Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Shyu S, Singhi AD. PRSS1 hereditary pancreatitis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreashereditary.html. Accessed April 20th, 2024.
Definition / general
- Autosomal dominant disorder caused by mutations of serine protease 1 (PRSS1), also known as the cationic trypsinogen gene, characterized by progressive irreversible lipomatous atrophy of the pancreatic parenchyma leading to pancreatic insufficiency (Am J Surg Pathol 2014;38:346)
- Most common subtype of familial hereditary pancreatitis (80%); other gene mutations associated with chronic pancreatitis include SPINK1, CFTR and CTRC
- Incomplete penetrance; 40 - 93% of mutation carriers develop chronic pancreatitis
- Patients may develop pancreatic intraepithelial neoplasia (PanIN) and have an increased lifetime risk of pancreatic cancer
Essential features
- Autosomal dominant mutation of PRSS1 with incomplete penetrance
- Recurrent episodes of acute pancreatitis that progress to chronic pancreatitis and lipomatous atrophy of the pancreas
- Increased risk of pancreatic cancer
Terminology
- Familial pancreatitis, hereditary chronic pancreatitis, autosomal dominant hereditary pancreatitis, recurrent or relapsing acute or chronic pancreatitis
ICD coding
- ICD-10: K86.1 - other chronic pancreatitis
Epidemiology
- Age of onset ranges from infancy to sixth decade of life
- Highest prevalence in the mid and southeastern regions of the United States, as well as parts of France, England and Germany (Clin Gastroenterol Hepatol 2004;2:252, Pancreatology 2001;1:439)
Pathophysiology
- R122H and N29I mutations in PRSS1 result in an increased propensity for trypsin-mediated trypsinogen activation, referred to as autoactivation, resulting in increased inappropriate intrapancreatic trypsin activity and digestion of the pancreatic parenchyma (Clin Exp Gastroenterol 2016;9:197)
- R122H mutation is a dual gain of function mutation that also prevents the autodegradation of active trypsin (Biochem Biophys Res Commun 2000;278:286)
- Obstruction from intraductal calculi may be responsible for lipomatous atrophy; exact mechanism unclear
Clinical features
