Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology / etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Grading | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Mastrosimini MG, Luchini C. Poorly differentiated neuroendocrine carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreasneuroendocrinecarcinoma.html. Accessed April 18th, 2024.
Definition / general
- Poorly differentiated, high grade, malignant epithelial neoplasm with neuroendocrine differentiation
- Poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs) are divided into small cell type (SC PanNEC) and large cell type (LC PanNEC)
Essential features
- Poorly differentiated neuroendocrine carcinomas represent a distinct entity from well differentiated (G1, G2, G3) neuroendocrine tumors (Am J Surg Pathol 2012;36:173)
- By morphology, PanNECs can be divided into small cell or large cell subtypes
- At the immunohistochemical level, the expression of neuroendocrine markers can be partial or weak (chromogranin A expression can even be absent)
- Tumor necrosis and high grade cytology
- Ki67 proliferation index > 20% (usually uniform and > 60%) (Ann Oncol 2013;24:152)
- Mitotic rate of > 20 mitoses/2 mm2
Terminology
- Neuroendocrine carcinoma refers to poorly differentiated neuroendocrine neoplasms, high grade by definition
- Neuroendocrine tumor is now reserved for well differentiated neoplasms with typical pathologic features of neuroendocrine lineage, truly resembling their nonneoplastic counterpart (cells of pancreatic islet), regardless of grade
- The current WHO (2019) classification groups pancreatic neuroendocrine neoplasms as follows:
- Pancreatic neuroendocrine microtumor (< 5 mm); previously known as neuroendocrine microadenoma
