Uterus
General
Staging-carcinoma and carcinosarcoma
Last author update: 1 March 2023
Last staff update: 14 November 2023
Copyright: 2002-2024, PathologyOutlines.com, Inc.
PubMed search: Staging - carcinoma and carcinosarcoma
Page views in 2023: 20,751
Page views in 2024 to date: 7,319
Cite this page: Parra-Herran C. Staging-carcinoma and carcinosarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uterusstaging.html. Accessed April 16th, 2024.
Definition / general
- Current staging of endometrial carcinoma is based on the International Federation of Obstetrics and Gynecology (FIGO for its name in French), most recently updated in 2021 (Int J Gynaecol Obstet 2021;155:45)
- The American Joint Committee on Cancer (AJCC) staging system currently harmonizes its tumor (T), lymph node (N) and metastasis (M) categories with the FIGO system (see Table 1) (AJCC: Cancer Staging Manual, 8th Edition, 2017)
- Initial stage should not be changed due to disease progression or recurrence or based on response to initial radiation therapy or chemotherapy that precedes primary tumor resection
- NCCN guidelines for management of uterine neoplasms utilize the 2018 FIGO staging criteria (J Natl Compr Canc Netw 2023;21:181)
- However, the 2023 update takes histologic and molecular features into account with the goal of creating substages that better align with prognosis and treatment options (see Tables 2 and 3) (Int J Gynaecol Obstet 2023;162:383)
- Management guidelines are likely to be updated in the relatively near future
- The International Collaboration on Cancer Reporting (ICCR) recently updated its reporting tool for endometrial carcinoma, incorporating core and optional elements of reporting and current staging elements (Int J Gynecol Pathol 2022;41:S90, ICCR: Endometrial Cancers [Accessed 9 February 2023])
- In addition, the International Society of Gynecological Pathologists (ISGyP) endometrial carcinoma consensus papers provide guidance in the evaluation of staging and other prognostic variables (Int J Gynecol Pathol 2019;38:S93)
Essential features
- AJCC 7th edition staging was sunset on December 31, 2017; as of January 1, 2018, use of the 8th edition is mandatory
- Primary tumor (T) staging depends on depth of myometrial invasion (absent or inner half stage T1a, outer half stage T1b), cervical involvement (stage T2), serosal or adnexal involvement (stage T3a) and parametrial or vaginal involvement (stage T3b)
- Regarding lymph node (N) staging, the AJCC system now includes staging categories for lymph node metastases according to metastatic tumor size: isolated tumor cells (ITCs), micrometastases and macrometastases; the FIGO stage does not recognize metastatic nodal tumor size for staging purposes
ICD coding
- ICD-10: C54.1 - malignant neoplasm of endometrium
Diagrams / tables
Table 1: Staging of endometrial carcinoma as per FIGO (2018 update) and AJCC 8th edition
| FIGO (2018)
| AJCC (2018)
| Description
|
| I
| T1
| Tumor confined to the uterus
