Table of Contents
Definition / general | Essential features | Nodal follicular / nodular proliferations | Nodal immunoblastic proliferations | Extranodal follicular proliferations | Extranodal immunoblastic proliferations | Case reports | Microscopic (histologic) images | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Segura-Rivera R, Marques-Piubelli ML, Miranda RN. Reactive B cell rich lymphoid proliferations that can mimic lymphoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphnodesreactivebcellrichlymphprolif.html. Accessed April 19th, 2024.
Definition / general
- Heterogeneous group of nonneoplastic proliferations that mimic B cell lymphomas and affect lymphoid tissue in nodal or extranodal sites
Essential features
- Heterogeneous group of nonneoplastic proliferations that mimic B cell lymphomas and affect lymphoid tissue in nodal or extranodal sites
- 3 different main patterns
- Nodal and extranodal follicular proliferations
- Nodal and extranodal nodular proliferations
- Nodal and extranodal immunoblastic proliferations
Nodal follicular / nodular proliferations
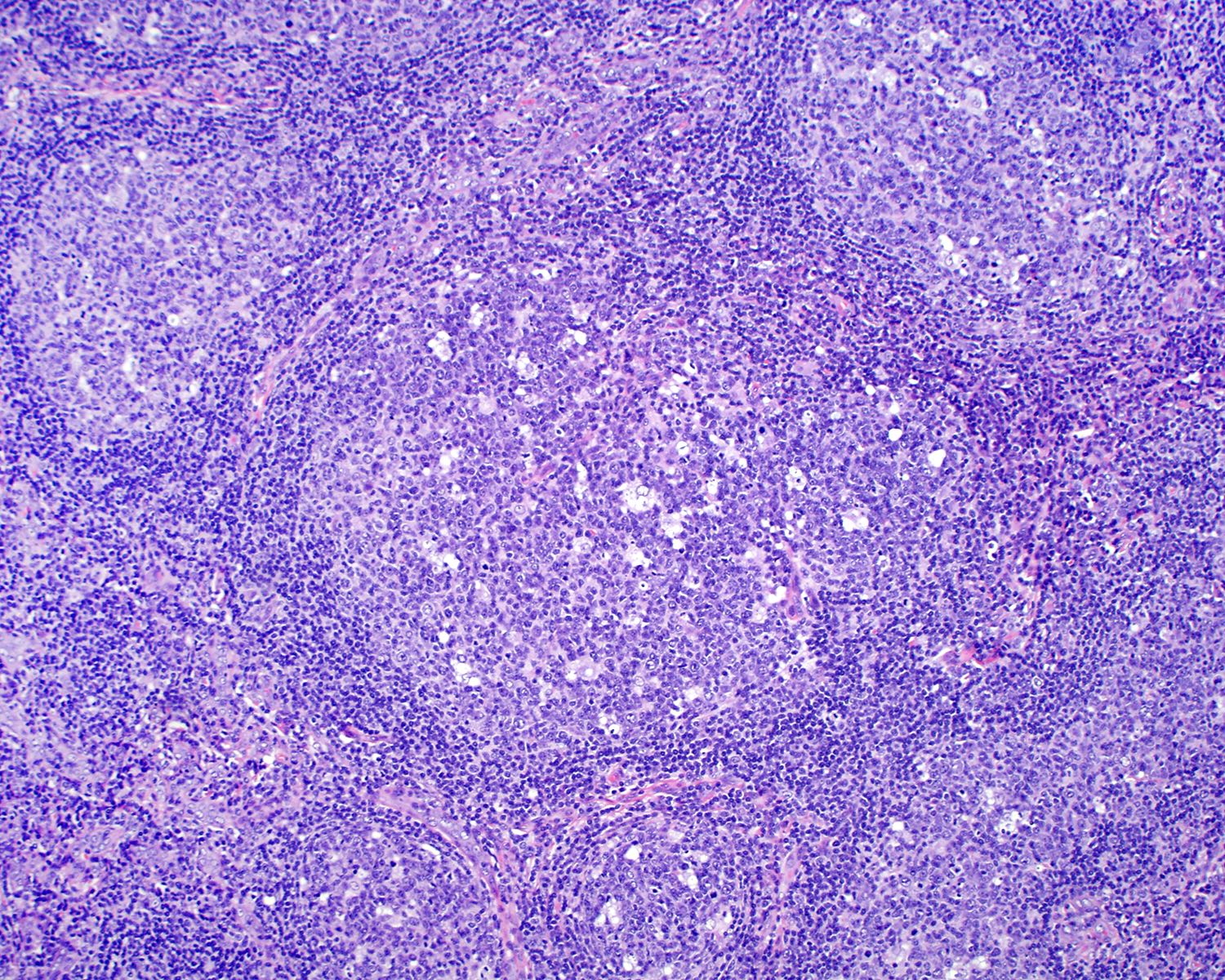
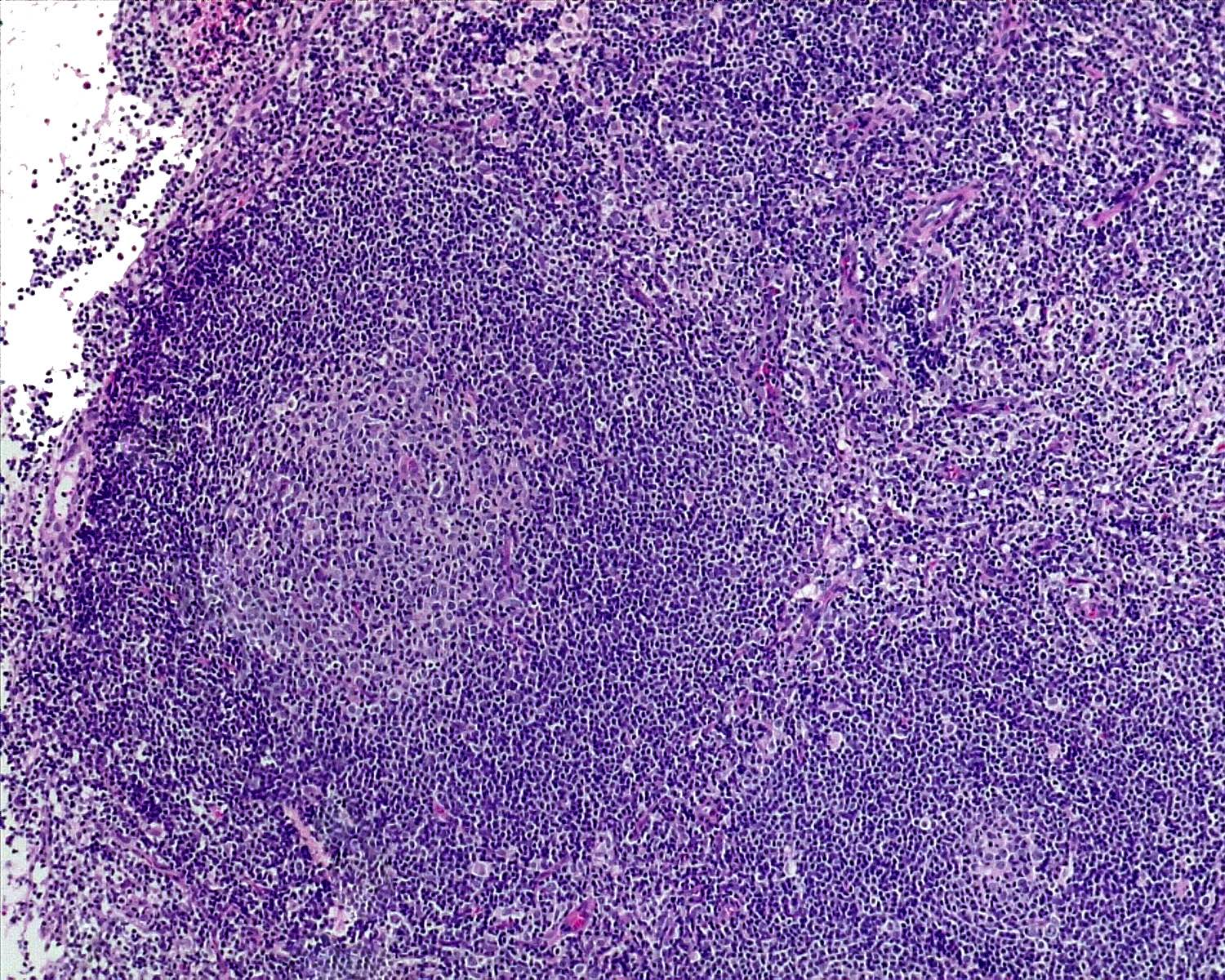
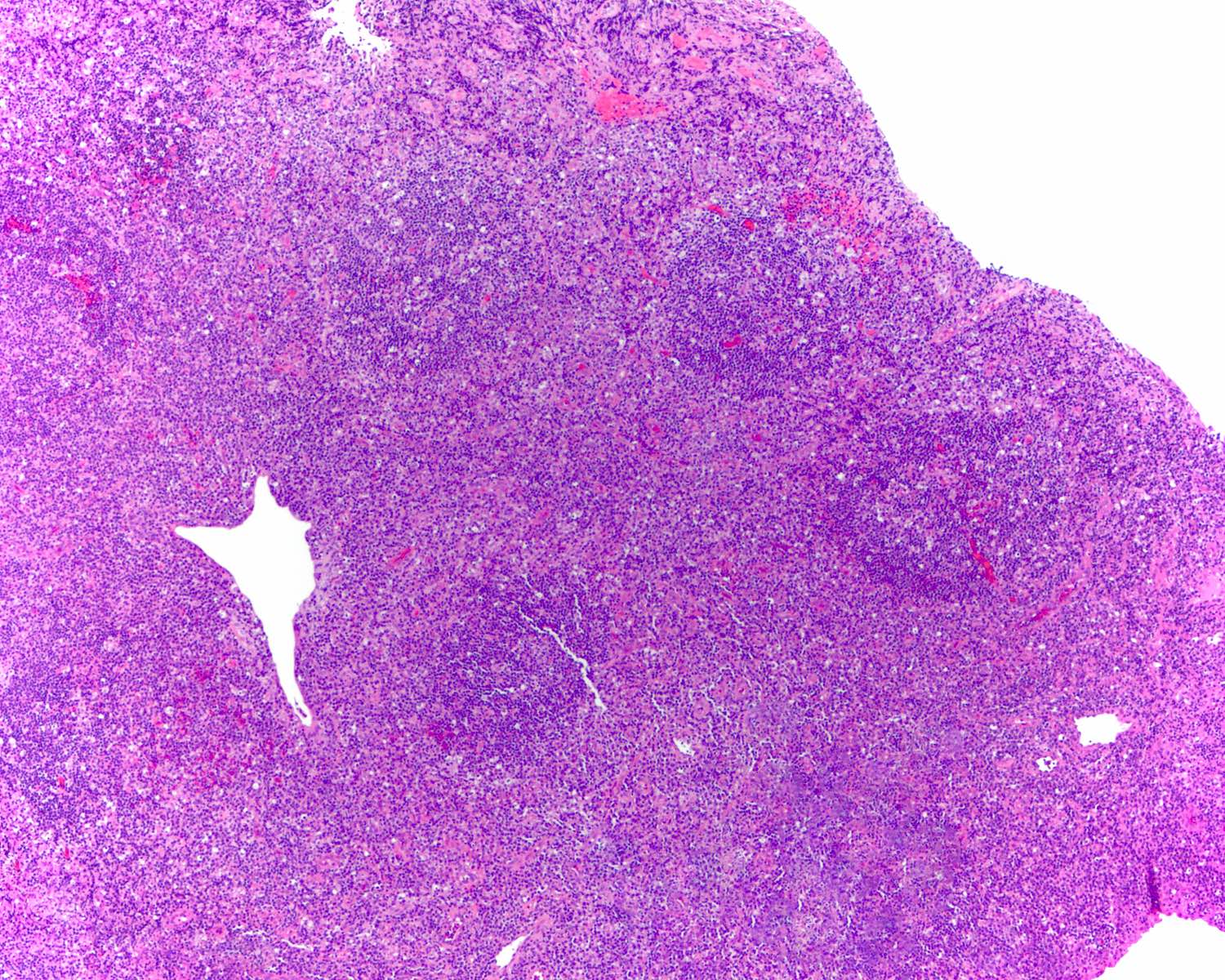
Florid follicular hyperplasia (Hematol Oncol Clin North Am 2009;23:729)
Progressive transformation of germinal centers (PTGC) (Virchows Arch 2019;475:771)
Hyaline vascular Castleman disease (HVCD)
- Increased number of widely spaced primary and secondary follicles
- Florid cases usually involve medulla
- Follicles show uneven size and shape
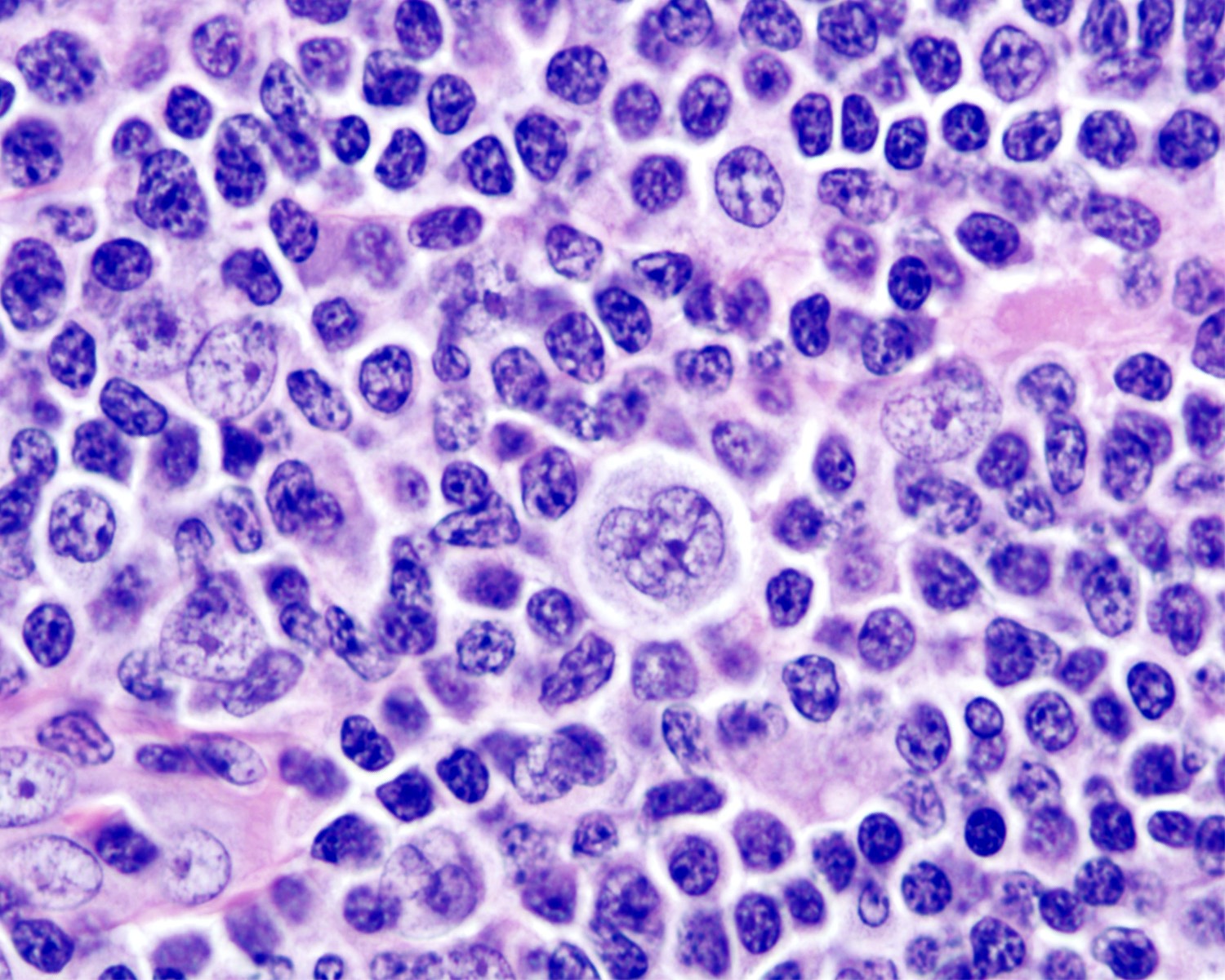
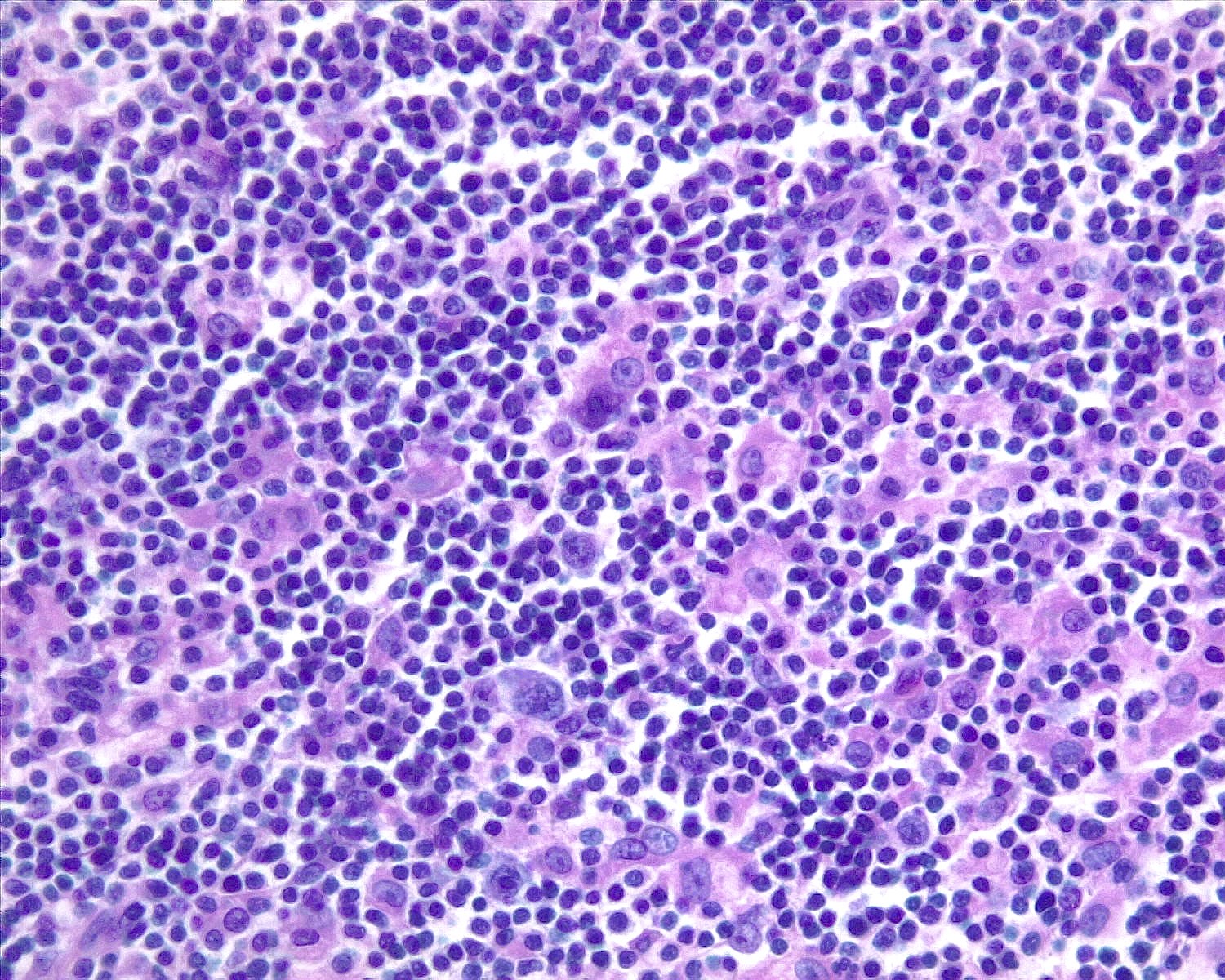
- Hyperplastic germinal centers (GC) comprise a mixture of centroblasts and centrocytes with brisk mitoses, reactive T cells, follicular dendritic cells (FDCs) and tingible body macrophages (Ann Diagn Pathol 2020;44:151421)
- B lymphocytes
- Centroblasts
- Mainly in the dark zone of GC
- 3 - 4x size of small lymphocytes
- Large vesicular nuclei, 1 - 3 peripheral nucleoli
- Centrocytes
- Mainly in the light zone of GC
- Small to intermediate sized cells with cleaved, hyperchromatic nuclei and small or absent nucleolus
- Both centroblasts and centrocytes are BCL6+, CD10+, LMO2+, HGAL / GCET+, OCT2+
- Centroblasts
- T lymphocytes
- Small and round
- CD3+ and BCL2+
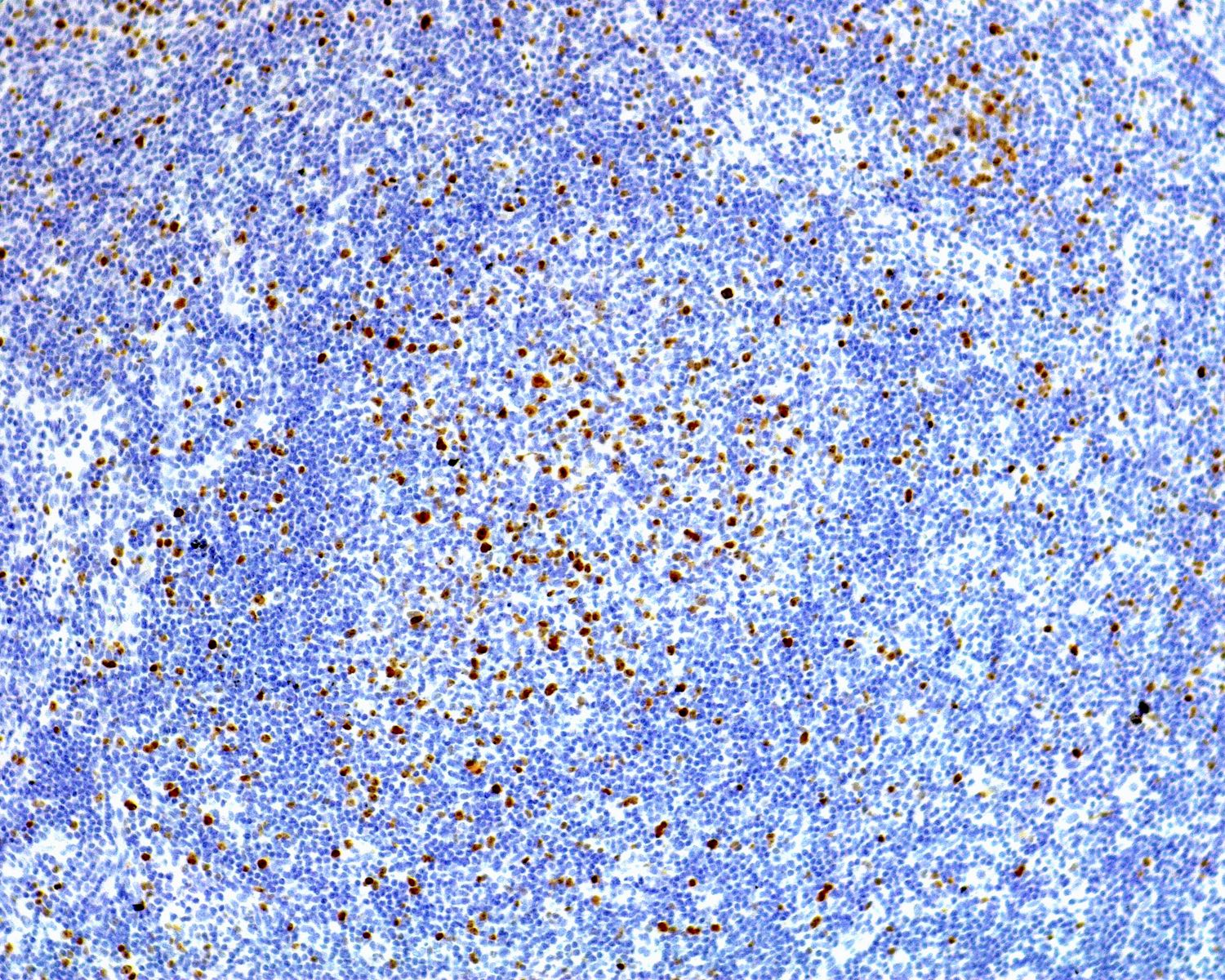
- Subset of T helper cells (TFH): polarized CD4+, PD-1 / CD279+, BCL6+, CD10+, CXCL13+, ICOS+
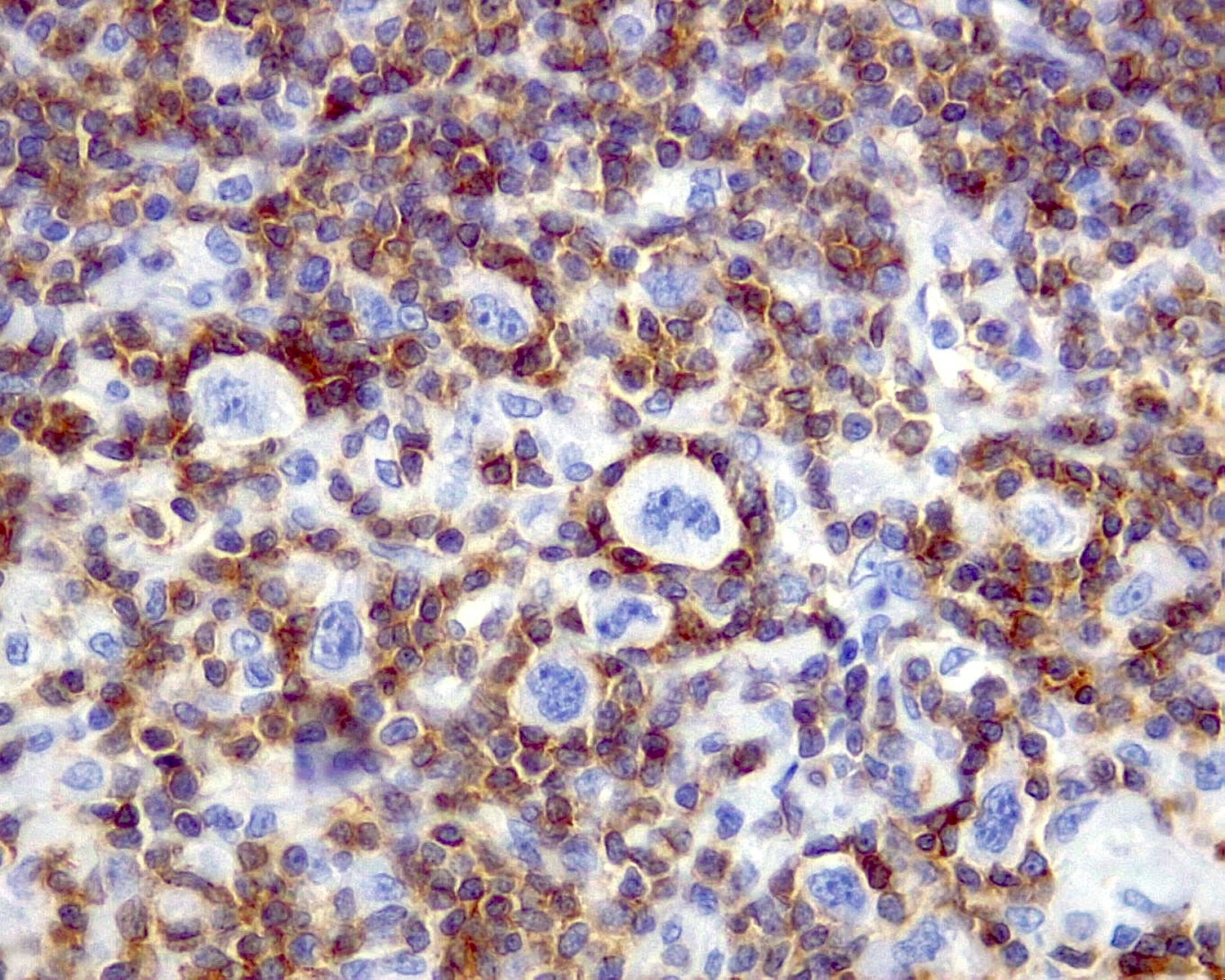
- Follicular dendritic cells (FDCs)
- Few (usually ~1%)
- 2 square shaped and adjacent nuclei (kissing cells) with vesicular chromatin
- May display small nucleolus
- Long cytoplasmic processes
- CD21+, CD23+ or CD35+
- Tingible body macrophages
- Display oval or twisted vesicular nuclei
- Abundant pale cytoplasm containing karyorrhectic nuclei
