Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Duarte-Neto AN. Candida auris. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/microbiologycauris.html. Accessed April 25th, 2024.
Definition / general
- Candida auris is an emergent, multidrug resistant fungal pathogen that causes infections with a high mortality rate; first described in Japan in 2009 (Arch Pathol Lab Med 2020;144:107, J Clin Microbiol 2011;49:3139)
Essential features
- Candida auris has a similar morphology to other Candida species in infected tissues, except C. glabrata, which does not form pseudohyphae or hyphae (Mycoses 2018;61:377)
- Incidence in the U.S. has been rising with outbreaks in healthcare facilities
ICD coding
Epidemiology
- Outbreaks of C. auris have happened in the U.S. since 2016, after the introduction of multiple strains from different continents; since then, local transmission has taken place (MMWR Morb Mortal Wkly Rep 2016;65:1234, Lancet Infect Dis 2018;18:1377)
- Major outbreaks occurred in Illinois, Chicago, New York and New Jersey (MMWR Morb Mortal Wkly Rep 2020;69:6, Ann Intern Med 2021;174:1554)
- Risks for C. auris infection include (MMWR Morb Mortal Wkly Rep 2017;66:514)
- Immunosuppression (malignancy, chemotherapy, neutropenia, high doses of corticosteroids, AIDS, chronic underlying diseases)
- Prolonged intensive care stays
- Abdominal surgery and anastomotic leak
- Pancreatitis
- Hemodialysis
- Use of broad spectrum antibiotics and azoles (previous fluconazole treatment)
- Total parenteral nutrition
- Injection of illicit drugs
- Preventive measures for healthcare infection transmission, such as contact isolation for colonized or infected patients and laboratory diagnostic surveillance to determine species and antifungal susceptibility / resistance in Candida isolates (MMWR Morb Mortal Wkly Rep 2017;66:514)
Sites
- Candida auris can infect any organ from any body system
- Bloodstream and disseminated infection must be investigated when C. auris is isolated from any sample (Clin Microbiol Rev 2017;31:e00029)
Pathophysiology
- Candida auris infection starts with colonization, followed by tissue invasion and then reaches the bloodstream
- Candida auris forms biofilm on catheter device surfaces (insertion or hub)
- Total parenteral nutrition is rich in lipid emulsions, which enhances the biofilm formation; broad spectrum antibiotics and intestinal / biliary surgery alter normal flora with Candida spp. overgrowth, predisposing to its intestinal translocation, followed by bloodstream dissemination
- Host factors predispose to disseminated C. auris infections (e.g., immune dysfunction [neutropenia, lymphopenia, denutrition, etc.] and mucositis after chemotherapy) (Clin Microbiol Rev 2017;31:e00029)
