Table of Contents
Definition / general | Essential features | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Practice question #1 | Practice answer #1 | Practice question #2 | Practice answer #2Cite this page: Alhalaseh Y, Mirza KM. Early T cell precursor lymphoblastic leukemia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomatcelllymphoblasticleukemia.html. Accessed August 27th, 2025.
Definition / general
- Aggressive T cell neoplasm with immunophenotypic markers of early T cell differentiation, including stem cell markers or myeloid differentiation markers
Essential features
- Histologic morphology of lymphoblastic leukemia / lymphoma
- Early T cell precursor (ETP) blasts have the following immunophenotype: positive for cytoplasmic CD3, absent (< 5% positive blasts) CD1a and CD8 expression, absent or dim (< 75% positive blasts) CD5 expression, positive (≥ 25% positive blasts) for ≥ 1 myeloid (CD11b, CD13, CD33, CD65, CD117) or stem cell (CD34, HLA-DR) marker and negative (< 3%) myeloperoxidase (Lancet Oncol 2009;10:147)
Epidemiology
- 12 - 17% of T cell acute lymphoblastic leukemia (TALL) (Lancet Oncol 2009;10:147, Blood 2016;127:1863)
- M:F = 4:1
- Incidence of 3 cases per million person years; does not vary by ethnicity
Sites
- Can involve the bone marrow, thymus, lymph nodes or extranodal sites
Pathophysiology
- Physiologically, early T cell precursors characteristically migrate from bone marrow to thymus
- They express lymphoid, myeloid and stem cell markers and are characterized by multilineage differentiation potential (Nature 2008;452:764, Nature 2008;452:768)
- Single cell RNA sequencing in ETP ALL demonstrates leukemic cell plasticity, with both stem cell and T cell signatures and can differentiate to stimulate an immunosuppressive microenvironment through expression of the LGALS9 checkpoint molecule and support of dysfunctional CD8 positive T cells (Blood 2021;137:2463)
- ETP ALL shows increased expression of MEF2C and decreased expression of BCL11B and GATA3 (Cancer Cell 2011;19:484, Blood 2021;138:773, Br J Haematol 2021;194:1034, J Exp Med 2009;206:2987, PLoS One 2013;8:e53190)
