Table of Contents
Definition / general | Essential features | Clinical features | Radiology description | Radiology images | Prognostic factors | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Sample pathology report | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Li JJX, Tse GM. Neoadjuvant chemotherapy. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignanttreatmenteffect.html. Accessed April 18th, 2024.
Definition / general
- Chemotherapy preceding surgical resection to downstage disease
- Associated with characteristic histological features on the resection specimen, with prognostic implications
Essential features
- The treatment effects associated with neoadjuvant chemotherapy can be seen in the primary tumor (tumor bed), axillary nodal metastases and normal breast tissue
- Neoadjuvant chemotherapy improves survival and functional outcome by allowing resection in initially nonresectable disease or by reducing the extent of surgical resection
- Degree of response (extent of residual disease) is predictive of outcome, in particular, a complete pathologic response without residual tumor detected is indicative of good prognosis (Mod Pathol 2015;28:1185)
Clinical features
- Indications for neoadjuvant chemotherapy include
- Locally advanced disease, to allow resection of unresectable disease
- Disease where breast conservation is not possible but potentially feasible if downstaging is achieved
- Temporary / treatable conditions that contraindicate surgery and thus require bridging therapy (Curr Oncol 2021;28:1338)
- Clinically, node positive disease with response to neoadjuvant chemotherapy and negative sentinel lymph nodes may be spared from axillary dissection (Br J Surg 2021;108:748)
- Grade, stage and biomarker status are additional considerations for neoadjuvant chemotherapy (J Clin Oncol 2021;39:1485)
- Clinical response to neoadjuvant chemotherapy is classified as
- Clinical complete response: complete disappearance of tumor
- Clinical partial response: ≥ 50% reduction greatest dimension of tumor
- Clinically positive nodes may also respond (reduce in size) after neoadjuvant chemotherapy
- Clinical assessment may overestimate tumor size due to posttreatment fibrosis (Radiology 2017;285:358)
- Clinical response is predictive of outcome and correlates with pathological response (J Surg Oncol 2002;80:4, Zhonghua Bing Li Xue Za Zhi 2010;39:734)
Radiology description
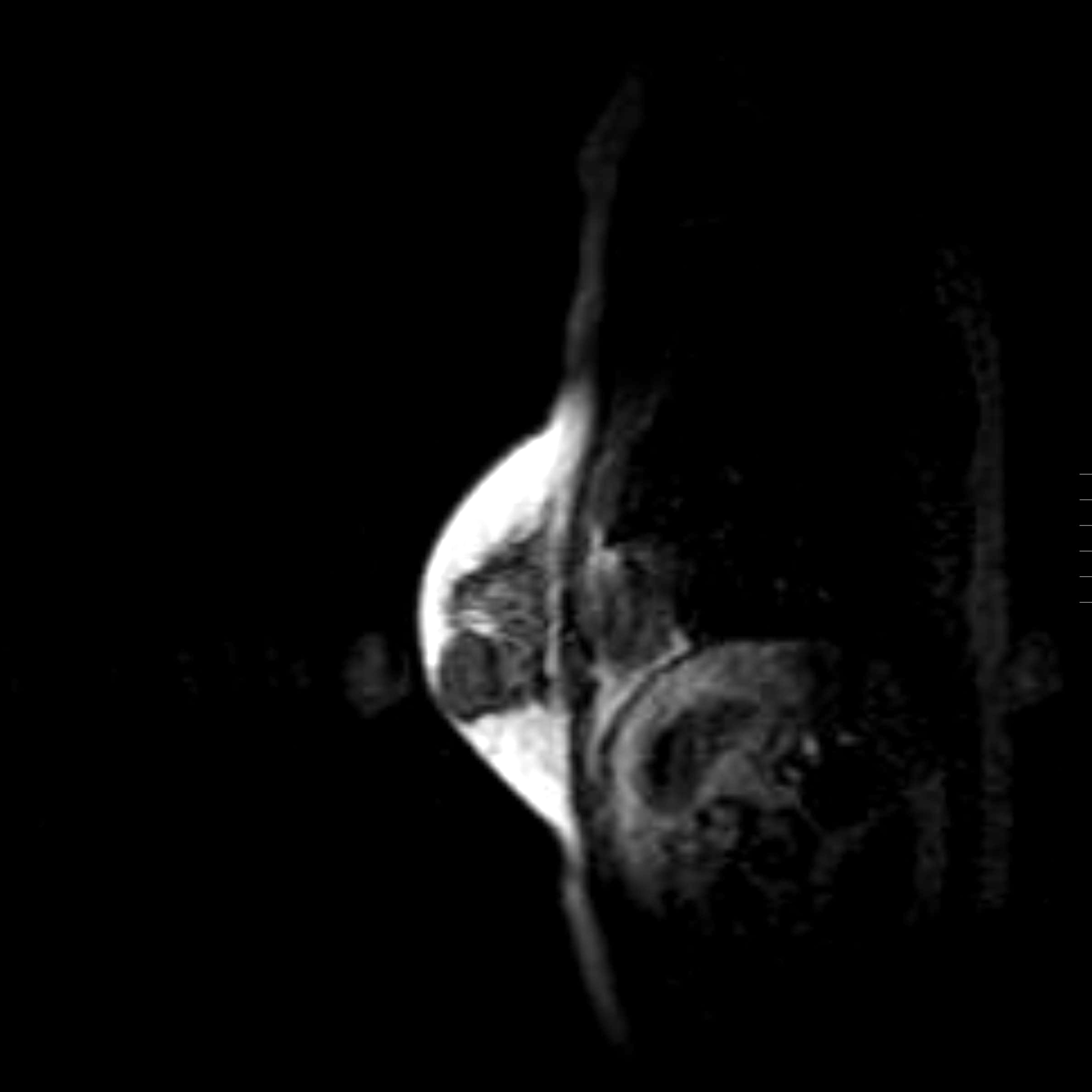
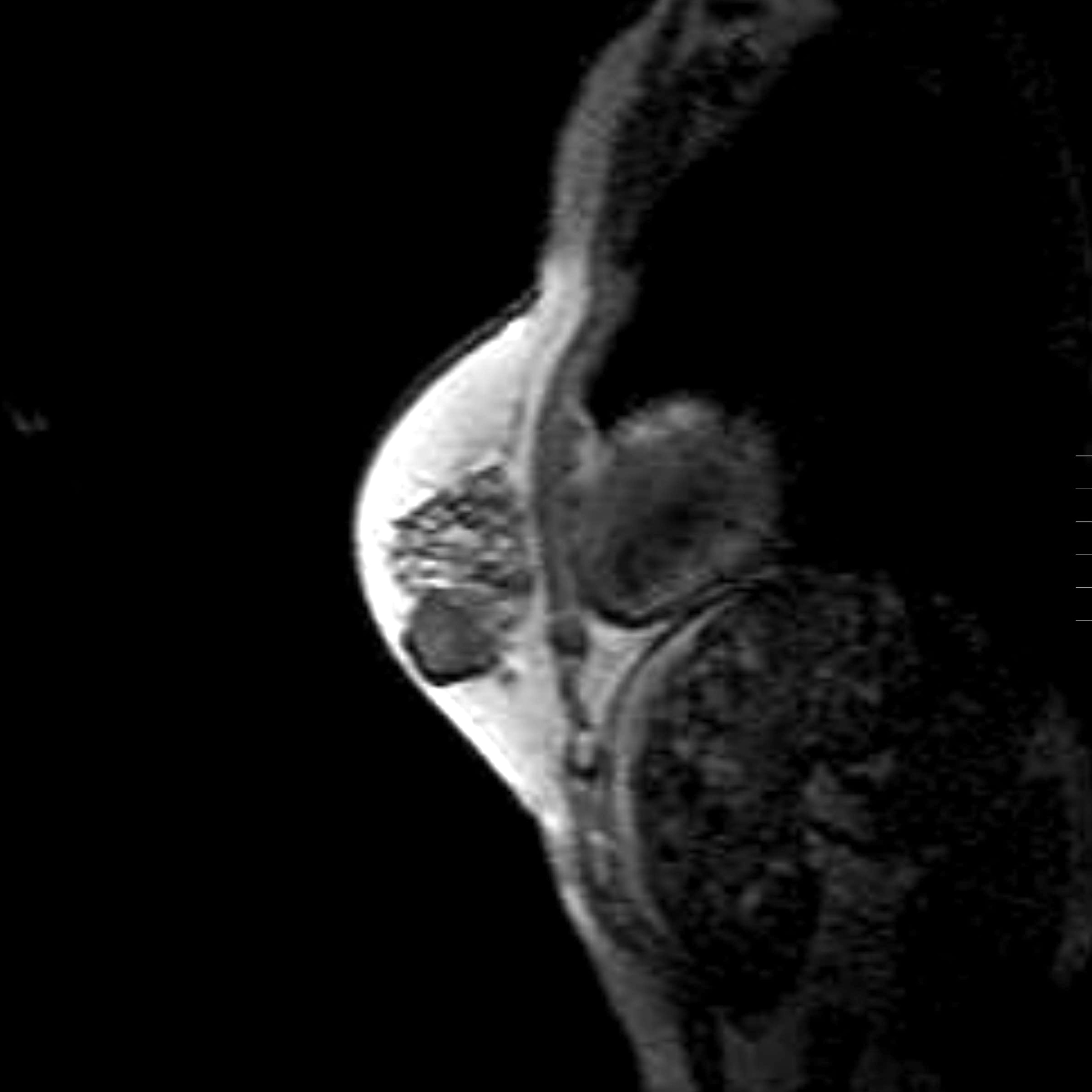
- Conventional imaging modalities for assessment of radiological response includes mammography, ultrasound, MRI
- Radiological response is defined by resolution of original imaging finding (complete response) or by change in lesion size
- Radiological response is predictive of outcome and correlates with pathological response (Eur J Surg Oncol 2021;47:232)
