Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Sample pathology report | Differential diagnosis | Additional references | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: George GV, Huber AR. Hyperplastic polyp. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/appendixhyperplasticpolyp.html. Accessed August 29th, 2025.
Definition / general
- Appendiceal hyperplastic polyps (HPs) are serrated proliferations in the appendiceal mucosa similar to colorectal hyperplastic polyps, that are devoid of architectural and cytologic dysplasia
- Epithelial serrations defined as crypt epithelium with sawtooth luminal infolding (Hum Pathol 2014;45:227)
Essential features
- Serrated polyp in the appendix similar to colorectal counterpart and by definition, lacks cytologic dysplasia
- Usually discovered incidentally in appendectomy specimens
- Frequently harbor KRAS mutations and are thought to be unrelated to the serrated pathway of colorectal neoplasia
- Entirely benign and cured with appendectomy
Terminology
- Nondysplastic serrated lesion
ICD coding
- ICD-11: 2E92.4Y & DB35.0 - other specified benign neoplasm of the large intestine & hyperplastic polyp of the large intestine
Epidemiology
- Occurs equally in both men and women
- Wide age range but mostly in older patients in the sixth to eighth decades of life
Sites
- May involve the tip or only the midportion, sparing the tip of the appendix (Hum Pathol 2014;45:227)
Pathophysiology
- Appendiceal serrated lesions commonly have KRAS mutations, while BRAF mutations are uncommon
- CpG island methylator phenotype (CIMP) is not usually identified
