Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Differential diagnosis | Childhood inflammatory myofibroblastic tumorCite this page: Wu R. Inflammatory myofibroblastic tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lungtumorinflammatorypseudotumor.html. Accessed April 20th, 2024.
Definition / general
- Category used to designate a variety of benign proliferative lesions forming a lung mass, including neoplastic and nonneoplastic entities
- Neoplastic: renamed inflammatory myofibroblastic tumor (IMT), may show clonal cytogenetic abnormalities involving 2p23 that encodes ALK gene, typically occurs in children and young adults, well demarcated but non encapsulated, usually solitary mass that replaces underlying lung tissue
- Nonneoplastic: older age, ill defined or irregular contour due to prominent organizing pneumonia component and fibrosis at edge (Arch Pathol Lab Med 2010;134:417)
Essential features
- Inflammatory pseudotumor is a heterogeneous group of benign lesions that may occur in the lung, with a variable mix of inflammatory cells and fibroblasts / myofibroblasts
- Inflammatory myofibroblastic tumor is recognized as a neoplastic subset that can be associated with ALK gene rearrangement and is typically seen in the younger age group
- The differential diagnosis is broad, and diagnosis typically requires surgical excision
Terminology
- Variable definitions in use of "inflammatory pseudotumor"; some use term to describe any circumscribed or irregular inflammatory nodule, mass or consolidation; others favor a more descriptive diagnosis
- Inflammatory myofibroblastic tumor means neoplastic, fibrohistiocytic, associated with ALK rearrangement
- Other names used in nonneoplastic lesions: plasma cell granuloma, hyalinizing granuloma, plasma cell pseudotumor, inflammatory myofibrohistiocytic proliferation
Epidemiology
- Incidence unclear since terminology / definition varies
- M = F, broad age range but usually < 30 if IMT
- 3% bilateral
Sites
- Typically parenchymal, but may involve pleura
- Rarely intrabronchial
Etiology
- Unknown, but may be autoimmune (IgG4) related or due to an infectious process
Clinical features
- 50% with cough, shortness of breath, chest pain, hemoptysis
Diagnosis
- Surgical excision with histologic examination
Radiology description
- Xray: single, well defined, round or oval mass
- CT: may show pleural retraction if lesion involves pleura, may show cavitation or calcification
Prognostic factors
- Usually remains stable or grows very slowly, may spontaneously resolve