- Concurrent EZH2 and RUNX1 inactivating mutations in a recent murine molecular pathogenesis model were hypothesized to be the initiating events that lead to expansion of early thymic progenitors with an ETP ALL gene expression signature
- Leukemic potential is further stimulated by acquisition of mutations (e.g., FLT3), resulting in constitutive activation of the RAS signaling pathway (Cancer Cell 2018;33:274)
- Pathogenesis in this model is similar to that of myeloid neoplasms, by harboring activating mutations in genes regulating cytokine receptor and RAS signaling (FLT3, NRAS, KRAS, IL7R, JAK3, JAK1, SH2B3 and BRAF), hematopoietic development (GATA3, ETV6, RUNX1, IKZF1) and histone modifications (EZH2, EED, SUZ12, SETD2, EP300) (Nature 2012;481:157, Blood 2013;121:4749, PLoS One 2013;8:e53190)
- NOTCH1 mutations are uncommon in ETP ALL, in contrast to other T lymphoblastic lymphoma / leukemia (T ALL) (PLoS One 2013;8:e53190)
- Frequency of CDKN2A / CDKN2B deletion is low in patients with ETP ALL (Haematologica 2020;105:e294, Am J Hematol 2021;96:312, Blood Adv 2021;5:2890)
- Nonrearranged TRG gene rearrangement is present in most ETP ALL patients; they may lack more frequent biallelic deletion of the TRG locus than non-ETP ALL, confirming early maturation stage (PLoS One 2013;8:e53190, Haematologica 2014;99:94)
Etiology
- Unknown etiology in most cases
- A study has demonstrated an association between germline RUNX1 variants and ETP ALL; it has been observed that mice with RUNX1 variants and mutated JAK3 induce ETP ALL (J Clin Invest 2021;131:e147898)
Clinical features
- Cervical, supraclavicular and axillary lymphadenopathy (50%)
- Mediastinal mass (50 - 75%) with associated compressive superior vena cava syndrome, tracheal obstruction and pericardial effusion (Blood Cancer J 2012;2:e55)
- CNS involvement (18%) (Blood Cancer J 2012;2:e55)
- Features related to extramedullary hematopoiesis (such as hepatosplenomegaly)
Diagnosis
- Morphologic features of lymphoblastic leukemia / lymphoma (Lancet Oncol 2009;10:147)
