Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Etiology | Clinical features | Clinical variants | Diagnosis | Laboratory | Laboratory images | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Minimal residual disease (MRD) testing | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Terzioglu M, Crane GM. Plasma cell myeloma (multiple myeloma). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomamyeloma.html. Accessed April 25th, 2024.
Definition / general
- Bone marrow based, multifocal plasma cell neoplasm usually associated with a monoclonal immunoglobulin (M protein) in serum or urine and evidence of organ damage related to the plasma cell neoplasm (J Natl Compr Canc Netw 2019;17:1154)
Essential features
- Diagnosis requires synthesis of clinical, laboratory, radiologic and histologic findings
- Assessment of serum or urine M protein, clonal plasma cells in bone marrow and presence of end organ damage related to the plasma cell neoplasm critical for diagnosis
Terminology
- Multiple myeloma
- Plasma cell myeloma
- Myeloma
- Medullary plasmacytoma
- Myelomatosis
- Kahler disease (no longer used) (Recent Results Cancer Res 2011;183:3)
- Smoldering (asymptomatic) plasma cell myeloma
- Nonsecretory myeloma
- Plasma cell leukemia
ICD coding
Epidemiology
- 1.8% of malignant tumor diagnoses in the U.S. annually, 19% of hematopoietic neoplasms, 21% of deaths from hematopoietic malignancies (CA Cancer J Clin 2021;71:7)
- M:F = 1.2:1
- Twice as frequent in African Americans compared to Caucasians
- Not seen in children, rarely seen in adults under 30; median age of diagnosis is ~70 years (Semin Oncol 2016;43:676, CA Cancer J Clin 2021;71:7)
- Potential genetic predisposition, relative risk of 2.1 to develop multiple myeloma among those with an affected first degree relative (Int J Cancer 2009;125:2147, Leukemia 2020;34:697)
Pathophysiology
- Bone marrow site of origin for nearly all cases
- Interactions between bone marrow stroma and neoplastic plasma cells directly influences disease with a potential key role of IL6 to support survival and expansion of myeloma cells (Leukemia 2014;28:1647, Cancer 2003;97:2440, Cancers (Basel) 2021;13:216)
- Role of IL6 and other cytokines in promoting osteoclastic activity and lytic bone lesions
- Multiple myeloma cells suppress the differentiation and proliferation of osteoblasts while inducing osteoclast differentiation and hyperfunction (Cancers (Basel) 2021;13:216)
Etiology
- Chronic antigen stimulation, exposure to radiation or toxins may result in increased risk; however, most patients do not have these associated factors (Cancer 1993;72:2148, Occup Environ Med 2011;68:391)
- Association with radiation may be weak and not identified in some studies (PLoS One 2016;11:e0162710)
- Almost all cases appear to arise in patients with precursor monoclonal gammopathy of undetermined significance (MGUS) (Blood 2009;113:5418)
Clinical features
- Bone disease is the most frequent disease defining clinical feature (Am Soc Clin Oncol Educ Book 2018;38:638)
