Table of Contents
Definition / general | Essential features | Overview | Common techniques and methods of RCA in pathology and laboratory medicine | Impact of RCA and resolution with process improvement | Videos | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Larsen MP, Le BH, Dintzis S. Causal analysis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/managementlabrootcauseanalysis.html. Accessed April 18th, 2024.
Definition / general
- Root cause analysis (RCA): a problem solving approach used to identify the principal underlying, originating (root) cause of an undesired or unintended outcome
- This topic is focused on applications in pathology and laboratory medicine
Essential features
- RCA involves structured methods to study near misses and negative events
- There are many methods of RCA analysis
- Findings are then applied to systems and processes to implement quality improvements
- While typically used retrospectively, the structured methods may be most effective when applied to near misses and used for prospective risk mitigation and process improvement
Overview
- Objective / goals of RCA: to study causes of undesired pathology and laboratory outcomes and prevent future occurrences
- An investigative tool to address a specific event, as opposed to a surveillance tool that monitors baseline activity
- Recognizes that negative outcomes are usually reflective of underlying system and process design flaws, not the actions of an individual or random event
- Allows for design and implementation of a quality improvement solution that aims to address the failure at its source
- Improves safety by reducing the risk of harm from similar future events
- Common themes for RCA in pathology and laboratory medicine:
- RCA can be performed after an unintended outcome (with harm or potential harm) and include all phases of laboratory testing
- Undesired outcomes are often a result of simultaneous failures in complex laboratory processes and have multiple root causes
- Laboratory errors may affect many patients
- RCA can be used to supplement risk assessment, quality control plans and quality control tools, in developing individualized quality control plans (IQCP) per CMS and CDC guidelines
- Roles of the pathologist in RCA:
- Investigative leader: the lead investigator must have working knowledge of laboratory testing and be able to facilitate the formulation of relevant questions, delegate responsibilities and ensure timely investigation
- Quality leader: the professional responsible for quality must have specific knowledge about critical aspects of the root cause(s) that are uncovered
- Collaborators: the lead investigator must gather information from all parties, both inside and outside of the laboratory, who may have been involved in the event which led to the incident
- References: Proc (Bayl Univ Med Cent) 2001;14:154, Appl Immunohistochem Mol Morphol 2019;27:329
Common techniques and methods of RCA in pathology and laboratory medicine
- Define the adverse event or undesired outcome
- Adverse event example: laboratory test resulted to incorrect patient
- Near miss example: labeling error identified and corrected by pathologist before test resulted to patient
- Identify all involved or knowledgeable personnel
- In the laboratory: frontline staff, support personnel, technologist, resident trainees, supervisors, managers and pathologists
- Nonlaboratory stakeholders: physicians or service lines involved (e.g., radiology, surgery), operating room (OR) staff, transport / courier staff, nurses
- Identify equipment, processes and policies that may be linked to the incident
- Gather information:
- Document objective information (who, what, where, when); question of "why" reserved for next step
- Interview and gather data from all personnel involved
- Create tools to help visually describe the components of the event (process map)
- References: Proc (Bayl Univ Med Cent) 2001;14:154, Appl Immunohistochem Mol Morphol 2019;27:329
5 "why's" method
- Iterative technique used to discover a problem source: ask "why" 5 consecutive times, each time using the previous answer as the starting point for the next question
- By the fifth "why", the root cause is often revealed
- Effective method to use when the root cause is likely internal to the laboratory (or organization)
- Example 1:
- I was late for work → why?
- Because I did not know about the accident on the highway → why?
- Because I did not turn on the radio while getting dressed → why?
- Because I woke up late and was rushing → why?
- Because I did not hear the alarm go off → why?
- Because I was out too late last night
- I was late for work → why?
- Example 2:
- The H&E stain has been suboptimal lately → why?
- Because the reagents have not been changed daily over the past week → why?
- Because the histology lab staff did not know daily routines → why?
- Because new histology staff did not receive adequate onboarding or supervision → why?
- Because there was no new staff orientation → why?
- Because the histology supervisor was absent and current operational policies and procedures do not specify who should supervise staff onboarding and orientation when the histology supervisor is absent
- The H&E stain has been suboptimal lately → why?
