Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Electron microscopy description | Sample pathology report | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Chan AWH. Drug / toxin induced hepatitis (DILI)-general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/liverdrugtoxingeneral.html. Accessed August 26th, 2025.
Definition / general
- Liver injury associated with exposure to certain drugs or toxins
- Drug / toxin reactions can be intrinsic or predictable when they are dose dependent (e.g., acetaminophen) or idiosyncratic and unpredictable when they are dose independent (e.g., isoniazid)
Essential features
- Diagnosis is based on the likelihood of DILI: time to onset, time to recovery, clinicopathological phenotype, exclusion of other causes, potentially offensive drug / toxin being a known cause of liver injury, and response to re-exposure
- Very wide range of clinical and pathological presentations
ICD coding
- ICD-10: K71.6 - toxic liver disease with hepatitis, not elsewhere classified
Epidemiology
- 10 - 20/100,000 based on prospective population based studies (Hepatology 2002;36:451, Am J Gastroenterol 2012;107:1380, Gastroenterology 2013;144:1419)
- Leading cause of acute liver failure and one of the major reasons for liver transplantation in the U.S. and Europe (Hepatology 2010;52:2065)
- Conventional medications are the most common cause of idiosyncratic DILI in the U.S. and Europe, whereas herbal medicine and dietary supplements are the major cause in Asia (Liver Int 2007;27:465)