Etiology
- Retrospective study has shown the first Candida auris isolate is from South Korea
- Sequencing of internal transcribed spacer (ITS) and D1 / D2 regions of ribosomal DNA has shown C. auris is similar to C. haemulonii and C. pseudohaemulonii in < 88% and 85%, respectively
- C. auris is geographically grouped into 4 clades: East Asia, South Asia, Africa and South America
- Molecular profiles of sequenced strains are more linked with strains from the same country (Clin Microbiol Rev 2017;31:e00029)
Clinical features
- Invasive healthcare associated infections with high mortality
- Pneumonia
- Vascular device associated bloodstream infections
- Skin lesions (papules, ulcers) in disseminated disease
- Pyelonephritis
- Biliary tract infections
- Inhospital sepsis
- Septic shock
- Panophthalmitis in immunocompromised host (AIDS) (Am J Ophthalmol Case Rep 2020;19:100738)
Diagnosis
- Definitive diagnosis of C. auris infection is performed with sequencing of 18S ITS regions or D1 / D2 regions of ribosomal DNA
- Real time polymerase chain reaction (RT PCR) may have high diagnostic accuracy
- Proteomic methods, such as MALDI TOF MS, can be useful
- Significant overlap with other Candida species on cultures, phenotypic and biochemical diagnostic systems (Clin Microbiol Rev 2017;31:e00029)
Laboratory
- Patients with C. auris infection may have neutropenia, anemia, lymphopenia, elevated C reactive protein or signs of multiorgan system failure with altered markers for organ dysfunction (azotemia, hypoxemia, elevated bilirubin, etc.) (Clin Microbiol Rev 2017;31:e00029)
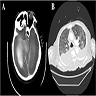
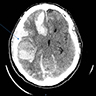
Radiology description
- Nonspecific
- Abscesses, pneumonia, pyelonephritis
- Infective foci suggestive of hematogenous spread
Prognostic factors
- Candida auris infection has a poor prognosis in general
Case reports
- 61 year old immunocompetent man with Candida auris candidemia after posttraumatic brain injury (Cureus 2020;12:e8850)
- 71 year old Japanese man with COVID-19 presented with Candida auris candidemia (J Infect Chemother 2023;29:713)
- First 7 reported cases of Candida auris in the U.S. (MMWR Morb Mortal Wkly Rep 2016;65:1234)
Treatment
- Echinocandins
- C. auris has a higher minimum inhibitory concentration (MIC) than other Candida species
- Lipid formulation amphotericin (Clin Microbiol Rev 2017;31:e00029)
- Often multidrug resistant
Gross description
- Tissue necrosis, abscesses, mucosal ulcers, mucosa covered with whitish or yellowish fibrinous exudate (Emerg Microbes Infect 2020;9:1160, Clin Microbiol Rev 2017;31:e00029)
