Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Diagrams / tables | Specimen | Principles | Laboratory | Interpretation of results | Pitfalls and preanalytic variables | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Seasor T, Moser KA. Mixing studies. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/coagulationmixingstudies.html. Accessed April 18th, 2024.
Definition / general
- Mixing studies are typically used to investigate abnormal clotting time results
- Mixing studies help distinguish clotting time prolongation due to a coagulation factor deficiency or an inhibitor (specific or nonspecific)
- Mixing study may direct further coagulation testing but it is not by itself diagnostic
Essential features
- Mixing studies help distinguish clotting time prolongation due to a coagulation factor deficiency or an inhibitor, e.g. lupus anticoagulant
- To perform a mixing study, mix patient plasma and normal pooled plasma and measure the clotting time that was initially prolonged
- If the clotting time corrects, this suggests a factor deficiency; if the clotting time does not correct, this suggests presence of a circulating inhibitor
- Mixing study may direct further coagulation testing but it is not by itself diagnostic, e.g. may aid in selecting factor assays
Terminology
- Mixing test
- Inhibitor screen
- Inhibitor assay
- Circulating anticoagulant
- Prothrombin time (PT) 1:1 mix
- Partial thromboplastin time (PTT) 1:1 mix
- Partial thromboplastin time (PTT) correction
ICD coding
Diagrams / tables
Contributed by Tori Seasor, M.D. and Karen A. Moser, M.D.
| Differential diagnosis of prolonged PT or aPTT incorporating mixing study results (correction versus noncorrection) | ||
| Clotting time prolonged | Mixing study result | Key differential diagnoses |
| Prothrombin time (PT) | Corrects |
|
| Does not correct |
| |
| Activated partial thromboplastin time (aPTT) | Corrects |
|
| Does not correct |
| |
| PT and aPTT | Corrects |
|
| Does not correct |
| |
Specimen
- Platelet poor plasma in 3.2% sodium citrate
Principles
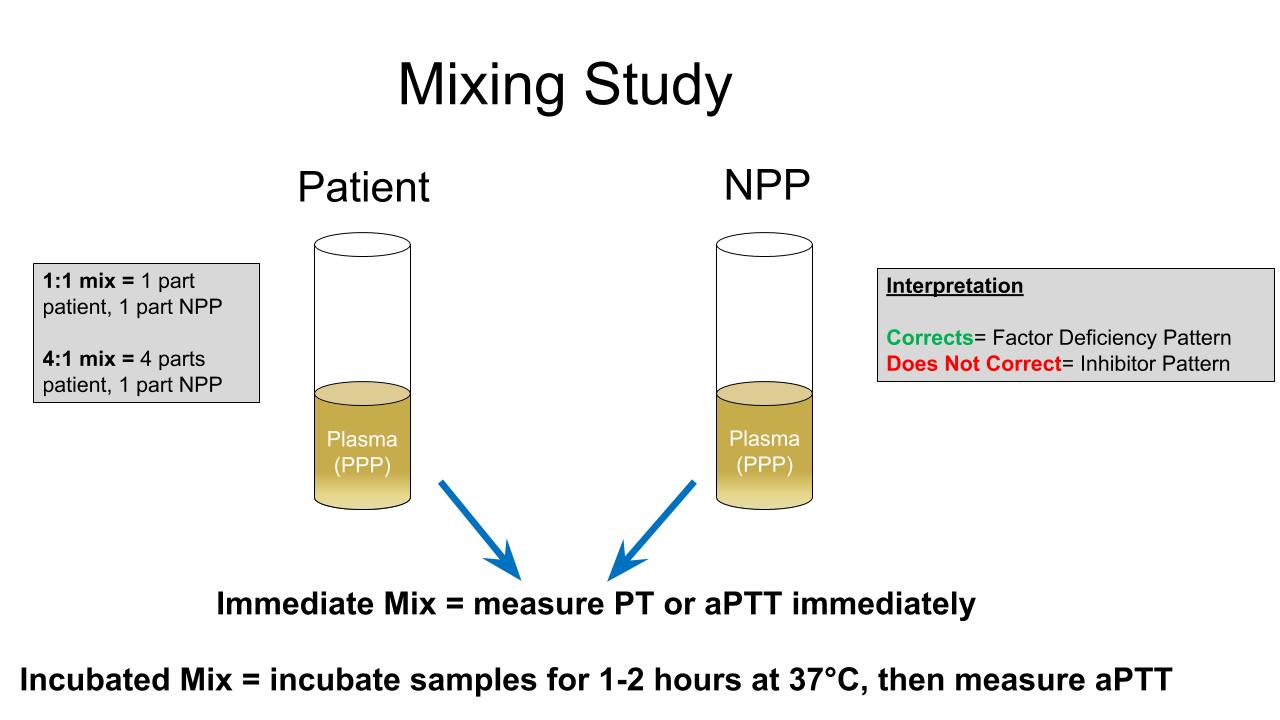
- To perform a mixing study, mix patient plasma and normal pooled plasma (NPP) and measure the clotting time that was initially prolonged
- In factor deficiency: NPP adds sufficient clotting factors to overcome the deficiency and correct the clotting time
- If an inhibitor is present, it typically inhibits the clotting factors in patient plasma and NPP so the clotting time remains prolonged
- Inhibitors can be specific inhibitors (antibodies directed against a specific coagulation factor), nonspecific inhibitors (e.g. lupus anticoagulant) or medications such as heparin, direct thrombin inhibitors or direct Xa inhibitors
- Mixing study results aid in selection of further coagulation testing, such as assays for specific factor deficiencies or inhibitors
Laboratory
- CLSI H47-A2 includes general guidance for the performance of PT and aPTT mixing studies (CLSI: H47-A2 - One-Stage Prothrombin Time (PT) Test and Activated Partial Thromboplastin Time (APTT) Test, 2nd Edition, 2008)
- Mixing study is performed for the prolonged clotting time only (e.g. if the PT is prolonged, a PT mixing study will be performed)
- Mixing studies vary in the ratio of patient plasma to normal pooled plasma (NPP) and in incubation time
- Patient plasma to NPP ratio
- 1:1 mixing study (equal parts patient plasma and NPP) is most commonly used
- 4:1 mixing study (4 parts patient plasma and 1 part NPP) is sometimes used to evaluate minimally prolonged aPTT as it may be more sensitive in this scenario (Am J Clin Pathol 2002;117:62)
- Incubation time
- Immediate: clotting time is evaluated immediately after mixing patient plasma and NPP
- Incubated: mixed patient plasma and NPP is incubated at 37 °C for 1 - 2 hours, depending on local laboratory protocols
- Incubation control consists of patient plasma and NPP incubated separately at 37 °C for same amount of time as mix; at end of incubation, patient plasma and NPP are mixed and clotting time is measured
