Table of Contents
Definition / general | Essential features | ICD coding | Diagrams / tables | Pathophysiology | Clinical features | Test indications | Laboratory | Immunofluorescence description | Immunofluorescence images | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Li JS, Shi J. Systemic lupus erythematosus (SLE). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/chemistrysle.html. Accessed April 20th, 2024.
Definition / general
- Systemic lupus erythematosus (SLE) is a chronic autoimmune disease marked by autoantibodies or immune complex deposits causing inflammation and damage in organs like the kidneys, heart, blood vessels, central nervous system (CNS), skin, lungs, muscles and joints; its severity varies from mild to life threatening (Ann Rheum Dis 2010;69:1603)
Essential features
- Antinuclear antibodies (ANAs) are present in systemic and organ specific diseases; ANA testing is important for diagnosing specific autoimmune conditions but not suitable for general screening due to poor sensitivity
- SLE patients typically have a strongly positive ANA test (≥ 1:80 titer); an ANA screening immunoassay or HEp-2 immunofluorescence with comparable performance is recommended
- Points are assigned based on 7 clinical categories and 3 immunologic test categories once the ANA criterion is met
- Exclusions apply if another cause is more likely than SLE and criteria need not occur simultaneously
- Score of ≥ 10 is indicative of SLE
ICD coding
- ICD-10: M32.9 - systemic lupus erythematosus, unspecified
Diagrams / tables
Pathophysiology
- In SLE, multiple organs are affected due to autoimmune related inflammation
- This damage results from immune system characteristics (specifically, the loss of nucleic acid tolerance and interferon system activation)
- SLE hallmarks are autoantibodies against nucleic acids (notably targeting dsDNA and RNA / RNA binding proteins) and an interferon stimulated gene (ISG) signature in blood and affected tissues
- Recent findings show that nucleic acids bound to SLE related autoantibodies trigger interferon production in plasmacytoid dendritic cells (pDCs) upon endocytosis
- This link highlights the role of endosomal Toll-like receptor (TLR) in SLE
- Additionally, interferon's diverse impact on dendritic cell (DC) and B cell differentiation has been reported (Ann Rheum Dis 2023;82:999)