Pathophysiology
- Underlying mechanisms include direct / indirect toxicity, aberrant metabolism producing toxic metabolites and immune mediated hypersensitivity
Etiology
- Drug dependent risk factors (J Hepatol 2019;70:1222):
- Dose
- Hepatic drug metabolism
- Lipophilicity
- Special moieties: toxic metabolite, oxidative stress, mitochondrial liability, hepatobiliary transport inhibition
- Concomitant drug / toxin
- Host dependent risk factors (J Hepatol 2019;70:1222):
- Age
- Gender
- Ethnicity
- Alcohol consumption
- Pregnancy
- Underlying liver disease or comorbidity
Clinical features
- Very wide range of clinical and pathological presentations can result; the time of onset after drug exposure varies from hours to months
- Clinical manifestations range from asymptomatic deranged liver function to fulminant hepatic failure and death
- Mimics all forms of acute, chronic, vascular or neoplastic liver diseases caused by other etiologies
- Reference: J Hepatol 2019;70:1222
Diagnosis
- Diagnosis is based on likelihood of DILI
- Time to onset
- Time to recovery
- Clinicopathological phenotype
- Exclusion of other causes
- Potentially offensive drug / toxin being a known cause of liver injury
- Response to re-exposure
- LiverTox, which is produced by NLM (National Library of Medicine) and NIDDK (National Institute of Diabetes and Digestive and Kidney Disease), provides up to date, accurate and easily accessed information on drug induced liver injury
