Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Laboratory | RhIG dosing | Case reports | Treatment | Peripheral smear images | Sample assessment & plan | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Potochny EM, George MR. Rh immune globulin . PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/transfusionmedrhimmuneglobulin.html. Accessed April 23rd, 2024.
Definition / general
- Rh (Rhesus) immune globulin (RhIG) is a derivative of pooled human plasma manufactured by cold ethanol fractionation to prevent alloimmunization (antibody formation) to RhD antigen following exposure, such as through a blood transfusion or pregnancy
- Treatment option for immune thrombocytopenia (ITP)
- Available via intramuscular (IM) or intravenous (IV) preparations
Essential features
- RhIG is used to prevent RhD alloimmunization (antibody formation) and as treatment option for ITP
- RhIG is typically administered in 300 μg IM vials as prophylaxis during pregnancy for RhD negative females and at time of delivery if neonate is RhD positive
- In cases of fetal maternal hemorrhage, a fetal screen is indicated and if positive, flow cytometry or Kleihauer-Betke testing is performed to estimate appropriate number of additional vials of RhIG
Terminology
- Rh immune globulin, Rh immunoglobulin, Rh0(D) immunoglobulin
- Trade name preparations of Rh immune globulin include Rhophylac®, RhoGAM®, MICRhoGAM®, WinRho SDF®, HyperRHO S/D®
Pathophysiology
- An RhD negative person exposed to RhD positive red blood cells, either through a blood transfusion or fetal maternal hemorrhage during a pregnancy, is at risk of developing antibodies to RhD
- RhD negative phenotype occurs in roughly 15% of whites
- Predominantly due to RHD gene deletion
- RhD negative phenotype in blacks is ~7 - 8%
- Most due to mutant RHD gene coding for premature stop codon
- RhD negative phenotype in Asians is < 1%
- RhD negative phenotype occurs in roughly 15% of whites
- RhIG can suppress the formation of these antibodies, preventing their development
- Exposure to Rh(D) positive red blood cells via massive transfusion protocol or whole blood exists when
- There is no policy to avoid Rh(D) positive red blood cells or whole blood to trauma patients of child bearing potential
- Or there is a lack of Rh(D) negative red blood cells due to inventory shortfalls
- Or via exposure through Rh(D) positive platelets administered emergently during a massive transfusion
- In the first two scenarios, there is the potential for exposure to large quantities of red blood cells such that the risk of hemolysis from RhIG may outweigh the risk of anti-D sensitization
- There is currently no consensus on management of Rh(D) positive red cell exposure from 1 or more units of red blood cells or whole blood
- Case reports exist of success of dosing RhIG following 1 - 2 units of red blood cells, with or without coinciding red blood cell exchange procedures
- Regarding exposure from residual Rh(D) positive red cells in platelet units, dosing is far easier
- If it is an apheresis platelet unit, there is likely ≤ 1 mL of residual red blood cells
- If a 300 µg vial of RhIG covers 30 mL of whole blood or 15 mL of packed red cells, this would adequately cover roughly 10 units of Rh(D) positive platelets