- Often presents with bone pain due to lytic bone lesions (thoracic vertebrae most common, also ribs, skull, shoulders, pelvis and long bones); spinal cord compression or peripheral neuropathy are less common presenting symptoms
- Renal failure / elevated creatinine / hyperuricemia (monoclonal light chain proteinuria results in renal tubular damage); hypercalcemia; hypoalbuminemia
- Recurrent infections due to impaired humoral immunity (immunoglobulin production, often < 50% normal)
- Anemia (bone marrow infiltration often in areas of most active hematopoiesis and renal failure causing loss of erythropoietin)
- Extramedullary involvement generally associated with advanced disease
- Historical fact: Bence Jones proteins were the first tumor marker (Clin Kidney J 2012;5:478)
Clinical variants
- Smoldering (asymptomatic):
- More likely to progress to symptomatic myeloma than monoclonal gammopathy of uncertain significance (MGUS)
- Both show gammopathy without myeloma defining events (hypercalcemia, anemia, bone lesions and renal insufficiency)
- Risk of progression 10% per year for the first 5 years
- Lower risk if no progression in the first 5 years after diagnosis (N Engl J Med 2007;356:2582)
- Nonsecretory myeloma (~1% of cases):
- Serum protein electrophoresis (SPE) / immunofixation electrophoresis (IFE) negative, 85% with impaired secretion and have cytoplasmic immunoglobulin (Ig) by IHC (Am J Clin Pathol 2011;136:168)
- 15% nonproducers, serum free light chain may still be detected
- Lower incidence of renal insufficiency, hypercalcemia and depression of normal IgG
- Must be distinguished from the rare IgD and IgE myelomas
- Plasma cell leukemia:
- Typically aggressive with short survival < 1 year
- > 2 x 109/L or 20% of the leukocyte count on differential are monoclonal plasma cells
- Primary plasma cell leukemia (0.6% of myeloma) or develop as a late stage transformation (secondary) (Curr Oncol Rep 2019;21:8)
- Usually lack CD56 (80% of PCLs), more frequent high risk genetic findings
- Bone pain and osteolytic lesions less common
- Typically associated with extramedullary lesions (e.g., body cavity effusions, lymphadenopathy and organomegaly)
Diagnosis
- Division into these categories will guide plan for therapy (Am Soc Clin Oncol Educ Book 2016;35:e418):
- Multiple (symptomatic) myeloma (J Natl Compr Canc Netw 2019;17:1154):
- Clonal bone marrow plasma cells ≥ 10% or biopsy proven bony or extramedullary plasmacytoma
- And ≥ 1 of the following myeloma defining events:
- Calcium > 1 mg/dL above upper limit of normal or > 11 mg/dL
- Renal insufficiency*; creatinine > 2 mg/dL or creatinine clearance < 40 mL/min (preferred)
- Hemoglobin < 10 g/dL or > 2 g/dL below the lower limit of normal
- 1 or more osteolytic bone lesions on skeletal radiography, CT or FDG PET / CT
- Clonal bone marrow plasma cells ≥ 60% of bone marrow cellularity
- Involved / uninvolved serum free light chain (FLC) ratio ≥ 100 and involved free light chain concentration 10 mg/dL or higher
- > 1 focal lesion on MRI studies ≥ 5 mm
