Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Immunofluorescence description | Positive stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Cazzaniga G, L'Imperio V. Checkpoint inhibitor associated tubulointerstitial nephritis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneynontumorcheckpointinhibitor.html. Accessed April 23rd, 2024.
Definition / general
- Definite immune checkpoint inhibitor associated acute kidney injury (ICI AKI) is confirmed by histological diagnosis and clinical consideration of risk factors in a patient who has undergone or is undergoing treatment with immune checkpoint inhibitor
- Criteria beyond histology indicate a probable or possible diagnosis (Kidney360 2020;1:130)
Essential features
- ICI AKI develops at a median of 14 - 16 weeks after ICI initiation (J Immunother Cancer 2021;9:e003467, J Am Soc Nephrol 2020;31:435)
- ICI AKI should be considered in cancer patients with AKI once other common causes, such as volume depletion, hemodynamic fluctuations and sepsis, have been ruled out (Front Med (Lausanne) 2022;9:964335)
- Kidney biopsy is recommended when feasible (J Immunother Cancer 2021;9:e002435)
- Histology shows an interstitial inflammatory infiltrate composed of lymphocytes, plasma cells and eventually neutrophils with frequent tubulitis (Kidney Int 2016;90:638)
Terminology
- ICI AKI, ICPI AKI: immune checkpoint inhibitor associated acute kidney injury (J Immunother Cancer 2021;9:e003467)
- Immune related adverse events (irAEs) (J Immunother Cancer 2021;9:e003467)
- Antiprogrammed cell death receptor 1 (anti-PD-1) antibodies, antiprogrammed cell death ligand 1 (anti-PDL1) antibodies and anticytotoxic T lymphocyte associated antigen 4 (anti-CTLA4) antibodies associated AKI
ICD coding
- ICD-10: N14.1 - nephropathy induced by other drugs, medicaments and biological substances
Epidemiology