- Impart a starry sky pattern when prominent
- CD4+, CD68+ or CD163+
- B lymphocytes
- Well developed mantle zones
- Distinction from germinal centers
- Composed predominantly of small lymphocytes and IgD+
- No or limited extension outside the capsule into perinodal soft tissue
- Location can suggest underlying disease and additional workup is necessary to confirm the diagnosis (Pediatr Clin North Am 2002;49:1009)
- Cervical: infectious mononucleosis
- Posterior cervical: toxoplasmosis
- Parotid, submaxillary, epitrochlear: HIV infection
- Cervical and axillary: cat scratch disease, dermatopathic lymphadenitis
- Inguinal: sexually transmitted diseases
- Immunoarchitecture (Ann Diagn Pathol 2020;44:151421, Clin Lab Med 2021;41:433)
- Lymphoid follicles express B cell antigens
- Primary follicles
- Small lymphocytes
- BCL2+, Ki67 (usually < 10%), BCL6- and CD10-
- Secondary follicles
- GCs are BCL2-
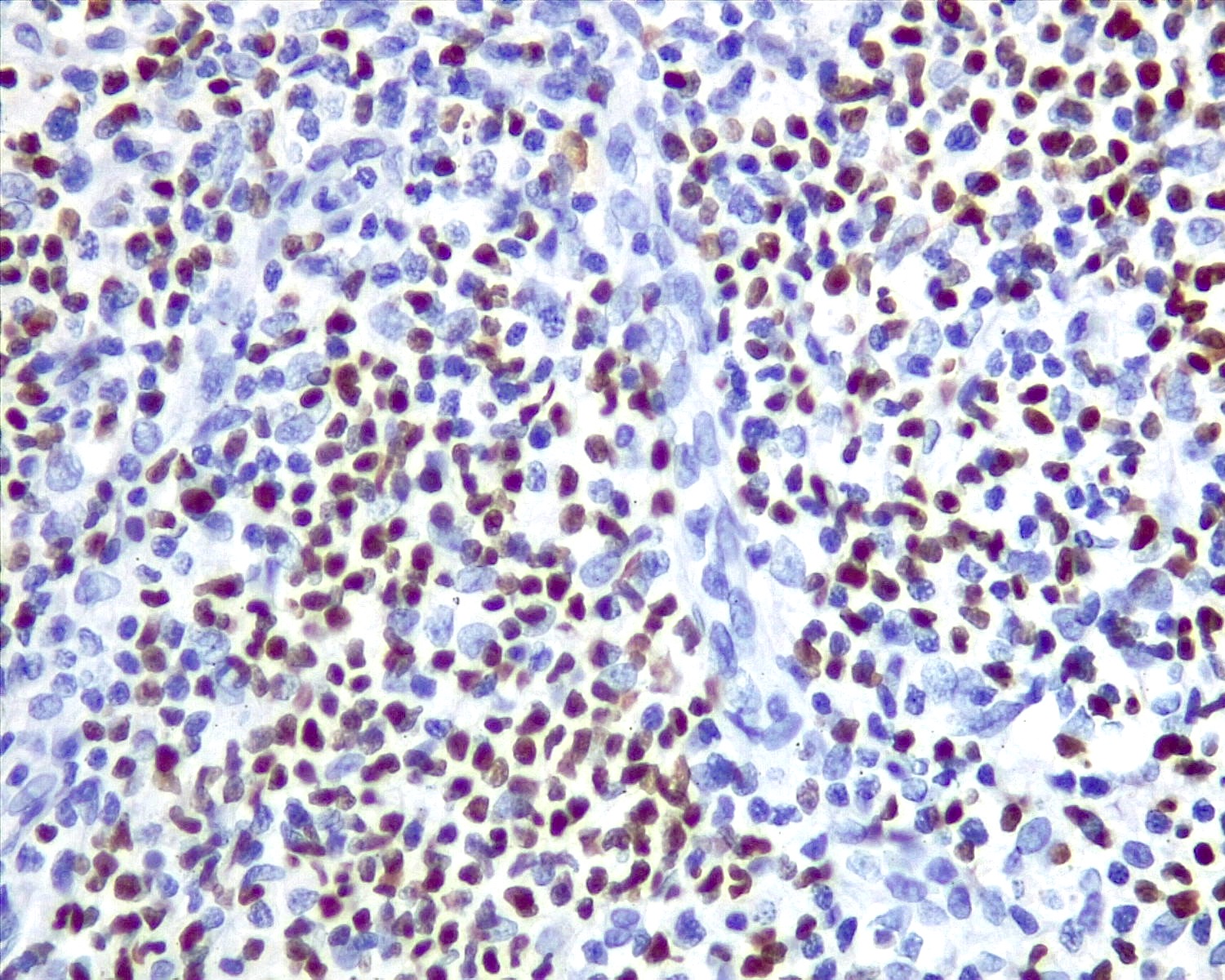
- TFH cells: subset of T cells in germinal centers are BCL2 positive and occasionally are increased
- May mimic follicular lymphoma
- Pediatric type follicular lymphoma (FL) is BCL2 negative
- FOXP1 is positive in GC cells of pediatric FL (Virchows Arch 2019;475:771)
- TFH cells: subset of T cells in germinal centers are BCL2 positive and occasionally are increased
- High proliferation Ki67
- Polarization (centroblast rich dark zone and a centrocyte rich pale zone)
- Low Ki67 proliferation rate within a germinal center should always be considered atypical and suspicious for follicular lymphoma
- BCL6+ and CD10+ are restricted to GCs, minimal in interfollicular areas
- GCs are BCL2-
- Flow cytometry
- Polytypic B cells; CD10+, T cell antigens-
- Polymerase chain reaction (PCR)
- Polyclonal immunoglobulin heavy chain gene rearrangement
- No evidence of t(14;18)(q32;q21) or IGH::BCL2 fusion
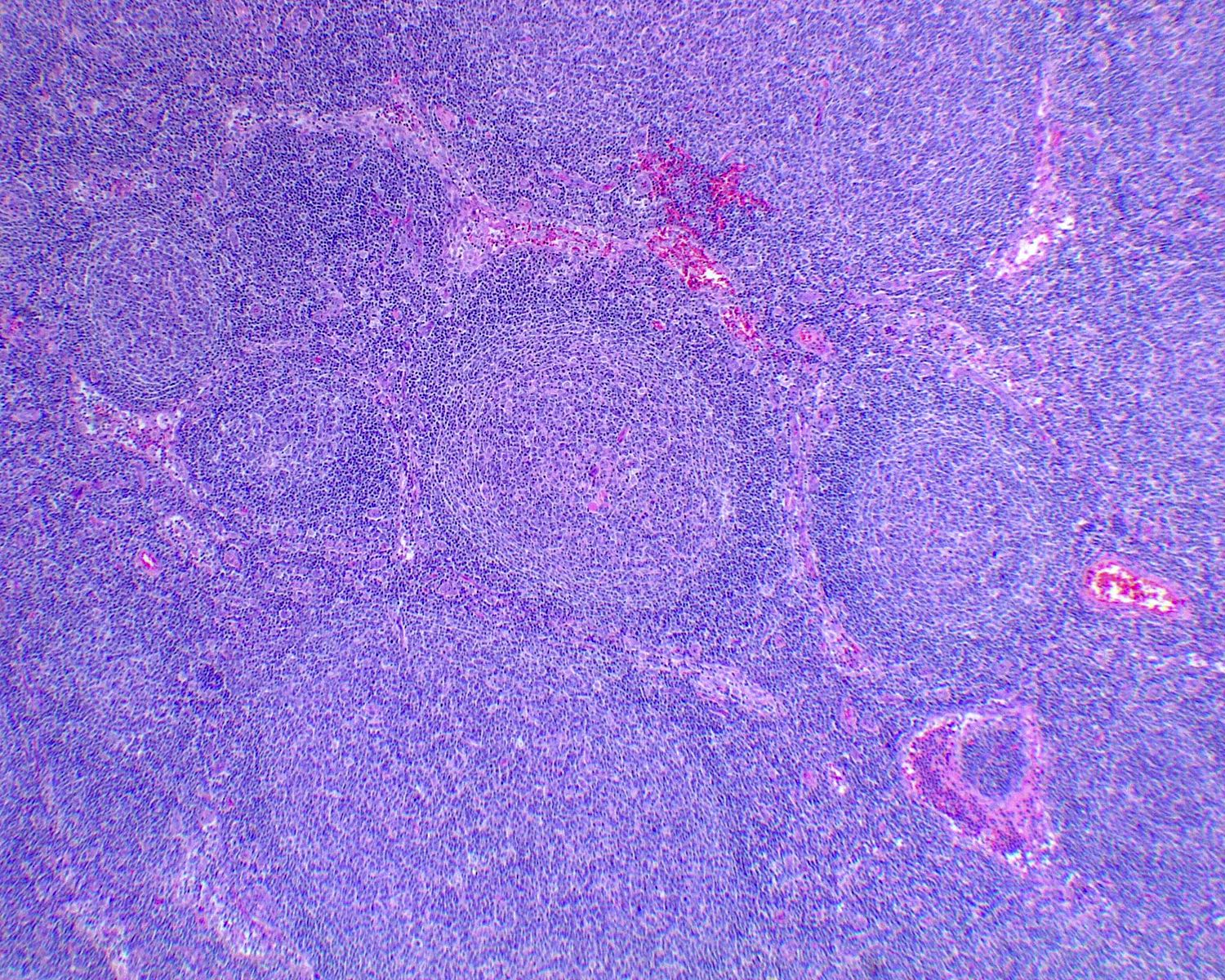
Progressive transformation of germinal centers (PTGC) (Virchows Arch 2019;475:771)
- Single or few large (4 - 5x normal) lymphoid follicles with mantle cells extensively indenting into GCs
- Background lymph node exhibits reactive follicular hyperplasia (RFH)
- Negative for rosetting by PD-1+ cells (TFH) around large B cells
- GCs remnants rarely show tingible body macrophages
- Morphological changes seem to proceed gradually
- Early stages show hyperplastic GCs
- Fusion of GCs within one single follicle (usually 2 - 3x size)
- Inward migration of mantle zone small B cells into the germinal center
- Later stages: dissolution of GCs results in islands or scattered centrocytes and centroblasts with follicular dendritic cells among small mantle zone B cells
- Immunoarchitecture
- Preserved B and T cell compartments of lymph node and prominent follicular pattern
- Both GC and mantle zone cells express pan-B cell antigens
