Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Vazzano J, Chen W. Ductal adenocarcinoma, NOS. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreasductal.html. Accessed April 25th, 2024.
Definition / general
- Pancreatic ductal adenocarcinoma (PDAC) is an invasive pancreatic epithelial neoplasm with glandular (ductal) differentiation, usually demonstrating luminal or intracellular mucin and without a substantial component of any other histological type
Essential features
- Adenocarcinoma derived from pancreatic ductal epithelia, with randomly arranged epithelial elements, intense stromal desmoplasia and variable necrosis
- Poor prognosis: 5 year survival rate of 6% (ranges from 2 - 9%) (Cancer Res 2014;74:2913, World J Gastroenterol 2016;22:9694)
- Precursors of invasive ductal adenocarcinoma:
Terminology
- Sometimes called tubular adenocarcinoma
ICD coding
- ICD-11: 2C10.0 - adenocarcinoma of pancreas
Epidemiology
- Fourth leading cause of cancer related deaths in the U.S. (predicted to rise to the second most common cause by 2030) (CA Cancer J Clin 2021;71:7, Cancer Res 2014;74:2913)
- Globally, 495,773 new cases and 466,003 new deaths in 2020 (CA Cancer J Clin 2021;71:209)
- M:F = 1.13:1 (CA Cancer J Clin 2021;71:209)
Sites
- Head of the pancreas: 60 - 70%; body: 5 - 15%; tail: 10 - 15%
- Head tumors: 50% have distention of biliary tree and progressive jaundice; 85% have extension beyond pancreas at diagnosis
- Body / tail tumors: typically larger at diagnosis since these tumors do not cause symptoms until late; 25% have peripheral venous thrombi; metastases common
- Rarely arises from heterotopic pancreatic tissue in the gastrointestinal tract
- Reference: Surg Pathol Clin 2016;9:547
