Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) images | Sample pathology report | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Katerji R, Huber AR. HER2 colon. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/stainsher2colon.html. Accessed April 19th, 2024.
Definition / general
- HER2 testing is performed on several cancer types to assess prognosis and to determine suitability for trastuzumab (Herceptin) therapy
- HER2 oncogene encodes for a 185 KD transmembrane glycoprotein receptor with intracellular tyrosine kinase activity
- HER2 amplification and overexpression lead to epithelial cell growth and tumorigenesis
Essential features
- HER2 is a member of the human epidermal growth factor receptor (HER / EGFR / ERBB) family
- Immunohistochemical testing gives a score of 0 - 3+ that measures the amount of HER2 receptor protein on the surface of cells in a sampled tissue
- Strong lateral or complete basolateral membrane staining of colorectal tumor cells is considered positive, similar to gastric and gastroesophageal junction adenocarcinomas, unlike breast cancers, where circumferential membrane reactivity is required for positivity
- Approximately 3 - 5% of colorectal cancers have amplification of the HER2 gene (PLoS One 2014;9:e98528)
Terminology
- Encoded by gene ERBB2 (erythroblastic oncogene B2)
- Also known as CD340 proto-oncogene Neu
Pathophysiology
- ERBB2, a known proto-oncogene, is located on the long arm of human chromosome 17 (17q12)