- These include traditional CRAB features associated with end organ damage (hypercalcemia, renal failure, anemia and osteolytic bone lesions) and biomarkers associated with ~80% risk of progression to end organ damage (≥ 60% clonal plasma cells in bone marrow, serum FLC ≥ 100 with FLC level ≥ 10 mg/dL or > 1 focal lesion by MRI) (Am Soc Clin Oncol Educ Book 2016;35:e418)
- *Only suspected or proven light chain cast nephropathy is considered a multiple myeloma defining event, consider renal biopsy to clarify if FLC < 500 mg/L (Am Soc Clin Oncol Educ Book 2016;35:e418)
- Smoldering (asymptomatic) myeloma (J Natl Compr Canc Netw 2019;17:1154):
- M protein in serum (IgG or IgA) at ≥ 3 g/dL
- Or Bence Jones protein ≥ 500 mg/24 h urine
- Or 10 - 59% clonal plasma cells in bone marrow
- And no related tissue damage / myeloma defining event or amyloidosis; if bone survey negative, bone disease should be assessed with whole body MRI, FDG PET / CT or low dose CT scan
- Multiple (symptomatic) myeloma (J Natl Compr Canc Netw 2019;17:1154):
Laboratory
- 97% have an M protein in serum or urine, 3% are nonsecretory
- IgG (50%), IgA (20%), light chain (20%), others < 10% (IgD, IgE, IgM and biclonal)
- SPEP: serum proteins normally separate into 5 major fractions based on electric charge and size (Am Fam Physician 2005;71:105):
- Albumin
- Alpha 1 globulins
- Alpha 2 globulins
- Beta 1 and beta 2 globulins
- Gamma globulins
- Gamma globulins include polyclonal antibodies and light chains, with a normal gamma zone appearing as a symmetrical smear; myeloma may appear as a spike in this region
- Urine protein electrophoresis (UPEP): monoclonal light chains in urine = Bence Jones protein
- IFE: used to characterize the M spike, by reacting with specific antisera to heavy chains IgG, IgA, IgM, IgD, IgE and kappa and lambda light chains
- Serum free light chain assay (SFLCA) (Freelite): more sensitive for monitoring light chain disease and nonsecretory myeloma (Blood Cancer J 2020;10:2)
- Mass spectrometry may have equivalent performance to IFE and better differentiate M protein from therapeutic antibodies (Blood Cancer J 2021;11:24)
Laboratory images
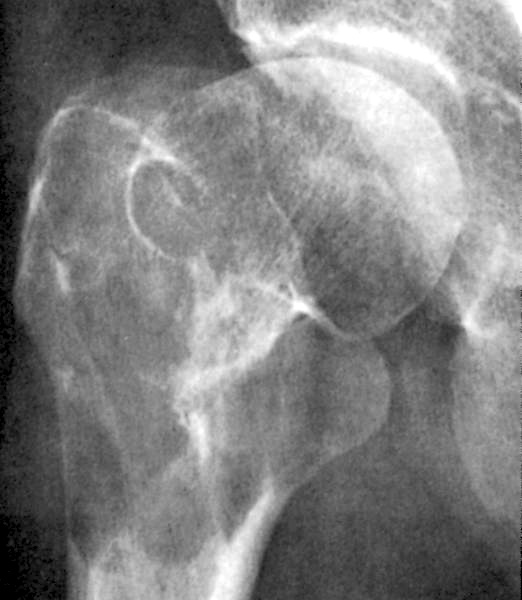
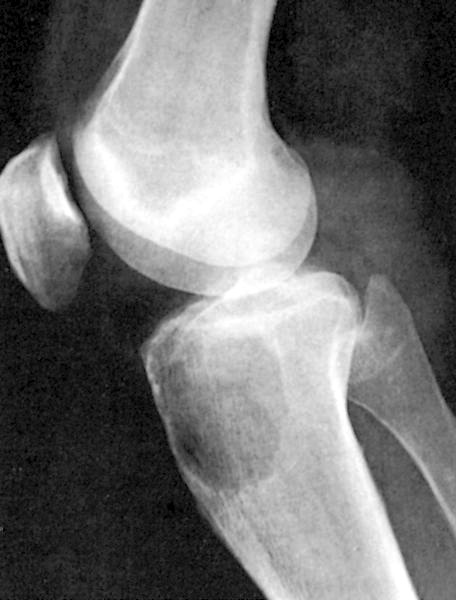
Radiology description
- Evidence of 1 or more sites of osteolytic bone destruction (at least 5 mm in size)
- Advanced methods include low dose whole body CT, MRI and (18F) fluorodeoxyglucose PET (FDG PET), and FDG PET with PET / CT (Am Soc Clin Oncol Educ Book 2016;35:e418)
- With greater sensitivity than radiographic bone survey and recommended prior to a diagnosis of smoldering multiple myeloma or solitary plasmacytoma (Am Soc Clin Oncol Educ Book 2016;35:e418)
- Increased uptake on PET / CT alone is not adequate without evidence of underlying osteolytic bone destruction; bone biopsy recommended if any doubt
Radiology images
Prognostic factors
- Usually incurable with median survival of ~5.5 years with a 5 year survival rate of 54% (ASCO: Multiple Myeloma - Statistics [Accessed 26 May 2022])
