Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Anderson D, Tretiakova M. Large cell neuroendocrine carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneytumormalignantlargecell.html. Accessed September 22nd, 2025.
Definition / general
- Extremely rare carcinoma composed of large cells with prominent nucleoli and neuroendocrine differentiation
Essential features
- High grade carcinoma with typical large cell neuroendocrine carcinoma (LCNEC) histology and positivity for ≥ 1 neuroendocrine immunohistochemical marker(s) (e.g., synaptophysin, chromogranin, CD56, INSM1, etc.)
- Criteria used to define LCNEC are similar to classifying the pulmonary counterpart, including (Adv Anat Pathol 2008;15:218)
- Neuroendocrine appearance by light microscopy
- Large polygonal cells with low N:C ratio
- Coarse chromatin and frequent prominent nucleoli
- High mitotic rate of > 10 mitoses per 10 high power fields accompanied by areas of necrosis
- Immunohistochemical or ultrastructural evidence of neuroendocrine differentiation
Terminology
- Large cell neuroendocrine carcinoma
ICD coding
- ICD-O: 8013/3 - large cell neuroendocrine carcinoma
- ICD-10:
- C64.1 - malignant neoplasm of right kidney, except renal pelvis
- C64.2 - malignant neoplasm of left kidney, except renal pelvis
- C66.1 - malignant neoplasm of right ureter
- C66.2 - malignant neoplasm of left ureter
- C67.0 - malignant neoplasm of trigone of bladder
- C67.1 - malignant neoplasm of dome of bladder
- C67.2 - malignant neoplasm of lateral wall of bladder
- C67.3 - malignant neoplasm of anterior wall of bladder
