Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Abdelzaher E. Cauda equina neuroendocrine tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumorparaganglioma.html. Accessed April 19th, 2024.
Definition / general
- Low grade, well circumscribed neuroendocrine neoplasm of cauda equina / filum terminale region
- CNS WHO grade 1
- Distinct from paragangliomas and pheochromocytomas outside the central nervous system (CNS) (Acta Neuropathol 2020;140:907)
Essential features
- Grade 1, slow growing neuroendocrine neoplasm of cauda equina / filum terminale region
- Rare entity with a benign clinical course (J Neurooncol 2015;122:539)
- Generally affects adults
- Distinct from paragangliomas and pheochromocytomas outside the CNS (Acta Neuropathol 2020;140:907)
Terminology
- Formerly, paraganglioma
- Acceptable: paraganglioma of the cauda equina, cauda equina paraganglioma (CEP)
- Also known as spinal paraganglioma, lumbar paraganglioma, paraganglioma of the filum terminale (Acta Neuropathol 2020;140:907, J Neurooncol 2015;122:539)
ICD coding
- ICD-O: 8693/3 - cauda equina neuroendocrine tumor (previously paraganglioma)
- ICD-11
- 2A02.0Y & XH1X68 - other specified gliomas of spinal cord, cranial nerves or other parts of the central nervous system & paraganglioma, benign
- 2A02.0Y & XH0EW6 - other specified gliomas of spinal cord, cranial nerves or other parts of the central nervous system & paraganglioma, NOS
Epidemiology
- Rare: < 3% of spinal tumors, 3.5% of cauda equina / filum terminale tumors (Radiol Case Rep 2019;14:1185, J Neurooncol 2015;122:539, J Surg Tech Case Rep 2012;4:46)
- Generally affects adults (fourth to sixth decades); mean age: 49 years (range: 17 - 75 years) (Neurosurg Rev 2022;45:103, J Neurooncol 2015;122:539)
- Slight male predominance (M:F = 1.5:1) (Neurosurg Rev 2022;45:103)
Sites
- Majority are located in the cauda equina / filum terminale region (at the L1 level or below) (Neurosurg Rev 2022;45:103)
- Most are intradural extramedullary and attached either to filum terminale or (less often) to a caudal nerve root (J Neurooncol 2015;122:539, J Surg Tech Case Rep 2012;4:46)
- Rarely reported in thoracic and cervical regions (Histopathology 1997;31:167)