- Estimated incidence of AKI in a cohort study with projected AKI rates ranges from 9.9% to 29% (Am J Nephrol 2017;45:160)
- Other studies report lower incidence of the disease (2.2%) (Kidney Int 2016;90:638)
- Patients with ICI AKI are older, have lower baseline estimated glomerular filtration rate (eGFR) and are more likely to have genitourinary cancer, proton pump inhibitor (PPI) exposure and to have received combination ICI therapy compared with patients without ICI AKI (J Immunother Cancer 2021;9:e003467, Clin Kidney J 2022;15:1881)
- Most recently approved class, PDL1 inhibitors (e.g., atezolizumab, durvalumab, avelumab), may have a favorable safety profile with lower rates of organ toxicity, including AKI, compared to other ICI classes (Kidney Med 2021;3:1074)
- Atezolizumab had the strongest association with renal adverse events among all ICI monotherapy regimens, whereas avelumab appeared to show a relatively weaker association with renal adverse effects (Cancer Med 2020;9:6576)
Sites
- Kidney
Pathophysiology
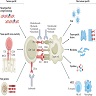
- Anti-CTLA4 and anti-PD-1 / anti-PDL1 reactivate the suppressor immune response through various mechanisms
- Mechanism by which ICIs induce AKI is not well established (Kidney Int Rep 2020;5:1139)
- One mechanism by which ICIs may induce kidney damage is related to the activation of autoreactive T cells normally kept dormant by immune checkpoint mechanisms under physiological conditions
- PD-1 is expressed after activation on T cells, B cells, natural killer T cells, activated monocytes and dendritic cells, whereas its ligand PDL1 is expressed on kidney tubules, especially the proximal tubular segments (Clin Immunol 2005;115:184)
- Another mechanism may involve the reactivation of exhausted drug specific T cells previously primed by nephritogenic drugs (e.g., PPIs, nonsteroidal anti-inflammatory drugs [NSAIDs]) (Cancers (Basel) 2023;15:1891)