- C67.4 - malignant neoplasm of posterior wall of bladder
- C67.5 - malignant neoplasm of bladder neck
- C67.6 - malignant neoplasm of ureteric orifice
- C67.7 - malignant neoplasm of urachus
- C67.8 - malignant neoplasm of overlapping sites of bladder
- C67.9 - malignant neoplasm of bladder, unspecified
- ICD-11:
- 2C94.Y & XH0NL5 - other specified malignant neoplasms of bladder & large cell neuroendocrine carcinoma
- 2C90.Y & XH0NL5 - other specified malignant neoplasms of kidney, except renal pelvis & large cell neuroendocrine carcinoma
- 2C9Y & XH0NL5 - other specified malignant neoplasms of urinary tract & large cell neuroendocrine carcinoma
Epidemiology
- Primary LCNEC comprises < 0.5% of urothelial neoplasms (Am J Surg Pathol 2021;45:1399, Anticancer Res 2020;40:2439)
- < 10 renal LCNECs have been reported in English literature so far and only a few of the ureters (Anticancer Res 2020;40:2439, Pathol Res Pract 2020;216:152788, J Endourol Case Rep 2016;2:204, Int J Clin Exp Pathol 2013;6:729)
- Bladder primary LCNEC shows M > F, mean age is 60.8 years old, range is 20 - 84 years old (Am J Surg Pathol 2021;45:1399)
- Renal LCNEC shows M > F with mean age is 52 years old (Anticancer Res 2020;40:2439)
Sites
- Can occur anywhere in the genitourinary tract including kidney, bladder, prostate, ureter and, rarely, penis (Histopathology 2012;61:319)
- Can occur anywhere in the bladder but in one study lateral wall was most common (Am J Surg Pathol 2021;45:1399)
Pathophysiology
- Origin of LCNEC of urothelial tract is uncertain but may arise from multipotent stem cell (BMC Cancer 2015;15:818, Urologia 2014;81:57, Cancer Manag Res 2018;10:4479)