- Well differentiated pancreatic neuroendocrine tumor (WD PanNETs), including nonfunctional PanNETs and functional PanNETs (with clinical evidence of hormone release, such as insulinoma, glucagonoma, gastrinoma, VIPoma)
- Poorly differentiated pancreatic neuroendocrine carcinomas (PD PanNECs), including SC PanNECs and LC PanNECs
- Mixed neuroendocrine nonneuroendocrine neoplasm (MiNEN)
- Mixed neoplasm with a neuroendocrine component combined with a nonneuroendocrine component (typically ductal adenocarcinoma or acinar cell carcinoma)
- Each component must account for ≥ 30% of the tumor cell population and both components should be morphologically recognizable
- Neuroendocrine component may be a tumor (less frequently) or a carcinoma (more frequently)
ICD coding
- ICD-O:
- 8246/3 - neuroendocrine carcinoma, NOS
- ICD-11:
- 2C10.1 & XH0U20 - neuroendocrine neoplasms of pancreas & neuroendocrine carcinoma, NOS
- XH9SY0 - small cell neuroendocrine carcinoma
- XH0NL5 - large cell neuroendocrine carcinoma
Epidemiology
- Rare (accounting for < 1% of all pancreatic tumors and no more than 2 - 3% of pancreatic neuroendocrine neoplasms)
- Mean patient age is 59 years (patients are usually aged 50 - 60 years but can occur in younger patients as well)
- M:F = 1.4:1
- Large cell variant is more commonly encountered (60%) (Am J Surg Pathol 2012;36:173)
Sites
- Pancreatic gland (head, neck, body, tail)
- Head of the pancreas is the most common site (Am J Surg Pathol 2012;36:173)
Pathophysiology / etiology
- Largely unknown
- Tobacco smoking
Diagrams / tables
Clinical features
- Back pain, jaundice (for PanNECs of pancreatic head) or nonspecific abdominal symptoms, likewise pancreatic ductal adenocarcinomas
- Neoplastic syndromes secondary to ectopic hormone production, such as ACTH (rare)