- Prepooled random donor platelets contain < 2 mL of residual red blood cells, thus a single vial of 300 µg is also sufficient
- References: Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 3rd Edition, 2019, Simon: Rossi's Principles of Transfusion Medicine, 5th Edition, 2016, J Clin Apher 2010;25:70, Transfusion 2008;48:1990
Clinical features
- Consequences of anti-D alloimmunization causing hemolytic disease of the fetus may include:
- Fetal anemia
- Organomegaly from extramedullary hematopoiesis
- Portal hypertension
- Fetal hydrops
- Death from high output cardiac failure
- Consequences of anti-D alloimmunization causing hemolytic disease of the newborn may include:
- Neonatal anemia
- Jaundice
- Kernicterus
Symptoms
- Potential adverse effects following RhIG administration prevention of anti-D formation:
- Nausea, dizziness, headache, pain at injection site, malaise
- Potential adverse effects following RhIG administration for ITP:
- Chills / rigors, fever, headache, hemolysis (rare)
- Reference: PDR: RhoD Immune Globulin Human - Drug Summary [Accessed 11 August 2020]
Laboratory
- Following delivery, an RhD negative female bearing an RhD positive neonate should undergo a fetal blood screen
- Negative fetal screen means only 1 vial RhIG required
- Indicates either no or minimal fetal maternal hemorrhage (< 30 mL)
- Positive fetal screen requires a quantitative test, to dose additional vials of RhIG, such as:
- Flow cytometry
- Kleihauer-Betke test
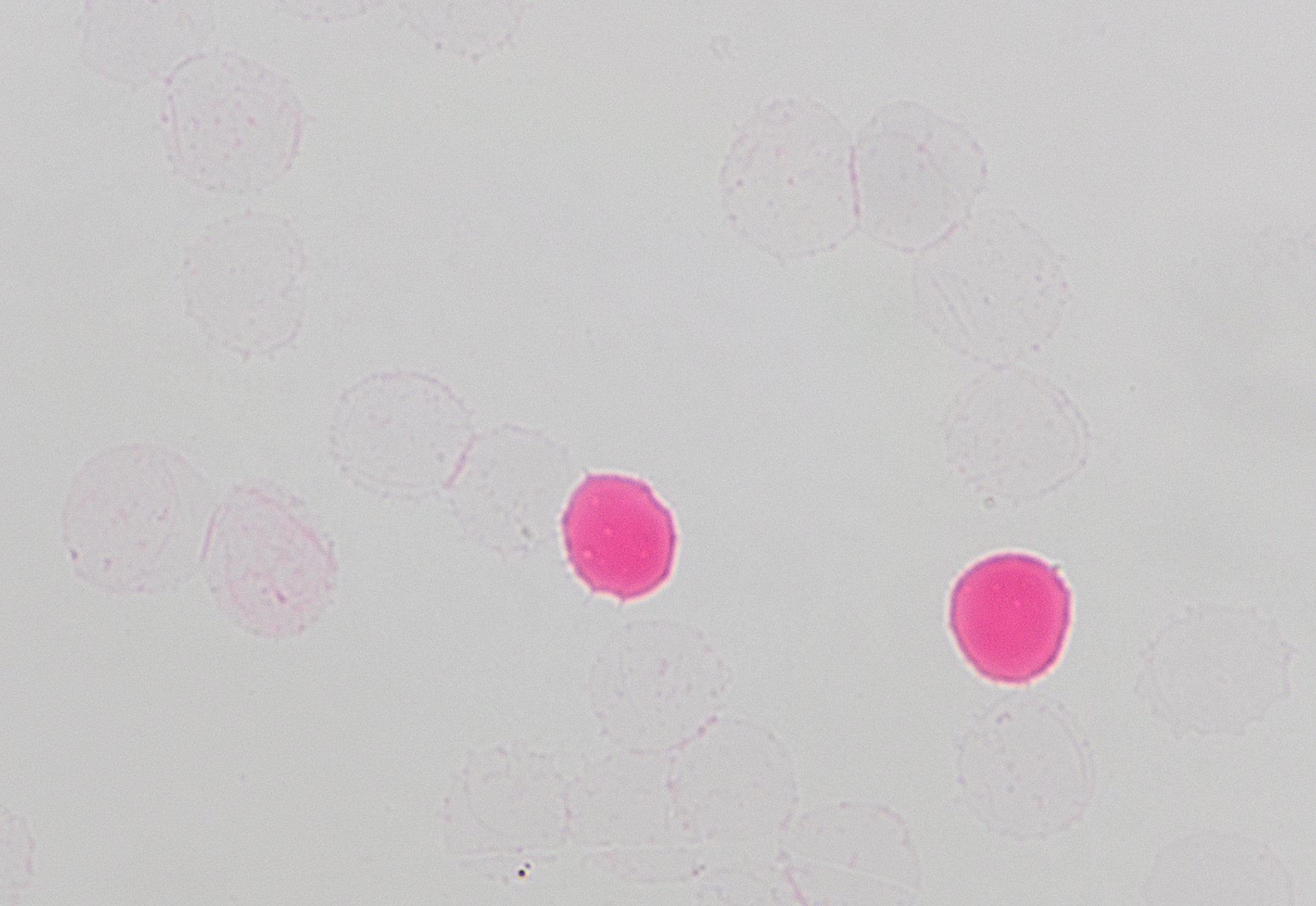
- Detects fetal red cells in maternal circulation by their pink color
- EDTA (pink / lavender top) specimen required, drawn from the mother following delivery
- Hemoglobin in fetal red cells is resistant to acid treatment (bright pink / red)
- Hemoglobin in maternal red cells is destroyed by acid treatment (clear / light pink “ghost” cells)
- Administration of RhIG will look like an anti-D on antibody identification and is generally called passive anti-D
- Pattern of reactivity is indistinguishable from true alloimmunization; blood bank interpretations may include cautionary statement
- Following administration of RhIG, serologic detection of passive anti-D may occur up to 3 - 4 months by tube testing or gel methodology
- Up to 5 - 6 months by solid phase red cell adherence assay (SPRCA)
- Patients injected with IM RhIG should not develop a positive antibody screen until 7 to 8 hours after administration
- Earlier detection raises concern for true alloimmunization
- Different manufactured brands of RhIG are comparable in strength and duration of reactivity across test methods (Transfusion 2015;55:1444)
- Special circumstance: detection of possible anti-D and anti-C in woman of childbearing age, as this may represent anti-G
- Anti-G is an antibody directed against epitopes on both the C and D antigens
- Antibody titering: anti-G is suspected when the anti-C titer is consistently higher than the anti-D titer (Transfusion 1997;37:493)
- Important to investigate via adsorption studies to determine eligibility for RhIG
- Various combinations of antibodies against C, D and G may be present
- If anti-D is not detected, patient (if woman of childbearing age) is eligible for RhIG
- If anti-D is detected, patient is not eligible to receive RhIG
RhIG dosing
- Calculate volume of fetal maternal hemorrhage (FMH) based on Kleihauer-Betke result:
- [# pink / red (fetal) cells ÷ 2,000 cells counted) x maternal blood volume (5,000 mL is average)
- A 300 µg vial of RhIG will cover 30 mL of whole blood
- Therefore, volume of FMH ÷ 30 = vials of RhIG
- If the number of calculated vials is < 0.5, round down to the nearest whole number and add 1 vial
- If the number of calculated vials is ≥ 0.5, round up to nearest whole number and add 1 vial
- For example, if 8 fetal red cells are detected of 2000 cells counted
- Volume of FMH = (8/2000 * 5000 mL) / 30 mL = 0.67 vials
- Round up to 1 and add 1 vial
- Answer: 2 vials should be administered
Case reports
- RhIG for variant RHD:
- 2 cases of variant RHD in pregnant women, misclassified as RhD positive, who did not receive RhIG when indicated and formed anti-D (Immunohematology 2017;33:60)
- RhIG after fetal maternal hemorrhage (FMH):
- 31 year old woman with large RhIG dose requirement following FMH (Am J Clin Pathol 2016;145:744)
- RhIG after RhD positive red cell transfusion following trauma:
- 25 year old woman involved in car accident (J Clin Apher 2010;25:70)
- RhIG for anti-G when clinically indicated - generally, female children and women with childbearing potential:
- 78 year old man with anti-G (Ann Lab Med 2018;38:280)
- RhIG for immune thrombocytopenia (ITP):
- 5 year old boy with platelet count of 2 x 109/L, a theoretical case for consultation of RhIG for ITP (Hematology Am Soc Hematol Educ Program 2013;2013:283)
Treatment
- Rh immune globulin is given to RhD negative women:
- At approximately 28 weeks estimated gestational age (EGA)
- Again within 72 hours of delivery if the neonate is RhD positive
- Following trauma
- After invasive procedures (amniocentesis, cordocentesis)
- Spontaneous or elective abortions
- Pregnancy inversions
- Pregnant women who are considered D variants (weak or partial D) may be capable of forming an anti-D
- RHD genotyping can distinguish weak from partial D and determine if RhD negative blood or RhIG is indicated
- PCR based test requiring specimen collected in EDTA tube
- Weak D types 1, 2, 3 can be managed as RhD positive
- Recent data suggests types 4.0 and 4.1 can also be managed as RhD positive (Transfusion 2020;60:855)
- Immune thrombocytopenia (ITP)
- Only used when patient is RhD positive
- Anti-D acts like an immune decoy to decrease body's formation of antiplatelet antibodies
- References: Fung: Technical Manual, 19th Edition, 2017, Shaz: Transfusion Medicine and Hemostasis - Clinical and Laboratory Aspects, 3rd Edition, 2019
Sample assessment & plan
- History: 35 year old woman presenting to hospital after motor vehicle accident
- Blood type: O negative
- Laboratory testing:
- Type and screen performed in the blood bank, followed by antibody identification which identifies reactivity consistent with anti-D plus anti-C
- Adsorption studies were performed at an immunohematology reference laboratory, identifying anti-G and anti-C; anti-D not identified
- Clinical significance:
- Anti-G is an antibody formed in almost all cases by Rh negative (D negative), G antigen negative patients. Anti-G reacts with epitopes on both the C antigen and the D antigen. Anti-G typically is seen in a D negative patient who has never knowingly been exposed to Rh positive blood, yet presents with an antibody that looks like a combination of both anti-D and anti-C.