- However, improvements in treatment are leading to longer survival times (Semin Oncol 2016;43:676)
- Higher risk:
- Elevated beta 2 microglobulin, lactate dehydrogenase, C reactive protein, serum soluble receptor for IL6 and plasma cell proliferation or bone marrow infiltration
- Reduced polyclonal (uninvolved) serum immunoglobulins
- Plasmablastic morphology
- Abnormalities by conventional cytogenetics
- Active myeloma can be staged using the International Staging System (ISS) or revised International Staging System (R-ISS) (J Natl Compr Canc Netw 2019;17:1154)
- Revised International Staging System:
- Stage I: serum beta 2 microglobulin < 3.5 mg/L, serum albumin ≥ 3.5 g/dL, standard risk chromosomal abnormalities by FISH [absence of del(17p), t(4:14) or t(14;16)] and normal serum LDH
- Stage II: not R-ISS stage I or III
- Stage III: serum beta 2 microglobulin ≥ 5.5 mg/L or either high risk chromosomal abnormalities [del(17p), t(4:14) or t(14;16)] and serum LDH > upper limit of normal
- Additional prognostic chromosomal abnormalities:
- Worse: t(4;14), MAF translocations t(14;16) and t(14;20), del(17p), del 13, aneuploidy, hypodiploidy
- Better: hyperdiploidy, t(11;14), t(6;14), cyclin D1 or D3 positive
- References: analysis of prognostic value of most frequent chromosomal changes in a large series of patients with newly diagnosed symptomatic myeloma (Blood 2007;109:3489)
- Model for identifying patients with increased risk of progression of smoldering myeloma (Mayo 2018 criteria or 20/2/20 criteria) with 3 independent risk factors:
- Serum monoclonal protein > 2 g/dL
- Involved to uninvolved serum free light chain ratio > 20
- Bone marrow plasma cells > 20%
- Low (0 factor), intermediate (1 factor) and high risk (2 - 3 factors) shown to have 2 year rates of progression to multiple myeloma of 5%, 17% and 46%, respectively (Am Soc Clin Oncol Educ Book 2020;40:1)
Case reports
- 49 year old woman with a history of severe, chronic, active sarcoidosis (Arch Pathol Lab Med 2002;126:365)
- 55 year old man with multiple myeloma and prognosis of undetermined significance (American Society of Hematology: Case Study [Accessed 26 May 2022])
- 56 year old woman diagnosed with multiple myeloma (type IgG kappa, 59 g/L) (J Hematother Stem Cell Res 2001;10:657)
- 61 year old woman with EBV+ multiple myeloma (Int J Clin Exp Pathol 2015;8:2090)
- 64 year old man with IgM multiple myeloma (Cancer Control 2018;25:1073274817744448)
- 67 year old man with acute kidney injury (J Hematol 2016;5:76)
- 77 year old woman with plasma cell leukemia with t(11;14)(q13;q32) simulating lymphoplasmacytic lymphoma (Rev Bras Hematol Hemoter 2017;39:66)
- Original description of Bence Jones proteinuria in 1850 (Med Chir Trans 1850;33:211)
Treatment
- Updated diagnostic criteria (see Diagnosis) from the International Myeloma Working Group to initiate therapy, including sensitive imaging, may help identify patients who would benefit from treatment before end organ damage occurs (CRAB features) (J Natl Compr Canc Netw 2019;17:1154, Lancet Oncol 2016;17:e328, Am Soc Clin Oncol Educ Book 2020;40:1)
