Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Merve A. Choroid plexus tumors (papilloma, atypical papilloma, carcinoma). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumorchoroidplexuspapillomas.html. Accessed April 19th, 2024.
Definition / general
- Choroid plexus tumors are rare neoplasms derived from the choroid plexus epithelium
- They occur within the ventricle system of the brain
Essential features
- Rare intracranial tumor arising in the ventricle, mainly occurring in children
- 3 histological grades (WHO grade 1, 2, 3): choroid plexus papilloma (CPP), atypical choroid plexus papilloma (aCPP), choroid plexus carcinoma (CPC)
- Histological classification is based on architecture (preservation of papillary pattern), cellular density, cytology (nuclear pleomorphism), proliferation (mitoses) and necrosis / brain invasion
- Diagnostically, transthyretin (TTR), KIR7.1, cytokeratin and Ki67 immunohistochemistry are most helpful
- Based on methylation profiling, these tumors are now categorized in to 3 subtypes
- In line with other CNS tumors, integrated phenotype genotype diagnosis is preferred, which will guide appropriate management
Terminology
- Conventionally, as per current WHO classification, these tumors are termed as choroid plexus papilloma (CPP, WHO grade 1), atypical choroid plexus papilloma (aCPP, WHO grade 2) and choroid plexus carcinoma (CPC, WHO grade 3) (Acta Neuropathol 2016;131:803)
- Based on recent DNA methylation profiling, they are being classified into 3 prognostically relevant epigenetic subgroups: supratentorial pediatric low risk choroid plexus tumors (CPP and aCPP), infratentorial adult low risk choroid plexus tumors (CPP and aCPP) and supratentorial pediatric high risk choroid plexus tumors (CPP and aCPP and CPC) (J Neurooncol 2020;148:39)
ICD coding
Epidemiology
- Predominantly occurs in children (J Neurooncol 2015;121:151)
- Accounts for 2 - 5% of all pediatric brain tumors (J Neurosurg Pediatr 2012;10:398)
- Relatively higher incidence in infants (J Neurooncol 2015;121:151)
- M:F = 1.2:1 (J Neurooncol 2015;121:151)
- Genetic susceptibility:
- Aicardi syndrome (probable X chromosome abnormalities) (Brain Dev 2005;27:164)
- Li-Fraumeni syndrome (germ line TP53 mutation) (J Clin Oncol 2010;28:1995)
Sites
- Lateral ventricle (common in children)
- Fourth ventricle (distributed across all age groups)
- Rarely in spinal cord or ectopic sites
