Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1Cite this page: Dasgupta AD, Satoskar AA. Granulomatosis with polyangiitis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneyGPA.html. Accessed April 25th, 2024.
Definition / general
- Necrotizing vasculitis of small to medium sized arteries with concomitant necrotizing granulomatous tissue inflammation that is frequently associated with positive antineutrophil cytoplasmic autoantibodies (ANCA)
- This disease can be systemic but usually involves the respiratory tract and kidney (90%) (Clin Exp Nephrol 2013;17:603, Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
Essential features
- Crescentic and necrotizing glomerular lesions, usually with concomitant fibrocellular and fibrous crescents
- With or without fibrinoid necrosis of intrarenal arteries
- Absent to few immune type deposits, mild IgA staining in some cases
- Important to rule out underlying bacterial infection, since histologic features can overlap and ANCA serology is positive in up to 20% cases of Staphylococcus infection associated glomerulonephritis (GN)
- Reference: Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
Terminology
- Granulomatosis with polyangiitis (GPA) is categorized as an ANCA associated vasculitis along with microscopic polyangiitis and renal limited vasculitis; due to their overlapping histologic findings on renal biopsy, the term ANCA associated glomerulonephritis may be encountered for these entities
- Since little or no evidence of immunoglobulin or complement deposition is seen on biopsy, the term pauci-immune glomerulonephritis may also be seen