- Mantle cells: BCL2+, IgD+, BCL6- and CD10-
- Disrupted GCs: BCL6+, CD10+, BCL2- and IgD-
- CD21, CD23 or CD35 show disruption of FDCs meshwork
- IgG4+ plasma cells: ~40 - 50% of cases
- Flow cytometry
- Polytypic B cells
- Genetic testing
- Polyclonal IGH rearrangements
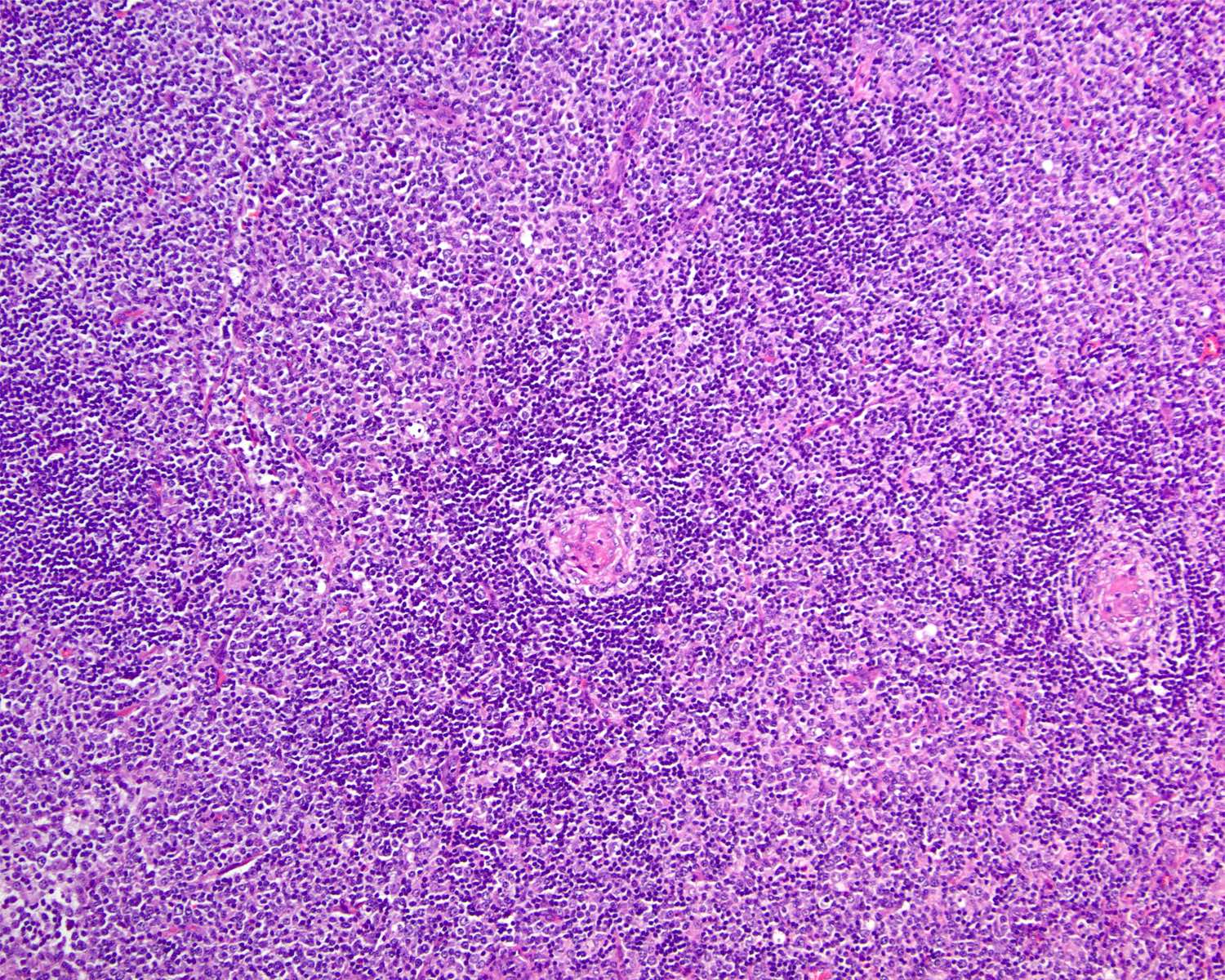
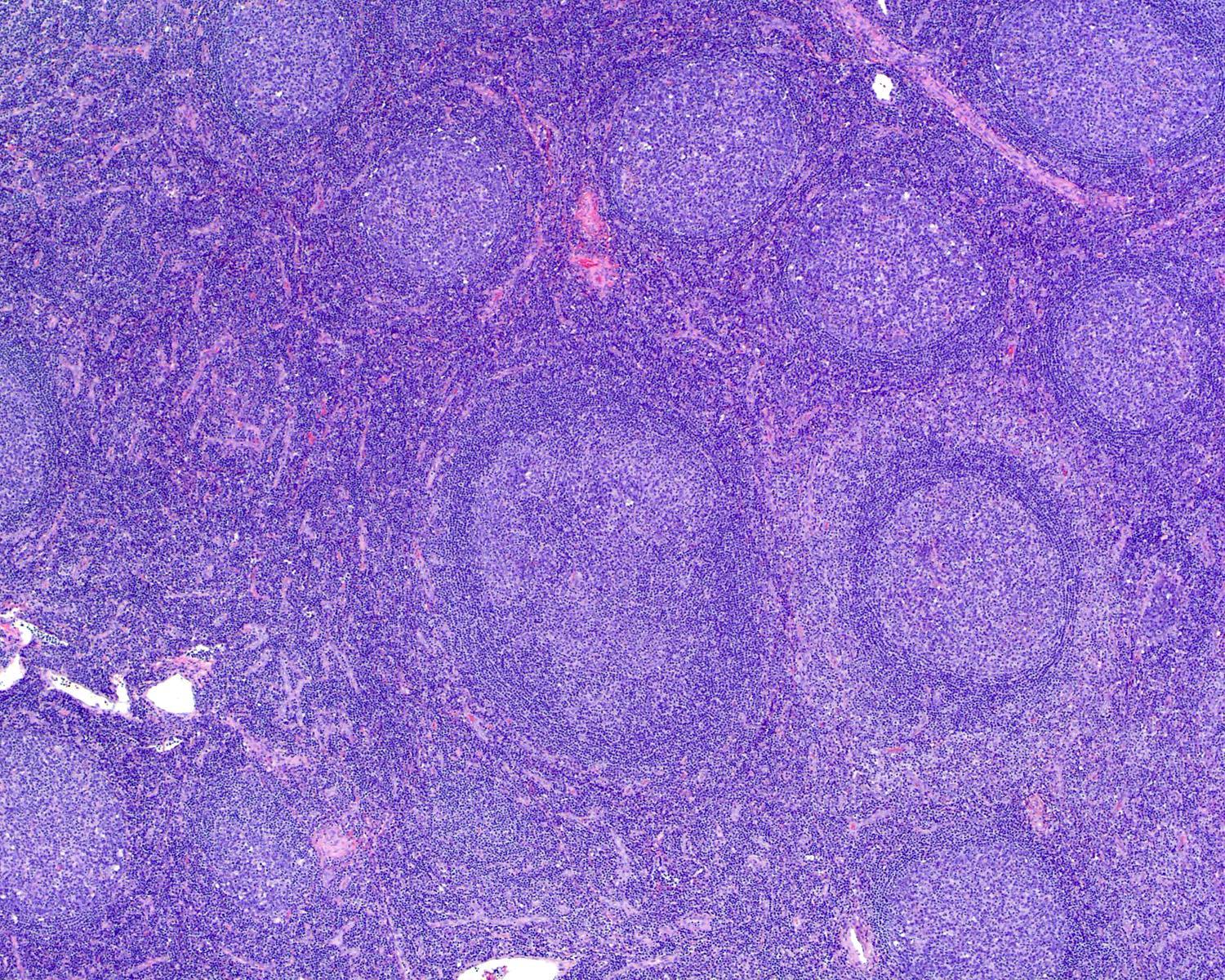
Hyaline vascular Castleman disease (HVCD)
- Young adults (Blood 2017;129:1658)
- Usually < 30 years old
- Can also affect children
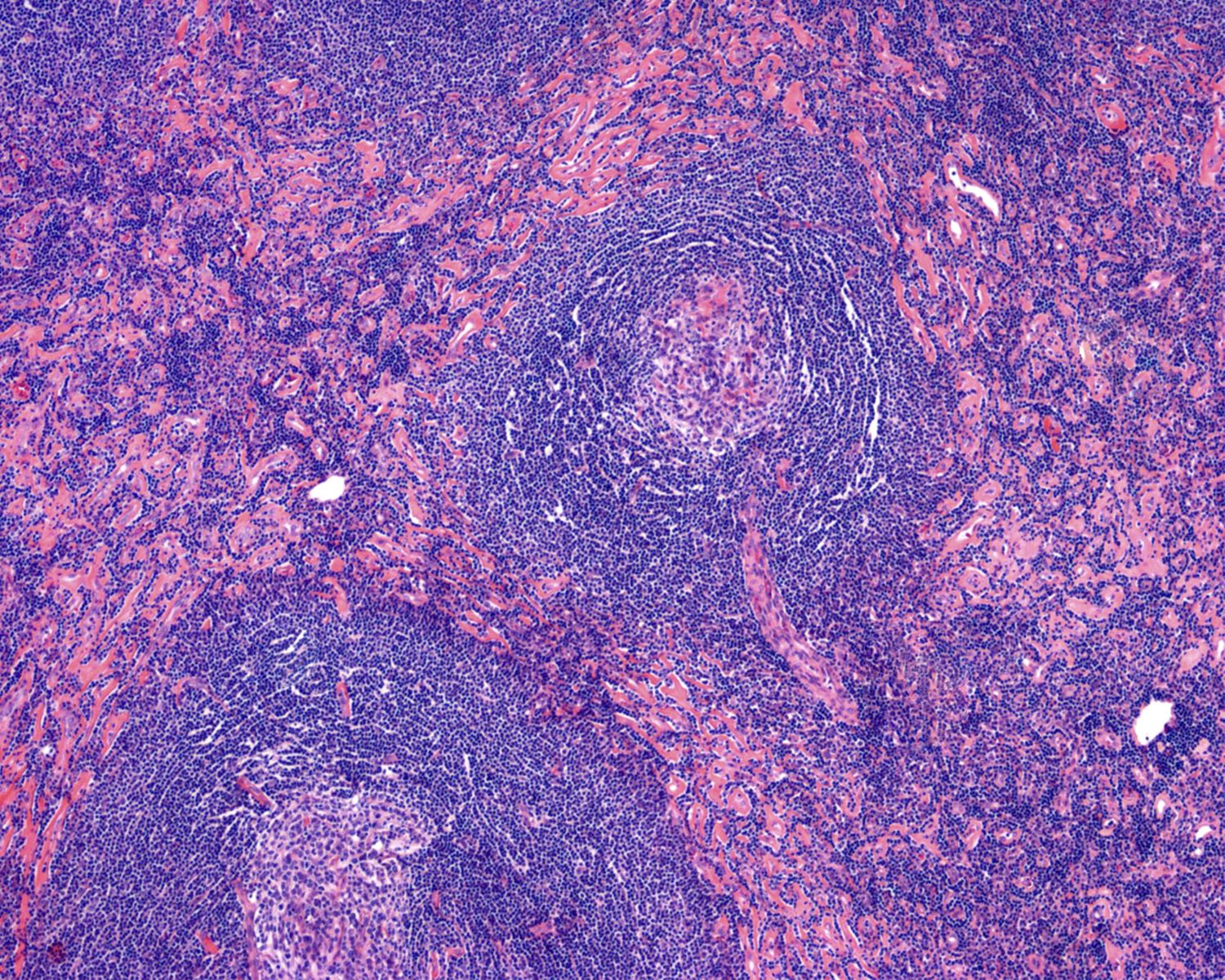
- Numerous follicles in cortex and medulla of lymph node or extramedullary sites
- Obliteration of subcapsular and interfollicular sinuses
- ≥ 2 germinal centers in follicle (also known as twinning)
- Follicles typically large with regressed (or involuted) germinal centers
- Mostly composed of FDCs with few lymphocytes
- Mantle zones prominent
- FDCs often hyperplastic and can show dysplasia
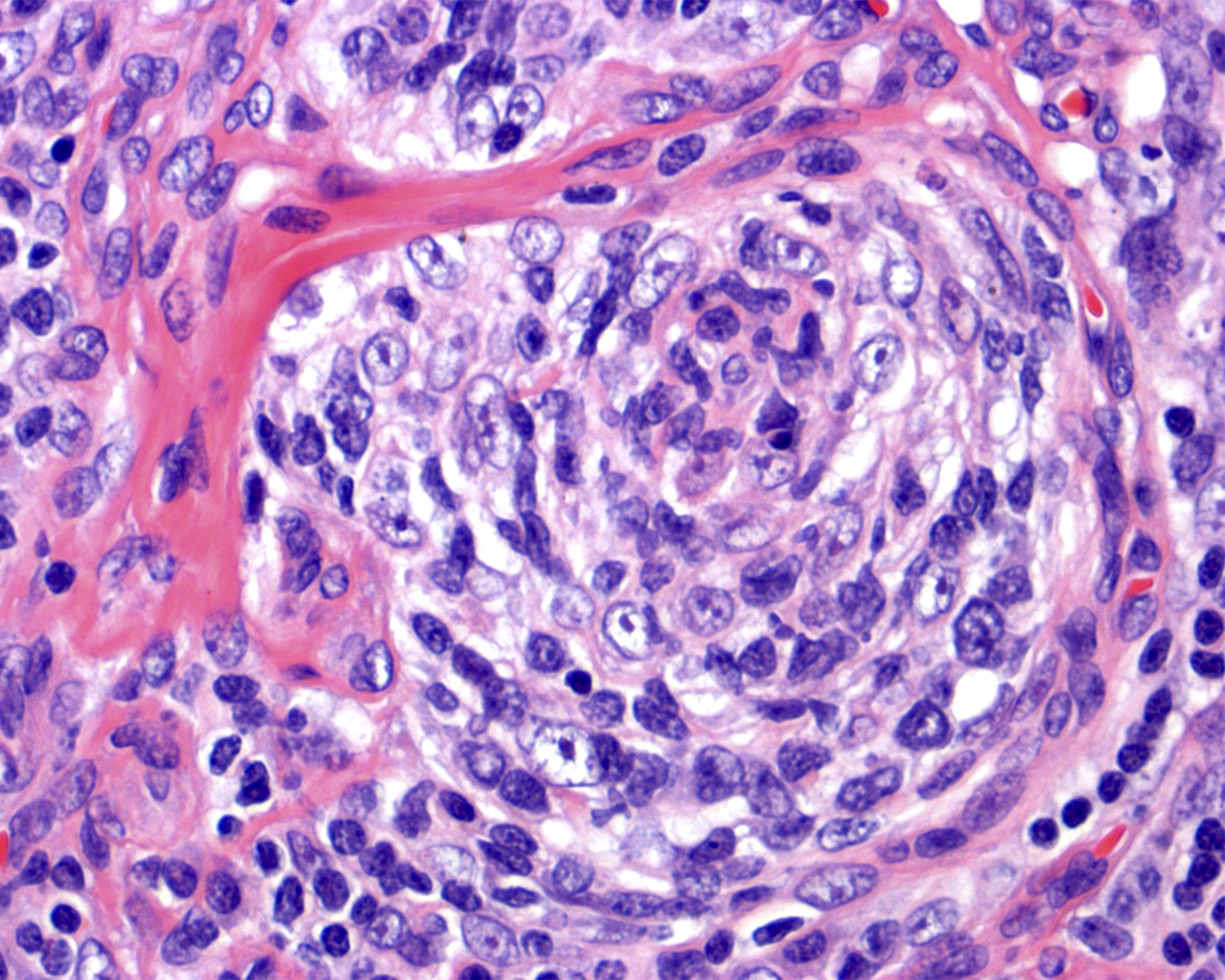
- Many follicles show so called lollipop features
- Concentric rings of mantle zone lymphocytes (onion skin appearance)
- Sclerotic blood vessels radially traversing into germinal center
- Interfollicular or stromal component is also important (J Clin Exp Hematop 2022;62:60)
- Increased number of high endothelial venules with hyalinized walls
- Stromal component can predominate with only a few hyaline vascular follicles
- Clusters of plasmacytoid dendritic cells can occur
- Plasma cells and immunoblasts are not abundant in HVCD
- More common and abundant in plasma cell Castleman disease (PCCD)
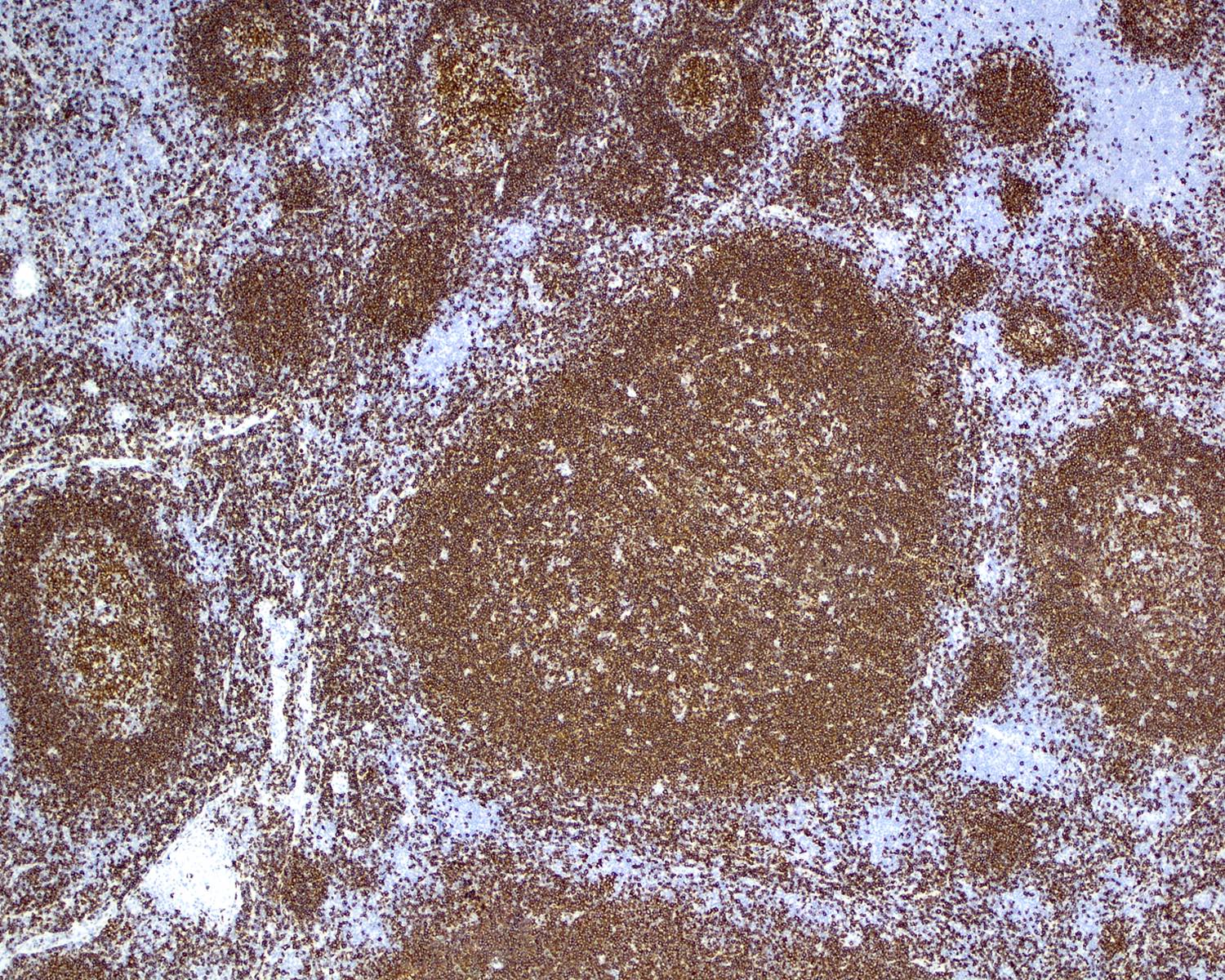
- Immunohistochemistry
- HHV8 is absent
- Polytypic B, T cells and plasma cells
- Increased FDCs in involuted germinal centers
- CD21+, CD23+, CD35+ or EGFR+
- Dysplastic FDCs often stain variably for FDC markers
- Differential diagnosis
- Classic Hodgkin lymphoma concurrent with Castleman disease (Virchows Arch 2020;477:437)
- Diagnosis is established upon identification of Hodgkin Reed-Sternberg (HRS) cells with usual phenotype
- Castleman-like follicles in infectious lymphadenopathies
- Changes are focal and sinuses are open
- Classic Hodgkin lymphoma concurrent with Castleman disease (Virchows Arch 2020;477:437)
| Table 1. Differential diagnosis between florid follicular hyperplasia and B cell lymphomas with follicular formation | ||||
| Feature | Florid follicular hyperplasia | Follicular lymphoma | Marginal zone lymphoma | Mantle cell lymphoma, mantle zone pattern |
| Follicles | Enlarged, with prominent GCs and distinct mantle zones | Variably sized surrounded by faint mantle zones | Nodules with remnants of GCs surrounded by monocytoid cells | Thickened mantle zones |
| Density | Low, widely spaced | Back to back | Variable; may be confluent | Variable |