Pathophysiology
- Invasive ductal adenocarcinoma arises in association with precursor lesions: PanIN, IPMN or MCN
Etiology
- Risk factors: smoking, alcohol abuse, obesity, high intake of dietary saturated fat, chronic pancreatitis, diabetes
- Hereditary syndromes: Peutz-Jeghers syndrome, hereditary pancreatitis, familial atypical multiple mole melanoma (FAMMM), familial pancreatic cancer, Lynch syndrome, familial breast cancer and other Fanconi anemia genes, familial adenomatous polyposis (FAP)
- Reference: World J Gastroenterol 2018;24:4846
Clinical features
- Back pain, weight loss, malaise, jaundice, diabetes mellitus
- Trousseau sign: migratory thrombophlebitis in 10% due to tumor or tumor necrosis producing platelet aggregating factors and procoagulants; causes arterial and venous thrombi
- Coexisting pancreatitis in 10%
- Metastases:
- Local lymph nodes (microscopic metastases found in 75% with T1 / T2 disease)
- Liver, lung, peritoneum, adrenal, bone, distal nodes
- Supraclavicular node metastasis may be a presenting symptom
- Tumor may track along biopsy needle path
- Metastases to ovary may simulate primary mucinous ovarian tumors (Am J Surg Pathol 1989;13:748)
Diagnosis
- Preoperative / pretreatment by endoscopic ultrasound guided fine needle aspiration (EUS-FNA)
- Surgical resection specimen
Laboratory
- Serum tests: CA19-9, CEA
Radiology description
- Hypodense mass on CT imaging in 92% of cases
- Double duct sign (dilation of both the biliary and pancreatic ducts) in pancreatic head mass
- Reference: Gastroenterology 2014;146:291
Prognostic factors
- Dependent on stage and tumor size
- Resectable and size < 4.5 cm are favorable factors
