Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Qasim S, Raghavan S. Malignant Spitz tumor (Spitz melanoma). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/skintumormelanocyticspitzoidmelanoma.html. Accessed April 24th, 2024.
Definition / general
- Spitz nevi are composed of epithelioid to spindled melanocytes with abundant homogeneous cytoplasm and large vesicular nuclei; they often display epidermal hyperplasia, clefts around and between melanocytes, Kamino bodies and maturation with descent
- The current classification of Spitz tumor includes:
- Benign Spitz nevi (SN)
- Atypical Spitz nevus / tumor (AST) as tumor of intermediate malignant potential
- Spitz melanoma (SM)
Essential features
- Spitz tumor refers to the spectrum of melanocytic tumors with histopathologic features of Spitz nevus that ranges from benign to malignant
- They may be challenging to classify as benign or malignant because histopathologic features that are commonly taken as indicators of malignancy (e.g., nuclear atypia, scatter of melanocytes in the upper epidermis, poor maturation within the dermis, deep extension and deep dermal mitoses) are commonly seen in Spitz tumors with benign biologic behavior
- WHO 2018 guideline differentiates Spitz versus spitzoid as follows: Spitz lesions are associated with at least a single specific genetic alteration (HRAS mutation, fusions in ROS1, ALK, NTRK1, NTRK3, MET and RET) and spitzoid lesions are lesions with morphological resemblance to Spitz nevi
- First described by Sophie Spitz in 1948 as melanomas of childhood
Terminology
- Spitzoid melanoma, malignant Spitz melanoma, spitzoid lesions