| Size | Uneven | Uniform | Variable | Uniform |
| Borders of GC | Sharp, well defined | Fainted, crack artifact | Blurry | Sharp, well defined |
| Distribution | Cortical predominance; florid cases involve medulla | Cortex and medulla | Cortex and medulla | Even distribution throughout cortex and medulla |
| Extension to perinodal fat | Absent or unusual | Often present | Often present | Uncommon |
| Mantle zone | Present, well developed | Attenuated to absent | Generally absent | Expanded in mantle zone pattern |
| Marginal zone | Can be hyperplastic | Absent | Expanded, may coalesce | Absent |
| Germinal center cells | Colonized by monocytoid cells | Absent | ||
| Tingible body macrophages | Present and common | Decreased or absent | Decreased or absent | Variable |
| Polarization | Present | Absent | Absent | Variable |
| Cytologic features | Centroblasts, centrocytes and macrophages | Centroblasts and centrocytes in various proportions | Monocytoid and plasmacytoid; scattered large cells | Small and intermediate centrocyte-like cells; few large cells |
| Immunoarchitecture | ||||
| BCL2 | Negative in GCs | Positive in germinal centers of FL grades 1 - 2; 50% in FL grade 3 | Positive in neoplastic cells; GC remnants are negative | Positive in mantle zones |
| BCL6, CD10 | Positive, restricted to GCs | Positive in GCs and interfollicular areas | Negative, GC remnants positive | Positive, restricted to GCs |
| Ki67 | High proliferation rate; polarized in GCs | Low, nonpolarized | Low, nonpolarized | Variable in mantle zones |
| CD21, CD23, CD35 | FDC meshworks preserved | FDC meshworks preserved | Distorted FDC meshworks | FDC meshworks preserved |
| Common positive markers | BCL6, LMO2, OCT2, HGAL | CD10, BCL2, BCL6, LMO2 | CD43, MNDA, CD5-/+ | Cyclin D1, SOX11, CD5 |
| Common negative markers | CD3, BCL2 | CD5, cyclin D1 | CD10, cyclin D1, SOX11 | CD10-, CD23- in mantle zones |
| Flow cytometry | Polytypic | Monotypic surface Ig; CD10 positive | Monotypic surface Ig; CD10 neg; CD5 neg or weak | Monotypic surface Ig; CD5 positive |
| Table 2. Differential diagnosis between progressive transformation of germinal centers and lymphomas with large nodules | |||
| Feature | Progressive transformation of germinal center | Nodular predominant Hodgkin lymphoma (patterns A / B) | Lymphocyte rich classic Hodgkin lymphoma, nodular variant |
| Follicles | |||
| Size | Scattered large nodules | Nodules larger than PTGC | Moderately enlarged |
| Germinal centers | Variably disrupted or involuted | Absent | Present within neoplastic nodules |
| Centrocytes and centroblasts | Decreased; scattered, BCL2- | Absent | In residual GCs |
| Mantle zone cells | Inward growth into GCs, BCL2+ and IgD+ | Absent | Expanded |
| Reactive follicular hyperplasia | Present at background | Absent or focal | Absent or focal |
