Table of Contents
Introduction | Prerequisites | Some Things to Do | Diagrams / tables | The Test - Lundberg's Loop | Phases of the Loop | Assessing and improving operationsCite this page: Horowitz R. Organization of the laboratory. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/managementlaboperationsorganization.html. Accessed April 19th, 2024.
Introduction
- The organization or systematization of the laboratory is enshrined in several documents: The Organizational Chart, The Policy Manual(s) and The Procedure Manuals
- These documents describe the integration of personnel, equipment, supplies and facilities for efficient and effective laboratory operation
Prerequisites
- Clear designation of authority and responsibility
- Precise identification of operations to be performed and goals to be achieved
- Accurate assessment of available personnel, skills, space, equipment and supplies needed to perform operations and implement the Strategic Plan
Some Things to Do
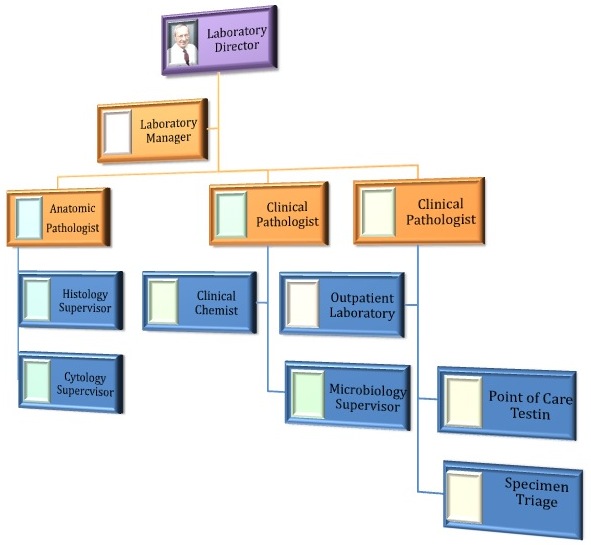
- The Organizational Chart:
- Establishes core authority and responsibility (JC Requirements)
- Clarifies different authorities and responsibilities for the Laboratory Director, the Pathologists, the Laboratory Manager and the Technical Supervisors (CLIA requirements)
- Identifies hierarchies and relationships
- Clarifies reporting lines
- Clarifies expectations
- Types include Hierarchical, Horizontal and Matrix
- To be of value, the organizational chart must be prominently displayed
- See example of hierarchical chart below
- The Policy Manual(s):
- Policies are the laws and rules of the organization and its units
- Employee Handbook is published by human resource department
- Safety Manual generally comes from the risk management department
- IT and Purchasing Manuals originate in these departments
- The Laboratory (User) Manual is the responsibility of the laboratory and contains information for the laboratory's users:
- Laboratory Staff - names, phones, expertise
- Accreditation Information - CAP, AABB, CLIA
- Location, hours of operation
- Test Ordering Information, STAT's
- Specimen requirements, collection information
- Result reporting systems
- How to use computer
- Listing of individual tests including test name, synonyms, when to order test, patient preparation, what specimen to collect, availability, TAT, methodology, result interpretation, reference ranges, sensitivity, specificity, predictive value, diagnostic efficiency
- Other important information, e.g. POC testing
- Procedure Manuals contain the instructions for performing specific tests or other laboratory specific tasks such as Equipment Maintenance Manuals
The Test - Lundberg's Loop
- The fundamental product of pathology and laboratory medicine is information based on the performance of tests
- In order to plan, organize and control lab operations the "test" must be the central element of concern
- George Lundberg, MD expanded the concept of a "test" from merely an analysis to the "brain-to-brain loop" wherein a "test" really commences when a clinician has a problem and is not completed until the problem is solved
- The terms "pre-analytic", "analytic" and "post-analytic" have been used to separate the phases of testing and to identify those phases which are clearly the responsibility of the laboratory and those that occur outside of the laboratory and are outside its control
- Regardless of where in the testing cycle a defect exists, or an error occurs, if the clinician's problem is not solved, it is considered a "lab error"
- Therefore, when designing (or assessing) laboratory operations, it is necessary to scrutinize all components of the "loop"
- In general, the analytic phase which is clearly under the control of the laboratory is well controlled by the laboratory's quality assurance and performance improvement systems and causes few slip-ups; the other phases of the "loop" are where errors most often occur and where the laboratory must take ownership and responsibility
- In this section the various sub-systems, or phases, which comprise the brain-to-brain loop are described
- The Brain-to-Brain Loop (JAMA 1981;245:1762, JAMA 1998;280:565, Am J Clin Pathol 2011;136:829)
- A physician has a problem
- The physician thinks of using a test to help solve the problem
- A test is ordered, verbally or in writing
- The test request is entered into an information system (HIS or Web)