- Treatment regimens are discussed in the National Comprehensive Cancer Network (NCCN) guidelines (J Natl Compr Canc Netw 2019;17:1154)
- May include proteasome inhibitors; immunomodulatory drugs, steroids, antibody based therapy (including elotuzumab [anti-SLAM7], daratumumab and isatuximab [CD38] and new BCMA targeting agents), traditional chemotherapeutic agents, radiation therapy (Multiple Myeloma Treatment Foundation: Standard Treatments [Accessed 26 May 2022])
- Autologous bone marrow transplant, particularly for young patients with newly diagnosed myeloma (Blood Cancer J 2019;9:44)
Minimal residual disease (MRD) testing
- Sensitive measure of response to therapy to guide treatment decisions
- Flow cytometry (Blood 2013;122:1088)
- Molecular methods monitoring immunoglobulin rearrangement by NGS (Blood Adv 2020;4:4573)
- Clonotypic peptides by mass spectrometry by liquid chromatography with tandem mass spectrometry (LC-MS / MS) (Clin Chem 2016;62:243)
- MRD negativity associated with better progression free survival (Blood Adv 2020;4:4573)
Gross description
- Bone defects are filled with a soft, gelatinous "fish flesh" hemorrhagic tissue
Frozen section description
- Osseous or extraosseous plasmacytomas, particularly if the patient is not known to have a history of plasma cell myeloma, may be sent for frozen section evaluation (Borczuk: Frozen Section Pathology, 1st Edition, 2021)
- Sites can include: mucosa of the upper respiratory tract, lymph nodes, thyroid, testes, breast, salivary gland and CNS (Int J Otolaryngol 2010;2010:302656)
- Morphology may range from monotonous plasma cells to more irregular, multinucleated or pleomorphic forms in more advanced myeloma
Frozen section images
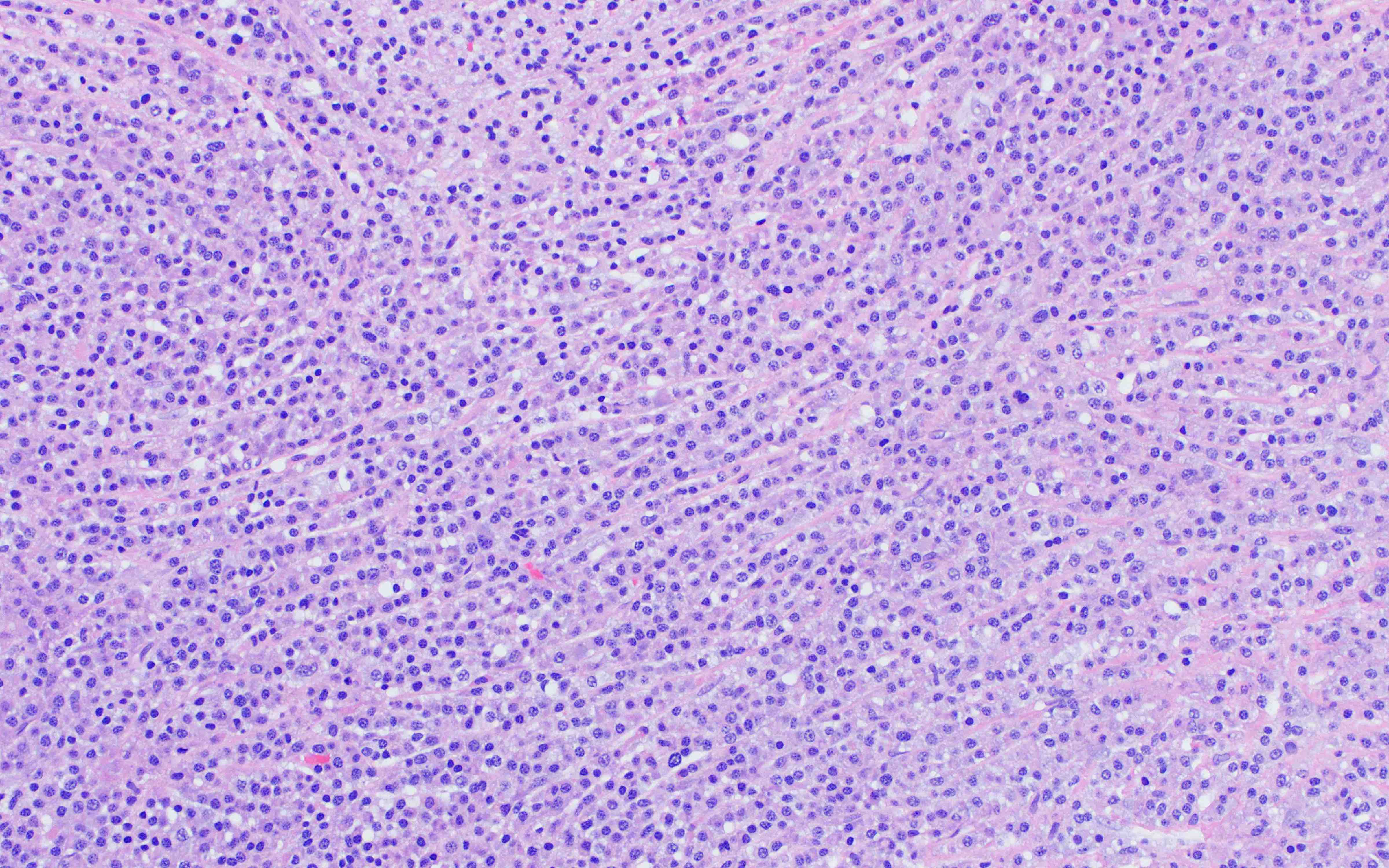
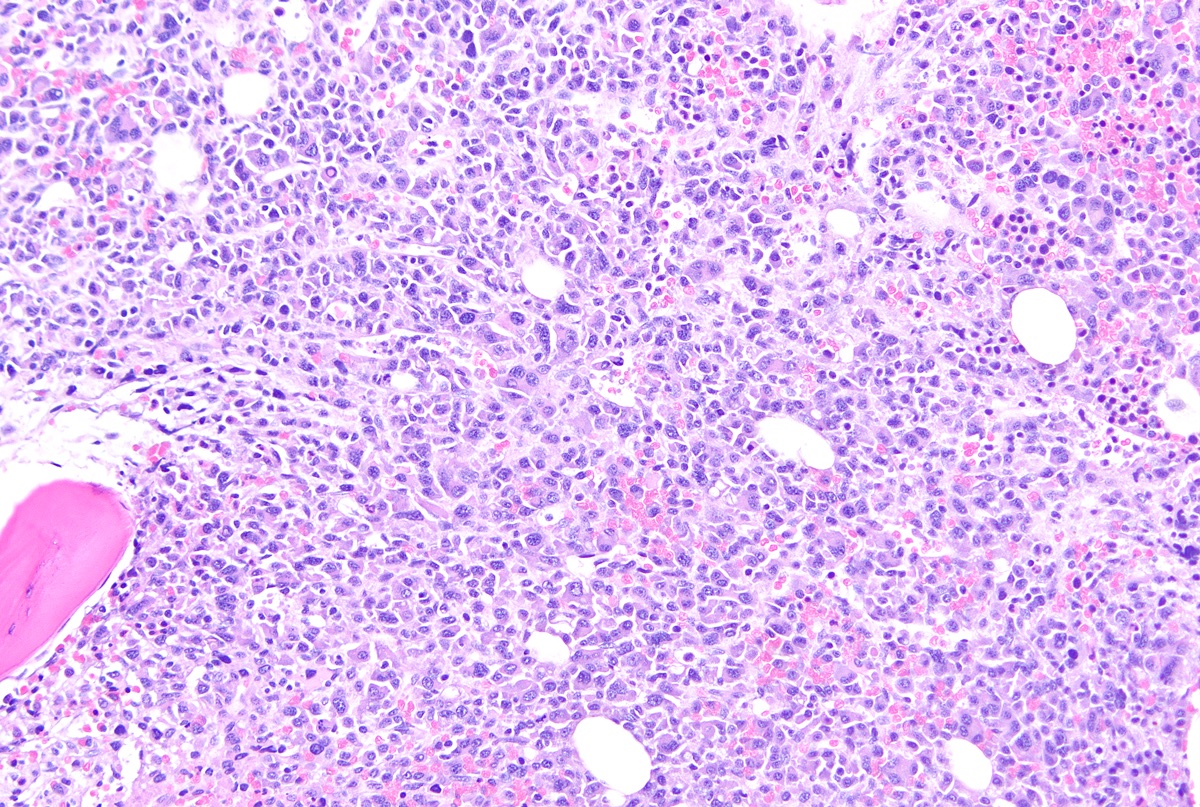
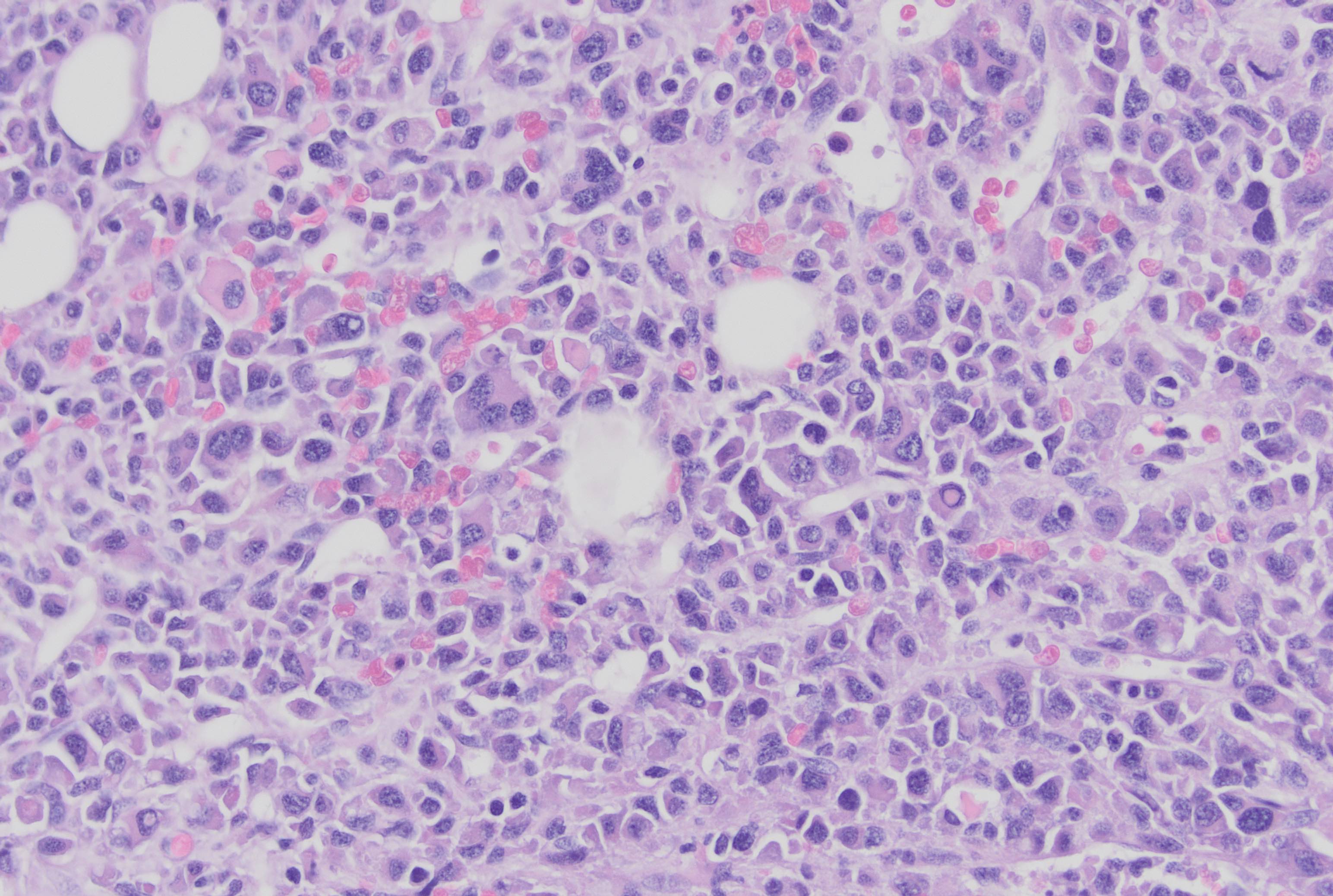
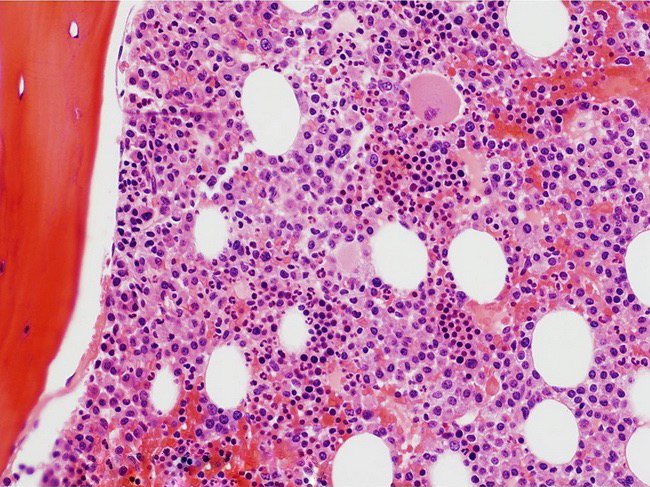
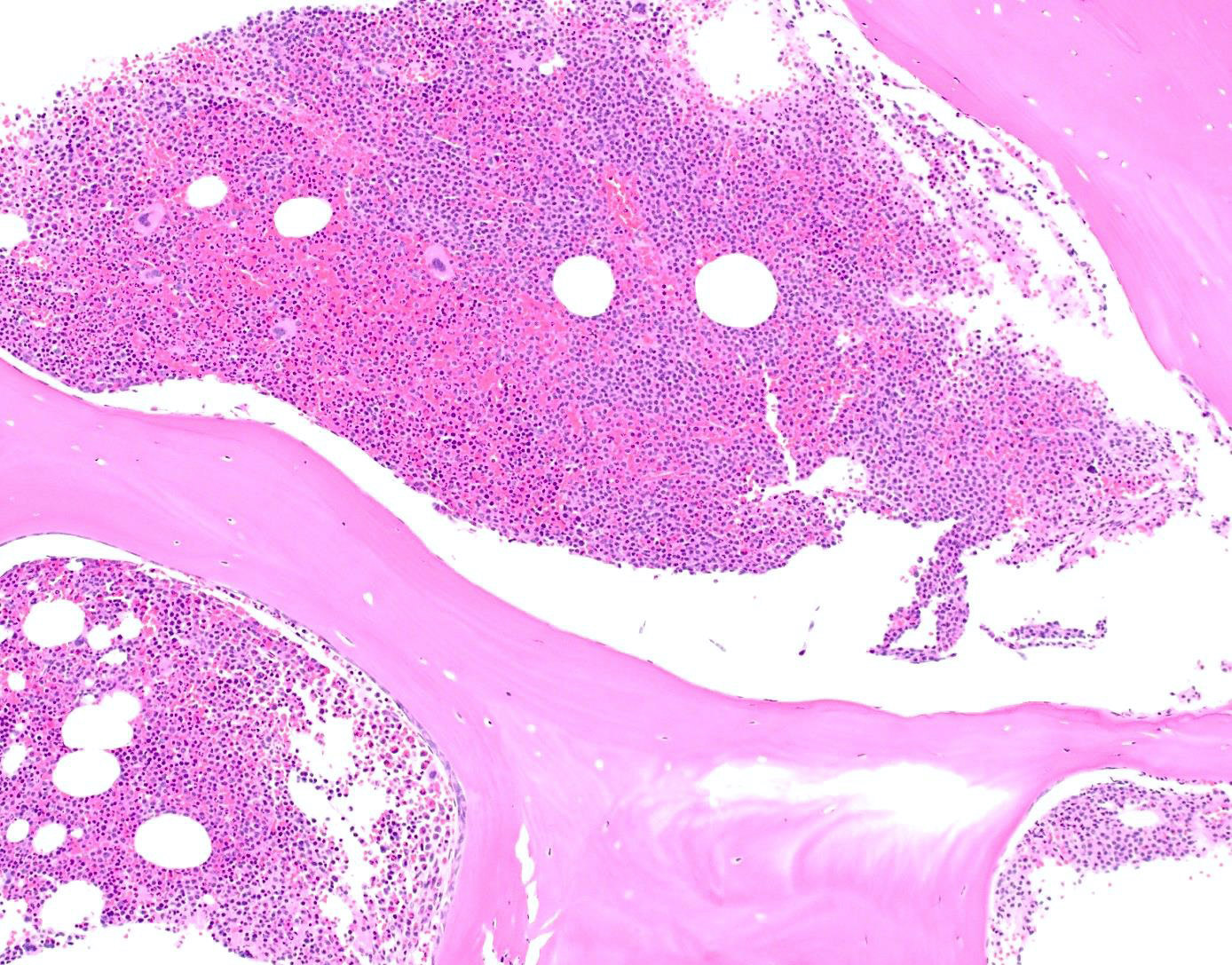
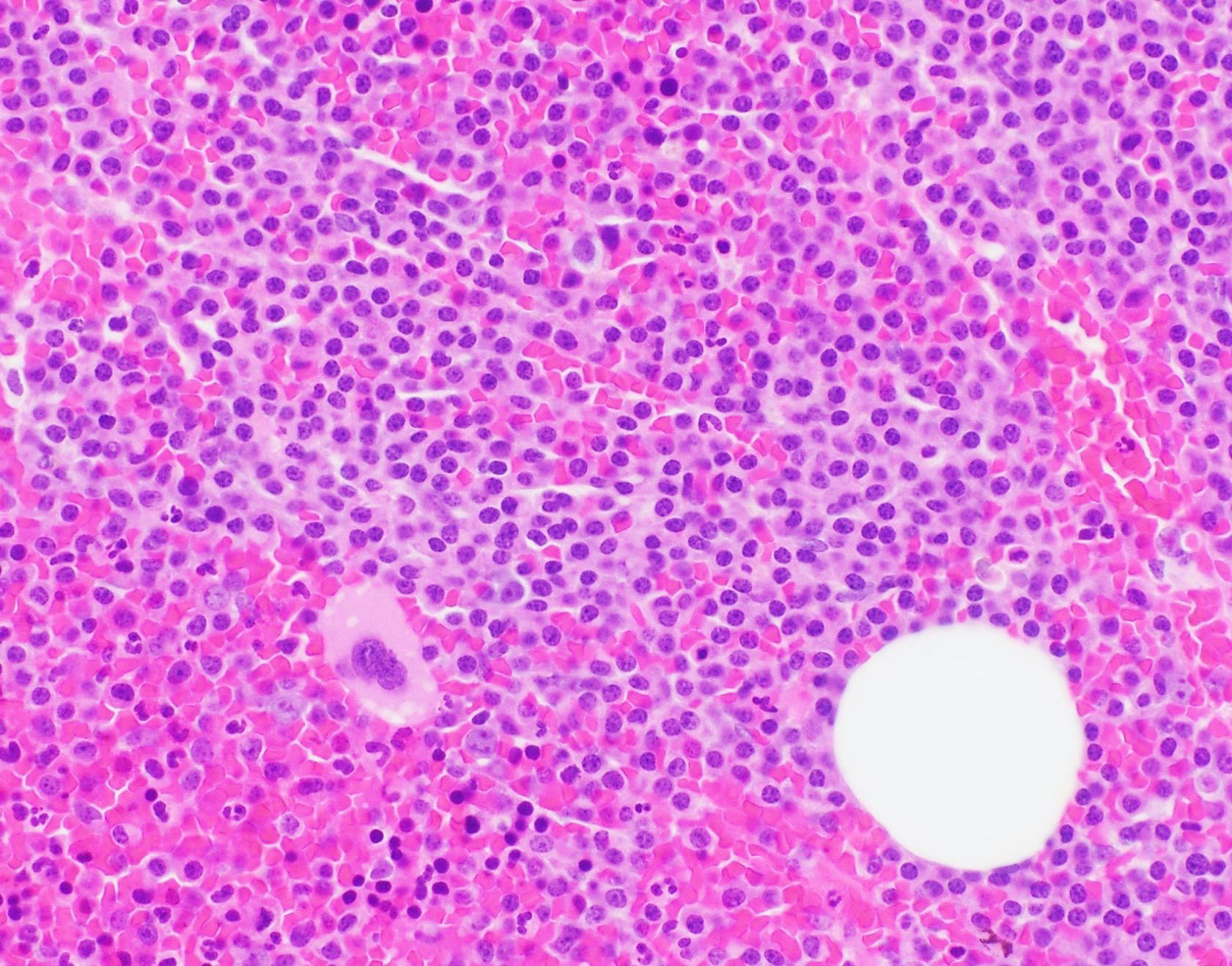
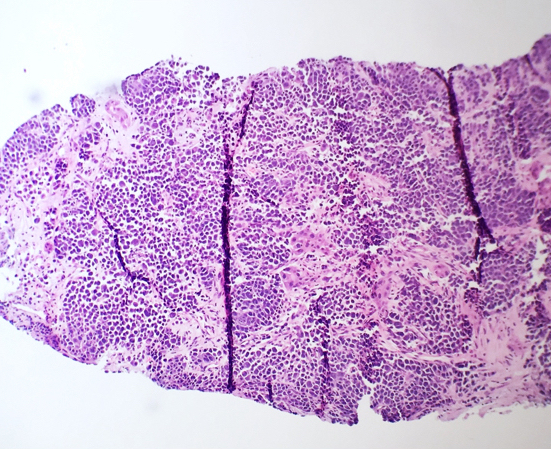
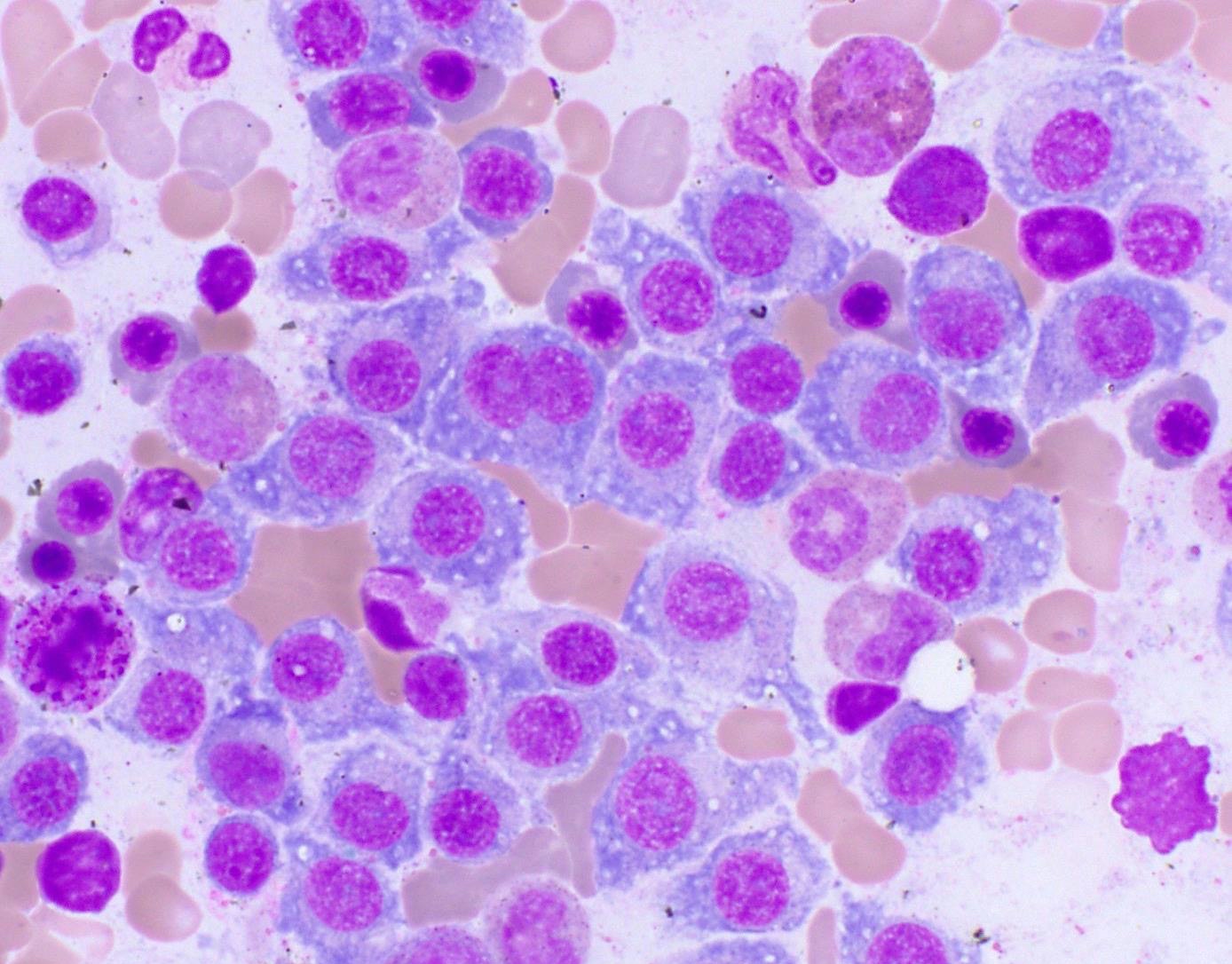
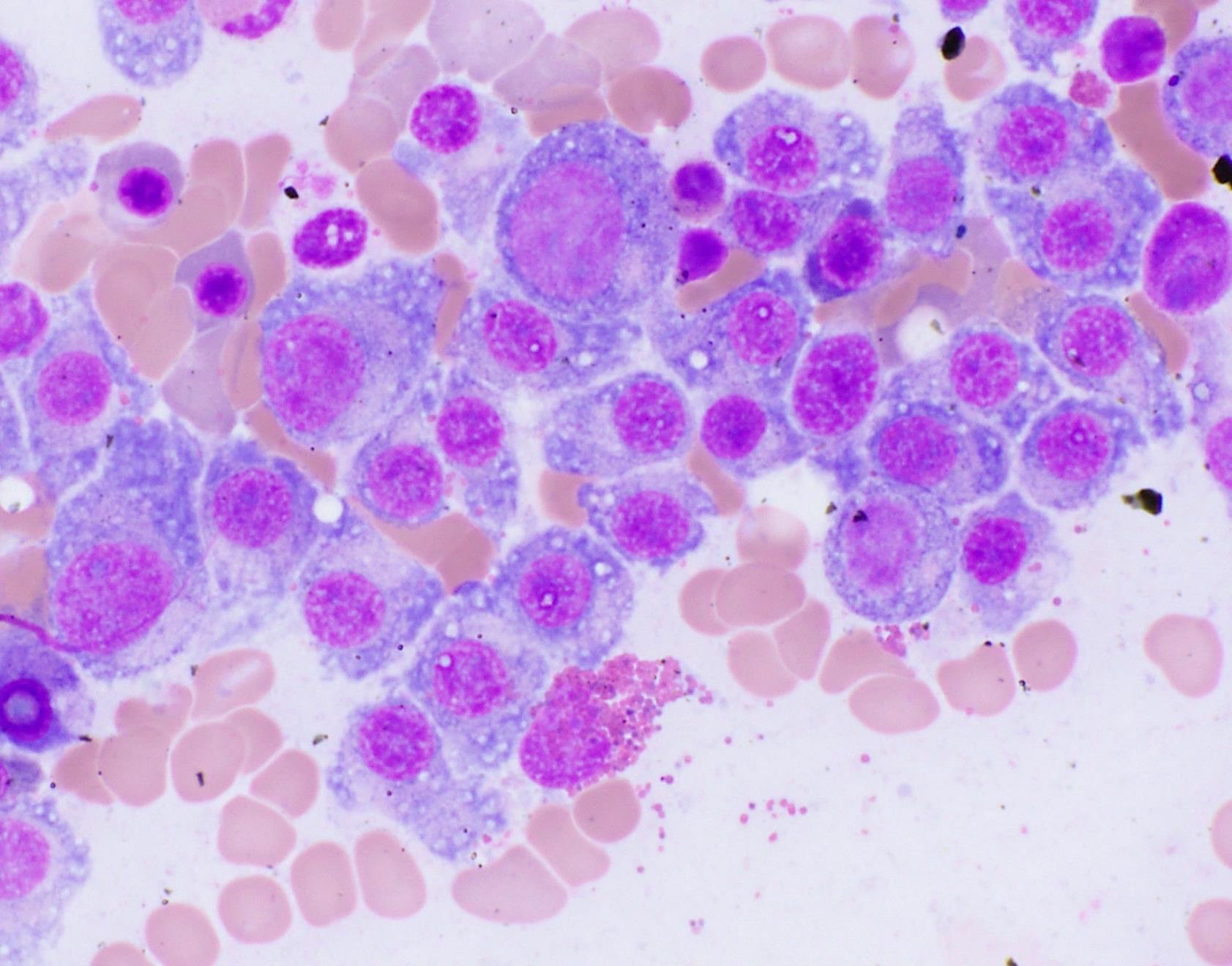
Microscopic (histologic) description
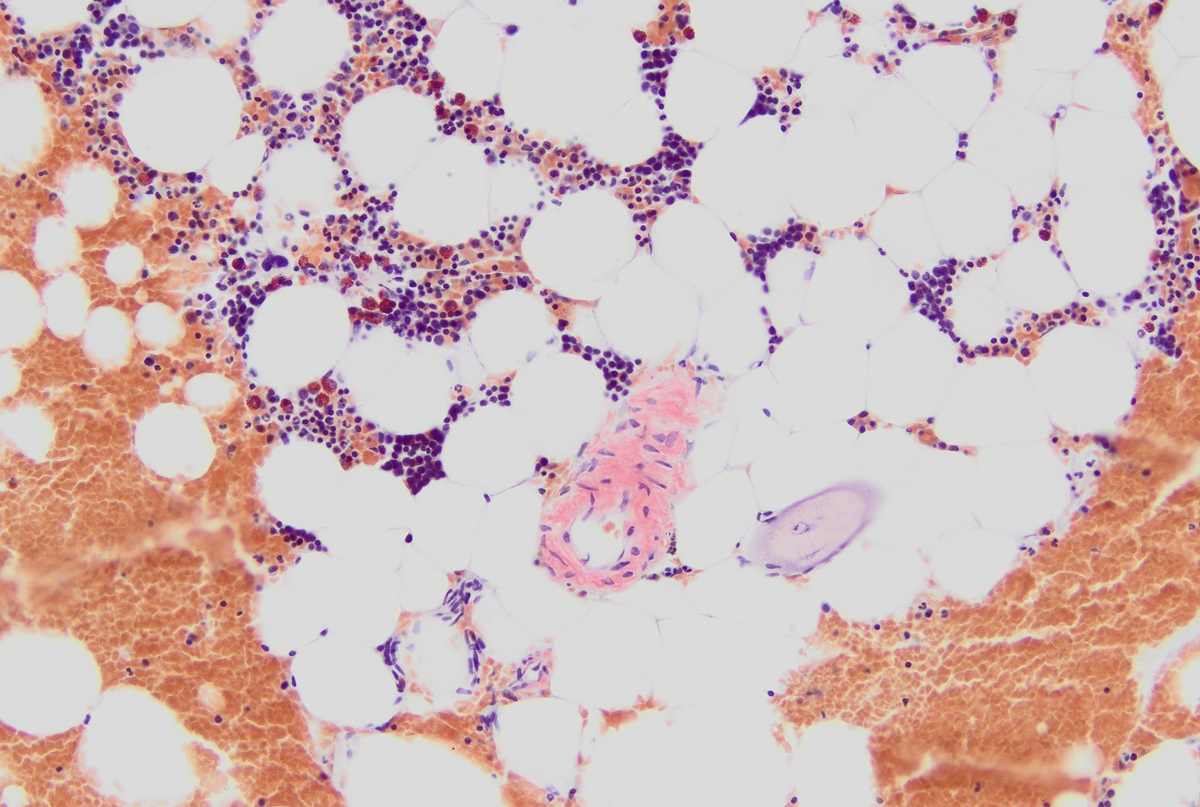
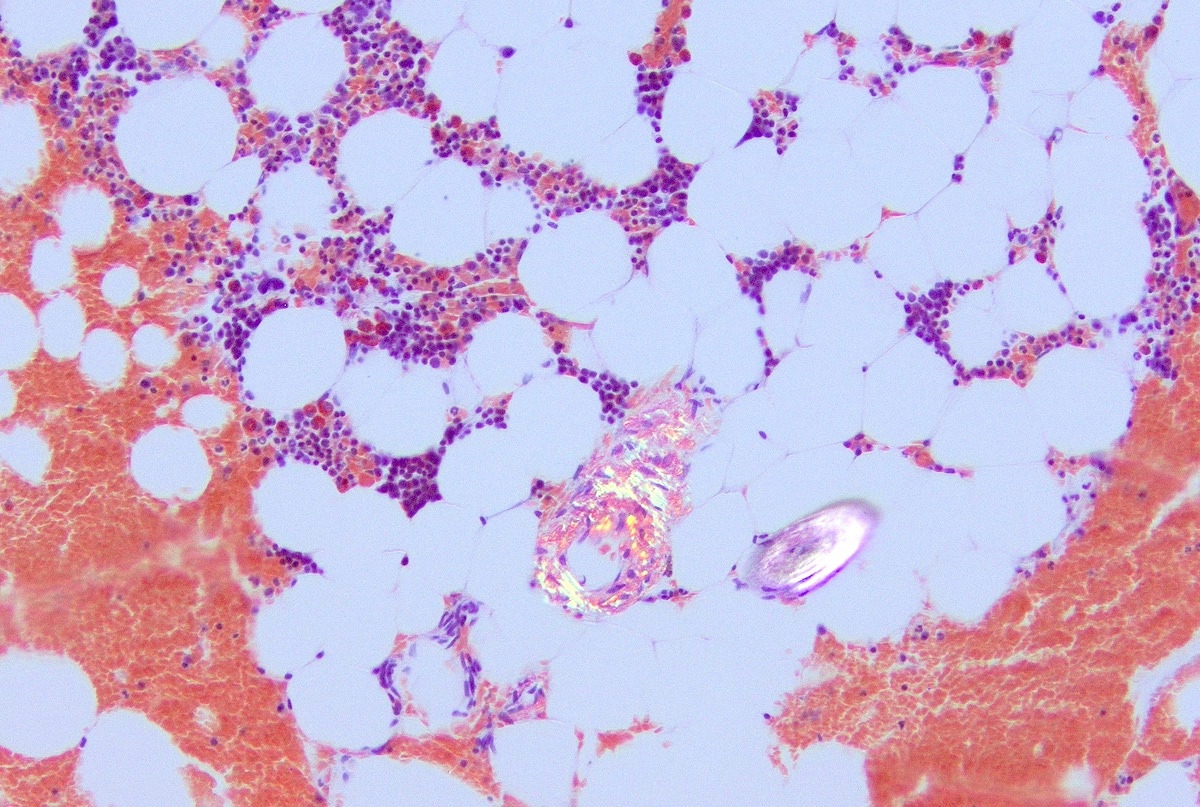
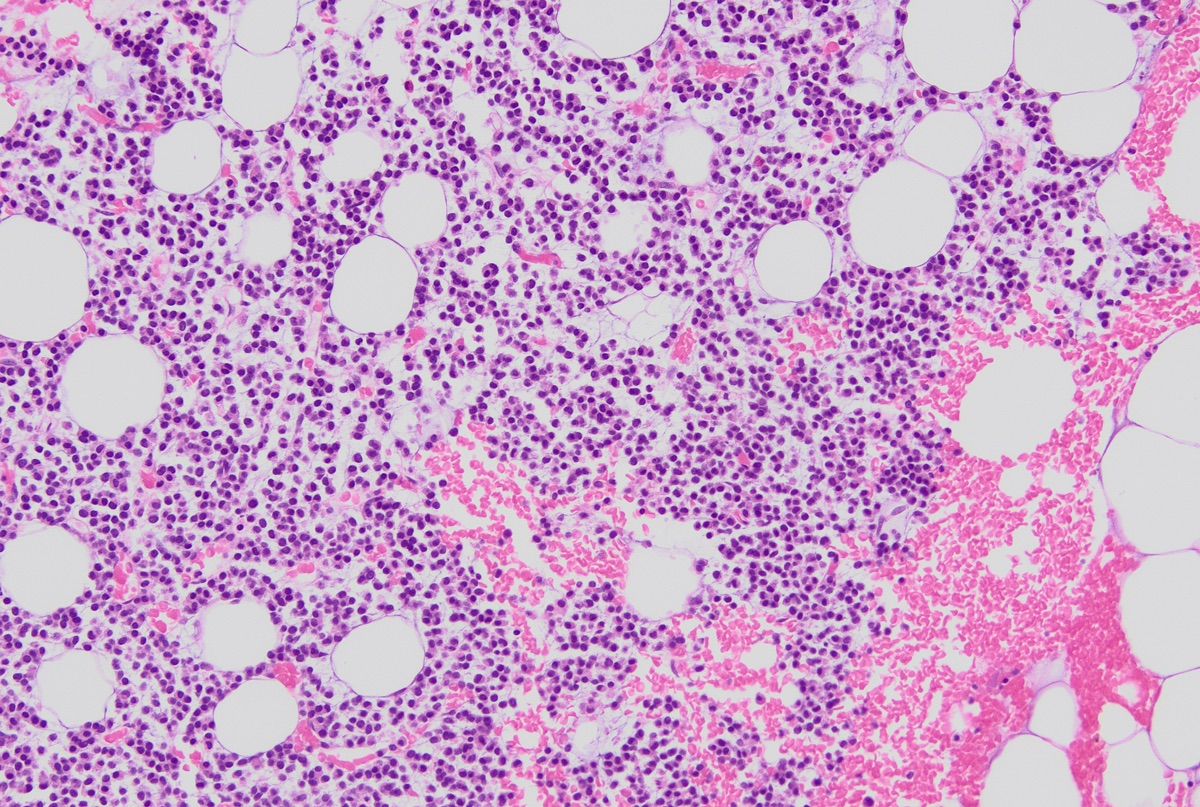
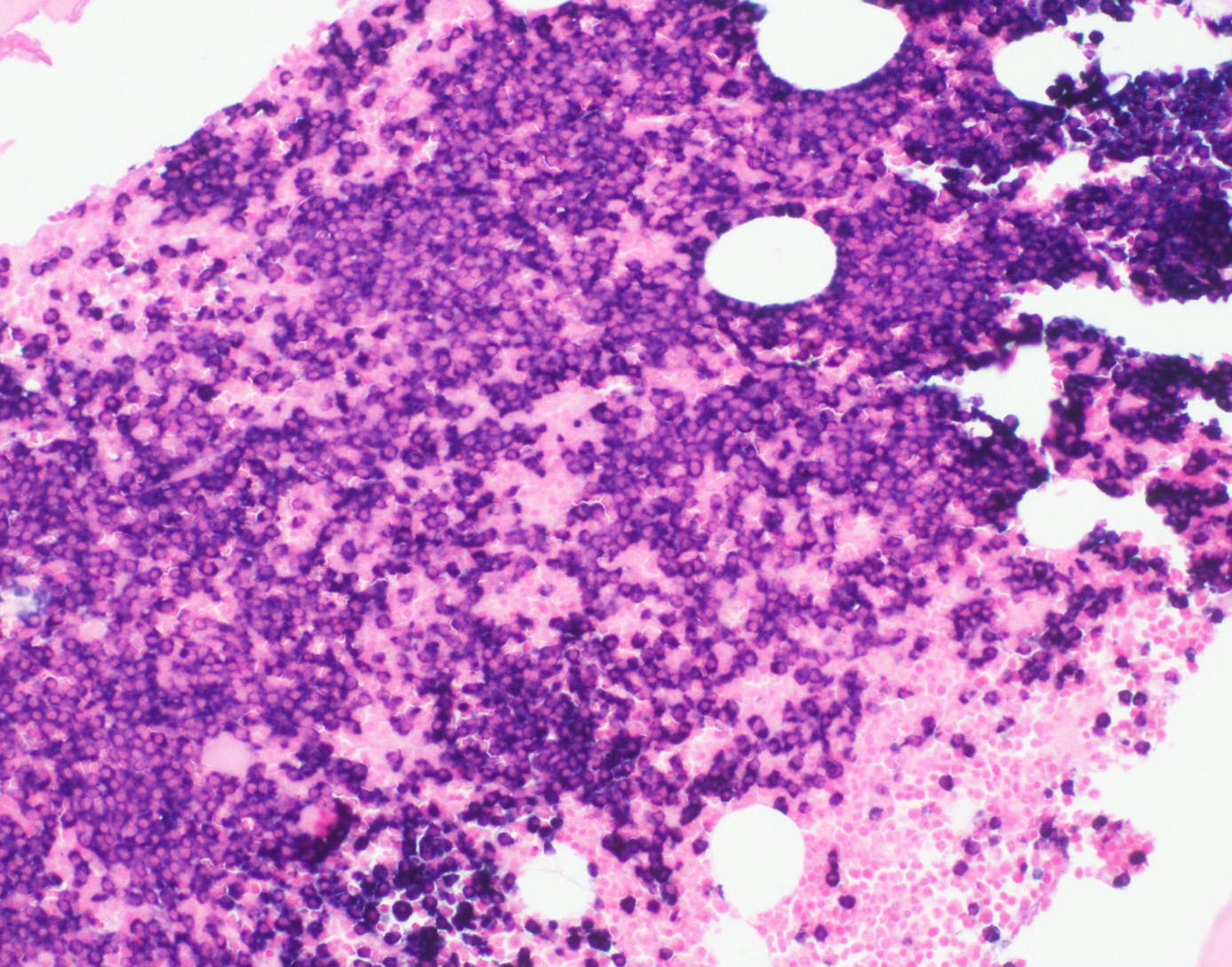
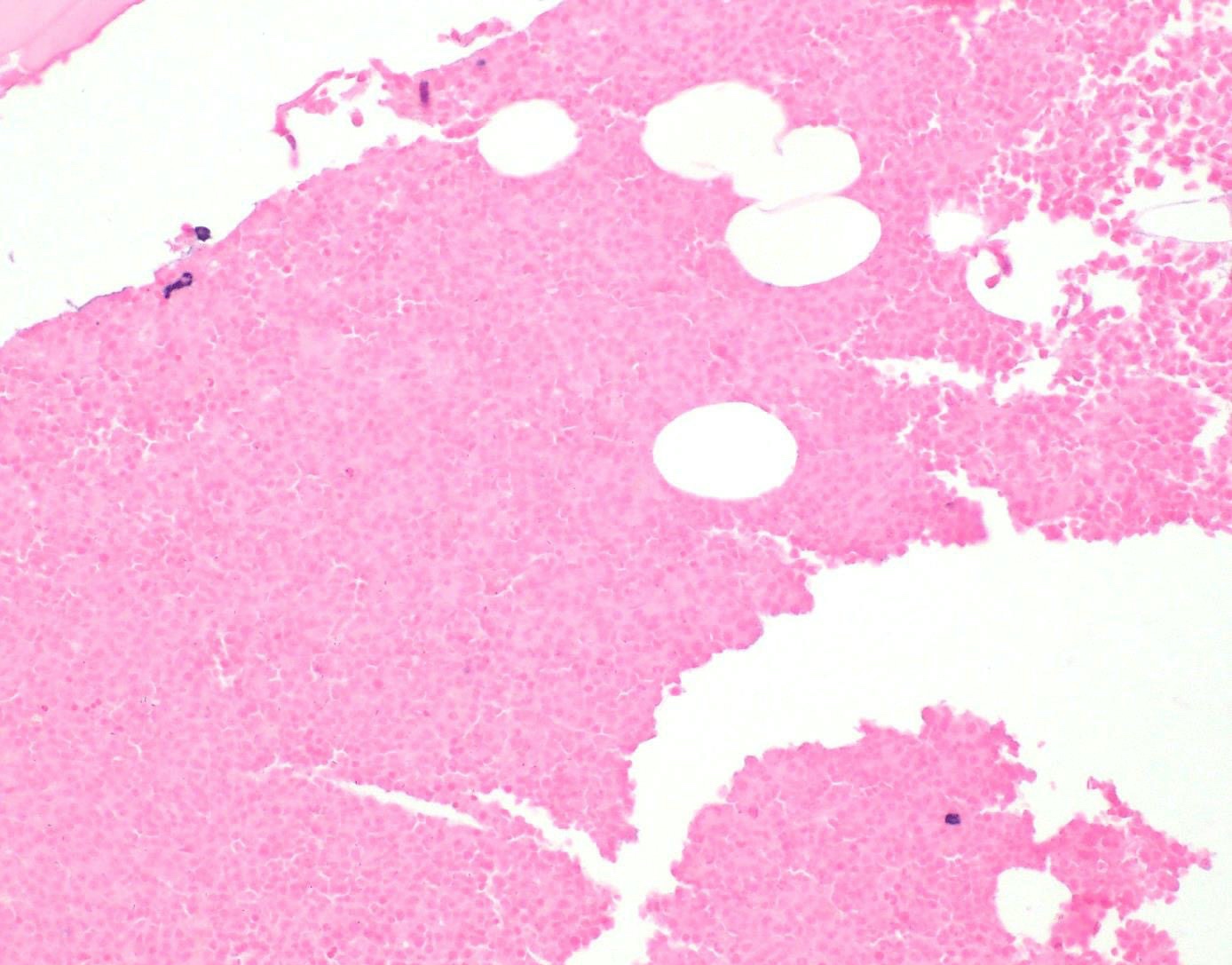
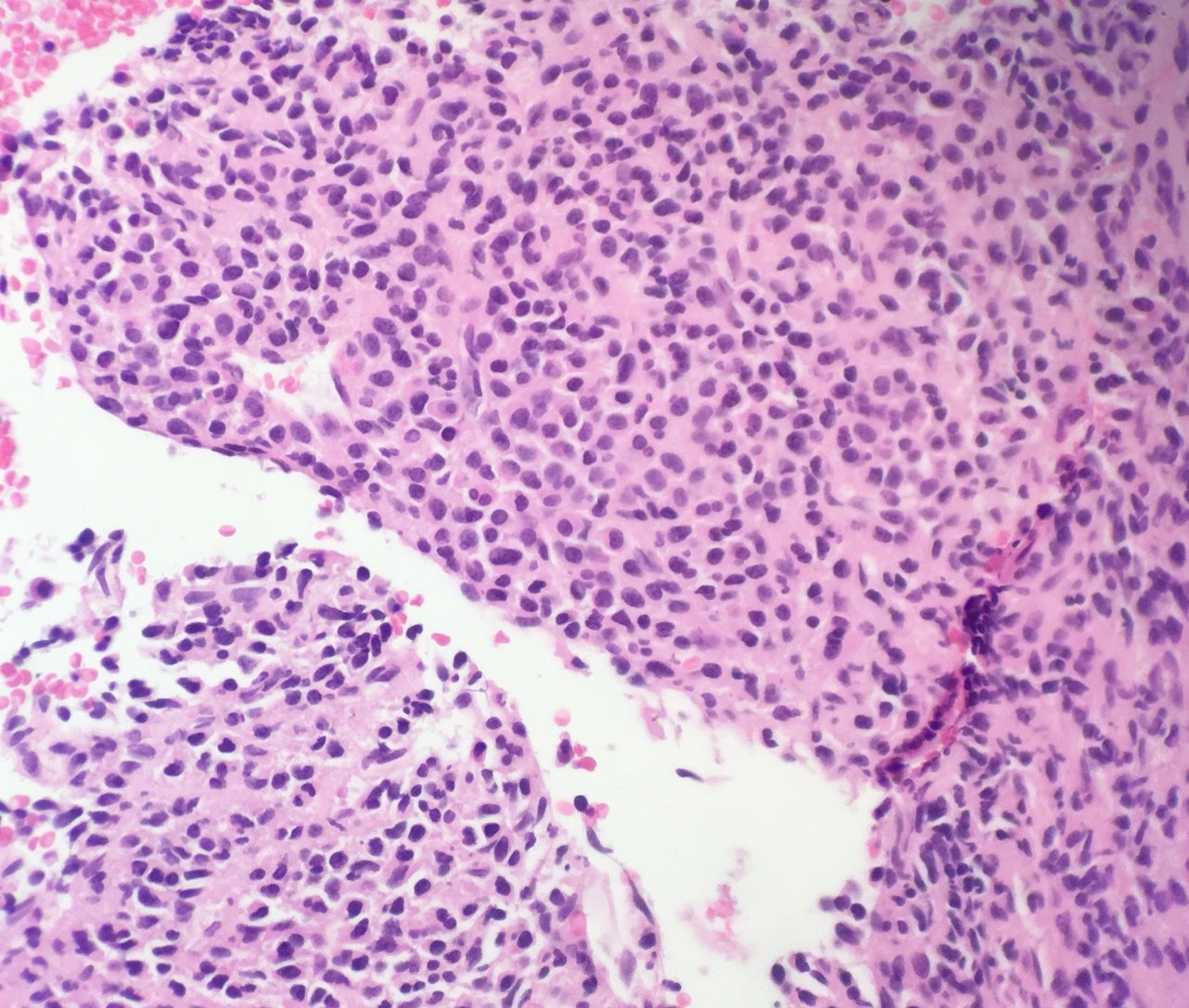
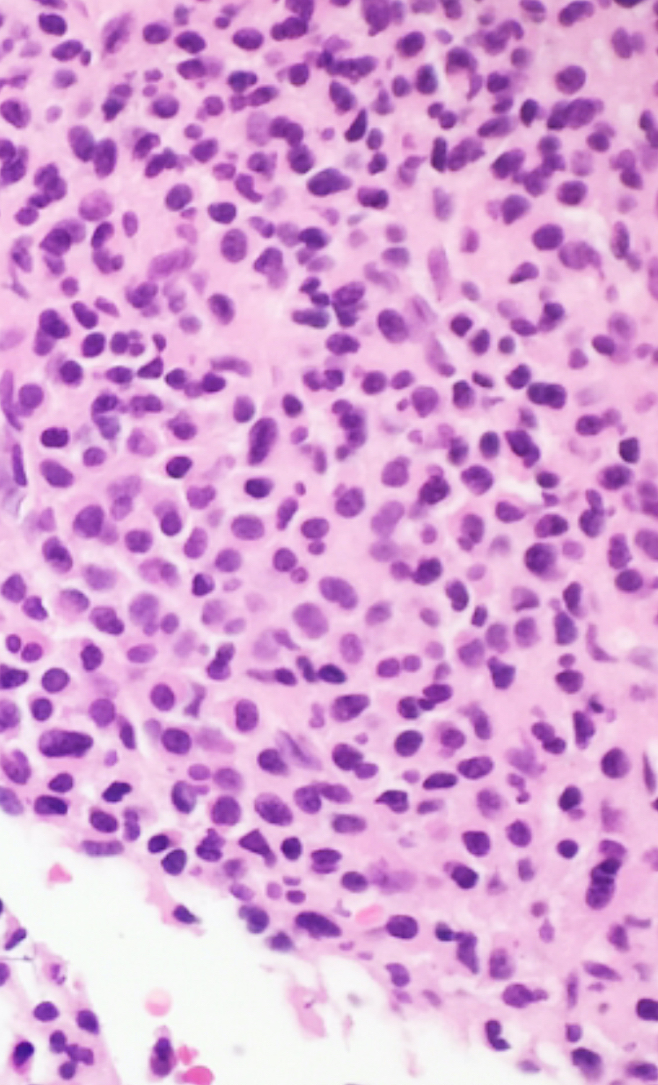
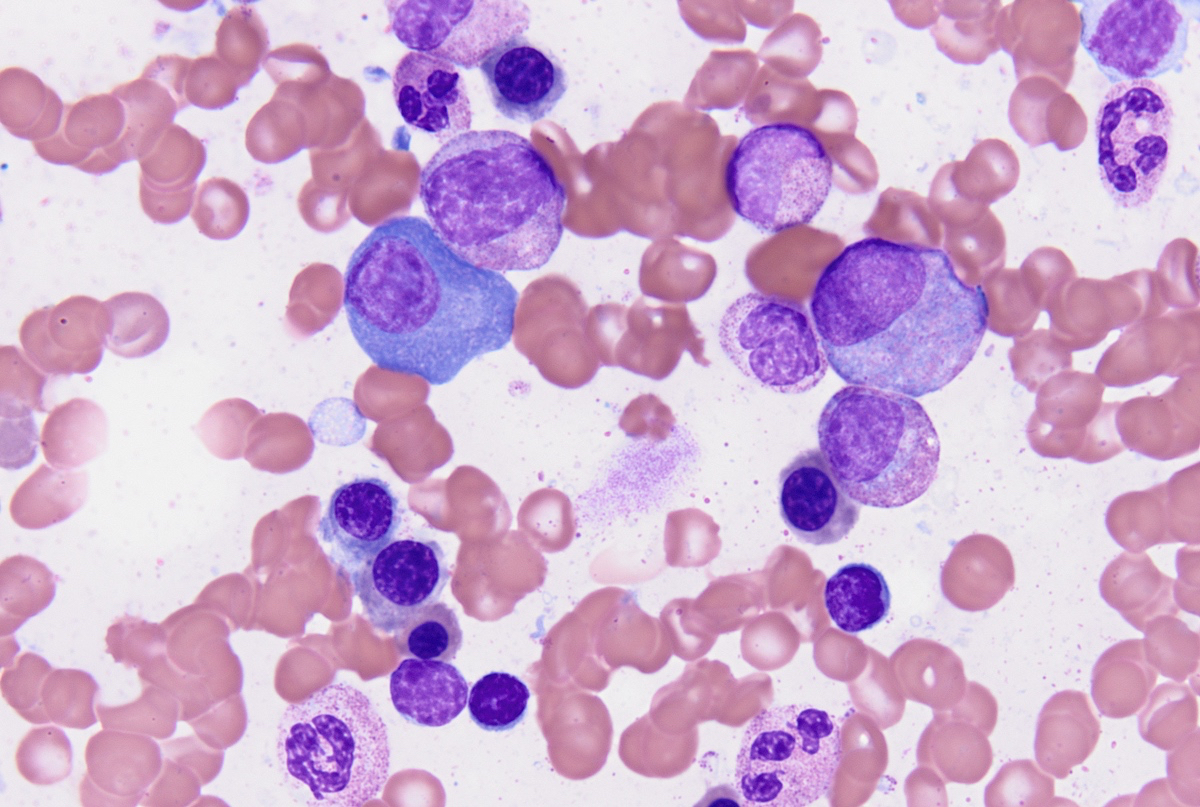
- Core biopsy (Am J Clin Path 1987;87:342):
- Interstitial clusters, nodules or sheets of plasma cells
- Areas of bone marrow may be spared with preserved hematopoiesis, other cases may have diffuse involvement and markedly suppressed hematopoiesis
- Prominent osteoclastic activity may be seen
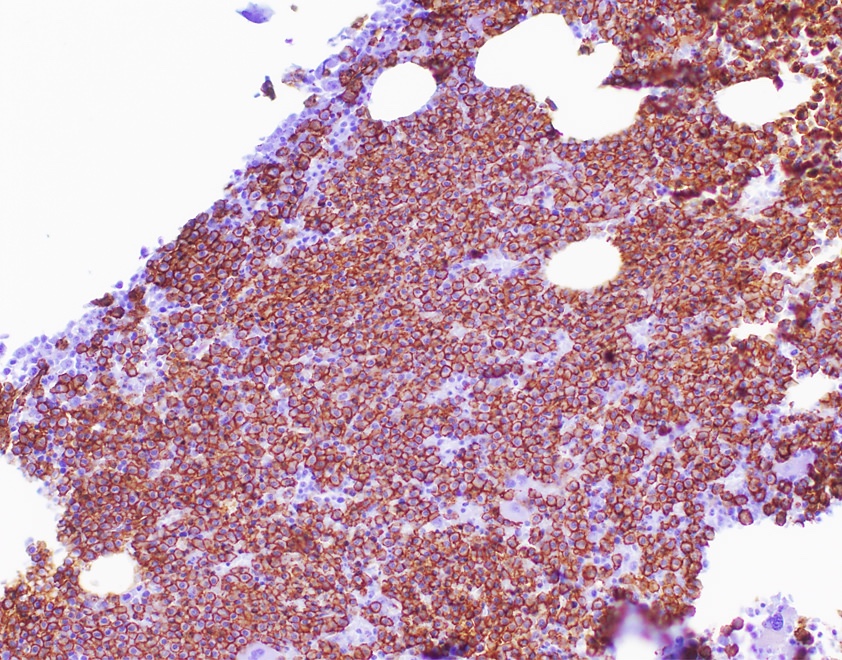
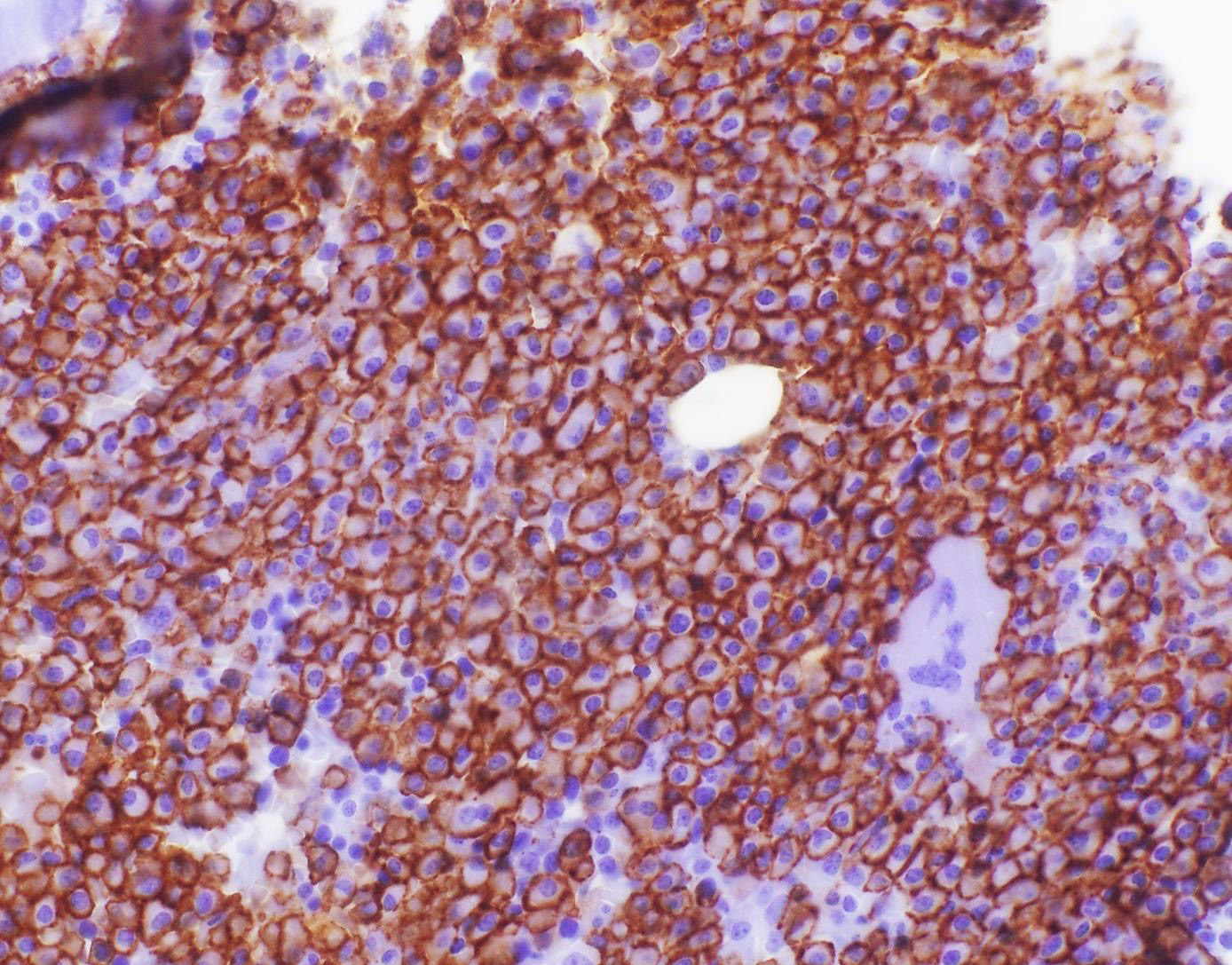
- IHC to quantify plasma cells (CD138), stains for Ig kappa and lambda to establish clonality
Microscopic (histologic) images
Contributed by Genevieve M. Crane, M.D., Ph.D. and Tapan Bhavsar, M.D., Ph.D.