Laboratory
- R value = [serum alanine aminotransferase (ALT) / upper limit of normal (ULN)] / [serum alkaline phosphatase (ALP) / ULN]
- Hepatocellular injury: R value ≥ 5 or ALT elevation ≥ 5 fold above ULN
- Cholestatic injury: R value ≤ 2 or ALP elevation ≥ 2 fold above ULN
- Mixed injury: R value between 2 and 5
- Liver biopsy may be considered in suspected DILI if:
- Elevated serum autoimmune markers
- Disease progresses or fails to resolve on withdrawal of suspected agent
- Reference: J Hepatol 2019;70:1222
Prognostic factors
- 5 - 20% may develop chronic DILI (Hepatology 2006;44:1581, Gastroenterology 2014;147:96, Gastroenterology 2019;156:2230)
- DILI severity grading (U.S. Drug Induced Liver Injury Network) (Hepatology 2010;52:730)
- Score 1 (mild): elevated ALT or ALP but total bilirubin < 2.5 mg/dL and international normalized ratio (INR) < 1.5
- Score 2 (moderate): elevated ALT or ALP and total bilirubin ≥ 2.5 mg/dL or INR ≥ 1.5
- Score 3 (moderate - severe): elevated ALT, ALP, total bilirubin or INR and hospitalization or ongoing hospitalization is prolonged because of a DILI episode
- Score 4 (severe): elevated ALT or ALP and total bilirubin ≥ 2.5 mg/dL and at least 1 of the following:
- Liver failure (INR > 1.5, ascites or encephalopathy)
- Other organ failure due to DILI
- Score 5 (fatal): death or liver transplantation due to DILI