- See Diagrams / tables for an overview of SLE pathogenesis
Clinical features
- SLE symptoms include fatigue, fevers, skin rashes and joint pain / swelling
- Flares and remissions occur variably
- Additional symptoms involve sun sensitivity, oral ulcers, arthritis, lung / heart / kidney problems, seizures, psychosis and blood / immune abnormalities
- See Diagrams / tables for a summary of clinical features
- Reference: Arthritis Rheumatol 2019;71:1400
Test indications
- Early SLE signs and symptoms lack specificity
- Rheumatologists need to use specific criteria for final diagnosis
- Women, especially ages 15 - 44, are at the highest risk for SLE (Arthritis Rheum 2012;64:2319)
- Black, Hispanic / Latino, Asian and American Indian people / Alaska Natives are more affected than White people (Imboden: Current Diagnosis & Treatment in Rheumatology, 3rd Edition, 2013)
- New 2019 European League Against Rheumatism (EULAR) / American College of Rheumatology (ACR) classification criteria allow earlier and more accurate SLE classification; these criteria are detailed in Diagrams / tables (Arthritis Rheumatol 2019;71:1400)
- ANA test positivity (≥ 1:80) is an entry criterion; ANA screening immunoassay or HEp-2 (human epidermoid carcinoma cells) immunofluorescence is recommended
- Points are assigned based on 7 clinical and 3 immunologic categories once the ANA criterion is met
- Exclusion happens if another cause seems more likely than SLE; criteria do not need to occur simultaneously
- Score ≥ 10 indicates SLE
Laboratory
- Common hematologic manifestations include anemia of chronic disease, autoimmune hemolytic anemia, leukopenia and thrombocytopenia
- Low complement C3 or C4 levels
- Antiphospholipid antibodies can be detected
- Strongly positive ANA is associated with SLE but it lacks specificity
- Can be seen in some healthy individuals (especially older female) as well as those with other autoimmune conditions, certain cancers and medications
- ANA detection relies on indirect immunofluorescence assay (IFA) using HEp-2 cells and solid phase assays
- Both methods have limitations that reduce antibody detection sensitivity; clinicians should understand these assays' strengths and weaknesses used in their laboratory
- In 2009, due to concerns regarding solid phase assay sensitivity, the ACR recommended initial ANA testing using IFA with HEp-2 cells (Ann Rheum Dis 2010;69:1420, Clin Chem Lab Med 2023;61:1167)
