Table of Contents
Definition / general | Essential features | LIS certification | Diagrams / tables | Components of an LIS | Information security | Test ordering | Specimen collection, accessioning and processing | Analytic phase | Result entry and validation | Result reporting | Videos | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Jonah UJ, Parwani A. LIS fundamentals. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/informaticslisfundamentals.html. Accessed April 19th, 2024.
Definition / general
- A laboratory information system (LIS) is computer software that processes, stores and manages data from all stages of medical processes and tests (TechTarget: Laboratory Information System [Accessed 10 May 2023])
- LIS manages the following systems
- Patient check in
- Order entry
- Specimen processing
- Results entry and patient demographics
- In summary, the LIS tracks and records the entire patient's encounter with the laboratory and stores such records in a database for future reference
- Data captured and processed in an LIS reduces the turnaround time of laboratory tests and significantly decreases the errors associated with the manual clinical data transcribing process
Essential features
- Laboratory information systems possess the following notable features
- Sample registration and monitoring
- Quality control assessments
- Workflow management
- Detailed reports and analytics
- Inventory tracking
- Equipment integrations
- Data management and storage
- Strict compliance with standard operating procedure (SOP) guidelines (see Videos)
- Reference: TrustRadius: Laboratory Information Management Systems [Accessed 10 May 2023]
LIS certification
- LIS is only certified to be of functional capacity when it meets the meaningful use program criteria of the 2009 Health Information Technology for Economic and Clinical Health (HITECH) Act (athenahealth: What is Meaningful Use? [Accessed 10 May 2023])
- Such certified LIS is usually referred to as an electronic health record [EHR] module
- Some of the meaningful use program measures include the
- Electronic submission of lab test results to public health agencies
- Incorporation of clinical lab test results into a certified EHR system
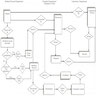
- Optimal functionality of an ideal LIS will depend on the modules in figure 1 (see Diagrams / tables)
Components of an LIS
- Components of an ideal LIS include
- Information security
- Test ordering
- Specimen collection, accessioning and processing
- Analytic phase
- Result entry and validation
- Result reporting
- Notification management
- Data mining and cross sectional reports
- Method validation
- Quality management
- Administrative and financial issues
- Operational issues (Arch Pathol Lab Med 2013;137:1129)
Information security
- Ideal LIS will prevent unauthorized access from internal and external threats (Arch Pathol Lab Med 2013;137:1129)
- This in turn will preserve the confidentiality of health records
- It also ensures that only legitimate users can be granted access to the system
- Physicians can access the information of their patients but are restricted from accessing information of other patients outside their care
- Quality assurance experts are allowed access only to certain information on all the patients
- Multistrata security system should be adopted, just as shown in table 1 below
- LIS should be fitted with secured interfaces and advanced capabilities (e.g., biometric recognition, radiofrequency identification devices [RFIDs])
- These capabilities will minimize keystrokes and login time
- They also provide quick automated logout upon leaving the workstation
- LIS should be capable of tracking and displaying the testing process live to minimize multiple login requirements by the management team
- Healthcare providers should be able to login remotely into the system using a secure web browser and from mobile and handheld devices (J Pathol Inform 2012;3:15, Clin Exp Med 2008;8:117)
- System should allow for the authentication of data and documents using efficient electronic signatures
Test ordering
- Test ordering (TO) is one of the laboratory steps that can benefit most from the LIS (Arch Pathol Lab Med 2013;137:1129)
- If managed by the LIS, TO will significantly improve laboratory utilization
- TO systems, when synchronized with intelligent decision support systems, will reduce turnaround times, decrease patient's length of stay and optimize test utilization among care providers (Stud Health Technol Inform 2009;150:527, J Pathol Inform 2011;2:35)
- For the TO system to be effective, immediate feedback must be relayed to the user
- Effective LIS allow providers to enter their test orders using the computerized provider order entry (CPOE) systems
- Computerized provider order entry systems must match the routine work processes of healthcare providers as well (Stud Health Technol Inform 2011;169:487)
- LIS should contain the following information from the ordering provider
- Ordering provider information
- Name (mandatory)
- Specialty
- Address (for providers in a different / remote location)
- Contact media (e.g., email addresses, mobile and desk telephone numbers, pagers, etc.) for routine feedback from the laboratory (mandatory)
- Additional recipients of patients' results (e.g., additional healthcare providers and other legally authorized individuals)
- Efficient notification protocols, such as
- Notifying providers when results are available
- Notifying providers when results exceed the reference range or custom threshold limit
- Ability to use multiple notification media effectively, such as
- Health information systems (HIS) overriding alert
- HIS alert on patient records
- Emails
- Short message service (SMS) text messages
- Automated phone calls
- Beeper
- Telefax, etc.