Pathophysiology
- Histogenetically, biologically and molecularly distinct from paragangliomas and pheochromocytomas outside the CNS (Acta Neuropathol 2020;140:907)
- Arises from specialized neural crest cells in the cauda equina / filum terminale region
- mRNA analyses revealed that cauda equina paragangliomas overexpress the transcription factor HOXB13 (which is developmentally expressed in the caudal extent of the spinal cord) as opposed to pheochromocytomas / paragangliomas of other regions of the body; this provides circumstantial evidence of their cellular origin (Neuropathol Appl Neurobiol 2021;47:889)
- Molecular alterations that drive tumorigenesis in cauda equina neuroendocrine tumors are unknown
Etiology
- Almost all are sporadic (Acta Neuropathol 2020;140:893)
- Not associated with hereditary paraganglioma / pheochromocytoma syndrome due to mutations in SDH subunit genes (Acta Neuropathol 2020;140:907)
Clinical features
- No distinct clinical presentation; signs and symptoms are similar to other tumors in the region
- Most common: lower back pain (94%) with radiculopathy, sciatica (J Neurooncol 2015;122:539, World Neurosurg 2020:142:e66, Neurosurg Rev 2022;45:103)
- Less common: sensorimotor deficits and sphincter dysfunction (Radiol Case Rep 2019;14:1185, J Neurooncol 2015;122:539)
- Uncommon: fully developed cauda equina syndrome, signs of increased intracranial pressure, papilledema, subarachnoid hemorrhage (Neurosurg Rev 2022;45:103, World Neurosurg 2020:142:e66)
- Extremely rare: functionally active tumors with sympathetic secretory signs and symptoms of catecholamine hypersecretion (J Neurooncol 2015;122:539, J Surg Tech Case Rep 2012;4:46)
Diagnosis
- Rarely a clinical or radiological diagnosis
- Neuroimaging: magnetic resonance imaging (MRI) (modality of choice) and computed tomography (CT) (Neurosurg Rev 2022;45:103)
- Biopsy
- WHO essential and desirable diagnostic criteria
- Essential
- Well demarcated tumor with zellballen architecture
- Synaptophysin or chromogranin immunoreactivity in chief cells
- Cauda equina location
- Methylation profile of cauda equina neuroendocrine tumor (for unresolved lesions)
- Desirable
- S100 positive sustentacular cells