- MRI is more sensitive and is preferred over other imaging modalities (Anticancer Res 2014;34:1219, Insights Imaging 2013;4:163)
- Functional imaging is also used for assessment of radiological response
- Response is defined by normalization of tracer uptake (complete response) or by change in tracer uptake (Radiology 2017;285:358)
- MRI is more accurate in predicting complete pathological response than PET / CT (Oncologist 2016;21:931)
Radiology images
Prognostic factors
- Complete pathologic response demonstrates good prognostic prediction in certain types of breast cancers but not all (J Clin Oncol 2012;30:1796)
- Strong positive correlation with survival in HER2 positive, triple negative and luminal B / HER2 negative (ER / PR positive, HER2 negative, grade 3) breast cancers
- Poor correlation with survival in luminal A (ER / PR positive, HER2 negative) and luminal B / HER2 positive (ER / PR positive, HER2 positive) breast cancers
- Multiple systems for reporting and evaluating response to neoadjuvant chemotherapy have been described (Drug Des Devel Ther 2020;14:2423)
- American Joint Committee on Cancer (classification after preoperative therapy, i.e., prefix "y")
- ypT0 denotes no residual cancer (including in situ carcinoma)
- ypTis denotes residual in situ carcinoma only
- ypT1-4 denotes presence of residual disease
- Residual cancer burden (Mod Pathol 2015;28:1185)