- Perineural invasion and vascular invasion are adverse prognostic factors (Eur J Surg Oncol 2007;33:892)
- High histologic grade unfavorable
- Any squamous component indicates poorer prognosis (median survival: 7 - 11 months)
Case reports
- Woman in her 50s with faint hypoechoic area in the body of the pancreas (J Med Ultrason 2018;45:617)
- 63 year old man with lobulated cystic lesion in the head of the pancreas with diffuse dilatation of the main pancreatic duct (Pancreas 2019;48:e24)
- 78 year old man with two solid tumors in the remnant pancreas after pancreaticoduodenectomy for acinar cell carcinoma (J Nippon Med Sch 2019;86:279)
Treatment
- Most (85%) tumors are not amenable to curative surgery
- For head / periampullary tumors: Whipple resection (subtotal pancreaticoduodenectomy); perioperative mortality: ~2%
- For body / tail tumors: distal pancreatectomy
- Resect retroperitoneal nerves and nodes if stage I / II to reduce local recurrence
- Palliative treatment: bypass operations, chemotherapy and radiation therapy
- Reference: World J Gastroenterol 2018;24:4846
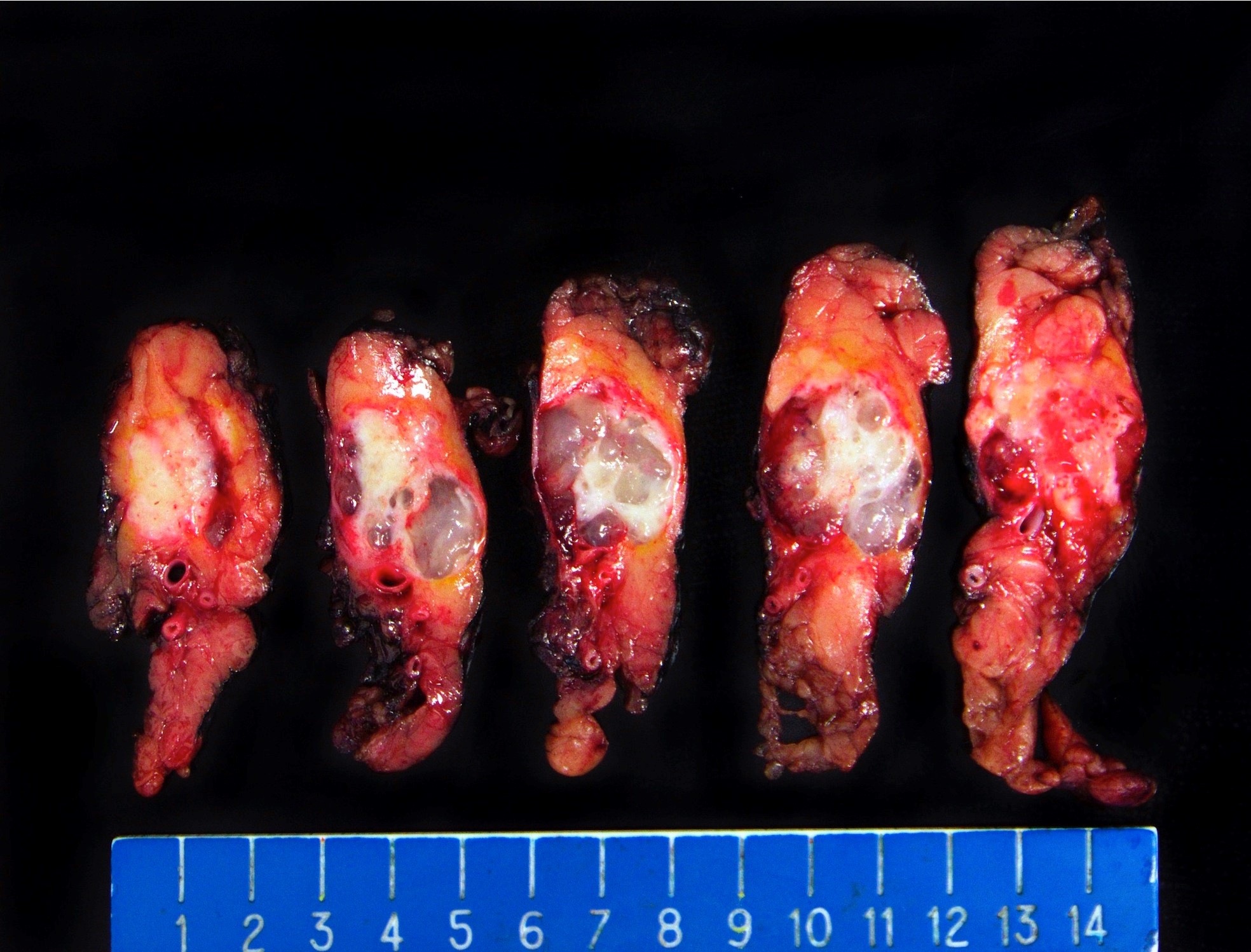
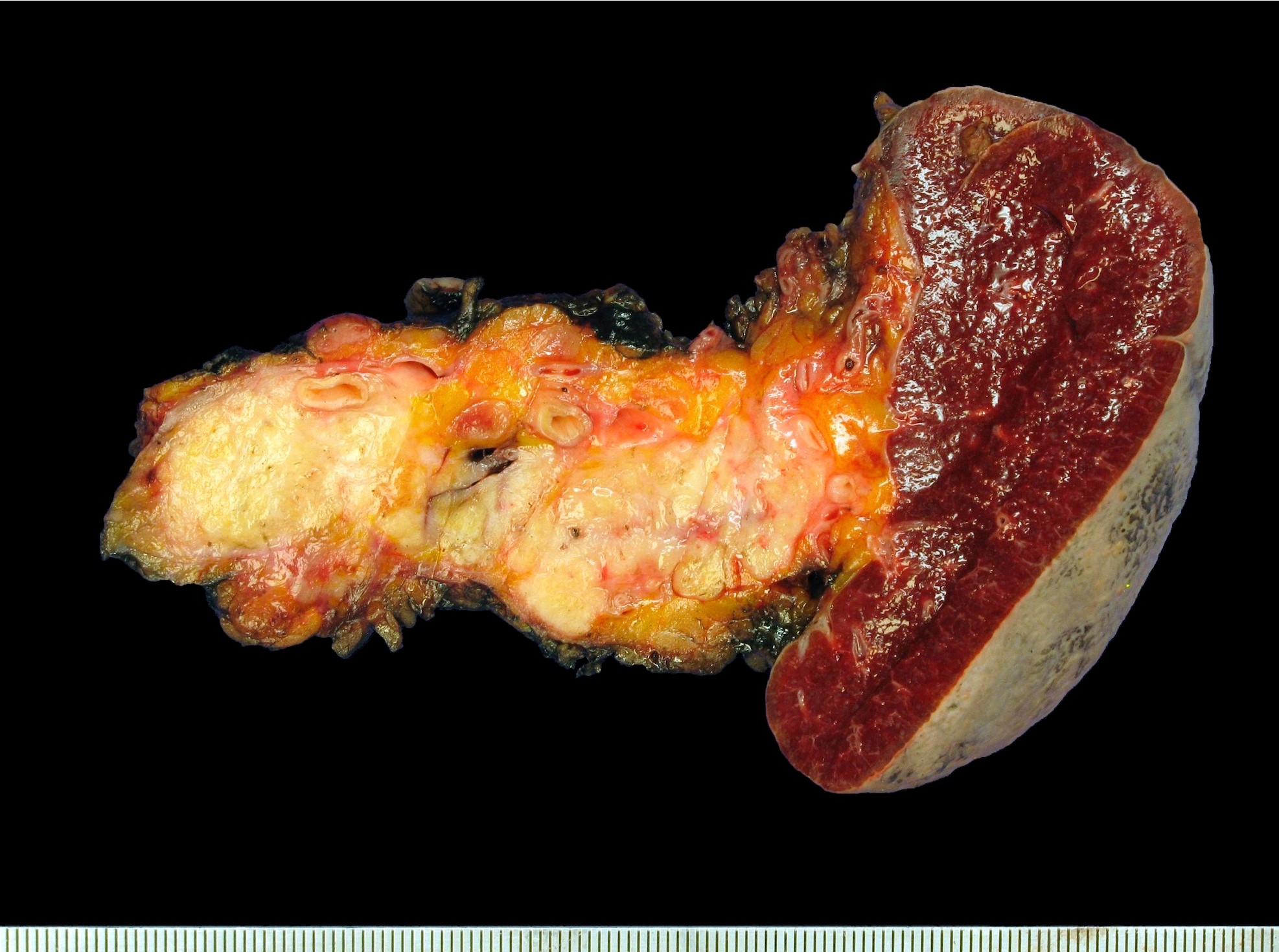
Gross description
- White gray, sclerotic, poorly defined mass
- > 75% are solid tumors
- 25% of head tumors extend to duodenal wall
- If advanced, may be difficult to determine site of origin between pancreas, ampulla and common bile duct
- 20% have multiple tumors
- Reference: Surg Pathol Clin 2016;9:547
Frozen section description
- Frozen sections used to confirm diagnosis and determine vascular resectability, margin positivity and status of extraregional lymph nodes / other metastatic sites
- Common margins evaluated: pancreatic neck / distal pancreatic parenchyma, bile duct and uncinate
