Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Differential diagnosis | Additional referencesCite this page: Wu R. Preinvasive-general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumordysplasiagen.html. Accessed April 19th, 2024.
Definition / general
-
Pulmonary preneoplastic changes include (Respir Res 2002;3:20):
- Bronchial squamous dysplasia and in situ carcinoma preceding invasive squamous cell carcinoma and basaloid carcinoma
- Atypical adenomatous hyperplasia preceding bronchioloalveolar carcinoma
- Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia, a proposed precursor for carcinoid tumor
Essential features
- Squamous dysplasia / CIS is a multifocal and clonal condition strongly associated with cigarette smoking i.e. “field cancerization”
- High grade squamous dysplasia / CIS is associated with an increased risk of invasive squamous cell carcinoma
- Squamous dysplasia / CIS tends to arise in large central airways
Terminology
- “Dysplasia” usually used for squamous bronchial lesions
- Angiogenic squamous dysplasia = capillary blood vessels closely juxtaposed to and projecting into metaplastic or dysplastic squamous bronchial epithelium (Clin Cancer Res 2000;6:1616)
ICD coding
- D02.20: Carcinoma in situ of unspecified bronchus and lung
- D02.21: Carcinoma in situ of right bronchus and lung
- D02.22: Carcinoma in situ of left bronchus and lung
Epidemiology
- Males appear to have higher prevalence of squamous dysplasia than females (J Natl Cancer Inst 1999;91:691)
Sites
- Squamous dysplasia / CIS with propensity for large central airways, usually around bifurcations, less commonly in trachea
Pathophysiology
- May precede mass by many years
- Pre invasive lesions and subsequent cancers are clonally related (J Pathol 2011;224:153)
- Evidence for stepwise progression relatively weak, but concept of field carcinogenesis is strongly supported (Cancer Metastasis Rev 2010;29:5)
Etiology
- Associated with smoking
- Possible progression from basal cells or metaplastic goblet cells to squamous metaplasia, dysplasia, CIS
Clinical features
- Usually not symptomatic on its own
Diagnosis
- Image enhanced endoscopy i.e. autofluorescence bronchoscopy (AFB), high magnification bronchovideoscopy (HMS), narrow band imaging (NBI), endobronchial ultrasonography (EBUS), optical coherence tomography (OCT) (Clin Chest Med 2013;34:373)
- Frequently encountered in resection specimens, but not often on endoscopic biopsy specimens
Prognostic factors
- Some preneoplastic lesions regress, while others progress
- No difference in progression rate and time to progression based on initial histologic grading; cannot differentiate the potentially more malignant lesions (Clin Cancer Res 2005;11:537)
- Persistence of dysplasia associated with development of invasive carcinoma (Cancer Prev Res 2016;9:96)
- Squamous dysplasia with high telomerase activity, increased Ki67 and p53 positivity tend to persist and might progress to carcinoma (Lung Cancer 2004;46:187)