| Immunoarchitecture | |||
| T follicular helper cells rosettes (PD-1+) | Absent | Present | May be present |
| IgG4+ plasma cells | In 40 - 50% of cases | Absent | Absent |
| Large B cells | Rare immunoblasts | LP cells (popcorn cells) | Present, HRS cells |
| Immunophenotype | IgG+ | CD20+, CD45+, OCT2+, EMA+/-, PAX5+ strong, IgD-/+ | CD30+, CD45-, CD15+, PAX5+ dim, OCT2-, EMA-, CD20- |
Nodal immunoblastic proliferations
Infectious mononucleosis (Semin Diagn Pathol 2018;35:54, Virchows Arch 2019;475:771)
Herpesvirus lymphadenitis (Virchows Arch 2019;475:771)
Cytomegalovirus lymphadenitis (Virchows Arch 2019;475:771)
- Caused by acute Epstein-Barr virus (EBV) infection
- Histologic changes are variable and depend on disease duration
- Early stage: reactive follicles, with mild paracortical expansion
- Progression: follicles sparse due to interfollicular expansion
- Advanced stage: follicles may become effaced, with interfollicular expansion
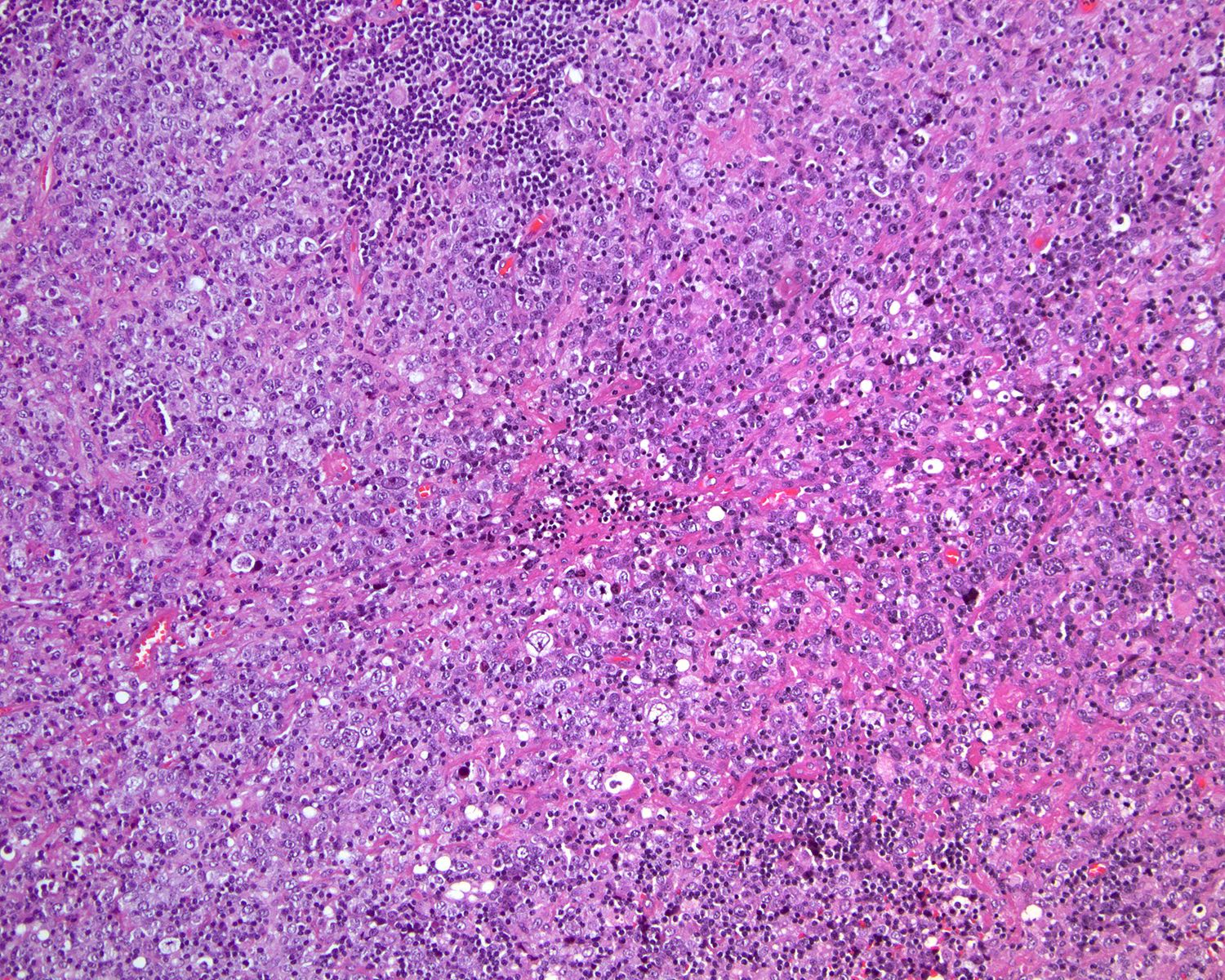
- Interfollicular areas with numerous immunoblasts or mixed infiltrate
- Immunoblasts can be dispersed or abundant
- Lymphocytes range from small to intermediate and large in size
- Plasma cells, plasmacytoid cells and histiocytes are variably present, less frequently accompanied by eosinophils
- Sinuses are regularly occupied by immunoblasts, monocytoid B cells and histiocytes
- Immunoblasts can be binucleated, mimicking HRS
- Intact nodal architecture admixed with B and T immunoblasts are indicative of a reactive process
- Focal necrosis may be present
- Immunohistochemistry
- HRS-like cells are CD45+, CD15-, CD30+ (weak) admixed with B and T immunoblasts
- EBV latency type 3 (Semin Diagn Pathol 2018;35:54)
- EBV encoded RNA (EBER) present in numerous infected cells, ranging from small and intermediate lymphocytes to HRS-like immunoblasts
- EBNA1+, EBNA2 variable and EBNA3+
- LMP1+ and LMP2+
- Most lymphocytes in the background are CD8+, TIA1+
Herpesvirus lymphadenitis (Virchows Arch 2019;475:771)
- Uncommon complication from either primary exposure or reactivation (more common)
- Prominent immunoblastic paracortical hyperplasia
- Similar to infectious mononucleosis
- Sharply circumscribed areas of necrosis and sinus histiocytosis
- Adjacent to areas of necrosis
- Multinucleated cells with ground glass chromatin and intranuclear viral inclusions (halo)
- Herpes simplex virus (HSV) immunohistochemistry confirms the diagnosis
Cytomegalovirus lymphadenitis (Virchows Arch 2019;475:771)
- Morphologic features overlapping with those described in other viral infections
- Monocytoid B cell hyperplasia is frequent
- Characteristic owl eye central intranuclear inclusions with a halo
- Cells infected with cytomegalovirus (CMV) often are T cells or endothelial cells
