Table of Contents
Definition / general | Essential features | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Hutchings D. Neuroendocrine neoplasms-general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreaspen.html. Accessed April 19th, 2024.
Definition / general
- Includes well differentiated neuroendocrine tumors (WDNET) and poorly differentiated neuroendocrine carcinomas (PDNEC)
- Express neuroendocrine markers (synaptophysin and chromogranin A) (see Positive stains)
- Mixed neuroendocrine nonneuroendocrine neoplasms (MiNEN) includes both neuroendocrine and nonneuroendocrine component (e.g., adenocarcinoma, acinar carcinoma), each ≥ 30%
- Functional tumors are associated with elevated serum hormone levels and a clinical hormonal syndrome (see Clinical features)
- Nonfunctional tumors are more common and are not associated with a clinical hormonal syndrome
- May still be associated with elevated serum hormone levels or tissue hormone expression on immunohistochemistry
- Nonfunctional tumors < 0.5 cm are termed pancreatic neuroendocrine microadenomas
Essential features
- Well differentiated neuroendocrine tumors (WDNET):
- Clumped chromatin (salt and pepper) nuclei
- Cellular uniformity, central ovoid nucleus
- Organoid architecture: ribbons / trabeculae, nesting, glandular, gyriform, pseudoacinar
- Necrosis is rare
- Amyloid deposition in insulinomas
- Psammoma bodies in somatostatinomas (also associated with NF1)
- Background of microadenomas in multiple endocrine neoplasia type 1 (MEN1) and von Hippel-Lindau (VHL) syndromes
- Clear cell morphology in von Hippel-Lindau syndrome
- Poorly differentiated neuroendocrine carcinomas (PDNEC):
- Diffuse sheets or solid nests of markedly atypical malignant cells with small cell or large cell morphology
- Abundant mitoses and apoptotic bodies
- Necrosis is frequent
Terminology
- Antiquated terms: islet cell tumor / carcinoma, carcinoid, APUDoma
- Classification based on WHO system:
- Per WHO 2019, neuroendocrine neoplasms with Ki67 > 20% are now regarded in 2 distinct groups, WDNET grade 3 or PDNEC, based on the morphologic characteristics
- Well differentiated neuroendocrine tumor grading:
- WHO grade 1: < 2 mitoses/2 mm2 or Ki67 index < 3%
