Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Hrones M, Tsang P. Acute lymphoblastic leukemia / lymphoma . PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphnodesALL.html. Accessed April 24th, 2024.
Definition / general
- Highly aggressive neoplasm of precursor lymphoid (blast) cells
- 2 main subtypes based on lymphoid lineage
- B lymphoblastic leukemia / lymphoma (B-ALL / LBL)
- T lymphoblastic leukemia / lymphoma (T-ALL / LBL)
Essential features
- Characterized by neoplastic proliferation of clonal precursor B cells or T cells that typically have blastic cytomorphology
- Lymphoblastic lymphoma generally refers to tissue mass lesion, while leukemia refers to bone marrow or blood involvement
- B-ALL / LBL has a better prognosis in children than in adults
- Childhood B lineage ALL / LBL has a better prognosis than childhood T lineage ALL / LBL
- Immature cell markers, such as CD34 and TdT, can help to differentiate lymphoblasts from Burkitt lymphoma which, is considered a mature high grade B cell lymphoma that mimics lymphoblastic lymphoma / leukemia
Terminology
- Lymphoblastic leukemia / lymphoma
- Acute lymphoblastic leukemia (ALL)
- Precursor B cell lymphoblastic leukemia / lymphoma or precursor T lymphoblastic leukemia / lymphoma
- Separation of lymphoblastic leukemias and lymphomas based on a measure of disease burden / stage
- Cases with tissue involvement + replacement of < 25% marrow involvement by lymphoid blasts = lymphoblastic lymphoma (LBL)
- Cases with ≥ 25% marrow involvement = lymphoblastic leukemia
ICD coding
Epidemiology
- Estimated annual incidence in the United States approximately 1.6 cases per 100,000 population (Blood Cancer J 2017;7:e577)
- Approximately 75 - 80% of ALL / LBL occurs in children, while the remainder occurs as aggressive disease in adults (J Natl Compr Canc Netw 2015;13:1240)
- Among LBL, T cell > B cell phenotype (~90% versus 10%) (Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Medicine), 4th Edition, 2017)
