duodenum / small bowel; variable findings can also be seen in the stomach and colon (Am J Surg Pathol 2014;38:1319)
- Severity of histologic findings variable among individuals and reversible with therapy
- Indirect immunofluorescence test performed using patient’s serum on normal frozen small intestinal tissue can assist in diagnosis; positive staining of apical and basolateral borders of enterocytes or goblet cell staining indicates the presence of anti-enterocyte and antigoblet cell antibodies (Pediatr Dev Pathol 1999;2:65)
- Not routinely used for diagnosis; if performed, helpful in eliminating congenital diarrheas that are in the differential diagnoses
- Duodenum, biopsy:
- Severe villous blunting with absence of goblet cells and Paneth cells, intraepithelial lymphocytosis and increased crypt apoptosis
- Duodenum, biopsy:
- Chronic active duodenitis with increased apoptosis (see comment)
- Comment: The biopsies demonstrate histologic features that raise the possibility of autoimmune enteropathy, in the correct clinical context. If celiac disease is excluded, serologic or molecular testing for autoimmune enteropathy is recommended.
- Celiac disease:
- Intact goblet and Paneth cells
- Typically more pronounced intraepithelial lymphocytes
- Typically responsive to gluten free diet
- Positive serologic tests (antiendomysial antibodies and tissue transglutaminase) for celiac disease
- Very early onset inflammatory bowel disease:
- Intact goblet and Paneth cells
- Granulomas (in Crohn’s disease)
- Infections:
- Stool polymerase chain reaction tests, cultures
- Acute onset of symptoms responsive to antibiotic medications
- Common variable immunodeficiency:
- Paucity of lamina propria plasma cells
- Congenital diarrheas:
- Tufting enteropathy:
- Epithelial tufts, EpCAM mutations, negative EpCAM immunohistochemical stain
- Microvillus inclusion disease:
- Enteroendocrine cell dysgenesis:
- Negative chromogranin, NEUROG 3 mutations
- Tufting enteropathy:
- Graft versus host disease
- History of stem cell transplant
- FOXO1
- FOXP3
- SS18
- TFE3
- Rare immune mediated systemic vasculitis that often presents with mucous membrane ulceration and ocular problems (Wikipedia: Behçet Disease [Accessed 12 February 2018])
- GI involvement in 10% of cases, usually ileum and cecum
- Punched out ulcers that may perforate
- Perivascular inflammation, necrotizing and nonnecrotising lymphocytic vasculitis affecting small veins and venules is often present
- Also aphthous stomatitis, genital ulcers, relapsing iritis
- 30 year old man with massive GI bleeding (Korean J Gastroenterol 2007;49:400)
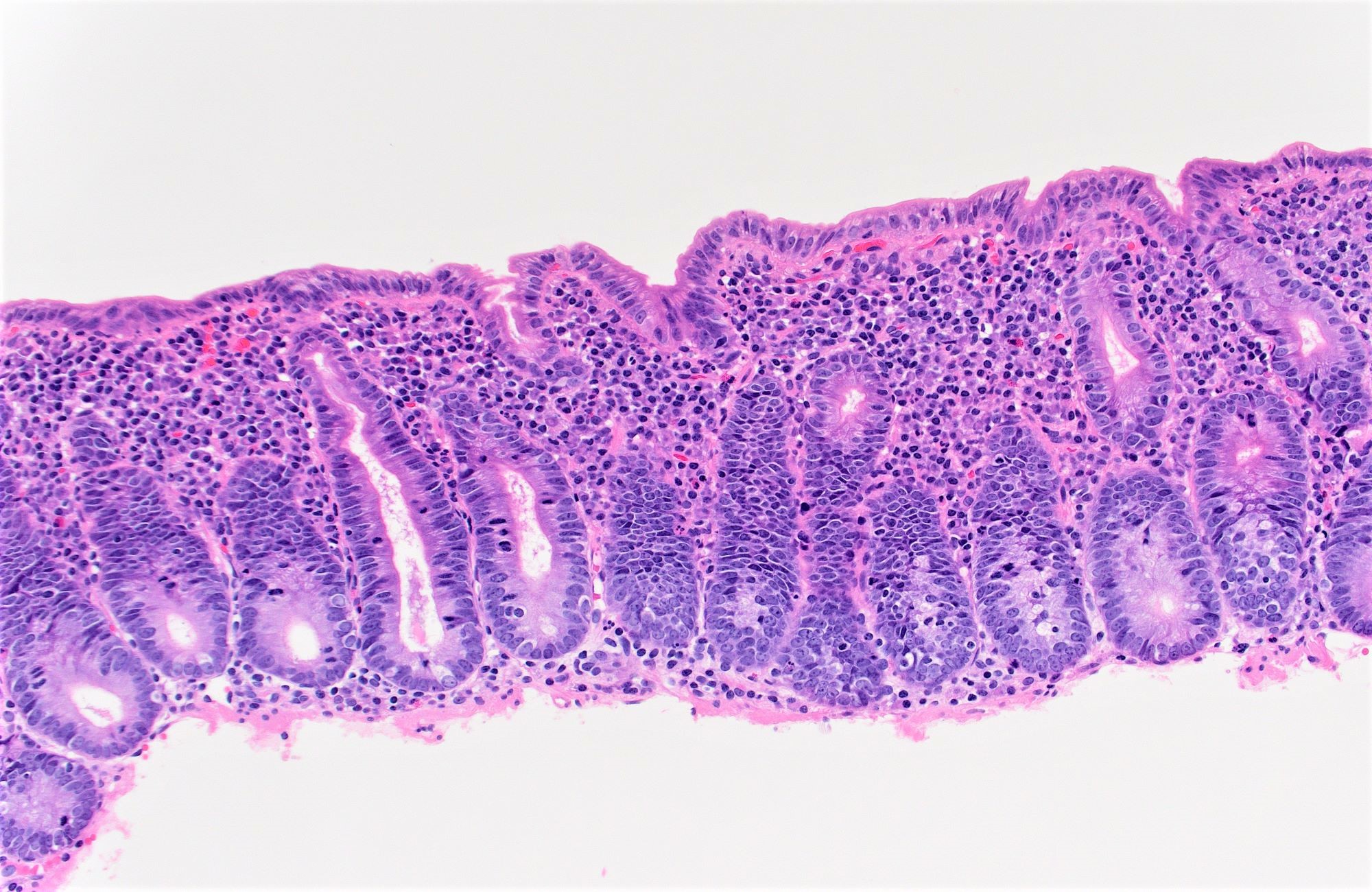
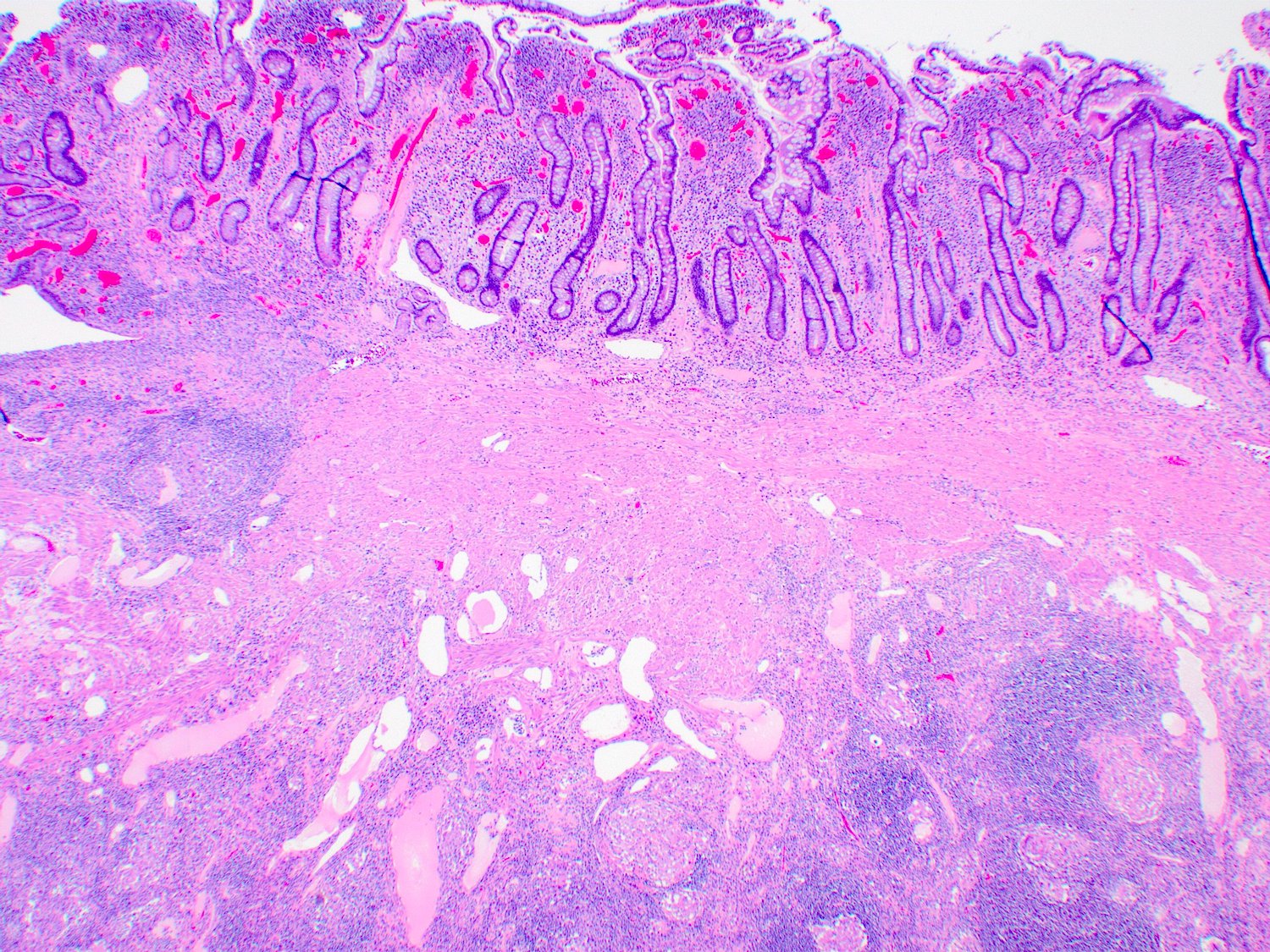
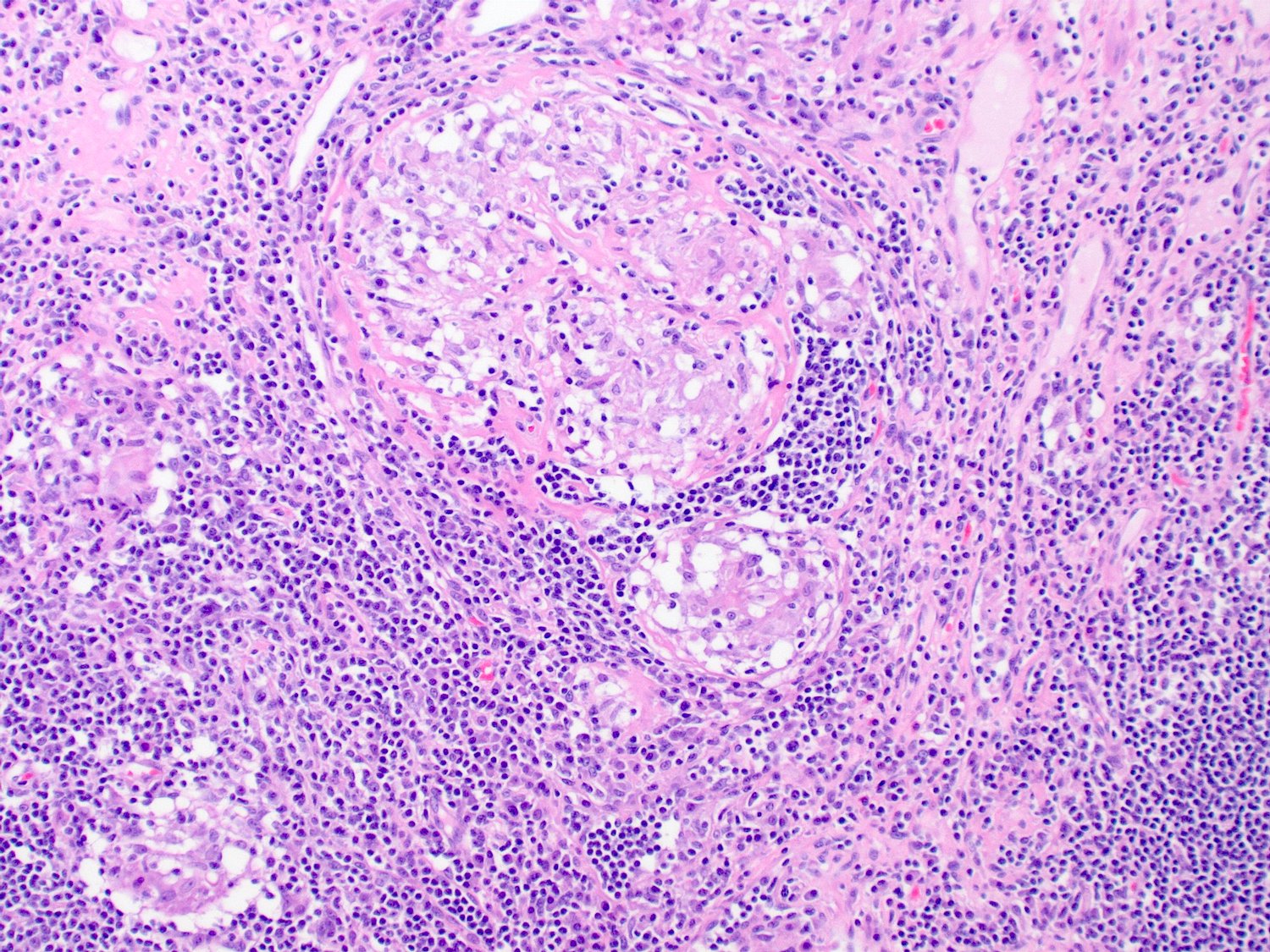
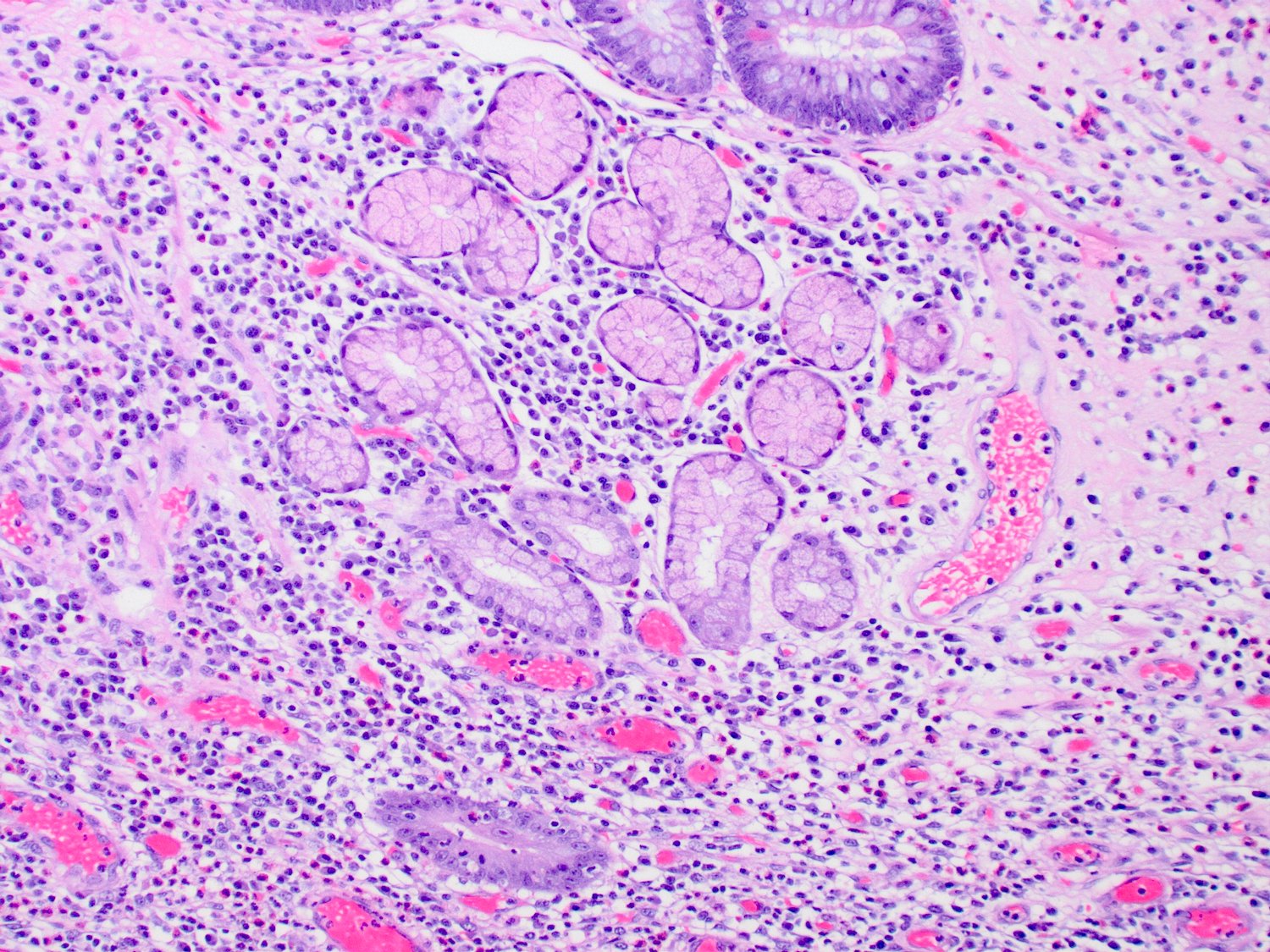
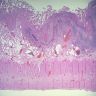
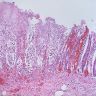
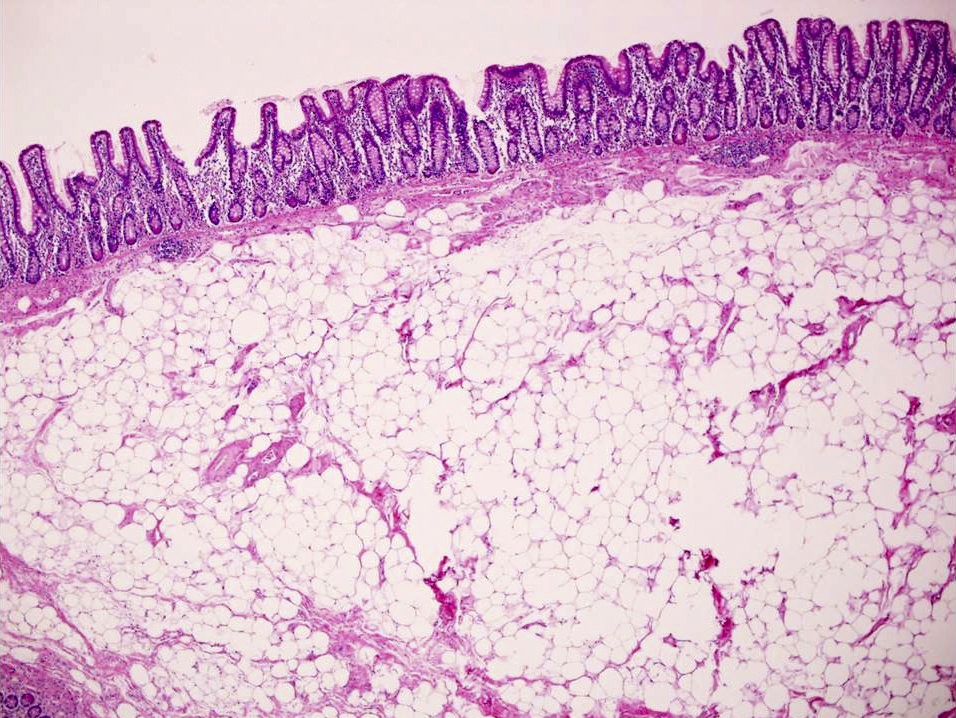
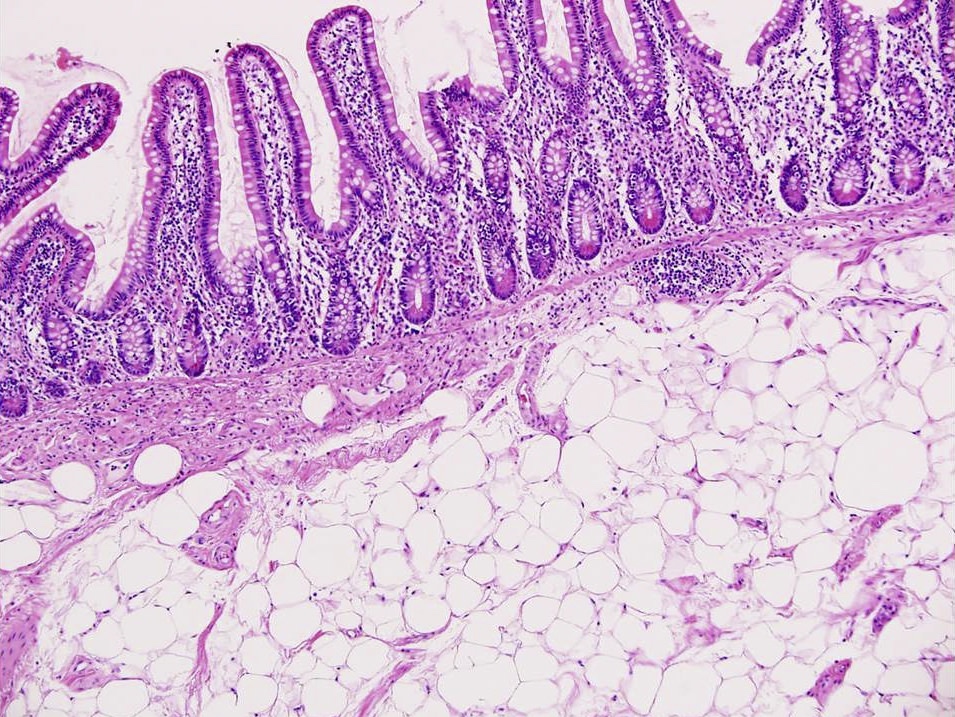
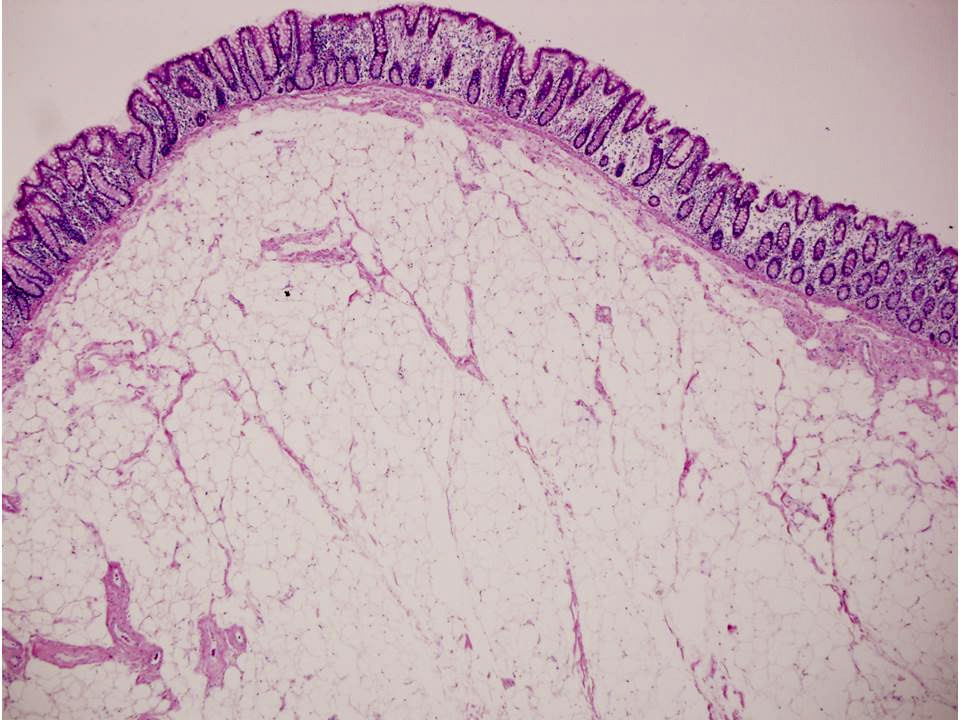
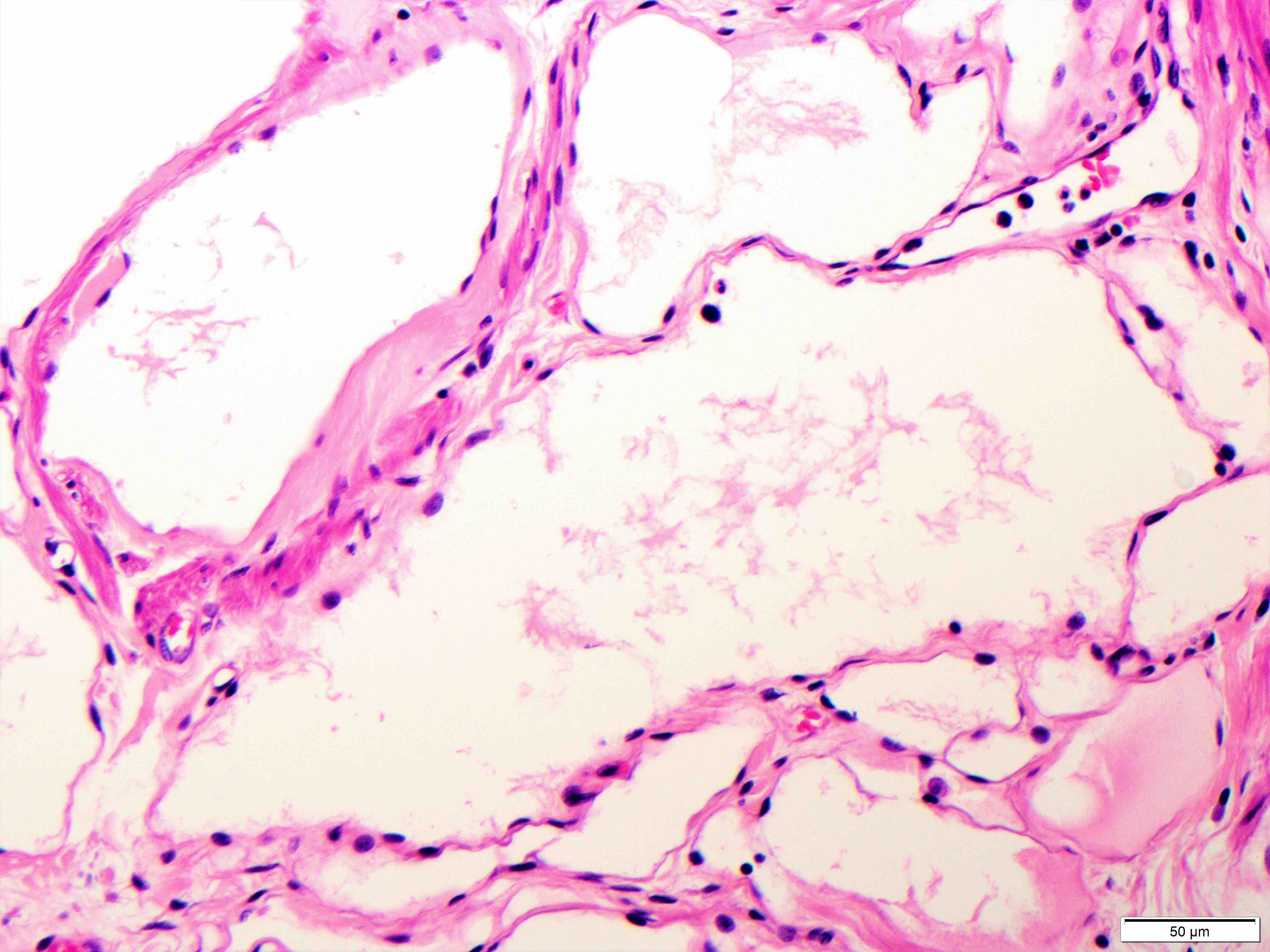
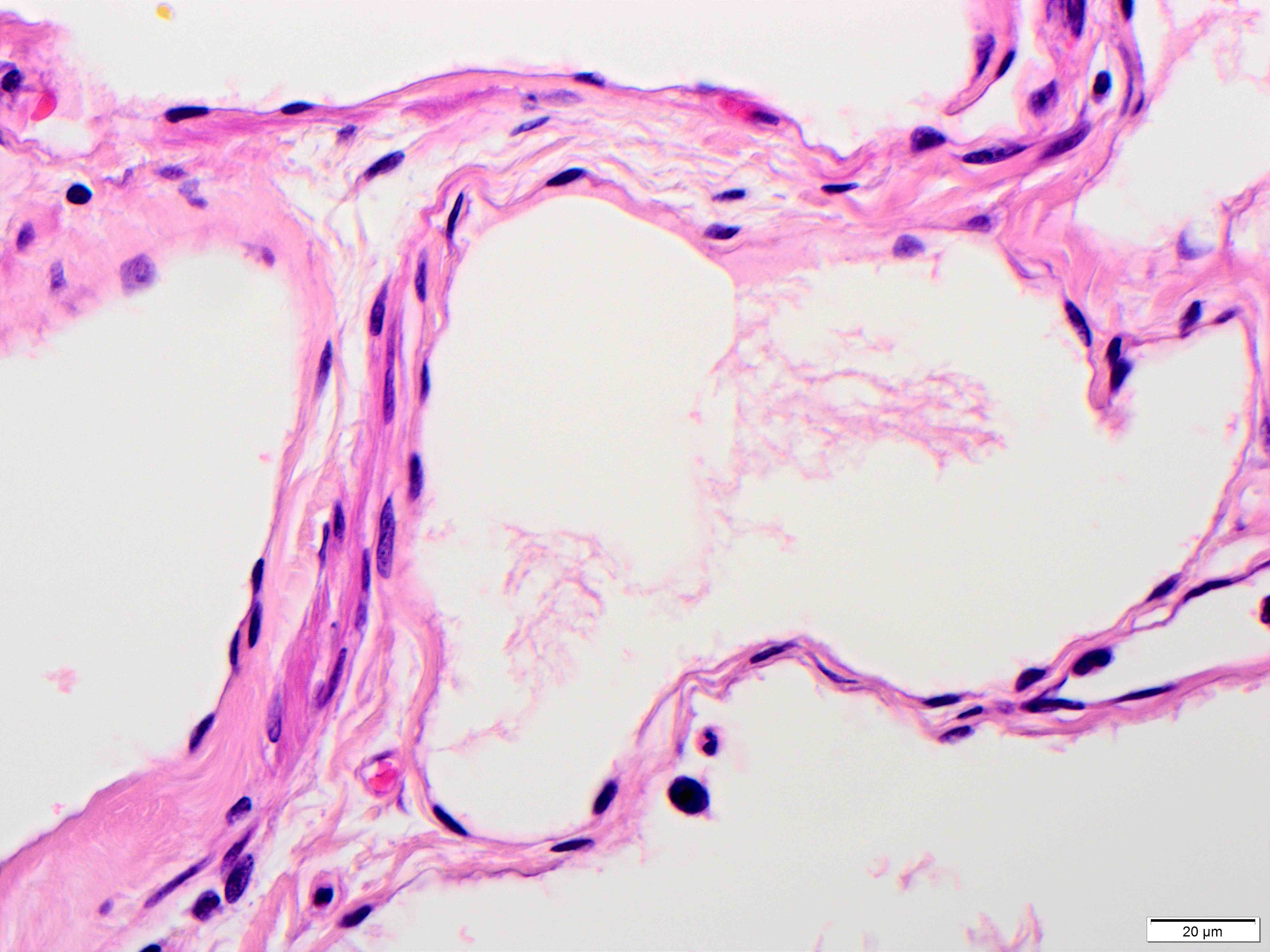
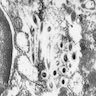
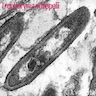
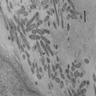
- Brunner gland hyperplasia (BGH) is defined as benign hyperplastic proliferation of mature Brunner glands, commonly in the duodenal bulb
- BGH clinical findings can be nonspecific; endoscopies show a polypoid / pedunculated mass that varies in size
- Histologic features show proliferation of Brunner glands that extends to the submucosa
- Smooth muscle proliferation and mature adipose tissue favors Brunner gland hamartoma
- Brunner gland hamartoma, Brunner gland adenoma and Brunneroma: > 0.5 cm with dilated glands and smooth muscle proliferation (Adv Ther 2021;38:2779, Rev Esp Enferm Dig 2022;114:124)
- ICD-11: DA53.Y - other specified duodenal polyp
- BGH represents < 1% of all gastrointestinal tumors and ~5% of benign duodenal tumors (Int J Clin Exp Pathol 2015;8:7565)
- More prevalent from 50 - 70 years old (World J Gastroenterol 2004;10:2616)
- Risk factors (World J Gastroenterol 2004;10:2616)
- High gastric acid secretion
- Helicobacter pylori infection
- Chronic pancreatitis
- Most common in proximal duodenum (57% in duodenal bulb) (Gastrointest Endosc 1998;47:403)
- Poorly understood
- Hypothesized to be embryonic dysplasia of the duodenum (Brunner gland hamartoma) (JNMA J Nepal Med Assoc 2019;57:50)
- High gastric acid secretion can cause BGH, increasing alkaline mucous secretion (Scand J Gastroenterol 1990;25:165)
- Relationship between H. pylori infection and BHG is not clearly understood
- Majority of cases have unclear etiology
- High acid environment in the duodenum, H. pylori and chronic pancreatitis may cause duodenal mucosal injury and trigger a repair process that includes foveolar metaplasia and BGH (BMC Gastroenterol 2014;14:14)
- Majority of patients are asymptomatic
- Nonspecific symptoms (J Surg Case Rep 2018;2018:rjy305)
- Dyspepsia, nausea and vomiting
- Abdominal pain and distension
- Rare cases present with gastrointestinal bleeding and iron deficiency anemia (especially in hamartomas and large lesions) (Endosc Ultrasound 2015;4:266)
- Few cases present with intestinal obstructions (lesions > 2 cm) (Am J Gastroenterol 1995;90:290)
- Ampullary lesions can present with biliary obstruction (Endoscopy 2000;32:998)
- Upper GI endoscopy (EGD)
- Some lesions (especially in posterior wall of duodenum) can be missed on EGD
- Sensitivity of EGD is 72 - 89% (Eur J Radiol 1993;16:115)
- Evaluates extent, size and origin of the lesion
- Duodenal nodule that can be covered by normal mucosa (Gastrointest Endosc 2006;64:464)
- Can mimic lipoma, endocrine tumor or gastrointestinal stromal tumor (GIST) (Trop Gastroenterol 2010;31:121)
- Radiology (barium Xray and abdominal CT)
- More sensitive for larger lesions (J Comput Assist Tomogr 2010;34:543)
- See Radiology description
- Barium Xray
- Sessile or pedunculated polypoid filling lesions (large Brunner gland hamartomas) (Gastroenterol Hepatol (N Y) 2008;4:473)
- Multiple small filling defects and cobblestone pattern (diffuse nodular BGH) (AJR Am J Roentgenol 2006;187:715)
- CT (Dig Liver Dis 2021;53:134)
- Mass in the duodenum with central low attenuation
- Shows the relationship with adjacent organs
- Overall good prognosis for most lesions
- Very rare cases of BGH have been reported showing dysplasia or invasive carcinoma (2% and 0.3%, respectively) (Am J Surg Pathol 2005;29:1442, J Gastroenterol 2002;37:293)
- 22 year old woman presented with duodenal intussusception due to BGH (Ann Surg 1959;150:160)
- 33 year old woman with large BGH presented with bleeding (Case Rep Surg 2021;2021:8861308)
- 57 year old man with pyloric obstruction due to Brunner gland hamartoma (J Surg Case Rep 2020;2020:rjaa191)
- 60 year old man with BGH mimicking malignant pathology (Int J Surg Case Rep 2021;81:105827)
- Endoscopic or surgical resection
- Pancreaticoduodenectomy has been reported for giant hamartomas and diffuse nodular BGH (J Korean Med Sci 2008;23:540)
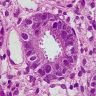
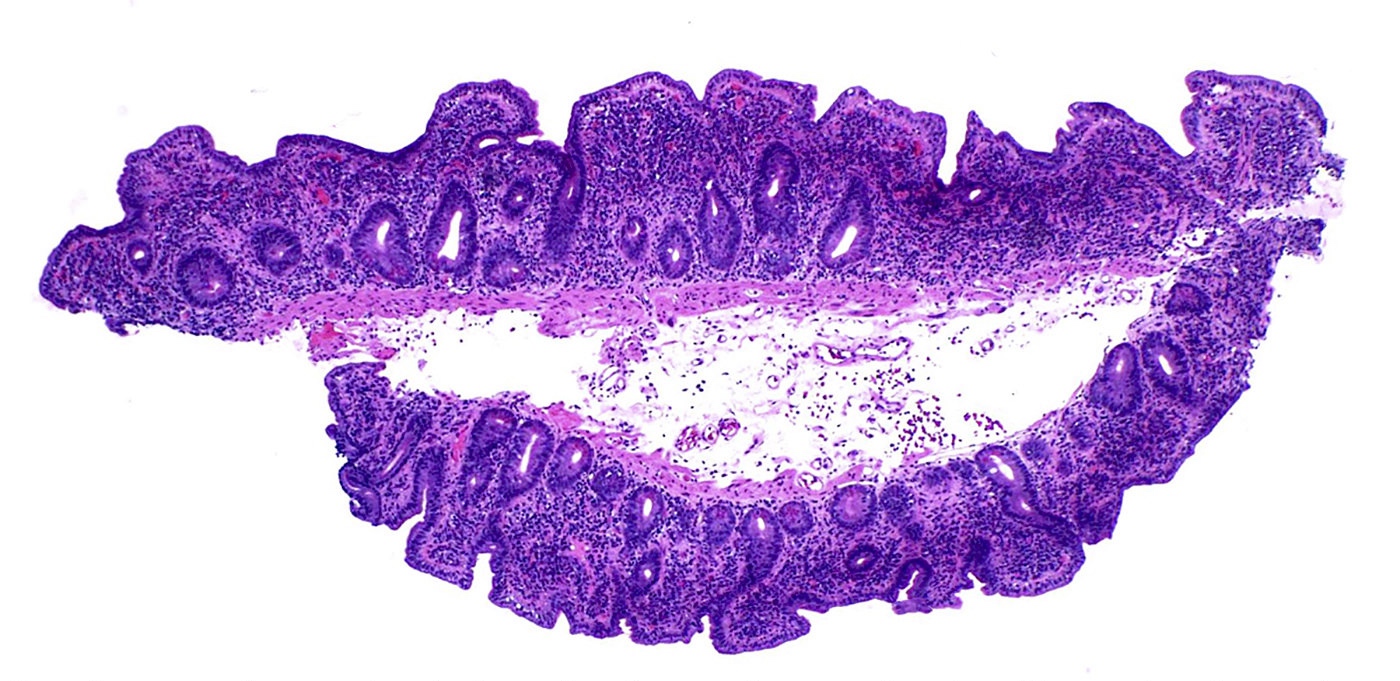
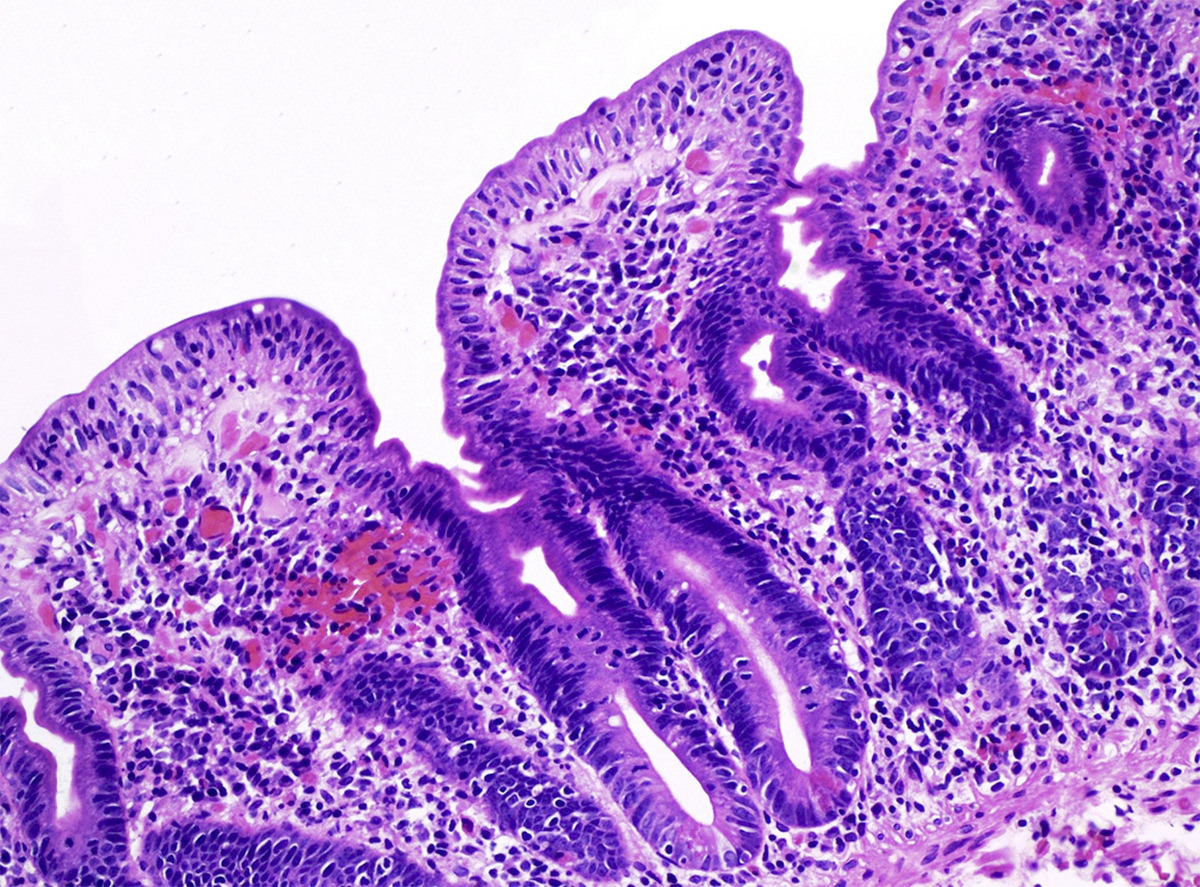
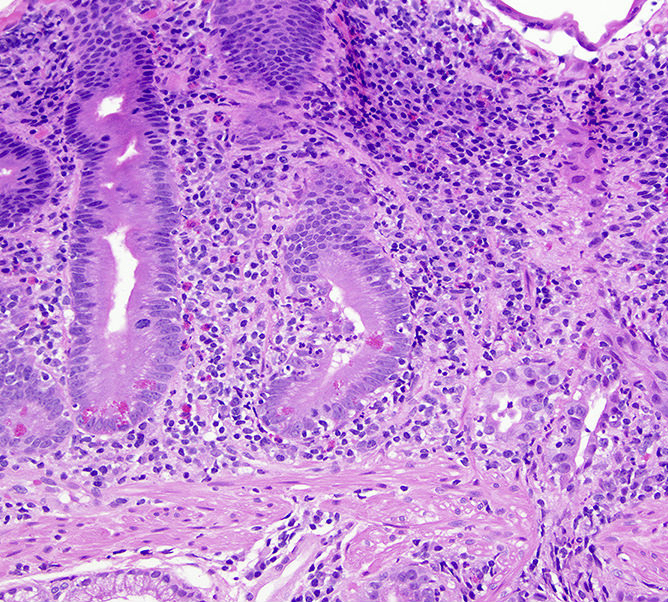
- Brunner gland hyperplasia
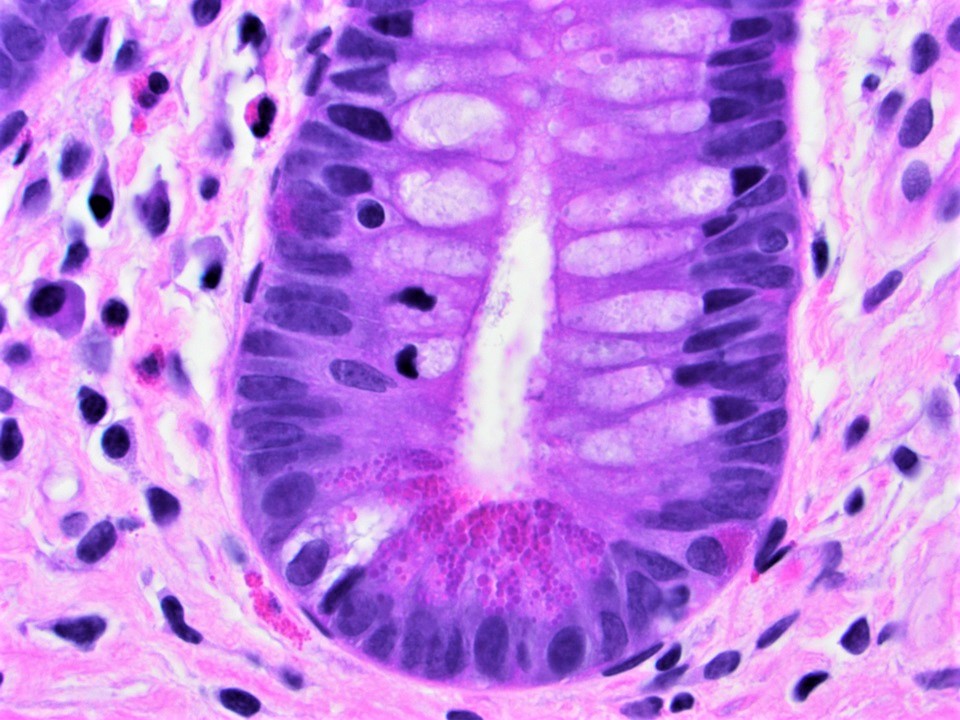
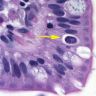
- Closely packed clusters of cuboidal cells with basal round to flat nuclei and foamy cytoplasm with neutral mucin
- Features of peptic duodenitis, foveolar metaplasia or mucosal injury can be seen (Scand J Gastroenterol 1990;25:165)
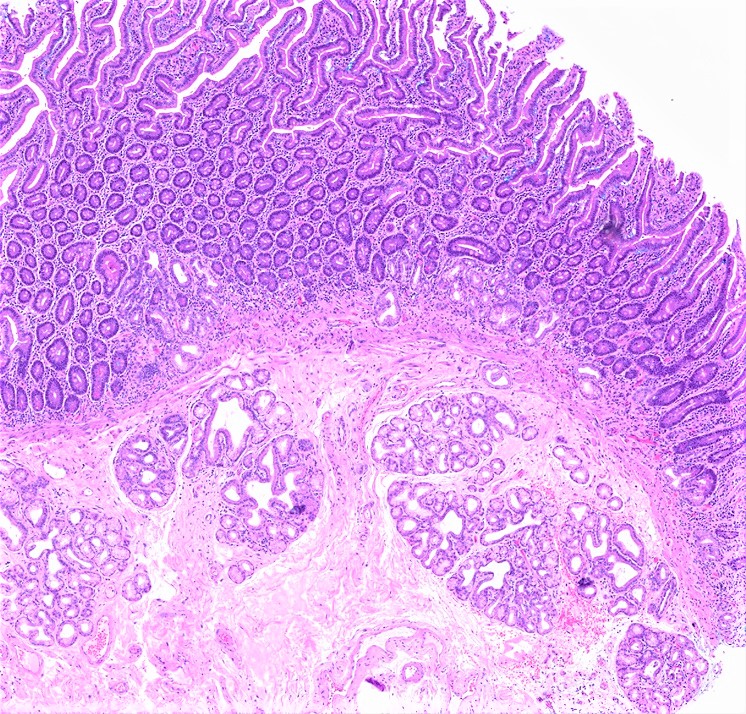
- Brunner gland hamartoma
- Typically, larger size: > 0.5 cm
- Smooth muscle and adipose tissue, intermixed with proliferating Brunner glands and cystically dilated ducts
- Brunner glands can be intermingled with mature adipose tissue / adipocytes
- Brunner gland adenoma
- Similar morphology to Brunner gland hyperplasia or hamartoma, with cytological atypia, nuclear enlargement and mitosis (Int J Clin Exp Pathol 2015;8:7565)
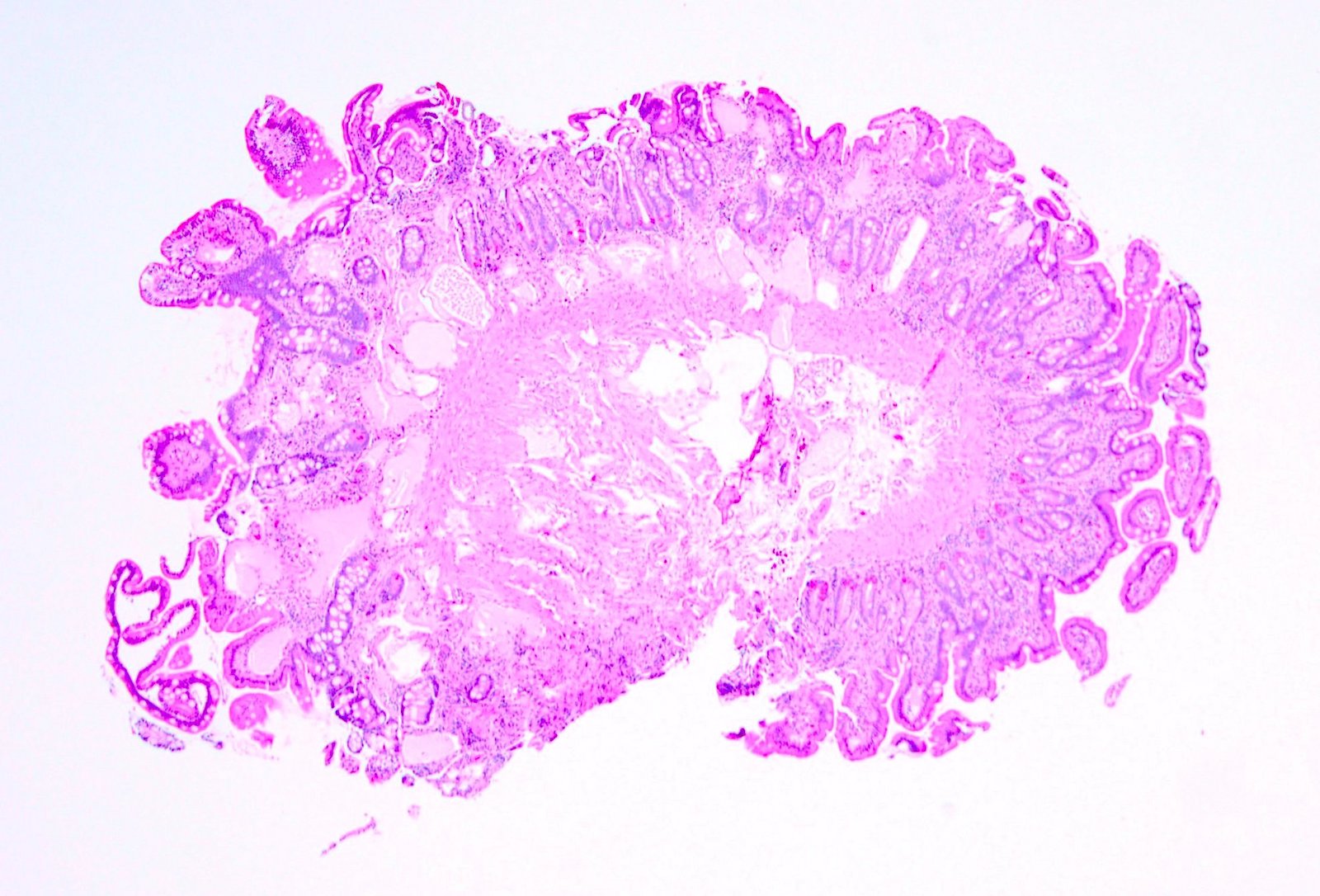
- Duodenum, polyp, biopsy:
- Polypoid duodenal mucosa with prominent Brunner glands consistent with Brunner gland hyperplasia
- Negative for dysplasia or malignancy
- Gastric heterotopia:
- Commonly in first and second parts of duodenum
- Gastric oxyntic glands with foveolar epithelium on the surface
- Pancreatic heterotopia:
- Variable mixture of elements of normal pancreatic tissue including acini, ducts or islet cells
- Neuroendocrine tumors:
- Commonly in first and second parts of duodenum, including the ampulla
- Nests of uniform cells with round to oval nuclei with salt and pepper chromatin
- Tumor cells are positive for chromogranin A and synaptophysin
- Duodenal adenoma:
- Common in the ampullary area
- Tubular or villous architecture with dysplasia of the surface epithelium
- Gastrointestinal stromal tumor (GIST):
- Leiomyoma:
- Incidental finding
- Can be merged with muscularis mucosa
- Positive for smooth muscle actin and desmin
- Lipoma:
- Rare, submucosal
- May be ulcerated
- Needs to be distinguished from fat rich Brunner gland hamartoma
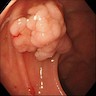
A 52 year old man with history of dyspepsia and abdominal pain. The upper GI endoscopy showed a 0.5 cm polyp in the duodenal bulb. Which of the following is a possible etiology of the findings depicted in the image above?
- Gastric atrophy
- Immunosuppression
- Increased duodenal acidic environment
- Inflammatory bowel disease
Comment Here
Reference: Brunner gland hyperplasia
The figure above is from a duodenal biopsy in a patient with duodenal polyp on upper GI endoscopy. Which of the following, if present, favors a diagnosis of Brunner gland hamartoma over Brunner gland hyperplasia?
- Lesion size < 0.5 cm
- Presence of foveolar metaplasia
- Smooth muscle proliferation
- Superficial lesion limited to the mucosa
Comment Here
Reference: Brunner gland hyperplasia
- Occurs in 1% with carcinoid tumors, 20% with widespread metastases
- Elevated levels of 5-hydroxyindoleacetic acid (5-HIAA), a metabolite, are found in blood and urine
- Normally liver deactivates vasoactive amines (serotonin, histamine, bradykinin, others) released from carcinoid tumors
- Clinical symptoms occur if liver metastases are present or if tumor venous blood flow bypasses the liver
- Vasomotor disturbances (cutaneous flushes, cyanosis of face and anterior chest, intermittent hypertension), palpitations, intestinal hypermotility (nausea, vomiting, diarrhea, cramps)
- Also asthmatic attacks with bronchospasm, fibrosclerosis of AV and tricuspid valves, elastotic sclerosis of mesenteric vessels causing ischemia, dermal sclerosis, hepatomegaly
- Right sided focal or diffuse plaques of thickened valvular or mural endocardium that may extend to the great veins, coronary sinus, pulmonary trunk and main pulmonary arteries
- Tricuspid and pulmonic valves are usually affected by plaque formation
- Left heart and left sided valves are less frequently affected
- Endocardial fibrosis is a reaction to serotonin or kinin peptide exposure
- Plaques are composed of fibroblasts, myofibroblasts and smooth muscle cells embedded in a collagenous matrix, covered by a layer of endothelium
- No elastic tissue is present within the plaque
- Celiac sprue / celiac disease (gluten sensitive enteropathy) is an immune mediated inflammatory disease of the small intestine seen in genetically predisposed individuals and is caused by sensitivity to prolamins, like wheat (gliadin), Barley (hordein), rye (secalin) and oats (avenin)
- Innate and adaptive immune response to prolamins leads to characteristic infiltration of the lamina propria and the epithelium with chronic inflammatory cells and villous atrophy, leading to malabsorption
- Immune mediated disease characterized by intraepithelial lymphocytosis in the small bowel secondary to gluten ingestion
- Partial or total atrophy of intestinal villi and hyperplasia of intestinal crypts with chronic inflammation in the lamina propria
- Serologic testing in combination with biopsy confirmation required for diagnosis
- Celiac disease
- Sprue
- Nontropical sprue
- Gluten sensitive enteropathy
- Gluten induced enteropathy
- ICD-10: K90.0 - celiac disease
- Seroprevalence rate: 1.4% worldwide (1.3% in South America; 1.8% in Asia) (Am J Gastroenterol 2021;116:1148)
- Prevalence of biopsy diagnosed celiac disease: 0.7% worldwide (Gastroenterology 2021;160:63)
- M:F = 1:1.85 (Dig Dis Sci 2018;63:184)
- Mean age at diagnosis is 8.4 years
- Bimodal distribution: first at 8 - 12 months and second in third to fourth decades
- Associated with autoimmune diseases (dermatitis herpetiformis, type 1 diabetes mellitus, Hashimoto thyroiditis, Graves disease, etc.), idiopathic diseases (dilated cardiomyopathy, epilepsy, multiple sclerosis, etc.) and chromosomal diseases (Down syndrome, Turner syndrome and William syndrome) (BMC Med 2019;17:142)
- Small bowel
- HLA class II genes HLA DQ2 and HLA DQ8 on chromosome 6 are implicated in the genetic susceptibility
- Up to 20% of the first degree relatives are affected by the disease indicating strong hereditary component
- HLA molecules on antigen presenting cells present the laminins to CD4+ T cells and activate them
- Environmental trigger is the ingestion of a diet containing gluten
- Fragments of gluten peptides resistant to degradation are transported across the epithelium by transcellular pathways
- Altered intestinal permeability and release of cellular contents, including the enzyme tissue transglutaminase (tTG)
- CD71 (transferrin) receptor is found to be upregulated in active celiac disease
- IFNγ and IL21 are released, which result in epithelial damage following activation of T cells by antigen presenting cells
- Innate immune response by intraepithelial lymphocytes (IELs), which express NK receptors MHC class I related chain A and B and HLE on epithelial cells and lead to destruction of epithelium
- IL15 upregulates NK receptors in cytotoxic intraepithelial lymphocytes and leads to T cell receptor independent killing
- Refractory sprue: where the disease persists despite avoidance of laminins, intraepithelial lymphocytes acquire a highly activated NK phenotype
- 2 types (Gastrointest Endosc Clin N Am 2012;22:639):
- Refractory celiac disease type I:
- Intraepithelial lymphocytes express CD3 and CD8, as well as TCRβ; similar to patients with celiac disease
- Good prognosis when combined with immunosuppressive therapy
- Refractory celiac disease type II:
- Lack CD8, CD4 and TCRαβ
- Have intracellular CD3ε and a clonal TCR gene rearrangement
- Carry dismal prognosis
- Refractory celiac disease type I:
- 2 types (Gastrointest Endosc Clin N Am 2012;22:639):
- Genetic factors: HLA DQ2 and HLA DQ8
- Ingestion of gluten containing diet
- Innate and adaptive immune responses
- Several research studies suggested a reduction in the diversity of gut microbiota and increase in Firmicutes / Bacteroidetes (Adv Nutr 2020;11:160)
Modified Marsh-Oberhuber classification of histologic findings in celiac disease:
| Marsh type | IEL/100 enterocytes: jejunum | IEL/100 enterocytes: duodenum | Crypt hyperplasia | Villi |
| 0 | < 40 | < 30 | Normal | Normal |
| 1 | > 40 | > 30 | Normal | Normal |
| 2 | > 40 | > 30 | Increased | Normal |
| 3a | > 40 | > 30 | Increased | Mild atrophy |
| 3b | > 40 | > 30 | Increased | Marked atrophy |
| 3c | > 40 | > 30 | Increased | Complete atrophy |
| 4 | > 40 | > 30 | Atrophic | Severe (flat) |
Images hosted on other servers:
- Celiac disease in pediatric population is characterized by diarrhea, loss of appetite, abdominal distension and failure to thrive
- Older children: diarrhea, bloating, constipation, abdominal pain or weight loss
- Adults: malabsorption syndrome with chronic diarrhea, weight loss and asthenia
- Extraintestinal manifestations:
- Iron deficiency anemia (microcytic)
- Macrocytic anemia due to vitamin B12 deficiency
- Osteopenia, osteoporosis due to altered absorption of calcium and vitamin D3
- Growth retardation; tooth enamel defects, aphthous stomatitis, hypertransaminasemia
- Dermatitis herpetiformis
- Nonspecific symptoms: headache, paresthesia, neuroinflammation, anxiety and depression
- Changes in reproductive system like late menarche, amenorrhea, recurrent miscarriages, premature birth, early menopause in females and changes in number and mobility of spermatozoa in males
- Refractory celiac disease (RCD) is a persistent malabsorption and villous atrophy despite strict adherence to a gluten free diet for at least 6 - 12 months in the absence of other causes and overt malignancy
- Type I and type II: severe complications like ulcerative jejunitis and enteropathy associated T cell lymphoma is seen in some patients with refractory celiac disease
- Gold standard for diagnosis is serology with confirmation of histology on duodenal biopsy
- Serologic testing: tTGA, EMA and IgA class antigliadin antibodies (AGA) are the serologic tests
- Endoscopy: hallmark endoscopic finding is patchy villous atrophy in the duodenum and is identified by magnification endoscopy or chromoendoscopy
- Scalloping or notching of mucosal folds can be seen
- Sensitivity is low
- For microscopic evaluation, 1 - 2 biopsy specimens should be taken from duodenal bulb and at least 4 specimens should be taken from postbulbar duodenum
- HLA DQ association test: identifies HLA DQ2 and HLA DQ8
- Negative test essentially rules out celiac disease but is also seen in populations without celiac disease
- See diagrams / tables
- tTGA has the highest sensitivity for celiac disease (98%); specificity is 90% (Arch Pathol Lab Med 2012;136:735)
- Positive even in individuals on gluten free diet
- EMA, while having a lower sensitivity, shows an almost absolute specificity (Arch Pathol Lab Med 2012;136:735)
- IgA class antigliadin antibodies (AGA) is now an obsolete test (Arch Pathol Lab Med 2012;136:735)
- Refractory celiac disease type 2 is characterized by loss of multiple surface T cell markers like CD3, CD7 and CD8 in more than 20% of the intraepithelial lymphocytes on flow cytometry
- Flow cytometry: quantification of intraepithelial lymphocytes by flow cytometry (called IEL lymphogram), demonstrates that the IELs are antigen experienced T lymphocytes and bear the αβ (> 90%) and γδ (< 10%) receptors
- In celiac disease, the total IELs are increased, along with a permanent increase in γδ IELs and a decrease in CD3 IELs (Auto Immun Highlights 2016;7:14)
- Fluoroscopy: small intestinal dilatation, dilution of contrast, multiple nonobstructing intussusceptions (coiled spring appearance), moulage sign (dilated jejunal loop with complete loss of jejunal folds) and flocculation (coarse clumps of disintegrated barium)
- CT / MRI: jejunoileal fold pattern reversal (highest specificity); ileal fold thickening, perienteric stranding, submucosal fat deposition in longstanding cases, lymphadenopathy and ascites (Eur J Radiol 2008;65:483)
- Excellent prognosis with gluten free diet
- Refractory celiac disease type 2 is refractory to gluten free diet and treatment
- Poor prognosis with severe complications like ulcerative jejunitis and enteropathy associated T cell lymphoma, with an incidence of 0.06 per 100,000 person years (95% confidence interval: 0.0 - 0.12)
- Enteropathy associated T cell lymphoma is seen within 5 years in refractory celiac disease type 2 (Gut 2010;59:547)
- Secondary enteropathy associated T cell lymphoma is seen in patients with long lasting, well controlled celiac disease
- Enteropathy associated T cell lymphoma observed in 80 - 90% of cases in relation to celiac disease and refractory celiac disease is linked to CD56-, gain of chromosomes 1q qnd 5q
- 5 year survival rate is 8 - 20%, irrespective of subtype of enteropathy associated T cell lymphoma
- Classic type of enteropathy associated T cell lymphoma is most commonly associated with celiac disease
- Higher risk of small bowel adenocarcinoma
- 6 year old girl presenting with celiac disease and aplastic anemia (J Med Case Rep 2018;12:16)
- 9 year old boy with celiac disease and aplastic anemia (Pediatr Dev Pathol 2014;17:470)
- 11 year old girl with complicated primary intestinal lymphangiectasia (Waldmann disease) successfully treated with octreotide (Ann Med Surg (Lond) 2021;68:102588)
- 39 year old man with undifferentiated chronic pulmonary disease, chronic anemia, celiac disease, atrial fibrillation and PLA2R positive membranous nephropathy (Respir Med Case Rep 2021;33:101446)
- 40 year old woman with cerebral arterial and venous sinus thrombosis revealing celiac disease (BMC Gastroenterol 2020;20:327)
