Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Chiu K, Schaeffer DF. Dysplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/colonibddysplasia.html. Accessed September 24th, 2025.
Definition / general
- Dysplasia of colonic epithelium identified in setting of colonic inflammatory bowel disease (IBD), usually in colonic biopsies from surveillance colonoscopies
- Precursor of invasive carcinoma
- Can be endoscopically visible or invisible
- Aim of surveillance is to reduce morbidity and mortality from colorectal carcinoma by identifying dysplasia (or early invasive carcinoma)
Essential features
- Precursor of invasive carcinoma in patients with inflammatory bowel disease
- Dysplasia status should be reported for biopsies from surveillance colonoscopies of patients with inflammatory bowel disease:
- Negative for dysplasia
- Indefinite for dysplasia
- Low grade dysplasia
- High grade dysplasia
- Management of dysplasia identified on colonoscopies is dependent on endoscopic appearance (visible [polypoid or nonpolypoid] or invisible) and resectability
Terminology
- Previously used term dysplasia associated lesion or mass (DALM) and related terms (e.g. adenoma-like or nonadenoma-like DALM) should be avoided due to the historical connotation of a high risk of malignancy (Mod Pathol 2018;31:1180)
- Endoscopic appearance based on standardized terminology as described by the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations (SCENIC) is recommended (Gastroenterology 2015;148:639)
ICD coding
- ICD-10:
- D12.0 - benign neoplasm of cecum
- D12.2 - benign neoplasm of ascending colon
- D12.3 - benign neoplasm of transverse colon
- D12.4 - benign neoplasm of descending colon
- D12.5 - benign neoplasm of sigmoid colon
- D12.6 - benign neoplasm of colon, unspecified
- D12.7 - benign neoplasm of rectosigmoid junction
- D12.8 - benign neoplasm of rectum
Sites
- Colon and rectum in areas of colitis
Pathophysiology
- Cytotoxic effect of inflammation leads to repeated cycles of epithelial wounding and repair
- Postulated selective pressure for mutant cells that can survive the inflammatory insult and rapidly repopulate the damaged mucosa
- Epigenetic and genetic changes accumulate in morphologically nondysplastic colonic mucosa long before development of neoplasia