ICD coding
- ICD-10: C43.9 - malignant melanoma of skin, unspecified
Epidemiology
- Though initially named as juvenile melanomas, Spitz tumors occur in individuals of all ages with the mean age of Spitz melanoma of 55 years in a 54 patient study (J Am Acad Dermatol 2014;71:1077)
- A study on the yearly incidence of Spitz nevi, atypical Spitz tumor and Spitz melanoma in the Netherlands between 1999 - 2014 demonstrated that the yearly incidence of Spitz nevi increased from 525 to 751 cases during this period; atypical Spitz tumor from 9 to 153 cases; Spitz melanoma from 8 to 40 cases (Br J Dermatol 2020;183:1121)
- Spitz melanoma occurs in all races, though the majority of Spitz melanoma cases have been reported in white patients (Pediatr Dermatol 2020;37:1073, Br J Dermatol 2019;181:366)
- WHO 2018 classification mentions a higher prevalence in men for Spitz melanoma; however, in other studies Spitz melanoma appears to exhibit a relatively equal distribution between sexes, though this conclusion could be limited by sample size (J Am Acad Dermatol 2014;71:1077, Am J Dermatopathol 2017;39:181)
Sites
- Almost all Spitz tumors occur in patients < 20 years old; older patients, particularly those > 20 or 30 years old, have a greater likelihood of malignancy (Clin Dermatol 2009;27:564)
- Spitz tumors commonly affect the extremities and face but atypical Spitz tumors may also, less frequently, affect other areas such as the back (J Am Acad Dermatol 2011;65:1073)
- Distribution of Spitz melanoma tends to be as follows: 50% located on the lower extremities, 22.2% located on the trunk, 20.4% the upper extremities and 7.4% the head and neck (J Am Acad Dermatol 2014;71:1077)