- Another potential mechanism is through haptenization, when low molecular weight drug compounds bind tubular antigens, thus creating a hapten that can be trapped in the parenchyma, leading to an immune response and tubular damage (Kidney Int Rep 2020;5:1139)
Etiology
- Renal damage caused by anti-CTLA4 leads to early lymphocyte infiltration of renal tissue, with rapid onset averaging 6 - 12 weeks (Front Med (Lausanne) 2022;9:1014257)
- Conversely, anti-PD-1 / anti-PDL1 treatment determines loss of tolerance and subsequent stimulation of immune response, resulting in nephrotoxicity onset at 3 - 12 months (Front Med (Lausanne) 2022;9:1014257)
- Combination of CTLA4 and PD-1 inhibitors carries a higher risk of ICI induced AKI (Kidney Med 2021;3:1074)
- Glomerular damage, including podocytopathy, membranous nephropathy and thrombotic microangiopathy, has been reported after administration of ipilimumab alone; ipilimumab has also been associated with systemic lupus erythematosus-like nephritis, characterized by diffuse tissue damage and glomerular sclerosis (Front Med (Lausanne) 2022;9:1014257)
Diagrams / tables
Clinical features
- ICI AKI: developed at a median of 14 - 16 weeks after ICI initiation (J Immunother Cancer 2021;9:e003467, J Am Soc Nephrol 2020;31:435)
- While hematuria (16%), eosinophilia (21%) and worsening renal hypertension (11%) were noted in some patients, rising serum creatinine (100%) and pyuria (68%) may be the only clinical clue in a large majority of the cases
- Only a few patients exhibit the classic symptoms of fever, eosinophilia or rash
- If distal renal tubule injury is involved, manifestations include hypokalemia and metabolic acidosis (Clin Kidney J 2019;13:42)
- 43% had a concomitant history of extrarenal irAEs, such as hypophysitis, thyroiditis, dermatitis, rash or colitis (J Am Soc Nephrol 2020;31:435)