- GPA was previously known as Wegener granulomatosis but this eponym was discarded in 2011
- Reference: Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
ICD coding
Epidemiology
- Incidence of 2.3 - 146 per 1,000,000 persons with higher frequencies seen in people of Northern European (white) ancestry (Nat Rev Dis Primers 2020;6:71)
- Usually seen in adults (50 - 70 years), although pediatric cases can occur
- M = F
Sites
- Respiratory and renal systems are the most frequently impacted
- GPA can affect the upper (nasopharynx) and the lower (lungs and trachea) respiratory systems
- Other organ systems, such as the eyes, skin and nervous system, can also be affected
- Reference: Am J Med 2004;117:39
Pathophysiology
- Although the trigger is unknown, autoantibodies to neutrophilic surface and cytoplasmic antigens are characteristically present and are pathogenic (antiproteinase 3 [PR3], myeloperoxidase [MPO], LAMP2, etc.)
- These antibodies interact with their respective receptors, usually on neutrophils or monocytes, causing further activation of inflammatory cell degranulation, formation of neutrophilic extracellular traps, autoantibodies to sequestered antigens and activation of complement pathway
- This leukocyte activation also leads to increased endothelial adhesion, which culminates in extensive microvascular injury
- Reference: Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
Etiology
- Unknown
Clinical features
- May present insidiously with nonspecific systemic symptoms (weight loss, malaise, fatigue, arthralgias)
- Respiratory symptoms vary and can include chronic rhinitis, epistaxis, nonproductive cough and hemoptysis