- Failsafe features must be built into important notifications (e.g., critical results)
- Providers should be able to provide acknowledgments of receipt of information from the laboratory
- Unacknowledged information should be escalated by the system using predetermined protocols (Clin Chem 2010;56:417)
- Patient information
- Patient identification
- Last and first names
- Institutional or social security number
- Unique coded audit trails for research or environmental specimens
- Patient demographics
- Date of birth
- Age
- Sex
- Race
- Ethnicity
- Prior names
- Patient address
- Permanent address
- Current location for hospitalized patients
- Codified diagnosis
- Preliminary diagnosis by the International Classification of Diseases (ICD)-9 or ICD-10
- Additional clinically relevant data (e.g., reason for the study)
- Codified results of nonlaboratory results
- Vital signs, height and weight
- Medications, including
- Dose of medication
- Date of prescription
- Mode and timing of administration
- Use of herbal medications and other supplements
- Diet and mealtimes to determine if the patient was fasting at the time of test order
- History of medical procedures (e.g., surgical procedures, radiologic procedures)
- Obstetric and gynecologic history
- Additional pertinent patient clinical information
- Patient identification
- Order information
- Requested test(s)
- Sources of requested specimen(s)
- Date and time of order
- Day and time of specimen collection including when specimen collection began and when it ended
- Test order repeat frequency (usually done for standing orders)
- Special patient preparation instructions prior to specimen collection
- Urgency of the test
- Those responsible for the test collection (e.g., patient mail in, point of care, ward or nursing unit, routine phlebotomy rounds, laboratory collection)
- Free text comments and instructions to the laboratory
- Ordering provider information
- LIS should be fitted with automated systems capable of harvesting patient information, previous test results and clinician’s input (including differential diagnosis) to suggest appropriate tests, test frequency and interpretative data (Stud Health Technol Inform 2011;169:487, Ann Intern Med 2001;134:274)
- LIS should contain a user friendly display of test catalog, which includes
- Tests performed by external reference laboratories
- Ability to group results in alternate ways (e.g., alphabetically, by laboratory discipline, by clinical situation)
- Menus on the interface must be consistent, complete and regularly updated
- Standard nomenclatures consistent with all HIS should be employed
- Items to be included in test catalog's entry should consist of those listed in table 2 below
- LIS should be able to restrict certain test ordering based on
- Location
- Diagnosis
- Healthcare provider’s specialty, etc.
- LIS should be able to specify tests that need approval from specialized health providers such as clinical or laboratory specialists
- This approval system should be able to automictically notify the approver
- It should be able to inform the ordering provider of pending tests needing approval
- LIS should be able to differentiate research specimens from patient care specimens
- It should enable different billing procedures
- Research orders should be attached to research management systems
- LIS should operate on an order appropriateness expert system as outlined in table 3 below
- LIS ordering system should be capable of relaying orders to different interfaced systems
- LIS at different institutions should be able to relay relevant information to one another without manual intervention
- Tests ordered at one institution should enable specimens to be collected and accessioned in another
- Reference laboratories should list their laboratory catalog online for easy access to ordering providers
- LIS should also enable participating institutions to implement institution specific restrictions and approval processes for ordering, testing and reporting
- For outgoing tests, LIS should be able to handle all information related to shipping the specimen (AMIA Annu Symp Proc 2010;2010:76)
- Ordering providers should have access to real time feedback from the ordering system of the LIS about each step of the process
- Real time progress can be reported as follows
- Order acknowledged by laboratory
- Specimen(s) collected
- Specimen(s) accessioned
- Accession(s) activated in laboratory
- Analysis completed
- Results verified
- Results reported; order completed
- LIS TO system should be able to split the laboratory orders such that one order can consist of multiple tests requiring multiple specimens and accessions
- Each component of an order should be tracked separately in real time as well
- Real time progress can be reported as follows
Specimen collection, accessioning and processing
- Quality of laboratory results is significantly determined by standard and appropriate specimen collection and processing (Arch Pathol Lab Med 2013;137:1129)
- Specimen collection produced by the LIS should meet institutional requirements (e.g., the LIS should produce preprinted accession labels using the appropriate list of patients for phlebotomy)
- List should indicate to patients the location and time of their phlebotomy (phlebotomy routes) in the most efficient manner possible