- Repletion of nutritional deficiencies
- Prevention of bone loss
- Treatment with sulfones for dermatitis herpetiformis
- Gluten (laminin) free diet
- Transglutaminase 2 inhibitor
- Hepatitis B and pneumococcal vaccination
- Flattening or blunting of villi in the small bowel
- Ulceration may be seen in severe cases
- Histological elementary lesions (Dig Liver Dis 2011;43:S385, Semin Diagn Pathol 2014;31:124):
- Increased intraepithelial T lymphocytes (IEL):
- 25 - 29 IEL/100 enterocytes is considered borderline
- > 30 IEL/100 enterocytes represents a pathological lymphocytosis
- Decreased enterocyte height, flattening of enterocytes, intracytoplasmic vacuolation and reduction or absence of brush border are suggestive but not specific
- Crypt hyperplasia:
- Extension of the regenerative epithelial crypts associated with changes in the presence of more than 1 mitosis per crypt
- Villous atrophy:
- Decrease in villous height, normal villous:crypt ratio (3:1) until total disappearance of villi
- This assessment requires proper orientation of the biopsies
- Increased intraepithelial T lymphocytes (IEL):
- Diagnostic categories are based on these elementary lesions:
- Modified Marsh-Oberhuber classification of histologic findings in celiac disease
- Simplified systems (Corazza & Villanaci or Ensari), which may be more reproducible (Arch Pathol Lab Med 2010;134:826, Pathol Res Pract 2016;212:1174)
- Different grades of duodenal mucosal lesions:
- Grade A / type 1: increased intraepithelial lymphocytes but no villous atrophy
- Grade B1 / type 2: villi still present but shortened
- Grade B2 / type 3: complete villous atrophy
Celiac Disease: A Fairly Advanced Lecture for Primary Healthcare Providers
- Tropical sprue:
- Variable villous blunting and contains more lamina propria eosinophils than celiac disease
- Typically involves proximal duodenum and spares terminal ileum
- Autoimmune enteropathy:
- Marked villous atrophy with apoptosis at bases of crypts and lymphocytes infiltrating the crypts but not surface epithelium
- Goblet cells, paneth cells and endocrine cells may be lost
- Common variable immunodeficiency:
- Apoptotic enterocytes with reduced or absent plasma cells
- Villous blunting and increased intraepithelial lymphocytes are present
- Food allergy:
- Hypersensitivity to food antigens other than gluten, including cows milk, soy protein, fish, rice and chicken
- May also be associated with increased numbers of intraepithelial lymphocytes, as well as architectural changes in the form of patchy or diffuse disease
- Crohn’s disease:
- Neutrophils in lamina propria and epithelium of duodenum with patchy involvement
- Collagenous sprue:
- Patchy, excessive, subepithelial collagen deposits
- Eosinophilic gastroenteritis:
- Dense eosinophilic and mast cell infiltrate
- Inflammatory bowel disease
- HIV enteropathy:
- Increased mitosis in glandular epithelial cells and increased number of apoptotic enterocytes at the surface
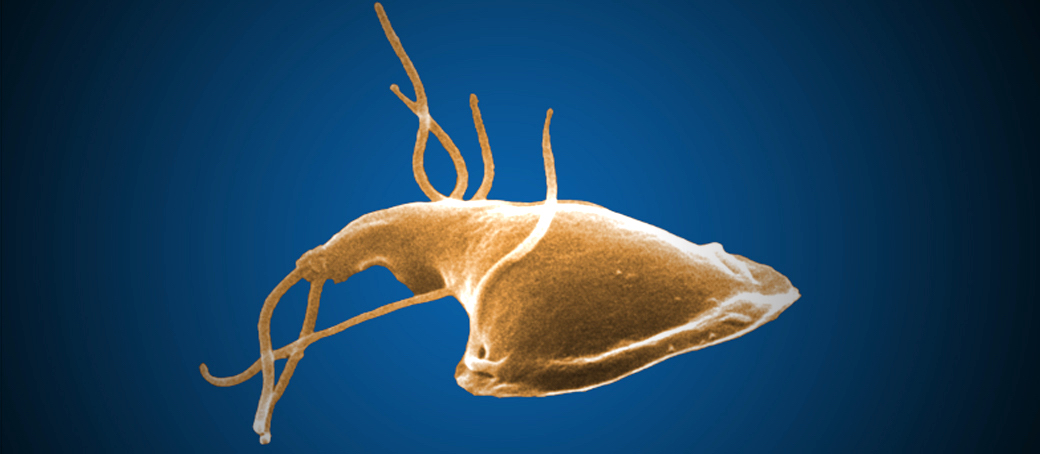
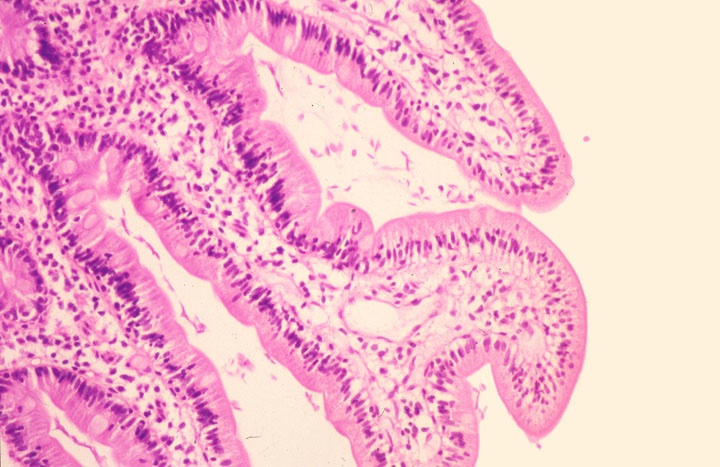
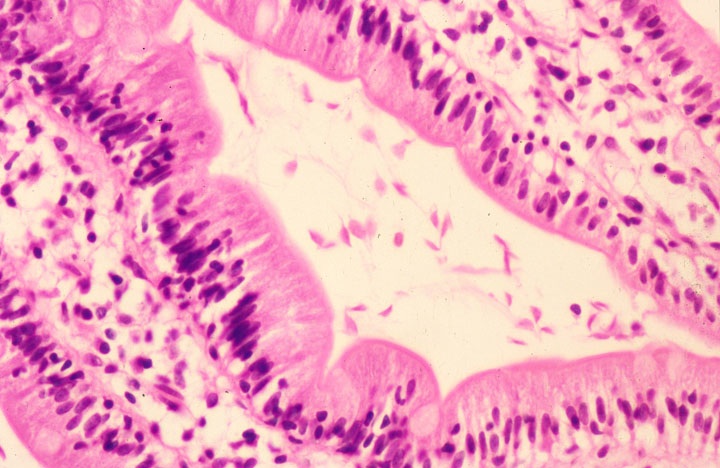
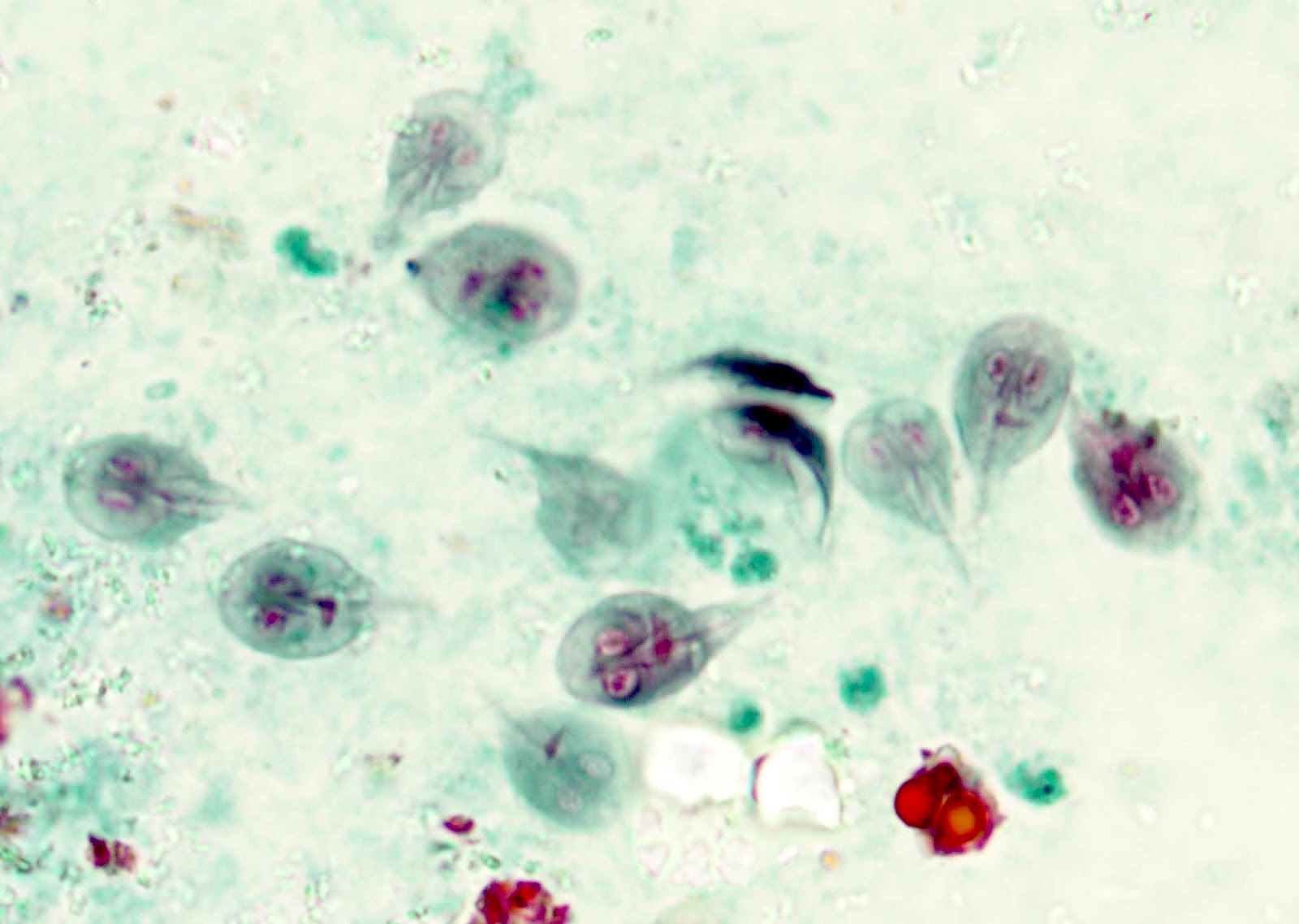
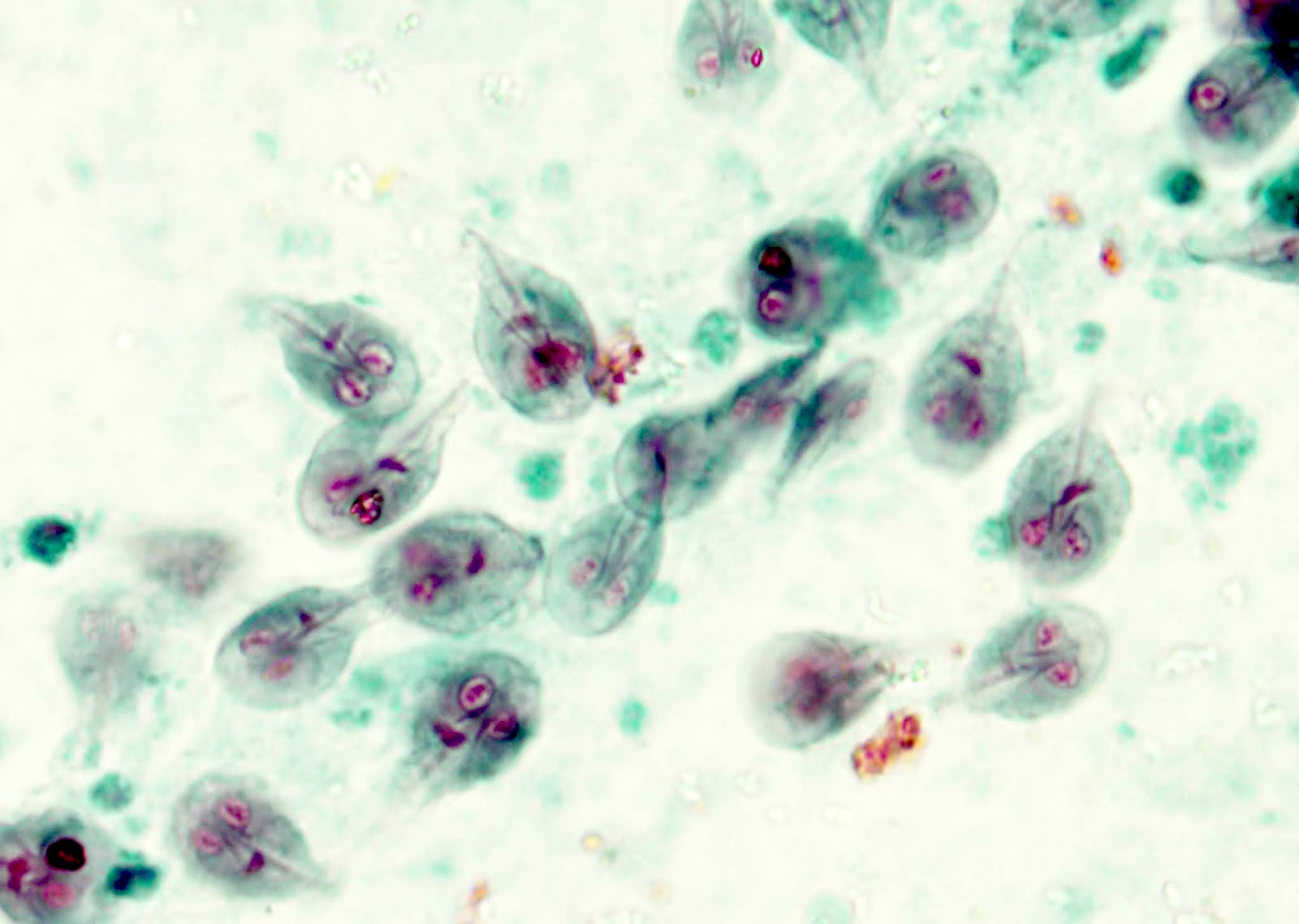
- Giardiasis:
- Variable villous blunting with tear drop shaped organisms with paired nuclei (size similar to enterocyte nuclei)
- Drugs (sartans, mycophenolate, NSAIDs):
- Serum tTg is not elevated
- Intestinal lymphoma:
- Monomorphic lymphocytes with irregular nuclear membranes
Comment Here
Reference: Celiac sprue
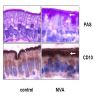
- CD2+, CD3-, CD45- aberrant T cell population
- CD3+, CD7+, CD103+, granzyme B and TCRαβ
- CD3+, T cells with αβ and γδ receptors
- CD4+, CD8+, CD3- T cells
- CD4+, T cells with γε and αδ receptors
- Primary immune defect (usually genetic) causing reduction in B cells and most immunoglobulin classes; also frequent bacterial infections (eMedicine: Common Variable Immunodeficiency [Accessed 16 February 2018])
- Chronic diarrhea, malabsorption, recurrent GI giardiasis
- 73 year old man with nonspecific abdominal pain (Case #398)
- Mucosa may resemble celiac sprue or be normal but always has reduced plasma cells and no IgA plasma cells
- May have lymphoid hyperplasia or apoptotic bodies in crypts
- Histologic patterns: lymphocytic colitis, collagenous enterocolitis, celiac disease, granulomatous disease, acute GVHD and IBD (Am J Surg Pathol 2007;31:1800)
Case #398
Contributed by @RaulSGonzalezMD on Twitter
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
Common variable immunodeficiency syndrome
- Idiopathic chronic inflammatory condition that may involve any part of the upper and lower gastrointestinal tract
- Tends to involve the distal ileum and proximal large intestine
- Diagnostic criterion: segmental disease, transmural inflammation, noncaseating granulomas, deep fissuring ulcers, ileal involvement
- Distal ileum is the most commonly involved part of the small intestine
- Risk of colorectal carcinoma increases with duration and extent of disease
- Inflammatory bowel disease (IBD), Crohn's disease (CD), Crohn's
- ICD-10: K50 - Crohn's disease of the small intestine
- Western counties, with the highest reported prevalence in Europe (322 per 100,000 in Germany) and North America (319 per 100,000 in Canada) (Lancet 2017;390:2769)
- Rising incidence in newly industrialized countries in Africa, Asia and South America (Lancet 2017;390:2769)
- Genetic predisposition through population and family based studies showed Crohn's disease with higher incidence among relatives with Crohn's disease, twins and Jewish population from middle European origin (Gastroenterology 2011;140:1704, Gastroenterology 1989;97:900, Med Clin North Am 1990;74:1)
- Bimodal age distribution, first peak between 15 - 30 years, where the majority of patients are diagnosed; the second peak is between 50 - 80 years of age (Am J Gastroenterol 2006;101:1559, Gastroenterology 1991;100:350, Med Clin North Am 1990;74:1)
- Slightly more common in women (F:M = 1.3:1)
- May occur anywhere in the GI tract, from oral cavity to perianal area
- Terminal ileum is the most commonly affected site (80%), with 33% having ileitis alone
- 50% of patients have ileocolitis (involvement of both terminal ileum and colon)
- About 20% of patients have limited colon disease, of which only half will have rectal sparing
- 33% of patients have perianal disease
- 5 - 15% can have oral gastroduodenal involvement, with significantly fewer patients developing esophageal and proximal small intestinal involvement
- Reference: UpToDate: Clinical Manifestations, Diagnosis, and Prognosis of Crohn Disease in Adults [Accessed 22 October 2021]
- Unclear, as overall pathogenesis remains poorly understood
- Inappropriate immune response has been implicated, with vast literature delineating the role of both host and microbial factors in its pathogenesis (Nat Rev Immunol 2008;8:458)
- Dysregulation in epithelial barriers, immune cells and secreted mediators have been shown to play a role (J Clin Invest 1983;72:142, Gut 2013;62:1734, Gastroenterology 2009;136:1261)
- Microbiota may induce inflammatory bowel disease if a concurrent underlying genetic defect is present (Nature 2012;491:119)
- More than 200 loci have been identified, many of which are shared among both Crohn's disease and ulcerative colitis (UC), indicating the overlap between these 2 entities; these loci lead to modulation of protein expression, rather than amino acid change, which supports that each locus confers an increased risk of developing Crohn's disease (Nature 2012;491:119, Inflamm Bowel Dis 2015;21:1166)
- Idiopathic
- Some associations have been demonstrated:
- Smoking, as shown in meta analysis studies (Mayo Clin Proc 2006;81:1462, Am J Gastroenterol 2012;107:1399)
- Physical activity is inversely related to developing Crohn's disease, with some limited data suggesting exercise can even reduce disease activity (BMJ 2013;347:f6633, Prev Med Rep 2016;3:177, Inflamm Bowel Dis 2015;21:1063)
- Sleep deprivation (Inflamm Bowel Dis 2013;19:2440, Sleep Breath 2020;24:971)
- Infection and immune response with Salmonella and Campylobacter gastroenteritis, as shown in population based cohort studies (Nat Rev Immunol 2008;8:458, Gastroenterology 2009;137:495)
- Mycobacterium paratuberculosis (Expert Opin Biol Ther 2019;19:79)
- Unclear medication association (Am J Gastroenterol 2011;106:2133, Am J Gastroenterol 2014;109:1728, Am J Gastroenterol 2010;105:2610, Lancet Gastroenterol Hepatol 2020;5:986)
- Abdominal pain, diarrhea (bloody or nonbloody), fatigue and weight loss are classic symptoms (Am J Gastroenterol 2000;95:3458, Clin Gastroenterol Hepatol 2006;4:614, Am J Gastroenterol 2018;113:481)
- Patients with distal terminal ileal involvement can present with right lower quadrant abdominal pain
- Distal ileum is the most commonly involved site
- Given the transmural inflammation nature in Crohn's disease, some advanced conditions can present with bowel obstruction due to fibrotic stricture formation
- Extraintestinal manifestations and associations include (Inflamm Bowel Dis 2011;17:471, J Crohns Colitis 2016;10:239, J Crohns Colitis 2019;13:541):
- Joint and bones: arthropathies and osteoporosis
- Eye: iritis, uveitis and episcleritis
- Skin: erythema nodosum, pyoderma gangerosum, Sweet syndrome
- Liver: primary sclerosing cholangitis, cholelithiasis
- Kidney: nephrolithiasis
- Lung: asthma, bronchiectasis, chronic bronchitis, interstitial lung disease, bronchiolitis obliterans with organizing pneumonia, sarcoidosis, necrobiotic lung nodules and pulmonary infiltrates
- Multimodality approach with clinicopathologic correlation and exclusion of other differential diagnoses
- Colonoscopy with ileoscopy, esophagogastroduodenoscopy (EGD) and endoscopic ultrasound are helpful in evaluating Crohn's disease as well as differentiating Crohn's disease from ulcerative colitis (Aliment Pharmacol Ther 2014;39:823)
- Endoscopy also plays a pivotal role in evaluating the severity of the disease and surveillance of neoplasms
- Characteristic endoscopic findings of Crohn's disease include (Med Clin North Am 1990;74:51, Gastrointest Endosc 1977;23:150, Gastrointest Endosc 1984;30:167):
- Aphthous ulcers
- Linear and serpiginous ulcers (giving a cobblestone appearance)
- Skip lesions
- Fistulas and strictures
- Isolated terminal ileum involvement, uninvolved rectum
- May be normal
- High white blood cells, anemia, elevated C reactive protein, electrolyte abnormalities, vitamin D deficiency and vitamin B12 deficiency may be found
- Elevated fecal calprotectin or lactoferrin (stool inflammatory markers), which can be used as a screening tool and determine the need for endoscopy (BMJ 2010;341:c3369)
- Not used as a primary diagnostic tool due to the wide availability of colonoscopy
- Magnetic resonance imaging (MRI), computed tomography (CT) and ultrasonography have shown good diagnostic accuracy by prospective meta analysis studies (Arch Dis Child 1996;74:22, Eur J Radiol 2006;58:140, Gut 2005;54:257)
- Disease course and severity may vary but is usually chronic and intermittent
- 50% of patients experience intestinal complications (strictures, fistula and abscess) 20 years after diagnosis (Intest Res 2015;13:19)
- Involvement of terminal ileum, ileocolonic and upper GI have higher risk of developing complications (Gastroenterology 2010;139:1147)
- Smoking, age < 40 years, perianal or rectal involvement and steroid requiring conditions are risk factors for disease progression (Gastroenterology 2006;130:650, Gut 2012;61:1140)
- Carcinoma may develop in longstanding disease with cumulative risk of 2.9% at 10 years, 5.6% at 20 years and 8.3% at 30 years after Crohn's disease diagnosis (World J Gastroenterol 2014;20:9872)
- 2 most important risk factors: duration and extent of disease
- 24 year old woman with Crohn's terminal ileitis in remission and recurrent ileal invagination (Dtsch Arztebl Int 2020;117:146)
- 41 year old man with Crohn's disease and mesenteric artery thrombosis (Ann Vasc Surg 2019;58:382.e15)
- 62 year old man with duodenal Crohn's disease with associated ampullary stenosis (Dig Dis Sci 2005;50:1118)
- 77 year old man with unusual initial presentation of elderly onset Crohn's disease (Cureus 2020;12:e10173)
- Multidisciplinary approach is recommended, including medications, surgery, nutritional, psychosocial support and cancer screening
- Assessing disease severity using Crohn's Disease Activity Index (CDAI) is helpful in guiding treatment approach (Gastroenterology 1979;77:829, J Pediatr Gastroenterol Nutr 1991;12:439, Gastroenterology 1999;116:527)
- Mild to moderately severe disease / low risk disease: sulfasalazine (colonic CD), budesonide (ileocecal CD) (Am J Gastroenterol 2018;113:481)
- Moderate to severe disease / moderate to high risk disease: corticosteroids, immunomodulators (e.g. azathioprine, 6-mercaptourin, methotrexate), anti-TNF agents (e.g. infliximab, adalimumab, certolizumab pegol), agents targeting leukocyte trafficking, agents targeting IL12 / 23 (anti-p40 antibody) (Am J Gastroenterol 2018;113:481)
- Severe / fulminant disease: intravenous corticosteroids, anti-TNF agents (Am J Gastroenterol 2018;113:481)
- Surgery is reserved for patients with limited disease, failure to response to treatment and complications of Crohn's disease (fistula, stricture, perforation) (Gastroenterology 1999;116:527, J Pediatr Surg 1997;32:1063, J Pediatr Gastroenterol Nutr 2015;60:347, Inflamm Bowel Dis 2013;19:7, Am J Gastroenterol 2018;113:481)
- Surveillance colonoscopy for dysplasia and colorectal carcinoma at 8 - 10 years after onset of symptoms with subsequent surveillance intervals of 1 - 2 years (Gastroenterol Hepatol (N Y) 2017;13:357)
- Discontinuous pattern of inflammation (segmental disease, skip lesions)
- Cobblestone appearance (areas of nonulcerated mucosa separated by deep ulcers)
- Adhesions, creeping fat / fat wrapping, strictures and fistulas
- References: Histopathology 2012;60:1034, Virchows Arch 2014;464:511, Virchows Arch 2018;472:81
- Features of activity: active inflammation with cryptitis, crypt abscess and ulceration
- Features of chronicity: pyloric gland metaplasia, architectural distortion, crypt loss, crypt atrophy, basal lymphoplasmacytosis, fibrosis and stromal hypertrophy
- Skip lesions (portions of normal appearing small intestine with scattered areas of disease)
- Transmural lymphoid aggregates (beading or rosary pattern)
- Noncaseating granulomas (not related to crypt injury)
- Deep fissuring ulcers
- Aphthous ulcers (small ulcers occurring over lymphoid aggregates)
- Obliterative muscularization of submucosa (Arch Pathol Lab Med 2001;125:1331)
- Dysplasia (low or high grade) and carcinoma may be present in patients with longstanding disease
- Note: Some features of Crohn’s disease may also be seen in fulminant, untreated ulcerative colitis, such as aphthous ulcers, deep ulceration with transmural inflammation and superficial fissuring ulcers
- References: Circulation 1965;32:332, Histopathology 2012;60:1034, Virchows Arch 2014;464:511, Virchows Arch 2018;472:81, Mod Pathol 2015;28:S30
- 71 susceptibility loci on 17 chromosomes (Nat Genet 2010;42:1118)
- Nucleotide binding oligodimerization domain 2 (NOD2) or caspase recruitment domain 15 (CARD15) on chromosome 16 (Nature 1996;379:821, Hum Mol Genet 1996;5:1679, Gastroenterology 2000;119:1483, Ann N Y Acad Sci 2006;1072:9)
- Autophagy related 16-like 1 (ATG16L1) and immunity related guanosine triphosphatase family member M (IRGM) gene on chromosome 5 (Nat Genet 2007;39:596, Gut 2008;57:717)
- Genetic polymorphisms in tumor necrosis factor receptors (TNFRSF1A / 1B) with differential treatment response to TNFα inhibitors (BMJ Open Gastroenterol 2019;6:e000246)
- Terminal ileum and cecum, ileocecectomy:
- Segment of terminal ileum with features consistent with history of Crohn's disease, including:
- Moderately to markedly active chronic ileitis with patchy ulcerations
- Fibromuscular stricture with decreased luminal circumference
- Scattered noncaseating granulomas
- No dysplasia identified
- Immunostain for CMV is negative
- Segment of terminal ileum with features consistent with history of Crohn's disease, including:
- Ulcerative colitis:
- Small bowel typically not involved except in case of backwash ileitis
- Absence of granulomas and segmental disease (these features are more specific for CD)
- Typically superficial inflammation with lack of transmural lymphoid aggregates
- Deep or serosal based lymphoid aggregates may be seen adjacent to ulceration in both UC and CD
- Absence of deep fissuring ulceration
- Superficial fissures may be seen in fulminant disease
- Infectious enteritis:
- May show overlapping features of inflammatory bowel disease
- Correlation with microbiologic studies is necessary for exclusion
- Drug associated enteritis (e.g. NSAIDs):
- Patchy active inflammation
- Tends to lack significant features of chronicity
- Behçet disease:
- Form of vasculitis
- Recurrent aphthous stomatitis and genital ulcers may be present
- Ischemia:
- Withering crypts and hyalinization of lamina propria
- References: Ann Gastroenterol 2011;24:271, Curr Gastroenterol Rep 2010;12:249
A 30 year old woman presents with abdominal pain and bloody diarrhea. The patient is refractory to medical management and a resection of the small intestine is performed. What diagnosis is most consistent with the histological findings?
- Backwash ileitis
- Crohn’s disease
- Drug associated enteritis
- Infections enteritis
- Deep fissuring ulceration
- Deep or serosal based lymphoid aggregates adjacent to ulceration
- Granulomas
- Segmental disease
Comment Here
Reference: Crohn's disease
- Small bowel pathology related to NSAID use, first described in 1988 (J Clin Pathol 1988;41:516)
- 46 year old woman with chronic aspirin use (Case of the Week #240)
- Cessation of NSAID use
- Often surgery (Colorectal Dis 2012;14:804) or endoscopic balloon dilation (Gastrointest Endosc 2006;64:1014)
- Segmentation of ileum by incomplete mucosal diaphragms, defined as thin circumferential membranes resembling the plica circularis, composed of mucosa and submucosa with accompanying fibrosis
- Also ulceration, strictures, perforation
- Thin circumferential membranes resembling plica circularis, composed of mucosa and submucosa with accompanying fibrosis
- Neuromuscular and vascular hamartoma-like changes, eosinophilic enteritis and acute inflammation (Am J Clin Pathol 2008;130:518)
- Cryptogenic multifocal ulcerous stenosing enteritis (CMUSE): small intestinal strictures and ulcerations of unknown origin (World J Gastroenterol 2011;17:2873), characterized as atypical vasculitis presenting with unexplained stricture and ulceration of the small bowel in young and middle aged patients but without systemic inflammation; treated with steroids
- Present in 1 - 2%, usually solitary and congenital, may cause obstructive jaundice, pancreatitis, fistulas, hemorrhage, perforation
- Usually penetrate the pancreas
- May project into lumen like a polyp
Jejunal:
- Present in 0.3 - 1.4% of autopsies
- Three times less frequent than duodenal but four times more likely to develop complications
- Usually proximal jejunum along mesenteric border
- Often multiple with thin wall
- Associated with diverticula elsewhere in GI tract
- Some are congenital but most are acquired
- Usually asymptomatic but may cause obstruction, hemorrhage, perforation, abscess, malabsorption or vitamin B12 deficiency, possibly due to bacterial overgrowth in the diverticula
- 88 year old man with acute ulcerative jejunal diverticulitis (World J Gastroenterol 2008;14:6265)
- Corrosive lesion caused by excessive gastric acid secretion, leading to defects in the mucosal surface of the duodenum
- Often results from heavy nonsteroidal anti-inflammatory drug (NSAID) use, Helicobacter pylori infection, gastric acid hypersecretion or stress
- Typically presents with epigastric pain but may be asymptomatic
- Histology shows ulceration and background foveolar metaplasia
- ICD-10: K26 - duodenal ulcer
- Subcodes based on hemorrhage, perforation and chronicity
- Incidence is 1 case per 1,000 person years (Pharmacoepidemiol Drug Saf 2011;20:718)
- 4 times more common than gastric ulcers (StatPearls: Peptic Ulcer Disease [Accessed 1 September 2023])
- M > F (StatPearls: Peptic Ulcer Disease [Accessed 1 September 2023])
- Incidence worldwide is decreasing in recent decades (Transl Res 2021;232:115)
- Most commonly involves the first part of the duodenum (bulb) (> 95%) (StatPearls: Duodenal Ulcer [Accessed 1 September 2023])
- Involvement of other parts of the duodenum is rare, particularly the fourth part
