Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Electron microscopy description | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Yoshikawa A, Fukuoka J. Acute interstitial pneumonia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungnontumoracuteinterstitialp.html. Accessed April 26th, 2024.
Definition / general
- In 1935, Hamman and Rich first reported autopsy cases of initially healthy individuals who developed a rapidly progressive and fatal type of interstitial lung disease, which differed from other interstitial pneumonia clinically and pathologically (Trans Am Clin Climatol Assoc 1935;51:154)
- Katzenstein et al. coined the term "acute interstitial pneumonia (AIP)" (Am J Surg Pathol 1986;10:256)
- In the multidisciplinary classification of idiopathic interstitial pneumonias by American Thoracic Society / European Respiratory Society, acute interstitial pneumonia is categorized as "acute / subacute interstitial pneumonia" (Am J Respir Crit Care Med 2013;188:733)
Essential features
- Rare and aggressive type of idiopathic interstitial pneumonia with diffuse alveolar damage (DAD), characterized by diffuse inflammation with hyaline membrane and fibroblastic proliferation
- Acute interstitial pneumonia shares common features with acute respiratory distress syndrome (ARDS) clinically and morphologically
Terminology
- Also called Hamman-Rich syndrome and idiopathic diffuse alveolar damage
ICD coding
Epidemiology
- Extremely rare (no conclusive epidemiological data available)
- Mean age 50 years but can occur at any age (7 - 83 years) (Eur Respir J 2000;15:412)
- No sex predilection
Sites
- Bilateral lung, usually in all five lobes of the lung
Pathophysiology
- Both endothelial and epithelial injury result in decreased integrity of the alveolar capillary membrane
- Imbalance of proinflammatory and anti-inflammatory mediators
- Neutrophils increase in alveoli and interstitium and release metabolites leading to lung injury
- Alveolar epithelial cells may go through epithelial - mesenchymal transition to become myofibroblasts, resulting in interstitial organization and fibrosis (BMC Pulm Med 2014;14:67)
Etiology
- No definite cause; no risk factors have been identified
Clinical features
- Influenza-like illness, followed by progressive shortness of breath (Am J Surg Pathol 1986;10:256)
- Vast majority of patients are previously healthy and lack history of lung disease
- Many clinical characteristics of acute interstitial pneumonia are similar to acute respiratory distress syndrome (Chest 2003;124:554)