- Diagnosis can be established utilizing CMV immunohistochemical stain
| Table 3. Differential diagnosis between nodal B cell immunoblastic proliferation and lymphomas | |||
| Feature | Infectious mononucleosis | Classic Hodgkin lymphoma | Diffuse large B cell lymphoma, EBV+ |
| Nodal compartment | Paracortical | Diffuse effacement | Diffuse effacement |
| Neoplastic cells | Not present | Scattered large cells; confluent in nodular sclerosis syncytial variant | Abundant large cells; diffuse pattern |
| Background lymphocytes | |||
| B cells | Small to intermediate | Small | Small |
| T cells | CD8+, small to intermediate | CD4+, small | CD8+, small |
| Monocytoid B cells hyperplasia | Often present | Absent | Absent |
| Plasma cells | Variably present | Few | Not frequent |
| Histiocytes | Variably present | Variable | Variable |
| Eosinophils | Rare | Present, often abundant | May be present |
| Necrosis | Focally present | Often present in nodular sclerosis subtype | May be present |
| Immunoarchitecture | |||
| EBV latency type | Type III | Type II | Type I |
| EBER | Positive in most cells | Positive in HRS cells | Positive in large cells |
| LMP1 | Positive, fewer small and large cells (less sensitive) | Restricted to HRS cells in positive cases | If positive, restricted to large neoplastic cells |
| EBNA2 | Mostly positive | Negative | Negative |
| EBNA3 | Positive | Negative | Negative |
| CD20 | Positive in large cells | Faint reactivity in HRS cells in ~20% of cases | Positive in large cells |
| CD3 | Positive in small lymphocytes | Negative in HRS cells | Negative in large cells |
| CD30 | Positive in HRS-like cells, usually dim | Positive in HRS cells, strong | May be positive in neoplastic cells |
| CD45 | Positive in most cells | Negative in HRS cells | Positive in neoplastic cells |
| CD4, PD-1, ICOS, CXCL13 | Negative in immunoblasts | Negative in HRS cells | Negative in neoplastic cells |
| CD21 | Present only in GCs | Present only in residual GCs | Absent |
| Diagnostic molecular testing | Polyclonal B and T cells | Polyclonal B and T cells | Monoclonal IGH gene rearrangements |
Extranodal follicular proliferations
Florid reactive lymphoid hyperplasia
- Most cases represent reactive tertiary lymphoid tissue and may be associated either with an infectious agent, autoimmune phenomena or chronic repetitive trauma; these processes may lead to extranodal marginal zone lymphoma (Pathology 2020;52:15)
- Stomach: Helicobacter pylori
- Skin: Borrelia burgdorferi
- Conjunctiva: Chlamydia psittaci
- Cervix: Chlamydia trachomatis
- Small intestine: Campylobacter jejuni
- Lung: Achromobacter xylosoxidans
- Gallbladder: extrahepatic biliary obstruction
- Thyroid: Hashimoto thyroiditis
- Salivary gland: Sjögren syndrome
- Differential diagnosis with marginal zone lymphoma can be challenging in some cases