- Recurrent episodes of acute pancreatitis, typically starting in childhood, with progression to chronic pancreatitis over time
- Presentation of acute and chronic pancreatitis is clinically indistinguishable from other etiologies for pancreatitis
Diagnosis
- Patients should be suspected of PRSS1 associated hereditary pancreatitis with the following: acute pancreatitis occurring in childhood; recurrent acute pancreatitis of unknown etiology; chronic pancreatitis of unknown cause (especially among individuals before age 25); a family history of recurrent acute pancreatitis and chronic pancreatitis following an autosomal dominant pattern of inheritance; and a family history of pancreatitis and pancreatic cancer
- Considering the aforementioned suspected clinical findings, a diagnosis is established by the identification of a heterozygous pathologic mutation in PRSS1
- Rare cases of PRSS1 R122H and A16V mutations have been reported in nonhereditary pancreatitis; therefore, family history prior to genetic testing is important to avoid misclassification and unnecessary testing (Clin Genet 2001;59:189, Pancreas 2001;22:18)
Prognostic factors
- R122H mutation may be associated with a more aggressive course, with earlier onset and increased severity of disease (Br J Surg 2000;87:170)
Case reports
- 38 month old boy with pancreaticopleural fistula (Pediatr Gastroenterol Hepatol Nutr 2019;22:601)
- 12 year old boy with recurrent abdominal pain (Tohoku J Exp Med 2001;195:191)
- 15 year old girl with painless, progressive abdominal distention (Postgrad Med J 1986;62:873)
- 11 family members with chronic pancreatitis (Arch Dis Child 1999;80:473)
Treatment
- Management is the same as for sporadic disease
- Total pancreatectomy may be considered in older patients due to the increased risk of cancer (World J Gastroenterol 2003;9:1)
- Relatives should undergo genetic counseling
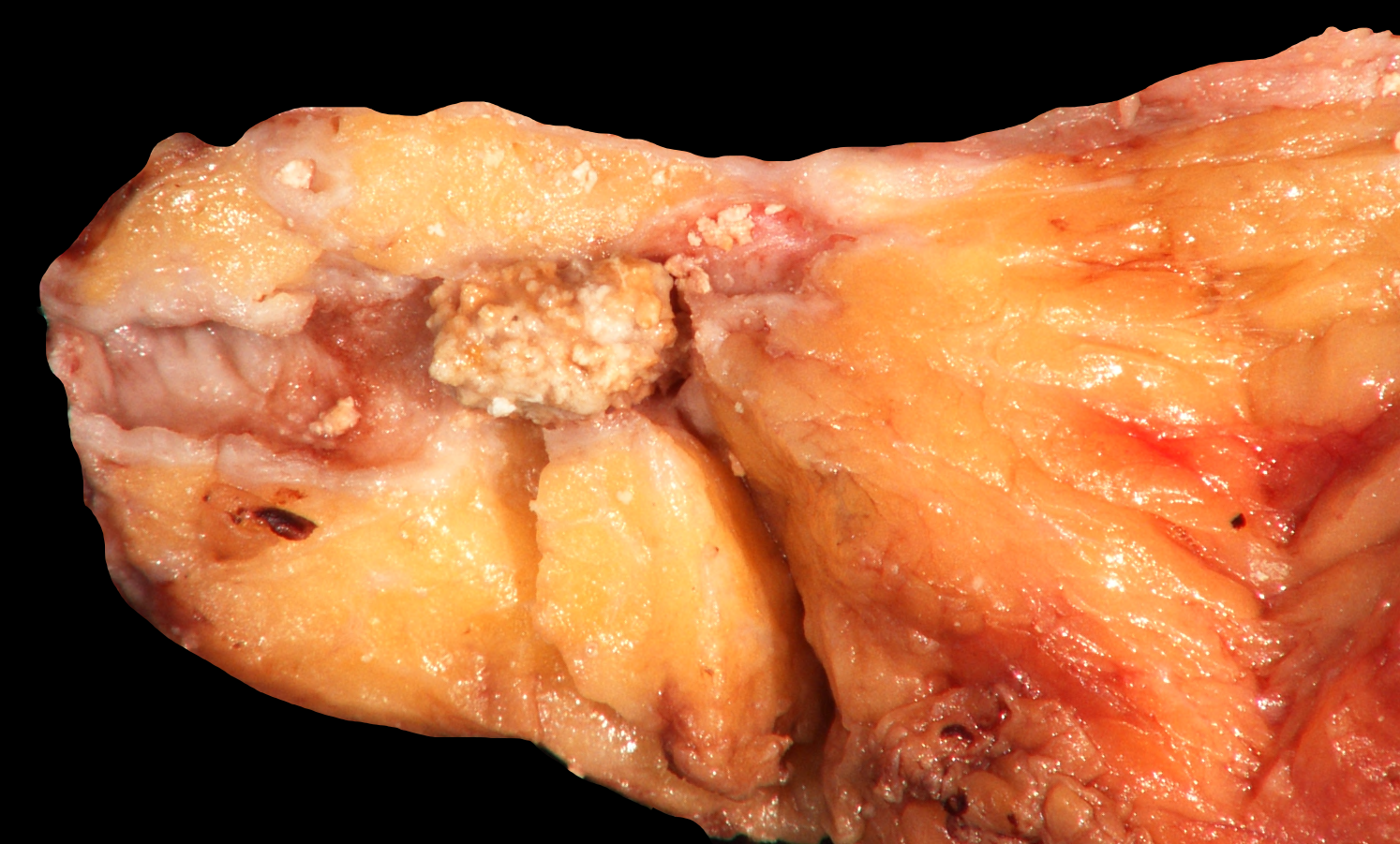
Gross description
- Pediatric specimens are typically unremarkable
- Adult specimens are soft and shrunken with marked atrophy and fat replacement, ductal dilatation and multiple intraductal calcifications
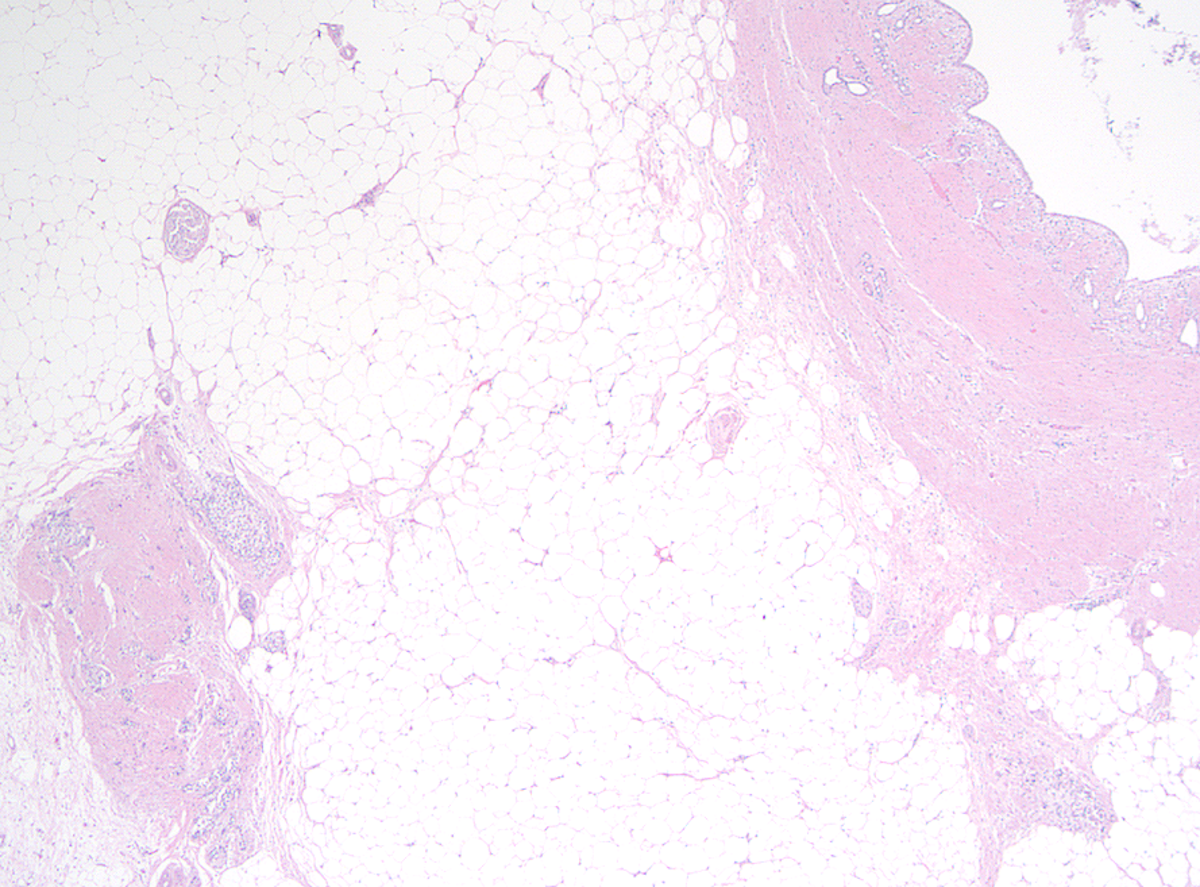
Microscopic (histologic) description
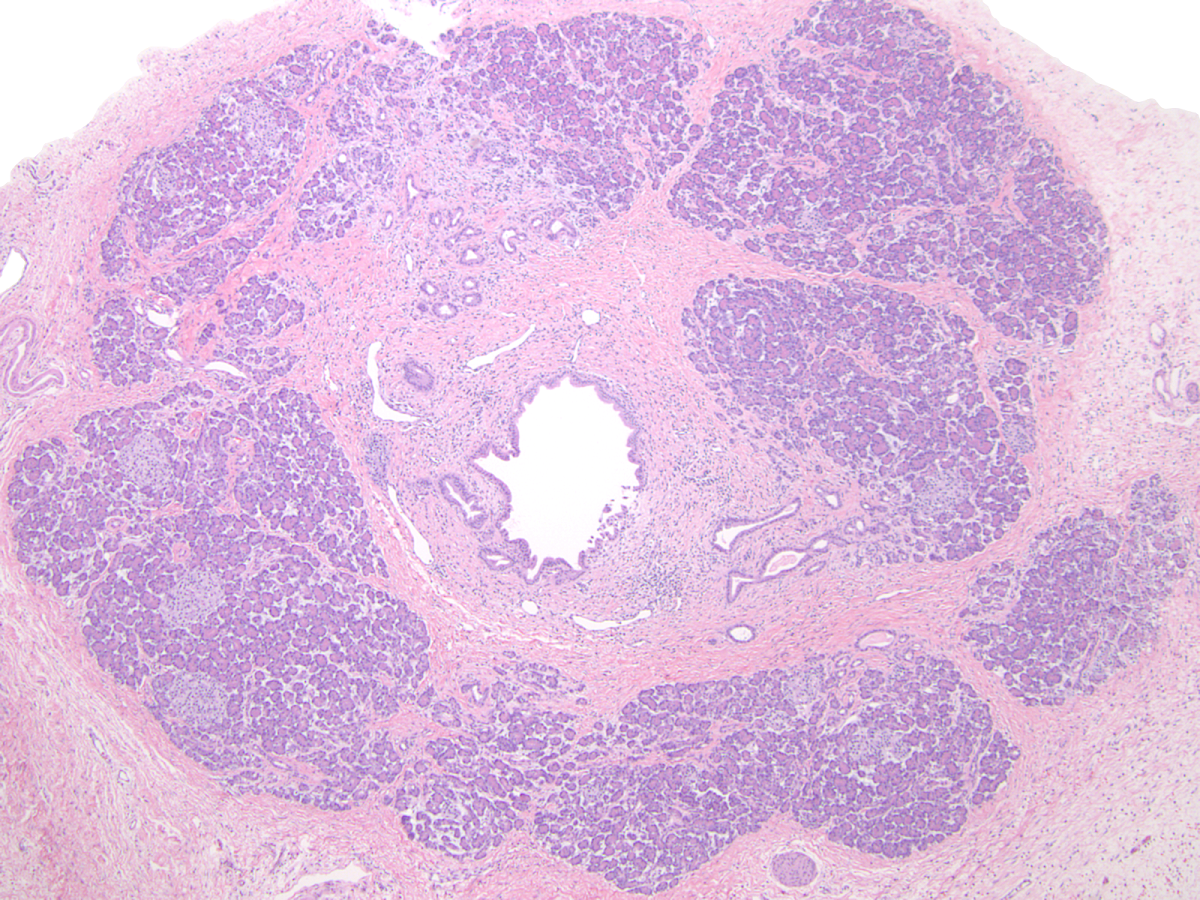