- Among ALL, B cell > T cell phenotype
- Children: 85% versus 15%
- Adults: 75% versus 25%
- T-ALL / LBL:
- More common in adolescents than children
- M:F = 2.2:1
- B-ALL / LBL:
- Bimodal age distribution with highest peak in children < 6 years old
- Second lower incidence peak in adults > 65 years old (Eur J Cancer Care (Engl) 2005;14:53)
- Slight male predominance, M:F = 1.2 - 1.8:1
Sites
- Bone marrow, peripheral blood or nodal involvement
- Anterior mediastinum
- Particularly T-LBL
- Compromised cardiac function and superior vena cava syndrome may result
- Less frequent sites involved
- Skin
- Soft tissues
- Central nervous system
Pathophysiology
- Clonal proliferation of precursor B cells in the bone marrow
- Clonal proliferation of precursor T cells in the thymus (thymocytes)
- Specific sequential pathologic mechanisms that follow appear to be variable, based on the varying genetic aberrations identified
Etiology
- Idiopathic and likely multifactorial
- Chromosomal aberrations with gene mutations and rearrangements commonly cited
- Specific risk factors seen in a minority of patients:
- Prenatal radiation exposure (Xradiation)
- Postnatal exposure to high doses of radiation (e.g. therapeutic radiation was previously used for tinea capitis and thymus enlargement)
- Genetic conditions, such as Down syndrome, neurofibromatosis and ataxia telangiectasia
- Inherited genetic polymorphisms
- Reference: Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Medicine), 4th Edition, 2017
Clinical features
- B-ALL / LBL
- Common presentation of fever, fatigue, bone or joint pain, bleeding or anorexia (signs of bone marrow infiltration)
- May present with widespread lymphadenopathy, hepatosplenomegaly, involvement of skin, soft tissue and testes, with predilection for the central nervous system
- Mediastinal mass uncommon
- T-ALL / LBL
