Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Electron microscopy description | Molecular / cytogenetics images | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Hiser W. Myotonic dystrophy. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/musclemyotonicdystrophy.html. Accessed April 19th, 2024.
Definition / general
- Inherited muscular dystrophy characterized by muscle weakness, myotonia and additional systemic manifestations including cardiac and neurologic
- Myotonic dystrophy type 1 (DM1) and myotonic dystrophy type 2 (DM2) are caused by differing nucleotide repeat expansions but have similar pathophysiologic mechanisms
- DM1 is the most common type of adult onset muscular dystrophy
Essential features
- Autosomal dominant (AD) muscular dystrophy caused by expansions of different nucleotide repeats which affect RNA splicing and processing, leading to muscle weakness, myotonia and systemic effects
- Variable clinical course, from late onset of mild symptoms to death in infancy
- Frequently involves cardiac conduction system and CNS
- Significantly increased internal nuclei on histologic examination
Terminology
- Dystrophia myotonica (DM)
- Classic type DM1 was first clinically described in 1909 by German physician Hans Steinert and termed, "Steinert's disease" (Biochim Biophys Acta 2015;1852:594)
ICD coding
- ICD-10: G71.11 - myotonic muscular dystrophy
Epidemiology
- Prevalence varies by region and is most common in individuals of European descent
- DM1 is more common than DM2 in the U.S. but some studies suggest similar prevalence of DM1 and DM2 in Europe (Neurol Clin 2014;32:705)
- Combined prevalence reported as 1 in 8,000 (12.5 per 100,000) but this is likely an underestimate due to clinical heterogeneity (Curr Opin Genet Dev 2017;44:30)
Sites
- DM1:
- Involvement of distal limb muscles with preferential involvement of finger, wrist and ankle flexors
- Diaphragm involvement can occur early in disease course