|
| 1A
| T1a
| Tumor confined to the endometrium or invades < 50% of the myometrial wall
|
| 1B
| T1b
| Tumor invades > 50% of the myometrial wall
|
| II
| T2
| Tumor infiltrates cervical stroma
|
| III
| T3, N1
| Tumor extends outside the uterus
|
| IIIA
| T3a
| Tumor involves serosa or adnexa (direct extension or metastases)
|
| IIIB
| T3b
| Tumor involves vagina or parametria (direct extension or metastases)
|
| IIIC
| N1
| Tumor with pelvic lymph node metastasis
|
| IIIC1
| N1 mi
| Micrometastases in pelvic lymph nodes (> 0.2 mm or 200 cells but ≤ 2 mm)
|
| N1a
| Macrometastases in pelvic lymph nodes (> 2 mm in size)
|
| IIIC2
| N2 mi
| Micrometastases in para-aortic lymph nodes (> 0.2 mm or 200 cells but ≤ 2 mm)
|
| N2a
| Macrometastases in para-aortic lymph nodes (> 2 mm in size)
|
| IV
| T4, M1
| Tumor invades bladder or bowel mucosa or involves distant organs
|
| IVA
| T4
| Tumor invades bladder mucosa or bowel mucosa
|
| IVB
| M1
| Distant metastases (including intra-abdominal metastases and inguinal lymph nodes;
excluding metastases to vagina, pelvic serosa or adnexa)
|
|
| N0i+
| Isolated tumor cells in regional lymph nodes*
|
* Isolated tumor cells category is recognized in the AJCC (TNM) system but not in the FIGO system
Table 2: 2023 FIGO staging of cancer of the endometrium
| Stage
| Description
|
| I
| Confined to the uterine corpus and ovary
|
| IA
| Disease limited to the endometrium or nonaggressive histological type (i.e., low grade endometroid, with invasion of < 50% of myometrium with no or focal lymphovascular space involvement [LVSI] or good prognosis disease)
|
| IA1
| Nonaggressive histological type limited to an endometrial polyp or confined to the endometrium
|
| IA2
| Nonaggressive histological types involving < 50% of the myometrium with no or focal LVSI
|
| IA3
| Low grade endometrioid carcinomas limited to the uterus and ovary
|
| IB
| Nonaggressive histological types with invasion of ≥ 50% of the myometrium and with no or focal LVSI
|
| IC
| Aggressive histological types limited to a polyp or confined to the endometrium
|
| II
| Invasion of cervical stroma without extrauterine extension or with substantial LVSI or aggressive histological types with myometrial invasion
|
| IIA
| Invasion of the cervical stroma of nonaggressive histological types
|
| IIB
| Substantial LVSI of nonaggressive histological types
|
| IIC
| Aggressive histological types with any myometrial involvement
|
| III
| Local or regional spread of the tumor of any histological subtype
|
| IIIA
| Invasion of uterine serosa, adnexa or both by direct extension or metastasis
|
| IIIA1
| Spread to ovary or fallopian tube (except when meeting stage IA3 criteria)
|
| IIIA2
| Involvement of uterine subserosa or spread through the uterine serosa
|
| IIIB
| Metastasis or direct spread to the vagina or to the parametria or pelvic peritoneum
|
| IIIB1
| Metastasis or direct spread to the vagina or the parametria
|
| IIIB2
| Metastasis to the pelvic peritoneum