- 2019 EULAR / ACR classification criteria strongly advocated IFA ANA testing as an entry criterion
- IFA and solid phase immunoassays for ANA testing involves the following steps
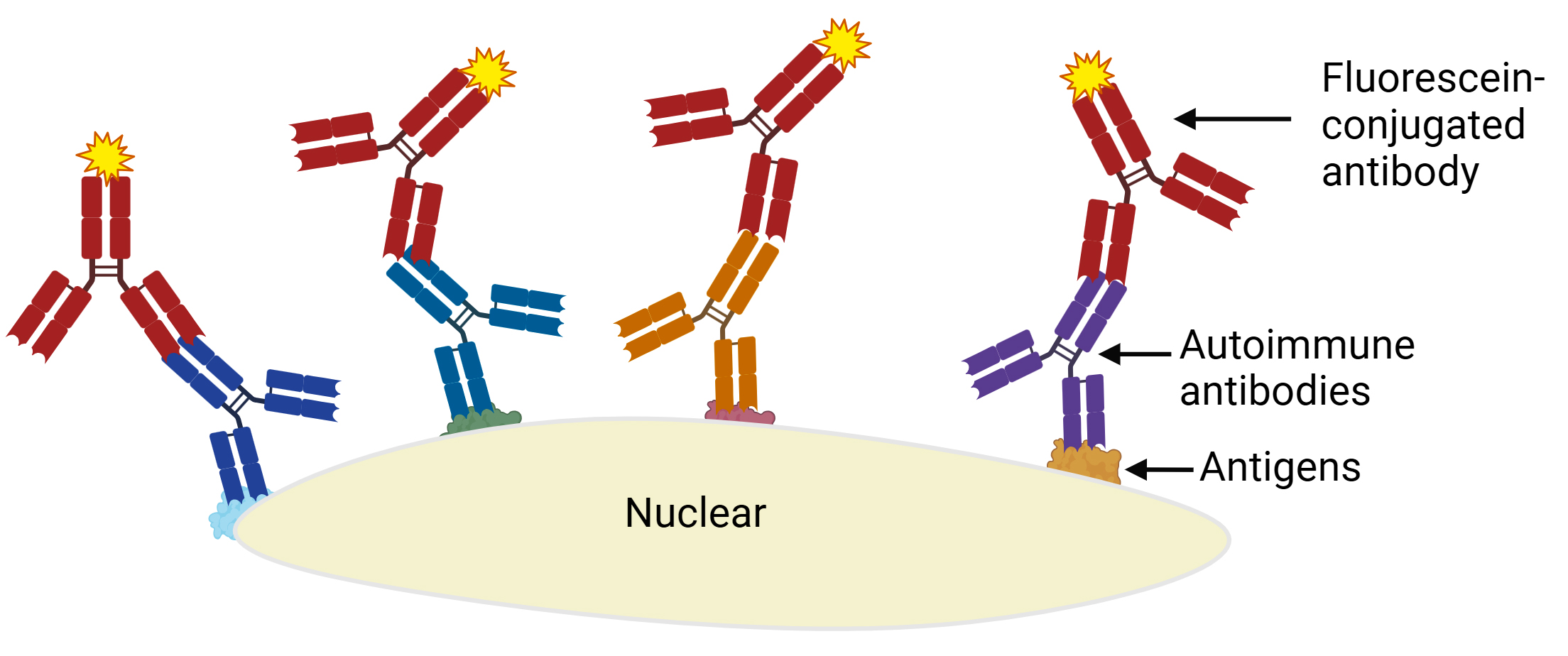
- IFA utilizes a HEp-2 cell line characterized by large nuclei, aiding in the visualization of staining patterns; these cells incorporate nearly all clinically relevant autoantigens, making them suitable for detecting corresponding autoantibodies
- Process involves fixing cells on glass slides, incubating them with patient serum, washing off nonadherent proteins and introducing fluorescein conjugated antibodies that bind to human antibodies interacting with HEp-2 cell antigens (see Diagrams / tables)
- ANA staining patterns in the nucleus, like homogeneous, speckled, nucleolar, dot and nuclear envelope patterns, are observed using an ultraviolet microscope (see Immunofluorescence images)
- Upon initial fluorescence detection at 1:80 dilution, serum undergoes serial dilution until fluorescence is detectable in less than half of the cells
- Advantages include broad autoantibody detection, aiding SLE diagnosis
- However, interpreting results requires skilled technicians; automated analyzers simplify the process but ANA patterns lack specificity, often requiring further solid phase testing
- Some autoantigens may not be detectable in HEp-2 cells, leading to potential false negatives
- Efforts to standardize ANA pattern nomenclature are ongoing through the International Consensus on Autoantibody Patterns (ICAP)
- Solid phase assays include enzyme linked immunosorbent assay (ELISA), fluorescent microsphere and immunoline assays, utilizing fixed autoantigens on surfaces to detect serum autoantibodies categorized as positive, indeterminate or negative
- These assays involve fixing autoantigens to a solid surface, incubating patient serum and using a labeled secondary antibody to detect bound autoantibodies
- They are efficient for high throughput ANA tests, identifying specific autoantibodies rather than patterns
- However, they might lack sensitivity compared to IFA, using fewer antigens and potentially reducing SLE diagnostic sensitivity (Arthritis Rheumatol 2019;71:1400)