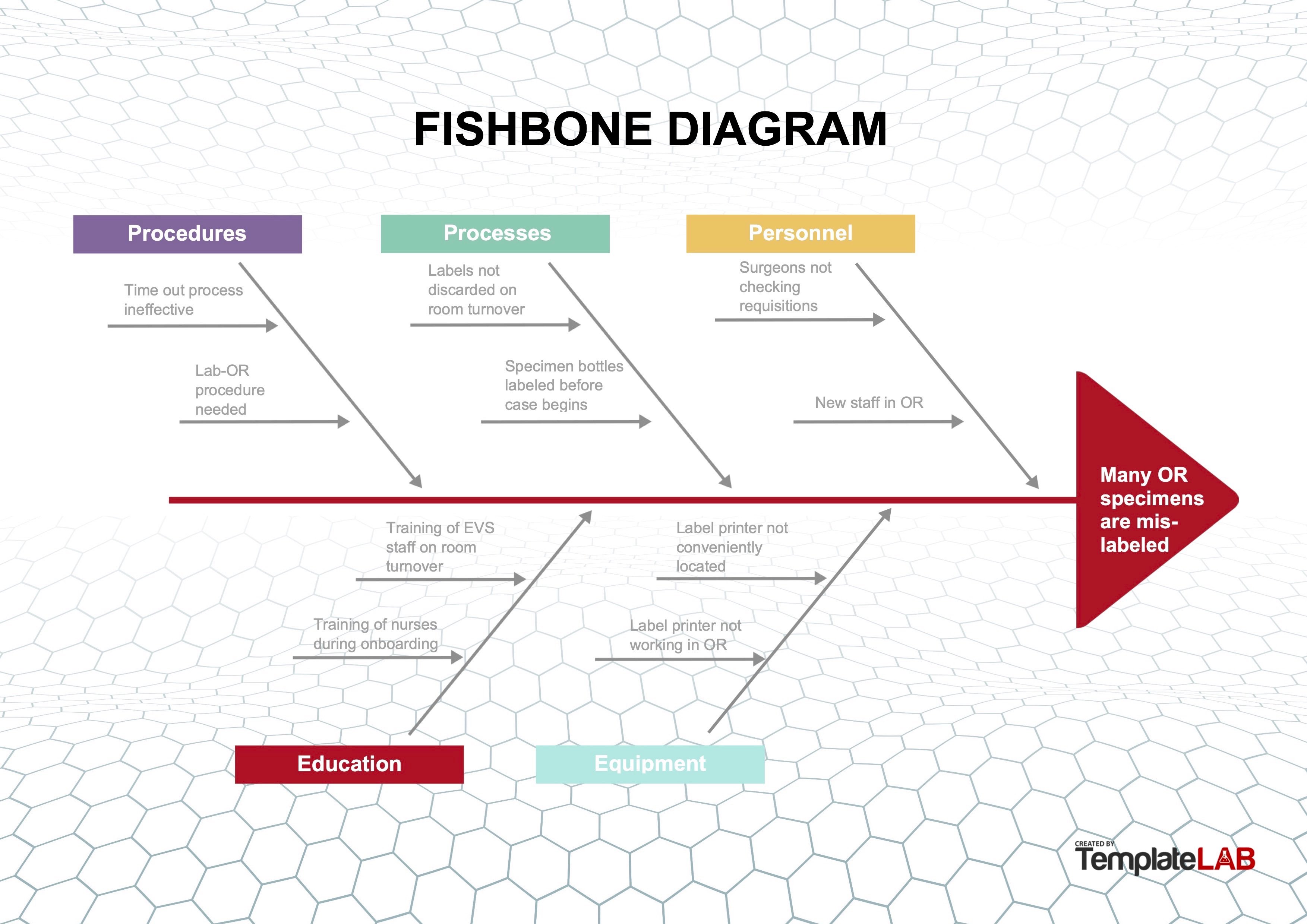
Fishbone or Ishikawa diagram
- Diagram used to identify possible causes of a problem and sort them into useful categories for investigation
- Problem to be addressed is the "head" of the fish and the categories of possible causes form the "bones," with various specific details along each of the bones
- Effective method to use when the root cause(s) is (are) deemed multifactorial or originating outside of the laboratory or department
- Example:
- During review of quality metrics, it was noted that the number of mislabeled specimens being received from the OR was above the metric threshold
- Because these specimens are critical, the standard re-education of OR staff regarding labeling was considered an insufficient measure to correct the problem
- Full RCA was undertaken
- Working group of stakeholders was convened to review the current processes; this included a pathologist, histotechnologist, specimen accessioning tech, nurse educator for the OR, circulating nurse and general surgeon
- Group implemented a fishbone diagram to review the processes and 5 areas of importance were investigated:
- Procedures: procedures for both the OR and the anatomic pathology department were reviewed and 2 major areas of potential weakness were identified
- Time out procedures for the OR required identification of the patient and procedure (operation) but did not include identification and confirmation of specimens for pathology
- Laboratory OR procedures did not detail the labeling and handoff process at each stage in relocating specimens from the OR, to the specimen drop off site, to collection of specimens, to accessioning
- Processes: a review of the OR processes showed that some staff were using shortcuts to "make things run more smoothly" or were not completing room turnover per protocol
- Specimen bottles were labeled with patient identifiers at the start of the surgery, rather than when specimens were acquired
- Prelabeled and unused bottles were not always discarded after patient surgery
