Table of Contents
Definition / general | Essential features | Excisions with palpable masses | Excisions for lesions localized with imaging studies | Cavity / shave margins performed simultaneously with primary excisions | Re-excision specimens | Mastectomy specimens | Postneoadjuvant specimens | Sentinel lymph node excisions | Axillary dissection specimens | Microdochectomy specimens | Microdochectomy images | Nipple resections | Specimens from oncoplastic procedures | Grossing implant specimens with suspicion of implant associated lymphoma | Reduction mammoplasty specimens | Specimens removed for gynecomastia | Gender reassignment specimens (top surgery) | Features to report | Gross images | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Guidi AJ. Grossing & features to report. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignantgrossing.html. Accessed April 20th, 2024.
Definition / general
- This summary describes how to sample breast resection specimens with documented, suspected or potential malignancy
- Essential clinical history:
- Indication
- Prior biopsy findings
- Imaging findings
- Presence of prior biopsy clips / localization devices
- Treatment history
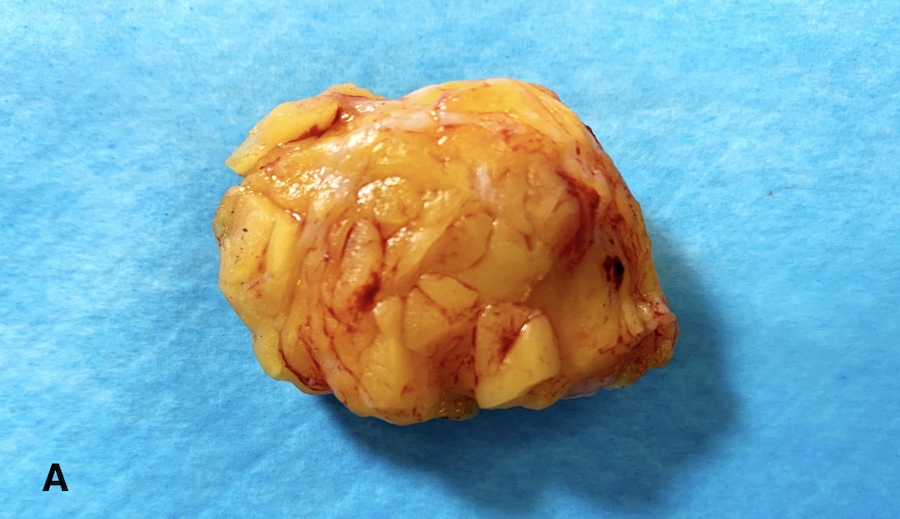
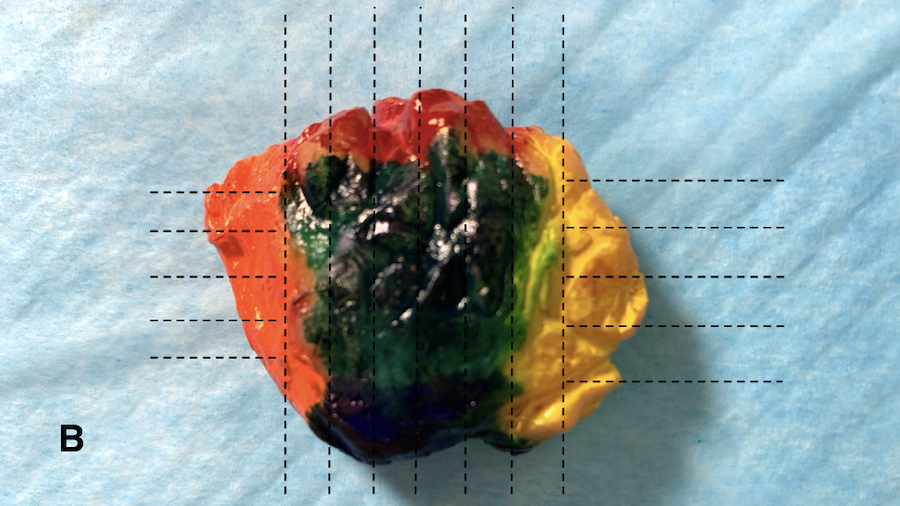
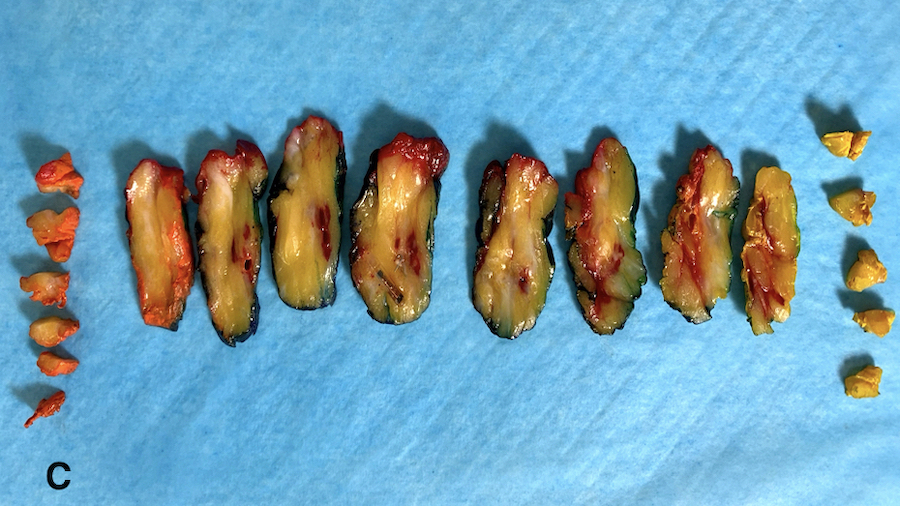
- Multicolor inks recommended for oriented specimens; sample inked margins with perpendicular, not tangential / en face, sections to provide optimal clinical information (Cancer 1997;79:1568, Arch Pathol Lab Med 2018;142:496)
- Inking may be performed by pathologists or surgeons; the latter may be more accurate (Ann Surg Oncol 2009;16:285, World J Surg Oncol 2010;8:4)
- Orange / yellow inks may be difficult to distinguish microscopically; apply to opposite ends
- Blot specimen dry, apply ink(s) and blot dry again prior to sectioning to prevent ink artifact; consider mordant (i.e., acetic acid) to optimize ink adherence
- Slice specimens as thinly as possible; palpate each section for lesions that may not be visible
- Change gloves if soiled to prevent ink artifact
- Diagrams or photographs that provide orientation / section keys facilitate accurate reporting
- Maintain specimen orientation following sampling in case additional sections required
- Ensure cold ischemic time < 1 hour and total fixation time 6 - 72 hours
Essential features
- Know indication for procedure, results of prior procedures, presence of biopsy clips
- Carefully ink specimens and blot dry to avoid inking artifact
- Sample all lesions and all margins (perpendicular, not tangential / en face, margins)
- For pure ductal carcinoma in situ lesions, submit all or as much fibrous tissue as practical to assess for invasion
- Consider utilizing specimen diagrams or photographs to illustrate orientation / section key
Excisions with palpable masses
- Gross exam:
- Measure, weigh specimen and describe orienting sutures
- Ink specimen as above; if fragmented, ink each fragment
- Describe / measure all lesions, including color / consistency / tumor border (i.e., well circumscribed, irregular, stellate); note presence of necrosis / hemorrhage
- If multiple lesions, measure the distance between each