Pathophysiology
- Pathogenesis and etiology of Spitz melanoma are unknown; discovery of kinase translocations and other mutations alludes to potential pathways involved in their pathogenesis
- Kinase fusions lead to a loss of a 3’ regulatory domain resulting in a constant activation of the kinase domain portion of the fusion, which is likely responsible for cellular proliferation in these lesions (Am J Surg Pathol 2019;43:538)
- Translocations include those involving ROS1, ALK, RET, BRAF, NTRK1, MET and NTRK3
Etiology
- Unknown at this time
Diagnosis
- Clinical and histopathologic characteristics are the gold standard in diagnosing Spitz lesions
- Ancillary studies including immunohistochemistry (IHC), array comparative genomic hybridization (aCGH), fluorescence in situ hybridization (FISH) and next generation sequencing (NGS) may be helpful for borderline lesions, such as atypical Spitz tumor, where ancillary studies can contribute to assessing malignant potential (Mod Pathol 2020;33:1122)
Prognostic factors
- Spitz melanoma has a poor prognosis compared with atypical Spitz tumor and Spitz nevi
- Prognosis of Spitz melanoma compared with melanoma is unclear
- Older age appears to correspond with negative outcomes
- 5 year survival rate for children < 10 years old is 88% and for children 11 - 17 years old is 49%
- Spitz melanoma with MAP3K8 kinase rearrangements may present with more advanced disease than those without
- References: JAMA Dermatol 2013;149:283, Hum Pathol 1998;29:1105, Cancer 2007;109:1579, Am J Surg Pathol 2019;43:1631