- Incubation control accounts for loss of labile factors during incubation
- Incubation performed if immediate mixing study does not show an inhibitor pattern
- Incubated aPTT mixing studies are useful to identify time dependent inhibitors (such as factor VIII inhibitors and some lupus anticoagulants)
- Incubated PT mixing studies are not typically useful
- Incubation control consists of patient plasma and NPP incubated separately at 37 °C for same amount of time as mix; at end of incubation, patient plasma and NPP are mixed and clotting time is measured
- Patient plasma to NPP ratio
- Definition of normal pooled plasma is also not standardized
- NPP can be purchased from a vendor
- Making NPP locally is not preferred but if necessary it is recommended to use platelet poor plasma from a minimum of 20 - 30 donors with coagulation factor levels of approximately 100% for NPP (Semin Thromb Hemost 2014;40:195)
Interpretation of results
- If a clotting time corrects in the immediate (PT) or immediate and incubated mixing study (aPTT), it is interpreted as a factor deficiency pattern
- Principle: at least 50% activity of a given factor is sufficient to produce a normal clotting time
- Example:
Patient factor activity NPP factor activity 1:1 mix factor activity Mixing study interpretation 2% 100% 51% Correction
- If a clotting time does not correct in the immediate (PT) or immediate or incubated mixing study (aPTT), it is interpreted as an inhibitor pattern
- Principle: the inhibitor binds all available factor, from both the patient and NPP and the clotting time remains prolonged
- There is no standard definition of correction of aPTT or PT mixing studies
- CLSI H60-A (CLSI: H60-A - Laboratory Testing for the Lupus Anticoagulant, 1st Edition, 2014) recommends interpreting mixing studies in the setting of lupus anticoagulant testing by using either
- 1:1 mix normalized ratio (ratio = 1:1 mix result(s) / 1:1 mix mean of reference interval(s))
- Rosner index (see below)
- CLSI H60-A (CLSI: H60-A - Laboratory Testing for the Lupus Anticoagulant, 1st Edition, 2014) recommends interpreting mixing studies in the setting of lupus anticoagulant testing by using either
- Strategy for interpretation should be determined and validated locally by individual laboratories
- Example strategies for determining mixing study correction (Semin Thromb Hemost 2013;39:283)
- Clotting time of mix returns to within laboratory's reference interval
- Clotting time of mix falls within a 2 - 3 standard deviation range of the mean normal clotting time
- Compare the clotting time of mix to the NPP clotting time (ratio)
- Estimated factor correction method takes into account effects of single versus multiple factor deficiencies (Blood Coagul Fibrinolysis 2016;27:90)
- Rosner index and Chang percentage are calculations created to assist in interpretation of mixing study results
- Rosner index is used in conjunction with a 1:1 mixing study; originally described for use in lupus anticoagulant testing
- Rosner index > 15 suggests presence of an inhibitor (Thromb Haemost 1987;57:144)
Rosner index = Clotting time of 1:1 mix − clotting time of NPP × 100 Clotting time of patient sample
- Rosner index > 15 suggests presence of an inhibitor (Thromb Haemost 1987;57:144)
- Chang percentage is used in conjunction with a 4:1 mixing study (optimal sensitivity) (Am J Clin Pathol 2002;117:62)
- Chang percentage result of < 50% suggests the presence of an inhibitor
Chang percentage = Initial patient clotting time − clotting time 4:1 mix × 100 Initial patient clotting time − clotting time NPP
- Chang percentage result of < 50% suggests the presence of an inhibitor
- Rosner index is used in conjunction with a 1:1 mixing study; originally described for use in lupus anticoagulant testing
Pitfalls and preanalytic variables
- Multiple factor deficiencies may cause mixing study noncorrection, which could be incorrectly interpreted as an inhibitor (Semin Thromb Hemost 2014;40:195)