- LIS should provide the specimen collector with an online or printed display of specimen collection instructions in an accessible step by step format
- Relevant online links containing a complete procedure guide should also be provided
- LIS should provide the specimen collector with a list of pending laboratory orders
- It should be capable of generating unique barcoded or RFID labels at patients' bedside upon scanning patients' identification wristband (J Med Syst 2011;35:1403, Ann Emerg Med 2010;56:630)
- Labels generated at the point of collection should include
- Minimum of 2 patient identifiers
- Date and time of specimen collection
- Collector identity
- Urgency of the order
- Abbreviated names of tests requested
- 2 dimensional bar codes or RFID labels should be used to allow for more significant amounts of information to be attached to the specimen
- After arriving at the laboratory, the LIS should be able to recognize specimens by scanning the labels attached to such specimen containers
- LIS in the laboratory should also initiate testing once a specimen arrives in the laboratory in a robotic specimen processing automation line where applicable
- LIS should allow the collector to enter pertinent patient information in codified or free text form to allow for proper interpretation of certain laboratory tests (see table 4 below)
- LIS should support bidirectional interfaces with portable devices for patient identification, specimen accessioning and point of care testing
- Point of care testing should be integrated with those from main analyzers
- Sources of test results should be identifiable as well
- LIS should also interface with a point of care management system to track instruments, reagents, quality control, user identity, training and competency records
- LIS should be able to record the accessioning of specimens separately (e.g., it should be capable of matching an order with a physical specimen)
- It should be able to acknowledge receipt of the specimen at the laboratory
- It should also be able to activate the specimen for analysis
- Mode of operation listed above can be summarized in the example below (adapted from Arch Pathol Lab Med 2013;137:1129)
- Phlebotomist scans the patient barcoded wristband and chooses an appropriate pending order
- System records the collection time and accessions the specimen
- Portable device carried by the phlebotomist prints an accession label
- Specimen is collected and labels are affixed to the specimen container in the presence of the patient
- Specimen is then sent to the laboratory
- Specimen accession labels are scanned at the laboratory reception desk upon arrival to confirm receipt by the lab
- They are then transported to the analytic section of the laboratory
- Labels are once more scanned when the specimen is placed in an automated robotic specimen processing line and the accession is then activated for analysis
- Entire process can be divided into order to collection to accession to receipt to activation to report
- Last component (activation to report) is referred to as the analytic time
- Previous components are referred to as the preanalytic time
- It is important to note that only the receipt to report processes are under complete control of the laboratory
- Using these time points, troubleshooting of the system can be efficiently done to resolve specimen collection, accessioning and processing issues
- LIS should allow institutions to customize their specimen processing systems in ways that are different from the processes outlined above; e.g.,
- Specimens received in the laboratory without an order but with appropriate patient identifiers should be acknowledged by the system pending the arrival of an appropriate corresponding order
- LIS should also allow the laboratory staff to enter a paper or verbal order into the system in certain cases
- When properly identified specimens without accession labels arrive at the laboratory with either a paper or electronic order, the laboratory should be able to verify the order and specimen
- Lab can then proceed to accession the specimen and apply appropriate labels or RFID tags to it
- LIS should be able to accession and process nonpatient specimens such as
- Animal specimens
- Research specimens
- Environmental specimens not associated with a patient
- Quality control and validation materials
- Proficiency testing materials
- LIS should also be able to codify and deidentify specimens for research purposes
- It should also be able to contain database management capabilities for biobanks and tissue repositories
- LIS should interface with the laboratory automation management software to enable the preanalytic requirements specified in the ordering process to be transmitted to the specimen processing system; examples of such preanalytic requirements include
- Centrifugation speed
- Time
- Number of aliquots
- Reflexive testing
- LIS should track the specimen location throughout the preanalytic, analytic and postanalytic phases of the specimen testing
- Transportation to various aspects of the laboratory and to external sites should also be tracked
- LIS should also keep track of the specimen storage management (J Med Syst 1998;22:137)
- Storage management should include the ability to easily retrieve the precise specimen storage location