|
| IIIC
| Metastasis to the pelvic or para-aortic lymph nodes or both
|
| IIIC1
| Metastasis to the pelvic lymph nodes
|
| IIIC1i
| Micrometastasis
|
| IIIC1ii
| Macrometastasis
|
| IIIC2
| Metastasis to para-aortic lymph nodes up to the renal vessels, with or without metastasis to the pelvic lymph nodes
|
| IIIC2i
| Micrometastasis
|
| IIIC2ii
| Macrometastasis
|
| IV
| Spread to the bladder mucosa or intestinal mucosa or distance metastasis
|
| IVA
| Invasion of the bladder mucosa or the intestinal / bowel mucosa
|
| IVB
| Abdominal peritoneal metastasis beyond the pelvis
|
| IVC
| Distant metastasis, including metastasis to any extra or intra-abdominal lymph nodes above the renal vessels, lungs, liver, brain or bone
|
- Disease limited to low grade endometrioid carcinomas involving the endometrium and ovaries (stage IA3) must be distinguished from extensive spread of the endometrial carcinoma to the ovary (stage IIIA1), by the following criteria
- No more than superficial myometrial invasion is present (< 50%)
- Absence of extensive / substantial LVSI
- Absence of additional metastases
- The ovarian tumor is unilateral, limited to the ovary, without capsule invasion / rupture (equivalent to pT1a)
- LVSI as defined in WHO 2021: extensive / substantial, ≥ 5 vessels involved
- Aggressive histological types include serous carcinomas, clear cell carcinomas, mesonephric-like carcinomas, gastrointestinal type mucinous endometrial carcinoma, undifferentiated carcinomas and carcinosarcomas
- For endometrial endometrioid carcinomas, grade is based on the proportion of solid areas: low grade = grade 1 (≤ 5%) and grade 2 (6 - 50%); and high grade = grade 3 (> 50%)
- Nuclear atypia excessive for the grade raises the grade of a grade 1 or 2 tumor by one
- Nonaggressive histological types are composed of low grade (grade 1 and 2) endometrial endometrioid carcinomas
Table 3: 2023 FIGO endometrial cancer stage with molecular classification (for patients with early endometrial cancer [stages I and II after surgical staging])
| Stage
| Description
|
| IAmPOLEmut
| POLEmut endometrial carcinoma, confined to the uterine corpus or with cervical extension, regardless of the degree of LVSI or histological type
|
| IICmp53abn
| p53abn endometrial carcinoma confined to the uterine corpus with any myometrial invasion, with or without cervical invasion and regardless of the degree of LVSI or histological type
|
Primary tumor (pT) and FIGO stages ()
- pTX: primary tumor cannot be assessed
- pT0: no evidence of primary tumor
- pT1 (I): tumor confined to corpus uteri
- pT1a (IA): tumor limited to endometrium or invades < 50% of the myometrium
- pT1b (IB): tumor invades ≥ 50% of the myometrium
- pT2 (II): tumor invades stromal connective tissue of the cervix but does not extend beyond uterus
- pT3 (III): tumor involving serosa, adnexa, vagina or parametrium
- pT3a (IIIA): tumor involves serosa or adnexa (direct extension or metastasis)