- Measure distance between lesions and closest inked margins
- Note biopsy site changes / fat necrosis
- See below for discussion of postneoadjuvant specimens
- Sections:
- Suspected benign lesions: 1 section per cm (2 sections per cassette)
- Suspected malignant lesions: submit entire lesion up to 4 cassettes
- Provide a full face section(s) of the tumor across the longest axis of the tumor where possible, using adjoining ink to indicate contiguous sections (Ann Diagn Pathol 2011;15:291)
- If the longest axis of the tumor is perpendicular to the plane of sectioning, tumor size can be calculated by multiplying the average slice thickness by the number of consecutive slices involved (Int J Surg Pathol 2021;29:39, Arch Pathol Lab Med 2009;133:15)
- Sample all grossly heterogeneous areas, including areas of necrosis
- If no discrete lesion, submit up to 12 cassettes of fibrous tissue as a screening step
- Sample intervening tissue between lesions
- Margins: document / sample closest margins; include lesion in section if possible
- Sample remaining margins with at least 2 sections (1 cassette) each
- Submit section of skin if present
- If specimen or lesion not entirely submitted, estimate proportion sampled
- For pure ductal carcinoma in situ, the College of American Pathologists recommends "when practical, the entire specimen should be submitted in a sequential fashion for histologic examination" (CAP: Protocol for the Examination of Resection Specimens from Patients With Ductal Carcinoma In Situ (DCIS) of the Breast [Accessed 18 March 2021])
- Nevertheless, there is significant variability in sampling between labs (Arch Pathol Lab Med 2018;142:496)
Excisions for lesions localized with imaging studies
- Gross exam:
- Specimen imaging typically performed
- Findings regarding targeted lesions, calcifications, biopsy clips and localization devices should be reconciled with gross exam
- Clips may be present and not grossly identified
- Lesions may or may not be grossly identified
- Same instructions for grossing specimens with palpable lesions should be followed
- Various localization devices are utilized; be familiar with devices at your institution:
- Localizing wires
- Radioactive seeds
- Tags using radiofrequency, infrared or electromagnetic waves
- Radiation safety officers should approve radioactive seed processing protocols
- See below for discussion of postneoadjuvant specimens
- Specimen imaging typically performed
- Sections:
- Visible lesions should be sampled in the same manner as palpable masses
- If no lesions grossly identified, use imaging studies, clips and localizing devices to guide sampling so that the targeted lesions are entirely submitted
- Submit up to 12 cassettes of fibrous tissue as a screening step
- Sample margins and skin in the same manner as palpable masses
Cavity / shave margins performed simultaneously with primary excisions
- To decrease need for subsequent re-excisions, surgeons often perform multiple cavity / shave margins simultaneously with primary excisions
- Gross exam:
- Primary excision may or may not be oriented
- If oriented, primary excision should be processed similarly to oriented primary excisions without cavity / shave margins