- IFA utilizes a HEp-2 cell line characterized by large nuclei, aiding in the visualization of staining patterns; these cells incorporate nearly all clinically relevant autoantigens, making them suitable for detecting corresponding autoantibodies
- SLE specific antibodies
- Anti-double stranded (dsDNA) antibodies
- Discovered in 1957
- Have long been established as diagnostic markers for systemic lupus erythematosus (SLE)
- Serve as reliable indicators of disease activity in SLE
- Current methodologies for detecting anti-dsDNA antibodies include Farr radioimmunoassay, Crithidia luciliae indirect immunofluorescence test (CLIFT), enzyme linked immunosorbent assay (ELISA), fluoroenzyme immunoassay (FEIA) and chemiluminescent immunoassay (CIA)
- Anti-Smith antibodies
- First identified in 1966
- Exhibit high specificity for SLE and are often detectable prior to diagnosis
- Several techniques are available for detecting anti-Sm antibodies
- While immunoprecipitation using a radioactive immunoassay (RIA) is considered the gold standard, in routine practice, alternative methods such as indirect immunofluorescence tests (IIFT), enzyme linked immunosorbent assays (ELISA), addressable laser bead immunoassays (ALBIA), line immunoassays (LIA), chemiluminescent immunoassays (CLIA) and fluorescent enzyme immunoassays (FEIA) are more commonly used
- Anti-double stranded (dsDNA) antibodies
Immunofluorescence description
- See Laboratory
Board review style question #1
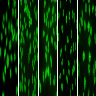
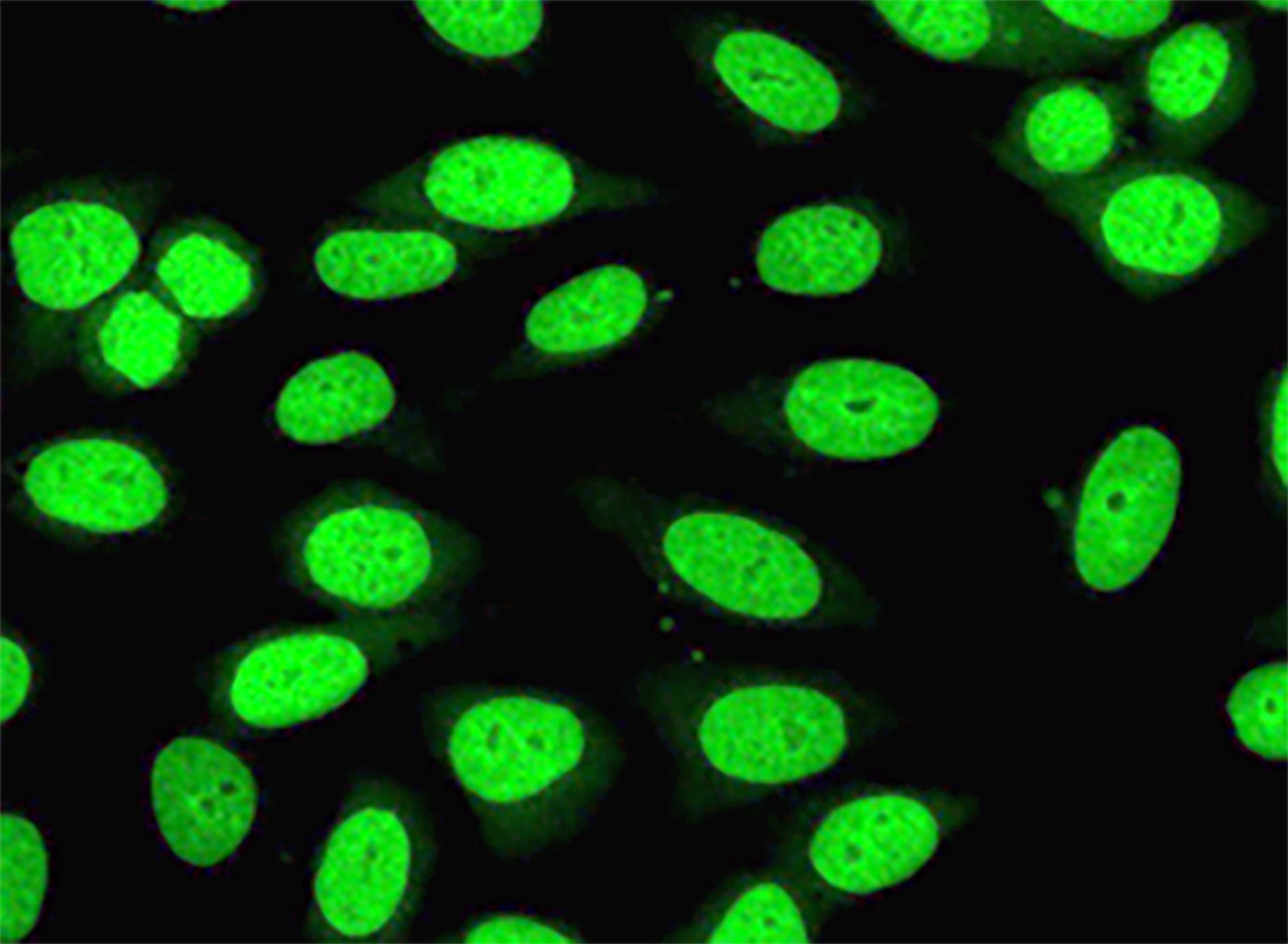
A 52 year old South American woman presented with fever (102 °F) and fatigue. She also has arthralgias and Raynaud phenomenon. Her primary care provider ordered an antinuclear antibody (ANA) screening test; the pattern on HEp-2 cells with serum diluted to 1:80 is shown in the figure. What is the ANA pattern?
- Centromere
- Homogeneous
- Negative
- Nucleolar
- Speckled
Board review style answer #1
B. Homogeneous. This is a homogeneous pattern, demonstrating homogenous and regular fluorescence across all nucleoplasms. The nucleoli may be stained or not stained depending on cell substrate. Mitotic cells (metaphase, anaphase and telophase) have the chromatin mass intensely stained in a homogeneous hyaline fashion (ICAP: ANA Patterns [Accessed 25 January 2024]). Answers A, C, D and E are incorrect because the pattern in the image does not match these choices.