- Prostate LCNEC is seen almost exclusively in the context of prior androgen deprivation therapy, possibly through neuroendocrine transdifferentiation (Anticancer Res 2020;40:2439, Ann Diagn Pathol 2019;42:48, Front Oncol 2020;10:1291, Am J Surg Pathol 2014;38:756)
Etiology
- Unknown
Clinical features
- May present with hematuria, dysuria, flank pain, flank mass or hydronephrosis, depending on location (BJU Int 2007;100:1030, Urologia 2014;81:57)
- Most bladder tumors present with advanced disease, involving the muscularis propria or beyond and are often metastatic at diagnosis (Am J Surg Pathol 2021;45:1399, Case Rep Oncol 2022;15:326)
Diagnosis
- Diagnosis is made through identification of typical morphology and demonstration of neuroendocrine differentiation through immunohistochemistry
- Rare neoplasm in the urinary tract which is essentially a diagnosis of exclusion
Prognostic factors
- Usually poor prognosis regardless of location and despite treatment
- Bladder LCNEC median survival < 1 year; 3 year survival is just over 20% (Front Oncol 2020;10:1291)
- Patient survival is associated with cancer stage
- In one study, the median cancer specific survival (CSS) was 3.6 months for pure LCNEC versus 40.5 months for mixed LCNEC of bladder (Am J Surg Pathol 2021;45:1399)
- Compared with small cell carcinoma, LCNEC has a worse prognosis when metastatic (stage IV) (Am J Surg Pathol 2021;45:1399)
- 75% are dead from the disease within 1 year if in kidney (Indian J Urol 2009;25:155, BJU Int 2007;100:1030)
Case reports