- It encodes a transmembrane glycoprotein receptor that functions as an intracellular tyrosine kinase
- HER2 protein has an extracellular ligand binding domain, a transmembrane domain and an intracellular tyrosine kinase catalytic domain
- HER2 is not involved in ligand binding of the growth factors unless it is overexpressed
- On ligand activation, the HER2 proteins undergo dimerization and transphosphorylation of their intracellular domains
- Heterodimerization results in the autophosphorylation of tyrosine residues within the cytoplasmic domain of the receptors and initiates a variety of signaling pathways, principally the mitogen activated protein kinase (MAPK), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and protein kinase C (PKC) resulting in cell proliferation, survival, differentiation, angiogenesis and invasion (Biochim Biophys Acta 1994;1198:165)
- As with other EGFR receptors, HER2 is critical in the activation of subcellular signal transduction pathways controlling epithelial cell growth and differentiation and possibly angiogenesis
Clinical features
- Rate of positivity in colon cancer is about 3 - 5%
- HER2 overexpression can be detected by immunohistochemical staining (IHC) for HER2 protein, fluorescence in situ hybridization (FISH) for ERBB2 gene amplification on paraffin embedded tissue or reverse transcription polymerase chain reaction (RT-PCR) for overexpression of ERBB2
- HER2 IHC assesses the level of expression of the membrane receptor; score range from 0 - 3+ (see table)
