Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Bidot S, Li X. Intraductal papilloma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastpapilloma.html. Accessed April 18th, 2024.
Definition / general
- Benign intraductal proliferation of epithelial cells with fibrovascular cores and underlying myoepithelial cells
Essential features
- Common benign breast lesion
- Distinction with malignant papillary lesions may be difficult on biopsy and immunohistochemistry may be necessary
- No consensus regarding management of papilloma without atypia
- Surgical excision of intraductal papilloma with atypical epithelial proliferation is recommended
Terminology
- Duct papilloma of the breast
- Intracystic papilloma of the breast (old terminology)
ICD coding
- ICD-10: D24 - benign neoplasm of the breast
Epidemiology
- 5% of benign breast lesions (Am J Surg Pathol 2006;30:665)
- Involves women of all ages (Arch Pathol Lab Med 2016;140:628)
- Uncommon in men
Sites
- Central intraductal papilloma: arises from large lactiferous ducts, usually solitary (Arch Pathol Lab Med 2016;140:628)
- Peripheral intraductal papilloma: involves terminal duct lobular unit, usually multiple (papillomatosis) (Arch Pathol Lab Med 2016;140:628)
Pathophysiology
- Poorly understood
- Intraductal papilloma might originate from bipotent progenitor cells that differentiate as luminal and myoepithelial cells (Am J Pathol 2018;188:1106)
Clinical features
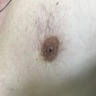
- Solitary central intraductal papilloma may present as a retroareolar mass with serous or serosanguineous nipple discharge
- Peripheral intraductal papilloma is often discovered incidentally
Diagnosis
- Screening mammography
- Core needle biopsy, vacuum assisted biopsy, excisional biopsy (Surg Pathol Clin 2018;11:1)
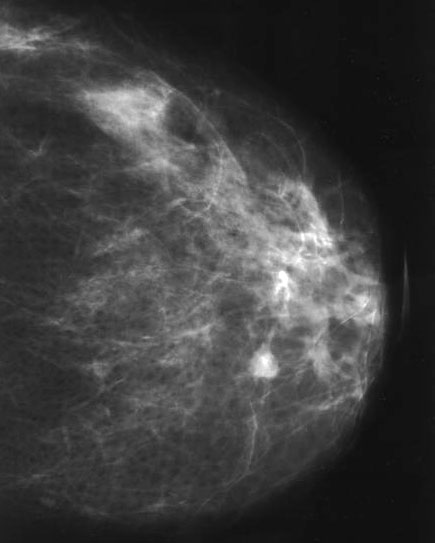
Radiology description
- Nonspecific findings
- Mammography (Diagn Interv Radiol 2013;19:471, AJR Am J Roentgenol 2012;198:264)
- Can be large (usually central papilloma), rounded or ovoid well circumscribed mass
- Calcifications may be seen
- Small lesions (usually peripheral papilloma) may not be visible
- Ultrasonography (Diagn Interv Radiol 2013;19:471, AJR Am J Roentgenol 2012;198:264)