- Childhood (early) changes
- Patchy loss of acinar and ductal tissue
- Loose perilobular and interlobular fibrosis
- Relative preservation and aggregation of islets of Langerhans (resulting in a hyperplastic appearance)
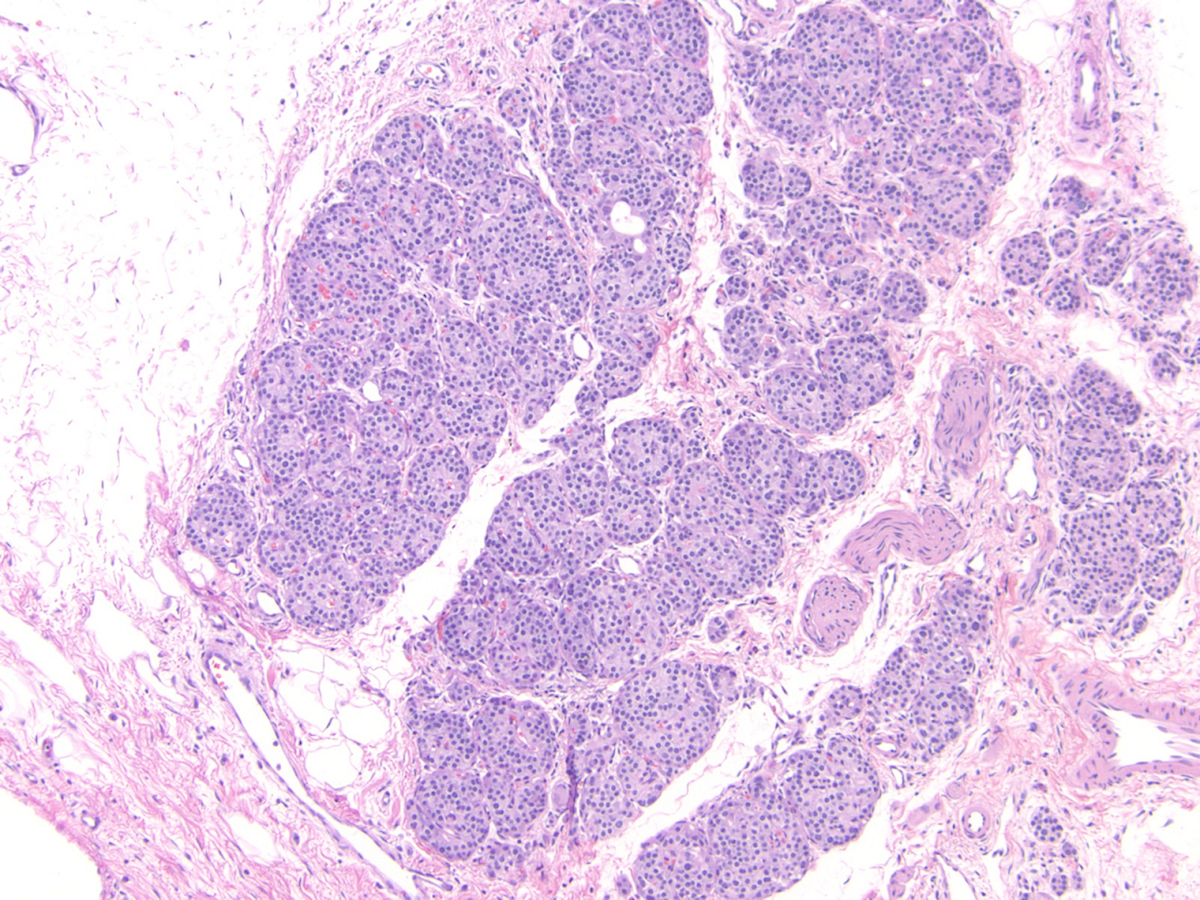
- Young adult (second to third decade of life) changes
- Progressive loss of acinar cell mass and ductal epithelium and increased fibrosis over time
- Fibrosis is increasingly intermingled with adipocytes
- Islets of Langerhans are still preserved
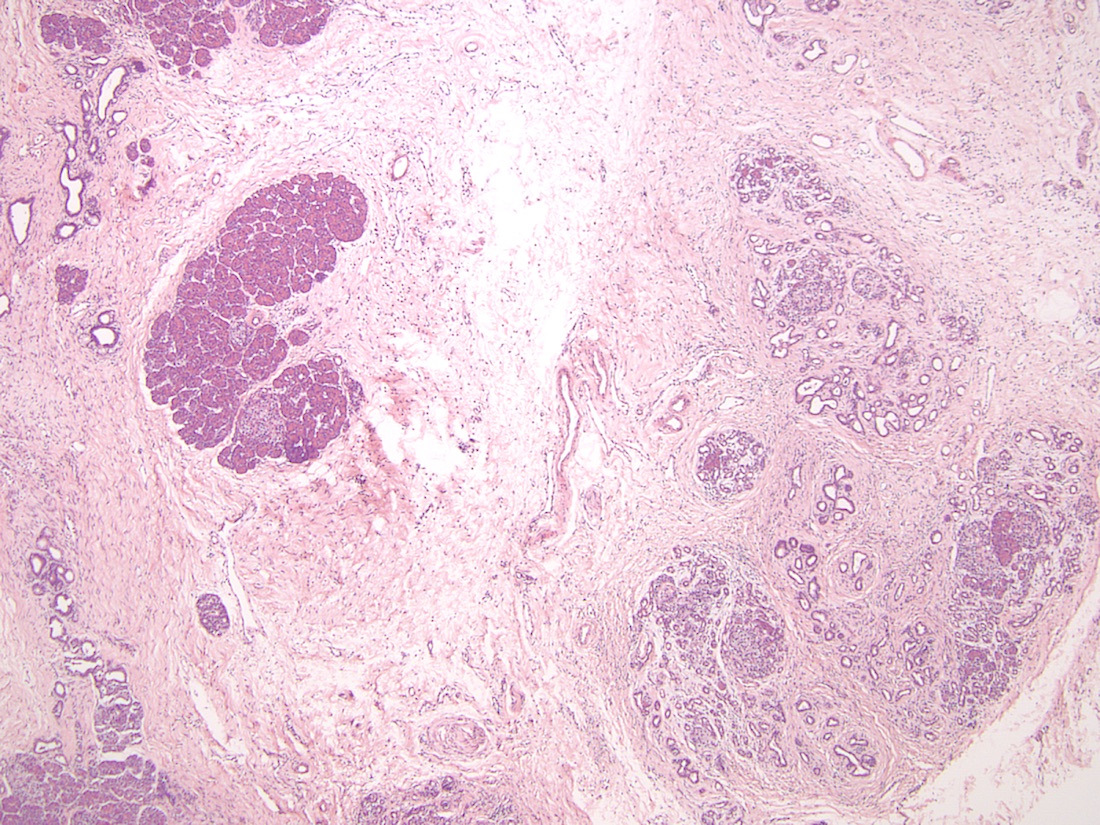
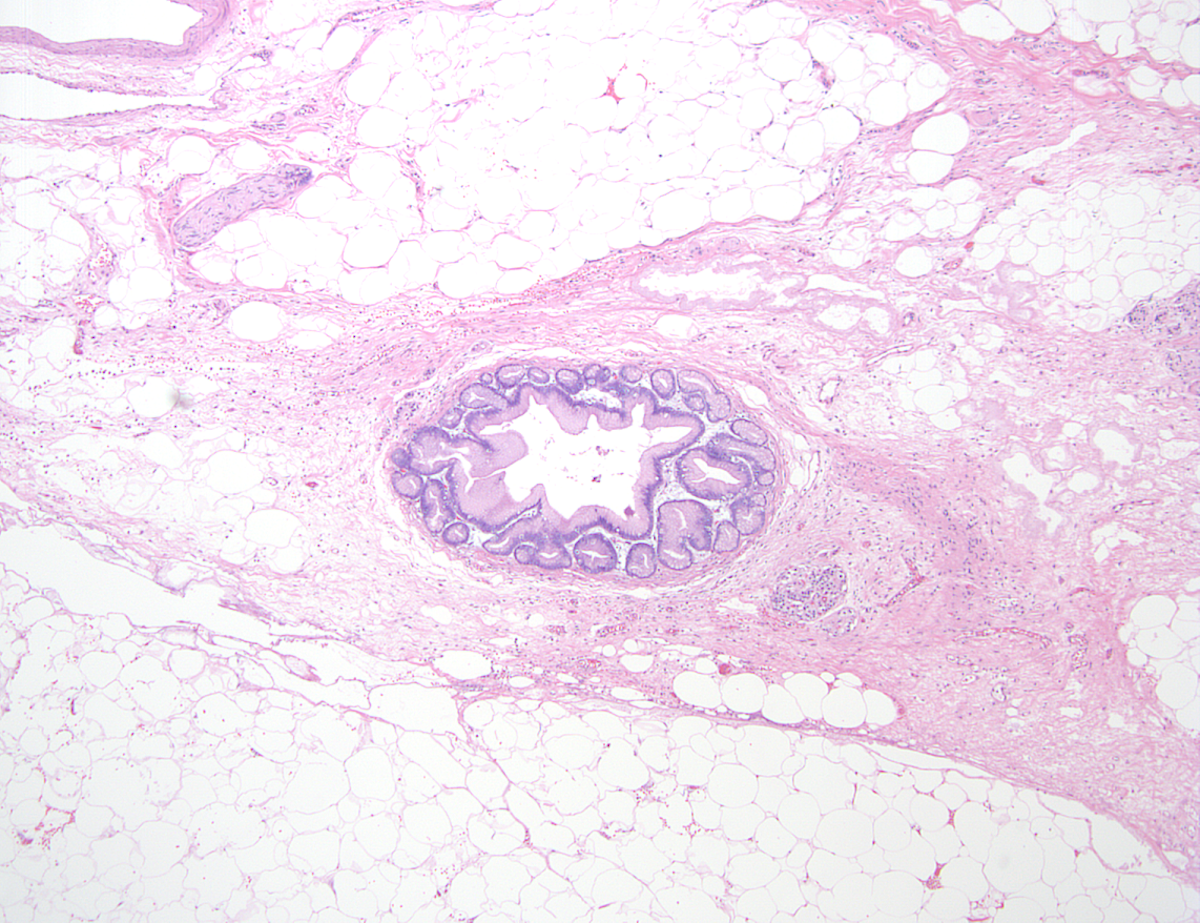
- Older adult (fourth to sixth decade of life) changes
- Near complete replacement of pancreatic parenchyma by mature adipose tissue with scattered islets of Langerhans and nerves
- Primarily periductal fibrosis
- Main pancreatic duct and larger interlobular ducts are more likely to be dilated, while smaller ducts are reduced or scarred
- Amyloid deposition may be seen
- Nonspecific ductal changes such as atrophy, dilatation, squamous metaplasia and intraductal calcifications