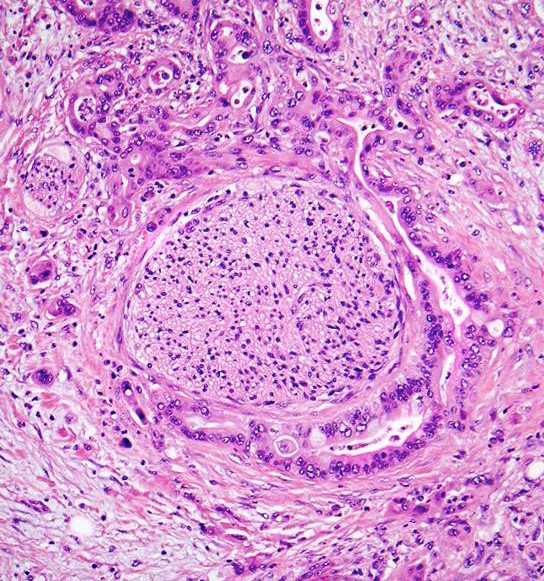
- Helpful features of PDAC: anisonucleosis in a single gland (at least 4:1), perineural invasion, tumor gland immediately next to large vessel, single infiltrating cells, low power evaluation for disorganized duct distribution with ill defined borders, necrosis and mitoses (Arch Pathol Lab Med 2021 Mar 26 [Epub ahead of print])
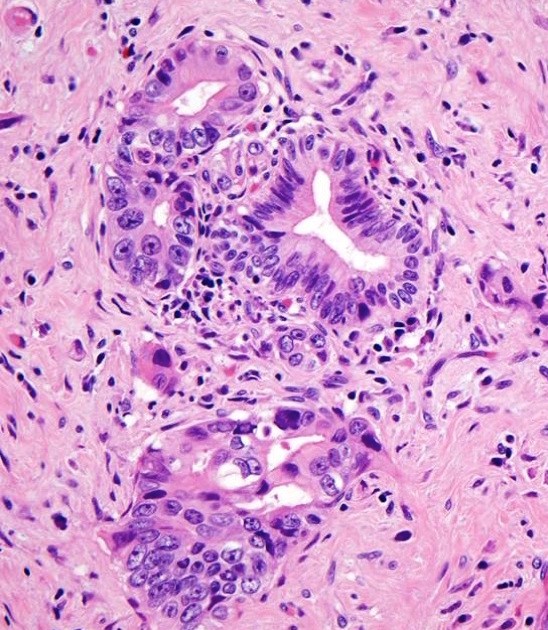
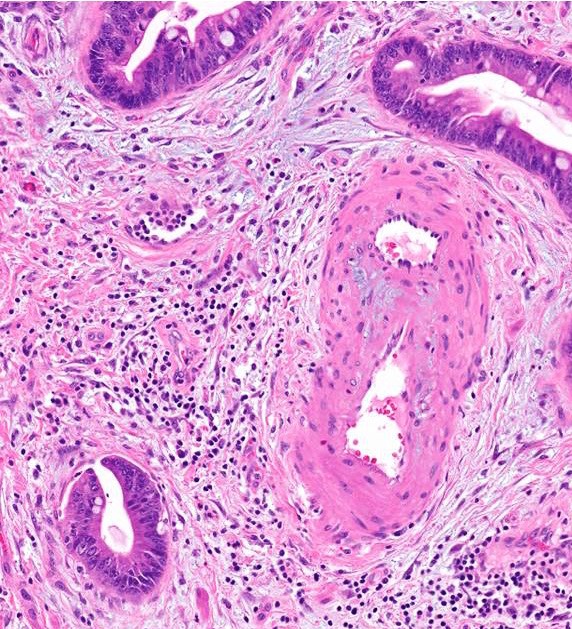
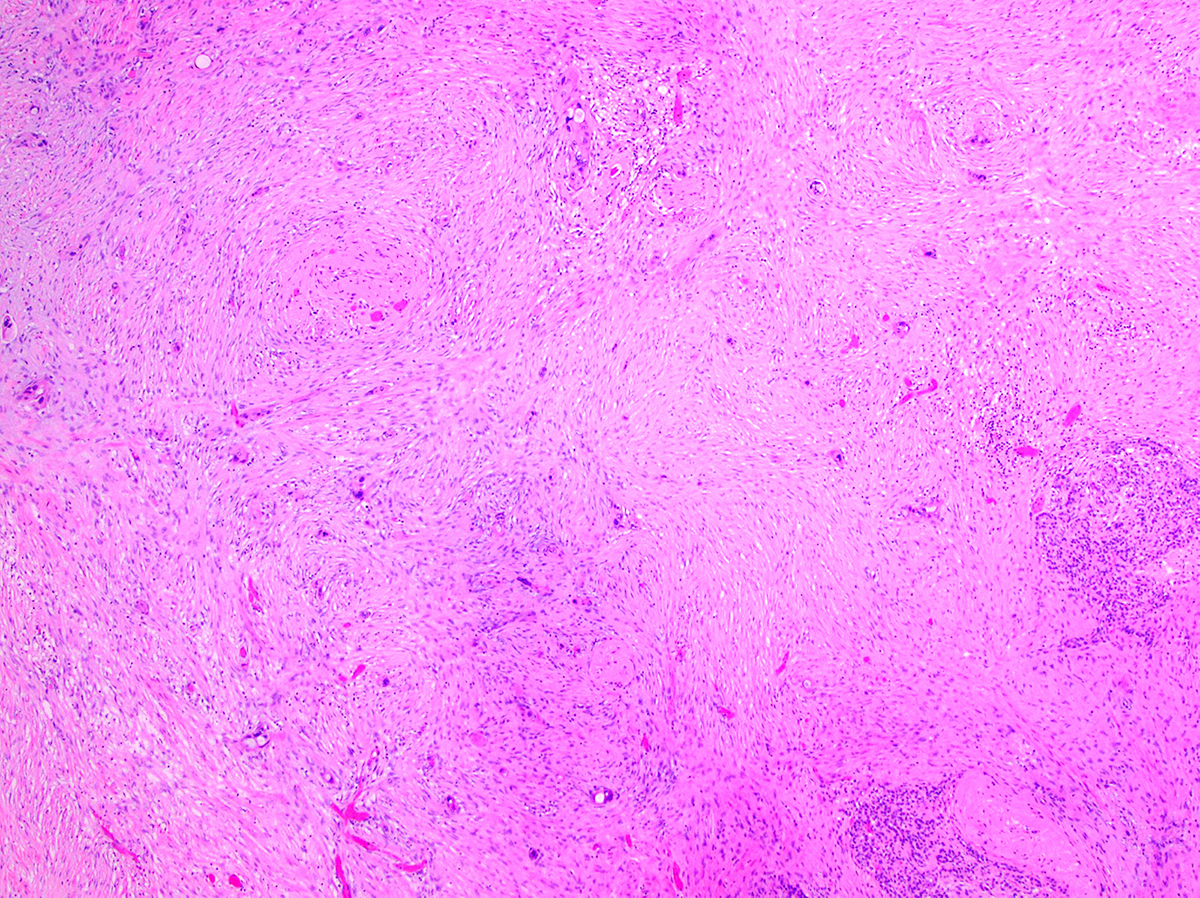
Microscopic (histologic) description
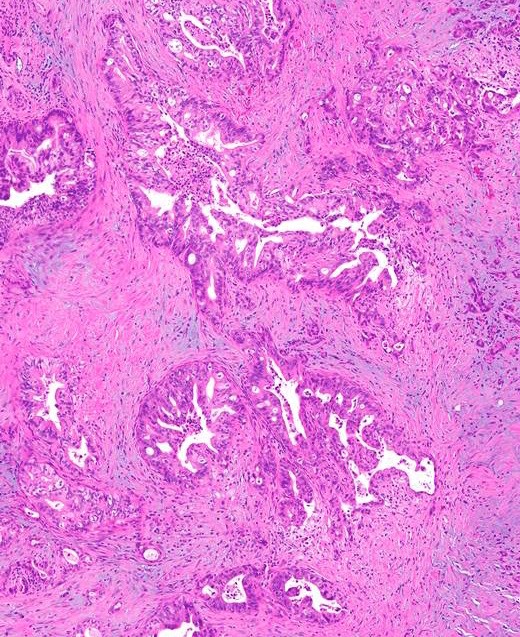
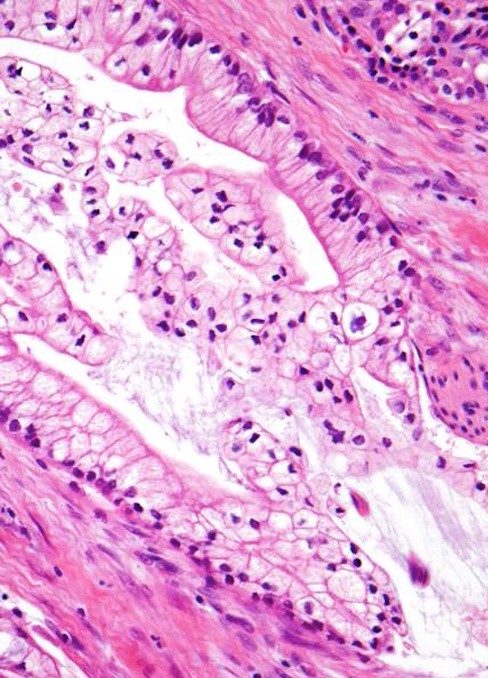
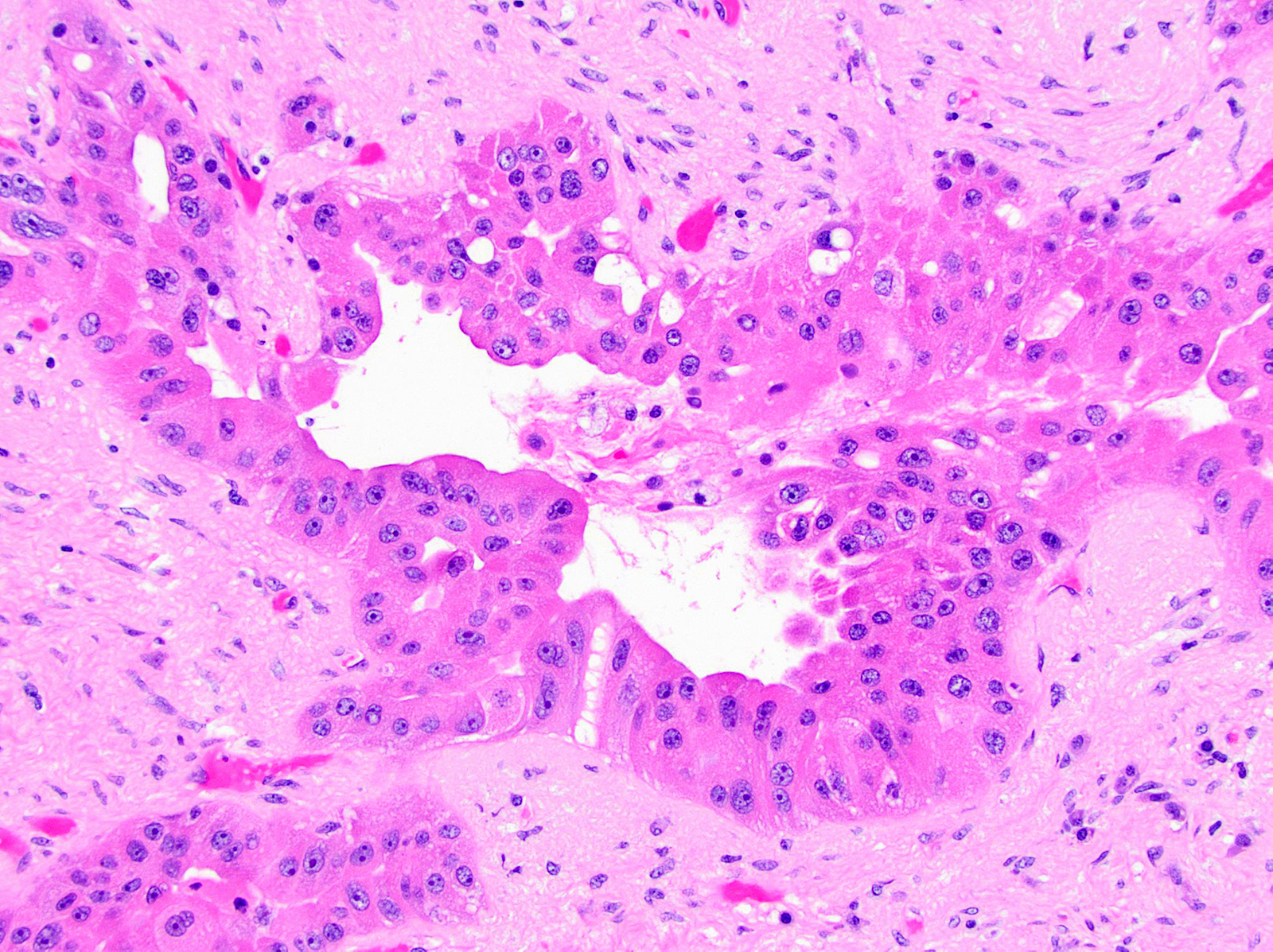
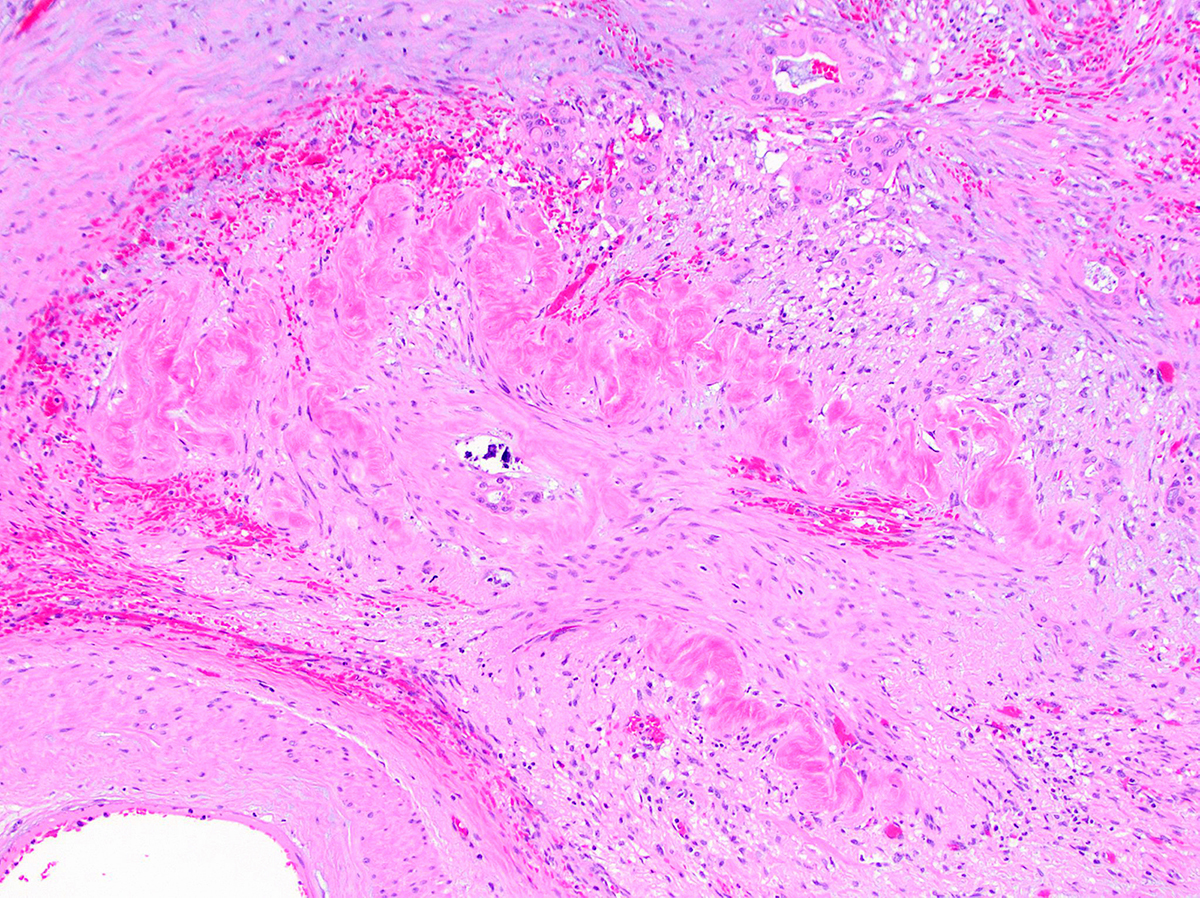
- Infiltrating well to poorly formed glandular / ductal structures surrounded by remarkably desmoplastic stroma
- Mucin production is specific for ductal origin versus acinar or neuroendocrine differentiation
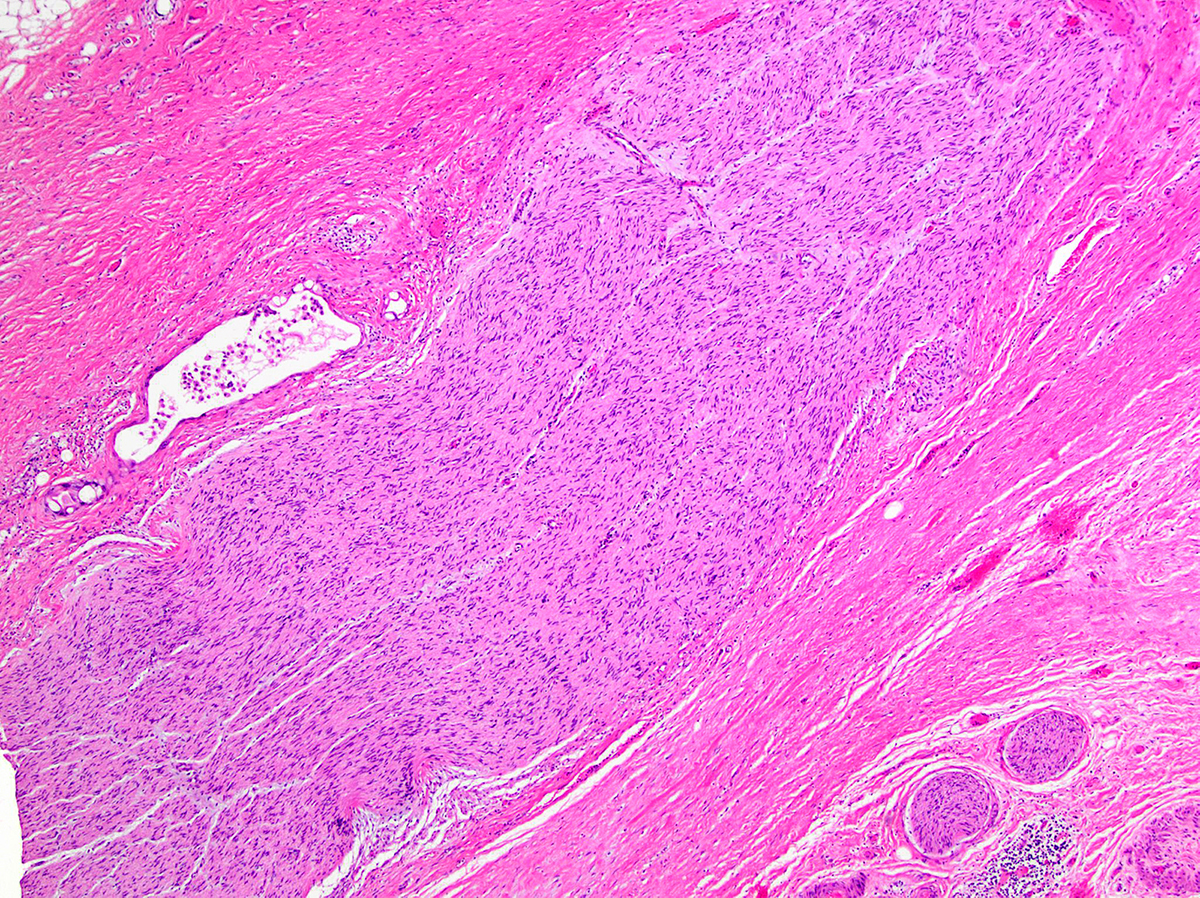
- Perineural invasion present in 90%, typically with better differentiated glands
- Angiolymphatic invasion in 50%; vascular invasion may mimic PanIN (Am J Surg Pathol 2012;36:235)
- Well differentiated: pink apical band composed of mucin granules, irregular shape and distribution; desmoplasia, marked nuclear pleomorphism with nucleoli, loss of polarity, mitotic figures
- Moderately to poorly differentiated (most tumors); abortive tubular structures, deeply infiltrative growth pattern, frequent mitosis, irregular and abortive mucin production
- TNM histologic grading system (College of American Pathologists), based on extent of glandular differentiation:
- G1 = well differentiated (> 95% tumor composed of glands)
- G2 = moderately differentiated (50 - 95% glands)