- Overall, the current data argue that appendiceal serrated lesions are not related to the colorectal serrated pathway of neoplasia, which is characterized by BRAF mutations, DNA methylation and microsatellite instability
- References: Am J Clin Pathol 2005;124:380, Nat Genet 2006;38:787, Hum Pathol 2014;45:227, Am J Surg Pathol 2007;31:1742
Etiology
- Unknown
- Hyperplastic polyps and mucosal hyperplasia may be seen in the postinflammatory reparative setting (i.e., after appendicitis, in diverticular disease involving the appendix or in interval appendectomy)
Clinical features
- Incidental finding detected in appendices removed for other reasons
- Rarely, large hyperplastic polyps may obstruct, leading to appendicitis and possible rupture
Diagnosis
- Usually incidental finding in appendices removed for other reasons
Prognostic factors
- Benign, not clinically relevant
Treatment
- Appendectomy is curative
Gross description
- May form a discrete lesion / polyp or may circumferentially involve the appendiceal mucosa
- Uncommonly recognized on gross examination as a discrete lesion
- Rarely associated with gross dilatation or luminal mucin
- Usually smaller than 1 cm
- References: Am J Surg Pathol 2007;31:1742, Hum Pathol 2014;45:227
Microscopic (histologic) description
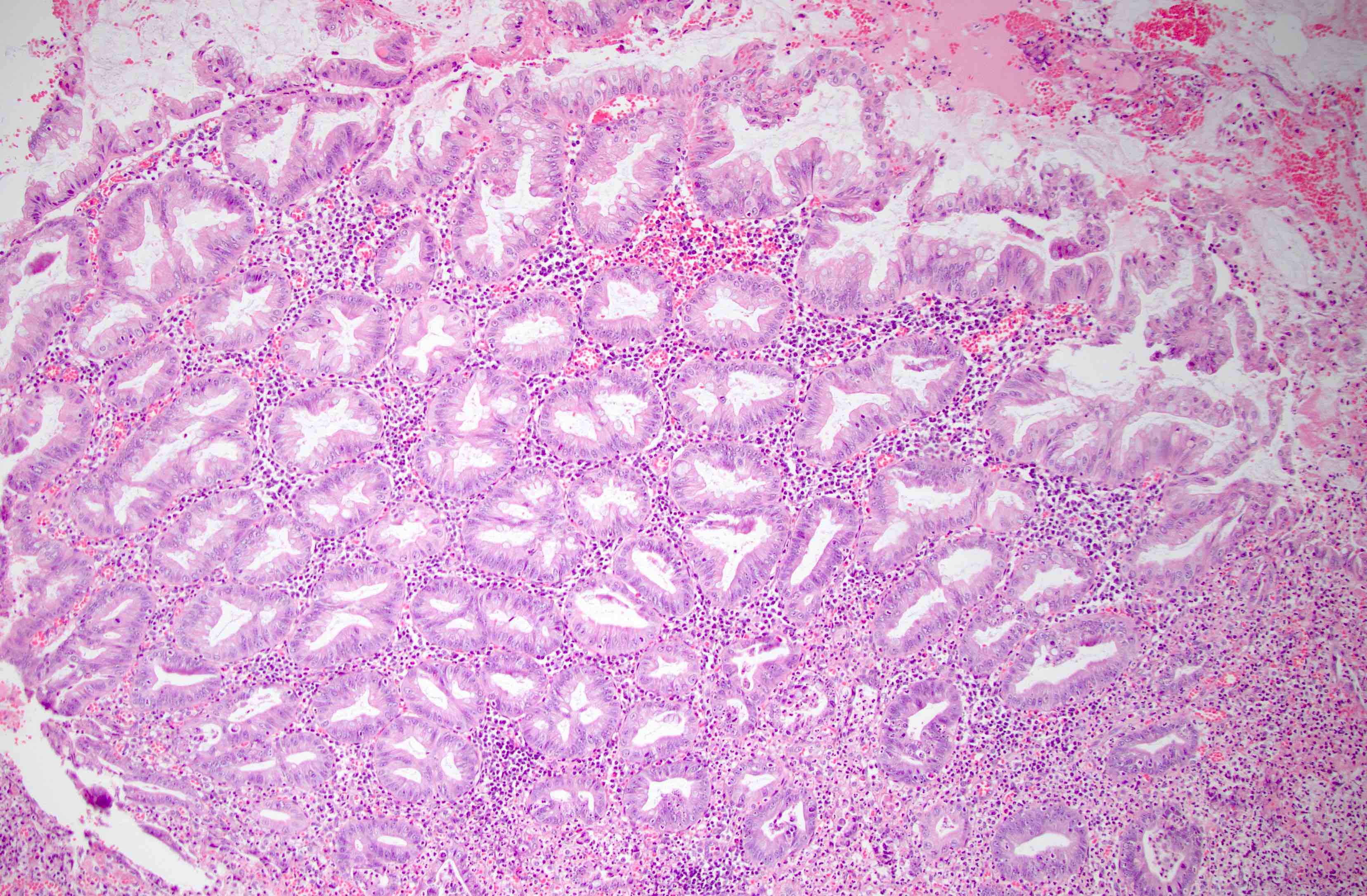
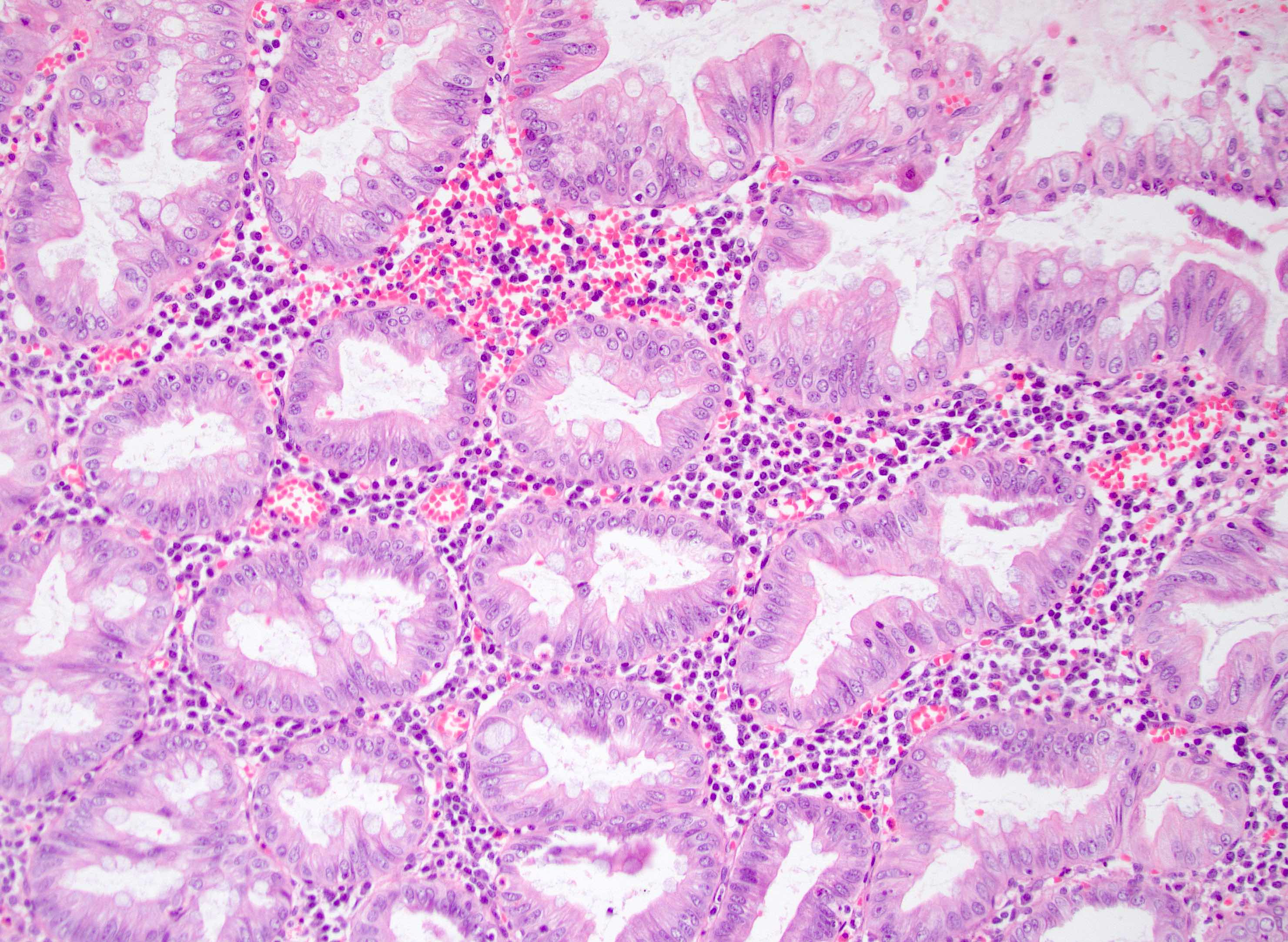
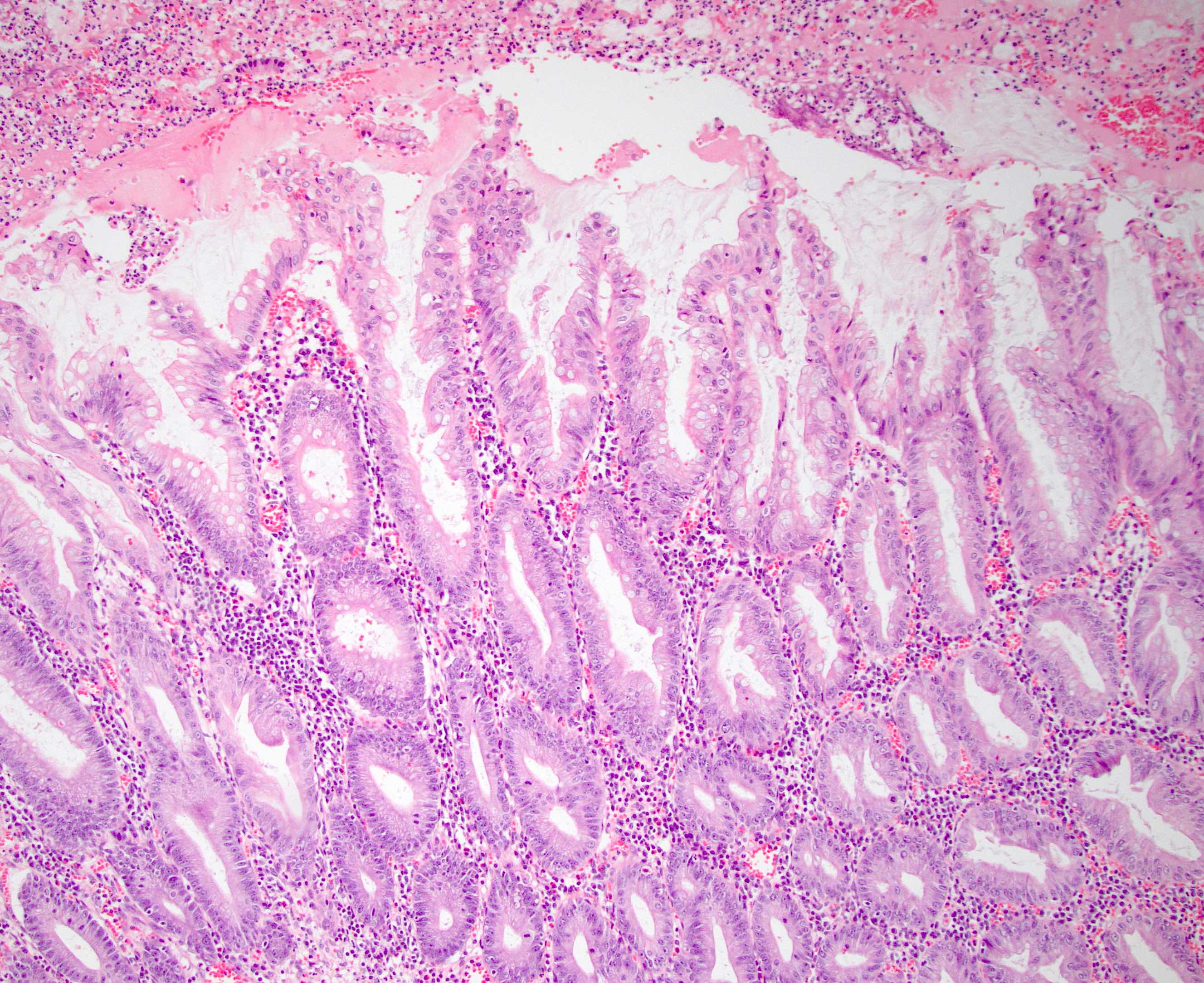
- Morphologically similar to colorectal hyperplastic polyps
- Increased number of goblet cells / mixture of goblet cells with columnar cells with abundant luminal mucin
- Elongated crypts with serration (sawtooth appearance) limited to the luminal aspect of the crypt
