Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Kostelecky N, Ahrendsen JT. Cerebral amyloid angiopathy. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnscerebralamyloid.html. Accessed April 19th, 2024.
Definition / general
- Vasculopathy characterized by the accumulation of beta amyloid protein within small to medium sized blood vessels of the brain and leptomeninges
- Renders affected blood vessels fragile, leading to intraparenchymal hemorrhage
- Distinct from other systemic forms of amyloidosis
Essential features
- Progressive disease of older individuals, predisposing them to intracerebral hemorrhage
- May coincide with or be independent of Alzheimer disease neuropathologic change (AD NC) or other small to medium vessel cerebrovascular disease (e.g., arteriolosclerosis)
- May present as noninflammatory or inflammatory subtypes
Terminology
- Congophilic angiopathy
- Dysphoric angiopathy
ICD coding
Epidemiology
- Sporadic disease (J Neurol Sci 2021;424:117425):
- More common in elderly patients, particularly in the seventh and eighth decades of life
- May coincide with (or be independent of) arteriolosclerotic cerebrovascular disease or Alzheimer disease neuropathologic change
- A large retrospective autopsy series detected sporadic disease in 73% of all subjects and 98% of patients with Alzheimer disease (J Neurol 2008;255:70)
- APOE mutations and cerebral amyloid angiopathy (CAA) (Lancet Neurol 2021;20:68):
- Mutations in the ε4 allele are associated with sporadic onset CAA and increased severity of disease
- Mutations in the ε2 allele may be protective
- Familial / hereditary disease (Arch Neurol 2010;67:987):
- Hereditary cerebral hemorrhage with amyloidosis - Dutch type (HCHWA D)
- Earlier onset (sixth decade) CAA symptoms with cerebral hemorrhages
- Autosomal dominant mutation of APP gene (E693Q) on chromosome 21
- Hereditary cerebral hemorrhage with amyloidosis - Icelandic type (HCHWA I)
- Cerebral hemorrhages in young to middle aged adults
- Mutations in cystatin C / gamma trace gene