- Blasts with expression of ETP immunophenotype: positive for cytoplasmic CD3, absent (< 5% positive blasts) CD1a and CD8 expression, absent or dim (< 75% positive blasts) CD5 expression, positive (≥ 25% positive blasts) for ≥ 1 myeloid (CD11b, CD13, CD33, CD65, CD117) or stem cell (CD34, HLA-DR) marker and negative (< 3%) myeloperoxidase (Lancet Oncol 2009;10:147, Blood 2016;127:1863)
Laboratory
- White blood count (WBC) may or may not be elevated and in some studies, ETP ALL patients tend to have lower WBC in comparison to those with non-ETP ALL (Pediatr Blood Cancer 2021;68:e28719)
- Anemia and thrombocytopenia are secondary to bone marrow involvement
Prognostic factors
- Several studies showed that long term outcomes for pediatric and adult ETP ALL patients were similar to those of other T ALL patients (Br J Haematol 2014;166:421)
- Favorable outcomes were reported despite higher rates of minimal residual disease (MRD) positive disease postinduction among ETP ALL patients; this can be because of treatment intensification for MRD positive ETP ALL patients and the increased rate of allogeneic stem cell transplantation after first remission (Pediatr Blood Cancer 2021;68:e28719, Blood Cancer Discov 2021;2:326)
- Because of BCL2 overexpression in ETP ALL, clinical trials are studying BCL2 inhibition as a potential treatment (Haematologica 2020;105:e294, Cancer Discov 2014;4:1074, Cancer Discov 2021;11:1440)
Case reports
- 42 year old woman presented with mild thrombocytopenia and generalized lymphadenopathy in the absence of constitutional symptoms (Hemasphere 2020;4:e379)
- 43 year old woman presented with fatigue, pancytopenia and increased blasts in the peripheral blood smear (Onco Targets Ther 2021;14:3795)
- 117 newly diagnosed children with T ALL with varying disease free survival rates based on treatment regimen (J Cancer Res Clin Oncol 2021;147:2775)
Treatment
- Myeloid oriented chemotherapy (as frontline induction treatment) along with gene targeting inhibitors, followed by allogeneic hematopoietic stem cell transplant