- In situ carcinoma is not considered residual disease but is recommended for reporting
- Miller-Payne (Breast 2003;12:320)
- Residual in situ carcinoma allowed for grade 5 (complete) response
- Chevallier (Am J Clin Oncol 1993;16:223)
- Fisher (J Clin Oncol 1998;16:2672)
- Japanese Breast Cancer Society (Breast Cancer 2008;15:5)
- Pinder (Histopathology 2007;50:409)
- Sataloff (J Am Coll Surg 1995;180:297)
- Sinn (Geburtshilfe Frauenheilkd 1994;54:552)
- American Joint Committee on Cancer (classification after preoperative therapy, i.e., prefix "y")
Gross description
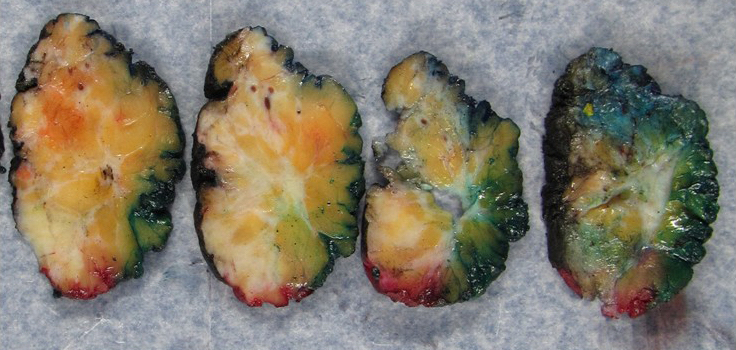
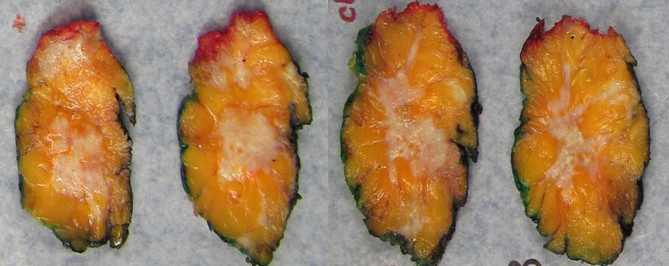
- Gross appearance of tumor may indicate degree of pathological response
- Tumors with poor response demonstrate a firm, gritty and spiculated appearance (i.e., no difference from typical untreated tumors)
- Response can be indicated by fibrosis or softening of the tumor bed (Mod Pathol 2015;28:1185)
- Tumors with partial response can be nodular, multifocal with and less well defined borders (Breast 2022;62:S25)
- In cases of complete response, the tumor bed may be soft and indistinguishable from normal breast tissue
- Tumor mapping with radiographs, photographs or drawings of the sectioned gross specimen is recommended (Mod Pathol 2015;28:1185)
- Considerations in tumor sampling
- Sampling should include all grossly residual disease, visible tumor bed, marker clips and adjacent tissue