- Also known as hereditary cystatin C amyloid angiopathy (HCCAA)
- Associated with mural deposition of gamma trace protein in arterioles
- Familial British and Danish forms
- Mutations in BR12 gene (chromosome 13)
- Less consistently associated with intraparenchymal hemorrhage
- Flemish form: APP gene mutations at codon 692 (Neurobiol Dis 1998;5:281)
- APP gene mutations at codon 694 (Alzheimer disease neuropathologic change with prominent CAA) (Ann Neurol 2001;49:697)
- Finnish type: autosomal dominant mutations in GSN gene with gelsolin deposition in vessels (Crit Rev Biochem Mol Biol 2012;47:282)
- Hereditary transthyretin amyloidosis (ATTR): systemic amyloidosis that can affect the central nervous system (Acta Neuropathol 2023;145:113)
- Additional familial cohorts have been described (Int J Mol Sci 2020;21:3435, Arch Neurol 2010;67:987)
- Hereditary cerebral hemorrhage with amyloidosis - Dutch type (HCHWA D)
Sites
- Central nervous system: small to medium sized arteries and veins of the cortex and leptomeninges
Pathophysiology
- Progressive deposition of beta amyloid protein in the arteries and veins of the cortex and leptomeninges
- Leads to weakness / fragility of affected vessel walls, predisposing individuals to intracerebral (lobar) hemorrhages (J Neurol Sci 2021;424:117425)
Etiology
- Deposition of beta amyloid in arterial media and adventitia
- Genetic factors (see Epidemiology) may predispose to the development of CAA, including more severe familial forms (Brain Pathol 2002;12:343)
Clinical features
- Intraparenchymal (lobar) hemorrhage (Neurology 1984;34:730)
- Additionally, there have been associations described with (Neurology 1993;43:2073)
- Cognitive decline
- Transient focal neurologic events
- Cortical atrophy
- Small cortical infarcts
- Leukoencephalopathy
- If coexisting Alzheimer disease neuropathologic change, CAA may act synergistically in its effects on clinical presentation, including memory disturbances (Ann Neurol 2011;69:320)
- Inflammatory CAA often presents more acutely with focal / multifocal neurologic deficits or seizure (J Neurol Sci 2021;424:117425)