- G3 = poorly differentiated (< 50% glands)
- Post neoadjuvant therapy (Hum Pathol 2021;109:1, Mod Pathol 2018;31:4, Arch Pathol Lab Med 2020;144:838):
- Variable amount of residual tumor in a densely fibrotic tumor bed
- Attention to perineural space and duodenal wall for residual tumor glands
- Blue-grey fibrosis is a useful clue to suggest the possibility of adjacent residual tumor (Hum Pathol 2021;109:1)
- May show vacuolated or eosinophilic tumor cell changes
- WHO variants:
- Adenosquamous carcinoma and squamous cell carcinoma
- Colloid carcinoma
- Hepatoid carcinoma
- Medullary carcinoma
- Invasive micropapillary carcinoma
- Signet ring cell (poorly cohesive cell) carcinoma
- Undifferentiated carcinoma (anaplastic, sarcomatoid, carcinosarcoma)
- Undifferentiated carcinoma with osteoclast-like giant cells
- Non-WHO variants / patterns:
- Clear cell:
- Glandular, ductal or nested structures with single layer of polygonal cells, distinct cell borders and variable nuclear atypia
- Not due to accumulation of glycogen or mucin
- Overexpression of HNF1β by IHC (Mod Pathol 2008;21:1075)
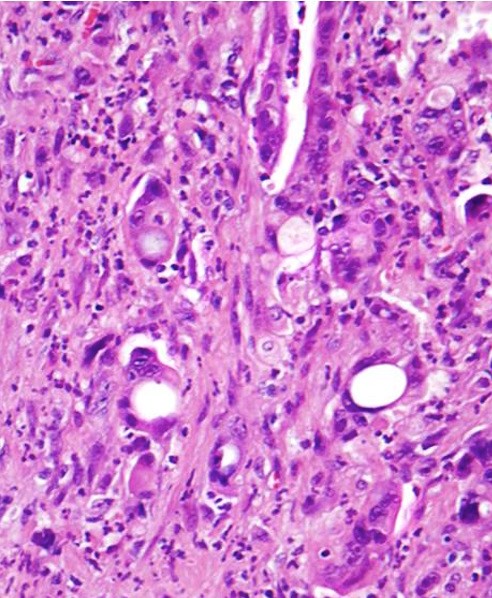
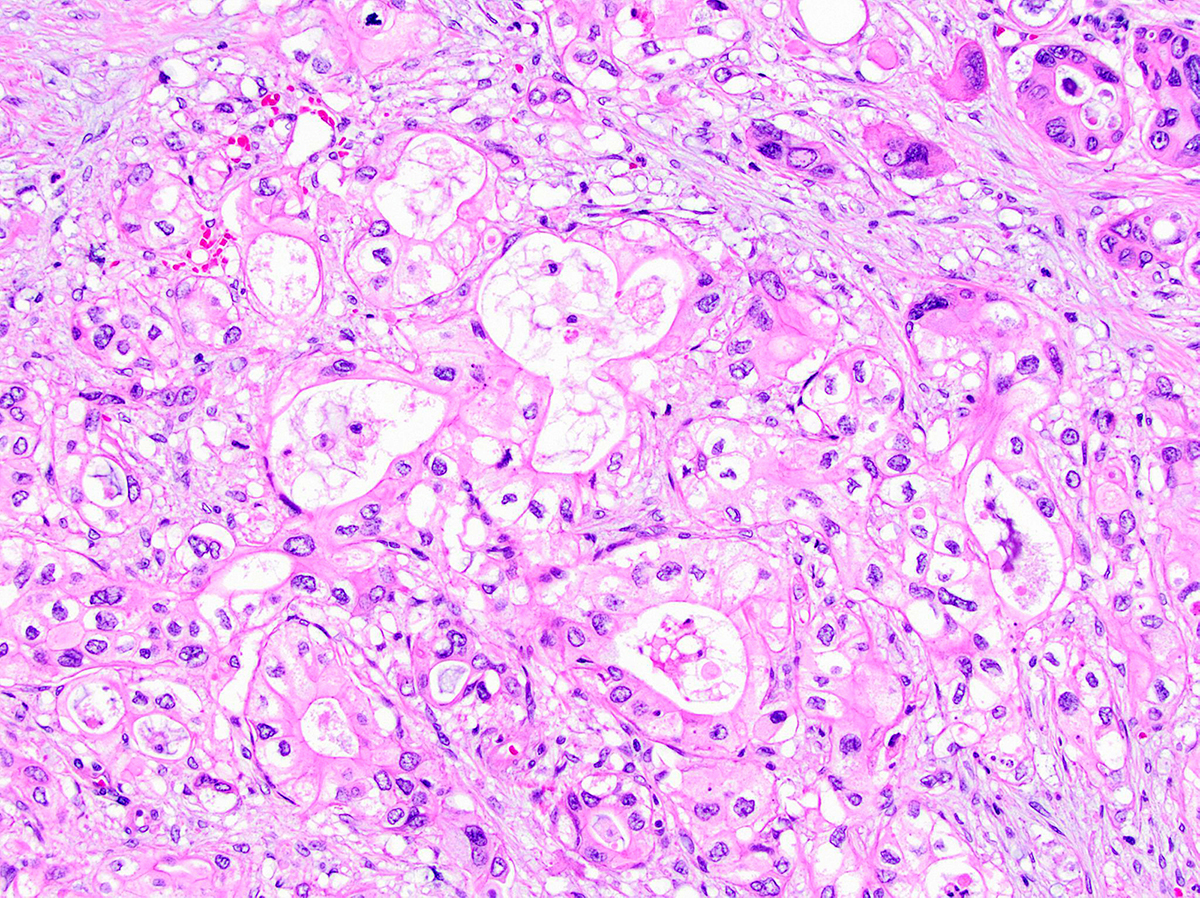
- Foamy gland:
- Deceptively benign appearing pattern
- Well formed glands with bland cells but subtle infiltration
- Cells have abundant microvesicular (white and crisply foamy) cytoplasm with distinct pink brush border-like zone at apical / luminal portion of the cell, nuclei are basal oriented, dense or wrinkled (raisinoid)
- Foamy material due to evenly sized mucigen granules that are mucin negative
- Foamy PanIN proposed to be the precursor lesion (Am J Surg Pathol 2000;24:493, Ann Diagn Pathol 2008;12:252)