- Neck flexors involved
- Facial muscles involved with wasting of temporalis muscles (hatchet appearance)
- DM2:
- Proximal musculature affected including limb girdle, neck flexors and elbow extensor muscles (Neurol Clin 2014;32:705)
- Less involvement of facial or respiratory musculature
Pathophysiology
- Both forms are a result of toxicity from abnormal mRNA caused by expanded repeats
- DM1 has been more extensively studied
- Mutant RNA with expanded repeats is not exported to cytoplasm but is retained in the nucleus where it forms multiple clumps or foci
- RNA binding proteins such as MBNL1 and DDX6 exhibit high affinity for the mutant RNA and become sequestered in the nucleus
- These proteins are normally involved in splicing, as well as mRNA transport, stability and decay
- Protein function is lost once sequestered, leading to incorrect splicing and defects of proteins including insulin receptor, dystrophin, BIN1 and ClC-1 and L type calcium channels
- Mutated RNA may also produce peptides which are directly cytotoxic (Neurol Clin 2014;32:705)
Etiology
- Autosomal dominant (AD) inheritance in both DM1 and DM2
- DM1 is caused by expansion of a CTG repeat in the 3' noncoding region of the DMPK gene on chromosome 19q13.3, which codes for myotonic dystrophy protein kinase
- Normal individuals have between 5 and 37 repeats but symptomatic patients typically have > 50 repeats
- Anticipation is frequently seen
- Symptoms appear earlier and with greater severity in successive generations
- Individuals with borderline elevated CTG repeats (> 50) may be asymptomatic but offspring are at risk
- Clinical presentation correlates with CTG repeat size (Neurol Clin 2014;32:705)
- DM2 is caused by expansion of a CCTG repeat in the first intron of the CNTB gene (previously ZNF9) on chromosome 3q21, which codes for CCHC type zinc finger nucleic acid binding protein
- Normal individuals typically have between 10 and 33 repeats but symptomatic patients usually have greater than 1,000 repeats (range, 75 to greater than 11,000)