- Most commonly presents with anterior mediastinal mass with or without superior vena cava syndrome
- Lymph node involvement on Xray / CT / MRI showing thymic enlargement or pleural effusion, resulting in symptoms of respiratory distress
- Generalized lymphadenopathy and hepatosplenomegaly also frequent
- Reference: Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Medicine), 4th Edition, 2017
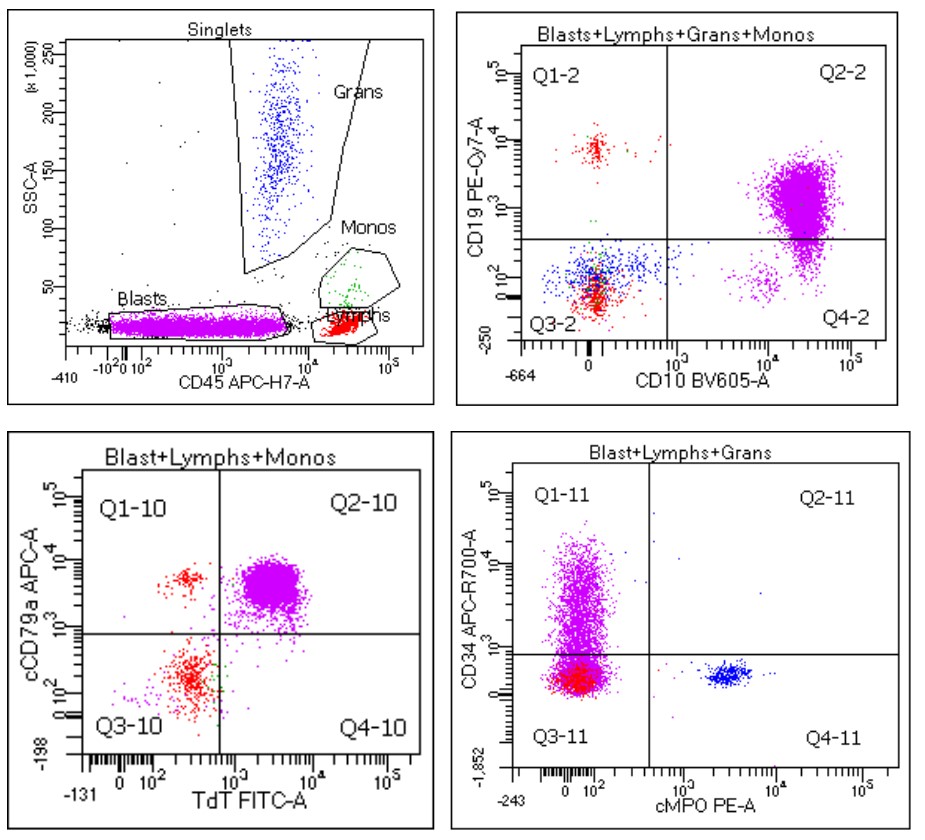
Diagnosis
- Multimodal pathologic evaluation with some combination of morphology, flow cytometry, immunohistochemistry, cytogenetics, FISH, PCR, NGS and basic clinical laboratory tests
- Nodal excisional biopsy may be the initial diagnostic procedure, although marrow involvement is frequently present simultaneously
- If marrow involvement suspected, initial approach may consist of complete blood count and peripheral blood smear, followed by bone marrow aspiration and biopsy
Laboratory
- Complete blood count
- Almost universally shows anemia and thrombocytopenia
- ~25% have severely decreased platelet count (< 20,000/uL)
- Total white blood cell (WBC) count variable but most commonly presents with neutropenia
- 50% show WBC count < 10,000/uL
- 30% show WBC count 5,000 - 50,000/uL
- 20% show WBC count > 50,000/uL
- 20% show preleukemic state with cytopenia < 1 year before overt leukemia
- Rare reports of asymptomatic hypereosinophilia (Cancer 1984;54:1058), most commonly seen in the setting of t(5;14) (q31;q32) or relatively less common recurrent abnormalities, such as translocation t(7;12)(q22;p13); also reported in the setting of hyperdiploidy (Blood 2020;135:974, Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Medicine), 4th Edition, 2017)
- Almost universally shows anemia and thrombocytopenia
Radiology description
- Lymphadenopathy
- T-LBL may show an anterior mediastinal mass due to thymic enlargement or presence of pleural effusion on Xray
Prognostic factors
- Prognosis generally determined by lineage (B cell or T cell phenotype), plus cytogenetic or molecular abnormalities detected