- WHO grade 2: 2 - 20 mitoses/2 mm2 or Ki67 index 3 - 20%
- WHO grade 3: > 20 mitoses/2 mm2 or Ki67 > 20%
- Ki67 count should be done on a minimum of 500 cells in hot spot areas
- Both mitotic count and Ki67 should be performed and grade is assigned based on largest value; usually Ki67 is higher than mitotic count (Am J Surg Pathol 2013;37:1671)
Epidemiology
- 1 - 5% of all pancreatic neoplasms; increasing incidence due to improved imaging techniques (Arch Pathol Lab Med 2019;143:1317, Cancer 2015;121:589)
- Incidence of 1 per 100,000 persons (Cancer 2015;121:589)
- Wide age range but most common in third to sixth decades (Arch Pathol Lab Med 2019;143:1317)
- M = F (Arch Pathol Lab Med 2019;143:1317)
- Most are sporadic
- 10 - 20% associated with syndrome (Arch Pathol Lab Med 2019;143:1317):
- 20 - 70% of multiple endocrine neoplasia 1 (MEN1) patients
- 20% von Hippel-Lindau (VHL) patients
- 10% neurofibromatosis type 1 (NF1) patients
- 1% of tuberous sclerosis complex (TSC) patients
Sites
- WDNET is more common in tail and body (Arch Pathol Lab Med 2019;143:1317)
- PDNEC is more common in head (Arch Pathol Lab Med 2019;143:1317)
- See Clinical hormonal syndromes
Pathophysiology
- Varies with clinical syndrome (see Clinical features)
Etiology
- Risk factors include smoking, diabetes and first degree relative with history of cancer (Sci Rep 2016;6:36073)
- Subset associated with MEN1 (MEN1 gene on 11q13), von Hippel-Lindau (VHL tumor suppressor gene on 3p25), neurofibromatosis 1 (microdeletion in neurofibromin gene on 17q11.2) and tuberous sclerosis complex (TSC1 tumor suppressor gene on 9q34 or TSC2 tumor suppressor gene on 16p13.3) (Arch Pathol Lab Med 2019;143:1317)
Clinical features
- Nonfunctional tumors are encountered incidentally and are usually larger at time of diagnosis
- Local obstruction / mass effect, if located in the pancreatic head
- Other clinical features of MEN1, VHL, NF1 or TSC (if applicable)
- Clinical hormonal syndromes in functioning tumors, 10 - 30% of tumors (Arch Pathol Lab Med 2009;133:350)
- Insulinoma:
- Most common
- Insulin secretion
- Whipple triad: symptoms of hypoglycemia, low plasma glucose, relief of symptoms with glucose administration
- Solitary tumor < 2 cm