- Cytokeratin positive chief cells
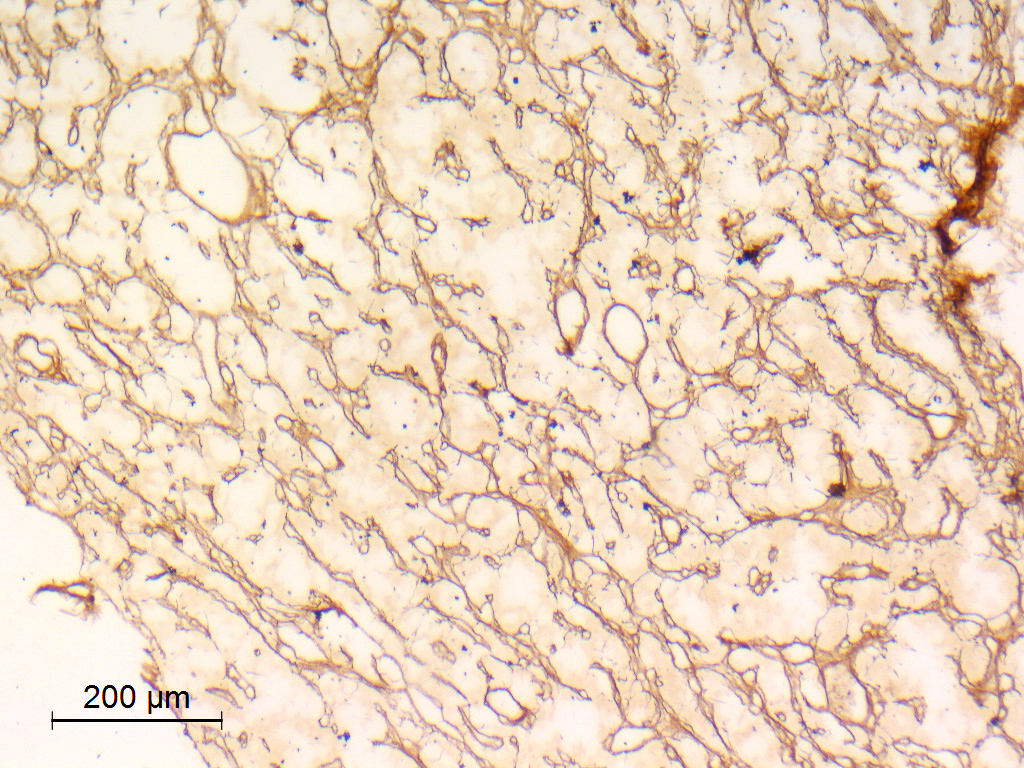
- Reticulin silver stain showing typical architecture
- Essential
Laboratory
- Cerebrospinal fluid (CSF) protein is usually markedly increased (Pract Neurol 2014;14:179)
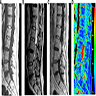
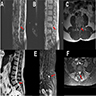
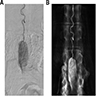
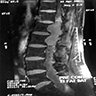
Radiology description
- MRI
- Well circumscribed, intradural extramedullary, sausage shaped mass at cauda equina / filum terminale region (Radiol Case Rep 2019;14:1185)
- Solid, occasionally partly cystic
- Generally T1 hypointense to isointense and T2 isointense to hyperintense, with intense post contrast enhancement (Neurosurg Rev 2022;45:103)
- Although MRI findings are nonspecific and indistinguishable from other solid tumors in cauda equina region, certain characteristic MRI findings could suggest the diagnosis, including a salt and pepper appearance on T2 weighted images related to the hypervascular nature (42.1%), a peripheral hypointense rim (cap sign) on T2 weighted images caused by subcapsular hemosiderin (47.3%) and serpiginous flow voids on all sequences (78.9%) (J Neurooncol 2015;122:539)
- Perfusion weighted MR images show increased blood flow, consistent with hypervascular tumor (Radiol Case Rep 2019;14:1185)
- Polar sign may be seen in T1 contrast enhanced and T2 weighted MR images, representing subacute to chronic polar intratumoral hematomas (J Spine Neurosurg 2014;3:4)
- Spinal angiography reveals a well defined hypervascular lesion with intense early blush that persists well into the arterial and venous phases (silk cocoon appearance), which helps presurgical planning and differentiates CEPs from other spinal tumors (Radiol Case Rep 2019;14:1185, Neurosurg Focus 2015;39:E16)
- CT: may show scalloping of the vertebral bodies (J Surg Tech Case Rep 2012;4:46)
Radiology images
Prognostic factors
- Excellent prognosis; the vast majority are clinically benign and curable by total excision
- < 1% are locally invasive (Histopathology 1997;31:167)
- Local recurrence after excision (7%) (Neurosurg Rev 2022;45:103, Histopathology 1997;31:167)