- Multifocal in exceptional circumstances
Pathophysiology
- So far, limited literature on pathogenesis or tumorigenesis
- Genetic or syndromic association present in a proportion of tumors
- Choroid plexus papilloma and atypical choroid plexus papilloma are genetically similar and are distinct from choroid plexus carcinoma (Clin Cancer Res 2015;21:184)
- Possible association with simian virus (SV40) reported (Virology 1995;212:710)
- Possible association with inflammatory pathway described (Acta Neuropathol Commun 2019;7:95)
Clinical features
- Hydrocephalus / raised intracranial pressure
- Headache, vomiting, papilledema
Diagnosis
- Clinical features, imaging (CT or preferably MRI)
- Biopsy with histologic examination for definitive diagnosis
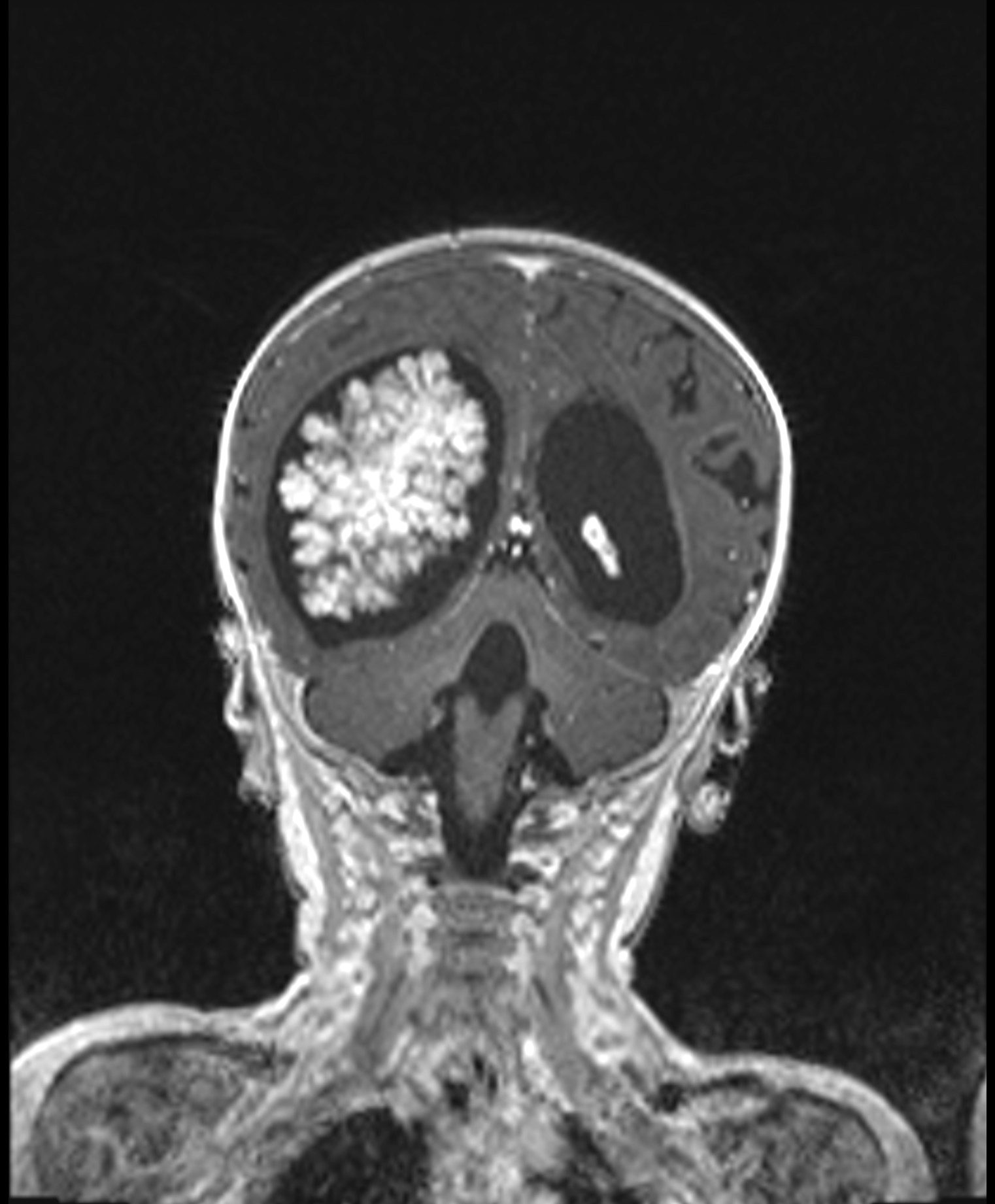
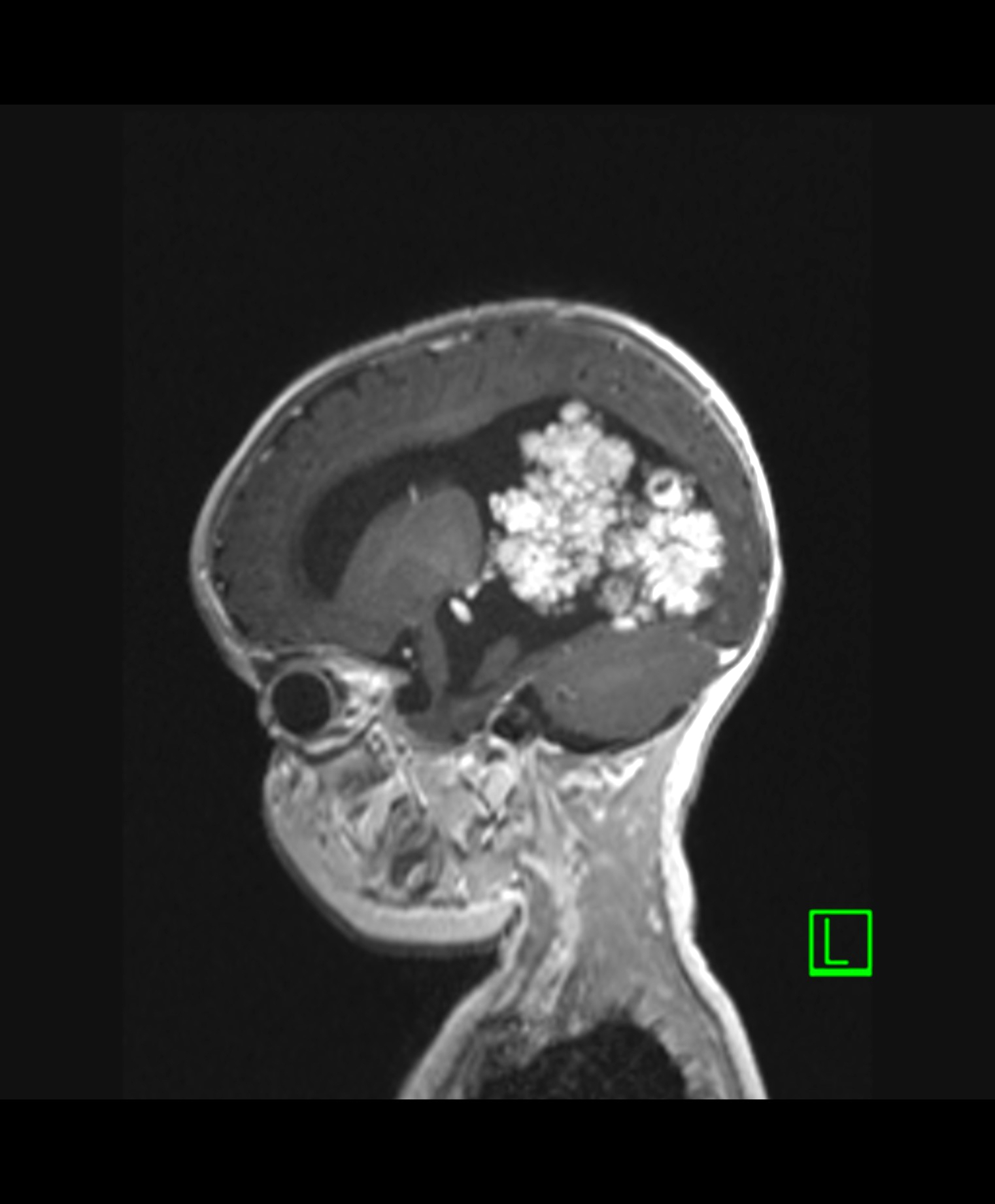
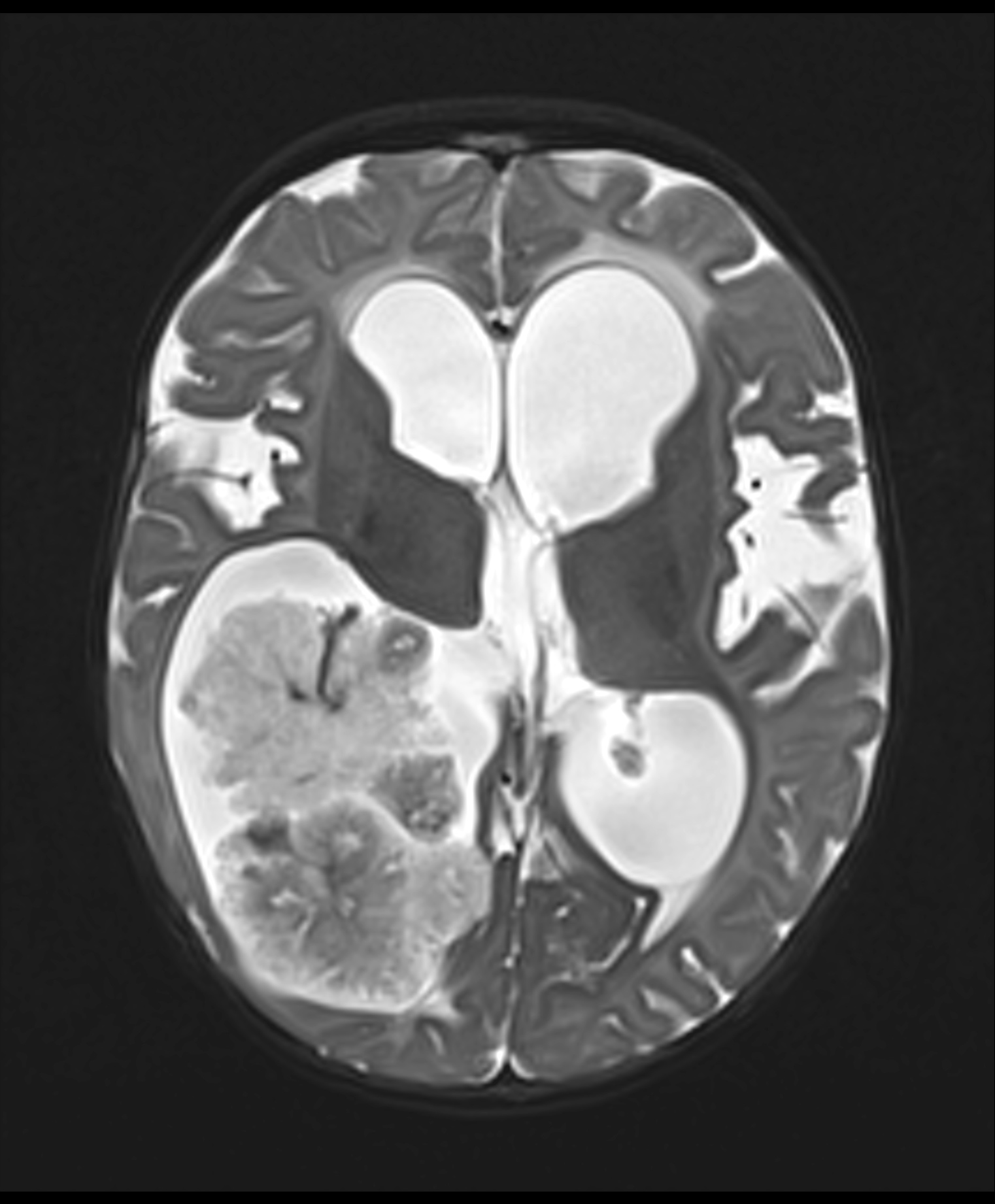
Radiology description
- Intraventricular papillary or lobulated lesions on MRI; hypo or isointense on T1, hyper or isointense on T2 and enhanced in postcontrast imaging (Cancer Imaging 2019;19:17)
Prognostic factors
- Historically, histological grade alone has been used for prediction of outcome