- 5 - 10% MEN1 and are usually multiple
- Generally indolent
- Gastrinoma:
- Second most common functioning pancreatic neuroendocrine tumor
- Gastrin secretion
- Zollinger-Ellison syndrome (peptic / duodenal ulcers, gastroesophageal reflux, diarrhea)
- Located in gastrinoma triangle (common bile duct, duodenum, pancreatic head)
- More commonly found in duodenum than pancreas
- 20 - 30% associated with MEN1
- Glucagonoma:
- 4 Ds: diabetes, dermatitis (necrolytic migratory erythema), deep vein thrombosis, depression
- Solitary, large
- Tail > head
- > 50% have metastasis at presentation
- VIPoma:
- Verner-Morrison syndrome: watery diarrhea, hypokalemia, achlorhydria / hypochlorhydria
- Solitary, large
- Tail > head
- Somatostatinoma:
- Diabetes mellitus, diarrhea or steatorrhea, anemia, malabsorption, cholelithiasis
- Very rare
- Solitary, large
- > 50% have metastasis at presentation
- Ectopic hormone producing neuroendocrine tumor:
- ACTH (Cushing syndrome), serotonin (atypical carcinoid syndrome), growth hormone
- Solitary, large
Laboratory
- Elevated serum hormone in functional tumors (see Clinical features)
- Elevated serum chromogranin A in 50 - 100%; correlates with tumor burden and metastasis but has limited utility due to low specificity and sensitivity (Arch Pathol Lab Med 2019;143:1317)
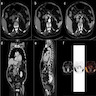
Radiology description
- Round, solid, hypervascular
- Subset may be cystic
- Well circumscribed
- Contrast enhanced computed tomography (CT) has decreased sensitivity in smaller (< 2 cm) tumors (Clin Endosc 2017;50:537)
- Magnetic resonance imaging (MRI) has better sensitivity, especially for small tumors compared with CT (Clin Endosc 2017;50:537)
- Gallium 68 dotatate positron emission tomography (PET) (Clin Endosc 2017;50:537):
- Uses radiolabeled somatostatin analog
- Useful for WDNETs, most of which express somatostatin receptor
- High sensitivity and specificity for somatostatin receptor expressing tumors
Radiology images
Prognostic factors
- WDNETs are usually slow growing with prolonged clinical course
- PDNECs are usually aggressive and rapidly progressive
- Ki67 index and mitotic count, as described for WHO grading