- Specimens with equivocal IHC results should then be validated using FISH
- FISH can be used to measure the number of copies of the gene which are present and is thought to be more reliable than IHC
- Results of FISH are reported as negative (not amplified) or positive (amplified)
- HER2 positive colorectal tumors have shown potential benefit from combination therapy with HER2 targeted therapy (trastuzumab plus lapatinib), which represents a standard treatment after failure of conventional therapy (Mod Pathol 2015;28:1481)
Interpretation
- Consensus panel recommendations on HER2 IHC scoring for colorectal cancer (HERACLES diagnostic criteria) (Mod Pathol 2015;28:1481)
| Immunohistochemistry staining | Immunohistochemistry expected pattern | Immunohistochemistry classification | FISH test |
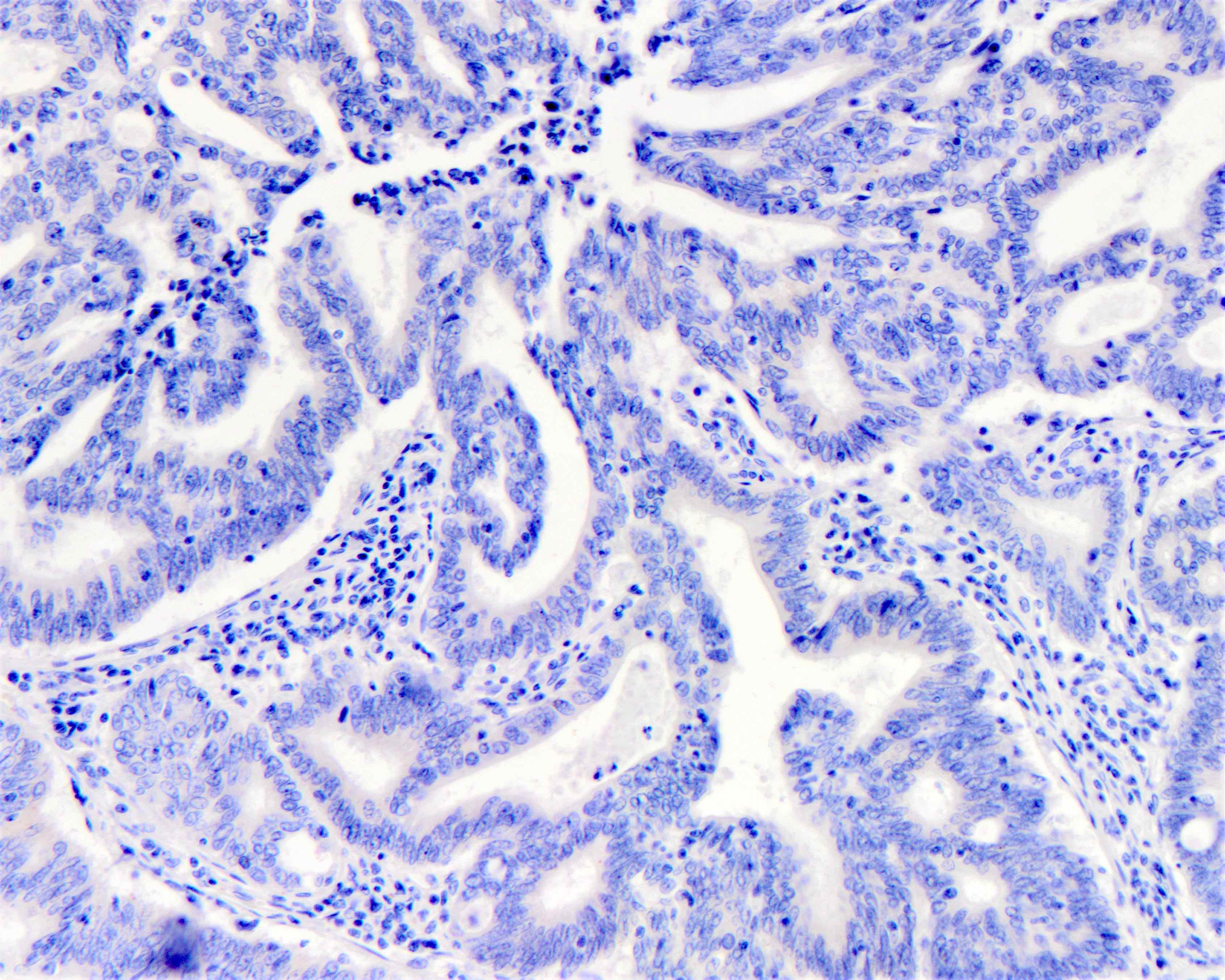
| No staining (0) | --- | Negative | No |
| Faint staining (1+) any cellularity | Segmental or granular | Negative | No |
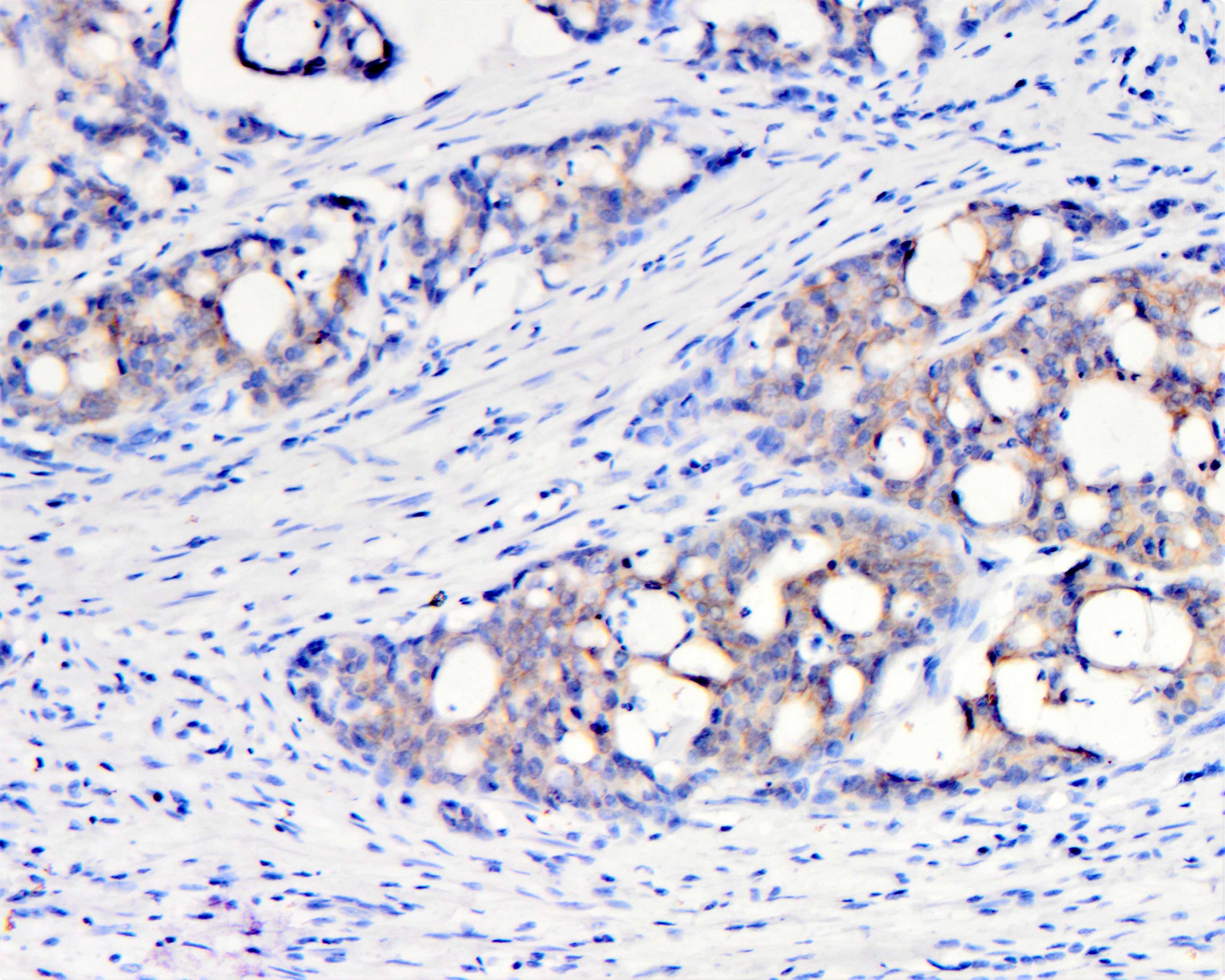
| Moderate (2+) in < 50% cells | Any | Negative | No |
| Moderate (2+) in ≥ 50% of cells | Circumferential, basolateral or lateral | Equivocal | Yes |
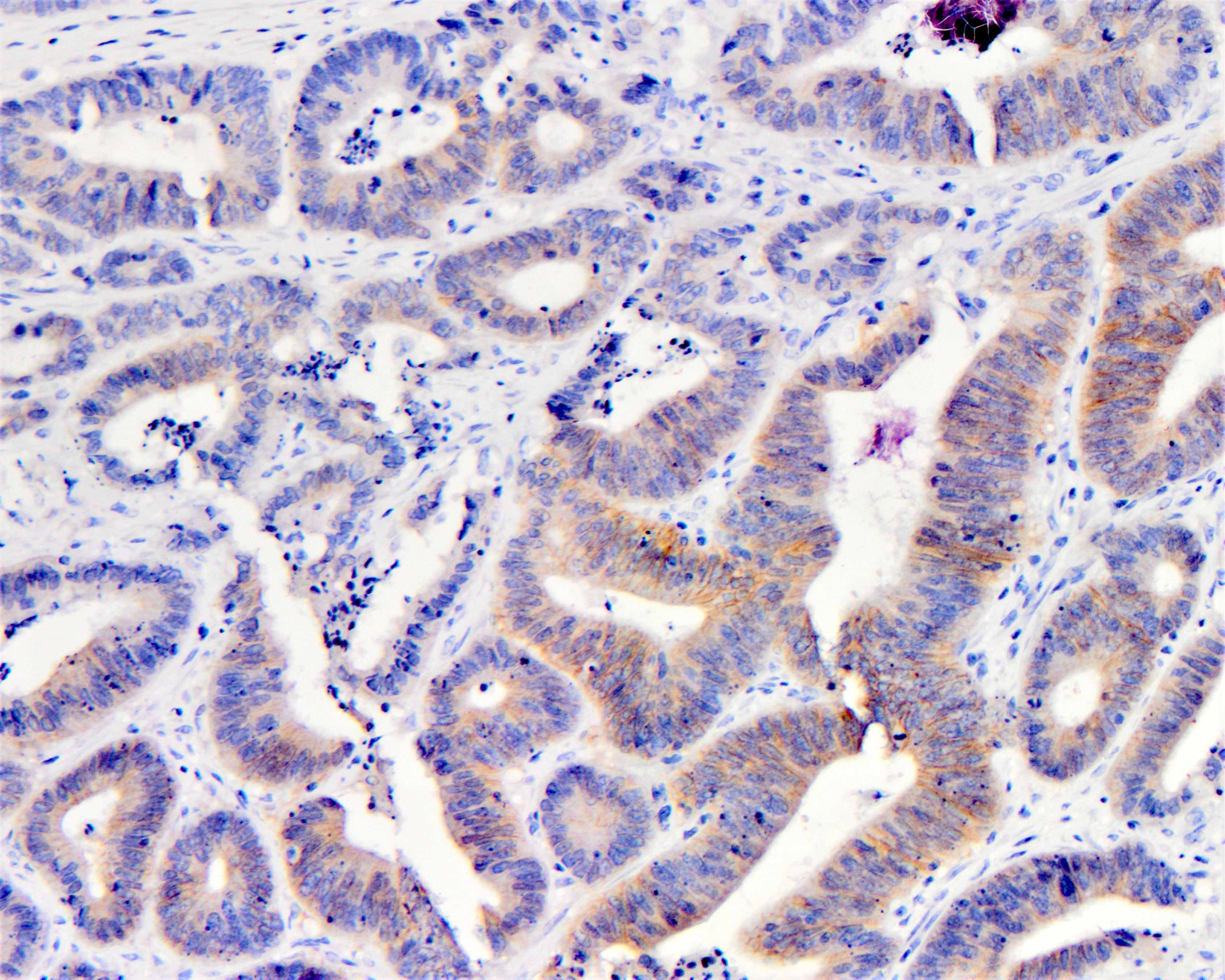
| Intense (3+) in ≤ 10% cells | Circumferential, basolateral or lateral | Negative | No |
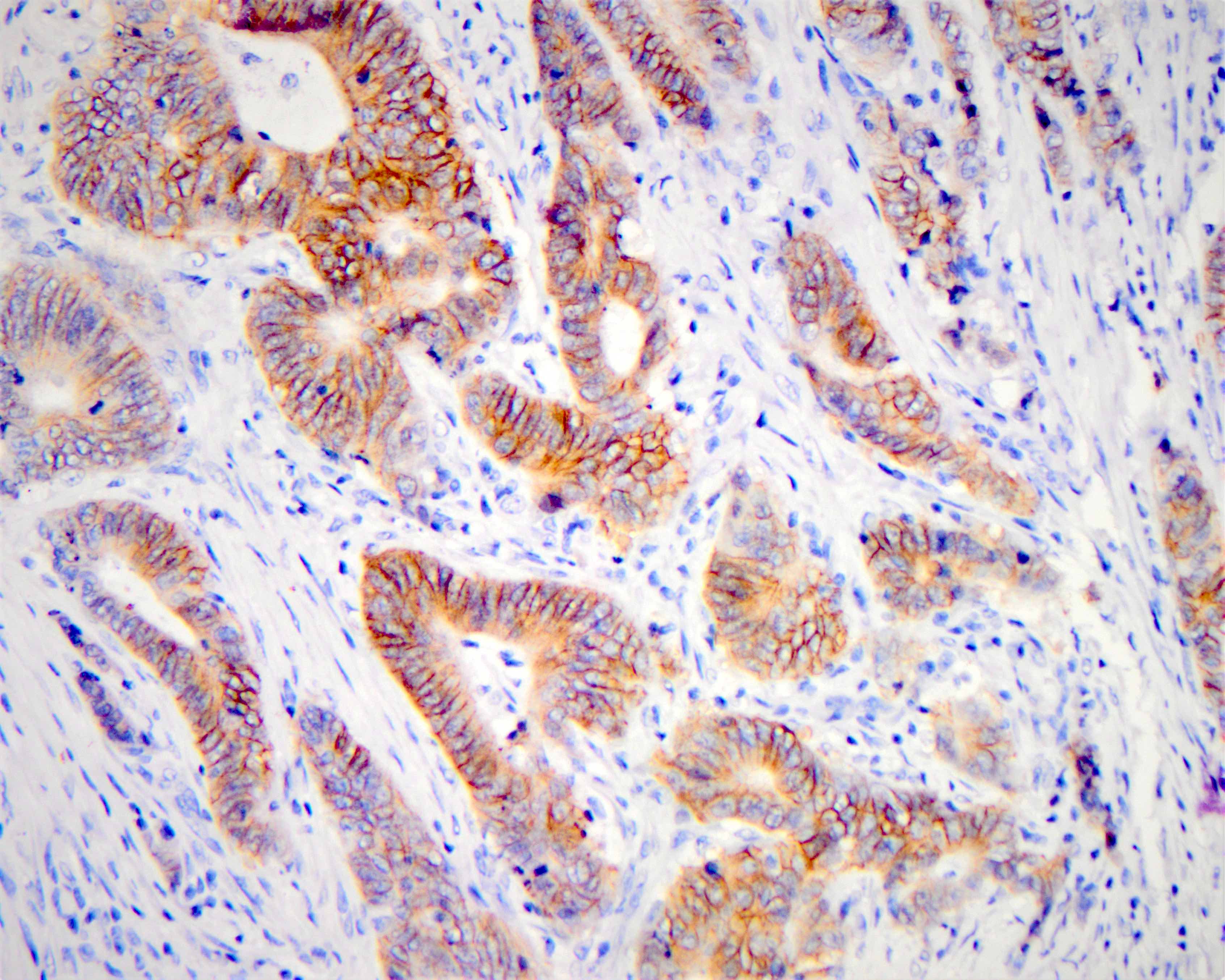
| Intense (3+) in > 10% | Circumferential, basolateral or lateral | Positive | No |
Uses by pathologists