- 5 year survival rate for carcinoma reported approximately 62% (Pediatr Blood Cancer 2015;62:784)
- 5 year survival rate for papilloma and atypical papilloma is up to 100% and 89%, respectively (J Neurooncol 2009;95:383)
- Recent studies suggest 3 prognostic risk groups based on epigenetics, patient age and tumor location (Neuro Oncol 2016;18:790):
- Supratentorial pediatric low risk choroid plexus tumors
- Infratentorial adult low risk choroid plexus tumors
- Supratentorial pediatric high risk choroid plexus tumors
- Cerebrospinal fluid spread, drop metastasis and tumor recurrence after treatment are known and when present, carry poorer prognosis (BMJ Case Rep 2012;2012:bcr0120125681)
Case reports
- 2 month old boy with recurrent choroid plexus carcinoma in the lateral ventricle (Childs Nerv Syst 2020;36:1601)
- 8 month old girl with choroid plexus papilloma and factor XIII deficiency (Pediatr Neurosurg 2018;53:413)
- 17 year old boy with an uncommon case of neck pain in choroid plexus papilloma (Medicine (Baltimore) 2018;97:e12466)
- 30 year old woman with cerebral intraparenchymal atypical choroid plexus papilloma (J Neurol Surg A Cent Eur Neurosurg 2019;80:53)