- TP53 mutations and aneuploidy are early events in colitis associated neoplasia and have been identified in nondysplastic colonic mucosa (Carcinogenesis 2018;39:11, Nat Rev Gastroenterol Hepatol 2017;14:218, Gastroenterology 2009;136:542)
- Accumulation of additional mutations, chromosomal abnormalities and epigenetic changes leads to dysplasia and invasive carcinoma
- In comparison, sporadic colorectal neoplasia tends to have APC mutations early and TP53 mutations later in tumorigenesis
Etiology
- Severe, extensive and longstanding chronic inflammation in the setting of ulcerative colitis is associated with an increased risk of colorectal neoplasia (Nat Rev Gastroenterol Hepatol 2017;14:218)
- Extensive colitis related to Crohn's disease is associated with an increased risk of dysplasia and neoplasia (Gastroenterology 2001;120:820)
Clinical features
- Surveillance colonoscopies typically start at 8 years after onset of inflammatory bowel disease (IBD) (Gastrointest Endosc 2015;81:1101, J Crohns Colitis 2017;11:649, Am J Gastroenterol 2019;114:384)
- Chromoendoscopy, the application of dye to help visualize lesions, can increase the sensitivity in detecting dysplasia
- Has been endorsed for IBD surveillance (Gastroenterology 2015;148:639, Gastrointest Endosc 2015;81:1101, J Crohns Colitis 2017;11:649)
- Most dysplasia identified in surveillance colonoscopies are endoscopically visible but some dysplasia is endoscopically invisible, i.e. indistinguishable from macroscopically unremarkable colonic mucosa (Gastrointest Endosc 2007;65:998)
- Endoscopic appearance of dysplasia based on descriptors from the Paris classification recommended by the SCENIC panel and other major societies, although other standardized classifications may be used (Gastrointest Endosc 2015;81:1101, J Crohns Colitis 2017;11:649, Gastroenterology 2015;148:639, Gastrointest Endosc 2003;58:S3, J Crohns Colitis 2021;15:1089):
- Visible dysplasia
- Polypoid: pedunculated or sessile
- Nonpolypoid: superficial elevated, flat or depressed
- Invisible dysplasia
- Visible dysplasia
- Visible lesions are endoscopically resected or biopsied
- Biopsies adjacent to endoscopically resected lesion may also be taken to ensure complete removal