- 23 year old man with primary renal large cell neuroendocrine carcinoma (J Clin Diagn Res 2014;8:ND08)
- 35 year old man and 75 year old woman who died within 6 and 5 months after surgery despite radical nephrectomy and chemotherapy (Ann Pathol 2000;20:357)
- 56 year old man with primary renal large cell neuroendocrine carcinoma (Pathol Oncol Res 2010;16:139)
- 59 year old man with renal LCNEC and cardiac metastasis (J Med Case Rep 2017;11:297)
- 78 year old man with left ureter LCNEC (Int J Clin Exp Pathol 2013;6:729)
- 82 year old woman with muscle invasive LCNEC of the bladder associated with urothelial carcinoma in situ (Case Rep Oncol 2022;15:326)
Treatment
- Due to rarity, no standard treatments have been approved for locally advanced disease or metastasis (Am J Surg Pathol 2021;45:1399)
- Many are treated with radical surgical resection, adjuvant or neoadjuvant etoposide, platinum based chemotherapy similar to lung LCNEC and possible radiotherapy (J Urol 2005;174:93, Case Rep Urol 2020;2020:8827646)
- Prompt diagnosis and early treatment with neoadjuvant chemotherapy followed by radical cystectomy may provide long term control of a localized tumor (Int J Urol 2008;15:1080, Am J Surg Pathol 2021;45:1399)
Gross description
- Tan to white tumors with gritty or necrotic cut surfaces
- In the bladder, median size is 4 cm (range: 1 - 9 cm) may be polypoid or nodular or less often flat or ulcerative and often invasive into the muscularis propria (Front Oncol 2020;10:1291)
- In the kidney, tumors are often large (frequently > 100 mm in diameter) and commonly extend into the renal sinus or perirenal tissue (Indian J Urol 2009;25:274, Urologia 2014;81:57)