- Therefore, these prelabeled bottles were at risk of being used incorrectly for a subsequent patient case
- Specimen label sheets were printed out for use at the start of a case and were not always discarded after the surgery
- Therefore, unused prelabeled specimen sheets were at risk of being used incorrectly for a subsequent patient case
- Specimen bottles were labeled with patient identifiers at the start of the surgery, rather than when specimens were acquired
- Personnel:
- Surgeons did not always perform a complete specimen count after case completion and did not sign the anatomic pathology requisition per protocol
- New personnel in the OR were not trained with standard procedures and practices
- Education:
- Onboarding of new staff in the OR and pathology required to understand the labeling and logging process for patient safety was incomplete
- Training OR staff and environmental care staff to identify material to be discarded during room turnover did not include empty labeled specimen containers or patient labels
- Equipment:
- Equipment limitations were encouraging staff to "prelabel" requisitions and containers as "shortcuts"
- Many specimen label printers were either inconveniently located outside the OR or not in working order
- Procedures: procedures for both the OR and the anatomic pathology department were reviewed and 2 major areas of potential weakness were identified
- All the above factors discovered during RCA contributed to specimen mislabeling; as a result, the following actions were taken:
- Procedures for specimen labeling, handoff and transfer were revised and clarified; all staff was retrained
- Procedures for room turnover were rewritten to emphasize items which must be discarded after case completion
- Unused patient labels and labeled specimen containers must be discarded at case completion
- Surgeons were mandated to review and sign laboratory specimen sheets at the end of a case
- IT was engaged to routinely round in the OR to check printer functionality