- Occasional leptomeningeal dissemination (Acta Neurochir (Wien) 1996;138:475, J Neurosurg Spine 2017;26:501)
- Rare metastasis outside the CNS (reported only once) (Histopathology 1997;31:167)
Case reports
- 42 year old man with gangliocytic paraganglioma of dorsolumbar spine (Asian J Neurosurg 2019;14:907)
- 50 year old man with functional conus cauda paraganglioma (J Surg Tech Case Rep 2012;4:46)
- 50 year old man with intradural paraganglioma of the thoracic spine (AJNR Am J Neuroradiol 1990;11:614)
- 61 year old woman with leptomeningeal dissemination of a low grade lumbar paraganglioma (J Neurosurg Spine 2017;26:501)
- 64 year old man with acute paraplegia due to hemorrhagic cauda equina paraganglioma (Asian J Neurosurg 2019;14:245)
Treatment
- Complete microsurgical resection remains the first choice (Neurosurg Rev 2022;45:103)
- Preoperative embolization may not be necessary (J Neurooncol 2015;122:539)
- Postoperative radiotherapy may be helpful on an individualized basis (J Neurosurg Spine 2017;26:501, J Neurooncol 2015;122:539)
- Follow up: there is a low risk of local recurrence and leptomeningeal dissemination
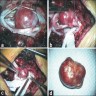
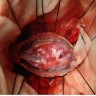
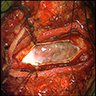
Clinical images
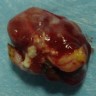
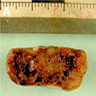
Gross description
- Well circumscribed, delicately encapsulated, oval to sausage shaped vascular mass
- Smooth surfaced, soft, red-brown and bleeds freely (Histopathology 1997;31:167)
- Size ranges from 10 mm to 112 mm in greatest dimension
- May show capsular calcification or cystic change
- Gross appearance resembles myxopapillary ependymoma
- If submitted with attached filum terminale, proximal and distal ends should be sampled as surgical margins
Frozen section description
- Discrete
- Lobular architecture
- Epithelioid cytologic features
- May show artifactual papillae mimicking myxopapillary ependymoma or ependymoma-like features with pseudorosettes (Folia Med (Plovdiv) 2022;64:1007, Turk Neurosurg 2012;22:353)
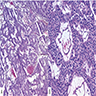
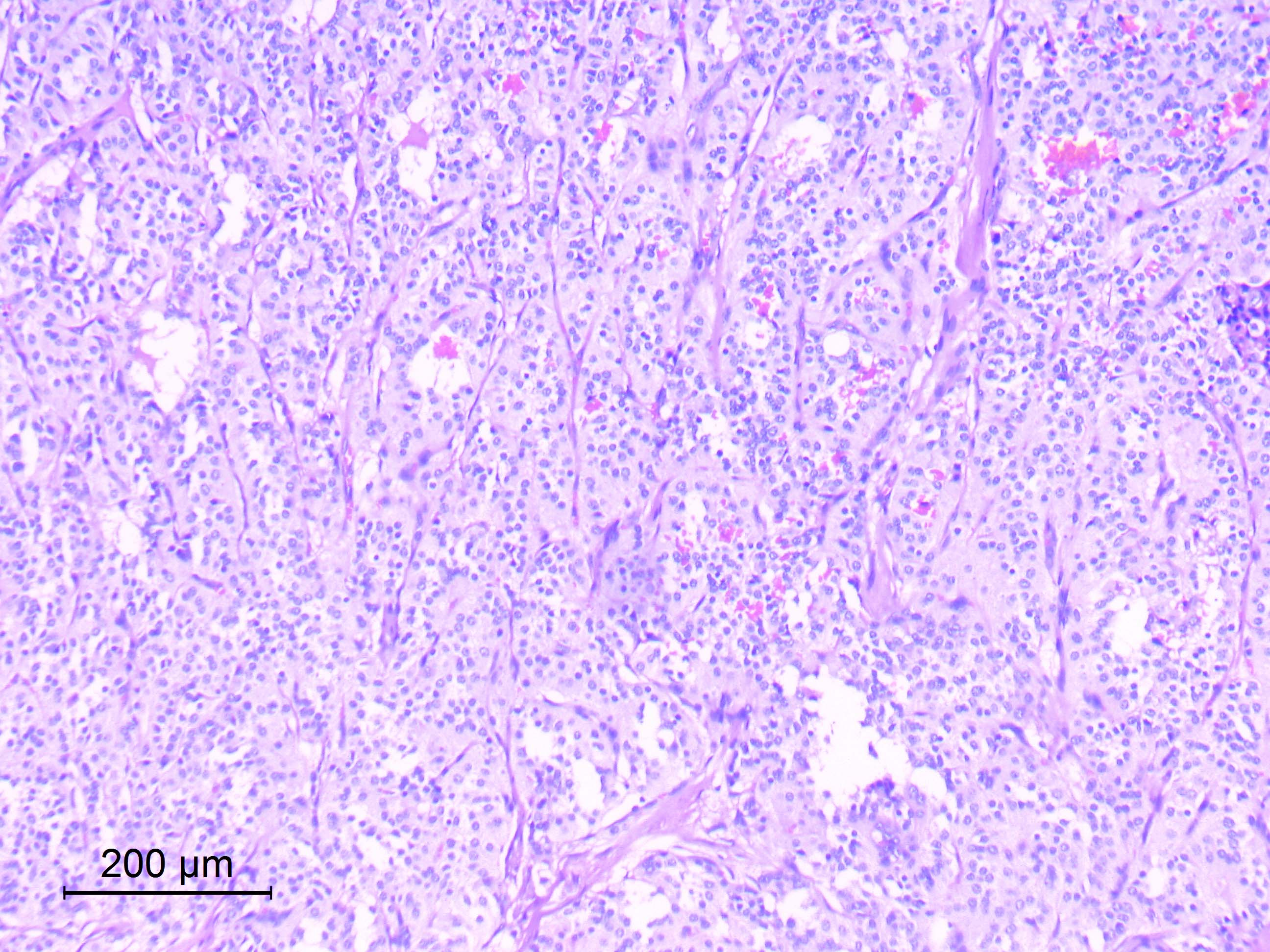
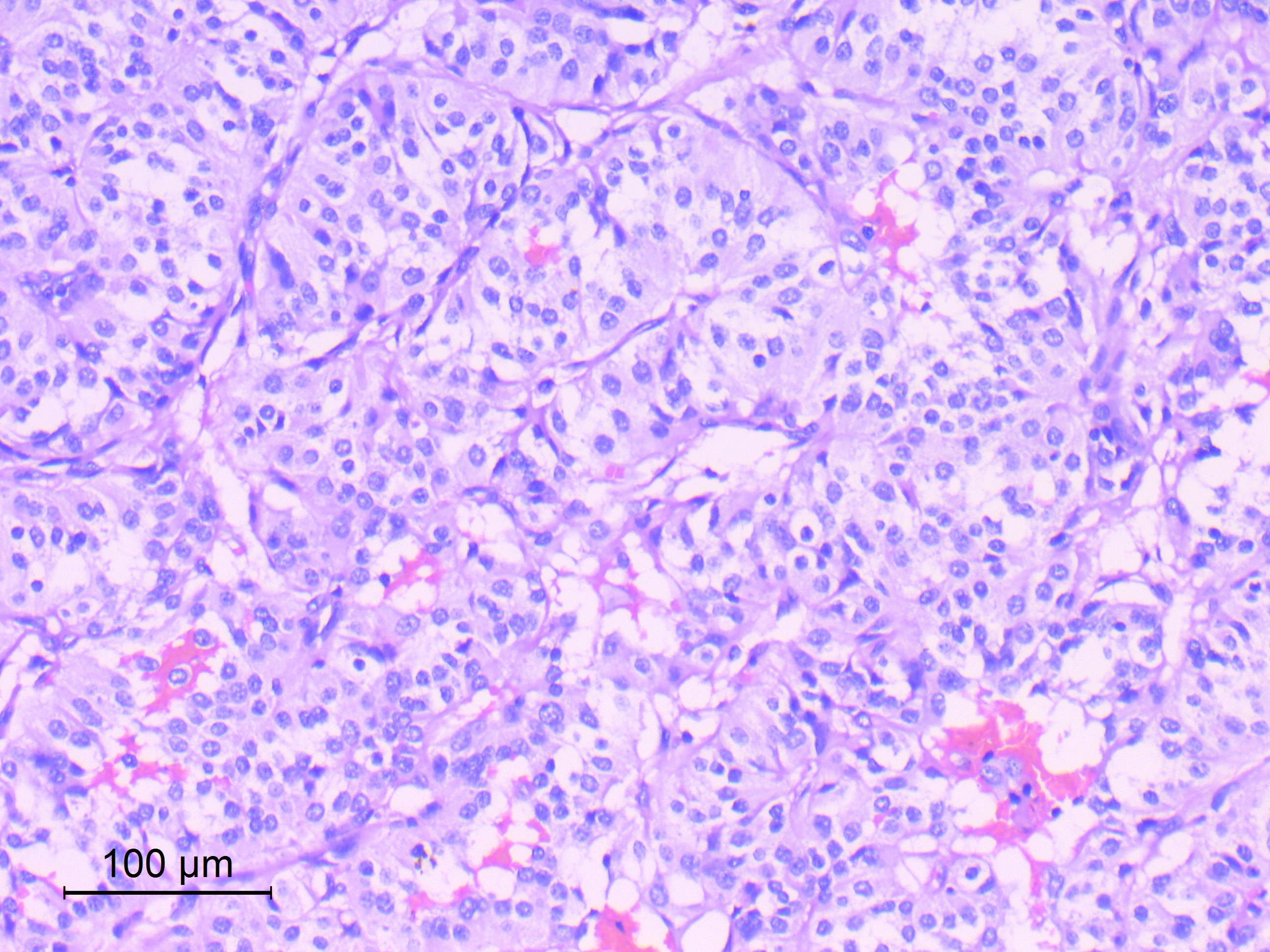
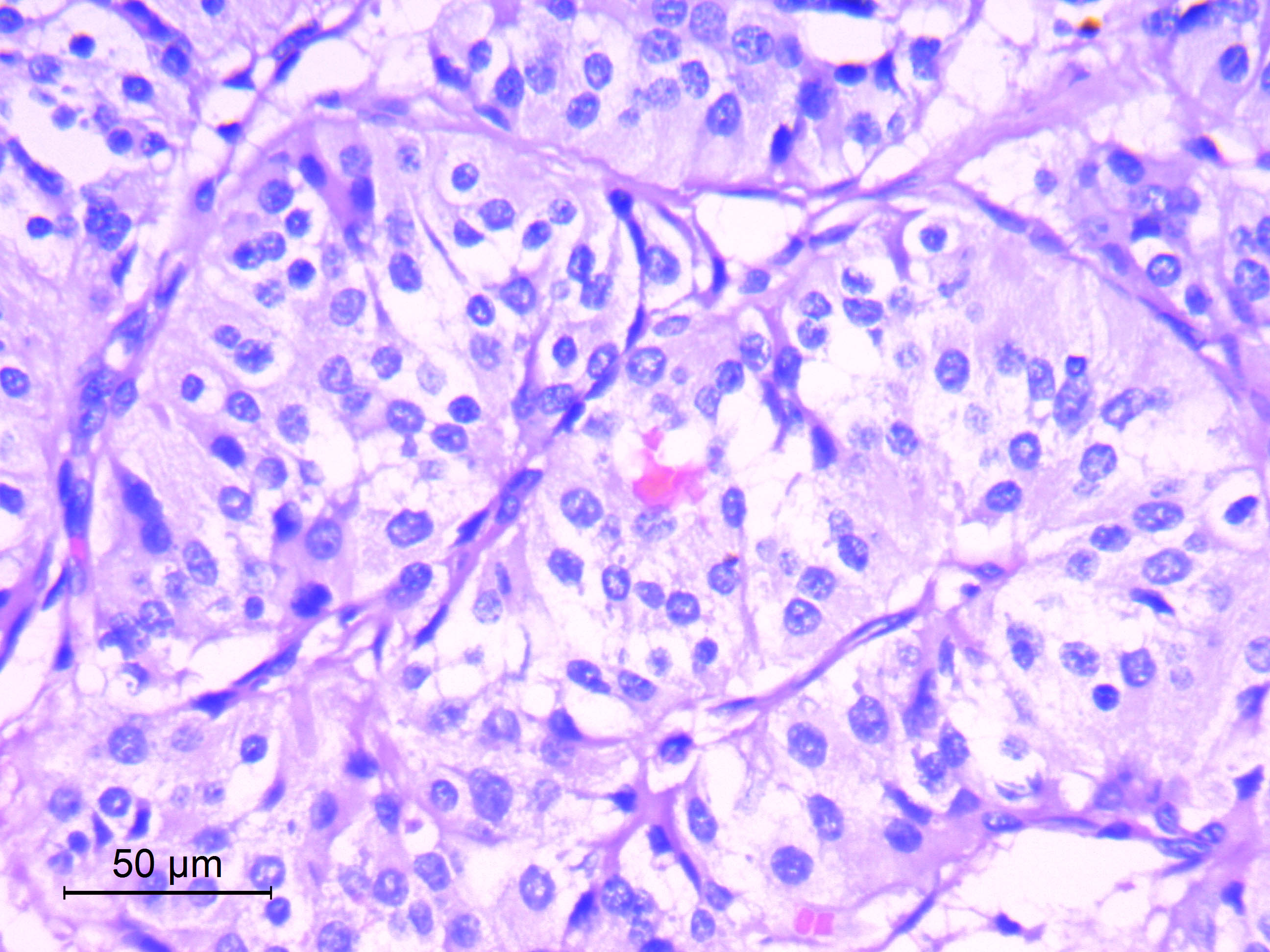