- Early T cell precursor acute lymphoblastic leukemia: diagnosis, updates in molecular pathogenesis, management and novel therapies (Front Oncol 2021;11:750789, Onco Targets Ther 2021;14:3795)
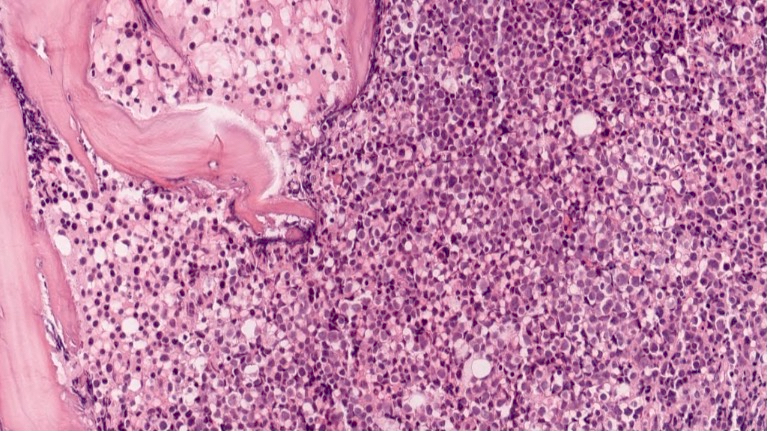
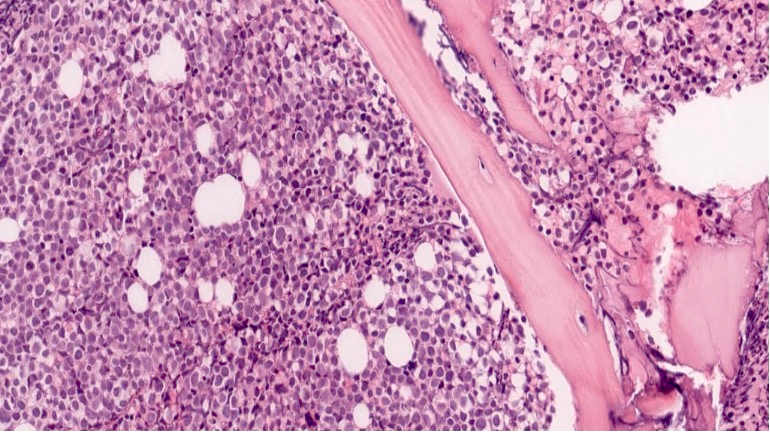
Microscopic (histologic) description
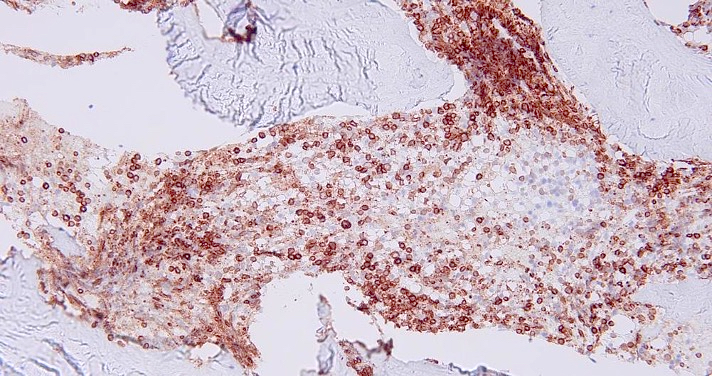
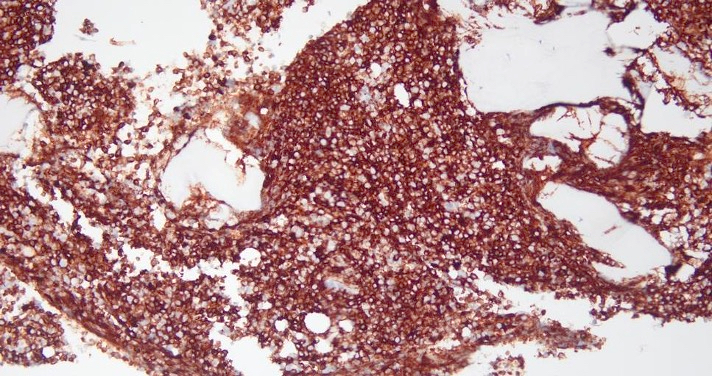
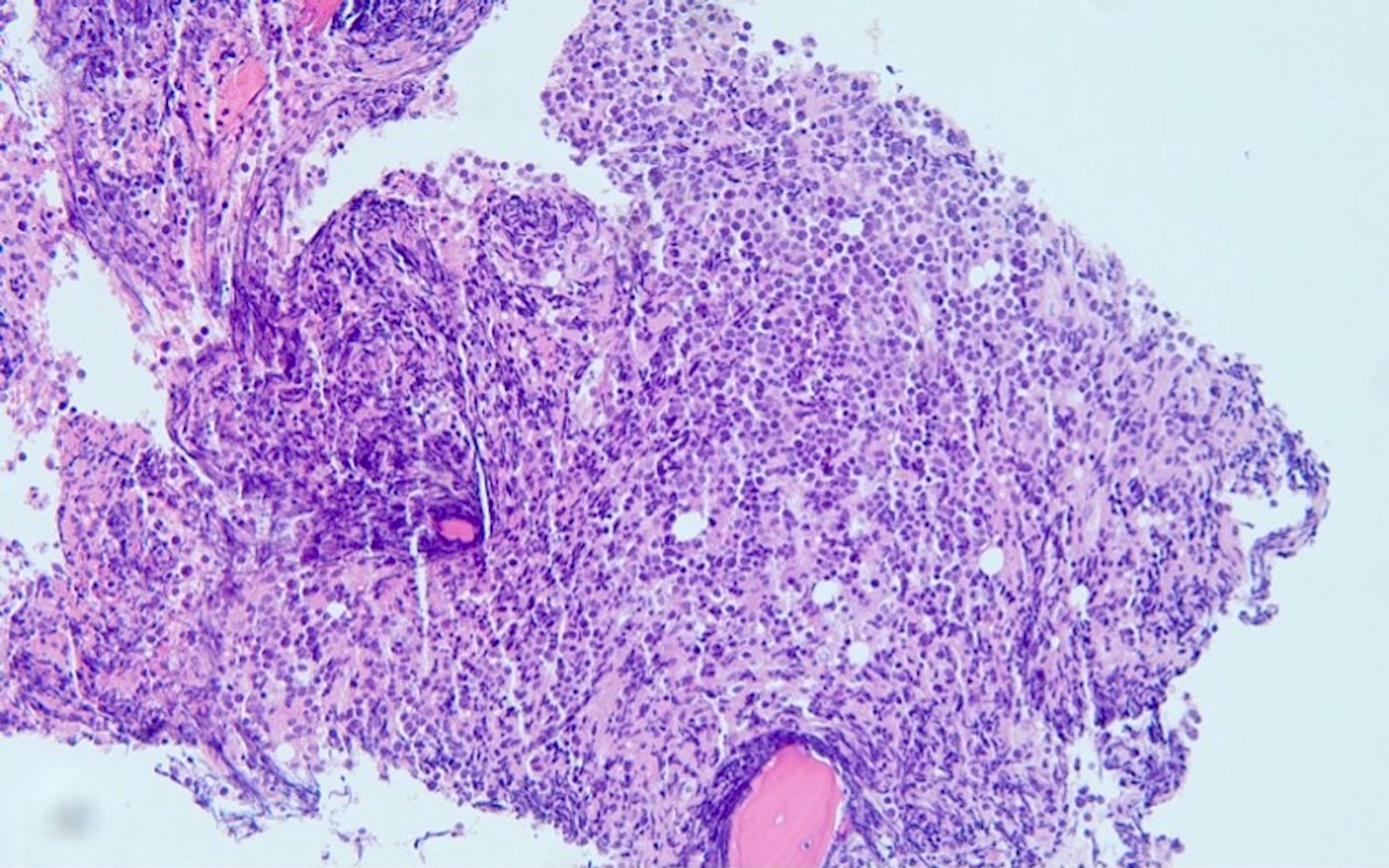
- As seen in patients with other ALL, blasts from patients with ETP ALL are small to medium sized with scant cytoplasm and inconspicuous nucleoli
Microscopic (histologic) images
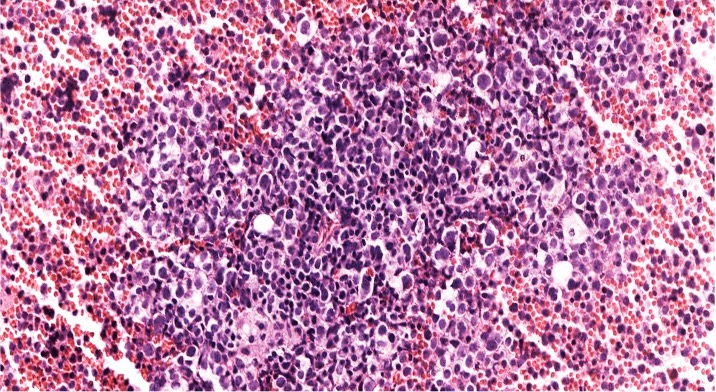
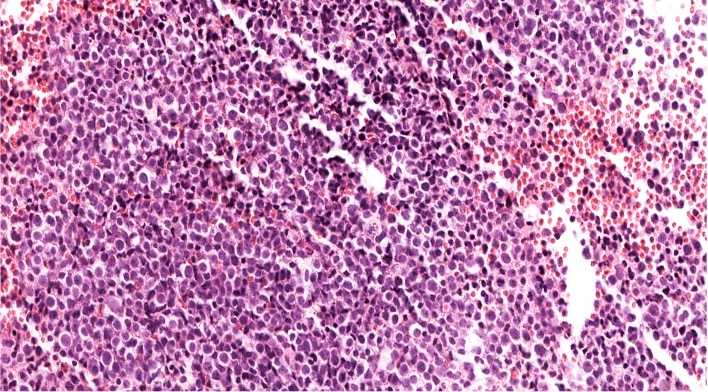
Peripheral smear description
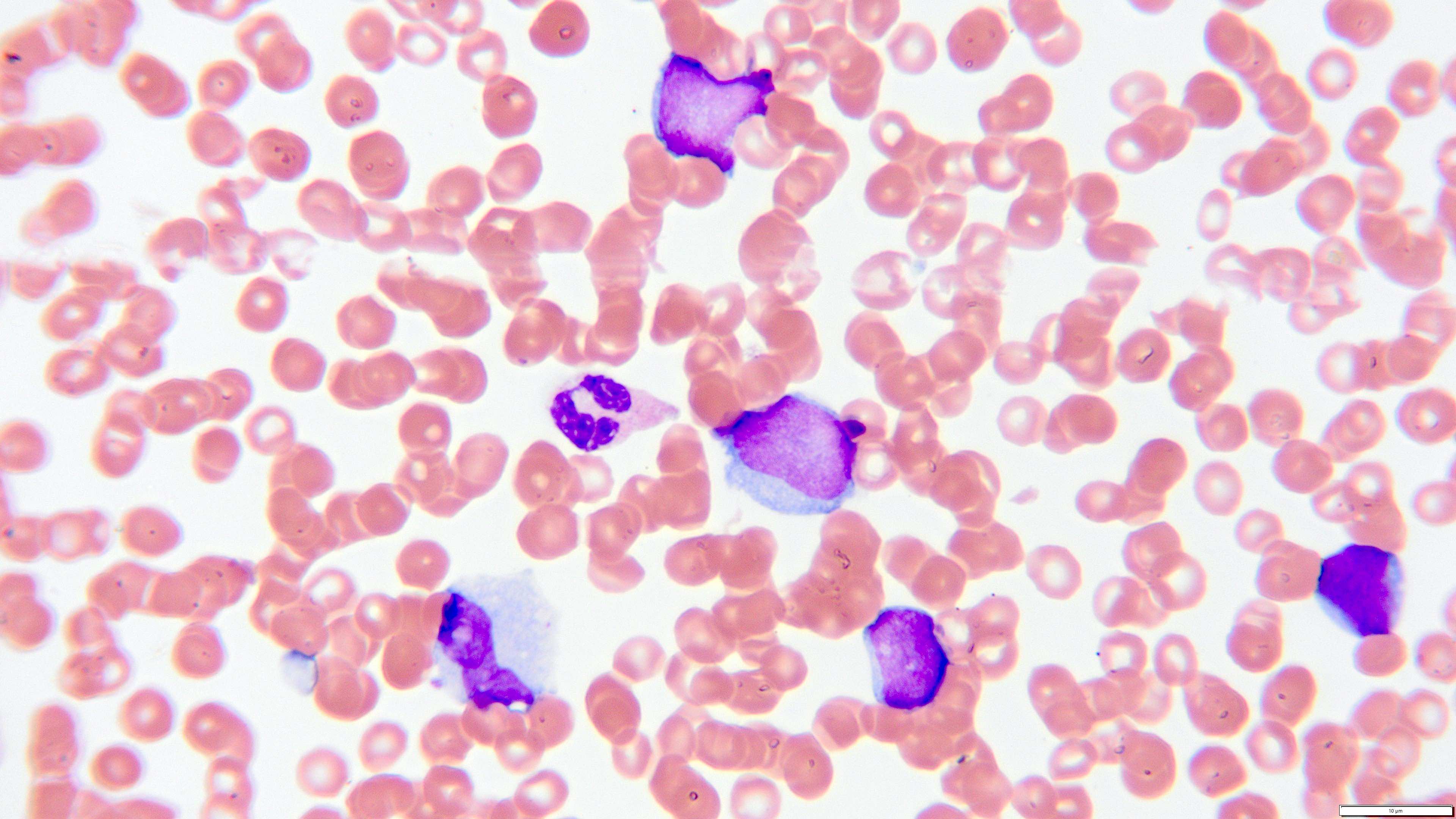
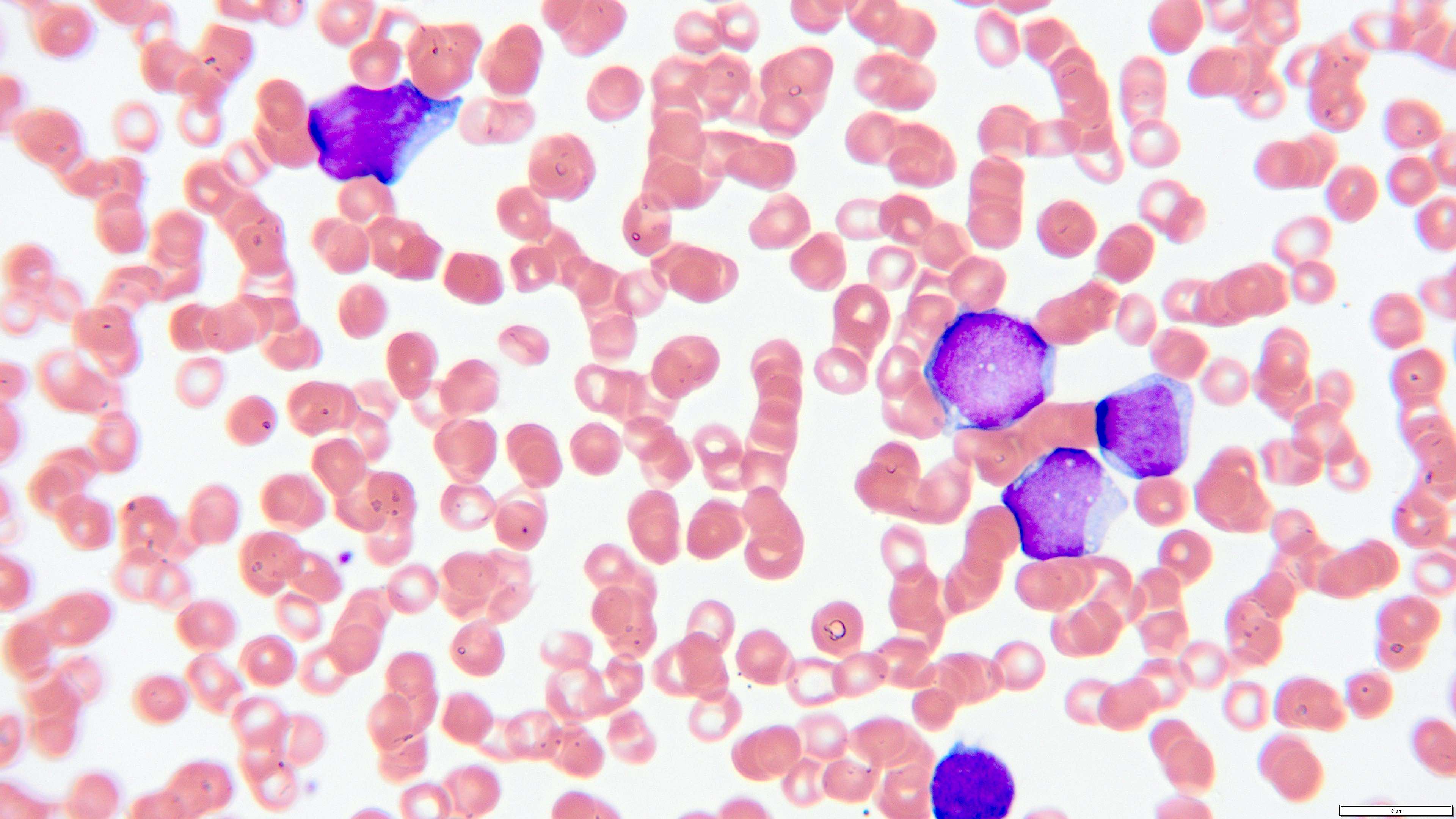
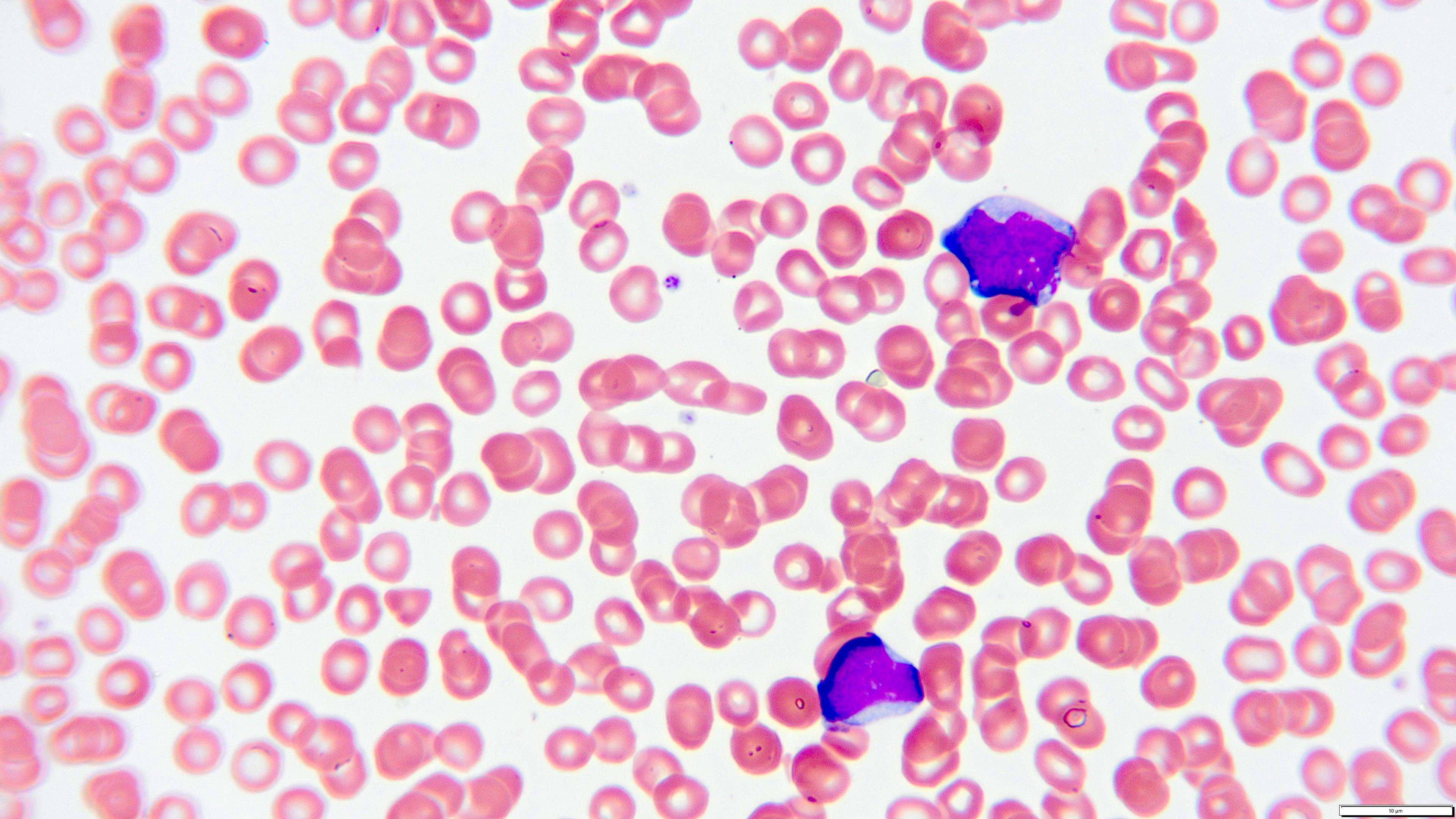
- Peripheral blood smear reveals increase in circulating blasts with high N:C ratio
Peripheral smear images
Positive stains
- Positive for cytoplasmic CD3, absent (< 5% positive blasts) CD1a and CD8 expression (Lancet Oncol 2009;10:147, Blood 2016;127:1863)
- Positive (≥ 25% positive blasts) for ≥ 1 myeloid (CD11b, CD13, CD33, CD65, CD117) or stem cell (CD34, HLA-DR) markers (Lancet Oncol 2009;10:147, Blood 2016;127:1863)
Negative stains
- Absent or dim (< 75% positive blasts), CD5 expression (Lancet Oncol 2009;10:147, Blood 2016;127:1863)
- Negative (< 3%) myeloperoxidase (MPO) (Lancet Oncol 2009;10:147, Blood 2016;127:1863)
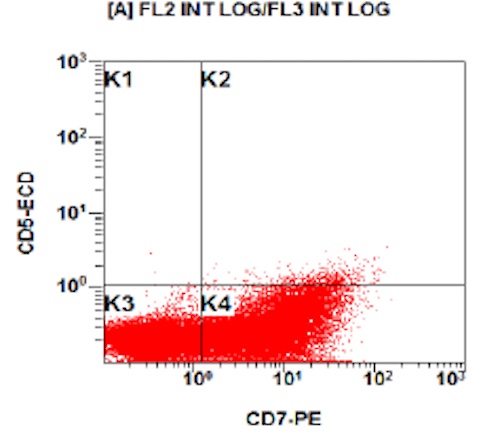
Flow cytometry description
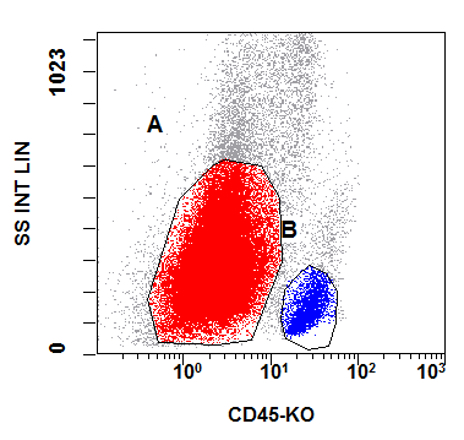
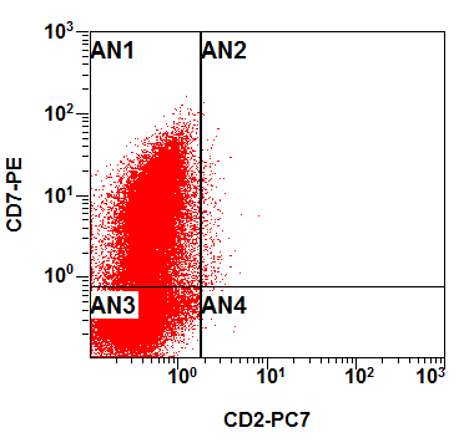
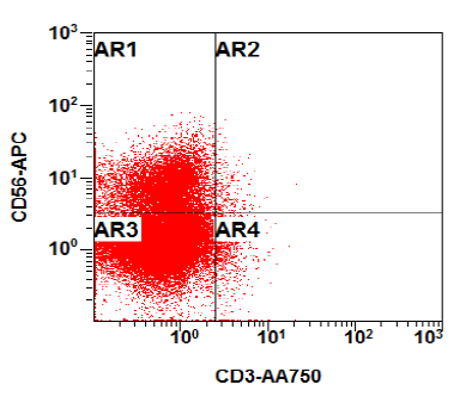
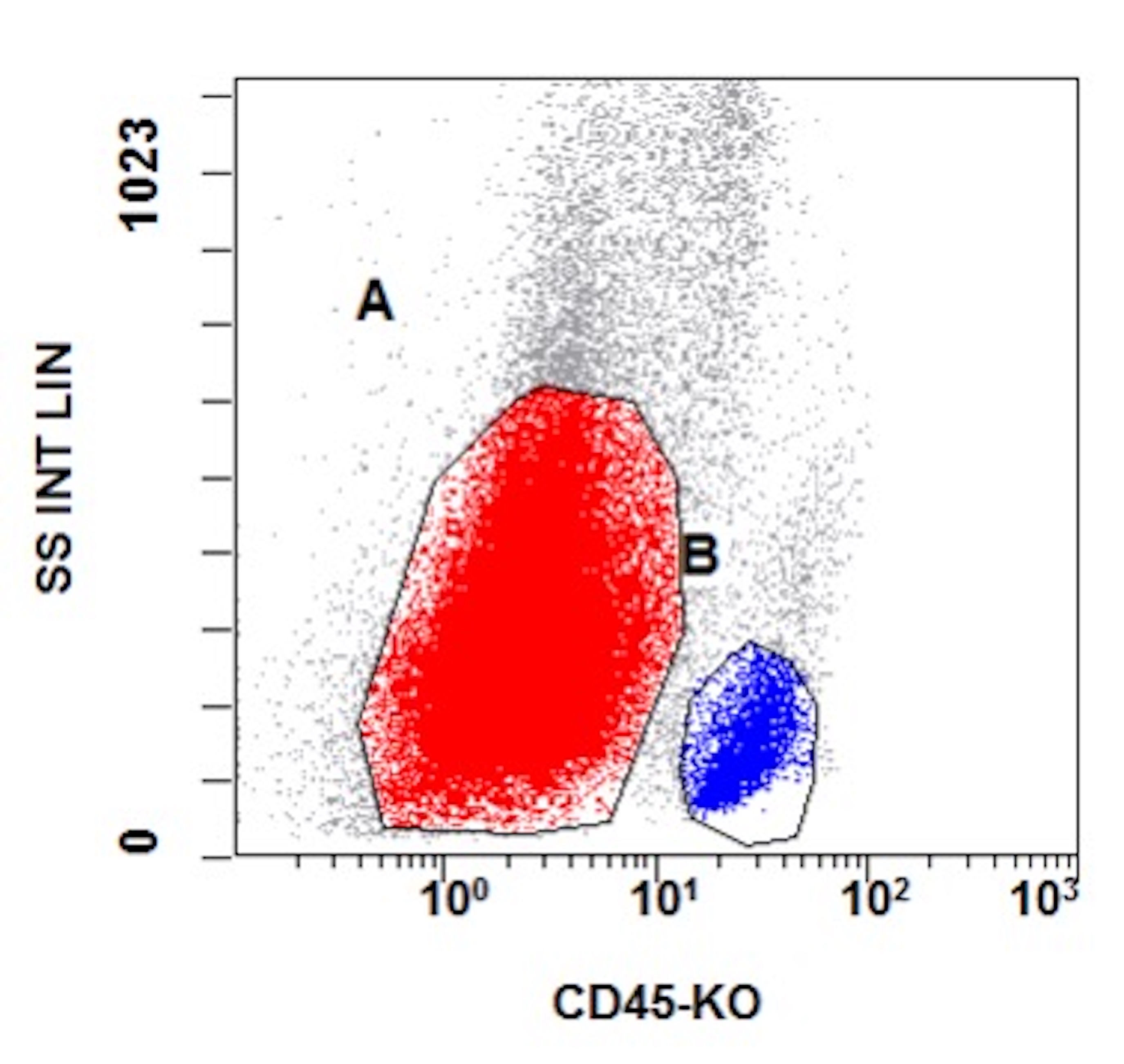
- Flow cytometry phenotyping of the blasts (dim CD45 gate) feature (Lancet Oncol 2009;10:147)
Flow cytometry images
Molecular / cytogenetics description
- Not relevant for diagnostic evaluation
Videos
ETP ALL case
Sample pathology report
- Right posterior iliac crest, core biopsy, clot section, aspirate smears and touch imprints:
- T lymphoblastic leukemia / lymphoma (T ALL phenotypically compatible with ETP ALL) (see comment)
- Hypercellular marrow with 87% blasts and markedly diminished trilineage hematopoiesis
- Correlation with cytogenetics is recommended for complete assessment
- Eosinophils (2%), lymphocytes (2%), plasma cells (1%) (500 cell count on aspirate smear)
- Comment: The peripheral blood film reveals 76% circulating blasts with high N:C ratio. The most recent hemogram shows leukocytosis, anemia and thrombocytopenia.
- The bone marrow core is fragmented and reveals sheets of blasts replacing normal bone marrow elements. The clot shows similar findings. Immunohistochemical stains (CD34, TdT, CD3) on the core and clot reveal blast positivity for CD34 and CD3. Stains for MPO and TdT are negative. Cytochemical analysis for MPO and alpha naphthyl acetate esterase (ANAE) stains are performed. The blasts are negative for MPO and ANAE by cytochemical as well.
- The marrow aspirate smears reveal markedly diminished normal trilineage hematopoiesis and blasts for 87% of a 500 cell differential count. The blasts have high N:C ratio, smudged chromatin with irregular nuclear membranes and occasional cytoplasmic vacuoles. Flow cytometry phenotyping of the blasts (dim CD45 gate) reveals positivity for cytoplasmic CD3, CD34, dim CD4, CD56, CD38, CD33, CD13 and CD117. The blasts are negative for MPO, surface CD3, CD5, CD8, CD22, CD10, CD20, HLA-DR, CD11c, CD64, CD14, TdT and cytoplasmic CD22. Gating on the mature lymphocytes reveals no monotypic B cell population or T cell abnormality based on markers assayed.
- Overall, the presence of cytoplasmic CD3 expression by flow cytometry and CD3 positivity by immunohistochemistry with concurrent lack of MPO (flow cytometry, immunohistochemistry and cytochemistry) monocytic markers and B cell antigens is diagnostic of T lymphoblastic leukemia / lymphoma. Please correlate with corresponding cytogenetics and FISH findings for complete assessment. The phenotype (CD3, CD4, CD7, CD33, CD13 positive, with negativity for MPO, CD8 and CD5) is compatible with ETP ALL (Nature 2012;481:157).