- It should also include periodic reports to facilitate batch disposal of specimens
- LIS should be able to generate multiple specimen aliquot labels
- These labels can then be scanned to execute the appropriate testing associated with each aliquot
- System should also be capable of tracking the management and storing of multiple aliquots or slides derived from 1 specimen
Analytic phase
- Analytic phase of the LIS has the most technological advancement in terms of clinical laboratory science and is hence least prone to errors in the clinical laboratory (Arch Pathol Lab Med 2013;137:1129)
- LIS can be interfaced with the analytic instrumentation software (also known as middleware) for the following processes
- Streamlining the processing of analytic requests
- Directing testing to the appropriate instruments
- Recalling specimens for repeated testing
- Directing specimen dilutions
- Performing reflexive testing
- Processing add on requests for additional tests
- Record test results and appropriate comments
- LIS should be able to track, record and store all the relevant information associated testing and assays as shown in table 5 below
- LIS should enable the analyst display on the screen or print out appropriate standardized testing procedure for each test (especially for manual assays)
- LIS should be able to oversee the maintenance of the testing instrument in the following ways
- Testing instruments record should be updated with each patient test
- Analyzer record should include all the information in table 6 below
- Analyzer should provide links to online preventive maintenance and service records
- In specified cases, the instrument manufacturer should be automatically notified of the instrument's functional status
- LIS should produce laboratory specific worklists capable of
- Facilitating batch processing
- Facilitating the production of manual and automated test results
- Tracking orders that are yet to be completed
- Expanding worklist after additional specimens are added by scanning the specimen's barcodes or RFID tags
- LIS should be able to display lists of accessioned but uncompleted tests, especially when such tests have exceeded their allotted time
- Display can either be live on the computer screen or on demand
- LIS should also be able to pinpoint at which point the failure occurred
- Incomplete lists should include tests that were sent to reference laboratories
- Displays can be done on large screens for instance in the emergency room
- Color coding can be added to the displays to bring attention to tests that have exceeded their allotted predefined time limit
- List should be able to be sorted by age of request and other demographic parameters
- Goal of such displayed lists is to alert staff to ensure that such test failures are quickly rectified and in so doing, improve the test turnaround time for patients
Result entry and validation
- LIS should guide analysts into providing high quality results that are accurate, reproducible and appropriate for the clinical situation (Arch Pathol Lab Med 2013;137:1129)
- LIS should allow for high quality results entry and validation
- It should be able to record results in various data formats (e.g., numbers, text with extended characters, images; the LIS data storage system should be flexible enough to avoid constraining limits on data size)
- Interfaced and noninterfaced analyzers as well as manual tests should allow for
- Automated entry of results
- Manual entry of results
- Application of standard security levels to results entry and reporting
- Options should be available for the following types of result entry
- Individual result entry
- Batch results entry
- Batch entry by exception
- Amended results
- Appended results
- Intermediate and final results
- Results can also be entered as individual tests or by panels with user definable panel configuration
- LIS should allow for different levels of results certification such as the ability to withhold release of results until approval is gotten from a higher level user (e.g., a supervisor)
- LIS should be able to receive results in different formats such as
- In the form of tables and graphs from other laboratories
- Via electronic interfaces for easy integration of the results into existing electronic records
- Receiving results from multiple laboratories in multiple formats will help diagnosis of diseases, like leukemia
- Diagnosis of leukemia requires clinical information, hematology, hematopathology, molecular and flow data reports
- LIS should have the ability to engage advanced expert decision support for autovalidation of results (Clin Chem 2000;46:1811, Eur J Clin Chem Clin Biochem 1997;35:711)
- Autovalidation significantly improves the efficient running of the laboratory by bypassing human intervention in the certification of laboratory results (Accredit Qual Assur 2002;7:431)
- System employed to perform autovalidation must be sophisticated enough to significantly reduce the probability of reporting erroneous results
- It should also allow adequate time for human specialists to examine exceptional results
- Artificial intelligence inputs needed to provide an autovalidation of test results involves
- Comparing the results with patients' previous test results the system's record in or database (temporal delta checks)