- High hematocrit or underfilled collection tubes can cause prolonged aPTT and PT due to excess citrate anticoagulant in the sample
- Anticoagulant effect that appears as an inhibitor should be taken into account when interpreting mixing studies
- Heparin: affects aPTT mixing studies
- May be detected with a thrombin time or anti-Xa activity assay and corrected by a neutralization procedure
- Direct thrombin inhibitor (DTI) may affect aPTT > PT mixing studies
- Direct Xa inhibitors may affect PT > aPTT mixing studies
- Heparin: affects aPTT mixing studies
- If a specimen is not properly centrifuged to ensure removal of platelets, platelets can interfere with clotting time results by neutralization of lupus anticoagulant effect (if present) by phospholipids present in platelet cell membranes (J Thromb Haemost 2009;7:1737)
- Rare occurrence of increased prolongation of aPTT (rather than shortening) when a patient's sample containing lupus anticoagulant is mixed with NPP (lupus cofactor effect) (Semin Thromb Hemost 2012;38:385)
- Lupus cofactor effect is reagent dependent and may represent a prozone effect (Am J Clin Pathol 2016;146:262)
- Decreased factor II in patient plasma may account for lupus cofactor effect in some cases (Am J Clin Pathol 2020;153:229, TH Open 2020;4:e40)
Additional references
Board review style question #1
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are performed on a 64 year old man. The patient is taking no anticoagulants or other medications. The results of a 1:1 mixing study are
Patient aPTT: 51 s (reference range 38 - 42 s)
Immediate 1:1 aPTT mixing study: 36 s
Which of the following is the next best test to order?
Patient aPTT: 51 s (reference range 38 - 42 s)
Immediate 1:1 aPTT mixing study: 36 s
Which of the following is the next best test to order?
- 4:1 mixing study
- Factor VIII activity
- Factor X activity
- von Willebrand factor activity
Board review style answer #1
B. Factor VIII activity. The mixing study indicates a corrected result which suggests a factor deficiency as a cause of the prolonged aPTT. The next step would be to investigate which factor deficiency is causing this, which can be accomplished with factor VIII, IX and XI activity testing. Factors VIII, IX and XI are factors in the intrinsic coagulation pathway that can prolong the aPTT and are associated with clinical bleeding. Lupus anticoagulant testing could also be considered, as some weak lupus anticoagulants may show apparent correction in mixing studies.
Comment Here
Reference: Mixing studies
Comment Here
Reference: Mixing studies
Board review style question #2
Coagulation laboratory tests are drawn on a 35 year old woman with the recent diagnosis of an autoimmune disease. The physician ordered a 1:1 mixing study after the original results revealed a prolonged activated partial thromboplastin time (aPTT).
Patient aPTT: 51 s (reference range 38 - 42 s)
Immediate 1:1 aPTT mixing study: 50 s
Which of the following do these laboratory results suggest?
Patient aPTT: 51 s (reference range 38 - 42 s)
Immediate 1:1 aPTT mixing study: 50 s
Which of the following do these laboratory results suggest?
- Factor V Leiden mutation
- Factor VII deficiency
- Lupus anticoagulant
- Vitamin K deficiency
Board review style answer #2
C. Lupus anticoagulant. The aPTT was prolonged in the patient sample and the addition of NPP in the immediate mixing study did not correct the aPTT to the normal reference range. This result suggests an inhibitor that overcomes the normal coagulation factors in the patient sample as well as the NPP. Lupus anticoagulants behave as nonspecific inhibitors in aPTT mixing studies and are a common cause of unexpected aPTT prolongation. The recent history of an autoimmune disorder diagnosed in a young woman would also raise concern for a lupus anticoagulant as an explanation for her prolonged aPTT.
Comment Here
Reference: Mixing studies
Comment Here
Reference: Mixing studies