Differential diagnosis
- Mixed phenotype acute leukemia (MPAL), T / myeloid:
- MPAL blasts express both T and myeloid lineage markers
- Cytoplasmic CD3, CD7 are expressed in the majority of cases
- CD2, CD4, CD5 and CD8 may also be present (Curr Oncol Rep 2021;23:22, Hum Pathol 2021;114:66)
- Surface CD3 expression is uncommon but may occur in an isolated population of T lineage blasts (Curr Oncol Rep 2021;23:22)
- Myeloid lineage markers: myeloperoxidase, CD13, CD15, CD33, CD65 and CD117
- Some cases meet the criteria for monocytic differentiation and express monocytic markers such as CD11c, CD14, CD36 and CD64 (Blood 2011;117:3163, Cytometry B Clin Cytom 2019;96:183, Curr Oncol Rep 2021;23:22)
- Bilineage (2 populations) or biphenotypic (single population) lineage expression patterns are identified in MPAL, including MPAL T / M
- A subset of early T precursor lymphoblastic leukemia / lymphoma (ETP ALL) may have immunophenotypic profiles that overlap with MPAL T / M
- In the initial definition of ETP ALL, a threshold of < 3% to define negative myeloperoxidase expression was adopted and may be a helpful distinctive feature (Lancet Oncol 2009;10:147)
- MPAL blasts express both T and myeloid lineage markers
Practice question #1
A 57 year old man presents to his primary care physician with a 2 month history of persistent fatigue. Vital signs are within normal limits. Physical examination is remarkable for pallor and hepatosplenomegaly. Laboratory workup reveals white blood cell (WBC) count of 17,000, hemoglobin of 9.0 and platelet count of 78,000. A peripheral blood smear shows 30% circulating blasts. A bone marrow core biopsy shows hypercellular bone marrow with 80% blasts. Immunohistochemical stains on the bone marrow core biopsy highlight the blast population that are positive for CD34 and CD3. Flow cytometry shows blasts that are positive for cytoplasmic CD3, CD34, dim CD4, CD56, CD33, CD13 and CD117. Genetic testing for BCR::ABL1 by cytogenetics was negative. Which of the following is the most likely diagnosis?