- Cutaneous reactive lymphoid hyperplasia must be also distinguished from primary cutaneous follicular center lymphoma (see table) (Arch Pathol Lab Med 2018;142:1313)
- Florid reactive lymphoid hyperplasia of the female reproductive tract
- Cervix is the most frequent affected site, followed by endometrium and vulva (Int J Gynecol Pathol 2022;41:459)
- Most cases show lymphoid infiltrate with superficial distribution, with or without ulceration
- Architecture varies from patchy and nodular to diffuse, with secondary follicles in most cases
- Tends to imitate lymph nodes organization, with a B follicular and T interfollicular compartments
- Cytologically composed by small lymphocytes and polytypic plasma cells, with variable number of granulocytes and histiocytes in the background
- Scattered large CD30+ immunoblasts are present in the majority of cases
- Clonal IGH rearrangement is not uncommon, although patients are free of lymphoma on follow up (Arch Pathol Lab Med 2018;142:1313)
| Table 4. Differential diagnosis between cutaneous follicular hyperplasia and B cell lymphomas | |||
| Feature | Reactive follicular hyperplasia | Primary cutaneous follicular center lymphoma | Primary cutaneous marginal zone lymphoma |
| Infiltrate | Denser in superficial dermis, wedge shaped | Denser in deep dermis, Grenz zone | Vertical orientation around follicles |
| Follicular architecture | Present, uneven shapes and size | Follicular or diffuse pattern | Pale nodules, some residual germinal centers |
| Mantle zone | Present, well developed | Attenuated to absent | Absent |
| Marginal zone | Generally absent | Absent | Expanded, may coalesce |
| Germinal center cells | Colonized by monocytoid cells | ||
| Tingible body macrophages | Present | Absent | Present in GC remnants |
| Polarization | Present | Absent | Absent |
| Cytologic features | Centrocytes and centroblasts | Centroblasts and centrocytes | Monocytoid cells, few large cells; plasma cells |
| Immunoarchitecture | B and T compartments preserved | Effaced, B cell predominant | Effaced, B cell predominant |
| BCL2 | Negative in germinal centers | Negative in germinal centers | Positive in neoplastic cells; GC remnants are negative |
| BCL6 | Positive, restricted to GCs | Positive in both GCs and interfollicular areas | Negative, GC remnants positive |
| Ki67 | High and polarized in GCs | Low, nonpolarized | Low, nonpolarized |
| CD21, CD23, CD35 | FDC meshworks preserved | FDC meshworks preserved | Distorted FDC meshworks |
| Light chain restriction | Absent | May be present | Present in plasmacytoid lymphocytes or mature plasma cells |
| Positive IgH / IgK clonality assays | No | Yes | Yes |
Extranodal immunoblastic proliferations
EBV positive mucocutaneous ulcer (Surg Pathol Clin 2023;16:213)