- Patients tend to be younger than in noninflammatory CAA
- Includes CAA related inflammation (CAAri) and Aβ related angiitis (ABRA)
- Subarachnoid hemorrhage and subpial hemosiderosis have been described in patients with leptomeningeal disease (AJNR Am J Neuroradiol 2008;29:184)
- Leukoencephalopathy may result from chronic hypoperfusion (Front Aging Neurosci 2022;14:1019088)
Diagnosis
- Combination of clinical findings, radiographic criteria (Boston criteria) and histology (biopsy or autopsy)
- Boston criteria: radiographic criteria for CAA diagnosis (Stroke 2018;49:491, J Clin Neurol 2011;7:1)
- Definite CAA
- Full postmortem examination demonstrating
- Lobar, cortical or cortical - subcortical hemorrhage
- Severe CAA with vasculopathy
- Absence of another diagnostic lesion
- Full postmortem examination demonstrating
- Probably CAA with supporting pathology
- Clinical data and pathologic tissue (evacuated hematoma or cortical biopsy) demonstrating
- Lobar, cortical or cortical - subcortical hemorrhage including intracerebral (ICH), cerebral microbleeds (CMB) or cortical superficial siderosis (cSS)
- Some degree of CAA in tissue specimen
- Absence of another diagnostic lesion
- Clinical data and pathologic tissue (evacuated hematoma or cortical biopsy) demonstrating
- Probable CAA
- Clinical data and MRI or CT demonstrating
- Multiple hemorrhages (intracerebral, cerebral microbleeds) restricted to lobar, cortical or cortical - subcortical regions (cerebellar hemorrhage allowed) or single lobar, cortical or cortical - subcortical hemorrhage and cortical superficial siderosis (focal or disseminated)
- Age: ≥ 55 years
- Absence of other causes of hemorrhage
- Clinical data and MRI or CT demonstrating
- Possible CAA
- Clinical data and MRI or CT demonstrating
- Single lobar, cortical or cortical - subcortical intracerebral, cerebral microbleeds or cortical superficial siderosis (focal or disseminated)
- Age: ≥ 55 years
- Absence of other cause of hemorrhage
- Clinical data and MRI or CT demonstrating