- Vast majority (> 90%) of patients present with metastasis at the time of diagnosis (Am J Surg Pathol 2012;36:173)
Diagnosis
- CT scan, MRI and ultrasonography are the preferred imaging modality
- FDG PET scans present high standardized uptake value
- Somatostatin receptor scintigraphy (SSRS) is often negative (or focal avidity) due to lack of expression of SSTR2 and SSTR5 (Arch Pathol Lab Med 2020;144:816)
- Diagnosis is by cytology / fine needle biopsy or on surgical specimens (Am J Surg Pathol 2016;40:1192)
Laboratory
- Serum hormone activity (such as chromogranin A) is very unusual (Cancer 1973;31:1523)
- Serum carcinoma associated markers (e.g., CEA, CA19-9, CA125) levels may be elevated (Am J Surg Pathol 2016;40:1192)
Radiology description
- CT and MRI features: irregular margins and frequent presence of necrotic foci within tumor mass
Grading
- PanNECs are, by WHO definition high grade, based on Ki67 (MIB1) index > 20% and mitotic rate > 20 mitoses/2 mm2
- WD PanNETs are graded from G1 to G3; but G3 PanNETs represent a distinct entity from PanNECs (Endocr Pathol 2022;33:115, Pathologica 2021;113:28)
Prognostic factors
- Very poor prognosis, compared to WD PanNETs
- Metastatic spread is present in most patients at the time of diagnosis
- MiNEN including a NEC component show an aggressive behavior: in these tumors, the Ki67 index of the NEC component is the most important prognostic driver (Endocr Relat Cancer 2018;25:583)
- Even in cases amenable to surgical resection and treated with adjuvant platinum based therapy, the median survival time is very short (< 1 year) and < 25% of patients survive beyond 2 years (Am J Surg Pathol 2012;36:173)
Case reports
- 27 year old woman with ACTH secreting PanNEC causing Cushing syndrome with pelvic and bilateral ovarian metastases (Int J Clin Exp Pathol 2015;8:15396)