- Can be associated with pancreatic intraepithelial neoplasia at any age (Am J Surg Pathol 2014;38:346, Dig Liver Dis 2012;44:8)
Microscopic (histologic) images
Molecular / cytogenetics description
- 2 most common PRSS1 mutations are the R122H and N29I missense mutations
- > 30 mutations within PRSS1 have been identified; other less common mutations include A16V, R122C, N29T, D22G and K23R (Clin Gastroenterol Hepatol 2004;2:252)
Sample pathology report
- Pancreas, biopsy:
- Pancreatic parenchyma with intralobular and interlobular fibrosis (history of hereditary pancreatitis)
- Pancreas, total pancreatectomy:
- Pancreatic parenchyma with intralobular and interlobular fibrosis, marked acinar parenchymal loss and pseudolipomatous atrophy (history of hereditary pancreatitis)
- Low grade pancreatic intraepithelial neoplasia
- Benign cystic dilation of small and large pancreatic ducts with associated reactive epithelial changes including squamous metaplasia
- Reactive bile duct mucosal changes with associated fibrosis
- 15 benign lymph nodes
- Negative for malignancy
Differential diagnosis
- Cystic fibrosis:
- Autosomal recessive disorder caused by CFTR mutation
- Progressive pancreatitis also characterized by lipomatous pseudohypertrophy; may share a common pathophysiological mechanism
- Majority of patients present with pancreatic insufficiency in utero or early infancy
- Alcoholic / obstruction associated chronic pancreatitis:
- Fibrosis in PRSS1 hereditary pancreatitis is relatively thinner and less compact (Am J Surg Pathol 2014;38:346)
- Lipomatous pseudohypertrophy of the pancreas:
- Common degenerative change in the obese and elderly
- Typically presents as a focal lesion and not as extensive as seen in PRSS1 pancreatitis
- Other conditions associated with lipomatous pseudohypertrophy include Cushing syndrome, steroid therapy, malnutrition, viral infection, main pancreatic duct obstruction and other genetic syndromes
Additional references
Board review style question #1
- A 20 year old man with a family history of recurrent pancreatitis and diabetes was found to have a PRSS1 mutation after being tested at age 5 during his first documented episode of pancreatitis. Regarding this entity, which of the following statements is true?