- Poor prognostic factors for IMT: metastases, necrosis > 15% of surface area examined, local recurrence, bizarre giant cells, > 3 mitotic figures / 50 HPF, advanced stage, high cellularity, poor circumscription
Case reports
- 27 year old man with IMT invading into GE junction (Ann Thorac Surg 2010;89:1659)
- 45 year old man with IMT and progressive dyspnea, cough, wheezing (J Cardiothorac Surg 2010;5:55)
- 58 year old man with inflammatory pseudotumor from Coxiella burnetti (Microbes Infect 2015;17:795)
Treatment
- Complete excision
- Rarely causes death due to local extension
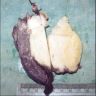
Gross description
- Variable size from < 1 cm to very large (> 30 cm)
- Well circumscribed, non encapsulated, usually solitary, white to gray, firm, fleshy parenchymal nodule
- May be yellow and friable or show hemorrhage, necrosis, calcification
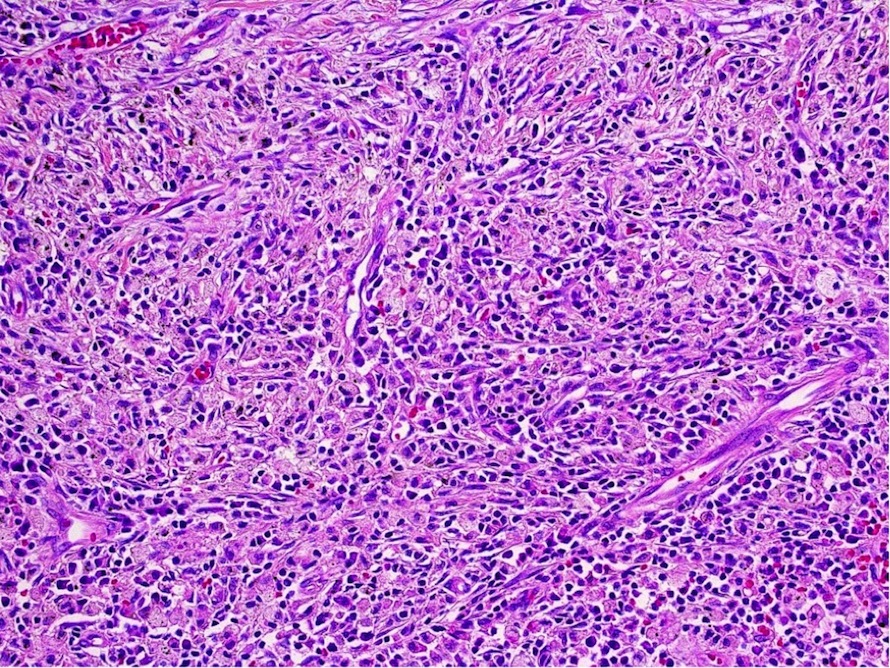
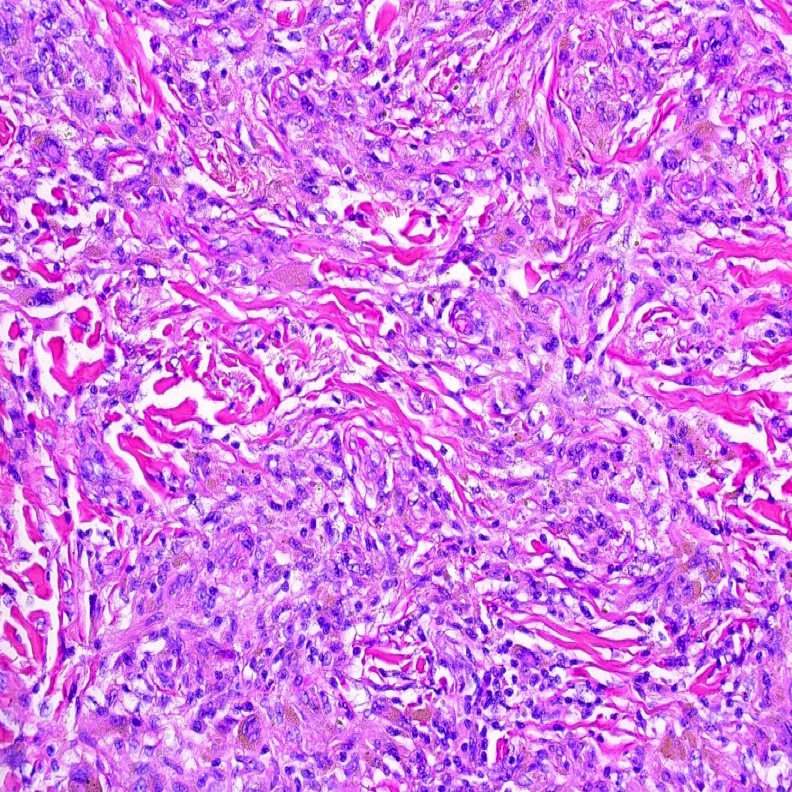
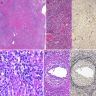
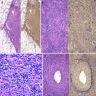
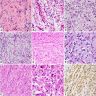
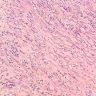
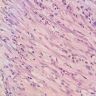
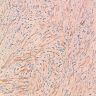
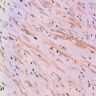
Microscopic (histologic) description
- Variable patterns, e.g. fibrohistiocytic, plasma cell granuloma, largely sclerosed, compact spindle cells, hypocellular fibrous, myxoid / vascular, fibroxanthomatous
- Generally fibroinflammatory with variable numbers of plasma cells, lymphocytes, histiocytes and myofibroblasts
- May show mast cells, eosinophils, neutrophils, multinucleated cells, hemosiderin, calcification
- May resemble nodular fasciitis, fibrous histiocytoma or fibromatosis
- May show features of organizing pneumonia including lymphohistiocytic inflammation and fibrosis with preservation of alveolar architecture in early lesions; also bordering alveoli with foamy macrophages and hyperplastic pneumocytes
- Lymphoplasmacytic variant with mostly plasma cells and lymphocytes, germinal centers, paucicellular collagen, endothelialitis, fibrinous pleuritis
Microscopic (histologic) images
Positive stains
- Spindle cells: vimentin, smooth muscle actin
- ALK1 staining in 40% of IMTs
- Polyclonal kappa and lamda light chain Ig in plasma cells
Electron microscopy description
- Elongated cytoplasmic processes with pinocytotic vesicles, subplasmalemmal plaques, thin filaments, abundant endoplasmic reticulum
Molecular / cytogenetics description
- ~50% of inflammatory myofibroblastic tumor have t(2;17) translocation