Case reports
- 34 year old woman with acute cholestatic hepatitis after Moderna vaccination (J Hepatol 2022 Apr 18 [Epub ahead of print])
- 39 year old woman with acute cholestatic hepatitis after herbal supplement containing ashwagandha root extract (J R Coll Physicians Edinb 2021;51:363)
- 51 year old man with vanishing bile duct syndrome after infliximab for malignant melanoma (Cureus 2022;14:e21940)
- 61 year old woman with secondary sclerosing cholangitis after apatinib and vinorelbine for metastasis breast cancer (Thorac Cancer 2021;12:1912)
- 80 year old woman with amiodarone induced submassive necrosis in background of cirrhosis (ACG Case Rep J 2015;2:116)
Treatment
- Most patients recover spontaneously after withdrawal of offensive agent
- Specific therapy targeted for specific forms of DILI:
- Steroid for drug induced autoimmune hepatitis or those with marked hypersensitivity features
- Ursodeoxycholic acid for drug induced chronic cholestasis
- N-acetylcysteine for acetaminophen hepatoxicity
- Carnitine for valproate hepatotoxicity
- Reference: J Hepatol 2019;70:1222
Microscopic (histologic) description
- Morphological patterns can be categorized into the following:
- Necroinflammatory injury:
- Acute hepatic necrosis: acetaminophen, carbon tetrachloride
- Drug induced autoimmune hepatitis: diclofenac, halothane, indomethacin, infliximab, methyldopa, minocycline, nitrofurantoin, statins (J Hepatol 2019;70:1222)
