10 June 2005 - Case #10
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
This case was contributed by Dr. David Lucas, University of Michigan Hospitals, Ann Arbor, Michigan, USA.

Case #10
Clinical history:
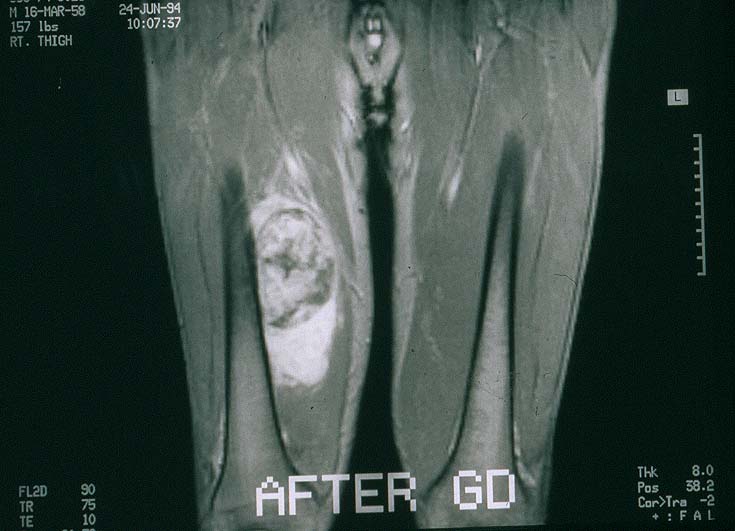
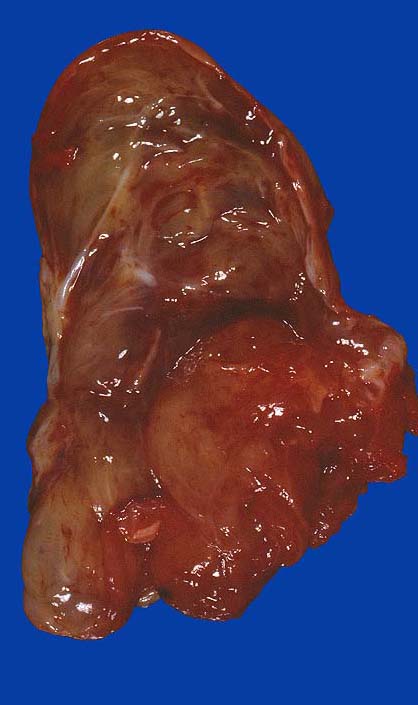
A 30 year old man presented with a mass in the inner thigh. MRI disclosed an enhancing, heterogeneous mass in the thigh, abutting the femur (image #1). Following open biopsy, he was treated with adriamycin based chemotherapy and the tumor was resected. Grossly, it was largely gelatinous but had areas that appeared fleshy (image #2). 18 months later he developed recurrent disease in the groin and he ultimately died of disseminated disease.
Radiology image:
Gross image:
Micro images (postchemotherapy):
Micro description:
The tumor showed variation from field to field. Some of the tumor was relatively hypocellular with myxoid stroma, low grade nuclear cytology, plexiform small vascular pattern and readily identified signet ring type lipoblasts (image #3). However, most of the tumor was more cellular, consisting of closely spaced, overlapping, anaplastic round cells with scant cytoplasm and readily identifiable mitotic activity. These areas had very little stroma and much fewer lipoblasts (image #4).
What is your diagnosis?
Diagnosis: High grade myxoid / round cell liposarcoma
Discussion:
Myxoid and round cell liposarcomas, although histologically different, actually represent two ends of a spectrum for a tumor which almost always has the same translocation, t(12;16)(q13;p11). They are common and together account for 50% of all liposarcomas. Tumors with pure myxoid features are considered to be well differentiated and to have a good prognosis. However, round cell features are considered high grade or poorly differentiated and are associated with a poorer outcome, even if they represent as little as 5% of the tumor volume. Thus, apparent myxoid liposarcomas should be sampled thoroughly to ensure that no significant round cell component is present. This is a particular problem with needle biopsies, which may entirely miss the round cell component.
Myxoid / round cell liposarcomas frequently metastasize, based in part on the percentage of round cells, with rates varying from 23% (0 - 5% round cells) to 58% (> 25% round cells). They metastasize to lung and bone, as well as to other soft tissue sites.
Histologically, pure myxoid liposarcomas resemble developing fetal adipose tissue, with a multinodular mass of low cellularity, particularly centrally. The tumor cells are bland, spindled or round and lie within a myxoid matrix. Numerous lipoblasts are present, with one or many vacuoles. There are no / rare mitotic figures. A delicate plexiform vasculature is present and helps differentiate this tumor from myxoma.
The round cell component is often separated from the myxoid component by a cellular transition area. The round cells are primitive, with minimal eosinophilic cytoplasm, a high N/C ratio and prominent nucleoli. Lipoblasts are less frequent than in myxoid areas. Often the cells are packed together with minimal stroma and blood vessels are difficult to find.
As noted, almost all myxoid / round cell liposarcomas share a common t(12;16)(q13;p11) translocation resulting in the TLS::CHOP fusion gene. Less common is t(12;22)(q13;p11) or an insertion between chromosomes 12 and 16, (12;16)(q13;p11.2p13).