- Recommendations on sampling extent are based on pretreatment size and whether residual tumor is grossly identifiable
- Residual carcinoma grossly present
- Usually 1 full face section of largest area and similar full face sections 1 cm apart to investigate the extent of the lesion (Surg Pathol Clin 2018;11:213)
- For very large tumors, 5 representative sections per full face are sufficient (Surg Pathol Clin 2018;11:213)
- No invasive carcinoma grossly identified
- Minimum of 1 block per cm of the pretreatment tumor size or at least 10 blocks in total, whichever is greater (Food and Drug Administration) (Surg Pathol Clin 2018;11:213)
- 5 blocks of a cross section per 1 - 2 cm, up to 25 blocks for very large tumors (Breast International Group and the North American Breast Cancer Group)
- Residual carcinoma grossly present
- In cases where the tumor bed cannot be identified, correlation with preoperative clinical and radiological findings is recommended for determining the location of sampling
Gross images
Microscopic (histologic) description
- Breast
- Multiple reporting systems are described but generally response is classified into
- Pathological complete response (pCR)
- Most widely agreed upon definition of pCR is no residual invasive carcinoma in both the breast and axillary lymph nodes, regardless of the presence of residual DCIS ypT0 ypN0 or ypTis ypN0 (Lancet 2014;384:164, Mod Pathol 2021;34:1271)