- Renal involvement usually presents acutely with hematuria, proteinuria and rising serum creatinine
- However, renal involvement can also be insidious with a slow decrease in renal function with mild proteinuria or hematuria
- Strongly associated with PR3 ANCA (c-ANCA, 75%) but MPO ANCA (p-ANCA, < 25%) is also seen
- Some cases are ANCA negative or associated with other autoantibodies (LAMP2, plasminogen and tissue plasminogen activator)
- Negative ANCA serology does not exclude a diagnosis of GPA (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
- Positive ANCA serologies can be seen in infections
- Exclusion of an underlying infection is paramount if clinical concern or worrisome biopsy findings (immune complex or complement deposition) are present
Diagnosis
- Clinical presentation
- Rapidly progressive glomerulonephritis with nephritic urine sediment
- Recent worsening of renal function, active urine sediment, subnephrotic proteinuria
- Subacute rise in serum creatinine over several months, sinus symptoms, worsening blood pressure control
Laboratory
- Renal involvement usually presents acutely with hematuria, proteinuria and rising serum creatinine
- However, renal involvement can also be insidious with a slow decrease in renal function with mild proteinuria or hematuria
- Strongly associated with PR3 ANCA (c-ANCA, 75%) but MPO ANCA (p-ANCA, < 25%) is also seen
- Some cases are ANCA negative or associated with other autoantibodies (LAMP2, plasminogen and tissue plasminogen activator)
- Negative ANCA serology does not exclude a diagnosis of GPA (Colvin: Diagnostic Pathology - Kidney Diseases, 3rd Edition, 2019)
Case reports