- Chronic myeloid leukemia
- Early T cell precursor lymphoblastic leukemia
- Myelodysplastic neoplasm
- Natural killer cell (NK) large granular lymphocytic leukemia
Practice answer #1
B. Early T cell precursor lymphoblastic leukemia. The patient presents with symptoms with concern for hematologic malignancy. Workup is notable for leukocytosis. The bone marrow biopsy shows hypercellular bone marrow with increased blasts. The immunohistochemical features are specific for early T cell precursor (ETP) given the CD3, CD56 and CD34 expression but absent CD1a, CD8 and myeloperoxidase.
Answer C (myelodysplastic neoplasm) is incorrect because the patient's blasts are significantly increased in the peripheral blood and bone marrow biopsy. Answer A (chronic myeloid leukemia) is incorrect because it can have increased blasts in the blast phase; however, the absence of BCR::ABL1 translocation rules out this diagnosis. Answer D (natural killer cell [NK] large granular lymphocytic leukemia) is incorrect because it is not characterized by increased CD34+ blasts.
Comment Here
Reference: Early T cell precursor lymphoblastic leukemia
Answer C (myelodysplastic neoplasm) is incorrect because the patient's blasts are significantly increased in the peripheral blood and bone marrow biopsy. Answer A (chronic myeloid leukemia) is incorrect because it can have increased blasts in the blast phase; however, the absence of BCR::ABL1 translocation rules out this diagnosis. Answer D (natural killer cell [NK] large granular lymphocytic leukemia) is incorrect because it is not characterized by increased CD34+ blasts.
Comment Here
Reference: Early T cell precursor lymphoblastic leukemia
Practice question #2
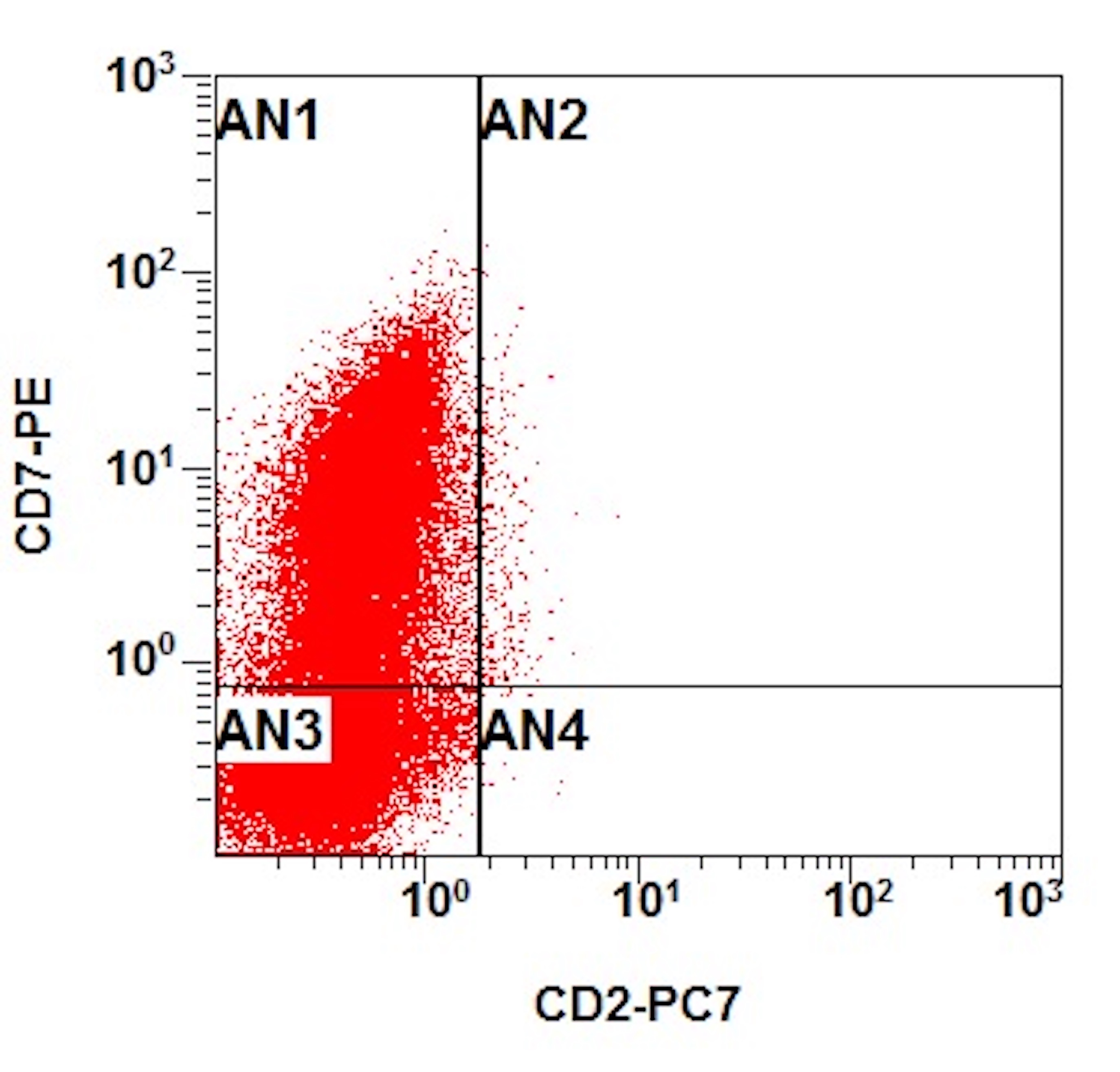
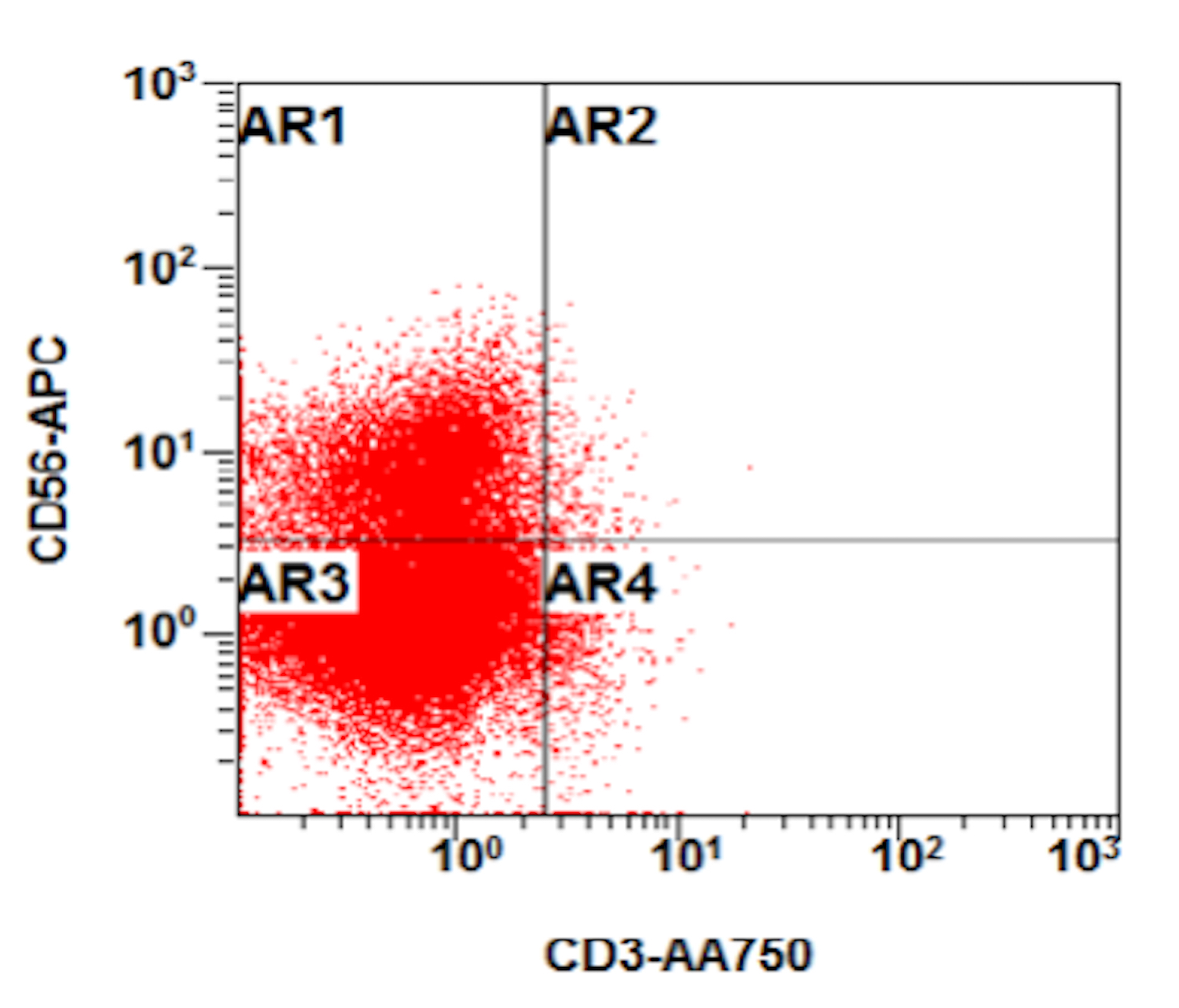
A 48 year old woman presents for evaluation of shortness of breath and fatigue over a 4 month period. She notes swelling in her neck and axillary region. Physical examination shows generalized lymphadenopathy and hepatosplenomegaly. Her complete blood count shows a white blood cell (WBC) count of 22,000, hemoglobin of 8.0 and platelet count of 100,000. A peripheral blood smear and flow cytometry on the peripheral blood are shown above. A bone marrow biopsy is performed as shown above and there is a neoplastic lymphoid population that is positive for cytoplasmic CD3, CD5 and CD34 and negative for CD1a, CD8 and myeloperoxidase. Which of the following is the most likely diagnosis?