- ICI can reactivate preexisting autoimmune diseases, such as rheumatoid arthritis, lupus and inflammatory bowel disease, in 27 - 50% of patients but adverse kidney outcomes in preexisting glomerulonephritis are less commonly reported (Kidney Med 2021;3:1074)
Diagnosis
- ICI AKI should be considered in cancer patients with AKI once other common causes, such as volume depletion, hemodynamic fluctuations and sepsis, have been ruled out (Front Med (Lausanne) 2022;9:964335)
- The Society for Immunotherapy of Cancer (SITC) recommends kidney biopsy when feasible (J Immunother Cancer 2021;9:e002435)
- Identification of non-ICPI induced kidney lesions, such as acute tubular injury, on biopsy may avoid unnecessary corticosteroid exposure and permit continued ICI therapy, especially in cases of suspected ICI related glomerulopathy
- If empiric corticosteroids are started before biopsy and the patient fails to respond, a biopsy should be considered to assess alternative etiologies of AKI (J Immunother Cancer 2021;9:e003467)
Laboratory
- Similar to those observed with AKI caused by other drug induced acute tubulointerstitial injury (ATIN)
- Bland urinalysis with subnephrotic range proteinuria, sterile pyuria, white blood cell casts, eosinophilia, unless a vascular and glomerular lesion is present (Front Med (Lausanne) 2022;9:964335, Kidney Int Rep 2020;5:1139)
- Normal complement levels (C3 and C4) (Kidney Int 2016;90:638)
- Noninvasive markers for the definite diagnosis of ICI AKI are not yet available
Radiology description
- Bilateral increase in kidney size, new / increasing perinephric stranding and bilateral wedge shaped hypoenhancing cortical foci can occur in immunotherapy related nephritis (Eur Radiol 2023;33:2227)
- On PET / CT, a diffuse increase in radiotracer uptake throughout the renal cortex and a decrease in radiotracer activity in the renal pelvis can be seen (Eur Radiol 2023;33:2227)
Prognostic factors
- During a median follow up of 30 weeks from ICI AKI diagnosis, 39.2% of patients died (J Immunother Cancer 2021;9:e003467)