- Residual tumor within lymphovascular spaces (lymphovascular invasion) is not allowed for pCR (Mod Pathol 2015;28:1185)
- Currently, presence of in situ cancer after treatment in the absence of residual invasive cancer in the breast and lymph nodes constitutes pCR
- However, there is debate on whether ductal carcinoma in situ should be considered as residual disease due to its association with shorter disease free survival but not overall survival (Mod Pathol 2015;28:1185, J Clin Oncol 2012;30:1796)
- Incomplete response / residual disease: evidence of tumor response but residual tumor cells are present
- Cutoffs for categories vary for different systems
- No response: treatment associated changes are not seen histologically
- Pathological complete response (pCR)
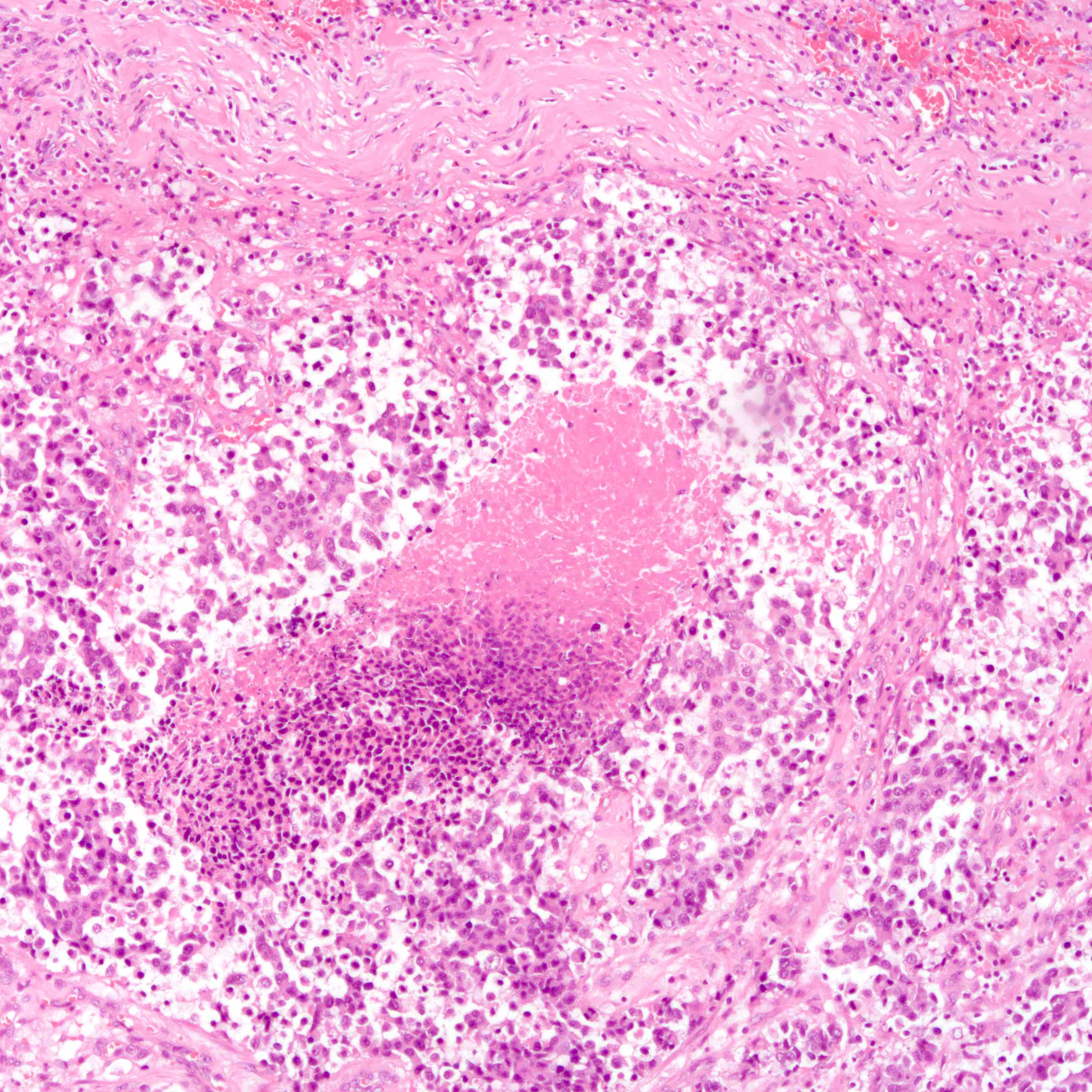
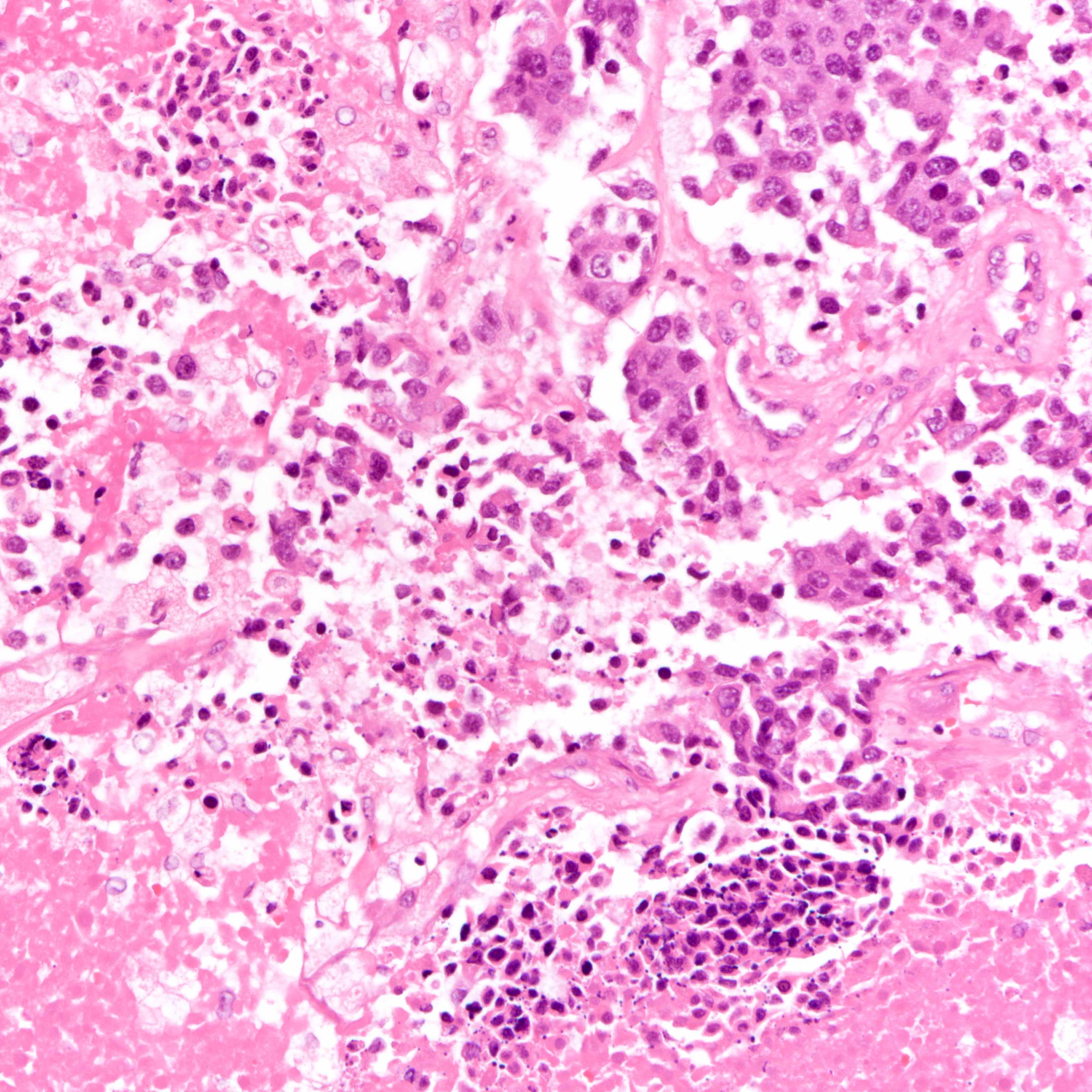
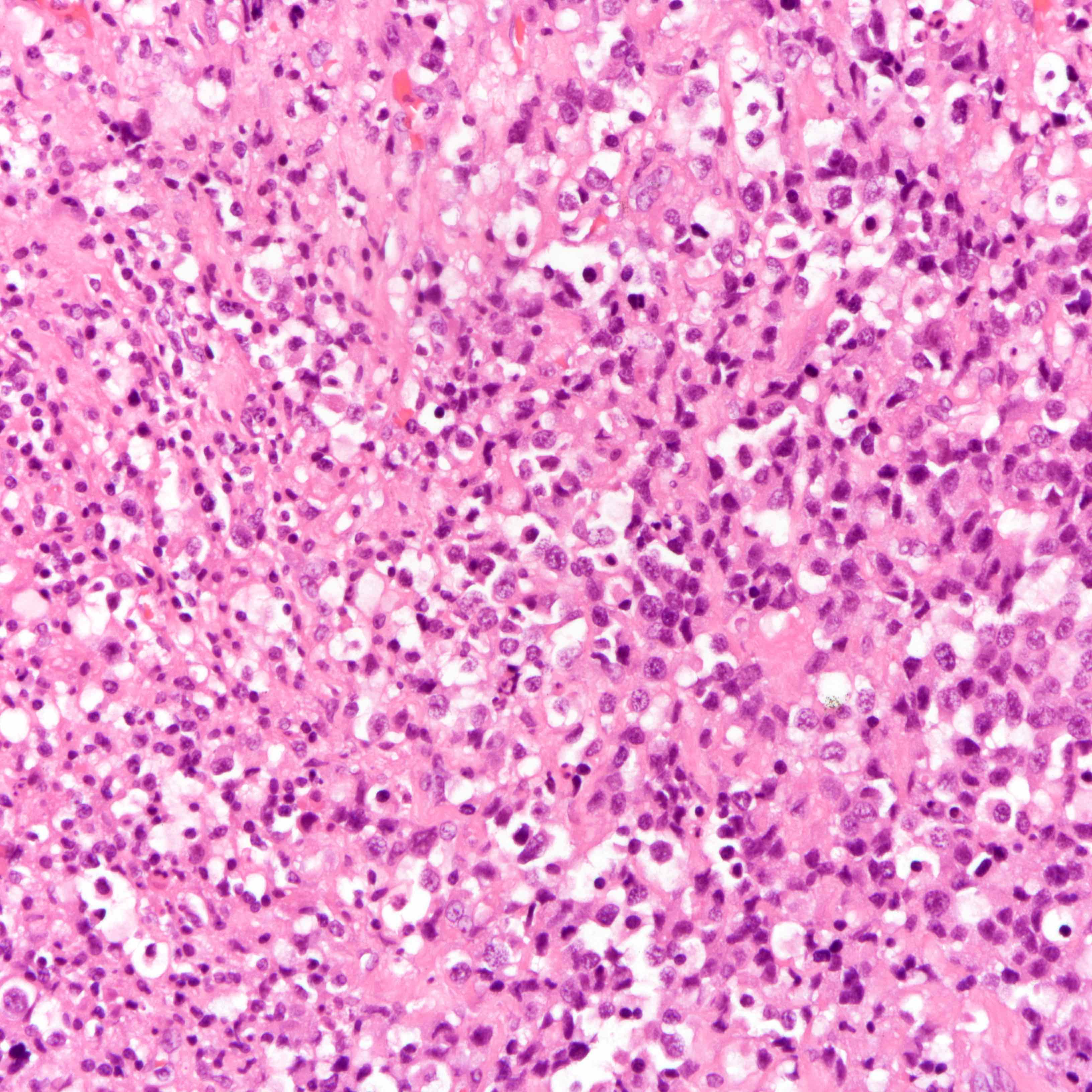
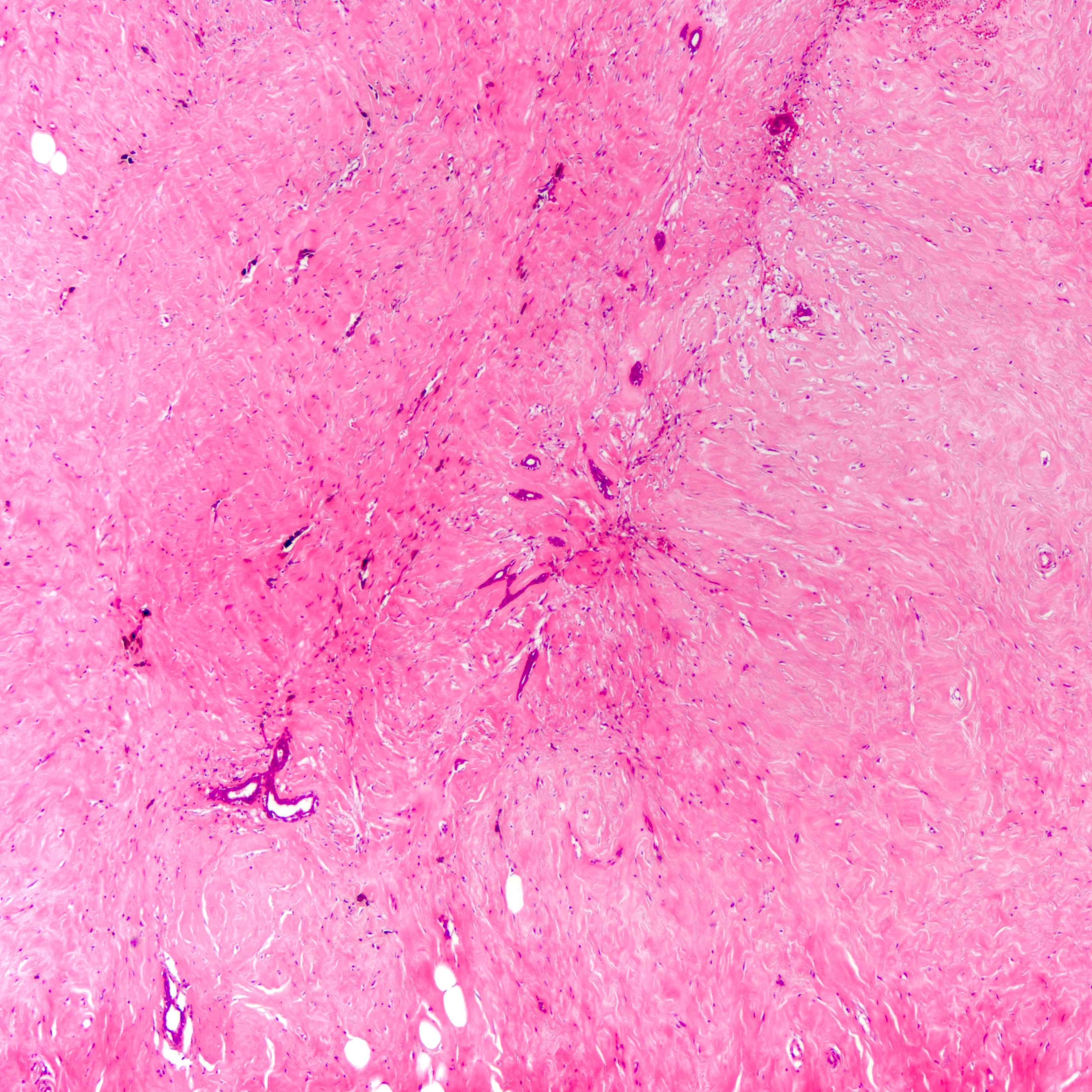
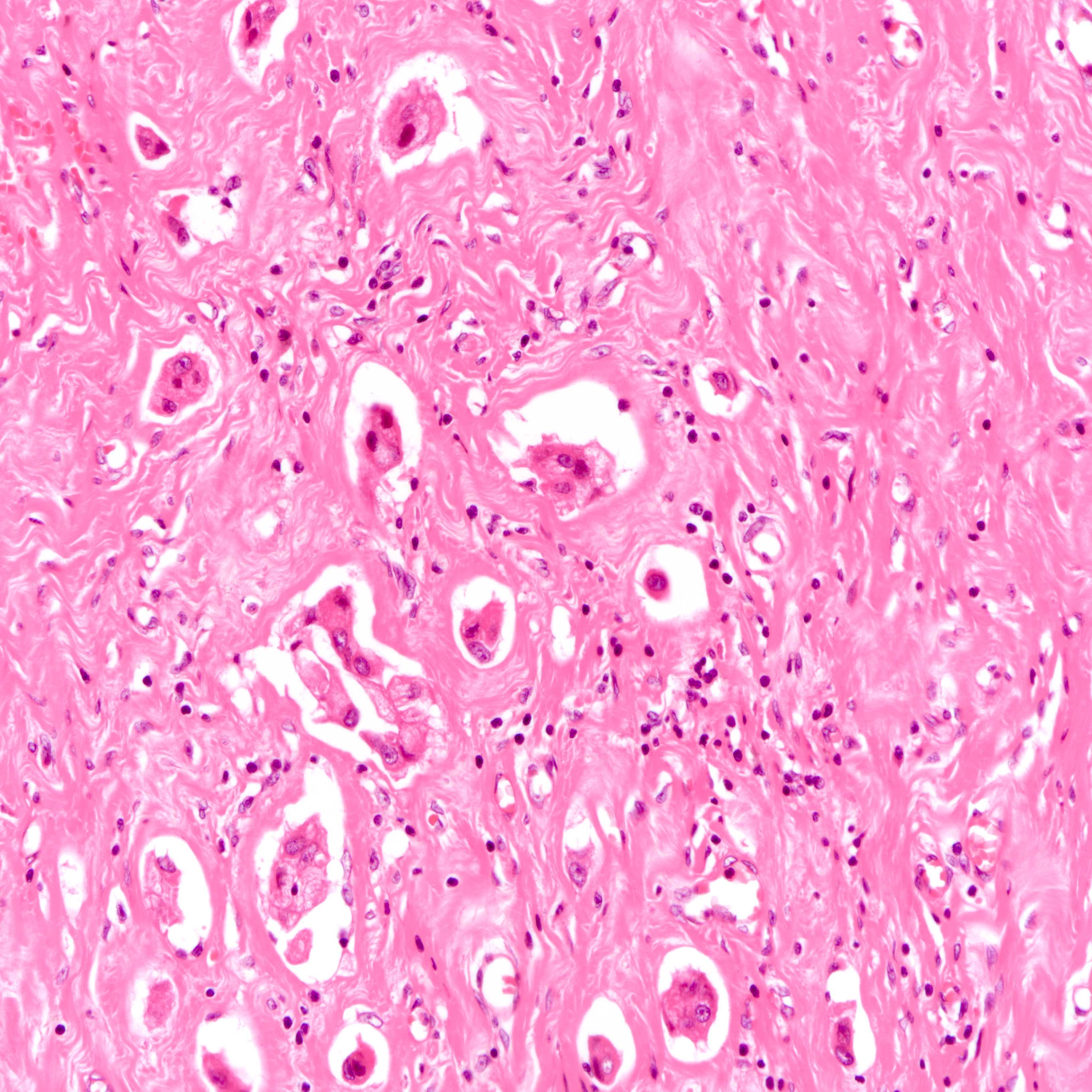
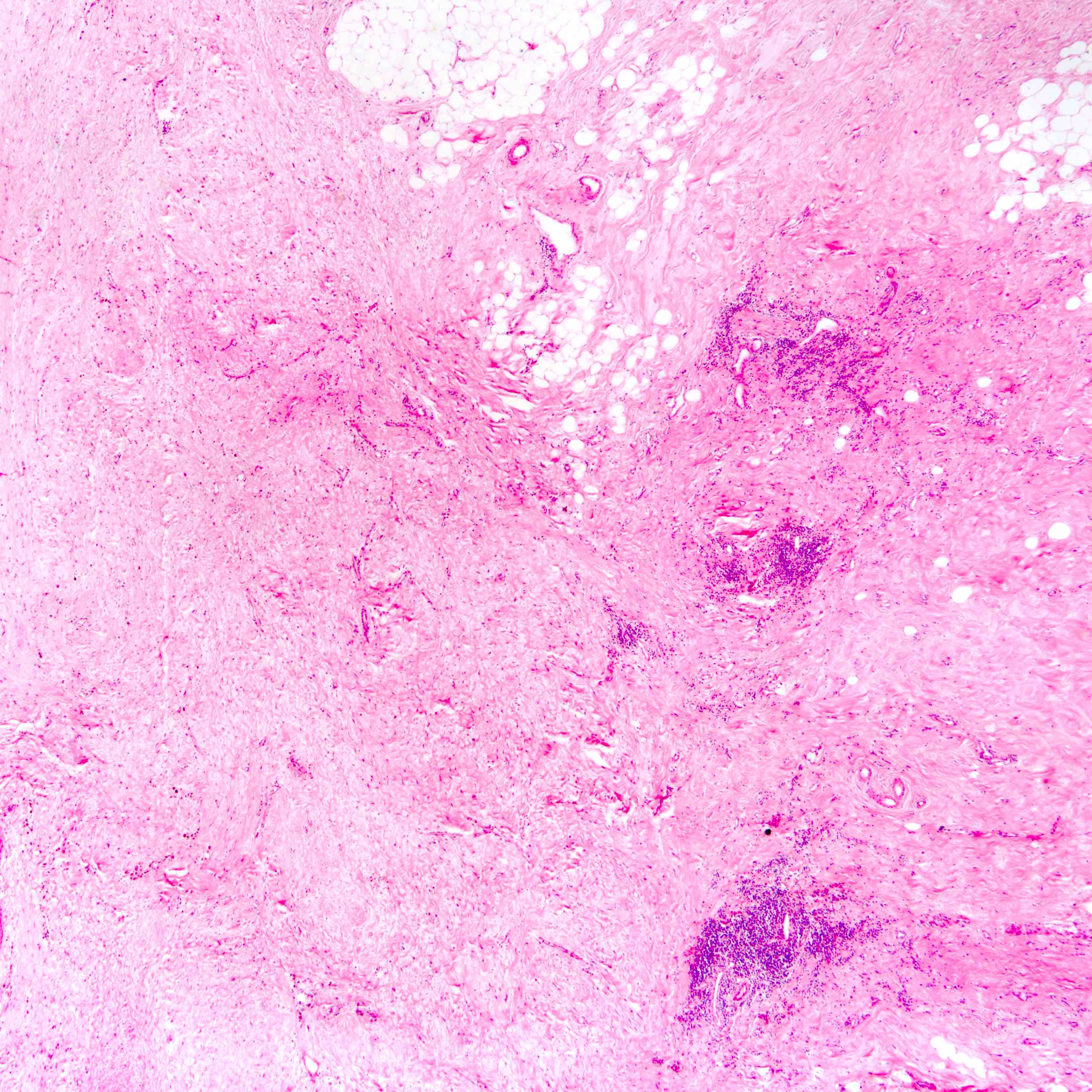
- Histologic evidence of tumor response includes fibrosis, hyalinization, lymphohistiocytic infiltration, hemosiderin laden macrophages, giant cell formation and vascular proliferation
- These histologic features are indicative of the tumor bed
- Patterns observed in partial response includes concentric tumor shrinkage, reduction in tumor cellularity and scattered multifocal tumor deposits (Mod Pathol 2015;28:1185)