Case reports
- 4 year old girl presented with multiple recurrent papules and inguinal lymphadenopathy diagnosed as metastatic Spitz tumor (Australas J Dermatol 2018;59:e234)
- 7 year old girl presented with a 12 mm nodule on the anterior right ankle and a 9 year old boy presented with a 14 mm nodule on the anterior left thigh diagnosed as ALK positive Spitz tumor (J Cutan Pathol 2018;45:136)
- 13 year old boy, 26 year old woman and 72 year old woman presented with melanocytic neoplasms with a spitzoid cytomorphology, variable nuclear atypia and harboring undescribed fusions involving RASGRF1 (Am J Surg Pathol 2022;46:655)
- 42 year old woman presented with a 1 cm sized, small brown macule on her right temple diagnosed as Spitz melanoma after a primary diagnosis of breast cancer (Ann Dermatol 2022;34:76)
Treatment
- No current consensus guidelines exist for the management of Spitz melanoma but given the resemblance to melanoma, the majority of clinicians perform a biopsy, especially in older patients (JAMA Dermatol 2013;149:1348)
- Excision is typically recommended to avoid recurrence and common practice is to re-excise a lesion with positive margins
- Sentinel lymph node biopsy may be recommended
- With the advent of a defined Spitz melanoma as a molecularly separate entity from conventional melanoma and the low incidence of recurrence / relapse associated with Spitz melanoma, treatment of Spitz melanoma may not require the same aggressive treatment protocol currently utilized in melanoma management (Melanoma Manag 2015;2:121)
- Ipilimumab, a monoclonal antibody to CTLA4 receptor and PD-1, is efficacious in treating advanced melanoma