- Classic pattern is solid mural nodule within a dilated duct
- Vascular pedicle may be seen on color Doppler
- MRI (Diagn Interv Radiol 2013;19:471, AJR Am J Roentgenol 2012;198:264)
- Well circumscribed round or ovoid intraductal mass with variable enhancement
- May be associated with ductal dilation
Radiology images
Prognostic factors
- Long term risk of developing breast carcinoma (Mod Pathol 2021;34:78)
- Papilloma without atypia: ~X2 if single; ~X3 if multiple
- Papilloma with atypia: ~X5 if single; ~X7 if multiple
- Risk of upgrade to malignancy (DCIS or invasive breast carcinoma)
- Upgrade rate of papilloma without atypia and with pathology / radiology concordance is low (Mod Pathol 2021;34:78, Am J Surg 2020;220:677, Cancer 2016;122:2819, Clin Imaging 2020;60:67, Breast Cancer Res Treat 2020;183:577, Ann Surg Oncol 2021;28:2573)
- Upgrade rate of intraductal papilloma with atypia is high (Hum Pathol 2021;110:43, Breast Cancer Res Treat 2020;183:577, Ann Surg Oncol 2021;28:2573)
Case reports
- 52 year old man with intraductal papilloma that progressed to ductal carcinoma in situ (Radiol Case Rep 2018;13:602)