References: Weiss: Enzinger and Weiss's Soft Tissue Tumors, 4th Edition, 2001, Am J Surg Pathol 1996;20:1047, Am J Surg Pathol 1996;20:171, Cancer 1996;77:1450
All cases are archived on our website. To view them sorted by case number, diagnosis or category, visit our main Case of the Month page. To subscribe or unsubscribe to Case of the Month or our other email lists, click here.
This case was contributed by Dr. David Lucas, University of Michigan Hospitals, Ann Arbor, Michigan, USA.

Website news:
(1) This week's case is sponsored by Invitrogen. The Zymed EGFr antibody clone 31G7 is the most widely referenced clone and the industry standard for EGFr detection in formalin-fixed, paraffin embedded (FFPE) tissue samples. Zymed's 31G7 antibody is extremely specific for EGFr and does not react with the highly homologous c-erbB-2 (HER2) protein. Invitrogen now offers the Zymed 31G7 antibody clone as part of a convenient, standardized immunohistochemical kit for the detection of EGFr protein expression in normal and neoplastic tissue. Invitrogen's Zymed EGFr Kit is compatible with manual or automated immunostainers and offers the greatest flexibility with FFPE tissue samples. For more information, visit www.invitrogen.com/antibodies. Note: sponsors do NOT have access in any manner to email addresses or other personal information in the possession of PathologyOutlines.com.
Visit and follow our Blog to see recent updates to the website.
(1) This week's case is sponsored by Invitrogen. The Zymed EGFr antibody clone 31G7 is the most widely referenced clone and the industry standard for EGFr detection in formalin-fixed, paraffin embedded (FFPE) tissue samples. Zymed's 31G7 antibody is extremely specific for EGFr and does not react with the highly homologous c-erbB-2 (HER2) protein. Invitrogen now offers the Zymed 31G7 antibody clone as part of a convenient, standardized immunohistochemical kit for the detection of EGFr protein expression in normal and neoplastic tissue. Invitrogen's Zymed EGFr Kit is compatible with manual or automated immunostainers and offers the greatest flexibility with FFPE tissue samples. For more information, visit www.invitrogen.com/antibodies. Note: sponsors do NOT have access in any manner to email addresses or other personal information in the possession of PathologyOutlines.com.
Visit and follow our Blog to see recent updates to the website.
Case #10
Clinical history:
A 30 year old man presented with a mass in the inner thigh. MRI disclosed an enhancing, heterogeneous mass in the thigh, abutting the femur (image #1). Following open biopsy, he was treated with adriamycin based chemotherapy and the tumor was resected. Grossly, it was largely gelatinous but had areas that appeared fleshy (image #2). 18 months later he developed recurrent disease in the groin and he ultimately died of disseminated disease.
Radiology image:
Gross image:
Micro images (postchemotherapy):
Micro description:
The tumor showed variation from field to field. Some of the tumor was relatively hypocellular with myxoid stroma, low grade nuclear cytology, plexiform small vascular pattern and readily identified signet ring type lipoblasts (image #3). However, most of the tumor was more cellular, consisting of closely spaced, overlapping, anaplastic round cells with scant cytoplasm and readily identifiable mitotic activity. These areas had very little stroma and much fewer lipoblasts (image #4).
What is your diagnosis?
Click here for diagnosis and discussion:
Diagnosis: High grade myxoid / round cell liposarcoma
Discussion:
Myxoid and round cell liposarcomas, although histologically different, actually represent two ends of a spectrum for a tumor which almost always has the same translocation, t(12;16)(q13;p11). They are common and together account for 50% of all liposarcomas. Tumors with pure myxoid features are considered to be well differentiated and to have a good prognosis. However, round cell features are considered high grade or poorly differentiated and are associated with a poorer outcome, even if they represent as little as 5% of the tumor volume. Thus, apparent myxoid liposarcomas should be sampled thoroughly to ensure that no significant round cell component is present. This is a particular problem with needle biopsies, which may entirely miss the round cell component.
Myxoid / round cell liposarcomas frequently metastasize, based in part on the percentage of round cells, with rates varying from 23% (0 - 5% round cells) to 58% (> 25% round cells). They metastasize to lung and bone, as well as to other soft tissue sites.
Histologically, pure myxoid liposarcomas resemble developing fetal adipose tissue, with a multinodular mass of low cellularity, particularly centrally. The tumor cells are bland, spindled or round and lie within a myxoid matrix. Numerous lipoblasts are present, with one or many vacuoles. There are no / rare mitotic figures. A delicate plexiform vasculature is present and helps differentiate this tumor from myxoma.
The round cell component is often separated from the myxoid component by a cellular transition area. The round cells are primitive, with minimal eosinophilic cytoplasm, a high N/C ratio and prominent nucleoli. Lipoblasts are less frequent than in myxoid areas. Often the cells are packed together with minimal stroma and blood vessels are difficult to find.
As noted, almost all myxoid / round cell liposarcomas share a common t(12;16)(q13;p11) translocation resulting in the TLS::CHOP fusion gene. Less common is t(12;22)(q13;p11) or an insertion between chromosomes 12 and 16, (12;16)(q13;p11.2p13).
References: Weiss: Enzinger and Weiss's Soft Tissue Tumors, 4th Edition, 2001, Am J Surg Pathol 1996;20:1047, Am J Surg Pathol 1996;20:171, Cancer 1996;77:1450