- ROS1 or RET gene rearrangements reported (Am J Surg Pathol 2015;39:957)
Differential diagnosis
- Amyloid tumor
- Benign fibrous histiocytoma, malignant fibrous histiocytoma
- Carcinoid tumor
- Histoplasmosis: see Am J Clin Pathol 2011;136:410
- Lipoid pneumonia
- Lymphoma
- Lymphoid hyperplasia
- Metastatic carcinoma
- Mycobacterial pseudotumor
- Organizing pneumonia
- Plasmacytoma
- Solitary fibrous tumor
- Tuberculosis: immunosuppressed patients
- Other spindle cell tumors
Childhood inflammatory myofibroblastic tumor
Definition / general
Essential features
Terminology
Epidemiology
Sites
Radiology description
Case reports
Treatment
Gross description
Gross images
Images hosted on other servers:
Microscopic (histologic) description
Microscopic (histologic) images
Images hosted on other servers:
Molecular / cytogenetics description
- Most common isolated lung lesion in children, usually asymptomatic
- Mostly neoplastic with frequent ALK gene rearrangements (in inflammatory myofibroblastic tumor)
- Benign, although rare cases of malignant behavior have been reported
Essential features
- Inflammatory myofibroblastic tumor (IMT) occurs more often in childhood and in young adults and shows morphology similar to the adult form
- IMT in children is more often associated with ALK gene rearrangement than in adults
Terminology
- Renamed inflammatory myofibroblastic tumor (formerly inflammatory pseudotumor)
Epidemiology
- Most common lung tumor in children age 16 and younger
Sites
- Lung, mediastinum, trachea, bronchus, abdomen, orbit
Radiology description
- Solid masses with homogeneous or heterogeneous enhancement, may contain calcification (Pediatr Radiol 2015;45:1672)
Case reports
- 3 year old child with cough and recurrent respiratory infections (Diagn Pathol 2012;7:83)
- 5 year old girl with recurrent pyrexia, dry cough and shortness of breath (Interact Cardiovasc Thorac Surg 2010;10:805)
Treatment
- Excision or radiation therapy
Gross description
- Solitary, small peripheral nodules, yellow, firm, covered by intact pleura or polypoid bronchial mass
Gross images
Images hosted on other servers:
Microscopic (histologic) description
- Plasma cells (often abundant), lymphocytes, histiocytes and myofibroblasts
- May have vascular proliferation, collagenous or hyalinized stroma, myxoid change, xanthoma cells, hemosiderin
- May resemble nodular fasciitis, fibrous histiocytoma or fibromatosis
Microscopic (histologic) images
Images hosted on other servers:
Molecular / cytogenetics description
- More often associated with ALK rearrangement than adult cases