- Acute interstitial pneumonia can progress to respiratory failure as profound as severe acute respiratory distress syndrome (PaO2/FIO2 ≤ 100 mm Hg) and almost all patients need mechanical ventilation and hospital care
- Respiratory failure usually appears 1 - 3 weeks from the onset, later than acute respiratory distress syndrome (16.8 days vs. 2.2 days)
- Multiple organ failure is less common in acute interstitial pneumonia
Diagnosis
- Diagnostic requirements
- Exclusion of any other causes of respiratory failure
- Histological diagnosis of diffuse alveolar damage
- Open lung biopsy, if possible, is recommended to reach the accurate diagnosis and to guide prompt treatment (Crit Care 2006;10:423)
- Transbronchial lung biopsy may be also helpful to find hyaline membranes of diffuse alveolar damage but it needs to be carefully distinguished from artifacts
Laboratory
- Hypoxia
- Increased serum ferritin, D dimer and C reactive protein
- KL-6 may increase slightly
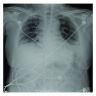
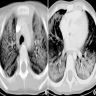
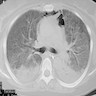
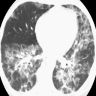
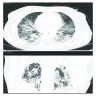
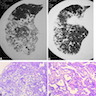
Radiology description
- Heterogeneous bilateral ground glass opacity due to pulmonary edema
- Chest radiograph
- Ground glass opacity
- Consolidation with air bronchogram
- Chest CT
- Ground glass opacity
- Airspace consolidation
- Bronchiolectasis / bronchiectasis; related to worse prognosis (Am J Respir Crit Care Med 2002;165:1551)
- Volume reduction
Radiology images
Prognostic factors
- Most patients die within 2 months unless appropriate treatment is provided (Eur Respir J 2000;15:412)
- High dose steroid therapy drastically improves the prognosis with long term survival of more than 80% (Chest 2006;129:753, Chest 2003;124:554)
- Survivors may suffer recurrences or develop chronic lung injury
Case reports
- 3 year old girl died of acute interstitial pneumonia (J Korean Med Sci 2008;23:529)
- 51 year old woman died of acute interstitial pneumonia (Case Rep Pulmonol 2012;2012:678249)
Treatment
- Oxygen therapy for respiratory failure
- Mechanical ventilation with positive end expiratory pressure
- High dose steroid pulse (Chest 2006;129:753)
- Direct hemoperfusion using polymyxin B immobilized fiber column was recently found to effectively improve the prognosis of acute interstitial pneumonia patients (Ther Adv Respir Dis 2017;11:261)
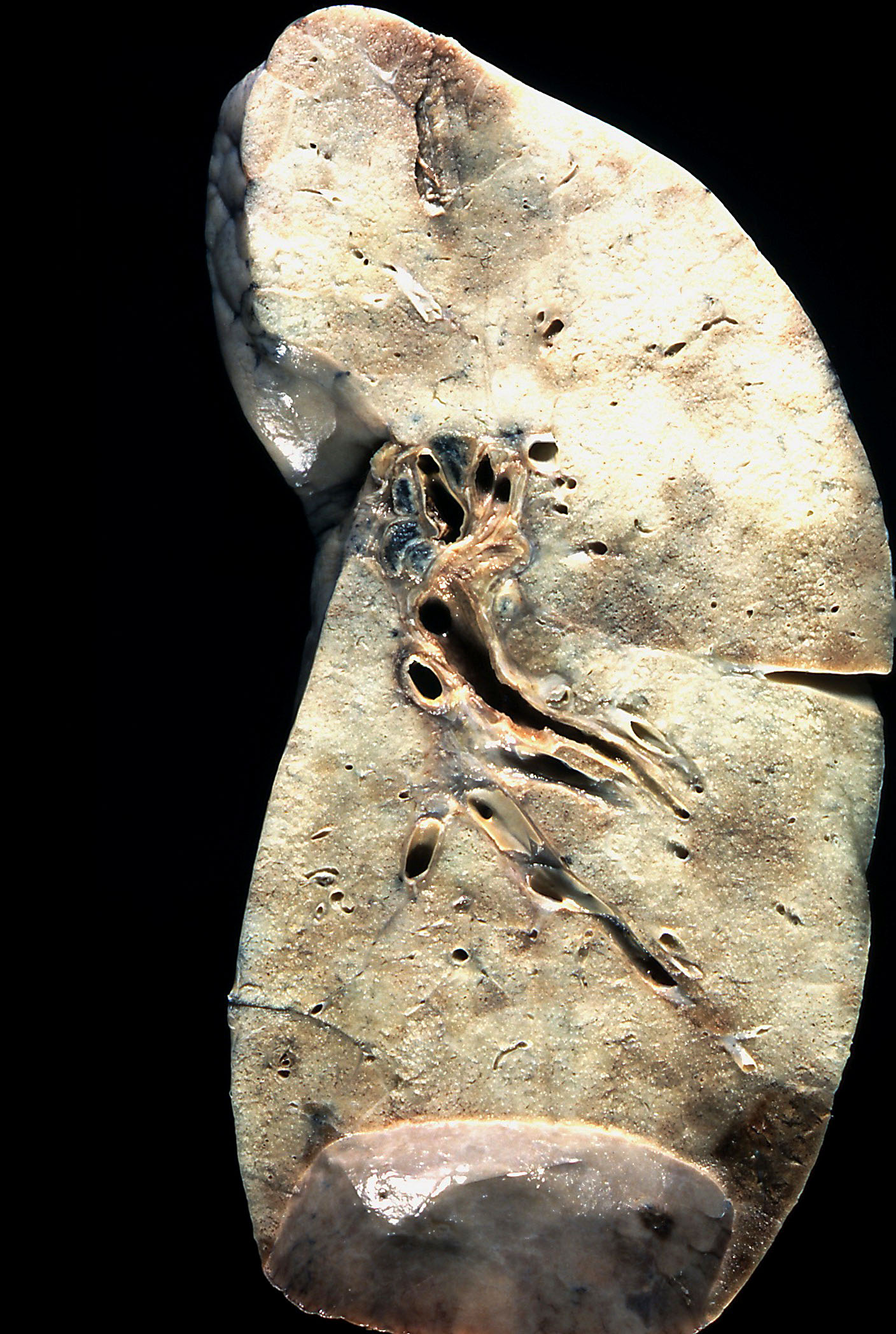
