Table of Contents
Definition / general | Terminology | Pathophysiology | Etiology of sideroblastic anemia (nonneoplastic) and anemia of chronic disease | Clinical features | Diagnosis | Laboratory: brief overview of iron status evaluation | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Positive stains | Electron microscopy description | Electron microscopy images | Additional referencesCite this page: Zhao X. Iron in nonneoplastic marrow. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowiron.html. Accessed April 16th, 2024.
Definition / general
- Normal forms of iron stores in bone marrow:
- Ferritin in erythroblasts
- Hemosiderin in macrophages (normally small and inapparent without special stains)
- Abnormal iron deposits in bone marrow:
- Due to excessive accumulation of hemosiderin within cells of mononuclear phagocyte system, associated with:
- Ringed sideroblasts: seen in nonneoplastic (see below) or neoplastic conditions
- Anemia of chronic disease
- Abnormal iron deposits in other cell types, e.g. plasma cells
Terminology
- Sideroblasts: normally present; erythroblasts with siderosome, an intracytoplasmic membrane bound ferritin molecule; bone marrow usually has 1 - 2 small siderotic granules in > 10% normoblasts
- Ringed sideroblasts: abnormal finding; 5+ iron granules encircling 1/3 or more of nuclear circumference in erythroid precursors, due to arrangement of iron granules in a ring form around the mitochondria
- Hemosiderin: may be normal or abnormal
- An intracellular iron storage complex which appears to be a complex of ferritin, denatured ferritin and other materials
- The iron within hemosiderin deposits is usually unavailable to supply iron when needed
Pathophysiology
- Iron is required by many biochemical reactions (i.e. oxidation reduction reactions) but may be toxic if not properly contained
- Approximately 60% of body iron is associated with hemoglobin in circulating red blood cells
- Daily erythropoiesis requires 25 - 30 mg iron per day, provided by macrophages through recycling of heme iron following phagocytosis of senescent red blood cells and heme catabolism
- Intestinal iron absorption (1 - 2 mg per day) only compensates for daily iron losses
Etiology of sideroblastic anemia (nonneoplastic) and anemia of chronic disease
- Sideroblastic anemia:
- Bone marrow produces ringed sideroblasts rather than healthy erythrocytes; although body has iron available, it cannot incorporate it into hemoglobin
- Anemia of chronic disease (ACD):
- Hepcidin, a 25 amino acid peptide synthesized in hepatocytes, has central role
- Secreted in plasma and rapidly removed in urine
- Negative regulator of intestinal iron absorption and heme iron recycling by macrophages
- Hepcidin synthesis is stimulated by iron or inflammation (mostly IL6)
- Hepcidin synthesis is repressed by iron deficiency and by all conditions that stimulate bone marrow erythropoiesis such as anemia, bleeding, hemolysis, dyserythropoiesis or erythropoietin injections
- Underlying mechanism for hemochromatosis (adult / child) is a defect in activation of hepcidin normally triggered by iron excess
- A defect in hepcidin repression is responsible for iron refractory iron deficiency anemia (IRIDA)
- Reduced hepcidin filtration in renal insufficiency contributes to associated anemia; stimulation of hepcidin synthesis by inflammation is a major cause of anemia of chronic disorders
- Inflammatory cytokines have important role:
- Decrease ferroportin expression and probably directly blunt erythropoiesis by decreasing the ability of bone marrow to respond to erythropoietin
- Promote production of white blood cells, which causes fewer stem cells to differentiate into red blood cells, even at normal erythropoietin levels and even aside from the effects of hepcidin
- Inhibit erythropoietin release from kidney and survival of circulating red cells is shortened
- Hepcidin, a 25 amino acid peptide synthesized in hepatocytes, has central role
Clinical features
- Sideroblastic anemia:
- Definition: > 5 iron granules, > 1/3 circumference, larger than normal
- Acquired, clonal: refractory anemia with ring sideroblasts (RARS): older adults, ineffective dyserythropoiesis, macrocytic or normocytic hypochromatic anemia, > 15% ringed sideroblasts in bone marrow
- Acquired, reversible: toxins and medications
- Alcohol: most common cause of acquired sideroblastic anemia; has direct toxic effect on erythroid precursors
- Copper deficiency: inadequate copper uptake or secondary to zinc overload; vacuoles in erythroid and myeloid precursors
- Isoniazid: inhibition of pyridoxine metabolism
- Lead: inhibition of δ-ALA dehydratase and heme synthetase
- Congenital: X Linked Sideroblastic Anemia (XLSA): defective aminolevulinic acid synthase (ALAS2) → ↑ affinity to cofactor, pyridoxal-5'-phosphate → ↑ protoporphyrin and heme; microcytic hypochromic anemia, some patients respond to pyridoxine (vitamin B6)
- Congenital: Pearson marrow pancreas syndrome: congenital large deletions or duplications of mitochondrial DNA → sideroblastic anemia and cytoplasmic vacuoles, acidosis and exocrine pancreatic insufficiency
- Anemia of chronic disease (ACD)
- Inflammatory cytokines promote production of white blood cells → fewer stem cells differentiating into red blood cells, even at normal erythropoietin levels and even aside from effects of hepcidin
- In short term, the above effect keeps more iron away from bacterial pathogens in human body (almost all bacteria are iron dependant for metabolism and reproduction) while producing more immune cells in response to infection