- Tumor cells show degenerative changes including nuclear and cytoplasmic vacuolation, karyorrhexis, karyolysis and pyknosis (Int J Appl Basic Med Res 2012;2:111)
- There may be a change in histologic grading after neoadjuvant therapy
- Normal breast tissue shows ductal and lobular atrophy, atypia and hyalinization of vessel wall
- Multiple reporting systems are described but generally response is classified into
- Lymph node
- Possible scenarios include
- Presence of tumor cells, with no evidence of histologic treatment response
- Presence of tumor cells, histologic evidence of treatment response seen
- Absence of tumor cells, histological evidence of treatment response seen
- Absence of tumor cells and histologic evidence of treatment (true negative nodes)
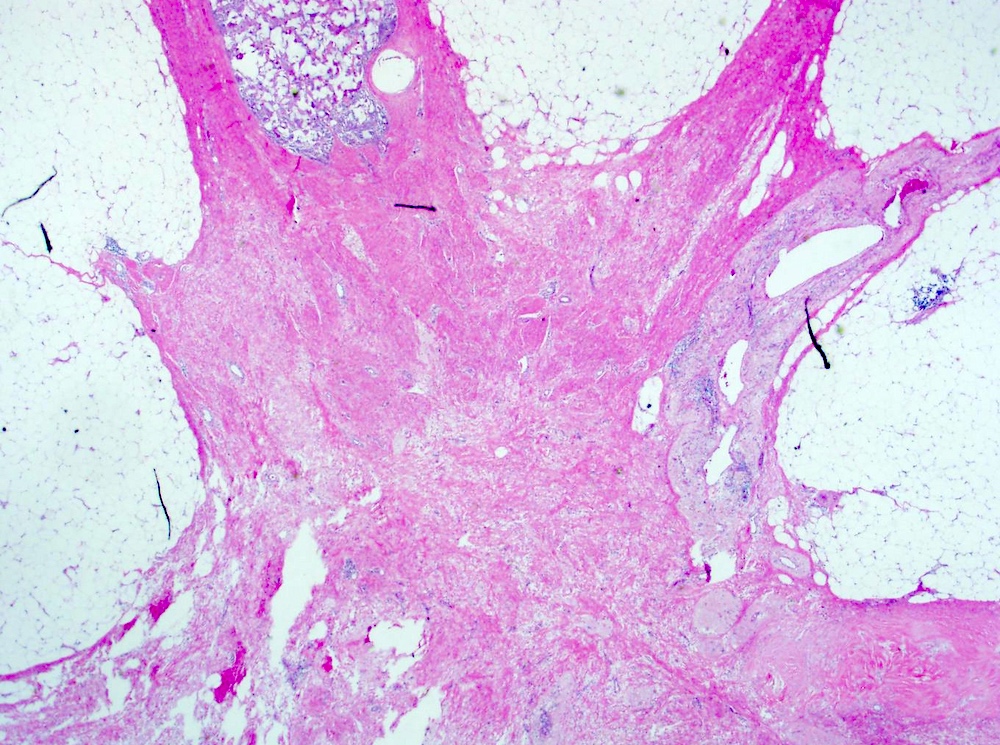
- Histological evidence of tumor response in the lymph node includes fibrosis, mucin pools, histiocytic infiltrates and lymphoid depletion
- Presence of tumor response indicates possible previous nodal involvement and is associated with prognosis intermediate between node negative and node positive patients (Ann Surg Oncol 2003;10:734)
- Residual nodal disease is associated with shorter disease free and overall survival (J Clin Oncol 2012;30:1796)
- Possible scenarios include
Microscopic (histologic) images
Contributed by Joshua J.X. Li, M.B.Ch.B., Gary M. Tse, M.B.B.S. and Emily S. Reisenbichler, M.D.