- Prognostic immunohistochemical markers (not widely used in routine clinical practice):
- Expression of COX2, p27, CD99 and PR immunostains (Pathol Int 2001;51:770, Am J Clin Pathol 2003;120:685, Am J Surg Pathol 2006;30:1588, Cancer 1992;70:2268)
- Expression of CK19 is associated with aggressive behavior (Am J Surg Pathol 2006;30:1588, Histopathology 2007;50:597, Am J Surg Pathol 2004;28:1145)
Case reports
- 45 year old woman with possible VHL syndrome with mixed serous neuroendocrine neoplasm (World J Clin Cases 2019;7:4119)
- 48 year old man with cystic pancreatic WDNET radiographically mimicking cystic mucinous neoplasm (Autops Case Rep 2020;10:e2020171)
- 53 year old man with pancreatic WDNET with rhabdoid features (Virchows Arch 2018;473:247)
- 58 year old woman with systemic mastocytosis incidentally found to have pancreatic WDNET (Int J Surg Case Rep 2020;77:397)
- 73 year old woman with breast carcinoma with unrecognized neuroendocrine differentiation metastasizing to the pancreas (Int J Surg Pathol 2016;24:463)
Treatment
- Surgical resection is the mainstay for WDNET, with resolution of both mass effect symptoms and symptoms associated with hormone secretion (Surg Clin North Am 2019;99:793)
- Benefit for both localized and metastatic disease
- Other therapies for metastatic WDNET (Surg Clin North Am 2019;99:793):
- Somatostatin analog therapy
- Molecular therapies: tyrosine kinase inhibitor, sunitinib; mTOR inhibitor, everolimus
- Chemotherapy (capecitabine and temozolomide [CAPTEM])
- Peptide receptor radionuclide therapy (PRRT)
- Liver directed therapy: ablation or hepatic artery embolization of liver metastasis
- PDNECs usually treated with platinum based chemotherapy (Surg Clin North Am 2019;99:793)
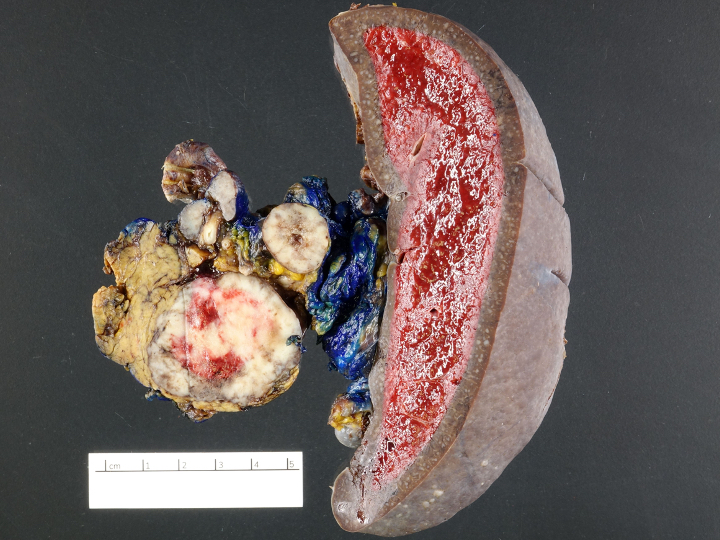
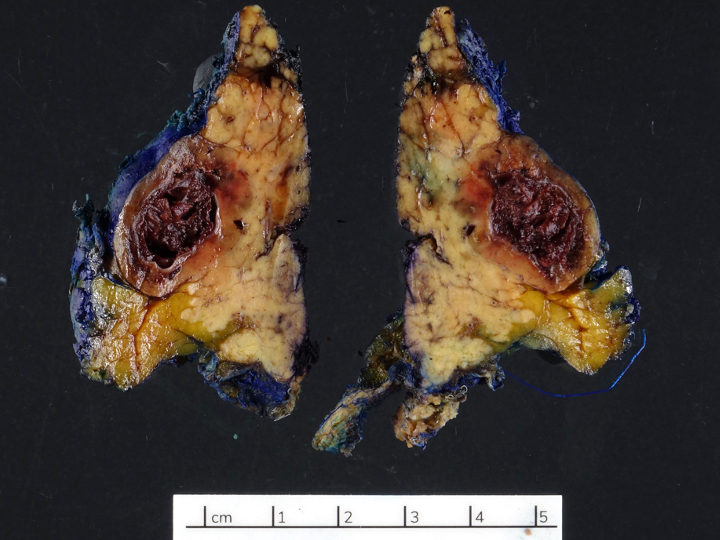
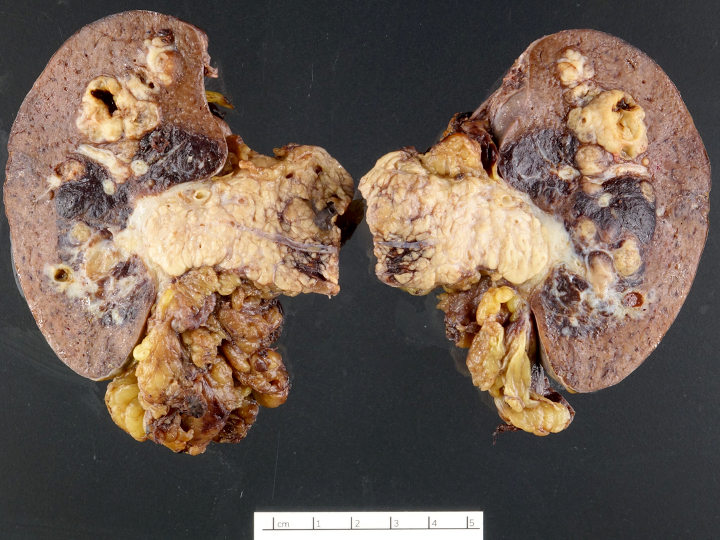
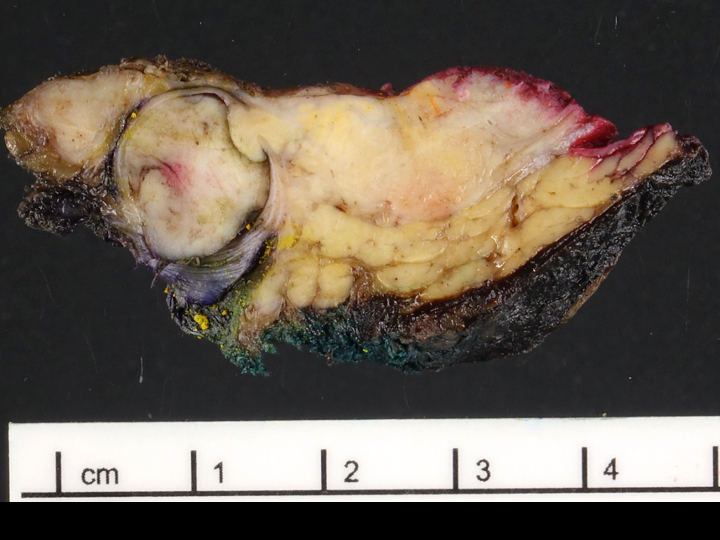
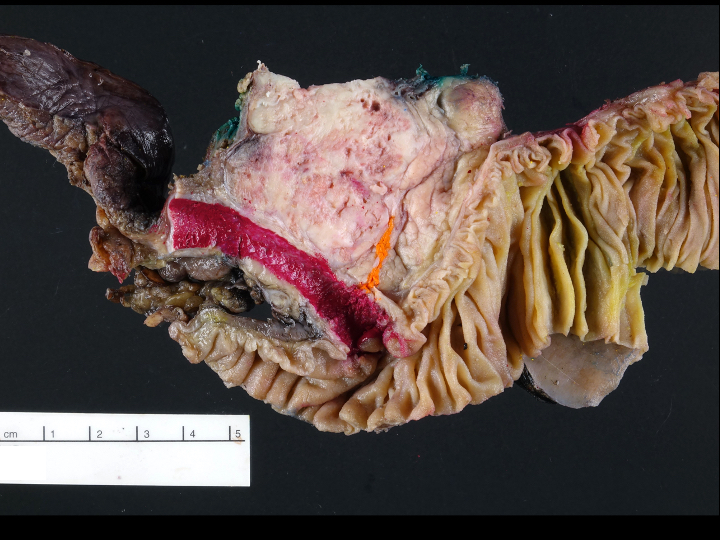
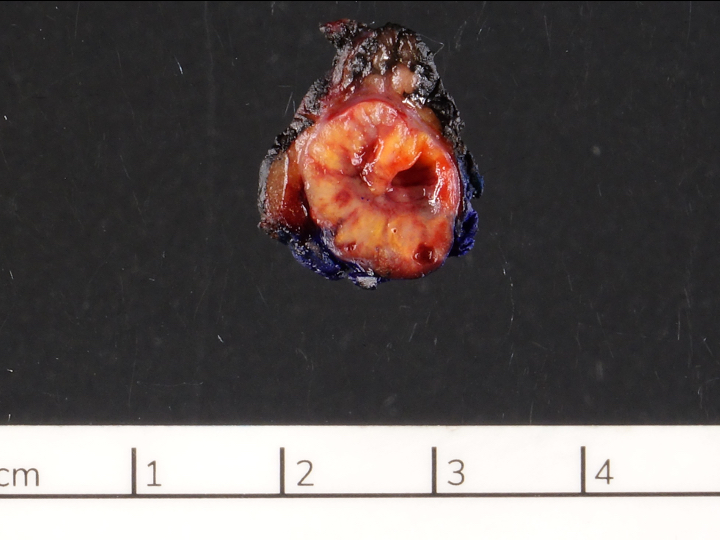
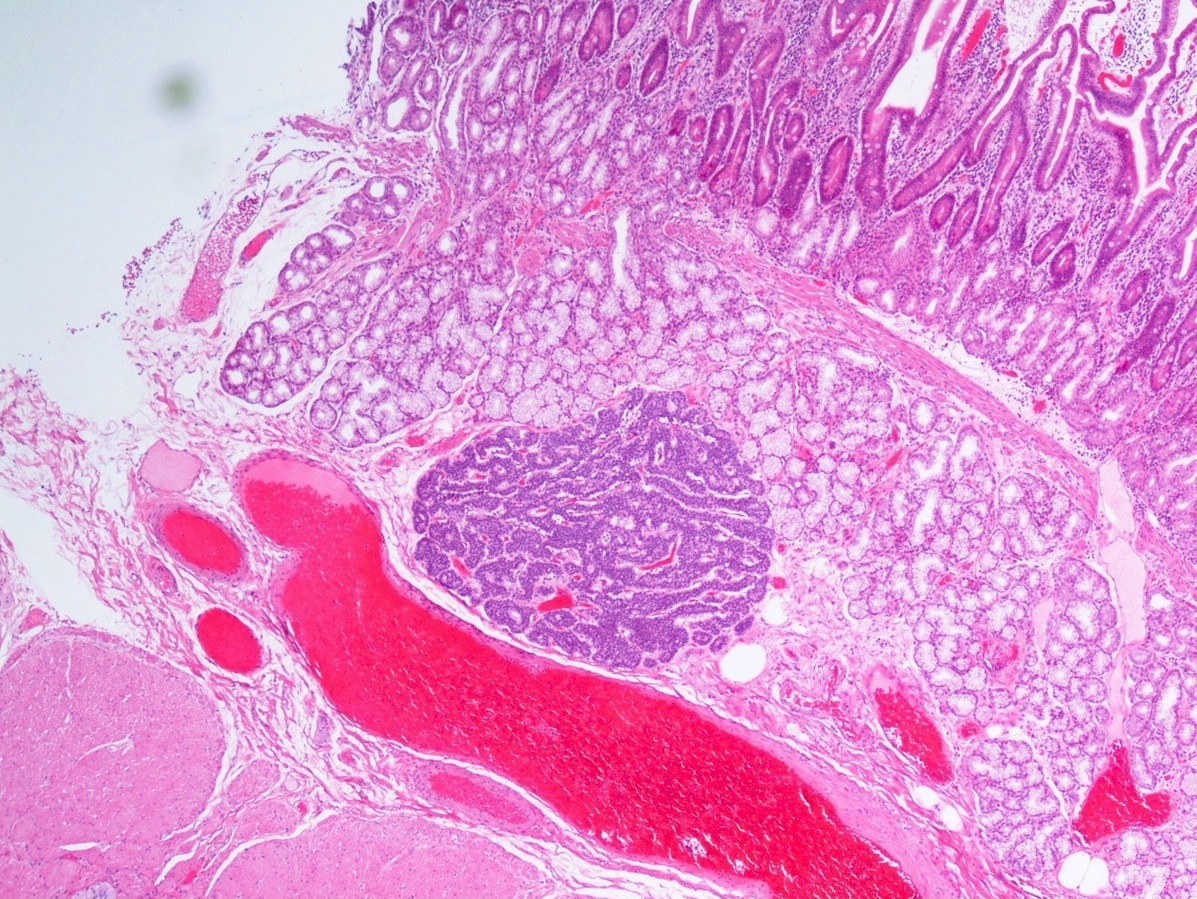
Gross description
- Usually well circumscribed, homogeneous
- May have cystic change
- Color and consistency varies according to degree of vascularity and stroma, ranges from white to pink to tan to brown; may be yellow if necrosis present (Arch Pathol Lab Med 2009;133:350)