Microscopic (histologic) description
- Well differentiated, encapsulated and richly vascular tumor
- Organoid (zellballen) architecture: nests or lobules of chief (type I) cells surrounded by sustentacular (type II) cells with intervening delicate capillary and reticulin networks (J Neurooncol 2015;122:539)
- Chief cells
- Uniform round or polygonal epithelioid cells
- Round to oval nuclei with salt and pepper chromatin pattern and inconspicuous nucleoli (J Surg Tech Case Rep 2012;4:46)
- Finely granular eosinophilic cytoplasm, may be amphophilic or clear (Acta Neuropathol 2020;140:907)
- Sharp cell borders, particularly around vessels
- No or mild degenerative nuclear pleomorphism (endocrine atypia), of no prognostic significance
- Sustentacular cells
- Spindle shaped cells with attenuated long processes
- Perilobular; surround chief cell lobules
- Inconspicuous by routine light microscopy (visible by IHC for S100)
- Mitotic activity is highly variable (0 - 5/10 high power fields), of no prognostic significance (Acta Neuropathol 2020;140:893)
- Other common features
- Ganglion cell metaplasia (gangliocytic neuroendocrine tumors) and Schwann cell (ganglioneuromatous) differentiation in 25% of cases (Acta Neuropathol 2020;140:907)
- Ependymoma-like perivascular formations; similar but less pronounced and less fibrillar than in ependymoma (Acta Neuropathol 2020;140:907, Turk Neurosurg 2012;22:353)
- Papillary formations (Acta Neuropathol 2020;140:893)
- Less common features
- Carcinoid-like architectural features, including angiomatous, adenomatous and pseudorosette patterns
- Melanotic cells (melanotic neuroendocrine tumors) (Histopathology 1997;31:167)
- Oncocytic change (oncocytic neuroendocrine tumors) (Histopathology 1997;31:167)
- Predominant spindle cell proliferation arranged in an ill defined storiform pattern (Histopathology 1997;31:167)
- Ribboning architecture
- Extensive sclerosis (Histopathology 1997;31:167)
- Focal hemorrhagic necrosis (of no prognostic significance)
- Capsular calcification
- Bone invasion (Histopathology 1997;31:167)
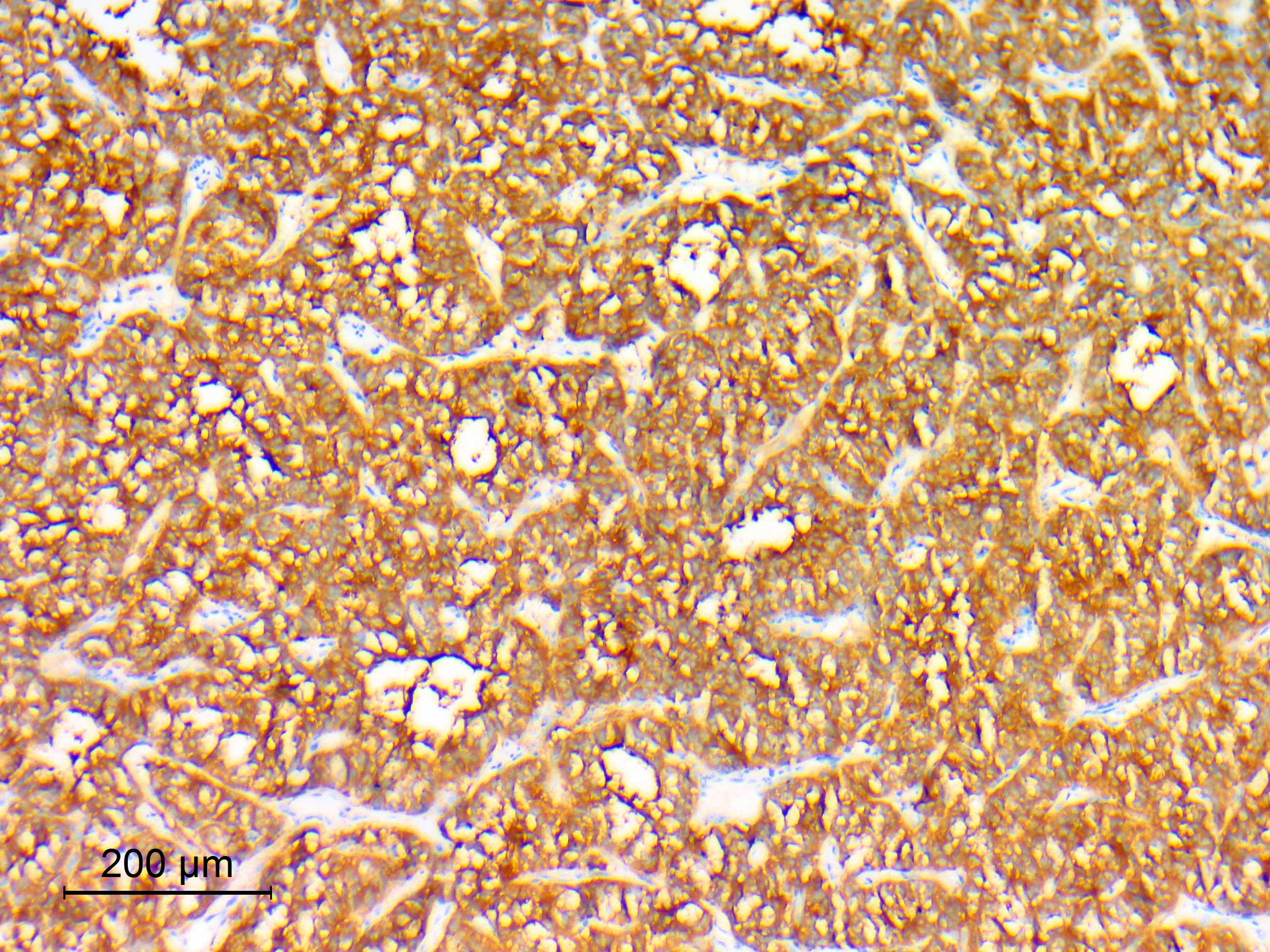
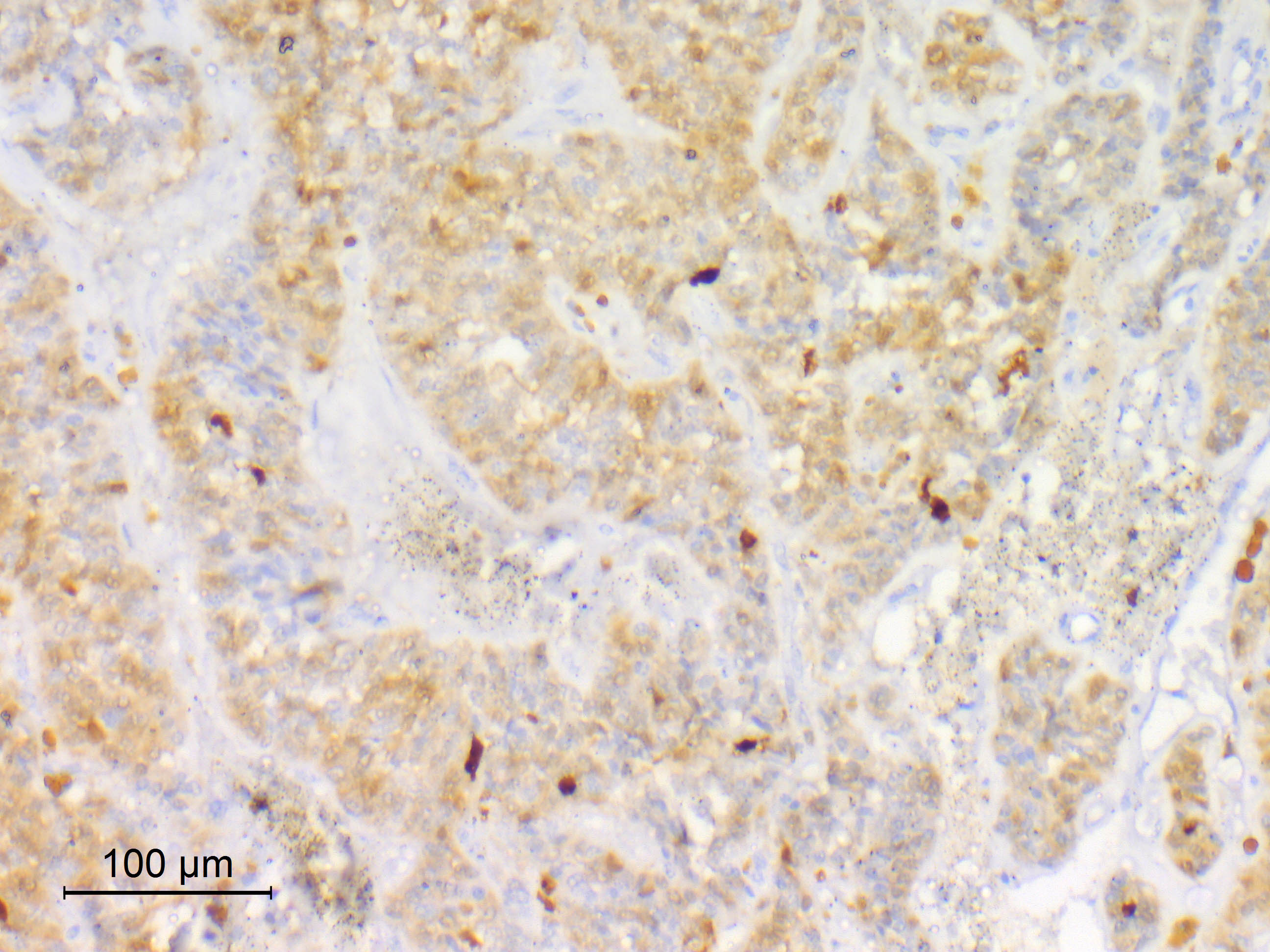
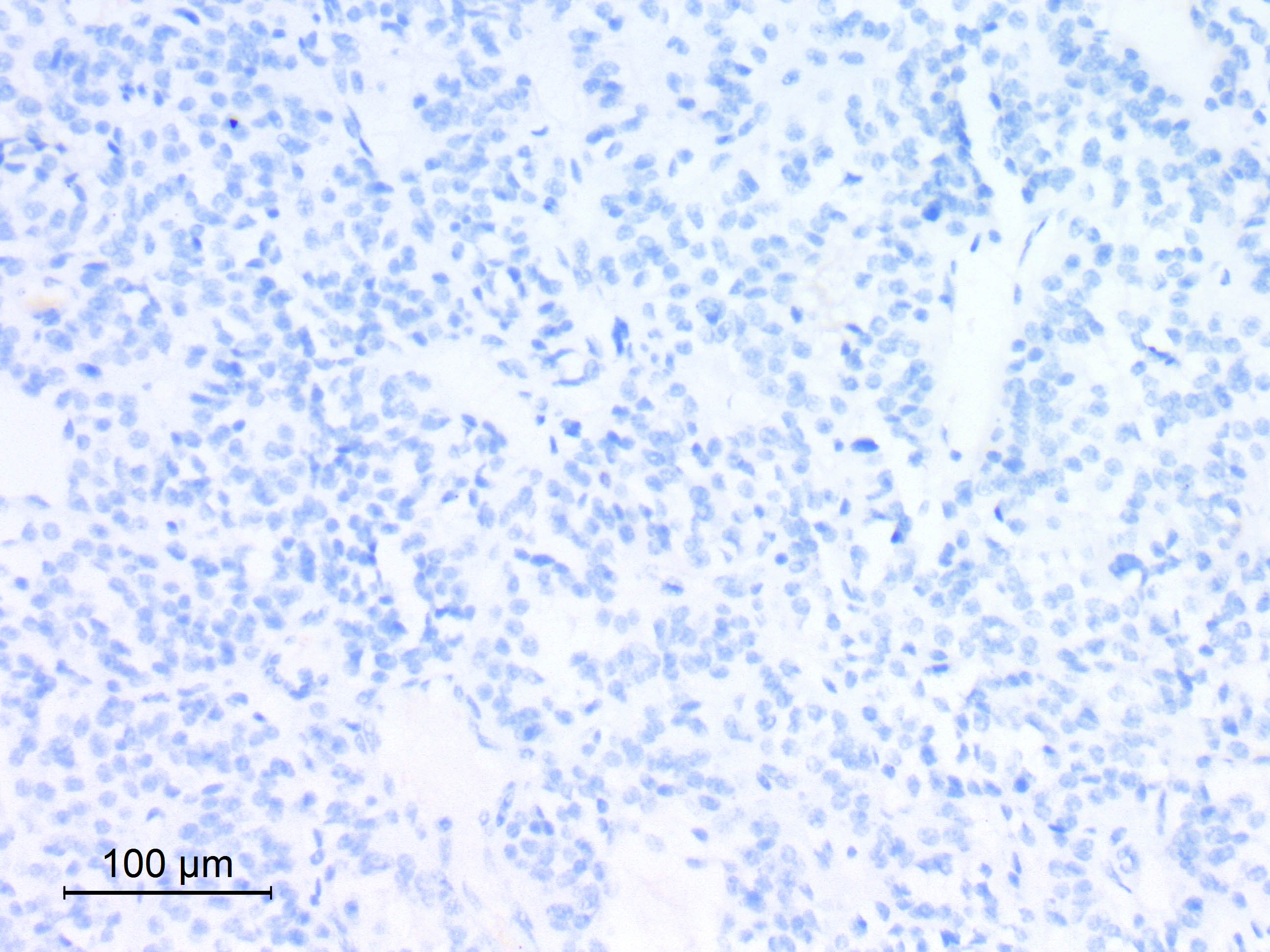
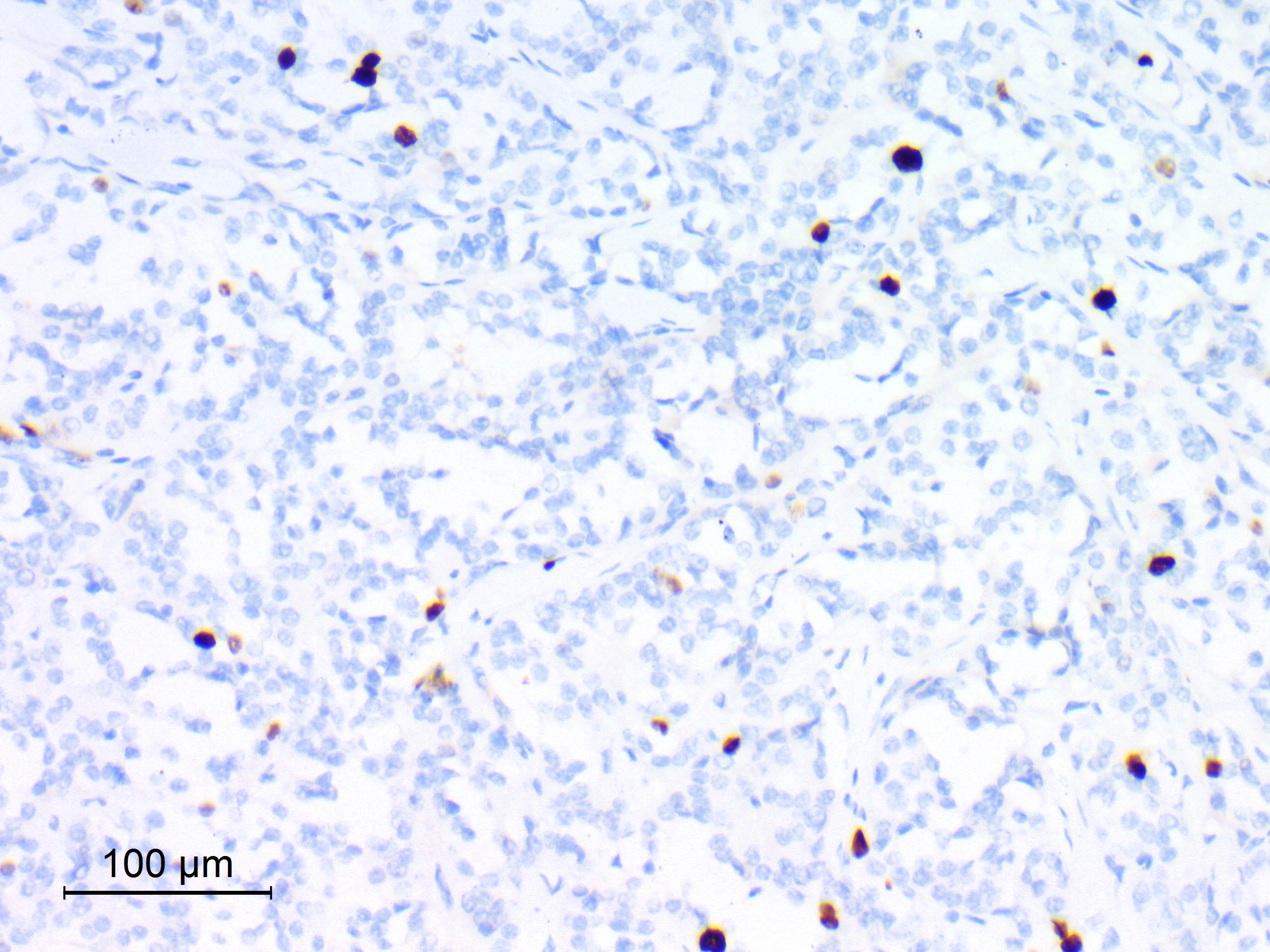