- 53 year old woman with a large intraductal papilloma (Int J Surg Case Rep 2019;55:1)
- 55 year old man with unilateral bloody discharge and intraductal papilloma (J Surg Case Rep 2019;2019:rjz023)
Treatment
- No consensus for intraductal papilloma without atypia
- Complete surgical excision often performed (Am J Surg 2020;220:677)
- Different management among institutions (Clin Imaging 2020;60:67)
- Complete surgical excision should be performed for intraductal papilloma with atypia
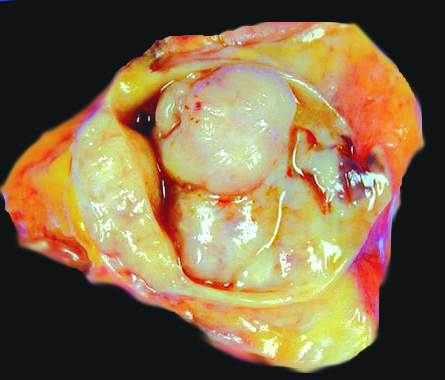
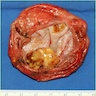
Gross description
- Central intraductal papilloma: well circumscribed polypoid nodule in a cystically dilated duct (Arch Pathol Lab Med 2016;140:628)
- Peripheral intraductal papilloma: not grossly visible (Arch Pathol Lab Med 2016;140:628)
Gross images
Frozen section description
- Frozen section not performed on lesions suspected to be intraductal papilloma
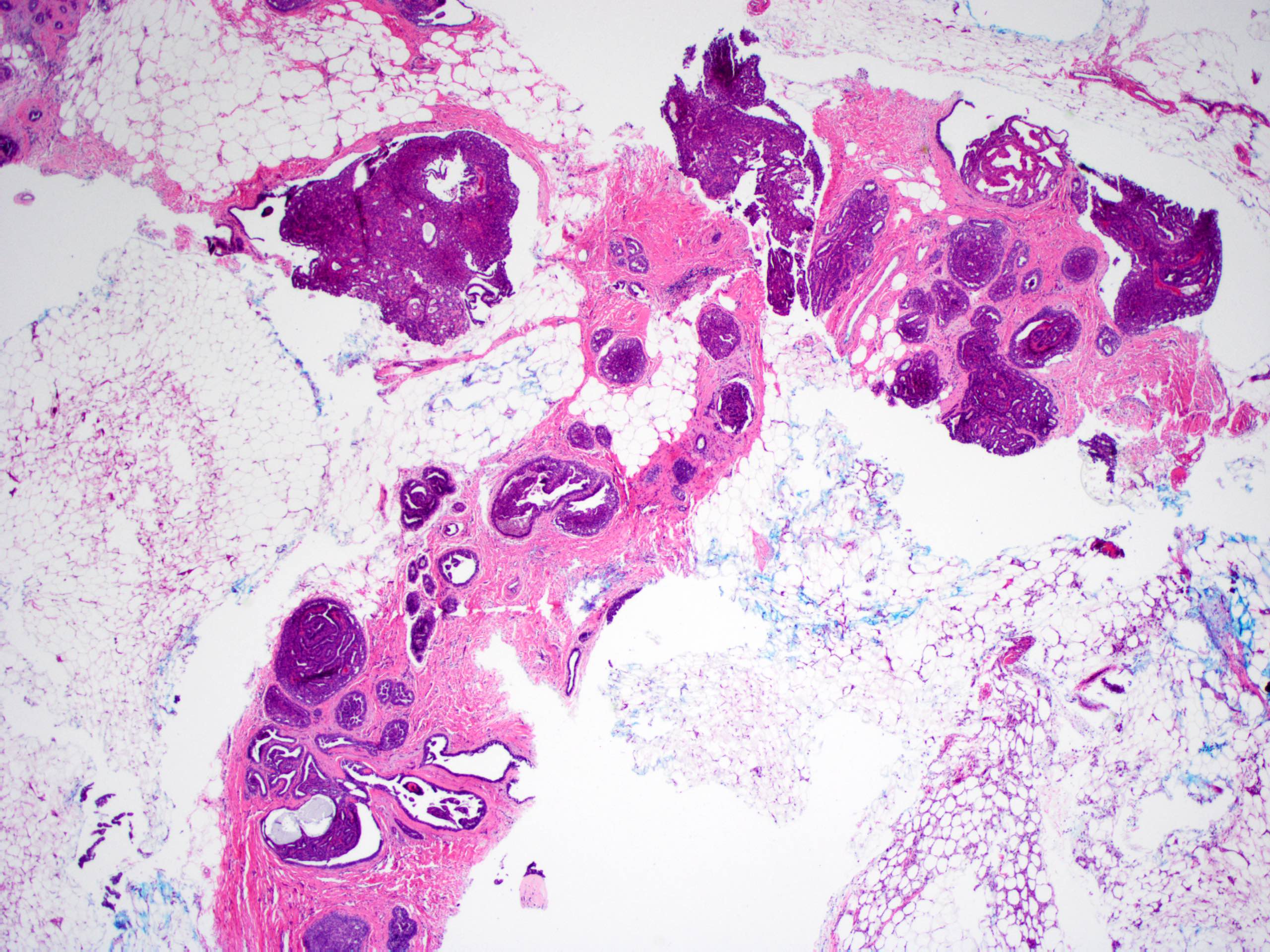
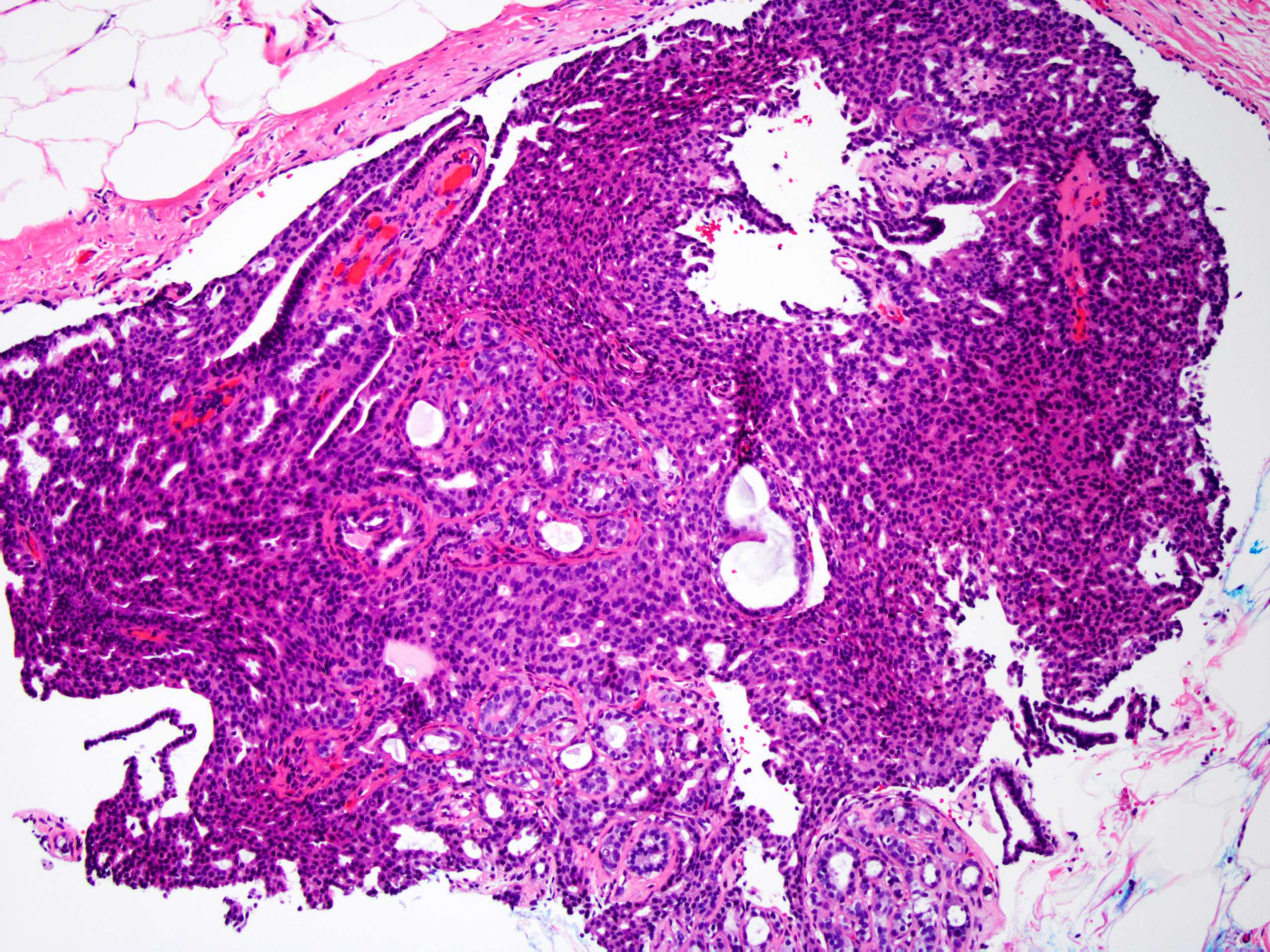
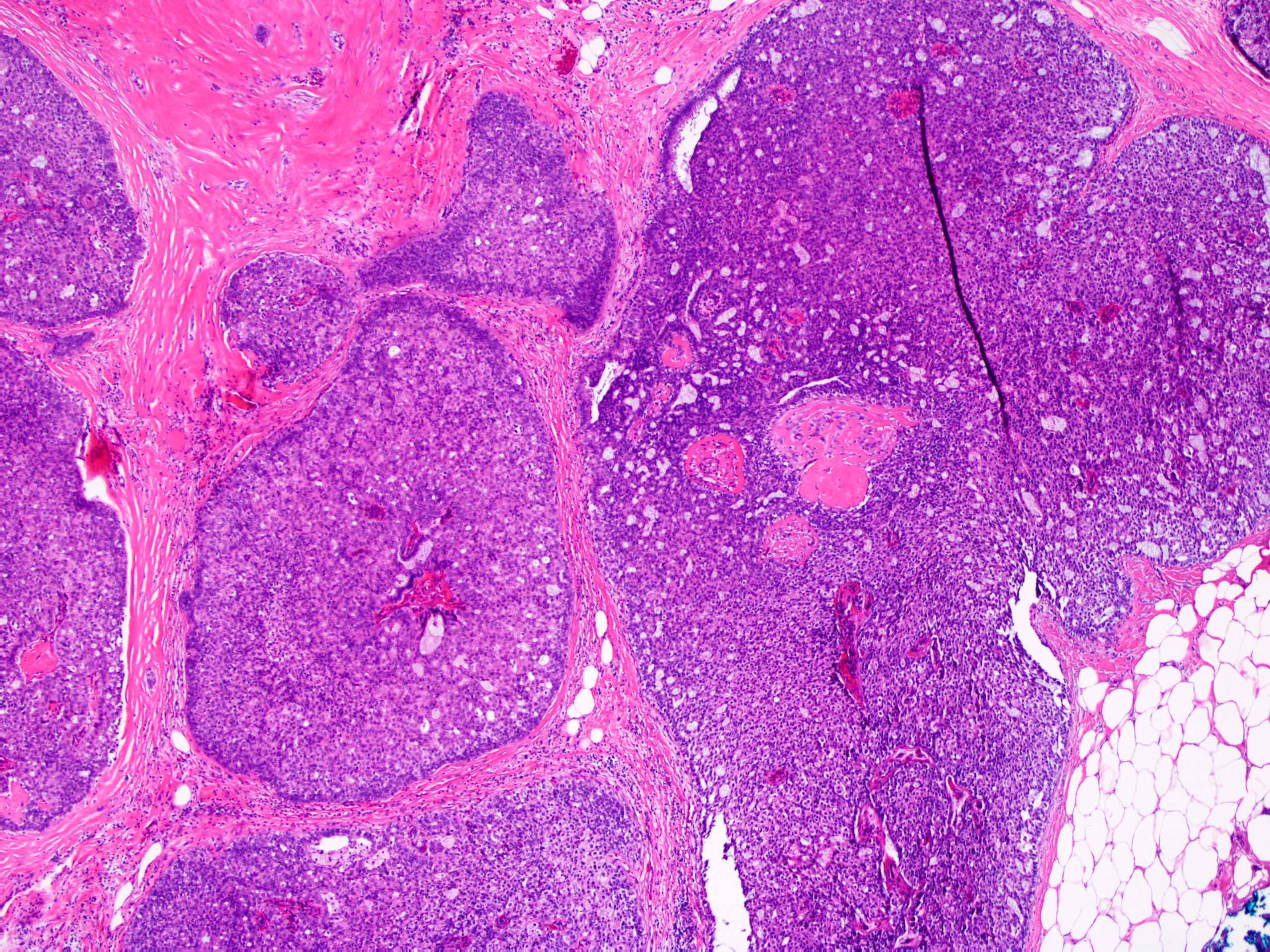