- Higher baseline eGFR, lung cancer and stage 3 AKI were each associated with lower odds of renal recovery, whereas concomitant treatment with ATIN causing medications was associated with higher odds of renal recovery (J Immunother Cancer 2021;9:e003467)
- Treatment with corticosteroids within 14 days following ICI AKI was also associated with a higher odds of renal recovery (J Immunother Cancer 2021;9:e003467)
- Critical role of the PD-1 pathway both in immune resistance by cancers arising in the context of long term immunosuppression and in the maintenance of partial adaptive tolerance to transplanted organs (N Engl J Med 2016;374:896)
- Outcomes for de novo ICI induced glomerular diseases are poor, with a limited ability to rechallenge with ICI, progression to end stage kidney disease and a significant mortality rate (Kidney Med 2021;3:1074)
Case reports
- 57 year old man with successfully treated late onset ICI associated membranous nephropathy (Front Immunol 2022;13:898811)
- 60 year old man with sarcoid-like granulomatous reaction after ICI treatment for renal cell carcinoma (Kidney Med 2023;5:100626)
- 62 year old man with renal cell carcinoma and nivolumab associated nephrotic syndrome (J Immunother 2017;40:345)
- 67 year old man with stage IV non-small cell lung cancer who developed kidney injury during treatment with the anti-PD-1 antibody nivolumab (BMC Nephrol 2018;19:48)
- 73 year old man with atezolizumab associated rapidly progressive pauci-immune glomerulonephritis (Antibodies (Basel) 2023;12:10)
Treatment
- Nephrology oncology approach is mandatory for treatment decisions
- When ICI related renal involvement is strongly suspected, the first step in management includes temporarily discontinuing ICI treatment and starting steroid therapies (Cancers (Basel) 2023;15:1891)
- Corticosteroid taper begins when creatinine improves to grade 1 or below