- 43 year old woman with a primary pigmented choroid plexus papilloma within the sella turcica (World Neurosurg 2017;105:1039.e13)
Treatment
- Surgery to achieve gross total resection
- Adjuvant radiotherapy or chemotherapy, based on the clinical need
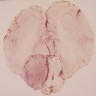
Gross description
- Papillomas are well circumscribed cauliflower-like masses
- Cysts, hemorrhages and calcifications may be present
- Carcinomas are invasive, solid and necrotic
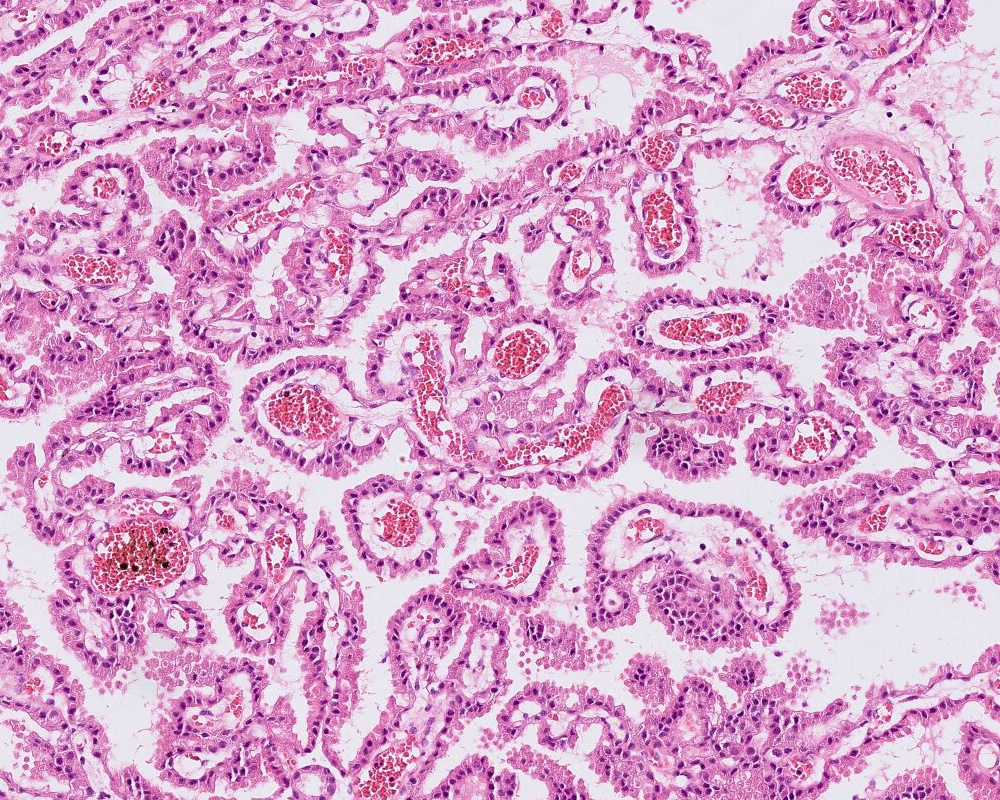
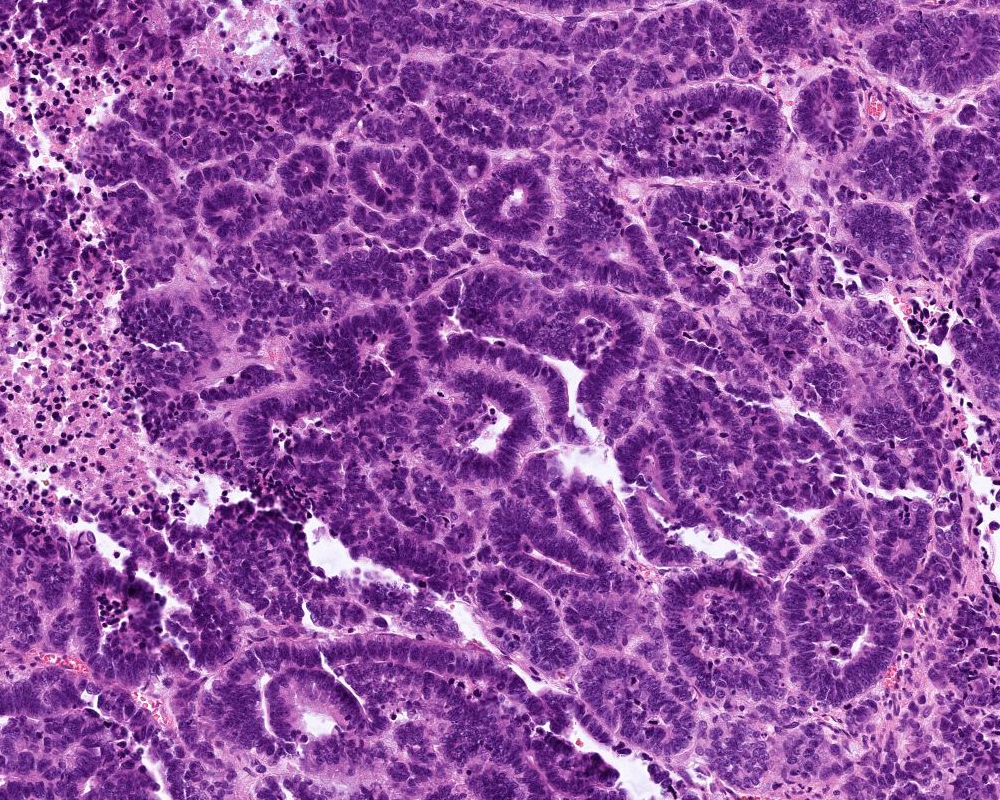
Microscopic (histologic) description
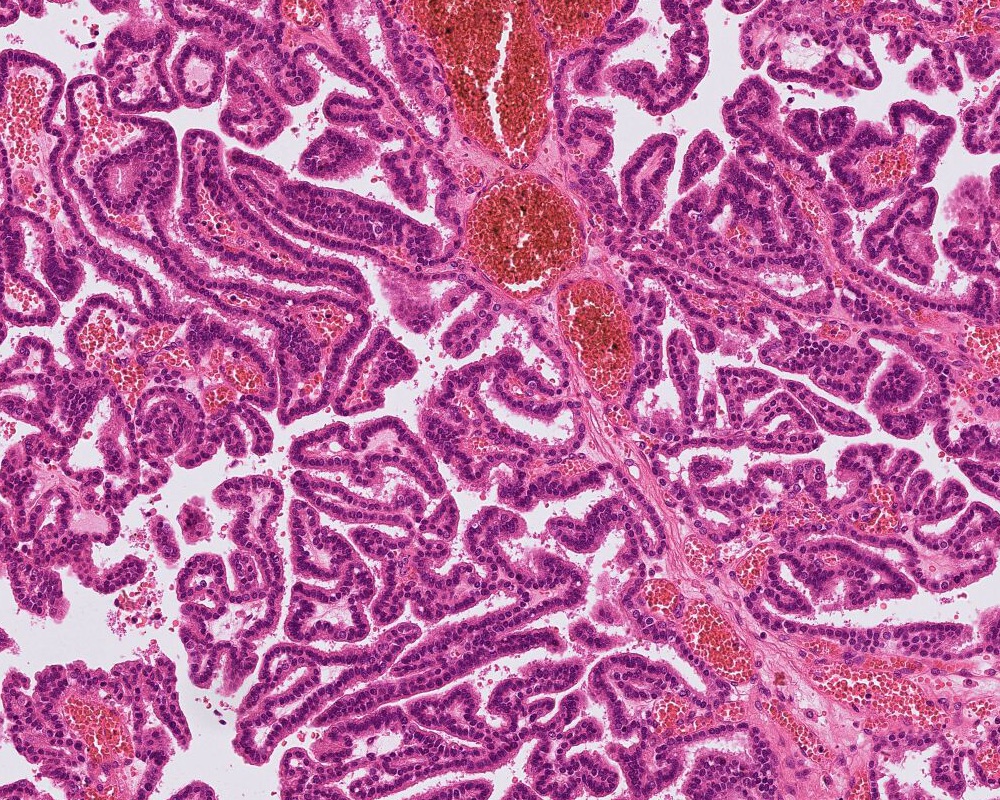
- Choroid plexus papilloma (CPP, WHO grade 1):
- Papillary (finger-like) architecture, resembling normal choroid plexus
- Single layer of cuboidal to columnar monomorphic cells
- Loss of cobblestone surface
- Mild nuclear pleomorphism, mitotic activity rare (< 2/10 high power fields), lacks necrosis
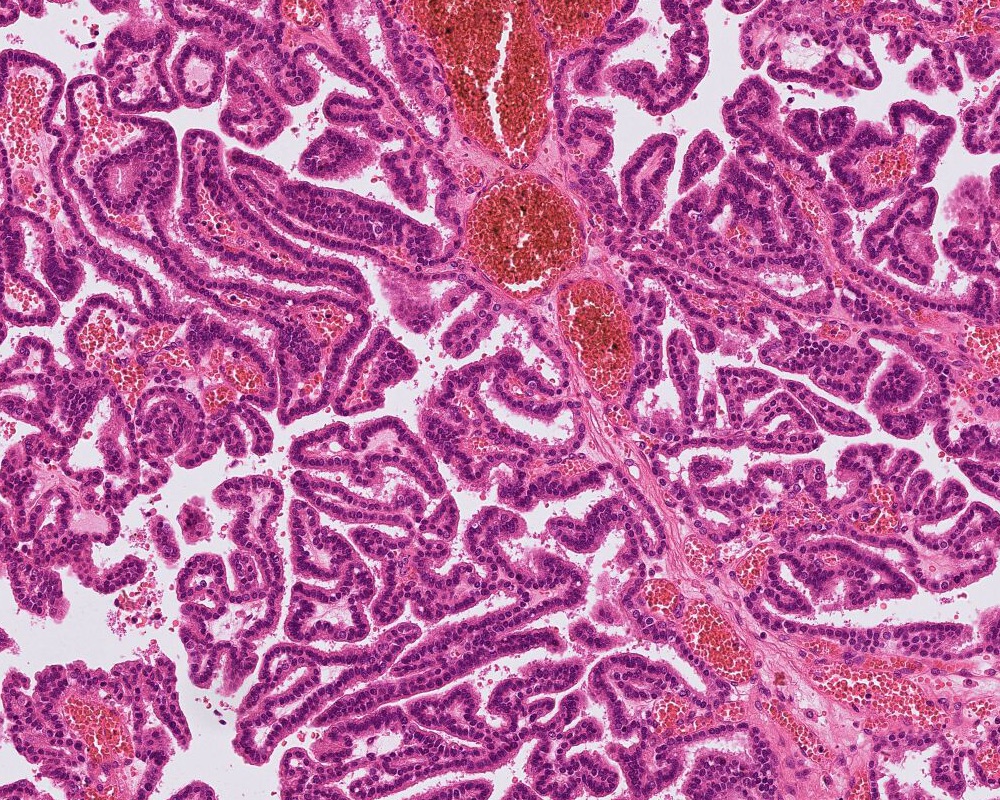
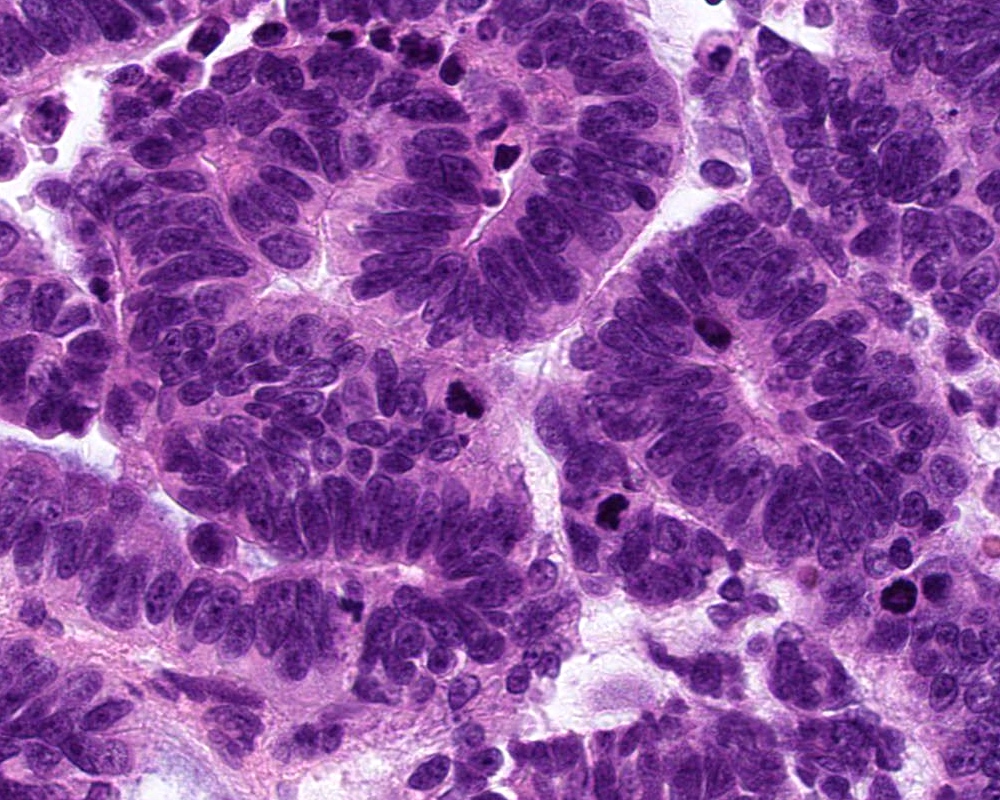
- Atypical choroid plexus papilloma (aCPP, WHO grade 2):
- Higher cellularity relative to CPP
- Moderate nuclear pleomorphism, blurring of papillary pattern
- Occasional mitoses (> 2/10 high power fields), with or without necrosis