- Anticipation is less prominent
- No correlation between repeat size and clinical presentation (Neurol Clin 2014;32:705)
Clinical features
- DM1 is typically broken down into four subtypes: congenital, childhood, classic and minimal / late onset
- Spectrum of clinical severity: from death in infancy to onset in late adulthood with extremely mild symptoms
- Congenital DM1: fetal onset, involving musculature and CNS; severe
- Prenatal features: decreased fetal movement, polyhydramnios
- Neonatal features: hypotonia with feeding or respiratory distress
- 79% require nasogastric feeding, 53% require ventilator support (Neurol Clin 2014;32:705)
- Childhood: delayed motor milestones, intellectual impairment, prominent oropharyngeal weakness with tenting of upper lip
- Degenerative features develop by second or third decade, resembling classic DM1
- More than half of mothers do not carry DM1 diagnosis so diagnosis can be delayed (Neurol Clin 2014;32:705)
- Childhood DM1: between 1 and 10 years of age
- Predominantly cognitive and behavioral issues
- Facial weakness and conduction abnormalities
- Approximately half with intellectual impairment
- Range of psychiatric disorders
- Classic DM1: onset usually between second and fourth decades
- Myotonia is the most common presenting symptom
- More pronounced after rest, improves with activity
- Involves forearms and hands (grip), tongue and jaw
- Muscle weakness of distal limbs and craniofacial muscles
- Wasting of fascial muscles with characteristic ptosis and hatchet appearance
- Respiratory distress secondary to diaphragmatic involvement
- Cardiac conduction abnormalities common
- Risk of sudden cardiac death as high as 1.1% per year
- Cataracts located on the posterior lens capsule with a multicolored, iridescent appearance on slit lamp examination (Neurol Clin 2014;32:705)
- Sleep disturbances (80% with daytime hypersomnolence)