- Duodenal ulcers develop due to a disruption in the equilibrium between aggressive factors (gastric acid) and protective factors (mucus and bicarbonate [HCO3-]) (StatPearls: Duodenal Ulcer [Accessed 1 September 2023])
- Erosion of the mucosal barrier exposes submucosal layers to gastric acid and enzymes
- Most patients have a history of H. pylori infection or heavy NSAID use
- H. pylori (StatPearls: Peptic Ulcer Disease [Accessed 1 September 2023])
- Inhibits somatostatin secretion, leading to increased gastrin secretion, which in turn increases hydrogen ion (H+) secretion and delivery to the duodenum
- Direct spread of H. pylori to the duodenum inhibits duodenal HCO3- secretion, leading to increased acid without adequate neutralization
- NSAIDs (StatPearls: Peptic Ulcer Disease [Accessed 1 September 2023])
- Inhibit COX1 and COX2, which decrease prostaglandin production, leading to erosion of the gastric mucosa (prostaglandins may have a protective effect, supporting blood flow and helping to maintain integrity of the mucosa)
- Decrease mucosal blood flow and mucosal cell proliferation
- H. pylori and NSAID use (see above) promote ulcer formation by interacting with additional risk factors such as smoking, heavy alcohol use, glucocorticoids and caffeine (StatPearls: Duodenal Ulcer [Accessed 1 September 2023])
- Diet, stress and genetic factors also play a role (StatPearls: Peptic Ulcer Disease [Accessed 1 September 2023])
- Rare causes of peptic ulcers include acid hypersecretory states (e.g., gastrinoma), non-NSAID medications (e.g., bisphosphonates), infections (e.g., cytomegalovirus [CMV]) or systemic inflammatory disease (StatPearls: Peptic Ulcer Disease [Accessed 1 September 2023])
- Up to 70% of patients with peptic ulcer disease are asymptomatic (StatPearls: Duodenal Ulcer [Accessed 1 September 2023])
- Characteristic symptoms include
- Dyspepsia: postprandial heaviness, early satiety and epigastric pain
- Epigastric pain described as burning or gnawing, often peaking during fasting periods and improving after meals
- Nausea and vomiting
- Abdominal bloating
- Weight gain due to improved symptoms after a meal
- Can cause occult blood in stool or signs of internal bleeding (e.g., anemia, hematemesis, melena)
- Presentation can vary based on the location and severity of the ulcer, including gastrointestinal bleeding, obstructions, perforation or fistula development
- Suspected in patients with dyspepsia and upper abdominal symptoms with a previous H. pylori diagnosis or a history of NSAID use
- Clinical guidelines for diagnosis exist (Am J Gastroenterol 2017;112:988)
- Patients < 60 years of age without red flags for dyspepsia begin with noninvasive testing for H. pylori (test and treat strategy) as successful eradication reduces unnecessary esophagogastroduodenoscopies (EGDs) and prolonged trials of acid suppression
- Urea breath test
- Stool antigen test
- Patients > 60 years of age, patients with red flags for dyspepsia or patients unresponsive to medical therapy are referred directly to EGD
- Red flags indicating the need for EGD in a younger patient include dysphagia, persistent vomiting, gastrointestinal (GI) bleeding, rapid weight loss and a family history of GI malignancy
- Barium endoscopy is an alternative for patients with contraindications to EGD
- Testing for rare causes is considered in cases of recurrent ulcers or unclear etiology (J Clin Endocrinol Metab 2022;107:e2110)
- Fasting serum gastrin
- Secretin stimulation test
- High levels in gastrinoma
- Parathyroid hormone (PTH) level (Gastroenterol Rep (Oxf) 2018;6:231)
- Testing for systemic inflammatory diseases
- Upper gastrointestinal (UGI) series: well defined, round or oval, lucent area with a crater-like appearance, often surrounded by edema (Insights Imaging 2021;12:94)
- Endoscopy (EGD): ulcer with a smooth base, rounded and regular edges, typically located on the anterior or posterior wall of the duodenal bulb, often accompanied by regular surrounding mucosa
- Double contrast barium meal: provides enhanced visibility of ulcer margins, especially for smaller ulcers (Insights Imaging 2021;12:94)
- Variable depending on the severity of the disease at the time of diagnosis
- Duodenal ulcers caused by H. pylori require treatment to eradicate the infection, with rates of resolution varying
- Duodenal ulcers caused by heavy NSAID use are treated by discontinuing the drug, with a generally favorable prognosis
- Patients with ulcer complications, such as perforation, gastric outlet obstruction and severe ulceration, experience high mortality rates
- Reference: StatPearls: Duodenal Ulcer [Accessed 1 September 2023]
- 12 year old boy with atypical perforated duodenal ulcer (BMJ Case Rep 2014;2014:bcr2014204716)
- 53 year old man with leukemia and perforated duodenal ulcer (J Med Case Rep 2013;7:257)
- 54 year old man with perforation of gastric and duodenal ulcers (J Med Case Rep 2010;4:272)
- Treatment is based on the severity of disease at time of diagnosis
- Lifestyle modifications
- Smoking cessation
- Avoid NSAIDs
- Alcohol reduction
- Stress management
- Acid suppression with proton pump inhibitors (PPIs) or histamine type 2 (H2) receptor antagonists
- Eradication of H. pylori infection using antibiotic regimens
- Triple therapy: 2 antibiotics (amoxicillin and clarithromycin) and 1 PPI (e.g., pantoprazole)
- Effective management involves addressing underlying causes and promoting mucosal healing
- Reference: StatPearls: Duodenal Ulcer [Accessed 1 September 2023]
- Benign peptic ulcer (StatPearls: Peptic Ulcer Disease [Accessed 1 September 2023])
- Smooth base
- Rounded, regular edges
- Regular surrounding mucosa
- Usually located on the anterior or posterior wall of the duodenal bulb
- Malignant ulcer (for comparison) (Radiology 2009;252:410)
- Base reveals an ulcerated mass protruding into the lumen
- Overhanging, irregular edges
- Nodular, irregular mucosa
- Atypical location
- Fibrinopurulent debris and granulation tissue at site of ulceration, extending at least into lamina propria if not into muscularis mucosae and submucosa
- Underlying inflammatory changes
- Severe examples may involve full thickness of wall
- Adjacent duodenal mucosa may show changes of peptic injury, such as foveolar metaplasia, villous blunting and Brunner gland hyperplasia
- H. pylori infection is rare within the duodenum itself and usually occurs in foveolar metaplasia (J Clin Pathol 2021;74:537)
Severe duodenal peptic ulcer
- Duodenum, ulcer, biopsy:
- Fibrinopurulent debris and granulation tissue, consistent with ulceration
- Background duodenal mucosa with foveolar metaplasia, consistent with peptic injury
- Negative for malignancy
- Celiac disease:
- Duodenal mucosa shows villous blunting, which may also occur in the setting of ulceration
- Duodenal mucosa shows increased intraepithelial lymphocytes, which should not occur in the setting of ulceration
- Peptic duodenitis:
- This change can be seen in the background of an ulcer but fibrinopurulent debris and granulation tissue should be absent
- Malignancy:
- Duodenal adenocarcinoma can also cause ulceration
- Microscopic examination reveals malignant glands or single cells
- Endoscopic appearance is also different (see above)
- Crohn's disease:
- May also cause severe duodenal injury, including ulceration
- Lower gastrointestinal involvement should occur
- Duodenal granulomas may be present
- Celiac disease
- Cytomegalovirus (CMV) infection
- Gastrinoma
- Heavy nonsteroidal anti-inflammatory drug (NSAID) use
- Stress
Comment Here
Reference: Duodenal peptic ulcer
Comment Here
Reference: Duodenal peptic ulcer
- Saccular to long, cystic structures which usually have shared muscular wall (so incomplete duplication)
- More common in ileum, near ileocaecal valve; rare in duodenum, where choledochocele (cystic or diverticular dilatation of terminal intramural portion of the common bile duct) is more common
- Present with abdominal mass, pain, vomiting and chronic rectal bleeding
- Associated with gastric heterotopia but not associated with vertebral body abnormalities
- 51 year old man with adenocarcinoma arising in a cystic duplication (World J Surg Oncol 2012;10:55)
- Resect entire duplication and segment of normal bowel attached to it
- Mucosal glands and mucinous cysts in submucosa or deeper layer (J R Soc Med 2004;97:29, Arch Pathol Lab Med 2010;134:378)
- Usually asymptomatic but may cause obstruction or intussuception
- Pathogenesis: herniation, implantation after ulceration, mucosal microdiverticula and reepithelialization of fistulae (e.g. in Crohn's disease)
- Mucosal glands and mucinous cysts in submucosa or deeper (subserosa) layer with hemosiderin, foreign body giant cells and lamina propria around the epithelium
- Found in wall of small bowel, mesentery, posterior mediastinum or rectorectal space
- May be associated with vertebral body abnormalities
- Lined by respiratory, small intestinal or gastric epithelium
- Wall composed of irregularly oriented smooth muscle
- Rare tumor of periampullary region and second part of duodenum
- Duodenal neoplasm that rarely metastasizes
- Triphasic histology (epithelioid, spindle, ganglion type cells) and immunoprofile are characteristic and distinctive
- ICD-10: D13.2 - Benign neoplasm of duodenum
- Almost all arise in the duodenum or around ampulla of Vater
- May very rarely arise elsewhere (such as in the nasopharynx, Arch Pathol Lab Med 2001;125:1098)
- May derive from endodermal neuroectodermal complexes in the embryonic ventral pancreas (Am J Surg Pathol 1985;9:31)
- 15 - 84 years (mean 52 years) (BMC Cancer 2011;11:187)
- M > F (1.5:1) (BMC Cancer 2011;11:187)
- Presents with GI bleeding or abdominal pain but may be incidental finding
- Usually benign and nonfunctional; very rarely produces hormones (J Gastrointest Oncol 2016;7:S107)
- Approximately 7% metastasize to lymph nodes (BMC Cancer 2011;11:187)
- Very rarely metastasizes widely and may cause patient death (World J Clin Cases 2017;5:222)
- May recur if incompletely excised
- Tissue diagnosis required
- Almost always benign but distant metastases can rarely occur
- 47 year old man with widely metastatic gangliocytic paraganglioma (World J Gastroenterol 2014;20:15454)
- 55 year old woman with gangliocytic paraganglioma of the thymus (J Pathol Transl Med 2016;50:165)
- 68 year old woman with gangliocytic paraganglioma metastatic to peripancreatic lymph node (Diagn Pathol 2017;12:57)
- 71 year old man with gangliocytic paraganglioma of the minor papilla (Intern Med 2017;56:1029)
- 73 year old woman with gangliocytic paraganglioma and concurrent ampullary adenocarcinoma (Intern Med 2018;57:2663)
- Endoscopic resection (Gastroenterol Hepatol 2016;39:605)
- Usually 1 - 3 cm, sessile or polypoid, no capsule
- Unencapsulated submucosal lesion
- Triphasic, with epithelioid, spindle cell (Schwann cell-like) and ganglion type cells of varying proportions
- May therefore resemble well differentiated neuroendocrine tumor, paraganglioma or ganglioneuroma
- Variable stromal amyloid (Am J Surg Pathol 1977;1:207)
- All 3 cell types may be visible in aspirated material (Diagn Cytopathol 2013;41:650)
- Epithelioid cells: pancreatic polypeptide, progesterone receptor, somatostatin, synaptophysin, chromogranin, CD56 (BMC Cancer 2015;15:269)
- Ganglion cells: S100, pancreatic polypeptide, somatostatin, synaptophysin, chromogranin, CD56
- Spindle cells: S100, BCL2 (67%)
- Epithelioid cells: S100, p53 (BMC Cancer 2015;15:269)
- Ganglion cells: progesterone receptor, pancytokeratin, estrogen receptor, p53
- Spindle cells: pancreatic polypeptide, progesterone receptor, somatostatin, synaptophysin (25%), chromogranin, CD56, estrogen receptor, p53
- May show HIF2A gain of function mutations (Endocr Relat Cancer 2016;23:L13)
- Ampulla, mass, resection:
- Gangliocytic paraganglioma (2.2 cm) (see comment)
- Margins of resection unremarkable.
- Comment: Immunohistochemical stains for S100 and synaptophysin highlight various components of this uncommon triphasic neoplasm, which is typically indolent but can rarely metastasize.
- Ganglioneuroma:
- Rare in the ampulla / duodenum
- Difficult to distinguish if ganglion cells are predominant in gangliocytic paraganglioma
- Paraganglioma:
- Rare in the ampulla / duodenum
- S100+ sustentacular cells are arranged around nests of epithelioid cells, rather than forming regions of the tumor
- Well differentiated neuroendocrine tumor:
- Can be difficult, depending on sampling
- Progesterone receptor and pancreatic polypeptide staining may help (Arch Pathol Lab Med 2017;141:1309)
- Blister, ganglion and stem
- Epithelioid, ganglion and spindle
- Epithelioid, ganglion and sustentacular
- Epithelioid, stem and spindle
Comment Here
Reference: Gangliocytic paraganglioma
- Protozoan disease caused by Giardia lamblia / Giardia intestinalis, a common parasite that can be detected in small bowel biopsies; it can also be detected in stool
- Clinical presentation ranges from asymptomatic shedding of giardial cysts to symptomatic giardiasis (i.e., malabsorption, chronic diarrhea)
- Protozoan disease with asymptomatic colonization or acute or chronic diarrheal illness; can be histologically confirmed by visualization of the organisms
- Giardia intestinalis
- Giardia duodenalis
- Giardia lamblia
- Beaver fever
- ICD-10: A07.1 - giardiasis (lambliasis)
- Found throughout the world but less common in developed countries (Int J Parasitol 2012;42:443)
- Common in areas with poor sanitation and limited water treatment facilities (Recent Pat Inflamm Allergy Drug Discov 2019;13:134)
- Infection is more common in children than in adults
- Equal in both sexes
- Persons at increased risk are children, travelers to endemic areas, workers and children in daycare settings, individuals with immunodeficiency, cystic fibrosis patients and individuals who practice unprotected oral - anal sex (Recent Pat Inflamm Allergy Drug Discov 2019;13:134)
- Found in the small intestine, classically duodenum
- Jejunum, ileum and stomach are other possible sites (Scand J Gastroenterol 1997;32:48)
- Giardia intestinalis infection causes enterocyte damage and loss of brush border of the epithelial cells of the small intestine, which leads to shortening of microvilli and altered epithelial barrier function (Curr Top Med Chem 2018;18:1287)
- Mucosal alterations cause watery diarrhea, malabsorptive steatorrhea, nausea, abdominal pain, vomiting and weight loss
- Main consequence of Giardia colonization is nutrient malabsorption
- Most infections are asymptomatic
- Causative agent is flagellate protozoan Giardia intestinalis (also known as G. lamblia or G. duodenalis) (Int J Parasitol 2012;42:443)
- Giardia exists in 2 forms: trophozoites and cysts
- Trophozoites: motile, 4 pairs of flagellates, pear shaped organism
- Cysts: elliptical infective form
- Infection is transmitted through feco-oral route, frequently through ingestion of contaminated water and food or person to person transmission through contaminated surfaces or objects (Recent Pat Inflamm Allergy Drug Discov 2019;13:134)
- Most infections are asymptomatic
- Symptomatic infections can present as acute or chronic infection; acute infection manifests as aqueous diarrhea, nausea, vomiting and fever, whereas chronic infection is associated with loose stools, malabsorption and resultant weight loss (Int J Surg Pathol 2021;29:257)
- Main consequence of Giardia colonization is nutrient malabsorption
- Diagnostic test of choice depends on clinical presentation and feasibility
- Demonstration by microscopy of Giardia trophozoites or cysts in stool specimens (sensitivity of 50 - 70%) (J Clin Diagn Res 2014;8:DC04)
- Detection of Giardia antigen in stool (sensitivity of 95 - 100%) (J Clin Diagn Res 2014;8:DC04)
- PCR assay for detecting Giardia in stool
- Histological demonstration of Giardia organisms in small intestinal biopsy; this is most important in chronic subset of patients
- Stool examination: detection of Giardia trophozoites or cysts in stool specimens by microscopic examination of wet mounts and concentrated samples or after preservation with polyvinyl alcohol or 10% formalin for permanent staining with trichrome or iron hematoxylin stains
- Sensitivity of stool test increases to > 90% with 3 serial specimens collected every 2 - 3 days
- Direct fluorescent antibody (DFA) test detects intact organisms
- Enzyme linked immunosorbent assay (ELISA) detects soluble antigens in the stool
- PCR assays can detect specific genes of the parasite in stool samples
- Direct aspiration of the duodenum or use of the commercially available string test (Enterotest)
- Duodenal biopsy (Recent Pat Inflamm Allergy Drug Discov 2019;13:134)
- Overall prognosis is good with treatment
- Dehydration can be life threatening for infants and pregnant women
- Chronic diarrhea might lead to rectal prolapse
- Reference: Recent Pat Inflamm Allergy Drug Discov 2019;13:134
- 31 year old woman with skin lesions due to giardiasis treated with antiparasitic treatment (Ann Agric Environ Med 2005;12:299)
- Woman in her 40s with intestinal pseudo-obstruction caused by Giardia lamblia infection (BMJ Case Rep 2022;15:e252319)
- 66 year old man with an acute respiratory distress syndrome due to SARS-CoV-2 infection and hypereosinophilia due to giardiasis reactivation (Parasitol Int 2021;80:102241)
- Commonly treated by antibiotics such as metronidazole, tinidazole and nitazoxanide
- Symptomatic management, such as avoiding dehydration in case of extensive diarrhea (Int J Surg Pathol 2021;29:257)
- Mostly normal appearing mucosa
- Blunting of villi resembling celiac sprue
- Congestion, erythema, erosion, nodular mucosa, rarely polyps (Int J Surg Pathol 2021;29:257)
- Wide range of histological features
- Range of normal villous architecture to variable villous blunting and expansion
- Increased intraepithelial lymphocytes
- Increased inflammatory cells and prominent lymphoid aggregates in the lamina propria (Int J Surg Pathol 2021;29:257)
- Demonstration of organisms is diagnostic; they float freely in the lumen between the duodenal villi, adhere to the mucosal surface or are located in the cytoplasm of the epithelial cells but not invading (Hum Pathol 2009;40:323)
- Organisms are pear shaped or have a falling leaves appearance and are about the size of an epithelial cell nucleus (often misinterpreted as sloughed epithelial cells or luminal contents), with 2 nuclei, mostly pale eosinophilic to translucent in crescent shaped trophozoites
- If the diagnosis of giardiasis is made, the pathologist should routinely check the lamina propria for plasma cells since chronic giardiasis can be seen with CVID and a paucity of plasma cells could be a clue to this second diagnosis
Contributed by Iresha Vithanage, M.B.B.S., M.D., Saroona Haroon, M.B.B.S., Bobbi Pritt, M.D.,
Emily Fernholz, MLS (ASCP), Suhail Muzaffar, M.B.B.S. and Bilal Karim Siddiqui, M.D. (Case #157)
- Duodenal fluid aspiration cytology shows pear or teardrop shaped organisms containing multiple flagella (Am J Surg Pathol 2017;41:570)
- Brush cytology of the duodenum detected giardiasis in a few cases with negative mucosal biopsy findings along with multiple negative stool examinations
- Trichrome with iron hematoxylin counterstain; methylene blue positively stains the trophozoites; Giemsa stain
- CD117 / KIT immunostain is positive (Hum Pathol 2009;40:323)
Giardiasis tutorial
- Duodenum, biopsy:
- Multiple pear shaped microorganisms seen on villous surface, consistent with Giardia
- Increased intraepithelial lymphocyte count noted
- Celiac disease:
- Strong morphological mimic; no Giardia organisms identified
- Normal duodenal biopsy:
- Sloughed epithelial cells and extruded mucin, common in normal duodenal biopsies, are in the differential diagnosis; however, the morphology will be different
- Deeper part of the small intestinal mucosa is frequently invaded by Giardia
- Duodenal villous architecture can never be normal in this condition
- Giardia organisms are pear shaped
- Giardia organisms cannot be seen by light microscope
- Immunohistochemical studies are mandatory for diagnosis
Comment Here
Reference: Giardia lamblia
A 39 year old man presents to a general physician with malaise, episodic watery diarrhea and mild weight loss for the past 3 months. There is no significant past medical or surgical history. His vitals are stable, apart from mild tachycardia. On upper GI endoscopy, there was duodenal nodularity. Biopsy is taken with the clinical suspicion of celiac disease. The histopathological section image is given above. What is the diagnosis?