Microscopic (histologic) images
Contributed by Eman Abdelzaher, M.D., Ph.D.
Cytology description
- Largely discohesive smears that spread easily
- Uniform cells with plasmacytoid, triangular and polygonal shape and round to oval nuclei with salt and pepper chromatin (Lacruz: Central Nervous System Intraoperative Cytopathology, 2014 Edition, 2013)
- May show nuclear atypia
- Sharp cell borders (no fibrillar processes)
- May show ganglion cells
Positive stains
- Chief cells
- Synaptophysin (91 - 100%) (J Neurooncol 2015;122:539, Histopathology 1997;31:167, Ann Diagn Pathol 2022;57:151887)
- Chromogranin A (91 - 100% with variable intensity) (J Neurooncol 2015;122:539, Acta Neuropathol 2020;140:907, Histopathology 1997;31:167)
- Neuron specific enolase (NSE) (100%) (J Neurooncol 2015;122:539, Histopathology 1997;31:167)
- Cytokeratin (CAM5.2, AE1 / AE3, CK18 and MNF116) (21 - 100% with variable staining patterns): diffuse, strong with occasional prominent paranuclear staining (65%), focal or rare positive cells (35%) (Histopathology 1997;31:167, Acta Neuropathol 2020;140:907, Acta Neuropathol 2020;140:893, Ann Diagn Pathol 2022;57:151887)
- Vimentin (58%) (J Neurooncol 2015;122:539)
- SDHB: retained (Acta Neuropathol 2020;140:907)
- HOXB13 (Neuropathol Appl Neurobiol 2021;47:889)
- Sustentacular cells
- S100: inconsistent (84 - 100%) (J Neurooncol 2015;122:539, Histopathology 1997;31:167, Ann Diagn Pathol 2022;57:151887, Acta Neuropathol 2020;140:907)
- SOX10
- Ki67: heterogeneous proliferative activity; mean: 5.7% (range: 1 - 10%) (World Neurosurg 2020:142:e66, Acta Neuropathol 2020;140:893)
- Reticulin histochemical stain: emphasizes the lobular architecture
Negative stains
- Epithelial membrane antigen (EMA) (J Neurooncol 2015;122:539)
- GATA3 (Acta Neuropathol 2020;140:907)
- Creatine kinase (J Neurooncol 2015;122:539)
- GFAP: may be focally positive in chief cells (9 - 30%) and sustentacular cells (Ann Diagn Pathol 2022;57:151887, Histopathology 1997;31:167, Brain Pathol 2005;15:169)
- S100: may be focally positive in chief cells (Ann Diagn Pathol 2022;57:151887)
- Serotonin (5-HT) and various neuropeptides: may be positive in chief cells (somatostatin [34%], leuenkephalin [47%] and metenkephalin) (Histopathology 1997;31:167)