- Diagnosis is made based primarily on radiology
- Most common PRSS1 mutations are N29I and A16V
- Patients are at an increased risk of pancreatic cancer
- Pattern of inheritance for this entity is autosomal recessive
Board review style answer #1
C. Patients are at an increased risk of pancreatic cancer. This picture shows a pancreas with partial replacement of normal parenchyma with loose perilobular and interlobular fibrosis with scattered adipocytes. In a patient with a family history of recurrent pancreatitis, identification of a pathologic mutation in PRSS1 in this patient establishes a diagnosis of PRSS1 hereditary pancreatitis. As the patient's disease progresses, the pancreas will continue to undergo replacement largely by adipocytes with primarily perilobuductal fibrosis. PanIN has been seen in several cases of hereditary pancreatitis and these patients are at an increased risk of pancreatic cancer.
Inheritance is in an autosomal dominant pattern with variable penetrance. The most common PRSS1 mutations are R122H and N29I. Patients' clinical, laboratory and radiological presentations of acute and chronic pancreatitis are the same as for other etiologies. Careful family history taking and testing for a PRSS1 mutation in a suspected individual is what establishes the diagnosis.
Comment Here
Reference: PRSS1 hereditary pancreatitis
Inheritance is in an autosomal dominant pattern with variable penetrance. The most common PRSS1 mutations are R122H and N29I. Patients' clinical, laboratory and radiological presentations of acute and chronic pancreatitis are the same as for other etiologies. Careful family history taking and testing for a PRSS1 mutation in a suspected individual is what establishes the diagnosis.
Comment Here
Reference: PRSS1 hereditary pancreatitis
Board review style question #2
- Which of the following statements is true regarding the histologic findings in PRSS1 hereditary pancreatitis?
- Definitive diagnosis can be made if lipomatous atrophy is present
- Islets of Langerhans are preferentially lost in early stages of the disease while acinar tissue is preserved
- Pancreatic intraepithelial neoplasia can be present at any age
- Presence of intraductal calcifications is associated with an increased risk of pancreatic cancer
Board review style answer #2
C. Pancreatic intraepithelial neoplasia can be present at any age. PRSS1 hereditary pancreatitis is a progressive disease. In early stages, fibrosis and loss of acinar and ductal tissue is mild and patchy while islets of Langerhans are relatively preserved, which can result in a hyperplastic appearance. As the disease progresses, the pancreatic parenchyma undergoes near complete replacement by mature adipose tissue and fibrosis. Lipomatous atrophy is a nonspecific finding, which can be seen as a degenerative change in the obese and elderly or in patients with cystic fibrosis or other genetic syndromes. Intraductal calcifications are not associated with an increased risk of cancer and are one of several nonspecific microscopic changes seen in hereditary pancreatitis, along with ductal dilatation, squamous metaplasia and amyloid deposition. Patients with PanIN are at an increased risk of pancreatic cancer and PanIN can be seen at any age.
Comment Here
Reference: PRSS1 hereditary pancreatitis
Comment Here
Reference: PRSS1 hereditary pancreatitis