- CIS is strong predictor of progression to invasive squamous cell carcinoma (Cancer Metastasis Rev 2010;29:5)
Case reports
- Three men ages 63, 65 and 71 years old with dysplastic lesions in bronchi which progressed to squamous cell carcinoma (Br J Cancer 1997;75:678)
Treatment
- Bronchoscopic followup of severe dysplasia and CIS, endobronchial or surgical techniques (Chest 2007;132:221S)
Gross description
- Either unremarkable mucosa or papillary and granular with loss of folds
Microscopic (histologic) description
- Histological patterns of bronchial epithelial dysplasia: basal cell dysplasia, columnar cell dysplasia, bronchial epithelial dysplasia with transitional differentiation and squamous dysplasia (Mod Pathol 2006;19:429)
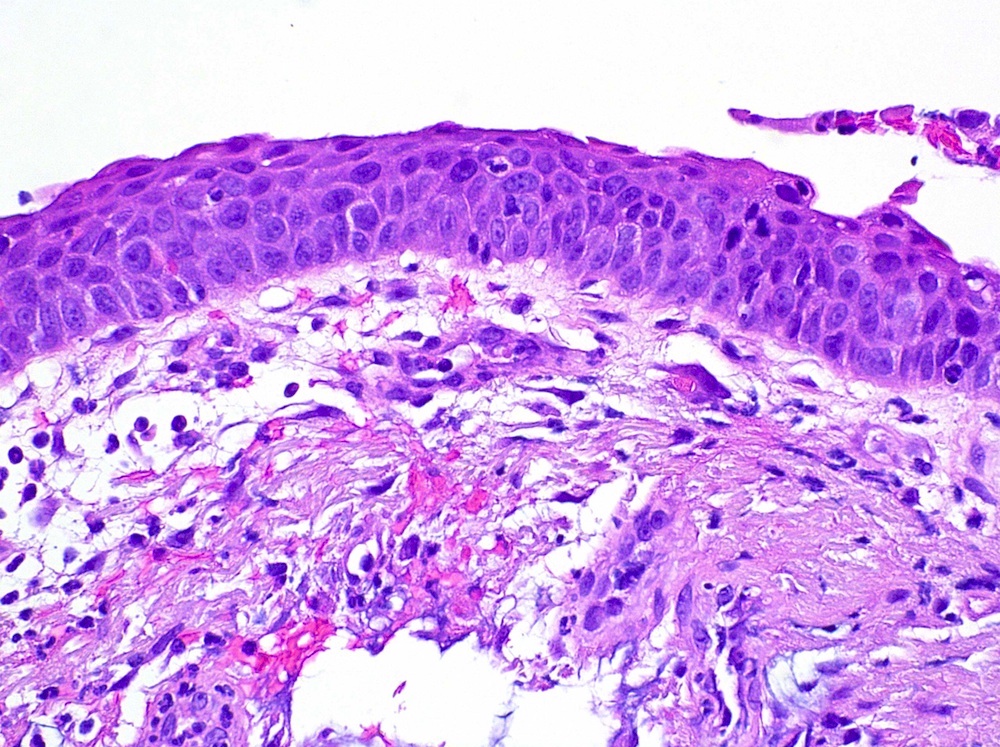
- Squamous dysplasia: focal to full thickness replacement of epithelium by squamous cells with increased nuclear to cytoplasmic ratio, nuclear pleomorphism, mitotic activity but intact basement membrane
- No invasive growth although may extend into ducts of submucosal glands
- Graded with a 4 tier (mild / moderate / severe / CIS) or 2 tier system (low grade / high grade) (J Clin Pathol 2001;54:257)
- Mild dysplasia: minimal abnormalities with basal expansion, increased cellularity, vertically oriented nuclei, limited to bottom third of epithelium, mitoses absent or rare, maturation present
- Moderate dysplasia: more abnormalities, partial maturation, extending to lower two thirds of epithelium, mitoses limited to lower two thirds
- Severe dysplasia: cellular pleomorphism, coarse chromatin, frequent nucleoli, basal zone to upper third, mitoses confined to lower two thirds, superficial cell flattening
- CIS: lack of maturation, significant cytologic abnormalities, coarse chromatin, inconsistent nuclear orientation, mitoses present in full thickness
- Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH): preinvasive proliferation of pulmonary neuroendocrine cells
- Proposed criteria for diagnosis on lung resection is multifocal neuroendocrine cell hyperplasia as defined by 5 or more pulmonary neuroendocrine cells in at least 3 separate small airways combined with 3 or more carcinoid tumorlets (Semin Diagn Pathol 2015;32:438, Lung 2015;193:659)
Microscopic (histologic) images
Positive stains
- Some cases of bronchial dysplasia show patchy or scattered staining for p53 and Ki67 (Mod Pathol 2006;19:429)
- Ki67 index significantly higher in severe dysplasia than in mild / moderate dysplasia (Pol J Pathol 2015;66:38)
Molecular / cytogenetics description
- Sequential changes in lung cancer pathogenesis include LOH, microsatellite alterations, telomerase dysregulation (Clin Cancer Res 2001;7:5, Histopathology 2009;54:43)
- Discordant genetics changes can exist in the same carcinogen exposed bronchial tissues (Clin Cancer Res 2001;7:259)
Differential diagnosis
- Squamous metaplasia and reactive / reparative atypia
- Basal cell hyperplasia
- Squamous papilloma
Additional references
- Concise Review: Preinvasive Lesions of the Bronchus (J Thorac Oncol 2009;4:545)