Gross description
- Dark blue lungs with hemorrhagic dots on pleural surface
- Heavy and firm due to edema and fibrosis
- Dilatation of alveolar ducts
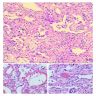
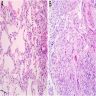
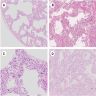
Microscopic (histologic) description
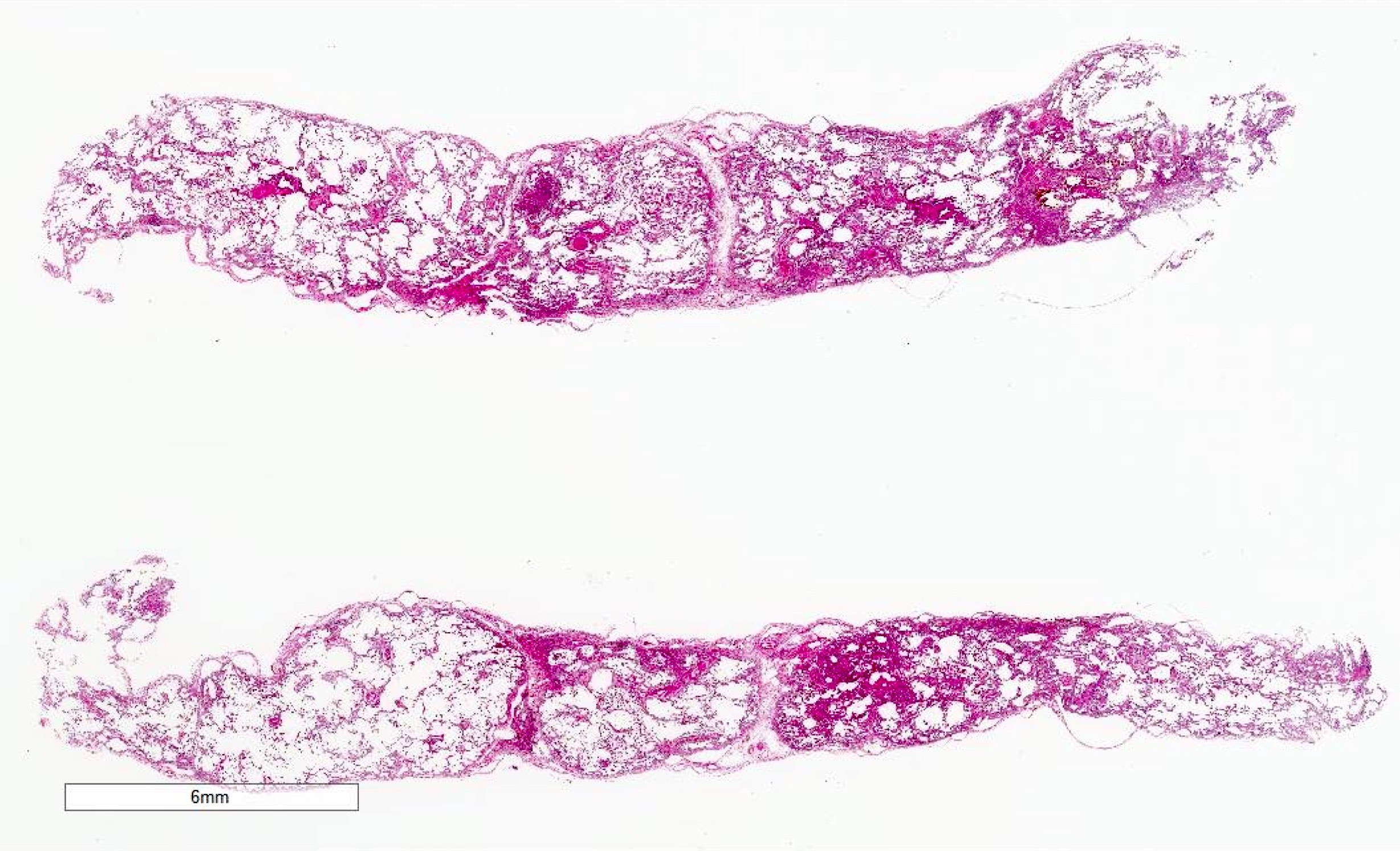
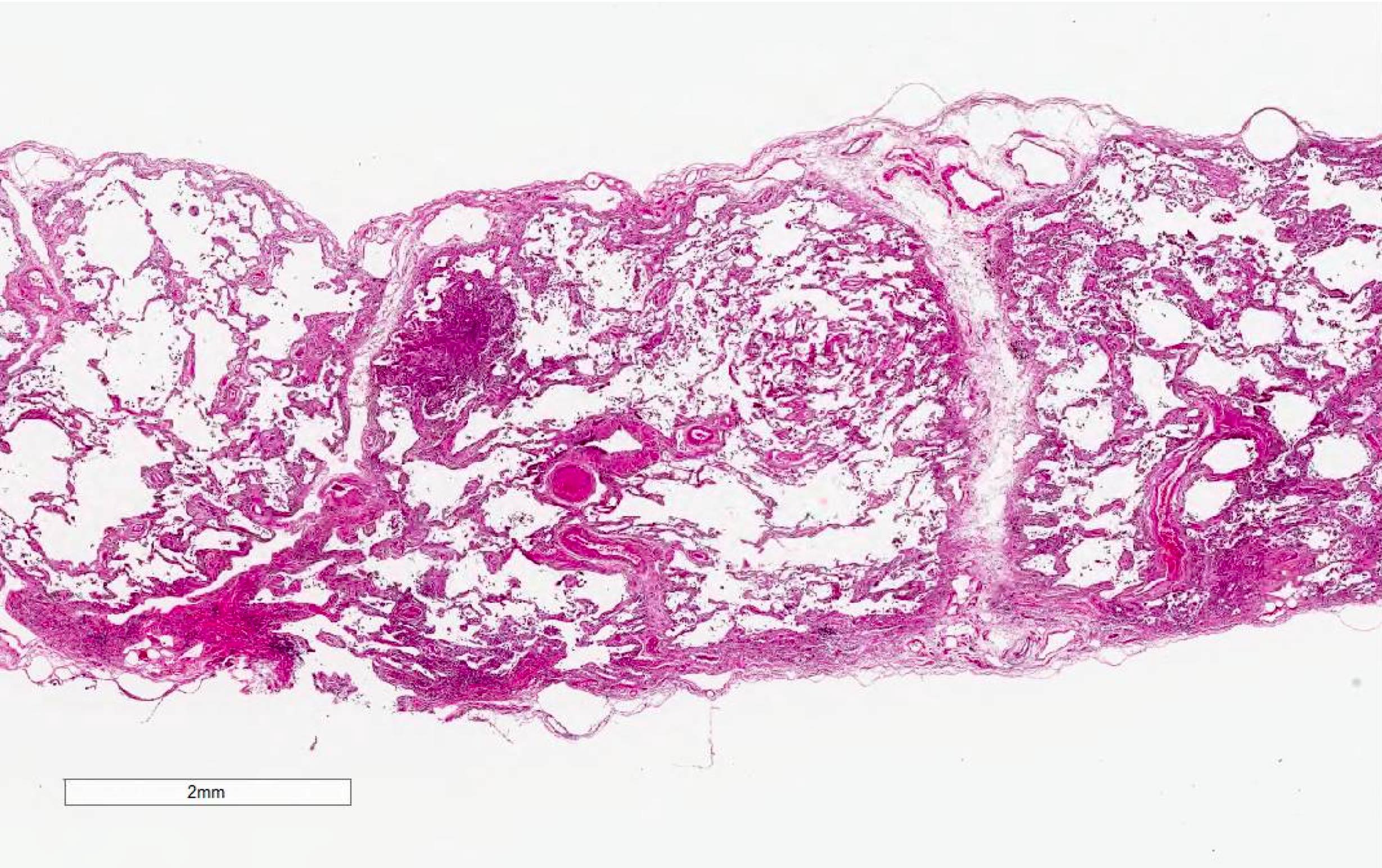
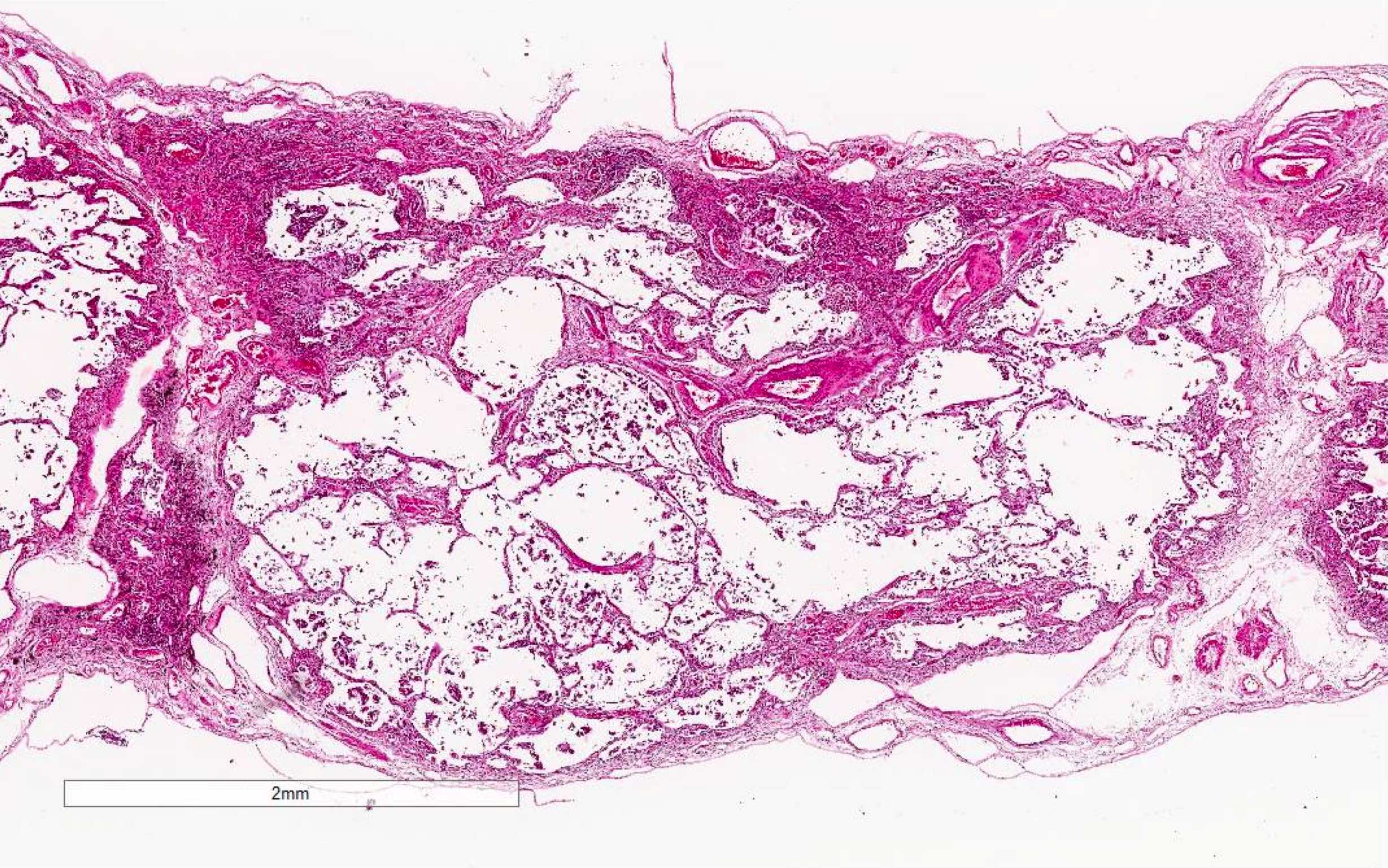
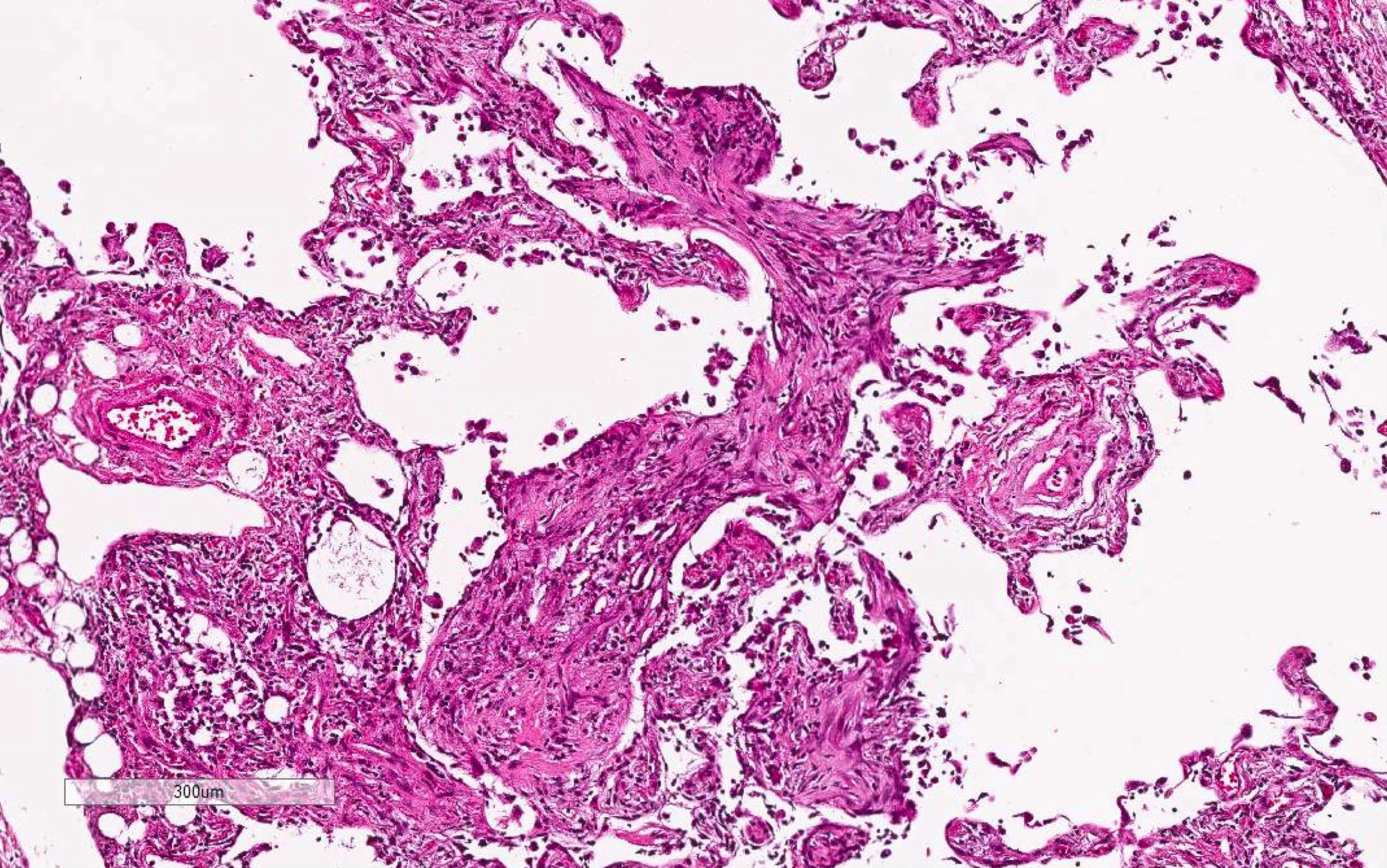
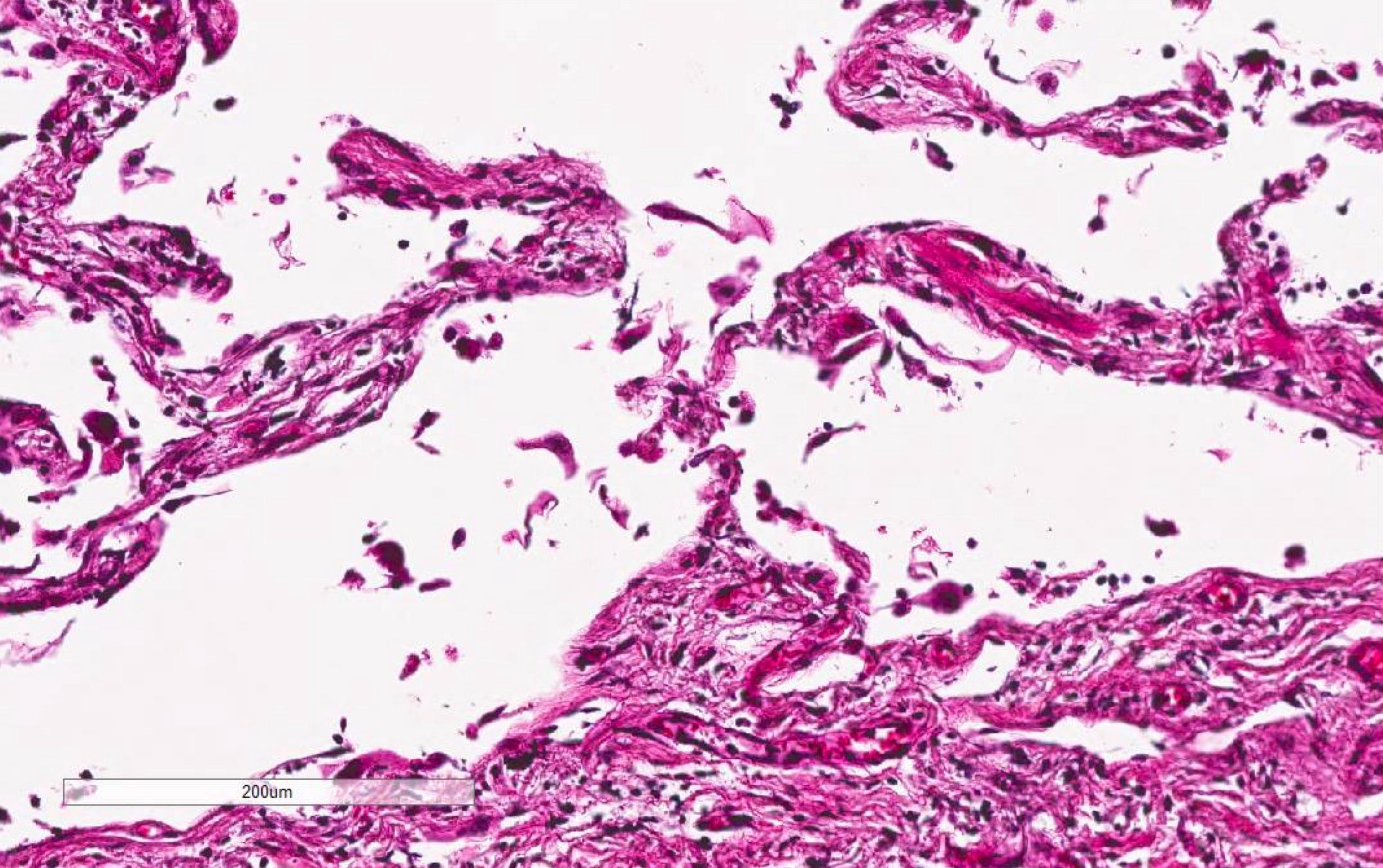
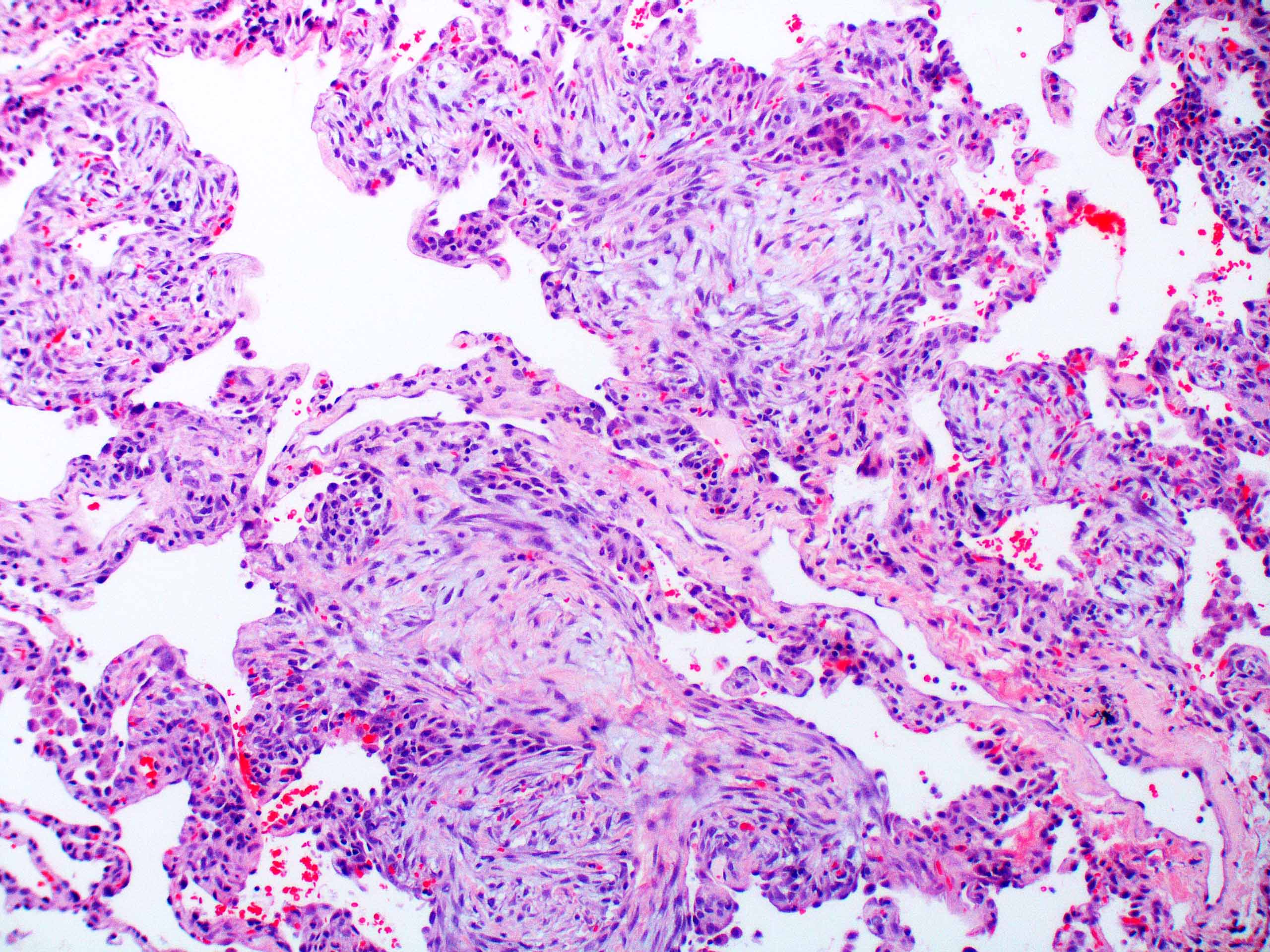
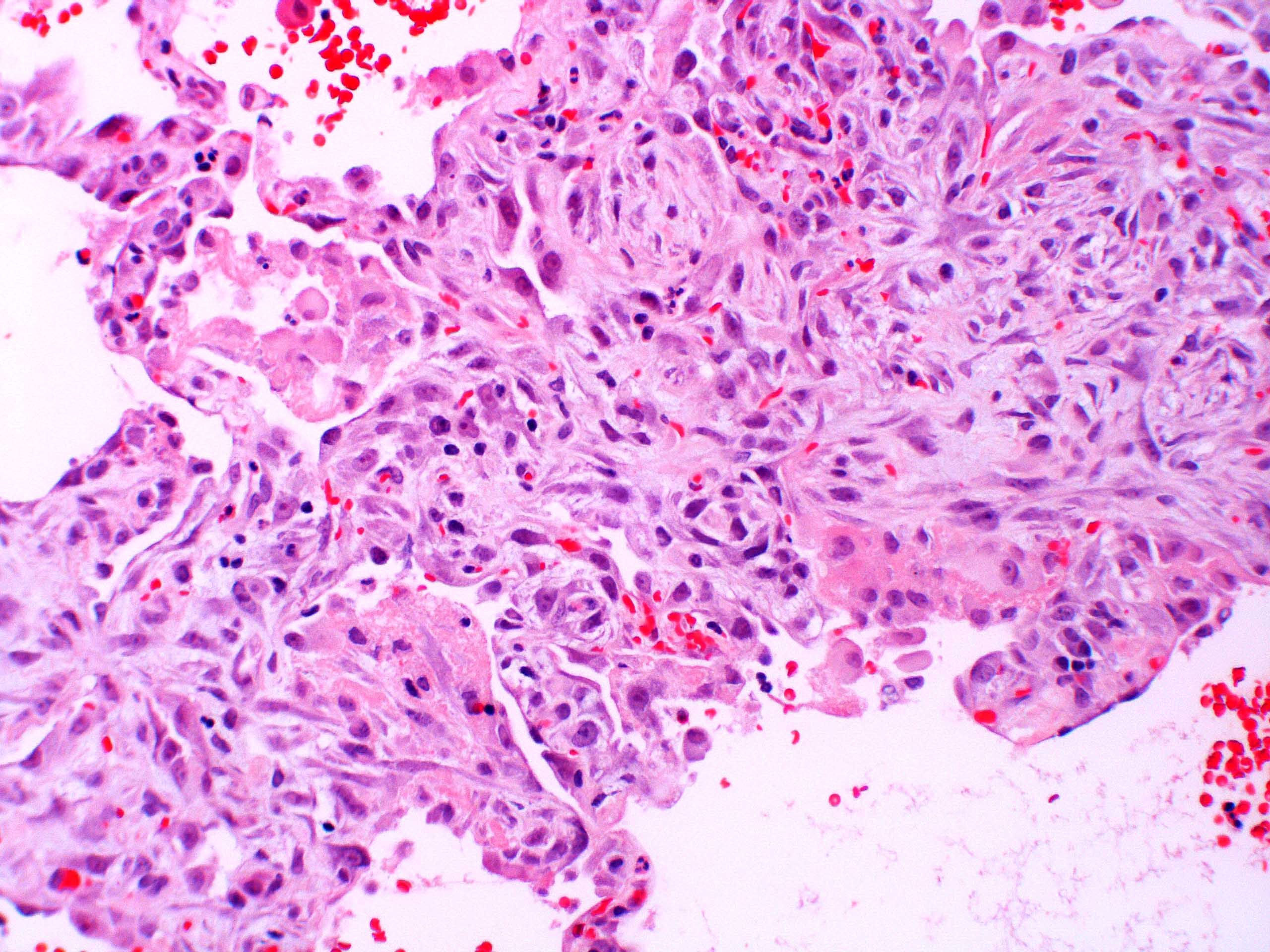
- Acute interstitial pneumonia shows diffuse alveolar damage, which is almost completely identical to acute respiratory distress syndrome / diffuse alveolar damage morphologically (Eur Respir J 2000;15:412)
- Proliferative / organizing (subacute) phase of diffuse alveolar damage is most common in acute interstitial pneumonia but also exudative (acute) phase and fibrotic (chronic) phase can be seen
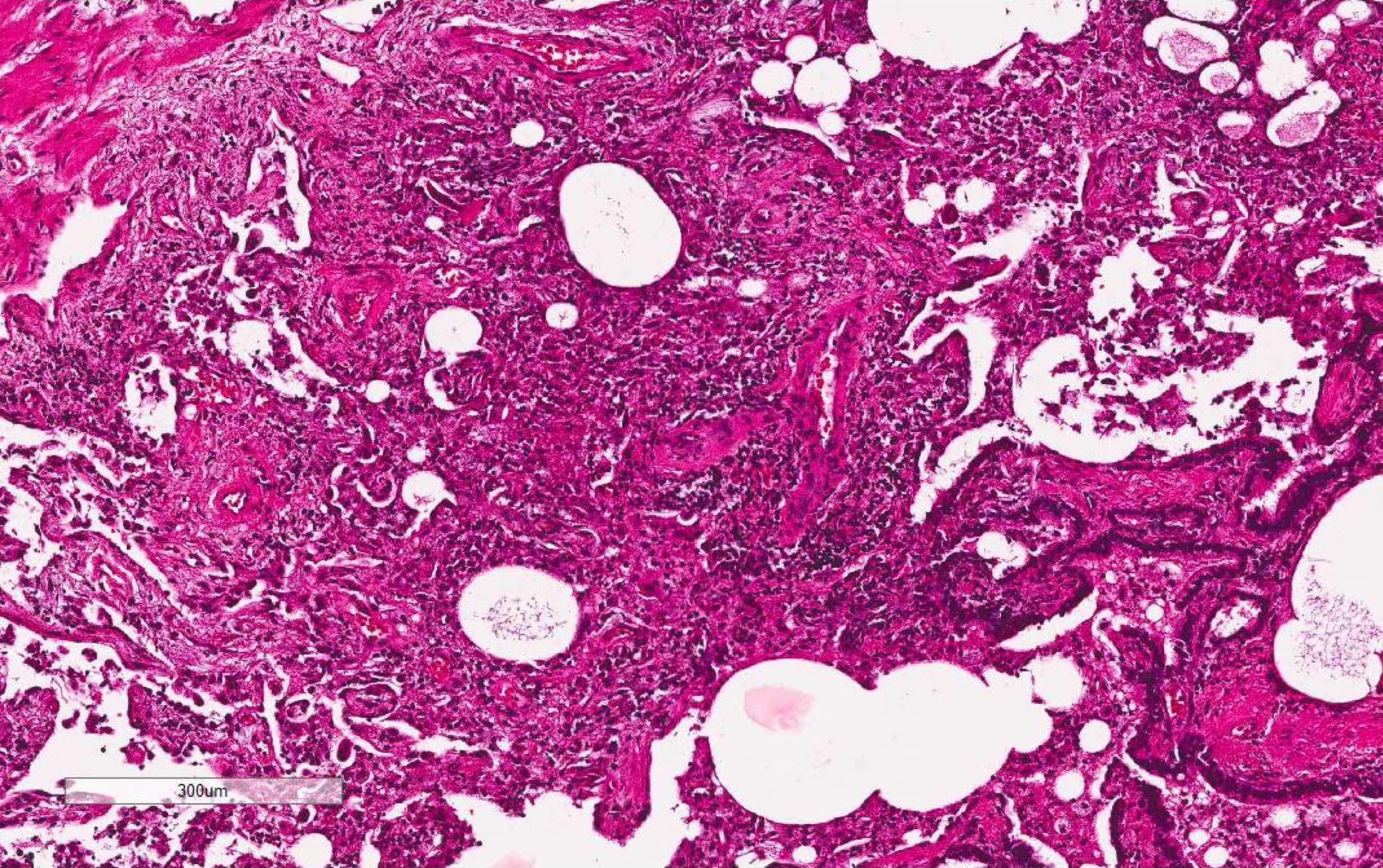
- Exudative phase
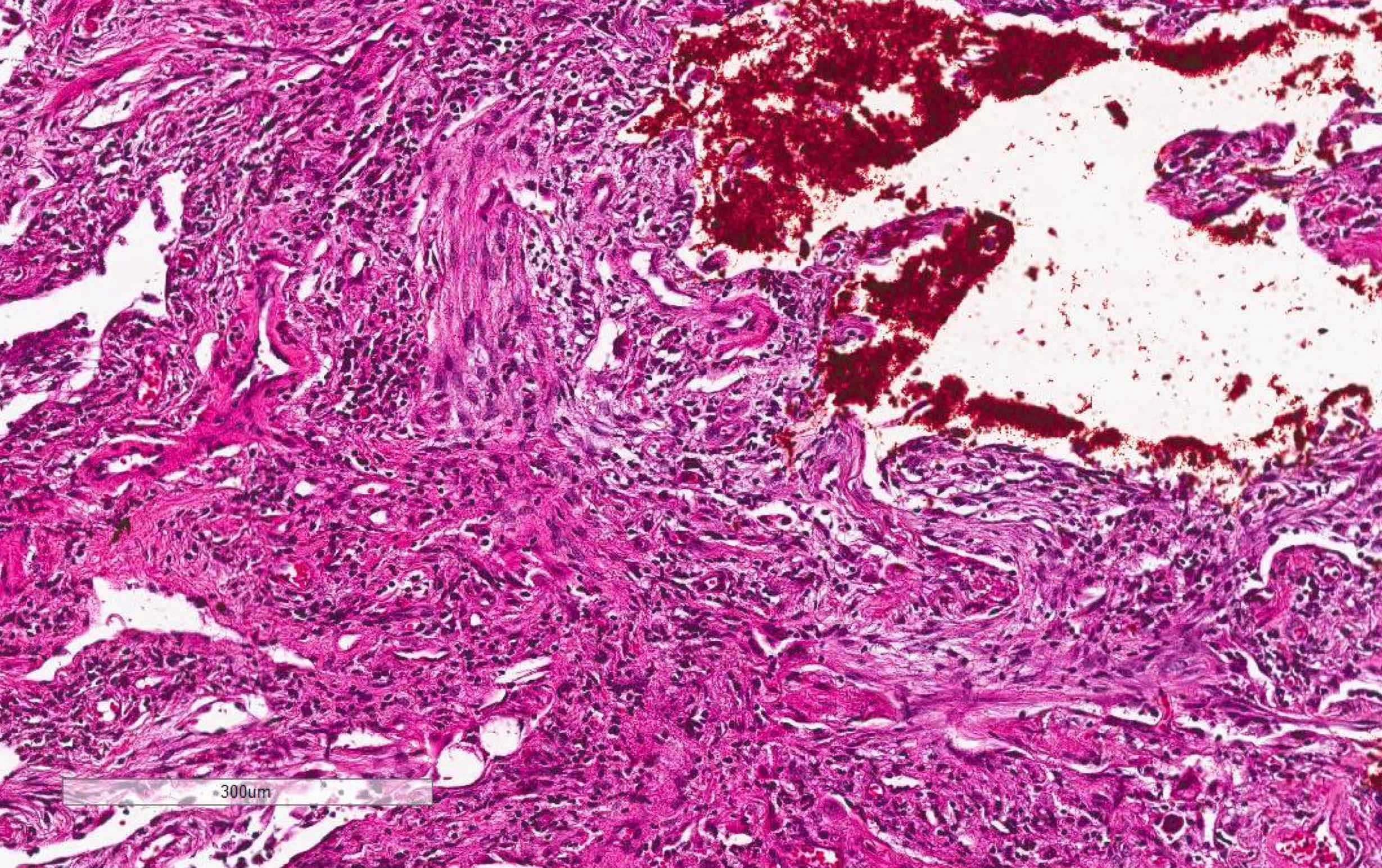
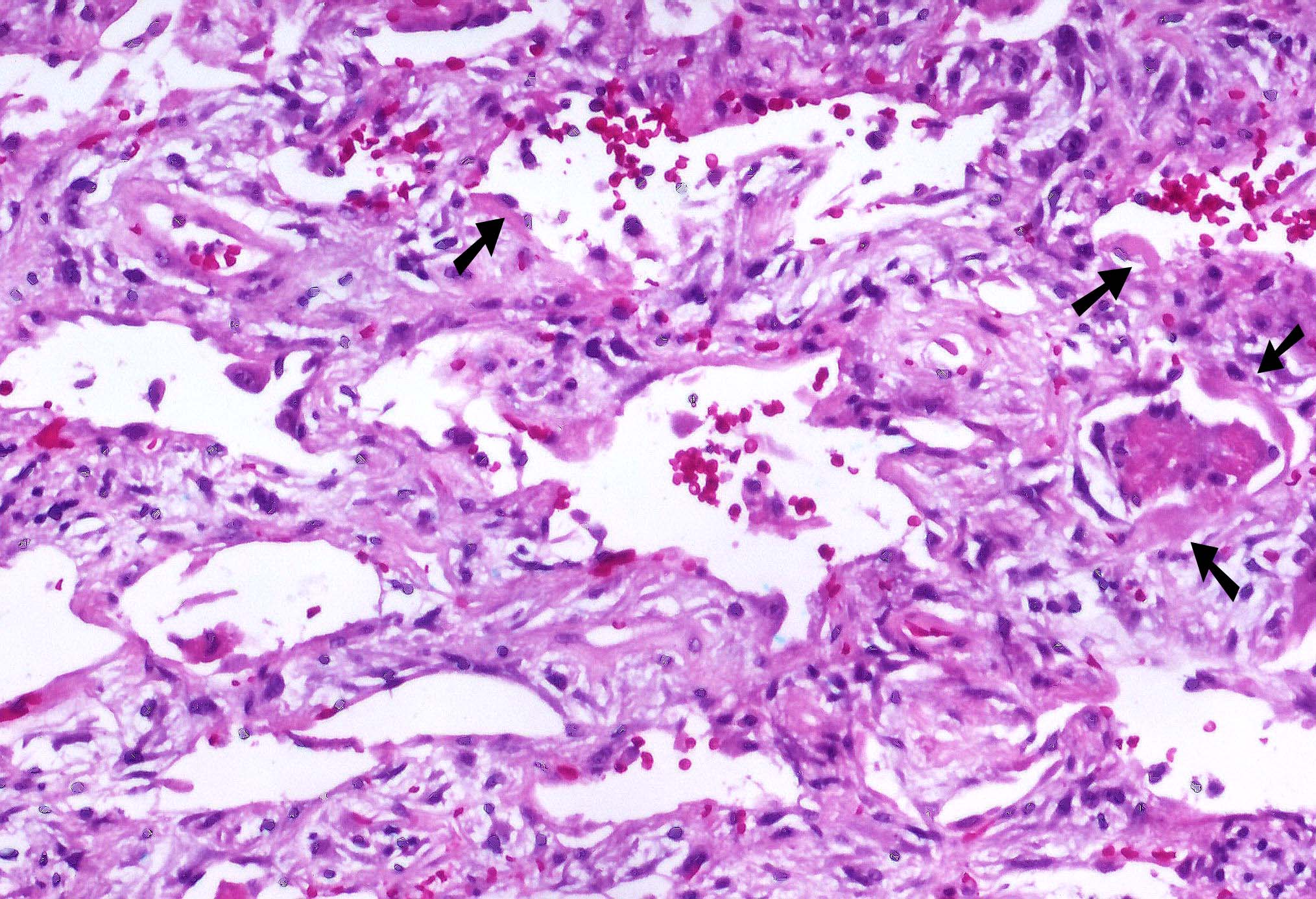
- Hyaline membranes in alveolar duct or sacs; scattered or not apparent, unlike in acute respiratory distress syndrome