- Celiac disease
- Duodenal amebiasis
- Duodenal giardiasis
- Helicobacter pylori infection
- These are nonspecific findings
Comment Here
Reference: Giardia lamblia
- Most common mesenchymal tumor of the gastrointestinal (GI) tract
- Derived from interstitial cells of Cajal
- Small bowel is second most common site of GISTs (stomach is first)
- NF1 and BRAF mutated GISTs are more common in small bowel versus other sites
- Prognosis depends on size, mitotic rate and location
- Gastrointestinal stromal tumor (GIST)
- ICD-10: C49.A3 - gastrointestinal stromal tumor of small intestine
- ICD-11: 2B5B&XH9HQ1 - gastrointestinal stromal tumor
- ICD-O: 8936/3 - gastrointestinal stromal tumor, malignant
- M:F = 1
- Median age is 60 - 65 years; rare in children and young adults
- May present in younger patients in neurofibromatosis type 1 (NF1) lesions (mean age 49 years), Carney triad (childhood), familial (middle age)
- Annual incidence of GIST is between 11 and 14.5 cases per million (Int J Cancer 2005;117:289, Cancer 2005;103:821)
- GISTs account for 1 - 3% of all GI neoplasms
- Occurs anywhere in tubular GI tract
- Small bowel is second most common site
- Stomach (60%) > jejunum and ileum (30%) > duodenum (4 - 5%) > rectum (4%) > colon and appendix (1 - 2%) > esophagus (< 1%) (Semin Diagn Pathol 2006;23:70)
- Extraintestinal GISTs occur in mesentery, omentum, retroperitoneum (Am J Surg Pathol 2005;29:52, Mod Pathol 2000;13:577)
- May represent a metastasis from an unrecognized primary or a detached mass from the GI tract
- Sporadic - vast majority
- Somatic mutations in KIT (70 - 85%), PDGFRA (5 - 10%, mutually exclusive with KIT mutations), NF1 and BRAF (Science 2003;299:708, Cancer Res 2001;61:8118)
- Sporadic tumor syndrome (nonhereditary)
- Carney triad (GIST, paraganglioma, pulmonary chondroma)
- SDH deficient but lacking SDH germline mutations
- Carney triad (GIST, paraganglioma, pulmonary chondroma)
- Autosomal dominant hereditary syndromes (5 - 10% of all GISTs)
- Neurofibromatosis type 1 (NF1) - commonly small bowel and often multifocal
- Germline KIT mutations
- Germline PDGFRA mutations
- Carney-Stratakis syndrome
- Germline mutations in SDHB, SDHC or SDHD subunit (Arch Pathol Lab Med 2020;144:655)
- Unknown, most cases are sporadic
- Small subset of cases can be familial (see Pathophysiology)
- Vague abdominal pain
- Symptoms related to mucosal ulceration, including bleeding (47%) (Ther Clin Risk Manag 2018;14:1467)
- Abdominal mass
- Smaller GISTs are discovered incidentally
- NF1 associated GISTs (Am J Surg Pathol 2006;30:90)
- Patients are more likely to develop multiple independent GISTs
- NF1 tumors have a strong predilection to arise in small bowel
- While NF1 associated GISTs have been estimated to account for only about 1 - 2% of tumors from all anatomic sites, they make up ~4 - 6% of small intestinal GISTs
- Double balloon enteroscopy (89%), CT angiography (71%) and CT (55%) are best imaging modalities (Ther Clin Risk Manag 2018;14:1467)
- Endoscopy with biopsy or fine needle aspiration
- Radiologic findings are variable, depending on size and time of presentation
- CT usually shows a solid, heterogeneous mass (reflecting the presence of hemorrhage or cystic degeneration)
- Endoscopic ultrasound reveals a hypoechoic solid mass
- References: Front Oncol 2021;11:582847, Front Oncol 2021;11:631927
- Prognosis depends on site, size, mitotic activity, molecular profile (Semin Diagn Pathol 2006;23:70)
- Small bowel GISTs are more likely to be malignant (35 - 40%) compared with gastric GISTs (25%) (Am J Surg Pathol 2006;30:477, Am J Surg Pathol 2005;29:52)
- NF1 mutated GISTs are usually grossly small and mitotically inactive, much like spontaneous low grade GISTs of the small intestine
- Their histologically benign appearance is reflected in their commonly nonaggressive clinical behavior
- NF1 associated tumors that do manifest as clinically malignant disease are generally bigger (> 5 cm in greatest dimension) and more proliferative (> 5 mitoses/5 mm2), adhering to the risk stratification provided by standard staging criteria
- 58 year old woman with small bowel GIST presenting as intussusception (GE Port J Gastroenterol 2016;23:279)
- 68 year old woman with small bowel GIST with diffuse omental and mesenteric implants (J Surg Case Rep 2020;2020:rjaa341)
- 74 year old man with small bowel GIST presenting as strangulated inguinal hernia (BMJ Case Rep 2017;2017:bcr2016217273)
- 75 year old woman with NF1 jejunal GIST ( J Surg Case Rep 2018;2018:rjy017)
- Most GISTs are treated with surgical resection
- Imatinib mesylate (Gleevec): tyrosine kinase inhibitor of KIT and PDGFRα
- Metastatic / recurrent / high risk GIST
- Sunitinib malate (Sutent): a tyrosine kinase inhibitor of KIT, PDGFRα, VEGFR
- Used in imatinib resistant GIST
- NF1 mutated GISTs - surgery is treatment of choice as these tumors do not respond to imatinib
- BRAF mutated GISTs do not respond to imatinib and BRAF inhibitors are used for therapy if indicated
- References: Am J Surg Pathol 2015;39:922, Surg Clin North Am 2017;97:437
- Well circumscribed masses of various sizes
- Cut surface may show hemorrhage, cystic change or necrosis
- Usually centered in the muscularis propria
- Small bowel GISTs more frequently present as external masses
- Reference: Am J Surg Pathol 2009;33:1267
- Spindle cell neoplasm
- 3 morphologic types: spindle (70%), epithelioid (20%), mixed (10%)
- Monotonous, bland cells with spindled or epithelioid cytoarchitecture, lightly eosinophilic to pale cytoplasm and vesicular chromatin residing within uniformly ovoid or round nuclei
- In spindle cell GISTs, the pink cytoplasm often has a fibrillary texture with indistinct cell borders
- Can show a degree of nuclear palisading reminiscent of schwannoma
- Small intestinal GISTs are most often spindled
- Epithelioid morphology in small intestinal GIST portends malignant behavior, in contrast to the stomach, where epithelioid morphology is not a significant prognostic feature
- Small intestine GISTs more commonly feature skeinoid fibers, stromal PAS positive globules of curved collagen fibrils, which, when present in a small intestinal tumor, correlate with less malignant clinical behavior
- Treated tumors can display a widely variable histomorphology that may significantly differ from treatment naive tumors
- Tumor may be necrotic or hypocellular; the stromal component, which may be hyalinized or calcified, becomes prominent
- Cytomorphology of the tumor cells can change, usually adopting a more epithelioid and even anaplastic or sarcomatoid appearance
- Histologic grade
- G1: low grade, mitoses < 5/5 mm2
- G2: High grade, mitoses > 5/5 mm2
- References: Am J Surg Pathol 2017;41:577, Arch Pathol Lab Med 2020;144:655
- Bland spindle and sometimes epithelioid cells
- EUS-FNA with IHC staining of cell block can lead to diagnosis
- If not enough material and large tumor size, there might not be enough for genotyping to guide neoadjuvant therapy
- Without enough tissue to perform immunohistochemical stains, it might be hard to get to a definitive diagnosis
- Reference: Diagn Cytopathol 2021 May 18 [Epub ahead of print]
- S100
- Desmin
- CK AE1 / AE3
- SMA - 34% in small bowel GISTs (Am J Surg Pathol 2006;30:477)
- NF1 (loss of expression of NF1; antibody specific to C terminus) - may help in identifying NF1 associated GISTs (Mod Pathol 2018;31:160)
- See Pathophysiology
Small bowel GIST
- Duodenum, resection:
- Gastrointestinal stromal tumor (GIST), spindle cell type, 3.0 cm, grade 1 (see synoptic report for risk stratification as well as biomarker report)
- Low grade spindle GISTs
- High grade spindle GISTs
- Leiomyosarcoma:
- Dedifferentiated liposarcoma:
- Spindle cell carcinoma:
- Positive for pancytokeratin and negative for CD117 and DOG1
Which of the following is true regarding gastrointestinal stromal tumors in NF1 patients?
- Always associated with KIT mutation
- Epithelioid in morphology with loss of SDHB immunostaining by immunohistochemistry
- More aggressive than sporadic GISTs and present with metastasis
- Tumor can be multiple, is spindle cell in morphology, located in small intestine and indolent in behavior
Comment Here
Reference: Gastrointestinal stromal tumor (GIST)
- BRAF
- IDH
- KRAS
- SDH
- Ink pancreatic duct and common bile duct margins, also retroperitoneal margin
- Probe common bile duct and pancreatic duct, and section along plane of both probes
- Should examine at least 12 lymph nodes in a pancreaticoduodenectomy specimen
- Tumor location
- Histologic type
- Histologic grade if relevant (well differentiated: > 95% glands; moderately: 50 - 95% glands; poorly: 5 - 49% glands; undifferentiated: < 5% glands)
- Tumor size
- Extent / depth of invasion
- Margin involvement (proximal, distal, radial [soft tissue closest to deepest tumor penetration]) and distance of tumor to margin
- Invasion of other structures
- Obstruction, perforation, ulceration, proximal dilation
- Nuclear grade, presence of necrosis
- Angiolymphatic invasion
- Other pathologic findings (polyps, Crohn's disease, celiac disease)
- Nodal involvement (number involved, number identified)
- Results of special studies
- Specimen type
- Tumor site
- Presumed tumor origin (intraampullary, periampullary duodenum, mixed, common bile duct, pancreatic)
- Histologic type
- Histologic grade (well, moderate, poor or undifferentiated)
- Tumor size (invasive carcinoma component)
- Depth of invasion, local extension
- Margins - invasive carcinoma; distance to closest margin and identify margin (recommended to ink posterior retroperitoneal surface of pancreas and submit sections of tumor closest to this margin)
- Margins - carcinoma in situ; involvement of pancreatic duct or common bile duct margin
- Margins - PanIn
- Pathologic staging
- Perineural invasion (for resections)
- Angiolymphatic invasion (for resections)
- Lymph nodes
- Additional findings: PanIN / dysplasia, adenoma, inflammation, adenomyosis, pancreatitis, gastritis, other
- Mature gastric tissue found outside the stomach
- Gastric fundic heterotopia represents a developmental anomaly and is frequently associated with Meckel diverticulum
- Developmental anomaly characterized by ectopic gastric mucosa outside the stomach
- Histologically composed of foveolar epithelium with specialized antral glands with or without oxyntic mucosa
- Clinical features can vary from asymptomatic to abdominal pain, obstruction, intussusception, perforation, palpable mass, ulceration, stricture, etc., depending on the site
- Treatment is surgical removal of ectopic mucosa for symptomatic lesions
- Heterotopic gastric mucosa (HGM) or ectopic gastric mucosa
- Incidence in the duodenum varies from 0.5 to 14.3% (Pathol Res Pract 2011;207:148)
- Can be found in oral cavity, esophagus, duodenum, jejunum, ileum, rectum, anal canal, gallbladder and bile duct
- Occurrence in the small intestine is rare unless associated with remnants of Meckel diverticulum (Case Rep Gastroenterol 2020;14:609)
- Commonly occurs in the first and second portion of the duodenum (Int J Clin Exp Pathol 2012;5:46)
- Error in the differentiation of pluripotent primitive endoderm stem cells could lead to the gastric mucosa being present anywhere throughout the gastrointestinal tract
- CDX2 stimulates markers of enterocyte differentiation; null mutation of CDX2 can lead to the development of ectopic lesions with a gastric phenotype in the midgut endoderm (J Cell Biol 1997;139:1553)
- Mainly a congenital malformation
- Symptoms caused by gastric heterotopia depend on location, as well as size of the heterotopic tissue
- In the majority of cases, patients are asymptomatic due to the small size of the lesion and diagnosis is made incidentally on endoscopy performed for other indications
- In both small and large intestines, it can give rise to abdominal pain, obstruction, intussusception, perforation, palpable mass, ulceration, stricture, bleeding per rectum and anemia (J Pediatr Surg 2008;43:e19)
- These heterotopic lesions cannot be accurately diagnosed by imaging or endoscopic visualization due to lack of specific features, rendering histopathologic examination as the gold standard for precise diagnosis
- CT scan, fluoroscopy, capsule endoscopy and 99mTc pertechnetate scan can usually detect heterotopic gastric mucosa
- Capsule endoscopies have been shown to be superior compared with other radiological investigations, including barium contrast studies, computed tomographic enteroclysis, push enteroscopy and MRI
- Reference: Medicine (Baltimore) 2017;96:e5854
- Patients with extensive heterotopia are at increased risk of adverse outcome, including massive hemorrhage or rarely death (Pediatr Dev Pathol 2000;3:277)
- 33 year old man with heterotopic gastric mucosa in ileum (Case Rep Gastroenterol 2020;14:609)
- 38 year old woman with gastric heterotopia (Case Rep Gastroenterol 2015;9:233)
- 61 year old man with pancreatic and gastric heterotopia (Case Rep Gastrointest Med 2017;2017:3126108)
- Surgery is the treatment of choice, whether it is carried out by laparoscopy or laparotomy
- Intraoperative gamma counter use has also been reported as a useful method of localizing bowel segments with nonpalpable areas of heterotopic gastric mucosa for resection (J Pediatr Surg 2001;36:1720)
- Can present as a polypoidal lesion leading to intestinal obstruction, intussusception
- Lesions consist of full mucosal thickness of specialized gastric glands characterized by chief and parietal cells and surface lined by gastric foveolar epithelium
- Histologically, oxyntic mucosa may not be seen in all cases of gastric heterotopia
- Prevalence and risk of malignant transformation of heterotopic gastric mucosa are not entirely understood (J Pediatr Gastroenterol Nutr 2010;50:329)
- Some reports have described heterotopic gastric mucosa progressing to malignancy in the esophagus, gallbladder and rectum (Korean J Pathol 2013;47:289)
- Presence of H. pylori organism in heterotopic gastric mucosa in Meckel diverticulum is a rare histological finding; in the last 22 years, 12 published reports were found that specifically looked into the presence of H. pylori in a heterotopic gastric mucosa in a Meckel diverticulum (Clin Med Insights Case Rep 2019;12:1179547619846088)
- Altered expression of cell cycle molecules (the CIP / KIP family) can be associated with heterotopic gastric mucosa (Ann Gastroenterol 2013;26:184)
- Polyp, duodenum, polypectomy:
- Consistent with gastric heterotopic mucosa
- Gastric metaplasia:
- Characterized by gastric foveolar epithelium and absence of specialized antral glands seen in heterotopic gastric mucosa
- Always a sequelae of chronic inflammation
- Can be associated with null mutation in CDX2 gene
- Microscopy shows gastric foveolar epithelium with absence of specialized gastric glands
- No malignant potential
Comment Here
Reference: Heterotopic gastric mucosa
Comment Here
Reference: Heterotopic gastric mucosa
- Also called adenomyoma, myoepithelial hamartoma (although without pancreatic tissue)
- Incidence of 1 - 14%, affects all ages but peaks in 4 - 6th decade
- Most common near ampulla of Vater; also stomach, jejunum
- May cause blockage of duct, leading to infection, cystic dilation and fat necrosis
- Usually incidental finding at surgery submitted for frozen section but carcinoma may arise from pancreatic heterotopia (Arch Pathol Lab Med 1999;123:707)
- Submucosal nodule, intramural mass
- Yellowish white, lobulated, 0.2 - 4 cm
- May have central mucosal dimple
- Widely separated pancreatic acini with minimally developed ducts, well formed acini may be seen (JOP 2007;8:588, Mod Pathol 2003;16:530)
- Pancreatic heterotopia may be total, only ducts, only acinar cells (exocrine heterotopia), only islet cells (endocrine heterotopia)
- Adenomyoma: predominance of pancreatic ducts with proliferation of thick smooth muscle bundles of the muscularis around the ducts (seen in periampullary region of the duodenum)
- Cystic change in pancreatic heterotopia from duplication
- Neuroendocrine tumor from endocrine heterotopia
- Well differentiated adenocarcinoma
- Formed by the union of the distal pancreatic duct and the intrapancreatic distal common bile duct
- Opens into the major duodenal papilla
- Complex anatomical structure generally lined by pancreatobiliary-type cells
- Ampullary mucosa forms prominent papillary folds
- Epithelium transitions from intestinal type (duodenal surface) to foveolar-like mucosa with occasional goblet cells (papilla) to pancreatobiliary epithelium (distal ducts) (Am J Surg Pathol 2012;36:1592)
- Lamina propria has only occasional lymphocytes, plasma cells and mast cells
- Small mucous glands / ductules underlie the mucosa
- Smooth muscle fibers (representing sphincter of Oddi) may extend into epithelial folds
- May have adjacent pancreatic acini, but usually no islets around major papillae
- Ampullary biopsy performed in autoimmune pancreatitis can help to differentiate from other "mass forming" pancreatitis by IgG4+ to IgG+ ratio (Am J Surg Pathol 2008;32:1770)
- Adenocarcinoma:
- Submucosal ducts / glands may appear malignant, especially if crushed or secondarily involved by an adenoma
- Which of the following may be seen or around in normal ampullary mucosa?
- Ciliated epithelium
- Islets of Langerhans
- Pancreatic acini
- Squamous epithelium
- Extends from gastric pylorus to ileocecal valve
- Layers include mucosa, submucosa, muscularis propria (externa), subserosa and serosa (described luminal to external)
- Functions relies on the structure of mucosal villi and crypts, lined by columnar cells
- Extends from gastric pylorus to ileocecal valve
- Composed of duodenum, jejunum and ileum
- Layers include mucosa, submucosa, muscularis propria (externa), subserosa and serosa (described luminal to external)
- Villi lined by columnar absorptive cells and goblet cells
- Crypts comprise the lower portion of mucosa and contain Paneth cells, endocrine cells and undifferentiated (immature) crypt cells
- Small bowel
- Duodenum, jejunum, ileum
- Villi are the location for digestion and absorption of food into the columnar cells (Gastrointest Endosc Clin N Am 2017;27:1)
- Crypts secrete ions and water and deliver IgA and antimicrobial peptides to the lumen
- Cell division and renewal occur in crypts
- Mucous cells generate adherent mucous layer that protects the epithelium and allows uptake of nutrients
- Terminal ileum absorbs intrinsic factor vitamin B12 complexes
- Small intestine is approximately 6 - 7 m in length (Gastrointest Endosc Clin N Am 2017;27:1)
- Duodenum:
- Retroperitoneal, except for first part
- Common bile duct and pancreatic duct enter the second part of the duodenum at the ampulla of Vater
- Suspensory duodenal ligament (ligament of Treitz) divides duodenum from jejunum
- Jejunum and ileum:
- Intraperitoneal
- Transition between the jejunum and ileum is not clearly defined
- Wall of jejunum is thicker due to prominent circular mucosal folds (folds of Kerckring / plicae circulares) (Gastrointest Endosc Clin N Am 2017;27:1)
- Small intestine ends at the ileocecal valve
- Mucosa:
- Contains villi (finger-like projections) with central blood vessels, lymphatics
- Layers are epithelium, lamina propria and muscularis mucosa
- Villi:
- Tallest in the jejunum, may be shorter or show more variability in height in duodenum
- Surface lined by microvilli
- Villus to crypt length ratio is 3 - 5:1
- Lined by primarily columnar absorptive cells and goblet cells
- Scattered intraepithelial lymphocytes (T cells), usually 1 lymphocyte per 5 enterocytes
- Villi may be shorter and distorted next to lymphoid aggregates
- In a biopsy, 4 normal villi in a row suggests normal villous architecture
- Each villus contains an arteriole with capillary network, veins and a central lymphatic with numerous nerve fibers
- Absorptive cells:
- Enterocytes
- Microvilli on luminal surface (brush border) and underlying mat of microfilaments (terminal web)
- Microvillus:
- 1.5 - 2 µm in length and 100 nm in diameter
- PAS positive, actin myosin complexes
- Goblet cells:
- Occur in crypts and surface absorptive cells
- Decrease towards villus tip, increase in frequency along small intestine (most numerous in lower ileum)
- Columnar in shape, mucus droplet in supranuclear area, secretes mucus, ions and water
- Paneth cells:
- Populate crypt bases and increase in number from proximal to distal intestine
- Strongly eosinophilic, pyramidal cells with zymogenic or secretory cell characteristics
- Supranuclear Golgi complex contains large, chunky apical membrane bound eosinophilic granules
- Granules contain various proteins involved in host defenses including lysozyme, secretory phospholipase A2 and alpha defensins / cryptdins (World J Gastrointest Pathophysiol 2017;8:150)
- Crypts of Lieberkühn:
- Lower 20% of epithelium, contain undifferentiated (immature) crypt cells, Paneth cells, scattered goblet cells and endocrine cells
- Surrounded by pericrypt fibroblast sheath
- Secrete ions, water, IgA, antimicrobial peptides into lumen
- Crypt cells take 3 - 8 days to migrate to surface
- Allows for rapid repair but also causes these cells to be sensitive to radiation therapy and chemotherapy
- Lamina propria:
- Contains loose connective tissue, lymphocytes, plasma cells, occasional eosinophils, macrophages and mast cells
- Submucosa:
- Contains connective tissue, blood vessels, lymphatics, submucosal (Meissner) plexus
- Brunner glands in duodenum
- Brunner glands:
- Submucosal mucous glands in duodenum
- Secrete bicarbonate ions, glycoproteins, pepsinogen II
- Resemble gastric pylorus mucous glands
- Muscularis propria (externa):
- Inner circular and outer longitudinal layer, with myenteric (Auerbach) plexus between these layers
- Plexus also contains interstitial cells of Cajal, ganglion cells, fibroblasts (Am J Surg Pathol 2003;27:228)
- Serosa:
- Contains mesothelial lining, loose connective tissue
- Endocrine cells:
- Contain fine eosinophilic granules with secretory proteins
- Cytoplasmic granules are subnuclear (versus supranuclear granules in Paneth cells)
- Peyer patch:
- Lymphoid aggregates randomly distributed around circumference of the small intestine (partially mucosal, partially submucosal) with central germinal center
- Peyer patch germinal centers are more common in children than adults
- Increase in number distally in the small bowel and become confluent in the ileum
- Exogenous dark brown granular pigment may be present within macrophages (Hum Pathol 1987;18:50, Gut 1996;38:390)
- Epithelium: CK20, CDX2 (Am J Surg Pathol 2004;28:1352, Int J Dev Biol 2005;49:867)
- Each microvillus contains a core bundle of vertically oriented, polarized actin filaments extending from the tip of the microvillus to the base of the terminal web
Small intestine: histology
What is the location and function of the submucosal mucous glands shown above?
- Duodenum, secrete bicarbonate ions, glycoproteins, pepsinogen II
- Duodenum, secrete proteins involved in host defense (e.g. alpha defensins / cryptdins)
- Ileum, secrete proteins involved in host defense (e.g. alpha defensins / cryptdins)
- Jejunum, secrete bicarbonate ions, glycoproteins, pepsinogen II
Comment Here
Reference: Histology - small intestine
- First part of the duodenum
- Ileum
- Jejunum
- Second part of the duodenum
Comment Here
Reference: Histology - small intestine
- Nonmalignant hyperplastic proliferation of small intestine
- Extremely rare benign duodenal / ampullary polyp
- Resembles microvesicular hyperplastic polyp microscopically
- ICD-10: D13.2 - benign neoplasm of duodenum
- Hyperplastic polyps are extremely rare anywhere in the duodenum
- Occurs in second part of duodenum and ampulla
- Uncertain malignant potential
- Mean age 52 years
- Usually incidentally detected
- 58 year old man with ridges in distal duodenum (J Clin Pathol 2006;59:1305)
- Similar to microvesicular hyperplastic polyp of the colorectum, with elongated crypts, superficial serration and decreased goblet cells (Hum Pathol 2011;42:1953)
- No evidence of basal crypt booting (as in sessile serrated adenoma of the colorectum) or gastric foveolar metaplasia
- May harbor KRAS or BRAF mutations
- Duodenum, polypectomy:
- Duodenal mucosa with bland hyperplastic and serrated architecture, most consistent with hyperplastic polyp (see comment)
- Comment: Hyperplastic polyps are rare in this location. It is unclear whether they possess premalignant potential.
- Adenoma:
- Far more common, especially in ampulla
- Dysplastic nuclei
- Peutz-Jeghers polyp:
- Contains arborizing musculature
- Goblet cell rich hyperplastic polyp
- Microvesicular hyperplastic polyp
- Mucin poor hyperplastic polyp
- Sessile serrated adenoma
This polyp was resected from the duodenum. Which of the following is true?
- Deeper levels would show conventional cytologic dysplasia
- These polyps are extremely rare in the small intestine
- This must be a traditional serrated adenoma, despite the lack of abundant eosinophilic cytoplasm
- This patient must have serrated polyposis syndrome
Comment here
Reference: Hyperplastic polyp
- Fibrosis which develops in retroperitoneum, often at aortic bifurcation
- Associated with drug methylsergine, other fibrotic lesions (sclerosis of major bile ducts, Riedel thyroiditis, inflammatory pseudotumor of orbit)
- IgG4 often involved in pathogenesis of idiopathic sclerosing lesion; associated with male gender
- Associated with lymphoma, which developed in 15% of patients
- 58 year old man with idiopathic sclerosing mesenteritis and retroperitoneal fibrosis (Korean J Gastroenterol 2011;58:221)
- With previous sclerosing mesenteritis and small bowel obstruction (Eur J Gastroenterol Hepatol 2006;18:1285)
- Not well circumscribed
- Widely scattered germinal centers and plasma cells in background of dense fibrosis
- Eosinophilic infiltration and oblitertaive phlebitis in IgG4 related cases (Am J Surg Pathol 2009;33:1833)
- Pouch is formed from connecting loops of terminal ileum for patients requiring total colectomy; used to create continence in an ileostomy or to preserve anal sphincter function
- Pouches are contraindicated in Crohn's disease because they are associated with fistulas and abscess
- Complications: fistula, obstruction, incontinence, leaks, pouchitis
- Incidence 8 - 46%
- Some cases are due to initially undiagnosed Crohn's disease
- Nausea, vomiting, malaise, fever, cramping
- Increased ileal stool that is bloody, watery, foul smelling
- Often with altered bacteria
- Antibiotics, pouch excision
- Decreased epithelial cell mucin, few / no lymphoid follicles
- Ulcers with granulation tissue, cryptitis, crypt abscesses and patchy neutrophils
- Rarely dysplasia
- CD10 staining confirms biopsy site - terminal ileum (CD10+) versus colon (CD10-), although negative staining also occurs in active enteritis (Mod Pathol 2011;24:1627)
- Also called Meditteranean lymphoma, Middle Eastern lymphoma
- More common among non-European Jews, Middle Eastern Arabs, South African blacks
- B cell lymphoma that arises in background of chronic diffuse mucosal plasmacytosis, with IgA heavy chain synthesis (no variable chain synthesized)
- Many have preexisting malabsorption
- May initially respond to antibiotics
- Often due to Campylobacter jejuni, another human pathogen responsible for immunoproliferative states (N Engl J Med 2004;350:239)
- 53 year old woman with immunoproliferative small intestinal disease (Turk J Gastroenterol 2012;23:91)
- Low grade: nonflattened mucosa
- High grade: diffusely thickened folds with small nodules in distal duodenum / proximal jejunum
- Heavy lymphoplasmacytic infiltrate by mature cells
High grade:
- Resembles diffuse large cell lymphoma with plasmacytoid features
- May have starry sky pattern, follicular hyperplasia
- Larvae of ascarids (Anisakidae) are found in sea animals
- After ingesting contaminated raw fish or other sea food, larvae attach to mucosa of stomach or small intestine and cause ulceration, penetration or perforation
Diagrams / tables
Images hosted on other servers:
Case reports
- 37 year old woman with epigastric pain two hours after ingesting fried hake and fish ova and 200 larvae obtained by endoscopy (J Investig Allergol Clin Immunol 2010;20:437)
- 50 year old man with abdominal pain and peripheral eosinophilia after eating raw salmon from Pacific Ocean (Am J Surg Pathol 2003;27:1167)
- Worm found in fresh salmon (Pritt: Creepy Dreadful Wonderful Parasites Blog - Case of the Week 557 [Accessed 7 September 2023])
Clinical images
Images hosted on other servers:
Gross images
Contributed by Bobbi Pritt, M.D.
Microscopic (histologic) description
- Serositis, mucosal edema, submucosal abscess with eosinophils surrounding parasite with unpaired excretory gland (renette cell), Y shaped lateral epidermal cords, no apparent reproductive system and a ventriculus (glandular esophagus)
- No lateral alae, no ventricular appendage, no intestinal cecum
Microscopic (histologic) description
Contributed by Bobbi Pritt, M.D.


Most likely a type I Anisakis or Pseudoterranova: anterior boring tooth (left); mucron (terminal spicule-like structure) (right)
Images hosted on other servers:
Additional references
- Bacterial related disease due to either ingestion of preformed toxin (Staphylococcus aureus, Vibrio cholera, Clostridium perfringens), infection by toxigenic organisms or infection by enteroinvasive organisms which invade and destroy mucosal epithelium cells (eMedicine: Bacterial Gastroenteritis [Accessed 7 September 2023])
- Bacterial adhere to mucosal epithelial cells, elaborate enterotoxins, have capacity to invade
- Adhere by plasmid mediated adhesins (E. coli, V. cholera), fimbriae or pili
- Adhesion destroys microvilli brush border
Clinical features
- Complications due to massive fluid loss and loss of mucosal barrier include dehydration, sepsis, perforation
- Salmonella: invades via transcytosis with minimal epithelial damage
- Yersinia enterocolitica: penetrates ileal mucosa, multiplies in Peyer patches and regional lymph nodes
- Insidious infection: Yersinia and Mycobacterium tuberculosis
- Cytotoxins: Shiga toxin, enterohemorrhagic E. coli
- Enterotoxins:
- Bind to cell membrane, enter cell, activates massive electrolyte secretion (cholera toxin, E. coli heat labile and heat stable toxins produce traveler's diarrhea)
- No white blood cells in stool
- Bacterial invasion:
- Enteroinvasive E. coli and Shigella have plasmid that mediates epithelial cell invasion via microbe simulated endocytosis; then intracellular proliferation, cell lysis, cell to cell spread
- Patients ingest preformed toxins:
- Symptoms within hours
- Explosive diarrhea and acute abdominal distress
- 1 - 2 days
- C. botulinum may produce rapid, fatal respiratory failure
- Infection with enteric pathogens:
- Incubation of hours - days
- Diarrhea and dehydration (secretory enterotoxin) or dysentery (cytotoxin or enteroinvasive)
- Traveler's diarrhea:
- Fecally contaminated water / food
- Begins abruptly, subsides in 2 - 3 days
Microscopic (histologic) description
- Decreased epithelial cell maturation, increased mitotic figures, hyperemia and edema of lamina propria, variable neutrophils, modest villus blunting of small bowel
- Late: lymphocytes, plasma cells, regenerative change
Differential diagnosis
- Acid fast protozoa which causes self limited disease in immunocompetent but severe watery diarrhea resistant to most therapy in immunocompromised (J Biomed Res 2012;25:1)
Diagnosis
- Acid fast infective oocyst in stool
Microscopic (histologic) description
- 2 - 5 micron basophilic spherical structures attached to microvillus surface of epithelium
- Variable villus abnormality, may have eosinophils infiltrating mucosa
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Differential diagnosis
- Cellular debris
- Mucin
- Dysentery causing protozoa that can also cause fulminant colitis; causes up to 100,000 deaths per year (Trop Biomed 2011;28:194)
- Increased incidence in homosexual men and AIDS patients
Pathophysiology
- Fecal oral spread
- Amoeba invade colonic crypts, burrow into lamina propria, create flask shaped ulcer with broad base
- 40% invade portal vessels, embolize to liver and cause abscesses up to 10 cm
- May activate apoptosis in target cells (Trends Parasitol 2011;27:254)
- Rare abscesses in lung, heart, kidneys, brain
Diagrams / tables
Images hosted on other servers:
Microscopic (histologic) images
Contributed by Hanni Gulwani, M.D.