- If cytological atypia is present, it is attributed to reactive changes in the deep crypts; cytologic dysplasia is absent
Microscopic (histologic) images
Sample pathology report
- Appendix, appendectomy:
- Hyperplastic polyp
Differential diagnosis
- Serrated lesion with or without cytologic dysplasia:
- Serrated lesions without dysplasia demonstrate serration and dilatation that extends to the base of the crypts with abnormal shapes (i.e., L or inverted T shapes)
- Serrated lesions with dysplasia demonstrate the same architectural features of a serrated lesion with conventional adenoma-like, serrated or traditional serrated adenoma-like dysplasia or a mixture of these subtypes of dysplasia in the same polyp (Am J Clin Pathol 2022;157:180)
- Dysplastic components are usually well demarcated or show a sharp transition from the nondysplastic epithelium
- Hyperplastic polyps, by definition, should not have cytologic dysplasia
- Given the relative morphologic ambiguity in classifying appendiceal serrated lesions, some authors have recommended simply classifying them as dysplastic or nondysplastic serrated lesion (Hum Pathol 2014;45:227, Am J Surg Pathol 2007;31:1742)
- Mucosal hyperplasia:
- May be seen in diverticular disease and postinflammatory settings (i.e., interval appendectomy) (Mod Pathol 2020;33:953)
- Histologically, there are increased numbers of goblet cells within the epithelium with scattered Paneth cells
- Historically associated with concomitant colorectal adenocarcinoma; the finding of mucosal hyperplasia of the appendix may justify excluding this possibility (Histopathology 1995;26:33)
- Mucosal hyperplasia is diffuse and nonpolypoid
Additional references
Practice question #1
Practice answer #1
