Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Vazzano J, Chen W. Colloid carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreascolloid.html. Accessed April 19th, 2024.
Definition / general
- Infiltrating ductal epithelial neoplasm of the pancreas characterized by the presence (in at least 80% of the neoplasm) of large extracellular stromal mucin pools containing suspended neoplastic cells (WHO)
Essential features
- > 80% of the tumor volume is composed of mucin pools with scanty floating tumor cells
- Associated with intraductal papillary mucinous neoplasm (almost always arises in an intestinal type IPMN), mucinous cystic neoplasm (MCN) and ampullary / duodenal tubulovillous adenomas
- Thought to have more favorable prognosis than conventional pancreatic ductal adenocarcinoma (cPDAC) (World J Gastroenterol 2018;24:4846)
Terminology
- Colloid carcinoma
- Mucinous noncystic carcinoma
- Gelatinous carcinoma
ICD coding
- ICD-10: C25 - malignant neoplasm of pancreas
Epidemiology
- 1 - 3% of malignant neoplasms of the exocrine pancreas
- 27 - 70% of IPMNs with associated invasive adenocarcinoma have a colloid component (Pathology 2008;40:655)
Sites
- Usually in the head of the pancreas
Pathophysiology
- Inverse polarization of cells (mucin glycoproteins in stroma facing surfaces instead of luminal surface)
- Expression of MUC2 (gel forming mucin, rare in cPDAC) and the absence of external lamina or basement membrane may lead to accumulation of extracellular mucin, which limits tumor spread and appears to have tumor suppressor activity (Am J Surg Pathol 2003;27:571, Mod Pathol 2002;15:1087)
Clinical features
- Mean age: 61 years
- M = F
- Abdominal / epigastric pain (in contrast to back pain of cPDAC), pancreatitis (50% of patients), diarrhea, jaundice, weight loss
- Larger (6.0 cm) compared with cPDAC, lower stage, better survival
- Incisional biopsy may contribute to thromboembolic complications (Am J Surg Pathol 2001;25:26)
- Pseudomyxoma peritonei can be a rare complication
Diagnosis
- Based on extensively sampled resection specimen
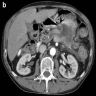
Radiology description
- Dilated ducts (sometimes filled with nodular lesions)
Prognostic factors
- 5 year survival is 57 - 72% (versus 12% for resectable cPDAC) (Am J Surg Pathol 2001;25:26, Dig Dis Sci 2011;56:1295)
- Tumor diameter, presence of IPMN / MCN precursor lesion, surgical margin status, vascular or perineural invasion, lymph node status, KRAS mutation or TP53 mutation are not prognostic factors (Am J Surg Pathol 2001;25:26)
Case reports
- 52 year old woman with history of necrotizing pancreatitis (Arch Pathol Lab Med 2005;129:255)
- 58 year old man with recurrent pancreatitis and 72 year old woman with pancreatic exocrine and endocrine insufficiency (Case Rep Pancreat Cancer 2016;2:40)
- 64 year old man with 15 cm tumor (JOP 2012;13:219)
- 65 year old man with pancreatic head tumor (Oncol Lett 2015;10:3195)
Treatment
- No specific treatment guidelines
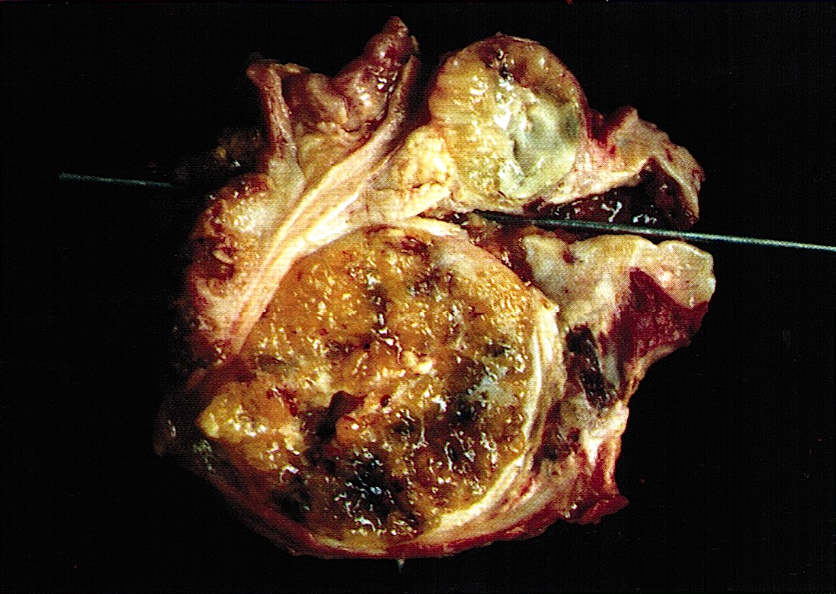
Gross description
- Large (mean 5 cm), well demarcated tumor
- Solid, firm, gelatinous cut surface
Microscopic (histologic) description