Images hosted on other servers:
- See Giardia lamblia
- Coccidian parasite that infects epithelial cells of small intestine
- Causes chronic diarrhea and acalculous cholecystitis in AIDS patients (Indian J Med Res 2011;134:878)
Diagnosis
- Cysts in stool, biopsy (H&E or EM)
Case reports
- 35 year old HIV+ man (Indian J Pathol Microbiol 2010;53:824)
Microscopic (histologic) description
- Cysts present in parasitophorous vacuole in lamina propria (Hum Pathol 2001;32:500)
- Ovoid developmental forms are identified in and beneath epithelial cells near villus tip
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
- Caused by Enterocytozoon bieneusi, an obligate intracellular protozoan that affects only enterocytes and Encephalitozoon intestinalis, which infects macrophages, fibroblasts, endothelial cells, enterocytes
- Both cause chronic diarrhea in patients with immunosuppression, HIV
Diagnosis
- Stool examination, PCR
Case reports
- 33 year old woman with myeloma, postautologous stem cell transplant and chemotherapy for relapse (Emerg Infect Dis 2012;18:1155)
- 43 year old man with pancreas / kidney transplant recipient and multiorgan system dissemination (Arch Pathol Lab Med 2004;128:e41)
Treatment
- Albendozole for E. intestinalis, nothing for E. bieneusi
Microscopic (histologic) description
- Minimal / no changes in mucosa but can find development spores as 1.5 mm dots in enterocytes
- May be surrounded by halos
- Also nucleated sporont present as 3 - 5 micron, rounded, basophilic structure often surrounded by a halo
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
Electron microscopy description
- Often helpful for diagnosis
- Primarily diarrheal illness caused by infection with rotavirus
Essential features
- Rotavirus is a segmented, nonenveloped double stranded RNA virus
- It is ubiquitous and infection is essentially universal first occurring in early childhood
- Transmission is fecal oral either via direct contact with stool or via fomites
- In the developed world, the vast majority of infections cause self limited diarrheal illness or in subsequent infections and vaccinated populations, are asymptomatic
- Most cases in the developing world are similar; however, the World Health Organization estimates that in 2013 ~215,000 children under 5 years old died from rotavirus infection (WHO: Rotavirus [Accessed 7 September 2023])
- Live attenuated vaccines are available and their use has resulted in a dramatic worldwide decrease of severe disease
- Intestinal intussusception is a rare but well recognized potential complication of vaccination
Terminology
- Rotavirus is a genus of the Reoviridae family
ICD coding
- ICD-10: A08.0 - rotaviral enteritis
Epidemiology
- Rotavirus is ubiquitous and infection is essentially universal in childhood in all socioeconomic groups
- Transmission is fecal oral either via direct contact with stool or via fomites
- Infection generally first occurs around 6 months of age as the gastrointestinal tract matures and passive immunity from breast milk wanes
- Infection may occur earlier in infants fed formula and in parts of India, Asia and sub-Saharan Africa where protection from maternal antibodies appears to be less effective
- Reinfection occurs throughout life and in most children older than 2 years and adults infection is minimally symptomatic or asymptomatic due to immunity either from prior infections or vaccination
- In the developed world, disease was more common in winter and spring; however, whether this pattern is still valid with widespread vaccination is unclear
- Transmission in the daycare and hospital setting is common
- Viral particles are hardy and resistant to chlorinated drinking water although they are vulnerable to alcohol based hand sanitizer and spray disinfectants
- The World Health Organization estimates that in 2013 ~215,000 children under 5 years old died from rotavirus infection, overwhelmingly in the developing world compared to 528,000 in 2000 (WHO: Global Illness and Deaths Caused by Rotavirus Disease in Children [Accessed 7 September 2023])
- According to the CDC in the United States rotavirus causes over 400,000 doctor visits, 200,000 emergency room visits, 55,000 to 70,000 hospitalizations and from 20 to 60 deaths each year (CDC: Rotavirus in the U.S. [Accessed 7 September 2023])
- Serologically rotavirus is classified into serogroups, subgroups and G and P serotypes
- Genotypically, it is classified into electropherotypes, genogroups and G and P genotypes
- There is a substantial geographic and temporal variation in strain distribution and antigenic drift as well as introduction and reassortment with animal viruses which increases genetic diversity
- Epidemiologically nucleic acid testing is increasingly replacing serologic testing
- Further information is available from the Rotavirus Classification Working Group's database (RotaC v2.0: Classification Tool for Rotaviruses Group A [Accessed 7 September 2023])
Sites
- Infection occurs in the small intestine
Pathophysiology
- Pathophysiology is complex and not fully understood
- Enterocytes at villous tips are initially infected; the virus binds surface carbohydrates and penetrates the cell membrane leading to transcription of viral RNA
- Damage to the mucosa leads to malabsorption
- Activity of disaccharidases and peptidases in the brush border is decreased and osmosis from unabsorbed nutrients contributes to the diarrhea
- Glucose cotransport of electrolytes is decreased but oral rehydration solutions are still affective (see Treatment below)
- Mature enterocytes are lost and initially replaced by immature secretory cells
- There is net secretion of water in part mediated by the enteric nervous system
- Clearance of infection is complex and involves overlapping elements of innate, cellular and humoral immunity
- Virus is usually shed for 6 to 10 days after patients become symptomatic
Clinical features
- There is an incubation period of 1 - 3 days
- Illness in infants and young children usually starts with vomiting and fever lasting 2 - 3 days, progressing to 4 - 5 days of profuse watery diarrhea
- In symptomatic older children and adults there is 1 - 4 days of diarrhea with anorexia, crampy abdominal pain and low grade fever
- Chronic infections may occur in immunosuppressed patients excepting HIV; isotonic dehydration and metabolic acidosis occur in severe cases
- Viremia is uncommon; extraintestinal manifestations are rare and described in the liver and kidney
- Intestinal intussusception is a rare but well recognized potential complication of vaccination
- Prior intussusception is a contraindication to vaccination
- Patient deaths are due to dehydration and electrolyte abnormalities that lead to cardiac failure or less commonly seizures or aspiration pneumonia
Diagnosis
- Enzyme immunoassay and PCR are the mainstays
- Latex agglutination is often used in resource poor areas
- Viral culture and electron microscopy are largely of historical interest
- Microbiologic diagnosis is rarely required
Laboratory
- Severe disease may cause metabolic acidosis and high urine specific gravity
- Mild elevation of transaminases and uric acid may occur
- Leukocytosis is rare and stool is usually negative for white blood cells and blood
Prognostic factors
- Disease is most severe in infants and young children
- Immunosuppressed patients often suffer from more severe disease and cannot receive the live attenuated vaccine although patients infected with HIV do not suffer more severe disease and should be vaccinated
- Infection in the developing world is more likely to lead to severe illness or death; this is related to less access to health care, malnutrition, the presence of other infections and possibly larger inoculums
Case reports
- 5 month old boy, 51, 54 and 71 year old men in a multiorgan transplant unit (Transpl Int 2005;18:470)
- 22 month old girl with opsoclonus myoclonus syndrome after rotavirus gastroenteritis (Pediatr Int 2014;56:e86)
- 8 year old Japanese girl with mild encephalopathy (BMJ Case Rep 2016;2016:bcr2016216895)
- 73 year old man with Henoch-Schönlein purpura (Am J Case Rep 2017;18:136)
Treatment
- Mild to moderate disease: oral rehydration
- Severe disease: intravenous lactated ringers or normal saline until oral rehydration can be tolerated
- Infants: return to breast feeding or formula as soon as it can be tolerated
- In the developing world, zinc supplementation and some probiotics in children older than 6 months may reduce disease duration
- Diosmectite, a type of aluminum magnesium clay silica, is available in parts of Europe and controls gastroenteritis
- Oral immunoglobulins have a possible role in chronic disease and for high risk patients where vaccination is unlikely to be effective (premature infants) or is contraindicated
- Antibiotics are not indicated
- Vaccination is recommended to commence at 2 months of age
Microscopic (histologic) description
- Biopsy is rarely performed
- Findings are nonspecific; villi are shortened, blunted and lined by cuboidal cells
- There is crypt hyperplasia and increased mononuclear cells within the lamina propria
- Apoptotic epithelial cells may be present
- Disease may be patchy in distribution
- Viral inclusions are not present
Electron microscopy images
Images hosted on other servers:
Differential diagnosis
- Other causes of infectious gastroenteritis and enterocolitis
Additional references
- Bacterial causing food poisoning and typhoid fever (S. typhi)
- Typhoid fever: systemic dissemination with bacteremia, fever, chronic infection of joints, biliary tree, bones and meninges
- From contaminated milk, eggs, beef, poultry
- Usually affects terminal ileum
Gross images
Case #362
Microscopic (histologic) description
- Ulcers overlying Peyer patches with minimal inflammatory cells, chiefly mononuclear (plasma cells, histiocytes and lymphocytes)
- Often histiocytes with erythrophagocytosis
- May lead to perforation or toxic megacolon
Microscopic (histologic) images
Case #362
- Nematode with complex life cycle that alternates between free living and parasitic cycles, with potential for autoinfection and multiplication within host
Life cycle
- Larvae burrow into mucosa of duodenum and jejunum, where they mature into adults
- Females lay eggs, which develop into larvae that pass into stool, where they mature and become infective
- Infective larvae in soil penetrate intact skin, usually through feet
- Larvae enter circulatory system, are transported to lungs, enter alveolar spaces, are carried to trachea and pharynx, are swallowed and enter intestinal tract, where process is repeated
- If larvae become infective before leaving body, they may invade intestinal mucosa or perianal skin, causing autoinfection
Diagrams / tables
Images hosted on other servers:
Clinical features
- Symptoms: none, diarrhea, malabsorption
- Severe / fatal infections in immunocompromised, due to worms moving from GI tract into other organs (WormBook 2007:1)
Diagnosis
- Larvae in stool
- Adult female or eggs in small bowel mucosa, often with eosinophilic or granulomatous inflammation
Case reports
- 43 year old Honduran man with diarrhea and abdominal pain (Case #133)
- 66 year old man with weakness and epigastric pain (Int J Prev Med 2012;3:370)
Treatment
- Antihelminths such as thiabendazole (Ann Pharmacother 2007;41:1992)
- Prevent by wearing shoes in endemic areas
Microscopic (histologic) images
Contributed by Josehp Christopher Castillo, M.D. and Case #133
Contributed by @liverwei on Twitter
Additional references
- See Whipple disease
- Characteristic viral inclusions are diagnostic
- Incidence has increased
- Infection typically affects the sigmoid colon and rectum
- Intussusception is a rare but recognized complication associated with vaccination
- Most deaths occur in the elderly
Comment Here
Reference: Infectious disorders-general
- Uncommon, benign polypoid submucosal mass with CD34+ spindle cells and granulation tissue, usually in distal stomach or terminal ileum (Arch Pathol Lab Med 2011;135:1311)
- Presents as obstructive mass or causing intussusception (Radiographics 1999;19:539)
- True neoplasms, usually with PDGFRA mutation (Pathologe 2009;30:117)
- 35 year old woman with concurrent appearance of Crohn's disease and inflammatory fibroid polyp (Ann Ital Chir 2005;76:395)
- Excision (does not recur)
- Polypoid (without stalk), submucosal mass, dumbbell shape in small intestine due to intussuception, mucosal ulceration and splitting and atrophy of muscularis propria
- Submucosal mixture of prominent capillaries, spindle cells (occasionally concentrically arranged around vessels / onion skinning) and inflammatory cells (particularly eosinophils, plasma cells and mast cells) in granulation tissue-like stroma (Arch Pathol Lab Med 2010;134:378)
- May be transmural
- May have lymphoid follicles
- Rare multinucleated giant cells, no atypia, no / rare mitotic figures
- Histologic patterns: classical, edematous, fibrovascular, nodular, sclerotic
- CD117 (although mast cells are positive)
- GIST: malignant in 30 - 50%, plump spindle cells with eosinophilic cytoplasm, variable skenoid fibers and muscular infiltration, CD117+ (Mod Pathol 2000;13:1134, Mod Pathol 2003;16:366)
- Inflammatory myofibroblastic tumor: usually children, may recur, plasma cells and lymphocytes with few eosinophils, mitotic activity in cellular areas, less prominent blood vessels, smooth muscle actin+, desmin+, ALK±, CD34- (Hum Pathol 2002;33:307)
- Inflammatory polyp of Crohn's or ulcerative colitis: similar histology but different clinical history and findings in remainder of bowel
- Also called inflammatory pseudotumor; previously called inflammatory fibrosarcoma
- Often in children but can occur at any age
- No clinical associations
- Benign but may recur; rarely has malignant behavior
- Rarely behaves malignant
- ALK+ tumors (22% of patients age 40 years or younger) have a more favorable prognosis (Am J Surg Pathol 2001;25:761)
- Complications: obstruction, intussusception
- 2 month old infant with intestinal obstruction (J Med Assoc Thai 2009;92:114)
- 2 year old girl with jejunal tumor (Pathol Int 2001;51:47)
- 29 year old woman with intramural ileal tumor showing ALK / TPM3 fusion using FISH (Hum Pathol 2006;37:112)
- Mean 3 - 4 cm, submucosal sessile polypoid mass or multiple masses with broad base
- Tan gray yellow
- Overlying mucosa may be ulcerated
- Usually limited to submucosa
- Plump ovoid myofibroblastic spindle cells with inflammatory infiltrate including plasma cells and lymphocytes
- May be sparsely cellular with myxoid stroma
- May have rarefaction around muscular walled blood vessels
- May be infiltrative
- Cellular areas may have up to 2 mitotic figures/HPF
- Variable vascular component
- S100, desmin (Mod Pathol 2000;13:1134), CD117 (endothelial cells may be CD117+), CD34
- Myofibroblasts
- Fibromatosis: invades bowel secondarily
- Polypoid inflammatory mucosa associated with Crohn's disease or ulcerative colitis
- Also amebiasis, schistosomiasis, ulcers, anastomotic sites
- Solitary small polyps of inflamed regenerating mucosa in a fibroblastic or granulation tissue stroma
- No / few mitotic figures, no atypical mitotic figures, often zonation
- May have bizarre stromal changes resembling sarcoma
- Intra-ampullary papillary tubular neoplasm (IAPN) is the name proposed in 2010 to unify the nomenclature for mass forming preinvasive (dysplastic) lesions that arise within the ampulla of Vater (Am J Surg Pathol 2010;34:1731)
- The precursor lesion of intra-ampullary adenocarcinomas (Am J Surg Pathol 2012;36:1592)
- Majority (78%) of IAPNs in one large series harbored an invasive component (Am J Surg Pathol 2010;34:1731)
- Analogous to pancreatic or biliary intraductal papillary and tubular neoplasms (Am J Surg Pathol 2010;34:1731)
- Must be localized within (or predominantly within) the ampulla (Am J Surg Pathol 2010;34:1731)
- Other / previous terms include intestinal type adenoma, noninvasive pancreatobiliary type neoplasm
- ICD-O: 8210/0 - adenomatous polyp, low grade dysplasia
- ICD-O: 8210/2 - adenomatous polyp, high grade dysplasia
- ICD-10: C24.1 - malignant neoplasm of ampulla of Vater
- ICD-11: 2E92.2 & XH3DV3 - benign neoplasm of duodenum, adenoma, NOS
- ICD-11: 2E92.2 & XH8MU5 - benign neoplasm of duodenum, adenomatous polyp, NOS
- Mean age 64; range: 27 - 85 years (Am J Surg Pathol 2010;34:1731)
- M:F = 2.4 (Am J Surg Pathol 2010;34:1731)
- Estimated prevalence base on autopsy studies is 0.04 - 0.12% (World J Gastrointest Endosc 2011;3:241)
- Ampulla of Vater: within the ampullary channel (common channel) or the intra-ampullary portions of the common bile duct or main pancreatic duct
- Given the prevalence of neoplasia in and around the ampulla, authors have speculated that bile acids may be carcinogenic (Mutat Res 2005;589:47)
- Patients may be asymptomatic or present with obstructive symptoms: jaundice, weight loss, abdominal pain, nausea / vomiting, pruritus, dark urine / light stools or tarry stools (Am J Surg Pathol 2010;34:1731)
- Rarely, patients present with cholangitis or pancreatitis
- A mass involving the ampullary region may be found on cross sectional imaging
- Tissue diagnosis is often made via endoscopic retrograde cholangiopancreatography (ERCP) biopsy
- Imaging modalities used to examine the ampulla include computed tomography (CT) and magnetic resonance imaging (MRI) (Am J Surg Pathol 2010;34:1731)
- Imaging generally shows a dilated common bile duct, intra / extrahepatic ducts; an ampullary, duodenal or pancreatic mass; or enlarged or irregular papillae (Am J Surg Pathol 2010;34:1731)
- Tumor size and degree of dysplasia correlate with risk of invasive component (Am J Surg Pathol 2010;34:1731)
- Overall 3 and 5 year survivals in tumors without an identified invasive component is 100% (Am J Surg Pathol 2010;34:1731)
- Overall 5 year survival in tumors with an invasive component is 45% (Am J Surg Pathol 2010;34:1731)
- Cases showing high grade dysplasia are more likely to be associated with an invasive carcinoma (Am J Surg Pathol 2010;34:1731)
- Surgery is the mainstay of treatment: transduodenal ampullectomy or pancreatoduodenectomy (Whipple procedure)
- Endoscopic resections are increasingly being used (J Clin Gastroenterol 2012;46:8, World J Gastrointest Endosc 2011;3:241, Gastrointest Endosc 2015;82:773)
- Intraluminal polypoid or papillary mass forming exophytic lesion within a dilated ampullary duct (Am J Surg Pathol 2010;34:1731)
- Lesion fills and may obstruct the ampullary channel (Am J Surg Pathol 2010;34:1731)
- Duodenal orifice is often widened, irregular and may be ulcerated (Am J Surg Pathol 2010;34:1731)
- Average tumor size was 2.7 cm in one large series (Am J Surg Pathol 2010;34:1731)
- Noninvasive exophytic tumor
- Growth pattern often shows a mixture of papillary and tubular architecture (Am J Surg Pathol 2010;34:1731)
- In one large series, cases showing predominantly papillary architecture were more likely to contain high grade dysplasia (Am J Surg Pathol 2010;34:1731)
- When considering the predominant phenotype, the majority show an intestinal phenotype, with a minority showing a gastric / pancreatobiliary phenotype (74% versus 26%, respectively) (Am J Surg Pathol 2010;34:1731, Hum Pathol 2014;45:1910)
- Intestinal type: tall columnar cells with elongated nuclei and inconspicuous nucleoli; Paneth, goblet or endocrine cells may be present; appear similar to conventional colonic adenomas
- Pancreatobiliary type: cuboidal cells with round nuclei arranged predominantly in a single layer; some resemble a pyloric gland adenoma histologically
- When taking into account all morphology present, 45% of cases show a mixed phenotype (Am J Surg Pathol 2010;34:1731)
- Many to most do not show significant mucin production (Ann Surg 2016;263:162, Am J Surg Pathol 2010;34:1731)
- At least focal high grade dysplasia is present in most cases (66.7 - 94%) (Am J Surg Pathol 2010;34:1731, Hum Pathol 2014;45:1910)
- Increased degree of architectural complexity and nuclear atypia
- Lesions with a pancreatobiliary phenotype are more likely to have more extensive high grade dysplasia (Am J Surg Pathol 2010;34:1731)
- The majority of IAPNs are accessible to endoscopic biopsy; however, exfoliative cytology is sometimes done and can be used to diagnose dysplasia (Cytojournal 2017;14:19)
- Intestinal phenotype: MUC2 (85%), CDX2 (94%),CK20 (93%), CK7 (63%) (Am J Surg Pathol 2010;34:1731)
- Gastric / pancreatobiliary phenotype: MUC1 / EMA (89%), MUC5AC (95%), MUC6 (83%), CK7 (89%) (Am J Surg Pathol 2010;34:1731)
- Intestinal phenotype: MUC1 / EMA (21%), MUC5AC (31%), MUC6 (24%) (Am J Surg Pathol 2010;34:1731)
- Gastric / pancreatobiliary phenotype: CDX2 (39%), CK20 (39%), MUC2 (22%) (Am J Surg Pathol 2010;34:1731)
- Ampulla, endoscopic ampullectomy:
- Intra-ampullary papillary tubular neoplasm, pancreatobiliary type, with multifocal high grade dysplasia
- No invasive carcinoma identified
- Resection margins are free of dysplasia
- Intraductal papillary mucinous neoplasm (IPMN):
- These lesions arise in the pancreatic ducts (main duct or side branch ducts) and generally have significant mucin production
- Proper grossing and sampling with specific location information of the tissue sampled are crucial in making the distinction between these entities, because without knowledge of the specific location of a given lesion, these can be histologically indistinguishable at microscopic level
- Duodenal / periampullary adenoma:
- These lesions will generally show an intestinal phenotype (will look like a colonic adenoma)
- Proper grossing and sampling with specific location information of the tissue sampled are crucial in making the distinction between these entities, because without knowledge of the specific location of a given lesion, these can be histologically indistinguishable at microscopic level
- Intra-ampullary adenocarcinoma:
- This is an adenocarcinoma that may arise from an IAPN but will show an invasive component
A 65 year old man presented with jaundice, weight loss and nausea. MRI showed a dilation of the intra and extrahepatic bile ducts with an ill defined fullness in the head of the pancreas; ERCP revealed a papillary mass in the ampullary channel. Forcep biopsy demonstrates histologic findings consistent with intra-ampullary papillary tubular neoplasm (IAPN) with high grade dysplasia including the pictured findings. The most likely immunoprofile for this lesion is
- Immunoreactive for CDX2, CK20 and MUC2; negative for MUC1 and MUC5AC
- Immunoreactive for CDX2, MUC1 and MUC2; negative for CK20 and MUC5AC
- Immunoreactive for MUC1 and MUC5AC; negative for CDX2, CK20 and MUC2
- Immunoreactive for MUC1, MUC2 and MUC5AC; negative for CDX2, CK20 and CK7
Comment Here
Reference: Intra-ampullary papillary tubular neoplasm (IAPN)
- Intestinal
- Oncocytic
- Pancreatobiliary
- Squamous
Comment Here
Reference: Intra-ampullary papillary tubular neoplasm (IAPN)
- Intra-ampullary papillary tubular neoplasm (IAPN) is the name proposed in 2010 to unify the nomenclature for mass forming preinvasive (dysplastic) lesions that arise within the ampulla of Vater (Am J Surg Pathol 2010;34:1731)
- The precursor lesion of intra-ampullary adenocarcinomas (Am J Surg Pathol 2012;36:1592)
- Majority (78%) of IAPNs in one large series harbored an invasive component (Am J Surg Pathol 2010;34:1731)
- Analogous to pancreatic or biliary intraductal papillary and tubular neoplasms (Am J Surg Pathol 2010;34:1731)
- Must be localized within (or predominantly within) the ampulla (Am J Surg Pathol 2010;34:1731)
- Other / previous terms include intestinal type adenoma, noninvasive pancreatobiliary type neoplasm
- ICD-O
- ICD-10
- C24.1 - malignant neoplasm of ampulla of Vater
- ICD-11
- 2E92.2 & XH3DV3 - benign neoplasm of duodenum & adenoma, NOS
- 2E92.2 & XH8MU5 - benign neoplasm of duodenum & adenomatous polyp, NOS
- Mean age: 64 - 67 years; range: 27 - 85 years (Am J Surg Pathol 2010;34:1731, Med Sci Monit 2019;25:7332)
- M:F = 2.4 in one surgical series and 42.5% men / 57.5% women in SEER analysis (Am J Surg Pathol 2010;34:1731, Med Sci Monit 2019;25:7332)
- Estimated prevalence based on autopsy studies is 0.04 - 0.12% (World J Gastrointest Endosc 2011;3:241)
- Incidence from SEER database study was 0.025 in 2014 (Med Sci Monit 2019;25:7332)
- Incidence has been decreasing annually since 2016 (Med Sci Monit 2019;25:7332)
- Ampulla of Vater: within the ampullary channel (common channel) or the intra-ampullary portions of the common bile duct or main pancreatic duct
- Unknown
- Given the prevalence of neoplasia in and around the ampulla, authors have speculated that bile acids may be carcinogenic (Mutat Res 2005;589:47)
- Patients may be asymptomatic or present with obstructive symptoms: jaundice, weight loss, abdominal pain, nausea / vomiting, pruritus, dark urine / light stools or tarry stools (Am J Surg Pathol 2010;34:1731)
- Rarely, patients present with cholangitis or pancreatitis
- Mass involving the ampullary region may be found on cross sectional imaging
- Tissue diagnosis is often made via endoscopic retrograde cholangiopancreatography (ERCP) biopsy
- Imaging modalities used to examine the ampulla include computed tomography (CT) and magnetic resonance imaging (MRI) (Am J Surg Pathol 2010;34:1731, Radiographics 2022;42:1320)
- Imaging generally shows a dilated common bile duct, intra / extrahepatic ducts; an ampullary, duodenal or pancreatic mass; or enlarged or irregular papillae (Am J Surg Pathol 2010;34:1731)
- Double duct sign is present in 52% of cases, with both biliary and pancreatic duct dilatation (Radiographics 2022;42:1320)
- It may be challenging to identify lesions < 1 cm due to the duodenal folds (Radiographics 2022;42:1320)
- Tumor size and degree of dysplasia correlate with risk of invasive component (Am J Surg Pathol 2010;34:1731)
- Overall 3 and 5 year survival rates in patients with tumors lacking an identified invasive component is 100% (Am J Surg Pathol 2010;34:1731)
- Overall 5 year survival rates in patients with tumors containing an invasive component is 45% (Am J Surg Pathol 2010;34:1731)
- Cases showing high grade dysplasia are more likely to be associated with an invasive carcinoma (Am J Surg Pathol 2010;34:1731)
- 61 year old woman presented with combined IAPN and ampullary neuroendocrine carcinoma (World J Clin Cases 2022;10:8045)
- 66 year old man presented with mucinous adenocarcinoma of the ampulla arising within an IAPN (Surg Case Rep 2021;7:25)
- 80 year old man presented with micropapillary adenocarcinoma of the ampulla arising within an IAPN (World J Clin Cases 2021;9:2671)
- Surgery is the mainstay of treatment: transduodenal ampullectomy or pancreatoduodenectomy (Whipple procedure)
- Endoscopic resections are increasingly being used (J Clin Gastroenterol 2012;46:8, World J Gastrointest Endosc 2011;3:241, Gastrointest Endosc 2015;82:773)
- Intraluminal polypoid or papillary mass forming exophytic lesion within a dilated ampullary duct (Am J Surg Pathol 2010;34:1731)
- Lesion fills and may obstruct the ampullary channel (Am J Surg Pathol 2010;34:1731)
- Duodenal orifice is often widened, irregular and may be ulcerated (Am J Surg Pathol 2010;34:1731)
- Average tumor size was 2.7 cm in one large series (Am J Surg Pathol 2010;34:1731)
- Noninvasive exophytic tumor
- Growth pattern often shows a mixture of papillary and tubular architecture (Am J Surg Pathol 2010;34:1731)
- In one large series, cases showing predominantly papillary architecture were more likely to contain high grade dysplasia (Am J Surg Pathol 2010;34:1731)
- When considering the predominant phenotype, the majority show an intestinal phenotype, with a minority showing a gastric / pancreatobiliary phenotype (74% versus 26%, respectively) (Am J Surg Pathol 2010;34:1731, Hum Pathol 2014;45:1910)
- Intestinal type
- Tall columnar cells with elongated nuclei and inconspicuous nucleoli
- Paneth, goblet or endocrine cells may be present
- Appears similar to conventional colonic adenomas
- Pancreatobiliary type
- Cuboidal cells with round nuclei arranged predominantly in a single layer
- Some resemble a pyloric gland adenoma histologically
- Intestinal type
- When taking into account all morphology present, 45% of cases show a mixed phenotype (Am J Surg Pathol 2010;34:1731)
- Many to most do not show significant mucin production (Ann Surg 2016;263:162, Am J Surg Pathol 2010;34:1731)
- At least focal high grade dysplasia is present in most cases (66.7 - 94%) (Am J Surg Pathol 2010;34:1731, Hum Pathol 2014;45:1910)
- Increased degree of architectural complexity and nuclear atypia
- Lesions with a pancreatobiliary phenotype are more likely to have more extensive high grade dysplasia (Am J Surg Pathol 2010;34:1731)
- Majority of IAPNs are accessible to endoscopic biopsy; however, exfoliative cytology is sometimes done and can be used to diagnose dysplasia (Cytojournal 2017;14:19)
- Intestinal phenotype: MUC2 (85%), CDX2 (94%), CK20 (93%), CK7 (63%) (Am J Surg Pathol 2010;34:1731)
- Gastric / pancreatobiliary phenotype: MUC1 / EMA (89%), MUC5AC (95%), MUC6 (83%), CK7 (89%) (Am J Surg Pathol 2010;34:1731)
- Intestinal phenotype: MUC1 / EMA (21%), MUC5AC (31%), MUC6 (24%) (Am J Surg Pathol 2010;34:1731)
- Gastric / pancreatobiliary phenotype: CDX2 (39%), CK20 (39%), MUC2 (22%) (Am J Surg Pathol 2010;34:1731)
- Ampulla, endoscopic ampullectomy:
- Intra-ampullary papillary tubular neoplasm, pancreatobiliary type, with multifocal high grade dysplasia
- No invasive carcinoma identified
- Resection margins are free of dysplasia
- Intraductal papillary mucinous neoplasm (IPMN):
- These lesions arise in the pancreatic ducts (main duct or side branch ducts) and generally have significant mucin production
- Proper grossing and sampling with specific location information of the tissue sampled are crucial in making the distinction between these entities because without knowledge of the specific location of a given lesion, these can be histologically indistinguishable at microscopic level
- Duodenal / periampullary adenoma:
- These lesions will generally show an intestinal phenotype (will look like a colonic adenoma)
- Proper grossing and sampling with specific location information of the tissue sampled is crucial in making the distinction between these entities because without knowledge of the specific location of a given lesion, these can be histologically indistinguishable at microscopic level
- Intra-ampullary adenocarcinoma:
- This is an adenocarcinoma that may arise from an IAPN but will show an invasive component
A 65 year old man presented with jaundice, weight loss and nausea. MRI showed a dilation of the intra and extrahepatic bile ducts with an ill defined fullness in the head of the pancreas; endoscopic retrograde cholangiopancreatography (ERCP) revealed a papillary mass in the ampullary channel. Forcep biopsy demonstrates histologic findings consistent with intra-ampullary papillary tubular neoplasm (IAPN) with high grade dysplasia including the pictured findings. What is the most likely immunoprofile for this lesion?
- Immunoreactive for CDX2, CK20 and MUC2; negative for MUC1 and MUC5AC
- Immunoreactive for CDX2, MUC1 and MUC2; negative for CK20 and MUC5AC
- Immunoreactive for MUC1 and MUC5AC; negative for CDX2, CK20 and MUC2
- Immunoreactive for MUC1, MUC2 and MUC5AC; negative for CDX2, CK20 and CK7
Comment Here
Reference: Intra-ampullary papillary tubular neoplasm (IAPN)
- Intestinal
- Oncocytic
- Pancreatobiliary
- Squamous
Comment Here
Reference: Intra-ampullary papillary tubular neoplasm (IAPN)
- Portion of bowel (intussuscipiens) swallows another length of bowel (intussusceptum)
- Usually occurs during first 5 years of life, 50% within first year (BMC Pediatr 2012;12:36)
- May be due to lymphoid hyperplasia in ileocecal region secondary to adenovirus or other viral infections
- In older patients, may be associated with HIV or due to pedunculated intraluminal lipoma or leiomyoma that serves as point of traction
- Complications: ischemic necrosis due to compression of vessels
- 75 year old man with giant ileal lipoma (Int J Surg Case Rep 2012;3:382)
- Manual reduction, barium enema, resection
- Decreased blood flow or lack of oxygen to the bowel that causes necrosis or damage
- Numerous arterial or venous causes of small bowel ischemia
- Early histologic findings: mucosal surface damage with hemorrhagic necrosis and lamina propria hyalinization
- Late histologic findings: necrosis, inflammation, loss of base crypts, crypt distortion
- Important to examine the vessels for evidence of thromboembolic disease or vasculitis
- Reference: Greenson: Diagnostic Pathology - Gastrointestinal, 3rd Edition, 2019
- Necrosis / necrotic bowel
- Mesenteric ischemia
- Mean age of 70 years old; less common before age 60
- M:F = 1:1
- Rare condition but high mortality rates (24 - 94%) (StatPearls: Bowel Ischemia [Accessed 9 August 2023])
- Decrease in mesenteric arterial blood flow is the most common cause
- Small bowel (duodenum, jejunum, ileum)
- Often has transmural infarction due to occlusion of superior mesenteric artery
- Decrease or lack of blood flow deprives the tissue of oxygen
- Lack of oxygen leads to cell death and necrosis
- Ischemia injury causes the release of toxic byproducts, production of free radicals and activation of neutrophils (StatPearls: Bowel Ischemia [Accessed 9 August 2023])
- Thrombosis can lead to full thickness infarction
- Artery occlusion: thrombosis, embolism, atherosclerosis, vasculitis, compression (adhesions, volvulus, tumor), radiation
- Venous occlusion: thrombosis (oral contraceptives, hypercoagulable states), compression (adhesions, volvulus, tumor)
- Low flow states: shock (blood loss, postsurgery), dehydration, heart failure, vasospasm (medications, drugs like cocaine)
- Intestinal obstruction: masses (neoplasms, diverticular disease), ileus, motility disorders
- Infection: enterohemorrhagic E. coli (E. coli O157:H7), cytomegalovirus
- Drugs / medications: NSAIDs, opioids, chemotherapy, digoxin, vasoconstrictors (cocaine, amphetamines, decongestants, ergot alkaloids)
- Reference: Am J Surg Pathol 1998;22:773
- Abdominal pain (usually sudden onset), nausea / vomiting, hematochezia and fever
- History and physical is most important
- Some patients have a leukocytosis
- CT abdomen with or without contrast (Curr Gastroenterol Rep 2019;21:27)
- Findings of underlying cause: CT angiography abdomen, magnetic resonance angiography (MRA) abdomen, infectious causes
- Endoscopic findings: edema and erythema, geographic ulcers, strictures (Greenson: Diagnostic Pathology - Gastrointestinal, 3rd Edition, 2019)
- Some patients present with a leukocytosis or other complete blood count (CBC) abnormalities (if bleeding)
- CT abdomen with and without contrast shows evidence of intestinal ischemia by bowel wall thickening, dilatation of bowel lumen, mesenteric fat stranding and ascites, bowel wall attenuation and possibly pneumatosis
- Depends on the underlying cause
- 90% mortality for mesenteric artery thrombosis, 10% mortality for nonocclusive disease (Turk J Surg 2017;33:104)
- 33 year old man presented with acute abdominal pain following amphetamine use (Radiol Case Rep 2020;15:2183)
- 34 year old man with history of right hepatectomy presented with diffuse abdominal pain, bloating and nausea (World J Gastrointest Pathophysiol 2019;10:29)
- 59 year old woman with history of peritonectomy (Int J Surg Case Rep 2020;76:247)
- Supportive treatment, hemodynamic stability and oxygen
- Surgical treatment if necessary
- Infarcted bowel mucosa and wall described as dusky, which is a dark red-brown color
- Bowel wall can be thin and friable, may contain bloody contents
- Geographic (sharp demarcation) between infarcted bowel and normal bowel
- Ulcers and pseudomembranes may be present (Greenson: Diagnostic Pathology - Gastrointestinal, 3rd Edition, 2019)
- Early histologic findings
- Mucosal surface with hemorrhagic necrosis and loss of surface crypts
- Base of crypts can remain intact but are atrophic and withered with marked regenerative atypia
- Lamina propria hyalinization with smudgy appearance
- Edematous submucosa, edema and subsequent splaying of muscularis mucosae
- Little inflammation
- Pseudomembranes may be present
- Later histologic findings
- Inflammation progresses with crypt distortion
- Hemosiderin laden macrophages
- Can have giant cells in areas of ulceration
- Vessels
- Evidence of thrombi, emboli or vasculitis; most commonly in resection specimens
- Do not over interpret thrombi or inflammation in ulcer bed
- Reference: Greenson: Diagnostic Pathology - Gastrointestinal, 3rd Edition, 2019
- Small bowel, biopsy:
- Enteric mucosa with changes consistent with ischemic type mucosal injury (see comment)
- Comment: Possible etiologies for this ischemic type mucosal injury include true ischemic colitis, infection (such as E. coli 0157:H7) and a drug reaction (including oral contraceptives, NSAIDs, digoxin and ergotamine derivatives). Clinical and endoscopic correlation is recommended.
- Small bowel, resection:
- Ischemic enteritis with transmural necrosis / hemorrhage / perforation
- Resection margins are viable
- Enterohemorrhagic E. coli infection:
- History of eating undercooked meat
- Fibrin thrombi are characteristic but not specific for E. coli
- Chronic inflammatory bowel disease:
- Chronic ischemia with stricture formation may look identical to quiescent Crohn's disease
- Presence of hemosiderin laden macrophages favors ischemia
- Presence of fistulae and granulomas favors Crohn's Disease
- Nonsteroidal anti-inflammatory drug damage:
- Can be histologically similar to ischemia
- Clinical history is important
- Dysplasia:
- Should appear top heavy; ischemia is bottom heavy
- Typically has more branched and complex architecture
- Regenerative crypts in ischemia are usually small and round
- Behçet disease:
- Scattered punched out ulcers in the GI tract, no intervening normal mucosa
- Patients have urogenital and ocular manifestations, which are required for diagnosis
- Clostridium difficile colitis:
- Usually, a more diffuse process than ischemia (geographic)
- Hyalinized lamina propria or withered crypts are not usually seen with C. difficile
- Reference: Greenson: Diagnostic Pathology - Gastrointestinal, 3rd Edition, 2019, Am J Surg Pathol 1998;22:773
Comment Here
Reference: Ischemia
- Branched and complex architecture in crypts
- Evidence of thrombi, emboli or vasculitis
- History of eating undercooked meat
- Scattered ulcers in the GI tract, ocular, oral and genital manifestations
Comment Here
Reference: Ischemia
- Mucosal damage (initially) due to vascular occlusion or reduced blood flow associated with shunting
- Usually not due to atherosclerosis because of widely anastomosing blood supply
- Damage varies from none to hemorrhagic infarction
- Also ulceration, mucosal plaque, strictures
- Grossing bowel specimens with possible ischemia: dissect blood vessels and submit several sections to detect inflammation and thrombosis
- Early: lamina propria hemorrhage, superficial epithelial necrosis, preservation of deep crypts
- Later: ulceration with minimal inflammation, villous distortion, pyloric gland metaplasia progressing to full thickness infarct
- Late: strictures affecting muscularis propria
- Crohn's disease: marked inflammatory infiltrate, no stricture of muscularis propria, no hemosiderin
- Juvenile polyposis (JP) of small intestine is a rare condition that is characterized by presence of multiple hamartomatous polyps in the small bowel typically presenting during childhood
- This topic describes features of juvenile polyps in the small intestine that differ from colonic juvenile (retention) polyp
- Clinical findings of sporadic juvenile polyps can be nonspecific
- Multiple juvenile polyps of small intestine with or without multiple colorectal juvenile polyps are required diagnostic criteria for juvenile polyposis syndrome (JPS)
- Juvenile polyps in small intestine can present with intussusception
- Histologic features show cystically dilated or branched glands with edematous and inflamed stroma with or without dysplasia
- Close surveillance and genetic testing are recommended for symptomatic cases
- Hamartomatous gastrointestinal polyposis
- ICD-11: DA98.Z - polyps of small intestine, unspecified
- Juvenile polyposis syndrome (JPS) has an incidence of 1 per 100,000 live births in western countries (Bull World Health Organ 1990;68:655)
- Incidence of small intestine JP is very low compared to colorectal JP and is most commonly located in the duodenum, ranging between 14 and 33% (Endoscopy 2009;41:1001)
- JPS is an autosomal dominant inherited disease associated with the presence of mutation in SMAD4 / DPC4 or BMPR1A genes (Clin Genet 2009;75:79)
- The most common site for small intestine JPS is the duodenum (14 - 33%) (Ann Surg Oncol 1998;5:751)
- Classical mutational profile in JPS involves either the bone morphogenetic protein receptor 1A (BMPR1A), located at 10q23 or SMAD4 gene, located at 18q21 (Clin Genet 2009;75:79, Gut 2008;57:623)
- Juvenile polyposis of infancy shows contiguous deletion of BMPR1A and PTEN genes at chromosome 10q23 (Am J Hum Genet 2006;78:1066)
- Neoplasia may arise in JPS from adenomatous epithelium within the polyp through a landscaper mechanism and abnormal stromal environment that affects TGFβ signaling (Science 1998;280:1036)
- Pathophysiology and etiology of sporadic / solitary juvenile polyps in small bowel is unknown
- See Pathophysiology
- Can be asymptomatic
- Clinical presentation includes (Am J Surg 1975;129:198, Folia Med (Plovdiv) 2022;64:693)
- Abdominal pain, nausea and vomiting
- Gastrointestinal bleeding and iron deficiency anemia
- Intermittent obstruction including intussusception
- Endoscopic findings
- Video capsule endoscopy
- Minimally invasive
- Can explore the entire small bowel
- Diagnostic sensitivity for epithelial and subepithelial polyps ≤ 1 cm is 75% and 83%, respectively (Endoscopy 2005;37:960)
- Double and single balloon enteroscopy
- Enables sampling and intervention
- Diagnostic sensitivity in double balloon enteroscopy for epithelial and subepithelial polyps ≤ 1 cm is 100% and 97%, respectively (Gastrointest Endosc 2012;76:344)
- Video capsule endoscopy
- JPS can present with duodenal adenocarcinoma; however, the risk analysis of cancer development in a juvenile polyp is unclear (Ann Surg Oncol 1998;5:751)
- Diagnostic criteria of JPS are based on these criteria (Histopathology 1988;13:619, J Pediatr Gastroenterol Nutr 2019;68:453)
- More than 3 - 5 juvenile polyps in colon or rectum
- Multiple juvenile polyps throughout gastrointestinal tract (including stomach and small intestine)
- Any number of juvenile polyps and positive family history
- Diagnostic criteria of isolated or single small intestine juvenile polyps are unclear (J Pediatr Gastroenterol Nutr 2020;71:491)
- Diagnosis can be reached by integrating histology, immunohistochemistry and next generation sequencing (Fam Cancer 2022;21:441)
- Genetic testing of the following gene mutations
- SMAD4 and BMPR1A: for juvenile polyposis syndrome
- PTEN: identified in juvenile polyposis of infancy and Cowden syndrome
- STK11 / LKB1: seen in Peutz-Jeghers syndrome
- Preferred for at risk patients, starting at age of 4 (Curr Mol Med 2007;7:29)
- Upper and lower endoscopy surveillance starting at age 12 if symptomatic (Dis Colon Rectum 2012;55:1038)
- Magnetic resonance enterography (MRE)
- Noninvasive
- Compared to video capsule endoscopy, magnetic resonance enterography showed comparable rates of detection for large (> 15 mm) polyps (Abdom Imaging 2012;37:279)
- Risk of upper gastrointestinal cancer, risk analysis is unclear
- 3 year old girl presented with chronic iron deficiency anemia caused by a single painless juvenile polyp in the small intestine (J Pediatr Gastroenterol Nutr 2020;71:491)
- 35 year old woman presented with a juvenile polyp complicated with intussusception of the small intestine (Folia Med (Plovdiv) 2022;64:693)
- 50 year old woman presented with severe iron deficiency anemia and numerous stomach and jejunum polyps (Fam Cancer 2022;21:441)
- 73 year old woman with an isolated benign hamartomatous (juvenile-like) polyp (Am J Case Rep 2023:24:e938929)
- Screening and management for small intestine JPS are based on symptoms (Am J Gastroenterol 2015;110:223)
- Goal is preventing malignancy through close surveillance and endoscopic resection if symptomatic
- Sirolimus studies showed decreased disease burden in JPS and juvenile polyposis of infancy (Pediatrics 2019;144:e20182922)
- Multiple polyps in JPS, up to 200 polyps, with variable size reaching up to a few centimeters in some cases (J Gastroenterol Hepatol 2005;20:1634)
- Surface of the polyp may show erosion or ulceration
- Solitary or multiple hamartomatous polyps
- Classic solitary polyps (Histopathology 1988;13:619)
- Eroded surface and reactive epithelium
- Abundant edematous stroma with inflammatory cells and granulation tissue
- Cystically dilated glands
- Hyperplastic glands can be seen
- Multilobulated / atypical polyp
- Finger-like lobules
- Increased epithelial - stromal ratio
- Budding and branching glands
- Focal dysplasia can be present (Am J Surg Pathol 2011;35:530)
Contributed by Divya Sharma, M.D., Rachel Sheridan, M.D. and Sara Szabo, M.D.
- Loss of SMAD4 expression in ~50% of JPS patients with germline SMAD4 mutation (somatic inactivation of the SMAD4 wild type allele) (Clin Cancer Res 2010;16:4126)
- Loss of SMAD4 is not seen in sporadic juvenile polyps or in patients with BMPR1A mutations
- Genetic testing for germline mutations in SMAD4, BMPR1A and PTEN genes
- Duodenum, polyp, endoscopic resection:
- Polypoid mucosa with edematous stroma and cystically dilated glands consistent with features of juvenile polyps
- Negative for dysplasia or malignancy
- Peutz-Jeghers polyp:
- Commonly in jejunum and ileum
- Papillary villous architecture with tree-like arborization of smooth muscles and normal overlying epithelium
- STK11 / LKB1 gene mutations
- Inflammatory polyp:
- Branched and cystically dilated glands with reactive atypia of the mucosa
- Lamina propria acute and chronic inflammation
- Hyperplastic polyp:
- Common in second part of duodenum
- Classically a solitary polyp
- Epithelial serrations and bland cytology
- Association with BRAF V600E and KRAS
- Cowden syndrome polyp:
- Dilated glands with expanded stroma
- Can be associated with intramucosal lipoma
- No dysplasia
- Histologically can be similar to juvenile polyp
- PTEN gene mutations
- Cronkhite-Canada polyp:
- Ill defined polyps
- Cystically dilated glands with edematous stroma
- Mononuclear and eosinophilic infiltrate
- Histologically can be similar to juvenile polyp
- Nonhereditary
- Ménétrier disease:
- Most commonly located in stomach
- Presentation with small bowel polyposis is rare
- Marked foveolar hyperplasia with no atypia
- Interstitial edema with mild inflammatory infiltrate
Upper esophagogastroduodenoscopy (EGD) in a 12 year old patient with history of abdominal pain and nausea showed multiple polyps in the second part of the duodenum. The largest polyp was biopsied and the microscopic findings demonstrated surface erosion and cystically dilated glands. Which of the following is a criterion for a diagnosis of juvenile polyposis syndrome (JPS) in this patient?
- 2 colon polyps from the same patient with similar morphology to the duodenal polyp
- Germline mutation in STK11 gene
- Multiple duodenal polyps that are at least 10 mm in size
- Positive family history
- More than 3 - 5 juvenile polyps in colon or rectum
- Multiple juvenile polyps throughout gastrointestinal tract (including stomach and small intestine)
- Any number of juvenile polyps and positive family history
Answer B is incorrect as STK11 mutations are seen in Peutz-Jeghers syndrome. Answer A is incorrect because the diagnostic criteria of JPS requires the presence of more than 3 - 5 colorectal polyps. Answer C is incorrect as there is no polyp size cut off criteria for the diagnosis of JPS.
Comment Here
Reference: Juvenile polyposis
The microscopic image above is from a biopsy of a duodenal polyp. Which of the following features, if present, favors a diagnosis of juvenile polyp over an inflammatory polyp?