- Neurofilament proteins: may be positive in chief cells with paranuclear globular staining (13%) (Histopathology 1997;31:167)
Electron microscopy description
- Chief cells
- Dense core (neurosecretory) granules with no evidence of catecholamine production (Neurosurg Rev 2022;45:103)
- Prominent, rough endoplasmic reticulum and Golgi apparatus (Neurosurg Rev 2022;45:103)
- Paranuclear intermediate filament whorls
- Sustentacular cells
- Electron dense with elongated processes
- Contain some intermediate filaments
- Lack neurosecretory granules
Electron microscopy images
Molecular / cytogenetics description
- Distinct DNA methylation and chromosomal copy number profiles as opposed to those of paragangliomas arising from other locations (Acta Neuropathol 2020;140:907)
- DNA methylation profiling and clustering analysis showed that CEPs are epigenetically distinct from extraspinal paragangliomas, pheochromocytomas, other neuroendocrine tumors and glial or ependymal neoplasms of the spinal cord (Acta Neuropathol 2020;140:907)
- Copy number analysis revealed diploid genomes in the vast majority of CEPs, whereas extraspinal paragangliomas were mostly aneuploid with recurrent trisomy 1q and monosomies of 1p, 3 and 11, none of which were present in the cohort of CEP (Acta Neuropathol 2020;140:907)
- RNA and DNA exome sequencing revealed that SDH mutations are absent in cauda equina paragangliomas (Acta Neuropathol 2020;140:893)
- CEPs are not driven by recurrent oncogenic gene fusions; fusion genes known to be relevant in paragangliomas / pheochromocytomas and associated with poor prognosis (e.g., MAML3 fusions) were not observed in CEPs (Acta Neuropathol 2020;140:893)
Molecular / cytogenetics images
Videos
Case 8: paraganglioma of the filum terminale
Sample pathology report
- Cauda equina mass, total resection:
- Cauda equina neuroendocrine tumor (cauda equina paraganglioma), CNS WHO grade 1 (see comment)
- Comment: Encapsulated tumor with zellballen architecture highlighted by reticulin histochemical stain. The tumor is composed of chief cells (synaptophysin positive) arranged in nests surrounded by S100 positive sustentacular cells.
