Table of Contents
Definition / general | Essential features | Diagrams / tables | Clinical features | Uses by pathologists | Prognostic factors | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Board review style question #1 | Board review style answer #1Cite this page: Ding Y. KRAS. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/molecularKRAS.html. Accessed April 19th, 2024.
Definition / general
- Kirsten RAt Sarcoma virus (KRAS) gene on chromosome 12p12.1
- Also known as K-RAS or K-Ras
- Member of Ras family (also includes NRAS, HRAS)
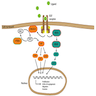
- Oncogene that acts as part of the RAS-MAPK pathway; KRAS protein includes 189 amino acids and belongs to the small GTPase superfamily
- Germline mutations are associated with cardiofaciocutaneous syndrome, Costello syndrome, Noonan syndrome and combinations of these syndromes (OMIM: Cardiofaciocutaneous Syndrome 1; CFC1 [Accessed 24 March 2022], OMIM: Costello Syndrome; CSTLO [Accessed 24 March 2022], OMIM: Noonan Syndrome 1; NS1 [Accessed 24 March 2022], Mol Cell Biol 2007;27:7765, Eur J Pediatr 2009;168:919)
Essential features
- KRAS is part of the RAS / MAPK pathway and plays critical roles in cell growth, proliferation and differentiation (Semin Cell Dev Biol 2016;58:127)
- Activating mutations in KRAS are driver mutations in various malignancies, with lung adenocarcinoma, pancreatic adenocarcinoma and colorectal carcinoma having the highest prevalence of KRAS mutations (Chin J Cancer 2013;32:63, Lancet 2016;388:73, J Hematol Oncol 2020;13:130, Swiss Med Wkly 2010;140:w13112)
Clinical features
- KRAS is one of the most frequently mutated oncogenes in human malignancies
- It took many years of drug discovery efforts before breakthrough achieved with covalent inhibitors targeting the KRAS G12C mutation
- Generally, mutually exclusive with EGFR mutations and ALK fusions
- Non small cell lung carcinoma (NSCLC):
- KRAS mutations account for 25 - 30% of driver mutations in lung adenocarcinomas
- Histologic association: usually less well differentiated, mucinous histotype
- Clinical association: strong association with smoking history in Western countries but less common in Asia (in contrast to EGFR mutations)
- NSCLC with KRAS G12C is currently considered a poor prognostic factor
- On 28 May 2021, the U.S. Food and Drug Administration approved Lumakras (sotorasib) as the first drug to target KRAS G12C mutation in adult patients with NSCLC (FDA: FDA Approves First Targeted Therapy for Lung Cancer Mutation Previously Considered Resistant to Drug Therapy [Accessed 27 October 2021])
- Colorectal carcinoma (CRC):
- KRAS mutations are early events in CRC formation, detected in ~45% of CRCs
- Tumors with a mutation in exons 2, 3 or 4 of either KRAS or NRAS genes are essentially insensitive to cetuximab or panitumumab therapy
- RAS (KRAS / NRAS) genotyping of tumor tissue in all patients with metastatic CRC is strongly recommended
- Other tumors:
- KRAS mutations are seen in up to 90% of pancreatic adenocarcinoma, with most frequent mutations: G12D > G12V > G12R > Q61H
- KRAS mutations occur early during carcinogenesis and are associated with poor prognosis (Cancer 2007;109:1561)
- KRAS mutations are seen in 11% of papillary thyroid carcinoma
- RAS (including KRAS) mutations are identified in approximately 15% of acute myeloid leukemia, 11% of adult T lineage acute lymphoblastic leukemia (T-ALL) and 30 - 40% of multiple myeloma
- Ovarian endometriosis associated low grade endometrioid adenocarcinomas (29%) (Hum Pathol 2012;43:1177)
- KRAS mutations are seen in up to 90% of pancreatic adenocarcinoma, with most frequent mutations: G12D > G12V > G12R > Q61H
Uses by pathologists
- Identification of KRAS mutation status
Prognostic factors
- KRAS mutation in lung cancer generally conveys a poor prognosis and resistance to EGFR tyrosine kinase inhibitor therapy
- Targeted therapy toward KRAS G12C mutated NSCLC can prolong survival
Molecular / cytogenetics description
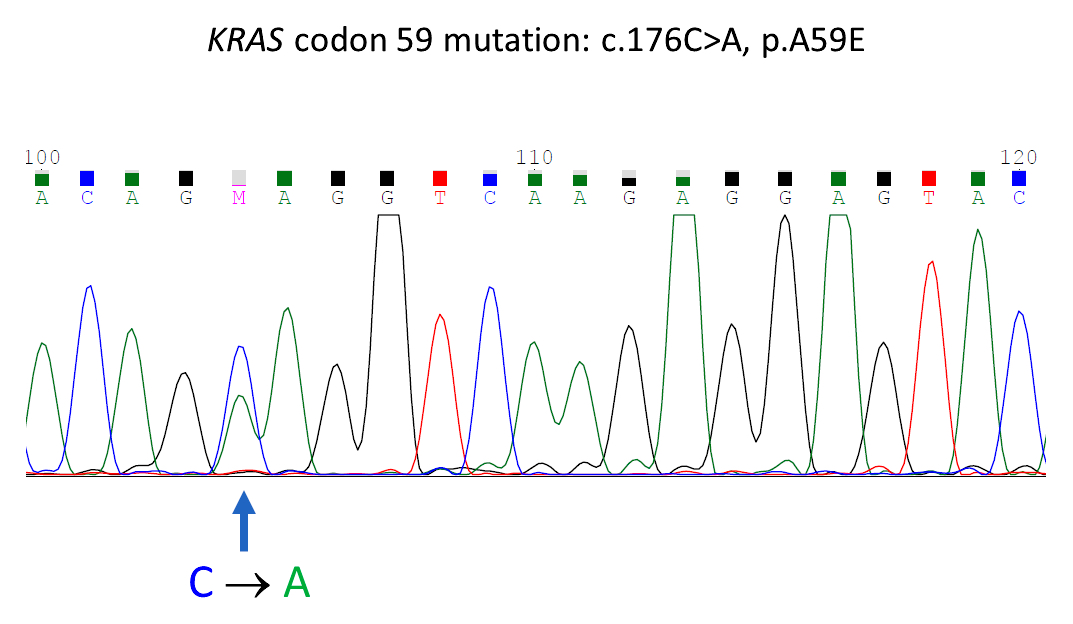