- If unoriented, primary excision can be inked black and processed similarly to unoriented primary excisions without cavity / shave margins; some labs do not ink unoriented primary excisions and report margins only on separate cavity / shave margins
- Cavity / shave margins should be measured in 3 dimensions
- True margin surface of cavity / shave margins should be inked with care to avoid artifactual ink seeping onto the nonmargin surface; some labs ink the nonmargin surface a different color to assist orientation microscopically
- Sections:
- Entire specimen can typically be submitted in < 10 cassettes
Re-excision specimens
- Subsequent procedures performed to obtain negative margins; may be global, surrounding the entire excision site or targeted, excising 1 or more specific margins
- Gross exam:
- True margins should be inked similarly to cavity / shave margins, avoiding artifactual ink seeping onto nonmargin surface
- Describe grossly evident or palpable lesions and biopsy cavity contents
- Measure range of thickness of cavity walls
- Sections:
- Global re-excisions:
- If re-excision is for invasive carcinoma, representative sections generally suffice
- If re-excision is for pure ductal carcinoma in situ, it is important to exclude invasive carcinoma; all parenchymal / fibrous tissue should be submitted if practical
- Submit all gross lesions and at least 4 sections from the biopsy cavity with closest margins
- Submit at least 2 sections from remaining margins (2 sections per cassette)
- If not completely submitted, include an estimate of proportion submitted
- Sample attached skin, if present
- Targeted re-excisions:
- Entire specimen can usually be submitted in < 10 cassettes
- For larger re-excisions, at least 1 section per 1 cm should be submitted
- For pure ductal carcinoma in situ lesions, all parenchymal / fibrous tissue should be submitted if practical
- Global re-excisions:
Mastectomy specimens
- Removal of most breast tissue to treat malignancy or to reduce risk of cancer (prophylactic)
- Mastectomy types:
- Simple: remove breast, skin and nipple / areola, typically without axillary nodes
- Modified radical: simple mastectomy and axillary dissection
- Radical: includes pectoralis muscle (rarely performed)
- Skin sparing: remove breast and nipple / areola leaving skin intact
- Subcutaneous: remove breast leaving skin and nipple intact; typically for benign indications
- Nipple sparing: remove breast leaving skin and nipple intact; typically for patients with cancer or at high risk; often includes biopsy of nipple duct margin
- Indications:
- Gross exam:
- Know indication, prior procedures, presence of biopsy clips and other clinical questions (i.e., residual calcifications)
- Intraoperative imaging often not performed; may need to obtain imaging to facilitate gross exam and document lesion / clip removal, particularly if no prior excision
- Weigh / measure specimen; describe orienting sutures
- Note presence / absence of skin, nipple / areola, skeletal muscle, axillary nodes; measure / describe each
- Describe skin, nipple / areolar lesions (retraction, ulceration, scars, etc.); palpate skin, as skin tumor satellites may not be visible
- Describe surgical defects (i.e., exposed biopsy cavity, etc.)