- Other causes of hemorrhage (differential diagnosis of lobar hemorrhages) to exclude for either probable or possible CAA:
- Antecedent head trauma, hemorrhagic transformation of an ischemic stroke, arteriovenous malformation, hemorrhagic tumor, warfarin therapy with international normalization ratio (INR) > 3, vasculitis
- Definite CAA
Laboratory
- Inflammatory CAA
- Cerebrospinal fluid studies may show elevated protein and pleocytosis (J Alzheimers Dis 2023;91:1173)
- Inflammatory markers (ESR / CRP) may be elevated (J Neurol Sci 2021;424:117425)
Radiology description
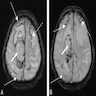
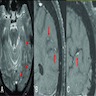
- Noninflammatory CAA (J Neurol Neurosurg Psychiatry 2012;83:275, J Med Imaging Radiat Oncol 2018 Mar 31 [Epub ahead of print])
- CAA related hemorrhages / cortical microbleeds typically occur in cortical or subcortical regions and tend to cluster
- If presenting with an intracerebral hemorrhage, they tend to be lobar and posteriorly located
- Hypointense or black on T2 weighted MRI
- Cortical hemosiderosis, acute sulcal subarachnoid hemorrhages and features of white matter disease may also be appreciated
- Utility of PET CT using amyloid binding compounds may be of some clinical utility (Ann Neurol 2004;55:306, J Neurosci 2001;21:RC189, PET Clin 2023;18:103)
- Inflammatory CAA (Neuroradiology 2014;56:283)
- Extensive T2 / FLAIR hyperintensity in cortical white matter and microhemorrhages
- Can be asymmetric (important to distinguish from symmetrical microvascular disease such as hypertension)
Radiology images
Prognostic factors
- Mutations in the ε4 allele are associated with sporadic onset CAA and increased severity of disease (Lancet Neurol 2021;20:68)
- Mutations in the ε2 allele may be protective (Lancet Neurol 2021;20:68)
Case reports
- 34 year old woman and 39 and 48 year old men with iatrogenic cerebral amyloid exposure (Ann Neurol 2019;85:284)
- 58 year old man presenting with seizures (Folia Neuropathol 2019;57:205)
- 72 year old woman with cerebral amyloid angiopathy mimicking intracranial metastases (J Med Case Rep 2018;12:133)