- Random biopsies may be taken to detect endoscopically invisible dysplasia
- Unconventional dysplasia may be more likely than conventional dysplasia to present as flat or invisible lesions (Histopathology 2021;78:814)
Diagnosis
- Detected with colonoscopies, typically as part of surveillance
- Targeted biopsies, endoscopic resections or polypectomies of visible lesions
- Random biopsies may be taken to detect endoscopically invisible dysplasia
Prognostic factors
- Polypoid dysplasia treated by endoscopic polypectomy:
- Low rate of invasive carcinoma on followup (Clin Gastroenterol Hepatol 2004;2:534, Gastroenterology 1999;117:1295, Clin Gastroenterol Hepatol 2014;12:756)
- Increased rate of dysplasia on followup (Clin Gastroenterol Hepatol 2014;12:756)
- Nonpolypoid dysplasia:
- In general, associated with a high rate of metachronous and synchronous carcinoma (J Crohns Colitis 2017;11:649, Am J Gastroenterol 2015;110:1461, Surg Endosc 2021;35:1534)
- Endoscopic resection can be achieved in subset of cases (Inflamm Bowel Dis 2018;24:1196, Gastrointest Endosc 2018;87:1079, Surg Endosc 2021;35:1534, Endoscopy 2017;49:1237, J Gastroenterol Hepatol 2019;34:1581)
- Invisible high grade dysplasia:
- High risk of synchronous and metachronous invasive carcinoma (Am J Gastroenterol 2015;110:1022)
- Invisible low grade dysplasia:
- Prognosis is controversial
- Some studies report a low rate of progression to carcinoma while others suggest a high rate (Clin Gastroenterol Hepatol 2017;15:665, Gastroenterology 2003;125:1311)
- Indefinite for dysplasia:
- Subset will show bonafide dysplasia on followup (Inflamm Bowel Dis 2010;16:1352)
- Unconventional dysplasia may be more likely than conventional dysplasia to be associated with increased risk of high grade dysplasia or carcinoma on followup, particularly hypermucinous, goblet cell deficient and crypt cell dysplasia variants (Histopathology 2021;78:814)
- Unconventional dysplasia may be more likely to present as flat or invisible lesions compared to conventional dysplasia (Histopathology 2021;78:814)
Case reports
- 19 year old woman with 6 year history of ulcerative colitis with invasive carcinoma and high grade dysplasia (BMJ Case Rep 2013;2013:bcr2013200172)
- 42 year old man with polypoid dysplasia in Crohn's colitis (Can J Gastroenterol 2009;23:477)