- Clinical history of inflammatory bowel disease
- Hyperplasia of the glands with gland branching and cystic dilatation
- Reactive atypia of the mucosal glands
- Solitary polyp and mucosal hyperemia seen on esophagogastroduodenoscopy (EGD)
Comment Here
Reference: Juvenile polyposis
- Benign smooth muscle tumors that arise from small bowel muscularis propria or muscular mucosa
- Duodenal leiomyomas of muscular mucosa are incidental, 1 - 2 mm, merge with muscularis mucosa; those not from muscularis mucosa are also often incidental but larger, 2 - 4 cm, with firm, white, whorled cut surface
- Low cellularity, no / rare mitotic figures, resembles esophageal leiomyoma
- GIST: often cytologically malignant in small bowel; CD117+, often CD34+ (Rom J Morphol Embryol 2011;52:1121, Am J Surg Pathol 2003;27:625)
- Rare, usually submucosal
- May cause ulceration or intussusception
- Lipohyperplasia often found near ileocecal valve
- 45 year old man with ileocecal intussusception due to lipoma (N Engl J Med 2009;361:e55)
- 52 year old woman with pedunculated lesion of terminal ileum (J Med Case Rep 2010;4:51)
- 77 year old man with ileocecal valve lipoma (JSLS 2009;13:80)
- Bright yellow, round, encapsulated
- Bulges into lumen, 5% multiple
- May have hemorrhage or necrosis
- Lipomatosis: bowel infiltrated by mature fat
- Mature adipocyte proliferation within the submucosal layer of the ileocecal valve not contained within a capsule (Iran J Radiol 2014;11:e4336)
- Rarely extends into serosa and mesenteric fat (Iran J Radiol 2014;11:e4336)
- Diffuse infiltration of the fatty tissue mainly in the submucosal layer is characteristic
- Usually asymptomatic and found incidentally
- Lack of encapsulation differentiates it from lipoma
- Cause of the fat deposition is not known
- Lipomatosis of the ileocecal valve
- Lipohyperplasia
- ICD-10: E88.2 - lipomatosis, not elsewhere classified
- M > F
- Uncommon under age 40
- More common with obesity
- Ileocecal valve and ileum
- Also can occur in cecum and ascending colon
- Benign mature adipocyte proliferation within the submucosal layer of the ileocecal valve
- Unknown
- Commonly asymptomatic
- May present with
- Right lower quadrant pain
- Constipation
- Diarrhea
- Obstruction
- Bleeding
- Hemorrhage
- Intussusception
- Can mimic appendicitis
- Risk factors: female, obese, age > 40
- Incidental diagnosis of right colon resection, which usually includes terminal ileum and ileocecal valve
- Commonly visualized on abdominal CT as well defined circumferential thickening of the lips of ileocecal valve with fat attenuation (Radiopedia: Lipomatosis of the ileocecal valve [Accessed 19 March 2019], Iran J Radiol 2014;11:e4336)
- Barium enema may show smooth mass-like enlargement of ileocecal valve
- Lips can be thickened with nodularity but margins are smooth (Am J Roentgenol Radium Ther Nucl Med 1973;119:323, Cancer 1972;119:323, J Korean Radiological Soc 2001;45:43)
- Ultrasound can show bulky and hyperechoic ileocecal valve with intussusception if present
- 45 year old man with melena and anemia (BMJ Case Rep 2013 Jul 8;2013)
- 50 year old woman with intestinal symptoms, anemia and positive fecal occult blood test (Tech Coloproctol 2007;11:278)
- 70 year old woman with abdominal pain (Iran J Radiol 2014;11:e4336)
- Usually asymptomatic
- Surgical resection if causing obstruction, significant symptoms or concern for malignancy
- Prominent and thickened ileocecal valve, usually an incidental finding during gross exam of right colectomy specimen
- Ileocecal valve mucosa with extensive submucosal proliferation of lipomatous mature adipocytes
- Fibrous septa and vascular congestion are usually present
- No surrounding fibrous capsule
- Not indicated
- Right hemicolectomy
- Adenocarcinoma of ascending colon (see synoptic report)
- Lipomatosis of ileocecal valve (incidental finding)
- 20 mesenteric lymph nodes negative for metastatic tumor (0/20)
- Lipoma:
- True lipoma of ileocecal valve is rare
- Has demarcating capsule around the fatty tissue and is confined to only one of the ileocecal lips
- Crohn's disease:
- Crohn's ileocolitis can be associated with lipomatosis of the ileocaecal valve
- Can cause difficulty in diagnostic imaging studies
- Gross and microscopic examination will show characteristic fissuring, skipping ulceration and transmural lymphoid aggregates
- Non-Hodgkin lymphoma (NHL):
- Can involve ileocecal valve and forming obstructive mass lesion or circumferential infiltration
- Microscopic examination will show malignant lymphoma cells with specific IHC staining patterns
- Adenomatous polyp:
- Can involve ileocecal valve
- Grossly, it is usually focal polypoid lesion rather than diffuse thickening
- Microscopic exam will show adenomatous tubules
- Adenocarcinoma of the ileocecal valve:
- Usually an irregular mass lesion with ulceration and obstruction
- Microscopic exam will show malignant tumor glands
A middle aged man underwent a right hemicoloectomy for cecal adenocarcinoma. Grossly, the ileocecal valve was prominent and diffusely thickened but no tumor was noted. What is your diagnosis?
- Crohn's disease
- Lipoma of the ileocecal valve
- Lipomatosis of the ileocecal valve
- Non-Hodgkin lymphoma
- Normal ileocecal valve
Comment here
Reference: Lipomatosis of the ileocecal valve
- Adipocyte proliferation in the muscularis propria not contained within a capsule
- Adipocyte proliferation in the submucosa contained within a capsule
- Adipocyte proliferation in the submucosa not contained within a capsule
- Diffuse gland proliferation
- Inflammatory cells predominately lymphocytes
Comment here
Reference: Lipomatosis of the ileocecal valve
- Dilated small intestinal lacteals, which may be primary or secondary
- Primary intestinal lymphangiectasia is a rare disorder resulting in lymph leakage into the small bowel lumen and responsible for protein losing enteropathy, leading to lymphopenia, hypoalbuminemia and hypogammaglobulinemia (Orphanet J Rare Dis 2008;3:5)
- Lymphagiectasia can be primary or secondary; primary intestinal lymphangiectasia is more common in children, though in rare cases it can present in early adulthood and is usually associated with malabsorption
- Secondary lymphangiectasia is often seen in adults and can be associated with various obstruction / constrictive conditions (described below), though exclusion of an underlying malignancy remains the main consideration
- Microscopy shows dilated lymphatic vessels in the mucosa and submucosa without significant villous atrophy or inflammation
- Waldmann disease
- ICD-10: K63.89 - Other specified diseases of intestine
- Primarily affects children (generally diagnosed before 3 years of age) and young adults but may be diagnosed later in adults (Orphanet J Rare Dis 2008;3:5)
- Very rare familial forms of Waldmann disease have been reported
- Small intestine
- To date, the etiology of primary intestinal lymphangiectasia is unknown
- Several genes, such as VEGFR3 (vascular endothelial growth factor receptor 3), prospero related homeobox transcriptional factor PROX1, forkhead transcriptional factor FOXC2 and SOX18 are implicated in the development of the lymphatic system (Orphanet J Rare Dis 2008;3:5)
- Mutations in the FOXC2 (MFH1) gene or deletion of the PIK3R1 gene may result in lymphangiectasia, arrested lymphatic sprouting and maturation defects (World J Gastrointest Endosc 2011;3:235)
- Etiology is unknown for primary intestinal lymphangiectasia
- Syndromes associated with primary intestinal lymphangiectasia include von Recklinghausen, Turner or Noonan, Klippel-Trenaunay and Hennekam (Turk J Gastroenterol 2018;29:714)
- Some secondary causes of intestinal lymphangiectasia include constrictive pericarditis, intestinal lymphoma, lymphenteric fistula, Whipple disease, Crohn's disease, sarcoidosis, intestinal tuberculosis, systemic sclerosis, radiation or chemotherapy with retroperitoneal fibrosis and human immunodeficiency virus related enteropathy (Orphanet J Rare Dis 2008;3:5)
- Classical symptoms are bilateral or unilateral lower limb edema and intermittent diarrhea (Am J Case Rep 2016;17:512)
- Some patients develop steatorrhea
- Accumulation of fluids in body cavities resulting in pleural effusion or ascites has been reported
- Lymphedema (Orphanet J Rare Dis 2008;3:5)
- In children, primary intestinal lymphangiectasia is generally diagnosed before 3 years of age and may be complicated by fatigue, abdominal pain, nausea, vomiting and weight loss, inability to gain weight and growth retardation; malabsorption may cause fat soluble vitamin deficiencies and hypocalcemia leading to convulsions (Orphanet J Rare Dis 2008;3:5)
- In secondary cases, the symptoms may be related to the underlying condition
- Endoscopy or capsule endoscopy can show swollen mucosa and enlarged whitish villi (World J Gastrointest Endosc 2011;3:235)
- Biopsy
- Hypoproteinemia, hypoalbunimia and hypogammaglobunimea due to protein loss (Orphanet J Rare Dis 2008;3:5)
- Exudative enteropathy is confirmed by high 24-h stool alpha-1 antitrypsin clearance due to enteric protein loss
- Functional absorption tests such as D-xylose test results are normal in primary intestinal lymphangiectasia
- Ultrasonographic findings may show dilation of the intestinal loops, regular and diffuse thickening of the walls, plical hypertrophy and severe mesenteric edema and, in some cases, ascites (Orphanet J Rare Dis 2008;3:5)
- CT scan can show diffuse, nodular small bowel wall thickening and edema, which are a consequence of the dilated lymphatics within the villi along with some degree of small bowel dilation with (in few cases) a halo sign due to swelling and edema (Orphanet J Rare Dis 2008;3:5)
- Primary intestinal lymphangiectasia is a chronic debilitating disorder requiring constraining long term dietary control based on a low fat regimen associated with supplementary medium chain triglycerides (Orphanet J Rare Dis 2008;3:5)
- Primary intestinal lymphangiectasia may be life threatening when malignant complications (lymphoma) or serous effusion(s) (pleural, pericardic) occur (Orphanet J Rare Dis 2008;3:5)
- 10 year old boy with pediatric localized intestinal lymphangiectasia treated with resection (Int Med Case Rep J 2019;12:23)
- 30 year old woman with primary intestinal lymphangiectasia causing intussusception and small bowel obstruction (ACG Case Rep J 2019;6:e00233)
- 47 year old patient with marginal zone lymphoma complicated by protein losing enteropathy (Case Rep Hematol 2016;2016:9351408)
- 60 year old woman with Waldmann disease (Rev Esp Enferm Dig 2017;109:385)
- Low fat diet associated with supplementary medium chain triglycerides is the cornerstone of primary intestinal lymphangiectasia medical management (Orphanet J Rare Dis 2008;3:5)
- Small bowel resection is useful in the rare cases in which intestinal lymphangiectasia is segmental and localized (duodenum)
- Steroids were prescribed to patients with intestinal lymphangiectasia secondary to inflammatory disease with variable efficacy and also to systemic lupus erythematosus patients with protein losing enteropathy
- Treatment of the underlying disease in secondary causes
- White granular surface (Turk J Gastroenterol 2018;29:714)
- Mucosa can be swollen
- May form a polypoid lesion
- Moderately to severely dilated mucosal and submucosal lymphatic vessels with polyclonal normal plasma cells (Orphanet J Rare Dis 2008;3:5)
- Intestinal lymphatics may be dilated in few or many villi
- No histologic difference between primary and secondary lymphangiectasia
- Lymphatic vessels are D2-40 positive
- Small intestine, biopsy:
- Small bowel lymphangiectasia (see comment)
- Comment: Small bowel lymphangiectasia can be primary or secondary to other conditions such as constrictive pericarditis, intestinal lymphoma, lymphenteric fistula, Whipple disease, Crohn's disease, sarcoidosis, intestinal tuberculosis, systemic sclerosis, radiation or chemotherapy with retroperitoneal fibrosis and human immunodeficiency virus related enteropathy.
- Lymphangioma (Am J Clin Pathol 2015;144:563):
- Presents as a discrete lesion
- Most reliable histologic features distinguishing lymphangioma from lymphangiectasia are the presence of smooth muscle surrounding the lymphatic spaces and complete circumferential lining of spaces by endothelial type cells present in lymphangioma
- Lymphoid aggregates may often be seen in lymphangiomas
A 10 year old presented with bilateral edema of the legs and diarrhea. Laboratory evaluation showed hypoalbuminemia and hypogammaglobinemia. No other abnormality was identified. Capsule endoscopy performed showed white granular surface in the small intestine. A biopsy was performed which showed dilated spaces in the mucosa and submucosa. PAS stain performed is negative. What is the most likely cause of the patient's underlying condition?
- Crohn's disease
- Primary lymphangiectasia
- Ulcerative colitis
- Whipple disease
- Nonencapsulated benign proliferation of lymphatic vessels
- Benign neoplasm of lymphatic origin (proved by immunohistochemical expression of lymphatic markers, such as D2-40)
- Usually detected as an incidental finding during procedures performed for other reasons but can be symptomatic
- Mostly presents as a sporadic single lesion and rarely can be syndromic and diffuse
- Prognosis is excellent; local recurrence may occur, especially for incompletely excised lesions
- Lymphangiomatosis (in cases of diffuse organ involvement)
- Lymphatic malformation / hamartoma
- Hemangiolymphangioma
- Simple (capillary) lymphangioma
- Mesenteric lymphangioma
- ICD-10: D18.1 - Lymphangioma, any site
- Mostly arises in children < 5 years old, with male predominance; rare in adults (Pediatr Radiol 2002;32:88)
- Accounts for 2% of GI tumors in adults and 6% of GI tumors in children
- Terminal ileum is most common GI site but can arise in any part of the GI tract (Gastrointest Endosc 2005;62:181)
- Environmental exposure: as a result of reactive lymphatic proliferation secondary to trauma
- Developmental anomaly: failure of development of lymphatic channels, resulting in lymphatic channel engorgement and dilation
- Genetics: Noonan syndrome, Maffucci syndrome, trisomies 13, 18, 21 and Turner syndrome (cystic hygroma)
- Variable clinical presentation, from asymptomatic to abdominal pain with mass effect (Keio J Med 1993;42:41)
- May present as an incidental finding during surgical procedures performed for other conditions (Int J Surg Case Rep 2020;66:319)
- Rarely in large lesions, complications include obstruction, infarct and perforation (J Clin Diagn Res 2017;11:PD01)
- May be associated syndromes (Turner, Noonan, Maffucci)
- Rarely is large, multiple or involves multiple organs (lymphangiomatosis)
- Initial examination is by ultrasound and CT; definitive diagnosis by histopathology and ancillary studies
- Ultrasound: cystic lesion; endoscopic ultrasound may be useful to determine depth of lesion
- CT: nonenhancing cystic lesions, multiplication, may have calcifications (Acta Radiol Open 2015;4:2047981614564911)
- Incompletely removed lesions may increase recurrence
- Excellent prognosis
- 22 year old man with lymphangioma causing intestinal malrotation (BMJ Case Rep 2013;2013:bcr2013008994)
- 37 year old man with recurrent intussusception (Int J Surg Case Rep 2020;75:126)
- 53 year old man with polypoid lymphangioma of the ileocecal valve (Saudi J Gastroenterol 2014;20:262)
- 57 year old woman with small intestinal lymphangioma who presented with persistent gastrointestinal bleeding (World J Gastroenterol 2012;18:2145)
- Complete surgical resection, especially for symptomatic lymphangioma
- Endoscopic mucosal resection can be used for mucosal lymphangioma (Ann Surg Treat Res 2018;94:52)
- Well defined lesions, polypoid or pedunculated mass with 3.0 cm average size
- Small lesions typically superficial (Gastrointest Endosc 2000;52:255)
- Large lesions may have calcifications and may involve deeper layers (World J Gastroenterol 2012;18:2145)
- Dilated and anastomosing thin walled blood vessels, lined by single layer of flat endothelial cells (Am J Clin Pathol 2015;144:563)
- Blood vessels are surrounded by lymphoid aggregate
- Lymphocytes, eosinophils and proteinaceous material often fill the vascular spaces
- Smooth muscle in blood vessel wall might be seen in large lesions
- No nuclear enlargement, hyperchromasia, pleomorphism or prominent nuclei should be seen in the lining endothelial cells
- Common endothelial markers: CD34, CD31 and factor VIII
- More specific markers: podoplanin (D2-40), VEGFR3 and LYVE1 (J Biol Chem 2001;276:19420)
- Small intestine, biopsy:
- Lymphangioma
- Secondary lymphangiectasia:
- Dilated lymphatics arise in response to trauma, obstruction or radiation
- Has identical histology (dilated lymphatics)
- Clinical history and presentation is necessary for distinction (Lymphat Res Biol 2009;7:75)
- Lymphangioma-like Kaposi sarcoma:
- Characteristic area of spindle cells with dissecting blood vessels diagnostic of Kaposi sarcoma should be present and HHV8 is positive
- Hemangioma:
- Smaller in size, has lobular contour and has fewer associated lymphocytes
- Angiosarcoma:
- Angulated and infiltrative blood vessels
- Nuclei of endothelial cells range from hyperchromatic to highly atypical with prominent nucleoli
A 34 year old man underwent upper gastrointestinal endoscopy for chronic anemia. A random biopsy from the duodenum shows dilated lymphatics lined by flatted endothelial cells. No atypia or mitosis is seen. Both CD31 and D2-40 are positive, highlighting the endothelial cells lining the lymphatic channels. HHV8 is negative. The most likely diagnosis is
- Angiosarcoma
- Hemangioma
- Kaposi sarcoma
- Lymphangioma
- Angelman syndrome
- DiGeorge syndrome
- Klinefelter syndrome
- Turner syndrome
- Refractory sprue-like condition associated with colonic mucosal abnormalities (Can J Gastroenterol 2008;22:771)
- Also called lymphoid polyp; formerly called pseudolymphoma
- Most common site is ileocecal region
- Causes intussusception in children
- Nodular lymphoid hyperplasia: nodules throughout bowel, associated with giardiasis or childhood viral infection
- 60 year old woman with florid reactive lymphoid hyperplasia of terminal ileum (BMJ Case Rep 2010;2010)
- Numerous lymphoid follicles with germinal centers in lamina propria or submucosa
- CD20, CD45; do not confuse BCL2 expression in marginal zone cells with BCL2 expression of germinal center cells in follicular lymphoma (Am J Surg Pathol 2003;27:888)
- Low grade lymphoma: often infiltrative, light chain restriction
- Standard site for biopsies is proximal jejunum, just distal to ligament of Treitz
- Mount specimen mucosal side up on solid substance, then embed perpendicular to mounting material, then step section or serial section
- Small bowel is important for absorption of fats, fat soluble vitamins, proteins, carbohydrates, electrolytes, minerals, water
- In U.S., most common malabsorption disorders are celiac sprue, pancreatic insufficiency and Crohn's disease
- Steatorrhea: bulky, greasy stools associated with weight loss, anorexia, muscle wasting
- Diarrhea, flatus, abdominal pain, weight loss, mucositis, anemia (iron, folate, vitamins B6, B12)
- Bleeding / purpura (vitamin K)
- Osteopenia, tetany (calcium, magnesium, vitamin D)
- Amenorrhea / impotence / infertility (generalized malnutrition)
- Hyperparathyroidism (calcium, vitamin D)
- Edema (albumin)
- Dermatitis (zinc, vitamin A, fatty acids, niacin)
- Peripheral neuropathy (vitamins A, B12)
- Disturbances related to:
- Intraluminal digestion: saliva, gastric peptic digestion, small bowel, bile salts
- Terminal digestion: hydrolysis of carbohydrates and peptides by disaccharidases and peptidases in brush border of small bowel
- Transepithelial transport: across small bowel epithelium to intestinal vasculature; fatty acids to triglycerides, cholesterol to chylomicrons
- Causes of defective intraluminal digestion:
- Digestion of fats / proteins: pancreatic insufficiency due to pancreatitis or cystic fibrosis, Zollinger-Ellison syndrome
- Defective bile secretion (fat solubilization): ileal dysfunction or resection with decreased bile salt uptake, cessation of bile flow (obstruction, hepatic dysfunction), nutrient preabsorption or modification by bacterial overgrowth
- Causes of abnormalities in terminal digestion or transepithelial transport:
- Disaccharidase deficiency (lactose intolerance), bacterial overgrowth, abetalipoproteinemia, defects in ileal bile acid transporter
- Rare; due to mutations in MTP gene encoding microsomal triglyceride transfer protein (MTP); autosomal recessive (Ann Hepatol 2011;10:221)
- Causes defect in synthesis and export of apoprotein B from intestinal mucosal cells
- As a result, free fatty acids and monoglycerides cannot be assembled into chylomicrons and become triglycerides stored within cells, causing lipid vacuolization
- Symptoms: failure to thrive, diarrhea, steatorrhea
Laboratory
- Lipid profile shows no chylomicrons, no VLDL, no LDL
- CBC smear shows acantholytic red blood cells (burr cells) due to lipid membrane abnormalities
Microscopic (histologic) description
- Marked fat vacuoles in apical villous cytoplasm, normal villi
Positive stains
- Fat stains highlight lipid vacuoles
Differential diagnosis
- See Celiac sprue
- Subtype of refractory sprue with patchy, excessive subepithelial collagen deposits (5 of 10 patients in one study, Am J Surg Pathol 2000;24:676)
- May eventually respond to gluten free diet but disease may also be fatal (Mod Pathol 2010;23:12)
Microscopic (histologic) description
- Small intestinal villous and crypt atrophy, subepithelial collagen deposit thicker than 12 μm that entraps lamina propria cellular elements (Arch Pathol Lab Med 2011;135:803)
- Also called congenital or familial microvillous atrophy
- Disorder of intestinal brush border that causes intractable watery diarrhea with steatorrhea in infants
- Patients require total parental nutrition and rarely live beyond age 2 years
- Villous atrophy may be due to apoptotic cell loss (Hum Pathol 2000;31:1404)
Treatment
- Small bowel transplant
Microscopic (histologic) description
- Severe villous abnormality with crypt hypoplasia, resembling celiac sprue but without lymphocytosis
- Increased enterocyte apoptosis and proliferation, bubbly vacuolated apical cytoplasm with extensive or patchy absence of brush border, absence of inflammation (Ultrastruct Pathol 2010;34:327)
Microscopic (histologic) images
Images hosted on other servers:
Positive stains
- CD10 (Am J Surg Pathol 2002;26:902), PAS, polyclonal CEA, alkaline phosphatase (cytoplasmic staining versus linear brush border staining in normals)
- Vacuoles - PAS, CEA
- Cytoplasmic CD10 staining of absorptive colonocytes can aid in the diagnosis in situations where only colonic biopsy could be obtained (Am J Surg Pathol 2010;34:970)
Electron microscopy description
- Abnormal microvillus structures at luminal border of enterocytes
- Apical intracytoplasmic inclusions lined by microvilli
- Also called postinfectious sprue
- Affects people living in or visiting the tropics, particularly Caribbean (not Jamaica), Africa, India, Southeast Asia, Central / South America (Case Rep Gastroenterol 2010;4:168)
- Has endemic and epidemic features
- May be due to E. coli or Haemophilus
- Symptoms: malabsorption within weeks of acute diarrheal enteric infection
Treatment
- Broad spectrum antibiotics (tetracycline), folic acid, vitamin B12
- No increased risk of intestinal lymphoma
Microscopic (histologic) description
- Variable villous atrophy (none, partial, total)
- Injury to entire small bowel (not proximal as in celiac sprue), inflammatory infiltrate, crypt hyperplasia
Microscopic (histologic) images
Contributed by Hanni Gulwani, M.B.B.S.

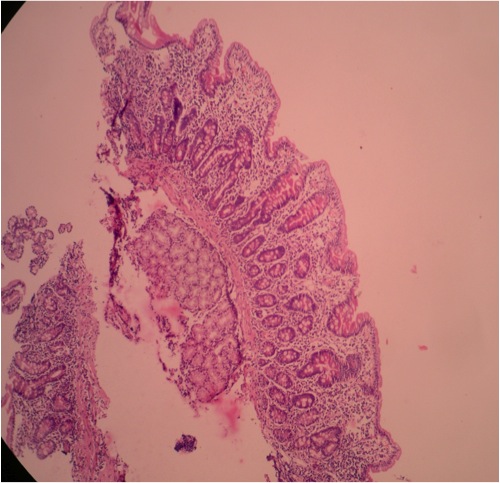
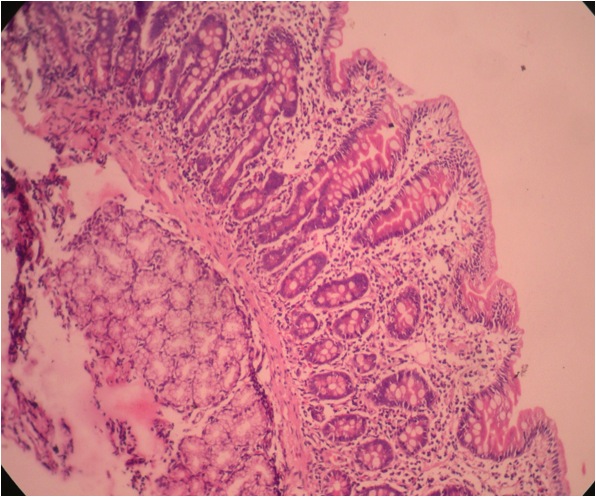
40 year old man with chronic diarrhea who improved after antibiotics; marked villous atrophy in duodenal biopsy but no increase in intraepithelial lymphocytes
Images hosted on other servers:
Additional references
- Intractable diarrhea syndrome, sometimes familial, beginning in neonates
- Also called intestinal epithelial dysplasia (Orphanet J Rare Dis 2007;2:20)
- Due to epithelial cell adhesion molecule gene (EpCAM) (Gastroenterology 2008;135:429, Gut Liver 2010;4:407)
Treatment
- Total parental nutrition
Microscopic (histologic) description
- Variable villus abnormality, epithelial crowding, disorganization and focal tufting
- No epithelial lymphocytosis
Microscopic (histologic) images
Images hosted on other servers:
Electron microscopy description
- Basement membrane abnormalities; increase in desmosome length and number; decreased number of microvilli of surface enterocytes with structural disorganization
Electron microscopy images
Images hosted on other servers:
- Rare malignant mesenchymal neoplasm of the gastrointestinal tract
- Mesenchymal malignancy that grows in sheets or cords and may have osteoclast-like giant cells
- Positive for S100 and synaptophysin; negative for HMB45 and MelanA
- Harbors EWSR1 translocation
- Also known as clear cell sarcoma-like tumor of the gastrointestinal tract
- Small bowel is most common site in gastrointestinal tract, followed by stomach (Am J Surg Pathol 2012;36:857)
- Median age 35 years; no sex predilection (Arch Pathol Lab Med 2015;139:407)
- Most common symptoms are abdominal pain and intestinal obstruction
- Tumor can spread to lymph nodes and liver and may cause death
- 17 year old boy with stomach mass (Oncol Lett 2014;8:2687)
- 18 year old man with small bowel mass (Diagn Pathol 2017;12:29)
- Surgery; possible adjuvant role for c-Met / ALK inhibitors and VEGF inhibitors (Oncology 2016;91:348)
- Large mass (mean 5 cm)
- Sheets or nests of oval / spindled mesenchymal cells with clear or eosinophilic cytoplasm, visible nucleoli and often mitoses
- Osteoclast-like giant cells may be seen
- Tumor often spans the entire wall
- HMB45, MelanA, CD117, DOG1, cytokeratin
- Secretory vesicles and dense core granules
- Translocation involving EWSR1 (most commonly EWSR1-ATF1 [t(12;22)] or EWSR1-CREB1)
- Jejunum, resection:
- Malignant gastrointestinal neuroectodermal tumor (6.1 cm) (see comment)
- Lymphovascular invasion present.
- Margins of resection unremarkable.
- Three of eight lymph nodes involved by metastatic tumor (3/8).
- Comment: Gastrointestinal neuroectodermal tumor is a rare malignancy that can metastasize distantly and has a poor prognosis. Immunohistochemical stains show the tumor is positive for S100 and synaptophysin.
- Alveolar rhabdomyosarcoma:
- Clear cell sarcoma:
- Gastrointestinal stromal tumor:
- Synovial sarcoma:
- Positive for keratins, harbors t(X;18) translocation
- It can demonstrate a t(12;22) EWSR1-ATF1 translocation
- It most commonly occurs in the colon
- Its preferred site of metastasis is the lungs
- It stains positive for S100 and cytokeratin by immunohistochemistry
- Most patients are in their sixth decade or older
Comment Here
Reference: Malignant GI neuroectodermal tumor
- Meckel diverticulum is a congenital disorder caused by incomplete obliteration of omphalomesenteric duct (also called vitelline duct), which results in a true diverticulum, typically located in the ileum within 2 feet from the ileocecal valve (Zhang: Surgical Pathology of Non-neoplastic Gastrointestinal Diseases, 1st Edition, 2019)
- Most common congenital malformation of the GI tract, affecting 2 - 4% of the population
- Most common cause of GI tract bleeding in children, usually before age 2
- Usually occurs within 2 feet from the ileocecal valve at the antimesenteric border of the ileum
- True diverticulum that consists of all layers of intestinal wall
- 50% with ectopic tissue, most common is gastric mucosa and then followed by pancreatic tissue
- Rule of 2s:
- Occurring in about 2% of infants
- Usually 2 inches long
- Located in the ileum approximately 2 feet from the ileocecal valve
- M:F = 2:1
- 2 types of ectopic tissue (gastric or pancreatic)
- ICD-10: Q43.0 - Meckel diverticulum (displaced) (hypertrophic)
- Most common congenital malformation of the gastrointestinal tract
- Difficult to determine the prevalence because many patients are asymptomatic
- Estimated to be in approximately 2 - 4% of the population
- 63% occur in men (Ann Surg 2005;241:529)
- Usually occurs within 2 feet from the ileocecal valve at the antimesenteric border of the ileum
- In children under 2 years old, the average distance is 34 cm from the ileocecal valve and for adults is 67 cm (J Antibiot (Tokyo) 1978;31:3)
- Remnant of the omphalomesenteric duct, which connects the yolk sac with the developing midgut in the fetal life
- Normally regresses by the seventh to eighth week of gestation
- Incomplete obliteration of the omphalomesenteric duct results in Meckel diverticulum
- Reference: Radiographics 2004;24:565
- Congenital disorder, caused by the incomplete obliteration of the omphalomesenteric duct
- Diverticulum results from fibrous obliteration of the umbilical end of the omphalomesenteric duct and complete patency of the ileal end of the duct
- Diverticulum is on the antimesenteric side of the ileum and may connect to the umbilicus by a fibrous band if the fibrous portion of the duct fails to be completely obliterated and absorbed
- 16% of patients may be symptomatic; among symptomatic patients, M:F ≈ 3:1 (Ann Surg 2005;241:529)
- Most frequent complications are bleeding from ectopic gastric mucosa and bowel obstruction due to the intussusception, volvulus or adhesive band
- Other uncommon complications include ulceration, diverticulitis, perforation and tumors
- Can also be present in an external hernia, typically on the right side, known as a hernia of Littre
- May be associated with other congenital anomalies
- Asymptomatic Meckel diverticulum is often incidentally diagnosed during laparoscopy or laparotomy
- However, preoperative diagnosis is still challenging
- Technetium 99m pertechnetate scan, also called Meckel scan, detecting gastric mucosa, has become the most common and accurate noninvasive test to diagnose Meckel diverticulum in children, with a 95% specificity and 85% sensitivity
- However, this test is only 9% specific and 62% sensitive in adults (Clin Anat 2011;24:416)
- Identified as a saccular, blind ending structure located on the antimesenteric border of the ileum
- Antimesenteric location can be confirmed from the position of the diverticulum, which faces away from the axis of the root of the small intestinal mesentery (Br J Surg 1980;67:417)
- Junction of the diverticulum with the ileum may show a mucosal triangular plateau or triradiate fold pattern, which represents the site of omphalomesenteric duct attachment to the ileum (AJR Am J Roentgenol 1980;134:925)
- Technetium 99m pertechnetate scan, also called Meckel scan, can detect gastric mucosa in Meckel diverticulum (Clin Anat 2011;24:416)
- 6 year old boy with desmoplastic small round cell tumor arising in Meckel diverticulum (J Clin Oncol 2007;25:3372)
- 11 year old boy with a giant Meckel diverticulum in distal ileum (Radiol Case Rep 2020;16:400)
- 16 year old girl with Meckel diverticulum presenting with necrotic diverticulitis attached to the bladder dome (Medicina (Kaunas) 2021;57:495)
- 64 year old man with Meckel diverticulum presenting with acute appendicitis (Int J Surg Case Rep 2021;83:105994)
- 70 year old man with Meckel diverticulum initially presented with left strangulated inguinal hernia (Int J Surg Case Rep 2021;79:271)
- Treatment for symptomatic Meckel diverticulum is surgical resection
- Prophylactic diverticulectomy is recommended for incidental asymptomatic Meckel diverticulum (Ann Surg 2005;241:529)
- Small bowel with blind pouch or diverticulum on antimesenteric aspect
- Average length is 3 cm but can be longer than 10 cm; giant Meckel diverticulum refers to those longer than 5 cm (Radiographics 2004;24:565)
- Diameters are usually smaller than ileum
- Other lesions may also be identified, such as umbilical bands or omphalomesenteric duct cyst
- Grossly specimen should be examined for evidence of perforation or presence of tumor (J Med Case Rep 2010;4:264)
- True diverticulum that consists of all layers of intestinal wall
- Common abnormalities in symptomatic Meckel diverticulum are ectopic tissue, diverticulitis, enterolith and tumors
- Most common ectopic tissue seen in Meckel diverticulum is gastric mucosa, which may consist of gastric foveolar or oxyntic glands
- Helicobacter pylori has also been seen in the ectopic gastric mucosa (Pathology 1998;30:7)
- Second most common ectopic tissue is pancreatic tissue
- Other rare ectopic tissue includes jejunal, duodenal mucosa or Brunner tissue, colonic mucosa, endometriosis or hepatobiliary tissue
- Tumors can occur in the ectopic tissue: the most common tumor is neuroendocrine tumor (Ann Med Surg (Lond) 2019;43:75)
- Other rare tumors include adenocarcinoma, gastrointestinal stromal tumor, intraductal papillary mucinous neoplasm or pancreatic intraepithelial neoplasm (within pancreatic heterotopia), leiomyosarcoma, peripheral nerve sheath tumor and desmoplastic small round cell tumor (Ann Med Surg (Lond) 2019;43:75)
- Small bowel, ileum, segmental resection:
- Meckel diverticulum identified measuring 3 x 1 cm
- Located 50 cm from the ileocecal valve
- Ectopic pancreatic tissue identified within the diverticulum, otherwise unremarkable
- Intestinal duplication or duplication cyst:
- Tubular or cystic
- Located at the mesenteric side
- Small bowel bleeding or perforation:
- No outpouching structures at the site of bleeding or perforation
- No ectopic tissue
- Allantois
- Ductus arteriosus
- Metanephric mesenchyme
- Omphalomesenteric duct
- Paramesonephric duct
Comment Here
Reference: Meckel diverticulum
Regarding the histologic finding in the section from a Meckel diverticulum, which of the following statements is true?