- pT3b (IIIB): vaginal involvement (direct extension or metastasis) or parametrial involvement
- pT4 (IVA): tumor invades bladder mucosa or bowel mucosa (bullous edema is not sufficient to classify a tumor as pT4)
Notes:
- Endocervical glandular involvement only should be considered as stage I and not stage II
- pTis is no longer a staging category
- Myometrial invasion:
- According to the ISGYP recommendations, this variable should be reported as none or less than half (< 50%) or half or more (≥ 50%) of the myometrial wall thickness
- This approach is relatively easy and obviates in most cases the need to locate the endomyometrial junction (which is difficult when the junction is completely obliterated or not sampled in the sections)
- In addition, other variables than can be reported include myoinvasion in terms of absolute numbers (invasive carcinoma depth in mm / myometrial thickness in mm) and the percentage of myometrial wall involved by tumor (a derivative of the absolute measurements in mm)
- Assessment should be performed at the deepest point of tumor invasion on a full thickness section of the wall spanning from mucosal surface to serosa (Figure 1)
- Involvement of adenomyosis by carcinoma does not by itself portend a worse prognosis
- These cases behave similarly to stage IA tumors (Gynecol Oncol 1990;37:401, Int J Gynecol Pathol 2010;29:445)
- Carcinoma is confined to the endometrium / adenomyosis when:
- Focus of carcinoma has a round or ovoid shape with a smooth and convex outer contour (regardless of its size) (Figure 2)
- Outer contour smoothly continues with adjacent uninvolved endomyometrial interface
- Residual endometrial type stroma or benign endometrial glands are present in the periphery or within the focus
- There is no significant desmoplastic reaction
- If myometrial invasion arises from areas of adenomyosis, the location of the deepest myoinvasive point with respect to the entire uterine wall should be recorded (inner versus outer wall) (Figure 3)
- There is controversy in whether to assign stage IA or IB to a carcinoma with foci of invasion in the outer wall arising from adenomyosis; the ISGyP endometrial carcinoma consensus recommends:
- If the foci are few and clearly arising from deep adenomyosis, it is advisable to categorize these lesions as stage IA; there is emerging evidence showing that these lesions behave more indolently, similar to stage IA tumors (Int J Gynecol Pathol 2019;38:S93, Mod Pathol 2019;32:1)
- If, on the other hand, myoinvasive carcinoma from adenomyosis is rather extensive and seen throughout the wall, it is prudent to classify the tumor as stage IB (Int J Gynecol Pathol 2019;38:S93)
- If the tumor is exophytic at the point of deepest myometrial invasion, the nearest endomyometrial junction should be identified and depth should be measured from that point
- Same recommendation applies to tumors arising from or involving an endometrial polyp