- 12 year old girl presented with cutaneous and renal features of IgA vasculitis subsequently diagnosed as GPA (Clin Med Insights Arthritis Musculoskelet Disord 2020;13:1179544120967371)
- 26 year old man presented with cough, epistaxis, weight loss and dark colored urine (Cureus 2021;13:e19814)
- 60 year old woman presented with a renal mass subsequently diagnosed as GPA (Indian J Nephrol 2021;31:406)
Treatment
- Immunosuppression is warranted in active cases of GPA (Arthritis Rheumatol 2021;73:1366)
- Induction therapy usually includes glucocorticoids in conjunction with rituximab or cyclophosphamide
- Both regimens appear to be similarly efficacious with remission rates varying between 50 - 90%
- Avacopan (C5a receptor inhibitor) can be used as an adjunctive induction agent when the standard dosage of glucocorticoids is contraindicated (J Am Soc Nephrol 2017;28:2756)
- After remission, maintenance therapy can begin using a variety of medications based on the organs involved and the tolerance of the patient
- Patients should be monitored for relapse through physical exam and laboratory values (serum creatinine, urinalysis)
- ANCA titers can be followed to assess for relapse, especially in patients with renal involvement, though they may not consistently reflect disease severity (J Am Soc Nephrol 2015;26:537)
- If end stage renal disease occurs, renal transplant can be performed with standard outcomes (Clin Transplant 2011;25:380)
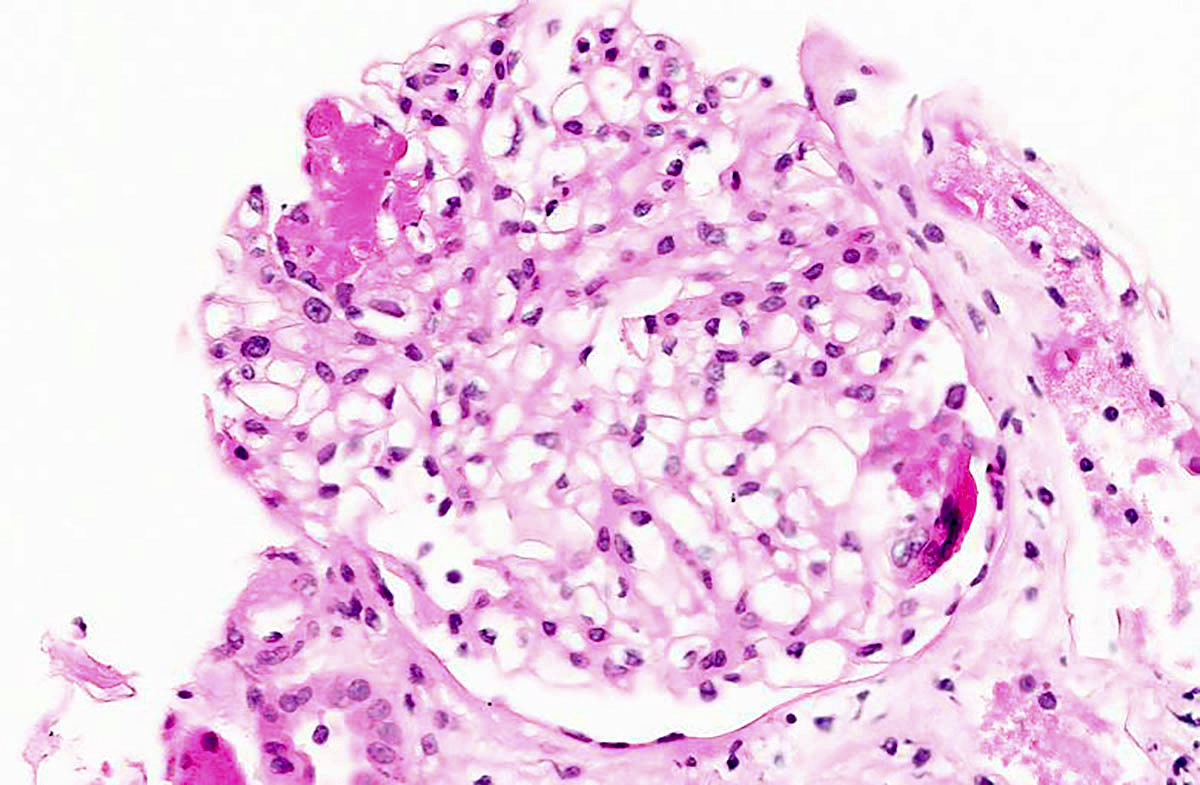
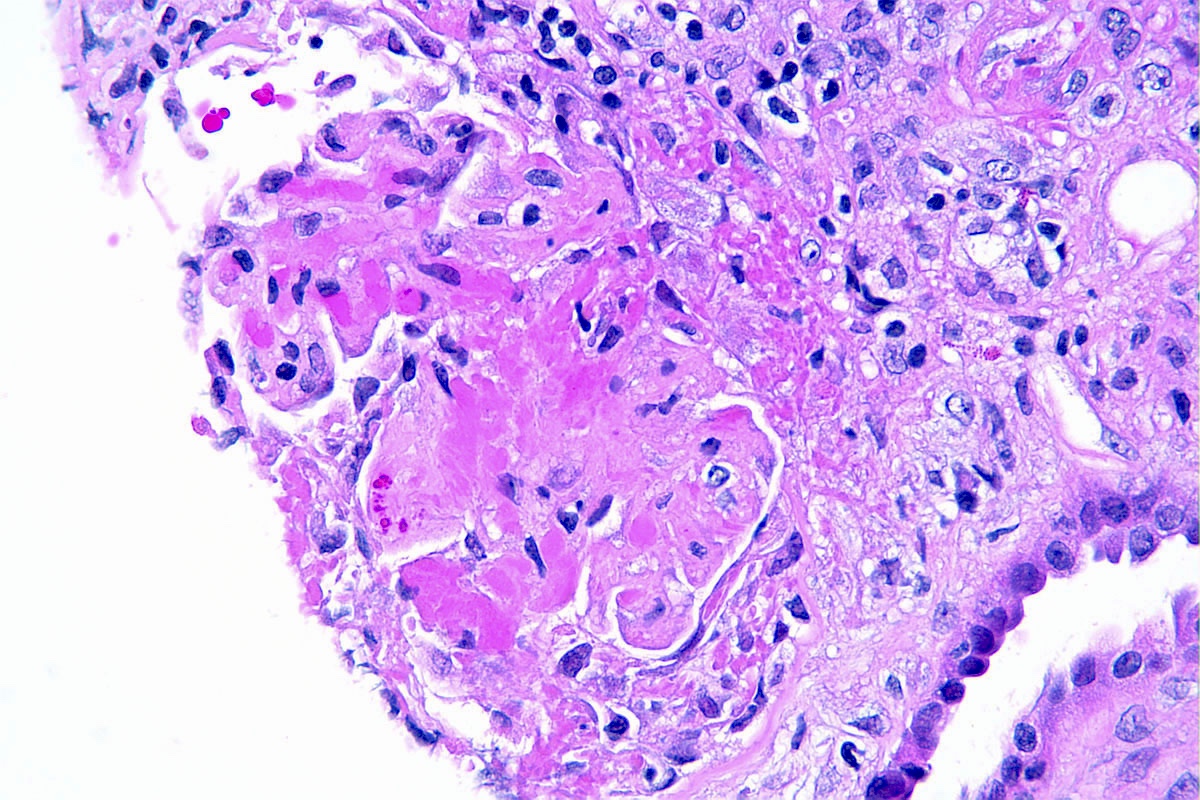
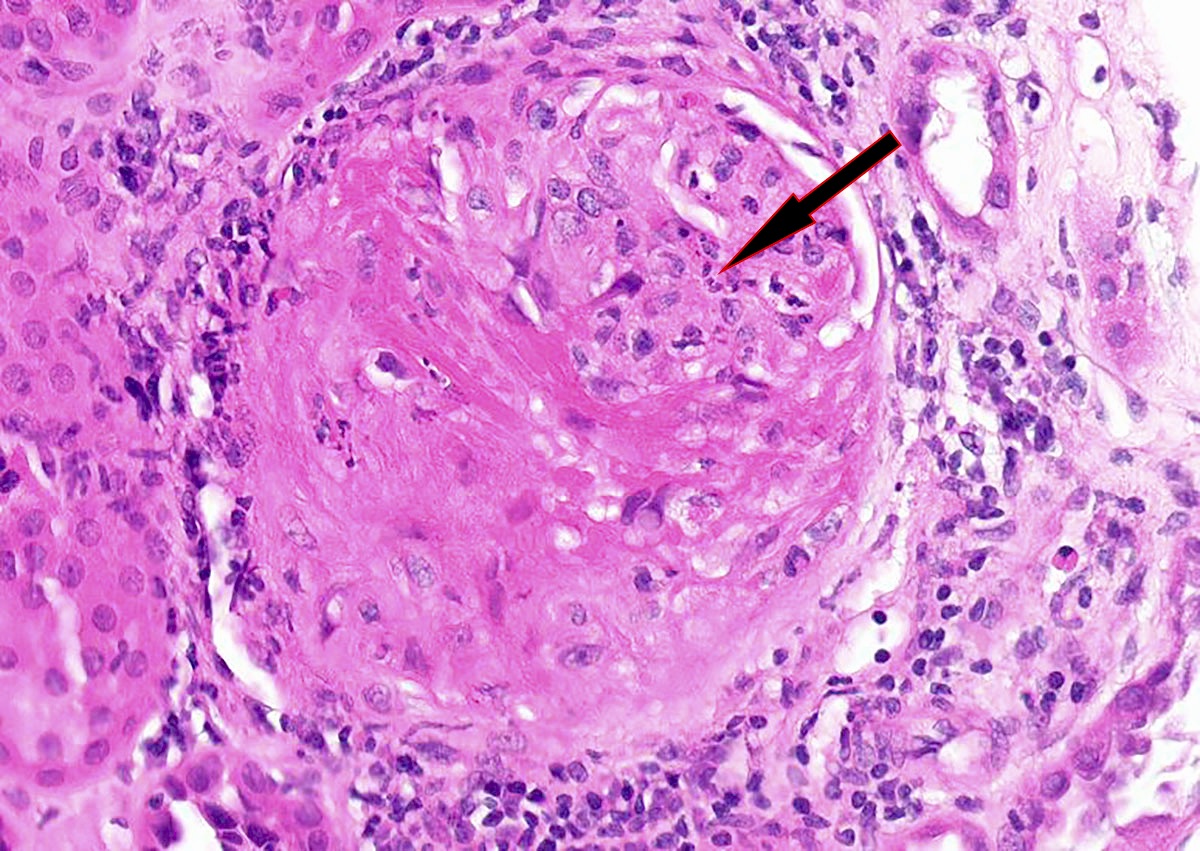
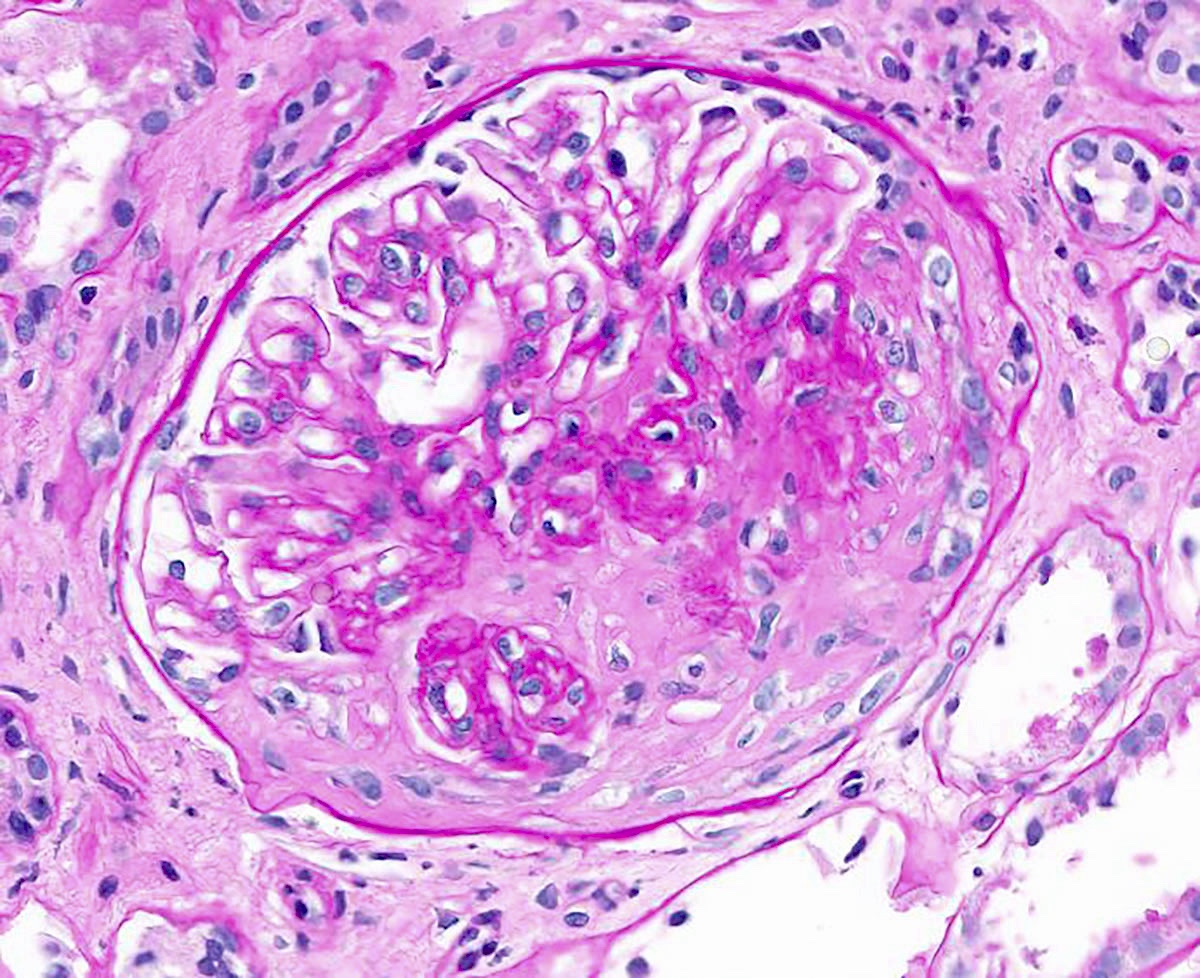
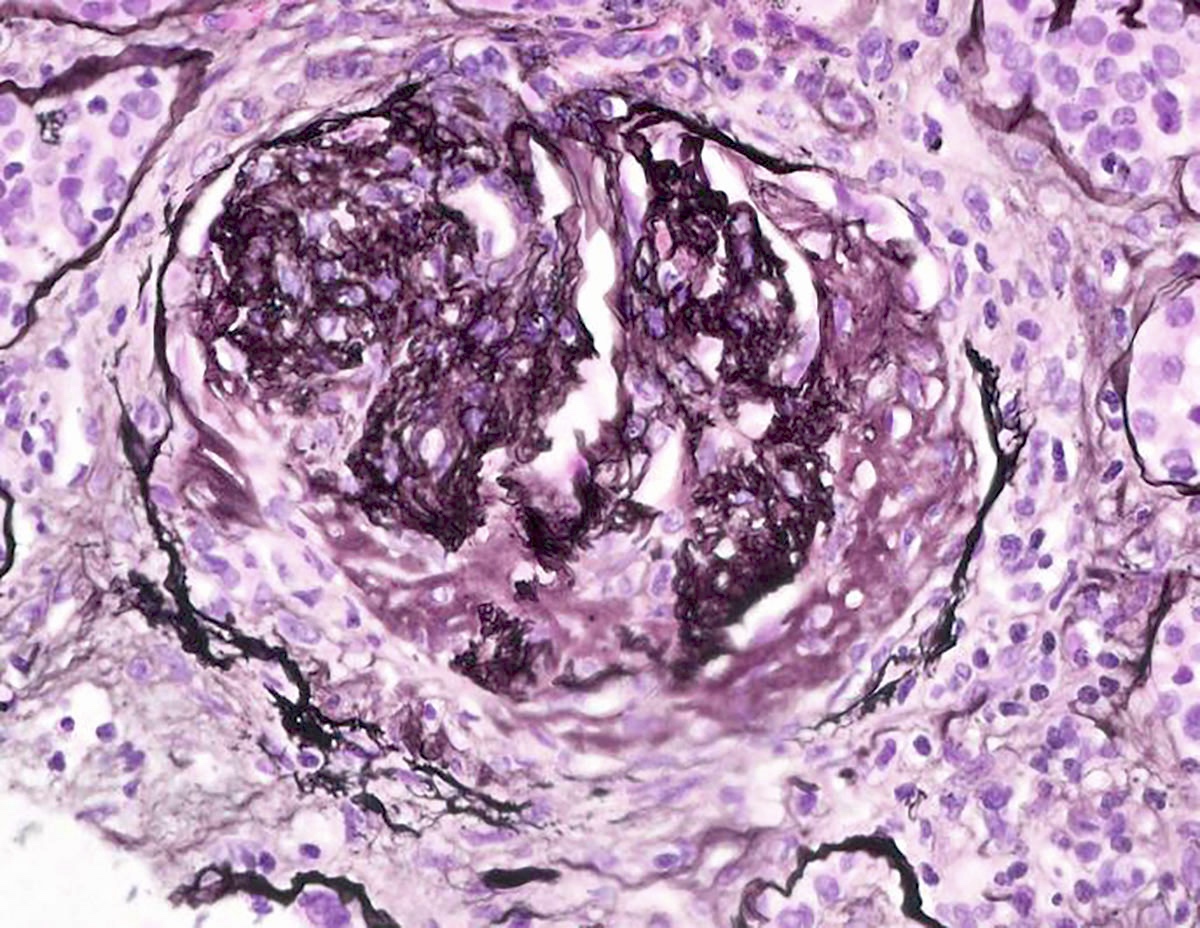
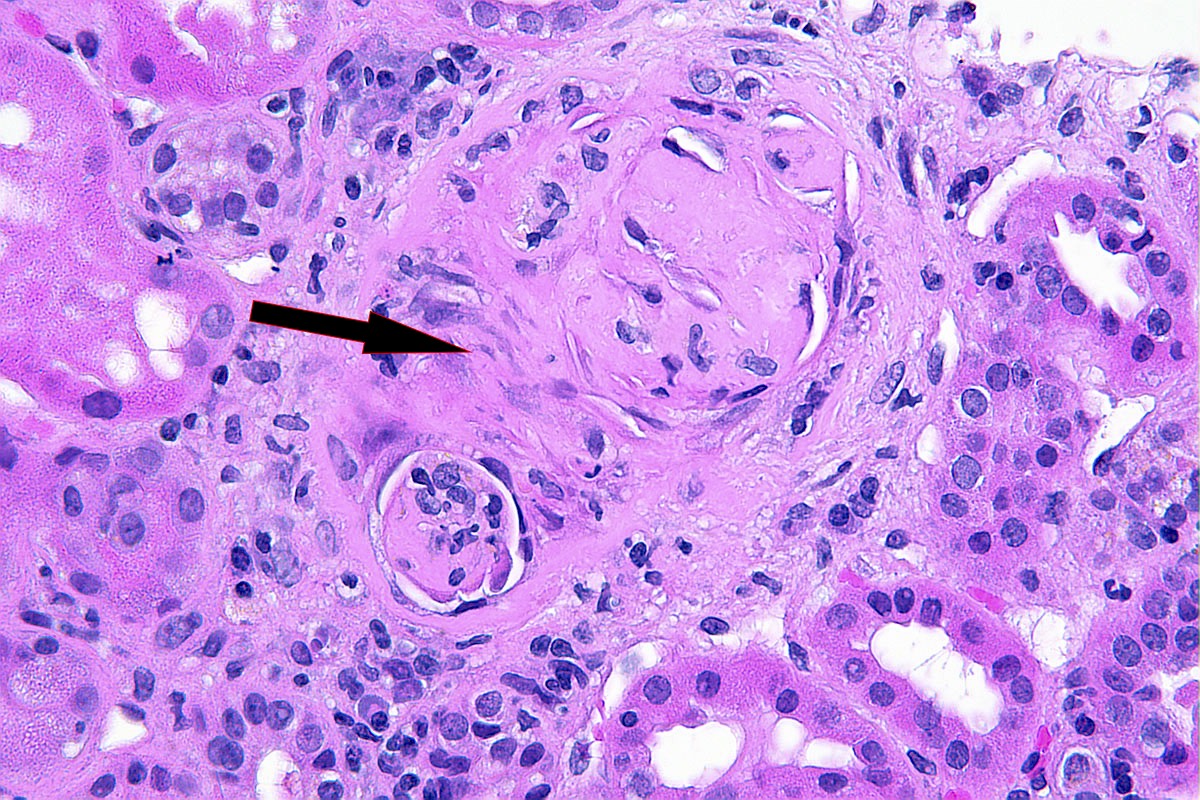