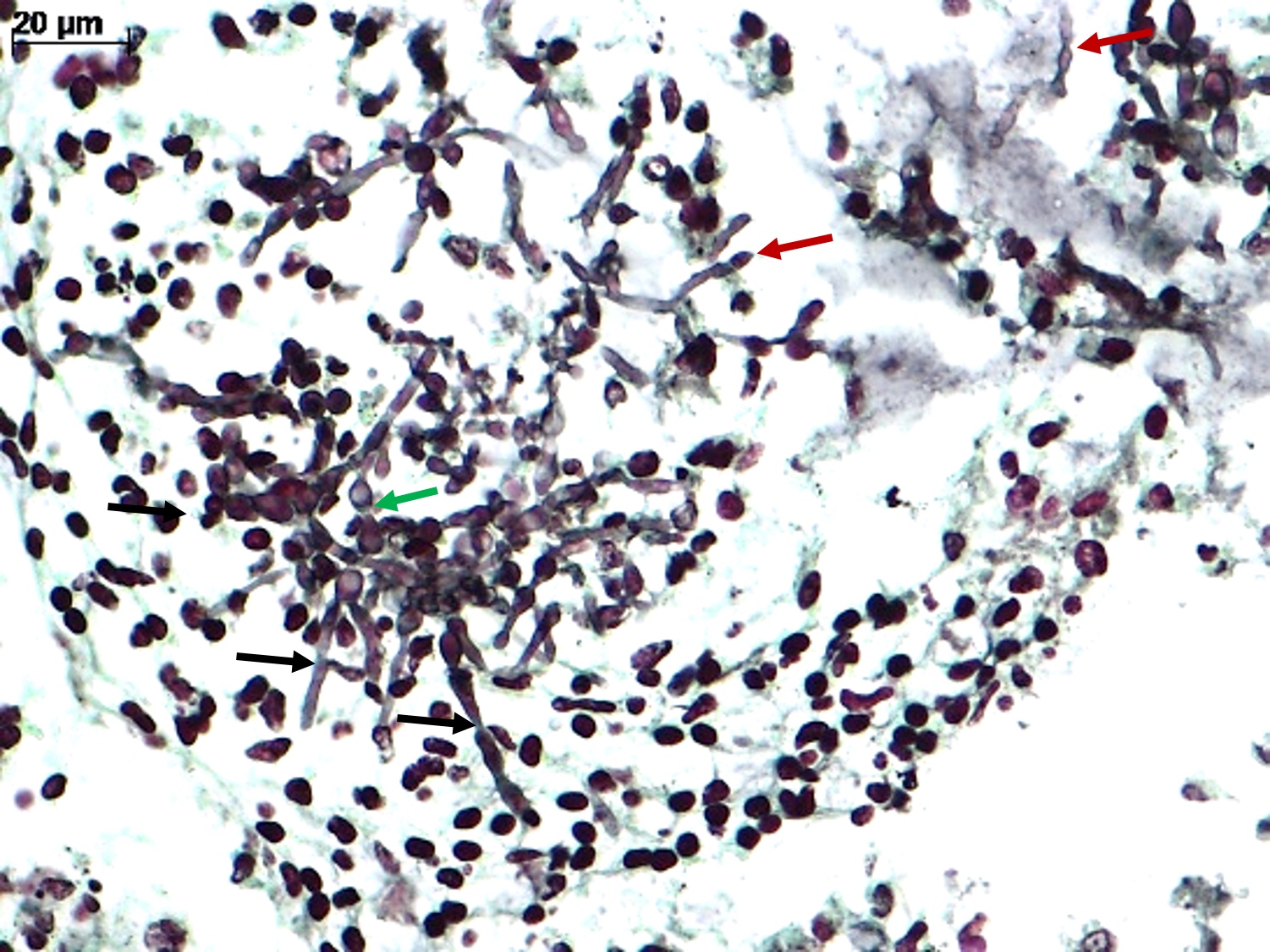
Microscopic (histologic) description
- Spores, pseudohyphae and hyphae (Mycoses 2018;61:377, Clin Microbiol Rev 2011;24:247)
- Yeasts measure 3 - 5 μm in diameter
- Candida auris has a similar morphology to other Candida species in infected tissues, except C. glabrata, which does not form pseudohyphae or hyphae
- Yeasts measure without capsule and with narrow neck budding
- Hyphae with erratic ramification (in general, < 45°) and absence of septa
- Tissue inflammatory reaction: variable, depending on host immune status; in general, necrosis, cell debris and mixed inflammatory reaction, with neutrophils, microabscesses
- Invasion of mucosa and vessels (angioinvasion); Candida invasive form: hyphae (Emerg Microbes Infect 2020;9:1160)
- Colonization of mucosa (mucosal surface, amid mucous, fibrinous exudate and cell debris), without epithelial invasion (Emerg Microbes Infect 2020;9:1160)
Microscopic (histologic) images
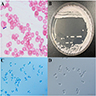
Cytology description
- Round to ovoid, isolated or grouped cells on smear; this method is not species specific and cannot differentiate colonization from invasive infection
Positive stains
- Candida auris has a similar histochemical profile to other Candida species: gram positive, PAS positive and argyrophilic yeasts; amphophilic on hematoxylin and eosin (H&E)
- Silver stains (such as Grocott-Gomori) can give more details about form and budding of the yeast
- Fuchsin (acid fast stains) may stain irregular fungal structures (Emerg Microbes Infect 2020;9:1160, Clin Microbiol Rev 2011;24:247)
Negative stains
- Mucicarmine: Candida spp. do not have cell capsule with mucopolysaccharide
- Fontana-Masson: Candida spp. do not express melanin on the cell wall (Emerg Microbes Infect 2020;9:1160, Clin Microbiol Rev 2011;24:247)
Electron microscopy description
- Ovoid cells with chlamydospore with bilayered cell wall formed by an outer electron transparent primary layer and an inner electron dense secondary layer; single large vacuole, several smaller vacuoles and cytoplasmic organelles (J Gen Microbiol 1981;125:199, J Electron Microsc (Tokyo) 2012;61:343)
Electron microscopy images
Molecular / cytogenetics description
- Multiplex PCR setup using specific primers for glycosylphosphatidylinositol (GPI) protein encoding genes; it is applicable for fluids and fresh tissue samples (Int J Med Microbiol 2018;308:812)
Sample pathology report
- Any tissue, biopsy or autopsy:
- Candida infection (see comment)
- Comment: The fungal structures show yeasts, pseudohyphae and hyphae forms, with single narrow neck budding, with (or without) angioinvasion.