- 45 year old man with ileorectal anastomosis, low grade dysplasia and invasive adenocarcinoma (SAGE Open Med Case Rep 2017;5:2050313X17692902)
- 76 year old woman with longstanding ulcerative colitis with low and high grade dysplasia (J Gastroenterol Hepatol 2016;31:1797)
- Dysplasia in 5 patients with ulcerative colitis (Intern Med 2016;55:911)
Treatment
- Depends on endoscopic appearance and resectability
- Polypoid dysplasia:
- Endoscopic polypectomy and followup with continued surveillance
- Nonpolypoid dysplasia:
- Endoscopically resectable:
- Endoscopic mucosal resection or submucosal dissection and followup with continued surveillance
- Not amenable to endoscopic resection:
- Cannot exclude underlying invasive carcinoma
- Should be surgically resected
- Endoscopically resectable:
- Invisible dysplasia:
- Refer for chromoendoscopy, if feasible and not already done, as this may help visualize endoscopically the dysplastic lesion (Gastroenterology 2015;148:639)
- Invisible high grade dysplasia:
- Resection, due to high risk of invasive carcinoma
- Invisible low grade dysplasia:
- Continued surveillance or colectomy; multifocal disease may be indication for surgery (Gastrointest Endosc 2015;81:1101, J Crohns Colitis 2017;11:649, Gastroenterology 2003;125:1311)
- Indefinite for dysplasia:
- Repeat endoscopy posttreatment to reduce inflammatory background (Gastrointest Endosc 2015;81:1101)
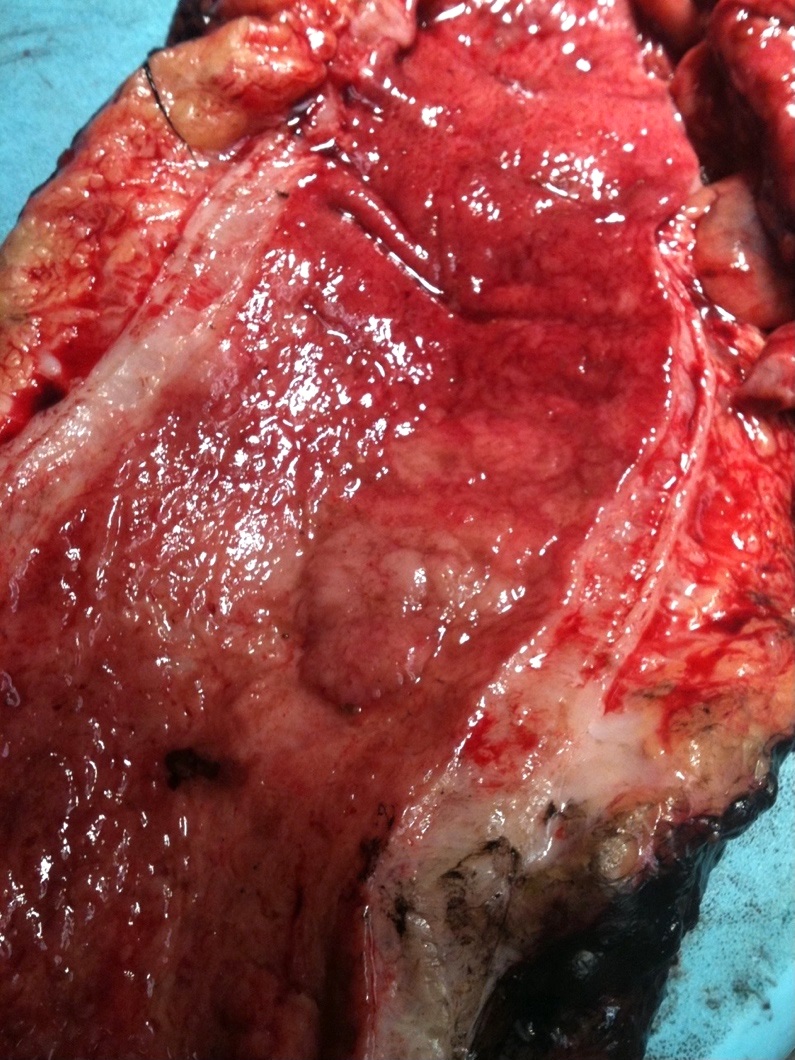
Clinical images
Gross description
- Can be macroscopically (endoscopically) unapparent
- Can be polypoid (pedunculated or sessile) or nonpolypoid (superficially elevated, flat or depressed)
Gross images
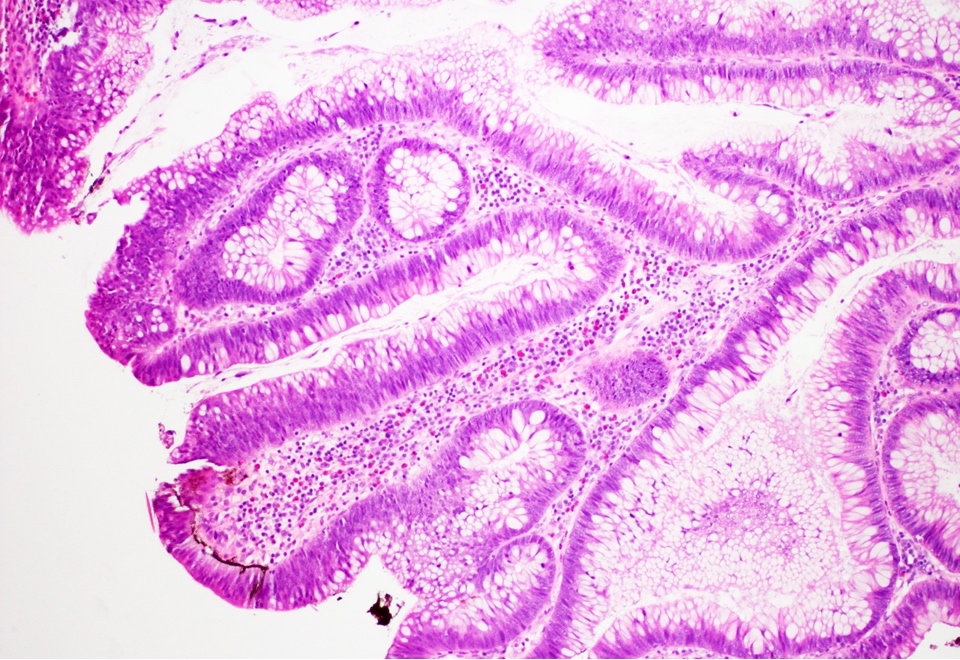
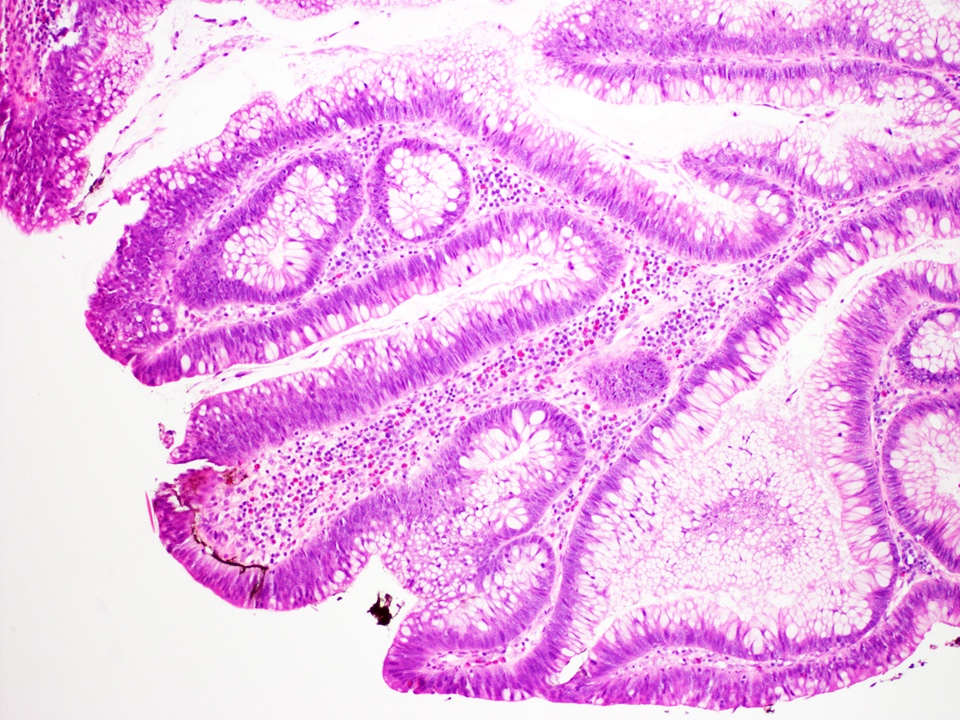
Microscopic (histologic) description
- Correlate with endoscopic appearance, as this influences management
- Visible dysplasia (polypoid or nonpolypoid)
- Invisible dysplasia
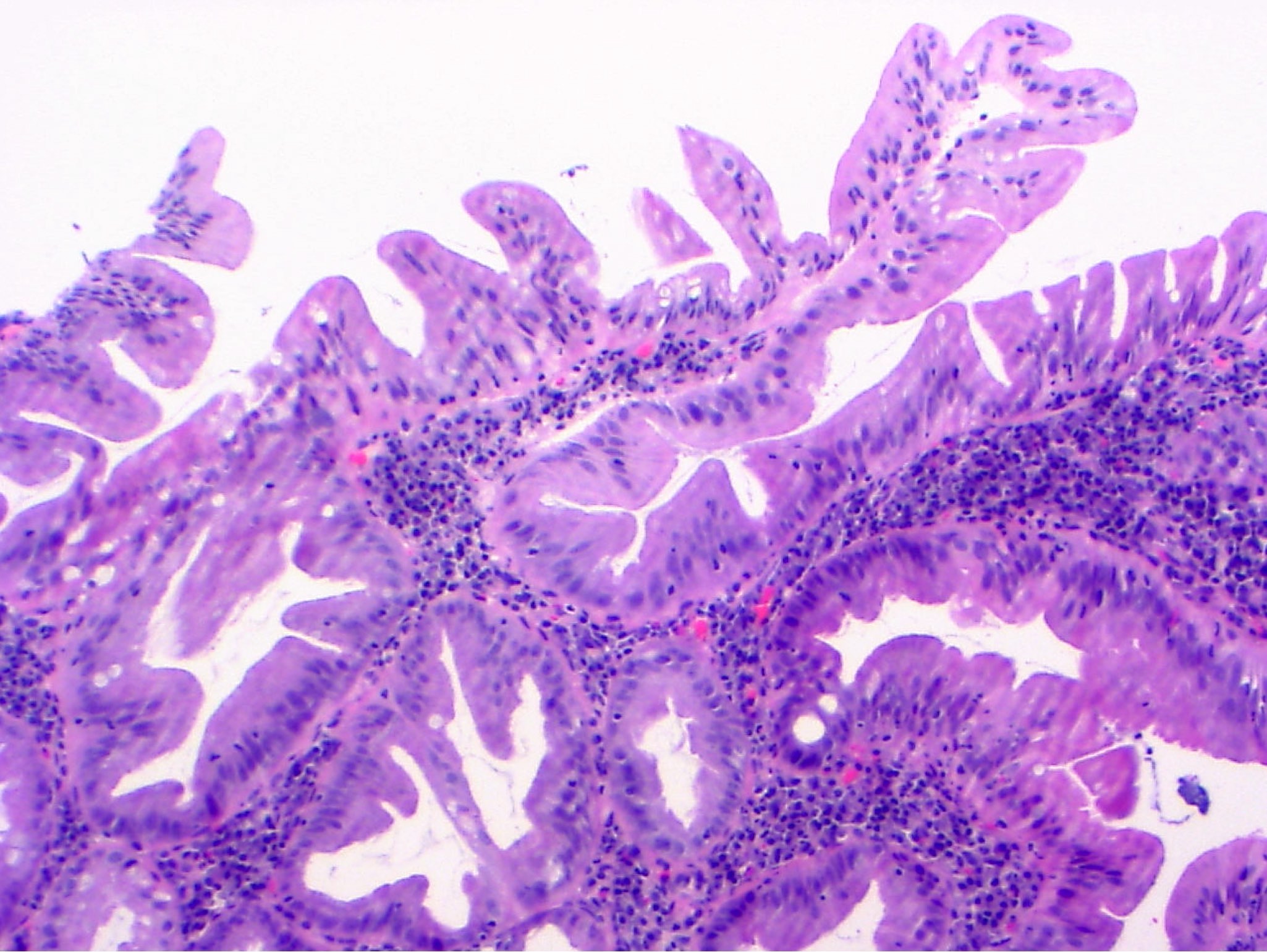
- Low grade dysplasia:
- Preserved nuclear polarity
- Pseudostratified, crowded, elongated and hyperchromatic nuclei
- Lack of surface maturation, i.e. abnormalities persist to surface
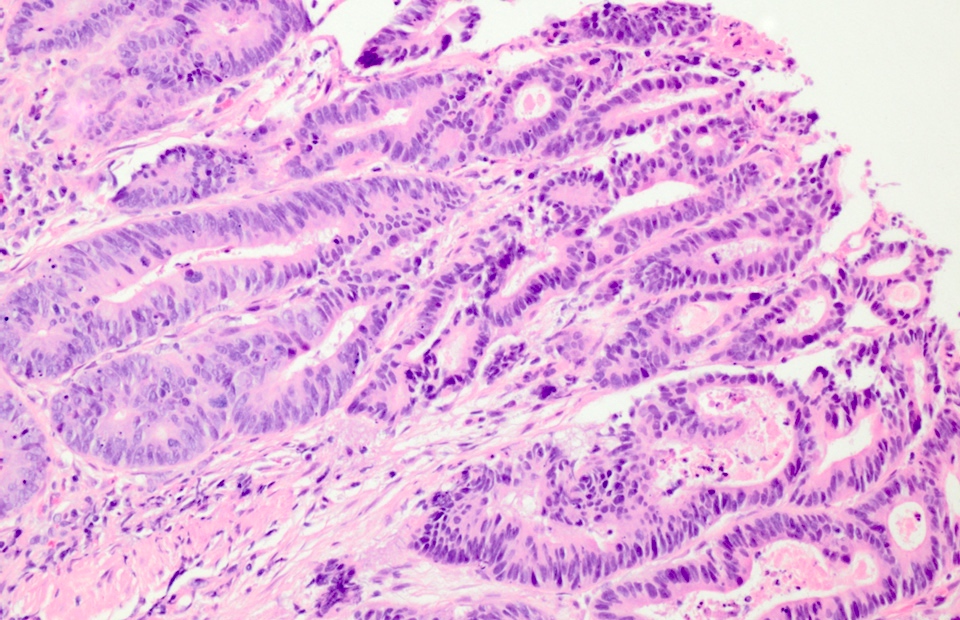
- High grade dysplasia:
- May show complex architecture, such as cribriform glands