- Testing for HER2 protein overexpression or gene amplification identifies patients who may benefit from HER2 targeted therapy with agents like trastuzumab
- HER2 amplification and KRAS, NRAS and BRAF mutations are mutually exclusive in advanced colorectal cancer (Nature 2012;487:330)
- HERACLES-A and MyPathway studies have shown benefit in a small number of patients with the use of combination trastuzumab-lapatinib and trastuzumab-pertuzumab, respectively (Mod Pathol 2015;28:1481, Lancet Oncol 2019;20:518)
- HER2 testing is now included in the National Comprehensive Cancer Network (NCCN) colon cancer treatment guidelines (NCCN: Colon Cancer Guidelines for Patients [Accessed 5 February 2021])
Prognostic factors
- HER2 overexpression has been controversially linked to prognosis
- Some studies showed no significant difference in disease free survival between patients whose cancers were HER2 positive and HER2 negative and is thought to be not a predictor of outcome; however, 5 year cancer specific survival was improved in patients whose cancers were HER2 positive versus negative (Am J Surg Pathol 2013;37:522)
- Other studies showed HER2 overexpression in colorectal cancer was associated with poorer 3 year and 5 year survival and correlated with advanced stage and high mitotic activity (Int J Colorectal Dis 2007;22:491, Histol Histopathol 1995;10:661)
Microscopic (histologic) images
Sample pathology report
- Colon, mass, biopsy:
- Moderately differentiated adenocarcinoma