- This antibody can form through previous exposure via pregnancy or transfusion. A patient with anti-G would be transfused in exactly the same way as a patient who has the combination of anti-D and anti-C: with Rh negative (D antigen negative) and C antigen negative blood.
- However, women of childbearing age with anti-G should receive Rh immune globulin during pregnancy to protect against the formation of a true alloimmunization against the D antigen, which could cause severe hemolytic disease of the newborn.
- Difficulty in finding compatible blood:
- This patient can receive O, RhD negative, C antigen negative packed red blood cells, which accounts for approximately 2% of the blood inventory
Board review style question #1
A 31 year old G2P1 woman presents to a new medical center after having moved from out of state. She is 16 weeks pregnant and was referred to maternal fetal medicine for a positive antibody screen. Antibody identification reveals a pattern consistent with anti-D and anti-C. Additional blood samples are requested by the blood bank for adsorption testing at an immunohematology reference laboratory, as anti-G is suspected. In which scenario is RhIG indicated for this patient?
- Anti-G, anti-C and anti-D are identified
- Anti-D and anti-C are identified but anti-G is not identified
- Anti-G and anti-C are identified but anti-D is not identified
- Anti-G and anti-D are identified but anti-C is not identified
Board review style answer #1
C. Anti-G and anti-C are identified but anti-D is not identified. Anti-G is an important consideration in the workup of a woman of childbearing age who appears to have anti-D and anti-C. Adsorption studies to discern the presence or absence of true anti-D should be undertaken to determine eligibility for RhIG in the interest of protecting against severe anti-D hemolytic disease of the newborn. Women of childbearing age who have not formed anti-D are eligible for RhIG. The presence or absence of of anti-G or anti-C does not influence RhIG administration.
Comment Here
Reference: Rh immune globulin
Comment Here
Reference: Rh immune globulin
Board review style question #2
A 27 year old G1P0 receives 300 μg RhIG at approximately 28 weeks gestational age because her blood type is A, RhD negative. She later delivers a full term healthy boy at 39 weeks gestational age. Cord blood testing of the infant reveals he is O, RhD positive. Additionally, a fetal screen performed on a postpartum sample from the mother is positive, indicating fetal maternal hemorrhage, and a Kleihauer-Betke is reflexively performed in order to properly dose RhIG. How many total vials of 300 μg RhIG are required if 2,000 total cells are counted and 8 fetal cells are identified (and a maternal blood volume of 5,000 mL is presumed)?
- 1 vial RhIG
- 2 vials RhIG
- 3 vials RhIG
- 4 vials RhIG
Board review style answer #2
B. 2 vials. Volume FMH identified by Kleihauer-Betke = (8 fetal cells / 2,000 total cells counted) x 5,000 mL maternal blood volume = 20 mL. 20 mL/30mL = 0.67 vial. In this case, 0.67 vial is rounded up to 1 vial and 1 additional vial is added, making the appropriate RhIG dose 2 vials.
Comment Here
Reference: Rh immune globulin
Comment Here
Reference: Rh immune globulin