- If the carcinoma at the point of deepest invasion involves a leiomyoma, the myometrial thickness should include the leiomyoma (Int J Gynecol Pathol 2019;38:S93)
- Lymphovascular space invasion (LVI) should not be included in the assessment of myometrial invasion
- Cervical stromal involvement:
- Presence of cervical stromal involvement defines stage II
- Depth of invasion into the cervical stroma and margin status should be reported, as it may influence adjuvant radiation treatment (Brachytherapy 2019;18:606)
- Assessment of cervical involvement is inconsistent between gynecologic pathologists (Am J Surg Pathol 2011;35:289)
- Uppermost endocervical mucinous gland in the section should be considered as the upper limit of the endocervix (Adv Anat Pathol 2018;25:71)
- If unsure whether tumor is in the cervix or lower uterine segment, identify the most proximal endocervical mucinous gland, which will define the boundary between these anatomic locations (Figures 4 and 5)
- Stromal invasion can be seen as obviously infiltrative glands or cells with desmoplasia or as complex architecture relative to the normal endocervical glandular compartment
- Carcinoma confined to the surface or to endocervical glands can be reported but does not modify stage or impact prognosis
- Reporting the depth of cervical stromal invasion by endometrial carcinoma is optional
- The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology Uterine Neoplasms lists deep cervical stromal invasion as an adverse risk factor in patients with stage II endometrial carcinoma (NCCN: Uterine Neoplasms [Accessed 9 February 2023])
- Estimation of depth of cervical wall invasion may be used to determine the type of adjuvant radiation (Brachytherapy 2019;18:606)
- Depth of invasion into the cervix can be measured in mm, providing the thickness of the cervical wall at that level in mm as well
- Alternatively, it can be reported in terms of invasion confined to 33% of the wall, 66% of the wall or extending to the outer third of the wall
- It is recommended to measure the distance between tumor and margin: distal cervical / vaginal mucosa and parametrial margins
- Serosal involvement:
- Involvement of the uterine serosa is an adverse prognostic factor and warrants a stage IIIA
- Involvement of the serosa is diagnosed when the tumor perforates through the mesothelium lined outer surface of the uterus; this is seen as an area of disruption of the serosa with tumor involvement, sometimes with soft tissue or other structures attached to it
- Sometimes a desmoplastic stromal reaction is present
- Locating the serosal plane flanking the area in question and extending the plane through the area of desmoplasia can be helpful; serosal involvement is considered present if there is disruption of that plane or carcinoma extends beyond the plane (Int J Gynecol Pathol 2022;41:S90)
- According to ISGYP guidelines, tumor that infiltrates the entire myometrial thickness and reaches submesothelial fibroconnective tissue should also be reported as serosal involvement (Int J Gynecol Pathol 2019;38:S93)