- Gastrointestinal involvement: cholelithiasis, intestinal dysmotility
- Insulin resistance, metabolic syndrome, frontal balding and hypogonadism in men
- Evidence for increased risk of malignancy (thyroid, ovarian, colorectal, endometrial, Mayo Clin Proc 2012;87:130, JAMA 2011;306:2480)
- Myotonia is the most common presenting symptom
- Minimal / late onset DM1: small expansions (70 - 100 repeat) with mild weakness, myotonia and development of cataracts, usually after age 40 (range 20 to 70 years, Neurol Clin 2014;32:705)
- DM2: overall milder disease; most often presents in the third decade of life (range second to sixth decades)
- Presents with proximal muscle weakness as well as myotonia
- May resemble limb girdle dystrophy
- Less muscle wasting and respiratory involvement than DM1
- Frontal balding, hypogonadism, cataracts and insulin resistance
- Less cardiac and CNS involvement than DM1 (Yachnis: Neuropathology - A Volume in the High Yield Pathology, 1st Edition, 2014)
- Some patients exhibit calf and thigh hypertrophy (true hypertrophy)
- Patients often have a history of unexplained pain and may have a diagnosis of fibromyalgia (Neurol Clin 2014;32:705)
- Presents with proximal muscle weakness as well as myotonia
Diagnosis
- Molecular testing is definitive and may be the only test performed in the appropriate clinical setting
- PCR is most commonly used for detection of repeat expansion
- Southern blot is sometimes utilized in addition to PCR testing (Eur J Hum Genet 2012;20:1203)
- Muscle biopsy is infrequently performed if clinical suspicion is high
Laboratory
- Not specific
- Liver function tests (LFTs) commonly abnormal
- Creatine kinase (CK) can be normal to slightly elevated
- Electromyography (EMG) shows combination of myotonic features and myopathic changes (Yachnis: Neuropathology - A Volume in the High Yield Pathology, 1st Edition, 2014)
Radiology description