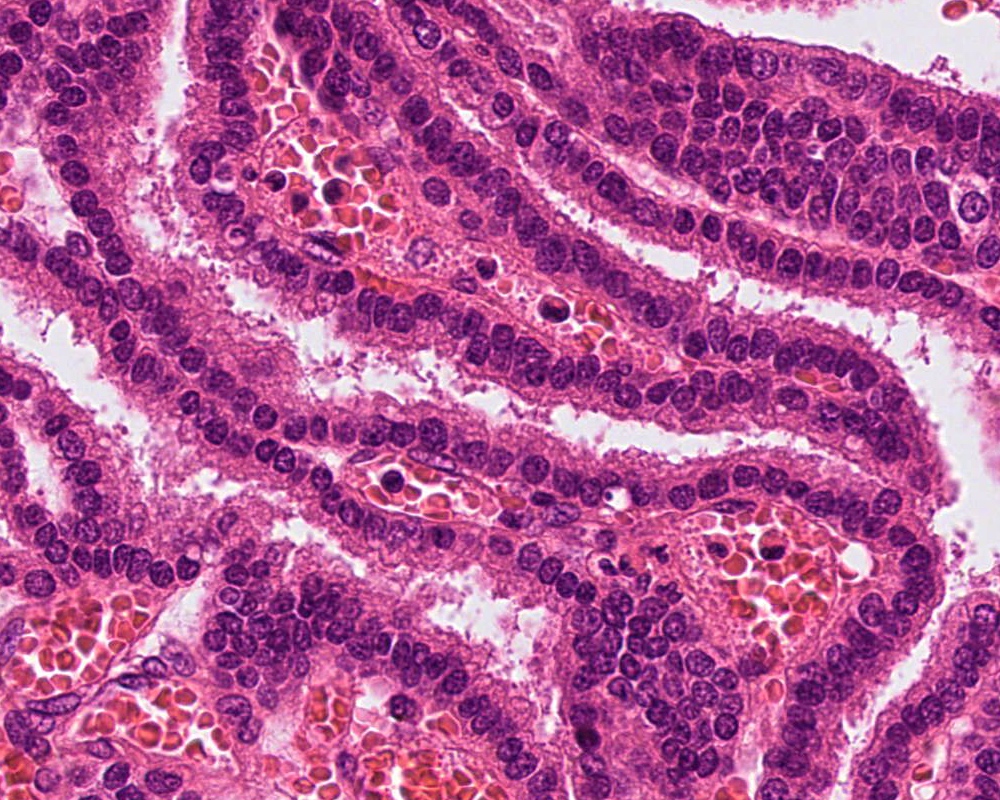
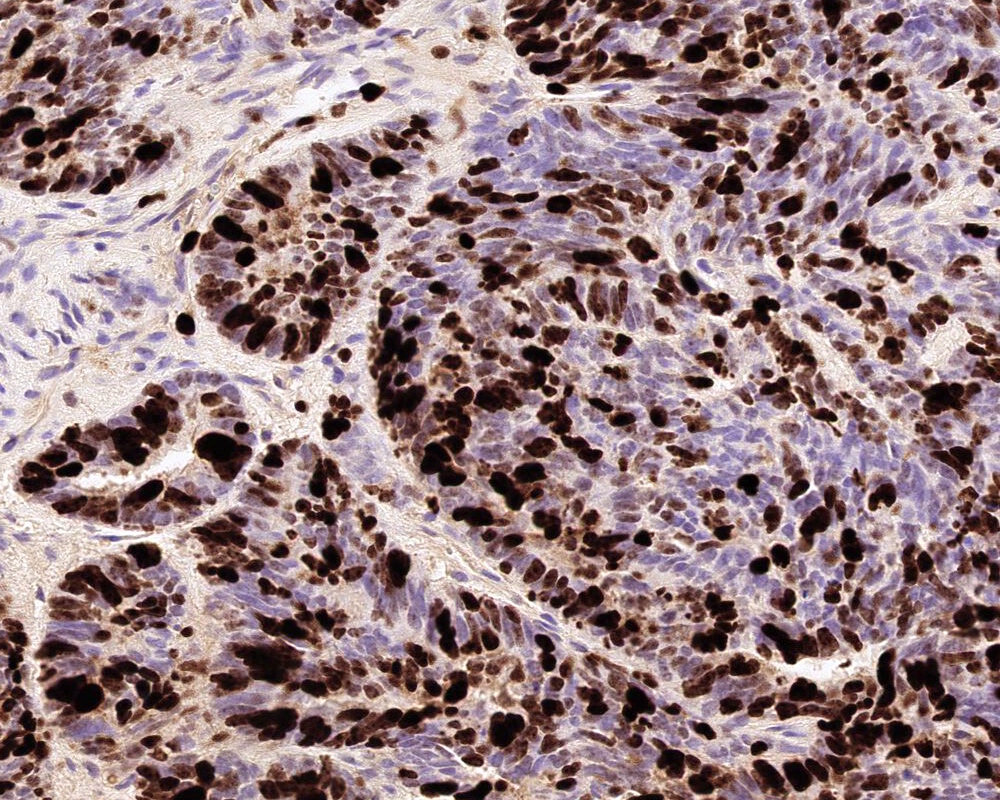
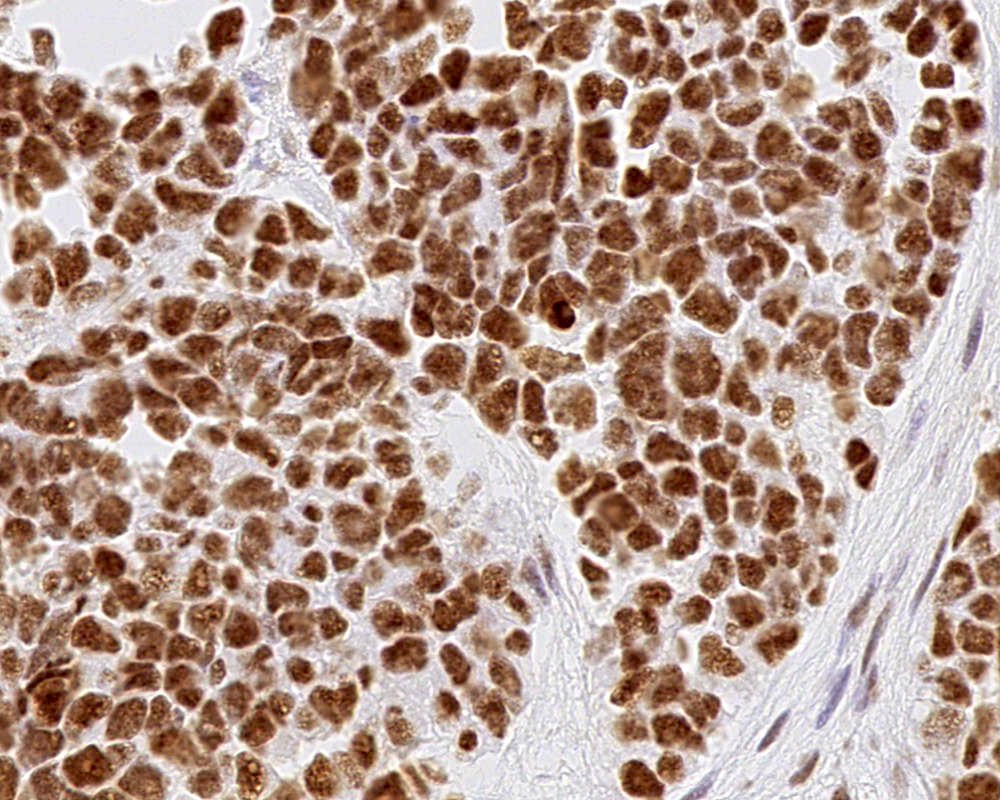
- Choroid plexus carcinoma (CPC, WHO grade 3):
- Frankly malignant
- High cellularity, hyperchromatic nuclei and nuclear pleomorphism
- Blurring of papillary pattern and solid arrangement
- Frequent mitoses (> 5/10 high power fields), necrosis, with or without brain invasion
Microscopic (histologic) images
Cytology description
- Intraoperative crush smear: papillary strands with fibrovascular cores
- Lesional cells exhibit relative cellular crowding and cytological atypia (in relation to normal choroid plexus epithelium)
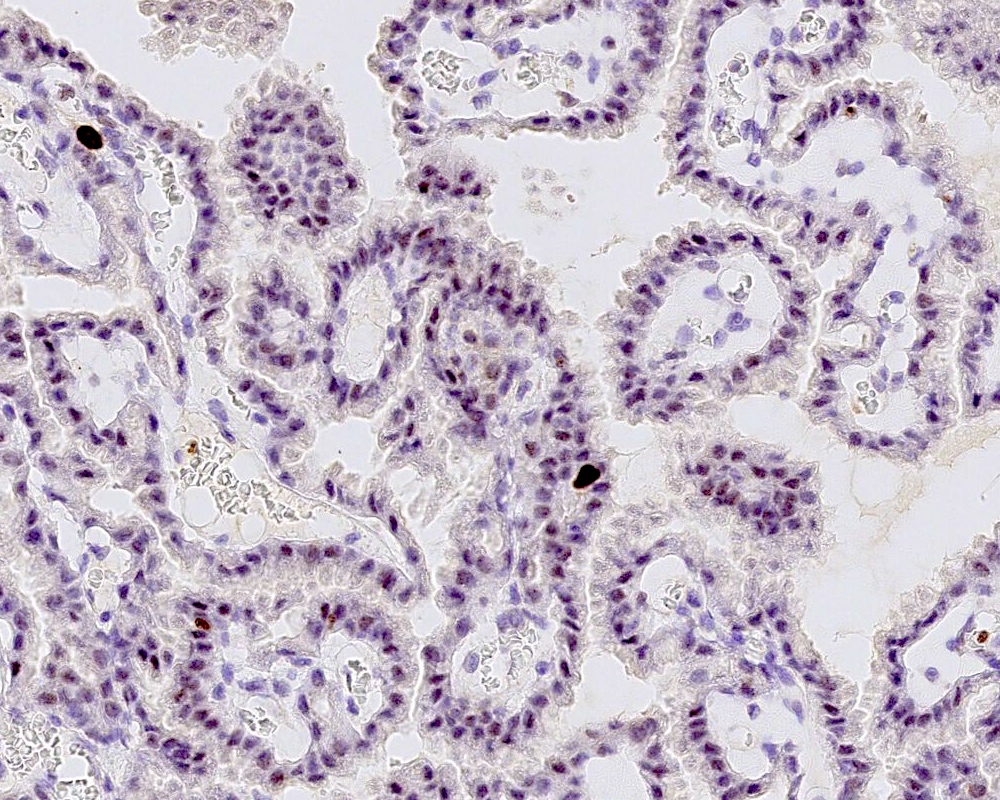
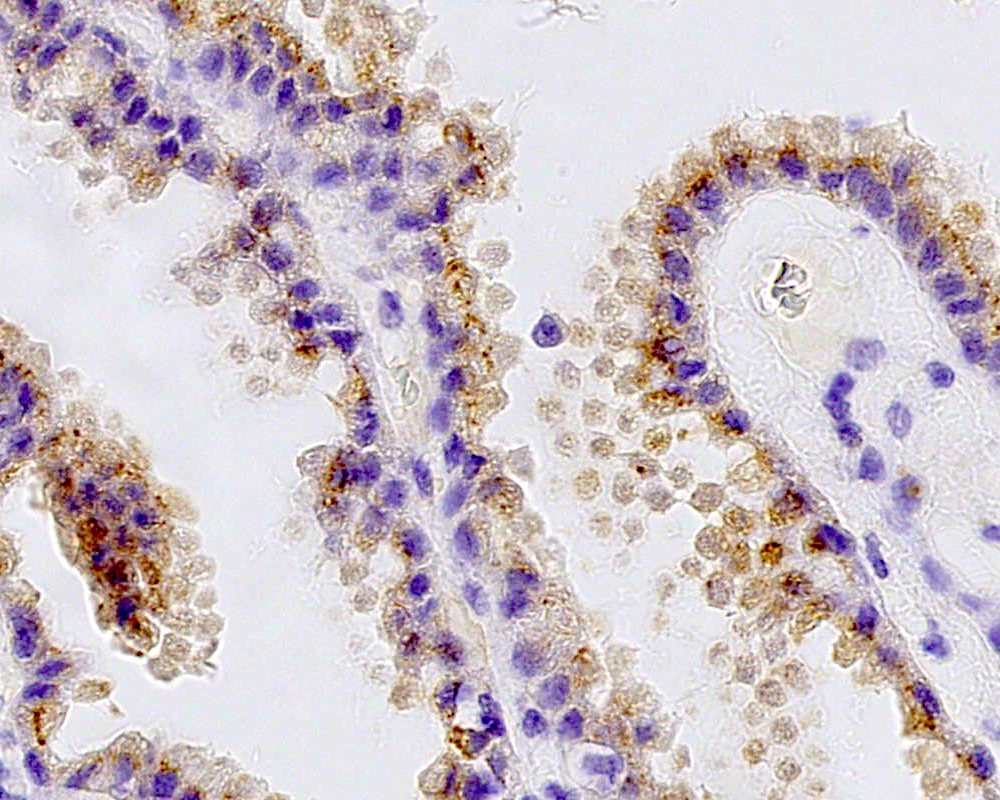
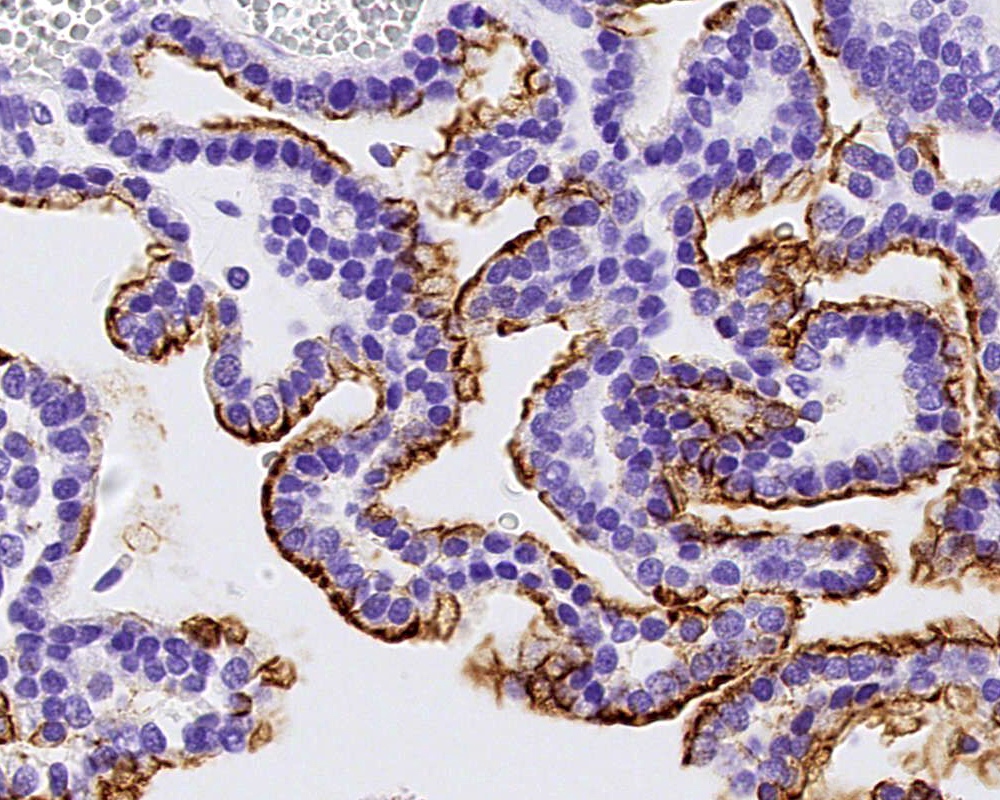
Positive stains
- Transthyretin, KIR7.1, EAAT1, S100, pancytokeratin (cytokeratin, MNF116, AE1 / AE3), CK7 > CK20
- Can be positive for GFAP, synaptophysin, S100
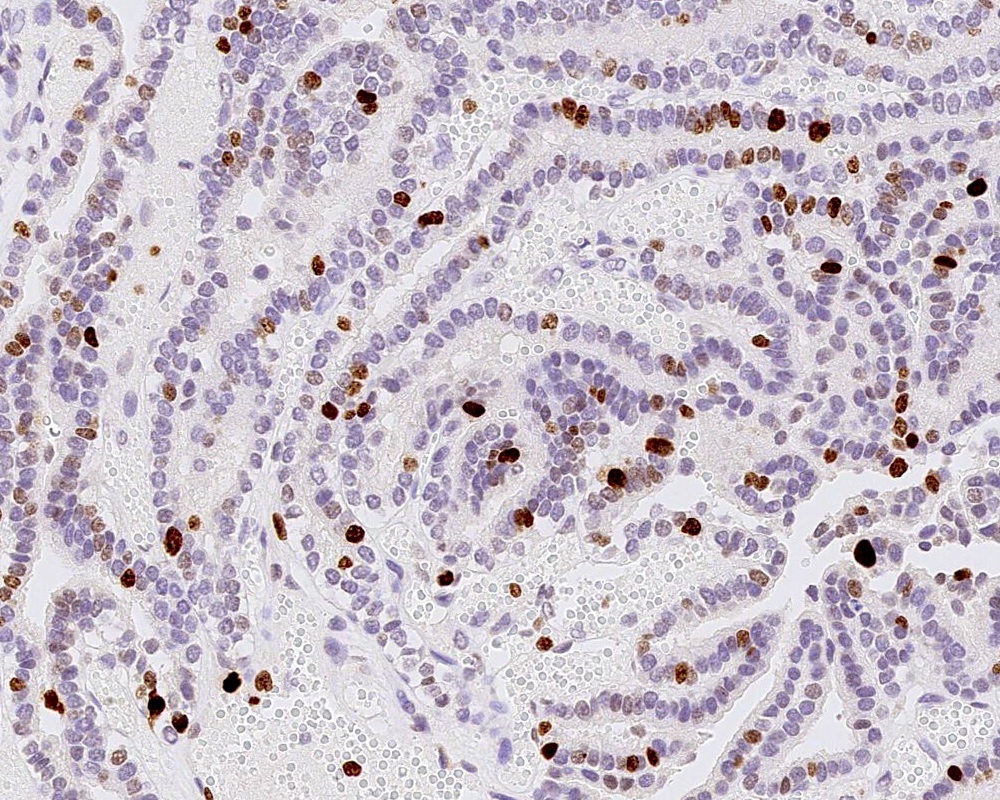
- Ki67 / MIB1 proliferation index may be used for grading (variable and subjective interpretation)
- Choroid plexus carcinoma: p53 variable, SMARCB1 / INI1 and SMARCA4 retained nuclear expression