- B-ALL / LBL
- Unfavorable prognostic factors:
- Infancy and adult age of diagnosis
- High white blood cell count
- Slow response to initial therapy
- Central nervous system involvement at the time of diagnosis
- Minimal residual disease after therapy
- Unfavorable prognostic factors:
- T-ALL / LBL
- Worse prognosis than B-ALL / LBL among pediatric population
- Better prognosis than B-ALL / LBL among adult population
- Reference: Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Medicine), 4th Edition, 2017
Case reports
- 2 year old boy with no significant medical history presented with lower extremity pain and fever (Ochsner J 2019;19:260)
- 31 year old man with unprecedented medical history who presented with constant back pain not responsive to pain meds, night sweats and fever (Stem Cell Investig 2019;6:17)
- 42 year old woman who presented with a 1 month history of paleness and fatigue (BMC Cancer 2018;18:1143)
- 44 year old woman with a secondary thymoma after chemotherapy treatment for T acute lymphoblastic leukaemia / lymphoma (Open Access Maced J Med Sci 2018;6:2373)
- 51 year old man presented with an increase in superficial lymph nodes (Medicine (Baltimore) 2018;97:e12743)
Treatment
- Multiagent chemotherapeutic regimen stratified based on epidemiology, presentation and other patient specific associated characteristics of the disease, as well as presence of specific cytogenetic abnormalities and therapeutic response to initial induction (Pediatr Int 2018;60:4)
- Intrathecal prophylactic therapy and radiation for those presenting with central nervous system involvement (Biol Blood Marrow Transplant 2016;22:575)
- Allogeneic stem cell / bone marrow transplantation in those demonstrating high risk findings (Am J Hematol 2017;92:18)
- Targeted tyrosine kinase inhibitors or JAK inhibitors may be used in patients with Ph-like ALL (Best Pract Res Clin Haematol 2018;31:351)