- No specific imaging features of the involved musculature have been identified
- Imaging of CNS may show alterations in the white matter signal intensity, most notable in the frontotemporal region
- Prenatal ultrasound in congenital DM1 patients may show borderline ventriculomegaly or talipes equinovarus (club foot) (Neurol Clin 2014;32:705)
Prognostic factors
- Classic DM1 has a slowly progressive course
- Respiratory failure is the leading cause of death in DM1, followed by sudden cardiac death (Neurol Clin 2014;32:705)
- Congenital DM1 patients may live to adulthood and typically die of cardiorespiratory complications (similar to classic DM1)
- DM2 patients typically have a milder clinical course
Case reports
- 38 year old man with myotonic dystrophy mimicking postpolio syndrome in a polio survivor (Am J Phys Med Rehabil 2009;88:161)
- 61 year old man with normal pressure hydrocephalus (Neurosurgery 2006;58:E796)
- Woman with gastric bypass surgery for obesity in DM1 (Neuromuscul Disord 2015;25:414)
- Patient with hydrocephalus and cognitive decline in myotonic dystrophy (Arch Phys Med Rehabil 1998;79:1022)
Treatment
- No curative treatment - supportive therapy only
- Many patients require nighttime respiratory support
- May require pacemaker or defibrillator placement
- Ongoing research into curative genetic therapies
Microscopic (histologic) description
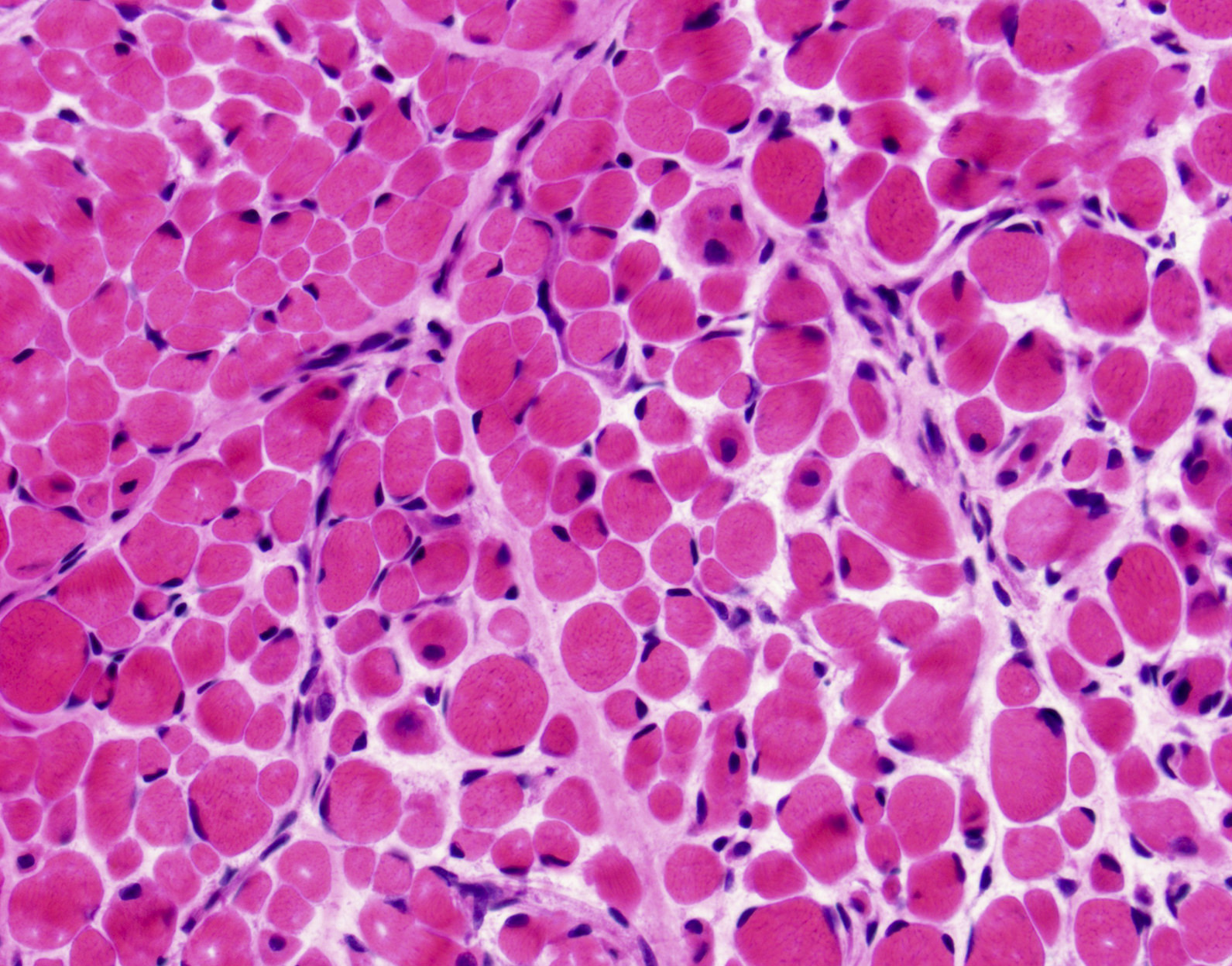
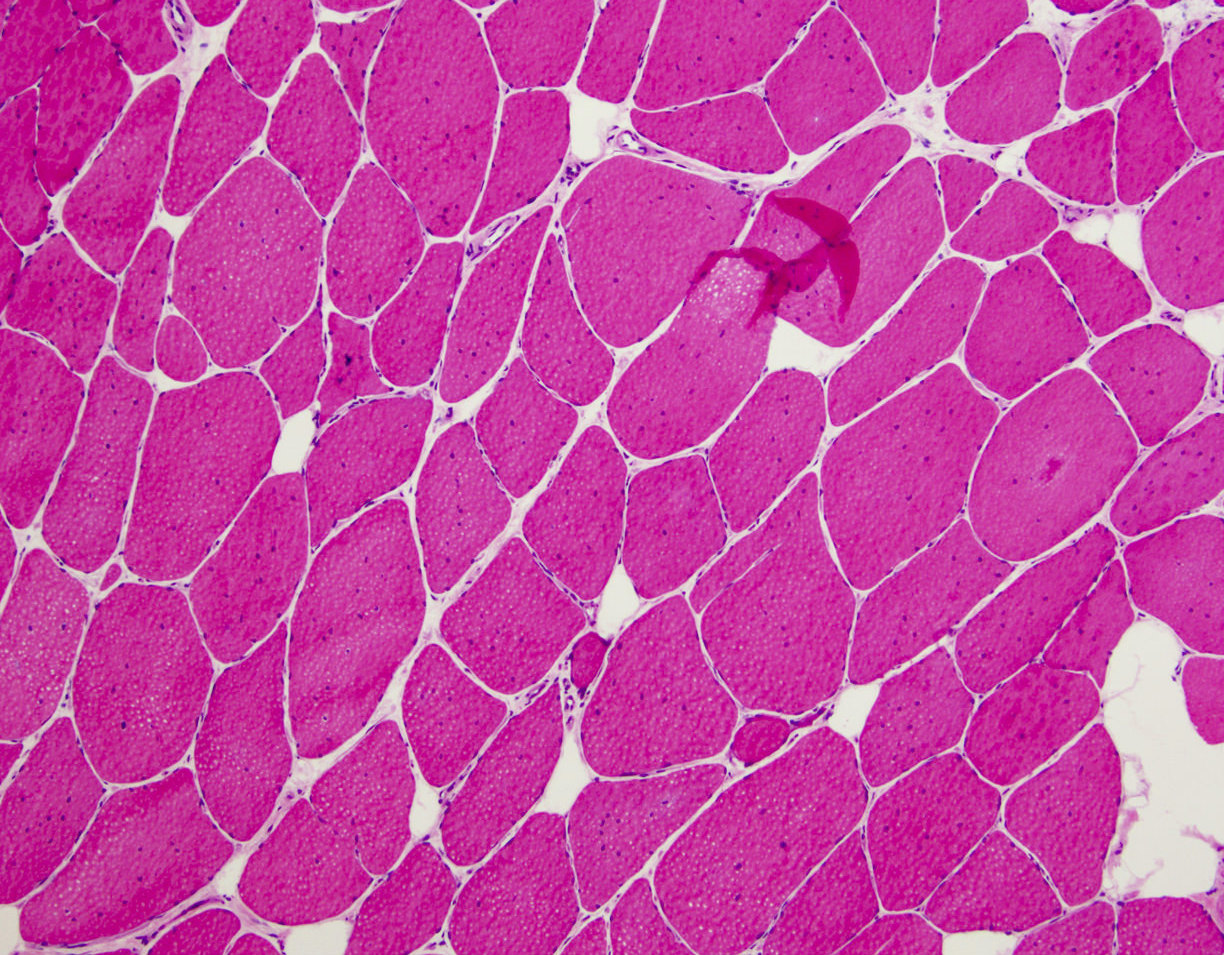
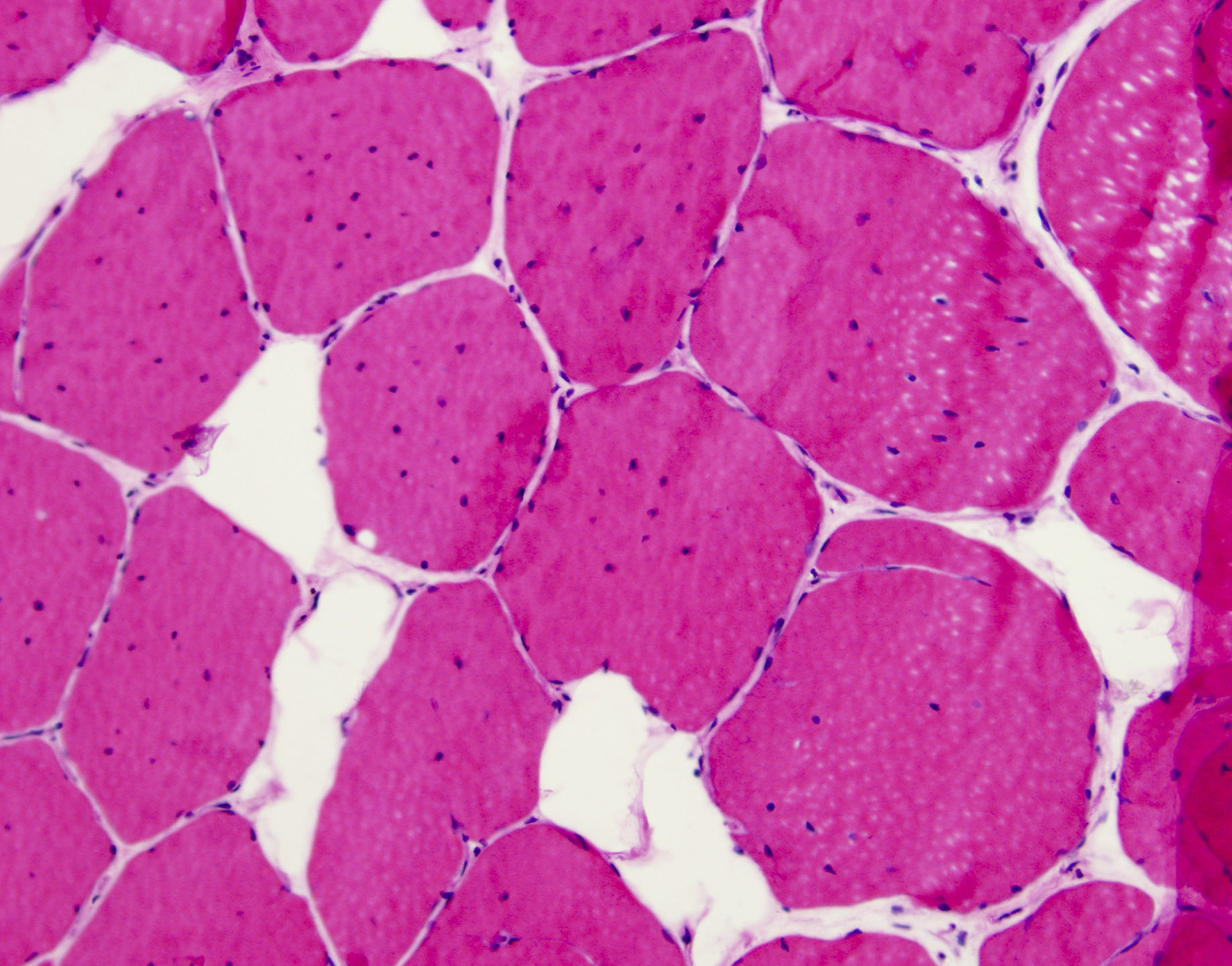
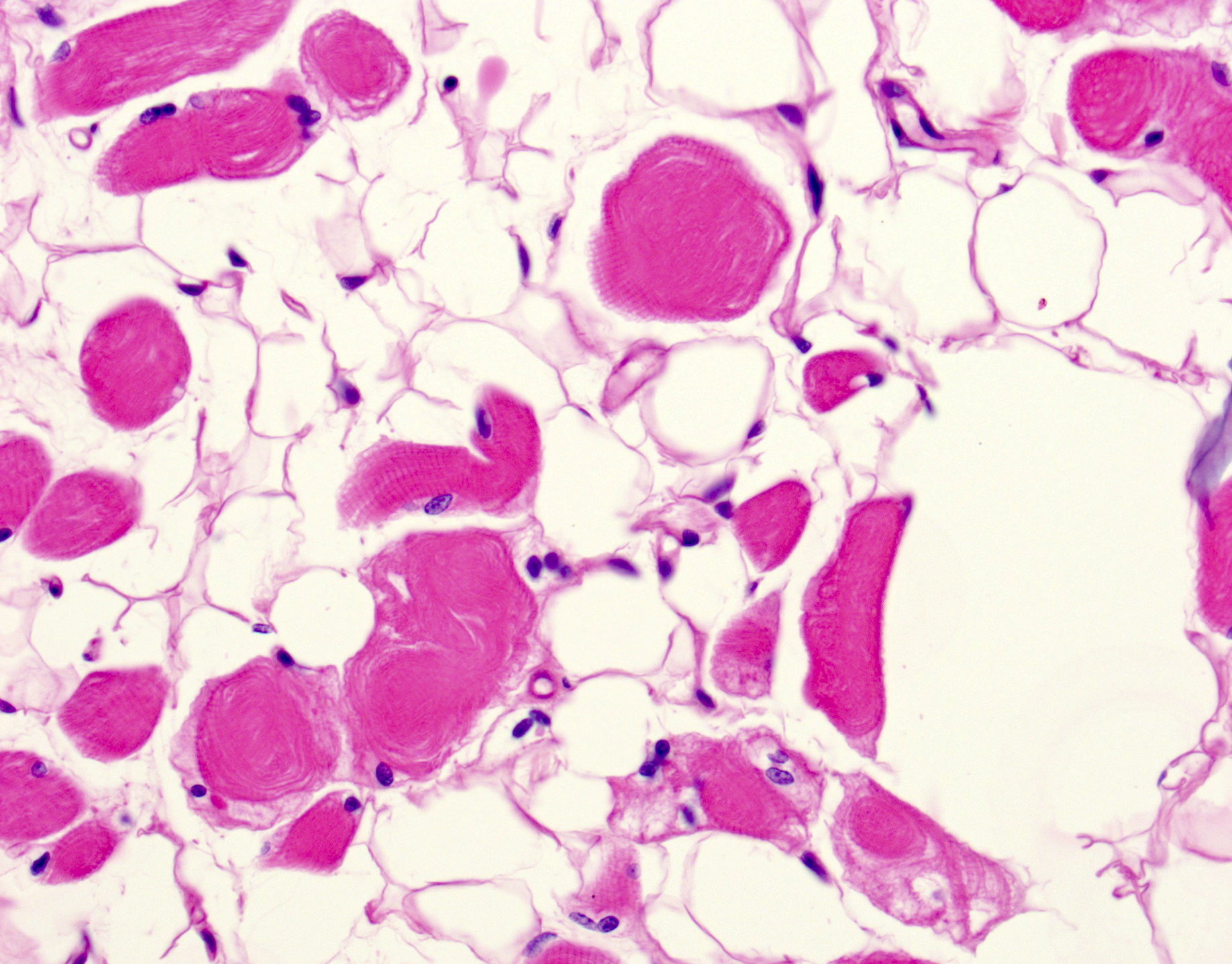
- Variation in myofiber size, ranging from 10 um to 100 um
- Ring fibers and sarcoplasmic masses (dark staining regions) are frequently seen in DM1
- DM1: type 1 myofiber atrophy with type 2 hypertrophy
- DM2: greater variation in both type 1 and 2 fibers with predominantly type 2 myofiber atrophy (Yachnis: Neuropathology - A Volume in the High Yield Pathology, 1st Edition, 2014)
- Pyknotic nuclear clumps in atrophic fibers
- May see moth eaten or whorled fibers