- Adult T cell leukemia
- Early T cell precursor lymphoblastic leukemia
- Mixed phenotype acute leukemia (MPAL), T / myeloid
- Natural killer cell (NK) large granular lymphocytic leukemia
Practice answer #2
B. Early T cell precursor lymphoblastic leukemia. The patient presents with history and exam with concern for hematologic malignancy. The peripheral blood smear demonstrates atypical lymphocytes. The third figure above depicts flow cytometry revealing blasts (red) with low side scatter and dim CD45. The fourth figure shows blasts are positive for CD7 and negative for CD2. The fifth figure demonstrates blasts are positive for CD56 and dim positive for CD3. The sixth figure shows that the blasts have no expression of CD5. In the first figure, bone marrow biopsy is notable for hypercellularity. Given these findings in the setting of positive cytoplasmic CD3, CD5 and CD34 and negative CD1a, CD8 and myeloperoxidase, early T cell precursor lymphoblastic leukemia is the most likely diagnosis.
Answer C (mixed phenotype acute leukemia [MPAL], T / myeloid) is incorrect because the tumor cells in this case lack the expression of CD8 and myeloperoxidase and the immunoprofile meets criteria for early T cell precursor acute lymphoblastic leukemia. Answer D (natural killer cell [NK] large granular lymphocytic leukemia) is incorrect because it is not characterized by increased CD34 positive blasts. Answer A (adult T cell leukemia) is incorrect because the tumor cells of this entity do not express stem cell markers such as CD34.
Comment Here
Reference: Early T cell precursor lymphoblastic leukemia
Answer C (mixed phenotype acute leukemia [MPAL], T / myeloid) is incorrect because the tumor cells in this case lack the expression of CD8 and myeloperoxidase and the immunoprofile meets criteria for early T cell precursor acute lymphoblastic leukemia. Answer D (natural killer cell [NK] large granular lymphocytic leukemia) is incorrect because it is not characterized by increased CD34 positive blasts. Answer A (adult T cell leukemia) is incorrect because the tumor cells of this entity do not express stem cell markers such as CD34.
Comment Here
Reference: Early T cell precursor lymphoblastic leukemia