Images hosted on other servers:
Virtual slides
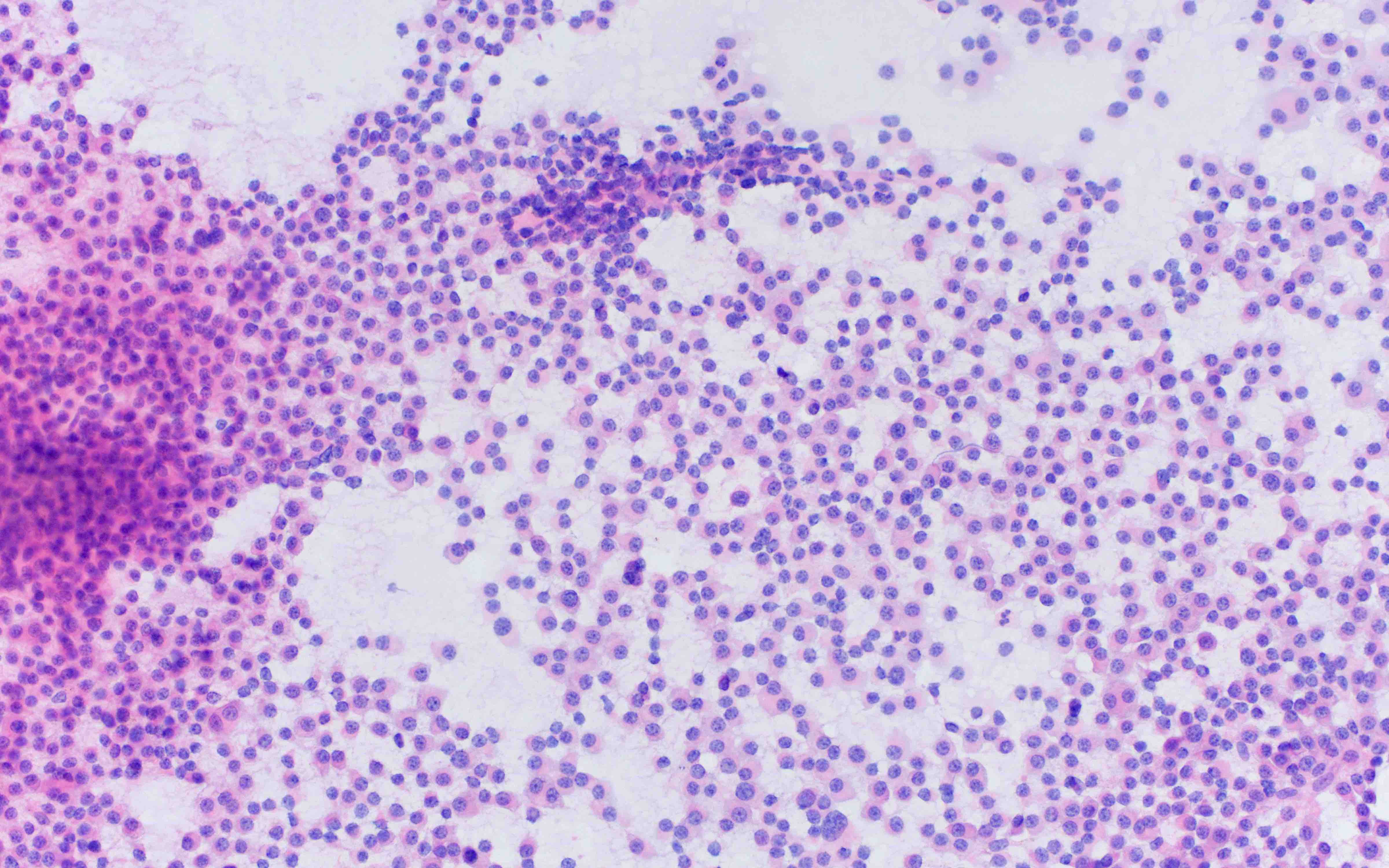
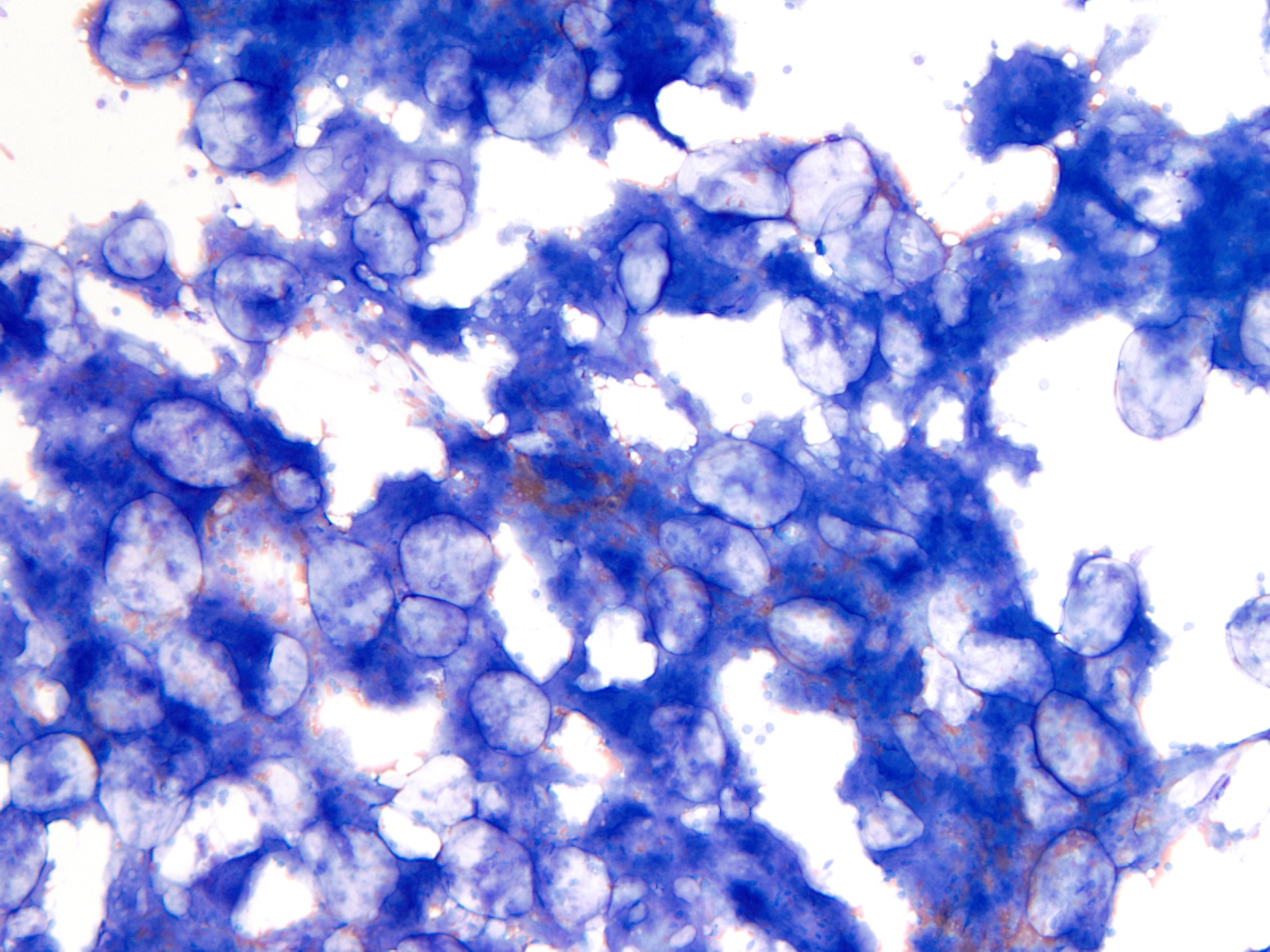
Cytology description
- Cytology can assess plasma cell morphology (e.g., mature, immature, plasmablastic) but number of plasma cells present may vary substantially from the core biopsy
- Mature plasma cells: oval with abundant basophilic cytoplasm, perinuclear hof, round eccentric nuclei, clock face chromatin and indiscernible nucleoli
- Immature plasma cells: higher nuclear to cytoplasmic ratio, more abundant cytoplasm and hof region compared to plasmablastic, more dispersed chromatin, often prominent nucleoli
- Plasmablastic: less abundant cytoplasm with little or no hof region, fine reticular chromatin, large nucleus (> 10 microns) or large nucleolus (> 2 microns) (Blood 1998;91:2501)
- Pleomorphic: multinucleated, polylobated
- Rare cases may have small, lymphoid appearing plasma cells or plasma cells with marked nuclear lobation
- Immature or pleomorphic features are rare in reactive plasma cell proliferations
- Morphologic features:
- Mott cells / morula cells: multiple grape-like cytoplasmic inclusions comprised of crystalized immunoglobulin
- Russell bodies: hyaline intracytoplasmic inclusions
- Flame cells: vermillion staining glycogen rich IgA in cytoplasmic projections (American Society of Hematology: Flame Cells in Multiple Myeloma [Accessed 26 May 2022])
- Pseudo-Gaucher cells / thesaurocytes: overstuffed fibrils (J Clin Pathol 1976;29:916)
- Cytoplasmic crystals: occasional in myeloma, common in adult Fanconi syndrome (Am J Clin Pathol 1983;80:224)
- Dutcher body: pale staining immunoglobulin filled cytoplasm invaginating into the nucleus and appearing as an intranuclear inclusion, single and usually large, more common in IgA myeloma
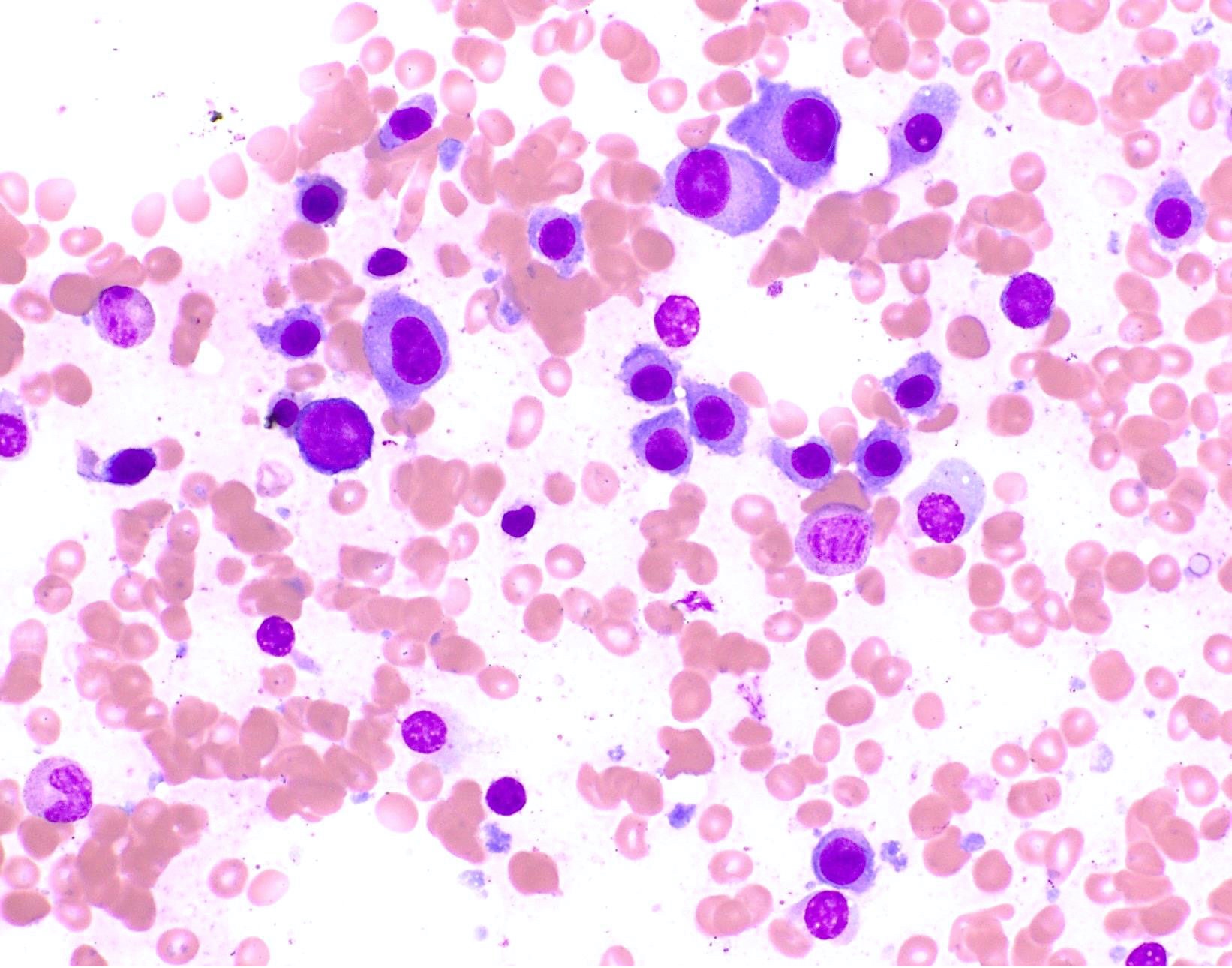
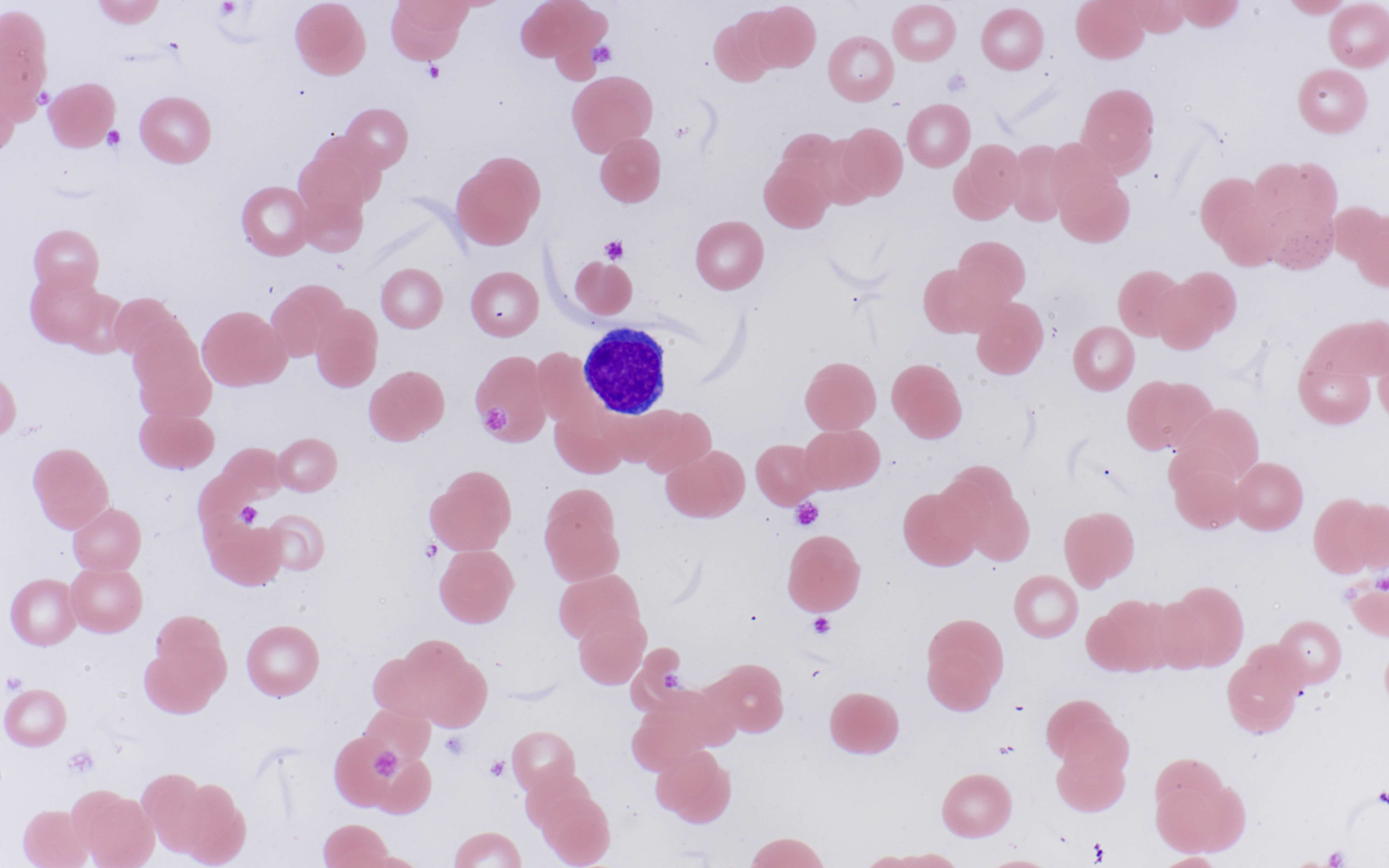
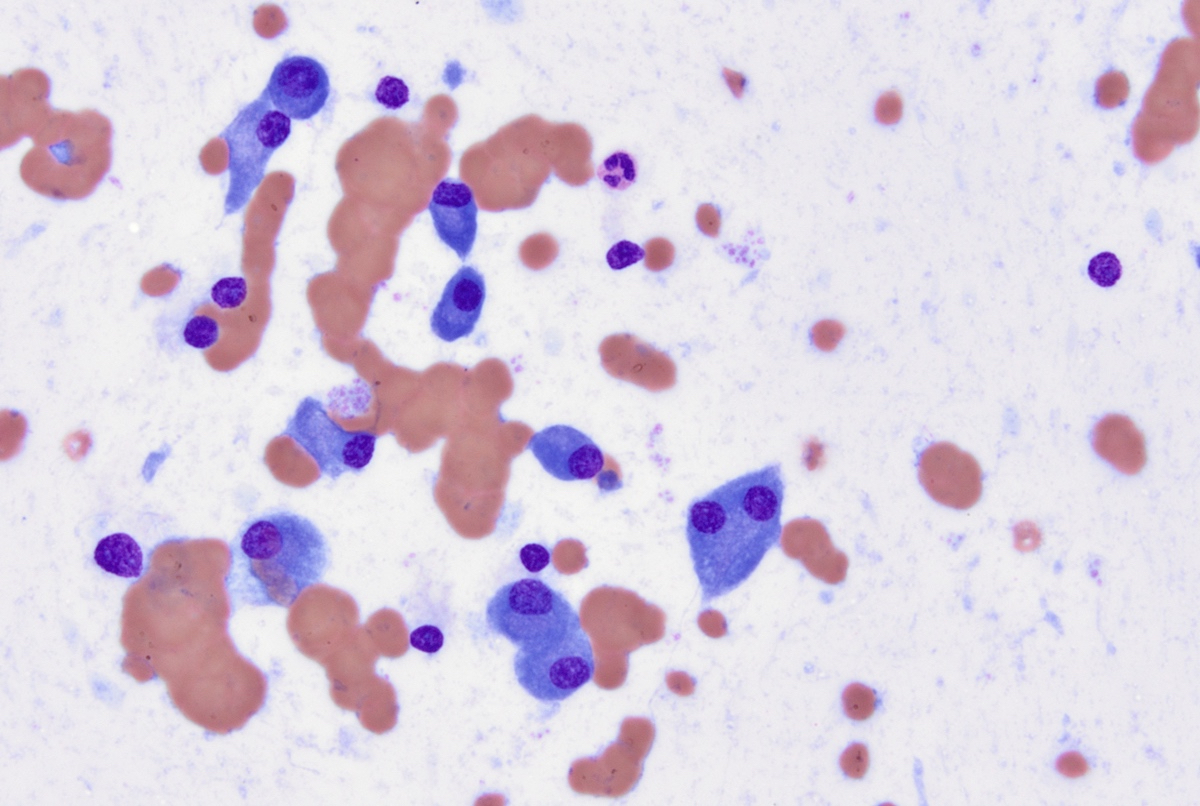
Peripheral smear description
- Rouleaux formation: erythrocytes resemble stacked coins; related to quantity and type of M protein, not specific and may be caused by alterations in other plasma proteins (Biophys J 2000;78:2470, American Society of Hematology: Rouleaux Formation [Accessed 26 May 2022])
- Leukoerythroblastic reaction can occur with extensive marrow involvement
- Circulating plasma cells can be seen in ~15% of cases, usually small numbers not meeting criteria for plasma cell leukemia (> 2 x 109/L or 20% of the leukocyte count)
Peripheral smear images
Positive stains
- CD38, CD138, VS38c
- MUM1, EMA, CD79a (variable)
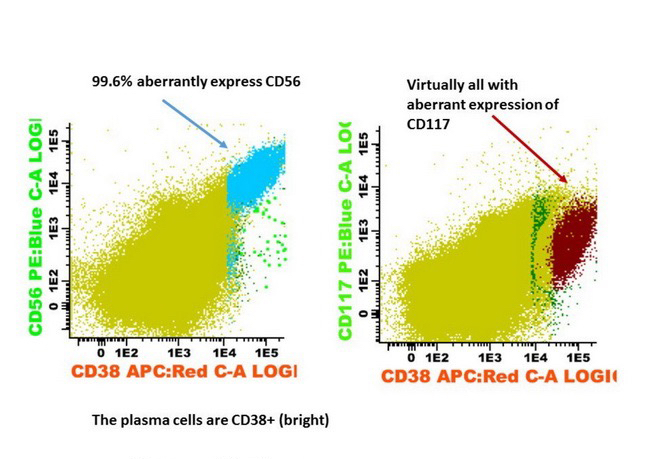
- May have aberrant expression of CD56 (75 - 80%), CD200 (60 - 75%), CD28 (~40%), CD117 / KIT (20 - 35%), CD20 (10 - 20%), CD52 (8 - 14%), CD10 and occasional myeloid or monocytic markers; may correlate with cytogenetics in some cases (Leuk Lymphoma 2015;56:426, Cytometry B Clin Cytom 2016;90:61)
- Monoclonal light chain
- Cyclin D1 positive in presence of t(11;14)(q13;q32), variable expression levels with hyperdiploidy or 11q13 amplification (Blood 2004;104:1120)
- t(11;14) translocation associated with expression of B cell markers on the clonal plasma cells including CD19, CD20, CD79a (Leuk Res 2013;37:1251)
- Increased MYC expression may be seen and potentially distinguish from MGUS (Am J Surg Pathol 2014;38:776)
Negative stains
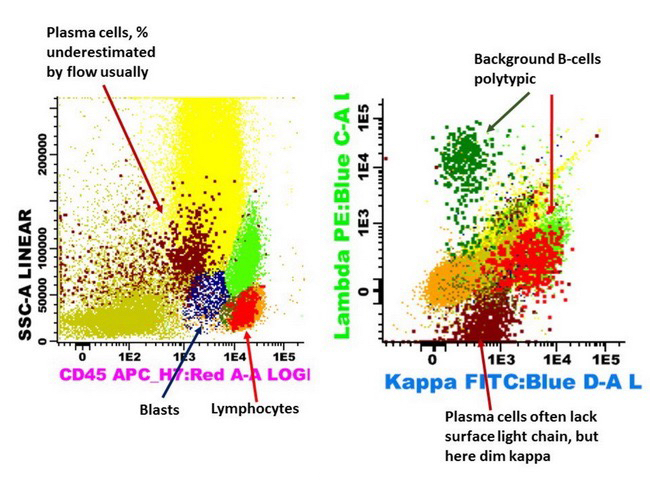
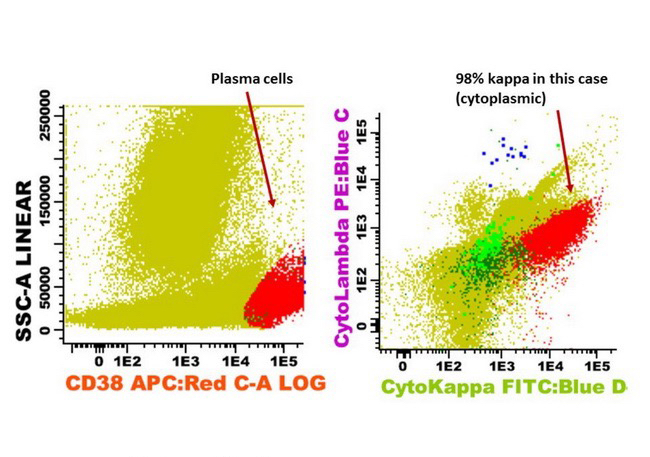
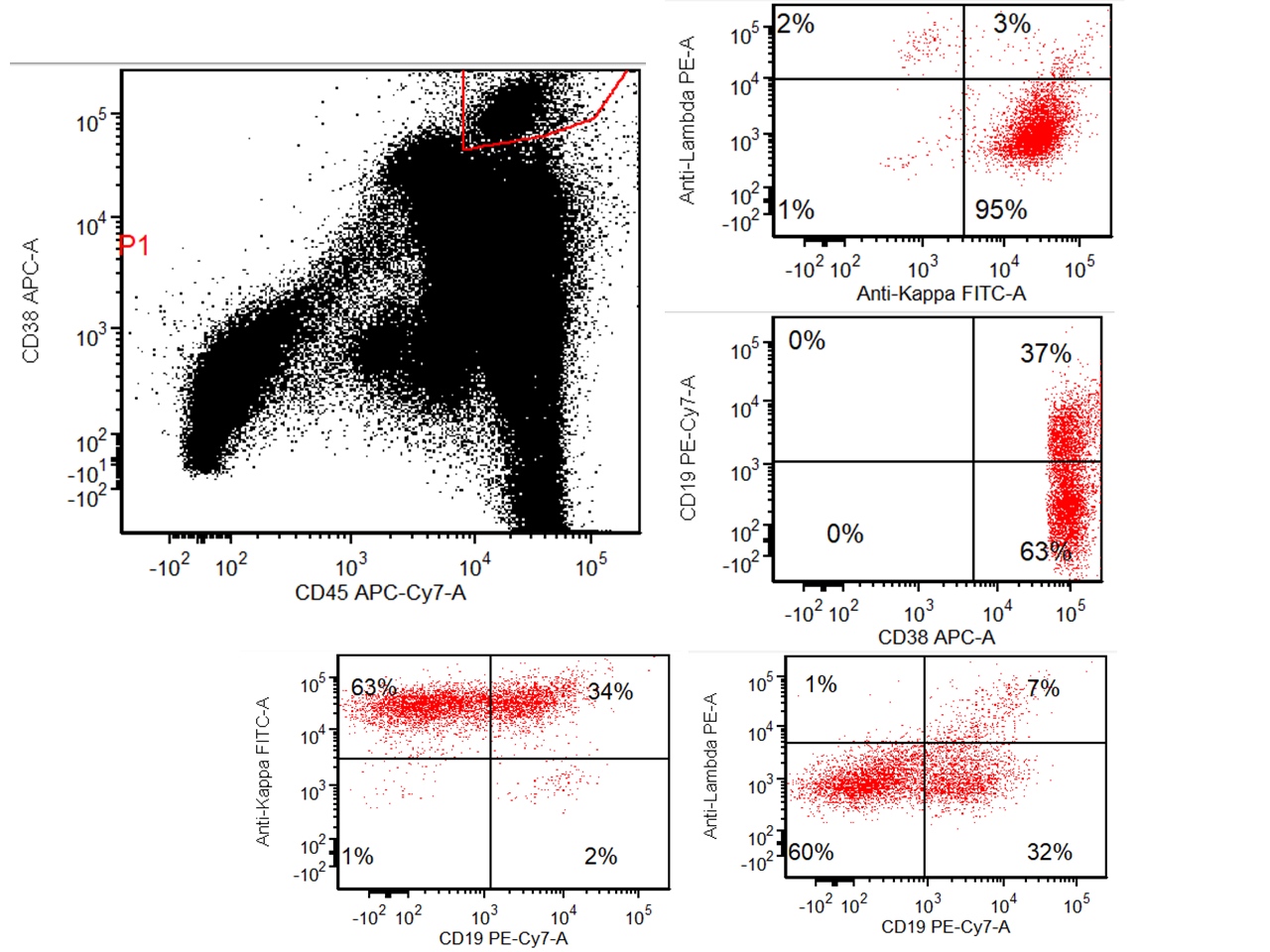
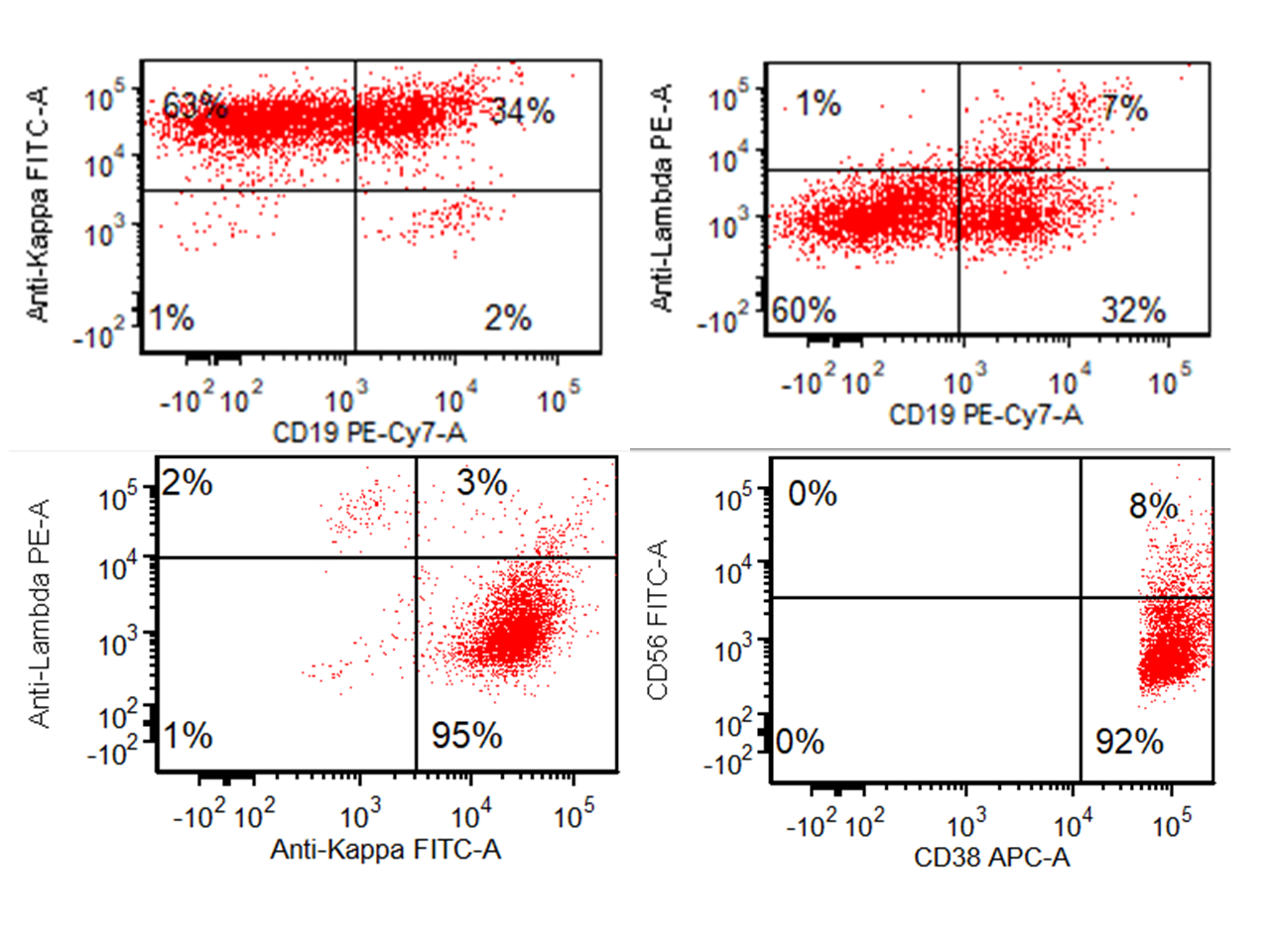
Flow cytometry description
- Monotypic cytoplasmic Ig and usually lack surface light chain