- Ink posterior / fascial aspect; the significance of soft tissue margins other than deep / fascial margin is unknown
- Examine lateral aspect for nodes; may occasionally be found in simple mastectomies
- Serially section fresh specimen from posterior anterior, without cutting through skin
- Estimate proportion of fatty to parenchymal / fibrous tissue
- Describe all lesions present similarly to excision protocol, including quadrants, distance between lesions and distance between lesions and margins
- Discrepancies between clinical and gross findings should be reconciled with surgeon
- See below for discussion of postneoadjuvant specimens
- Sections:
- Follow protocol for excision specimens regarding sampling lesion(s) and biopsy site(s)
- Section perpendicular deep / fascial margin closest to lesion(s)
- Sample uninvolved quadrants (2 sections / 1 cassette each)
- Sample skeletal muscle (1 perpendicular section closest to tumor or biopsy site)
- Sample nipple / areola; if no lesions, 1 central perpendicular section; if lesion or suspected Paget disease, more extensive sampling is recommended
- Sample skin lesions, including scars (1 section)
- For nipple sparing mastectomies, nipple / areolar margin insertion site on anterior surface should be inked / sampled (2 perpendicular sections, 1 cassette)
- Sample all lymph nodes, as detailed below
- For prophylactic mastectomies, sample all gross lesions as above; if no lesions, submit 2 sections per quadrant and sample nipple / areola and deep / fascial margins, as above
Postneoadjuvant specimens
- Neoadjuvant chemo or endocrine therapy may be used to shrink tumors prior to definitive excision or mastectomy
- Assessment of treatment response in the breast / nodes provides useful prognostic information
- Review imaging reports describing tumor location, local extent, pre / posttherapy characteristics and presence of biopsy clip(s)
- Gross exam:
- If significant treatment response, may be difficult to identify tumor bed
- Coordinated gross / microscopic evaluation required to define tumor bed in at least 2 dimensions (Surg Pathol 2018;11:213)
- For multiple discrete primary tumors, tumor bed estimates should be provided for each
- Sections:
- In addition to standard sections for excision / mastectomy specimens, submit sections spanning largest span of tumor bed to assess overall tumor cellularity
- Amount of sampling is dictated by pretreatment tumor size and presence of visible tumor bed / gross residual tumor
- Recommended minimum of 1 block per cm of pretreatment tumor size (Int J Surg Pathol 2021;29:39)
Sentinel lymph node excisions
- Gross exam:
- Quantitate discrete nodes / node candidates
- Comment on blue dye, if present
- If previously biopsied, document clips (specimen imaging may be required)
- Slice each node as thinly as possible, 2 mm or thinner; comment on size, color, consistency and presence / absence of grossly evident tumor or scarring
- If tumor evident, comment on tumor border / evidence of extranodal extension
- Sections:
- Entirely submit each node
- Provide detailed slide key regarding number of nodes in each cassette
Axillary dissection specimens
- Gross exam:
- Quantitate discrete nodes / node candidates
- If nodes previously biopsied, document clips (specimen imaging may be required)
- Section / describe each node as described above
- Multiple positive nodes may be fused / matted; estimate number of fused nodes, if possible
- Full axillary dissections should yield 10 or more nodes; if < 10 nodes, consider utilizing clearing solutions (i.e., Carnoy fixative) or sample surrounding adipose tissue
- Sections:
- Entirely submit each node
- Large grossly positive nodes may be representatively sampled; include any irregular tumor borders to assess for extranodal extension
Microdochectomy specimens
- Usually small excisions performed to evaluate patients with nipple discharge (with or without abnormal ductogram)
- Gross exam:
- If oriented, maintain orientation; ink using the protocol above
- If dilated duct grossly evident, serially section perpendicular to the duct, as thinly as possible; examine for intraductal lesions
- Sections:
- Entirely submit
Microdochectomy images
Nipple resections
- Usually performed for Paget disease of the nipple
- Gross exam:
- Measure in 3 dimensions; describe orienting sutures
- If oriented, apply multiple inks to maintain orientation, similarly to skin excisions
- Describe color and consistency of skin surface; measure and describe lesions and distance to inked margins
- Sections:
- Serially section perpendicular to skin surface
- Entirely submit in a manner allowing assessment of all perpendicular lateral and deep margins
Specimens from oncoplastic procedures
- Procedures involve removal of tumor simultaneously with plastic surgery techniques designed to obtain cosmetically optimal results
- Typically results in large, irregular and difficult to orient specimens
- Gross exam:
- Communicate with the surgeon to identify appropriate margins to sample
- Surgeon may submit multiple separate shave margins to ensure optimal margin evaluation; process similarly to targeted re-excision specimens, as above