Gross description
- Clinically, Spitz melanomas are usually evolving amelanotic nodular lesions and can grow to a diameter of ≥ 1 cm; they can often go clinically undiagnosed because of their wide range of clinical appearances and a lack of pigmentation
- Spitz melanoma is typically larger than atypical Spitz tumor, with a mean of 1.05 cm, however, Spitz melanoma < 6 mm can also be seen
- Spitz melanoma tumors are more likely to be nodular than Spitz nevi but can also present as flat lesions
- Spitz melanomas exhibit a wide range of color, with 80% containing red / pink, 53.3% containing dark brown, 35.7% containing gray and 33.3% containing light brown
- References: J Am Acad Dermatol 2014;71:1077, J Am Acad Dermatol 2018;78:278, J Am Acad Dermatol 2015;72:47
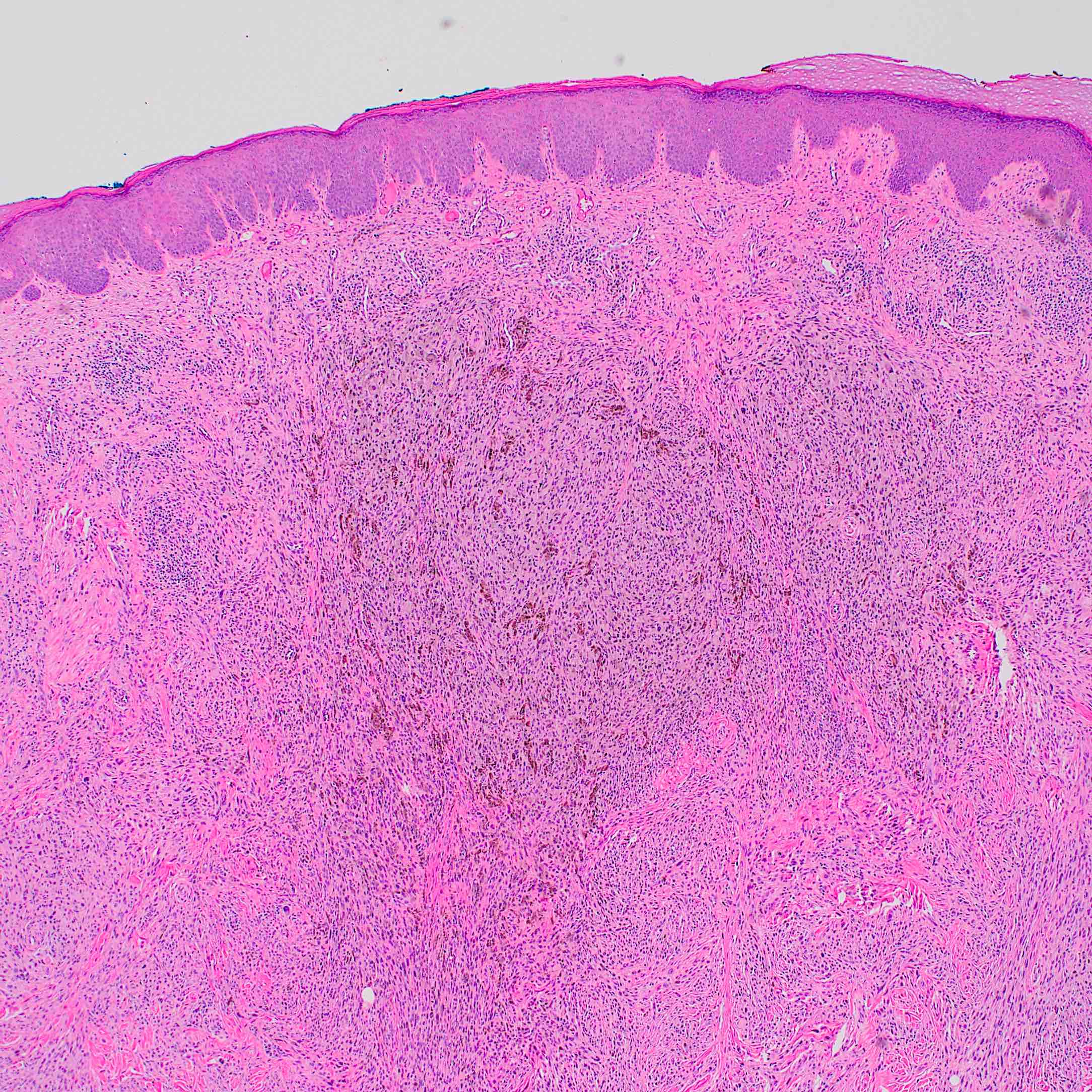
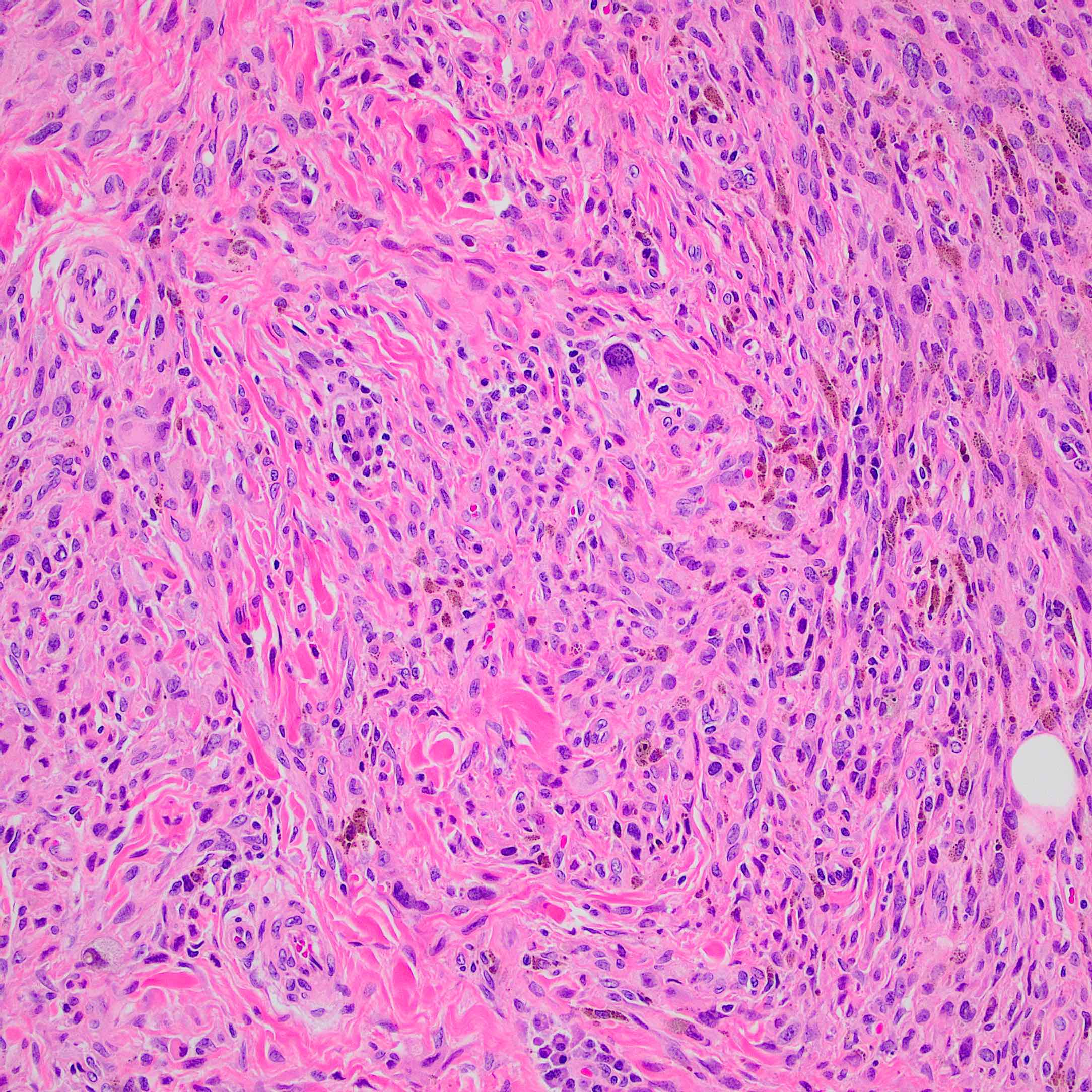
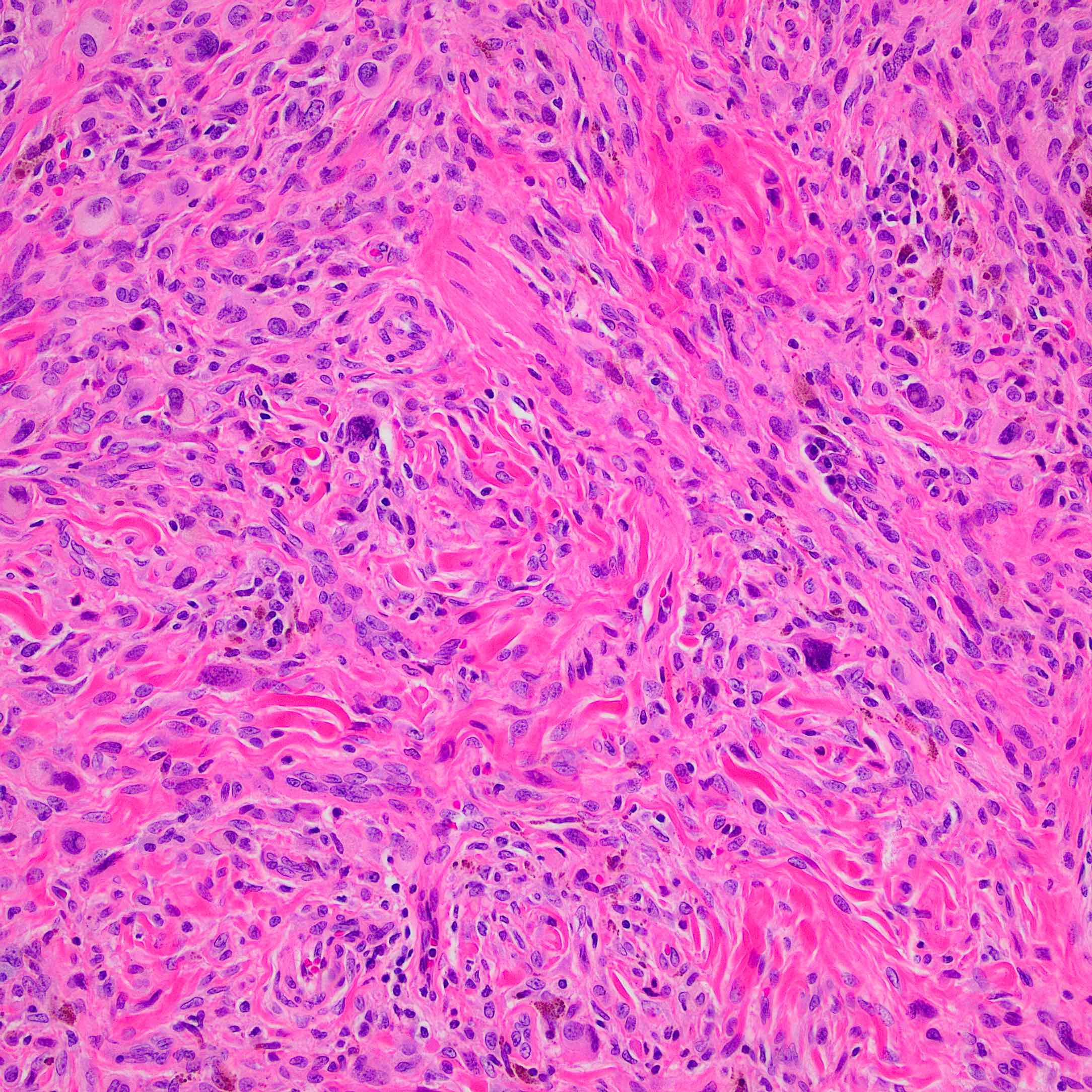
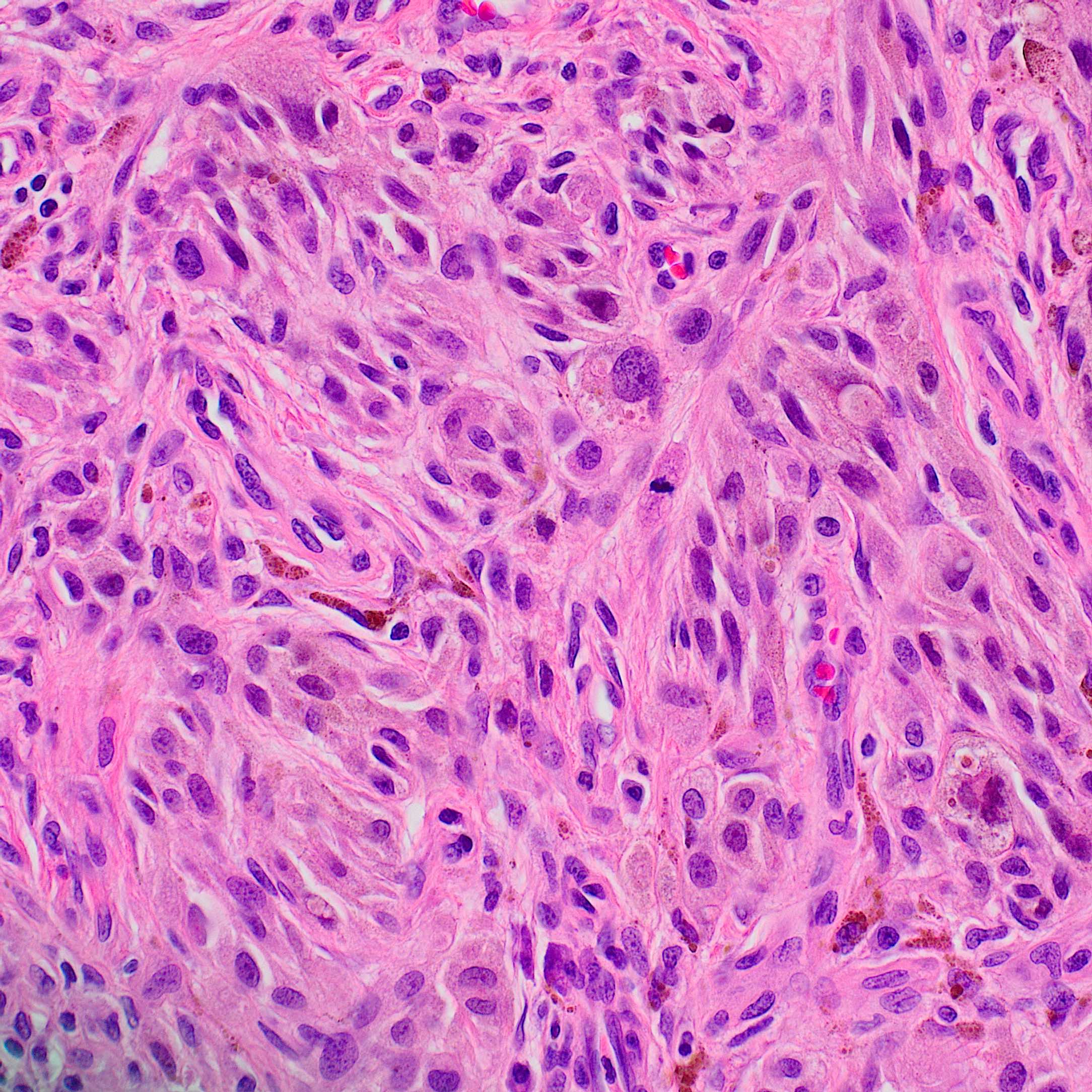
Microscopic (histologic) description
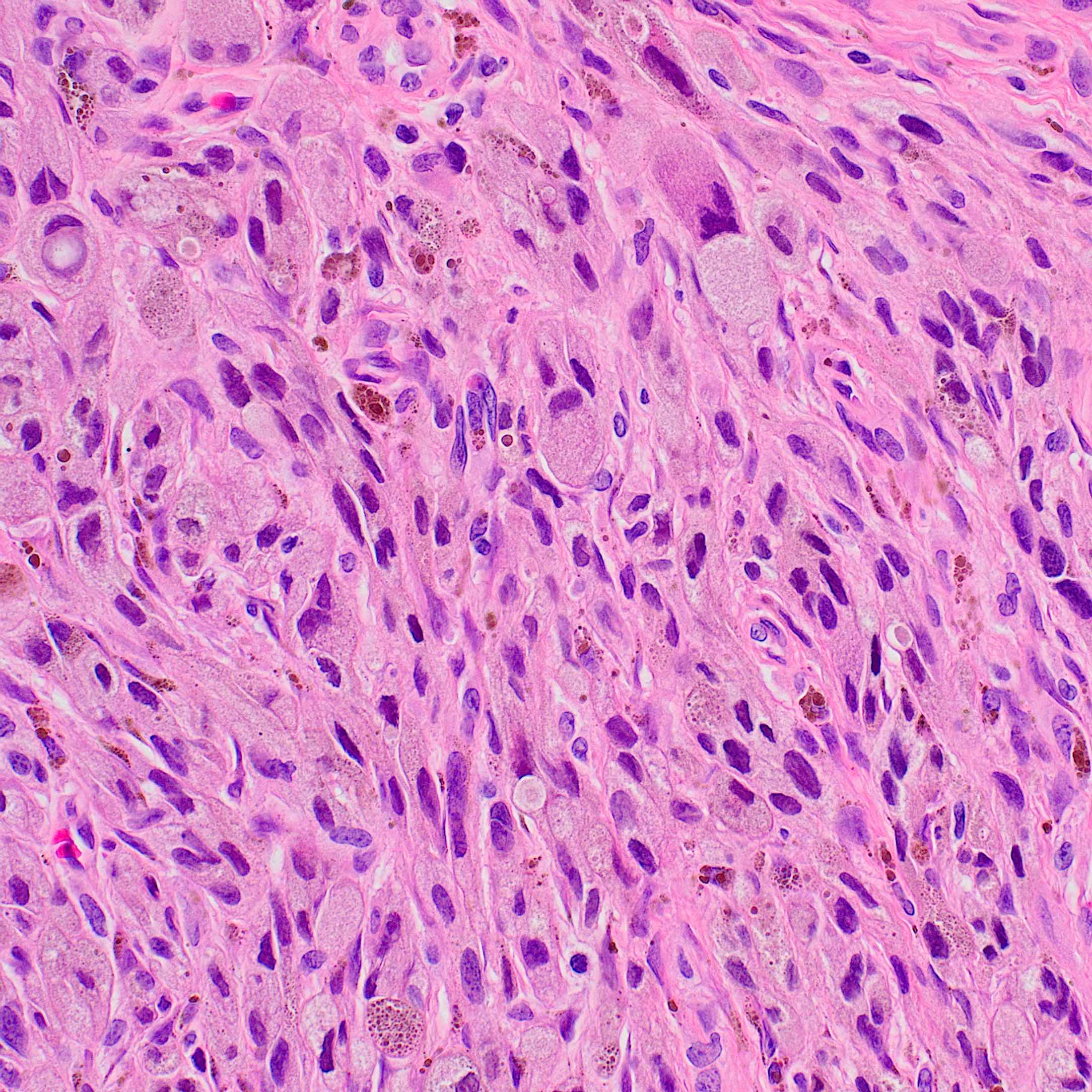
- According to WHO 2018 guidelines, compared with Spitz nevi, Spitz melanomas are much larger (> 1 cm) and like atypical Spitz tumor, they show asymmetry, irregular nesting patterns, lack of maturation, poor circumscription, ulceration, increased mitotic rate (> 6 mm2) and deep or atypical mitoses (Am J Dermatopathol 2012;34:478, Pathology 2002;34:6)
- These lesions also display high rates of cytological atypia
- Some Spitz melanomas retain histopathologic features of Spitz nevi, including loss of p16 in those with HRAS mutations, fascicular melanocyte nests and increased dermal mitoses in those with ALK fusions and dermal rosette-like structures in those with NTRK1 fusions (Mod Pathol 2020;33:1122)