- References: Neurosurgery 1988;23:384, Acta Neuropathol 1990;80:635, Am J Surg Pathol 2006;30:66, J Neuropathol Exp Neurol 1999;58:398
Negative stains
- CEA, EMA (weak or negative), LIN28a
- Reference: Acta Neuropathol 1990;80:635
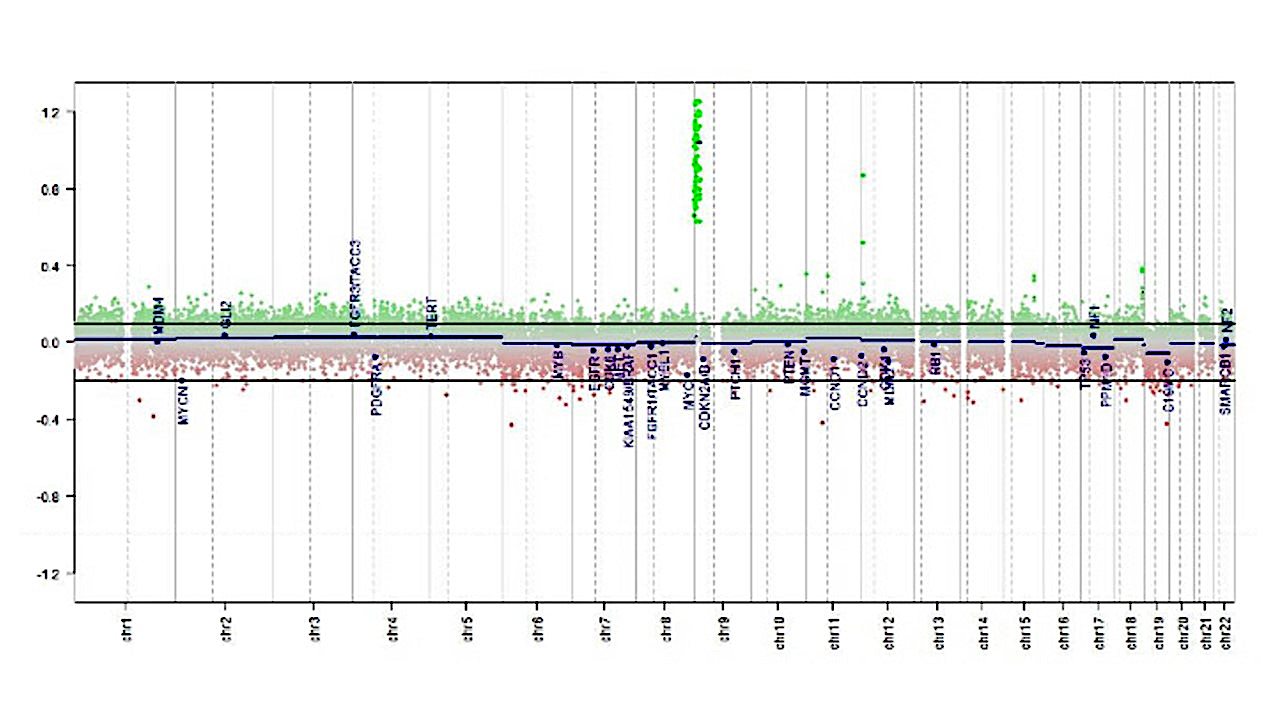
Molecular / cytogenetics description
- The following 3 methylation classes of choroid plexus tumors are recognized based on the German Cancer Research Center (DKFZ) Heidelberg classification:
- Plexus tumor, subclass adult
- Plexus tumor, subclass pediatric A
- Plexus tumor, subclass pediatric B (closely related to methylation cluster 3 described in Neuro Oncol 2016;18:790)
- TP53 sequencing and testing for germ line TP53 mutation is advisable in children with choroid plexus carcinoma
Molecular / cytogenetics images
Sample pathology report
- Lateral ventricle, tumor, excision:
- Atypical choroid plexus papilloma (WHO grade 2)
- Methylation class: Plexus tumor, subclass pediatric B, family class PLEX_T
- Molecular tests (sequencing): No TP53 mutation
- Comment: The tumor shows moderate atypia, histologically equivalent to WHO grade 2. The methylation array has classified as subclass B, which is closely related to methylation cluster 3 described in Neuro Oncol 2016;18:790.