- Immunohistochemistry HER2 analysis
- Results: negative
- Interpretation: 0
- Staining results: no reactivity
- Methodology
- Antibody and assay methodology: rabbit anti-human HER2, Herceptest™ (FDA approved test kit) (Dako, Carpinteria, CA)
- Control slides examined: external kit slides provided by manufacturer (cell lines with high, low and negative HER2 protein expression) and in house known HER2 amplified control tissue was evaluated along with the test tissue and showed appropriate staining
- Interpretation based on recommendations put forth by Valtorta et al. (Mod Pathol 2015;28:1481)
Interpretation criteria:
3+ Positive Strong complete, lateral or basolateral membranous reactivity in more than 10% of cells 2+ Equivocal Moderate complete, lateral or basolateral reactivity in 50% of cells 1+ Negative Faint barely perceptible membranous reactivity any cellularity 0 Negative No reactivity
Additional references
Board review style question #1
While reviewing the HER2 immunohistochemical stains of a biopsy specimen of colon adenocarcinoma, the pathologist observes that ≥ 50% of cells have moderate lateral staining. Repeated immunohistochemical stains showed the same pattern. The correct interpretation and next step of this finding according to the HERACLES trial is
- Equivocal because there is moderate lateral or basolateral staining in ≥ 50% of tumor cells; FISH is the next step