- Spitz melanomas with MAP3K8 fusions exhibit similar morphology to atypical Spitz tumor with MAP3K8 mutations, including epithelioid morphology, high grade cytologic atypia, multinucleated giant cells and p16 loss (Virchows Arch 2022;480:369)
Microscopic (histologic) images
Positive stains
- Compared with melanoma (where usually the Ki67 proliferation index is > 10% of melanocytes), Spitz melanoma tends to have a high and diffuse expression throughout the entire lesion and like other melanomas, tends to display a higher proliferative index (> 10% of melanocytes) (Front Med (Lausanne) 2018;5:344)
- Staining pattern of HMB45 to distinguish Spitz nevus from Spitz melanoma, as Spitz nevus displays a progressively diminished HMB45 staining pattern; atypical Spitz tumor displays either diminished or variable staining in the dermis; deep staining of the dermis is common in Spitz melanoma
- Combinations of different IHC stains may assist in algorithmically differentiating between Spitz nevi, atypical Spitz tumor and Spitz melanoma, and supplement histopathological differential diagnoses
- It has been proposed that if IHC of a lesion displays deep dermal cell proliferation through Ki67 in association with complete loss of p16 or HMB45, the lesion is less likely a Spitz nevus and more likely Spitz melanoma / atypical Spitz tumor; this staining pattern should prompt additional molecular investigations (Mod Pathol 2016;29:656)
Molecular / cytogenetics description