- 73 year old woman with multiple cortical microbleeds (Medicine (Baltimore) 2019;98:e18296)
Treatment
- Noninflammatory CAA (Int J Stroke 2021;16:356, Int J Mol Sci 2021;22:3869)
- Blood pressure control to prevent intracerebral hemorrhage
- Evacuation of hemorrhage can be performed in some cases of lobar hemorrhage to relieve intracranial pressure
- Experimental therapies currently under review
- Inflammatory CAA (J Neurol Sci 2021;424:117425):
- Not fully established but includes immunosuppressive therapy such as corticosteroids
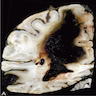
Gross description
- Grossly visible lesions will consist of several different types (J Neurol Sci 2021;424:117425, Neurosurg Focus 2012;32:E7):
- Lobar hemorrhages which may extend into adjacent lobes or ventricles
- Petechial microhemorrhages
- Superficial cortical siderosis
- Distinguishing features between noninflammatory and inflammatory CAA is made histologically (J Neurol Sci 2021;424:117425)
Microscopic (histologic) description
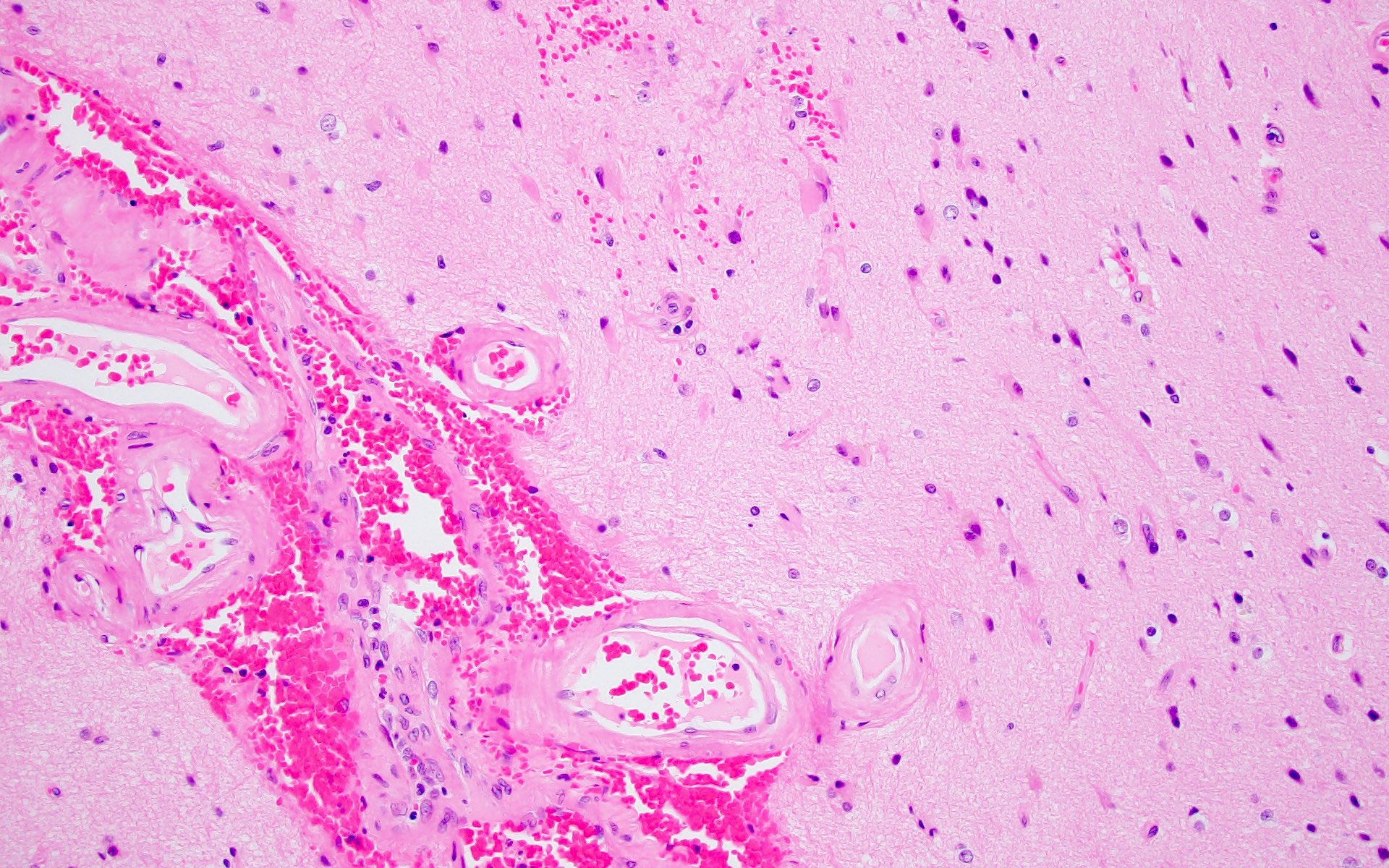
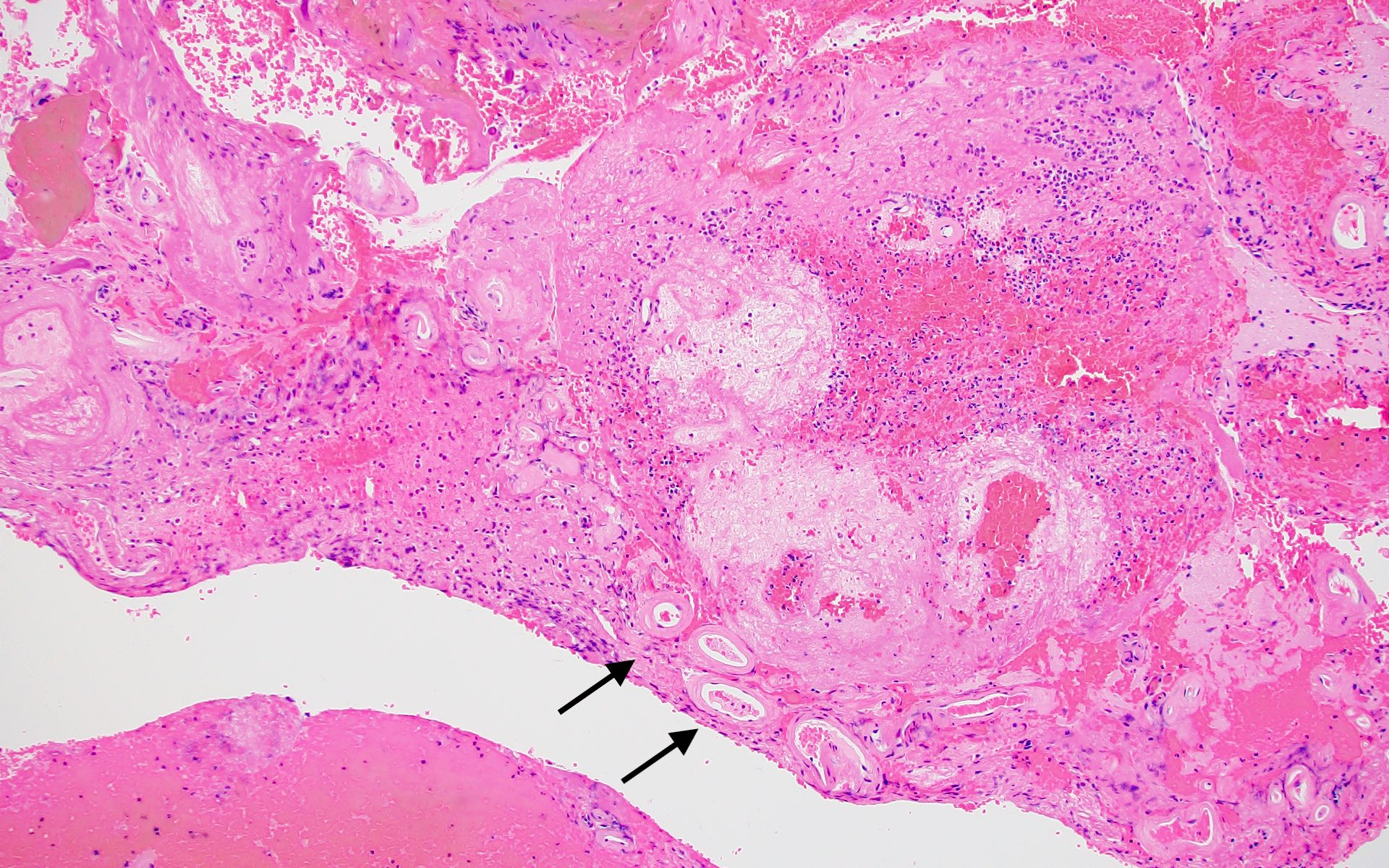
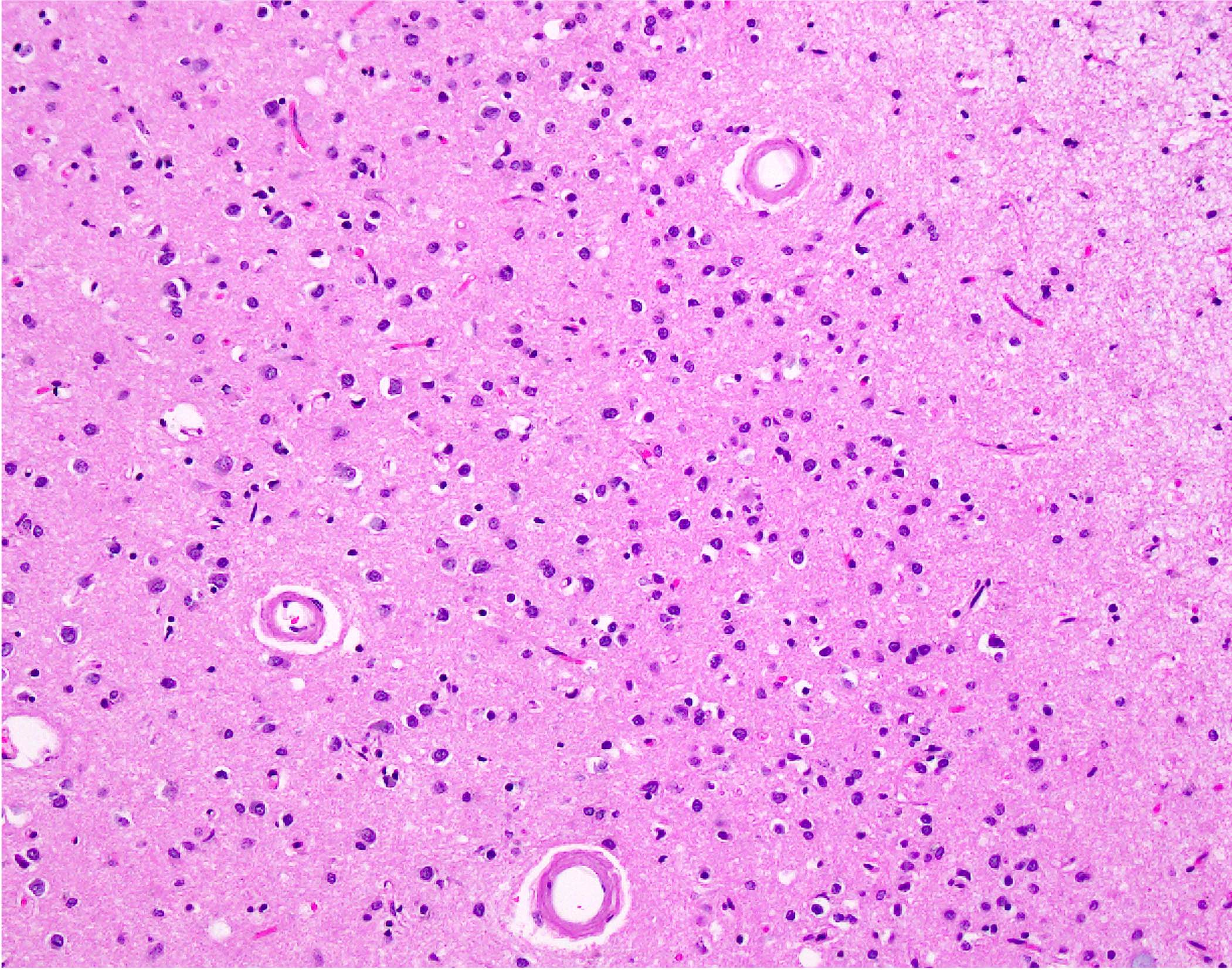
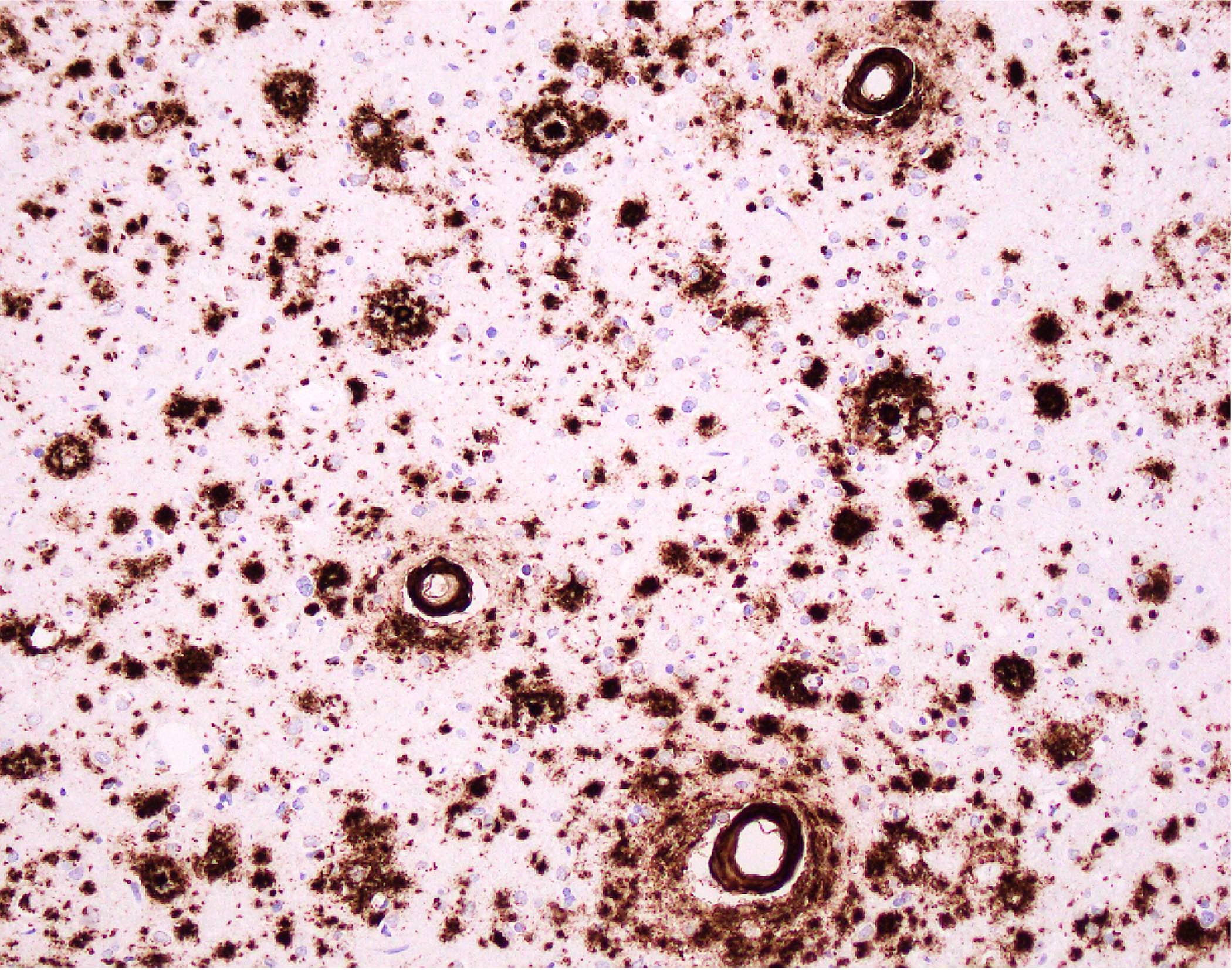
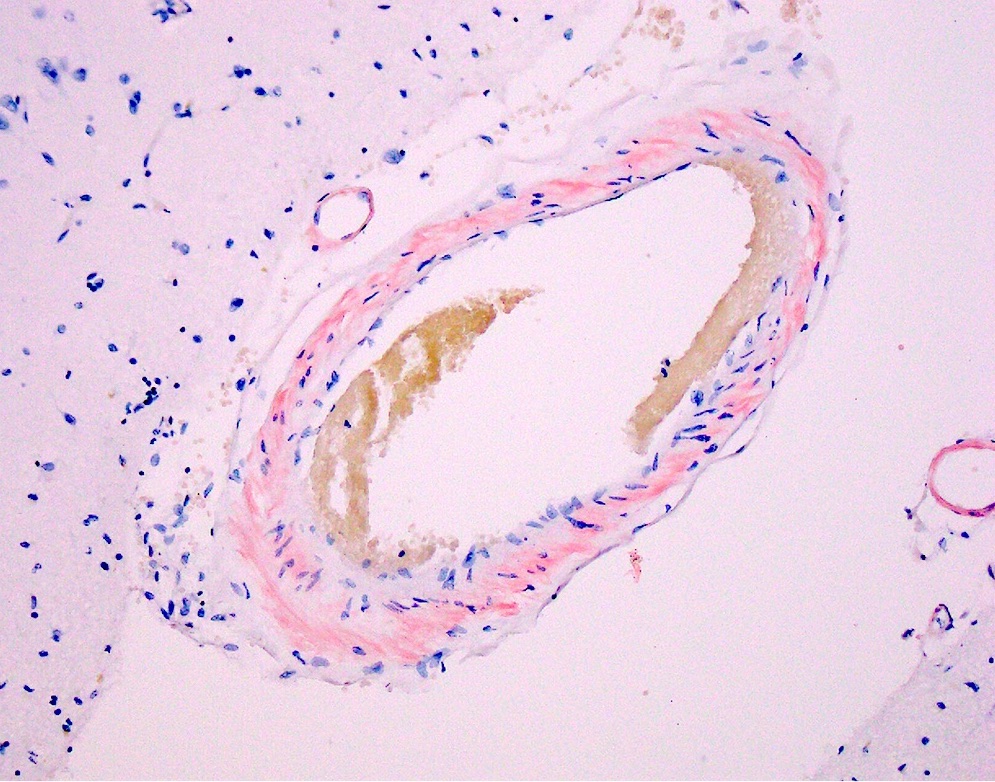
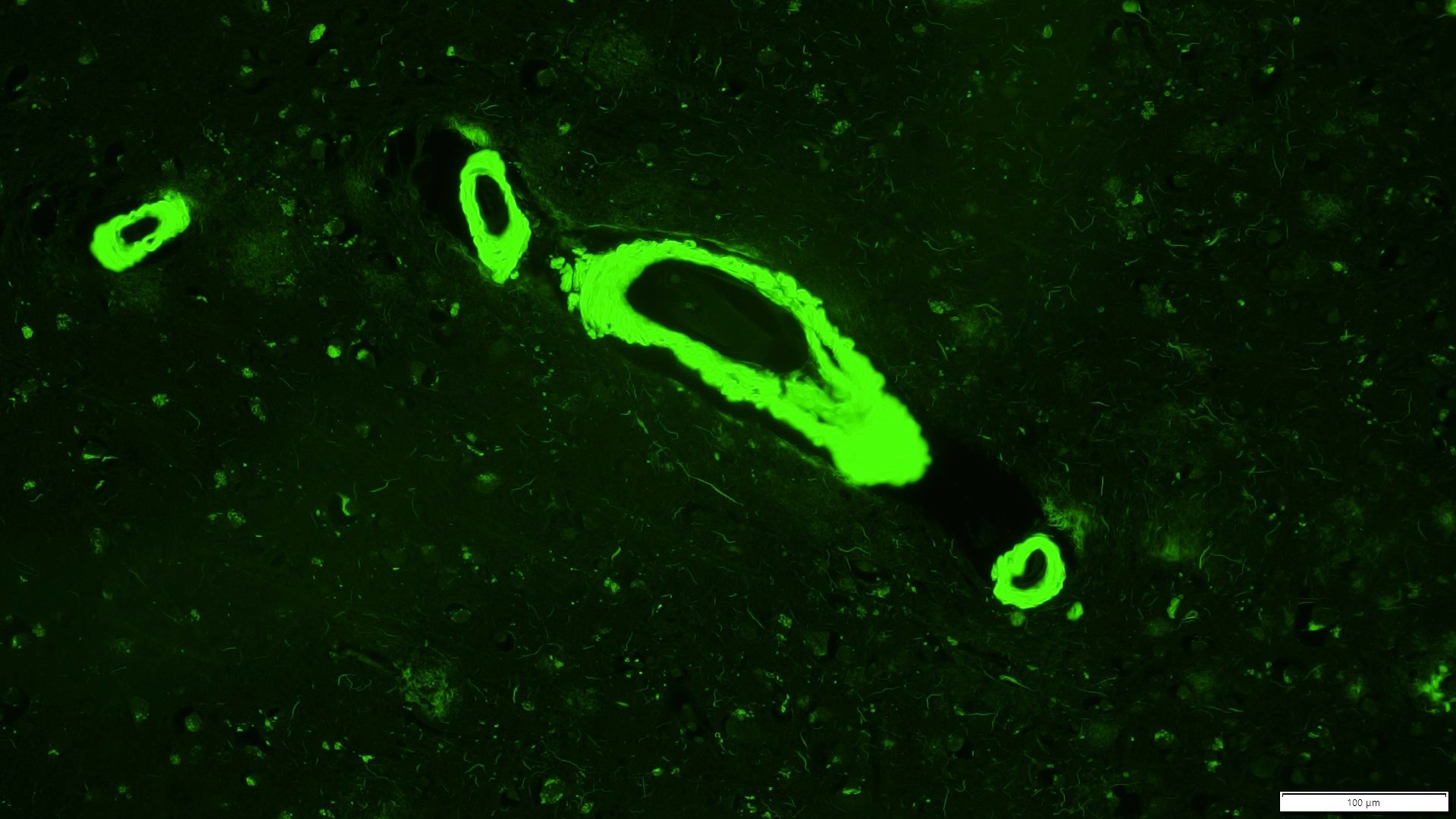
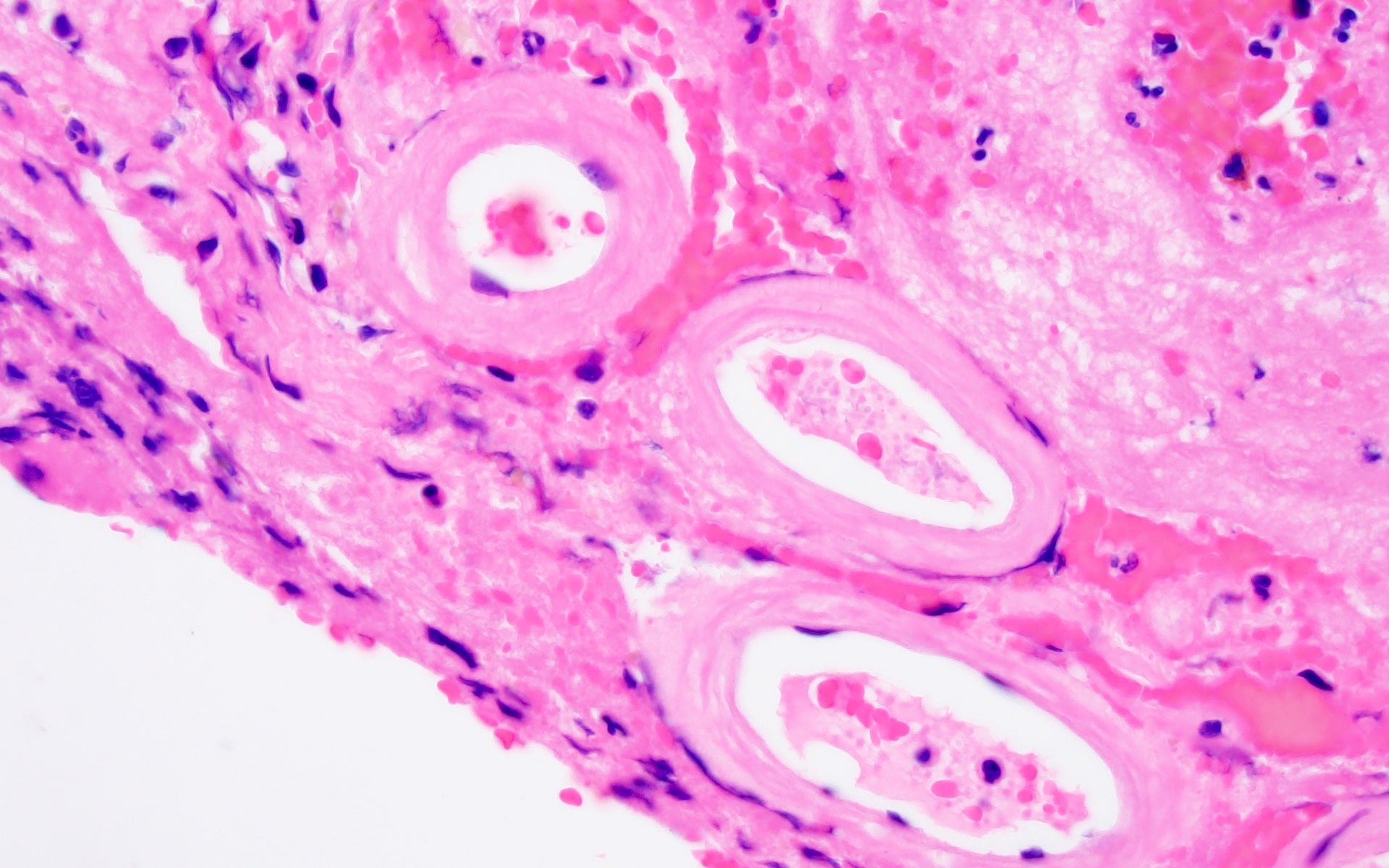
- Small to medium sized cortical and leptomeningeal vessels with mural thickening and deposition of homogenous, acellular, eosinophilic material in vessel walls (Ann Neurol 1991;30:637, Handb Clin Neurol 2017;145:79, J Clin Neurol 2011;7:1)
- Noninflammatory CAA with 2 historical subtypes (J Neurol Sci 2021;424:117425)
- CAA type 1 (microangiopathy, drusige Endartung, plaque-like angiopathy of Scholz)
- Affects cortical parenchymal capillaries
- Perivascular parenchymal involvement by amyloid
- CAA type 2 (Congophilic angiopathy of Pantelakis)
- Affects leptomeningeal or superficial cortical perforating arteries
- Advanced disease may result in double barrel appearance due to the separation of intima from media (Int J Mol Sci 2021;22:3869)