- CAR-T cell immunotherapy in relapsed patients (Curr Hematol Malig Rep 2018;13:396)
- Supportive therapy for cytopenias
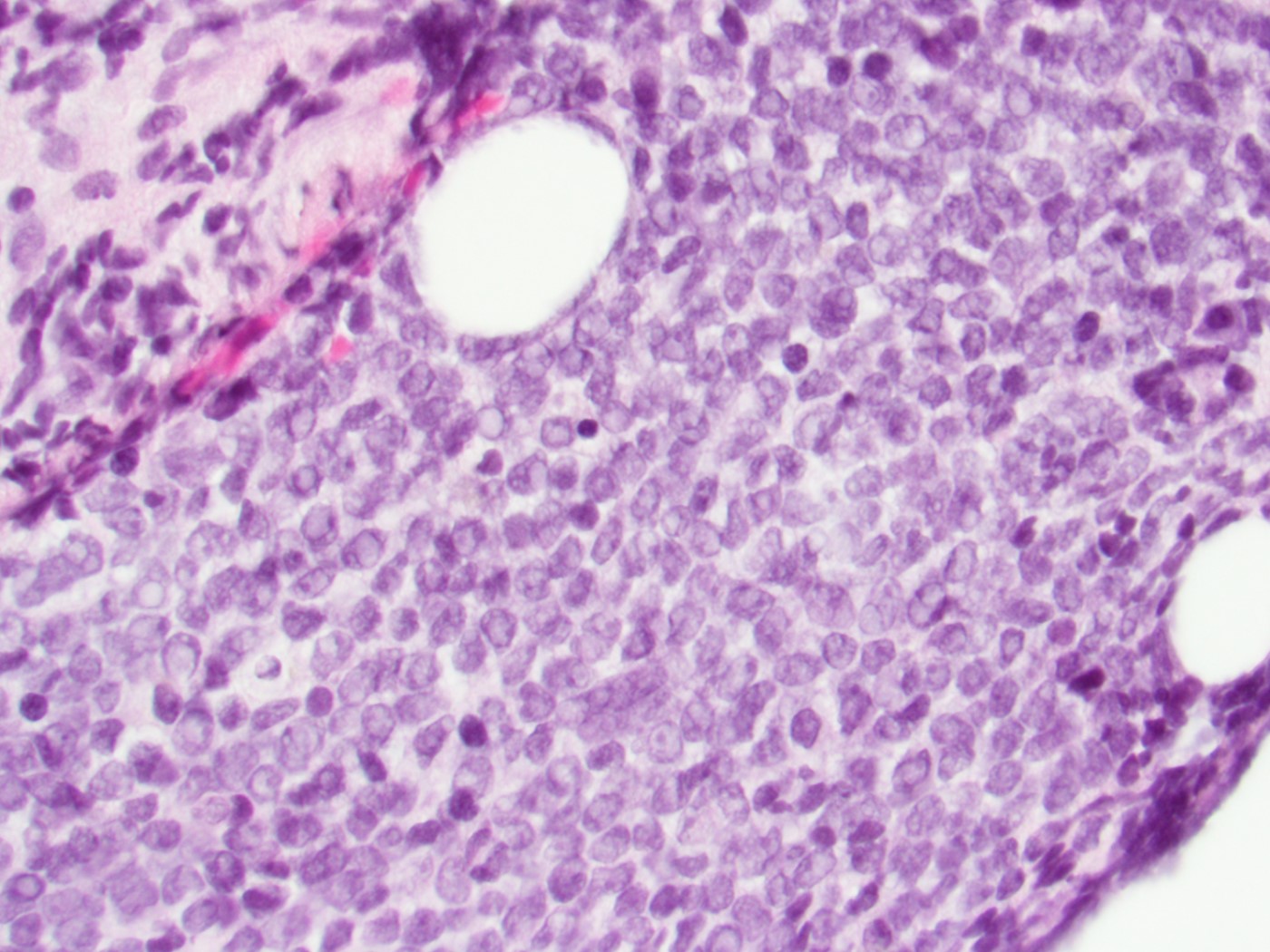
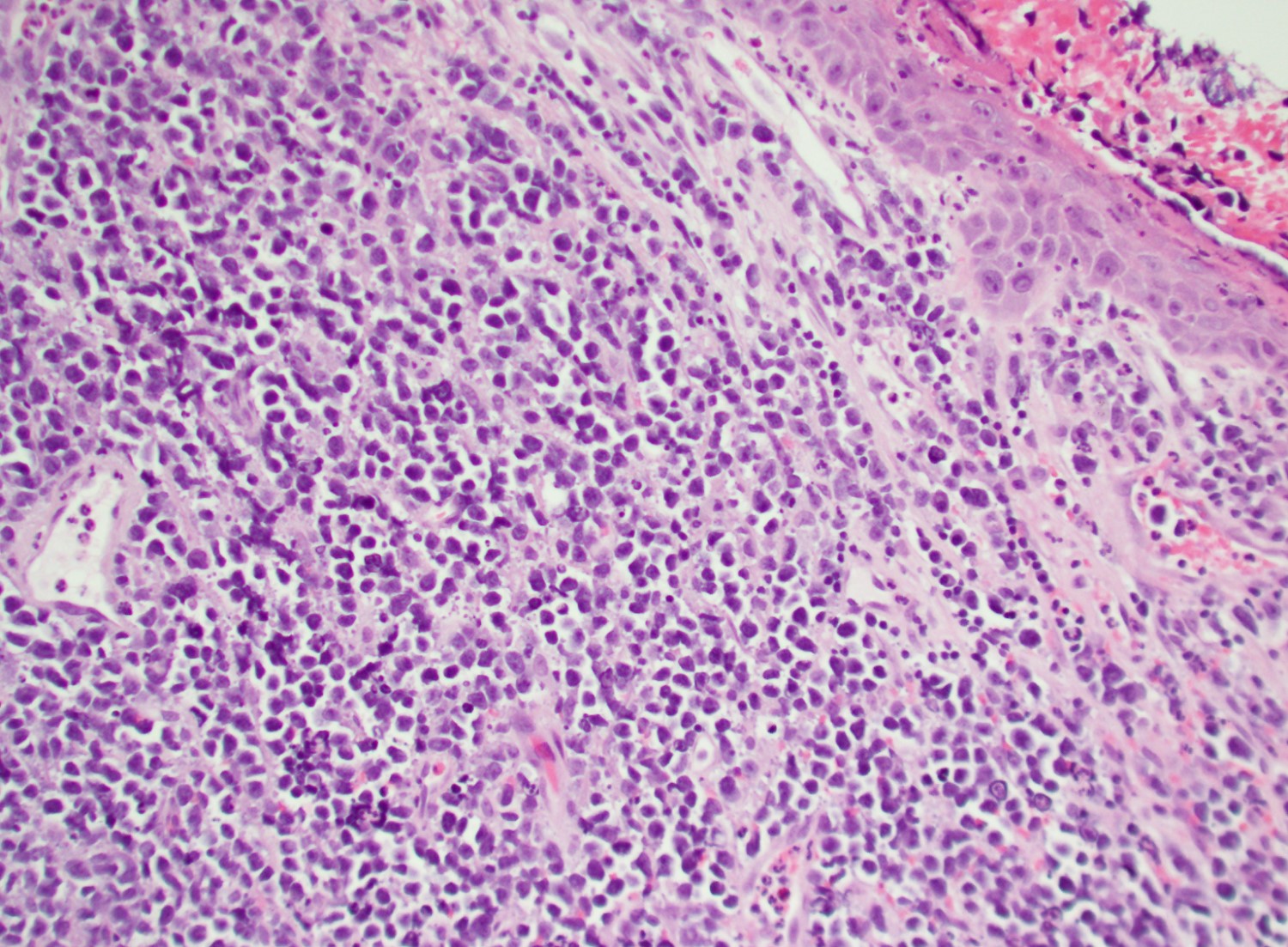
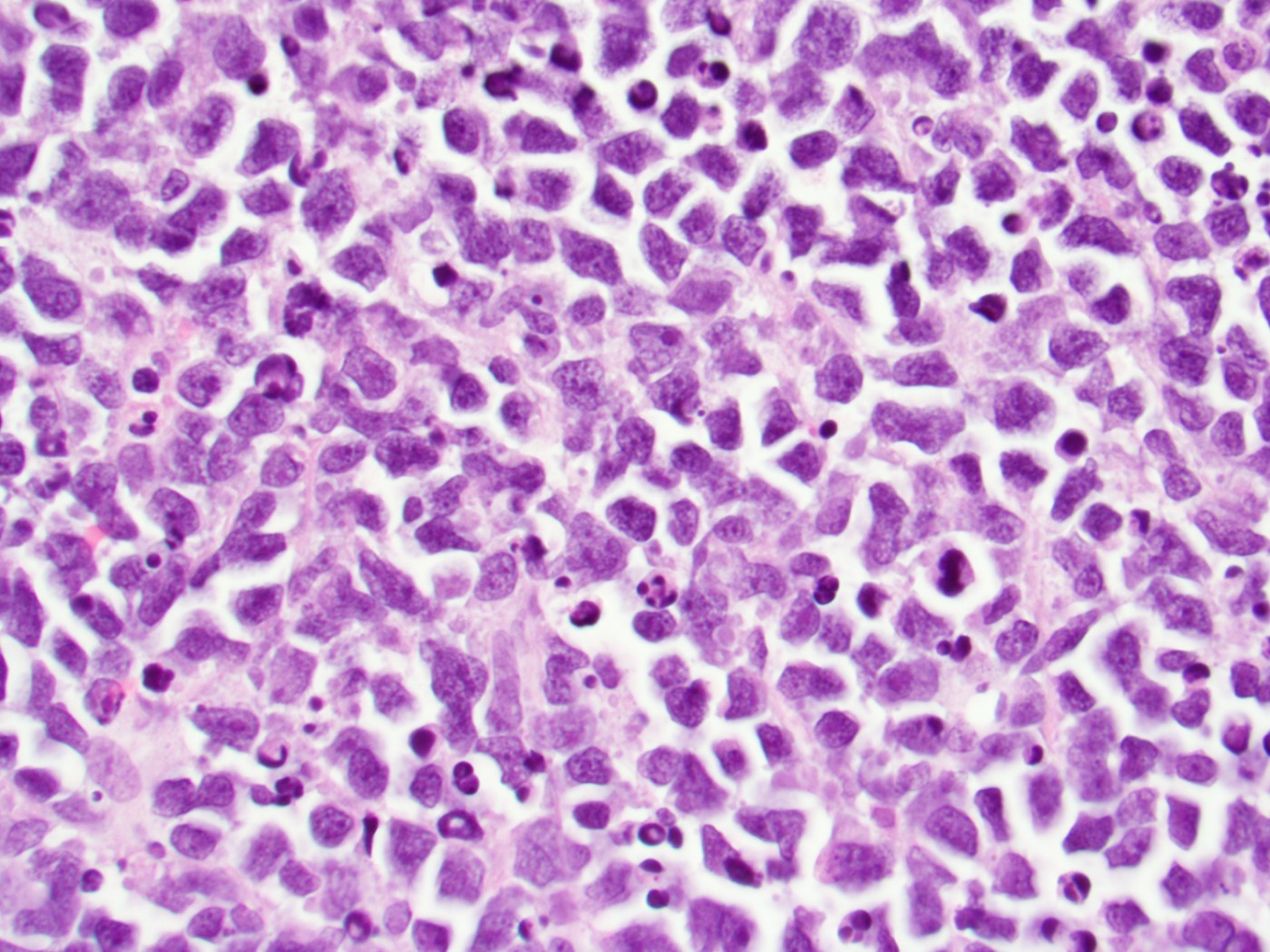
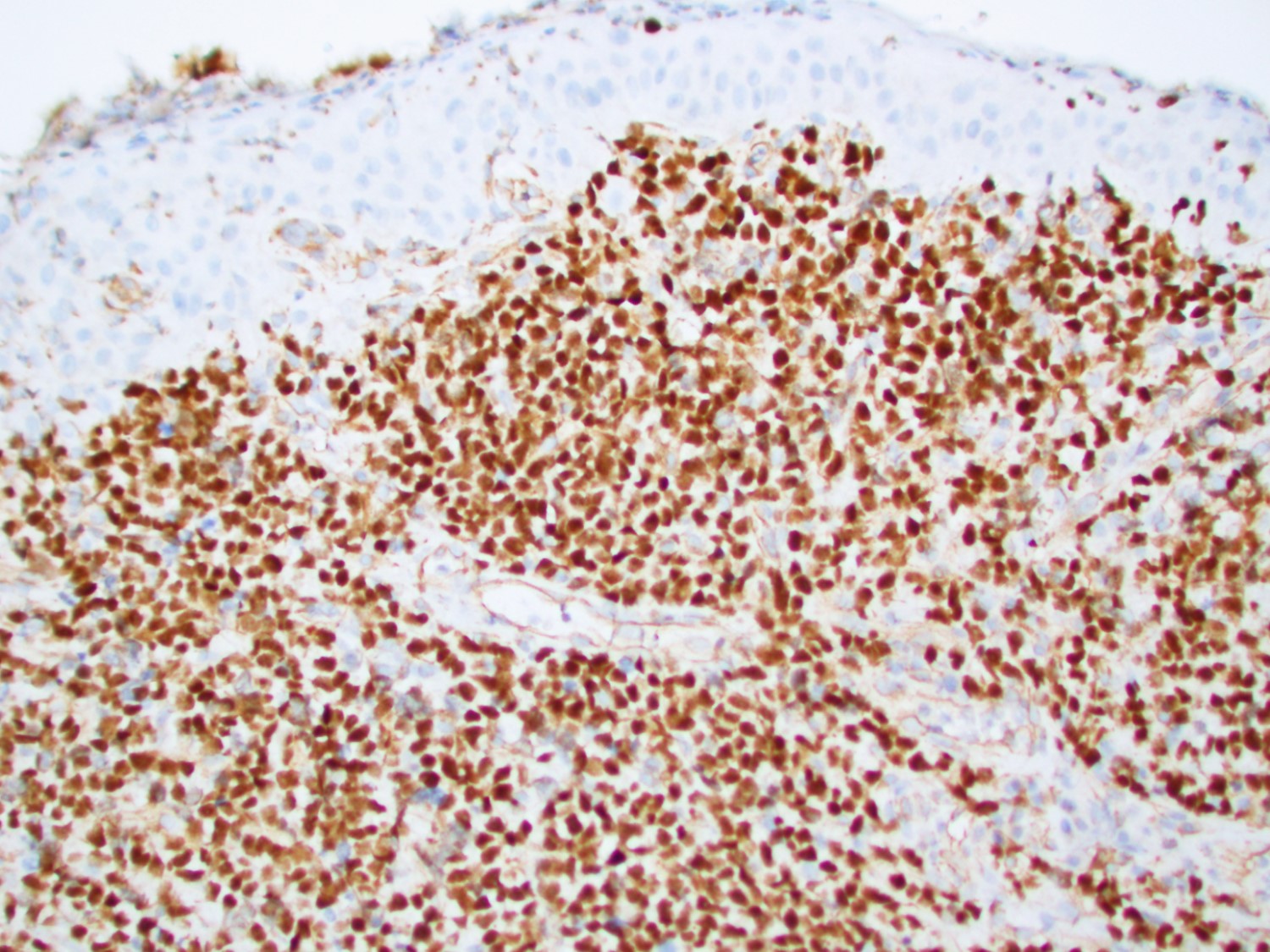
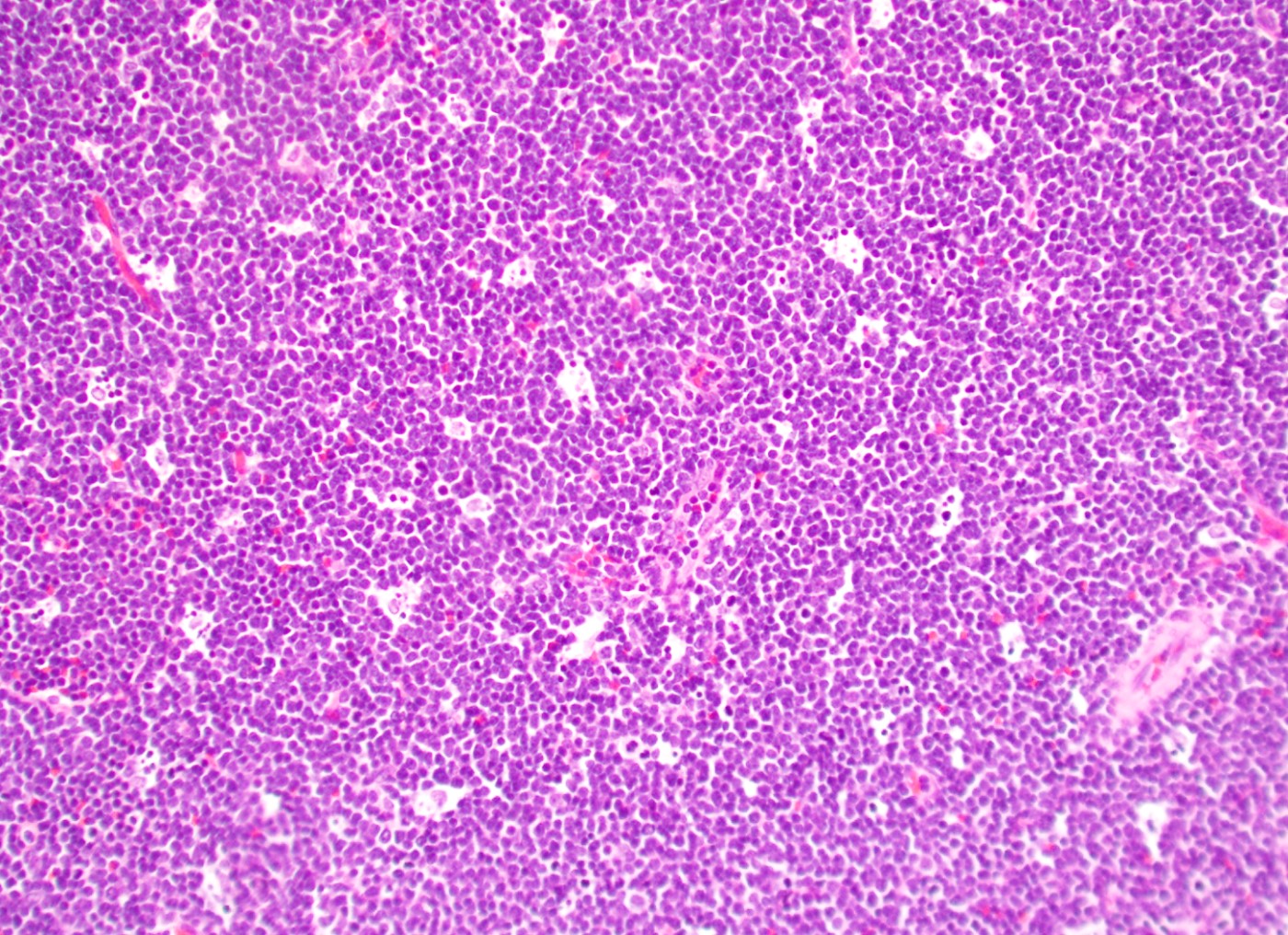
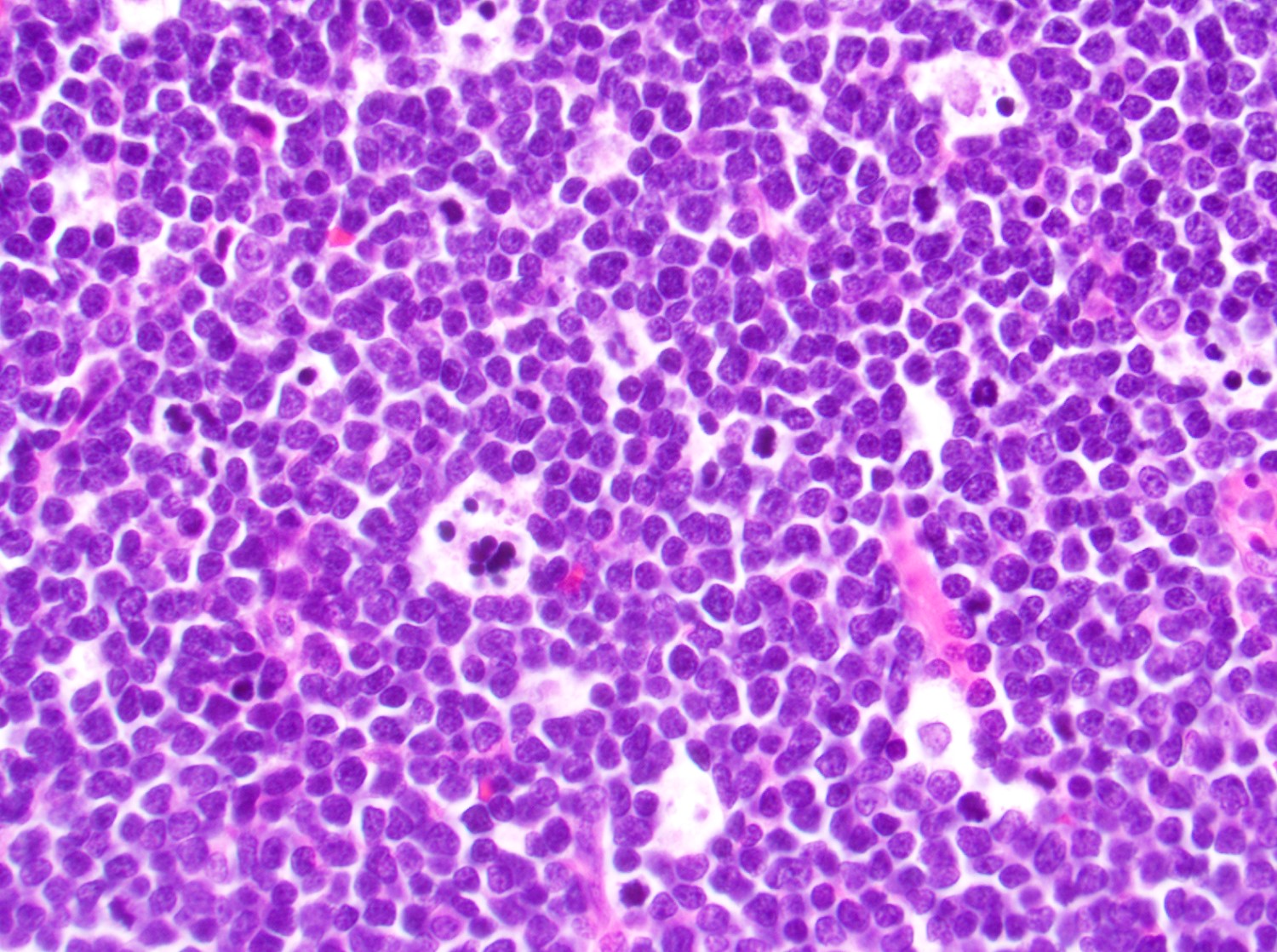
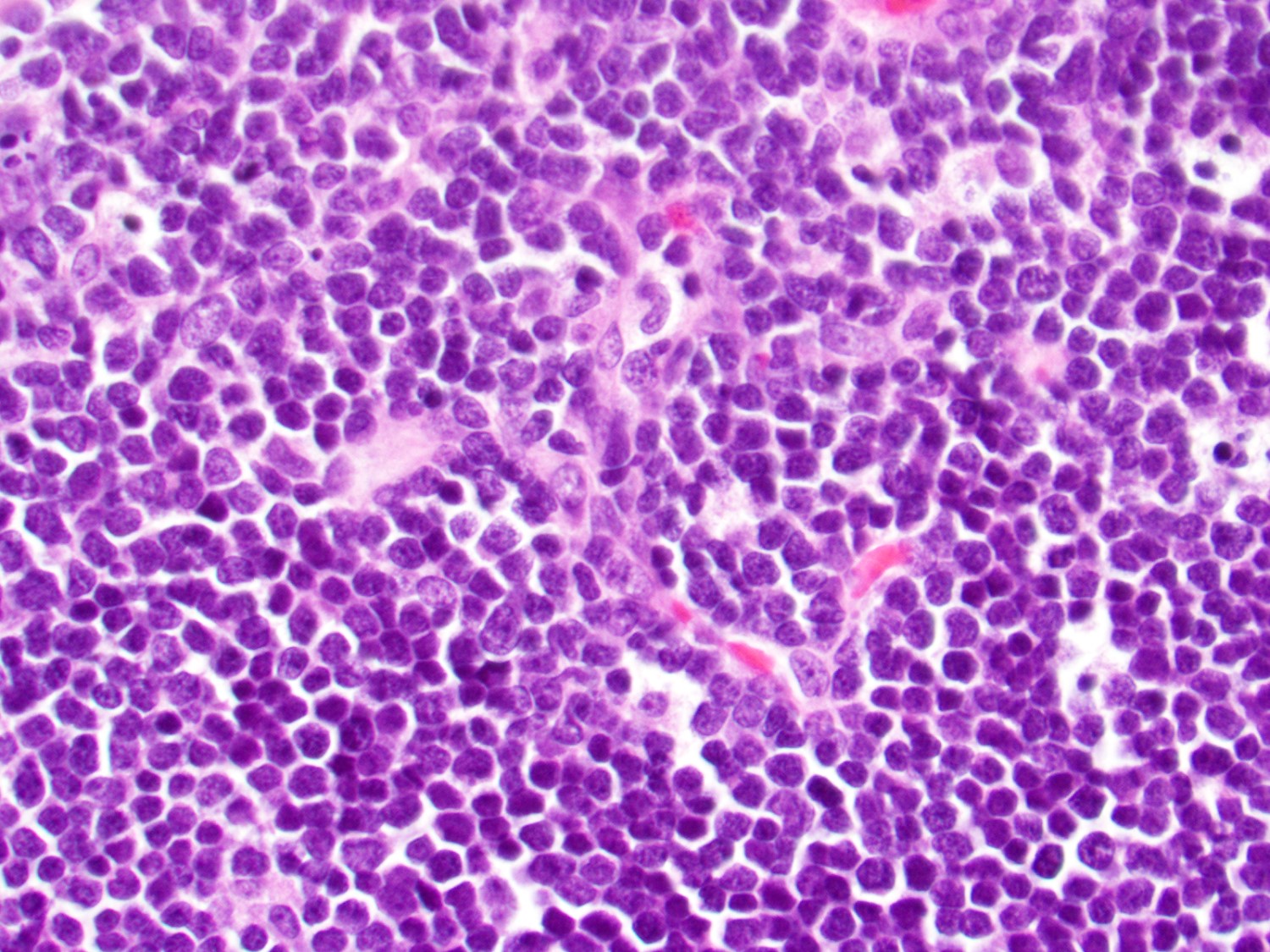
Microscopic (histologic) description
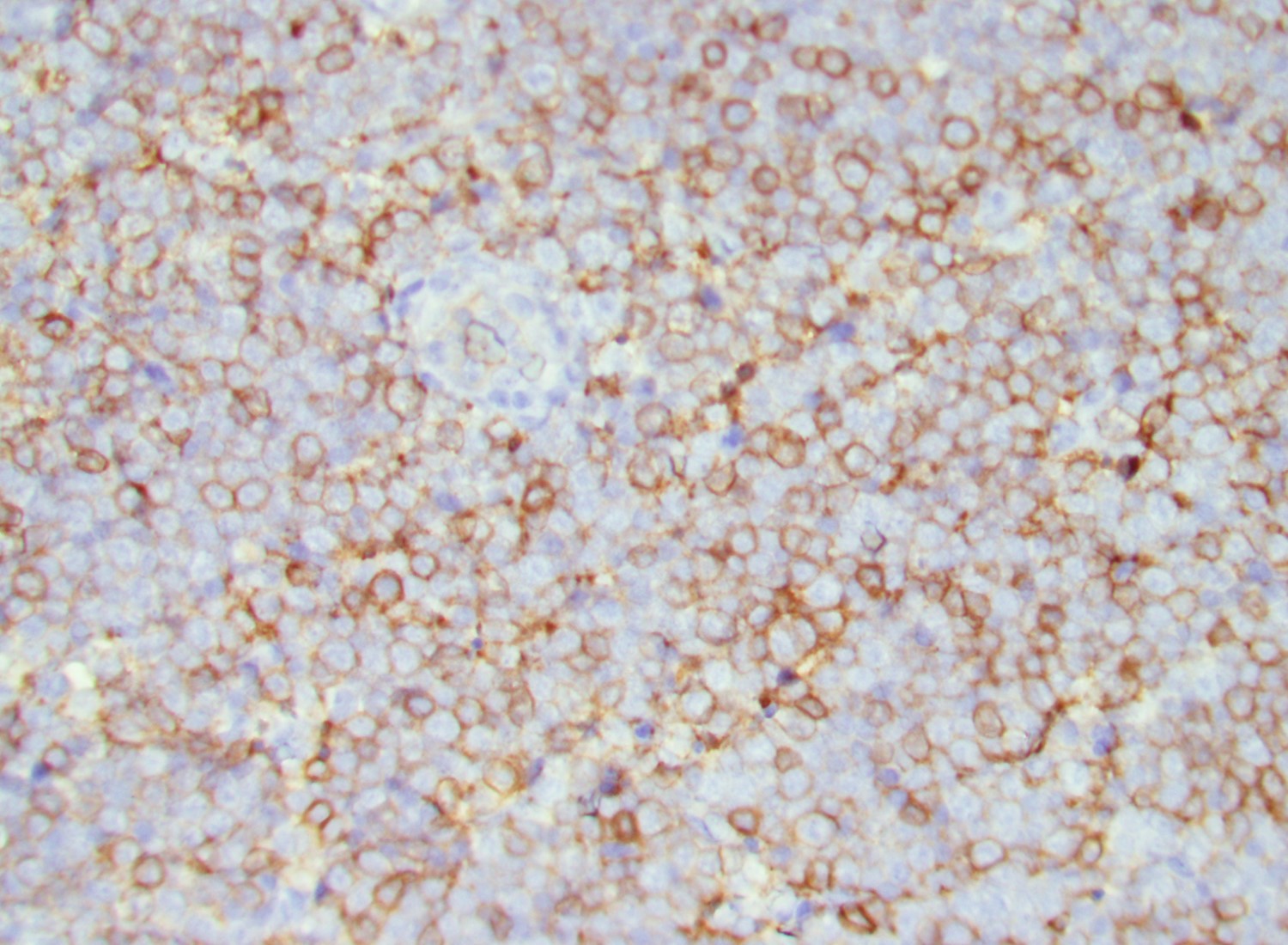
- In tissue masses or marrow, effacement of architecture by round blue cells raises the differential diagnoses of ALL / LBL versus neuroblastoma, Ewing sarcoma and other mimickers
- Proliferation of typically small to medium sized primitive cells but some tumor cells may appear as larger blasts containing cytoplasmic vacuoles
- B and T cell precursors are morphologically indistinguishable
- In nodal involvement, blasts usually display a diffuse growth pattern
- Partial nodal involvement of T-LBL may have a predominantly interfollicular distribution
- Lymphoblasts may be oval in shape and display an indented nucleus with finely dispersed chromatin and inconspicuous nucleoli
- Revised criteria to the original French American British (FAB) classification of ALL cytologic subtypes
- L1 and L2 subtype segregation not shown to correlate with clinical or biologic behavior; thus, most commonly used for descriptive purpose at this time
- L1 subtype: most common, with intermediate size, uniform features, scant basophilic cytoplasm, slightly condensed chromatin with inconspicuous / absent nucleoli
- L2 subtype: more heterogeneous appearance, with slightly more cytoplasm and more prominent nucleoli, resembling myeloblasts
- L3 subtype: now corresponds to leukemic phase of Burkitt lymphoma, a high grade mature B cell lymphoma (Blood Cancer J 2017;7:e577)
- L1 and L2 subtype segregation not shown to correlate with clinical or biologic behavior; thus, most commonly used for descriptive purpose at this time