- Granulomatous hepatitis: allopurinol, carbamazepine, methyldopa, phenytoin, quinidine, sulphonamides (J Hepatol 2019;70:1222)
- Cholestatic injury:
- Bland cholestasis: oral contraceptives
- Acute cholestatic hepatitis: amoxicillin / clavulanate, phenothiazines (Gut 1992;33:368)
- Chronic cholestatic injury: chlorpromazine, azathioprine, androgens, amoxicillin clavulanate, carbamazepine, chlorpromazine, erythromycin, estradiol, flucloxacillin, phenytoin, terbinafine, cotrimoxazole (Hepatology 1994;20:1437, J Hepatol 2019;70:1222)
- Steatosis and steatohepatitis:
- Microvesicular steatosis: amiodarone, didanosine, stavudine, valproate, zalcitabine, aspirin (Reye syndrome) (J Hepatol 2019;70:1222)
- Macrovesicular steatosis and steatohepatitis: corticosteroids, 5-fluorouracil, irinotecan, methotrexate, tamoxifen (J Hepatol 2019;70:1222)
- Vascular lesion:
- Sinusoidal obstruction syndrome: antineoplastic cytotoxic agents (Paediatr Drugs 2010;12:277)
- Budd-Chiari syndrome: oral contraceptives, dacarbazine (Postgrad Med J 2015;91:692)
- Nodular regenerative hyperplasia: azathioprine, busulphan, bleomycin, cyclophosphamide, chlorambucil, cysteine arabinoside, carmustine, doxorubicin, 6-thioguanine, oxaliplatin (J Hepatol 2019;70:1222)
- Sinusoidal dilatation and peliosis: oral contraceptives (Dig Liver Dis 2015;47:e10)
- Neoplasm and neoplasm-like lesion:
- Focal nodular hyperplasia (FNH): oral contraceptives (not associated with FNH pathogenesis; associated with larger and more symptomatic FNH)
- Hepatocellular adenoma: oral contraceptives, anabolic steroids (J Gastroenterol 2000;35:557)
- Angiosarcoma and cholangiocarcinoma: thorotrast (Hepatology 1995;22:1674, Cancer 1982;49:2161)
- Adaptive change:
- Pseudo ground glass inclusion: barbiturates, diazepam, cyanamide, transplant patients on multiple immunosuppressive drugs (steroids, tacrolimus and mycophenolate mofetil) (J Clin Pathol 2009;62:481)
- Necroinflammatory injury:
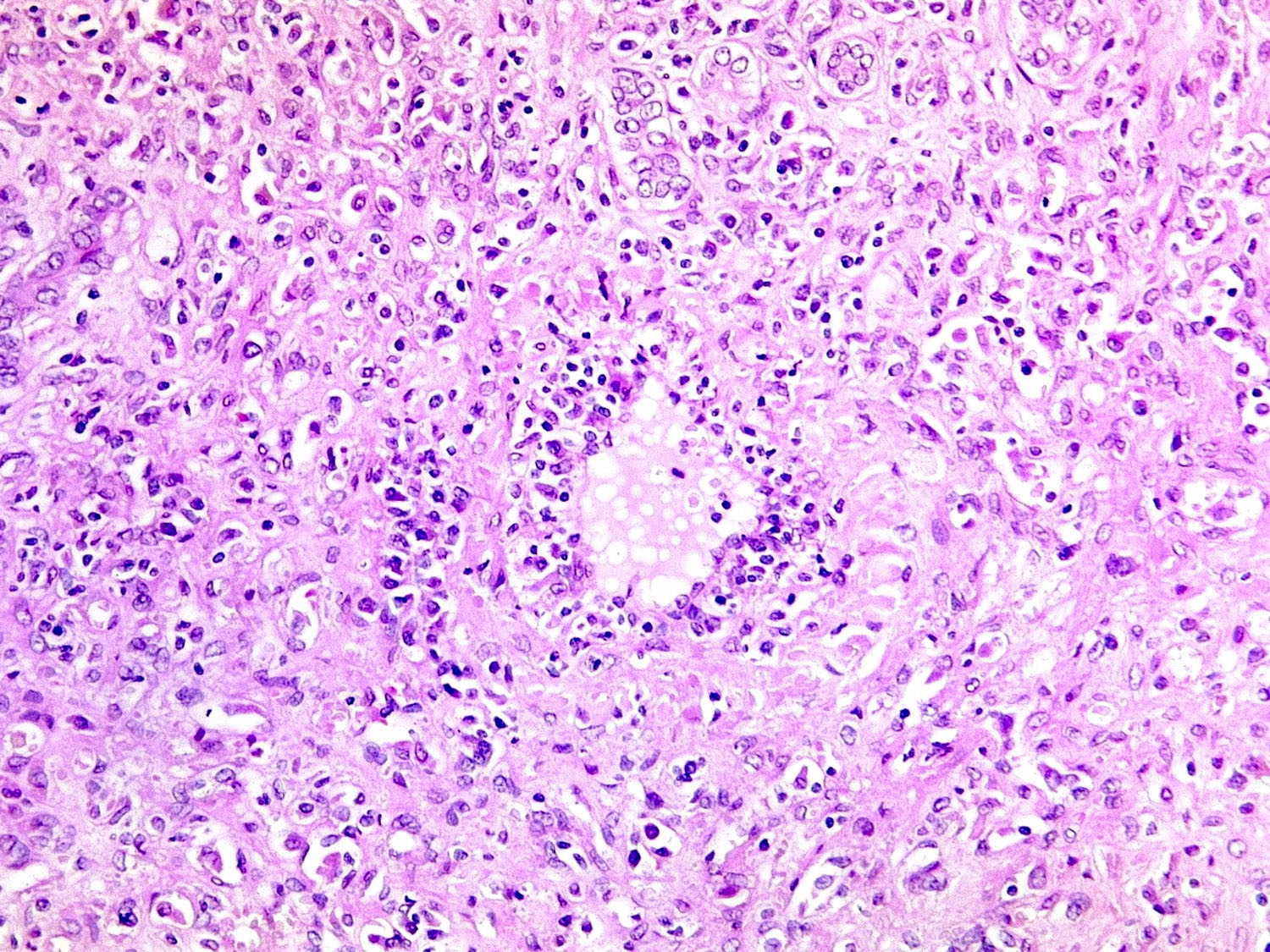
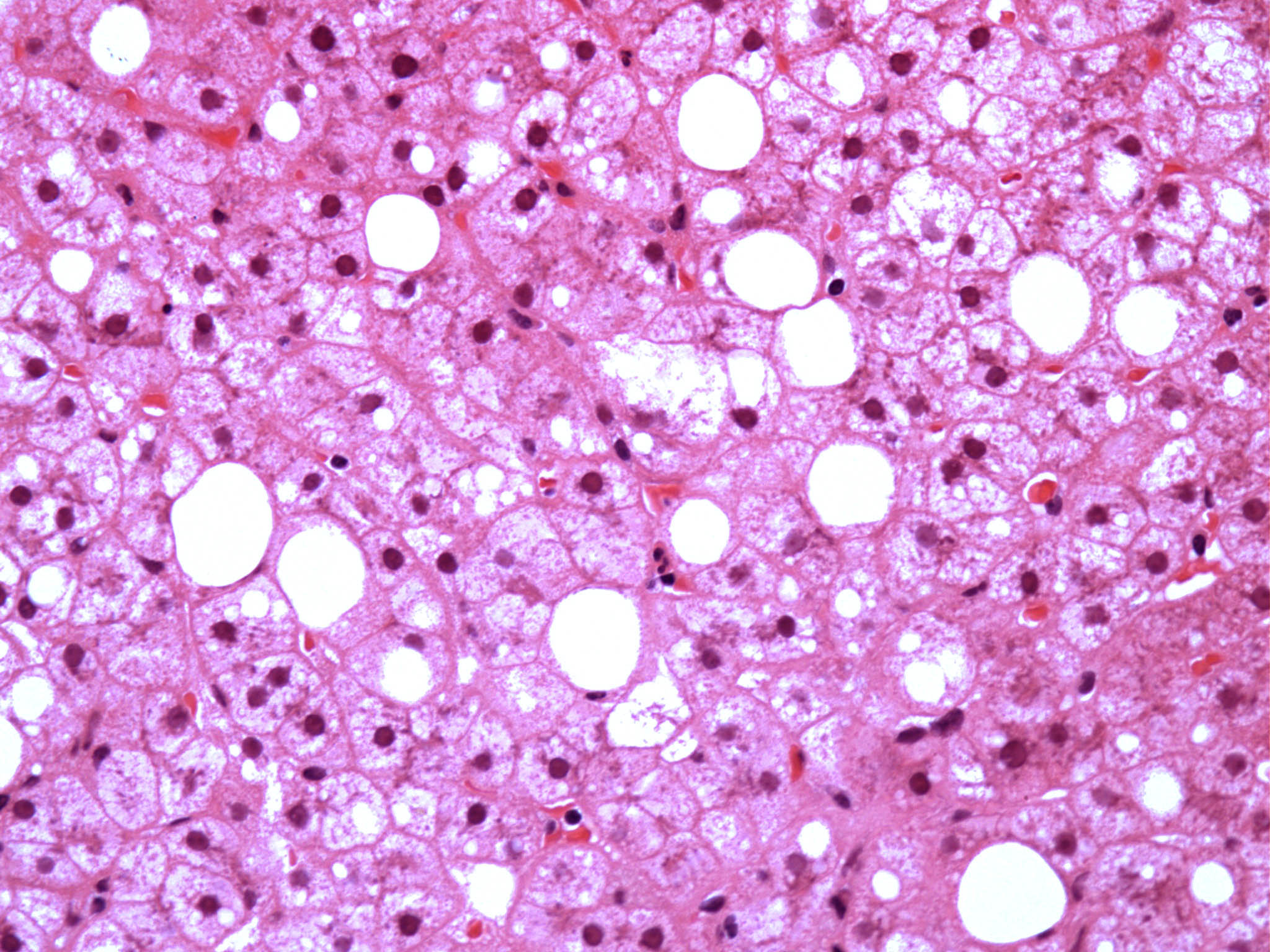
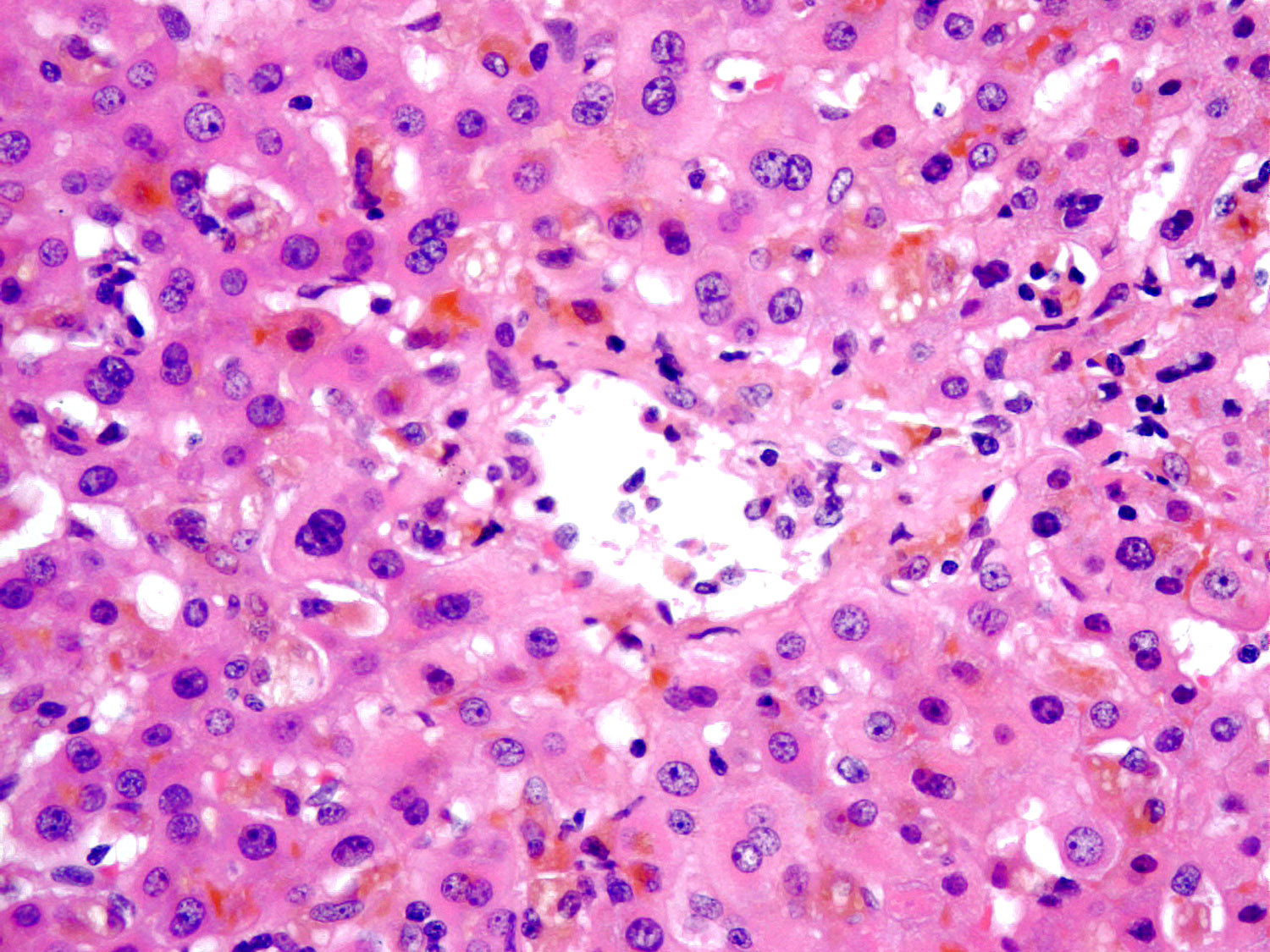
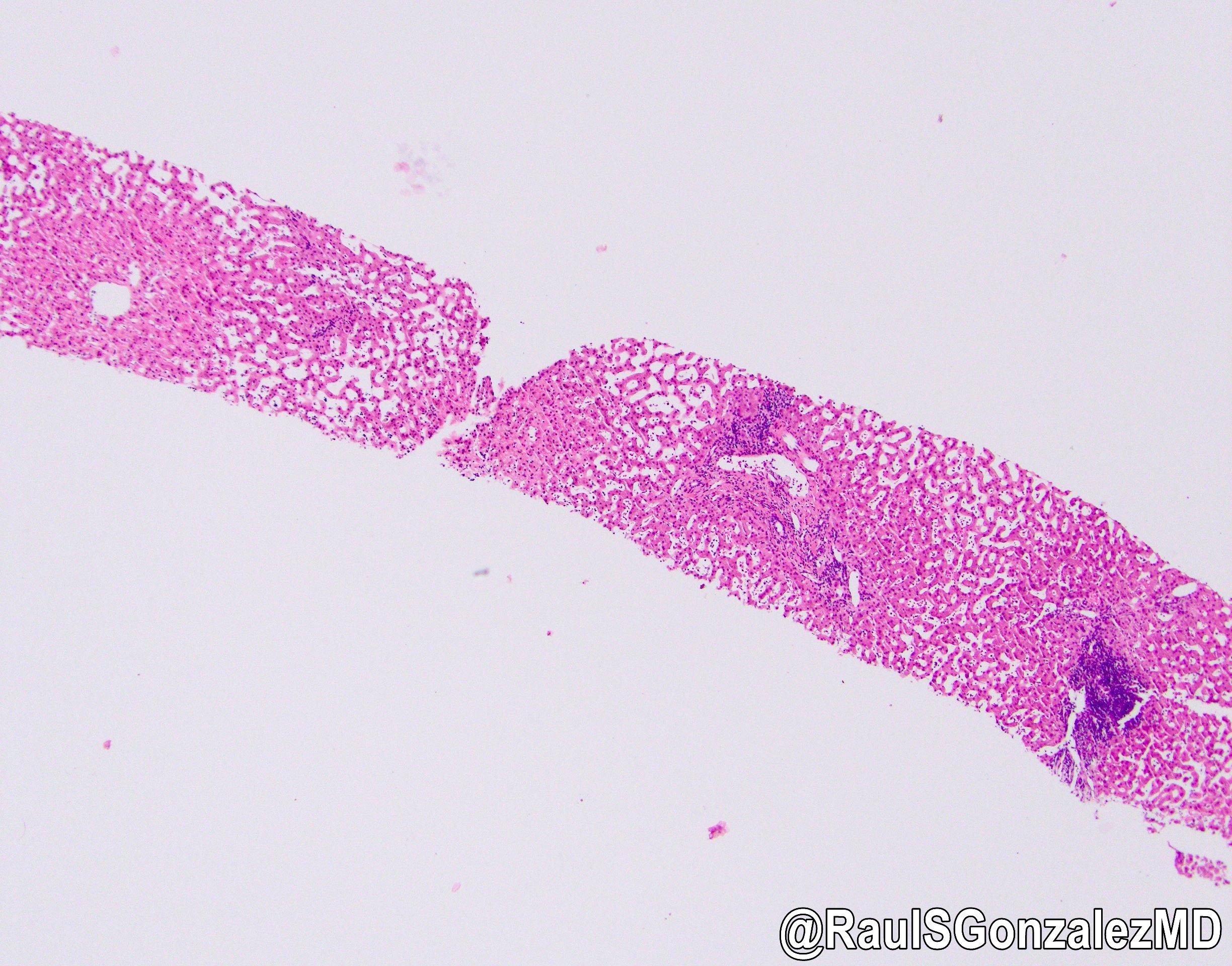
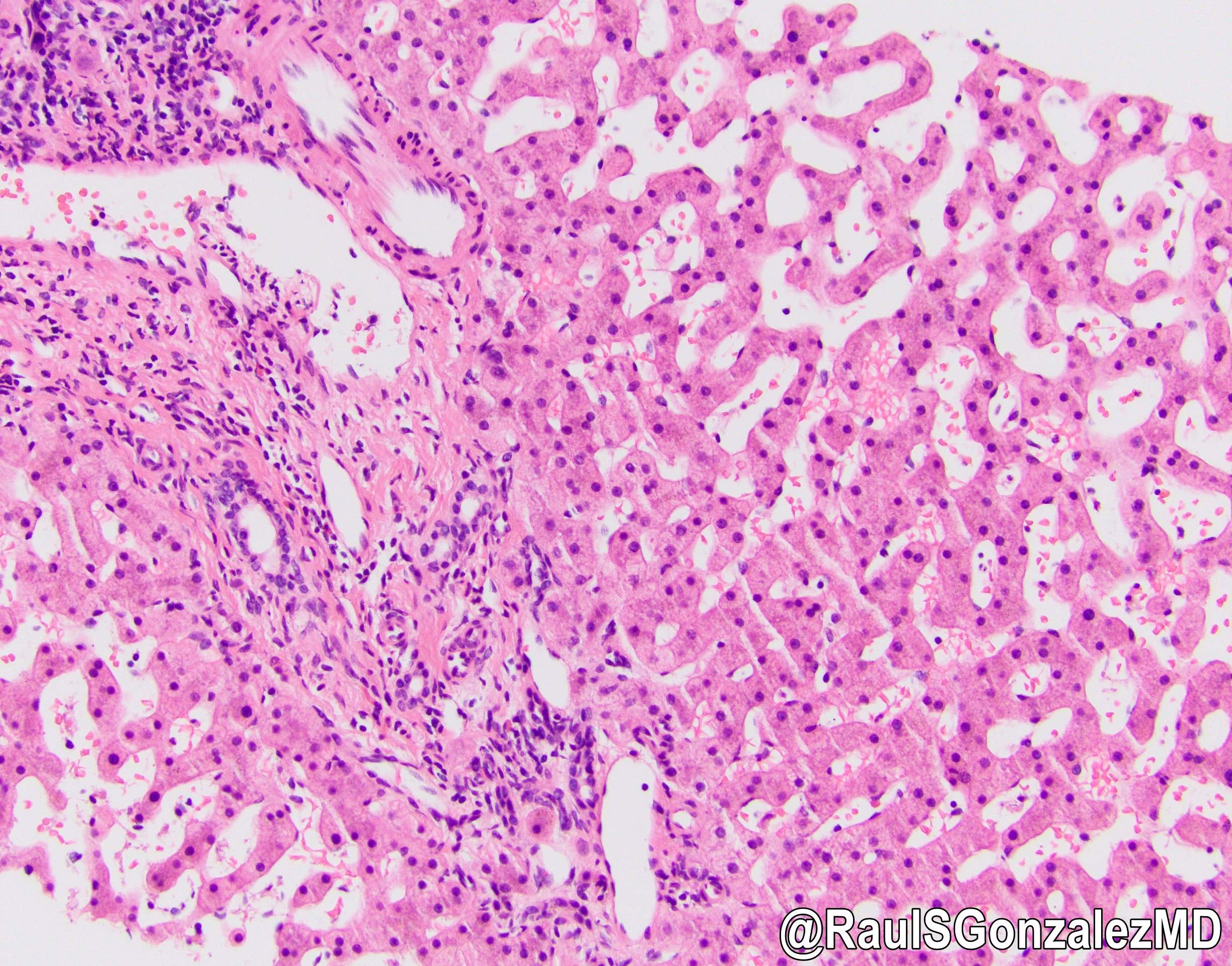
Microscopic (histologic) images
Electron microscopy description
- Amiodarone: lamellar lysosomal inclusion bodies representing phospholipidosis (Hum Pathol 1990;21:59)
Sample pathology report
- Liver, biopsy:
- Intrahepatic cholestasis without any significant necroinflammatory activity or fibrosis (see comment)
- Comment: The histological features are those of bland cholestasis. Differential diagnoses include drug induced liver injury, sepsis, shock, paraneoplastic syndrome, intrahepatic cholestasis of pregnancy and familial disorders of bile acid transporter. According to the case note, the patient has used oral contraceptive pills for a few months. Oral contraceptive pills are one of the most common medications associated with drug induced bland cholestasis. Recommend correlation with clinical impression, drug history and disease progress after withdrawal of the oral contraceptive pill.
Practice question #1
Which medication is associated with microvesicular steatosis?
- Corticosteroid
- Didanosine
- Methotrexate
- Tamoxifen
Practice answer #1
B. Didanosine. Didanosine is associated with microvesicular steatosis, whereas the other 3 drugs are associated with macrovesicular steatosis.
Comment Here
Reference: Drug / toxin induced hepatitis (DILI) - general
Comment Here
Reference: Drug / toxin induced hepatitis (DILI) - general
Practice question #2
A patient was found to have an incidental liver tumor during routine checkup. You received the wedge excision of the liver tumor. Microscopic examination revealed a well differentiated hepatocellular neoplasm. The hepatocytes were bland and arranged in trabeculae of up to 2 cells with intact reticulin framework. Unpaired arterioles were noted. Which medication is associated with this hepatic mass lesion?
- Azathioprine
- Ethanol
- Oral contraceptive pill
- Thorotrast
Practice answer #2
C. Oral contraceptive pill. The description of the tumor is typical for hepatocellular adenoma. Oral contraceptive pills are a risk factor associated with hepatocellular adenoma.
Comment Here
Reference: Drug / toxin induced hepatitis (DILI) - general
Comment Here
Reference: Drug / toxin induced hepatitis (DILI) - general