- Comparing the results with results of other related tests in the same or closely related specimens (cross sectional check) (e.g., creatinine versus urea)
- Examining specimens for predefined limits of hemolysis, lipemia or icterus
- Making use of clinically relevant demographic information such as location (inpatient versus outpatient), type of clinic, diagnoses, medications, procedures
- Making use of internal and external quality control results
- Making use of statistical data on result distribution (Clin Chem 2000;46:1811)
- LIS temporal delta checks abilities should include
- Analysis of temporal data
- Calculation of rates of change and absolute changes in reference to predetermined limits subject to variations based on patient demographics, diagnoses, therapies and other relevant patients' clinical records (Clin Lab Med 2008;28:83, J Am Med Inform Assoc 2007;14:674)
- LIS expert system should possess the ability to order reflexive testing based on results analysis and clinical data
- Policies regarding such reflexive testing should be set by the institution or laboratory
- Reflexible testing should also be customizable by the ordering provider
- It should also interface with the specimen processing and analytic systems
- In addition, the LIS should be able to append appropriate codes or comments to the results (Arch Pathol Lab Med 2013;137:1129)
Result reporting
- LIS should be capable of providing a variety of test results that can be organized as follows
- Test
- Test group
- Date
- Date range
- Ordering provider
- Provider group
- Clinic or specialty
- Sequential worksheets
- Tabulated cumulative worksheets (Arch Pathol Lab Med 2013;137:1129)
- LIS should measure the actual value of test results and display numeric values in the following format
- Units of measurement
- Reference interval of the appropriate patient population such as ambulatory patients, recumbent patients, sex, age, race, body mass, gestational age, menstrual cycle phase
- Above reference populations should be user configurable when used as clinical inputs
- There should be a measure of individuality to guide the interpretation of reference ranges (Clin Chem 2002;48:395)
- Tests with high individuality usually have a within subject variability that is much lower than between subject variability
- In such cases, an appendage should be provided highlighting the fact that individual based reference changes are more appropriate than population based reference intervals
- In reference ranges with individuality coupled with enough recorded data of the patient, the system should be able to calculate and display individual specific reference ranges
- E.g., the system could plot a normal distribution curve based the patient's previous results to enable the healthcare provider to identify outliers
- LIS should put into consideration the confidence interval of the results based on analytical variability observed at a corresponding level
- LIS should also be capable of displaying reference change values (RCV)
- Reference change value refers how much a test result has changed from the patient's known usual or basal value
- RCV may help pick up patients with rapidly rising levels of creatinine for instance (from 0.3 mg/dL to 0.9 mg/dL within 48 hours) even when the new value is within the reference range of the general population (0.4 - 1.4 mg/dL) (Lablogatory: Reference Intervals vs. Reference Change Values [Accessed 10 May 2023])
- Factors that may be affecting the RCV include analytic imprecision, within subject biologic variability, number of repeated tests performed (Clin Chem 2002;48:395, Arch Pathol Lab Med 2010;134:81, Clin Chem 2011;57:1635)
- Users should be allowed to customize the RCV by selecting any of the following
- Confidence threshold (e.g., 95%)
- Appropriate Z value for decisions that involve 1 sided (e.g., increase) versus 2 sided (either increase or decrease) changes
- LIS should also be able to display results associated flags with user predefined thresholds as shown in table 7 below
- LIS should also display relevant comments appended by result analysts
- Report generation by the LIS should have the following qualities
- Flexibility
- Configurable by users of the test information, such as report producers like the laboratorians and report recipients like the healthcare providers and their patients
- LIS should endeavor to make reports available by a variety of options such as
- User configurable automated secure faxing
- Emailing and other electronic text transmission mechanisms
- LIS should allow for sophisticated graphing of laboratory results with the integration of the following clinical information
- Vital signs
- Biometrics
- Medication dose / timing (Clin Chem 2003;49:41, Methods Inf Med 2000;39:88, J Am Med Inform Assoc 2010;17:416)
- Graphing dynamics should also allow for
- Dynamic changes in axis and scales
- Histograms
- Conditional formatting
- Color coding
- User definable formulas for calculated results
- Display of multidimensional data
- Colored displays
Board review style question #1
What component of the laboratory information system (LIS) has the tremendous potential of reducing test turnaround times for patients, decreasing their length of stay and optimizing test utilization by healthcare providers?