Virtual slides
Positive stains
- Cytokeratins are also used in assessment of sentinel lymph nodes in a postneoadjuvant chemotherapy setting (Breast 2022;62:S25)
- Entrapped normal breast epithelium in the tumor bed is positive to cytokeratins and should not be confused for residual tumor cells
- Myoepithelial markers may help in distinguishing normal breast epithelium and residual in situ carcinoma from residual invasive carcinoma (Arch Pathol Lab Med 2011;135:422)
- Histiocytic markers (CD68, CD163) may help distinguish residual invasive carcinoma from infiltrating histiocytes in the tumor bed (Histopathology 2015;67:279)
- Endothelial markers (CD31, ERG) may help distinguish residual invasive carcinoma from lymphovascular invasion
- Entrapped normal breast epithelium in the tumor bed is positive to cytokeratins and should not be confused for residual tumor cells
- Biomarker status may change after neoadjuvant chemotherapy (Pathobiology 2022;89:297)
Sample pathology report
- Left breast, local excision:
- No residual tumor (complete pathologic response) (see comment)
- Comment: Sections of the tumor bed shows fibrosis and atrophic changes, with lymphohistiocytic infiltrates and hemosiderin deposition. No viable tumor cells are seen, indicating a pathologic complete response to neoadjuvant chemotherapy. The remaining breast tissue is unremarkable.
- Left breast, local excision:
- Residual invasive breast carcinoma, NST type, with therapy effect status postneoadjuvant chemotherapy, indicating partial / incomplete response (see comment)
- Comment: Sections show residual invasive carcinoma of ______ (histotype) and _______ (grade). Fibrosis, lymphohistiocytic infiltrates and hemosiderin deposition in keeping with treatment effects are / are not noted. The residual tumor cellularity is ______%. In situ carcinoma is / is not noted and comprises ______% of the residual tumor cellularity. Lymphovascular invasion is / is not identified. The tumor measures ______ mm and is clear from (specify if < 1 cm ____) / involves the resection margins.
- Left axillary dissection:
- __ out of ___ lymph nodes involved by metastatic carcinoma (see comment)
- Comment: Sections show a total of ____ lymph nodes. ____ lymph nodes show metastatic carcinoma with ____ showing fibrosis, mucin pools and histiocytic infiltrates, suggestive of treatment response. Extracapsular spread is / is not seen. Similar features are seen / not seen in ___ of the remaining benign lymph nodes.
Board review style question #1
Which of the following cases is considered a complete pathologic response to neoadjuvant chemotherapy?
- Absence of invasive carcinoma in the tumor bed but isolated tumor cells present in an axillary lymph node
- Absence of invasive carcinoma in the tumor bed but with viable tumor cells present in lymphovascular spaces of quadrant samplings
- Fibrous tumor bed with adjacent lobules showing atypical epithelial lining cells
- Significant tumor necrosis in the tumor bed with rare viable invasive carcinoma
- Small clusters of invasive carcinoma constituting < 5% of cellularity among a fibrous tumor bed
Board review style answer #1
C. Fibrous tumor bed with adjacent lobules showing atypical epithelial lining cells. Complete pathologic response is defined as the absence of residual tumor cells. Presence of any viable tumor cells, regardless of cellularity, in the tumor bed, lymph nodes or lymphovascular spaces should not be considered as complete pathologic response. Chemotherapy can result in atypia in normal breast epithelium and should not be confused with residual disease.
Comment Here
Reference: Neoadjuvant chemotherapy
Comment Here
Reference: Neoadjuvant chemotherapy
Board review style question #2
In which of the following scenarios is neoadjuvant chemotherapy generally considered?
- Early stage, low grade, hormone positive / HER2 negative invasive breast cancer
- Patient with ductal carcinoma in situ of the breast
- Patient with local invasive breast cancer and active hepatitis B infection
- Patient with radiological evidence of bone metastasis with biopsy proven stage IV breast cancer
- Patient with suspected muscle invasive breast cancer
Board review style answer #2
E. Patient with suspected muscle invasive breast cancer. General contraindications to chemotherapy apply to neoadjuvant chemotherapy, including active hepatitis. Patients with unresectable disease or bulky nodal disease are also not indicated for neoadjuvant chemotherapy. The current role of adjuvant therapy in phyllodes tumor is unclear and is generally not offered (Histopathology 2016;68:5). T4 disease (muscle invasion) is not a contraindication to neoadjuvant therapy.
Comment Here
Reference: Neoadjuvant chemotherapy
Comment Here
Reference: Neoadjuvant chemotherapy