- Adnexal involvement (versus synchronous ovarian carcinoma):
- Distinguishing between the following is relevant for staging and treatment:
- Synchronous ovarian and endometrial tumors
- Primary endometrial with metastatic ovarian involvement
- Primary ovarian with metastatic endometrial involvement
- However, it is often difficult to separate these scenarios on pathologic examination and one may only suggest one possibility over the others
- Synchronous ovarian carcinoma is present in up to 30% of reproductive age women with endometrial cancer (Int J Womens Health 2014;6:691, Obstet Gynecol 2005;106:693)
- When present, synchronous ovarian and endometrial carcinomas usually have the same histologic type (vast majority are low grade endometrioid)
- Synchronous endometrial and ovarian endometrioid carcinoma have good prognosis, similar to those with only endometrial or only ovarian stage I cancer (Int J Gynecol Cancer 2014;24:54, Clin Cancer Res 2008;14:5840)
- Features that support 2 synchronous carcinomas include:
- Different histologic types or tumor grades
- Endometrial carcinoma has absent or only superficial myometrial invasion
- Ovarian carcinoma is confined to the ovary
- Absence of lymphovascular space invasion
- Different patterns of mismatch repair (MMR) or hormone receptor expression by IHC
- Conversely, secondary ovarian involvement by a primary endometrial tumor should be considered when:
- Endometrial tumor has deep myometrial invasion, lymphovascular space invasion, serosal or parametrial involvement
- Bilateral ovarian involvement
- Ovarian tumor involves surface and has extensive lymphovascular space invasion
- Both tumors show similar MMR and hormone receptor expression by IHC
Regional lymph nodes (pN)
- pNX: regional lymph nodes cannot be assessed
- pN0: no regional lymph node metastasis
- pN0(i+): isolated tumor cells in regional lymph node(s) ≤ 0.2 mm
- pN1 (IIIC1): regional lymph node metastasis to pelvic lymph nodes
- pN1mi: regional lymph node metastasis (> 0.2 mm but ≤ 2 mm in diameter) to pelvic lymph nodes
- pN1a: regional lymph node metastasis (> 2 mm in diameter) to pelvic lymph nodes
- pN2 (IIIC2): regional lymph node metastasis to para-aortic lymph nodes, with or without positive pelvic lymph nodes
- pN2mi: regional lymph node metastasis (> 0.2 mm but ≤ 2 mm in diameter) to pelvic lymph nodes
- pN2a: regional lymph node metastasis (> 2 mm in diameter) to pelvic lymph nodes
Notes:
- Emerging data shows that patients with low volume disease (isolated tumor cells and micrometastases) have better recurrence free survival compared to those with macrometastatic nodal disease (Ann Surg Oncol 2016;23:1653, Gynecol Oncol 2017;146:240)
- When measuring size of tumor within lymph nodes, tumor foci should be measured separately; then the largest individual size is reported
- It is recommended to report extranodal extension if present (although it is not a mandatory staging criterion)