- KRAS mutations are usually detected by molecular technology, including PCR, Sanger sequencing and next generation sequencing (NGS) (BMC Med Genomics 2019;12:162, Curr Mol Med 2015;15:418)
- Utility of immunohistochemistry (IHC) as surrogate marker for detection of KRAS mutations is still under investigation
Sample pathology report
- Brain, biopsy:
- Metastatic adenocarcinoma consistent with lung primary (see comment)
- KRAS gene mutation G12C detected, resulting in p.Gly12Cys. Nucleotide change of c.34G>T. (Block A1 used for testing.)
- Comment: Microscopic examination of the tissue block used for KRAS mutation analysis shows ≥ 10% viable tumor.
- Clinical significance:
- The KRAS gene encodes the human cellular homolog of a transforming gene isolated from the Kirsten rat sarcoma virus. Normally, wild type (WT) KRAS plays a critical role in cellular signaling processes, including proliferation, differentiation and senescence.
- Mutant KRAS is a potential oncogene that is found in many human cancers. In non small cell lung cancers (NSCLC), KRAS mutations are common oncogenic drivers and generally exclusive of other driver mutations in genes such as EGFR, ALK and ROS1. KRAS mutations are often early truncal mutations that can persist during disease progression. Approximately 20% of human tumors have activating RAS point mutations, mainly in codons 12, 13 or 61. KRAS G12C is the single most prevalent emerging biomarker in NSCLC and is detected in approximately 13% of such cases. Patients with NSCLC being considered for KRAS G12C inhibitor therapy (such as sotorasib) should have tumor tissue genotyped for its mutation status. Please refer to ClinicalTrials.gov for more information regarding clinical trials targeting KRAS mutations.
- Methodology:
- Tissue sections are reviewed by a pathologist and relevant tumor is selected for analysis. FFPE tissue section(s) or extracted DNA is tested using the ____ KRAS assay (real time PCR assay) tested on the _____ system. This assay detects KRAS G12C mutation in exon 2.
- Clinical indications for use of this assay include but not limited to: (1) guideline recommended mutation evaluation for metastasized non small cell lung cancers; (2) evaluation of potential gene targets for possible clinical trials; (3) interrogating actionable mutations in other human tumor types or poorly differentiated tumor / uncertain origin based on clinical pathological evaluation. The lower limit of detection (LLoD) of this assay is 5% allelic frequency in a background of wild type genomic DNA.
- The performance characteristics of this assay have been validated by the Molecular Diagnostics Laboratory at ___________. It has not been cleared or approved by the FDA. The laboratory is regulated under CLIA as qualified to perform high complexity testing. This test is used for clinical purposes. It should not be regarded as investigational or for research.
Board review style question #1
Which of the following statements regarding KRAS G12C mutation in non small cell lung cancer (NSCLC) is correct?
- More commonly seen in Asians
- Lung adenocarcinoma with mucinous features is a reliable predictor for KRAS G12C mutation
- Never smokers are significantly more likely to have G>A transition mutation, as seen in KRAS G12C
- G>T transversion, a known tobacco smoke induced mutation, is the most common nucleotide change in former and current smokers, as seen in KRAS G12C
- NSCLC with KRAS G12C is a favorable prognostic factor
Board review style answer #1
D. G>T transversion, a known tobacco smoke induced mutation, is the most common nucleotide change in former and current smokers, as seen in KRAS G12C. KRAS G12C is less common in Asians (A). Lung adenocarcinoma with mucinous features is associated with but not a reliable predictor for KRAS G12C mutation (B). Never smokers are significantly more likely to have G>A transition mutation, as seen in KRAS G12D (C). NSCLC with KRAS G12C is currently considered a poor prognostic factor (E).
Comment Here
Reference: KRAS
Comment Here
Reference: KRAS