Microscopic (histologic) description
- In the active phase, glomeruli are primarily normocellular with fibrinoid necrosis or cellular crescents, which can range from segmental and focal to global and diffuse (Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014)
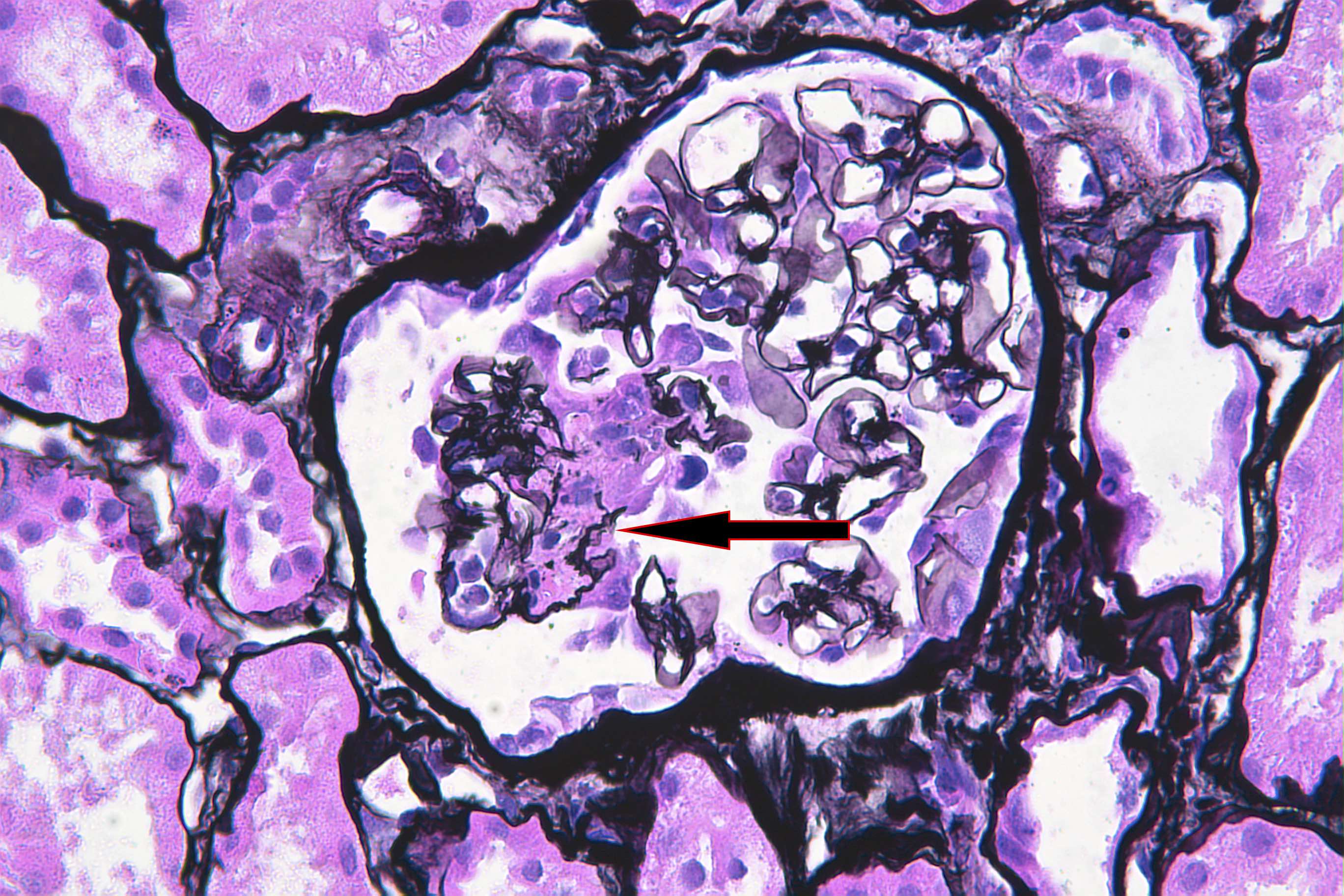
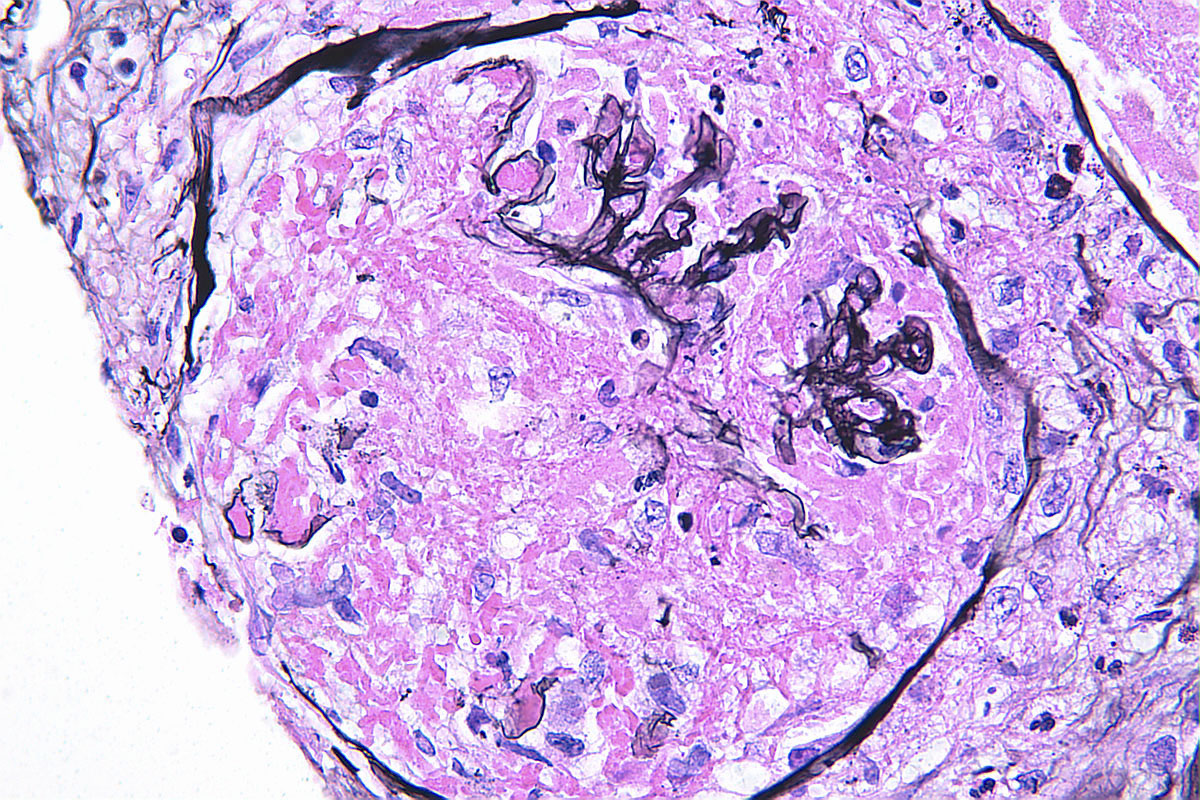
- Silver and PAS stains can highlight the breaks in the glomerular basement membrane (GBM) in these areas
- Trichrome stain can highlight the fuchsinophilic fibrinoid necrosis
- In areas of GBM rupture, segmental to severe capillary tuft thrombosis can occur with extension into the afferent arteriole
- Patients can have recurrent episodes of glomerular injury with fibrocellular or fibrous crescents frequently encountered in cases with concomitant features of active injury
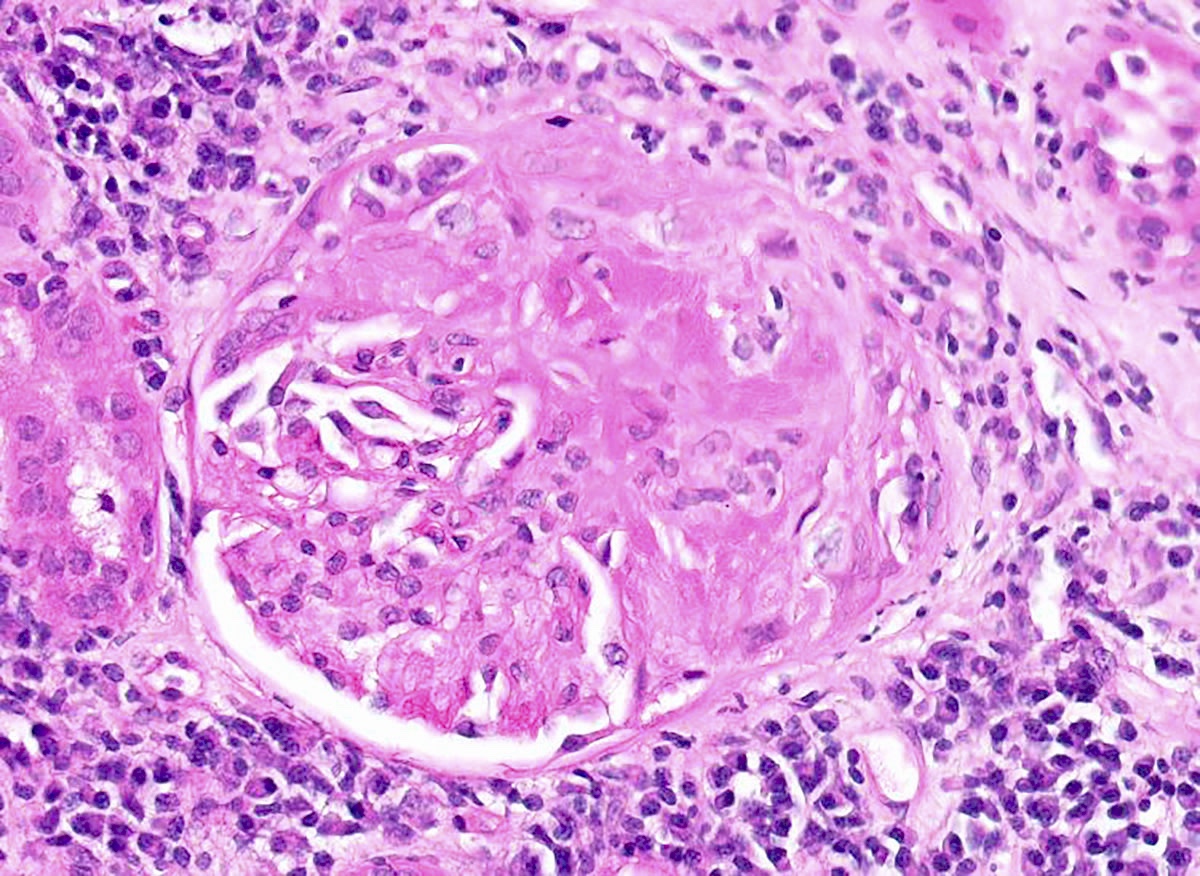
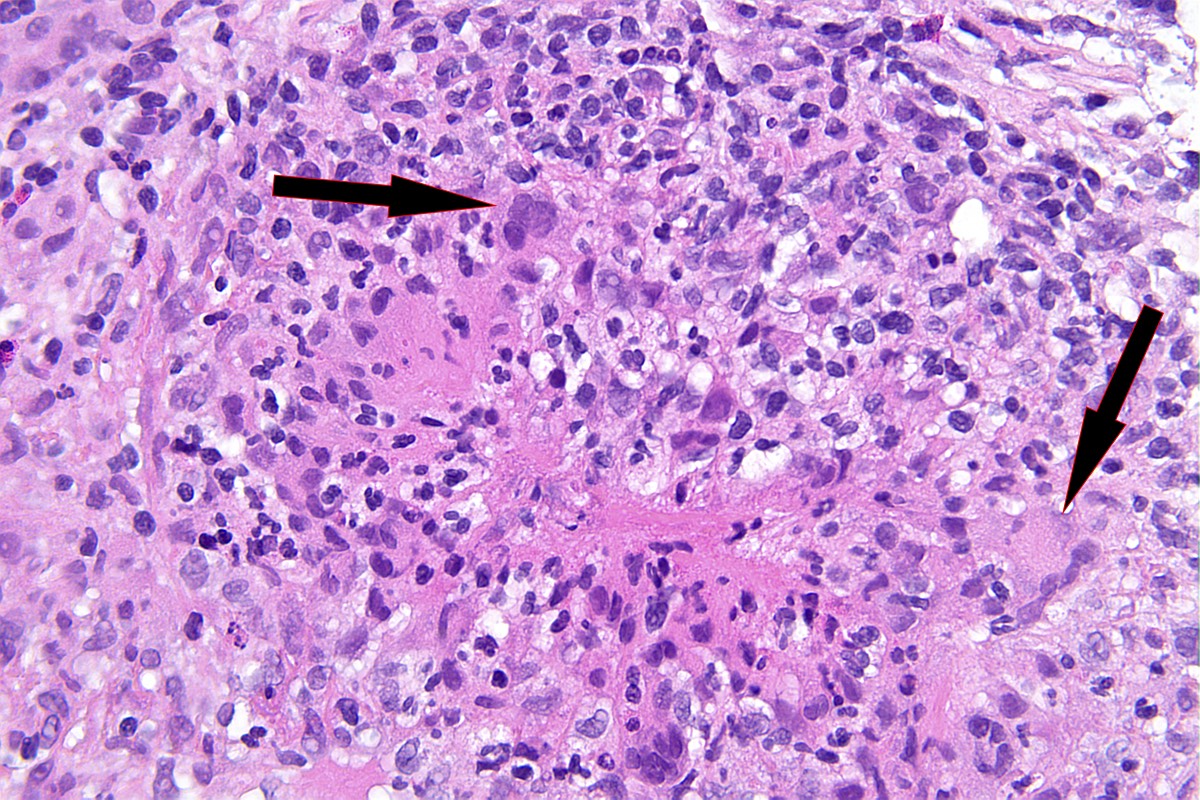
- Destruction of Bowman capsule by crescent formation may result in conspicuous periglomerular inflammation with occasional granuloma formation
- In active cases, features of acute tubular injury (epithelial simplification, flattening) and lymphocytic tubulitis can be seen
- Red blood cells and red blood cell casts can be identified within tubular lumens
- Interstitial edema and inflammation can be seen, occasionally with a prominent eosinophilic or neutrophilic component
- In rare cases, necrotizing neutrophilic granulomatous interstitial inflammation is present, representing a component of the systemic necrotizing granulomatosis
- Review of the special stains is recommended to ensure these are not foci of exuberant cellular crescent formation