- Grading (J Clin Neurol 2011;7:1):
- Grading severity of the disease is made on histologic grounds, though ancillary staining techniques may aid in the identification of affected vessels and characterize source of injury
- Method 1 (proposed by Olichney et al.) (Arch Neurol 1995;52:702)
- 0: no amyloid beta positive blood vessels
- 1: scattered amyloid beta positivity in either leptomeningeal or intracortical blood vessels
- 2: strong, circumferential amyloid beta positive in either some leptomeningeal or intracortical blood vessels
- 3: widespread, strong, circumferential amyloid beta positivity in leptomeningeal and intracortical blood vessels
- 4: same as 3 but with additional leakage into surrounding parenchyma
- Method 2 (proposed by Vonsattel et al.) (Ann Neurol 1991;30:637)
- Mild: amyloid restricted to tunica media without significant destruction of smooth muscle cells
- Moderate: tunica media is replaced by amyloid and thickened
- Severe: extensive amyloid deposition with focal wall fragmentation or double barreling of the vessel wall, microaneurysms, fibrinoid necrosis or leakage of blood
- CAA type 1 (microangiopathy, drusige Endartung, plaque-like angiopathy of Scholz)
- Inflammatory CAA (Stroke 2015;46:e210)
- CAAri
- Perivascular inflammation that does not involve the vessel wall
- Lymphocytes may cuff the vessel and be associated with foreign body giant cells lying internal and external to the deposits
- Amyloid beta related angiitis
- Vasculitis with inflammation involving the vessel wall and often associated granuloma formation
- May result in complete destruction of vessel wall in severe cases
- Inflammatory infiltrates are variably composed of lymphocytes, plasma cells, epithelioid histocytes and occasionally multinucleated giant cells
- CAAri
Microscopic (histologic) images
Positive stains
- Amyloid beta: also highlights parenchymal amyloid plaques, if present; highly sensitive and reliable marker
- Congo red: apple green birefringence under polarized light; can give equivocal or unreliable results