- Loss of nuclear polarity
- Nuclear pleomorphism, vesicular nuclei and prominent nucleoli
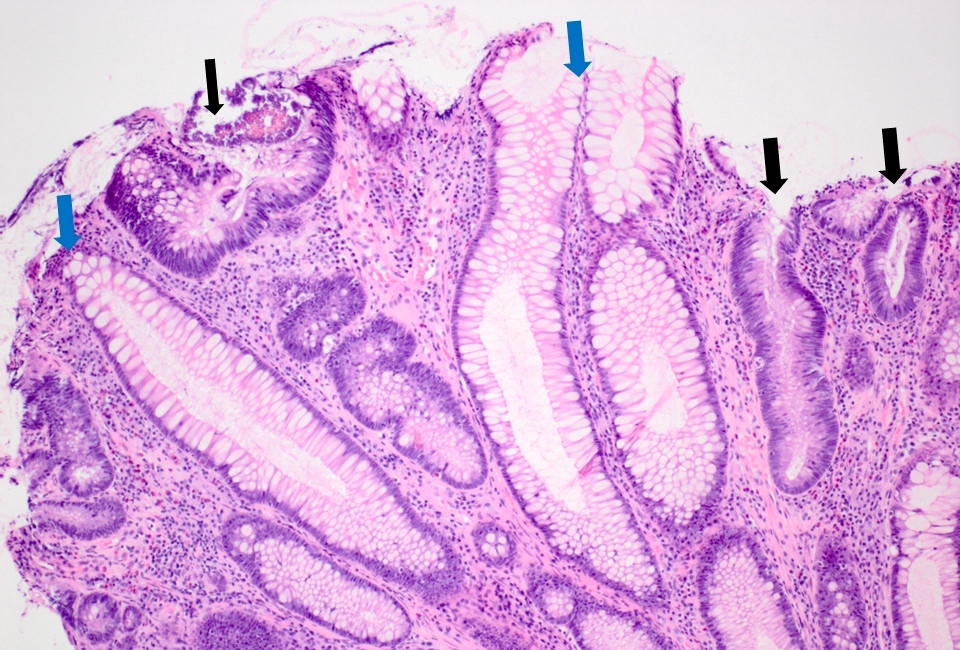
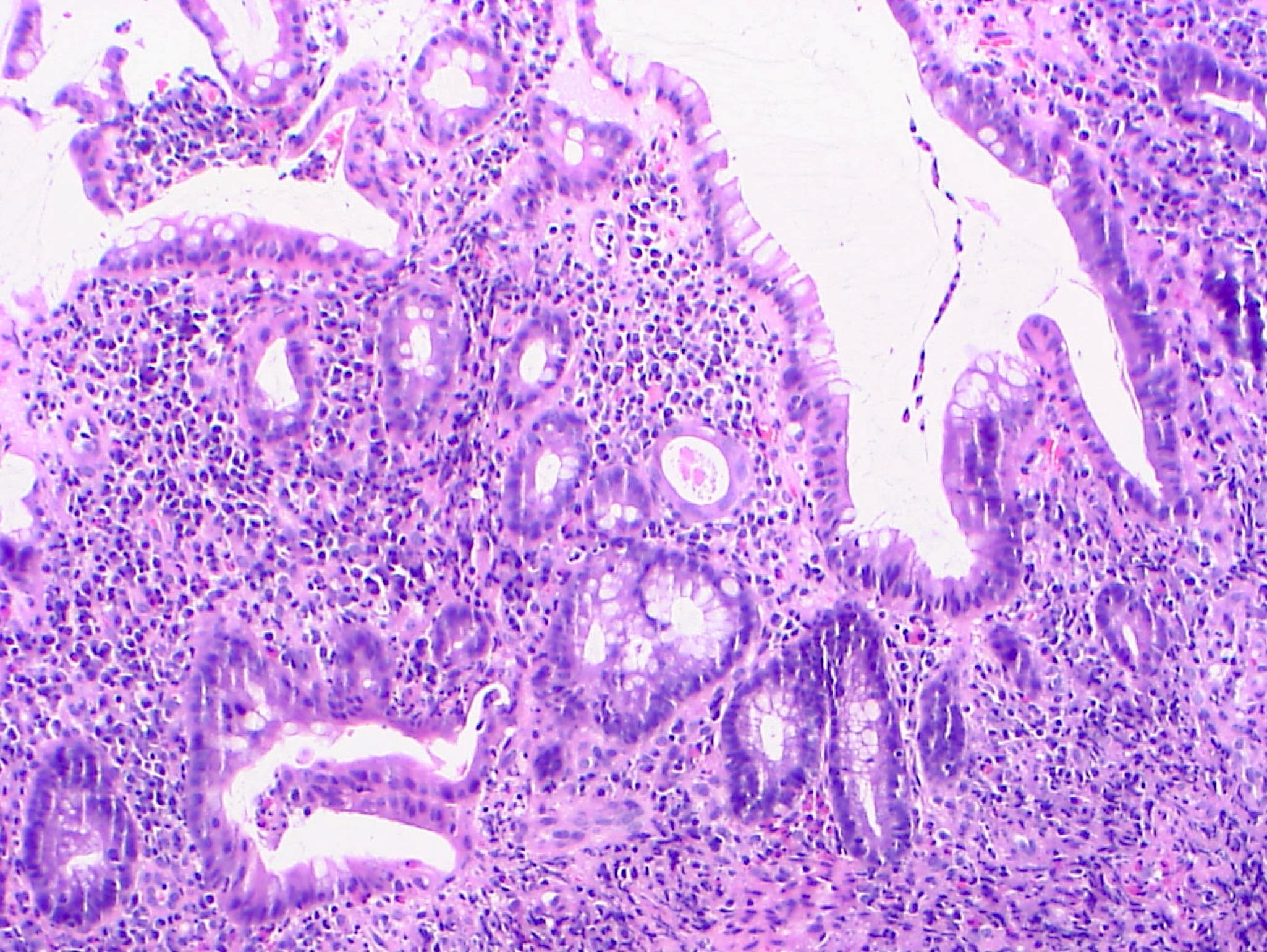
- Indefinite for dysplasia:
- Reserved for cases when distinction between dysplasia and reactive epithelial atypia cannot be made
- Mucosal erosion or ulceration (precluding assessment of surface maturation) or prominent inflammation may be sources of difficulty
- Features distinguishing IBD associated polypoid dysplasia and sporadic adenomas have been described but are not reliable and have no therapeutic implications
- Include lamina propria inflammation, mixture of benign and dysplastic glands at the surface and dysplasia in stalk of polyp (Hum Pathol 1983;14:931, Am J Surg Pathol 1998;22:275)
- Distinction is also not clinically significant as endoscopic polypectomy suffices either way
- More recently, Gui et al. reported features that can be seen in IBD associated nonpolypoid dysplasia but only rarely seen in polypoid dysplasia or sporadic adenomas (Hum Pathol 2020;100:24):
- Dysplastic epithelium associated with a background of inflammatory polyp or granulation tissue, micropapillary or hobnailing surface epithelial cells, nuclear pleomorphism and disarray, and microvesicular or bubbling cytoplasm
- IBD associated nonconventional dysplasia (i.e. not intestinal type) has been described and is relatively common in IBD patients:
- 45% of IBD patients with colorectal cancer and of those, 46% did not have associated conventional dysplasia as reported by Choi et al. (Mod Pathol 2020;33:933)
- 33% of IBD patients with dysplasia as reported by Lee et al. (Histopathology 2021;78:814)
- 38% of carcinoma related lesions and 41% of nonpolypoid dysplasia compared to 7% of polypoid dysplasia as reported by Gui et al. (Hum Pathol 2020;100:24)
- Nonconventional dysplasia subtypes (Histopathology 2021;78:814, Hum Pathol 2020;100:24, Mod Pathol 2020;33:933):
- Hypermucinous (mucinous) dysplasia:
- Tubulovillous or villous architecture with prominent mucinous differentiation
- Usually mild nuclear atypia, especially towards the surface
- Dysplasia with increased Paneth cell differentiation:
- Tubular architecture with elongated, hyperchromatic nuclei