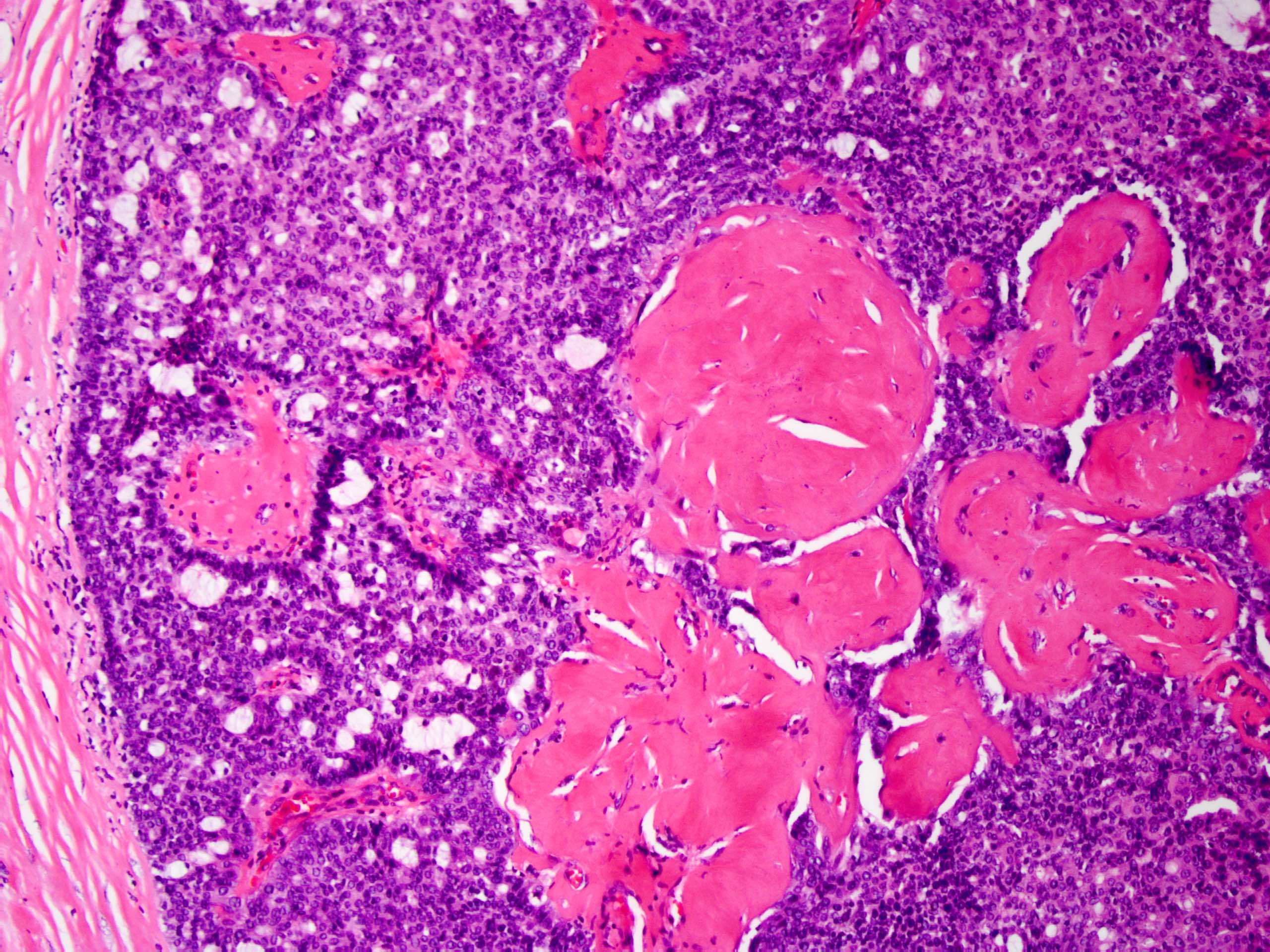
Microscopic (histologic) description
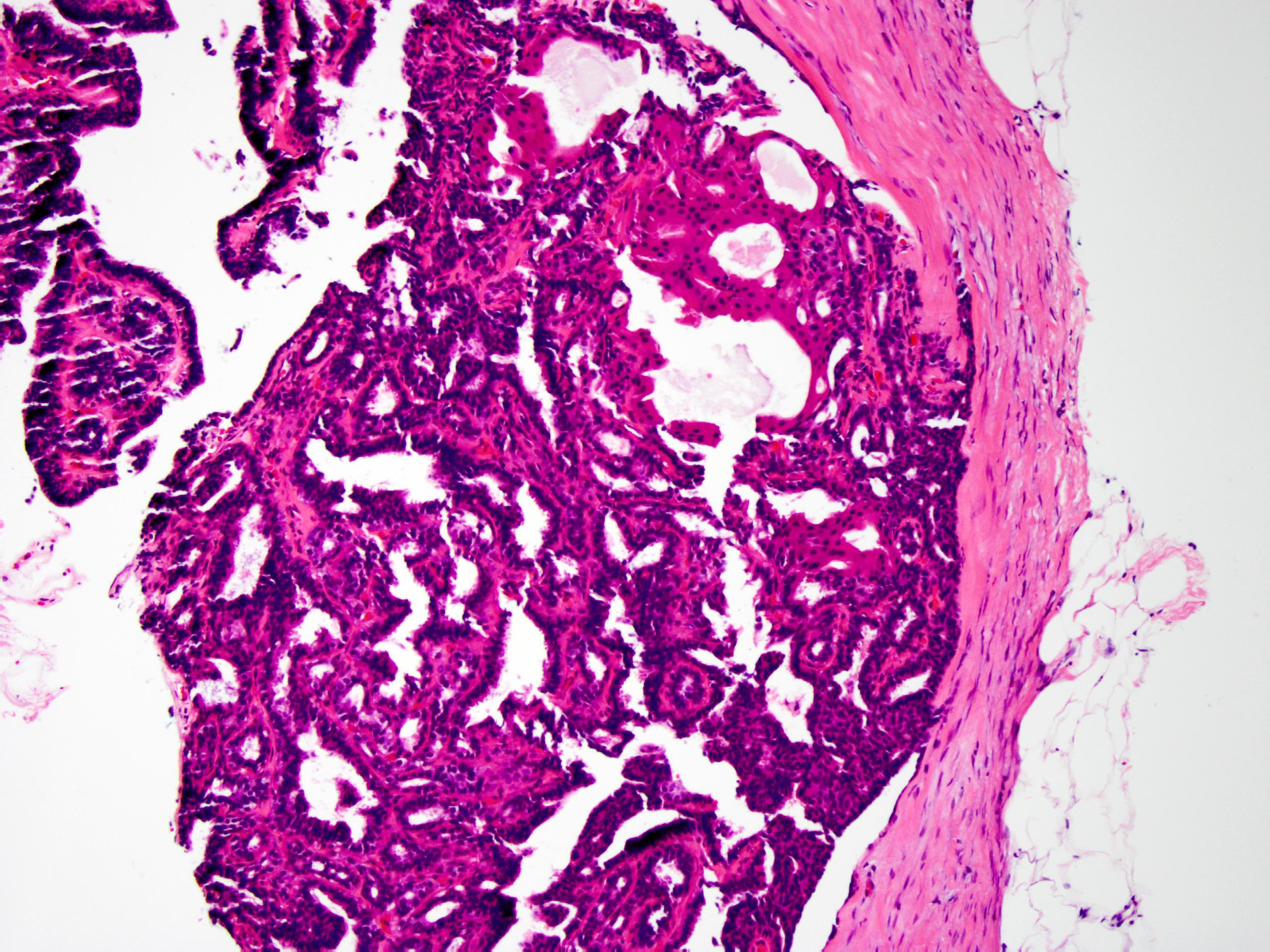
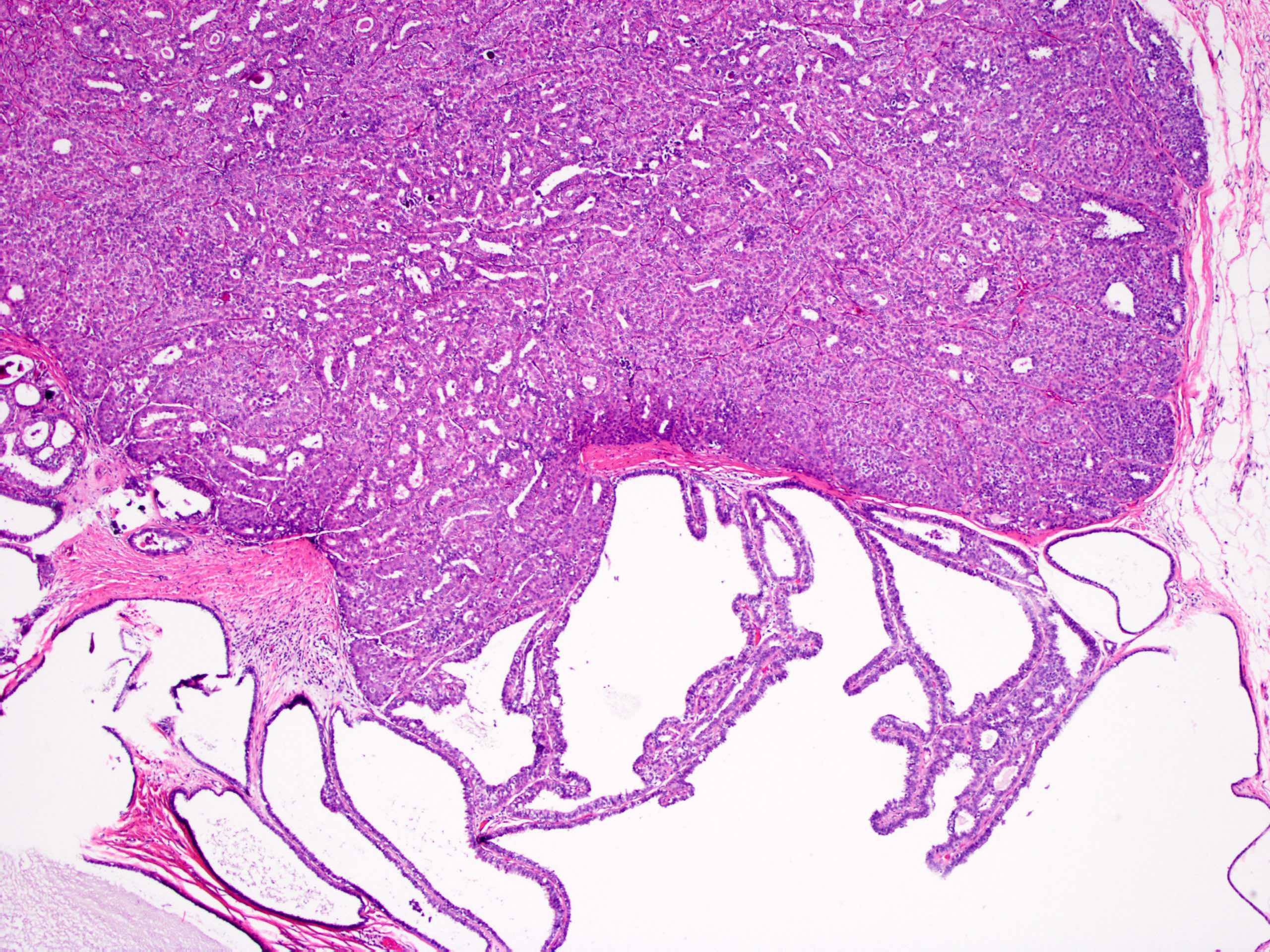
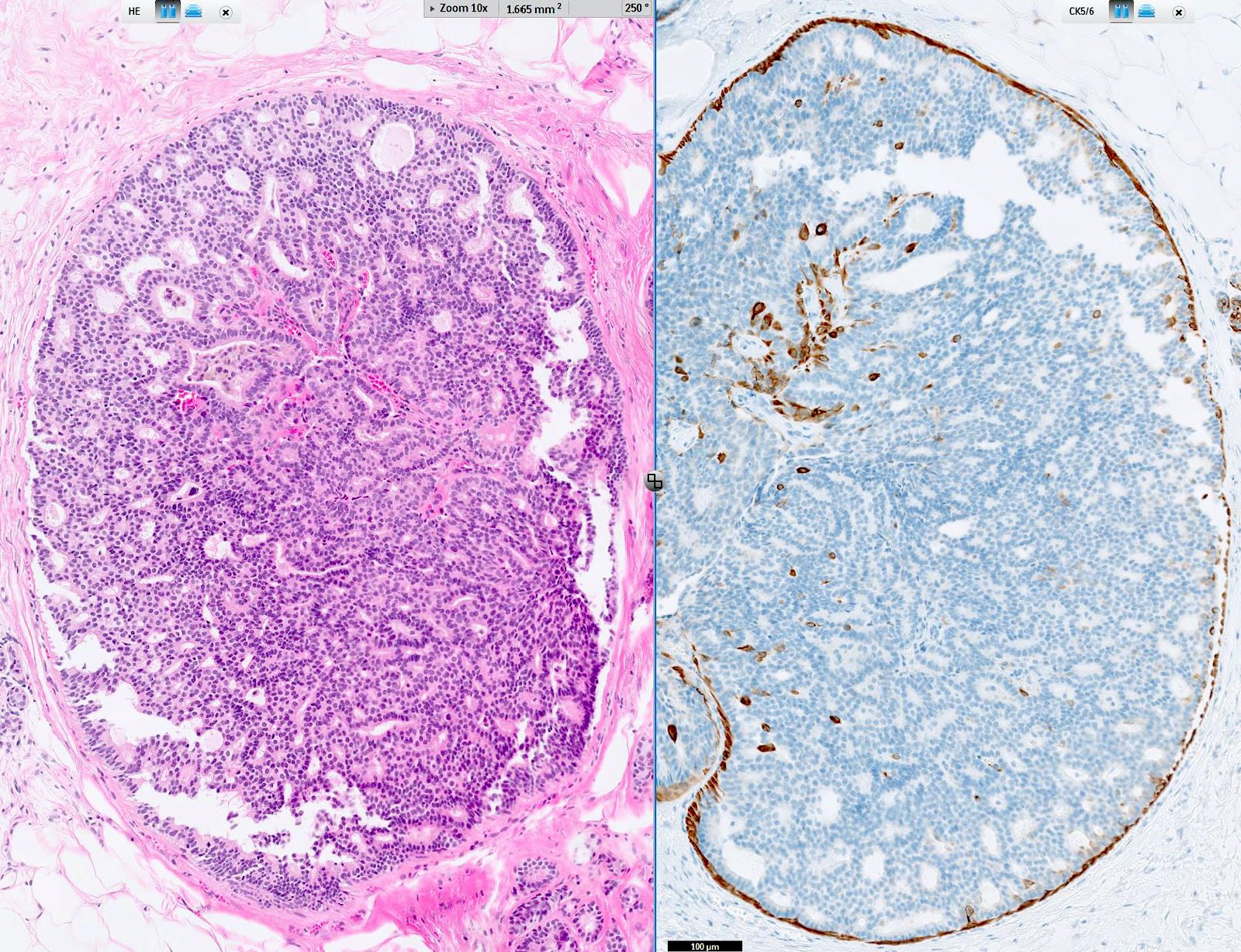
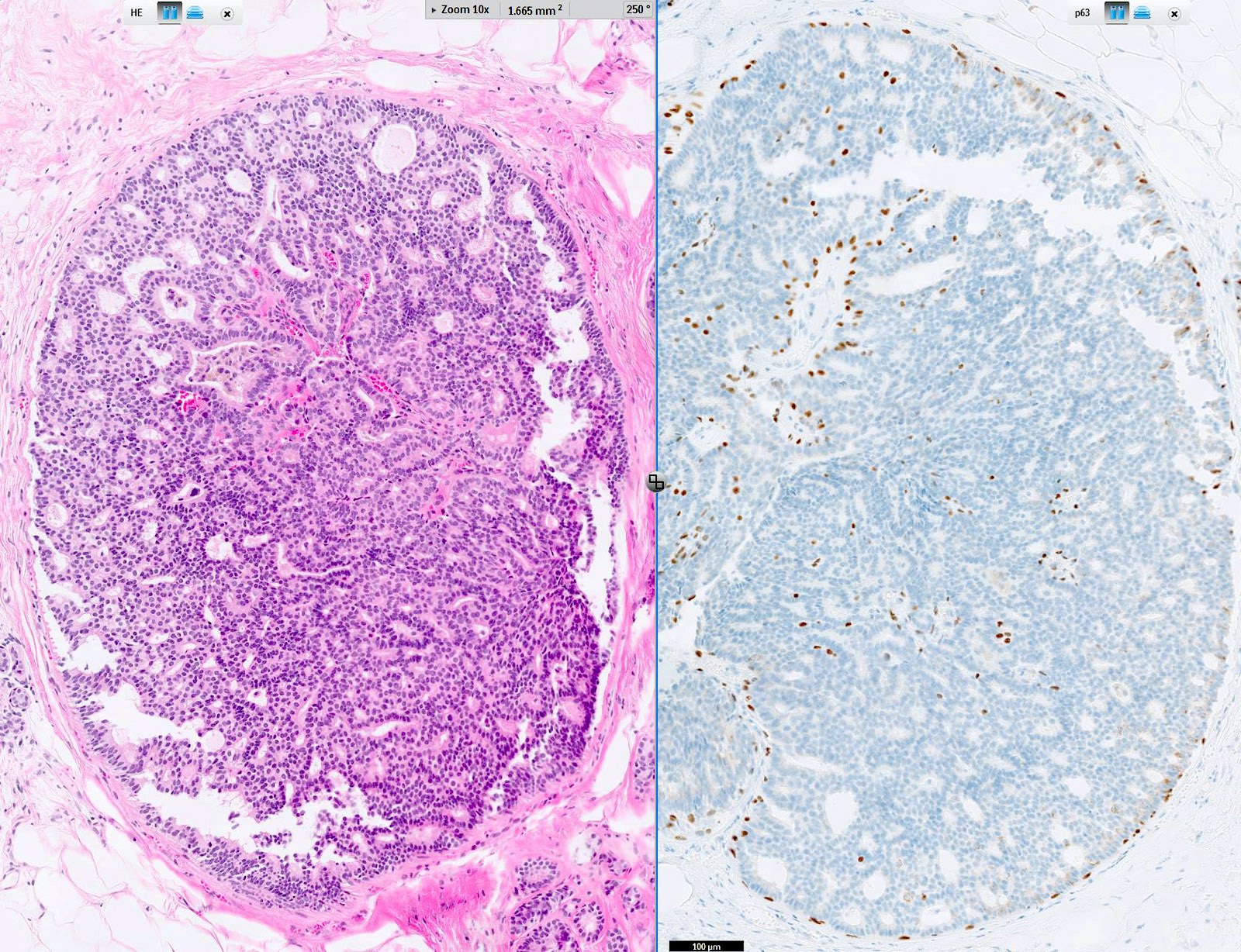
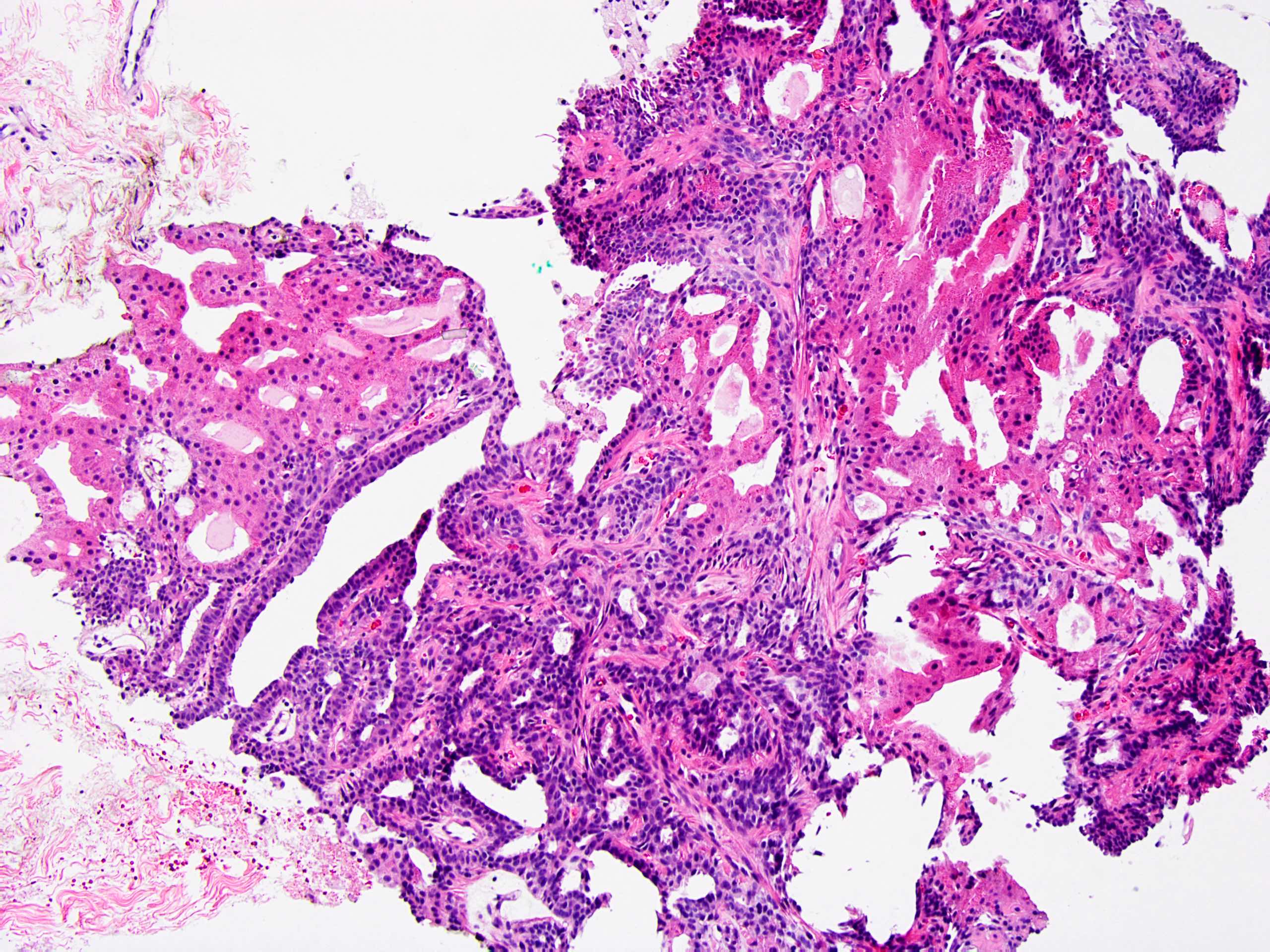
- Circumscribed intraductal proliferation comprised of arborizing fibrovascular cores lined by outer layer of luminal cells and an inner layer of myoepithelial cells (Histopathology 2016;68:22)
- Myoepithelial cells might be inconspicuous in sclerotic areas
- Myoepithelial cells also present at the periphery of the involved duct
- Frequently associated with usual type ductal hyperplasia and apocrine metaplasia, less frequently squamous, osseous or chondroid metaplasia may be seen
- May undergo ischemic or hemorrhagic changes, either spontaneously or secondary to biopsy / trauma
- May show prominent fibrosis / sclerosis which may contained entrapped glands (sclerosing papilloma)
- May be involved by:
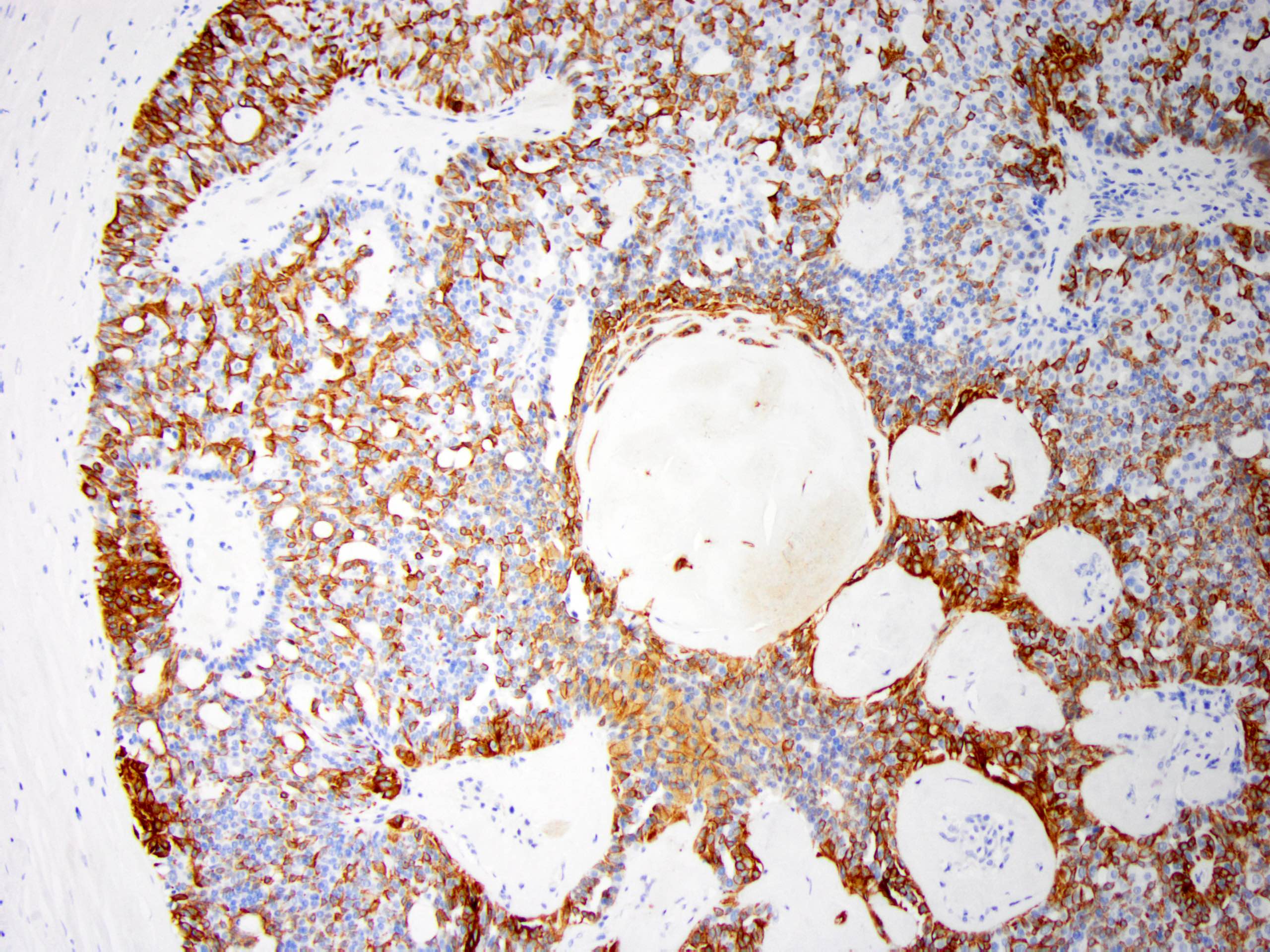
Microscopic (histologic) images
Contributed by Xiaoxian (Bill) Li, M.D., Ph.D.