- Due to specimen complexity, utilize diagrams / photographs with orientation / section key
- Sections:
- Sample all lesions using excision protocol, above
- Sample all relevant perpendicular inked margins indicated by surgeon
Grossing implant specimens with suspicion of implant associated lymphoma
- Implants (intact or ruptured) are typically sent to pathology for gross exam, often with attached or separate peri-implant capsular tissue that should be sampled for histologic evaluation
- Implant associated anaplastic large cell lymphoma is a rare entity most commonly associated with textured implants (silicone or saline), usually associated with a peri-implant effusion and rarely associated with a grossly evident mass (Plast Reconstr Surg 2019;143:65S)
- Lymphoma may arise many decades following implant placement
- If lymphoma is clinically suspected, determine if there is associated effusion; if so, fluid should be aspirated / sent for cytologic evaluation and flow cytometry; could be performed by surgeon intraoperatively or by pathologist if effusion is resected intact
- Gross exam:
- All specimens should be photographed, including implant manufacturer information
- Weigh specimen and measure in 3 dimensions
- Describe the surface of the implant (adherent tissue / capsule, smooth or textured surface, intact or disrupted, color of effusion) and implant contents
- Sections:
- If effusion received intact, aspirate and send for fluid for cytology and flow cytometry
- Extensively sample tissue surrounding the effusion; may require sampling material scraped from implant surface
- Sample adherent tissue and peri-implant capsule (multiple sections for each of 6 margins if possible)
Reduction mammoplasty specimens
- Typically includes unoriented breast tissue and skin
- Tissue weights often important for billing purposes
- Unsuspected atypical and malignant lesions more commonly discovered in older patients (Arch Pathol Lab Med 2017;141:1523)
- Gross exam:
- Report weights provided by surgeon as these may differ from lab weights due to blood / fluid loss and fixation
- Measure specimens in aggregate
- Describe skin surfaces (lesions, etc.)
- If oriented, ink specimen / maintain orientation when sectioning
- Serially section as thinly as possible; inspect and palpate slices for lesions
- Sections:
- Sample all grossly evident breast and skin lesions
- In no lesions, submit sections of fibrous parenchyma:
- Women < 40: 4 sections in 2 cassettes
- Women ≥ 40 or known risk factors: 8 sections in 4 cassettes
Specimens removed for gynecomastia
- Subcutaneous mastectomies, often bilateral and unoriented
- Gross exam:
- Weigh, measure and ink each specimen
- Serially section as thinly as possible, examining for visible and palpable lesions
- Sections:
- Submit all lesions, with nearest inked margins
- If no lesions, submit 4 sections (2 cassettes) of each specimen focusing on fibrous parenchyma
Gender reassignment specimens (top surgery)
- There is lack of evidence for specific guidelines at this time
Features to report
- Click here for recommendations from the College of American Pathologists based on the specimen type
Gross images
Board review style question #1
Regarding breast excision specimens for ductal carcinoma in situ, the College of American Pathologists recommends which of the following?
- Measuring the specimen and sampling 1 section per cm, focusing on fibrous / parenchymal tissue
- Sampling up to 10 sections as a screening step
- Serially sectioning the specimen and submitting every other slice
- Serially sectioning the specimen and submitting 1 section per slice
- Submitting the entire specimen in a sequential fashion, when practical
Board review style answer #1
E. Submitting the entire specimen in a sequential fashion, when practical. If this is not possible, at least the entire region of the targeted lesion should be examined microscopically (CAP: Protocol for the Examination of Resection Specimens from Patients With Ductal Carcinoma In Situ (DCIS) of the Breast [Accessed 14 February 2022]).
Comment Here
Reference: Breast - Grossing & features to report
Comment Here
Reference: Breast - Grossing & features to report
Board review style question #2
Which statement pertaining to breast specimen processing is true?
- Biopsy clips may be present in excision or mastectomy specimens by specimen imaging but not grossly evident
- For breast cancer specimens, tangential / en face margins provide similar clinically relevant information as margins taken perpendicular to inked surfaces
- It is rarely necessary to correlate the gross examination of breast specimens with specimen imaging studies for specimens excised for image detected lesions
- Knowledge of the indications for mastectomy is rarely required to optimally process these specimens
Board review style answer #2
A. Biopsy clips may be present in excision or mastectomy specimens by specimen imaging but not grossly evident
Comment Here
Reference: Breast - Grossing & features to report
Comment Here
Reference: Breast - Grossing & features to report