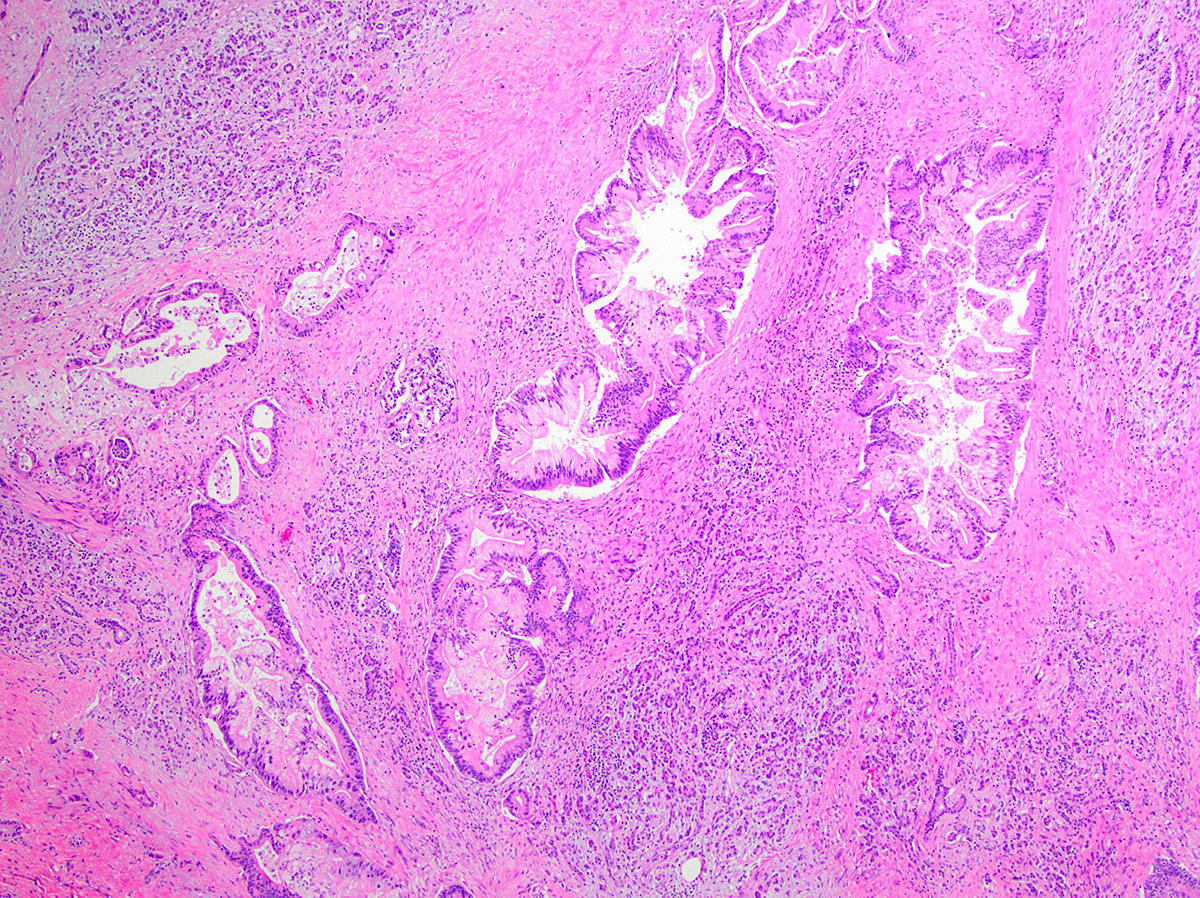
- Large duct:
- Seen in < 10% of PDAC
- Clustered ducts with irregular jagged contours (diameter: 0.5 mm - 1 cm)
- Desmoplastic and myxoid stroma, intraluminal neutrophils and granular debris
- Focal microcystic appearance due to marked ectasia of infiltrating neoplastic glands, particularly near duodenal muscularis propria
- May have papillary pattern (Mod Pathol 2012;25:439, Am J Surg Pathol 2012;36:696)
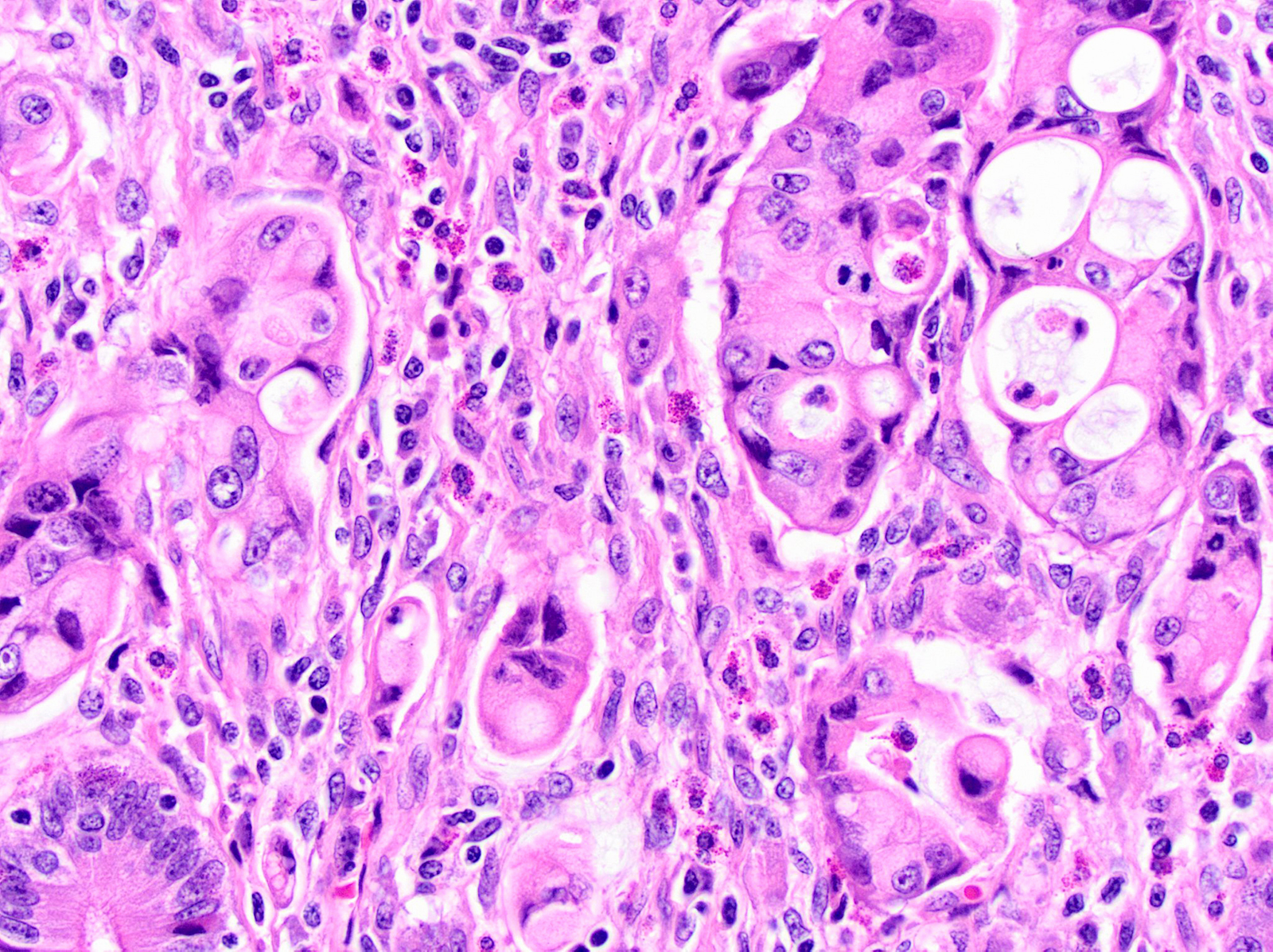
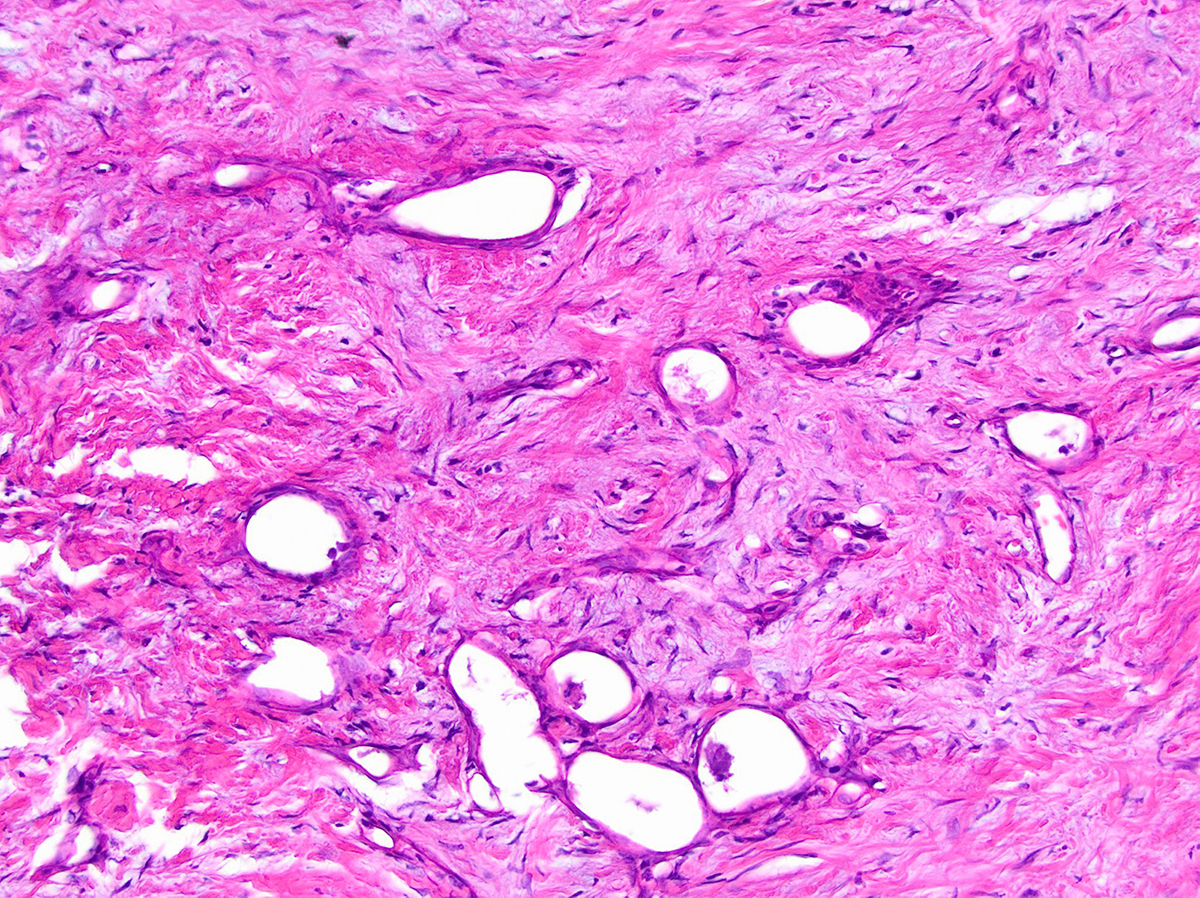
- Vacuolated:
- High grade tumors, clusters of tumor cells containing large vacuoles imparting cribriform architecture (reminiscent of adipocytes or signet ring cells)
- Microcysts contain cellular debris and mucin
- Can resemble fat necrosis or lipogranulomas in lymph node
- Recognizing the atypical, enlarged, hyperchromatic nuclei is key (Virchows Arch 2010;457:643)
- Clear cell:
Microscopic (histologic) images
Contributed by Wei Chen, M.D., Ph.D.
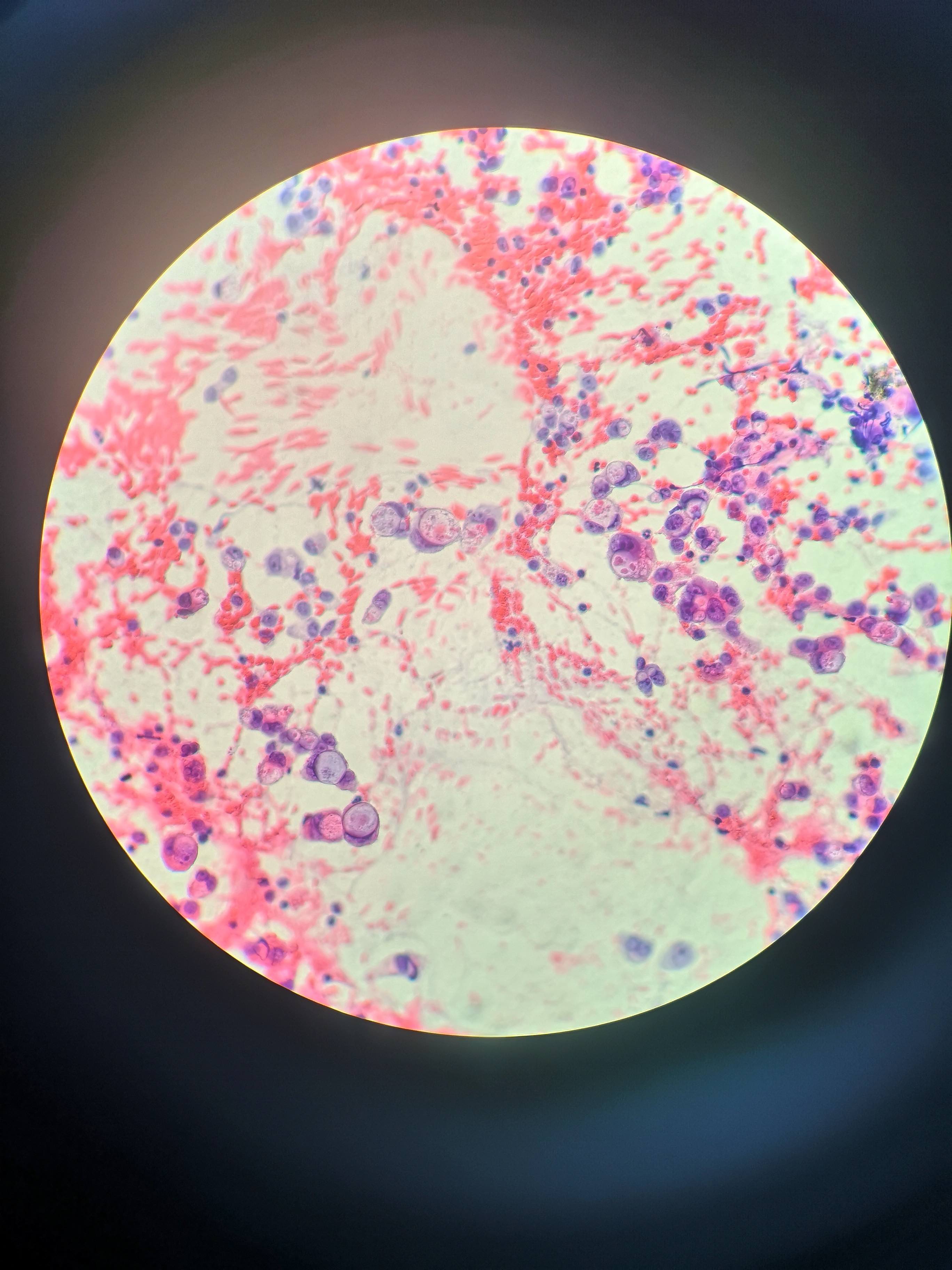
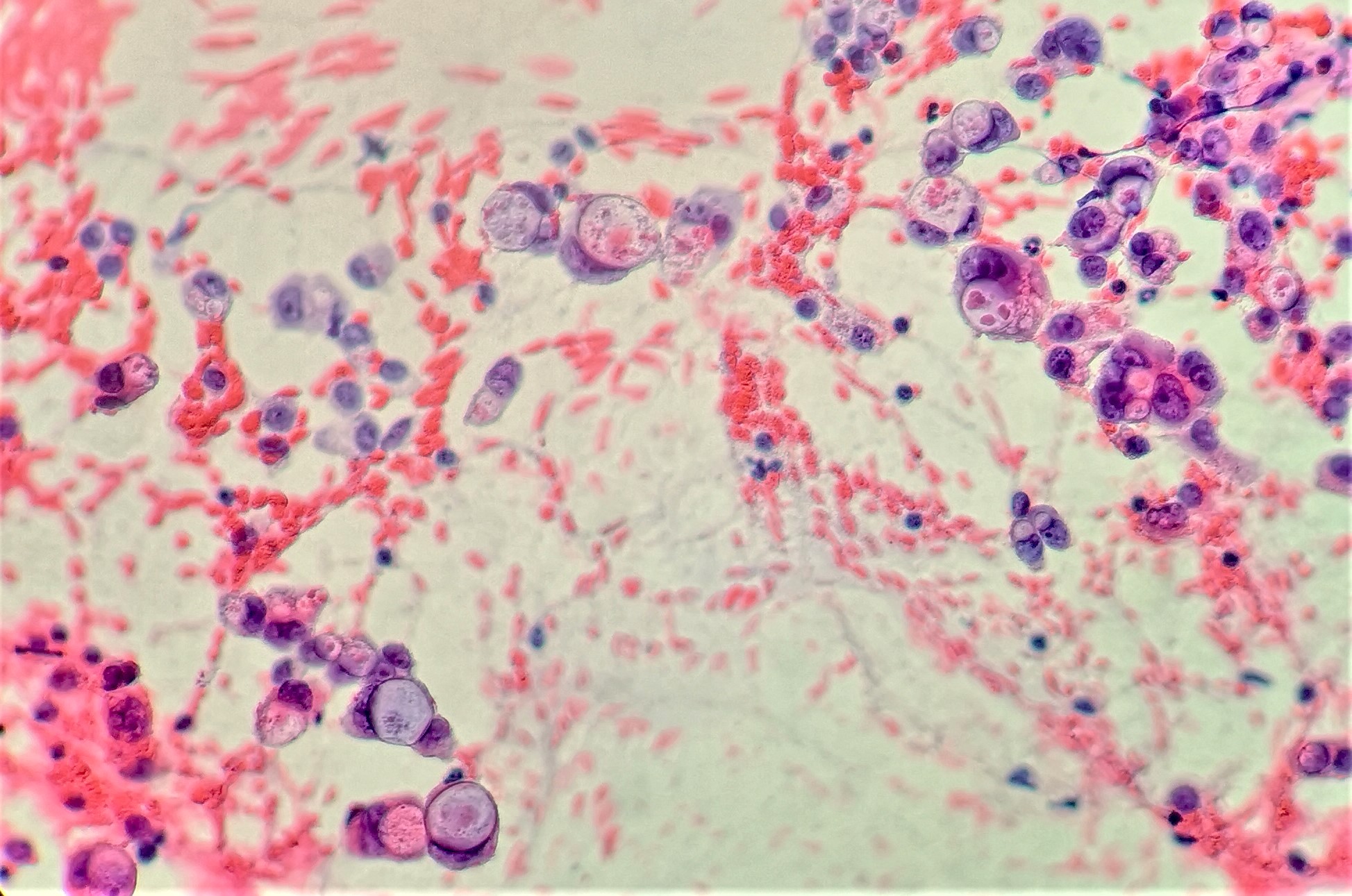
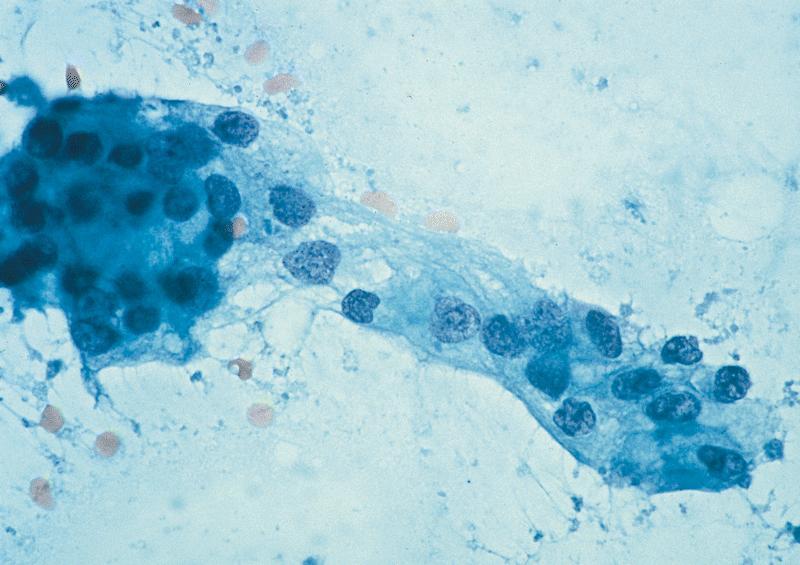
Cytology description
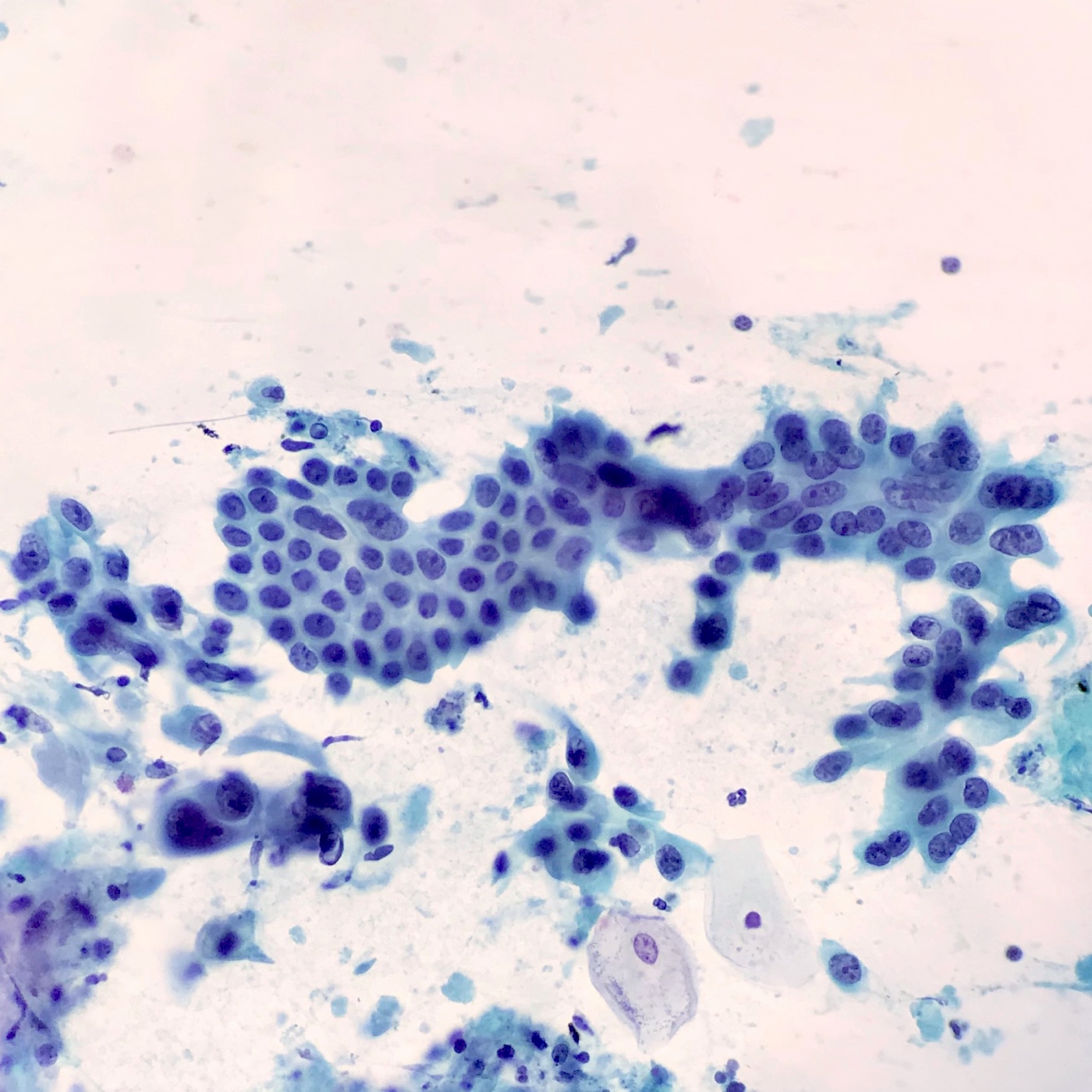
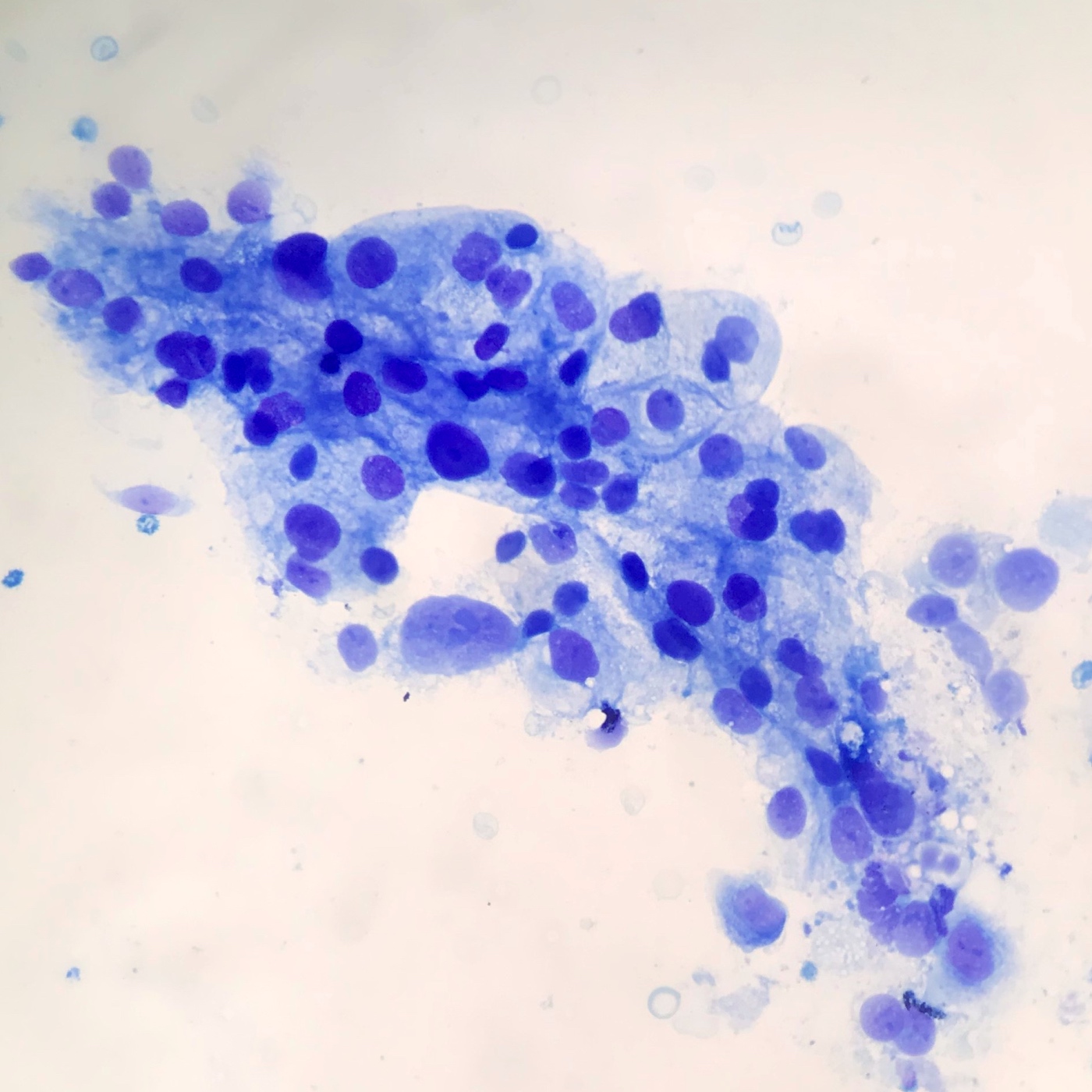
- EUS-FNA has sensitivity and specificity of > 90% and 100%, increased sensitivity with ThinPrep, brushings are 50% sensitive (repeat if inconsistent with clinical or radiologic findings) (Arch Pathol Lab Med 2000;124:387)
- Aspirates are cellular, without acinar cells, with atypical ductal cells in sheets (drunken honeycomb), clusters or singly; anisonucleosis (4:1 variation)
- Signet ring cells and mitotic figures are helpful when present