- Guidelines from the National Comprehensive Cancer Network are similar and specify a starting dose and duration for the corticosteroid taper at 0.5 - 1 mg/kg/day for grade 2 and 1 - 2 mg/kg/day for grade 3 AKI with the dose being tapered over 4 - 6 weeks after creatinine decreases to less than or equal to grade 1
- Compared with nonrechallenged patients, rechallenged patients were less likely to initially have had ICI AKI stage 3 and were more likely to have had renal recovery following the initial ICI AKI episode; survival was similar among patients rechallenged versus not rechallenged in the first 90 days and in the first 180 days following ICI AKI
- Consider additional immunosuppression (cyclophosphamide, azathioprine, cyclosporine, infliximab or mycophenolate) if AKI does not improve to less than grade 2 within 1 week (Kidney Med 2021;3:1074)
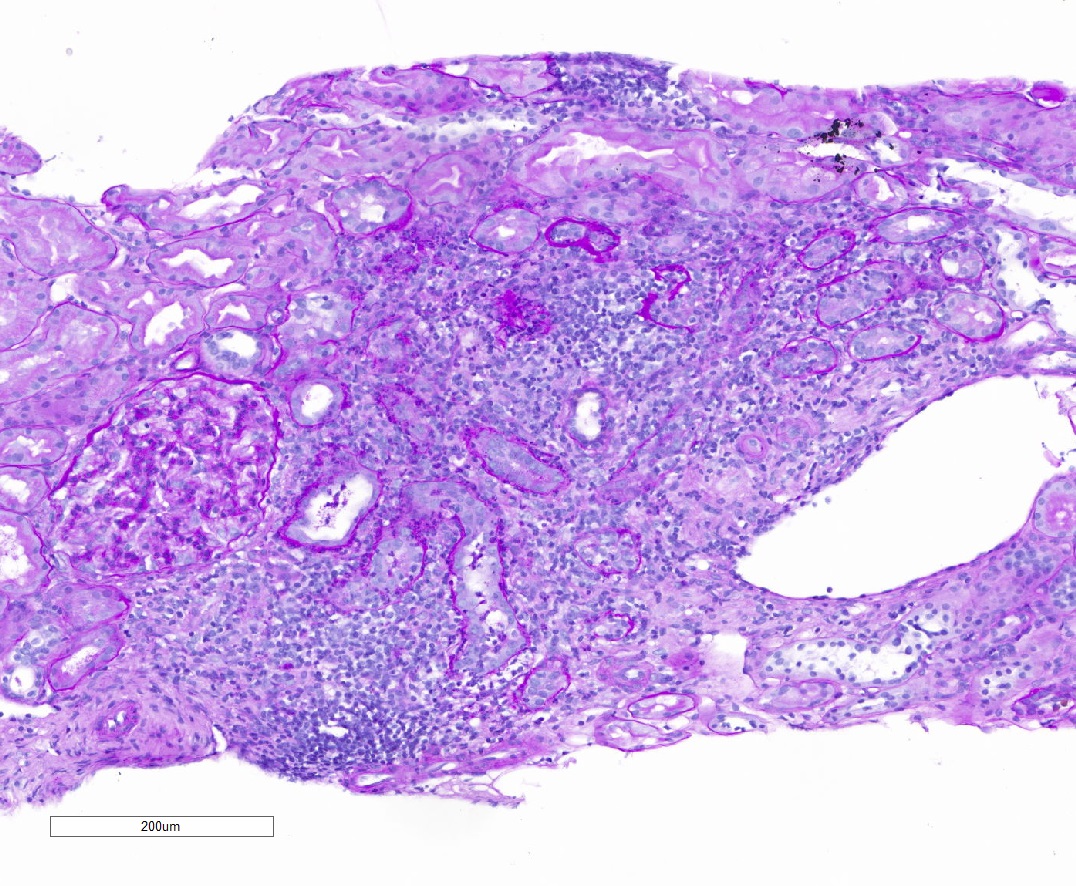
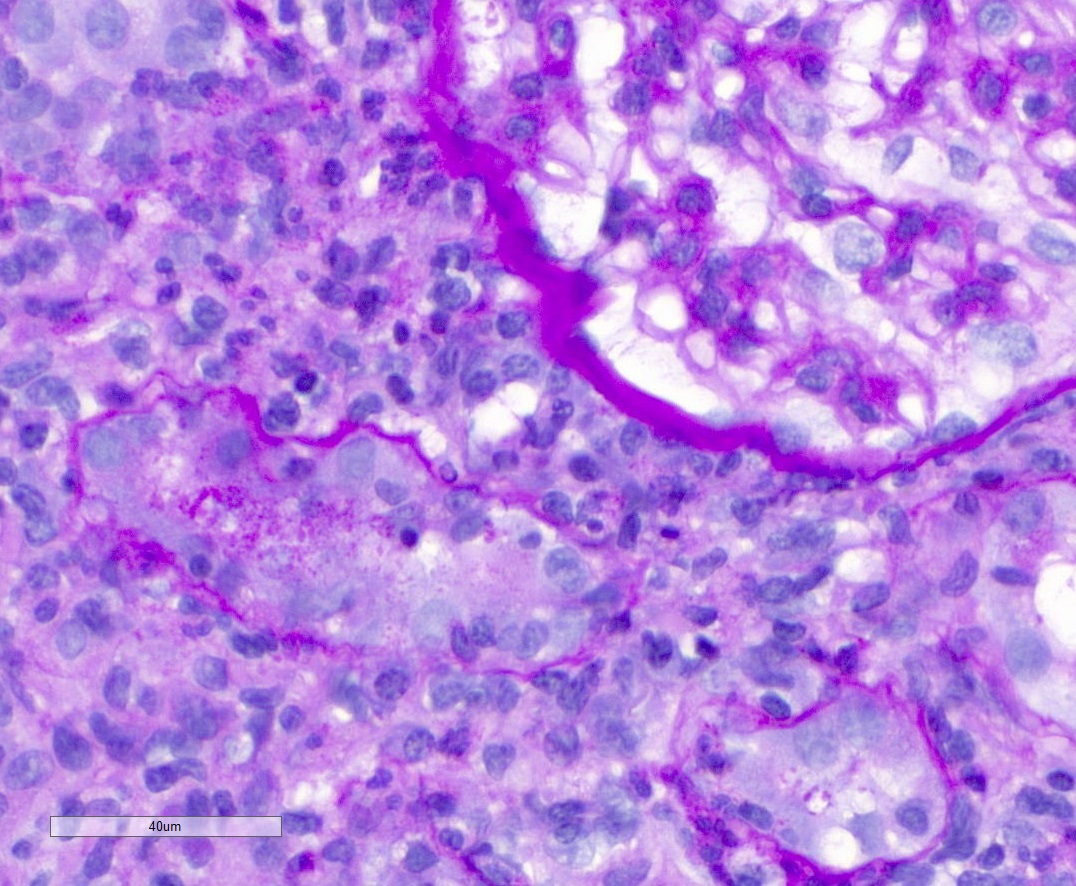
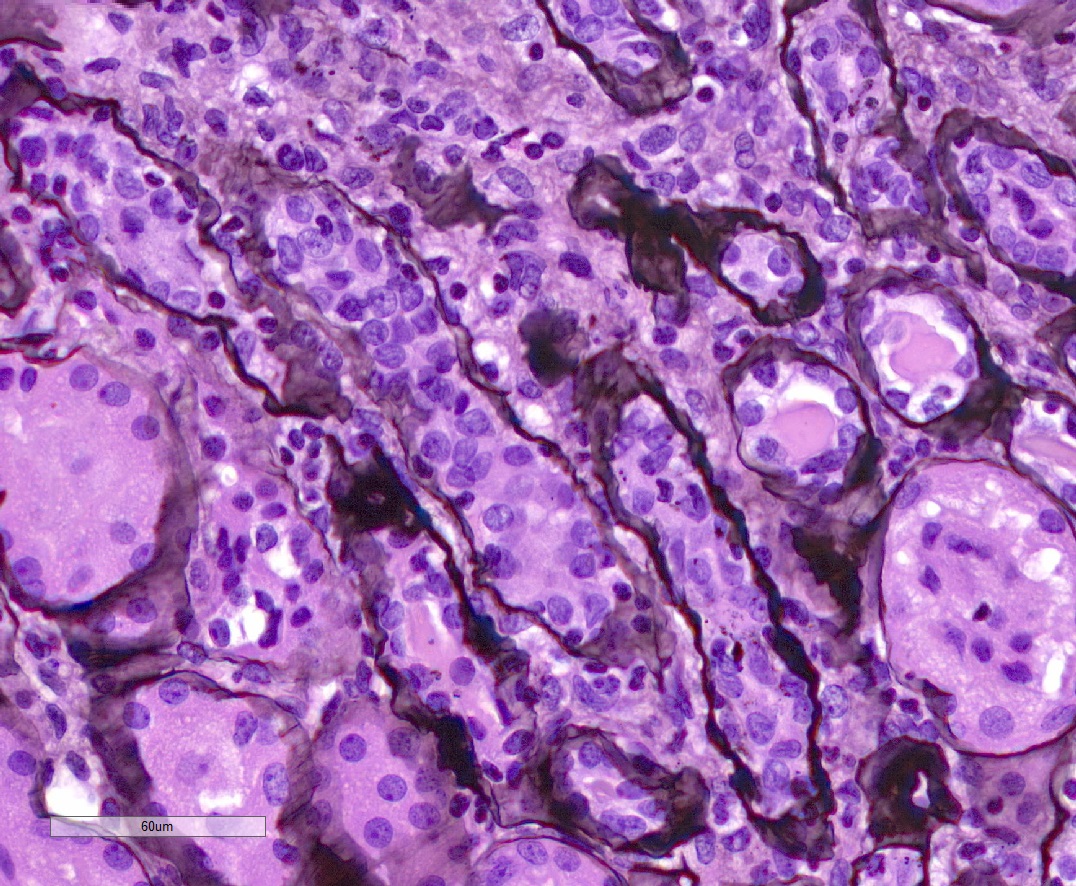
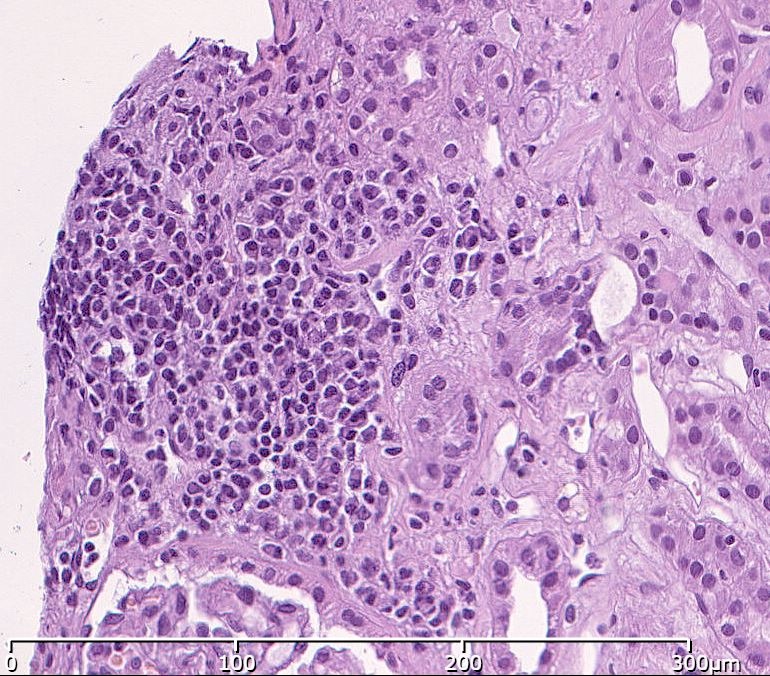
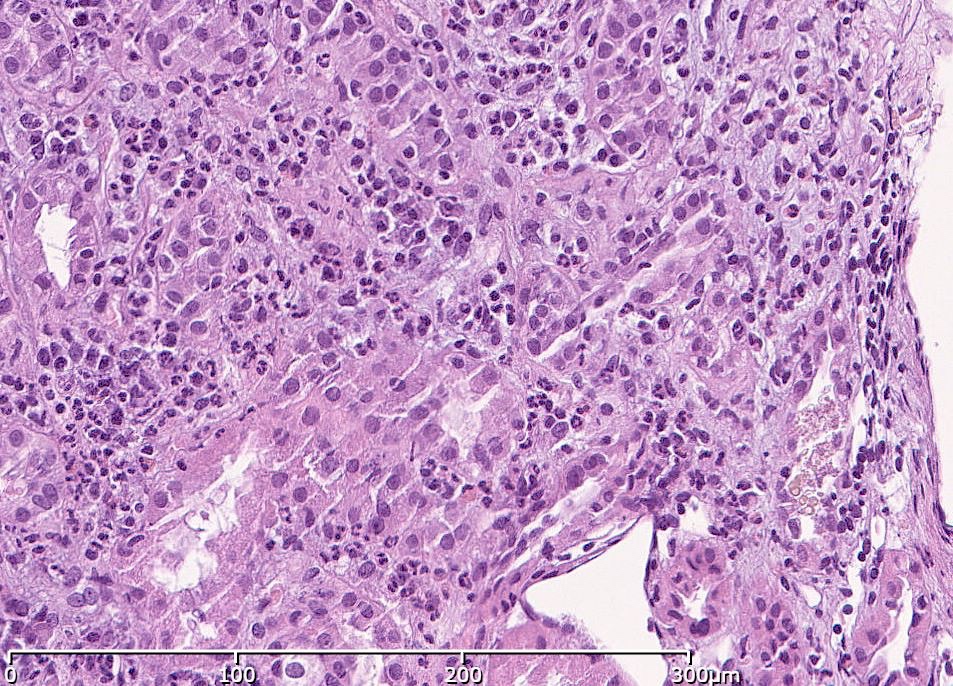
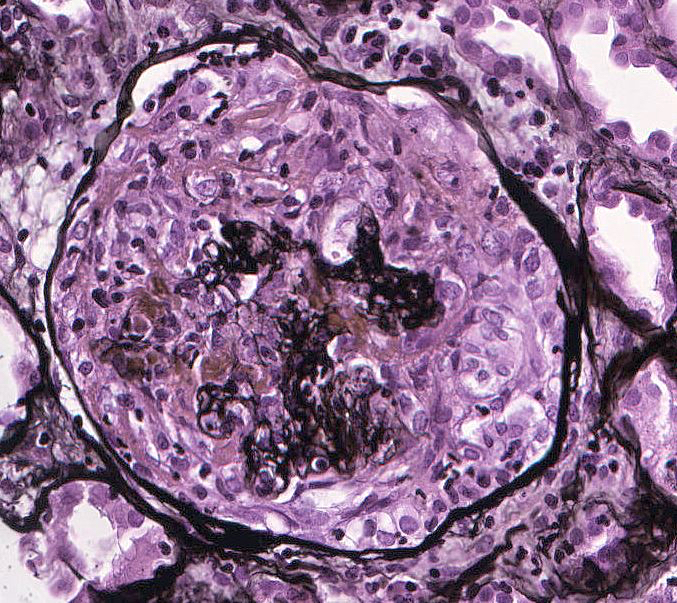
Microscopic (histologic) description
- Acute tubulointerstitial nephritis (ATIN) is the most common observation in ICI related AKI, histologically indistinguishable from ATIN secondary to other drugs (Kidney Int 2016;90:638)
- ATIN affects > 90% of patients who undergo biopsy (J Am Soc Nephrol 2020;31:435)
- Infiltrate is predominantly composed of lymphocytes, with varying degrees of plasma cells, eosinophils and neutrophils (Kidney Int 2016;90:638)
- Further characterization of the lymphocyte infiltrate shows a predominance of CD3+ T lymphocytes
- It can occur either alone or in combination with other glomerular or tubular pathologies (Kidney Med 2021;3:1074)
- Granulomatous formations with multinucleated giant cells
- Thrombotic microangiopathy
- Lupus nephritis
- Focal segmental glomerulosclerosis