- At least 80% of the neoplasm consists of large extracellular stromal mucin pools
- Scanty carcinoma cells suspended within these mucin pools
- Usually cuboidal or columnar cells
- Cribriform or stellate clusters, strips of columnar cells, small tubules or signet ring cells
- Incomplete lining of mucin lakes common
- Mucin tends to be retained during histologic processing (in contrast to IPMN and MCN)
- Muconodular invasive component of 1 cm or more
- Usually arise in association with IPMN, MCN or tubular / tubulovillous adenoma
- Perineural invasion and regional lymph node metastasis common
- Reference: Am J Surg Pathol 2001;25:26
Microscopic (histologic) images
Cytology description
- Difficult to spread thinly on slides due to abundant mucus
- Cellularity of malignant component may be low
Positive stains
- Strong expression of CDX2 and MUC2 (indicating intestinal differentiation); cPDAC usually MUC2-
- CEA shows accentuated staining in basal aspects of tumor cells (luminalization), in addition to luminal staining
- May show focal reactivity for synaptophysin or chromogranin
- Reference: Am J Surg Pathol 2001;25:26
Electron microscopy description
- Mucigen granules on stromal surface, no basement membrane
Molecular / cytogenetics description
- KRAS codon 12 mutation (33%); less frequent than cPDAC (> 90%)
- TP53 mutation (22%)
- High prevalence of somatic mutations in GNAS
- Microsatellite stable, unlike mucinous carcinomas of colon (Mod Pathol 2003;16:537)
Sample pathology report
- Distal stomach, duodenum and pancreatic head, pancreaticoduodenectomy:
- Colloid carcinoma of the pancreas, arising in a background of high grade intraductal papillary mucinous neoplasm (IPMN)
- Carcinoma involves the muscularis propria of the duodenum
- Positive for perineural invasion
- Negative for lymphovascular invasion
- All margins of resection, negative for carcinoma or high grade dysplasia
- Metastatic carcinoma involving one of seventeen lymph nodes (1/17)
- Portion of benign stomach with chronic inactive gastritis, negative for Helicobacter pylori on H&E stained sections
Differential diagnosis
- Extravasated benign stromal mucin:
- Mucus lakes limited to periductal location, devoid of neoplastic cells
- Frequently inflamed
- Intraductal papillary mucinous neoplasm:
- Smooth contours, complete epithelial lining, intraluminal mucin lost during processing
- Mucinous cystic neoplasm:
- Ovarian type stroma
- Usually occurs in women
- Conventional ductal adenocarcinoma:
- Intracytoplasmic and luminal mucin
- No or scant stromal mucin
Additional references
Board review style question #1
A 62 year old woman presented with a pancreatic head mass. Histologic sections demonstrate areas of irregular mucin pools with suspended clusters of malignant cells and also floating signet ring cells. However, no individual infiltrating signet ring cells are identified in the stroma. There is an intestinal type intraductal papillary mucinous neoplasm (IPMN) in the background; no conventional ductal adenocarcinoma component is seen. What is the diagnosis?
- Colloid carcinoma
- IPMN with rupture and extruded stromal mucin
- Mucinous cystadenocarcinoma
- Signet ring cell carcinoma
Board review style answer #1