A. Hyperplastic polyp. This is an incidentally identified hyperplastic polyp in the appendix. There is background acute inflammation indicative of acute appendicitis. Appendiceal hyperplastic polyps are usually found in appendices removed for reasons other than a polypoid lesion. Answers B and D are incorrect because there is no cytologic dysplasia, so this is not a serrated lesion with dysplasia or a tubulovillous adenoma. Answer C is incorrect because a serrated lesion without dysplasia should have architectural features of a serrated lesion, including serrations involving the full thickness of the epithelium and base of the crypts with abnormal inverted T or L shaped crypts.
Comment Here
Reference: Hyperplastic polyp
Comment Here
Reference: Hyperplastic polyp
Practice question #2
Which of the following is true regarding the current molecular understanding of the possible pathogenesis of hyperplastic polyps in the appendix?
- They are clearly related to and caused by the serrated pathway of colorectal neoplasia
- They commonly demonstrate a CpG island methylator (CIMP) phenotype
- They commonly have microsatellite instability
- They more often have KRAS rather than BRAF mutations
Practice answer #2
D. Appendiceal hyperplastic polyps more often harbor a KRAS gene mutation versus a BRAF mutation. Appendiceal hyperplastic polyps do not appear to develop via the serrated pathway of colorectal neoplasia, which is characterized by BRAF mutations, DNA methylation and microsatellite instability. Appendiceal hyperplastic polyps do not typically have a CIMP phenotype.
Comment Here
Reference: Hyperplastic polyp
Comment Here
Reference: Hyperplastic polyp