- Pigmented black pancreatic neuroendocrine tumor contains intracytoplasmic lipfuscin and mimics metastatic melanoma (Arch Pathol Lab Med 2009;133:350)
- Lipid rich pancreatic neuroendocrine tumor appears yellow and may mimic adrenal cortical neoplasia (Arch Pathol Lab Med 2009;133:350)
- Large tumors may appear lobulated and infiltrative
- Can invade adjacent fibroadipose tissue, other organs and large vessels
Gross images
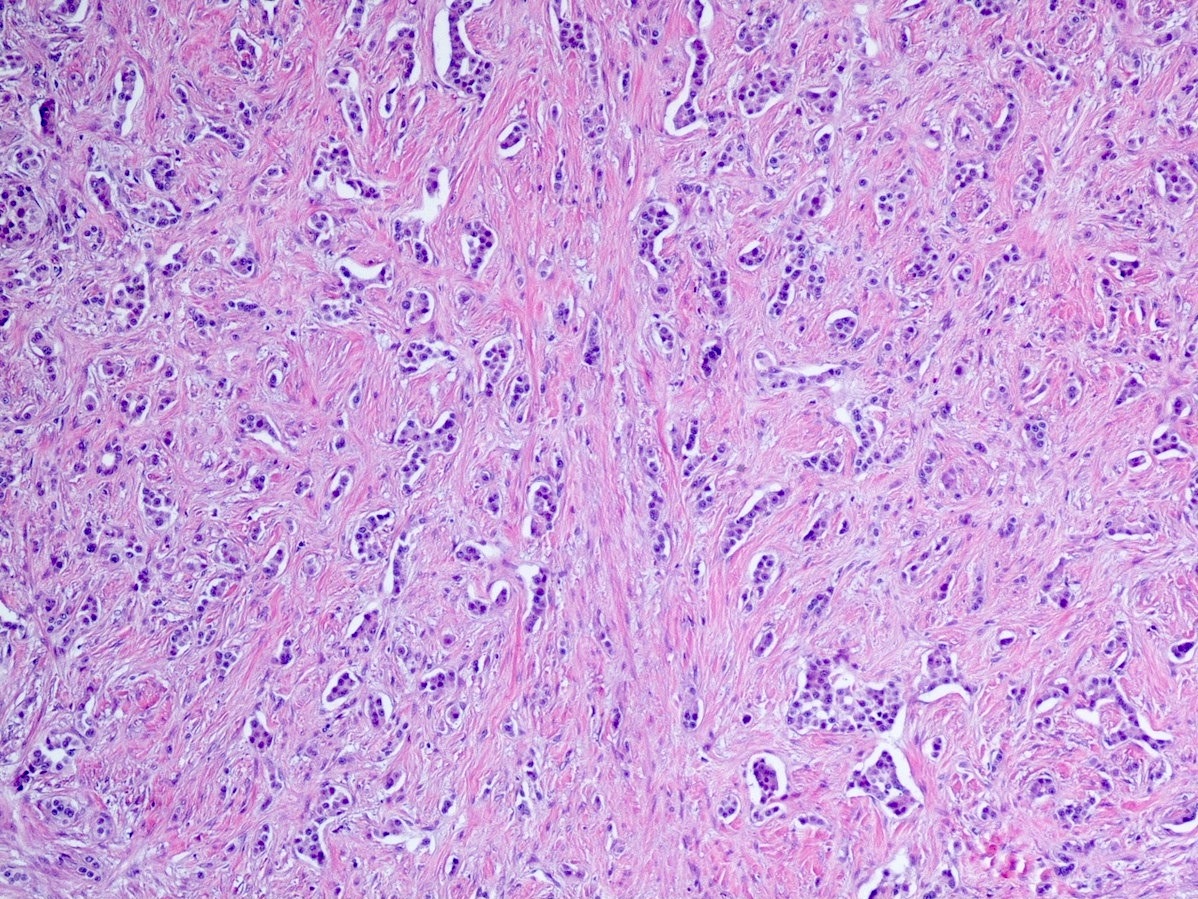
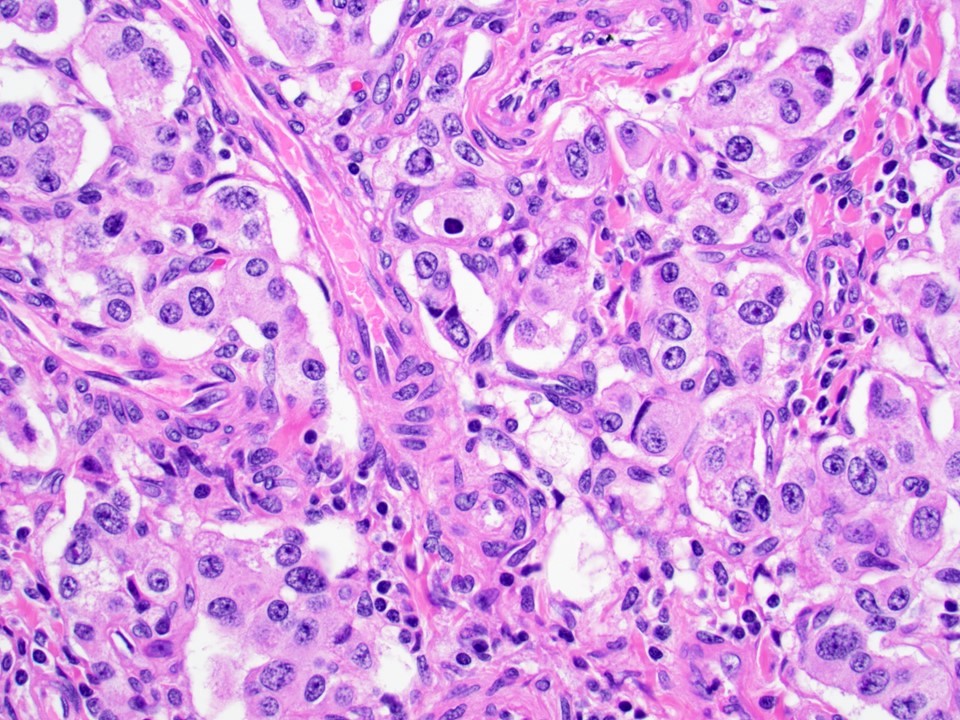
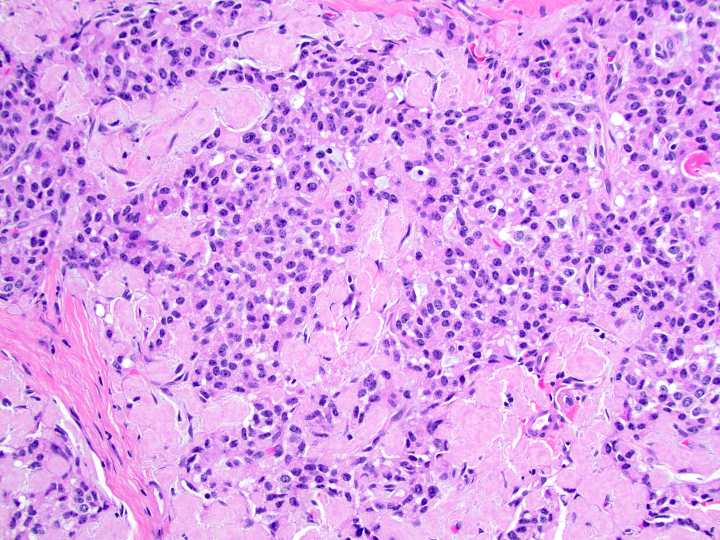
Microscopic (histologic) description
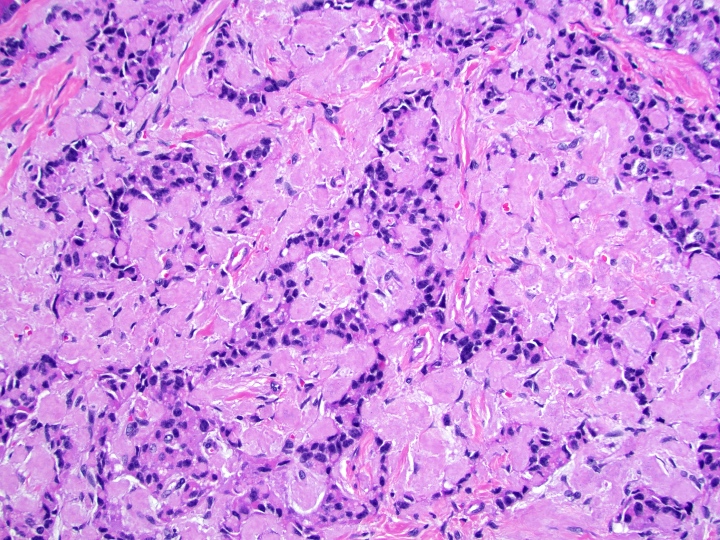
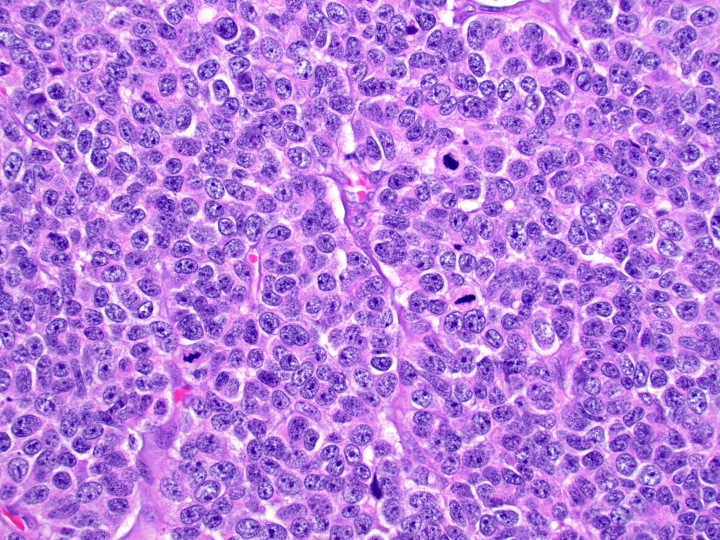
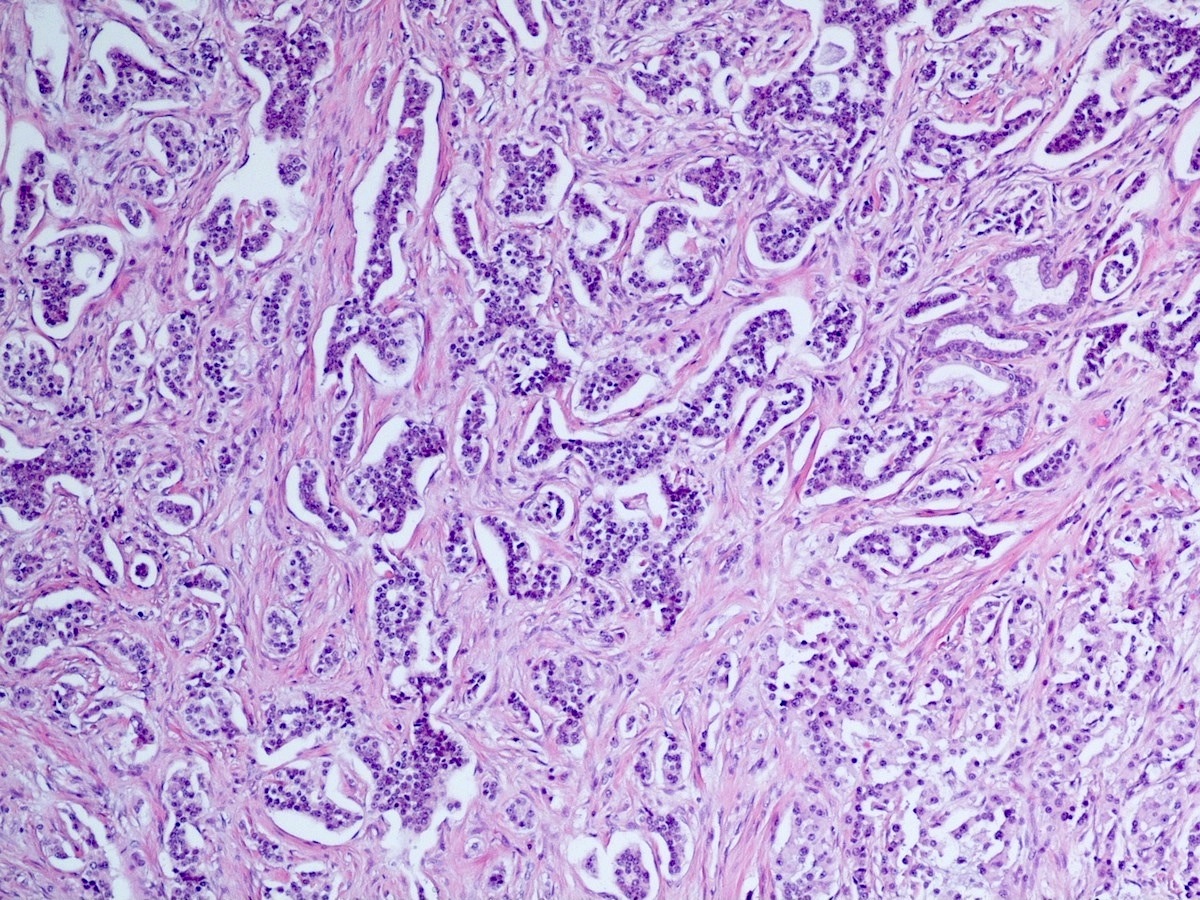
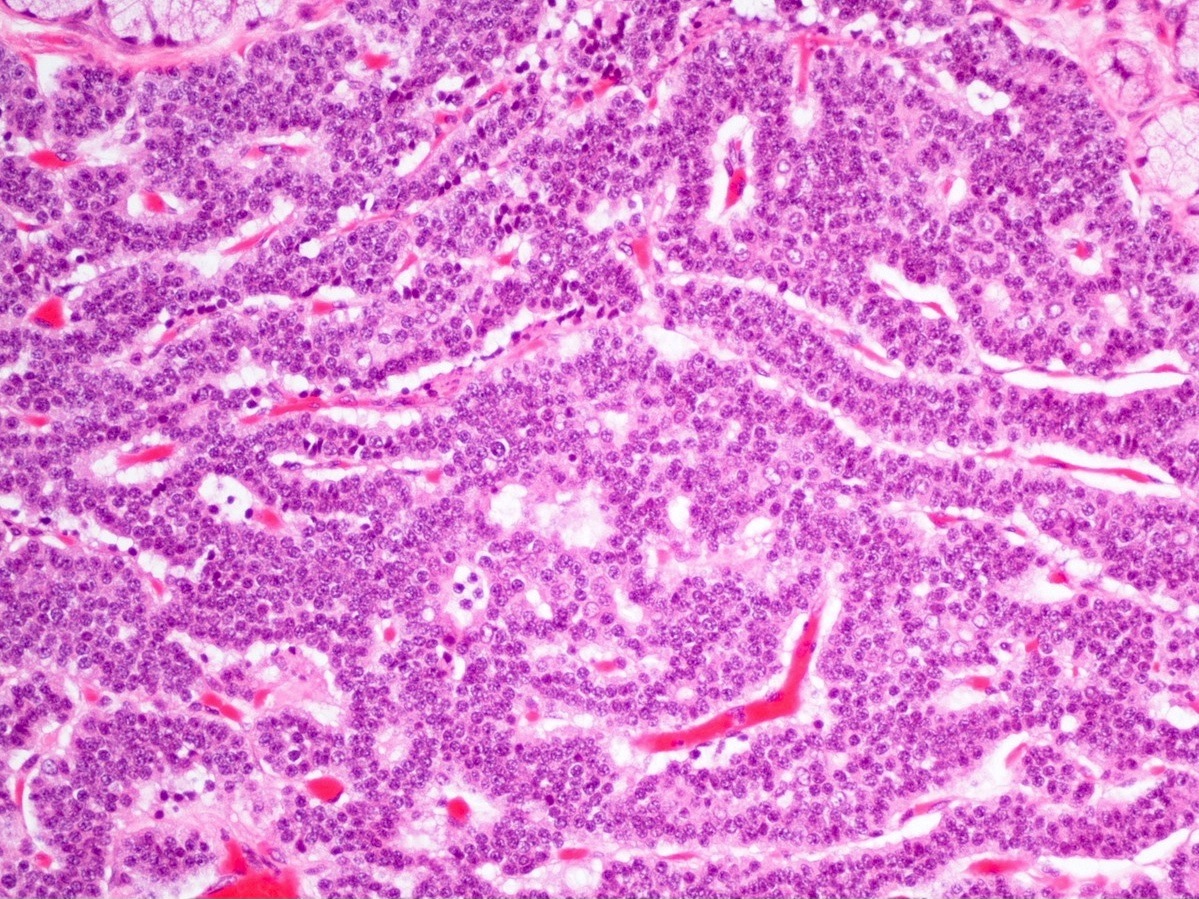
- Well differentiated neuroendocrine tumors:
- Organoid architecture: solid nests, trabeculae, gyri, cords, festoons, ribbons, glandular, tubuloacinar
- Small to medium cells with eosinophilic to amphophilic and finely granular cytoplasm; nuclei are uniform, central, round / oval, with salt and pepper (finely stippled) chromatin; no or inconspicuous nucleoli
- Rich vascular network
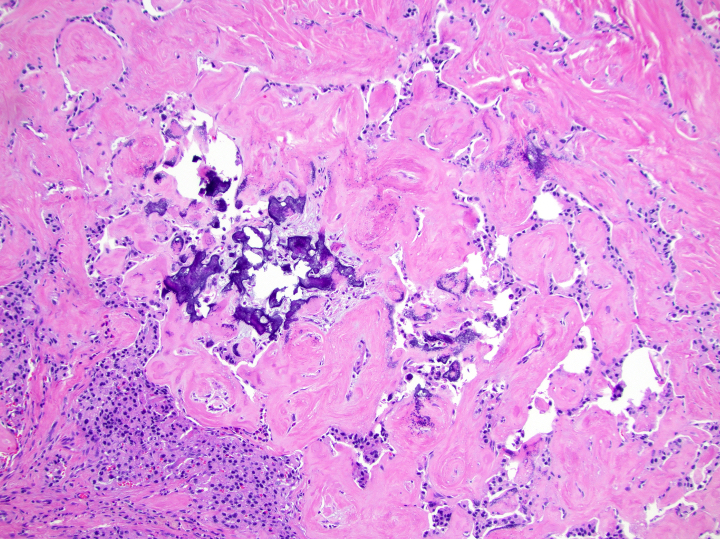
- Amyloid deposition in insulinomas
- Psammoma bodies in somatostatinomas
- May have hyaline globules (Am J Surg Pathol 2011;35:981)
- Variants include clear cell, lipid rich, oncocytic, rhabdoid and pigmented black neuroendocrine neoplasm (Arch Pathol Lab Med 2019;143:1317, Virchows Arch 2018;473:247, Cancer 2001;92:1984)
- Poorly differentiated neuroendocrine carcinomas:
- Diffuse sheets or solid nests of markedly atypical malignant cells
- Salt and pepper chromatin is lost
- Abundant mitoses and apoptotic bodies
- Necrosis is frequent
- May be small cell (high N/C ratio, nuclear hyperchromasia and molding) or large cell (moderate to abundant amphophilic cytoplasm, may have prominent nucleoli) morphology
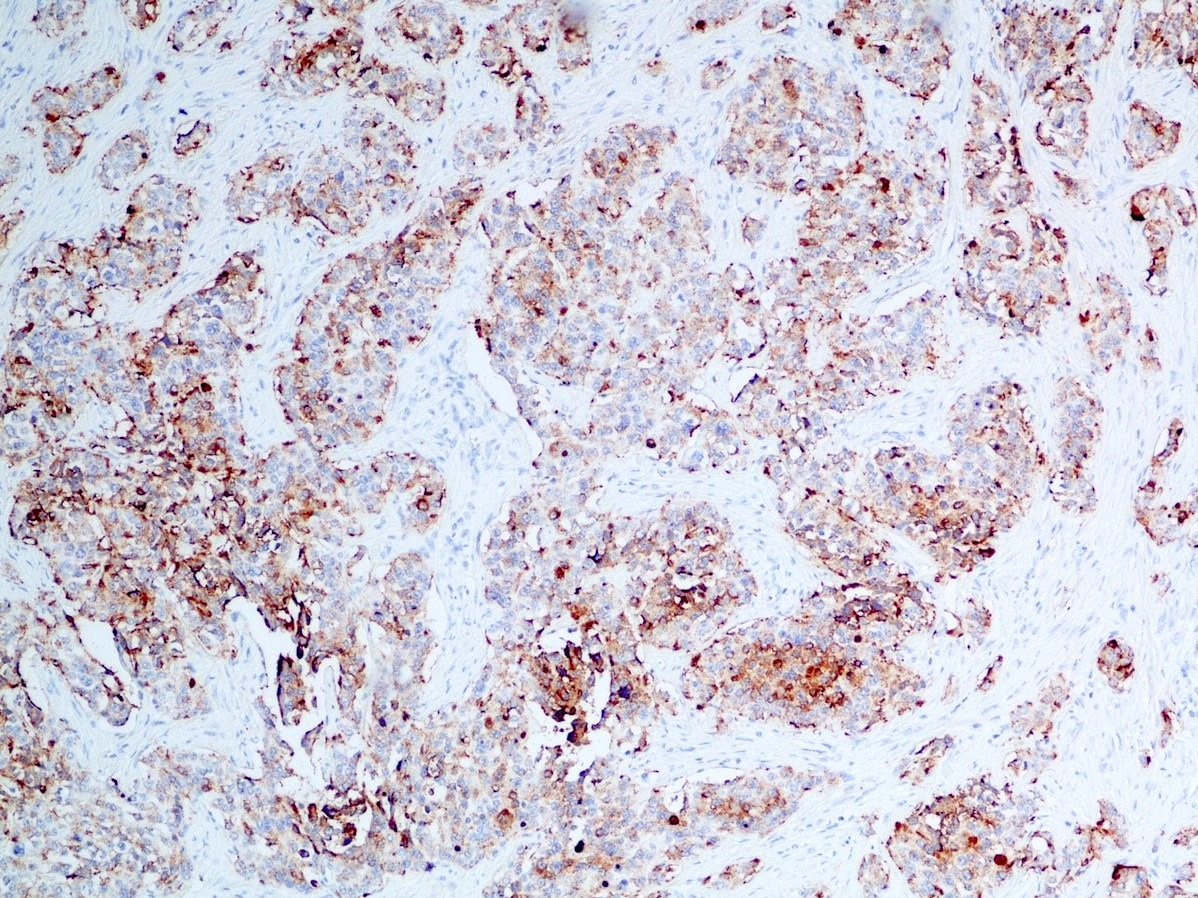
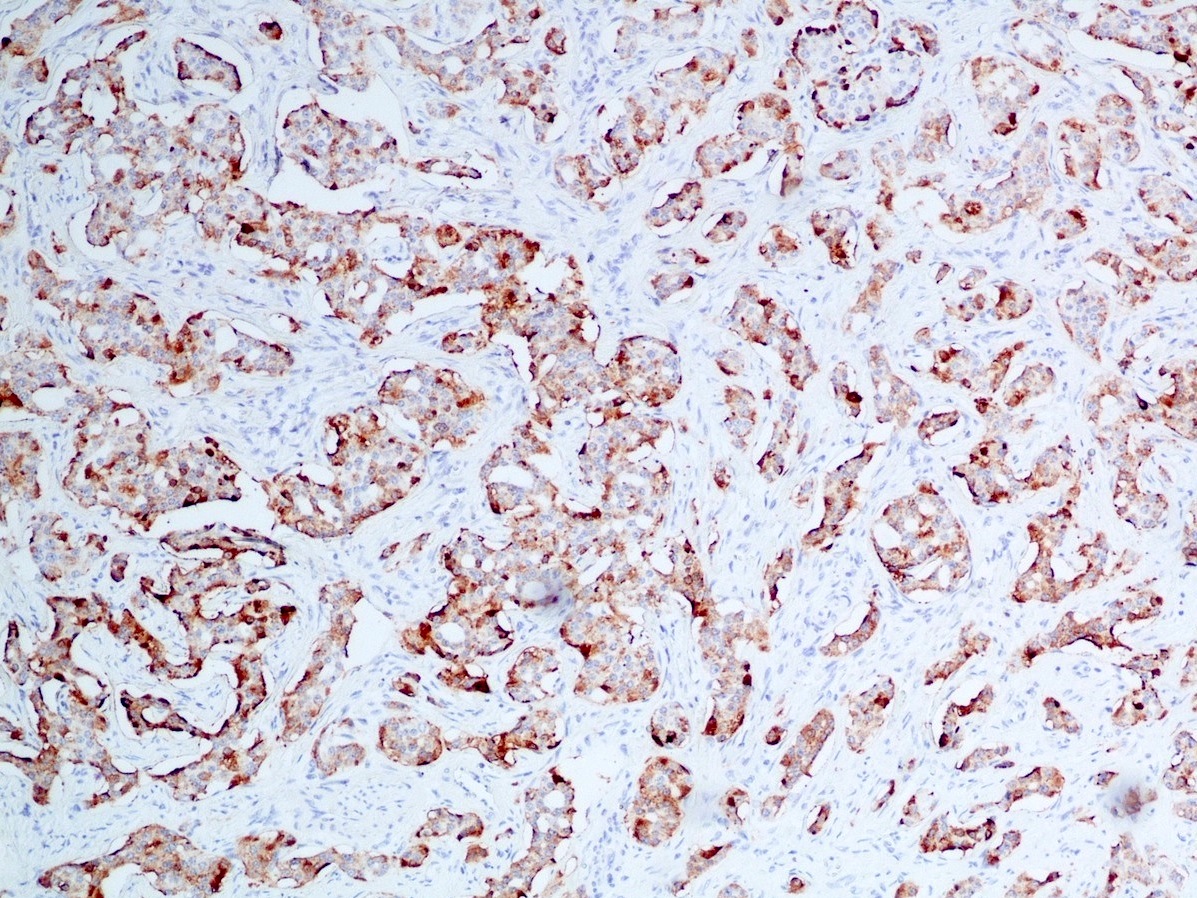
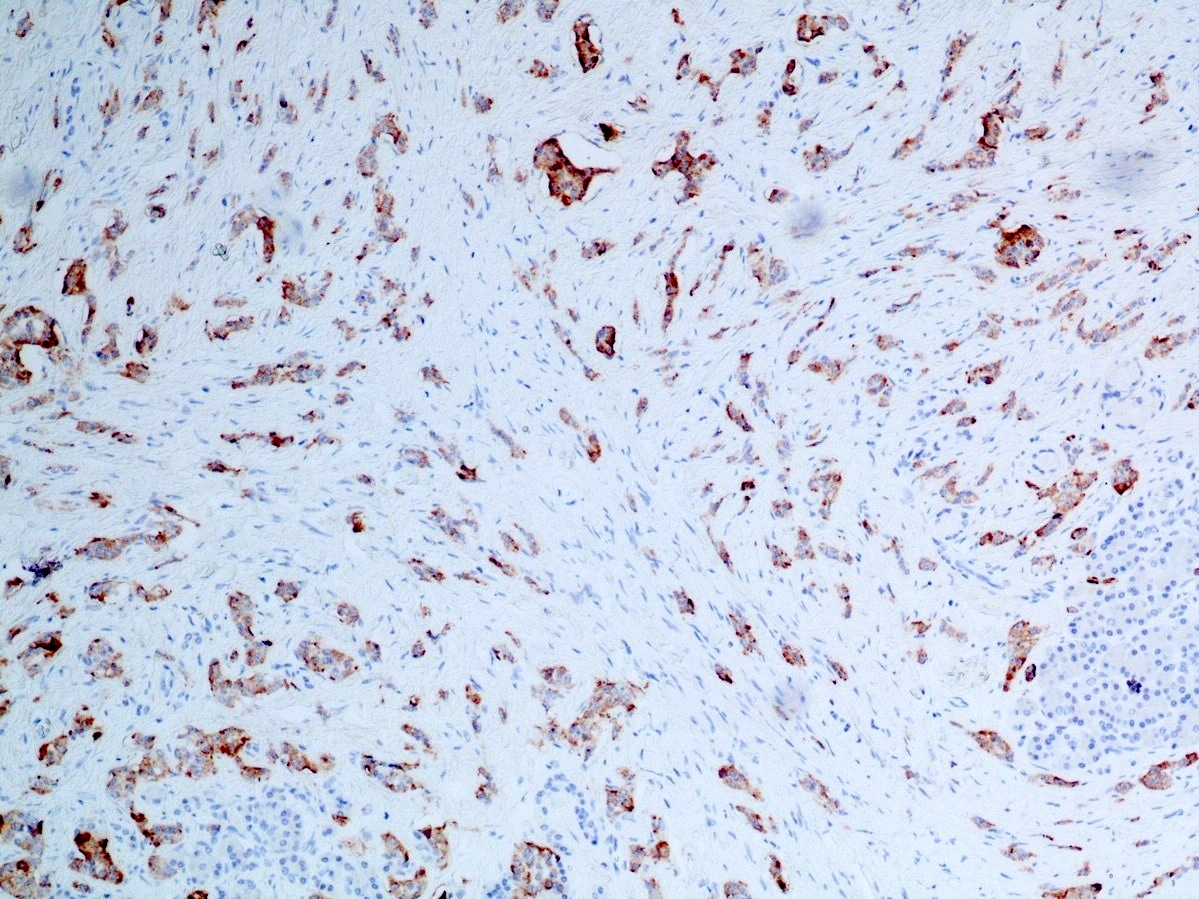
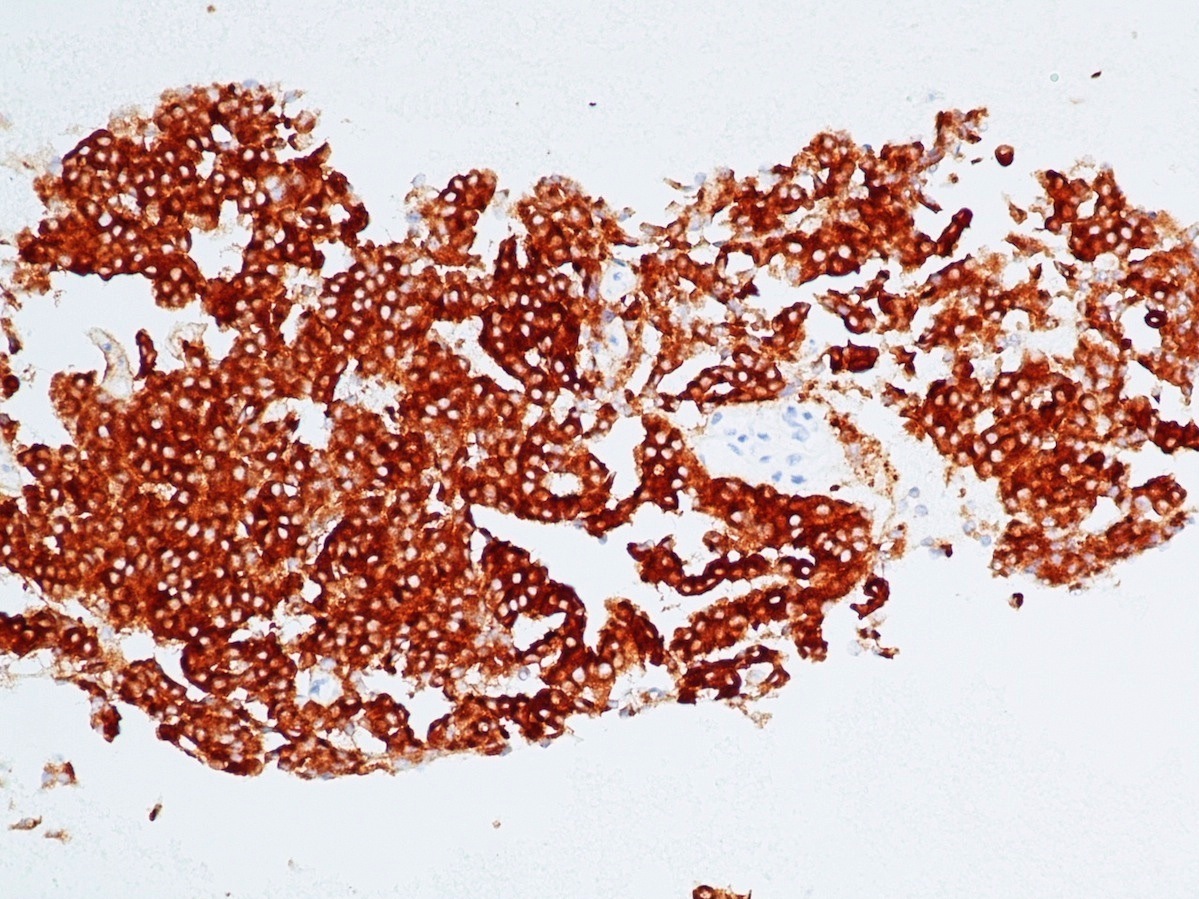
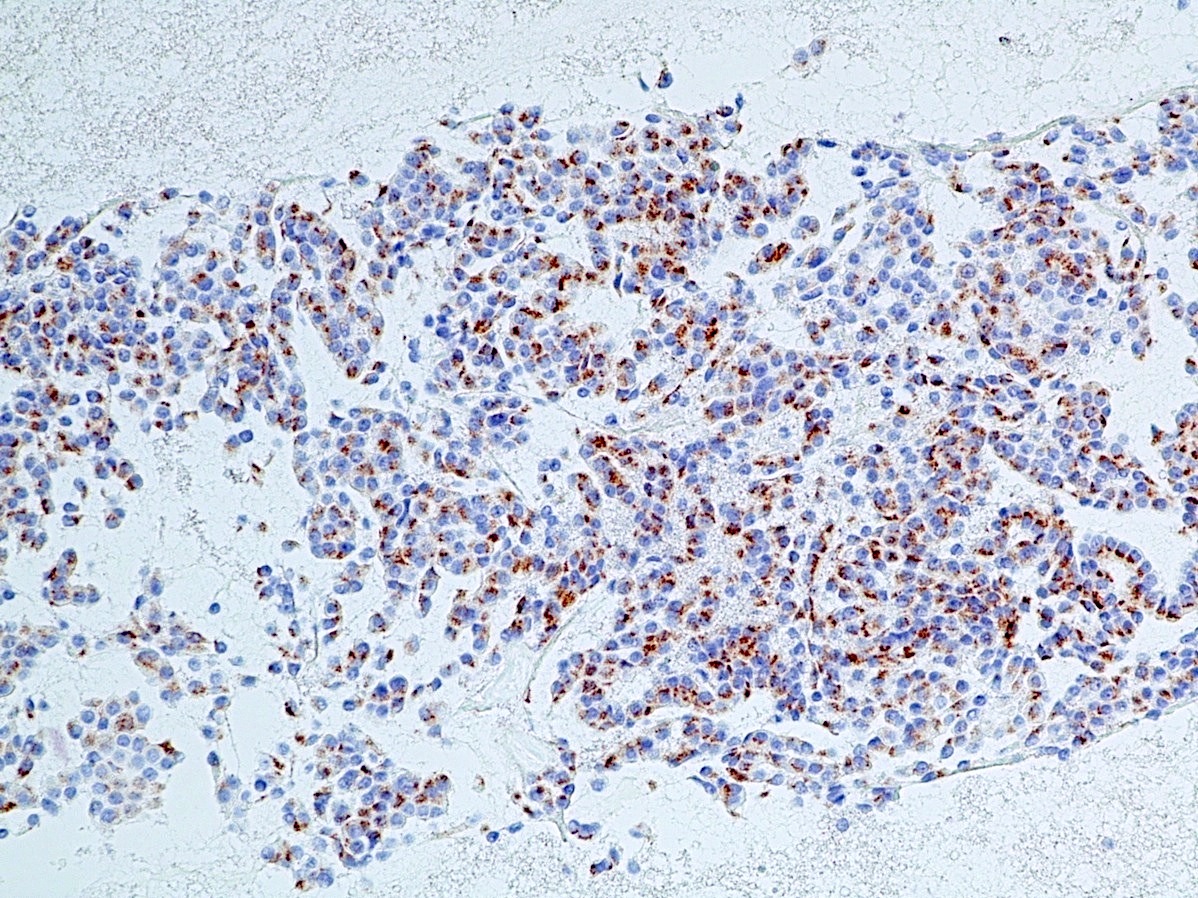
Microscopic (histologic) images
Contributed by Danielle Hutchings, M.D. and Sabrina Sopha, M.D.