Microscopic (histologic) images
Cytology description
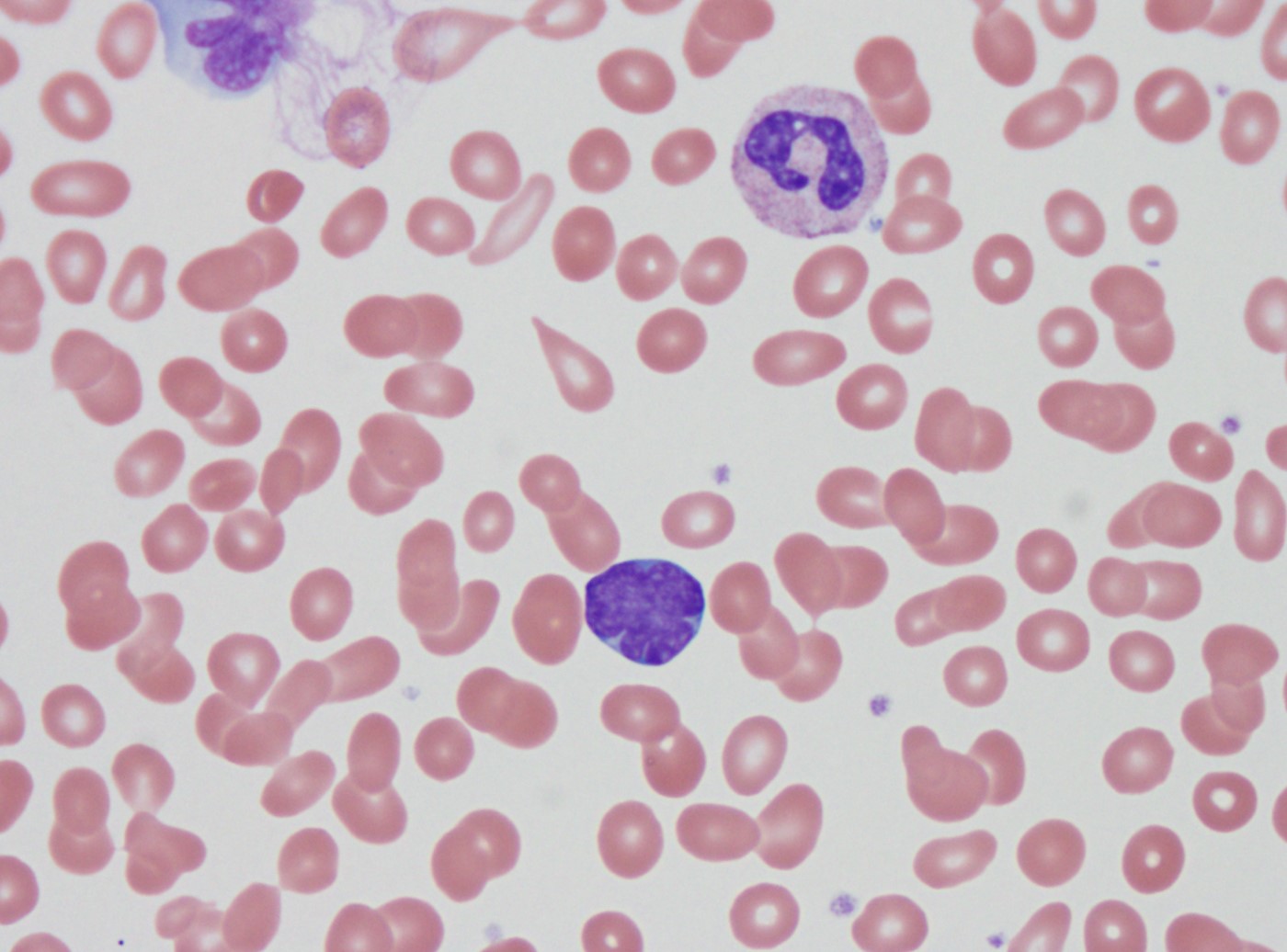
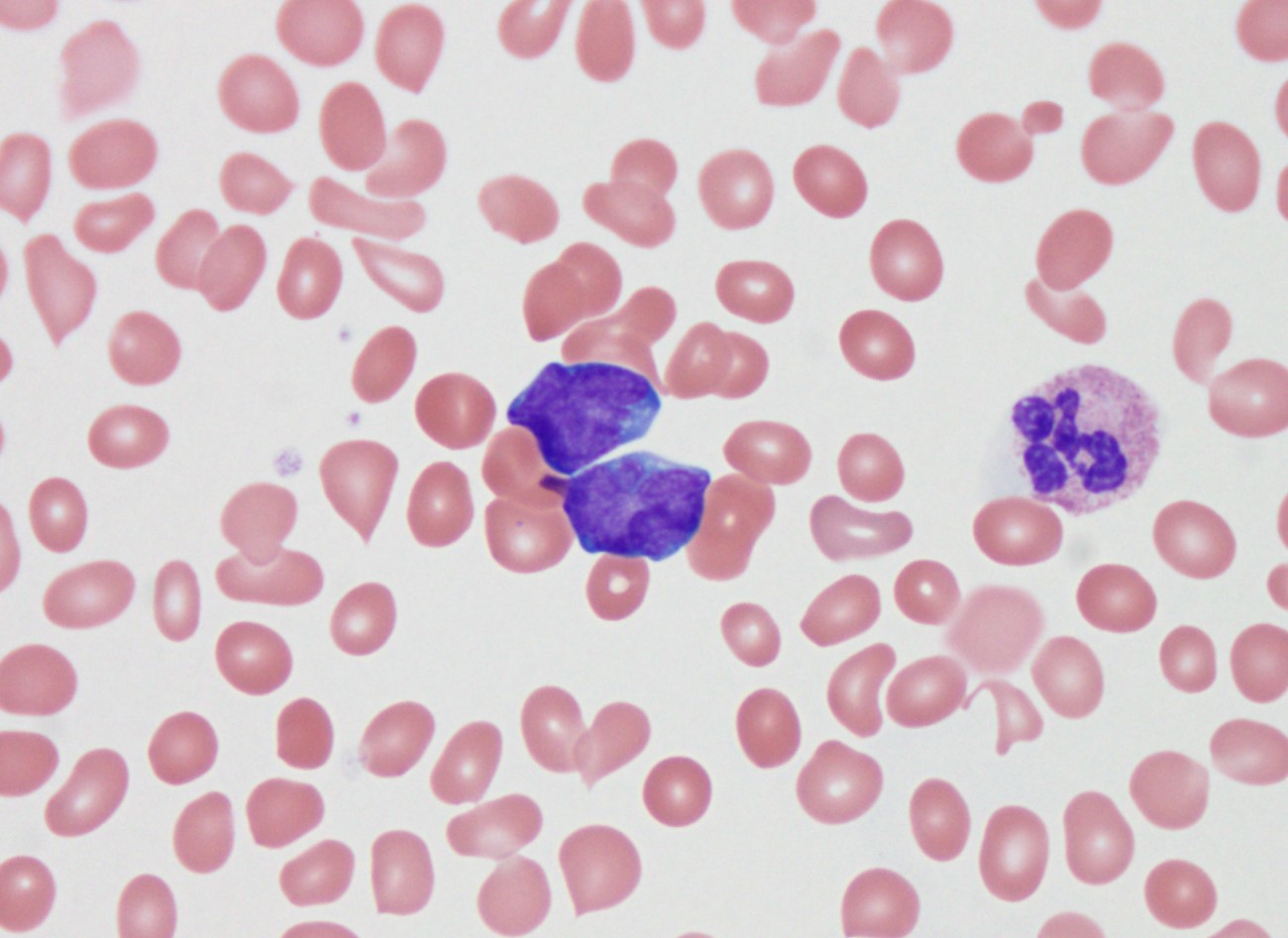
- Lymphoblasts may have variable cell sizes and typically show a high N/C ratio
- May range from small blasts with a condensed chromatin pattern and inconspicuous nucleoli to larger blasts with an open chromatin pattern, prominent nucleoli and bluish cytoplasm
- Reference: Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Medicine), 4th Edition, 2017
Peripheral smear description
- Predominance of lymphoblasts
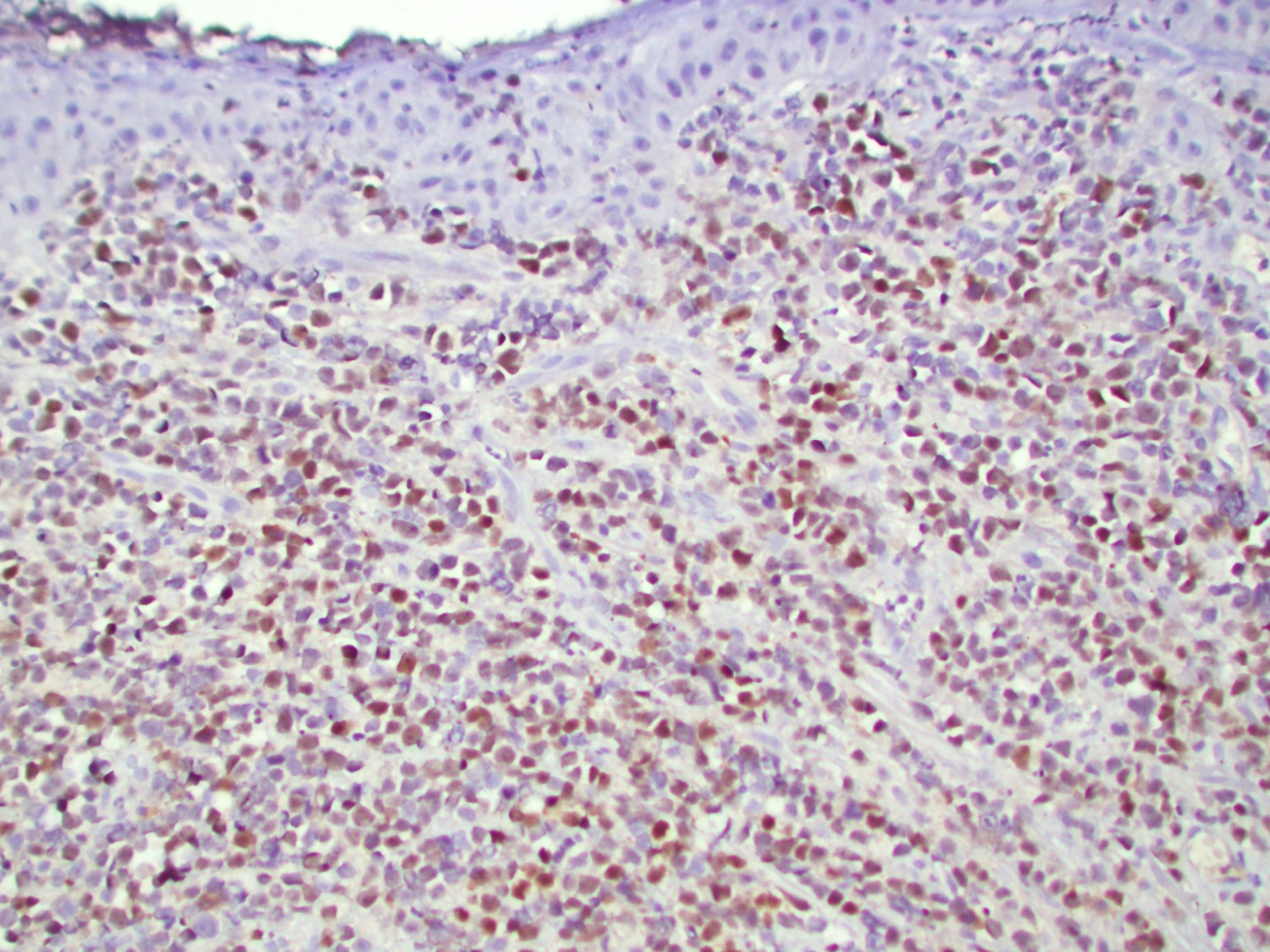
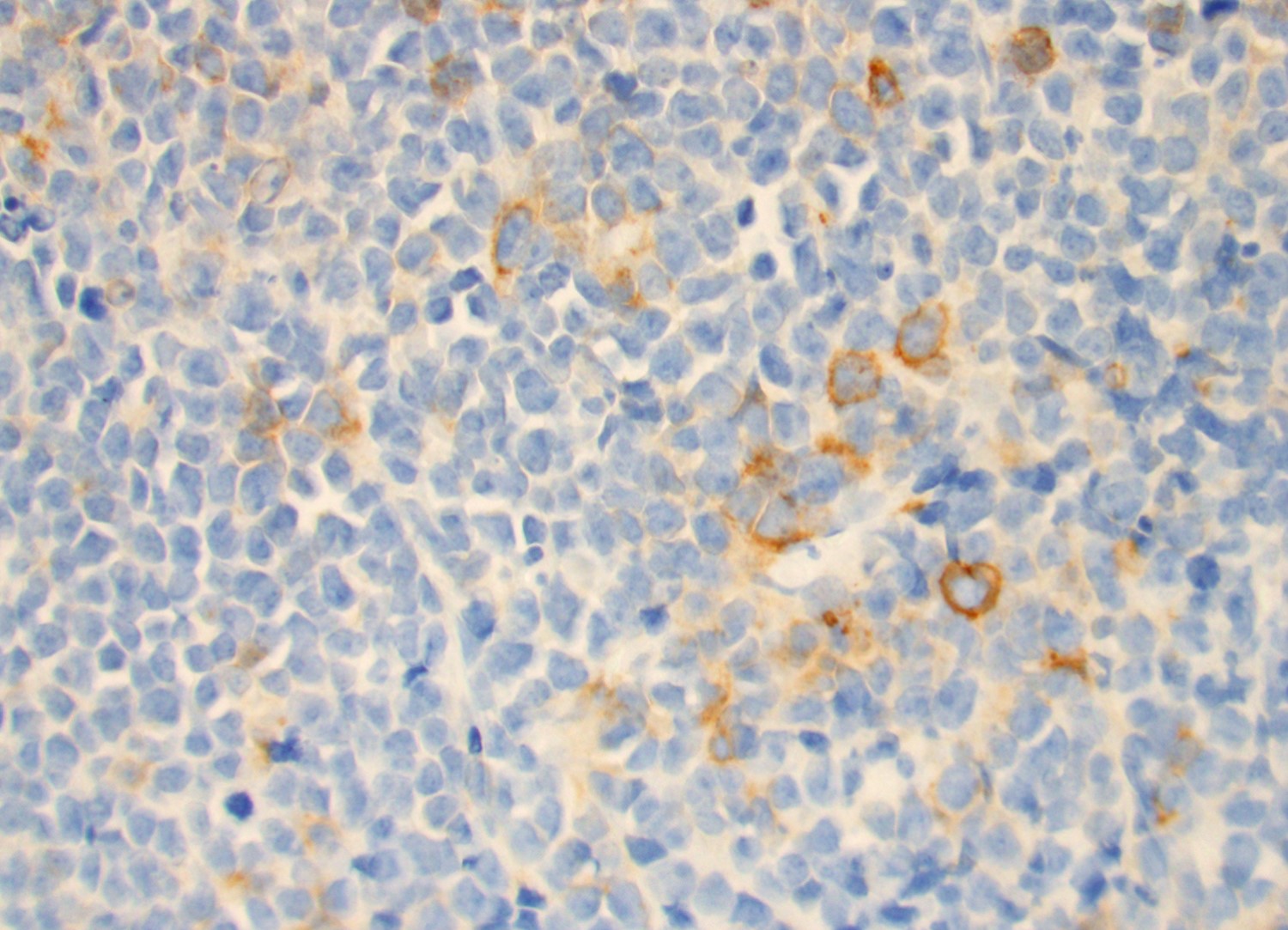
Positive stains
- B-ALL / LBL
- T-ALL / LBL
- Reference: Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Medicine), 4th Edition, 2017
Negative stains
- Myeloperoxidase and lysozyme should not be expressed
- B-ALL / LBL: surface immunoglobulin is usually absent
Molecular / cytogenetics description
- Most B-ALL show clonal IgH (immunoglobulin heavy locus) gene rearrangement; simultaneous T cell receptor (TCR) gene rearrangement present in up to 70%
- Recurrent genetic abnormalities in B-ALL often have prognostic significance:
- t(12;21) TEL-AML: very favorable prognosis; most common rearrangement in childhood B-ALL / LBL (25%), with cures of > 90%
- Hyperdiploidy: favorable prognosis; trisomies 4, 10 and 17 have the best prognosis
- t(9;22) BCR-ABL1 (Philadelphia chromosome): very poor prognosis; incidence increases with age, 3% of childhood cases and 25% of adult
- t(v;11q23) MLL gene rearrangement: poor prognosis
- Hypodiploidy: poor prognosis
- BCR-ABL1-like IGH-CRLF2 translocation (and less commonly EPOR): CRLF2 translocation frequent in Down syndrome; standard risk in children and high risk in adolescents and adults; more frequent in Hispanics and Native Americans
- t(5;14) IL3-IGH: uncertain prognostic significance; reactive eosinophilia is characteristic