- Atypical glands suspicious for a malignancy
- Clinically not significant
- Commonly seen in Meckel diverticulum
- Metaplastic change
- Small bowel mucosa with no significant pathological changes
Comment Here
Reference: Meckel diverticulum
- Rare prenatal complication in 1 per 30,000 live births
- GI perforation releases meconium into abdominal cavity, inducing sterile inflammatory reaction and calcium deposition
- Perforation may be due to anoxia leading to bowel ischemia, atresia, congenital bands, Hirschprung disease, internal hernia, meconium ileus, stenosis, volvulus or idiopathic
- Presents with fetal distress, maternal polyhydramnios, abdominal distention or a mass
- Newborns with perforation should be evaluated for cystic fibrosis (Pediatr Surg Int 2003;19:75)
- Prenatal ultrasound shows dilated bowel, ascites, polyhydramnios, intra-abdominal calcifications (Prenat Diagn 2005;25:676)
- Ultrasound findings have prognostic value (Fetal Diagn Ther 2003;18:255, Prenat Diagn 2007;27:960)
- 35 week old girl with intrauterine distress (Case of the Week #106)
- Surgical
- Gestational age at diagnosis does not predict postnatal outcome (J Pediatr Surg 1995;30:979)
- Organized peritonitis with fibrosis, calcifications, dense intestinal adhesions
- Meconium pseudocyst (fibrous wall) may form
- Peritoneal surface shows fibrinous exudate with microcalcifications, bile pigment-like debris, histiocytes, chronic inflammatory cells
- Vernix caseosa peritonitis: cheesy white exudate coats the visceral organs after cesarean section (J Obstet Gynaecol 2007;27:660)
- Acute, necrotizing inflammation of small bowel and colon in patients with myelosuppression
- Also called typhlitis, neutropenic enterocolitis, ileocecal syndrome
- Most common acquired GI emergency of neonates (eMedicine: Necrotizing Enterocolitis [Accessed 13 February 2018])
- Common in premature or low birth weight infants, particularly when they start on oral foods at 2 - 4 days
- Also adults with various myeloproliferative disorders, solid malignancies, posttransplant immunosuppression (eMedicine: Neutropenic Enterocolitis [Accessed 13 February 2018])
- Affects terminal ileum, ascending colon
- Intestinal bacteria invade immature intestinal epithelium, causing subsequent inflammation and tissue necrosis
- Bacteria in food produce more cytokines and injure mucosa
- May be due to TNF receptor 1 dependent depletion of mucus which occurs in immature small intestine (Am J Physiol Gastrointest Liver Physiol 2011;301:G656)
- May also be due to deranged intestinal blood flow (J Pediatr Surg 2011;46:1023)
- Symptoms: mild GI disturbance or fulminant illness with intestinal gangrene, perforation, sepsis, shock
- Complications: short bowel syndrome, malabsorption (due to ileal resection), strictures, recurrence
- Fluids and surgery if gangrene / perforation
- Early: mucosal edema, hemorrhage, necrosis
- Late: hemorrhagic and gangrenous bowel wall, fibrous strictures; often pneumatosis cystoides intestinalis
- After recovery, Paneth cell hyperplasia; colon also shows metaplastic Paneth cells (Pediatr Res 2011;69:217)
- Usually fatal
- 50 year old man with obstructive jaundice with an ampullary mass: collision tumor-mixed adenoneuroendocrine carcinoma (Case #322)
- 52 year old man with collision tumor of primary adenocarcinoma and neuroendocrine carcinoma of duodenum (Rare Tumors 2012;4:e20)
- 55 year old woman with large cell neuroendocrine carcinoma with glandular differentiation (J Clin Pathol 2004;57:1098)
- 73 year old woman with large cell neuroendocrine carcinoma with squamous cell and glandular components (Jpn J Clin Oncol 2011;41:434)
- 74 year old man with small cell neuroendocrine carcinoma with villous adenoma (World J Gastroenterol 2008;14:4709)
- 74 year old with large cell neuroendocrine carcinoma (Arch Pathol Lab Med 2003;127:221)
- Ampullary tumors are rare, present with progressing jaundice
- Aggressive with poor prognosis (Hepatobiliary Pancreat Dis Int 2008;7:422)
- Small cell carcinoma is rare; in few cases reported, prognosis better than for small cell lung tumors (Arch Pathol Lab Med 2003;127:e357, J Clin Oncol 2004;22:2730, J Clin Pathol 2010;63:620)
- Marked pleomorphism, large irregular hyperchromatic nuclei, prominent nucleoli, tumor necrosis, frequent mitotic figures
- Large cell carcinoma:
- Islands and trabeculae of large cells with brisk mitotic activity and extensive necrosis
- Cells have more cytoplasm than small cell carcinoma, irregular chromatin, frequent nucleoli
- Small cell carcinoma:
- Sheets and nests of small, round cells with scanty cytoplasm, hyperchromatic nuclei, stippled chromatin, indistinct nucleoli, numerous mitotic figures and apoptotic cells
- Foci of necrosis and vascular invasion common
- Resembles pulmonary tumor
- Pure or mixed with adenocarcinoma
- Small cell carcinoma: membrane bound dense core granules
- Well differentiated duodenal, jejunal and ileal epithelial neoplasms with neuroendocrine differentiation
- Subtypes:
- Neuroendocrine tumor, grades 1 - 3
- Gastrinoma, NOS
- Somatostatinoma, NOS
- Enterochromaffin cell carcinoid
- Neuroendocrine tumors (NETs) of the small intestine and ampulla are rare, although the incidence is increasing
- Majority of tumors are nonfunctioning; functional (hormone secreting) neuroendocrine tumors are rare
- Histologic features include monotonous tumor cells with finely stippled chromatin arranged in nests, trabeculae or tubuloglandular architecture
- Grading is based on mitotic rate and Ki67 index
- Intestinal NET: carcinoid (not preferred), well differentiated neuroendocrine tumor
- Prevalence increased from 0.006% in 1993 to 0.048% in 2012
- NETs are more prevalent in women than in men (2.5:1)
- Incidence of GI-NET 3.56 per 100,000 population
- Incidence of small intestinal NET 1.05 per 100,000 persons
- References: World J Gastrointest Oncol 2020;12:791, JAMA Oncol 2017;3:1335
- > 95% duodenal NETs located in part 1 or 2 (those in part 2 are predominantly in the ampullary region)
- Gastrin expressing NETs: duodenum
- Somatostatin expressing NETs: ampulla
- Majority of jejunoileal NETs are located in the distal ileum, with only 11% of jejunal origin:
- Serotonin expressing enterochromaffin cell NETs: Meckel diverticula
- Poorly understood at this time
- Etiology of sporadic intestinal NETs is unknown
- Minority of NETs arise in setting of hereditary cancer predisposition syndromes
- Multiple endocrine neoplasia (MEN) type 1: develop multiple duodenal gastrinomas (Neuroendocrinology 2017;104:112)
- 10% of ampullary somatostatinomas arise in the setting of neurofibromatosis type 1 (NF1) (J Gastrointest Surg 2010;14:1052)
- Nonfunctioning NETs: patients may present with symptoms of mass effect (small bowel obstruction, jaundice)
- Functioning NETs: can cause symptoms related to hormone secretion
- Gastrinomas: cause Zollinger-Ellison syndrome (hypergastrinemia, gastric hypersecretion, refractory peptic ulcer disease, diarrhea) (Int J Endocr Oncol 2017;4:167)
- Patients with Zollinger-Ellison syndrome have worse prognosis and higher rate of metastasis
- Somatostatinomas: rarely cause somatostatinoma syndrome (diabetes mellitus, diarrhea, steatorrhea, hypohydrea or achlorhydia, anemia, gallstones) (Endocr Relat Cancer 2008;15:229)
- Carcinoid syndrome (diarrhea, bronchospasms, flushing, tricuspid valve fibrosis) occurs in < 10% of cases and only if liver metastasis is present (Endocr Relat Cancer 2008;15:229)
- Gastrinomas: cause Zollinger-Ellison syndrome (hypergastrinemia, gastric hypersecretion, refractory peptic ulcer disease, diarrhea) (Int J Endocr Oncol 2017;4:167)
- Most small intestine and ampullary NETs are detected on endoscopy and diagnosed with biopsy
- Well differentiated NETs are associated with elevated serum chromogranin A
- Increases with larger tumor burden
- Not recommended as a screening test due to low specificity
- May be used to assess disease progression, response to therapy or recurrence in patients with established advanced NET
- Clinical importance decreasing due to day to day variation in chromogranin levels and lack of a standard assay
- 24 hour urinary excretion of 5-HIAA is the preferred initial diagnostic test for carcinoid syndrome (sensitivity > 90%, specificity 90%)
- 5-HIAA is the end product of serotonin metabolism
- Most useful for jejunoileal, appendiceal and ascending colon NETs because they secrete the highest levels of serotonin
- CT or MRI considered mandatory for radiologic staging of NETs
- CT and MRI have poor sensitivity for detection of primary small bowel NETs
- Somatostatin receptor scintigraphy has been used for decades to image and survey small intestinal NETs
- Currently, PET and concomitant CT with 68Ga-DOTA somatostatin analogs (SSAs) are preferred, given the better detection of distant metastases
- Ampullary NETs:
- 5 year survival rate of 82%; 10 year survival rate 71% (Arch Pathol Lab Med 2010;134:1692)
- Tumors < 2 cm or limited to ampulla have better prognosis
- Lymph node metastases are frequent but do not influence overall and disease free survival rates (Langenbecks Arch Surg 2012;397:933)
- Liver is the most common site of metastasis
- Small intestine NETs:
- Often present with metastatic disease (locoregional lymph nodes and liver most common)
- Most have prolonged survival due to low proliferative rate (World J Surg 2012;36:1419)
- Localized disease 5 year overall survival rate: 70 - 100%
- Distant metastasis 5 year overall survival rate: 35 - 60%
- Long term recurrence rates are ~50% (HPB (Oxford) 2010;12:427)
- Nodal metastasis, mesenteric involvement, lymphovascular invasion and perineual invasion increase risk of recurrence
- 38 year old woman with occult duodenal microgastrinoma with lymph node metastases (Am J Surg Pathol 2008;32:1101)
- 53 year old woman undergoing papillectomy for ampullary adenoma found to have accompanying ampullary NET (World J Gastroenterol 2016;22:3687)
- 58 year old man diagnosed with ampullary NET, pheochromocytoma and multiple gastrointestinal stromal tumors as first manifestation of NF1 (Diagn Pathol 2019;14:77)
- 60 year old man with periampullary neuroendocrine tumor presenting with acute pancreatitis (Am J Case Rep 2018;19:1063)
- Surgical excision of tumor and regional lymph nodes
- Duodenal and periampullary NETs are small polypoid lesions within the submucosa (usually < 2 cm); rarely can be large and infiltrating
- Jejunoileal NETs can be multifocal; deep infiltration of muscularis propria and subserosa is common
- Composed of uniform tumor cells showing round to oval nuclei with salt and pepper chromatin
- Varying architectural patterns
- Gastrin expressing G cell NETs: trabecular growth pattern
- Somatostatin expressing D cell NETs: tubuloglandular pattern; may have psammoma bodies
- Serotonin expressing EC cell NETs: nests of tumor cells with peripheral palisading, often minor pseudoglandular component
- Grading:
- Well differentiated neuroendocrine neoplasms:
- Neuroendocrine tumor, grade 1: low grade, < 2 mitoses/2 mm2, Ki67 index: < 3%
- Neuroendocrine tumor, grade 2: intermediate grade, 2 - 20 mitoses/2 mm2, Ki67 index: 3 - 20%
- Neuroendocrine tumor, grade 3: high grade, > 20 mitoses/2 mm2, Ki67 index: > 20%
- Well differentiated neuroendocrine neoplasms:
- AE1 / AE3, CAM5.2
- Chromogranin A, synaptophysin
- Somatostatin expressing NETs less likely to express chromogranin A
- CDX2 and SSTR2A are positive in serotonin producing EC cell NETs
- Well formed, membrane bound, dense core secretory granules
- Sporadic duodenal and ampullary neuroendocrine tumor: no molecular data available
- Small intestinal NETs have a complex genomic landscape:
- 60 - 90% of jejunoileal NETs show chromosome 18 deletion (Mod Pathol 2005;18:1079)
- Chromosome 14 gain in advanced lesions and metastases (Endocr Relat Cancer 2009;16:953)
- CDKN1B mutations in ≤ 10% of ileal NETs (Ann Surg Oncol 2015;22:S1428)
- Epigenetic changes are common (Epigenomics 2015;7:1245)
- > 50% of ileal NETs are CpG island methylator phenotype
- Substantial epigenetic dysregulation found in 70 - 80% of tumors
- 3 molecular subgroups with potential prognostic impact (Clin Cancer Res 2016;22:250):
- Favorable prognosis: small intestinal NET with chromosome 18 loss (55%)
- Correlates with CpG island methylator phenotype negativity and CDKN1B mutation
- Intermediate prognosis: small intestinal NET with no whole arm copy number variation (19%)
- Correlates with CpG island methylator phenotype positivity
- Poor prognosis: small intestinal NET with multiple whole arm copy number variations (26%)
- Favorable prognosis: small intestinal NET with chromosome 18 loss (55%)
- Ileum, resection:
- Well differentiated neuroendocrine tumor (2.4 cm), WHO grade 2 of 3 (see synoptic report)
- Ki67 proliferation index: 6%
- Tumor involves mucosa up to subserosa
- Lymphovascular invasion present
- Resection margins are negative for tumor
- 10 lymph nodes, negative for tumor
- Neuroendocrine carcinoma:
- Poorly differentiated neoplasm with sheets of tumor cells or poorly formed trabeculae or nests that is almost exclusive to the ampullary region
- Pleomorphic cells with large cell or small cell patterns
- Chromogranin A and synaptophysin may be negative
- Loss of RB1 and aberrant p53 expression support the diagnosis of neuroendocrine carcinoma
- Poorly differentiated neoplasm with sheets of tumor cells or poorly formed trabeculae or nests that is almost exclusive to the ampullary region
- Mixed neuroendocrine - nonneuroendocrine neoplasms (MiNENs):
- Exocrine (usually adenocarcinoma) and neuroendocrine components, each ≥ 30%
- Adenocarcinoma:
- Negative or focally positive for neuroendocrine markers
- More prominent nuclear atypia
- Gangliocytic paraganglioma:
- Triphasic (neuroendocrine, Schwannian and ganglion cell-like components)
- Predominantly in ampullary region
A 49 year old woman presented with epigastric abdominal pain, diarrhea and significant weight loss in the last 2 years. Clinical examination revealed the presence of a few café au lait spots located on the upper limbs, large, poorly circumscribed areas of hyperpigmentation on the trunk, bilateral axillary freckling and multiple asymptomatic, soft, flesh colored, sessile or dome shaped cutaneous tumors 1 - 2 cm in diameter, nonadherent to the underlying structures, distributed on the trunk and limbs. An abdominal MRI revealed an infiltrative duodenal mass. Microscopy and immunohistochemistry uncovered a well differentiated neuroendocrine tumor (Ki67 = 1%) with psammoma bodies. A mutation in which of the following genes is most likely responsible for the patient’s disease?
- MEN1
- RET
- MLH1
- APC
- NF1
MEN1 (answer A) is incorrect because mutations in the MEN1 gene cause multiple endocrine neoplasia type 1 (MEN 1). MEN1 is a hereditary syndrome characterized by hyperplasia or sometimes adenomas of the parathyroid glands, pancreatic neuroendocrine tumors or pituitary gland tumors. RET (answer B) is incorrect because the RET proto-oncogene mutations are responsible for multiple endocrine neoplasia (MEN) type 2. MEN2 syndrome is divided into three subtypes (MEN2A, MEN2B and FMTC). People with all subtypes of MEN2 syndrome have an increased risk of medullary thyroid cancer, pheochromocytoma and parathyroid gland cancer. MLH1 (answer C) is incorrect because MLH1 is one of the mismatch repair gene mutations that have been implicated in the development of hereditary nonpolyposis colon cancer (Lynch) syndrome. This patient does not have colon cancer. APC (answer D) is incorrect because the autosomal dominant APC gene mutation is responsible for familial adenomatous polyposis (FAP). This condition causes multiple adenomatous polyps in the colon of affected individuals.
Comment Here
Reference: Neuroendocrine tumor
- A sprue-like enteropathy associated with olmesartan, initially reported in 2012 by Rubio-Tapia et al (Mayo Clin Proc 2012;87:732)
- Although less frequent, it can be seen with other angiotensin II receptor antagonists
- Reversible sprue-like enteropathy with chronic diarrhea and weight loss
- Negative celiac serology
- Variable villous atrophy (with or without collagen deposition), increased intraepithelial lymphocytes, crypt hyperplasia and variable active inflammation
- No clinical response to gluten free diet
- Fast clinical and histologic improvement after suspension of olmesartan
- Sprue-like enteropathy associated with olmesartan
- ICD-10: K63.9 - disease of intestine, unspecified
- Frequency not really known; in a large study done by DeGaetani et al, several cases of unclassified sprue were later reclassified as olmesartan enteropathy (Am J Gastroenterol 2013;108:647)
- Mainly duodenum but stomach (lymphocytic or collagenous gastritis) and colon (lymphocytic or collagenous colitis) also can be involved (Mayo Clin Proc 2012;87:732)
- IL15 expression is increased in response to olmesartan medoxomil treatment
- ZO-1, a tight junction protein, is disrupted in olmesartan medoxomil treated Caco-2 cells (human colonic epithelial line) (Aliment Pharmacol Ther 2015;42:1303)
- Olmesartan medoxomil (trade names of Benicar, Benicar HCTZ, Azor and Tribenzor)
- Most taking 40 mg/day before onset of diarrhea (mean: 3.1 years) (Mayo Clin Proc 2012;87:732)
- Severe diarrhea (often following a dose increase; sudden onset in some cases), steatorrhea, weight loss, nausea, vomiting, abdominal pain, bloating and fatigue (Virchows Arch 2017;470:245)
- High index of suspicion is needed; diagnosis can be made in hypertensive patients on olmesartan who have clinical, endoscopic and histologic sprue-like enteropathy who have negative celiac serology and no clinical improvement following a gluten free diet
- May be misdiagnosed as refractory celiac disease if medication induced injury is not considered
- May be misdiagnosed as refractory celiac disease if medication list is not reviewed by the pathologist
- Negative IgA tissue transglutaminase antibody testing
- Negative endomysial antibodies
- Anti-enterocyte antibody can be rarely positive (linear / apical pattern)
- HLA DQ2 more frequently positive than in general population (68% versus 25%) (Mayo Clin Proc 2012;87:732)
- Normocytic normochromic anemia
- Hypoalbuminemia
- Small bowel bacterial overgrowth can be seen in a subset of cases
- No patients have recurrence of symptoms after restarting a diet containing gluten once olmesartan is discontinued
- 59 year old Caucasian man admitted to the hospital with complaints of intractable diarrhea, vomiting and considerable weight loss (Am J Case Rep 2019;20:111)
- 68 year old Caucasian woman with a history of hypertension with recurrent episodes of acute intermittent diarrhea, nausea, vomiting, renal failure and 15 lbs weight loss (BMJ Case Rep 2018 Oct 2;2018)
- 72 year old woman with a 6 month clinical history of nonbloody diarrhea and weight loss (Eur J Case Rep Intern Med 2019;6:001093)
- 74 year old woman who presented with mainly upper gastrointestinal symptoms (BMJ Case Rep 2018;11:pii:e226133)
- 84 year old woman with 15 months of chronic diarrhea without improvement despite multiple empiric treatments (BMJ Case Rep 2015 Sep 14;2015)
- Discontinuation of olmesartan
- Antibiotics not helpful even if small bowel bacterial overgrowth is noted
- Mucosal scalloping of duodenal mucosa can be seen (World J Gastroenterol 2013;19:6928)
- Partial or total villous atrophy
- Increased intraepithelial lymphocytes (> 25 - 30 IEL/100 enterocytes)
- Crypt hyperplasia
- Variable active inflammation
- With or without thick band of subepithelial collagen deposition (collagenous sprue pattern)
- H&E is sufficient for the diagnosis
- Significant increase in the numbers of CD8+ cells and the number of cells that are FoxP3+ (Aliment Pharmacol Ther 2015;42:1303)
- Duodenum, biopsy:
- Duodenal mucosa with (mild / moderate / severe) villous blunting, crypt hyperplasia, increased intraepithelial lymphocytes and increase in lymphoplasmacytic infiltrate within lamina propria (see comment)
- Comment: The above histopathologic findings are in keeping with a malabsorption pattern of injury. The findings can be seen in medication induced injury (such as olmesartan, NSAIDs, methotrexate, azathioprine, colchicine), gluten sensitive enteropathy, reactive duodenopathy, bacterial overgrowth, tropical sprue, chronic malnutrition, hypersensitivity reaction to nongluten proteins (cow's milk, soy, egg, etc.) and immune related disorders (including CVID and autoimmune enteropathy). Correlation with the clinical, endoscopic and serologic findings (including IgA TTG antibodies in non-IgA deficient patients) is needed.
- Gluten sensitive enteropathy
- Responds to gluten free diet
- Celiac serology frequently positive, particularly tTGA (sensitivity: 98%)
- Tropical sprue
- GI symptoms with temporal relationship to travel history to tropical regions
- Responds to antibiotics
- Collagenous sprue
- Can be a pattern of injury seen in olmesartan enteropathy (exclusion of celiac disease is key)
- Autoimmune enteropathy
- Marked reduction of goblet cells or Paneth cells
- Crypt apoptoses more prominent
- Anti-enterocyte antibody can be positive in both entities
- Responds to immusuppressive agents
- Common variable immunodeficiency
- 2/3 of patients show loss of plasma cells in duodenal biopsy
- Prominent lymphoid aggregates commonly seen
- Immunoglobulin replacement indicated (does not improve olmesartan enteropathy)
- Medication associated injury (eg. mycophenolate mofetil, NSAIDs)
- Mycophenolate mofetil most prominent feature is increase in apoptotic bodies, while celiac-like features are unusual in duodenal biopsies (12%); history of kidney heart or liver transplant is key (Am J Surg Pathol 2009;33:1355)
- NSAIDs are associated with mucosal erosions and ulcerations in jejunum and ileum and GI bleeding
An 84 year old woman presents to your hospital with chronic watery diarrhea (15 months) and 30 lbs of unintentional weight loss. Celiac serology is negative. The patient is on 20 mg on Benicar (olmesartan medoxomil) 1 tablet by mouth daily for her hypertension. She tried a gluten free diet for one year with no response. A duodenal biopsy demonstrates the following findings (see image). After switching to valsartan the patient has a dramatic improvement of her diarrhea within days. Which of the following is true about this entity?
- Histopathologic findings can mimic gluten sensitive enteropathy
- HLA DQ2 is always negative in these patients
- IgA tissue transglutaminase antibody testing is positive in most patients
- Onset of diarrhea is typically within days or weeks after initiation of olmesartan
Reference: Small intestine - Olmesartan enteropathy
Comment here
- Clinical and histologic resolution is slow and it takes years for the duodenal mucosa to recover after discontinuation of olmesartan
- It is an important diagnostic consideration in patients with presumed refractory celiac disease
- Olmesartan associated injury can only be seen in duodenum
- Subset of patients with bacterial overgrowth respond to antibiotics
Comment Here
Reference: Small intestine - Olmesartan enteropathy
- Histologic findings indicative of duodenal mucosa injury as a result of chronic exposure to increased gastric secretion
- Primarily involves proximal duodenum
- Histologic features include gastric foveolar metaplasia and Brunner gland hyperplasia
- Usually mild and regresses with treatment
- Also termed chronic nonspecific duodenitis
- ICD-10: K29.80 - duodenitis without bleeding
- Median age: 58 years (Hum Pathol 2010;41:1593)
- Foveolar metaplasia can be seen in up to 26.3% of patient population (Lab Invest 2016;96 Suppl 1:187A)
- Duodenum, usually proximal segment
- Chronic exposure of the duodenal mucosa to excessive gastric acidity in proportion to duodenal bicarbonate contents, resulting in injury to the lining mucosa (Monogr Pathol 1990;31:69)
- Medications (in particular NSAIDs) (Aliment Pharmacol Ther 2012;36:48)
- Helicobacter pylori infection (though recent data suggests H. pylori might not be implicated as much as it was believed) (Hum Pathol 2010;41:1593, Lab Invest 2016;96 Suppl 1:187A)
- Idiopathic (Aliment Pharmacol Ther 2013;38:946)
- May be asymptomatic (incidental finding) or cause dyspepsia, abdominal pain, hematemesis
- In advanced cases: symptoms related to gastric outlet obstruction or acute abdominal pain (clinical features of peritonitis) secondary to duodenal perforation
- Endoscopy with biopsy
- Negative celiac sprue serology (antitissue transglutaminase, endomysial antibodies, deamidated gliadin peptide)
- Mucosal erythema
- Mucosal nodularity (Mod Pathol 2005;18:1134)
- Mucosal erosion and ulceration
- Good prognosis as most cases regress with treatment
- 56 year old man with foveolar gastric metaplasia of atypical appearance (J Med Case Rep 2016;10:355)
- 72 year old man with duodenal obstruction secondary to Brunner gland hyperplasia (Pathologica 2017;109:414)
- 80 year old man with carcinoma arising from gastric foveolar metaplasia in the duodenum after 9 years of observation (Clin J Gastroenterol 2018;11:391)
- Stop the offending medication
- Proton pump inhibitors
- Treat underlying infection, like H. pylori (Histopathology 2006;48:417)
- Surgical intervention for perforated peptic duodenitis or gastric outlet obstruction
- Foveolar metaplasia of the surface duodenal epithelium
- Brunner gland hyperplasia (Brunner glands seen above the muscularis mucosae)
- Expansion of the lamina propria by mixed inflammatory cell infiltrate, including few neutrophils that usually do not infiltrate the epithelium
- Mildly increased intraepithelial lymphocytes, usually corresponding to Marsh 1 lesion (Mod Pathol 2005;18:1134)
- Mild villous blunting can be seen (World J Gastroenterol 2005;11:686)
- Severe cases may show mucosal erosion, ulceration or regenerative changes, like mucin depletion, nuclear hyperchromasia and increased mitotic activity
- H. pylori very rarely present in metaplastic epithelium
Contributed by Mohamed Mostafa, M.D.
Contributed by @RaulSGonzalezMD on Twitter
Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
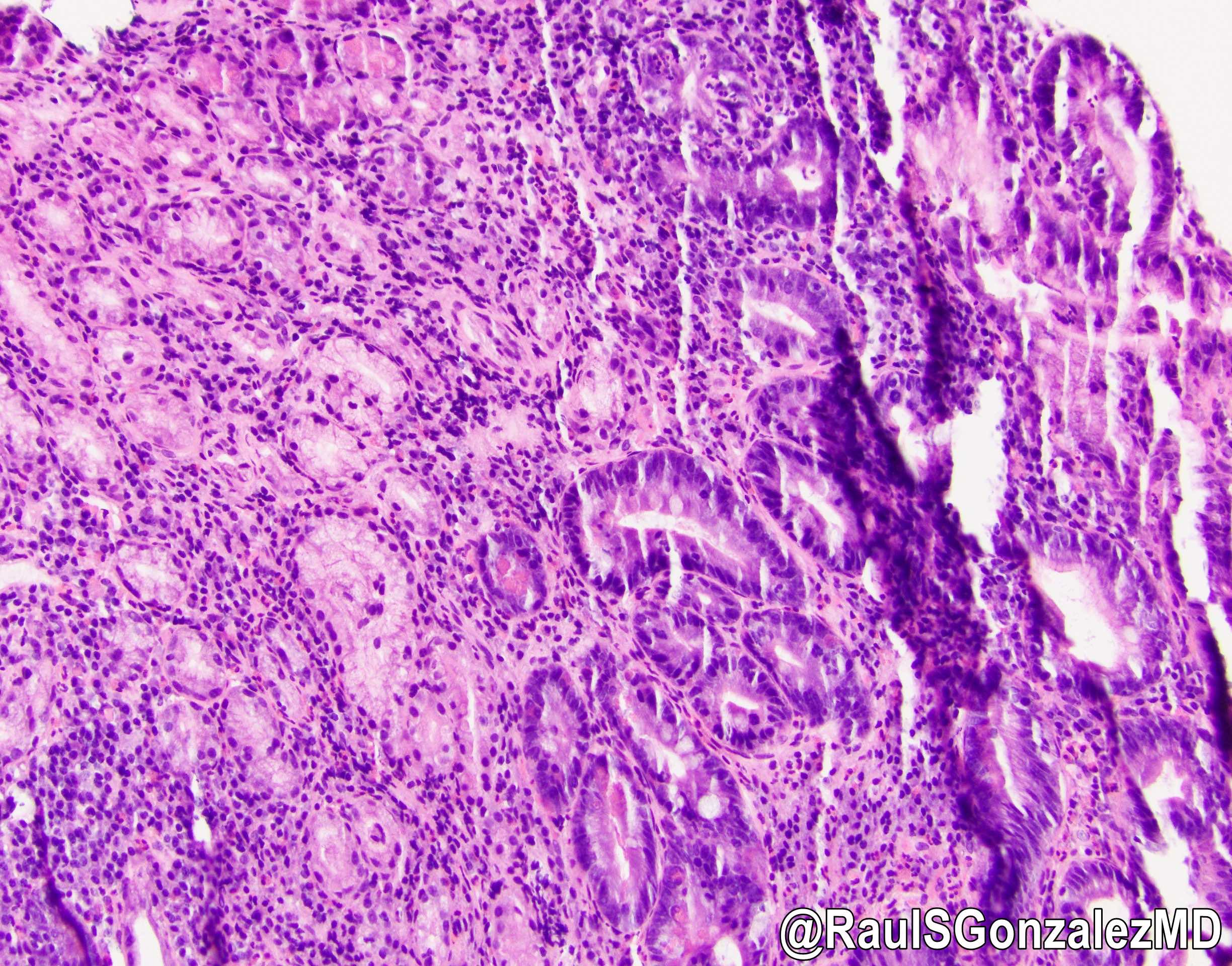 Helicobacter pylori colonizing foveolar metaplasia in the duodenum? bit.ly/38K3WT0 #pathology #PathTwitter #PathOutPic"
Helicobacter pylori colonizing foveolar metaplasia in the duodenum? bit.ly/38K3WT0 #pathology #PathTwitter #PathOutPic"Contributed by @RaulSGonzalezMD on Twitter (see original post here)">
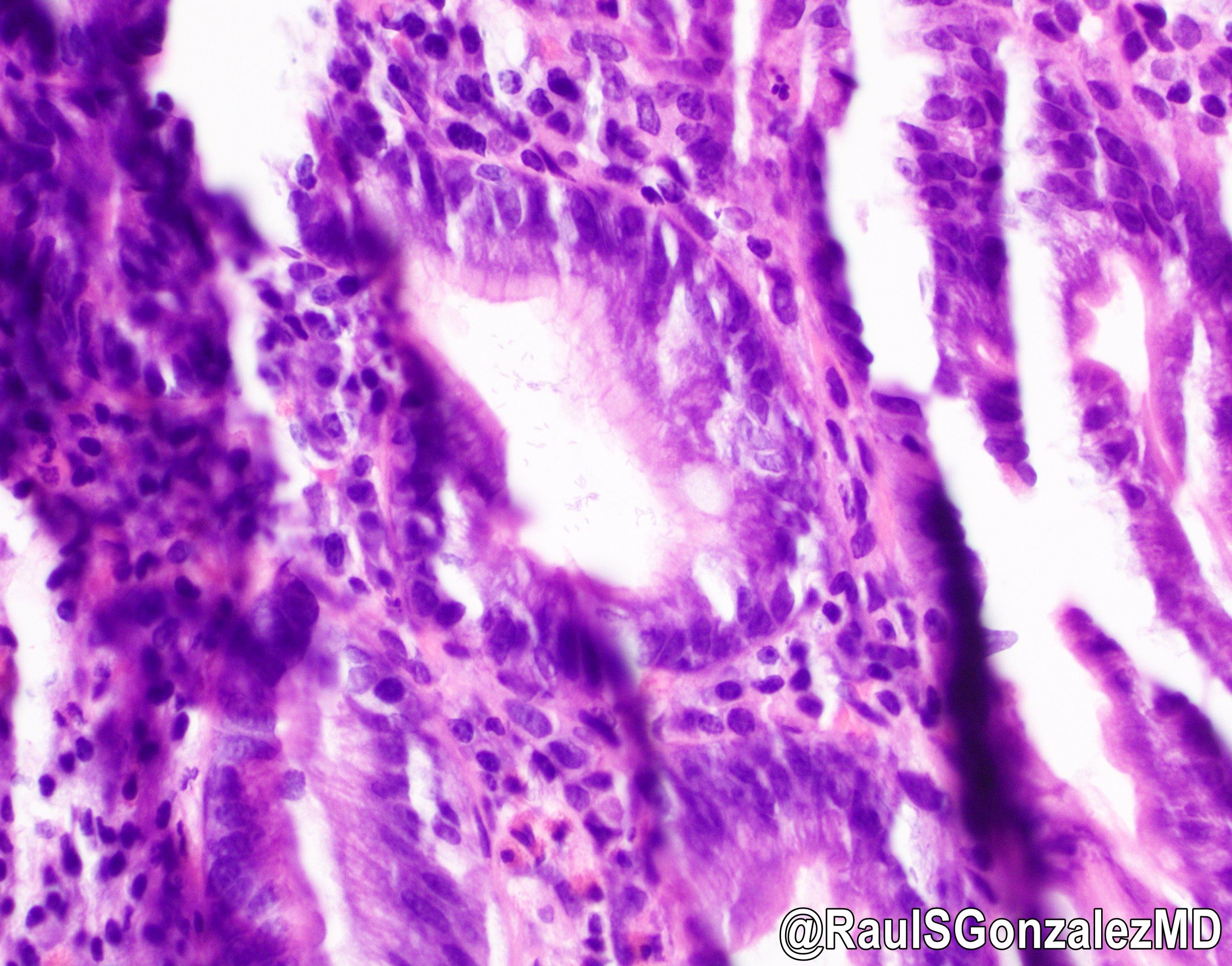
Peptic duodenitis
- Duodenum (D1 / D2), biopsy:
- Duodenal mucosa with preserved villous architecture and gastric metaplasia, suggestive of peptic injury
- Celiac sprue:
- Positive celiac serology
- Marked increased intraepithelial lymphocytes and prominent villous blunting
- Improvement with gluten elimination
- May also show concomitant peptic injury / gastric metaplasia
- Crohn’s disease:
- Nonnecrotizing granulomas
- Involvement of other locations in the gastrointestinal tract
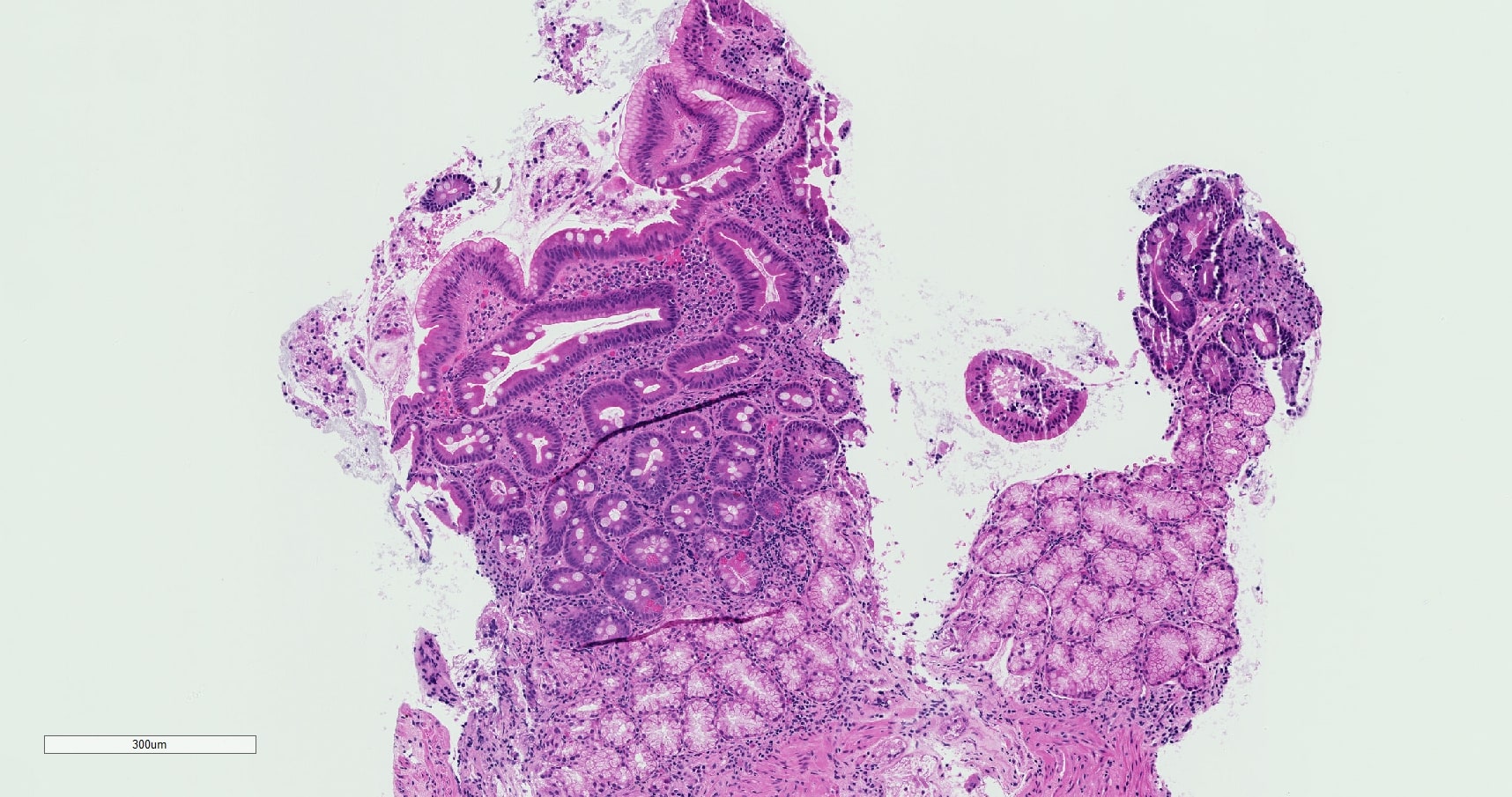
- Cryptitis and crypt abscess formation
- Lamina propria expansion and nonnecrotizing granulomas
- Marked intraepithelial lymphocytosis and moderate villous blunting
- Scattered lymphoid follicles in the lamina propria
- Surface foveolar metaplasia and Brunner gland hyperplasia
Comment Here
Reference: Peptic duodenitis
- Affects primarily duodenal mucosa distal to ampulla of Vater
- Always associated with Helicobacter pylori gastritis
- Histologic findings result from excessive bile reflux
- Medication is always a culprit
- Presents with mild symptoms and regresses with treatment
Comment Here
Reference: Peptic duodenitis
- Hamartomatous polyp associated with Peutz-Jeghers syndrome (PJS) and characterized by papillary architecture with arborizing compact bundles of smooth muscle
- > 90% of patients with Peutz-Jeghers polyposis syndrome have an autosomal dominant germline mutation in STK11 (formerly LKB1) (Clin Genet 2007;72:568)
- Most commonly affects the small bowel but can occur anywhere along the GI tract
- Morphologically characterized by central cores of arborizing smooth muscle that divide lobules of relatively normal glandular mucosa
- Moderate to high lifetime cumulative risk of malignancies in Peutz-Jeghers syndrome, with a variable risk for malignancy of the GI tract, breast, pancreas, ovary, lung, cervix, uterus and testes (Gastroenterology 2000;119:1447, Am J Gastroenterol 2010;105:1258)
- Peutz-Jeghers polyp (PJP)
- Peutz-Jeghers polyposis syndrome (PJPS)
- Peutz-Jeghers polyposis syndrome is an autosomal dominant syndrome with variable penetrance and an incidence of 1 case in 50,000 - 200,000 births (Clin Gastroenterol Hepatol 2006;4:408)
- ~67% of patients present in the second to third decades of life and 33% present in first decade of life
- Small bowel (in ~95% of patients with Peutz-Jeghers polyposis syndrome), most commonly in jejunum and ileum
- Colon and stomach (in ~25% of patients with Peutz-Jeghers polyposis syndrome) (Am J Gastroenterol 2000;95:596, Int J Surg Pathol 2016;24:185)
- Rarely, these polyps can be seen in the respiratory tract, urogenital tract and gallbladder (Int J Colorectal Dis 2000;15:118)
- Direct precursor is unknown
- May represent a genetic predisposition to epithelial prolapse and polyp formation (Gut 2006;55:1)
- Dysplasia in Peutz-Jeghers polyps is rare; however, sections of unaffected colon in Peutz-Jeghers syndrome patients have shown protracted clonal evolution in the crypts which may explain increased cancer risk (Gut 2012;61:839)
- Autosomal dominant inheritance pattern
- Germline mutations in tumor suppressor gene serine / threonine kinase 11 (STK11) (formerly called LKB1) located on gene 19p13.3 are found in up to 90% of cases (J Med Genet 2006;43:e18)
- ~25% of cases are sporadic and are likely due to de novo STK11 mutations or low penetrance variants (Gastroenterology 2000;119:1447)
- STK11 gene is postulated to be involved in cell polarity, chromatin remodeling, cell cycle arrest and Wnt signaling; mutations in STK11 lead to dysregulation of mTOR pathway
- Rare cases of Peutz-Jeghers polyp in patients that do not meet criteria for Peutz-Jeghers syndrome
- Mucocutaneous hyperpigmented macules around the lips, eyes, nostrils, perianal area, mouth and of the buccal mucosa
- Often presents with abdominal pain due to polyp related obstruction, intussusception or prolapse (Curr Mol Med 2007;7:29)
- May also develop chronic gastrointestinal bleeding, hematochezia, hematemesis and anemia
- Diagnosis of a Peutz-Jeghers polyp requires endoscopy with polypectomy and histologic evaluation
- WHO diagnostic criteria for Peutz-Jeghers syndrome includes:
- ≥ 3 histologically confirmed Peutz-Jeghers polyps
- Any number of Peutz-Jeghers polyps with a family history of Peutz-Jeghers syndrome
- Characteristic, prominent mucocutaneous pigmentation with a family history of Peutz-Jeghers syndrome
- Any number of Peutz-Jeghers polyps and characteristic, prominent mucocutaneous pigmentation
- Reference: Gut 2010;59:975
- Variably sized polyps that often occur in clusters
- Larger polyps commonly have a more lobular appearance
- Prognosis for Peutz-Jeghers syndrome is mostly determined by the associated cumulative risk of malignancy, with the lifetime cumulative risk for all types of cancer being > 90%
- Most common sites of developing malignancy are colorectum (39% cumulative risk), stomach (29%), small intestine (13%), pancreas (100x risk, 11 - 36%), breast (32 - 54%), ovary (21%), cervix (10 - 23%), uterus (9%), testis (9%), lung (7 - 17%) (Gastroenterology 2000;119:1447)
- Unusual gonadal lesions such as sex cord tumor with annular tubules (SCTAT), calcifying Sertoli cell tumor of the testes and HPV independent gastric type adenocarcinoma of the cervix (formerly known as adenoma malignum) are associated with Peutz-Jeghers polyposis syndrome (Am J Gastroenterol 2005;100:476)
- 7 year old boy with Peutz-Jeghers syndrome and jejunoileal intussusception with invasive adenocarcinoma (BMJ Case Rep 2018;11:e225076)
- 33 year old woman with Peutz-Jeghers syndrome and bilateral ovarian SCTATs (J Obstet Gynaecol Res 2022;48:492)
- 36 year old woman with Peutz-Jeghers syndrome and gastric type cervical adenocarcinoma (Obstet Gynecol Sci 2019;62:474)
- 60 year old man with a Peutz-Jeghers polyp of the appendix (Case Rep Pathol 2019;2019:7584070)
- U.S. Multi-Society Task Force (USMSTF) recommends polypectomies when seen on endoscopy, are symptomatic or are ≥ 10 mm on imaging (Gastroenterology 2022;162:2063)
- Also recommended are scheduled surveillance of GI tract, breast, pancreas, ovaries, testes and lungs
- Genetic testing of all asymptomatic first degree relatives
- Variable size of polyps; small polyps can be sessile
- Larger polyps are usually pedunculated with a multilobulated / cerebriform smooth surface and a thick stalk
- Peutz-Jeghers polyps of the small bowel have distinctive papillary villous architecture with tree-like arborization of compact smooth muscle bundles and relatively normal overlying epithelium (Mod Pathol 2013;26:1235)
- Epithelial component is usually arranged in a distinct lobular configuration, separated by cores of smooth muscle
- Epithelium often shows glandular dilation and distortion
- Secondary erosion and ulceration can be present
- Epithelial misplacement (pseudoinvasion) can be seen in the submucosa, muscularis propria and even to the serosa due to prolapse and peristaltic kneading; pseudoinvasion is commonly associated with pedunculated polyps
- This can be mistaken for invasive carcinoma
- Dysplasia is rare in Peutz-Jeghers polyps but if present, is characterized by complex cribriform architecture, nuclear hyperchromasia, loss of polarity, nuclear crowding and nuclear stratification extending to the surface
- Cytogenetic evaluation for STK11 mutation via germline sequencing may be helpful in subtle cases
Small bowel, Peutz-Jeghers polyp
- Small bowel, jejunum, polypectomy:
- Hamartomatous polyp, Peutz-Jeghers type (see comment)
- Comment: Histologic sections show a hamartomatous polyp with arborizing smooth muscle cores dividing lobular compartments of mucosa, consistent with Peutz-Jeghers polyp. There are no adenomatous changes or malignancy identified.