- The requisition is transferred to the laboratory computer
- Laboratory computer generates pick-up lists, work lists, billing data, checks for duplicate test orders, duplicate names and checks for appropriateness of order (in terms of Admitting Diagnosis or ICD codes)
- Phlebotomist or nurse obtains specimen and sends it to the laboratory
- Specimen is triaged in laboratory central receiving area
- Test is performed and verified (the old concept of what a test is)
- Pathologist interprets test as needed
- Test results are transferred to the originating information system
- Test results are available at the nursing station (paper or CRT)
- Test results are placed in patient chart and sent to physician's office
- Physician uses the test results to help solve the problem
Phases of the Loop
- Requirements of the Specimen Acquisition Phase
- This is the next phase; the LIS generates pick-up lists, work lists, billing data, checks for duplicate test orders, duplicate names and checks for appropriateness of order based on Admitting Diagnosis or ICD codes
- A phlebotomist or nurse obtains specimen and sends it to the laboratory and the specimen is triaged in laboratory central receiving area
- This phase of the "TEST" has many requirements, most not in, or related to, the laboratory; these include:
- Patient and specimen identification system (bar code, RFID)
- Hospital computer system that has the capability for duplicate name check
- Laboratory computer system that has capability for duplicate test order checking
- Personnel training for proper specimen acquisition; phlebotomists, nurses, couriers - all need training - particularly nurses and orderlies who are responsible for specimen acquisition, e.g., sputum or urine or ICU specimens from IV lines
- Special training for personnel outside the hospital, particularly doctor's office personnel, surgicenter personnel, remote outpatient phlebotomy sites, etc.
- Equipment: computer, cars, pneumatic tubes, bar code printers, phlebotomy carts, robots for specimen transport must all be checked for proper functioning; for example, does the car used by the courier service have appropriate refrigeration for specimens? Does the pneumatic tube system cause hemolysis?
- Specimen triage in the laboratory's Central Receiving and Processing area is often the source of errors, it requires fail-safe systems and protocols for:
- Test ordered lists (on paper or on computer)
- Specimen receipt list
- Specimen splitting and distribution
- Work lists
- Send-out list
- Overdue test lists
- Re-draw lists
- Requirements of the Testing Phase
- This is the next phase of the "Test Loop" and the one usually thought of by laboratorians as constituting the "test"
- Some general requirements are listed below - these obviously differ for the various tests done in the laboratory, but must be kept in mind at all times:
- Test development and selection requires assessment of clinical utility, cost analysis and comparison, technology assessment, impact on workload, need for information management and employee education and test validation
- Test performance is dependent on: competent personnel, functioning equipment, adequate space and proper reagents
- A Standard Operating Procedure (SOP) for every test needs detailed descriptions of:
- Purpose and principles of the test
- Specimen requirements
- Reagents and equipment
- Testing procedure
- Calibration
- Quality control
- Calculations
- Interpretation
- References
- History of the test in the laboratory
- Quality assurance (encompassing QA, QC, quality management, proficiency testing, performance measurement, process improvement, outcomes management, etc.) is an essential component of the Testing Phase
- Point of care and ancillary testing is generally the responsibility of the laboratory and needs special attention with regard to:
- Justifying the point of care testing
- Developing simple methods for non-lab personnel
- Acquiring fail-safe instruments
- Selecting methods and equipment
- Training personnel
- Quality management and proficiency testing
- Billing
- Transfer of result data from POC to LIS
- Pathologist's interpretation and verification is required on all anatomic and selected clinical pathology tests
- Requirements of the Reporting Phase
- This is the final phase of the "Loop" and consists of: results transfer to the originating information system, to the nursing station, to the patient chart, to the ordering physician's office and to the physician's brain so he can use the information to solve his original problem