- If inflammation continues, the inability of bone marrow to produce red blood cells interferes with normal body functions, as red blood cells require massive amounts of iron for hemoglobin and oxygen transportation
- ACD can also result from Hodgkin lymphoma, lung and breast carcinomas; also noninfectious inflammatory diseases (rheumatoid arthritis, systemic lupus erythematosus)
- Note: all anemia in those with chronic disease is NOT anemia of chronic disease; ACD is often considered separate from anemia of renal failure (due to poor production of erythropoietin) or associated with AZT or other medications
Diagnosis
- Laboratory tests (see below)
- Bone marrow biopsy and aspiration
- Cytogenetic and molecular analysis to rule out acquired clonal disorders (RARS in MDS) and congenital disorders
Laboratory: brief overview of iron status evaluation
- Reticulocyte count: best test to differentiate abnormalities of production and survival
- Ferritin:
- Storage form of iron; levels directly correlate with total body iron storage
- Most sensitive lab test for iron deficiency anemia
- Also an acute phase reactant, so must obtain CRP (detects inflammation) to rule out elevated ferritin due to inflammation
- Transferrin: iron binding blood plasma proteins for transportation of iron
- Total iron binding capacity (TIBC): blood capacity to bind iron with transferrin
- Percent transferrin saturation: serum iron/TIBC x 100%
- Serum soluble transferrin receptor (STFR):
- Carrier protein for transferrin
- Truncated soluble receptors, shed into blood from erythroblasts in the marrow
- Correlate with iron status of erythroblasts and total mass of erythron; inversely related to iron supply to cells; not altered by inflammation
- STFR is significantly increased in iron deficiency anemia but is normal / only mildly increased in ACD
- Free erythroid protoporphyrin (FEP): increased if decreased iron supply or decreased iron incorporation into heme; normal in thalassemia
- Sideroblastic anemia: ↑ serum iron, ↑ marrow iron, ↑↑ percent saturation, ↑↑ ferritin, ↓ TIBC, ↓ STFR
- ACD: ↓ serum iron, ↓ marrow iron, > 15% percent saturation, ↑ ferritin, ↓ TIBC, normal or ↑ STFR
Case reports
- 52 year old woman with sideroblastic anemia secondary to zinc toxicity (Blood 2013;122:311)
Treatment
- Sideroblastic anemia associated with nonneoplastic conditions
- Transfusion may be required in severe cases if patients do not respond to erythropoietin
- Desferrioxamine, a chelating agent, is used for iron overload from transfusions; also therapeutic phlebotomy
- In certain severe cases, bone marrow transplant may be an option although data on success rates are limited
- For isoniazid induced sideroblastic anemia, moderate / high levels of pyrodoxine (vitamin B6) correct the anemia
- Anemia of chronic disease (ACD)
- Treament of underlying chronic disease is optimal although this is only rarely possible with existing knowledge / treatments
- Parenteral iron is increasingly used
- Commercially produced erythropoietin can be helpful in some circumstances but is costly and may be dangerous
- Transfusions if patients are hemodynamically unstable (usually from complications, not the ACD alone)
- New therapeutic perspectives include agonists or antagonists of hepcidin or siRNA to reduce hepcidin synthesis
Microscopic (histologic) description
- Sideroblastic anemia associated with nonneoplastic conditions
- BM biopsy: erythroid hyperplasia with normoblastic to megaloblastic maturation and occasional dysplastic changes
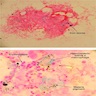
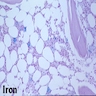
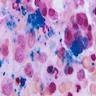
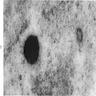
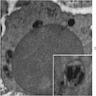
- BM aspirate: Prussian blue stain demonstrates increase in ringed sideroblasts
- Anemia of chronic disease (ACD)
- BM biopsy: may show minimal morphological changes initially; when condition progresses, bone marrow may appear hypocellular with decreases in erythropoietic cells, M:E ratio may be increased with a shift in maturation sequence to later stages
- BM aspirate: Prussian blue stain demonstrates storage iron in macrophages and absence of iron in erythroid precursors
Microscopic (histologic) images
Peripheral smear description
- Sideroblastic anemia associated with nonneoplastic conditions:
- Dimorphic (hypochromic and normochromic) red blood cells, basophilic stippling and Pappenheimer bodies (multiple blue dots in inregular sizes and irregularly located or in small clusters)
- Anemia of chronic disease (ACD):
- Often mild normocytic anemia while can sometimes be more severe and can sometimes be microcytic
- Morphologically, ACD often closely resembles iron deficiency anemia and many people with chronic disease are also iron deficient
- The combination of both causes of anemia produces a more severe anemia
- Both ACD and iron deficiency anemia show low reticulocyte production index
Positive stains
- Prussian blue stain (iron stain)
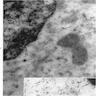
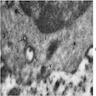
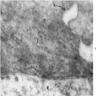
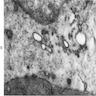
Electron microscopy description
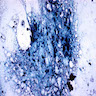
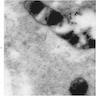
- Sideroblastic anemia: bone marrow EM demonstrates excessive accumulation of iron in macrophages as well as iron granules within erythroblasts and erythrocytes, in particular within mitochondria
Electron microscopy images
Additional references