- HRAS mutations can be present in both Spitz nevi and Spitz tumors and are associated with a good clinical outcome (Am J Pathol 2000;157:967)
- ROS1 translocations are present in up to 17% of Spitz nevi or atypical Spitz tumor; Spitz melanoma usually does not have this translocation
- BRAF mutations exclude a diagnosis of Spitz tumor but these lesions can have a translocation in this gene as the driver oncogenic event
- MAPK activating mutations identified in Spitz melanoma and spitzoid melanoma
Videos
Spitz nevi and spitzoid melanocytic lesions
Sample pathology report
- Skin, right forehead, biopsy:
- Malignant Spitz tumor
Differential diagnosis
- Spitz nevus:
- More common in children and young adults
- < 1 cm, symmetrical with sharply demarcated borders, no deep extension, rare mitoses, Kamino bodies
- Atypical Spitz nevus:
- Mostly in adults
- > 1 cm, asymmetrical with irregular lateral borders, expansile nodule with deep extension, frequent mitoses, no Kamino bodies
- Low Ki67 nuclear staining
Additional references
Board review style question #1
Board review style answer #1
B. Asymmetry. Spitz melanomas are differentiated from Spitz nevi on the basis of asymmetry, lack of maturation, poor circumscription, ulceration, increased mitotic rate as well as high rates of cytological atypia.
Comment Here
Reference: Malignant Spitz tumor (Spitz melanoma)
Comment Here
Reference: Malignant Spitz tumor (Spitz melanoma)
Board review style question #2
Which of the following mutations is seen in Spitz melanoma but not in superficial spreading melanoma?
- BRAF fusion
- BRAF mutation
- NF1 mutation
- NRAS mutation
Board review style answer #2