- Negative because a positive result is strong circumferential membrane staining in > 70% of tumor cells, FISH is not required
- Negative because only moderate complete basolateral (and not lateral) membrane reactivity is a considered equivocal result, FISH is not required as a next step
- Positive because a positive result is moderate lateral or basolateral reactivity in at least 10% of neoplastic cells
- Positive because there is intense lateral membrane positivity in ≥ 50% of cells
Board review style answer #1
A. An equivocal result of HER2 staining in colorectal adenocarcinoma is moderate (2+) in ≥ 50% of cells in circumferential, basolateral or lateral. First retest with IHC is mandatory to make sure you have > 50% cellularity; if confirmed the next step is to do FISH. If amplified, the results are interpreted as positive. The remaining answer choices are incorrect because they deviate from this criterion. Choice B is incorrect because you need moderate (2+) in ≥ 50% of cells to have equivocal results and the next step is FISH. Choice C is incorrect because moderate circumferential lateral or basolateral staining in more than 50% is considered equivocal. Choice D is incorrect because positive result is when you have intense staining (3+) and not moderate. Choice E is incorrect because the image doesn’t show intense staining in ≥ 50% of cells; this choice describes the positive results of HER2 IHC test and FISH is not mandatory as a next step.
Comment Here
Reference: HER2 colon
Comment Here
Reference: HER2 colon