- 56 year old man presenting with pure, alpha fetoprotein producing PanNEC without another coexisting malignant component, such as adenocarcinoma or hepatoid carcinoma (BMC Gastroenterol 2015;15:16)
- 65 year old man with SC PanNEC with similar genetic alterations to invasive ductal adenocarcinoma (KRAS mutation, altered expressions of TP53 and SMAD4 / DPC4) (Clin J Gastroenterol 2016;9:261)
- 67 year old woman with cutaneous metastases of a PanNEC with CK20 positivity (G Ital Dermatol Venereol 2018;153:722)
Treatment
- Surgical resection
- Chemotherapy (no established protocol): platinum based regimen (Pancreas 2021;50:138)
Gross description
- Large neoplasms with infiltrative borders
- Brownish to whitish color, fleshy or hard consistency and typically with grossly visible necrotic areas (Endocr Pathol 2022;33:115, Pathologica 2021;113:28)
Frozen section description
- High grade and hypercellular neoplasm, with necrotic areas
- Diagnosis should be postponed to definitive examination after formalin fixation and also supported by ancillary methods (Endocr Pathol 2022;33:115, Pathologica 2021;113:28)
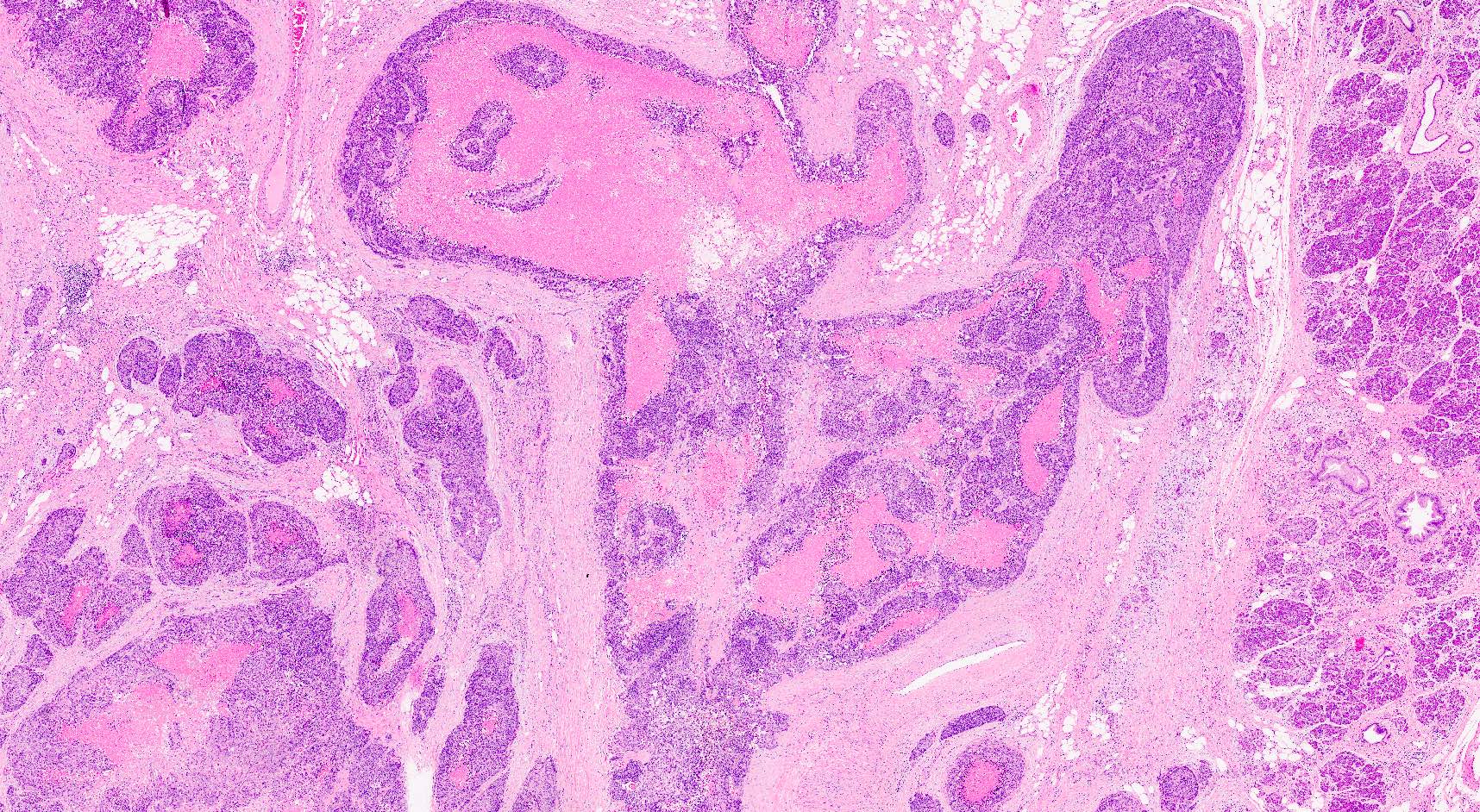
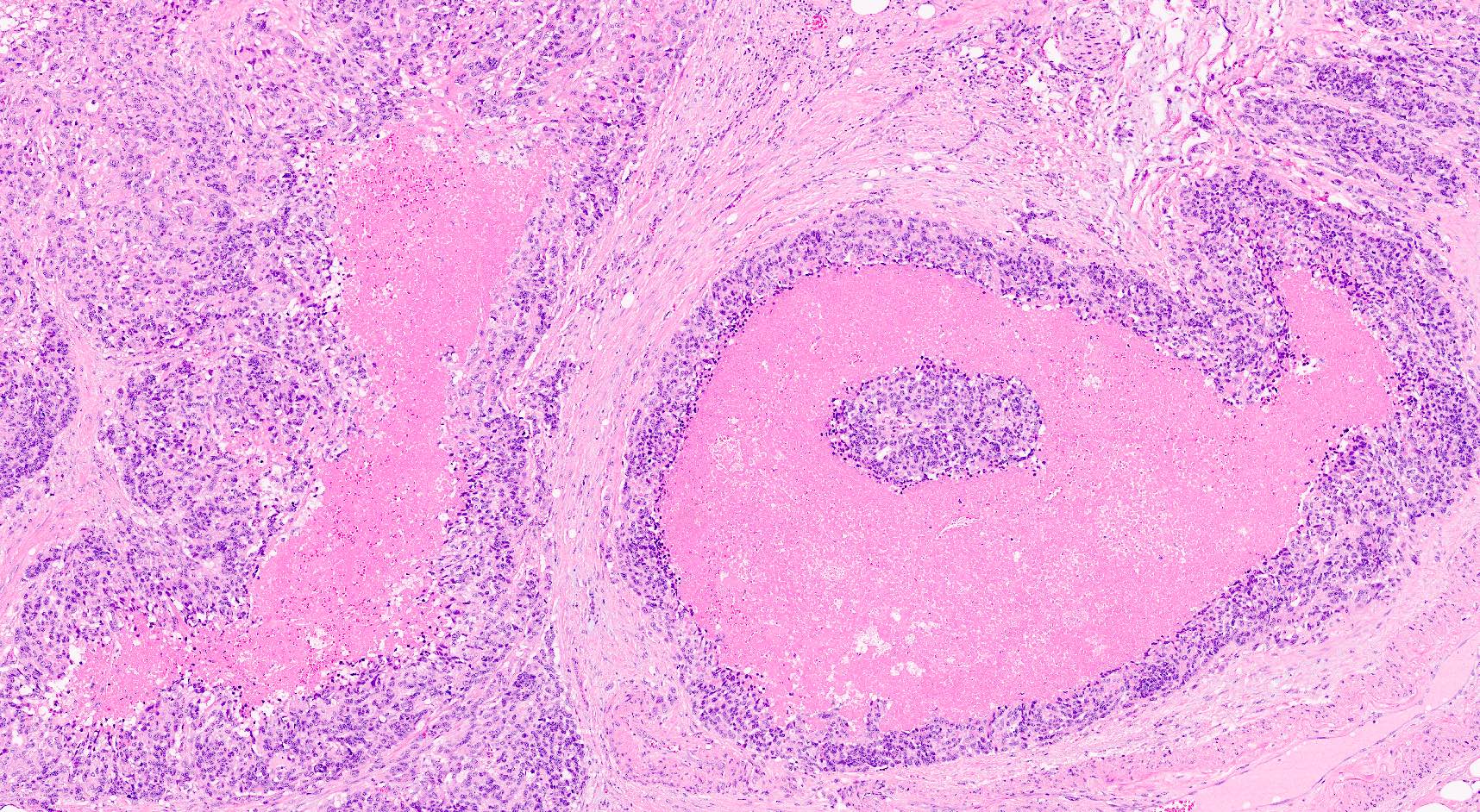
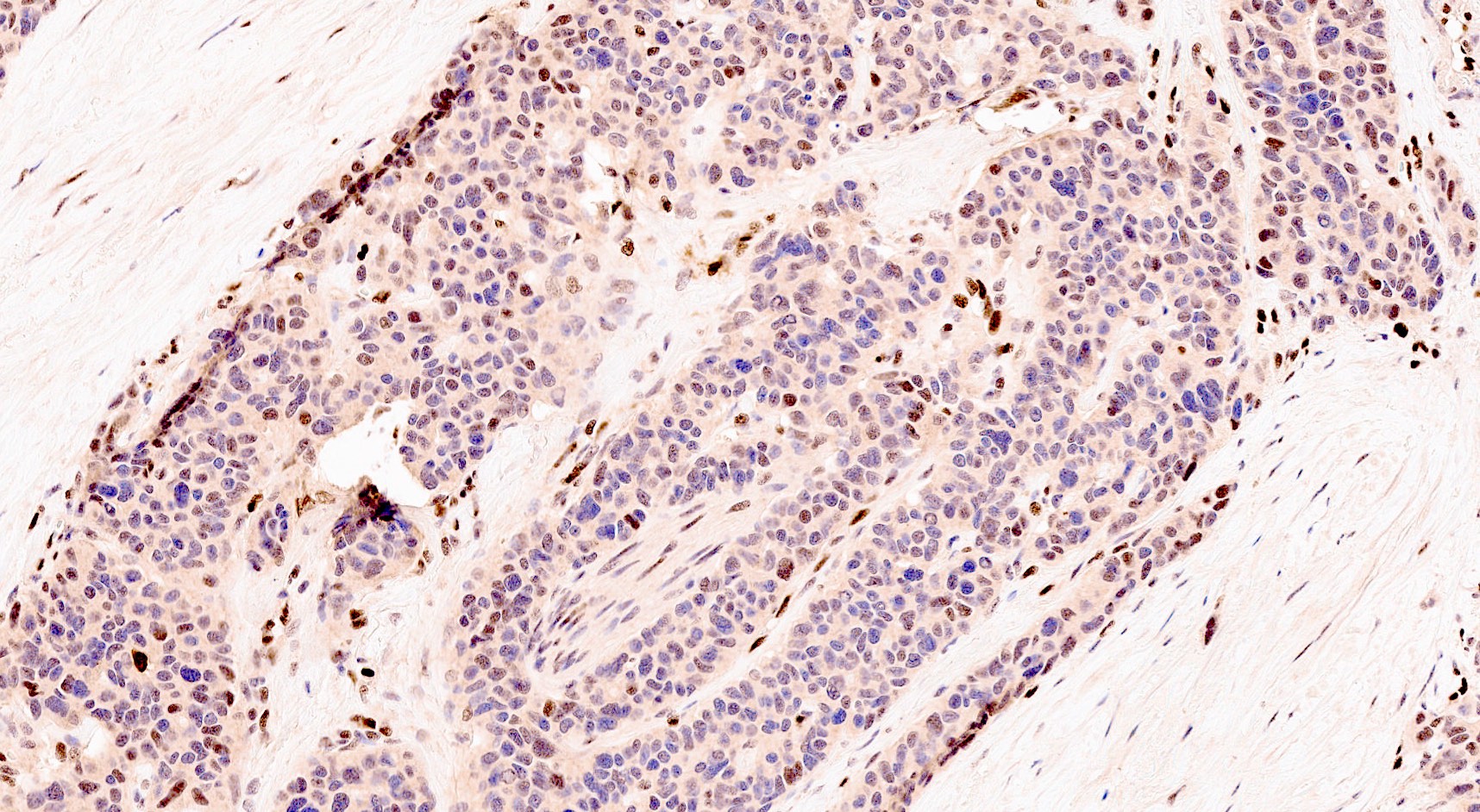
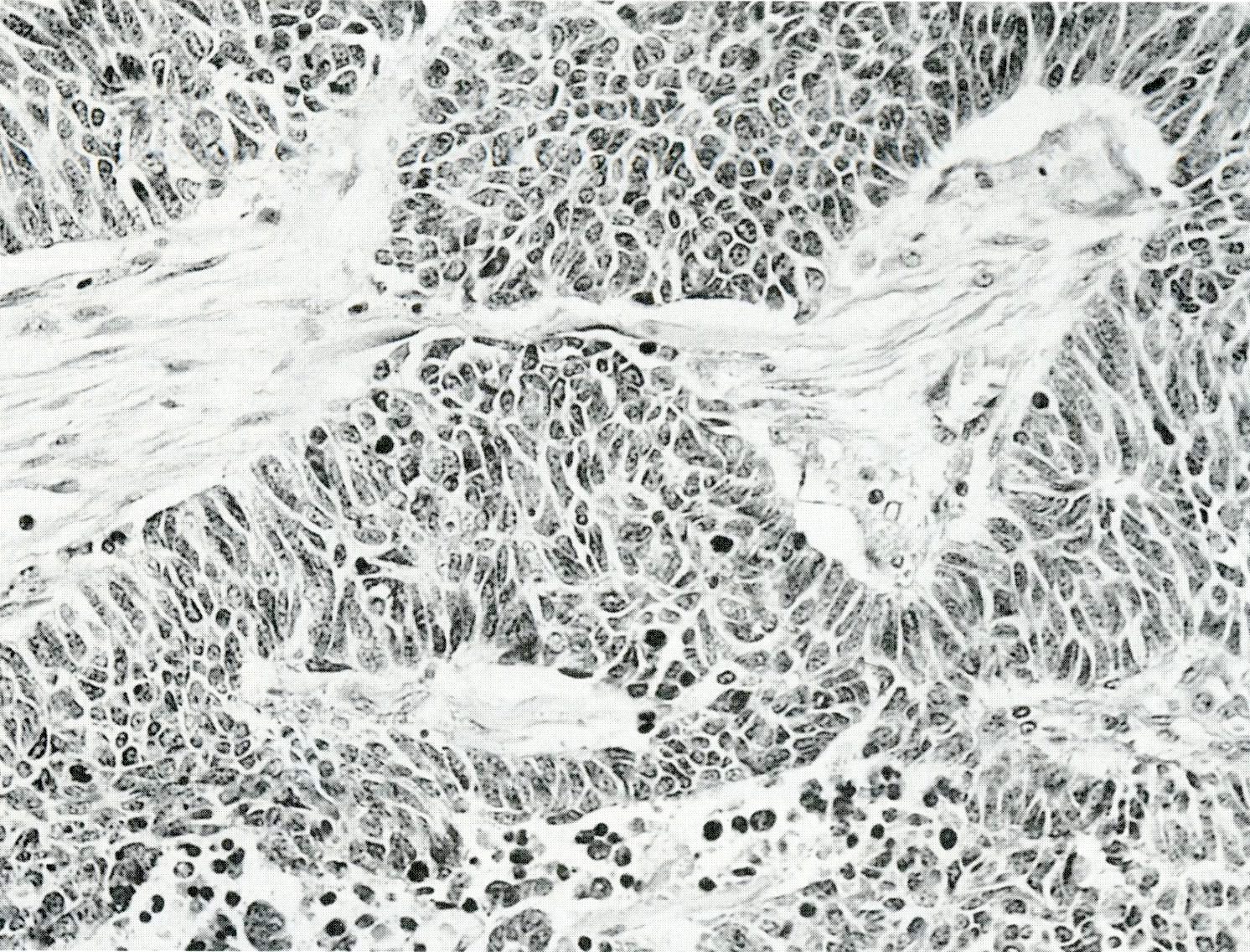
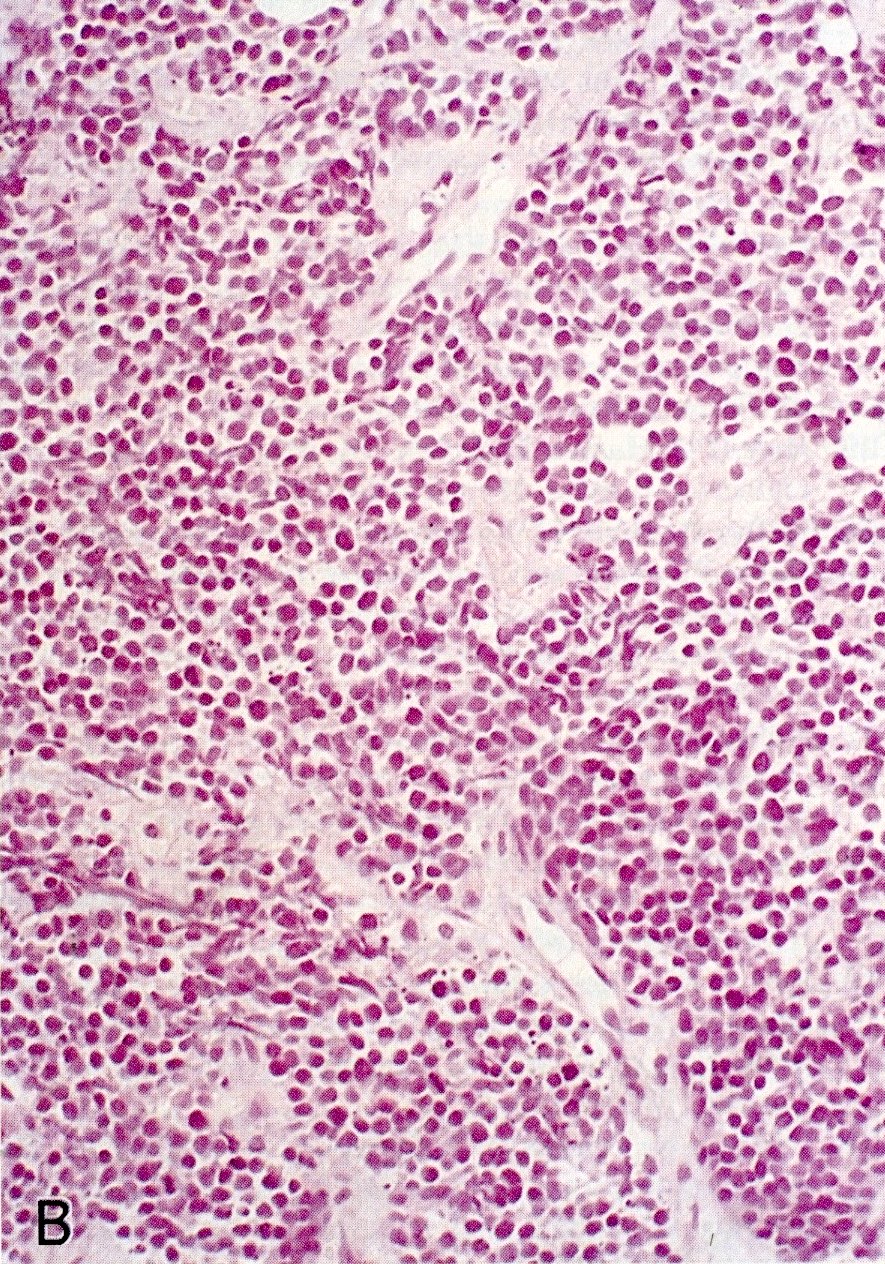
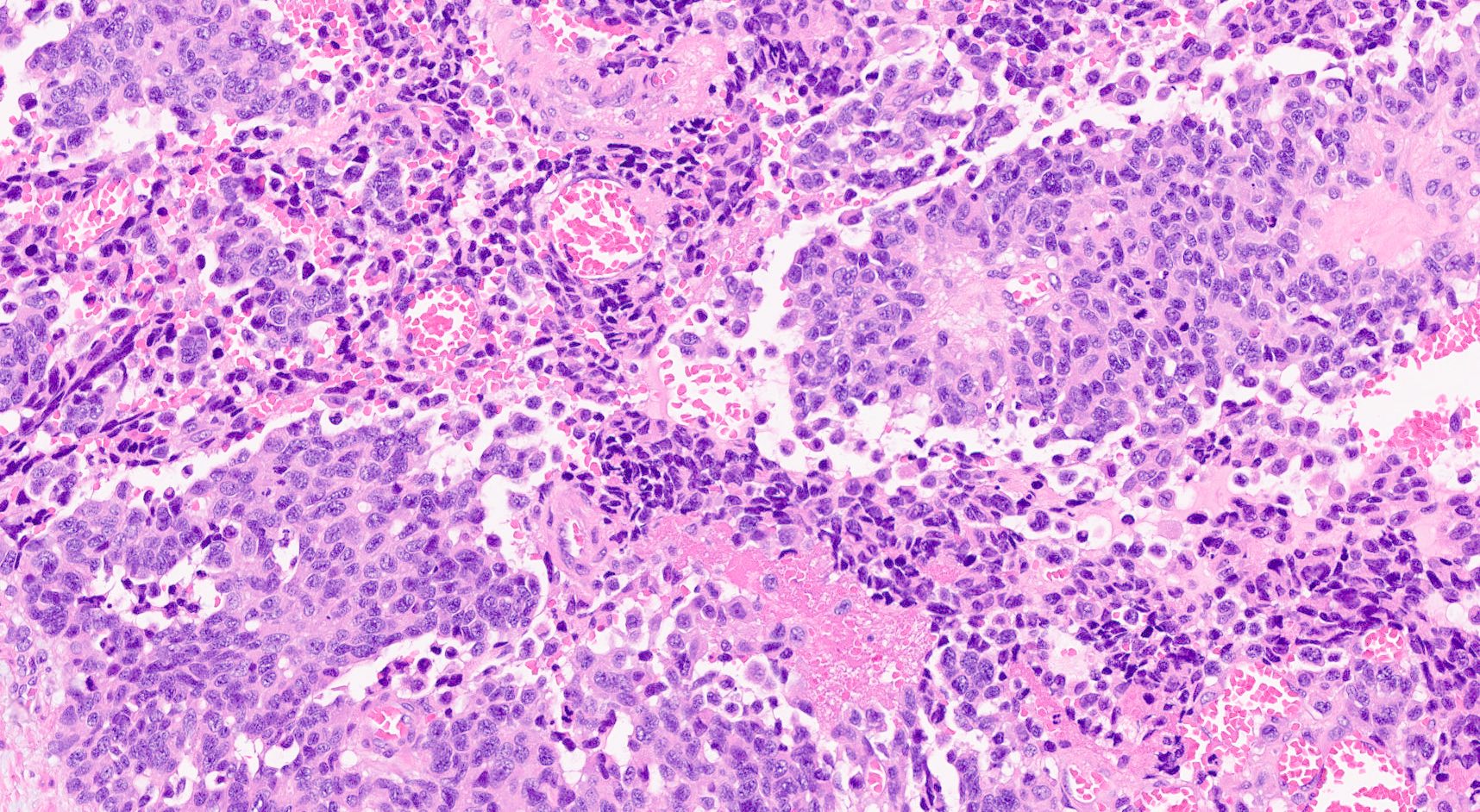
Microscopic (histologic) description
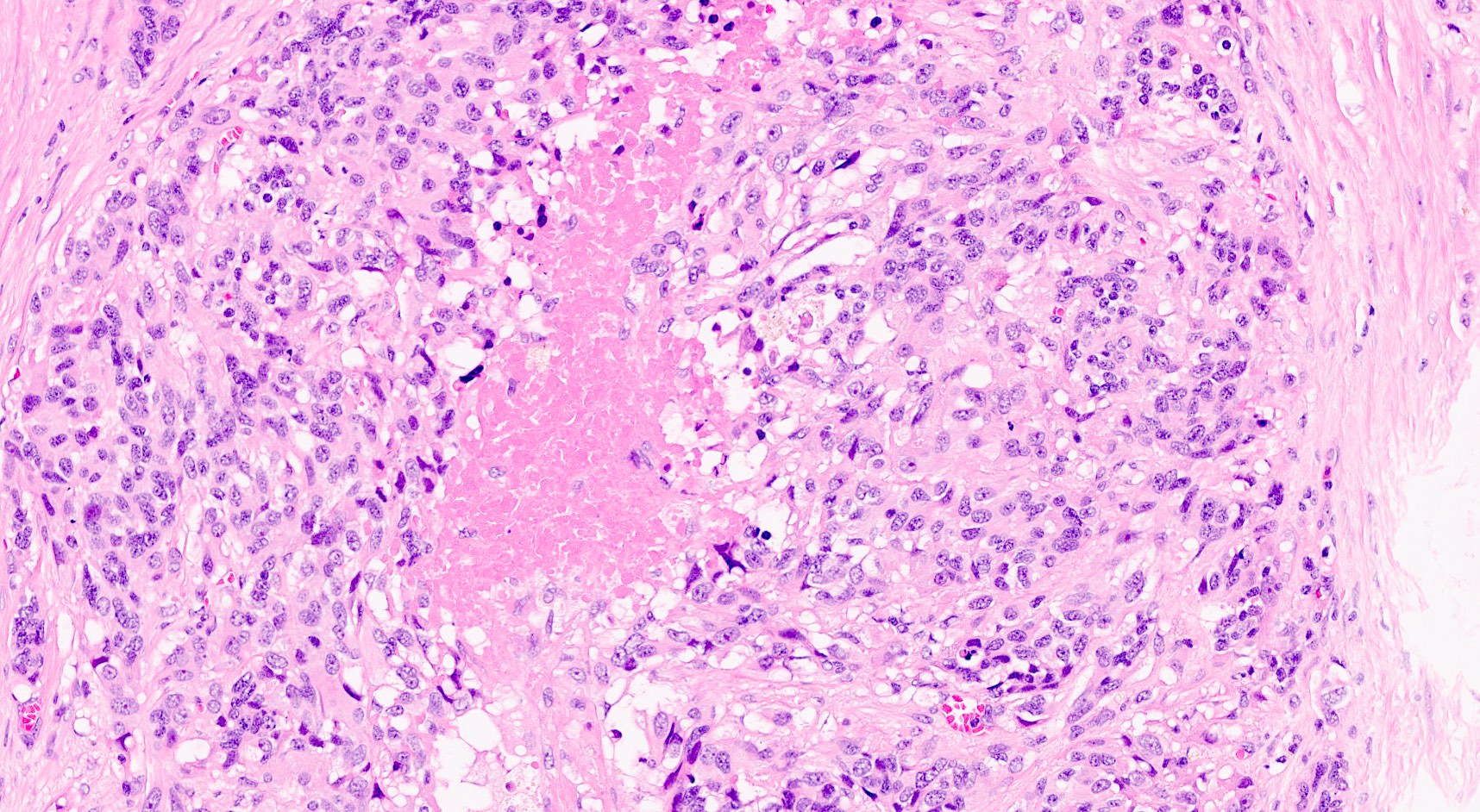
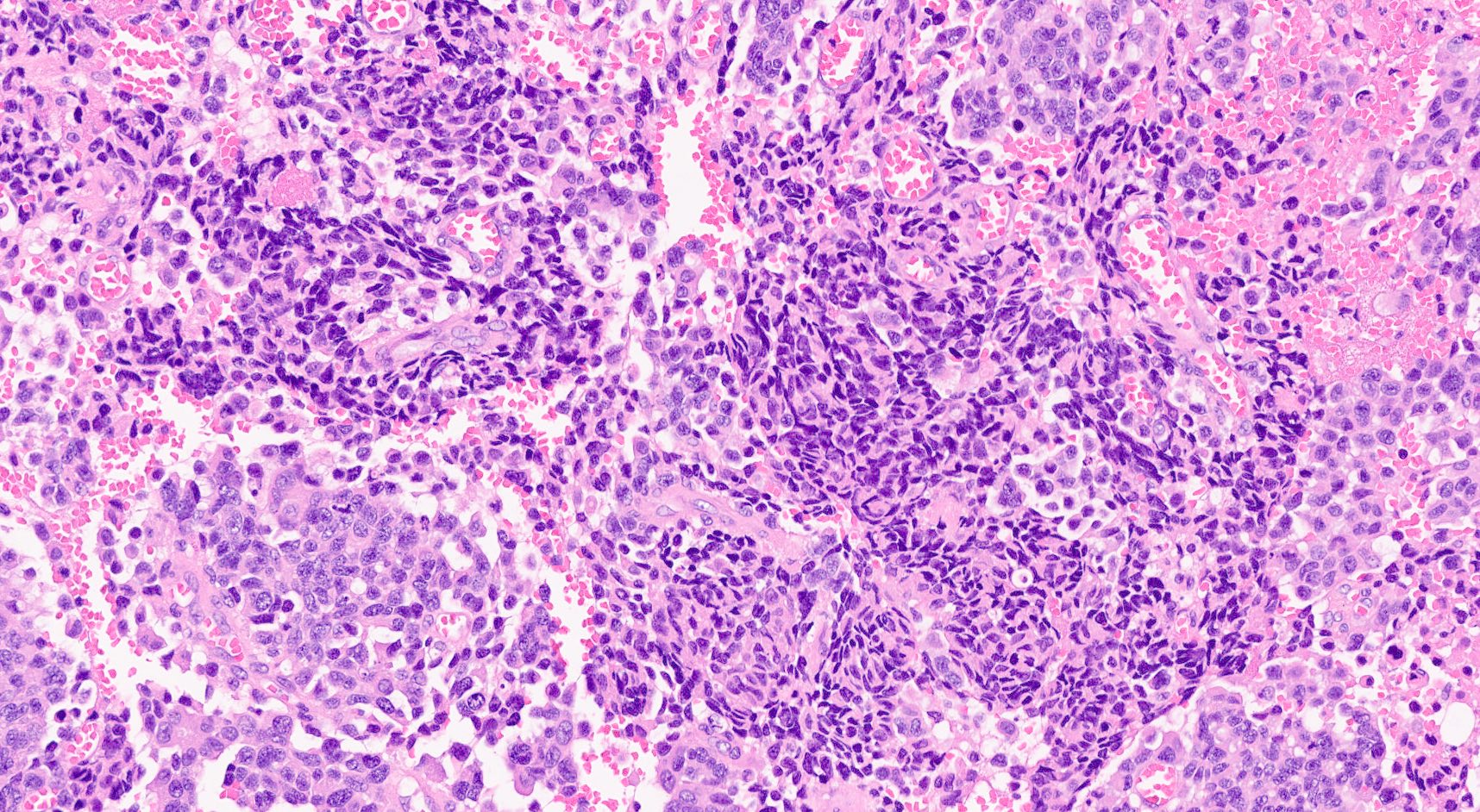
- Hypercellular neoplasm, with large and irregular nests, infiltrative growth pattern with randomly oriented large vascular structures and desmoplastic type fibrosis
- Necrosis with geographic pattern and comedo-like appearance