- Foci of increased Paneth cell differentiation, defined by Choi et al. as involving at least 2 contiguous crypts in 2 different foci and increased relative to background mucosa (Mod Pathol 2020;33:933)
- Goblet cell deficient (eosinophilic) dysplasia:
- Goblet cells are nearly or completely absent
- Eosinophilic cytoplasm
- Serrated dysplasia, which includes:
- Traditional serrated adenoma (TSA)-like
- Sessile serrated lesion (SSL)-like
- Serrated lesion not otherwise specified: does not meet criteria for SSL-like or TSA-like dysplasia
- Dysplasia with terminal epithelial differentiation (differentiated dysplasia) (Hum Pathol 2020;100:24, J Clin Pathol 2020;73:391)
- Nuclei are enlarged (can be mildly so), round to oval, slightly irregular, hyperchromatic and mostly nonstratified
- Nucleoli can be present, inconspicuous and occasionally prominent
- Some authors consider at least a subset of differentiated dysplasia as crypt cell dysplasia (see next heading) (Histopathology 2021;78:814, Hum Pathol 2020;100:24, J Clin Pathol 2020;73:391)
- Crypt cell dysplasia:
- Recently proposed to represent a high risk dysplastic lesion but the diagnosis based on histologic features alone is subject to significant interobserver variability and high rates of nondysplastic diagnoses, particularly indefinite for dysplasia (Histopathology 2021;78:814, Histopathology 2019;75:578)
- Atypia is limited to crypt base and does not involve surface epithelium
- Nuclei are round or oval, mildly enlarged, hyperchromatic and show slightly irregular contours
- Hypermucinous (mucinous) dysplasia:
- Dysplasia can show mixed histologic patterns (including mixed nonconventional and conventional dysplasia)
Microscopic (histologic) images
Virtual slides
Positive stains
- Various immunohistochemical stains have been evaluated to help distinguish dysplasia from nondysplastic epithelium but lack of sensitivity and specificity and limited data limit their use
- p53 and Ki67: increased nuclear staining extending to the surface has been suggested to favor dysplasia over regenerative atypia but lack of specificity limits their diagnostic use (Arch Pathol Lab Med 2013;137:338, Semin Diagn Pathol 2015;32:334)
- CK7: positive staining may be a marker of dysplasia but sensitivity and specificity are limited (Mod Pathol 2014;27:303)