Differential diagnosis
- Metastatic adenocarcinoma with papillary structures:
- Variable morphology depending on the primary
- GATA3+ (breast carcinoma), TTF1+ (lung adenocarcinoma), PAX8 (renal, Müllerian thyroid origin carcinomas)
- Choroid plexus tumors are often diffusely synaptophysin+, which differs from metastatic papillary carcinoma (J Neuropathol Exp Neurol 1999;58:398)
- Ependymoma:
- Ependymal perivascular pseudo rosettes
- GFAP+, KIR7.1-, transthyretin-
- Intraventricular meningioma:
- Whorl formation, vesicular nuclei and syncytial cytoplasm
- SSTR2A+, KIR7.1-, transthyretin-
- Central neurocytoma:
- Uniform round cells with neuronal differentiation; no papillary structure
- NeuN+, MAP2+
- Germ cell tumor:
- Medulloblastoma:
- Small round blue cell tumor with or without Homer-Wright rosettes
- Synaptophysin+
- Embryonal tumor with multilayered rosettes (ETMR):
- Biphasic pattern with abundant neuropil and embryonal cells in multilayered rosettes
- LIN28a+
- Atypical teratoid / rhabdoid tumor (AT / RT):
- Poorly differentiated embryonal tumor
- INI1-
- Cribriform neuroepithelial tumor (CRINET) (J Neuropathol Exp Neurol 2009;68:1249):
- Intraventricular and papillary
- INI1-
Additional references
Board review style question #1
A 3 month old boy presented with restless crying and vomiting. His head size was larger than expected for his age. Imaging showed an 8 cm, lobulated, enhancing lesion in the right lateral ventricle. Histology is as above. Which of the following is true?
- Choroid plexus tumors are common in adults
- Recent studies suggest 3 distinct molecular entities based on methylation profiling, patient age and tumor location
- This is a choroid plexus carcinoma (WHO grade 3)
- TP53 mutation is strongly associated with choroid plexus papilloma
Board review style answer #1
B. Recent studies suggest 3 distinct molecular entities based on methylation profiling, patient age and tumor location. An atypical choroid plexus tumor is shown in the image.
Choroid plexus tumors are rare intracranial tumors arising in the choroid plexus epithelium of the ventricles. They are more common in children. Histologically, they are classified into 3 categories: choroid plexus papilloma (CPP, WHO grade 1), atypical choroid plexus papilloma (aCPP, WHO grade 2) and choroid plexus carcinoma (CPC, WHO grade 3). Although any of these tumors can have CSF spread and recur following treatment, the latter is associated with poor outcome and higher recurrence rate. Recent studies have shown that CPP and aCPP are genetically similar but CPP and aCPP are genetically distinct from CPC. The CPCs are mostly driven by loss of function or mutations of tumor suppressor gene TP53 and may be associated germ line mutations (Li-Fraumeni syndrome). Furthermore, based on methylation profiling, tumor location and age, 3 distinct subgroups with prognostic significance have been described. The advances in understanding of genetics may help deliver appropriate personalized treatment.
Comment Here
Reference: Choroid plexus tumors (papilloma, atypical papilloma, carcinoma)
Choroid plexus tumors are rare intracranial tumors arising in the choroid plexus epithelium of the ventricles. They are more common in children. Histologically, they are classified into 3 categories: choroid plexus papilloma (CPP, WHO grade 1), atypical choroid plexus papilloma (aCPP, WHO grade 2) and choroid plexus carcinoma (CPC, WHO grade 3). Although any of these tumors can have CSF spread and recur following treatment, the latter is associated with poor outcome and higher recurrence rate. Recent studies have shown that CPP and aCPP are genetically similar but CPP and aCPP are genetically distinct from CPC. The CPCs are mostly driven by loss of function or mutations of tumor suppressor gene TP53 and may be associated germ line mutations (Li-Fraumeni syndrome). Furthermore, based on methylation profiling, tumor location and age, 3 distinct subgroups with prognostic significance have been described. The advances in understanding of genetics may help deliver appropriate personalized treatment.
Comment Here
Reference: Choroid plexus tumors (papilloma, atypical papilloma, carcinoma)
Board review style question #2
Which of the following is true regarding the histological differential diagnosis for choroid plexus carcinoma?
- Diffuse synaptophysin expression and Homer-Wright rosettes are features of choroid plexus carcinoma
- IDH1 is positive in choroid plexus carcinoma
- LIN28a is positive in choroid plexus carcinoma
- Nuclear INI1 (SMARB1) expression is retained in choroid plexus carcinoma but lost in atypical teratoid / rhabdoid tumor
Board review style answer #2
D. Nuclear INI1 (SMARB1) expression is retained in choroid plexus carcinoma but lost in atypical teratoid / rhabdoid tumor
Choroid plexus carcinomas can mimic other malignant tumors of the childhood, such as CNS embryonal tumors, and it is important to exclude them to guide appropriate treatment. INI1 (SMARCB1) and SMARCA4 immunohistochemistry expression is retained in CPC, which helps to differentiate from aggressive tumor atypical teratoid / rhabdoid tumor (AT / RT). A negative LIN28a differentiates from embryonal tumor with multilayered rosettes (ETMR, bearing C19MC alterations). Diffuse synaptophysin expression with patchy GFAP and presence of Homer-Wright rosettes should raise the possibility of medulloblastoma. Diffuse GFAP expression with possible IDH1 expression (in younger adults) with microvascular endothelial proliferation and necrosis would indicate a high grade glioma / glioblastoma. DNA methylation array and classification of CNS tumors will help in definitive classification. Nevertheless, it is important to have a solid histological diagnosis in case the array fails to confidently classify the tumor.
Comment Here
Reference: Choroid plexus tumors (papilloma, atypical papilloma, carcinoma)
Choroid plexus carcinomas can mimic other malignant tumors of the childhood, such as CNS embryonal tumors, and it is important to exclude them to guide appropriate treatment. INI1 (SMARCB1) and SMARCA4 immunohistochemistry expression is retained in CPC, which helps to differentiate from aggressive tumor atypical teratoid / rhabdoid tumor (AT / RT). A negative LIN28a differentiates from embryonal tumor with multilayered rosettes (ETMR, bearing C19MC alterations). Diffuse synaptophysin expression with patchy GFAP and presence of Homer-Wright rosettes should raise the possibility of medulloblastoma. Diffuse GFAP expression with possible IDH1 expression (in younger adults) with microvascular endothelial proliferation and necrosis would indicate a high grade glioma / glioblastoma. DNA methylation array and classification of CNS tumors will help in definitive classification. Nevertheless, it is important to have a solid histological diagnosis in case the array fails to confidently classify the tumor.
Comment Here
Reference: Choroid plexus tumors (papilloma, atypical papilloma, carcinoma)