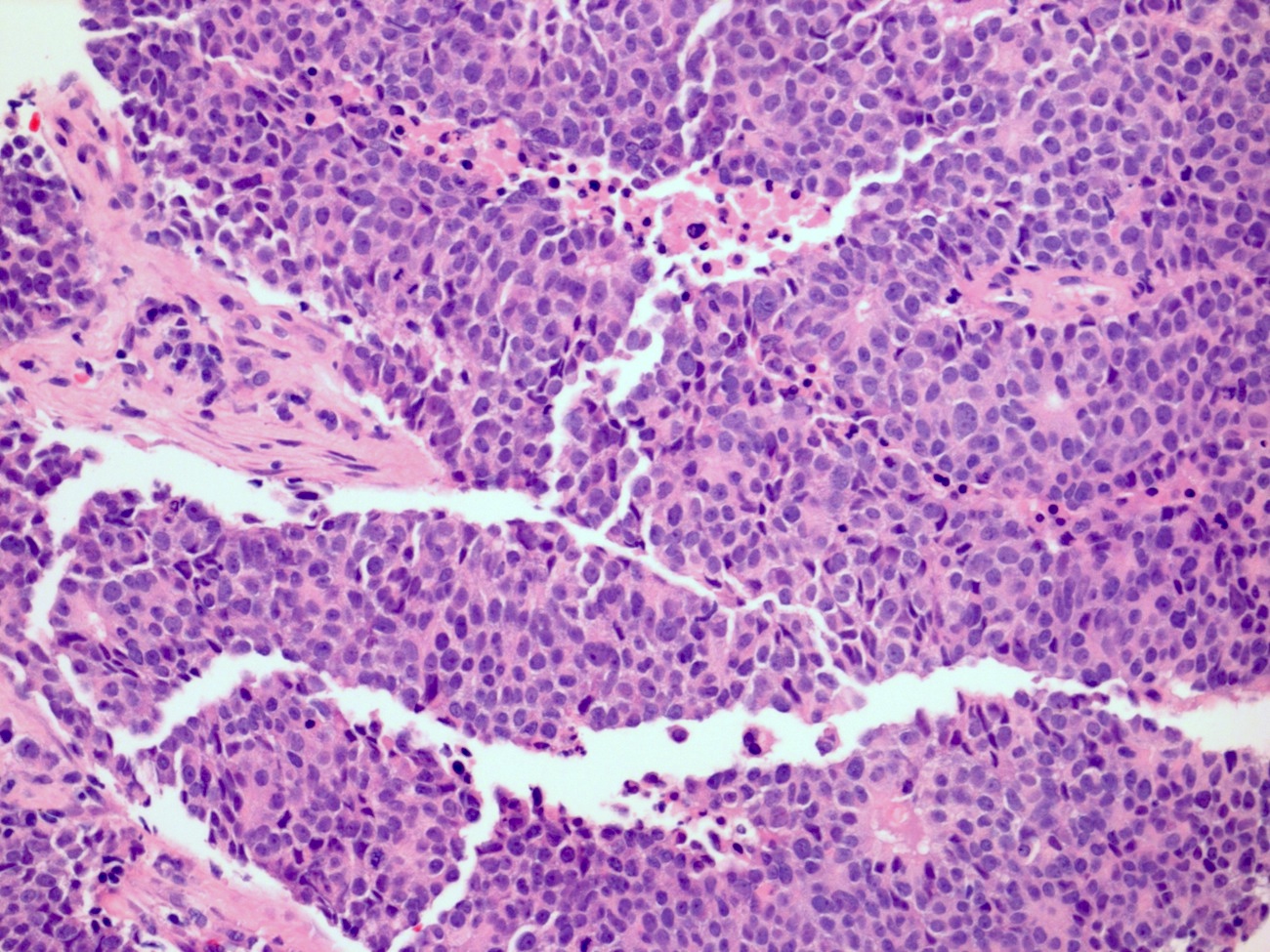
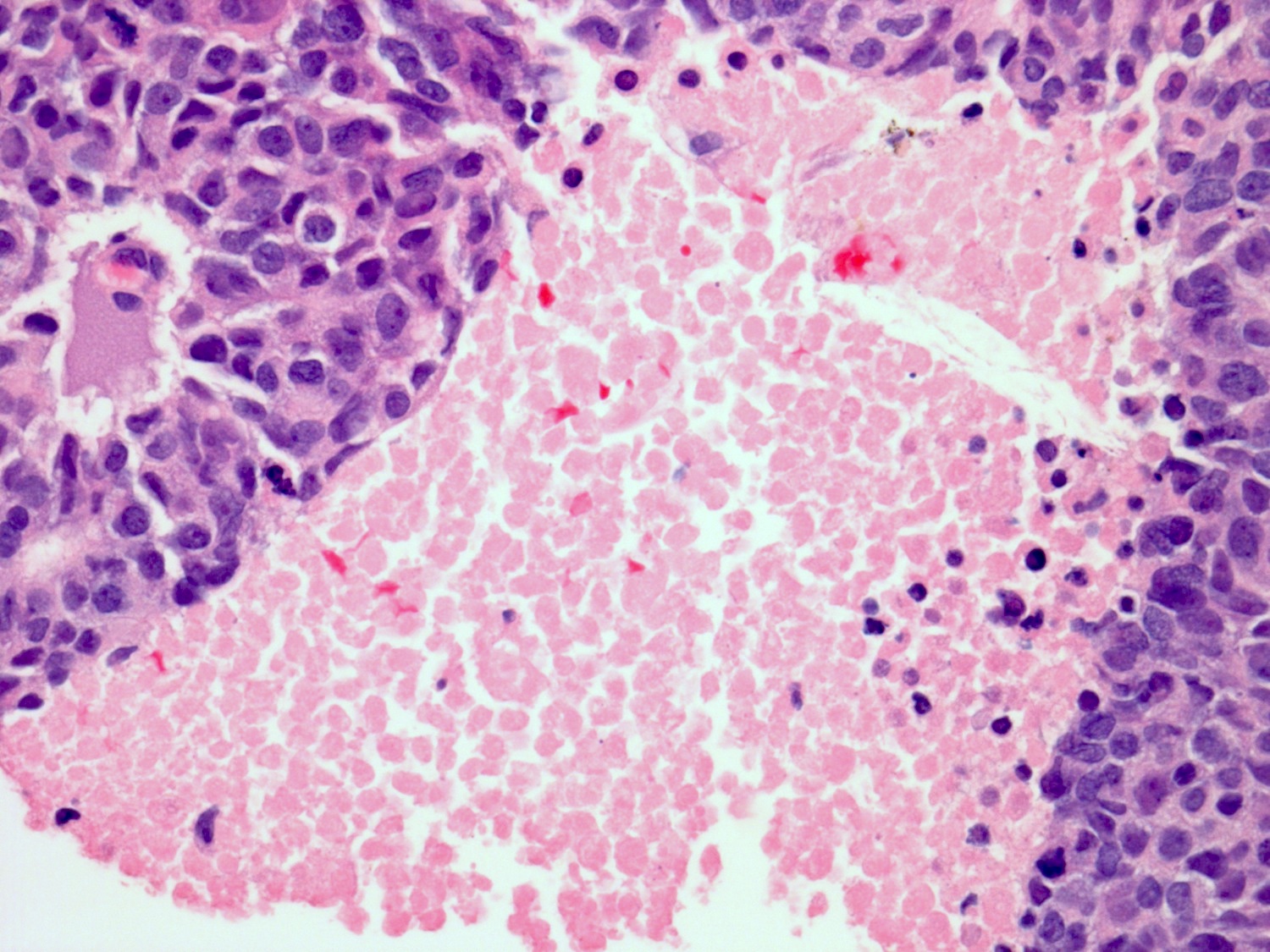
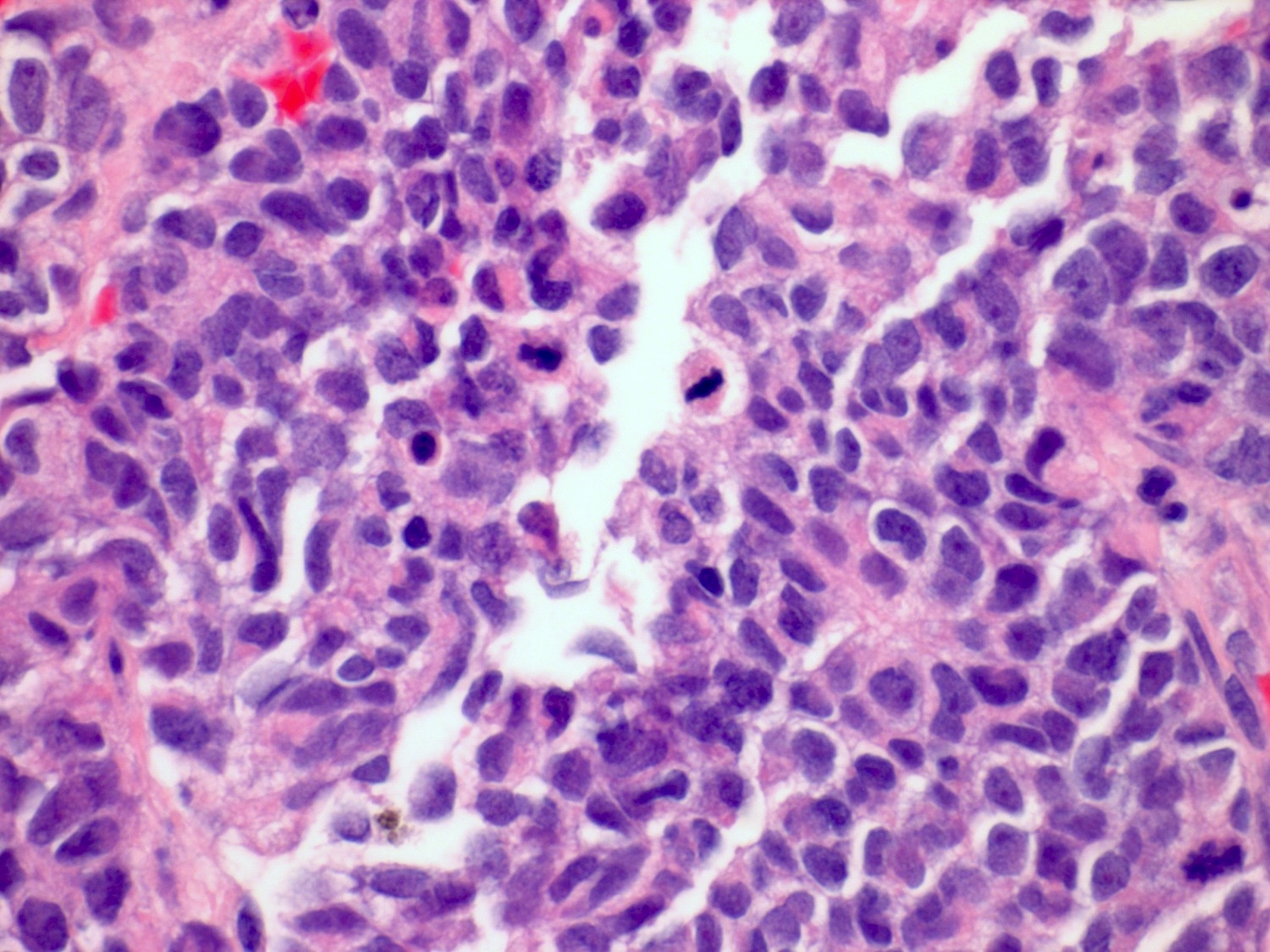
Microscopic (histologic) description
- Medium to large cell size with round to polygonal nuclei, low N:C ratio, vesicular / fine chromatin, frequent nucleoli and nuclear pleomorphism (Am J Surg Pathol 2021;45:1399, Mod Pathol 2009;22:S96)
- No clear nuclear or cell size cutoff between LCNEC and small cell carcinoma (Am J Clin Pathol 2001;116:466)
- Neuroendocrine growth pattern such as organoid, nesting, palisading, acini, trabeculae, sheet-like or solid growth pattern; peripheral palisading and rosettes may be occasionally seen
- Usually > 10 mitosis per high power fields (Am J Surg Pathol 2021;45:1399)
- Frequent necrosis, apoptotic bodies and often with lymphovascular invasion and lymph node metastasis
- May be pure or mixed with other histologic subtypes; subtypes include conventional urothelial carcinoma, carcinoma in situ, small cell carcinoma, glandular, squamous, etc. or prostatic adenocarcinoma if from the prostate gland (Am J Surg Pathol 2021;45:1399, Mod Pathol 2009;22:S96)
- Little guidance on terminology but when mixed, may be labeled as mixed tumors with types and percentages of neuroendocrine and nonneuroendocrine components
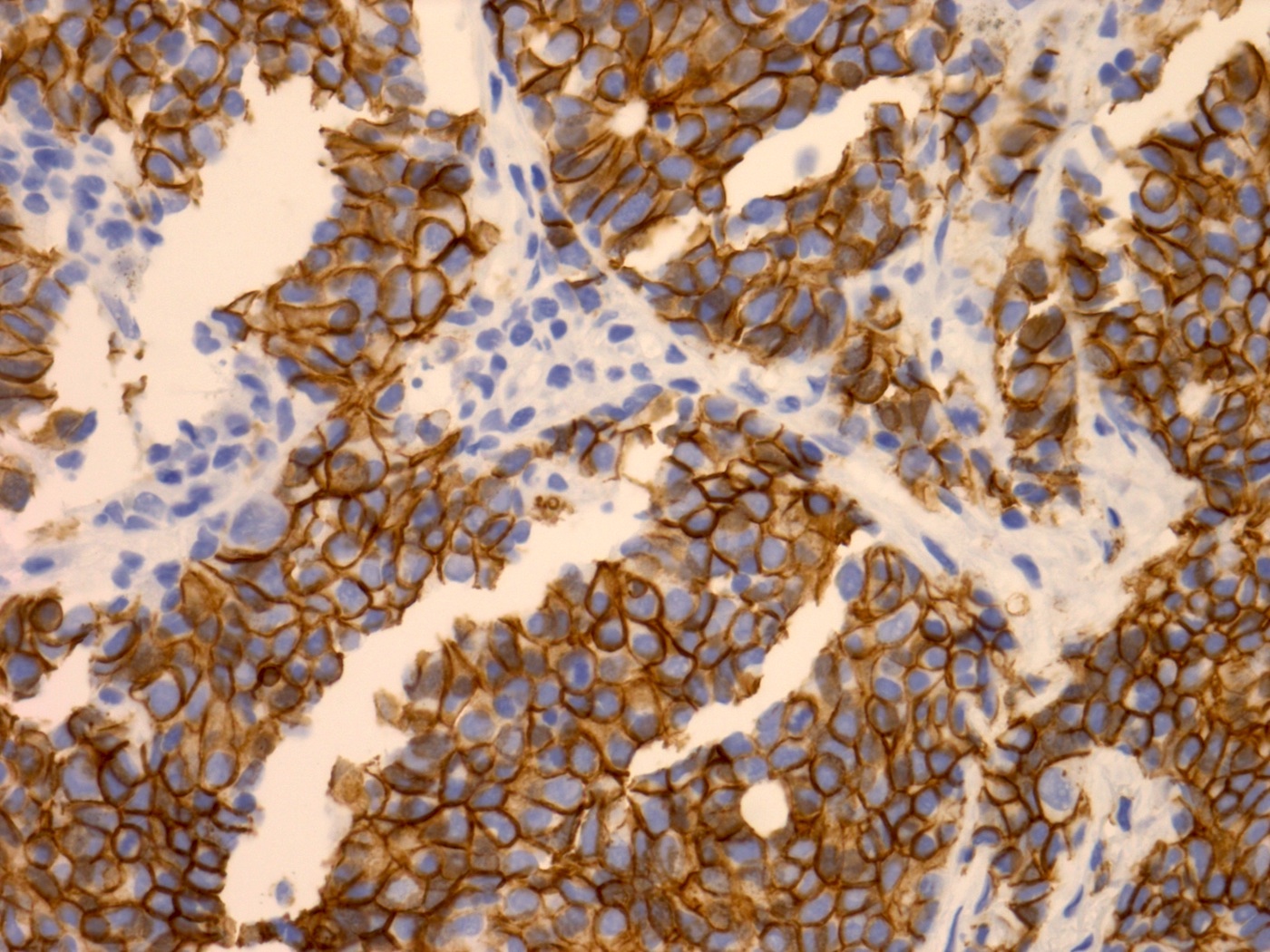
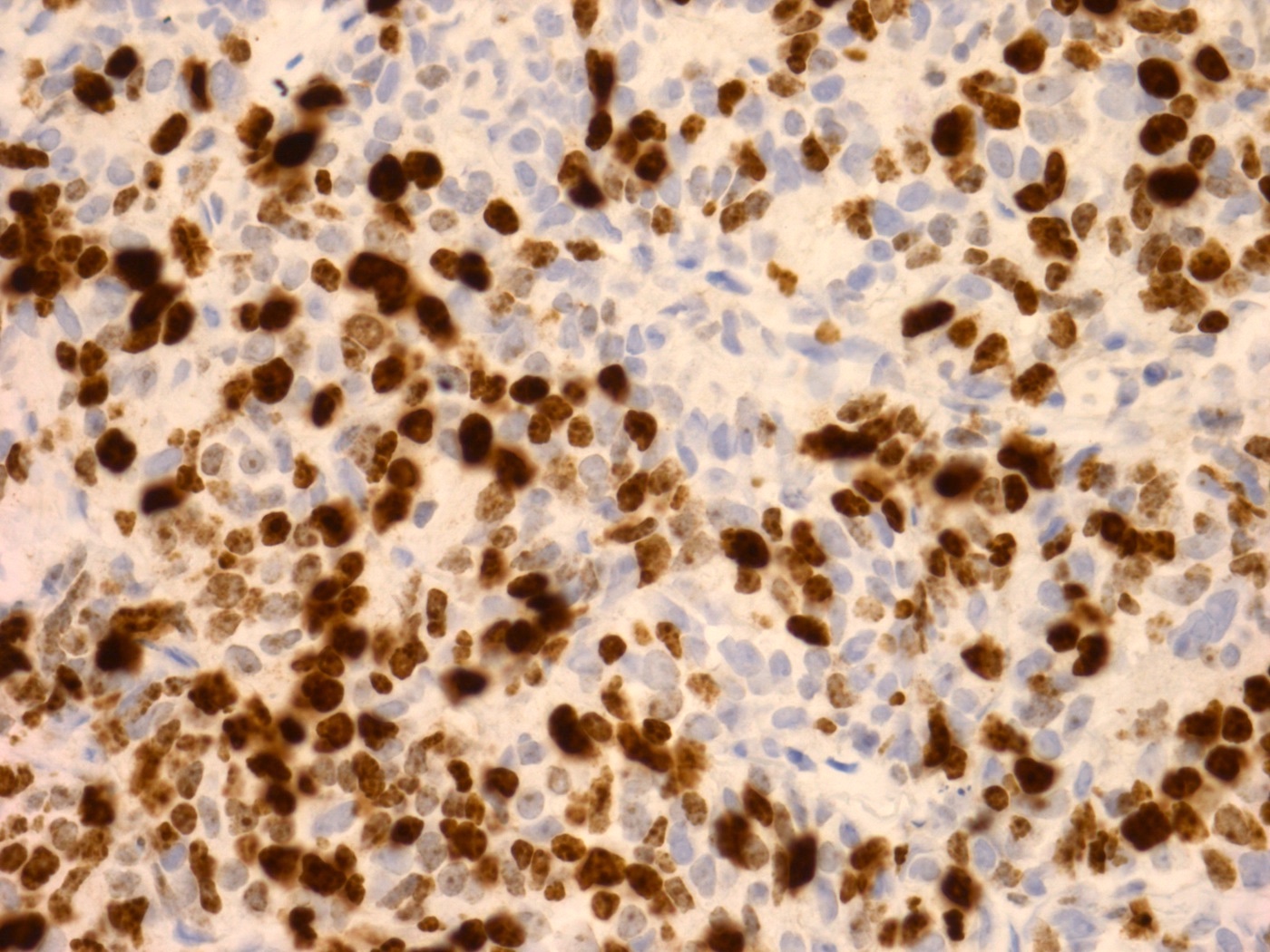
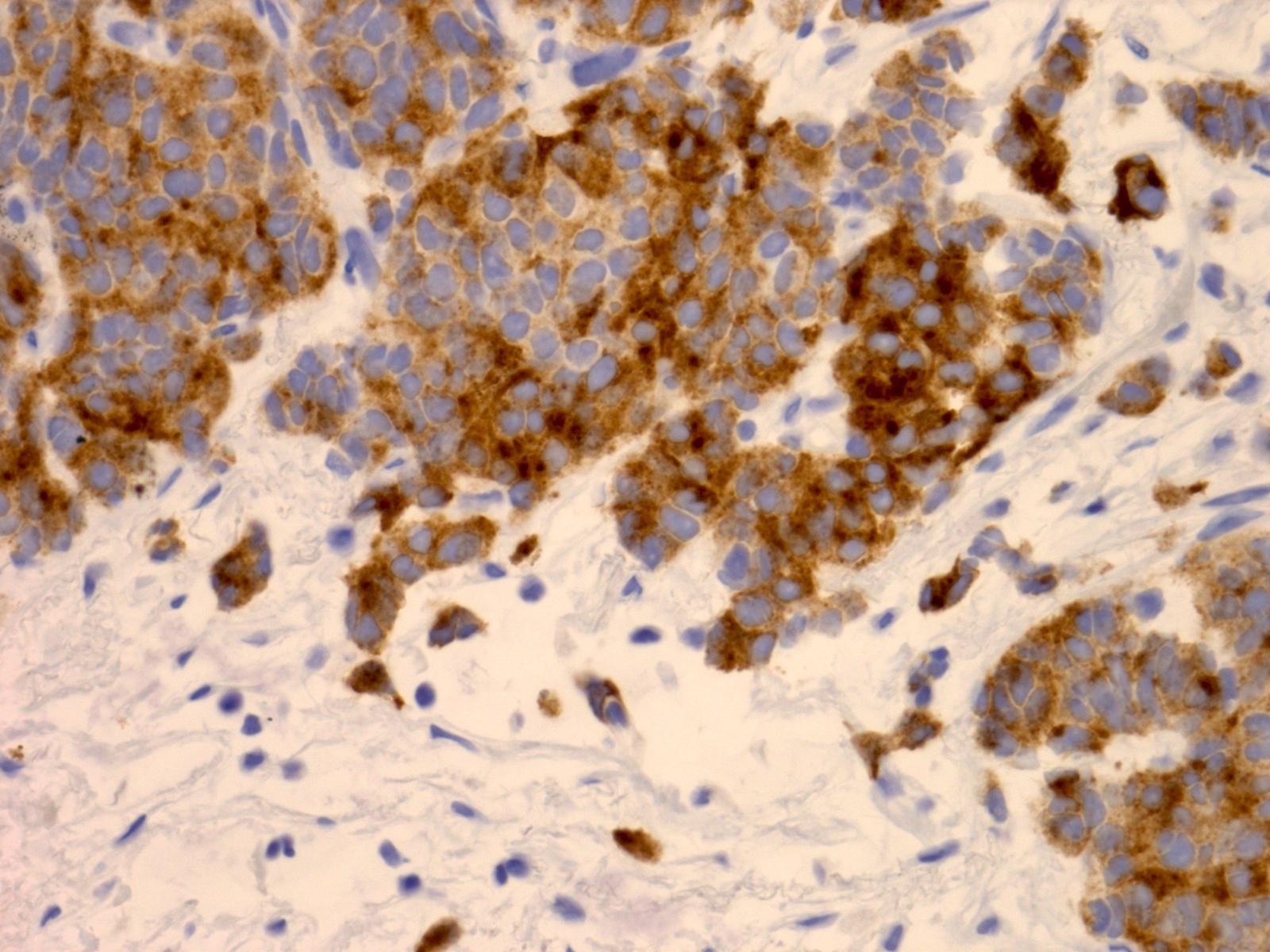
Microscopic (histologic) images
Cytology description
- Similar to LCNEC of the lung displaying the following features:
- Pleomorphic medium to large cells
- Cells are round or polygonal in shape with abundant cytoplasm
- Nuclei round, oval or polygonal with thin and smooth nuclear membranes
- Chromatin finely or coarsely granular
- Nucleoli may be prominent or inconspicuous
- Cells may appear in clusters, rosettes or singly
- Necrosis in background and nuclear streaking may be present (Lung Cancer 2005;48:331)
- Nuclear pleomorphism, molding and mitosis, peripheral nuclear palisading and naked nuclei may also be present (Cancer 2008;114:180)
Positive stains
- Synaptophysin, CD56, CD57, chromogranin (Pathol Oncol Res 2010;16:139)
- Caution with CD56 if the only positive stain as the stain is nonspecific
- NSE and Leu7 (Indian J Urol 2009;25:274)
- INSM1 (Am J Surg Pathol 2021;45:1399)
- AE1 / AE3, CK7 usually weak / focal (Am J Surg Pathol 2021;45:1399)
- LCNEC often shows more diffuse cytoplasmic staining for pancytokeratin and CK7, compared with the dot-like staining pattern seen in small cell carcinoma (Am J Surg Pathol 2021;45:1399)
- Vimentin and CD10 rarely reported to be positive (BJU Int 2007;100:1030)
- Ki67 index may vary from 25% to ~100% (Am J Surg Pathol 2021;45:1399, Pathology 2015;47:533)
- Ki67 index > 40% has been shown to be 80% sensitive and 86% specific in distinguishing LCNEC and urothelial carcinoma, which usually features a Ki67 proliferation rate as high as 25% (Am J Surg Pathol 2021;45:1399)
- TTF1 may be expressed (Anticancer Res 2017;37:4529, Am J Surg Pathol 2021;45:1399)
- p53 and p16 (cell cycle proteins) may be expressed at higher levels by bladder high grade neuroendocrine carcinoma compared with conventional urothelial carcinoma (Anticancer Res 2020;40:2439)