- Thioflavin S: strong green fluorescence
- Reference: Neuropathol Appl Neurobiol 2003;29:106
Sample pathology report
- Brain and leptomeninges, left parieto-occipital lobe, biopsy:
- Cerebral amyloid angiopathy (see comment)
- Comment: Amyloid deposition is present in small and medium sized blood vessels of the cortex and leptomeninges. There is no granuloma formation or significant inflammation. Additionally, occasional diffuse and neuritic type amyloid plaques are present in the cortical parenchyma. While not diagnostic, these changes could indicate Alzheimer disease neuropathologic change. Clinical correlation is recommended.
Differential diagnosis
- Hypertensive cerebrovascular disease (Handb Clin Neurol 2017;145:79):
- Hypertensive hemorrhagic strokes generally occur in deep seated brain regions (thalamus, basal ganglia, pons, cerebellum)
- Thickened blood vessels are more commonly found in white matter and deep gray matter nuclei (rather than superficial cortex and leptomeninges) and are negative for beta amyloid deposition
- Primary angiitis of the CNS (PACNS) (J Neurol Sci 2021;424:117425, Neurol Sci 2020;41:3135):
- Clinical syndrome of gradual onset headache, altered mental status and focal neurologic deficits
- MRI shows bilateral, multifocal supratentorial lesions with restricted diffusion
- Shows overlapping clinical features with inflammatory CAA
- Histology shows necrotizing vasculitis without beta amyloid deposition in vessels
Additional references
Board review style question #1
A 67 year old man in otherwise good health presents with a catastrophic lobar hemorrhage. Microscopic sections at brain autopsy show thickened vessel walls with a double barrel appearance, as shown in the photomicrograph above. What stain will confirm your histologic suspicion?
- Alpha synuclein
- Beta amyloid
- GFAP
- Luxol fast blue
- Tau
Board review style answer #1