- See Arch Pathol Lab Med 2008;132:1608, Arch Pathol Lab Med 2008;132:84, Arch Pathol Lab Med 2009;133:942
- This phase requires a report which may be paper, computer screen, verbal or other
- Pathology is an information business; our product is information in the form of reports, numbers, graphs, pictures, descriptions and diagnoses
- Our various report formats are the "gift wrap" for our product; this includes the classical paper report, the telephone report and the report appearing on the computer screen
- The design of the various report formats is the responsibility of the laboratory working with the clinicians and other customers, e.g., nurses, ward clerks
- Essentials of a good form
- Visual appeal - include logo - get design help
- Easy data entry and readability
- Customized report formats for different customers
- Zoning, spacing, sequencing, emphasizing and elimination of unnecessary data
- Establish a system of forms control
- If there are too many forms, too much paper, poor readability, filing problems
- Collect all forms, index and classify them, analyze the need for each form, eliminate, consolidate and redesign the essential forms
- Pathologists' special reporting functions - the TELEPHONE
- Call the surgeon and attending physician on all malignant, interesting or strange surgical diagnoses
- Call attending physician about all new panic values or unusual clinical laboratory test results
- STAT and critical values reporting
- Criteria for inclusion and ranges by clinicians
- Reporting methods: phone, computer, text message
- Hierarchical escalation of reporting
- HIPAA
- Privacy protection standards
- Billing Subsystem
- Although not included in the original Lundberg Loop, billing is an essential component of laboratory operations and has many requirements that will be discussed in subsequent chapters on financial management
- References: JAMA 1981;245:1762, JAMA 1998;280:565, Am J Clin Pathol 2011;136:829
Assessing and improving operations
- Work Flow Analysis
- Synonyms: process analysis, flow charting, time motion study, function sequencing; this is the best way to learn how your laboratory operates
- How to do work flow analysis:
- Direct observation by Laboratory Director and/or Chief Tech of one work station at a time, for a representative time span
- Include every step in the testing process
- Assess all involved personnel, equipment, functions including machine set up time, reagent storage, batching, when work arrives, how work is done, how results are reported, breaks, backup personnel and equipment, interactions with others in the laboratory, distance to storeroom, to computer terminal, availability of terminals, etc.
- Simple observation and charting of above and reviewing it with the involved personnel can lead to remarkable efficiencies
- It is essential to perform a work flow analysis for the entire laboratory before embarking on a major new program like installation of a laboratory computer, a collaborative venture or before designing a new laboratory or when assigned to a new laboratory
- The critical evaluation of the work flow analysis will result in improved efficiency by eliminating duplication, improving staffing and scheduling of personnel, better space and equipment utilization, better computer interfaces, improved reagent and supply management
- Other project management techniques
- Gannt Charts - a simple bar chart showing tasks, projects, startup and completion dates; overlapping bars show interrelationship of the various tasks
- PERT Charts - Program Evaluation and Review Technique: a method for setting time goals, particularly for research and development projects
- CPM - Critical Path Method: defines the tasks that need to be done, analyzes the sequence in which the tasks must be completed and estimates the time needed for completion; the method uses a diagram which consist of "nodes" representing activities connected by arrows showing the relationships among the activities
- How to Assess a Laboratory
- Is management system evident
- Is an organizational chart visible?
- Are policy and procedure manuals evident?
- Safety, environment, cleanliness, order
- Good lighting, air quality, noise levels
- Scheduling system
- Teamwork and motivation
- Are performance goals and achievements posted and visible?