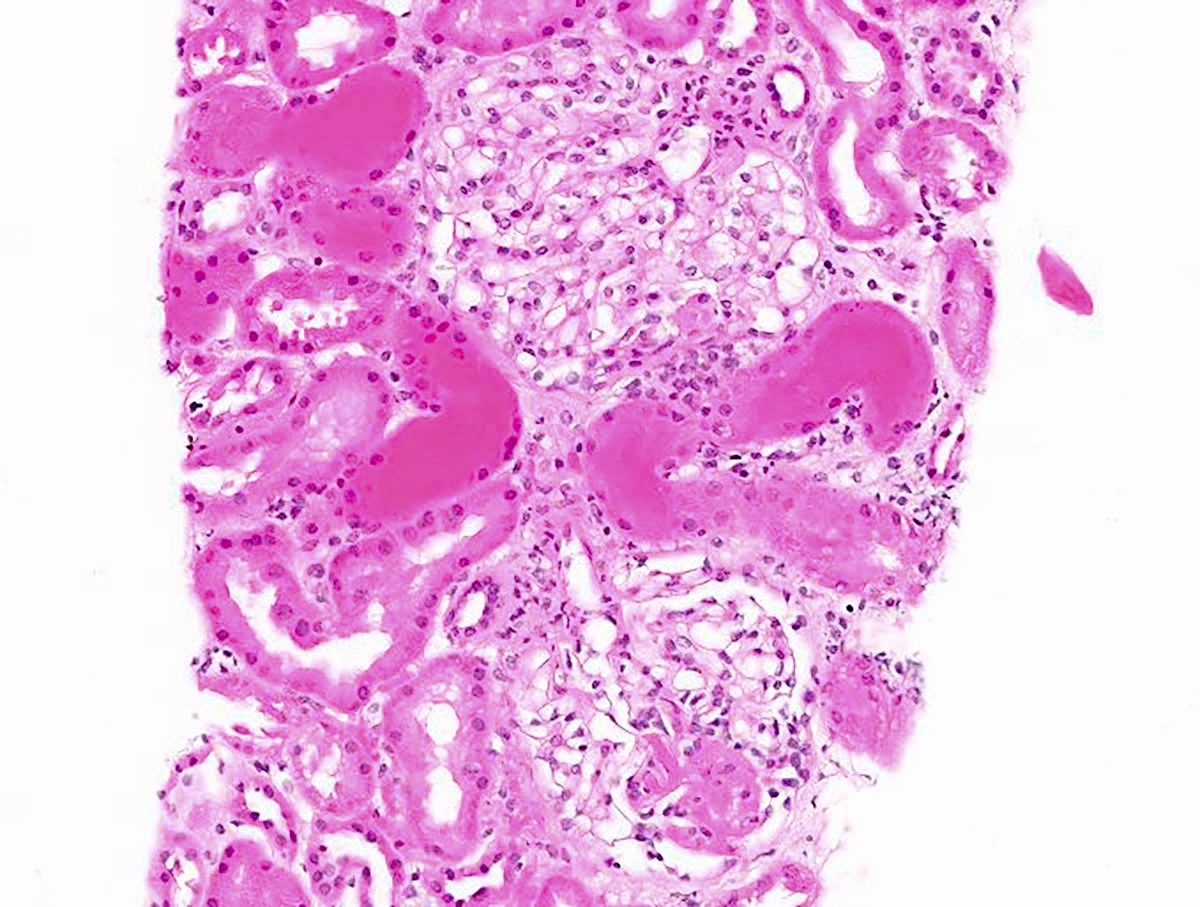
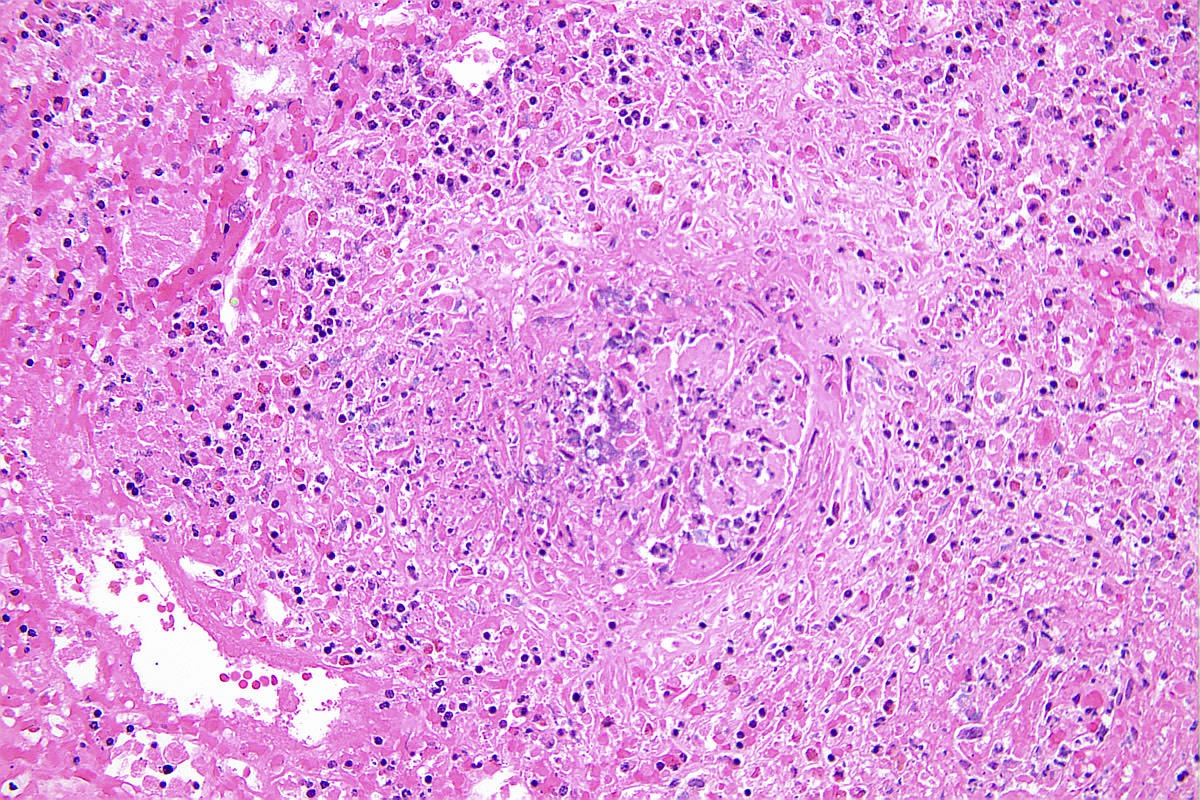
- Vasculitis may affect vessels of any size within the biopsy but interlobular arteries are the most frequently involved
- Features of vasculitis include segmental to circumferential fibrinoid necrosis with associated mural and perivascular mononuclear and neutrophilic inflammation
- Occasional granuloma formation can also be seen
- In the chronic phase, disruption of the internal elastic lamina with extension of asymmetrical scarring into the muscularis suggests a prior vasculitic injury
Microscopic (histologic) images
Contributed by Anjali A. Satoskar, M.D.
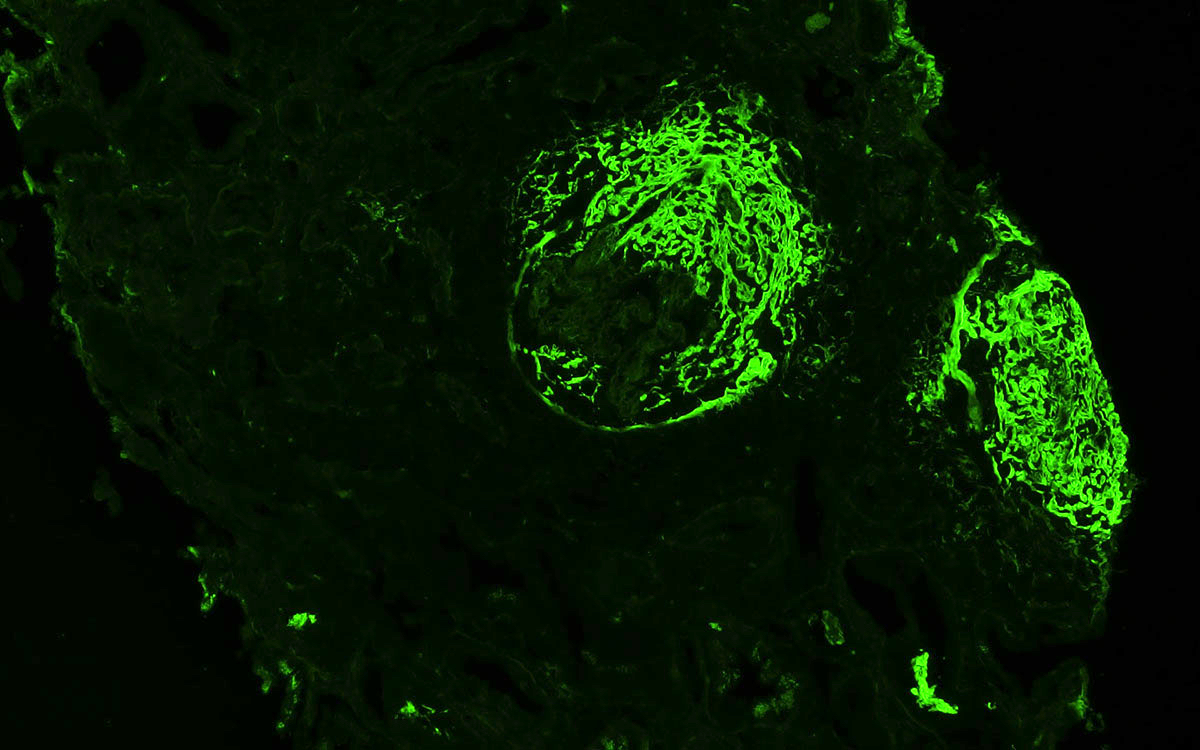
Immunofluorescence description
- GPA is a pauci-immune glomerulonephritis
- Specimens usually show no immunofluorescence staining for immunoglobulins or complement
- However, mild staining (up to 2+ on a 0 - 4+ scale) may be seen
- In areas of fibrinoid necrosis, bright staining for fibrin can be seen
- These areas can also show staining for immunoglobulins and complement and should not be over interpreted as true immunoglobulin or complement deposition
- In areas of glomerular scarring, irregular staining for IgM, C3 and C1q can occur
- This finding should also not be over interpreted as true immunoglobulin or complement deposition
- If strong staining (3 - 4+) for immunoglobulin or complement is seen in areas without sclerosis or necrosis, the diagnosis of pauci-immune glomerulonephritis should be reconsidered
- Reference: Jennette: Heptinstall's Pathology of the Kidney, 7th Edition, 2014
Positive stains