Contributed by Jijgee Munkhdelger, M.D., Ph.D. and Andrey Bychkov, M.D., Ph.D.
Cytology description
- Large stellate tissue fragments of benign ductal cells in a proteinaceous background (Diagn Cytopathol 2007;35:386)
Positive stains
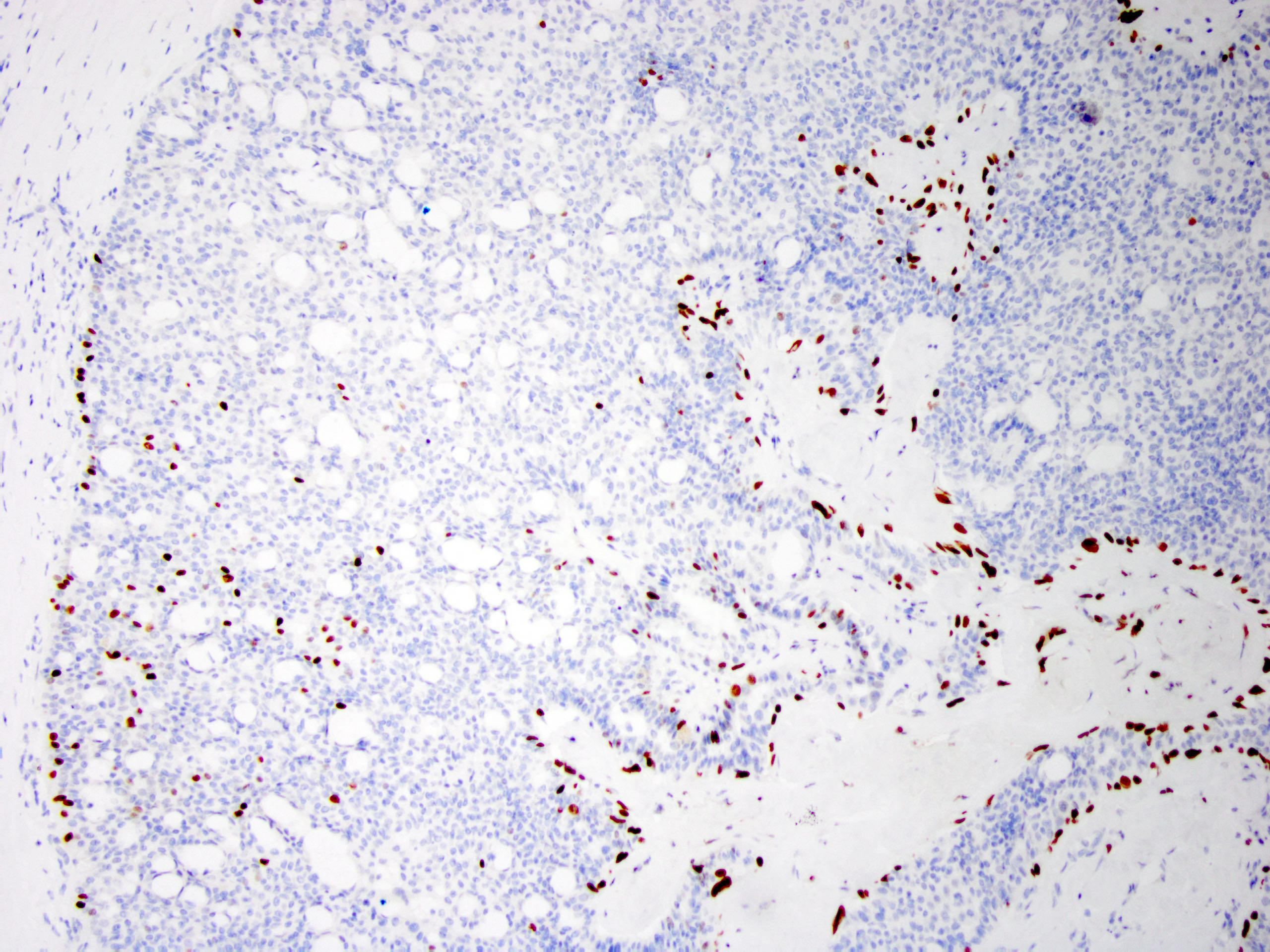
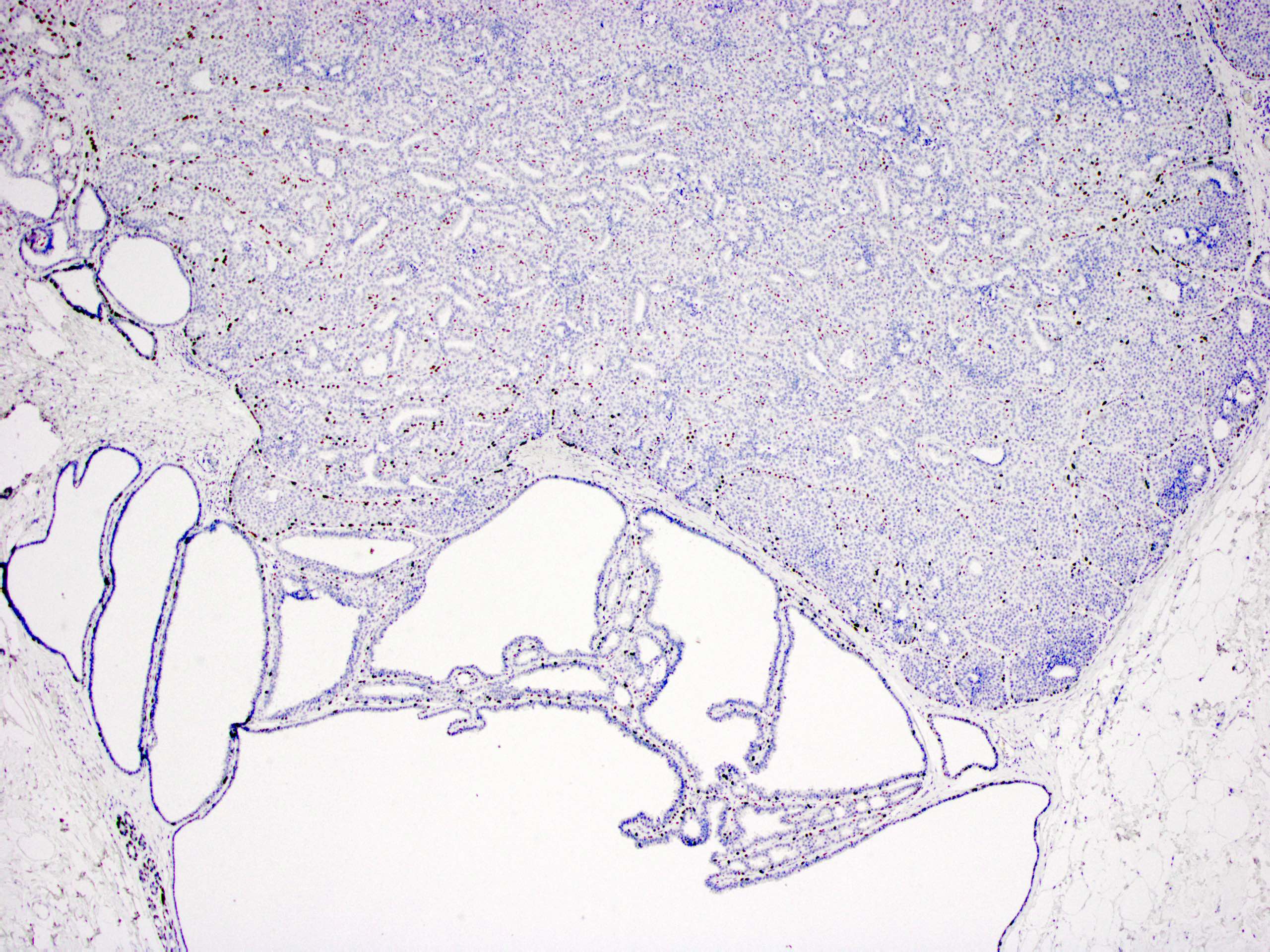
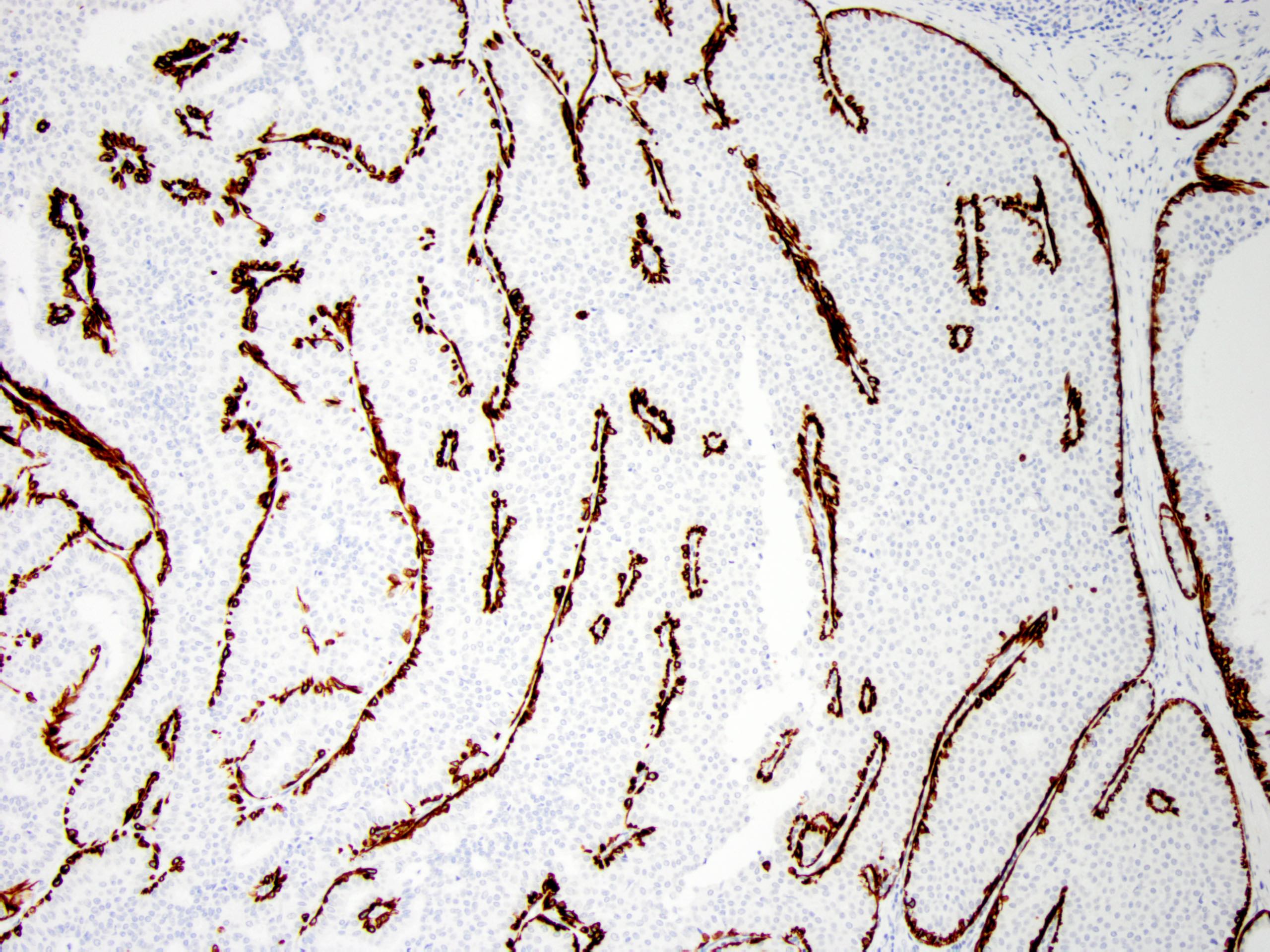
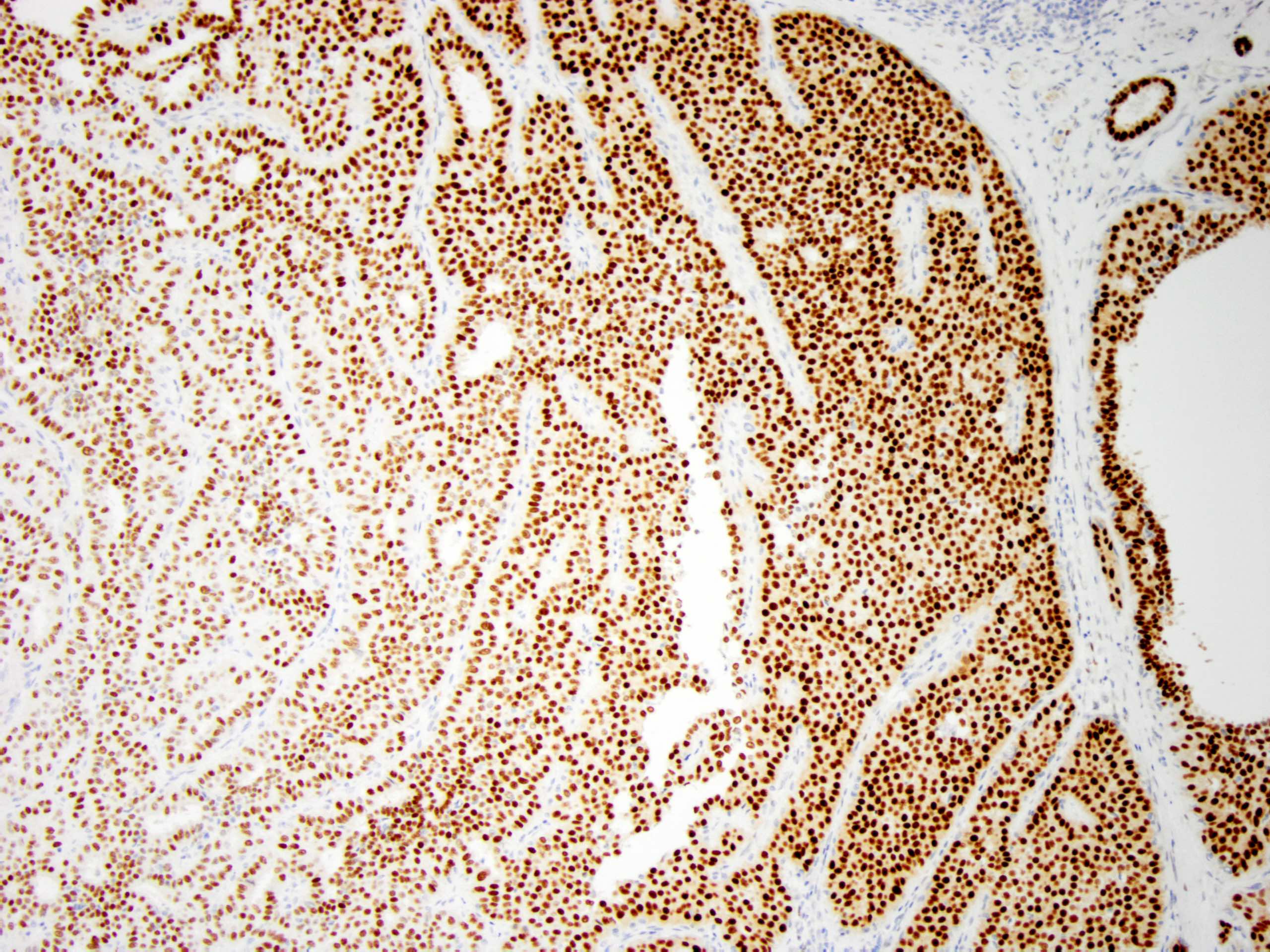
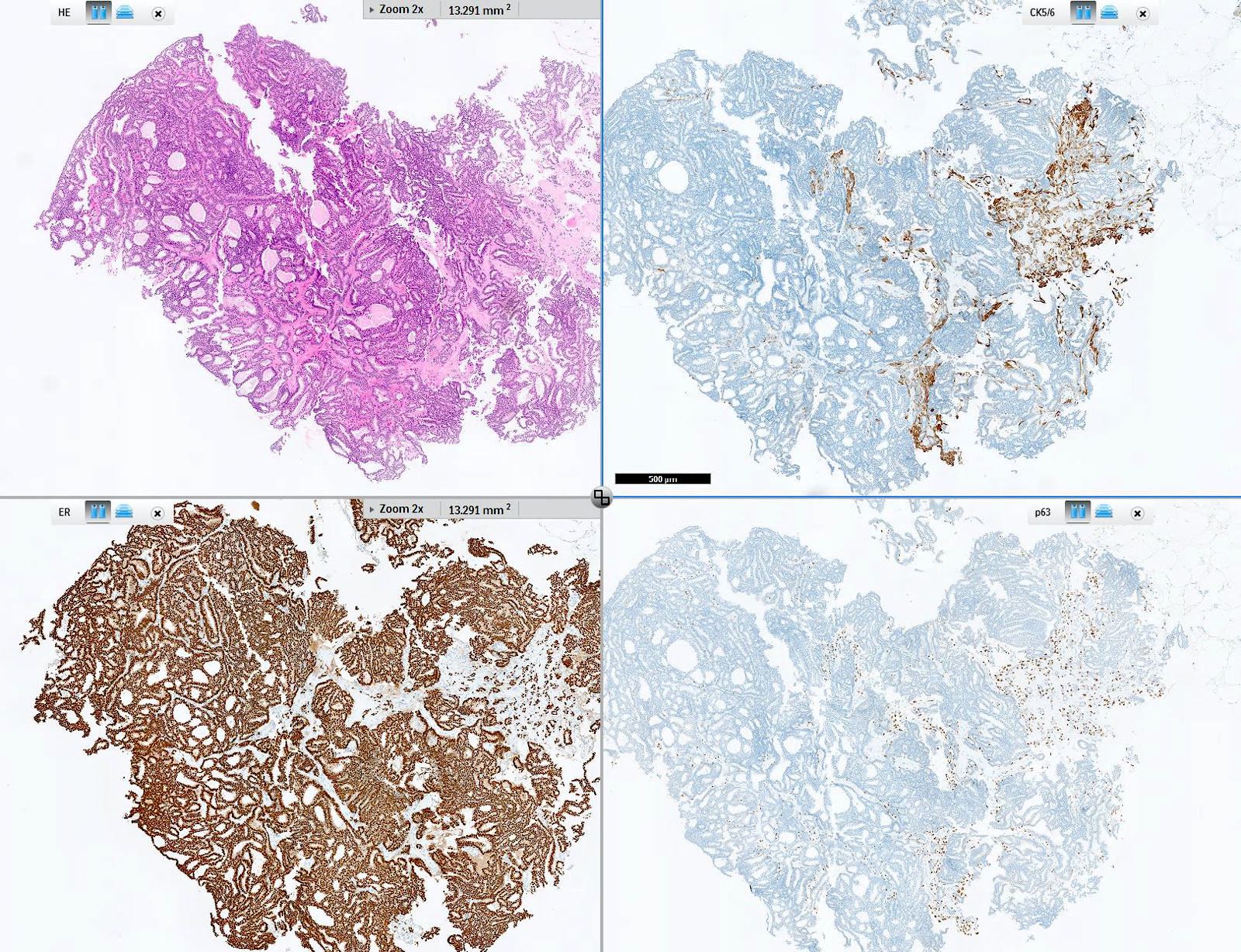
- Myoepithelial cell markers (CK5, p63, p40, calponin, alpha smooth muscle actin, smooth muscle myosin heavy chain / SMMHC) show myoepithelial cells in fibrovascular cores and at the periphery of the involved duct (Arch Pathol Lab Med 2016;140:628)
- In intraductal papilloma involved by usual ductal hyperplasia
- ER is patchy positive (Pathol Int 2012;62:381)
- CK5 is patchy positive in a mosaic pattern (Pathol Int 2012;62:381)
Molecular / cytogenetics description
- PIK3CA / AKT1 pathway mutations commonly involved (Mod Pathol 2010;23:27, Mod Pathol 2021;34:1044):
- AKT1 mutations are commonly seen in papilloma without (54%) or with mild hyperplasia (15%)
- PIK3CA mutations can be seen in 21 - 42% in papilloma with or without atypia
Videos
Intraductal papilloma
Sample pathology report
- Right breast, 9 o'clock, 1 cm from the nipple, ultrasound guided core biopsy:
- Intraductal papilloma with apocrine metaplasia
- Comment: Patchy positivity for CK5 and ER supports the above diagnosis.
Differential diagnosis
- Atypical ductal hyperplasia involving an intraductal papilloma (atypical papilloma) (Histopathology 2016;68:22):