- Space utilization, movement of materials, products
- Well labeled storage
- Crowding, use of hallways
- Maintenance of equipment and tools
- Are preventive maintenance schedules posted and visible?
- Levels of inventory?
- Has there been a work flow analysis?
- Commitment to quality and customer satisfaction
- Are customer surveys and QA data displayed?
- Is there a complaint management program?
- Is management system evident
- Signs and Symptoms of Poor Laboratory Operations
- See Wagar, Horowitz, and Siegal: Laboratory Administration for Pathologists (chapter 3)
- Recurrent overload crises
- Supervisors are unable to handle their sections
- Excessive overtime
- Prolonged turnaround time
- Delays due to low supplies
- Skilled workers doing menial tasks
- Supervisors doing bench work
- Excessive traffic, noise, crowding, talking
- Too many phone calls
- Too many STATs
- Complaints, external
- Complaints, internal
- Too many notices or rules posted in the laboratory
- Too many forms
- Frequent equipment failure
- Excess employee turnover
- Decreasing productivity (Productivity = Output/Input)
- Total Tests/FTE or Billable Tests/FTE or WLU/FTE or Billable Tests /Total Labor Expense
- Decreasing efficiency
- Total Revenue $/Test or /Admission or /Month
- Total Expense $/Test or /Admission or /Month
- How to Change a Laboratory
- See Wagar, Horowitz, and Siegal: Laboratory Administration for Pathologists (chapter 3)
- Identify current dissatisfactions or perceptions
- Independently verify and validate those perceptions and dissatisfactions and confirm that change is necessary
- Examine the various possible changes and choose the ideal one
- Define the IDEAL: What are the components? What space, equipment, personnel, supplies, etc., are needed to achieve the IDEAL?
- What are the implications and consequences of the IDEAL (on the laboratories personnel, on other programs and activities)? What are the costs? Where are the areas of resistance to change? Could the IDEAL be modified and still achieve the desired result?
- How can IDEAL be implemented? Who will communicate and direct the changes? What needs to be done? What is the time frame?
- How will the change be evaluated? By whom? According to what standards? Over what period of time?
- Note: to minimize the need to change, there should be an ongoing, monthly evaluation of operations; this is the equivalent of QC in chemistry - it is QC of the management of the laboratory; it is the sine qua non of operational success and needs to be incorporated into the monthly staff meeting with review of marketing, finance, space and equipment, human resources, productivity, efficiency, and bottom line (achievement of goals)
- How to Change People
- In order to change a laboratory, people must change - very difficult
- Any suggestion or request to change implies that what is currently being done is inadequate or "bad"
- People generally think of themselves as "whole" or 100%
- Change takes a hunk out of that self perception, perhaps 20%
- Leadership must fill in the gap with 20% of something new
- Resistance to change can be reduced by:
- Leadership support
- Getting everyone involved in decision making
- Sell change as decreasing difficulties
- Sell change as increasing opportunities and experience
- Other
- Obsolete: TQM, CQI, Quality Circles, Benchmarking
- Currently in Vogue: PDSA, DMAIC, Lean, Six Sigma and RCA
- PDSA is used by Systems Engineers "Plan-Do-Study-Act"
- DMAIC is "Define, Measure, Analyze, Improve, Control"
- Lean was first implemented at Toyota Motors in Japan as a means for creating more value for customers by eliminating waste; the goal is to create processes that need less human effort, less space, less capital, and less time to make products and services at far less costs and with fewer defects; Lean empowers employees to improve the processes
- Six Sigma is a business management strategy, initially implemented at Motorola, that seeks to improve quality by identifying and removing the causes of defects (errors) and variability in manufacturing and business processes (six sigma refers to six standard deviations or 3.4 errors/million)
- Root Cause Analysis (RCA) is a problem solving method which identifies the root causes of problems or events; the practice of RCA is predicated on the belief that problems are best solved by attempting to correct or eliminate multiple root causes, as opposed to merely addressing the immediately obvious symptoms; there is usually more than one potential contributing factor that causes any given problem
- References: Gaithersburg: Medical Laboratory Management: Forms, Checklists & Guidelines