- Interstitial and intra-alveolar edema
- Collapsed alveoli
- Denudation and necrosis of type I pneumocytes
- Hemorrhage, usually mild
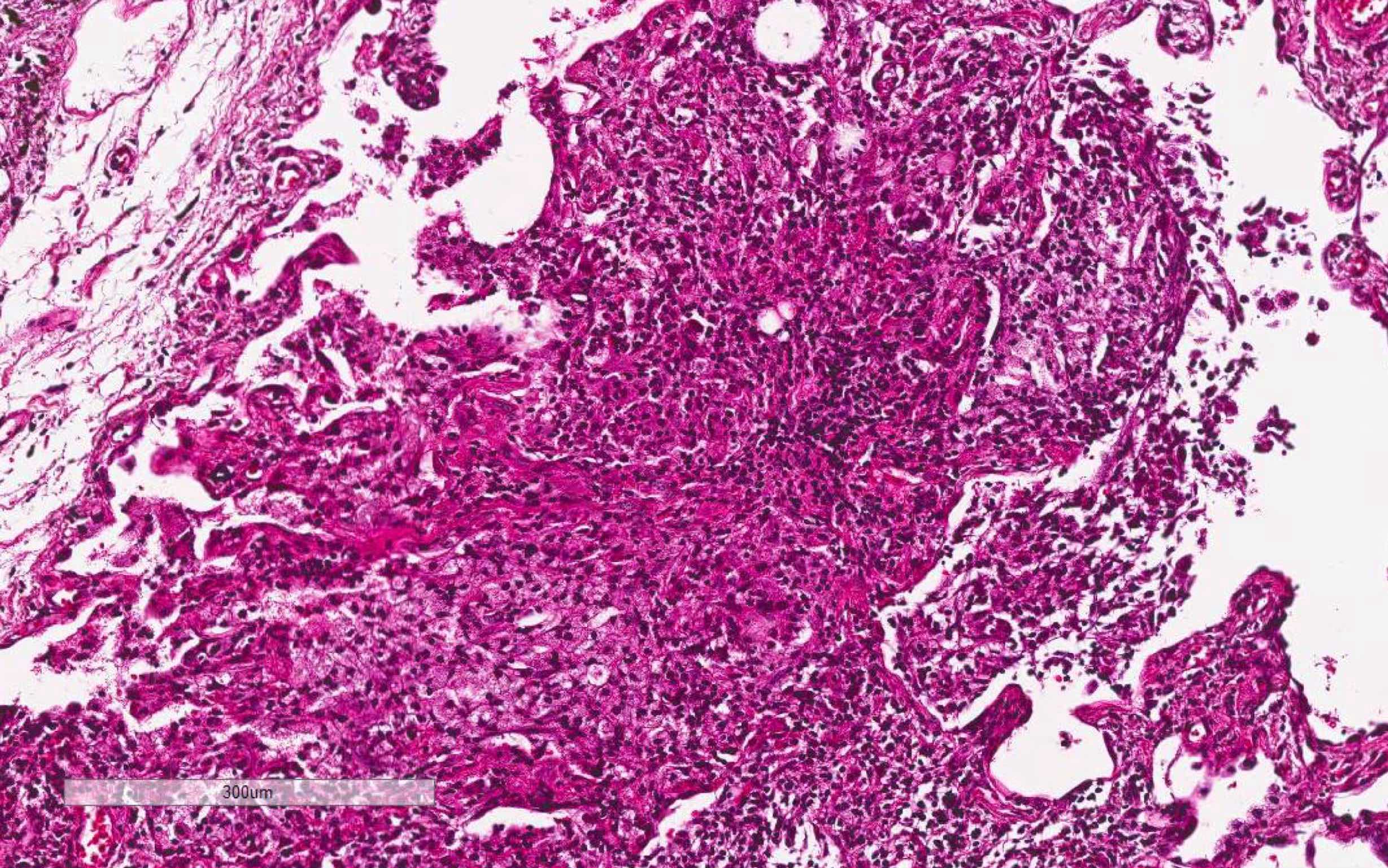
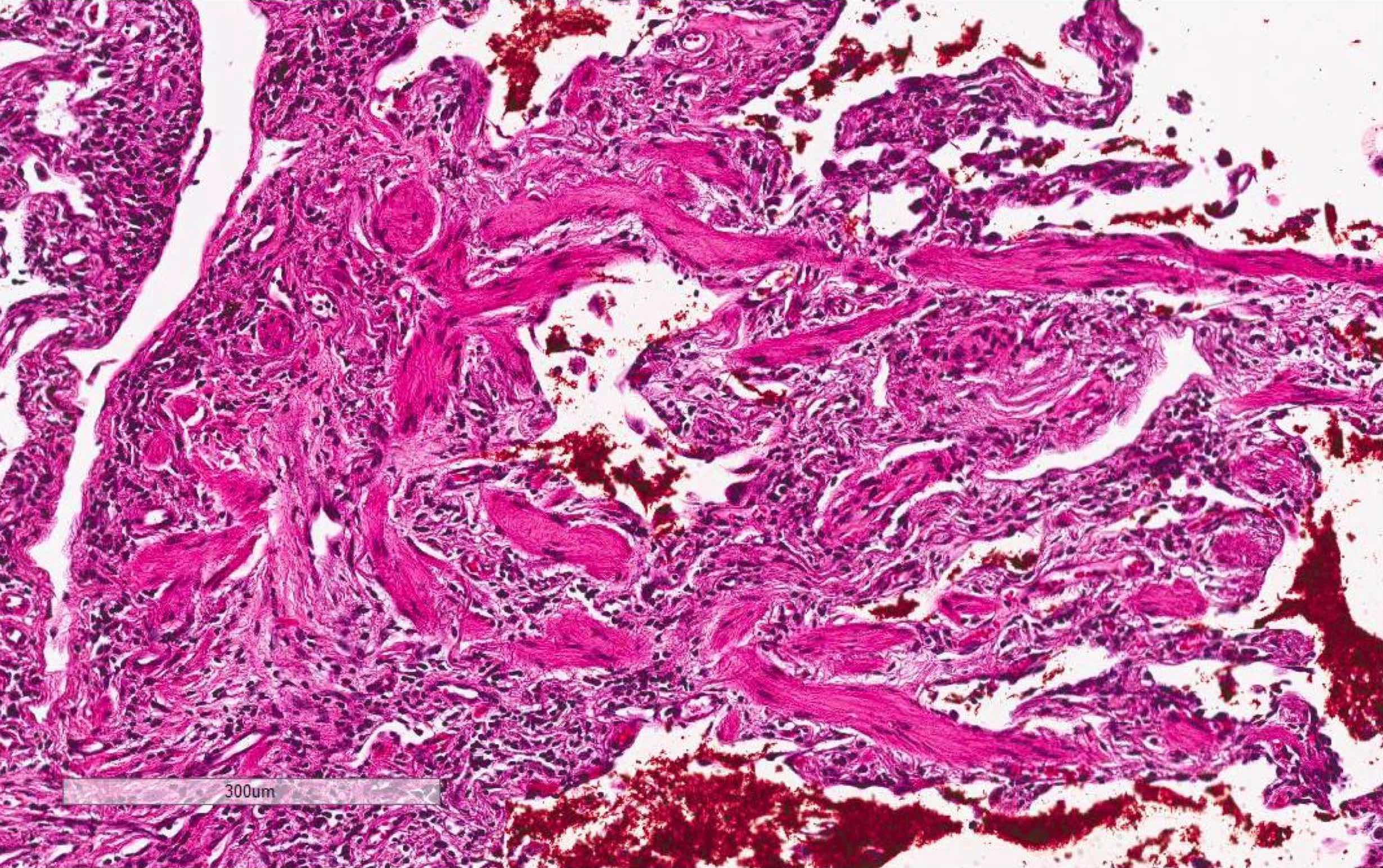
- Proliferative / organizing phase (Am J Surg Pathol 1986;10:256, Eur Respir J 2003;21:187)
- Organizing pneumonia with / without remnants of hyaline membrane
- Interstitial and intra-alveolar proliferation of fibroblasts / myofibroblasts
- Lymphocytic infiltration; usually more prominent than in acute respiratory distress syndrome
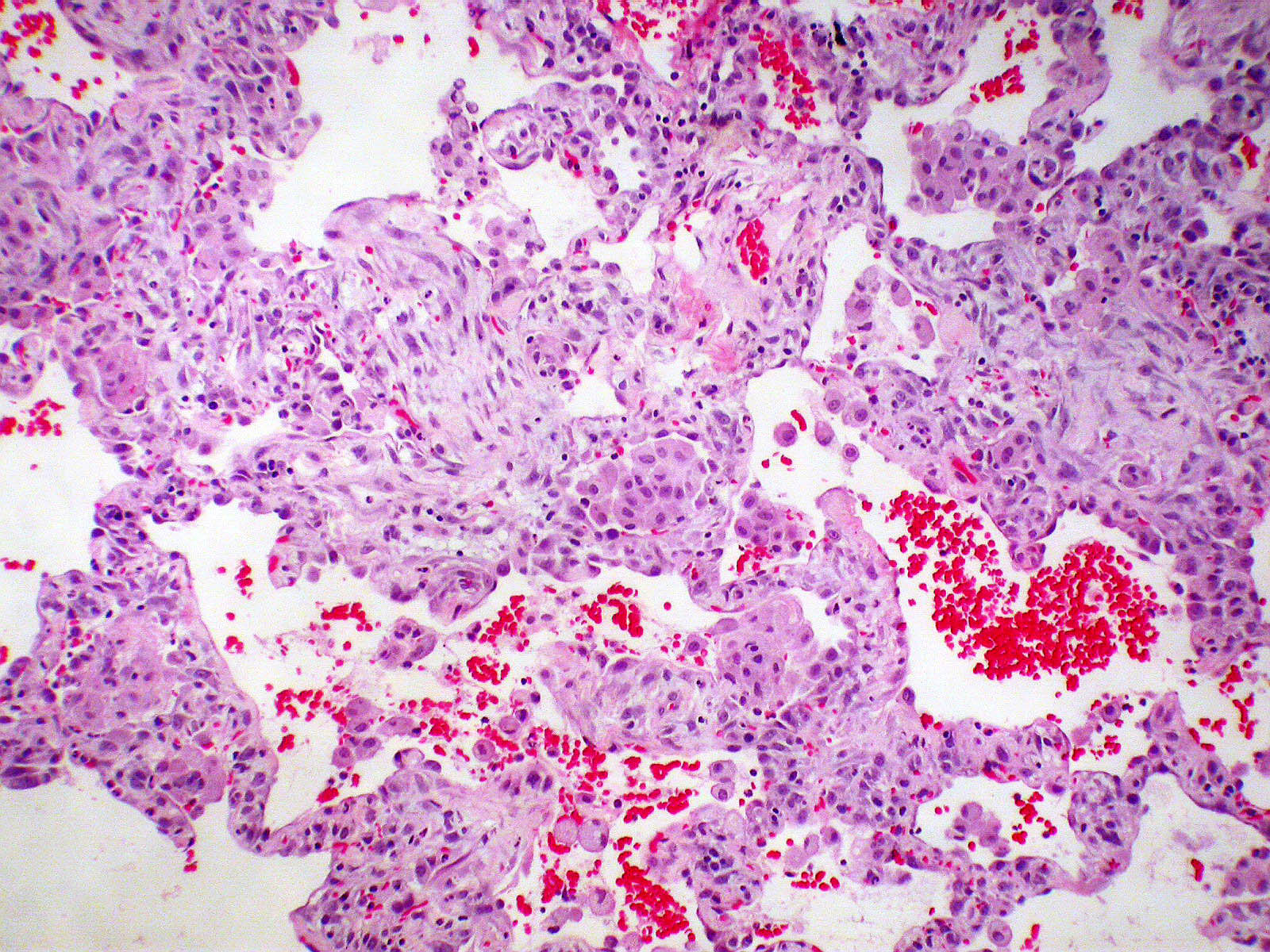
- Proliferation of type II pneumocytes with occasional cellular atypia
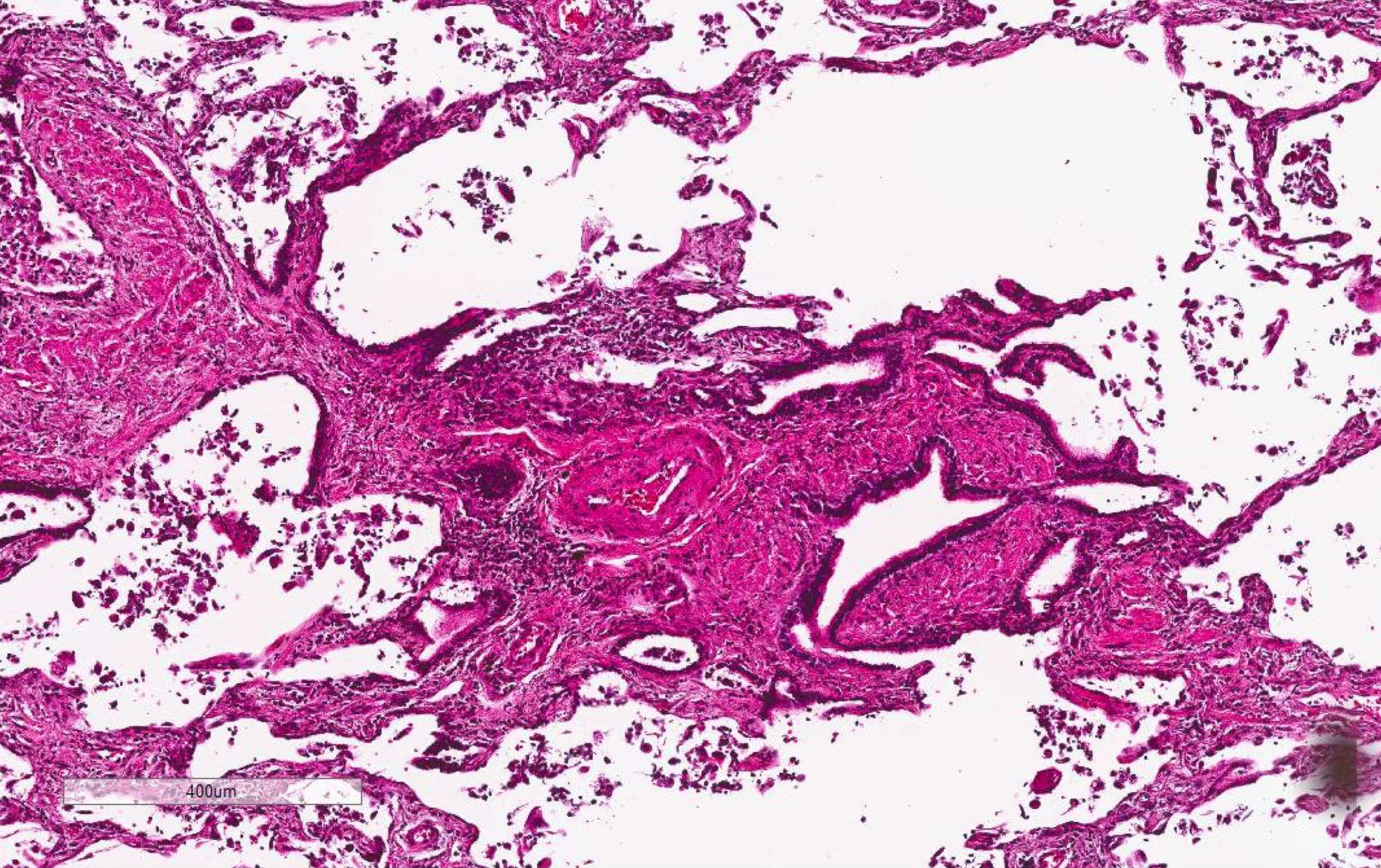
- Endothelial injury and fibrinous thromboembolism in arterioles / arteries
- Fibrosis phase
- Diffuse collagenous fibrosis
- Microscopic honeycomb-like change
- Traction bronchiolectasis
- Squamous metaplasia
- Organized thrombus
- Thickening of pleura with dilatation of lymphatic / blood vessels
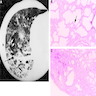
Microscopic (histologic) images
Contributed by Akira Yoshikawa, M.D. and Yale Rosen, M.D.
Images hosted on other servers:
Cytology description
- Bronchoalveolar lavage (BAL) fluid
- Increased neutrophils (Eur Respir J 2000;15:412)