- High rate of cellular turnover (high mitotic rate and high apoptotic rate) (Front Oncol 2013;3:2)
- SC PanNEC: diffuse sheets of relatively small cells with round or elongated hyperchromatic nuclei, finely granular chromatin and lacking nucleoli; typical feature of nuclear molding (sharing with the pulmonary counterpart) (Front Oncol 2013;3:2)
- LC PanNEC: nesting / trabecular pattern, with round to polygonal medium to large sized cells, with amphiphilic cytoplasm and atypical nuclei with either coarse chromatin or conspicuous nucleoli; the nuclear to cytoplasmic (N/C) ratio of LCNECs is lower than that of SCNECs
Microscopic (histologic) images
Contributed by Claudio Luchini, M.D., Ph.D. and AFIP images
Cytology description
- Scant cytoplasm, slightly granular and high N/C ratio
- SC PanNEC: small cells with large nuclei, nuclear molding, finely speckled and dark chromatin with inconspicuous nucleoli, prominent background degeneration and typical crush artifact with nuclear streaming (Am J Surg Pathol 2012;36:173)
- LC PanNEC: large undifferentiated cells with bizarre forms or syncytial aggregates, irregular overlapping nuclei with prominent nucleoli, vesicular chromatin and abundant cytoplasm (delicate, dense or granular) (Am J Surg Pathol 2012;36:173)
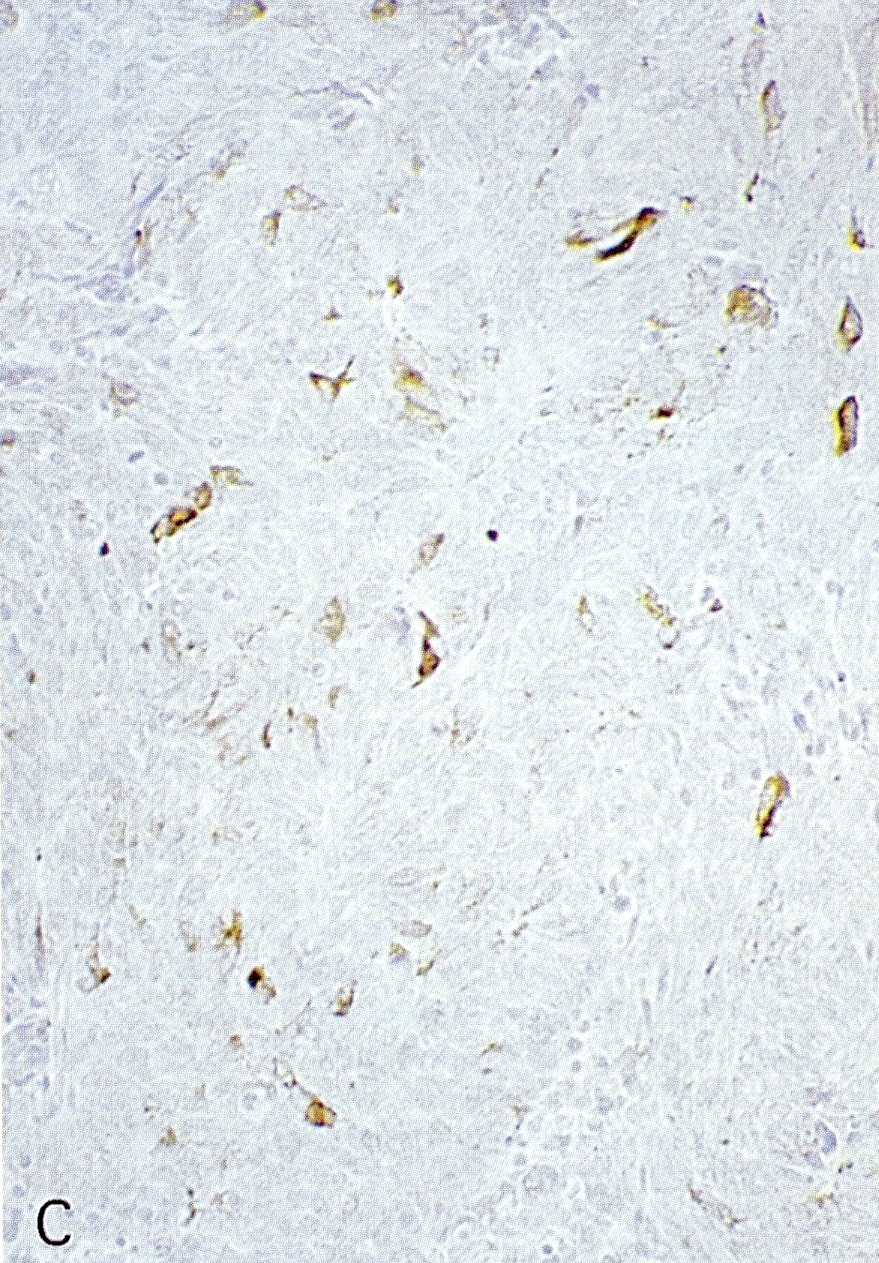
Positive stains
- Cytokeratins (CK8 / CK18, CK AE1 / AE3)
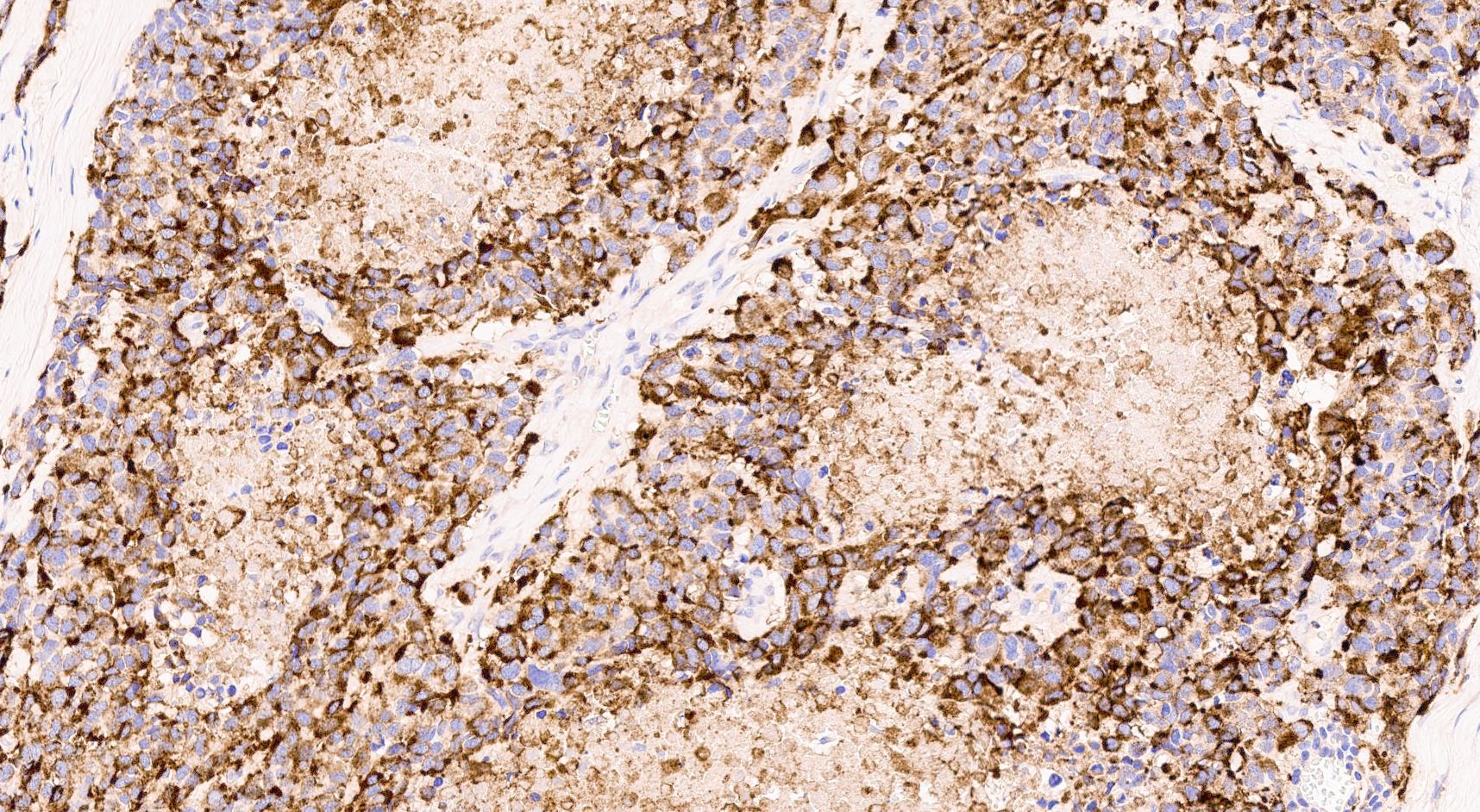
- Synaptophysin: usually positive, may be rarely weak or focal (Am J Surg Pathol 2012;36:173)
- Chromogranin A: usually positive or weakly positive; can be also almost absent; SC PanNEC can also show a paranuclear dot-like immunoreactivity (Endocr Pathol 2018;29:150)