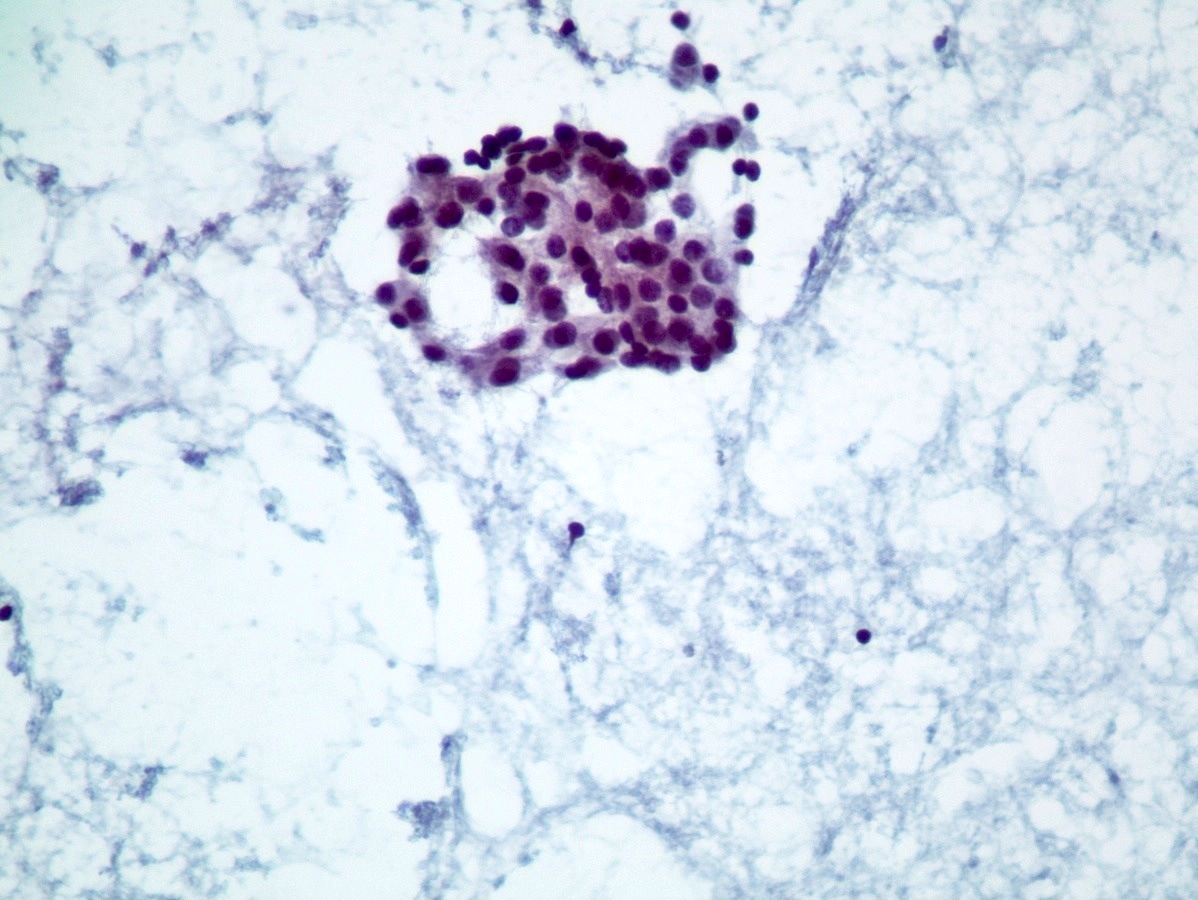
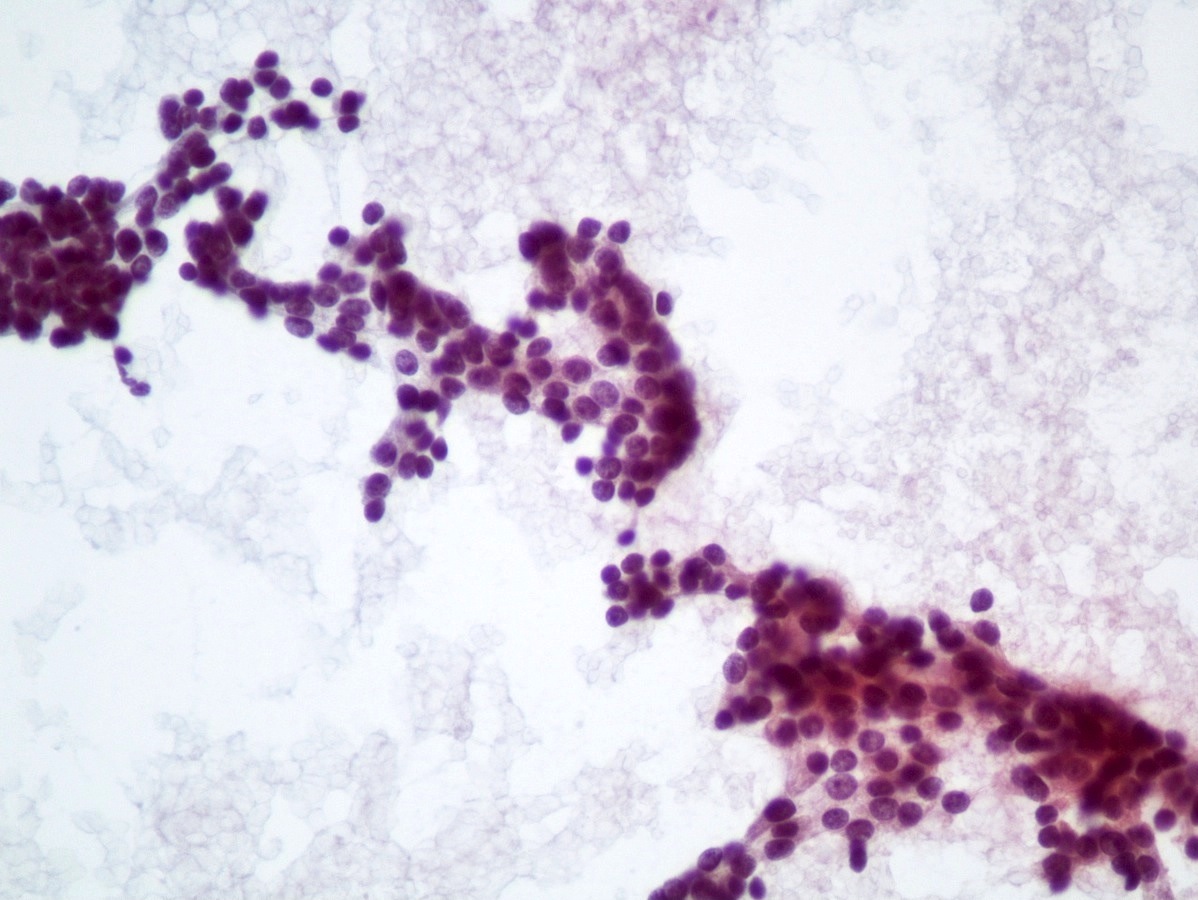
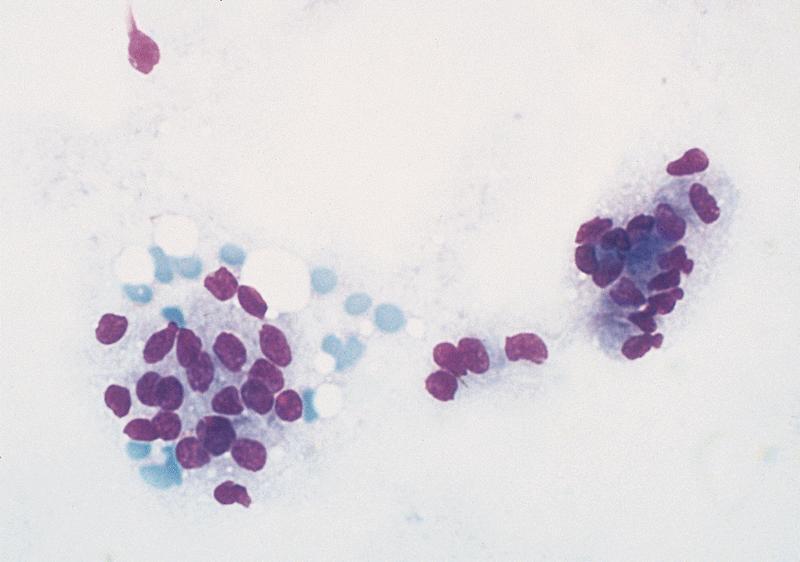
Cytology description
- Well differentiated neuroendocrine tumors (Diagn Cytopathol 2006;34:649):
- Cellular specimen with clean background
- Clusters and individual cells
- Uniform round oval, small / medium sized cells
- Frequently plasmacytoid
- Amphophilic, finely granular cytoplasm (neurosecretory capability)
- Poorly differentiated neuroendocrine carcinomas (Diagn Cytopathol 2006;34:649):
- Overtly malignant cells with small cell or large cell morphology
- Necrosis
Cytology images
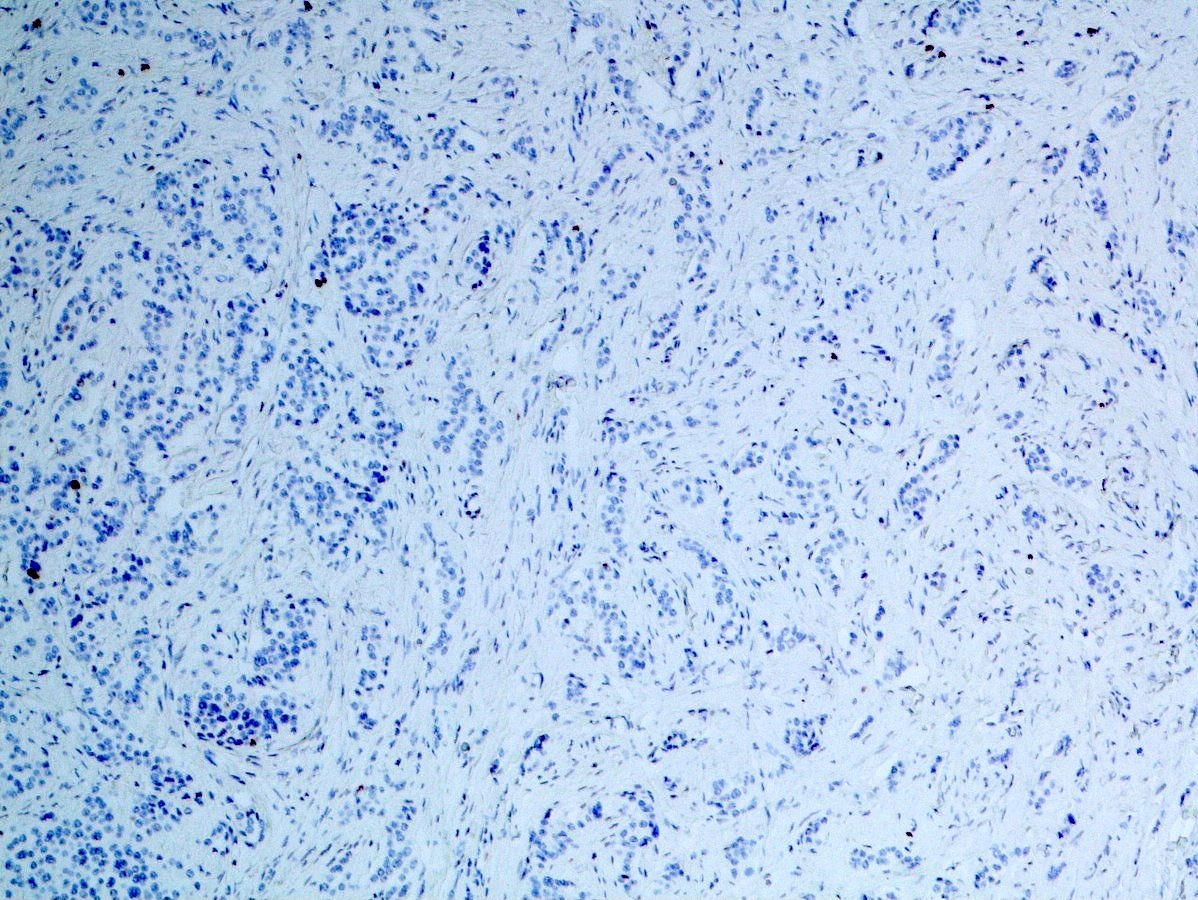
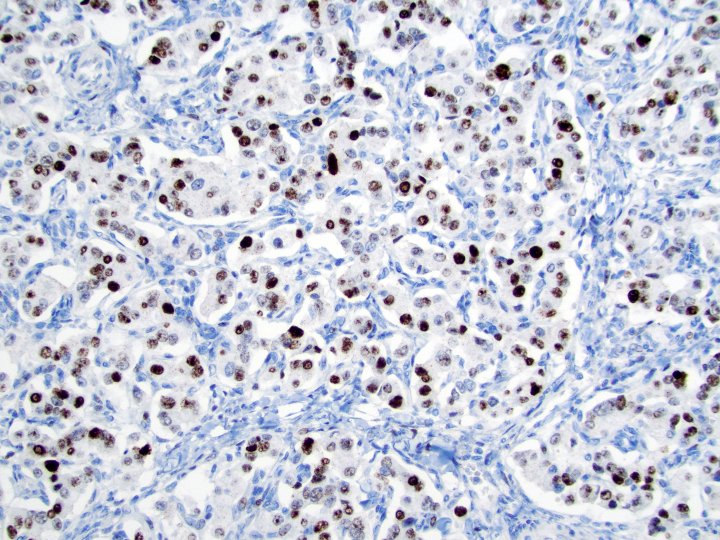
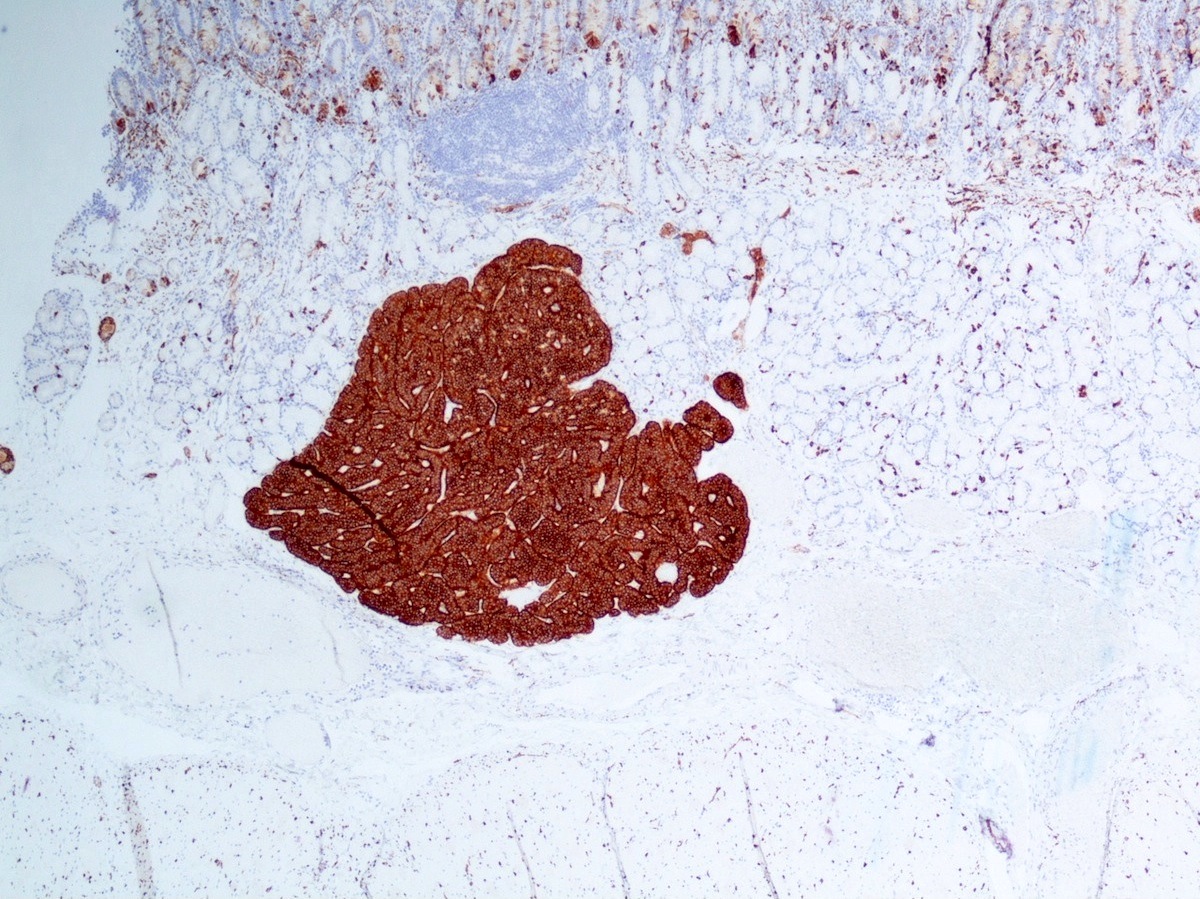
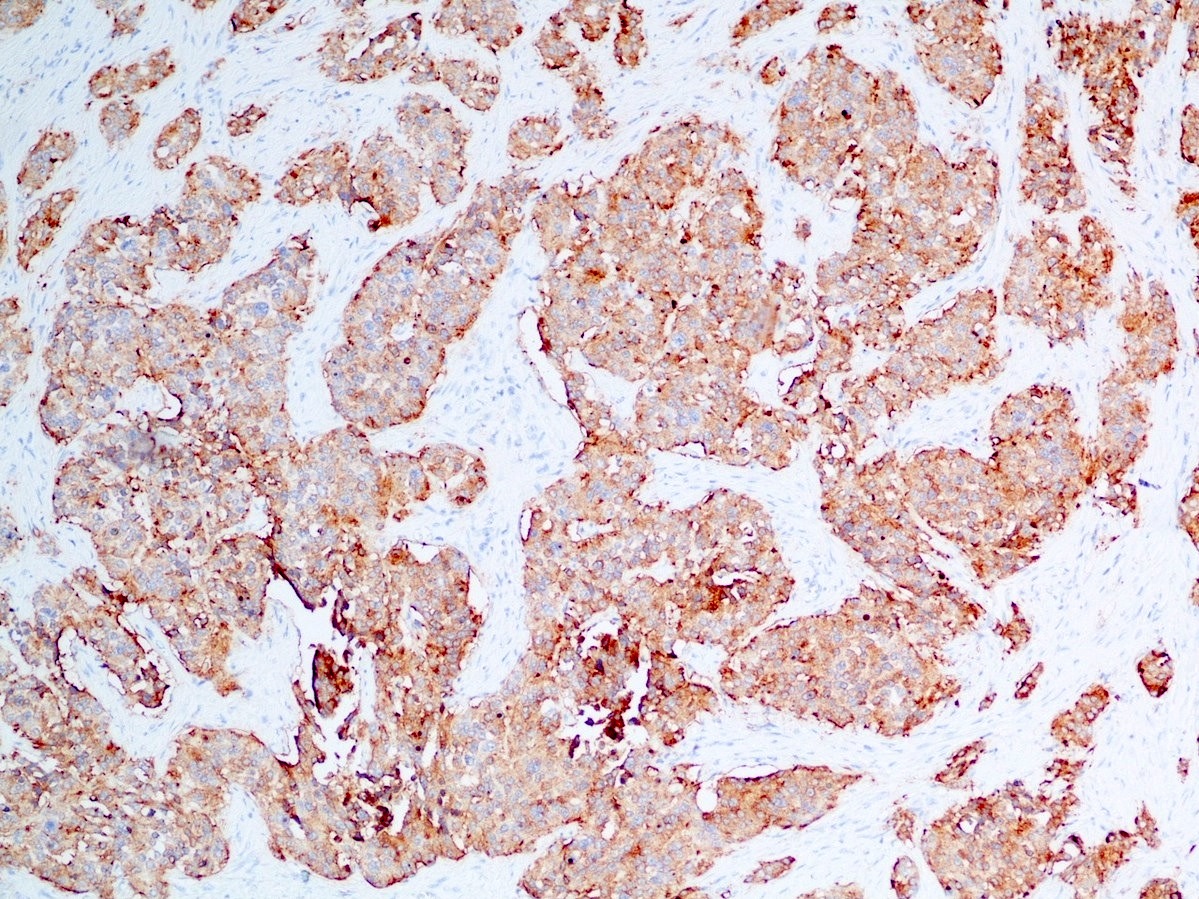
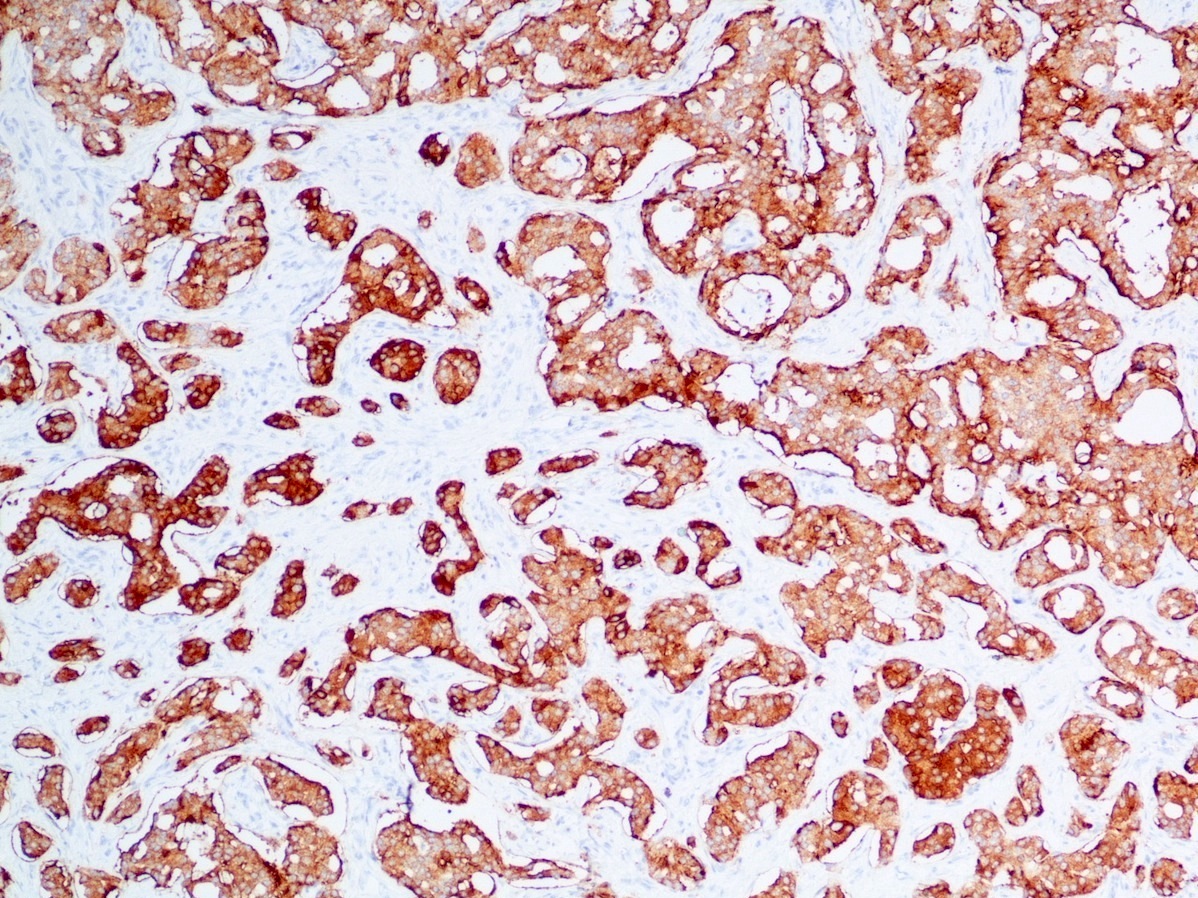
Positive stains
- Epithelial markers: CK8 / CK18, CAM 5.2, OSCAR, AE1 / AE3 (labels 50% of tumors) (Virchows Arch A Pathol Anat Histopathol 1986;409:609)
- Neuroendocrine markers:
- Synaptophysin: strong and diffuse in WDNET; may be weak, patchy or negative in PDNEC (Hum Pathol 2020;96:8)
- Chromogranin A: may be more focal and 10 - 20% of WDNET may be negative; may be focal or negative in PDNEC (Hum Pathol 2020;96:8)
- INSM1: newer marker with high sensitivity and specificity (Am J Clin Pathol 2015;144:579)
- CD56, neuron specific enolase (NSE), CD57: less specific, limited utility (Hum Pathol 2020;96:8)
- Panel to determine pancreatic origin for WDNET: Islet1+, PAX6+, TTF1-, CDX2 usually negative, SATB2-
- Panel is nonspecific in the setting of PDNEC
- TTF1 is positive in 40% of extrapulmonary PDNEC (Hum Pathol 2020;96:8)
- Panel to differentiate WDNET, grade 3 from PDNEC (Arch Pathol Lab Med 2019;143:1317)
- WDNET: somatostatin receptor+, loss of DAXX / ATRX
- PDNEC: abnormal expression of p53, loss of RB1, loss of SMAD4 / DPC4
- Hormone expression (insulin, glucagon, gastrin, somatostatin, VIP, pancreatic polypeptide): may be seen but does not indicate functional tumor