- Atypical epithelial cells are rarely present (Eur Respir J 2003;21:187)
Positive stains
- Elastica van Gieson (fiber staining) is helpful to evaluate architectural destruction of alveoli
Electron microscopy description
- Proliferation of type II pneumocyte with cytoplasmic projection into alveolar septa, abnormally large lamellar bodies or denudation from basement membrane (Am J Surg Pathol 1986;10:256)
Differential diagnosis
- Acute exacerbation of interstitial lung disease, especially idiopathic pulmonary fibrosis (IPF): history of IPF, background of dense fibrosis and honeycombing
- Acute hypersensitivity pneumonitis: history of exposure to causative antigens, remission of symptoms after antigen removal, lymphocytosis ( > 30%) in bronchoalveolar lavage, nonnecrotizing granulomas, strong bronchocentric accentuation
- Acute respiratory distress syndrome: predisposition of pulmonary or systemic insult, an onset within 7 days, PaO2/FIO2 ≤ 300 mm Hg
- Collagen tissue disease associated interstitial lung disease
- Several collagen tissue disease are known to rarely present with acute interstitial pneumonia-like symptoms and diffuse alveolar damage (Mod Rheumatol 2012;22:243, Chest 2006;130:553)
- Clinical manifestation and serum autoantibody tests are helpful for the diagnosis
- Drug induced lung injury: history of causative drug, remission of symptoms after drug withdrawal, marked eosinophils, foamy changes in type II cells
- Eosinophilic pneumonia: smoking history, eosinophilia ( > 25%) in bronchoalveolar lavage, degranulation of eosinophils in the lung tissue, pink macrophages, marked gumball airspace fibrin rather than hyaline membranes
- Organizing pneumonia: exposure to causative particles, migratory shadows on radiology, preservation of alveolar architecture
Additional references
Board review style question #1
Which of the following findings is not required for the diagnosis of acute interstitial pneumonia?
- Absence of exposure to causative factors of respiratory failure
- Absence of prior history of lung disease
- Bilateral shadows on chest radiograph
- Diffuse alveolar damage on histology
- PaO2/FIO2 ≤ 300 mm Hg
Board review style answer #1
E. PaO2/FIO2 ≤ 300 mm Hg is one of the diagnostic criteria of acute respiratory distress syndrome. Acute interstitial pneumonia can also cause the similar severe respiratory failure, however it is not a diagnostic requirement for acute interstitial pneumonia.
Comment Here
Reference: Acute interstitial pneumonia
Comment Here
Reference: Acute interstitial pneumonia