Differential diagnosis
- Cryptococcus spp. (Clin Microbiol Rev 2011;24:247):
- Measure 4 - 10 μm in diameter
- May form germinative tube in highly proliferative infections
- Small yeasts in small tissue samples (e.g., pulmonary biopsies) may not produce a large capsule or mucopolysaccharide, rendering the diagnosis difficult
- Fontana-Masson stain may be helpful (stain melanin on Cryptococcus capsule)
- Histoplasma capsulatum (Clin Microbiol Rev 2011;24:247):
- Small yeasts (measure 2 - 4 μm in diameter) in small samples can be problematic for diagnosis
- Histoplasma spp. may have a central black dot on the Grocott stain
- Thick pseudocapsule on H&E may be helpful
- Malassezia furfur (Clin Microbiol Rev 2011;24:247):
- Fungic structures with spaghetti and meatball appearance on the stratum corneum of the skin
- Hyphomycetes (Clin Microbiol Rev 2011;24:247):
- Wide hyphae with spores and pseudohyphae
- Isolated conidia of Aspergillus in small lung biopsies may be difficult when it is not possible to identify Aspergillus conidial heads; those conidias are amphophilic on H&E, Grocott positive, PAS positive and gram positive, mimicking Candida spp. spores, mainly C. glabrata
Additional references
Board review style question #1
A premature newborn (33 weeks of pregnancy) was hospitalized in the intensive care unit after birth with a low Apgar score and respiratory insufficiency. He was maintained on mechanical ventilation; central venous lines were set and he was fed with a nasogastric tube. On the eighth day, he developed abdominal distension, had bloody stool and was diagnosed with necrotizing colitis, which required large spectrum antibiotics and surgical treatment. He was then started on total parenteral nutrition. After a week, the newborn developed a new sepsis with alveolar - perivascular infiltrates on the lungs. A bronchoalveolar lavage showed mixed inflammatory infiltrate surrounding structures, which are shown in the image above. What is the most likely etiological agent of this sepsis?
- Aspergillus spp.
- Candida spp.
- Gram positive cocci
- Histoplasma capsulatum
Board review style answer #1
B. Candida spp. There are spores, pseudohyphae and hyphae in the figure. Candida spp. are the most common nosocomial fungal infections and their main characteristic is forming spore, pseudohyphae and hyphae in tissues. Answer D is incorrect because H. capsulatum is a yeast in tissue and rarely produces pseudohyphae, even in cases with high fungal burden; moreover, it is not a common fungal nosocomial infection. Answer C is incorrect because gram positive cocci do not have a yeast-like aspect in tissues, as they are much smaller, without budding. Gram positive cocci form colonies in tissues. Answer A is incorrect because Aspergillus spp. are hyaline hyphae fungal agents, with acute dichotomous branching and multiple regular septa. Candida spp. can form hyphae but they are associated with spores and pseudohyphae. The Candida spp. hyphae are amphiphilic, randomly branching and without septa.
Comment Here
Reference: Candida auris
Comment Here
Reference: Candida auris
Board review style question #2
A man with idiopathic pulmonary fibrosis requiring high dose corticosteroids was hospitalized with fever and sepsis. The blood culture identified Candida spp. and in a few days, the strain was identified as C. auris by sequencing the D1 / D2 region of the 28s ribosomal DNA. In parallel, the patient developed bilateral pulmonary infiltrates with respiratory insufficiency and was put under mechanical ventilation. A bronchoalveolar lavage and biopsy were performed, showing round to oval yeasts, with thick walls and narrow based single budding, associated with mononuclear inflammatory reaction. Rare forms showed germinative tubes. Which stain should be requested to confirm the etiology of this fungal infection?
- Gram stain
- Mucicarmine stain
- Von Kossa stain
- Ziehl-Neelsen stain
Board review style answer #2
B. Mucicarmine stain. Cryptococcus spp. has mucicarmine positive capsule. It causes opportunistic infections in patients who receive high doses of corticoids, mainly pneumonia and meningitis. Cryptococcosis can occur with other opportunistic infections in the same patient. When there is high C. neoformans tissue burden, some yeasts can form germ tubes, which mimic Candida spp. pseudohyphae. Answer A is incorrect because Gram stain can label all fungal species (as they are all gram positive) and does not give a specific diagnosis. Answers C and D are incorrect because these stains (von Kossa and Ziehl-Neelsen) do not stain fungal forms.
Comment Here
Reference: Candida auris
Comment Here
Reference: Candida auris