- Membranous nephropathy
- IgA nephropathy
- Minimal change disease
- Renal tubular acidosis
- Acute tubular necrosis
- Non-ATIN patterns of damage likely cause < 10% of all ICI induced AKI (Kidney Med 2021;3:1074)
- Pauci-immune glomerulonephritis / vasculitis (27%), podocytopathies (20%) and C3 glomerulopathy (11%) are the most common non-ATIN manifestation of ICI toxicity (Kidney Int Rep 2020;6:66)
- ICIs may reactivate preexisting autoimmune diseases (Kidney Med 2021;3:1074)
Microscopic (histologic) images
Immunofluorescence description
- Immunostaining for IgA, IgG, IgM, C3, C1q, fibrinogen and kappa and lambda chains is usually negative or unspecific (Br J Cancer 2016;115:1457)
- Immunofluorescence may show a background staining for C3 along vessel walls, with no significant tubular basement membrane or glomerular staining (Kidney Int 2016;90:638)
Positive stains
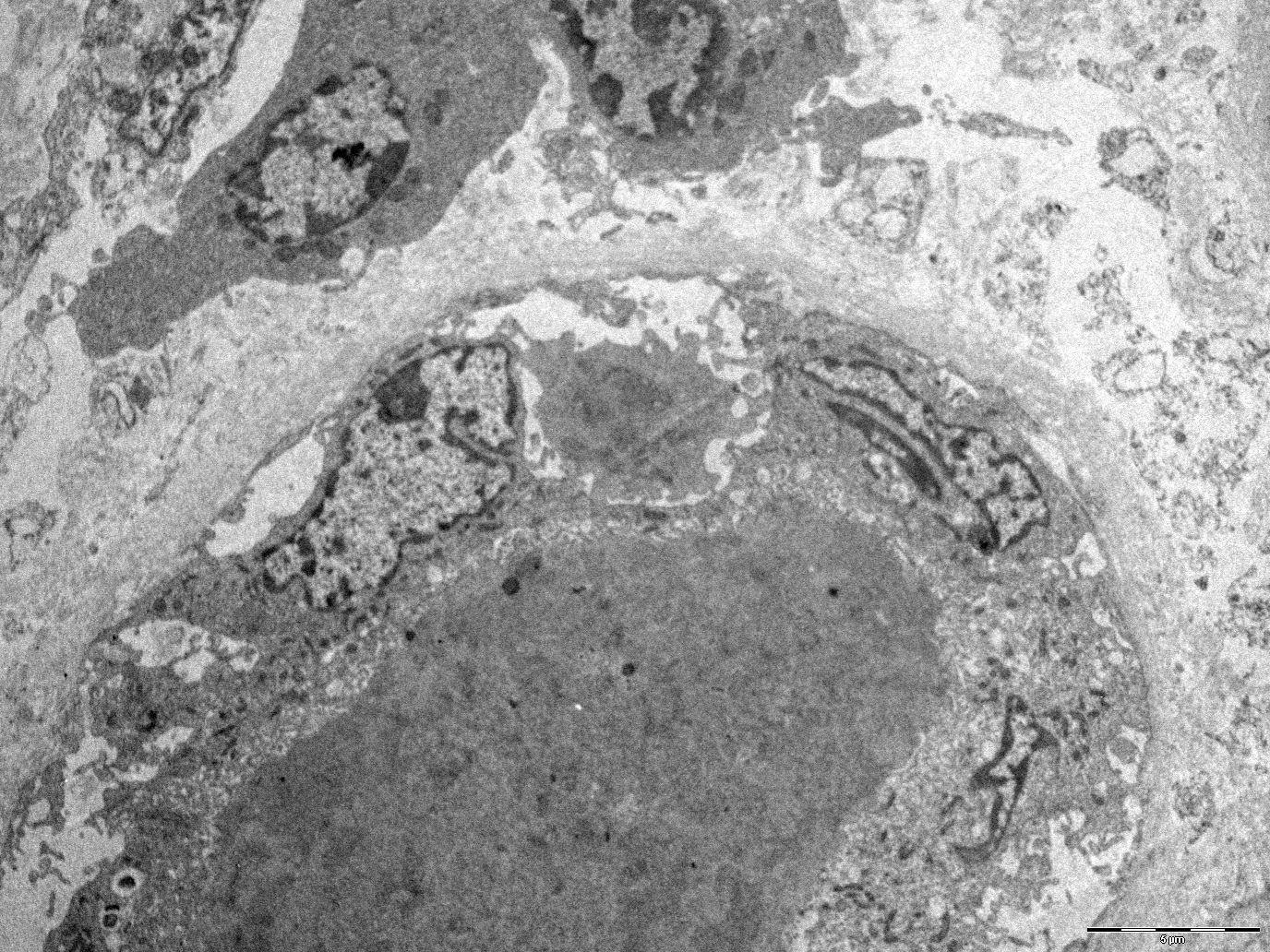
Electron microscopy description
- In electron microscopy, no specific findings are observed unless a concomitant glomerular pathology, such as focal segmental glomerulosclerosis (FSGS), IgA or membranous nephropathy, develops due to ICI therapy (Kidney Int 2016;90:638)
- Nonspecific inflammatory changes such as tubulitis can be observed
Electron microscopy images
Sample pathology report
- Left kidney, needle core biopsy:
- Acute tubulointerstitial nephritis with predominant neutrophilic component
- Microscopic description: 7 glomeruli, 2 of which are globally sclerotic. Glomeruli show no evidence of mesangial or endo / extracapillary hypercellularity. No evidence of segmental sclerosis of the tuft. Tubular atrophy and interstitial fibrosis involving ~10% of the cortex. Focal areas of inflammation with lymphocytes and numerous neutrophils, extending to nonfibrotic areas with frequent tubulitis. Mild intimal fibrosis of medium size arteries.