- AMACR: strong cytoplasmic staining reported to favor dysplasia (Semin Diagn Pathol 2015;32:334, Hum Pathol 2009;40:166)
Negative stains
- Loss of SATB2 expression described in a subset of IBD associated dysplasia (J Clin Pathol 2020;73:391)
Molecular / cytogenetics description
- TP53 mutations and aneuploidy occur early in IBD associated neoplasia (Nat Rev Gastroenterol Hepatol 2017;14:218)
- Aneuploidy reported to be more common in nonconventional dysplasia (particularly hypermucinous and goblet cell deficient types) than conventional dysplasia (Histopathology 2021;78:814)
- Aneuploidy frequent with invisible and flat dysplasia (J Pathol Transl Med 2021;55:83)
- Case series of crypt cell atypia or dysplasia reported aneuploidy in all 14 cases (Histopathology 2021;78:814, Histopathology 2019;75:578)
- Conventional and nonconventional dysplasia frequently show p53 immunohistochemical mutant pattern expression and other molecular alterations (e.g. KRAS mutations, MLH1 deficiency, etc.) (J Clin Pathol 2020;73:391)
- TSA-like dysplasia in colectomy specimens more likely to show KRAS mutations and less likely to show BRAF mutations than endoscopically resected TSA-like lesions in IBD patients (Hum Pathol 2020;97:19)
Molecular / cytogenetics images
Sample pathology report
- Presence or absence of dysplasia should be reported for biopsies from surveillance colonoscopies as follows (Hum Pathol 1983;14:931, Gut 2000;47:251):
- Negative for dysplasia
- Indefinite for dysplasia
- Low grade dysplasia
- High grade dysplasia
- Polypoid lesion within colitic mucosa, endoscopically resected:
- Colon (sigmoid), polypectomy:
- Polypoid low grade dysplasia
- Colon (sigmoid), polypectomy:
- Nonpolypoid lesion, endoscopically resected piecemeal:
- Colon (ascending), biopsy:
- High grade dysplasia
- Completeness of excision cannot be determined (specimen fragmented)
- Colon (ascending), biopsy:
- Invisible dysplasia identified on random biopsy:
- Rectum, biopsy:
- Low grade dysplasia
- Rectum, biopsy:
- Note: a comment noting that endoscopically resectable lesions that are completely removed can be managed with continued surveillance rather than colectomy may be helpful
Differential diagnosis
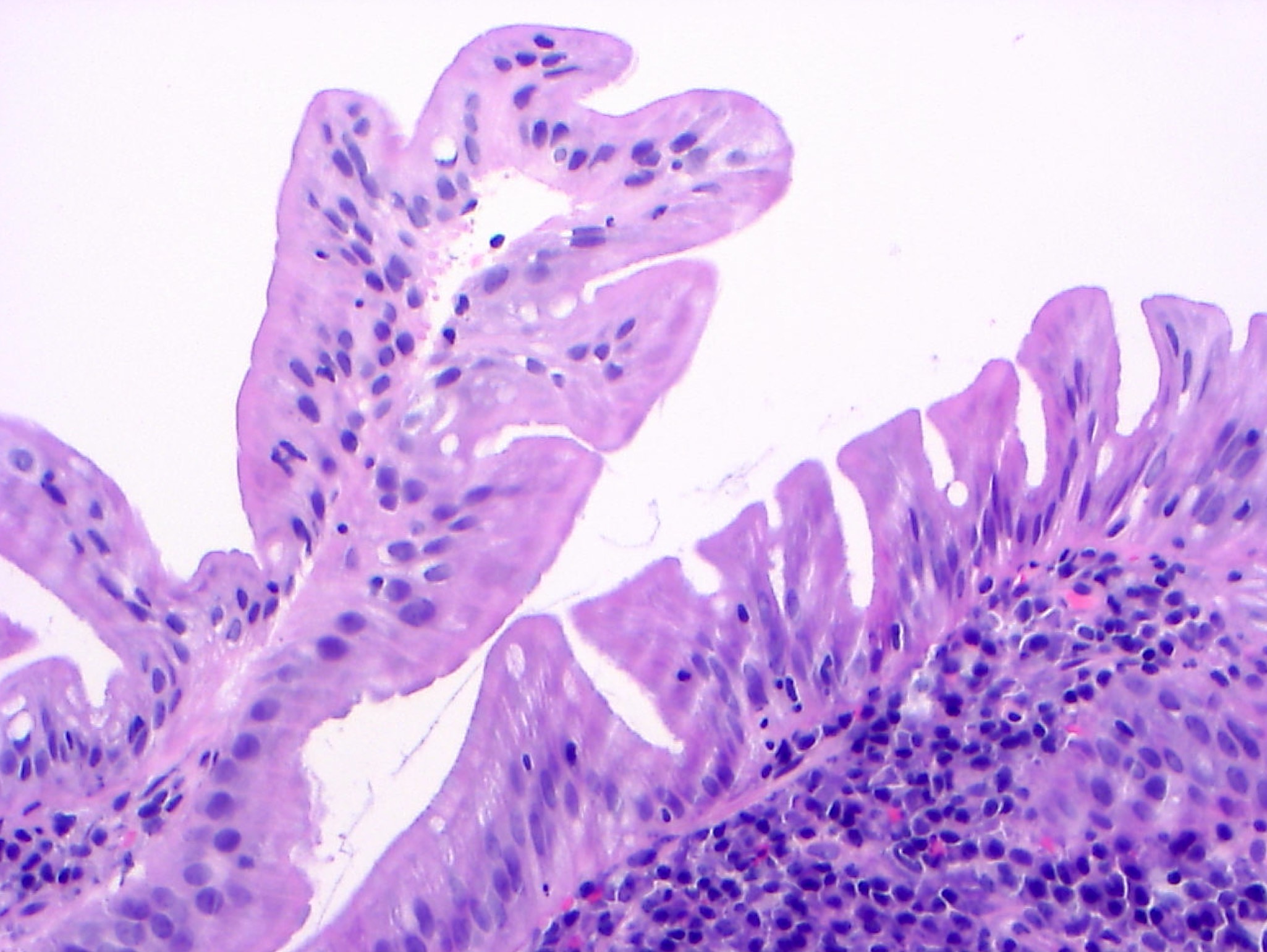