- Papanicolaou Society of Cytopathology's 6 tiered system for pancreatobiliary cytology: nondiagnostic, negative for malignancy, atypical, neoplastic, suspicious and positive / malignant (Cytojournal 2014;11:3)
- Duodenal secretions are 80% sensitive in head tumors, 33% sensitive in tail tumors; endoscopic retrograde cholangiopancreatography (ERCP) juice is 50 - 85% sensitive
Cytology images
Positive stains
Negative stains
- pVHL negative / loss seen in > 90% PDACs (Arch Pathol Lab Med 2012;136:601, Hum Pathol 2013;44:503)
- Loss of SMAD4 / DPC4 seen in 55%; specific for malignancy (in situ or invasive) (Am J Clin Pathol 2001;116:831)
- CK20, MUC2, vimentin, synaptophysin, chromogranin, trypsin, chymotrypsin, lipase
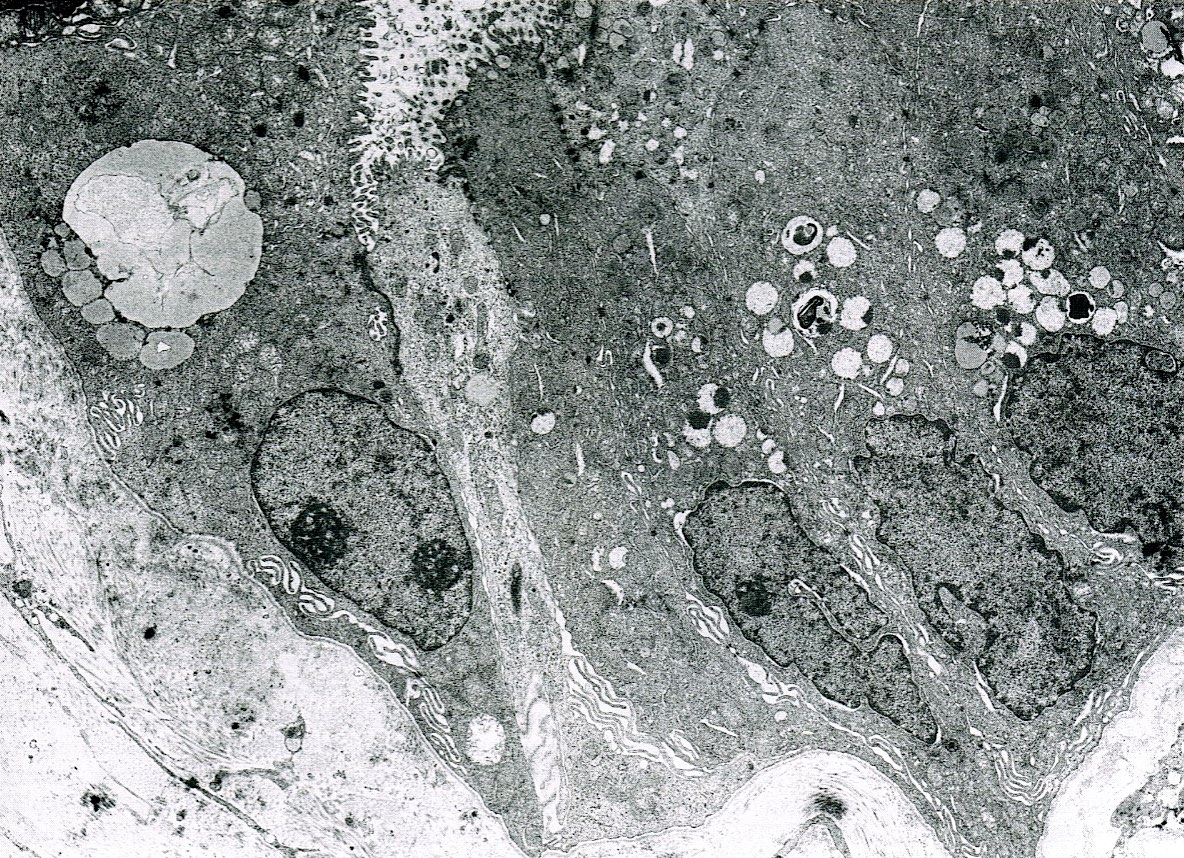
Electron microscopy description
- Mucigen granules
Molecular / cytogenetics description
- 4 key driver gene mutations (Arch Pathol Lab Med 2011;135:716, Hum Pathol 2009;40:612, Cancer Cell 2017;32:185)
- KRAS: > 95%, early event during carcinogenesis (Cancer 2007;109:1561)
- CDKN2A (p16): 95%
- TP53: 50 - 80%
- SMAD4: 55%
- 50% overexpress HER2
- Many core signaling pathways involved
- ~10% of pancreatic cancers have a familial basis
Sample pathology report
- Distal stomach, duodenum and pancreatic head, pancreaticoduodenectomy:
- Moderately differentiated pancreatic ductal adenocarcinoma, 2.2 cm
- Adenocarcinoma involves distal bile duct
- All margins negative for carcinoma or high grade dysplasia
- Positive for lymphovascular invasion and perineural invasion
- Metastatic adenocarcinoma involving 2 of 23 lymph nodes (2/23)
- Portion of benign stomach with chronic inactive gastritis, negative for Helicobacter pylori on H&E
- Benign duodenum with no significant pathologic change
Differential diagnosis