B. Beta amyloid will be positive in small to medium caliber blood vessels in the cerebral cortex and leptomeninges in patients with cerebral amyloid angiopathy (CAA). Alpha synuclein can be helpful to highlight Lewy bodies. GFAP is a marker of astrocytes and glial tumors. Luxol fast blue highlights myelin and can be helpful to identify areas with demyelination and tau can be helpful to highlight neurofibrillary tangles. Tau can be helpful to highlight neurofibrillary tangles.
Comment Here
Reference: Cerebral amyloid angiopathy
Comment Here
Reference: Cerebral amyloid angiopathy
Board review style question #2
Postmortem examination of a patient who died of intracerebral hemorrhage reveals cortical vessels with associated vessels. Beta amyloid immunohistochemistry establishes this to be a case of cerebral amyloid angiopathy (CAA). How might the inflammatory subtypes of CAA (including cerebral amyloid angiopathy related inflammation [CAAri] and amyloid beta related angiitis [ABRA]) be distinguished?
- ABRA and CAAri are indistinguishable on histopathologic findings alone
- ABRA with fibrinoid vessel necrosis; CAAri with perivascular lymphocytic cuffing with extravascular foreign body giant cell reaction
- ABRA with noncaseating granulomatous inflammation; CAAri with fibrinoid vessel necrosis
- ABRA with predilection for optic chiasm; CAAri with a predilection for cerebellum
Board review style answer #2
B. ABRA with fibrinoid vessel necrosis; CAAri with perivascular lymphocytic cuffing with extravascular foreign body giant cell reaction. Answer A is incorrect, as these subtypes are distinguishable by the following features: fibrinoid necrosis and granuloma formation (ABRA) and perivascular inflammation and foreign body giant cell reaction without vessel involvement by inflammation (CAAri). Answer C is incorrect because the inflammation does not involve the vessel wall in CAAri. Answer D is incorrect because in ABRA, there is a predilection for the posterior aspect of the brain. The optic chiasm is more commonly affected in neurosarcoidosis. In CAAri, there is a predilection for the posterior aspect of the brain.
Comment Here
Reference: Cerebral amyloid angiopathy
Comment Here
Reference: Cerebral amyloid angiopathy