Negative stains
- High molecular weight keratin, CK5/6, p40 / p63, uroplakin, CK20 typically negative (Am J Surg Pathol 2021;45:1399)
- Androgen receptor usually negative (Ann Diagn Pathol 2019;42:48)
- GATA3 variable, ranging from completely negative to weakly / moderately positive depending on if mixed with conventual urothelial carcinoma or if pure LCNE (Am J Surg Pathol 2021;45:1399)
- Calretinin, serotonin, somatostatin, gastrin, calcitonin, glucagon, insulin (Pathol Oncol Res 2010;16:139)
- EMA (BJU Int 2007;100:1030)
- S100, HMB45 and LCA (Anticancer Res 2017;37:4529)
Molecular / cytogenetics description
- Molecular features are unclear due to low prevalence
Sample pathology report
- Bladder, tumor, transurethral resection:
- Invasive carcinoma with large cell neuroendocrine carcinoma features; the tumor invades the muscularis propria (detrusor muscle) (see comment)
- Comment: Immunohistochemistry was performed. The carcinoma cells are positive for synaptophysin, chromogranin and ISM1 and negative for GATA3, NKX3.1, CDX2, p63, LCA (CD45), S100, TTF1, CDX2 and CK20. AE1 / AE3 and CK7 show focal to variable staining in the carcinoma cells. Features of primary conventional urothelial carcinoma and in situ carcinoma are not identified within this specimen. Clinical and radiologic correlation is required to exclude a metastasis. If this tumor is determined to be primary to the bladder, a definitive diagnosis of large cell neuroendocrine carcinoma requires review of the resection specimen.