- Analytic phase
- Information security
- Result entry and validation
- Specimen collection, accessioning and processing
- Test ordering system (TOS)
Board review style answer #1
E. Test ordering system. The test ordering system is a component of the LIS that can negatively impact the rest of the process if it is done incorrectly. Failure in the test ordering system will increase patient test turnaround time (TAT), delay hospital inpatients' discharge and significantly jack healthcare expenses. The LIS test ordering system, coupled with intelligent decision support systems such as the computerized provider order entry (CPOE) systems, can potentially reduce laboratory test turnaround times, decrease hospital inpatients' length of stay and guide providers toward optimized test utilization (Stud Health Technol Inform 2009;150:527, J Pathol Inform 2011;2:35).
Answer A is incorrect because the analytic phase of the LIS is the least error prone part of the LIS. It is highly automated and would not significantly impact the turnaround time of test results compared to the test ordering system. Answer B is incorrect because although good information security integrated into the LIS will minimize internal and external threats, the test ordering system (a part of the preanalytical phase of laboratory testing) remains one of the steps that if properly utilized will cut down the turnaround times of a test. Answer C is incorrect because while an integral part of the LIS, result entry and validation do not influence test turnaround times compared to the test ordering system. Answer D is incorrect because specimen collection, accessioning and processing is a useful part of LIS but does not contribute to the overall reduction in test turnaround time when compared to the test ordering system.
Comment Here
Reference: LIS fundamentals
Answer A is incorrect because the analytic phase of the LIS is the least error prone part of the LIS. It is highly automated and would not significantly impact the turnaround time of test results compared to the test ordering system. Answer B is incorrect because although good information security integrated into the LIS will minimize internal and external threats, the test ordering system (a part of the preanalytical phase of laboratory testing) remains one of the steps that if properly utilized will cut down the turnaround times of a test. Answer C is incorrect because while an integral part of the LIS, result entry and validation do not influence test turnaround times compared to the test ordering system. Answer D is incorrect because specimen collection, accessioning and processing is a useful part of LIS but does not contribute to the overall reduction in test turnaround time when compared to the test ordering system.
Comment Here
Reference: LIS fundamentals
Board review style question #2
A laboratory information system (LIS) that harbors a result entry system capable of receiving internal and external laboratory results in various formats, results formats like tables and graphs that can be integrated into patients' electronic records will have the most benefit in the diagnosis of which of the following diseases?
- Acute lymphoblastic leukemias
- Acute pancreatitis
- Gonococcal urethritis
- Helminthiasis
- Non-ST elevation myocardial infarction (NSTEMI)
Board review style answer #2
A. Acute lymphoblastic leukemias. LIS results entry system that can present results in various formats, such as tables and graphs, will significantly improve the diagnosis of diseases like leukemias. Leukemias require the patient's clinical information alongside hematology, hematopathology, molecular and flow data reports to enable the oncologist to make an accurate diagnosis. An LIS that can present all these results data to the healthcare provider in various formats will be invaluable in making precise and accurate diagnoses.
Answer B is incorrect because acute pancreatitis can easily be confidently diagnosed using clinical history and elevated serum amylase and lipase levels without the need for multiple complex result formats and interpretations like leukemias. Answer C is incorrect because gonococcal urethritis diagnosis is based on clinical history and a urethral swab and not a series of complex test results in need of multiple formats of reporting and interpretation like acute lymphoblastic leukemias. Answer D is incorrect because a simple gross and microscopic stool ova and parasite examination will easily diagnose helminthiasis. Answer E is incorrect because NSTEMI can be easily diagnosed using ECG findings, clinical history and elevated cardiac enzyme levels without the need for multiple complex result formats and interpretations like leukemias.
Comment Here
Reference: LIS fundamentals
Answer B is incorrect because acute pancreatitis can easily be confidently diagnosed using clinical history and elevated serum amylase and lipase levels without the need for multiple complex result formats and interpretations like leukemias. Answer C is incorrect because gonococcal urethritis diagnosis is based on clinical history and a urethral swab and not a series of complex test results in need of multiple formats of reporting and interpretation like acute lymphoblastic leukemias. Answer D is incorrect because a simple gross and microscopic stool ova and parasite examination will easily diagnose helminthiasis. Answer E is incorrect because NSTEMI can be easily diagnosed using ECG findings, clinical history and elevated cardiac enzyme levels without the need for multiple complex result formats and interpretations like leukemias.
Comment Here
Reference: LIS fundamentals