- Monotonous low grade cellular proliferation
- Atypical proliferation is small (≤ 3 mm) (Cancer 1996;78:258, Am J Surg Pathol 2006;30:665)
- Decreased myoepithelial cells
- CK5/6 negative, ER diffusely positive
- Monotonous low grade cellular proliferation
- Intraductal papillary carcinoma (papillary DCIS) (Histopathology 2016;68:22):
- Encapsulated papillary carcinoma (Histopathology 2016;68:22):
- Adenomyoepithelioma (Mod Pathol 2021;34:1044, Mod Pathol 2020;33:1764, Am J Surg Pathol 1991;15:554):
- Rare, usually benign, well circumscribed nonencapsulated biphasic tumor with myoepithelial and epithelial components
- Multiple patterns (spindle cell, tubular, lobulated, papillary)
- Papillary pattern might be difficult to differentiate from intraductal papilloma
- In adenomyoepithelioma, myoepithelial cells are more conspicuous
- Invasive papillary carcinoma (Mod Pathol 2021;34:1044):
- Uncommon subtype of breast carcinoma
- Invasive carcinoma with > 90% papillary architecture
- Negative for myoepithelial cells
- Tall cell carcinoma with reverse polarity (Mod Pathol 2021;34:1044):
- Rare papillary lesion with uncertain malignant potential
- Distinctive morphology:
- Columnar epithelial cells with apically located nucleus with papillary, solid and follicular architecture
- Nuclei with clearing, groove and pseudoinclusion (reminiscent of tall cell variant of papillary thyroid carcinoma)
- Recurrent IDH2 R172 hotspot mutation
- Solid papillary carcinoma (Mod Pathol 2021;34:1044):
- Uncommon subtype of breast carcinoma
- Expansile solid nodules with interspersed fibrovascular cores composed of monotonous atypical cells (low or intermediate grade)