- Smudgy fibrinogen staining around disrupted glomerular capillary tuft and Bowman capsule on immunofluorescence workup can help to confirm presence of crescents
Negative stains
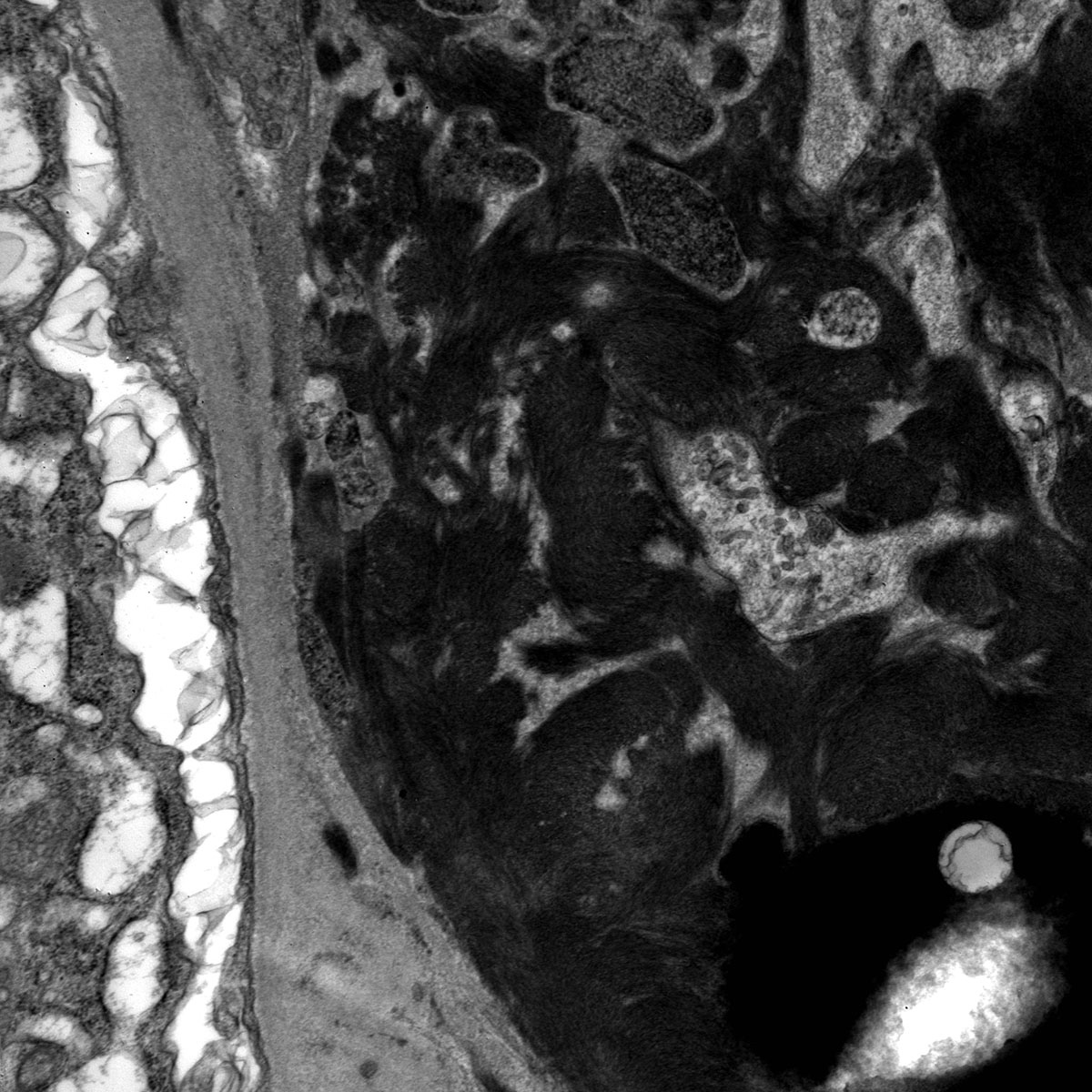
Electron microscopy description
- Similar to the immunofluorescence findings, patients with GPA should have no to very little immune complex type electron dense deposits
- Fibrin tactoids and inflammatory cells may be seen in areas of fibrinoid necrosis or crescent formation
Electron microscopy images
Sample pathology report
- Kidney, native, biopsy:
- Active ANCA associated pauci-immune crescentic and necrotizing glomerulonephritis (see comment)
- Comment: Active cellular crescents and necrotizing lesions with absent or only a few electron dense immune type deposits, in the absence of underlying bacterial infection with positive ANCA serology are together supportive of ANCA associated glomerulonephritis. Concomitant focal fibrocellular and fibrous crescents can be present. Necrotizing vasculitis involving interlobular or arcuate sized intrarenal arteries can be present but can be subject to sampling bias and therefore may not be seen in every biopsy. In the presence of peripheral eosinophilia, the possibility of eosinophilic granulomatosis with polyangiitis emerges; however, histologically, it may not be possible to distinguish from granulomatosis with polyangiitis or microscopic polyangiitis. 10 - 20% of the patients can have negative ANCA serology (ANCA negative small vessel vasculitis). A few small electron dense deposits may be seen and mild IgA staining also can occasionally be present.
Differential diagnosis
- Infection associated glomerulonephritis:
- Anti-glomerular basement membrane disease:
- Crescents all are similar age
- Bright linear staining of the GBM for IgG
- Anti-GBM titers positive
- Drug induced ANCA vasculitis:
- Clinical history should include drugs associated with ANCA vasculitis, such as hydralazine, propylthiouracil, minocycline, anti-TNFα agents, levamisole and cocaine
- Anti-MPO antibodies are more likely to be elevated
- Other pauci-immune vasculitis:
- Can be impossible to distinguish between these entities
- Requires correlation with lab findings and clinical history to suggest the appropriate differential diagnoses
Board review style question #1
In a patient with renal involvement by granulomatosis with polyangiitis, what immunofluorescence staining pattern would be seen in their glomeruli?
- Bright (2 - 3+) fibrinogen staining in active crescents with no or little immunoreactivity in uninvolved glomeruli
- Bright (3+) linear staining of the basement membrane by IgG
- Bright (3+) mesangial C3 staining
- Bright (3+) mesangial IgA staining
Board review style answer #1
A. Bright (2 - 3+) fibrinogen staining in active crescents with no or little immunoreactivity in uninvolved glomeruli. Granulomatosis with polyangiitis is a pauci-immune glomerulonephritis. In active crescents with focal necrosis, bright fibrinogen staining in areas of fibrin deposition can be seen. Answers B - D are incorrect because little to no immunofluorescence staining for IgG, IgA, IgM, C3, C1q, kappa light chain or lambda light chain should be seen.
Comment Here
Reference: Granulomatosis with polyangiitis
Comment Here
Reference: Granulomatosis with polyangiitis