Contributed by Moira P. Larsen, M.D., M.B.A.
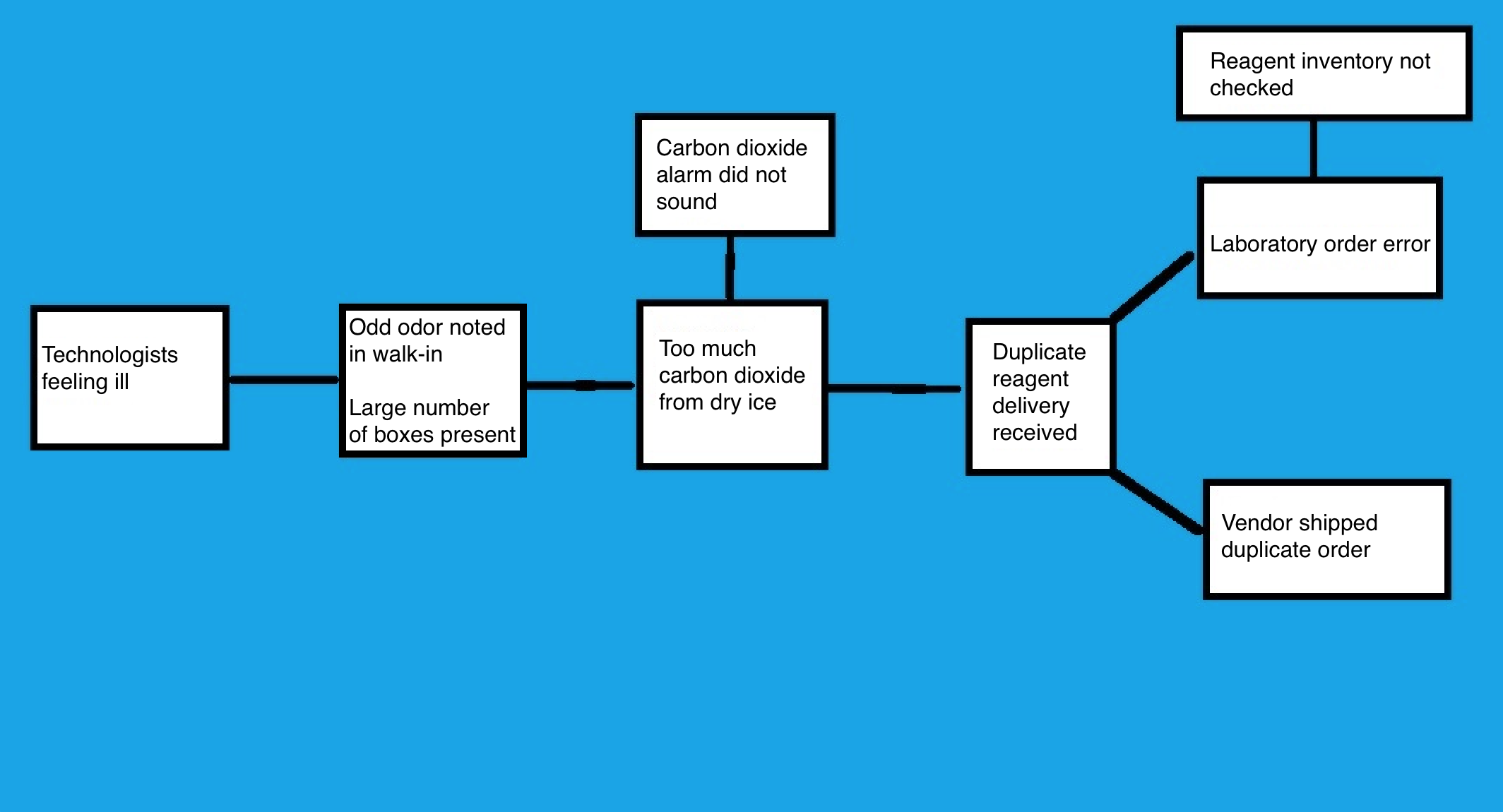
Cause map analysis
- Cause and effect diagram, similar to a fishbone diagram but organized from left to right on the page
- Start with the problem, then draw an arrow to the next box that answers the question of "why" or "was caused by"
- Continue with iterations until the root cause is revealed
- Example:
- Multiple medical technologists in the core laboratory reported to occupational health over the course of 2 days with symptoms including headaches and nausea
- Symptoms, which developed at work, were reported to laboratory leadership
- Investigation regarding the illnesses began and was conducted by the occupational health nurse, chemistry supervisor and laboratory administrative director
- Medical technologists were interviewed and all reported an odd odor in the walk-in freezer
- As the investigating team checked the freezer, they also noted the odor and began developing symptoms
- Chemistry supervisor discovered that there were twice as many reagent supply boxes in the walk-in as usual and when opened and inspected, all of the boxes contained dry ice
- Team suspected that excessive dry ice was leading to high levels of CO2 in the enclosed space and producing the symptoms
- Dry ice was removed and an RCA investigation was conducted
- Facilities were asked to check and confirm the high levels of CO2, then address the CO2 alarm
- Functional CO2 alarm should sound when CO2 levels are dangerously elevated
- Investigation revealed that the alarm had not sounded and was not functioning
- Alarm was repaired
- Chemistry supervisor contacted the vendor to determine why the standing reagent order had been doubled
- Investigation found an error in lab ordering
- Lab ordered the supplies, as inventory had not been appropriately checked
- To prevent recurrence of this error, additional logs and inventory management were implemented
Contributed by Moira P. Larsen, M.D., M.B.A.