- Most T-ALL / LBL harbor T cell receptor gene rearrangement; simultaneous IGH gene rearrangement present in about 20%
- In T-ALL / LBL, recurrent cytogenetic abnormalities can also be seen:
- TCR loci at 14q11.2, 7p35 and 7p14-15, with various partner genes
- Del (9p) with loss of tumor suppressor gene CDKN2A occurs in at least 30%
- NOTCH1 gene mutations can lead to shorter survival in adult T-ALL / LBL
- Reference: Swerdlow: WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (Medicine), 4th Edition, 2017
Sample pathology report
- Base of tongue, biopsy:
- B lymphoblastic lymphoma, not otherwise specified (see microscopic description and FISH)
- Microscopic description: Biopsy of the tongue shows benign squamous mucosa with an extensive and diffuse subepithelial infiltrate of medium sized to large neoplastic cells, some containing a prominent nucleolus. Mitoses and apoptotic bodies are readily observed. Immunohistochemistry demonstrates that the neoplastic cells are of precursor B cell origin (CD45+, PAX5+, CD79a+, TdT+, CD10+, CD43+, CD34 subset +, CD20 few scattered cells +). Not expressed are CD117, CD3, CD30, myeloperoxidase, lysozyme, EBV (EBER) and MYC. Proliferative fraction is > 95% based on Ki67.
- FISH: Interphase FISH analysis performed on formalin fixed paraffin embedded tissue shows the following results
- Positive for tetraploidy, consistent with a favorable prognostic implication in B-ALL / LBL.
- Negative for MYC-IGH t(8;14), BCR-ABL t(9;22), BCL2 rearrangement and BCL6 rearrangement.
- No specific genetic abnormalities were identified in the FISH ALL panel to fulfill the diagnostic criteria of B-ALL/LBL with recurrent genetic abnormalities.
Differential diagnosis
- Burkitt lymphoma, a high grade B cell lymphoma:
- Hematogones, non-clonal B cell precursors in bone marrow, can be confused with B-ALL, especially in minimal residual disease:
- Typically smaller than lymphoblasts