Microscopic (histologic) images
Positive stains
- No specific special or immunohistochemical stains are typically utilized for the diagnosis
- In situ hybridization (ISH) specific for CTG or CCTG repeats may be utilized in some laboratories to allow for direct visualization of aberrant mRNA in muscle biopsies (Acta Myol 2013;32:154)
- Staining for MBNL1 is also available in some laboratories (Neuromuscul Disord 2012;22:225)
- Involved neurons of the limbic system and brain stem contain tau protein positive neurofibrillary tangles (Yachnis: Neuropathology - A Volume in the High Yield Pathology, 1st Edition, 2014)
Electron microscopy description
- Sarcoplasmic masses composed of disorganized myofibrils, dilated sarcoplasmic reticulum and free ribosomes (Yachnis: Neuropathology - A Volume in the High Yield Pathology, 1st Edition, 2014)
Molecular / cytogenetics images
Differential diagnosis
- Limb girdle dystrophy (DM2): often has significantly increased CK; molecular testing and immunohistochemistry on muscle biopsy can also be utilized to make the diagnosis
- Other muscular dystrophies: molecular studies will aid in differentiation; myotonic dystrophy shows a greater number of and more consistent internal nuclei
- Myotubular myopathy (in congenital DM1): greater number of internal nuclei in DM1 without peripheral halos seen in myotubular myopathy; can also look for MTM1 gene mutation
- Myopathic conditions (i.e. inflammatory myopathies): degenerating and regenerating fibers, as well as inflammatory cell infiltrates, are not commonly seen in myotonic dystrophy
Additional references
Board review style question #1
Myotonic dystrophy type 2 (DM2) is inherited in what pattern?
- Autosomal dominant
- Autosomal recessive
- Mitochondrial
- X linked
Board review style answer #1
A. Both DM1 and DM2 are inherited in an autosomal dominant pattern.
Comment Here
Reference: Myotonic dystrophy
Comment Here
Reference: Myotonic dystrophy