- Trypsin: scattered positive cells may be seen but if > 25%, the tumor is classified as mixed (Am J Surg Pathol 1994;18:765, Am J Surg Pathol 2002;26:893, Dig Dis Sci 2002;47:2254)
Negative stains
- Beta catenin (labels cell membrane only) (Arch Pathol Lab Med 2019;143:1317)
- Trypsin (negative or only few positive cells) (Am J Surg Pathol 1994;18:765, Am J Surg Pathol 2002;26:893, Dig Dis Sci 2002;47:2254)
- Chymotrypsin (Arch Pathol Lab Med 2019;143:1317)
- BCL10 (Arch Pathol Lab Med 2019;143:1317)
Molecular / cytogenetics description
- WDNETs:
- MEN1 inactivation 44% (Arch Pathol Lab Med 2019;143:1317)
- DAXX / ATRX mutation or loss 43% (Arch Pathol Lab Med 2019;143:1317)
- mTOR pathway alterations (PTEN, TSC2, PIK3CA) 14% (Arch Pathol Lab Med 2019;143:1317)
- VHL small subset (Semin Diagn Pathol 2014;31:498)
- Poorly differentiated neuroendocrine carcinomas and mixed neuroendocrine nonneuroendocrine neoplasms:
- TP53, RB, KRAS, APC, BRAF, SMAD4 / DPC4 (more like adenocarcinomas) (Arch Pathol Lab Med 2019;143:1317, Am J Surg Pathol 2012;36:173)
Videos
Solid tumors of the pancreas
Sample pathology report
- Pancreas, distal pancreatectomy:
- Well differentiated neuroendocrine tumor, WHO grade 1 of 3 (3 cm) (see comment)
- Ki67 proliferation index: 2%
- 1 mitosis per 2 mm2
- Tumor confined to the pancreas
- Lymphovascular and perineural invasion identified
- Proximal pancreatic parenchyma margin is uninvolved
- Metastatic tumor in 2/17 lymph nodes
- Comment: The tumor cells are positive for synaptophysin and chromogranin A.
- Well differentiated neuroendocrine tumor, WHO grade 1 of 3 (3 cm) (see comment)
Differential diagnosis
- Solid pancreatic tumors:
- Acinar cell carcinoma:
- Adults, high mitotic rate, labeling with BCL10, trypsin, chymotrypsin
- May have focal positivity with synaptophysin but chromogranin is negative
- Pancreatoblastoma:
- Children, squamoid nests
- Solid pseudopapillary neoplasm:
- Women, degenerated pseudopapillae, hyaline globules
- Nuclear labeling with beta catenin
- May have focal positivity with synaptophysin but chromogranin is negative
- Usual ductal adenocarcinoma:
- Desmoplastic stroma
- Invasive mucin producing glands
- Paraganglioma:
- Very rare, nested zellballlen architecture
- Cytokeratin negative, S100 highlights sustentacular cells, GATA3 positive (Mod Pathol 2013;26:1365)
- Acinar cell carcinoma:
- Clear cell tumors:
- Clear cell sarcoma:
- Labels with melanoma markers and has t(12;22)
- Metastatic renal cell carcinoma:
- PAX8+
- PEComa:
- Labels with melanoma markers, inhibin, cathepsin K and TFE3, melanoma and other clear cell tumors
- Solid variant of serous cystadenoma:
- Labels with inhibin (Am J Surg Pathol 2004;28:339)
- Clear cell sarcoma:
Additional references
Board review style question #1
A 58 year old man presents with a 2.8 cm solid mass in the tail of the pancreas. Based on the morphology below, you suspect a well differentiated neuroendocrine tumor (WDNET). Which of the following immunoprofiles would be most characteristic of pancreatic WDNET?
- Synatophysin-, chromogranin-, BCL10+, trypsin+, beta catenin cytoplasmic
- Synatophysin-, chromogranin-, BCL10-, trypsin-, beta catenin nuclear
- Synatophysin+, chromogranin+, BCL10-, trypsin-, beta catenin cytoplasmic
- Synatophysin+, chromogranin-, BCL10-, trypsin-, cytokeratin-
Board review style answer #1
C. Synatophysin+, chromogranin+, BCL10-, trypsin-, beta catenin cytoplasmic
Comment Here
Reference: Neuroendocrine neoplasms-general
Comment Here
Reference: Neuroendocrine neoplasms-general
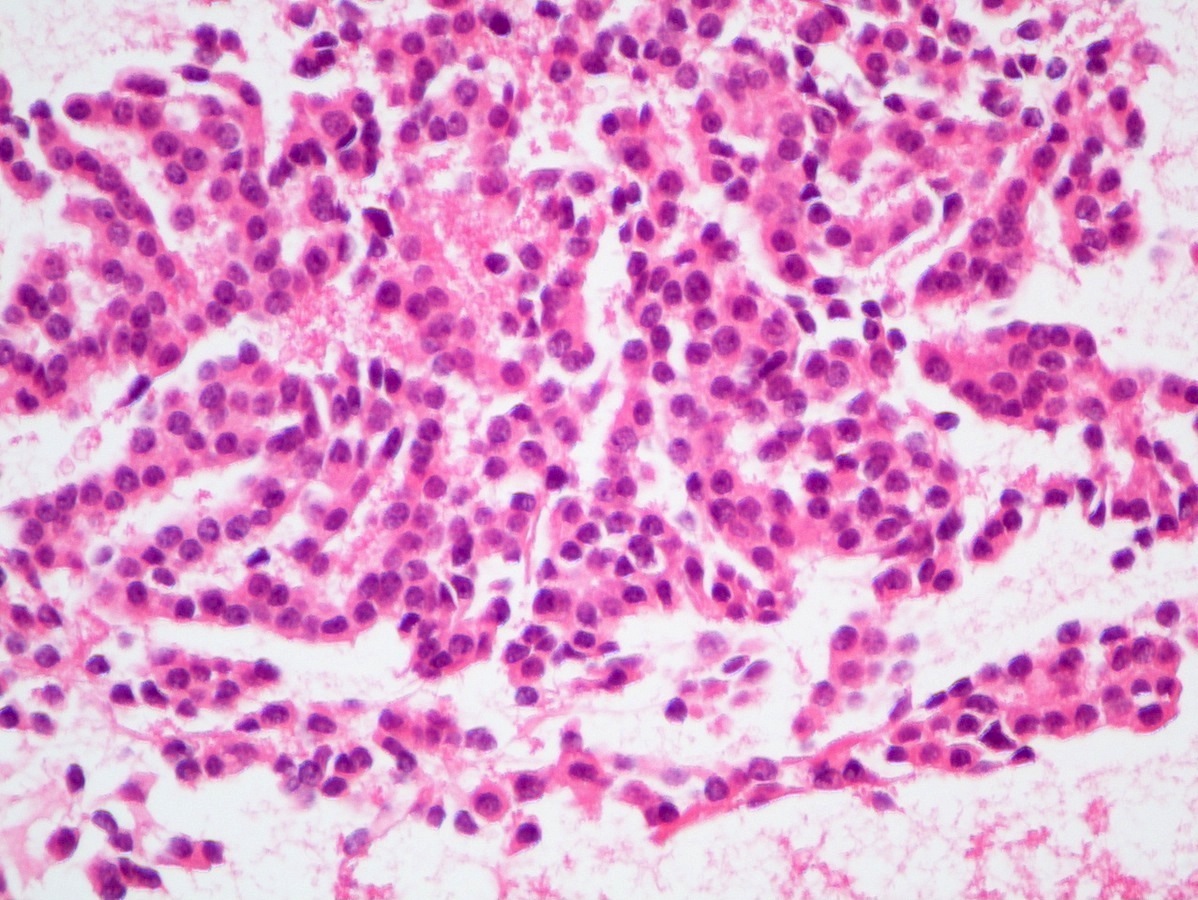
Board review style question #2
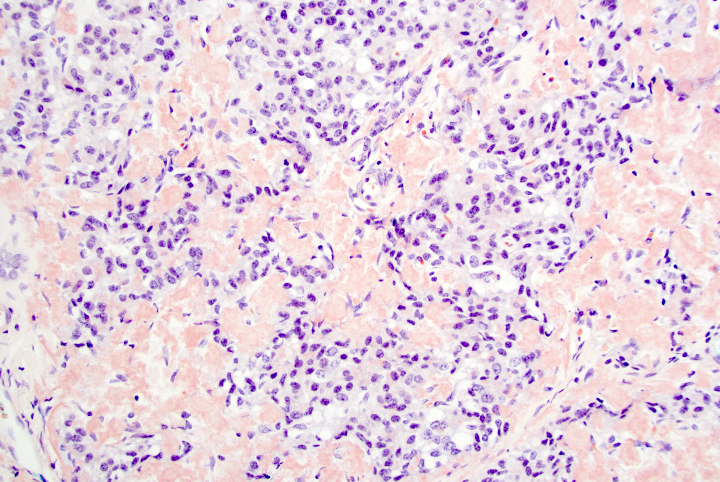
A 45 year old woman presents recurrent episodes of dizziness, palpitations and sweating, which she reports usually resolve with drinking orange juice. She is found to have a 2.0 cm solid mass in the body of the pancreas. Resection specimen is shown in the image above. What is the most likely diagnosis based on H&E and congo red stains?
- Acinar cell carcinoma
- Gastrinoma
- Insulinoma
- Pancreatic ductal adenocarcinoma
- Well differentiated pancreatic neuroendocrine tumor, nonfunctional
Board review style answer #2