Comment Here
Reference: Systemic lupus erythematosus (SLE)
Comment Here
Reference: Systemic lupus erythematosus (SLE)
Board review style question #2
The IFA ANA result (> 1:80) was interpreted as homogeneous pattern in a 52 year old South American woman who presented with fever (102 °F) and fatigue, with arthralgias and Raynaud phenomenon. The provider ordered routine extractable nuclear antigen (ENA) profile test (Ro [SSA], La [SSB], Sm [Smith], RNP, Scl-70 and Jo1). Both Sm and RNP were found to be positive. What condition or diagnosis would you suspect for this patient?
- Rheumatoid arthritis
- Scleroderma
- Sjögren syndrome
- Systemic lupus erythematosus (SLE)
Board review style answer #2
D. Systemic lupus erythematosus (SLE). According to the 2019 EUALR / ACR new classification criteria, a positive ANA test with a titer of ≥ 1:80 and a score of ≥ 10 is indicative of SLE.
Answer A is incorrect because it remains uncertain how this information could contribute to the clinical management of rheumatoid arthritis. Currently, ANA positivity is not utilized as a screening tool for diagnosing rheumatoid arthritis. There has been no extensive population based cohort study investigating ANA positivity specifically in rheumatoid arthritis patients.
Answer B is incorrect because there is no clear evidence supporting a diagnosis of systemic sclerosis, based on this patient's testing results and clinical presentation. When scleroderma affects both the skin and internal organs, it is referred to as systemic sclerosis. Antinuclear antibodies (ANAs) are present in ~95% of systemic sclerosis patients, making ANA testing a crucial initial step in disease assessment. The observed ANA patterns in systemic sclerosis can vary widely, including homogeneous, centromere, nucleolar, speckled nuclear patterns or reticular / ANA cytoplasmic pattern; however, the results of an ANA test alone are not sufficient for a scleroderma diagnosis. According to the European League Against Rheumatism (EULAR) / American College of Rheumatology (ACR) criteria for the classification of systemic sclerosis, positivity for anticentromere, antitopoisomerase I or anti-Sd 70 antibodies is typical.
Answer C is incorrect because there is no clear evidence supporting a diagnosis of Sjögren syndrome, based on this patient's testing results and clinical presentation. For individuals presenting with persistent symptoms of dry eyes or mouth, an unexplained increase in dental caries, parotid gland enlargement or abnormal results of specific serologic tests (e.g., anti-Ro / SSA antibodies with or without anti-La / SB antibodies, rheumatoid factor and hyperglobulinemia), Sjögren syndrome should be considered.
Comment Here
Reference: Systemic lupus erythematosus (SLE)
Answer A is incorrect because it remains uncertain how this information could contribute to the clinical management of rheumatoid arthritis. Currently, ANA positivity is not utilized as a screening tool for diagnosing rheumatoid arthritis. There has been no extensive population based cohort study investigating ANA positivity specifically in rheumatoid arthritis patients.
Answer B is incorrect because there is no clear evidence supporting a diagnosis of systemic sclerosis, based on this patient's testing results and clinical presentation. When scleroderma affects both the skin and internal organs, it is referred to as systemic sclerosis. Antinuclear antibodies (ANAs) are present in ~95% of systemic sclerosis patients, making ANA testing a crucial initial step in disease assessment. The observed ANA patterns in systemic sclerosis can vary widely, including homogeneous, centromere, nucleolar, speckled nuclear patterns or reticular / ANA cytoplasmic pattern; however, the results of an ANA test alone are not sufficient for a scleroderma diagnosis. According to the European League Against Rheumatism (EULAR) / American College of Rheumatology (ACR) criteria for the classification of systemic sclerosis, positivity for anticentromere, antitopoisomerase I or anti-Sd 70 antibodies is typical.
Answer C is incorrect because there is no clear evidence supporting a diagnosis of Sjögren syndrome, based on this patient's testing results and clinical presentation. For individuals presenting with persistent symptoms of dry eyes or mouth, an unexplained increase in dental caries, parotid gland enlargement or abnormal results of specific serologic tests (e.g., anti-Ro / SSA antibodies with or without anti-La / SB antibodies, rheumatoid factor and hyperglobulinemia), Sjögren syndrome should be considered.
Comment Here
Reference: Systemic lupus erythematosus (SLE)