- Express CD38, CD138, often CD56+ or CD117+; may have partial CD45, usually negative for CD20, CD19 and CD10 (Cytometry B Clin Cytom 2016;90:61)
- Exception in myeloma with t(11:14) where plasma cells more often express B cell markers including CD19 and CD20
Flow cytometry images
Electron microscopy description
- Prominent rough endoplasmic reticulum, often with Russell bodies budding off of it (Acta Haematol 1977;58:173)
Molecular / cytogenetics description
- Immunoglobulin heavy and light chain genes are clonally rearranged with a high load of IGHV gene somatic hypermutation; abnormalities in IGH rearrangement may be seen with light chain only disease (Blood 2004;103:3869)
- Cytogenetics: abnormalities by conventional cytogenetics seen in 33% of cases but identified by FISH in > 90%, including trisomies, deletions and translocations (Blood Cancer J 2015;5:e365)
- FISH studies often performed by enriching the plasma cell fraction using magnetic cell sorting (MACS) and fluorescence activated cell sorting (FACS) to increase detection of cytogenetic abnormalities in interphase cells (Am J Clin Pathol 2011;136:712)
- IGH (14q32) translocations in 40% of tumors, recurrent oncogenes:
- t(4;14) FGFR3 / MMSET (NSD2) (4p16.3); 15%
- t(6;14) cyclin D3 (6p21); 4%
- t(11;14) cyclin D1 (11q13); 20%
- t(14;16) MAF (16q23); 4%
- t(14;20) MAFB (20q11); 1%
- MAFA (8q24)
- Cyclin D2 (12p13)
- Tumors without 1 of the frequent IGH are more often hyperdiploid with gains of odd numbered chromosomes (3, 5, 7, 9, 11, 15, 19, 21); 50%
- Monosomy or partial deletion of chromosome 13 (13q14) found in nearly 50% by FISH
- MYC rearrangements in nearly half of tumors
- Activating mutations of KRAS, NRAS or BRAF in 40% of tumors
- Other: TP53 deletion or mutation, gain of 1q, loss of 1p, NF kappa B pathway activation, inactivation of CDKN2C, RB1, FAM46C, DIS3 and DNA methylation changes
Videos
Sample pathology report
- Bone marrow aspirate smears, touch imprints, core biopsy and clot section with peripheral smear:
- Plasma cell myeloma (see comment)
- Normocytic anemia, rouleaux formation
- Comment: The patient is a 65 year old man who recently presented with back pain, anemia and hypercalcemia. Xrays demonstrated evidence of lytic bone lesions and serum protein analysis demonstrated an IgG kappa M protein. The bone marrow shows sheets of atypical plasma cells in a hypercellular bone marrow overall comprising 80% of the intertrabecular space. Flow cytometry as reported separately, demonstrates a CD56+, CD19- kappa restricted plasma cell population. Please correlate with forthcoming cytogenetics and myeloma FISH panel, which will be reported as an addendum.
Differential diagnosis
- Mature B cell lymphoma with extensive plasmacytic differentiation (marginal zone or lymphoplasmacytic lymphoma):
- Monoclonal gammopathy of undetermined significance (MGUS):
- No myeloma defining events, < 10% bone marrow plasma cells and < 3 g/dL M protein
- Plasmablastic lymphoma:
- Differential consideration from myeloma with plasmablastic features with presence of associated lymphadenopathy, oral mass in absence of myeloma defining signs helpful to distinguish plasmablastic lymphoma
- EBER more often positive in PBL but can be seen in myeloma or plasmacytoma (Histopathology 2015;67:225, J Clin Pathol 2017;70:775)
- Primary effusion lymphoma:
- May express plasma cell markers, particularly MUM1 but universally HHV8 positive, majority coexpress EBER (J Cutan Pathol 2014;41:928)
- Polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy and skin changes (POEMS) syndrome:
- Rare disorder, VEGF correlates with disease activity, predominantly lambda, if other symptoms are not recognized may be more likely to be diagnosed as MGUS (Am J Hematol 2019;94:812)
- Reactive plasmacytosis:
- Rare cases may reach up to 30% plasma cells, will be polytypic
- Solitary plasmacytoma of bone:
- Focal bone symptoms, single lesion on imaging, may have M spike, no bone marrow plasmacytosis (< 10% clonal plasma cells in bone marrow), no CRAB (hypercalcemia, renal failure / insufficiency, anemia, lytic bone lesions)
- Systemic AL amyloidosis:
- Evidence of a clonal plasma cell disorder, demonstration of amyloid (Congo red) and that the amyloid is light chain related, amyloid can occur in association with myeloma
- Telangiectasias, elevated erythropoietin and erythrocytosis, monoclonal gammopathy, perinephric fluid collection and intrapulmonary shunting (TEMPI) syndrome:
- Very rare, identified by constellation of symptoms, responds to plasma cell directed therapy (Blood 2020;135:1199)
Additional references
- Swerdlow: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th Edition, 2017, Leukemia 2021;35:18, Hematology Am Soc Hematol Educ Program 2020;2020:264, Leukemia 2020;34:985, Curr Hematol Malig Rep 2016;11:111, Nat Rev Dis Primers 2017;3:17046, Nat Rev Clin Oncol 2021;18:71, Acta Med Acad 2019;48:57, Morphologie 2015;99:38, Nat Rev Cancer 2012;12:335, Lancet Oncol 2019;20:e302
Board review style question #1
Board review style answer #1
C. IgG lambda M protein in serum of 3.5 g/dL. In smoldering myeloma, end stage organ damage should be absent (no CRAB features), so the elevated serum calcium excludes answer E and the osteolytic lesion excludes option D. Plasma cells in excess of 60% or light chain ratio (involved to uninvolved) of ≥ 100 would qualify as active plasma cell myeloma excluding answers A and B. In the diagnosis of smoldering myeloma the patient may have M protein in serum (IgG or IgA) at ≥ 3 g/dL or Bence Jones protein ≥ 500 mg/24 h urine or 10 - 59% clonal plasma cells in bone marrow.
Comment Here
Reference: Plasma cell myeloma (multiple myeloma)
Comment Here
Reference: Plasma cell myeloma (multiple myeloma)
Board review style question #2
Board review style answer #2
D. t(11:14). The presence of CD19 positive clonal plasma cells may favor the presence of a small B cell lymphoma with plasmacytic differentiation as opposed to a plasma cell neoplasm in many cases. These entities are important to differentiate as the treatments are generally distinct. However, in the setting of a t(11;14) translocation, the plasma cells are cyclin D1+, typically retain CD19 expression and may express additional B cell markers including CD20. This can create potential diagnostic pitfalls (Leuk Res 2013;37:1251).
Comment Here
Reference: Plasma cell myeloma (multiple myeloma)
Comment Here
Reference: Plasma cell myeloma (multiple myeloma)
Board review style question #3
Which of the following findings would most strongly favor the presence of a neoplastic plasma cell process rather than a reactive plasma cell proliferation?
- Interstitial plasma cells comprising 10% of cellularity
- Plasma cells with prominent clock face chromatin
- Russell bodies
- Scattered immature plasma cells
- Scattered Mott cells with grape-like inclusions
Board review style answer #3
D. Scattered immature plasma cells are more specific to a neoplastic process compared to binucleation, Russell bodies or mild plasmacytosis.
Comment Here
Reference: Plasma cell myeloma (multiple myeloma)
Comment Here
Reference: Plasma cell myeloma (multiple myeloma)








%20skull%20xray.jpg)
%20skull%20xray2.jpg)
%20tibia%20xray.jpg)