Differential diagnosis
- As in the lung, LCNEC may be underreported and diagnosed as undifferentiated urothelial carcinoma in the urinary tract if the diagnosis is not considered
- Metastatic LCNEC, including from the prostate and lung:
- Histologically similar to primary tumors
- Multifocality and lymphovascular emboli may indicate metastasis
- Clinical and radiologic correlation helpful
- Lack of conventional primary carcinoma and in situ carcinoma are sometimes helpful
- TTF1 stain may not be helpful as primary urothelial LCNEC can be positive
- Poorly differentiated / undifferentiated urothelial carcinoma, lymphoepithelioma-like urothelial carcinoma (Pathol Res Pract 2003;199:559)
- Usually lacks neuroendocrine markers such as synaptophysin, chromogranin, CD56, INSM1 and usually expresses urothelial markers, such as uroplakin and CK20
- GATA3 is not helpful as LCNEC may express
- Paraganglioma / pheochromocytoma:
- Usually classic zellenballen (nested) architecture and well circumscribed
- May display endocrine atypia
- Typically lacks the degree of necrosis, abundant mitotic activity and cellular anaplasia seen in LCNEC
- Positive for S100 (sustentacular cells) and neuroendocrine markers; negative for cytokeratin
- Lymphoma:
- Clinical history
- Expression of hematolymphoid markers (e.g., LCA / CD45)
- Lack of cytokeratin and neuroendocrine markers
- Malignant melanoma:
- Clinical history
- Expression of MART1 / MelanA, HMB45, S100
- Lack of neuroendocrine markers
- Small cell carcinoma:
- Will have different morphologic features and overlapping but not identical staining pattern (Am J Surg Pathol 2021;45:1399)
- Low grade neuroendocrine tumor:
- More cytologic uniformity, lacks necrosis, lower mitotic rate / Ki67 index
- Alveolar rhabdomyosarcoma:
- Stains for muscle markers (myogenin, MyoD1, desmin)
- Subset may express cytokeratin and neuroendocrine markers
- Metastatic Merkel cell carcinoma:
- Clinical history
- CK20 dot-like and Merkel cell polyomavirus (MCPyV) are usually positive
- Other poorly differentiated / undifferentiated high grade tumors
Practice question #1
A 63 year old man presents to the urologist with hematuria. Cystoscopy shows a 3 cm polypoid mass in the right lateral wall of the urinary bladder. The tumor is subsequently biopsied which shows a high grade tumor with prominent nucleoli, necrosis, abundant mitosis, low N:C ratio, neuroendocrine differentiation (synaptophysin, CD56, chromogranin positive) and TTF1 positivity by immunohistochemistry. Which is the most correct answer?
- Advanced stage is not common
- CK20 shows dot-like cytoplasmic positivity
- May display conventional urothelial carcinoma or in situ carcinoma
- Must be metastatic from the lung due to TTF1 positivity
Practice answer #1
C. Primary large cell neuroendocrine carcinomas may display conventional urothelial carcinoma or in situ carcinoma. The question describes a large cell neuroendocrine carcinoma, a very rare neoplasm of the bladder and urinary tract. In one study, ~64% of urinary LCNEC were mixed with other histologic types of carcinoma, most commonly urothelial carcinoma or urothelial carcinoma in situ (Am J Surg Pathol 2021;45:1399). These tumors can closely mimic other carcinomas which involve the urinary tract including through metastasis. TTF1 can be positive in primary urothelial large cell neuroendocrine carcinoma and is not a specific marker to determine the origin of the tumor. LCNEC of the bladder usually is diagnosed in an advanced stage and has an overall poor prognosis. CK20 is usually not expressed by LCNEC. Dot-like cytoplasmic CK20 positivity is more common in metastatic Merkel cell carcinoma, a rare neoplasm of the skin, many of which are associated with infection from Merkel cell polyomavirus.
Comment Here
Reference: Large cell neuroendocrine carcinoma
Comment Here
Reference: Large cell neuroendocrine carcinoma
Practice question #2
Practice answer #2
B. Show a worse prognosis when metastatic. Small cell carcinoma and large cell neuroendocrine carcinoma are the 2 recognized histologic subtypes of neuroendocrine carcinomas that are primary to the bladder. These tumors are often mixed with a nonneuroendocrine carcinoma component(s). In small cell carcinomas, the estimate of mixed cases varies from 32 to 67% (Cancer 2004;101:957, Hum Pathol 2018;79:57). The percent of large cell neuroendocrine carcinomas cases with mixed histology vary between 43 and 64% (Front Oncol 2020;10:1291, Am J Surg Pathol 2021;45:1399). According to one study, when compared with a cohort of small cell carcinoma cases of the bladder, LCNEC had a worse median overall survival (8.5 versus 29.0 months, p = 0.26) as well as a worse cancer specific survival (8.9 versus 53 months, p = 0.039) (Am J Surg Pathol 2021;45:1399). The cancer specific survival was not significantly different between LCNEC and small cell carcinoma in stage I through III; however, LCNEC had significantly shorter median survival time compared with small cell carcinoma for patients with distant metastasis (stage IV) (1.6 versus 15.0 months, p = 0.02). Like LCNEC, primary urinary bladder small cell carcinoma may demonstrate TTF1 expression / positivity (Hum Pathol 2005;36:718). Therefore, TTF1 is an unreliable marker to distinguish between a metastatic lung neuroendocrine carcinoma and a primary urinary bladder neuroendocrine carcinoma. Though there is some overlap between small cell carcinoma and LCNEC, as opposed to small cell carcinoma, LCNEC will show lower N:C ratio, prominent nucleoli, more cytoplasm and may show more diffuse rather than dot-like cytokeratin staining.
Comment Here
Reference: Large cell neuroendocrine carcinoma
Comment Here
Reference: Large cell neuroendocrine carcinoma