- Multiple medical technologists in the core laboratory reported to occupational health over the course of 2 days with symptoms including headaches and nausea
Impact of RCA and resolution with process improvement
- Identification and resolution of the underlying cause of sentinel event
- Framework for working with other departments to evaluate events as process issues, avoid personal accusations and allow the development of robust solutions
- Pathology example: RCA investigation of mislabeled specimens from the OR revealed → incomplete room turnover, resulting in → leaving patient labels from the previous case in the room to potentially be used in error, leading to → the addition of label disposal process to room turnover checklist
- Intradepartmental studies place emphasis on process rather than the individual facilitating sharing of details
- Laboratory medicine example: RCA investigation of the release of incorrect type blood revealed → safety risks in test tube handling and manual computer data entry when working on multiple first time patient specimens in the same test tube rack, leading to → implementation of single patient flow process for new patients with no transfusion history
- Framework for working with other departments to evaluate events as process issues, avoid personal accusations and allow the development of robust solutions
- Identification of underlying systemic problems and issues
- Focus on the process allows for development of "just culture" and self reporting of events before they become sentinel events
- Routine review of reported internal events at quality meetings to look for patterns, for example:
- Standard operating procedure violations
- Preanalytic errors: mislabeled specimens, label printer malfunctions, incorrect specimen tube selection
- Technical interpretation errors: Gram stain culture mismatch rate, identification of crystals in synovial fluid
- Manual data entry errors for noninterfaced tests: pregnancy tests, COVID-19 assays, point of care test results
- Prevent future new and recurrent adverse events
- Develop acute sense of what could go wrong to allow for proactive process improvement
- Constant assessment of preanalytic, analytic and postanalytic processes to allow for the avoidance of human error
- Pathology example: assuring that when accessioning and grossing specimens, the same specimen types (i.e., colon biopsy, tonsils, placenta) are separated by different specimen types to reduce risk of mix up
- Laboratory medicine example: require second technologist review and sign off on manual entry of COVID-19 test results that are not interfaced to avoid errors
- Risk mitigation
- Increased attention to potential errors with proactive process improvement efforts reduces risk
- Rapid cycle changes to evaluate possible solutions and monitor impact thus generating the best solutions (Kaizen events)
- Culture shift from personal blame to process improvement leads to development of "just culture"
- Breaks down hierarchy
- Makes staff comfortable speaking up for safety
- Includes self reporting of errors made
- Optimization of patient safety, compliance and operational efficacy
- Ultimately, culture of continuous process improvement reduces serious safety events
- Allows for more attention to be paid to the work at hand
- More efficient operations with fewer interruptions and corrections
- High quality and compliant work and test resulting
- Right test at the right time for the right patient allows safer patient care and more efficient flow through the healthcare system
- References: Proc (Bayl Univ Med Cent) 2001;14:154, Appl Immunohistochem Mol Morphol 2019;27:329
Videos
5 why's technique
Fishbone diagram
RCA2
Additional references
- TemplateLAB: 25 Great Fishbone Diagram Templates & Examples [Accessed 4 February 2022], PSNet: Root Cause Analysis [Accessed 4 February 2022], Cancer Cytopathol 2017;125:79, Institute for Healthcare Improvement: RCA2 - Improving Root Cause Analyses and Actions to Prevent Harm [Accessed 4 February 2022], Institute for Healthcare Improvement: 5 Whys - Finding the Root Cause [Accessed 7 July 2022], Institute for Healthcare Improvement: Cause and Effect Diagram [Accessed 7 July 2022], ThinkReliability: Cause Mapping [Accessed 7 July 2022]
Board review style question #1
Which of the following scenarios is least likely to benefit from a root cause analysis?
- Addressing the cause of blood product administration to the wrong patient
- Daily monitoring of personnel punctuality to ensure adequacy of shift coverage
- Explaining the reason for difficulties in personnel recruitment and retention
- Increased turnaround time for surgical pathology reports
- Investigating a complaint regarding frequent specimen mislabeling
Board review style answer #1
B. Daily monitoring of personnel punctuality to ensure adequacy of shift coverage. RCA is most practical when used as an investigative tool to uncover and address the chief underlying reason(s) for an undesired event, trend or outcome. It has less utility as a proactive surveillance tool. The daily monitoring of personnel punctuality to ensure adequacy of shift coverage is an ongoing surveillance activity; unless a negative trend is discovered, RCA is not likely to be directly applicable. The underlying reason (root cause) for increased turnaround time in surgical pathology, specimen mislabeling or incorrect blood product administration may originate from systemic issues beyond what may be immediately visible and thus these scenarios may benefit from RCAs. Similarly, difficulties in personnel recruitment and retention may be due to reasons other than labor market forces (such as institutional / departmental reputation, conflicting personalities, safety concerns, onboarding problems, human resource conflicts) and as such, may benefit from RCA.
Comment Here
Reference: Causal analysis
Comment Here
Reference: Causal analysis
Back to top