- Immunofluorescence on the biopsy containing up to 2 glomeruli, negative for all antisera.
- Electron microscopy: no significant ultrastructural modifications affecting glomeruli, sparse mononuclear and polymorphonuclear inflammatory cells in the interstitium with occasional tubulitis foci.
Differential diagnosis
- Use of nephritogenic drugs (PPIs, NSAIDs):
- Generally ATIN with prominent eosinophilic infiltrate sometimes associated with the formation of nonnecrotizing interstitial granulomas
- Urinary tract infections:
- Generally neutrophil dominant interstitial infiltrate with frequent intratubular neutrophilic plugs
Board review style question #1
Which of the following immune checkpoint inhibitors is known to have the fastest toxicity onset?
- Avelumab
- Ipilimumab
- Nivolumab
- Pembrolizumab
Board review style answer #1
B. Ipilimumab. Renal damage caused by anti-CTLA4 leads to early lymphocyte infiltration of renal tissue, with rapid onset averaging 6 - 12 weeks. Answers A, C and D are incorrect because anti-PD-1 / anti-PDL1 treatment determines nephrotoxicity with onset at 3 - 12 months.
Comment Here
Reference: Checkpoint inhibitor associated tubulointerstitial nephritis
Comment Here
Reference: Checkpoint inhibitor associated tubulointerstitial nephritis
Board review style question #2
Board review style answer #2
D. Pauci-immune vasculitis. Pauci-immune vasculitis is described as the most common non-ATIN manifestation of ICI related toxicity, accounting for up to 27% of cases. Answers A and B are incorrect because fibrillary glomerulonephritis and IgG4 related disease are also possible causes but are very rare presentations of ICI related toxicity. Answer C is incorrect because oxalate nephropathy is not a known pattern of injury related to ICI.
Comment Here
Reference: Checkpoint inhibitor associated tubulointerstitial nephritis
Comment Here
Reference: Checkpoint inhibitor associated tubulointerstitial nephritis