- Reactive epithelial atypia:
- May show mild nuclear stratification and crowding, nuclear enlargement and hyperchromasia and nucleoli
- Surface maturation is present
- Nuclear polarity is preserved
- Inflammatory background may be present
- Nonconventional dysplasia, e.g. increased Paneth cell differentiation or goblet cell deficient variant, can mimic reactive, metaplastic changes (Mod Pathol 2020;33:933)
- Features such as lack of surface maturation and cytoarchitectural atypia help establish a diagnosis of dysplasia
- Sporadic adenoma:
- Occurs in colonic segments outside colitic mucosa
- Not related to IBD
- Otherwise similar epithelial changes as those in (conventional) IBD associated dysplasia
Practice question #1
Which of the following statements regarding surveillance colonoscopies for patients with inflammatory bowel disease is true?
- Complete endoscopic resection of colitis associated polypoid high grade dysplasia can be followed up with continued surveillance
- Most colorectal dysplasia identified in surveillance colonoscopies is endoscopically invisible
- Nonpolypoid dysplasia is never endoscopically resectable and should therefore prompt colectomy
- Polyps with dysplasia present in mucosa outside of mucosa affected by chronic colitis are not considered sporadic adenomas
Practice answer #1
A. Complete endoscopic resection of colitis associated polypoid high grade dysplasia can be followed up with continued surveillance.
Comment Here
Reference: Dysplasia
Comment Here
Reference: Dysplasia
Practice question #2
Which of the following statements regarding dysplasia associated with inflammatory bowel disease is true?
- Early genetic changes present in colitis associated neoplasia are similar to those in sporadic neoplasia
- Indefinite for dysplasia may be diagnosed if the distinction between reactive atypia and dysplasia cannot be established
- Loss of nuclear polarity is a feature of low grade dysplasia
- Only patients with ulcerative colitis and not Crohn's disease have an increased risk of colorectal carcinoma
Practice answer #2
B. Indefinite for dysplasia may be diagnosed if the distinction between reactive atypia and dysplasia cannot be established
Comment Here
Reference: Dysplasia
Comment Here
Reference: Dysplasia