Prefixes
- c: clinical classification
- p: pathological classification
- y: post therapy classification
- r: recurrence or retreatment classification
- a: autopsy classification
AJCC prognostic stage groups
| Stage 0: | Tis | N0 | M0
|
| Stage I: | T1 | N0 | M0
|
| Stage IA: | T1a | N0 | M0
|
| Stage IB: | T1b | N0 | M0
|
| Stage II: | T2 | N0 | M0
|
| Stage III: | T3 | N0 | M0
|
| Stage IIIA: | T3a | N0 | M0
|
| Stage IIIB: | T3b | N0 | M0
|
| Stage IIIC1: | T1 - 3 | N1 | M0
|
| Stage IIIC2: | T1 - 3 | N2 | M0
|
| Stage IVA: | T4 | any N | M0
|
| Stage IVB: | any T | any N | M1
|
Histologic grade (G)
- FIGO grading applies to endometrial carcinomas of the endometrioid type:
- Grade 1: ≤ 5% of a nonsquamous or nonmorular solid growth pattern
- Grade 2: 6 - 50% of a nonsquamous or nonmorular solid growth pattern
- Grade 3: > 50% of a nonsquamous or nonmorular solid growth pattern
Notes:
Microscopic (histologic) images
Contributed by Carlos Parra-Herran, M.D.
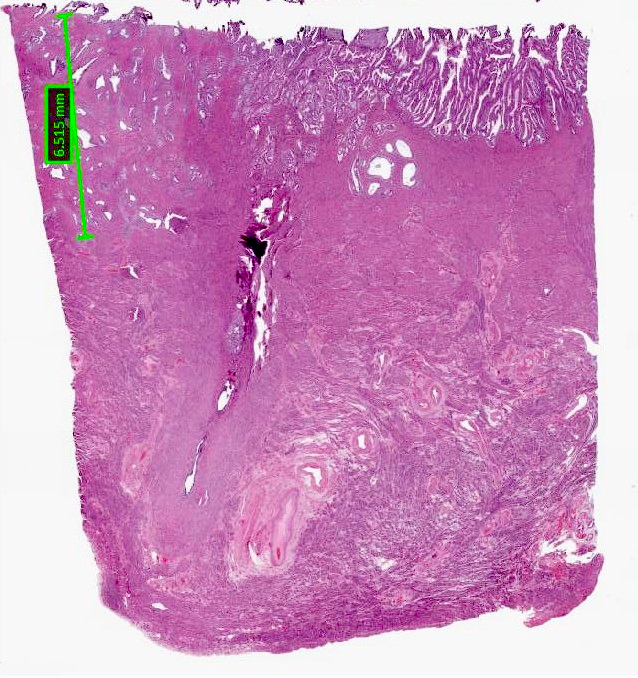
Depth of myometrial invasion
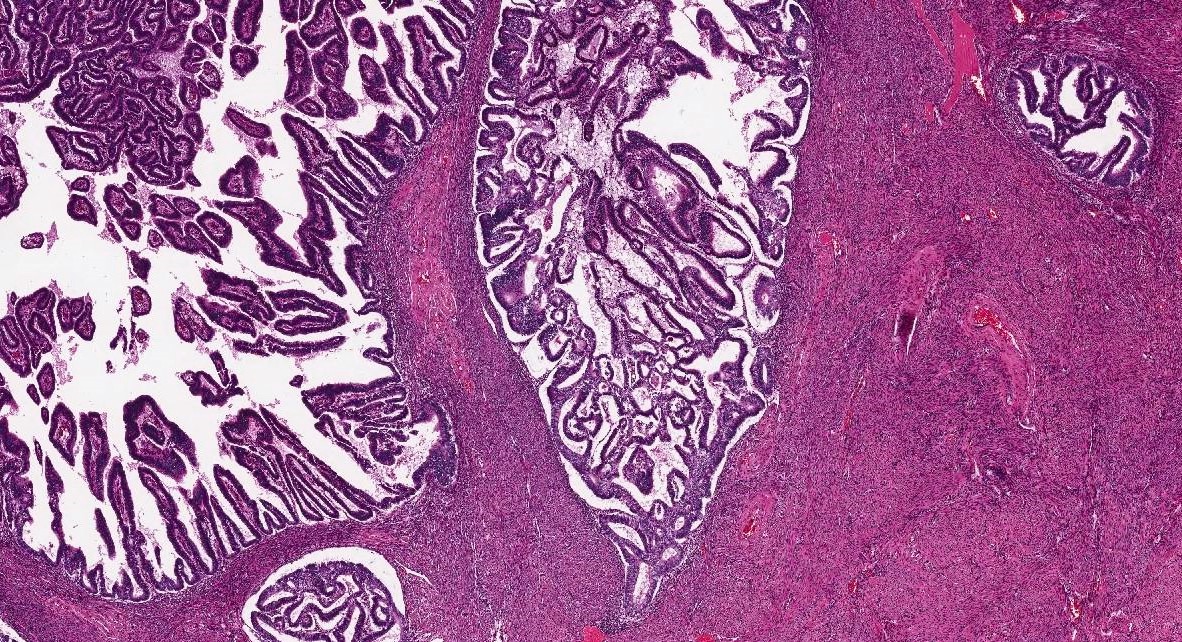
Endometrial carcinoma involving adenomyosis
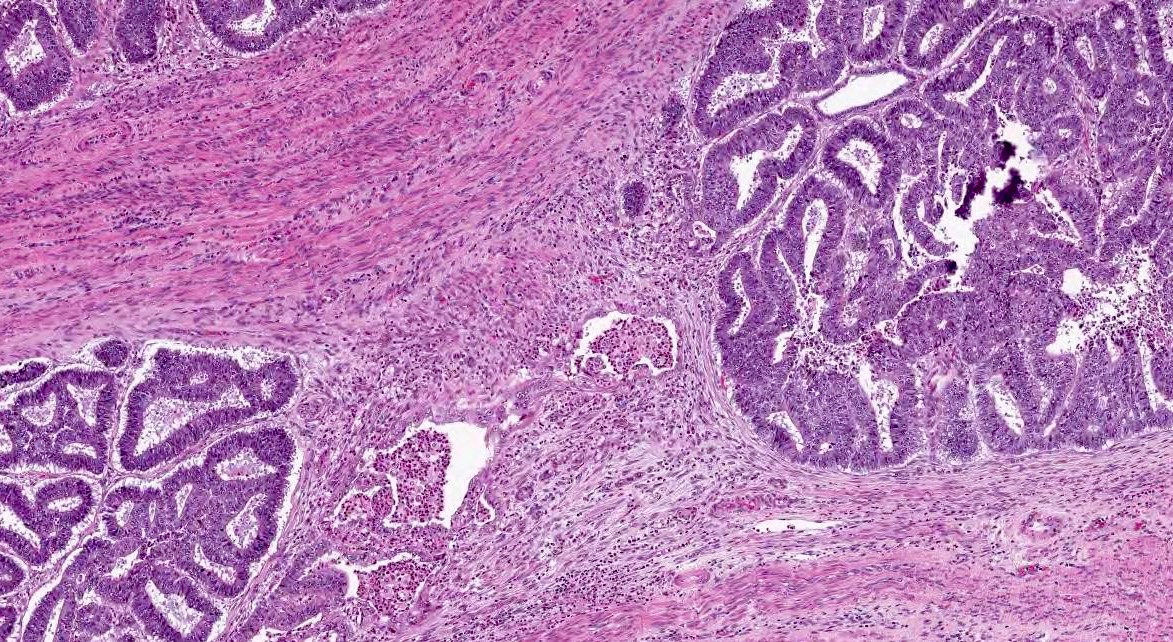
Myometrial invasion from adenomyosis
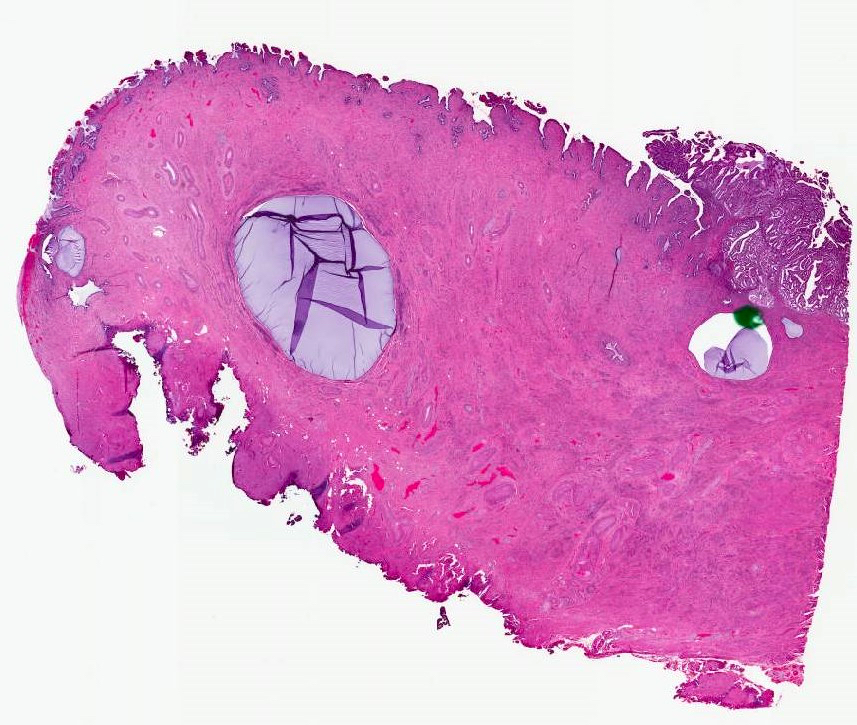

Cervical involvement by endometrial carcinoma (pT2)
Board review style question #1
What is the defining feature of stage T3a / IIIA carcinoma of the uterine corpus?
- Involvement of bladder or rectal mucosa
- Involvement of cervical stromal tissue
- Involvement of uterine serosa or adnexa
- Involvement of vagina or parametrial tissue
- Pelvic lymph node metastases
Board review style answer #1
C. Involvement of uterine serosa or adnexa. Serosal or adnexal involvement by endometrial carcinoma is the defining feature of stage T3a (AJCC) / IIIA (FIGO). Bladder / rectal mucosal involvement, cervical stromal involvement, vaginal / parametrial involvement and lymph node metastases are the defining features of stages T4 / IVA, T2 / II, T3b / IIIB and N1 / IIIC1, respectively.
Comment here
Reference:
Uterus - Staging-carcinoma and carcinosarcoma
Board review style question #2
What is the defining feature of stage T2 / II carcinoma of the uterine corpus?
- Involvement of bladder or rectal mucosa
- Involvement of cervical epithelium
- Involvement of cervical stromal tissue
- Involvement of vagina or parametrial tissue
- Myometrial invasion involving 50% of the uterine wall or more
Board review style answer #2
C. Involvement of cervical stromal tissue. Cervical stromal involvement by endometrial carcinoma is the defining feature of stage T2 (AJCC) / II (FIGO). Bladder / rectal mucosal involvement, vaginal / parametrial involvement and outer half myometrial invasion are the defining features of stages T4 / IVA, T3b / IIIB and T1b / IB, respectively. Cervical involvement confined to the epithelium does not affect staging.
Comment here
Reference:
Uterus - Staging-carcinoma and carcinosarcoma
Back to top