- Lymphoproliferative disorder characterized by EBV+ large B cells / Reed-Sternberg-like cells (RS-like cells) cells
- Oral mucosa is most common site, followed by skin
- Multiple small lesions within same anatomic region can occur
- No systemic symptoms, lymphadenopathy, hepatosplenomegaly or bone marrow involvement
- Well circumscribed superficial ulcer with a subjacent polymorphous infiltrate
- Deep base shows a sharp demarcation by a dense rim of small lymphocytes
- Angioinvasion and high proliferation index may be present
- Monoclonal IGH or TCR gene rearrangements can occur in up to a third of cases
- Immunohistochemistry
- HRS-like cells: CD45+, CD15- and CD30+ (usually weak)
- Background: B and T cells
- EBV latency type 3 (Semin Diagn Pathol 2018;35:54)
- EBV encoded RNA (EBER) present in numerous infected cells, ranging from small and intermediate lymphocytes to HRS-like immunoblasts
- LMP1+, EBNA2+
- Basal rim of T cells are CD8+
Case reports
- 24 year old man with follicular lymphoid hyperplasia with aggressive behavior in maxilla (Oral Maxillofac Surg 2017;21:475)
- 40 year old man with infectious mononucleosis that presented as a lymphadenopathy with geographic necrosis (Pathol Res Pract 2004;200:53)
- 57 year old woman with idiopathic multicentric Castleman disease (TAFRO subtype) (Br J Haematol 2022;196:461)
- 70 year old woman with atypical follicular hyperplasia after COVID-19 booster (Virchows Arch 2023;482:905)
Microscopic (histologic) images
Contributed by Roberto N. Miranda, M.D. and Roman Segura-Rivera, M.D.
Board review style question #1
Which of the following statements is true about progressive transformation of germinal center (PTGC)?
- Centrocytes are increased and have a BCL2+ phenotype
- IgG4+ plasma cells are usually absent
- Reactive follicular hyperplasia is usually absent in the background
- Rosettes of T follicular helper (TFH) cells are usually absent
Board review style answer #1
D. Rosettes of TFH cells are usually absent. Answer B is incorrect because PTGC cases usually present with IgG4+ plasma cells in up to 50% of cases. Answers A and C are incorrect because there is a decrease of centrocytes and reactive follicular hyperplasia in the background.
Comment Here
Reference: Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Comment Here
Reference: Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Board review style question #2
Board review style answer #2
D. Type III: EBER+, LMP1+, EBNA2+. Answers A, B and C are incorrect because the most common latency pattern in infectious mononucleosis is the pattern III, which is characterized by the positivity of EBER, LMP1 and EBNA2.
Comment Here
Reference: Reactive B cell rich lymphoid proliferations that can mimic lymphoma
Comment Here
Reference: Reactive B cell rich lymphoid proliferations that can mimic lymphoma