- Ampullary adenocarcinoma:
- Epicenter at ampulla, presence of preinvasive component at ampulla
- Chronic pancreatitis:
- Lobular architecture at low power with central ectatic branched ductules and clusters of round ductules surrounded by cuff of stroma (Arch Pathol Lab Med 2009;133:382)
- Features of PDAC to differentiate from chronic pancreatitis (Arch Pathol Lab Med 2015;139:848):
- Haphazard architecture and glands at abnormal locations in interlobular areas, next to vessels and naked glands in fat
- Anisonucleosis (4:1 variation), loss of cell polarity, perineurial invasion, individual cell infiltration, budding into lumen; has p53 mutations and loss of SMAD4 / DPC4
- Distal bile duct adenocarcinoma:
- Epicenter at bile duct, circumferential / symmetrical involvement of the bile duct, presence of in situ component (BilIN or biliary intraductal papillary neoplasm)
Additional references
Board review style question #1
Among the entities below, which one is considered a precursor lesion for invasive ductal adenocarcinoma of pancreas?
- Intraductal papillary mucinous neoplasm
- Lymphoepithelial cyst
- Serous cystadenoma
- Solid pseudopapillary neoplasm
Board review style answer #1
Board review style question #2
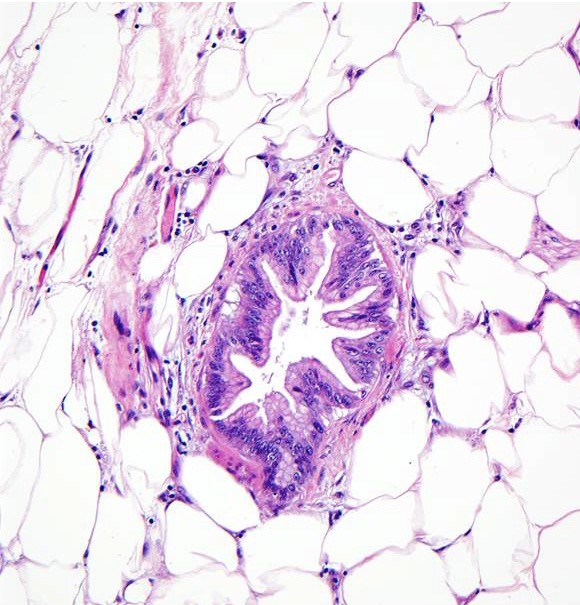
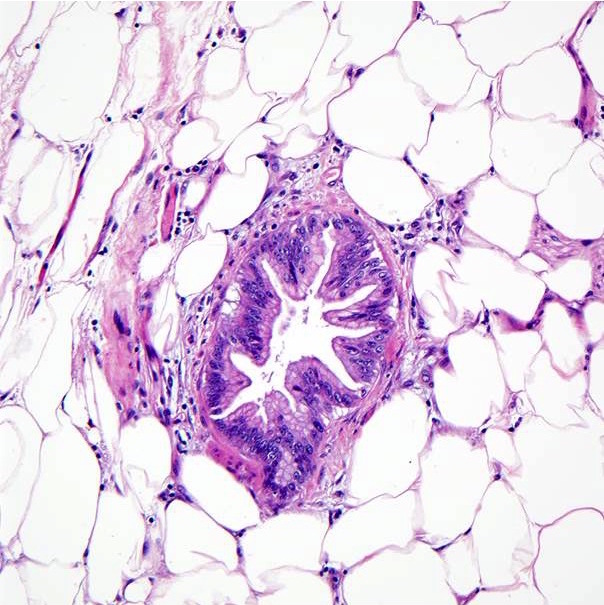
- The above microphotograph demonstrates a section from peripancreatic adipose tissue in a patient with pancreatic ductal adenocarcinoma. What is the lesion in the center of the microphotograph?
- Intraductal papillary mucinous neoplasm
- Pancreatic ductal adenocarcinoma
- Pancreatic intraepithelial neoplasia, high grade
- Pancreatic intraepithelial neoplasia, low grade
Board review style answer #2