- CD56, neuron specific enolase (NSE), protein gene product 9.5 (PGP9.5) / ubiquitin C terminal hydrolase 1 protein (UCHL1): general neuroendocrine markers, less specific, limited utility
- Insulinoma associated protein 1 (INSM1) has emerged as an additional general neuroendocrine marker (Am J Clin Pathol 2015;144:579)
- Achaete-scute homolog 1 (ASH1) (Hum Pathol 2013;44:1391)
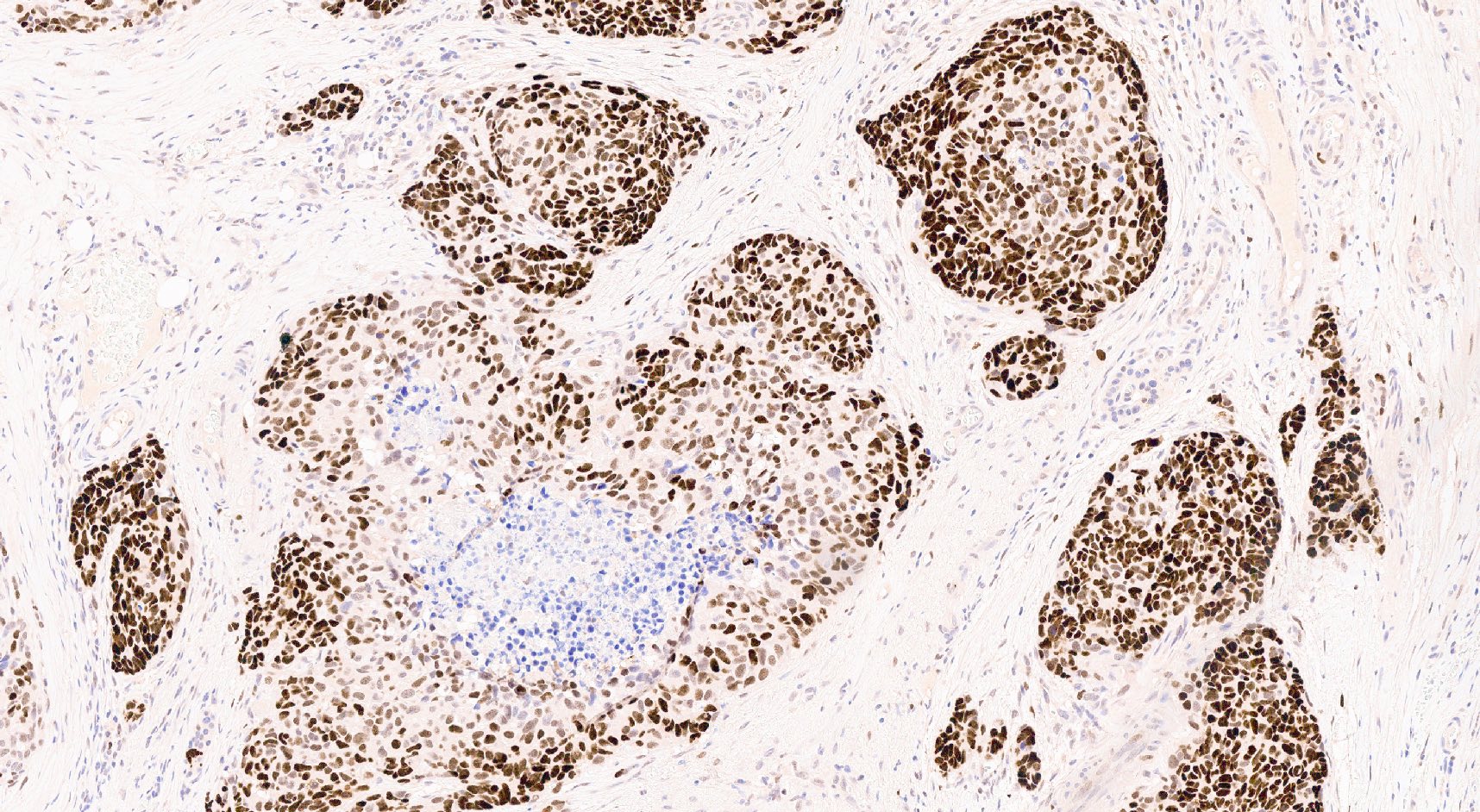
- p53: abnormal expression pattern / nuclear accumulation (Pathologica 2021;113:28)
- Useful (but neither ideal nor absolute) panel for determining pancreatic origin is PDX1+, ISL1+, PAX8+, CDX2+, TTF1- (Pathologica 2021;113:28)
Negative stains
- Rb (classic loss of Rb protein) (Am J Surg Pathol 2012;36:173)
- SMAD4 (Am J Surg Pathol 2016;40:1192)
- BCL10 (helping in excluding an acinar cell carcinoma or the presence of a focal acinar differentiation)
- Beta catenin (no nuclear staining; normal positivity of cell membrane)
Molecular / cytogenetics description
- Alterations of TP53 and Rb represent the most typical molecular events in PanNEC (Cancer Treat Rev 2017;56:28, Cancer Discov 2022;12:692)
- KRAS is also mutated in a significant proportion of cases (up to 30%) (Am J Surg Pathol 2012;36:173)
- Inactivation of SMAD4 / DPC4 is also reported (5%) (Am J Surg Pathol 2012;36:173)
- Microsatellite instability can be present in up to 5 - 8% of cases and should be assessed at the time of diagnosis (Hum Pathol 2022 Jun 14 [Epub ahead of print])
Sample pathology report
- Duodenum and pancreatic head, pancreaticoduodenectomy:
- Pancreatic neuroendocrine carcinoma, large cell type (see comment)
- Pancreatic head with the presence of a high grade neuroendocrine neoplasm
- Ki67 (MIB1) index 70%; mitotic rate: 50 mitoses/2 mm2
- Presence of tumor necrosis
- The neoplasm infiltrates distal choledochus, adipose tissue and duodenum