- Considered as an in situ disease
- Myoepithelial cells may be attenuated at the periphery
Board review style question #1
Board review style answer #1
B. This intraductal papilloma shows typical features of apocrine metaplasia.
Comment Here
Reference: Intraductal papilloma
Comment Here
Reference: Intraductal papilloma
Board review style question #2
What is the staining pattern of p63 for intraductal papilloma of the breast without atypia?
- At the periphery of involved duct only
- In the fibrovascular cores only
- Intraductal papillomas do not stain for p63
- At the periphery and in the fibrovascular cores
Board review style answer #2
D. p63 stain for myoepithelial cells is positive throughout the lesion, including in the fibrovascular cores and at the periphery of the involved duct.
Comment Here
Reference: Intraductal papilloma
Comment Here
Reference: Intraductal papilloma
Board review style question #3
What is the typical staining pattern of atypical epithelial proliferation involving an intraductal papilloma of the breast?
- Diffusely positive for ER and patchy positive for CK5/6, negative for p63
- Diffusely positive for ER, negative for p63 and CK5/6
- Diffusely positive for ER, p63 and CK5/6
- Negative for ER, p63 and CK5/6
- Patchy positive for ER, negative for p63 and CK5/6
Board review style answer #3
B. The atypical low grade epithelial proliferation is diffusely positive for ER, negative for CK5/6 and negative for p63.
Comment Here
Reference: Intraductal papilloma
Comment Here
Reference: Intraductal papilloma