- Molecular genetics: methylation profile of cauda equina neuroendocrine tumor
Differential diagnosis
- Consider the possibility of paraganglioma for any cauda equina / filum terminale mass, as CEP is rarely diagnosed preoperatively
- Myxopapillary ependymoma:
- Perivascular myxoid change (Alcian blue+) (Histopathology 1997;31:167)
- Little reticulin
- Diffuse GFAP+ (Appl Immunohistochem Mol Morphol 2013;21:485)
- Synaptophysin- (Diagn Pathol 2008;3:40)
- Smears: tissue fragments, cells with fibrillar processes and smaller, darker nuclei
- Spinal ependymoma (including tanycytic pattern):
- Intramedullary, commonly cervical or cervicothoracic, demarcated tumors
- No capsule
- Little reticulin
- Tanycytic pattern characterized by spindle shaped cells with fascicular architecture
- GFAP+, EMA+ (dot-like microlumina) (Turk Neurosurg 2012;22:353, Ann Diagn Pathol 2022;57:151887)
- Chromogranin- and synaptophysin- (J Neurooncol 2005;74:1)
- Smears: tissue fragments, cells with fibrillar processes and smaller, darker nuclei
- Schwannoma:
- Biphasic with Antoni A and B areas
- Pericellular reticulin
- Strongly and diffusely S100+, SOX10+ (Arq Bras Neurocir 2018;37:105)
- Synaptophysin- and chromogranin- (Hum Pathol 2012;43:650)
- Smears: tissue fragments, spindle cells
- Hemangioblastoma:
- Vacuolated stromal cells
- Stromal cells variably lipid laden
- Oil red O+ staining
- Inhibin alpha+
- Chromogranin- and synaptophysin- (Vojnosanit Pregl 2004;61:273)
- Meningioma:
- Whorls, psammoma bodies
- EMA+ (J Neuropathol Exp Neurol 2017;76:289)
- Metastatic carcinoma:
- Pilocytic astrocytoma:
- Biphasic piloid and spongy areas (Acta Neuropathol 2015;129:775)
- GFAP+
- Little reticulin
- Melanocytoma:
- Most spinal melanocytomas arise at cervical and thoracic levels
- Positive for melanocytic markers (e.g., S100, SOX10, HMB45, MelanA)
- Chromogranin- and synaptophysin- (Radiol Case Rep 2015;10:1010)
- Cytokeratin- (Radiol Case Rep 2015;10:1010)
Additional references
- WHO Classification of Tumours Editorial Board: Central Nervous System Tumours, 5th Edition, 2022, Burger: Diagnostic Pathology - Neuropathology, 2nd Edition, 2016, Burger: Tumors of the Central Nervous System (AFIP Atlas of Tumor Pathology), 1st Edition, 2007, Burger: Surgical Pathology of the Nervous System and its Coverings, 4th Edition, 2001
Board review style question #1
A 49 year old man presented with lower back pain and sciatica for a duration of 3 months. MRI showed cauda equina intradural extramedullary enhancing vascular mass. The received gross specimen was a delicately encapsulated oval mass with a red-brown cut section. The images shown above depict the histological features of the lesion and synaptophysin immunostain. What is the most likely diagnosis?
- Cauda equina paraganglioma
- Hemangioblastoma
- Myxopapillary ependymoma
- Schwannoma
Board review style answer #1
A. Cauda equina paraganglioma. The location of the tumor at the cauda equina region together with the histologically distinctive zellballen architecture and synaptophysin positive chief cells are diagnostic of cauda equina paraganglioma. Answer B is incorrect because hemangioblastomas have vacuolated stromal cells and are synaptophysin negative. Answer C is incorrect because myxopapillary ependymomas show characteristic perivascular myxoid change and are synaptophysin negative. Answer D is incorrect because schwannomas do not have a zellballen architecture and are synaptophysin negative.
Comment Here
Reference: Cauda equina neuroendocrine tumor
Comment Here
Reference: Cauda equina neuroendocrine tumor
Board review style question #2
Which of the following statements about cauda equina paraganglioma is correct?
- Encapsulated tumors with zellballen architecture and synaptophysin immunoreactivity
- It is commonly functioning with clinical features of catecholamine hypersecretion
- It shares common genetic profile with extraspinal paragangliomas and pheochromocytomas
- Recurrence rate after total resection is high
Board review style answer #2
A. Encapsulated tumors with zellballen architecture and synaptophysin immunoreactivity. Zellballen architecture and synaptophysin positive chief cells are characteristic of cauda equina paraganglioma. Answer B is incorrect because functional activity is extremely rare in cauda equina paraganglioma. Answer D is incorrect because recurrence rate after total resection is low (around 7%). Answer C is incorrect because cauda equina paragangliomas are molecularly distinct from paragangliomas and pheochromocytomas outside the CNS.
Comment Here
Reference: Cauda equina neuroendocrine tumor
Comment Here
Reference: Cauda equina neuroendocrine tumor