- Metastasis in 3/12 lymph nodes
- Surgical margins without tumor involvement
- Comment: The integration of morphology with the immunohistochemical profile (and in particular, CK AE1 / AE3 and CK8 / CK18 positive, synaptophysin positive, chromogranin A weakly positive, TP53: aberrant pattern; Rb: negative) is consistent with the diagnosis of pancreatic neuroendocrine carcinoma.
Differential diagnosis
- Well differentiated G3 neuroendocrine tumor (WD PanNET):
- Nonfunctional PanNETs are identified incidentally and have no symptoms
- PanNETs G3 show avidity on SSRS imaging (Octreoscan and Gallium 68 Dotatate PET / CT)
- Cytology: PanNETs G3 show abundant granular cytoplasm, low N/C and stippled chromatin
- Resection specimens: in PanNETs G3, the G3 component is usually not homogenous and mixed with a lower grade counterpart and the same for Ki67, which is heterogeneous
- Positive stains: in PanNETs, there are diffuse and strong chromogranin A (more specific) and synaptophysin (more sensitive) staining
- Molecular profile: in PanNETs, TP53 and Rb are usually wild type; there are recurrent and mutually exclusive mutations of DAXX (death domain associated protein, 25%) and ATRX (alpha thalassemia / intellectual disability syndrome X linked, 17.6%), usually wild type in PanNECs (Science 2011;331:1199)
- High grade neuroendocrine carcinomas of other sites (pancreatic metastasis):
- Other pancreatic primaries:
- Acinar cell carcinoma:
- Mixed neuroendocrine nonneuroendocrine neoplasms:
- Different components should be identified by morphology and not only by immunohistochemistry
- Medullary carcinoma:
- Syncytial growth pattern, intratumor inflammatory cells
- Negative for neuroendocrine markers
- Lymphoma:
- Melanoma:
- Small blue cell tumors in young adults:
- Desmoplastic small cell tumor (Am J Surg Pathol 2004;28:808):
- Large nests and broad bands of small blue cells, separated by fibrous stroma
- Positive stains: cytokeratins (AE1 / AE3 and CAM5.2), NSE, desmin and WT1
- Negative stains: chromogranin A, S100 and CD99
- Primitive neuroectodermal tumors (Am J Surg Pathol 2002;26:1040):
- Sheets and lobules of small cells with round to oval nuclei, nuclear molding and scant cytoplasm; no Homer-Wright rosettes
- Positive stains: CD99, cytokeratins (AE1 / AE3 and CAM5.2), NSE, chromogranin A or synaptophysin (may be focal or diffuse)
- Negative stans: desmin, actin, S100 protein, insulin, glucagon or somatostatin
- Desmoplastic small cell tumor (Am J Surg Pathol 2004;28:808):
Additional references
Board review style question #1
Which are the molecular hallmarks of PanNEC?
- CCND1 amplification
- DAXX and ATRX mutation
- EGFR alteration
- KRAS mutation
- TP53 and Rb alteration
Board review style answer #1
E. TP53 and Rb alteration. Indeed, the most typical molecular events in PanNEC are TP53 and Rb alteration. Immunohistochemistry is a good surrogate of their molecular status and in PanNEC it generally shows an aberrant expression pattern (usually a hyperaccumulation) for p53 and Rb loss.
Comment Here
Reference: Neuroendocrine carcinoma
Comment Here
Reference: Neuroendocrine carcinoma
Board review style question #2
Board review style answer #2
B. Focal tumor necrosis and nuclear features. Tumor necrosis is a very important morphological feature for supporting the diagnosis of PanNEC but the immunohistochemical confirmation of the neuroendocrine nature is also very important.
Comment Here
Reference: Neuroendocrine carcinoma
Comment Here
Reference: Neuroendocrine carcinoma