- Mucosal prolapse polyp:
- More common in rectosigmoid colon
- Smooth muscle shows more disarray and is less compact than Peutz-Jeghers polyp
- Smooth muscle wrapping around individual crypts favors mucosal prolapse
- Juvenile polyp:
- Loose, edematous lamina propria with inflammation is a characteristic feature
- Smooth muscle arborization is not a prominent feature
- Marked cystic dilation of epithelial component is more common
- Genetic testing shows germline mutations in SMAD4 or BMPR1A
- Inflammatory polyp:
- Usually associated with branching and cystic dilatation of epithelial component with regenerative mucosa with reactive atypia
- Edematous lamina propria with acute and chronic inflammatory infiltrate
- Sporadic hyperplastic polyp:
- Uncommon, typically occurs in the second portion of the duodenum
- Histologically resembles colonic microvesicular hyperplastic polyps with epithelial serrations, crypts with narrow bases, mucin droplets and bland cytology
- Small case series showing association with mutations in BRAF V600E and KRAS (Hum Pathol 2011;42:1953)
- In addition to histology, clinical and family history often aid in the diagnosis; for Peutz-Jeghers polyps, as for other hamartomatous polyps with syndromic considerations, consultation with genetic counseling is recommended to gather family history and consider germline mutation analysis
Which of the following is true about the syndrome associated with the jejunal polyp shown above?
- Associated with autosomal recessive inheritance pattern
- Associated with germline mutations in SMAD4
- Dysplasia is seen in a majority of these polyps
- Histology is characterized by papillary architecture with arborizing compact bundles of smooth muscle
- More commonly seen in the sigmoid colon
Comment Here
Reference: Peutz-Jeghers polyp
Comment Here
Reference: Peutz-Jeghers polyp
- Submucosal gas filled cysts in GI tract
- Called mucosal pseudolipomatosis if it resembles lipomatosis
- Part of differential diagnosis of acute abdomen (World J Gastroenterol 2012;18:453)
- In infants, associated with necrotizing enterocolitis and may be fatal; also associated with cystic fibrosis, congenital heart defects
- In adults, either idiopathic or varied clinical settings including chemotherapy, chronic lung disease, drugs, ischemic colitis, obstruction, scleroderma (World J Gastroenterol 2011;17:4932, Arch Pathol Lab Med 2010;134:378)
- Often indolent clinical course, although radiographically resembles carcinoma
- Considered a finding, not a diagnosis
- 6 year old girl with ulcerative colitis (Arch Pathol Lab Med 1999;123:354)
- 32 year old woman with idiopathic myointimal hyperplasia of mesenteric veins and pneumatosis intestinalis (J Crohns Colitis 2011;5:239)
- 73 year old woman with dysplasia in perforated intestinal pneumatosis (World J Gastroenterol 2009;15:4189)
- Polypoid grape-like masses protrude through mucosa
- Soft, bluish and often sessile, "bubble wrap" crackling noise while handling the specimen
- Submucosal cysts lined by multinucleated giant cells
- Mucosa contains cryptitis, crypt abscesses, granulomas
- May also resemble lipomatosis
- Brownish black pigment in lamina propria macrophages of proximal duodenum
- Also known as melanosis duodeni
- Due to iron and sulfur (J Formos Med Assoc 1995;94:632) and may be associated with oral iron intake, hypertension, diabetes or end stage renal disease (Endoscopy 2008;40:165, World J Gastroenterol 2012;18:1414)
- Not associated with laxative abuse (J Clin Gastroenterol 1988;10:127)
- Not associated with lipofuscin pigment, which is identified in melanosis coli
- Often identified at endoscopy
- Pigment may originate in atmosphere or diet
- No known clinical significance
- 94 year old woman with GI bleed and spotty hyperpigmentation at endoscopy (Case #231)
- Observed acutely or years after radiation therapy for cervical carcinoma, Wilms tumor, lymphoma or other peritoneal tumors
- Acute: anorexia, cramps, diarrhea due to mucosal injury and malabsorption
- Chronic: may present as inflammatory colitis or indolent; also vascular injury, ischemic fibrosis, stricture
- Treatment: resection if severe
- Thickened bowel wall
- Varies from mild epithelial damage to massive necrosis and ulceration (BMC Surg 2004;4:10)
- Loss of columnar shape and nuclear polarity of enterocytes, nuclear pyknosis, bizarre nuclei, mucin depletion and decreased mitoses
- Also fibroblasts and endothelium with submucosal edema but low nuclear to cytoplasmic ratio, preservation of architecture
- No desmoplasia, no infiltrative pattern
Chronic:
- Atrophic and ulcerated mucosa, ectatic blood vessels, fibrosis of submucosa and muscular wall, vascular wall thickening, vascular stenosis
- Possible fistula formation, atherosclerosis-like changes of vasculature (subintimal lipid laden macrophages, calcification, thrombosis), hyalinization of lamina propria
- First described in 2003 in 5 patients (4 men), mean age 56 years (Am J Surg Pathol 2003;27:532)
- May present as obstruction (Am Surg 2011;77:790) but otherwise benign clinical course
- Firm, tan white, 4 - 6 cm, solitary / multiple, in small bowel or colon
- Low / moderate cellularity
- Spindled fibroblasts arranged haphazardly or in intersecting fascicles
- Often infiltrative borders
- Stroma rich in wire-like, keloidal or hyalinized collagen
- Peripheral lymphoid aggregates
- Vimentin, CD117 or muscle specific actin (80%), smooth muscle actin or desmin (60%) (Int J Surg Pathol 2004;12:365)
- Small bowel transplantation is a surgical option for patients who have intestinal failure and have failed parenteral nutrition
- Rejection of the transplanted bowel occurs through cellular or antibody mediated mechanisms and is responsible for a significant portion of graft failures
- Rejection is a major complication of small bowel transplantation, with 30 - 40% of patients developing an episode of acute cellular rejection (ACR) within the first year
- ACR is characterized by increased crypt apoptotic figures, increased lamina propria mixed inflammation and epithelial injury
- Repeated episodes of ACR can lead to chronic rejection and graft dysfunction, which shows nonspecific histologic findings, including ischemic changes and lamina propria fibrosis
- Acute cellular rejection (ACR)
- Antibody mediated rejection (AMR)
- Chronic rejection
- Small bowel transplants are uncommon; < 100 performed in the U.S. per year since 2019 (Am J Transplant 2023;23:S264)
- 30 - 40% of adult transplants develop acute cellular rejection within the first year posttransplant (Am J Transplant 2021;21:1705)
- Chronic rejection was documented in 15% of transplants in 1 series (Ann Surg 2009;250:567)
- Rejection is the primary cause of graft loss in ~13% of cases (sepsis causes ~50% and cardiac complications account for ~8%) (Am J Transplant 2015;15:210)
- Transplants performed as small intestine only, with liver or multivisceral (with liver, stomach, pancreas, colon, spleen or kidney)
- Direct pathway
- Donor antigen presenting cells using donor major histocompatibility complex (MHC) class II and class I molecules present allogenic molecules from the graft to recipient CD4+ and CD8+ lymphocytes
- Activated CD4+ and CD8+ lymphocytes are predominantly responsible for early acute cellular rejection occurring within the first several months posttransplant
- Semidirect pathway
- Donor membrane bound MHC molecules are transferred to recipient antigen presenting cells and presented to CD4+ and CD8+ lymphocytes without processing
- Responsible for delayed acute cellular rejection occurring years after transplant
- Indirect pathway
- Donor MHC antigens are internalized and processed by recipient antigen presenting cells and presented to recipient CD4+ lymphocytes
- Responsible for antibody mediated rejection and delayed acute cellular rejection
- Inverted direct pathway
- Donor CD4+ T cells are activated by recipient B cells presenting recipient MHC class II molecules
- Responsible for early antibody mediated rejection
- Reference: Int J Mol Sci 2023;24:4541
- Rejection of allograft through cellular mediated response or antibody mediated response involving activation of CD4+ and CD8+ lymphocytes (Int J Mol Sci 2023;24:4541)
- Symptoms are typically nonspecific: fever, diarrhea / increased ostomy output, nausea and abdominal pain
- Acute cellular rejection
- Onset is typically between 1 and 9 weeks posttransplant
- Antibody mediated rejection
- Typically occurs during the first 2 weeks posttransplant
- Detection of lymphocytotoxic antibodies by serologic testing
- Chronic rejection
- Typically occurs after repeated episodes of acute cellular rejection
- Also associated with nutritional deficiency and generalized edema (associated with protein losing enteropathy)
- Reference: Arch Pathol Lab Med 2012;136:761
- Mucosal biopsies of the graft are the only current reliable method (Scand J Gastroenterol 2021;56:13)
- No specific laboratory markers are routinely used to diagnose or monitor rejection
- Biomarkers such as decreased serum citrulline and increased fecal calprotectin are seen in moderate to severe rejection but are nonspecific (Scand J Gastroenterol 2021;56:13)
- Detection of lymphocytotoxic antibodies may support antibody mediated rejection
- Imaging findings are nonspecific
- Noncontrast computed tomography (CT): diffuse wall thickening, mucosal hyperenhancement or hypoenhancement and moderate ascites
- Magnetic resonance imaging (MRI): wall thickening and mucosal hyperenhancement with contrast on T1 sequence
- Fluoroscopy: wall thickening, loss of mucosal fold patterns and hypomotility
- Reference: Br J Radiol 2018;91:20180173
- Increased number of episodes of ACR, higher grade of ACR and earlier onset of ACR are associated with an increased risk of chronic rejection (Pediatr Dev Pathol 2003;6:240)
- 5 year graft survival for patients < 18 years old is better than adults (62% versus 43%) (Am J Transplant 2023;23:S264)
- 18 year old man with small bowel transplant and acute cellular rejection (World J Gastroenterol 2005;11:5332)
- 19 year old woman with small bowel transplant and severe acute rejection (Dig Dis Sci 2010;55:3336)
- 20 year old woman with a history of small bowel transplant and severe late onset acute cellular rejection (Transplant Proc 2019;51:3181)
- 20 year old man with small bowel transplant and acute cellular rejection associated with an elevation of donor specific HLA antibodies (Transplant Proc 2016;48:525)
- 31 year old woman with small bowel transplant, acute cellular rejection with white exudates on endoscopy (Transplant Proc 2017;49:2419)
- Induction immunosuppression with antithymocyte globulin or interleukin 2 receptor blocking antibody
- Maintenance immune suppression with tacrolimus and mycophenolate mofetil or corticosteroids
- Reference: Am J Transplant 2023;23:S264
- Endoscopically, acute cellular rejection shows an erythematous and friable mucosa with or without erosion or ulceration
- No endoscopic / macroscopic findings have been identified that are specific for chronic ACR or AMR
- Reference: Am J Transplant 2021;21:1705
- Standardized histologic criteria were developed at the pathology workshop of the VIII International Small Bowel Transplant Symposium (Transplant Proc 2004;36:335)
- Acute cellular rejection
- Grading based on apoptotic bodies per 10 consecutive crypts, amount of lamina propria mixed inflammation (lymphocytes, eosinophils and neutrophils) and amount of epithelial injury (crypt dropout, erosions or ulceration) (see table below)
- Other features include edema, villous blunting, reactive epithelial changes and vascular congestion
- Antibody mediated rejection
- Capillaritis, lamina propria inflammation, mucosal erosion / ulceration, fibrin thrombi within lamina propria vasculature and edema (Am J Transplant 2018;18:2250)
- Chronic rejection
- Nonspecific findings: mild ischemic changes, apoptotic bodies (< 6/10 consecutive crypts) and mild fibrosis of the lamina propria
Grading of acute cellular rejection (adapted from Arch Pathol Lab Med 2012;136:761, Transplant Proc 2004;36:335)
| Grade | Apoptotic bodies / 10 consecutive crypts | Crypt dropout | Amount of lamina propria inflammation | Surface epithelial damage | Additional findings |
| 0 (no ACR) | < 2 | None | Not increased | None | Unremarkable mucosa |
| Indeterminate (grade ind) | < 6 | None | Focal minimal | None | Focal mild villous blunting |
| Mild (grade 1) | ≥ 6 | None | Mild | Focal damage / erosion | Mucin depletion, nuclear enlargement and hyperchromasia |
| Moderate (grade 2) | ≥ 6; possibly confluent | Focal | Moderate to severe | Erosion | Moderate to marked architectural distortion and villous blunting, edema and congestion |
| Severe (grade 3) | Variable | Extensive | Moderate to severe | Extensive erosion / ulceration | Moderate to marked architectural distortion, granulation tissue formation |
- Complement 4d (C4d) is usually positive in antibody mediated rejection (not routinely performed) (Am J Transplant 2018;18:2250)
- Cytomegalovirus, adenovirus and Epstein-Barr virus stains
- Small intestine, transplant, biopsy:
- Moderate mixed lamina propria inflammatory cells, increased crypt apoptotic figures and foci of crypt distortion, crypt loss and ulceration, consistent with moderate, acute cellular rejection (see comment)
- Comment: Up to 8 crypt apoptotic figures per 10 consecutive crypts are identified. This finding, in combination with lamina propria inflammatory infiltrate and focal epithelial injury, is compatible with moderate acute cellular rejection.
- Infectious enteritis (Arch Pathol Lab Med 2012;136:761):
- Most commonly caused by adenovirus and cytomegalovirus (CMV); other etiologies including bacteria, other viruses, fungi and protozoa are possible
- Apoptotic figures are generally less prominent, viral inclusions may be seen
- Positive immunohistochemistry for adenovirus or CMV useful for confirmation
- Correlation with cultures and serologic studies may be helpful
- Medication effect (commonly mycophenolate mofetil) (Transplant Proc 2010;42:82):
- Can display increased crypt apoptotic figures, increased lamina propria inflammation and epithelial erosion / ulceration
- Will affect both transplanted and native bowel
- Correlation with medication history and supportive endoscopic findings is helpful
- Graft versus host disease (Nat Rev Gastroenterol Hepatol 2017;14:711):
- Commonly affects multiple sites: skin, liver, gastrointestinal tract
- Small bowel involvement characterized by increased crypt apoptotic figures and crypt dropout
- Only affects the native tissues (bowel)
A 35 year old patient with a history of small bowel transplantation presents with diarrhea, abdominal pain and weight loss. Out of concern for acute cellular rejection (ACR), endoscopic biopsies of both the transplanted small bowel and segment of native small bowel are obtained. Histologic examination of the transplanted bowel shows the findings displayed in the representative photomicrograph. What additional findings, if present, would be most supportive of the diagnosis of acute cellular rejection of the transplant bowel in this patient?
- Identification of eosinophilic nuclear inclusions
- Increased mitotic rate
- Occasional crypt dropout and apoptotic figures in the native small bowel
- Occasional crypt dropout and erosion of the surface epithelium in the transplanted small bowel
Comment Here
Reference: Rejection
- Frequent surveillance biopsies
- History of multiple acute cellular rejection episodes
- Long term immunosuppressive therapy
- Short duration of cold ischemia time during transplant surgery
- Younger age at transplant
Comment Here
Reference: Rejection
- Inflammatory and fibrosing process affecting visceral peritoneum that can encase small bowel (World J Gastroenterol 2012;18:1999)
- Infiltrates mesentery at attachment site with peritoneum
- Usually spares retroperitoneum
- Associated with dry eyes and itchy scaly skin lesions
- Associated with beta blockers; chronic peritoneal dialysis
- Rare causes include abdominal surgery, abdominal tuberculosis, cirrhosis, dermoid cyst rupture, endometriosis, familial Mediterranean fever, fibrogenic foreign material, GI malignancy, intraperitoneal chemotherapy, liver transplantation, luteinized ovarian thecomas, pertioneoventricular shunting, protein S deficiency, recurrent peritonitis, sarcoidosis, subclinical primary viral peritonitis
- 76 year old woman with rectal cancer and a suspected enteric duplication cyst in the distal ileum (Case of the Month #482)
- Sclerosing peritonitis associated with luteinized adult granulosa cell tumor (Int J Gynecol Pathol 2012;31:141)
A. Colon
B. Ovary
C. Small bowel
D. Stomach
Pathologic TNM staging of carcinomas of the Ampulla of Vater, AJCC 8th edition
- All carcinomas of the Ampulla of Vater or the duodenal papilla, including poorly differentiated neuroendocrine carcinomas, are covered by this staging system
- Not covered by this staging system are well differentiated neuroendocrine tumors at this location (use the neuroendocrine tumor staging system instead)
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- ICD-10: C24.1 - malignant neoplasm of ampulla of Vater
- NX: regional lymph nodes cannot be assessed
- N0: no regional lymph node involvement
- N1: metastasis to one to three regional lymph nodes
- N2: metastasis to four or more regional lymph nodes
- Notes:
- Regional lymph nodes include peripancreatic, hepatic artery and portal vein nodes
- Minimum of 12 lymph nodes must be recovered for lymph node staging to be considered accurate in curative resections
- M0: no distant metastasis
- M1: distant metastasis
- y: preoperative radiotherapy or chemotherapy
- r: recurrent tumor stage
- Stage 0:Tis N0 M0
- Stage I:T1a N0 M0
- Stage IB:T1b - T2 N0 M0
- Stage IIA:T3a N0 M0
- Stage IIB:T3b N0 M0
- Stage IIIA:T1a - T3b N1 M0
- Stage IIIB:T4 any N M0
- any T N2 M0
- Stage IV:any T any N M1
- Tumor size
- Lymph node status
- Margin status
- Histologic differentiation
- Histologic subtype
- Preoperative or pretreatment CEA
- Preoperative or pretreatment CA 19 - 9
- Adjuvant therapy
- GX: grade cannot be assessed
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
- Carcinoma in situ
- Adenocarcinoma
- Adenocarcinoma, invasive intestinal type
- Adenocarcinoma, pancreatobiliary type
- Clear cell adenocarcinoma
- Hepatoid adenocarcinoma
- Mucinous carcinomas
- Signet ring cell carcinoma
- Squamous cell carcinoma
- Adenosquamous carcinoma
- Neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Small cell neuroendocrine carcinoma
- Mixed adenoneuroendocrine carcinoma
- Undifferentiated carcinoma
- Undifferentiated carcinoma with osteoclast-like giant cells
- Noninvasive pancreatobiliary papillary neoplasm with high grade dysplasia
- Papillary carcinoma, invasive
- 0.2 cm
- 0.5 cm
- 1.0 cm
- 2.0 cm
Pathologic TNM staging of neuroendocrine tumors of the duodenum and ampulla of Vater, AJCC 8th edition
- Well differentiated neuroendocrine tumors of the ampulla ("carcinoids") and of the duodenum are covered by this staging system
- Not covered by this staging system are poorly differentiated neuroendocrine carcinomas at this location (use the adenocarcinoma staging system instead)
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- Hormone secretion by the tumor (gastrin, somatotatin, etc.) is not incorporated into staging
- "Carcinoid” is no longer an acceptable term
- ICD-10: C7A.019 - Malignant carcinoid tumor of the small intestine, unspecified portion
- TX: primary tumor cannot be assessed
- T1: tumor invades the mucosa or submucosa only and is ≤ 1 cm (duodenal tumors); tumor ≤ 1 cm and confined within the sphincter of Oddi (ampullary tumors)
- T2: tumor invades the muscularis propria or is ≥ 1 cm (duodenal); tumor invades through sphincter into duodenal submucosa or muscularis propria or is ≥ 1 cm (ampullary)
- T3: tumor invades the pancreas or peripancreatic adipose tissue
- T4: tumor invades the visceral peritoneum (serosa) or other organ
- Notes: Tis is not a valid category under AJCC 8th edition staging for these tumors
- NX: Regional lymph nodes cannot be assessed
- N0: No regional lymph node involvement
- N1: Regional lymph node involvement
- Notes: Regional lymph nodes include duodenal, hepatic, pancreatoduodenal, infrapyloric, gastroduodenal, pyloric, superior mesenteric and pericholedochal nodes
- M0: No distant metastasis
- M1: Distant metastasis
- M1a: Metastasis confined to liver
- M1b: Metastasis in at least one extrahepatic site (e.g., lung, ovary, nonregional lymph node, peritoneum, bone)
- M1c: Both hepatic and extrahepatic metastases
- r: recurrent tumor stage
- Stage I: T1 N0 M0
- Stage II: T2 - T3 N0 M0
- Stage III: T4 N0 M0
- any T N1 M0
- Stage IV: any T any N M1
- Size of tumor
- Maximum depth of invasion
- Number of tumors
- Lymph node status
- Grade
- Mitotic count
- Ki67 labeling index
- Perineural invasion
- Lymphovascular invasion
- Margin status
- Functional status
- Genetic syndrome
- Location in duodenum
- Type of surgery
- Preoperative chromogranin A level
- Preoperative pancreastatin level
- Preoperative neurokinin level
- Age of patient
- Histologic variants
- Grading is not formally part of the staging system
- Most pathologists use the ENETS / WHO grading criteria:
- G1: mitotic rate < 2 per 10 high power fields and Ki67 rate < 3%
- G2: mitotic rate 2 - 20 per 10 high power fields or Ki67 rate 3 - 20%
- G3: mitotic rate > 20 per 10 high power fields or Ki67 rate > 20%
- Well differentiated neuroendocrine tumor
- Glandular duodenal neuroendocrine tumor (i.e., somatostatinoma)
- Gangliocytic paraganglioma
- Regarding neuroendocrine tumors of the ampulla, which T category in the 8th edition AJCC staging guide includes size as a factor?
- T1 only
- T1 and T2
- T1, T2, and T3
- T2, T3, and T4
Pathologic TNM staging of carcinomas of small bowel, AJCC 8th edition
- Carcinomas, including neuroendocrine carcinomas, of the duodenum, jejunum and ileum are covered by this staging system
- Use this system for duodenal / ampullary neuroendocrine tumors and this system for jejunum / ileum neuroendocrine tumors
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the AJCC, 8th Edition, 2018 is mandatory
- C17.9: malignant neoplasm of small intestine, unspecified
- TX: primary tumor cannot be assessed
- T0: no evidence of primary tumor
- Tis: high grade dysplasia / carcinoma in situ
- T1: tumor invades the lamina propria or submucosa
- T1a: tumor invades the lamina propria
- T1b: tumor invades the submucosa
- T2: tumor invades the muscularis propria
- T3: tumor invades through the muscularis propria into the subserosa or extends into nonperitonealized perimuscular tissue (mesentery or retroperitoneum) without serosal penetration
- T4: tumor perforates the visceral peritoneum or directly invades other organs or structures (e.g., other loops of small intestine, mesentery of adjacent loops of bowel and abdominal wall by way of serosa; for duodenum only, invasion of pancreas or bile duct)
Notes:
- For jejunal / ileal pT3 tumors, the nonperitonealized perimuscular tissue is part of the mesentery
- For duodenal pT3 tumors in areas lacking serosa, the nonperitonealized perimuscular tissue is part of the interface with the pancreas
- NX: regional lymph nodes cannot be assessed
- N0: no regional lymph node metastasis
- N1: metastasis in one or two regional lymph nodes
- N2: metastasis in three or more regional lymph nodes
Notes:
- Regional lymph nodes for the nonampullary duodenum include the retropancreatic, hepatic artery, inferior pancreaticoduodenal and superior mesenteric nodes
- Regional lymph nodes for the jejunum or ileum include the mesenteric and superior mesenteric nodes; for the terminal ileum, the cecal and ileocolic nodes are also included
- M0: no distant metastasis
- M1: distant metastasis
- Stage 0:Tis N0 M0
- Stage I:T1 - 2 N0 M0
- Stage IIA:T3 N0 M0
- Stage IIB:T4 N0 M0
- Stage IIIA:any T N1 M0
- Stage IIIB:any T N2 M0
- Stage IV:any T any N M1
- Primary tumor site (duodenum, jejunum, ileum)
- Number of lymph nodes examined
- Presurgical CEA
- LVI
- Microsatellite instability
- Tumor grade
- Presence of disease
- Personal or family history of familial GI malignancies (familial adenomatous polyposis, Lynch syndrome, Peutz-Jeghers syndrome)
- GX: grade cannot be assessed
- G1: well differentiated
- G2: moderately differentiated
- G3: poorly differentiated
- Adenocarcinoma
- Mucinous adenocarcinoma
- Mucin producing adenocarcinoma
- Adenocarcinoma in adenomatous polyp
- Adenocarcinoma in villous adenoma
- Adenocarcinoma in tubulovillous adenoma
- Signet ring cell carcinoma
- Carcinoma, NOS
- Adenosquamous carcinoma
- Squamous cell carcinoma
- Large cell neuroendocrine carcinoma (NEC)
- Small cell neuroendocrine carcinoma (NEC)
- Mixed adenoneuroendocrine carcinoma
- Undifferentiated carcinoma
- Medullary carcinoma
- High grade dysplasia
- pT1
- pT2
- pT3
- pT4
Pathologic TNM staging of neuroendocrine tumors of the jejunum and ileum, AJCC 8th edition
- Well differentiated neuroendocrine tumors of the jejunum and ileum are covered by this staging system
- Not covered by this staging system are well differentiated neuroendocrine tumors of the ampulla or duodenum (use ampulla staging) or poorly differentiated neuroendocrine carcinomas of the small intestine (use small bowel carcinoma staging)
- TX: primary tumor cannot be assessed
- T0: no evidence of primary tumor
- T1: invades lamina propria or submucosa and ≤ 1 cm in size
- T2: invades muscularis propria or > 1 cm in size
- T3: invades through the muscularis propria into subserosal tissue without penetration of overlying serosa
- T4: invades visceral peritoneum (serosa) or other organs or adjacent structures
- NX: regional lymph nodes cannot be assessed
- N0: no regional lymph node metastasis
- N1: regional lymph node metastasis in < 12 nodes
- N2: large mesenteric masses (> 2 cm) or extensive nodal deposits (≥ 12), especially those that encase the superior mesenteric vessels
Notes:
- Regional lymph nodes include superior mesenteric and mesenteric nodes; posterior cecal nodes also apply for terminal ileum lesions
- 2 cm cutoff for including mesenteric masses in N categorization may be suboptimal (Mod Pathol 2018 May 24 [Epub ahead of print])
- M0: no distant metastasis
- M1: distant metastasis
- M1a: metastasis confined to liver
- M1b: metastasis in at least one extrahepatic site (e.g., lung, ovary, nonregional lymph node, peritoneum, bone)
- M1c: both hepatic and extrahepatic metastasis
- (m): multiple primary lesions (provide stage for the most advanced lesion)
- r: recurrent tumor stage
- Stage I:T1 N0 M0
- Stage II:T2 - 3 N0 M0
- Stage III:T4 N0 M0
- any T N1 - 2 M0
- Stage IV:any T any N M1
- Size of tumor (value or unknown)
- Tumor focality (unifocal or multifocal)
- Depth of invasion
- Nodal status and number of nodes involved, if applicable
- Sites of metastasis, if applicable
- NKA (neurokinin A) level
- Pancreastatin level
- Ki67 index
- Mitotic count
- Histologic grading (from Ki67 and mitotic count): G1, G2, G2
- Grading is not formally part of the staging system
- Most pathologists use the European Neuroendocrine Tumor Society (ENETS) / WHO grading criteria:
- Grade 1: mitotic rate < 2 per 10 high power fields and Ki67 rate < 3%
- Grade 2: mitotic rate 2 - 20 per 10 high power fields or Ki67 rate 3 - 20%
- Grade 3: mitotic rate > 20 per 10 high power fields or Ki67 rate > 20%
- Neuroendocrine tumor (NET) G1 (carcinoid)
- Neuroendocrine tumor (NET) G2
- Separate complete staging for each primary focus
- pT1/T2/T3
- pT1(1)T2(1)T2(1)
- pT3(m) or pT3(3)
- Severe UC is associated with contiguous backwash ileitis and appendix involvement
- UC also associated with postcolectomy pouchitis
- Rarely diffuse duodenitis is associated with ulcerative colitis but it does not behave as Crohn's disease (Am J Surg Pathol 2000;24:1407)
- Diffuse chronic duodenitis in ulcerative colitis patients who have colectomy is a strong predictor of pouchitis (Am J Surg Pathol 2010;34:1672)
- Recommended to restrict use of term to active enteritis involving ileum contiguously from cecum that has a similar or greater degree of active inflammation; mild cases predominantly involves the superficial mucosa in a contiguous pattern
- Focal isolated ileal erosions, mucous gland metaplasia or patchy edema with mild active inflammation are features of Crohn's disease (Am J Clin Pathol 2006;126:365)
Images hosted on other servers:
Mild backwash ileitis in moderately active cecal chronic ulcerative colitis resection specimen; mucosa
of distal ileum is edematous and villi are slightly wider and flatter than normal; most of the active colitis
stops abruptly at transition point between the two mucosae; the two most distal villi have the greatest
degree of injury, including blunting, edema and neutrophils in the lamina propria and surface epithelium
- Rare systemic infection caused by intracellular rod shaped actinobacterium Tropheryma whipplei
- Whipple disease is a rare, multisystem infection due to Tropheryma whipplei that most commonly affects the small bowel but may involve any part of the gastrointestinal tract
- Histologically, small intestinal biopsies show infiltration of the lamina propria by foamy macrophages containing PAS positive, AFB negative bacteria
- Electron microscopy shows intact and degenerated rod shaped bacterial forms
- PCR for 16S ribosomal genes of T. whipplei is often diagnostically useful and can be performed on fresh or paraffin embedded, formalin fixed tissue
- Treatment consists of long term antimicrobial therapy
- Tropheryma whipplei is also known as the Whipple bacillus
- Formerly called Tropheryma whippelii
- Historical term: intestinal lipodystrophy (Clin Gastroenterol Hepatol 2004;2:849)
- ICD-10: K90.81 - Whipple disease
- M:F = 8:1
- More common in middle aged white males
- Extremely rare, annual incidence of approximately 1 - 6 new cases per 10,000,000 persons per year worldwide (Scand J Gastroenterol 1997;32:52)
- Multisystem infection
- Most often involves gastrointestinal tract
- Small intestine > esophagus / stomach / appendix / colorectum
- May involve mesenteric lymph nodes, joints, heart, lungs, eyes and central nervous system
- Most often involves gastrointestinal tract
- Many individuals carry antibodies against Tropheryma whipplei, despite the extremely low incidence of Whipple disease
- Disease presumably develops in individuals with predisposing immune factors
- Patients with Whipple disease often have immunomodulatory conditions, such as alcohol abuse, sarcoidosis, ankylosing spondylitis, Crohn's disease and rheumatoid arthritis (Am J Surg Pathol 2012;36:1066)
- Association with HLA-B27 (Eur J Clin Invest 1979;9:385)
- Organism laden macrophages mechanically compress lymphatics in small bowel, leading to malabsorption
- Causative agent is Tropheryma whipplei
- Actinomycete, related to mycobacteria
- Clinical presentation is highly variable
- Most common symptoms are malabsorption, weight loss, diarrhea, arthralgias and abdominal pain
- Generalized lymphadenopathy may be present (particularly in the abdomen)
- Often referred to as protean and the great mimicker, like syphilis, because of nonspecific clinical presentation (Am J Surg Pathol 2012;36:1066)
- Approximately 15% of patients do not have typical symptoms
- Neurological symptoms (such as cognitive changes, ophthalmoplegia, nystagmus and myoclonia) present in ~24% of patients (Medicine (Baltimore) 2015;94:e714)
- Tropheryma whipplei accounts for ~6.3% of culture negative endocarditis (J Clin Microbiol 2012;50:216)
- Rarely, can present in retroperitoneum, mimicking a neoplasm (Braz J Infect Dis 2014;18:346)
- Esophagogastroduodenoscopy findings:
- Unremarkable (majority of cases) (Medicine (Baltimore) 2015;94:e714)
- Pale yellow, shaggy mucosa
- Thickened mucosal folds
- Yellow-white plaques
- Erosions, erythema, friability
- Upper endoscopy with biopsies of small intestine
- H&E histology
- Periodic acid-Schiff (PAS) staining
- PCR on paraffin embedded, formalin fixed tissue for 16S ribosomal genes of Tropheryma whipplei
- PCR testing can also be performed on fresh tissue, vitreous fluid and cardiac valves (N Engl J Med 1992;327:293, N Engl J Med 1995;332:363, J Infect Dis 2004;190:935)
- PCR can be negative after antibiotic treatment (Ann Intern Med 1997;126:520)
- Immunohistochemical stain: polyclonal rabbit antibody against T. whipplei, available from reference center, such as Centers for Disease Control and Prevention (Am J Clin Pathol 2002;118:742)
- Electron microscopy
- Cerebrospinal fluid and synovial fluid
- Cytology
- PAS staining
- PCR
- Laboratory abnormalities seen in chronic inflammatory conditions are common (Medicine (Baltimore) 2015;94:e714):
- Anemia (81% of patients)
- Leukocytosis (48% of patients)
- Thromobocytosis (56% of patients)
- Elevated C reactive protein (69% of patients)
- Radiological findings are nonspecific (Clin Gastroenterol Hepatol 2004;2:849)
- Abdominal computed tomography and magnetic resonance imaging:
- Thickened small bowel mucosal folds
- Mesenteric lymphadenopathy
- Small bowel contrast radiology may also show thickened mucosal folds
- Response to antimicrobial treatment is unpredictable
- Relapse is common after treatment
- 4 year old girl with chronic bloody diarrhea and growth retardation (Intest Res 2021;19:119)
- 49 year old man with weakness, loss of appetite, weight loss and joint pain (Med Princ Pract 2020;29:90)
- 52 year old man with diarrhea, marked weight loss and vision changes (GE Port J Gastroenterol 2020;27:283)
- Antimicrobial treatment (Clin Microbiol Rev 2017;30:529)
- Optimal treatment regimen is uncertain and without consensus but most regimens involve an initial phase followed by a maintenance phase
- Initial phase (2 - 4 weeks): ceftriaxone or penicillin G
- Maintenance phase (one year): trimethoprim sulfamethoxazole
- Doses and durations of therapy depends on presence or absence of central nervous system disease or endocarditis
- Lamina propria expanded by foamy macrophage infiltrate (Am J Surg Pathol 2012;36:1066)
- Infiltrate may be diffuse or patchy
- Blunt or distended villi (Dig Dis Sci 2019;64:213)
- Scattered dilated lacteals (Am J Surg Pathol 2012;36:1066)
- Fat vacuoles within lamina propria (Dig Dis Sci 2019;64:213)
- Overlying enterocyte vacuolization due to intracytoplasmic lipid accumulation
- Acute inflammation (variable)
- Mononuclear inflammation usually absent
- Epithelioid granulomas (minority of cases) (Virchows Arch A Pathol Anat Histol 1979;382:227)
- Limited clinical utility
- Cytospin stained with PAS reagent may be used for fluid samples (i.e. cerebrospinal fluid [CSF], synovial fluid)
- Histiocytes with numerous intracellular PAS positive, granular particles
- Intracellular particles may appear sickle shaped, resulting in sickleform particle containing (SPC) cells (Gastroenterology 1997;113:434)
- CSF cytology may be positive in patients without neurological symptoms (Gastroenterology 1997;113:434)
- PAS and PASD (granular staining)
- Stains the glycoprotein wall of the bacteria
- Can stain both viable and nonviable organisms
- Tropheryma whipplei immunostain (granular staining) (Am J Clin Pathol 2002;118:742)
- Can stain both viable and nonviable organisms
- Rod shaped bacterium (bacillary bodies) within macrophages and lamina propria
- Intracellular forms may be associated with phagocytic vacuoles (Am J Surg Pathol 1981;5:507)
- After treatment, bacterial breakdown products and degenerating organisms may be seen
- PCR for 16S ribosomal genes of T. whipplei is an important diagnostic tool for diagnosing Whipple disease
- In patients with histologically confirmed disease: sensitivity 96.6% and specificity 100% (Ann Intern Med 1997;126:520)
- Conversion from positive to negative PCR results can occur before histologic resolution of disease because bacterial remnants and cell wall structures often persist in macrophages after treatment (Gastroenterology 1996;110:1735)
- Positive PCR can be seen in asymptomatic colonization of patients without disease
- Duodenum, endoscopic biopsy:
- Duodenal mucosa with abundant foamy macrophages in lamina propria (see comment)
- Special stains for PAS and PAS with diastase (PASD) show strong, granular positivity within macrophages
- AFB stain (Ziel-Neelsen method) is negative
- Comment: In the appropriate clinical setting, the findings are concerning for Whipple disease. Immunohistochemical stain for Tropheryma whipplei as well as PCR on the paraffin embedded block have been requested, results of which will be reported in a follow up addendum.
- Mycobacterium avium intracellulare complex:
- Histoplasmosis:
- Malakoplakia:
- Lamina propria macrophage infiltrate showing targetoid Michaelis-Gutmann bodies (PAS positive, von Kossa positive)
- False positivity with Tropheryma whipplei immunostain has been documented (Am J Surg Pathol 2020;44:1251)
- Rhodococcus:
- Lamina propria macrophage infiltrate containing intracellular coccobacillary organisms
- Gram positive on tissue Gram stain
- PAS positive (typically more granular than T. whipplei)
- T. whipplei immunostain negative
- Gaucher disease:
- Lamina propria macrophage infiltrate with fibrillary cytoplasm (tissue paper-like)
- PAS positive
- Electron microscopy shows elongated lysosomes with tubular inclusions
- Crushed Brunner glands:
- Interpret in context of adjacent more intact Brunner glands
- PAS positive
A 54 year old man presents to his primary care physician with a 5 month history of diarrhea and abdominal pain. He is referred for esophagogastroduodenoscopy, which demonstrates diffuse yellow-white plaques of the duodenal mucosa. Multiple biopsies are performed. Representative H&E of the duodenal biopsy is shown above. Which of the following pairs of ancillary stains would be most helpful in establishing a diagnosis?
- AFB and CD68 immunostain
- GMS and AFB
- PAS and AFB
- PAS and CD68 immunostain
- PAS and GMS
Comment Here
Reference: Whipple disease
- In a majority of patients with confirmed Whipple disease, EGD is unremarkable
- On H&E stain, infiltration of the duodenal lamina propria by macrophages is pathognomonic of Whipple disease
- PASD stain is usually negative due to the glycogen containing cell wall of the causative organism
- PCR for 16S ribosomal genes of Tropheryma whipplei cannot be performed on paraffin embedded formalin fixed tissue
- Stomach biopsies are superior to duodenal biopsies for establishing a tissue diagnosis
Comment Here
Reference: Whipple disease
- WHO classification of tumors of the small intestine and ampulla
- Currently on 5th edition, published in 2019
- Based on histologic appearance, not molecular characteristics
-
Benign epithelial tumors and precursors ICD-O codes
- Adenomatous polyp, low grade dysplasia 8210/0
- Adenomatous polyp, high grade dysplasia 8210/2
- Intestinal type adenoma, low grade 8144/0
- Intestinal type adenoma, high grade 8144/2
- Serrated dysplasia, low grade 8213/0
- Serrated dysplasia, high grade 8213/2
- Noninvasive pancreatobiliary papillary neoplasm with low grade dysplasia 8163/0
- Noninvasive pancreatobiliary papillary neoplasm with high grade dysplasia 8163/2
- Intra-ampullary papillary tubular neoplasm
-
Malignant epithelial tumors ICD-O codes
- Adenocarcinoma, NOS 8140/3
- Mucinous adenocarcinoma 8480/3
- Signet ring cell carcinoma 8490/3
- Medullary carcinoma, NOS 8510/3
- Adenocarcinoma, intestinal type 8144/3
- Pancreatobiliary type adenocarcinoma 8163/3
- Tubular adenocarcinoma 8211/3
- Neuroendocrine tumor, NOS 8240/3
- Neuroendocrine tumor, grade 1 8240/3
- Neuroendocrine tumor, grade 2 8249/3
- Neuroendocrine tumor, grade 3 8249/3
- Gastrinoma, NOS 8153/3
- Somatostatinoma, NOS 8156/3
- Enterochromaffin cell carcinoid 8241/3
- Extra-adrenal paraganglioma, NOS 8693/3
- Neuroendocrine carcinoma, NOS 8246/3
- Large cell neuroendocrine carcinoma 8013/3
- Small cell neuroendocrine carcinoma 8041/3
- Mixed neuroendocrine - nonneuroendocrine neoplasm (MiNEN) 8154/3
Find related Pathology books: GI, liver, molecular