- Have characteristic immunophenotypic expression pattern by flow cytometry
- Thymoma with reactive thymic T cells exhibiting CD4+ CD8+ immature T cell phenotype, resembling T-LBL in anterior mediastinal mass:
- Immunohistochemistry highlights the neoplastic epithelial cell component
- Lobulated architecture
- May be associated with myasthenia gravis in adults
- Incidental indolent T lymphoproliferative proliferations (Cytometry B Clin Cytom 2020;98:282)
Additional references
Board review style question #1
A 4 year old boy is brought to the physician by his mother due to a 5 week history of lethargy, a progressively enlarging left sided neck mass and a 5 day onset of unexplainable bilateral diffuse lower leg petechial hemorrhage. On exam, the left posterior cervical lymph node is enlarged and nontender to palpation. A lymph node biopsy shows a predominance of interfollicular infiltrate containing numerous blast cells and focal necrosis. Immunohistochemistry is positive for PAX5, CD10 and TdT. Upon further workup, which of the following translocations would be associated with a good prognosis?
- t(12;21)
- t(14;18)
- t(8;14)
- t(9;22)
Board review style answer #1
Board review style question #2
A 58 year old Hispanic woman comes to the physician because of a 2 month history of bilateral axillary lymphadenopathy and a new onset right breast mass at 12 o'clock. The patient has a previous history significant for Down syndrome and a diagnosis of acute lymphoblastic leukemia 20 years ago for which she received treatment and has since been free of disease. Needle core biopsy of the breast mass reveals extensive involvement by monotonous medium sized lymphoid cells with a high N/C ratio and scant cytoplasm, consistent with a diagnosis of B lymphoblastic lymphoma. Which of the following genetic mutations is most likely detected in blast cells?
- BCR-ABL1
- CRLF2
- ETV6-RUNX1
- FLT3
Board review style answer #2